Notes
Article history
The research reported in this issue of the journal was commissioned by the HTA programme as project number 08/25/02. The contractual start date was in May 2009. The draft report began editorial review in April 2010 and was accepted for publication in September 2010. As the funder, by devising a commissioning brief, the HTA programme specified the research question and study design. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
DWP has been an occasional advisor to the Nucleus™ Corporation (parent company of Entific® Medical Systems/Cochlear™), has had several research grants from the Nucleus Corporation, and runs a surgical training course annually for the company for which he is paid a fee.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2011. This work was produced by Colquitt et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2011 Queen’s Printer and Controller of HMSO
Chapter 1 Aim and background
Aim
The aim of this report is to synthesise the evidence assessing the clinical effectiveness and cost-effectiveness of bone-anchored hearing aids (BAHAs) for people who are bilaterally deaf.
The evaluation will consider BAHAs compared with conventional hearing aids, ear surgery and the unaided condition, and the use of unilateral or bilateral BAHAs. If the systematic review of economic evaluations shows that there are no appropriate good-quality economic evaluations, an economic model relevant to the UK setting is to be developed. The study aims to identify areas where further research is required.
Description of underlying health problem
Deafness and hearing loss can be described as mild, moderate, severe or profound (Table 1), and are defined according to the quietest sound a person can hear across a range of frequencies. The greater this threshold is, measured in units of decibels hearing level (dB HL), the worse the hearing loss is. Hearing loss that occurs in both ears is described as bilateral, and may be the same or different in each ear. Single-sided (unilateral) deafness is excluded from this evaluation.
| Audiometric descriptor1 | Hearing threshold level (dB)a |
|---|---|
| Mild hearing loss | 20–40 |
| Moderate hearing loss | 41–70 |
| Severe hearing loss | 71–95 |
| Profound hearing loss | > 95 |
Normal hearing occurs when sound waves travel through the external, middle and inner ear and are translated into nerve impulses, which are interpreted by the brain. The external ear acts as a sound-collecting funnel, with the passing sound waves causing the eardrum to vibrate. These vibrations (sound waves) are then passed on by the eardrum to three small bones (ossicles) in the middle ear, which amplify the vibrations and pass them on to the cochlea (inner ear). Movement of tiny hair-like cells in the fluid-filled cochlea convert the sound waves into nerve impulses, which are transmitted to the brain by the auditory nerve. Disturbances at any point in this pathway can cause hearing loss.
The main types of hearing loss are sensorineural loss and conductive loss,2 and the presence of both types is referred to as mixed hearing loss. Sensorineural hearing loss (SNHL) is caused by damage to the outer or inner hair cells of the cochlea or the auditory nerve. 3 SNHL involves a loss of both the ability to detect quiet sound (acuity) and the ability to make sense of sound (discrimination) and is the most common form of hearing loss in more developed countries4 (approximately 90%). 3,5 It is often attributed to natural deterioration with ageing and prolonged noise exposure. 3 SNHL can have almost any frequency configuration and extent (from mild to profound).
Conductive hearing loss (CHL) involves a loss of acuity only and is the result of damage or blockage in the outer or middle ear due to a variety of causes such as infection, fluid (otitis media with effusion), ostosclerosis (growth of extra bone tissue) or trauma/damage to the eardrum. CHL may be caused by congenital abnormalities, which can affect any or all of the outer and middle ear structures,6 or may be part of a syndrome such as Treacher Collins, Crouzon, branchio-oto-renal or Goldenhar syndrome. 7 It can also occur following mastoid surgery or in Down syndrome8,9 (although SNHL can also occur with Down syndrome). The most common cause of CHL in children is otitis media with effusion and although this hearing loss is often only temporary,10 it may be permanent in a very small number of cases. 6 CHL is most commonly of a flat frequency configuration, and its maximum extent can only be that of the contribution of the conductive pathway to audition [40–50 decibels (dB)].
A less common type of hearing loss is neural deafness, caused by the absence of, or damage to, the auditory nerve. This type of hearing loss does not benefit from sound amplification as the nerve is unable to pass on any or enough sound information,2 and is therefore not considered further in this review.
The majority of people with hearing loss benefit from conventional air conduction hearing aids (ACHAs). These aids receive, amplify and transmit sound down the ear canal to the cochlea and are fitted behind the ear, in the ear or in the ear canal. However, people with an obstructed conduction process (via air) are unable to benefit fully or at all from ACHAs. For those with an infected ear, ACHAs may prevent adequate ventilation of the ear and thereby exacerbate the infection, whereas congenital abnormality or atresia of the pinna (external ear) may prevent an ACHA being fitted. 6 Some people with CHL can be treated with surgery in the form of repairing perforated eardrums, reconstruction or stapedectomy (surgical removal of the stapes ossicle of the middle ear),3,6,10 but for those for whom surgery is not an option, bone conduction hearing aids (BCHAs) may be an alternative.
Conventional BCHAs use a vibrator pressed firmly against the skin of the skull via a spring headband or special spectacles to conduct sound directly through the bone to the cochlea of the inner ear, bypassing the impaired or diseased external or middle ear. 11 However, BCHAs are associated with a number of drawbacks: they are uncomfortable to wear owing to the pressure needed to apply the device effectively and can cause skin irritations and headaches; they have poor aesthetics and are difficult to hide; and speech recognition can be affected by insecure positioning or shifting of the transducer and by the attenuation of sound by tissue layers between the vibrator and the skull. 12–14
An alternative type of hearing aid which utilises bone conduction (BC) is the BAHA, where contact with the skull is maintained by a surgical implant. It should be noted that the term ‘Baha®’ is a registered trademark of Cochlear Bone Anchored Solutions AB, a Cochlear™ group company; however, reference to BAHA in this report applies to all such BC devices and not to the manufacturer, supplier or trade name. BAHAs are used to help people with conductive or mixed hearing loss who cannot benefit from conventional hearing aids or from ear surgery, or in some cases as an alternative to surgery (stapedectomy). BAHAs have undergone a number of developments since they were first introduced in 1977, and are discussed in further detail in Description of BAHAs.
Epidemiology of hearing loss
Although an understanding of the epidemiology of hearing loss is key to the assessment of the clinical effectiveness and cost-effectiveness of a technology and to the subsequent development of guidance on its provision and use, limited research has been undertaken. 15 Assessments of the epidemiology of hearing loss have tended to focus on retrospective cohort studies of its prevalence and have been limited in the type of hearing loss considered. They often use surrogate measures of prevalence such as use of health services, rather than population-based studies, resulting in the potential to underestimate needs. In addition, studies have tended to be affected by differences in the methods for assessing and diagnosing hearing loss (e.g. self-assessment) and variations in definitions and classification of hearing loss (including arbitrary nature of thresholds). Several studies have been undertaken within the UK and elsewhere and those most relevant to this evaluation are discussed in the following section. As there is little evidence focusing on CHL, the epidemiology of hearing loss in general is discussed to give some context to this evaluation.
Prevalence of hearing loss
Children
The prevalence of hearing loss in children has been assessed in several population-based surveys within the UK and elsewhere. These studies have shown variations in prevalence, with rates differing depending on the type of loss, its severity, temporal factors and its aetiology. Within the UK a series of retrospective studies (population surveys) has been undertaken by the Medical Research Council Institute for Hearing Research, providing prevalence data for several cohorts within cities, regions and nationally. In a retrospective survey of providers of health and educational services to children with a hearing loss conducted in 1995, Fortnum and Davis16 assessed the prevalence of permanent hearing loss [≥ 40 dB HL averaged over 0.5, 1.0, 2.0 and 4.0 kilohertz (kHz)] in children resident in the Trent Health Region (UK) who were born between 1985 and 1990. They found an overall prevalence of permanent hearing loss of 1.33 cases per 1000 live births. For those with severe hearing loss (70–94 dB HL) the prevalence was 0.28 per 1000 and for profound hearing loss (≥ 95 dB HL) 0.31 per 1000. Congenital hearing loss was more prevalent than acquired hearing loss, accounting for 1.12 cases per 1000 live births (Table 2). Fortnum and Davis found that SNHL was more common than purely conductive loss. 16 The prevalence of congenital and acquired SNHL (≥ 40 dB HL) was 1.27 per 1000 live births compared with 1.33 per 1000 for all hearing losses. The higher prevalence of impairments that were congenital compared with acquired, and of sensorineural compared with conductive impediments, was evident for different levels of severity of hearing loss (see Table 2).
| Study | Hearing loss (kHz) | ||||
|---|---|---|---|---|---|
| All | Moderate | Severe | Profound | ||
| Fortnum and Davis 199716 | (≥ 40 dB HL) | (40–69 dB HL) | (70–94 dB HL) | (≥ 95 dB HL) | |
|
Retrospective cohort survey Population: children born in Trent health region 1985–90, survey 1995 Outcome: prevalence per 1000 live births (95% CI) |
All cases | ||||
| Congenital and acquired | 1.33 (1.22 to 1.45) | 0.74 (0.65 to 0.83) | 0.28 (0.23 to 0.35) | 0.31 (0.26 to 0.37) | |
| Congenital | 1.12 (1.01 to 1.23) | 0.64 (0.56 to 0.73) | 0.23 (0.19 to 0.29) | 0.24 (0.20 to 0.30) | |
| Permanent SNHL | |||||
| Congenital and acquired | 1.27 (1.16 to 1.39) | 0.68 (0.61 to 0.78) | 0.28 (0.23 to 0.34) | 0.31 (0.26 to 0.37) | |
| Congenital | 1.06 (0.96 to 1.17) | 0.59 (0.52 to 0.68) | 0.23 (0.18 to 0.28) | 0.24 (0.20 to 0.30) | |
| Fortnum et al. 200117 | (≥ 40 dB HL) | (41–70 dB HL) | (71–95 dB HL) | (> 95 dB HL) | |
|
Retrospective cohort survey Population: children born in the UK 1980–95, survey 1998 Outcome: prevalence per 1000 live births (95% CI) |
Children aged 3 years |
0.91 (0.85 to 0.98) 1.07 (1.03 to 1.12) |
0.45 (0.40 to 0.50) 0.60 (0.54 to 0.66) |
0.20 (0.17 to 0.24) 0.22 (0.21 to 0.24) |
0.26 (0.22 to 0.29) 0.27 (0.26 to 0.29) |
| Children aged 9–16 years |
1.65 (1.62 to 1.68) 2.05 (2.02 to 2.08)a |
0.89 (0.86 to 0.91) 1.21 (1.18 to 1.24)a |
0.35 (0.33 to 0.36) 0.41 (0.40 to 0.42)a |
0.39 (0.38 to 0.41) 0.44 (0.43 to 0.44)a |
|
| Fortnum et al. 200218 | (≥ 40 dB HL) | (41–70 dB HL) | (71–95 dB HL) | (> 95 dB HL) | |
|
Retrospective cohort survey Population: children born in the UK 1980–95, survey 1998 Outcome: percentage of total study population with aetiology |
Total known aetiology | 50.6 | 47.7 | 50.9 | 57.5 |
| Genetic | 20.2 | 18.3 | 20.8 | 24.1 | |
| Syndromal | 9.5 | 11.6 | 6.6 | 7.4 | |
| Prenatal | 4.1 | 2.4 | 5.2 | 6.8 | |
| Perinatal | 8.0 | 7.4 | 11.4 | 6.7 | |
| Postnatal | 6.9 | 5.3 | 6.3 | 11.3 | |
| Other | 1.9 | 2.8 | 0.6 | 1.2 | |
| MacAndie et al. 200319 | (≥ 40 dB HL) | ||||
|
Retrospective cohort survey Population: children born in the UK 1985–94, survey 2000 Outcome: prevalence per 1000 live births |
All | 1.23 | |||
| Congenital | 1.09 | ||||
Fortnum and colleagues17 undertook a similar study to estimate the prevalence of permanent bilateral hearing loss (greater than 40 dB averaged over pure-tone threshold of 0.5, 1.0, 2.0 and 4.0 kHz in the better hearing ear) in children born between 1980 and 1995 who were resident in the UK in 1998. The retrospective survey of all health and educational providers for hearing-impaired children identified 17,160 cases, finding a prevalence of 0.91 per 1000 live births for children aged 3 years and a prevalence of 1.65 per 1000 for those aged 9–16 years (data for children aged 4–8 years were not provided numerically). Adjustment of these rates for underascertainment resulted in an increase to 1.07 and 2.05 per 1000 live births for children aged 3 years and 9–16 years, respectively. When comparing the prevalence by the severity of hearing loss, rates were higher among those children with a moderate loss than among those with a severe or profound loss. Some 0.45 per 1000 children aged 3 years and 0.89 per 1000 children aged 9–16 years had a hearing loss of 41–70 dB HL compared with 0.20 per 1000 and 0.35 per 1000 for children aged 3 years and 9–16 years, respectively, for a loss of 71–95 dB HL (severe) and 0.26 per 1000 and 0.39 per 1000 for children aged 3 years and 9–16 years, respectively, for a loss of ≥ 95 dB HL (profound).
Comparisons between the studies in the Trent health region and the UK showed limited difference between the prevalence rates when cohorts were matched for age. In the Trent health region the overall prevalence rate was 1.33 per 1000 [95% confidence interval (CI) 1.22 to 1.45] for children born in the period 1985–90 compared with 1.44 per 1000 (95% CI 1.41 to 1.48) for children born in the period 1988–93 in the UK survey. Similar prevalence rates were evident when comparing the different severities of hearing loss. In the Trent health region prevalence rates were 0.74 (95% CI 0.65 to 0.83) for moderate, 0.28 (95% CI 0.23 to 0.35) for severe and 0.31 (95% CI 0.26 to 0.37) for profound hearing loss compared with 0.80 (95% CI 0.77 to 0.82) for moderate, 0.29 (95% CI 0.28 to 0.31) for severe and 0.34 (95% CI 0.32 to 0.35) for profound hearing loss in the UK study.
Fortnum and colleagues18 assessed the annual prevalence of hearing loss and profound hearing loss among children born between 1980 and 1995 in the UK to see if there were any temporal patterns. They found that the prevalence of hearing loss increased from 634 cases in 1980 to 1342 cases in 1987, declining to 669 cases in 1995. The proportion of children with profound hearing loss has ranged from 31.5% of children with hearing loss in 1980 to 20.4% in 1989.
Fortnum and colleagues18 compared the aetiology for the different levels of severity of hearing loss. It was evident that for around 50% of all children with hearing loss, the cause was not known or specified. For all hearing losses, 20.2% were genetic, 9.5% syndromal, 8.0% perinatal, 6.9% postnatal and 4.1% prenatal. When comparing the aetiology for the different severities of hearing loss it was evident that there were significant differences. Fortnum and colleagues found that children with moderate hearing loss were more likely to have an unknown aetiology than those with severe loss (moderate 52.3%, severe 49.1%, profound 42.5%, p < 0.001) or a syndromal aetiology (moderate 11.6%, severe 6.6%, profound 7.4%, p < 0.001). Also, severely impaired children were more likely to have a perinatal cause (moderate 7.4%, severe 11.4%, profound 6.7%, p < 0.001) and profoundly impaired children to have a genetic (moderate 18.3%, severe 20.8%, profound 24.1%, p < 0.001), prenatal (moderate 2.4%, severe 5.2%, profound 6.8%, p < 0.001) or postnatal aetiology (moderate 5.3%, severe 6.3%, profound 11.3%, p < 0.001) than the other groups.
Similar prevalence rates for hearing loss were shown by MacAndie and colleagues19 in a retrospective study in Greater Glasgow (UK). The study focused on children born between 1985 and 1994 who were identified from the Educational Audiology database. Of the 105,517 live births in Greater Glasgow between 1985 and 1994, 130 children had a permanent hearing loss (≥ 40 dB HL), which equates to an incidence of 1.23 cases per 1000 live births. Some 116 children had a congenital hearing loss (1.09 per 1000 live births), with only 14 children having a hearing loss that was postnatally acquired or progressive. When assessing the aetiology of bilateral hearing loss, MacAndie and colleagues found that 31% of children had a family history of congenital hearing loss, 12% craniofacial syndrome, 15% had an admission to neonatal intensive care unit that may have contributed to their hearing loss, 7% had a postnatal infection, 3% a prenatal infection and 28% had an unknown or uncategorised aetiology.
Adults
Davis20 surveyed the prevalence of hearing loss among a cohort of 35,330 people within four cities in the UK between 1980 and 1986. The study found that 16.1% of people aged 17–80 years had mild (≥ 25 dB HL), 3.9% moderate (≥ 45 dB HL) and 1.1% severe (≥ 65 dB HL) hearing loss in both ears. The prevalence of bilateral hearing loss was shown to increase with age. Prevalence rates for moderate bilateral loss increased from 0.2% for those aged 17–30 years to 1.1% for 31- to 40-year-olds, 1.7% for 41- to 50-year-olds, 4.0% for 51- to 60-year-olds, 7.4% for 61- to 70-year-olds and 17.6% for 71- to 80-year-olds. Similar variations by age group were evident for those people with a severe bilateral hearing loss, although the prevalence rates were approximately a quarter of those for people with moderate hearing loss. For those with severe bilateral loss the rates varied from less than 0.1% for those aged 17–30 years to 0.7% for 31- to 40-year-olds, 0.3% for 41- to 50-year-olds, 0.9% for 51- to 60-year-olds, 2.3% for 61- to 70-year-olds and 4.0% for 71- to 80-year-olds.
Davis20 assessed the effects of age, sex, occupational group and occupational noise on hearing loss through logistic regression analysis. The prevalence of hearing loss (≥ 45 dB HL) was shown to significantly increase with a person’s age [odds ratios (OR) 7.6 (p < 0.05) for 41–50 years; 17.3 (p < 0.005) for 51–60 years; 32.1 (p < 0.005) for 61–70 years; 95.4 (p < 0.005) for 71–80 years], occupation [OR 2.2 (p < 0.005) for manual occupations] and exposure to occupational noise [OR 2.3 (p < 0.01) for ≥ 91 dB(A) equivalent continuous sound level (Leq].
Lee and colleagues21 assessed the prevalence of self-reported hearing loss among adults (107,100 White and 17,904 African-American people aged ≥ 18 years) in the USA using the National Centre for Health Statistics National Health Interview Survey between 1986 and 1995 (annual survey of approximately 50,000 civilian households). The annual age-adjusted rates for ‘some hearing impairment’ and ‘severe bilateral impairment’ were higher among Whites than among African Americans. The rates for ‘some hearing impairment’ ranged from 11.0% to 12.7% for Whites and from 5.9% to 8.5% for African Americans. The prevalence of ‘severe bilateral impairment’ was lower for both groups, with rates ranging from 0.7% to 1.1% for Whites and from 0.1% to 0.5% for African Americans. Although the rates varied temporally during the 10 years, there were no significant upward or downward trends in prevalence.
Unsurprisingly, analysis of the prevalence of ‘any hearing impairment’ among different age groups showed that the older age groups had a higher prevalence of impairment. This was evident for both the White and African American groups, although the prevalence was higher for all age groups among Whites than among African Americans. Comparison of the prevalence of impairment for the different age groups during the 10-year period showed limited variation for all the age groups in the White population and among the 18–39, 40–49, 50–59 and 60–69 years age groups in the African-American population. In contrast, the 70–79 and ≥ 80 years age groups in the African-American population showed considerable variation, although there were no discernible trends in the prevalence data.
Estimates of burden of hearing loss in England and Wales
Using the studies of the prevalence of hearing loss and population estimates for England and Wales,16,17,19,22,23 it is possible to provide a provisional estimate of the burden of bilateral hearing loss in England and Wales (Table 3). These estimates should be interpreted with caution owing to the differences in the nature of the studies and the classifications of hearing loss used. Estimates of the prevalence of bilateral hearing loss among children in England and Wales indicate that there could be between 900 and 1000 children in each annual birth cohort with a bilateral hearing loss of ≥ 40 dB HL. Although the majority of children would have a moderate hearing loss of 41–70 dB HL, around 400 would have either a severe (71–95 dB HL) or profound loss (≥ 95 dB HL). It was evident that the majority of impairments among children would be congenital in origin, accounting for hearing impairment in around 750–775 children per annual birth cohort in England and Wales. 16,19 Most of these congenital hearing impairments are thought to be permanent sensorineural (approximately 730 per annual birth cohort). 16 It was estimated that among adults in England and Wales there would be around 1.6 million people aged 17–80 years with a hearing loss of ≥ 45 dB, with around a quarter of these having a loss of ≥ 65 dB (Table 4). Around 60% (275,000) of those with a hearing loss ≥ 65 dB HL would be aged 60–80 years.
| Severity of hearing loss | Range of prevalence of bilateral hearing loss (rate per 1000 live births)16,17 | Range of estimated number of children with a bilateral hearing loss in England and Walesa |
|---|---|---|
| Moderate (41–70 dB) | 0.74–0.80 | 511–552 |
| Severe (71–95 dB) | 0.28–0.29 | 193–200 |
| Profound (≥ 95 dB) | 0.31–0.34 | 214–235 |
| All impairments (≥ 40 dB) | 1.33–1.44 | 918–994 |
| Age group (years) | Population in England and Wales (mid-2008) (000s)23 | Prevalence of bilateral hearing loss (%)20 | Estimated number of people with hearing loss in England and Wales | ||
|---|---|---|---|---|---|
| Severity of loss | Severity of loss | ||||
| ≥ 45 dB | ≥ 65 dB | ≥ 45 dB | ≥ 65 dB | ||
| 17–30 | 10,186.1 | 0.2 | 0.1 | 20,372 | 10,186 |
| 31–40 | 7516.0 | 1.1 | 0.7 | 82,676 | 52,612 |
| 41–50 | 7907.4 | 1.7 | 0.3 | 134,426 | 23,722 |
| 51–60 | 6563.6 | 4.0 | 0.9 | 262,544 | 59,072 |
| 61–70 | 5411.4 | 7.4 | 2.3 | 400,444 | 124,462 |
| 71–80 | 3718.8 | 17.6 | 4.0 | 654,509 | 148,752 |
| Total | 41,303.3 | 3.9 | 1.1 | 1,610,829 | 454,336 |
Impact of hearing loss
For those people with hearing loss who identify with the ‘Deaf community’ (people whose first or preferred language is British Sign Language), being deaf is seen as part of their total identity and not as a deficiency. 24 However, deafness and hearing loss can have a profound effect on individuals and have been associated with a range of negative consequences, including educational and employment disadvantages, social isolation and stigmatisation. 25,26 According to a report by the World Health Organization, hearing loss is the second leading cause globally of ‘years lived with disability’ and has a larger non-fatal burden than alcohol use disorders. 26 The impact of hearing loss is influenced by the severity of the loss and age at onset. Deafness present at birth or during early childhood (the pre-lingual period) has considerable effects on speech acquisition and cognitive and psychosocial development. 27 Deafness acquired post-lingually requires the individual to adopt new communication strategies and often an entirely different lifestyle,24 and can result in isolation and compromised quality of life (QoL). 27 Hearing loss affects not only individuals, but also the people around them such as family and co-workers. 28 These people have to put more effort into communication with the individual, for example speaking more slowly and with better articulation, turning their face to allow lip-reading and moving closer. 28 As a consequence, there is a risk that people will make less contact and the individual will become more isolated.
Early hearing loss delays the development of basic auditory skills, including auditory detection, discrimination, recognition, comprehension and attention, which negatively affects the child’s ability to learn and use an auditory–oral language system. 29 Difficulties with the rules of language, the meaning of words and the use of language in social contexts lead to comprehension, expressive communication and learning problems, and can result in reduced academic achievement. 29 In contrast, a number of studies have shown that children with hearing loss who are raised by parents with hearing loss often have psychosocial advantages over those who are born to hearing families, as they grow up in an environment where communication is naturally dependent on visual, not oral, cues. 24
A recent study used both parent-report and videotaped data from 116 severely and profoundly deaf and 69 hearing preschool-age children, and demonstrated that hearing-impaired children displayed more behaviour problems and greater difficulties with oral language, parent–child communication and sustained attention than hearing children. 30 High rates of behavioural and emotional problems and a high rate of social maladjustment according to general population norms were also found by a cross-sectional study of 84 children and adolescents (age 2–18 years) attending schools for the deaf. 31 According to parents’ descriptions, children were socially isolated and not participating in structured activities. Similar results were demonstrated by a study in Upper Austria to evaluate mental health and QoL in a representative sample of deaf pupils with a bilateral impairment of at least 40 dB, from both mainstream schools and a school for the hearing impaired. 32 Using the strengths and difficulties questionnaire,33 deaf children scored higher for conduct, emotional and peer problems than children from a normative sample, though differences were less marked for hyperactivity/inattention. Whereas parents of deaf children had a generally positive view of their child’s QoL, deaf children provided a more complex picture, stressing areas of dissatisfaction.
A non-systematic review of mild bilateral hearing loss described studies demonstrating that many children with even mild hearing loss do not perform at expected academic levels, especially in the areas of vocabulary, reading comprehension and language use, and that they expend more effort in listening to speech in quiet and in the presence of background noise than children with normal hearing. 34 The author suggests that children with even a relatively mild degree of bilateral hearing loss may exert more energy than their normal-hearing peers to listen in a classroom setting, leaving them with less energy or attention capacity for processing what they hear, taking notes and other activities required of school children. 34
There is evidence to suggest that the effects of hearing loss in adults differ according to age group. For example, a large Norwegian health-screening survey examined the association between hearing loss, measured by pure-tone audiometry, and self-report symptoms of mental health and well-being in a normal population sample of over 50,000 people aged between 20 and 101 years. 35 The survey found a moderate but clear effect of hearing loss on anxiety, depression, self-esteem and well-being among young and middle-aged people. The strongest effects were found for depression and self-esteem among young men; however, the effects were almost absent among elderly people.
These findings are supported by a recent cross-sectional study which used the internet to determine both hearing status and self-reported psychosocial health in 1511 adults aged 18–70 years. 36 Hearing status was assessed using the ‘National Hearing test’, an online speech-in-noise screening test (also available via telephone) that has been implemented in the UK and the Netherlands. The study found significant associations between hearing status and distress, somatisation, depression and loneliness, but not between hearing status and self-efficacy or anxiety. For every dB signal-to-noise ratio (SNR) reduction in hearing status, the odds for developing moderate or severe depression increased by 5%, and the odds for developing severe or very severe loneliness increased by 7%. The study also found that different age groups exhibit different associations between hearing status and psychosocial health; increased loneliness was an issue for the 18–29 years group and the 40- to 49-year-olds had the greatest number of significant associations (distress, somatisation, self-efficacy, depression and anxiety), but in the 60–70 years group none of the adjusted associations reached statistical significance. The authors suggest that the differences in age groups could be due to differences in the time of onset of hearing loss, in the use of health care, or in the way hearing loss is regarded; it may be considered as part of the normal ageing process by older adults,36 whereas younger people may suffer from being different in terms of not being fully able to function as expected for people of their age. 35 Nevertheless, hearing loss can still affect the lives of older adults, as demonstrated by a population-based longitudinal study of 2688 participants aged 53–97 years. 37 This study used pure-tone audiometry to assess hearing loss, and reported a significant association between severity of hearing loss and reduced QoL.
Current service provision
In the UK, babies are screened for hearing loss as part of the NHS Newborn Hearing Screening Programme (within 26 days) and further monitoring and tests can confirm any diagnosis of hearing loss. Although there is no national school-based hearing screening programme in the UK, a 2007 survey found that most areas (over 90% of state schools) apply a hearing test at school entry,38 and the UK National Screening Committee recommended in 2006 that screening for hearing loss in school-age children should continue. 38 Those whose hearing loss develops during later childhood or adulthood generally present to their general practitioner, who will undertake tests and refer on to an ear, nose and throat (ENT) department for assessment and treatment if necessary. In many cases people are referred on to an audiology department, where treatment is the supply of a hearing aid.
As described previously, there are different hearing aid options available to those with deafness, including ACHs, BCHAs and BAHAs. In the UK NHS, most ACHAs are now digital and the types prescribed are typically behind-the-ear types; hearing aids that sit in the ear are less often prescribed in the NHS but people may purchase their own privately. For those with congenital hearing loss, or who cannot wear ACHAs owing to infection, BCHAs can be used. However, as discussed previously, they can be uncomfortable and so many people do not use them in all situations, and some prefer not to use them at all. In some cases of bilateral deafness surgical procedures (such as stapedectomy) are considered and can lead to improved hearing, but for many there are no surgical options. In these instances BAHAs may be considered.
Bone-anchored hearing aids are available on the NHS, but are usually fitted at a specialised centre rather than in a local ENT department. 2 In general, a referral for a BAHA will come to a specialist centre from an ENT surgeon; however, in some cases an audiologist will make this referral. In either situation, an audiological assessment to ascertain suitability for a BAHA will be made. Thereafter, BAHA availability can depend on local reimbursement policies (see Variation in services below). Follow-up visits are required to assess if the healing process and BAHA fixture are satisfactory. There are currently 89 BAHA centres in the UK, with around 10 more planned. 39
Quality standards in BAHAs for children and young people suggest that a child with a significant hearing loss must be provided with suitable amplification soon after diagnosis, prior to the referral to the BAHA service. 6 For some children, an ACHA may be tried in the first instance, although where a chronic CHL is present, BCHAs should always be considered, tried and evaluated and children should be provided with the opportunity to be referred for assessment to the BAHA service. 6 Until the child is old enough for BAHA surgery, a BAHA® Softband (Cochlear Bone Anchored Solutions, Sydney, Australia) may be used. This is an elastic band with a BAHA sound processor connected to a plastic snap connector disc sewn into the band. The plastic snap connector disc is held against the skin behind the ear, or at another bony location of the skull, through the pressure from the band, and works in the same way as a conventional bone conductor.
Variation in services
The number of BAHAs in use is unknown as there are no formal records, but it is thought that there are about 6000–7000 BAHAs in current use in the UK (David Proops, Birmingham Children’s NHS Hospital Trust, March 2010, personal communication). Services for BAHA users vary throughout the NHS and funding is not universally available. Primary care trusts (PCTs) differ in policy on the provision of BAHAs, the eligibility criteria for funding BAHAs and sometimes the number of aids funded. 40 For most children with bilateral hearing loss, PCTs will fund a unilateral BAHA as long as a range of criteria around the nature of the hearing loss, the indication and the social and psychological impact have been met. Few PCTs, however, will fund bilateral BAHAs. Furthermore, as stated previously, not all NHS hospitals have an audiology department or one that specialises in the fitting of BAHAs;2 therefore, people referred for BAHAs may have to travel a considerable distance for treatment. 41
Current service cost
Bone-anchored hearing aid funding is recovered on an individual cost-per-case basis via the PCT. 39 BAHAs are more costly than other hearings aids,42 with the sound processor having to be replaced every 3–5 years. 13 The NHS reference costs 2007/08,43 report that a day-case admission for one-stage insertion of fixture for a BAHA costs £1918. This does not include the cost of fixtures, surgical consumables and the BAHA sound processor, which are reimbursed separately, through a high-cost low-volume top-up payment. Prices from Cochlear UK suggest that the product cost for an implant, abutment and processor ranges from £2700 to £3800, this price being dependent on the type of processor used. The 2010 price list from Oticon Medical AB (William Demant Holding) gives a package deal (processor, implant and abutment) for the Ponto (Oticon Medical, Askim, Sweden) and Ponto Pro (Oticon Medical, Askim, Sweden) of £2654.64 and £2886.60, respectively. Prices for surgery, inpatient episode and internal device cost published by the Nottingham University Hospitals44 in 2007–8 were £2683 for adults and £1588 for children. Additional maintenance costs amounted to £3800 in the first year, reducing to £1250 annually, and these did not differ for adults or children. 44
People with BAHAs require lifelong rehabilitation. Every individual should be on a rolling maintenance programme, and therefore funding is required for ongoing maintenance and replacement. These costs, however, need to be considered in the light of the often considerable costs of hearing loss to the person, the NHS and wider society.
Description of BAHAs
Criteria for treatment
Bone-anchored hearing aids are indicated for people with conductive or mixed hearing loss who can benefit from amplification of sound. BAHAs are also indicated for unilateral sensorineural deafness, also known as single-sided deafness, which is beyond the scope of this report. Otological indications for BAHAs include:45
-
congenital malformation of the middle/external ear or microtia
-
chronically draining ear or other infective state that does not allow use of an ACHA (e.g. external otitis, draining mastoid activity)
-
patients with bilateral CHL due to ossicular disease (and not appropriate for surgical correction) or unable to be aided by conventional hearing aid devices.
Chronic suppurative otitis media and recurrent ear canal infections are the most common diagnoses for adults fitted with BAHAs, as these make it difficult to wear conventional ACHAs. 46 For children, the most common diagnoses are congenital ear malformations, with the BAHA often used instead of a conventional BCHA. 46 Bone thickness is critical for implant integration47 and is often insufficient in children under 4 years of age. 48 While it has been suggested that children as young as 3 years can be fitted with a BAHA,2 the devices are indicated for children aged ≥ 5 years. 49,50
Intervention
The BAHA consists of:
-
A permanent titanium implant (3–4 mm), which is surgically placed in the mastoid bone behind the ear, where it fuses with the living bone (osseointegration). The implant transfers sound vibrations to the functioning cochlea.
-
An abutment, which protrudes through the skin and connects the titanium implant to the sound processor, transferring sound vibrations.
-
A small sound processor, which picks up sound vibrations and transfers them to the abutment. The processor can be attached to the abutment and disconnected by the user. Some processors are at head level, although the more powerful are body worn.
Fitting a BAHA requires surgery and can involve either a one-stage or two-stage surgical procedure, with each stage taking around 1 hour. In the one-stage procedure, the implant and abutment are placed at the same time, whereas in the two-stage procedure, the abutment is fitted after a period of around 3 months in adults or 4–6 months in children to allow osseointegration (where bone fuses with the implant) to occur. 51 The advantage of one-stage surgery is that it requires only one surgical procedure, but it risks transmission of forces through the abutment to the fixture before osseointegration has occurred, resulting in a failure of osseointegration and loss of the fixture. The two-stage procedure is therefore most commonly used for young children, adults who may not be able to protect the abutment adequately (e.g. adults with learning difficulties) or adults with poor bone quality (e.g. irradiated bone following radiotherapy in cancer patients). The one-stage procedure is, however, being trialled in children in some centres and has been found to be safe for children as well as adults,52,53 and can be considered for the older child aged 14–16 years. Finally, the sound processor is connected to the abutment after a period of about 1 month. 54
Bone-anchored hearing aid surgery is generally uncomplicated. The most common potential side effects are soft tissue reactions (with poor hygiene being the most frequent reason for adverse skin reactions)55 and loss of fixture. 54 Failures in children tend to occur soon after implantation as, relative to the adult skull, the infant skull is lower in mineral and higher in water content. 49 Re-operation rates are more common in children than adults, for example a Health Technology Assessment review13 for the Ontario Ministry of Health and Long-Term Care found that re-operation rates for tissue reduction or repositioning were generally under 10% for adults but as high as 25% for children. Similarly, an association between younger age and increasing adverse outcomes, such as requiring revision surgery or experiencing fixture loss, was reported by a UK review of 71 children with BAHAs. 54
If trauma or failure of osseointegration occurs, a reserve or ‘sleeper’ implant may be fitted during the first procedure as a backup. This allows a new vibrating part to be fitted into the second implant as soon as a problem occurs with the first, without the need for a repeated first-stage procedure and subsequent 4- to 6-month wait for osseointegration to occur, during which time the individual would be without any hearing aid. It has been usual practice to fit the sleeper approximately 5 mm from the primary fixture; however, the sleeper is rarely needed and, as the bone is thinner, it is less likely to osseointegrate successfully. 57 For bilateral hearing loss it has been recommended that the sleeper should be placed on the contralateral side at the time of the primary surgery, where it is located in an optimum position and could be used if the decision is made to proceed to bilateral BAHA placement, reducing the number of procedures needed. 57
In the past the BAHA was fitted on just one side (unilaterally), which could be either the better hearing side if the two cochleae differ in acuity58 or the side preferred by the individual. The vibratory patterns of bone-conducted sound would suggest that one BAHA should be sufficient for good hearing amplification in bilateral hearing loss, as sound is transmitted to both the ipsilateral (same side) and contralateral (opposite side) cochleae. 59 However, it has been suggested that people with bilateral BAHAs benefit in terms of greater stimulation levels at the cochlea, better directional hearing and space perception, and better speech recognition in noise. 14,59–61 A potential advantage of this includes road safety, especially for children. A further benefit of bilateral BAHAs is that in the event of a problem with one side, for example an infected site or malfunctioning processor, the individual still has one functioning BAHA rather than being without any hearing aid while the problem is resolved. In a consensus statement from BAHA experts in 2005,42 bilateral application with thorough counselling was advocated in young children with severe congenital conductive hearing impairment.
However, the application of bilateral BAHAs is still debatable. Although the benefits of bilateral stimulation through air conduction (AC) are well established, the benefits with BC are less clear. One consequence of BC stimulation is crossover transmission, where the signal presented to one side of the head is transmitted to the contralateral cochlea. When bilateral stimulation occurs, the signals from each side are transmitted to both cochleae and thus interfere, potentially leading to the cancelling of the differences in signals arriving from the two ears and removing the benefits of binaural hearing. 62 The term binaural hearing ‘denotes our faculty for taking advantage from comparisons of the acoustic signals at the two ears’,63 implying the involvement of specialised brain processing that compares the neural correlates of the acoustic signals at the two ears. While empirical evidence suggests that some people with two BAHAs can use some available cues for localisation of sound, the processes remain unclear.
Past BAHA models
The BAHA technique was introduced in 1977, with the first BAHA device made by Branemark and Kuikka. 64 Since then, BAHAs have undergone a series of developments. The first generation of BAHAs, HC 100 (1981–6, Wennberg finmekanik),65 were serially produced but handmade. The second generation of BAHAs, HC 200 [1987–91, Nobel Biotech, Zurich, Switzerland (previously Nobelpharma, Göteborg, Sweden)],65 incorporated a number of improvements such as a damped transducer and a new amplifier system. A more powerful body-worn version, known as the Superbass HC 220 (1987–97, Nobel Biotech, Zurich, Switzerland/Nobelpharma, Göteborg, Sweden) was also developed for people with poorer nerve loss.
The third generation of BAHAs included the HC 300 (1987–97, Nobel Biotech, Zurich, Switzerland/Nobelpharma, Göteborg, Sweden), later named the Classic 300 (1991–9, Nobelpharma). 65 The first Cordelle (Cochlear Bone Anchored Solutions, Sydney, NSW, Australia) (previously Mega base HC 380) was introduced in 1999, described as having the most powerful sound processor,50 with a functional gain that exceeded older BAHAs in higher (5–7 dB) and lower (10–15 dB) frequencies. 55 This was followed by the Compact (Cochlear Bone Anchored Solutions, Sydney, NSW, Australia) (previously HC 360) in 2000. 65 Although the simple signal processing used by models such as the Classic 300 and the Compact benefitted users with CHLs, newer models use more complex signal processing schemes that also benefit users with sensorineural loss. 66 The third-generation BAHA devices marketed by Entific Medical Systems (now Cochlear Bone Anchored Solutions AB) have US Food and Drug Administration (FDA) clearance and carry the CE mark. 67
The Xomed Audiant® (Xomed-Treace, Florida, FL, USA) was introduced in 1985 and manufactured by Xomed and Treace, but was never CE marked. 68 It was a transcutaneous type of BAHA, which used electromagnetic energy from an external processor. The Audiant did not perform well at lower frequencies and is no longer manufactured. 47
The above models are no longer sold in the UK and users should have received an upgrade to one of the devices described below.
Current BAHA models
There are six BAHA devices that are currently manufactured: four from Cochlear and two from Oticon Medical.
The Baha Divino™, Baha Intenso™ and Cordelle II™ were initially manufactured by Entific Medical Systems (Gothenburg, Sweden), which was acquired by the Cochlear Corporation in 2005. The Divino is described as being suitable for people with moderate-to-severe mixed hearing or symmetrical conductive loss [defined as ≤ 10 dB difference (pure-tone average, PTA) or ≤ 15 dB difference at individual frequencies]. Bilateral fitting is suitable for people with moderate-to-severe bilateral symmetrical conductive and/or mixed hearing loss. The processor’s digital technology and built-in directional microphone operate entirely at head level. 69
The Intenso device also has digital technology and operates entirely at head level. 69 It has a larger sound processor than the Divino and hence needs a larger battery. The device is indicated for people with mixed or conductive hearing loss with BC thresholds in the 0–45 dB range across speech frequencies. 70 It is also indicated for bilateral implantation in people with bilaterally symmetrical conductive or mixed hearing loss. The function gain of BC for both the Divino and the Intenso, defined as the difference between BC thresholds measured with a standard audiometer and aided sound field thresholds (expressed in dB HL), is between 5 and 10 dB. 71 The Divino is described as having good sound clarity with reduced feedback.
The Cordelle II reportedly offers even more amplification for people with a severe hearing loss and is on average 13 dB stronger than the discontinued Classic 300 model. It is indicated for CHL and mixed hearing loss, in individuals with average BC thresholds better than 45 dB (across 0.5, 1.2 and 3.0 kHz). It connects directly to external equipment such as television, MP3 players and hi-fi systems, without disconnecting the environmental microphone, and a built-in telecoil receiver allows wearers to connect to teleloop facilities. This device has a body-worn amplifier unit that powers an ear-level transducer. 69
The latest generation of devices from Cochlear and Oticon Medical are even more sophisticated. They are digital with computer-based fitting allowing adjustment to the person’s individual hearing requirements, whereas BAHAs such as the Divino are adjusted using a screwdriver. The devices also have improved quality and advanced features such as directionality.
The most recently launched BAHA sound processor by Cochlear is the BP100 (Cochlear Bone Anchored Solutions, Sydney, NSW, Australia). It is indicated for people with conductive and mixed hearing loss or single-sided sensorineural deafness and average BC thresholds of ≤ 45 dB (across 0.5, 1.0, 2.0 and 3.0 kHz). It is also indicated for bilateral implantation in people with bilaterally symmetric conductive or mixed hearing loss. The device is reported to offer improved audibility, sound quality and speech understanding owing to various automatic systems and has been attributed with a more than 25% improvement in speech understanding in noise. 72
The Ponto and Ponto Pro processors were released in the UK in autumn 2009 by Oticon Medical. The range complies with all European medical device regulatory requirements and has FDA approval. The processors are indicated for people with conductive and mixed hearing loss with an average BC threshold better than 45 dB HL (across 0.5, 1.0, 2.0 and 3.0 kHz), and for single-sided deafness with a PTA AC threshold of the hearing ear better than 20 dB HL (across 0.5, 1.0, 2.0 and 3.0 kHz). Bilateral fitting is applicable for most people with a symmetrical BC threshold. The Pronto Pro model contains additional advanced features such as automatic multiband adaptive directionality, noise reduction and learning volume control.
Chapter 2 Methods for the systematic review of clinical effectiveness and cost-effectiveness
The a priori methods for systematically reviewing the evidence of clinical effectiveness and cost-effectiveness are described in the research protocol (see Appendix 1), which was sent to experts for comment. Although helpful comments were received relating to the general content of the research protocol, there were none that identified specific problems with the methodology of the review. The methods outlined in the protocol are briefly summarised below.
Search strategy
A comprehensive search strategy was developed, tested and refined by an experienced information scientist. Separate searches were conducted to identify studies of clinical effectiveness, cost-effectiveness, QoL, resource use and costs, and epidemiology. Sources of information and search terms are provided in Appendix 2. The most recent search was carried out in November 2009.
A total of 19 electronic resources were searched: 13 databases listing published papers and abstracts and six databases listing ongoing studies. Searches were from database inception to the current date with no language restrictions. The following electronic databases were searched: MEDLINE (Ovid); MEDLINE In-Process & Other Non-Indexed Citations; EMBASE; The Cochrane Library including Cochrane Central Register of Controlled Trials and Cochrane Database of Systematic Reviews; Centre for Reviews and Dissemination including Health Technology Assessment Database, Database of Abstracts of Reviews of Effects and National Health Service Economic Evaluation Database; EconLit; Science Citation Index and Conference Proceedings Citation Index (Web of Science); BIOSIS; Health Management Information Consortium; National Institute for Health Research (NIHR) CRN Portfolio; Current Controlled Trials; Clinical trials.gov; CenterWatch; Health Services Research Projects in Progress; and Computer Retrieval of Information on Scientific Projects. In addition, society websites and conferences were searched for recent abstracts and ongoing studies (see Appendix 2). Bibliographies of retrieved articles were checked for any additional references, and the expert advisory group and BAHA manufacturers were contacted to identify additional published and unpublished studies.
Inclusion and data extraction process
Studies were selected for inclusion in the systematic review of clinical effectiveness through a two-stage process using predefined and explicit criteria. The full literature search results were independently screened by two reviewers to identify all citations that possibly met the inclusion criteria. Full papers of relevant studies were retrieved and assessed independently by two reviewers using a standardised eligibility form. As far as possible, full papers or abstracts describing the same study were linked together, with the article reporting key outcomes designated as the primary publication.
Data were extracted by one reviewer using a standard data extraction form and checked by a second reviewer. At each stage, any disagreements between reviewers were resolved by consensus or, if necessary, by arbitration by a third reviewer.
Titles and abstracts identified by the search strategy for the systematic review of cost-effectiveness were assessed for potential eligibility by two health economists using predetermined inclusion criteria. Full papers were formally assessed for inclusion by one health economist with respect to their potential relevance to the research question.
Quality assessment
The methodological quality and the quality of reporting of the included clinical effectiveness studies were assessed following guidelines by Thomas and colleagues,73 which were modified to accommodate the types of studies included in this review (see Appendices 6–10). Quality criteria were applied by one reviewer and checked by a second reviewer, with any differences in opinion resolved by consensus or by arbitration by a third reviewer.
Quality assessment for the systematic review of cost-effectiveness was based on a checklist for economic evaluation publications74 and guidelines for good practice in decision-analytic modelling in health technology assessment. 75
Inclusion and exclusion criteria
Participants
-
Adults and children with bilateral deafness were included.
-
Single-sided deafness was excluded.
-
Studies reporting both bilateral and unilateral hearing loss were included only if the groups were reported separately or if the majority of participants had bilateral hearing loss.
Interventions
-
Bone-anchored hearing aids, consisting of a surgically implanted titanium fixture. Devices in current use and devices no longer manufactured were included. BAHAs could be fitted unilaterally or bilaterally.
Comparisons
-
Bone-anchored hearing aids versus:
-
– conventional hearing aids (ACHA or BCHA)
-
– unaided hearing
-
– ear surgery (tympanoplasty, myringoplasty, ossiculoplasty, stapedectomy and stapedotomy).
-
-
Unilateral versus bilateral BAHAs.
-
Studies comparing different BAHA models were excluded.
Outcomes
-
Hearing measures, aided hearing thresholds, speech recognition scores.
-
Validated measures of QoL and patient satisfaction.
-
Adverse events.
-
Measures of cost-effectiveness [i.e. cost per quality-adjusted life-year (QALY), cost per life-year saved] and consequences in terms of health service resources.
Study design
For the systematic review of clinical effectiveness, studies were classified according to the criteria by Thomas and colleagues,73 with some adaptations to meet the requirements of this review. The following study designs were eligible:
-
randomised controlled trials (RCTs)
-
controlled clinical trials
-
prospective cohort analytic studies (two groups pre and post, i.e. assessments made before and after BAHA surgery in the intervention group and the control group)
-
prospective cohort one-group pre and post studies (no control group, assessments made before and after BAHA surgery)
-
cross-sectional ‘audiological comparison studies’ [no control group, assessments with intervention and comparator(s) made at one point in time, after BAHA surgery]
-
prospective case series (no comparator condition, outcomes reported with BAHA only).
Where evidence from different types of study design was identified for each of the above comparisons, only studies with the most rigorous designs were included. Where higher level evidence was limited to BAHA models no longer in current use, lower level evidence for models in current use (Divino, Intenso, Cordelle II, BP100, Ponto, Ponto Pro) was considered.
Studies published as abstracts or conference presentations were included only if sufficient details were presented to allow an appraisal of the methodology and the assessment of results to be undertaken.
Only full economic evaluations, those reporting both costs and outcomes, were eligible for inclusion in the systematic review of cost-effectiveness evidence. Conference abstracts were not eligible for inclusion in the cost-effectiveness section.
Data synthesis
Studies of clinical effectiveness and cost-effectiveness were synthesised through a narrative review with full tabulation of the results of all included studies. It was considered inappropriate to combine the results of the studies in a meta-analysis owing to differences in the outcome measures and patient populations. Within Chapter 3, results are discussed according to the comparison to aid interpretation. Where studies report outcomes for more than one comparison (e.g. BAHA vs ACHA and BAHA vs unaided), these are discussed in each relevant section. Care should therefore be taken to avoid double-counting the BAHA data, which are repeated. This is noted where appropriate. Outcome measures are discussed throughout the review of clinical effectiveness as reported by the included studies, including the use of descriptions such as ‘improvement’ or ‘deterioration’. To aid interpretation of the data, lower hearing thresholds are considered to be ‘better’ than higher thresholds, but it is acknowledged that this is a simplistic approach and, while true in many cases, it may not necessarily be so. The methods for the economic model are described in Chapter 4, Southampton Health Technology Assessments Centre economic analysis.
Chapter 3 Clinical effectiveness results
Quantity and quality of research available
Searching identified 665 references after de-duplication. The number of references excluded at each stage of the systematic review is shown in Figure 1. Selected references which were retrieved but later excluded are listed in Appendix 3 with reasons for exclusion. Studies were often excluded for more than one reason; the most common reason being study design (16 studies), followed by outcomes (12 studies), intervention (six studies), comparator (10 studies) or participants (one study). Although not formally assessed, the level of agreement between reviewers for screening was good. Twenty-eight relevant non-English references were identified by the searches and can be seen in Appendix 4. After examination of the titles and English abstracts (where available) it was unclear whether or not any of these studies met the inclusion criteria, and none appeared to have a concurrent control group. Because it was anticipated the studies would add limited value to the review, and in view of limited resources, translation and full screening of the papers were not undertaken. Searches did not identify any eligible ongoing studies.
FIGURE 1.
Flow chart of identification of studies.
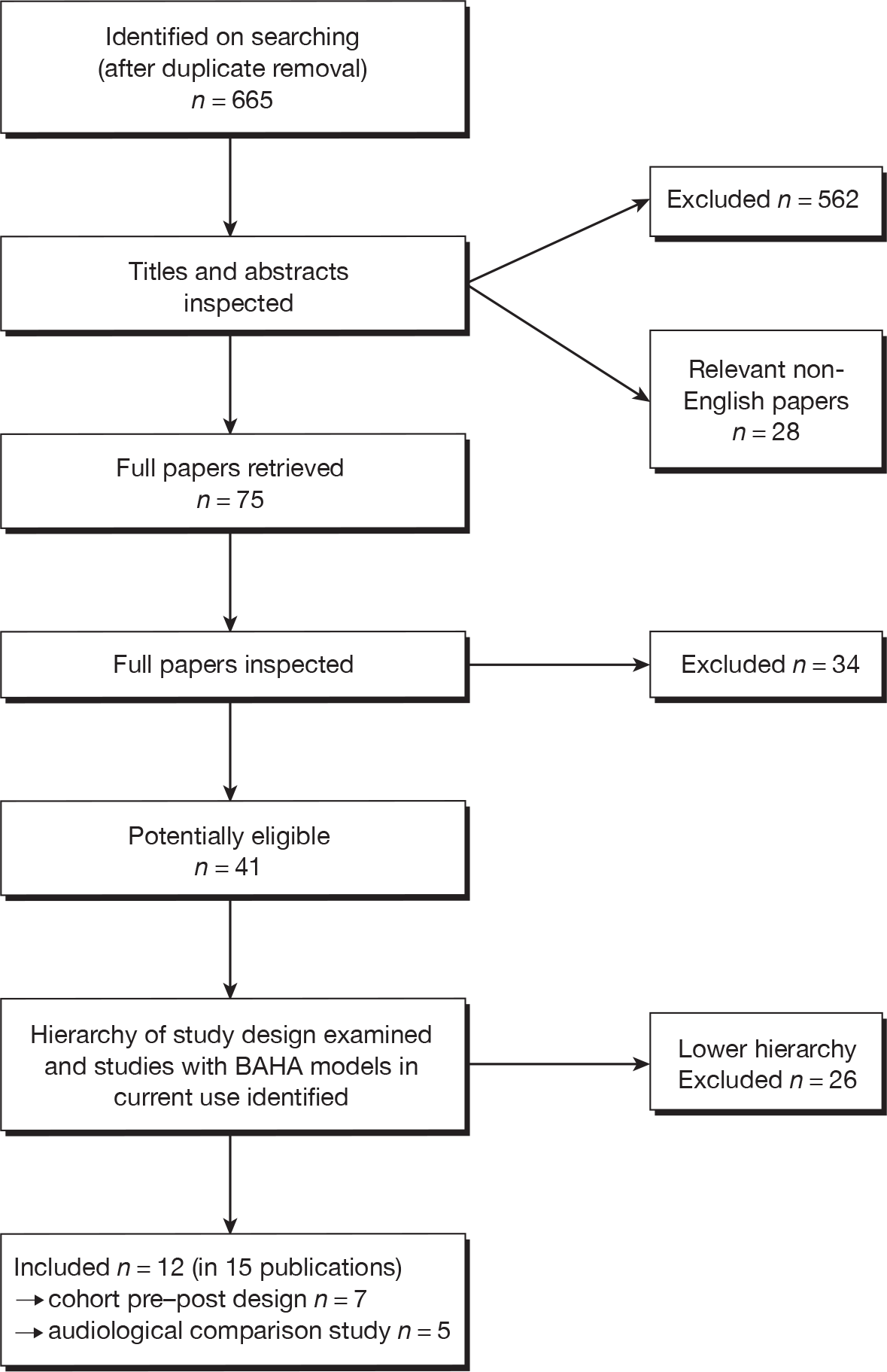
Forty-one potentially eligible studies were identified. After selecting the highest level of evidence available for each comparison (BAHA vs BCHA, ACHA, unaided hearing or ear surgery, unilateral vs bilateral BAHA) and checking the remaining studies for BAHA models in current use, 12 studies (in 15 publications) were included in the systematic review of clinical effectiveness. 59,60,66,76–87 The included studies were either one-group cohort pre and post studies or cross-sectional ‘audiological comparison’ studies (study design is discussed further in Quality assessment); no RCTs, controlled clinical trials or prospective cohort analytic studies were identified. Only two studies included BAHA models that are in current use. 66,76 A summary of the highest level of evidence available and the current availability of the BAHAs used (whether or not currently manufactured) for each comparison can be seen in Table 5. The remaining 26 lower evidence studies are listed in Appendix 5, and were described by reviewers as audiological comparison studies (using BAHAs no longer manufactured, 20 studies) or prospective case series with no comparator (six studies). No eligible studies comparing BAHAs with ear surgery were identified.
| Comparison | Highest level of evidence identified and current availability of BAHA (no. of studiesa) |
|---|---|
| BAHA vs BCHA | CPP and BAHA in current use: 0 |
| CPP and BAHA no longer manufactured: 477–82 | |
| ACS and BAHA in current use: 0 | |
| ACS and BAHA no longer manufactured: 14 (see Appendix 5) | |
| BAHA vs ACHA | CPP and BAHA in current use: 0 |
| CPP and BAHA no longer manufactured: 578–84 | |
| ACS and BAHA in current use: 176 | |
| ACS and BAHA no longer manufactured: 13 (see Appendix 5) | |
| BAHA vs unaided | CPP and BAHA in current use: 166 |
| CCP and BAHA no longer manufactured: 377,78,83 | |
| ACS and BAHA in current use: 0 | |
| ACS and BAHA no longer manufactured: 6 (see Appendix 5) | |
| BAHA vs ear surgery | 0 eligible studies |
| Unilateral vs bilateral | CPP and BAHA in current use: 0 |
| CPP and BAHA no longer manufactured: 0 | |
| ACS and BAHA in current use: 0 | |
| ACS and BAHA no longer manufactured: 459,60,85–87 |
Characteristics of included studies
BAHAs versus BCHA or ACHA
Study design
Seven studies (one study had three associated publications79–81) comparing BAHAs with conventional aids, either BCHAs,77 ACHAs76,83,84 or both (in separate subgroups),78–82 were included (Table 6, Appendices 6–8). Three of the studies also tested participants unaided77,78,83 (see BAHA versus unaided hearing). Six77–84 of the studies were described by reviewers as cohort pre- and post-studies (before and after studies) and either assessed BAHAs models that are no longer manufactured77–83 or did not report the model used (although this study was published in 1998 so is unlikely to have used a BAHA that is currently manufactured84). Only one study was identified that assessed a BAHA model in current use;76 this study compared the BAHA Intenso with an ACHA, and was described by reviewers as a cross-sectional audiological comparison study.
| Study | Intervention and timing of audiology | Participants indication and characteristics |
|---|---|---|
| BAHAs vs BCHA | ||
|
Béjar-Solar et al. 200077 Mexico Cohort pre–post |
One group: n = 11
|
Inoperable bilateral congenital microtia atresia. BC PTA ≥ 45 dB HL with 100% speech discrimination. Low socioeconomic background Age, mean years (range): 10 (5–17) Sex (M : F): 7 : 4 PTA thresholds (1.25–3.00 kHz), mean, dB HL: AC right ear 69, left ear 69; BC right ear 20, left ear 14; sound field PTA 64 |
| BAHA vs ACHA | ||
|
UK Cohort pre–post |
One group: n = 9
|
Otosclerosis. Average BC thresholds (0.5–4.0 kHz) < 40 dB HL for ear level BAHA, < 60 dB HL for body-worn Superbass Age, mean years (range): NR for study sample Sex (M : F): NR for study sample Average BC thresholds (0.5–4.0 kHz), dB HL: NR for study sample |
|
Flynn et al. 200976 Sweden Audiological comparison study |
One group: n = 10
|
Mixed hearing loss, no further details. Sensorineural component ≥ 25 dB HL plus air-bone gap > 30 dB Age, mean years (range): 59 (32–75) Sex (M : F): 5 : 5 PTA thresholds (0.5, 1.0 and 2.0 kHz), mean dB HL (range): AC 77 (55–80); BC 41 (25–66) |
|
Netherlands Cohort pre–post |
One group: n = 34
|
Bilateral conductive or mixed hearing loss with chronic otitis. No audiological criteria stated Age, mean years (range): 48 (26–72) Sex (M : F): 12 : 22 PTA thresholds (0.5, 1.0, 2.0 and 4.0 kHz), mean dB HL (range):d AC 60 (25–90); BC 26 (6–46) |
| BAHA vs BCHA and ACHA (in separate subgroups) | ||
|
UK Cohort pre–post |
Four subgroups [previous aid AC or BC, aetiology congenital (CON) or CSOM]:
|
CSOM or congenital aetiology. Average BC thresholds (0.5–4.0 kHz) < 40 dB HL (ear level) or < 60 dB HL (body-worn), speech discrimination score ≥ 60% Age, mean years:e CSOM/ACHA 58; CSOM/BCHA 61; CON/ACHA 30; CON/BCHA 24 Sex (M : F): NR PTA thresholds (0.5–4.0 kHz), mean dB HL:e AC: CSOM/ACHA 58, CSOM/BCHA 65, CON/ACHA 70, CON/BCHA 60; BC: CSOM/ACHA 24, CSOM/BCHA 30, CON/ACHA 20, CON/BCHA 13 |
|
Netherlands Cohort pre–post |
Two subgroups (previous aid AC or BC):
|
Acquired conductive or mixed hearing loss, no further details Age, mean years (range): ACHA 47.9 (24–73), BCHA 62 (42–82) Sex (M : F): ACHA 12 : 24; BCHA 9 : 11 PTA thresholds (0.5, 1.0 and 2.0 kHz), mean dB HL (range): AC: ACHA 63.2 (30–103), BCHA 76.5 (40–107); BC: ACHA 26.8 (9–51), BCHA 43.4 (17–63) |
|
cSnik et al. 1992,79 1994,80 199881 Netherlands Cohort pre–post |
Two subgroups (previous aid AC or BC):79
|
Recurrent otorrhoea. Severe mixed hearing loss with sensorineural components of 45–60 dB HL79 Age, mean (SD, range): (i) 60.6 (18.8, 34–84) years; (ii) 62 (13.9, 46–78) years Sex (M : F): NR PTA thresholds (frequencies NR), mean dB HL (SD, range):f,g AC (i) 91.1 (14.3, 70–108), (ii) 84.8 (12.3, 72–100); BC (i) 46.2 (12.6, 28 to > 62), (ii) 49.6 (7.3, 40–57) |
Four subgroups (previous aid AC or BC, current BAHA HC200 or HC220):80
|
Chronic otitis media/externa, aural atresia. Both normal to moderate and more severe SNHL80 Age, range: 10–77 (mean NR) years Sex (M : F): NR PTA thresholds (0.5, 1.0 and 2.0 kHz) for BC, range dB HL: HC 200 (n = 42) 0–44; HC 220 (n = 16) 33–63 |
|
Two subgroups:81
|
Conductive or mixed binaural hearing loss, SNHL of ≤ 30 dB HL. No details of aetiology81 Age, range: 10–70 (mean 43) years Sex (M : F): NR PTA thresholds (0.5, 1.0, 2.0 kHz), mean dB HL (range): AC 55 (30–90); BC 16 (0–28) |
|
| BAHA vs unaided (see also three studies from above: Béjar-Solar et al. 2000,77 Burrell et al. 199683 and Cooper et al. 199678) | ||
|
Kompis et al. 200766 Switzerland Cohort pre–post |
One group: n = 7
|
Bilateral CHL, some mild-to-moderate SNHL. No further details. All had at least 2 years’ experience with BAHAs Age, mean (range): 49 (19–66) years Sex (M : F): 3 : 4 PTA AC and BC thresholds: IPD presented in figure but could not be extracted |
| Unilateral vs bilateral BAHAs | ||
|
Netherlands Audiological comparison study |
One group: n = 25(HC 200 or Classic 300) Assessments at same session |
Recurrent otorrhoea, otitis externa, congenital atresia Age, mean (range): 44.3 (12–74) years Sex (M : F): 14 : 11 BAHA experience, mean (range):f unilateral 49.1 (18–105) months; bilateral 13.6 (3–105) months PTA thresholds (0.5, 1.0 and 2.0 kHz), mean dB HL (SD, range): AC first fitted side 59.5 (13.7, 32–82),f second fitted side 63.6 (10.9, 38–82);f BC first fitted side 21.0 (10.7, –5 to 36);g second fitted side 21.9 (12.4, –8 to 48)g |
|
Dutt et al. 200286 UK Audiological comparison study |
One group: n = 11(Compact) Assessments at same session |
Treacher Collins syndrome, Goldenhar syndrome, bilateral: mastoid cavities, CON, chronic otitis media, microtia, acquired otosclerosis Age, mean (range):f 42.3 (22–54) years Sex (M : F): 3 : 9 (one patient chose not to participate) BAHA experience, mean (range):f unilateral 6.3 (3–12) years; bilateral 2.2 (1–5) years PTA AC and BC thresholds: NR |
|
Priwin et al. 200487 Sweden Audiological comparison study |
One group: n = 12(Compact and Classic 300) Assessments at same session |
Chronic otitis, otosclerosis, congenital ear canal atresia Age, mean (range):f 51.7 (27–68) years Sex (M : F): 3 : 9 BAHA experience, mean (range):f unilateral 14.3 (5.8–21) years; bilateral 6.8 (1–19.6) years PTA thresholds (0.5, 1.0 and 2.0 kHz), mean dB HL (SD, range):f,g AC first fitted side 58.3 (15.3, 38–87), second fitted side 59 (20.7, 27–102); BC first fitted side 29.8 (15.2, 8–53),h second fitted side 30.9 (13.4, 7– 50) |
|
Priwin et al. 200759 Sweden Audiological comparison study |
Two groups:(Compact and Classic) Assessments at same session |
Majority had symmetrical maximal or near-maximal conductive bilateral hearing loss Age, mean (range): 11.3 (6–17) years Sex (M : F): 3 : 6 BAHA experience: at least 3 months (no further details) PTA (M4) thresholds (0.5, 1.0, 2.0 and 4.0 kHz) mean dB HL (SD): AC better ear 61.3 (15.5), worse ear 72.1 (12.1); BC better ear 14.1 (12.7); worse ear 13.8 (10.7) |
Post-operative assessment with the BAHA was undertaken after either 4–6 weeks79,80,84 or 6 months. 77,78,82 The post-operative duration was not reported in one study. 83 Participants in the cross-sectional audiological comparison study had ≥ 12 months experience with the BAHA before being assessed with a BAHA and an ACHA at the same time point. 76
Participants
The study from the Netherlands by Snik and colleagues79–81 was associated with three eligible publications, which had considerable overlap of participants. It appears that the participants who formed the BAHA HC 200 subgroup (n = 42) and the HC 220 subgroup (n = 16) in the 1994 study by Snik and colleagues80 were also reported in the 1998 study (BAHA HC 200, n = 41)81 and the 1992 study (BAHA HC 220, n = 12),79 respectively. Participants from another centre were also included in the 1992 study. To avoid double-counting of participants these three publications have been considered as one study with the appropriate publication referenced when discussed. It is not clear whether there is an overlap with the participants from the studies by Mylanus and colleagues84 or Hol and colleagues,82 which were also conducted by the same group in the Netherlands. There may also be some overlap of participants between two of the UK studies, which were undertaken by the Birmingham group, but again this is not clear. 78,83
The studies comparing BAHAs with conventional aids included participants with inoperable bilateral congenital microtia atresia,77 otosclerosis,83 chronic otitis media/externa or otorrhoea,78,79,81,84 aural atresia80 and congenital hearing loss. 78 Details of aetiology were not reported in the studies by Flynn and colleagues76 or Hol and colleagues. 82
All seven studies76–84 reported PTA thresholds for AC and/or BC, although the frequencies over which these were determined varied between the studies (see Table 6). Mean PTA thresholds for AC varied from 55 dB HL (range 30–90 dB HL) at 0.5, 1.0 and 2.0 kHz in the 1998 publication by Snik and colleagues81 to 91.1 dB HL [standard deviation (SD) 14.3, range 70–108 dB HL] (frequencies not reported) in the BCHA subgroup of the 1992 publication by Snik and colleagues. 79 Similarly, mean PTA thresholds for BC varied from 16 dB HL (range 0–28 dB HL) at 0.5, 1.0 and 2.0 kHz in the 1998 publication by Snik and colleagues84 to 49.6 dB HL (SD 7.3, range 40–57 dB HL) (frequencies not reported) in the ACHA subgroup of the 1992 publication by Snik and colleagues. 79
One study included children only (age 5–17 years)77 and five studies76,78,79,82,84 included adults only (mean age from approximately 4378 to 5982 years). In the study by Snik and colleagues, two of the publications included children and adults (age range from 10 to 7081 or 7780 years), while the 1992 publication77 included adults only (age range 34–78 years). The proportion of men and women varied between those studies reporting characteristics of the study sample. 76,77,82,84
These studies are generalisable only to people with bilateral conductive or mixed hearing loss who had previously used either an ACHA or a BCHA.
Outcomes
Data were reported in a variety of ways in the studies. Five of the studies reported pure-tone or warble-tone average thresholds,76–78,83,84 and one study reported the average difference between warble-tone thresholds. 79 The range of frequencies over which these were assessed varied between the studies, and although in the USA it is mandatory to include 3 kHz, this is not required in Europe. One study did not report any audiological measures at follow-up. 82 Outcomes for speech audiometry included 100% speech audiometry discrimination with background noise at 65 dB HL,77 speech recognition threshold (level at which 50% of the presented phonemes were repeated properly by the participant)79 and speech discrimination score at 63 dB. 78,83 One study reported the speech-to-noise ratio with BAHAs and ACHAs,76 whereas two other studies reported only the change in speech-to-noise ratio81,84 or the change in speech reception threshold (SRT) in quiet. 81 The maximum phoneme score was reported by two studies. 79,84 One study reported the number of participants with a statistically significant change in the speech recognition in quiet score and speech-to-noise ratio score. 80 Accurate directional identification of location of a sound source was evaluated by one study. 78
Two studies77,82 reported using a validated measure of QoL, although limited details and data were reported in one of these. 77 The second study80 did not report any audiological measures, but presented before and after data from the Short Form questionnaire-36 items (SF-36), European Quality of Life-5 Dimensions (EQ-5D) and the Hearing Handicap and Disability Index (HHDI). Five studies reported the results of subjective questionnaires on patient preference,77,81,83,84 satisfaction,78 comfort79,83 and opinions on speech recognition in noise and quiet,78–81 but none of these questionnaires appears to have been validated.
In five of the seven studies,76,78,80,81,83,84 some data were reported only in figures and had to be estimated by reviewers, which increases potential for error. In two of the studies,79,83 individual patient data without any summary statistics were reported for some outcomes, therefore means and SDs presented in this review for these studies were calculated by reviewers. Data estimated from figures or summary statistics calculated by reviewers are indicated in Tables 6, 8 and 10.
BAHA versus unaided hearing
Four studies included a comparison of BAHAs with unaided hearing (see Table 6 and Appendices 6–9). 66,77,78,83 Three of these studies have been described above, as they compared BAHAs with conventional aids but also reported outcomes unaided. The BAHAs used in these studies are no longer manufactured. Audiological assessment was undertaken 6 months post-operatively in two studies,77,78 but this was not reported in the other study. 83
A study by Kompis and colleagues66 was identified that compared BAHAs with the unaided condition and included a BAHA model in current use (BAHA Divino). This study is defined by reviewers as a pre and post cohort study, but differs from the other included studies as the participants had prior experience with BAHAs when tested unaided at baseline. The aim of the study was to compare the BAHA Divino with the BAHA Compact (this comparison did not meet the inclusion criteria of the review), and the participants had at least 2 years’ experience with a BAHA Compact or Classic 300 prior to the study. However, the study also assessed the unaided condition at baseline and then assessed the BAHA Divino after 3 months’ use, so these data were included in the systematic review (see Appendix 9).
Participants
Kompis and colleagues66 included participants described as having substantial bilateral CHL, some combined with mild-to-moderate SNHL. No details of aetiology were reported. PTA thresholds for AC and BC for each ear of individual participants were presented in figures in this study, but data could not be extracted. The participants in this study were adults with a mean age of 48.6 years. 66 The studies by Burrell and colleagues,83 Cooper and colleagues78 and Béjar-Solar and colleagues77 have already been described in BAHAs versus BCHA or ACHA.
The generalisability of the study by Kompis and colleagues66 may be limited to people with bilateral conductive or mixed hearing loss with previous experience of BAHAs.
Outcomes
Kompis and colleagues66 reported the average improvement in sound field pure-tone thresholds over all frequencies compared with unaided. Outcomes for speech audiometry included mean speech recognition threshold and speech recognition scores in quiet, and speech recognition in noise when noise was presented from the front or back. QoL was not reported. Outcomes reported by Burrell and colleagues,83 Cooper and colleagues78 and Béjar-Solar and colleagues77 have already been described in BAHAs versus BCHA or ACHA.
Data had to be estimated from figures by reviewers for the study by Kompis and colleagues;66 this is indicated in Tables 6 and 12.
Country
The study by Kompis and colleagues66 was conducted in Switzerland and, as described in BAHAs versus BCHA or ACHA, two studies were conducted in the UK78,83 and one in Mexico. 77
Funding
The BAHA Divinos used in the study by Kompis and colleagues66 were provided by Entific Medical Systems. The other three studies are reported in BAHAs versus BCHA or ACHA. 77,78,83
Unilateral versus bilateral BAHAs
Four studies59,60,85–87 comparing unilateral and bilateral BAHAs were included, and all were described as audiological comparison studies by reviewers (see Table 6, Appendix 10). None of these studies (or any eligible study from a lower level of evidence) compared unilateral and bilateral BAHAs using a model in current use.
Participants
The studies comparing unilateral with bilateral BAHAs included participants with various diagnoses, including recurrent otorrhoea,60 chronic otitis,60,86,87 congenital atresia,60,87 otosclerosis,86,87 congenital syndromes and hearing loss,86 mastoid cavities86 and microtia. 86 One study did not describe aetiology, simply stating that the majority of participants had symmetrical maximal or near-maximal conductive bilateral hearing loss.
Three of the four studies reported PTA thresholds (of frequencies 0.2, 1.0 and 2.0 kHz60,87 or 0.5, 1.0, 2.0 and 4.0 kHz59) for AC and BC. Mean PTA thresholds for AC were ∼ 60 dB HL in these studies, and mean PTA thresholds for BC ranged from ∼ 14 dB HL59 to ∼ 30 dB HL. 87
Some of the participants in the study by Bosman and colleagues60 had previously used a unilateral BCHA and had been deprived of binaural cues since early life,85 whereas participants in the study by Dutt and colleagues86 were required to have previous experience of binaural hearing. Previous experience of binaural hearing was not explicitly stated by the other two studies. 59,87
Implantation of the bilateral BAHAs was not simultaneous in three of the studies;60,85–87 the participants in two of these studies consisted of self-selected volunteers applying for a second BAHA. 60,86 Participants had on average 4.160 to 14.387 years experience with unilateral BAHAs, and 1.160 to 6.887 years experience with bilateral BAHAs at the time the studies were conducted. It is not clear whether bilateral implantation was simultaneous or sequential in the fourth study, which simply stated that participants had at least 3 months’ experience with BAHAs. 60
One study included children only (mean age 11.3 years, range 6–17 years)59 and two studies included adults only [mean age 42.3 years (SD 10 years)86 and 51.7 years (SD 13.3 years)87]. Bosman and colleagues60 included both adults and children, with a mean age of 44.3 years (SD 16.3 years, range 12–74 years). Three studies had a higher proportion of women than of men. 59,86,87
Three of these studies60,85–87 are generalisable only to people with bilateral conductive or mixed hearing loss and previous experience with unilateral and bilateral BAHAs. The fourth study is relevant to a paediatric population who have at least 3 months’ experience with unilateral and/or bilateral BAHAs. 59
Outcomes
Two of the four studies reported data on sound field tone thresholds; one of these reported data separately for unilateral and bilateral BAHAs where sound was presented from the front59 and one reported average improvement with bilateral BAHAs where sound was presented from the front, at best side, at shadow side and from behind the participant. 87 Outcomes for speech audiometry included SRT in quiet;60,87 SNR with noise from baffle and shadow side;60 change in the SNR with bilateral BAHAs with noise at best side, shadow side and as surrounding noise;87 scores for speech in quiet at a range of intensity levels;86 and speech-in-noise scores with noise presented at front, left and right. 86 Three studies reported directional hearing, including correct localisation,59,60 localisation within 30°60,87 and correct lateralisation. 59,60 The set-up of the loudspeakers for the assessment of directional hearing was different in each of the three studies, using either five loudspeakers positioned at 45° intervals in a frontal semicircle,59 seven or nine loudspeakers positioned at 30° intervals in an arc spanning 180° or 240° with a 1-m radius,60 or using 12 loudspeakers positioned at 30° in a circle with a 1-m radius87 (see data extraction forms in Appendix 10 for further details). The ‘binaural masking level difference’ was reported by two studies in an attempt to demonstrate the existence of binaural hearing (defined as the ability to use binaural cues, i.e. use the different sound information at the two cochleae to improve hearing) with bilateral BAHAs. 60,87 In this test, a pure-tone signal is presented in noise, and the task is to detect the tone. Three conditions were tested: for the first condition, the pure-tone signal and noise were presented equally at both ears; for the second condition, the phase of the tones presented at the two sides had an opposite phase (180° out of phase), but the noises were in phase (the levels were equal at both sides); and for the third condition, the noises at both sides were 180° out of phase, but the tones were in phase.
One study59 reported data from the validated Meaningful Auditory Integration Scale (MAIS) and Meaningful Use of Speech Scale (MUSS)45 to assess hearing skills in ‘meaningful, real world situations’, and the International Outcome Inventory for Hearing Aids (IOI-HA)88 to assess hearing aid outcomes.
All four studies59,60,85–87 presented individual patient data without any summary statistics for some outcomes, while other outcomes were presented in figures only. Data estimated from figures or summary statistics calculated by reviewers are indicated in Tables 6 and 13.
Quality assessment
All 12 included studies were rated overall as ‘weak’ for their methodological quality and quality of reporting (Table 7). 59,60,66,76–87 The studies were not described using recognised study types or descriptions, and therefore were termed ‘cohort pre and post studies’ (seven studies66,77–84) or cross-sectional ‘audiological comparison’ studies (five studies59,60,76,85–87) by reviewers. In the cohort pre and post studies, baseline measurements were undertaken with the individual’s previous aid or unaided or both, before BAHA surgery, and measurements with the BAHA were undertaken after a given period of use. In the audiological comparison studies, the measurements were undertaken with the intervention and comparator at the same point in time. With both of these designs, a potential source of bias is that, as with any hearing aid trial, participants are likely to prefer the second hearing aid tested. 89,90 As the study design method was not described by any of the included studies, all were assigned a rating of ‘weak’, although in the hierarchy of evidence, cohort pre and post studies are ranked higher than audiological comparison studies.
| Study | Selection bias | Study design (description) | Confounders | Blinding | Data collection methods | Withdrawals and dropouts | Intervention integrity | Analysis appropriate to question? | Global rating | |
|---|---|---|---|---|---|---|---|---|---|---|
| Appropriate statistical methods? | Handling of missing data reported? | |||||||||
| BAHAs vs BCHA | ||||||||||
| Béjar-Solar et al. 200077 | Weak | Weak (CPP) | Weak | Weak | Strong | Weak | No | No | No | Weak |
| BAHA vs ACHA | ||||||||||
| Burrell et al. 199683 | Moderate | Weak (CPP) | Weak | Weak | Strong | Weak | No | No | No | Weak |
| Flynn et al. 200976 | Weak | Weak (ACS) | Weak | Weak | Strong | Weak | No | Can’t tell | No | Weak |
| Mylanus et al. 199884 | Moderate | Weak (CPP) | Weak | Weak | Strong | Weak | Can’t tell | Yes | No | Weak |
| BAHA vs BCHA/ACHA | ||||||||||
| Cooper et al. 199678 | Weak | Weak (CPP) | Weak | Weak | Strong | Weak | Yes | Yes | No | Weak |
| Hol et al. 200482 | Moderate | Weak (CPP) | Weak | Weak | Strong | Weak | Yes | Yes | Yes | Weak |
| Snik et al. 199879–81 | Moderate | Weak (CPP) | Weak | Weak | Strong | Strong | Can’t tell | Can’t tell | No | Weak |
| BAHA vs unaided (see also three studies from above: Béjar-Solar et al. 2000,77 Burrell et al. 199683 and Cooper et al. 199678) | ||||||||||
| Kompis et al. 200766 | Weak | Weak (CPP) | Weak | Weak | Strong | Weak | No | Yes | No | Weak |
| Unilateral vs bilateral BAHAs | ||||||||||
| Bosman et al. 200160,85 | Moderate | Weak (ACS) | Weak | Weak | Strong | Weak | Can’t tell | No | No | Weak |
| Dutt et al. 200286 | Weak | Weak (ACS) | Weak | Weak | Strong | Strong | Can’t tell | No | No | Weak |
| Priwin et al. 200487 | Moderate | Weak (ACS) | Weak | Weak | Strong | Weak | Can’t tell | Yes | No | Weak |
| Priwin et al. 200789 | Weak | Weak (ACS) | Weak | Weak | Strong | Weak | No | Yes | No | Weak |
Six studies were rated as ‘moderate’ for selection bias,60,81–84,87 indicating that the selected individuals are at least somewhat likely to be representative of the target population, and at least 60% of those identified agreed to participate. The remaining six studies were rated as ‘weak’ for selection bias,59,66,76–78,86 as participants may not be representative of the target population if they are self-referred, or because selection method or the level of participation were not described.
Although some studies described some baseline characteristics of the participants, none of the included studies reported these as potential confounding variables or described how these may be causally related to the outcomes of interest. All 12 studies were therefore rated as ‘weak’ for the assessment of potential confounders.
It was assumed that outcome assessors were not blinded to the intervention as this was not reported by any of the studies. This can lead to bias in the care provided (performance bias) and how the outcomes are assessed (measurement or detection bias). Participants were blinded to the use of unilateral or bilateral BAHAs in one study;87 however, it was assumed that participants were not blinded to the research question in the remaining studies, leading to reporting bias. While it may be difficult to blind participants to the intervention, it is important to note that this bias is present when interpreting the results.
All 12 studies were rated as strong for data collection methods. The 11 studies that reported audiometric data all used methods known to be valid and reliable; however, some of these studies also reported self-reported subjective outcomes using methods that are not valid and reliable and are therefore not discussed in the systematic review (but can be viewed in Appendices 6–10). The study by Hol and colleagues82 used QoL measures that are known to be valid and reliable.
Two studies were rated ‘strong’ for withdrawals and dropouts,81,86 as they described both the numbers and reasons for withdrawals and dropouts, and had a follow-up rate of ≥ 80%. This information could not be deduced from the remaining studies, which may therefore be at risk from attrition bias.
Intervention integrity describes whether all participants received the intervention in the same way, for example the same frequency or duration of use. Only two studies78,82 reported the amount of use with the BAHA (such as number of hours per day), five studies reported that the BAHA was used ‘daily’ with no further description,60,79–81,84,86,87 and the remaining five studies did not report how often the BAHA was used during the study period. 59,66,76,77,83
Statistical analysis of the results was not undertaken by four studies. 60,77,83,86 The remaining eight studies reported statistical analyses, six of which were judged appropriate to the research question59,66,78,82,84,87 and in two studies this was not clear. 76,81 Only one of the studies reported on how missing data were dealt with in the analysis. 82
Assessment of clinical effectiveness: BAHA versus BCHA
Audiological measures
Three included studies (reported in five publications)77–81 provided a comparison of audiological measures between BAHA and BCHA (Table 8). All three studies were cohort pre and post studies and none used BAHA models that are in current use. The high risk of bias in all three included studies should be considered when interpreting the results.
| Study and outcomes | BCHA | BAHA model | Comparison |
|---|---|---|---|
| Béjar-Solar et al. 200077 (n = 11) | BCHA | Classic 300 | Difference |
| Sound field PTA (125–3000 Hz), dB HL | 30 | 19 | –11 (37% improvement) |
| Sound field 100% speech audiometry discrimination, background noise at 65 dB, dB HL | 62 | 48 | –14 (23% improvement) |
| Accurate directional identification of location of a sound source (% of cases) | 0 | 80 | |
| Cooper et al. 199678 two subgroups: CSOM (n = 19), CON (n = 16) | BCHA | HC 200, 300, 220 | p-value |
| Mean sound field warble-tone thresholds, dB [dB(A), 500–4000 Hz)]a |
CSOM 42 CON 31 |
CSOM 35 CON 26 |
p < 0.01 p < 0.01 |
| Mean sound field speech discrimination score (at 63 dB),% correcta |
CSOM 65 CON 86 |
CSOM 72 CON 85 |
p = NS p = NS |
| Snik et al. 1992,79 1994,80 199881 | BCHA | HC 200, 220 | p-value |
| Snik et al. 199279 (n = 7) | BCHA | HC 220 | |
| Maximum phoneme score,%, mean (SD) rangeb | 36.1 (28.9), 0–85 | 48.7 (31.7), 0–100 | NR |
| Speech recognition threshold, dB(A), mean (SD) rangeb |
(n = 2) 40 (7.1), 35–45 |
(n = 4) 38.8 (11.1), 25–50 |
NR |
| Average difference between the sound field warble thresholds (BCHA minus BAHA), dBa,c | |||
| 250 Hz | 2 | ||
| 500 Hz | 3 | ||
| 1000 Hz | –2 | ||
| 2000 Hz | –10 | ||
| 4000 Hz | –14 | ||
| 8000 Hz | NR | ||
| Snik et al. 199480 (n = 44) | HC 220, HC 200 | ||
| Patients with a statistically significant change in: | HC 220 (n = 11) | ||
| speech recognition in quiet score with BAHAd | Improved: 6 of 11 (54%); deteriorated: 0 of 11 | ||
| speech-to-noise ratio score with BAHAd | Improved: 5 of 11 (44%); deteriorated: 0 of 11 | ||
| Patients with a statistically significant change in: | HC 200 (n = 33) | ||
| speech recognition in quiet score with BAHAd | Improved: 4 of 33 (12%); deteriorated: 0 of 33 | ||
| speech-to-noise ratio score with BAHAd | Improved: 20 of 33 (60%); deteriorated: 0 of 33 | ||
| Snik et al. 199881 (n = 33) | HC 200 | ||
| Change in SRT in quiet (BCHA minus BAHA), mean dB (SD) | 2.7 (4.4), improvement p < 0.05 | ||
| Change in speech-to-noise ratio, mean dB (SD) | 2.5 (2.2), improvement p < 0.05 | ||
Audiometry
All three included studies reported data on hearing threshold tests, two78,79 used sound field warble-tone thresholds and the remaining study used sound field pure-tone thresholds (see Table 8). 77
Béjar-Solar and colleagues77 report meaned sound field PTA thresholds in their 11 participants (at 0.125–3.000 kHz) with inoperable bilateral congenital microtia atresia. The study found a 37% improvement with the Classic 300 BAHA compared with the BCHA (BCHA 30 dB HL, BAHA 19 dB HL), although statistical analysis was not undertaken.
Cooper and colleagues78 tested two subgroups: 19 participants who had chronic suppurative otitis media and 16 participants who had congenital hearing loss. Mean sound field warble-tone threshold (over a 0.5–4.0 kHz range) was reported to be statistically significantly better with the BAHA than with the BCHA in the CSOM subgroup [42 dB(A) BCHA vs 35 dB(A) BAHA, p < 0.01]. In the CON subgroup the BAHA was also seen to be statistically significantly better than the BCHA [31 dB(A) BCHA vs 26 dB(A) BAHA, p < 0.01]. In this study the BAHA tested was one of the HC 200, 300 or 220.
Snik and colleagues79 presented data from seven participants using the HC 220 on the average difference between the sound field warble thresholds (BCHA minus BAHA) across a range of frequencies, where tones were presented from the front through a loudspeaker. At 0.25 and 0.50 kHz, positive values indicate that hearing was better with the BCHA; at 1, 2 and 4 kHz, negative values indicate that hearing was better with the BAHA. The study did not report the results for testing at 8 kHz. No statistical analysis was undertaken.
Speech audiometry
All three77–81 included studies provide details of speech discrimination using BCHA and BAHAs (see Table 8). Tests varied between the studies and are discussed in turn below.
Béjar-Solar and colleagues77 reported sound field 100% speech audiometry discrimination at a background noise of 65 dB. The authors adapted a test by Håkansson and colleagues, using colloquial language common to Mexico City and sentences with a high degree of difficulty. This showed a 23% improvement with the BAHA compared with the BCHA (BCHA 62 dB HL, BAHA 48 dB HL, difference –14). In addition, accurate directional identification of the location of a sound was demonstrated in 80% of cases with the BAHA (0% with the BCHA).
Cooper and colleagues78 reported mean sound field speech discrimination scores, using Boothroyd word lists undertaken at 63 dB, for their two subgroups. The proportion of correct scores was not statistically significant between BCHA and BAHA in the chronic suppurative otitis media subgroup (n = 19; BCHA 65% vs BAHA 72%) or in the congenital hearing loss group (n = 16; BCHA 86% vs BAHA 85%).
Snik and colleagues79 reported both the maximum phoneme score and the speech recognition threshold (presentation level at which 50% of presented phonemes were repeated properly by the individual), which were determined using standard Dutch phonetically-balance (PB) word lists consisting of 10 monosyllables. The study showed that the maximum phoneme score was better with the BAHA HC 220 [48.7% (SD 31.7)] than with the BCHA [36.1% (SD 28.9)] although no statistical significance testing was undertaken and the sample size was small (n = 7). The mean speech recognition threshold in four participants with the BAHA HC 220 [38.8 (SD 11.1)] was similar to that with the BCHA in two participants [40.0 (SD 7.1)]; however, as noted previously, no statistical significance testing of these differences was undertaken. Two later publications reported additional outcomes and also a group of participants using the BAHA HC 200. 80,81 In the 1994 publication,80 the proportion of participants with a statistically significant improvement or deterioration with the BAHA in speech recognition in quiet and speech-to-noise ratio was reported. The speech recognition in quiet value in this study was the maximum phoneme score obtained using standard phonetically balanced lists of monosyllables, presented at 60, 70 and 80 dB. The speech-to-noise ratio was the difference between the SRT (established with an adaptive procedure, using the test described by Plomp and Mimpen91,92 with lists of 13 sentences) and the steady-state, speech-shaped noise presented at a fixed level of 65 dB). The study reports results separately for those using the HC 200 (n = 33) BAHA and those using the HC 220 (n = 11). This showed that four participants (12%) with the HC 200 and six (54%) with the HC 220 improved on their speech recognition in quiet score. For speech-to-noise ratio, 20 participants (60%) with the HC 200 and five (44%) with the HC 220 improved. No participants had a statistically significant deterioration in either outcome with the HC 200 or 220. No further details were reported. In the 1998 publication,81 a statistically significant improvement with the BAHA HC 200 (n = 33) was seen in mean SRT in quiet [2.7 dB (SD 4.4 dB), p < 0.05] and speech-to-noise ratio [2.5 dB (SD 2.2 dB), p < 0.05] compared with the BCHA. This publication reported using the tests as described in the 1994 publication above. 80
Self-reported measures
Béjar-Solar and colleagues77 reported QoL using a validated measure described as the Coop/Dartmouth test. 93 However, limited details were reported; the authors simply stated that ‘results uniformly showed the response “hardly could have done better” (options: hardly could have done better, pretty good, indifferent, pretty bad, hardly could have done worse)’. They also stated that physical and emotional condition was reported as very improved. Given that limited details were reported, these results should be interpreted with caution.
Hol and colleagues82 assessed QoL using a BCHA prior to BAHA surgery and again after 6 months’ experience with a BAHA. There were no statistically significant differences found by the SF-36 or EQ-5D (Table 9). The SF-36 showed increased emotional problems [indicated by a decrease in role limitations (emotional) score, p = 0.19], more pain experienced (p = 0.3) and improved mental health (p = not significant) with the BAHA, but the clinical effects were small (0.33, 0.24 and –0.36, respectively). Scores from the EQ-5D suggested that participants were slightly less mobile (p = 0.26) and experienced more pain/discomfort (p = 0.26) with the BAHA, but again the effect sizes were small (–0.30 and –0.28, respectively). However, statistically significant improvements in disability (p < 0.01) and handicap (p < 0.01) were found with the BAHA by the HHDI, and effect sizes indicated a large clinical impact (1.42 and 0.79, respectively).
| Study and outcomes | BCHA | BAHA model: Classic, Cordelle | Mean difference | Effect sizea |
|---|---|---|---|---|
| Hol et al. 200482 (n=20) | ||||
| SF-36 mean (SD) | ||||
| Physical functioning | 69.2 (25.4) | 70.8 (24.6) | 1.4 (p = NS) | –0.06 |
| Role limitations (physical) | 61.3 (40.1) | 57.5 (45.2) | –3.8 (p = NS) | 0.09 |
| Role limitations (emotional) | 76.7 (39.1) | 63.3 (41.8) | –13.4 (p = 0.19) | 0.33 |
| Vitality | 60.8 (16.6) | 61.0 (21.9) | 0.2 (p = NS) | –0.01 |
| Mental health | 68.4 (17.6) | 74.2 (14.2) | 5.8 (p = NS) | –0.36 |
| Social functioning | 80.6 (17.9) | 82.2 (18.3) | 1.6 (p = NS) | –0.09 |
| Pain | 73.8 (20.0) | 67.9 (27.9) | –5.9 (p = 0.30) | 0.24 |
| General health | 61.0 (19.8) | 59.5 (20.3) | –1.5 (p = NS) | 0.07 |
| EQ-5D mean (SD) | ||||
| EFive domains (score 1–3) | ||||
| Mobility | 1.35 (0.49) | 1.50 (0.51) | 0.15 (p = 0.26) | –0.3 |
| Self-care | 1.20 (0.41) | 1.10 (0.31) | –0.10 (p = NS) | 0.28 |
| Usual activities | 1.60 (0.68) | 1.55 (0.60) | –0.05 (p = NS) | 0.08 |
| Pain/discomfort | 1.70 (0.57) | 1.85 (0.49) | 0.15 (p = 0.26) | –0.28 |
| Anxiety/depression | 1.26 (0.45) | 1.20 (0.41) | –0.06 (p = NS) | 0.13 |
| Utility (score 0–1) | 0.71 (0.23) | 0.70 (0.19) | –0.01 | 0.05 |
| Visual analogue scale (0–100) | 74.0 (16.0) | 72.4 (17.4) | –1.6 | 0.10 |
| HHDI mean (SD) | ||||
| Disability | 31.0 (6.0) | 20.8 (8.2) | –10.2 (p < 0.01) | 1.42 |
| Handicap | 27.4 (6.2) | 21.8 (8.0) | –5.6 (p < 0.01) | 0.79 |
Hol and colleagues82 also reported the number of otolaryngology visits over the preceding 6 months for draining ears, and found these to reduce from a mean of 5.40 (SD 4.19, range 0–20) visits with BCHA to a mean of 1.50 (SD 2.10, range 0–6) visits with the BAHA (statistical significance not reported).
Four studies reported the results of subjective questionnaires on patient preference,77,81,82 satisfaction,78 comfort79 and opinions on speech recognition in noise and quiet. 78–81 None of these questionnaires appeared to have been validated and are therefore not discussed here. The data from these studies can be viewed in the data extraction forms in Appendices 6 and 8; however, care should be taken when interpreting results because of the issues associated with non-validated questionnaires.
Assessment of clinical effectiveness: BAHA versus ACHA
Audiological measures
Five included studies (in seven publications)76,78–81,83,84 provided a comparison of audiological measures between a BAHA and an ACHA (Table 10). Four studies were pre–post comparisons and the BAHAs used in these studies are no longer manufactured. One audiological comparison study assessed a BAHA model in current use (BAHA Intenso). 76 The high risk of bias in all five included studies should be considered when interpreting the results.
| Study and outcomes | ACHA | BAHA model | Comparison |
|---|---|---|---|
| aBurrell et al. 199683 (n = 9) | Model NR | ||
| Average sound field warble-tone thresholds (0.5–4.0 kHz), mean (SD), range,b dB(A) | 33 (5.4), 28–40 | 30.6 (8.1), 22–43 | NR |
| Sound field speech discrimination at 63 dB(A), % correct, mean (SD), rangeb | 91.6 (14.7), 60–100 | 84 (22.3), 30–100 | NR |
| aCooper et al. 199678 two subgroups: CSOM (n = 24), CON (n = 6) | HC 200, 300, 220 | p-value | |
| Mean sound field warble-tone thresholds, [dB(A), 500–4000 Hz]c |
CSOM 40 CON 41 |
CSOM 33 CON 28 |
p < 0.01 p < 0.01 |
| Mean sound field speech discrimination score (at 63 dB),% correctc |
CSOM 69 CON 57 |
CSOM 72 CON 82 |
p = NS p < 0.05 |
| Flynn et al. 200976 (n = 10) | Intenso | Difference | |
| Average aided warble-tone thresholds (dB SPL)c | p < 0.01 overall | ||
| 250 Hz | 39 | 47 | |
| 500 Hz | 42 | 39 | |
| 1 kHz | 37 | 30d | |
| 2 kHz | 43 | 31d | |
| 3 kHz | 46 | 39d | |
| 4 kHz | 50 | 41d | |
| 6 kHz | 75 | 53 | |
| 8 kHz | 68 | 55 | |
| Speech-in-noise ratio, dB | 3.44 | 0.88 | 2.56 |
| eMylanus et al. 199884 (n = 34) | Model NR | Difference and p-value | |
| Mean sound field threshold, dB HLa (SDf) | |||
| 0.25 kHz | 40 | 39 | p = NS |
| 0.50 kHz | 36 | 36 | p = NS |
| 1 kHz | 28 | 22 (8.3) | p < 0.01 |
| 2 kHz | 22 (11.9) | 25 | p = NS |
| 4 kHz | 37 | 33 | p = NS |
| 8 kHz | 55 (21.3) | 43 (22.3) | p < 0.001 |
| Maximum phoneme score (mean ± SD) | Data NR | Data NR | 1.0% ± 5.4%, p = NS |
| Speech-to-noise ratio improvement | Data NR | Data NR | 1.1 ± 2.1 dB p < 0.01 |
| eSnik et al. 1992,79 1994,80 199881 | HC 200, 220 | ||
| Snik et al. 199279 (n = 5) | ACHA | HC 220 | |
| Maximum phoneme score,%, mean (SD), rangeg | 81.6 (8.7), 70–90 | 67.6 (22.2), 43–90 | |
| Speech recognition threshold, dB(A), mean (SD), rangeg | 39 (10.8), 20–45 |
(n = 3) 45 (5), 40–50 |
|
| Average difference between the sound field warble thresholds, dBc (ACHA minus BAHA) | |||
| 250 Hz | –6 | ||
| 500 Hz | –5 | ||
| 1000 Hz | 3 | ||
| 2000 Hz | 4 | ||
| 4000 Hz | 15 | ||
| 8000 Hz | 0 | ||
| Snik et al. 199480 (n = 14) | HC 220, HC 200 | ||
| Patients with a statistically significant change in: | HC 220 (n = 5) | ||
| • speech recognition in quiet score with BAHAh | Improved: 2 of 5 (40%); deteriorated: 1 of 5 (20%) | ||
| • speech-to-noise ratio score with BAHAh | No results for HC 220 | ||
| Patients with a statistically significant change in: | HC 200 (n = 9) | ||
| • speech recognition in quiet score with BAHAh | Improved: 0 of 9; deteriorated: 1 of 9 (11%) | ||
| • speech-to-noise ratio score with BAHAh | Improved: 5 of 9 (56%); deteriorated: 1 of 9 (11%) | ||
| Snik et al. 199881 (n = 8) | HC 200 | ||
| Change in SRT in quiet (ACHA minus BAHA), mean dB (SD) | –6.4 (3.7), p < 0.05 significant deterioration | ||
| Change in speech-to-noise ratio, mean dB (SD) | 1.6 (1.0), p < 0.05 significant improvement | ||
Audiometry
All five included studies reported data on hearing threshold tests (see Table 10); four used sound field warble-tone thresholds76,78,79,83 and the remaining study states that it used sound field thresholds, but whether this was pure-tone or warble-tone was not reported. 84
Cooper and colleagues78 reported mean sound field warble-tone thresholds over a 0.5–4.0 kHz range. In this study two subgroups were tested, those whose condition was caused by chronic suppurative otitis media (n = 19) and those whose condition was congenital hearing loss (n = 16). Mean sound field warble-tone threshold was reported to be statistically significantly better with the BAHA than with the ACHA in the chronic suppurative otitis media subgroup [40 dB(A) ACHA vs 33 dB(A) BAHA, p < 0.01]. In the congenital hearing loss subgroup the BAHA was also seen to be statistically significantly better than the ACHA [41 dB(A) ACHA vs 28 dB(A) BAHA, p < 0.01]. In this study the BAHA tested was one of the HC 200, 300 or 220. Flynn and colleagues76 reported data on the average aided sound field warble-tone thresholds at a range of frequencies (0.25–8.0 kHz). Testing 10 participants with either an ACHA or the BAHA Intenso, this study reports that, clinically, audibility improved with the BAHA by 5–15 dB at 1, 2, 3 and 4 kHz and that the BAHA provided a statistically significant improvement (p < 0.01) in audibility. All participants in this study had used BAHAs for at least 1 year prior to assessment with the ACHA.
Snik and colleagues79 presented data from five participants on the difference between the sound field warble thresholds (ACHA minus HC 220 BAHA) across a range of frequencies. At 0.25 and 0.50 kHz, negative values indicate that hearing was better with the BAHA, while at higher frequencies (1–4 kHz), the positive values indicate that hearing was better with the ACHA (with a zero difference at 8 kHz). However, there is no statistical significance testing to aid interpretation of these data. Participants in this study had severe mixed hearing loss (mean hearing aid use of 23 years). Burrell and colleagues83 reported mean sound field warble-tone thresholds over a 0.5–4.0 kHz range. Participants in this study had otosclerosis for which stapedectomy was declined, not indicated or had previously failed. In the nine participants tested, the mean (SD) sound field warble-tone thresholds (0.5–4.0 kHz) were 33.0 dB(A) (5.4) with the ACHA compared with 30.6 dB(A) (8.1) with the BAHA. No statistical analyses are presented; however, the authors state that results were comparable to the ACHA and ‘significantly’ better in one case. The BAHA model used in this study, which was conducted in 1996, was not reported.
Mylanus and colleagues84 tested mean sound field thresholds over a 0.25–8.00 kHz range in 34 participants. The BAHA (model not reported) was seen to be statistically significantly better than the ACHA at 1 kHz and at 8 kHz. There were no statistically significant differences between the two devices at the other frequencies tested.
Speech audiometry
All five76,78–81,83,84 included studies provide details of speech discrimination using ACHA and BAHAs (see Table 10). A variety of different tests were used, as discussed below.
Cooper and colleagues78 report mean sound field speech discrimination scores, using Boothroyd word lists undertaken at 63 dB, for their two subgroups (chronic suppurative otitis media n = 24, congenital hearing loss n = 9). The difference in proportion of correct scores between ACHA and BAHA in the chronic suppurative otitis media subgroup was not statistically significant [ACHA 69% vs BAHA 72%, p = not significant (NS)], but in the COM subgroup there was a statistically significant difference in favour of BAHA (ACHA 57% vs BAHA 82%, p < 0.05). Burrell and colleagues83 also reported mean sound field speech discrimination scores, using Boothroyd word lists at 63 dB(A). The proportion of correct scores was not tested for statistical significance; however, scores were better (91.6%, SD 14.7) with the ACHA than with the BAHA (84.0%, SD 22.3).
Flynn and colleagues76 measured speech understanding in noise using the adaptive procedures from the Swedish version of the Hearing In Noise Test,94 and presented speech through a loudspeaker from 0° and noise from 180°. They found a 2.56 dB improvement in the speech-to-noise ratio with the BAHA compared with the ACHA in their sample of 10 (ACHA 3.44 dB vs BAHA 0.88 dB). No statistical analysis was presented for this difference, but the authors suggested that the improvement in speech understanding with BAHA was ‘large and clinically significant’. In this cross-sectional audiological comparison study, all 10 participants had experience with BAHAs prior to testing with the ACHA.
Mylanus and colleagues84 reported both the maximum phoneme score, which was calculated from the sound field speech audiogram, and the speech-to-noise ratio improvement. The speech-to-noise ratio was determined according to the criteria of Plomp and Mimpem91 at a fixed noise level of 65 dB. In this study of 34 participants it was shown that there was no statistically significant difference between devices on the maximum phoneme score (difference 1.0% ± 5.4%), but a statistically significant improvement in the speech-to-noise ratio between ACHA and BAHA (difference 1.1 ± 2.1 dB, p < 0.01) in favour of the BAHA.
Snik and colleagues79 found improved outcomes with the ACHA compared with the BAHA HC 220 for maximum phoneme score [ACHA 81.6% (SD 8.7%), BAHA 67.6% (SD 22.2%); n = 5)] and speech recognition threshold [ACHA 39.0 (SD 10.8) db (A), BAHA 45.0 (SD 5.0) db (A); n = 3], which were determined using standard Dutch PB word lists consisting of 10 monosyllables. However, no statistical significance testing was undertaken and the sample size was small, therefore the meaningfulness of the data is uncertain. Two later publications reported additional outcomes and also a group of participants using the BAHA HC 200. 82,83 The 1994 publication80 simply reported the proportion of participants with a statistically significant improvement or deterioration with the BAHA in speech recognition in quiet and speech-to-noise ratio, determined using methods previously described in Audiological measures. This showed that with the HC 200 (n = 9) no participants improved on their speech recognition in quiet score, whereas 11% deteriorated, and with the HC 220 (n = 5) 40% of participants improved and 20% showed a deterioration. No further detail was reported. For speech-to-noise ratio, 55% of participants with the HC 200 improved and 11% deteriorated. No results were presented for the HC 220 on this measure. The 1998 publication81 reported the change in SRT in quiet and the change in speech-to-noise ratio. The authors reported a statistically significant deterioration in mean SRT in quiet with the BAHA HC 200 [–6.4 dB (SD 3.7), p < 0.05] among their eight participants, although there was a statistically significant improvement in speech-to-noise ratio [1.6 dB (SD 1.0), p < 0.05]. The authors reported that these ambiguous results do not mean that the ACHA would be used in preference to the BAHA in their sample, owing to their chronic draining ears.
Self-reported measures
Hol and colleagues82 assessed QoL using an ACHA prior to BAHA surgery and again after 6 months’ experience with a BAHA. There were no statistically significant differences found by the SF-36 (Table 11), and although mental health improved slightly with the BAHA (p = not significant), the effect size was small (–0.28). The EQ-5D found a statistically significant increase in anxiety/depression (p < 0.01) with the BAHA, but again the clinical effect was small (–0.3). There were no other statistically significant differences in the EQ-5D. However, statistically significant improvements in disability (p < 0.01) and handicap (p < 0.01) were found with the BAHA by the HHDI, and effect sizes indicated a large clinical impact (0.79 and 0.86, respectively).
| Study and outcomes | ACHA | BAHA model: Classic, Cordelle | Mean difference | Effect sizea |
|---|---|---|---|---|
| Hol et al. 200482 (n = 36) | ||||
| SF-36 mean (SD) | ||||
| Physical functioning | 80.3 (21.8) | 79.8 (22.4) | –0.5 (p = NS) | 0.02 |
| Role limitations (physical) | 71.5 (39.7) | 68.9 (40.5) | –2.6 (p = NS) | 0.06 |
| Role limitations (emotional) | 76.2 (40.1) | 73.2 (38.1) | –3.0 (p = NS) | 0.07 |
| Vitality | 60.4 (20.0) | 59.9 (19.9) | –0.5 (p = NS) | 0.02 |
| Mental health | 62.4 (18.0) | 67.9 (21.3) | 5.5 (p = NS) | –0.28 |
| Social functioning | 69.8 (28.3) | 75.0 (27.8) | 5.2 (p = NS) | –0.19 |
| Pain | 74.7 (25.2) | 79.2 (25.0) | 4.5 (p = NS) | –0.18 |
| General health | 63.2 (21.4) | 63.6 (21.2) | –0.4 (p = NS) | –0.18 |
| EQ-5D mean (SD) | ||||
| Five domains (score 1–3) | ||||
| Mobility | 1.29 (0.46) | 1.31 (0.47) | 0.02 | –0.04 |
| Self-care | 1.03 (0.17) | 1.03 (0.17) | 0.00 | 0.00 |
| Usual activities | 1.47 (0.66) | 1.44 (0.50) | –0.03 (p > 0.05) | 0.05 |
| Pain/discomfort | 1.49 (0.51) | 1.47 (0.51) | –0.02 (p > 0.05) | 0.04 |
| Anxiety/depression | 1.26 (0.44) | 1.42 (0.60) | 0.16 (p < 0.01) | –0.30 |
| Utility (score 0–1) | 0.78 (0.17) | 0.77 (0.17) | –0.01 | 0.06 |
| Visual analogue scale (0–100) | 76.1 (14.1) | 73.4 (17.1) | –2.7 | 0.17 |
| HHDI mean (SD) | ||||
| Disability | 25.8 (6.5) | 20.9 (6.2) | –5.0 (p < 0.01) | 0.79 |
| Handicap | 25.0 (5.9) | 19.6 (6.7) | –5.4 (p < 0.01) | 0.86 |
Hol and colleagues82 also reported the number of otolaryngology visits over the preceding 6 months for draining ears, and found these to reduce from a mean of 12.7 (SD 10.5, range 0–30) visits with ACHA to a mean of 3.3 (SD 4.8, range 0–25) visits with the BAHA (statistical significance not reported).
Five studies reported the results of subjective questionnaires on patient preference,81–84 satisfaction,78 comfort79,83 and opinions on speech recognition in noise and quiet. 78–81 None of these questionnaires appeared to have been validated and they are therefore not discussed here. The data from these studies can be viewed in the data extraction forms in Appendices 7 and 8, although care should be taken when interpreting results owing to the issues associated with non-validated questionnaires.
Assessment of clinical effectiveness: BAHA versus unaided hearing
Audiological measures
Four cohort pre and post studies were identified that provide a comparison between BAHAs and unaided hearing (Table 12). 66,77,78,83 Three studies used BAHA models that are no longer manufactured. 77,78,83 The study by Kompis and colleagues66 included the BAHA Divino, which is in current use. As discussed in Characteristics of included studies, this study differs from the other included studies as the participants had prior experience with BAHAs when tested unaided at baseline. The high risk of bias in all four included studies should be considered when interpreting the results.
| Study and outcomes | Unaided | BAHA model | Comparison |
|---|---|---|---|
| Béjar-Solar et al. 200077 (n = 11) | BAHA Classic 300 | ||
| Sound field PTA threshold (1.25–3.00 kHz), dB HL | 64 | 19 | |
| aBurrell et al. 199683 (n = 9) | Model NR | ||
| Average sound field warble-tone thresholds (0.5–4.0 kHz), mean (SD), rangeb, dB(A) | 49.4 (11.9), 40–78 | 30.6 (8.1), 22–43 | |
| Sound field speech discrimination at 63 dB(A), % correct, mean (SD), rangeb | 74 (19.5), 50–98c | 84 (22.3), 30–100 | |
| aCooper et al. 1996,78 four subgroups: previous aid BC, CSOM (n = 19), CON (n = 16); previous aid AC, CSOM (n = 24), CON (9) | HC 200, 300, 220 | p-value | |
| Mean sound field warble-tone thresholds, dB [dB(A) 0.5–4.0 kHz]d | Previous aid BC | Previous aid BC | |
| CSOM 63 | CSOM 35 | p < 0.01 | |
| CON 62 | CON 26 | p < 0.01 | |
| Previous aid AC | Previous aid AC | ||
| CSOM 60 | CSOM 33 | p < 0.01 | |
| CON 68 | CON 28 | p < 0.01 | |
| Mean sound field speech discrimination score (at 63 dB), % correctd | Previous aid BC | Previous aid BC | |
| CSOM 17 | CSOM 72 | p = NR | |
| CON 3 | CON 85 | p = NR | |
| Previous aid AC | Previous aid AC | ||
| CSOM 19 | CSOM 72 | p = NR | |
| CON 17 | CON 82 | p = NR | |
| Kompis et al. 200766 (n = 7) | Divino | p-value | |
| Average improvement in sound field thresholds over all frequencies compared with unaided, dB | 28.0 | p < 0.0001 | |
| Speech recognition thresholds in quiet using two-digit numbers, dB (assume value is mean)d | 54 | 23 | p = NR |
| Speech recognition scores for monosyllabic words in quiet, % correct (assume mean)d | |||
| 50 dB SPL | 5 | 45 | p = NR |
| 65 dB SPL | 15 | 90 | |
| 80 dB SPL | 50 | 95 | |
| Speech recognition threshold in noise (noise presented from front or back), dBd | Omnidirectional/directional mode | ||
| Front | 12 | 3/4 | |
| Back | 9 | 3/1 | |
The studies by Béjar-Solar and colleagues,77 Burrell and colleagues83 and Cooper and colleagues78 also reported data comparing the BAHA with the hearing aids previously used, BCHAs,77 ACHAs83 or ACHAs and BCHAs. 78 In order to aid the narrative synthesis of studies for similar comparators, the BAHA data from these studies are repeated in Tables 8, 10 and 12. Care should therefore be taken to ensure that data for BAHAs are not ‘double-counted’ when interpreting the results. The entire data set for each study can be seen in the data extraction forms in Appendices 6–9.
Audiometry
All four studies66,77,78,83 included data on audiometry: two78,83 stated using sound field warble-tone thresholds and one used sound field pure-tone. 77 All four studies found an improvement with BAHAs compared with the unaided condition (see Table 12). 66,77,78,53 Mean sound field PTA threshold improved from 64 dB HL unaided to 19 dB HL with the BAHA in 11 participants with inoperable bilateral congenital atresia in the study by Béjar-Solar and colleagues. 77 Burrell and colleagues83 included nine participants with otosclerosis who had previously used an ACHA. Average sound field warble-tone thresholds (0.5–4.0 kHz) improved from 49.4 (SD 11.9) dB(A) unaided to 30.6 (SD 8.1) dB(A) with the BAHA, although no statistical analysis was reported. It should be noted that the unaided condition was assessed pre- and post-operatively in this study, and it is not clear which of these data are presented. Another study from the same centre (and therefore note the possibility of overlap of participants) by Cooper and colleagues78 included a total of 68 participants split into four subgroups according to previous aid (ACHA or BCHA) and aetiology (chronic suppurative otitis media or congenital hearing loss). In all subgroups, the mean sound field warble-tone thresholds showed statistically significant improvement with the BAHA (p < 0.01 for each comparison; see Table 12). The models used in this study were the HC 200, 220 or 300.
The study by Kompis and colleagues66 included seven participants who had at least 2 years’ experience of a BAHA Compact or Classic 300. Sound was presented through two loudspeakers placed just off one diagonal axis, which the participant sat in between. The study found a statistically significant improvement of 28 dB (p < 0.001) in average sound field thresholds over all frequencies after 3 months with the BAHA Divino compared with unaided.
Speech audiometry
Three studies66,78,83 reported data on speech audiometry. In nine participants with otosclerosis, Burrell and colleagues83 demonstrated an improvement in sound field speech discrimination scores, using Boothroyd word lists at 63 dB(A), from 74% (SD 19.5%) unaided to 84% (SD 22.3%) with a BAHA (model not reported), although no statistical analysis was conducted. Cooper and colleagues78 also found improvements in this outcome using either a BAHA HC 200, 300 or 220 compared with unaided in each of their subgroups (see Table 12), but again no statistical analysis was undertaken.
Speech recognition thresholds in quiet (levels required for 50% speech understanding, using Freiburger two-digit numbers) improved from 54 dB unaided to 23 dB with the BAHA Divino in the study by Kompis and colleagues. 66 There was also an improvement in speech recognition scores in quiet, which were defined as the percentage of correctly repeated Freiburger monosyllabic words at 50 dB SPL (decibel sound pressure level) (unaided 5%, BAHA Divino 45%), 60 dB SPL (unaided 15%, BAHA Divino 90%) and 80 dB SPL (unaided 50%, BAHA Divino 95%). Speech audiometry in noise was assessed using the Basler sentence test,95 an adaptive test in which speech was presented at 70 dB from a loudspeaker in front of the participant and noise was emitted either from the same direction or from the back of the participant (180°). The SNR, in dB, at which 50% of the key words were understood correctly, was measured. The speech recognition threshold in noise was reduced approximately from 12 dB unaided to 3 dB with the Divino in the omnidirectional model and to 4 dB in directional mode when noise was presented from the front; and when noise was presented from the back the threshold was reduced approximately from 9 dB unaided to 3 and 1 dB, respectively. However, no statistical analysis was undertaken for any of these comparisons.
Self-reported measures
No self-reported measures were described by the included studies comparing BAHAs with unaided hearing.
Assessment of clinical effectiveness: unilateral versus bilateral BAHAs
Audiological measures
Four audiological comparison studies59,60,85–87 compared unilateral and bilateral BAHAs (one study was reported in two publications60,85) (Table 13, Appendix 10). None of these studies assessed a BAHA model that is currently available. In each of the four studies, participants had several months’ experience with bilateral BAHAs before being tested with unilateral and bilateral BAHAs during the same session, which could lead to bias, as discussed in Quality assessment. The high risk of bias in all four included studies should be considered when interpreting the results.
| Study and outcomes | Unilateral | Bilateral | Comparison (p-value) | |
|---|---|---|---|---|
| Bosman et al. 2001,60,85 BAHA HC 200 or Classic 300 (n = 25) | Unilateral | Bilateral | ||
| SRT in quiet [dB(A)] | 41.5 | 37.5 | p < 0.001 | |
| SNR ([dB(A)], noise from the baffle side | –0.7 | –3.2 | p < 0.001 | |
| SNR [dB(A)], noise from shadow side | –3.4 | –4.0 | p > 0.05 | |
| Directional hearing at 500 Hz, %a | ||||
| Correct localisation | 23 | 42b | ||
| Localisation within 30° | 56 | 90b | ||
| Lateralisation | 54 | 85b | ||
| Directional hearing at 2 kHz, %a | ||||
| Correct localisation | 24 | 45b | p < 0.001 across all observations | |
| Localisation within 30° | 58 | 89b | ||
| Lateralisation | 64 | 87b | ||
| Proportion of responses corresponding to the fitted BAHA side at | ||||
| 500 Hz | 75.3% | 45.7% | ||
| 2 kHz | 70.3% | 48.8% | ||
| Binaural masking level difference SNRa,c | Bilateral BAHAs (n = 9) | |||
| S0N0 | SπN0 | S0Nπ | ||
| 125 Hz | 2.2 | 3.8 | –3.7 | p < 0.001 |
| 250 Hz | 0.1 | –6.0 | –5.1 | p < 0.001 |
| 500 Hz | 0.4 | –5.9 | –3.9 | p < 0.001 |
| 1 kHz | 0.4 - | –3.3 | –4.9 | p > 0.05 (NS) |
| Dutt et al. 2002,86 BAHA Compact (n = 11) | Unilateral | Bilateral | ||
| Speech-in-quiet (Arthur-Boothroyd word list cumulative scores, 30 words) ata | Best response | |||
| 30 dB intensity levels | 1 | 5 | ||
| 40 dB intensity levels | 13 | 19 | ||
| 50 dB intensity levels | 20 | 24 | ||
| 60 dB intensity levels | 25 | 28 | ||
| 70 dB intensity levels | 27 | 29 | ||
| 80 dB intensity levels | 30 | 30 | ||
| Speech-in-quiet (Bamford–Koval–Bench sentences) | All 11 patients scored 100% with right, left and bilateral BAHAs | |||
| Speech-in-noise (Bamford–Koval–Bench cumulative sentence scores) ata | Best response | |||
| Plus 10 SNR | 99 | 100 | ||
| Zero SNR | 80 | 81 | ||
| Minus 10 SNR | 0 | 1 | ||
| Plomp test,% correct score [mean (SD), range]d | ||||
| Sound front, noise front | Left side: 76 (11.7), 56–93; right side: 77.3 (11.7), 58–90 | 82.4 (13.3), 60–97 | ||
| Sound front, noise left | Left side: 40.1 (25.3), 2.–71; right side: 84.1 (11.2), 55–97 | 71.1 (14.9), 44–95 | ||
| Sound front, noise right | Left side: 88.2 (9), 72–100; right side: 45.8 (22.1), 13–88 | 79.5 (11.6), 58–93 | ||
| Priwin et al. 200487 BAHA Compact or Classic (n = 12) | ||||
| Average difference in sound field tone thresholds (at 0.25–8 kHz), dB | ||||
| Sound presented in front, at best side and from behind patients | 2–7 dB improvement with bilateral | |||
| Sound presented at shadow side | 5–15 dB improvement with bilateral | |||
| Speech recognition in quiet, average threshold, dB HL | 38.7 | 33.3 | p = 0.001 | |
| Speech-in-noise (change in SNR with bilateral BAHA), masking noise presented: | ||||
| At best side | 3.1 dB improvement | |||
| At shadow side | 1.0 dB deterioration | |||
| As surrounding noise | 2.8 dB improvement | |||
| Directional hearing | Best/shadow side | |||
| Per cent of correct answersa,e | ||||
| 0.5 kHz | 12/11 | 25 | ||
| 2.0 kHz | 8/10 | 23 | ||
| Per cent of answers within 30° of correct responsea,e | ||||
| 0.5 kHz | 23/30 | 53 | ||
| 2.0 kHz | 28/27 | 51 | ||
| Binaural masking level difference (relative threshold change in dB from the condition ‘signal and noise in phase at both sides’) | Bilateral BAHAs (n = 12) | |||
| 0.25 kHz | ||||
| SπN0 | Threshold changes within 3 dB except for two patients | |||
| S0Nπ | Threshold changes between –18 and 3 dB, mean –5 dB | |||
| 0.5 kHz | ||||
| SπN0 | Average threshold change 2 dB | |||
| S0Nπ | Average threshold change –4 dB | |||
| 1 kHz | ||||
| SπN0 | Average threshold change 3 dB | |||
| S0Nπ | Average threshold change –3 dB | |||
| Priwin et al. 200759 BAHA Classic or Compact. Two groups: (unilateral BAHA n = 6, bilateral BAHA n = 3) | One BAHA (unilateral n = 6/bilateral n = 3) | Two BAHAs (bilateral n = 3) | ||
| Sound field average tone thresholds, dB HL;d mean (SD, range) | 24 (5, 20–32)/30 (5, 25–35) | 25 (5, 20–30) | ||
| Speech recognition in noise, median score (%)a | ||||
| SNR 0 dB | 87/69 | 88 | ||
| SNR 4 dB | 92/79 | 93 | ||
| SNR 6 dB | 98/97 | 90 | ||
| Localisation of sound at 0.5 kHz,a mean % | ||||
| Correct scoref | ||||
| 50 dB | 20/20 | 50 | ||
| 60 dB | 28/20 | 50 | ||
| Lateralisations scoref | ||||
| 50 dB | 68/60 | 86 | ||
| 60 dB | 70/68 | 94 | ||
| Localisation of sound at 3 kHz,a mean % | ||||
| Correct scoref | ||||
| 50 dB | 28/16 | 50 | ||
| 60 dB | 37/18 | 57 | ||
| Lateralisations scoref | ||||
| 50 dB | 60/68 | 80 | ||
| 60 dB | 72/56 | 96 | ||
Audiometry
Two included studies reported data on hearing thresholds, one in an adult population87 and one in children59 (see Table 13). Priwin and colleagues87 included 12 adults and found an average improvement of 2–7 dB with bilateral BAHAs in free sound field tone thresholds (at 0.25, 1, 1.5, 2, 3, 4, 6 and 8 kHz) when sound was presented in front, at the best side (usually aid first implanted) and from behind participants, and an improvement of 5–15 dB when sound was presented at the shadow side. A comparative strength of this study is that the BAHAs, which were either Compact or Classic models, were electronically controlled by research personnel (BAHAs could be switched on and off by the investigator without the participant’s knowledge) and tests were randomised so that participants were blinded to unilateral or bilateral use of BAHAs.
A later study by the same group59 included two small groups of children, unilateral BAHA users (n = 6) and bilateral BAHA users (n = 3), although no statistical comparison was made between the groups. The bilateral BAHA users group were tested using one BAHA and two BAHAs, but the unilateral BAHA users group were only tested with one BAHA. Mean sound field average-tone thresholds were 24 (SD 5) dB HL for the unilateral BAHA user group, 30 (SD 5) dB HL for the bilateral group using one BAHA and 25 (SD 5) dB HL for the bilateral group using two BAHAs.
Speech audiometry
All four included studies59,60,85–87 reported speech audiometry with unilateral and bilateral BAHAs (see Table 13).
In a study of 25 consecutive patients by Bosman and colleagues,60,85 mean SRTs in quiet were measured with sentences by Plomp and Mimpen91 and Smoorenburg,96 with speech presented in front of the participant and an adaptive procedure used to determine the presentation level providing a whole-sentence correct score of 50%. SRTs in quiet were found to be significantly lower with bilateral than unilateral BAHAs [37.5 dB(A) vs 41.5 dB(A), p < 0.001]. A statistically significant difference was also found in favour of bilateral BAHAs in the SNR for noise presented at 65 dB(A) from the baffle side [–3.2 dB(A) vs –0.7 dB(A), p < 0.001], but not for noise presented from the shadow side [–4.0 dB(A) vs –3.4 dB(A), p > 0.05]. This study used the BAHA HC 200 or Classic 300.
Dutt and colleagues86 assessed 11 adults whose ‘professional needs warranted binaural hearing’ and used a BAHA Compact. All had voluntarily applied for the second BAHA and all had previous experience of binaural hearing. No statistical comparison was made between unilateral and bilateral use of BAHAs; therefore, comments are based on observation of the data. For sound field speech in quiet using Bamford–Kowal–Bench sentences, all 11 participants scored 100% with right, left and bilateral BAHAs. For speech in quiet using Arthur Boothroyd word list cumulative scores at 30–80 dB intensity levels, bilateral BAHAs appeared to be slightly better than the best unilateral response at lower intensities (see Table 13). Speech-in-noise cumulative sentence scores appeared similar between the best unilateral response and bilateral BAHAs at SNRs of + 10, 0 and –10 dB. When using the Modified Plomp multitalker noise test, similar results were obtained with unilateral and bilateral BAHAs when both sound and noise were presented from the front. However, when the noise was presented from the BAHA side (baffle situation, i.e. noise from left and using left BAHA only, or noise from right and using right BAHA only), scores were lower with a unilateral BAHA than with bilateral BAHAs, and when noise was presented from the opposite side (shadow side, i.e. noise from right and using left BAHA only, or noise from left and using right BAHA only), scores were better with a unilateral BAHA than a bilateral BAHA.
In their study of 12 adults, Priwin and colleagues87 found the average threshold for speech recognition in quiet (measured with phonetically balanced three-word sentences extracted from Hagerman97 and presented at 0°) was statistically significantly lower with bilateral BAHAs than the best unilateral side (33.3 dB HL vs 38.7 dB HL, p = 0.001). SRT in noise was also tested, where speech was presented at the participant’s most comfortable level, between 65 and 80 dB HL, and noise was speech weighted. An improvement of around 3 dB in the SNR was found with bilateral BAHAs when masking noise was presented at the best side and as surrounding noise. A deterioration of 1.0 dB in the SNR was found with bilateral BAHAs when noise was presented at the shadow side.
Similar scores for speech recognition in noise were found between the unilateral BAHA users group (n = 6) and the bilateral BAHA users group (n = 3) in the study of children by Priwin and colleagues59 (see Table 13), although the group of bilateral BAHA users had lower scores when tested with just one BAHA at 0 and 4 dB SNR. However, as the sample sizes were very small and no statistical analysis was undertaken, this should be interpreted with caution. This study used phonemically balanced Swedish three-word sentences extracted from Hagerman. 97 Speech and noise were presented at 0°, with speech presentation level set at 60 dB SPL and noise presented at SNRs of 0, 4 and 6 dB; thus, noise was presented at 60, 56 and 54 dB SPL.
Directional hearing
Directional hearing was assessed in three included studies59,60,85,87 (see Unilateral versus bilateral BAHAs and Appendix 10 for details on methods). Correct localisation, localisation within 30° and lateralisation measured at 0.5 and 2.0 kHz were significantly better than chance (p < 0.05) with bilateral BAHAs, but not with unilateral BAHAs, in the study by Bosman and colleagues. 60,85 Bilateral scores were statistically significantly better than unilateral scores across all observations (p < 0.001) (see Table 13). The study also found that sounds appeared to come from the fitted side when just one BAHA was in use. The proportion of responses corresponding to the fitted (baffle) side for unilateral BAHAs was 75.3% at 0.5 kHz and 70.3% at 2.0 kHz, whereas for bilateral BAHAs the responses were more symmetrical at 45.7% at 0.5 kHz and 48.8% at 2.0 kHz.
Priwin and colleagues59 found similar results in their studies of 12 adults87 and nine children, although no statistical analyses were undertaken. In the first study, the proportion of correct answers with a unilateral BAHA on the best or shadow side (scores between 8% at 2.0 kHz and 12% at 0.5 kHz) were close to the chance level of 8.3%, while with a bilateral BAHA the proportion of correct answers increased to 25% at 0.5 kHz and 23% at 2.0 kHz. The results for the proportion of answers within 30° of a correct response followed the same pattern (see Table 13). 87 This suggests that sound localisation was better with bilateral BAHAs. Similarly, the second study59 found an improvement in sound localisation and sound lateralisation ability with bilateral BAHAs, while with unilateral BAHAs the results were close to chance levels (see Table 13).
Binaural hearing
Two studies used the masking-level difference test to investigate binaural hearing with bilateral BAHAs,60,87 and both claimed that their results indicate that binaural hearing with bilateral BAHAs is possible, at least in some situations. However, the interpretation of this test with BC, being more complex than the interpretation with AC, remains to be established. 98
Self-reported measures
Priwin and colleagues59 reported the validated MAIS and MUSS to assess hearing skills in ‘meaningful, real world situations’, and the IOI-HA to assess hearing aid outcomes. Scores appeared similar between unilateral and bilateral BAHA users for most items; however, given the very small sample sizes (n = 2–6), these results should be interpreted with caution (Table 14).
| Study and outcomes | Unilateral BAHA users (n = 6) | Bilateral BAHA users (n = 3) | p-value |
|---|---|---|---|
| Priwin et al. 200759 | |||
| MAIS and MUSS, a mean (SD) | (n = 6) | (n = 3b) | p = NR |
| Hearing aid use | 3.4 (1.3) | 4.0 | |
| Reaction to sounds | 3.1 (0.9) | 3.5 | |
| Sound discrimination | 3.5 (0.8) | 3.8 | |
| Verbal communication | 3.8 (0.6) | 3.7 | |
| Speech intelligibility | 3.1 (1.2) | 3.3 | |
| IOI-HA, c mean (SD) | (n = 6) | (n = 2b) | p = NR |
| Use | 5.0 (0.0) | 5.0 | |
| Benefit | 5.0 (1.0) | 5.0 | |
| Residual activity limitation | 4.2 (0.5) | 4.0 | |
| Satisfaction | 4.3 (1.0) | 5.0 | |
| Residual participation | 4.2 (1.3) | 3.0 | |
| Impact on others | 4.8 (0.4) | 2.5 | |
| QoL | 4.8 (0.4) | 5.0 | |
Adverse effects
Included studies
Only 3 of the 12 included studies reported any data on adverse effects77,81,84 (Table 15). Béjar-Solar and colleagues77 reported that no irritation was present at most follow-up visits (71 of 82 visits); however, it is not clear how many of the 11 participants experienced irritation. No participants in this study experienced infection leading to loss of the implant or any major complications. Osseointegration could not be achieved in one participant following an impact to the mastoid area. Re-operations to remove or replace implants or to reduce the thickness of the subcutaneous layer around the implant were required in 15% of participants in the study by Snik and colleagues. 81 Two of 34 participants in the study by Mylanus and colleagues84 stopped using their BAHA owing to pain of unknown cause; this was after 3 months’ use in one participant and 2.5 years’ in the second participant.
| Study | Results |
|---|---|
| Béjar-Solar et al. 200077 | BAHA Classic 300 (n = 11) |
| Unable to obtain osseointegration (following impact to mastoid area 24 hours after discharge from first stage) | 1/11 |
| Major complications | 0/11 |
| Types of skin reactions, n of observations (%) | |
| No irritation | 71/82 (87) |
| Slight erythema | 7/82 (8) |
| Erythema and moisture | 3/82 (4) |
| Red and moist with granulation tissue | 1/82 (1) |
| Infection leading to loss of implant | 0 |
| Total number of observations | 82 (71 at scheduled visits, 11 at unscheduled visits) |
| Mylanus et al. 199884 | BAHA model NR (n = 34) |
| States surgery was uneventful in all patients. Two stopped using their BAHA after 3 months and 2.5 years respectively, owing to pain – no explanation for this found | |
| Snik et al. 199881 | BAHA HC 200 (n = 39) |
| Lost implant owing to inflammation after 2 years of use | 1 – implant not replaced |
| Requested implant removal owing to pain after 3 years | 1 |
| Implants loss due to inflammation | 1 – implant replaced |
| Lost implant owing to trauma | 2 – implant replaced |
| Reduction of thickness of the subcutaneous layer around implant to minimise risk for inflammation | 2 |
| Total re-operations | 6/39 (15.4%) |
| Rejections of BAHA due to insufficient amplification | 0 |
| Severe deterioration in sensorineural hearing (25–65 dB HL) after surgery for cholesteatoma in cerebellopontine angle and refitted with more powerful BAHA (NBC-HC-220). However, result poor owing to severe deterioration of cochlear function | 1 |
| Non-users after at least 4.5 years (all others using BAHA on daily basis) | 2/39 (5%) |
Prospective case series
To supplement the limited data from the included studies, prospective case series reporting adverse events were identified from the list of potentially eligible studies (see Appendix 5). As they were not included in the systematic review, these studies did not undergo the same process of data extraction and quality assessment. Six prospective case series reporting adverse events were identified; five of these can be seen in Table 16. Further examination of the remaining study revealed no useful data for the purposes of this review so it is not discussed further. 99 The discussion below focuses on loss of implants and skin reactions as these were identified as being the most relevant to this report.
| Study details and patient characteristics | Adverse events | Results | ||||
|---|---|---|---|---|---|---|
| Bonding 2000100 | ||||||
| Denmark | Implants lost | 7 (19.4%) in 6 patients | ||||
| Prospective case series | After 27–78 months (median 42 months) | |||||
| Study period: 1986–98 | ||||||
| Length of follow-up (years): median 6, range 1–12 | Estimated causes of loss |
Minor trauma (3) Malignant disease with emaciation (1) |
||||
| Number of participants: 31 | ||||||
| Sample attrition/dropout: NR | Severe psoriasis with granulation (1) | |||||
| Indication for treatment: | ||||||
| Chronic otitis media (28) | Uncertain (2) | |||||
| External otitis (2) | Success rate of implants, expressed in a life table sample a | |||||
| Congenital atresia of ear canal (1) | Time period (years) | n | Implants lost | Success rate (%) | ||
| Age (years): median 58, range 36–80 | Within-group b | Cumulative | ||||
| 1 | 31 | 0 | 100 | 100.0 | ||
| Sex (M : F): 17 : 14 | 2 | 29 | 0 | 100 | 100.0 | |
| Funding: NR | 3 | 26 | 3 | 88.5 | 88.5 | |
| 4 | 21 | 1 | 95.2 | 84.3 | ||
| 5 | 19 | 1 | 94.7 | 79.8 | ||
| 6 | 15 | 0 | 100 | 79.8 | ||
| 7 | 14 | 1 | 92.9 | 74.1 | ||
| > 7 | 10 | 0 | 100 | 74.1 | ||
| Håkansson et al. 1990101 | ||||||
| Sweden | 167 implants in 147 participants | |||||
| Prospective case series | Type of skin reactions c | Observations, % (n) | ||||
| Study period: 1977–87 | 0: no irritation | 93.2 | ||||
| Length of follow-up: 1 month to 11.5 years | 1: slight redness | 4.1 | ||||
| 2: red and moist tissue | 1.3 (16 in 16 patients) | |||||
| Number of participants: 147 | 3: granulation tissue | 1.3 (16 in 13 patients) | ||||
| Sample attrition/dropout: 0 | 4: infection and removal of abutment | 0.1 (1) | ||||
| Indication for treatment | Total observations | 1236 | ||||
| Otitis media/external (107) | Abutments removed | 16 | ||||
|
External ear canal/ossicular malformation (24) |
• Skin infection | 1 | ||||
| • Discomfort, psychological | 5 | |||||
| Otosclerosis (9) | • No hearing improvement | 7 (sensorineural hearing loss) | ||||
| SNHL (7) | • Trauma | 2 | ||||
|
Age (years): mean 50.8 (SD 17.4), range (5–82) |
• Implant not integrated | 1 | ||||
| • Abutments changed owing to inadequately hygienic and loose coupling | 9 (all early in study) | |||||
| Sex (M : F): 78 : 69 | ||||||
| Funding: NR | ||||||
| • Tissue reduction, thick and moveable skin | 10 (all early in study) | |||||
| Jacobsson et al. 1992103 | ||||||
| Sweden | Grade of skin reactions e | Observations, n (%) | ||||
| Prospective case series | 0: no irritation | 99 (91.7) | ||||
| Study period: NR | 1: slight redness | 7 (6.5) | ||||
| Length of follow-up: 40 (range 1–144)d months | 2: red and moist, no granulation | 1 (0.9) | ||||
| 3: as in 2, with granulation tissue | 0 | |||||
| Number of participants: 16 | 4: revision of skin-penetration necessary | 0 | ||||
| Sample attrition/dropout: NR | ||||||
| Indication for treatment: NR | R: removal of implant owing to non-integration | 1 (0.9) | ||||
| Age (years): mean 10 (range 3–16)d | ||||||
| Total observations | 108 | |||||
| Sex: NR | ||||||
| Funding: NR | ||||||
| Mylanus et al. 1994102 | ||||||
| Netherlands | 33 implants | |||||
| Prospective case series | Type of skin reactions c | |||||
| Study period: 1991–2 | 0: no irritation | NR | ||||
| Length of follow-up (months): 9–25 | 1: slight redness | 11 (in 8 implants) | ||||
| 2: red and moist tissue | 7 (in 6 implants) | |||||
| Number of participants: 33 | 3: red and moist tissue and/or granulation, revision surgery | 0 | ||||
| Indication for treatment: | 4: infection and removal of abutment | 1 | ||||
| Chronic otitis media (28) | Implants lost | 2/33 | ||||
| Chronic otitis externa (3) | Cause of loss | Severe inflammatory reaction around implant site (1), trauma (1) | ||||
| Congenital anomaly (2) | ||||||
| Age (years): mean 50, range 15–76 | ||||||
| Life table: cumulative proportion of implants which did not suffer from any skin reaction in follow-up period | ||||||
| Sex (M : F): 13 : 20 | ||||||
| Funding: reportede | Interval months | No. followed | No. of reactions | Proportion within interval (%) | Cumulative proportion size | Effective sample size |
| 0–4 | 33 | 7 | 79 | 79 | 26 | |
| 4–8 | 26 | 3 | 88 | 70 | 18 | |
| 8–12 | 17 | 1 | 94 | 66 | 11 | |
| 12–16 | 10 | 0 | 100 | 66 | 7 | |
| 16–20 | 6 | 0 | 100 | 66 | 4 | |
| 20–24 | 3 | 0 | 100 | 66 | 3 | |
| Portmann et al. 1997104 | ||||||
| France | Type of skin reactionsc,e | Observations, n (%) | ||||
| Prospective case series | 0: no irritation | 232 (87.5) | ||||
| Time period: 1991–6 | 1: slight redness | 22 (8.3) | ||||
| Length of follow-up: 6 months to 5 years | 2: red and slightly moist tissue | 8 (3) | ||||
| 3: reddish and moist, granulation, revision may be indicated | 1 (0.4) | |||||
| Number of participants: 36 | ||||||
| Sample attrition/dropout: 1 | 4: removal of abutment due to infection | 2 (0.8) | ||||
| Indication for treatment: | ||||||
| Bilateral agenesia of ear (16) | Total observations | 265 | ||||
| Chronic otitis (20) | Implants removed | 3 [osseointegration did not occur (2), head trauma (1)] | ||||
| (44 fixtures: five with bilateral implants, three re-implantation) | ||||||
| Age (years): 5.5–62.0 | ||||||
| Sex: NR | ||||||
| Funding: NR | ||||||
Loss of implants
Bonding100 followed 31 adults for a median of 6 years (range 1–12 years). Seven (19.4%) implants were lost in six participants after a median of 42 months (range 27–78 months); the estimated causes were minor trauma (three), malignant disease with emaciation (one), severe dermatitis (psoriasis) with granuloma at the point of skin penetration (one), and uncertain causes (two). The success rate of the implants, expressed in a life table, was 100% at 2 years, reducing to 85% at 3–4 years and about 75% after 7 years (see Table 16).
In the study by Håkansson and colleagues,101 147 participants with 167 implants were followed for between 1 month and 11.5 years during 1977–87. Sixteen (9.6%) of the abutments were removed, owing to no hearing improvement (in seven participants with SNHL), unexplained discomfort or for psychological-cosmetic reasons (five), trauma (two), skin infection (one) and the bone implant not integrated (one). Nine abutments were changed owing to inadequate hygiene and loose coupling, and in 10 participants the subcutaneous tissue reduction had not been extensive enough, although these all occurred early in the study while improvements were still being made.
Mylanus and colleagues102 followed 33 participants with 33 implants for 9–25 months. Two (6.1%) of the implants were lost owing to a severe inflammatory reaction around the implant site (one) and trauma (one). Jacobsson and colleagues103 followed 16 children for a median of 40 months (range 1–144 months). One (6.3%) implant was removed owing to non-integration. Portmann and colleagues104 followed 36 participants with 44 fixtures (five with bilateral implants and three re-implantation) for between 6 months and 5 years. Three (6.8%) implants were removed, two because of lack of osseointegration and one because of head trauma.
Skin reactions
Skin reaction definitions used by the studies can be seen in Table 16. From a total of 1236 observations of skin reactions, Håkansson and colleagues101 found that 93.2% had no skin irritation and 4.1% had slight redness. Red and moist tissue, and granulation tissue, were each observed on 1.3% of occasions. Infection leading to the removal of the abutment occurred in one case (0.1% of observations). A total of 265 observations were made in the study by Portmann and colleagues;104 87.5% of these had no irritation, 8.3% had slight redness and 3.0% had red and slightly moist tissue. Skin reactions Type 3 and Type 4 were observed on 0.4% and 0.8% of occasions, respectively. Mylanus and colleagues102 did not report the total number of observations made, but reported that skin reaction Type 1 was observed 11 times in eight implants and Type 2 was observed seven times in six implants. Skin reaction Type 3 was never observed during the study, and a skin reaction leading to the loss of an implant was observed once. A life table of the cumulative proportion of implants that did not suffer from any skin reaction can be seen in Table 16. In the small study of 16 participants by Jacobsson and colleagues,103 91.7% of 108 observations had no irritation and 6.5% had slight redness. No grade 3 or 4 skin reactions were observed.
Summary of clinical effectiveness
No trials with a concurrent control group were identified; the 12 included studies59,60,66,76–87 were either one-group cohort pre and post studies or cross-sectional audiological comparison studies. The methodological quality and quality of reporting of the included studies was weak, putting them at high risk of bias.
BAHA versus BCHA
Four cohort pre and post studies77–82 provided a comparison of BAHAs and BCHAs. Improvements in sound field PTA and warble-tone thresholds were found with a BAHA by all three studies77–81 that reported this outcome, but statistical analysis was reported by only one study (p < 0.01). 78 A statistically significant improvement in SRT in quiet and speech-to-noise ratio was found by one study,81 while another study found no statistically significant difference in speech discrimination score. 78 Statistical analysis was not reported for other results, which included a 23% improvement in 100% speech audiometry discrimination in noise,77 but little difference in speech recognition threshold. 79 Statistically significant improvements with large clinical effects were found with BAHAs compared with BCHAs for disability and handicap using the HHDI. 82
BAHA versus ACHA
Five cohort pre and post studies78–84 and one cross-sectional audiological comparison study76 provided a comparison of BAHAs and ACHAs. Results for sound field pure-tone or warble-tone thresholds were inconsistent among the five studies reporting audiological data. 76,78–81,83,84 Where statistical analysis was undertaken, one study83 found a statistically significant improvement in mean warble-tone thresholds (0.5–4.0 kHz) with a BAHA, while a different study76 comparing each frequency individually found a statistically significant improvement with a BAHA at 1 and 8 kHz, but not at 0.25, 0.5, 2 or 4 kHz. The remaining three studies79,80,83 did not compare data statistically; one study found that the ACHA was better at 0.25 kHz but there was improvement with BAHA at other frequencies,76 another study found that the BAHA was better at 0.25 and 0.50 kHz, but the ACHA was better at higher frequencies,79 while in another study data on average warble-tone thresholds (0.2–4.0 kHz) were described as ‘comparable’ between BAHAs and ACHAs. 83
The direction of the effect was also unclear for speech audiometry, with some studies finding improved outcomes with the ACHA and some with the BAHA. One study reported better outcomes with the ACHA for speech discrimination scores,83 and another for maximum phoneme score79 or speech recognition threshold,79 although statistical analysis was not conducted. A later publication by the same authors as the latter study found a statistically significant deterioration in SRT in quiet with BAHA (p < 0.05), but statistically significant improvement in speech-to-noise ratio (p < 0.05). 81 One study found no statistically significant difference in maximum phoneme score, but a statistically significant improvement in speech-to-noise ratio with BAHA. 84 Speech discrimination score was statistically significantly better with the BAHA in the congenital hearing loss group but not the chronic suppurative otitis media group in one study. 78 The final study reported an improvement in speech-in-noise with the BAHA described as ‘large and clinically significant’. 84
Statistically significant improvements with large clinical effects were found with BAHAs compared with ACHAs for disability and handicap using the HHDI. 82
BAHA versus unaided hearing
Four studies66,77,78,83 reported improvements in sound field thresholds and speech audiometry (where reported) with BAHA compared with unaided hearing, which were statistically significant where analysis was undertaken.
Unilateral versus bilateral BAHAs
An improvement in sound field average tone thresholds with bilateral BAHAs compared with unilateral BAHAs was found in adults87 and a small group (n = 3) of children59 with previous experience of BAHAs. Two studies found that speech recognition thresholds in quiet were statistically significantly lower with bilateral BAHAs,60,87 although one study found similar results between unilateral and bilateral BAHAs. 86 Bilateral BAHAs produced better results when noise was presented from baffle/best side, but not when noise was presented from the shadow side. 60,86,87 Three studies found that localisation of sound was improved with bilateral BAHAs. 59,60,87 Two studies60,87 reported the binaural masking level difference test and suggested that BAHAs give binaural hearing, although the validity of their methods is uncertain.
Adverse events
The included studies reported very limited data on adverse events. Prospective case series reported rates of loss of implants between 6.1% (9–25 months follow-up)102 and 19.4% (median 6 years’ follow-up). 100 The vast majority of participants in the prospective case series experienced no or minor skin reactions.
Chapter 4 Economic analysis
The aim of this section is to assess the cost-effectiveness of BAHAs with respect to conventional hearing aids or unaided hearing, and unilateral and bilateral BAHAs in adults or children with bilateral deafness who would be considered suitable for a BAHA. The economic analysis comprises:
-
a systematic literature review of economic evaluations, QoL and cost studies in BAHAs and other potentially relevant comparator hearing aids
-
the development of a de novo economic model and presentation of cost-effectiveness results.
Systematic review
Published economic evaluations
A systematic literature search was undertaken to identify full economic evaluations and cost studies that included BAHAs and other potentially relevant hearings aids (BCHAs and ACHAs). The methods for the systematic review are described in Chapter 2. Details of the inclusion and exclusion criteria are shown in Chapter 2, Inclusion and exclusion criteria and the search strategies are documented in Appendix 2.
A total of 225 potentially relevant publications were identified by the searches. No relevant full economic evaluations involving BAHAs were found after screening titles and abstracts. The searches identified 29 economic evaluations in hearing aids. None of the identified economic evaluations (19 in cochlear implants and 10 in other hearing aids) was found to be directly applicable to the aim of this study.
Two studies107,108 that reported costs, but not outcomes, associated with BAHAs were reviewed for their relevance to estimating resource use and costing in our economic model. Catalano and colleagues107 assessed the costs (in terms of participants’ and physicians’ time, as well as fees for treatment) of outpatient and inpatient insertion of BAHAs in a retrospective study with 19 US participants. However, the costs in the study were not directly applicable to the development of our economic model because of the US perspective of the study. Moreover, day case surgery would be the current service standard for surgical implantation, preparatory to fitting of the BAHA processor, in the NHS. Therefore, this study was not used to inform the economic model.
Watson and colleagues108 in a retrospective analysis compared service use before and after BAHA insertion in 26 adults with suppurative otitis media exacerbated by behind-the-ear hearing aids. They identified a reduction in the number of treatments and visits after BAHA insertion compared with behind-the-ear hearing aids. As part of the analysis they outlined treatment protocols for people undergoing surgical implantation and post-surgical management (within the ENT clinic), as well as audiological management to fit and commission the BAHA processor in a UK district general hospital. This study was used, in conjunction with current service standards109 relevant to the NHS and expert opinion, to identify the management pathway for individuals considered eligible for a BAHA as a basis for costing the intervention in our economic model (see Resource use and cost data for more details).
Unpublished economic evaluations
An unpublished, UK-based, economic evaluation of BAHAs conducted for an MSc thesis was identified. 110 This study does not strictly meet the inclusion criteria for the review, as it appears that a minority of participants had bilateral hearing loss (33.3% were reported as having bilateral hearing loss, the remainder were unilateral/single-sided or not stated). However, given that this is the only economic evaluation of BAHAs compared with conventional hearing aids that was identified, it is briefly reviewed below.
The initial sampling frame for the study was all adults (greater than 16 years old) undergoing primary BAHA implantation by the Departments of Audiology and Otolaryngology at University Hospitals Birmingham NHS Foundation Trust (UHB) between April 2007 and June 2008. All patients were invited to participate in the study and were sent the Health Utilities Index (HUI) 15-item self-completion questionnaire, along with a set of questions concerning current hearing aid use and duration of hearing impairment. Of the 147 eligible participants, 89 returned the first questionnaire (61% response rate). Of those 89 who completed the baseline questionnaire, 70 completed a second questionnaire (at least 3 months after the fitting of their BAHA).
The mean age of participants responding to the initial questionnaire was 55 years, with 44% of respondents being male. The mean time from surgery to receiving their BAHA was 2.8 months and the mean time from fitting of their BAHA to receiving the second questionnaire was 6.1 months.
Costs for BAHA provision were based on charges by the provider to PCTs. These were £5689 for the first year, to cover surgical and audiological assessment, implantation surgery, post-surgical care and acquisition and fitting of the BAHA sound processor, and a cost for the annual maintenance contract. The contribution of each component to this total charge was not reported. For subsequent years, the continuing cost of providing BAHAs was based on the annual maintenance contract fee of £1004. The analysis appears not to take account of costs associated with adverse events or treatment failure in BAHA users. The comparator group for the analysis was modelled by assumption, using participants’ reported usage of hearing aids prior to receiving their BAHA [39/70 (56%) reported using one or more hearing aids prior to receiving their BAHA]. Costs of conventional hearing aids were based on provision of an NHS digital hearing aid (£260) replaced every 5 years.
Outcomes in the analysis were based on differences in utility (before and after BAHA provision), scored using the HUI3 and HUI2 algorithms, and participants’ age-sex-specific life expectancy, derived from UK life tables. The mean HUI3 utility at baseline was 0.57 (95% CI 0.52 to 0.62) for all 89 respondents to the initial questionnaire and 0.59 (95% CI 0.53 to 0.65) for the 70 participants who responded to both outcome questionnaires. The mean HUI3 utility score for participants post BAHA was 0.66 (95% CI 0.60 to 0.72). It is unclear whether individual utility values or the mean values were used to derive QALYs, as the study report refers both to using ‘pooled [utility] results’ and to ‘each subject[s]… utility scores’ in calculating QALYs.
Table 17 reports the costs, QALYs and incremental cost-effectiveness ratio (ICER) for BAHAs compared with participants’ previous hearing aid provision. Costs and outcomes are discounted at 3.5%.
| Previous hearing aid | BAHA | Difference | ICER (£/QALY gained) | |
|---|---|---|---|---|
| Costs (£) (95% CI) | 827 (644 to 1022) | 21,430 (20,263 to 22,535) | 20,604 | 17,610 |
| QALYs (95% CI) | NR | NR | 1.17 (0.50 to 1.91) |
A series of scenario analyses were reported, assuming that all participants in the modelled comparator group were using standard hearing aids (rather than the observed proportion, 56%) and adopting shorter time horizons. The study110 also reported a subgroup analysis, breaking the total population of participants down by the main indication for treatment. The ICER results were generally robust to variables in the scenario analyses, with the ICER reducing fractionally (to £17,224 per QALY gained) for increasing the proportion of the modelled comparator group using conventional hearing aids. Reducing the time horizon of the model led to higher ICERs (£18,820 at 20 years, £22,097 at 10 years and £28,928 at 5 years). In the analysis by indication for treatment, BAHAs were least cost-effective in participants with bilateral CHL (£32,331 compared with £7459 for unilateral CHL and £19,391 for single-sided deafness). However, the number of cases included for each comparison was low (13–31 cases).
Published quality-of-life studies
In addition to the searches for economic evaluations and cost studies, a systematic literature search was undertaken to identify QoL studies of BAHAs and other potentially relevant hearing aids (see Appendices 2 and 11 for details).
A total of 322 potentially relevant publications were identified by the searches. After screening titles and abstracts, only one relevant study (by Hol and colleagues,82 discussed in Chapter 3, Self-reported measures) was identified. This study reported statistically non-significant differences before and after BAHA implantation using generic measures (SF-36 and EQ-5D), but statistically significant differences using a condition-specific measure (HHDI). The difference between the condition-specific and the generic health-related QoL instruments was probably due to a lack of hearing dimension on the EQ-5D and SF-36 questionnaires. As has been noted elsewhere,112,113 the HUI3 appears to be a more suitable generic measure for QoL in a population with hearing difficulties rather than the EQ-5D or SF-36. Owing to a lack of sensitivity in the EQ-5D and SF-36 it was decided that the study by Hol and colleagues82 should not be used in the decision model.
Southampton Health Technology Assessments Centre economic analysis
We developed a new model to estimate the cost-effectiveness of BAHAs in separate cohorts of eligible adults and children. In view of the lack of relevant clinical data (see Chapter 3), expert advice was sought to determine the comparator most appropriate to clinical practice. This suggested that the model should be limited to comparing BAHAs against BCHAs. The outcomes used in the model are in terms of cost per case and cost per successful implantation. An exploratory analysis using cost per QALY is also presented. Scenario and sensitivity analyses were undertaken to consider the impact of parameter and structural uncertainty.
Model type and rationale for model structure
The management pathway for individuals considered eligible for a BAHA, as outlined in the Bone Anchored Hearing Aids Service Standards109 (discussed further in Resource use and cost data), indicates an initial phase of intensive activity (to assess eligibility, perform surgical implantation, fit and commission the BAHA processor) involving care from a multidisciplinary team, followed by a less intensive phase of long-term maintenance using the device. The clinical effectiveness section of this report (see Chapter 3, Adverse effects) has highlighted the risk of periodic occurrence of adverse outcomes including skin reactions or failure of the titanium implant, which may lead to revision surgery, a repeat of the original implantation procedure or possibly to people stopping using the BAHA. In addition to these longer term adverse event risks, there may also be short-term adverse events associated with the initial implantation procedure. 54,114 To take account of the changes in intensity of management, the periodic occurrence of adverse events and the potential for users to abandon the use of their BAHA, we developed a simple state transition model.
The model includes three states:
-
success
-
success with adverse outcome
-
failure.
Individuals in the success state may experience an adverse event and move to the success with adverse outcome state, or they may remain in the success state. Individuals in the success with adverse outcome state may undergo surgical or non-surgical management (depending on the nature of the adverse event) and may move to the success state (if the adverse outcome resolves), may move to the failure state (if they choose not to continue with the BAHA) or may remain in the success with adverse outcome state. Those in the failure state may remain there or may elect for a repeat of the original implantation procedure (with success, success with adverse outcome or failure as possible outcomes).
This conceptual model was implemented as a decision tree with embedded Markov processes using the software package Treeage Pro (Williamstown, MA, USA), as shown in Figure 2. People enter the model with bilateral deafness, currently managed using BCHA, but are considered potentially suitable for BAHA. If they choose to accept this option, they follow the branch from the root node of the decision tree marked BAHA and will immediately be assigned all the costs related to assessing eligibility for BAHA and the costs of surgical implantation. The BAHA node is associated with a Markov process containing four health states (the failure state described above has been split into a temporary ‘failure’ state, where people make a decision whether to accept a re-operation, and a CeaseBAHA state for those who decide not to undergo a repeat operation), plus an absorbing state marked death. This does not indicate that BAHA use or implantation surgery is expected to be associated with a significant risk of death, but is included to take account of general, all-cause, mortality over time in the population being modelled. People undergoing initial implantation surgery are allocated to potential short-term outcomes (success, success with adverse outcome and failure) on the basis of probabilities estimated from the literature. Success with adverse outcome or failure might be expected to incur higher costs or poorer outcomes (in terms of QoL) than successful surgery. Individuals whose initial implantation procedure was successful are subject to risks of adverse events (in the model these are limited to skin reactions at site of implantation and loss of integration of titanium implant) for each cycle of the model. Skin reactions may be treated non-surgically (grades 1 or 2, assumed managed by cleaning regimes and antibiotics) or surgically (grade 3 requiring revision surgery and grade 4 resulting in removal of implant). Loss of bone integration is managed by a repeat operation, although the model allows for people to choose not to undergo repeat surgery, with a similar range of outcomes as for the initial operation.
FIGURE 2.
Outline of model.
People whose initial surgery resulted in success, but with adverse outcome, may choose to cease treatment, in which case they move to the CeaseBAHA state. If they continue treatment, the adverse outcome may resolve, in which case they would move to the success state. If the adverse outcome is not resolved they continue similarly to those in the success state, but will stay in the success with adverse outcome arm of the model.
Individuals whose initial surgery was a failure (defined as failure to achieve bone integration) or whose implants subsequently lose bone integration may choose to have a re-operation with a similar possible range of outcomes as initial surgery.
The model has an annual cycle and the principal outcome for the model is the incremental cost of BAHAs compared with BCHA. BAHA users who choose not to undergo repeat operations, owing to loss of bone integration or severe skin reactions, are identified as treatment failures and are assumed either to revert to BCHA or to continue unaided.
Baseline cohort
The population in the base-case analysis are those with bilateral deafness who were already provided with BCHAs, but are considered for BAHA owing to convenience and improved wearability. For the purposes of the model, adults are considered to be aged ≥ 18 years. Children are those less than 18 years of age. The sex composition of the included cohorts reflects the general population and is applicable only in calculation of death rates.
Data sources used in the model
Clinical effectiveness data
Gain in hearing
Chapter 3 of this report, Assessment of clinical effectiveness: BAHA versus BCHA, presents the systematic review of outcomes for BAHAs compared with BCHA in terms of audiological outcomes. No quantitative summary of the reported outcomes could be produced and there was little consistency of reporting between included studies. Consideration was given to using one of these outcomes for inclusion in the economic model, but it is not clear from the included studies what these outcomes mean to those receiving BAHAs. We were not able to identify any robust methods to map from audiological outcome measures to QoL measures. As a result, the outcomes in the model are based on potential gains using a generic QoL scale that is sensitive to changes in hearing. Quality of life reports the approach adopted for outcome assessment (potential QoL gain) in the model.
Adverse events
Table 18 presents estimates of the annual risk of implant failure (loss of bone integration), using data reported and discussed in Chapter 3, Prospective case series. Data in columns 1–5 of Table 18 were extracted from the study by Bonding,100 which reported the number of patients followed up for up to 7 years and the count of implants lost each year. Annual risk of failure (column 6) has been estimated from the reported cumulative success proportions, using the declining exponential approximation to life expectancy (DEALE) method,115 which estimates a constant risk over time. The estimated annual risk of failure, beyond year 2, varies between 3.8% and 4.5%.
| Time (years) | n | Implants lost | Success (% success per year) | Cumulative success (%) | Annual risk of failurea (%) |
|---|---|---|---|---|---|
| 0 | 31 | 0 | 100.0 | 100.0 | |
| 1 | 31 | 0 | 100.0 | 100.0 | 0.00 |
| 2 | 29 | 0 | 100.0 | 100.0 | 0.00 |
| 3 | 26 | 3 | 88.5 | 88.5 | 4.09 |
| 4 | 21 | 1 | 95.2 | 84.2 | 4.28 |
| 5 | 19 | 1 | 94.7 | 79.8 | 4.51 |
| 6 | 15 | 0 | 100.0 | 79.8 | 3.76 |
| 7 | 14 | 1 | 92.9 | 74.1 | 4.28 |
| > 7 | 10 | 0 | 100.0 | 74.1 |
Figure 3 illustrates the fit of the predicted annual risk [based on the cumulative success proportion at 7 years (0.741)] to the data reported by Bonding,100 treating the cumulative success proportions as Kaplan–Meier survival estimates. This figure suggests that applying the constant risk may overestimate the failure rate in the first 2 years from implantation. For subsequent years there appears to be reasonable agreement between the Kaplan–Meier estimate and the line of fit using the DEALE method. The annual failure risk estimated at year 7 (4.28%) is applied in the base-case analysis.
FIGURE 3.
Goodness of fit of annual risk of titanium implant failure (derived using the DEALE method) and Kaplan–Meier estimates.
The clinical data discussed in Chapter 3, Prospective case series, typically report the proportion of BAHA users experiencing skin reactions, rather than an estimate of rates. The latter would be more appropriate given the differential follow-up periods shown for participants in all of the included studies. Table 19 reports our estimates of the rates of skin reactions by grade for people using BAHAs, based on the reported counts and cumulative person-years of observation in each study shown in Chapter 3, Prospective case series (95% CIs have been estimated using exact confidence limits for Poisson counts116). The estimated rates show considerable variation, with substantially higher rates of all skin reactions, except grade 3, reported by Portmann and colleagues104 and Mylanus and colleagues,102 than in other studies. It is difficult to interpret the significance of the results of these studies or suggest explanations for variation between studies, as little information is provided on characteristics of populations in the studies [for example, the proportion of paediatric cases or the proportion of subjects (adult or children) with learning disability]. The studies are comparatively small and are not all consistent in the categorisation of grade of skin reaction. We therefore adopted rates estimated from the study by Håkansson and colleagues101 in our model, as this is the largest study using standard definitions for grades of skin reaction. The sensitivity of results to this assumption is addressed in scenario analyses, in which estimates based on other included studies are applied.
| Source | Person-months at risk (n) | Count by grade | Rate per 100 person-years at risk (PYAR) (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| Grades 1 and 2 | Grade 3 | Grade 4 | Grades 1 and 2 | Grade 3 | Grade 4 | ||
| Holgers et al. 1988105 | 1515 (64) | 15 | 5 | 1 | 11.88 (6.65 to 19.60) | 3.96 (1.29 to 9.24) | 0.79 (0.02 to 4.41) |
| Håkansson et al. 1990101 | 5542 (164) | 67 | 16 | 1 | 14.51 (11.24 to 18.42) | 3.46 (1.98 to 5.63) | 0.22 (0.01 to 1.21) |
| Jacobsson et al. 1992103 | 586 (15) | 8 | 0 | 0 | 16.38 (7.07 to 32.28) | 0.00 (0.00 to 7.55) | 0.00a (0.00 to 7.55) |
| Mylanus et al. 1994102 | 476 (33) | 18 | 0 | 1 | 45.34 (26.89 to 71.72) | 0.00 (0.00 to 9.30) | 2.52 (0.06 to 14.05) |
| Portmann et al. 1997104 | 1338 (41) | 30 | 1 | 2 | 26.91 (18.15 to 38.41) | 0.90 (0.02 to 5.00) | 1.79 (0.22 to 6.48) |
The impact of these adverse events is included in cost estimates used in the model (see Resource use and cost data for discussion of the costs of managing and treating adverse events). No direct estimate of the impact of adverse events on QoL was found in our literature searches and these are not included in the base-case analysis.
All-cause mortality
The most recent UK life tables were used in the model to estimate the percentage of the cohort dependent on age that dies in each cycle of the model. No increase in the mortality rate was assumed to be applicable to the baseline cohort.
Quality of life
Exploratory analysis of hearing improvements from HUI3
The lack of useable QoL data for people using BAHAs in the studies discussed earlier in this chapter (see Systematic review) and the absence of any robust methods to map from outcomes identified in Chapter 3, Assessment of clinical effectiveness: BAHA versus BCHA, to QoL/health-state utility led to further methods being sought to link potential benefits from the use of BAHAs. An exploratory analysis was undertaken using the difference between the levels of the hearing attribute in the HU13 classification system.
The HUI is a generic, preference-based system for measuring health status and comprises a health-state classification system and formulae for calculating utility scores. The classification systems consist of a number of attributes, each representing a particular dimension of health status (such as pain or emotion). The attributes are divided into levels of increasing impact on health status. The HUI is available in three versions which, although they have some common attributes, have some notable differences. These determine the version that is most appropriate for a given study group. For example, the HUI2 contains a single ‘sensation’ attribute relating to sight, hearing and speech, whereas the HUI3 contains separate attributes for each of these senses. As a result of the inclusion of separate attributes for the sensations and demonstrated improved sensitivity over other generic QoL measures,112 the HUI3 has been used in previous economic evaluations of hearing aid devices117–119 and for descriptive studies of QoL before and after hearing aid provision. 113 As was noted in our review of QoL studies, the HUI appears to be a more suitable generic measure for QoL in a population with hearing difficulties than the EQ-5D or SF-36. Table 20 presents the level descriptions for the hearing attribute for the HUI3 classification system.
| Level | Description |
|---|---|
| 1 | Able to hear what is said in a group conversation with at least three other people, without a hearing aid |
| 2 | Able to hear what is said in a conversation with at least one other person in a quiet room without a hearing aid, but requires a hearing aid to hear what is said in a group conversation with at least three other people |
| 3 | Able to hear what is said in a conversation with one other person in a quiet room with a hearing aid and able to hear what is said in a group conversation with at least three other people with a hearing aid |
| 4 | Able to hear what is said in a conversation with one other person in a quiet room without a hearing aid, but unable to hear what is said in a group conversation with at least three other people even with a hearing aid |
| 5 | Able to hear what is said in a conversation with one other person in a quiet room with a hearing aid, but unable to hear what is said in a group conversation with at least three other people even with a hearing aid |
| 6 | Unable to hear at all |
The HUI3 has structural independence of the attributes in its classification system. 74 This is important for the explanatory analysis of QoL benefit as it is possible to strip out the other attributes and concentrate on potential changes in hearing gain as described in the classification system. However, any results derived from these methods should be interpreted with caution owing to their weak methodological basis. Furthermore, severe hearing loss and associated improvement in hearing and wearability from the use of BAHA could possibly affect other attributes in the HUI3 classification system, such as cognition. 112 Therefore, it is possible that this will underestimate the real gain in QoL that may be experienced from using a BAHA.
An estimate of potential utility gain was calculated from the difference in utility between levels in the hearing attribute of the HUI3, using the scoring algorithm for the multiattribute utility function on the Dead–Healthy Scale reported by Furlong and colleagues. 121 This was calculated while keeping all other attributes fixed at ‘level 1’. For example, the associated gain in utility for moving from level 6 of the HUI3 hearing attribute, at which the respondent is ‘unable to hear at all’, to level 5, at which the respondent is ‘able to hear what is said in conversation with one other person in a quiet room with a hearing aid, but unable to hear what is said in a group conversation with at least three other people with a hearing aid’, is 0.178. Put in terms of overall health-state utility, this is a move from 0.465 to 0.644 on the scale, where 0 is dead and 1 is full health. In Table 21 the difference in utility gains for moving between each of the hearing levels is calculated.
| Hearing level on the HUI3 hearing attribute (health state utility) | Utility gain from BAHA or hearing aid | |
|---|---|---|
| Before BAHA or hearing aid | After BAHA or hearing aid | |
| Level 6 (0.465) | Level 6 (0.465) | 0.000 |
| Level 6 (0.465) | Level 5 (0.644) | 0.178 |
| Level 6 (0.465) | Level 3 (0.849) | 0.384 |
We assumed that the QoL benefit from improved hearing could be proxied by levels of the hearing dimension in the HUI3, that identical gains were potentially achievable through use of BAHA and BCHA, and that BAHA-eligible patients were initially at level 6 (unable to hear at all). The minimum utility gain is therefore 0.178 (level 6 to level 5) and the maximum 0.384 (level 6 to level 3). Differences between the utility associated with use of BAHA or BCHA were assumed to be realised through differences in the wearability of alternative devices. For this we required information on participants’ use of their previous device and the BAHA.
Included studies were reviewed for information on post-implantation use of BAHAs (hours per day and days per week that the device was used) and for use of previous hearing aids. Additional publications reporting use of BAHAs and BCHAs were identified from the reference lists of included studies and using targeted searches. The results of these searches are summarised in Table 22, indicating (where reported) the key characteristics of participants included in the studies.
| Study | Participant characteristics | Questionnaire | Usage | |
|---|---|---|---|---|
| de Wolf et al. 2009122 | 135 (of 211) BAHA compact users aged 18–77 years. 100 with bilateral conductive/mixed hearing loss; 23 with unilateral conductive/mixed hearing loss; 12 with unilateral conductive/mixed hearing loss/other ear deaf | IOI-HA | BAHA use for greater than 8 hours per day | |
| Age range (years) | % | |||
| 18–40 | 82.1 | |||
| 41–60 | 84.1 | |||
| > 60 | 70.7 | |||
| Badran et al. 2006123 | 117 (of 152) adults who ‘underwent BAHA procedure for greater than 6 months’. 64% chronic otitis media and 21% chronic otitis externa and/or acquired stenosis | Entific Medical Systems questionnaire | 81% reported using BAHA everyday | |
| Hours BAHA used per day | % | |||
| > 8 | 78 | |||
| 4–8 | 15 | |||
| 2–4 | 3 | |||
| < 2 | 3 | |||
| Hol et al. 200482 | 56 consecutive adult patients with acquired conductive or mixed hearing loss (20 using conventional BCHA) | Not stated | Of those previously using BCHA, 100% reported using their BAHA for greater than 8 hours per day compared with 90% for their previous aid | |
| Dutt et al. 2002124 | 227 (of 351) children and adults implanted at Birmingham implant otology unit.a Cause of hearing loss not stated in this publication | Entific Medical Systems questionnaire | 95% reported using BAHA everyday | |
| Hours BAHA used per day | % | |||
| > 8 | 86 | |||
| 4–8 | 10 | |||
| 2–4 | 5 | |||
| < 2 | 3 | |||
| Cooper et al. 199678 | 68 (of 106) adults. 43 with CSOM (24 using ACHA and 19 using BCHA prior to BAHA) and 25 with congenital CHL (9 using ACHA and 16 using BCHA prior to BAHA) | Not stated | 95.5% reported using BAHA greater than 8 hours per day | |
Only one study,82 of relatively small size, reported results for both BAHA and BCH and this reported very limited information, simply the proportion of people using the hearing aid for more than 8 hours per day. For the base-case analysis it was assumed that this was a reasonable characterisation of the relative use of BCHA and BAHA, with BAHA use being approximately 10% greater than use of BCHA. The sensitivity of results to this assumption was tested in deterministic sensitivity analyses using the upper and lower values for the exact binomial CIs as shown in Table 23 and in scenario analyses.
| Hearing aid type | n | Reported usage (%) | 95% CI (exact binomial) |
|---|---|---|---|
| BCHA | 20 | 90 | 68.3% to 98.8% |
| BAHA | 20 | 100 | 83.2% to 100% |
The utility associated with each device, in the model, was therefore a weighted average of the unaided utility (uunaided) and the aided utility (uaided), with the weight based on the proportion of time users were able to wear their device (pwear). Hence the total utility for a given time period was defined as in Equation 1.
Another potentially relevant dimension of the HUI is pain and this was incorporated in scenario analyses. This item has five levels as described in Table 24.
| Level | Description |
|---|---|
| 1 | Free of pain and discomfort |
| 2 | Mild-to-moderate pain that prevents no activities |
| 3 | Moderate pain that prevents a few activities |
| 4 | Moderate-to-severe pain that prevents some activities |
| 5 | Severe pain that prevents most activities |
In the scenario analysis we assumed that the BCHA may be associated with ‘mild to moderate’ pain (level 2 of the HUI pain attribute) for the majority of users. In this scenario analysis pain is also included for BAHA users who experience pain requiring removal of the BAHA implant, assuming HUI level 5 pain for the cycle following implantation and leading to removal (in effect a very large penalty of 0.55 arising from intolerable pain).
Resource use and cost data
Both resource use and cost data are taken from an NHS perspective. The resource use associated with the implantation of BAHAs was identified using Watson and colleagues’s108 retrospective study of resource use and the Bone Anchored Hearing Aid Service Standards. 109 These sources were used to develop a resource use protocol (illustrated in Figure 4) which was discussed with clinical experts.
FIGURE 4.
Resource use protocol for patients considered eligible for a BAHA.
Four distinct phases of costs associated with BAHAs were identified. These cost phases were defined as being associated with:
-
assessment of surgical and audiological eligibility
-
surgery
-
post-surgical management (up to 12 months following the initial surgical procedure and included fitting of external processor)
-
long-term management.
All cost data and relevant sources are given and discussed in turn below. All unit costs and cost-effectiveness results are expressed in 2009 pound sterling.
Assessment of surgical and audiological eligibility costs
Potential BAHA users have an initial consultation with the specialist BAHA surgeon. This includes an examination of the middle and external ears and determination of the aetiology of hearing loss, as well as an assessment of the individual’s general medical status and suitability for BAHAs. The cost of consultation with the surgeon was taken from the NHS reference costs. 43 An ENT outpatient first attendance cost of £110.78 was used for adults and a paediatric ENT outpatient first attendance cost of £131.69 was used for children.
An audiological assessment is carried out by an audiologist, who assesses current middle and external ear status. AC and BC are tested using pure-tone audiometry. There may be an evaluation of the user’s current hearing aid provision, if applicable. The cost of the audiological assessment (£57.48) is taken from the NHS reference costs. 43
The final stage of the assessment phase involves a multiprofessional consultation (£147.36) to agree to the individual’s eligibility for a BAHA and to gain consent for surgery.
The breakdown of unit costs for the initial assessment of surgical and audiological eligibility for adults and children is given in Table 25. The total cost of the assessment of surgical and audiological eligibility cost was £315.63 for adults and £336.53 for children.
| Item | Adult unit costs (£) | Paediatric unit costs (£) |
|---|---|---|
| Initial consultation with surgeona | 110.78 | 131.69 |
| Audiological assessmentb | 57.48 | 57.48 |
| Multiprofessional consultationc | 147.36 | 147.36 |
| Total cost | 315.63 | 336.53 |
Surgery costs
The cost of one-stage surgery to implant the BAHA was taken from the NHS reference costs for a day-case BAHA operation with a cost of £2004.57 for adults. The paediatric two-stage method for implantation of a BAHA was assumed to cost twice the NHS reference cost for BAHA surgery and therefore had a cost of £4009.14. 43 Additional costs including the fixture and abutment costs and additional consumables from the surgery are given in Table 26. These had a total cost of £989.50. These costs are current list prices and were provided by the UHB. The breakdown of unit cost of surgery for adults and children is given in Table 26. The total cost for surgery in adults was estimated at £2994.07 in adults and £4998.64 in children.
| Item | Adult unit costs (£) | Paediatric unit costs (£) |
|---|---|---|
| BAHA surgery procedurea | 2004.57 | 4009.14 |
| Guide drillb | 15.50 | 15.50 |
| Countersinkb | 29.50 | 29.50 |
| Fixtureb | 310.00 | 310.00 |
| Abutmentb | 520.00 | 520.00 |
| Cover screwb | 62.00 | 62.00 |
| Healing capb | 23.50 | 23.50 |
| Dermatome blade b | 29.00 | 29.00 |
| Total | 2994.07 | 4998.64 |
Post-operative costs
Watson and colleagues108 identified three obligatory visits to ENT and audiology after surgery. This was verified by our experts. These visits consist of changing the dressing (removal of the mastoid bandages after 24 hours), removal of healing disc and stitches and an assessment to ensure osseointegration at around 3 months. The cost for these three visits was taken from the NHS reference costs. 43 There appears to be practice variation at this stage with either the surgeon or a specialist nurse undertaking the assessments. It was assumed that in the base case the surgeon undertook the assessment. This uncertainty was explored in a scenario analysis. Therefore, the cost of each consultation was taken from the NHS reference costs for an ENT outpatient follow-up attendance with a cost of £72.11 for adults and £90.93 for paediatric ENT43 (Table 27).
| Item | Unit costs (£) | |
|---|---|---|
| Adults | Paediatrics | |
| ENT outpatient visit to change dressinga,b | 72.11 | 90.93 |
| ENT outpatient visit to remove healing disk and stitchesa,b | 72.11 | 90.93 |
| ENT outpatient visit to ensure osseointegrationa,b | 72.11 | 90.93 |
| Initial fitting of the BAHA by an audiologistc | 64.80 | 64.80 |
| 4-week audiological follow-up of BAHA fittingd | 50.17 | 50.17 |
| 3-month performance of processor by an audiologistd | 50.17 | 50.17 |
| Total costs | 381.47 | 437.91 |
The first audiological attendance is to fit the BAHA device. This includes adjusting the device settings based on audiometric results and the user’s response, and recording the output of the device using electro-acoustic methods. Furthermore, consideration is given to fitting BAHA accessories, discussing the user’s expectation and providing information on the management of the device. The second audiological follow-up occurs at around 4 weeks and includes a site and abutment inspection when the tightness of the abutment is checked using a torque driver. A record of the output from the device using electro-acoustic methods is taken and this is compared with the measure taken at the time of fitting. The third audiological follow-up occurs at 3 months and includes a record of the output from the device to further assess performance and outcome measurement. An unaided and aided sound field audiometry test is undertaken and post-operative questionnaires are administered. The costs of the three audiological visits were taken from the NHS reference costs and were assumed to consist of a fitting cost of £64.80 and two follow-up visits of £50.17 each. 43 The breakdown of unit cost of post-operative follow-up for adults and children is given in Table 27. The total cost of post-operative follow-up for adults is £381.47 and £437.91 for children.
The cost of the BAHA processor is also included in this phase. The average cost of four of the BAHA processors currently used in the NHS and the cost of their maintenance plans are reported in Table 28. The average cost for both the processor and the maintenance plan was used in the model owing to uncertainty over which processors are currently used most in the NHS. A sensitivity analysis of the range of costs of the processor and first year maintenance plan was used to explore this uncertainty.
| BAHA model | Cost of processor (£) | Cost of maintenance plan (£) | Total cost (£) |
|---|---|---|---|
| Divino | 1820.00 | 610.00 | 2430.00 |
| Intenso | 1980.00 | 665.00 | 2645.00 |
| Cordelle | 1970.00 | 670.00 | 2640.00 |
| BP 100 | 2995.00 | 1000.00 | 3995.00 |
| Average cost | 2191.25 | 736.25 | 2927.50 |
The total cost of the post-operative follow-up and the processor (and maintenance plan) after surgery for adults is £3308.97 for adults and £3365.41 for children.
The cost per user at each stage of a successful implantation of a BAHA in the first year is given in Table 29. This includes the cost of audiological assessment, surgery costs and post-operative surgery costs with a total cost estimated at £6618.68 for adults and £8700.59 for children per successful implantation of a unilateral BAHA.
| Stage of BAHA implantation | Total cost adults (£) | Total cost paediatrics (£) |
|---|---|---|
| Total initial audiological assessment cost (see Table 25) | 315.63 | 336.53 |
| Total surgery costs (see Table 26) | 2994.07 | 4998.64 |
| Total post-operative surgery costs, first year (see Tables 27 and 28) | 3308.97 | 3365.41 |
| Total cost for BAHA implantation | 6618.68 | 8700.59 |
Long-term follow-up costs
Expert opinion and current service standards109 suggest that BAHA users will have periodic audiological follow-ups. In addition to the annual audiological follow-up, those who continue to use a BAHA will have the sound processor replaced every 3 years under the annual maintenance plan. At the annual audiological assessment a record of output from the device is measured using an electro-acoustic test and this is compared with the measure taken at time of fitting. Performance and outcome measurements are undertaken with unaided and aided sound field audiometry tests. Post-operative questionnaires may also be administered at this stage (Table 30).
| Item | Frequency | Unit costs adults (£) | Unit costs paediatrics (£) |
|---|---|---|---|
| Audiological assessment | Every year | 50.17a | 50.17a |
| BAHA maintenance plan | Every year | 736.25b | 736.25b |
| Total long-term costs every year | 786.42 | 786.42 |
Adverse events costs
Two types of adverse events were identified from the review of the literature and confirmed by our clinical experts: skin irritation associated with the implantation of the BAHA and a loss of osseointegration of the fixture. The resource use associated with grade 1 or grade 2 skin irritation (using the Holgers and colleagues grading system105) was assumed to consist of an outpatient visit for dermatological care and antibiotics to treat the reaction. The costs of an outpatient dermatology visit was taken from the NHS reference costs, with a cost £118.10 for adults and £134.82 for children. 43 The antibiotic steroid cost of £5.40 was taken from the British National Formulary (BNF). 125 The total estimated cost of treating a grade 1 or grade 2 skin reaction is £123.50 for adults and £140.22 for children (Table 31). Grade 3 skin reactions were assumed to require surgical revision and were costed as intermediate skin procedures, provided as day cases. 43 Grade 4 skin reactions were assumed to require removal of the skin-penetrating implant and were costed using the reference cost applied for the initial day case surgery (see Table 26), excluding the costs of surgical consumables required for the initial implantation.
| Skin reaction | Cost, adults (£) | Cost, paediatrics (£) | |
|---|---|---|---|
| Grades 1 and 2 | Dermatologista | 118.10 | 134.82 |
| Antibiotic steroids | 5.40 | 5.40 | |
| Total | 123.50 | 140.22 | |
| Grade 3 | Revision surgeryb | 663.66 | 663.66 |
| Grade 4 | Day case surgery to remove implantc | 2004.57 | 2004.57 |
| Loss of bone integration | |||
| Re-operationd | 2994.07 | 4998.64 | |
| Post-operative follow-up, year 1e | 1117.72 | 1174.16 | |
| Total | 4111.80 | 6172.80 | |
If loss of bone integration occurs, then there is a cost of re-operation surgery to re-implant the fixture and an associated post-operative surgery cost. This was assumed to be the same as the initial surgical operation and post-operative follow-up, as calculated above (see Table 29). The breakdown of costs for skin irritation and loss of osseointegration are reported in Table 31. The overall total cost of a loss of osseointegration was £4111.80 for adults and £6172.80 for children.
Costs associated with comparator pathways
People considered eligible for provision of BCHA were assumed to undergo an initial audiological assessment (including assessment of middle and external ear status as well as testing of AC and BC using pure-tone audiometry) similar to that for those considered suitable for BAHAs. People receiving a BCHA were assumed to have a single attendance to fit the BCHA with a follow-up visit at 3 months to assess the performance of the hearing aid. In subsequent years individuals were assumed to attend once a year for follow-up, with replacement of the hearing aid occurring every 5 years.
A range of costs was provided for the BCHA device itself, from a low cost of £117–183 for a body-worn aid (which is attached to the transducer via a cord) to £250 for a more cosmetically appealing option, incorporating a behind-the-ear hearing aid constructed onto the headband to drive the vibrator/transducer (using an NHS digital power aid). A higher cost of £300–350 was provided for proprietary behind-the-ear devices (Table 32). These costs were supplied by two NHS providers (see Table 32) and reflect current NHS purchasing arrangements for BCHA, rather than manufacturers’ list prices for any particular devices.
| Item | Unit costs, adults (£) | Unit costs, paediatrics (£) | |
|---|---|---|---|
| Initial audiological assessment costsa | 57.48 | 57.48 | |
| Fitting costsb | 64.80 | 64.80 | |
| Cost of BCHA device (£) | Low (body-worn)c | 117–183 | 117–183 |
| Low (behind-ear worn)c | 250 | 250 | |
| Highd | 300–350 | 300–350 | |
| Post-fitting, follow-up assessment (3 months) of device by an audiologiste | 50.17 | 50.17 | |
| Total | Low (body-worn) | 289–355 | 289–355 |
| Low (behind-ear worn) | 422 | 422 | |
| High | 472–522 | 472–522 | |
The total cost of providing a BCHA was estimated as between £289 and £522 for the first year (depending on the type of aid provided), with long-term costs of £50.17 (for an annual audiological assessment) and a replacement cost (for a new device, fitting and post-fitting assessment) of between £232 and £465 every 5 years.
Perspective and time horizon
The perspective of the cost-effectiveness analysis is that of the NHS and Personal Social Services (PSS). The analysis has adopted a medium-term horizon of 10 years. This is shorter than the lifetime horizon proposed in the protocol for this review. However, it is long enough for differences between the two cohorts to become apparent, but avoids extrapolating too far beyond the available clinical data (for example, clinical data on adverse events report outcomes at 7 years, for implant survival, and a maximum of 14 years for other outcomes).
Discounting
Both costs and outcomes were discounted using a 3.5% discounting rate, as currently recommended by the UK Treasury for public sector appraisal. 126
Assessment of uncertainty
The purpose of this analysis is to test the robustness of the cost-effectiveness results to variation in structural assumptions and parameter inputs. A deterministic sensitivity analysis (DSA) was used to address particular areas of uncertainty in the model. We investigated the uncertainties around the probability, resource use and cost estimates that were expected, a priori, to have a disproportionate impact on the study results, by applying ranges around the point estimates used in the base-case analysis. Scenario analysis was used to address the uncertainty associated with the choice of data source adopted for parameter values in the base case and the structural assumption that patients who stop using their BAHA switch to an alternative hearing aid or continue unaided.
Parameter uncertainty was addressed using probabilistic sensitivity analysis (PSA). Probability distributions are assigned to the point estimates used in the base-case analysis. Variables included in the PSA, the sampling distribution and the parameterisation of the sampling distributions are reported in Appendix 13.
Summary of assumptions and input parameters used in the model
Table 33 summarises the probabilities included as input parameters to the model and is predominantly concerned with adverse events associated with BAHA provision (for full details of potential data sources and selection of parameter inputs see Clinical effectiveness data). The table includes 95% CIs, used as upper and lower limits in deterministic analyses.
| Input parameter | Base-case value | 95% CI | Source | |
|---|---|---|---|---|
| Lower limit | Upper limit | |||
| Proportion of cohort that is male | ||||
| Children (aged < 16 years) | 0.5121 | NA | NA | ONS127 |
| Adults (aged > 50 years) | 0.4639 | NA | NA | ONS127 |
| Probability of adverse outcome from initial surgery | 0.0970 | See (a) and (b) below | ||
| (a) Probability of bleeding within 24 hours of surgery | 0.0121 | 0.0015 | 0.0431 | Badran et al.114 |
| (b) Probability of surgical removal of skin growth or soft tissue thickening around the abutment | 0.0848 | 0.0472 | 0.1383 | Badran et al.114 |
| Probability of failure of initial surgery | 0.0332 | See (c) and (d) below | ||
| (c) Probability of intolerable pain requiring removal of abutment and flange fixture | 0.0272 | 0.0075 | 0.0682 | Badran et al.114 |
| (d) Probability of failure to integrate | 0.0060 | 0.0002 | 0.0329 | Håkansson et al.101 |
| Probability of ceasing treatment in patient with adverse outcome from initial surgery | 0.0000 | NA | NA | Assumption |
| Probability of resolution of adverse outcome of surgery | 1.0000 | NA | NA | Assumption |
| Probability of skin reaction (all grades) | 0.1819 | See (e), (f) and (g) below | ||
| (e) Probability of grade 1 or 2 skin reaction | 0.1451 | 0.1124 | 0.1842 | Håkansson et al.101 |
| (f) Probability of grade 3 skin reaction | 0.0346 | 0.0198 | 0.0563 | Håkansson et al.101 |
| (g) Probability of grade 4 skin reaction | 0.0022 | 0.0001 | 0.0121 | Håkansson et al.101 |
| Probability of losing bone integration | 0.0428 | 0.0188 | 0.1028 | Bonding100 |
| Probability of re-operation | 0.9474 | 0.7397 | 0.9987 | Proops54 |
| Probability of death from all causes | Age-specific | NA | NA | ONS128 |
Table 34 summarises the input parameters used to estimate potential utility gain from aided hearing and usage of BAHA and comparator hearing aids. These assumptions are relevant only to the augmented base case used in the exploratory cost-effectiveness analysis reported in Exploratory cost-effectiveness analysis. Full details and the rationale for adopting these input values are presented in Quality of life.
| Input parameter | Base-case value | 95% CI | Source | |
|---|---|---|---|---|
| Lower limit | Upper limit | |||
| Utility associated with hearing levels (HUI3) | ||||
| Unable to hear at all (level 6) | 0.465 | NA | NA | Furlong et al.120,121 |
| Able to hear conversation with one person but not group (level 5) | 0.644 | |||
| Able to hear conversation with one person and with group (level 3) | 0.849 | |||
| Proportion of cohort using devices | ||||
| BCHA | 0.90 | 0.683 | 0.988 | Hol et al.82 |
| BAHA | 1.00 | 0.832 | 1.000 | |
Table 35 summarises the costs associated with BAHA provision included as input parameters to the model (for full details of resource use assumptions and unit costs, see Resource use and cost data). The majority of unit costs have been taken from NHS reference costs 2007/08. 43 However, costs for BAHA sound processors and surgical consumables were supplied by NHS providers, as these data are not routinely reported. The BAHA costs reported in Table 35 are based on list prices and do not take account of discounts that may be available to individual hospital trusts.
| Input parameter | Base-case value (£) | Source | |
|---|---|---|---|
| Costs of BAHA provision | |||
| Cost of assessments prior to initial surgery | |||
| Adult | 315.63 | NHS reference costs 2007/08 43 | |
| Paediatric | 336.53 | ||
| Cost of initial surgery | |||
| One-stage | 2994.07 | NHS reference costs 2007/08 43 | |
| Two-stage | 4998.64 | ||
| Post-surgical costs (in year following surgery) | |||
| Adult | 381.47 | NHS reference costs 2007/08 43 | |
| Paediatric | 437.91 | ||
| Cost of BAHA processor | 2191.25 | UHB; SUHT | |
| Long-term costs of BAHA | 786.42a | ||
| Adverse outcomes | |||
| Bleeding within 24 hours of operation | 332.35b | NHS reference costs 2007/08 43 | |
| Surgical removal of tissue round abutment | 663.66c | ||
| Removal of abutment due to intolerable pain | 2004.57 | ||
| Adverse events | |||
| Grade 1 or 2 skin irritation (adult) | 123.50 | NHS reference costs 2007/08,43 BNF125 | |
| Grade 1 or 2 skin irritation (paediatric) | 140.22 | ||
| Grade 3 skin irritation | 663.66c | NHS reference costs 2007/08 43 | |
| Grade 4 skin irritation | 2004.57d | ||
| Repeat operation owing to loss of bone integration (adult) | 4111.80 | See Table 31 | |
| Repeat operation owing to loss of bone integration (paediatric) | 6172.80 | ||
| Costs of BCHA provision | |||
| Cost of assessments prior to fitting of BCHA | 57.48 | NHS reference costs 2007/08 43 | |
| Cost of fitting BCHA | 64.80 | ||
| Cost of audiological assessment post-BCHA fitting | 50.17 | ||
| Cost of BCHA device | Low (body-worn) | 117–183 | SUHT |
| Low (behind-ear worn) | 250 | ||
| High | 300–350 | UHB | |
| Long-term costs of BCHAe | 75–123 | ||
Cost analysis
Base case: cost analysis
Table 36 reports the modelled cost per case for providing a BAHA or BCHA to a cohort of children and adults, using a time horizon of 10 years. In both cases, BAHA is the more costly strategy – increasing costs in children by approximately 94% over BCHA, and increasing costs in adults by 93%. The differences between the modelled costs for paediatric and adult cases principally arise in assumptions regarding the use of two-stage surgery for paediatric cases and also higher unit costs for paediatric outpatient assessments. The same assumptions regarding treatment failure, loss of bone integration and skin reactions were applied to both cohorts. There are slight differences between the paediatric and adult cohorts in terms of the general mortality probabilities applied (based on age-specific death rates for the general population in the UK).
| Strategy | Paediatric (£) | Adult (£) |
|---|---|---|
| BC hearing aid | 1105 | 1084 |
| BAHA | 17,514 | 14,533 |
| Incremental cost per case | 16,409 | 13,449 |
Table 37 reports a breakdown of the modelled cost per case for BAHA provision, using the phases of the management pathway identified in Resource use and cost data. The most costly phase identified in Table 37 is long-term maintenance, constituting 36% and 42% of total costs in paediatric and adult cases, respectively. The high cost of the long-term maintenance is primarily the result of costs associated with maintenance plans for the BAHA sound processors and the periodic replacement of the processors.
| Cost breakdown | Paediatric (£) | Adult (£) |
|---|---|---|
| Initial assessment | 337 | 316 |
| Surgical costs | 4999 | 2994 |
| Post-surgery costs, including fitting of sound processor | 3253 | 3189 |
| Long-term maintenance costs | 6241 | 6114 |
| Adverse event costs | 2684 | 1921 |
In the base-case analyses reported in Tables 36 and 37 it is assumed that individuals modelled as treatment failures with a BAHA, either because of intolerable pain or because they choose not to have a re-operation following loss of bone integration, are not provided with an alternative hearing aid. These constitute approximately 6% of the original cohort and a proportion might be expected to revert to their previous hearing aid on failure with a BAHA. Table 38 presents an alternative base-case analysis with all individuals who experience treatment failure with a BAHA switching to BCHA in the year following treatment failure. The effect of this is to marginally increase the cost per case for the BAHA cohort, by 0.8% for children and 0.9% for adults.
| Strategy | Paediatric (£) | Adult (£) |
|---|---|---|
| BC hearing aid | 1105 | 1084 |
| BAHA | 17,649 | 14,666 |
| Incremental cost per case | 16,545 | 13,582 |
Table 39 reports a breakdown of the modelled cost per case for BAHA provision, similar to that in Table 37, but including the cost of people switching to BCHA on failure with a BAHA. As this change in management occurs as a result of experiencing adverse events, the cost of providing BCHA and continued audiological management is classified under adverse event costs in Table 39.
| Cost breakdown | Paediatric (£) | Adult (£) |
|---|---|---|
| Initial assessment | 337 | 316 |
| Surgical costs | 4999 | 2994 |
| Post-surgery costs, including fitting of sound processor | 3253 | 3189 |
| Long-term maintenance costs | 6241 | 6114 |
| Adverse event costs | 2820 | 2054 |
Average cost per case successfully treated with a BAHA
Given that a proportion of participants (up to 6%) are assumed, in the base case, to choose not to continue with their BAHAs, the results in Table 36 could be presented slightly differently – as cost per case successfully treated with a BAHA. To derive this figure we calculated the proportion of the cohort still using a BAHA at the end of the modelled time horizon by subtracting those who had died (0.1% for children and 1.9% for adults) and those who had chosen not to continue with the BAHA owing to adverse events (6.1% for children and 6% for adults). This equates to 93.8% successfully treated children and 92.1% successfully treated adults. The average cost per successfully treated patient was derived by dividing the average costs in Table 36 (average cost per patient, assuming treatment failures do not receive an alternative device) by the estimated proportion of cases successfully treated. Under these assumptions the cost per patient successfully treated with a BAHA is £18,681 for paediatric cases and £15,785 for adults.
Cost analysis: deterministic sensitivity analysis
We conducted a series of univariate sensitivity analyses, varying one parameter at a time from its base-case value while leaving all other variables unchanged. Probability parameters were varied between their 95% confidence limits, calculated as exact binomial CIs (see Table 33). In the absence of appropriate measures of variability in NHS reference costs,43 cost parameters were varied by plus or minus 25%, except for the costs of the BAHA sound processor and associated maintenance plans, which were varied between the lowest and highest model costs (as supplied by UHB and SUHT; see Table 28).
Table 40 reports the results of a DSA for paediatric cases, assuming that BAHA treatment failures do not switch to an alternative hearing aid. The value of the input parameter for each analysis is shown in the second column of the table. The table contains two rows for each input parameter – the first of which reports the results at the lower limit (either lower 95% confidence limit or the lower limit of the assumed range) and the second of which reports the results at the upper limit (either upper 95% confidence limit or the upper limit of the assumed range). The table also shows the base-case value for each input parameter, following the description of the parameter in the first column of the table. Table 41 reports similar analyses, assuming that participants switch to using BCHA on treatment failure.
| Parameter (base-case value) | Input value | BCHA (£) | BAHA (£) | Incremental cost per case (£) |
|---|---|---|---|---|
| Probability of bleeding within 24 hours of surgery (0.0121) | 0.00147 | 1105 | 17,513 | 16,408 |
| 0.04310 | 1105 | 17,518 | 16,413 | |
| Probability of surgical reduction of skin growth/soft tissue thickening around abutment (0.0848) | 0.04716 | 1105 | 17,509 | 16,404 |
| 0.13826 | 1105 | 17,525 | 16,420 | |
| Probability of failure to integrate (0.006) | 0.00015 | 1105 | 17,513 | 16,409 |
| 0.03291 | 1105 | 17,501 | 16,397 | |
| Probability of intolerable pain requiring removal of abutment and flange fixture (0.0272) | 0.00746 | 1105 | 17,840 | 16,735 |
| 0.06820 | 1105 | 17,445 | 16,341 | |
| Probability of re-operation (0.9474) | 0.73972 | 1105 | 16,668 | 15,563 |
| 0.99867 | 1105 | 17,732 | 16,627 | |
| Cost of initial ENT consultation (£131.69) | 98.77 | 1105 | 17,481 | 16,376 |
| 164.61 | 1105 | 17,547 | 16,442 | |
| Cost of audiological assessment (£57.48) | 43.11 | 1105 | 17,499 | 16,395 |
| 71.85 | 1105 | 17,528 | 16,424 | |
| Cost of ENT multiprofessional assessment (£147.36) | 110.52 | 1105 | 17,477 | 16,372 |
| 184.21 | 1105 | 17,551 | 16,446 | |
| Cost of day case surgery for implantation (£4009.14) | 3006.86 | 1105 | 16,159 | 15,055 |
| 5011.43 | 1105 | 18,868 | 17,764 | |
| Cost of fixture and abutment (£830.00) | 622.50 | 1105 | 17,338 | 16,234 |
| 1037.50 | 1105 | 17,689 | 16,585 | |
| Cost of surgical consumables (£159.50) | 119.63 | 1105 | 17,460 | 16,355 |
| 199.38 | 1105 | 17,568 | 16,463 | |
| Cost of follow-up ENT consultations (£90.93) | 68.20 | 1105 | 17,424 | 16,319 |
| 113.66 | 1105 | 17,604 | 16,499 | |
| Cost of audiological consultation to fit/commission sound processor (£64.80) | 48.60 | 1105 | 17,493 | 16,388 |
| 81.00 | 1105 | 17,535 | 16,431 | |
| Cost of audiology follow-up in year of surgery (£50.17) | 37.62 | 1105 | 17,481 | 16,376 |
| 62.71 | 1105 | 17,547 | 16,442 | |
| Cost of BAHA sound processor (£2191.25) | 1820.00 | 1105 | 17,147 | 16,042 |
| 2995.00 | 1105 | 18,308 | 17,204 | |
| Cost of BAHA sound processor maintenance plan (£736.25) | 610.00 | 1105 | 16,346 | 15,242 |
| 1000.00 | 1105 | 19,953 | 18,849 | |
| Cost of surgical revision for grade 3 skin reaction (£663.66) | 497.75 | 1105 | 17,459 | 16,354 |
| 829.58 | 1105 | 17,569 | 16,464 |
| Parameter (base-case value) | Input value | BCHA (£) | BAHA (£) | Incremental cost per case (£) |
|---|---|---|---|---|
| Probability of bleeding within 24 hours of surgery (0.0121) | 0.00147 | 1105 | 17,648 | 16,544 |
| 0.04310 | 1105 | 17,654 | 16,549 | |
| Probability of surgical reduction of skin growth/soft tissue thickening around abutment (0.0848) | 0.04716 | 1105 | 17,644 | 16,540 |
| 0.13826 | 1105 | 17,661 | 16,556 | |
| Probability of failure to integrate (0.006) | 0.00015 | 1105 | 17,649 | 16,545 |
| 0.03291 | 1105 | 17,653 | 16,549 | |
| Probability of intolerable pain requiring removal of abutment and flange fixture (0.0272) | 0.00746 | 1105 | 17,881 | 16,777 |
| 0.06820 | 1105 | 17,601 | 16,496 | |
| Probability of re-operation (0.9474) | 0.73972 | 1105 | 16,950 | 15,846 |
| 0.99867 | 1105 | 17,830 | 16,725 | |
| Cost of initial ENT consultation (£131.69) | 98.77 | 1105 | 17,616 | 16,512 |
| 164.61 | 1105 | 17,682 | 16,578 | |
| Cost of audiological assessment (£57.48) | 43.11 | 1105 | 17,635 | 16,530 |
| 71.85 | 1105 | 17,664 | 16,559 | |
| Cost of ENT multiprofessional assessment (£147.36) | 110.52 | 1105 | 17,612 | 16,508 |
| 184.21 | 1105 | 17,686 | 16,582 | |
| Cost of day case surgery for implantation (£4009.14) | 3006.86 | 1105 | 16,295 | 15,190 |
| 5011.43 | 1105 | 19,004 | 17,899 | |
| Cost of fixture and abutment (£830.00) | 622.50 | 1105 | 17,474 | 16,369 |
| 1037.50 | 1105 | 17,825 | 16,720 | |
| Cost of surgical consumables (£159.50) | 119.63 | 1105 | 17,595 | 16,491 |
| 199.38 | 1105 | 17,703 | 16,599 | |
| Cost of follow-up ENT consultations (£90.93) | 68.20 | 1105 | 17,559 | 16,455 |
| 113.66 | 1105 | 17,740 | 16,635 | |
| Cost of audiological consultation to fit/commission sound processor (£64.80) | 48.60 | 1105 | 17,628 | 16,524 |
| 81.00 | 1105 | 17,671 | 16,566 | |
| Cost of audiology follow-up in year of surgery (£50.17) | 37.62 | 1105 | 17,616 | 16,512 |
| 62.71 | 1105 | 17,682 | 16,578 | |
| Cost of BAHA sound processor (£2191.25) | 1820.00 | 1105 | 17,282 | 16,178 |
| 2995.00 | 1105 | 18,444 | 17,339 | |
| Cost of BAHA sound processor maintenance plan (£736.25) | 610.00 | 1105 | 16,482 | 15,377 |
| 1000.00 | 1105 | 20,089 | 18,984 | |
| Cost of surgical revision for grade 3 skin reaction (£663.66) | 497.75 | 1105 | 17,595 | 16,490 |
| 829.58 | 1105 | 17,704 | 16,600 |
The DSA suggests that the cost results are generally robust to variation in the value of input parameters. The results are most sensitive to variation in the probability of re-operation when implants lose bone integration, the cost of surgical implantation, the cost of the BAHA processor maintenance plan and, to a lesser extent, the initial cost of the BAHA processor and the probability of intolerable pain requiring removal of the BAHA fixture.
Table 42 reports the results of a DSA for adult cases, assuming that BAHA treatment failures do not switch to an alternative hearing aid. Table 43 reports similar analyses assuming that participants switch to using BCHA on treatment failure. As with the previous analysis for paediatric cases, the DSA suggests that the results are generally robust to variation in the value of input parameters, with costs of BAHA provision being most sensitive to variation in the probability of re-operation (for loss of bone integration), the cost of surgical implantation and the cost of the BAHA processor maintenance plan and, to a lesser extent, the initial cost of the BAHA processor and the probability of intolerable pain requiring removal of the BAHA fixture.
| Parameter (base-case value) | Input value | BCHA (£) | BAHA (£) | Incremental cost per case (£) |
|---|---|---|---|---|
| Probability of bleeding within 24 hours of surgery (0.0121) | 0.00147 | 1084 | 14,532 | 13,448 |
| 0.04310 | 1084 | 14,538 | 13,453 | |
| Probability of surgical reduction of skin growth/soft tissue thickening around abutment (0.0848) | 0.04716 | 1084 | 14,529 | 13,444 |
| 0.13826 | 1084 | 14,545 | 13,460 | |
| Probability of failure to integrate (0.006) | 0.00015 | 1084 | 14,533 | 13,449 |
| 0.03291 | 1084 | 14,531 | 13,447 | |
| Probability of intolerable pain requiring removal of abutment and flange fixture (0.0272) | 0.00746 | 1084 | 14,828 | 13,744 |
| 0.06820 | 1084 | 14,472 | 13,387 | |
| Probability of re-operation (0.9474) | 0.73972 | 1084 | 13,877 | 12,792 |
| 0.99867 | 1084 | 14,702 | 13,618 | |
| Cost of initial ENT consultation (£110.78) | 83.09 | 1084 | 14,506 | 13,422 |
| 138.48 | 1084 | 14,561 | 13,477 | |
| Cost of audiological assessment (£57.48) | 43.11 | 1084 | 14,519 | 13,435 |
| 71.85 | 1084 | 14,548 | 13,464 | |
| Cost of ENT multiprofessional assessment (£147.36) | 110.52 | 1084 | 14,497 | 13,412 |
| 184.21 | 1084 | 14,570 | 13,486 | |
| Cost of day case surgery for implantation (£2004.57) | 1503.43 | 1084 | 13,860 | 12,776 |
| 2505.71 | 1084 | 15,207 | 14,123 | |
| Cost of fixture and abutment (£830.00) | 622.50 | 1084 | 14,359 | 13,275 |
| 1037.50 | 1084 | 14,708 | 13,624 | |
| Cost of surgical consumables (£159.50) | 119.63 | 1084 | 14,480 | 13,396 |
| 199.38 | 1084 | 14,587 | 13,503 | |
| Cost of follow-up ENT consultations (£72.11) | 54.09 | 1084 | 14,462 | 13,378 |
| 90.14 | 1084 | 14,604 | 13,520 | |
| Cost of audiological consultation to fit/commission sound processor (£64.80) | 48.60 | 1084 | 14,512 | 13,428 |
| 81.00 | 1084 | 14,555 | 13,470 | |
| Cost of audiology follow-up in year of surgery (£50.17) | 37.62 | 1084 | 14,501 | 13,417 |
| 62.71 | 1084 | 14,566 | 13,482 | |
| Cost of BAHA sound processor (£2191.25) | 1820.00 | 1084 | 14,168 | 13,084 |
| 2995.00 | 1084 | 15,325 | 14,241 | |
| Cost of BAHA sound processor maintenance plan (£736.25) | 610.00 | 1084 | 13,388 | 12,303 |
| 1000.00 | 1084 | 16,927 | 15,843 | |
| Cost of surgical revision for grade 3 skin reaction (£663.66) | 497.75 | 1084 | 14,480 | 13,396 |
| 829.58 | 1084 | 14,587 | 13,503 |
| Parameter (base-case value) | Input value | BCHA (£) | BAHA (£) | Incremental cost per case (£) |
|---|---|---|---|---|
| Probability of bleeding within 24 hours of surgery (0.0121) | 0.00147 | 1084 | 14,665 | 13,580 |
| 0.04310 | 1084 | 14,670 | 13,586 | |
| Probability of surgical reduction of skin growth/soft tissue thickening around abutment (0.0848) | 0.04716 | 1084 | 14,661 | 13,577 |
| 0.13826 | 1084 | 14,677 | 13,593 | |
| Probability of failure to integrate (0.006) | 0.00015 | 1084 | 14,666 | 13,582 |
| 0.03291 | 1084 | 14,679 | 13,595 | |
| Probability of intolerable pain requiring removal of abutment and flange fixture (0.0272) | 0.00746 | 1084 | 14,869 | 13,784 |
| 0.06820 | 1084 | 14,623 | 13,539 | |
| Probability of re-operation (0.9474) | 0.73972 | 1084 | 14,152 | 13,068 |
| 0.99867 | 1084 | 14,798 | 13,714 | |
| Cost of initial ENT consultation (£110.78) | 83.09 | 1084 | 14,638 | 13,554 |
| 138.48 | 1084 | 14,694 | 13,609 | |
| Cost of audiological assessment (£57.48) | 43.11 | 1084 | 14,651 | 13,567 |
| 71.85 | 1084 | 14,680 | 13,596 | |
| Cost of ENT multiprofessional assessment (£147.36) | 110.52 | 1084 | 14,629 | 13,545 |
| 184.21 | 1084 | 14,703 | 13,618 | |
| Cost of day case surgery for implantation (£2004.57) | 1503.43 | 1084 | 13,992 | 12,908 |
| 2505.71 | 1084 | 15,339 | 14,255 | |
| Cost of fixture and abutment (£830.00) | 622.50 | 1084 | 14,491 | 13,407 |
| 1037.50 | 1084 | 14,841 | 13,756 | |
| Cost of surgical consumables (£159.50) | 119.63 | 1084 | 14,612 | 13,528 |
| 199.38 | 1084 | 14,719 | 13,635 | |
| Cost of follow-up ENT consultations (£72.11) | 54.09 | 1084 | 14,595 | 13,511 |
| 90.14 | 1084 | 14,737 | 13,653 | |
| Cost of audiological consultation to fit/commission sound processor (£64.80) | 48.60 | 1084 | 14,645 | 13,561 |
| 81.00 | 1084 | 14,687 | 13,603 | |
| Cost of audiology follow-up in year of surgery (£50.17) | 37.62 | 1084 | 14,633 | 13,549 |
| 62.71 | 1084 | 14,699 | 13,614 | |
| Cost of BAHA sound processor (£2191.25) | 1820.00 | 1084 | 14,300 | 13,216 |
| 2995.00 | 1084 | 15,458 | 14,373 | |
| Cost of BAHA sound processor maintenance plan (£736.25) | 610.00 | 1084 | 13,520 | 12,436 |
| 1000.00 | 1084 | 17,060 | 15,976 | |
| Cost of surgical revision for grade 3 skin reaction (£663.66) | 497.75 | 1084 | 14,612 | 13,528 |
| 829.58 | 1084 | 14,719 | 13,635 |
Exploratory cost-effectiveness analysis
An exploratory, augmented base-case analysis was developed to incorporate potential benefits from improved hearing resulting from BAHA provision and improvements in wearability, compared with BCHA. In this analysis we assumed that the QoL benefit from improved hearing could be proxied by levels of the hearing attribute in the HUI3 and that identical gains were potentially achievable through use of BAHA and BCHA. Differences between the utility associated with use of BAHA or BCHA were assumed to be realised through differences in the proportion of time that members of the modelled cohorts used each device, as outlined in Quality of life.
The results are reported in terms of total costs and total QALYs for each treatment strategy, incremental costs and benefits and the ICERs. Costs and outcomes are discounted at 3.5%.
The results of the augmented base-case analysis in paediatric cases are reported in Table 44. The average costs estimated for each cohort are identical to those reported in Table 38 (average costs, assuming BAHA treatment failures revert to BCHA). The QALY outcomes for BCHA and BAHA have been estimated on two different potential levels of hearing gain associated with the use of hearing aids (based on items in the HUI3 hearing domain). Under the first assumption (QALY1) the utility gain from aided hearing is 0.178, based on attaining level 5 on the HUI hearing domain (‘able, when using hearing aid, to hear conversation with one other person, but unable to hear what is said in a group conversation’; see Table 21 and Quality of life for more details), while under the second assumption (QALY2) the utility gain from aided hearing is 0.384, based on attaining level 3 on the HUI3 hearing domain (‘able to hear both conversation with one other person and what is said in a group conversation, when using hearing aid’; see Table 21 and Quality of life for more details). These assumptions apply equally to BCHA and BAHA. However, different assumptions apply to the proportion of the cohort using the relevant hearing aid (see Table 34), which give rise to different QALY estimates for the two modelled cohorts (BAHA vs BCHA).
| Cost (£) | QALY1a | QALY2b | |
|---|---|---|---|
| BCHA | 1105 | 5.20 | 6.74 |
| BAHA | 17,649 | 5.34 | 7.04 |
| Difference | 16,545 | 0.14 | 0.30 |
| ICER (£) | 119,367 | 55,642 |
For paediatric cases, the QALY gain associated with providing BAHA ranges from 0.14 to 0.30 (depending on the assumed level of utility associated with aided hearing), resulting in ICERs of £119,367 and £55,642, respectively.
The results of the augmented base-case analysis in adults, applying the same assumptions on potential utility gain from aided hearing and usage of hearing aids, are reported in Table 45. The estimated QALY gain from use of BAHA is similar to that for children, ranging from 0.14 to 0.29 depending on the assumed level of utility associated with aided hearing. However, the ICERs are lower (£100,029 and £46,628, respectively) given the lower incremental costs estimated for adults (resulting from the use of one-stage surgery in adults and lower costs for adult outpatient attendances).
| Cost (£) | QALY1a | QALY2b | |
|---|---|---|---|
| BCHA | 1084 | 5.10 | 6.60 |
| BAHA | 14,666 | 5.23 | 6.89 |
| Difference | 13,582 | 0.14 | 0.29 |
| ICER (£) | 100,029 | 46,628 |
Deterministic sensitivity analysis
Table 46 reports the results of a series of univariate sensitivity analyses, indicating the effect of variation in input parameters on the ICER. Results for each parameter are reported on two lines – the first gives the results at the lower limit of the input parameter and the second gives the result at the upper limit. Probabilities are varied between the lower and upper limits of the 95% CI, while unit costs derived from NHS reference costs43 are varied by plus or minus 25% of their average values. Costs of the BAHA processor and annual maintenance contract are varied from the lowest to highest reported values. As for the base-case analysis, ICERs are reported for two outcome scenarios (QALY1 and QALY2) in which the utility gain from aided hearing is estimated based on levels of the hearing domain of the HUI3. In the final two analyses reported in Table 46, two scenarios are considered. Firstly, the proportion of the BCHA cohort using their aid for ≥ 8 hours is varied between the lower and upper limits of the 95% CI for the value adopted in the base case [18/20 (90%)], while keeping the proportion of the BCHA cohort using their aid for ≥ 8 hours at 100%. Secondly, in a bivariate sensitivity analysis in which the proportion of people using their BCHA and the proportion of people using their BAHA are varied simultaneously, the ICERs are estimated at the lower limit of the 95% CI for hearing aid usage in the BCHA and BAHA cohorts (0.683 and 0.832, respectively). No analysis is reported for the upper limit of the CI, as that would simply repeat the results presented in the previous row. The majority of the input variables included in the DSA have minimal impact on the QALY outcomes for both BCHA and BAHA (full results are reported in Appendix 12).
| Input parameter (base-case value) | Input value | ICER (£ per QALY gained) | |||
|---|---|---|---|---|---|
| Paediatric | Adult | ||||
| QALY1a | QALY2b | QALY1a | QALY2b | ||
| Probability of bleeding within 24 hours of surgery (0.0121) | 0.00147 | 119,358 | 55,638 | 100,020 | 46,624 |
| 0.04310 | 119,397 | 55,656 | 100,060 | 46,642 | |
| Probability of surgical reduction of skin growth/thickening around abutment (0.0848) | 0.04716 | 119,331 | 55,626 | 99,993 | 46,611 |
| 0.13826 | 119,448 | 55,680 | 100,111 | 46,667 | |
| Probability of failure to integrate (0.006) | 0.00015 | 119,461 | 55,686 | 100,110 | 46,666 |
| 0.03291 | 124,361 | 57,970 | 104,302 | 48,620 | |
| Probability of intolerable pain requiring removal of abutment and flange fixture (0.0272) | 0.00746 | 117,042 | 54,559 | 98,172 | 45,763 |
| 0.06820 | 119,872 | 55,878 | 100,433 | 46,816 | |
| Probability of re-operation (0.9474) | 0.73972 | 119,907 | 55,894 | 100,909 | 47,038 |
| 0.99867 | 119,248 | 55,587 | 99,825 | 46,533 | |
| Cost of initial ENT consultation [£131.69 (paediatric) and £110.78 (adult)] | 98.77 (P), 83.09 (A) | 119,129 | 55,532 | 99,825 | 46,533 |
| 164.61 (P), 138.48 (A) | 119,604 | 55,753 | 100,233 | 46,723 | |
| Cost of audiological assessment (£57.48) | 43.11 | 119,263 | 55,594 | 99,923 | 46,579 |
| 71.85 | 119,471 | 55,691 | 100,135 | 46,678 | |
| Cost of ENT multiprofessional assessment (£147.36) | 110.52 | 119,101 | 55,518 | 99,758 | 46,502 |
| 184.21 | 119,633 | 55,766 | 100,301 | 46,755 | |
| Cost of day case surgery for implantation (£2004.57) | 1503.43 | 109,596 | 51,088 | 95,068 | 44,316 |
| 2505.71 | 129,138 | 60,197 | 104,990 | 48,941 | |
| Cost of fixture and abutment (£830.00) | 622.50 | 118,099 | 55,052 | 98,742 | 46,028 |
| 1037.50 | 120,634 | 56,233 | 101,316 | 47,228 | |
| Cost of surgical consumables (£159.50) | 119.63 | 118,978 | 55,461 | 99,634 | 46,444 |
| 199.38 | 119,756 | 55,824 | 100,424 | 46,812 | |
| Cost of follow-up ENT consultations [£90.93 (paediatric) and £72.11 (adult)] | 68.20 (P), 54.09 (A) | 118,716 | 55,339 | 99,506 | 46,385 |
| 113.66 (P), 90.14 (A) | 120,018 | 55,946 | 100,552 | 46,872 | |
| Cost of audiological consultation to fit/commission sound processor (£64.80) | 48.60 | 119,213 | 55,571 | 99,874 | 46,556 |
| 81.00 | 119,520 | 55,714 | 100,185 | 46,701 | |
| Cost of audiology follow-up in year of surgery (£50.17) | 37.62 | 119,129 | 55,532 | 99,789 | 46,516 |
| 62.71 | 119,604 | 55,753 | 100,270 | 46,740 | |
| Cost of BAHA sound processor (£2191.25) | 1820.00 | 116,719 | 54,408 | 97,336 | 45,373 |
| 2995.00 | 125,099 | 58,314 | 105,861 | 49,347 | |
| Cost of BAHA sound processor maintenance plan (£735.25) | 610.00 | 110,943 | 51,715 | 91,589 | 42,694 |
| 1000.00 | 136,966 | 63,846 | 117,661 | 54,847 | |
| Cost of surgical revision for grade 3 skin reaction (£663.66) | 497.75 | 118,971 | 55,458 | 99,634 | 46,444 |
| 829.58 | 119,762 | 55,827 | 100,424 | 46,812 | |
| Proportion of cohort using BCHA for > 8 hours per day (0.90) | 0.683 | 37,025 | 17,259 | 31,027 | 14,463 |
| 0.988 | 1,216,561 | 567,095 | 1,019,479 | 475,226 | |
| Proportion using BCHA at lower limit of 95% CI, 0.683, and proportion using BAHA at lower limit of 95% CI, 0.832 (BCHA = 0.90; BAHA = 1.00) | 0.683/0.832 | 82,287 | 38,358 | 68,948 | 32,140 |
The DSA suggests that the results are generally robust to variation in input probabilities and unit costs. In terms of input probabilities, the greatest variation in ICER relates to initial failure of bone integration, failure of BAHA implantation owing to intolerable pain and the probability of re-operation because of loss of bone integration. In contrast to other input probabilities, the ICER reduces as the probability of re-operation increases. This occurs because, although costs increase (associated with additional surgical procedures), the QALY gained from BAHA also increases (with the proportionate QALY gain being greater than the proportionate increase in cost). With respect to cost inputs, the greatest variation in ICER relates to the cost of day surgery for implantation and the cost of components of the BAHA system (fixture and abutment, BAHA sound processor and the cost of the maintenance plan), with the cost of the maintenance plan having the greatest impact. The variable that has the greatest influence on the cost-effectiveness results is the proportion of each cohort using their hearing aids. Very high ICER values are associated with high usage of BCHA (98.8% at the upper limit of the 95% CI, resulting in a small difference in usage between BCHA and BAHA), but more acceptable values are associated with lower BCHA usage (than for BAHA). Threshold values for differences in use of hearing aids and the underlying utility gain from aided hearing are explored further in a range of scenario analyses in the following section of this report.
Scenario analysis
Scenario 1: alternative cost assumptions for BCHA
Tables 47 and 48 report a scenario analysis in which the base-case analysis is re-run for alternative assumptions regarding the cost of BCHA. Four possible costs, two related to body-worn hearing aids (ranging from £117 to £183) and two related to behind-the-ear aids (from £250 for a device incorporating an NHS digital aid to £350 for a proprietary device), were identified by NHS providers for conventional BCHA (see Table 32). In the base case, the mid-point of £250 was adopted. The scenario analysis examines the robustness of the model results to alternative assumptions regarding the cost of the comparator device. The values reported in Tables 47 and 48 suggest that the ICERs are robust to alternative assumptions regarding the cost of BCHA. The costs of providing BCHA vary by approximately £600 between the highest and lowest cost options, over the modelled 10-year time horizon. The difference between BCHA and BAHA ranges from £16,337 to £16,821 for paediatric cases and from £13,337 to £13,853 for adults.
| Unit cost of BCHA (£) | Strategy | Cost (£) | QALY1a | ICER1 (£ per QALY gained) | QALY2b | ICER2 (£ per QALY gained) |
|---|---|---|---|---|---|---|
| 117 | BCHA | 765 | 5.20 | 6.74 | ||
| BAHA | 17,587 | 5.34 | 121,362 | 7.04 | 56,573 | |
| 183 | BCHA | 934 | 5.20 | 6.74 | ||
| BAHA | 17,618 | 5.34 | 120,372 | 7.04 | 56,111 | |
| 250 | BCHA | 1105 | 5.20 | 6.74 | ||
| BAHA | 17,649 | 5.34 | 119,367 | 7.04 | 55,642 | |
| 350 | BCHA | 1360 | 5.20 | 6.74 | ||
| BAHA | 17,696 | 5.34 | 117,866 | 7.04 | 54,943 |
| Unit cost of BCHA (£) | Strategy | Cost (£) | QALY1a | ICER1 (£ per QALY gained) | QALY2b | ICER2 (£ per QALY gained) |
|---|---|---|---|---|---|---|
| 117 | BCHA | 765 | 5.20 | 6.74 | ||
| BAHA | 17,587 | 5.34 | 121,362 | 7.04 | 56,573 | |
| 183 | BCHA | 917 | 5.10 | 6.60 | ||
| BAHA | 14,635 | 5.23 | 101,038 | 6.89 | 47,098 | |
| 250 | BCHA | 1084 | 5.10 | 6.60 | ||
| BAHA | 14,666 | 5.23 | 100,029 | 6.89 | 46,628 | |
| 350 | BCHA | 1334 | 5.10 | 6.60 | ||
| BAHA | 14,712 | 5.23 | 98,524 | 6.89 | 45,927 |
Scenario 2: alternative assumptions regarding the proportion of the cohort using a BCHA for more than 8 hours per day
Tables 49 and 50 report scenario analyses in which the proportion of the cohort using a BCHA for more than 8 hours per day is varied from 0% to 100%, while holding the proportion using a BAHA at 100%. As indicated in the DSA, reported above, the QALY gain from aided hearing and hence the ICERs are highly sensitive to assumptions regarding the usage of hearing aids. The ICERs for QALY1 (able to hear one-to-one conversation) are low and below conventional thresholds for acceptable cost-effectiveness for low-to-medium proportions using a BCHA – up to approximately 60% in both paediatric cases and adults. When the assumed utility for aided hearing is greater (i.e. QALY2, in which members of the modelled cohorts can hear group conversation as well as one-to-one conversation), even relatively high proportions using a BCHA (80% usage or an assumed difference of 20% compared with BAHA usage) yield ICERs below conventionally adopted thresholds.
| Proportion using BCHA | Device | Cost (£) | QALY1a | ICER1a (£ per QALY gained) | QALY2b | ICER2b (£ per QALY gained) |
|---|---|---|---|---|---|---|
| 0.0 | BCHA | 1105 | 3.87 | 3.87 | ||
| BAHA | 17,649 | 5.28 | 11,675 | 6.91 | 5442 | |
| 0.1 | BCHA | 1105 | 4.01 | 4.18 | ||
| BAHA | 17,649 | 5.29 | 12,976 | 6.92 | 6049 | |
| 0.2 | BCHA | 1105 | 4.16 | 4.50 | ||
| BAHA | 17,649 | 5.30 | 14,603 | 6.93 | 6807 | |
| 0.3 | BCHA | 1105 | 4.31 | 4.82 | ||
| BAHA | 17,649 | 5.30 | 16,697 | 6.95 | 7783 | |
| 0.4 | BCHA | 1105 | 4.46 | 5.14 | ||
| BAHA | 17,649 | 5.31 | 19,491 | 6.96 | 9085 | |
| 0.5 | BCHA | 1105 | 4.61 | 5.46 | ||
| BAHA | 17,649 | 5.32 | 23,408 | 6.98 | 10,911 | |
| 0.6 | BCHA | 1105 | 4.76 | 5.78 | ||
| BAHA | 17,649 | 5.32 | 29,295 | 6.99 | 13,656 | |
| 0.7 | BCHA | 1105 | 4.91 | 6.10 | ||
| BAHA | 17,649 | 5.33 | 39,140 | 7.01 | 18,245 | |
| 0.8 | BCHA | 1105 | 5.06 | 6.42 | ||
| BAHA | 17,649 | 5.34 | 58,951 | 7.02 | 27,480 | |
| 0.9 | BCHA | 1105 | 5.20 | 6.74 | ||
| BAHA | 17,649 | 5.34 | 119,367 | 7.04 | 55,642 | |
| 1.0 | BCHA | 1105 | 5.35 | 7.06 | ||
| BAHA | 17,649 | 5.35 | Dominated | 7.05 | Dominated |
| Proportion using BCHA | Device | Cost (£) | QALY1a | ICER1a (£ per QALY gained) | QALY2b | ICER2b (£ per QALY gained) |
|---|---|---|---|---|---|---|
| 0.0 | BCHA | 1084 | 3.79 | 3.79 | ||
| BAHA | 14,666 | 5.17 | 9784 | 6.76 | 4561 | |
| 0.1 | BCHA | 1084 | 3.93 | 4.10 | ||
| BAHA | 14,666 | 5.18 | 10,874 | 6.78 | 5069 | |
| 0.2 | BCHA | 1084 | 4.08 | 4.41 | ||
| BAHA | 14,666 | 5.19 | 12,237 | 6.79 | 5704 | |
| 0.3 | BCHA | 1084 | 4.22 | 4.72 | ||
| BAHA | 14,666 | 5.19 | 13,992 | 6.81 | 6522 | |
| 0.4 | BCHA | 1084 | 4.37 | 5.04 | ||
| BAHA | 14,666 | 5.20 | 16,333 | 6.82 | 7614 | |
| 0.5 | BCHA | 1084 | 4.51 | 5.35 | ||
| BAHA | 14,666 | 5.21 | 19,616 | 6.83 | 9144 | |
| 0.6 | BCHA | 1084 | 4.66 | 5.66 | ||
| BAHA | 14,666 | 5.21 | 24,549 | 6.85 | 11,444 | |
| 0.7 | BCHA | 1084 | 4.81 | 5.97 | ||
| BAHA | 14,666 | 5.22 | 32,799 | 6.86 | 15,289 | |
| 0.8 | BCHA | 1084 | 4.95 | 6.29 | ||
| BAHA | 14,666 | 5.23 | 49,400 | 6.88 | 23,028 | |
| 0.9 | BCHA | 1084 | 5.10 | 6.60 | ||
| BAHA | 14,666 | 5.23 | 100,029 | 6.89 | 46,628 | |
| 1.0 | BCHA | 1084 | 5.24 | 6.91 | ||
| BAHA | 14,666 | 5.24 | Dominated | 6.90 | Dominated |
Threshold values (of the proportion using a BCHA) for cost-effectiveness, at a willingness to pay (WTP) of £30,000 per QALY gained, were 61% and 67% for QALY1 and 82% and 85% for QALY2 in paediatric and adult cases, respectively. This means that, when the difference in usage between BCHA and BAHA is greater than 39% in paediatric cases and greater than 33% in adults and the utility gain from aided hearing (with BAHA or BCHA) is 0.178, the BAHA may be a cost-effective option. If functional gain (and hence the utility gain) is greater, then the minimum difference is lower (18% in paediatric cases and 15% in adults). Similar threshold values can be calculated for a WTP threshold of £20,000 per QALY gained. These are 42% and 51% for QALY1 and 73% and 77% for QALY2 in paediatric and adult cases, respectively. BAHA usage is assumed to be 100%, as reported by Hol and colleagues,82 in these scenarios.
Scenario 3: include pain associated with BCHA use
Tables 51 and 52 report scenario analyses in which the base-case analysis is re-run, but including an assumption that use of a BCHA is associated with some discomfort (proxied by level 2 on the HUI3 pain dimension; see Quality of life for details). This has the effect of reducing total QALYs for BCHAs from 5.2 to 4.9 (for QALY1) and from 6.74 to 6.37 (for QALY2). Given that the outcome model for BAHAs incorporates some patients having their implants removed owing to intolerable pain, we included a utility loss (proxied by level 5 of the HUI3 pain dimension; see Quality of life for details) for these participants, in the cycle in which the removal was modelled to occur. Including pain associated with the BAHA implant results in a reduction in the total QALYs for BAHAs from 5.34 to 5.33 (QALY1) and from 7.04 to 7.02 (QALY2). This has the effect of increasing the QALY difference between BAHAs and BCHAs and leads to substantially lower ICER values.
| Cost (£) | QALY1a | QALY2b | |
|---|---|---|---|
| BCHA | 1105 | 4.90 | 6.37 |
| BAHA | 17,649 | 5.33 | 7.02 |
| Difference | 16,545 | 0.43 | 0.65 |
| ICER | 38,348 | 25,559 |
| Cost (£) | QALY1a | QALY2b | |
|---|---|---|---|
| BCHA | 1084 | 4.80 | 6.24 |
| BAHA | 14,666 | 5.22 | 6.87 |
| Difference | 13,582 | 0.42 | 0.63 |
| ICER | 32,136 | 21,419 |
Scenario 4: threshold analysis on utility difference
A final series of threshold analyses were conducted to estimate the utility difference (the utility gain from aided hearing) required to meet conventional cost-effectiveness thresholds, if the difference in use between BCHAs and BAHAs is 10% (as reported by Hol and colleagues82). For a WTP threshold of £30,000 per QALY, the required utility difference in adults is 0.597. Using the anchor points adopted in our base-case analysis (see Table 34) of 0.644 for being able to hear one-to-one conversation and 0.849 for being able to hear one-to-one and group conversation, a utility difference of 0.597 implies a utility value of 0.047 for deafness (approximately equal to that for death) at the lower anchor point and of 0.252 for the higher anchor point. Repeating this analysis for paediatric cases gives a larger required utility difference of 0.712.
Table 53 reports similar analyses undertaken at varying values for the difference in the proportion using their BCHA (compared with BAHA). As with the earlier DSA, this analysis suggests that the required utility difference (to achieve acceptable cost-effectiveness) reduces as the proportion using BCHA reduces (compared with the proportion using BAHA).
| Difference in proportion of cohort using BCHA (compared with BAHA) | Threshold WTP (£ per QALY gained) | |
|---|---|---|
| £20,000 | £30,000 | |
| 0.10 | NA | 0.597 |
| 0.15 | 0.592 | 0.395 |
| 0.20 | 0.442 | 0.295 |
| 0.25 | 0.353 | 0.235 |
| 0.30 | 0.294 | 0.196 |
| 0.35 | 0.251 | 0.168 |
| 0.40 | 0.220 | 0.146 |
| 0.45 | 0.195 | 0.130 |
| 0.50 | 0.176 | 0.117 |
Probabilistic sensitivity analysis
In a PSA in which the difference in proportion of participants using their hearing aid (BAHA–BCHA), hearing aid costs, the probability of adverse events as well as surgical and long-term maintenance costs were sampled probabilistically, mean costs and QALY outcomes were similar to those reported for the deterministic analysis [see Table 44 (paediatric cases) and Table 45 (adults)]. Providing BAHAs for children is associated with increased QALYs [with a range from 0.01 to 0.36 for the lower utility gain (QALY1) and from 0.03 to 0.79 for the higher utility gain (QALY2)], but also increased costs (ranging from £13,804 to £21,496) in all simulations, when compared with BCHAs (Table 54).
| Lifetime costs (£) (95% CI) | QALY1a (95% CI) | QALY2b (95% CI) | |
|---|---|---|---|
| BCHA | 1039 (765 to 1360) | 5.20 (4.97 to 5.33) | 6.74 (6.22 to 7.01) |
| BAHA | 17,700 (14,895 to 22,496) | 5.34 (5.33 to 5.35) | 7.03 (7.00 to 7.05) |
| Incremental | 16,661 (13,804 to 21,496) | 0.14 (0.01 to 0.36) | 0.30 (0.04 to 0.79) |
In this analysis, providing BAHAs in place of BCHAs in paediatric cases had a probability of being cost-effective of 0% at a WTP threshold of £20,000 per QALY gained and of 0.1% at a WTP threshold of £30,000 per QALY gained, if the utility gain from aided hearing was assumed to be 0.178 (moving from level 6 to level 5 of the HUI hearing attribute). If the utility gain from aided hearing was assumed to be 0.384 (moving from level 6 to level 3 of the HUI hearing attribute), providing BAHAs in place of BCHA in paediatric cases had a probability of being cost-effective of 2.3% at a WTP threshold of £20,000 per QALY gained and 12.0% at a WTP threshold of £30,000 per QALY gained (Figure 5).
FIGURE 5.
Augmented base case: cost-effectiveness acceptability curve for paediatric cases receiving a BAHA.
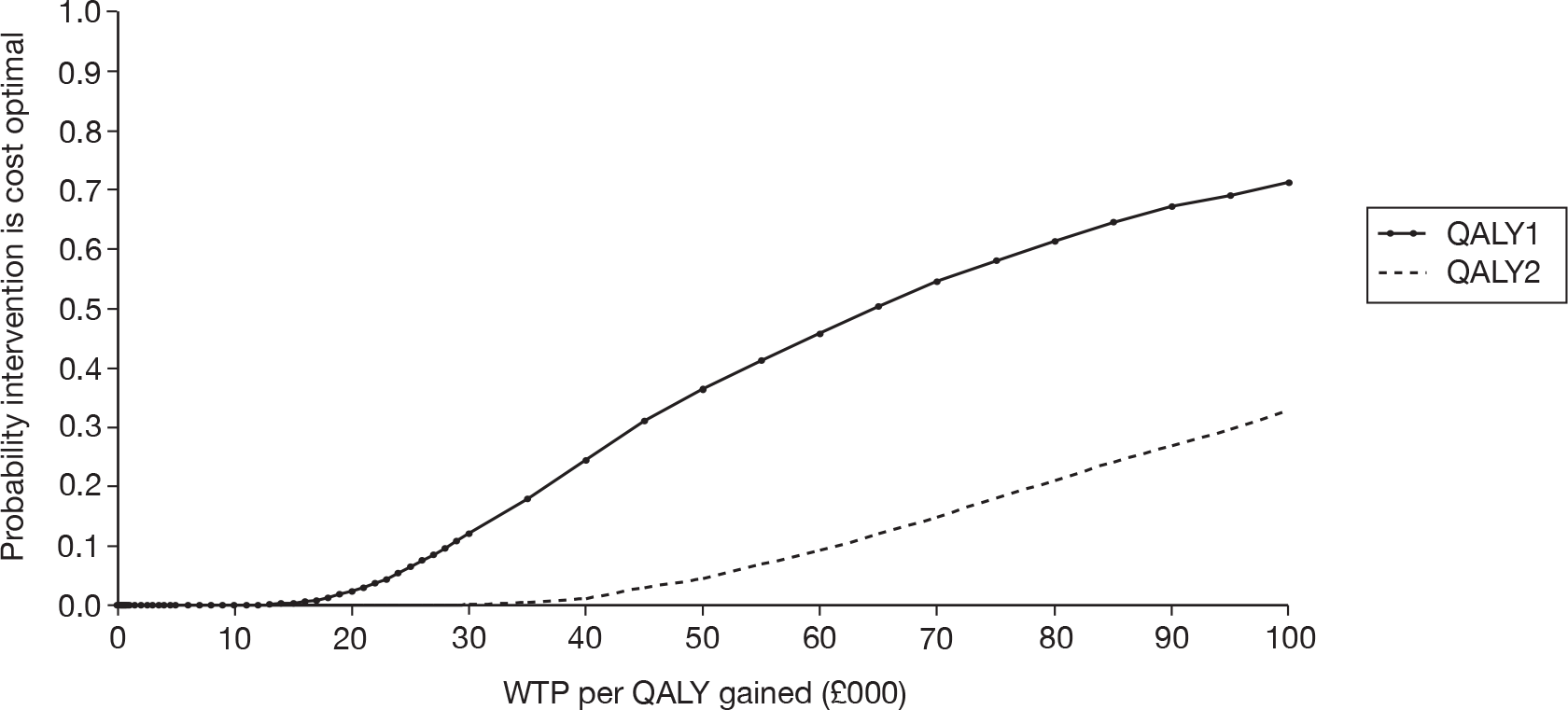
Conducting the same analysis for adults yielded similar results, with BAHAs being associated with increased QALYs [with a range from 0.01 to 0.36 for the lower utility gain (QALY1) and from 0.03 to 0.77 for the higher utility gain (QALY2)], but also increased costs (ranging from £11,362 to £17,851) compared with BCHAs (Table 55). BAHA costs are higher in children than in adults, owing to the use of two-stage surgery and higher costs for paediatric outpatient consultations.
| Lifetime costs (£) (95% CI) | QALY1a (95% CI) | QALY2b (95% CI) | |
|---|---|---|---|
| BCHA | 1023 (751 to 1334) | 5.10 (4.86 to 5.22) | 6.60 (6.10 to 6.87) |
| BAHA | 14,722 (12,440 to 18,813) | 5.23 (5.22 to 5.24) | 6.89 (6.86 to 6.90) |
| Incremental | 13,699 (11,362 to 17,851) | 0.14 (0.01 to 0.36) | 0.29 (0.03 to 0.77) |
Providing BAHAs in place of BCHAs for adults had a probability of being cost-effective of 0% at a WTP threshold of £20,000 per QALY gained and of 0.6% at a WTP threshold of £30,000 per QALY gained, for the lower utility gain (QALY1, moving from level 6 to level 5 of the HUI hearing dimension). For the higher utility gain (QALY2, moving from level 6 to level 3 of the HUI hearing dimension), providing BAHAs had a probability of being cost-effective of 5.0% at a WTP threshold of £20,000 per QALY gained and of 19.0% at a WTP threshold of £30,000 per QALY gained (Figure 6).
FIGURE 6.
Augmented base case: cost-effectiveness acceptability curve for adults receiving a BAHA.
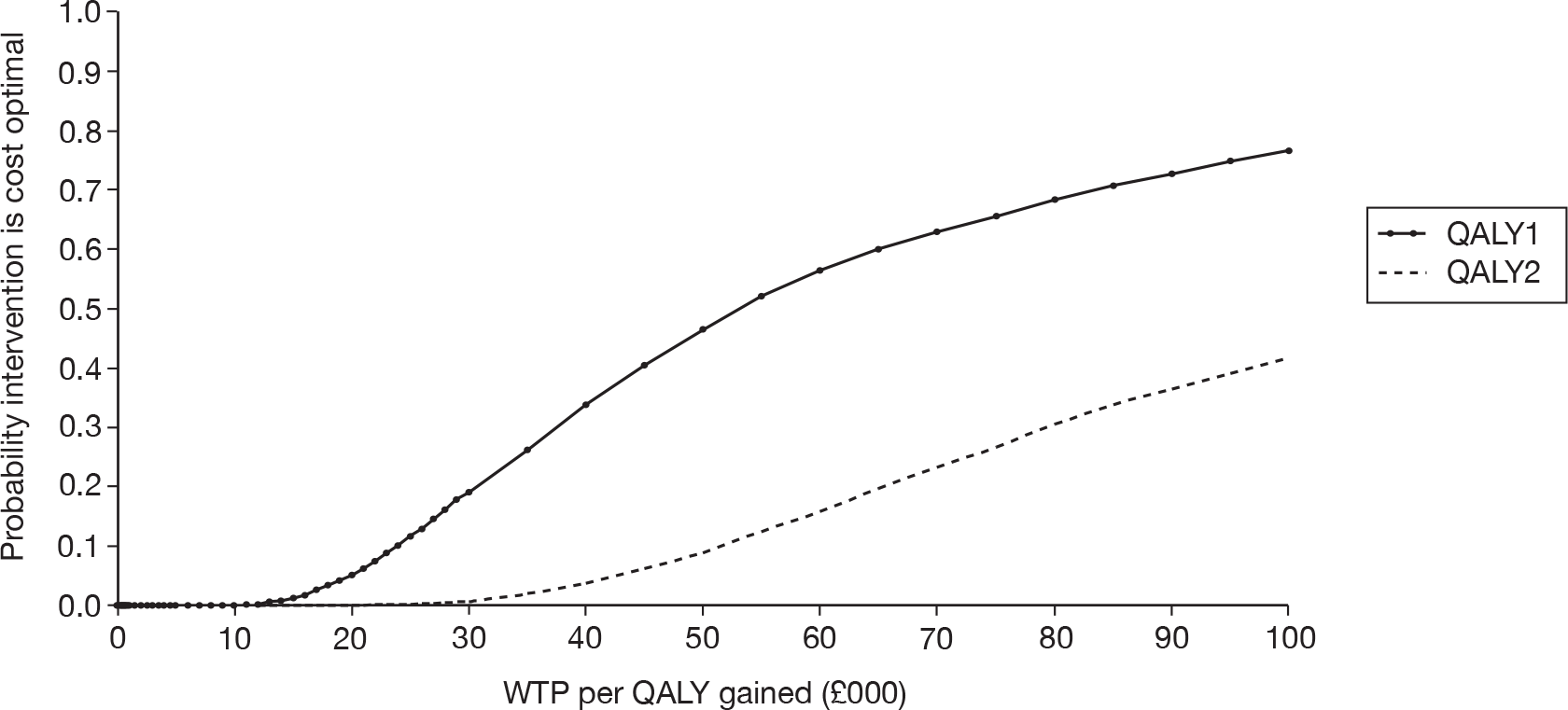
The PSA was re-run for small changes in the difference of the proportion of participants using their BCHA compared with BAHA. In the base-case analysis, this was assumed to be 10%, based on figures reported by Hol and colleagues. 82 In the PSA reported in Tables 54 and 55, the mean value for this difference was also 10%, sampled from a beta distribution that was parameterised as having two non-users of their BCHA in a population of 20 (as reported by Hol and colleagues82). The PSA was re-run for two alternative scenarios, in which the number of people not using their BCHA was set to three in a population of 20 (yielding an average difference of 15%) and in which the number of people not using their BCHA was set to four in a population of 20 (yielding an average difference of 20%). Figures 7 and 8 illustrate the cost-effectiveness acceptability curves derived from these PSAs for children, assuming that the QALY gain from aided hearing is 0.178 (QALY1) and 0.384 (QALY2), respectively. Figures 9 and 10 report the results of the same analysis for adults.
FIGURE 7.
Cost-effectiveness acceptability curves for alternative assumptions on the difference in use of BCHA and BAHA in children (QALY1).
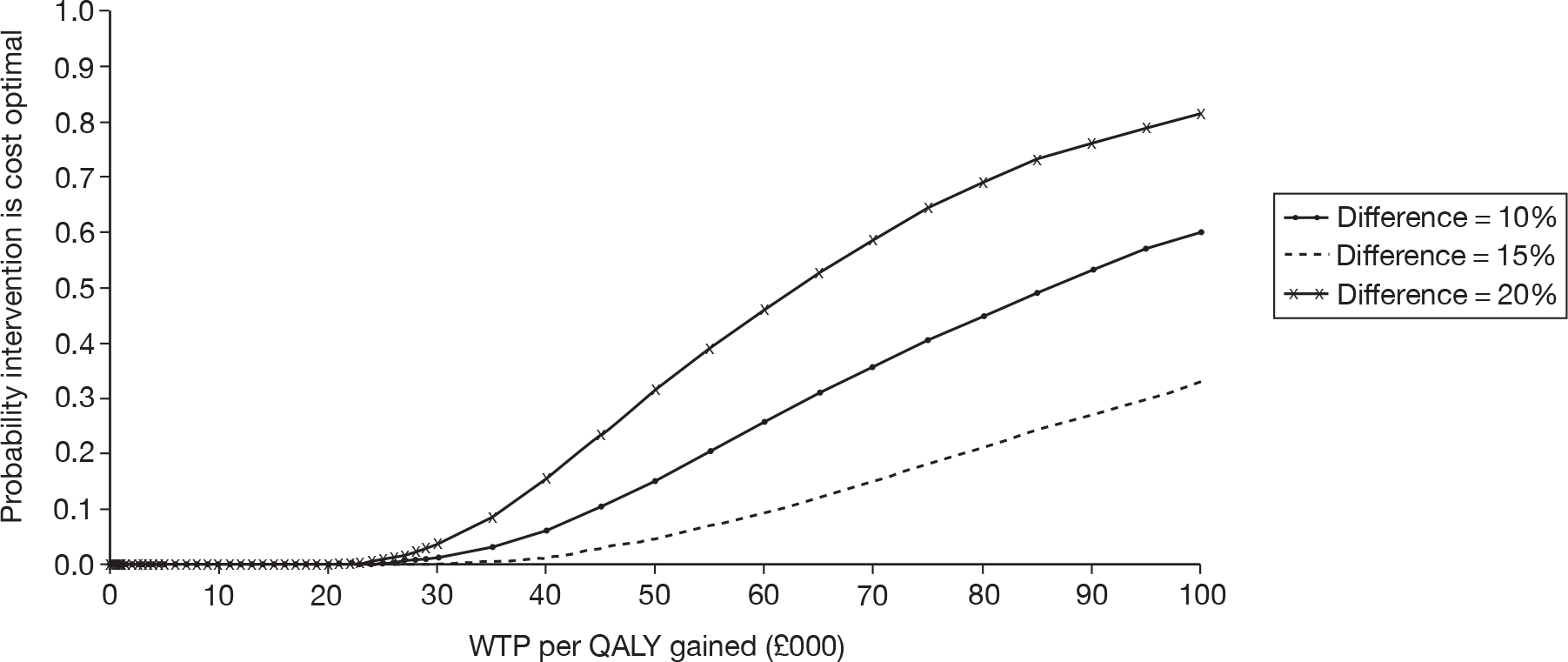
FIGURE 8.
Cost-effectiveness acceptability curves for alternative assumptions on the difference in use of BCHA and BAHA in children (QALY2).
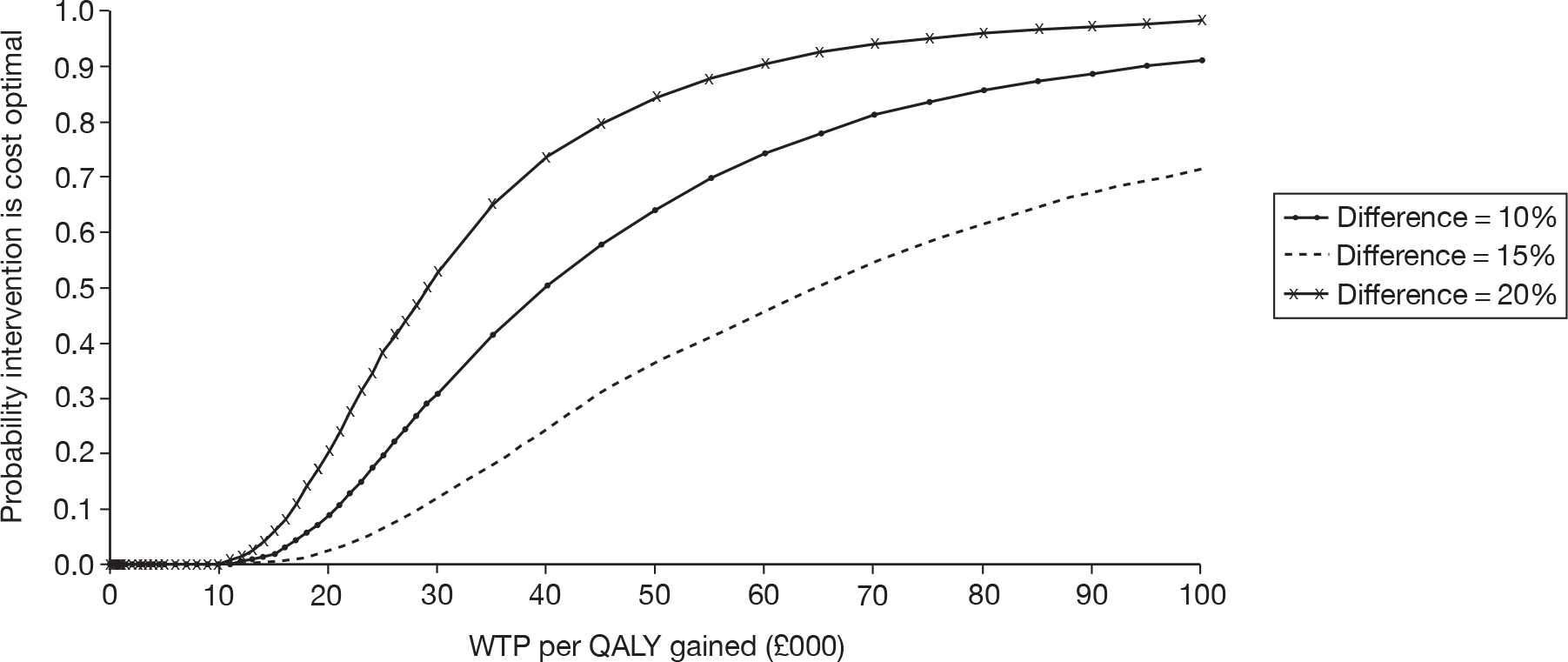
FIGURE 9.
Cost-effectiveness acceptability curves for alternative assumptions on the difference in use of BCHAs and BAHAs in adults (QALY1).
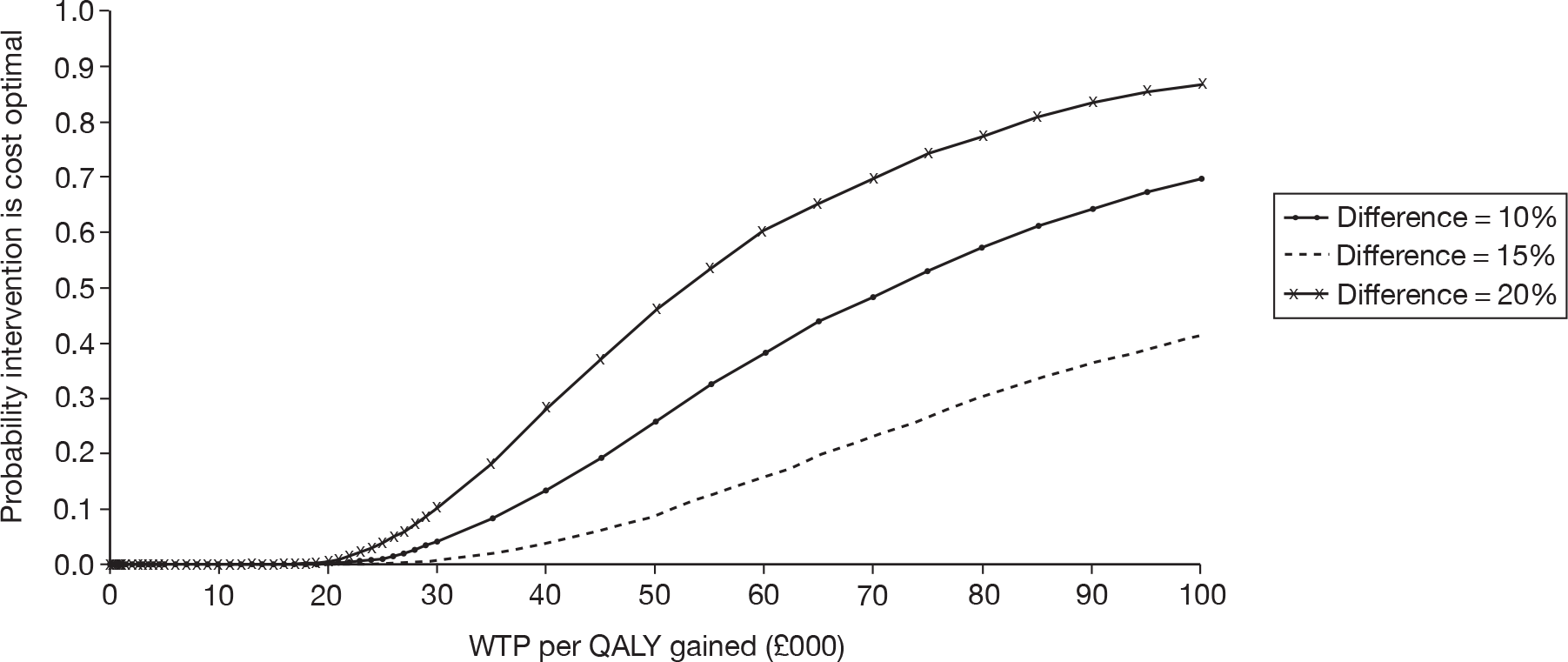
FIGURE 10.
Cost-effectiveness acceptability curves for alternative assumptions on the difference in use of BCHAs and BAHAs in adults (QALY2).
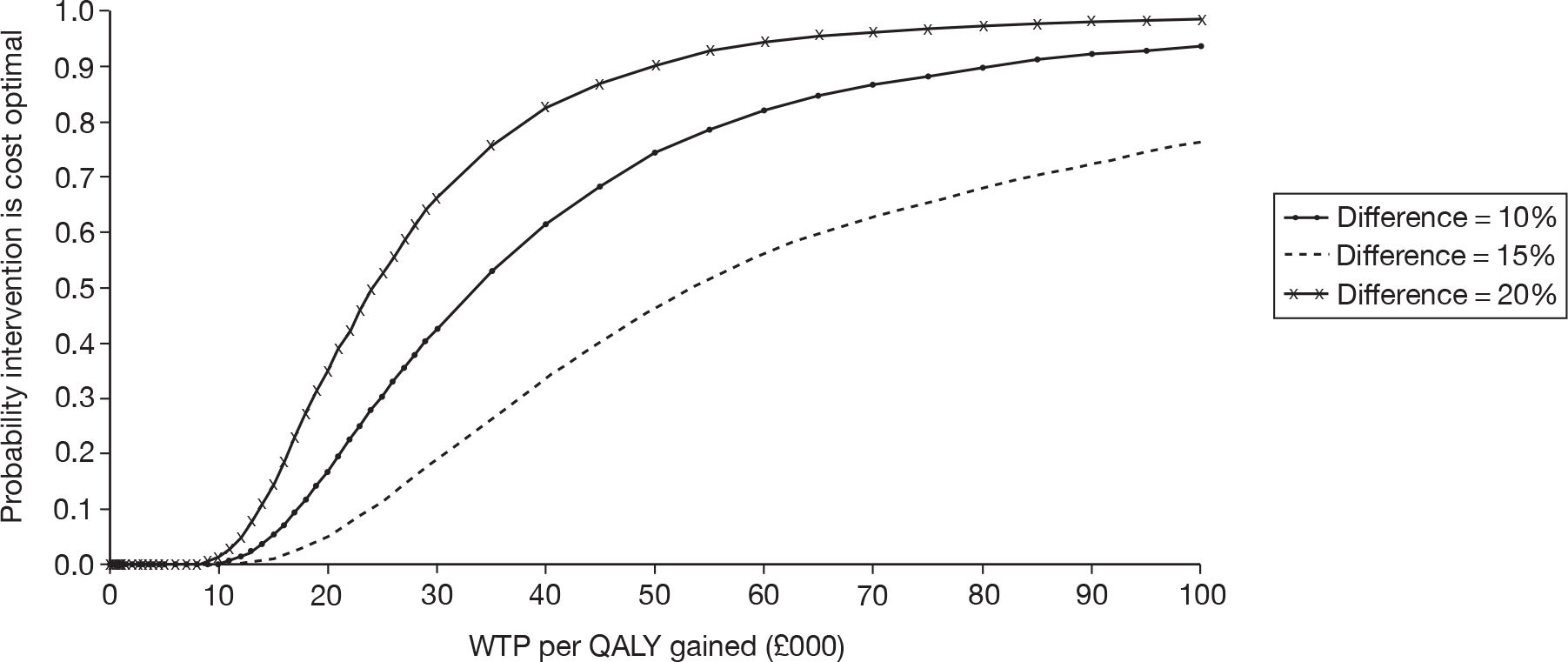
Table 56 summarises the probability of BAHAs being a cost-effective option at conventionally adopted thresholds for acceptable cost-effectiveness, from each of the PSAs. These results reinforce the impact of the assumed difference in the proportion of individuals using their comparator hearing aid on the cost-effectiveness of BAHAs.
| Patient group | Utility gain from aided hearing | Threshold WTP (£ per QALY gained) | Difference in use | ||
|---|---|---|---|---|---|
| 10% (2/20) | 15% (3/20) | 20% (4/20) | |||
| Children | QALY1a | 20,000 | 0.00 | 0.00 | 0.06 |
| 30,000 | 0.10 | 1.26 | 3.66 | ||
| QALY2b | 20,000 | 2.30 | 8.58 | 20.36 | |
| 30,000 | 11.96 | 30.64 | 52.80 | ||
| Adults | QALY1a | 20,000 | 0.00 | 0.14 | 0.56 |
| 30,000 | 0.64 | 4.00 | 10.18 | ||
| QALY2b | 20,000 | 5.00 | 16.68 | 35.02 | |
| 30,000 | 18.96 | 42.52 | 66.20 | ||
Summary of cost-effectiveness
The results of our cost analysis demonstrate that BAHAs are a significantly more costly strategy than conventional aids for people with bilateral hearing loss. These additional costs are not restricted to the initial process of surgical implantation, followed by the acquisition and fitting of the BAHA sound processor, but will continue while individuals remain using their BAHAs. Our exploratory cost-effectiveness analysis suggests that, where the benefits (in terms of hearing improvement) are similar for BAHAs and their comparators and where the probability of using alternative aids for ≥ 8 hours per day is similar, BAHAs are unlikely to be a cost-effective option. The greater the benefit from BAHA-aided hearing and, in particular, the greater the difference in the proportion of people using the BCHA or BAHA for ≥ 8 hours per day, the more likely BAHAs are to be a cost-effective option. The inclusion of other dimensions of QoL may also increase the likelihood of BAHAs being a cost-effective option.
Chapter 5 Discussion
Statement of principal findings
Clinical effectiveness
The extensive search strategy did not identify any studies with a concurrent control group. The studies included in the systematic review of clinical effectiveness were rated weak overall for methodological quality and quality of reporting, and, thus, they have a high risk of bias and caution is required when interpreting the results.
BAHAs versus BCHAs
Four cohort pre and post studies included a comparison of BAHAs and BCHAs. The people included in these studies (where reported) were described as having inoperable congenital microatresia,77 either chronic suppurative otitis media or a congenital aetiology,78 or recurrent otorrhoea or aural atresia. 79–81 The BAHAs used in these studies are no longer manufactured and, although there should be little difference in terms of amplification between older and newer models, the newer models provide greater convenience and more flexible controls and have more benefits for mixed hearing loss. Improvements in the sound field PTA and warble-tone thresholds were found with a BAHA by two studies,77,78 but statistical analysis was reported by only one study (p < 0.01). 78 The third study did not report thresholds averaged across frequencies, but found improved thresholds with the BCHA at 0.25 and 0.50 kHz, and with the BAHA at higher frequencies. A statistically significant improvement in SRT in quiet and speech-to-noise ratio was found in people with a SNHL of less than 30 dB HL,81 while another study found no statistically significant difference in speech discrimination score in patients with either chronic suppurative otitis media or a congenital aetiology. 78 Statistical analysis was not reported for other results, which included a 23% improvement in 100% speech audiometry discrimination in noise,77 but little difference in speech recognition threshold. 79
Two studies reported using a validated measure of QoL,77,82 although limited data were reported by one of the studies. 77 The second study found no statistically significant differences between BCHAs and BAHAs using the SF-36 and EQ-5D; however, a statistically significant improvement with a large clinical impact was found for handicap and disability with the HHDI. 82 The HHDI is specific to hearing loss, whereas the SF-36 and EQ-5D are generic measures that do not have a hearing dimension, which may explain the difference in outcomes between the different instruments.
In summary, while there appear to be some audiological benefits of BAHAs when compared with BCHAs, the limited evidence base of studies with a high risk of bias does not provide a reliable estimate of the degree of benefit. Improvements in QoL were identified by the hearing-specific instrument but not the generic QoL measures. Other issues such as wearability have not been adequately addressed by the included studies.
BAHAs versus ACHAs
Five cohort pre and post studies78–81,83,84 and one cross-sectional ‘audiological comparison’ study76 included a comparison of BAHAs and ACHAs. The people included in these studies (where reported) were described as having otosclerosis,83 mixed hearing loss,76 chronic otitis,84 either chronic suppurative otitis media or a congenital aetiology,78 or recurrent otorrhoea or aural atresia. 79–81 Only one study assessed a BAHA model in current use. 76 The direction of effect for sound field pure-tone or warble-tone thresholds was inconsistent between the studies. One study found a statistically significant improvement in mean warble-tone thresholds (0.5–4.0 kHz) with a BAHA,78 while in another study data on average warble-tone thresholds (0.2–4.0 kHz) were described as ‘comparable’ between BAHAs and ACHAs, but no statistical analysis was provided. 83 Three studies presented thresholds at each frequency individually, but there was no clear pattern as to the comparative benefits of ACHAs and BAHAs. 76,79,84
The direction of the effect was also unclear for speech audiometry, with some studies finding improved outcomes with the ACHA and some with the BAHA. Two studies, which included patients with otosclerosis83 or recurrent otorrhoea with severe hearing loss,79 reported better outcomes with the ACHA for speech discrimination scores,83 maximum phoneme score79 or speech recognition threshold,79 although statistical analysis was not conducted. A later publication by the same authors as the latter study, but with a different patient group (less severe hearing loss), found a statistically significant deterioration in SRT in quiet with BAHA (p < 0.05), but a statistically significant improvement in speech-to-noise ratio (p < 0.05). 81 One study of participants with conductive or mixed hearing loss with chronic otitis found no statistically significant difference in maximum phoneme score, but a statistically significant improvement in speech-to-noise ratio with BAHA. 84 Speech discrimination score was statistically significantly better with the BAHA in the congenital aetiology group, but not the chronic suppurative otitis media group, in one study. 78 The final study reported an improvement in speech-in-noise with the BAHA described as ‘large and clinically significant’ in participants with mixed hearing loss. 76 Although the ACHA may produce better audiometric results in some situations, it should be noted that the most appropriate hearing aid may not necessarily be the one with the best performance, as other factors such as the ability to wear the aid and reduced susceptibility to infections need to be considered. These issues have not been adequately addressed by the included studies.
One study reported using a validated measure of QoL. 82 A statistically significant increase in anxiety/depression with BAHAs was found by the EQ-5D, but the clinical effect was small. No other statistically significant differences were found between ACHAs and BAHAs by the EQ-5D or the SF-36; however, a statistically significant improvement with a large clinical impact was found for handicap and disability with the HHDI. 82
One study reported the number of otolaryngology visits over the preceding 6 months for draining ears, and found a reduction with BAHAs compared with ACHAs [mean visits 12.7 (SD 10.5) vs 3.3 (SD 4.8)]; however, statistical analysis was not undertaken. 82
In summary, the limited evidence base suggests improvements in speech understanding in noise with the BAHA compared with the ACHA; however, the ACHA may produce better audiological results for other outcomes. Improvements in QoL were identified by the hearing-specific instrument but not the generic QoL measures. Other issues such as improvement of discharging ears have not been adequately addressed by the included studies.
BAHAs versus unaided hearing
Four cohort pre and post studies included a comparison of BAHAs with unaided hearing. 66,77,78,83 The people included in these studies were described as having inoperable bilateral congenital microtia atresia,77 otosclerosis,83 chronic suppurative otitis media or a congenital aetiology,78 or CHL with some mild-to-moderate sensorineural loss (details of the aetiology not reported). 66 One of the studies assessed a BAHA model in current use. 60 All four studies found improvements in sound field thresholds with the BAHA compared with unaided hearing, and these improvements were statistically significant in the two studies that conducted analysis. Improvements were also found in speech discrimination66,78,83 and speech recognition thresholds in quiet and noise,66 although statistical analysis was not undertaken. No self-reported measures were reported by these four included studies. In summary, the limited evidence suggests that hearing is improved with BAHAs compared with no hearing aid.
Unilateral versus bilateral BAHAs
Four cross-sectional ‘audiological comparison’ studies compared unilateral with bilateral BAHAs. 59,60,86,87 The people included in these studies were described as having recurrent otorrhoea,60 chronic otitis,60,86,87 congenital atresia,60,87 otosclerosis,86,87 congenital syndromes or congenital hearing loss,86 mastoid cavities,86 microtia, or86 symmetrical maximal or near-maximal conductive bilateral hearing loss (details of aetiology not reported). 59 The BAHAs used in these studies are no longer manufactured. The participants in the included studies all underwent sequential (separate operations) implantation of the bilateral BAHAs. The timing of bilateral implantation, whether sequential or simultaneous, is a major issue in cochlear implant research. It has been suggested that simultaneous cochlear implantation enables both ears to adjust to the new form of sound processing simultaneously129 and leads to better sound localisation130 in young children, but the timing in adults appears to be less critical. 130 However, as BAHAs do not selectively stimulate each side, these issues may not be so important.
Sound field average tone thresholds were improved with bilateral BAHAs compared with unilateral BAHAs in adults87 and a small group (n = 3) of children59 with previous experience of bilateral BAHAs, but statistical analysis was not undertaken. Two studies found that speech recognition thresholds in quiet were statistically significantly lower with bilateral BAHAs,60,87 although another study found similar results between unilateral and bilateral BAHAs. 86 Bilateral BAHAs produced better results than one BAHA when noise was presented from the baffle/best side (the side with the BAHA in the unilateral condition), but not when noise was presented from the shadow side (the side opposite to the BAHA in the unilateral condition);60,86,87 this is explained by the increase in noise transmitted to the ears with an extra BAHA on the shadow (noise) side. Three studies found that localisation of sound was improved with bilateral BAHAs. 59,60,87 Two studies reported the binaural masking level difference test and suggested that BAHAs give binaural hearing,60,87 although the validity of the methods is uncertain. One study described self-reported measures using a validated tool (MAIS and MUSS, and IOI-HA), but the sample size was very small (n = 2 or n = 3 for the bilateral users group). In summary, the limited evidence suggests that there are benefits of bilateral BAHAs in many, but not all, situations, and the presence of binaural hearing with bilateral BAHAs remains uncertain.
Adverse events
As the included studies reported very limited data on adverse events, additional data from prospective case series were described. It should be noted that these studies did not undergo the same process of data extraction and quality assessment. Five prospective case series were discussed and reported loss rates of implants between 6.1% (9–25 months’ follow-up)102 and 19.4% (median 6 years’ follow-up). 100 The vast majority of patients in the prospective case series experienced no or minor skin reactions.
Cost-effectiveness
Published economic evaluations
The search strategy did not identify any fully published economic evaluations of BAHAs. Two studies107,108 reporting resource use or cost data for patients receiving BAHAs were reviewed for their relevance. One cost study,107 focusing on outpatient BAHA implantation and conducted in the US, was not relevant to the perspective of the current review. The second cost study,108 a UK retrospective analysis of service use before and after BAHA implantation, was used in conjunction with current service standards to identify the management pathway for individuals considered eligible for a BAHA, and as a basis for costing the intervention in our economic model.
Unpublished economic evaluations
An unpublished, UK-based, economic evaluation of BAHAs was identified. 110 Although not meeting the inclusion criteria for this review (33.3% of included participants were stated as having bilateral hearing loss, the remainder were unilateral/single-sided or not stated); the methods and data inputs of the model were briefly reviewed. The analysis was based on patient-level data for adult patients undergoing primary BAHA implantation at UHB. Unit costs for surgery were based on PCT charges for BAHAs and for digital (non-BAHA) hearing aids. Health outcomes were assessed using QALYs, based on each patient’s age-sex-specific life expectancy with utility values based on responses to the HUI3. Mean HUI3 utility at baseline was reported as 0.59 (95% CI 0.53 to 0.65) and post-BAHA was 0.66 (95% CI 0.60 to 0.72). The incremental cost for BAHA provision was £20,604. The mean discounted QALY gain was 1.17, yielding an ICER of £17,610.
Southampton Health Technology Assessments Centre economic model
We developed a new model to estimate the cost-effectiveness of BAHAs in separate cohorts of eligible adults and children with bilateral deafness. Owing to data limitations identified in the clinical effectiveness section above, the model was limited to comparing BAHAs against BCHAs.
Owing to limitations of the evidence base, the model did not incorporate direct measures of gain in hearing from included studies. As a result, the model reports a cost comparison – of BAHAs compared with BCHAs – and an exploratory cost-effectiveness analysis using estimated potential utility gains based on levels of the hearing dimension of the HUI3 and limited data on the use of BCHAs compared with BAHAs. The analysis assumes that the utility gain from aided hearing is the same for both BAHAs and BCHAs and that differences in outcome for the devices arise from differences in the proportion of individuals using the devices.
Included studies were reviewed for information on the incidence of adverse events, to populate the economic model. Adverse events in the model were limited to perioperative complications (bleeding), failure of initial bone integration, pain leading to removal of the implant, skin reactions and loss of bone integration. We did not identify sources that reported separate incidence of adverse events for children and adults; hence the same event probabilities are used for both populations.
Resource use in the model was estimated based on published reports,108 current audiology service standards for BAHAs109 and discussion with clinical experts. We identified a management pathway from an initial consultation with an ENT surgeon, through to surgical implantation and long-term management. Unit costs were derived based on NHS reference costs43 – where available – and from NHS providers (for costs of components of the BAHA system and comparator hearing aids).
In the cost analysis, the BAHA is the more costly strategy, increasing costs in children by approximately 94% over the BCHA (from £1105 to £17,514) and increasing costs in adults by 93% (from £1084 to £14,533). The higher costs for BAHA provision in children arise from the use of two-stage surgery and from higher outpatient costs for paediatric cases. The single most costly phase of BAHA provision is long-term maintenance (at £6241 for paediatric cases and £6114 for adults), although the majority of BAHA cost is incurred in the first year (for implant surgery and the cost of the BAHA sound processor). An average cost per case successfully treated was estimated, allowing for a proportion of participants choosing not to continue with their BAHA owing to pain from the implant or choosing not to have a re-operation following late failure due to loss of bone integration or a severe skin reaction leading to implant removal. It was estimated that up to 6% of the initial cohort would have ceased using their BAHA by the end of the 10-year time horizon. Under these assumptions the cost per case successfully treated is £18,681 for children and £15,785 for adults. In a DSA, the results of the cost analysis were generally robust to variation in the value of input parameters. The results were most sensitive to variation in the probability of re-operation when implants lose bone integration, the cost of surgical implantation and the cost of the BAHA processor maintenance plan.
In the absence of usable QoL data for people with bilateral hearing loss or methods to map changes in hearing measures reported in included studies to QoL, we conducted an exploratory cost-effectiveness analysis. This incorporated assumptions regarding the potential gains from aided hearing in people with bilateral hearing loss, who are unable to hear one-to-one or group conversation without a hearing aid, and limited data on use of BCHAs and BAHAs in BAHA-eligible subjects (indicating a 10% increase in the proportion of patients using their BAHA compared with usage of BCHAs). Under these assumptions, provision of BAHAs resulted in a QALY gain of between 0.14 and 0.30 for paediatric cases (depending on the assumed level of utility associated with aided hearing). Combined with the incremental cost estimates described above, these yielded ICERs of £119,367 and £55,642 per QALY gained, respectively. Applying the same analysis to adults yields similar QALY gains from BAHA provision (0.14 and 0.29 QALYs), resulting in lower ICERs (£100,029 and £46,628 per QALY gained, respectively), given the lower incremental costs estimated for adults. These ICERs are high and above conventionally adopted thresholds for acceptable cost-effectiveness in an NHS decision-making context.
Deterministic sensitivity analyses for the exploratory cost-effectiveness analysis suggest that the results are generally robust to variation in input probabilities and unit costs. The variable that has the greatest influence on the cost-effectiveness results is the proportion of each cohort using their hearing aids for 8 or more hours per day. Very high ICER values are associated with a high proportion of people using BCHA for 8 or more hours per day (98.8% at the upper limit of the 95% CI, resulting in a small difference in usage between BCHA and BAHA), but more acceptable values are associated with a lower proportion using BCHA for 8 or more hours per day (compared with BAHA).
Threshold values for differences in use of hearing aids, the presence of pain/discomfort associated with the use of BCHA and the underlying utility gain from aided hearing were explored in a range of scenario analyses. Where the utility gain from aided hearing is related to the ability to hear one-to-one conversation in quiet, the difference in the proportion of people using their hearing aid for 8 hours or more per day needs to be greater than 33% in adults (greater than 39% in children) for BAHAs to be a cost-effective option (at a WTP threshold of £30,000 per QALY). Where the utility gain from aided hearing is related to the ability to hear both one-to-one conversation in quiet and group conversation, the required difference in the proportion of people using their hearing aid for 8 hours or more per day for BAHAs to be a cost-effective option is lower (greater than 15% in adults, 18% in children). Where pain/discomfort is included in the analysis, the ICERs fall substantially, with BAHAs appearing to be a cost-effective option (if the utility gain from aided hearing is related to the ability to hear one-to-one conversation in both quiet and group conversation).
General discussion
The findings of the systematic review of clinical effectiveness are in line with those of a previous systematic review,11 which assessed the non-acoustic benefits of BAHAs. The earlier review, however, included studies of unilateral as well as bilateral deafness and also included retrospective studies. The authors concluded that there is limited statistically supported, empirically controlled evidence supporting the non-acoustic benefits of BAHAs relative to more conventional hearing aids or no hearing aids at all. No other systematic reviews of BAHAs for bilateral hearing loss were identified.
The conclusions drawn from the present systematic review of clinical effectiveness are constrained by the limitations of the available evidence. Despite conducting a wide-ranging and systematic search of the literature, no trials with a concurrent control group (either RCTs, controlled clinical trials or prospective cohort analytic studies) were identified. The included studies were rated overall as weak, therefore there is a high risk of bias in the studies. The outcome measures reported by the studies also have limitations, and it is not always clear what is clinically significant or meaningful to the patient. Audiological measures such as hearing threshold levels or speech reception levels in quiet may be too simplistic, and a measure such as ‘match to target’ may be more meaningful; however, this was not reported by the included studies. Lower hearing thresholds are considered to be better than higher thresholds throughout the review, but it is acknowledged that this is a simplistic approach and may not necessarily be the case if they are below the target value. The review uses the study authors’ descriptions such as ‘improvement’ or ‘deterioration’ where available.
Only three studies59,77,82 reported using a validated measure of QoL; thus, it is difficult to make any judgement about the impact of BAHAs on the QoL of a person with bilateral hearing loss. An important issue for the individual is comfort and the ability to wear the hearing aid, especially if he or she cannot wear conventional hearing aids. For example, if the aid cannot be worn owing to discomfort or a discharging ear, then it is not appropriate. These issues are not considered by audiological comparisons of BAHAs with conventional aids. Although some included studies reported patient preference, the tools used were not validated and were likely to be biased, especially considering evidence that suggests that patients report preferring the second hearing aid tested, even if it is in fact an identical aid. 130 Synthesis of the included studies was through narrative review; although 12 studies were included in the review of clinical effectiveness, differences in participants, comparator (ACHA, BCHA or unaided) and outcome measures meant that meta-analysis was inappropriate. No prospective studies comparing BAHAs with ear surgery were identified; thus, no conclusions could be drawn.
There are potentially many benefits of BAHAs for individuals and their families, but these are difficult to quantify and there is little evidence available. In children, improved speech and language development may lead to a reduced need for specialist schooling and involvement of teachers for the deaf. Children may perform better at school, potentially leading to better employment opportunities in the future. For adults, an improvement in the discharging ear may mean attending fewer ENT clinics, less absence from work and again better employment opportunities. There may also be benefits in terms of improved road safety with bilateral BAHAs. These factors could not be addressed in the present report.
Bone-anchored hearing aid technology is continuously evolving and as a result the majority of the evidence in this review is based on BAHA devices that are no longer manufactured. The newer models may have greater benefits for mixed hearing loss than for CHL. They provide more convenience with flexible controls and a directional microphone.
With regards to service provision, BAHAs are likely to remain a specialist service as a part of a comprehensive audiological rehabilitation, with a small number of centres. Children may be more appropriately cared for in specialised children’s units because of comorbidities and anaesthetic difficulties. The number of people who meet the existing BAHA criteria and who could potentially benefit from BAHAs is unclear. There is also currently a lack of awareness about BAHAs, both in primary care and in audiology departments where there is no BAHA programme,131 so current provision is likely to be below potential. It is thought that growing awareness will lead to increased referrals for BAHA services, which in turn will lead to an escalating number of BAHAs that need repairing, replacing or upgrading. 132 Commissioners of services in the NHS will therefore need to make decisions as to how they support this need. Potential benefits to the NHS may include a decrease in ENT attendance for discharging ears.
Strengths and limitations of the assessment
This review has the following strengths:
-
It is independent of any vested interest.
-
It has been undertaken following the principles for conducting a systematic review. The methods were set out in a research protocol (see Appendix 1), which defined the research question, inclusion criteria, quality criteria, data extraction process and methods to be employed at different stages of the review.
-
A multidisciplinary advisory group has informed the review from its initiation. The research protocol was informed by comments received from the advisory group and the advisory group has reviewed and commented on the final report.
-
The review brings together the evidence for the clinical effectiveness and cost-effectiveness of BAHAs for people who are bilaterally deaf. This evidence has been critically appraised and presented in a consistent and transparent manner.
-
An economic model has been developed de novo following recognised guidelines, and systematic searches have been conducted to identify data for the economic model. The main results have been summarised and presented.
In contrast, this review also has certain limitations:
-
Twenty-eight relevant non-English references were identified by the searches, and although titles and English abstracts (where available) were examined, the papers were not translated and screened. However, none of the papers appeared to present higher level evidence and it is unlikely that the inclusion of more low-level evidence would change the conclusions of this report.
-
The rigorous methods of the review meant that many of the available data on QoL, patient preference, patient satisfaction, comfort and wearability did not meet the inclusion criteria for the systematic review of clinical effectiveness owing to study design and the use of tools that were not validated.
-
Limited data on adverse events were reported in the included studies; thus, data from eligible prospective case series were described. Although data from these studies were extracted by one reviewer and checked by a second reviewer, these studies did not undergo the same process of quality assessment.
-
The economic evaluation presented in this report is severely limited by a lack of robust evidence on the outcome of hearing aid provision. This has led to a more restricted analysis than was originally anticipated (limited to a comparison of BAHA with BCHA). In the absence of usable QoL data, the cost-effectiveness analysis is based on potential utility gains from hearing that have been inferred using a QoL instrument rather than measures reported by hearing aid users themselves. As a result, the analysis is regarded as exploratory and the reported results should be interpreted with caution.
-
Given the exploratory nature of the economic evaluation (particularly the fact that the utility data used to estimate QALYs), we felt that it was not appropriate to extend this to include a value of information analysis (as we originally proposed).
Chapter 6 Conclusions
Implications for service provision
The findings suggest that hearing is improved with BAHAs compared with no hearing aid and, although there are audiological benefits of BAHAs when compared with conventional BCHAs, the audiological comparisons with ACHAs are more equivocal. However, candidates for BAHAs may not be able to use these conventional aids, or be able to use them for only a limited time, for example owing to discomfort or infections. Limited data suggest an improvement in QoL with BAHAs when compared with conventional aids, but there is an absence of evidence regarding other potential benefits, such as length of time the aid is able to be worn and improvement of discharging ears. The evidence suggests that there are some benefits of bilateral BAHAs compared with unilateral BAHAs. BAHAs are significantly more costly than conventional hearing aids. The additional costs continue while individuals remain using their BAHAs and are not restricted to initial surgical procedures and acquisition of the BAHA sound processor. Our exploratory cost-effectiveness analysis suggests that BAHAs are most likely to be cost-effective in people with the greatest benefit from aided hearing and, in particular, with greater difference in usage of BAHAs compared with conventional aids. Inclusion of other dimensions of QoL (other than hearing) may also increase the likelihood of BAHAs being a cost-effective option. The conclusions are limited by the quality of the available evidence.
Suggested research priorities
-
There are many areas of uncertainty surrounding BAHAs, including, but not limited to: the need for BAHA services; resource implications (both costs and potential savings through reduced ENT attendance); type of service provision (e.g. specialist centres); benefits of BAHAs; usage of conventional hearing aids in patients eligible for BAHAs; and adverse events. A national audit of BAHAs should be implemented, which will provide clarity on these issues. The collection of such data would significantly increase the robustness of economic evaluation of BAHAs and would, potentially, broaden the scope of comparators beyond BCHAs.
-
Further research is required into the non-audiological benefits of BAHAs, including QoL, improvement of discharging ears and time wearing the aid. While an RCT would be preferable, many patients are referred for BAHAs as a last resort when conventional aids are unsuitable; therefore, a controlled clinical trial or a prospective cohort analytic study (two groups’ before and after study) is suggested.
-
A randomised crossover study comparing unilateral and bilateral BAHAs is required. The order of BAHA (unilateral or bilateral) should be randomised, and participants should have at least 12 weeks’ experience with each aid before assessment.
-
The number of people who are potentially eligible for BAHAs is not known. Further research into the incidence and prevalence of hearing loss, and of conductive and mixed hearing loss in particular, is required.
-
Empirical studies into the masking level difference with BC in people with normal hearing and CHL are required.
Acknowledgements
We would like to thank members of our advisory group who provided expert advice and comments on the protocol and/or a draft of this report: Mark Lutman, Professor of Audiology, Institute of Sound and Vibration Research, University of Southampton; Stephen Palmer, Senior Research Fellow in Health Economics, University of York; and Lyn Kolsteron, Service User and Chairperson of BAHA Users Support (Kent) (BUSK). In addition we acknowledge advice or information provided by Daniel Rowan (Institute of Sound and Vibration Research, University of Southampton), Mark Flynn (Cochlear Limited), Dianne Darbyshire (Oticon Medical), Steve Worrollo (Maxillofacial Prosthetics Manager, UHB), Fiona Boyle (Finance Manager – Service Line Reporting, Southampton University Hospitals) and Susan Robinson (Audiology Service Manager, Southampton University Hospitals Trust).
We are also grateful to Jackie Bryant, Principal Research Fellow (Southampton Health Technology Assessments Centre, University of Southampton) for reviewing a draft of this report.
Contributions of authors
JL Colquitt (Senior Research Fellow) developed the original research grant application, developed the research protocol, drafted the background section, assisted in the development of the search strategy, assessed studies for inclusion, extracted data from and quality assessed included studies, synthesised evidence, drafted and edited the final report, and project managed the study.
J Jones (Principal Research Fellow) developed the original research grant application, developed the research protocol, assessed studies for inclusion, extracted data from and quality assessed included studies, synthesised evidence, developed the economic evaluation and drafted the report.
P Harris (Research Fellow) developed the research protocol, assisted with drafting the background section, assisted in the development of the search strategy, assessed studies for inclusion, extracted data from and quality assessed included studies, and drafted the report.
E Loveman (Senior Research Fellow) developed the original research grant application, developed the research protocol, assisted with drafting the background section, assessed studies for inclusion, extracted data from and quality assessed included studies, synthesised evidence, and drafted the report.
A Bird (Research Fellow) developed the research protocol, assessed studies for inclusion, extracted data from and quality assessed included studies, synthesised evidence, developed the economic evaluation and drafted the report.
AJ Clegg (Professor/Director of Southampton Health Technology Assessments Centre) developed the original research grant application, developed the research protocol, drafted the background section, assessed studies for inclusion, provided methodological guidance, drafted the report and provided quality assurance.
DM Baguley (Head of Audiology/Consultant Grade Audiological Scientist) developed the original research grant application, developed the research protocol, provided expert advice, assisted in the interpretation of the data and commented on the draft report.
DW Proops (Consultant ENT Surgeon) developed the original research grant application, developed the research protocol, provided expert advice, assisted in the interpretation of the data and commented on the draft report.
TE Mitchell (Consultant Otolaryngologist) developed the original research grant application, developed the research protocol, provided expert advice, assisted in the interpretation of the data and commented on the draft report.
PZ Sheehan (Consultant Paediatric Otolaryngologist) developed the research protocol, provided expert advice, assisted in the interpretation of the data and commented on the draft report.
K Welch (Information Specialist) developed the research protocol, developed the search strategy, conducted the searches and commented on the draft report.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- British Society of Audiology . Recommended Procedure. Pure Tone Air and Bone Conduction Threshold Audiometry With and Without Masking and Determination of Uncomfortable Loudness Levels 2004. www.thebsa.org.uk/docs/RecPro/PTA.pdf (accessed 20 January 2010).
- Royal National Institute for Deaf People . Types and Causes n.d. www.rnid.org.uk/information_resources/aboutdeafness/causes (accessed 23 December 2009).
- Yueh B, Shapiro N, MacLean CH, Shekelle PG. Screening and management of adult hearing loss in primary care. JAMA 2003;289:1976-85.
- Smith RJ, Bale J, White KR. Sensorineural hearing loss in children. Lancet 2005;365:879-90.
- Parmet S, Lynm C, Glass RM. Adult hearing loss. JAMA 2009;289.
- National Deaf Children’s Society (NDCS) . Quality Standards in Bone Anchored Hearing Aids for Children and Young People 2003.
- Shprintzen R J. Genetics, syndromes and communication disorders. San Diego, CA: Singular Publishing Group, Inc.; 1997.
- Miller JF, Leddy M, Leavitt LA. Evaluating communication to improve speech and language skills. Improving the communication of people with Down’s syndrome. Baltimore, MD: Paul H Brookes Publishing Co; 1999.
- Roizen N. Hearing loss in children with Down’s syndrome: a review. Downs Syndr Quarterly 1997;2:1-4.
- Nadol JB. Hearing loss. N Engl J Med 1993;329:1092-102.
- Johnson CE, Danhauer JL, Reith AC, Latiolais LN. A systematic review of the nonacoustic benefits of bone-anchored hearing aids. Ear Hear 2006;27:703-13.
- Browning GG, Gatehouse S. Estimation of the benefit of bone-anchored hearing aids. Ann Otol Rhinol Laryngol 1994;103:872-8.
- Medical Advisory Secretariat . Bone anchored hearing aid: an evidence-based analysis. OHTAS 2002;2.
- Snik AF, Bosman AJ, Mylanus EA, Cremers CW. Candidacy for the bone-anchored hearing aid. Audiol Neurotol 2004;9:190-6.
- Davis A, Davis K, Smith P, Graham J, Baguley D. Ballantyne’s deafness. Oxford: John Wiley & Son Ltd; 2009.
- Fortnum H, Davis A. Epidemiology of permanent childhood hearing impairment in Trent Region, 1985–1993. Br J Audiol 1997;31:409-46.
- Fortnum HM, Summerfield AQ, Marshall DH, Davis AC, Bamford JM. Prevalence of permanent childhood hearing impairment in the United Kingdom and implications for universal neonatal hearing screening: questionnaire based ascertainment study. BMJ 2001;323:1-6.
- Fortnum H, Marshall D, Summerfield A. Epidemiology of the UK population of hearing-impaired children, including characteristics of those with and without cochlear implants – audiology, aetiology, comorbidity and affluence. Int J Audiol 2002;41:170-9.
- MacAndie C, Kubba H, McFarlane M. Epidemiology of permanent childhood hearing loss in Glasgow, 1985–1994. Scott Med J 2003;48:117-19.
- Davis AC. The prevalence of hearing impairment and reported hearing disability among adults in Great Britain. Int J Epidemiol 1989;18:911-17.
- Lee D, Gomez-Marin O, Lam B, Zheng D. Trends in hearing impairment in United States adults: the national health interview survey, 1986–1995. J Gerontol A Biol Sci Med Sci 2004;59:1186-90.
- Office for National Statistics . Birth Statistics: Births and Patterns of Family Building England and Wales. FM1 No. 36–2007 2008. www.statistics.gov.uk/downloads/theme_population/FM1_36/FM1-No36.pdf (accessed 1 December 2009).
- Office for National Statistics . Mid-2008 Population Estimates: United Kingdom; Estimated Resident Population by Single Year of Age and Sex 2009. www.statistics.gov.uk/statbase/Product.asp?vlnk=15106 (accessed 1 December 2009).
- Jambor E, Elliott M. Self-esteem and coping strategies among deaf students. J Deaf Stud Deaf Educ 2005;10:63-81.
- Smith A. Preventing deafness – an achievable challenge. The WHO perspective. ICS 2003;1240:183-91.
- Mathers C, Smith A, Concha M. Global Burden of Hearing Loss in the Year 2000 2000. www.who.int/healthinfo/statistics/bod_hearingloss.pdf (accessed 13 April 2009).
- Kalatzis V, Petit C. The fundamental and medical impacts of recent progress in research on hereditary hearing loss. Hum Mol Genet 1998;7:1589-97.
- Arlinger S. Negative consequences of uncorrected hearing loss – a review. Int J Audiol 2003;42:17-20.
- Matkin ND, Wilcox AM. Considerations in the education of children with hearing loss. Pediatr Clin North Am 1999;46:143-52.
- Barker DH, Quittner AL, Fink NE, Eisenberg LS, Tobey EA, Niparko JK, et al. Predicting behavior problems in deaf and hearing children: the influences of language, attention, and parent-child communication. Dev Psychopathol 2009;21:373-92.
- Vostanis P, Hayes M, Feu M, Warren J. Detection of behavioural and emotional problems in deaf children and adolescents: comparison of two rating scales. Child Care Health Dev 1997;23:233-46.
- Fellinger J, Holzinger D, Sattel H, Laucht M. Mental health and quality of life in deaf pupils. Eur Child Adolesc Psychiatry 2008;17:414-23.
- Woerner W, Becker A, Friedrich C, Klasen H, Goodman R, Rothernberger A. Normal values and evaluation of the German parent’s version of strengths and difficulties questionnaire (SDQ): results of a representative field study. Z Kinder Jugendpsychiatr Psychother 2002;30:105-12.
- Tharpe A. Unilateral and mild bilateral hearing loss in children: past and current perspectives. Trends Amplifi 2008;12:7-15.
- Tambs K. Moderate effects of hearing loss on mental health and subjective well-being: results from the Nord-Trondelag Hearing Loss Study. Psychosom Med 2004;66:776-82.
- Nachtegaal J, Smit JH, Smits C, Bezemer PD, van Beek JH, Festen JM, et al. The association between hearing status and psychosocial health before the age of 70 years: results from an internet-based national survey on hearing. Ear Hear 2009;30:302-12.
- Dalton DS, Cruickshanks KJ, Klein BEK, Klein R, Wiley TL, Nondahl DM. The impact of hearing loss on quality of life in older adults. Gerontologist 2003;43:661-8.
- National Screening Committee . The UK NSC Policy on Hearing Screening in Children 2006. www.screening.nhs.uk/hearing-child (accessed 30 November 2009).
- World of Sound . UK Baha Implant Centres 2008. www.worldofsound.org/campaigns/baha/baha-implant-centres.html (accessed 21 October 2009).
- BNSSG Commissioning Advisory Forum . Commissioning Policy for Bone Anchored Hearing Aids 2008. www.avon.nhs.uk/phnet/Publications/baha_caf_version210808.doc (accessed 13 April 2009).
- Select Committee on Health Written Evidence . Evidence Submitted by John Beadle (AUDIO6) 2007. www.publications.parliament.uk/pa/cm200607/cmselect/cmhealth/392/392we09.htm (accessed 13 April 2009).
- Snik AF, Mylanus EA, Proops DW, Wolfaardt JF, Hodgetts WE, Somers T, et al. Consensus statements on the BAHA system: where do we stand at present?. Ann Otol Rhinol Laryngol 2005;195:2-12.
- Department of Health . NHS Reference Costs 2007 08 2009. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_098945 (accessed 8 May 2009).
- Nottingham University Hospital NHS Trust . Nottingham Cochlear Implant and Bone Anchored Hearing Aid Programmes n.d. www.nuh.nhs.uk/ncip/Documents/prices_200708.pdf (accessed 8 April 2009).
- Robbins AM, Renshaw JJ, Berry SW. Evaluating meaningful auditory integration in profoundly hearing-impaired children. Am J Otol 1991;12:144-50.
- Priwin C, Granstrom G. A long-term evaluation of bone-anchored hearing aid (BAHA) in children. Cochlear Implants Int 2005;6:81-3.
- Cox R, Stephens D, Kramer SE. Translation of the international outcome inventory for hearing aids (IOI-HA). Int 2002;41:3-26.
- Granstrom G, Bergstrom K, Odersjo M, Tjellstrom A. Osseointegrated implants in children: experience from our first 100 patients. Otolaryngol Head Neck Surg 2001;125:85-92.
- Davids T, Gordon KA, Clutton D, Papsin BC. Bone-anchored hearing aids in infants and children younger than 5 years. Otolaryngol Head Neck Surg 2007;133:51-5.
- Spitzer JB, Ghossaini SN, Wazen JJ. Evolving applications in the use of bone-anchored hearing aids. Am J Audiol 2002;11:96-103.
- Wazen JJ, Gupta R, Ghossaini S, Spitzer J, Farrugia M, Tjellstrom A. Osseointegration timing for Baha system loading. Laryngoscope 2007;117:794-6.
- Kohan D, Morris LG, Romo T. Single-stage BAHA implantation in adults and children: is it safe?. Otolaryngol Head Neck Surg 2008;138:662-6.
- Somers T, De CJ, Daemers K, Govaerts P, Offeciers FE. The bone anchored hearing aid and auricular prosthesis. Acta Oto-Rhino-Laryngologica Belgica 1994;48:343-9.
- Proops DW. The Birmingham bone anchored hearing aid programme: surgical methods and complications. J Laryngol Otol 1996;21:7-12.
- Tjellstrom A, Håkansson B, Granstrom G. Bone-anchored hearing aids: current status in adults and children. Otolaryngol Clin North Am 2001;34:337-64.
- Lloyd S, Almeyda J, Sirimanna KS, Albert DM, Bailey CM. Updated surgical experience with bone-anchored hearing aids in children. J Laryngol Otol 2007;121:826-31.
- Bernstein J, Sheehan P. An approach to bilateral bone-anchored hearing aid surgery in children: contralateral placement of sleeper fixture. J Laryngol Otol 2009;123:555-7.
- Stenfelt S. Bilateral fitting of BAHAs and BAHA fitted in unilateral deaf persons: acoustical aspects. Int J Audiol 2005;44:178-89.
- Priwin C, Jonsson R, Hultcrantz M, Granstrom G. BAHA in children and adolescents with unilateral or bilateral conductive hearing loss: a study of outcome. Int J Pediatr Otorhinolaryngol 2007;71:135-45.
- Bosman AJ, Snik AF, van der Pouw CT, Mylanus EA, Cremers CW. Audiometric evaluation of bilaterally fitted bone-anchored hearing aids. Audiol 2001;40:158-67.
- Stenfelt S. Physiological aspects regarding bilateral fitting of BAHAs. Cochlear Implants Int 2005;6:83-6.
- Rowan D, Gray M. Lateralization of high-frequency pure tones with interaural phase difference and bone conduction. Int J Audiol 2008;47:404-11.
- Akeroyd MA. The psychoacoustics of binaural hearing. Int J Audiol 2006;45:S25-S33.
- Branemark R, Branemark PI, Rydevik B, Myers RR. Osseointegration in skeletal reconstruction and rehabilitation. J Rehabil Res Dev 2001;38:175-81.
- Håkansson B. Birth of Baha 2006. www.baha-users-support.com/birth_of_baha.php (accessed 6 April 2009).
- Kompis M, Krebs M, Hausler R. Speech understanding in quiet and in noise with the bone-anchored hearing aids Baha Compact and Baha Divino. Acta Otolaryngol 2007;127:829-35.
- US Food and Drug Administration . Section 2. Summary and Certification 2002. www.accessdata.fda.gov/cdrh_docs/pdf2/K021837.pdf (accessed 2 June 2009).
- Declau F, Cremers C, Van de HP. Diagnosis and management strategies in congenital atresia of the external auditory canal. Study Group on Otological Malformations and Hearing Impairment. Br J Audiol 1999;33:313-27.
- Cochlear . Baha® Bone Conduction Implants n.d. www.cochlear.com/sea/baha-bone-conduction-implants (accessed 6 April 2009).
- Cochlear . Intenso Data Sheet n.d. http://professionals.cochlearamericas.com/cochlear-products/baha/baha-support-materials/baha-product-specs/baha-intenso (accessed 1 April 2009).
- Carlsson PU, Håkansson BE. The bone-anchored hearing aid: reference quantities and functional gain. Ear Hear 1997;18:34-41.
- Flynn M, Sadeghi A, Halvarsson G. Results of the First Clinical Evaluation of Cochlear Baha BP100 White Paper 2009. http://bp100.cochlear.com/sites/default/files/E81511%20Results%20of%20the%20first%20clinical%20evaluation%20of%20Cochlear%20Baha%20BP100%20whitepaper.pdf (accessed 27 April 2009).
- Thomas BH, Ciliska D, Dobbins M, Micucci S. A process for systematically reviewing the literature: providing the research evidence for public health nursing interventions. Worldviews Evid Based Nurs 2004;1:176-84.
- Drummond MF, O’Brien BJ, Torrance GW, Stoddart GL. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 1997.
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8.
- Flynn MC, Sadeghi A, Halvarsson G. Baha solutions for patients with severe mixed hearing loss. Cochlear Implants Int 2009;10:43-7.
- Béjar-Solar I, Rosete M, de Jesus MM, Baltierra C. Percutaneous bone-anchored hearing aids at a pediatric institution. Otolaryngol Head Neck Surg 2000;122:887-91.
- Cooper HR, Burrell SP, Powell RH, Proops DW, Bickerton JA. The Birmingham bone anchored hearing aid programme: referrals, selection, rehabilitation, philosophy and adult results. J Laryngol Otol 1996;21:13-20.
- Snik AF, Jorritsma FF, Cremers CW, Beynon AJ, van den Berge NW. The super-bass bone-anchored hearing aid compared to conventional hearing aids. Audiological results and the patients’ opinions. Scand Audiol 1992;21:157-61.
- Snik AF, Mylanus EA, Cremers CW. Speech recognition with the bone-anchored hearing aid determined objectively and subjectively. Ear Nose Throat J 1994;73:115-17.
- Snik AF, Dreschler WA, Tange RA, Cremers CW. Short- and long-term results with implantable transcutaneous and percutaneous bone-conduction devices. Otolaryngol Head Neck Surg 1998;124:265-8.
- Hol MK, Spath MA, Krabbe PF, van der Pouw CT, Snik AF, Cremers CW, et al. The bone-anchored hearing aid: quality-of-life assessment. Otolaryngol Head Neck Surg 2004;130:394-9.
- Burrell SP, Cooper HC, Proops DW. The bone anchored hearing aid – the third option for otosclerosis. J Laryngol Otol 1996;21:31-7.
- Mylanus EA, van der Pouw KC, Snik AF, Cremers CW. Intraindividual comparison of the bone-anchored hearing aid and air-conduction hearing aids. Otolaryngol Head Neck Surg 1998;124:271-6.
- van der Pouw KT, Snik AF, Cremers CW. Audiometric results of bilateral bone-anchored hearing aid application in patients with bilateral congenital aural atresia. Laryngoscope 1998;108:548-53.
- Dutt SN, McDermott AL, Burrell SP, Cooper HR, Reid AP, Proops DW. Speech intelligibility with bilateral bone-anchored hearing aids: the Birmingham experience. J Laryngol Otol 2002;116:47-51.
- Priwin C, Stenfelt S, Granstrom G, Tjellstrom A, Håkansson B. Bilateral bone-anchored hearing aids (BAHAs): an audiometric evaluation. Laryngoscope 2004;114:77-84.
- Cox RM, Alexiades GC, Beyer CM. Norms for the international outcome inventory for hearing aids. J Am Acad Audiol 2003;14:403-13.
- Baguley DM, Bird J, Humphriss RL, Prevost AT. The evidence base for the application of contralateral bone anchored hearing aids in acquired unilateral sensorineural hearing loss in adults. Clin Otolaryngol 2006;31:6-14.
- Green R, Day S, Bamford J. A comparative evaluation of four hearing-aid selection procedures. II – quality judgements as measures of benefits. Br J Audiol 1989;23:201-6.
- Plomp R, Mimpen AM. Improving the reliability of testing the speech reception threshold for sentences. Int J Audiol 1979;18:43-52.
- Plomp R. A signal-to-noise ratio model for the speech-reception threshold of the hearing impaired. J Speech Hear Res 1986;29:146-54.
- Lopez GA, Valois L, Arias J, Alonzo F, Cardenas R, Villasis MA, et al. Validation of the Coop/Dartmouth questionnaire to evaluate the functional/biopsicosocial condition of children and teenagers with chronic diseases. Bol Med Hosp Infant Mex 1996;53:606-15.
- Hällgren M, Larsby B, Arlinger S. A Swedish version of the Hearing In Noise Test (HINT) for measurement of speech recognition. Int J Audiol 2006;45:227-37.
- Niklès JM, Tschopp K. Audiologische Grundlagen des Basler Satztests. Audiol Akust 1996;2.
- Smoorenburg GF. Speech reception in quiet and in noisy conditions by individuals with noise-induced hearing loss in relation to their tone audiogram. J Acoust Soc Am 1992;91:421-37.
- Hagerman B. Sentences for testing speech intelligibility in noise. Scand Audiol 1982;11:79-87.
- Rowan D, Tompkins L. Binaural masking-level difference with bone conduction: implications for bilateral BAHAs (Oral Presentation) n.d.
- Soo G, Tong MC, Tsang WS, Wong TK, To KF, Leung SF, et al. The BAHA hearing system for hearing-impaired postirradiated nasopharyngeal cancer patients: a new indication. Otol Neurotol 2009;30:496-501.
- Bonding P. Titanium implants for bone-anchored hearing aids--host reaction. Acta Otolaryngol 2000;543:105-7.
- Håkansson B, Liden G, Tjellstrom A, Ringdahl A, Jacobsson M, Carlsson P, et al. Ten years of experience with the Swedish bone-anchored hearing system. Ann Otol Rhinol Laryngol 1990;151:1-16.
- Mylanus EA, Cremers CW. A one-stage surgical procedure for placement of percutaneous implants for the bone-anchored hearing aid. J Laryngol Otol 1994;108:1031-5.
- Jacobsson M, Albrektsson T, Tjellstrom A. Tissue-integrated implants in children. Int J Pediatr Otorhinolaryngol 1992;24:235-43.
- Portmann D, Boudard P, Herman D. Anatomical results with titanium implants in the mastoid region. Ear Nose Throat J 1997;76:231-4.
- Holgers KM, Tjellstrom A, Bjursten LM, Erlandsson BE. Soft tissue reactions around percutaneous implants: a clinical study of soft tissue conditions around skin-penetrating titanium implants for bone-anchored hearing aids. Am J Otol 1988;9:56-9.
- Holgers KM, Tjellstrom A, Bjursten LM, Erlandson B-E. Soft tissue reactions around percutaneous implants: a clinical study of soft tissue conditions around skin-penetrating titanium implants used for abone-anchored auricular proshteses. Scand J Plast Reconstr Surg 1987;21:225-8.
- Catalano PJ, Choi E, Cohen N. Office versus operating room insertion of the bone-anchored hearing aid: a comparative analysis. Otol Neurotol 2005;26:1182-5.
- Watson GJ, Silva S, Lawless T, Harling JL, Sheehan PZ. Bone anchored hearing aids: a preliminary assessment of the impact on outpatients and cost when rehabilitating hearing in chronic suppurative otitis media. Clin Otolaryngol 2008;33:338-42.
- BAHA professionals group . Bone Anchored Hearing Aids Service Standards n.d. www.baha-professionals.org.uk/documents/BAHA%20best%20practice%20standards.pdf (accessed 11 March 2008).
- Monksfield P. Cost Effectiveness (utility) Analysis of Bone Anchored Hearing Aids 2009.
- Efron B, Tibshirani RJ. An introduction to the bootstrap. London: Chapman and Hall; 1993.
- Grutters JP, Joore MA, van der HF, Verschuure H, Dreschler WA, Anteunis LJ. Choosing between measures: comparison of EQ-5D, HUI2 and HUI3 in persons with hearing complaints. Qual Life Res 2007;16:1439-49.
- Barton GR, Bankart J, Davis AC, Summerfield QA. Comparing utility scores before and after hearing-aid provision: results according to the EQ-5D, HUI3 and SF-6D. Appl Health Econ Health Policy 2004;3:103-5.
- Badran K, Arya AK, Bunstone D, Mackinnon N. Long-term complications of bone-anchored hearing aids: a 14-year experience. J Laryngol Otol 2009;123:170-6.
- Beck JR, Pauker SG, Gottlieb JE, Klein K, Kassirer JP. A convenient approximation of life expectancy (The “DEALE”) II. Use in medical decision-making. Am J Med 1982;73:889-97.
- Daly LE, Bourke GJ. Interpretation and uses of medical statistics. Oxford: Blackwell Science; 2000.
- Cheng AK, Rubin HR, Powe NR, Mellon NK, Francis HW, Niparko JK. Cost-utility analysis of the cochlear implant in children. JAMA 2000;284:850-6.
- Summerfield Q. Criteria of candidacy for unilateral cochlear implantation is postlingually defeaned adults II: cost-effectiveness analysis. Ear Hear 2004;25:336-60.
- Barton GR, Stacey PC, Fortnum HM, Summerfield AQ. Hearing-impaired children in the United Kingdom, IV: cost-effectiveness of pediatric cochlear implantation. Ear Hear 2006;27:575-88.
- Health Utilities Inc . Health Utilities Index Mark 3 (HUI3) n.d. www.healthutilities.com/ (accessed 8 May 2009).
- Furlong W, Feeny D, Torrance GW, Goldsmith CH, DePauw S, Zhu Z, et al. Multiplicative Multi-Attribute Utility Function for the Health Utilities Index Mark 3 (HUI3) System: A Technical Report 1998.
- de Wolf MJ, Leijendeckers JM, Mylanus EA, Hol MK, Snik AF, Cremers CW. Age-related use and benefit of the bone-anchored hearing aid compact. Otol Neurotol 2009;30:787-92.
- Badran K, Bunstone D, Arya AK, Suryanarayanan R, Mackinnon N. Patient satisfaction with the bone-anchored hearing aid: a 14-year experience. Otol Neurotol 2006;27:659-66.
- Dutt SN, McDermott AL, Jelbert A, Reid AP, Proops DW. Day to day use and service-related issues with the bone-anchored hearing aid: the Entific Medical Systems questionnaire. J Laryngol Otol 2002;116:20-8.
- British Medical Association and Royal Pharmaceutical Society of Great Britain . British National Formulary 2009.
- HM Treasury . The Green Book. Appraisal and Evaluation in Central Government 2003. www.hm-treasury.gov.uk/data_greenbook_index.htm (accessed 1 April 2009).
- Office for National Statistics . Key Population and Vital Statistics. 2007 2009. www.statistics.gov.uk/downloads/theme_population/KPVS34–2007/KPVS2007.pdf (accessed 24 April 2009).
- Office for National Statistics . United Kingdom, Interim Life Tables, 1980–82 to 2006–08 2009. www.statistics.gov.uk/downloads/theme_population/Interim_Life/ILTUK0608Reg.xls (accessed 8 May 2009).
- Litovsky R, Parkinson A, Arcaroli J, Sammeth C. Simultaneous bilateral cochlear implantation in adults: a multicenter clinical study. Ear Hear 2006;27:714-31.
- Papsin BC, Gordon KA. Bilateral cochlear implants should be the standard for children with bilateral sensorineural deafness. Curr Opin Otolaryngol Head Neck Surg 2008;16:69-74.
- Proops D. The Success of BAHA 2008. www.worldofsound.org/campaigns/baha/success.html (accessed 21 October 2009).
- Worrollo S. How to Run an Effective Baha Programme 2008. www.worldofsound.org/campaigns/baha/running_a_programme.html (accessed 21 October 2009).
- NHS Centre for Reviews and Disseminaiton . Undertaking Systematic Reviews of Research on Effectiveness 2001.
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ 1996;313:275-83.
- Deeks J, Dinnes J, D’Amico R, Sowden A, Sakarovitch C, Song F, et al. Evaluating non-randomised intervention studies. Health Technol Assess 2003;7.
- National Institute for Health and Clinical Excellence . Guide to the Methods of Technology Appraisal 2004.
- Curtis L, Netten A. Unit costs of health and social care. Canterbury: Personal Social Services Research Unit, University of Kent; 2006.
- Department of Health . NHS Reference Costs 2006–07 2008. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/D_08257.
- Department of Health . National Tariff 2006 07 2006. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_4127649 (accessed 8 May 2009).
- British Medical Association and Royal Pharmaceutical Society of Great Britain . British National Formulary 2008.
- Claxton K, Ginnelly L, Sculpher M, Philips Z, Palmer S. A pilot study on the use of decision theory and value of information analysis as part of the NHS Technology Assessment Programme. Health Technol Assess 2004;8.
- Girling AJ, Freeman G, Gordon J, Poole-Wilson P, Scott DA, Lilford R. Modelling payback from research into the efficacy of left-ventricular devices. Int J Technol Assess Health Care 2007;23:269-77.
- Eckermann S, Willan AR. Expected value of information and decision making in HTA. Health Econ 2007;16:195-209.
- Morgan DE, Dirks DD, Bower DR. Suggested threshold sound pressure levels for frequency modulated (warble) tones in the sound field. J Speech Hear Disord 1979;44:37-54.
- Mylanus EAM, Snik AFM, Cremers CWRJ. Patient’s opinions of bone anchored vs conventional hearing aids. Arch Otolarynhol Head Neck Surg 1995;121:421-5.
- Thornton AR, Raffin MJM. Speech discrimination scores modeled as a binominal variable. J Speech Hear Res 1978;21:507-18.
- Snik AF, Beynon AJ, Mylanus EA, van der Pouw CT, Cremers CW. Binaural application of the bone-anchored hearing aid. Ann Otol Rhinol Laryngol 1998;107:187-93.
Appendix 1 Protocol methods
Research methods for synthesis of evidence of clinical effectiveness and cost-effectiveness
A systematic review will be undertaken in accordance with the NHS Centre for Reviews and Dissemination guidelines,133 published guidelines on meta-analysis133 and criteria for appraising economic evaluations. 134
Search strategy
A search strategy will be developed and tested by an experienced information scientist. Literature will be identified from several sources including electronic databases, bibliographies of articles, grey literature sources and hand searching of specialist journals. A comprehensive database of relevant published and unpublished articles will be constructed using Reference Manager (refman) software (Thomson Reuters, London). Searches to identify studies will be carried out via a number of routes:
-
general health and biomedical databases including MEDLINE, EMBASE, Science Citation Index, BIOSIS
-
specialist electronic databases: Database of Abstracts of Reviews of Reviews of Effects, The Cochrane Library
-
grey literature and conference proceedings
-
contact with individuals with an interest in the field
-
checking of reference lists
-
research in progress databases: NIHR Clinical Research Network Portfolio (formerly UK Clinical Research Network website, Current Controlled Trials), Clinical trials.gov.
The draft search strategy for MEDLINE is shown in Appendix 2. This will be adapted for other databases. All databases will be searched from inception to the current date with no language restrictions. Hand searching will focus on key meeting abstracts published in the past 2 years identified in consultation with experts and analysis of searches.
Planned inclusion/exclusion criteria
The planned inclusion/exclusion criteria for the systematic review are shown in Table 57.
| Participants |
Adults or children with bilateral deafness Papers reporting both bilateral and unilateral hearing loss will be included if the groups are reported separately or if the majority of participants have bilateral hearing loss Single-sided deafness will be excluded |
| Interventions |
|
| Comparators |
|
| Outcomes |
|
| Study design |
|
Studies will be selected for inclusion through a two-stage process using the predefined and explicit criteria. The full literature search results will be screened by two reviewers to identify all citations that may meet the inclusion criteria. Full manuscripts of all selected citations will be retrieved and assessed by two reviewers against the inclusion criteria. An inclusion flow chart will be developed and used for each paper assessed. Any disagreements over study inclusion will be resolved by consensus or if necessary by arbitration by a third reviewer.
Data extraction and quality assessment
Data extraction and quality assessment will be undertaken by one reviewer and checked by a second reviewer using a predesigned and piloted data extraction form to avoid any errors. The methodological quality of all included studies will be appraised using recognised quality assessment tools73 and criteria for appraising economic evaluations. 75,134 The tool selected for assessing the quality of primary studies of clinical effectiveness has been recognised as one of the more comprehensive sets of criteria for assessing the quality of different study designs. 135 Where possible, missing information will be obtained from investigators. Any disagreements between reviewers will be resolved by consensus or if necessary by arbitration by a third reviewer.
Data synthesis
Studies will be synthesised through a narrative review with tabulation of results of included studies. Where possible, the results from individual studies will be synthesised through meta-analysis, with causes of heterogeneity of results examined. The specific methods for meta-analysis and for the detection and investigation of heterogeneity will depend upon the summary measure selected.
Southampton Health Technology Assessments Centre economic model
If the systematic review of cost-effectiveness of BAHAs for people who are bilaterally deaf finds any relevant high-quality economic evaluations, the feasibility of adapting and updating these existing models will be investigated. In the absence of relevant high-quality, model-based economic evaluations, a de novo decision analytic model will be developed. The model will be structured using published evidence on the epidemiology and natural history of bilateral deafness, and will be informed by guidance from clinical advisors, to reflect the natural course of bilateral deafness and the impact of alternative interventions. Accepted guidelines for good practice in decision-analytic modelling and the general principles outlined in the National Institute for Health and Clinical Excellence (NICE) ‘reference case’75,136 will be followed. The model will be used to provide a cost–consequence analysis, reporting the costs of interventions included in the systematic review and their consequences in terms of hearing measures, QoL, complications, and health service resource use for bilaterally deaf patients receiving standard hearing aids (including BC hearing aids), surgery, unilateral BAHAs and bilateral BAHAs. The model will also be used to estimate the longer term consequences in terms of quality-adjusted life expectancy. The model will adopt a UK NHS and PSS perspective. The time horizon for the long-term model will be the patients’ lifetime, with health outcomes expressed in terms of QALYs – costs and QALYs will be discounted at an annual rate of 3.5%.
Development of the structure of the model will be informed by several sources including previous models identified in the systematic review of cost-effectiveness, evidence on the epidemiology and natural history of bilateral deafness and guidance from clinical and methodological advisors. The economic model will only include clinically relevant comparators found to be clinically effective by the systematic review. Evidence of effectiveness will originate from the systematic review. Specific targeted literature searches will be required to populate other parameters in the model, including baseline characteristics of the population requiring intervention, age-condition-specific life expectancy and the impact of deafness on patient satisfaction and health-related QoL. Information on adverse events and complications will come from the systematic review of effectiveness.
Resource use and unit costs, including consultations (e.g. ENT surgeon, audiologist), treatments, adverse events and complications will be obtained from published evidence, official sources such as Unit costs of health and social care137 and NHS Reference Costs,138 and from the Costing Unit at Southampton General Hospital. Costs of hearing aids, both BAHAs and conventional devices, will be taken from published tariff prices for the UK. 139,140 Costs will be inflated to current prices as necessary. If no published data are available, we will consult with expert advisors to obtain estimates for the parameters relating to resource use.
The results of the economic model will be presented as a cost–consequence analysis, clearly specifying the direct costs associated with each intervention and consequences in terms of hearing, QoL and adverse events of each intervention, and as a cost–utility analysis. The results of the cost–utility analysis will be presented as ICERs for the base case and using CEACs to show the probability of each device being cost-effective at different WTP thresholds. Uncertainty will be examined using deterministic sensitivity analysis and PSA. The importance of the underlying model assumptions will be assessed through an analysis of different scenarios. Value of information analysis will be undertaken to help inform payback in terms of reduced parameter uncertainty from additional research, identifying which parameters most contribute to decision uncertainty and should therefore be the focus of future research. 141–143
The model will be constructed in Microsoft Excel 2003 (Microsoft Corporation, Redmond, WA, USA) to ensure transparency. All stages in the development of the model, analysis of data and interpretation of results will be undertaken by one health economist and checked by a second. All model assumptions and data sources will be clearly specified and their effects on outcomes checked through sensitivity analysis, to ensure model results accurately reflect the inputs used. Internal consistency will also be assessed through the replication of the model in different software to compare results. External consistency will be assessed through comparing results with the previously published analyses.
Appendix 2 Search strategy
All databases searched for the systematic review are presented below.
| Database searched | Clinical effectiveness searches | Cost-effectiveness and QoL searches |
|---|---|---|
| BIOSIS (Web of Science) | 1990–2009 | 1990–2009 |
| Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library) | All available years | All available years |
| Cochrane Database of Systematic Reviews (The Cochrane Library) | All available years | All available years |
| Database of Abstracts of Reviews of Effects (Centre for Reviews and Dissemination) | All available years | All available years |
| EconLit (American Economic Association’s electronic bibliography, Ebsco) | All available years | Searched 11 November 2009 |
| EMBASE | 1980–2009 | 1980–2009 |
| Health Technology Assessment Database (Centre for Reviews and Dissemination) | All available years | Searched 11 November 2009 |
| Health Management Information Consortium | All available years | Searched 11 November 2009 |
| MEDLINE (Ovid) | 1950–2009 | 1950–2009 |
| MEDLINE In-Process & Other Non-Indexed Citations | 1950–2009 | 1950–2009 |
| NHS Economic Evaluation Database (Centre for Reviews and Dissemination) | All available years | Searched 11 November 2009 |
| Searched for ongoing trials | ||
| Web of Science: Proceedings Citation Index (Web of Knowledge) | 1970–2009 | 1970–2009 |
| Web of Science: Science Citation Index (Web of Knowledge) | 1970–2009 | 1970–2009 |
| National Institute for Health Research Clinical Research Network Portfolio (formerly UK Clinical Research Network website) | ||
| Current Controlled Trials | ||
| Clinical trials.gov | ||
| Center Watch | ||
| Computer Retrieval of Information on Scientific Projects | ||
| Health Services Research Projects in Progress | ||
The MEDLINE search strategy (presented below) for the systematic review of clinical effectiveness was adjusted as necessary for other electronic databases for both clinical effectiveness and cost-effectiveness (including QoL information) searches. Search strategies for the systematic review are available from the authors on request. Citations identified by the searches were added to a Reference Manager database.
MEDLINE search strategy
-
exp Deafness/ (20,783)
-
((mixed adj5 deaf*) or (mixed adj5 hearing adj loss*)).ti,ab. (392)
-
(sensorineural* adj5 deaf*).ti,ab. (1277)
-
(bilateral* adj5 deaf*).ti,ab. (724)
-
exp Hearing Loss/ (42,445)
-
Hearing Disorders/ (12,599)
-
Hearing Impaired Persons/ (689)
-
(hearing loss* adj5 bilateral*).ti,ab. (1301)
-
(hearing loss* adj5 conductive).ti,ab. (1506)
-
(hearing loss* adj5 sensorineural).ti,ab. (5810)
-
Hearing Loss, Sensorineural/ (9843)
-
Hearing Loss mixed conductive sensorineural/ (55)
-
Hearing Loss, Bilateral/ (1324)
-
Hearing Loss, Conductive/ (2348)
-
(hearing adj2 loss*).ti,ab. (21,232)
-
hearing loss noise induced/ (5132)
-
“Rehabilitation of Hearing Impaired”/ (1203)
-
(hearing adj5 impair*).ti,ab. (8111)
-
or/1-18 (61,735)
-
Bone Conduction/ (1998)
-
exp Osseointegration/ (4946)
-
osseointegrat*.ti,ab. (3525)
-
exp hearing aids/ (9717)
-
23 and (20 or 21 or 22) (320)
-
(divino or intenso or cordelle).ti,ab. (9)
-
(divino or intenso or cordelle).mp. (38)
-
(classic adj1 “300”).ti,ab. (6)
-
(HC adj1 “300”).ti,ab. (3)
-
(HC adj1 “100”).ti,ab. (28)
-
(HC adj1 “200”).ti,ab. (13)
-
(HC adj1 “210”).ti,ab. (1)
-
(HC adj1 “220”).ti,ab. (1)
-
(HC adj1 “300”).ti,ab. (3)
-
(HC adj1 “360”).ti,ab. (0)
-
(HC adj1 “380”).ti,ab. (0)
-
(HC adj1 “400”).ti,ab. (2)
-
temporal bone/ (7731)
-
prosthesis implantation/ (4922)
-
bone anchored.mp. (387)
-
23 and 37 (228)
-
23 and 38 and 39 (40)
-
or/24–36,40-41 (584)
-
19 and 42 (323)
-
(bone anchor* and (hear* or deaf*)).ti,ab. (259)
-
23 and (BAHA or BAHAs or “BAHA’s”).ti,ab. (164)
-
19 and (BAHA or BAHAs or “BAHA’s”).ti,ab. (138)
-
((BAHA or BAHAs or “BAHA’s”) and (hear* or deaf*)).ti,ab. (172)
-
19 and 38 and 39 (28)
-
(bone anchor* adj5 hearing aid*).ti,ab. (246)
-
43 or 44 or 45 or 46 or 47 or 48 (470)
-
(letter or comment or editiorial).pt. (769,498)
-
50 not 51 (458)
-
limit 52 to humans (453)
-
from 53 keep 1-453 (453)
Update searches run on 18 November 2009.
Reference lists
The reference lists of retrieved articles were examined for additional studies.
Other searches
The experts advisory group and BAHA manufacturers were contacted in order to obtain information about additional references and any ongoing studies.
British societies and conferences (sources checked on 25 November 2009)
American Academy of Otolaryngology-Head and neck Surgery.
American Otological Society.
American Society of Pediatric Otolaryngology.
Association for Research in Otolaryngology.
BAHA Professionals Group.
Baha User Group.
British Academy of Audiology.
British Association of Paediatric Otolaryngology.
British Society of Hearing Aid Audiologists.
Canadian Society of Otolaryngology.
Deafness Research.
Ear Foundation.
European Academy of Otorhinolaryngology.
European Academy of Otology & Neuro-Otology.
European Academy of Otorhinolaryngolgy, Head and Neck Surgery.
European archives of Oto-Rhino-Laryngology.
European Federation of Audiology Societies.
European Federation of Oto-Rhino-Laryngological Societies.
European Society of Pediatric Otorhinolaryngology.
Hearing Aid Council.
Institute of Hearing Research.
National Deaf Children’s Society.
Royal National Institute for Deaf People.
Scottish Otolaryngological Society.
Appendix 3 List of excluded studies
Albrektsson T, Branemark PI, Jacobsson M, Tjellstrom A. Present clinical applications of osseointegrated percutaneous implants. Plast Reconstr Surg 1987;79:721–31. Reason for exclusion: design.
Arthur D. The Vibrant Soundbridge™ Trends Amplif 2002;6:67–72. Reason for exclusion: intervention.
Blackmore KJ, Kernohan MD, Davison T, Johnson IJ. Bone-anchored hearing aid modified with directional microphone: do patients benefit? J Laryngol Otol 2007;121:822–5. Reasons for exclusion: intervention; comparator.
Bonding P, Nielsen LH, Pedersen U, Brask T. The Danish BAHA file – preliminary results. In Portman M, Boudard P, Portmann D, editors. Transplants and implants in otology – III. New York, NY: Kugler Publications; 1996. pp. 297–9. Reason for exclusion: paper not available from British Library (conference proceedings dated 1996).
Bosman AJ, Snik AFM, Mylanus EAM, Cremers CWRJ. Fitting range of the BAHA Intenso. Int J Audiol 2009;48:346–52. Reason for exclusion: comparator.
Browning GG. The British experience of an implantable, subcutaneous bone conduction hearing aid (Xomed Audiant). J Laryngol Otol 1990;104:534–8. Reasons for exclusion: intervention; comparator; outcomes; design.
Buratti C, Romagnoli M, Galli A, Parmigiani F. Bone-anchored hearing-aid – our experience in children with congenital external and middle-ear malformations. Child and the Environment – Present and Future Trends 1993;1012:256–8. Reason for exclusion: outcomes.
Cano FAC, Blass FA. Branemark bone-anchored implants for hearing-aid adaptation. Otolaryngol Head Neck Surg 1991;1221–9. Reason for exclusion: design.
Carlsson P, Håkansson B. The bone-anchored hearing-aid. Child and the Environment – Present and Future Trends 1993;1012:247–50. Reasons for exclusion: outcomes; design.
Davison T, Marley S, Leese D, Johnson I. Clinical impressions of a new bone anchored hearing aid processor. Unpublished. 2009. Reason for exclusion: comparator.
Dunham ME, Friedman HI. Audiologic management of bilateral external auditory canal atresia with the bone conducting implantable hearing device. Cleft Palate J 1990;27:369–73. Reason for exclusion: intervention.
Durvasula VS, Patel H, Mahendran S, Gray RF. Bone anchored hearing aids: a second fixture reduces auditory deprivation in Cambridge. Eur Arch Otorhinolaryngol 2007;264:991–4. Reason for exclusion: design.
Dutt SN, McDermott AL, Burrell SP, Cooper HR, Reid AP, Proops DW. Patient satisfaction with bilateral bone-anchored hearing aids: the Birmingham experience. J Laryngol Otol 2002:37–46. Reason for exclusion: study design.
Flynn MC, Sadeghi A. Results of the first clinical evaluation of Cochlear TM Baha BP100. 2009. URL: http://bp100.cochlear.com/sites/default/files/E81511%20Results%20of%20the%20first%20clinical%20evaluation%20of%20Cochlear%20Baha%20BP100%20whitepaper.pdf (accessed 16 September 2009). Reason for exclusion: comparator.
Forton GEJ, Van De Heyning PH. Bone anchored hearing aids (BAHA). B-ENT 2007:45–50. Reason for exclusion: design, outcomes.
Gatehouse S, Browning G. An evaluation of the role of bone-anchored hearing aids in the management of hearing impairment. Clin Otolaryngol 1992;17:462. Reason for exclusion: outcomes.
Goodyear PW, Raine CH, Firth AL, Tucker AG, Hawkins K. The Bradford bone-anchored hearing aid programme: impact of the multidisciplinary team. J Laryngol Otol 2006;120:543–52. Reason for exclusion: design.
Granstrom G, Bergstrom K, Tjellstrom A. The bone-anchored hearing aid and bone-anchored epithesis for congenital ear malformations. Otolaryngol Head Neck Surg 1993;109:46–53. Reasons for exclusion: outcomes; design; participants.
Granstrom GPB, Bergstrom KM, Tjellstrom AMR. Some considerations regarding the rehabilitation of patients with congenital ear malformations using the osseointegration concept. In Portman M, Boudard P, Portmann D, editors. Transplants and implants in otology – III. New York, NY: Kugler Publications; 1996. pp. 91–8. Reasons for exclusion: outcomes; design.
Håkansson B, Tjellstrom A, Rosenhall U. Hearing thresholds with direct bone conduction versus conventional bone conduction. Scand Audiol 1984;13:3–13. Reason for exclusion: outcomes.
Håkansson B, Tjellstrom A, Rosenhall U. Acceleration levels at hearing threshold with direct bone conduction versus conventional bone conduction. Acta Otolaryngol 1985;100:240–52. Reason for exclusion: outcomes.
Holgers KM, Tjellstrom A, Bjursten LM, Erlandsson BE. Soft tissue reactions around percutaneous implants: a clinical study of soft tissue conditions around skin-penetrating titanium implants for bone-anchored hearing aids. Am J Otol 1988;9:56–9. Reason for exclusion: design.
Johnson RM, Schleuning A. Evaluation of the new behind-the-ear audiant(TM) bone conductor(TM). Semin Hear 1992;13:325–30. Reason for exclusion: intervention.
Kunst SJ, Hol MK, Cremers CW, Mylanus EA. Bone-anchored hearing aid in patients with moderate mental retardation: impact and benefit assessment. Otol Neurotol 2007;28:793–7. Reasons for exclusion: comparator; design.
Liepert DR, DiToppa JC. The Nobelpharma auditory system bone-anchored hearing aid: the Edmonton experience. J Otolaryngol 1994;23:411–18. Reason for exclusion: design.
Negri S, Bernath O, Hausler R. Bone conduction implants: Xomed Audiant bone conductor vs. BAHA. Ear Nose Throat J 1997;76:394–6. Reasons for exclusion: outcomes; design.
Niehaus HH, Helms J, Muller J. Are implantable hearing devices really necessary? Ear Nose Throat J 1900;74:271–4. Reasons for exclusion: intervention; comparator; outcomes.
Priwin C, Granstrom G. A long-term evaluation of bone-anchored hearing aid (BAHA) in children. Cochlear Implants Int 2005;6:81–3. Reason for exclusion: design.
Ringdahl A, Israelsson B, Caprin L. Paired comparisons between the Classic 300 bone-anchored and conventional bone-conduction hearing aids in terms of sound quality and speech intelligibility. Br J Audiol 1995;29:299–307. Reason for exclusion: outcomes.
Roper A, Hobson J, Green K. Combined bone anchored hearing aid and mastoidectomy. Mediterr J Otol 2008;4:138–42. Reason for exclusion: paper not available from British Library (reports four cases only of BAHA and mastoidectomy combined).
Rosenbom T, Specht Petersen A. Clinical study of a direct bone conductor. Askim, Sweden: Oticon Medical; 2010. Reason for exclusion: comparator.
Snik AFM, Mylanus EAM, Cremers CWRJ. The bone-anchored hearing aid (BAHA) versus air-conduction hearing aids. In Portman M, Boudard P, Portmann D, editors. Transplants and implants in otology – III. New York, NY: Kugler Publications; 1996. pp. 309–12. Reason for exclusion: paper not available from British Library (conference proceedings dated 1996).
van der Pouw CT, Carlsson P, Cremers CW, Snik AF. A new more powerful bone-anchored hearing aid: first results. Scand Audiol 1998;27:179–82. Reason for exclusion: comparator.
Wade PS, Halik JJ, Chasin M. Bone conduction implants: transcutaneous vs. percutaneous. Otolaryngol Head Neck Surg 1992;106:68–74. Reasons for exclusion: comparator; design.
Appendix 4 List of relevant non-English-language publications identified by searches
Aguado BF, ntoli Candela CF. [Branemarck bone-anchored implants for the adaptation of conductive hearing aids.] [Spanish.] Acta Otorrinolaringologica Espanola 1990;41:169–72.
Belus JF, Sarabian A, Triglia JM, Zanaret M. [Bone anchored auditory prosthesis. Indications, clinical and audiometric results.] [French.] Annales d Oto-Laryngologie et de Chirurgie Cervico-Faciale 1996;113:79–85.
Bonding P, Jonsson MH, Salomon G. [Bone-anchored hearing aids. Preliminary results.] [Danish.] Ugeskrift for Laeger 1990;152:667–70.
Bonding P. [Permanent, percutaneous osseointegrated titanium implants. A review and preliminary results.] [Danish.] Ugeskrift for Laeger 1990;152:664–7.
Bonding P, Jonsson MH, Salomon G, Ahlgren P. [The bone-anchored hearing aid. Host-reaction and audiological effect.] [Danish.] Ugeskrift for Laeger 1993;155:1183–5.
Candela Cano FA, Aguado BF, Sada Garcia-Lomas J. [Branemark-type osteo-integrated implants for the adaptation of endosseous hearing aids.] [Spanish.] Acta Otorrinolaringologica Espanola 1990;41:61–4.
Cremers CWRJ, Snik AFM, Beynon AJ. [A hearing aid anchored in the cranial bone to amplify bone conduction.] [Dutch.] Nederlands Tijdschrift voor Geneeskunde 1991;135:468–71.
Federspil P, Kurt P, Koch A. [Bone-anchored epitheses and audioprostheses: 4 years’ experience with the Branemark system in Germany.] [French.] Revue de Laryngologie Otologie Rhinologie 1992;113:431–7.
Federspil PA, Plinkert PK. [Bone-anchored hearing aids: always bilateral!.] [German.] HNO 2002;50:405.
Grunder I, Seidl RO, Ernst A, Todt I. [Relative value of BAHA testing for the postoperative audiological outcome.] [German.] HNO 2008;56:1020–4.
Hamann C, Manach Y, Roulleau P. [Bone anchored hearing aid. Results of bilateral applications.] [French.] Revue de Laryngologie Otologie Rhinologie 1991;112:297–300.
Healthcare Insurance Board. ]Evaluation of bone-anchored hearing aids – primary research.] [Dutch.] URL: www.cvz.nl (accessed March 2008).
Hoelzl M, Caffer P, Jungk J, Scherer H, Schrom T. [The Ti-Epiplating system in bone anchored hearing aids.] [German.] Laryngo-Rhino-Otologie 2007;86:193–9.
Jankowski R, Pialoux R, Labaeye P, Simon C. [Bone anchored hearing aid (BAHA): clinical evaluation.] [French.] Annales d Oto-Laryngologie et de Chirurgie Cervico-Faciale 1998;115:315–20.
Kitamura K, Tokano H. Bone-Anchored Hearing Aid: BAHA. [Japanese.] Oto-Rhino-Laryngology Tokyo 2004;47:8–16.
Klaiber S, Weerda H. [BAHA (bone-anchored hearing aid) in bilateral external ear dysplasia and congenital ear atresia.] [German.] HNO 2002;50:949–59.
Kondoh K, Matsushiro N, Satoh T, Kuramasu T, Kubo T. [Audiological effect of bone-anchored hearing aid.] [Japanese.] Nippon Jibiinkoka Gakkai Kaiho 2005;108:1144–51.
Lyberg T, Tjellstrom A. [Craniofacial prostheses. Clinical application of titanium implants for retention of facial prostheses and bone-anchored hearing aids.] [Norwegian.] Tidsskrift for Den Norske Laegeforening 1988;108:2009–12.
Machida S, Shimakura Y, Okamoto M, Nonomura E. [Fitting of bone-conduction hearing aids for persons suffering from mixed hearing loss.] [Japanese.] Audiology Japan 1983;26:27–33.
Negri S, Bernath O, Hausler R. [Implantable bone conduction hearing aids: Audiant(TM) vs. BAHA(TM).] [German.] Oto-Rhino-Laryngologia Nova 1996;6:82–8.
Nystrand A. [Bone-anchored hearing aids and implants in the cochlea improve hearing.] [Swedish.] Lakartidningen 143;88:137–8.
Portmann D, Boudard P, Vdovytsya O. [Bone-anchored hearing aids BAHA: 10 years’ experience.] [French.] Revue de Stomatologie et de Chirurgie Maxillo-Faciale 2001;102:274–7.
Portmann D, Bourdin M. [Bone anchored hearing aid: the Bordeaux experience.] [French.] Revue de Laryngologie Otologie Rhinologie 1995;116:299–300.
Portmann D, Dutkiewicz J, Boudard P. [The use of osseointegration of hearing aids.] [Polish.] Otolaryngologia Polska 1995;49:543–8.
Sanchez-Camon I, Lassaletta L, Castro A, Gavilan J. [Quality of life of patients with BAHA.] [Spanish.] Acta Otorrinolaringologica Espanola 2007;58:316–20.
Shrom T, Siegert R. [Problems with the BAHA abutment.] [German.] Laryngo- Rhino- Otologie 2008;87:764–7.
Schupbach J, Kompis M, Hausler R. [Bone anchored hearing aids (B.A.H.A.).] [German.] Therapeutische Umschau 2004;61:41–6.
Zhang Q, Gao X. [Bone anchored hearing aid.] [Chinese.] Chin J Clin Rehabil 2006;10:124–8.
Appendix 5 List of potentially eligible studies but lower level of evidence
Abramson M, Fay TH, Kelly JP, Wazen JJ, Liden G, Tjellstrom A. Clinical results with a percutaneous bone-anchored hearing aid. Laryngoscope 1989;99:707–10. Design: audiological comparison study.
Bance M, Abel SM, Papsin BC, Wade P, Vendramini J. A comparison of the audiometric performance of bone anchored hearing aids and air conduction hearing aids. Otol Neurotol 2002;23:912–19. Design: audiological comparison study.
Bonding P, Jonsson MH, Salomon G, Ahlgren P. The bone-anchored hearing aid. Osseointegration and audiological effect. Acta Otolaryngol 1992;492:42–5. Design: audiological comparison study.
Bonding P. Titanium implants for bone-anchored hearing aids – host reaction. Acta Otolaryngol 2000;543:105–7. Design: prospective case series.
Bosman AJ, Snik AF, Mylanus EA, Cremers CW. Fitting range of the BAHA Cordelle. Int J Audiol 2006;45:429–37. Design: prospective case series.
Browning GG, Gatehouse S. Estimation of the benefit of bone-anchored hearing aids. Ann Otol Rhinol Laryngol 1994;103:872–8. Design: audiological comparison study.
Carlsson P, Håkansson B, Rosenhall U, Tjellstrom A. A speech-to-noise ratio test with the bone-anchored hearing aid: a comparative study. Otolaryngol Head Neck Surg 1986;94:421–6. Design: audiological comparison study.
Cremers CW, Snik FM, Beynon AJ. Hearing with the bone-anchored hearing aid (BAHA, HC 200) compared to a conventional bone-conduction hearing aid. Clin Otolaryngol 1992;17:275–9. Design: audiological comparison study.
Cremers CWRJ, Snik AFM, Beynon AJ. The bone anchored hearing-aid versus the previous conventional bone conduction hearing-aid – a preliminary-report of a clinical-trial. In Charachan R, Garcaibanez E, editors. Long-term results and indications in otology and otoneurosurgery. Amsterdam: Kugler Publications; 1991. pp. 461–4. Design: audiological comparison study.
Håkansson B, Liden G, Tjellstrom A, Ringdahl A, Jacobsson M, Carlsson P, et al. Ten years of experience with the Swedish bone-anchored hearing system. Ann Otol Rhinol Laryngol 1990;151:1–16. Design: audiological comparison study.
Håkansson B, Tjellstrom A, Rosenhall U, Carlsson P. The bone-anchored hearing aid. Principal design and a psychoacoustical evaluation. Acta Otolaryngol 1985;100:229–39. Design: audiological comparison study.
Håkansson BE, Carlsson PU, Tjellstrom A, Liden G. The bone-anchored hearing aid: principal design and audiometric results. Ear Nose Throat J 1994;73:670–5. Design: audiological comparison study.
Hartland SH, Proops D. Bone anchored hearing aid wearers with significant sensorineural hearing losses (borderline candidates): patients’ results and opinions. J Laryngol Otol 1996;21:41–6. Design: audiological comparison study.
Hickson L, Mackenzie D, Gordon J, Neall V, Wu D, Wu J. The outcomes of bone anchored hearing aid (BAHA) fitting in a paediatric cohort. ANZJA 2006;28:75–89. Design: audiological comparison study.
Hol MK, Snik AF, Mylanus EA, Cremers CW. Long-term results of bone-anchored hearing aid recipients who had previously used air-conduction hearing aids. Arch Otolaryngol Head Neck Surg 2005;131:321–5. Design: audiological comparison study.
Jacobsson M, Albrektsson T, Tjellstrom A. Tissue-integrated implants in children. Int J Pediatr Otorhinolaryngol 1992;24:235–43. Design: prospective case series.
Lindeman P, Tengstrand T. Clinical experience with the bone-anchored hearing aid. Scand Audiol 1987;16:37–41. Design: audiological comparison study.
Mylanus EA, Cremers CW. A one-stage surgical procedure for placement of percutaneous implants for the bone-anchored hearing aid. J Laryngol Otol 1994;108:1031–5. Design: prospective case series.
Mylanus EA, Snik AF, Jorritsma FF, Cremers CW. Audiologic results for the bone-anchored hearing aid HC220. Ear Hear 1994;15:87–92. Design: audiological comparison study.
Mylanus EA, Snik FM, Cremers CW, Jorritsma FF, Verschuure H. Audiological results of the bone-anchored hearing aid HC200: multicenter results. Ann Otol Rhinol Laryngol 1994;103:368–74. Design: audiological comparison study.
Mylanus EA, Beynon AJ, Snik AF, Cremers CW. Percutaneous titanium implantation in the skull for the bone-anchored hearing aid. J Invest Surg 1994;7:327–32. Design: audiological comparison study.
Portmann D, Boudard P, Herman D. Anatomical results with titanium implants in the mastoid region. Ear Nose Throat J 1997;76:231–4. Design: prospective case series.
Powell RH, Burrell SP, Cooper HR, Proops DW. The Birmingham bone anchored hearing aid programme: paediatric experience and results. J Laryngol Otol 1996;21:21–9. Design: audiological comparison study.
Priwin C, Stenfelt S, Edensvard A, Granstrom G, Tjellstrom A, Kansson H. Unilateral versus bilateral bone-anchored hearing aids (BAHAs). Cochlear Implants Int 2005;6:79–81. Design: audiological comparison study.
Soo G, Tong MC, Tsang WS, Wong TK, To KF, Leung SF, et al. The BAHA hearing system for hearing-impaired postirradiated nasopharyngeal cancer patients: a new indication. Otol Neurotol 2009;30:496–501. Design: prospective case series.
Stenfelt S, Håkansson B, Jonsson R, Granstrom G. A bone-anchored hearing aid for patients with pure sensorineural hearing impairment: a pilot study. Scand Audiol 2000;29:175–85. Design: audiological comparison study.
Appendix 6 Data extraction: BAHA versus BCHA
| Reference and design | Intervention | Participants | Outcome measures |
|---|---|---|---|
|
Béjar-Solar et al. 200077 Mexico Design: cohort (one group pre and post) Study setting: tertiary referral centre for patients of low socioeconomic status Number of centres: single centre Funding: grant from Hospital’s Board of Patrons |
|
Indication for treatment: inoperable bilateral congenital microtia atresia Number of participants: 11 Sample attrition/dropout: implant was rejected in one patient Inclusion criteria for study entry: BC PTA 45 dB HL or better with 100% speech discrimination; high resolution CT demonstrating inoperable bilateral congenital microtia atresia; age at least 5 years; current use of a conventional BCHA Exclusion criteria: sensineural hearing loss with a BC PTA < 45 dB HL; lack of hygiene facilities to properly clean the skin around the implant; insufficient score on the psychological evaluation (minimal standards for intelligence and family support); economic capability to purchase batteries (approximately cost US$1); accessibility to hospital for follow-up visits |
Primary and secondary outcome: audiological benefit; complications; patient satisfaction (not validated); QoL;Coop/Dartmouth test (validated) Method of assessing outcomes: pre-operative audiologic evaluation: PTA (125–3000 Hz) in both air and BC; free-field PTA with and without BCHA; 100% speech audiometry discrimination in dB HL; 100% speech audiometry discrimination with background noise at 65 dB HL with BCHA Test adapted to their group, using sentences with a high degree of difficulty, using colloquial language common to Mexico City Patient, wearing BCHA, asked to pinpoint the position of natural vocal speech in an area with high background noise, at a distance of 3m Tests repeated with BAHA at 6 months’ follow-up. PTA (125–3000 Hz) in both AC and BC measured for use as a control Subjective patient satisfaction questionnaire, no further details reported QoL psychological test evaluating emotional condition, impact on daily chores and social activities, social support and pain (Coop/Dartmouth test) Condition of skin evaluated on 0–4 scale (0 = normal, 1 = slight erythema, 2 = red and moist, 3 = red and moist with granulation tissue, 4 = infection leading to loss or removal of implant) Length of follow-up: states that follow-up was 24 months. Visits scheduled at 1, 2, 4, 6, 12, 18 and 24 months Audiological tests were scheduled at 6 months |
| Characteristics of participants | |||
| Age, years | 5–17 | ||
| Sex (M : F) | 7 : 4 | ||
| No. with mandibulofacial dysotosis (Treacher Collins syndrome) | 4/11 | ||
| Mean age at implantation, years | 10 | ||
| Results | |||
| Attained successful use of BAHA at 2-year follow-up | 10/11 | ||
| Audiologic results, dB HL | Before surgery (unaided) | After surgery (unaided) | Difference in threshold |
| AC PTA, RE | 69 | 71 | ± 2 |
| AC PTA, LE | 69 | 68 | ± 1 |
| BC PTA, RE | 20 | 18 | ± 2 |
| BC PTA, LE | 14 | 15 | ± 1 |
| Free-field PTA, dB HL (1.25–3.00 kHz) | 64 | 63 | ± 1 |
| Level at which right ear 100% speech audiometry discrimination was achieved | 87 | 90 | ± 3 |
| Level at which left ear 100% speech audiometry discrimination was achieved | 84 | 83 | ± 1 |
| BCHA (before surgery) | BAHA | ||
| Free-field PTA dB HL (1.25–3.00 kHz) | 30 | 19 | –11 (37% improvement) |
| Free-field 100% speech audiometry, background noise at 65 dB | 62 | 48 | –14 (23% improvement) |
| BCHA (before surgery) | BAHA | ||
| Accurate directional identification of location of a sound source (% of cases) | 0 | 80% | |
| Patient satisfaction questionnaire | Data not presented. States that compared with the BCHA ‘all patients preferred the BAHA, believed there had been an excellent improvement in aesthetic appearance, and would choose to have it done again (scale: excellent, good, fair, poor, very poor)’ | ||
| QoL | Data not presented. States that ‘tests results uniformly showed the response “hardly could have done better” (options: hardly could have done better, pretty good, indifferent, pretty bad, hardly could have done worse). Physical and emotional condition was reported as very improved. Positive family support was confirmed in all cases’ | ||
| Adverse effects | |||
| Unable to obtain osseointegration (following impact to mastoid area 24 hours after discharge from first stage) | 1/11 | ||
| Major complications | 0/11 | ||
| Types of skin reactions, n of observations (%) | |||
| • No irritation | 71/82 (87) | ||
| • Slight erythema | 7/82 (9) | ||
| • Erythema and moisture | 3/82 (4) | ||
| • Red and moist with granulation tissue | 1/82 (1) | ||
| • Infection leading to loss of implant | 0 | ||
| • Total number of observations | 82 (71 at scheduled visits, 11 at unscheduled visits) | ||
| Methodological comments | |||
|
|||
| General comments | |||
|
|||
Quality assessment for primary studies
| A. Selection bias | |||||
|---|---|---|---|---|---|
| 1. Are the individuals selected to participate in the study likely to be representative of the target population? | Very likely |
Somewhat likely x |
Not likely | Can’t tell | |
| 2. What percentage of selected individuals agreed to participate? | 80–100% | 60–79% | < 60% | Not applicable |
Can’t tell x |
| Summary of selection bias (methodological strength of study) | Strong | Moderate |
Weak x |
||
| B. Study design | |||||
| 1. What was the study design? (Please tick appropriate and specify design in No. 7) | RCT | ||||
| Controlled clinical trial | |||||
| Cohort analytic (two group pre and post) | |||||
| Case–control | |||||
| Cohort [one group pre and post (before and after)] | x reviewer’s opinion | ||||
| Interrupted time series | |||||
| Other – specify | |||||
| Can’t tell | |||||
| 2. Was the study described as randomised? | Yes |
No x |
|||
| If answer to No. 2 is no, go to section on Section C Confounders. If answer yes, answer No. 3 and No. 4 below. | |||||
| 3. If answer was yes, was the method of randomisation described? | Yes | No | |||
| 4. If answer was yes, was the method appropriate? | Yes | No | |||
| Summary of study design (methodological strength of study) | Strong | Moderate |
Weak x |
||
| C. Confounders | |||||
|
• If there are two groups included in the study: ‘are confounders reported AND controlled for in the analysis?’ • If there is one group of participants in the study: ‘are potential confounding variables reported?’ |
Yes |
No x |
Can’t tell | ||
| Summary of confounders (methodological strength of study) | Strong | Moderate |
Weak x |
||
| D. Blinding | |||||
| 1. Was the outcome assessor aware of the intervention or exposure status of participants? |
Yes x |
No | Can’t tell | ||
| 2. Were the study participants aware of the research question? |
Yes x |
No | Can’t tell | ||
| Summary of blinding (methodological strength of study) | Strong | Moderate |
Weak x |
||
| E. Data collection methods | |||||
| 1. Were data collection tools shown to be valid? |
Yes x |
No | Can’t tell | ||
| 2. Were data collection tools shown to be reliable? |
Yes x |
No | Can’t tell | ||
| Summary of data collection (methodological strength of study) |
Strong x |
Moderate | Weak | ||
| F. Withdrawals and dropouts | |||||
| 1. Were withdrawals and dropouts reported in terms of numbers and reasons per group? | Yes |
No x |
Can’t tell | ||
| 2. Indicate the percentage of participants completing the study (if the percentage differs by groups, record the lowest) | 80–100% | 60–79% | < 60% |
Can’t tell x |
|
| Summary of withdrawals and dropouts (methodological strength of study) | Strong | Moderate |
Weak x |
||
| G. Intervention integrity | |||||
| 1. Was the consistency of the intervention measured? | Yes |
No x |
Can’t tell | ||
| H. Analysis | |||||
| 1. Are the statistical methods appropriate for the study design? | Yes |
No x |
Can’t tell | ||
| 2. Does the study report how missing data are dealt with in the analysis? | Yes |
No x |
Can’t tell | ||
|
Global rating for study a (Overall methodological strength of study – based on sections A–F) |
Strong | Moderate |
Weak x |
||
Appendix 7 Data extraction: BAHA versus ACHA
| Reference and design | Intervention | Participants | Outcome measures |
|---|---|---|---|
|
Burrell et al. 199683 UK Design: cohort pre–post Study setting: secondary care Number of centres: single centre Funding: NR |
BAHA (model not stated) ACHA (states ‘old aid’ in paper. Information from author) Unaided Other interventions used: NR |
Indication for treatment: otosclerosis Number of participants: 32 assessed over 5-year period 19 suitable for BAHA 10 fitted with BAHAs (nine waiting for surgery) data available for nine Sample attrition/dropout: missing data for two patients for one outcome Inclusion criteria for study entry: audiological criteria: average BC thresholds (0.5–4 kHz) < 40 dB HL (ear level BAHA); average BC thresholds < 60 dB HL (body-worn Superbass); speech discrimination > 60% (AB wordlists via headphones); realistic expectations; good support. Final decision to proceed with BAHA taken by a multidisciplinary team including ENT surgeons, audiologists and a specialist speech therapist |
Primary and secondary outcomes: audiological performance; free-field warble-tone audiometry; free-field speech audiometry; subjective evaluation of sound quality and comfort Method of assessing outcomes: pre-operative audiologic evaluation unaided and with any existing hearing aids. Free-field speech audiometry using Boothroyd list, aided and unaided Post-operative evaluation unaided and with BAHA (duration not stated) Sound quality and comfort rated pre-operatively for their old aid, and post-operatively for BAHA, on a scale of 1–10 (sound quality: 1 = distorted, 10 = clear and natural; comfort: 1 = so uncomfortable the aid cannot be worn, 10 = so comfortable you are unaware of its presence) Length of follow-up: NR |
| Characteristics of participants | |||
| Patients suitable for BAHA (n = 19) | |||
| Age, years (mean) | 45.7, range 25.0–76.0 | ||
| Sex (M : F) | 4 : 15 | ||
| Average BC thresholds (0.5–4.0 kHz) | 24 dB HL | ||
| Patients unsuitable for BAHA (n = 13) | |||
| Reasons for being unsuitable: | Hearing too bad = 8 | ||
| Hearing too good = 1 (unilateral otosclerois) | |||
| Declined = 4 | |||
| Results (n = 9) | |||
| Outcomes | Unaided | ACHA | BAHA |
| Average free-field warble-tone thresholds (0.5–4.0 kHz), mean (SD), rangea | 49.4 (11.9), 40–78 dB(A) | 33.0 (5.4), 28–40 dB(A) | 30.6 (8.1), 22–43 dB(A) |
| Comments: all patients gained improvements in threshold using their ACHA compared with unaided. BAHA results were comparable with the ACHA, but ‘significantly’ better in only one case | |||
| Free-field speech discrimination at 63 dB(A), mean (SD), rangea | 74.0 (19.5), 50–98 dB(A)b | 91.6 (14.7), 60–100 dB(A) | 84.0 (22.3), 30–100 dB(A) |
| Comments: improvements were observed from unaided to ACHA, however, comparisons between ACHA and BAHA showed no improvement | |||
| Subjective assessment | ACHA | BAHA | p–value |
| Patients’ rating of sound quality, mean (SD), rangea | 4.6 (2.1), 2–8 | 7.9 (2.4), 2–10 | |
| Patients’ rating of comfort, mean (SD), rangea | 4.1 (2.7), 1–10 | 9.4 (1.0), 7–10 | |
| Cosmetic preference | BAHA: 8/9 (89%) | ||
| No difference: 1/9 (11%) | |||
| Patient preference in background noise | BAHA: 55% | ||
| ACHA: 11% | |||
| No difference: 34% | |||
| Methodological comments | |||
| Allocation to treatment groups: one group tested pre- and post-operatively, aided and unaided | |||
Blinding: none
|
|||
| General comments | |||
|
|||
Quality assessment for primary studies
| A. Selection bias | |||||
|---|---|---|---|---|---|
| 1. Are the individuals selected to participate in the study likely to be representative of the target population? | Very likely |
Somewhat likely x |
Not likely | Can’t tell | |
| 2. What percentage of selected individuals agreed to participate? |
80–100% x |
60–79% | < 60% | Not applicable | Can’t tell |
| Summary of selection bias (methodological strength of study) | Strong |
Moderate x |
Weak | ||
| B. Study design | |||||
| 1. What was the study design? (Please tick appropriate and specify design in No. 7) | RCT | ||||
| Controlled clinical trial | |||||
| Cohort analytic (two group pre and post) | |||||
| Case-control | |||||
| Cohort [one group pre and post (before and after)] | x reviewer’s opinion | ||||
| Interrupted time series | |||||
| Other – specify | |||||
| Can’t tell | |||||
| 2. Was the study described as randomised? | Yes |
No x |
|||
| If answer to No. 2 is no, go to section on Section C Confounders. If answer yes, answer No. 3 and No. 4 below. | |||||
| 3. If answer was yes, was the method of randomisation described? | Yes | No | |||
| 4. If answer was yes, was the method appropriate? | Yes | No | |||
| Summary of study design (methodological strength of study) | Strong | Moderate |
Weak x |
||
| C. Confounders | |||||
|
• If there are two groups included in the study: ‘are confounders reported AND controlled for in the analysis?’ • If there is one group of participants in the study: ‘are potential confounding variables reported?’ |
Yes |
No x |
Can’t tell | ||
| Summary of confounders (methodological strength of study) | Strong | Moderate |
Weak x |
||
| D. Blinding | |||||
| 1. Was the outcome assessor aware of the intervention or exposure status of participants? |
Yes x |
No | Can’t tell | ||
| 2. Were the study participants aware of the research question? |
Yes x |
No | Can’t tell | ||
| Summary of blinding (methodological strength of study) | Strong | Moderate |
Weak x |
||
| E. Data collection methods | |||||
| 1. Were data collection tools shown to be valid? |
Yes x |
No | Can’t tell | ||
| 2. Were data collection tools shown to be reliable? |
Yes x |
No | Can’t tell | ||
| Summary of data collection (methodological strength of study) |
Strong x |
Moderate | Weak | ||
| F. Withdrawals and dropouts | |||||
| 1. Were withdrawals and dropouts reported in terms of numbers and reasons per group? | Yes |
No x |
Can’t tell | ||
| 2. Indicate the percentage of participants completing the study (if the percentage differs by groups, record the lowest) |
80–100% x |
60–79% | < 60% | Can’t tell | |
| Summary of withdrawals and dropouts (methodological strength of study) | Strong | Moderate |
Weak x |
||
| G. Intervention integrity | |||||
| 1. Was the consistency of the intervention measured? | Yes |
No x |
Can’t tell | ||
| H. Analysis | |||||
| 1. Are the statistical methods appropriate for the study design? | Yes |
No x |
Can’t tell | ||
| 2. Does the study report how missing data are dealt with in the analysis? | Yes |
No x |
Can’t tell | Not applicable | |
|
Global rating for study a (Overall methodological strength of study – based on sections A–F) |
Strong | Moderate |
Weak x |
||
| Reference and design | Intervention | Participants | Outcome measures |
|---|---|---|---|
|
Flynn et al. 200976 Sweden Design: audiology comparison study Study setting: unclear Number of centres: unclear Funding: Cochlear Bone Anchored Solutions |
|
Indication for treatment: mixed hearing loss Number of participants: 10 Sample attrition/dropout: NR Inclusion/exclusion criteria for study entry: had worn BAHA for at least 1 year at time of study and had previous experience of ACHA. Mixed hearing loss defined as an average sensorineural component > 25 dB HL in addition to an air-bone gap ≥ 30 dB |
Primary and secondary outcomes: aided warble-tone free-field thresholds Speech understanding in noise (speech-to-noise ratio) Method of assessing outcomes: aided free-field thresholds (warble tones) measured as described by Morgan et al. 144 Speech understanding in noise measured using adaptive procedures from Swedish version of Hearing In Noise Test (Hällgren et al. 200694). Two loud speakers used at 1 m from subject. Speech presented from 0˚ and noise from 180˚ Length of follow-up: appears that assessments were undertaken at same session |
| Characteristics of participants | |||
| Mean age, years (range) | 59 (32–75) | ||
| Sex (M : F) | 5 : 5 | ||
| Unaided PTA AC thresholds (0.5, 1.0 and 2.0 kHz), mean | 77 (range 55–80) dB HL | ||
| Unaided PTA BC thresholds (0.5, 1.0 and 2.0 kHz), mean | 41 (range 25–66) dB HL | ||
| Results | |||
| Outcomes | BAHA Intenso (n = 10) | ACHA (n = 10) | p-value |
| Average aided thresholds (dB SPL) a | |||
| 250 Hz | 47 | 39 | |
| 500 Hz | 39b | 42 | |
| 1 kHz | 30b | 37 | |
| 2 kHz | 31b | 43 | |
| 3 kHz | 39b | 46 | |
| 4 kHz | 41b | 50 | |
| 6 kHz | 53 | 75 | |
| 8 k Hz | 55 | 68 | |
| Speech-to-noise ratio, dB (SNR) | 0.88 | 3.44 | Difference 2.56 |
| Methodological comments | |||
|
|||
| General comments | |||
|
|||
Quality assessment for primary studies
| A. Selection bias | |||||
|---|---|---|---|---|---|
| 1. Are the individuals selected to participate in the study likely to be representative of the target population? | Very likely | Somewhat likely | Not likely |
Can’t tell x |
|
| 2. What percentage of selected individuals agreed to participate? | 80–100% | 60–79% | < 60% | Not applicable |
Can’t tell x |
| Summary of selection bias (methodological strength of study) | Strong | Moderate |
Weak x |
||
| B. Study design | |||||
| 1. What was the study design? (Please tick appropriate and specify design in No. 7) | RCT | ||||
| Controlled clinical trial | |||||
| Cohort analytic (two group pre and post) | |||||
| Case–control | |||||
| Cohort [one group pre and post (before and after)] | |||||
| Interrupted time series | |||||
| Other – specify: audiology comparison study | x reviewer’s opinion | ||||
| Can’t tell | |||||
| 2. Was the study described as randomised? | Yes |
No x |
|||
| If answer to No. 2 is no, go to section on Section C Confounders. If answer yes, answer No. 3 and No. 4 below. | |||||
| 3. If answer was yes, was the method of randomisation described? | Yes | No | |||
| 4. If answer was yes, was the method appropriate? | Yes | No | |||
| Summary of study design (methodological strength of study) | Strong | Moderate |
Weak x |
||
| C. Confounders | |||||
|
• If there are two groups included in the study: ‘are confounders reported AND controlled for in the analysis?’ • If there is one group of participants in the study: ‘are potential confounding variables reported?’ |
Yes |
No x |
Can’t tell | ||
| Summary of confounders (methodological strength of study) | Strong | Moderate |
Weak x |
||
| D. Data collection methods | |||||
| 1. Was the outcome assessor aware of the intervention or exposure status of participants? |
Yes x |
No | Can’t tell | ||
| 2. Were the study participants aware of the research question? |
Yes x |
No | Can’t tell | ||
| Summary of blinding (methodological strength of study) | Strong | Moderate |
Weak x |
||
| E. Data collection methods | |||||
| 1. Were data collection tools shown to be valid? |
Yes x |
No | Can’t tell | ||
| 2. Were data collection tools shown to be reliable? |
Yes x |
No | Can’t tell | ||
| Summary of data collection (methodological strength of study) |
Strong x |
Moderate | Weak | ||
| F. Withdrawals and dropouts | |||||
| 1. Were withdrawals and dropouts reported in terms of numbers and reasons per group? | Yes | No |
Can’t tell x |
||
| 2. Indicate the percentage of participants completing the study (if the percentage differs by groups, record the lowest) | 80–100% | 60–79% | < 60% |
Can’t tell x |
|
| Summary of withdrawals and dropouts (methodological strength of study) | Strong | Moderate |
Weak x |
||
| G. Intervention integrity | |||||
| 1. Was the consistency of the intervention measured? | Yes |
No x |
Can’t tell | ||
| H. Analysis | |||||
| 1. Are the statistical methods appropriate for the study design? | Yes | No |
Can’t tell x |
||
| 2. Does the study report how missing data are dealt with in the analysis? | Yes | No | Can’t tell x | ||
|
Global rating for study a (Overall methodological strength of study – based on sections A–F) |
Strong | Moderate |
Weak x |
||
| Reference and design | Intervention | Participants | Outcome measures |
|---|---|---|---|
|
Mylanus et al. 199884 Netherlands Design: cohort, pre and post Study setting: outpatient (secondary care) Number of centres: one Funding: not stated |
|
Indication for treatment: bilateral conductive or mixed hearing loss and chronic ear problems. Blockage of the ear canal with the ear mould of ACHA had caused or exacerbated chronic ‘otitis’ (assume otitis media) Number of participants: 34 Sample attrition/dropout: no attrition reported, one participant did not complete the questionnaire Inclusion/exclusion criteria for study entry: not stated explicitly. Previously fitted ACHA |
Primary outcomes: not defined as primary or secondary outcomes; free-field aided thresholds, speech recognition in quiet, speech recognition in noise, MPS, speech-to-noise ratio, subjective questionnaire Secondary outcomes: also functional gain (not data extracted) Method of assessing outcomes: before testing aids checked for normal functioning and adjusted to the patients preferred setting The MPS was calculated from the free-field speech recognition-intensity function (speech audiogram) Speech-to-noise ratio was determined according to criteria of Plomp and Mimpen89 at a fixed noise level of 65 dB The subjective questionnaire concerned ear infection, frequency of visits to outpatient clinic, ‘handling and feedback’, also speech recognition in quiet and noise, quality of sound, cosmetic appearance, patient preference advantages and disadvantages. Sent after BAHA use between 9 months and 7 years (mean 32 months) and compared with a questionnaire sent out after 5 months of BAHA fitting (this aspect not data extracted) Length of follow-up: 4–6 weeks after fitting of BAHA (for objective outcomes) |
| Characteristics of participants | |||
| BAHA | p-value | ||
| Age, years | Average age 48 years, range 26–72 years | ||
| Sex (M : F) | 12 : 22 | ||
| PTA for AC at 0.5, 1.0, 2.0 and 4.0 kHza |
12 with linear, medium-power ACHA: between 25 and 65 dB HL 22 with linear, high-power ACHA: between 40 and 90 dB HL Overall group mean: 60 dB HL (range 25–90 dB HL) |
||
| PTA for BC at 0.5, 1.0, 2.0 and 4.0 kHza | Mean: 26 dB HL (range 6–46 dB HL) | ||
| Air-bone gapa | Mean 34 dB HL (range 11–54 dB HL); 15 (44%) had one totally deaf ear | ||
| Results | |||
| Outcomes | BAHA (4–6 weeks post fitting) | ACHA (pre-op) | Difference and p-value |
| Mean free-field threshold, dB HL (SD) |
0.25 kHz: 39 0.50 kHz: 36 1.00 kHz: 22 (8.3) 2.00 kHz: 25 4.00 kHz: 33 8.00 kHz: 43 (22.3) |
0.25 kHz: 40 0.50 kHz: 36 1.00 kHz: 28 2.00 kHz: 22 (11.9) 4.00 kHz: 37 8.00 kHz: 55 (21.3) |
p = NS p = NS p < 0.01 p = NS p = NS p < 0.001 |
|
Comments: estimated from figure by reviewer SDs for each frequency NR. States that the SD varied between 11.9 dB at 2 kHz and 21.3 dB at 8 kHz for ACHA, and between 8.3 dB at 1 kHz and 22.3 dB at 8 kHz for BAHA |
|||
| MPS (mean ± SD) | Data NR | Data NR | 1.0% ± 5.4%, p = NS |
| Comments: states 16 participants obtained an MPS of 100% with both types of hearing aid. Individual participant data of improvement in MPS by air-bone gap reported in a figure but not data extracted | |||
| Speech-to-noise ratio improvement | Data NR | Data NR | 1.1 ± 2.1 dB, p < 0.01 |
|
Comments: when a 1.4 dB criterion for significance was used on an individual basis, the speech-on-noise ratio with the BAHA improved significantly in 15 patients, it did not change in 14 patients and it deteriorated significantly in five patients Individual participant data presented for speech-to-noise ratio by air-bone gap in a figure but not data extracted. A significant correlation was found between the change in speech-to-noise ratio and the width of the air-bone gap (r = 0.59, p < 0.01) |
|||
| Speech recognition in quiet | |||
| Comments: despite this being an outcome measure no mention is made of this in the results | |||
| Preference of device based on (n = 33) | BAHA (%) | ACHA (%) | No preference (%) |
| Ear infections | 32 (97) | 0 (0) | 1 (3) |
| Speech-in-quiet | 20 (61) | 5 (15) | 8 (24) |
| Speech-in-noise | 10 (30) | 9 (27) | 14 (42) |
| Quality of sound | 20 (61) | 6 (19) | 7 (21) |
| Visibility | 15 (45) | 8 (24) | 10 (30) |
| Handling | 13 (39) | 5 (15) | 15 (45) |
| Feedback | 25 (76) | 4 (12) | 4 (12) |
| ENT visits | 21 (64) | 4 (12) | 8 (24) |
| Overall preference | 27 (82) | 5 (15) | 1 (3) |
| Comments: all estimated from figure by reviewer. One participant did not complete the questionnaire. No statistical significance testing undertaken. Also reports most important advantage and disadvantage of BAHA but data not extracted | |||
| Adverse effects | |||
| Comments: states surgery was uneventful in all patients. Two stopped using their BAHA after 3 months and 2.5 years respectively, owing to pain – no explanation for this found | |||
| Methodological comments | |||
|
|||
| General comments | |||
|
|||
Quality assessment for primary studies
| A. Selection bias | |||||
|---|---|---|---|---|---|
| 1. Are the individuals selected to participate in the study likely to be representative of the target population? | Very likely |
Somewhat likely x |
Not likely | Can’t tell | |
| 2. What percentage of selected individuals agreed to participate? |
80–100% x |
60–79% | < 60% | Not applicable | Can’t tell |
| Summary of selection bias (methodological strength of study) | Strong |
Moderate x |
Weak | ||
| B. Study design | |||||
| 1. What was the study design? (Please tick appropriate and specify design in No. 7) | RCT | ||||
| Controlled clinical trial | |||||
| Cohort analytic (two group pre and post) | |||||
| Case–control | |||||
| Cohort [one group pre and post (before and after)] | x reviewer’s opinion | ||||
| Interrupted time series | |||||
| Other – specify | |||||
| Can’t tell | |||||
| 2. Was the study described as randomised? | Yes | No x | |||
| If answer to No. 2 is no, go to section on Section C Confounders. If answer yes, answer No. 3 and No. 4 below. | |||||
| 3. If answer was yes, was the method of randomisation described? | Yes | No | |||
| 4. If answer was yes, was the method appropriate? | Yes | No | |||
| Summary of study design (methodological strength of study) | Strong | Moderate |
Weak x |
||
| C. Confounders | |||||
|
• If there are two groups included in the study: ‘are confounders reported AND controlled for in the analysis?’ • If there is one group of participants in the study: ‘are potential confounding variables reported? |
Yes |
No x |
Can’t tell | ||
| Summary of confounders (methodological strength of study) | Strong | Moderate |
Weak x |
||
| D. Blinding | |||||
| 1. Was the outcome assessor aware of the intervention or exposure status of participants? |
Yes x |
No | Can’t tell | ||
| 2. Were the study participants aware of the research question? |
Yes x |
No | Can’t tell | ||
| Summary of blinding (methodological strength of study) | Strong | Moderate |
Weak x |
||
| E. Data collection methods | |||||
| 1. Were data collection tools shown to be valid? | Yes x | No | Can’t tell | ||
| 2. Were data collection tools shown to be reliable? | Yes x | No | Can’t tell | ||
| Summary of data collection (methodological strength of study) |
Strong x |
Moderate | Weak | ||
| F. Withdrawals and dropout | |||||
| 1. Were withdrawals and dropouts reported in terms of numbers and reasons per group? | Yes | No |
Can’t tell x |
||
| 2. Indicate the percentage of participants completing the study (if the percentage differs by groups, record the lowest) | 80–100% | 60–79% | < 60% |
Can’t tell x |
|
| Summary of withdrawals and dropouts (methodological strength of study) | Strong | Moderate |
Weak x |
||
| G. Intervention integrity | |||||
| 1. Was the consistency of the intervention measured? | Yes | No |
Can’t tell x |
||
| H. Analysis | |||||
| 1. Are the statistical methods appropriate for the study design? |
Yes x |
No | Can’t tell | ||
| 2. Does the study report how missing data are dealt with in the analysis? | Yes |
No x |
Can’t tell | ||
|
Global rating for study a (Overall methodological strength of study – based on sections A–F) |
Strong | Moderate |
Weak x |
||
Appendix 8 Data extraction: BAHA versus BCHA/ACHA
| Reference and design | Intervention | Participants | Outcome measures | ||||
|---|---|---|---|---|---|---|---|
|
Cooper et al. 199678 UK Design: cohort (one group pre and post) Study setting: secondary care Number of centres: single Funding: NR |
BAHA model: Nobel Biocare HC200/300/220 Other interventions used: candidates discuss surgical procedure, pros/cons of BAHAs and other options. Patients must meet another BAHA patient in order to have realistic expectations If existing aid was old or inadequate, a new appropriate aid/s was fitted prior to testing, so testing was against previous optimal aiding |
Indication for treatment: adults with bilateral hearing loss Number of participants: 68 Subgroups (aetiology and previous aid):Sample attrition/dropout: 68/106 successfully follow up Inclusion/exclusion criteria for study entry: minimum age 17 years. Audiological criteria: |
Outcomes: PTA, free-field speech results, free-field warble-tone threshold and questionnaire Method of assessing outcomes: PTAs are calculated from thresholds at 500, 1000, 2000 and 4000 Hz. Free-field speech results (%) discrimination at 63 dB(A) and obtained under three conditions (without aid, with existing aid, with BAHA) and frequencies are the same as for PTA Average free-field warble-tone threshold at same frequencies and conditions Questionnaire: 11 questions on usage and satisfaction, scored pre- and post-BAHA fittingLength of follow-up: pre-op assessment and 6 months post-BAHA fitting assessment |
||||
| Characteristics of participantsa | |||||||
| CSOM/ACHA, n = 24 | CSOM/BCHA, n = 19 | CON/ACHA, n = 9 | CON/BCHA, n = 16 | p-value | |||
| Age, mean years | ∼ 58 | ∼ 61 | ∼ 30 | ∼ 24 | p < 0.01b | ||
| Mean PTA threshold, AC 500–4000 Hz (db HL) | ∼ 58 | ∼ 65 | ∼ 70 | ∼ 60 | p > 0.05b | ||
| Mean PTA threshold, BC 500–4000 Hz (db HL) | ∼ 24 | ∼ 30 | ∼ 20 | ∼ 13 | p < 0.01b | ||
| Air-bone gap (500–4000 Hz) (db HL) | ∼ 33 | ∼ 32 | ∼ 52 | ∼ 49 | |||
| CON overall group mean for BC threshold = 17.2 dB HL – just inside the normal range, both CSOM groups are outside the normal range | |||||||
| Results | |||||||
| Questionnaire (BAHA compared with old aid): | CSOM/ACHA, n = 24 | CSOM/BCHA, n = 19 | p-value | ||||
| Worst | Same | Better | Worst | Same | Better | ||
| Hearing in quiet, n | 3 | 9 | 9 | 0 | 7 | 9 | CSOM/BCHA p < 0.01c |
| Hearing in noise, n | 2 | 5 | 12 | 1 | 4 | 11 | CSOM/ACHA p < 0.01,c CSOM/BCHA p < 0.01c |
| Hearing TV/radio, n | 4 | 9 | 9 | 1 | 4 | 11 | CSOM/BCHA p < 0.01c |
| Feelings about BAHAs, n | 3 | 3 | 15 | 1 | 5 | 10 | CSOM/ACHA p < 0.01,c CSOM/BCHA p < 0.01c |
| Overall satisfaction, n | 5 | 5 | 10 | 2 | 3 | 12 | CSOM/ACHA p = NS, CSOM/BCHA p < 0.01c |
| Questionnaire (BAHA compared with old aid): | CON/ACHA, n = 9 | CON/BCHA, n = 16 | p-value | ||||
| Worst | Same | Better | Worst | Same | Better | ||
| Hearing in quiet, n | 0 | 3 | 3 | 0 | 6 | 6 | CON/BCHA p < 0.05c |
| Hearing in noise, n | 1 | 0 | 5 | 3 | 4 | 5 | |
| Hearing TV/radio, n | 0 | 1 | 5 | 0 | 5 | 7 | CON/ACHA p < 0.01,c CON/BCHA p < 0.05c |
| Feelings about BAHAs, n | 0 | 0 | 8 | 2 | 1 | 9 | CON/ACHA p < 0.05,c CON/BCHA p < 0.01c |
| Overall satisfaction, n | 0 | 2 | 5 | 0 | 5 | 9 | CON/ACHA p < 0.05,c CON/BCHA p < 0.01c |
| Questionnaire: sound quality (BAHA compared with old aid),% of patients with: | Old aid | BAHA | p-value | ||||
| Positive responses | 44 | 67 | |||||
| Negative responses | 63 | 50 | |||||
| Comments: 95.5% of patients used BAHA for > 8 hours a day, 89.7% of these reporting sufficiently amplified sound | |||||||
| Hearing measures | CSOM/ACHA, n = 24 | CSOM/BCHA, n = 19 | p-value | ||||
| No aid | Old aid | BAHA | No aid | Old aid | BAHA | ||
| Mean free-field warble-tone thresholds [dB(A), 500–4000 Hz] | ∼ 60 | ∼ 40 | ∼ 33 | ∼ 63 | ∼ 42 | ∼ 35 | CSOM/ACHA p < 0.01,d CSOM/BCHA p < 0.01d |
| Mean free-field speech discrimination score (at 63 dB),% correct | ∼ 19 | ∼ 69 | ∼ 72 | ∼ 17 | ∼ 65 | ∼ 72 | CSOM/ACHA p = NS,e CSOM/BCHA p = NSe |
| Worst | Same | Better | Worst | Same | Better | ||
| Mean free-field warble-tone threshold (BAHA compared with previous aid), number of patients | ∼ 6 | 0 | ∼ 18 | ∼ 1 | ∼ 1 | ∼ 15 | |
| Speech discrimination scores at 63 dB (BAHA compared with old aid), number of patients | ∼ 12 | ∼ 2 | ∼ 9 | ∼ 5 | ∼ 1 | ∼ 12 | |
| CON/ACHA, n = 9 | CON/BCHA, n = 16 | p-value | |||||
| No aid | Old aid | BAHA | No aid | Old aid | BAHA | ||
| Mean free-field warble-tone thresholds, dB (500–4000 Hz) | ∼ 68 | ∼ 41 | ∼ 28 | ∼ 62 | ∼ 31 | ∼ 26 | CON/ACHA p < 0.01,d CON/BCHA p < 0.01d |
| Mean free-field speech discrimination score (at 63 dB),% correct | ∼ 17 | ∼ 57 | ∼ 82 | ∼ 3 | ∼ 86 | ∼ 85 | CON/ACHA p < 0.05,f CON/BCHA p = NSf |
| Worst | Same | Better | Worst | Same | Better | ||
| Mean free-field warble-tone threshold (BAHA compared with previous aid), number of patients | 0 | 0 | ∼ 9 | ∼ 3 | 0 | ∼ 11 | |
| Speech discrimination scores at 63 dB (BAHA compared with old aid), number of patients | ∼ 0 | ∼ 3 | ∼ 5 | ∼ 3 | ∼ 5 | ∼ 5 | |
| Methodological comments | |||||||
|
|||||||
| General comments | |||||||
|
|||||||
Quality assessment for primary studies
| A. Selection bias | |||||
|---|---|---|---|---|---|
| 1. Are the individuals selected to participate in the study likely to be representative of the target population? | Very likely |
Somewhat likely x |
Not likely | Can’t tell | |
| 2. What percentage of selected individuals agreed to participate? | 80–100% | 60–79% | < 60% | Not applicable |
Can’t tell x |
| Summary of selection bias (methodological strength of study) | Strong | Moderate |
Weak x |
||
| B. Study design | |||||
| 1. What was the study design? (Please tick appropriate and specify design in No. 7) | RCT | ||||
| Controlled clinical trial | |||||
| Cohort analytic (two group pre and post) | |||||
| Case–control | |||||
| Cohort [one group pre and post (before and after)] | x reviewer’s opinion | ||||
| Interrupted time series | |||||
| Other – specify | |||||
| Can’t tell | |||||
| 2. Was the study described as randomised? | Yes | No x | |||
| If answer to No. 2 is no, go to section on Section C Confounders. If answer yes, answer No. 3 and No. 4 below. | |||||
| 3. If answer was yes, was the method of randomisation described? | Yes | No | |||
| 4. If answer was yes, was the method appropriate? | Yes | No | |||
| Summary of study design (methodological strength of study) | Strong | Moderate |
Weak x |
||
| C. Confounders | |||||
|
Two groups: are confounders reported AND controlled for in the analysis? OR if one group: are potential confounding variables reported? |
Yes |
No x |
Can’t tell | Not applicable | |
| Summary of confounders (methodological strength of study) | Strong | Moderate |
Weak x |
Not applicable | |
| D. Blinding | |||||
| 1. Was the outcome assessor aware of the intervention or exposure status of participants? |
Yes x |
No | Can’t tell | ||
| 2. Were the study participants aware of the research question? |
Yes x |
No | Can’t tell | ||
| Summary of blinding (methodological strength of study) | Strong | Moderate |
Weak x |
||
| E. Data collection methods | |||||
| 1. Were data collection tools shown to be valid? |
Yes x |
No | Can’t tell | ||
| 2. Were data collection tools shown to be reliable? |
Yes x |
No | Can’t tell | ||
| Summary of data collection (methodological strength of study) |
Strong x |
Moderate | Weak | ||
| F. Withdrawals and dropouts | |||||
| 1. Were withdrawals and dropouts reported in terms of numbers and reasons per group? | Yes |
No x |
Can’t tell | ||
| 2. Indicate the percentage of participants completing the study (if the percentage differs by groups, record the lowest) | 80–100% |
60–79% x |
< 60% | Can’t tell | |
| Summary of withdrawals and dropouts (methodological strength of study) | Strong | Moderate |
Weak x |
||
| G. Intervention integrity | |||||
| 1. Was the consistency of the intervention measured? |
Yes x |
No | Can’t tell | ||
| H. Analysis | |||||
| 1. Are the statistical methods appropriate for the study design? |
Yes x |
No | Can’t tell | ||
| 2. Does the study report how missing data are dealt with in the analysis? | Yes |
No x |
Can’t tell | N/A | |
|
Global rating for study a (Overall methodological strength of study – based on sections A–F) |
Strong | Moderate |
Weak x |
||
| Reference and Design | Intervention | Participants | Outcome measures | |
|---|---|---|---|---|
|
Hol et al. 200482 Netherlands Design: cohort pre and post Study setting: otorhino-laryngology department Number of centres: one Funding: NR |
|
Indication for treatment: acquired conductive or mixed hearing loss Number of participants: n = 56 (ACHA, n = 36; BCHA, n = 20) Sample attrition/dropout: NR Inclusion/exclusion criteria for study entry: consecutive adult patients with acquired conductive or mixed hearing loss and listed for BAHA surgery |
Primary outcomes: quality of life Secondary outcomes: number of hours of daily BAHA use; number of visits to otolaryngologist due to otorrhoea or skin irritations; frequency of episodes of otorrhoea; prevalence of skin irritations Method of assessing outcomes: SF-36; EQ-5D: 5 domains (1 = no problems to 3 = severe problems); utility index (0 = worse than death to 1 = perfect health); EQ-5D visual analogue scale (0 = worst imaginable state of health to 100 = best imaginable state of health); HHDI Note: data for whole group also reported but not extracted Length of follow-up: questionnaires completed with previous aid (ACHA or BCHA) before surgery and after 6 months experience with BAHA |
|
| Characteristics of participants | ||||
| Total (n = 56) | ACHA (n = 36) | BCHA (n = 20) | ||
| Age, years, mean (range) | 52.9 (24–82) | 47.9 (24–73) | 62.0 (42–82) | |
| Sex,% male | 39 | 33 | 45 | |
| Hearing loss at 0.5, 1.0 and 2.0 kHz, dB HL, mean (range): | ||||
| AC | 68.1 (30–107) | 63.2 (30–103) | 76.5 (40–107) | |
| BC | 31.8 (9–63) | 26.8 (9–51) | 43.4 (17–63) | |
| Air-bone-gap, dB, mean (range) | 36.3 (13–60) | 36.4 (16–60) | 36.1 (13–53) | |
| Results: previous aid ACHA | ||||
| SF-36 mean (SD) | ACHA (n = 36) | BAHA (n = 36) | Mean difference | Effect size |
| Physical functioning | 80.3 (21.8) | 79.8 (22.4) | –0.5 (p = NS) | 0.02 |
| Role limitations (physical) | 71.5 (39.7) | 68.9 (40.5) | –2.6 (p = NS) | 0.06 |
| Role limitations (emotional) | 76.2 (40.1) | 73.2 (38.1) | –3.0 (p = NS) | 0.07 |
| Vitality | 60.4 (20.0) | 59.9 (19.9) | –0.5 (p = NS) | 0.02 |
| Mental health | 62.4 (18.0) | 67.9 (21.3) | 5.5 (p = NS) | –0.28 |
| Social functioning | 69.8 (28.3) | 75.0 (27.8) | 5.2 (p = NS) | –0.19 |
| Pain | 74.7 (25.2) | 79.2 (25.0) | 4.5 (p = NS) | –0.18 |
| General health | 63.2 (21.4) | 63.6 (21.2) | –0.4 (p = NS) | –0.18 |
| No statistically significant changes in any domain (better functioning leads to a higher score on a specific item). Mental health improved but not statistically significantly and effect size was small (–0.28) | ||||
| EQ-5D mean (SD) | ACHA (n = 36) | BAHA (n = 36) | Mean difference | Effect size |
| 5 domains (score 1–3): | ||||
| Mobility | 1.29 (0.46) | 1.31 (0.47) | 0.02 | –0.04 |
| Self-care | 1.03 (0.17) | 1.03 (0.17) | 0.00 | 0.0 |
| Usual activities | 1.47 (0.66) | 1.44 (0.50) | –0.03 (p > 0.05) | 0.05 |
| Pain/discomfort | 1.49 (0.51) | 1.47 (0.51) | –0.02 (p > 0.05) | 0.04 |
| Anxiety/depression | 1.26 (0.44) | 1.42 (0.60) | 0.16 (p < 0.01) | –0.30 |
| Utility (score 0–1) | 0.78 (0.17) | 0.77 (0.17) | –0.01 | 0.06 |
| Visual analogue scale (score 0–100) | 76.1 (14.1) | 73.4 (17.1) | –2.7 | 0.17 |
| Anxiety/depression increased (p < 0.01), but the clinical effect was small (–0.3) | ||||
| HHDI mean (SD) | ACHA (n = 36) | BAHA (n = 36) | Mean difference | Effect size |
| Disability | 25.8 (6.5) | 20.9 (6.2) | –5.0 (p < 0.01) | 0.79 |
| Handicap | 25.0 (5.9) | 19.6 (6.7) | –5.4 (p < 0.01) | 0.86 |
| Statistically significant improvements in disability and handicap, large clinical impact | ||||
| ACHA (n = 36) | BAHA (n = 36) | |||
| Number of otolaryngology visits over preceding 6 months for draining ears, mean (SD) | 32 patients, 12.7 (10.5) visits, range 0–30 | 33 patients, 3.3 (4.8), range 0–25 | ||
| Patient preference in regard to: | ||||
| Otorrhoea | 1 (3%) | 17 (47%) | ||
| Skin irritation | 6 (17%) | 14 (39%) | ||
| Proportion using aid > 8 hours per day | 78% | 100% | ||
| Results: previous aid BCHA | ||||
| SF-36 mean (SD) | BCHA (n = 20) | BAHA (n = 20) | Mean difference | Effect size |
| Physical functioning | 69.2 (25.4) | 70.8 (24.6) | 1.4 (p = NS) | –0.06 |
| Role limitations (physical) | 61.3 (40.1) | 57.5 (45.2) | –3.8 (p = NS) | 0.09 |
| Role limitations (emotional) | 76.7 (39.1) | 63.3 (41.8) | –13.4 (p = 0.19) | 0.33 |
| Vitality | 60.8 (16.6) | 61.0 (21.9) | 0.2 (p = NS) | –0.01 |
| Mental health | 68.4 (17.6) | 74.2 (14.2) | 5.8 (p = NS) | –0.36 |
| Social functioning | 80.6 (17.9) | 82.2 (18.3) | 1.6 (p = NS) | –0.09 |
| Pain | 73.8 (20.0) | 67.9 (27.9) | –5.9 (p = 0.30) | 0.24 |
| General health | 61.0 (19.8) | 59.5 (20.3) | –1.5 (p = NS) | 0.07 |
| No statistically significant changes in any domain. With BAHA, role limitations (emotional) deteriorated (meaning increased emotional problems) and pain scores were lower (meaning more pain experienced), but not statistically significant. Mental health improved, but not statistically significant. The clinical effect was small (–0.36) | ||||
| EQ-5D mean (SD) | BCHA (n = 20) | BAHA (n = 20) | Mean difference | Effect size |
| 5 domains (score 1–3): | ||||
| Mobility | 1.35 (0.49) | 1.50 (0.51) | 0.15 (p = 0.26) | –0.3 |
| Self-care | 1.2 (0.41) | 1.10 (0.31) | –0.10 (p = ns) | 0.28 |
| Usual activities | 1.60 (0.68) | 1.55 (0.60) | –0.05 (p = ns) | 0.08 |
| Pain/discomfort | 1.7 (0.57) | 1.85 (0.49) | 0.15 (p = 0.26) | –0.28 |
| Anxiety/depression | 1.26 (0.45) | 1.20 (0.41) | –0.06 (p = ns) | 0.13 |
| Utility (score 0–1) | 0.71 (0.23) | 0.70 (0.19) | –0.01 | 0.05 |
| Visual analogue scale (score 0–100) | 74.0 (16.0) | 72.4 (17.4) | –1.6 | 0.10 |
| Scores on mobility and pain/discomfort increased, meaning patients were slightly less mobile and experienced more pain/discomfort. Effect size small | ||||
| HHDI mean (SD) | BCHA (n = 20) | BAHA (n = 20) | Mean difference | Effect size |
| Disability | 31.0 (6.0) | 20.8 (8.2) | –10.2 (p < 0.01) | 1.42 |
| Handicap | 27.4 (6.2) | 21.8 (8.0) | –5.6 (p < 0.01) | 0.79 |
| Statistically significant improvements in disability and handicap, large clinical impact | ||||
| BCHA (n = 20) | BAHA (n = 20) | |||
| Number of otolaryngology visits over preceding 6 months for draining ears, mean (SD) | 19 patients, 5.4 (4.9) visits, range 0–20 | 20 patients, 1.5 (2.1), range 0–6 | ||
| Patient preference in regard to: | ||||
| Otorrhoea (%) | 0 (0) | 5 (25) | ||
| Skin irritation (%) | 2 (10) | 10 (50) | ||
| Per cent using aid > 8 hours per day | 90 | 100 | ||
| Methodological comments | ||||
|
||||
| General comments | ||||
|
||||
Quality assessment for primary studies (modified for BAHAs)
| A. Selection bias | |||||
|---|---|---|---|---|---|
| 1. Are the individuals selected to participate in the study likely to be representative of the target population? | Very likely |
Somewhat likely x |
Not likely | Can’t tell | |
| 2. What percentage of selected individuals agreed to participate? |
80–100% x |
60–79% | < 60% | N/A | Can’t tell |
| Summary of selection bias (methodological strength of study) | Strong |
Moderate x |
Weak | ||
| B. Study design | |||||
| 1. What was the study design? (Please tick appropriate and specify design in No. 7) | RCT | ||||
| Controlled clinical trial | |||||
| Cohort analytic (two group pre and post) | |||||
| Case–control | |||||
| Cohort [one group pre and post (before and after)] | x reviewers opinion | ||||
| Interrupted time series | |||||
| Other – specify | |||||
| Can’t tell | |||||
| 2. Was the study described as randomised? | Yes |
No x |
|||
| If answer to No. 2 is no, go to section on Section C Confounders. If answer yes, answer No. 3 and No. 4 below. | |||||
| 3. If answer was yes, was the method of randomisation described? | Yes | No | |||
| 4. If answer was yes, was the method appropriate? | Yes | No | |||
| Summary of study design (methodological strength of study) | Strong | Moderate |
Weak x |
||
| C. Confounders | |||||
|
• If there are two groups included in the study: ‘are confounders reported AND controlled for in the analysis?’ • If there is one group of participants in the study: ‘are potential confounding variables reported?’ |
Yes |
No x |
Can’t tell | ||
| Summary of confounders (methodological strength of study) | Strong | Moderate |
Weak x |
||
| D. Blinding | |||||
| 1. Was the outcome assessor aware of the intervention or exposure status of participants? |
Yes x |
No | Can’t tell | ||
| 2. Were the study participants aware of the research question? |
Yes x |
No | Can’t tell | ||
| Summary of blinding (methodological strength of study) | Strong | Moderate |
Weak x |
||
| E. Data collection methods | |||||
| 1. Were data collection tools shown to be valid? |
Yes x |
No | Can’t tell | ||
| 2. Were data collection tools shown to be reliable? |
Yes x |
No | Can’t tell | ||
| Summary of data collection (methodological strength of study) |
Strong x |
Moderate | Weak | ||
| F. Withdrawals and dropout | |||||
| 1. Were withdrawals and dropouts reported in terms of numbers and reasons per group? | Yes | No | Can’t tell x | ||
| 2. Indicate the percentage of participants completing the study (if the percentage differs by groups, record the lowest) | 80–100% | 60–79% | < 60% |
Can’t tell x |
|
| Summary of withdrawals and dropouts (methodological strength of study) | Strong | Moderate |
Weak x |
||
| G. Intervention integrity | |||||
| 1. Was the consistency of the intervention measured? |
Yes x |
No | Can’t tell | ||
| H. Analysis | |||||
| 1. Are the statistical methods appropriate for the study design? |
Yes x |
No | Can’t tell | ||
| 2. Does the study report how missing data are dealt with in the analysis? |
Yes x |
No | Can’t tell | ||
|
Global rating for study a (Overall methodological strength of study – based on sections A–F) |
Strong | Moderate |
Weak x |
||
| Reference and design | Intervention | Participants | Outcome measures | |||
|---|---|---|---|---|---|---|
|
Snik et al. Three linked studies: 1998,81 199480 and 199279 Netherlands Design: cohort (one group pre and post) Study setting: hospital otorhinolaryn-gology department Number of centres: one;80,81 two79 Data on TBS from a second centre presented but not extracted79 Funding: grants from the Fund of the Investigative Medicine of the Ziekenfonds-raad;81 NR79,80 |
BCHA ACHA BAHA HC 200,81 HC 200 and HC 220,80 HC 22079 One patient did not want to use a body-level hearing aid so a behind-the-ear combined with the HC 200 was used instead79 |
1998:81 Indication for treatment: conductive or mixed type binaural hearing loss, with SNHL of ≤ 30 dB HL Number of participants: n = 41 (BCHA, n = 33; ACHA: n = 8) Sample attrition/dropout: n = 2 (15%) Sample dropout/attrition: two unrelated deaths – long-term follow-up n = 39 Inclusion/exclusion criteria for study entry:Note: these participants appear to be the same as those in the HC 200 group of the 1994 study80 1994:80 Indication for treatment: chronic otitis media, chronic otitis externa, aural atresia Number of participants: n = 58 (HC200, n = 42; HC220, n = 16) Sample attrition/dropout: five did not complete speech-in-noise test Inclusion/exclusion criteria for study entry: all patients at the Nijmegen clinic who were fitted with a BAHA between 1988 and 1992. No other details reported Note: six of the participants from the HC 220 group are reported in the 1992 study79 The participants in the HC 200 group appear to be the same as those in the 1998 study81 1992:79 Indication for treatment: severe mixed hearing loss Number of participants: 12 Previous aid: BCHA, 7; ACHA, 5 Sample attrition/dropout: speech recognition thresholds could not be obtained for some patients Inclusion/exclusion criteria for study entry: all had recurrent otorrhoea preventing use of occluding ear moulds Note: six of these participants are also reported in the 1994 study80 |
1998:81 Primary outcome: SRT in quiet and noise Secondary outcome: subjective opinion questionnaire on device use and speech recognition in quiet and noise Method of assessing outcomes: Speech Recognition in Noise Test91 consisted of 13 sentences and a steady-state, speech-shaped noise presented at a fixed level. SRT of the sentences established with an adaptive procedure. The critical speech-to-noise ratio (difference between SRT and noise level in decibels) was determined Speech-to-noise ratio is independent of the volume setting of the hearing aid, as long as the speech level is above the patient’s threshold. The difference in speech-to-noise ratio between the old and new device was expressed as a change in the percentage of correctly repeated sentences (i.e. change of 1 dB in the speech-to-noise ratio equals 17% change in sentence recognition) SRT also determined in quiet SRT results calculated by deducting the new device from the old device and averaging it for each subgroup The questionnaire was marked on a 1–10 scale (impossible to excellent), administered pre- and post-surgery (details previously described in Mylanus et al. 145). Questions on recognition of speech in relatively quiet surrounding (five subquestions) and noisy situations (nine subquestions) were considered. An average score on both sets of subquestions was calculated for each patient. During follow-up, patients were regularly asked about actual use of the BAHA, and any relevant medical and technical problems were documented Length of follow-up: ACHA and BCHA data obtained before surgery. BAHA evaluated after at least 6 weeks. Questionnaire pre surgery and 3–5 months post BAHA fitting. Long-term evaluation exceeded 4.5 years 1994:80 Primary and secondary outcomes: percentage of patients whose SQ score (speech recognition in quiet) and speech-to-noise ratio (speech-to-noise ratio) improved or deteriorated significantly Questionnaire on speech recognition in quiet and noisy situations Method of assessing outcomes: SQ: free-field phoneme recognition score obtained using standard phonetically balanced lists of monosyllables, presented at 60 dB. If phoneme score < 100%, phoneme scores also obtained at 70 dB and 80 dB. SQ value is the maximum phoneme score obtained Speech recognition in noise: used test by Plomp and Mimpen89 (see above). Noise presented at 65 dB(A). SRT and speech-to-noise ratio determined as above Questionnaire answers rated on a scale 1–10. Five subquestions for speech in quiet, nine subquestions for speech-in-noise Length of follow-up: tests on previous aids performed 1–8 weeks prior to fitting BAHA Tests on BAHA after at least a 4-week period of daily use Questionnaire completed before surgery and 5 months after BAHA fitted 1992:79 Primary and secondary outcomes: maximum phoneme score; speech recognition threshold; average difference between free-field warble thresholds; patient’s opinions (questionnaire, not validated) Method of assessing outcomes: warble-tones use to obtain free-field thresholds, generated by the standard audiometer, with a frequency modulation of 5%. Sound was presented from the front via loudspeaker Free-field speech audiogram used standard Dutch PB word lists consisting of 10 monosyllables. The level of the (fluctuating) signal was read at the slow speed, using the ‘A’ filter. The readings for 40 words were averaged and the free-field speech levels are presented in dB(A) Phoneme scores as a function of the presentation levels were recorded separately for the BAHA and ACHA, and the maximum phoneme score and speech recognition threshold were determined, the later being the presentation level in dB(A) at which 50% of the presented phonemes were repeated properly by the patient Average difference between the free-field warble thresholds obtained by subtracting the thresholds obtained with the BAHA from those obtained with the previous aid Questionnaire: patient’s opinions rated on scale 1 to 10. Three scores were calculated from this: speech recognition in quiet, speech recognition in noise, and comfort. The scores are the average of the rated scores of the questions involved per topic. Positive scores indicate better score with BAHA Length of follow-up: tests performed after at least 4 weeks of daily use Questionnaire after at least 4 months of daily use with BAHA |
|||
| 199881 characteristics of participants | ||||||
| BAHA (n = 41) | p-value | |||||
| Age, mean, years (range) | 43 (10–70) | |||||
| PTA AC, dB HL (range) | 55 (30–90) | |||||
| PTA BC, dB HL (range) | 16 (0–28) | |||||
| Comments: PTA indicates average hearing loss at 0.5, 1.0 and 2.0 kHz | ||||||
| 199881 results | ||||||
| Previous aid BCHA (n = 33) | Previous aid ACHA (n = 8) | p-value | ||||
| Change in SRT in quiet (previous aid minus BAHA), mean dB (SD) | 2.7 (4.4)a | –6.4 (3.7)b | ||||
| Improvement in speech-to-noise ratio, mean dB (SD) | 2.5 (2.2)a | 1.6 (1.0)a | ||||
| Questionnaire | Previous aid BCHA (n = 33) | Previous aid ACHA (n = 8) | p-value | |||
| Speech recognition in quiet surroundings, median change in questionnaire score (range) | 1.4 (–0.6–5.6) | 0.2 (–1.4–3.3) | ||||
| Speech recognition in noisy surroundings, median change in questionnaire score (range) | 1.6 (–0.8–7.0) | 0 (–1.5–4.0) | ||||
| Note: change in score from previous aid NS for both subgroups | ||||||
| Previous aid BCHA (n = 33) | Previous aid ACHA (n = 8) | |||||
| Proportion of patients who preferred each device with regard to: | New device | No preference | Old device | New device | No preference | Old device |
| Speech recognition in noisy surroundings,% of patientsc | ~ 76 | ~ 12 | ~ 12 | ~ 37 | ~ 26 | ~ 37 |
| Speech recognition in quiet surroundings,% of patientsc | ~ 70 | ~ 20 | ~ 12 | ~ 50 | ~ 24 | ~ 24 |
| Comments: after the trial, all patients chose to use the BAHA, not their previous aid | ||||||
| Adverse events | BAHA (n = 39)d | p-value | ||||
| Lost implant due to inflammation after 2 years of use | 1 (implant not replaced) | |||||
| Requested implant removal due to pain after 3 years | 1 | |||||
| Implants loss owing to inflammation | 1 (implant replaced) | |||||
| Lost implant due to trauma | 2 (implant replaced) | |||||
| Reduction of thickness of the subcutaneous layer around implant to minimise risk for inflammation | 2 | |||||
| Total re-operations | 6 | |||||
| Rejections of BAHA due to insufficient amplification | 0 | |||||
| Severe deterioration in sensorineural hearing (25–65 dB HL) after surgery for cholesteatoma in the cerebellopontine angle and refitted with a more powerful BAHA (NBC-HC-220). However, result was poor owing to severe deterioration of cochlear function | 1 | |||||
| Non-users after at least 4.5 years (all others using BAHA on daily basis) | 2/39 (5%) | |||||
| 199478 characteristics of participants | ||||||
| Age, years | Range 10–77 | |||||
| Average hearing loss at 0.5, 1.0 and 2.0 kHz in best ear | Range 30–100 dB HL | |||||
| Average bone-conduction thresholds at 0.5, 1.0 and 2.0 kHz (PTAb,c) | HC200, 0–44 dB HL; HC220, 33–63 dB HL | |||||
| History of patients | Chronic otitis media, 86%; chronic otitis externa, 5%; aural atresia, 9% | |||||
| Previous hearing aid | BC 44/58 (76%); AC 14/58 (24%) | |||||
| 199478 results | ||||||
| Outcomes | Previously used BC | |||||
| Percentage of patients with a statistically significant improvement or deterioration in: | HC 200 (n = 33) | HC220 (n = 11) | p-value | |||
| SQ score | Improved, 12; deteriorated, 0 | Improved, 54; deteriorated, 0 | ||||
| Speech-in-noise score | Improved, 60; deteriorated, 0 | Improved, 44; deteriorated, 0 | ||||
| Speech recognition in quiet (questionnaire) | Improved, 63; deteriorated, 9 | Improved, 91; deteriorated, 0 | ||||
| Speech recognition in noise (questionnaire) | Improved 75; deteriorated, 12 | Improved, 91; deteriorated, 0 | ||||
| Comments: in the total group of patients who previously used BC, the average subjective improvement with the BAHA on the speech recognition-in-quiet and in-noise was > 1.3 points | ||||||
| Previously used AC | p-value | |||||
| Percentage of patients with a statistically significant improvement or deterioration in: | HC 200 (n = 9) | HC220 (n = 5) | ||||
| SQ score | Improved, 0; deteriorated, 11 | Improved, 40; deteriorated, 20 | ||||
| Speech-in-noise score | Improved, 55; deteriorated, 11 | Improved, no results; deteriorated, no results | ||||
| Speech recognition in quiet (questionnaire) | Improved, 22; deteriorated, 44 | Improved, 80; deteriorated, 20 | ||||
| Speech recognition in noise (questionnaire) | Improved, 11; deteriorated, 44 | Improved, 80; deteriorated, 20 | ||||
| 199279 characteristics of participants | ||||||
| Previous aid BCHA (n = 7) | Previous aid ACHA (n = 5) | |||||
| Age, years mean (SD), rangee | 60.6 (18.8), 34–84 | 62 (13.9), 46–78 | ||||
| PTA for BC, dB HL, mean (SD) rangee | 46.2 (12.6), 28 to > 62 | 49.6 (7.3), 40–57 | ||||
| PTA for AC, dB HL, mean (SD) rangee | 91.1 (14.3), 70–108 | 84.8 (12.3), 72–100 | ||||
| Hearing aid use (years) | 23 (range 7–40) | |||||
| 199279 results | ||||||
| Previous aid BCHA ( n = 7) | ||||||
| BCHA | BAHA | p-value | ||||
| Maximum phoneme score,%, mean (SD) rangee | 36.1 (28.9), 0–85 | 48.7 (31.7), 0–100 | ||||
| Speech recognition threshold, dB(A), mean (SD) rangee | (n = 2); 40 (7.1), 35–45 | (n = 4); 38.8 (11.1), 25–50 | ||||
| Average difference between the free-field warble thresholds, dBc | (BCHA minus BAHA) | |||||
| 250 Hz: 2 | ||||||
| 500 Hz: –3 | ||||||
| 1000 Hz: –2 | ||||||
| 2000 Hz: –10 | ||||||
| 4000 Hz: –14 | ||||||
| 8000 Hz: NR | ||||||
|
Comments: the maximum phoneme score with the BAHA was equal to the BCHA in three patients and better in four patients (range of improvement 15–28%) Speech recognition threshold values could be compared only in two patients: in one patient the value was 10 dB better with the BAHA; in one patient the values were equal At higher frequencies, the average difference in warble-tone thresholds was negative, indicating that the hearing in this region was, on average, better with the BAHA than BCHA |
||||||
| Change scores from questionnaire, mean (SD), range:e | BAHA minus BCHA | p-value | ||||
| Speech recognition in quiet | 0.7 (2.0), –1.2–4.4 | |||||
| Speech recognition in noise | 0.4 (2.0), –3.0–2.8 | |||||
| Comfort | 1.0 (1.0), 0.0–2.7 | |||||
| Previous aid ACHA ( n = 5) | ||||||
| ACHA | BAHA | |||||
| Maximum phoneme score,%, mean (SD) rangee | 81.6 (8.7), 70–90 | 67.6 (22.2), 43–90 | ||||
| Speech recognition threshold, dB(A), mean (SD) rangee | 39 (10.8), 20–45 | (n = 3); 45 (5), 40–50 | ||||
| Average difference between the free-field warble thresholds, dBc | (ACHA minus BAHA) | |||||
| 250 Hz: –6 | ||||||
| 500 Hz: –5 | ||||||
| 1000 Hz: 3 | ||||||
| 2000 Hz: 4 | ||||||
| 4000 Hz: 15 | ||||||
| 8000 Hz: 0 | ||||||
|
Comments: maximum phoneme scores with the BAHA were better in one patient (+ 10%), equal in one patient and worse in three patients (–13–40%) Speech recognition threshold values could be compared in three patients: values obtained with the ACHA and BAHA were equal within 5 dB At higher frequencies, the average difference in warble-tone thresholds was positive, indicating that the hearing in this region was, on average, better with the ACHA than the BAHA |
||||||
| Change scores from questionnaire, mean (SD), range:e | BAHA minus ACHA | |||||
| Speech recognition in quiet | –1.0 (4.6), –5.8–3.5 | |||||
| Speech recognition in noise | 0.1 (3.3), –4.2–3.7 | |||||
| Comfort | 0.6 (3.2), –3.2–5.4 | |||||
| Methodological comments | ||||||
|
||||||
| General comments | ||||||
|
||||||
Quality assessment for primary studies (Snik et al. 199881)
| A. Selection bias | |||||
|---|---|---|---|---|---|
| 1. Are the individuals selected to participate in the study likely to be representative of the target population? | Very likely |
Somewhat likely x |
Not likely | Can’t tell | |
| 2. What percentage of selected individuals agreed to participate? |
80–100% x |
60–79% | < 60% | Not applicable | Can’t tell |
| Summary of selection bias (methodological strength of study) | Strong |
Moderate x |
Weak | ||
| B. Study design | |||||
| 1. What was the study design? (Please tick appropriate and specify design in No. 7) | RCT | ||||
| Controlled clinical trial | |||||
| Cohort analytic (two group pre and post) | |||||
| Case–control | |||||
| Cohort [one group pre and post (before and after)] | x reviewer’s opinion | ||||
| Interrupted time series | |||||
| Other – specify | |||||
| Can’t tell | |||||
| 2. Was the study described as randomised? | Yes | No x | |||
| If answer to No. 2 is no, go to section on Section C Confounders. If answer yes, answer No. 3 and No. 4 below | |||||
| 3. If answer was yes, was the method of randomisation described? | Yes | No | |||
| 4. If answer was yes, was the method appropriate? | Yes | No | |||
| Summary of study design (methodological strength of study) | Strong | Moderate |
Weak x |
||
| C. Confounders | |||||
|
Two groups: are confounders reported AND controlled for in the analysis? OR if one group: are potential confounding variables reported? |
Yes |
No x |
Can’t tell | ||
| Summary of confounders (methodological strength of study) | Strong | Moderate |
Weak x |
||
| D. Blinding | |||||
| 1. Was the outcome assessor aware of the intervention or exposure status of participants? |
Yes x |
No | Can’t tell | ||
| 2. Were the study participants aware of the research question? |
Yes x |
No | Can’t tell | ||
| Summary of binding (methodological strength of study) | Strong | Moderate |
Weak x |
||
| E. Data collection methods | |||||
| 1. Were data collection tools shown to be valid? |
Yes x |
No | Can’t tell | ||
| 2. Were data collection tools shown to be reliable? |
Yes x |
No | Can’t tell | ||
| Summary of data collection (methodological strength of study) |
Strong x |
Moderate | Weak | ||
| F. Withdrawals and dropouts | |||||
| 1. Were withdrawals and dropouts reported in terms of numbers and reasons per group? |
Yes x |
No | Can’t tell | ||
| 2. Indicate the percentage of participants completing the study (if the percentage differs by groups, record the lowest) |
80–100% x |
60–79% | < 60% | Can’t tell | |
| Summary of withdrawals and dropouts (methodological strength of study) |
Strong x |
Moderate | Weak | ||
| G. Intervention integrity | |||||
| 1. Was the consistency of the intervention measured? | Yes | No | Can’t tell x | ||
| H. Analysis | |||||
| 1. Are the statistical methods appropriate for the study design? | Yes | No |
Can’t tell x |
||
| 2. Does the study report how missing data are dealt with in the analysis? | Yes |
No X |
Can’t tell | ||
|
Global rating for study a (Overall methodological strength of study – based on sections A–F) |
Strong | Moderate |
Weak x |
||
Appendix 9 Data extraction: BAHA versus unaided hearing
| Reference and design | Intervention | Participants | Outcome measures |
|---|---|---|---|
|
Kompis et al. 200766 Switzerland Design: cohort pre–post Study setting: secondary care Number of centres: single centre Funding: Divinos provided by Entific Medical Systems |
Other interventions used: none Note: BAHA Compact also assessed but not extracted. See General comments |
Indication for treatment: substantial bilateral CHL, some combined with mild-to-moderate SNHL Number of participants: seven Sample attrition/dropout: one did not complete questionnaires Inclusion/exclusion criteria for study entry: NR |
Primary and secondary outcomes: sound field thresholds using narrow-band noise; speech audiometry in quiet and noise; APHAB questionnaire (data not presented in paper) Method of assessing outcomes: All speech materials were pre-recorded in German, presented to the participant who was sat in between two loudspeakers, placed just off one diagonal axis Speech audiometry in quiet: speech recognition thresholds in quiet (levels required for 50% speech understanding) measured using Freiburger two-digit numbers; the percentage of correctly repeated words at 50, 65 and 80 dB SPL measured using Freiburger monosyllabic words Speech audiometry in noise used Basler sentence test, speech was presented at 70 dB and the SNR in dB, at which 50% of the key words were understood correctly, was measured. The speech signal was emitted from a loudspeaker in front of the listener and noise was emitted either from the same direction or from the back (180˚) Length of follow-up: unaided and Compact assessed at month 0. Then 3 months use with Divino. Divino and Compact assessed at 3 months |
| Characteristics of participants | |||
| Age, years, mean (range) | 48.6 (19–66) | ||
| Sex (M : F) | 3 : 4 | ||
| No other hearing aid used in contralateral ear | |||
|
Comments: five used a Compact, two had experience with a Compact but were regular users of a Classic 300. At least 2 years use with BAHA Compact or Classic 300 prior to study AC and BC thresholds in both ears presented for individual patients in a figure (not data extracted). PTA ‘yielded essentially the same results at 0 months and 3 months’ [average of the differences: AC 0.3 dB (SD 5.0); BC –1.2 dB (SD 4.2)] |
|||
| Results | |||
| Outcomes | Unaided (0 months) | Divino (3 months) | p-value |
| Average improvement in sound field thresholds over all frequencies compared with unaided, dB | 28.0 | p < 0.0001 vs unaided | |
| Speech recognition thresholds in quiet using two-digit numbers, dBa | 54 | 23 | NR |
| Speech recognition thresholds in quiet using two-digit numbers: average improvement between unaided and Compact at 0 months, and between unaided and Compact or Divino at 3 months = 29.0–30.3 dB, p = 0.016 | |||
| Unaided (0 months) | Divino (3 months) | p-value | |
| Speech recognition scores for monosyllabic words in quiet,% correcta | 50 dB SPL: 5 | 50 dB SPL: 45 | |
| 65 dB SPL: 15 | 65 dB SPL: 90 | ||
| 80 dB SPL: 50 | 80 dB SPL: 95 | ||
| Comments: the average gain in speech understanding over all presentation levels (50–80 dB) is 52%. The improvement is statistically significant (p < 0.001) for each of the three aided conditions (Compact 0 months, Compact 3 months, Divino 3 months) | |||
| Unaided (0 months) | Divino (3 months) | p-value | |
| Speech recognition threshold in noise (noise presented from front or back), dBa | Front: 12 | Omnidirectional mode | |
| Back: 9 | Front: 3 | ||
| Back: 3 | |||
| Directional mode | |||
| Front: 4 | |||
| Back: 1 | |||
| Comments: Compact and Divino (omnidirectional and directional mode) had significantly better speech recognition threshold in noise than unaided, when noise arrives from both the front and rear (average improvement of 8.7–12.0 dB, p = 0.03 for all comparisons) | |||
| Methodological comments | |||
|
|||
| General comments | |||
|
|||
Quality assessment for primary studies
| A. Selection bias | |||||
|---|---|---|---|---|---|
| 1. Are the individuals selected to participate in the study likely to be representative of the target population? | Very likely |
Somewhat likely x |
Not likely | Can’t tell | |
| 2. What percentage of selected individuals agreed to participate? | 80–100% | 60–79% | < 60% | Not applicable |
Can’t tell x |
| Summary of selection bias (methodological strength of study) | Strong | Moderate |
Weak x |
||
| B. Study design | |||||
| 1. What was the study design? (Please tick appropriate and specify design in No. 7) | RCT | ||||
| Controlled clinical trial | |||||
| Cohort analytic (two group pre and post) | |||||
| Case–control | |||||
| Cohort [one group pre and post (before and after)] | x reviewer’s opinion | ||||
| Interrupted time series | |||||
| Other – specify | |||||
| Can’t tell | |||||
| 2. Was the study described as randomised? | Yes |
No x |
|||
| If answer to No. 2 is no, go to section on Section C Confounders. If answer yes, answer No. 3 and No. 4 below | |||||
| 3. If answer was yes, was the method of randomisation described? | Yes | No | |||
| 4. If answer was yes, was the method appropriate? | Yes | No | |||
| Summary of study design (methodological strength of study) | Strong | Moderate |
Weak x |
||
| C. Confounders | |||||
|
• If there are two groups included in the study: ‘are confounders reported AND controlled for in the analysis?’ • If there is one group of participants in the study: ‘are potential confounding variables reported? |
Yes |
No x |
Can’t tell | ||
| Summary of confounders (methodological strength of study) | Strong | Moderate |
Weak x |
||
| D. Blinding | |||||
| 1. Was the outcome assessor aware of the intervention or exposure status of participants? |
Yes x |
No | Can’t tell | ||
| 2. Were the study participants aware of the research question? |
Yes x |
No | Can’t tell | ||
| Summary of blinding (methodological strength of study) | Strong | Moderate |
Weak x |
||
| E. Data collection methods | |||||
| 1. Were data collection tools shown to be valid? |
Yes x |
No | Can’t tell | ||
| 2. Were data collection tools shown to be reliable? |
Yes x |
No | Can’t tell | ||
| Summary of data collection (methodological strength of study) |
Strong x |
Moderate | Weak | ||
| F. Withdrawals and dropouts | |||||
| 1. Were withdrawals and dropouts reported in terms of numbers and reasons per group? | Yes | No |
Can’t tell x |
||
| 2. Indicate the percentage of participants completing the study (if the percentage differs by groups, record the lowest) | 80–100% | 60–79% | < 60% |
Can’t tell x |
|
| Summary of withdrawals and dropouts (methodological strength of study) | Strong | Moderate |
Weak x |
||
| G. Intervention integrity | |||||
| 1. Was the consistency of the intervention measured? | Yes |
No x |
Can’t tell | ||
| H. Analysis | |||||
| 1. Are the statistical methods appropriate for the study design? |
Yes x |
No | Can’t tell | ||
| 2. Does the study report how missing data are dealt with in the analysis? | Yes |
No x |
Can’t tell | ||
|
Global rating for study a (Overall methodological strength of study – based on sections A–F) |
Strong | Moderate |
Weak x |
||
Appendix 10 Data extraction: unilateral versus bilateral BAHAs
| Reference and design | Intervention | Participants and outcome measures | ||
|---|---|---|---|---|
|
Netherlands Design: audiological comparison study Study setting: clinic Number of centres: one Funding: Entific Audiological comparison study |
|
Participants Indication for treatment: contraindication to ACHA due to either recurrent otorrhoea or otitis externa, or to congential aural atresia Number of participants: 25 Only nine participants undertook the binaural masking level difference (BMLD) assessments Sample attrition/dropout: NR Inclusion/exclusion criteria for study entry: all bilaterally fitted patients from the clinic; at least 3 months’ experience with two BAHAs. Initial series of participants had to have symmetry of BC thresholds (average BC thresholds across 0.5, 1.0, 2.0 and 4.0 kHz to not differ by more than 10 dB; thresholds at individual frequencies should lie within 15 dB) but criterion of symmetry relaxed slightly for latter phase of the study (no details of the new criterion or the numbers of participants) Outcome measures Primary outcomes: directional hearing, speech reception in quiet and noise, SRT, BMLD Method of assessing outcomes: directional hearing with either seven or nine loudspeakers arranged in a circle with a 1-metre radius, distributed with 30° intervals, spanning 180° or 240°. One placed to the front and three (or four) to the left and right. Stimuli consisted of 1-second noise bursts. The BAHA volume control was kept in the position used by the patient in everyday life. All stimuli were presented two or three times to estimate test–retest reliability. To obtain equivalent results for unilateral left- and right-sided fittings, data from right sided fitting were mirrored to left-sided fittings before pooling results Speech recognition was measured with the sentence material of Plomp and Mimpen91 with a female speaker and Smoorenburg96 with a male speaker. Each sentence contained eight or nine syllables and was representative of everyday speech In quiet, the speech material was presented in front of the participant, in noise, speech presented at the front, with masking noise at + 90° or –90° at baffle side (side participant used unilateral BAHA) or shadow side (opposite side) Sounds were presented at 65 dB(A) [except for one patient, 60 dB(A) was used as 65 dB(A) was ‘too loud’] SRT used the adaptive one-up one-down procedure of Plomp and Mimpen to determine the presentation level, to provide a whole-sentence correct score of 50%. Each list contained 13 sentences; first three to obtain an initial estimate of the SRT, the next 10 were averaged to produce the SRT for the condition. Participants had to repeat the sentences as accurately as possible. Effects of quiet and noise also tested. SNR = value relative to the noise level, where better performance equals a more negative SNR. A 1 dB change in SRT corresponds to a change in score of about 15% for normally hearing listeners. Both stimulus conditions and sentence lists were varied to a counterbalanced design. In the quiet conditions, four lists uttered by the male speaker were used, and in the noise conditions, eight lists by the female speaker were used. Noise conditions were measured twice to allow for test–retest estimation BMLD stimuli were generated by an audiometer, the output of which was connected to the external input of the BAHA 300 (two ‘matched’ devices were used which had been checked by Entific for phase and amplitude characteristics). BMLDs were measured with pure-tones of 125, 250, 500 and 1000 Hz masked by 1/3 octave bands of filtered white noise centred at the stimulus frequency. Thresholds obtained for (1) in-phase tone stimuli and noise bands (S0N0); (2) 180° out-of-phase tone stimuli and in-phase noise bands (SπN0); and (3) in-phase tone stimuli and 180° out-of-phase noise bands (S0Nπ). Noise bands were presented at the participants most comfortable level. The tone stimulus had a rhythmic pattern with symmetric on and off intervals of 0.5 seconds. Detection thresholds were measured with manual procedures employing 1 dB steps and measured twice for test–retest reliability. The level difference between S0N0 and SπN0 was taken as the BMLD estimate. The BAHA volume controls were kept at their maximum value Length of follow-up: same day. Two 45-minute testing sessions with a break in between |
||
| Characteristics of participants | ||||
| BAHA | ||||
| Age, years mean (standard deviation, SD), rangea | 44.3 (16.3) 12–74 | |||
| Sex (M : F) | 14 : 11 | |||
| Diagnosis |
Six congenital atresia (four with Treacher Collins syndrome) of which five had bilateral aural atresia. Four of these have been published previously85 Nineteen recurrent otorrhoea [eight with cholesteatoma; 10 with chronic otitis (externa); one with cheilognato-palato schisis]. Three of these have been published previously147 All of the cholesteatoma patients had a previous radical mastoidectomy |
|||
| BAHA experience, unilateral, mean (SD) months, rangea | 49.1 (26.2), 18–105 | |||
| BAHA experience, bilateral, mean (SD) months, rangea | 13.6 (9.2), 3–39 | |||
| PTA (PTA) at 0.5, 1.0 and 2.0 kHz, dB HL, first side, air conduction (AC), mean (SD), rangea | 59.5 (13.7), 32–82 | |||
| PTA at 0.5, 1.0 and 2.0 kHz, dB HL, first side, BC, mean (SD), rangeb | 21.0 (10.7), –5–36 | |||
| PTA at 0.5, 1.0 and 2.0 kHz, dB HL, second side, AC, mean (SD), rangea | 63.6 (10.9), 38–82 | |||
| PTA at 0.5, 1.0 and 2.0 kHz, dB HL, second side, BC, mean (SD), rangeb | 21.9 (12.4), –8–48 | |||
| Results | ||||
| Directional hearing at 500 Hz, %c n = 25 | Unilateral | Bilateral | p-value unilateral vs bilateral | |
| Correct localisation | 23 | 42d | Across all observations p < 0.001 | |
| Localisation within 30° | 56 | 90d | ||
| Lateralisation | 54 | 85d | ||
| Directional hearing at 2 kHz, %c n = 25 | ||||
| Correct localisation | 24 | 45d | ||
| Localisation within 30° | 58 | 89d | ||
| Lateralisation | 64 | 87d | ||
|
Comments: the effect of stimulus frequency was not statistically significant, p > 0.1 Paper also states that many participants had difficulty localising sound with one BAHA, that all sounds appeared to come from the fitted side. The bias of responding to the fitted (baffle) side is shown only when aggregating individual response matrices to a group matrix. With unilateral fittings, 75.3% and 70.3% of the responses (for the 500 Hz and 2 kHz stimuli, respectively) correspond to the fitted side. With bilateral fittings, the response patterns are more symmetrical (45.7% and 48.8% of responses corresponded to the fitted side, respectively) Also reports data for a subgroup of participants with congenital atresia (n = 6) but not extracted here |
||||
| Speech recognition n = 25 | Unilateral | Bilateral | p-value | |
| SRT in quiet [dB(A)] | 41.5 | 37.5 | p < 0.001 | |
| Speech-to-noise ratio [dB(A], noise from the baffle side | –0.7 | –3.2 | p < 0.001 | |
| Speech-to-noise ratio [dB(A)], noise from shadow side | –3.4 | –4.0 | p > 0.05 | |
|
Comments: standard error based on test–retest data also presented in a figure but not estimated here Also reports data for a subgroup of participants with congenital atresia (n = 6) but not extracted here |
||||
| BMLD SNR, (n = 9)c | Condition: S0N0 | Condition: SπN0 | Condition: S0Nπ | p-value |
|
125 Hz 250 Hz 500 Hz 1 kHz |
2.2 0.1 0.4 0.4 |
–3.8 –6.0 –5.9 –3.3 |
–3.7 –5.1 –3.9 –4.9 |
p < 0.001 at 125, 250 and 500 Hz. Not significant (p > 0.05) at 1 kHz |
| Comments: text states that in the SπN0 condition the SNR’s (‘release from masking’) were 6.1, 6.0, 6.6 and 4.1 for the frequencies 125, 250, 500 and 1000 Hz respectively; however, the figure does not appear to support this | ||||
| Methodological comments | ||||
|
||||
| General comments | ||||
|
||||
Quality assessment for primary studies (modified for BAHAs)
| A. Selection bias | |||||
|---|---|---|---|---|---|
| 1. Are the individuals selected to participate in the study likely to be representative of the target population? | Very likely |
Somewhat likely x |
Not likely | Can’t tell | |
| 2. What percentage of selected individuals agreed to participate? |
80–100% x |
60–79% | < 60% | Not applicable | Can’t tell |
| Summary of selection bias (methodological strength of study) | Strong |
Moderate x |
Weak | ||
| B. Study design | |||||
| 1. What was the study design? (Please tick appropriate and specify design in No. 7) | RCT | ||||
| Controlled clinical trial | |||||
| Cohort analytic (two group pre and post) | |||||
| Case–control | |||||
| Cohort [one group pre and post (before and after)] | |||||
| Interrupted time series | |||||
| Other – specify: audiological comparison study | x reviewer’s opinion | ||||
| Can’t tell | |||||
| 2. Was the study described as randomised? | Yes | No x | |||
| If answer to No. 2 is no, go to section on Section C Confounders. If answer yes, answer No. 3 and No. 4 below | |||||
| 3. If answer was yes, was the method of randomisation described? | Yes | No | |||
| 4. If answer was yes, was the method appropriate? | Yes | No | |||
| Summary of study design (methodological strength of study) | Strong | Moderate |
Weak x |
||
| C. Confounders | |||||
|
• If there are two groups included in the study: ‘are confounders reported AND controlled for in the analysis?’ • If there is one group of participants in the study: ‘are potential confounding variables reported?’ |
Yes |
No x |
Can’t tell | ||
| Summary of confounders (methodological strength of study) | Strong | Moderate |
Weak x |
||
| D. Blinding | |||||
| 1. Was the outcome assessor aware of the intervention or exposure status of participants? |
Yes x |
No | Can’t tell | ||
| 2. Were the study participants aware of the research question? |
Yes x |
No | Can’t tell | ||
| Summary of blinding (methodological strength of study) | Strong | Moderate |
Weak x |
||
| E. Data collection methods | |||||
| 1. Were data collection tools shown to be valid? |
Yes x |
No | Can’t tell | ||
| 2. Were data collection tools shown to be reliable? |
Yes x |
No | Can’t tell | ||
| Summary of data collection (methodological strength of study) |
Strong x |
Moderate | Weak | ||
| F. Withdrawals and dropouts | |||||
| 1. Were withdrawals and dropouts reported in terms of numbers and reasons per group? | Yes | No |
Can’t tell x |
||
| 2. Indicate the percentage of participants completing the study (if the percentage differs by groups, record the lowest) | 80–100% | 60–79% | < 60% |
Can’t tell x |
|
| Summary of withdrawals and dropouts (methodological strength of study) | Strong | Moderate |
Weak x |
||
| G. Intervention integrity | |||||
| 1. Was the consistency of the intervention measured? | Yes | No |
Can’t tell x |
||
| H. Analysis | |||||
| 1. Are the statistical methods appropriate for the study design? | Yes |
No x |
Can’t tell | ||
| 2. Does the study report how missing data are dealt with in the analysis? | Yes |
No x |
Can’t tell | ||
|
Global rating for study a (Overall methodological strength of study – based on sections A–F) |
Strong | Moderate |
Weak x |
||
| Reference and design | Intervention | Participants | Outcome measures |
|---|---|---|---|
|
Dutt et al. 200286 UK Design: audiology comparison study Study setting outpatient: Number of centres: one Funding: NR |
|
Indication for treatment: unilateral BAHA users whose professional needs warranted binaural hearing Number of participants: n = 15 with bilateral BAHAs; n = 12 eligible; n = 11 participated Sample attrition/dropout: one chose not to participate Inclusion/exclusion criteria for study entry: used second-side BAHA for at least 12 months. Paper states criteria not stringent: previous knowledge and experience with binaural hearing (conventionally aided or unaided); bilaterally symmetrical hearing loss (interaural threshold difference of < 15 dB four-time average); professional needs of users, e.g. businessmen, teachers, nurses; motivation – voluntarily applied for a second-side BAHA; age (limited to adults) |
Primary outcomes: speech recognition in quiet and in noise; modified Plomp test Method of assessing outcomes: right, left and bilateral BAHAs evaluated Unaided sound field levels [dB(A)] and aided thresholds Sound field speech used Arthur Boothroyd word lists Speech-in-quiet and speech-in-noise evaluated with BKB sentences, at SNRs of + 10 dB, 0 dB and –10 dB Modified Plomb Multitalker Noise Test used to evaluate speech-in-noise with open-set speech recognition. Three speakers used. Speech presented from front speaker at 70 dB(A). Speech babble noise (20 talkers/cocktail party noise) presented from left or right at a SNR of 0 dB Length of follow-up: audiological evaluations undertaken at same session |
| Characteristics of participants (n = 12) | |||
| Age, years, mean (SD), rangea | 42.3 (10), 22–54 | ||
| Sex (M : F) | 3 : 9 | ||
| Duration with one BAHA, years, mean (SD), rangea | 6.3 (3.2), 3–12 | ||
| Duration with two BAHAs, years, mean (SD), rangea | 2.2 (1.1), 1–5 | ||
| Diagnosis | Treacher Collins syndrome (2) | ||
| Bilateral mastoid cavities (3) | |||
| Bilateral CON | |||
| Bilateral chronic otitis media (3) | |||
| Goldenhar syndrome | |||
| Bilateral microtia | |||
| Bilateral acquired otosclerosis | |||
| Results (n = 11) | |||
| Outcomes | Best-unilateral response | Bilateral | p-value |
| Speech-in-quiet – cumulative Arthur Boothroyd word (30 words) list scoresb at: | |||
| 30 dB intensity levels | 1 | 5 | |
| 40 dB intensity levels | 13 | 19 | |
| 50 dB intensity levels | 20 | 24 | |
| 60 dB intensity levels | 25 | 28 | |
| 70 dB intensity levels | 27 | 29 | |
| 80 dB intensity levels | 30 | 30 | |
| Speech-in-quiet (BKB sentences) | Data not presented. All 11 patients scored 100% with right, left and bilateral BAHAs | ||
| Speech-in-noise – cumulative BKB sentence scoresb at: | Best-unilateral response | Bilateral | p-value |
| + 10 SNR | 99 | 100 | |
| Zero SNR | 80 | 81 | |
| – 10 SNR | 0 | 1 | |
| Plomp test,% correct score [mean (SD), range]:a | Left BAHA | Right BAHA | Bilateral BAHA |
| Sound front, noise front | 76 (11.7), 56–93 | 77.3 (11.7), 58–90 | 82.4 (13.3), 60–97 |
| Sound front, noise left | 40.1 (25.3), 2–71 | 84.1 (11.2), 55–97 | 71.1 (14.9), 44–95 |
| Sound front, noise right | 88.2 (9.0), 72–100 | 45.8 (22.1), 13–88 | 79.5 (11.6), 58–93 |
| Methodological comments | |||
|
|||
| General comments | |||
|
|||
Quality assessment for primary studies
| A. Selection bias | |||||
|---|---|---|---|---|---|
| 1. Are the individuals selected to participate in the study likely to be representative of the target population? | Very likely | Somewhat likely |
Not likely x |
Can’t tell | |
| 2. What percentage of selected individuals agreed to participate? |
80–100% x |
60–79% | < 60% | Not applicable | Can’t tell |
| Summary of selection bias (methodological strength of study) | Strong | Moderate |
Weak x |
||
| B. Study design | |||||
|
1. What was the study design? (Please tick appropriate and specify design in No. 7) |
RCT | ||||
| Controlled clinical trial | |||||
| Cohort analytic (two group pre and post) | |||||
| Case–control | |||||
| Cohort [one group pre and post (before and after)] | |||||
| Interrupted time series | |||||
| Other – specify: audiology comparison study | x reviewer’s opinion | ||||
| Can’t tell | |||||
| 2. Was the study described as randomised? | Yes |
No x |
|||
| If answer to No. 2 is no, go to section on Section C Confounders. If answer yes, answer No. 3 and No. 4 below | |||||
| 3. If answer was yes, was the method of randomisation described? | Yes | No | |||
| 4. If answer was yes, was the method appropriate? | Yes | No | |||
| Summary of study design (methodological strength of study) | Strong | Moderate |
Weak x |
||
| C. Confounders | |||||
|
• If there are two groups included in the study: ‘are confounders reported AND controlled for in the analysis?’ • If there is one group of participants in the study: ‘are potential confounding variables reported?’ |
Yes |
No x |
Can’t tell | ||
| Summary of confounders (methodological strength of study) | Strong | Moderate |
Weak x |
||
| D. Blinding | |||||
| 1. Was the outcome assessor aware of the intervention or exposure status of participants? |
Yes x |
No | Can’t tell | ||
| 2. Were the study participants aware of the research question? |
Yes x |
No | Can’t tell | ||
| Summary of blinding (methodological strength of study) | Strong | Moderate |
Weak x |
||
| E. Data collection methods | |||||
| 1. Were data collection tools shown to be valid? |
Yes X |
No | Can’t tell | ||
| 2. Were data collection tools shown to be reliable? |
Yes x |
No | Can’t tell | ||
| Summary of data collection (methodological strength of study) |
Strong x |
Moderate | Weak | ||
| F. Withdrawals and dropout | |||||
| 1. Were withdrawals and dropouts reported in terms of numbers and reasons per group? |
Yes x |
No | Can’t tell | ||
| 2. Indicate the percentage of participants completing the study (if the percentage differs by groups, record the lowest) |
80–100% x (11/12) |
60–79% | < 60% | Can’t tell | |
| Summary of withdrawals and dropouts (methodological strength of study) |
Strong x |
Moderate | Weak | ||
| G. Intervention integrity | |||||
| 1. Was the consistency of the intervention measured? | Yes | No |
Can’t tell x |
||
| H. Analysis | |||||
| 1. Are the statistical methods appropriate for the study design? | Yes |
No x |
Can’t tell | ||
| 2. Does the study report how missing data are dealt with in the analysis? | Yes |
No x |
Can’t tell | ||
|
Global rating for study a (Overall methodological strength of study – based on sections A–F) |
Strong | Moderate |
Weak x |
||
| Reference and design | Intervention | Participants | Outcome measures | |
|---|---|---|---|---|
|
Priwin et al. 200487 Sweden Design: audiological comparison study Study setting: secondary care Number of centres: one Funding: in part by Swedish Research Council for Enginerring Sciences (TFR 299–2000–576) |
|
Indication for treatment: combined symmetric or slight asymmetric sensorineural and conductive hearing level, and primarily conductive hearing level Number of participants: 12 Sample attrition/dropout: assume none Inclusion/exclusion criteria for study entry: all patients fitted with bilateral BAHAs at ENT clinic at least 1 year before study |
Primary outcomes: directional hearing; SRT; binaural hearing Method of assessing outcomes: BAHAs were electronically controlled by research personnel without patient’s knowledge. Tests randomised and patients blinded to unilateral or bilateral use of BAHAs Pure AC (using earphones) and BC tone thresholds (using bone transducer) were measured using standard audiometric procedures and equipment. Details reported. 12 loudspeakers spaced at 30° intervals placed in a circle with a 1-m radius from patient and at height equivalent to head of sitting patient. Free-field warble tones recorded and presented using the Békésy sweep method, thresholds tested at four directions (front, left, right and behind) Directional hearing: same speaker set up as tone thresholds. Narrow-band (1/3 octave) noise centred at 0.5 or 2.0 kHz presented at 65 dB HL for 1-second duration. Three BAHA options tested: unilateral on best side (usually the aid first implanted), unilateral on shadow side (opposite side) and bilateral. Stimuli presented three times from each speaker and for each option, according to a randomised sequence of three presentations from each speaker. Data presented visually in figure (not possible to extract data) and also presented as correct score or within 30° of stimulation angle SRTs: measured in quiet and noise with phonetically balanced three-word sentences extracted from Hagerman. 97 Each test list comprised of 10 three-word sentences, presented by female voice. Aim was to find the noise level giving a 50% correct score. Three lists (one practice and two test lists) presented for two BAHA options: unilateral on best side and bilateral. Test list randomised among patients. Speech presented at 0° For SRT in noise, noise was speech weighted, presentation at either + 90°, –90° or from all 11 remaining loudspeakers simultaneously (with practice and test lists and for unilateral and bilateral, as noted above) BMLD test: sensitive to proving existence of binaural hearing, carried out with bilateral BAHAs. A pure-tone signal is presented in noise and the task is to detect tone. Details reported. Test conducted at 0.25, 5.00 and 1.00 kHz, combined with a narrow and noise centred on the corresponding signal frequency at 65 dB hearing level Length of follow-up: unilateral BAHAs and bilateral BAHAs tested at same session |
|
| Characteristics of participants | ||||
| Age, mean (SD), range, yearsa | 51.7 (13.3), 27–68 | |||
| Sex (M : F) | 3 : 9 | |||
| Chronic otitis, further underlying etiology unknown, number of patients | 8/12 | |||
| Recurrent external otitis and otosclerosis, number of patients | 1/12 | |||
| Congenital ear canal atresia, number of patients | 3/12 (one part of Treacher Collins syndrome) | |||
| Duration with unilateral BAHAs at time of study, mean (SD), range, yearsa | 14.3 (4.1), 5.8–21.0 | |||
| Duration with bilateral BAHAs at time of study, mean (SD), range, yearsa | 6.8 (6.0), 1.0–19.6 | |||
| Use of bilateral BAHAs, number of patients | Daily: 11/12 | |||
| Occasionally: 1/12 | ||||
| BAHA model, number of patients | Compact in both ears: 4 | |||
| Classic 300 in both ears: 7 | ||||
| Compact and Classic 300: 1 | ||||
| Pure-tone average thresholds of frequencies 0.5, 1.0 and 2.0 kHz [mean (SD)],a range, dB HL for: | ||||
| AC (first fitted side) | 58.3 (15.3), 38–87 | |||
| BC (first fitted side) | 29.8 (15.2), 8–53 (in three patients, at one or more frequency no fixed value was attained, highest measurable value used for mean) | |||
| AC (second fitted side) | 59 (20.7), 27–102 | |||
| BC (second fitted side) | 30.9 (13.4), 7–50 | |||
| Symmetric BC thresholds (difference ≤ 10 dB), number of patients | 10/12 | |||
| Asymmetric BC thresholds, number of patients | 2/12 (although did not differ by > 20 dB) | |||
| Results | ||||
| Improvement of free sound field tone thresholds ( n = 12) | ||||
|
Comments: data presented in figure for sound presented at front, best side, shadow side and behind, for frequencies 0.25, 1.00, 1.50, 2.00, 3.00, 4.00, 6.00 and 8.00 kHz. Data not estimated and extracted by reviewer When sound presented in front, at best side and from behind patient, the average improvement with BAHAs fitting was between 2 and 7 dB (at 0.25–8.00 kHz) When sound presented at shadow side, the average improvement with bilateral BAHAs was between 5 and 15 dB (at 0.25–8.00 kHz) States that the results differed greatly among the patients, that the SD of the improvement (data not presented) was almost as great as the average improvement, and that consequently the SD of the improvement when sound was at the shadow side was greater than the other three sides |
||||
| Directional hearing | Unilateral at best side | Unilateral at shadow side | Bilateral | p-value |
| Score (% of correct answers) b | ||||
| 0.5 kHz | 12 | 11 | 25 | |
| 2.0 kHz | 8 | 10 | 23 | |
| (Chance level for correct answer 8.3%) | ||||
| Score (% of answers within 30° of correct response) b | ||||
| 0.5 kHz | 23 | 30 | 53 | |
| 2.0 kHz | 28 | 27 | 51 | |
| (Chance level for correct answer 25%) | ||||
| Comments: states that with a unilateral BAHA, results are close to change level, but ‘there is a significant increase in the ability to localise the sound source’ with bilateral BAHAs | ||||
| Unilateral at best side | Bilateral | p-value | ||
| Speech recognition in quiet, average threshold, dB HL | 38.7 | 33.3 | p = 0.001 | |
| Speech-in-noise | Difference between bilateral and unilateral BAHAs | |||
| Masking noise presented: | ||||
| At best side | 3.1 dB improvement in SNR with bilateral | |||
| At shadow side | 1.0 dB deterioration in SNR with bilateral | |||
| As surrounding noise (remaining 11 speakers) | 2.8 dB improvement in SNR with bilateral | |||
| BMLD (relative threshold change in dB from the condition ‘signal and noise in phase at both sides’) | Bilateral BAHAs | |||
| 0.25 kHz: | ||||
| Signal 180° out of phase and noise in phase | Threshold changes within 3 dB except for two patients. | |||
| Signal in phase and noise 180° out of phase | Threshold changes between –18 and 3 dB, mean –5 dB. | |||
| 0.5 kHz: | ||||
| Signal 180° out of phase and noise in phase | Average threshold change 2 dB | |||
| Signal in phase and noise 180° out of phase | Average threshold change –4 dB | |||
| 1 kHz: | ||||
| Signal 180° out of phase and noise in phase | Average threshold change 3 dB | |||
| Signal in phase and noise 180° out of phase | Average threshold change –3 dB | |||
| Methodological comments | ||||
|
||||
| General comments | ||||
|
||||
Quality assessment for primary studies
| A. Selection bias | |||||
|---|---|---|---|---|---|
| 1. Are the individuals selected to participate in the study likely to be representative of the target population? | Very likely |
Somewhat likely x |
Not likely | Can’t tell | |
| 2. What percentage of selected individuals agreed to participate? |
80–100% x |
60–79% | < 60% | Not applicable | Can’t tell |
| Summary of selection bias (methodological strength of study) | Strong |
Moderate x |
Weak | ||
| B. Study design | |||||
| 1. What was the study design? (Please tick appropriate and specify design in No. 7) | RCT | ||||
| Controlled clinical trial | |||||
| Cohort analytic (two group pre and post) | |||||
| Case–control | |||||
| Cohort [one group pre and post (before and after)] | |||||
| Interrupted time series | |||||
| Other – specify: audiological comparison study | x reviewer’s opinion | ||||
| Can’t tell | |||||
| 2. Was the study described as randomised? | Yes |
No x |
|||
| If answer to No. 2 is no, go to section on Section C Confounders. If answer yes, answer No. 3 and No. 4 below | |||||
| 3. If answer was yes, was the method of randomisation described? | Yes | No | |||
| 4. If answer was yes, was the method appropriate? | Yes | No | |||
| Summary of study design (methodological strength of study) | Strong | Moderate |
Weak x |
||
| C. Confounders | |||||
|
• If there are two groups included in the study: ‘are confounders reported AND controlled for in the analysis?’ • If there is one group of participants in the study: ‘are potential confounding variables reported?’ |
Yes |
No x |
Can’t tell | ||
| Summary of confounders (methodological strength of study) | Strong | Moderate |
Weak x |
||
| D. Blinding | |||||
| 1. Was the outcome assessor aware of the intervention or exposure status of participants? |
Yes x |
No | Can’t tell | ||
| 2. Were the study participants aware of the research question? |
Yes x |
No | Can’t tell | ||
| Summary of blinding (methodological strength of study) | Strong | Moderate |
Weak x |
||
| E. Data collection methods | |||||
| 1. Were data collection tools shown to be valid? |
Yes x |
No | Can’t tell | ||
| 2. Were data collection tools shown to be reliable? |
Yes x |
No | Can’t tell | ||
| Summary of data collection (methodological strength of study) |
Strong x |
Moderate | Weak | ||
| F. Withdrawals and dropout | |||||
| 1. Were withdrawals and dropouts reported in terms of numbers and reasons per group | Yes | No |
Can’t tell x |
||
| 2. Indicate the percentage of participants completing the study (if the percentage differs by groups, record the lowest) | 80–100% | 60–79% | < 60% |
Can’t tell x |
|
| Summary of withdrawals and dropouts (methodological strength of study) | Strong | Moderate |
Weak x |
||
| G. Intervention integrity | |||||
| 1. Was the consistency of the intervention measured? | Yes | No |
Can’t tell x |
||
| H. Analysis | |||||
| 1. Are the statistical methods appropriate for the study design? |
Yes x |
No | Can’t tell | ||
| 2. Does the study report how missing data are dealt with in the analysis? | Yes | No |
Can’t tell x |
||
|
Global rating for study a (Overall methodological strength of study – based on sections A–F) |
Strong | Moderate |
Weak x |
||
| Reference and design | Intervention | Participants | Outcome measures | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Priwin et al. 200759 Sweden Design: audiological comparison study Study setting: ENT clinic and audiologic units Number of centres: two Funding: Acta Oto-laryngologica foundation |
|
Indication for treatment: children with bilateral CHL Number of participants: n = 9a (bilateral BAHA, n = 6; unilateral BAHA, n = 3) Sample attrition/dropout: none reported. One patient did not complete questionnaire Inclusion/exclusion criteria for study entry: patients aged 6–18 years with bilateral CHL and fitted unilaterally or bilaterally with BAHAs. Appropriate school attendance and proficiency in Swedish for age |
Outcomes: tone thresholds; speech recognition in noise; localisation of sound; questionnaires [MAIS and MUSS (validated), IOI-HA (validated)] Method of assessing outcomes: all participants were tested in a soundproof booth, with stimuli of sound presented through headphones, bone conductors, and/or loudspeakers. Five speakers were placed at 45° intervals in a frontal semicircle at head-height, with patients facing frontal speaker Tone thresholds were presented as PTA (M4) for the frequencies 0.5, 1.0, 2.0 and 4.0 kHz. Patients with one aid were tested with and without it; patients with two aids were tested without aid, with both BAHAs and unilaterally on their best side. Sound field thresholds were considered clinically normal at 20 dB HL Speech recognition in noise was measured with phonemically balanced Swedish three-word sentences (both speech and noise were presented from front speaker). Sentences were extracted from five-word sentence test97 with first two words removed. After a practice list, test included two lists of 10 three-word sentences (60 words) for three different speech/noise ratios (0, 4 and 6 dB). Speech material was CD pre-recorded (female voice) and had a with fixed SNRs and modulated noise, speech presentation level set at 60 dB SPL. Noise test was presented at 60, 56 and 54 dB SPL for all patients with one to three hearing options depending on hearing aid use; (1) no amplification, (2) unilateral BAHA and (3) bilateral BAHA Localisation of sound (five loudspeakers set up): all loudspeakers were numbered and marked in different colours. A narrowband (1/3 octave) noise centred at 0.5 or 3.0 kHz was presented at 50 and 60 dB SPL for 1 second. Stimuli was presented three times from each speaker at each presentation level for each hearing option in a randomised sequence and patients had to identify the speaker presenting the sound IOI-HA to assess hearing aid outcome: seven domains (daily use, benefit, residual activity limitations, satisfaction, residual participation restrictions, impact on others and quality of life, QoL) scored 1–5 (worst to best outcome). Youngest patients allowed some assistance from parent. When comparing group data, mean scores < 3.5 indicate poor habilitation outcome in patients with mild-to-moderate hearing impairment unaided and < 3.6 in patients with moderate-to-severe hearing impairment MAIS and MUSS: 21 questions dealing with hearing and communication (scored 1–5, never to always) completed by children’s guardian and their usual classroom teacher independently. Outcomes were then thematically grouped into hearing aid use, reaction to sounds, sound discrimination, verbal communication and speech intelligibility Length of follow-up: none reported, tests appear to have been undertaken at same session |
|||||||
| Characteristics of participants | ||||||||||
| Unilateral BAHA (n = 6) | Bilateral BAHA (n = 3) | p-value | ||||||||
| Age, mean years (SD, range)b | 11.3 (4.0, 6–16) | 11.3 (5.5, 6–17) | ||||||||
| Sex: M : F | 3 : 3 | 3 : 0 | ||||||||
| Syndrome, number of patients: | ||||||||||
| Suspected syndrome | 1 | |||||||||
| Branchio-oto-renal syndrome | 1 | |||||||||
| Goldenhar syndrome | 1 | |||||||||
| Crouzon | 1 | |||||||||
| Treacher Collins | 1 | 1 | ||||||||
| CHARGE (not defined) | ||||||||||
| Type of malformation/surgery number of patients: | ||||||||||
| Microtia and ear canal atresia | 4c | 2 | ||||||||
| Ear canal atresia | 1 | |||||||||
| Bilateral modified radical | 1 | |||||||||
| Mastoidectomy | ||||||||||
| Diagnosis, number of patients: | ||||||||||
| Ear malformation | 5 | 2 | ||||||||
| Chronic otitis media | 1 | 1 | ||||||||
| Pure-tone thresholds, mean (SD) dB HL: | All bilateral hearing loss patients ( n = 9) | |||||||||
| AC PTA (M4) in better ear | 61.3 (15.5) | |||||||||
| BC PTA (M4) in better ear | 14.1 (12.7) | |||||||||
| AC PTA (M4) in worse ear | 72.1 (12.1) | |||||||||
| BC PTA (M4) in worse ear | 13.8 (10.7) | |||||||||
|
All experienced BAHA users with a minimum use of 3 months The majority of children with bilateral hearing loss had symmetrical maximal or near-maximal CHL |
||||||||||
| Results | ||||||||||
| Sound field average-tone thresholds, dB HLb | Unaided | One BAHA | Two BAHAs | p-value | ||||||
| Unilateral BAHA (n = 6), mean (SD, range) | 53 (15, 25–68) | 24 (5, 20–32) | N/A | p = 0.046 | ||||||
| Bilateral BAHAs (n = 3), mean (SD, range) | 62 (8, 55–70) | 30 (5, 25–35) | 25 (5, 20–30) | |||||||
| Comments: in the unilateral BAHA, thresholds were significantly improved with one BAHA compared with none, but thresholds with hearing amplification still significantly differed from the norm of 20 dB HL (p = 0.028). Trend similar in bilateral BAHA group, where fitting of a unilateral BAHA improved sound field threshold to almost normal. No extra gain was found with additional BAHA | ||||||||||
| Speech recognition in noise, median score (%)d | Unaided | One BAHA | Two BAHAs | |||||||
| S/N 0 dB | S/N 4 dB | S/N 6 dB | S/N 0 dB | S/N 4 dB | S/N 6 dB | S/N 0 dB | S/N 4 dB | S/N 6 dB | ||
| Unilateral BAHA (n = 6) | 0 | 0 | 0 | 87 | 92 | 98 | N/A | N/A | N/A | |
| Bilateral BAHA (n = 3) | 0 | 0 | 0 | 69 | 79 | 97 | 88 | 93 | 90 | |
| Comments: without hearing amplification all patients lacked open speech recognition. A trend towards lower test performance in children fitted with bilateral BAHAs when tested with one BAHA compared with two BAHAs was noted. Speech recognition ability diminished with increasing noise level, and with speech-in-noise approaching 0 dB | ||||||||||
| Localisation of soundd | Unilateral BAHA (n = 6) | Bilateral BAHAs (n = 3) | ||||||||
| 0.5 kHz | 3 kHz | 0.5 kHz | 3 kHz | |||||||
| 50 dB | 60 dB | 50 dB | 60 dB | 50 dB | 60 dB | 50 dB | 60 dB | |||
| Correct score, mean % (chance level 20%) | ||||||||||
| One BAHA | ~ 20 | ~ 28 | ~ 28 | ~ 37 | ~ 20 | ~ 20 | ~ 16 | ~ 18 | ||
| Two BAHAs | N/A | N/A | N/A | N/A | ~ 50 | ~ 50 | ~ 50 | ~ 57 | ||
| Lateralisations score, mean % (chance level 68%) | ||||||||||
| One BAHA | ~ 68 | ~ 70 | ~ 60 | ~ 72 | ~ 60 | ~ 68 | ~ 68 | ~ 56 | ||
| Two BAHAs | N/A | N/A | N/A | N/A | ~ 86 | ~ 94 | ~ 80 | ~ 96 | ||
|
Comments: authors note that scores could be obtained only with aids as the levels of stimulus presented in the speech recognition test were around 50 or 60 dB SPL, meaning that children with bilateral moderate-to-severe hearing loss received cues at subthreshold levels without their hearing amplification Sound localisation ability was poor and close to chance level with one BAHA, but there was a trend towards improved sound localisation ability in children with bilaterally fitted BAHAs. Bilateral BAHAs improved sound lateralisation ability to near normal |
||||||||||
| IOI-HA, mean (SD) | Unilateral BAHA (n = 6) | Bilateral BAHAs (n = 2e) | p-value | |||||||
| Use | 5.0 (0) | 5.0 | ||||||||
| Benefit | 5.0 (1.0) | 5.0 | ||||||||
| Residual activity | 4.2 (0.5) | 4.0 | ||||||||
| Limitation: | ||||||||||
| Satisfaction | 4.3 (1.0) | 5.0 | ||||||||
| Residual participation | 4.2 (1.3) | 3.0 | ||||||||
| Impact on others | 4.8 (0.4) | 2.5 | ||||||||
| QoL | 4.8 (0.4) | 5.0 | ||||||||
| MAIS and MUSS, mean (SD) | Unilateral BAHA (n = 6) | Bilateral BAHAs (n = 3)f | p-value | |||||||
| Hearing-aid use | 3.4 (1.3) | 4.0 | ||||||||
| Reaction to sounds | 3.1 (0.9) | 3.5 | ||||||||
| Sound discrimination | 3.5 (0.8) | 3.8 | ||||||||
| Verbal communication | 3.8 (0.6) | 3.7 | ||||||||
| Speech intelligibility | 3.1 (1.2) | 3.3 | ||||||||
| Methodological comments | ||||||||||
|
||||||||||
| General comments | ||||||||||
|
||||||||||
Quality assessment for primary studies
| A. Selection bias | |||||
|---|---|---|---|---|---|
| 1. Are the individuals selected to participate in the study likely to be representative of the target population? | Very likely |
Somewhat likely x |
Not likely | Can’t tell | |
| 2. What percentage of selected individuals agreed to participate? | 80–100% | 60–79% | < 60% | Not applicable |
Can’t tell x |
| Summary of selection bias (methodological strength of study) | Strong | Moderate |
Weak x |
||
| B. Study design | |||||
| 1. What was the study design? (Please tick appropriate and specify design in No. 7) | RCT | ||||
| Controlled clinical trial | |||||
| Cohort analytic (two group pre and post) | |||||
| Case–control | |||||
| Cohort [one group pre and post (before and after)] | |||||
| Interrupted time series | |||||
| Other – specify: audiological comparison study | x reviewer’s opinion | ||||
| Can’t tell | |||||
| 2. Was the study described as randomised? | Yes | No x | |||
| If answer to No. 2 is no, go to section on Section C Confounders. If answer yes, answer No. 3 and No. 4 below | |||||
| 3. If answer was yes, was the method of randomisation described? | Yes | No | |||
| 4. If answer was yes, was the method appropriate? | Yes | No | |||
| Summary of study design (methodological strength of study) | Strong | Moderate |
Weak x |
||
| C. Confounders | |||||
|
Two groups: are confounders reported AND controlled for in the analysis? OR if one group: are potential confounding variables reported? |
Yes |
No x |
Can’t tell | ||
| Summary of confounders (methodological strength of study) | Strong | Moderate |
Weak x |
||
| D. Blinding | |||||
| 1. Was the outcome assessor aware of the intervention or exposure status of participants? |
Yes x |
No | Can’t tell | ||
| 2. Were the study participants aware of the research question? |
Yes x |
No | Can’t tell | ||
| Summary of blinding (methodological strength of study) | Strong | Moderate |
Weak x |
||
| E. Data collection methods | |||||
| 1. Were data collection tools shown to be valid? |
Yes x |
No | Can’t tell | ||
| 2. Were data collection tools shown to be reliable? |
Yes x |
No | Can’t tell | ||
| Summary of data collection (methodological strength of study) |
Strong x |
Moderate | Weak | ||
| F. Withdrawals and dropouts | |||||
| 1. Were withdrawals and dropouts reported in terms of numbers and reasons per group? | Yes | No | Can’t tell x | ||
| 2. Indicate the percentage of participants completing the study (if the percentage differs by groups, record the lowest) | 80–100% | 60–79% | < 60% |
Can’t tell x |
|
| Summary of withdrawals and dropouts (methodological strength of study) | Strong | Moderate |
Weak x |
||
| G. Intervention integrity | |||||
| 1. Was the consistency of the intervention measured? | Yes |
No x |
Can’t tell | ||
| H. Analysis | |||||
| 1. Are the statistical methods appropriate for the study design? |
Yes x |
No | Can’t tell | ||
| 2. Does the study report how missing data are dealt with in the analysis? | Yes |
No x |
Can’t tell | ||
|
Global rating for study a (Overall methodological strength of study – based on sections A–F) |
Strong | Moderate |
Weak x |
||
Appendix 11 Inclusion/exclusion criteria for systematic review of quality of life
| Yes | Unclear | No | |
|---|---|---|---|
|
1. Before and after study, or randomised comparison (a) reporting health-state utilities (b) quality of life QoL/health-related QoL (c) reviews 1(a), 1(b) or both |
Exclude | ||
|
2. Intervention • BAHA |
|||
|
3. Population/indication • adults or children with a conductive or mixed hearing loss |
|||
|
Retrieve? If 1(a) or 1(b) = yes; 2 = yes; and 3 = yes THEN include in review |
Appendix 12 Deterministic sensitivity analyses: full results
Results for each parameter are provided on two lines – the first gives the results at the lower limit of the output parameter and the second gives the results at the upper limit.
Cost analysis
BCHA device cost = £117
DSA – paediatric cases, with treatment failures not switching to alternative hearing aid
| Parameter (base-case value) | Input value | BCHA (£) | BAHA (£) | Incremental cost per case (£) |
|---|---|---|---|---|
| Probability of bleeding within 24 hours of surgery (0.0121) | 0.00147 | 765 | 17,513 | 16,747 |
| 0.04310 | 765 | 17,518 | 16,753 | |
| Probability of surgical reduction of skin growth/soft tissue thickening around abutment (0.0848) | 0.04716 | 765 | 17,509 | 16,743 |
| 0.13826 | 765 | 17,525 | 16,760 | |
| Probability of failure to integrate (0.006) | 0.00015 | 765 | 17,513 | 16,748 |
| 0.03291 | 765 | 17,501 | 16,736 | |
| Probability of intolerable pain requiring removal of abutment and flange fixture (0.0272) | 0.00746 | 765 | 17,840 | 17,075 |
| 0.06820 | 765 | 17,445 | 16,680 | |
| Probability of re-operation (0.9474) | 0.73972 | 765 | 16,668 | 15,902 |
| 0.99867 | 765 | 17,732 | 16,966 | |
| Cost of initial ENT consultation (£131.69) | 98.77 | 765 | 17,481 | 16,715 |
| 164.61 | 765 | 17,547 | 16,781 | |
| Cost of audiological assessment (£57.48) | 43.11 | 765 | 17,499 | 16,734 |
| 71.85 | 765 | 17,528 | 16,763 | |
| Cost of ENT multiprofessional assessment (£147.36) | 110.52 | 765 | 17,477 | 16,711 |
| 184.21 | 765 | 17,551 | 16,785 | |
| Cost of day-case surgery for implantation (£4009.14) | 3006.86 | 765 | 16,141 | 15,376 |
| 5011.43 | 765 | 18,887 | 18,121 | |
| Cost of fixture and abutment (£830.00) | 622.50 | 765 | 17,338 | 16,573 |
| 1037.50 | 765 | 17,689 | 16,924 | |
| Cost of surgical consumables (£159.50) | 119.63 | 765 | 17,460 | 16,694 |
| 199.38 | 765 | 17,568 | 16,802 | |
| Cost of follow-up ENT consultations (£90.93) | 68.20 | 765 | 17,424 | 16,658 |
| 113.66 | 765 | 17,604 | 16,839 | |
| Cost of audiological consultation to fit/commission sound processor (£64.80) | 48.60 | 765 | 17,493 | 16,727 |
| 81.00 | 765 | 17,535 | 16,770 | |
| Cost of audiology follow-up in year of surgery (£50.17) | 37.62 | 765 | 17,481 | 16,715 |
| 62.71 | 765 | 17,547 | 16,781 | |
| Cost of BAHA sound processor (£2191.25) | 1820.00 | 765 | 17,147 | 16,381 |
| 2995.00 | 765 | 18,308 | 17,543 | |
| Cost of BAHA sound processor maintenance plan (£736.25) | 610.00 | 765 | 16,346 | 15,581 |
| 1000.00 | 765 | 19,953 | 19,188 | |
| Cost of surgical revision for grade 3 skin reaction (£663.66) | 497.75 | 765 | 17,459 | 16,694 |
| 829.58 | 765 | 17,569 | 16,803 |
DSA – paediatric cases, with treatment failures switching to alternative hearing aid (BCHA)
| Parameter (base-case value) | Input value | BCHA (£) | BAHA (£) | Incremental cost per case (£) |
|---|---|---|---|---|
| Probability of bleeding within 24 hours of surgery (0.0121) | 0.00147 | 765 | 17,586 | 16,820 |
| 0.04310 | 765 | 17,591 | 16,826 | |
| Probability of surgical reduction of skin growth/soft tissue thickening around abutment (0.0848) | 0.04716 | 765 | 17,582 | 16,816 |
| 0.13826 | 765 | 17,598 | 16,833 | |
| Probability of failure to integrate (0.006) | 0.00015 | 765 | 17,586 | 16,821 |
| 0.03291 | 765 | 17,583 | 16,818 | |
| Probability of intolerable pain requiring removal of abutment and flange fixture (0.0272) | 0.00746 | 765 | 17,862 | 17,097 |
| 0.06820 | 765 | 17,529 | 16,764 | |
| Probability of re-operation (0.9474) | 0.73972 | 765 | 16,819 | 16,054 |
| 0.99867 | 765 | 17,785 | 17,019 | |
| Cost of initial ENT consultation (£131.69) | 98.77 | 765 | 17,554 | 16,788 |
| 164.61 | 765 | 17,620 | 16,854 | |
| Cost of audiological assessment (£57.48) | 43.11 | 765 | 17,572 | 16,807 |
| 71.85 | 765 | 17,601 | 16,836 | |
| Cost of ENT multiprofessional assessment (£147.36) | 110.52 | 765 | 17,550 | 16,785 |
| 184.21 | 765 | 17,624 | 16,858 | |
| Cost of day-case surgery for implantation (£4009.14) | 3006.86 | 765 | 16,214 | 15,449 |
| 5011.43 | 765 | 18,960 | 18,194 | |
| Cost of fixture and abutment (£830.00) | 622.50 | 765 | 17,411 | 16,646 |
| 1037.50 | 765 | 17,762 | 16,997 | |
| Cost of surgical consumables (£159.50) | 119.63 | 765 | 17,533 | 16,767 |
| 199.38 | 765 | 17,641 | 16,875 | |
| Cost of follow-up ENT consultations (£90.93) | 68.20 | 765 | 17,497 | 16,731 |
| 113.66 | 765 | 17,677 | 16,912 | |
| Cost of audiological consultation to fit/commission sound processor (£64.80) | 48.60 | 765 | 17,566 | 16,800 |
| 81.00 | 765 | 17,608 | 16,843 | |
| Cost of audiology follow-up in year of surgery (£50.17) | 37.62 | 765 | 17,554 | 16,788 |
| 62.71 | 765 | 17,620 | 16,854 | |
| Cost of BAHA sound processor (£2191.25) | 1820.00 | 765 | 17,220 | 16,454 |
| 2995.00 | 765 | 18,381 | 17,616 | |
| Cost of BAHA sound processor maintenance plan (£736.25) | 610.00 | 765 | 16,419 | 15,654 |
| 1000.00 | 765 | 20,026 | 19,261 | |
| Cost of surgical revision for grade 3 skin reaction (£663.66) | 497.75 | 765 | 17,532 | 16,767 |
| 829.58 | 765 | 17,642 | 16,876 |
DSA – adult cases, with treatment failures not switching to alternative hearing aid
| Parameter (base-case value) | Input value | BCHA (£) | BAHA (£) | Incremental cost per case (£) |
|---|---|---|---|---|
| Probability of bleeding within 24 hours of surgery (0.0121) | 0.00147 | 751 | 14,532 | 13,781 |
| 0.04310 | 751 | 14,538 | 13,786 | |
| Probability of surgical reduction of skin growth/soft tissue thickening around abutment (0.0848) | 0.04716 | 751 | 14,529 | 13,777 |
| 0.13826 | 751 | 14,545 | 13,793 | |
| Probability of failure to integrate (0.006) | 0.00015 | 751 | 14,533 | 13,782 |
| 0.03291 | 751 | 14,531 | 13,779 | |
| Probability of intolerable pain requiring removal of abutment and flange fixture (0.0272) | 0.00746 | 751 | 14,828 | 14,077 |
| 0.06820 | 751 | 14,472 | 13,720 | |
| Probability of re-operation (0.9474) | 0.73972 | 751 | 13,877 | 13,125 |
| 0.99867 | 751 | 14,702 | 13,951 | |
| Cost of initial ENT consultation (£110.78) | 83.09 | 751 | 14,506 | 13,754 |
| 138.48 | 751 | 14,561 | 13,810 | |
| Cost of audiological assessment (£57.48) | 43.11 | 751 | 14,519 | 13,768 |
| 71.85 | 751 | 14,548 | 13,796 | |
| Cost of ENT multiprofessional assessment (£147.36) | 110.52 | 751 | 14,497 | 13,745 |
| 184.21 | 751 | 14,570 | 13,819 | |
| Cost of day-case surgery for implantation (£2004.57) | 1503.43 | 751 | 13,842 | 13,090 |
| 2505.71 | 751 | 15,225 | 14,474 | |
| Cost of fixture and abutment (£830.00) | 622.50 | 751 | 14,359 | 13,607 |
| 1037.50 | 751 | 14,708 | 13,957 | |
| Cost of surgical consumables (£159.50) | 119.63 | 751 | 14,480 | 13,728 |
| 199.38 | 751 | 14,587 | 13,836 | |
| Cost of follow-up ENT consultations (£72.11) | 54.09 | 751 | 14,462 | 13,711 |
| 90.14 | 751 | 14,604 | 13,853 | |
| Cost of audiological consultation to fit/commission sound processor (£64.80) | 48.60 | 751 | 14,512 | 13,761 |
| 81.00 | 751 | 14,555 | 13,803 | |
| Cost of audiology follow-up in year of surgery (£50.17) | 37.62 | 751 | 14,501 | 13,749 |
| 62.71 | 751 | 14,566 | 13,815 | |
| Cost of BAHA sound processor (£2191.25) | 1820.00 | 751 | 14,168 | 13,416 |
| 2995.00 | 751 | 15,325 | 14,574 | |
| Cost of BAHA sound processor maintenance plan (£736.25) | 610.00 | 751 | 13,388 | 12,636 |
| 1000.00 | 751 | 16,927 | 16,176 | |
| Cost of surgical revision for grade 3 skin reaction (£663.66) | 497.75 | 751 | 14,480 | 13,728 |
| 829.58 | 751 | 14,587 | 13,836 |
DSA – adult cases, with treatment failures switching to alternative hearing aid (BCHA)
| Parameter (base-case value) | Input value | BCHA (£) | BAHA (£) | Incremental cost per case (£) |
|---|---|---|---|---|
| Probability of bleeding within 24 hours of surgery (0.0121) | 0.00147 | 751 | 14,604 | 13,852 |
| 0.04310 | 751 | 14,609 | 13,858 | |
| Probability of surgical reduction of skin growth/soft tissue thickening around abutment (0.0848) | 0.04716 | 751 | 14,600 | 13,849 |
| 0.13826 | 751 | 14,616 | 13,865 | |
| Probability of failure to integrate (0.006) | 0.00015 | 751 | 14,605 | 13,853 |
| 0.03291 | 751 | 14,611 | 13,860 | |
| Probability of intolerable pain requiring removal of abutment and flange fixture (0.0272) | 0.00746 | 751 | 14,850 | 14,098 |
| 0.06820 | 751 | 14,553 | 13,802 | |
| Probability of re-operation (0.9474) | 0.73972 | 751 | 14,024 | 13,273 |
| 0.99867 | 751 | 14,754 | 14,003 | |
| Cost of initial ENT consultation (£110.78) | 83.09 | 751 | 14,577 | 13,826 |
| 138.48 | 751 | 14,633 | 13,881 | |
| Cost of audiological assessment (£57.48) | 43.11 | 751 | 14,590 | 13,839 |
| 71.85 | 751 | 14,619 | 13,868 | |
| Cost of ENT multiprofessional assessment (£147.36) | 110.52 | 751 | 14,568 | 13,817 |
| 184.21 | 751 | 14,642 | 13,890 | |
| Cost of day-case surgery for implantation (£2004.57) | 1503.43 | 751 | 13,913 | 13,161 |
| 2505.71 | 751 | 15,297 | 14,545 | |
| Cost of fixture and abutment (£830.00) | 622.50 | 751 | 14,430 | 13,679 |
| 1037.50 | 751 | 14,780 | 14,028 | |
| Cost of surgical consumables (£159.50) | 119.63 | 751 | 14,551 | 13,800 |
| 199.38 | 751 | 14,658 | 13,907 | |
| Cost of follow-up ENT consultations (£72.11) | 54.09 | 751 | 14,534 | 13,782 |
| 90.14 | 751 | 14,676 | 13,924 | |
| Cost of audiological consultation to fit/commission sound processor (£64.80) | 48.60 | 751 | 14,584 | 13,832 |
| 81.00 | 751 | 14,626 | 13,875 | |
| Cost of audiology follow-up in year of surgery (£50.17) | 37.62 | 751 | 14,572 | 13,821 |
| 62.71 | 751 | 14,637 | 13,886 | |
| Cost of BAHA sound processor (£2191.25) | 1820.00 | 751 | 14,239 | 13,488 |
| 2995.00 | 751 | 15,397 | 14,645 | |
| Cost of BAHA sound processor maintenance plan (£736.25) | 610.00 | 751 | 13,459 | 12,707 |
| 1000.00 | 751 | 16,999 | 16,247 | |
| Cost of surgical revision for grade 3 skin reaction (£663.66) | 497.75 | 751 | 14,551 | 13,800 |
| 829.58 | 751 | 14,658 | 13,907 |
BCHA device cost = £183
DSA – paediatric cases, with treatment failures not switching to alternative hearing aid
| Parameter (base-case value) | Input value | BCHA (£) | BAHA (£) | Incremental cost per case (£) |
|---|---|---|---|---|
| Probability of bleeding within 24 hours of surgery (0.0121) | 0.00147 | 934 | 17,513 | 16,579 |
| 0.04310 | 934 | 17,518 | 16,584 | |
| Probability of surgical reduction of skin growth/soft tissue thickening around abutment (0.0848) | 0.04716 | 934 | 17,509 | 16,575 |
| 0.13826 | 934 | 17,525 | 16,591 | |
| Probability of failure to integrate (0.006) | 0.00015 | 934 | 17,513 | 16,580 |
| 0.03291 | 934 | 17,501 | 16,567 | |
| Probability of intolerable pain requiring removal of abutment and flange fixture (0.0272) | 0.00746 | 934 | 17,840 | 16,906 |
| 0.06820 | 934 | 17,445 | 16,512 | |
| Probability of re-operation (0.9474) | 0.73972 | 934 | 16,668 | 15,734 |
| 0.99867 | 934 | 17,732 | 16,798 | |
| Cost of initial ENT consultation (£131.69) | 98.77 | 934 | 17,481 | 16,547 |
| 164.61 | 934 | 17,547 | 16,613 | |
| Cost of audiological assessment (£57.48) | 43.11 | 934 | 17,499 | 16,566 |
| 71.85 | 934 | 17,528 | 16,594 | |
| Cost of ENT multiprofessional assessment (£147.36) | 110.52 | 934 | 17,477 | 16,543 |
| 184.21 | 934 | 17,551 | 16,617 | |
| Cost of day-case surgery for implantation (£4009.14) | 3006.86 | 934 | 16,141 | 15,207 |
| 5011.43 | 934 | 18,887 | 17,953 | |
| Cost of fixture and abutment (£830.00) | 622.50 | 934 | 17,338 | 16,404 |
| 1037.50 | 934 | 17,689 | 16,756 | |
| Cost of surgical consumables (£159.50) | 119.63 | 934 | 17,460 | 16,526 |
| 199.38 | 934 | 17,568 | 16,634 | |
| Cost of follow-up ENT consultations (£90.93) | 68.20 | 934 | 17,424 | 16,490 |
| 113.66 | 934 | 17,604 | 16,670 | |
| Cost of audiological consultation to fit/commission sound processor (£64.80) | 48.60 | 934 | 17,493 | 16,559 |
| 81.00 | 934 | 17,535 | 16,601 | |
| Cost of audiology follow-up in year of surgery (£50.17) | 37.62 | 934 | 17,481 | 16,547 |
| 62.71 | 934 | 17,547 | 16,613 | |
| Cost of BAHA sound processor (£2191.25) | 1820.00 | 934 | 17,147 | 16,213 |
| 2995.00 | 934 | 18,308 | 17,375 | |
| Cost of BAHA sound processor maintenance plan (£736.25) | 610.00 | 934 | 16,346 | 15,412 |
| 1000.00 | 934 | 19,953 | 19,019 | |
| Cost of surgical revision for grade 3 skin reaction (£663.66) | 497.75 | 934 | 17,459 | 16,525 |
| 829.58 | 934 | 17,569 | 16,635 |
DSA – paediatric cases, with treatment failures switching to alternative hearing aid (BCHA)
| Parameter (base-case value) | Input value | BCHA (£) | BAHA (£) | Incremental cost per case (£) |
|---|---|---|---|---|
| Probability of bleeding within 24 hours of surgery (0.0121) | 0.00147 | 934 | 17,617 | 16,683 |
| 0.04310 | 934 | 17,622 | 16,688 | |
| Probability of surgical reduction of skin growth/soft tissue thickening around abutment (0.0848) | 0.04716 | 934 | 17,613 | 16,679 |
| 0.13826 | 934 | 17,629 | 16,695 | |
| Probability of failure to integrate (0.006) | 0.00015 | 934 | 17,618 | 16,684 |
| 0.03291 | 934 | 17,618 | 16,684 | |
| Probability of intolerable pain requiring removal of abutment and flange fixture (0.0272) | 0.00746 | 934 | 17,872 | 16,938 |
| 0.06820 | 934 | 17,565 | 16,631 | |
| Probability of re-operation (0.9474) | 0.73972 | 934 | 16,884 | 15,951 |
| 0.99867 | 934 | 17,807 | 16,873 | |
| Cost of initial ENT consultation (£131.69) | 98.77 | 934 | 17,585 | 16,651 |
| 164.61 | 934 | 17,651 | 16,717 | |
| Cost of audiological assessment (£57.48) | 43.11 | 934 | 17,603 | 16,670 |
| 71.85 | 934 | 17,632 | 16,698 | |
| Cost of ENT multiprofessional assessment (£147.36) | 110.52 | 934 | 17,581 | 16,647 |
| 184.21 | 934 | 17,655 | 16,721 | |
| Cost of day-case surgery for implantation (£4009.14) | 3006.86 | 934 | 16,245 | 15,311 |
| 5011.43 | 934 | 18,991 | 18,057 | |
| Cost of fixture and abutment (£830.00) | 622.50 | 934 | 17,442 | 16,508 |
| 1037.50 | 934 | 17,794 | 16,860 | |
| Cost of surgical consumables (£159.50) | 119.63 | 934 | 17,564 | 16,630 |
| 199.38 | 934 | 17,672 | 16,738 | |
| Cost of follow-up ENT consultations (£90.93) | 68.20 | 934 | 17,528 | 16,594 |
| 113.66 | 934 | 17,708 | 16,774 | |
| Cost of audiological consultation to fit/commission sound processor (£64.80) | 48.60 | 934 | 17,597 | 16,663 |
| 81.00 | 934 | 17,639 | 16,705 | |
| Cost of audiology follow-up in year of surgery (£50.17) | 37.62 | 934 | 17,585 | 16,651 |
| 62.71 | 934 | 17,651 | 16,717 | |
| Cost of BAHA sound processor (£2191.25) | 1820.00 | 934 | 17,251 | 16,317 |
| 2995.00 | 934 | 18,412 | 17,479 | |
| Cost of BAHA sound processor maintenance plan (£736.25) | 610.00 | 934 | 16,450 | 15,516 |
| 1000.00 | 934 | 20,057 | 19,123 | |
| Cost of surgical revision for grade 3 skin reaction (£663.66) | 497.75 | 934 | 17,563 | 16,629 |
| 829.58 | 934 | 17,673 | 16,739 |
DSA – adult cases, with treatment failures not switching to alternative hearing aid
| Parameter (base-case value) | Input value | BCHA (£) | BAHA (£) | Incremental cost per case (£) |
|---|---|---|---|---|
| Probability of bleeding within 24 hours of surgery (0.0121) | 0.00147 | 917 | 14,532 | 13,616 |
| 0.04310 | 917 | 14,538 | 13,621 | |
| Probability of surgical reduction of skin growth/soft tissue thickening around abutment (0.0848) | 0.04716 | 917 | 14,529 | 13,612 |
| 0.13826 | 917 | 14,545 | 13,628 | |
| Probability of failure to integrate (0.006) | 0.00015 | 917 | 14,533 | 13,617 |
| 0.03291 | 917 | 14,531 | 13,614 | |
| Probability of intolerable pain requiring removal of abutment and flange fixture (0.0272) | 0.00746 | 917 | 14,828 | 13,912 |
| 0.06820 | 917 | 14,472 | 13,555 | |
| Probability of re-operation (0.9474) | 0.73972 | 917 | 13,877 | 12,960 |
| 0.99867 | 917 | 14,702 | 13,786 | |
| Cost of initial ENT consultation (£110.78) | 83.09 | 917 | 14,506 | 13,589 |
| 138.48 | 917 | 14,561 | 13,645 | |
| Cost of audiological assessment (£57.48) | 43.11 | 917 | 14,519 | 13,603 |
| 71.85 | 917 | 14,548 | 13,631 | |
| Cost of ENT multiprofessional assessment (£147.36) | 110.52 | 917 | 14,497 | 13,580 |
| 184.21 | 917 | 14,570 | 13,654 | |
| Cost of day-case surgery for implantation (£2004.57) | 1503.43 | 917 | 13,842 | 12,925 |
| 2505.71 | 917 | 15,225 | 14,309 | |
| Cost of fixture and abutment (£830.00) | 622.50 | 917 | 14,359 | 13,442 |
| 1037.50 | 917 | 14,708 | 13,792 | |
| Cost of surgical consumables (£159.50) | 119.63 | 917 | 14,480 | 13,563 |
| 199.38 | 917 | 14,587 | 13,671 | |
| Cost of follow-up ENT consultations (£72.11) | 54.09 | 917 | 14,462 | 13,546 |
| 90.14 | 917 | 14,604 | 13,688 | |
| Cost of audiological consultation to fit/commission sound processor (£64.80) | 48.60 | 917 | 14,512 | 13,596 |
| 81.00 | 917 | 14,555 | 13,638 | |
| Cost of audiology follow-up in year of surgery (£50.17) | 37.62 | 917 | 14,501 | 13,584 |
| 62.71 | 917 | 14,566 | 13,650 | |
| Cost of BAHA sound processor (£2191.25) | 1820.00 | 917 | 14,168 | 13,251 |
| 2995.00 | 917 | 15,325 | 14,409 | |
| Cost of BAHA sound processor maintenance plan (£736.25) | 610.00 | 917 | 13,388 | 12,471 |
| 1000.00 | 917 | 16,927 | 16,011 | |
| Cost of surgical revision for grade 3 skin reaction (£663.66) | 497.75 | 917 | 14,480 | 13,563 |
| 829.58 | 917 | 14,587 | 13,671 |
DSA – adult cases, with treatment failures switching to alternative hearing aid (BCHA)
| Parameter (base case value) | Input value | BCHA (£) | BAHA (£) | Incremental cost per case (£) |
|---|---|---|---|---|
| Probability of bleeding within 24 hours of surgery (0.0121) | 0.00147 | 917 | 14,634 | 13,717 |
| 0.04310 | 917 | 14,639 | 13,723 | |
| Probability of surgical reduction of skin growth/soft tissue thickening around abutment (0.0848) | 0.04716 | 917 | 14,630 | 13,714 |
| 0.13826 | 917 | 14,646 | 13,730 | |
| Probability of failure to integrate (0.006) | 0.00015 | 917 | 14,635 | 13,719 |
| 0.03291 | 917 | 14,645 | 13,728 | |
| Probability of intolerable pain requiring removal of abutment and flange fixture (0.0272) | 0.00746 | 917 | 14,859 | 13,942 |
| 0.06820 | 917 | 14,588 | 13,672 | |
| Probability of re-operation (0.9474) | 0.73972 | 917 | 14,088 | 13,171 |
| 0.99867 | 917 | 14,776 | 13,859 | |
| Cost of initial ENT consultation (£110.78) | 83.09 | 917 | 14,607 | 13,691 |
| 138.48 | 917 | 14,663 | 13,746 | |
| Cost of audiological assessment (£57.48) | 43.11 | 917 | 14,621 | 13,704 |
| 71.85 | 917 | 14,649 | 13,733 | |
| Cost of ENT multiprofessional assessment (£147.36) | 110.52 | 917 | 14,598 | 13,682 |
| 184.21 | 917 | 14,672 | 13,755 | |
| Cost of day-case surgery for implantation (£2004.57) | 1503.43 | 917 | 13,943 | 13,027 |
| 2505.71 | 917 | 15,327 | 14,411 | |
| Cost of fixture and abutment (£830.00) | 622.50 | 917 | 14,460 | 13,544 |
| 1037.50 | 917 | 14,810 | 13,893 | |
| Cost of surgical consumables (£159.50) | 119.63 | 917 | 14,582 | 13,665 |
| 199.38 | 917 | 14,689 | 13,772 | |
| Cost of follow-up ENT consultations (£72.11) | 54.09 | 917 | 14,564 | 13,648 |
| 90.14 | 917 | 14,706 | 13,790 | |
| Cost of audiological consultation to fit/commission sound processor (£64.80) | 48.60 | 917 | 14,614 | 13,697 |
| 81.00 | 917 | 14,656 | 13,740 | |
| Cost of audiology follow-up in year of surgery (£50.17) | 37.62 | 917 | 14,602 | 13,686 |
| 62.71 | 917 | 14,668 | 13,751 | |
| Cost of BAHA sound processor (£2191.25) | 1820.00 | 917 | 14,269 | 13,353 |
| 2995.00 | 917 | 15,427 | 14,510 | |
| Cost of BAHA sound processor maintenance plan (£736.25) | 610.00 | 917 | 13,489 | 12,573 |
| 1000.00 | 917 | 17,029 | 16,113 | |
| Cost of surgical revision for grade 3 skin reaction (£663.66) | 497.75 | 917 | 14,581 | 13,665 |
| 829.58 | 917 | 14,689 | 13,772 |
BCHA device cost = £350
DSA – paediatric cases, with treatment failures not switching to alternative hearing aid
| Parameter (base-case value) | Input value | BCHA (£) | BAHA (£) | Incremental cost per case (£) |
|---|---|---|---|---|
| Probability of bleeding within 24 hours of surgery (0.0121) | 0.00147 | 1360 | 17,513 | 16,153 |
| 0.04310 | 1360 | 17,518 | 16,158 | |
| Probability of surgical reduction of skin growth/soft tissue thickening around abutment (0.0848) | 0.04716 | 1360 | 17,509 | 16,149 |
| 0.13826 | 1360 | 17,525 | 16,166 | |
| Probability of failure to integrate (0.006) | 0.00015 | 1360 | 17,513 | 16,154 |
| 0.03291 | 1360 | 17,501 | 16,142 | |
| Probability of intolerable pain requiring removal of abutment and flange fixture (0.0272) | 0.00746 | 1360 | 17,840 | 16,480 |
| 0.06820 | 1360 | 17,445 | 16,086 | |
| Probability of re-operation (0.9474) | 0.73972 | 1360 | 16,668 | 15,308 |
| 0.99867 | 1360 | 17,732 | 16,372 | |
| Cost of initial ENT consultation (£131.69) | 98.77 | 1360 | 17,481 | 16,121 |
| 164.61 | 1360 | 17,547 | 16,187 | |
| Cost of audiological assessment (£57.48) | 43.11 | 1360 | 17,499 | 16,140 |
| 71.85 | 1360 | 17,528 | 16,169 | |
| Cost of ENT multiprofessional assessment (£147.36) | 110.52 | 1360 | 17,477 | 16,117 |
| 184.21 | 1360 | 17,551 | 16,191 | |
| Cost of day-case surgery for implantation (£4009.14) | 3006.86 | 1360 | 16,141 | 14,781 |
| 5011.43 | 1360 | 18,887 | 17,527 | |
| Cost of fixture and abutment (£830.00) | 622.50 | 1360 | 17,338 | 15,979 |
| 1037.50 | 1360 | 17,689 | 16,330 | |
| Cost of surgical consumables (£159.50) | 119.63 | 1360 | 17,460 | 16,100 |
| 199.38 | 1360 | 17,568 | 16,208 | |
| Cost of follow-up ENT consultations (£90.93) | 68.20 | 1360 | 17,424 | 16,064 |
| 113.66 | 1360 | 17,604 | 16,244 | |
| Cost of audiological consultation to fit/commission sound processor (£64.80) | 48.60 | 1360 | 17,493 | 16,133 |
| 81.00 | 1360 | 17,535 | 16,176 | |
| Cost of audiology follow-up in year of surgery (£50.17) | 37.62 | 1360 | 17,481 | 16,121 |
| 62.71 | 1360 | 17,547 | 16,187 | |
| Cost of BAHA sound processor (£2191.25) | 1820.00 | 1360 | 17,147 | 15,787 |
| 2995.00 | 1360 | 18,308 | 16,949 | |
| Cost of BAHA sound processor maintenance plan (£736.25) | 610.00 | 1360 | 16,346 | 14,987 |
| 1000.00 | 1360 | 19,953 | 18,594 | |
| Cost of surgical revision for grade 3 skin reaction (£663.66) | 497.75 | 1360 | 17,459 | 16,099 |
| 829.58 | 1360 | 17,569 | 16,209 |
DSA – paediatric cases, with treatment failures switching to alternative hearing aid (BCHA)
| Parameter (base-case value) | Input value | BCHA (£) | BAHA (£) | Incremental cost per case (£) |
|---|---|---|---|---|
| Probability of bleeding within 24 hours of surgery (0.0121) | 0.00147 | 1360 | 17,695 | 16,336 |
| 0.04310 | 1360 | 17,701 | 16,341 | |
| Probability of surgical reduction of skin growth/soft tissue thickening around abutment (0.0848) | 0.04716 | 1360 | 17,691 | 16,332 |
| 0.13826 | 1360 | 17,708 | 16,348 | |
| Probability of failure to integrate (0.006) | 0.00015 | 1360 | 17,696 | 16,337 |
| 0.03291 | 1360 | 17,706 | 16,346 | |
| Probability of intolerable pain requiring removal of abutment and flange fixture (0.0272) | 0.00746 | 1360 | 17,896 | 16,536 |
| 0.06820 | 1360 | 17,654 | 16,295 | |
| Probability of re-operation (0.9474) | 0.73972 | 1360 | 17,049 | 15,689 |
| 0.99867 | 1360 | 17,863 | 16,504 | |
| Cost of initial ENT consultation (£131.69) | 98.77 | 1360 | 17,663 | 16,304 |
| 164.61 | 1360 | 17,729 | 16,370 | |
| Cost of audiological assessment (£57.48) | 43.11 | 1360 | 17,682 | 16,322 |
| 71.85 | 1360 | 17,711 | 16,351 | |
| Cost of ENT multiprofessional assessment (£147.36) | 110.52 | 1360 | 17,659 | 16,300 |
| 184.21 | 1360 | 17,733 | 16,374 | |
| Cost of day-case surgery for implantation (£4009.14) | 3006.86 | 1360 | 16,324 | 14,964 |
| 5011.43 | 1360 | 19,069 | 17,710 | |
| Cost of fixture and abutment (£830) | 622.50 | 1360 | 17,521 | 16,161 |
| 1037.50 | 1360 | 17,872 | 16,512 | |
| Cost of surgical consumables (£159.50) | 119.63 | 1360 | 17,642 | 16,283 |
| 199.38 | 1360 | 17,750 | 16,391 | |
| Cost of follow-up ENT consultations (£90.93) | 68.20 | 1360 | 17,606 | 16,247 |
| 113.66 | 1360 | 17,787 | 16,427 | |
| Cost of audiological consultation to fit/commission sound processor (£64.80) | 48.60 | 1360 | 17,675 | 16,316 |
| 81.00 | 1360 | 17,718 | 16,358 | |
| Cost of audiology follow-up in year of surgery (£50.17) | 37.62 | 1360 | 17,663 | 16,304 |
| 62.71 | 1360 | 17,729 | 16,370 | |
| Cost of BAHA sound processor (£2191.25) | 1820.00 | 1360 | 17,329 | 15,970 |
| 2995.00 | 1360 | 18,491 | 17,131 | |
| Cost of BAHA sound processor maintenance plan (£736.25) | 610.00 | 1360 | 16,529 | 15,169 |
| 1000.00 | 1360 | 20,136 | 18,776 | |
| Cost of surgical revision for grade 3 skin reaction (£663.66) | 497.75 | 1360 | 17,641 | 16,282 |
| 829.58 | 1360 | 17,751 | 16,392 |
DSA – adult cases, with treatment failures not switching to alternative hearing aid
| Parameter (base-case value) | Input value | BCHA (£) | BAHA (£) | Incremental cost per case (£) |
|---|---|---|---|---|
| Probability of bleeding within 24 hours of surgery (0.0121) | 0.00147 | 1334 | 14,532 | 13,198 |
| 0.04310 | 1334 | 14,538 | 13,203 | |
| Probability of surgical reduction of skin growth/soft tissue thickening around abutment (0.0848) | 0.04716 | 1334 | 14,529 | 13,194 |
| 0.13826 | 1334 | 14,545 | 13,210 | |
| Probability of failure to integrate (0.006) | 0.00015 | 1334 | 14,533 | 13,199 |
| 0.03291 | 1334 | 14,531 | 13,196 | |
| Probability of intolerable pain requiring removal of abutment and flange fixture (0.0272) | 0.00746 | 1334 | 14,828 | 13,494 |
| 0.06820 | 1334 | 14,472 | 13,137 | |
| Probability of re-operation (0.9474) | 0.73972 | 1334 | 13,877 | 12,542 |
| 0.99867 | 1334 | 14,702 | 13,368 | |
| Cost of initial ENT consultation (£110.78) | 83.09 | 1334 | 14,506 | 13,171 |
| 138.48 | 1334 | 14,561 | 13,227 | |
| Cost of audiological assessment (£57.48) | 43.11 | 1334 | 14,519 | 13,185 |
| 71.85 | 1334 | 14,548 | 13,213 | |
| Cost of ENT multiprofessional assessment (£147.36) | 110.52 | 1334 | 14,497 | 13,162 |
| 184.21 | 1334 | 14,570 | 13,236 | |
| Cost of day-case surgery for implantation (£2004.57) | 1503.43 | 1334 | 13,842 | 12,507 |
| 2505.71 | 1334 | 15,225 | 13,891 | |
| Cost of fixture and abutment (£830.00) | 622.50 | 1334 | 14,359 | 13,024 |
| 1037.50 | 1334 | 14,708 | 13,374 | |
| Cost of surgical consumables (£159.50) | 119.63 | 1334 | 14,480 | 13,145 |
| 199.38 | 1334 | 14,587 | 13,253 | |
| Cost of follow-up ENT consultations (£72.11) | 54.09 | 1334 | 14,462 | 13,128 |
| 90.14 | 1334 | 14,604 | 13,270 | |
| Cost of audiological consultation to fit/commission sound processor (£64.80) | 48.60 | 1334 | 14,512 | 13,178 |
| 81.00 | 1334 | 14,555 | 13,220 | |
| Cost of audiology follow-up in year of surgery (£50.17) | 37.62 | 1334 | 14,501 | 13,166 |
| 62.71 | 1334 | 14,566 | 13,232 | |
| Cost of BAHA sound processor (£2191.25) | 1820.00 | 1334 | 14,168 | 12,833 |
| 2995.00 | 1334 | 15,325 | 13,991 | |
| Cost of BAHA sound processor maintenance plan (£736.25) | 610.00 | 1334 | 13,388 | 12,053 |
| 1000.00 | 1334 | 16,927 | 15,593 | |
| Cost of surgical revision for grade 3 skin reaction (£663.66) | 497.75 | 1334 | 14,480 | 13,145 |
| 829.58 | 1334 | 14,587 | 13,253 |
DSA – adult cases, with treatment failures switching to alternative hearing aid (BCHA)
| Parameter (base-case value) | Input value | BCHA (£) | BAHA (£) | Incremental cost per case (£) |
|---|---|---|---|---|
| Probability of bleeding within 24 hours of surgery (0.0121) | 0.00147 | 1334 | 14,711 | 13,376 |
| 0.04310 | 1334 | 14,716 | 13,381 | |
| Probability of surgical reduction of skin growth/soft tissue thickening around abutment (0.0848) | 0.04716 | 1334 | 14,707 | 13,372 |
| 0.13826 | 1334 | 14,723 | 13,388 | |
| Probability of failure to integrate (0.006) | 0.00015 | 1334 | 14,712 | 13,377 |
| 0.03291 | 1334 | 14,731 | 13,396 | |
| Probability of intolerable pain requiring removal of abutment and flange fixture (0.0272) | 0.00746 | 1334 | 14,883 | 13,548 |
| 0.06820 | 1334 | 14,676 | 13,341 | |
| Probability of re-operation (0.9474) | 0.73972 | 1334 | 14,248 | 12,914 |
| 0.99867 | 1334 | 14,831 | 13,497 | |
| Cost of initial ENT consultation (£110.78) | 83.09 | 1334 | 14,684 | 13,350 |
| 138.48 | 1334 | 14,739 | 13,405 | |
| Cost of audiological assessment (£57.48) | 43.11 | 1334 | 14,697 | 13,363 |
| 71.85 | 1334 | 14,726 | 13,392 | |
| Cost of ENT multiprofessional assessment (£147.36) | 110.52 | 1334 | 14,675 | 13,340 |
| 184.21 | 1334 | 14,749 | 13,414 | |
| Cost of day-case surgery for implantation (£2004.57) | 1503.43 | 1334 | 14,020 | 12,685 |
| 2505.71 | 1334 | 15,404 | 14,069 | |
| Cost of fixture and abutment (£830.00) | 622.50 | 1334 | 14,537 | 13,203 |
| 1037.50 | 1334 | 14,886 | 13,552 | |
| Cost of surgical consumables (£159.50) | 119.63 | 1334 | 14,658 | 13,324 |
| 199.38 | 1334 | 14,765 | 13,431 | |
| Cost of follow-up ENT consultations (£72.11) | 54.09 | 1334 | 14,641 | 13,306 |
| 90.14 | 1334 | 14,783 | 13,448 | |
| Cost of audiological consultation to fit/commission sound processor (£64.80) | 48.60 | 1334 | 14,691 | 13,356 |
| 81.00 | 1334 | 14,733 | 13,398 | |
| Cost of audiology follow-up in year of surgery (£50.17) | 37.62 | 1334 | 14,679 | 13,345 |
| 62.71 | 1334 | 14,744 | 13,410 | |
| Cost of BAHA sound processor (£2191.25) | 1820.00 | 1334 | 14,346 | 13,012 |
| 2995.00 | 1334 | 15,504 | 14,169 | |
| Cost of BAHA sound processor maintenance plan (£736.25) | 610.00 | 1334 | 13,566 | 12,231 |
| 1000.00 | 1334 | 17,106 | 15,771 | |
| Cost of surgical revision for grade 3 skin reaction (£663.66) | 497.75 | 1334 | 14,658 | 13,324 |
| 829.58 | 1334 | 14,765 | 13,431 |
Cost-effectiveness analysis
BCHA device cost = £117
DSA – impact on ICER
| Input parameter (base-case value) | Input value | ICER (£ per QALY gained) | |||
|---|---|---|---|---|---|
| Paediatric (P) | Adult (A) | ||||
| QALY1a | QALY2b | QALY1a | QALY2b | ||
| Probability of bleeding within 24 hours of surgery (0.0121) | 0.00147 | 121,353 | 56,568 | 102,022 | 47,557 |
| 0.04310 | 121,393 | 56,587 | 102,062 | 47,576 | |
| Probability of surgical reduction of skin growth/thickening around abutment (0.0848) | 0.04716 | 121,327 | 56,556 | 101,995 | 47,544 |
| 0.13826 | 121,444 | 56,610 | 102,113 | 47,600 | |
| Probability of failure to integrate (0.006) | 0.00015 | 121,457 | 56,617 | 102,112 | 47,599 |
| 0.03291 | 126,383 | 58,913 | 106,330 | 49,565 | |
| Probability of intolerable pain requiring removal of abutment and flange fixture (0.0272) | 0.00746 | 119,273 | 55,599 | 100,408 | 46,805 |
| 0.06820 | 121,817 | 56,784 | 102,384 | 47,726 | |
| Probability of re-operation (0.9474) | 0.73972 | 121,480 | 56,628 | 102,491 | 47,776 |
| 0.99867 | 121,346 | 56,565 | 101,928 | 47,513 | |
| Cost of initial ENT consultation [£131.69 (P) and £110.78 (A)] | 98.77 (P) | 121,125 | 56,462 | 101,827 | 47,466 |
| 83.09 (A) | |||||
| 164.61 (P) | 121,600 | 56,683 | 102,235 | 47,656 | |
| 138.48 (A) | |||||
| Cost of audiological assessment (£57.48) | 43.11 | 121,259 | 56,524 | 101,925 | 47,512 |
| 71.85 | 121,466 | 56,621 | 102,137 | 47,611 | |
| Cost of ENT multiprofessional assessment (£147.36) | 110.52 | 121,097 | 56,449 | 101,760 | 47,435 |
| 184.21 | 121,628 | 56,697 | 102,302 | 47,688 | |
| Cost of day-case surgery for implantation (£2004.57) | 1503.43 | 111,458 | 51,956 | 96,935 | 45,186 |
| 2505.71 | 131,267 | 61,190 | 107,127 | 49,937 | |
| Cost of fixture and abutment (£830.00) | 622.50 | 120,095 | 55,982 | 100,744 | 46,961 |
| 1037.50 | 122,630 | 57,163 | 103,318 | 48,161 | |
| Cost of surgical consumables (£159.50) | 119.63 | 120,974 | 56,391 | 101,636 | 47,377 |
| 199.38 | 121,751 | 56,754 | 102,426 | 47,745 | |
| Cost of follow-up ENT consultations [£90.93 (P) and £72.11 (A)] | 68.20 (P) | 120,712 | 56,269 | 101,508 | 47,318 |
| 54.09 (A) | |||||
| 113.66 (P) | 122,013 | 56,876 | 102,554 | 47,805 | |
| 90.14 (A) | |||||
| Cost of audiological consultation to fit/commission sound processor (£64.80) | 48.60 | 121,209 | 56,501 | 101,875 | 47,489 |
| 81.00 | 121,516 | 56,644 | 102,186 | 47,634 | |
| Cost of audiology follow-up in year of surgery (£50.17) | 37.62 | 121,125 | 56,462 | 101,790 | 47,449 |
| 62.71 | 121,600 | 56,683 | 102,272 | 47,673 | |
| Cost of BAHA sound processor (£2191.25) | 1820.00 | 118,715 | 55,338 | 99,337 | 46,306 |
| 2995.00 | 127,094 | 59,245 | 107,863 | 50,280 | |
| Cost of BAHA sound processor maintenance plan (£735.25) | 610.00 | 112,938 | 52,646 | 93,591 | 43,627 |
| 1000.00 | 138,962 | 64,776 | 119,663 | 55,780 | |
| Cost of surgical revision for grade 3 skin reaction (£663.66) | 497.75 | 120,967 | 56,388 | 101,636 | 47,377 |
| 829.58 | 121,758 | 56,757 | 102,426 | 47,745 | |
| Proportion of cohort using BCHA for > 8 hours per day (0.90) | 0.683 | 37,644 | 17,548 | 31,648 | 14,752 |
| 0.988 | 1,236,900 | 576,576 | 1,039,880 | 484,736 | |
| Proportion using BCHA at lower limit of 95% CI, 0.683, and proportion using BAHA at lower limit of 95% CI, 0.832 (BCHA = 0.90; BAHA = 1.00) | 0.683 | 83,663 | 38,999 | 70,328 | 32,783 |
| 0.832 | |||||
BCHA device cost = £183
DSA – impact on ICER
| Input parameter (base-case value) | Input value | ICER (£ per QALY gained) | |||
|---|---|---|---|---|---|
| Paediatric (P) | Adult (A) | ||||
| QALY1a | QALY2b | QALY1a | QALY2b | ||
| Probability of bleeding within 24 hours of surgery (0.0121) | 0.00147 | 120,363 | 56,107 | 101,028 | 47,094 |
| 0.04310 | 120,402 | 56,125 | 101,068 | 47,113 | |
| Probability of surgical reduction of skin growth/thickening around abutment (0.0848) | 0.04716 | 120,337 | 56,094 | 101,001 | 47,081 |
| 0.13826 | 120,453 | 56,149 | 101,120 | 47,137 | |
| Probability of failure to integrate (0.006) | 0.00015 | 120,467 | 56,155 | 101,118 | 47,136 |
| 0.03291 | 125,379 | 58,445 | 105,324 | 49,096 | |
| Probability of intolerable pain requiring removal of abutment and flange fixture (0.0272) | 0.00746 | 118,166 | 55,083 | 99,299 | 46,288 |
| 0.06820 | 120,852 | 56,335 | 101,416 | 47,274 | |
| Probability of re-operation (0.9474) | 0.73972 | 120,699 | 56,264 | 101,706 | 47,410 |
| 0.99867 | 120,305 | 56,080 | 100,884 | 47,027 | |
| Cost of initial ENT consultation [£131.69 (P) and £110.78 (A)] | 98.77 (P) | 120,135 | 56,000 | 100,834 | 47,003 |
| 83.09 (A) | |||||
| 164.61 (P) | 120,610 | 56,222 | 101,242 | 47,193 | |
| 138.48 (A) | |||||
| Cost of audiological assessment (£57.48) | 43.11 | 120,268 | 56,063 | 100,932 | 47,049 |
| 71.85 | 120,476 | 56,159 | 101,143 | 47,148 | |
| Cost of ENT multiprofessional assessment (£147.36) | 110.52 | 120,106 | 55,987 | 100,766 | 46,972 |
| 184.21 | 120,638 | 56,235 | 101,309 | 47,225 | |
| Cost of day-case surgery for implantation (£2004.57) | 1503.43 | 110,468 | 51,494 | 95,941 | 44,723 |
| 2505.71 | 130,277 | 60,728 | 106,134 | 49,474 | |
| Cost of fixture and abutment (£830.00) | 622.50 | 119,105 | 55,520 | 99,751 | 46,498 |
| 1037.50 | 121,640 | 56,702 | 102,324 | 47,698 | |
| Cost of surgical consumables (£159.50) | 119.63 | 119,983 | 55,930 | 100,643 | 46,914 |
| 199.38 | 120,761 | 56,292 | 101,432 | 47,282 | |
| Cost of follow-up ENT consultations [£90.93 (P) and £72.11 (A)] | 68.20 (P) | 119,721 | 55,808 | 100,515 | 46,855 |
| 54.09 (A) | |||||
| 113.66 (P) | 121,023 | 56,414 | 101,560 | 47,342 | |
| 90.14 (A) | |||||
| Cost of audiological consultation to fit/commission sound processor (£64.80) | 48.60 | 120,219 | 56,039 | 100,882 | 47,026 |
| 81.00 | 120,526 | 56,183 | 101,193 | 47,171 | |
| Cost of audiology follow-up in year of surgery (£50.17) | 37.62 | 120,135 | 56,000 | 100,797 | 46,986 |
| 62.71 | 120,610 | 56,222 | 101,278 | 47,210 | |
| Cost of BAHA sound processor (£2191.25) | 1820.00 | 117,725 | 54,877 | 98,344 | 45,843 |
| 2995.00 | 126,104 | 58,783 | 106,869 | 49,817 | |
| Cost of BAHA sound processor maintenance plan (£735.25) | 610.00 | 111,948 | 52,184 | 92,598 | 43,164 |
| 1000.00 | 137,971 | 64,315 | 118,670 | 55,317 | |
| Cost of surgical revision for grade 3 skin reaction (£663.66) | 497.75 | 119,977 | 55,927 | 100,643 | 46,914 |
| 829.58 | 120,768 | 56,295 | 101,433 | 47,282 | |
| Proportion of cohort using BCHA for > 8 hours per day (0.90) | 0.683 | 37,337 | 17,404 | 31,340 | 14,609 |
| 0.988 | 1,226,807 | 571,871 | 1,029,756 | 480,016 | |
| Proportion using BCHA at lower limit of 95% CI, 0.683, and proportion using BAHA at lower limit of 95% CI, 0.832 (BCHA = 0.90; BAHA = 1.00) | 0.683 | 82,980 | 38,681 | 69,643 | 32,464 |
| 0.832 | |||||
BCHA device cost = £350
DSA – impact on ICER
| Input parameter (base-case value) | Input value | ICER (£ per QALY gained) | |||
|---|---|---|---|---|---|
| Paediatric (P) | Adult (A) | ||||
| QALY1a | QALY2b | QALY1a | QALY2b | ||
| Probability of bleeding within 24 hours of surgery (0.0121) | 0.00147 | 117,857 | 54,939 | 98,515 | 45,922 |
| 0.04310 | 117,897 | 54,957 | 98,555 | 45,941 | |
| Probability of surgical reduction of skin growth/thickening around abutment (0.0848) | 0.04716 | 117,831 | 54,926 | 98,488 | 45,910 |
| 0.13826 | 117,948 | 54,981 | 98,606 | 45,965 | |
| Probability of failure to integrate (0.006) | 0.00015 | 117,960 | 54,987 | 98,604 | 45,964 |
| 0.03291 | 122,840 | 57,262 | 102,776 | 47,909 | |
| Probability of intolerable pain requiring removal of abutment and flange fixture (0.0272) | 0.00746 | 115,365 | 53,777 | 96,491 | 44,979 |
| 0.06820 | 118,410 | 55,197 | 98,966 | 46,133 | |
| Probability of re-operation (0.9474) | 0.73972 | 118,724 | 55,343 | 99,719 | 46,484 |
| 0.99867 | 117,671 | 54,852 | 98,244 | 45,796 | |
| Cost of initial ENT consultation [£131.69 (P) and £110.78 (A)] | 98.77 (P) | 117,629 | 54,832 | 98,320 | 45,832 |
| 83.09 (A) | |||||
| 164.61 (P) | 118,104 | 55,054 | 98,728 | 46,022 | |
| 138.48 (A) | |||||
| Cost of audiological assessment (£57.48) | 43.11 | 117,763 | 54,895 | 98,418 | 45,877 |
| 71.85 | 117,970 | 54,991 | 98,630 | 45,976 | |
| Cost of ENT multiprofessional assessment (£147.36) | 110.52 | 117,601 | 54,819 | 98,253 | 45,800 |
| 184.21 | 118,132 | 55,067 | 98,795 | 46,053 | |
| Cost of day-case surgery for implantation (£2004.57) | 1503.43 | 107,962 | 50,326 | 93,428 | 43,551 |
| 2505.71 | 127,771 | 59,560 | 103,620 | 48,302 | |
| Cost of fixture and abutment (£830.00) | 622.50 | 116,599 | 54,352 | 97,237 | 45,327 |
| 1037.50 | 119,134 | 55,534 | 99,811 | 46,527 | |
| Cost of surgical consumables (£159.50) | 119.63 | 117,478 | 54,762 | 98,129 | 45,743 |
| 199.38 | 118,255 | 55,124 | 98,919 | 46,111 | |
| Cost of follow-up ENT consultations [£90.93 (P) and 72.11 (A)] | 68.20 (P) | 117,216 | 54,640 | 98,001 | 45,683 |
| 54.09 (A) | |||||
| 113.66 (P) | 118,517 | 55,246 | 99,047 | 46,170 | |
| 90.14 (A) | |||||
| Cost of audiological consultation to fit/commission sound processor (£64.80) | 48.60 | 117,713 | 54,871 | 98,369 | 45,854 |
| 81.00 | 118,020 | 55,014 | 98,680 | 45,999 | |
| Cost of audiology follow-up in year of surgery (£50.17) | 37.62 | 117,629 | 54,832 | 98,283 | 45,814 |
| 62.71 | 118,104 | 55,054 | 98,765 | 46,039 | |
| Cost of BAHA sound processor (£2191.25) | 1820.00 | 115,219 | 53,709 | 95,830 | 44,671 |
| 2995.00 | 123,598 | 57,615 | 104,356 | 48,645 | |
| Cost of BAHA sound processor maintenance plan (£735.25) | 610.00 | 109,442 | 51,016 | 90,084 | 41,992 |
| 1000.00 | 135,466 | 63,147 | 116,156 | 54,146 | |
| Cost of surgical revision for grade 3 skin reaction (£663.66) | 497.75 | 117,471 | 54,759 | 98,129 | 45,742 |
| 829.58 | 118,262 | 55,127 | 98,919 | 46,111 | |
| Proportion of cohort using BCHA for > 8 hours per day (0.90) | 0.683 | 36,560 | 17,042 | 30,560 | 14,245 |
| 0.988 | 1,201,269 | 559,966 | 1,004,140 | 468,075 | |
| Proportion using BCHA at lower limit of 95% CI, 0.683, and proportion using BAHA at lower limit of 95% CI, 0.832 (BCHA = 0.90; BAHA = 1.00) | 0.683 | 81,253 | 37,876 | 67,911 | 31,656 |
| 0.832 | |||||
Appendix 13 Variables included in the probabilistic sensitivity analyses
Results for each parameter are provided on two lines – the first gives the results at the lower limit of the output parameter and the second gives the results at the upper limit.
| Input variable | Mean | Variability | Distribution | Sampling method/parameters of distribution |
|---|---|---|---|---|
| BCHA cost (£) | N/A | N/A | N/A | Sample from table of possible values (117, 183, 250, 350) with equal probability |
| BAHA sound processor cost (£) | N/A | N/A | N/A | Sample from table of possible values (1820, 1980, 1970, 2995) with equal probability |
| BAHA sound processor maintenance cost (£) | N/A | N/A | N/A | Sample from table of possible values (610, 665, 670, 1000) with equal probability |
| BAHA implant day-case surgery (£) | 1918 | ± 25% of mean | Gamma | α = 126.0710; β = 15.2136 |
| Loss of bone integration | 0.741 (at 7 years) | 95% CI of Kaplan–Meier estimate (0.4868 to 0.8767) | Beta | α = 14.3726; β = 5.0210 |
| Difference in use of hearing aid (BAHA–BCHA) | 0.1 | N/A | Beta | r = 2; n = 20 |
| Probability of initial failure of bone integration | 0.0060 | N/A | Beta | r = 1; n = 167 |
| Probability of failure owing to intolerable pain | 0.0272 | N/A | Beta | r = 4; n = 147 |
| Probability of re-operation following failure of bone integration or removal of implant due to grade 4 skin reaction | 0.9474 | N/A | Beta | r = 18; n = 19 |
| Probability of grade 1/2 skin reaction | 0.1188 | 95% CI (0.0665 to 0.1960) | Beta | α = 11.4037; β = 84.5776 |
| Probability of grade 3 skin reaction | 0.0396 | 95% CI (0.0129 to 0.0924) | Beta | α = 3.6565; β = 88.6690 |
| Probability of grade 4 skin reaction | 0.0079 | 95% CI (0.0002 to 0.0441) | Beta | α = 0.4956; β = 62.0683 |
| Cost of surgery for grade 3 skin reaction (£) | 635 | ± 25% of mean | Gamma | α = 126.0710; β = 5.0368 |
| Cost of outpatient attendance for grade 2 skin reaction (£) | 113 | ± 25% of mean | Gamma | α = 126.0710; β = 0.8963 |
| Cost of initial ENT consultation (paediatric) (£) | 126 | ± 25% of mean | Gamma | α = 126.0710; β = 0.9994 |
| Cost of follow-up ENT consultation (paediatric) (£) | 87 | ± 25% of mean | Gamma | α = 126.0710; β = 0.6901 |
| Cost of initial ENT consultation (adult) (£) | 106 | ± 25% of mean | Gamma | α = 126.0710; β = 0.8408 |
| Cost of follow-up ENT consultation (adult) (£) | 69 | ± 25% of mean | Gamma | α = 126.0710; β = 0.5473 |
| Cost of initial audiological assessment (£) | 55 | ± 25% of mean | Gamma | α = 126.0710; β = 0.4363 |
| Cost of attendance to fit BAHA (£) | 62 | ± 25% of mean | Gamma | α = 126.0710; β = 0.4918 |
| Cost of follow-up audiological assessments (£) | 48 | ± 25% of mean | Gamma | α = 126.0710; β = 0.3807 |
Glossary
- Aided thresholds
- The softest sounds that a person can hear while wearing hearing aid(s).
- Baffle side
- The side on which the hearing aid is worn.
- Deaf people
- The Royal National Institute for Deaf People uses the term ‘deaf people’ in a general way when talking about people with all degrees of deafness. Similarly, throughout this report we use the term ‘people who are bilaterally deaf’ to describe people with all degrees of deafness and hearing loss but affecting both ears to the same or differing degrees.
- Decibel (dB)
- Logarithmic unit of sound intensity. Letters A, B or C following dB in parenthesis [e.g. dB(A)] indicate the frequency weightings on a sound level meter.
- Decibels hearing level (dB HL)
- Decibel scale referenced to accepted standards for normal hearing (0 dB is average normal hearing for each audiometric test frequency).
- Decibels sound pressure level (dB SPL)
- Decibel scale referenced to a physical standard for pressure intensity.
- Directional hearing (auditory localisation)
- The ability to locate the direction from which a sound is coming, making use of differences in intensity and/or phase between the two ears.
- Free field
- A sound field whose boundaries exert a negligible effect on the sound waves, but often used synonymously with sound field. Note that the term ‘sound field’ testing is used throughout the report, despite some included studies using the term ‘free field’ testing, as this is unlikely for most studies.
- Masking
- The process by which the threshold of audibility for one sound is raised by the presence of another (masking) sound.
- Masking level difference (binaural masking level difference)
- The difference in threshold of the signal when the signal and a masker have the same phase and level relationships at the two ears and when the interaural phase and/or level relationships of the signal and masker differ.
- Maximum phoneme score
- The highest score obtained in a phoneme test irrespective of presentation level.
- Phoneme
- The minimal unit of sound in a language that is distinct from other sounds. Phoneme identification is used in speech perception and auditory language comprehension, testing phonological awareness or vowel identification abilities.
- Plomp test
- A test measuring speech reception threshold in quiet or noise.
- Pure tone
- A sound whose instantaneous sound pressure follows a sinusoidal function of time. Such a sound has only a single frequency component. Pure-tone stimuli are used to measure hearing sensitivity in audiometry.
- Pure-tone audiometry
- The procedure most commonly used for the measurement of hearing impairment. Pure tones are presented via air conduction and bone conduction and the individual’s sensitivity to discrete frequencies is measured.
- Shadow side
- The side opposite to the hearing aid (head shadow refers to the reduction of sounds as they travel from one side of the head to the other).
- Signal-to-noise ratio, speech-to-noise ratio
- Relationship between the sound levels of the signal and the noise of the listener’s ear, commonly reported as the difference in decibels between the intensity of the signal and the intensity of the background noise (e.g. if the speech signal is measured at 70 dB and the noise is 64 dB, the signal-to-noise ratio is + 6 dB). The higher the signal-to-noise ratio, the more difficult an individual finds it to hear in noise.
- Sound field
- A space where sound is propagated. In sound field testing, calibrated auditory signals are presented through loudspeakers into a sound-isolated room rather than through headphones to test hearing, often used when testing children who will not tolerate headphones and in evaluating hearing performance. Often used synonymously with free field. Note that the term ‘sound field’ testing is used throughout the report despite some included studies using the term ‘free field’ testing, as this is unlikely for most studies.
- Speech audiometry
- Measurement of speech perception skills including speech awareness and speech recognition, one component of an audiometric test battery.
- Speech detection threshold (speech awareness threshold)
- The lowest intensity level at which a person can detect the presence of a speech signal, it approximates the best hearing level in the 250–800 hertz (Hz) audiometric frequency region. Used clinically with children or others who have such a poor speech understanding that a speech recognition threshold cannot be obtained.
- Speech-in-noise
- A functional hearing test assessing how well an individual can understand speech in a noisy environment.
- Speech-in-quiet
- A functional hearing test assessing how well an individual can understand speech in a quiet environment.
- Speech recognition threshold (speech reception threshold)
- The lowest intensity level at which a person can detect the presence of a speech signal, it approximates the best hearing level in the 250–800 Hz audiometric frequency region.
- Threshold
- The intensity at which an individual can just barely hear a sound 50% of the time; all sounds louder than threshold can be heard, but sounds below threshold cannot be detected.
- Warble-tone thresholds
- An acoustic signal produced by modifying a pure tone with small and rapid changes in frequency; used in sound field audiometry to minimise the likelihood of standing waves from reflective surfaces.
- Word recognition test (speech discrimination test)
- A speech audiometry measure that typically uses monosyllabic words presented at a suprathreshold level in an open-set format; provides an assessment of a person’s speech understanding as a per cent correct score.
List of abbreviations
- AC
- air conduction
- ACHA
- air conduction hearing aid
- BAHA
- bone-anchored hearing aid
- BC
- bone conduction
- BCHA
- bone conduction hearing aid
- BNF
- British National Formulary
- CHL
- conductive hearing loss
- CI
- confidence interval
- DEALE
- declining exponential approximation to life expectancy
- dB
- decibel(s)
- dB(A)
- decibels A-weighted (decibels measured using an A scale sound filter)
- dB HL
- decibels hearing level
- dB SPL
- decibels sound pressure level
- DSA
- deterministic sensitivity analysis
- ENT
- ear, nose and throat
- EQ-5D
- European Quality of Life-5 Dimensions
- FDA
- Food and Drug Admistration
- HHDI
- Hearing Handicap and Disability Index
- HUI
- Health Utilities Index
- Hz
- hertz (unit of frequency)
- ICER
- incremental cost-effectiveness ratio
- IOI-HA
- International Outcomes Inventory for Hearing Aids
- kHz
- kilohertz
- MAIS
- Meaningful Auditory Integration Scale
- MUSS
- Meaningful Use of Speech Scale
- NIHR
- National Institute for Health Research
- OR
- odds ratio
- PCT
- primary care trust
- PSA
- probabilistic sensitivity analysis
- PSS
- Personal Social Services
- PTA
- pure-tone average
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- SD
- standard deviation
- SF-36
- Short Form questionnaire-36 items
- SNHL
- sensorineural hearing loss
- SNR
- signal-to-noise ratio
- SRT
- speech reception threshold
- SUHT
- Southampton University Hospitals NHS Trust
- UHB
- University Hospitals Birmingham NHS Foundation Trust
- WTP
- willingness to pay
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Department of Public Health and Epidemiology, University of Birmingham
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, University of Liverpool
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Peter Brocklehurst, Professor of Women’s Health, Institute for Women’s Health, University College London
-
Professor Jenny Donovan, Professor of Social Medicine, University of Bristol
-
Professor Jonathan Green, Professor and Acting Head of Department, Child and Adolescent Psychiatry, University of Manchester Medical School
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Freddie Hamdy, Professor of Urology, Head of Nuffield Department of Surgery, University of Oxford
-
Professor Allan House, Professor of Liaison Psychiatry, University of Leeds
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor Irwin Nazareth, Professor of Primary Care and Head of Department, Department of Primary Care and Population Sciences, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norrie, Chair in Clinical Trials and Biostatistics, Robertson Centre for Biostatistics, University of Glasgow
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Martin Underwood, Professor of Primary Care Research, Warwick Medical School, University of Warwick
-
Professor Marion Walker, Professor in Stroke Rehabilitation, Associate Director UK Stroke Research Network, University of Nottingham
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Tom Foulks, Medical Research Council
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Diana Baralle, Consultant and Senior Lecturer in Clinical Genetics, University of Southampton
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Public contributor
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Nicola Lennard, Senior Medical Officer, MHRA
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Public contributor
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Public contributor
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Matthew Thompson, Senior Clinical Scientist and GP, Department of Primary Health Care, University of Oxford
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Joanna Jenkinson, Board Secretary, Neurosciences and Mental Health Board (NMHB), Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Public contributor
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire County Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Mrs Jean Thurston, Public contributor
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Public contributor
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Public contributor
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Public contributor
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Public contributor
-
Mr Sankaran Chandra Sekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Public contributor
-
Mr Seumas Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Dr Matthew Wilson, Consultant Anaesthetist, Sheffield Teaching Hospitals NHS Foundation Trust
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan, Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Public contributor
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer, Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Public contributor
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Public contributor
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Public contributor
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Expert Advisory Network
-
Professor Douglas Altman, Professor of Statistics in Medicine, Centre for Statistics in Medicine, University of Oxford
-
Professor John Bond, Professor of Social Gerontology & Health Services Research, University of Newcastle upon Tyne
-
Professor Andrew Bradbury, Professor of Vascular Surgery, Solihull Hospital, Birmingham
-
Mr Shaun Brogan, Chief Executive, Ridgeway Primary Care Group, Aylesbury
-
Mrs Stella Burnside OBE, Chief Executive, Regulation and Improvement Authority, Belfast
-
Ms Tracy Bury, Project Manager, World Confederation of Physical Therapy, London
-
Professor Iain T Cameron, Professor of Obstetrics and Gynaecology and Head of the School of Medicine, University of Southampton
-
Professor Bruce Campbell, Consultant Vascular & General Surgeon, Royal Devon & Exeter Hospital, Wonford
-
Dr Christine Clark, Medical Writer and Consultant Pharmacist, Rossendale
-
Professor Collette Clifford, Professor of Nursing and Head of Research, The Medical School, University of Birmingham
-
Professor Barry Cookson, Director, Laboratory of Hospital Infection, Public Health Laboratory Service, London
-
Dr Carl Counsell, Clinical Senior Lecturer in Neurology, University of Aberdeen
-
Professor Howard Cuckle, Professor of Reproductive Epidemiology, Department of Paediatrics, Obstetrics & Gynaecology, University of Leeds
-
Professor Carol Dezateux, Professor of Paediatric Epidemiology, Institute of Child Health, London
-
Mr John Dunning, Consultant Cardiothoracic Surgeon, Papworth Hospital NHS Trust, Cambridge
-
Mr Jonothan Earnshaw, Consultant Vascular Surgeon, Gloucestershire Royal Hospital, Gloucester
-
Professor Martin Eccles, Professor of Clinical Effectiveness, Centre for Health Services Research, University of Newcastle upon Tyne
-
Professor Pam Enderby, Dean of Faculty of Medicine, Institute of General Practice and Primary Care, University of Sheffield
-
Professor Gene Feder, Professor of Primary Care Research & Development, Centre for Health Sciences, Barts and The London School of Medicine and Dentistry
-
Mr Leonard R Fenwick, Chief Executive, Freeman Hospital, Newcastle upon Tyne
-
Mrs Gillian Fletcher, Antenatal Teacher and Tutor and President, National Childbirth Trust, Henfield
-
Professor Jayne Franklyn, Professor of Medicine, University of Birmingham
-
Mr Tam Fry, Honorary Chairman, Child Growth Foundation, London
-
Professor Fiona Gilbert, Consultant Radiologist and NCRN Member, University of Aberdeen
-
Professor Paul Gregg, Professor of Orthopaedic Surgical Science, South Tees Hospital NHS Trust
-
Bec Hanley, Co-director, TwoCan Associates, West Sussex
-
Dr Maryann L Hardy, Senior Lecturer, University of Bradford
-
Mrs Sharon Hart, Healthcare Management Consultant, Reading
-
Professor Robert E Hawkins, CRC Professor and Director of Medical Oncology, Christie CRC Research Centre, Christie Hospital NHS Trust, Manchester
-
Professor Richard Hobbs, Head of Department of Primary Care & General Practice, University of Birmingham
-
Professor Alan Horwich, Dean and Section Chairman, The Institute of Cancer Research, London
-
Professor Allen Hutchinson, Director of Public Health and Deputy Dean of ScHARR, University of Sheffield
-
Professor Peter Jones, Professor of Psychiatry, University of Cambridge, Cambridge
-
Professor Stan Kaye, Cancer Research UK Professor of Medical Oncology, Royal Marsden Hospital and Institute of Cancer Research, Surrey
-
Dr Duncan Keeley, General Practitioner (Dr Burch & Ptnrs), The Health Centre, Thame
-
Dr Donna Lamping, Research Degrees Programme Director and Reader in Psychology, Health Services Research Unit, London School of Hygiene and Tropical Medicine, London
-
Professor James Lindesay, Professor of Psychiatry for the Elderly, University of Leicester
-
Professor Julian Little, Professor of Human Genome Epidemiology, University of Ottawa
-
Professor Alistaire McGuire, Professor of Health Economics, London School of Economics
-
Professor Neill McIntosh, Edward Clark Professor of Child Life and Health, University of Edinburgh
-
Professor Rajan Madhok, Consultant in Public Health, South Manchester Primary Care Trust
-
Professor Sir Alexander Markham, Director, Molecular Medicine Unit, St James’s University Hospital, Leeds
-
Dr Peter Moore, Freelance Science Writer, Ashtead
-
Dr Andrew Mortimore, Public Health Director, Southampton City Primary Care Trust
-
Dr Sue Moss, Associate Director, Cancer Screening Evaluation Unit, Institute of Cancer Research, Sutton
-
Professor Miranda Mugford, Professor of Health Economics and Group Co-ordinator, University of East Anglia
-
Professor Jim Neilson, Head of School of Reproductive & Developmental Medicine and Professor of Obstetrics and Gynaecology, University of Liverpool
-
Mrs Julietta Patnick, Director, NHS Cancer Screening Programmes, Sheffield
-
Professor Robert Peveler, Professor of Liaison Psychiatry, Royal South Hants Hospital, Southampton
-
Professor Chris Price, Director of Clinical Research, Bayer Diagnostics Europe, Stoke Poges
-
Professor William Rosenberg, Professor of Hepatology and Consultant Physician, University of Southampton
-
Professor Peter Sandercock, Professor of Medical Neurology, Department of Clinical Neurosciences, University of Edinburgh
-
Dr Philip Shackley, Senior Lecturer in Health Economics, Sheffield Vascular Institute, University of Sheffield
-
Dr Eamonn Sheridan, Consultant in Clinical Genetics, St James’s University Hospital, Leeds
-
Dr Margaret Somerville, Director of Public Health Learning, Peninsula Medical School, University of Plymouth
-
Professor Sarah Stewart-Brown, Professor of Public Health, Division of Health in the Community, University of Warwick, Coventry
-
Dr Nick Summerton, GP Appraiser and Codirector, Research Network, Yorkshire Clinical Consultant, Primary Care and Public Health, University of Oxford
-
Professor Ala Szczepura, Professor of Health Service Research, Centre for Health Services Studies, University of Warwick, Coventry
-
Dr Ross Taylor, Senior Lecturer, University of Aberdeen
-
Dr Richard Tiner, Medical Director, Medical Department, Association of the British Pharmaceutical Industry
-
Mrs Joan Webster, Consumer Member, Southern Derbyshire Community Health Council
-
Professor Martin Whittle, Clinical Co-director, National Co-ordinating Centre for Women’s and Children’s Health, Lymington