Notes
Article history
The research reported in this issue of the journal was commissioned by the HTA programme as project number 09/23/01. The contractual start date was in August 2009. The draft report began editorial review in April 2010 and was accepted for publication in September 2010. As the funder, by devising a commissioning brief, the HTA programme specified the research question and study design. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2011. This work was produced by Carroll et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2011 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Description of health problem
Stickler syndrome, also known as hereditary progressive arthro-ophthalmopathy, is an inherited progressive disorder of the collagen connective tissues which was first described in 1965. 1–3 It is indicated by a variety of symptoms and can affect the formation of the eyes, ears, palate, jaw and joints. 1,2,4–9 Manifestations can include short-sightedness, cataracts, retinal problems leading to retinal detachment (RD) and possible blindness, hearing loss, facial abnormalities including cleft palate and joint problems. 1,2,8 Stickler syndrome is the most commonly identified, inherited cause of RD in childhood. 1 RD is a separation of the sensory retina from the retinal pigment epithelium, with an accumulation of vitreous fluid in the potential space between them.
There are no agreed diagnostic criteria for Stickler syndrome,1 but diagnosis can be confirmed by genetic analysis. Stickler syndrome is genetically heterogeneous with at least five subgroups, some with a high risk of ocular complications, others with no ocular involvement at all. The majority of patients have type 1 Stickler syndrome (MIM 108300), which is caused by mutation in the single gene which encodes type II collagen and has ocular, auditory, oro-facial and skeletal manifestations. 10,11 This gene is called COL2A1. Types 2 and 3 Stickler syndrome are caused by mutations in the genes encoding type XI collagen. 6,12,13 Unlike type II collagen there are three genes encoding type XI collagen and they are COL11A1, COL11A2 and COL2A1. Type 2 Stickler syndrome (MIM 604841) is due to mutations in the COL11A1 gene and has ocular, auditory, oro-facial and skeletal manifestations. 6,12–14 The COL11A2 gene (mutations of which are responsible for type 3 Stickler syndrome – MIM 104840) is not expressed in the eye and therefore this group of patients do not suffer eye problems and are more properly referred to as suffering from otospondylomegaepiphyseal dysplasia. 14 Given that these patients have no ocular involvement, they are not considered further in this review. Both type 1 and type 2 Stickler syndrome are autosomal dominant disorders, but recently a fourth recessive variety of Stickler syndrome has been identified due to mutations affecting both alleles of the gene encoding the a1 chain of type IX collagen (COL9A1) (MIM 120210). In other families, all known candidate genes have been excluded, so that there is at least a fifth genetic variation, and further heterogeneity remains to be resolved.
About 75% of people diagnosed with Stickler syndrome suffer from type 1. Types 1 and 2 both indicate ‘full’ Stickler syndrome. 11 ‘Full’ Stickler syndrome affects the eyes, joints and hearing; patients with type 1 have an increased incidence of cleft abnormalities and those with type 2 an increased incidence of deafness. 15 Type 2 may also have a reduced risk of RD. 2,5,6 There can be a great deal of variability in the number and type of systemic or non-ocular symptoms in Stickler syndrome patients. 2,8,16 A subgroup of individuals have been identified who have type 1 Stickler syndrome, confirmed by genetic analysis, but with no or very few systemic features. 17–20 In the absence of genetic testing, the diagnosis of Stickler syndrome can therefore be problematic. Diagnosis may also be delayed (e.g. until the first RD has occurred), especially in children, who may not report symptoms. 2,8,21,22 Clinical advice also suggests that a diagnosis of Stickler syndrome may not even be considered for adults experiencing an RD. Consequently, the number of individuals with Stickler syndrome may be higher than currently diagnosed or reported. No figures on prevalence are available for the UK, but it has been reported previously to be approximately one case in 10,000 people for types 1 and 2 in the USA. 8,23 However, given the difficulties with diagnosis, this figure may not be reliable: for these reasons prevalence is estimated to be higher by the UK Genetic Testing Network (www.ukgtn.org).
The rate of RD, potentially leading to loss of vision, in patients with Stickler syndrome has been found in adults to be as high as 57%,20 60%2 or 61%8 in one eye or 40% in both eyes. 2 RD is ‘a separation of the sensory retina from the retinal pigment epithelium, with an accumulation of fluid in the potential space between them’. 24 Whereas RD can occur at any age and the risk is life-long,2,25,26 the first RD has been found to occur most commonly in adolescence or early adulthood, between the ages of 10 and 30 years. 2,27 For example, the mean age of those presenting with a first RD (and therefore being diagnosed as having Stickler syndrome) has been reported by one study to be between 21 and 25 years. 27 However, clinical advice also suggests that a diagnosis of Stickler’s syndrome is not always considered for adults presenting with an RD, so the mean age of first RD may be higher still. Children may therefore be more likely to be diagnosed with Stickler syndrome but represent a different problem as they may be unlikely to report symptoms and so are diagnosed only after the first RD or other irreparable damage has occurred. Given the more likely diagnosis of Stickler syndrome in children, there is therefore a potential case for early prophylactic intervention in type 1 and type 2 Stickler syndrome patients, especially as the treatment of RD in this population is complex and difficult to manage: success rates for reattachment have been reported to be 78.57% (22/28 patients), but with an average time to redetachment of < 4 months in 73% of cases. 27 The risk of RD progressing to blindness, i.e. the loss of sight in both eyes, in Stickler syndrome is also uncertain as there are very little published data. A survey of members of Stickler syndrome support groups from the UK and the USA reported that 11% and 8% respectively were registered as legally blind (i.e. both eyes). 2 Sixteen per cent of the UK sample was also categorised as ‘partially sighted’, i.e. complete loss of sight in one eye and reduced vision in the fellow eye. However, this sample was composed of individuals diagnosed with various types of Stickler syndrome, and it is known that the risk of RD, and therefore blindness, is higher for those with type 1. The proportion of this published sample with type 1 is unknown. It is also unclear how many of this sample had suffered and been treated for an RD prior to blindness or how many who received treatment for RD were not classified as legally blind. The long-term success of RD surgery is therefore unknown for this population and the risk of subsequent blindness is uncertain.
Current service provision
Current service provision in the UK in terms of prophylaxis for RD in Stickler syndrome populations consists of no treatment, with or without monitoring; prophylaxis using 360° cryotherapy; or prophylaxis using laser treatment. In both cases the procedure forms a scar with the aim of increasing adhesion and reducing the likelihood of tears or holes leading to a detachment. There is currently a lack of certainty regarding best practice. There are no current guidelines on prophylactic interventions for this population either in the UK or elsewhere.
Description of technology under assessment
The technologies under assessment are primary prophylactic interventions to reduce the risk of RD in eyes that have not previously had a detachment, and, thus, to reduce the potential for loss of vision. The possible interventions include cryotherapy, laser photocoagulation and scleral buckling. Cryotherapy uses intense cold, applied via a freezing probe at the peripheral retina throughout 360°, to destroy choroidal and retinal tissue in order to form a chorioretinal scar. The scar increases adhesion between the neurosensory retina and the retinal pigment epithelium. 28 Different areas of the eye can be treated in this way: at the post-oral retina and at the equator. Laser photocoagulation involves applying multiple small laser burns to the peripheral retina throughout 360° to create a chorioretinal scar and thus increase retinal adhesion. As with cryotherapy, this treatment can be applied to different areas of the eye. 29 Scleral buckling involves the application of a 360° silicone band around the eyeball at the equator or over affected areas. However, these prophylactic interventions are not without the possibility of unwanted side effects or adverse events, such as discomfort, lid swelling or epiphora.
A possible relevant subgroup for primary prophylactic intervention may be children, because the risk of a first RD has been reported to be highest in Stickler syndrome populations between the ages of 10 and 30 years: the percentage of individuals with Stickler syndrome experiencing RD increases from 8% (aged 0–9 years) to 26% (aged 10–19 years) to 61% (aged 20–29 years), then it levels out (57%–65% for those aged ≥ 30 years). 2 Given that children are also arguably the most likely to be diagnosed with Stickler syndrome, albeit perhaps only after an RD has already occurred, it therefore makes sense to perform prophylaxis at an earlier rather than a later age. There are currently no data publicly available on the current levels of use of each or any of these technologies in the NHS.
Chapter 2 Definition of the decision problem
Decision problem
The assessment will address the question ‘Can prophylactic surgery reduce the risk of RD and blindness in Stickler syndrome, especially in children?’.
Overall aims and objectives of assessment
-
To evaluate the clinical effectiveness of prophylactic retinal interventions for the primary prevention of RD in children and adults with Stickler syndrome.
-
To evaluate the safety (numbers of types of adverse events or complications) of interventions for the primary prevention of RD.
-
To identify key areas for primary research.
It is not the aim of this assessment to evaluate the relative effectiveness of interventions using indirect comparison methods.
Chapter 3 Assessment of clinical effectiveness
Methods for reviewing effectiveness
A review of the evidence for clinical effectiveness has been undertaken systematically following the general principles recommended in the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement. 29 English- and non-English-language studies were included (where translation is available) and there was no limit by date.
Identification of studies
A comprehensive search was undertaken in October 2009 to identify, systematically, both clinical effectiveness and adverse events literature pertaining to prophylactic retinal interventions to prevent RD in populations reported specifically to comprise participants with Stickler syndrome or populations that may include participants with Stickler syndrome. This search was performed by an information specialist (AR). Searches were not restricted by language or publication date. The MEDLINE search strategy is reported in Appendix 1.
The following electronic databases and online conference proceedings were searched from inception for published and unpublished research evidence:
-
MEDLINE (Ovid) 1950–October 2009
-
MEDLINE in process (Ovid) October 2009
-
EMBASE 1980–October 2009
-
Cumulative Index to Nursing and Allied Health Literature (via EBSCO) 1982–October 2009
-
The Cochrane Library including the following databases 1991–October 2009: Cochrane Systematic Reviews Database, Cochrane Controlled Trials Register, Database of Abstracts of Reviews of Effects (DARE), Health Technology Assessment (HTA) and NHS Economic Evaluation Database (NHS EED)
-
Biological Abstracts [via Thomson Reuters (formerly ISI) Web of Science®] 1969–October 2009
-
Science Citation Index (via ISI Web of Science) 1900–October 2009
-
UK Clinical Trials Research Network (UKCRN) and the National Research Register archive up to October 2009
-
Current Controlled Trials up to October 2009
-
Clinical Trials.gov up to October 2009
-
Annual Meeting of the Association for Research in Vision and Ophthalmology up to 2009.
All citations were imported into reference manager, version 12, software (Thomson Reuters, New York, NY, USA) and duplicates were deleted (AR). Titles and abstracts of all unique citations were then double-screened by two reviewers (CC and DP) using the inclusion criteria outlined below. Any disagreements concerning possible inclusion were resolved by discussion between the reviewers or with reference to the full paper itself. The full papers of all potentially relevant citations were retrieved so that an in-depth assessment concerning inclusion could be made. Again, both reviewers independently screened full papers for relevance and any disagreements concerning possible inclusion were resolved by discussion. In the event that published papers did not report potentially relevant data, corresponding authors were contacted by letter. If relevant data were made available by this route, they were included in the analysis.
Inclusion and exclusion criteria
Population
Children (up to the age of 18 years) and adults diagnosed with type 1 or type 2 Stickler syndrome or ‘Wagner–Stickler’ syndrome with non-ocular features. There are no universally agreed diagnostic criteria for Stickler syndrome, but it is expected that study participants would demonstrate the presence of a typical vitreous phenotype (type 1 or 2) and/or COL2A1/COL11A1 mutation. Criteria of diagnosis were recorded. The protocol originally stated that individuals with Wagner–Stickler syndrome were to be excluded (see Appendix 6). It is recognised that Wagner and Stickler syndromes are quite distinct genetically, and in terms of systemic features. 10,17,19,30,31 For example, Wagner syndrome is accepted to have only ocular abnormalities and no other systemic features. 10,17,19,30 However, Stickler syndrome has a highly variable degree of systemic features (a subgroup has been identified with no or very few systemic features). 17–20 The differences between the two syndromes have become clinically apparent only in recent years, so, despite the previously ‘confusing’ nomenclature of ‘Wagner–Stickler’ syndrome,17 studies of this population have also been included in this review if their study samples exhibit non-ocular symptoms (i.e. consistent with Stickler syndrome). This is because there is little published research evaluating primary prophylaxis in populations specifically diagnosed with Stickler syndrome, and study samples diagnosed with Wagner–Stickler syndrome may be composed of individuals diagnosed with Stickler syndrome, in part at least. Clinical advice was divided on the relevance of including these studies, but the majority opinion was that they offered some interesting supporting but not pivotal information, as long as the issues regarding the reported diagnosis of these populations in these studies were highlighted. Any studies of Wagner–Stickler patients with non-ocular symptoms have therefore not been presented as pivotal evidence but are alluded to as supporting evidence only. Children form a possible relevant subgroup, as the risk of RD, although life-long, has been reported to be highest between the ages of 10 and 30 years in Stickler syndrome populations. Individuals with conditions or syndromes other than Stickler syndrome or Wagner–Stickler syndrome with non-ocular features, but who have a predisposition to RD, e.g. retinopathy of prematurity or Marfan syndrome, were excluded.
Interventions
Any intervention aimed at the primary prevention of RD. Interventions must involve surgical procedures or settings, such as the use of a sterile environment or anaesthesia.
Comparators
No prophylactic treatment (there is no defined usual care for this population).
Settings
Secondary care.
Outcomes
Primary outcome
Retinal detachment in the eye(s) exposed to prophylactic intervention.
Secondary outcomes
-
Adverse events relating to the intervention.
-
Blindness (by self-assessment, or being registered or legally blind).
-
Time to RD.
-
Presence and type of lesions or retinal tears (as these may constitute a precursor for RD).
Study design
Any study design with a control or comparator group.
Data extraction strategy
Data were extracted independently from all included studies by two reviewers (CC and DP) using a data extraction form developed for this review and piloted on two studies (see Appendix 2). Any discrepancies between extractions were resolved by discussion and referral to the full paper.
Quality assessment strategy
Assessment of study quality was undertaken using an appropriate study design checklist, in this case the Critical Appraisal Skills Programme checklist for cohort studies. 32 A copy of the full checklist is included in Appendix 3. The critical appraisal of study quality was again conducted for each study independently by two reviewers (CC and DP) and any discrepancies resolved by discussion. The aim of the quality assessment process was to address issues regarding the appropriate recruitment of the sample, controlling for possible confounders (including comparability of groups), the length of follow-up, and the preciseness and external validity of the results. Studies were not excluded on the basis of their assessed quality. The purpose of this appraisal was to assess both the internal validity of the included studies and the potential risk of bias across studies included in the review.
Methods of analysis/synthesis
Data were tabulated and, given the small number of studies identified (two pivotal studies33,34 and two supporting studies36–38) and the heterogeneity of the evaluated interventions, a narrative synthesis rather than a meta-analysis was performed. The relative risk or risk ratio (RR) measure of relative effect was not reported in any of the published papers and also has not been reported in the main body of this report. This is because of the high risk of bias found in both studies33,34 (see Quality assessment below), especially concerning the comparability of treatment and control groups, which would adversely affect the reliability and validity of any such estimates of effect. 35 The between-group differences reported in the published papers are therefore the only statistical results reported here.
Results
Quantity and quality of research available
The search of electronic databases identified 1444 unique citations. One hundred and twenty-two full papers were retrieved after double-screening to determine whether they were relevant to this review. After double-screening of the full papers, only two studies explicitly satisfied all of the inclusion criteria: Ang et al. 33 and Leiba et al. 34 A further two studies (three papers)36–38 were identified as being of potential relevance as supporting studies because the study population had Wagner–Stickler syndrome. The diagnostic criteria described in these two studies included non-ocular features, and so were possibly consistent with a diagnosis of Stickler rather than Wagner syndrome. No additional relevant papers were identified from either reference tracking (two potential papers were unattainable, but appeared to concern Wagner syndrome patients only)39,40 or contact with expert advisors.
Seventy full papers were double-screened and excluded because they clearly failed to satisfy one or more of the criteria relating to the population, intervention or outcomes (these studies are listed in Appendix 4). The full papers of three citations were not available for screening. 41–43 A total of 44 further papers were excluded because they evaluated primary prophylactic surgical interventions for RD but did not provide sufficient details to be certain that there were no Stickler syndrome patients within the study population. These studies are listed in Appendix 5. Eight of these studies stated that a family history of RD was either an indication for prophylactic intervention or a characteristic of the study population. 44–51 Details of the screening and inclusion process are provided in the PRISMA flow diagram (Figure 1).
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram.
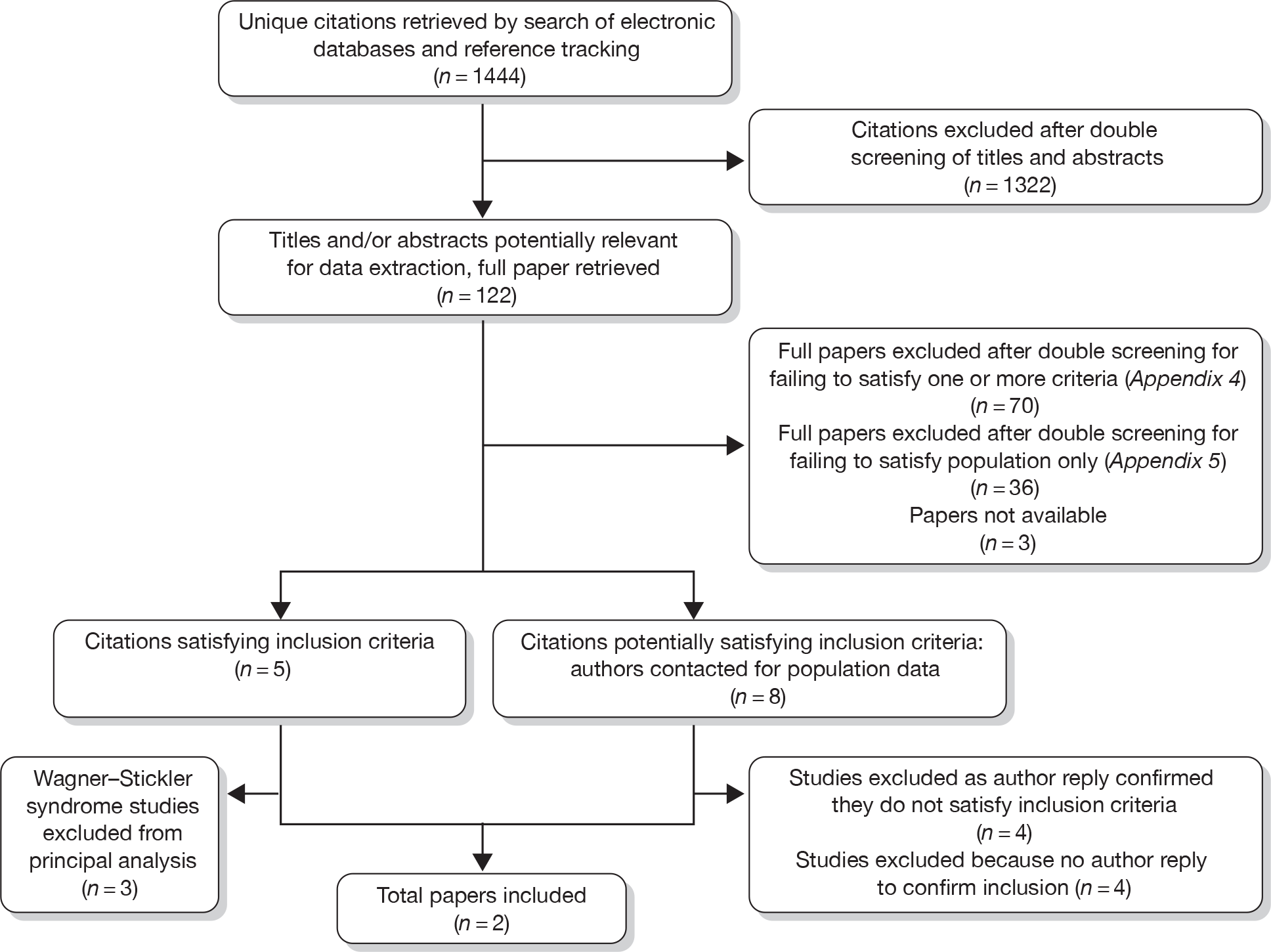
The reviewers therefore contacted the authors of these eight papers44–51 to ascertain whether there were any Stickler syndrome patients in their study sample (November 2009) and the results of any intervention for this subgroup. However, at the time of this report, only four authors had communicated with the review team, and all reported either that there was no known Stickler syndrome patients in their samples45–47 or that the data were no longer available to determine whether or not Stickler patients had been included. 48 The total number of studies therefore included in the principal analysis was two controlled cohort studies of prophylactic surgical interventions in type 1 Stickler syndrome populations. Details of two studies (three papers)36–38 of cohorts with comparator groups evaluating prophylactic surgical interventions in ‘Wagner–Stickler’ syndrome populations are also summarised as supporting evidence.
Summary of studies
Two studies were identified that assessed primary prophylactic surgical interventions in populations diagnosed with type 1 Stickler syndrome (Table 1). 33,34 The diagnostic criteria applied in both studies were consistent with Stickler syndrome. In both studies, the diagnosis was confirmed ‘where possible’ with genetic analysis, but this does not appear to have been applied to all participants.
| Study | Study design | Population, age and gender | Diagnostic criteria | Inclusion criteria | Intervention (n = patients) | Control (n = patients) | Follow-up |
|---|---|---|---|---|---|---|---|
|
Ang et al. 200833 UK |
Retrospective cohort study with controls |
Type 1 Stickler syndrome patients with GRTs and RD in one eye or no eye (n = 204) Age range 2–92 years Gender: 109 male; 95 female |
Mutation analysis, where possible, with gene COL2A1, plus congenital vitreous anomaly and any three of myopia with onset before age 6 years; RRD or paravascular pigmented LD; joint hypermobility with an abnormal Beighton score with or without radiological evidence of joint degeneration; audiometric confirmation of hearing defect; midline clefting | Diagnostic criteria or individuals with type 1 previously seen or still under active management of Addenbrooke’s Hospital, Cambridge, UK |
Bilateral and unilateral surgical prophylaxis ‘Standard prophylaxis’: 360° cryotherapy on the post-oral retina Group 1: bilateral, i.e. both eyes (n = 62) Group 2: unilateral, fellow eye only (n = 31) |
Group 3: No prophylaxis or ‘non-standard prophylaxis’, which included ‘treating isolated areas of lattice more posteriorly or using laser retinopexy’ (n = 111) |
Group 1: range 1–27 years (mean 11.5 years) Group 2: range 1–33 years (mean 15.5 years) Group 3: ‘data on the timing of events were either unreliable or missing’ |
|
Leiba et al. 199634 Israel |
Not reported; appears to be retrospective cohort study with controls |
A family group of type 1 Stickler Syndrome patients with ocular abnormalities (n = 22) Age range: NR Gender: 11 male; 11 female |
High myopia, retinal degeneration, midface hypoplasia and retrognathia; definite history of family members. Diagnosis was confirmed by mutation analysis on gene COL2A1 |
Intervention group Diagnostic criteria and (1) ocular abnormalities: extensive peripheral retinal degeneration, i.e. at least 5 continuous hours of LD with or without retinal breaks; or (2) isolated foci of LD with one or more of the risk factors for RD: family member with inherited vitreoretinal disease; previous RD in fellow eye; family history of RD; myopia Control group Diagnostic criteria only |
Bilateral and unilateral surgical prophylaxis (n = 6) Circumferential laser treatment for eyes with extensive contiguous retinal lesions where lesions were present in at least three quadrants of the retina Focal laser treatment for eyes with small localised lesions of LD or isolated breaks |
No prophylaxis (n = NR; reviewers calculate n = 16) | Range: 1–15 years |
In the Ang et al. study,33 the intervention was 360° cryotherapy on the post-oral retina to prevent progression to RD of the posterior flap of giant retinal tears (GRTs). The study by Leiba et al. 34 evaluated circumferential or focal laser treatment. The circumferential treatment consisted of confluent laser burns 360° around the peripheral retina, with four to eight laser burns applied circumferentially at the junction between the posterior border of the lesions and the unaffected retina. In the focal treatment, small localised lesions of lattice degeneration or isolated breaks were encircled by three to six rows of laser burns. The Ang et al. 33 study was conducted in the UK and the Leiba et al. 34 study in Israel. Both studies employed retrospective case review of data from a cohort exposed to the intervention and a cohort of controls. In both studies, bilateral and unilateral prophylaxis was performed. In the Leiba et al. 34 study, the control group does not appear to have received any specific form of prophylaxis. However, in the study by Ang et al. ,33 an unknown number of procedures of laser retinopexy or ‘treating isolated areas of lattice more posteriorly’ may have been performed on members of the control group. The length of follow-up for the intervention groups ranged from 1 to 33 years in the Ang et al. 33 study and from 1 to 15 years in the Leiba et al. 34 study. There was no reported length of follow-up for the controls in either study.
Quality assessment
Both the Ang et al. 33 and Leiba et al. 34 studies recruited relevant populations diagnosed with type 1 Stickler syndrome, although confirmatory genetic analysis appears to have been used only ‘where possible’ in the study by Ang et al. 33 Therefore, the diagnosis was made by clinical criteria only, and not confirmed by mutation analysis, for an unknown number of participants in the intervention and control groups in the Ang et al. 33 study. However, the clinical examination used in this study, i.e. to identify the relevant membranous vitreous phenotype, has been shown to have a high degree of sensitivity in predicting the results of genetic analysis. 30 Neither study justified the size of the sample (204 in Ang et al. 33 and 22 in Leiba et al. 34) or considered its implications in analysis, although the Ang et al. 33 study does evaluate the largest published sample of any study of prophylactic interventions in Stickler syndrome or other potentially relevant populations. The intervention and outcome (RD) appear to be measured accurately in both studies (although an unknown number of participants in the control group in the Ang et al. 33 study may have been exposed to some form of prophylaxis). It is unclear in both studies whether possible participants had been excluded.
The risk of RD is life-long,2 so the longer the follow-up, the better. The Ang et al. 33 study had a mean follow-up for both intervention groups of between 11 and 15 years, which is substantial. However, there is no reported follow-up for the control group. The follow-up of the intervention group in the Leiba et al. 34 study was as much as 15 years, but was also as little as 1 year, which may not be long enough to demonstrate effectiveness reliably. However, the follow-up for three patients in the intervention group (6 of the 10 eyes) was between 8 and 15 years, which is more reliable. The length of follow-up for the control group was not reported. Neither Ang et al. 33 nor Leiba et al. 34 reported the relative risk [or confidence intervals (CIs)] of experiencing the outcome when exposed to the intervention compared with the control. Both studies reported only whether there was a significant difference in rates of RD between the intervention and control groups. There was therefore no estimate of effect. Also, Leiba et al. 34 did not report the test used to determine a statistically significant difference between the two groups. The external validity of the Ang et al. 33 study was good in comparison with Leiba et al. :34 the population and setting were highly applicable to the decision problem, being type 1 Stickler syndrome patients, compared with Leiba et al. ’s34 consideration of a single family group of individuals with type 1 Stickler syndrome.
The results of both studies are subject to a high risk of bias. Both were retrospective cohort studies and so were limited by the bias inherent in that design. 52 The study reported by Ang et al. 33 had a number of strengths, including sample size, length of follow-up for the intervention groups and the reporting of data on the principal confounding factor of age. However, the control group presents a number of major problems. It does not represent a homogeneous group in terms of being exposed either to a single comparator intervention or to no intervention at all: an unknown number in the sample appear to have received some sort of prophylaxis that was not cryotherapy. The study correctly reports the potential confounding factor of age, but does not control for this in the results or analysis. The rate of RD in the control group is high in comparison with the intervention groups and is also higher than reported elsewhere for other Stickler syndrome populations not exposed to prophylaxis (but unconfirmed as type 1 only, and therefore potentially not at the highest risk of RD, unlike most if not all of the type 1 individuals in the Ang et al. 33 study): 73% per patient compared with 57%–61% per patient reported in surveys. 2,8,20 There is also a substantial difference between the intervention groups and the control group in terms of ‘follow-up’: the former has a maximum of 33 years with a mean of between 11 and 15 years, while there is no reported ‘follow-up’ at all for the latter, the controls. This further adversely affects the reliability of any comparison of event data between intervention and control groups. Also, the mean age of the controls was 49 years (range 5–92 years), the mean age of the bilateral prophylaxis group was 21 years (3–61 years) and the mean age of the unilateral group was 36 years (2–75 years). Given that age and, consequently, follow-up are both recognised to be important confounders, i.e. the likelihood of RD increases over time, with age, then the likelihood of the control group having experienced the outcome is inherently much higher than for the intervention groups. The relative effect of the intervention on the outcome of RD may therefore have been exaggerated when compared with the control group based on the event data reported in this study. It is also unclear whether the study was sufficiently powerful to generate a reliable effect size for the primary outcome. The risk of bias in this study was therefore high.
Leiba et al. 34 considered the potential confounding factors of age at first RD and the presence or absence of RD in the primary eye. Differences between intervention and control groups were not reported, although only those participants who were considered eligible for treatment actually received prophylaxis; the control group may therefore have had a different (possibly higher) level of risk of RD. The control group in the Leiba et al. 34 study is homogeneous as the subjects all appear to have received no form of prophylaxis at all. However, this study had more weaknesses than Ang et al. :33 the reported follow-up was shorter (a minimum of 1 year and a maximum of 15 years); the sample was much smaller and narrower (i.e. from a single pedigree); and the mean age of the intervention and control groups was not reported, although the data reported enable the comparison to be made that 9/10 individuals in the control group experienced an RD before the age of 30 years, and 5/6 patients exposed to prophylaxis received the treatment before 30 years of age. The risk of bias in this study was therefore also high.
Assessment of effectiveness
No estimates of effect were reported in the published papers or calculated by the authors of this report (owing to the high risk of bias in the two studies33,34). The papers themselves appear to test for and report only between-group differences (see Table 2). The Ang et al. 33 study reported a statistically significant difference between groups both for eyes [χ2 = 119.2, degrees of freedom (df) = 1, p < 0.001] and for patients (χ2 = 37, df = 1, p < 0.001), and the Leiba et al. 34 study reported a statistically significant difference between intervention and control groups for RD (p < 0.0025), but the test used was not reported and it is unclear whether this was for eyes or patients. Relative estimates of effect (relative risks), calculated by the authors of this report and based on the event data reported by these studies, are not reported in the main body of the report because their validity is affected by the high risk of bias within the included studies. However, these relative risks are reported in Appendix 8.
| Study | Intervention vs control, N (eyes) | RD post bilateral and unilateral prophylaxis, n/N (eyes) | RD post bilateral prophylaxis | RD post unilateral prophylaxis | Time to treatment failure | Blindnessa | Location of tears, lesions etc. likely to have caused RD. Other tears and lesions |
|---|---|---|---|---|---|---|---|
|
Ang et al. 200833 UK |
360° cryotherapy (N = 155) vs no prophylaxis (N = 222) |
7/155 vs 134/222 Difference between groups based on eyes: χ2 = 119.2, df = 1, p < 0.001 |
4/124 vs 134/222 No analysis reported |
3/31 vs 134/222 No analysis reported |
Group 1: range 2 months to 15 years (mean 7.7 years) Group 2: range 49 months to 15 years (mean 11.6 years) |
NR |
RDs in treated area Group 1: 3/4; group 2: 1/3 RDs posterior to treated area Group1: 1/4; group 2: 2/3 Group 1: three posterior holes requiring top-up retinopexy Other tears or lesions: NR |
|
Leiba et al. 199634 Israel |
Circumferential (N = 4) and focal (N = 6) laser treatment vs no prophylaxis (N = 34) |
1/10 vs 15/34b Difference between groups: p < 0.0025 (test not reported) |
1/8 vs 15 or 18/34 No analysis reported |
0/2 vs 15/34b No analysis reported |
5 years | 0/10 vs 16/34c |
One RD occurred ‘owing to a new lesion’ in an untreated area of the eye Three eyes required new focal laser treatment because they developed new lesions (location and type NR) |
Neither study reported details of any retinal tears or lesions which did not lead either to an RD or to further surgery. Only Leiba et al. 34 reported data on blindness due to RD: the intervention group had only one RD and no resulting blindness; 10 members of the control group experienced RD in one or both eyes (18 eyes), and 16 of these 18 eyes proceeded to blindness post RD surgery (time to failure not reported). Only two eyes had not re-detached by the time of the study (duration of follow-up not reported).
Subgroups: children
Only Leiba et al. 34 performed a subgroup analysis based on age. The study reported that 0/6 eyes treated prophylactically in children aged ≤ 13 years detached compared with 1/4 eyes treated prophylactically in children aged ≥ 13 years. The findings of this study may also indicate an increase in the likelihood of RD in adolescence and young adulthood. In the control group, who did not receive any prophylaxis, the retina detached in 6/13 (46%) eyes in children aged ≤ 13 years, but detached in 9/15 (60%) in adolescents and adults aged ≥ 13 years. However, this sample is very small.
Safety
None of the studies reported any serious or long-term adverse events or complications associated with cryotherapy, focal or circumferential laser treatment or scleral buckling. Only minor and temporary complications were reported by any of the studies. For cryotherapy, Ang et al. 33 reported transient epiphora, lid swelling and temporary accommodative paresis, but no cases of choroidal haemorrhage, macular pucker or unexplained loss of vision. However, the study did not report the number of patients experiencing any complications, so the proportion of patients experiencing these or any other complications, and the duration of any side effects, is unknown. Leiba et al. 34 reported that there were no ocular complications associated with the laser prophylaxis performed and visual acuity was unaffected.
Supporting studies
There are two studies (three papers), by Monin et al. 36,37 and Fritsch et al. ,38 reporting evaluations of primary prophylactic interventions in populations diagnosed as having ‘Wagner–Stickler’ syndrome (Tables 3 and 4). Both studies reported that all participants in their sample had ‘Wagner–Stickler’ syndrome, although the diagnostic criteria were not reported in the study by Monin et al. 36,37 However, in this study by Monin et al. , as well as being diagnosed with Wagner–Stickler syndrome, a number of participants had either a ‘family history’ of RD or ‘systemic abnormalities (cleft palate)’ in addition to ocular abnormalities stated as being consistent with Wagner or Stickler syndrome. 36 In the study by Fritsch et al. ,38 in addition to ocular abnormalities, all participants had non-ocular symptoms, which may be suggestive of Stickler rather than Wagner syndrome. However, neither chromosome nor genetic analysis was performed in either study to clarify diagnosis. It therefore cannot be stated categorically that the populations in these studies had Stickler syndrome. However, the reported, published diagnosis of Wagner–Stickler syndrome for these patients, and the greater consistency of symptoms with Stickler rather than Wagner syndrome, suggest that there are reasons to consider that these studies may provide possible relevant supporting evidence to this review. They have therefore been included, but are not considered as principal evidence.
| Study | Study design | Population, age and gender | Diagnostic criteria | Inclusion criteria | Intervention (n = patients) | Control | Follow-up |
|---|---|---|---|---|---|---|---|
|
Monin et al. 199436 and 199337 France |
Retrospective cohort study with controls |
Wagner–Stickler patients (n = 22) Age: NR 16 male, 6 female |
NR Some participants have a ‘family history’ of RD or ‘systemic abnormalities (cleft palate)’ |
RD in first eye and had not received any prophylaxis in the fellow eye |
Unilateral surgical prophylaxis Group 1: argon laser photocoagulation with a ‘barrage circulaire large’ or a ‘plaque’ posterior to the ‘equateur’ (n = 10) Group 2: Encircling scleral buckling (n = 8) (1993) |
‘Other treatments’ (n = 4) 1 = cryotherapy; 1 = focal laser photocoagulation; 1 = circular laser photocoagulation; 1 = vitrectomy |
Range: 3–67 months |
|
Fritsch et al. 198938 France |
Cohort study without controls |
Wagner–Stickler patients (n = 26) Age = NR Gender = NR |
|
Diagnostic criteria (1), (2) and (3) or (1) and (2) only; RD in one eye (n = 7) or no RD (n = 19) |
Bilateral and unilateral surgical prophylaxis Exact numbers for each intervention NR: Focal laser treatment or cryotherapy for patients without RD (n = 22; bilateral = 19; unilateral = 3); |
Unilateral prophylaxis: Scleral buckling (n = 2) Focal laser treatment plus scleral buckling (n = 2) |
Range: 2–8 years |
| Study | Intervention vs control, n = eyes | RD post bilateral and unilateral prophylaxis, n/N (eyes) | RD post bilateral prophylaxis | RD post unilateral prophylaxis | Time-to-treatment failure | Location of tears, lesions, etc. likely to have caused RD. Other tears and lesions |
|---|---|---|---|---|---|---|
|
Monin et al. 199436 and 199337 France |
ALP (N = 10) vs encircling scleral buckling (N = 8) vs various other interventions (N = 4) | N/A | N/A |
5/10 vs 0/7b vs 4/4 No analysis reported |
Group 1: 3–24 months (mean 12 months) Group 2: N/A Group 3: 18–67 months |
NR |
|
Fritsch et al. 198938 France |
Various interventions (N = 45) No control |
0/45 | 0/38 | 0/7 | N/A | Three eyes required additional treatment in the monitoring period because they developed new lesions |
In the Monin et al. 36,37 study, only participants who had already experienced RD in the primary eye were included; prophylaxis was performed on the fellow eye (i.e. the eye that had not experienced a detachment). The study was conducted in France. There were three intervention groups, each exposed to different forms of primary prophylaxis: argon laser photocoagulation; scleral buckling; and a group exposed to four different interventions: focal cryotherapy, focal or circular laser photocoagulation, or vitrectomy. No group was designated as the primary intervention group or as controls. This study employed retrospective case review of data from cohorts exposed to the various interventions. Follow-up was reported to range from 3 to 67 months. In the study by Fritsch et al. ,38 participants received either bilateral or unilateral prophylaxis. This was a cohort study conducted in France. It is unclear whether the study was prospective or retrospective. Groups in the cohort were exposed to one of the following interventions: focal laser treatment or cryotherapy, and scleral buckling or focal laser treatment with scleral buckling. Follow-up was reported to range from 2 to 8 years.
Monin et al. 36,37 reported that scleral buckling appeared to be effective as none of the seven participants exposed to this unilateral intervention in the fellow eye had experienced an RD at follow-up (9 months to 3 years) (Table 4). 37 However, 5 of 10 individuals exposed to unilateral argon laser photocoagulation had an RD in the fellow eye in the follow-up period, as did all four individuals exposed to cryotherapy, focal or circular laser photocoagulation, or vitrectomy. The mean age at first RD was 8 years in the laser group failures, 11 years for the laser group ‘successes’ and 16 years for the successful scleral buckling group participants. The age at first RD may therefore be a confounding factor. In the Fritsch et al. 38 study, none of the individuals exposed to cryotherapy (number unknown), focal laser treatment (number unknown), scleral buckling (n = 2) or focal laser treatment with scleral buckling (n = 2) experienced an RD. Monin et al. 36,37 reported lid swelling and chemosis immediately post operation for scleral buckling, as well as a single case of longer-term sero-haemorrhagic choroid detachment, which spontaneously resolved. The Fritsch et al. 38 study did not report any complications with any intervention.
Both the Monin et al. 36,37 and Fritsch et al. 38 studies had a high risk of bias. They appear to be retrospective cohort studies and had very small samples (22 and 26 respectively); it is unclear if some possible participants had been excluded and the diagnosis itself may be flawed. There is no justification of the sample size in either study. In the absence of clearly reported diagnostic or treatment criteria, it is not possible to determine whether the populations in the treatment groups in the study by Monin et al. 36,37 are in fact all the same. Fritsch et al. 38 was a cohort study with comparator groups, but did not report the exact number of participants exposed to either focal laser treatment or cryotherapy in the principal group. The effect of each of the reported interventions therefore could not be determined. Neither Monin et al. 36,37 nor Fritsch et al. 38 reported any differences between groups. The follow-up in both studies (maximum 8 years) is almost certainly insufficient to demonstrate effect. Neither study performed any analysis on the results or calculated an estimate of effect. Fritsch et al. 38 reported that no participant experienced the outcome of interest. This seems unlikely given the population and length of follow-up (up to 8 years): Monin et al. 36,37 evaluated similar interventions in a similar population over a shorter length of time and reported a high incidence of RD in two of the three intervention groups. The external validity of both studies is limited because the populations were diagnosed as Wagner–Stickler syndrome rather than Stickler syndrome (although reported symptoms suggest a majority may have had Stickler syndrome) and neither was conducted in the UK, and techniques may differ by location.
Chapter 4 Assessment of factors relevant to the NHS and other parties
Stickler patients may present to the NHS in one of three ways. Firstly, individuals may present with an RD in the primary eye and it is noted that they have systemic features consistent with Stickler syndrome, e.g. cleft lip or joint abnormalities. 8,27 Secondly, they may be referred to a consultant ophthalmologist (Hospital Eye Service) on account of poor vision, high myopia or amblyopia (‘lazy eye’) and are found also to have other ocular and systemic features that are consistent with Stickler syndrome. Given the issues with diagnosis outlined above, molecular genetic analysis would be required to confirm the presence and type of Stickler syndrome. It has been reported that the efficiency of mutation detection after vitreoretinal assessment is 96.5% for the membranous phenotype COL2A1. 53 Currently, the cost of diagnostic genetic testing is reported to be approximately £1000 (East Anglian Medical Genetics Service, Addenbrooke’s Hospital, Cambridge, UK, 1 July 2010). Finally, family members of an individual (index case) diagnosed with Stickler syndrome could be approached and offered molecular genetic analysis to confirm the presence and type of Stickler syndrome (pre-symptomatic tests £162 for sequence of one exon: East Anglian Medical Genetics Service, 1 July 2010). An assessment of ocular and non-ocular features of Stickler syndrome would also need to be made for these individuals, and the risk of RD determined. All groups for whom mutation of the relevant genes has been detected may also benefit from genetic counselling. 8,19,54 Published figures estimate the lifetime costs associated with congenital visual loss in childhood or adolescence to be up to £257,000 per person, with 61% of this cost attributable to productivity losses. 55 In the event that a form of prophylaxis was found to be definitely relatively more effective than others (though no particular treatment is demonstrably and certainly more effective based on current published evidence), then that form of prophylaxis could be offered to these groups.
In order to quantify or assess the implications for the NHS, more reliable estimates or data are needed on the prevalence of Stickler syndrome in the UK, the risk of blindness in individuals diagnosed with type 1 and type 2 Stickler syndrome, i.e. those types at highest risk of RD, with and without treatment for RD, and the efficacy of prophylaxis. If these elements are established, then there may also be a case for screening programmes in order to identify individuals both with and without a recognised family history (i.e. a new mutation) before they present with an RD.
Chapter 5 Discussion
Statement of principal findings
Two studies were identified that assessed the effectiveness of interventions for the primary prophylaxis of RD in type 1 Stickler syndrome populations. 33,34 Both studies were retrospective cohort studies. The study by Ang et al. 33 evaluated the efficacy of 360° cryotherapy for the prevention of GRTs progressing to RD. The intervention was applied to both eyes or one eye only, i.e. as bilateral or unilateral prophylaxis, and compared with either no prophylaxis or, for some controls, alternative but unknown forms of prophylaxis. This study had 204 participants. The Leiba et al. 34 study evaluated focal and 360° circumferential laser treatment in bilateral or unilateral prophylaxis compared with no prophylaxis. This study had only 22 participants, from a single family group. The primary outcome in both studies was the incidence of RD in eyes without any prior RD and receiving prophylaxis. Both studies reported a significant difference between the number of RDs in the intervention and control groups. The reduction in the risk of RD was statistically significant for cryotherapy prophylaxis compared with non-cryotherapy prophylaxis or no prophylaxis both for individuals with no previous RD and for those with an RD in the primary eye.
There was clinical heterogeneity between these two studies,33,34 so their results could not be combined statistically to offer a potentially more robust and precise estimate of effect. Both studies included only patients diagnosed as having type 1 Stickler syndrome, but all participants in the intervention groups in the study by Ang et al. 33 had GRTs, while none of the participants exposed to the intervention in the study by Leiba et al. 34 had any GRTs. The indications for prophylaxis were therefore different in the two studies. The interventions being evaluated were also different – 360° cryotherapy alone33 or focal or circumferential laser treatment34 as was the control – no prophylaxis or prophylaxis other than cryotherapy33 – or no prophylaxis. 34 Both studies had reasonable follow-up of the intervention groups. Although the risk of RD is life-long, all reported RDs occurred in < 6 years in the Leiba et al. 34 and Monin et al. 36,37 studies, and at a mean of 7.7 years for the bilateral prophylaxis population in the Ang et al. 33 study; so, follow-up of up to 15 or 33 years, which was achieved for some participants in the Leiba et al. 34 and Ang et al. 33 studies respectively, may potentially capture a sizeable number of RDs subsequent to prophylaxis. However, longer follow-up would offer much more reliable results. According to the studies by Ang et al. 33 and Leiba et al. ,34 neither 360° cryotherapy nor focal or circumferential laser treatment appears to be associated with major or long-term complications. However, the number of patients experiencing minor or temporary complications or side effects was not reported in either study.
There is a high risk of bias within both studies. The lack of comparability between the intervention and control groups is the principal source of bias affecting the reliability and validity of the findings of the study by Ang et al. 33 The control group is different from the intervention groups. It is substantially older than the intervention groups (mean age of 49 years compared with 21 or 36 years) and, given that the risk of RD is life-long,2,8 these controls were therefore inherently much more likely to have experienced the outcome of interest (RD). The study acknowledges the problem of the lack of comparability, stating that the control group offered ‘a useful estimate of the prevalence of RD’ without cryotherapy. However, the percentage of patients with RD in either eye in the control group is also higher than the figure commonly cited in the literature for rates of RD in Stickler syndrome populations not exposed to prophylaxis (73% vs a rate of 57% in 165 members of a family with Stickler syndrome,20 and 60% or 61% in two studies sampling individuals in Stickler syndrome support groups in either the UK and North America2 or the UK only8). As noted above, these surveys are not explicitly limited to individuals with type 1 Stickler syndrome, who are at the highest risk of RD, only individuals principally diagnosed by ophthalmologists or geneticists as having Stickler syndrome. There may also be a risk of misdiagnosis, but this has never been quantified. The sampling of both the controls in the Ang et al. 33 study and the participants in the surveys is at risk of bias, so neither figure is a reliable estimate of prevalence of RD in untreated Stickler syndrome populations. However, these studies offer the only currently reported relevant comparative data on this outcome within this population.
By contrast, members of the intervention group receiving bilateral prophylaxis in the Ang et al. 33 study, with a mean age of 21 years, will have been the least likely to experience the outcome by the age of intervention or follow-up, as the risk of RD is reported to increase in young adulthood up to 30 years of age2 and if the primary eye has already experienced a detachment. 50,56–58 Given the life-long risk of RD in individuals with Stickler syndrome, age is a major confounding factor in any comparison of primary prophylaxis for RD, and should be controlled. Ang et al. 33 also recognise that a substantially increased rate of RD beyond the existing follow-up period might potentially negate the findings of the study. 21 A higher rate of RD beyond the study duration is possible, given that the mean age of the group receiving bilateral prophylaxis was 21 years; and it has been reported elsewhere that the first RD occurred between the mean ages of 21 and 25 years in a group of Stickler syndrome patients presenting over a 40-year period. 27
The data for the control group in the Ang et al. 33 study are also cross-sectional, i.e. they are reported only for a single point in time (the time of the study’s data collection), unlike the data for the intervention groups. The intervention groups have a baseline (the time of the exposure to the intervention) and an end point (the time of the study’s data collection). The control group has not been exposed to an intervention and so lacks the ‘baseline’; there is therefore no reported length of follow-up. This therefore also introduces further risk of bias into any comparison between the intervention and control groups. The generation of a potentially more comparable control group, from within the Ang et al. 33 controls, may be possible if age at first RD was known, i.e. those who had not experienced an RD by the mean age at which the bilateral prophylaxis group were exposed to the intervention (10 years), and those who had experienced RD in only one eye by the mean age at which the unilateral prophylaxis group were exposed to the intervention (21 years). This would offer a baseline for comparability between groups: the age of the controls at the time of data collection would represent the follow-up, and the incidence of RD (including if there was bilateral RD) would be more comparable to any reported incidence in the intervention groups. However, it is stated in the study by Ang et al. 33 that ‘data on the timing of events [in the control group] were either unreliable or missing’ and the confounding factors of age and heterogeneity in the exposure of controls to prophylaxis would remain. This lack of comparability between the intervention and control groups therefore introduces a high risk of bias into this study; consequently, there is considerable uncertainty regarding the relative efficacy of this intervention.
The reliability of any estimate of the relative effect of the intervention would be further adversely affected by heterogeneity in the control group in terms of the comparator intervention as some subjects were exposed to no intervention at all and an unknown number received some form of some prophylaxis. Finally, it is also unclear whether the study would be powerful enough to generate a reliable estimate of effect.
The study by Leiba et al. 34 was smaller and shorter. It considered only 22 individuals from a single family, and the follow-up of the intervention group was as little as 1 year and a maximum of only 15 years, which overall may not be long enough to reliably demonstrate effectiveness. There was no power calculation, so it is uncertain whether this small study would be powerful enough to generate a reliable estimate of effect. Leiba et al. 34 also failed to report differences between intervention and control groups, including the potential major confounding factor of age, and only those participants who were considered eligible for treatment actually received prophylaxis, so the relative likelihood of the control group experiencing RD is unknown.
Despite the high risk of bias in both studies, the rate of RD in the intervention groups is lower than the rate experienced in the study control groups. The rate of RD is 4/124 (3%) per eye and 4/62 (6%) per patient in those exposed to bilateral cryotherapy prophylaxis, and 3/31 (10%) per eye and per patient in those exposed to unilateral cryotherapy prophylaxis. This compares with 134/222 (60%) per eye in the study control group or, excluding those who experienced bilateral RD, 28/116 (24%). 33 The rate of RD is 1/8 (13%) in those exposed to bilateral laser prophylaxis and 0/2 in those exposed to unilateral laser prophylaxis, compared with 15/34 (44%) in the untreated control group. 34 The rates reported for the intervention groups are also lower than the 57%, 60% or 61% reported for rates of RD in surveys of Stickler syndrome populations not exposed to prophylaxis. 2,8,20 These studies do not report a mean age for these figures but, again, it is likely to be higher than the mean age reported for the intervention groups in the study by Ang et al. ,33 so there exists the same problem of comparability. Also, the rates of RD in the largest study sample increased from 26% to 61% from the 10–19 years to the 20–29 years age group. 2 This again highlights a problem with the mean age of 21 years for the bilateral prophylaxis group in the Ang et al. 33 study, as this group is likely to be at inherently lower risk of having experienced a first RD. However, in the two surveys sampling a similar population base, the percentage of patients experiencing RD was 16% (n = 27/164) in those < 20 years of age2 and 20% (n = 15/74) for those < 16 years of age,8 which are both higher than the rates reported for the bilateral and unilateral prophylaxis intervention groups with mean ages of 21 or 36 years in the study by Ang et al. :33 6% and 10% respectively.
The incidence of first RD appears to rise substantially after the age of 20 years in both of these surveys (from ≤ 26% to 60% or 61%). 2,8 Therefore, the ongoing reporting of rates of RD in the intervention groups of the study by Ang et al. 33 would permit a further, more robust evaluation of the relative efficacy of cryotherapy in the primary prophylaxis of RD in type 1 Stickler syndrome. This is because both the mean age of the intervention groups (currently a major confounder introducing a risk of bias into the study results) and the duration of follow-up (a second important confounder) would increase with the consequence that the risk of bias would be reduced. However, the problems with the study’s control group would remain.
Two further studies were identified that assessed the effectiveness of interventions for primary prophylaxis of RD in populations designated as ‘Wagner–Stickler’ syndrome, but in which some or all of the participants had systemic features that may be consistent with Stickler syndrome. 36–38 Both were small cohort studies with a number of comparable intervention groups. Neither study reported significant differences between the number of RDs in the intervention groups. Monin et al. 36,37 reported 5/10 RDs in the argon laser photocoagulation group, 0/7 in the scleral buckling group and 4/4 in the group exposed to cryotherapy, focal or circumferential laser treatment, or vitrectomy. Fritsch et al. 38 reported no RDs in any group. However, there is a high risk of bias within both studies: neither was definitely conducted on a majority of Stickler syndrome individuals; neither controlled for confounding factors such as age; neither had follow-up of > 8 years for any individual; neither had large samples; and the numbers exposed to specific interventions were not reported in the principal intervention group in the study of Fritsch et al. 38
In the absence of head-to-head studies of the stated interventions in this population, there may be scope for undertaking a form of indirect comparison. It is unfortunate that there are insufficient data to make a robust comparison between individual techniques. Further research is required to produce a definitive conclusion on the most effective clinical approach. Nevertheless, there may also be value in quantifying the uncertainty regarding the relative effectiveness of each intervention, if only to use it as a basis for designing a prospective randomised controlled trial (RCT) comparing potential interventions (J Stevens, Lecturer and Director of the Centre for Bayesian Statistics in Health Economics, March 2010, personal communication).
Strengths and limitations of the assessment
Strengths
-
There is no other published review of primary prophylactic interventions for RD in either Stickler syndrome or Wagner–Stickler syndrome individuals.
-
The literature search: a sensitive search was performed to identify published and unpublished comparative studies that satisfied the inclusion criteria. No formal assessment of publication bias has been made for this review, but the effect of any such bias is likely to be minimal given the absence of any date or language limits on the search, the inclusion of non-English-language journal articles, and the inclusion of supporting studies reporting inconsistent results for the interventions, including no effect. 36,59 The likelihood of a relevant study having been missed is therefore low.
-
Authors of papers were contacted if a family history of RD was cited as a characteristic of study participants being exposed to primary prophylactic interventions for RD, but without any specific reference to Stickler syndrome. The aim was to identify any additional relevant data on Stickler syndrome subgroups in studies that did not otherwise specify that participants did or did not have this condition. No further data were forthcoming.
-
The review process: all titles and abstracts of citations retrieved by the search of electronic databases were screened independently for inclusion and exclusion by two reviewers; and all data extraction and quality assessment of included studies were performed independently by two reviewers, and any discrepancies identified and resolved.
-
The identification of two principal studies satisfying the inclusion criteria with populations diagnosed with type 1 Stickler syndrome patients (the subtype at highest risk of RD) and confirmed ‘where possible’ by genetic analysis.
Weaknesses
-
The absence of any relevant studies with a robust comparative design to limit the risk of bias, e.g. RCTs. The only pivotal studies identified were retrospective cohort studies.
-
The absence of any good-quality studies or data with which to answer the research question.
-
The small number of relevant studies identified: two principal studies of type 1 Stickler syndrome individuals,33,34 and two supporting studies of ‘Wagner–Stickler’ syndrome individuals,36–38 with issues surrounding this diagnosis.
-
Despite efforts to identify all published and unpublished research satisfying the inclusion criteria, publication bias as a result of the non-publication of studies of any of the various prophylactic interventions but which demonstrate no effect cannot entirely be discounted.
Uncertainties
The review identified only two principal studies that satisfied the inclusion criteria,33,34 and the risk of bias in both studies is high. The study designs used (retrospective cohorts with comparator groups) are inherently at greater risk of bias than alternative study designs, such as randomised or prospective controlled trials. 52 These two studies also had major weaknesses in the conduct of the study, including major differences between the intervention and control groups in terms of potential confounding factors, a possible lack of power, limited follow-up and, in one case, a small and narrow sample. The data reported by these studies therefore cannot generate a robust or reliable estimate of the effect for 360° cryotherapy or focal or circumferential laser treatment compared with no intervention as primary prophylaxis for RD in type 1 Stickler syndrome.
It is likely that future trials with greater comparability between treatment groups, longer follow-up, and a lower risk of bias, would not only enable the calculation of a valid and reliable estimate of effect, but also generate a reliable estimate of the relative risk of RD when exposed to either another primary prophylactic intervention or no intervention at all. The ongoing reporting of data from the intervention groups in the Ang et al. 33 study should partially address some of these issues. In the absence of good-quality trials comparing interventions within Stickler syndrome populations, or sufficient data to permit a robust indirect comparison, it is also uncertain which if any of the interventions of cryotherapy or focal or circumferential laser treatment is relatively the most effective.
It is uncertain whether other primary prophylactic interventions may be potentially effective in reducing rates of RD in this population. Scleral buckling, for example, has not been evaluated in confirmed type 1 Stickler syndrome populations, but Monin et al. 36,37 reported positive results for this technique in an intervention arm of a study of ‘Wagner–Stickler’ individuals. However, the number of participants in this group was very small (n = 7) and the follow-up was very short (3 years). The risk of bias in the studies of ‘Wagner–Stickler’ populations was also high, preventing reliable conclusions being drawn from the efficacy results relating to a range of different prophylactic interventions.
There appear to be few major or long-term complications or adverse events associated with 360° cryotherapy or focal or circumferential laser treatment, but the number of individuals likely to experience either minor or major short-term complications is uncertain. The clinical advice elicited for this report suggests that cryotherapy is likely to produce greater pain and swelling than laser therapy.
The interventions evaluated by the principal studies of Stickler syndrome individuals are 360° cryotherapy on the post-oral retina by Ang et al. 33 and focal or circumferential laser treatment by Leiba et al. 34 The efficacy of both interventions in different areas of the eye, such as 360° cryotherapy at the posterior border of the vitreous base60 and at the equator, has not been assessed in type 1 Stickler syndrome populations. The evidence identified by this review also does not permit a conclusion to be drawn on whether there is an optimal intervention for particular indications, i.e. whether cryotherapy and/or focal or circumferential laser treatment are likely to be effective in Stickler syndrome populations presenting with indications for treatment different from those evaluated in the studies. Prophylactic cryotherapy has been evaluated only in type 1 Stickler syndrome patients with GRTs, and focal and circumferential laser treatment only in type 1 Stickler syndrome individuals with lattice degeneration with or without retinal breaks, or isolated foci of lattice degeneration with at least one of the following: myopia, previous RD and a family history of RD or vitreoretinal disease.
It is uncertain what the optimal indications are for prophylaxis in Stickler syndrome populations, that is if any such optimal indications exist, e.g. GRTs or other retinal lesions, lattice degeneration or high myopia. It is also unclear whether there are indications for which one or both of the interventions should not be used. Clinical advice suggests that the choice of intervention is currently determined by the clinician’s preference only. The optimal age for treatment is also uncertain. It has been suggested that early intervention, in childhood, may be advisable given that the first RD has been found to occur more frequently in the 10–30 years age group, and that children may not be able to report symptoms until it is too late. 21 Leiba et al. 34 performed a subgroup analysis based on age and found a smaller number of postprophylaxis RDs in children or adolescents aged ≤ 13 years old compared with those aged > 13 years. However, this sample was very small (n = 11).
It is uncertain how effective the interventions are at preventing or reducing the presence or type of retinal tears or lesions (possible precursors of RDs) in untreated areas of the eye. These data are reported by only one study for the intervention groups and not for the control group. 33 It is therefore also uncertain how frequently an intervention may need to be performed, given that neither procedure appears to prevent all tears or lesions that may lead to detachment. It has also been suggested that cryotherapy may cause or accelerate the development of new tears or lesions. 50,61 Supplementary prophylactic intervention (or ‘top-up retinopexy’) may need to be used to treat such tears or breaks that occur posterior to the treated area or secondary to GRTs. 33
Other relevant factors
None reported.
Chapter 6 Conclusions
Implications for service provision
Only 360° cryotherapy33 and focal and circumferential laser34 treatment have been evaluated for the type 1 Stickler syndrome population, and by only a single retrospective, controlled, cohort study in each case. Both of these studies do report a significant difference between intervention and control groups (principally no treatment) and no major or long-term side effects or complications. However, there is a high risk of bias within both studies, so the relative effectiveness of either 360° cryotherapy or focal and circumferential laser treatment in comparison with no treatment is uncertain. There is also no head-to-head trial comparing the two interventions, so their relative effectiveness in comparison with each other is also uncertain. It is necessary to determine whether or not an individual has type 1 or type 2 Stickler syndrome, as this determines the risk of RD and therefore possible eligibility for any form of prophylaxis. Genetic analysis is required to establish the presence and type of Stickler syndrome. The groups for whom this may be necessary are described in Chapter 4. Continued follow-up and analysis of study data, and data collection from relevant sample populations, are required to assess the long-term risks of blindness, RD and prophylaxis. Therefore, given the uncertainties found by this report regarding the relative efficacy of the evaluated interventions, the implications for existing service provision are very limited, especially as continued follow-up and analysis of data being generated from existing services is a recommendation of this report.
Suggested research priorities
As a result of the high risk of bias in the studies included in this report, more reliable data may be generated from two sources. Firstly, the ongoing reporting and analysis of data from the study by Ang et al. 33 could potentially offer more reliable findings, but will still be affected by the risk of bias inherent in the design and control group of this study, unless the latter in particular was addressed.
Secondly, a new study could be undertaken that addresses the current uncertainties in the evidence base. Given that there are uncertainties concerning both the efficacy of cryotherapy and laser therapy compared with no treatment, and also uncertainty regarding the relative efficacy of the two principal, evaluated interventions, then a three-armed study comparing all of these options is to be recommended. A prospective RCT comparing the current treatment options would obviously offer the optimal study design for controlling for the effect of the principal confounding variables of age, comparable follow-up between groups, RD in the primary eye, and pathology and/or indications for treatment, as these factors should be present in comparable or equal numbers across groups.
However, as such a trial would be both costly and impractical, given the rarity of the condition and the likely number of centres involved, and also given that such strong opinions are held within the ophthalmology community on the prophylactic efficacy or otherwise of the two interventions,21 some referring clinicians are unlikely to accept the randomisation of eligible patients under their care to a study arm, and either a treatment or no treatment that they consider to be ineffective or around which there is too much uncertainty. Consequently, despite being inherently at a greater risk of bias than an RCT, a potential priority for research might be a prospective cohort comparison study, comparing three cohorts exposed to cryotherapy, laser therapy and no treatment with participants satisfying specific inclusion criteria on diagnosis, age and pathology. Relevant referring clinicians could then enter eligible patients into the study arm of their choice. Individuals exposed to bilateral or unilateral prophylaxis (i.e. having already experienced an RD in the primary eye) would be entered into the study and would be analysed separately, given the possible non-independence of eyes from the same person. These data could be used to supplement the ongoing report of data from the Ang et al. 33 retrospective study. The primary outcome is RD.
Participants would then be followed over time until a difference between treatments, or between treatment and no treatment, became apparent through interim analysis of available data (e.g. at 5, 10 and 15 years), when the study could be discontinued. Alternatively, the study could also be discontinued if no such difference was demonstrated through such interim analysis of available data. In both cases, an appropriate ‘stopping rule’ which was deemed clinically and statistically robust would need to be determined, i.e. what constitutes a clinically meaningful difference between groups and a sufficiently meaningful length of follow-up. The relative effect of the treatments or the non-treatment would be determined by a calculation of the RR using the dichotomised data of there being either an event (RD) or no event at a single point in time (e.g. 5, 10 and 15 years).
The resulting study may lack power, especially if it was discontinued early, as the sample size may be small. For the purpose of providing context only, the following power calculations are presented to give an idea of possible sample sizes, and their power, required by such a study. Using a sample size formula for binary data (i.e. the risk of having or not having an RD),62 98 participants (196 eyes) exposed to bilateral prophylaxis in each arm could detect a reduction in the relative risk of RD from 20% in one group to 10% in the other groups at 80% power and 5% (α = 0.05) two-sided level of significance. Given the rarity of the condition, and potential problems with recruiting to the study, 80% power is preferred to 90% power, as the latter would require a larger number of participants in each arm to detect this difference in the reduction of the relative risk of RD between arms (i.e. 131 participants, 262 eyes). Clinical advice has suggested that this reduction in the relative risk of an RD would be clinically important: the figure of 20% being similar to the rates of RD in individuals up to 20 years of age reported in two surveys,2,8 the age group most likely to present for prophylaxis. These figures are for bilateral prophylaxis only; for 80% power, unilateral prophylaxis would require 196 participants in each arm.
Given that a majority of the likely participants might be children or adolescents, there would be ethical issues surrounding consent and participation in any such study. Also, while it should be noted that the studies evaluating the interventions reported no major or long-term adverse events or complications, the frequency of minor complications is unknown and further studies would need to take into account the potential complications of both laser therapy and cryotherapy and include these in the patient information sheet and ethics application. Clinical advice also suggests that there is a need to identify reliable prevalence data on type 1 or type 2 Stickler syndrome, as this would provide a context for assessing the implications of the efficacy of any form of prophylaxis.
Acknowledgements
Dr Jennifer Evans, Lecturer and member of Cochrane Eyes and Vision Group (CEVG), London School of Hygiene and Tropical Medicine, London, UK.
Mr Alistair Laidlaw, Consultant Ophthalmologist, St Thomas’ Hospital, London, UK.
Mr Richard Sheard, Consultant Ophthalmologist, Royal Hallamshire Hospital, Sheffield, UK.
Dr Martin Snead, Consultant Vitreoretinal Surgeon, Addenbrooke’s Hospital, Cambridge, UK.
Gill Rooney provided administrative support in preparing and formatting the report.
Contributions of authors
Christopher Carroll acted as Principal Investigator for this assessment, he also designed, co-ordinated and wrote the review. Christopher Carroll and Angie Rees designed the searches, and Angie Rees undertook the searches. Christopher Carroll and Diana Papaioannou screened search results, assessed the quality of included papers, extracted data from papers and undertook analysis of and interpreted the data. Eva Kaltenthaler read and approved the review.
About ScHARR
The School of Health and Related Research (ScHARR) is one of the nine departments in the Faculty of Medicine, Dentistry and Health at the University of Sheffield. ScHARR specialises in health services and public health research, and the application of health economics and decision science to the development of health services and the improvement of public health.
The ScHARR Technology Assessment Group (ScHARR-TAG) synthesises research on the clinical and cost-effectiveness of health-care interventions for the National Institute of Health Research HTA programme on behalf of a range of policy-makers, including the National Institute of Health and Clinical Excellence. ScHARR-TAG is part of a wider collaboration of six units from other regions. The other units are the Southampton Health Technology Assessment Centre (SHTAC), University of Southampton; Aberdeen HTA Group, University of Aberdeen; Liverpool Reviews & Implementation Group, University of Liverpool; Peninsular Technology Assessment Group, University of Exeter; NHS Centre for Reviews and Dissemination, University of York; and West Midlands Health Technology Assessment Collaboration, University of Birmingham.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- Snead M. Clinical and molecular genetics of Stickler syndrome. J Med Genet 1999;36:353-9.
- Stickler G. Clinical features of hereditary progressive arthroophthalmopathy (Stickler syndrome): A survey. Genet Med 2001;3:192-6.
- Stickler G, Belau P, Farrell F, Jones J, Pugh D, Steinberg A, et al. Hereditary progressive arthro-ophthalmopathy. Mayo Clin Proc 1965;40:433-55.
- Nowak C. Genetics and hearing loss: a review of Stickler syndrome. J Commun Disord 1998;31:437-53.
- Poulson A, Hooymans J, Richards A, Bearcroft P, Murthy R, Baguley DM, et al. Clinical features of type 2 Stickler syndrome. J Med Genet 2004;41.
- Annunen S. Splicing mutations of 54-bp exons in the COL11A1 gene cause Marshall syndrome, but other mutations cause overlapping Marshall/Stickler phenotypes. Am J Hum Genet 1999;65:974-83.
- Richards A, Laidlaw M, Meredith S, Shankar P, Poulson A, Scott J, et al. Missense and silent mutations in COL2A1 result in Stickler syndrome but via different molecular mechanisms. Hum Mutat 2007;28.
- Webb A, Markus A. The diagnosis and consequences of Stickler syndrome. Br J Oral Maxillofac Surg 2002;40:49-51.
- Popkin J, Polomeno R. Stickler’s syndrome (hereditary progressive arthro-opthalmopathy). Can Med Assoc J 1974;111:1071-6.
- Snead M. Stickler syndrome type 2 and linkage to the COL11A1 gene. Ann N Y Acad Sci 1996;785.
- Snead M. Stickler syndrome: Correlation between vitreoretinal phenotypes and linkage to COL 2A1. Eye 1994;8:609-14.
- Snead M, Richards A. Stickler syndrome: Further mutations in COL11A1 and evidence for additional locus heterogeneity. Eur J Hum Genet 1999;7:807-14.
- Richards A. A family with Stickler syndrome type 2 has a mutation in the COL11A1 gene resulting in the substitution of glycine 97 by valine in alpha1(XI) collagen. Hum Mol Genet 1996;5:1339-43.
- Sirko-Osadsa D, Murray M, Scott J, Lavery M, Warman M, Robin N. Stickler syndrome without eye involvement is caused by mutations in COL11A2, the gene encoding the alpha2 (XI) chain of type XI collagen. J Pediatr 1998;132:368-71.
- Hughes W. Stickler Syndrome: What is it? And how can we help? An introduction to the condition and the support group (for patients, carers and families). Stickler Syndrome Support Group; 2006.
- Lansford M. Focus on the physical assessment of the infant with Stickler syndrome. Adv Neonatal Care 2008;8:308-14.
- Richards A. COL2A1 exon 2 mutations: Relevance to the Stickler and Wagner syndromes. Br J Ophthalmol 2000;84:364-71.
- Parma E. Radial perivascular retinal degeneration: A key to the clinical diagnosis of an ocular variant of Stickler syndrome with minimal or no systemic manifestations. Am J Ophthalmol 2002;134:728-34.
- Parke D. Stickler syndrome: Clinical care and molecular genetics. Am J Ophthalmol 2002;134:746-8.
- Donoso L. Identification of a stop codon mutation in exon 2 of the collagen 2A1 gene in a large stickler syndrome family. Am J Ophthalmol 2002;134:720-7.
- Snead M. Author reply. Ophthalmology 2008;115:1637-8.
- Monin C, Le Mer Y, Van Effenterre G, Ammar N. Non-traumatic retinal detachment in children under 15 years of age. Ophtalmologie 1989;3:31-4.
- Vandenburg P. Molecular basis of hereditable connective tissue disease. Biochem Med Metab Biol 1993;49:1-12.
- Wilkinson C. Interventions for asymptomatic retinal breaks and lattice degeneration for preventing retinal detachment. Cochrane Database Syst Rev 2001.
- Knobloch W. Inherited hyaloideo-retinopathy and skeletal-dysplasia. Trans Am Ophthalmol Soc 1976;73:417-51.
- Liberfarb R. Stickler syndrome resulting from seven mutations in the type II collagen gene locus COL2A1. Genet Med 2003;5:21-7.
- Abeysiri P. Outcomes of surgery for retinal detachment in patients with Stickler syndrome: A comparison of two sequential 20-year cohorts. Graefes Arch Clin Exp Ophthalmol 2007;245:1633-8.
- Ang GS, Townend J, Lois N. Interventions for prevention of giant retinal tear in the fellow eye. Cochrane Database Syst Rev 2009.
- Moher D, Liberati A, Tetzlaff J, Altman D. PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:1-16.
- Richards A. High efficiency of mutation detection in type 1 stickler syndrome using a two-stage approach: vitreoretinal assessment coupled with exon sequencing for screening COL2A1. Hum Mutat 2006;27:696-704.
- Brown D, Graemiger R, Hergersberg M, Schinzel A, Messmer E, Niemeyer G, et al. Genetic linkage of wagner disease and erosive vitreoretinopathy to chromosome 5q13–14. Arch Ophthalmol 1995;113:671-5.
- Critical Apprasial Skills Programme (CASP) 12 questions to help you make sense of a cohort study. Oxford: Public Health Research Unit; 2004.
- Ang A, Poulson A, Goodburn S, Richards A, Scott J, Snead M. Retinal detachment and prophylaxis in type 1 Stickler syndrome. Ophthalmology 2008;115:164-8.
- Leiba H, Oliver M, Pollack A. Prophylactic laser photocoagulation in Stickler syndrome. Eye 1996;10:701-8.
- Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions 2009. www.cochrane-handbook.org (accessed 3 March 2011).
- Monin C, Van Effenterre G, Andre-Sereys P, Haut J. Prevention of retinal detachment in Wagner–Stickler disease. Comparative study of different methods. Apropos of 22 cases. J Fr Ophtalmol 1994;17:167-74.
- Monin C, Allagui M, Larricart P, Ameline B, Haut J. Prevention of non-traumatic retinal detachment by surgical encircling in a series of 20 cases. J Fr Ophtalmol 1993;16:247-53.
- Fritsch D, Vallat M, Lagoutte F, Verin P. Prevention of retinal detachment in Wagner–Stickler syndrome. Bull Soc Ophtalmol Fr 1989;89:657-64.
- Khayrallah M, Kamoun N, Malouch N, Nouira F, Garsallah R, Chachia N. Limites du laser dans la prevention du decollement de retine de la Maladie de Wagner. Bull Soc Tun Ophthalmol 1991;3:82-6.
- Limon S, Pattoret M. Maladie de Wagner et decollement de retine. A propos de 10 cas. Ophtalmologie 1987;1:223-5.
- Corcostegui G. Personal experience of prophylactic treatment of retinal detachment by means of argon laser photocoagulation. Arch Soc Esp Oftalmol 1978;38:795-803.
- Juhas T, Klisenbauer D. The argon laser in the prevention of retinal detachment. Cesk Oftalmol 1992;48:402-5.
- Zeidan A, Bedda A, El Habrouk M, Rashad K, Galal A. Management of the fellow eye in patients with rhegmatogenous retinal detachment. J Med Res Inst 1995;16:76-83.
- Boen-Tan T. Analysis of prophylactic laser coagulation of retinal defects and degenerations performed in patients of the Free University of Amsterdam during the period from 1982 to July 1986. Doc Ophthalmol 1987;67:83-8.
- Levin M, Naseri A, Stewart J. Resident-performed prophylactic retinopexy and the risk of retinal detachment. Ophthalmic Surg Laser Imag 2009;40:120-6.
- Mody K, Saxena A. Laser photocoagulation and prophylactic treatment for retinal detachment of the lesion. Indian J Ophthalmol 1983;31:961-3.
- Chignell A, Shilling J. Prophylaxis of retinal detachment. Br J Ophthalmol 1973;57:291-8.
- Pollack A, Milstein A, Oliver M, Zalish M. Circumferential argon laser photocoagulation for prevention of retinal detachment. Eye 1994;8:419-22.
- McPherson A, O’Malley R, Beltangady S. Management of the fellow eyes of patients with rhegmatogenous retinal detachment. Ophthalmology 1981;88:922-34.
- Freeman H. Fellow eyes of giant retinal breaks. Trans Am Ophthalmol Soc 1978;76:343-82.
- Boniuk I, Okun E, Johnston GP, Arribas N. Xenon photocoagulation vs. cryotherapy in the prevention of retinal detachment. Mod Probl Ophthalmol 1974;12:81-92.
- Mann C. Observational research methods. Research design II: cohort, cross-sectional and case-control studies. Emerg Med J 2003;20:54-60.
- Richards A, McNinch A, Martin H, Oakhill K, Rai H, Waller S. Stickler syndrome and the vitreous phenotype: Mutations in COL2A1 and COL11A1. Hum Mutat 2010;31:E1461-71.
- Wilson M. Long-term follow-up of ocular findings in children with Stickler’s syndrome. Am J Ophthalmol 1996;122:727-8.
- Guide Dogs for the Blind Association . The Costs of Blindness. An Analysis of the Costs of Visual Impairment and Blindness in the United Kingdom 2003.
- Gonzales C, Gupta A, Schwartz S, Kreiger A. The fellow eye of patients with rhegmatogenous retinal detachment. Ophthalmology 2004;111:518-21.
- Mastropasqua L, Carpineto P, Ciancaglini M, Falconio G, Gallenga P. Treatment of retinal tears and lattice degenerations in fellow eyes in high risk patients suffering retinal detachment: A prospective study. Br J Ophthalmol 1999;83:1046-9.
- American Academy of Ophthalmology . Preferred Practice Patterns: Management of Posterior Vitreous Detachment, Retinal Breaks and Lattice Degeneration 2003:1-20.
- Dickersin K, Rothstein H, Sutton A, Borenstein M. Publication bias in meta-analysis: prevention, assessment, and adjustments. New York, NY: Wiley; 2005.
- Wolfensberger T. Prophylactic 360 degrees cryotherapy in fellow eyes of patients with spontaneous giant retinal tears. Ophthalmology 2003;110:1175-7.
- Sabates N. Macular changes after retinal detachment surgery. Am J Ophthalmol 1989;108:22-9.
- Campbell M, Machin D, Walters S. Medical Statistics: A textbook for the health sciences. Chichester: Wiley; 2007.
Appendix 1 Literature search strategies
Example Search Strategy: Database: Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) (1950 to Present)
-
stickler.mp. (256)
-
progressive arthro-opthalmopathol*.tw. (0)
-
progressive arthroopthalmopath*.tw. (0)
-
or/1-3 (256)
-
exp cryotherapy/ (17,686)
-
exp Light Coagulation/ (9473)
-
exp laser coagulation/ (4980)
-
exp Scleral Buckling/ (2085)
-
cryotherap*.tw. (4115)
-
((laser or light) adj2 (coagulat* or photocoagulat*)).tw. (4771)
-
(scleral* adj2 (buckl* or encircl*)).tw. (1437)
-
encircling band.tw. (110)
-
or/5-12 (34,143)
-
prophyla*.tw. (97,339)
-
prevent*.tw. (704,485)
-
ameliorat*.tw. (35,737)
-
or/14-16 (805,028)
-
13 and 17 (2425)
-
4 or 18 (2677)
-
exp RD/ (14,335)
-
exp retinal perforations/ (2955)
-
(retinal adj2 (detach* or tear* or break* or perforat*)).tw. (12,624)
-
or/20-22 (16,095)
-
23 and 19 (427)
Appendix 2 Data abstraction tables
Characteristics of included studies
| Reference Manager ID | Study reference Author, date, country |
Study design | Diagnostic criteria | Inclusion criteria (including criteria for diagnosis) | Exclusion criteria (including number excluded) | Intervention group and population characteristics Number, age, gender, ethnicity, retinal status, comorbidities, etc. |
Comparison group and population characteristics Number, age, gender, ethnicity, retinal status, comorbidities, etc. |
Prophylactic intervention Description of technique and setting |
Control/comparison (e.g. no treatment) |
|---|---|---|---|---|---|---|---|---|---|
Study outcomes
| Reference Manager ID | Study reference Author, date |
Study duration/follow-up | Measurement details How, by whom |
Intervention group: No. enrolled No. included in analysis No. excluded, withdrew |
Comparison group: No. enrolled No. included in analysis No. excluded, withdrew |
Intervention group Primary outcome: No. eyes (patients) unilateral RD No. eyes (patients) bilateral RD Secondary outcomes: No with total vision loss No. with unilateral vision loss No with retinal tear/lesions Time to RD or tear/lesions |
Comparison group Primary outcome: No. eyes (patients) unilateral RD No. eyes (patients) bilateral RD Secondary outcomes: No with total vision loss No. with unilateral vision loss No with retinal tear/lesions Time to RD or tear/lesions |
Adverse effects Descriptions and frequency |
Notes and new references |
|---|---|---|---|---|---|---|---|---|---|
Appendix 3 Quality assessment
CRITICAL APPRAISAL SKILLS PROGRAMME
making sense of evidence
12 questions to help you make sense of a cohort study
General comments
Three broad issues need to be considered when appraising a cohort study:
-
Are the results of the study valid?
-
What are the results?
-
Will the results help locally?
The 12 questions on the following pages are designed to help you think about these issues systematically.
The first two questions are screening questions and can be answered quickly. If the answer to those two is ‘yes’, it is worth proceeding with the remaining questions.
There is a fair degree of overlap between several of the questions.
You are asked to record a ‘yes’, ‘no’ or ‘can’t tell’ to most of the questions.
A number of italicised hints are given after each question. These are designed to remind you why the question is important. There will not be time in the small groups to answer them all in detail!
Critical Appraisal Skills Programme (CASP) 2004. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise without the prior permission of CASP. However, organisations may reproduce or use the publication for non-commercial educational purposes provided the source is acknowledged. Enquiries concerning reproduction or use in other circumstances should be addressed to CASP.
Appendix 4 List of studies excluded because they clearly failed to satisfy one or more of the designated population, intervention or outcome criteria
-
Alexander P, Ang A, Poulson A, Snead M. Scleral buckling combined with vitrectomy for the management of rhegmatogenous retinal detachment associated with inferior retinal breaks. Eye (Basingstoke) 2008;22:200–3.
-
Althaus C. Prophylactic argon laser coagulation for rhegmatogenous retinal detachment in AIDS patients with cytomegalovirus retinitis. Graefes Arch Clin Exp Ophthalmol 1998;236:359–64.
-
Amaro M. Prophylactic photocoagulation in acute retinal necrosis. Rev Bras Oftalmol 1993;52:53–6.
-
Arevalo J. Retinal complications after laser-assisted in situ keratomileusis (LASIK). Curr Opin Ophthalmol 2004;15:184–91.
-
Arroyo J, Postel E, Stone T, McCuen B, Egan K. A matched study of primary scleral buckle placement during repair of posterior segment open globe injuries. Br J Ophthalmol 2003;87:75–8.
-
Avitabile T, Longo A, Lentini G, Reibaldi A. Retinal detachment after silicone oil removal is prevented by 360 degrees laser treatment. Br J Ophthalmol 2008;92:1479–82.
-
Baron A, Michel G, Baron A, Michel G. Preventive cryotherapy of retinal detachment. Bull Soc Ophtalmol Fr 1972;72:635.
-
Belda J, Ruiz-Moreno J, Perez-Santonja J, Alio J. Scleral buckle and corneal ectasia after LASIK. Ophthalmology 2002;109:1950–1.
-
Bergamini F. Laser photocoagulation of the peripheral fundus lesions. Ann Ottalmol Clin Ocul 1995;121:717–19.
-
Binder S, Riss B. Prophylactic treatment of retinal detachment. Klin Monbl Augenheilkd 1981;179:78–80.
-
Bochkareva A, Ivanishko I. Use of photocoagulation for preventing and treating traumatic retinal detachments. Vestn Oftalmol 1981;1:17–19.
-
Boke W, Voigt G. Results of prophylactic cryoretinopexy. Klin Monbl Augenheilkd 1971;159:12–21.
-
Bonnet M, Aracil P. Retinal detachment after preventive treatment with an argon laser. Bull Soc Ophtalmol Fr 1988;88:621–3.
-
Bonnet M, Ducournau D. Retinal detachment following preventive cerclage using argon laser photocoagulation. Bull Mem Soc Fr Ophtalmol 1981;93:58–62.
-
Bonnet M. Rhegmatogenous retinal detachment after prophylactic argon laser photocoagulation. Graefes Arch Clin Exp Ophthalmol 1987;225:5–8.
-
Bonnet M. Retinal detachment after prophylactic argon laser photocoagulation cerclage. Bull Mem Soc Fr Ophtalmol 1982;93:58–62.
-
Brasseur G, Charlin J, Langlois J. The fellow eye in cases of giant tears of the retina. Preventive attitude. Bull Soc Ophtalmol Fr 1985;85:215–18.
-
Bregeat P, Regnault F. Prophylactic treatment of retinal detachment. Annee Ther Clin Ophtalmol 1970;21:345–55.
-
Brihaye-van G, Watillon M. Prevention and therapy of retinal detachment. Arch Ophtalmol Rev Gen Ophtalmol 1972;32:687–704.
-
Brown S, Bloom S. Spontaneous expulsion of a radial miragel scleral buckle. Retina 2004;24:306–7.
-
Chabot J, Bouchet G. Use of the Essel laser photocoagulator in prevention of retinal detachment on localized alterations or holes. Bull Soc Ophtalmol Fr 1970;70:797–808.
-
Chang T. Prophylactic scleral buckle for prevention of retinal detachment following vitrectomy for macular hole. Br J Ophthalmol 1999;83:944–8.
-
Chang T, Hay D. (Untitled). Br J Ophthalmol 2000;84(6).
-
Chauhan D. Failure of prophylactic retinopexy in fellow eyes without a posterior vitreous detachment. Arch Ophthalmol 2006;124:968–71.
-
Condon P, Jampol L, Farber M, Rabb M, Serjeant G. A randomized clinical trial of feeder vessel photocoagulation of proliferative sickle cell retinopathy. II. Update and analysis of risk factors. Ophthalmology 1984;91:1496–8.
-
Cooper H. Spontaneous regression and successful laser prophylaxis in progressive outer retinal necrosis syndrome. Am J Ophthalmol 1996;121:723–4.
-
Coscas G. Prevention of retinal detachment by laser photocoagulation. Bull Soc Ophtalmol Fr 1989;89:653–4.
-
Davis J. Laser photocoagulation prophylaxis for CMV retinal detachments – Reply. Ophthalmology 1998;105:1354–5.
-
Francois P, Madelain F, Constantinides G. Inefficacy of the prevention of retinal detachment by traction using pan-retinal photocoagulations. Bull Soc Ophtalmol Fr 1978;78:615–16.
-
Goezinne F, La Heij EC, Berendschot TT, Gast ST, Liem AT, Lundqvist IL, et al. Low redetachment rate due to encircling scleral buckle in giant retinal tears treated with vitrectomy and silicone oil. Retina 2008;28:485–92.
-
Gross-Jendroska M, Owens S, Flaxel C, Guymer R, Bird A. Treatment to fellow eyes of unilateral retinal pigment epithelial tears with prophylactic laser. Invest Ophthalmol Vis Sci 1996;37(3).
-
Han DPL. Laser photocoagulation in the acute retinal necrosis syndrome. Arch Ophthalmol 1987;105:1051–4.
-
Haut J, Van Effeenterre G, Monin C, Fleury P. Analysis of 8 cases of retinal detachment occurring shortly after argon laser photocoagulation for prevention of retinal detachment. Bull Soc Ophtalmol Fr 1981;81:65–9.
-
Haut J, Allagui M, Lepvrier N, Morel C. Preventive surgical scleral buckling of retinal detachment after severe ocular injuries. Journal Francais d’Opthalmologie 1993;16:668–72.
-
Herzeel R. Prophylactic cryotherapy and cataract surgery. Journal Francais d’Ophtalmologie 1989;12:433–7.
-
Hudde T. Argon laser photocoagulation to prevent rhegmatogenous retinal detachment in patients with acute retinal necrosis (ARN) syndrome. Ophthalmologe 1998;95:473–7.
-
Hudson JR, Kanski JJ. Prevention of aphakic retinal detachment by circumferential cryotherapy. Mod Probl Ophthalmol 1977;18:530–7.
-
Hudson J. The prevention of retinal detachment. Isr J Med Sci 1972;8:1410–14.
-
Itakura HO. Cases of retinal detachment in spite of prophylactic photocoagulation for lattice degeneration. Japanese Journal of Clinical Ophthalmology 2002;56:847–51.
-
Izumi N, Sugimoto M, Matsubara H, Kuze M, Uji Y. Long-term outcome after photocoagulation for Coats disease in infants and children. Rinsho Ganka 2005;59:61–4.
-
Jin D-LD. Preventive photocoagulation treatment of retinal degeneration and tear before LASIK. International Journal of Ophthalmology 2009;9:1175–6.
-
John J. Significance of preventive photocoagulation. Cesk Oftalmol 1978;34:45–50.
-
Johnson D, Nieto J, Ip M. Retinal detachment due to an outer retinal tear following laser prophylaxis for retinoschisis. Arch Ophthalmol 2008;126:1775–6.
-
Kaluzny J. Complications following laser coagulation used in prevention and treatment of retinal detachment. Klin Oczna 1973;43:523–527.
-
Kanski J, Daniel R. Prophylaxis of retinal detachment. Am J Ophthalmol 1975;79:197–205.
-
Kazahaya M. Prophylaxis of retinal detachment. Semin Ophthalmol 1995;10:79–86.
-
Kikuchi M, Iwaki M, Fukao R, Okinami S. Treatment of cytomegalovirus retinitis with ganciclovir and laser photocoagulation. Nippon Ganka Kiyo 1991;42:2350–6.
-
Kosmides P, Ladas I, Koulios P, Dadoush G, Nicolaidou S, Liarikos S, et al. Prophylactic laser photocoagulation following ganciclovir treatment of CMV retinitis. IXth International Conference on AIDS in affiliation with the IVth STD World Congress, 1993, Berlin.
-
Kovacevic D. Long-term results of argon laser retinal photocoagulation for retinal ruptures. Acta Med Croatica 2006;60:149–52.
-
Lippera S, Esente S. Prevention of retinal detachment in the fellow eye with laser photocoagulation. Ann Ottalmol Clin Ocul 1987;113:317–23.
-
Meffert S, Luckie A, Mansour S, Ai E. Laser photocoagulation in cmv retinis as prophylaxis for retinal detachment: A pilot study. Iovs 1995;36:ARVO.
-
Meffert S. Laser photocoagulation prophylaxis for CMV retinal detachments. Ophthalmology 1998;105:1353–5.
-
Mester U, Volker B, Kroll P, Berg P. Complications of prophylactic argon laser treatment of retinal breaks and degenerations in 2,000 eyes. Ophthalmic Surg 1988;19:482–4.
-
Morris R, Kuhn F, Witherspoon CD, Mester V. Prophylactic scleral buckle for prevention of retinal detachment following vitrectomy for macular hole. Br J Ophthalmol 2000;84:673.
-
Ospina L, Lyons C, Matsuba C, Jan J, McCormick A. Argon laser photocoagulation for retinopathy of prematurity: long-term outcome. Eye (Basingstoke) 2005;19:1213–18.
-
Pecoldowa K. Prophylaxis of retinal detachment in cases of retinoschisis using photocoagulation. Ann Ophthalmol 1980;12:199–200.
-
Ranta P. Retinal breaks and detachment after neodymium: YAG laser posterior capsulotomy: Five-year incidence in a prospective cohort. J Cataract Refract Surg 2004;30:58–66.
-
Rauber M, Mester U. Incidence and prophylaxis of retinal detachment following pars plana vitrectomy. Ophthalmologe 2006;103:673–6.
-
Robertson D, Norton E. Cause of failure in prophylactic treatment of retinal breaks. Mod Probl Ophthalmol 1974;12:74–80.
-
Robertson D. 360 degrees prophylactic cryoretinopexy. A clinical and experimental study. Arch Ophthalmol 1979;97:2130–4.
-
Sakaue E. A report of three cases with retinal detachment following prophylactic photocoagulation. Nippon Ganka Kiyo 1970;21:395–403.
-
Sanderson P. To push? Or not to push?: Second stage management in a patient with Stickler syndrome at risk of retinal detachment. J Obstet Gynaecol 2009;29:61–2.
-
Saracco J, Estachy G, Gastaud P, Maymard I. Prophylactic treatment of aphakic retinal detachment by argon laser photocoagulation. Study on 600 cases. Ophthalmologica 1980;181:142–8.
-
Schimkat M, Althaus C, Sundmacher R. Prophylactic argon-laser coagulation for rhegmatogenous retinal detachment in AIDS-patients with cytomegalovirus-retinitis. Invest Ophthalmol Vis Sci 1996;37:1711.
-
Schroeder W. Retinal detachment after prophylactic coagulation. Ophthalmologe 1996;93:144–8.
-
Stein R, Pinchas A, Treister G. Prevention of retinal detachment by a circumferential barrage prior to lens extraction in high-myopic eyes. Ophthalmologica 1972;165:125–36.
-
Sternberg J. Photocoagulation to prevent retinal detachment in acute retinal necrosis. Ophthalmology 1988;95:1389–93.
-
Vallat M, Detre J, Van Coppenolle F, Goudoud M. Limitations of argon laser photocoagulation in the preventive treatment of retinal detachment. Bull Soc Ophtalmol Fr 1986;86:1307–11.
-
Vallat M, Barthe J, Mathon C, Fritsch D, Van Coppenolle F, Detre J, et al. Prevention of retinal detachment. Whom to treat? Ophtalmologie 1989;3:297–8.
-
Yoshizumi M, Kreiger A, Sharp D. Massive peri-retinal proliferation following prophylactic treatment of retinal breaks. Trans Ophthalmol Soc N Z 1983;35:33–6.
Appendix 5 List of studies excluded on basis of lack of details on population alone
Studies evaluating primary prophylaxis interventions for RD, but with insufficient details on population to exclude categorically as including no Stickler syndrome patients.
*Indicates papers stating that a family history of RD was a characteristic of the population or an indication for treatment. The authors of these papers were contacted to ascertain if their sample included any Stickler or Wagner–Stickler syndrome patients.
-
Abujamra S. Prophylactic argon laser photocoagulation. Rev Bras Oftalmol 1984;43:204–10.
-
Avitabile T, Bonfiglio V, Reibaldi M, Torrisi B, Reibaldi A, Avitabile T, et al. Prophylactic treatment of the fellow eye of patients with retinal detachment: a retrospective study. Graefes Arch Clin Exp Ophthalmol 2004;242:191–6.
-
*Boen-Tan T. Analysis of prophylactic laser coagulation of retinal defects and degenerations performed in patients of the Free University of Amsterdam during the period from 1982 to July 1986. Doc Ophthalmol 1987;67:83–8.
-
Boguszakova J. Prophylactic use of the argon laser in the patients with imminent retinal detachments. Cesk Oftalmol 1984;40:272–5.
-
*Boniuk I, Okun E, Johnston GP, Arribas N, Boniuk I, Okun E, et al. Xenon photocoagulation vs. cryotherapy in the prevention of retinal detachment. Mod Probl Ophthalmol 1974;12:81–92.
-
*Chignell A, Shilling J. Prophylaxis of retinal detachment. Br J Ophthalmol 1973;57:291–8.
-
Constantinides G, Francois P, Madelain F. Prevention of retinal detachment. Bull Soc Ophtalmol Fr 1973;73:1213–16.
-
Eliseeva R, Makarskaia N, Malashenkova E, Moiseeva I, Nesterov S, et al. Importance of photocoagulation in the prevention of retinal detachment. Vestn Oftalmol 1978;4:52–7.
-
Folk J. Prophylactic treatment to the fellow eye of patients with phakic lattice retinal detachment: Analysis of failures and risks of treatment. Retina 1990;10:165–9.
-
Franchuk A, Linnik L, Pukhlik E, Ganichenko I, Rasskazova N. Degree of the risk of occurrence of bilateral retinal detachment and the role of laser coagulation in its prevention (late observations). Oftalmol Zh 1981;36:67–70.
-
*Freeman H. Fellow eyes of giant retinal breaks. Trans Am Ophthalmol Soc 1978;76:343–82.
-
Girard P. Long-term follow-up of the unaffected eye following retinal detachment: Study of 1,148 cases. Journal Francais d’Ophtalmologie 1982;5:681–5.
-
Glasspool M, Kanski J. Prophylaxis in giant tears. Trans Ophthalmol Soc U K 1973;93:363–71.
-
Haut J, Monin C, ner-Nedey S, Van Effenterre G. Prevention of bilateralization of idiopathic retinal detachment by treatment with argon laser. Journal Francais d’Opthalmologie 1987;10:717–22.
-
Haut J, Monin C, ner-Nedy S, Van Effenterre G, Lacotte J. Value of the preventive treatment of the 2d eye when the 1st has developed retinal detachment. Bull Soc Ophtalmol Fr 1987;87:1055–6.
-
Haut J. Prophylactic treatment of retinal detachment of the fellow eye. Results of 5 years follow-up of 109 eyes. J Fr Ophtalmol 1991;14:397–404.
-
Karel I. Argon laser photocoagulation in prevention of retinal detachment (author’s transl). Klin Oczna 1981;83:213–15.
-
Karel I. Argon laser coagulation as prophylaxis of retinal detachment. Cesk Oftalmol 1980;36:1–6.
-
Kreissig I, Robert Y. Prevention, in the contralateral eye, of a giant tear ablation. Klin Monbl Augenheilkd 1983;182:121–4.
-
La T, Carelli F, Galassi F. Retinal detachment preventing treatment by argon-laser photocoagulation about retinal tears from one papillar diameter upwards statistical review 3 years. Ann Ottalmol Clin Ocul 1986;112:289–93.
-
Laatikainen L. The fellow eye in patients with unilateral RD: Findings and prophylactic treatment. Acta Ophthalmol 1985;63:546–51.
-
Labrune P, Theron HP, Massin M. Argon laser in the prevention of retinal detachment. Arch Ophtalmol Rev Gen Ophtalmol 1975;35:725–34.
-
Lanzetta L. Prophylaxis of RD with Argon-Laser photocoagulation of regmatogenous retinal lesions. Ann Ottalmol Clin Ocul 1994;120:465–9.
-
*Levin M. Resident-performed prophylactic retinopexy and the risk of retinal detachment. Ophthal Surg Laser Imag 2009;40:120–6.
-
Luo Y. Prophylactic retinopexy for asymptomatic retinal breaks in fellow eyes of rhegmatogenous retinal detachment. Int J Ophthalmol 2008;8:164–6.
-
Manganelli CF. Prophylactic argon laser treatment of retinal detachment in fellow eyes and peripheral degenerations. Ann Ottalmol Clin Ocul 1990;116:651–6.
-
Manys-Kubacka K, Krause A, Finke S, Switek-Tyma B, Kociecki J. Results of prevention of retinal detachment by using photocoagulation. Klin Oczna 1991;93: 315–16.
-
Massin M. Five-year results of prophylaxis of retinal detachment by means of argon laser. Bull Mem Soc Fr Ophtalmol 1979;91:170–5.
-
Mastropasqua L. Treatment of retinal rears and lattice degenerations in fellow eyes in high risk patients suffering retinal detachment: A prospective study. Br J Ophthalmol 1999;83:1046–9.
-
*McPherson A. Management of the fellow eyes of patients with rhegmatogenous retinal detachment. Ophthalmology 1981;88:922–34.
-
*Mody K, Saxena A. Laser photocoagulation and prophylactic treatment for retinal detachment of the lesion. Indian J Ophthalmol 1983;31S:961–3.
-
Okinami S.Matsumura M. Prophylactic treatment of lattice degeneration of the retina without retinal breaks. Nihon Ganka Gakkai 1979;83:2251–4.
-
Okinami S, Matsumura M, Ishigooka H, Tanaka Y. Prophylactic treatment of retinal breaks (author’s transl). Nihon Ganka Gakkai 1980;84:56–62.
-
Okinami S, Matsumura M. Prophylactic treatment of retinal detachment and complemental treatment of retinal detachment surgery by Argon Laser Photocoagulation. Nihon Ganka Gakkai 1984;88:1311–17.
-
*Pollack A. Circumferential argon laser photocoagulation for prevention of retinal detachment. Eye 1994;8:419–22.
-
Pollak A. Argon laser photocoagulation of symptomatic flap tears and retinal breaks of fellow eyes. Br J Ophthalmol 1981;65:469–72.
-
Reuter U. Complications of prophylactic treatment of retinal holes and retinal degenerations by cryopexy in 1000 eyes. Ophthalmologe 1996;93:139–43.
-
Sakaue E. Long-term follow-up study of the treatment with photocoagulation in the prophylaxis of retinal detachment. Nippon Ganka Kiyo 1972;23:303–10.
-
Sinno W. Prophylaxis of retinal detachment with the argon laser. Report on 804 treated eyes. Bull Mem Soc Fr Ophtalmol 1982;93:122–3.
-
Tashiro T. Prophylactic treatment of retinal detachment by photocoagulation. Josai Shika Daigaku Kiyo 1983;12:477–83.
-
Vatne H. Prophylactic argon laser photocoagulation in retinal detachment fellow eyes. Acta Ophthalmol 1982;60:505–10.
-
Wolfensberger T. Prophylactic 360 degrees cryotherapy in fellow eyes of patients with spontaneous giant retinal tears. Ophthalmology 2003;110:1175–7.
-
Yanoff M. Prophylactic cryotherapy of retinal breaks. Ann Ophthalmol 1977;9:283–6.
-
Zayed A, el-Guindi N. The prophylactic repair of retinal breaks using cryopexy and a full thickness segmental scleral buckle. Bull Ophthalmol Soc Egypt 1972;65:395–9.
Appendix 6 Protocol
Can prophylactic surgery reduce the risk of RD and blindness in Stickler syndrome, especially in children?
HTA 09/23/01
13 August July 2009
Title of the project
The clinical effectiveness and safety of prophylactic retinal interventions to reduce the risk of RD and subsequent vision loss in adults and children with Stickler syndrome
Project lead
The University of Sheffield, School of Health and Related Research (ScHARR)
Dr Christopher Carroll, Research Fellow
ScHARR, University of Sheffield, Sheffield, UK
Plain English summary (all references omitted)
Stickler syndrome, also known as hereditary progressive arthro-ophthalmology, is an inherited progressive disorder of the collagen connective tissues. It is indicated by a variety of symptoms and can affect the formation of the eyes, ears, palate, jaw and joints. Signs and symptoms can include short-sightedness, retinal problems, cataracts, blindness, hearing loss, facial abnormalities, including cleft palate, and joint problems. Stickler syndrome is the most common identified, inherited cause of RD in childhood. The exact prevalence of Stickler syndrome is unknown owing to variability in symptoms and under-diagnosis, but has been reported to be approximately 1 in 10,000 in the USA. The actual prevalence of Stickler syndrome may therefore be higher. No figures on prevalence are available for the UK.
There are no agreed diagnostic criteria for Stickler syndrome, but two principal types of Stickler syndrome have been identified. In type 1 Stickler syndrome there appear to be defects in the vitreous phenotype and a mutation in the type II collagen (COL2A1 gene), and, in type 2, defects in the vitreous phenotype but mutation in the type XI collagen (COL11A1 gene). Type 1 is responsible for Stickler syndrome in about 75% of people diagnosed with the condition. Types 1 and 2 both indicate ‘full’ Stickler syndrome. ‘Full’ Stickler syndrome affects the eyes, joints and hearing; patients with type 1 have an increased incidence of cleft abnormalities, and those with type 2 an increased incidence of deafness. The genes responsible for a third type of Stickler syndrome, which also affects the eyes, joints, hearing and mid-line clefting of lip and palate, have yet to be identified. The rate of RD, potentially leading to loss of vision, in patients with Stickler syndrome has been suggested to be as high as 60%. Type 1 Stickler syndrome has been found to have a higher risk of RD than type 2. Whereas RD can occur at any age, it most commonly occurs in adolescence or early adulthood.
Prophylactic retinal interventions aim to reduce the risk of RD and thus the potential for loss of vision. Such interventions include cryotherapy (application of intense cold to create a scar that increases retinal adhesion), scleral buckling (use of a 360-degree silicone band around the eye ball) and laser photocoagulation (light energy from the laser is used to create a scar and thus increase retinal adhesion). There is some evidence that prophylactic interventions may prevent RD in the Stickler syndrome population, thus reducing the risk of blindness. However, these prophylactic interventions are not without the possibility of unwanted side effects or adverse events.
The aim of this review is to systematically evaluate and appraise the safety and clinical effectiveness of prophylactic retinal interventions in comparison with usual care (no treatment or routine care) for the primary prevention of RD in adults and children with Stickler syndrome.
Decision problem
4.1 Purpose of the decision to be made
The assessment will address the question ‘Can prophylactic surgery reduce the risk of RD and blindness in Stickler syndrome, especially in children?’.
4.2 Clear definition of the intervention
Prophylactic retinal interventions aimed at preventing RD. This includes scleral buckling, cryotherapy and laser photocoagulation.
4.3 Place of the intervention in the treatment pathway(s)
This review will focus on the use of retinal interventions as primary prevention for RD. This will be before RD has occurred or if retinal attachment has occurred in one eye only and prophylactic treatment is administered to the non-affected eye.
4.4 Relevant comparators
No treatment/usual care.
4.5 Population and relevant sub-groups
The population for the assessment is children and adults with all types of Stickler syndrome, who have no history of RD or in one eye only.
4.6 Key factors to be addressed
-
Evaluate the clinical effectiveness of prophylactic retinal interventions for prevention of RD among children and adults with Stickler syndrome.
-
Evaluate the safety of prophylactic retinal interventions for prevention of RD.
-
Identify key areas for primary research.
5. Report methods for synthesis of evidence of clinical effectiveness
A review of the evidence for clinical effectiveness will be undertaken systematically following the general principles recommended in the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement, formally QUOROM (quality of reporting of meta-analyses). English and non-English language studies will be included (where translation is available), and there will be no limit by date (although Stickler syndrome was first described in 1965).
5.1 Population
5.1.1 Inclusion criteria
Children and adults diagnosed with Stickler syndrome (any type). There are no universally agreed diagnostic criteria for Stickler syndrome, but it is expected that study participants would demonstrate either the presence of a typical vitreous phenotype (type 1 or 2) and/or COL2A1/COL11A1 mutation. Criteria of diagnosis will be recorded.
5.1.2 Exclusion criteria
Individuals with other syndromes leading to a predisposition to RD, e.g. Wagner–Stickler syndrome, Marfan syndrome.
5.2 Interventions
Any intervention aimed at primary prevention of RD. This includes:
-
cryotherapy
-
laser photocoagulation
-
scleral buckling.
5.3 Comparators
No treatment/usual care (there is no defined usual care for this population).
5.4 Settings
Secondary care.
5.5 Outcomes
5.5.1 Primary outcome
-
Number of RDs (RD) post prophylactic intervention: unilateral or bilateral.
5.5.2 Secondary outcomes
-
Adverse events relating to the intervention.
-
Blindness (by self-assessment, or being registered or legally blind).
-
Time to RD.
-
Number of lesions or retinal tears (a pre-cursor for RD).
5.6 Search strategy
The search strategy will comprise the following main elements:
-
searching of electronic databases
-
contact with experts in the field
-
scrutiny of bibliographies of retrieved papers.
5.6.1 Electronic searches
A comprehensive search will be undertaken to identify systematically both clinical effectiveness and adverse events literature pertaining to prophylactic retinal interventions to prevent RD. Search strategies will be used to identify relevant studies (as specified under the inclusion criteria, above) and systematic reviews/meta-analyses (for identification of additional studies). Searches will not be restricted by language or publication date. An example of the MEDLINE search strategy is shown in Appendix 10.1 (on pp. 57). The aim of the strategy is to identify all studies that report on interventions to prevent RD in either populations reported specifically to comprise participants with Stickler syndrome or populations that may include participants with Stickler syndrome. Only data relating to participants with Stickler syndrome will be extracted and analysed. Authors of studies that do not specify whether or not participants have Stickler syndrome will be contacted, and, if these data are available, they will be included in the analysis.
5.6.2 Databases
The following electronic databases will be searched from inception:
-
MEDLINE (Ovid)
-
MEDLINE in process (Ovid);
-
EMBASE;
-
The Cochrane Library including the Cochrane Systematic Reviews Database, Cochrane Controlled Trials Register, DARE, NHS EED and HTA databases;
-
Science Citation Index (via ISI Web of Science)
-
UKCRN and the National Research Register archive
-
Current Controlled Trials
-
Clinical Trials.gov.
In addition, relevant conference proceedings will be searched, for example: The proceedings of the Annual Meeting of the Association for Research in Vision and Ophthalmology.
5.7 Inclusion criteria
The inclusion criteria are as reported in 5.1–5.5 above. For the review of clinical effectiveness and safety, it is unlikely that RCTs will exist in this area. In the absence of RCT evidence, other study designs will be included. These include prospective and retrospective studies such as cohort studies and case–control studies, and case studies/series.
Titles and abstracts will be examined for inclusion by two reviewers independently. Disagreement will be resolved by consensus, or with reference to a third reviewer when necessary.
5.8 Exclusion criteria
Reviews of primary studies will not be included in the analysis, but will be retained for discussion and identification of additional trials. The following publication types will be excluded from the review: animal models, preclinical and biological studies, narrative reviews, editorials, opinions and those in which insufficient methodological details are reported to allow critical appraisal of study quality. The authors of studies of mixed populations (i.e. individuals with Stickler syndrome combined with non-Stickler syndrome individuals), or unspecified populations undergoing prophylactic intervention for RD, but that do not present separate event data for individuals with Stickler syndrome, will be contacted to ascertain if there are any such data on patients in their sample. If these data are not available, then the study will be excluded and listed under ‘excluded studies’. If these data are available, they will be included in the analysis.
5.9 Data extraction strategy
Data will be extracted independently from all studies by two reviewers using a standardised data extraction form (see Appendix 2). Discrepancies will be resolved by discussion, with reference to a third reviewer if necessary.
5.10 Quality assessment strategy
Owing to the likelihood of inclusion of non-RCT evidence, study quality assessment will be tailored according to the study’s design. This will be undertaken by using an appropriate study design checklist for each study design. Likely study designs include cohort studies, case–control studies and case series or case studies. An example of the latter is included in Appendix 3.
Consideration of study quality will include the following study characteristics:
-
appropriateness of study design
-
recruitment and selection (including inclusion and exclusion criteria)
-
comparability of groups
-
numbers followed up
-
is the length of follow-up appropriate?
-
is the outcome measure appropriate and valid?
-
consideration of confounding variables
-
appropriateness of form of analysis
-
validity of results.
Critical appraisal will be performed by two reviewers independently. Discrepancies will be resolved by discussion, with involvement of a third reviewer when necessary.
5.11 Methods of analysis/synthesis
Data will be tabulated and, if appropriate, meta-analysis will be employed to estimate a summary measure of effect on relevant outcomes based on intention-to-treat analyses. However, it is anticipated that heterogeneity of study designs and interventions, and the type of data available, may mean that it is not appropriate to perform meta-analysis. The likely form of analysis will be narrative synthesis.
All preliminary analyses will be performed based on the intervention and primary outcome, with populations combined (regardless of age group or type of Stickler syndrome). If possible, subgroup analysis will also be performed on these data, according to age group (child or adult) and type of Stickler syndrome, to explore whether different treatment effects or adverse events are apparent in different groups. Where possible, analysis will be performed on secondary outcomes also, such as number of retinal tears.
5.12 Methods for estimating qualify of life
Quality of life will not be assessed in this report.
6. Report methods for synthesising evidence of cost-effectiveness
A review of cost effectiveness literature is not commissioned and therefore will not be undertaken for this review.
7. Expertise in this TAR team
TAR Centre
The ScHARR Technology Assessment Group (ScHARR-TAG) undertakes reviews of the effectiveness and cost-effectiveness of health-care interventions for the NHS R&D HTA programme on behalf of a range of policy-makers in a short timescale, including the National Institute for Health and Clinical Excellence. A list of our publications can be found at www.sheffield.ac.uk/scharr/sections/heds/collaborations/scharr-tag/reports.
Much of this work, together with our reviews for the international Cochrane Collaboration, underpins excellence in health care worldwide.
Team members’ contributions
Christopher Carroll, Research Fellow, ScHARR, has extensive experience in systematic reviews of health technologies. CC will lead the project and undertake the systematic reviewing. He will co-ordinate the review process, protocol development, abstract assessment for eligibility, quality assessment of trials, data extraction, data entry, data analysis and review development of background information and clinical effectiveness.
Diana Papaioannou, Research Associate, ScHARR, has experience in systematic reviews of health technologies. DP will assist CC with the project and undertake the systematic reviewing. She will be involved in protocol development, abstract assessment for eligibility, quality assessment of trials, data extraction, data entry, data analysis and review development of background information and clinical effectiveness.
Angie Rees, Systematic Reviews Information Officer, ScHARR, has extensive experience of undertaking literature searches for the ScHARR Technology Assessment Group systematic reviews and other external projects. AR will be involved in the protocol development and she will develop the search strategy and undertake the electronic literature searches.
Gill Rooney, Project Administrator, will assist in the retrieval of papers and in preparing and formatting the report.
Clinical and expert advisors
Dr Jennifer Evans, Lecturer and member of Cochrane Eyes and Vision Group (CEVG), London School of Hygiene and Tropical Medicine, London, UK.
Mr Alistair Laidlaw, Consultant Ophthalmologist, St Thomas’ Hospital, London, UK.
Mr Richard Sheard, Consultant Ophthalmologist, Royal Hallamshire Hospital, Sheffield, UK.
Dr Martin Snead, Consultant Vitreoretinal Surgeon, Addenbrooke’s Hospital, Cambridge, UK.
8. Competing interests of authors
The authors do not have any competing interests.
Clinical advisors:
-
Jennifer Evans: none
-
Alistair Laidlaw: none
-
Richard Sheard: none.
Martin Snead is the lead applicant of a bid to the National Commissioning Group to provide multi-disciplinary team service for patients and families with Stickler syndrome.
9. Timetable/milestones
The project is expected to run from 4 August 2009 to 31 March 2010.
| Milestone | |
|---|---|
| Draft protocol | 4 August 2009 |
| Final protocol | 14 August 2009 |
| Start review | 7 September 2009 |
| Progress report | 3 March 2010 |
| Assessment report | 31 March 2010 |
10. Protocol appendices
10.1 Appendix 1: Draft MEDLINE search strategy
Database: Ovid MEDLINE(R) <1950 to July Week 2 2009> Search strategy
-
stickler.mp. (248)
-
progressive arthro-opthalmopathol*.tw. (0)
-
progressive arthroopthalmopath*.tw. (0)
-
or/1-3 (248)
-
exp Cryotherapy/ (17,290)
-
exp Laser Coagulation/ (4910)
-
exp Light Coagulation/ (9394)
-
exp Scleral Buckling/ (2075)
-
cryotherap*.tw. (3926)
-
((laser or light) adj2 (coagulat* or photocaogulat*)).tw. (1369)
-
(scleral adj2 (buckl* or encircl*)).tw. (1411)
-
encircling band.tw. (108)
-
or/5-12 (32,125)
-
prophyla*.tw. (92,101)
-
prevent*.tw. (658,496)
-
prevent*.tw. (658,496)
-
ameliorat*.tw. (32,765)
-
or/15-17 (685,228)
-
13 and 18 (1941)
-
4 or 19 (2187)
-
exp RD/ (14,246)
-
exp Retinal Perforations/ (2927)
-
(retinal adj2 (detach* or tear* or break* or perforat*)).tw. (12,260)
-
or/21-23 (19,348)
-
20 and 24 (352)
Appendix 7 Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2009 Checklist
| Section/topic | # | Checklist item | Reported on page # |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both | 1 |
| ABSTRACT | |||
| Structured summary | 2 | Provide a structured summary including, as applicable, background; objectives; data sources; study eligibility criteria, participants and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number | 2–7 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known | 8–10 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS) | 12 |
| METHODS | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g. web address), and, if available, provide registration information including registration number | Appendix 6 |
| Eligibility criteria | 6 | Specify study characteristics (e.g. PICOS, length of follow-up) and report characteristics (e.g. years considered, language, publication status) used as criteria for eligibility, giving rationale | 14–16 |
| Information sources | 7 | Describe all information sources (e.g. databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched | 13–14 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated | Appendix 1 |
| Study selection | 9 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated | 13–14 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g. piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators | 14 |
| Data items | 11 | List and define all variables for which data were sought (e.g. PICOS, funding sources) and any assumptions and simplifications made | Appendix 2 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis | 15–16 |
| Summary measures | 13 | State the principal summary measures (e.g. risk ratio, difference in means) | 16 |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g. I2) for each meta-analysis | 16 |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g. publication bias, selective reporting within studies) | 16 |
| Additional analyses | 16 | Describe methods of additional analyses (e.g. sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified | 16 |
| RESULTS | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram | 16–18 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g. study size, PICOS, follow-up period) and provide the citations | 17,19,23 |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12) | 19–21 |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms) present for each study: (a) simple summary data for each intervention group and (b) effect estimates and confidence intervals, ideally with a forest plot | 21–22,24–28 |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency | N/A |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15) | 21–22 |
| Additional analysis | 23 | Give results of additional analyses, if done ]e.g. sensitivity or subgroup analyses, meta-regression (see Item 16)] | 22 |
| DISCUSSION | |||
| Summary of evidence | 24 | Summarise the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g. health-care providers, users and policy-makers) | 29–35 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g. risk of bias) and at review-level (e.g. incomplete retrieval of identified research, reporting bias) | 30–38 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research | 30–41 |
| FUNDING | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g. supply of data); role of funders for the systematic review | 1–2 |
Appendix 8 Relative estimates of effect based on the published event data
The relative risk or RR measure of relative effect was not reported in any of the published papers, so has been generated for both fixed- and random-effects models by the authors of this report using revman version 5.0 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). The primary outcome was the binary variable of RD or no RD at a single point in time (the one follow-up). The RR is the standard measure of effect used in HTA reports and is used in preference to the odds ratio because, for interventions such as prophylaxis that aim to reduce the chances of events, the odds ratio may be smaller than the RR and this may lead to an overestimation of the effect of the intervention. To calculate the RR, the risk of an event in the intervention group (i.e. the number of RDs in the intervention group, divided by the number of eyes in that group) is divided by the risk of the event in the control group (i.e. the number of RDs in the control group, divided by the number of eyes in that group). These calculations were performed on the data from the principal studies only. Separate analyses were conducted for participants who were exposed to bilateral prophylaxis (prophylaxis in both eyes of an individual) and unilateral prophylaxis (prophylaxis in only one eye of an individual who had already experienced an RD in the primary eye) and, where possible, for both sets of study participants combined. These relative risks were calculated using the event data provided in each of the published papers, but for which only chi-squared analyses or an equivalent had been performed to test for differences between groups (see Chapter 3, Assessment of effectiveness).
Based on the event data reported in the two studies, there was a statistically significant reduction in the risk of RD for those exposed to cryotherapy for bilateral prophylaxis compared with the controls (RR 0.05, 95% CI 0.02 to 0.14, p < 0.0001), as well as for unilateral prophylaxis (RR 0.16, 95% CI 0.05 to 0.47, p = 0.0009). There was also a reduction in the risk of RD for those exposed to laser treatment for bilateral prophylaxis compared with the controls (RR 0.28, 95% CI 0.04 to 1.84, p = 0.19), as well as for those exposed to unilateral prophylaxis (RR 0.13, 95% CI 0.01 to 1.90, p = 0.45), but neither was statistically significant (see table below), possibly because, given the small sample and small number of events in the intervention group (1/10), the study was underpowered or the effect was due to chance. The validity and reliability of these relative estimates of effect, generated using the event data reported for the intervention and control groups of these studies, must be considered in light of the high risk of bias within both studies, especially affecting the comparability between groups.
| Study | Intervention vs control | RD post bilateral and unilateral prophylaxis n/N (eyes) |
RD post bilateral prophylaxis | RD post unilateral prophylaxis |
|---|---|---|---|---|
|
Ang et al. 200833 UK |
360° cryotherapy (N = 155) vs no prophylaxis (N = 222) |
7/155 vs 134/222 RR 0.07 (95% CI 0.04 to 0.16), p < 0.0001 |
4/124 vs 134/222 RR 0.05 (95% CI 0.02 to 0.14), p < 0.0001 |
3/31 vs 134/222 RR 0.16 (95% CI 0.05 to 0.47), p = 0.0009 |
|
Leiba et al. 199634 Israel |
Circumferential and focal laser treatment vs no prophylaxis |
1/10 vs 15/34 RR 0.23 (0.03 to 1.51), p = 0.13 |
1/8 vs 15/34 RR 0.28 (0.04 to 1.84), p = 0.19 |
0/2 vs 15/34 RR 0.13 (0.01 to 1.90), p = 0.45 |
Glossary
- Retina
- The membrane or interior surface at the back of the eyeball.
- Retinal detachment
- A separation of the sensory retina from the retinal pigment epithelium, with an accumulation of vitreous fluid in the potential space between them.
- Retinal pigment epithelium
- The pigmented cell layer just outside the neurosensory retina, which is firmly attached to the underlying choroid and overlying retinal visual cells.
- Vitreous
- A transparent, colourless mass of soft, gelatinous material filling the eyeball behind the lens.
List of abbreviations
- CI
- confidence interval
- DARE
- Database of Abstracts of Reviews of Effects
- df
- degrees of freedom
- GRT
- giant retinal tear
- HTA
- Health Technology Assessment
- NHS EED
- NHS Economic Evaluation Database
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RCT
- randomised controlled trial
- RD
- retinal detachment
- RR
- risk ratio
- UKCRN
- UK Clinical Trials Research Network
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Professor of General Practice, Department of Primary Health Care, University of Oxford Programme Director,
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, University of Liverpool
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Deborah Ashby, Professor of Medical Statistics and Clinical Trials, Queen Mary, Department of Epidemiology and Public Health, Imperial College London
-
Professor Peter Brocklehurst, Director, National Perinatal Epidemiology Unit, University of Oxford
-
Professor John Cairns, Professor of Health Economics, London School of Hygiene and Tropical Medicine
-
Professor Peter Croft, Director of Primary Care Sciences Research Centre, Keele University
-
Professor Jenny Donovan, Professor of Social Medicine, University of Bristol
-
Professor Jonathan Green, Professor and Acting Head of Department, Child and Adolescent Psychiatry, University of Manchester Medical School
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Freddie Hamdy, Professor of Urology, Head of Nuffield Department of Surgery, University of Oxford
-
Professor Allan House, Professor of Liaison Psychiatry, University of Leeds
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norris, Chair in Clinical Trials and Biostatistics, Robertson Centre for Biostatistics, University of Glasgow
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor James Raftery, Chair of NETSCC and Director of the Wessex Institute, University of Southampton
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Martin Underwood, Warwick Medical School, University of Warwick
-
Professor Marion Walker, Professor in Stroke Rehabilitation, Associate Director UK Stroke Research Network, University of Nottingham
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norris Chair in Clinical Trials and Biostatistics, Robertson Centre for Biostatistics, University of Glasgow
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor James Raftery, Chair of NETSCC and Director of the Wessex Institute, University of Southampton
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Martin Underwood, Warwick Medical School, University of Warwick
-
Professor Marion Walker, Professor in Stroke Rehabilitation, Associate Director UK Stroke Research Network, University of Nottingham
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Public contributor
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Public contributor
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Public contributor
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Catherine Moody, Programme Manager, Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Public contributor
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire Country Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Professor Carol Tannahill, Glasgow Centre for Population Health
-
Mrs Jean Thurston, Public contributor
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Public contributor
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Public contributor
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Public contributor
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Sarah Tyson, Senior Research Fellow & Associate Head of School, University of Salford
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Public contributor
-
Mr David P Britt, Public contributor
-
Mr Sankaran ChandraSekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Public contributor
-
Mr Seamus Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor David Taggart, Consultant Cardiothoracic Surgeon, John Radcliffe Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Professor Nicholas James, Professor of Clinical Oncology, School of Cancer Sciences, University of Birmingham
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/ Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Mr John Chapman, Public contributor
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Ms Kylie Gyertson, Oncology and Haematology Clinical Trials Manager, Guy’s and St Thomas’ NHS Foundation Trust London
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Public contributor
-
Dr Martin Shelly, General Practitioner, Silver Lane Surgery, Leeds
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Public contributor
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Public contributor
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Public contributor
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Expert Advisory Network
-
Professor Douglas Altman, Professor of Statistics in Medicine, Centre for Statistics in Medicine, University of Oxford
-
Professor John Bond, Professor of Social Gerontology & Health Services Research, University of Newcastle upon Tyne
-
Professor Andrew Bradbury, Professor of Vascular Surgery, Solihull Hospital, Birmingham
-
Mr Shaun Brogan, Chief Executive, Ridgeway Primary Care Group, Aylesbury
-
Mrs Stella Burnside OBE, Chief Executive, Regulation and Improvement Authority, Belfast
-
Ms Tracy Bury, Project Manager, World Confederation of Physical Therapy, London
-
Professor Iain T Cameron, Professor of Obstetrics and Gynaecology and Head of the School of Medicine, University of Southampton
-
Professor Bruce Campbell, Consultant Vascular & General Surgeon, Royal Devon & Exeter Hospital, Wonford
-
Dr Christine Clark, Medical Writer and Consultant Pharmacist, Rossendale
-
Professor Collette Clifford, Professor of Nursing and Head of Research, The Medical School, University of Birmingham
-
Professor Barry Cookson, Director, Laboratory of Hospital Infection, Public Health Laboratory Service, London
-
Dr Carl Counsell, Clinical Senior Lecturer in Neurology, University of Aberdeen
-
Professor Howard Cuckle, Professor of Reproductive Epidemiology, Department of Paediatrics, Obstetrics & Gynaecology, University of Leeds
-
Professor Carol Dezateux, Professor of Paediatric Epidemiology, Institute of Child Health, London
-
Mr John Dunning, Consultant Cardiothoracic Surgeon, Papworth Hospital NHS Trust, Cambridge
-
Mr Jonothan Earnshaw, Consultant Vascular Surgeon, Gloucestershire Royal Hospital, Gloucester
-
Professor Martin Eccles, Professor of Clinical Effectiveness, Centre for Health Services Research, University of Newcastle upon Tyne
-
Professor Pam Enderby, Dean of Faculty of Medicine, Institute of General Practice and Primary Care, University of Sheffield
-
Professor Gene Feder, Professor of Primary Care Research & Development, Centre for Health Sciences, Barts and The London School of Medicine and Dentistry
-
Mr Leonard R Fenwick, Chief Executive, Freeman Hospital, Newcastle upon Tyne
-
Mrs Gillian Fletcher, Antenatal Teacher and Tutor and President, National Childbirth Trust, Henfield
-
Professor Jayne Franklyn, Professor of Medicine, University of Birmingham
-
Mr Tam Fry, Honorary Chairman, Child Growth Foundation, London
-
Professor Fiona Gilbert, Consultant Radiologist and NCRN Member, University of Aberdeen
-
Professor Paul Gregg, Professor of Orthopaedic Surgical Science, South Tees Hospital NHS Trust
-
Bec Hanley, Co-director, TwoCan Associates, West Sussex
-
Dr Maryann L Hardy, Senior Lecturer, University of Bradford
-
Mrs Sharon Hart, Healthcare Management Consultant, Reading
-
Professor Robert E Hawkins, CRC Professor and Director of Medical Oncology, Christie CRC Research Centre, Christie Hospital NHS Trust, Manchester
-
Professor Richard Hobbs, Head of Department of Primary Care & General Practice, University of Birmingham
-
Professor Alan Horwich, Dean and Section Chairman, The Institute of Cancer Research, London
-
Professor Allen Hutchinson, Director of Public Health and Deputy Dean of ScHARR, University of Sheffield
-
Professor Peter Jones, Professor of Psychiatry, University of Cambridge, Cambridge
-
Professor Stan Kaye, Cancer Research UK Professor of Medical Oncology, Royal Marsden Hospital and Institute of Cancer Research, Surrey
-
Dr Duncan Keeley, General Practitioner (Dr Burch & Ptnrs), The Health Centre, Thame
-
Dr Donna Lamping, Research Degrees Programme Director and Reader in Psychology, Health Services Research Unit, London School of Hygiene and Tropical Medicine, London
-
Professor James Lindesay, Professor of Psychiatry for the Elderly, University of Leicester
-
Professor Julian Little, Professor of Human Genome Epidemiology, University of Ottawa
-
Professor Alistaire McGuire, Professor of Health Economics, London School of Economics
-
Professor Neill McIntosh, Edward Clark Professor of Child Life and Health, University of Edinburgh
-
Professor Rajan Madhok, Consultant in Public Health, South Manchester Primary Care Trust
-
Professor Sir Alexander Markham, Director, Molecular Medicine Unit, St James’s University Hospital, Leeds
-
Dr Peter Moore, Freelance Science Writer, Ashtead
-
Dr Andrew Mortimore, Public Health Director, Southampton City Primary Care Trust
-
Dr Sue Moss, Associate Director, Cancer Screening Evaluation Unit, Institute of Cancer Research, Sutton
-
Professor Miranda Mugford, Professor of Health Economics and Group Co-ordinator, University of East Anglia
-
Professor Jim Neilson, Head of School of Reproductive & Developmental Medicine and Professor of Obstetrics and Gynaecology, University of Liverpool
-
Mrs Julietta Patnick, Director, NHS Cancer Screening Programmes, Sheffield
-
Professor Robert Peveler, Professor of Liaison Psychiatry, Royal South Hants Hospital, Southampton
-
Professor Chris Price, Director of Clinical Research, Bayer Diagnostics Europe, Stoke Poges
-
Professor William Rosenberg, Professor of Hepatology and Consultant Physician, University of Southampton
-
Professor Peter Sandercock, Professor of Medical Neurology, Department of Clinical Neurosciences, University of Edinburgh
-
Dr Philip Shackley, Senior Lecturer in Health Economics, Sheffield Vascular Institute, University of Sheffield
-
Dr Eamonn Sheridan, Consultant in Clinical Genetics, St James’s University Hospital, Leeds
-
Dr Margaret Somerville, Director of Public Health Learning, Peninsula Medical School, University of Plymouth
-
Professor Sarah Stewart-Brown, Professor of Public Health, Division of Health in the Community, University of Warwick, Coventry
-
Dr Nick Summerton, GP Appraiser and Codirector, Research Network, Yorkshire Clinical Consultant, Primary Care and Public Health, University of Oxford
-
Professor Ala Szczepura, Professor of Health Service Research, Centre for Health Services Studies, University of Warwick, Coventry
-
Dr Ross Taylor, Senior Lecturer, University of Aberdeen
-
Dr Richard Tiner, Medical Director, Medical Department, Association of the British Pharmaceutical Industry
-
Mrs Joan Webster, Consumer Member, Southern Derbyshire Community Health Council
-
Professor Martin Whittle, Clinical Co-director, National Co-ordinating Centre for Women’s and Children’s Health, Lymington