Notes
Article history
The research reported in this issue of the journal was commissioned by the HTA programme as project number 02/37/03. The contractual start date was in May 2005. The draft report began editorial review in December 2009 and was accepted for publication in July 2010. As the funder, by devising a commissioning brief, the HTA programme specified the research question and study design. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© 2011 Queen’s Printer and Controller of HMSO. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2011 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Venous leg ulcers
Leg ulceration is a chronic, recurring condition, affecting 15–18/1000 adults in industrialised countries,1 with venous leg ulcers representing up to 84% of all leg ulcer cases in developed countries. 2 Venous insufficiency occurs when the foot or calf muscle pumps are unable to empty the veins effectively, causing the pressure within the veins and capillaries to rise above normal limits. 3 This raised pressure leads to numerous changes in the capillaries and surrounding tissues, manifested by signs and symptoms such as venous flare, oedema, and hardening and staining of the dermal tissue (lipodermatosclerosis), the progressive stages of which can eventually lead to ulceration. 4 These ulcers have a significant personal impact on health and quality of life (QoL). 2,5
Treating venous leg ulcers
The only therapy so far shown to be clearly effective in the treatment of venous leg ulcers is the application of compression therapy, as either bandaging or hosiery, with high compression (around 40 mmHg pressure at the ankle) being more effective than lower levels of compression [relative risk of healing 1.5, 95% confidence interval (CI) 1.2 to 2.0]. 6 We know from both trials of compression therapy and other prognostic studies that small ulcers [< 5 cm2 in area] and new ulcers (< 6 months in duration) treated with high compression heal quickly. For example, in a large trial of compression bandaging the median time to healing of ulcers with pre-trial duration of < 6 months was 77 days. 7 New ulcers treated with high compression, therefore, can be described as generally healing without the need for adjuvant therapies. One high-quality prognostic study has found that 95% of venous ulcers that are both small (< 5 cm2 in area ) and new (< 6 months in duration), if treated with high compression, can be expected to heal within 6 months (95% CI 75% to 99%). 8 Audits of healing times using four-layer high compression (widely used in the UK) confirm the importance of ulcer area and duration in predicting healing at 6 months. 9,10 One of the remaining clinical challenges is how to increase healing in those ulcers that are not ‘easy to heal’ (i.e. the big/old ulcers rather than the small/new ulcers). The goal is both to increase the proportion of ulcers healed (20% remained unhealed in a large bandaging trial at 12 months)7 and to decrease the time to healing among people with longstanding ulceration or large ulcers.
Cost of venous leg ulceration
Leg ulceration is a condition associated with large financial costs to health-care providers. The cost of leg ulcer management in the UK in 1989 was estimated to be between £150M and £600M per annum, with > 60% of this cost attributed to community-based nursing services. 11 Recent studies have estimated the annual cost of treating a venous leg ulcer patient as being approximately £700–900, which increases the longer it takes for the ulcer to heal or the larger it becomes. 12,13 The 2004 Healthcare Commission estimated that NHS leg ulcer treatments cost £300–600M per annum and wound dressings accounted for 5 million community prescriptions in England during 2006 at a cost of £122M. 14 With the majority of leg ulcer patients in the UK treated within the community,15 such patients often constitute a large proportion of community nurses’ caseloads. 16
Therapeutic ultrasound
Ultrasound therapy is a well-recognised treatment option for soft tissue injuries in the physiotherapy clinic, but has been more recently used in some specialist centres for the management of chronic wounds. 3
Mechanical vibrations transmitted at a frequency > 20,000 Hz are above the level of human hearing and are known as ultrasonic. 17 Ultrasound may be divided into two classes: those using ‘high’ intensities (> 3 W/cm2), used for ultrasonic scalpels, and those using ‘low’ intensities (up to 3 W/cm2), used to stimulate normal physiological responses to injury to aid repair. 18 The type of ultrasound used is dependent on the target tissues (structure and depth) and the intended effect (i.e. heating the tissues or not). Tissues with a higher protein content (e.g. ligament and tendon) are able to absorb ultrasound to a greater extent than those with a low protein content (e.g. blood and fat). 19 Therapeutic ultrasound has a frequency of 0.75–3.00 MHz, and most machines used to deliver it are set at a frequency of either 1 or 3 MHz. 20 The absorption coefficient of ultrasound in soft tissue increases linearly with frequency, so using higher frequencies (say 3 rather than 1 MHz) reduces the penetration depth by about one-third (from 37 to 12 mm in skin). 21
Presumed mechanisms of action
There are two types of mechanisms commonly thought to explain the effects produced by therapeutic ultrasound, and these are classed as thermal and non-thermal effects,18 although ter Haar18 and Baker et al. 21 argue that both effects may be present to varying degrees. They agree that it is difficult to identify the mechanisms involved, let alone separate the thermal and non-thermal effects. Investigators have described the effects of ultrasound on tissues in vitro and in vivo, but it is not clear whether these are responsible for reported clinical effects, or merely incidental.
Many physiological responses to the biophysical effects of therapeutic ultrasound have been described, and the research has been reviewed by Baker et al. 21 and others. 18,20
When ultrasound travels through tissue, a percentage of it is absorbed, which leads to the generation of heat within the tissue. 19 The beneficial effects thought to arise from this heating include an increase in blood flow, reduction in muscle spasm, increased extensibility of collagen fibres and a proinflammatory response. 20 Any problem of excess heat is reduced by using pulsed ultrasound (which has an on/off cycle), as the effective intensity is lower, with the heat being dissipated between the pulses. 22 While ultrasound has historically been used primarily for its professed thermal effects, it is suggested that ‘non-thermal’ mechanisms play a role in producing therapeutic effects. 19 Watson19 concludes that although the therapeutic benefits of tissue heating are well known, the ability of ultrasound to generate sufficient thermal change in tissues to achieve these effects is doubtful.
Purported non-thermal mechanisms are predominantly attributed to ‘cavitation’ – the production and vibration of micron-sized bubbles within the tissue fluids which, as the bubbles move and oscillate within the fluids, can cause changes in the cellular activities of the targeted tissues. 17 An additional outcome of ultrasound is ‘acoustic streaming’, which is described as the ‘localised liquid flow in the fluid around the vibrating bubble’. 22 Baker et al. ,21 however, argue that there is no evidence from in vivo studies in humans that cavitation occurs at the ultrasound doses used for tissue repair. Given the absence of cavitation, except in gas-filled cavities (such as the lungs), it is further argued that acoustic streaming does not occur in vivo. The way in which cavitation or acoustic streaming might contribute to tissue repair is not obvious, but it is postulated that they might lead to a reversible increase in cell membrane permeability and increased protein synthesis. 17
Ultrasound application
Ultrasound is reflected at the skin, as the air–skin interface presents a barrier to the transmission of ultrasound; thus, it is necessary to provide a coupling medium to allow the ultrasound to be transmitted into the tissues. 19 Suitable coupling mediums include gel with a high water content, a film dressing, water or saline. 17
The dose at which ultrasound is delivered at is related to its frequency (Hz), power (W/cm2), duty cycle (pulsed or continuous) and the duration of treatment, which produce a large number of possible combinations. 19
Ultrasound is contraindicated in people with ankle prostheses/metal anywhere in the foot (e.g. pin and plate, shrapnel), because both metal and the bone cement used in the replacement of joints have a high absorption capacity. The application of ultrasound to the ankle area may lead to heat damage of the local area and any prosthetic joints. 23 Ultrasound is also contraindicated for people with suspected thrombophlebitis (the mechanical vibrations may cause an embolism), people with active cellulitis (potential risk of accelerated growth and dissemination of bacteria throughout the body), in cases of suspected or confirmed local cancer/metastatic disease and in cases of obvious ulcer infection. 23
Existing evidence for the effect of ultrasound on healing
A number of studies have investigated the impact of ultrasound on skin cells (in vitro) and chronic wounds (in vivo). In general, there have been few good-quality studies demonstrating that any of the ‘in vitro’ effects have any clinical importance. 21
Before designing this trial we were aware of seven trials24–30 of ultrasound for treating venous leg ulcers, and one31 became available during this period (Table 1). These were summarised in a systematic review. 32 The sample sizes in these trials ranged from 12 to 108 patients, and four trials25,26,28,29 used true randomisation with allocation concealment. The trials made various comparisons of ultrasound versus sham (four trials24–27) or ultrasound as an adjunct to standard care versus standard care alone. Various types of ultrasound at different dose were used. Frequency of ultrasound ranged between 0.03 and 3 MHz: 0.03 MHz was used in two trials28,30 (applied via a water bath), 1 MHz was used in four trials24,25,29,31 and 3 MHz was used in another two trials26,27 (1 MHz has greater depth penetration than 3 MHz). Ultrasound doses ranged between 0.1 and 1.0 W/cm2. In two trials28,30 in which a water bath ultrasound device was used, 0.1 W/cm2 was used. Doses of 0.5 W/cm2 were used in three trials25,29,31 and 1.0 W/cm2 in four trials24,26,27,31 (one trial31 compared both 0.5 and 1 W/cm2 against standard care). In the seven trials available during the study design phase the following therapeutic regimens were used:
-
One24 reported an evaluation of ultrasound at 1 MHz and 1.0 W/cm2 (38 people).
-
Two28,30 reported evaluations of ultrasound with a water bath at 30 kHz and 0.1 W/cm2 (61 people).
-
Two26,27 reported evaluations of ultrasound at 3 MHz and 1.0 W/cm2 (53 people).
-
Two25,29 reported evaluations of ultrasound at 1 MHz and 0.5 cm2 (152 people).
| First author of trial (number in trial) | Comparison (sham/standard care) | Ultrasound regimen (frequency/dose) | Times per week | Duration of each treatment (minutes) | Period of ultrasound therapy (weeks) |
|---|---|---|---|---|---|
| Eriksson24 (n = 38) | Sham | 1 MHz/1.0 W/cm2 | Two | 10 | 8 |
| Lundeberg25 (n = 44) | Sham | 1 MHz/0.5 W/cm2 | Three, two, one | 10 | 12 |
| Roche26 (n = 26) | Sham | 3 MHz/1.0W/cm2 | Three | ? | 4 |
| Dyson27 (n = 25) | Sham | 3 MHz/1.0W/cm2 | Three | 10 | 4 |
| Weichenthal28 (n = 37) | Standard care | 0.3 MHz/0.1W/cm2 | Three | 10 | 8 |
| Callam29 (n = 108) | Standard care | 1 MHz/0.5 W/cm2 | One | 5–10 | 12 |
| Peschen30 (n = 12) | Standard care | 0.3 MHz/0.1 W/cm2 | Three | 3 | 12 |
Given that the most evidence was available for the combination of ultrasound at 1 MHz and 0.5 cm2, and this included the study with only moderate risk of bias, we decided to use this regimen for the trial. No trials reported that they confirmed ultrasound equipment output.
The largest trial29 (108 people) evaluated ultrasound administered weekly, but the other trials administered ultrasound two or three times a week, with one25 having a reducing frequency from three to one time(s) a week. Four trials25,29–31 used ultrasound for 12 weeks, two24,28 for 8 weeks and two26,27 for 4 weeks. The five trials that described duration of ultrasound regimen used 10 minutes (three trials24,28,30) or 5–10 minutes, depending on ulcer area (two trials26,27).
The heterogeneity of these trials with respect to the delivery mode, dose, duration, treatment length and frequency used means that meta-analysis of all these trials may not be reliable. Another problem with synthesising these studies is the likely difference in the ultrasound actually delivered, even when treatment regimens appear similar, owing to the differences in output between machines and over time (drift). Not all these trials restricted their recruitment to people with ‘hard-to-heal ulcers’ and some were performed without high-compression therapy as the standard care system; hence, their applicability to current care challenges faced by clinicians is unclear. Given that standard care of venous ulcers, using high compression and simple dressings, heals around 80% of all ulcers within 12 months, ultrasound as an adjuvant therapy is likely to be reserved for those resistant to standard therapy, or whose ulcers are identified at the outset as ‘hard-to-heal’.
The Cochrane review32 concluded that there was tentative evidence that ultrasound increased the healing of venous ulcers, but that the trials identified had moderate to high risk of bias. The reviewers, therefore, called for a large, methodologically robust trial of ultrasound to be undertaken; hence, we set out to determine whether adding ultrasound to best practice for venous ulcers (appropriate compression therapy) increased the chance and rate of healing.
Summary of main points
The majority of venous leg ulcers heal in 6–12 months, but those with a duration of > 6 months, or which are larger than average, are harder to heal. With the best available standard care – simple dressings and high-compression bandages – these ‘harder to heal’ ulcers usually take > 6 months to heal. With the burden, in terms of both patients’ finances and QoL, attributed to leg ulcers being significant, increasing the numbers of ulcers healing and decreasing the time taken for ulcers to heal is important.
Ultrasound has been used for many years to help tissue repair and it is commonly given by physiotherapists to help joint and muscle repair, using ultrasound treatment regimens of treatment three to seven times a week. It is not feasible to administer ultrasound with this frequency when treating venous ulcers in the community as delivering ultrasound to an ulcer can be done only when bandages are removed, for example during regular dressing changes. Compression bandages stay in place for up to a week and current thinking about wound healing suggests that wounds should be disturbed as little as possible to avoid damaging delicate tissues at each dressing change. The costs of having daily appointments with the nurse for dressing changes for the purpose of administering ultrasound is likely to be prohibitive (even if nurse workload allowed daily dressings for this purpose, which is unlikely).
There is some evidence that delivering ultrasound to the wound can help heal leg ulcers, although the previous studies were methodologically weak, small in size and varied widely in application regimens, and not all used high-compression therapy as standard care. In addition, the previous trials included people with small, new ulcers, which heal relatively quickly using compression bandaging, and therefore the results of these studies may not be applicable to ‘hard-to-heal’ ulcers.
Research objectives
To compare the clinical effectiveness and cost-effectiveness of low-dose ultrasound (0.5 W/cm2 spatial average and temporal peak) delivered at 1 MHz in conjunction with standard care against standard care alone in the treatment of hard-to-heal venous ulcers.
Primary objective
-
To compare the effects of low-dose ultrasound plus standard care with standard care alone on the time to healing of the reference (largest) ulcer.
Secondary objectives
-
To compare the cost-effectiveness of ultrasound plus standard care with standard care alone.
-
To compare the effects of ultrasound plus standard care with standard care alone on the proportion of patients with ulcers healed at 12 months.
-
To compare the effects of ultrasound plus standard care with standard care alone on percentage and absolute change in ulcer size.
-
To compare the effects of ultrasound plus standard care with standard care alone on health-related quality of life (HRQoL).
-
To compare the effects of ultrasound plus standard care with standard care alone on reported adverse events, withdrawals and loss to follow-up.
-
To compare the effects of ultrasound plus standard care with standard care alone on the proportion of time patients are ulcer free.
Chapter 2 Methods
Trial design
The Venus Ulcer Study (VenUS) III trial was a pragmatic, multicentre, two-armed, randomised controlled, open trial with equal randomisation. Participants with ‘hard-to-heal’ ulcers (> 6 months’ duration and/or larger than 5 cm2) were randomised (1 : 1) to receive either:
-
low-dose (0.5 W/cm2) ultrasound, 1 MHz, and a pulsed pattern of 1 : 4 weekly for up to 12 weeks, plus standard care, or
-
standard care alone.
The study protocol can be seen in Appendix 1.
Sample size
The sample size calculation was based on median time to healing. Lambourne et al. 9 and Vowden et al. 10 found that 60% of large ulcers (> 10 cm2) and 60% of ulcers of > 6 months’ duration (treated with four-layer compression) healed in 24 weeks. This represents a median time to heal of 15–22 weeks (estimated from published survival curve). Our sample was to include some smaller ulcers, but importantly it would also include people with both high ulcer duration and large area (of whom between 13% and 37% heal at 24 weeks with high compression),8 and therefore, overall we estimated that 50% of ulcers in the standard care group would heal within 22 weeks (compared with 11 weeks for small, new ulcers as determined in VenUS I). 7
Estimating that clinicians and patients would value a reduction in healing time of 7 weeks (i.e. from 22 weeks to 15 weeks), we based our sample size calculation on this premise. This moderately sized effect was deemed as being required before clinicians would introduce a new therapy, with the consequent requirements to undergo training, arrange for purchase and servicing of machines, etc. To give us 90% power to detect this 7-week difference in median healing time we required a sample size of 306 patients, or 336 patients allowing for 10% attrition. This sample size also gave us 80% power to detect an 8-week reduction in median time to healing from 24 weeks and 90% power to detect an 8-week reduction from 26 weeks.
Approvals obtained
The York Multicentre Research Ethics Committee (MREC) approved the study on 4 February 2005. The details of the MREC, Local Research Ethics Committees (LREC) and Research and Development Department approvals are provided in Appendix 2. The trial was assigned the International Standard Randomised Controlled Trial Number (ISRCTN) of ISRCTN21175670; EudraCT number 2004-004911-51 and National Research Register number N0484162339.
Trial sites
The study was conducted in 11 UK sites and one Irish site (Dublin). These sites were recruited throughout the duration of the study and represented a range of urban and rural settings plus a number of different types of leg ulcer service. Details of the study sites are provided in Appendix 3.
Participant eligibility
Only people with ‘hard-to-heal’ venous leg ulcers were to be recruited into this study. For the purpose of this study a hard-to-heal venous leg ulcer was considered to be any break in the skin on the leg (below the knee), which either (a) had been present for > 6 weeks or (b) occurred in a person with a history of venous leg ulceration. A participant was considered to have a venous leg ulcer where there was no other evident causative aetiology, the ulcer appeared clinically venous (moist, shallow, irregular shape, venous eczema, ankle oedema, and/or lipodermatosclerosis, not confined to the foot), and the study participant had an Ankle–Brachial Pressure Index (ABPI) of ≥ 0.8. An ABPI of < 0.8 indicates that there is a high probability that arterial insufficiency is present and that the ulcer should not be regarded as venous. 33
Prognostic studies have found that patients with ulcers larger than 5 cm2 and with duration of > 6 months are less likely to heal within 24 weeks. 34
Inclusion criteria
All people with venous leg ulcers were potentially eligible for inclusion in the proposed trial if they met the following criteria:
-
They were receiving care from community/leg ulcer/outpatients nurses in trial centres.
-
They were able to give written informed consent to participate in the study. Information sheets and consent forms were to be provided in languages other than English if required.
-
The primary cause of their ulcer was chronic venous insufficiency. This diagnosis was made using the same diagnostic criteria currently employed by caregivers in the community, namely the clinical appearance of the ulcer, patient history and an ABPI to rule out arterial insufficiency. 33
-
They had ‘hard-to-heal ulcers’ as defined by the presence of at least one of these criteria:
-
a venous ulcer of > 6 months’ duration
-
a venous ulcer > 5 cm2.
-
-
They had Doppler-determined ABPI of ≥ 0.8 measured within last 3 months.
-
Those with an ulcer infection (based on a clinical signs and symptoms checklist) at baseline were eligible to participate once the infection had been resolved. 35
-
Those who were unable to self-complete the English language QoL tools were still eligible to participate, but we did not collect QoL data from them [the Short Form questionnaire-12 items (SF-12) is validated in English, Spanish, Italian, French and German and we anticipated that the number of non-English speakers who use these languages would be very small, based on previous trial experience].
Exclusion criteria
Potential participants were excluded if they met the following criteria:
-
Their leg ulcer was due to causes other than venous insufficiency (e.g. arterial insufficiency, malignancy, pyoderma gangrenosum, etc.).
-
They had poorly controlled diabetes, as evidenced by a glycated haemoglobin (HbA1c) of > 10%.
-
They had ankle prostheses/metal anywhere in the foot (e.g. pin and plate): because bone cement used in the replacement of joints has a high absorption capacity, the application of ultrasound to the ankle area may lead to heat damage of the prosthetic joint. 23
-
They had suspected thrombophlebitis: because the mechanical vibrations may cause an embolism. 23
-
They had active cellulitis: because of the potential risk of accelerated growth and dissemination of bacteria throughout the body. 23
-
They had suspected or confirmed local cancer/metastatic disease. 23
Patients previously enrolled into the study who had become ulcer free were not eligible for rerandomisation into the trial if their ulcer(s) recurred.
Recruitment into the trial
Local nursing staff, community nurses, leg ulcer nurses and trial nurses identified potential participants and supplied them with an information sheet about the trial (see Appendix 4). Patients were given a minimum of 24 hours to read the information sheet and consider participation. All potential venous leg ulcer patients were screened using a pre-trial screening form (see Appendix 5) which listed the main eligibility criteria. Patients potentially eligible and interested in participating were seen by their local trial nurse, who would discuss the study if more detail was requested, obtain written informed consent, record baseline data and administer the first allocated treatment. Participants’ general practitioners (GPs) were notified of their involvement in VenUS III after recruitment. Information provided on the pre-trial screening forms was collected centrally at York Trials Unit to allow reasons for non-eligibility to be collated.
Baseline assessment
After written informed consent had been obtained, baseline data were collected on the patient using a Patient Record Form (see Appendix 5). The following data were collected.
Ulcer history and initial assessment
If the patient had more than one ulcer, nurses were asked to define which ulcer was the ‘reference ulcer’ (and therefore the reference leg), which was classed as the largest eligible ulcer. The outline of the reference ulcer was traced onto an acetate film grid marked with 1 cm2 squares and an assessment was then made by the recruiting nurse of whether the reference ulcer area was ≤ 5 cm2 or > 5 cm2. The actual area was calculated at the York Trials Unit at a later date using the mouseyes computer program, Version 3.1 (Dr Robert John Taylor, Salford). 36 The reference ulcer was labelled according to leg (R for right; L for left) and the number 1 given to it (e.g. reference ulcer present on right leg = R1). Nurses completed tracings of all ulcers a patient had and labelled them in descending order of area (R2, R3, etc.) to allow follow-up of all ulcers on the limb.
Measurement of ankle–brachial pressure index
The result of a Doppler-determined ABPI measurement for the reference leg was noted along with the date it was measured. For inclusion in the study, this reading must have been obtained in the last 3 months as readings change over time. 37
Number of ulcer episodes on leg with reference ulcer
Data from VenUS I7 suggested that a greater number of ulcer episodes are prognostic of a longer healing time; we therefore recorded the number of ulcer episodes on the leg of the reference ulcer since the first episode.
Duration of reference ulcer and duration of oldest ulcer
Duration of reference ulcer was recorded based on participant report along with the duration of the oldest ulcer on the reference leg.
Patient mobility
Reduction in mobility can potentially contribute to ulceration by means of reduced venous return, secondary to calf muscle wastage. 38 It was recorded whether the patient was able to move freely, with difficulty or was immobile.
Ankle mobility, patient’s height and weight
The VenUS I7 reported that, as well as ulcer area, duration and number of episodes, ankle mobility (full, reduced range or fixed), and body weight were also prognostic for healing. These data were all collected at baseline.
Ulcer position and image
For monitoring purposes a record of the position of all ulcers on the trial leg including the reference ulcer was made. A dated digital photograph was also taken of the reference ulcer.
Pain
Patients were asked to indicate how intense the pain had been in their reference ulcer during the past 24 hours using a visual analogue scale (VAS) ranging from ‘no pain’ to ‘worst pain imaginable’. The minimum value on the scale was 0 mm and the maximum was 150 mm.
Date of birth
Date of birth was recorded at recruitment, allowing age at recruitment to be calculated. Increased age was associated with slower healing rates in one study. 38
Gender
The gender of participants was recorded, allowing a comparison with the existing information on the population of people with venous ulcers, in which women outnumber men. 39
Compression level
Whether the participant was currently being treated with two-, three- or four-layer high-compression bandaging was recorded.
Randomisation
Participants were randomised equally between the two trial arms: ultrasound plus standard care and standard care alone. Randomisation was carried out using varying block sizes of four and six participants. To maintain allocation concealment the generation of the randomisation sequence and subsequent treatment allocation were performed by an independent, secure, remote, telephone randomisation service (York Trials Unit). The computerised randomisation system was checked periodically during the trial following standard operating procedures. Owing to the nature of the intervention, it was not possible to conceal the treatment allocation from either the patient or the nurse.
Trial interventions
Participants were randomised to receive either:
-
Low-dose (0.5 W/cm2) ultrasound, 1 MHz, with a pulsed pattern of 1 : 4 as per trials by Callam et al. 29 and Lundeberg et al. 25 The ultrasound was to be applied to periulcer skin, weekly for up to 12 weeks, at regular dressing changes, plus standard care (see below for the definition of standard care). After the 12 weeks of ultrasound, patients returned to standard care alone.
or:
-
Standard care: this was defined as a simple low-adherent dressing and high-compression, four-layer bandaging, reduced compression or no compression, according to the clinical assessment of the level of pressure tolerated by the patient. This is based upon UK Clinical Practice Guidelines. 33
Preparation for ultrasound treatment
Prior to the application of the ultrasound the leg would be washed and any loose skin from around the ulcer and remnants of emollients removed. The ultrasound was applied directly to the skin surrounding the ulcer, using a water-based contact gel to ensure passage of the waveform from the transducer to the tissues.
Target ulcer
The ultrasound treatment was applied for 5–10 minutes to the previously defined reference ulcer (see Appendix 6).
Calculating ultrasound treatment time
Previous studies had applied ultrasound for varying amounts of time. We sought to balance the need for larger ulcers to have more treatment time, the need for treatment time to be feasible as an addition to standard practice, and the need for a simple system to help nurses titrate ultrasound to the various patients she or he would see.
Ulcers of area < 5 cm2 received 5 minutes’ ultrasound; those of 10 cm2 or larger received 10 minutes’ ultrasound. For ulcer areas between 5 and 10 cm2, the treatment time in minutes was calculated as the ulcer area in square centimetres (for example: an ulcer calculated as 6 cm2 received 6 minutes of ultrasound). Ulcer area was recalculated every 4 weeks using acetate film grids with pre-printed 1 cm2 areas.
Concurrent therapy
All dressings and bandages were to be replaced at each visit, as per standard practice. Concurrent therapy for all patients was low-adherent dressings and four-layer high-compression bandaging, reduced compression or no compression, according to the clinical assessment of the level of pressure tolerated by the patient. Should a change from the low-adherent dressing or the prescribed level of compression be required, in the opinion of the clinician, then this was recorded. The patient did not withdraw from the trial should the concurrent therapy change.
Nurse training
Prior to the trial starting, participating nurses attended a training programme on the rationale for the trial, patient eligibility, recruitment procedures (including consent and randomisation), ultrasound treatment application, data collection (completion of trial documentation and tracing ulcer outlines), handling participant withdrawal and adverse event reporting (see Appendix 7). Competency in ultrasound administration was assessed at the end of the training. The trained trial nurses were also permitted to cascade training in delivering ultrasound for the purposes of the trial to other local nurses so that treatments could be maintained during holiday periods or staff absences.
Checks were made by the local trial nurses regarding the use of ultrasound within the trial, to ensure that the ultrasound was being delivered as per protocol.
Ultrasound machines
The ultrasound machines were supplied, at discounted price, by EMS Physio Ltd, the largest UK manufacturer of ultrasound machines. The machine chosen was the EMS Therasonic 355 Physiotherapy system (EMS Physio Ltd, Wantage) which delivers only 1 MHz ultrasound: effective radiation area 4 cm2, with a large transducer head, collimated beam, and beam non-uniformity ratio (BNR) < 5. We used a pulsed mode; each pulse lasted 2 milliseconds and the pulse ratio was 1 : 4. Prior to acceptance, all machines were tested at the National Physical Laboratory (NPL) to confirm that they were delivering ultrasound at the necessary frequency and intensity.
Auditing ultrasound machine performance
The ultrasound machines were assessed after 3 months’ recruitment and 3-monthly thereafter to check the intensity of ultrasound delivered. Checks were undertaken by the ultrasound machine suppliers. These originally took place at each clinical site by the ultrasound machine suppliers except where there were no available engineers (Northern Ireland and Scotland). Previous studies have indicated that there are differences between the ‘nominal’ dose and that actually delivered by the machines. 42 Some of this is apparent at machine delivery, and some is due to drift or step changes in output. 43 Each ultrasound machine was numbered so that patients who received treatment from individual machines could be identified. This check took place when a site had been recruiting for 3 months and 3-monthly thereafter.
Ultrasound therapy arm
During the 12 weeks of ultrasound therapy, the date of every nurse visit was recorded in an Ultrasound Treatment Log Booklet by the treating nurse, along with location and whether or not ultrasound had been applied and, if so, for how long, machine number and signature of the nurse applying it for (see Appendix 5). Information relating to dressings and bandaging applied was also recorded. Reasons for any changes in these from the previous visit were requested. If for any reason the patient was not given his or her weekly ultrasound treatment (infection, illness, non-attendance, etc.), that week was not carried over. Thus, the maximum number of ultrasound treatments a patient could receive was 13 (one at baseline plus one per week for a total of 12 weeks).
After the patient’s ultrasound treatment period had finished, treatment reverted to standard care alone with the visit dates, locations, dressings and bandage changes recorded in a separate booklet – Dressing Log Booklet (see Appendix 5). Follow-up then continued as per the protocol.
Standard care arm
Visits to patients allocated to standard care alone with the visit dates, locations, dressings and bandage changes were recorded in the Dressing Log Booklet (see Appendix 5). Follow-up continued throughout their treatment period as per the protocol.
For all patients, acetate film tracings of all ulcers and digital photographs of the reference ulcer were taken every 4 weeks until 12 months after randomisation (or between 6 and 12 months in some cases depending on when they were recruited in the trial recruitment period) or the patient became ulcer free, whichever happened first.
Participant follow-up
Appendix 8 shows a summary of VenUS III.
Trial completion
Participants were deemed to have exited the trial when:
-
The participant became ulcer free.
-
The participant had been in the trial for 12 months (or between 6 and 12 months in some cases depending on when they were recruited in the trial recruitment period).
-
The participant wished to exit the trial fully.
-
The participant’s doctor or nurse withdrew him or her from the trial.
-
The participant was lost to follow-up.
-
The participant died.
Participants whose reference ulcer had healed but who still had a venous ulcer (because the reference ulcer was not their only ulcer) continued to have routine clinical data collected about them until they became ulcer free or they had been in the trial for > 12 months (or between 6 and 12 months for cases recruited during the last months).
Instead of withdrawing fully from the trial, participants had the option of:
-
withdrawing only from nurse data collection by the nurse
-
withdrawing only from receiving the trial treatment
-
withdrawing only from postal questionnaires; or
-
any combination of the above.
Patients who elected to withdraw from all three (data collection, trial treatment and postal questionnaires) were deemed to be full withdrawals (trial exit). Nurses were able to indicate any change in the patient’s level of participation by completing a Change of Circumstances Form (see Appendix 5). This ensured appropriate follow-up from the Trials Unit.
Measurement and verification of primary measure
The primary end point was time to healing of the reference ulcer.
Secondary outcomes were cost-effectiveness of ultrasound, proportion of patients with ulcers healed at 3, 6 and 12 months, percentage and absolute change in ulcer size, HRQoL and adverse events.
Determination of reference ulcer healing
Healing was defined as ‘full epithelial cover without a scab, where a scab is a thick crust over the wound’. This meant that an ulcer in which there was full epithelialisation overlaid by a thin layer of dry skin could be classified as healed. Clinically, this type of wound would not require a primary contact layer.
When the treating nurse deemed the reference ulcer to be healed, she or he completed an Ulcer Healed Form (see Appendix 5) and took a digital photograph of the healed ulcer (see Appendix 9). In addition, nurses were asked to take another digital photograph 7 days after they had considered the reference ulcer healed.
Two blinded assessors independently assessed all photographs for each participant to determine a date for healing. This assessment followed the same successful process as that used in VenUS II. 44 The assessors discussed any discrepancies with referral to a third assessor for a final decision if required. The primary outcome was calculated using the date of healing as decided by the blind assessors. However, if no photographs were available for a participant, then the date of healing decided by the treating nurse was taken as the healed date.
Measurement and verification of secondary outcomes
Collection of resource use data
During their treatment period within the study, data were recorded (see Appendix 5) about treatment received, location of the visit and the level of compression bandaging applied.
At recruitment and 3, 6, 9 and 12 months after randomisation, patients were asked to complete a questionnaire on health and social care resource use during the previous month (see Appendix 5). The questionnaire was designed for participant completion and was returned to the trial office using a reply-paid envelope. Participants indicated how many times in the previous month they had used health services (e.g. seen a GP or nurse or received hospital care) and whether any health service use was ulcer related. The collection of self-reported resource use data was continued until the patient had been in the study for 12 months (or between 6 and 12 months for cases recruited during the last months).
Proportion of ulcers healed at 3, 6 and 12 months
The proportion of reference ulcers healed at 3 and 6 months post randomisation was reported to summarise the effects of the two regimens. Proportions healed are commonly used in wound care trials and hence this allowed us to compare our results directly with other trials which have rarely used time to event data.
Proportions of time patients are ulcer free
Reduction in recurrence would help reduce the prevalence of this condition and thus costs. Crude recurrence rates are potentially biased by any difference in healing rates associated with the two groups (ultrasound or standard care). As the treatment group which experiences faster healing is, by definition, exposed to more risk of recurrence if one group has more rapid healing, then people in that group are at risk of earlier recurrence. To account for this we used the proportion of time that patients were ulcer free. Patients with healed ulcers were contacted by telephone at 6, 9 and 12 months (up to June 2009) after healing in order to obtain recurrence data.
Percentage and absolute change in ulcer size
Data collected at 1, 3 and 12 months post randomisation allowed us to determine the reduction in ulcer area in patients who did not achieve complete ulcer healing. If the ultrasound and standard care groups achieved similar times to complete healing but one resulted in larger changes in ulcer area, then this may be clinically important as smaller ulcers may exude less and therefore require less frequent dressing changes. Furthermore, the recording of ulcer area at these time points allowed further study of the trajectory of healing for venous leg ulcers and the relationship between the reduction in ulcer area and eventual healing.
One study found that increased ulcer area at 1 month after initiation of treatment is a useful predictor for non-healing. 45 Identifying patients who are likely to fail to heal early in treatment allows these patients to have prompt referral to specialist centres for further assessment and treatment. Measurement of ulcer size involved taking tracing using acetate wound grids and a fine-nibbed, indelible pen, taking the outer edge of the ulcer rim as the outer edge of the tracing line (i.e. ulcer area = area enclosed by tracing and area of line). Ulcer area, as determined by acetate tracing, is an accurate and reliable measure. 46
Health-related quality of life
Participants were asked to answer questions relating to their HRQoL throughout the study, when they were asked to complete two generic instruments (EQ-5D and SF-12). Generic instruments were used to measure participants’ perceptions of health outcome in this trial, which have previously been shown to be sensitive to changes venous ulcer healing status. 47 In addition, these instruments are also particularly useful for comparing groups of participants, while also having a broad capacity for use in economic evaluation. Their generic nature also makes them potentially responsive to side effects or unforeseen effects of treatment.
Each participant’s perception of his or her general health was assessed using the acute version of the SF-1248 and the EQ-5D (EuroQol). 41 The SF-12 is a reliable and well-validated questionnaire,49 and has been used in UK populations including older people and leg ulcer patients. 50,51 We used a layout of the SF-12 shown in previous work to yield improved response rates and quality. 52 The EQ-5D is a generic measure of health status, in which health is characterised on five dimensions (mobility, self-care, ability to undertake usual activities, pain, anxiety/depression). 41 Participants were asked to describe their level of health on each dimension using one of three levels: no problems, moderate problems and severe problems. Each response locates a person into one of 243 mutually exclusive health states, each of which has previously been valued on the 0 (equivalent to dead) to 1 (equivalent to perfect health) ‘utility’ scale based on interviews with a sample of 3395 members of the UK public. 53 The EQ-5D has been validated in the UK and questionnaires containing both instruments were administered to patients by postal survey at baseline and at 3, 6, 9 and 12 months.
Optimising questionnaire response rates is of vital importance, and a recent systematic review has investigated strategies to increase questionnaire response rates. 54 The review reported that response rates to postal questionnaires doubled (odds ratio = 2.02, 95% CI 1.79 to 2.27) when a financial reward was included with the questionnaire compared with rates obtained with no incentive. The response rate increased further when the incentive was not conditional on response versus upon return of questionnaire (odds ratio = 1.71, 95% CI 1.29 to 2.26). Based on these data, we enclosed an unconditional payment to participants along with their final (usually the 12th month) questionnaire. They received notification in advance that would receive £5 in recognition of their commitment to our study and the time they spent completing questionnaires.
Adverse events
An adverse event is defined as ‘any undesirable clinical occurrence in a subject, whether it is considered to be device related or not’. 55 Both treatment-related and -unrelated adverse events were reported to the trial office (see Appendix 5). The reporting nurse indicated whether, in his or her opinion, the event was related to trial treatment or not. Events were also classed as serious or non-serious. Serious adverse events (SAEs) were defined as death, life-threatening risk, hospitalisation, persistent or significant disability/incapacity and patient being newly diagnosed as diabetic. For other events the treating nurse made a clinical decision about the seriousness. A list of possible treatment-related adverse events was established a priori, based on reports in the literature and VenUS I and VenUS II. These were pressure damage, infection, skin damage surrounding ulcer, new ulcer, ulcer deterioration and also adverse reaction to the ultrasound treatment or gel.
All nurses were asked to report any SAEs within 24 hours of the event occurring, or within 24 hours of becoming aware of the event. Sites were also provided with review forms in order to follow up all events so that information on eventual outcomes was recorded.
Clinical analysis
The objective of the clinical analyses was to compare the clinical effectiveness of low-dose ultrasound (0.5 W/cm2 spatial average and temporal peak) delivered at 1 MHz plus standard care with standard care alone in the treatment of hard-to-heal venous ulcers (i.e. those with duration of > 6 months and/or surface area > 5 cm2). Our aim was to assess whether the addition of 5–10 minutes of ultrasound (depending on ulcer area) to a package of best available standard care had any affect on:
-
time to ulcer healing
-
complete ulcer healing (the patient is completely healed of all ulcers)
-
HRQoL
-
adverse events.
Outcomes
Primary
-
Time to complete healing of the reference ulcer.
The reference ulcer was the largest ulcer on either leg (as assessed at the time of trial entry). The date of healing was recorded by the research nurses on the Ulcer Healed Form and the photographs of the reference ulcer as assessed independently by two people blind to treatment group. Any disagreements were resolved by discussion or referral to a third blinded assessor. The primary outcome was calculated using the date of healing as decided by the blind assessors. If the blinded assessors did not agree on a healing date, then the date as recorded on the Ulcer Healed Form was used. Time to healing was derived as the number of days between randomisation and the first date that healing was confirmed. Patients who withdrew unhealed from the trial or died prior to healing were treated as censored in the analysis. Their time to censoring was derived using the date of trial exit, the date of their last ulcer assessment or the date of trial closure. Patients who completed the full 12-month follow-up without their reference ulcer healing were also treated as censored and their time to censoring was calculated as 12 months (365 days).
Secondary
-
Reduction in ulcer area.
-
HRQoL (SF-12) (baseline and 3, 6, 9 and 12 months).
-
Complete healing of all ulcers during the trial period (up to 12 months).
-
Adverse events.
-
Ulcer recurrence.
Analyses
Primary statistical analyses were performed on an intention-to-treat basis using a two-sided, 5% significance level.
Primary outcome
The primary analysis compared the time to complete healing of the reference ulcer between the two randomised groups using a Cox regression model. 56 The analysis adjusted for centre as a random effect, ulcer area (from baseline tracing), ulcer duration and whether or not the patient was treated with high-compression bandaging. If centres recruited a small number of patients (five or fewer) then the analysis was repeated with no adjustment for centre, as a sensitivity analysis. Hazard ratios and corresponding 95% CIs were presented. The assumption of proportional hazards was checked using log–log plots, inclusion of interaction terms in the model (for each term with time) and by looking at plots of Schoenfeld residuals. The linearity assumption of continuous terms in the model (ulcer area and duration) was assessed using plots of the Martingale residuals and, if necessary, a suitable transformation used.
Kaplan–Meier survival curves were produced for the two groups and the median time to healing with 95% CI, as well as the Kaplan–Meier estimates of the proportions of ulcers healed at 12 and 24 weeks (for comparison with the results of other venous leg ulcer trials) presented.
A sensitivity analysis was performed to repeat the primary analysis using only the data of healing as recorded by the research nurses, and not using the data from the photographs.
Agreement on date of healing
The Cohen’s kappa measure of inter-rater agreement was used to assess agreement between the two assessors of the photographs for time to healing. This was repeated to look at agreement between the final decision from the photograph assessment and the nurses’ recorded date of healing.
Secondary outcomes
Ulcer area
Ulcer area tracing data were measured using a specialist computer package (mouseyes). 36 The areas at baseline and each assessment were summarised using descriptive statistics [mean, standard deviation (SD), median, range]. Previous research has shown that the initial reduction in ulcer area (percentage reduction after 4 weeks of good wound care) is predictive of eventual healing. 45 Therefore, we compared the initial healing rates between the ultrasound and standard care groups. The ulcer area at week 4 was compared between treatment groups using analysis of covariance to adjust for baseline ulcer area, centre, ulcer duration and use of compression.
Quality of life
Quality of life was measured using the SF-12 questionnaire [measured at baseline (0), and at 3, 6, 9 and 12 months]. The scores for the physical and mental health components were analysed using a multilevel regression model approach. The outcomes at each time point were used in a single model, with time points nested within patients to account for within-patient correlation in scores. The model was used to estimate the difference between treatments over the whole 12-month follow-up period. The outcome modelled was the score at each follow-up assessment and the covariates included in the model were centre, baseline health component score, baseline ulcer area, ulcer duration, use of compression and time (indicators for 0, 3, 6, 9 and 12 months). Whether the pattern in QoL scores over time is different between the two treatments was assessed by including an interaction term between treatment and time in the model.
The assumption of normally distributed data was assessed and, if necessary, log transformations or other analysis methods used.
The numbers of patients with missing data at each time point were summarised together with reasons for missing data (if available). We summarised the proportions of missing questionnaires at each time point by healing status and used joint modelling of repeated QoL data and time to ulcer healing. 57
Complete healing of all ulcers at 12 months
The proportions of patients completely healed of all ulcers on both legs by the end of trial follow-up (12 months or earlier) were summarised by treatment group. A Kaplan–Meier plot of the time to complete healing and estimates of the median time to complete healing and 95% CI for each group was presented. Any patient not completely healed by the end of the trial was treated as censored.
Ulcer recurrence
The proportions of patients whose reference ulcer healed and then recurred was summarised by treatment group.
Adverse events
The number of adverse events experienced by each patient was compared between treatment groups using a negative binomial model adjusting for the same covariates as the primary analyses (centre, ulcer size, ulcer duration, use of compression). This analysis summarised all adverse events and then SAEs and non-serious adverse events (NSAEs) separately. The number of patients with one or more adverse events and the numbers of adverse events, their severity and whether they were considered treatment related or not, were summarised descriptively by treatment group.
Economic analysis
Economic evaluation of health interventions is a tool used to assist decision makers in prioritising and allocating resources in the health-care sector, by assessing the value for money (cost-effectiveness) of alternative interventions. 58
The aim of the economic evaluation was to assess cost-effectiveness of low-dose ultrasound in conjunction with standard care compared with standard care alone in the treatment of hard-to-heal venous leg ulcers. Using individual-level data, cost-effectiveness analysis and cost–utility analysis were performed. These analyses are expected to provide evidence, taking into account both comparative cost and effectiveness simultaneously, to assist the decision on whether or not to adopt ultrasound in conjunction with standard care for the treatment of hard-to-heal venous leg ulcers.
The analysis was conducted on an ‘intention-to-treat’ basis, comparing incremental costs with incremental ulcer-free days (cost-effectiveness analysis) and incremental quality-adjusted life-years (QALYs) (cost–utility analysis). The perspective of NHS and Personal Social Services was adopted in the current analysis considering that the findings will be used to inform the decision maker in the NHS. Discounting for the future cost and health outcome was not necessary as the time frame of the trial was 12 months after randomisation. The year of pricing was 2007. The analyses were conducted using stata 10 (StataCorp, College Station, TX, USA). 59
Data
Cost and health benefits data used in the economic analysis were extracted from the current trial. There were two sources of cost data: nurse-completed and patient-completed questionnaires. Nurse-completed questionnaires provide detailed information on each treatment the patients received, such as the choice of compression therapy, the duration of ultrasound treatment (if applied) and the treatment setting, whereas patient-completed data, derived from patient self-reported questionnaires at 3-, 6-, 9- and 12-month follow-up, provided information on frequency of health-care consultations, including doctor, nurse and hospital outpatient visits. Two different measures of health benefit were used: the time to healing and QALY. Time to healing was recorded by nurses and assessed by blinded investigators, whereas QALYs, representing years living in full health, were calculated based on information obtained from patient self-reported questionnaires every 3 months. Details of each constituent component of the economic analysis are discussed in the following sections.
Owing to the restricted follow-up period (1 year) as well as death and loss to follow-up, censoring occurred in both cost and health benefits data. To adjust for this censoring issue, in order to obtain more accurate estimation, the inverse probability weight (IPW) approach60 was applied. This method applies a set of different weights to account for the censoring. The same approach was used in VenUS II. 61 Taking the time to healing as an example, in this IPW approach only participants with observed time to healing data contribute with non-zero terms, but their contributions are inversely weighted by the probability of being observed. 61 Consequently, individuals who are less likely to be observed are weighted most heavily. The censoring distribution was estimated through the Kaplan–Meier estimator. Similarly, an IPW approach was adopted in the estimation of mean costs and QALYs to adjust for the censoring issues in the cost and QALY data, respectively.
Outcomes of the economic analysis
The economic analysis used incremental costs and incremental health benefits, estimated from the trial data, to evaluate the value for money of the ultrasound with standard care treatment. Time to healing of the reference ulcer and QALYs were the units of health benefit in the cost-effectiveness analysis and cost–utility analysis, respectively.
Health benefits
Time to healing estimation
The mean time to healing was estimated from the IPW regression using the same covariates as in the clinical analysis to adjust for the nature of the censoring, possible baseline imbalances and randomisation stratification, namely baseline ulcer duration (logarithmic), ulcer area (logarithmic), use or not of compression therapy and centres (aggregating centres with fewer than five cases). The centre effect was treated as fixed effect in all subsequent analyses. In order to facilitate the interpretation of the cost-effectiveness analysis, the effectiveness outcome was reported as ‘gain in ulcer-free days’. The gain in ulcer-free days is the same as the difference in mean time to healing between two trial arms (same absolute value), but with the opposite sign (–/+).
Utility scores and quality-adjusted life-year estimation
Utilities of patients were measured by the EQ-5D62 questionnaire at baseline and at each follow-up of 3, 6, 9 and 12 months, independent of healing status. EQ-5D is one of the most widely used HRQoL measures and provides summary index (utility) scores of health states for the purpose of economic evaluation. The EQ-5D description system contains five dimensions – mobility, self-care, usual activities, pain/discomfort and anxiety/depression; each dimension has three levels – no, some and severe problems. The utility, also known as EQ-5D index score, of each patient at each observed time point was derived from patient’s responses to the EQ-5D description system at that time point and a predefined weight was assigned accordingly. The predefined weight represents the social preference of the general population in England and Wales towards EQ-5D health states. 63 These utility scores, 1 representing full health and 0 indicating death, were then used in QALY calculation.
According to the data collection, the study time horizon was partitioned in homogeneous subintervals (quarterly intervals). Quarterly QALYs were calculated by multiplying an individual’s utility score with survival time using the area under the curve approach, which was defined by linearly interpolating the utility scores measured over time. 64 However, not all participants were followed up for a full trial period because of censoring. To account for the impact of censoring, thus avoiding biased estimates, mean quarterly QALYs were estimated using the IPW method. To ensure comparability with the clinical analysis, linear regression was used to adjust QALYs by the same clinical covariates and also baseline utility. In the presence of imbalance, failure to include baseline utility results in biased results and a misrepresentation of uncertainty. 65 QALYs were weighted by the inverse of the probability of an individual not being censored. In the case of participants who died, utility values were recoded as zero. Mean total QALYs, over the 12-month follow-up period were then estimated as the sum of the estimated mean QALYs per period for each trial arm given the mean value of the rest of the covariates observed in the whole sample.
Resource use and unit costs
A cost for each trial participant was calculated as the product of resources used and their relevant costs. The analysis was costed at 2007 prices (mid-point of the trial). Three components were considered in the estimation of leg ulcer treatment cost: cost of ultrasound machine, cost of compression therapy and cost of health-care consultations (focusing on cost of health-care provider’s time). Other treatments, such as primary and secondary dressing or skin preparations, were assumed to be used equally across treatment arms; these resources were not included in the economic analyses. Incorporating costs categorised as irrelevant in distinguishing treatments into the analysis will increase the variance (and the standard error) of total costs for each arm and, thus, misrepresent uncertainty in the incremental costs. 66
Cost of ultrasound machine
We purchased 40 ultrasound machines at a cost of £26,267.87 including the purchasing and testing fees as listed in Table 2. These fees are regarded as one-off equipment costs. Following the suggested method58 for dealing with one-off equipment costs in economic evaluation, the study calculated the equivalent annual equipment cost, taking into account depreciation of the ultrasound machine and its opportunity cost. By assuming that the average lifespan of an ultrasound machine is 7 years and considering linear depreciation with the interest rate for opportunity cost at 3.5%, the equivalent annual equipment cost over the period of 7 years is £4659.15. The information on lifespan and cost of ultrasound machines was provided by the machine supplier (EMS Physio Ltd). The interest rate chosen here was based on the discounting rate suggested in the reference case for technology appraisal recommend by National Institute for Health and Clinical Excellence (NICE). 67 Note that discounting was not applied in the current analysis.
| Item | Total cost (£) | Note | Source |
|---|---|---|---|
| Machine purchasing fee | 19,180.00 | Forty machines | EMS Physio Ltd |
| Testing fee | 7087.87 | One-off at machine set-up | NPL |
| Equivalent annual equipment cost | 4659.15 | Assuming 7-year lifespan and an interest rate of 3.5% | |
| Servicing fee | 10,133.71 | Once every 3 months | EMS Physio Ltd |
| Cost of ultrasound machine per patient | 88.05 | Dividing the sum of the servicing fee and equivalent annual equipment cost by the number of participants in the ultrasound arm |
The cost of ultrasound machines per patient in the treatment arm was estimated to be £88.05. This was calculated by summing the equivalent annual equipment cost of ultrasound machines with their servicing fee through a year (occurring every 3 months), then dividing by the number of participants in the treatment arm. This cost was added to each participant in the treatment arm to calculate their total costs of leg ulcer treatment.
As detailed, the costs associated with the ultrasound machine were based on the cost of the current trial, and might not reflect the ‘real’ cost in other, more generalised, settings. For instance, if the ultrasound machines are also used for other purposes (therefore more patients use them), the cost of ultrasound machine per patient could be lower (as a result of a larger denominator). The impact of this estimation was explored in the sensitivity analysis.
Cost of compression therapy
Table 3 shows the unit cost of each compression bandaging system. The composition of the compression bandaging system was obtained from VenUS II and updated based on the supply and prices as listed in the British National Formulary (BNF). 68 The choice of the class of compression therapies was recorded by a nurse at each treatment (high compression: four-layer, three-layer, two-layer and short-stretch high compression; low compression: three-layer reduced compression; light compression). As no information on the size or brand of the system was available, an arithmetic average cost for commercially available systems was used to cost the class recorded. The cost of compression therapy per patient/per visit was then calculated accordingly.
| Bandaging system | Example | Cost (£) (from BNF) |
|---|---|---|
| High-compression bandaging: 4LB | K-Four® | 6.25 |
| Profore® | 8.35 | |
| System 4® | 7.77 | |
| Ultra Four® | 5.67 | |
| Mean cost | 7.01 | |
| High-compression bandaging: SSB | Actiban® | 3.18 |
| Actico® | 3.21 | |
| Comprilan® | 3.12 | |
| Rosidal K® | 3.36 | |
| Silkolan® | 3.39 | |
| Mean cost | 3.25 | |
| High compression: two-layer kits | ProGuide® | 8.49 |
| Coban® | 8.08 | |
| Mean cost | 8.29 | |
| Layer 1 | Advasoft® | 0.39 |
| Cellona Undercast padding® | 0.42 | |
| Flexi-Ban® | 0.44 | |
| K-Soft® | 0.4 | |
| Ortho-Band Plus® | 0.37 | |
| Softexe® | 0.58 | |
| Ultra Soft® | 0.42 | |
| Velband® | 0.66 | |
| Surerpress® | 0.53 | |
| Profore #1® | 0.62 | |
| Mean cost | 0.48 | |
| Class 2 | Neosport® | 0.99 |
| Soffcrepe® | 1.14 | |
| Setocrepe® | 1.18 | |
| Profore #2® | 1.16 | |
| K-Lite® | 0.89 | |
| Mean cost | 1.07 | |
| Class 3A | Elset® | 3.06 |
| Elset S® | 5.13 | |
| K-Plus® | 2.17 | |
| Profore #3® | 3.46 | |
| Mean cost | 3.46 | |
| Cohesive | Coban® | 3.61 |
| Profore #4® | 2.86 | |
| Hospifour # 4® | 1.93 | |
| Mean cost | 2.80 | |
| Class 3C | Setopress® | 3.46 |
| Tensopress® | 3.47 | |
| Profore Plus® | 3.24 | |
| Mean cost | 3.39 | |
| Compression systems (using mean costs presented) | Three-layer high compression: 1, 3C, cohesive | 6.67 |
| Three-layer reduced compression: 1, 2, cohesive | 4.36 | |
| Light compression: 1, cohesive | 3.28 |
Cost of health-care consultation
Both nurse- and patient-reported data regarding visits to or from health-care providers were available. Unless patients refused, nurse-reported data were intended to record every single nurse-administered ulcer treatment a participant received until healed, death or end of follow-up. Therefore, nurse-reported data comprised ulcer-related resource use only. Health-care consultations in patient self-reported data were extracted from the follow-up questionnaires (every 3 months). Participants were asked how many times they had seen a doctor or nurse and had been to hospital as an outpatient in the past 4 weeks and how many consultations were related to their ulcer. There were a large number of missing data regarding health-care consultations (for instance, the missing rates for patient-reported nurse visits are 28%, 32%, 37% and 38% in the 3, 6, 9 and 12-month follow-up questionnaires, respectively) so we used the nurse-reported data for the base case, while the use of patient-reported data were explored in a sensitivity analysis.
The cost per nurse visit was calculated by multiplying the duration of each visit by the cost per minute of nurse consultations, which varied depending on the location of treatment (Table 4). There was, however, no direct information available in the current trial for the duration of each nurse visit. We followed the approach used in VenUS I,7 in which the duration of a home visit was assigned as 40 minutes and a clinic visit as 22 minutes. The number was taken from the national estimates and was in agreement with a survey conducted within VenUS I to investigate the duration of time nurses spent on treating ulcer patients. Therefore, in the current study it is assumed that the duration of a nurse home visit was 40 minutes and all other visits were 22 minutes. When the visits included ultrasound treatment, the amount of time to apply ultrasound, as recorded by nurses, was added to the duration of visits.
| Item | Mean unit value (£ or minutes) | Source |
|---|---|---|
| Nurse consultation (per minute) | ||
| Community | 1.00 | PSSRU |
| Home visit | 1.07 | PSSRU |
| Travel cost for home visit | 1.40 | PSSRU |
| GP surgery | 0.48 | PSSRU |
| Hospital | 0.63 | PSSRU |
| Duration of nurse consultation | ||
| Home visit | 40 | VenUS I |
| Other visit | 22 | VenUS I |
All cost units/values regarding nurse consultation are listed in Table 4. The sources of costing include the VenUS I trial and the costs of health and social care published by the Personal Social Services Research Unit (PSSRU), University of Kent. 69
In the current trial, adverse events were recorded by the nurse. However, there was too little information available to allow for an accurate estimation of costs associated with these episodes. Therefore, the cost of adverse events was ignored in the base-case analysis. In sensitivity analysis, however, further resources used, e.g. hospital visits, were self-reported by patients. Thus, in this alternative analysis possible resource consumptions due to adverse event might be captured.
Total cost estimation
Resource use data are also subject to censoring, and estimating the mean total cost based on complete case analysis will underestimate the true expected costs. An IPW regression (as described above) was used to account for censoring, possible baseline imbalances and randomisation stratification variables. Mean cost estimation was made by partitioning the study period into quarterly periods and the IPW regression estimates obtained for each period were then summed to obtain the total expected costs.
Cost-effectiveness
To assess cost-effectiveness, the mean difference in costs between trial arms was compared with the mean difference in health benefits. For instance, if ultrasound plus standard care is more expensive and is associated with fewer health benefits than standard care alone, then ultrasound with standard care is dominated by standard care alone and the decision whether or not to adopt ultrasound is straightforward (not adopt). The decision arising from the converse situation (in which ultrasound plus standard care is dominant, i.e. less costly and more beneficial than standard care alone) is similarly straightforward (adopt). However, if ultrasound is more costly and more beneficial or less costly and less beneficial than standard care, we need to assess whether the increased cost of the ultrasound is worth the increased benefit, or whether the reduced benefit conferred by the ultrasound is justified by the reduced cost.
To ascertain the cost-effectiveness of a health-care intervention relative to another in the absence of dominance, an incremental analysis of cost-effectiveness is conducted. The incremental cost-effectiveness ratio (ICER) is the most commonly used cost-effectiveness measure and combines costs and health benefit in a single measure to which a decision rule for cost-effectiveness can be applied. It combines costs and benefits in a ratio of the mean difference in cost between the alternative treatments being compared with the mean difference in health benefits:
where C1 and B1 are, respectively, the mean costs and mean health benefits associated with the technology under evaluation (ultrasound with standard care in the current analysis), while C0 and B0 are the mean costs and mean health benefits associated with the comparator technology (standard care alone).
The decision rule for cost-effectiveness on the basis of the ICER indicates that a treatment strategy can be considered cost-effective only if the decision maker’s willingness to pay for an additional unit of health benefit (QALYs and ulcer-free days) is greater (or equal) to the ICER. According to NICE, the willingness to pay for an additional QALY ranges between £20,000 and £30,000. 67 Therefore, if the results of a cost–utility analysis (the estimated cost per QALY) is below this threshold, the intervention will be considered cost-effective.
Cost-effectiveness analysis in which the outcome is expressed in a natural unit, for example cost per ulcer-free day, may be more intuitive for clinicians. Despite this, and for the purpose of informing adoption decisions, the results of cost-effectiveness analysis are harder to interpret because there is no information on the willingness to pay for an additional ulcer-free day. Without this information we cannot determine whether or not the new intervention is cost-effective. To inform a decision based on this measure of health benefit, a threshold has to be established, and the decision maker is responsible for establishing this. Another reason to include this type of analysis is to facilitate the comparisons between relevant studies.
Uncertainty assessment
There is uncertainty in the incremental cost-effectiveness between two interventions, and this can be summarised graphically in a cost-effectiveness plane. By plotting the non-parametric bootstrapping results – 4000 replicates of difference in costs and health benefits (QALYs and ulcer-free days), the position of the estimates can indicate the degree of certainty that one of the interventions is dominant (north-west or south-east quadrants).
The ICER is associated with uncertainty because its numerator (expected costs) and its denominator (effectiveness) are estimated with sampling uncertainty. Therefore, a decision on whether or not to adopt a treatment based on the ICER estimate is also associated with uncertainty. In other words, if the trial were repeated, we might observe a different mean incremental cost and effectiveness, resulting in a different ICER estimate. The analysis of the consequences of this uncertainty and the extent to which it impacts on the adoption decision should be investigated to inform whether further research is needed. 70 Thus, the uncertainty around incremental costs and effects estimates and its impact on the adoption decision were investigated here.
Confidence intervals
Uncertainty around the decision to adopt the treatment under evaluation was assessed through a non-parametric bootstrap resampling technique. 71 This is a common methodology used to construct CIs around the incremental costs and health benefits from sampled cost and effectiveness random variables. Firstly, the bootstrap technique was used to sample (with replacement) from the observed cost and effectiveness pairs, maintaining the correlation structure. For each bootstrap resample, an IPW estimate of expected total costs, expected QALYs and expected time to healing was calculated, which allowed computation of cost-effectiveness and cost–utility outcome replicates. The 95% CIs for the differential costs and QALYs were then calculated using bias-corrected non-parametric bootstrapping.
Cost-effectiveness acceptability curves
To explore the decision uncertainty regarding the cost-effectiveness of ultrasound treatment, cost-effectiveness acceptability curves (CEACs) were plotted. The CEAC expresses the probability that an intervention is cost-effective in relation to the comparator intervention, as a function of the threshold willingness to pay. 72 Thus, the CEAC summarises, for every value of willingness to pay, the evidence in favour of the intervention being cost-effective. In this case, given the trial data, the CEAC for ultrasound with standard care represents the probability of this treatment being cost-effective compared with standard care alone for a range of willingness-to-pay values for an additional ulcer-free day/QALY.
Sensitivity analysis
Scenario 1
To explore the impact of the source of health-care consultation on cost-effectiveness/cost–utility analysis, a sensitivity analysis was conducted. In this analysis, patient-reported, instead of nurse-reported, data were used to calculate leg ulcer-related costs. Costs unrelated to leg ulcers were assumed to be equal between arms and were thus not considered in this analysis. 66 In contrast to the base-case analysis, in which only nurse consultations were costed, in this scenario we also considered ulcer-related hospital visits and doctor consultations. The cost per visit was calculated assuming different duration of home and clinic visits, as stated in Table 4. The self-reported data collected information about the visit setting (home or clinic). However, this information was recorded for all doctor/nurse/outpatient visits rather than for ulcer-related visits only. Consequently, we assumed that the setting of ulcer-related visits from self-reported data would follow the same pattern as that reported for all visits, for which data were available.
As shown in Table 5, the additional unit cost applied in this sensitivity analysis included doctor visit at GP surgery and at home, £31 and £50 per visit, respectively. Furthermore, the cost per hospital outpatient was charged as a non-consultant-led follow-up attendance fee, £71.
| Item | Mean unit value (£) | Source |
|---|---|---|
| Doctor visit | ||
| GP surgery | 31 | PSSRU |
| Home visit | 50 | PSSRU |
| Hospital outpatient | 71 | PSSRU |
Scenario 2
One-way sensitivity analysis was also carried out to investigate the impact of the cost of the ultrasound machine on the total cost estimation. In the base-case analysis, the cost of the ultrasound machine was borne by the trial participants only (those in the treatment arm). In reality (such as the NHS setting), ultrasound machines are used for other purposes as well, e.g. tennis elbow and plantar fasciitis, therefore the cost of equipment per patient should be lower (shared by more users). This implied that in the current trial the average cost of the treatment arm was overestimated.
Chapter 3 Protocol changes
Recruitment period
As stated in the original protocol, we planned to recruit 336 patients over an 18-month period with all participants being followed up for 12 months. As the study progressed, a low recruitment rate (despite the recruitment of extra study sites; Appendix 3 details participation length of sites), led us to apply to the funder for an 18-month extension to the recruitment period (July 2007 to December 2008). The original sample size of 336 participants remained unchanged.
Follow-up
The extension to the recruitment period resulted in a variable duration of follow-up, depending on when the patient was randomised. The extension required that the last day of any follow-up was set as 30 June 2009 so that patients recruited between July 2008 and 31 December 2008 were followed up for periods ranging from 6 to 12 months. Patients were followed up, where possible, until they became ulcer free, in order to provide economic end points. In patients with only one ulcer, and in those in whom smaller ulcers healed before the reference ulcer healed, the date of healing of the reference ulcer was deemed to be the date of complete healing.
Inclusion and exclusion criteria
The study investigators felt that many people may have venous ulcers in the presence of rheumatoid arthritis and that an ulcer is not necessarily due to their rheumatoid disease. We had initially excluded this population as people with arterial inflammation may be more prone to compression damage, but given that the clinicians caring for these patients reported that they commonly used high compression, we decided to drop the exclusion criterion ‘rheumatoid arthritis’.
Similarly, clinical collaborators argued that people can have venous ulcers in the presence of diabetes mellitus, and that their ulcer may not be secondary to diabetes. We had initially excluded this population as, according to National Clinical Practice Guidelines, they would not be suitable for high compression. However, we were informed that in some clinical centres expert practitioners treated people with well-controlled diabetes and an expert vascular assessment, with high compression therapy. With well-controlled diabetes defined as a recent HbA1c level of < 10% we dropped the exclusion criterion ‘diabetes’ and replaced it with the requirement that anyone with diabetes had an HbA1c of < 10%.
It was decided to drop the exclusion criterion of peripheral arterial disease, as it was felt to be unnecessary given that the inclusion criterion states that the ulcer must be primarily due to venous disease. If the recruiting nurse had considered someone for trial entry owing to the patient’s clinical diagnosis of ‘ulcer primarily due to venous insufficiency’ and the ABPI reading confirmed the lack of significant arterial insufficiency, it was felt appropriate to drop the exclusion criterion of ‘peripheral arterial disease’.
The original protocol for which we gained ethical approval was to recruit people with venous ulceration and an ABPI of at least 0.8 who are able to tolerate high-compression therapies. Again, our clinical collaborators argued that some people who are tolerant of only reduced compression therapy represent a particular challenge, as at present high-compression therapy is the single most effective element of treatment. It was then felt appropriate to drop the inclusion criterion of ‘willing and able to wear four-layer high-compression bandaging’, as these patients (people with hard-to-heal venous ulcers unable to tolerate high compression) could be a group who may benefit from any effect of therapeutic ultrasound.
Outcome measures
In light of advice from the Trial Steering Committee (20 January 2006) and the Trial Management Group (3 March 2006) we decided to amend the primary outcome measure.
The rationale for this was that we had initially set the primary outcome measure as ‘complete healing of all ulcers’. This is, clinically, the time at which leg ulcer treatment can be said to have achieved its ultimate aim, and the patient no longer requires dressings, bandages or nurses visits. However, in this trial the ultrasound was to be delivered only to the reference (i.e. largest) ulcer and, therefore, any outcome measure which relied on the healing of other ulcers remote from this would have the potential to dilute any treatment effect.
We therefore amended the primary outcome as ‘complete healing of the ulcer treated with ultrasound (the reference ulcer)’ and recorded the time to complete healing of all ulcers as a secondary outcome measure.
Digital images
The original protocol did not require the use of digital photographs of the reference ulcer to follow healing progress or to verify healing. However, following the success of this in VenUS II44 it was decided to include such digital photographs, to be taken every 4 weeks, on healing and 7 days post healing in order to validate the outcome of healing. These photographs were to be assessed ‘blind’ by assessors for confirmation of healing.
Questionnaire response rate
We had identified that the patient questionnaire return rates in the previous VenUS trials7,44 could be improved and, therefore, we obtained agreement from collaborators and the Ethics Committee to send £5 as a token ‘thank you’ payment to patients at the end of the trial, with the final questionnaire. This was not mentioned in the patient information sheet, so that any possibility that it would be interpreted as a financial incentive to taking part in the trial was minimised. The final questionnaire, at 12 months post randomisation, was preceded by a letter notifying the patient that their final trial questionnaire was due to arrive shortly. This letter also stated that the questionnaire would be accompanied by a £5 note as a thank you for their taking part in the trial and completing the questionnaires. In the letter it was made explicit that the £5 was not conditional on the patient returning the questionnaire. This approach was based on the results of a systematic review which had investigated ways of increasing questionnaire response rates54 and on its successful use in two previous studies co-ordinated by the York Trials Unit. 73,74
Chapter 4 Clinical results
This chapter presents the statistical analysis of the clinical results of VenUS III. In the first section of the chapter the clinical data are described, including tables and figures of the data summaries. In the second section the statistical models fitted to the data are presented. Before the results of each statistical model, exploratory data analysis specific to that modelling framework will be presented to show the rationale for this modelling framework.
Trial recruitment
Twelve centres participated in the study from across the UK and Ireland. The number of patients recruited per site ranged from 2 to 102. These were Western Health and Social Care Trust (WHSCT), Leeds Community, Scarborough, Bradford, Bolton, Nottingham, Hull, South Essex, Birmingham, Dublin, Cumbria and Dunfermline.
Figure 1, the consolidated standards of reporting trials (CONSORT) flowchart, shows the flow of patients through the trial.
FIGURE 1.
Venous Ulcer Study III consolidated standards of reporting trials diagram.
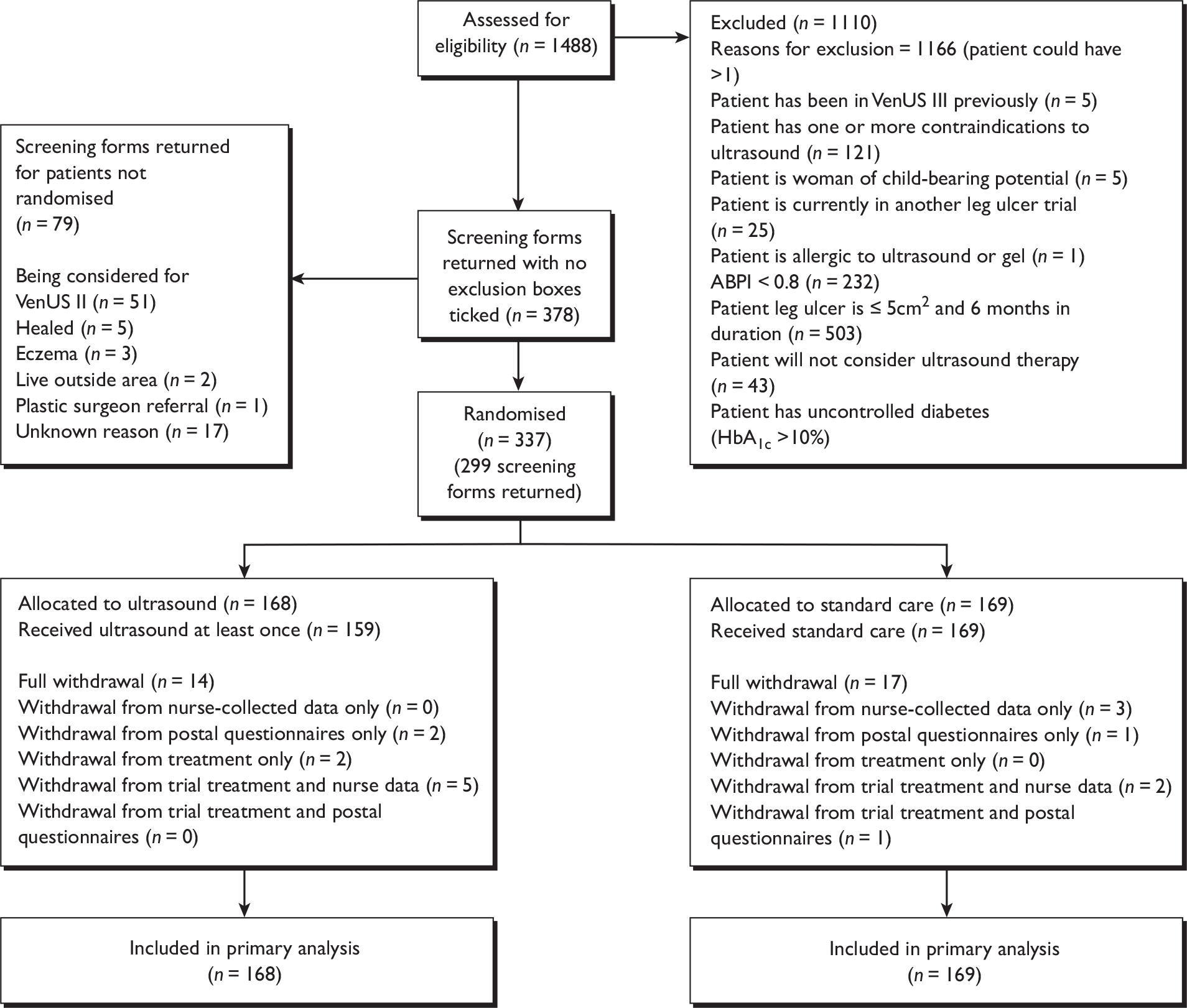
Clinical data
Baseline patient characteristics
In total, 337 patients were recruited to the study: 168 in the ultrasound group and 169 in the standard care group. The baseline characteristics are shown in Table 6.
| Ultrasound (n = 168) | Standard care (n = 169) | Overall (N = 337) | |
|---|---|---|---|
| Gender | |||
|
Male (%) Female (%) |
64 (46.72) 104 (52.00) |
73 (53.28) 96 (48.00) |
137 (40.65) 200 (59.35) |
| Age (years) | |||
| Mean (SD) | 68.91 (14.80) | 69.92 (14.21) | 69.44 (14.50) |
| Median (min, max) | 71.64 (26.66, 95.76) | 71.91 (20.57, 98.81) | 71.85 (20.57, 98.81) |
| Missing (%) | 0 (0) | 0 (0) | 0 (0) |
| Height (m) | |||
| Mean (SD) | 1.69 (0.11) | 1.69 (0.11) | 1.69 (0.11) |
| Median (min, max) | 1.68 (1.40, 1.96) | 1.68 (1.40, 2.01) | 1.68 (1.40, 2.01) |
| Missing (%) | 5 (2.98) | 5 (2.96) | 10 (2.97) |
| Weight (kg) | |||
| Mean (SD) | 86.88 (23.81) | 88.71 (27.44) | 87.80 (25.66) |
| Median (min, max) | 82.23 (38.10, 180.98) | 85.50 (38.10, 184.16) | 82.55 (38.10, 184.16) |
| Missing (%) | 6 (3.57) | 7 (4.14) | 13 (3.86) |
| BMI | |||
| Mean (SD) | 30.42 (7.50) | 30.72 (8.28) | 30.57 (7.89) |
| Median (min, max) | 28.98 (17.19, 57.01) | 29.38 (15.36, 65.53) | 29.28 (15.36, 65.53) |
| Missing (%) | 7 (4.17) | 8 (4.73) | 15 (4.45) |
| Patient mobility | |||
| Walks freely (%) | 90 (48.65) | 95 (51.35) | 185 (54.90) |
| Walks with difficulty (%) | 69 (48.94) | 72 (51.06) | 141 (41.84) |
| Immobile (%) | 5 (71.43) | 2 (28.57 ) | 7 (2.10) |
| Missing (%) | 4 (2.38) | 169 (0.00) | 4 (1.19) |
The majority of patients in the study were female (59%) and the two groups were well balanced for gender: 52% females in the ultrasound versus 48% in the control arm. The mean age of the patients in the study was 69 (SD 15) years, and randomisation resulted in well balanced groups for age, gender, body mass index (BMI), and mobility.
Table 7 shows the baseline ulcer data for both groups. This confirms the chronic, ‘hard-to-heal’ nature of this population. Ulcer duration was high, as expected with the inclusion criteria (> 6 months duration and/or > 5 cm2), with a median of 12 months. Median ulcer area was 12 cm2 (mean 28 cm2). The median worst ulcer pain score in the previous 24 hours baseline was 31 (range 0–100). The groups were well balanced for ulcer area, duration and level of pain.
| Ultrasound (n = 168) | Standard care (n = 169) | Overall (N = 337) | |
|---|---|---|---|
| Reference ulcer area categorised | |||
| ≤ 5 cm2 N and (%) | 43 (51.19) | 41 (48.81) | 84 (24.93) |
| > 5 cm2 N and (%) | 125 (49.41) | 128 (50.59) | 253 (75.07) |
| Reference ulcer area in cm2 | |||
| Mean (SD) | 27.95 (44.47) | 27.18 (41.96) | 27.56 (43.17) |
| Median (min, max) | 12.0 (1.0, 268.0) | 12.0 (1.0, 283.5) | 12.0 (1.0, 283.5) |
| Reference ulcer duration | |||
| ≤ 6 months, n (%) | 42 (42.86) | 56 (57.14) | 98 (29.08) |
| > 6 months, n (%) | 126 (52.72) | 113 (47.28) | 239 (70.92) |
| Ulcer duration (months) | |||
| Mean (SD) | 32.19 (64.14) | 31.82 (53.96) | 32.01 (59.17) |
| Median (min, max) | 12.0 (1.0, 516.0) | 11.0 (1.0, 360.0) | 12.0 (1.0, 516.0) |
| Worst pain from ulcer in previous 24 hours (VAS: 0–100) | |||
| Mean (SD) | 38.16 (29.36) | 32.44 (26.72) | 35.28 (28.17) |
| Median (min, max) | 34.51 (0.00, 100.00) | 27.46 (0.00, 100.00) | 30.80 (0.00, 100.00) |
| Missing (%) | 7 (4.17) | 6 (3.55) | 13 (3.86) |
| Reference leg | |||
| Left (%) | 99 (53.51) | 86 (49.49) | 185 (54.90) |
| Right (%) | 66 (44.59) | 82 (55.41) | 148 (43.92) |
| Missing (%) | 3 (1.79) | 1 (0.60) | 4 (1.19) |
| Treated with high compression | |||
| Yes (%) | 145 (49.49) | 148 (50.51) | 293 (86.94) |
| No (%) | 23 (52.27) | 21 (47.73) | 44 (13.06) |
| ABPI | |||
| Mean (SD) | 1.06 (0.19) | 1.08 (0.21) | 1.07 (0.20) |
| Median (min, max) | 1.06 (0.00, 1.69) | 1.10 (0.08, 1.64) | 1.08 (0.00, 1.69) |
| Missing (%) | 7 (4.17) | 3 (1.78) | 10 (2.97) |
| Duration of oldest ulcer (months) | |||
| Mean (SD) | 33.93 (66.91) | 33.58 (56.38) | 33.75 (61.76) |
| Median (min, max) | 12.0 (0.0, 516.0) | 11.0 (0.0, 36.0) | 12.0 (0.0, 516.0) |
| Missing (%) | 4 (2.38) | 4 (2.37) | 8 (2.37) |
| Number of ulcers on reference leg | |||
| Mean (SD) | 2.01 (1.59) | 1.81 (1.49) | 1.90 (1.54) |
| Median (min, max) | 1 (1, 10) | 1 (1, 9) | 1 (1, 10) |
| Missing (%) | 3 (1.79) | 0 (0.00) | 3 (0.90) |
| Number of ulcer episodes | |||
| Mean (SD) | 3.55 (5.03) | 3.71 (8.88) | 3.63 (7.18) |
| Median (min, max) | 2 (0, 30) | 1 (0, 99) | 1.5 (1, 99) |
| Missing (%) | 7 (4.17) | 12 (7.10) | 19 (5.64) |
| Ankle mobility | |||
| Full range of motion (%) | 112 (53.08) | 99 (46.92) | 211 (62.61) |
| Reduced range of motion (%) | 38 (41.30) | 54 (58.70) | 92 (27.30) |
| Fixed ankle (%) | 6 (60.00) | 4 (40.00) | 10 (2.97) |
| Missing (%) | 12 (7.14) | 12 (7.10) | 24 (7.12) |
| Ankle circumference (cm) | |||
| Mean (SD) | 24.72 (2.91) | 24.56 (3.06) | 24.64 (2.99) |
| Median (min, max) | 24.5 (17.0, 36.0) | 24.0 (19.5, 37.0) | 24.0 (17.0, 37.0) |
| Missing (%) | 7 (4.17) | 0 (0.00) | 7 (2.08) |
The distribution of baseline ulcer area was highly skewed (coefficient of variation 157%); therefore, to reduce this high variability, in the subsequent analysis the logarithm of baseline ulcer area was used. The baseline ulcer duration was also highly skewed (coefficient of variation of 185%) therefore the logarithm of ulcer duration was used.
Table 7 also shows that the two groups are similar in terms of ulcer history on the affected leg, number of previous ulcer episodes, time since first ulcer on this leg and ankle mobility. There seemed to be no difference between the two treatment arms in the distribution of patients in high compression, the mean ABPI, the median duration of the oldest ulcer and number of ulcers per participant. The median number of ulcer episodes was 1.5 (range: 0–99), and the majority of the patients’ ankles were described as having a full range of motion (63%); only 3% of patients’ ankles were described as ‘fixed’. The median ankle circumference was 24 cm (range 17–37cm), and the two groups were comparable to each other: ultrasound: 25 (SD 3) and standard care: 25 (SD 3).
Ulcer healing
Determination of healing date
The time to healing (survival time) of the reference ulcer was the defined as the time from the date of randomisation to the date of healing (with healing date decided by two independent, blinded adjudicators looking at photographs of the reference ulcers). Any disagreements were resolved by discussion or referral to a third blinded assessor. If no photographs were available, or if they were unclear, then the date recorded on the Ulcer Healed Form was used. Those patients who were lost to follow-up, withdrew from the study and were followed up until the end of the study without experiencing the event (healing of the reference leg ulcer) were treated as censored observations. Their survival time was the last date they were observed in the study. The explanatory variables – ulcer area, ulcer duration, ulcer compression and participating centre – were recorded at baseline.
The kappa measure of agreement was used to assess the agreement between the two assessors of the blinded photographs as to whether or not the wound had healed. This was also repeated by looking at the agreement between the final decision from the blinded photographs and the nurse’s decision on healing status of the patients.
Table 8 shows the data comparing the outcomes from two independent assessors for photographs of 331 ulcers/ulcer sites. Of these, 185 ulcers were deemed by both assessors to be ‘not healed’ while both agreed that 120 had healed. The assessors disagreed in 26 cases: assessor 1 classified 13 ulcers as healed while assessor 2 did not; similarly, assessor 2 classified 13 ulcers as healed while assessor 1 classified them as not healed. To quantify the strength of this association, the kappa measure of agreement was estimated as 0.84 (standard error 0.03, 95% CI 0.78 to 0.90). This indicates an almost perfect agreement. Where the two assessors disagreed on the date of healing, a third assessor was asked to evaluate the blinded photographs and 13 more patients were found to have healed, bringing the total number of healed patients to 133.
| Case I | Case II | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Assessor 2 | Blinded photographs | ||||||||
| Not healed | Healed | Total | Not healed | Healed | Total | ||||
| Assessor 1 | Not healed | 185 | 13 | 198 | Nurse decision | Not healed | 164 | 1 | 165 |
| Healed | 13 | 120 | 133 | Healed | 40 | 132 | 172 | ||
| Total | 198 | 133 | 331 | Total | 204 | 133 | 337 | ||
In Table 8 we also summarise the extent to which the nurse decision agreed with the blinded photographs. There was agreement between nurses and the photographic assessment (by two assessors – above) for 164 unhealed and 132 healed ulcers. There was disagreement between the nurse judgement and blindly assessed photographs in 41 cases: in 40 cases, the nurse judged that ulcers had healed but the blinded assessors considered the same photographs to indicate that ulcers had not yet healed. In one case the nurse described the ulcer as not healed when the two independent assessors agreed that it had. To quantify the strength of this association the kappa measure of agreement was estimated as 0.76 (standard error 0.03, 95% CI 0.69 to 0.83). This indicates a substantial agreement.
Healing data by site and treatment group
Twelve centres participated in the study (Table 9). In our analysis, centres that recruited fewer than five patients were combined. Consequently, Dunfermline, Cumbria and Dublin were combined with a total enrolment of nine patients (2.67%).
| Centre name | Ultrasound (n = 168) (%) | Standard care (n = 169) (%) | Overall N = 337 (%) | Percentage healed | Adverse events: total (NSAE, SAE) |
|---|---|---|---|---|---|
| WHSCT | 50 (49) | 52 (51) | 102 (30.27) | 57 (58/102) | 38 (27, 11) |
| Leeds Community | 22 (50) | 22 (50) | 44 (13.06) | 50 (22/44) | 109 (101, 8) |
| Scarborough | 21 (49) | 22 (51) | 43 (12.76) | 47 (20/43) | 129 (100, 29) |
| Bradford | 22 (52) | 20 (48) | 42 (12.46) | 33 (14/42) | 73 ( 62, 11) |
| Bolton | 19 (53) | 17 (47) | 36 (10.68) | 19 (7/36) | 10 (3, 7) |
| Nottingham | 15 (50) | 15 (50) | 30 (8.90) | 17 (5/30) | 51 (40, 11) |
| Hull | 8 (50) | 8 (50) | 16 (4.75) | 31 (5/16) | 111 (103, 8) |
| South Essex | 5 (56) | 4 (44) | 9 (2.67) | 0 (0/9) | 0 ( 0, 0) |
| Birmingham | 3 (50) | 3 (50) | 6 (1.78) | 17 (1/6) | 7 ( 6, 1) |
| Dublin | 2 (50) | 2 (50) | 4 (1.19) | 11 (1/9) | 5 (3, 2) |
| Cumbria | 1 (33) | 2 (67) | 3 (0.89) | 11 (1/9) | 5 (3, 2) |
| Dunfermline | 1 (50) | 1 (50) | 2 (0.59) | 11 (1/9) | 5 (3, 2) |
In total, 133 out of the 337 patients (39%) had a healed reference ulcer during follow-up while 204 out of the 337 patients (61%) did not heal. The percentage of healed patients in each centre was highest in WHSCT (57%) and Leeds Community (50%). The number of adverse events (serious or non-serious) by trial centre is given in Table 8. There were a total of 533 adverse events (88 SAEs and 445 NSAEs).
Health-related quality of life data
The SF-12v2 (4-week recall) questionnaire was used to assess self-reported HRQoL at baseline and at 3, 6, 9 and 12 months. The descriptive statistics for the physical component score (PCS) and mental component score (MCS) are presented in Table 10. The descriptive statistics for the other component scores (physical functioning, role physical, bodily pain, general health, vitality, role emotional, social functioning and mental health) are presented later. Analysis was done only on the PCS and MCS. All the other scores have been presented descriptively. The minimum score was 0 and the maximum score was 100, with 0 as the worst score.
| PCS | MCS | |||||
|---|---|---|---|---|---|---|
| Ultrasound (n = 168) | Standard care (n = 169) | Overall (N = 337) | Ultrasound (n = 168) | Standard care (n = 169) | Overall (N = 337) | |
| Baseline (n) | 160 | 167 | 327 | 160 | 167 | 327 |
| Mean (SD) | 36.55 (11.32) | 35.33 (11.47) | 35.93 (11.40) | 46.72 (11.52) | 47.11 (11.29) | 46.92 (11.39) |
| Median (range) | 36.75 (12.15–58.80) | 35.78 (7.10–58.05) | 36.28 (7.10–58.80) | 47.43 (19.85–67.93) | 46.94 (18.71–66.14) | 47.25 (18.71–67.93) |
| Missing (%) | 8 (4.76) | 2 (1.18) | 10 (2.97) | 8 (4.76) | 2 (1.18) | 10 (2.97) |
| 3 months | 143 | 142 | 285 | 143 | 142 | 285 |
| Mean (SD) | 33.87 (11.49) | 34.96 (11.39) | 34.41 (11.43) | 45.95 (12.22) | 46.83 (11.38) | 46.39 (11.80) |
| Median (range) | 33.24 (5.90–57.49) | 33.76 (8.67–58.05) | 33.35 (5.90–58.05) | 46.36 (12.61–72.78) | 48.61 (17.55–68.49) | 47.43 (12.61–72.78) |
| Missing (%) | 25 (14.88) | 27 (15.98) | 52 (15.43) | 25 (14.88 ) | 27 (15.98) | 52 (15.43) |
| 6 months | 127 | 135 | 262 | 127 | 135 | 262 |
| Mean (SD) | 34.65 (12.59) | 34.93 (12.24) | 34.79 (12.39) | 46.05 (11.69) | 46.83 (10.84) | 46.46 (11.25) |
| Median (range) | 33.55 (4.64–59.11) | 34.21 (8.37–61.52) | 33.97 (4.64–61.52) | 48.28 (14.29–66.77) | 47.79 (19.75–67.51) | 47.85 (14.29–67.51) |
| Missing (%) | 41 (24.40) | 34 (20.12) | 75 (22.26) | 41 (24.40) | 34 (20.12) | 75 (22.26) |
| 9 months | 116 | 117 | 233 | 116 | 117 | 233 |
| Mean (SD) | 34.51 (11.81) | 34.55 (12.31) | 34.53 (12.03) | 46.86 (12.42) | 46.88 (11.18) | 46.87 (11.78) |
| Median (range) | 33.98 (9.43–58.05) | 33.45 (10.29–60.18) | 33.50 (9.43–60.18) | 47.72 (14.48–65.58) | 46.92 (15.33–65.20) | 47.50 (14.48–65.58) |
| Missing (%) | 52 (30.95) | 52 (30.77) | 104 (30.86) | 52 (30.95) | 52 (30.77) | 104 (30.86) |
| 12 months | 118 | 111 | 229 | 118 | 111 | 229 |
| Mean (SD) | 34.61 (12.09) | 35.57 (1.88) | 35.07 (11.97) | 47.51 (11.54) | 45.41 (12.15) | 46.49 (11.86) |
| Median (range) | 33.19 (8.17–60.39) | 35.07 (13.93–57.49) | 34.36 (8.17–60.39) | 48.50 (15.48–67.69) | 44.67 (12.52–71.22) | 47.08 (12.52–71.22) |
| Missing (%) | 50 (29.76 ) | 58 (34.32) | 108 (32.05) | 50 (29.76) | 58 (34.32) | 108 (32.05) |
The mean PCS and MCS for the study population at baseline were compared with the SF-12v2 standard norms from the 1998 general US population. The median age of the VenUS III population was 72 years so we compared the mean baseline scores of the participants with the US norm-based scores for individuals aged between 65 and 74 years. In VenUS III the PCS means (SD) were 36.55 (11.32) for the ultrasound group and 35.33 (11.47) for the standard care group, compared with 43.93 (9.29) for the general US population. For the MCS, the baseline means were 46.72 (11.52) for the ultrasound group and 47.11 (11.29) for the standard care group, compared with 51.57 (8.36) for the general US population. Therefore, mean PCS and MCS for our trial population were lower than the mean values of the general US population, suggesting that our trial participants had lower QoL in terms of physical and mental health compared with a similar age group in the USA.
The changes in HRQoL as measured by the SF-12v2 are summarised in Table 10, and illustrated in Figures 2 and 3.
FIGURE 2.
Short Form questionnaire-12 items physical component scores over time (mean and 95% CI).
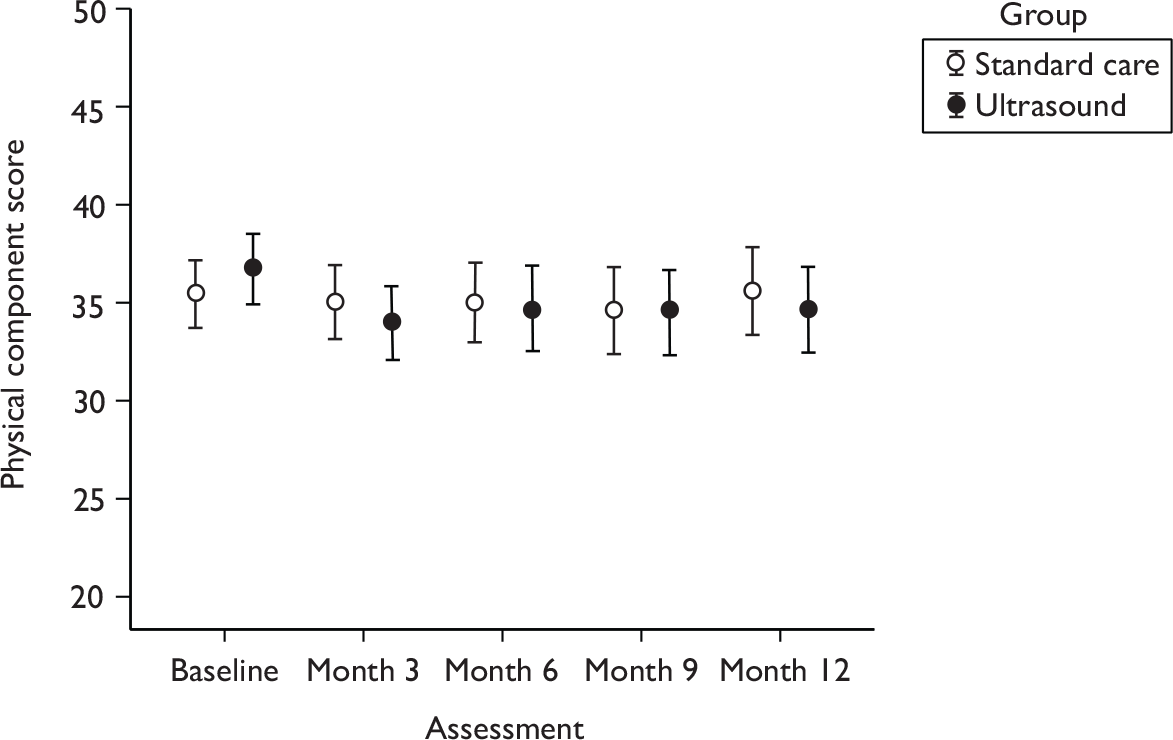
FIGURE 3.
Short Form questionnaire-12 items mental component scores over time (mean and 95% CI).
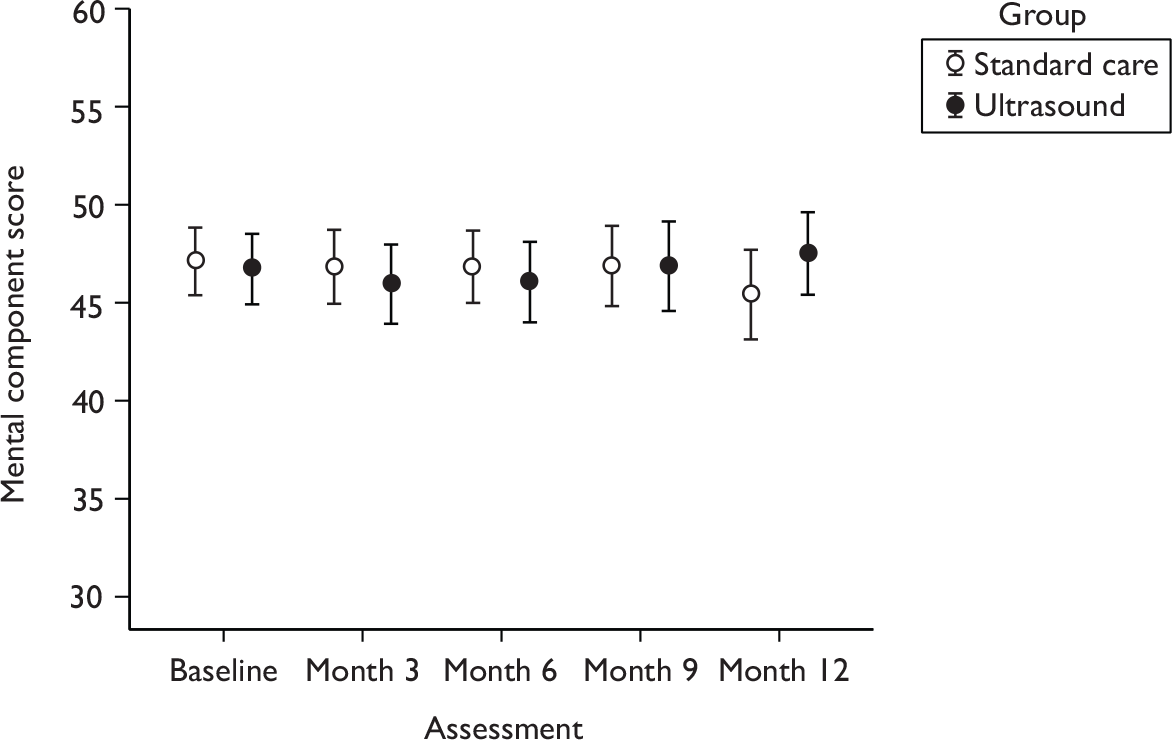
Overall there seemed to be no change in the PCS or the MCS during follow-up. The eight individual subscales of SF-12v2 are shown in Table 11.
| Time point | Physical scores, mean (SD) | Mental scores, mean (SD) | ||||
|---|---|---|---|---|---|---|
| Ultrasound (n = 168) | Standard care (n = 169) | Overall (N = 337) | Ultrasound (n = 168) | Standard care (n = 169) | Overall (N = 337) | |
| Physical functioning | Vitality | |||||
| Baseline | 36.54 (11.62) | 36.23 (12.15) | 36.38 (11.88) | 44.16 (11.36) | 43.05 (12.44) | 43.60 (11.92) |
| 3 months | 34.28 (11.94) | 34.81 (12.54) | 34.54 (12.23) | 42.26 (11.56) | 43.00 (11.03) | 42.63 (11.29) |
| 6 months | 34.27 (11.98) | 34.81 (12.34) | 34.55 (12.14) | 41.73 (10.48) | 42.64 (10.74) | 42.20 (10.61) |
| 9 months | 34.23 (12.21) | 34.74 (12.20) | 34.48 (12.18) | 42.46 (10.98) | 42.85 (11.41) | 42.65 (11.18) |
| 12 months | 34.69 (12.49) | 34.85 (12.37) | 34.76 (12.40) | 42.25 (10.78) | 42.44 (10.97) | 42.34 (10.84) |
| Role physical | Social functioning | |||||
| Baseline | 39.01 (11.85) | 34.70 (11.21) | 38.35 (11.53) | 39.61 (14.47) | 39.09 (14.34) | 39.49 (14.39) |
| 3 months | 37.53 (11.67) | 38.36 (11.40) | 37.94 (11.52) | 37.57 (14.46) | 40.10 (13.94) | 38.83 (14.23) |
| 6 months | 37.86 (11.32) | 37.93 (10.89) | 37.90 (11.07) | 38.28 (14.44) | 39.76 (13.62) | 39.04 (14.01) |
| 9 months | 38.39 (10.96) | 37.68 (11.50) | 38.04 (11.22) | 39.24 (14.70) | 39.65 (13.71) | 39.45 (14.18) |
| 12 months | 38.57 (11.18) | 39.08 (10.95) | 38.82 (11.05) | 39.20 (14.09) | 38.74 (13.95) | 38.97 (13.99) |
| Bodily pain | Role emotional | |||||
| Baseline | 38.60 (14.05) | 38.52 (13.53) | 38.56 (13.77) | 42.99 (13.08) | 44.10 (12.69) | 43.55 (12.87) |
| 3 months | 36.41 (13.53) | 38.57 (12.84) | 37.49 (13.21) | 41.52 (14.10) | 42.84 (12.79) | 42.18 (13.45) |
| 6 months | 38.02 (14.15) | 38.95 (13.36) | 38.49 (13.73) | 42.93 (13.49) | 43.24 (12.09) | 43.09 (12.77) |
| 9 months | 38.65 (12.92) | 38.29 (13.91) | 38.47 (13.40) | 42.53 (13.56) | 42.09 (13.36) | 42.31 (13.43) |
| 12 months | 38.55 (13.79) | 38.54 (12.49) | 38.55 (13.14) | 43.11 (13.78) | 41.37 (12.85) | 42.27 (13.33) |
| General health | Mental health | |||||
| Baseline | 40.50 (11.90) | 39.30 (12.41) | 39.88 (12.02) | 46.32 (11.40) | 46.40 (10.82) | 46.36 (11.09) |
| 3 months | 37.23 (11.77) | 37.91 (11.25) | 37.56 (11.50) | 46.13 (12.05) | 45.91 (10.96) | 46.02 (11.50) |
| 6 months | 38.27 (12.09) | 37.94 (12.04) | 38.10 (12.04) | 45.37 (11.80) | 46.01 (11.06) | 45.70 (11.41) |
| 9 months | 37.47 (11.93) | 37.93 (11.33) | 37.70 (11.61) | 46.73 (12.04) | 46.78 (10.70) | 46.75 (11.36) |
| 12 months | 38.57 (11.99) | 37.82 (12.21) | 38.21 (12.08) | 47.91 (10.95) | 45.60 (12.17) | 46.79 (11.59) |
Record of patient treatment
Table 12 summarises the treatment details of the two groups: for the standard care patients during follow-up and for the ultrasound patients during both the 12 weeks on ultrasound therapy and the post-ultrasound period. For the standard care patients during follow-up, the median number of nurse consultations during study follow-up was 28 (range 1–354). There were a total of 6508 consultations in the home, leg ulcer clinic, nursing home, GP surgery, leg ulcer club and other specified locations. The majority of consultations took place in the leg ulcer clinic (39%), while the fewest were in a leg ulcer club (0.02%). In 40% of consultations knitted viscose dressings were applied (e.g. non-adherent). There were a total of 6473 bandages used during follow-up ranging from four-layer high-compression to non-compressive regimens. The four-layer high-compression system was most frequently applied (35% of consultations).
| Group | Standard care (n = 169) | Ultrasound (n = 168) | |
|---|---|---|---|
| Through trial follow-up | Initial 12 weeks of ultrasound | Post 12 weeks’ ultrasound | |
| Number of nurse consultations | |||
| Mean (SD) | 41.34 (46.76) | 15.36 (8.88) | 39.23 (38.06) |
| Median (min, max) | 28 (1, 354) | 13 (2, 51) | 33 (1, 273) |
| Missing (%) | 10 (5.92) | 9 (5.34) | 47 (27.98) |
| Setting of treatment consultation | |||
| Home (%) | 2344 (36.02) | 817 (33.87) | 1717 (36.45) |
| Leg ulcer clinic (%) | 2545 (39.11) | 1077 (44.65) | 1806 (38.34) |
| Nursing home (%) | 13 (0.20) | 20 (0.83) | 29 (0.62) |
| GP surgery (%) | 1071 (16.46) | 324 (13.43) | 783 (16.62) |
| Leg ulcer club (%) | 1.0 (0.02) | 1 (0.04) | 0 (0.00) |
| Other (%) | 534 (8.21) | 173 (7.17) | 375 (7.96) |
| Total (%) | 6508 (100) | 2412 (100) | 4710 (100) |
| Knitted viscose dressing applied | |||
| Yes (%) | 2556 (39.86) | 1181 (54.55) | 1889 (40.33) |
| No (%) | 3856 (60.14) | 984 (45.45) | 2795 (59.67) |
| Total (%) | 6412 (100) | 2165 (100) | 4684 (100) |
| Compression bandages applied | |||
| Four-layer high compression (%) | 2241 (34.62) | 875 (36.83) | 1276 (22.64) |
| Three-layer high compression (%) | 1543 (23.84) | 488 (20.54) | 1837 (32.59) |
| Three-layer reduced compression (%) | 668 (10.32) | 188 (7.91) | 875 (15.52) |
| Two-layer high compression (%) | 857 (13.24) | 297 (12.50) | 624 (11.07) |
| Short-stretch compression (%) | 805 (12.44) | 319 (13.43) | 529 (9.38) |
| Low compression (%) | 139 (2.15) | 82 (3.45) | 168 (2.98) |
| No compression (%) | 220 (3.40) | 127 (5.35) | 328 (5.82) |
| Total (%) | 6473 (100) | 2376 (100) | 5,637 (100) |
| Number of ultrasound applications | |||
| Mean (SD) | – | 10.25 (2.96) | – |
| Median (min, max) | – | 11 (1, 15) | – |
| Missing (%) | – | 9 (5.34) | – |
| Proportion of consultations where ultrasound applied | |||
| Mean (SD) | – | 0.77 (0.24) | – |
| Median (min, max) | – | 0.86 (0.14, 1.00) | – |
| Missing (%) | – | 9 (5.34) | – |
| Duration of ultrasound application (minutes) | |||
| Mean (SD) | – | 73.94 (31.69) | – |
| Median (min, max) | – | 69.0 (9.0, 130.0) | – |
| Missing (%) | – | 9 (5.34) | – |
The median number of nurse consultations during the initial 12 weeks of ultrasound therapy was 13 (range 2–51; total of 2412 consultations). The majority of consultations took place at the leg ulcer clinic (45%) and the fewest at a leg ulcer club (0.04%). The median number of ultrasound applications was 11 (range 1–15). Ultrasound was delivered during most treatment consultations during the initial 12-week period: the median proportion of visits receiving ultrasound was 0.86 (range 0.14–1). In total, patients received more than 1 hour of ultrasound: the median duration of total ultrasound application was 69 minutes (range 9–130). During the post-ultrasound therapy period, the median number of nurse consultations was 33 (range 1–273) mainly in leg ulcer clinics (38%) with no consultations in leg ulcer clubs (0%). Knitted viscose dressings were applied during 40% of these consultations while three-layer high compression was applied in 33%.
Analysis of clinical results
This portion of the report presents the results of the statistical models fitted to the data. It is arranged into three main sections as follows. The first section presents the results of the modelling of the primary outcome: time to healing of the reference leg ulcer. The second section presents the results of the modelling of the secondary outcomes: ulcer areas, QoL, complete healing of all ulcers at 12 months, ulcer recurrence and adverse events. The third section presents the conclusions of the statistical analysis of the clinical outcomes.
Modelling of the primary outcome (time to healing of leg ulcers)
The primary analysis compared the time to healing of the reference ulcer between the two randomised groups: ultrasound plus standard care and standard care alone. This was accomplished by fitting a Cox proportional hazard regression model with the main covariate as treatment adjusting for baseline ulcer area, baseline ulcer duration, ulcer compression and centre.
As seen in Table 9, the number of patients healing in each centre varied. We assumed that the 10 centres in this study were a random sample drawn from a population of centres on which we would like to make inferences. Therefore, centre was treated as a random effect in the Cox proportional hazard regression (shared-frailty effect). Observations from the same centre share the same frailty effect. Hence, observations from the same centre are correlated because they share the same frailty effect. The frailty effect is a latent random effect that enters multiplicatively on the hazard function.
Figure 4 shows that there was a high level of variability in the healing rates between centres, with patients from WHSCT seemingly more frail (healing more) than patients from other centres. Similarly, patients from South Essex were seemingly less frail (healed less) than those from other centres. No patients healed from South Essex. There was a significant difference in the survival time among centres (log-rank test 36.19, p < 0.0001) unadjusted for other covariates in the model. This variability in the healing rates between centres will be accounted for by including a centre frailty effect in the model. So patients from the same centre are correlated because they share the same frailty effect.
FIGURE 4.
Unadjusted Kaplan–Meier survival curves by participating centre showing high variability in healing rates between centres.
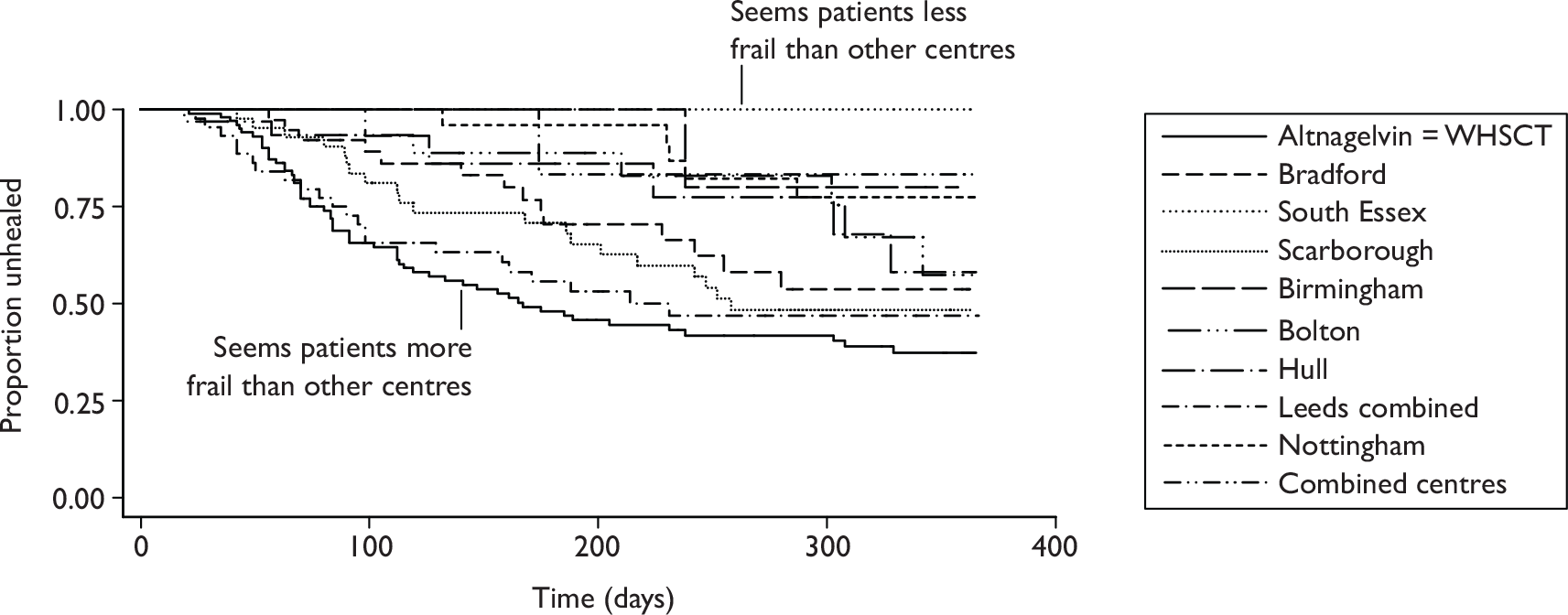
There was no evidence of a difference in survival (time to healing) between the two treatment groups using the log-rank statistic (0.2544, p = 0.6140) and the Wilcoxon test (0.3350, p = 0.5628) (Figure 5). In other words, we have identified no evidence that low-dose ultrasound significantly affects the time to healing of hard-to-heal venous leg ulcers.
FIGURE 5.
Unadjusted Kaplan–Meier survival curves by treatment randomised.
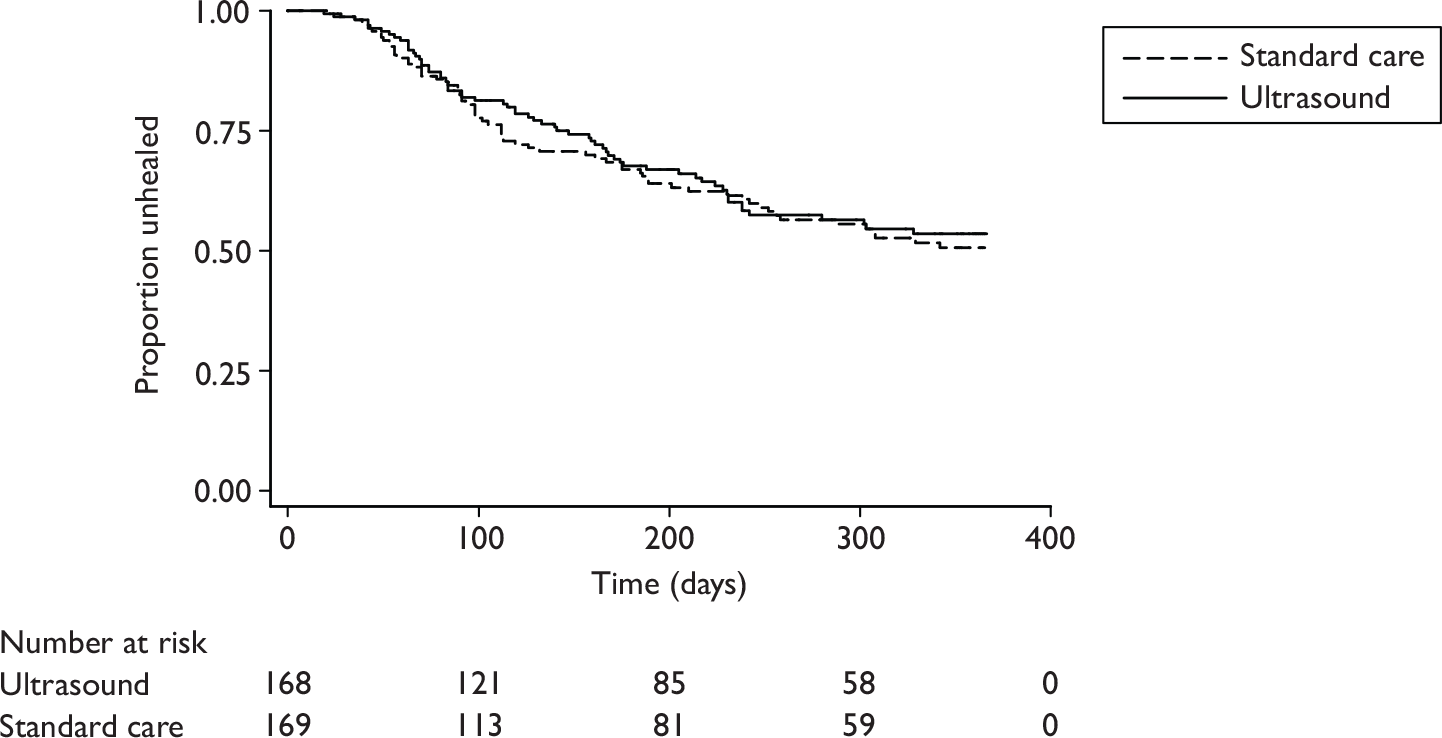
Table 13 shows the median time to healing and lower boundary of the 95% CI. Note that the upper 95% CI could not be estimated from the data because fewer than half the patients in each group healed (64 out of 168 patients in the ultrasound group and 69 out of 169 in the standard care group).
| Number of adverse events | Ultrasound (n = 168) | Standard care (n = 169) |
|---|---|---|
| Number healing/total number healed (%) | 64/133 (48) | 69/133 (52) |
| Unadjusted Kaplan–Meier estimates | ||
| Median time to healing (days, 95% CI) | Inestimable (238, inestimable) | Inestimable (252, inestimable) |
| Log-rank test statistic; (p-value) | 0.2544 (p = 0.6140) | |
| Wilcoxon test statistic; (p-value) | 0.3350 (p = 0.5628) | |
The results of fitting the Cox proportional hazard regression model without and with the centre frailty parameter are given in Table 14. For the model without the frailty effect, the hazard ratio of ultrasound versus standard care is 1.01 (p = 0.9520) adjusting for log(area), log(duration) and ulcer compression. There was no evidence that the survival experience (healing time) of patients randomised to ultrasound and standard care is different. The hazard ratio for log(area) is 0.66 (p < 0.0001) adjusting for treatment, log(duration) and ulcer compression. Therefore, there was a significant effect of area on the healing time experience of both groups in that small ulcers healed more quickly than large ulcers. The hazard ratio for log(duration) was 0.57 (p < 0.0001) adjusting for treatment, log(area) and ulcer compression. Therefore, there was a significant effect of log(duration) on the time to healing for all patients in that newer ulcers healed faster than older ulcers. The hazard ratio for compression versus no compression was 0.93 (p = 0.7740). Therefore, there was no effect of compression usage at baseline on the survival experience of the patients in the two arms of the study.
| Parameter | Estimate (standard error) | Hazard ratio (95% CI) | p-value | Test of PH assumption p-value |
|---|---|---|---|---|
| Without centre frailty effect | ||||
| Ultrasound vs standard care | 0.01 (0.16) | 1.01 (0.72 to 1.43) | 0.9520 | 0.8677 |
| Log(area) | –0.41 (0.08) | 0.66 (0.57 to 0.77) | 0.0001 | 0.1147 |
| Log(duration) | –0.56 (0.09) | 0.57 (0.48 to 0.68) | 0.0001 | 0.2914 |
| High compression (yes vs no) | –0.07 (0.24) | 0.93 (0.58 to 1.49) | 0.7740 | 0.1270 |
| Global test of PH assumption | – | – | – | 0.2548 |
| With centre frailty effect | ||||
| Ultrasound vs standard care | –0.01 (0.18) | 0.99 (0.70 to 1.40) | 0.9690 | 0.7230 |
| Log(area) | –0.44 (0.08) | 0.64 (0.55 to 0.75) | 0.0001 | 0.1467 |
| Log(duration) | –0.51 (0.09) | 0.59 (0.50 to 0.71) | 0.0001 | 0.2938 |
| High compression (yes vs no) | –0.10 (0.25) | 0.90 (0.56 to 1.46) | 0.673 | 0.2030 |
| Theta | 0.26 (0.20) | – | – | – |
| Global test of PH assumption | – | – | – | 0.3536 |
The proportional hazard (PH) assumption assumes that the hazard ratios above do not depend on time (i.e. they are constant over time). This assumption was tested via a statistical test on each covariate separately then globally over all the four covariates in the model. The idea behind this test is that if the PH assumption is valid then the Schoenfeld residuals for the global test and Schoenfeld scaled residuals for separate tests with each covariate should not be correlated with survival time. If, on the other hand, the residuals tend to be positive for subjects who heal at relatively earlier time and negative for subjects who heal at a relatively late time (or vice versa) then there is evidence that the hazard ratio is not constant over time (i.e. PH assumption is violated). 75 The test of the PH assumption was insignificant (ultrasound vs standard care, p = 0.8677; log(area), p = 0.1147; log(duration), p = 0.2914; high compression, p = 0.1270; and global test, p = 0.2548) meaning that the PH assumption was not violated separately for each covariate and globally for all covariates. Hence, the hazard ratios were assumed to be constant over time.
For the model with the centre frailty effect, there was a significant centre frailty effect (p < 0.0001), meaning that the correlation of patients within a study centre could not be ignored. All hazard ratios have the same interpretation, but conditioned on centre (i.e. compares patients from the same centre). The hazard ratio of ultrasound versus standard care was 0.99 (p = 0.9690) adjusting for log(area), log(duration) and ulcer compression for patients from the same study centre.
In the estimation of the centre frailty effect, the significance of the centre frailty effect was tested under the null hypothesis that its variance was equal to zero (centre frailty effects were assumed to be distributed as gamma random variables with mean 1 and variance theta). So the null hypothesis is on the boundary of the parameter space (as variances can never be negative); hence the null distribution of the likelihood ratio test statistic is not just a chi-squared [degrees of freedom (df): 1], but a 50 : 50 mixture of chi-squared (df 0), point mass at 0 and a chi-squared (df 1). As such, the p-value was calculated from a mixture of these two chi-squared distributions. Similarly, as before, the PH assumption was tested here. The results show that the assumption was not violated separately for each covariate [ultrasound vs standard care, p = 0.7230; log(area), p = 0.1467; log(duration), p = 0.2938; high compression, p = 0.2030; or globally, p = 0.3548]. Comparing the models without and with the centre frailty effect we can see that the results are similar; however, the advantage of the model with the centre frailty effect is that it takes into account the fact that participants within each study centre are highly correlated.
Analysis of secondary outcomes
In this section, the results of the secondary analysis of ulcer area, QoL, complete healing of all ulcers at 12 months, rates of ulcer recurrence and finally adverse events are presented.
Ulcer area
Previous research has shown that the initial reduction in ulcer area (percentage reduction after 4 weeks of good wound care) is predictive of eventual healing. 45 Therefore, we compared the initial healing rates between the ultrasound and standard care groups. The ulcer area at week 4 was compared between treatment groups using a linear mixed model to adjust for baseline ulcer area, centre, ulcer duration and use of compression. The model is linear because we have a continuous response and mixed because we have fixed effects (baseline ulcer area, ulcer duration and use of compression) and a random effect (centre).
The mean ulcer area at baseline was 27.56 cm2 (SD 43.17 cm2). The median was 12.0 cm2 (range 1.0–283.5 cm2). The mean ulcer area after 4 weeks was 22.65 cm2 (SD 43.14 cm2), median 8.00 cm2 (range 0.11–368.50 cm2). There seemed to be a reduction in the mean and median ulcer area after 4 weeks. The ulcer area at 4 weeks was modelled as the response variable and the main covariate in the model was the treatment group randomised adjusted for baseline ulcer area, centre, ulcer duration and use of compression.
The results in Table 15 show that there was a non-significant treatment effect (0.05, p = 0.4979) and a non-significant ulcer compression effect (0.02, p = 0.8361). There was a significant baseline log(area) effect (0.77, p = 0.0001) and baseline log(duration) effect (0.13, p = 0.0001). Therefore, there was no evidence that therapeutic low-dose ultrasound significantly increased the rate of change of venous ulcer area over the first 4 weeks of treatment and no evidence that the application of compression significantly influenced the rate of change of ulcer area over 4 weeks. As there was a significant log(area) effect, then for a unit increase in baseline log(area), the log(area) at week 4 increases by 0.77 cm2 (95% CI 0.72 to 0.84 cm2). Similarly, for the log(duration) effect there was an increase of 0.13 cm2 (95% CI 0.07 to 0.19 cm2) in log(area) after 4 weeks for each unit increase in log(duration) at baseline. After adjusting for the other covariates in the model, there was a non-significant centre random effect (0.01, p = 0.1489). So log(area) scores after 4 weeks did not significantly vary among centres.
| Parameter | Estimate (standard error) | p-value | 95% CI |
|---|---|---|---|
| Fixed effects | |||
| Intercept | –0.02 (0.15) | 0.8752 | –0.33 to 0.28 |
| Ultrasound vs standard care | 0.05 (0.07) | 0.4979 | –0.09 to 0.19 |
| Baseline log(area) | 0.77 (0.03) | 0.0001 | 0.72 to 0.84 |
| Baseline log(duration) | 0.13 (0.03) | 0.0001 | 0.07 to 0.19 |
| Ulcer compression (yes vs no) | 0.02 (0.11) | 0.8361 | –0.19 to 0.24 |
| Covariance parameters | |||
| Random centre effect | 0.01 (0.01) | 0.1489 | – |
| Measurement error | 0.39 (0.03) | 0.0001 | – |
Complete healing of all ulcers at 12 months
The number of leg ulcers that had completely healed by 12 months was based on nurse-reported data and not on blinded photographs as photographs were not taken after the reference ulcer had healed. After 12 months of follow-up there were 72 patients (48%) in the ultrasound group and 78 patients (52%) in the standard care group whose ulcers had all healed. There is little difference in the proportions of patients with all ulcers healed after 12 months between the two groups.
Kaplan–Meier survival curves for time to healing are shown in Figure 6. The median time to complete healing of all ulcers in the standard care group was 328 days (95% CI 235 days, inestimable) and for the ultrasound group was 365 days (95% CI 224 days, inestimable). Under the null hypothesis of no difference in the survival experience of the two groups of patients, the log-rank test (p = 0.6051) and the Wilcoxon test (p = 0.6357) were not significant; hence, there was no statistically significant difference in the time to complete healing of all ulcers in the two treatment groups. A formal analysis, such as Cox regression analysis, was not performed here as for the primary outcome as any other ulcers that the patient may have had were not receiving ultrasound treatment.
FIGURE 6.
Kaplan–Meier plot of time to complete healing of all ulcers by treatment group.
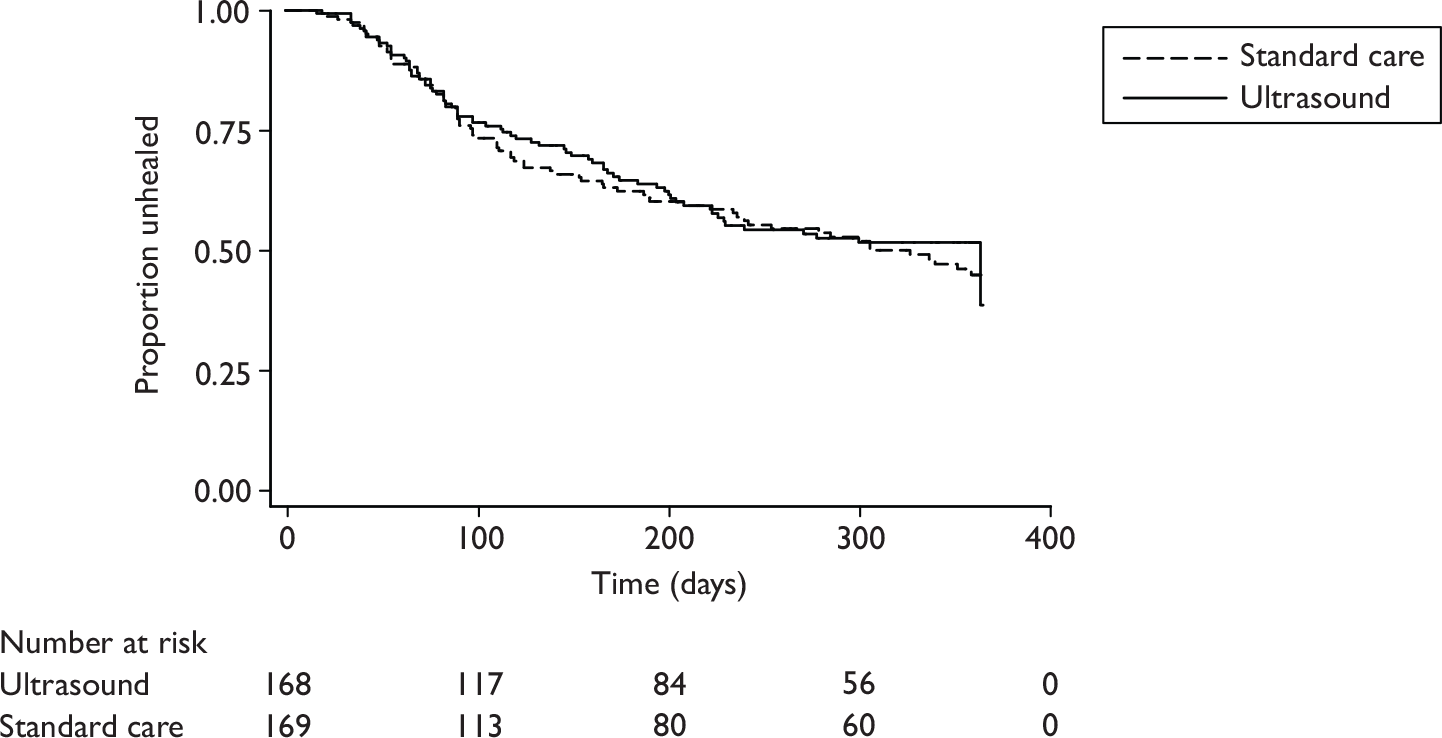
Ulcer recurrence
Patients were contacted by telephone at 6, 9 and 12 months after healing to ascertain if their healed reference ulcers had recurred. The proportions of patients whose healed reference ulcer had recurred are summarised by treatment group. We were able to contact 124 patients out of the 133 patients that healed in the study; the remaining patients were not contacted for a variety of reasons including invalid telephone numbers, no telephone number provided, telephone unanswered, patients seemed confused and the patients had moved to a different location.
The number of patients whose reference ulcer had recurred was 31 out of 124 (25%), whereas 93 out of 124 (75%) had no reference ulcer recurrence. Of the 31 patients with ulcer recurrence, 14 patients (45%) were in the standard care group, while 17 patients (55%) were in the ultrasound group. There was no statistically significant difference in the recurrence rates between the two groups (Fisher’s exact test, p = 0.6803).
Quality of life using the Short Form questionnaire-12 items
The HRQoL was measured using the SF-12 questionnaire over time [at baseline (0), 3, 6, 9 and 12 months]. There was very little change in the scores over time (constant linear trend of PCS and MCS against time; see Figures 2 and 3). Also, there was no increase in the SDs (constant standard errors) of the mean scores against time of follow-up as seen from the SDs at months 0, 3, 6, 9 and 12. There was high variability in PCS and MCS at baseline so random intercepts were included in the model. Thus, the modelling strategy that was adopted was to fit two linear mixed models with response variables PCS and MCS separately and covariates were treatment, baseline ulcer area, ulcer duration, compression usage, time (0, 3, 6, 9 and 12 months) and an interaction between time and treatment to assess whether QoL scores differed over time for the two treatments. We included centre as a random effect. We used a linear mixed model as time was nested within patients, so there was need to account for within-patient correlation in the PCS and MCS.
Table 16 shows the results of the linear mixed model fitted to the PCS and MCS. In the model with PCS as the response variable there were non-significant effects of ultrasound versus standard care, time, log(area), an interaction between treatment and time and ulcer compression. There was a significant effect of log(duration). There was not enough evidence of a significant difference in the mean PCS scores of the patients between the two treatment groups (0.69, p = 0.5773) and there was not enough evidence of a significant difference between the two treatment groups during follow-up (–0.11, p = 0.1944). There was not enough evidence of a significant difference in PCS mean scores for patients with different ulcer log(area) at baseline (–0.81, p = 0.0840). However, adjusting for all the other covariates in the model, there was a significant effect of log(duration) (–1.22, p = 0.0145). This implies that, adjusting for other covariates in the model, there was a 1.22 reduction in mean PCS scores for a unit increase in log(duration). Hence, longer ulcer duration was associated with lower mean PCS scores than was shorter ulcer duration. There was a borderline ulcer compression effect (3.25, p = 0.0661). This implies that, adjusting for all other covariates in the model, those who were using compression at baseline had PCS mean scores 3.25 higher than those who were not using compression.
| Parameter | PCS | MCS | ||||
|---|---|---|---|---|---|---|
| Estimate (standard error) | p-value | 95% CI | Estimate (standard error) | p-value | 95% CI | |
| Fixed effects | ||||||
| Intercept | 38.14 (2.45) | 0.0001 | 36.73 to 44.59 | 49.83 (2.22) | 0.0001 | 46.45 to 54.20 |
| Treatment (ultrasound vs standard care) | 0.69 (1.23) | 0.5773 | –1.79 to 3.08 | –0.93 (1.21) | 0.4395 | –3.30 to 1.44 |
| Time | –0.03 (0.06) | 0.6657 | –0.15 to 0.09 | –0.10 (0.07) | 0.1760 | –0.24 to 0.04 |
| Log(area) | –0.81 (0.46) | 0.0840 | –1.72 to 0.10 | –0.13 (0.43) | 0.7702 | –0.97 to 0.72 |
| Log(duration) | –1.22 (0.49) | 0.0145 | –2.12 to –0.17 | –1.00 (0.46) | 0.0312 | –1.91 to –0.09 |
| Treatment × time | –0.11 (0.09) | 0.1944 | –0.29 to 0.06 | 0.16 (0.10) | 0.1249 | –0.04 to 0.36 |
| Ulcer compression (yes vs no) | 3.25 (1.76) | 0.0661 | –0.22 to 6.71 | 0.23 (1.63) | 0.8860 | –2.97 to 3.44 |
| Covariance parameters | ||||||
| Random intercept | 94.96 (8.90) | 0.0001 | – | 80.56 (7.99) | 0.0001 | – |
| Centre | 3.77 (3.92) | 0.1681 | – | 0.28 (1.26) | 0.4125 | – |
| Autoregressive | 0.30 (0.05) | 0.0001 | – | 0.15 (0.05) | 0.0040 | – |
| Measurement error (residual variance) | 37.24 (2.67) | 0.0001 | – | 53.17 (3.02) | 0.0001 | – |
As much of the variation in the data was at baseline, this was modelled through random intercepts. This means that each individual patient’s baseline intercept varies around an average value of 38.14 (p = 0.0001) with the total random intercept variation estimated as 94.96 (p = 0.0001). This implies that there were baseline differences that needed to be accounted for in the data, and this was adequately modelled by using random intercepts. The centre effect entered the model as a random effect. The estimate of the variance of the centre effect was 3.77 (p = 0.1681). This implies that after adequately modelling the data, adjusting for all the other covariates in the model, the variability in the mean PCS scores between centres was not statistically significant. The correlation between measurements on the same individual was modelled by the autoregressive correlation. This assumes that the measurements close together are highly correlated (i.e. month 0 vs month 3), while measurements far apart are less correlated (i.e. month 0 vs month 6). In other ways the correlation decreases as the time lag between measurements increases. The autoregressive correlation estimate was 0.30 (p = 0.0001), i.e. two measurements from the same subject 3 months apart have a correlation of 0.30. For measurements 6 months apart, the correlation is estimated as 0.302 = 0.09, and so on. This implied that the correlation between the longitudinal patient’s measurements could not be ignored and that it needed to be modelled in the data. To arrive at the autoregressive correlation as the best one here, different correlation structures were compared by the likelihood ratio test, and this correlation structure was the one that increased the likelihood more with respect to the number of parameters in the model. The measurement error was estimated as 37.24 (p = 0.0001). In conclusion, considering all the random effects in the model, much variability was attributed to differences in baseline PCS scores. No CIs are presented for the covariance parameters.
In the model with MCS as the response variable, there were non-significant effects of ultrasound versus standard care, time, log(area), an interaction between treatment and time and ulcer compression There was a significant effect of log(duration). This implies that there was no statistically significant difference in the mean MCS scores of the patients between the two treatment groups (–0.93, p = 0.4395) and, similarly, there was no statistically significant difference between the two treatment groups during follow-up (with time) (0.16, p = 0.1249). There was no statistically significant difference in mean MCS scores for patients with different ulcer log(area) at baseline (–0.13, p = 0.7702). However, adjusting for all the other covariates in the model, there was a significant effect of log(duration) (–1.00, p = 0.0312). This implies that, adjusting for other covariates in the model, there was a 1.00 reduction in mean MCS scores for a unit increase in log(duration). Hence, longer ulcer duration was associated with lower mean MCS scores than was shorter ulcer duration. There was an insignificant ulcer compression effect (0.23, p = 0.8860). This implies that, adjusting for all other covariates in the model, there was no statistically significant difference in mean MCS scores between those who were using compression at baseline and those who were not.
As much of the variation in MCS scores was at baseline, this was modelled through random intercepts. This means that each individual patient’s baseline intercept varies around an average value of 49.83 (p = 0.0001), with the total random intercept variation estimated as 80.56 (p = 0.0001). This implied that there were baseline differences that needed to be accounted for in the data, and this was adequately modelled by using random intercepts. The centre effect was entered into the model as a random effect. The estimate of the variance of the centre effect is 0.28 (p = 0.4125). This implies that after adequately modelling the data, adjusting for all the other covariates in the model, the variability in the mean MCS scores between centres was not significant. The correlation between measurements on the same individual was modelled by the autoregressive correlation. The autoregressive correlation estimate was 0.15 (p = 0.0001). This implies that the correlation between the longitudinal patient’s measurements could not be ignored and needed to be modelled in the data. Different correlation structures were compared with this by the likelihood ratio test and this correlation structure was the best. The measurement error was estimated as 53.17 (p = 0.0001). Therefore, in conclusion, as for the PCS scores, considering all the random effects in the model, much variability was attributed to differences in baseline MCS scores.
Adverse events
Here we present the analysis of the adverse events data in the form of descriptive statistics and then random effects negative binomial regression models of the adverse events. To account for the excess zeros (many patients had no adverse events), alternative zero-inflated random effects negative binomial regression models will be considered.
Serious adverse events
There was a total of 88 SAEs in 64 patients. Of these, 29 patients (45%) were in the standard care group and 35 (55%) were in the ultrasound group (Table 17). For all the subjects in the study, the mean number of SAEs per patient was 0.26 (SD 0.62). The median number of SAEs was 0.00 (range 0.00–4.00). The coefficient of variation was approximately 238%: hence, there was too much variability in the number of SAEs per patient. The data are overdispersed (i.e. variance much larger than the mean).
| Treatment group | Total (%) | ||
|---|---|---|---|
| Standard care (%) | Ultrasound (%) | ||
| Number of SAEs | |||
| 0 | 140 (51.28) | 133 (48.72) | 273 (81.01) |
| 1 | 20 (43.48) | 26 (56.52) | 46 (13.65) |
| 2 | 9 (64.29) | 5 (35.71) | 14 (4.15) |
| 3 | 0 (0.00) | 2 (100.00) | 2 (0.59) |
| 4 | 0 (0.00) | 2 (0.00) | 2 (0.59) |
| Number of NSAEs | |||
| 0 | 102 (55.43) | 82 (44.57) | 184 (54.60) |
| 1 | 28 (53.85) | 24 (46.15) | 52 (15.43) |
| 2 | 9 (23.08) | 30 (76.92) | 39 (11.57) |
| 3 | 9 (52.94) | 8 (47.06) | 17 (5.04) |
| 4 | 11 (52.38) | 10 (47.62) | 21 (6.23) |
| 5 | 3 (42.86) | 4 (57.14) | 7 (2.08) |
| 6 | 4 (66.67) | 2 (33.33) | 6 (1.78) |
| 7 | 0 (0.00) | 3 (100.00) | 3 (0.89) |
| 8 | 0 (0.00) | 2 (100.00) | 2 (0.59) |
| 9 | 1 (100.00) | 0 (0.00) | 1 (0.30) |
| 10 | 0 (0.00) | 1 (100.00) | 1 (0.30) |
| 11 | 0 (0.00) | 1 (100.00) | 1 (0.30) |
| 13 | 0 (0.00) | 1 (100.00) | 1 (0.30) |
| 14 | 1 (100.00) | 0 (0.00) | 1 (0.30) |
| 15 | 1 (100.00) | 0 (0.00) | 1 (0.30) |
Non-serious adverse events
There were 445 NSAEs in 153 patients (45%). Of these patients, 67 (44%) were in the standard care group and 86 (56%) were in the ultrasound group (Table 17). Considering all 337 patients in the study, the mean number of NSAEs per patient was 1.32 (SD 2.23). The median number of NSAEs was 0.00 (range 0.00–15.00). The coefficient of variation was approximately 169%; hence, there was relatively too much variability (overdispersion) in the number of NSAEs per patient.
The outcome variables (number of NSAEs, number of SAEs and number of NSAEs and SAEs combined) have been regressed against treatment group, baseline ulcer area, baseline ulcer duration, use of compression and centre in a negative binomial regression model. Centre will be treated as a random effect. We assumed that the 10 centres in the study were randomly chosen from a population of centres where inference has to be made. The negative binomial regression model has been chosen as opposed to the Poisson regression so that we can account for overdispersion (variance greater than the mean).
The adverse events were assessed by the nurse by categorising their relationship with treatments (Table 18). The majority of the adverse events (81% and 86% for NSAEs and SAEs, respectively) were categorised as unrelated to the treatment received. None of the NSAEs was classified as ‘not able to assess if related’, while only one SAE (1%) in the ultrasound group was classified as ‘not able to assess if related’. There were two SAEs and one NSAE that were not categorised (missing data). Percentages are calculated on the adverse events that were categorised.
| Relationship | NSAE | SAE | ||||
|---|---|---|---|---|---|---|
| Standard care | Ultrasound | Total (%) | Standard care | Ultrasound | Total (%) | |
| Unrelated | 183 | 178 | 361 (81.31) | 33 | 41 | 74 (86.05) |
| Unlikely to be related | 11 | 46 | 57 (12.84) | 2 | 7 | 9 (10.47) |
| Possibly related | 0 | 17 | 17 (3.83) | 0 | 0 | 0 (0.00) |
| Probably related | 0 | 6 | 6 (1.35) | 1 | 0 | 1 (1.16) |
| Definitely related | 0 | 3 | 3 (0.68) | 1 | 0 | 1 (1.16) |
| Not able to assess if related | 0 | 0 | 0 (0.00) | 0 | 1 | 1 (1.16) |
| Total | 194 | 250 | 444 | 37 | 49 | 86 |
In the random effects negative binomial regression model, in which the response was the number of NSAEs per patient during the entire time of follow-up in the study, the explanatory variables in the model were the fixed effects [treatment, log(area), log(duration), ulcer compression] and the random effect (centre). The results show that there was a significant effect of treatment (p = 0.0411). There was a non-significant effect of log(area) (p = 0.4247), log(duration) (p = 0.2395) and ulcer compression (p = 0.9882). Therefore, the number of NSAEs was significantly associated with the treatment received, with more NSAEs episodes in the ultrasound group than in the standard care group. The number of NSAEs was not related to baseline ulcer area, baseline ulcer duration or baseline compression usage. The estimate of random centre effect SD was 1.39 (standard error 0.38, p = 0.0051). Therefore, the correlation of patients within a centre could not be ignored. Owing to overdispersion in the data, an ordinary Poisson regression did not fit the data very well, but this random effects negative binomial model did. The dispersion parameter was estimated as 0.57 (standard error 0.13, p = 0.0016), giving strong evidence of zero inflation (more patients with no NSAEs than those modelled under negative binomial regression model). A zero-inflated random effects negative binomial model was fitted, but results did not differ much from those above so the random effects negative binomial model without considering zero inflation was considered the best model here.
In the random effects negative binomial regression model in which the response was the number of SAEs per patient during the entire time of follow-up in the study, the explanatory variables in the model were the fixed effects [treatment, log(area), log(duration), ulcer compression] and the random effect (centre). The results show that there was a non-significant effect of treatment (p = 0.3904) and ulcer compression (p = 0.6585). There was a borderline effect of log(area) (p = 0.0781) and log(duration) (p = 0.0647). Therefore, there was no statistically significant difference in the number of SAEs due to treatment received or baseline record of compression used. There was relatively weak evidence that baseline ulcer area and baseline ulcer duration are associated with number of SAEs. The estimate of random centre effect SD was 0.51 (standard error 0.18, p = 0.0211). Therefore, the correlation of patients within a centre could not be ignored. A zero-inflated random effects negative binomial model was fitted to the data but the results did not differ much from those above; hence, the random effects negative binomial model without considering zero inflation was considered the best model here (dispersion parameter 0.91, standard error 0.47, p = 0.0884, giving weak evidence of zero inflation).
In the random effects negative binomial regression model in which the response was the number of NSAEs plus SAEs (adverse events) per patient during the entire time of follow-up in the study, the explanatory variables in the model were the fixed effects [treatment, log(area), log(duration), ulcer compression] and the random effect (centre). The results (Table 19) show that there was a significant effect of treatment (p = 0.0446). There was a non-significant effect of log(area) (p = 0.1963) and ulcer compression at baseline (p = 0.9882).There was a borderline significant effect of log(duration) (p = 0.0843). Therefore, the number of adverse events was significantly associated with the treatment received, with more adverse events episodes in the ultrasound group than in the standard care group. There was no statistically significant association between the number of adverse events and baseline ulcer area, or baseline compression usage, and a weak but significant association with baseline ulcer duration. The estimate of random centre effect SD was 1.18 (standard error 0.32, p = 0.0053). Therefore, the correlation of patients within a centre could not be ignored. A zero-inflated random effects negative binomial model was fitted, but results did not differ much from those above, so the random effects negative binomial model without considering zero inflation was considered the best model here (dispersion parameter 0.49, standard error: 0.11, p = 0.0015), giving strong evidence of zero inflation.
| Parameter | NSAEs | SAEs | NSAEs and SAEs combined | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate (standard error) | p-value | 95% CI | Estimate (standard error) | p-value | 95% CI | Estimate (standard error) | p-value | 95% CI | |
| Fixed effects | |||||||||
| Intercept | –0.84 (0.55) | 0.1599 | –2.08 to 0.40 | –2.55 (0.52) | 0.0008 | –3.72 to –1.37 | –0.63 (0.47) | 0.2157 | –0.69 to 0.44 |
| Treatment (ultrasound vs standard care) | 0.35 (0.14) | 0.0411 | 0.02 to 0.67 | 0.23 (0.25) | 0.3904 | –0.34 to 0.79 | 0.30 (0.13) | 0.0446 | 0.01 to 0.60 |
| Log(area) | 0.05 (0.05) | 0.4247 | –0.08 to 0.17 | 0.19 (0.09) | 0.0781 | –0.03 to 0.40 | 0.07 (0.05) | 0.1963 | –0.04 to 0.18 |
| Log(duration) | 0.08 (0.06) | 0.2395 | –0.06 to 0.22 | 0.22 (0.10) | 0.0647 | –0.02 to 0.45 | 0.11 (0.06) | 0.0843 | –0.02 to 0.24 |
| Ulcer compression (yes vs no) | –0.003 (0.21) | 0.9882 | –0.46 to 0.47 | –0.16 (0.36) | 0.6585 | –0.98 to 0.65 | –0.01 (0.19) | 0.9481 | –0.44 to 0.41 |
| Covariance parameters | |||||||||
| Centre effect | 1.39 (0.38) | 0.0051 | 0.54 to 2.26 | 0.51 (0.18) | 0.0211 | 0.10 to 0.93 | 1.18 (0.32) | 0.0053 | 0.45 to 1.91 |
| Dispersion parameter | 0.57 (0.13) | 0.0016 | 0.28 to 0.88 | 0.91 (0.47) | 0.0884 | –0.17 to 1.98 | 0.49 (0.11) | 0.0015 | 0.25 to 0.74 |
Summary
There was no evidence of a statistically significant effect of therapeutic ultrasound on the healing of hard-to-heal venous leg ulcers when compared with standard care alone. There was a significant centre effect observed in the trial, such that rates of ulcer healing differed significantly between centres.
Patients with smaller ulcers at baseline, those receiving high compression at baseline and those with shorter duration ulcers at baseline all healed more quickly than those with larger or older ulcers or ulcers in people that were not receiving high compression at baseline, after adjusting for all other factors.
There was no evidence of a statistical significant effect of low-dose ultrasound on QoL (as measured by SF-12) etc.; however, patients with older ulcers at baseline reported significantly poorer QOL as measured by the PCS and MCS component scores of the SF-12. Significantly more people receiving therapeutic ultrasound experienced adverse events than those receiving standard care; most adverse events were non-serious.
Chapter 5 Economic analysis
Three hundred and thirty-seven participants were recruited to VenUS III. There were 169 participants allocated to standard care and 168 to ultrasound plus standard care.
Outcomes of the economic analysis
Resource use and cost
Ultrasound plus standard care
Over the 12 weeks of ultrasound treatment, participants in the treatment arm had an average of 15.35 nurse consultations and 10.25 applications of ultrasound (the trial protocol suggested no more than one ultrasound application per week). Each ultrasound application lasted, on average, 7.21 minutes. Patients received therapeutic ultrasound in 77% of consultations. The details can be found in Table 20.
| Treatment recorded | Ultrasound (n = 168) |
|---|---|
| Number of nurse consultations, per patient | |
| Mean (SD) | 15.35 (8.88) |
| Median (min, max) | 13 (2, 51) |
| Missing | 9 |
| Number of ultrasound applications, per patient | |
| Mean (SD) | 10.25 (2.96) |
| Median (min, max) | 11 (1, 15) |
| Missing | 9 |
| Duration of ultrasound application (minutes), per patient | |
| Mean (SD) | 7.21 (2.32) |
| Median (min, max) | 7 (1, 10) |
| Missing | 9 |
| Proportion of visits receiving ultrasound, per patient | |
| Mean % (SD) | 77 (24) |
| Median % (min, max) | 86 (14, 100) |
| Missing | 9 |
Compression therapy
Table 21 presents the total number of times high compression was applied in both arms over the period of trial: at 84% of nurse consultations in the standard care arm and 78% of nurse consultations in the ultrasound arm.
| Compression therapy | % (N) | |
|---|---|---|
| Ultrasound (n = 168) | Standard care (n = 169) | |
| Compression bandages applied | ||
| Four-layer high compression | 30.67 (2151) | 34.62 (2241) |
| Three-layer high compression | 18.89 (1325) | 23.84 (1543) |
| Two-layer high compression | 15.16 (1063) | 13.24 (857) |
| Short-stretch | 13.13 (921) | 12.44 (805) |
| Three-layer reduced compression | 12.09 (848) | 10.32 (668) |
| Low compression | 3.56 (250) | 2.15 (139) |
| No compression | 6.49 (455) | 3.4 (220) |
| Knitted viscose dressing applied | ||
| Yes | 44.82 (3070) | 39.98 (2556) |
| No | 55.18 (3779) | 60.14 (3856) |
Health-care consultations
The number of consultations recorded by nurses for the full follow-up period is shown in Table 22; standard care patients received, on average, 41.34 nurse consultations compared with 44.90 in the ultrasound arm. Most nurse consultations took place in the leg ulcer clinic or patients’ homes (75% and 76% for standard care and ultrasound arms, respectively). Note that we received no information regarding treatments received for 18 patients (10 in the standard care arm and 8 in the ultrasound arm) and they were, therefore, recorded as missing throughout the economic analysis. There was also one participant in the ultrasound arm for whom no information about the duration of ultrasound treatments was available.
| Nurse therapy | Ultrasound (n = 168) | Standard care (n = 169) |
|---|---|---|
| Number of nurse consultations, per patient | ||
| Mean (SD) | 44.90 (43.16) | 41.34 (46.76) |
| Median (min, max) | 30 (2, 307) | 28 (1, 354) |
| Missing (%) | 8 (4.8) | 10 (5.9) |
| Location of consultations, n (%)a | ||
| Home | 2534 (35.58) | 2344 (36.02) |
| Leg ulcer clinic | 2883 (40.48) | 2545 (39.11) |
| GP surgery | 1107 (15.54) | 1071 (16.46) |
| Other | 548 (7.69) | 534 (8.21) |
| Nursing home | 49 (0.69) | 13 (0.20) |
| Leg ulcer club | 1 (0.01) | 1 (0.02) |
Total costs
The total cost for each participant was calculated by summing the cost of compression therapy, cost of health-care consultation and the cost of ultrasound machine where applicable. The cost of health-care consultation was the main contributor to the total cost. Quarterly and annual (unadjusted) figures are presented in Table 23.
| Time point | Ultrasound (n = 168) | Standard care (n = 169) |
|---|---|---|
| Months 1–3 | ||
| Mean (SD) | 572.8 (538.6) | 456.7 (538.9) |
| Median (min, max) | 355.5 (64.6, 4544.9) | 272.8 (20.6, 4413.9) |
| Missing (%) | 8 (4.8) | 10 (5.9) |
| Months 4–6 | ||
| Mean (SD) | 436.5 (580.8) | 343.4 (605.5) |
| Median (min, max) | 271.1 (0.0, 4681.3) | 207.7 (0.0, 4258.5) |
| Missing (%) | 25 (14.3) | 24 (14.2) |
| Months 7–9 | ||
| Mean (SD) | 333.8 (555.5) | 291.9 (547.9) |
| Median (min, max) | 182.8 (0.0, 4524.3) | 142.8 (0.0, 3785.3) |
| Missing (%) | 35 (20.2) | 34 (20.1) |
| Months 10–12 | ||
| Mean (SD) | 290.2 (570.3) | 283.2 (607.6) |
| Median (min, max) | 63.9 (0.0, 4985.4) | 31.1 (0.0, 4431.5) |
| Missing (%) | 45 (26.2) | 46 (27.2) |
| Total costs | ||
| Mean (SD) | 1471.0 (1884.7) | 1237.4 (2055.7) |
| Median (min, max) | 902.0 (135.6, 16,288.6) | 670.3 (20.61, 16,889.3) |
| Missing (%) | 8 (4.8) | 10 (5.9) |
Table 24 shows the adjusted annual cost for each arm and their mean difference. To account for the censored nature of the data, possible baseline imbalances and stratification variables, the inverse probability-weighted multiple regression was used. This allows estimation of the mean cost difference between the two arms adjusting for covariates: baseline ulcer duration (logarithmic), ulcer area (logarithmic), use or not of compression therapy and centres (aggregating centres with fewer than five cases). The results of the base-case analysis show that adding ultrasound treatment to standard care costs, on average, £197.88 more per participant per year (95% bias-corrected CI –£35.19 to £420.32). This difference was not statistically significant.
| Arm | Mean cost (£) | 95% bias-corrected CI (£) |
|---|---|---|
| Ultrasound | 1583.39 | 1427.51 to 1728.70 |
| Standard care | 1385.51 | 1223.84 to 1549.21 |
| Differenceb | 197.88 | –35.19 to 420.32 |
Health benefits
Time to healing
The estimated mean time to healing (over 12 months) was 245.0 days for standard care and 259.7 days for ultrasound plus standard care (Table 25). The result was in favour of the control arm (standard care alone). However, the difference (14.7 days) was not statistically significant (95% bias-corrected CI –32.7 to 56.8 days). The estimation was based on the IPW regression with covariates of baseline ulcer duration (logarithmic), ulcer area (logarithmic), use or not of compression therapy and centres (aggregating centres with fewer than five cases).
| Arm | Mean (days) | 95% bias-corrected CI (days) |
|---|---|---|
| Ultrasound | 259.7 | 232.7 to 302.5 |
| Standard care | 245.0 | 230.6 to 282.3 |
| Differenceb | 14.7 | –32.7 to 56.8 |
Utility and quality-adjusted life-years
The unadjusted utility (EQ-5D) scores are shown in Table 26. The average utility scores at baseline for control and treatment arms were 0.537 and 0.509, respectively, indicating some imbalance. Based on individuals’ utility scores, the total QALY was calculated and the results are shown in Table 27. The mean annual unadjusted QALY for the standard care arm was 0.568 and for ultrasound was 0.550 (Table 28).
| Time point | Ultrasound (n = 168) | Standard care (n = 169) |
|---|---|---|
| Baseline | ||
| Mean (SD) | 0.509 (0.340) | 0.537 (0.322) |
| Median (min, max) | 0.620 (–0.349, 1.000) | 0.620 (–0.484, 1.000) |
| Missing (%) | 19 (11) | 12 (7) |
| 3 months | ||
| Mean (SD) | 0.472 (0.365) | 0.527 (0.342) |
| Median (min, max) | 0.587 (–0.426, 1.000) | 0.62 (–0.239, 1.000) |
| Missing (%) | 34 (20) | 34 (20) |
| 6 months | ||
| Mean (SD) | 0.497 (0.362) | 0.55 (0.335) |
| Median (min, max) | 0.587 (–0.594, 1.000) | 0.62 (–0.239, 1.000) |
| Missing (%) | 44 (26) | 34 (20) |
| 9 months | ||
| Mean (SD) | 0.497 (0.373) | 0.546 (0.346) |
| Median (min, max) | 0.620 (–0.429, 1.000) | 0.620 (–0.181, 1.000) |
| Missing (%) | 55 (33) | 53 (31) |
| 12 months | ||
| Mean (SD) | 0.534 (0.359) | 0.544 (0.36) |
| Median (min, max) | 0.620 (–0.594, 1.000) | 0.620 (–0.181, 1.000) |
| Missing (%) | 47 (28) | 60 (36) |
| Time point | Ultrasound (n = 168) | Standard care (n = 169) |
|---|---|---|
| 1–3 months | ||
| Mean (SD) | 0.126 (0.079) | 0.133 (0.074) |
| Median (min, max) | 0.147 (–0.087, 0.250) | 0.151 (–0.025, 0.250) |
| Missing (%) | 47 (28) | 43 (25) |
| 4–6 months | ||
| Mean (SD) | 0.124 (0.083) | 0.134 (0.078) |
| Median (min, max) | 0.138 (–0.104, 0.250) | 0.151 (–0.052, 0.250) |
| Missing (%) | 54 (32) | 48 (28) |
| 7–9 months | ||
| Mean (SD) | 0.130 (0.083) | 0.137 (0.082) |
| Median (min, max) | 0.155 (–0.118, 0.250) | 0.155 (–0.052, 0.250) |
| Missing (%) | 67 (40) | 58 (34) |
| 10–12 months | ||
| Mean (SD) | 0.136 (0.082) | 0.141 (0.081) |
| Median (min, max) | 0.155 (–0.118, 0.250) | 0.155 (–0.045, 0.250) |
| Missing (%) | 67 (40) | 73 (43) |
| Annual (complete cases only) | ||
| Mean (SD) | 0.550 (0.301) | 0.568 (0.287) |
| Median (min, max) | 0.625 (–0.342, 1.000) | 0.629 (–0.160, 1.000) |
| Missing (%) | 89 (53) | 88 (52) |
| Arm | Mean QALYs (years) | 95% bias-corrected CI (years) |
|---|---|---|
| Ultrasound | 0.515 | 0.488 to 0.537 |
| Standard care | 0.525 | 0.499 to 0.545 |
| Differenceb | –0.009 | –0.042 to 0.024 |
After adjusting for the censored nature of data, imbalance of utility score at baseline and other covariates, the mean QALY was 0.525 for standard care and 0.515 for ultrasound. The difference (–0.009 QALYs) was not statistically significant (95% bias-corrected CI –0.042 to 0.024 QALYs).
Cost-effectiveness and uncertainty
The base-case analysis shows that ultrasound with standard care is expected to be more costly and less beneficial (as measured by both QALY and time to healing) than standard care alone. As summarised in Table 29, compared with standard care alone, individuals who received ultrasound plus standard care took an average of 14.7 days longer to heal, had 0.009 fewer QALYs and higher treatment costs (by £197.88). Ultrasound therapy plus standard care for leg ulcers was therefore dominated by standard care alone and should not be adopted. However, none of the differences in costs and benefits between these alternative treatments were statistically significant, indicating uncertainty which should be examined using bootstrapping.
| Variables | Mean annual costs (95% CIa) | QALYs (95% CIa) |
Time to healing (95% CIa) |
Gain in ulcer-free dayc (95% CIa) |
ICER (cost per QALY/cost per ulcer-free day) |
|---|---|---|---|---|---|
| Ultrasound | 1583.39 | 0.515 | 259.7 | – | Dominated |
| Standard care | 1385.51 | 0.525 | 245.0 | – | – |
| Differenceb |
197.88 (–35.19 to 420.32) |
–0.009 (–0.042 to 0.024) |
14.7 (–32.7 to 56.8) |
–14.7 (–56.8 to 32.7) |
– |
The incremental cost-effectiveness plane, shown in Figure 7, was drawn to demonstrate the uncertainty associated with the mean difference in cost and health benefits between two trial arms, by plotting the non-parametric bootstrapping results – 4000 replicates of difference in costs and health benefits (QALYs and ulcer-free days). As shown in Figure 7, in the cost–utility (cost per additional QALY) analysis, the majority of points (67%) fall into the north-west quadrant, indicating that ultrasound with standard care is dominated by standard care alone. The cost-effectiveness analysis (i.e. cost per additional ulcer-free day) similarly indicates standard care to be dominant, with 74% of the estimates falling in the north-west quadrant.
FIGURE 7.
Cost-effectiveness plane.
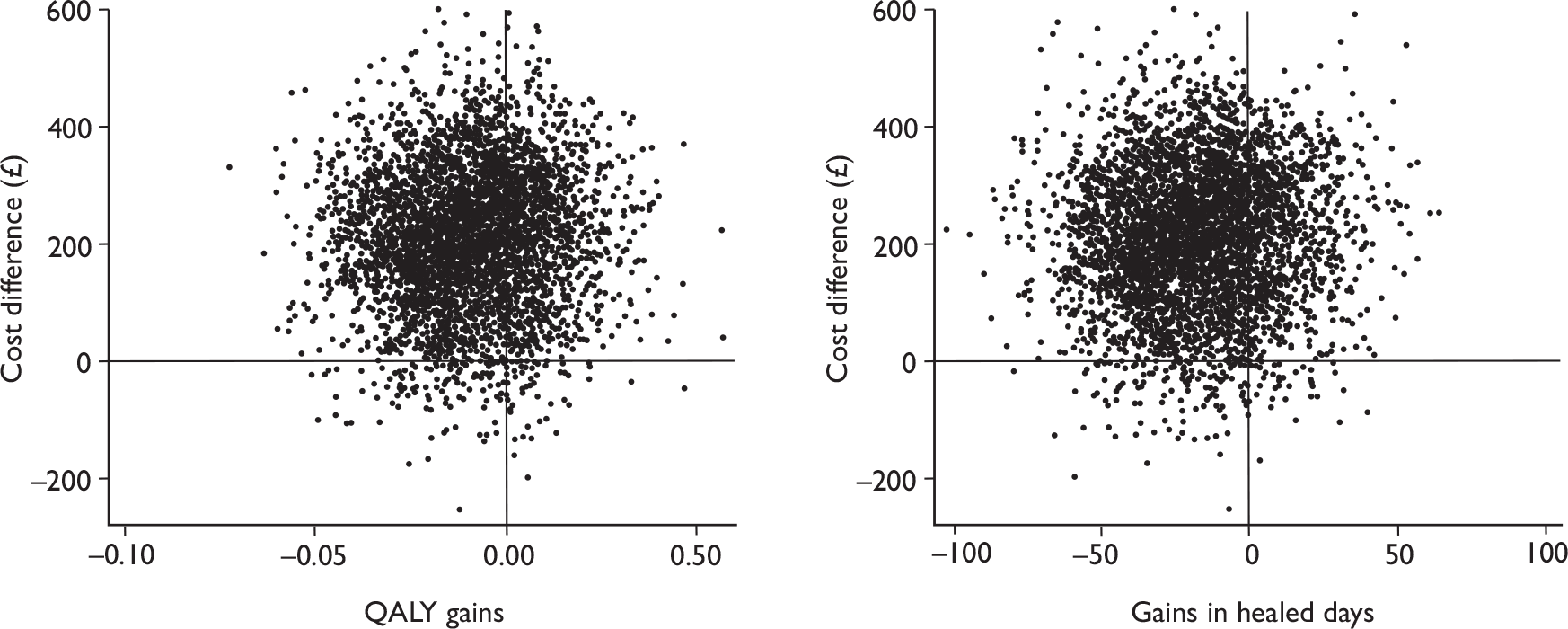
Figure 8 presents the CEACs. For the cost per QALY analysis, according to the figure, the probability of ultrasound treatment being cost-effective is < 20% given the willingness to pay for additional QALY up to £30,000. Therefore, based on the current trial evidence, it is unlikely that ultrasound with standard care is cost-effective. Similarly, in the cost per ulcer-free day analysis, the probability of ultrasound treatment being cost-effective is around 20% maximum for any willingness to pay for an additional ulcer-free day.
FIGURE 8.
Cost-effectiveness acceptability curve (base-case analysis). WTP, willingness to pay.
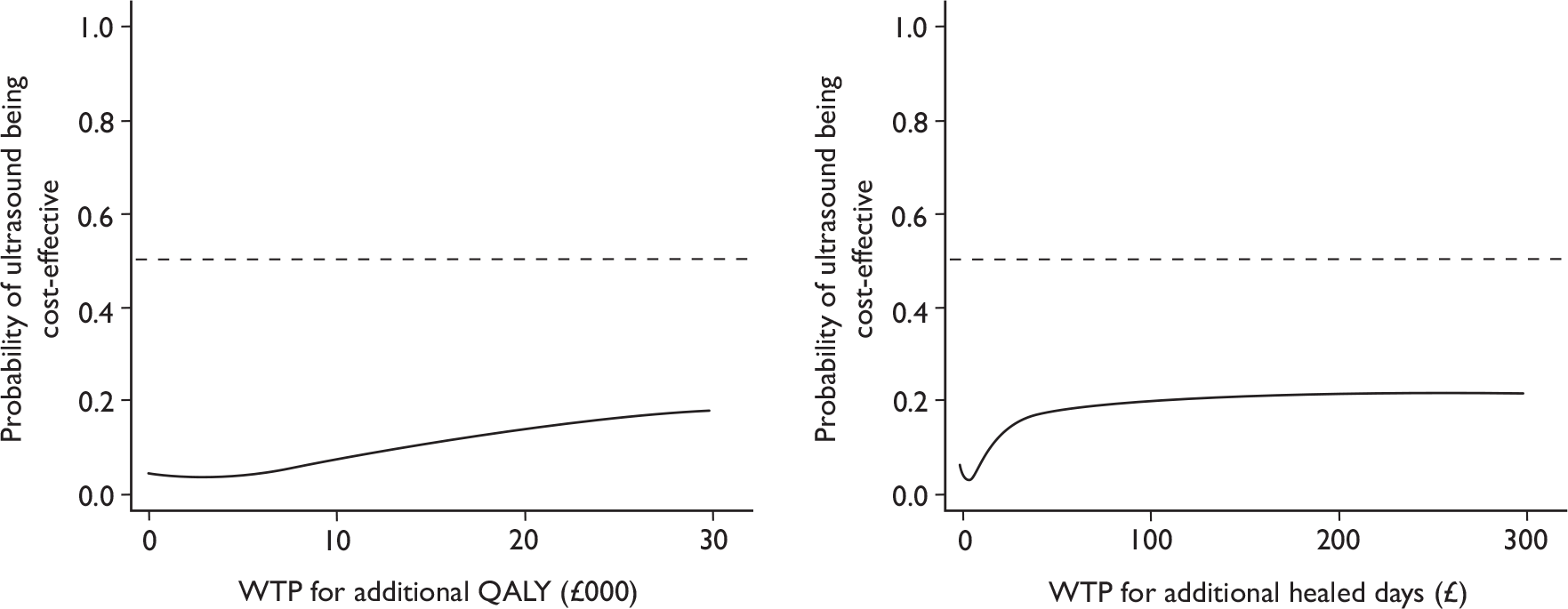
Sensitivity analysis
Scenario 1
Sensitivity analysis was conducted to investigate the impact on the cost-effectiveness results of using patient-reported data regarding the health-care consultations. Ulcer-related doctor, nurse and hospital outpatient visits were extracted from postal questionnaires received from patients every 3 months during the follow-up period.
The number of ulcer-related doctor/nurse/hospital outpatient visits is shown in Table 30. Patients reported far fewer health-care consultations than the nurses (see Table 22). On average, patients reported a total of 25 nurse consultations in the standard care arm and 37 consultations in the ultrasound arm whereas the nurses reported 41 (standard care) and 45 (ultrasound) consultations.
| Time | Event | Ultrasound (n = 168) | Standard care (n = 169) | |
|---|---|---|---|---|
| 3 months | Doctor visits related to ulcers | Mean (SD) | 0.8 (2.7) | 0.6 (2.2) |
| Median (min, max) | 0 (0, 24) | 0 (0, 15) | ||
| Missing | 41 (25.0) | 41 (24.3) | ||
| Nurse visits related to ulcers | Mean (SD) | 11.1 (16.3) | 8.9 (12.0) | |
| Median (min, max) | 3 (0, 84) | 0 (0, 57) | ||
| Missing | 39 (23.8) | 55 (32.5) | ||
| Hospital visits related to ulcer | Mean (SD) | 3.7 (6.9) | 3.4 (7.0) | |
| Median (min, max) | 0 (0, 36) | 0 (0, 36) | ||
| Missing | 27 (16.7) | 31 (18.3) | ||
| 6 months | Doctor visits related to ulcers | Mean (SD) | 0.7 (2.5) | 0.7 (2.3) |
| Median (min, max) | 0 (0, 15) | 0 (0, 15) | ||
| Missing | 57 (34.5) | 49 (29.0) | ||
| Nurse visits related to ulcers | Mean (SD) | 9.9 (15.5) | 8.8 (14.2) | |
| Median (min, max) | 0 (0, 84) | 0 (0, 84) | ||
| Missing | 56 (33.9) | 52 (30.8) | ||
| Hospital visits related to ulcers | Mean (SD) | 2.9 (8.3) | 2 (6.4) | |
| Median (min, max) | 0 (0, 66) | 0 (0, 60) | ||
| Missing | 43 (26.2) | 34 (20.1) | ||
| 9 months | Doctor visits related to ulcers | Mean (SD) | 1 (3.3) | 0.8 (2.2) |
| Median (min, max) | 0 (0, 21) | 0 (0, 12) | ||
| Missing | 58 (35.1) | 68 (40.2) | ||
| Nurse visits related to ulcers | Mean (SD) | 8.4 (15.4) | 8.6 (15.3) | |
| Median (min, max) | 0 (0, 84) | 0 (0, 84) | ||
| Missing | 59 (35.7) | 67 (39.6) | ||
| Hospital visits related to ulcers | Mean (SD) | 2 (5.8) | 2.1 (5.4) | |
| Median (min, max) | 0 (0, 48) | 0 (0, 30) | ||
| Missing | 51 (31.0) | 52 (30.8) | ||
| 12 months | Doctor visits related to ulcers | Mean (SD) | 1 (3.5) | 1.4 (5.9) |
| Median (min, max) | 0 (0, 30) | 0 (0, 45) | ||
| Missing | 55 (33.3) | 67 (39.6) | ||
| Nurse visits related to ulcers | Mean (SD) | 8.1 (14.0) | 5.9 (13.9) | |
| Median (min, max) | 0 (0, 84) | 0 (0, 84) | ||
| Missing | 60 (36.3) | 72 (42.6) | ||
| Hospital visits related to ulcers | Mean (SD) | 1.4 (4.9) | 1.7 (5.2) | |
| Median (min, max) | 0 (0, 36) | 0 (0, 36) | ||
| Missing | 43 (26.2) | 56 (33.1) | ||
| Total (completed cases only) | Doctor visits related to ulcers | Mean (SD) | 2.91 (6.74) | 2.32 (7.60) |
| Median (min, max) | 0 (0, 36) | 0 (0, 96) | ||
| Missing | 98 (58.3) | 94 (55.6) | ||
| Nurse visits related to ulcers | Mean (SD) | 36.73 (50.56) | 25.32 (35.29) | |
| Median (min, max) | 15 (0, 294) | 12 (0, 153) | ||
| Missing | 94 (56.0) | 103 (60.9) | ||
| Hospital visits related to ulcers | Mean (SD) | 8.26 (16.82) | 8.84 (19.01) | |
| Median (min, max) | 0 (0, 90) | 0 (0, 96) | ||
| Missing | 71 (42.3) | 76 (45.0) |
The unadjusted costs for each arm based on patient-reported data are shown in Table 31. The adjusted mean costs for the standard care arm were £1650.50 per participant per year (95% bias-corrected CI £1439.20 to £1888.30) and £1790.70 for the ultrasound arm (95% bias-corrected CI £1553.60 to £2018.40). The difference in means between two arms was £140.20 and was not significant (95% bias-corrected CI –£186.60 to £438.20). The estimated difference in this scenario was lower than that in the base case.
| Total costs (£) | Ultrasound (n = 168) | Standard care (n = 169) |
|---|---|---|
| Mean (SD) | 1756.1 (2272.7) | 1478.9 (2023.7) |
| Median (min, max) | 906.7 (107.8, 14,882.9) | 698.3 (0.0, 12,252.7) |
| Missing (%) | 9 (4.8) | 10 (5.9) |
As only the cost of ultrasound was subjected to sensitivity analysis, the health benefit estimate is equivalent to base case. Similar to the results observed in the base-case analysis, in this scenario ultrasound with standard care is expected to be dominated by standard care alone (as shown in Figure 9) and ultrasound with standard care treatment is unlikely to be cost-effective based on the current trial evidence (as shown in Figure 10).
FIGURE 9.
Cost-effectiveness plane (sensitivity analysis).
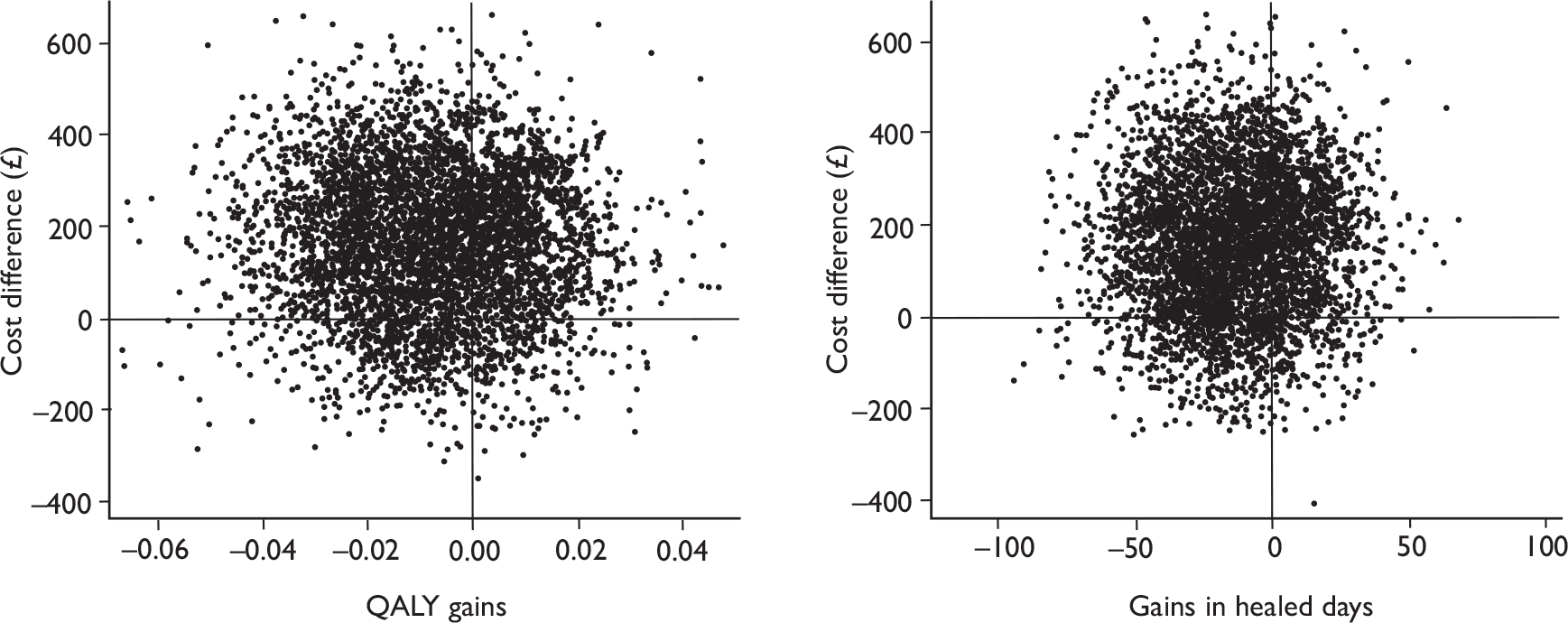
FIGURE 10.
Cost-effectiveness acceptance curve (sensitivity analysis). WTP, willingness to pay.
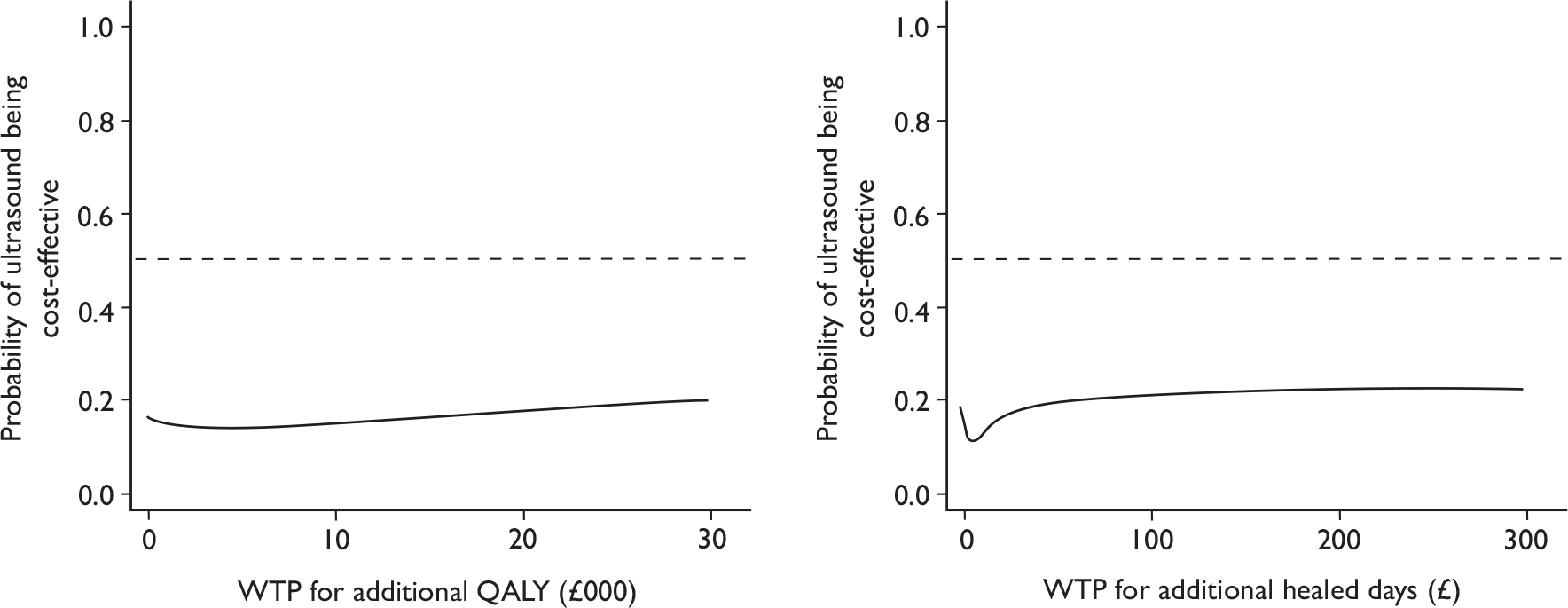
Scenario 2
One-way sensitivity analysis was conducted to investigate the influence of the equipment cost of ultrasound machines on the adjusted cost difference, shown in Figure 11. With a decrease (from 88.05 to 0.00 with every 10% decrease) in the equipment cost of ultrasound machine, the adjusted cost difference between two arms also decreased. The adjusted cost difference dropped from £197.88 with the full equipment cost as used in the base-case analysis to £110.60 with no equipment cost involved. The remaining difference was mostly caused by the incurred cost of extra nurse time in applying ultrasound treatments. However, none of these estimates on cost difference was statistically significant. Thus, the uncertainty around the cost difference remained. The results here were based on 4000 bootstrap replicates and bias-corrected 95% CIs were reported.
FIGURE 11.
One-way sensitivity analysis result (bias-corrected 95% CI based on 4000 bootstrap replicates).
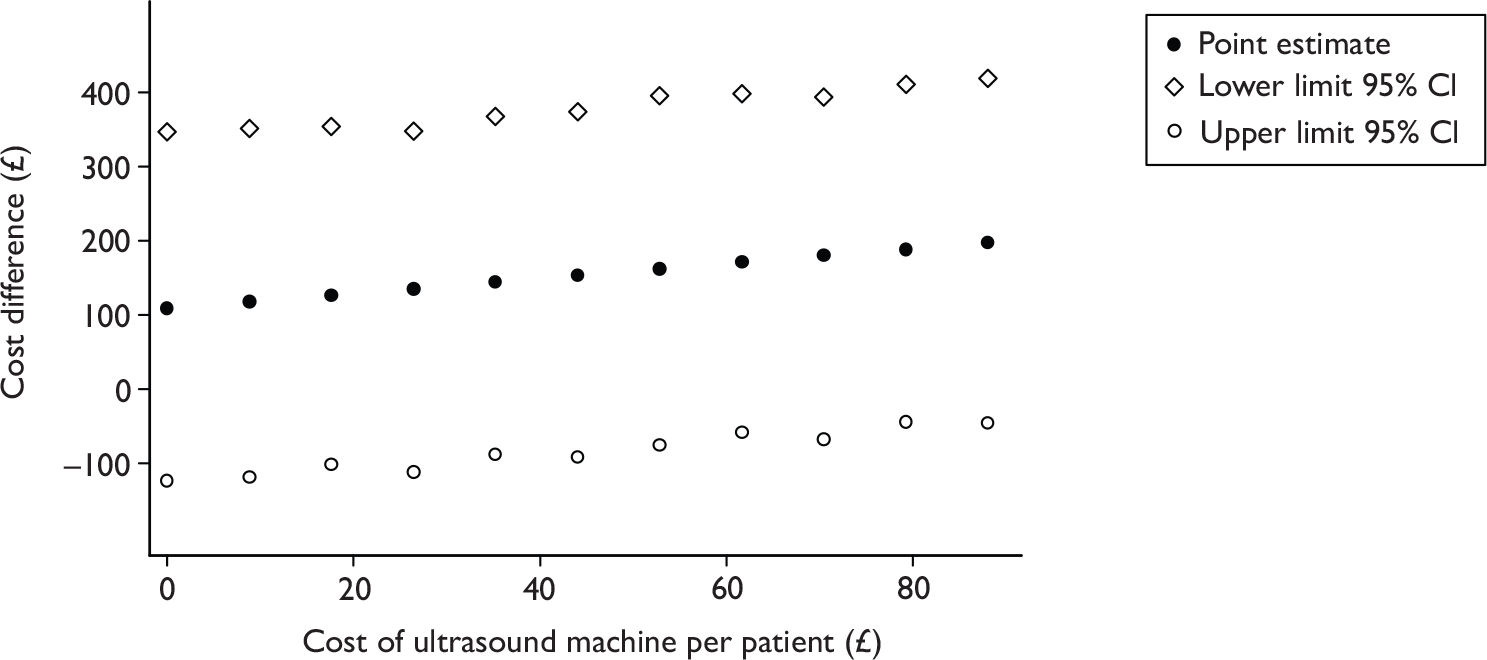
Summary
Evidence from VenUS III suggests that low-dose ultrasound plus standard care is expected to be more costly and slightly less beneficial than standard care alone for the treatment of hard-to-heal venous leg ulcers. The analysis of uncertainty also shows that it is unlikely that this treatment strategy is cost-effective.
Based on these data, low-dose ultrasound is deemed not to be cost-effective and thus should not be recommended for adoption in the NHS. The results remained unchanged when the patient-reported health-care consultation data were used.
Decreasing the equipment cost of ultrasound machine did not affect the overall conclusion.
Chapter 6 Ultrasound output verification test and servicing
A total of 40 therapeutic ultrasound machines (EMS Therasonic 355 Physiotherapy systems) were purchased for use in VenUS III. Given that previous studies have indicated that there can be differences between the ‘nominal’ dose and that which is actually delivered by the machines,42 the study team wanted to ensure that the machines were functioning accurately. Since some of these differences can be apparent at machine delivery and some are due to drift or step changes in output,43 two forms of testing were deemed necessary.
Firstly, prior to the commencement of the study, all machines were tested by the NPL in order to ascertain whether they were delivering ultrasound at the necessary frequency and intensity.
Secondly, the ultrasound machines were serviced by the manufacturer 3 months after the site had recruited its first patient allocated to ultrasound and 3-monthly thereafter.
Initial testing by the National Physical Laboratory
The NPL was asked to conduct acceptance testing of the machines (including retesting of any that fail initially), which was to include:
-
measurement of total acoustic power at 0.5 W/cm2 on continuous wave mode and also on a specified pulsed wave setting, using NPL absorbing target radiation force balance
-
measurement of effective radiating area (AER) at 1 MHz, using absorbing apertures for the majority of machines
-
pass/fail criteria consistent with the current versions of International Electrotechnical Commission (IEC) 60601-2-5 and IEC 61689.
In order for a machine to pass, any deviation from nominal had to be 20% or less for all of the tests conducted. The methods used by the NPL to obtain the necessary measurements can be found in Appendix 10.
The machines were initially sent for testing in two batches following consecutive delivery from the suppliers. The first batch contained two machines and the second batch consisted of the remaining 38 machines.
Results of the acceptance testing
The number of machines acceptance tested and passed is shown in Table 32.
| Acceptance test | Number tested | Number failed | NPL test report reference | Resultsa shown in |
|---|---|---|---|---|
| Initial testing | 40 | 11 | U2263 and U2310 | Appendix 10 |
| Retesting | 11 | 1 | U2419 | Appendix 10 |
| Second retesting | 1 | 1 | U2617 | Appendix 10 |
Auditing ultrasound machine performance
The ultrasound machines were assessed to check the intensity of ultrasound delivered throughout the study (see Appendix 10). This originally was conducted by the ultrasound machine suppliers at each clinical site except for those sites in regions where there was no engineer (Northern Ireland and Scotland). Each ultrasound machine was numbered so that patients who received treatment from individual machines could be identified. This check was first carried out 3 months after a site randomised its first ultrasound patient and 3-monthly thereafter.
Thirteen of the machines developed a problem at some time during the study, the most common being a discrepancy with the output requiring repair or adjustment, followed by transducer heads failing to operate (immediately obvious) owing to the pins in the connecting lead being bent as a result of incorrect/forceful insertion into machine.
The digital screen of one machine was broken and required replacing, while one further machine was stolen.
Chapter 7 Discussion
Here we report the largest trial ever undertaken of therapeutic ultrasound for wound treatment. 76–78
This trial is also much larger than any trial of ultrasound for fractures included in a recent systematic review79 and was conducted and reported in accordance with international guidelines for research excellence. 80,81
We were stimulated to conduct this trial by summaries of the pre-existing trial evidence for the effects of ultrasound in wound healing generally78 and leg ulcers specifically. 76 The evidence suggested that this trial was conducted following systematic reviews which identified a potential benefit of ultrasound therapy for venous ulcer healing; however, a definitive trial was needed because previous trials were small and had methodological weaknesses and/or incomplete reporting; hence, the results of the reviews were not conclusive and at high risk of bias. Furthermore, the clinical contexts in which the previous trials had taken place (e.g. before the widespread introduction of high-compression therapy) meant that their relevance to today’s leg ulcer patients was dubious. The largest previous trial was undertaken prior to the introduction of high-compression therapy and, hence, ultrasound was used against a background of lower healing rates. We now know from prognostic studies and trials research that have established that high-compression therapy such as four-layer bandages heals the vast majority (80%) of venous ulcers within 12 months, and that ulcers which are < 5 cm2 and of < 6 months’ duration are more likely to heal quickly. 8 In designing this trial we therefore felt that adding an adjuvant therapy such as ultrasound to the treatment of small or new ulcers was unlikely. There is likely to be little benefit to adding an adjuvant therapy to the treatment of venous ulcers that are small or new. The sample size of a trial to find a benefit of adding ultrasound to the treatment of most venous ulcers would be very large given the high healing rate. This study, therefore, evaluated the addition of low-dose ultrasound to modern compression techniques for ‘hard-to-heal’ ulcers. We evaluated the effect of 12 weeks of low-dose ultrasound, delivered during the regular nurse visits consultations, on time to healing, cost-effectiveness of this treatment and adverse effects. The discussion summarises the key findings, considers possible mechanisms, compares these results with published studies, considers the limitations of the present study and, finally, summarises the clinical and research implications of the work.
Key findings
We compared the effects of low-dose ultrasound delivered alongside standard care with standard care alone on the time to healing of the reference (largest) ulcer. We found no evidence that adding low-dose ultrasound treatment to the standard package of care (low-adherent dressings and high-compression therapy) reduced the time to leg ulcer healing compared with standard care alone (log-rank statistic 0.25, p = 0.61). The final statistical model, which included a frailty component that accounted for a centre effect and the difference in healing rates between centres, and adjusted for baseline ulcer area, duration and the use of compression, resulted in a hazard ratio for healing (with ultrasound vs standard care alone) of 0.99 (95% CI 0.70 to 1.40, p = 0.969).
A small, statistically not significant difference in the median time to complete healing of all ulcers was observed. Median time to healing of the standard care group was 328 days, (95% CI 235 days, inestimable) compared with the median time to healing of the ultrasound group (median 365 days, 95% CI 224 days, inestimable). Wound triallists traditionally report healing outcomes in a plethora of different ways,78 and there was no evidence of a difference between low-dose ultrasound and standard care for any measure of healing in this trial.
There was no difference between low-dose ultrasound plus standard care and standard care alone in the proportion of patients with ulcers healed at 12 months (72/168 in ultrasound vs 78/169 standard care), nor in the change in ulcer size at 4 weeks.
We did, however, confirm our earlier findings7,44 that baseline ulcer area and ulcer duration were statistically significant predictors of time to healing (p < 0.0001), with larger ulcers and those of longer duration taking longer to heal. 7,61
There was no evidence of a difference in recurrence of healed ulcers and few people had a recurrence within trial follow-up.
We investigated changes in HRQoL from baseline using the SF-12v2. 40
Our results confirm the previous findings that leg ulcer patients have a poorer physical health (PCS) than age norms. There was little change in the PCS score during the trial in both groups. There was no evidence of a statistically significant change in mental health scores (MCS) over time. We cannot conclude that ultrasound has any impact on HRQoL. As the SF-12 is a generic tool it is possible that it may have missed changes in ulcer-specific dimensions; however, previous work has demonstrated that the SF-12 and EQ-5D are sensitive to, and thus able to measure, change in venous leg ulcer patients. 47
There were more adverse events in the ultrasound group than in the standard care group. Given that this trial was open, and ultrasound is not currently used in practice, then it is possible that nurses were more likely to attribute adverse events (such as ulcer deterioration) to a treatment with which they are relatively unfamiliar. The overall adverse event rate (the number of people reporting any SAE or NSAE) was similar to that in VenUS I,7 in which the adverse event rate was approximately 40%.
We evaluated whether adding ultrasound to standard care was a cost-effective strategy for the management of venous leg ulceration in relation to standard care alone. The intervention requires an increase in treatment time and, hence, costs associated with nursing time. In addition, there is the need to the purchase ultrasound machines. We found that ultrasound therapy as an adjuvant to standard care was found not a cost-effective treatment when compared with standard care alone. In the base-case analysis, while mean QALYs were very similar between groups, the mean cost of ultrasound was £197.88 (bias-corrected 95% CI –£35.19 to £420.32) higher than the mean cost of standard care per participant per year. The analysis of uncertainty surrounding these estimates shows that it is unlikely that this treatment strategy is cost-effective, while health benefits were lower.
Sensitivity analyses were conducted for the cost-effectiveness. These assessed the impact of altering various assumptions such as nurse-reported rather than patient-reported data on consultation rates and reducing the costs of the ultrasound treatment (lower cost machine or used more widely). Neither approach had any impact on the findings.
Consideration of possible explanations
There are two explanations of our findings: that there is a treatment effect but this trial failed to detect it (either by chance or due to methodological problems) or ultrasound at this dose and frequency does not accelerate healing of venous ulcers of long duration/large area. We minimised the play of chance accounting for the lack of an effect by having a large sample size. We minimised detection bias by using confirmed healing date by photographic assessment. The possibility that ultrasound machines did not deliver the prescribed dose was reduced by having them serviced and calibrated regularly, and we recorded all treatments given to ascertain if there was evidence of performance bias (e.g. extra treatments in the standard care group), which there was not. The decision to use this form of ultrasound was based upon the previous evidence (the largest trial) and the need to balance the need to deliver leg ulcer care in an efficient manner, with weekly visits to renew dressings and bandages, and the desire to deliver ultrasound more frequently. We stopped delivering ultrasound at 12 weeks as we felt this was a pragmatic approach to evaluating the addition of a novel therapy that took account of the extra treatment time required by the nurse. We felt that asking nurses to apply ultrasound for the whole trial period would have resulted in disillusionment if nurses felt that there was no discernible benefit, as this would increase the workload significantly: this would have hampered recruitment. The lack of any treatment effect difference, as seen in the Kaplan–Meier curves, seems consistent with there being no effect on healing rate within the first 12 weeks.
Comparison with other studies/reviews
The explanation for the difference between our results and those of previous trials probably lies in the much lower risk of bias in our study and our larger sample size, ensuring that chance differences were less likely to manifest as type 1 errors. We designed this trial in 2003, at which time there were seven trials summarised in a systematic review,32 two of which were not randomised. 26,27 Of the remaining five randomised controlled trials (RCTs), three reported healing data for a 12-week time point, all of which were at medium or high risk of bias. 25,29,30 Individually these all identified a benefit associated with ultrasound for the outcome of proportion of ulcers healed at 12 weeks, though none of the differences was statistically significant when analysed by the numbers randomised rather than by complete case analysis. When the three trials were pooled, however (heterogeneity was minimal with an I2 = 0), a statistically significant benefit associated with ultrasound, over sham or standard care, was apparent (relative risk 1.5, 95% CI 1.0 to 2.3, p = 0.047) (Figure 12).
FIGURE 12.
Plot of meta-analysis for venous ulcers trials before Venous Ulcer Study III (numbers healed at 12 weeks). Std, standard; US, ultrasound.
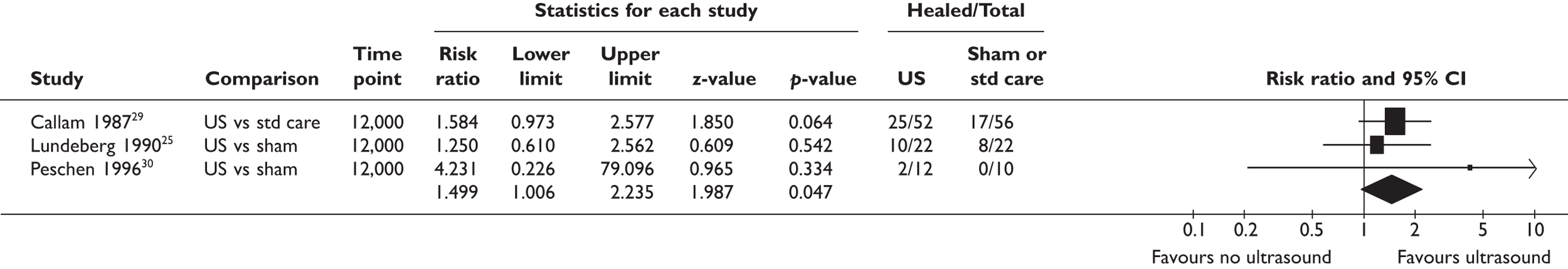
We can now add the VenUS III data into this meta-analysis, by taking the numbers healed at 12 weeks (Figure 13, fixed effect model, I2 = 0). Overall, although more people healed with ultrasound than with sham ultrasound or standard care, this difference was not statistically significant (p = 0.095). Furthermore, since VenUS III is the only trial which met all the validity criteria of adequate randomisation, full allocation concealment, blinded outcome assessment and intention to treat analysis, we would argue that the VenUS III result is the most valid. The conclusion we would therefore draw from all the evidence is that low-dose therapeutic ultrasound does not confer any benefit on the healing of hard-to-heal venous leg ulcers.
FIGURE 13.
Plot of meta-analysis for venous ulcers healing with ultrasound after VenUS III (numbers healed at 12 weeks).
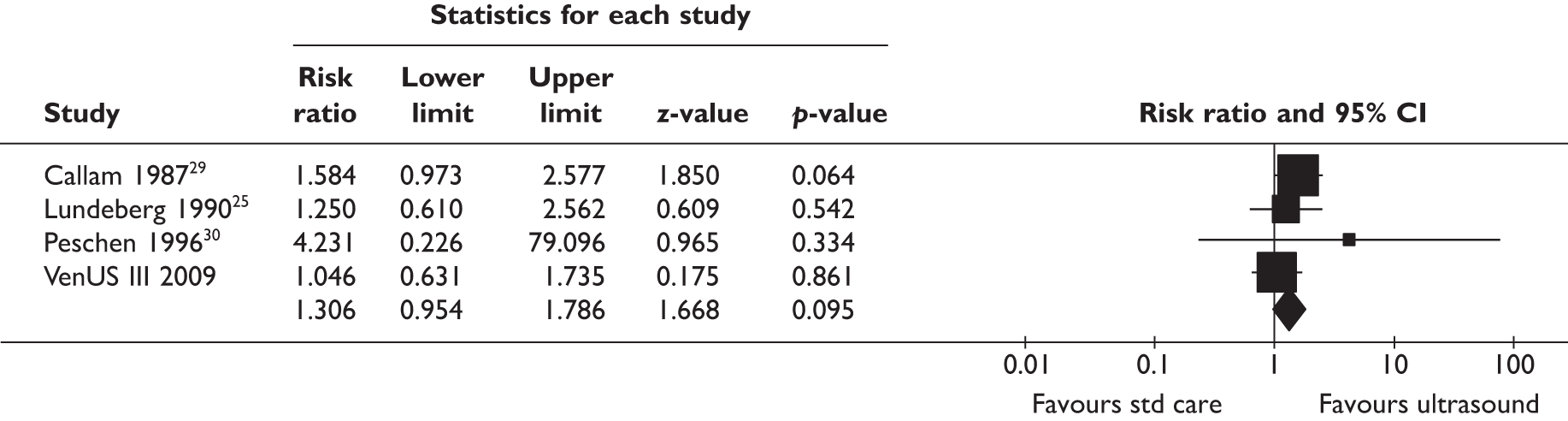
Strengths and limitations of the study
We did recruit people who had hard-to-heal ulcers and, therefore, in whom clinicians might consider adding additional interventions, above standard care packages of dressing and bandages, to aid healing. Ulcers in the people recruited were, on the whole, large (12 cm2) and chronic (1 year duration). The people recruited were representative of the venous ulcer population in terms of age and gender. 1
We had originally estimated that we would recruit 336 people from six sites in 18 months. It took twice this time and 12 centres to meet our recruitment target. This allowed us to detect a 7-week difference in time to healing (with 80% power, 5% alpha). Our finding of no evidence of difference is not likely to be due to lack of power (type 2 error).
In common with most trials of wound care in the community, we were not able to arrange simultaneous, blinded outcome assessment by an independent assessor (blinded to the use of ultrasound or not) owing to logistics and costs. Like VenUS II, we used remote, blinded assessment of serial photographs and confirmed the agreement between two clinical nurses in making this judgement, and in agreement with the local clinical nurse.
Our experience of using cameras for outcome assessment, akin to VenUS II, to obtain blinded outcome assessments was not without challenge. In some cases, we did not receive photographs (e.g. where a camera was stolen or camera data cards lost) and we had to fall back on unblinded data.
As with VenUS I and VenUS II, questionnaire response rates fell over time (they were 89%, 84%, 81% and 86% at 3, 6, 9 and 12 months, respectively). In addition, the questionnaire completion rate (i.e. the proportion of each questionnaire that was fully completed) fell. This meant that at 12 months almost 50% of HRQoL data were unavailable. This was in spite of our efforts to ensure the return of questionnaires (reminders and financial incentives).
Nurse-reported resource data was used in the base case of the economic analysis for several reasons. These data are prospectively and comprehensively collected by the health-care professional and are thus expected to be more accurate and unbiased. In contrast, patient-reported data are subject to individuals’ recollection of health-care resource use over the past 3 months. Additionally, patient self-reported data were found to have a high rate of missing values, especially as follow-up time increased. Patient data do not provide enough detail to allow direct calculation of costs associated with treatments, and this was derived from nurse-reported data. However, patient-reported resource use included other information on health-care consultations, e.g. doctor consultations and hospital outpatient visits. Therefore patient data may be more comprehensive in evaluating ulcer-related costs. For this reason, the use of patient-reported data to inform resource use (health-care consultations) was explored in sensitivity analyses to ensure that the results were not due to us using either nurse or patient data alone.
We identified in the ‘Executive summary, Existing evidence for the effect of ultrasound healing’ that a number of ultrasound regimens have been evaluated in the past, and in designing this study we sought to identify a regimen that was credible. As most evidence was available for the combination of ultrasound at 1 MHz and 0.5 W/cm2, including the best-quality study to date (with only moderate risk of bias), we selected this regimen. We were unable to base our selection of regimen on dose–response studies (as none exist) or other robust evidence, and, hence, we can only report on the effects of this particular ultrasound regimen and not higher doses or longer durations of treatment.
Generalisability of the results
VenUS III recruited from 12 centres across England, Scotland, Northern Ireland and Ireland. The sites had various models of delivering leg ulcer care. The inclusion of outpatient clinics, community tissue viability services and district nursing teams means that we can be confident that these results are broadly generalisable, i.e. that the study has external validity across the UK and probably Europe.
Implications for health care
There is no evidence from this trial that low-dose ultrasound should be used for venous leg ulcers that are > 5 cm2 and/or older than 6 months, i.e. those described as ‘harder to heal’. We evaluated only a specific ultrasound treatment regimen and therefore these results cannot be extrapolated to other regimens.
Implications for research
We identified a large variation in healing rates according to trial centres, with those centres recruiting more patients to the trial having higher healing rates overall. This centre effect may be a manifestation of a relationship between the volume of throughput and the quality of care, such that centres with higher patient throughput may have the opportunity to develop greater expertise in patient assessment, in compression bandage application and in accessing specialist services of vascular surgery, dermatology, orthotics, etc. We controlled for ulcer area and duration; hence, the difference in healing rates across centres is not likely to be due to these prognostic factors being distributed differently across sites (i.e. larger/old ulcers in one site). This interesting finding is worthy of more exploration in future research.
Acknowledgements
Huge thanks are due to the participants for taking part in this trial, research nurses, tissue viability teams, district nurses and hospital outpatient staff for recruiting participants and completing the trial documentation, principal investigators at each site for co-ordinating participant recruitment and the Trial Steering Committee (TSC) and Data Monitoring and Ethics Committee (DMEC) members for overseeing the study.
We would specifically like to thank
Una Adderley, Paul Allcoat, Jacqui Ashton, Annemarie Brown, Carol Dealey, Sharon Hailes, Linda McDermott-Scales, Janet McGowan, Angie Oswald, Sarah Pankhurst, Nikki Stubbs and Anne Witherow who were the nurse leads in each site. In addition, we would like to thank Bernie Boyle, Susan Bradley, Melanie Burrows, Margaret Crookes, Joanne Dakin, Patricia Davies, Erica Denton, Mary Doherty, Sarah Fiori, Gemma Hancock, June Jones, Peter Jones, Denise Nightingale, Lesley Marland, Jeanette Marshall, Jane Mayes, Jane Megson, Sue Merryweather, Carol Muir, Pam Ross, Rebecca Stubbs, Debra Vickery, Victoria Warner, Shirley Williams and Lindsey Worstenholme for recruiting patients into the study.
Una Adderley, Gemma Hancock and Susan O’Meara undertook the blinded outcomes assessment of clinical ulcer photographs and Mei-See Man carried out the ulcer tracing measurements.
Collaborations and contributions of the authors
The VenUS III collaborators (current and past) are Martin Bland, Sue Collins, Ling-Hsiang Chuang, Ben Cross, Nicky Cullum, Jo Dumville, Kate Flemming, Liz Holey, Cynthia Iglesias, Arthur Kang’ombe, Andrea Nelson, Stephen Pye, Elizabeth Scanlon, Marta Soares, Gerben ter Riet, David Torgerson, Kath Vowden, Peter Vowden, Michael Walker, Shernaz Walton, Val Wadsworth, Judith Watson and Gill Worthy.
EAN was the chief investigator, chaired the Trial Management Group and edited and approved the final draft of the report. JMW was the trial co-ordinator. GW and MB designed the clinical analysis. MB oversaw the conduct of the analysis. AK conducted the clinical analysis. MS contributed to the trial management group, designed the economic analyses and oversaw the conduct of the analysis. L-HC undertook the economic analysis. CI participated in the design and conduct of the study. NAC and DJT contributed to the study design and co-ordination.
Trial Steering Commimttee members
Professor S Homer-Vanniasinkam (Chairperson of TSC January 2006 to December 2006).
Dr Su Mason (member of TSC January 2006 to December 2006, then Chairperson of TSC January 2007 to end).
Professor Kevin Burnand (member of TSC January 2006 to August 2008).
Mrs Denise Howell (member of TSC January 2006 to end).
Dr Timothy Rowlands (member of TSC January 2007 to end).
Mrs Nikki Stubbs (member of TSC December 2007 to end).
Consumer representatives
Mrs Joyce Goulton.
Mr Geoffrey Farmery.
Data Monitoring and Ethics Committee Members
Professor Robin Prescott (Chairperson).
Professor Andrew Bradbury.
Ms Brenda King.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- Graham ID, Harrison MB, Nelson EA, Lorimer K, Fisher A. Prevalence of lower-limb ulceration: a systematic review of prevalence studies. Adv Skin Wound Care 2003;16:305-16.
- Dale JJ, Callam MJ, Ruckley CV, Harper DR, Berrey PN. Chronic ulcers of the leg: a study of prevalence in a Scottish community. Health Bull (Edinb) 1983;41:311-14.
- Moffatt C, Martin R, Smithdale R. Leg ulcer management. Oxford: Blackwell Publishing Ltd; 2007.
- Ågren MS, Gottrup F, Morison MJ, Moffatt CJ, Franks PJ. Leg ulcers – a problem-based learning approach. Edinburgh: Mosby Elsevier; 2007.
- Roe B, Cullum N, Hamer C, Cullum NA, Roe BH. Leg ulcers: nursing management. London: Balliere Tindall; 1995.
- O’Meara S, Cullum NA, Nelson EA. Compression for venous leg ulcers. Cochrane Database Syst Rev 2008. 10.1002/14651858.CD000265.pub2.
- Iglesias C, Nelson EA, Cullum NA, Torgerson DJ. for the VenUS Team . The VenUS I trial: a randomised comparison of 4-layer and short-stretch bandages for the treatment of venous leg ulcers. Health Technol Assess 2004;8.
- Margolis DJ, Berlin JA, Strom BL. Which venous leg ulcers will heal with limb compression bandages?. Am J Med 2000;109:15-9.
- Lambourne LA, Moffatt CJ, Jones AC, Dorman MC, Franks PJ. Clinical audit and effective change in leg ulcer services. J Wound Care 1996;5:348-51.
- Vowden KR, Barker A, Vowden P. Leg ulcer management in a nurse-led, hospital-based clinic. J Wound Care 1997;6:233-6.
- Laing W. Chronic venous diseases of the leg. London: Office of Health Economics; 1992.
- Morrell CJ, Walters SJ, Dixon S, Collins KA, Brereton LM, Peters J, et al. Cost effectiveness of community leg ulcer clinics: randomised controlled trial. BMJ 1998;316:1487-91.
- Ragnarson Tennvall G, Hjelmgren J. Annual costs of treatment for venous leg ulcers in Sweden and the United Kingdom. Wound Repair Regen 2005;13:13-8.
- Department of Health . Prescription Cost Analysis Data England, 2006 2007.
- Callam MJ, Ruckley CV, Harper DR, Dale JJ. Chronic ulceration of the leg: extent of the problem and provision of care. Br Med J 1985;290:1855-6.
- Hickie S, Ross S, Bond C. A survey of the management of leg ulcers in primary care settings in Scotland. J Clin Nurs 1998;7:45-50.
- Dyson M, Adjuvant therapies: ultrasound, laser therapy, electrical stimulation, hyperbaric oxygen and vacuum-assisted closure therapy. In Morison MJ, Moffatt CJ, Franks PJ. Leg ulcers – a problem-based learning approach. Edinburgh: Mosby Elsevier; 2007.
- ter Haar G. Therapeutic ultrasound. Eur J Ultrasound 1999;9:3-9.
- Watson T. Ultrasound in contemporary physiotherapy practice. Ultrasonics 2008;48:321-9.
- Speed CA. Therapeutic ultrasound in soft tissue lesions. Rheumatology 2001;40:1331-6.
- Baker KG, Robertson VJ, Duck FA. A review of therapeutic ultrasound: biophysical effects. Phys Ther 2001;81:1351-8.
- ter Haar G. Basic physics of therapeutic ultrasound. Physiotherapy 1987;73:110-13.
- Enraf Nonius . Ultrasound Therapy 1995.
- Eriksson SV, Lundeberg T, Malm M. A placebo controlled trial of ultrasound therapy in chronic leg ulceration. Scand J Rehabil Med 1991;23:211-13.
- Lundeberg T, Nordstrom F, Brodda-Jansen G, Eriksson SV, Kjartansson J, Samuelson UE. Pulsed ultrasound does not improve healing of venous ulcers. Scand J Rehabil Med 1990;22:195-7.
- Roche C, West J. A controlled trial investigating the effect of ultrasound on venous ulcers referred from general practitioners. Physiotherapy 1984;70:475-7.
- Dyson M, Franks C, Suckling J. Stimulation of healing of varicose ulcers by ultrasound. Ultrasonics 1976;14:232-6.
- Weichenthal M, Mohr P, Stegmann W, Brietbart EW. Low-frequency ultrasound treatment of chronic venous ulcers. Wound Repair Regen 1997;5:18-22.
- Callam MJ, Harper DR, Dale JJ, Ruckley CV, Prescott RJ. A controlled trial of weekly ultrasound therapy in chronic leg ulceration. Lancet 1987;330:204-6.
- Peschen M, Vanscheidt W. Low Frequency Ultrasound of Chronic Venous Leg Ulcers As Part of an Out-Patient Treatment n.d.
- Franek A, Chmielewska D, Brzezinska-Wcislo L, Slezak A, Blaszczak E. Application of various power densities of ultrasound in the treatment of leg ulcers. J Dermatolog Treat 2004;15:379-86.
- Flemming K, Cullum N. Therapeutic ultrasound for venous leg ulcers. Cochrane Database Syst Rev 2000. 10.1002/14651858.CD001180.
- RCN Institute, Centre for Evidence Based Nursing, University of York and School of Nursing Midwifery and Health Visiting, University of Manchester . Clinical Practice Guidelines. The Management of Patients With Venous Leg Ulcers. Recommendations for Assessment, Compression Therapy, Cleansing, Debridement, Dressing, Contact Sensitivity, Training Education and Quality Assurance 1998.
- Margolis DJ, Berlin JA, Strom BL. Risk factors associated with the failure of a venous leg ulcer to heal. Arch Dermatol 1999;135:920-6.
- Gardner SE, Frantz RA, Troia C, Eastman S, MacDonald M, Buresh K, et al. A tool to assess clinical signs and symptoms of localized infection in chronic wounds: development and reliability. Ostomy Wound Manage 2001;47:40-7.
- Taylor R. ‘Mouseyes’: an aid to wound measurement using an computer. J Wound Care 1997;6:123-6.
- Simon D, Freak L, Williams I, McCollum C. Progression of arterial disease in patients with healed venous ulcers. J Wound Care 1994;3:179-80.
- Skene AI, Smith JM, Dore CJ, Charlett A, Lewis JD. Venous leg ulcers: a prognostic index to predict time to healing. BMJ 1992;305:1119-21.
- Callam M. Prevalence of chronic leg ulceration and severe chronic venous disease in western countries. Phlebology 1992;1:6-12.
- Jenkinson C, Layte R. Development and testing of the UK SF-12 (short form health survey). J Health Serv Res Policy 1997;2:14-8.
- Kind PT, Spilker B. Quality of life and pharmacoeconomics in clinical trials. Philadelphia, PA: Lippincott-Raven; 1996.
- ter Riet G. Problems in the conduct of a randomised controlled trial. J Wound Care 1998;7:259-62.
- Pye S. Ultrasound therapy equipment: does it perform?. Physiotherapy 1996;78:263-7.
- Dumville JC, Worthy G, Bland JM, Cullum N, Dowson C, Iglesias C, et al. Larval therapy for leg ulcers (VenUS II): randomised controlled trial. BMJ 2009;338. 10.1136/bmj.b773.
- Kantor J, Margolis DJ. A multicentre study of percentage change in venous leg ulcer area as a prognostic index of healing at 24 weeks. Br J Dermatol 2000;142:960-4.
- Etris MB, Pribble J, LaBrecque J. Evaluation of two wound measurement methods in a multi-center, controlled study. Ostomy Wound Manage 1994;40:44-8.
- Iglesias CP, Birks Y, Nelson EA, Scanlon E, Cullum NA. Quality of life of people with venous leg ulcers: a comparison of the discriminative and responsive characteristics of two generic and a disease specific instruments. Qual Life Res 2005;14:1705-18.
- Ware JE, Kosinski N, Keller SD. The 12-Item Short-Form Health Survey (SF-12): construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220-33.
- Jenkinson C, Layte R, Jenkinson D, Lawrence K, Peterson S, Paice C, et al. A shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies?. J Public Health Med 1997;19:179-96.
- Iglesias CP, Birks YF, Torgerson DJ. Improving the measurement of quality of life in older people: the York SF-12. QJM 2001;94:695-8.
- Walker NK. Epidemiological Studies of Leg Ulcers in Auckland, New Zealand 2000.
- Iglesias CP, Birks YF, Torgerson DJ. Increasing response rates to postal questionnaires. Changing layout of questionnaires increases response rates. BMJ 2002;325.
- Kind P, Hardman G, Macran S. UK population norms for EQ-5D. Centre for Health Economics discussion paper 172. York: Centre for Health Economics, University of York; 1999.
- Edwards P, Roberts I, Clarke M, DiGuiseppi C, Pratap S, Wentz R, et al. Methods to increase response rates to postal questionnaires. Cochrane Database Syst Rev 2007. 10.1002/14651858.MR000008.pub4.
- Majeske C. Reliability of wound surface area measurements. Phys Ther 1992;72:138-41.
- Collett D. Modelling survival data in medical research. Boca Raton, FL: Chapman & Hall/CRC Press LLC; 2003.
- Billingham LJ, Abrams KR. Simultaneous analysis of quality of life and survival data. Stat Methods Med Res 2002;11:25-48.
- Drummond M, Sculpher M, Torrance G, O’Brien B, Stoddart G. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2005.
- StataCorp . STATA Statistical Software: Release 10.1 2008.
- Willan AR, Lin DY, Manca A. Regression methods for cost-effectiveness analysis with censored data. Stat Med 2005;24:131-45.
- Dumville J, Worthy G, Soares M, Bland JM, Cullum M, Dowson C, et al. VenUS II: a randomised controlled trial of larval therapy in the management of leg ulcers. Health Technol Assess 2009;13.
- Brook R. EuroQol: the current state of play. Health Policy 1996;37:53-72.
- The MVH Group . The Measurement and Valuation of Health: First Report on the Main Survey 1994.
- Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ 1990;300:230-5.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96.
- Gold M, Siegel J, Russell L, Weinstein M. Cost-effectiveness in health and medicine. Oxford: Oxford University Press; 1996.
- National Institute for Health and Clinical Excellence . Guide to the Methods of Technology Appraisal 2008.
- British Medical Association/Royal Pharmaceutical Society of Great Britain . British National Formulary 2007.
- Personal Social Service Research Unit (PSSRU) . Unit Costs of Health and Social Care 2008 2008.
- Claxton K, Culyer A. Health economics: critical perspectives on the world economy. London: Routledge; 2007.
- Efron B, Tibshirani R. An introduction to the bootstrap. London: Chapman & Hall; 1993.
- Van Hout BA, Al MJ, Gordon GS, Rutten FF. Costs, effects and c/e-ratios alongside a clinical trial. Health Econ 1994;3:309-19.
- Brealey S, Atwell C, Bryan S, Coulton S, Cox H, Cross B, et al. The DAMASK trial protocol: a pragmatic randomised trial to evaluate whether GPs should have direct access to MRI for patients with suspected internal derangement of the knee. BMC Health Serv Res 2006;6.
- Watson J, Helliwell P, Morton V, Adebajo A, Dickson J, Russell I, et al. Shoulder acute pain in primary healthcare: is retraining effective for GP principals? SAPPHIRE – a randomised controlled trial. Rheumatology 2008;47:1795-802.
- Kleinbaum D, Klein M. Survival analysis, a self-learning text. New York, NY: Springer Science + Business Media, Inc.; 1997.
- Al-Kurdi D, Bell-Syer SEM, Flemming K. Therapeutic ultrasound for venous leg ulcers. Cochrane Database Syst Rev 2008.
- Akbari Sari A, Flemming K, Cullum NA, Wollina U. Therapeutic ultrasound for pressure ulcers. Cochrane Database Syst Rev 2006. 10.1002/14651858.CD001275.pub2.
- Cullum N, Nelson EA, Flemming K, Sheldon T. Systematic reviews of wound care management: (5) beds; (6) compression; (7) laser therapy, therapeutic ultrasound, electrotherapy and electromagnetic therapy. Health Technol Assess 2001;5.
- Busse JW, Kaur J, Mollon B, Bhandari M, Tornetta P III, Schünemann HJ, et al. Low intensity pulsed ultrasonography for fractures: systematic review of randomised controlled trials. BMJ 2009;338. 10.1136/bmj.b351.
- Altman DG, Schulz KF, Moher D, Egger M, Davidoff F, Elbourne D, et al. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med 2001;134:663-94.
- Department of Health . Medicines for Human Use (clinical Trials) Regulations 2004 n.d. www.uk-legislation.hmso.gov.uk/si/si2004/20041031.htm (accessed March 2005).
Appendix 1 Study protocol
VenUS III (Venous Ulcer Studies III)
Ultrasound for venous leg ulcers
Protocol version 6.0
March 2006
Chief Investigator: Dr E Andrea Nelson
School of Healthcare
University of Leeds
Baines Wing
Leeds LS2 9UT
1 Key Contacts
Dr E Andrea Nelson, Chief Investigator
School of Healthcare, University of Leeds,
Baines Wing
Leeds LS2 9UT
Tel: 0113 343 1373
Fax: 0113 343 7560
Email: e.a.nelson@leeds.ac.uk
Dr Judith Watson, Trial Manager
York Trials Unit, University of York,
Seebohm Rowntree Building (area 4)
York YO10 5DD
Tel: 01904 321306
Fax: 01904 321 387
Email: jmw19@york.ac.uk
Mrs Sue Collins, Trial Secretary
York Trials Unit, University of York,
Seebohm Rowntree Building (area 4)
York YO10 5DD
Tel: 01904 321 727
Fax: 01904 321 387
Email: sc27@york.ac.uk
Professor Martin Bland, Statistician
Department of Health Sciences, University of York
Seebohm Rowntree Building (area 1)
York YO10 5DD
Tel: 01904 321 334
Fax:
Email: mb55@york.ac.uk
Miss Cynthia Iglesias, Health Economist
Department of Health Sciences, University of York
Seebohm Rowntree Building (area 2)
York YO10 5DD
Tel: 01904 321 346
Fax:
Email: cpiu1@york.ac.uk
Mr Ben Cross, Data Manager
Department of Health Sciences, University of York
Seebohm Rowntree Building (area 4)
York YO10 5DD
Tel: 01904 321 364
Fax: 01904 321 387
Email: bc8@york.ac.uk
2 Amendments to protocol since August 2004
2.1 Inclusion/exclusion
-
We have dropped the exclusion criterion ‘rheumatoid arthritis’. Investigators made a strong case that many people can have venous ulcers in the presence of rheumatoid arthritis and that ulcer is not necessarily due to their rheumatoid disease. We initially excluded this population as they may be more prone to compression damage but given that the clinicians caring for these patients commonly use high compression, then we decided to include them.
-
We have dropped the exclusion criterion ‘diabetes’. Clinical collaborators have argued that people can have venous ulcers in the presence of diabetes mellitus, and that their ulcer may not be secondary to diabetes. We initially excluded this population as according to National Clinical Practice Guidelines, they would not be suitable for high compression, but we are informed that in some clinical centres expert practitioners will treat people with well-controlled diabetes, who have had a vascular assessment, with high compression therapy. Well-controlled diabetes is defined as a recent HbA1c level of < 10%.
-
We have dropped the exclusion criterion peripheral arterial disease, as this is unnecessary as the inclusion criterion states that the ulcer must be primarily due to venous disease. The clinician has considered someone for the trial as the patients has a clinical diagnosis of ‘ulcer primarily due to venous insufficiency’, and the ABPI reading confirms the lack of significant arterial insufficiency.
-
We have gained ethical approval to recruit people with venous ulceration and an ABPI of at least 0.8 who are unable to tolerate high compression therapies. Our clinicians argue that some people are tolerant of reduced compression therapy and this population represent a particular challenge to heal, as high compression therapy is the single most effective element of treatment.
2.2 Outcome measures
-
We decided to amend the primary outcome measure in the light of advice from the Trial Steering Committee (20 January 2006) and the Trial Management Group (3 March 2006).
Rationale:
Initially we had the primary outcome measure as complete healing of all ulcers, as this is clinically the time at which leg ulcer treatment can be said to have achieved its ultimate aim, and the patient no longer requires dressings, bandages or nurses visits. However, in this trial the ultrasound is delivered only to the reference (i.e. largest) ulcer, then any outcome measure which relied on the healing of other ulcers remote from this would have the potential to dilute any treatment effect.
-
We will therefore have the primary outcome as complete healing of the ulcer treated with ultrasound (the reference ulcer) and record the time to complete healing of all ulcers as a secondary outcome measure.
-
We have added a digital photograph for confirmation of healing at day of healing and 7 days later. This photograph will be assessed ‘blind’ at the Trials Unit, for confirmation of healing. We did not ask nurses to take a digital photograph at every visit as we felt this was onerous. Digital photography was not budgeted for in the trial and we have limited resources to provide cameras, however, as many centres have these for the VenUS II trial, we felt that taking healing photographs was important.
-
We confirmed that the patients are followed up until all ulcers are healed as costs to the patient and provider continue until the patient is ulcer free, therefore the economic end points require that we have data on date of complete ulcer healing. In patients with one ulcer, and in those in whom smaller ulcers heal before the largest ulcer heals, then the date of healing of the reference ulcer will be the date of complete ulcer healing.
-
We identified that patient questionnaire return rates in the previous VenUS trials could be improved and therefore we have obtained agreement from collaborators to send £5 as a token ‘thank you’ payment to patients at the end of the trial, with the final questionnaire. This will not be mentioned in the patient information sheet, so that any possibility that it would be interpreted as a financial incentive to taking part in the trial will be minimised. The final questionnaire, at 12 months post-randomisation will be preceded by a letter notifying the patient that their final trial questionnaire is due to arrive shortly, and that it will be accompanied by a £5 note as a thank you for their taking part in the trial and completing the questionnaires. This letter will make it explicit that the £5 is not conditional on the patient retuning the questionnaire.
-
We identified, after discussion with the manufacturers of the ultrasound machines, that 6 monthly checks of the ultrasound machines may be unnecessary as the amount of drift is related to usage of the machines, and each machine will be used for an average of 9 hours (over 2 years) during the trial. They therefore suggested yearly testing was sufficient. We propose to test machines at 3 months, using an ultrasound balance, and if the readings indicate that the machine output is within tolerance, then recheck every 6 months.
2.3 Minor amendments/typographical errors
-
Protocol clarified to reflect that an ankle brachial pressure index (ABPI) of 0.8 or greater is acceptable for definition of non-clinically significant arterial insufficiency. The previous protocol stated ABPI had to be > 0.8. National clinical practice guidelines recommend that compression is used on people with venous ulceration and an ABPI of 0.8 or greater, and this amendment reflects national guidance and local treatment protocols.
-
Protocol amended to clarify that the research objective proportion of ulcers healed at 12 months should read ‘the proportion of patients with ulcers healed at 12 months’.
3 Trial identifier
3.1 Full title of trial
Randomised controlled trial of cost-effectiveness of ultrasound for ‘hard-to-heal’ venous ulcers
3.2 Acronym
VenUS III (Venous Ulcer Studies III)
4 Background to the trial
4.1 Leg ulceration
Leg ulceration is a chronic, relapsing, and remitting condition, affecting 15–18/1000 adults in industrialised countries. 1 It has a significant personal impact on older people’s health and quality of life (QoL). 2,3 Venous leg ulcers represent up to 84% of all leg ulcer cases in developed countries. 3 The total cost of leg ulcer management in the UK in 1989 was estimated to be between £150M and £600M per annum, with more than 60% of this cost attributed to community-based nursing services. 4 The only therapy so far shown to be clearly effective in the treatment of venous leg ulcers is compression bandaging or hosiery, with high compression being more effective than low compression (relative risk of healing 1.5, 95% CI 1.2 to 2.0). 5 Small (< 5 cm2 area) and new (< 6 months duration) ulcers treated with high compression heal quickly; in our recent trial of compression, the median time to healing of ulcers with pre-trial duration of < 6 months, was 77 days. 6 New ulcers treated with high compression, therefore, heal without the need for adjuvant therapies. One high-quality prognostic study has found that 95% of venous ulcers that are both small (< 5 cm2) and new (< 6 months duration), if treated with high compression (Unna’s boot, the standard system in the USA), can be expected to heal within 6 months (95% CI 75% to 99%). 7 Audits of healing times using the UK standard compression system (four-layer high compression) confirm the importance of ulcer area and duration in predicting healing at 6 months. 8,9 The challenge is now to increase the proportion of ulcers healed (20% remained unhealed in VenUS I at 12 months)6 and to decrease the time to healing, particularly amongst people with longstanding ulceration or large ulcers.
4.2 Ultrasound
Longitudinal waves with a frequency between 20 hertz (Hz) and 20,000 Hz can be heard, however humans cannot detect frequencies below 20 Hz; these are described as ‘subsonic’, nor those above 20,000 Hz, described as ‘ultrasonic’. In clinical practice, the frequencies for ultrasound treatment are typically between 700,000 Hz and 4,000,000 Hz [0.7–4.0 megahertz (MHz)]. As ultrasound penetrates the skin tissues, absorption of the energy wave means that the intensity of ultrasound decreases as the wave travels into the tissues. The amount of absorption depends on the nature of the tissues and on the intensity of the ultrasound. The absorption coefficient of ultrasound in soft tissue increases linearly with frequency, so using higher frequencies (say 3 MHz rather than 1 MHz), reduces the penetration depth, by about one third (from 37 mm to 12 mm in skin). 10
4.2.1 Effect of ultrasound on tissues
When skin is exposed to ultrasound (insonated), there is a transfer of the energy from the ultrasound waveform to the tissues. Researchers have described a number of physiological responses to the biophysical effects of therapeutic ultrasound, and the research has been critically reviewed by Baker et al. 11 Many of the investigations of the biophysical effects of ultrasound have been in vitro studies and there is relatively little evidence that these changes occur in vivo. Biophysical effects of ultrasound have traditionally been separated into thermal and non-thermal effects, though Baker et al. points out that this distinction is artificial, as at low doses, where non-thermal effects are said to predominate, there will always be some transfer of thermal energy. 11 At high doses, where thermal effects are said to predominate there will always be both non-thermal and thermal effects.
Non-thermal effects
Ultrasound vibrations transmitted to the skin cause compression and expansion in the tissues at the same frequency as the applied ultrasound, leading to areas of high and low pressure in the tissues. The effects of these small movements in the tissues are poorly understood. Ultrasound is also said to cause cavitation (development of small gas bubbles in the tissues)10 and acoustic streaming (localised liquid flow around a vibrating bubble). 12 Baker et al. argues that there is no evidence from in vivo studies in humans that cavitation occurs at the ultrasound doses used for tissue repair. 10 Given the absence of cavitation, except in gas filled cavities (such as the lungs), it is further argued that acoustic streaming does not occur in vivo. The way in which cavitation or acoustic streaming might contribute to tissue repair is not obvious; it is postulated that they might lead to reversible changes in the cell membrane permeability. 13 In vitro studies have demonstrated that there are changes in cell membrane permeability during ultrasound exposure, but it is not clear if these findings also occur in vivo, or what impact they would have on healing. 13
Thermal effects
Absorption of ultrasound in the tissues may lead to frictional heat, which in animal models has been shown to increase the local temperature by up to 5 °C. 14 Clearly too much heating could lead to local burns, and it is unclear whether a heating effect is beneficial, and if so, how much local heating is effective and safe. The problem of excess heat is reduced when using pulsed ultrasound as the effective intensity is lower and some of the heat is dissipated between the pulses. 12
4.2.2 Ultrasound application
There are a number of ways of delivering ultrasound to the skin tissues, mainly treatment under water or direct contact, viz.:
-
directly to the area of injury – the ultrasound is directed at the area of tissue for healing
-
indirect – the ultrasound is applied to an area away from the target point, and is transmitted to that area by direct transmission, reflection, and refraction (e.g. application of ultrasound to opposite side of leg from an ulcer; application in a water bath with transmission through water).
4.2.2.1 Dose
Dose of ultrasound delivered is related to both stimulus strength [intensity, expressed as watts (W)/ centimetre (cm)2] and the duration of treatment. A number of factors make it difficult to apply precise doses of ultrasound to the tissues. The output wave is not uniform across the width of the beam; the degree of variation across the beam is described in the beam non-uniformity ratio (BNR). Also, because of differences in the ability of different tissues to absorb ultrasound and because of reflection and refraction of the ultrasound beam in the tissues, the amount of ultrasound energy delivered to the treated area is not easily predicted from the applied dosage. The treatment head is kept in motion in an effort to minimise the variations in ultrasound energy delivered throughout the target area. In this trial we will use a standard duration of treatment (according to the area insonated) and deliver a stimulus strength of 0.5 W/cm2. This will allow us to describe accurately the ‘effective intensity’ of ultrasound. Effective intensity will be measured according to current international standards. 15,16
4.2.2.2 Contraindications
Ultrasound is contraindicated in people with ankle prostheses/metal anywhere in the foot (e.g. pin and plate, shrapnel), because bone cement used in the replacement of joints has a high absorption capacity, the application of ultrasound to the ankle area may lead to heat damage of the prosthetic joint. 17 Ultrasound is also contraindicated for people with suspected thrombophlebitis (the mechanical vibrations may cause an embolism);17 people with active cellulitis (potential risk of accelerated growth and dissemination of bacteria throughout the body);17 in cases of suspected or confirmed local cancer/metastatic disease,17 and cases of obvious ulcer infection. 17
4.3 Ultrasound and wound healing: the need for a trial
A number of studies have investigated the impact of ultrasound on skin cells (in vitro) and chronic wounds (in vivo). In general there have been few good quality studies demonstrating that any of the ‘in-vitro’ effects have any clinical importance. 11
There have been eight randomised controlled trials (RCTs) of ultrasound for treating venous leg ulcers. Seven of these were summarised in a systematic review by some of the applicants18 and one additional trial has been published subsequently. 19 The sample sizes in these trials ranged from 12–108 patients and five trials used true randomisation with allocation concealment. The trials made various comparisons of ultrasound versus sham (four trials) or ultrasound as an adjunct to standard care versus standard care alone. Various types of ultrasound at different dose were used. Frequency of ultrasound ranged between 0.3 and 3.0 MHz: 0.3 MHz was used in two trials (applied via water bath), 1.0 MHz was used in four trials and 3.0 MHz was used in another two trials. 1.0 MHz has greater depth penetration than 3.0 MHz. Ultrasound doses ranged between 0.1 and 1.0 W/cm2. In two trials in which a water bath ultrasound device was used, 0.1 W/cm2 was used. Doses of 0.5 W/cm2 were used in three trials and 1.0 W/cm2 in four trials (one trial compared 0.5 and 1.0 W/cm2 against standard care). No trials reported that they confirmed ultrasound equipment output.
The largest trial (108 people) evaluated weekly ultrasound but the other trials administered ultrasound at two- or three-times a week, with one having a reducing frequency from three-to one-time(s) a week. Four trials used ultrasound for 12 weeks, two for 8 weeks and two for 4 weeks. The five trials that described duration of ultrasound regimen used 10 minutes (three trials) or 5–10 minutes, depending on ulcer area (two trials).
The heterogeneity in these trials with respect to the delivery mode, dose, duration, treatment length and frequency used, means that meta-analysis of all these trials may not be reliable. Another problem with synthesising these studies is the likely difference in the ultrasound actually delivered, even when treatment regimens appear similar due to the differences in output between machines and over time (drift). The Cochrane review undertook meta-analysis of the four trials that reported data on proportion of ulcers healed at 8–12 weeks found that the relative risk of healing with ultrasound was 1.44 (95% CI 1.01 to 2.05). The absolute difference in the risk of ulcers healing in the trials against sham ultrasound was 10% (95% CI –10% to 30%), while in the trials comparing against standard care alone it was 15% (95% CI 0% to 30%). Given that data from only four of the eight trials were pooled, and the potential heterogeneity in the interventions, this meta-analysis must be interpreted cautiously.
Given that standard care of venous ulcers, using high compression and simple dressings heals around 80% of all ulcers with 12 months, then ultrasound as an adjuvant therapy is likely to be reserved for those resistant to standard therapy, or are identified at the outset as ‘hard to heal’.
5 Research objectives
To compare the clinical effectiveness and cost-effectiveness low-dose ultrasound (0.5 W/cm2 spatial average and temporal peak) delivered at 1 MHz in conjunction with standard care against standard care alone in the treatment of hard-to-heal venous ulcers. The trial will assess whether the addition of 5–10 minutes of ultrasound (depending on ulcer area) to a package of best available practice affects:
-
the time to healing of venous leg ulcers
-
the proportion of patients with ulcers healed at 12 months
-
health-related quality of life (HRQoL)
-
the costs of caring for venous leg ulcers.
Ultrasound machines with regularly verified output will be used to allow valid inferences of the effect of the applied dose.
5.1 Research methods
5.2 Study design
A multicentre, pragmatic, RCT with an economic evaluation, comparing low-dose ultrasound with standard care in hard-to-heal venous ulcers.
5.2.1 Case definition
Only people with ‘hard-to-heal’ venous leg ulcers will be recruited into this study.
Venous ulceration
For the purpose of this study a leg ulcer will be considered to be any break in the skin on the leg (below the knee), which has either (a) been present for more than 6 weeks or (b) occurs in a person with a history of venous leg ulceration. A participant will be considered to have a purely venous leg ulcer where there is no other causative aetiology, the ulcer appears clinically venous (moist, shallow, irregular shape, venous eczema, ankle oedema, and/or lipodermatosclerosis, not confined to the foot), and the study participant has an ABPI of > 0.8. An ABPI < 0.8 indicates that there is a high probability that arterial insufficiency is present and that the ulcer should not be regarded as venous. 20
Hard-to-heal ulceration
Prognostic studies have found that patients with ulcers > 5 cm2 and duration > 6 months are less likely to heal within 24 weeks. 21 For a person to be included in the trial they must either:
-
have a venous ulcer of > 6 months duration (determined by asking the patient), or
-
have a venous ulcer larger than 5 cm2 (estimated by tracing the ulcer outline onto a transparent grid with 1 cm lines; nurse training will include standard tracing techniques/calculation of area).
Patients with ulcers that fulfil both criteria (> 5 cm2 and present of more than 6 months) are also eligible.
5.2.2 Inclusion criteria
All people with venous leg ulcers are potentially eligible for inclusion in the proposed trial if they meet the following criteria:
-
Currently receiving care from community/leg ulcer/out-patients nurses in trial centres.
-
Able to give written informed consent to participate in the study. Information sheets and consent forms will be provided in languages other than English if required.
-
The primary cause of their ulcer is chronic venous insufficiency. This diagnosis will be made using the same diagnosis criteria currently employed by caregivers in the community, namely the clinical appearance of the ulcer, patient history and an ABPI to rule out arterial insufficiency. 20
-
Have ‘hard-to-heal’ ulcers as defined by the presence of at least one of these criteria:
-
a venous ulcer of > 6 months duration,
-
a venous ulcer larger than 5 cm2.
-
Doppler-determined ABPI of at least 0.8 within last 3 months.
-
People with an ulcer infection (based on a clinical signs and symptoms checklist) at baseline will be eligible to participate once the infection has resolved. 24
-
People who are unable to self-complete the English language QoL tools will still be eligible to participate, but we will not collect QoL data from them [the Short Form questionnaire-12 items (SF-12) is validated in English, Spanish, Italian, French and German and we anticipate that the number of non-English speakers who use these languages will be very small].
5.3 Exclusion criteria
Potential participants will be excluded if they meet the following criteria:
-
Their leg ulcer is due to causes other than venous insufficiency (e.g. arterial insufficiency, malignancy).
-
The patient has poorly controlled diabetes, as evidence by a glycolated haemoglobin (HbA1c) of > 10%.
-
People with ankle prostheses/metal anywhere in the foot (e.g. pin and plate): because bone cement used in the replacement of joints has a high absorption capacity, the application of ultrasound to the ankle area may lead to heat damage of the prosthetic joint. 17
-
People with suspected thrombophlebitis: the mechanical vibrations may cause an embolism. 17
-
People with active cellulitis: because of the potential risk of accelerated growth and dissemination of bacteria throughout the body. 17
-
In cases of suspected or confirmed local cancer/metastatic disease. 17
5.4 Patient recruitment
Patients with venous leg ulcers will be recruited from the following clinical centres:
-
Hull
-
Leeds
-
West Cumbria
-
Bradford
-
Altnagelvin (Londonderry)
-
Selby and York
-
Bolton
-
other centres as required.
Local nursing staff or clinical research nurses (CRNs) will identify potential participants, and will supply them with an information sheet about the trial. Patients will be given a minimum of 24 hours to read the information sheet and consider participation. A research or community/leg ulcer nurse will visit those patients that agree to participate, and at the enrolment visit will:
-
obtain written consent from them to participate in the trial
-
record baseline data
-
telephone the freephone randomisation service to randomise patient
-
administer first ultrasound treatment, if appropriate, and reapply compression bandages.
5.5 Randomisation
Research or community nurses from each study centre will enter patients into the trial by calling a freephone central randomisation service provided by the Trials Unit in York. The following information will be collected at randomisation from the nurse:
-
patient details including full name, gender, date of birth, full postal address
-
trial centre
-
whether ulcer is smaller or larger than 5 cm2
-
whether ulcer has been present for more or < 6 months
-
confirmation of eligibility (including use of high compression therapy)
-
confirmation of written informed consent.
Participants will be randomised by computer in equal proportions, block sizes randomly of size four and six. There will be no stratification.
5.5.1 Non recruitment
Clinical research nurses will be asked to complete a screening form for all patients with venous ulcers who present to the local service. For people who are not eligible to enter the trial, these forms will be returned to the York Trials Unit. Information collected will be all reasons patient is not eligible/decided not to consider trial recruitment, as well as patient date of birth, gender, and date of consideration for trial entry.
6 Data collection
Research, community/leg ulcer or outpatients nurses will collect baseline data from each participant, prior to randomisation. The patient’s regular nurse will undertake the assessment of the primary outcome (time to healing) and take a digital photograph at this time, every 4 weeks, at healing (or 12 months, whichever is sooner) and after 7 days post-healing (if healed). Research nurses will collect recurrence data at 6 and 12 months.
Health-related quality of life (HRQoL) data will be collected via postal survey at 3, 6, 9 and 12 months. We will monitor response rates in VenUS II and VenUS III trials and if necessary, reduce the number of assessments in order to increase response rates (Table 1).
| Visit | Time | Assessments |
|---|---|---|
| Pre | –7 to 0 days | Screening, baseline assessment, including ulcer assessment by digital photograph and tracing |
| 1 | 0 days | Randomisation and commencement of ultrasound treatment |
| 2 | 3 months | Assessment of QoL, end of ultrasound treatment |
| 3 | 6 months | Assessment of recurrence and QoL |
| 4 | 9 months | Assessment of QoL |
| 5 | 12 months | Assessment of recurrence and QoL |
| Monthly until ulcer healed or 12 months (which ever is sooner) | Assessment of ulcer area by digital photographs and tracings, costs, non-trial treatments, and adverse events |
6.1.1 Baseline measurements
Study centres: Altnagelvin, Bradford, Hull, Leeds, West Cumbria etc.
Demographic data: age, sex.
Clinical history: incident or recurrent ulcer, duration of ulcer disease, duration of current ulcer (oldest ulcer and reference ulcer if different), mobility, height, weight, ankle circumference.
Prognostic variables: current ulcer duration and ulcer size, as they are predictive of ulcer healing within 24 weeks. 7 Ulcer area will be determined from a leg ulcer tracing.
Ankle brachial pressure index: a Doppler-determined ABPI will be obtained from clinical records for each participant. ABPI are routinely obtained for all leg ulcer patients. All groups use non-directional Doppler with 8 MHz probes to record arm (brachial) and ankle pressure measurements according to the method described by Vowden et al. 27 For inclusion in the study, this reading must have been obtained in the last 3 months as readings change over time. 28
Health-related quality of life questionnaires: SF-12 and European Quality of Life-5 Dimensions (EQ-5D).
6.1.2 Primary outcome measure
The primary outcome measure in this trial will be time to reference ulcer healed. Healing will be defined as complete epithelialisation in the absence of scab/eschar. Time to healing data will be collected by the local nurse, who will notify the CRN both when the reference ulcer (the largest at recruitment) and when the last ulcer has healed. A photograph of the reference/last ulcer site will be taken at healing and at 7 days post-healing for validation purposes. These photographs will be assessed blind at the York Trials Unit to confirm healing.
6.1.3 Secondary outcome measures
A number of secondary outcome measures will be investigated, viz.:
6.1.3.1 Proportion of patients healed
Measured at 3 and 6 months post-randomisation. This will allow direct comparison of the results with other trials.
6.1.3.2 Percentage and absolute change in ulcer size
Measured at 1, 3 and 12 months post-randomisation. The data collected will allow the determination of reduction in ulcer area in patients who do not achieve complete ulcer healing. If the ultrasound and standard care groups achieve similar times to complete healing but one resulted in larger changes in ulcer area, then this may be clinically important as smaller ulcers are thought to exude less and therefore require less frequent dressing changes. Furthermore the recording of ulcer area at these time points will allow further study of the trajectory of healing for venous leg ulcers and the relationship between the reduction in ulcer area and eventual healing. One study found that increased ulcer area at 1 month after initiation of treatment is a useful predictor for non-healing. 29 Identifying patients who are likely to fail to heal early on in treatment allows these patients to have prompt referral to specialist centres for further assessment and treatment. Measurement of ulcer size will involve taking a leg ulcer tracing according to standard procedure – using a comfortable, transparent acetate sheet and a fine-nibbed, indelible pen, taking the outer edge of the ulcer rim as the outer edge of the tracing line (i.e. ulcer area = area enclosed by tracing and area of line). Ulcer area, as determined by acetate tracing, is an accurate and reliable measure. 30
6.1.3.3 Proportion of time patients are ulcer free
Reduction in recurrence would help reduce the prevalence of this condition and thus cost. Crude recurrence rates are potentially biased by any difference in healing rates associated with the two groups (ultrasound or standard care), since if one group has more rapid healing, then people in that group are at risk of earlier recurrence. To account for this we will use the proportion of time that patients are ulcer free as the clinically important measure since it is a function of both healing and recurrence and is important for patients. Patients with healed ulcers will be contacted by telephone at 6, 9 and 12 months in order to obtain recurrence data.
6.1.3.4 Costs
Recorded at each visit by a community nurse until the ulcer has healed or for 12 months, whichever is sooner. The nurse will record at each visit the ultrasound delivered (time, dose etc.), the number and type of dressing products, and compression bandages used. This process will facilitate an incremental analysis of the costs of ultrasound with a view to determining cost-effectiveness of ultrasound. Direct costs (hire of ultrasound machine, dressing product, compression bandages, antibiotic use) will not vary by centre, while indirect costs (e.g. depreciation of capital, mileage) and salary will and therefore will not be recorded.
6.1.3.5 Health-related quality of life (HRQoL)
Each person’s perception of his or her general health will be assessed using the acute version of the SF-1232 and the EQ-5D. 25 The SF-12 is a reliable and well-validated questionnaire,33 and has been used in UK populations including with older people and leg ulcer patients. 34,35 SF-12 will be completed at baseline, 3, 6, 9 and 12 months. The EQ-5D is a generic measure of health status, where health is characterised on five dimensions (mobility, self-care, ability to undertake usual activities, pain, anxiety/depression). 25 Patients are asked to describe their level of health on each dimension using one of three levels: no problems, moderate problems and severe problems. Each response locates a person into one of 245 mutually exclusive health states, each of which has previously been valued on the 0 (equivalent to dead) to 1 (equivalent to good health) ‘utility’ scale based on interviews with a sample of 3395 members of the UK public. 36 The EQ-5D has been validated in the UK. The QoL questionnaires will be administered to patients by postal survey. The EQ-5D will be administered at baseline, 3, 6, 9 and 12 months.
6.1.3.6 Adverse events
Recorded at each visit by a nurse until the patient is ulcer free or for 12 months, whichever is sooner. Both device related and unrelated events will be recorded. Serious device related adverse events will be reported to the trial co-ordinator within 24 hours and reported to both the trial sponsor and Multicentre Research Ethics Committee (MREC) (as per EN 540). 37
6.1.4 Withdrawal
Withdrawal may refer to the following situations; where the patient wishes to withdraw from the study treatment but is prepared to continue answering questions about their ulcer and it’s effect on their life, and where the patient wishes to withdraw from both the trial treatment and the follow-up. We will ensure that the local nurses and CRNs are aware of the difference in these situations, and that they are explicit about whether patients wish to withdraw from treatment or follow-up.
6.1.5 Loss to follow-up
Loss to follow-up occurs when there is no further data available on a patient during the 12 months post-randomisation. As this population is relatively stable, we anticipate a low loss to follow-up rate (for example VenUS I trial). Despite this, follow-up rates for the competition of questionnaires can drop as patients progress through the trial. However, recent evidence from Edwards et al. 38 shows that the odds of response to postal questionnaires doubles when a monetary incentive is used. This almost doubled again when the incentive was non-conditional on response. In addition, the authors found that contacting participants before sending the questionnaire also increased the response. Based on this evidence, we propose to send a letter to participants two weeks prior their final questionnaire informing them of its forthcoming arrival. This final questionnaire will then be posted along with a £5 note as recognition of their commitment to the study. The receipt of this £5 is not conditional on the return of the questionnaire.
7 Planned interventions
Participants will be randomised to receive:
-
low-dose (0.5 W/cm2) ultrasound, 1 MHz, with a pulsed pattern of 1 : 4. The ultrasound will be applied to periulcer skin, weekly for up to 12 weeks, at regular dressing changes, or
-
standard care: this will be a simple low-adherent dressing and high compression, four-layer bandaging, reduced compression or no compression, according to the clinical assessment of the level of pressure tolerated by the patient. The nurses will decide on the frequency of bandage change according to clinical need.
7.1 Ultrasound therapy
7.1.1 Preparation for treatment
Prior to the application of the ultrasound the leg will be washed (often immersed in a bucket of tap water). Any loose skin from around the ulcer and remnants of emollients will be removed (these can accumulate on the ultrasound head, making cleaning, and infection control, more difficult). The ultrasound will be applied directly to the skin surrounding the ulcer, with a water based contact gel to ensure passage of the waveform from the transducer to the tissues (ultrasound is reflected from air pockets).
7.1.2 Target ulcer
The patient’s regular nurse will administer each ultrasound treatment for 5–10 minutes to the reference ulcer. The reference ulcer is defined as the largest eligible ulcer at the baseline visit.
7.1.3 Concurrent therapy
All dressings and bandages will be replaced at these visits. Concurrent therapy for all patients will be low-adherent dressings and four-layer high compression bandaging, reduced compression or no compression, according to the clinical assessment of the level of pressure tolerated by the patient. Additional visits for ultrasound therapy should not be required as this would increase the number of visits and of bandage applications required (and hence the cost of care).
7.2 Training in and monitoring of application of ultrasound intervention
Prior to the trial starting, participating community nurses will attend a full day training programme on the rationale for the trial, patient eligibility, recruitment procedures (including consent and randomisation), ultrasound treatment application, data collection (completion of trial documentation and tracing ulcer outlines), handling participant withdrawal and adverse event reporting. Competency in ultrasound administration will be assessed at the end of the training day. The CRNs will also cascade training in delivering ultrasound for the purposes of the trial to other local nurses so that treatments can be maintained during holiday periods/staff absences.
Clinical Research Nurses will audit the use of ultrasound within the trial, to check that the ultrasound is being delivered as per protocol, i.e. assessment of area of insonation, preparation of skin, application of ultrasound, recording treatment delivered, assessment of unwanted effects, etc.
7.3 Calculating ultrasound treatment time
Ulcers of area < 5 cm2 will receive 5 minutes ultrasound, those of 10 cm2 or < 10 cm2 will receive 10 minutes ultrasound (the maximum time of treatment). For ulcer areas between 5 and 10 cm2, the treatment time in minutes equals the ulcer area in square cm (6 cm2 means 6 minutes etc.). Ulcer area will be recalculated every 4 weeks.
7.4 Ultrasound machines
The ultrasound machines are supplied, at discounted price, by EMS Physio Ltd, the largest UK manufacturer of ultrasound machines. Their EMS Therasonic 355 Physiotherapy machine delivers only 1 MHz ultrasound.
7.5 Auditing performance of ultrasound machines
The ultrasound machines will be assessed for a check of the intensity of ultrasound delivered. This will take place at each clinical site, by the ultrasound machine suppliers, and takes approximately one day for all the machines at one site. Previous studies have indicated that there are differences between the ‘nominal’ dose and that actually delivered by the machines. 22 Some of this is apparent at machine delivery, and some is due to drift or step-changes in output. 23 This will allow us to determine whether the ultrasound machine output has changed over the duration of the trial. Should significant change in ultrasound output occur, then a per protocol analysis will exclude patients who have not received the prescribed dose of ultrasound (± 20%). Each ultrasound machine will be numbered so that patients who have received treatment from individual machines can be identified. This check will take place when a site has been recruiting for 3 months and 3 monthly thereafter.
8 Ethical arrangements
8.1 Adverse effects and anticipated benefits to participants and society
Given the chronic nature of venous leg ulceration, the identification of an intervention that increases healing rates at a reasonable cost would be highly beneficial to leg ulcer patients and the NHS. The known adverse effects associated with ultrasound are pain, erythema, allergy to conducting jelly, and pinhead bleeding in the skin around the ulcer. In previous trials these were reported in 5%–10% of patients, and none were classed as serious adverse events. The local nurse, using a proforma, will routinely record any adverse events associated with any of the trial treatments during the trial. Training will emphasise the need to record and report adverse effects.
8.2 Informing trial participants of possible benefits and risks of intervention
All trial participants will be provided with a patient information sheet prior to their giving consent. The information sheet will outline fully the potential benefits and risks of being involved in the trial. This information sheet will meet all the requirements of the local ethics committees.
8.3 Informed consent
Maintenance of confidentiality and compliance with the UK Data Protection Acts will be emphasised to all study participants. Participation in the study will be entirely voluntary and written consent will be sought. All data will be treated with the strictest confidence. A variety of ethnic groups are likely to be involved in this trial. Contact with individuals from all cultures will be handled with suitable care. We will translate information sheets/consent forms and use local translators to negotiate consent in sites where a significant proportion of people with ulcers speak languages other than English (e.g. Leeds/Bradford).
8.4 Proposed action if informed consent is not possible
One of the inclusion criteria is that people are able to provide written informed consent to participate in the study. If a clinician does not feel that the potential participant meets this requirement (e.g. if they have a diagnosis of cognitive impairment) then they would not be eligible for inclusion in the study.
8.5 Proposed time period for retention of trial documents
All paper copies of patient information will be kept in a locked room at the University of York with identifying information kept separate from the coded data collection forms. Computerised data will be password protected on a computer at the University of York. The Trials Unit will retain all study treatment disposition records in a secure data archive for 5 years from the end of the trial.
9 Statistical considerations
9.1 Proposed sample size
The majority of data on ulcer healing is presented as proportion of ulcers healed at 12 or 24 weeks but the choice of an arbitrary end point fails to capture the time course of healing and can be misleading. We will therefore base the sample size on median time to healing. There is evidence from audits of healing rates, and a prognostic study of ulcer healing, that ‘hard-to-heal’ ulcers take approximately twice as long to heal as new/small ulcers when treated with four-layer compression. Lambourne et al. 8 and Vowden et al. 9 found that 60% of ulcers > 10 cm2, and 60% of ulcers of > 6 months duration (treated with four-layer compression) healed in 24 weeks (168 days). This represents a median time to heal of 15–22 weeks (estimated from survival curve). Our sample will include some smaller ulcers, but importantly it will include people with both high ulcer duration and large area (of whom between 13% and 37% heal at 24 weeks with high compression),7 and therefore, overall we have estimated that 50% of ulcers in the standard care group will heal within 22 weeks.
We estimate that clinicians and patients would value a reduction in healing time of 7 weeks (a 32% reduction in healing time from 22 weeks to 15 weeks) and have based our sample size calculation on this premise. To detect a difference in median healing time of 7 weeks (from 22 weeks to 15 weeks), we require 306 patients in total. When we allow for 10% attrition, this brings the total sample size to 336. A 10% dropout rate has been allowed for in this trial, although VenUS I had no attrition in primary outcome data. Based on this figure and current caseloads, it is estimated that it will take 15 months to recruit sufficient people for the trial, with each area expected to recruit around 50 patients – three patients per month (22 patients total per month). We have allowed 18 months overall for recruitment.
A sample size of 336 patients also gives us 80% power to detect an 8-week reduction in median time to healing from 24 weeks and 90% power to detect this difference from 26 weeks, see Table 2.
| Median time to heal in standard care | Median time to heal in Rx | Difference in weeks | Difference in days (% of baseline) | Total sample size for 80% power | Total sample size for 90% power |
|---|---|---|---|---|---|
| 22 weeks | 14.5 | 7.5 | 52 (34) | 198 | 258 |
| 22 weeks | 15.0 | 7.0 | 47 (30) | 228 | 306 |
| 22 weeks | 15.5 | 6.5 | 45 (29) | 274 | 366 |
| 22 weeks | 16.0 | 6.0 | 42 (27) | 332 | 444 |
| 24 weeks | 15.5 | 8.5 | 59 (35) | 344 | 460 |
| 24 weeks | 16.0 | 8.0 | 56 (33) | 288 | 384 |
| 24 weeks | 16.5 | 7.5 | 52 (31) | 242 | 326 |
| 26 weeks | 18.0 | 8.0 | 56 (31) | 256 | 344 |
| 26 weeks | 19.0 | 7.0 | 49 (27) | 354 | 476 |
| 26 weeks | 20.0 | 6.0 | 42 (23) | 510 | 682 |
9.2 Recruitment rate
Experience from VenUS I has informed the likely recruitment rate. In VenUS I, Cumbria, Leeds and Southport each recruited 36–60 patients per year with venous ulcers (sustained over 1–2 years). A smaller ‘pool’ of people will be eligible for VenUS III as we are excluding people who have small, non-chronic ulcers. In VenUS I, 60% of participants had an ulcer that was both ‘small’ and ‘new’; these would not be eligible for inclusion in this trial. We anticipate that patient and clinician interest will be greater for this trial as it offers an opportunity to improve healing rates in a group of patients in whom standard therapy has a low success rate (and centres report increasing numbers of ‘hard-to-heal’ ulcers). This suggests that each centre could be confidently expected to recruit at least 40% of the patients for VenUS III, which they recruited for VenUS I. The contraindications for ultrasound therapy are unlikely to exclude many patients (few have ankle prostheses, or local cancer) and people with an ulcer infection can be recruited into the trial once the infection resolves.
We have also considered that during the proposed recruitment phase for VenUS III we are also co-ordinating a concurrent HTA funded trial (VenUS II – larval therapy) which is recruiting people with venous or arterial/venous ulcers in whom at least 25% of the ulcer is covered in slough. This is likely to reduce, by a small proportion, the number of people available for the ultrasound trial but we anticipate that the benefits of having a CRN already in place in the sites, identifying people eligible for the larval therapy or ultrasound trials, will increase the efficiency of the recruitment process and reduce some of the start-up costs. Furthermore, at the start of recruitment to the ultrasound trial there will be a cohort of patients with ulcers who did not wish to take part in the larval therapy trial who may be eligible for inclusion in this trial. Throughput data from the sites not involved in VenUS I (these were Cumbria, Southport and Leeds) confirms their ability recruit patients into the trial. Hull has around 140 new ulcers per year, Altnagelvin has 150 new patients per year, and Bradford has more than 300 new ulcers per year; the majority of which are venous.
We will assess recruitment problems by having regular monitoring of recruitment to identify problems, such as needing to extend the catchment area served by a recruitment centre and having monthly newsletters to CRNs. We will also invite CRNs to update meetings at the Trials Unit to encourage sharing of good practice and engender esprit de corps.
In order to recruit 336 patients over 18 months, we require 19 patients per month across all sites. For sites with CRN staffing of 2 days per week this equates to three patients per month, and for sites with 1 day per week, 1.5 patients per month.
10 Statistical analysis
10.1.1 Data management
All data from the trial will be collected using paper-based forms (case record forms, CRFs). Research nurses will be responsible for ensuring the completeness and reliability of the data from their site, and then for conveying paper records to the University of York Trials Unit. Data from CRFs will then be entered into a master database for the trial using optical scanning techniques.
10.1.2 Analysis of clinical data
Data on baseline demographic characteristics such as gender, age, ulcer duration and size, and clinical signs of infection will be summarised and descriptive summary statistics provided. All tests for significance will be based on two-tailed tests. Simple incidence rates, relative risks and 95% CIs will be obtained for all binary variables in the first instance, with subsequent multiple logistic regression analysis conducted if important confounding is shown to exist. The effectiveness of the interventions on time-to-event outcomes, such as time to healing, will be analysed using Kaplan–Meier curves and log-rank test to compare the differences between the two groups. Cox proportional hazards regression analysis will be used to assess time-to-event data, taking into account known covariates. The proportionality assumption will be checked using standard graphical techniques and interval censoring will be employed where appropriate (e.g. analysis of time to healing where the exact day of healing is not known). The initial comparison will be between the survival (time to healing) curves for the two groups (ultrasound and standard care). Sensitivity analysis will be carried out to determine the effect of missing data from patients that are lost to follow-up. All randomised participants who receive study treatment will be included in an analysis of the tolerability of treatment. The numbers of participants discontinuing treatment prematurely for any reason will be summarised by treatment group and by reasons for discontinuation. The incidence of all suspected adverse treatment reactions will be summarised by treatment group.
A per protocol analysis will be undertaken in which only patients receiving ultrasound from machines which were found to be delivering 80%–120% of the prescribed ultrasound dose, will be included.
10.1.3 Analysis of economic and quality of life data
Cost and clinical health benefits associated with the different dressings being compared will be combined in two different types of economic evaluation analysis. First, a simple marginal cost-effectiveness ratio of cost per ulcer free days will be estimated. Second, a cost per quality-adjusted life-year (QALY) gained will also be calculated. The perspective of the economic analyses will be that of the UK National Health Service. Health benefits will be measured in terms of both Kaplan–Meier estimates of the mean time to healing after 12 months per trial arm, and QALYs. The EQ-5D will be used to elicit patient utility values at different points in time. 25 These utility values will then be used to ‘quality adjust’ each patient’s survival time (if the patient dies, a zero value is applied after the point of death). QALYs will be calculated for each patient using the area under the curve of the patient’s utility scores versus time, QALYs will also be adjusted for any imbalances in the EQ-5D scores between groups at baseline. Information regarding patient’s resource use may be truncated at any point in time before the end of the study, i.e. cost data are naturally censored. Consequently, the Lin et al. method26 will be used to estimate mean total treatment cost for each treatment arm. Given the likely skewness of the distribution of the cost data, bootstrapping techniques will be used to estimate a 95% CI of the average mean cost difference between trial arms.
11 Supervision of trial
This trial will be run according to the Medical Research Council (UK) Good Clinical Practice Guidelines. 39 A Study Management Committee will be established to oversee the conduct of this trial. The committee will consist of the study co-ordinator and data management staff, the principal investigator and the trial statistician. Meetings to discuss the data will be held by on a quarterly basis. The committee will provide 6 monthly reports of the progress, or completion, termination or discontinuation of the study to the local ethics committees.
A Trial Steering Committee consisting of the principal investigator of the study, an independent chair and at least two other independent members will be established to discuss on a 6 monthly basis progress with the trial. The trial co-ordinator and the study statistician will attend the meetings as required.
A Data Safety and Monitoring Committee made up of experts independent from the principal investigators and host institutions will monitor the study data. This committee will monitor the data after the first 100 patients. This committee will monitor the progress of the trial, adherence to the trial protocol, and the consideration of any new information and will focus on maintaining the dignity, rights, safety and well being of all study participants. The study data will be provided to the committee members in the form of a data report, including information on any adverse events.
12 Project timetable and milestones
The trial will take 3 years to complete, with 18 months recruitment and 12 months follow-up.
| Prior to trial start (not funded) | Start date to be arranged | Notification from HTA/amendment of study protocol if required/investigators meeting for protocol and data collection tools sign-off |
|---|---|---|
| 3 months prior to trial start | Apply for MREC/advertise trial co-ordination and research nurse posts | |
| 1–2 months prior to trial start | Interview and appoint trial co-ordination and research nurse posts | |
|
Uplift grant Months 1–2 |
Apply for LREC. Trial co-ordinator will develop study materials and training materials for CRNs | |
| Months 3–20 | CRNs start in post. Train CRNs then local nurses in ultrasound use / trial documentation. Commence recruitment and randomisation of participants, ongoing data entry and cleaning | |
| Month 8 | 112 patients recruited | |
| Month 14 | 224 patients recruited | |
| Month 20 | 336 patients recruited | |
| Months 21–32 | Complete follow-up of all patients; ongoing data entry and cleaning; drafting final report | |
| Months 33–36 | Analysis and final draft report |
13 Staff roles
Trial Co-ordinator. This person will be responsible for the day to day running of the trial. She or he will help recruit CRNs in each site, provide training (with CRNs) to all community and hospital nurses involved in recruiting to the trial; draft 6 monthly reports to HTA; compile newsletters for clinical sites; liaise with Local Research Ethics Committee (LREC) and MREC regarding study progress; visit trial sites for source data verification; support CRNs in achieving their recruitment targets and ensure the quality of their work; raise the profile of the trial by writing articles describing the study for professional journals; submit the study to the National Research Register and Clinical Trials Registers, and contributing to the drafting of the final report.
Secretary (UK). This person will be the initial point of contact for CRNs, collaborators and all external queries regarding the trial. She or he will undertake general trial-related secretarial duties including submissions to Ethics and Clinical Governance committees, case record filing, organisation of study days and meetings; provision of data collection tools to sites; arrangement of Trial Management and Steering Group meetings (including preparation of agendas, minutes), compilation of final draft report.
Clinical Research Nurses. At each clinical site a CRN will identify patients potentially eligible for participation in the trial; approach potential trial participants and invite them to participate; support local nurses in recruiting their patients into the trial, undertake initial clinical assessments; audit ultrasound treatments locally; undertake follow-up assessments; participate in trial-related training of community nurses; support local community nurses in trial participation; maintain a high profile for the trial locally; check the completeness and accuracy of all data forms; return completed forms to York.
Data manager. This person will be responsible for data entry and cleaning of all UK-derived clinical, economics and QoL. She ore he will be responsible for generating reminders for nurses/patients to complete the QoL data (every 3 months), will receive and log all completed clinical, QoL and economic data, prepare recruitment and data completion reports for the Trial Steering Committee, run data checks, and preparing summary reports for the final report.
Statistician. This person will conduct all analyses of the clinical data under supervision of Professor Bland.
Principal investigator. The named lead investigator has overall responsibility within the team of researchers for the design, conduct and reporting of the study.
14 Investigators
Ms Shernaz Walton
Consultant Dermatologist
Hull & East Yorkshire Hospitals NHS Trust
Princess Royal Hospital
Salthouses Road
Hull HU8 9HE
Ms June Jones
Clinical Nurse Specialist
Southport & Formby Community NHS Trust
Southport
Mr Peter Vowden
Consultant
Bradford Royal Infirmary, Bradford Hospitals
NHS Trust
Duckworth Lane
Bradford BD9 6RJ
Ms Katherine Vowden
Clinical Nurse Specialist
Bradford Royal Infirmary, Bradford Hospitals NHS Trust
Duckworth Lane
Bradford BD9 6RJ
Mr Michael A Walker
Consultant Surgeon
West Cumberland Hospital
Whitehaven, Cumbria
Mrs Elizabeth Scanlon
Nurse Consultant in Tissue Viability
Leeds General Infirmary
Leeds
LS2
Ms Anne Witherow
Clinical Effectiveness/Tissue Viability Nurse Specialist
Altnagelvin Hospitals HSS Trust
Glenshane Road
Londonderry BT47 6SB
Dr Gerben Ter Riet
A/Professor
Academic Medical Centre
University of Amsterdam
Room J3-354, Academic Medical Center
Meibergdreef 9
Amsterdam Zuidoost
Ms Kate Flemming
Department of Health Sciences
University of York
Seebohm Rowntree Building
York, YO10 5DD
Professor Nicky Cullum
Department of Health Sciences
University of York
Seebohm Rowntree Building – area 2
York YO10 5DD
Professor Martin J Bland
Department of Health Sciences
University of York
Seebohm Rowntree Building – area 1
York, YO10 5DD
Dr Stephen Pye
Consultant Medical Physicist
Lothian University Hospitals NHS Trust
Western General Hospital
Edinburgh, EH4 2XU
Ms Liz Holey
Principal Lecturer, Head of Physiotherapy
University of Teesside
Middlesbrough, TS1 3BA
Professor David J Torgerson
Director of York Trials Unit
Department of Health Sciences
University of York
Seebohm Rowntree Building – area 4
York YO10 5DD
Ms Cynthia Iglesias
Research Fellow
Dept. Health Sciences & Centre for Health Economics
University of York
Seebohm Rowntree Building – area 2
York YO10 5DD
15 References
- Callam MJ, Harper DR, Dale JJ, Ruckley CV, Prescott RJ. A controlled trial of weekly ultrasound therapy in chronic leg ulceration. Lancet 1987;2:204-6.
- Roe B, Cullum N, Hamer C, Cullum NA, Roe BH. Leg ulcers: nursing management. London: Balliere Tindall; 1995.
- Dale JJ, Callam MJ, Ruckley CV, Harper DR, Berrey PN. Chronic ulcers of the leg: a study of prevalence in a Scottish community. Health Bull (Edinb) 1983;41:311-14.
- Laing W. Chronic venous diseases of the leg. London: Office of Health Economics; 1992.
- Cullum N, Fletcher AW, Nelson EA, Sheldon TA. Cochrane Database Syst Rev. Oxford: Update Software; 2000.
- Iglesias C, Nelson EA, Cullum NA, Torgerson DJ. for the VenUS Team . The VenUS I trial: a randomised comparison of 4-layer and short-stretch bandages for the treatment of venous leg ulcers. Health Technol Assess 2004;8.
- Margolis DJ, Berlin JA, Strom BL. Which venous leg ulcers will heal with limb compression bandages?. Am J Med 2000;109:15-9.
- Lambourne LA, Moffatt CJ, Jones AC, Dorman MC, Franks PJ. Clinical audit and effective change in leg ulcer services. J Wound Care 1996;5:348-51.
- Vowden KR, Barker A, Vowden P. Leg ulcer management in a nurse-led, hospital-based clinic. J Wound Care 1997;6:233-6.
- Baker KG, Robertson VJ, Duck FA. A review of therapeutic ultrasound: biophysical effects. Phys Ther 2001;81:1351-8.
- Low J, Reed A. Electrotherapy explained: principles and practice. Oxford, England: Butterworth Heinemann; 1994.
- ter Haar G. Basic physics of therapeutic ultrasound. Physiotherapy 1987;73:110-3.
- Dyson M, McCulloch JM, Kloth LC, Fudar JA. Wound healing: alternatives in management. Philadelphia: FA Davis; 1995.
- Draper DO, Sunderland S, Kirkendall DT, . A comparison of temperature rise in human calf muscle following applications of underwater and topical gel ultrasound. J Orthop Sports Phys Ther 1993;17:247-51.
- IEC 60601-2-5: Medical Electrical Equipment – Part 2-5 n.d.
- IEC 61689: Ultrasonics – Physiotherapy Systems – n.d.
- Enraf Nonius . Ultrasound Therapy 1995.
- Flemming K, Cullum N. Therapeutic ultrasound for venous leg ulcers. Cochrane Database Syst Rev 2000;4.
- Swist-Chmielewska D, Franek A, Brzezinska-Wcislo L, Blaszczak E, Polak A, Krol P. Experimental selecytion of best physical and application parameters of ultrasound in the treatment of venous crural ulceration. Pol Merk Lek 2002;12:500-5.
- RCN Institute, Centre for Evidence Based Nursing, University of York and School of Nursing Midwifery and Health Visiting, University of Manchester . Clinical Practice Guidelines. The Management of Patients With Venous Leg Ulcers. Recommendations for Assessment, Compression Therapy, Cleansing, Debridement, Dressing, Contact Sensitivity, Training Education and Quality Assurance 1998.
- Margolis DJ, Berlin JA, Strom BL. Risk factors associated with the failure of a venous leg ulcer to heal. Arch Dermatol 1999;135:920-6.
- ter Riet G. Problems in the conduct of a randomised controlled trial. J Wound Care 1998;7:259-62.
- Pye S. Ultrasound therapy equipment: does it perform?. Physiotherapy 1996;78:263-7.
- Gardner SE, Frantz RA, Troia C, Eastman S, MacDonald M, Buresh K, et al. A tool to assess clinical signs and symptoms of localized infection in chronic wounds: development and reliability. Ostomy Wound Manage 2001;47:40-7.
- Kind P, Spilker B. The EuroQoL instrument: an index of health-related quality of life. Philadelphia: Lippincott-Raven; 1996.
- Lin DY, Feuer EJ, Etzioni R, Wax Y. Estimating medical costs from incomplete follow-up data. Biometrics 1997;53:419-34.
- Vowden K, Goulding V, Vowden P. Hand-held Doppler assessment for peripheral arterial disease. J Wound Care 1996;5:125-7.
- Simon D, Freak L, Williams I, McCollum C. Progression of arterial disease in patients with healed venous ulcers. J Wound Care 1994;3:179-80.
- Kantor J, Margolis DJ. A multicentre study of percentage change in venous leg ulcer area as a prognostic index of healing at 24 weeks. Br J Dermatol 2000;142:960-4.
- Etris MB, Pribble J, LaBrecque J. Evaluation of two wound measurement methods in a multi-center, controlled study. Ostomy Wound Manage 1994;40:44-8.
- Ware JE, Kosinski N, Keller SD. The 12-Item Short-Form Health Survey (SF-12): construction of scales and preliminary tests of reliability and validity. Medical Care 1996;34:220-33.
- Jenkinson C, Layte R, Jenkinson D, Lawrence K, Peterson S, Paice C, et al. A shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies?. J Public Health Med 1997;19:179-96.
- Iglesias CP, Birks YF, Torgerson DJ. Improving the measurement of quality of life in older people: the York SF-12. QJM Monthly Journal of the Association of Physicians 2001;94:695-8.
- Walker NK. Epidemiological Studies of Leg Ulcers in Auckland, New Zealand 2000.
- Kind P, Hardman G, Macran S. UK population norms for EQ-5D. Centre for Health Economics discussion paper 172. York: Centre for Health Economics, University of York; 1999.
- European Committee for Standardisation (CEN) . Clinical Investigation of Medical Devices for Human Subjects EN 540 1993.
- Edwards P, Roberts I, Clarke M, DiGuiseppi C, Pratap S, Wentz R. Increasing response rates to postal questionnaires: systematic review. BMJ 2002;324.
- Medical Research Council . Guidelines for Good Clinical Practice in Clinical Trials 1998.
Appendix 2 Regulatory approvals
The MREC approval was obtained for the study from the York Research Ethics Committee on 4 February 2005. The LRECs were also approached in each recruitment area prior to recruitment as were the relevant Research and Development departments. Approval was given at the following meetings.
| Site | PCT/trust | LREC | Approved | Research and development approval |
|---|---|---|---|---|
| Western Trust | Altnagelvin Health and Social Services Trust | Health and Social Care REC 1 | 26 April 2005 | 5 December 2005 |
| Bolton | Bolton PCT/Bolton Hospitals NHS Trust | Bolton REC | 19 August 2005 | 1 November 2005 |
| Bradford | Bradford Teaching Hospitals NHS Trust | Bradford REC | 11 February 2005 | 29 June 2005 |
| Cumbria | West Cumberland Hospital | Cumbria and Lancashire A | 26 April 2005 | 4 May 2005 |
| Dunfermline | NHS Fife | Fife and Forth Valley REC | 14 March 2006 | 4 May 2006 |
| Hull | Hull and East Yorkshire Hospitals NHS Trust | Hull and East Riding REC | 14 March 2005 | 15 May 2006 |
| South Essex | Castle Point and Rochford PCT; Southend on Sea PCT | Essex 2 REC | 4 May 2006 | 7 July 2006 |
| Leeds Community | Leeds PCTs Research Consortium | Leeds (East) REC | 27 April 2005 | 6 October 2005 |
| Scarborough | Scarborough , Whitby and Ryedale PCT | York REC | 26 July 2005 | 20 August 2005 |
| Nottingham | Nottinghamshire County Teaching PCT; Nottingham City PCT; Gedling PCT; Rushcliffe PCT; Broxtowe and Hucknall PCT | Nottingham REC 1 | 17 May 2006 | 10 July 2006 |
| Birmingham | University Hospital Birmingham NHS Foundation Trust | South Birmingham REC | 19 September 2006 | 17 October 2006 |
| Dublin | The Aldelaide and Meath Hospital, Dublin | St. James’s Hospital/Adelaide and Meath Hospital incorporating the National Children’s Hospital | 30 November 2006 | Not required at time |
Appendix 3 Details of the study sites
| Site | Site organisation and recruitment dates |
|---|---|
| Bolton | Outpatient leg ulcer clinics (recruited first patient March 2006; last patient September 2008) |
| Scarborough | Leg ulcer clinics/community tissue viability service/district nurse teams (recruited first patient March 2006; last patient December 2008) |
| Cumbria | Outpatient leg ulcer clinics (recruited first patient May 2006; last patient November 2007) |
| Western Trust | Outpatient leg ulcer clinic/community service (recruited first patient March 2006; last patient October 2008) |
| Leeds Community | Community tissue viability service (recruited first patient April 2006; last patient December 2008) |
| Hull | Outpatient leg ulcer clinics (recruited first patient February 2007; last patient December 2008) |
| Dunfermline | Outpatient leg ulcer clinics (recruited first patient February 2007; last patient July 2008) |
| Nottingham | Outpatient leg ulcer clinics/community service (recruited first patient October 2006; last patient December 2008) |
| South Essex | Leg ulcer clinics/leg ulcer clubs (recruited first patient October 2006; last patient November 2006) |
| Birmingham | Outpatient leg ulcer clinics (recruited first patient January 2007; last patient September 2008) |
| Bradford | Outpatient leg ulcer clinics (recruited first patient November 2006; last patient December 2008) |
| Dublin | Outpatient wound clinics (recruited first patient January 2007; last patient February 2007) |
Appendix 4 Information sheet for patients
Patient Information Sheet Local headed paper
Please read this document carefully.
We would like to invite you to take part in this study of ultrasound therapy used in the treatment of leg ulcers. Ultrasound therapy is the application of inaudible sound energy to help with healing.
Leg ulcers are common, costly to the NHS and can be very distressing for patients. There are a range of treatments currently being used to do this, for example, wound dressings and bandages. The use of compression bandages is very effective however, this kind of treatment can work quite slowly, and it is thought that ultrasound therapy may speed up the rate of healing.
Ultrasound therapy involves the use of sound waves to the skin around the ulcer. A watery jelly is applied to the skin so that the sound waves can travel into the skin at the edge of the ulcer.
The purpose of this study is to find out if adding ultrasound therapy to the best possible care (firm compression bandages applied by your nurse) helps in healing leg ulcers. We are interested in how quickly the ulcers heal, and also in your opinion about the treatment you receive. In order to compare the treatments we need to treat approximately 168 patients with the ultrasound therapy and bandages, and 168 patients with bandages. If you agree to take part in this study you will be allocated to one of these two treatments. The decision regarding which treatment you will receive will be made after you agree to take part. The choice of treatment will be determined at random, that is we cannot predict which treatment you will receive. You will have an equal chance of receiving each treatment, in the same way that tossing a coin gives an equal chance of getting ‘heads’ or ‘tails’.
Your leg ulcer dressings will be carried out, as normal, by your community nurse or clinic staff. The ulcer will be traced and photographed at the start of the study and then regularly to see if the ulcer is reducing in size. The study will last for 12 months, but you would receive ultrasound for a maximum of 12 weeks; if your ulcer heals in the first 12 weeks of treatment, you will only receive ultrasound therapy up to the date of healing. During the trial you will be asked to complete five short questionnaires. We envisage that each of these questionnaires will take less than 15 minutes to complete. We will continue to send you questionnaires about your leg and how it affects you, even if your ulcer has completely healed. We do not anticipate that you will have to see the nurse or attend your clinic more frequently than would normally be required and we will therefore not be able to pay any travel expenses incurred.
Why do the study?
Ultrasound therapy given once a week, for 5 to 10 minutes, in addition to bandages, may heal more ulcers than using bandages alone. It is therefore important to carry out this study so that patients with leg ulcers can be provided with the most appropriate and effective care. We are also interested in how you feel having the leg ulcer treatment so that nurses, doctors and patients can make future decisions about which treatments are comfortable. Without this information patients may receive inefficient care.
Can I change my mind later?
Participation in this study is entirely voluntary. You can change your mind at any time. Your future care and treatment will not be influenced by your decision to take part or not. If you do agree to take part in this study and decide at a later time to withdraw then you are free to do so at any time without influencing your future care or treatment.
Are there any side-effects from the ultrasound therapy?
There may be some tingling pain around the ulcer edge from the ultrasound therapy, or redness. A small proportion of people – fewer than 1 in 10 – have reported pinhead bleeding in the skin around the ulcer. We do not anticipate that you will be harmed by being on this trial. Should this occur, however, normal NHS negligence procedures will apply. If you have any problems you should contact your community nurse or clinic nurse. The name of a contact nurse and telephone number where they can be reached is provided below.
What do I do now?
If you are interested in taking part please tell your nurse. They will give you a consent form to sign and a questionnaire to be completed.
Where can I get more information about the study?
If you do not understand anything on this information sheet or would like further information please contact your local research nurse on the telephone number below.
Local Nurse: Telephone number:
Local Investigator: Telephone number:
Research Co-ordinator: Telephone number:
Address:
Thank you for taking the time to read this information sheet
Appendix 5 Data collection forms
Appendix 6 Ultrasound protocol
Appendix 7 Site training
| Site | Date of training session (number trained) | Date of training session (number trained) | Date of training session (number trained) |
|---|---|---|---|
| Bolton | 23 November 2005 (10) | ||
| Scarborough | 9 January 2006 (9) | ||
| Cumbria | 7 February 2006 (2) | ||
| WHSCT | 20 February 2006 (2) | ||
| Leeds | 17 March 2006 (24) | 22 September 2006 (1) | |
| Hull | 11 May 2006 (3) | 19 May 2006 (2) | 23 October 2007 (1) |
| Dunfermline | 9 June 2006 (3) | ||
| Nottingham | 31 July 2006 (3) | ||
| South Essex | 11 September 2006 (7) | ||
| Birmingham | 20 October 2006 (3) | 3 July 2007 (1) | 11 February 2008 (1) |
| Bradford | 6 November 2006 (2) | ||
| Dublin | 9 January 2007 (4) |
Appendix 8 Flow chart for VenUS III trial
Appendix 9 Digital image and tracing protocol
VenUS III ultrasound trial
Protocol for digital photographs and tracings of ulcers
At baseline (week 1 – first day of treatment equals day 0):
-
please take a digital photograph of the reference ulcer (largest) AND
-
tracings of all the ulcers the patient has.
Every 4 weeks from the first trial treatment:
-
please take a digital photograph of the reference ulcer AND
-
tracings of all the ulcers the patient has.
i.e. each time you take a photograph of the reference ulcer you should also take tracings of all the ulcers.
If the reference ulcer heals:
-
please take a digital photograph to confirm this on the day of healing AND
-
take another digital photograph 7 days later.
If there are still other unhealed ulcers on the patient’s leg:
-
please continue to take tracings of the ulcers every 4 weeks as usual and complete dressing log booklets at each visit until they become ulcer free OR one year has passed since they first started in the trial.
NOTE
Please remember to always include a colour target card in the photograph, with the date, patient’s trial number and ulcer ID (e.g. R1 or R2) written on it.
AND
Please write the same information on the acetate grid when you take the tracings.
Appendix 10 Ultrasound machine acceptance testing and 3-monthly servicing
| Machine ID number | Acceptance testing result (pass/fail) | Re-testing result (pass/fail) | 3-monthly servicing and result | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Pass | Pass | 24 August 2006 | 14 November 2006 | 6 March 2007 | 6 June 2007 | 23 August 2007 | 19 November 2007 | 20 February 2008 | 22 May 2008 | 22 August 2008 | 25 November 2008 | 18 February 2008 |
| 2 | Pass | Pass | 24 August 2006 | 14 November 2006 | 6 March 2007 | 6 June 2007 | 23 August 2007 | 19 November 2007 | 20 February 2008 | 22 May 2008 | 22 August 2008 | 25 November 2008 | 18 February 2008 |
| 3 | Pass | Pass | 29 August 2006 | 20 November 2006 | 23 April 2007 | 9 August 2007 | 4 December 2007 | 23 April 2008 | 29 July 2008 | ||||
| 4 | Fail | Pass | 17 January 2008 | 15 April 2008 | 5 September 2008 | ||||||||
| 5 | Pass | Pass | 29 August 2006 | 20 November 2006 | 23 April 2007 | 9 August 2007 | 4 December 2007 | 23 April 2008 | 29 July 2008 | ||||
| 6 | Pass | Pass | 10 August 2006 | 10 November 2006 | 15 February 2007 | 3 May 2007 | 2 August 2007 | 1 November 2007 | 29 January 2008 | 28 April 2008 | 17 July 2008 | 24 October 2008 | 21 January 2009 |
| 7 | Fail | Pass | 29 August 2006 | 20 November 2006 | 23 April 2007 | 9 August 2007 | 4 December 2007 | 23 April 2008 | 29 July 2008 | ||||
| 8 | Pass | Pass | 10 August 2006 | 10 November 2006 | 15 February 2007 | 3 May 2007 | 2 August 2007 | 1 November 2007 | 29 January 2008 | 28 April 2008 | 17 July 2008 | 24 October 2008 | 21 January 2009 |
| 9 | Pass | Pass | 31 October 2006 | 22 March 2007 | 9 August 2007 | 3 December 2007 | 23 April 2008 | ||||||
| 10 | Pass | Pass | 31 October 2006 | 22 March 2007 | 9 August 2007 | N/A | 23 April 2008 | 12 August 2008 | |||||
| 11 | Pass | Pass | 30 October 2006 | 23 January 2007 | 26 April 2007 | 23 August 2007 | 6 November 2007 | 10 January 2008 | 14 April 2008 | 31 July 2008 | |||
| 12 | Pass | Pass | 30 October 2006 | 23 January 2007 | 26 April 2007 | 23 August 2007 | 6 November 2007 | 10 January 2008 | 14 April 2008 | 29 July 2008 | 8 December 2008 | ||
| 13 | Fail | Pass | 15 April 2008 | 18 August 2008 | |||||||||
| 14 | Fail | Pass | 15 September 2008 | ||||||||||
| 15 | Fail | Pass | Not returned for servicing | ||||||||||
| 16 | Pass | Pass | 24 August 2006 | 14 November 2006 | 6 March 2007 | 6 June 2007 | 23 August 2007 | N/A | 20 February 2008 | 22 May 2008 | 22 August 2008 | 25 November 2008 | 18 February 2008 |
| 17 | Pass | Pass | 19 January 2007 | 12 September 2007 | 7 January 2008 | 11 April 2008 | 18 July 2008 | 15 October 2008 | 26 January 2009 | 29 May 2009 | |||
| 18 | Pass | Pass | 19 January 2007 | ||||||||||
| 19 | Fail | Pass | 4 January 2007 | 16 March 2006 | 25 June 2007 | 24 September 2007 | 17 December 2007 | 17 March 2008 | 9 June 2008 | ||||
| 20 | Pass | Pass | 4 January 2007 | 16 March 2006 | 25 June 2007 | 24 September 2007 | 17 December 2007 | 17 March 2008 | 9 June 2008 | ||||
| 21 | Pass | Pass | 23 July 2008 | Sent out for site use when main units being serviced | |||||||||
| 22 | Pass | Pass | Sent out for site use when main units being serviced | ||||||||||
| 23 | Fail | Fail | Not given out for site use | ||||||||||
| 24 | Pass | Pass | 18 February 2008 | 21 December 2008 | 26 February 2009 | ||||||||
| 25 | Pass | Pass | Not given out for site use – fault with transducer head | ||||||||||
| 26 | Pass | Pass | 18 February 2008 | 21 December 2008 | 26 February 2009 | ||||||||
| 27 | Pass | Pass | 22 January 2007 | 23 April 2007 | 6 November 2007 | 4 January 2008 | 14 April 2008 | 15 September 2008 | |||||
| 28 | Pass | Pass | 23 April 2007 | 6 November 2007 | 4 January 2008 | 28 January 2008 | 14 April 2008 | 15 September 2008 | |||||
| 29 | Fail | Pass | Not returned for servicing – site withdrew | ||||||||||
| 30 | Pass | Pass | Not returned for servicing – site withdrew | ||||||||||
| 31 | Pass | Pass | 3 December 2007 | 23 July 2008 | 14 January 2009 | ||||||||
| 32 | Pass | Pass | 7 August 2008 | 8 December 2008 | |||||||||
| 33 | Pass | Pass | 6 November 2007 | 10 January 2008 | 14 April 2008 | 13 August 2008 | 14 January 2009 | ||||||
| 34 | Pass | Pass | 7 January 2008 | 11 April 2008 | 18 July 2008 | 15 December 2008 | 26 January 2009 | 29 May 2009 | |||||
| 35 | Pass | Pass | 19 November 2008 | ||||||||||
| 36 | Fail | Pass | Not given out for site use | ||||||||||
| 37 | Fail | Pass | Not given out for site use | ||||||||||
| 38 | Pass | Pass | Not given out for site use | ||||||||||
| 39 | Pass | Pass | Not given out for site use | ||||||||||
| 40 | Fail | Pass | Not given out for site use | ||||||||||
List of abbreviations
- ABPI
- ankle–brachial pressure index
- AER
- effective radiating area
- BNF
- British National Formulary
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- df
- degrees of freedom
- EQ-5D
- European Quality of Life-5 Dimensions
- GP
- general practitioner
- HbA1c
- glycated haemoglobin
- HRQoL
- health-related quality of life
- ICER
- incremental cost-effectiveness ratio
- IPW
- inverse probability weight
- ISRCTN
- International Standard Randomised Controlled Trial Number
- LREC
- Local Research Ethics Committee
- MCS
- mental component score
- MREC
- Multicentre Research Ethics Committee
- NICE
- National Institute for Health and Clinical Excellence
- NPL
- National Physical Laboratory
- NSAE
- non-serious adverse event
- PCS
- physical component score
- PH
- proportional hazard
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SD
- standard deviation
- SF-12
- Short Form questionnaire-12 items
- VAS
- visual analogue scale
- VenUS
- Venous Ulcer Study
- WHSCT
- Western Health and Social Care Trust
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Professor of General Practice, Department of Primary Health Care, University of Oxford Programme Director,
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, University of Liverpool
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Deborah Ashby, Professor of Medical Statistics and Clinical Trials, Queen Mary, Department of Epidemiology and Public Health, Imperial College London
-
Professor Peter Brocklehurst, Director, National Perinatal Epidemiology Unit, University of Oxford
-
Professor John Cairns, Professor of Health Economics, London School of Hygiene and Tropical Medicine
-
Professor Peter Croft, Director of Primary Care Sciences Research Centre, Keele University
-
Professor Jenny Donovan, Professor of Social Medicine, University of Bristol
-
Professor Jonathan Green, Professor and Acting Head of Department, Child and Adolescent Psychiatry, University of Manchester Medical School
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Freddie Hamdy, Professor of Urology, Head of Nuffield Department of Surgery, University of Oxford
-
Professor Allan House, Professor of Liaison Psychiatry, University of Leeds
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norris, Chair in Clinical Trials and Biostatistics, Robertson Centre for Biostatistics, University of Glasgow
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor James Raftery, Chair of NETSCC and Director of the Wessex Institute, University of Southampton
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Martin Underwood, Warwick Medical School, University of Warwick
-
Professor Marion Walker, Professor in Stroke Rehabilitation, Associate Director UK Stroke Research Network, University of Nottingham
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norris Chair in Clinical Trials and Biostatistics, Robertson Centre for Biostatistics, University of Glasgow
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor James Raftery, Chair of NETSCC and Director of the Wessex Institute, University of Southampton
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Martin Underwood, Warwick Medical School, University of Warwick
-
Professor Marion Walker, Professor in Stroke Rehabilitation, Associate Director UK Stroke Research Network, University of Nottingham
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Public contributor
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Public contributor
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Public contributor
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Catherine Moody, Programme Manager, Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Public contributor
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire Country Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Professor Carol Tannahill, Glasgow Centre for Population Health
-
Mrs Jean Thurston, Public contributor
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Public contributor
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Public contributor
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Public contributor
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Sarah Tyson, Senior Research Fellow & Associate Head of School, University of Salford
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Public contributor
-
Mr David P Britt, Public contributor
-
Mr Sankaran ChandraSekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Public contributor
-
Mr Seamus Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor David Taggart, Consultant Cardiothoracic Surgeon, John Radcliffe Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Professor Nicholas James, Professor of Clinical Oncology, School of Cancer Sciences, University of Birmingham
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/ Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Mr John Chapman, Public contributor
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Ms Kylie Gyertson, Oncology and Haematology Clinical Trials Manager, Guy’s and St Thomas’ NHS Foundation Trust London
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Public contributor
-
Dr Martin Shelly, General Practitioner, Silver Lane Surgery, Leeds
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Public contributor
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Public contributor
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Public contributor
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Expert Advisory Network
-
Professor Douglas Altman, Professor of Statistics in Medicine, Centre for Statistics in Medicine, University of Oxford
-
Professor John Bond, Professor of Social Gerontology & Health Services Research, University of Newcastle upon Tyne
-
Professor Andrew Bradbury, Professor of Vascular Surgery, Solihull Hospital, Birmingham
-
Mr Shaun Brogan, Chief Executive, Ridgeway Primary Care Group, Aylesbury
-
Mrs Stella Burnside OBE, Chief Executive, Regulation and Improvement Authority, Belfast
-
Ms Tracy Bury, Project Manager, World Confederation of Physical Therapy, London
-
Professor Iain T Cameron, Professor of Obstetrics and Gynaecology and Head of the School of Medicine, University of Southampton
-
Professor Bruce Campbell, Consultant Vascular & General Surgeon, Royal Devon & Exeter Hospital, Wonford
-
Dr Christine Clark, Medical Writer and Consultant Pharmacist, Rossendale
-
Professor Collette Clifford, Professor of Nursing and Head of Research, The Medical School, University of Birmingham
-
Professor Barry Cookson, Director, Laboratory of Hospital Infection, Public Health Laboratory Service, London
-
Dr Carl Counsell, Clinical Senior Lecturer in Neurology, University of Aberdeen
-
Professor Howard Cuckle, Professor of Reproductive Epidemiology, Department of Paediatrics, Obstetrics & Gynaecology, University of Leeds
-
Professor Carol Dezateux, Professor of Paediatric Epidemiology, Institute of Child Health, London
-
Mr John Dunning, Consultant Cardiothoracic Surgeon, Papworth Hospital NHS Trust, Cambridge
-
Mr Jonothan Earnshaw, Consultant Vascular Surgeon, Gloucestershire Royal Hospital, Gloucester
-
Professor Martin Eccles, Professor of Clinical Effectiveness, Centre for Health Services Research, University of Newcastle upon Tyne
-
Professor Pam Enderby, Dean of Faculty of Medicine, Institute of General Practice and Primary Care, University of Sheffield
-
Professor Gene Feder, Professor of Primary Care Research & Development, Centre for Health Sciences, Barts and The London School of Medicine and Dentistry
-
Mr Leonard R Fenwick, Chief Executive, Freeman Hospital, Newcastle upon Tyne
-
Mrs Gillian Fletcher, Antenatal Teacher and Tutor and President, National Childbirth Trust, Henfield
-
Professor Jayne Franklyn, Professor of Medicine, University of Birmingham
-
Mr Tam Fry, Honorary Chairman, Child Growth Foundation, London
-
Professor Fiona Gilbert, Consultant Radiologist and NCRN Member, University of Aberdeen
-
Professor Paul Gregg, Professor of Orthopaedic Surgical Science, South Tees Hospital NHS Trust
-
Bec Hanley, Co-director, TwoCan Associates, West Sussex
-
Dr Maryann L Hardy, Senior Lecturer, University of Bradford
-
Mrs Sharon Hart, Healthcare Management Consultant, Reading
-
Professor Robert E Hawkins, CRC Professor and Director of Medical Oncology, Christie CRC Research Centre, Christie Hospital NHS Trust, Manchester
-
Professor Richard Hobbs, Head of Department of Primary Care & General Practice, University of Birmingham
-
Professor Alan Horwich, Dean and Section Chairman, The Institute of Cancer Research, London
-
Professor Allen Hutchinson, Director of Public Health and Deputy Dean of ScHARR, University of Sheffield
-
Professor Peter Jones, Professor of Psychiatry, University of Cambridge, Cambridge
-
Professor Stan Kaye, Cancer Research UK Professor of Medical Oncology, Royal Marsden Hospital and Institute of Cancer Research, Surrey
-
Dr Duncan Keeley, General Practitioner (Dr Burch & Ptnrs), The Health Centre, Thame
-
Dr Donna Lamping, Research Degrees Programme Director and Reader in Psychology, Health Services Research Unit, London School of Hygiene and Tropical Medicine, London
-
Professor James Lindesay, Professor of Psychiatry for the Elderly, University of Leicester
-
Professor Julian Little, Professor of Human Genome Epidemiology, University of Ottawa
-
Professor Alistaire McGuire, Professor of Health Economics, London School of Economics
-
Professor Neill McIntosh, Edward Clark Professor of Child Life and Health, University of Edinburgh
-
Professor Rajan Madhok, Consultant in Public Health, South Manchester Primary Care Trust
-
Professor Sir Alexander Markham, Director, Molecular Medicine Unit, St James’s University Hospital, Leeds
-
Dr Peter Moore, Freelance Science Writer, Ashtead
-
Dr Andrew Mortimore, Public Health Director, Southampton City Primary Care Trust
-
Dr Sue Moss, Associate Director, Cancer Screening Evaluation Unit, Institute of Cancer Research, Sutton
-
Professor Miranda Mugford, Professor of Health Economics and Group Co-ordinator, University of East Anglia
-
Professor Jim Neilson, Head of School of Reproductive & Developmental Medicine and Professor of Obstetrics and Gynaecology, University of Liverpool
-
Mrs Julietta Patnick, Director, NHS Cancer Screening Programmes, Sheffield
-
Professor Robert Peveler, Professor of Liaison Psychiatry, Royal South Hants Hospital, Southampton
-
Professor Chris Price, Director of Clinical Research, Bayer Diagnostics Europe, Stoke Poges
-
Professor William Rosenberg, Professor of Hepatology and Consultant Physician, University of Southampton
-
Professor Peter Sandercock, Professor of Medical Neurology, Department of Clinical Neurosciences, University of Edinburgh
-
Dr Philip Shackley, Senior Lecturer in Health Economics, Sheffield Vascular Institute, University of Sheffield
-
Dr Eamonn Sheridan, Consultant in Clinical Genetics, St James’s University Hospital, Leeds
-
Dr Margaret Somerville, Director of Public Health Learning, Peninsula Medical School, University of Plymouth
-
Professor Sarah Stewart-Brown, Professor of Public Health, Division of Health in the Community, University of Warwick, Coventry
-
Dr Nick Summerton, GP Appraiser and Codirector, Research Network, Yorkshire Clinical Consultant, Primary Care and Public Health, University of Oxford
-
Professor Ala Szczepura, Professor of Health Service Research, Centre for Health Services Studies, University of Warwick, Coventry
-
Dr Ross Taylor, Senior Lecturer, University of Aberdeen
-
Dr Richard Tiner, Medical Director, Medical Department, Association of the British Pharmaceutical Industry
-
Mrs Joan Webster, Consumer Member, Southern Derbyshire Community Health Council
-
Professor Martin Whittle, Clinical Co-director, National Co-ordinating Centre for Women’s and Children’s Health, Lymington