Notes
Article history
The research reported in this issue of the journal was commissioned by the HTA programme as project number 03/46/09. The contractual start date was in June 2005. The draft report began editorial review in June 2010 and was accepted for publication in October 2010. As the funder, by devising a commissioning brief, the HTA programme specified the research question and study design. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2011. This work was produced by Gregory et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2011 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction to the DEPICTED study
Diabetes
Diabetes is the third most common chronic disease in childhood, with 1–2 per 1000 children and adolescents in the UK receiving prescriptions of insulin in recent years (1998 and 2005). 1 Since 1989, the incidence has doubled, with a particularly marked increase noted in the preschool age group. 2 In childhood, the vast majority of affected children experience autoimmune-mediated destruction of their insulin-secreting pancreatic β-cells, which leads to insulin deficiency (type 1 diabetes). As a consequence of insulin deficiency, children develop raised blood glucose concentrations (hyperglycaemia), which lead to excess urinary losses (polyuria) and therefore increased thirst (polydipsia). In addition, insulin deficiency leads to uncontrolled breakdown of fat (lipolysis), as demonstrated by marked weight loss over relatively short time periods. Lipolysis in the presence of insulin deficiency results in ketosis, which, if uncontrolled, may lead to potentially life-threatening episodes of acute diabetic ketoacidosis. The presence of vomiting or development of ketoacidosis are common reasons for children with diabetes to require hospital admission. The acute metabolic consequences of insulin deficiency may be reversed or prevented by the administration of an insulin treatment.
Complications of diabetes
In the short term, excess insulin for requirements may cause hypoglycaemia (low blood glucose levels), which, if severe, may lead to loss of consciousness. By contrast, inadequate insulin therapy may cause symptoms similar to those at diagnosis (see above). In the longer term, chronically elevated blood glucose concentrations leads to an increased risk of clinical complications. In childhood, poor glycaemic control causes growth failure and pubertal delay, which may be reversible with improved clinical management including optimisation of insulin therapy. In the longer term, more serious and eventually irreversible microvascular complications arise. These include sight-threatening retinopathy and renal disease. Initially, renal disease is asymptomatic and detected by increased protein (albumin) excretion in the urine but, if untreated, will eventually deteriorate leading to renal failure and the need for dialysis. A further devastating complication is neuropathy, which may produce a range of symptoms such as impaired peripheral sensation and pain or gastrointestinal and genitourinary problems if the autonomic system is affected, resulting in major adverse effects on quality of life (QoL). In addition to the microvascular complications, macrovascular disease is common, with increased risks of myocardial infarction and strokes in later life. Microvascular and macrovascular complications are rarely seen in childhood, but occur with increasing frequency in young adult life. There is clear evidence, however, that the quality of blood glucose control through childhood is a significant risk factor for the development of many of these complications in later adult life. 3
Psychosocial aspects of diabetes
The management of diabetes is complex, requiring significant practical expertise to optimise outcomes and, unsurprisingly, may result in significant psychological difficulties for young people with diabetes and their families. Variations in blood glucose concentrations, particularly overnight, have been shown to affect mood and behaviour. 4 The difficulties of adhering to a practically demanding regimen may result in overdependence of children on their parents5 or adverse effects on behaviour, including an increase in suicidal thoughts. 6 For the family, managing childhood diabetes brings particular pressures, including the grief experienced by parents at diagnosis. 7 In relation to the challenges of the day-to-day management of the diabetes, problems may occur in communication between parent and child, and there is a risk of increased family conflict with the experience of frustration and guilt at failure to achieve optimal outcomes. 8 Existing psychological issues within families involving functioning, coping and interpersonal relationships may be exacerbated. Psychiatric and psychological problems (including eating disorders and effects on body image, etc., exacerbated by the inter-relationship with insulin and other aspects of diabetes management) are therefore unsurprisingly seen more commonly in young people with diabetes than in the non-diabetic population. 9,10
It is well recognised11 that psychosocial and educational influences play a key role in determining management outcomes in children with diabetes. For example, a large audit in Scotland has shown that throughout childhood family structure is associated with glycaemic control. 12 During adolescence, rapid physical change (puberty) leads to relative resistance to the effects of insulin. 13 Concurrent major developmental changes include increasing independence, emerging sexuality and increased stress from peer and academic pressures. These factors together are often associated with deteriorating glycaemic control. Knowledge and skills imparted by the diabetes teams are especially important tools for the child and their family to achieve optimal glycaemic control during this crucial period.
Diabetes management
The management of diabetes by patients and their family requires them to develop an understanding of the complex interaction of the effects of insulin, food and physical activity on blood glucose concentrations. Treatment of diabetes involves the regular administration of insulin, most commonly by two to four subcutaneous injections daily or through the use of an insulin pump, which provides a continuous infusion of insulin through a subcutaneously sited catheter. A healthy lifestyle is recommended, including regular physical activity and a diet that regulates carbohydrate and fat intake. To optimise diabetes management, it is recommended14 that the patient and his/her family develop a sophisticated understanding of the carbohydrate content of food so that the amount of insulin administered can be finely tuned (so-called ‘carbohydrate counting’). The efficacy of management is monitored in the short term by regular self-measurement (ideally four or more times daily) of blood glucose concentrations and in the longer term by monitoring (3–4 monthly) glycosylated haemoglobin (HbA1c) levels in blood and regular review in paediatric diabetes clinics.
In the UK, clinical care is usually delivered by paediatric diabetes services established in secondary care. Such services require the multidisciplinary input of doctors with expertise in both paediatrics and childhood diabetes, nurse specialists who liaise between the clinic, the child’s home and school, dietitians, child psychologists, podiatrists and social workers. There also needs to be close collaboration between paediatric and adult services to ensure that as children progress through their teens arrangements are made for their care to be handed over from paediatric to adult services. This is a time when particular difficulties may be encountered by clinical services, as teenagers with diabetes take increasing responsibility for their self-management and also encounter the problems caused by increased insulin resistance during puberty. 15
Adherence to diabetes management
The aims of paediatric diabetes services are to support and educate children and their parents in the care of diabetes, to manage diabetes in a manner that optimises clinical outcomes and to prepare teenagers for young adult life by helping them to become increasingly independent in their self-management. Given the complexities of diabetes management described above, it is unsurprising that many children and their families struggle to adhere to optimal treatment strategies, resulting in adverse consequences for diabetes outcomes in both the short and longer term. In the UK, the National Institute for Health and Clinical Excellence (NICE) has recommended that parents and children be informed that the target for optimal HbA1c concentrations is values < 7.5%. 16 However, an audit of outcomes in 2002 for children treated in the UK demonstrated that, depending on age, only 14–20% of children cared for in clinical services in the UK achieve these outcomes. 17
The landmark Diabetes Control and Complications Trial (DCCT study) has shown that provision to a group of teenagers and young adults of very high levels of support from the multidisciplinary team to facilitate intensification of their diabetes treatment can produce dramatic improvements in blood glucose and HbA1c concentrations. 18 After a mean of 6.5 years’ follow-up, the group who received intensification of their diabetes management experienced – by comparison with the control group receiving conventional treatment – a reduction in their risk for the development of retinopathy of 76%, microalbuminuria of 39% and clinical neuropathy of 60%, albeit at a cost of a two- to threefold increase in severe hypoglycaemia. Subgroup analysis has shown similar benefits for the younger participants in this study. Interestingly, even after the discontinuation of the DCCT study when both arms experienced similar HbA1c concentrations, those who had undergone intensified therapy continued to experience a longer-term benefit of a reduced risk of developing diabetes-related complications, including a near 50% reduction in serious adverse cardiovascular disease event. 19–21
The challenge for paediatric diabetes clinical services, therefore, is how to facilitate patients and their families to make changes in their diabetes management that result in similar improvements in HbA1c level to those achieved in the DCCT study, with subsequent reduced risks of diabetes-related complications.
Behaviour change
Theories of health behaviour change (e.g. reasoned action theory, the health action process approach) and the research associated with them have clarified the need to look beyond a simple approach to adherence and change based upon the delivery of expert information. 22 As Marteau and Lerman23 have put it, ‘Just telling people they are at risk of developing a disease is rarely sufficient to change behaviour’. Two variables run through many of the theoretical models as predictors of health behaviour change: beliefs about the value of change and beliefs about one’s capacity to succeed (self-efficacy). Thus, for example, the efficacy of theory-based interventions such as cognitive behavioural therapy (CBT) has largely been attributed to their capacity to enhance self-efficacy. 24 Using a skills-based approach to counselling has been found to be effective in a number of fields. 24. 25 So, too, brief interventions have been found to be effective in changing a number of risky health behaviours. 26
A second line of research has focused on how the therapeutic relationship either hinders or promotes motivation to change. For example, an early effort to understand the effective ingredients of motivational interviewing (MI)27 identified a correlation between confrontational interviewing and resistance, and between ‘change talk’ and behaviour change. 28 A meta-analysis of MI29 found consistent evidence for effectiveness in some (e.g. alcohol, drug use), but not in all behavioural domains. Interest in the field of diabetes among young people has also emerged. 30–33 One of the challenges in much of this research, however, has been to clarify exactly what elements of a complex method were used by the interventionists. It does appear that some of the principles of MI can be realised in brief health-care consultations, and that helping patients to clarify for themselves why and how they might change their behaviour (MI) can be more effective than brief advice-giving. 34,35 One recent development has been the first effort to integrate this method with CBT. 36 Put simply, this body of work calls attention to both the direction of consultations about change (towards enhancing coping skills) and the way patients are spoken to (eliciting motivation and solutions from them).
Psychoeducational interventions in diabetes
An NHS health technology assessment (HTA) systematic review of the effects of educational and psychosocial interventions for adolescents with diabetes, which led to the commissioning brief for this study, reported that there were no results from randomised controlled trials (RCTs) of psychoeducational interventions in the UK. 37 However, the review did identify an ongoing study evaluating the effects of MI on behaviour change in teenagers. 32 This trial was based on positive findings in a pilot study in children33 and an RCT involving adults with type 2 diabetes. 38,39 The review commented that small to medium-sized beneficial effects on a variety of diabetes management outcomes have been demonstrated mostly in North American studies. 40 It concluded that there is a need for well-designed clinical trials that recognise the inter-relatedness of various aspects of diabetes management and assess outcomes that are specifically targeted for change, at an appropriate time after the intervention. In particular, the review recommended that such research be developed by a consultation process with stakeholders including patients, their families, health-care professionals (HCPs) and health economists. The commissioning brief for this research project further refined these principles in that effort should be directed towards a generic intervention that does not require delivery by trained clinical psychologists, given their relative scarcity in paediatric diabetes services. 41
Overview of the DEPICTED study
The study described in this report was delivered in two phases. The first phase involved six developmental components required to inform the development of the emerging intervention (health-care staff trained to modify their consultation approach to help them discuss behaviour change skilfully in their patients and families), and was followed by a second phase in which the intervention and training programme were trialled. This overview starts with a brief consideration of the issues relevant at the time to modelling and complex intervention development.
Modelling and complex intervention development
This research did not start out with a fixed position on the best psychosocial approach on which to base the intervention. However, a number of principles and conceptual aids were brought to the development process for consideration by the research team and associated stakeholders (Box 1).
The need to integrate behaviour change within routine clinical care encounters
Consideration and balancing of multiple behaviours
Matching intervention components to individual need
An intervention that addresses common clinical problems, delivered by non-specialist (i.e. psychologist) diabetes practitioners
First, there was the need to integrate talk about lifestyle change, self-control and QoL with routine care when patients are at the receiving end of a range of medical and nursing interventions. Practitioners would need to find ways of moving between providing medical care on the one hand and ‘letting go responsibility’ on the other,39 to encourage children and teenagers to take control of their health with assistance from others. Of relevance, therefore, was a model developed by one of the co-applicants with practitioners in the coronary heart disease field, which described the value of moving flexibly between directing, listening and guiding communication styles when talking about behaviour change. 42
A second conceptual and clinical challenge was the need to move beyond thinking about change as involving an isolated, single behaviour, a limitation in much of the theory of behaviour change in health psychology. The challenge was to help patients find a balance between multiple and inter-related health behaviours and lifestyle choices. 37,39,43 How to negotiate a complex behaviour change agenda would be one useful starting point in intervention development. 44
Thirdly, the possibility of targeting or matching interventions to the needs of patients would need to be borne in mind. Efforts to match interventions to patients in other fields45,46 have proved difficult; therefore, the feasibility of targeting would be a particular focus for the stakeholders to consider. Among the key targets might be interventions, for example, for different age groups or for talking to parents in a constructive way. Another view of targeting would be to regard this as something that happens not across interventions but within the consultation, as the practitioner shifts style and topic according to the needs of the patient. 47 To this end, there was some evidence for the acceptability and feasibility of using a targeting approach based on a flexible menu of strategies in which the practitioner and patient selected a topic according to need. 46,48 This intervention framework had been developed in efforts to train health-care professionals to use elements of MI, and an application among drug-abusing young people had produced promising results. 49 In the present context, however, it was not the intervention approach (MI) or content that might have been useful, but the use of a framework or methodology for targeting within the consultation based on a menu of topics for discussion.
Finally, the intervention development process would benefit from a clear understanding of who would be providing what and to whom. To this end a conceptual approach at the outset helped to distinguish between:
-
psychological therapy provided by a therapist, using a wide range of skills in a relatively long consultation
-
brief counselling provided by any health-care professional that involves setting aside some time, perhaps 10–15 minutes, to discuss specific issues of importance to the patient
-
psychosocial intervention as part of routine care and consultations; this third level of intervention required the use of a much narrower range of therapeutic skills, but carried the advantage of use in a relatively much large number of consultations.
The level of intervention in this research would fit within points (2) and (3) above, and its exact nature would emerge from the various developmental studies in the first phase of this research.
In summary, it was essential to move beyond the use of a simple model of compliance that assumed that patients merely need expert information to encourage behaviour change. Theory and research on behaviour change and development work already carried out clearly indicated that the dynamics of talk about behaviour change were more complex.
Phase I
Intervention development
The modelling stage in developing a complex intervention uses appropriate exploratory methods to identify and clarify the effective components of the intervention, as well as considering factors such as acceptability and feasibility. Modelling may also be used to better understand the processes operating with the normal (usual care) setting. 50 Phase I of this research would follow this guidance using a variety of research methods, and, combined with the review of the literature,37 would build on approaches found to be useful in development work in other areas. For example, patients would be used not only to understand the issues,51–53 but also to receive the emerging intervention and provide feedback. 34 We also planned that the emergent intervention once developed would be thoroughly documented. 48,54 Materials developed in the study for use by health-care professionals would draw heavily upon clinical examples (including lay study participants and practitioners), providing face validity to the intervention. Practitioners would also be part of the intervention development process; a survey of current practice and promising interventions would be accompanied by interviews with them51 and simulated consultations would be used to refine the intervention. 55
Practitioner training and skill acquisition/assessment
A similar approach would be required to develop an acceptable and feasible method for helping practitioners to learn new skills. Training practitioners to change their behaviour, to use a complex intervention of the kind described above, would clearly need to move beyond the delivery of guidelines for good practice56 or the production of a training manual. Even if the intervention was relatively simple when compared with specialist delivery of psychological therapy, some form of face-to-face training would probably be essential. The development work in phase I would seek to model a training programme that itself is acceptable and feasible for practitioners. Among the approaches to be used were:
-
surveys (telephone and postal) for establishing current practice and the acceptability of training options
-
provision of time for practitioners themselves to contribute the training outline via a specially constituted group of lay and professional stakeholders – the Stakeholder Action Group (SAG)
-
pilot training to refine its structure and content in the light of change in competencies and feedback from participants themselves.
Similar work among general practitioners has led to the development of what has been called context-bound learning,55,57 in which everyday clinical scenarios form the basis for learning new skills and for monitoring their use in practice.
The core research team would work with the SAG and other contributors to develop aspects of the intervention and training programme. For the latter, a resource that they could consider adopting was the use of simulated patients. Other available resources included an existing software architecture designed to host training content for health practitioners (Talking Sense, Cardiff University, Cardiff and Smile-On Ltd, London), which could be adapted to suit varying health or social-care settings.
If the intervention to be used involved face-to-face with patients, a measure of practitioner competence would be an essential adjunct to assessing the efficacy of training and for monitoring the quality of intervention delivery in the RCT. This project would utilise the team’s recent experience of developing an instrument to measure shared decision-making in primary care58 and another on the subject of behaviour change counselling in health-care settings. 59,60 It was expected that initial development work would commence in phase I, but would continue through the course of phase II.
Preparation for the randomised controlled trial
The modelling process informed the development of the intervention prior to the trial. Outcomes were to be compared with those arising from ‘control’ centres delivering ‘usual care’. The stakeholders and user consultation process would identify the most relevant established outcomes to be targeted by the intervention. In addition to the process for assessing professional performance just described, a survey for assessing patient and carer preferences within the consultation was developed in this phase. This included the identification of attributes for a discrete choice experiment (DCE) in collaboration with the SAG (described further in Chapter 6).
Phase II
The effectiveness of the intervention developed in phase I would be assessed using a pragmatic cluster RCT design described more fully later in this report (see Chapters 8–13). The primary outcome in this trial was to be the change in blood HbA1c concentrations in patients with type 1 diabetes. Following a 12-month study period, comparisons between intervention and control groups would also include the following secondary outcomes.
Patients
-
Clinical measures such as body mass index (BMI).
-
Patient-reported outcomes, such as generic and specific QoL, self-care/management activities, perceptions of health-care providers and preferences for care.
-
Service usage measures, such as hospital admissions (particularly with ketoacidosis), attendance at diabetes clinic, other health service contacts.
Carers
-
Self Generic QoL, perceptions of health care provided and preferences for care.
-
Proxy Generic and specific QoL for the younger child, school absences.
Professionals
-
Performance of techniques taught during the training programme.
A cost-effectiveness analysis would be undertaken assessing costs against the primary outcome measure (levels of HbA1c).
Presentation of this report
The next six chapters present the component studies of the development phase of the DEPICTED (Development and Evaluation of a Psychosocial Intervention for Children and Teenagers Experiencing Diabetes) study. Chapter 2 presents the overarching methodological framework adopted for these studies and Chapters 3–6 report the individual studies. Chapter 7 describes how these were integrated within the intervention and training programme, which are also described in detail, and it also serves to summarise the body of work conducted in the developmental phase. Chapters 8–10 describe the introduction, methods and results of the trial, respectively. The DCE, which explores patient and carer preferences, is described in entirety in Chapter 11, whereas the trial process evaluation (PE) is presented in Chapter 12. Finally, the results from the DEPICTED study as a whole are discussed, with conclusions, in Chapter 13.
Chapter 2 Phase I of the DEPICTED study: overview and framework of developmental studies
In this first chapter describing the work conducted within the developmental phase of the DEPICTED study, we present an update of the existing evidence base and the theoretical rationale underpinning our approach. First, the previous HTA systematic review of the effects of educational and psychosocial interventions for adolescents with diabetes mellitus61 was updated. Second, we provide details of the MI approach, which underwrote many aspects of our developing intervention. Finally, we summarise the framework for our methodological approach.
Updating the systematic review
In 2001, the NHS research and development (R&D) HTA programme published a systematic review of the effects of educational and psychosocial interventions for adolescents with diabetes mellitus. 37 In summary, this review identified 62 studies, of which 25 were RCTs, mostly conducted in the USA (none from the UK). The mean (pooled) effect size was 0.37 for psychosocial outcomes and 0.33 for HbA1c with outliers (0.08 without outliers), suggesting that these interventions have small to medium beneficial effects on diabetes management outcomes. The authors concluded that future studies should be evaluated by assessing outcomes that the intervention specifically targets for changes, at an appropriate point in time post intervention to reflect the impact and durability of the intervention. The lack of cost-effectiveness analyses of published studies was highlighted.
When our study was initiated, we undertook an update to this systematic review. At the time of analysis of papers identified, similar structured and systematic review updates of the effectiveness of psychoeducational interventions in children with diabetes were published. 62–64 These published reviews identified largely similar manuscripts and drew similar conclusions to those that we were developing at that time and, therefore, we will draw largely upon their findings.
A further 27 papers had been published describing the evaluation of 24 psychoeducational interventions. 64 As before, routine clinical care seems to produce inadequate metabolic outcomes. Education seems most effective when integrated into routine care, where continued parental involvement65,66 and adolescent self-efficacy is encouraged. Although there was evidence of a methodological improvement in published trials, there was no evidence of improved effectiveness of the interventions. Although psychological interventions seemed more effective in children and adolescents than in adults,63 few studies have investigated the effectiveness of interventions in younger children and most trials remained underpowered to demonstrate modest, but clinically significant improvements in HbA1c level. An estimated sample size of 360 is required to show an HbA1c concentration difference of 0.5%, and a sample size of 350 is required to detect a small psychological effect size of 0.3 with 80% power. 64 Most psychological interventions were based around CBT and the limited understanding of the potential of MI in childhood diabetes32,33 was highlighted. 63 No specific psychoeducational intervention could be deemed superior to others. Specifically, there were no interventions that seemed effective when targeting those with poor glycaemic control, and concerns have been expressed62 that targeting only those demonstrating ‘readiness to change’ patterns of thinking may overestimate the effectiveness of certain interventions. 33
Hampson and colleagues37 concluded that agreement was required on appropriate, valid outcome measures for trials of psychoeducational interventions, but little progress has been made in this respect. The need to agree measures that include glycaemic control using common reference methodology, age-validated questionnaires for psychosocial variables and service utilisation and cost measures is clear. Unresolved issues include the relative importance of the content of the intervention as opposed to contact time with the interventionist and whether or not interventions should be combined with other efforts to intensify insulin therapy. 67 Given the increasing importance ascribed to education and the wider number of HCPs, including physicians, providing such education to patients and their families, the importance of understanding the role of self-efficacy, the principles of education and its delivery have been highlighted as priorities for training. 64 Future interventions should be theoretically grounded, with clearly described protocols to allow adequate analysis and reproduction62 and greater priority given to patient preferences. 63
Motivational interviewing
The starting point
When this research was awarded funding, there was consensus within the team that MI might inform the emergence of the intervention to be developed. This consensus was based on two features of MI: first, its purposeful focus on behaviour change, which seemed suited to the lifestyle challenges faced by children with type 1 diabetes and, second, its focus on using the professional relationship to enhance motivation for change.
These two features of MI lie at the centre of a method originally developed in the addictions field in the early 1980s as a form of psychotherapy. The central feature of this method is the use of empathic listening rather than confrontation when speaking to people struggling with ambivalence about behaviour change; specific listening techniques are developed to encourage clients to express their own arguments for change (phase I) and to formulate a plan of action that feels achievable (phase II). 27
Refinement of motivational interviewing for health-care settings
From its origins in the addictions field, MI was adapted and refined in a number of ways over the following 20 years. To begin with, attention focused on a series of research studies that examined the process and outcome of feeding back test results to people with drinking problems. Thus, for example, outcome was significantly better if these results were fed back in an empathic style compared with a more usual ‘confrontational’ style. 68 This led to the development of a four-session variant of MI called motivational enhancement therapy. Other research confirmed the importance of counsellor style on behaviour change outcomes. For example, in the delivery of behaviour therapy, counsellor empathy accounted for over two-thirds of the variance in outcome. 69 MI delivered prior to treatment (inpatient and outpatient), with adults and adolescents, produced better outcomes of subsequent treatment and also improved retention in treatment (see Miller and Rose70 for a review of this research).
By the mid-1990s the most striking refinement was in the development of brief forms of MI suitable for application in health-care and other settings. Development work and a series of outcome studies were published in a number of fields, for example among drinkers in a hospital setting,46 smokers in primary care34 and among adults with type 2 diabetes. 38
Among the innovations that emerged from this health-care development work were:
-
‘agenda-setting’ – tested in the diabetes field,39 this is a strategy for helping patients make choices about the kind of behaviour change on which they and the practitioner feel it is advisable to focus
-
the ‘elicit–provide–elicit’ sequence for exchanging information
-
the ‘pros and cons’ strategy for resolving ambivalence about behaviour change
-
the ‘importance and confidence’ strategy for conducting a rapid assessment of motivation to change, in which scaling questions are used to encourage patients to articulate why and how they might change.
Much of this work was documented in a practical text for clinicians,44 and the first systematic review of brief forms of MI in four behavioural domains was published in 2001. 71
By the time this research study was funded, two other significant developments had occurred: first, the research base had broadened, with four further reviews and meta-analyses confirming the effectiveness of MI in many settings and problem areas, although not all. 29,72–75 The last of these reviews embraced 72 randomised trials. The current record presents over 200 trials to date across a wide range of clinical settings. 70
A second, more recent, development was a conceptual one, designed to explain the link between MI and everyday practice. To this end, it was suggested that better practice in consultations about change might be promoted through a switch in style from directing to guiding,42 with MI being conceptualised simply as a refined form of the guiding style. As such, learning a guiding style in health-care consultations might provide the foundation for more specialist or complex MI practice.
Application of motivational interviewing in the diabetes field in Cardiff
On the initiation of this study, development work and position papers had earmarked MI as a potential intervention in the diabetes field. 44,61,76,77
Within the School of Medicine, Cardiff University, a Medical Research Council (MRC)-funded trial had examined the ability of general practitioners and nurses to use an agenda-setting chart to elicit meaningful changes areas from patients with type 2 diabetes. 38,39 Attention then turned to children with type 1 diabetes in a series of studies that led up to the current research project. Initially, an encouraging pilot feasibility study was conducted that explored the potential of counsellor-delivered MI for lowering HbA1c levels;33 this was followed by a larger randomised, multisite trial in which a nurse counsellor trained in MI produced significantly better outcomes than routine care supported by non-directive support counselling. 32 Of particular interest here was the use of agenda-setting in both of the above studies. Finally, a study by Viner and colleagues31 seemed to support the robustness of MI for adaptation in the diabetes field, leaving open the question of whether or not it was possible to adapt the method further for use by any clinician involved in the routine care of children with type 1 diabetes.
Some questions about motivational interviewing for the development phase
Among the questions about MI taken into the development phase of this research were the following:
-
What training experience and aspirations held by clinicians might lend themselves to which elements of MI?
-
What are the priorities of parents and children in consultations with clinicians, and how might these be blended with what elements of MI?
-
How feasible is it to train everyday diabetes practitioners in the finer arts of listening skills, apparently central to MI?57,70
-
How attractive is the idea of the guiding style to clinicians, parents and children?
-
Could the idea of ‘agenda-setting’ prove attractive to all participants involved?
Framework for the methodological approach
The approach of the team to the research development of the intervention mirrored many of the principles they felt could underpin the resulting clinical intervention itself. As a group of experts in the field, we felt we had some ideas that might be useful, but needed to explore how the target practitioners and patients would receive these ideas and what they would find useful.
These questions provided the starting point for the intervention development process, in which the systematic study of the views and experiences of clinicians would be brought to a multidisciplinary group of stakeholders that included parents and children. This stakeholder group would work with the research team to design the guiding principles and structure of an intervention that responded to the needs of all involved. Chapters 3–7 provide an account of this unfolding development process.
Chapter 3 Telephone survey of professionals: the challenges faced in meeting psychological needs in routine care
Introduction
This section is a description of a survey of practitioners, one of the stakeholder consultation activities designed to elicit information about their experiences of meeting psychosocial needs in clinical practice. The aims of the survey were to understand practitioners’ own assessment of challenges in delivering routine care and their existing approaches to encouraging behaviour change. This information would inform the development of the intervention and the training programme for teams.
Method
A random sample of 112 hospital centres stratified by region was selected from an augmented list of 216 UK hospitals (excluding Northern Ireland) providing services to children and young people with diabetes in the UK. 78 No more than one hospital per trust was selected for inclusion. Consultants (or nominated alternatives) responding positively to an initial postal approach were followed up by telephone interview.
A telephone interview schedule was developed by the research team with additional input from Diabetes UK. Survey domains covered included current and past service innovation and educational approaches, routine care provision, psychological support and clinic characteristics (Box 2).
Clinic characteristics (e.g. size, specialist nursing sessions, access to psychiatric and/or psychology support, routine clinic structure)
Past and present psychosocial support initiatives
Education programmes within the service
Target outcomes for children and adolescents
Main challenges in providing care
Gaining awareness of patients’ psychosocial needs
Current approaches to behaviour change
The survey was anchored on patients at least 12 months post diagnosis. The survey instrument was piloted in six interviews by two members of the research team with four local practitioners. The interview was planned for 20 minutes’ duration, included several open-ended items with standardised probes, and was audio-recorded with respondents’ verbal consent. Two interviewers completed the interviews.
Analysis
Responses to quantitative items were analysed and reported using percentages. Responses to key open-ended items were transcribed, analysed and coded according to emergent themes. A priori categories were not used. Coding of the narrative data was agreed by two researchers (HH and KB) who both independently coded three interviews and then the coding was completed by one researcher (HH) supported by the use of a Microsoft Access 2003 database (Microsoft Corporation, Redmond, WA, USA). Illustrative extracts will be used to support the description of emergent themes with the coding of ‘D’ for doctor and ‘N’ for nurse, followed by their identity number.
Results
Seventy (63%) practitioners responded to the initial approach and 51 clinicians completed the interview, of whom 22 (43%) were from teaching hospitals. Forty-four respondents (86%) were doctors and seven (14%) were nurses. Characteristics of responding practitioners and clinics are summarised in Table 1.
| Respondent | n (%) | |
|---|---|---|
| Gender | ||
| Male | 35 (69) | |
| Female | 16 (31) | |
| Profession | ||
| Medical | 44 (86) | |
| Nursing | 7 (14) | |
| Previous training | ||
| Postgraduate communication skills | 16 (31) | |
| Psychology-based training | 15 (29) | |
| Clinic sizea | ||
| Mean (SD) no. of nursing sessions (per 100 clients) | ||
| Small (≤ 70) | 10 (20) | 15.7 (7.7) |
| Medium (71–150) | 25 (49) | 9.9 (6.0) |
| Large (> 150) | 15 (29) | 8.1 (7.7) |
| Psychology/psychiatry support | ||
| Mean (SD) no. of clients per service | ||
| Provided | 27 (53) | 151.4 (86.5) |
| Not provided | 23 (45) | 116.8 (79.3) |
The main responses to the four open questions are summarised in Box 3. The key themes to emerge are described in two sections: Challenges of providing care and Managing behaviour change.
Medical (e.g. low HbA1c levels, glycaemic control, growth)
Experiential (e.g. accepting diabetes as a way of life)
Behavioural (e.g. able to manage diabetes, school attendance)
How do you gain awareness of psychosocial need?Nurse contact with family
Physical symptoms (e.g. admissions)
School nurse
Team discussions
What are the main challenges of providing care?Integrating diabetes into everyday life
Managing diabetes in a family context
Imposition of a rigid lifestyle
Teenage rebellion
Overprotectiveness of young children
Communication about complications
How do you encourage behaviour change?Giving advice
Pointing out positives of change
Information about complications
Shared goal-setting
Discussing barriers to change
Individualised approach
Challenges of providing care
In considering the practitioners’ views of the challenges faced by teams in providing psychosocial care, the dominant theme was the issue of engagement and communication, but within this there were two key areas: the integration of diabetes into everyday life and meeting the needs of different ages.
Engagement and communication
The capacity to engage patients and their families with the process of, for example, self-care, clinic attendance, education, etc., was regarded as a significant challenge. This was related to the complexities of meeting the needs of families and different age groups, but it also encompassed communication skills including balancing different priorities, conveying health messages sensitively, and cultural and language issues. Respondents talked about dealing with educational and emotional issues: ‘engaging them and helping them to understand what diabetes is about and trying to get across the longer term for them without frightening them’ (N17).
There was variation in the amount of training and supervision respondents had received in communication skills: postgraduate generic communication skills training was the most common (16 practitioners, 31%) and two (4%) practitioners had received diabetes-specific communication skills training. Fifteen (29%) had received psychology-based training, of whom three (6%) had received diabetes-specific training and five (10%) had trained in behaviour change methods such as MI. Supervision by a mental health professional had been received by 12 practitioners.
Integrating diabetes into their everyday life
One of the key target outcomes identified by respondents was for diabetes to affect the patients ‘day-to-day as little as possible’ (N21). However, it was recognised that this presents a challenge of integrating the diabetes regime into the ‘very variable lifestyles’ (D1) of patients within the service. For example, one clinician stated ‘we are trying to impose quite a rigid lifestyle on individuals … it’s about the constraint of diabetes lifestyle’ (D16). This was also mentioned in relation to the family context, which clinicians identified as a specific challenge to providing care.
Meeting needs of different ages
Age was frequently mentioned as a factor, for example the ‘challenges of various age groups’ (D4) within their service and patients’ changing ‘developmental stages and educational needs’ (D4).
When working with families with younger children, practitioners raised the issue of parents’ ‘guilt complex’ (D5) and being ‘overprotective’ (D26). The most frequent age-related comments were in respect of working with teenagers (Box 4), referencing the impact of the peer group, their changing emotional relationship with diabetes and their need for independence.
‘A lot don’t want to know about their diabetes, its not top of their priority due to peer pressure’ (N31)
‘Difficulties with teenagers are the emotional factors, and if they’ve had it a long time, there can be an element of denial that they’ve got it, and sometimes going through a grieving reaction with their diabetes’ (D2)
‘They have ‘a feeling of omnipotence’ … it’ll never happen to me’ (D44)
‘The adolescent group want to try things and do things differently so they don’t comply’ (D18)
Managing behaviour change
When asked to describe their approaches to encouraging patients and families to change behaviour (Table 2), there were two broad categories of response: some were more focused on education and advice-giving using a didactic style, whereas others were more exploratory and included shared goal-setting. It was also recognised that each individual presents a unique set of issues and so the approach needs to be individualised. This question about encouraging behaviour change was one that some clinicians expressed difficulty in answering (5) and others (7) gave very vague responses, such as ‘through discussion’ (N31) or ‘it would take a week to go through all the possibilities, I don’t think I can say in a nutshell’ (D37) and did not elaborate further.
| Education and advice | ‘I try to motivate them to do better, pointing out their positive abilities, pointing out where they can do better and improve things’ (D4) |
| ‘Usually just to learn more about diabetes and the complications, not to the point to frighten them but you need to stress to them why it is important for them to do that’ (D18) | |
| Exploratory, including shared goal-setting | ‘My personal way of doing it is looking at what I think is ideal, or they think is ideal, then ask them what things they need to do to move in that direction, and how we could achieve it … what could be done, what are they willing to do rather than giving them a list of things that they haven’t agreed to and which they are very unlikely to do’ (D4) |
| ‘We discuss the situation and try and work out why it is difficult and try and come up with a workable solution specific to that family’ (D42) | |
| Individualised | ‘I think it depends on the individual family … it’s about anticipating those difficulties and giving them advice about trying to prevent that becoming a problem’ (D15) |
Discussion
Completing the survey engaged stakeholders in thinking about their services, the challenges they face in relation to providing routine care and their experience of facilitating behaviour change. The high rate of response to this survey demonstrated that this is an area that practitioners recognise as a priority. Respondents outlined the complexity of engaging patients and their families and the importance of communication skills in trying to meet the needs of many different ages, developmental stages and cultural backgrounds within a range of family contexts. They described using a combination of advice, education, listening and shared goal-setting to help encourage their patients to change.
From the responses it would seem that the clinicians were using the three core skills of asking, listening and informing in their communication. There was also implicit recognition that behaviour change is at the heart of the interaction: practitioners described trying to engage patients in making those shifts between the competing demands, yet that process of change was very difficult for the majority to conceptualise or articulate. In considering the most appropriate patient–practitioner interactional model for the intervention in DEPICTED, it had to be effective in addressing behaviour change and incorporate the practitioners’ existing key consultation skills. One model of communication with potential for improving practitioners’ confidence and skilfulness in dealing with behaviour change in routine consultations was to incorporate more of a guiding style into their consultations, encouraging patients to explore their own views of the behaviour and making their own decisions – an approach that has been shown to make change more likely. 42
Although many respondents had received communication skills training in various guises, it would seem that training in communication skills and behaviour change may have been too distant, too general, or not tailored to the context in which they work, to be of use in helping practitioners have a conceptual map of the work and tools available to enable them to function confidently.
The survey contributed significant information to help plan the training programme. It was clear that any intervention had to have a broad application that was flexible enough to respond to the differing needs of a very mixed patient population. It had to facilitate the balancing of the often different priorities of patient, family and practitioner in the consultation process. For practitioners to be able to grasp the relevance of any such training programme to behaviour change, the training needed to be conceptually clear and specific to the context of delivering clinical care within a paediatric diabetes service. By ensuring that the training was more context bound, with the focus on everyday scenarios that have meaning for the practitioners and with the communication skills aspects of training woven into the practice, the aim was to increase the relevance and retention of the information. 55 As well as giving guidance in relation to the development of the DEPICTED study, the findings of this survey underline the importance of the style of training at undergraduate and postgraduate levels across disciplines.
Chapter 4 Questionnaire survey of communication skills of health-care professionals in paediatric diabetes services
Introduction
The attitudes and experience of professionals in the UK in communicating with children and teenagers with diabetes and their families are unknown. For children and teenagers with type 1 diabetes, consultations are complicated by family dynamics and developmental issues. In the UK, notable attempts to train HCPs in communication skills have occurred in specialties such as oncology and general practice and have met with mixed success. 38,43,79,80 However, there is little published evidence regarding the acquisition and proficiency of communication skills of HCPs in paediatric disciplines. Staff involved in the management of child and adolescent obesity in the USA have reported low levels of self-perceived proficiency in the use of behavioural management strategies, delivering guidance in parenting strategies and in addressing family conflict. 81 This suggests that additional training would be beneficial in improving confidence and skills in these areas. This survey aimed to assess communication experiences, attitudes and training opportunities for HCPs to inform the development of the programme.
Blended learning approaches, which provide a mixture of learning opportunities, have been effective in delivering communication skills programmes. 79,82–84 More recent technological advances, such as CD-ROM (compact disc read-only memory) and web-based programmes, provide a flexible method of education delivery and have been used with some success to teach clinical communication skills. 85 However, such technologies have not been evaluated on a large scale in a multidisciplinary clinical environment in the UK and their potential acceptability to paediatric clinical staff is unknown. Despite the obvious advantages of e-learning (such as the potential to reach large numbers of learners), barriers to the use of e-learning in continuing professional development (such as lack of time and confidence) have been reported and may restrict such developments. 86. 87 However, the use of technology by HCPs in everyday practice is rapidly developing. It is possible that such problems may have been resolved and preferences for training may have moved to embrace such approaches. Therefore, this element of the developmental work also aimed to assess the perceived feasibility of and preferences for various methods of learning among staff working in paediatric diabetes services.
Methods
Sample
In April 2006, consultants from 67 paediatric diabetes services were asked to distribute questionnaires for completion by all doctors, nurses, dietitians, psychologists and other HCPs working in their paediatric diabetes teams. These consultants had previously taken part in the telephone survey reported in the previous chapter. Lead consultants for the services were clarified by telephone contact with listed services and were approached to participate. Sixty-seven consultants who had expressed an interest in taking part in the previous survey also agreed to be contacted in relation to this postal survey. Questionnaires were distributed to 383 professionals in total, including 150 doctors, 124 nurses, 77 dietitians and 32 psychologists or therapists.
Questionnaire
The survey covered three broad areas (1) previous experience in communication skills training and its delivery; (2) a scenario-based assessment of attitudes towards addressing different topics in routine consultations; and (3) perceived feasibility of different options for training delivery and skill maintenance. The overall content domain and individual items were developed by a research team comprising psychologists, communication skills trainers and clinical practitioners in paediatric diabetes, among others, and piloted with 11 practitioners working in two paediatric diabetes centres in south Wales. Consultants’ responses to a previous telephone survey of psychosocial service provision for children with diabetes also contributed to the content of the questionnaire.
Scenario-based assessments
To represent commonly encountered challenges within routine paediatric and adolescent diabetes practice, three clinical case scenarios were constructed for use in the questionnaire. Each scenario was constructed to contain clinically relevant medical and psychosocial topics (e.g. elevated levels of HbA1c, health-threatening behaviour), each of which then formed the basis of subsequent questions (Box 5). Respondents were asked to rate the importance they gave to addressing that topic within the consultation, and their confidence in addressing it. Respondents used a rating scale from 1 to 5, where a score of 1 represented ‘not at all important/confident’ and 5 ‘very important/confident’. These importance and confidence ratings were developed on the basis of behaviour change theory,44 with an aim to identify areas of training need and clinicians’ motivation to learn new skills. Scores across the three scenarios were combined to form aggregate ‘importance’ and ‘confidence’ summary scores for both ‘psychosocial’ and ‘medical’ topics. Internal consistency of the summary scores was assessed using Cronbach’s alpha.
Emma, a 14-year-old girl, comes to see you with her mother. Her HbA1c result is 13.5% and she has lost 5 kg of weight since her last clinic visit. Her mother has told you in confidence that Emma has been feeling low lately and is concerned that Emma has been losing weight deliberately.
How much importance would you give to addressing the following topics?
How confident would you feel addressing these topics?
-
her loss in weight
-
her HbA1c result
-
her insulin regimen
-
her diet
-
her low mood
-
her mother’s concern about her weight
-
Emma’s views on life with diabetes
Respondents were asked to rate the feasibility of a variety of possible training options on a scale of 1–5, where 1 represented ‘not at all feasible’ and 5 ‘very feasible’. Options included traditional training, such as off-site workshops, as well as the applications of newer technology, such as internet ‘chat rooms’.
Follow-up procedure
If a questionnaire had not been received back from a centre within 3 weeks of distribution, the consultant was followed up by telephone to establish whether or not the questionnaires had been received, whether or not any further questionnaires were required and to encourage distribution and completion.
Data analysis
Data are presented as frequencies, means and medians. Differences in responses to scenarios were analysed using t-tests and analysis of variance (ANOVA). Standard deviations were adjusted to account for clustering of responses within services through inflation by the intracluster correlation coefficient (ICC). 88 Responses from services with just one team member in the sample were excluded from analyses of scenario responses to minimise distortion of the ICC (n = 11). Associations between variables were examined by calculating Pearson’s coefficient. For analyses of responses to all questions on previous experience of training in communication skills and the case scenarios, psychologists and other therapists were excluded from the analysis (n = 14). All data were analysed using Spss version 14.0 (SPSS Inc., Chicago, IL, USA).
Results
Survey sample
In total, 266 completed questionnaires were received from 65 services – a response rate of 69.5%. Respondents included consultants, doctors in specialist training, dietitians, specialist diabetes and paediatric nurses, psychologists, psychotherapists, counsellors and play therapists (Table 3). The majority of respondents were female (74.1%). Respondents’ experience of working with children and teenagers with diabetes ranged from < 1 year to 44 years (median 9 years).
| Professional group | No. | Percentage | Median years’ experience in paediatric diabetes (25th, 75th percentiles) |
|---|---|---|---|
| Doctor | 109 | 41.0 | 10 (5, 20) |
| Nurse | 91 | 34.2 | 11 (6, 16) |
| Dietitian | 50 | 18.7 | 5 (2.4, 10) |
| Psychology/other (therapist) | 14 | 5.3 | 4 (2.5, 8.5) |
| Not reported | 2 | 0.8 | |
| Total | 266 | 100 | 9 (4.5, 16) |
Previous training in communication skills
Almost one-quarter of nurses and 41 (16.4%) of all professionals had received no previous training in communication skills. One hundred and fifty-four (61.6%) professionals received training as an undergraduate, 122 (48.8%) had received postgraduate training and 70 (28.0%) had received specialist training, with a minority of dietitians having received training in behaviour change counselling techniques, such as MI (Table 4).
| Professional group | Training | Training in specialist communication skills | ||||||
|---|---|---|---|---|---|---|---|---|
| None (%) | Undergraduate (%) | Postgraduate (%) | Diabetes specific (%) | MI (%) | CBT (%) | Family therapy (%) | Other counselling (%) | |
| Doctor | 15.5 | 53.2 | 67.9 | 14.0 | 4.5 | 0 | 1.9 | 4.7 |
| Nurse | 23.1 | 57.1 | 27.5 | 20.4 | 12.1 | 0 | 3.4 | 11.4 |
| Dietitian | 6.0 | 88.0 | 46.0 | 6.0 | 28.0 | 6.0 | 0 | 6.0 |
| All groups | 16.4 | 61.6 | 48.8 | 14.6 | 12.0 | 1.2 | 2.0 | 7.3 |
Importance and confidence ratings for communicating with patients
The internal consistency of aggregate scores was high for ‘confidence’ ratings (medical issues α = 0.91; psychosocial issues α = 0.83) and reasonable for ‘importance’ ratings (medical issues α = 0.81; psychosocial issues α = 0.69). Internal consistency of aggregate scores was optimised by excluding those topics not falling exclusively into a ‘medical’ or ‘psychosocial’ category, such as a girl’s weight.
For the case scenarios presented, respondents rated both ‘medical’ and ‘psychosocial’ issues as either important or very important to address during routine consultations {mean [standard deviation (SD)] ratings 4.0 (0.68) and 4.5 (0.50), respectively}. Psychosocial issues were given higher importance ratings to address within a routine consultation than medical issues (t = 8.93, p < 0.001). Confidence to address medical issues was high [mean rating = 4.3 (0.66)], particularly among doctors and nurses, but confidence to address psychosocial issues was significantly lower across all disciplines [mean 3.5 (0.75), t = 15.85, p < 0.001; Figure 1)]. The biggest discrepancy between importance ratings for a specific topic and confidence ratings related to the topic of a teenage girl’s low mood (see Box 5). Other issues which respondents rated as ‘important’ or ‘very important’, but had less confidence to address included the impact of parental conflict on a young girl and talking about a teenage girl’s views of living with diabetes. Sixty-eight (27.0%) respondents said they would not consider addressing the impact of parental conflict on a young girl themselves (Table 5).
| Topic | Percentage rating as either ‘important’ or ‘very important’ | Mean (SDa) important rating (all) | Percentage rating as either ‘confident’ or ‘very confident’ | Mean (SDa) confident rating (all) | Percentage stating they would not attempt to address topic themselves | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Doctor | Nurse | Dietitian | All | Doctor | Nurse | Dietitian | All | Doctor | Nurse | Dietitian | |||
| A teenage girl’s … | ||||||||||||||
| HbA1c resultb | 75.2 | 78.0 | 72.5 | 74.0 | 4.1 (0.98) | 89.2 | 93.6 | 89.0 | 80.0 | 4.3 (0.77) | 1.2 | 0.9 | 0 | 4.0 |
| Insulin regimenb | 65.0 | 64.2 | 69.2 | 59.1 | 3.9 (1.03) | 80.0 | 93.6 | 86.8 | 80.0 | 4.1 (0.98) | 9.3 | 1.9 | 1.1 | 56.2 |
| Low moodc | 95.6 | 98.1 | 97.8 | 85.7 | 4.7 (0.56) | 42.8 | 47.7 | 46.1 | 26.0 | 3.3(1.10) | 14.9 | 18.5 | 8.9 | 18.0 |
| Views on life with diabetesc | 98.4 | 98.1 | 98.9 | 98.0 | 4.8 (0.46) | 66.8 | 69.7 | 76.9 | 42.0 | 3.7 (0.93) | 5.6 | 2.7 | 5.5 | 12.0 |
| A teenage boy’s … | ||||||||||||||
| HbA1c resultb | 70.1 | 72.6 | 72.5 | 62.0 | 4.0 (0.97) | 89.6 | 94.4 | 93.4 | 72.0 | 4.4 (0.80) | 1.6 | 0.9 | 0 | 6.0 |
| Insulin regimen (encouraging him to talk about)b | 81.9 | 85.0 | 85.7 | 70.0 | 4.3 (0.79) | 87.6 | 92.6 | 94.5 | 64.0 | 4.3 (0.78) | 2.4 | 0.9 | 1.1 | 8.0 |
| Life at schoolc | 91.5 | 90.7 | 93.4 | 89.6 | 4.5 (0.69) | 77.5 | 72.2 | 89.0 | 68.0 | 4.0 (0.77) | 3.2 | 3.7 | 3.3 | 2.0 |
| Drinking behaviourc | 90.0 | 87.0 | 94.5 | 88.0 | 4.4 (0.69) | 69.9 | 63.8 | 81.3 | 62.0 | 3.9 (0.82) | 6.0 | 9.3 | 3.3 | 4.0 |
| A young girl’s … | ||||||||||||||
| HbA1c resultb | 76.5 | 80.5 | 79.8 | 62.0 | 4.0 (0.81) | 90.3 | 92.2 | 93.5 | 80.0 | 4.4 (0.80) | 0.8 | 0.9 | 0 | 2.0 |
FIGURE 1.
Importance (a) and confidence (b) ratings for medical and psychosocial issues. a, t-statistics are for the whole sample.
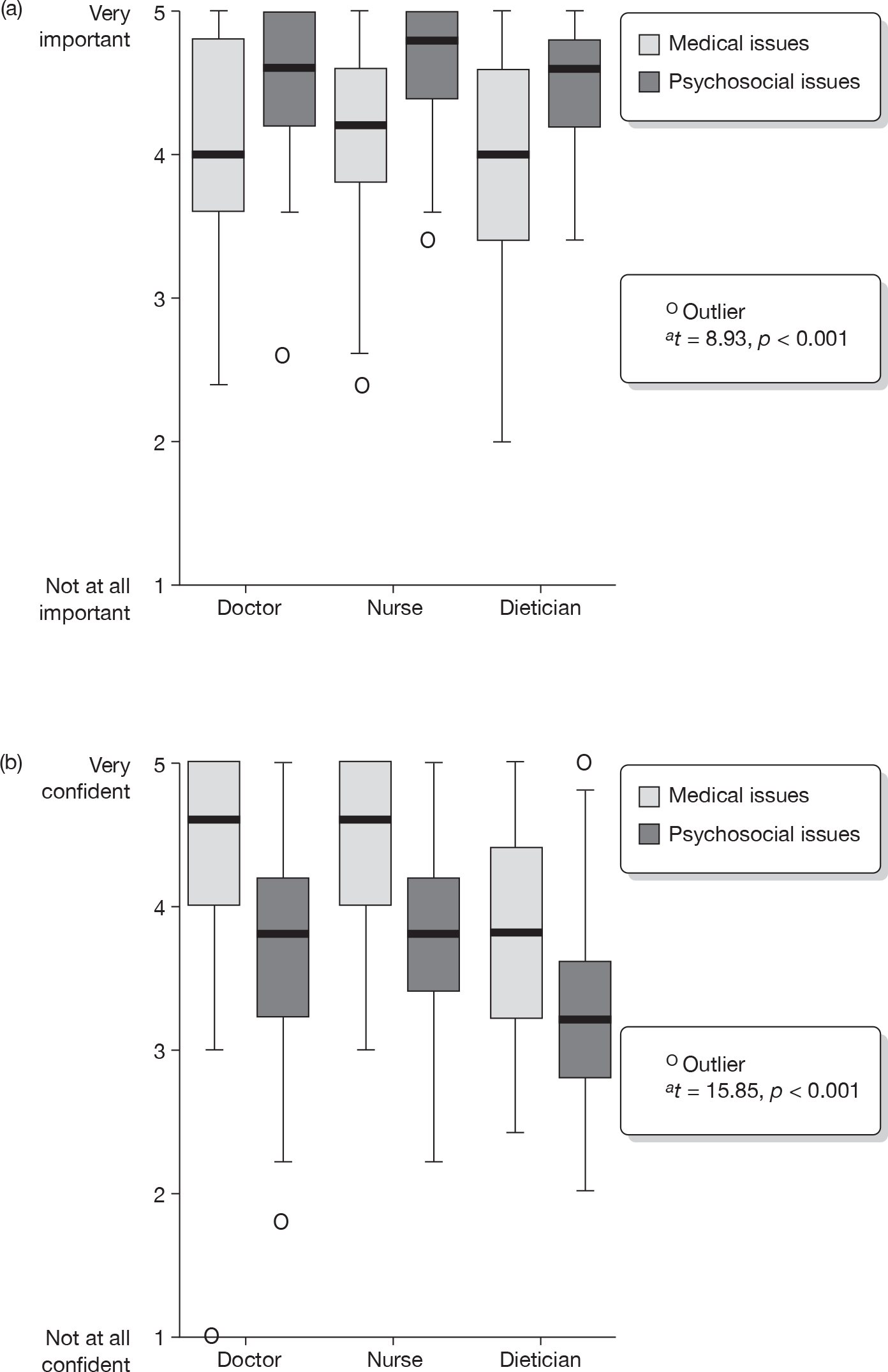
There were no interprofessional group differences in importance given to addressing psychosocial and medical topics within the consultation, but there were interprofessional differences in confidence, with dietitians expressing less confidence across all issues (F2,229 = 4.12, p = 0.018; Figure 1). Confidence ratings for addressing both medical and psychosocial issues were correlated with years of experience working in diabetes (r = 0.30 and r = 0.36, respectively, both p < 0.001). A weak correlation was found between importance ratings for addressing psychosocial issues and years’ experience working in diabetes (r = 0.15, p = 0.026). The correlation between importance ratings given to medical issues and years’ experience in diabetes was not significant (r = 0.13, p = 0.059). Those who had received specialist communication skills training, such as MI and CBT, reported slightly higher mean confidence ratings for psychosocial issues than those who had not received specialist training (mean = 3.8 and 3.6, respectively), although this difference was not statistically significant (t = 1.6, p = 0.103). No other differences were found between attitudes towards addressing psychosocial issues and previous communication skills training. There was some clustering of importance and confidence ratings within services, particularly for confidence ratings to address psychosocial issues (ICC = 0.08), indicating a tendency for members of the same team to self-rate in similar fashion. The same was true for importance ratings given to medical issues (ICC = 0.04).
Motivating factors for participating in a communication skills learning programme included helping patients talk about their needs more easily, helping to change patient behaviour and learning skills that can be used in life beyond diabetes care (80.0%, 79.2% and 72.8% of respondents agreed with these statements). A total of 19.6% of respondents expressed finding talking with patients sometimes quite difficult as a reason for participation. Agreement with this statement was correlated with fewer years’ experience working in diabetes (r = 0.15, p = 0.016).
Training delivery
Face-to-face training
The most common formats for communication skills training previously experienced by respondents were small-group discussions (n = 56, 21.0%), lectures (n = 19, 7.1%) and role play (n = 33, 12.4%). Respondents considered the most feasible options for training in communication skills to be meeting together as a team once per month for 30 minutes and attending a 1-day off-site workshop (Table 6). Attending a 3-day off-site workshop was rated unfeasible by 143 (54.1%) respondents.
| Training options | Percentage rating ‘feasible’ (scored 4 or 5) | Percentage rating ‘unfeasible’ (score 1 or 2) |
|---|---|---|
| Team meeting once per month | 77.3 | 6.9 |
| One-day off-site workshop | 64.8 | 12.5 |
| CD-ROM | 53.6 | 16.7 |
| Website | 56.4 | 16.6 |
| Three-day off-site workshop | 17.5 | 54.1 |
Technology-based training
Nine respondents reported experience of learning with video and audio materials and just one respondent had interacted with web-based materials. However, 149 (56.4%) respondents rated engaging with materials on a website as a feasible training option. Perceived barriers to accessing web-based material at work included lack of time (n = 143, 55.4%), lack of privacy or a busy office (n = 87, 33.9%), inconvenient location (n = 47, 18.5%) and slow internet connection (n =31, 12.2%). Few respondents considered insufficient computer skills and lack of interest to be barriers to either web-based or CD-ROM learning (n = 14, 5.5%, and n = 6, 2.4%, respectively). A total of 178 (66.3%) respondents reported that they would consider accessing web-based learning materials on their computer at home.
Practising skills
Discussing experiences with colleagues once per month and allowing an experienced coach or colleagues to observe and feed back on consultations were all rated as feasible options for encouraging skills in practice by most respondents. The least feasible option was discussing experiences with other practitioners on the internet, rated unfeasible by 154 (58.8%). Writing up reports of challenging consultations was rated unfeasible by one-quarter of respondents (Table 7).
| Learning activities | Percentage rating ‘feasible’ (4 or 5) | Percentage rating ‘unfeasible’ (1 or 2) |
|---|---|---|
| Discussing experiences with: | ||
| Colleagues (once per month) | 65.5 | 9.3 |
| A visiting coach | 62.2 | 11.9 |
| An experienced coach by e-mail | 53.4 | 22.9 |
| Other providers in a 1-day workshop | 44.3 | 24.1 |
| An experienced coach by telephone | 42.1 | 36.3 |
| Other providers in an internet ‘chat room’ | 17.0 | 58.8 |
| An experienced coach observing your consultations | 65.2 | 10.7 |
| A colleague observing your consultations | 65.0 | 13.4 |
| Audio-recording a consultation to reflect on yourself | 57.4 | 20.5 |
| Audio-recording a consultation to share with a coach | 57.8 | 18.4 |
| Audio-recording a consultation to share with colleagues | 49.4 | 20.3 |
| Writing up reports of challenging consultations | 46.0 | 25.3 |
Discussion
Although some professionals had undertaken specialist training in communicating with patients, almost one-quarter of nurses had received no such training and half of all professionals surveyed had received no training since graduating. Confidence among doctors and nurses to address medical issues in consultations involving young people with diabetes was high, but confidence to address psychosocial issues was significantly lower. Given that addressing psychosocial needs is perceived as important by both patients89 and professionals, these low confidence ratings reflect a training need and motivation to learn new skills among professionals working in paediatric diabetes and a gap in current care provision.
It is clear that some practitioners feel unprepared to address psychosocial challenges that are commonly found in practice, and it may be that some feel that it is outside their remit to do so. Referral to psychology services may be an answer for some patients with particularly pressing concerns, but access to such support is limited. 90 In many services, practitioners have little option but to address complex psychological and emotional topics themselves. The clustering of confidence and importance ratings found within individual services may be a reflection of differing ‘cultures’ and variations in the availability of specialist support. Establishing models of care to meet families’ psychological and emotional needs that are applied across services is therefore a priority.
High importance ratings given to addressing psychosocial issues, coupled with low confidence ratings, suggest a role for communication skills education to support routine care. In addition, reasons given by practitioners for participating in a communication skills learning programme demonstrate the clinical challenge of behavioural self-management in diabetes and emphasise the perceived importance of effective communication between family and professional.
Among the strengths of this study was the development of the survey instrument by a team of clinical and research professionals with a particular interest in training, enhancing both the face and content validity of the final survey instrument. Furthermore, the scenario-based assessments were developed on the basis of clinical experience and conceptually driven. This was borne out by the high level of internal consistency for three of the four resulting aggregate scales, with scores exceeding the benchmark Cronbach’s alpha of 0.7. 91 Similarly, associations found with other related variables, such as the positive relationships between confidence ratings and years of experience in diabetes, support the construct validity of these measures. Potential weaknesses of the study include the response rate and coverage of the survey. The sample of respondents may be biased towards professionals who are interested in, or seeking training in, communication skills. Therefore, caution should be taken before suggesting that these findings represent the attitudes and training needs of all staff working in paediatric diabetes. However, given that clinicians from approximately one-quarter of all services in the UK took part in the survey, the sample includes a significant proportion of professionals working with children and teenagers with diabetes in the UK.
What messages are there for training provision in this field from this part of the developmental work? There was support for multiple methods of delivery of a learning programme with monthly team-based learning activities rated as most feasible and support given for face-to-face learning, case reflection, colleague and coach observation, and feedback. Training within teams at regular intervals may prove a valuable method of learning, particularly in context. Given the lack of previous experience of online learning in communication skills, there was considerable support for the use of web-based or CD-ROM materials, although potential barriers – such as lack of time – continue to be reported. 85 Unlike previous findings,85,86 lack of skills was barely reported as a potential barrier to accessing web materials and is a likely reflection of the rapid increase in skills in and use of information technology by health professionals in everyday practice. Given potential barriers such as lack of time, electronically delivered learning programmes must ensure flexible delivery, minimal technical demands of the user, and timely support.
Chapter 5 Incorporating users’ experiences in the development of training materials for the DEPICTED study
Introduction
This section describes part of the preparation for the development of a clinical intervention to improve communication in clinic consultations, deliverable within the context of routine care by the diabetes team. As part of the development of this intervention, the DEPICTED team used focus group methodology to gather contextual information from children and young people with diabetes (and their parents or guardians) about the way diabetes affected their lives and how they felt the doctor–patient relationship worked for them in clinical encounters.
Methods
Focus group methods were adapted for paediatric settings, using previously published guidelines. 92,93 The discussion framework used in the focus group discussions is described in Box 6. These discussions aimed to enable participants to describe the issues that took prominence in their lives, their hopes and aspirations, how these were identified and dealt with by HCPs in the clinic setting, and what patients and families wanted from clinic consultations.
What’s the hardest thing about living with diabetes?
What would you most like to change about living with diabetes?
What’s most helpful about the diabetes clinic?
What would you most like to change about the diabetes clinic?
What is communication like with the clinic staff and how would you want it to be?
Six audio-recorded focus groups were comoderated by two non-clinical researchers. Potential participants (parents, children and young people), who were identified by a clinician working in a paediatric diabetes service, were sent information sheets and forms for consent to researcher contact. Same-gender and related-age-range discussion groups were arranged, as recommended by earlier research on conducting focus groups with children. 92 Participants were selected to achieve a range of treatment regimens (two, three or four injections per day – insulin pump), family structures (single- or two-parent families, siblings or parents with diabetes) and coping/treatment adherence (e.g. ‘doing well’ or ‘struggling’ from a clinician perspective). Children (aged 7–11 years) and young people (aged 12–16 years) with type 1 diabetes and their parents were invited to participate.
All potential participants expressing an interest in the study were telephoned a few days before the focus groups for the researchers to introduce themselves, reiterate the purpose and format of the groups, re-confirm their decision to participate and to respond to any questions. A specialist nurse from the paediatric diabetes service, familiar to the participants, greeted them on arrival, and was available after the discussions to answer any medical concerns that may have arisen. The specialist nurse was not present during the focus group discussions themselves. Written informed consent was taken before the focus groups started. All participants received refreshments on arrival and a £10 gift voucher as token appreciation. All parents, those participating and those accompanying their children/young people to the venue, completed an information sheet documenting their own age, occupation, family size, child’s age, duration of diabetes, other family members with diabetes and, for accompanying parents, how well they believed diabetes management was going at that time for their child.
To encourage greater openness, children and young people took part in the groups without their parents present in the room. Basic ground rules (e.g. everyone getting an opportunity to talk, one person speaking at a time) were introduced at the start of the focus groups, followed by an introductory session for the parents’ and young persons’ groups, and ice-breaker games for the children’s groups. In addition, the children and young persons groups used a ‘pick a postcard’ strategy to start discussion about what living with diabetes was like for them, based on their choice of picture. During the focus groups, one researcher assumed the role of the main facilitator, whereas the other provided interim and final summaries, logged comments, wrote bullet points on the flip chart, and handled recording equipment and refreshments.
Group discussions lasted between 66 and 98 minutes, including a short comfort break. The discussions were audio-recorded and notes made by the support facilitator. They were transcribed verbatim and loaded into NVivo 2 (QSR International, Doncaster, VIC, Australia), a qualitative software package. Transcripts were coded and emerging themes were identified by one researcher. Two others then read the transcripts independently and agreed the coding and themes. Themes were further developed and linked through discussion between researchers.
Results
Forty-eight patients and parents were invited to participate initially, of whom 39 returned ‘consent to contact’ slips, and 32 eventually participated in one of the six focus groups held (Table 8). The range of duration of diabetes in participating patients was 18 months to 7 years. The ice-breaker activities generally worked well, but were more difficult to control in the younger age groups, particularly the boys’ group, which was less inclined to focus on the task.
| Group | Participants | Male | Female | Age range (years) |
|---|---|---|---|---|
| 1 | Children | 5 | 7–11 | |
| 2 | Children | 4 | 7–11 | |
| 3 | Young people | 7 | 12–16 | |
| 4 | Young people | 5 | 12–16 | |
| 5 | Parents of younger children | 1 | 3 | Children < 12 |
| 6 | Parents of teenagers | 2 | 5 | Children > 12 |
Themes arising from the data
Discussion was subdivided into two main areas (1) personal accounts of the experiences and relationships built up with HCPs in the paediatric diabetes clinic and (2) what it was like living with diabetes on a daily basis as a young person or a parent. There were no important differences in the identification of subthemes by those involved in the thematic analysis, although there was considerable discussion about children’s day-to-day experiences in school settings and with their peer groups.
Experiences from the paediatric diabetes clinic
The clinic process and perceptions of the multidisciplinary diabetes team
Some children and young people found coming to clinic boring and some found being examined as part of routine annual reviews intrusive (especially the girls). Waiting for painful blood tests (often taken from the back of the hand) and the waiting times between seeing the different HCPs at annual reviews were especially disliked. The practice of measuring growth (weight and height) in relatively public areas was particularly unpopular with teenage girls.
Both teenagers and younger children said they had at times felt annoyed by the lack of consultation with them as individuals. Examples were given, such as future appointments being made through discussion with the parent rather than with the child, and repetition of tests or referrals without paying attention to the child’s assertion that these had been recently undertaken. Although some children relished ‘getting out of school’ as one upside of having to attend clinics, they also acknowledged that clinics in school time meant they had to catch up with study notes later. For older children, attending evening clinics at times interfered with after-school activities.
An emergent finding was that service users perceived differences between professions and their communication styles. Both children’s and parents’ evaluations of their contacts with the diabetes team differentiated clearly between doctors and nurses. They noted that the nursing staff were ‘realistic’ and gave simple and understandable explanations about diabetes. They were ‘always there’ when participants had needed someone on the end of a telephone. There was general agreement on this in all the discussion groups. Support from nurses and home visits were highly valued.
By contrast, communication with the doctors in clinic was seen as more formal and rushed, and felt less supportive. When they had to account for high blood glucose concentrations, some children felt uncomfortable, as they thought poor control was not necessarily their fault. Their perception was that different professionals reacted differently in this situation. All four groups of children wanted more constructive talk about ways to make glucose readings better. They found it easier to talk to nurses in general, and this was especially so if the nurse was someone who had supported the family from diagnosis.
All participants disliked the lack of continuity with doctors and wanted to see the same doctor each time they attended (although, as one young boy pointed out, if you didn’t like a particular doctor, it was good to have alternatives). Having other people present in a consultation was not liked (e.g. medical or nursing students), but they did not feel able to refuse or sometimes were just not asked for their consent. Members in all groups commented on the poor communication skills they had experienced. This started with non-verbal messages – doctors in formal suits, sitting behind desks, showing greater interest in medical records or the biochemical results than the child, and arranging further tests or appointments without consultation. Some children felt that the doctors talked down to them, whereas others said they had been treated like adults, but then had struggled to understand the language used. Most teenagers and children felt disengaged from the management decisions doctors made with their parents then, if a new regime did not work, or a new insulin pen was more painful, they felt very annoyed (Box 7). However, especially in the discussion groups with younger children, there was a sense of inevitability and acceptance regarding their peripheral status in consultations with doctors. They did not really want to become more involved in interactions for which they did not feel equipped to participate. Some felt ‘put on the spot’ by attempts to include them in the conversation. Teenagers were especially sensitive to being asked questions about their personal lives that they felt did not arise from ‘genuine’ interest.
‘Sometimes when I go into the consultant’s room, I basically sit there and they say lots of stuff you don’t understand and you try to say something but then your parents just say “shhhhh!” You can, so you can’t say anything. They come out and say, oh that was good, did you understand that, you say no, they say, you should have asked them, and then you say, oh you didn’t let me, they say “rubbish!” ’ (boy, aged 10 years)
‘If they like, if we should put your insulin up, they say to you, how do you feel about that, and it’s like what am I meant to say to that, it’s like you don’t feel that there’s much option’ (girl, aged 13 years)
Experiences of having diabetes as a child or teenager
The controlling effect of diabetes on day-to-day activities
Children were managing their own insulin injections and glucose monitoring from an early age, with all of the younger age groups giving their own insulin and most of them checking blood glucose as well. The apparent random nature of swinging levels made some children feel frustrated and out of control, as they felt they could not plan or predict how the day would unfold, even although they were testing and injecting regularly. Sometimes, such blood glucose swings stopped them from activities such as swimming, attending after-school sports or clubs and walking home.
Reactions to blood glucose recordings
There was emphatic agreement in all children’s groups that they did not like doing their blood tests, but liked having to record their levels even less. Most said they resented the time it took to write down both the glucose level and insulin dosage in their charts. When asked for the recordings at clinics, many admitted giving evasive answers or ‘forgetting’ their booklet on purpose. Some used the memory on the monitor to collect their data and put it into their record books at regular intervals, and a few admitted to making up readings. Although most of the children and teenagers were aware that the readings were needed for the recognition of patterns of high or low blood glucose, most did not use the data to look for these interpretations themselves, and some did not understand why it needed to be done. Recording blood glucose levels was therefore felt to be a thankless and mostly needless chore.
The discussion groups with teenage and young girls voiced feelings of being a ‘disappointment’ to parents and HCPs (Box 8). In the parent groups, participants described the checking of insulin given at the right times, co-ordinating this with blood glucose monitoring and eating, as tiring, frustrating and a continuing grind.
‘And if they’re high, I don’t wanna write it down, I don’t want my mum to see it, yeah I think mum is worse than the doctors … it just makes me feel really upset and down, cause I know if I go and tell my mum she will be in a bit of a mood or disappointed with me for like, not controlling myself when it comes to food – I’d just like never to do blood sugars again …’ (girl, aged 16 years)
‘If you don’t write them down it’s not, they don’t shout at you, it’s just they look a bit disappointed and it’s like it’s worse than being shouted at’ (girl, aged 15 years)
Parents‘When I actually flicked through her monitor I just didn’t believe that she’d lie to me that constantly’ (mother of 12-year-old girl)
‘She’s had the opportunity to have monitors and she hasn’t done it … I mean diabetes is just something she has to deal with and she only deals with it when she has to. She doesn’t want to do it a quarter of an hour before a meal because that makes her meal such an issue … I mean I’ll nag her but it makes no difference. It just causes tension the whole time’ (mother of 14-year-old girl)
‘Having to keep an eye on that all the time is a real pain, because it’s us who are worrying about the future rather than them. They are not worried about at the moment, are they? They are just thinking “Oh shut up” ’ (mother of 15-year-old boy)
The way schools reacted to children with diabetes
Participants in the teenager groups agreed that it was tiresome to continually have to explain their diet, blood testing and insulin routines to others and bemoaned a general lack of knowledge about type 1 diabetes. Their non-diabetic peers were not always very sympathetic. In the groups with younger children, coming to terms with restrictions on sweets and chocolate while watching their friends and peers eat them on a daily basis was a difficult experience to which all participants could relate. Children mentioned books that they had been given on diabetes, which emphasised that having diabetes did not make them different from anyone else, but this did not concur with how they felt, with exclusions from sports and treats, and their intense experiences of hospitalisations and injections.
Some children told of teachers who had been unhelpful and unsympathetic towards them. For example, teachers would question how long it should take them to give themselves insulin injections and the need for frequent toilet breaks. Children felt that teachers often had no idea how diabetes emergencies, such as hypoglycaemic attacks, should be managed. Parents and children requested greater dialogue between schools and paediatric diabetes services to raise both awareness and skills. There appeared to be some variation in the way teachers responded to children who were experiencing hypoglycaemic attacks and sometimes children were left to sort it out for themselves (Box 9).
‘As a reward in school we were given sweets. But she stopped in front of me and said “I don’t know whether you’re allowed to have them” so she didn’t give me any!’ (girl, aged 15 years)
‘I almost got my pump confiscated once because the teacher thought it was an MP3 player and she only realised when there was like a tube attached and she was pulling it and she was going, why isn’t it coming off? I was like, because it’s attached’ (girl, aged 13 years)
‘And then you leave it to the last moment, you’re walking up the steps like, to get to your locker and you’re like falling all over the place’ (girl, aged 15 years)
‘I was having a hypo and the teacher didn’t believe me … She was “why aren’t you doing your work?” She kept shouting at me. I just got up and shouted “shut up” and I got a detention for it’ (boy aged 12 years)
‘I fainted on my desk once in school cause I forgot to eat lunch … apparently just like hit the desk and I broke my nose while I was doing it. The teacher was trying to wake me up and didn t know what was happening, she thought I’d gone to sleep, so they phoned my parents’ (boy, aged 13 years)
‘In year 9 you do a lot of work on diet, healthy eating and everything, and it always says in all the booklets if you eat too much sugar, you will become diabetic, and everyone stared at me like you’ve eaten so much that you’ve become diabetic, and it’s like, no’ (girl, aged 13 years)
‘In primary school my friends were like, oh I wish I had diabetes, and I was like no you really, you really don’t, (yeah) everything in it is horrible’ (girl, aged 15 years)
Further themes arising from the parents’ focus groups
For parents, the most difficult aspects of having a child with diabetes were witnessing the discomfort of injecting and glucose monitoring, and the constant vigilance on blood glucose levels. Parent groups strongly expressed the feeling that the spontaneity of childhood was lost through diabetes. For some, the fear of hypoglycaemic attacks while their children were out of sight meant they did not allow them to go on sleepovers or impromptu outings. Issues such as school trips, alcohol and smoking worried many of the parents of older children. The difficulty of deciding when to treat them as ‘normal’, ‘special’ or ‘different’ needed constant evaluation and recalibration.
Many parents said they had used the internet, support groups and Diabetes UK to get information on new advances in diabetes management. Asked about suggestions for improvement, some parents wished for clinic staff to be more forthcoming about cutting-edge innovations. A few parents expressed concerns that staff were possibly constrained by cost considerations, and appeared resistant to introduce new ideas.
However, very positive views about the diabetes specialist nurses were expressed. Some parents described how nurses had lived through the initial diagnosis period with them (described by one parent as if she had been ‘hit by a train’) and that shared significant life experience was very important. The nurses’ constant availability and continuity of care was much appreciated and was a very important factor in their acceptability to service users. By comparison, doctors were seen as formal and distant, and some parents questioned their usefulness in a clinic setting. As the turnover of doctors was high, some parents felt they had to explain themselves over and over to ‘new’ trainee doctors, who often gave the impression that they were less knowledgeable than the parents themselves (Box 10). General practitioners and hospital-ward staff inspired even less confidence.
‘You’d think that they’d have all this information coming to them before we’d hear about it, because we’re just normal people … they’d know more what was going on and be able to tell us straight away about it’ (mother of 15-year-old boy)
‘The doctors are really busy, they are not there to be waffly, The specialist nurses are there to give you a bit more time, a bit more information, and you go to the doctor just to have an overview’ (mother of 13-year-old boy)
‘But the bang, bang, bang that I’ve had from behind the desk with consultants, I’ve had at the same time, if I’d wanted it from the nurses out there. So I wonder why we go in there, into a different situation, which is a little more alien to the relaxed atmosphere out there to have it in a more formal and more, sort of meeting the head teacher situation’ (mother of 14-year-old girl)
‘And they (nurses) seem to be more in touch with the children and their personalities. They are a bit more understanding. Whereas the doctors are a little bit more alien’ (mother of 15-year-old boy)
‘It’s funny because although we all sit out there none of us talk to each other unless one of the nurses introduces us’ (mother of 12-year-old girl)
Discussion
A broad set of issues associated with their diabetes will occupy the minds of patients and their parents, which may only be partially addressed by a narrow clinical focus on glycaemic management. These can sometimes cause conflicting expectations of the function of clinic visits by children, parents and health professionals, who all have their own personal agendas, however implicit or apparently modest.
The data from the focus group discussions clearly described children’s experiences of poor communication and marginalisation, anxiety experienced waiting in clinic for blood tests and to see ‘the doctor’. Many children expressed their dislike of being measured or examined by people they hardly knew and the ‘adult’ acceptance of medical jurisdiction was not a paradigm they found easy to accept. Most children (especially the younger ones) relied on their parents taking over. Additional barriers to effective communication include duration and frequency of contact, gender, perceived attitudes to children and adolescents, and the presence of other people in the consulting room. This results in children and adolescents taking a passive role in consultations, reluctant to raise personal or sensitive issues or to ask questions that might reveal poor adherence. 89
There were clear differences in expressed preferences for the typical ‘nurse-style’ consultation over the ‘doctor-style’ consultation, as nurses were seen as more approachable and more realistic in their expectations of patients, and often had a longstanding relationship with patients.
Health professionals are expected to work in partnership with young people94 and their parents, but often lack the communication techniques with which to engage them81 in the discussion and management of their illnesses. This is particularly important when applied to the care of chronic conditions such as type 1 diabetes, where day-to-day self-management involving children and their families is crucial in maintaining optimal control and good clinical outcomes. A recent survey showed that 16% of doctors, nurses and dietitians working in UK paediatric diabetes services had received no training in communication skills, and 47% had had no training since graduating. 95 The balance between achieving biomedical outcomes while acknowledging and listening to children’s daily experiences (referred to graphically by Barry and colleagues96 as their ‘lifeworld’) is often unequal, resulting in suppression of their contextualised accounts and a reduction in their autonomy and engagement. This is especially the case if communication is held mainly with the attending parent(s). This approach ignores and contrasts with the considerable role of children in their own diabetes management. 97 In contrast, skill-based approaches that more actively engage patients in their consultation (e.g. MI) can facilitate behavioural changes and improve glycaemic control in teenagers with diabetes, but it takes time to do and requires specialist input. 32
Clarification and understanding of agreed agendas in the clinic setting at the start of a consultation is a clearly important conclusion from the data presented here. So is the communication style adopted by the HCP. A direct consequence of the insights gained from the focus groups has been the development of an interactive agenda-setting tool within the learning programme of the DEPICTED trial. The aim of this tool has been to ensure that everyone in the consultation can raise issues that are important to them.
Many of the themes developed from the data are familiar, but we have developed them to produce guidance in the form of a ‘conceptual approach’ for professionals working in a paediatric context in the UK (although they might need to be adapted for other cultural settings) (Box 11). This approach emphasises that how the patient is involved in the consultation is as important as what is communicated during it.
Check you know what you want to get from the consultation – but remember that in order to do that you need to address the families’ agendas as well
Concentrate less on the negative aspects of biochemical values be prepared to be flexible and work with the child’s needs
Take gender, age, developmental level of the child into account. Try to understand the child/parents’ journey, e.g. determine what the child’s issues are likely to be – school, sleepovers, sport, social activities, etc.
Attitudes and atmosphereRead the clinical notes before the family come in
Decrease formality and distance – let the child decide where to sit
Appear less busy
Use your own style to get to know the child a little first. Just saying ‘how’s school?’ isn’t good enough
You can be authoritative without being authoritarian
Be realistic in your expectations of the child and his/her family
You can create a relationship in which you are still approachable and seen to be realistic while still emphasising the importance of improving self-management practices such as blood sugar measurements
Show respect for the child/adolescent
Show the child that your interest is genuine
Remember that children find it difficult to accept being examined – always ask for consent and ensure that the child knows what you are going to do
Ensure dignity and privacy for the child
Attempt to ensure continuity if at all possible
Chapter 6 The Stakeholder Action Group
Introduction
Major UK funding bodies and Research Ethics Committees (RECs) actively support public involvement in research. 50 The National Institute for Health Research HTA programme has developed an evidence-based approach to involving service users in research and development agenda-setting, which includes approaches to reducing barriers to meaningful participation. 98 Similarly, the James Lind Alliance was established in 2004 to encourage patients, carers and clinicians to work collaboratively to identify research questions. 99 The commissioning brief for the DEPICTED study emphasised the need for key stakeholders, including children and teenagers with diabetes and parents/carers, to be actively involved in the development of the research intervention, not surprisingly, given that the intervention had to be ultimately deliverable within the context of routine care without the need for additional clinical support, and acceptable to all stakeholders. This chapter concerns stakeholder involvement, particularly lay stakeholder involvement, at key stages of the research as members of a SAG. Their involvement is described, and the issues and challenges arising are identified and explored within the literature on service-user involvement in health-care research.
The Stakeholder Action Group
The DEPICTED research team worked with a specially constituted SAG that was to advise on the developing research intervention and on the formal trial evaluating the intervention. The SAG was responsible for reviewing relevant evidence provided by the researchers, considering and advising on developing ideas for the intervention, and guiding the research team about plans for evaluation in the subsequent trial. The group was so named to confirm the active role it had in working with the research team.
Lay representatives included teenagers and young adults with type 1 diabetes, and parents of affected individuals approached through independent support groups outside the catchment area of the clinical researchers. This safeguarded confidentiality and professional relations by avoiding the inclusion of patients and parents known to professional attendees. Parents were invited to attend meetings with their children or on their own. In addition, the research team included the mother of a teenager with diabetes in her capacity as a user representative, and a representative from Diabetes UK was invited.
The professional group was selected to represent key professionals with an interest in children and teenagers with diabetes. Professions represented included paediatrics, specialist diabetes nursing, general practice, child psychiatry, paediatric dietetics, clinical psychology, school nursing and social work.
Setting and context
The SAG met on three full days over 10 months. An independent facilitator – a consultant clinical psychologist not involved in diabetes care – was employed to run the meetings, and met with the research team before and after each SAG meeting to clarify roles and responsibilities and review events, respectively. Various research team members facilitated small-group sessions and hosted other plenary sessions. Each SAG meeting was constructed around plenary and parallel small-group sessions. Plenary sessions included presentations by the research team about study activities and developmental concepts of the research intervention. The composition of groups in the small-group sessions varied, for example mixed stakeholder groups addressed ‘What makes for a successful consultation?’, whereas lay and professional stakeholders were separated to consider ‘Choices about service delivery’. Lay stakeholders were further divided into parent and teenager groups to discuss the development of separate patient and parent/carer questionnaires for use in the subsequent trial. Additional materials were made available to view during breaks and over lunch (e.g. scientific and ‘meet the team’ posters). In all meetings, presentations included examples of the developing intervention and made use of both audio and video materials encouraging further input of ideas and comments.
All lay stakeholders provided written informed consent at the start of each SAG meeting. All stakeholders received reimbursement of travel expenses, and lay stakeholders also received £30 vouchers for each meeting they attended. Newsletters were used between meetings to update stakeholders about study progress. At the final meeting, stakeholders were asked whether or not they wanted to continue being informed about the study as it progressed into its trial phase.
Evaluation of stakeholder action group meetings
Stakeholders’ views were audio-recorded, and the data transcribed and analysed following each SAG meeting. Summary written notes taken by researchers observing the group sessions were made available to stakeholders during the day and were subsequently formally transcribed as additional data. Each SAG meeting was evaluated using an anonymous stakeholder feedback form that nevertheless identified whether or not the respondent was a patient, parent or professional. This form addressed expectations about the day, whether or not these had been met, what they did/did not enjoy, their views on information provision, meeting format and practical arrangements. Suggestions for how future SAG meetings could be modified were sought. The evaluation form for the third meeting asked how stakeholders would like to continue being involved in the study.
Outcomes
Between 13 and 17 lay stakeholders (teenagers and adults with diabetes, and parents) and 10 or 11 professional stakeholders attended each meeting. In addition, there were between 13 and 15 research team members present.
How the stakeholder action group influenced the design of the research intervention
Three key outputs arose from the SAG meetings, all of which were implemented in the trial phase of the DEPICTED study:100
-
The Talking Diabetes course. 57
-
The shared agenda-setting tool (3T: TimeToTalk). The concept of a shared agenda-setting tool arising from the focus groups with lay stakeholders was reinforced during SAG meetings, where lay stakeholders helped the research team with choices related to the tool design (Table 9). Advice from both lay and professional stakeholders was used to develop guidelines for subsequent use of the tool in trial centres. Finally, at the third meeting, a naming competition was held for the tool in which all stakeholders and researchers had the opportunity to suggest and select names for the new tool (3T).
-
A DCE questionnaire (see Chapter 11).
| Comments on their most favoured design | Comments on their least favoured design |
|---|---|
|
Patient 4 (11 years) ‘People look funny. I like the little pictures on it. Make all the children’s clothes colourful not have some plain’ Patient 3 (13 years) ‘I liked the colour of my first choice because it’s bright and makes you feel happy. I also like the pictures. To improve my first choice I would use a little less space and have more blank boxes because there might be quite a lot of things someone wants to talk about’ Patient 1 (adult) ‘I like this design because it’s colourful and would be appealing to all children of different backgrounds and sizes. It doesn’t emphasise the ideal body image that young teenagers and children are so concerned about these days. Maybe it could include a Muslim girl in a headscarf and may be as an option you could use the buzzword relationships. This may help to open up the child’s mind. They may feel more comfortable talking about it but it gives them the option’ Parent 2 ‘The thought bubbles jump out at me and is saying think about what you like to talk about. More striking than the other designs. The empty bubbles are good as again they make you think what other subjects can I think of?’ Parent 4 ‘Colourful quirky format. Have the first little boy smiling’ Professional 4 ‘Liked the human pictures which represented all ages. Yes, we were unanimous in agreeing that the pictures were all too happy! And smiley! If someone wants to talk about something that makes them sad or serious – smiley, happy pictures may be offputting’ Professional 2 ‘This is clear with pleasant faces. Thought bubbles will encourage thinking and perhaps help a young person to believe they are not alone. I would like to see a younger face amongst them. Not keen on such a smiley face when thinking about blood tests!’ |
Patient 1 (adult) ‘I don’t like design A because I feel that it’s very bland. It’s not very appealing to the eye’ Patient 2 (13 years) ‘I don’t like the pictures – not colourful enough’ Patient 3 (13 years) ‘I didn’t like the colour because it’s just black and white. I don’t like the writing because it’s boring so people wouldn’t want to read it’ Patient 5 (9 years) ‘It was not in colour’ Parent 4 ‘Don’t like the fact that the heads are in colour and the bodies are not’ Parent 2 ‘Design B didn’t inspire me to think about anything. Didn’t jump out at me’ Professional 6 ‘It’s not as colourful as the others. I’m not sure about the doodles – in their own way they stimulate ideas of topics that may be selected – is this what is intended?’ Professional 1 ‘It had an unfinished appearance with the line-drawn cartoon bodies – not attractive’ |
Evaluation of the stakeholder action group process
Feedback from stakeholders in the first two SAG meetings (Table 10) resulted in longer discussion groups and shorter formal presentation slots in subsequent SAG meetings. The timing of the meeting (at a weekend) was unpopular with several professional stakeholders, but this was not an issue raised by lay stakeholders.
| Meeting 1 | Meeting 2 |
|---|---|
| Suggested changes | Suggested changes |
|
Maybe just make the small-group discussions longer (parent) Give more time for fuller discussion and working through issues (professional) Broader selection of the groups (i.e. from more areas) (parent) More service users at the meeting (professional) Vouchers for HMV or somewhere else (patient) Less presentations so there is more time to give our points across (patient) Quieter venue (professional) |
Would like day to be longer as felt rushed in afternoon discussions (parent) Making the day longer (parent) |
| What enjoyed least | What enjoyed least |
|
The presentations (patient) It being on a Saturday so having to give up part of my weekend (professional) Probably that it was a Saturday (professional) I still remain a little unsure of the aims of the group (professional) Not enough time (professional) |
Early start (patient) I felt uncomfortable when I was put in the spotlight to talk (patient) Travelling (professional) Discussion prior to afternoon session about how to engage teams as wasn’t aware of background to project (setting up RCT) (professional) |
The second meeting started and finished earlier because some lay stakeholders were tired or less engaged towards the end of day 1. However, some disagreed with this change, requesting longer discussion and a longer day. One commented on the unease felt when being ‘put on the spot’ in a discussion session. In subsequent meetings, more attention was given to the facilitation process to ensure avoidance of discomfort in stakeholders when eliciting their views.
After the third meeting, feedback on stakeholder involvement in the study as a whole, focusing on what aspects of the study they enjoyed most and what they would have liked the research team to have done differently, was collated and the findings are summarised descriptively in Table 11. For most stakeholders, it was a positive experience. Lay stakeholders valued the opportunity to meet others in the same situation and to be listened to by others. Overall, parents wanted greater opportunity to discuss their views and some expressed dissatisfaction about the level of lay/professional integration. When asked whether or not they wished to have any further involvement in the study, only one lay stakeholder declined. Both lay and professional stakeholders valued exposure to each other’s views and perspectives and knowing that they were contributing to a worthwhile endeavour. Professional stakeholders mentioned a desire to experience the research intervention. Some professional stakeholders would have preferred a choice about group allocation. An initial challenge related to engagement of some professional stakeholders with the behavioural science behind the proposed intervention and its clinical utility. The involvement of practitioners in the research team who had been involved in the developmental work underlying the research intervention was important in overcoming this challenge.
| What you enjoyed most | What would you do differently? |
|---|---|
|
Lay stakeholders Chance for child to reflect on own condition ‘Involving my daughter in thinking about her condition, which hopefully will impact positively’ (parent) Meeting other service users ‘Meeting other people in similar situations’ (parent) Being actively listened to ‘All of the group work and feeling you are helping towards a worthwhile goal. Having my ideas listened to and taken on board’ (parent) Professional stakeholders Enthusiastic and generous researchers ‘The programme team’s enthusiasm and generosity of spirit’ Learning about the study ‘Hearing how the project is evolving’ Both lay and professional stakeholders Being involved/contributing to a process ‘Putting ideas across that can hopefully be helpful’ (patient) A feeling that your views will have an impact on future patient care’ (parent) ‘I’ve enjoyed being a part of something that ultimately could improve communication between service users and providers’ (professional) Meeting and hearing from others ‘Listening to other people’s ideas and helping design the tool’ (patient) ‘Listening to feedback from groups and hearing similar themes to those of your own AND new ideas’ (parent) ‘Knowing opinions of adolescent patients and their parents’ (professional) |
Lay stakeholders Longer day to do more ‘I don’t think so – no – it has all been well organised – maybe have a longer day to get more in’ (parent) Greater lay and professional integration ‘The clinical ‘experts’ did not integrate very much with youngsters/parents; they seemed generally more interested in catching up with each other. They are not the ones living day/day with diabetes, despite their experience’ (parent) More notice of session content ‘A better idea of what would be involved on the meeting days’ Professional stakeholders Present evidence base ‘A little more on the behaviour change methodology and evidence it works over time!’ Choice about group allocation ‘More choice of which discussion groups to take part in’ |
Discussion
The requirement by the National Institute for Health Research HTA programme to actively involve key stakeholders, including children and teenagers with diabetes and their parents/carers, reflects the increasing focus on patients and the public being involved at all stages of the research process. 101,102 Kirby103 proposes that service user involvement can occur at three different levels: consultation, collaboration and user-controlled research. Service-user involvement in DEPICTED was primarily at the second level – collaboration – and was viewed as a reciprocal partnership between stakeholders, including service users and researchers. In DEPICTED, stakeholder involvement resulted in major contributions to the design of the research intervention, including reassurance to the research team of the intervention’s utility by ensuring that practical aspects proved acceptable to practitioners, patients and parents. Furthermore, stakeholders’ identification of the need for, and contribution to the design of, an agenda-setting tool (3T), and their specific contribution to the patient preference questionnaire (DCE), have been shown to be valuable given their successful piloting and the subsequent central nature of 3T to the intervention. Involving stakeholders was not simply a philosophical driver in this process; it also directly addressed the validity of the research being delivered. 104,105
These findings confirm that research proposals may benefit from service-user involvement at an early stage in their development rather than simple identification of the research topic and dissemination of research findings, which are the levels of service-user involvement in research most commonly reported106 and are in contrast to the conclusions of a recent consensus study. 107
A survey in 2007 concluded that only a small proportion of NHS researchers were actively involving service users when evaluated against eight consensus-derived indicators of successful service-user involvement. 107,108 As recognised by Barber and colleagues,108 this is partly due to the evaluated projects being undertaken at an early stage in the development of policies on service-user involvement. Nevertheless, although principles for successfully involving service users in health research have been clarified by Telford and Faulkner,107 there is less reported evidence of actual user involvement in NHS research,107,108 although this deficiency has been partly addressed in a formal evaluation of patient and public involvement in the UK Clinical Research Collaboration. 102 The lack of reported evidence may be due to the fact that service-user involvement in research is patchy and inconsistent. 109,110 It is still in its relative infancy, with many practical, ethical, moral, methodological and philosophical questions unanswered. 110
There is lack of clarity concerning the extent to which service users can influence and benefit the research process and low levels of consensus about what it means to involve service users successfully in research. 108,111 The work by Telford and Faulkner107 in identifying clear and valid principles to guide good practice, and the subsequent survey by Barber and colleagues,108 are important developments in an under-researched area, but raise questions regarding how ‘successful’ involvement can or should be measured. Furthermore, the eight indicators relate only to process and do not attempt to measure the impact of successful user involvement upon research outcomes. This concern is raised by other researchers working in the field of involvement, who purport that the lack of an evidence base concerning the impact of involving service users can mean that this endeavour is seen as relatively low status and labelled as an ‘add-on’. 112 They further suggest that the existence of a strong evidence base would significantly contribute to the ‘business case’ for involvement, encourage more general recognition and help protect continued funding.
The experience of the DEPICTED research team demonstrates that stakeholders can make a significant contribution to the design of a complex research intervention, even at relatively early developmental stages. This finding provides evidence that involving lay and professional stakeholders can produce relevant and valid interventions that benefit substantially from their combined experiences. This is consistent with outcomes reported by others investigating the benefits of involving people with diabetes in research. 113 Furthermore, the experiences of the DEPICTED research team, and of the lay stakeholders involved in the study, seem to conflict with the belief that one of the fundamental barriers to the empowerment of service users is researchers’ fear of losing their power and status as ‘experts’. 109 Of course, it is important that equality of power is considered at the planning stage of research. For example, in DEPICTED, an adequate number of service users in the SAG balanced the influence that professionals might exert on the process,114 particularly when a question (e.g. what are the characteristics of a ‘successful’ consultation?) potentially posed a tension between lay and professional viewpoints. The nature of stakeholder involvement reported here strongly suggests that lay stakeholders and researchers can work together as experts, but in complementary dimensions of the research project: expertise by experience and expertise by profession. 115
An indicator of successful service-user involvement in DEPICTED is that despite travel and time commitments no collaborating young person or parent withdrew from the study. This could reflect their inherent interest in the topic under investigation or perhaps the value they placed on being able to influence service improvements, a significant motivating factor for people involved in research. 107,116 It could be argued, therefore, that the lay stakeholders felt empowered through their involvement at this level in the research process. They had views about their experiences of clinic consultations and identified strategies to improve existing services. These views were listened to and directly informed the development of the 3T and DCE questionnaire, as well as strongly influencing the design of the research intervention and the subsequent trial design, all of which confirmed for lay stakeholders the value and purpose of their involvement.
There were specific issues related to working with families. The decision to include parents of participating teenagers was made partly for logistical reasons (parents needed to transport/accompany their youngsters to the meetings). In most cases, teenagers were accompanied by two parents, resulting in excess numbers of parents contributing to the discussion groups. Furthermore, it became clear that teenagers should be in separate discussion groups from their parents to promote their active engagement, to allow them to step outside their usual generational roles and to disclose information that they might not wish to share with their parents. This arrangement proved successful, allowing teenagers to become more vocal within the groups, and is an important lesson for research teams proposing to work with young people and their families in the future.
Although problems were not encountered, it is important to anticipate tensions when research is steered by service users (collaboration) as opposed to seeking their opinion (consultation), as occurred, for example, in the naming of the agenda-setting tool. Although consensus was reached with little difficulty, this process could have proved problematic if there had been disparity between the research team’s choice of name and that of other stakeholders. Greater transparency in decision-making, including clarity about individual roles, should ensure that all can make a valid and recognised contribution. Establishing a consensus-based ‘terms of reference’ for the group at the outset would be a useful future strategy.
Although guidelines for service user involvement exist in terms of ethical and practical issues, there is a dearth of research evaluating this phenomenon, and different challenges inevitably arise when service users are involved in research as active, rather than passive, participants. 103,117,118 One such issue, particularly when health research focuses on a specific condition such as diabetes, concerns the ability of service users to see beyond their own experiences to view the ‘bigger picture’. Although service-user involvement could be used as a platform for airing particular grievances about service provision, such experiences are also part of the value of public engagement. 119 In DEPICTED, some lay stakeholders attended poorly resourced diabetes clinics and their initial contributions were understandably focused by their own experiences of the adverse effects of such limited resources. However, with a greater understanding over time of the purpose of their involvement, assisted by careful facilitation of discussion groups whose leaders were aware of this issue, the lay stakeholders were able to consider the implications of the research and its effects on patients in a wider context other than their own clinic, which significantly increased the value of their contribution to this part of the research project.
Stakeholder involvement is more broadly represented in this study by inclusion in the research management team of a mother of a teenage patient who participated in a number of roles throughout the study including cofacilitator in SAG meetings. Despite her enthusiasm and commitment, it is important to recognise that such an unfunded contribution requires her to prioritise competing commitments with consequent risks of guilt at being unable to respond to overwhelming numbers of requests from the research team. In retrospect, although her involvement was not tokenistic, it would have been better to have more than one service user at this level of involvement. Consideration should also have been given to including adequate funding of her time commitment in the grant proposal, which others suggest is important in maximising the potential of user involvement in research. 119
Reflections on the process
The research team have learnt important lessons for the future (Table 12). Such engagement has to be well planned and resourced (note that up to 15 researchers worked on each day). Meeting formats should vary to accommodate a range of stakeholder interests and preferences, and to suit the task requirements. We found an external facilitator especially helpful as he provided a challenge to the potentially insular perspective of the research team, helped the research team clarify their objectives for each SAG meeting and had expertise in managing mixed group dynamics. Support for the stakeholders (e.g. exploring and providing for their requirements, providing plenty of information before and during meetings) also seemed to be successful and acknowledged. What we would do differently includes initial eliciting of role expectations from both stakeholders and the research team, which could be formalised in a jointly developed terms of reference. This would include clarity about responsibility for decision-making and safeguarding all stakeholders’ valid contributions. Integration of all stakeholder groups should be addressed, perhaps by an explicit initial exercise, and supported thereafter by adequate opportunities to mix. Finally, working with families requires even further consideration to enable optimal involvement and experience for both children and their parents.
| What worked well | What we would do differently |
|---|---|
| Detailed planning | Explore stakeholder expectations more fully |
| Varying meeting formats | Agree explicit roles and terms of reference |
| External facilitator | Facilitate greater integration of all stakeholders |
| Support for stakeholders | Give greater consideration to family and professional dynamics |
Summary
Service-user involvement in health-care research is increasingly being required by funding bodies. However, the evidence base concerning the impact of involvement, particularly on research outcomes, is minimal. This is an important issue that needs addressing if involvement in health-care research is to achieve higher status and attract continuing funding. This chapter describes the active involvement of lay and professional stakeholders in the developmental stage of DEPICTED. Challenges encountered during the course of the research are identified and reflected upon. Importantly, it is also clearly demonstrated how stakeholder involvement positively affected the study outcomes: that a research intervention was successfully developed only because stakeholders, including teenagers with diabetes, were actively involved.
Chapter 7 Talking diabetes and 3T: integration of developmental activities and description of finalised intervention
Introduction
Overview
In this chapter we describe how the individual components of the developmental phase of the DEPICTED study were integrated in the design of the Talking Diabetes intervention. Where outputs from these component studies have been previously discussed in this report, only summary points will be presented in so far as they clarify their contribution to intervention development. Further details are provided about additional activity contributing to intervention design and to the development of the learning programme designed to train practitioners in the intervention. The intervention and the training programme are presented in this chapter. Finally, the chapter also synthesises the key messages arising from the developmental phase studies and describes how the work has contributed to the design and delivery of the evaluation phase presented in the next section of the study report.
Developing a complex intervention
Identifying the existing evidence base (see Chapter 2) represented by the Hampson and Murphy reviews37,50,64 was the first part of the developmental phase of a complex intervention. Main messages for the design of our intervention were the need for the intervention to be feasible for an NHS context, for it to be accepted by practitioners as an integral component of care, and for the developmental process to engage with all key stakeholders (including patients, their families, HCPs and health economists). The commissioning brief for this research project further favoured a generic intervention not requiring delivery by trained clinical psychologists, given their relative scarcity in paediatric diabetes services. The conclusion of Murphy and colleagues’ review,64 that education appears to be more effective when integrated within routine care when parents are involved in their child’s care and when self-efficacy is promoted, was also a key driver in intervention design.
Chapter 2 also presented the structuring principles guiding intervention development. Theoretical predictors of behaviour – patient beliefs about the value of change and their beliefs about their capacity to change – indicate the value of consultations that enhance coping skills. The way that patients are spoken to may hinder or promote intrinsic motivation to change and distilling the effective components of complex relational approaches such as MI for use in brief health-care encounters is gathering a supportive evidence base. In clarifying the theoretical rationale, the conceptual model of flexible shifting between consultation styles when talking about behaviour change may be useful, with a move towards a guiding style that promotes autonomous self-management. 42 A framework that distinguishes between levels of intervention (specialist psychological therapy, brief counselling by HCPs and interventions as part of routine care) may be additionally useful for structuring consideration of intervention options.
Modelling the intervention design to identify and clarify effective components and to assess acceptability and feasibility is the third major developmental component prior to experimental evaluation. Modelling the learning programme being considered to deliver the intervention is equally important.
Summary
The development process in the DEPICTED study aimed for an intervention that would improve support to children, teenagers and their families and help them cope better with restrictions associated with diabetes management. This involved developing a training programme for paediatric diabetes staff which would enable them to provide such support as part of routine care (i.e. not requiring extra staff or extra sessions). This chapter summarises the modelling process leading to the finalisation of the intervention and its associated learning programme, and describes how the developmental phase studies have contributed to trial design.
Framework for DEPICTED study intervention development
The framework for developing the DEPICTED study intervention is outlined in Figure 2 and summarises the empirical work planned in the first phase of the study. Existing theoretical approaches and the relevant evidence base have already been described, and the figure therefore focuses upon the modelling process, which is the third substantive component within the ‘Development’ element in the MRC guidance framework for complex intervention development. 120
FIGURE 2.
Intervention development model.
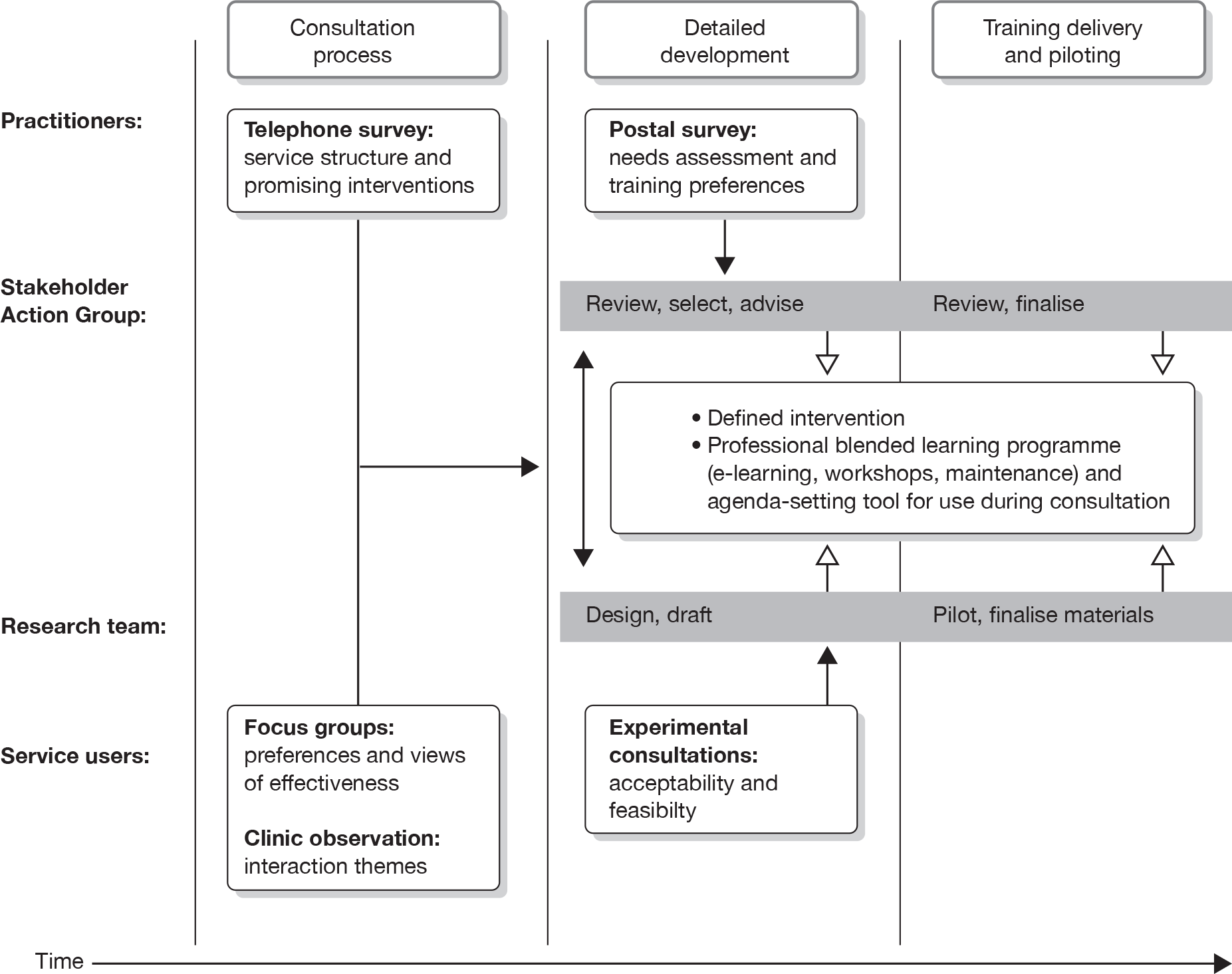
The model describes the three phases that proceeded from an initial consultation process with relevant stakeholders, through to a detailed development stage during which time the intervention starts to take definitive form and, finally, a stage of training delivery and piloting. Research activity within and across each phase is described in the model. The model further identifies which stakeholder group (practitioners, the formally constituted SAG, service users) and research team contributed to each phase and activity. A time line is indicated, although it was also expected that the overall process may not actually occur in such a well-prescribed linear fashion. 120
The research team’s role in addition to co-ordinating and undertaking the component activities was to work with a SAG to define the intervention and develop the training programme that would deliver it in the trial. This is indicated in the detailed development phase in Figure 2 and proceeds into a third phase at which point the intervention was confirmed and training programme finalised. The work of the SAG is more fully described in Chapter 6. Essentially their collaborative role was to provide input and guidance to the research team at key development stages of the intervention. Meeting at three full-day workshops over 10 months, preliminary ideas and outline strategies were presented and discussed in a mixture of small- and large-group formats. Guidance was provided from an overarching ‘in principle’ level through to more detailed input, for example about the design of intervention materials. Although the workshops and intervening contact were organised by the study management team, an independent facilitator was responsible for on-site co-ordination.
Brief description of developmental activities
Consultation phase
During the consultation phase, three activities were planned. First, a telephone survey was undertaken with providers of paediatric diabetes services in the UK (excluding Northern Ireland). This survey addressed the following domains: current and past service innovation and educational approaches, routine care provision, psychological support and clinic characteristics. Clinical representatives from a sample of 112 services were approached for interview and 51 completed the interview (44 doctors and seven nurses). Narrative responses were analysed thematically. Full details are provided in Chapter 3.
A second major activity in the consultation phase was a focus group study involving service users (young people with diabetes and their parents). Six focus groups with a purposive sample of service users from one local service were conducted to explore experiences of living with diabetes and of attending diabetes clinic. A particular focus was upon the interaction that patients and parents had with staff and aspects of their clinic experience that could be improved. Data were analysed thematically and the study is described fully in Chapter 5.
Finally, a small observational study was conducted at three local diabetes clinics (Cardiff, Newport and Bristol), which involved two researchers independently sitting in on routine consultations and associated team meetings. This allowed the non-clinical intervention development lead (KB) to gain increased familiarity with the relevant clinical setting and to directly observe consultation interactions with families.
Detailed development phase
Two core activities were undertaken. The first was a postal survey of practitioners, which aimed to identify perceived educational needs related to communication skills and to review preferences for training approach. Questionnaires were disseminated to all team members by the 67 consultants who initially responded positively to the request for a telephone interview described in Chapter 3. The postal study itself is fully described in Chapter 4. In short, the survey addressed three areas of interest: experience in communication skills training and its delivery; an assessment of attitudes towards addressing different topics in routine consultations; and perceived feasibility of different options for training delivery.
Secondly, role play and experimental consultations were conducted to test the acceptability, feasibility and face validity of the developing intervention from both patient and practitioner perspectives. The former involved two paediatric diabetes practitioners (JG and LL) and two clinical psychologists (SR and SC) expert in MI approaches. The patient roles were played by young actors from the drama club of a local high school. The experimental consultations were also an opportunity to gather recorded data to inform the detailed learning programme design (e.g. to facilitate the scripting of simulated dialogue). Families registered with the local paediatric diabetes service in Cardiff were invited to attend an experimental consultation. In preparation for the consultations, one paediatrician and study team member (JG) was trained in the intervention strategies by a clinical psychologist (SC). Consultations were audio- or video-recorded with family consent. From early in development consultations involved the use of the agenda-setting tool and other intervention strategies as appropriate. SC and two other study team members (EC and KaH) were involved in conducting experimental consultations, although only the former simulated the intervention. It was requested that consultations should involve discussion of a behaviour change and that consultation length should be kept within 20 minutes to reflect local practice.
Experimental consultation participants were debriefed by a researcher (KB). For families, this involved a discussion that could be informed by playback of the actual consultation. A debrief schedule addressed families’ perception of consultation style and comparison with their routine consultations. Those in the intervention consultation were additionally asked about the agenda-setting tool and, for example, whether or not they had any suggestions for redesign or instructions for use. Professional debrief interviews addressed reflections of the effectiveness of the strategies used, any concerns raised and comparison with routine consultations.
Developing a blended learning programme
The emergent intervention was to be delivered via a blended learning programme of online experiences and face-to-face workshops.
e-Learning programme
A common architecture for the web-based learning programme was developed alongside two other professional training programmes (directed towards primary care practitioners). A core team was responsible for developing the design of intervention and the e-learning programme (KB, SR, MR) in conjunction with an educational designer from a commercial company that specialised in producing training materials for health professionals [HLC Ltd (Smile-On Ltd), London, UK]. Throughout the development process this core team was responsible for engaging with other members of the management team (in particular, clinical members JG, SC and LL) as well as co-ordinating with external advisors including the SAG.
Developing e-learning required a technical outline and detailed design specification (Box 12). A thread running through the programme was the depiction of three common clinical challenges, demonstrated by individual case scenarios. This was a vehicle to facilitate learner engagement and to enhance authenticity. Although the strategic content of the learning programme has general clinical applicability, it was important that the programme started from the challenges facing practitioners in routine paediatric diabetes consultations. The cases represented different behaviour change scenarios, were introduced to learners at the outset and were returned to at different points of the programme. Scripting of recorded material drew upon the insights gained from the observational and qualitative work, and involved iterative writing input from clinical team members as well as the core development team.
Internal database communication (e.g. tracking learner progress)
User interaction (e.g. accessing static programme resource)
Administrative functions
Outline designFunctional and build elements
Specifying learning objectives
Specifying core learning objects (e.g. video, audio modules)
Detailed designSpecific module aims and learning objectives
Full content storyboarding
Design conceptsEnhancing practitioner engagement by:
-
common clinical challenges and case studies
-
clinically meaningful script
Seminars
Two face-to-face seminars were to be integrated into the learning programme and were designed to address specific aims and learning objectives. The seminars aimed to provide the opportunity to practise intervention strategies and receive feedback within a broader group of learners, including others from the same and other clinical services.
Developing a shared agenda-setting tool
The role for a mechanism to promote shared agenda-setting emerged in the development phase. A design brief was developed which identified what consultation obstacles it was intended to address, its intended function and desired qualities (Box 13). Outline physical designs were discussed with a local graphic design company (Escape to… Design Ltd, Cardiff, UK), which was then commissioned to generate further designs and, once the final design was chosen, to produce the final tool. As mentioned above, the SAG was influential in helping the research team develop ideas about the tool’s design and functional application.
Silent/disengaged children and teenagers
Clinicians telling patients what to do instead of helping them find their own solutions
Intended functions of the talking aid/agenda-setting toolGet patients (and their parents) involved in the consultation process
Support communicative processes that might be difficult to bring off otherwise
Support clinician’s use of a ‘guiding style’ when talking about behaviour change
Legitimise introduction of non-diabetes topics (patient’s lifeworld)
Help create spirit of curiosity
Desired qualitiesSimple and not overly ‘psychologising’
Self-explanatory in its use before the consultation (although clinicians will be trained on how to use in consultation interactions)
Same design for all ages (appropriate for younger children, but not patronising to older ones)
Option to choose from a menu of topics, but also add your own
Inexpensive (to enable broad applicability and rollout)
Synthesis and integration of emergent messages from modelling studies
Full results from the consultation phase activities and postal survey are provided elsewhere in this report and therefore greater emphasis is placed here upon the experimental consultations and description of the intervention and learning programme. Nevertheless, key findings from each are summarised in Table 13, as are salient messages for intervention design and learning programme development. Key messages emerge across activity area. First, a clear conclusion was the need to better engage children and their families so that they are more active and in control of their diabetes management. From the telephone survey, practitioners reported this to be a common clinical challenge, as did families in the focus groups. It was clear though that engagement needed to by sensitively delivered and that attempts should reflect genuine curiosity on behalf of practitioners.
| Activity | Relevant findings | Key design messages |
|---|---|---|
| Telephone survey |
Challenges in engaging children/families in process of care Current training remote from actual clinical practice Integrating diabetes into everyday life Meeting needs of different patients (e.g. different ages) |
An emphasis on family engagement Locating the training material in familiar clinical context – authenticity and conceptual clarity Understanding the patient/family agenda – what’s important for them Flexibility of approach |
| Focus group study |
Clinic attendance seen as for benefit of clinicians rather than families, and children in particular may feel marginalised Children want a more realistic and positive approach which appreciates their lifeworld |
An emphasis on enabling for sincere engagement A consultation approach that goes beyond narrowly focused clinical agenda |
| Observational study | Children underinvolved in key parts of the consultation | An emphasis on engagement for both children and their parents |
| Postal survey |
Practitioners recognise importance of addressing psychosocial issues, but lack confidence to do so Paucity of previous training in communication skills Support for workshop web-based training, but concerns about time |
Gap in communication skills recognised particularly in relation to non-medical aspects of consultation To recognise existing experience and also previous training Recognition that one approach will not be universally popular and therefore supportive of a blended approach to training. Heightened awareness of the need for feasible and flexible learning options |
Practitioners had little formal training in relevant communication skills and the training that they did have had not necessarily been rooted in the reality of their daily clinical world. Therefore, there appeared to be enthusiasm for clinically relevant training that reflect the common challenges that practitioners face. Practitioners were nevertheless experienced in consulting with families, and had in many cases developed their own approach. Therefore, the learning programme should build upon such experience and provided further conceptual clarity.
Related to engagement was the clarification of the importance to families of the non-medical agenda. The agenda-setting approach should therefore enable this and help the consultation retain a realistic feel for families. An agenda-setting approach should also be able to accommodate families with diverse perspectives and concerns. The intervention as a whole should also be flexibly adaptable to a wide range of consultation scenarios. Lastly, for flexibility in training delivery and options for learners, it is important to maintain engagement and to reflect the restrictions imposed by busy clinical workloads.
Experimental consultations
Several topics arose in both the initial practitioner role-play sessions and the experimental consultations. These are summarised in Table 14 and involve some general observations about the intervention approach as well as strategy-specific topics. The former includes reflection following training about the practitioner’s natural tendency to initiate problem-solving early in the consultation and the effort to resist this. The latter includes observations about the enthusiastic response to the agenda-setting approach.
| Observation | Implication for intervention and/or learning programme |
|---|---|
| Problem-solving | |
| Natural initial inclination for practitioner to problem solve | Highlight opportunities to elicit or provide solutions following exploration of problem |
| Time taken to listen | |
| Listening to families may feel longer than it actually is in practice | Acknowledge this observation and show the benefits of open questions |
| Summarising | |
| The value of summarising for patients (feeling heard) and for practitioners (as a device for managing the direction of the consultation | Bring this consultation skill to the foreground of the intervention rather than simply as a component of an existing strategy |
| The ‘guiding style’ | |
|
Surprised by the approach, but positively received by families – less formal and more discussion about them than their diabetes Impact on consultation length |
Supportive of the approach – but raise awareness that this represents a change for families in the approach to their consultations Be honest about initial impact of acquisition and rehearsal of new skills on consultation length |
| Agenda-setting approach | |
| Enthusiastically reviewed by families who would have preferred access prior to attending clinic. Some discomfort among younger children with greater attention on them as a result of its use | Clear instructions in learning programme on how agenda-setting is introduced to families at the outset |
| Information provision | |
| Some agenda items represented requests for information rather than a behaviour change element | Learning programme to highlight that agenda-setting may involve a variety of topics and communication styles in responding. Add some background guidance on providing information |
| Pros and cons strategy | |
| Worked well from practitioner perspective, but can risk appearing insincere to patients who may be suspicious about being asked about the ‘pros’ and deleterious behaviour | Attention to careful use of language to emphasise the intention to understand patient (not what the patient thinks practitioner wants to hear) |
| Importance and confidence strategy | |
| Not actually observed in consultation | Appropriate use related to whether or not there is a behaviour change being considered. Review weight attached to this in learning programme |
| Goal-setting strategy | |
| Positively reviewed by families, particularly brainstorming; jointly arriving at realistic solution enhanced motivation and empowerment | Following goal-setting, need to review original agenda to close down consultation and plan for subsequent consultations |
Although the feedback from both practitioners and families was generally positive, there were also some concerns raised. These included some wariness on behalf of younger patients about being the focus of attention in the consultation, surprise about the greater patient-centred approach and potential doubts about the sincerity of the practitioner adopting these new approaches. From the practitioners’ perspective there were also some areas to be addressed, for example the probable impact upon consultation length when introducing the new strategies into their practice.
These observations and reflections were used to further guide the detailed design of the intervention and its associated learning programme (see Table 14). In some cases this involved simply being transparent about the implications of strategy use (e.g. that implementing newly acquired skills may lengthen consultations initially). Other feedback indicated greater attention to be paid to clear instructions or explanation for families or practitioners (e.g. in using the agenda-setting approach). A more fundamental change to the intervention itself was the promotion of summarising skills to the foreground of the learning programme because of their general perceived value.
Description of the Talking Diabetes intervention model
The intervention model is described in Figure 3 and consists of the 3T agenda-setting tool, a menu of strategies to support behaviour change and a conceptual framework that identifies the importance of flexible shifting between consultation styles, with an emphasis upon guiding. Agenda-setting helps patients/families prioritise their concerns prior to the consultation and all parties to agree what to cover in the consultation. It aims to promote parity in deciding what to talk about, to more fully engage patients in their consultation and make them more active partners in the management of their diabetes. Practitioners can select from a menu of strategies that can be used when behaviour change is a possibility. ‘Pros and cons’ may be most useful when a patient appears reluctant to consider a particular change. ‘Importance and confidence’ can help explore a patient’s apparent ambivalence about the ‘why’ and ‘how’ of changing behaviour. Both strategies can help indicate whether or not it will be productive to continue discussing behaviour change within the consultation or move on to other topics. Where patients are ready to plan for change, shared goal-setting through brainstorming encourages them to be active partners in planning and reviewing progress. The value of each of the three consultation styles for different consultation demands provides an overarching framework. All may be appropriate and skilful practice may be defined by flexible shifting between styles. Guiding may be most effective for behaviour change and the use of open questions and effective summarising can support such a consultation focus.
FIGURE 3.
Talking Diabetes intervention model.
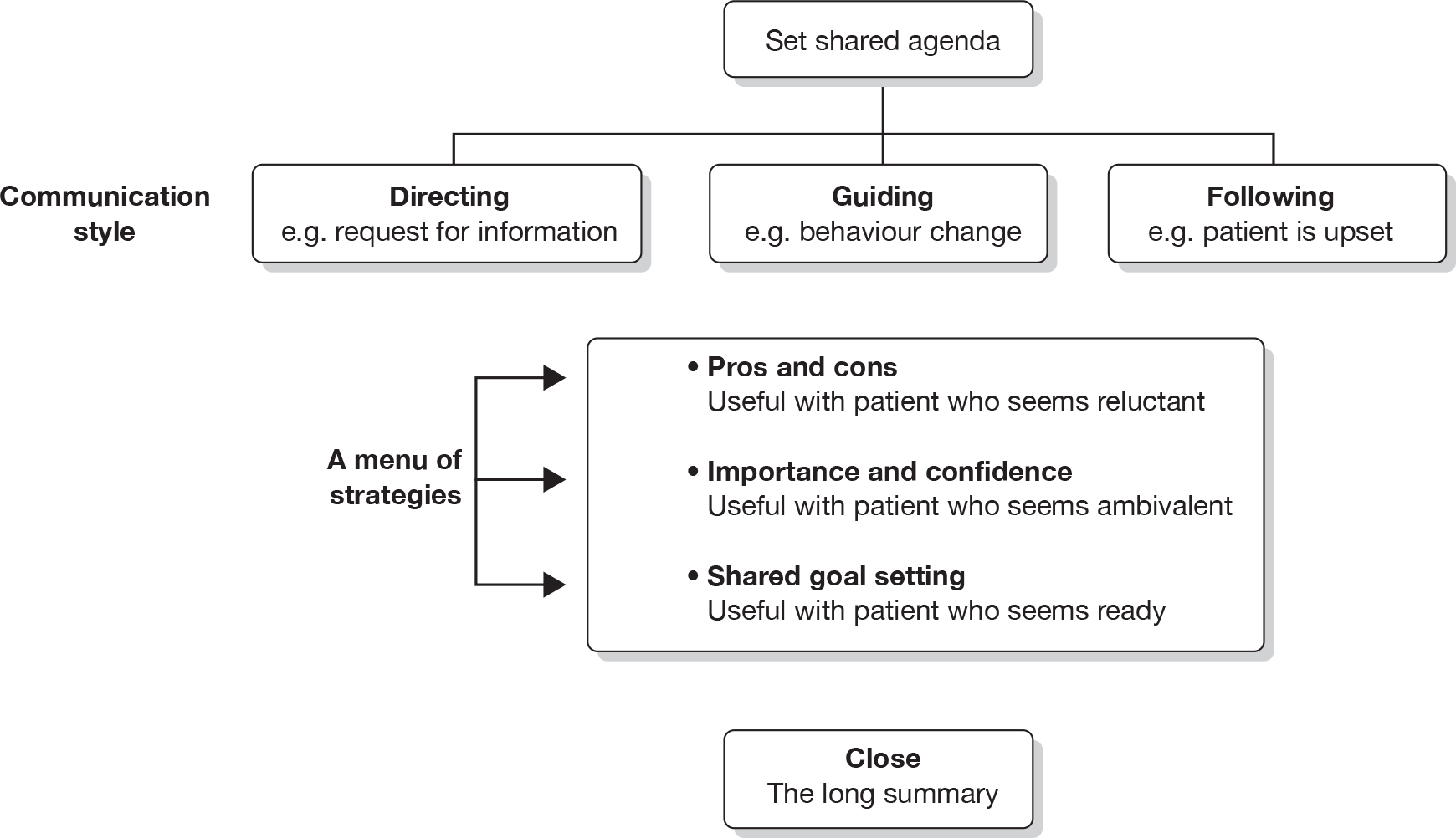
Description of the Talking Diabetes intervention and learning programme
The shared agenda-setting tool: 3T
The tool consists of an A4-sized gummed pad of 28 agenda sheets with images of children and young people, and encircled discussion topics that vary by sheet in a sequence of four sheets (Figure 4). The colourful photographic images portray individuals of different gender, ethnicity and apparent mood. Entitled ‘I think I’d like to talk about …’, the sheet offers plenty of blank space and blank topic circles for patients to add their thoughts. The pad’s inner sleeve provides a rationale and instructions for use and a diagrammatic example of a completed sheet. The pad is presented in a rigid plastic folder (Figure 5) of matching design, which incorporates insert pockets (e.g. for storing papers and pen holders).
FIGURE 4.
Sample agenda sheet from 3T.

FIGURE 5.
Front cover of the 3T folder.

The Talking Diabetes learning programme
The learning programme is aimed at all members of the clinical paediatric diabetes team and is delivered in eight parts, blending two face-to-face workshops with online activities (Table 15). Each programme part comprises separate modules that represent the specific programme and learning objectives. The environment of the 3T website provides access through a verified registration to the learning programmes. A top-level menu page provides access to each online part and module. Learners proceed sequentially through the online learning programme, which tracks module completion status. Nevertheless, once completed, learners can move back and forth through programme modules. ‘Help’ menus and additional learning material not covered in the foreground of the programme modules (provided in a ‘Resources’ section) are accessible from the top-level menu.
| Part | Modules | Duration |
|---|---|---|
| Part 1: E-learning | ||
| ‘Three common challenges’ | Welcome and introduction/how they see it/how you see it/wrap up/what are your thoughts? | 15 minutes |
| Part 2: E-learning | ||
| ‘Three styles and agenda-setting’ | Introduction/three styles/agenda-setting/the 3T tool/what are your thoughts? | 30 minutes |
| Part 3: Seminar | ||
| ‘Three styles and 3T’ | Why agenda-setting?/practical agenda-setting/adopting agenda to own service/behaviour change and communication style | One day |
| Part 4: Online reflection | ||
| ‘Impact of seminar on me’ | Portfolio task | 10 minutes |
| Part 5: E-learning | ||
| ‘Into the heart of behaviour change’ | Introduction/problems and principles in behaviour change talk/core behaviour change strategies and skills/wrap-up/what are your thoughts? | 45 minutes |
| Part 6: Seminar | ||
| ‘Improving consultations’ | ‘Strategies in the consultation/behaviour change constructs/‘pros and cons’ /‘importance and confidence’/‘shared goal-setting’/closing the consultation | One day |
| Part 7: Practical work | ||
| ‘Three real cases’ | Three real cases | Variable |
| Part 8: Exchanging experiences | Web forum | Variable |
The e-learning parts vary in length, with the longest being Part 5 at approximately 45 minutes. Learner engagement is initiated through the use of cases studies that depict common clinical challenges and which track through the learning programme. Materials presented through a variety of media (including audio, video, text, graphics) provide a learning experience which combines didactic with self-directed components. Typically, the presentation of theoretical rationale is followed by practical demonstration (e.g. using simulated consultations) and opportunities for self-assessment, further reflection and further reading.
The first half of the learning programme including the first seminar focuses upon preparing for constructive consultations, whereas the second half emphasises practical strategies for facilitating behaviour change. The two seminars are an opportunity using role play to practise skills presented online and learners are encouraged to attend with their other team members. Each seminar is facilitated by two trainers (a clinician and a psychologist) and lasts approximately 5 hours. Activity is undertaken in both large plenary groups and smaller working groups. The seminars are described by a formal manual that was developed iteratively by the trainers.
Learners are encouraged to reflect upon the training, in particular by reporting their clinical experiences when attempting to implement newly acquired skills. Following the second seminar, Part 7 requires learners to reflect upon such attempts with three real cases. Once completed, their reflections are forwarded to the training team, who provide specific feedback and further guidance to the learner via e-mail. Following completion of the programme, learners are encouraged to reaccess the online programme to review their learning and also to contribute to a web forum made available to all learners. A course certificate and a personalised portfolio documenting the programme content and learner responses can be downloaded and kept for personal development portfolios.
Piloting the learning programme
Piloting took two forms. First, a group review of online training materials was conducted with a selection of practitioners and researchers. Secondly, the two face-to-face workshops were piloted in full with members of a single paediatric diabetes team from south Wales. All five trainers were involved in the pilot and, within each workshop, rotated between acting as facilitator and observer. Piloting was a learning opportunity for the trainers; it enabled evaluation and refinement of the teaching strategies (e.g. use of role play) and materials, contributed to developing a trainer’s manual and allowed review of learners’ experience of the online learning. The trainers met in planning and review meetings before, in between and after the two workshops. Feedback from piloting indicated the need for more theory about behaviour change to enhance programme credibility, the need to emphasise the background development work to the intervention and the need for more theory within the first workshop in particular. The role play had worked well. Problems with the online software were reported, with some learners being unable to download the player software that was required to run video components. It was therefore planned that learners would be provided with minimum running requirements and encouraged to access the programme sufficiently in advance of the workshops to allow remedial support. Two further pilot workshops were run following such modifications with a second professionally heterogeneous group of practitioners to confirm the validity of such changes and to provide further experience for the trainers. The balance within each trainer pair (clinician and psychologist) was intended to ensure clinical and theoretical credibility and the value of this appeared to be borne out in practice.
Discussion
A process of complex intervention development has delivered a theoretically driven intervention and associated learning programme for professionals working in paediatric diabetes teams. Development has included modelling intervention and programme components and assumptions within a planned iterative process that has involved a broad multidisciplinary research team and a collaborative group of lay and professional stakeholders. The intervention seeks to improve outcomes for patients by changes in their behaviour facilitated by a modified consultation approach that emphasises engagement and a guiding style supported by specific consultation strategies.
Emergent messages from across the developmental phase studies included the challenges for practitioners in engaging with families – their awareness of the importance of psychosocial issues in helping families, but a lack of confidence in addressing this. Current training opportunities for practitioners have not adequately provided skills that may be useful in negotiating self-management with families, but practitioners were also concerned about not adding to their existing full schedule of work. A lack of engagement by young patients in their own clinical care was borne out by observational findings and by the reports of children and families themselves, who felt marginalised by a clinic agenda that does not fully recognise the concerns of children. Children reported that demands on them were not realistic and not sufficiently positive.
How have phase I studies contributed to intervention and learning programme design?
The development of the Talking Diabetes intervention has been guided by the existing empirical evidence for educational and psychosocial intervention in young people with diabetes. This has resulted in an intervention that can be applied in routine consultations by all practitioners engaging with families and which, beyond the Talking Diabetes training, does not require a specialist (psychological) background or extended consultation time. The intervention would provide the practitioner with a menu of strategies and skills to support patient self-efficacy and engage patients in their own care. A flexible approach to implementation is core to the design and is essential for the intended application across diverse settings, patients with differing requirements and practitioners with varying experience and confidence.
The conceptual model clarified that the intervention is attempting to address not the more severe end of problems that practitioners encounter in clinic but the common clinical challenges found in practice. The learning programme was designed with that focus and uses everyday practice as its framework. Material used in the e-learning was drawn from real-life observation and, in particular, feedback from patients, carers and practitioners. It was felt important that learning could not be all remote, hence the face-to-face workshops would encourage teams to attend rather than individual practitioners. We recognise that this then makes a substantial demand upon practitioners and would be an important component of PE in the trial.
Developing a shared consultation agenda is a good example of how evidence has been integrated in our approach. Previous experience by the research team in the value of this broad approach was influential, but it was also clear from the qualitative work with families that engagement and the dominance of a clinical agenda was problematic. Along with a commercial design company, the stakeholder group of both professional and lay members helped the research team develop their design and implementation of ideas. Piloting showed the intuitive appeal of the approach to both patients and practitioners. The blended learning programme foregrounded the approach in both its e-learning and practical components.
How have phase I studies contributed to trial design?
The design of the intervention and of the professional training programme had clear implications for the design of the trial. Although the primary trial outcome would be a measure of glycaemic control, we would expect the intervention to be effective by a process of initial behaviour change by practitioners leading to an attitudinal shift in patient and families, and a subsequent change in behavioural self-management. It would therefore be important to assess the extent to which these impacts are observed in practice.
First, practitioner engagement with the training itself would be assessed in terms of both contact and response. Key questions would be to what extent do practitioners attend and engage with the training offered. Furthermore, to what extent could we observe the intended behaviours by practitioners in actual practice – would the guiding style be evident in practice and would the strategies such as agenda-setting be implemented as intended? More broadly, what would be the costs and operational requirements of this intervention and training – especially important if this is to be broadly rolled out into routine NHS practice? These issues would be addressed as part of the PE.
Second, what evidence from patients and carers would support the hypothesised pathway of effect? Agenda-setting seeks to better engage families, and the strategies are intended to enhance confidence and self-efficacy among patients who are considering changes in self-management. The detailed design of the trial would need to include measures of process and outcome that can shed some light on the black box of change.
Summary
The Talking Diabetes intervention places patients at the heart of their own consultation, aims to engage them in their own health care and supports all members of the health-care team with behaviour change strategies that can be flexibly deployed in routine clinical encounters. The detailed evaluation of the intervention in a formal trial of effectiveness and cost-effectiveness is presented in the following chapters.
Chapter 8 Introduction to trial phase
Intervention development
Developmental work informing the trial
The primary aim of the DEPICTED study was to develop a training programme for HCPs working in children’s diabetes services, helping them talk more skilfully with their patients and families, with the aim of improving outcomes by facilitating behaviour change. The developmental phase of this project, described in detail in previous chapters, was informed by a previous systematic review of psychoeducational interventions37 and identification of more recently published papers, surveys of current clinical practice and of how practitioners might learn and apply such training and discussions with children and their parents about what they would like to experience in their consultations with clinic staff. The views of patients, their parents, practitioners and other stakeholders were then sought in a series of consultation exercises designed to model, develop and evaluate the training package for practitioners in order to produce a set of time-efficient and patient-centred interventions, which were then tested in experimental consultations. The next step following ethical approval (phase II) was to test this new method and training programme (Talking Diabetes) in a pragmatic trial of this complex intervention, measuring outcomes in children with diabetes attending a variety of paediatric diabetes services in England and Wales. Although HbA1c levels were determined by the commissioning brief to be the primary outcome for this trial, a particular challenge was to identify a range of appropriate measures of psychosocial outcomes and the costs of the intervention.
Complex intervention development and process evaluation
Complex interventions include several components, and a challenge in their evaluation is to identify and reproduce their ‘effective’ elements. 121 Other factors contributing to complexity include targeting the intervention at multiple groups; variability in, and number of, outcomes assessed; and the number of behaviours or components within an intervention and associated difficulty in delivery. 50 The development of the Talking Diabetes intervention was informed by the MRC framework for developing and evaluating (non-pharmacological) interventions. 120. 122
The MRC guidance recommends that complex intervention development be driven by a coherent theoretical approach: expected outcomes should be clearly defined and sufficient description of the development process should be provided to facilitate replication or roll-out. Assessments of feasibility (e.g. of recruitment within the target population) and cost-effectiveness are also key elements of development, as are ensuring adequate assessment (i.e. appropriate experimental design) and evaluation (i.e. inclusion of a PE). Clarification and extension of the more recent guidance120 includes a recommendation for greater attention to development and pilot work, a less linear approach to PE, increased emphasis on integration of process and outcome evaluation, and recognition that complex interventions may work best when tailored in some way to account for local contextual factors, rather than being uniformly standardised. 50
The current trial
Consideration of appropriate experimental design for evaluation within a given context is a key feature of complex intervention development. 120 When evaluating population-level interventions, individually randomised trials are often inappropriate owing to likely contamination of the control group and biased estimates of effect size. 121,123 A cluster, randomised design minimises this source of contamination and is therefore the most appropriate method of evaluation for the current, clinic-level intervention.
Empirical and consultative work during the intervention development phase helped formulate and operationalise the Talking Diabetes intervention (described in Chapters 2–7 of this report). The development stage provided evidence that the intervention is feasible for teams managing care, and is acceptable to patients and carers. A RCT was therefore needed to test its effectiveness. The aims, objectives and methodology used in the trial are described in the following chapter.
Chapter 9 Trial phase methods
Trial design and objectives
Trial design
The study was a pragmatic, cluster RCT (Figure 6 – an overview of trial design). Twenty-six teams were randomised to receive training at the start (intervention group) or the end (control group) of the 1-year study period. Multicentre approval was granted by Berkshire REC (07/MRE12/9) (see Appendix 1) and site-specific approval was granted by local RECs at all trial sites and all participating acute trusts.
FIGURE 6.
Trial design.
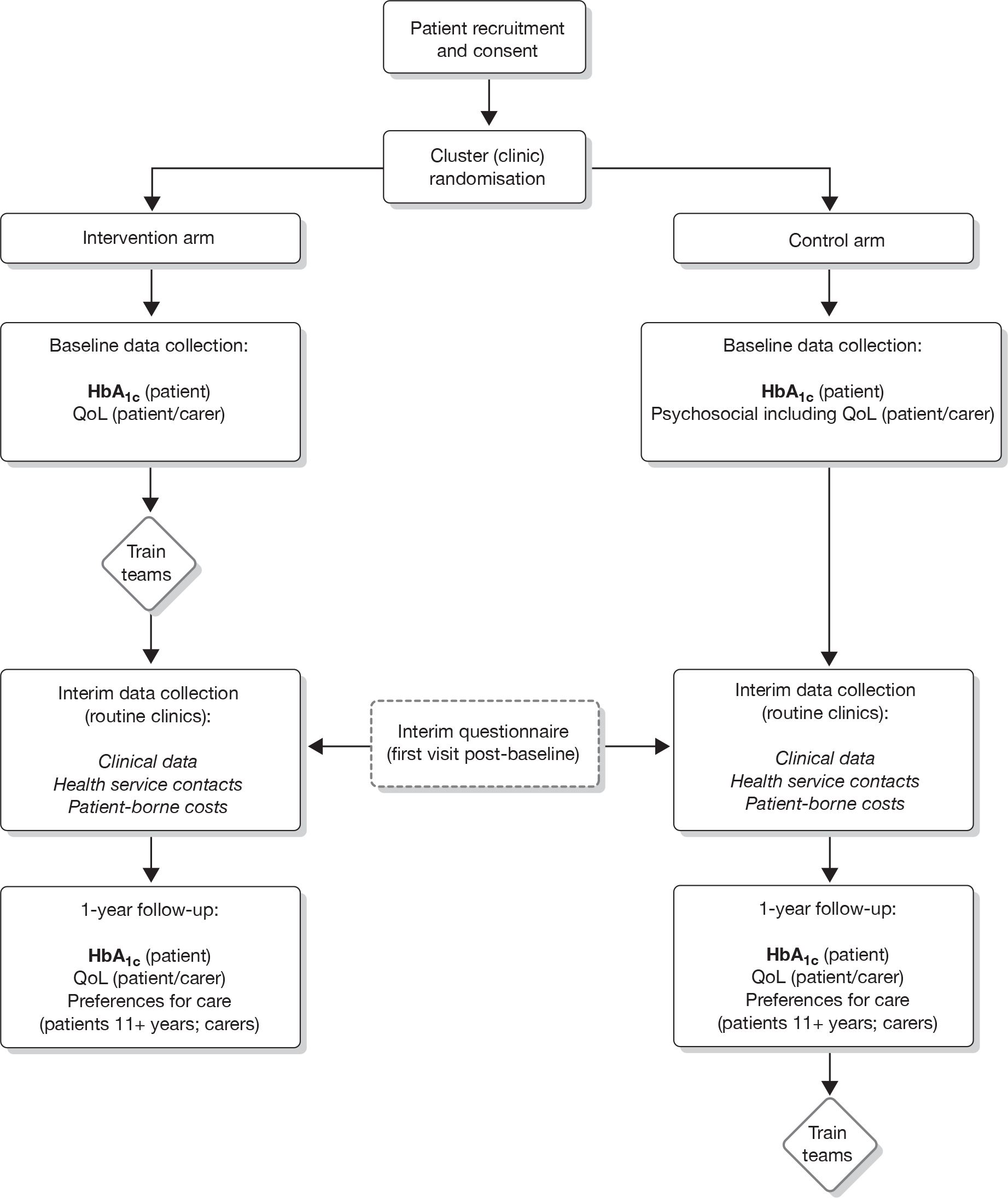
Trial objectives
The primary trial objective was to determine whether or not a multifaceted communication skills training intervention (incorporating a shared agenda-setting component) delivered at clinic level for non-psychologist members of a paediatric diabetes team would improve clinical outcomes (HbA1c levels) for young people with type 1 diabetes. Secondary objectives included measuring intervention impact upon psychosocial outcomes (including QoL) and assessing cost-effectiveness. A PE was undertaken to assess skill retention and performance of clinical team members in delivering the intervention and to examine any systemic changes to service delivery.
Participants
Centre recruitment
Potential clinics were approached using a variety of recruitment methods. Flyers outlining the nature of the study were distributed to members of the British Society for Paediatric Endocrinology and Diabetes (BSPED) and consultants and diabetes specialist nurses on a database compiled as a result of the surveys carried out during the development phase. Flyers were also distributed at professional and scientific conferences. Expressions of interest were received from 54 UK clinics. Thirty centres were formally approached to participate, based on clinic size and geographical location (see below) – 26 centres agreed to take part and were able to meet contractual requirements. All team members undergoing training were consented prior to randomisation and the incentive of receiving training at the end of the study was provided to reduce the risk of differential levels of dropout or engagement between the two groups of teams.
Participant recruitment
All eligible patients were identified from clinic lists by the research nurse, and a random sample of 40 patients was selected by the research team (from an anonymised list) and approached en bloc by the research nurse to obtain a target sample of 30 recruited patients per clinic. Written informed consent was obtained in all cases from a parent and, as appropriate, either written informed consent or assent was obtained from the child (both parent and patient had to be in agreement in order to take part in the study). Where the carer or research nurse felt that the participant was too young to give assent, a missing assent form was completed by the research nurse. Recruitment and randomisation of clinics was undertaken in three phased blocks (according to their governance readiness to commence the study):124 however, patients within each centre were approached en bloc by letter.
Inclusion and exclusion criteria
Each included clinic had to be staffed by at least one paediatrician with an interest in diabetes and a diabetes specialist nurse, and had to comprise 40 or more potentially eligible children and adolescents. Eligibility criteria for participants are provided in Table 16.
| Inclusion criteria | Exclusion criteria |
|---|---|
|
Type 1 diabetes Aged 4–15 years Under care of paediatric/adolescent diabetes team for duration of trial Diabetes diagnosed > 12 months earlier Parental or carer (and child when able) consent given Ability of patients (aged 7–15 years) and at least one parent or carer to complete study materials (questionnaires) |
Not under care of parent or guardian (i.e. a looked-after child) Co-morbid chronic illness likely to impact on HbA1c levels independent of patient’s ability to manage diabetes (e.g. condition requiring steroid treatment, cystic fibrosis, renal failure) In receipt of ongoing psychiatric/psychological therapy at the start of the study Other patients judged by clinical carer to be vulnerable because of existing medical or social condition |
Withdrawal and loss to follow-up
We allowed for a 22% loss to follow-up in the sample size calculation. The upper age limit of 15 years was set to maximise the likelihood of participants remaining under the care of the paediatric team for the duration of the study.
Trial procedures
Intervention
The intervention was delivered at clinic level. Members of clinical teams allocated to the intervention arm received a blended training programme comprising web-based material and face-to-face seminars (the Talking Diabetes programme). The training course aimed to prepare practitioners for constructive behaviour change conversations with patients and to provide practitioners with strategies and skills for encouraging behaviour change and is described in Chapter 7. Following the second face-to-face workshop, participants were invited to submit reports of three consultations in which they used their newly acquired skills and feedback was provided by pre-assigned trainers.
The training programme introduced practitioners to the shared agenda-setting device (3T). Practitioners had the option to complete a proforma on which general topics discussed at clinic visits could be recorded and kept with patient notes, to facilitate clinical record keeping and communication between HCPs. Copies of the paper agenda-setting pad (without folder) were made available to each clinic to refill or replace folders as required and for patients who were not otherwise recruited to the study.
Frequency and duration of follow-up
Patients provided blood samples, and patients and carers completed questionnaires immediately post recruitment, following their first clinic visit during the trial phase (questionnaire only) and at 1 year. Professionals’ consultation performance was measured post training and after 1 year to determine acquisition and maintenance of new skills. Professionals also provided attitudinal self-rating (importance and confidence) at the start and end of the training programme.
Training for research nurses
On-site nurses were trained to conduct study procedures (patient approach and consent, data collection) via pre-study group training sessions (all nurses were required to attend a single workshop for training in study procedures and good clinical practice). Research nurses were supported throughout the study period by the central research team.
Baseline data collection
Baseline data were collected by the research nurse in the clinic at a routine visit, during an ad hoc recruitment clinic (patients and parents were reimbursed for any travel expenses incurred) or in the patient’s home. The research nurse provided patients and carers with a copy of an age-appropriate questionnaire (7–10 or 11–15 years) assessing QoL and other self-reported psychosocial outcomes, which was to be returned directly to the research team (who followed up non-responders directly). Self-reported questionnaire data were not collected for the youngest patients (< 7 years old). The research nurse also completed a baseline Case Record Form (CRF), recording demographic information and clinical data (such as years since diagnosis, insulin type, dose and regimen, taken from patient notes on receipt of consent). Participants’ general practitioners were informed in writing of their patient’s trial participation by local clinic staff.
Capillary HbA1c samples were collected by research nurses and sent to a single UK laboratory (Diabetes Research Network Wales Laboratory, Llandough Hospital, Cardiff, UK) for measurement of HbA1c concentrations. Samples were collected in 5-µl glass capillary tubes and stored in a plastic-lidded tube (prefilled with diluent and preservative). Samples were securely packaged according to the laboratory manual and sent via Royal Mail (identified as a biological substance, category B). HbA1c assays were carried out using a Menarini HA-8160 instrument and results were reported directly to the research team, following adjustment against the DCCT international standard. When a sample was lost or spoilt in transit, the research nurse approached the patient and carer for consent to provide a second sample. In the event that a patient HbA1c sample was > 15.0% (considered to be indicative of a patient at significant acute clinical risk), local diabetes teams that were responsible for patient care were informed so that comparison could be made with the most recent HbA1c sample taken and analysed locally. Any patient contact resulting from notification of a high HbA1c value was at the discretion of the patient’s diabetes care team: the research team had no direct contact with patients in connection with HbA1c levels.
Interim data collection
Clinical patient details (HbA1c levels, height, weight, BMI, insulin regimen), health service contacts and patient-borne costs were recorded by the local research nurse at each clinic visit on the CRF. The research nurse also recorded who patients consulted with, for how long, and whether or not patients consulted on their own at each visit. At the first clinic visit, questions on the CRF were anchored to the baseline assessment. For future visits throughout the year, questions on the CRF referred to the period since the previous clinic visit. Patients and carers were also asked to complete an interim questionnaire (assessing patient enablement, or feelings towards clinic visit for younger patients aged 7–10 years) at their first clinic visit following the start of the trial.
Follow-up data collection
Capillary HbA1c samples for patients and questionnaires for patients and carers were repeated at 1 year. Where possible, primary outcome data (HbA1c levels) were collected 2 weeks either side of the expected date of follow-up (i.e. within a 1-month window). Follow-up questionnaires were sent to patients and carers directly by the central research team. Follow-up questionnaires also assessed preferences for care using a DCE (see Chapter 11) not previously included at baseline. If completed questionnaires were not returned to the research team within 4 weeks of the initial mailshot, participants received a follow-up telephone call from the research team and further copies of the questionnaires were sent out. Families were also sent a letter 2 weeks prior to their 1-year follow-up to remind them that they would shortly receive the questionnaires and that their local research nurse would be contacting them to arrange an appointment to collect a follow-up HbA1c sample. All families who were sent follow-up questionnaires were entered into a prize draw, as a thank you for trial participation and data returned to date. Ten families were selected at random at the end of follow-up, each of which was sent a £30 gift voucher.
Primary and secondary outcomes
The primary trial outcome was glycaemic control, assessed at the individual level using HbA1c value. Secondary trial outcomes included QoL, other clinical (including BMI) and psychosocial outcomes (assessed at participant level) and cost (assessed at clinic level), and are detailed in the following sections.
Piloting participant outcome measures
Participant questionnaires were piloted with patients and carers attending the paediatric diabetes clinic in Cardiff (University Hospital of Wales). Patients registered at the Cardiff clinic were identified and recruited by their clinical carer (diabetes specialist nurse). Measures included in the questionnaires have previously been validated in other populations, although some minor modifications had been made to some. The purpose of the pilot study was, therefore, to determine overall acceptability of the measures in this patient group, particularly in terms of presentation and design. A sample of six children (five aged > 11 years and one aged 7–10 years) and five carers completed age-appropriate questionnaires. Following completion, a researcher (HH) conducted a semistructured cognitive debrief interview. Questions assessed acceptability of the questionnaire items, ease of understanding and length. For older children (11–15 years), this process took place on a one-to-one basis with the researcher. For the younger child, a carer was also present for the interview. Cognitive interviews were conducted using standard probes that were related to particular areas of interest within the questionnaires. Analysis indicated that questionnaires were generally acceptable to both patients and carers. However, respondents had some difficulty completing the DCE component of the questionnaire. Piloting and further development of the DCE is described in Chapter 11.
Patient outcomes
Measure selection was informed by two HTA systematic reviews37,125 and through consultation with the SAG in the intervention development phase. Patient-reported outcomes (assessed via an age-appropriate questionnaire at baseline and follow-up) included demographic characteristics (age, gender, ethnic origin: baseline only), measures of diabetes-specific QoL,126,127 self-care [mismanagement questions relating to diet, number of injections and monitoring,128 patient enablement129 and patient perceptions of the diabetes team,130,131 importance of, and confidence in, their ability to undertake diabetes care and monitoring activities (patients aged > 11 years only)] and preferences for care (DCE: follow-up only). 132 Biochemical and clinical measures for patients comprised HbA1c levels, BMI, insulin type, dose and number of injections and self-reported frequencies of moderate and severe hypoglycaemic episodes (all recorded on a CRF at each clinic visit).
Carer outcomes
Carer outcomes included demographic information (age, gender, ethnic origin, socioeconomic status: baseline only), parent measures of QoL, anxiety and perceptions of the diabetes team,130 including items relating to communication between practitioners, feelings towards the next visit and continuity of care,131 enablement,129 and importance of, and confidence in, their ability to undertake diabetes care and monitoring activities. Proxy outcomes (patients aged 4–11 years) comprise diabetes-specific QoL126,127 and self-care. 128 Patient and carer outcome measures are summarised in Table 17.
| Domain | Measure and modifications | No. of items, response scale | Respondents | Assessment | Subscales |
|---|---|---|---|---|---|
| Diabetes-specific QoL |
PedsQL – type 1 diabetes module only: UK version126 Minor wording change: ‘fatigue’ changed to ‘tired’ |
28, five-point scale (0–4) | Patients (> 7 years), carers | B, F | Five subscales: barriers (four items), symptoms (11 items), adherence (seven items) and worry (three items), communication (three items) |
|
PAID Scale 127 From recently adapted adult version (Weissberg-Benchall, unpublished). Modified for DEPICTED from six- to five-point response scale for consistency with other measures. Minor wording change in carers’ version: ‘He/she’ to ‘my child’ (single occurrence) |
23, five-point scale (1–5) | Patients (> 11 years), carers | B, F | None for modified version | |
| General QoL (three single items) | 1, five-point scale (1–5) | Patients (> 7 years), carers | B, F | None | |
|
Compared to this time last year … living with my diabetes has become In general I feel … |
1, five-point scale (1–5) | ||||
| Compared to this time last year I feel | 1, five-point scale (1–5) | ||||
| Perceptions of health-care provider |
HCCQ 130 Items adapted to refer to ‘the diabetes team’ rather than ‘my physician’. Original seven-point response scale modified to five-point scale and scale numbers (1–7) changed to written response options (no, not at all; not much; a little; yes, quite a bit; yes, very much) for consistency with other response formats in questionnaire One item (The diabetes team … have confidence in my ability to look after my diabetes) removed for younger patients |
6 (5 for younger patients), five-point scale (1–5) | Patients (> 7 years), carers | B, F | None |
|
DCCS 131 Four out of six items in a subscale of the DCCS removed (subscale: communication between HCPs); remaining items refer to team communication and current information about care Wording of response options changed from strongly disagree/disagree/no opinion/agree/strongly agree to be consistent with HCCQ |
2, five-point scale (1–5) | Patients (> 11 years), carers | B, F | None | |
|
Patient Enablement Inventory 129 Three-point scale adapted to five-point for consistency with other measures Three-item version: understand illness, cope with illness and keep healthy |
6 (3), five-point scale (1–5) | Patients (> 11 years), carers | B, I, F | No subscale, but three-item version | |
|
Emotions prior to clinic visit Developed specifically for DEPICTED (fed up, excited, guilty, good, worried) |
5, five-point scale (1–5) | Patients (> 7 years), carers | B, F (I for patients ≤ 10) | None | |
| Self-care |
Diabetes MISMANAGEMENT Questionnaire 128 The item on diary entries was replaced with one on monitoring. Seven items were removed in total (original scale 10 items) ‘Shots’ has been changed to ‘injections’ and a ‘don’t’ know’ response option was included in the parent version. ‘N/A’ option for two items for those on an insulin pump added ‘Frequently’ response option removed Response options changed from ‘in last 10 days’ to ‘in last week’ |
4, four-point scale (1–4) | Patients (> 11 years), carers | B, F | None |
|
Importance score Additional questions developed to assess the ‘importance’ patients give to carrying out diabetes care behaviours |
6, five-point scale (1–5) | Patients (> 11 years), carers | B, F | N/A | |
|
Confidence score Additional questions developed to assess confidence in carrying out diabetes care behaviours |
6, five-point scale (1–5) | Patients (> 11 years); carers | B, F | N/A |
Resource use
The cost of the intervention included the cost of training intervention teams. The following training data were recorded: travel costs to seminars, time spent on offline learning activities (i.e. discussion of training content in pairs, reported online), time spent at seminars and time spent online (automatically recorded on website). Other training costs (venue, training materials, cost of trainer) were also calculated. Secondary costs are represented by between-group differences in service use, including in-patient admissions (including intensive therapy unit and high dependency unit care), accident and emergency unit attendances, clinic attendances, contacts with the diabetes team (home, telephone, face to face, electronic), other health service contacts (general practitioner attendances, any other) and medication or equipment use (insulin type and dose). Other costs assessed included travel to clinic, school absences and time taken off work by carers.
Process evaluation outcomes
The embedded PE is described in Chapter 12.
Statistical methods
Sample size
For an individually randomised trial to have 80% power to detect a moderate effect size of 0.4 for HbA1c levels at a 5% significance level, 200 patients would be required. Audit data from a Welsh Paediatric Diabetes Interest Group (the Brecon Group) relating to 750 children from all 13 centres in Wales indicate an ICC of 0.08 for HbA1c levels in patients aged 4–15 years. With 24 centres recruiting an average of 23 patients each, this inflates the total sample size required to 550. To allow for a 22% loss to follow-up, the intention was to recruit 700 patients. Twenty-six centres were recruited to allow for any subsequent centre dropout.
Randomisation
Allocation was based on clusters (i.e. paediatric diabetes teams). Half of the trial centres were randomised to the intervention arm and the other half to the control arm. Teams were recruited and then randomisation was optimally balanced133 for population (patient list) size. After the first block of randomisations, each subsequent block incorporated the balance from the previous allocation(s). 124
Recruitment bias is common in cluster randomised trials134 and therefore it was planned that patients would be approached and recruited before teams knew to which arm of the study they had been allocated. However, in practice this was not always possible. Allocation was revealed to all centres approximately 2 weeks prior to the first face-to-face training workshop for intervention teams, even at centres where recruitment was incomplete, to allow sufficient time for intervention teams to complete the e-learning component of the training. In all of the cases, however, eligible patients were identified by teams and a sample randomly selected by the research team for approach prior to clinic randomisation.
Main analysis
The primary analysis was an intention-to-treat comparison of HbA1c values between the two groups of patients at 1 year, using multilevel modelling to allow for cluster (centre) and individual effects (including baseline concentrations of HbA1c as a covariate). The primary analysis involved a two-level linear model. The influence of missing data was examined by replacing missing laboratory HbA1c measurements with routine clinic HbA1c measurements where possible.
Intention-to-treat analysis was used for all secondary analyses. Psychological outcome measures were derived from baseline and follow-up questionnaires and analysed using a two-level linear model incorporating baseline scores as covariates. Individual questionnaire items with proportional outcome data were analysed using multilevel logistic models.
No interim analyses were undertaken. Further exploratory analyses to be carried out, but not reported here will include a dose–response analysis conducted to explore associations between the amount of patient contact and an intervention effect. The two groups will also be compared for non-attendance as the intervention may improve motivation to attend. A review of patient outcome measures used in diabetes, predominantly in adults, concluded that, although most have been shown to have content validity, there is less available evidence regarding reliability and responsiveness to change. 135 Responsiveness of the specific measures used will be assessed using both effect sizes and correlation to clinical variables and self-rated change. Short- and long-term impacts of the intervention will be analysed within the intervention group only using repeated-measures ANOVA.
Economic evaluation
Interventions that involve training are inevitably resource intensive. Given the demands made on NHS resources, it is important to identify at what costs any benefits are achieved and to assess whether or not the intervention is cost-effective as well as clinically effective. Our survey of existing evidence regarding the effectiveness of psychoeducational interventions applied in paediatric diabetes services found no previous economic evaluations relevant to UK practice. As the main objective is to inform decision-making in the NHS, the economic evaluation adopted an NHS perspective. Direct costs include training and 3T (agenda-setting tool). All development and evaluation associated costs were excluded. Training resources, including the time of those being trained, were monitored prospectively and valued using relevant unit costs. In the base case it was assumed that all training activity took place during work hours, which reflects how the intervention is likely to be delivered if rolled out across the NHS. The base case also assumed that the intervention did not affect organisational factors such as number of clinics held. Validity checks on this were made during site interviews (see Chapter 12) and by comparing times spent with different members of the diabetes teams as reported on CRFs. As training is a one-off investment producing a flow of benefits into the future, training costs were annuitised over 5 years at a rate of 3.5%136 and expressed per eligible patient by site.
Indirect costs were patients’ differential use of NHS resources as recorded on CRFs at each clinic visit and valued using relevant unit costs. All unit costs and their sources are shown in Appendix 2. All costs are in 2009 prices, uplifted, where necessary, using the NHS Hospital and Community Health Services index. As follow-up was for 12 months only, discounting has not been applied. Non-NHS costs, i.e. patients’ time off school for any health reason and carers’ time off work in relation to their child’s health, were analysed and reported separately.
A series of one-way sensitivity analyses were undertaken to show the effect of changing base-case assumptions. These included the assumption that all online training occurred during leisure time and took place during a mix of work/leisure time based on the proportion of logins initiated during work time for each trainee. Further sensitivity analyses assessed (1) the effect of a 10-year life of training; (2) amortising at 7%; and (3) DEPICTED increasing clinic time by 20%.
The same methods for dealing with missing data were used in the economic analysis as in the main analyses. The only exception was the method used to impute insulin regime, which had been reported in total units or expressed as a ratio of units to grams of carbohydrate intake. As preliminary analysis showed the proportion reported using the second approach to be small, a regression method was used to impute the daily units of insulin. Age, gender, weight and HbA1c levels were used as predictors. Five imputations were carried out and the average of these was used to impute the missing data. Analysis was undertaken using Spss (version 17).
Given the skewed distribution of costs and resource units data, 95% confidence intervals (CIs) of mean difference were calculated using the bootstrap technique,137 1000 replications were performed and the cluster command was used to account for any correlation at centre level. The Stata version 10 (StataCorp LP, College Station, TX, USA) package was used for the analysis.
Chapter 10 Trial-phase results
Participant flow
Of the 30 diabetes centres formally approached to participate, 26 centres were recruited into the DEPICTED trial (Figure 7). Half were randomised to the intervention arm and the other half to the control arm, balanced by list size. Each centre was asked to recruit a minimum of 30 patients in order to achieve our desired sample size. Of the 1673 eligible patients, 1262 were approached. Control centres recruited a total of 334 patients and intervention centres recruited 359 patients, totalling 693 subjects. Baseline HbA1c measurements were obtained for 356/359 (99.2%) subjects in the intervention arm and 333/334 (99.7%) in the control arm. At 12 months’ follow-up, HbA1c measurements were obtained for 342/359 (95.3%) subjects in the intervention arm and 318/334 (95.2%) in the control arm.
FIGURE 7.
DEPICTED centre and participant flow chart.
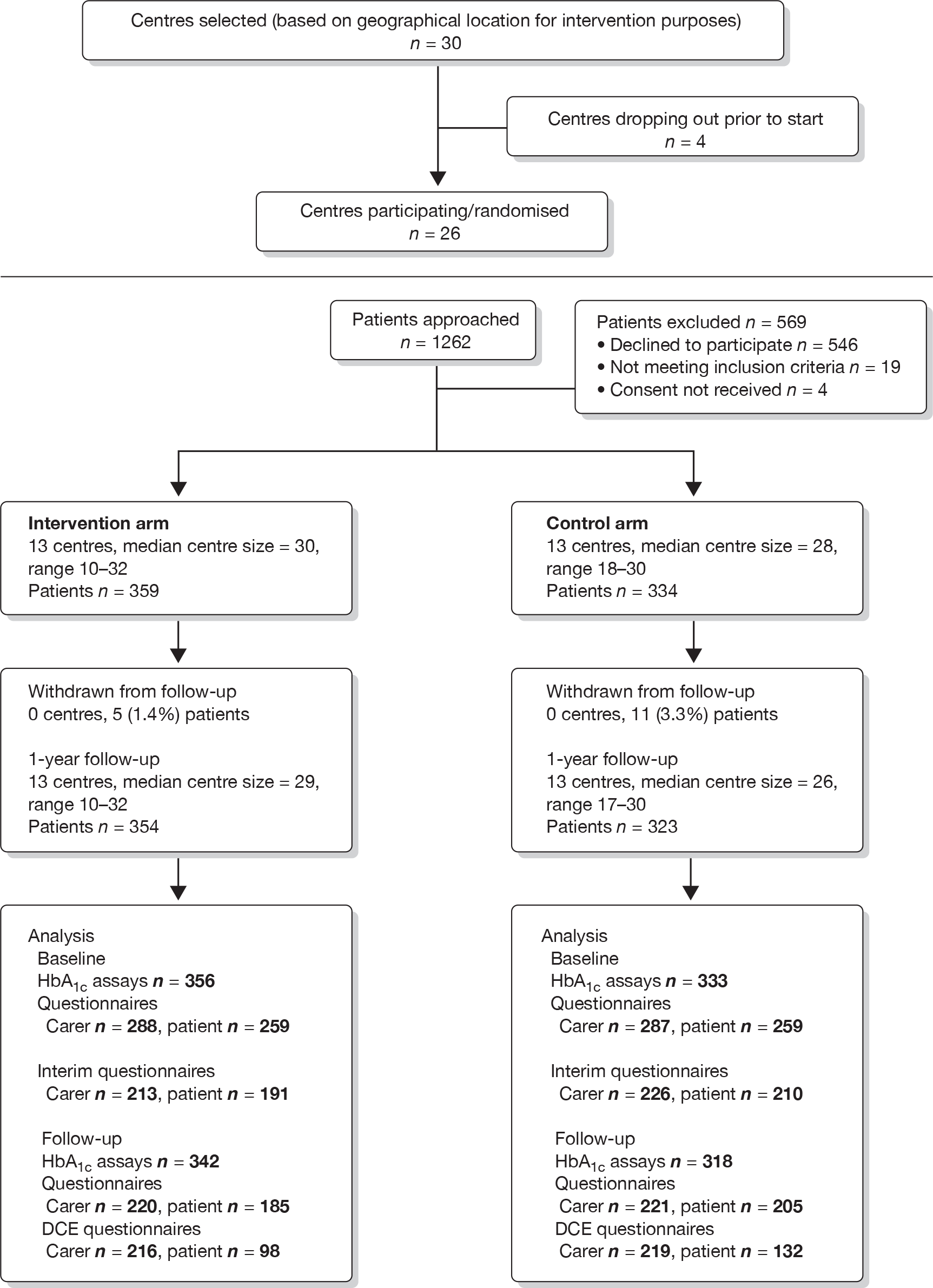
Response rates for baseline questionnaires were 83% for patients and 82% for carers and dropped to 64% and 65%, respectively, at the 12-month follow-up. Numbers completing individual outcome measures are given later in this chapter (see Tables 24–31). Response rates for the DCE questionnaire were 65% for patients and 63% for carers.
Recruitment
Centres were recruited between 30 August 2007 and 2 April 2008, and patients between 30 January and 25 September 2008.
Baseline data
Table 18 summarises baseline demographic information for control and intervention groups. Variable counts are due to missing item data that were assumed to be missing at random.
| Control | Intervention | Overall | |||
|---|---|---|---|---|---|
| n | Mean (SD) or % | n | Mean (SD) or % | Mean (SD) or % | |
| Patients | |||||
| HbA1c (%) | 333 | 9.2 (1.8) | 356 | 9.4 (1.7) | 9.3 (1.8) |
| Age in years | 334 | 10.7 (2.8) | 359 | 10.4 (2.8) | 10.6 (2.8) |
| Age at diagnosis in years | 253 | 6.3 (3.0) | 255 | 5.7 (3.0) | 6.0 (3.0) |
| Length of time had diabetes (years) | 253 | 5.0 (2.7) | 255 | 5.2 (2.8) | 5.1 (2.7) |
| Gender male | 334 | 155 (46) | 359 | 187 (52) | 342 (49) |
| BMI (kg/m2) | 329 | 19.2 (3.1) | 356 | 19.5 (3.2) | 19.4 (3.2) |
| Ethnicity | |||||
| White British | 259 | 91 | 262 | 91 | 91 |
| Other white | 7 | 2 | 5 | 2 | 2 |
| Mixed | 11 | 4 | 12 | 4 | 4 |
| Black or black British | 1 | <1 | 4 | 1 | 1 |
| Asian or Asian British | 7 | 2 | 6 | 2 | 2 |
| Other | 1 | < 1 | 0 | 0 | < 1 |
| Carer providing data | |||||
| Carer status: mothers | 286 | 93 | 286 | 93 | 93 |
| Usually attends clinic: yes | 284 | 99 | 286 | 99 | 99 |
| Provides majority of care: yes | 284 | 97 | 281 | 99 | 98 |
| Generally see same doctor at clinic: yes | 286 | 71 | 286 | 69 | 70 |
| Generally see same nurse at clinic: yes | 285 | 89 | 284 | 93 | 91 |
| Socioeconomic class | |||||
| Managerial and professional occupations | 139 | 54 | 134 | 54 | 57 |
| Intermediate occupations | 31 | 12 | 38 | 15 | 14 |
| Small employers and own-account workers | 26 | 10 | 23 | 9 | 10 |
| Lower supervisory and technical occupations | 22 | 9 | 28 | 11 | 10 |
| Semiroutine and routine occupations | 40 | 16 | 27 | 11 | 13 |
Baseline data indicate that the randomisation achieved adequate balance for all demographic variables including the primary outcome, HbA1c levels. There were slightly more males in the intervention arm, but adjusting for gender in the primary analysis did not influence the result.
Cluster-level balance was examined for patient demographic data. Summary data for HbA1c levels, age, age at diagnosis and gender of patients are given in Table 19 and indicate adequate balance across centres. There was variation in the number of patients recruited by each centre. More centres in the intervention arm achieved the minimum recruitment targets than in the control arm. As both the number of centres and patients required were originally inflated to account for dropout rates that were not observed, these variations do not affect the overall power of the study.
| Participating centres | n | Baseline HbA1c levels (%), mean (SD) | Age in years, mean (SD) | Age at diagnosis in years, mean (SD) | Gender, % male |
|---|---|---|---|---|---|
| Control centres | |||||
| 1 | 27 | 8.5 (1.99) | 11.2 (2.22) | 6.0 (2.63) | 48 |
| 2 | 28 | 10.1 (2.21) | 11.8 (2.49) | 6.3 (3.39) | 39 |
| 3 | 19 | 10.3 (1.53) | 10.7 (2.56) | 7.4 (3.77) | 53 |
| 4 | 24 | 9.7 (1.87) | 11.1 (2.29) | 5.7 (2.60) | 46 |
| 5 | 30 | 9.4 (2.63) | 10.3 (3.08) | 6.0 (3.53) | 53 |
| 6 | 18 | 9.5 (1.40) | 9.8 (2.58) | 5.5 (3.26) | 39 |
| 7 | 29 | 8.9 (2.13) | 10.9 (2.54) | 6.7 (3.23) | 66 |
| 8 | 19 | 9.3 (1.37) | 11.3 (2.47) | 6.4 (2.35) | 26 |
| 9 | 20 | 9.3 (1.08) | 10.8 (3.24) | 5.6 (2.95) | 60 |
| 10 | 30 | 8.6 (1.17) | 9.9 (2.96) | 6.1 (3.40) | 37 |
| 11 | 30 | 8.4 (1.77) | 9.7 (2.99) | 6.6 (3.31) | 67 |
| 12 | 30 | 9.2 (1.47) | 10.7 (2.85) | 7.3 (3.01) | 30 |
| 13 | 30 | 9.5 (1.43) | 11.5 (3.17) | 6.8 (2.31) | 37 |
| Intervention centres | |||||
| 14 | 30 | 9.2 (1.55) | 9.9 (2.85) | 5.1 (2.94) | 50 |
| 15 | 30 | 9.0 (1.71) | 10.1 (2.18) | 4.5 (2.61) | 53 |
| 16 | 20 | 9.5 (1.36) | 10.9 (3.02) | 5.6 (3.02) | 45 |
| 17 | 32 | 9.2 (1.31) | 10.7 (2.72) | 5.0 (2.81) | 38 |
| 18 | 30 | 9.8 (1.70) | 11.2 (2.60) | 6.3 (3.03) | 67 |
| 19 | 29 | 10.0 (1.66) | 9.9 (2.88) | 6.2 (2.55) | 52 |
| 20 | 32 | 9.0 (1.56) | 9.3 (2.94) | 6.4 (3.07) | 59 |
| 21 | 29 | 9.5 (1.10) | 10.4 (3.18) | 5.7 (3.20) | 48 |
| 22 | 29 | 8.6 (1.53) | 10.6 (2.87) | 5.7 (3.14) | 52 |
| 23 | 31 | 9.9 (1.86) | 10.4 (2.42) | 5.9 (2.72) | 42 |
| 24 | 27 | 9.8 (3.02) | 11.3 (2.84) | 5.3 (3.63) | 67 |
| 25 | 10 | 9.1 (1.48) | 10.3 (2.50) | 4.3 (1.83) | 70 |
| 26 | 30 | 9.8 (1.48) | 10.6 (2.59) | 6.6 (3.72) | 47 |
Numbers analysed
All analyses were carried out according to the intention-to-treat principle and all centres and participants were analysed as randomised. All 26 centres (13 in each arm) were included in all the primary and secondary analyses. For the primary outcome, 657 patients had HbA1c measurements at both baseline and follow-up and were included in the analysis. Sensitivity analysis was carried out using routine clinic HbA1c measurement to replace missing central laboratory levels where possible (HbA1c, if measured, taken within 100 days of baseline or 12-month follow-up). All four missing baseline values and 7 out of 32 missing follow-up HbA1c levels were included for this analysis. For each of the secondary outcomes, numbers included are given (see Tables 24–31).
Checks for bias
As there were missing HbA1c data for the primary outcome, baseline summary patient demographic data were also tabulated for the group that had complete HbA1c data at baseline and follow-up compared with those with missing follow-up HbA1c data. Table 20 indicates that those patients missing follow-up measurements had slightly higher baseline HbA1c levels, had slightly lower BMI and were more likely to be female.
| Complete cases | Missing follow-up HbA1c | |||
|---|---|---|---|---|
| n | Mean (SD) or % | n | Mean (SD) or % | |
| HbA1c (%) | 657 | 9.3 (1.75) | 32 | 9.9 (2.29) |
| Age (years) | 657 | 10.6 (2.77) | 32 | 10.4 (3.06) |
| Age at diagnosis (years) | 488 | 6.0 (3.07) | 18 | 5.8 (2.88) |
| Length of time had diabetes (years) | 488 | 5.1 (2.79) | 18 | 4.5 (2.41) |
| BMI (kg/m²) | 650 | 19.4 (3.17) | 31 | 18.4 (2.80) |
| Gender (% male) | 657 | 329 (50) | 32 | 12 (38) |
| Arm (% intervention) | 657 | 339 (52) | 32 | 17 (53) |
In order to reduce allocation knowledge bias, unblinded cluster randomised trials should aim to recruit all subjects before the allocation of the centres is revealed. In practice this was not always possible. In DEPICTED, 213/693 (30.7%) of subjects on centre eligibility lists were approached and consented prior to revealing allocation to the centres. Baseline data for the groups recruited before and after revealing allocation to the centres for training purposes are given in Table 21. There was no evidence of allocation knowledge bias for patient demographic data.
| Consent taken blind | Consent taken unblinded | |||
|---|---|---|---|---|
| n | Mean (SD) or % | n | Mean (SD) or % | |
| HbA1c (%) | 212 | 9.1 (1.88) | 477 | 9.5 (1.73) |
| Age (years) | 213 | 10.4 (2.85) | 480 | 10.7 (2.74) |
| Age at diagnosis (years) | 160 | 6.3 (3.23) | 350 | 5.9 (2.97) |
| Length of time had diabetes (years) | 160 | 4.6 (2.77) | 350 | 5.2 (2.75) |
| BMI (kg/m²) | 212 | 19.0 (2.98) | 473 | 19.5 (3.21) |
| Gender (% male) | 213 | 97 (46) | 480 | 245 (51) |
| Arm (% intervention) | 213 | 91 (43) | 480 | 268 (44) |
Primary outcome
The distribution of the HbA1c data was examined and was slightly positively skewed. A natural log transformation was performed for multilevel regression analysis. Summary data have been tabulated in the original scale for ease of interpretation (Table 22). The HbA1c levels in both arms increased by a similar amount from baseline to follow-up. The intervention effect in the log scale can be interpreted as percentage change and it can be seen that there was a 1% increase in HbA1c levels in the intervention arm compared with control, which was not statistically significant. The addition of HbA1c data from routine clinic visits to replace missing central laboratory values did not alter the result (see Table 23). Although gender was significantly associated with follow-up HbA1c levels, adjusting for age and gender did not alter the results and there were no significant interactions between intervention arm and age or gender. These data are also shown in Table 23.
| Primary outcome | n | Control | n | Intervention | Adjusted for baseline HbA1c (log-transformed data) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline mean (SD) | Follow-up mean (SD) | Baseline mean (SD) | Follow-up mean (SD) | ICC | Intervention effect and 95% CI | p-value | |||
| HbA1c (%) | 318 | 9.2 (1.8) | 9.5 (1.7) | 339 | 9.4 (1.7) | 9.7 (1.7) | 0.057 | 0.01 (–0.02 to 0.04) | 0.50 |
| Adjusted primary outcome | n | Control | n | Intervention | Adjusted for baseline HbA1c (log-transformed data) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline mean (SD) | Follow-up mean (SD) | Baseline mean (SD) | Follow-up mean (SD) | ICC | Intervention effect and 95% CI | p-value | |||
| Missing data | |||||||||
| HbA1c (%) | 321 | 9.2 (1.8) | 9.5 (1.7) | 346 | 9.4 (1.7) | 9.7 (1.7) | 0.059 | 0.01 (–0.02 to 0.04) | 0.47 |
| Gender | |||||||||
| HbA1c (%) | 318 | 9.2 (1.8) | 9.5 (1.7) | 339 | 9.4 (1.7) | 9.7 (1.7) | 0.063 | 0.01 (–0.02 to 0.04) | 0.47 |
| Age | |||||||||
| HbA1c (%) | 318 | 9.2 (1.8) | 9.5 (1.7) | 339 | 9.4 (1.7) | 9.7 (1.7) | 0.060 | 0.01 (–0.02 to 0.05) | 0.39 |
Secondary patient outcomes
Validation was carried out on all secondary outcome scores. Factor analysis indicated that for all outcome scores individual items contributed to a single construct in each case. A table listing the Cronbach’s alpha statistics is given in Appendix 3. The distributions of all patient and carer secondary outcomes were examined. A degree of negative skew was observed for some of the scores, including Health Care Climate Questionnaire (HCCQ) and Diabetes Continuity of Care Scale (DCCS) and ‘Importance’, whereas the Patient Enablement score was slightly positively skewed. The degree of non-normality was within the limits of the methods used and all scores were left untransformed for multilevel analyses.
Health Care Climate Questionnaire
The HCCQ score ranges from 1 to 5, with higher scores indicating more positive feelings about the diabetes team. Overall, 10.1% of the variation in (HCCQ) score was attributable to centres, but there was no effect of the intervention to improve the HCCQ score (Table 24).
| Outcome | n | Control | n | Intervention | Adjusted for baseline score | ||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline mean (SD) | Follow-up mean (SD) | Baseline mean (SD) | Follow-up mean (SD) | ICC (%) | Intervention effect and 95% CI | p-value | |||
| HCCQ | 182 | 4.1 (0.60) | 4.0 (0.62) | 164 | 4.0 (0.69) | 4.0 (0.71) | 10.1 | 0.04 (–0.2 to 0.2) | 0.66 |
| QoL | |||||||||
| Barriers | 186 | 69.3 (19.6) | 73.3 (18.2) | 167 | 66.8 (22.0) | 67.5 (21.2) | 0.9 | –4.6 (–8.5 to –0.6) | 0.03 |
| Symptoms | 185 | 56.5 (13.6) | 57.2 (14.3) | 167 | 54.4 (15.0) | 55.3 (15.3) | 3.3 | –0.9 (–4.2 to 2.4) | 0.60 |
| Adherence | 183 | 77.9 (15.1) | 80.6 (15.4) | 166 | 76.4 (17.2) | 76.8 (17.4) | 0 | –3.1 (–6.3 to –0.01) | 0.05 |
| Worry | 181 | 67.3 (22.0) | 69.8 (20.2) | 162 | 68.8 (23.8) | 67.2 (23.2) | 0 | –3.4 (–7.4 to 0.7) | 0.10 |
| Communication | 181 | 66.0 (23.8) | 69.1 (22.2) | 162 | 63.3 (26.9) | 62.3 (26.9) | 0.1 | –5.4 (–11.1 to 0.3 | 0.06 |
Health-related quality of life
Table 24 also gives the QoL scores related to fives domains: barriers, symptoms, adherence, worry and communication. The scores for each range from 0 to 100 and a higher score represents a better QoL domain score. The ICC values given in Table 24 indicate that there is little variation between centres for any QoL scores apart from ‘symptoms’. For the ‘barriers’ score there is an increase between baseline and follow-up in the control arm, indicating an improvement, whereas in the intervention arm there was no change. This difference between the arms was statistically significant. There was no intervention effect on the ‘symptoms’ scores and the mean scores remained unchanged. The ‘adherence score’ also indicates an improvement in the control arm and no change in the intervention arm. This difference was borderline statistically significant. There was little change in the QoL ‘worry’ scores and a slight improvement in the ‘communication’ scores for the control arm only, although not reaching conventional significance.
The HCCQ and QoL outcomes were available for all patients, whereas the remaining scores were applicable only to the older age group (11–16 years).
Diabetes Continuity of Care Scale
The DCCS score ranges from 1 to 5, with higher scores indicating better continuity of care. There was no difference between the control and intervention arms, with scores remaining at a similar level at baseline and follow-up. In total, 7.8% of the variation in the DCCS follow-up score was attributable to centres (Table 25).
| Outcome | n | Control | n | Intervention | Adjusted for baseline score | p-value | |||
|---|---|---|---|---|---|---|---|---|---|
| Baseline mean (SD) | Follow-up mean (SD) | Baseline mean (SD) | Follow-up mean (SD) | ICC (%) | Effect and 95% CI | ||||
| DCCS | 121 | 4.5 (0.52) | 4.3 (0.66) | 88 | 4.3 (0.72) | 4.2 (0.76) | 7.8 | –0.02 (–0.3 to 0.2) | 0.85 |
| PAID | 123 | 33.6 (19.2) | 36.4 (20.6) | 89 | 35.0 (21.7) | 38.9 (20.8) | 0 | 1.8 (–3.0 to 6.6) | 0.46 |
| Importance | 123 | 4.2 (0.66) | 4.0 (0.67) | 89 | 4.1 (0.62) | 4.0 (0.69) | 0 | 0.2 (–0.13 to 0.17) | 0.81 |
| Confidence | 123 | 3.7 (0.73) | 3.7 (0.72) | 89 | 3.7 (0.70) | 3.5 (0.77) | 0 | –0.2 (–0.4 to 0) | 0.06 |
| Diabetes care/mismanagement | 110 | 1.6 (0.53) | 1.8 (0.66) | 80 | 1.6 (0.46) | 1.8 (0.60) | 0 | 0.03 (–0.12 to 0.18) | 0.72 |
| Patient enablement (interim follow-up) | 116 | 28.0 (28.8) | 19.7 (25.4) | 83 | 28.5 (30.4) | 30.1 (32.6) | 6.4 | 10.4 (0.5 to 20.4) | 0.04 |
| Patient enablement (12-month follow-up) | 122 | 29.1 (30.9) | 26.4 (30.9) | 88 | 28.4 (29.4) | 21.3 (27.7) | 9.0 | –5.2 (–16.1 to 5.7) | 0.34 |
Problem Areas in Diabetes
Problem Areas in Diabetes (PAID) scores ranged from 0 to 100, with a higher score indicating more problems with diabetes. There was very little variation between centres in the PAID scores. In both arms there was a slight increase in the score, indicating more problems with diabetes, in both control and intervention arms, but there was no difference between the arms at follow-up.
Importance
The importance score is a six-item scale with scores ranging from 1 to 5. Validation of this new score was carried out using baseline questionnaire data from patients and carers. Factor analysis indicated that all items contributed to a single construct. Cronbach’s alpha was 0.87 for both patients and carers items, indicating high internal consistency. Higher importance scores indicate a higher level of importance associated with diabetes self-care. There was little variation between centre for importance scores and the scores remained unchanged at follow-up compared with baseline in both arms.
Confidence
The confidence score was also a six-item score ranging from 1 to 5. Validation of the confidence score was also carried out. Factor analysis indicated that all items contributed to a single construct. Cronbach’s alpha scores for patients and carers were 0.84 and 0.90, respectively. Higher confidence scores indicate a higher level of confidence with diabetes self-care. There was little variation between centres in confidence scores. Baseline scores for confidence remained unchanged in the control group, but were slightly reduced in the intervention group at follow-up. This difference was close to conventional statistical significance.
Diabetes care/mismanagement
The diabetes care score ranges from 1 to 5, and higher scores indicate greater mismanagement of diabetes care. In both arms the scores increased by a similar amount, indicating increased mismanagement of diabetes at follow-up. However, there was no difference between the control and intervention arms.
Patient enablement
Patient enablement was measured at one interim time point as well as at final follow-up. The interim score is a three-item scale and higher scores indicate an improved ability to cope with diabetes. Scores in the control group were lower at follow-up than at baseline, whereas in the intervention group enablement improved. A statistically significant positive effect of the intervention was observed at the interim time point, which was not observed at final follow-up. This may be due, however, to a printing error in the questionnaires, which meant that one item had to be dropped from the scale score.
Individual items
Patients were asked how often they checked their blood glucose. At baseline 52.8% and 47.6% in the control and intervention groups, respectively, reporting checking four or more times per day (Table 26). At 12 months’ follow-up the proportion in the control arm remained unchanged at 51.7%, whereas in the intervention group the portion had reduced to 42.9%.
| Individual item scores | n | Control | n | Intervention | Adjusted for baseline, reference group = control | p-value | |||
|---|---|---|---|---|---|---|---|---|---|
| Baseline, n (%) | Follow-up, n (%) | Baseline, n (%) | Follow-up, n (%) | ICC (%) | OR and 95% CI | ||||
| Check glucose ≥ 4 times per day | 118 | 62 (51) | 61 (51.7) | 84 | 40 (47.6) | 36 (42.9) | 0 | 0.7 (0.39 to 1.32) | 0.29 |
| Experiencing emotion itemsa | |||||||||
| Fed up | 179 | 62 (35) | 70 (39) | 163 | 55 (34) | 77 (47) | 1.0 | 1.5 (0.95 to 2.42) | 0.08 |
| Excited | 180 | 59 (33) | 42 (23) | 161 | 64 (40) | 54 (34) | 0.5 | 1.6 (0.93 to 2.65) | 0.09 |
| Guilty | 177 | 23 (13) | 16 (9) | 161 | 16 (10) | 20 (12) | 0 | 1.6 (0.79 to 3.41) | 0.18 |
| Good | 181 | 130 (72) | 115 (64) | 162 | 108 (67) | 104 (64) | 0 | 1.1 (0.71 to 1.86) | 0.57 |
| Worried | 179 | 61 (34) | 47 (26) | 165 | 64 (39) | 56 (34) | 6.4 | 1.4 (0.85 to 2.29) | 0.19 |
Patients were also asked if they were experiencing various emotions prior to their last clinic visit. At baseline, less than half of the patients reported ‘negative’ emotions, namely ‘fed up’, ‘guilty’ and ‘worried’. At follow-up the proportion reporting that they were worried decreased in both the control and intervention arms, whereas those reporting feeling fed up increased in both arms. Those reporting feeling guilty decreased in the control arm, but increased in the intervention arm. The positive emotion ‘good’ was reported by two-thirds of the patients at baseline and decreased slightly in both arms, whereas ‘excited’ was reported by less than half and also decreased in both arms. None of the odd ratios observed reached conventional statistical significance.
Global quality-of-life questions
Patients were asked how easy it was living with their diabetes compared with the previous year. Just over half responded that it was ‘easier’ or ‘much easier’ in the control and intervention arms at baseline, and these proportions did not change significantly in either arm. The proportions reporting that in general they were ‘happy’ or ‘very happy’ were higher in the control arm than in the intervention arm, but did not change at follow-up in relation to baseline. When asked about their QoL compared with the previous year, proportions reporting that they were ‘happier’ or ‘much happier’ were not different between arms (Table 27).
| Individual item scores | n | Control | n | Intervention | Adjusted for baseline, reference group = control | p-value | |||
|---|---|---|---|---|---|---|---|---|---|
| Baseline, n (%) | Follow-up, n (%) | Baseline, n (%) | Follow-up, n (%) | ICC (%) | OR and 95% CI | ||||
| Living with diabetesa | 183 | 99 (54) | 93 (51) | 159 | 85 (54) | 83 (52) | 0 | 1.07 (0.69 to 1.66) | 0.77 |
| General QoLb | 181 | 135 (75) | 134 (74) | 161 | 107 (67) | 106 (66) | 0 | 0.74 (0.45 to 1.22) | 0.24 |
| QoL compared with last yearc | 180 | 85 (47) | 83 (46) | 163 | 70 (43) | 72 (44) | 0 | 0.96 (0.62 to 1.48) | 0.84 |
Secondary carer outcomes
The scores for the carer data have been calculated as for the patient scores, with the same ranges and direction of effects and are interpreted in the same way.
Health Care Climate Questionnaire
No significant intervention effect was observed for HCCQ score, high scores in both control and intervention groups indicating that carers were equally happy with the diabetes team at baseline and follow-up (Table 28).
| Outcome | n | Control | n | Intervention | Adjusted for baseline score | p-value | |||
|---|---|---|---|---|---|---|---|---|---|
| Baseline, mean (SD) | Follow-up, mean (SD) | Baseline, mean (SD) | Follow-up, mean (SD) | ICC | Effect and 95% CI | ||||
| HCCQ | 209 | 4.3 (0.57) | 4.2 (0.59) | 202 | 4.2 (0.64) | 4.3 (0.62) | 3.0 | 0.1 (0 to 0.2) | 0.13 |
| QoL | |||||||||
| Barriers | 208 | 61.3 (17.5) | 62.3 (17.5) | 203 | 60.4 (18.7) | 59.0 (20.2) | 0 | –2.9 (6.1 to 0.4) | 0.08 |
| Symptoms | 209 | 58.9 (13.3) | 60.0 (13.6) | 202 | 56.1 (13.9) | 57.1 (14.9) | 0 | –1.0 (–3.1 to 1.1) | 0.36 |
| Adherence | 208 | 74.9 (16.3) | 75.0 (15.9) | 203 | 73.9 (16.4) | 73.2 (17.3) | 0.6 | –1.4 (4.4 to 1.6) | 0.35 |
| Worry | 205 | 54.6 (20.8) | 52.9 (22.2) | 201 | 50.9 (24.1) | 51.9 (21.9) | 0 | 0.8 (–2.9 to 4.6) | 0.67 |
| Communication | 204 | 66.8 (26.8) | 67.7 (25.3) | 199 | 63.7 (28.0) | 64.4 (29.0) | 0 | –1.7 (–6.2 to 2.8) | 0.46 |
Health-related quality of life
Table 28 also shows that the scores for all of the QoL domains followed a similar pattern to those of the patients. The carers had slightly lower ‘Barriers’ scores at baseline than patients, which were slightly higher in the intervention arm than in the control arm, although this difference was not statistically significant. None of the other QoL sores demonstrated an intervention effect.
Diabetes Continuity of Care Scale
There was a statistically significant positive effect of the intervention on the DCCS scores for carers. Table 29 shows that the intervention group follow-up scores improved, whereas the control group scores were reduced compared with baseline.
| Outcome | n | Control | n | Intervention | Adjusted for baseline | p-value | |||
|---|---|---|---|---|---|---|---|---|---|
| Baseline, mean (SD) | Follow-up, mean (SD) | Baseline, mean (SD) | Follow-up, mean (SD) | ICC | Effect and 95% CI | ||||
| DCCS | 208 | 4.4 (0.59) | 4.2 (0.73) | 203 | 4.3 (0.69) | 4.4 (0.63) | 0 | 0.2 (0.1 to 0.3) | 0.01 |
| PAID | 209 | 41.4 (17.6) | 43.0 (19.4) | 203 | 45.6 (18.7) | 45.2 (20.2) | 3.0 | –0.9 (–3.7 to 2.0) | 0.55 |
| Importance | 208 | 4.7 (0.42) | 4.7 (0.40) | 202 | 4.6 (0.41) | 4.7 (0.41) | 0 | 0.02 (–0.1 to 0.1) | 0.61 |
| Confidence | 208 | 3.7 (0.66) | 3.8 (0.76) | 203 | 3.7 (0.74) | 3.8 (0.73) | 0 | –0.02 (–0.1 to 0.1) | 0.78 |
| Care/mismanagement | 186 | 1.5 (0.43) | 1.6 (0.57) | 183 | 1.5 (0.47) | 1.6 (0.51) | 0 | –0.01(–0.10 to 0.09) | 0.87 |
| Patient enablement (interim) | 209 | 18.3 (27.8) | 16.3 (25.2) | 190 | 25.1 (31.5) | 23.5 (28.4) | 3.0 | 5.2 (–1.3 to 11.6) | 0.11 |
| Patient enablement (follow-up) | 207 | 22.3 (29.7) | 23.9 (32.1) | 201 | 24.3 (32.5) | 28.7 (35.4) | 3.4 | 4.4 (–3.5 to 12.3) | 0.27 |
Other secondary outcome scores
Table 29 shows that there were no other significant effects of the intervention on the secondary outcomes listed.
Individual items
Tables 30 and 31 give results for the additional individual items asked in carer questionnaires. The intervention had a statistically significant positive effect on the proportion feeling excited when thinking about how they felt before their last clinic visit. Comparing Table 30 with patients’ data in Table 26, it can be seen that carers were less fed up, more guilty, more worried and less excited than patients prior to their last clinic visit. No other items demonstrated a significant intervention effect. The global QoL item scores for carers were lower than patient scores overall, indicating that carers are finding it harder living with their child’s diabetes.
| Individual item scores | n | Control | n | Intervention | ICC (%) | (Reference group = control), OR and 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|---|---|
| Baseline, n (%) | Follow-up, n (%) | Baseline, n (%) | Follow-up, n (%) | ||||||
| Check glucose ≥ 4 times per day | 202 | 132 (65) | 129 (63.9) | 191 | 104 (54.5) | 103 (53.9) | 1.5 | 0.25 (0.16 to 1.37) | 0.37 |
| Experiencing emotion itemsa | |||||||||
| Fed up | 200 | 55 (28) | 59 (30) | 195 | 51 (26) | 60 (31) | 0 | 1.13 (0.69 to 1.85) | 0.63 |
| Excited | 196 | 30 (15) | 23 (12) | 190 | 30 (16) | 37 (20) | 3.3 | 1.90 (1.05 to 3.43) | 0.03 |
| Guilty | 198 | 49 (25) | 55 (28) | 190 | 49 (26) | 57 (30) | 0 | 1.11 (0.68 to 1.83) | 0.67 |
| Good | 200 | 132 (66) | 120 (60) | 191 | 133 (70) | 124 (65) | 2.7 | 1.19 (0.76 to 1.85) | 0.44 |
| Worried | 200 | 98 (49) | 103 (52) | 193 | 101 (52) | 108 (56) | 0 | 1.16 (0.75 to 1.79) | 0.50 |
| Individual item scores | n | Control | n | Intervention | ICC (%) | Reference group = control | p-value | ||
|---|---|---|---|---|---|---|---|---|---|
| Baseline, n (%) | Follow-up, n (%) | Baseline, n (%) | Follow-up, n (%) | OR and 95% CI | |||||
| Living with diabetesa | 206 | 77 (37) | 63 (31) | 201 | 71 (35) | 61 (30) | 1.2 | 0.91 (0.57 to 1.45) | 0.69 |
| General QoLb | 207 | 126 (61) | 121 (59) | 197 | 130 (66) | 104 (53) | 1.4 | 0.65 (0.41 to 1.03) | 0.06 |
| QoL compared with last yearc | 207 | 51 (25) | 51 (25) | 198 | 66 (33 | 49 (25) | 2.4 | 1.01 (0.66 to 1.55) | 0.97 |
Exploratory analyses
Attendance data were examined via CRF completion rates and it was found that in the control arm 11/334 (3.3%) patients did not attend at all, whereas in the intervention arm 4/359 (1.1%) did not attend any clinic sessions [difference and 95% CI 2.2 (–0.1 to 4.8)]. The mean (SD) number of clinic visits during the period of the intervention was 3.5 (1.1) for both the intervention arm and the control arm. In order to investigate any possible dose effect, the number of clinic visits was added to the multilevel model for the primary outcome. The number of visits was not statistically significant and there was no significant interaction between number of visits and trial arm.
Direct costs: DEPICTED training
The costs of training 79 trainees across 13 intervention sites are shown in Table 32. The total cost was £170,895. Of this, £46,377 (£3567 per site) was preparation and delivery costs incurred by the DEPICTED team.
| Activity | Units | Unit cost (£) | Cost (£) |
|---|---|---|---|
| Administration | |||
| Preparing seminar packs (hours) | 39 | 12.53 | 488.67 |
| Organising locations, etc. (hours) | 32.5 | 12.53 | 407.23 |
| Responding to queries (hours) | 13 | 12.53 | 162.89 |
| Telephone support (hours) | 4.25 | 12.53 | 53.25 |
| Delegate packs (items) | 79 | 25.00 | 1975.00 |
| Postage (items) | 996 | 0.24 | 239.04 |
| Total administration costs £3326.08 | |||
| Seminar preparation (trainers) | |||
| Seminar planning meetings (hours) | 30 | Various | 1217.34 |
| Pilot training sessions (hours) | 120 | Various | 4869.36 |
| Total seminar preparation costs £6086.70 | |||
| Seminar delivery | |||
| Venue hire (£) | 7143.73 | Amounts paid | 7143.73 |
| Trainers’ travel time (hours) | 113.03 | Various | 4512.71 |
| Trainers’ travel costs (£) | 1840.01 | Amounts paid | 1840.01 |
| Trainers’ accommodation, etc. (£) | 4346.61 | Amounts paid | 4346.61 |
| Trainers’ time at seminars (hours) | 420.00 | Various | 17,340.72 |
| Total seminar delivery costs £35,183.78 | |||
| Follow-up | |||
| Administration (hours) | 39.5 | 12.53 | 494.94 |
| Trainer (hours) | 39.5 | 32.55 | 1285.73 |
| Total follow-up costs £1780.67 | |||
| Total cost of preparation and delivery (A) £46,377.23 | |||
| [Cost of preparation and delivery per site (n = 13) £3567.49] | |||
| Trainee costs | |||
| Trainee travel time (hours) | 189.65 | Various | 11,644.10 |
| Trainee travel costs (£) | Fares paid | 3515.00 | |
| Trainee time at seminar (hours) | 1464.00 | Various | 89,148.00 |
| Total trainee seminar cost = £104,307.10 | |||
| Online training | |||
| Total time logged on (hours) | 300.15 | Various | 20,210.90 |
| Total cost of receiving DEPICTED training (B) £124,518.00 | |||
| Total cost of DEPICTED training (A + B) £170,895.23 | |||
A breakdown of costs incurred by intervention sites is shown in Table 33. The mean (SD) cost per site was £9575 (£4831). The number of trainees per site varied between 3 and 12. The mean (SD) cost per trainee was £1614 (£463). The final column shows the cost per site including the cost of preparing and delivering DEPICTED training (£3567 per site). The total mean (SD) cost per site is £13,146 (£11,698). The number of staff per site and the degree of their engagement with training (both entering and completing) were factors that varied according to site. Variations in clinic list size would be reflected in differing number of staff across sites available for training. Practitioners who completed all modules of training would have incurred more cost than those who maybe did not.
| Site (no. trained) | Seminar hours | Seminar time cost (£) | Travel hours | Travel time cost (£) | Login hours | Login time cost (£) | Other costs (£) | Total cost for site (£) | Cost per trainee (£) | Total cost/site, including trainer’s cost (£) |
|---|---|---|---|---|---|---|---|---|---|---|
| 11 (4) | 72.00 | 3636.00 | 5.80 | 190.40 | 12.63 | 820.48 | 54.00 | 4700.88 | 1175.22 | 8268.15 |
| 13 (12) | 192.00 | 16,260.00 | 16.75 | 1381.25 | 60.42 | 4616.62 | 145.00 | 22,402.87 | 1866.91 | 25970.14 |
| 14 (3) | 60.00 | 3996.00 | 16.00 | 944.00 | 14.97 | 679.10 | 665.00 | 6284.10 | 2094.70 | 9851.37 |
| 18 (7) | 108.00 | 5976.00 | 6.80 | 453.50 | 28.00 | 1675.22 | 26.00 | 8130.72 | 1161.53 | 11,697.99 |
| 22 (6) | 132.00 | 6624.00 | 10.25 | 447.50 | 33.33 | 2885.78 | 72.00 | 10,029.28 | 1671.55 | 13,596.55 |
| 23 (4) | 84.00 | 5400.00 | 4.55 | 239.25 | 14.38 | 791.42 | 31.00 | 6461.67 | 1615.42 | 10,028.94 |
| 26 (9) | 204.00 | 10,728.00 | 8.10 | 403.20 | 25.97 | 1538.42 | 37.00 | 12,706.62 | 1411.85 | 16,273.89 |
| 28 (5) | 108.00 | 7968.00 | 34.00 | 2766.00 | 16.13 | 1142.33 | 1615.00 | 13,491.33 | 2698.27 | 17,058.60 |
| 35 (8) | 108.00 | 4932.00 | 21.00 | 935.50 | 25.95 | 1263.83 | 27.00 | 7158.33 | 894.79 | 10,725.60 |
| 37 (8) | 144.00 | 8964.00 | 25.50 | 1451.00 | 22.85 | 1788.53 | 507.00 | 12,710.53 | 1588.82 | 16,277.80 |
| 42 (3) | 72.00 | 4416.00 | 4.55 | 251.50 | 12.77 | 678.23 | 33.00 | 5378.73 | 1792.91 | 8946.00 |
| 43 (5) | 84.00 | 4920.00 | 10.10 | 552.50 | 15.05 | 971.02 | 42.00 | 6485.52 | 1297.10 | 10,052.79 |
| 44 (5) | 96.00 | 5328.00 | 26.25 | 1628.50 | 17.70 | 1359.92 | 261.00 | 8577.42 | 1715.48 | 12,144.69 |
| Mean (SD) | 112.62 (44.77) | 6857.54 (3492.16) | 14.59 (9.57) | 895.70 (746.28) | 23.09 (13.03) | 1554.68 (1099.49) | 270.38 (452.85) | 9578.31 (4831.25) | 1614.20 (463.03) | 13,145.58 (11,697.99) |
Table 34 shows the annuitised training cost per site and per eligible patient. The latter figure, which varied from £14 to £71 (mean £49, SD £15), together with the cost of one 3T agenda-setting tool (£18.04), represents the direct per-patient costs of DEPICTED.
| Site | Eligible patients (n) | Cost of training (£) | Annuitised training costa (£) | Annuitised training costa per patient (£) |
|---|---|---|---|---|
| 11 | 55 | 8268.37 | 1830.99 | 33.29 |
| 13 | 125 | 25,970.36 | 5751.00 | 46.01 |
| 14 | 154 | 9851.59 | 2181.58 | 14.17 |
| 18 | 55 | 11,698.21 | 2590.51 | 47.10 |
| 22 | 53 | 13,596.77 | 3010.93 | 56.81 |
| 23 | 51 | 10,029.16 | 2220.90 | 43.55 |
| 26 | 51 | 16,274.11 | 3603.82 | 70.66 |
| 28 | 72 | 17,058.82 | 3777.59 | 52.47 |
| 35 | 56 | 10,725.82 | 2375.18 | 42.41 |
| 37 | 63 | 16,278.02 | 3604.68 | 57.22 |
| 42 | 50 | 8946.22 | 1981.09 | 39.62 |
| 43 | 36 | 10,053.01 | 2226.19 | 61.84 |
| 44 | 41 | 12,144.91 | 2689.43 | 65.60 |
| Mean (SD) | 66.31 (34.17) | 13,145.58 (11,697.99) | 2911.07 (1069.85) | 48.52 (14.95) |
Indirect costs: patients’ use of NHS resources
Data on indirect costs were from CRFs. The number of CRFs completed reflects the number of clinic visits post recruitment during the follow-up period, which varied between 1 and 6. Patients’ use of NHS resources is the sum across all CRFs.
Data were bootstrapped (1000 replications) taking account of clustering effects at the centre level. Table 35 shows the mean (SD) number of contacts by group. There was virtually no difference in number of (post-recruitment) clinic attendances between patients in intervention and control sites. For the remaining variables the low means (apart from contacts with nurses on the diabetes team) are due largely to most patients having zero contacts for that resource item. The intervention group had significantly fewer contacts with community/GP nurses (p = 0.01) and more home attendances by ambulance crews (p = 0.05).
| Intervention (n = 352), mean (SD) | Controls (n = 323), mean (SD) | p-value (95% CI) | |
|---|---|---|---|
| Clinic visits | 2.66 (1.01) | 2.67 (0.87) | 0.95 (–0.39 to 0.37) |
| Contacts with diabetes team excluding routine clinic visitsa | |||
| Doctor | 0.57 (1.33) | 0.47 (1.01) | 0.72 (–0.44 to 0.63) |
| Nurse | 5.09 (6.04) | 4.28 (6.71) | 0.22 (–0.50 to 2.12) |
| Dietitian | 0.38 (0.96) | 0.36 (0.84) | 0.80 (–0.16 to 0.21) |
| Other | 0.28 (1.12) | 0.14 (0.61) | 0.30 (–0.12 to 0.39) |
| Hospital contacts | |||
| Accident and emergency visits | 0.25 (0.60) | 0.22 (0.56) | 0.71 (–0.10 to 0.15) |
| Paediatric assessment unit visits | 0.15 (0.48) | 0.11 (0.38) | 0.46 (–0.06 to 0.13) |
| Ambulance journeys | 0.07 (0.31) | 0.03 (0.18) | 0.08 (–0.005 to 0.08) |
| Ambulance home attendances | 0.05 (0.38) | 0.01 (0.11) | 0.05 (0.00 to 0.08) |
| Intensive therapy unit inpatient days | 0.02 (0.32) | 0.01 (0.08) | 0.51 (–0.02 to 0.04) |
| High dependency unit inpatient days | 0.03 (0.21) | 0.07 (0.49) | 0.37 (–0.13 to 0.05) |
| Other ward inpatient days | 0.60 (3.73) | 0.18 (0.68) | 0.12 (–0.11 to 0.95) |
| Day visits | 0.13 (0.54) | 0.17 (0.63) | 0.54 (–0.19 to 0.10) |
| Other NHS contacts | |||
| General practitioner surgery/home visits | 0.84 (1.62) | 0.73 (1.13) | 0.37 (–0.12 to 0.33) |
| Practice/community nurse (surgery/home visits) | 0.11 (0.51) | 0.28 (0.71) | 0.01 (–0.29 to –0.05) |
| Other | 1.85 (1.26) | 2.16 (1.71) | 0.03 (–1.05 to –0.07) |
Mean (SD) total costs by study group are shown in Table 36. The first four rows show patients’ total NHS resource over 12 months, including clinic visits. The amortised per-patient costs of DEPICTED training (£35) and the cost of the 3T tool (£18) are added for patients in the intervention group.
| Resource use | Intervention (n = 352), mean (SD) | Control (n = 322), mean (SD) | p-value (95% CI) |
|---|---|---|---|
| Insulin | 430.49 (207.22) | 425.57 (175.41) | 0.84 (–42.01 to 51.85) |
| Contacts with diabetes team | 552.97 (227.35) | 535.36 (216.20) | 0.59 (–46.10 to 81.31) |
| Hospital contacts and investigations | 287.86 (1194.38) | 189.07 (566.49) | 0.29 (–82.46 to 280.05) |
| Other NHS contacts | 54.99 (105.90) | 58.30 (73.56) | 0.76 (–24.19 to 17.47) |
| DEPICTED training cost | 48.52 (14.95) | N/A | |
| 3T tool | 18.04 | N/A | |
| Total cost | 1393.38 (1298.69) | 1209.42 (676.41) | 0.10 (–32.22 to 402.14) |
Total costs for the intervention group were higher than for controls, but the difference was not statistically significant (p = 0.10; 95% CI –32.22 to 402.14).
With regard to non-NHS costs, a comparison of cost of carer time off work in relation to their child’s health showed no significant differences (intervention mean £100, SD £273 vs control mean £86, SD £221; p = 0.61). Similarly, there was no difference in patient’s time off school for any health reason (intervention mean 3.6 days, SD 5.4 days, vs control mean 3.9 days, SD 7.5 days; p = 0.73).
Sensitivity analyses
Consultation length
The base case assumed that the DEPICTED intervention did not increase the length of clinic consultation. This was supported by the PE (see Chapter 12), which showed time being a major issue in both intervention and control sites, and was further supported by summing the times recorded on the CRFs in which patients and carers reported time that they spent either individually (i.e. parent only or child only) or together across various health professionals at clinic visits. The mean (SD) reported total time was 99.92 (66.59) minutes for intervention clinics and 104.79 (56.30) minutes for control clinics. The difference was not statistically significant (p = 0.32).
Nevertheless, concerns had been expressed that the intervention might increase consultation times. As these were not directly monitored, a sensitivity analysis was undertaken to show what the effect would be if the costs of intervention clinic visits were increased by 20%. The effect was to raise mean (SD) total costs from £1393 (£1299) to £1484 (£1307) and to increase the statistical significance of the mean cost difference to p = 0.01 (95% CI £55.79 to £493.16).
Training time online
The base case assumed that all online training was undertaken during work time. A sensitivity analysis changing this to all log-on time being undertaken during leisure time reduced mean (SD) online training costs from £1555 (£1099) to £389 (£275), but this had little effect on the comparison of total costs (p = 0.10; 95% CI –£36.50 to £397.78). A second sensitivity analysis examined the effect of login time being split between work and leisure. This showed mean (SD) online training costs to be £1104 (£596), again with little effect on overall results (p = 0.10; 95% CI –£33.65 to £400.71).
Annuitisation
Sensitivity analyses were also undertaken to show the effect of altering the base-case assumption of a 5-year life of training to 10 years (both at 3.5%). This reduced mean (SD) total costs from £1393 (£1299) to £1371 (£1299), although the difference between groups was not statistically significant (p = 0.14, 95% CI –£54.55 to £379.55). A further sensitivity analysis altering the annuitisation rate to 7% showed little effect on results (p = 0.09; 95% CI –£27.27 to £407.15).
Future work
The economic evaluation plan for DEPICTED included a cost-effectiveness analysis assessing costs against the primary outcome (HbA1c levels). However, the results showed the difference in the primary outcome to be close to zero and not statistically significant. As DEPICTED training costs applied only to the intervention arm, the control arm had lower overall costs. The absence of a statistically significant difference in effect, however, is no longer a justification to adopt a cost-minimisation analysis approach and a cost-effectiveness plane should still be produced. 138,139
In the analysis reported above we used a bootstrap technique to account for any cluster effects on costs. Production of a cost-effectiveness plane when there are cluster effects poses methodological difficulties and a number of different analytical techniques are available. Bachmann and colleagues140 compared these techniques, but their own data had relatively good characteristics (a balanced cluster with 50 observations available for each cluster). Before producing a cost-effectiveness plane, the DEPICTED data will be used to further examine the performance of these techniques, while at the same time dealing with challenges posed by the near-zero effect, which is conventionally the denominator in the cost-effectiveness ratio.
Chapter 11 A discrete choice experiment of family preferences for routine consultations in paediatric diabetes
Introduction
A recent methodological development to elicit patient preferences is known as ‘stated choices’. Stated choice studies in health care describe services in terms of collections of attributes. 132 By varying the levels (ranges) of these attributes, different ‘treatment profiles’ are created. Patients are asked either to order (ranking experiments) or to choose between a set of choices (DCEs) to infer the relative importance of different attributes. The researcher can manipulate attributes and levels to study how patients react to actual treatment options or processes of care. However, choices are made using hypothetical scenarios and may not reflect actual behaviour. It is therefore important that the treatment profiles are realistic and rigorously developed to permit valid inferences about behaviour. 141–143
Modifying the clinical encounter to engage patients and families better, to enhance their clinic experience and to support self-management are aims of the Talking Diabetes intervention. In this DCE we aimed to formally identify the key components sought in a routine consultation in paediatric diabetes and determine the relative importance that patients and carers attach to these components.
Furthermore, the DEPICTED study offered an important opportunity to investigate two methodological issues in the use of DCEs. First, it allowed exploration of the feasibility of using a DCE with young respondents and, second, it enabled comparisons of patients’ and parents’ preferences. Only a few studies have included young participants144,145 and fewer have compared health professionals and carers. 146 However, no study was found that compared children’s and carers’ preferences.
Methods
A DCE involves five steps: (1) identifying attributes and their levels; (2) designing the experiment (identifying the choice sets to use); (3) piloting the questionnaire (e.g. to address cognitive burden); (4) administering the questionnaire; and (5) analysing and interpreting data. 147 Steps 1–3 are fully described in Appendix 4, and more briefly summarised in this chapter. Step 4 is reported in Chapter 9 of this report and step 5 is described in full below.
Steps 1–4
Focus groups conducted with parents (n = 11) and patients (n = 12) as part of the SAG (see Chapter 6) were used to identify and rank suitable consultation attributes and levels. Attributes that could be influenced by the trial intervention were selected for use (Table 37).
| Attributes | Levels for patient (design coding) | Levels for carer (design coding) |
|---|---|---|
| Who the doctor talks to | Talks mainly with my parent (0) | Talks mainly with me (0) |
| Talks mainly with me (1) | Talks mainly with my child (1) | |
| Talks with both me and my parent (2) | Talks with both me and my child (2) | |
| The amount of information I am given | A little bit of information (0) | A little bit of information (0) |
| Some information (1) | Some information (1) | |
| A lot of information (2) | A lot of information (2) | |
| Who sets the goals on how to look after my child’s diabetes | The doctor (0) | The doctor (0) |
| Me (1) | My child and I (1) | |
| The doctor and me together (2) | The doctor, my child and I together (2) | |
| Which doctor do I see | A different doctor each time (0) | A different doctor each time (0) |
| The same doctor most of the time (1) | The same doctor most of the time (1) | |
| The same doctor each time (2) | The same doctor each time (2) | |
| In the consultation I have time for | A few of my questions (0) | A few of my questions (0) |
| Most of my questions (1) | Most of my questions (1) | |
| All my questions (2) | All my questions (2) |
A fractional factorial design of 27 treatment profiles (35–2) was used to achieve a practical number of scenarios. 148 The treatment profiles were represented in two separate questionnaire booklets, each containing 15 profiles. Each pair of choices of treatment profiles used a constant comparator scenario constructed from the middle term of each attribute. The final booklet asked respondents to describe the attribute levels of their normal consultation, to rank attributes by importance and to complete the 15 pair-wise choice exercise. The draft questionnaire was piloted with families in two rounds of cognitive interviewing conducted face to face or on the telephone. In response to piloting, important changes in the content and presentation were made to the draft questionnaire booklet. Most fundamentally, the DCE questionnaire was finally presented in its own booklet, separate from the main trial follow-up questionnaire, but concurrently. The administration of the DCE questionnaire and main trial follow-up questionnaire is described in Chapter 9.
Step 5: data analysis and interpretation
Responders and non-responders were compared on the basis of clinical and sociodemographic characteristics. A multilevel logistic regression model using MlwiN software version 1.1 (MLwiN, Centre for Multilevel Modelling, Bristol, UK) accounted for correlations at site level (level 3) and individual level (level 2) and the multiple responses from within each individual (level 1). Two models were estimated: one for carers and one for patients (aged 12–16 years). The dependent variable represented the probability of choosing the alternative scenario. The explanatory variables included the attributes, the randomisation group and a range of relevant clinical and sociodemographic characteristics. Dummy variables were used for the attributes levels to avoid assuming that the changes between attributes options were ordinal.
The analysis firstly used default settings for distributional assumptions (binomial), linearisation (first order) and estimation type (marginal quasi-likelihood). These assumptions were later relaxed and the extra binomial, second-order and penalised quasi-likelihood estimation type were used as they had a better fit (measured with the log-likelihood function). Explanatory variables such as age and gender were added one at the time and removed before adding new ones if not significant.
The carer and the patient DCE questionnaire data were analysed and presented separately, and for each group results are presented in the following order: (1) response rate and representativeness of the sample; (2) current consultation style; (3) ranking exercise; (4) pair-wise choices trading and non-trading choices pattern; (5) pair-wise choices main effect model; and (6) pair-wise choices – interactions with key variables.
Results
The DCE questionnaire was administered to all the carers (n = 693) participating in the study and to patients aged 12 years and over (n = 355), with 435 and 230 questionnaires being returned, respectively, giving a response rate of 63% and 65% for carers and patients, respectively.
Patients
Response rate and representativeness
Respondents and non-responders were balanced in terms of age, whereas patients in the intervention arm and with a higher HBA1c level were less likely to return the questionnaire (Table 38). The two versions of the questionnaire were balanced in terms of response rate, so no weighting was applied to the analysis of the data.
| Item | Responders | Non-responders | p-value |
|---|---|---|---|
| Mean (SD) age (years) at follow-up | 13.77 (1.3) | 13.70 (1.4) | 0.64a |
| Mean (SD) HbA1c levels at follow-up | 9.67 (1.8) | 10.50 (2.2) | < 0.001a |
| Gender, n (%) | |||
| Male | 107 (63.3) | 62 (36.7) | 0.66b |
| Female | 123 (66.1) | 63 (33.9) | |
| Trial allocation, n (%) | |||
| Control group | 132 (73.3) | 48 (26.7) | < 0.001b |
| Intervention group | 98 (66.0) | 77 (44.0) | |
| DCE version, n (%) | |||
| A | 119 (67.6) | 57 (32.4) | 0.32b |
| B | 111(62.0) | 68 (38.0) | |
Five respondents did not answer six or more pairs of choices and were removed from the analysis. Four additional respondents did not answer one of the 15 pair-wise choices and, for these participants, only these choices were eliminated from the analysis. This left 3386 useable observations.
Current consultation style
The consultation style experienced by respondents from intervention and control centres was slightly different (Table 39), but none of the items reached statistical significance.
| Attributes | Control, n (%) | Intervention, n (%) |
|---|---|---|
| Who the doctor talks toa | ||
| Talks mainly with me | 20 (15.4) | 6 (6.6) |
| Talks with both me and my parent | 105 (80.8) | 82 (90.1) |
| Talks mainly with my parent | 5 (3.8) | 3 (3.3) |
| The amount of information I am givenb | ||
| A little bit of information | 12 (9.2) | 9 (10) |
| Some information | 61 (46.9) | 49 (54.4) |
| A lot of information | 57 (43.8) | 32 (35.6) |
| Who sets the goals on how to look after my child’s diabetesa | ||
| Me | 2 (1.5) | 3 (3.3) |
| The doctor | 22 (16.9) | 23 (25.3) |
| The doctor and me together | 106 (81.6) | 65 (71.4) |
| Which doctor do I seea | ||
| A different doctor each time | 14 (10.8) | 10 (11) |
| The same doctor most of the time | 78 (60) | 62 (68.1) |
| The same doctor each time | 38 (29.2) | 19 (20.9) |
| In the consultation I have time forb | ||
| A few of my questions | 20 (15.5) | 16 (17.6) |
| Most of my questions | 26 (20.1) | 27 (29.7) |
| All my questions | 83 (63.4) | 48 (52.7) |
Ranking exercise
Control and intervention group patients ranked the attributes similarly: ‘who sets the goals’ was the most important attribute and ‘continuity of care’ was the least important attribute (Table 40).
| Attributes | Control | Intervention | ||
|---|---|---|---|---|
| n (%) | Rank | n (%) | Rank | |
| Who the doctor talks to | 31 (24.2) | Fourth | 28 (30.4) | Fourth |
| Amount of information given | 46 (36) | Second | 32 (34.4) | Second |
| Who sets the goals on how to manage the diabetes | 40 (32) | First | 39 (41.9) | First |
| Who you see at the consultation | 44 (36.7) | Fifth | 24 (26.1) | Fifth |
| Time for your questions | 35 (27.3) | Third | 28 (30.1) | Third |
Trading and non-trading choices pattern
The repetitiveness of the DCE task might deter respondents from reading each set of choices, leading to repeatedly making the same choice (‘not trading’), particularly if one visit option is constant throughout (as in this study). In the patient group, only one respondent chose the constant scenario for all choices, suggesting that children can manage this type of questionnaire and that 15 sets of choices seem acceptable.
Pair-wise choices: main model and interactions
All attributes were statistically significant, implying that the qualitative work correctly identified the factors that are relevant to patients when presenting for a clinic consultation in paediatric diabetes (Table 41). The betas represent the probability of moving away from the constant scenario and in economic terms predicts the utility (if accompanied by a positive sign) or the disutility (if accompanied by a negative sign) that the attribute bears. For instance, in Table 41, the beta values for ‘the doctor talks mainly with me’ and ‘the doctor talks with both me and my parent’ are 0.332 and 1.507, respectively. This indicates that any move away from ‘the doctor talks mainly to my parent’ is preferred by the patients (i.e. bears higher utility) and that the option ‘the doctor talks with both me and my parent’ is preferred much more to ‘the doctor talks mainly with me’.
| Attributes | β | SE | p-value |
|---|---|---|---|
| Who the doctor talks to | |||
| Talks mainly with my parent | 0.000 | Ref. | < 0.001 |
| Talks mainly with me | 0.332 | 0.102 | |
| Talks with both me and my parent | 1.507 | 0.103 | |
| The amount of information I am given | |||
| A little bit of information | 0.000 | Ref. | < 0.001 |
| Some information | 0.457 | 0.104 | |
| A lot of information | 0.888 | 0.103 | |
| Who sets the goals on how to look after my diabetes | |||
| The doctor | 0.000 | Ref. | < 0.001 |
| Me | 0.083 | 0.102 | |
| The doctor and me together | 0.931 | 0.101 | |
| Which doctor do I see | |||
| A different doctor each time | 0.000 | Ref. | < 0.001 |
| The same doctor most of the time | 1.690 | 0.112 | |
| The same doctor each time | 1.658 | 0.112 | |
| In the consultation I have time for | |||
| A few of my questions | 0.000 | Ref. | < 0.01 |
| Most of my questions | 0.136 | 0.103 | |
| All my questions | 0.337 | 0.105 | |
| Constant term | –2.791 | 0.163 | |
| Centre level | 0.013 | 0.044 | |
| Patient level | 0.885 | 0.126 | |
| Observation level | 0.958 | 0.024 | |
| Extra-binomial, second order, PQL; –2 log likelihood = 3245.45; n = 3386 | |||
Table 41 gives some evidence that the patient wants the doctor to address both him/her and the carer, that the goals on how to manage diabetes should be jointly set with the doctor and that continuity of care is preferred to seeing a different doctor each time. Finally, the amount of information and consultation time are relatively less important, yet still significant, with the sign indicating that more information and enough time to get answers to all questions are preferred.
Using the betas from Table 41, we can rank the set of scenarios (treatment profiles) and determine the ones that bear the highest predicted utility. This study included 27 scenarios (see Appendix 4 for study design) and Table 42 lists the 10 treatment options with the highest predicted utility.
| Scenario | Who the doctor talks to | The amount of information I am given | Who sets the goals on how to look after my diabetes | Which doctor do I see | In the consultation I have time for | Predicted utilities |
|---|---|---|---|---|---|---|
| 1 | Talks with both me and my parent | Some information | The doctor and me together | The same doctor each time | Most of my questions | 4.689 |
| 2 | Talks with both me and my parent | A little bit of information | The doctor and me together | The same doctor most of the time | All my questions | 4.465 |
| 3 | Talks mainly with me | A lot of information | The doctor and me together | The same doctor each time | All my questions | 4.146 |
| 4 | Talks with both me and my parent | A lot of information | Me | The same doctor each time | A few of my questions | 4.136 |
| 5 | Talks with both me and my parent | A lot of information | The doctor | The same doctor most of the time | A few of my questions | 4.085 |
| 6 | Talks with both me and my parent | Some information | Me | The same doctor most of the time | Most of my questions | 3.873 |
| 7 | Talks mainly with my parent | A lot of information | The doctor and me together | The same doctor most of the time | Most of my questions | 3.645 |
| 8 | Talks with both me and my parent | A little bit of information | The doctor | The same doctor each time | All my questions | 3.502 |
| 9 | Talks mainly with me | Some information | The doctor and me together | The same doctor most of the time | A few of my questions | 3.410 |
| 10 | Talks mainly with me | A lot of information | Me | The same doctor most of the time | All my questions | 3.330 |
From Table 42 it can be seen that if a scenario includes ‘the doctor talks with me and my parent’, ‘the doctor and me together set the goals on how to look after my diabetes’ and ‘I see the same doctor every time’, which are attributes leading to the highest level of utility, the patient sacrifices (trades off) the amount of information and consultation time. In fact, a reduction in any of these options can be compensated only by the presence of one or both of the other attributes.
The interaction between main attributes and study group was not significant, which suggests that the intervention did not generate a shift in patients’ preferences. Patient preferences did not show any interaction with gender. There was a significant interaction between attributes and questionnaire version, which is expected from creating two orthogonal designs (alias blocks).
Carers
Response rate and representativeness
As shown in Table 43, there were no differences in terms of patient age and gender, but carers of patients with higher HbA1c levels at follow-up were less inclined to respond.
| Item | Responders | Non-responders | p-value |
|---|---|---|---|
| Mean (SD) patient age (years) at follow-up | 11.6 (2.8) | 11.5 (2.8) | 0.673a |
| Mean (SD) patient HbA1c levels at follow-up | 9.42 (1.6) | 9.93 (1.8) | 0.001a |
| Patient gender, n (%) | 0.70b | ||
| Male | 212 (48.7) | 130 (50.4) | |
| Female | 223 (51.3) | 128 (49.6) | |
| Trial arm, n (%) | 0.157b | ||
| Control | 219 (50.3) | 115 (46.6) | |
| Intervention | 216 (49.7) | 143(55.4) | |
| DCE version, n (%) | 0.43b | ||
| A | 213 (49) | 135 (52.3) | |
| B | 222 (51) | 123 (47.7) |
There was also good balance in terms of control and intervention group and versions A and B of the questionnaire, the latter implying that no weighting needed to be applied for the analysis.
In total, 409 respondents (94%) answered all choices. Of the remaining 26, 10 did not answer six or more choices and these participants were removed from the sample. Data from those answering three or fewer choices were analysed, eliminating the missing choices. HbA1c concentration at follow-up was missing for three children and the values at baseline were imputed. In total, 6356 observations were available for analysis.
Current consultation style
At 1-year follow-up, both control and intervention group respondents experienced similar clinical consultation style (Table 44). Over 80% of both groups reported seeing the same doctor either ‘most of the time’ or ‘always’.
| Attributes | Control, n (%) | Intervention, n (%) |
|---|---|---|
| Who the doctor talks to | ||
| Talks mainly with me | 18 (8.2) | 19 (8.9) |
| Talks mainly with my child | 176 (80.4) | 163 (76.2) |
| Talks with both me and my child | 25 (11.4) | 32 (14.9) |
| The amount of information I am given | ||
| A little bit of information | 26 (11.9) | 27 (12.6) |
| Some information | 102 (46.8) | 93 (43.4) |
| A lot of information | 90 (41.3) | 94 (43.9) |
| Who sets the goals on how to look after my child’s diabetes | ||
| The doctor | 7 (3.3) | 4 (1.9) |
| My child and I | 31 (14.4) | 27 (12.7) |
| The doctor, my child and I together | 177 (82.3) | 181 (85.4) |
| Which doctor do I see | ||
| A different doctor each time | 39 (17.8) | 30 (14) |
| The same doctor most of the time | 118 (53.9) | 121 (56.5) |
| The same doctor each time | 62 (28.3) | 63 (29.4) |
| In the consultation I have time for | ||
| A few of my questions | 22 (10) | 13 (6.1) |
| Most of my questions | 35 (16) | 40 (18.7) |
| All my questions | 162 (74) | 161 (76.2) |
Ranking exercise
At 1-year follow-up, there was no difference between study groups in the ranking of the top two attributes (‘continuity of care’ and ‘who the doctor talks to’) (Table 45). The order of the remaining attributes was slightly different.
| Attributes | Control | Intervention | ||
|---|---|---|---|---|
| n (%) | Rank | n (%) | Rank | |
| Who the doctor talks to | 98 (45.8) | Fifth | 87 (41.2) | Fifth |
| Amount of information given | 68 (31.8) | Second | 60 (28.4) | Second |
| Who sets the goals on how to manage the diabetes | 60 (28) | Third | 41 (19.4) | Fourth |
| Who you see at the consultation | 89 (41.6) | First | 78 (37) | First |
| Time for your questions | 36 (16.7) | Fourth | 62 (29.3) | Third |
Trading and non-trading choices
Only three carers (two with version A) chose the constant scenario throughout, thus providing evidence that respondents read all questions and were willing to trade between attributes.
Pair-wise choices: main model and interactions
All attributes were statistically significant, with ‘continuity of care’ being the most important attribute relative to the others, followed by ‘who the doctor talks to’ and ‘who sets the goals’ (Table 46). Carers showed a strong preference for their child to be part of the consultation and involved in setting goals on how to look after his or her diabetes.
| Attributes | β | SE | p-value |
|---|---|---|---|
| Who the doctor talks to | |||
| Talks mainly with me | 0 | Ref. | < 0.001 |
| Talks mainly with my child | 0.737 | 0.100 | |
| Talks with both me and my child | 1.849 | 0.084 | |
| The amount of information I am given | |||
| A little bit of information | 0 | Ref. | < 0.001 |
| Some information | 0.782 | 0.088 | |
| A lot of information | 0.862 | 0.091 | |
| Who sets the goals on how to look after my child’ diabetes | |||
| The doctor | 0 | Ref. | < 0.001 |
| My child and I | 0.434 | 0.085 | |
| The doctor, my child and I together | 1.608 | 0.088 | |
| Which doctor do I see | |||
| A different doctor each time | 0 | Ref. | < 0.001 |
| The same doctor most of the time | 2.202 | 0.099 | |
| The same doctor each time | 2.328 | 0.103 | |
| In the consultation I have time for | |||
| A few of my questions | 0 | Ref. | < 0.001 |
| Most of my questions | 0.684 | 0.088 | |
| All my questions | 1.050 | 0.090 | |
| Constant term | –4.415 | 0.162 | |
| Centre level | 0.002 | 0.036 | |
| Carer level | 1.576 | 0.149 | |
| Observation level | 1.047 | 0.019 | |
| Extra-binomial, second order, PQL; –2 log likelihood = 4440.54; n = 6356 | |||
Table 47 lists the 10 scenarios with the highest expected utility. Carers show a strong preference for their child to be part of the consultation and setting the goals on how to look after the diabetes.
| Scenario | Who the doctor talks to | The amount of information I am given | Who sets the goals on how to look after my diabetes | Which doctor do I see | In the consultation I have time for | Predicted utilities |
|---|---|---|---|---|---|---|
| 1 | Talks with both me and my child | Some information | The doctor, my child and I together | The same doctor each time | Most of my questions | 7.251 |
| 2 | Talks with both me and my child | A little bit of information | The doctor, my child and I together | The same doctor most of the time | All my questions | 6.709 |
| 3 | Talks mainly with my child | A lot of information | The doctor, my child and I together | The same doctor each time | All my questions | 6.585 |
| 4 | Talks with both me and my child | Some information | My child and I | The same doctor most of the time | Most of my questions | 5.951 |
| 5 | Talks with both me and my child | A lot of information | My child and I | The same doctor each time | A few of my questions | 5.473 |
| 6 | Talks mainly with me | A lot of information | The doctor, my child and I together | The same doctor most of the time | Most of my questions | 5.356 |
| 7 | Talks mainly with my child | Some information | The doctor, my child and I together | The same doctor most of the time | A few of my questions | 5.329 |
| 8 | Talks mainly with my child | A lot of information | My child and I | The same doctor each time | All my questions | 5.285 |
| 9 | Talks with both me and my child | A little bit of information | The doctor | The same doctor each time | All my questions | 5.227 |
| 10 | Talks with both me and my child | A lot of information | The doctor | The same doctor most of the time | A few of my questions | 4.913 |
The interaction between main attributes and study group was not significant. There were no significant interactions between parents’ preferences and patients’ HbA1c levels at follow-up or with the child’s age.
Discussion
A rigorously developed DCE questionnaire with five categorical attributes of three levels each modelled both patients’ and carers’ preferences for the clinic consultation. In terms of consultation style, reportedly experienced patients in intervention sites report that their doctor addresses both them and their parent more frequently than do patients in control sites. Patients in intervention sites also report that their doctor alone sets goals more frequently than do patients in control sites. The former is consistent with the trial results, but the latter is not what would have been expected if trained practitioners are attempting to share decision-making with patients. Neither, however, reached statistical significance at the conventional level.
The ranking exercise showed no significant differences between control and intervention groups. However, the ranking differed between patients and carers, with patients listing ‘who sets the goals’ and ‘continuity of care’ as most and least important, respectively, whereas ‘continuity of care’ and ‘who the doctor talks to’ were listed by carers as the most and least important, respectively.
All attributes and levels were statistically significant, and both patient and carers wanted the other to be part of the consultation as well as part of the goal-setting. Trial allocation was not associated with any difference in either patient or carer preferences.
Strengths and weaknesses of the discrete choice experiment
A strength of this DCE study was that it was conducted alongside an RCT, which enabled assessment of any preference changes produced by the intervention. It also offered an opportunity to investigate the feasibility and acceptability of this type of questionnaire in a young population. The complete data sets supplied by over 90% of responding patients, coupled with the fact that only one respondent constantly chose the constant scenario, support the use of DCE questionnaires with this cohort of participants.
The response rate was good for this type of questionnaire. However, both groups were less representative of the cohort at baseline, which needs to be accounted for when interpreting the results.
Only a small number of DCE surveys have been conducted in the field of diabetes (including three with UK participants). 149–151 One investigated doctors’ preferences for a report card,152 whereas the others focused on patient preferences for alternative routes of insulin administration,150 patients’ willingness to pay for insulin delivery systems149,153,154 and treatment preferences and medication adherence. 151 However, none of these studies related to the clinic consultation and none of them was limited to people with type 1 diabetes or included children.
Future work
The current data set will be used to investigate how other reported psychosocial outcomes relate to DCE choices. It will also explore the feasibility of combining both patient and carer data sets for analysis and the result of using effects coding as opposed to using the dummy coding.
Conclusions
This study presents the first example of using a DCE to explore young patients’ and their carers’ preferences for clinic consultations in the management of diabetes. The approach was shown to be practical, with piloting vital to ensure feasibility and interpretation of the results (see Appendix 4).
The results show that both patients and carers prefer the doctor to address both parties and carers prefer their children to be involved in deciding goals on how to manage their diabetes, whereas the children want the doctor to be involved in the goal-setting process. This is consistent with the aims of the DEPICTED trial, although the DCE did not identify any trial effects. Future work will carry out subgroup analysis, which may produce a clearer picture of respondent preferences and intervention effects.
From a policy perspective, the study identified the key attributes of a routine consultation in paediatric diabetes services. However, the results might not reflect entirely the preferences of patients with higher concentrations of HbA1c, or of their carers.
Chapter 12 Process evaluation
Introduction
The intervention was intended to influence how the diabetes team interacts with patients and carers through strategies and skills used by trained practitioners within the consultation. It was not intended to impact directly on structural issues (e.g. number of clinics held, length of consultations, the physical space provided, number of routine home visits, school visits or telephone/text contacts). Nonetheless, it was also important to determine how the intervention was delivered in practice and whether the particular context of the trial may have influenced implementation (either facilitative or inhibitive). A third major element in evaluating the trial process was to assess the impact of training upon practitioner performance. Therefore, the aims of the embedded PE were to explore:
-
clinicians’ perceptions about how the intervention was received by clinical teams and by families and, in particular, factors that may have facilitated or hindered implementation and effectiveness
-
systematic changes within services during the study period, which may have resource usage implications
-
evidence of training impact upon practitioner performance.
The first two aims were addressed through interviews with informants from each clinical team, and the third by the rating of routine consultation recordings.
Local researcher interviews
Methods
Design and sample
Telephone interviews were conducted at the end of the trial with informants (local principal investigator or research nurse) from each trial centre (n = 26 staff in total). The interviews would address organisational changes that had occurred during the period of study at each site and identify whether or not the economic evaluation needed to test its assumption of no changes being due to the intervention through sensitivity analyses.
Interview
The semi-structured interview schedule (see Appendix 5) included initial general questions about service structure and changes during the study period. A series of closed probes were used within this section of the schedule to capture resource relevant data. The remainder of the interview included questions about the implementation of the Talking Diabetes intervention (omitted for control centre informants). Two members of the research team piloted the schedule with a local practitioner. The interview was intended to last approximately 30 minutes.
Procedure
Participants were invited to take part by letter, which briefly described the purpose and content of the interview. Appointments were made at a time suitable to the interviewee. Interviewees were encouraged to discuss the subject matter of the planned interview with colleagues to allow reflections from the broader team to be reported. Those unwilling to be interviewed were asked to nominate a local alternative. Interviews were conducted by two interviewers (ET-J and NB) and recorded following verbal consent.
Analysis
Interviews were transcribed and anonymised. Analysis was supported by the use of the NVivo software package. Data on systemic service changes were summarised descriptively according to the structured schedule. Data generated in the later part of the interview were coded inductively and emergent themes identified. Coding and analysis was primarily conducted by one interviewer (ET-J), who discussed analysis with two experienced social researchers (HP and MR).
Results
All participating centres took part, respondents including 23 nurses, two doctors and one dietitian. Eight interviewees were the nominated study principal investigators for their centre. Interviews lasted approximately 15–38 minutes.
Effects of intervention on direct costs of service delivery
Planning for major restructuring was undertaken at three intervention centres during the trial period. In two cases the changes were organisational: one involving a merger of two trusts and the other a move of the service to a different trust. The third case involved the physical relocation of the service to a new children’s hospital. Attributing any changes in service delivery to DEPICTED during such periods of restructuring would inevitably be difficult.
Number of clinics
Nine intervention centres reported no change in the number of clinics. One reported an increase in clinics from two to three per month, one introduced a new dietitian-led clinic for carbohydrate counting and one implemented a new nurse-led clinic for insulin pump users. One control centre reported the addition of a new teenage clinic.
Consultation time
None of the intervention centres increased the scheduled time for a clinic visit, although nine reported an increase in the actual consultation times, leading clinics to over-run. Of these, three attempted to quantify the increase, two reporting an average increase of 5–10 minutes and one an average increase of 15 minutes. Two further centres reported that consultations had increased initially but, as participants became more adept with their new skills, had now returned to their original length (Box 14). Interviews with control centres, however, revealed a similar pattern, with eight centres reporting that clinics usually – or in two cases ‘always’ – over-ran.
ID 26: ‘I think it [consultation times] has increased, but I think it is also something that we get better at … in terms of length of time they’re in, it is still longer … it does depend on the engagement side of things as well’
Frequency of clinic visits
Only one intervention centre reported an increase in the frequency of clinic visits (not quantified), but only for patients with the poorest glycaemic control. One control centre reported an increase in the frequency of nurse-led clinics.
Space
Three intervention centres indicated that additional room was needed, but none reported that additional room had been provided as a result of the intervention. Five control centres also stated that they needed additional room.
Frequency of non-clinic contact by the diabetes team
Three intervention centres reported a change in the frequency of non-clinic contact with patients; one involved an increase in telephone contacts, but this was due to the amalgamation of services, one involved an increase in text messaging and one involved an increase in school visits, but at the same time also a reduction in home visits. Three control centres also reported an increase in the frequency of school visits and one reported an increase in home visits.
Implementing the intervention
The results described below focus on the implementation and resulting outcomes of the Talking Diabetes intervention, and only data from intervention centre interviews are presented.
How was the intervention delivered?
Evidence from the majority of the intervention centres is that the use of 3T and the implementation of the skills and strategies provided by the training team were delivered across the whole-clinic population (Box 15). A few centres, however, reported that the intervention was delivered to trial participants only. Consequently, this might suggest potential inconsistencies in the delivery of the intervention with implications for the fidelity of intervention implementation.
ID 18: ‘We used it not just for the children that were on the DEPICTED study, but we gave it to everyone … and used the same skills all the way through’
ID 28: ‘Rolling it out to everybody you know, not just sticking, the DEPICTED study – you know the patients on the study, I think that made things, we kind of got to grips with things a bit easier’
ID 42: ‘Maybe we could have grouped them better in the clinics … said OK today we’re having all these patients coming on DEPICTED’
3T agenda-setting tool
Centres adopted a variable approach to implementing 3T, some providing folders to all their patients and others distributing the pads to trial participants only. Some centres utilised many methods to distribute the pads (e.g. posting to patients prior to appointments, giving them to patients as they arrived at the clinic, as well as having pads available in the waiting area). Other centres made the pads available to patients at the clinic only.
The use of the 3T pads (Box 16) by families was varied; some respondents reported that patients utilised the folder a great deal initially but recorded fewer agenda items as time progressed. Respondents felt that this was due in part to many of the issues raised by patients having been addressed at previous clinic visits. In general, most reported that the use of the pads simply declined as the study progressed. Of those who reported a decline, most inferred that the approach of the consultation nevertheless remained very much focused on ‘is there anything you’d like to talk about today?’ Many interviewees also commented that they used the ‘rate your diabetes’ question to initiate the consultation, in the absence of any written items on the 3T pad.
ID 18: ‘Some children will come in and they still bring their folders with them and they still have agenda on it, which is fantastic’
ID 37: ‘Some people find it really hard to think of things to put down on their pad, and interestingly people that use the pads well, actually their agenda items got less because they felt that so many things had been answered previously’
In terms of the age group of participants who utilised the 3T notepads (Box 17), many respondents felt that the younger age group were more likely to record their thoughts, with fewer older children and teenagers using the pads.
ID 44: ‘We did find that the reaction to the sheet varied hugely, we did find that girls were better than boys, we had a lot of younger-end girls particularly, fill it in completely’… ‘we’ve found some of the strategies useful with the boys, you know, “one to ten how is your diabetes this week”, we found quite useful’
ID 23: ‘The younger ones opened up a lot faster… they were better with the tools, the writing things down’ … There was certainly more resistance from the adolescents and the teenagers’
A few centres indicated that they would continue to use the agenda-setting pads, although one particular centre [ID 22] had implemented its own version of the 3T pad (post study period), adapted from a combination of the Talking Diabetes pad and an adult goal-setting tool.
Feedback on practitioner training
The feedback on the training programme for DEPICTED was generally positive (Box 18). A few interviewees suggested that the structured team approach, with the whole team being trained together, was beneficial. In addition, one centre suggested that interim training sessions to reiterate the skills and strategies learnt would have been of benefit to the clinical team.
ID 28: ‘ Having the training together, and thinking the same way in that respect and encouraging each other as well, has helped’
ID 44: ‘I think what might have been very useful was interim sessions … but an interim session on site … I found one of the most useful things the feedback from the psychologist on the three case studies, so if you could almost have that at intervals throughout or someone come to the centre and say oh yeah you know that was the right way, or we didn’t cross that at all’
Facilitators: what promoted the use of the intervention?
Promotional materials provided to the teams to act as prompts for the practitioners proved to be useful (Box 19), with many respondents reporting that they were used as a reminder of the strategies (see Appendix 6). Many also reported that support and ‘buy-in’ from the whole team, with opportunities for team reflection, encouraged individuals within the team. Evidence that clinicians had adapted their overall style in routine practice was also presented by some interviewees. Many of the nurses interviewed reported that they felt better able to use the skills and promote agenda-setting outside of the clinic setting, for example during home or school visits, as the patients were generally more relaxed in their own environment.
ID 18: ‘We had to have that little thing [intervention prompt sheet] in front of us to remind us about the different sections and skills’
ID 22: ‘I think actually learning from each other as well has been useful’
ID 11: ‘Dr (surname) used some of these [3T] in some other clinics … he’s used the ideas and stuff quite a bit in his general work’
ID 13: ‘Both the nurse and the dietitian who do home visits both felt that they could do better agenda-setting in the home than they could do in the clinic’
Barriers: what inhibited the use of the intervention?
A number of factors inhibited intervention implementation, some of which related to skill acquisition by practitioners and some to the structure and process of the clinic. The change in consultation style indicated by the study intervention had evidently taken most practitioners time to master (Box 20). Nevertheless, many of those felt that their confidence in their ability to implement the new skills increased with time.
ID 22: ‘I think we’ve sort of, like, got towards the end of the study before really the nurses are really using those skills in the clinic’
ID 23: ‘I was taking far too long so I’ve got sleeker and faster at it, (yeah) and it did take longer to start off with … so yeah more confident, I feel I’m doing it better’
Many reported variability in the consistency of the intervention delivery, as well as the level of skilfulness among the clinical team (Box 21), with differences between doctors and nurses. A few reported that their dietitian found the skills to ‘be less useful’, given that their consultations were perceived to be more directive in nature.
ID 11: ‘When you have sort of junior doctors changing all the time, but sort of making sure that everybody has had the training, which was difficult, at some points, there might not have been a doctor in clinic who’d actually been through the training process … so depending on who was the doctor in clinic that day you could have an excellent person who had the training and had all the skills and you could have somebody who was the complete opposite and I suspect for some of the patients that became extremely noticeable’
ID 22: ‘… I’m not convinced that everybody used it either … I think doctors particularly find it difficult to change the way that they consult you know because they’re used to a different style of consultation aren’t they … diabetes is my job, that’s what I do all the time, you know our consultant, it’s a tiny part of his work … so I think it probably is easier for nurses to change’
One of the key inhibiting factors seemed to be time constraints (Box 22). Many reported that during busy clinics, which often over-ran, they found it particularly difficult to maintain the guiding approach, and some confessed to reverting back to a more directive approach when pushed for time. However, given enough time, it was felt that the intervention worked.
ID 28: ‘It worked well from the beginning, cause the clinics lasted for ages, it was really, really tough to keep that up, and its very tempting to then adopt a directive approach to get people out when you’re looking at the time’
In addition to time constraints, one centre felt that inflexibility in service provision (interval between clinic visits of 3–4 months) would have limited exposure to the intervention (Box 23). One interviewee suggested that more nurse-led clinics could potentially address this issue. Another factor influencing the implementation was attrition of trained staff, as well as availability of trained staff within the clinic setting.
ID 22: ‘It’s a new way of consulting … and I think that change was probably a bit difficult for them to get used to and then of course they didn’t come back for 3 months… so if I had my time over again I would definitely be seeing them more often … so when they’ve set some goals not waiting 3 months to see how they’ve got on’
Changes in practice
Notwithstanding factors inhibiting implementation, interviewees from all intervention centres clearly felt that the focus of the consultation had shifted away from clinical outcomes (i.e. maintaining an acceptable HbA1c level) to a greater focus on the needs of the child (Box 24). Many felt that this was achieved through more structured and open consultations, and engaging with the patient in a less directive manner. Many reported that if the young person was engaged with the consultation, they were more likely to contribute to problem-solving. A few interviewees commented that this approach was potentially not applicable in all situations, for example when control was stable or where the patient was clearly not ready to change behaviour. The majority of interviewees reported that shared agenda-setting was evident within their practice.
ID 13: ‘ I think we probably do all spend a bit more time trying to explore’ … the biggest thing is recognising that people, allowing people to say I’m not ready to do this yet’
ID 22: ‘Because of the study there’s much more of a focus on the child from an earlier stage’
ID 35: ‘Having done the study, it does equip you with more of the skills to, sort of, empower the patient more, and for them it tends to, sort of, be more, they’ve just seemed more involved with coming up with solutions, rather than relying on you’
ID 37: ‘About the agenda-setting tool, I think without exception everybody said that was a useful tool’
ID 44: ‘It has made us more aware and better at involving the child as number one … the child being first in the consultation, and we felt that generally there were three agendas, child’s, parents’ and ours, and we all learnt something new from that … when we used the strategies and the children engaged then we felt that we had very positive outcomes’
Patient feedback
The focus more towards the child was also echoed in the mainly positive feedback from patients (Box 25), albeit as reported by interviewees. In general, most reported that the patient felt ‘listened to’, with children perceiving the consultation as less stressful and especially the teenagers finding the process less confrontational as a consequence of enhanced patient engagement.
ID 14: ‘Some of the teenagers, particularly, have appreciated the change in the way that the clinics have been run and their role in presenting what they want to discuss and how they’ve been approached’
ID 26: ‘In terms of what their expectations are that has hugely shifted and the fact they’re bringing something to us rather than just taking something away’
ID 44: ‘I think people were positive because I think the children appreciated that you were at least trying to get their perspective first’
Relationship with patients
Many interviewees felt positive that their relationship with the children and young people had improved, and some commented that the change in their approach to consulting with teenagers especially had been successful (Box 26). This perception of success was considered as an improvement in patient engagement, following the shift in emphasis of the consultation to a more open, patient-focused style of communication.
ID 18: ‘Some of the teenagers felt that because we’d focused away from this wonderful HbA1c test, they quite liked the fact that we were not jumping in straight away with that’
ID 26: ‘It’s their voice that we’re hearing more of, and I think that has been the consensus in terms of the patient engagement now is greater’ … ‘it’s definitely given them a louder voice’
ID 28: ‘I think they’re probably a lot more open’ … ‘because you’re talking about things they’re interested in, and they’re making the decisions’
In response to the question ‘would you say that you’ve changed the way in which you practise’ the overall consensus from the intervention centres was that some elements of their practice had changed, but clearly the skill level was variable, and that changes in personal practice of this nature would take time to implement consistently and with confidence (Box 27).
ID 18: ‘It will be a style that obviously we will still continue to use’
ID 28: ‘I think as a team, I think it has made us a bit stronger’
ID 35: ‘I do feel that there’s still some learning to be done … that it could be better’
Practitioner performance assessment
Methods
Design
To examine the impact of the training upon practitioner performance, a new scale was developed to address two questions: did experimental practitioners demonstrate better training-related skills than shown by controls and did experimental practitioners continue to use the intervention in the year following training?
Participants and audio-recording procedure
Practitioners were asked to audio-record a sample of their clinical sessions, following written informed consent from practitioners, patients and carers. Families were informed about the study and approached for consent to the recording prior to entering the consultation room. Patients approached for consent included those already recruited to the trial and other patients attending the normal clinic session. Practitioners nominated up to two consultations where a behaviour change issue was discussed (analysis was not restricted to these consultations). During any one clinic session, only one team member was recorded for logistical reasons and to enable patients to speak freely to other members of staff. A maximum of three randomly selected team members per clinic were recorded.
Recordings returned
A total of 171 valid consultation recordings (i.e. downloadable and with valid consent) were returned (Table 48) – not all centres or practitioners approached returned useable data. At time point 1 (T1) (post training for the intervention group only) seven clinicians from 7 of the 13 trial intervention centres returned useable recordings. At time point 2 (T2) (12 months post training for the intervention group and pretraining for controls), 31 clinicians from 14 of the 26 trial centres returned useable data.
| Group | No. of centres | No. of practitioners | Total no. of consultations | Recordings by professional group | |||
|---|---|---|---|---|---|---|---|
| Doctor | Nurse | Dietitian | Joint | ||||
| T1 intervention | 7 | 7 | 49 | 16 | 20 | 6 | 7 |
| T2 intervention | 7 | 16 | 61 | 23 | 23 | 15 | 0 |
| T2 controls | 7 | 15 | 61 | 30 | 22 | 9 | 0 |
Where available, two consultations per practitioner were selected for further analysis (two per practitioner per time point for the intervention group). Where more than two consultations were available, two were randomly selected from those available. This yielded a total of 86 consultations (28 intervention at T1, 29 intervention and 29 control group at T2), all of which were rated by one rater and a random sample of 20 also rated by a second rater to assess inter-rater agreement.
Rating scale development
The 86 recordings were rated on a scale developed to reflect the consultation skills and strategies addressed by the Talking Diabetes learning programme. The domains covered were:
-
overall adherence to a guiding style
-
agenda-setting
-
pros and cons of change
-
importance and confidence about change
-
brainstorming solutions (goal-setting).
The intervention drew upon some elements of MI and represented a format uniquely tailored to the context and study. As such, only items measuring domain no. 1 were derived directly from an existing reliable and valid scale of MI called the MITI (Motivational Interviewing Treatment Integrity) code. 155 MITI consists of three items about collaboration, evocation and autonomy support. Items for the remaining domains were constructed afresh, directly from the content of the learning programme, resulting in an additional global judgement for each of four domains: agenda-setting, ‘pros and cons’, ‘importance and confidence’ and ‘goal-setting’.
The programme development team and trainers met a number of times to review the aims, background and psychometric challenges, leading up to the final scale described below. The starting point was a decision to focus on practitioner skills only, given the primary aim of assessing their adherence to the learning programme content. Following the lessons learned in the validation of the MITI scale,155 a decision was also made to use global ratings of domains rather than actual behaviour counts. It then became a question of what domains to assess and how they might be designed so that raters broadly familiar with MI would be able to listen to a recording, and conduct their assessment.
Items and scoring (see Appendix 7 for the scale used)
The guiding style (from the MITI scale)
A global rating on each of three items (evocation, autonomy support and collaboration) on a five-point scale reflects the degree to which this element was present in the interview. Raters also provide a global judgement about adherence to a guiding style, called ‘guiding style’ in the analysis below.
Agenda-setting, pros and cons, importance and confidence and brainstorming
Rating these domains used the same rationale and scoring system in which:
-
Whether or not the task was carried out and the skilfulness with which it was delivered were scored separately.
-
Task ratings used a three-point scale (from 0, ‘no evidence’, to 2, ‘good evidence’). Skilfulness ratings used a seven-point scale (from 0–6, with implicit anchors ranging from ‘not at all skilful’ to ‘very skilful’).
-
Rating was assisted by a visual guide for both task (a breakdown of task components) and skills (a breakdown of component skills). The guides helped frame initial judgement and were not analysed further.
Training of raters
A manual was constructed to guide the two raters (both trainers in MI), one of whom had been trained as a MITI rater. A single pass of a recording was considered adequate for rating all items.
Analysis plan
The analysis aimed to establish inter-rater reliability and to answer the two primary questions: did experimental practitioners demonstrate better training-related skills than controls and did experimental practitioners continue to use the intervention in the year following training? Of secondary-level interest was the question about whether or not performance differed across professional groups.
As the guiding scores (‘evocation’, ‘collaboration’, ‘autonomy supportive’ and ‘guiding style’ itself) were positively skewed, non-parametric tests were used (Mann–Whitney U-test for tests between two groups and Kruskal–Wallis H-test for tests between three professional groups). For the ‘task’ scores, there were not enough data in all of the cells of the cross-tabulation to validly apply a chi-squared test. Thus, proportions of those doing or partially doing the ‘task’ were calculated instead, along with the CI for the difference between groups. For the ‘skilfulness’ scores, the differences between the intervention at the two time points was tested using the Mann–Whitney U-test. To test the types of ‘professional’ in each group, a chi-squared test was used because of the categorical nature of the data.
A variance components analysis was done to assess the levels of variance in ‘guiding style’ score attributable to individual practitioners. This was done by fitting a linear mixed-effects model to the ‘guiding style’ score with ‘group’ as a fixed effect and ‘practitioner’ as a random effect. This was carried out twice: first to compare the control group and the intervention at T2 and then to compare the intervention group at the two time points.
Results
Inter-rater reliability
With the exception of agenda-setting, the level of agreement on ratings ranged from 0.49 to 0.88, (i.e. moderate to excellent) (Table 49). Raw data for κ-statistics are available in Appendix 9.
| Rating domain | Quadratic weighted kappa | |
|---|---|---|
| Evocation | 0.65 | |
| Collaboration | 0.49 | |
| Autonomy supportive | 0.58 | |
| Guiding style | 0.58 | |
| Task | Skilfulness | |
| Shared agenda-setting | 0.27 | 0.30 |
| Pros and cons | 0.72 | 0.66 |
| Importance and confidence | 0.70 | 0.75 |
| Brainstorming | 0.50 | 0.88 |
Number of recordings from different professional groups
The number of recordings for each professional group was not significantly different between groups (Table 50: χ2 = 2.88, df = 4, p = 0.578).
| Group | Profession | |||
|---|---|---|---|---|
| Doctor | Nurse | Dietitian | Total | |
| Control | 14 | 9 | 6 | 29 |
| Intervention (T1) | 13 | 11 | 4 | 28 |
| Intervention (T2) | 10 | 10 | 9 | 29 |
| Total | 37 | 30 | 19 | 86 |
Performance: guiding style
For each scale, higher scores represent a greater adherence to a guiding style. On all of the scores there was a significant difference (p < 0.001) between control and intervention groups at T2 (Table 51), with the latter scoring higher. In the intervention group, all of the guiding style scores are higher at T1 than at T2 (Table 52), but this difference is statistically significant only for ‘evocation’. There was no difference in ‘guiding style’ score between professional groups (Table 53).
| Strategy | Control | Intervention (T2) | Difference in means | p-value | ||
|---|---|---|---|---|---|---|
| n | Mean (SD), median | n | Mean (SD), median | |||
| Guiding style | 29 | 1.2 (0.47), 1 | 29 | 2.3 (0.85), 2 | –1.14 | < 0.001 |
| Evocation | 29 | 1.1 (0.44), 1 | 29 | 2.0 (1.02), 2 | –0.90 | < 0.001 |
| Collaboration | 29 | 1.5 (0.63), 1 | 29 | 2.3 (0.84), 2 | –0.83 | < 0.001 |
| Autonomy supportive | 29 | 1.3 (0.60), 1 | 29 | 2.4 (0.82), 3 | –1.07 | < 0.001 |
| Strategy | Intervention (T1) | Intervention (T2) | Difference in means | p-value | ||
|---|---|---|---|---|---|---|
| n | Mean (SD), median | n | Mean (SD), median | |||
| Guiding style | 28 | 2.6 (0.91), 3 | 29 | 2.3 (0.85), 2 | 0.33 | 0.128 |
| Evocation | 28 | 2.6 (1.06), 3 | 29 | 2.0 (1.02), 2 | 0.61 | 0.039 |
| Collaboration | 28 | 2.6 (0.92), 3 | 29 | 2.3 (0.84), 2 | 0.30 | 0.188 |
| Autonomy supportive | 28 | 2.6 (0.83), 3 | 29 | 2.4 (0.82), 3 | 0.26 | 0.171 |
| Strategy | Doctor | Nurse | Dietitian | p-value | |||
|---|---|---|---|---|---|---|---|
| n | Mean (SD), median | n | Mean (SD), median | n | Mean (SD), median | ||
| Guiding style | 37 | 2.0 (1.11), 2 | 30 | 2.1 (0.94), 2 | 19 | 2.0 (0.85), 2 | 0.477 |
The variance components analysis for control group and intervention at T2 shows that there is almost no variance (40.4 × 10–9) attributable to the practitioner level for ‘guiding style’ score, with the residuals containing most (0.470) of it. The p-value obtained is < 0.001, which mirrors the result shown in Table 51 of a statistically significant difference in ‘guiding style’ score between control group and the intervention at T2. The variance components analysis for the intervention group at T1 and T2 shows that a fair proportion of the variance (0.216) in ‘guiding style’ score is attributable to the practitioner level, though the majority (0.571) is explained by the residuals. The p-value obtained is 0.123, which mirrors the result shown in Table 52 of no statistically significant difference in ‘guiding style’ score between the intervention groups at the two time points.
Performance: tasks
The two most frequently used of the four strategies were shared agenda-setting and brainstorming (Table 54). Between T1 and T2 there was a reduction in frequency of use of all of the strategies, although agenda-setting is still evident in half of the rated consultations at T2 and brainstorming in one-quarter of consultations (Table 55).
| Task | Proportion with task done or partially done, n (%) | Difference in proportions and 95% CI | |
|---|---|---|---|
| Control | Intervention (T2) | ||
| Shared agenda-setting | 2/29 (6.9) | 15/29 (51.7) | –0.45 (–0.62 to –0.22) |
| Pros and cons | 0/29 (0.0) | 3/29 (10.3) | –0.10 (–0.26 to 0.03) |
| Importance and confidence | 0/29 (0.0) | 3/29 (10.3) | –0.10 (–0.26 to 0.03) |
| Brainstorming | 1/29 (3.5) | 7/29 (24.1) | –0.21 (–0.39 to –0.03) |
| Task | Proportion with task done or partially done, n (%) | Difference in proportions (95% CI) | |
|---|---|---|---|
| Intervention (T1) | Intervention (T2) | ||
| Shared agenda-setting | 20/28 (71.4) | 15/29 (51.7) | 0.20 (–0.05 to 0.42) |
| Pros and cons | 8/28 (28.6) | 3/29 (10.3) | 0.18 (–0.03 to 0.38) |
| Importance and confidence | 6/28 (21.4) | 3/29 (10.3) | 0.11 (–0.08 to 0.30) |
| Brainstorming | 16/28 (57.1) | 7/29 (24.1) | 0.33 (0.08 to 0.53) |
Performance: skilfulness
As relatively few of the control group consultations involved using one of the four strategies, it was only worthwhile assessing the differences in skilfulness over time for the intervention group. There was a small reduction in skilfulness score for the ‘pros and cons’ strategy, which was of borderline statistical significance. There were no significant differences in skilfulness score for the other three strategies over time (Table 56).
| Skilfulness domain | Intervention at T1 | Intervention at T2 | Difference in means | p-value | ||
|---|---|---|---|---|---|---|
| n | Mean (SD), median | n | Mean (SD), median | |||
| Shared agenda-setting | 20 | 2.5 (0.89), 2 | 15 | 2.3 (0.80), 2 | 0.23 | 0.440 |
| Pros and cons | 8 | 3.1 (1.13), 3 | 3 | 1.7 (0.58), 2 | 1.46 | 0.053 |
| Importance and confidence | 6 | 2.2 (1.17), 2 | 3 | 1.7 (0.58), 2 | 0.50 | 0.583 |
| Brainstorming | 16 | 2.4 (0.89), 2 | 7 | 2.6 (0.54), 3 | –0.13 | 0.639 |
Discussion
Overall, there was a positive response to the training and to the skills and strategies of the intervention, but also some important messages about how implementation could be improved. Some centres chose to implement the intervention in a more limited group of just trial patients and there was some reversion to more directive approaches possibly driven by time pressures in clinic. Inherent within this is also an indication that some practitioners had not fully accepted guiding as a generically applicable approach. How the intervention was actually used in practice was, in part, consistent with the intended flexible menu approach, but it does raise questions about programme fidelity and how that may be assessed. Finally, informants described a clear shift in the emphasis of the consultation that was consistent with the intervention goals, with increased patient engagement in agenda-setting and problem-solving.
Service-level impact
Clinic time is a real constraint in both intervention and control sites, although the perception that the intervention may have increased consultation length was not borne out by the available consultations recordings. Nevertheless, a sensitivity analysis was performed as part of the cost-effectiveness assessment to show the effect of assuming a 20% increase in consultation time in intervention sites (see Chapter 9). There was no indication from the interviews that the intervention had impacted on any of the other service-level factors and no additional sensitivity analyses were undertaken.
Intervention implementation
Centres reported a variety of approaches to distributing 3T to patients, but not all received the tool in advance of the consultation. Use of 3T notepads declined over time, although the ability of practitioners to utilise the concepts without written prompts was nevertheless evident and consistent with the spirit of agenda-setting from the training. The apparent differential uptake of 3T by patients of different ages was anticipated at the design stage, which is why both a pad and a separate folder were provided.
That the Talking Diabetes approach was not considered suitable in all consultations is consistent with the conditional application of strategies using a flexible menu. Services adapted the menu of strategies for use in their own settings, serving to enhance local ‘ownership’ of the intervention by teams. Although the menu approach has advantages for service implementation, it is also more difficult to accurately determine programme fidelity with increasing levels of flexibility. A shared agenda-setting approach and the emphasis of guiding in behaviour change consultations are the part of the intervention that would nevertheless have the most general applicability.
Access to training will impact upon intervention effectiveness. Training was well received by clinical teams, both for online learning and for face-to-face workshops. The feedback with regards to the training was insightful, as training the whole team was our intention. However, we were not always able to achieve this (owing to individuals being unable to attend). We offered online training to newly arriving team members (i.e. after initial team training), but could not run workshop sessions outside our original planned sessions. Further support to teams following the training sessions would need careful consideration in the future.
Exposure of participants to the intervention was varied, with on average quarterly clinic visits. Frequency of clinic visits (including non-attendance), actual presence of Talking Diabetes-trained staff and consultation length are all factors that will vary the effective dose available to patients. This remains a challenge for an intervention required to be deliverable within routine consultations with no additional resource and time in clinic being an overarching concern for some practitioners.
Practitioner performance
Practitioners in the intervention group were applying the skills that they had learned in the training programme. Compared with the control group they demonstrated greater use of a guiding style and implementation of the four intervention key strategies. This was the case across the three professional groups, with no significant differences between them. The results suggest that practitioners used some of the strategies more than others, with more frequent use of ‘shared agenda-setting’ and ‘brainstorming’ than ‘pros and cons’ and ‘importance and confidence’. There was some evidence that the use of the strategies in the intervention group diminished over time, but the size of the available sample meant that it was not possible to judge whether or not there was a change in skilfulness with which the tasks were delivered.
Some limitations with the performance assessment need to be considered. It was difficult to engage some teams and some practitioners with the recording, resulting in fewer available recordings than had been envisaged. Although there was a spread of the professions and a spread of centres in the analysed sample, any systematic differences between those returning and not returning tapes requires further exploration.
The new rating measure was developed by the researchers who were responsible for defining the training programme to specifically measure the key components of the curriculum. Although this could limit broader applicability, it does ensure a high degree of content validity. Expected differences detected between intervention and control groups support the validity of the new measure. Although some changes over time were observed, further work will be required to determine whether or not the measure is sensitive to change. The levels of inter-rater agreement were generally satisfactory, but some further work should address agenda-setting in particular. Further development of the rater training and training manual should enhance validity and reliability of the assessment, although if it added to the complexity of the rating task this could negatively impact on assessment feasibility. However, as the tool has been developed for application in a research context and can be applied following a single pass through a recording this should not unduly limit its application.
Further detailed analysis of recordings from trained practitioners with the highest and lowest guiding scores may provide additional insights about how the training programme has been implemented in practice. This would help refine the development of the training, for example by clarifying which elements have been less successful and also what additional dimensions may need to be added.
Chapter 13 Discussion and conclusions
Key trial findings
Training HCPs to improve their consultation skills, particularly in relation to behaviour change, as developed in DEPICTED, could not be shown to impact beneficially on glycaemic control as measured by HbA1c levels. Furthermore, patients in clinics where staff had undergone training may have experienced a reduction in confidence in their ability to manage their diabetes, whereas those in the control arm showed, surprisingly, a reduction in barriers and improvement in adherence to their diabetes management. However, patients in intervention centres did report an increased ability to cope with their diabetes as a result of their clinic visit, although this effect was only found in the short term. By contrast, parents of those in the intervention arm experienced greater excitement about clinic visits and an improvement in the continuity of care without the adverse effects seen in their offspring.
DEPICTED has demonstrated that a high-quality, complex, pragmatic trial of health service delivery can be successfully conducted in a currently challenging clinical environment, recruiting teams and patients from clinical services reflecting a wide range of philosophies and research experience within England and Wales. Evidence from this study has demonstrated that paediatric health-care teams can be successfully trained to improve their consultation skills using a combination of workshop and internet-based training, albeit with evidence to suggest that reinforcement of these skills remains an important need. The workshop component of this training represented a significant contribution to the overall costs (both time and financial) of the intervention.
In the following discussion, we initially focus on the findings of the trial phase, highlighting the strengths and weaknesses of the study, including generalisability of the findings. Thereafter, we consider the interpretation of the study results, taking into account the hypothesis and statistical issues relevant to the methodology. 156 Considerations for future delivery of clinical services and research are subsequently addressed in the final chapter.
Strengths and limitations
A cluster design was used to good effect in this study. We provided for the additional number of subjects required and accounted for possible dropout by over-recruiting both centres and patients. All centres recruited into the study completed their participation and the study dropout rate was extremely low, especially for HbA1c blood samples, providing additional statistical power for both primary and secondary analyses.
Internal validity
Measurement bias was minimised by the use of a central laboratory for all HbA1c assays. The two trial arms were well balanced at baseline, and checks on the small number of non-completers revealed that they were more likely to be female, have lower BMI and have slightly higher baseline HbA1c levels. It was also shown that the average number of visits that patients made to their clinic was not significantly different between trial arms.
One limitation of pragmatic cluster trials is possible bias due to trial arm allocation knowledge. Allocation was revealed to all centres approximately 2 weeks prior to the first face-to-face training workshop for intervention teams, to allow sufficient time for professionals to complete the initial e-learning component. At this stage, only 30% of participants had formally consented. However, all of the participants were approached to participate before teams knew to which arm of the trial they had been allocated. Furthermore, checks for bias revealed no obvious imbalances in demographic data. It is possible that allocation knowledge bias may have affected recruitment rates, as more of the intervention centres recruited the target number of patients than control centres. Recruitment and randomisation of clinical teams was also undertaken in blocks for unavoidable logistical reasons, for example highly variable turnaround times in obtaining necessary governance approvals. This trial preceded the introduction of the current co-ordinated system for gaining NHS permissions in England (Coordinated System for Gaining NHS Permission), which should benefit future studies with similar requirements.
Diabetes-specific issues
A strength of the DEPICTED trial has been its incorporation of recommendations from previous systematic reviews of psychoeducational interventions in childhood diabetes. 37 Specifically, in the developmental phase, considerable effort was made to build on previous findings from similar interventions, both through the systematic review of the literature, but also through an extensive survey of clinical experience of similar initiatives in UK clinics, findings from which may not have been published. The similarity in conclusions from our own independent review to those published by other groups at the same time62,64 suggests that we did not fail to identify important interventions which should have influenced our study design. Furthermore, DEPICTED had a firm theoretical basis that drew significantly on the principles of MI, which have been shown previously to have potential in childhood diabetes. 31,33
The training developed in DEPICTED highlighted skills necessary for consultations involving both child and parents, recognising evidence from previous studies that outcomes for adolescents may be better where parents remain involved in a negotiated manner in their care. 157,158 It is of interest that parents in the intervention arm reported a greater experience of continuity of care and did not reflect the reduction in confidence reported by their children. This suggests that parents may have benefited more from the intervention than their children did and would be better placed to provide ongoing support to their children.
The trial design had a number of potential strengths with respect to the analysis. The counselling of patients by a large number of practitioners in the intervention arm allowed intervention impact to be evaluated without the confounding influence of an individual practitioner’s skills and personality. The selection of secondary outcomes included several which have been shown to perform well in measuring QoL in children with diabetes in a range of contexts. 159 Although some minor changes had been made to secondary outcome measures, partly to ensure consistency of the overall outcomes package, preliminary analysis supported the validity of the revised measures. Finally, the assessment of practitioner performance used routine consultation recordings and benefited from an existing validated measure drawn from the field of MI as well as a newly validated measure tailored to the specific intervention. 155
Previous reviews have highlighted the need to embrace the views of both lay and professional stakeholders. In DEPICTED, this input occurred at many levels. For example, the parent of a child with diabetes was a member of the study management team, lay views were sought via the focus group work and a formal SAG with lay and professional membership met to guide the developing intervention. The effect of this on the research process can be seen, for example, in the development of an agenda-setting tool that extended the original research design. Our findings suggest that stakeholders can make a valid contribution to a research programme such as DEPICTED to ensure an output that is likely to be feasible and practical in an NHS paediatric diabetes context.
Feasibility and training exposure
The trial intervention appears to be deliverable within the context of routine care without major impacts on service structure. Length of consultations, for example, did not appear to differ between intervention and control centres. The training programme was designed to reflect the learning preferences expressed by professionals during the development phase and motivation to attend face-to-face workshops was high, as evidenced by attendance rates. Most practitioners at each study site attended both workshops. However, the variable way in which teams defined their membership (e.g. some including adult physicians) and the inevitable rotation of clinical staff meant that potential trainees could not always be either identified or provided for.
Training exposure
Exposure to all modules of the online learning programme was not as high as hoped (e.g. case reporting to trainers). However, the PE indicated significant skill acquisition, particularly agenda-setting and skill maintenance at 1 year. Given no significant effect on the main outcome, it may be that the less frequently used intervention elements (such as eliciting importance and confidence) may be critical components for effectiveness. Determining the effective elements of complex interventions is a general challenge. It is also possible that a low existing base rate for agenda-setting or guiding provides the potential for improved performance that is statistically significant, but clinically ineffective.
Generalisability
Centres recruited into the study were geographically spread and were balanced for list size in the randomisation. This provides very good generalisability of the results for diabetes patients in the UK aged 4–16 years and their carers. The very low dropout rate and the spread of attendance noted (from no visits up to six visits between baseline and follow-up in both arms) also strengthens the findings of the study.
In DEPICTED, exclusion criteria were kept to a minimum and the heterogeneity of the paediatric diabetes population is well reflected by trial participants. Pragmatic RCTs therefore tend to demonstrate high external validity and assess effectiveness rather than efficacy. It would be difficult to draw conclusions about the impact of the same intervention in specific population subgroups. For example, there may be a dose–response effect that cannot be determined using an intention-to-treat approach: patients with poor glycaemic control are also likely to be poor clinic attenders and therefore may have had little exposure to the current intervention (approximately half of the current sample visited the clinic only twice post baseline and prior to follow-up).
There are no a priori reasons why the reported training costs should not be generalised to other centres. As the study was pragmatic, centres could decide how many members of the diabetes team would be trained. The resulting variation in numbers trained is the main reason for the variation in training costs, but given the relatively large number of centres in the intervention group (13) this is likely to reflect what would be seen in other centres. At the same time, there is probably scope for reducing overall training costs by having more seminars delivered on site and making greater us of local trainers.
Changing the clinic culture
The DEPICTED intervention was intended to embrace the whole clinical team. The goal was to shift the orientation of consultations towards more active parents and children, using the agenda-setting tool as the fulcrum for this shift in the culture of service provision. Despite enthusiasm in workshops and evidence that intervention practitioners used some of the skills required, it seems reasonable to conclude that this pragmatic trial failed to effect the shift in treatment culture that ran through intervention development discussions and subsequent learning programme.
One of the anticipated strengths of the intervention – a pragmatic focus on readily useable guiding skills, but without training attention to listening skills – might have been a weakness. The failure of the DEPICTED study to demonstrate an effect on HbA1c level is surprising, given the influence of MI on its design and the previous evidence that MI can have beneficial effects on glycaemic control. 32,33 However, one MI ingredient missing from the DEPICTED study was attention to listening skills; practitioners were not taught about them, and this could well account for the positive results in the studies by Channon and colleagues. 32,33
The pragmatic nature of this intervention was important to test its utility in a wide range of service environments. However, this did not allow us to specifically assess whether or not these techniques would have been more effective if combined with other efforts to intensify diabetes management. Concerns were expressed by trainees that incorporation of the techniques into routine care would produce longer consultations, although there was no clear evidence to support this view. However, the health economics analysis of the DEPICTED trial shows this to be a relatively expensive intervention, given the costs of training.
Interpretation of results
The failure of the intervention to impact on the primary outcome – HbA1c levels – demonstrates that the training as encompassed in the DEPICTED trial is not effective in changing glycaemic control over a 1-year follow-up after team training. It is possible that this disappointing outcome has been due to one or more of the following influences: (1) the improvement in practitioner expertise in communication may have remained insufficient to achieve beneficial changes in patient behaviour; (2) the extent of contact between HCPs and patients over the course of the 1-year follow-up (typically three to four brief clinic visits) may have been insufficient for improved communication skills to have impacted on patient behaviour; (3) the effect of training may have been diluted by the impact on patients of practitioners within the team who had not undergone training; and (4) the training, as developed in the DEPICTED trial, did not induce the skills required to facilitate behaviour change in the patient. In retrospect, an extended-length pilot study of the training might have proved useful in highlighting some of these issues. However, on the basis of the results, the training as defined in the DEPICTED trial cannot be recommended to produce clinically significant changes in children with diabetes.
Figure 8 portrays training received by practitioners to enhance their skilful consultation practice. It is more likely that the training will be effective in changing practitioner behaviour if they subsequently value the importance of the guiding approach and feel confident in applying the strategies and skills taught. Skills acquired in training need to be deployed in routine consultations with families. Within the trial there was evidence of high levels of initial exposure of practitioners to the training – good levels of attendance of workshops and, despite some logistical challenges, good engagement with the web-based training. The DEPICTED PE also provided evidence that trained practitioners do adopt a more guiding style when consulting with families about behaviour change, and there is further evidence of the use of some of the strategies in these consultations (particularly agenda-setting).
FIGURE 8.
Model of potential intervention pathway.
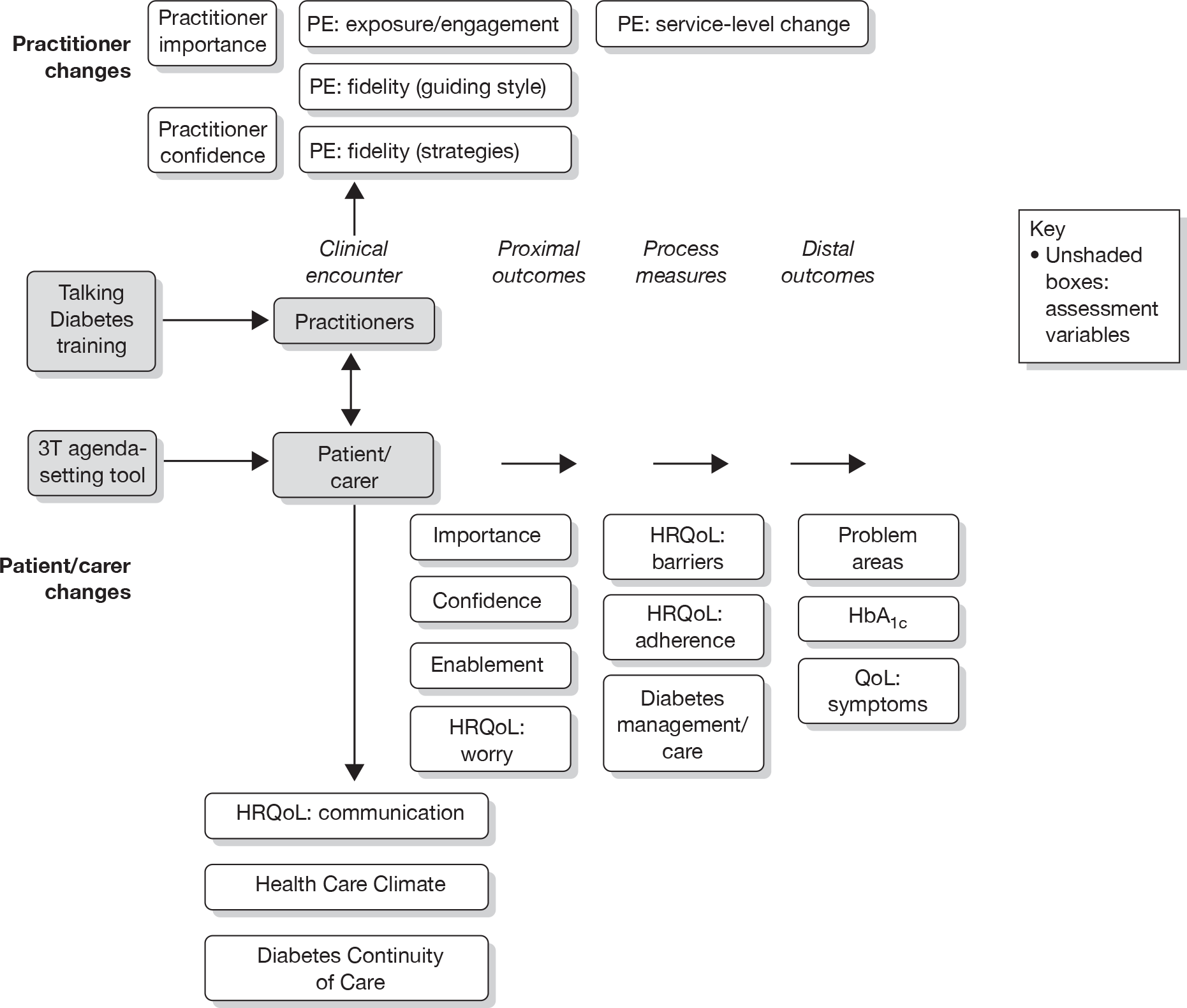
The impact of a trained practitioner can be conceived as ranging from proximal to distal. Among the former would be enablers for behaviour change, such as perceived importance of behaviour change and confidence in making such changes (e.g. self-efficacy). In practice, most patients and carers reported the value of change, even at baseline, so this was unlikely to be further modified by the intervention. At 12 months’ follow-up, confidence in managing diabetes was actually reduced for patients in the intervention arm, despite the increased ability to cope with diabetes as a consequence of the clinic visit reported in the short term (another proximal outcome). Physical outcomes for patients included HbA1c levels – the primary trial outcome – but which is clearly a distal outcome dependent upon several intervening steps. Similarly, the health-related QoL (HRQoL) subscale ‘symptoms’ reports experience of the effects of poorly controlled diabetes and is also a distal outcome. Evidence of the intervening processes leading to these outcomes is provided by the QoL subscales ‘barriers’ and ‘adherence’, and the diabetes ‘care/mismanagement scale’. These record the patients’ appraisal of tasks and behaviours that are associated with self-management. A change in psychosocial factors for patients (e.g. confidence, enablement) may be expected to lead to attempts at behavioural change that these three self-report measures would assess. However, there was no direct attempt to measure behaviour in DEPICTED, for example by recording diet, exercise, medication use or self-monitoring behaviour. Although trial evidence shows that control patients experienced reduced barriers and improved adherence is unexpected, it is consistent with their higher levels of confidence.
Other impacts of the intervention, but ones which may not be directly implicated in behaviour change or physical outcomes, are the patient’s and carer’s appraisal of the clinic team. Perhaps most surprising is the absence of a difference between patients in each study arm in their appraisal of the clinical teams. The HCCQ includes items that are directly relevant to the intervention (e.g. providing choices and options, encouraging question asking). The improvement in continuity of care for carers in the intervention arm may reflect an enhancement in team communication as a result of explicit agenda-setting and greater attempts to engage with and understand the patient and family. Using Figure 8 as a guide, there is evidence of a change in practitioner behaviour following exposure to training, and of an impact upon proximal patient and carer outcomes. There is some evidence of an impact on patients’ appraisal of their own self-management, although no direct evidence of behaviour change. There is no evidence that such changes then led to objective physical outcomes for patients which would be considered as distal outcomes given the training intervention being trialled.
Building on the model (Figure 8) and following the logic of the MRC guidance on complex interventions, it is advisable to interpret the results of this study within two domains. These are firstly, efforts to change the behaviour of practitioners (and the team as a whole) and secondly, their subsequent efforts to change the behaviour of patients (children and parents). The DEPICTED development process paid attention to both domains: the learning programme to the first, and 3T with guiding and useful strategies to the second. Figure 8 illustrates how these domains might be linked together in the treatment of paediatric diabetes.
The economic evaluation showed that, although training costs appear high, when considered as an investment, the cost per patient receiving care from a trained team is modest. Overall, costs were only marginally and not statistically significantly higher for the intervention group, even when taking account of training costs.
Change in practice behaviour
DEPICTED produced mixed results when it came to changes in practice behaviour among those in the intervention group. On the one hand, there was evidence of improved skilfulness from the process analyses and, on the other, there was some doubt about the ability and willingness of whole teams to embrace the shift in style, attitude and skills promoted in the learning programme. A lack of evidence for complex interventions of this kind within the paediatric diabetes field provided the rationale for this study. In contrast, there is quite a sizeable body of evidence about the value of adapting MI in health-care settings. 70,74 It appears that practitioners can learn this kind of complex skill set and produce good outcomes. What, then, might be the implications of the mixed findings from DEPICTED for future research and the training of practitioners in paediatric diabetes?
It might be tempting to allow the failure to produce positive primary outcome data to suggest that no further development of this kind is needed in paediatric diabetes. However, there were numerous data sets and findings within the DEPICTED study that pointed to the value of helping practitioners to engage more effectively with children and parents. It could be ill-advised to allow the main results from one study to downgrade the importance of providing staff with the training support to achieve better engagement and outcomes in their work. Rather, the findings of this trial suggest that limited training in this area, such as half- or 1-day courses, is not enough, and without ongoing supervision and skill development is likely to have very limited impact on patient outcomes. One positive message that may emerge from the trial is that practitioners need to invest time in developing these skills if the training is going to make a difference and that perhaps it is not for everybody.
At least two lines of research and development on practitioner training and behaviour change might be worth considering: first, more attention could be paid to learning programmes that encourage the whole team to initiate and maintain changes in their consulting. One lesson from this study was that other and more long-term learning devices and opportunities might indeed produce better results. A second line of development might be to fill the gap in this study in the listening skills development process that could produce better patient outcomes, a hypothesis strongly supported in the MI field. 74 Here, practitioners could be taught these skills early on in their careers and/or as they enter the paediatric field as matter of priority. Upon this foundation it should then be possible to help practitioners use the strategies embedded in the DEPICTED learning programme with greater frequency and hypothesised effectiveness.
Change in patient and family behaviour
Levels of HbA1c, the primary trial outcome, were established in the research commissioning brief because glycaemic control is crucial to the short- and long-term well-being of patients with type 1 diabetes. Effective glycaemic control is consequent upon several contributing factors, some of which are reflected in Figure 8 (model of potential intervention pathway). It is therefore important to focus attention on the secondary outcomes, given their potential position in the intervention pathway.
There is evidence from DEPICTED that, by comparison with those in the control arm, patients in the intervention arm may experience a reduction in their confidence to manage their diabetes. This finding could be interpreted as suggesting discomfort induced by a greater insight into the challenges of self-care, a necessary first step to then identifying ways of making positive changes. This is particularly understandable given the pre-existing and consistently high value placed on self-management by both patients and carers (‘importance’ score). The initial positive impact on self-efficacy for intervention patients is not inconsistent with this explanation. Patients feel better supported by their consultation and encouraged to consider change initially, but with the resulting dissonance (between current behaviour and personal goals) creating discomfort. Similar effects were seen in patients in an earlier pilot study of MI. 33 Given that the subsequent randomised controlled study of MI32 showed benefits on glycaemic control 2 years after the start of the intervention, DEPICTED may therefore have failed to follow up patients for long enough to demonstrate the effects of the change in clinicians’ counselling styles.
Conclusions
Summary points
This study has shown that a training package for HCPs working in paediatric diabetes services to help them counsel their patients more skilfully, particularly with respect to issues around behaviour change, failed to result in improvements in glycaemic control of patients 1 year after the training.
Given evidence of the improved skilfulness of practitioners, this finding most likely suggests that either the skill levels achieved were insufficient to impact on glycaemic control, unlike the outcomes of previous trials of MI, or there was insufficient contact between HCPs and patients to effect this change. These conclusions imply that investment of significantly greater resources in training and patient contact than those utilised within this trial may be required.
In the short term, patients in the intervention centres felt more able to cope with their diabetes following their clinic consultations but, later, they experienced reduced confidence in managing diabetes compared with the control group. By contrast, and surprisingly, patients in control centres reported fewer barriers to treatment and problems adhering to diabetes management at follow-up, perhaps suggesting less insight into the difficulties of their situation.
The developed learning programme for practitioners blended web-based training with face-to-face workshops. In piloting and in the main trial, training was well evaluated, with the workshops being particularly well received. However, the workshops added to the cost of the training with the requirement for two whole-day attendances for all team members. Nevertheless, costs were only marginally higher in the intervention group, even when taking into account training costs, and the difference was not statistically significant.
Practitioners can be trained to undertake agenda-setting and to use a guiding style of consultation in routine practice though other elements from the menu of strategies were less evident in practice. Maintaining skills of health-care professionals following training, such as in the DEPICTED trial, remains a particular challenge.
On the positive side, this study has demonstrated that a robust stakeholder engagement process in the design of a complex, pragmatic intervention, such as the DEPICTED study, is deliverable and can ensure that lay and professional opinions influence study designs.
Several key messages emerged from the developmental studies and are of importance for future psychosocial interventions. These include the challenge for practitioners to engage families in the process of care, the recognition of the difficulties of integrating diabetes into everyday life and the requirement to meet the needs of different patients, the need to alter the perception that clinics are for practitioner rather than patient benefit, the provision of a more realistic and positive approach which recognises children’s lifeworld and the need to engage children more in their own consultations. Health-care professionals recognise the importance of dealing with these issues but feel relatively underskilled in tackling them.
Implications for clinical services
Communication skills training
From the development phase surveys through to the early stages of recruiting clinics in the trial phase, there was a clear interest and perceived need for the training in communication skills developed in DEPICTED,95 consistent with the importance placed by regulatory bodies on effective communication underlying high-quality clinical care for children. 160 The PE demonstrated that it is possible to train practitioners, although some of the intervention strategies seemed more popular than others. The evidence of improving skills in eliciting the child’s agenda is important as, traditionally, clinicians are poor at involving children in triadic consultations161 and children resent being ‘left out’ of discussions about their medical care. 162 Workshop attendance was excellent, aided by the availability of alternative training dates. Some elements of the web-based methods seemed less popular, although practitioners generally felt that it had a high degree of clinical validity. Most practitioners completed the didactic web-based training, but only half completed one or more of the three planned web-based case studies following the training, and there was no engagement with web-based discussion thereafter. Some logistical problems contributed to this level of activity (e.g. trust firewalls hindering access to some or all web content).
Resource implications
The failure of the improved communication skills that health-care professionals demonstrated to impact on metabolic control may imply that additional training is required to both increase and reinforce these skills further. However, another impediment to such skills impacting positively on outcomes may have arisen from the limited contact that most children and their families would have had with their clinical services. Whereas most patients with established diabetes are seen by clinic staff three or four times annually, studies of successful interventions such as MI have involved typically four additional contacts per year, lasting 20–60 minutes each, to meet the therapist. 32 This represents significant extra contact time with a HCP and would require substantial investment in resources to reproduce for all patients attending paediatric diabetes clinical services.
Despite using largely convenient locations, the training seminars required two full days’ attendance which is a substantial resource issue. On the other hand, the web-based training required much less time and in theory offered the trainee greater flexibility, given the widespread availability of computers in the NHS with access to the World Wide Web. Although learning opportunities were blended rather than optional, further training will need to accommodate preferred ‘ways of learning’ as well as cost. A notable part of the unintended added value for practitioners of attending workshops was interacting with their own and other teams.
There is some evidence that practitioner skill levels and application deteriorated over time, and that ongoing follow-up after initial training is essential if practitioners are to maintain their skills. This is the component of the training programme that we felt could have been most improved and may have contributed to the lack of effectiveness of our intervention on outcomes. Additional top-up sessions were considered by the trainers and favoured by some trainees, as was individualised mentoring support from the training team. This would have further added to the cost of programme delivery and hence affected the feasibility of broader implementation. A few centres initiated in their own local mechanisms to sustain and support skilful practice, and such local ownership (with or without remote guidance) may be important for sustaining the longer-term take-up of programmes such as Talking Diabetes.
Future developments
DEPICTED has identified professional demand for communication skills training and provided an evidence base from which to develop. If a derivation of DEPICTED is found to be effective in the future, key considerations will be where responsibility lies for the provision and regulation (e.g. with NHS trusts or professional bodies such as the Royal Colleges or General Medical Council). It is worth reflecting that some trainees thought they already used the skills taught in the programme, and that many thought that professionally they should be practising in this manner, but that actually few demonstrated this in assessment. That pressure of time in clinic may dictate a directive approach that is considered less satisfactory is an important message and challenge for future researchers. In an age of increased interest in validation, development of appropriate forms of assessment such as those used to assess the recordings of consultations or similar to that published recently163 will be required. Consideration will also need to be given to parents and children participating in this process,164 as lay members of the SAG (see Chapter 6) demonstrated considerable interest in the process and expressed strong views about how clinicians’ communication skills could be improved. 165
Implications for future research
The discussion in this chapter alludes to a central issue that pervades much of the process and outcome research on MI. Specifically, evidence for effectiveness is widespread, although not uniformly positive in every setting, whereas process research points to the central value of using reflective listening to evoke change talk, which, in turn, predicts behaviour change. 166 Researchers in paediatric diabetes might respond to this by following the model of Channon and colleagues32 in using counsellors or psychologists to deliver MI-linked therapy, no doubt with selected patients. Alternatively, they might set out to train teams or team members in the requisite skills to deliver an intervention to the required standard, including reflective listening. In retrospect, the DEPICTED study tried to steer a path between these two options; this might be one reason for the results obtained. In the end, the intervention used was not MI as such, but a menu of guiding skills that did not include listening at its core.
Further work may explore the effectiveness and added value of incorporating reflective listening into the existing training package. A curriculum design consideration in DEPICTED was not to add so much training content that it became unfeasible to teach. However, there is some redundancy within the current training programme that would allow the addition of such content. Acquiring mastery and maintaining skills should also be a focus for further work. Many practitioners did not fully engage with activities following the second workshop, which were intended to support further learning and maintenance. Some practitioners and some teams demonstrated particular creativity and determination in trying to apply and retain their skills. How skills can be practised and maintained in routine clinical practice with its time and other constraints, in a cost-effective manner, can usefully be further explored. Finally, follow-up of HbA1c levels within the current trial cohort at 2 years may help to explore whether or not differences in psychosocial outcomes at 12 months resolve into subsequent differences in metabolic control. It is recognised that a straight group comparison will be confounded by subsequent training for control centre practitioners at the end of the trial, but exploring the longitudinal relationship between psychosocial and metabolic variables has value.
Acknowledgements
Contributions of authors
All of the following named authors contributed to the development of the research question and study design, study implementation (including membership of study management group), analysis and/or interpretation of data and submission of the final report. Contributions to individual study outputs/particular study contributions are denoted below in publication citations.
Professor John Gregory (Professor & Honorary Consultant in Paediatric Endocrinology) Co-principal investigator, lead applicant and study guarantor; led the development of research question and study design, study implementation and report submission; and contributor to stakeholder meetings and learning programme trainer.
Dr Mike Robling [Senior Lecturer, Associate Director, South East Wales Trials Unit (SEWTU)] Co-principal investigator, co-applicant and study guarantor; led the development of research question and study design, study implementation, report submission; chaired study management group; joint lead on learning programme development and process evaluation; and contributor to stakeholder meetings and learning programme trainer.
Dr Kristina Bennert (Research Associate) Qualitative lead responsible for co-ordinating stakeholder involvement, development and field testing of the intervention; and contributor to stakeholder meetings.
Dr Sue Channon (Consultant Clinical Psychologist) Co-applicant; contributed to intervention development; joint lead for process evaluation; and contributor to stakeholder meetings and learning programme trainer.
Professor David Cohen (Professor of Health Economics) Co-applicant; designed and led the economic evaluation; and contributor to stakeholder meetings.
Dr Elizabeth Crowne (Consultant in Paediatric Endocrinology and Diabetes) Co-applicant, contributor to stakeholder meetings and report submission.
Helen Hambly (Research Psychologist) Implemented surveys in the development phase; developed outcome measures for trial questionnaire and for assessing practitioner competencies; and trial data management and contributor to stakeholder meetings.
Dr Kamila Hawthorne (Reader, Primary Care & Public Health) Contributor to stakeholder meetings and trainer for learning programme.
Professor Kerenza Hood (Professor of Medical Statistics, Director of SEWTU) Co-applicant, lead on the quantitative elements and contributor to stakeholder meetings.
Mirella Longo (Research Fellow) Co-applicant, contributed to design of economic evaluation, lead for discrete choice experiment and contributor to stakeholder meetings.
Dr Lesley Lowes (Senior Lecturer/Practitioner (Paediatric Diabetes) Co-applicant, advised on stakeholder involvement, contributor to stakeholder meetings and learning programme trainer.
Dr Rachel McNamara (Senior Trial Manager) Led trial management, chaired project team meetings and contributor to stakeholder meetings.
Mr Tim Pickles (Junior Statistician) Data management programmer and statistician for performance assessment and other trial data.
Dr Rebecca Playle (Senior Statistician) Responsible for the design of the randomisation protocol and the statistical analysis of the primary and secondary trial outcomes.
Professor Stephen Rollnick (Professor of Healthcare Communication) Co-applicant, lead for intervention development and joint lead for learning programme development; joint lead for process evaluation; and contributor to stakeholder meetings and learning programme trainer.
Dr Emma Thomas-Jones (Trial Manager) Managed 12-month follow-up phase and led the service change assessment (design, conduct and analysis of interviews).
The report authors gratefully acknowledge the substantial contribution of the following in the conduct and delivery of the DEPICTED study:
Administrative, methodological and technical support Mrs Veronica Silva Dunning (trial administrator), Professor Chris Butler (co-applicant), Professor Ian Russell (co-applicant), Ms Helen Stanton, Mr Tim Harris, Mrs Cathy Lisles, Mr Neil Burt, Mr Fasih Alam, Mr Benedict Hodge, Karen Marsh and Nina Gobat (consultation raters), Mrs Carolyn Boston, and members of the administrative team at SEWTU.
Members of the Trial Steering Committee Professor Steve Greene (Chairperson), Dr Julie Edge, Professor Tim Peters and Professor Frank Snoek.
Participating clinical centres and support groups The local principal investigators, members of each clinical team and local UK Clinical Research Network research staff participating in the 26 trial centres, and to all the practitioners and families who contributed to the development and implementation of the Talking Diabetes intervention (particularly from clinics and support groups in south Wales and south-west England). The local principal investigators and participating NHS trusts were:
Mr Paul Langridge (Children’s Community Specialist Nurse) at Countess of Chester Hospital NHS Foundation Trust; Dr Tracy Tinklin (Consultant Paediatrician) at Derby Hospitals NHS Foundation Trust; Ms Francesca Annan (Specialist Paediatric Dietitian) at Royal Liverpool Children’s NHS Trust; Professor Timothy Barratt (Professor of Paediatrics) at Wellcome Trust Clinical Research Facility at Birmingham Children’s Hospital NHS Trust; Miss Caroline Spence (Paediatric Diabetes Specialist Nurse) at Brighton and Sussex University Hospitals NHS Trust; Dr Claire Smith (Consultant Paediatrician) at East Lancashire Hospitals NHS Trust; Dr Fiona Regan (Consultant Paediatrician) at The Hillingdon Hospital NHS Trust; Dr Rakesh Amin (Consultant Paediatric Endocrinologist) at Central Manchester & Manchester Children’s University Hospitals NHS Foundation Trust; Dr Mark Bone (Paediatric Diabetologist) at Central Manchester & Manchester Children’s University Hospitals NHS Trust; Dr Loretta Davis-Reynolds and Dr Sunil Bhimsaria (Consultant Paediatrician) at Barnsley Hospital NHS Foundation Trust; Ms Marie Marshall (Paediatric Diabetes Nurse) at Central Manchester & Manchester Children’s University Hospitals NHS Foundation Trust; Dr Shaun Gorman (Consultant Paediatrician) at Bradford Teaching Hospitals NHS Foundation Trust; Dr Neil Hopper (Consultant Paediatrician) at City Hospitals Sunderland NHS Foundation Trust; Dr Jo Mannion (Consultant Paediatrician) at York Hospitals NHS Trust; Dr Anuja Natarajan (Consultant Paediatrician) at Doncaster & Bassetlaw Hospitals NHS Foundation Trust; Dr Peter Stutchfield (Consultant Paediatrician) at Conway and Denbighshire NHS Trust; Dr Jerry Wales (Senior Lecturer in Paediatric Endocrinology) at Sheffield Children’s NHS Trust; Dr Fran Ackland (Consultant Paediatrician) at Northampton General Hospital NHS Trust; Dr Shailini Bahl (Consultant Paediatrician) at Ashford and St Peter’s Hospitals NHS Trust; Ms Sue Courtman (Diabetes Nurse) at East and North Hertfordshire NHS Trust; Dr Ivor Lewis (Consultant Paediatrician) at Surrey and Sussex Healthcare NHS Trust; Dr Ahmed Massoud (Consultant Paediatrician) at North West London Hospitals NHS Trust; Ms Roma Romano-Morgan (Paediatric Diabetes Nurse Specialist) at The Whittington Hospital NHS Trust; Dr Joanna Stanley (Consultant Paediatrician) at The Lewisham Hospital NHS Trust; Ms Victoria Surrell (Diabetes Specialist Nurse) at Hinchingbrooke Healthcare NHS Trust; Dr Tim Taylor (Consultant Paediatrician) at Royal West Sussex NHS Trust; Dr Nicola Trevelyan (Consultant Paediatrician) at Southampton University Hospitals NHS Trust; Dr Tabitha Randell (Consultant in Paediatric Endocrinology and Diabetes) at Nottingham University Hospitals NHS Trust; Dr Fiona Campbell (Clinical Director) at The Leeds Teaching Hospitals NHS Trust; and Dr Ruby Mathew (Consultant Paediatrician) at Chesterfield Royal Hospital NHS Foundation Trust.
Members of the SAG The young patients, parents, adult patients and diabetes professionals participating in the SAG who critically helped design the trial intervention. To Professor Neil Frude who acted as SAG facilitator.
User representative We particularly acknowledge the dedicated input of our parent and patient representative Mrs Charlotte Crawley (co-applicant) in particular during the development phase of the study.
Other contributors to programme development Students and staff at Whitchurch High School (Drama Club), escape to Design (3T: TimeToTalk), the HealthCare Learning Company Ltd (Talking Diabetes online learning programme, in particular Pat Cannon and Valerie Dougall), Philippa Thomas and Dafydd Gape (learning programme actors).
Institutional support We acknowledge with thanks the trial funders, the UK National Institute for Health Research Health Technology Assessment programme (NIHR HTA), and the financial contribution of Novo Nordisk who provided partial funding for the production of some study materials. SEWTU is funded by the Wales Assembly Government through the National Institute of Social Care & Health Research and the authors gratefully acknowledge SEWTU’s contribution to study implementation.
We thank all those not otherwise mentioned above who have contributed to the DEPICTED study.
Publications
Hambly H, Robling M, Crowne E; Hood, K; Gregory, JW Communication skills of health-care professionals in paediatric diabetic services. Diabetic Med 2009;26: 502–9.
Channon S, Hambly H, Robling M, Bennert K, Gregory JW. Meeting the psychosocial needs of children with diabetes within routine clinical practice. Diabetic Med 2010;10:1209–11.
Lowes L, Robling MR, Bennert K, Crawley C, Hambly H, Hawthorne K, et al. Involving lay and professional stakeholders in the development of a research intervention for the DEPICTED Study. Health Expect 2010. doi: 10.1111/j.1369-7625.2010.00625.x. [Epub ahead of print.]
McNamara R, Robling M, Hood K, Bennert K, Channon S, Cohen D, et al. (DEPICTED): a protocol for a cluster randomised controlled trial of the effectiveness of a communication skills training programme for healthcare professionals working with young people with type 1 diabetes. BMC Health Serv Res 2010;10:36.
Hawthorne K, Bennert K, Lowes L, Channon S, Robling M, Gregory JW; on behalf of the DEPICTED Study team. The experiences of children and their parents in paediatric diabetes services should inform the development of communication skills for healthcare staff (the DEPICTED Study). Diabet Med 2011. doi: 10.1111/j.1464-5491.2011.03292.x. [Epub ahead of print.]
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- Hsia Y, Neubert AC, Rani F, Viner RM, Hindmarsh PC, Wong IC. An increase in the prevalence of type 1 and 2 diabetes in children and adolescents: results from prescription data from a UK general practice database. Br J Clin Pharmacol 2009;67:242-9.
- Patterson CC, Dahlquist GG, Gyurus E, Green A, Soltesz G. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet 2009;373:2027-33.
- Amin R, Widmer B, Prevost AT, Schwarze P, Cooper J, Edge J, et al. Risk of microalbuminuria and progression to macroalbuminuria in a cohort with childhood onset type 1 diabetes: prospective observational study. BMJ 2008;336:697-701.
- Matyka KA, Wigg L, Pramming S, Stores G, Dunger DB. Cognitive function and mood after profound nocturnal hypoglycaemia in prepubertal children with conventional insulin treatment for diabetes. Arch Dis Child 1999;81:138-42.
- Evans CL, Hughes IA. The relationship between diabetic control and individual and family characteristics. J Psychosom Res 1987;31:367-74.
- Goldston DB, Kelley AE, Reboussin DM, Daniel SS, Smith JA, Schwartz RP, et al. Suicidal ideation and behavior and noncompliance with the medical regimen among diabetic adolescents. J Am Acad Child Adolesc Psychiatry 1997;36:1528-36.
- Lowes L, Lyne P, Gregory JW. Childhood diabetes: parents’ experience of home management and the first year following diagnosis. Diabet Med 2004;21:531-8.
- Jacobson AM. The psychological care of patients with insulin-dependent diabetes mellitus. N Engl J Med 1996;334:1249-53.
- Blanz BJ, Rensch-Riemann BS, Fritz-Sigmund DI, Schmidt MH. IDDM is a risk factor for adolescent psychiatric disorders. Diabetes Care 1993;16:1579-87.
- Kovacs M, Goldston D, Obrosky DS, Bonar LK. Psychiatric disorders in youths with IDDM: rates and risk factors. Diabetes Care 1997;20:36-44.
- La Greca A, Follansbee D, Skyler J. Developmental and behavioral aspects of diabetes management in youngsters. J Child Health Care 1990;19:132-9.
- Scottish Study Group for the Care of the Young Diabetic . Factors influencing glycemic control in young people with type 1 diabetes in Scotland: a population-based study (DIABAUD2). Diabetes Care 2001;24:239-44.
- Amiel SA, Sherwin RS, Simonson DC, Lauritano AA, Tamborlane WV. Impaired insulin action in puberty. A contributing factor to poor glycemic control in adolescents with diabetes. N Engl J Med 1986;315:215-19.
- Waldron S, Hanas R, Palmvig B. How do we educate young people to balance carbohydrate intake with adjustments of insulin?. Horm Res 2002;57:S62-5.
- Allen D, Gregory J. The transition from children’s to adult diabetes services: understanding the ‘problem’. Diabet Med 2009;26:162-6.
- National Collaborating Centre for Women’s and Children’s Health . Type 1 Diabetes: Diagnosis and Management of Type 1 Diabetes in Children and Young People 2004.
- Diabetes UK. The National Paediatric Diabetes Audit: results from the audit year 2002. Diabetes UK; 2004.
- The Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977-86.
- Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group . Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA 2003;290:2159-67.
- Nathan D, Cleary P, Backlund J, Genuth S, Lachin J, Orchard T, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643-53.
- Martin C, Albers J, Herman W, Cleary P, Waberski B, Greene D, et al. Neuropathy among the diabetes control and complications trial cohort 8 years after trial completion. Diabetes Care 2006;29:340-4.
- Butler C, Rollnick S, Stott N. The practitioner, the patient and resistance to change: recent ideas on compliance. CMAJ 1996;154:1357-62.
- Marteau TM, Lerman C. Genetic risk and behavioural change. BMJ 2001;322:1056-9.
- Jepson R. The Effectiveness of Interventions to Change Health-Related Behaviours: A Review of Reviews 2000.
- Miller W, Andrews N, Wilbourne P, Bennett M, Miller W, Heather N. Treating addictive behaviours. New York, NY: Wiley; 1998.
- Kok G, van den Borne B, Mullen PD. Effectiveness of health education and health promotion: meta-analyses of effect studies and determinants of effectiveness. Patient Educ Couns 1997;30:19-27.
- Miller WR, Rollnick S. Motivational interviewing: preparing people to change addictive behaviour. New York, NY: Guildford Press; 1991.
- Amrhein PC, Miller WR, Yahne CE, Palmer M, Fulcher L. Client commitment language during motivational interviewing predicts drug use outcomes. J Consult Clin Psychol 2003;71:862-78.
- Burke BL, Arkowitz H, Menchola M. The efficacy of motivational interviewing: a meta-analysis of controlled clinical trials. J Consult Clin Psychol 2003;71:843-61.
- Knight KM, Bundy C, Morris R, Higgs JF, Jameson RA, Unsworth P, et al. The effects of group motivational interviewing and externalizing conversations for adolescents with Type-1 diabetes. Psychol Health Med 2003;8:149-57.
- Viner RM, Christie D, Taylor V, Hey S. Motivational/solution-focused intervention improves HbA1c in adolescents with type 1 diabetes: a pilot study. Diabet Med 2003;20:739-42.
- Channon SJ, Huws-Thomas MV, Rollnick S, Hood K, Cannings-John RL, Rogers C, et al. A multicenter randomized controlled trial of motivational interviewing in teenagers with diabetes. Diabetes Care 2007;30:1390-5.
- Channon S, Smith VJ, Gregory JW. A pilot study of motivational interviewing in adolescents with diabetes. Arch Dis Child 2003;88:680-3.
- Butler C, Rollnick S, Cohen D, Bachmann M, Stott NC. Motivational consulting versus brief advice for smokers in general practice: a randomized trial. Br J Gen Pract 1999;49:611-16.
- Rollnick S. Consultations about behaviour change. Medicine 2000:28-6.
- Westra HA, Arkowitz H, Dozois DJ. Adding a motivational interviewing pretreatment to cognitive behavioral therapy for generalized anxiety disorder: a preliminary randomized controlled trial. J Anxiety Disord 2009;23:1106-17.
- Hampson SE, Skinner TC, Hart J, Storey L, Gage H, Foxcroft D, et al. Effects of educational and psychosocial interventions for adolescents with diabetes mellitus: a systematic review. Health Technol Assess 2001;5.
- Pill R, Stott NC, Rollnick SR, Rees M. A randomized controlled trial of an intervention designed to improve the care given in general practice to Type II diabetic patients: patient outcomes and professional ability to change behaviour. Fam Pract 1998;15:229-35.
- Pill R, Rees ME, Stott NC, Rollnick SR. Can nurses learn to let go? issues arising from an intervention designed to improve patients’ involvement in their own care. J Adv Nurs 1999;29:1492-9.
- Satin W, La Greca AM, Zigo MA, Skyler JS. Diabetes in adolescence: effects of multifamily group intervention and parent simulation of diabetes. J Pediatr Psychol 1989;14:259-75.
- Jefferson IG, Swift PG, Skinner TC, Hood GK. Diabetes services in the UK: third national survey confirms continuing deficiencies. Arch Dis Child 2003;88:53-6.
- Rollnick S, Butler CC, McCambridge J, Kinnersley P, Elwyn G, Resnicow K. Consultations about changing behaviour. BMJ 2005;331:961-3.
- Kinmouth AL, Woodcock A, Griffin S, Spiegal N, Campbell MK. Randomised controlled trial of patient centred care of diabetes in general practice: impact on current well-being and future disease risk. BMJ 1998;317:1202-8.
- Rollnick S, Mason P, Butler C. Health behaviour change: a guide for practitioners. Edinburgh: Churchill Livingstone; 1999.
- Mattson M, Miller W, Heather N. Treating addictive behaviours. New York, NY: Wiley; 1998.
- Heather N, Rollnick S, Bell A, Richmond R. Effects of brief counselling among male heavy drinkers identified on general hospital wards. Drug Alcohol Rev 1996;15:29-38.
- Rollnick S, Miller W, Heather N. Treating addictive behaviours. New York, NY: Plenum; 1998.
- Rollnick S, Heather N, Bell A. Negotiating behaviour change in medical settings: the development of brief motivational interviewing. J Ment Health 1992;1:25-39.
- McCambridge J, Strang J. The efficacy of single-session motivational interviewing in reducing drug consumption and perceptions of drug-related risk and harm among young people: results from a multi-site cluster randomized trial. Addiction 2004;99:39-52.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337.
- Butler CC, Rollnick S, Pill R, Maggs-Rapport F, Stott N. Understanding the culture of prescribing: qualitative study of general practitioners’ and patients’ perceptions of antibiotics for sore throats. BMJ 1998;317:637-42.
- Rollnick S, Seale C, Rees M, Butler C, Kinnersley P, Anderson L. Inside the routine general practice consultation: an observational study of consultations for sore throats. Fam Pract 2001;18:506-10.
- Butler CC, Pill R, Stott NC. Qualitative study of patients’ perceptions of doctors’ advice to quit smoking: implications for opportunistic health promotion. BMJ 1998;316:1878-81.
- Rollnick S, Butler CC, Stott N. Helping smokers make decisions: the enhancement of brief intervention for general medical practice. Patient Educ Couns 1997;31:191-203.
- Rollnick S, Seale C, Kinnersley P, Rees M, Butler C, Hood K. Developing a new line of patter: can doctors change their consultations for sore throat?. Med Educ 2002;36:678-81.
- Grimshaw JM, Shirran L, Thomas R, Mowatt G, Fraser C, Bero L, et al. Changing provider behavior: an overview of systematic reviews of interventions. Med Care 2001;39:II2-45.
- Miller W, Rollnick S. Motivational interviewing: preparing people for change. New York, NY: The Guilford Press; 2002.
- Elwyn G, Edwards A, Wensing M, Hood K, Atwell C, Grol R. Shared decision making: developing the OPTION scale for measuring patient involvement. Qual Saf Health Care 2003;12:93-9.
- Lane C, Johnson S, Rollnick S, Edwards K, Lyons M. Consulting about lifestyle change: evaluation of a training course for specialist diabetes nurses. Prac Diab Int 2003;20:204-8.
- Lane C, Huws-Thomas M, Hood K, Rollnick S, Edwards K, Robling M. Measuring adaptations of motivational interviewing: the development and validation of the behavior change counseling index (BECCI). Patient Educ Couns 2005;56:166-73.
- Clark M, Hampson SE. Implementing a psychological intervention to improve lifestyle self-management in patients with type 2 diabetes. Patient Educ Couns 2001;42:247-56.
- Northam EA, Todd S, Cameron FJ. Interventions to promote optimal health outcomes in children with Type 1 diabetes: are they effective?. Diabet Med 2006;23:113-21.
- Winkley K, Ismail K, Landau S, Eisler I. Psychological interventions to improve glycaemic control in patients with type 1 diabetes: systematic review and meta-analysis of randomised controlled trials. BMJ 2006;333.
- Murphy HR, Rayman G, Skinner TC. Psycho-educational interventions for children and young people with Type 1 diabetes. Diabet Med 2006;23:935-43.
- Wysocki T, Harris MA, Greco P, Bubb J, Danda CE, Harvey LM, et al. Randomized, controlled trial of behavior therapy for families of adolescents with insulin-dependent diabetes mellitus. J Pediatr Psychol 2000;25:23-3.
- Wysocki T, Greco P, Harris MA, Bubb J, White NH. Behavior therapy for families of adolescents with diabetes: maintenance of treatment effects. Diabetes Care 2001;24:441-6.
- Franklin V, Waller A, Pagliari C, Greene S. Sweet Talk: text messaging support for intensive insulin therapy for young people with diabetes. Diabetes Technol Ther 2003;5:991-6.
- Miller WR, Benefield RG, Tonigan JS. Enhancing motivation for change in problem drinking: a controlled comparison of two therapist styles. J Consult Clin Psychol 1993;61:455-61.
- Miller WR, Baca LM. Two-year follow-up of bibliotherapy and therapist-directed. Behav Ther 1983;14:441-8.
- Miller WR, Rose GS. Toward a theory of motivational interviewing. Am Psychol 2009;64:527-37.
- Dunn C, Deroo L, Rivara FP. The use of brief interventions adapted from motivational interviewing across behavioral domains: a systematic review. Addiction 2001;96:1725-42.
- Burke B, Arkowitz H, Dunn C, Miller W, Rollnick S. Motivational interviewing: preparing people for change. New York, NY: The Guilford Press; 2002.
- Britt E, Hudson SM, Blampied NM. Motivational interviewing in health settings: a review. Patient Educ Couns 2004;53:147-55.
- Rubak S, Sandbaek A, Lauritzen T, Christensen B. Motivational interviewing: a systematic review and meta-analysis. Br J Gen Pract 2005;55:305-12.
- Hettema J, Steele J, Miller WR. Motivational interviewing. Annu Rev Clin Psychol 2005;1:91-111.
- Smith DE, Heckemeyer CM, Kratt PP, Mason DA. Motivational interviewing to improve adherence to a behavioral weight-control program for older obese women with NIDDM. A pilot study. Diabetes Care 1997;20:52-4.
- Trigwell P, Grant PJ, House A. Motivation and glycemic control in diabetes mellitus. J Psychosom Res 1997;43:307-15.
- CMA Medical Data . Directory of Diabetes Care 2005.
- Fallowfield L, Jenkins V, Farewell V, Saul J, Duffy A, Eves R. Efficacy of a Cancer Research UK communication skills training model for oncologists: a randomised controlled trial. Lancet 2002;359:650-6.
- Eastlaugh A, Higginson I, Webb D. Palliative care communication workshops for general practitioners and district nurses. Educ Gen Pract 1998;9:331-6.
- Story MT, Neumark-Stzainer DR, Sherwood NE, Holt K, Sofka D, Trowbridge FL, et al. Management of child and adolescent obesity: attitudes, barriers, skills, and training needs among health care professionals. Pediatrics 2002;110:210-14.
- Ammentorp J, Sabroe S, Kofoed PE, Mainz J. The effect of training in communication skills on medical doctors’ and nurses’ self-efficacy. A randomized controlled trial. Patient Educ Couns 2007;66:270-7.
- Cegala DJ, Lenzmeier Broz S. Physician communication skills training: a review of theoretical backgrounds, objectives and skills. Med Educ 2002;36:1004-16.
- Hulsman RL, Ros WJ, Winnubst JA, Bensing JM. Teaching clinically experienced physicians communication skills. A review of evaluation studies. Med Educ 1999;33:655-68.
- Hulsman RL, Ros WJ, Winnubst JA, Bensing JM. The effectiveness of a computer-assisted instruction programme on communication skills of medical specialists in oncology. Med Educ 2002;36:125-34.
- Docherty A, Sandhu H. Student –perceived barriers and facilitators to e-learning in continuing professional development in primary care. Educ Prim Care 2006;17:343-53.
- Wharrad HJ, Cook E, Poussa C. Putting post-registration nursing students on-line: important lessons learned. Nurse Educ Today 2005;25:263-71.
- Campbell MK, Fayers PM, Grimshaw JM. Determinants of the intracluster correlation coefficient in cluster randomized trials: the case of implementation research. Clin Trials 2005;2:99-107.
- Beresford BA, Sloper P. Chronically ill adolescents’ experiences of communicating with doctors: a qualitative study. J Adolesc Health 2003;33:172-9.
- Innove . How PCTs Are Implementing the Diabetes NSF: Findings from DiabetesE, First National Report 2006.
- Nunnally J. Psychometric theory. New York, NY: McGraw-Hill; 1978.
- Heary CM, Hennessy E. The use of focus group interviews in pediatric health care research. J Pediatr Psychol 2002;27:47-5.
- Cote-Arsenault D, Morrison-Beedy D. Maintaining your focus in focus groups: avoiding common mistakes. Res Nurs Health 2005;28:172-9.
- Newbould J, Smith F, Francis SA. ‘I’m fine doing it on my own’: partnerships between young people and their parents in the management of medication for asthma and diabetes. J Child Health Care 2008;12:116-28.
- Hambly H, Robling M, Crowne E, Hood K, Gregory JW. Communication skills of healthcare professionals in paediatric diabetes services. Diabet Med 2009;26:502-9.
- Barry CA, Stevenson FA, Britten N, Barber N, Bradley CP. Giving voice to the lifeworld. More humane, more effective medical care? A qualitative study of doctor–patient communication in general practice. Soc Sci Med 2001;53:487-505.
- Alderson P, Sutcliffe K, Curtis K. Children as partners with adults in their medical care. Arch Dis Child 2006;91:300-3.
- Oliver S, Clarke-Jones L, Rees R, Milne R, Buchanan P, Gabbay J, et al. Involving consumers in research and development agenda setting for the NHS: developing an evidence-based approach. Health Technol Assess 2004;8.
- Buckley B, Grant A, Firkins L, Greene A, Frankau J. Working together to identify research questions. Continence 2007;1:76-81.
- McNamara R, Robling M, Hood K, Bennert K, Channon S, Cohen D, et al. Development and Evaluation of a Psychosocial Intervention for Children and Teenagers Experiencing Diabetes (DEPICTED): a protocol for a cluster randomised controlled trial of the effectiveness of a communication skills training programme for healthcare professionals working with young people with type 1 diabetes. BMC Health Serv Res 2010;10.
- NIHR Health Technology Assessment programme . Planning Patient and Public Involvement in HTA n.d. www.hta.ac.uk/PPIguidance/index.shtml (accessed 28 July 2011).
- TwoCan Associates . An Evaluation of the Process and Impact of Patient and Public Involvement in the Advisory Groups of the UK Clinical Research Collaboration 2009.
- Kirby P. A guide to actively involving young people in research: for researchers, research commissioners, and managers. Eastleigh: INVOLVE; 2004.
- Entwistle VA, Renfrew MJ, Yearley S, Forrester J, Lamont T. Lay perspectives: advantages for health research. BMJ 1998;316:463-6.
- Hanley B, Truesdale A, King A, Elbourne D, Chalmers I. Involving consumers in designing, conducting, and interpreting randomised controlled trials: questionnaire survey. BMJ 2001;322:519-23.
- Chambers R, O’Brien L, Linnell S, Sharp S. Why don’t health researchers report consumer involvement?. Qual Prim Care 2004;12:151-7.
- Telford R, Faulkner A. Learning about service user involvement in mental health research. J Ment Health 2004;13:549-59.
- Barber R, Boote JD, Cooper CL. Involving consumers successfully in NHS research: a national survey. Health Expect 2007;10:380-91.
- Hanley B. Research As Empowerment? Report of a Series of Seminars Organised by the Toronto Group 2005.
- Minogue V, Boness J, Brown A, Girdlestone J. The impact of service user involvement in research. Int J Health Care Qual Assur Inc Leadersh Health Serv 2005;18:103-12.
- Boote J, Telford R, Cooper C. Consumer involvement in health research: a review and research agenda. Health Policy 2002;61:213-36.
- Staniszewska S, Herron-Marx S, Mockford C. Measuring the impact of patient and public involvement: the need for an evidence base. Int J Qual Health Care 2008;20:373-4.
- Lindenmeyer A, Hearnshaw H, Sturt J, Ormerod R, Aitchison G. Assessment of the benefits of user involvement in health research from the Warwick Diabetes Care Research User Group: a qualitative case study. Health Expect 2007;10:268-77.
- Jones J, Hunter D. Consensus methods for medical and health services research. BMJ 1995;311:376-80.
- Faulkner A, Thomas P. User-led research and evidence-based medicine. Br J Psychiatry 2002;180:1-3.
- Wright D, Corner J, Hopkinson J, Foster C. Listening to the views of people affected by cancer about cancer research: an example of participatory research in setting the cancer research agenda. Health Expect 2006;9:3-12.
- Steel R. A guide to paying members of the public who are actively involved in research: for researchers and research commissioners (who may also be people who use services). Eastleigh: INVOLVE; 2003.
- Lowes L, Hulatt I. Involving service users in health and social care research. London: Routledge; 2005.
- Trivedi P, Wykes T. From passive subjects to equal partners: qualitative review of user involvement in research. Br J Psychiatry 2002;181:468-72.
- Medical Research Council . Developing and Evaluating Complex Interventions: New Guidance 2008.
- Campbell M, Fitzpatrick R, Haines A, Kinmonth AL, Sandercock P, Spiegelhalter D, et al. Framework for design and evaluation of complex interventions to improve health. BMJ 2000;321:694-6.
- Medical Research Council . A Framework for the Development and Evaluation of RCTs for Complex Interventions to Improve Health 2000.
- Campbell MK, Elbourne DR, Altman DG. CONSORT statement: extension to cluster randomised trials. BMJ 2004;328:702-8.
- Carter BR, Hood K. Balance algorithm for cluster randomized trials. BMC Med Res Methodol 2008;8.
- Eiser C, Morse R. Quality-of-life measures in chronic diseases of childhood. Health Technol Assess 2001;5.
- Varni JW, Burwinkle TM, Jacobs JR, Gottschalk M, Kaufman F, Jones K. Reliability and validity of the Pediatric Quality of Life Inventory Generic Score Scales and Type 1 Diabetes Module. Diabetes Care 2003;18:631-7.
- Polonsky WH, Anderson BJ, Lohrer PA, Welch G, Jacobson AM, Aponte JE, et al. Assessment of diabetes-related distress. Diabetes Care 1995;18:754-60.
- Weissberg-Benchell J, Glasgow AM, Tynan WD, Wirtz P, Turek J, Ward J. Adolescent diabetes management and mismanagement. Diabetes Care 1995;18:77-82.
- Howie JG, Heaney DJ, Maxwell M, Walker JJ. A comparison of a Patient Enablement Instrument (PEI) against two established satisfaction scales as an outcome measure of primary care consultations. Fam Pract 1998;15:165-71.
- Williams GC, Freedman ZR, Deci EL. Supporting autonomy to motivate patients with diabetes for glucose control. Diabetes Care 1998;21:1644-51.
- Dolovich LR, Nair KM, Ciliska DK, Lee HN, Birch S, Gafni A, et al. The Diabetes Continuity of Care Scale: the development and initial evaluation of a questionnaire that measures continuity of care from the patient perspective. Health Soc Care Community 2004;12:475-87.
- Ryan M, Gerard K, Amaya-Amaya M. Using discrete choice experiments to value health and health care. Dordrecht: Springer; 2008.
- Rabb G, Butcher I. Balance in cluster randomized trials. Stat Med 2001;20:351-65.
- Puffer S, Torgerson D, Watson J. Evidence for risk of bias in cluster randomised trials: review of recent trials published in three general medical journals. BMJ 2003;327:785-9.
- Garratt AM, Schmidt L, Fitzpatrick R. Patient-assessed health outcome measures for diabetes: a structured review. Diabet Med 2002;19:1-11.
- Curtis L. Unit costs of health and social care. Canterbury: Personal Social Services Research Unit, University of Kent; 2008.
- Efron B, Tibshirani RJ. An introduction to the bootstrap. London: Chapman & Hall; 1993.
- Briggs AH, O’Brien BJ. The death of cost-minimization analysis?. Health Econ 2001;10:179-84.
- Dakin H, Wordsworth S. CMA or CEA for Non-Inferiority Trials and Those Observing No Significant Difference in Efficacy? n.d.
- Bachmann MO, Fairall L, Clark A, Mugford M. Methods for analyzing cost effectiveness data from cluster randomized trials. Cost Eff Resour Alloc 2007;5.
- Ryan M, Gerard K. Using discrete choice experiments to value health care programmes: current practice and future research reflections. Appl Health Econ Health Policy 2003;2:55-64.
- DeShazo JR, Fermo G. Designing choice sets for stated preference methods: the effects of complexity on choice consistency. Journal of Environmental Economics and Management 2002;44:123-43.
- Presser S, Rothgeb JM, Couper MP, Lessler JT, Martin E, Martin J, et al. Methods for testing and evaluating survey questionnaires. Oxford: Wiley; 2004.
- Gibson C, Nelson K. Obtaining adolescents’ views about inpatient facilities using conjoint analysis. Paediatr Nurs 2009;21:34-7.
- Espelid I, Cairns J, Askildsen JE, Qvist V, Gaarden T, Tveit AB. Preferences over dental restorative materials among young patients and dental professionals. Eur J Oral Sci 2006;114:15-21.
- Gidman W, Elliott R, Payne K, Meakin GH, Moore J. A comparison of parents and pediatric anesthesiologists’ preferences for attributes of child daycase surgery: a discrete choice experiment. Paediatr Anaesth 2007;17:1043-52.
- Lancsar E, Louviere J. Conducting discrete choice experiments to inform healthcare decision making: a user’s guide. Pharmacoeconomics 2008;26:661-77.
- Street D, Burgess L. The construction of optimal stated choice experiments: theory and methods. Oxford: Wiley; 2007.
- Aristides M, Weston AR, FitzGerald P, Le Reun C, Maniadakis N. Patient preference and willingness-to-pay for Humalog Mix25 relative to Humulin 30/70: a multicountry application of a discrete choice experiment. Value Health 2004;7:442-54.
- Chancellor J, Aballea S, Lawrence A, Sheldon R, Cure S, Plun-Favreau J, et al. Preferences of patients with diabetes mellitus for inhaled versus injectable insulin regimens. Pharmacoeconomics 2008;26:217-34.
- Hauber AB, Mohamed AF, Johnson FR, Falvey H. Treatment preferences and medication adherence of people with Type 2 diabetes using oral glucose-lowering agents. Diabet Med 2009;26:416-24.
- Chen TT, Chung KP, Huang HC, Man LN, Lai MS. Using discrete choice experiment to elicit doctors’ preferences for the report card design of diabetes care in Taiwan – a pilot study. J Eval Clin Pract 2010;16:14-20.
- Guimaraes C, Marra CA, Colley L, Gill S, Simpson SH, Meneilly GS, et al. A valuation of patients’ willingness-to-pay for insulin delivery in diabetes. Int J Technol Assess Health Care 2009;25:359-66.
- Guimaraes C, Marra CA, Colley L, Gill S, Simpson S, Meneilly G, et al. Socioeconomic differences in preferences and willingness-to-pay for insulin delivery systems in type 1 and type 2 diabetes. Diabetes Technol Ther 2009;11:567-73.
- Moyers TB, Martin T, Catley D, Harris KJ, Ahluwalia JS. Assessing the integrity of motivational interventions: Reliability of the Motivational Interviewing Skills Code. Behavioural and Cognitive Psychotherapy 2003;31:177-84.
- Zwarenstein M, Treweek S, Gagnier JJ, Altman DG, Tunis S, Haynes B, et al. Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ 2008;337.
- Anderson BJ, Brackett J, Ho J, Laffel LM. An office-based intervention to maintain parent-adolescent teamwork in diabetes management. Impact on parent involvement, family conflict, and subsequent glycemic control. Diabetes Care 1999;22:713-21.
- Laffel LM, Connell A, Vangsness L, Goebel-Fabbri A, Mansfield A, Anderson BJ. General quality of life in youth with type 1 diabetes: relationship to patient management and diabetes-specific family conflict. Diabetes Care 2003;26:3067-73.
- de Wit M, van de Waal HA, Pouwer F, Gemke RJ, Snoek FJ. Monitoring health related quality of life in adolescents with diabetes: a review of measures. Arch Dis Child 2007;92:434-9.
- General Medical Council . 0–18 Years: Guidance for All Doctors 2007:8-9.
- Pantell RH, Stewart TJ, Dias JK, Wells P, Ross AW. Physician communication with children and parents. Pediatrics 1982;70:396-402.
- Lewis C, Knopf D, Chastain-Lorber K, Ablin A, Zoger S, Matthay K, et al. Patient, parent, and physician perspectives on pediatric oncology rounds. J Pediatr 1988;112:378-84.
- Howells RJ, Davies HA, Silverman JD, Archer JC, Mellon AF. Assessment of doctors’ consultation skills in the paediatric setting: the Paediatric Consultation Assessment Tool. Arch Dis Child 2010;95:323-9.
- Crossley J, Eiser C, Davies HA. Children and their parents assessing the doctor–patient interaction: a rating system for doctors’ communication skills. Med Educ 2005;39:820-8.
- Lowes L, Robling MR, Bennert K, Crawley C, Hambly H, Hawthorne K, et al. Involving lay and professional stakeholders in the development of a research intervention for the DEPICTED Study. Health Expect 2010. 10.1111/j.1369-7625.2010.00625.x.
- Moyers TB, Martin T, Christopher PJ, Houck JM, Tonigan JS, Amrhein PC. Client language as a mediator of motivational interviewing efficacy: where is the evidence?. Alcohol Clin Exp Res 2007;31:S40-7.
- Patton MQ. Qualitative research and evaluation methods. London: Sage Publications; 2001.
- Tourangeau R, Rips LJ, Rasinski K. The psychology of survey response. Cambridge: Cambridge University Press; 2007.
- Collins D. Pretesting survey instruments: an overview of cognitive methods. Qual Life Res 2003;12:229-38.
Appendix 1 Letter of favourable ethical opinion from the Thames Valley Research Ethics Committee
Appendix 2 Unit costs
Contacts with diabetes team
| Contact | Unit cost (£) | Source |
|---|---|---|
| Clinic visit | 167.85 |
157.00 paediatric face to face (follow-up attendance) uplifteda |
| Doctor | ||
| Home | 46.80 |
Client contact hourly rates: senior house officer, £38; registrar, £51 and consultant, £166. Length of contact. b Ratio home visit: 60% consultant and 40% registrar (23.4 minutes). Ratio clinic contact: 50% consultant, 40% registrar and 10% senior house officer (11.7 minutes). Ratio e-mail/phone/text: 40% consultant, 50% registrar and 10% senior house officer (7.1 minutes) |
| Clinic | 20.90 | |
| E-mail, telephone, text | 11.32 | |
| Nurse | ||
| Home visit | 34.32 |
Community-based specialist nurse: client contact hourly rate £88. Length of contactb |
| Clinic visit | 17.16 | |
| E-mail, telephone, text | 10.41 | |
| Dietitian | ||
| Home visit | 23.01 |
Length of contact. b Home visit: 23.4 minutes at £59/hour. Clinic visit: 11.7 minutes at £33/hour. E-mail/telephone/text: 7.1 minutes at £34/hour |
| Clinic visit | 6.44 | |
| E-mail, telephone, text | 4.02 | |
Investigations and appointments
| Investigation/consultation | Unit cost (£) | Source |
|---|---|---|
| HbA1c | 14.99 | Khunti (2006). 2003 prices £12 uplifteda |
| Total cholesterol | 4.88 | Marks and colleagues (2002). £3.77 uplifteda |
| High-density lipoprotein cholesterol | 5.10 | Marks and colleagues (2002). £3.94 uplifteda |
| Triglycerides | 5.32 | Marks and colleagues. £4.11 uplifteda |
| Lipid profile | 15.29 | Marks and colleagues (2002). £11.82 uplifteda |
| Coeliac or thyroid test | 5.32 | As triglycerides |
| Urine test for microalbuminuria | 4.88 | As total cholesterol |
| Anti-EMA test | 13.59 | Holmes (2001). £10, approximately, uplifteda |
| Anti-tTG test | ||
| AGA IgA or AGA IgG | 13.06 | Dretzke and colleagues (2004). £11 uplifted.a Includes additional £4/test cost of obtaining a sample |
| tTG IgA | ||
| Chest radiograph | 18.00 | www.nice.org.uk/media/59D/3B/Preoperative_tests_rec_reminders_4 _costing.xls (accessed 2 June 2010) |
| ECG (12 lead) | 41.00 |
National Schedule of Reference Costs 2008–09 http://download.lww.com/wolterskluwer_vitalstream_com/PermaLink/FPC/A/FPC_2009_08_11_PIRMOHAMED_201056_SDC4.doc National average from PCTs Direct Access Diagnostic Services: ECG (DA01), echocardiogram (DA02), EEG and EMG (DA14) |
| Echocardiogram | 105.00 | |
| EEG or EMG | 135.00 | |
| Blood glucose self-monitoring | 25.88 | Farmer and colleagues (2009). Intervention costs nurse visit at surgery £8, meter £17.50, lancet £0.03, test strip £0.35 |
| Lactose intolerant (breath hydrogen test) | 115.00 | www.doctormyhill.co.uk/wiki/Lactoseintolerance_ (accessed 2 June 2010) |
| Consultant | 47.59 | Curtis (2009). £166 per patient-related hour. Length of contactb (17.2 minutes) |
| Optician | 27.72 | Eccles and colleagues (2006) £25 per contact uplifteda |
| Retinopathy screening | 20.35 | www.jobs.nhs.uk/cgi-bin/advsearch (accessed 7 May 2010): diabetic retinopathy screening manager, band 7 average annual salary £35,308.50. Curtis (2009). Hourly rate from similar salary: £71/hour patient contact. Length of contactb |
| Audiology | 33.00 | www.jobs.nhs.uk/cgi-bin/advsearch (accessed 7 May 2010): Band 5 audiologist, average annual salary £24,355. Curtis (2009). Band 5 dietitian on similar salary: unit cost £33/hour in clinic |
Hospital contacts
| Hospital contact | Unit cost (£) | Source |
|---|---|---|
| A&E attendance | 93.00 | Curtis (2009). Treatment not leading to admission |
| Paediatric assessment unit | 167.85 | Curtis (2007). £157 uplifteda |
| Ambulance to hospital | 56.11 | Curtis (2008). Patient transport service £54 uplifteda |
| Ambulance team treatment at home | 357.42 | Curtis (2008) (paramedic unit). £344 uplifteda |
| ITU bed-day | 1710.61 | Hutchings and colleagues (May 2009). £1600 2007 uplifteda |
| HDU bed-day | 908.76 | Hutchings and colleagues (May 2009). £850 2007 uplifteda |
| Other ward bed-day | 238.40 | Curtis (2007). Weighted average for inpatient rehabilitation services. £223 uplifteda |
| Day care | 127.23 | Curtis (2007). Day-care services (non-stroke/non-elderly). £119 uplifteda |
Other NHS contacts
| Contact | Unit cost (£) | Source | |
|---|---|---|---|
| GP | Home | 117.00 | Curtis (2009). Including direct care staff and qualification costs. Home visit: 23.4 minutes (including travel). Consultation:17.2 minutes |
| Surgery | 52.00 | ||
| Practice nurse | Home | 16.38 | Curtis (2009). Length of contact.a Home visit: 23.4 minutes at £42/hour. Clinic visit: 11.7 minutes at £34/hour |
| Surgery | 6.63 | ||
| Clinical psychologist | Home | 29.25 | Curtis (2009). Client contact rate £75/hour. Length of contacta |
| Clinic | 14.63 | ||
| Telephone/e-mail/text | 8.88 | ||
| Podiatrist | Home | 21.00 | Curtis (2009) |
| Surgery | 11.00 | ||
| Consultant | Home | 64.74 | Curtis (2009). As consultant/patient-related hour. Length of contacta |
| Surgery | 32.67 | ||
| Dentist | Surgery | 25.50 | www.jobs.nhs.uk/cgi-bin/advsearch salaries average £52,917.50. Post of similar salary level in Curtis (2009): registrar (p. 168) £51/hour. Average length of appointment 30 minutes |
| Eye test | Consultation | 27.72 | £25 per consultation. Average from Eccles upliftedb |
| Ultrasonographer | Visit | 25.67 | www.jobs.nhs.uk/cgi-bin/advsearch advanced practitioner in ultrasound. Post of similar salary in Curtis (2009): nurse team manager (p. 157) £77.00/hour patient contact. Average length of visit 20 minutes ( p. 151) |
| Radiographer | Visit | 16.00 | Curtis (2009). Client contact rate. Average visit 20 minutes (p. 151) |
| NHS Direct | Call | 29.00 | Snooks (2006) |
Unit costs: time off from work
| Unit | Unit cost (£) | Source |
|---|---|---|
| Time off work (per working day) | 95.80 | Curtis (2008) and NHS Economic Evaluation Database Handbook (April 2007) |
Hospital and Community Health Services inflation rates
| Uplift for 1992–3 = 6.9% | Uplift for 1997–8 = 1.7% | Uplift for 2002–3 = 3.5% | Uplift for 2007–8 = 2.9% |
| Uplift for 1993–4 = 3.4% | Uplift for 1998–9 = 4.0% | Uplift for 2003–4 = 5.2% | Uplift for 2008–9 = 3.9% |
| Uplift for 1994–5 = 2.6% | Uplift for 1999–2000 = 4.5% | Uplift for 2004–5 = 3.3% | |
| Uplift for 1995–6 = 4.0% | Uplift for 2000–1 = 4.2% | Uplift for 2005–6 = 3.7% | |
| Uplift for 1996–7 = 2.8% | Uplift for 2001–2 = 5.1% | Uplift for 2006–7 = 3.7% |
References
- Curtis L. Unit Costs of Health and Social Care. University of Kent, Canterbury: PSSRU; 2007.
- Curtis L. Unit Costs of Health and Social Care. University of Kent, Canterbury: PSSRU; 2008.
- Curtis L. Unit Costs of Health and Social Care. University of Kent, Canterbury: PSSRU; 2009.
- Dretzke J, Cummins C, Sandercock J, Fry-Smith A, Barrett T, Burls A. Autoantibody testing in children with newly diagnosed type 1 diabetes mellitus. Health Technol Assess 2004;8.
- Eccles MP, Hawthorne G, Whitty P, Steen N, Vanoli A, Grimshaw JM, et al. A Cluster Randomized Controlled Trial of a Diabetes Recall and Management System: The DREAM Trial 2006.
- Farmer A, Wade A, French D, Simon J, Yudkin P, Gray A, et al. Blood glucose self-monitoring in type 2 diabetes: a randomised controlled trial. Health Technol Assess 2009;13.
- Holmes GKT. Coeliac disease and type 1 diabetes mellitus – the case for screening. Diabetes UK 2001;18:169-77.
- Hutchings A, Durand MA, Grieve R, Harrison D, Rowan K, Green J, et al. Evaluation of modernisation of adult critical care services in England: time series and cost effectiveness analysis. BMJ 2009;339.
- Khunti K, Stone MA, Burden AC, Turner D, Raymond NT, Burden M, et al. Randomised controlled trial of near patient testing for glycated haemoglobin in people with type 2 diabetes mellitus. Br J Gen Pract 2006;56:511-17.
- Marks D, Wonderling D, Thorogood M, Lambert H, Humphries SE. Cost effectiveness analysis of different approaches of screening for familial hypercholesterolaemia. BMJ 2002;324.
- National Schedule of Reference Costs Year 2008–09. PCTs Direct Access Diagnostic Services n.d. URL: www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/@ps/documents/digitalasset/dh_111608.xls.
- Craig D, Rice S. NHS Economic Evaluation Database Handbook. University of York: UK Centre for Reviews and Dissemination; 2007.
- Snooks H. NHS Direct Wales Evaluation Report 2006.
Appendix 3 Secondary outcome internal consistency summary results
| Outcome | Cronbach’s alpha from baseline questionnaire items | ||
|---|---|---|---|
| Patients, 7–10 years old | Patients, 11–16 years old | Carers of all patients | |
| HCCQ | 0.72 | 0.82 | 0.89 |
| HRQoL: | |||
| Barriers | 0.65 | 0.64 | 0.47 |
| Symptoms | 0.68 | 0.83 | 0.80 |
| Adherence | 0.58 | 0.66 | 0.67 |
| Worry | 0.69 | 0.74 | 0.75 |
| Communication | 0.76 | 0.80 | 0.88 |
| DCCS | 0.67 | 0.80 | |
| PAID | 0.93 | 0.92 | |
| Importance | 0.87 | 0.87 | |
| Confidence | 0.84 | 0.90 | |
| Diabetes care/mismanagement | 0.52 | 0.44 | |
| Patient enablement: | |||
| Interim follow-up | |||
| 12-month follow-up | 0.89 | 0.93 | |
Cronbach’s alpha statistics for secondary outcome scores indicate a high level of internal consistency.
Appendix 4 Detailed description of initial steps of the discrete choice experiment
The DEPICTED DCE is presented in Chapter 11. The initial development of the DCE (steps 1–3) is described in full below and in brief in Chapter 11.
Step 1: identification of attributes and levels
The attributes of routine clinic consultations that are of importance to patients and their carers have to be realistic, tradable and relevant to respondents. 141 This was achieved via a series of focus groups. Focus groups can inhibit some quieter participants. Therefore, principles of nominal group technique (whereby contributions from individuals are solicited prior to group discussion) were used to structure and facilitate input from all participants. 114,167 The focus groups involved individual working, group discussions and a formal ranking exercise of key attributes. Separate focus groups were conducted for carers and patients, held as part of the second stakeholder workshop (see Chapter 6).
Each group was run by two facilitators using a semistructured guide to identify key attributes and realistic levels, to ascertain whether or not any attributes dominated and to assess completeness of attributes identification. Discussions were audio-recorded and transcribed. Content analysis was used to identify the attributes and levels and also used data from individual participants’ response sheets. The patient group included 12 individuals (seven boys) aged between 12 and 16 years and the carer group included 11 carers (four with children aged ≤ 11 years).
The final selection of attributes and levels, involved combining some attributes and rejecting those that could not be affected by the DEPICTED intervention (e.g. distance to clinic). A final list of five attributes with three levels each was produced. The same attributes and levels were identified for both groups, with wording adjusted to fit either patients or carers.
| Rank | Patient | Total points allocated | Carer | Total points allocated |
|---|---|---|---|---|
| 1 | Get me involved | 24 | Putting the patient first | 35 |
| 2 | Listen to me | 18 | Interaction skills of the clinician | 27 |
| 3 | Useable, understandable advice information | 15 | Continuity of care | 25 |
| 4 | Problems solved | 13 | Time | 22 |
| 5 | Time (according to need) | 8 | Setting objectives/action plan | 8 |
| 6 | Continuity | 7 | Guidance (including info provision) | 7 |
| 7 | Body language | 6 | Having a positive approach | 4 |
| 8 | Goal-setting – realistic/individualised | 6 | Clinic organisation | 3 |
Step 2: designing the experiment
The resultant attributes and levels generate 243 (35) profiles (full factorial design). As this would require a large number of respondents, a regular fraction factorial design was used whereby only a subset of the combinations are used to model respondents’ choices (35–x). 148 However, the smallest regular fraction is defined by the number of parameters that need to be estimated, including the interactions to be investigated. As the above attributes are all categorical and have three levels each, 11 parameters (10 for the attributes and one for the constant term – explained below) would be needed to estimate the main effect of the model and four additional parameters to estimate each two-way interaction.
The present study determined a regular fraction design of 27 treatment profiles (35–2), where three attributes are the generator factors of the other two attributes. Attribute D is derived by the defining equation D = A + B + C and attribute E is derived by the defining equation E = A + 2B.
As 27 treatment profiles is still a considerable number for each individual to answer, it was decided to use a two-level attribute to generate two orthogonal questionnaires (version A and version B). Each questionnaire included the lowest and the highest treatment combinations (00000 and 22222) producing a total of 15 profiles for each version of the questionnaire. In order to construct a pair-wise choice set, it was decided to compare each treatment option with a constant scenario. 132 In this study the middle term of each attribute (11111) was used as a constant scenario.
To facilitate interpretation of the parameters to be estimated by the model, we report the utility model function for the main model only (Figure 1).
Where:
-
Vi = the change in utility in moving from consultation A to consultation B
-
β0 = constant term
-
β1 – β10 = the beta coefficients of the model to be estimated
-
ε1 error term at centre level = error because of differences across centres
-
ε2 error term at respondent level = error because of differences across respondents
-
ε3 error term at observation level = error because of differences across observation (each respondent was asked to answer 15 pair-wise choices).
In this multilevel logistic regression equation model, the betas represent the probability of moving away from the constant scenario. For example, if the respondent prefers a lot of information to some or little information, then it would be expected that when the alternative treatment option includes a lot of information, the respondent will choose this over the constant scenario and β4 would be positive [all other attributes’ levels being (or perceived) similar between visit options].
The DCE questionnaire was presented in its own booklet, which included three sections. In part 1, the list of attributes and levels were presented and respondents were asked to report what usually happens in their clinic consultation. This enabled respondents to familiarise themselves with the attributes and levels, and also provided a baseline picture of respondents’ current experience of consultations. In part 2, respondents were asked to rank attributes in order of importance. Part 3 included the 15 pair-wise choices. Different sets of choices were presented in two questionnaire versions (A or B) in otherwise identical booklets.
Step 3: piloting the questionnaire
Cognitive interviews using retrospective probing were used to pilot the questionnaire to ensure that respondents understood the task as intended and that the task was not too burdensome. 168,169 A structured interview guide was designed to probe possible problem areas.
Two rounds of pilots were carried out and 55 invitation packs were sent to families attending the paediatric diabetes clinical service in Cardiff. In the first round of piloting, participants were given the DCE questionnaire (incorporated into the main trial outcome questionnaire form) to complete and were interviewed afterwards for approximately 15 minutes. Interviews were audio-recorded and responses were summarised and coded.
Eleven families expressed interest in taking part in the piloting during the next clinic visit. Interviews were carried out with six patients (aged 10–14 years, five female) and five carers (all female). Key findings (problems) were completion of only a single pair-wise choice, completion based on actual rather than preferred consultation, inattention to provided instructions, misinterpretation of some terms and problems understanding the ranking exercise.
Subsequent changes to the questionnaire included (1) clearer instructions and modified layout for the ranking exercise; (2) modified instructions for the pair-wise choices; (3) presenting the questionnaire in a separate booklet to the main trial outcome questionnaire; (4) emphasising the hypothetical nature of the consultation styles used; (5) emphasising that the pair-wise choices might seem very similar; and (6) various wording changes. The number of changes suggested that the revised version should be piloted.
Following revisions to the questionnaire, a second round of piloting was carried out with lay stakeholders via telephone. The cognitive debriefing interview was concurrent with the participant answering the questionnaire. A series of vignettes indicating to pause were included at key parts of the questionnaire (e.g. ranking exercise) to indicate this. Each interview was audio-recorded and transcribed for analysis. Three parents and two teenagers took part.
None of the participants reported any problems with the instructions, wording and ranking exercise. One parent mentioned that the repetitiveness of the task was challenging and questioned the need for 15 pairs of choices. Also, from the probing, it emerged that this participant’s choices were mainly based on a current as opposed to hypothetical situation. Finally, two parents noticed that the questionnaire did not include any reference to the diabetic nurse (one mentioned the dietitian also).
No changes were made to the questionnaire.
Appendix 5 Semi-structured interview schedule for intervention
Appendix 6 Practitioner ‘prompt card’
Appendix 7 Talking Diabetes: consultation score sheet
-
Use one score sheet to rate each consultation.
-
Enter the consultation ID and your initials and the date at the top of the sheet.
-
For each of the strategies, tick the associated box if there is evidence of an attempt at a task and circle a number 0–2, where 0 = no evidence of strategy, 1 = some evidence and 2 = good evidence of strategy use.
-
For skills, mark the visual analogue scales and use them as a tool to make a global judgement about practitioners’ skilfulness, where 0 = not at all skilful and 6 = very skilful.
-
To assess guiding style, use the visual scales in the same way to give scores for ‘evocation’, ‘collaboration’ and ‘autonomy-supportive’ between 1 and 5, and a global ‘guiding’ score between 1 and 5.
-
Rating consultations is not easy. There may be occasions where you are not 100% confident in your scoring decision. Do not worry, just go with your instincts. If you are very unsure how to make a judgement on a particular occasion, refer your query to ……………………………
Appendix 8 DEPICTED protocol
Principal investigators
Professor John W Gregory
Professor in Paediatric Endocrinology, Department of Child Health, Cardiff University
Dr Mike Robling
Associate Director, South East Wales Trials Unit, Department of Primary Care & Public Health, Cardiff University
Co-investigators
Professor Christopher Butler
Professor of Primary Care Medicine & Head of Department, Department of Primary Care & Public Health, Cardiff University
Dr Susan Channon
Consultant Clinical Psychologist in Paediatrics, Cardiff & Vale NHS Trust
Professor David Cohen
Professor of Health Economics, School of Care Sciences, University of Glamorgan
Mrs Charlotte Crawley
Cardiff Support Group for Parents of Diabetic Children
Dr Elizabeth Crowne
Consultant in Paediatric Diabetes, United Bristol Hospitals Trust
Dr Kerenza Hood
Director, South East Wales Trials Unit, Centre for Health Sciences Research, Cardiff University
Ms Mirella Longo
Research Fellow, Health Economics, School of Care Sciences, University of Glamorgan
Dr Lesley Lowes
Research Fellow/Practitioner, Department of Nursing & Midwifery Studies, Cardiff University
Professor Steve Rollnick
Professor of Healthcare Communication, Department of Primary Care & Public Health, Cardiff University
Professor Ian Russell
Professor of Public Health & Director, Institute of Medical & Social Care Research, Bangor University
|
Trial statistician Dr Kerenza Hood Director, South East Wales Trials Unit Cardiff University 7th floor, Neuadd Meirionnydd Heath Park, Cardiff |
Trial coordinator Rachel McNamara South East Wales Trials Unit Cardiff University 7th floor, Neuadd Meirionnydd Heath Park, Cardiff |
Please contact the Trial Coordinator for general queries and supply of trial documentation.
Glossary of abbreviations
- AE
- adverse event
- CRF
- Case Record Form
- CTIMP
- Controlled Trial of an Investigational Medicinal Product
- DCE
- Discrete Choice Experiment
- DMC
- Data Monitoring Committee
- HCCQ
- Health Care Climate Questionnaire
- MI
- motivation interviewing
- NCCHTA
- NHS R&D National Coordinating Centre for Health Technology Assessment
- PAID
- Problem Areas in Diabetes
- PedsQoL
- Pediatric Quality of Life Inventory
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- SAE
- serious adverse event
- SSA
- Site Specific assEssment
- SSC
- Study Steering Committee
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
Keywords type 1 diabetes, children, teenagers, young people, clinician training, psychosocial intervention, cluster randomised trial, blood glucose, HbA1c, quality of life, clinical outcomes, psychosocial outcomes, cost-effectiveness
Trial summary and schema
An overview of the trial is presented in the following flow charts demonstrating the study design and flow of participants through the study. A summary of the trial is also presented.
Trial schema
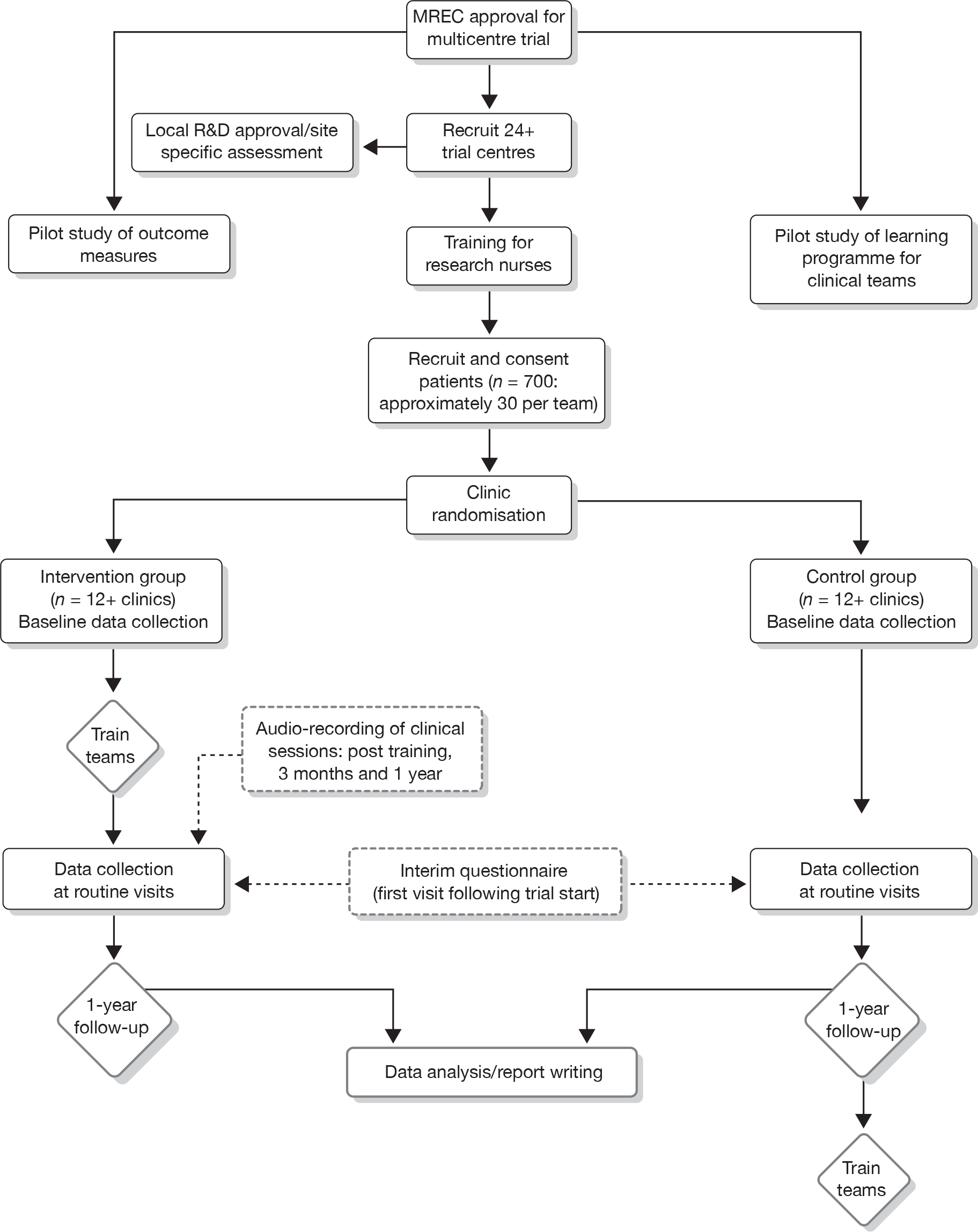
Patient flow diagram
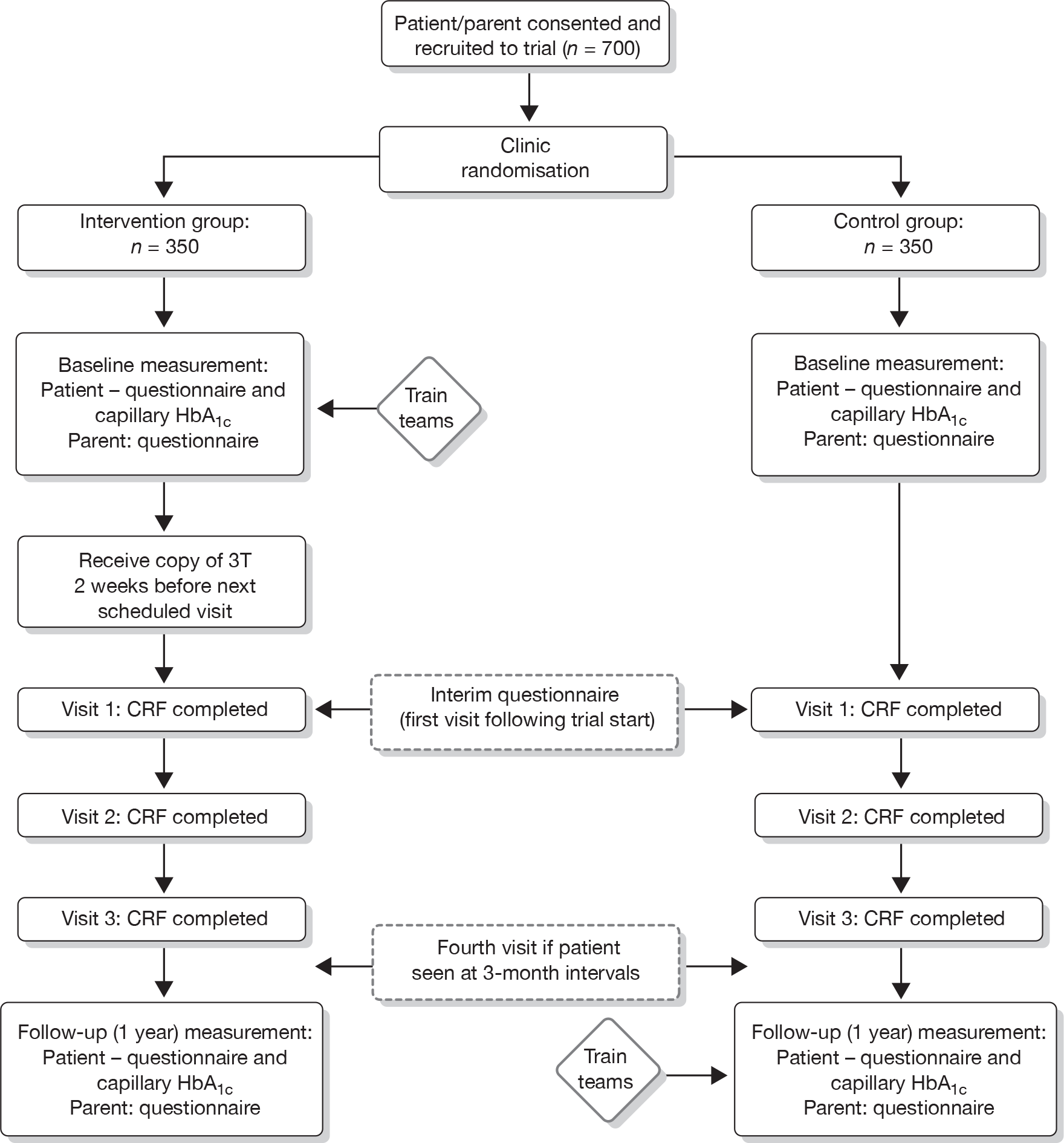
Trial summary
Diabetes is the third most common chronic condition in childhood and the incidence has doubled over the last 20 years. Patients are at risk of both long- and short-term complications that may affect their development and life expectancy, such as eye, circulatory, kidney and cardiovascular problems. To reduce the risk of these complications, regular treatment with insulin, exercise and a healthy diet are required. It is well known, however, that psychosocial and educational factors also play a key role in the successful management of young people with diabetes.
A recent review concluded that there are currently no educational or psychosocial interventions proven to be effective in adolescents with diabetes. The review stated that well-designed clinical trials recognising the inter-relatedness of various aspects of diabetes management are required. In particular the recommendation was for research developed in consultation with key stakeholders [including patients, families and health-care professionals (HCPs)]. Interventions also need to be suitable for use by non-specialist care professionals (i.e. not psychologists) to enable widespread application throughout the NHS.
The body of research and theory on health behaviour change highlights the importance of enhancing coping skills amongst patients and eliciting motivation and solutions relating to the way in which patients are spoken to. Following a development phase involving key stakeholders (patients, parents, clinicians), a psychosocial intervention for use in paediatric diabetes clinics by non-specialist staff has been developed (‘Talking Diabetes’), along with a training programme for health professionals. Approaches that have informed the development of the current intervention include (1) flexible consulting styles that integrate as appropriate directing, following and guiding communication; (2) balancing multiple and inter-related health behaviours and negotiating complex behaviour change; and (3) the possibility of matching or targeting interventions to the needs of individual patients using a flexible ‘menu’ of strategies: an approach that has shown promise in other clinical settings.
The effectiveness of the current psychosocial intervention will be evaluated in a cluster randomised trial. The primary outcome is glycaemic control [glycosylated haemoglobin (HbA1c)] at 1 year for patients with type 1 diabetes aged 4 5 years (and at least 1 year post diagnosis). Secondary outcomes include quality of life (QoL), for both patients and parents, and cost-effectiveness. A minimum of 24 paediatric diabetes care teams will take part in the trial (approximately 700 patients in total). Half of participating teams will be randomly assigned to the intervention group and will receive the training at the start of the trial. Remaining teams will be allocated to the control arm, and offered training at the end of the 1-year trial period. Outcomes for patients and parents will be compared between the two groups at 1 year.
Introduction
Overview
The DEPICTED study was reviewed and funded for two phases – an intervention development phase (18 months’ duration) and an intervention evaluation phase (2 years’ duration). This document describes the protocol for the evaluation phase, which is a pragmatic cluster randomised controlled trial (RCT) of a communication skills-based training intervention for UK clinical teams caring for children and adolescents with diabetes. The training programme intervention is called ‘Talking Diabetes’.
Clinical background
Diabetes: the challenge with children and young people
Diabetes is the third most common chronic disease in childhood, with new cases affecting at least 13.5 per 100,000 children per year in the UK. 1 The incidence had doubled in the preceding 20 years. In childhood, the vast majority of affected children experience autoimmune-mediated destruction of their insulin-secreting pancreatic β-cells, which leads to insulin deficiency (type 1 diabetes). As a consequence, they develop hyperglycaemia and ketosis, which require insulin treatment to prevent potentially life-threatening episodes of ketoacidosis. In the longer term, chronically elevated blood glucose concentrations lead to an increased risk of complications, which include growth failure and pubertal delay in childhood, and sight-threatening retinopathy, nephropathy, neuropathy and cardiovascular disease in later life. Treatment requires the regular administration of insulin, most commonly by two to four injections daily in conjunction with promotion of a healthy lifestyle, including exercise and a diet, which regulates carbohydrate and fat intake. The efficacy of management is monitored in the short term by regular self-measurement (ideally four or more times daily) of blood glucose concentrations and in the longer term by monitoring (3–4 monthly) HbA1c levels in the blood and regular review in paediatric diabetes clinics. This is facilitated in most diabetes services by nurse specialists who liaise with the clinic, the child’s home and school.
Psychosocial aspects of diabetes
It is well recognised2 that psychosocial and educational influences play a key role in determining management outcomes in children with diabetes. For example, throughout childhood, a large audit in Scotland has shown that family structure is associated with glycaemic control. 3 During adolescence, rapid physical change (puberty) leads to relative resistance to the effects of insulin. 4 Concurrent major developmental changes include increasing independence, emerging sexuality and increased stress from peer and academic pressures. These factors together are often associated with deteriorating glycaemic control. Knowledge and skills imparted by the diabetes teams are especially important tools for the child and their family to achieve optimal glycaemic control during this crucial period.
A recent NHS Health Technology Assessment (HTA) systematic review of the effects of educational and psychosocial interventions for adolescents with diabetes reported that there were no results from RCTs of such interventions in the UK. 5 However, the review did identify an ongoing study from our own group evaluating the effects of motivational interviewing (MI) on behaviour change in teenagers. This trial is based on positive findings in a pilot study in children6 and an RCT involving adults with type 2 diabetes. 7,8 We have recently completed an RCT of MI in children, which demonstrates persisting improvements in HbA1c level up to 2 years after the start of a 1-year MI intervention when compared with a group receiving non-specific counselling. 9 Small- to medium-sized beneficial effects on a variety of diabetes management outcomes have been demonstrated mostly in North American studies. 10 The HTA review concluded that there is a need for well-designed clinical trials that recognise the inter-relatedness of various aspects of diabetes management and assess outcomes that are specifically targeted for change, at an appropriate time after the intervention. In particular, the review recommends that such research is developed by a consultation process with stakeholders, including patients, their families, HCPs and health economists. The commissioning brief for this research project has further refined these principles in that effort should be directed towards a generic intervention that does not require delivery by trained clinical psychologists, given their relative scarcity in paediatric diabetes services. 11
Behaviour change: what theory and research tell us
Theories of health behaviour change (e.g. reasoned action theory, the health action process approach), and the research associated with them have clarified the need to look beyond a simple approach to compliance and change based upon the delivery of expert information. 12 As Marteau and Lerman13 have put it, ‘Just telling people they are at risk of developing a disease is rarely sufficient to change behaviour’. Two variables run through many of the models as predictors of health behaviour change: beliefs about the value of change and beliefs about one’s capacity to succeed (self-efficacy). The efficacy of theory-based interventions like cognitive behaviour therapy (CBT) have largely been attributed to their capacity to enhance self-efficacy. 14 Using a skills-based approach to counselling has been found to be effective in a number of fields. 14,15 So too, brief interventions have been found to be effective in changing a number of risky health behaviours. 16
A second line of research has focused on how the therapeutic relationship either hinders or promotes motivation to change. For example, an early effort to understand the effective ingredients of MI17 identified a correlation between confrontational interviewing and resistance, and between ‘change talk’ and behaviour change. 18 A recent meta-analysis of MI19 found consistent evidence for effectiveness in some (e.g. alcohol, drug use), but not in all, behavioural domains. Interest in the field of diabetes among young people has also emerged. 6,20,21 One of the challenges in much of this research, however, has been to specify exactly what elements of a complex method were used by the interventionists. It does appear that some of the principles of MI can be realised in brief health-care consultations, and that helping the patient to clarify for themselves why and how they might change their behaviour (MI) can be more effective than brief advice-giving. 22,23 One recent development has been the first effort to integrate this method with CBT. 24
Put simply, this body of work calls attention to both the direction of consultations about change (towards enhancing coping skills) and the way patients are spoken to (eliciting motivation and solutions from them).
Development of the Talking Diabetes intervention
Consistent with the original commissioning brief, the development of the Talking Diabetes intervention did not start out with a predetermined position on the best form of psychosocial therapy as its basis. However, a number of principles and conceptual aids were brought to the intervention development, including the process for consideration by stakeholders.
Firstly, there is the need to integrate talk about lifestyle change, self-control and QoL with routine care, where patients are at the receiving end of a range of medical and nursing interventions. Practitioners need to find ways of moving between providing medical care on the one hand, and ‘letting go responsibility’ on the other,8 to encourage children and teenagers to take control of their health, with assistance from others. A consultative stakeholder reference group established for the development stage of the study considered a model developed by one of the co-applicants (Professor Rollnick) with practitioners in the coronary heart disease field, which describes the value of moving flexibly between instructing, listening and guiding communication styles when talking about behaviour change.
A second conceptual and clinical challenge is the need to move beyond thinking about change as involving an isolated, single behaviour, a limitation in much of the theory of behaviour change in health psychology. The challenge is to help patients find a balance between multiple and inter-related health behaviours and lifestyle choices. 5,7,8,25 How to negotiate a complex behaviour change agenda was another useful starting point in intervention development. 26
Thirdly, targeting or matching interventions to the needs of patients was a design consideration efforts to match interventions to patients in other fields27,28 have proved difficult. One approach to targeting is to regard this as something that happens not across interventions but within the consultation, as the practitioner shifts style and topic according to the needs of the patient. 29 To this end, there is some evidence for the acceptability and feasibility of using a targeting approach based on a flexible menu of strategies in which the practitioner and patient select a topic according to need. 28,30 This intervention framework has been developed in efforts to train HCPs to use elements of MI, and a recent application among drug abusing young people has produced promising results. 31 In the present context, however, it is not the intervention approach (MI) or content that is considered useful, but the use of a framework or methodology for targeting within the consultation based on a menu of topics for discussion.
Empirical and consultative work during the intervention development phase helped formulate and operationalise the Talking Diabetes intervention. These activities included (1) a telephone survey of 51 clinicians working in UK paediatric diabetes clinics, conducted to explore existing psychosocial practice; (2) a postal survey of 266 UK clinicians exploring experiences and preferences for training in health communication skills (including acceptability and feasibility of different training options); (3) focus groups with young patients with diabetes and with parents of young patients; (4) observational work of consultations within three paediatric diabetes clinics (Wales and England); (5) experimental role play with clinicians and young people; (6) an ongoing stakeholder consultation process built around three 1-day workshops attended by a multidisciplinary and lay constituency; and (7) local piloting of approaches with young patients. The finalised intervention consists of a blended learning programme for clinicians working in the paediatric diabetes field. The programme provides training in a number of communication strategies and skills to prepare patients for behaviour change conversations and for conducting such consultations. A key element of this strategic approach is the use of a patient agenda-setting device [‘3T: TimeToTalk’ (3T)].
The current trial
The HTA programme systematic review demonstrated the absence of high quality UK-based studies of educational and psychosocial interventions in adolescents with diabetes. Since Hampson’s review, at least two such studies funded by Diabetes UK (RCTs) have been undertaken. However, both were established interventions (MI by members of this group in Cardiff and cognitive behavioural therapy in Bristol Children’s Hospital) delivered by trained psychologists. The HTA programme seeks to explore the efficacy of interventions not requiring the involvement of a trained psychologist, maximising the feasibility and practicality of delivering such interventions in an NHS context in which clinical psychology services are very limited.
Following the development of the intervention during our initial study phase, a RCT is needed to test its efficacy. The development phase has provided evidence that the intervention is feasible for the teams managing care and acceptable to the patients and their parents.
Trial objectives
The aims of the trial are to determine the effectiveness and cost-effectiveness of the Talking Diabetes intervention in children and teenagers with type 1 diabetes.
Primary objectives
Does a multifaceted communication skills training intervention (incorporating a patient agenda-setting component) for non-psychologist members of a paediatric diabetes team improve clinical and psychological outcomes for children and teenagers with type 1 diabetes?
Is a multifaceted communication skills training intervention (incorporating a patient agenda-setting component) for non-psychologist members of a paediatric diabetes team cost-effective?
Secondary objective
A secondary objective of the study is to assess skill retention, competency and confidence of non-psychologist members of the paediatric diabetes team, in delivering the intervention.
Trial design
The current study is a pragmatic cluster RCT. A minimum of 24 teams (approximately 700 patients) will be randomised to receive training at the start (intervention group) or the end of the study period (control group).
Treatment period
Outcomes for patients being cared for by trained teams will be compared to outcomes for patients being cared for by untrained teams over the year following training. Team members will be asked to self rate how frequently they feel they have used the training during this year.
Frequency and duration of follow-up
Patients will provide blood samples, and patients and parents will complete questionnaires immediately post recruitment, following their first clinic visit during the trial phase and at 1 year. Consent from participants will also be sought to allow the possibility of longer-term follow-up (i.e. clinic HbA1c level at 2 years).
Professionals will have their competencies measured post training, at 3 months and after 1 year, to assess acquisition and maintenance of new skills. Professionals will also provide attitudinal self-rating (importance and confidence) at the start and end of the training programme and at 1 year.
Primary and secondary outcomes
The primary trial outcome will be glycaemic control, assessed using HbA1c level. Secondary trial outcomes will include QoL, cost other clinical outcomes [including body mass index (BMI)] and psychosocial outcomes.
Clinic and patient selection
All eligible patients will be identified from the clinic list by the research nurse. A random sample of 40 patients will be selected by the research team (from an anonymised eligible list) and approached en bloc by clinics in order to obtain the target sample of 30 recruited patients per clinic. Should this target not be reached in the first instance, further patients will be randomly selected by the researchers for approach by the clinical team until approximately 30 patients are recruited. It is envisaged that this can be achieved across all centres over a 3-month period.
It is envisaged that patients will be approached and recruited before the team knows to which arm of the study it has been allocated. However, randomisation will be revealed to all centres approximately 2 weeks prior to the first face-to-face training workshop for intervention teams, even if recruitment is incomplete, to allow sufficient time for intervention teams to complete the e-learning.
All teams will be consented prior to randomisation and the incentive of receiving training at the end of the study is provided to avoid differential levels of dropout/engagement between the two groups of teams. Recruitment bias is common in cluster randomised trials. 32 In this study, all patients will be identified and approached by a member of the local team (likely to be a nurse, and to be trained in recruitment) during the period prior to randomisation and intervention training. The broad entry criteria mean that the majority of their patient group will be eligible. Details of numbers excluded in each category will be collected.
Inclusion and exclusion criteria
Criteria for teams
-
Inclusion criteria Teams include a paediatrician with an interest in diabetes and a diabetes specialist nurse.
-
Exclusion criteria Fewer than 40 potentially eligible children/adolescents (diagnosed more than 1 year ago) attending the clinic.
Criteria for patients
Inclusion criteria:
-
type 1 diabetes
-
4–15 years old
-
under care of paediatric/adolescent diabetes team for duration of trial
-
diabetes diagnosed > 12 months earlier
-
parental or carer (and child, when able) consent given
-
ability of patient and at least one parent or carer to complete study materials (questionnaires).
Exclusion criteria:
-
not under care of parent or guardian (i.e. a looked after child)
-
comorbid chronic illness likely to impact on HbA1c level, independent of patient’s ability to manage their diabetes (e.g. condition requiring steroid treatment, cystic fibrosis, renal failure)
-
in receipt of ongoing psychiatric/psychological therapy at the start of the study
-
other patients judged by their clinical carer to be vulnerable due to existing medical or social condition.
Recruitment
Procedures for clinic and patient recruitment are outlined in the following sections.
Recruitment process
A research nurse (likely a member of the local diabetes team) will be employed to write to eligible patients identified from clinic registers. Information sheets for both parent and child (age appropriate) and consent forms will be enclosed. This approach will be followed up by a telephone call approximately 1 week later to enable any questions about the study to be answered. The parents and patient will be asked to return the consent forms to the clinic (in a prepaid, freepost envelope) if they wish to take part. Both parent and child will be sent a questionnaire to complete by the research nurse, and an appointment will be made to take a capillary blood sample. At the patient’s preference, this can be done either in their own home, or in clinic at the time of routine venesection/capillary testing required for normal clinical care. Trial centres will also have the option to run an additional session within the clinic setting for baseline and follow-up data collection (patients and parents will be reimbursed for any travel expenses incurred as result of attending these data collection clinics). Patients/parents who do not return questionnaires following their baseline data collection visit will be followed up directly by the research team.
Patient recruitment will be undertaken in blocks (maximum of three) should delays in gaining research governance approvals in some centres occur. However, patients within each centre will be approached en bloc and it is envisaged that recruitment will be complete within a 3-month period.
Informed consent
All members of the diabetes care team within participating clinics will be asked to consent to take part in the trial.
Informed consent will be taken by the research nurse from patients and parents/carers. All patients will be asked to sign either a consent or assent form, and the parent/carer will be asked to consent to their own participation in the study, as well as consenting on behalf of their child. Consent will be sought (at the discretion of the research nurse and/or a parent/carer) from children aged 11 years and above. Assent will be sought as a matter of course for younger children. Should the parent/carer and/or research nurse feel that the patient is too young to give assent then a missing assent form will be completed by the research nurse. Parent and patient must be in agreement to take part in the study: if either party is not happy to consent then neither the child nor the parent/carer will take part in the study.
Registration
All eligible patients will be identified by the clinic-based research nurse from the clinic list. Anonymised details will be passed to the research team for the selection of the random sample (appropriate identifiers will be used in discussion with each clinic). Once returned to the research nurse the list can be screened by the clinic team to further exclude vulnerable or otherwise ineligible patients (as defined in exclusion criteria) prior to being approached by the research nurse. Patients will continue to be approached until approximately 30 patients have consented.
Non-registration
Patients will be screened for eligibility prior to being approached for recruitment. Patients and/or parents who are eligible but do not wish to take part in the study will be assured that their treatment will not be adversely affected by this decision.
Withdrawal and loss to follow-up
Patients and parents will be informed that they are free to withdraw from the study at any time, without stating a reason, and without any detrimental effect on clinical care.
Compliance
All eligible patients will be identified before the teams are trained. This will incorporate some patients who do not regularly attend their routine appointments and who may not receive much psychosocial intervention from the teams. However, in practice, patients who are poorly controlled have greater contact with teams both within clinic and informally (e.g. by telephone). All patients will be followed up to give a comprehensive picture of this patient group.
Loss to follow-up
We have allowed for a 22% loss to follow-up in the sample size calculation. This is a relatively ‘captive group’ to follow-up. The upper age limit of 15 years is set to maximise the likelihood of them remaining under the care of the paediatric team for the duration of the study. Questionnaire non-responders will be followed up directly by the research team.
Trial intervention
The clinician learning programme
All members of each clinical team allocated to the intervention arm of the study will undergo a blended training programme comprising of web-based material and face-to-face seminars (the Talking Diabetes programme). The training course aims to prepare clinicians and their patients for constructive behaviour change conversations and provide clinicians with strategies and skills for encouraging behaviour change. The training emphasises a guiding style when consulting with patients about behaviour change and draws upon the MI approach.
Clinicians will access the secured website using a unique username and password and work their way through a number of distinct programme parts with an approximate total duration of 1.5 hours (delivered via three main e-learning modules). In addition, more interactive web-based components of the course will allow clinicians to record their thoughts and experiences as they proceed through the programme. Two face-to-face seminars with combined clinical teams (approximately three teams) will be held as part of the training course. Time spent on offline learning activities, such as discussing the training content in pairs, will be recorded online. Clinicians will be asked to state whether online learning activities were completed in work or leisure time to give an indication of feasibility in rolling out the training programme. Trial centres in the intervention arm will be given a number of options to facilitate ongoing development and maintenance of the skills acquired during the learning programme. Centres will be given the option of regular contact with a trainer via e-mail or telephone from a member of the training team at an agreed period following the second face-to-face seminar.
3T: An agenda-setting tool for patients
The training programme will show clinicians how to use a device for promoting shared agenda setting during clinical encounters with patients (3T). Clinicians will be asked to incorporate 3T within their normal practice. It consists of a rigid folder and an inserted paper agenda pad of tear-off sheets. The paper pad can be used by patients and parents to record topics of importance to be raised within forthcoming consultations. Patients will be sent their own copy of 3T by the research nurse following consenting to the trial so that they receive it approximately 2 weeks before their next scheduled clinic appointment. Consent and baseline data collection will be timed so that it will be completed before the first face-to-face training sessions for clinicians. 3T remains the property of the patient both for the duration of the trial and after the study. While they are encouraged to bring 3T to each consultation, they do not have to leave a copy of their completed agenda chart (from the 3T paper pad) with the clinic. Copies of the agenda sheet will not be collected by the research team. However, clinicians will have the option to complete a proforma, on which general topics discussed at clinic visits can be recorded and kept with patient notes, to facilitate clinical record-keeping.
Copies of the paper agenda-setting pad (without folder) will be made available to each clinic to refill/replace folders as required and for other patients not otherwise recruited to the study.
Trial outcomes
Outcome measures and assessment instruments
Patient outcomes
Careful selection of outcome measures for children is important to ensure appropriateness, feasibility (not overloading the participant) and acceptability. Measure selection has been informed by two HTA systematic reviews (one specific to this clinical area5 and the other on measures for children with chronic diseases generally33) and consultation with the stakeholder reference group in our intervention development phase.
Clinical measures:
-
level of HbA1c
-
body mass index (height, weight) [recorded on a Case Record Form (CRF) at each clinic visit]
-
insulin type, dose and number of injections (CRF at each visit)
-
self-reported moderate and/or severe hypoglycaemic episodes (CRF at each visit).
Patient-reported outcomes (measured using age-appropriate questionnaire at baseline and follow-up):
-
demographic: age, gender, ethnic origin (baseline only)
-
diabetes-specific QoL [Pediatric Quality of Life Inventory (PedsQL) diabetes module;34 Problem Areas in Diabetes (PAID) (emotional impact); global questions]
-
diabetes self-care35 (mismanagement questions relating to diet, number of injections and monitoring)
-
self-efficacy36 (perceived competency scale)
-
patient enablement37 (coping anchored to clinic visit)
-
perceptions of diabetes team [Health Care Climate Questionnaire (HCCQ)36 and items regarding communication between clinicians, feelings towards next visit, continuity of care38]
-
importance of, and confidence in, ability to undertake diabetes care and monitoring activities (patients aged 11+ only)
-
preferences for care [Discrete Choice Experiment (DCE): follow-up only].
Parent/carer outcomes (measured at baseline and follow-up)
Self:
-
demographic: age, gender, ethnic origin, socioeconomic status (baseline only)
-
parent QoL/anxiety (PAID, additional global questions)
-
perceptions of diabetes team (HCCQ36 and items regarding communication between clinicians, feelings towards next visit, continuity of care38)
-
importance of, and confidence in ability to undertake diabetes care and monitoring activities
-
preferences for care (DCE: follow-up only).
Proxy (for patients aged 5–1 years):
-
diabetes-specific QoL (Pediatric Quality of Life Inventory (PedsQL) diabetes module,34 Problem Areas in Diabetes (PAID) (emotional impact), global questions)
-
diabetes self-care35 (mismanagement questions).
Service usage measures/indirect costs (recorded on CRF at each clinic visit unless otherwise specified):
-
travel to each clinic (measured at baseline and follow-up in parent questionnaire)
-
school absences
-
time off work (parent)
-
inpatient admissions (including intensive therapy unit and high-dependency unit, particularly with ketoacidosis)
-
Accident and Emergency attendances
-
clinic attendances
-
contacts with the diabetes team (home, telephone, face to face, electronic)
-
other health service contacts (general practitioner attendances, any other)
-
medication/equipment use (insulin type and dose; insulin needles; lancets, testing strips; hypostop/glucogel/glucagon).
Interim questionnaire (completed by patients aged 7+ years and carers at first clinic visit following trial start)
-
Patient enablement37 (coping anchored to clinic visit: patients 11+ years and carers only).
-
Feeling towards current clinic visit (7–10 years only).
Outcomes for clinical teams
Professionals’ communication skills training involvement and cost:
-
clinician job title/grade, gender
-
travel to seminars (reported at seminars and calculated by project team)
-
time spent on offline learning activities, i.e. discussion of training content in pairs (reported online)
-
time spent at seminars (recorded by project team)
-
time spent online (automatically recorded on website).
Other training costs:
-
venue/training materials cost
-
cost of trainer (time, job title, grade).
Training outcomes:
-
Behaviour change consultation competencies (immediately after training and at 1 year). Competencies for clinicians in the control group will be assessed at the end of the study period, prior to training. 38
-
Confidence and importance in behaviour change consultations (before and after training – 1 year after training).
-
Systemic service changes (including consultation times, telephone/home contacts: at baseline and follow-up).
Trial procedures
Trial procedures relating to data collection and assessment, piloting and procedures for reporting adverse events (AEs) are outlined in the following section.
Participant data collection
Baseline data collection
Baseline data will be collected by the research nurse in clinic at the time of a routine clinic visit or in the patient’s home if requested. Home visits will also be made if the patient is not due for a routine clinic visit within 2 weeks of the follow-up telephone call. Trial centres will also be given the option to run one-off data collection clinics at baseline and follow-up: patients and parents will be reimbursed for any travel expenses incurred as a result of attending these clinics. Informed consent will be taken by the research nurse, and patients and parents provided with a copy of an age-appropriate questionnaire to be returned to the research team (note: non-responders will be followed up directly by the research team). The research nurse will also complete a baseline CRF, comprising demographic information and clinical data (taken from patient notes on receipt of consent) such as years since diagnosis, insulin type, dose and regimen. Patients’ general practitioners will be informed in writing of their patient’s participant in the trial by clinic staff (research nurse).
Blood samples will be collected by research nurses and sent to a single UK laboratory. Samples will be collected in a 5-µl glass capillary tube, and stored in a plastic-lidded tube (prefilled with diluent and preservative). Samples will be securely packaged according to the laboratory manual and sent via Royal Mail (identified as biological substance, category B). HbA1c assays will be carried out using a Menarini HA-8160 instrument calibrated by the manufacturer to be traceable to the Diabetes Control and Complications Trial (DCCT) international standard. Results will be reported directly to the research team.
In the event that a sample is lost or spoilt in transit to the central laboratory, the research nurse will approach the patient and carer (in writing) and ask if they would be willing to provide a second sample. Written consent from parents and consent/assent from patients will be sought. Patients and carers will be reassured that refusal to provide a second sample will not impact on their participation in the trial or future clinical care. In the event that a patient HbA1c sample is in excess of 15.0% (considered to be clinically significant) local diabetes teams responsible for patient care will be informed, for comparison with the most recent HbA1c sample taken in clinic. Any patient contact resulting from notification of a high HbA1c value will be at the discretion of the patient’s diabetes care team. The research team will not contact patients directly in connection with HbA1c results.
Data collection during the trial phase
Clinical patient details (to include HbA1c level, height, weight, BMI, insulin regimen), health service contacts and patient-borne costs will be recorded by the local research nurse at each clinic visit on the CRF. The research nurse will also record who patients consulted with, for how long, and whether patients consulted on their own, on the CRF at each visit. At the first clinic visit, questions on the CRF will be anchored to the baseline blood sample. For future visits throughout the year, questions on the CRF will refer to the period since the previous clinic visit.
Patients and carers will also be asked to complete an interim questionnaire (assessing patient enablement) at their first clinic visit following the start of the trial.
Follow-up data collection
Capillary HbA1c samples for patients, and questionnaires for both patients and carers will be repeated at 1 year (note: the same carer will be asked to complete questionnaires at both baseline and follow-up). Follow-up questionnaires will be sent to patients and carers directly by the research team at 1 year, and will include a covering letter stating that all families will automatically be entered into a prize draw, as a thank you for trial participation and data returned to date. Ten prizes of £30 gift vouchers (for a store of the families’ choice) will be available, and the draw will take place in September 2009 at the end of the follow-up period. If questionnaires are not received within 4 weeks of this mailshot, participants will receive a follow-up call from the research team and further copies of the questionnaires will be sent out. Families will also be sent a letter 2 weeks prior to their 1-year follow-up, to remind participants that they will shortly receive the follow-up questionnaires and that their local research nurse will be in touch to arrange for a second HbA1c sample to be taken from patients.
Where patients are lost to follow-up without withdrawing consent for use of data already provided, or where it is not possible to obtain a follow-up HbA1c sample within the specified time frame (2 weeks either side of the baseline sample date), routinely collected clinic HbA1c data will be analysed in place of a follow-up capillary HbA1c sample. Patient and parent information sheets and consent forms will also inform participants that longer-term follow-up based on routine assessments (e.g. clinic HbA1c measurements) may be undertaken up to 2 years following the start of the trial (subject to additional funding: the procedure for collecting this data will be submitted as an ethical amendment).
Training outcomes for clinical teams
Professional competencies will be assessed following training (i.e. following the second face-to-face training seminar), at 3 months and at the end of the 1-year period (for the intervention group this will be a reassessment and for the control group it will be their pretraining assessment). Following advice from our stakeholder reference group, clinicians will be asked to audio-record clinical sessions, for which written informed consent will be sought. Patients will be informed about the study and approached for consent to the recording prior to entering the consultation room, and provided with the option to withdraw their consent following the consultation. Patients approached for consent will include those already recruited to the trial, and other patients attending the normal clinic session. A log of patients attending the session will be kept by the research nurse, to record patient name, presence of parent(s) and the names of all clinicians in attendance. Clinicians will be asked to nominate up to two consultations where there was a behaviour change issue discussed, although analysis will not necessarily be restricted to these consultations. Clinicians will also be asked to rate their performance in the consultations they select. During any one session only one team member will be recorded for logistical reasons and to enable patients to speak freely to other members of staff. A maximum of three randomly selected team members per clinic will be recorded. Recordings will be collected by the research team and stored securely. Ratings will be conducted using a modified version of the Behaviour Change Counselling Index (BECCI) rating scale,39 adapted to reflect the competencies addressed by the Talking Diabetes programme. Items will cover agenda-setting, ‘pros and cons’ of the intervention, goal-setting and global judgements. Anonymised transcripts will be made of sampled recordings for further validation of rating materials and for training of raters.
Clinicians in the intervention group will also complete a short questionnaire about their confidence in using the intervention, their perception of its efficacy and their perception of the broad impact of the intervention on their service.
Cost-effectiveness
Preferences for delivery of care will be assessed using a DCE to be administered as a separate questionnaire at 1-year only.
A DCE works by presenting individuals with hypothetical scenarios involving different levels of defined attributes and asking them to make discrete pairwise choices. During the development phase of the current study the patient and parent focus groups and the stakeholder reference groups were used to identify the most relevant attributes of diabetes care and to clarify levels. In the trial phase, the DCE will be administered to patients aged 11 years and above, and carers of all patients, as part of the follow-up questionnaires. This will allow exploration of (1) the use of DCEs with children and teenagers (there is currently little evidence as to a lower age limit for use of a DCE, i.e. at which point the parent must be used as a surrogate) and (2) possible differences between children’s preferences and their parents.
The DCE has been designed with five attributes, each with three levels. 40 This results in a full factorial design of 243 scenarios. An orthogonal fractional design results in 28 scenarios and still allows for the assessment of main effects. A constant comparator will be chosen, which represents the mid-point of each scenario. The remaining scenarios will be randomly divided into two questionnaires (A and B), with both containing the comparison of the two extremes to the constant scenario. Each questionnaire will contain 15 scenarios. Patient and parents will be randomised to one of two groups to receive either questionnaire A or B at follow-up.
Follow-up
Primary and secondary outcome measures will be reassessed at 1 year: preferences for care (DCE) will be measured at 1 year only. Training competencies however, will be measured at two time points: immediately post-training and at 1 year. Consent will also be sought to assess clinical outcomes (routine HbA1c level) up to 2 years.
Training for research nurses
Onsite nurses (or other staff where appropriate) will be trained to conduct study procedures (patient approach and consent, data collection) via prestudy group training sessions. Research nurses will be supported throughout the trial period by the research team.
Piloting: outcome measures and the training programme
Piloting: patient and parent outcome measures
A pilot study of participant questionnaires was undertaken with patients and parents attending the paediatric diabetes clinic in Cardiff (University Hospital of Wales). Patients registered at the Cardiff clinic were identified and recruited by their clinical carer (diabetes specialist nurse).
Measures included in study questionnaires have been previously validated in other populations. The purpose of this pilot study was therefore to determine overall acceptability of the measures in this patient group, particularly in terms of presentation and design. A sample of six children (five aged 11+ years and one aged 7–10 years) and five carers completed age-appropriate questionnaires. Following completion, a nominated member of the research team carried out cognitive debriefing using a semistructured interview. Questions assessed acceptability of the questionnaire items, ease of understanding and length. For older children (11–15 years) this process took place on a one-to-one basis with the researcher. For younger children, a parent or carer was also present for the interview. Cognitive interviews were conducted using standard probes related to particular areas of interest in the questionnaires. Informal analysis of these probes indicated that questionnaires are generally acceptable to both patients and carers. However, respondents generally had some difficultly in completing the DCE component of the questionnaire. This measure will therefore not be included as part of the baseline assessment, and will form a separate questionnaire at follow-up only. Further piloting of the DCE using modified instructions has been carried out with two additional patients and three carers, recruited through patient/carer support groups and members of the Stakeholder Action Group (SAG) convened during the development phase of the study. Having obtained written consent and following completion of the DCE, a nominated member of the research team undertook cognitive debriefing interviews as outlined above. No further changes to the DCE were made following the second round of piloting. If indicated by the data, formal coding of transcript extracts may be undertaken. 41
Piloting the clinician training programme
The clinician training programme (including both face-to-face seminars and the online learning component) has been piloted with staff at the paediatric diabetes clinics in Cardiff (University Hospital of Wales), Newport (Royal Gwent Hospital), Salisbury (District Hospital) and Bristol (Children’s Hospital).
Serious adverse events
The current study is not a Controlled Trial of an Investigational Medicinal Product (CTIMP), but an evaluation of a psychosocial intervention. Therefore, no additional risks for patients are expected to occur as a result of participation, over and above those incurred during routine clinical care. However, data relating to AEs and serious adverse events (SAEs) occurring as a result of the condition under study (type 1 diabetes) will be routinely monitored via study CRFs and reported quarterly to the Trial Management Team and Trial Steering Committee where appropriate.
-
Adverse event any untoward medical occurrence in a study participant
-
Serious adverse event any untoward and unexpected medical occurrence or effect that:
-
– results in death
-
– is life-threatening (refers to an event during which the participant was at risk of death at the time of the event; it does not refer to an event which might have caused death had it been more severe in nature)
-
– requires hospitalisation, or prolongation of existing hospitalisation
-
– results in persistent/significant disability or incapacity
-
– is a congenital abnormality or birth defect.
-
Statistical considerations
Randomisation
Half of the trial centres will be randomised to the intervention arm, and half to the control arm. Teams will be recruited and then randomisation will be optimally balanced42 for:
-
population (patient list) size
-
whether care is provided by a paediatric-trained specialist nurse (i.e. presence or absence of any paediatric-trained nurse within the clinic)
-
specialist adolescent (e.g. a separate transition clinic) or child clinics.
Sample size
In order for an individually randomised trial to have 80% power to detect a moderate effect size of 0.4 for HbA1c at a 5% significance level, 200 patients would be required. Audit data from the Wales Paediatric Diabetes Interest Group consisting of data on 750 children from all 13 centres in Wales indicates an intracluster correlation coefficient of 0.08 for HbA1c in patients aged 4–15 years. With 24 centres recruiting an average of 23 patients each, this inflates the total sample size required to 550. To allow for loss to follow-up, we intend to recruit 700 patients (78% follow-up). Currently, 29 centres have been recruited to allow for any subsequent dropout.
Analysis
Proposed methods of analysis are outlined in the following section.
Main analysis
The main analysis will be intention to treat comparing the two groups of patients on HbA1c values at 1 year. This will use multilevel modelling to allow for cluster (centre) and individual effects (including baseline levels of HbA1c as a covariate). Secondary analysis on other outcomes such as QoL and cost will also be conducted using multilevel modelling incorporating baseline scores as covariates. A dose–response analysis will be conducted to explore associations between the amount of patient contact and an intervention effect. The two groups will also be compared on the non-attendance rate as the intervention may improve motivation to attend.
A recent review of patient outcome measures used in diabetes, although predominantly in adults, concluded that whilst most have been shown to have content validity, considerably fewer have been shown to be reliable and none had been shown to be responsive to change. 43 Responsiveness of the specific measures used will be assessed using both effect sizes and correlation to clinical variables and self-rated change.
Competence, confidence and importance of behaviour change counselling will be compared between the two groups at 1 year, using a two-level linear regression model controlling for profession. Short- and long-term impact of the intervention will be analysed within the intervention group only at three time points using repeated measures analysis of variance. Analysis of reliability of the competence inventory will be conducted on the trial data. Generalisability theory44 will be used to identify key sources of variability and generate reliability statistics relating to raters, professional (nurse/doctor/other) and occasion.
Subgroup and interim analysis
No formal subgroup analyses are planned. However, exploratory analysis of the impact of patient level factors (e.g. age and gender) and clinic level factors (e.g. size of clinic and level of specialist psychological support) on the effect of the intervention will be carried out.
No interim analyses are planned.
Cost-effectiveness analysis
The aim of the economics in the project is twofold. First, a cost-effectiveness analysis will be undertaken assessing costs against the primary outcome measure (HbA1c level). An NHS perspective will be adopted. Costs will include the extra cost of the intervention (including training) as well as differences in cost generating events such as hospital admissions, contacts with the diabetes team and clinic attendances. If non-dominance occurs, an incremental cost-effectiveness ratio will be produced. A series of sensitivity analyses will assess robustness of the results. Patient-borne and other costs will also be determined but reported separately.
The DCE will be analysed separately for patients and parents using a multilevel logistic model to allow for clustering at the level of clinic and multiple scenarios. The analysis will focus on the main effect of the attributes used. Exploratory analysis will compare paired parent and patient utilities at an individual level and assess the impact of age of patient on this.
Data storage and retention
All data will be kept for 15 years in line with Cardiff University’s Research Governance Framework Regulations for clinical research. Electronic data will be stored confidentially on password-protected servers maintained on the Cardiff University Network. Paper copies of participant information and/or data will be kept in lockable filing cabinets, to which only members of the research team will have access.
Trial closure
The end of the trial will be considered as the date on which the last patient has completed their follow-up assessment (end of September 2009).
Regulatory issues
Ethical approval
The Chief Investigator has obtained approval from the Thames Valley Research Ethics Committee (REC). The study must be submitted for Site Specific Assessment (SSA) at each participating NHS Trust. The Chief Investigator will require a copy of the SSA approval letter before accepting participants into the study. The study will be conducted in accordance with the recommendations for physicians involved in research on human subjects adopted by the 18th World Medical Assembly, Helsinki 1964, and later revisions.
Consent
Consent to participate in the study must be sought from each patient and parent only after they have had sufficient time to read the information sheet and consider the implications of taking part in the study. Signed consent forms must be obtained from all parents, and signed consent/assent from children. All participants are free to withdraw from the study at any time without stating a reason and without prejudicing further treatment.
Confidentiality
The Chief Investigator and the research team will preserve the confidentiality of participants in accordance with the Data Protection Act 1998.
Indemnity
Cardiff University will provide indemnity and compensation in the event of a claim by, or on behalf of participants, for negligent harm as a result of the study design and/or in respect of the protocol authors/research team. Cardiff University does not provide compensation for non-negligent harm.
All participants will be recruited at NHS sites and therefore the NHS indemnity scheme/NHS professional indemnity will apply with respect to claims arising from harm to participants at site management organisations.
Trial sponsorship
Cardiff University will act as sponsor for trial. Delegated responsibilities will be assigned to the NHS trusts taking part in the this study.
Funding
The DEPICTED study is funded by the UK Department of Health through the NHS Health Technology Assessment (HTA) programme.
Audits and inspections
The trial is subject to inspection by NHS R&D National Coordinating Centre for Health Technology Assessment (NCCHTA) as the funding organisation. The study may also be subject to inspection and audit by Cardiff University under their remit as sponsor.
Trial management
A Trial Management Group (TMG) consisting of all coapplicants, researchers and the Chief Investigator, has been set up and will meet monthly. The day-to-day management of the trial will be coordinated by the core project team, consisting of the two Chief Investigators, trial statistician, trial coordinator and data manager.
Data monitoring and quality assurance
TSC
A Trial Steering Committee (TSC) has also convened, consisting of a Chair, an independent statistician and a number of independent members (see Appendix C). The TSC also served as the Study Steering Committee (SSC) during the development phase of this trial. The TSC will meet before the trial commences, and at least once a year, at their discretion.
Data Monitoring Committee
The TSC will initially fulfil the role of a Data Monitoring Committee (DMC), unless they decide to constitute a separate DMC.
Publication policy
DEPICTED is a two-phase study of intervention development and evaluation within the field of paediatric diabetes. Study coapplicants and collaborators are drawn from a number of academic, clinical and patient organisations. Criteria based on British Medical Journal rules on authorship and contributorship (http://bmj.bmjjournals.com/advice; appendix A) will be used to acknowledge the level and nature of contribution of key individuals in publications arising from the project. In particular, the TMG must agree all proposals for publication using DEPICTED data. These publications fall into three categories:
-
level 1 publications central to DEPICTED
-
level 2 publications clearly related to DEPICTED but not central to it
-
level 3 publications of work derived from DEPICTED but not part of it.
Further detail relating to these categories is provided in Appendix A.
Milestones
A Gantt chart for the trial is appended to this report.
References
- Metcalfe MA, Baum JD. Incidence of insulin dependent diabetes in children aged under 15 years in the British Isles during 1988. BMJ 1991;302:960-1.
- La Greca A, Follansbee D, Skyler J. Developmental and behavioural aspects of diabetes management in youngsters. J Child Health Care 1990;19:132-9.
- Scottish Study Group for the Care of the Young Diabetic . Factors influencing glycaemic control in young people with type 1 diabetes in Scotland: a population-based study (DIABAUD2). Diabetes Care 2001;24:239-4.
- Amiel SA, Sherwin RS, Simonson DC, Lauritano AA, Tamborlane WV. Impaired insulin action in puberty. A contributing factor to poor glycaemic control in adolescents with diabetes. N Engl J Med 1986;31:215-19.
- Hampson SE, Skinner TC, Hart J. Effects of educational and psychosocial interventions for adolescents with diabetes mellitus: a systematic review. Health Technol Assess 2001;5.
- Channon S, Smith VJ, Gregory JW. A pilot study of motivational interviewing in adolescents with diabetes. Arch Dis Child 2003;88:680-3.
- Pill R, Stott N, Rollnick S, Rees M. A randomized controlled trial of an intervention to improve the care given in general practice to Type II diabetic patients: patient outcomes and professional ability to change behaviour. Fam Pract 1998;15:229-35.
- Pill R, Rees M, Stott N, Rollnick S. Can nurses learn to let go? Issues arising from an intervention designed to improve patients’ involvement in their own care. J Adv Nurs 1999;29:1492-9.
- Channon SJ, Huws-Thomas MV, Rollnick S, Hood K, Cannings-John RL, Rogers C, et al. A multicenter randomized controlled trial of motivational interviewing in teenagers with diabetes. Diabetes Care 2007;30:1390-5.
- Satin W, La Greca AM, Zigo MA, Skyler JS. Diabetes in adolescence: effects of a multifamily group intervention and parent simulation of diabetes. J Pediatr Psychol 1989;14:259-75.
- Jefferson IG, Swift PGF, Skinner TC, Hood GK. Diabetes services in the UK: third national survey confirms continuing deficiencies. Arch Dis Child 2003;88:53-6.
- Butler C, Rollnick S, Stott N. The practitioner, the patient and resistance to change: recent ideas on compliance. Can Med Assoc J 1996;154:1357-62.
- Marteau T, Lerman C. Genetic risk and behaviour change. BMJ 2001;322:1056-9.
- Jepson R. The Effectiveness of Interventions to Change Health-Related Behaviours: A Review of Reviews 2000.
- Miller W, Andrews N, Wilbourne P, Bennett M, Miller W, Heather N. Treating addictive behaviours. New York: Wiley; 1998.
- Kok G, van den Borne B, Dolan Mullen P. Effectiveness of health education and health promotion: meta-analyses of effect studies and determinants of effectiveness. Patient Educ Counsel 1997;30:19-27.
- Miller W, Rollnick S. Motivational interviewing: preparing people to change. New York: Guilford Press; 2002.
- Amrhein PC, Miller WR, Yahne CE, Palmer M, Fulcher L. Client commitment language during motivational interviewing predicts drug use outcomes. J Consult Clin Psychol 2003;71:862-78.
- Burke BL, Arkowitz H, Menchola M. The efficacy of motivational interviewing: a meta-analysis of controlled clinical trials. J Consult Clin Psychol 2003;71:843-61.
- Knight KM, Bundy, C, Morris R, Higgs JF, Jameson RA, Unsworth P, et al. The effects of group motivational interviewing and externalizing conversations for adolescents with type-1 diabetes. Psychol Health Med 2003;8:149-57.
- Viner RM, Cristie D, Taylor V, Hey S. Motivational/solution-focused intervention improves HbA1c in adolescents with Type 1 diabetes: a pilot study. Diabet Med 2003;20:739-42.
- Butler C, Rollnick S, Cohen, D, Russell I, Bachmann M, Stott N. Motivational consulting versus brief advice for smokers in general practice: A randomized trial. Br J Gen Pract 1999;49:611-16.
- Rollnick S. Consultations about behaviour change. Resp Med 2000;94:24-6.
- Arkowitz H, Westra H. Integrating motivational interviewing and cognitive behavioural therapy in the treatment of depression and anxiety. J Cognitive Psychother 2004;18.
- Kinmouth A, Woodcock A, Griffin S, Spiegal N, Campbell M. Randomised controlled trial of patient centred care of diabetes in general practice: impact on current well-being and future disease risk. BMJ 1998;317:1202-8.
- Rollnick S, Mason P, Butler C. Health Behaviour change: a guide for practitioners. Edinburgh: Churchill Livingstone; 1999.
- Mattson M, Miller W, Heather N. Treating addictive behaviours. New York: Wiley; 1998.
- Heather N, Rollnick S, Bell A, Richmond R. Effects of brief counselling among heavy drinkers identified on general hospital wards. Drug Alcohol Rev 1996;5:29-38.
- Rollnick S, Miller W, Heather N. Treating addictive behaviours. New York: Plenum; 1998.
- Rollnick S, Heather N, Bell A. Negotiating behaviour change in medical settings: the development of brief motivational interviewing. J Ment Health 1992;1.
- McCambridge J, Strang J. The efficacy of single session motivational interviewing in reducing drug consumption and perceptions of drug-related risk and harm among young people: results from a multi-randomised trial. Addiction 2004;99:39-52.
- Puffer S, Torgerson D, Watson J. Evidence for risk of bias in cluster randomised trials: review of recent trials published in three general medical journals. BMJ 2003;327:299-302.
- Eiser C, Morse R. Quality-of-life measures in chronic disease of childhood. Health Technol Assess 2001;5.
- Varni J, Burwinkle T, Jacobs J, Gottschalk M, Kaufman F, Jones K. Reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales and Type 1 Diabetes Module. Diabetes Care 2003;26:631-7.
- Weissberg-Benchell J, Glasgow A, Tynan W, Wirtz P, Turek J, Ward J. Adolescent diabetes management and mismanagement. Diabetes Care 1995;18:77-82.
- Williams G, Freedman Z, Deci E. Supporting autonomy to motivate glucose control in patients with diabetes. Diabetes Care 1998;21:1645-51.
- Howie J, Heaney D, Maxwell M, Walker J. A comparison of a Patient Enablement Instrument (PEI) against two established satisfaction scales as an outcome measure of primary care consultations. Fam Pract 1998;15:165-71.
- Dolovich L, Nair K, Ciliska D, Lee H, Birch S, Gafni A, et al. The Diabetes Continuity of Care Scale: the development and evaluation of a scale that measures continuity from the patient perspective. Health Soc Care Community 2004;12:475-87.
- Lane C, Huws-Thomas M, Hood K, Rollnick S, Edwards K, Robling M. Measuring adaptations of motivational interviewing: the development and validation of the Behaviour Change Counselling Index (BECCI). Patient Educ Couns 2005;56:166-73.
- Louviere J, Hensher D, Swait J. Stated choice methods, analysis and application. Cambridge: Cambridge University Press; 2003.
- Willis G. Cognitive interviewing: a tool for improving questionnaire design. Thousand Oaks, CA, USA: Sage; 2005.
- Rabb G, Butcher I. Balance in cluster randomised trials. Stat Med 2001;20:351-65.
- Garratt AM, Schmidt L, Fitzpatrick R. Patient-assessed health outcome measures for diabetes: a structured review. Diabet Med 2002;19:1-11.
- Brennan R. Statistics for social science and public policy: generalizability theory. New York: Springer; 2001.
Appendices
-
Appendix A DEPICTED publication policy
-
Appendix B Trial-phase Gantt chart
-
Appendix C TSC membership.

Appendix A: DEPICTED publication policy
Development and Evaluation of Psychosocial Interventions for Children and Teenagers Experiencing Diabetes (The DEPICTED study): publication policy
Scope
This document relates to publications arising from the DEPICTED study.
Publication policy
Many people will contribute to the DEPICTED study during its course, including members of the core project team, management team, steering committee, participating clinicians, South East Wales Trials Unit (SEWTU) staff and others. This document addresses how individuals contribute to the publication process to ensure timely study outputs in an equitable, efficient and transparent manner.
Principles regarding authorship and writing
-
All proposals for publications using DEPICTED data must be approved by the study management team.
-
A lead author and wider writing team will be established for each identified paper.
-
All potential contributors will have the opportunity to opt in to a writing team.
-
It is the responsibility of the Principal Investigators to ensure balance and inclusivity in writing teams across the range of likely study publications.
-
It is the responsibility of the Principal Investigators in conjunction with the lead author to decide authorship order.
-
All named authors must meet authorship criteria (detailed below).
-
Each author should have participated sufficiently to take public responsibility for the publication’s content.
-
A timetable for publication will be agreed with each lead author and approved by the management team and will include a start date (for drafting) and target submission date.
-
Publication timetabling must account for appropriate review by the funding body.
-
For any one paper, each substantive new draft will be circulated by the lead author to the writing team to ensure opportunity to contribute.
The following criteria based on British Medical Journal rules on authorship and contributorship (see http://resources.bmj.com/bmj/authors/article-submission/authorship-contributorship) will be used to acknowledge the level and nature of contribution of key individuals in publications arising from the project. Note that this states the following.
Authorship
The uniform requirements for manuscripts submitted to medical journals state that authorship credit should be based only on substantial contribution to:
-
conception and design, or analysis and interpretation of data
-
drafting the article or revising it critically for important intellectual content
-
and final approval of the version to be published.
All of these conditions must all be met. Participation solely in the acquisition of funding or the collection of data does not justify authorship.
The lead author and/or one of the Principal Investigators will be identified as guarantors of the paper. The guarantor accepts full responsibility for the work and/or the conduct of the study, had access to the data and controlled the decision to publish.
Publication level and authorship listing
Publications fall into two categories, which will be agreed by the study management team:
-
Level 1 Publications central to DEPICTED study: authorship will take the form ‘A, B, C … and the DEPICTED study team’. Members of the management team (including coapplicants) would be able to list such publications in their CVs.
-
Level 2 Publications derived from DEPICTED study, but not central to it: authorship will take the form ‘A, B, C … in collaboration with the DEPICTED study team’. In normal circumstances other members of the management team would not list such publications in their CVs.
Contributorship and acknowledgements
Contributors to the DEPICTED study will be acknowledged on each publication and on the study website. Where journal restrictions apply, it may be that readers are simply directed to the study website for full details of contribution. Contributorship relates to the DEPICTED study as a whole, not necessarily individual study outputs. Contributors may also be already listed as authors on individual papers. Two levels of contributorship are distinguished:
Major contributor Members of the study team who have made a major scientific contribution to design, data collection, analysis or reporting, over a period of at least 6 months. Whilst it is likely that an individual’s contribution will be continuous, for some it may have been appropriately intermittent. They should have devoted a modicum of their employed time to the study during each month of that period. Acknowledgement as a major contributor is reserved for those people who have invested heavily in the study.
Other contributors (organisational, clinical or administrative) These should have made a major non-scientific contribution to implementing the protocol over a period of at least 6 months, for example administrative staff, research nurses, lead clinicians, clinical collaborators.
Acknowledgements
We shall acknowledge all others who have played a part in the study but do not fulfil the criteria for contributors.
-
date of current version: v1.3
-
previous version date: 17.11.06.
Planned publications
| Study component/outline paper | Aims of paper/notes | Possible target journal/s (impact factor) | Lead writer | Other writers | Publication level | Priority | |
|---|---|---|---|---|---|---|---|
| Development phase | |||||||
| 1 | Clinician views and experiences of psychosocial support provision within UK paediatric diabetes care: a telephone survey |
Possibly short report Possible as companion paper to #7 |
Archives of Diseases in Childhood (IF: 2.09) Diabetic Medicine (IF: 2.484) |
SC | HH, MR, JG, LL, LC | 1 | Medium |
| 2 | Patient and parent views on paediatric diabetes care: a focus group study | Exploring patient/parent views of: (1) involvement in diabetes consultations and (2) communication with clinicians |
Practical Diabetes (IF:) Diabetic Medicine (IF: 2.484) Quality and Safety In Healthcare (IF: 2.382) |
KHa | MR, HH, JG, LL | 1 | Medium/low |
| 3 | Developing a complex training intervention/learning programme | Describe the conceptual basis, development and piloting of ‘Talking Diabetes’ learning programme |
Patient Education & Counselling (IF: 1.429) Medical Education (IF: 2.467) |
MR | KB, HH, JG, SR, SC | 1 |
High In place for main trial paper |
| 4 | Developing a patient and parent agenda-sharing tool | Describe the conceptual basis, development and piloting of 3T |
Patient Education & Counselling (IF: 1.429) Health Expectations (IF: 2.089) |
HH | KB, MR, SR, JG, CC | 1 (note: not described in original protocol) |
High In place for main trial paper |
| 5 | Engaging stakeholders in research process | To describe experiences of engaging stakeholders (particularly patients/families) in research process/intervention development | Health Expectations (IF: 2.089) | LL, MR | CC, KB, HH, KHa | 2 | Medium |
| 6 | Surveying training needs and preferences of diabetes HCPs (postal survey) | Possible companion paper to #1 |
Diabetic Medicine (IF: 2.484) ? |
HH | MR, KH, JG, LC | 1 |
Medium Pref. in place for main trial paper |
| 7 | A discourse analysis of experimental consultations | Addressing patient activation and involvement from patient perspective |
Patient Education & Counselling (IF: 1.429) Health Communication (IF: 1.277) |
KB | HH, LC | Medium | |
| Intervention phase | |||||||
| 8 | Study protocol paper | Description of detailed study protocol | BMC Health Services Research (IF: 1.198) | RMcN | JG, MR, KH | 1 | High (prior to submission of main trial paper) |
| 9 | Patient preferences in paediatric diabetes: a discrete choice experiment | Development and results of DCE | Medical Decision Making (IF: 1.736) | ML | KH, MR, DC, KB | 1 | High |
| 10 | Main trial results metabolic and psychological | Metabolic, psychological | JG/MR | RMcN, DC, KH, SC, BP | 1 | High | |
| 11 | Main trial results cost-effectiveness | Economic evaluation | ?? | DC | JG, MR, ML, KH, BP | 1 | High |
| 12 | PE | RMcN, HH | MR, KH, JG, SC, SR, LC | 2 | Med | ||
| 13 | Assessing clinical competencies | Re-validation, results of clinician assessments from trial | SR | MR, JG, SC, KH, HH, RMcN, LC | 1 | Medium/high | |
| Other written output | |||||||
| Main report to HTA | JG, MR | All | |||||
Written output
Milestones for main writing activity/submission dates (during funded timescale of study).
| 2008 | 2009 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8 | 9 | 10 | 11 | 12 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| Interim | |||||||||||||||||
| Final | |||||||||||||||||
| #1: Telephone survey | SC | ||||||||||||||||
| #2: Focus group study | |||||||||||||||||
| #3: Learning programme | MR | ||||||||||||||||
| #4: Agenda-setting | HH | ||||||||||||||||
| #5: Stakeholder paper | MR | ||||||||||||||||
| #6: Postal survey | HH | ||||||||||||||||
| #7: Discourse analysis | |||||||||||||||||
| #8: Protocol paper | RMc | ||||||||||||||||
| #9: DCE | |||||||||||||||||
| #10: Trial results (clinical) | JG | ||||||||||||||||
| #11: Trial results (economic) | DC | ||||||||||||||||
| #12: Process evaluation | |||||||||||||||||
| #13: Clinical competencies | |||||||||||||||||
| Report | |||||||||||||||||
| Initial drafting | |||||||||||||||||
| Formal review and revision | |||||||||||||||||
General model for paper writing in DEPICTED study
Conferences
| Status | Date of conference | Conference | Venue | Title | Presenter | Type | |
|---|---|---|---|---|---|---|---|
| 1 | Completed | June 2005 | CHSR launch | Cardiff | ‘Talking diabetes: The DEPICTED Study – a CHSR collaboration between General Practice and Child Health’. | MR | Poster |
| 2 | Completed | March 2006 | SAPC (regional meeting) | Birmingham | ‘Putting the package together’: developing a complex intervention through multiprofessional and patient collaboration. | MR | Poster |
| 3 | Completed | July 2006 | COMET | Cardiff | ‘Orchestrating the voices of children, parents and clinicians in paediatric diabetes care’. | KB | Oral |
| 4 | Completed | September 2006 | ISPAD | Cambridge | Surveying psychosocial practice in paediatric diabetes care: Preparation for the DEPICTED study. | HH | Poster |
| 5 | Completed | September 2006 | ISPAD | Cambridge | ‘Talking Diabetes’ in routine care encounters: Development of a complex intervention to support behaviour change (The DEPICTED study). | KB | Poster |
| 6 | Completed | September 2006 | BSPED | Cork | Engaging clinicians in course design for the DEPICTED trial: a professional survey of communication skills needs and training preferences. | HH | Oral |
| 7 | Completed | September 2006 | BSPED | Cork | ‘Talking Diabetes’ in routine care encounters: Development of a complex intervention to support behaviour change (The DEPICTED study). | KB | Poster |
| 8 | Invited | March 2007 | Diabetes UK | Glasgow | TBC | JG | Oral |
| 9 | Accepted | June 2007 | COMET | Lugano | Patient activation with 3T: What is the impact of agenda-setting on paediatric diabetes consultations? | KB | Oral |

Appendix B: Trial-phase Gantt chart
| Year | 2008 | 2009 | ||||||||||||||||||||
| Calendar Month | Mar | Apr | May | Jun | Jul | Aug | Sept | Oct | Nov | Dec | Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sept | Oct | Nov | Dec |
| Project Month | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 | 42 | 43 | 44 | 45 | 46 | 47 | 48 | 49 | 50 | 51 | 52 | 53 | 54 | 55 |
| Obtain local R&D/ethical approval (including contracts) | B3 | |||||||||||||||||||||
| Further DCE pilot work | ||||||||||||||||||||||
| Prepare documents/equipment for trial centres | ||||||||||||||||||||||
| Recruit patients, collect baseline data | ||||||||||||||||||||||
| Identify & select patients | B3 | |||||||||||||||||||||
| Recruit & consent patients | B1&2 | B3 | ||||||||||||||||||||
| Collect baseline data | B1&2 | B3 | ||||||||||||||||||||
| Randomise clinical teams | B3 | |||||||||||||||||||||
| Train teams (intervention) | B1 | B2 | B3 | B3 | ||||||||||||||||||
| Intervention phase | B1 | B2 | B3 | B1 | B2 | B3 | ||||||||||||||||
| Clinician competency assessment | B1 | B2 | B3 | B1 | B2 | B3 | B3 | B1 | B2 | B3 | ||||||||||||
| 1 year follow up (including DCE) | B1 | B2 | B3 | |||||||||||||||||||
| Train teams (control) | ||||||||||||||||||||||
| Data cleaning | ||||||||||||||||||||||
| DCE analysis | ||||||||||||||||||||||
| Data analysis/report writing | ||||||||||||||||||||||
| Submit final report | ||||||||||||||||||||||

Appendix C: Trial Steering Committee membership
Trial Steering Committee independent members
Professor Frank Snoek
Professor of Medical Psychology
Medical Centre, Vrije Universiteit
Dr Julie Edge
Consultant Paediatrician
Dept of Paediatrics, John Radcliffe Hospital
Prof Tim Peters
Professor of Primary Care Health Services Research
Community Based Medicine, The Grange
Dr Steve Greene
Reader in Child & Adolescent Health
Maternal & Child Health Sciences, University of Dundee
Appendix 9 Raw data for κ-statistics calculated in consultation ratings
| Evocation | Score | Second rater | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| First rater | 1 | 3 | 4 | 0 | 0 | 0 |
| 2 | 0 | 4 | 2 | 1 | 0 | |
| 3 | 0 | 1 | 0 | 2 | 1 | |
| 4 | 0 | 0 | 1 | 0 | 1 | |
| 5 | 0 | 0 | 0 | 0 | 0 | |
| Collaboration | Score | Second rater | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| First rater | 1 | 3 | 3 | 0 | 1 | 0 |
| 2 | 2 | 2 | 0 | 0 | 1 | |
| 3 | 0 | 1 | 3 | 1 | 2 | |
| 4 | 0 | 0 | 0 | 0 | 1 | |
| 5 | 0 | 0 | 0 | 0 | 0 | |
| Autonomy supportive | Score | Second rater | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| First rater | 1 | 5 | 1 | 0 | 1 | 0 |
| 2 | 3 | 1 | 1 | 0 | 0 | |
| 3 | 0 | 3 | 1 | 2 | 1 | |
| 4 | 0 | 0 | 0 | 0 | 1 | |
| 5 | 0 | 0 | 0 | 0 | 0 | |
| Guiding style | Score | Second rater | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| First rater | 1 | 4 | 2 | 0 | 1 | 0 |
| 2 | 2 | 3 | 1 | 0 | 0 | |
| 3 | 0 | 2 | 1 | 2 | 1 | |
| 4 | 0 | 0 | 0 | 0 | 1 | |
| 5 | 0 | 0 | 0 | 0 | 0 | |
| Shared agenda-setting tasks | Score | Second rater | ||
|---|---|---|---|---|
| 0 | 1 | 2 | ||
| First rater | 0 | 7 | 2 | 3 |
| 1 | 2 | 0 | 6 | |
| 2 | 0 | 0 | 0 | |
| Shared agenda-setting skills | Score | Second rater | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | ||
| First rater | 0 | 9 | 0 | 1 | 1 | 1 | 2 | 0 |
| 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |
| 2 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | |
| 3 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Pros and cons tasks | Score | Second rater | ||
|---|---|---|---|---|
| 0 | 1 | 2 | ||
| First rater | 0 | 17 | 0 | 0 |
| 1 | 1 | 1 | 1 | |
| 2 | 0 | 0 | 0 | |
| Pros and cons skills | Score | Second rater | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | ||
| First rater | 0 | 17 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 2 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Importance and confidence tasks | Score | Second rater | ||
|---|---|---|---|---|
| 0 | 1 | 2 | ||
| First rater | 0 | 16 | 0 | 0 |
| 1 | 3 | 0 | 0 | |
| 2 | 0 | 0 | 1 | |
| Importance and confidence skills | Score | Second rater | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | ||
| First rater | 0 | 16 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 3 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Brainstorming tasks | Score | Second rater | ||
|---|---|---|---|---|
| 0 | 1 | 2 | ||
| First rater | 0 | 12 | 0 | 1 |
| 1 | 1 | 0 | 4 | |
| 2 | 1 | 0 | 1 | |
| Brainstorming skills | Score | Second rater | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | ||
| First Rater | 0 | 12 | 1 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 3 | 0 | 0 | 1 | 0 | 4 | 0 | 0 | |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
List of abbreviations
- 3T
- 3T: TimetoTalk (agenda-setting tool)
- A&E
- accident and emergency
- ANOVA
- analysis of variance
- BMI
- body mass index
- CBT
- cognitive behavioural therapy
- CD-ROM
- compact disc read-only memory
- CI
- confidence interval
- CRF
- case record form
- DCCS
- Diabetes Continuity of Care Scale
- DCCT
- Diabetes Control and Complications Trial
- DCE
- discrete choice experiment
- DEPICTED
- Development and Evaluation of a Psychosocial Intervention for Children and Teenagers Experiencing Diabetes
- HbA1c
- glycosylated haemoglobin
- HCCQ
- Health Care Climate Questionnaire
- HCP
- health-care professional
- HRQol
- health-related quality of life
- HTA
- health technology assessment
- ICC
- intracluster correlation coefficient
- MI
- motivational interviewing
- MITI
- Motivational Interviewing Treatment Integrity
- MRC
- Medical Research Council
- NICE
- National Institute for Health and Clinical Excellence
- PAID
- Problem Areas in Diabetes
- PE
- process evaluation
- QoL
- quality of life
- R&D
- research and development
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- SAG
- Stakeholder Action Group
- SD
- standard deviation
- SEWTU
- South East Wales Trials Unit
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Department of Public Health and Epidemiology, University of Birmingham
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, University of Liverpool
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Peter Brocklehurst, Professor of Women’s Health, Institute for Women’s Health, University College London
-
Professor Jenny Donovan, Professor of Social Medicine, University of Bristol
-
Professor Jonathan Green, Professor and Acting Head of Department, Child and Adolescent Psychiatry, University of Manchester Medical School
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Freddie Hamdy, Professor of Urology, Head of Nuffield Department of Surgery, University of Oxford
-
Professor Allan House, Professor of Liaison Psychiatry, University of Leeds
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor Irwin Nazareth, Professor of Primary Care and Head of Department, Department of Primary Care and Population Sciences, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norrie, Chair in Clinical Trials and Biostatistics, Robertson Centre for Biostatistics, University of Glasgow
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Martin Underwood, Professor of Primary Care Research, Warwick Medical School, University of Warwick
-
Professor Marion Walker, Professor in Stroke Rehabilitation, Associate Director UK Stroke Research Network, University of Nottingham
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Tom Foulks, Medical Research Council
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Diana Baralle, Consultant and Senior Lecturer in Clinical Genetics, University of Southampton
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Public contributor
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Nicola Lennard, Senior Medical Officer, MHRA
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Public contributor
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Public contributor
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Matthew Thompson, Senior Clinical Scientist and GP, Department of Primary Health Care, University of Oxford
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Joanna Jenkinson, Board Secretary, Neurosciences and Mental Health Board (NMHB), Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Public contributor
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire County Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Mrs Jean Thurston, Public contributor
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Public contributor
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Public contributor
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Public contributor
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Public contributor
-
Mr Sankaran Chandra Sekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Public contributor
-
Mr Seumas Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Dr Matthew Wilson, Consultant Anaesthetist, Sheffield Teaching Hospitals NHS Foundation Trust
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan, Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Public contributor
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer, Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Public contributor
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Public contributor
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Public contributor
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Expert Advisory Network
-
Professor Douglas Altman, Professor of Statistics in Medicine, Centre for Statistics in Medicine, University of Oxford
-
Professor John Bond, Professor of Social Gerontology & Health Services Research, University of Newcastle upon Tyne
-
Professor Andrew Bradbury, Professor of Vascular Surgery, Solihull Hospital, Birmingham
-
Mr Shaun Brogan, Chief Executive, Ridgeway Primary Care Group, Aylesbury
-
Mrs Stella Burnside OBE, Chief Executive, Regulation and Improvement Authority, Belfast
-
Ms Tracy Bury, Project Manager, World Confederation of Physical Therapy, London
-
Professor Iain T Cameron, Professor of Obstetrics and Gynaecology and Head of the School of Medicine, University of Southampton
-
Professor Bruce Campbell, Consultant Vascular & General Surgeon, Royal Devon & Exeter Hospital, Wonford
-
Dr Christine Clark, Medical Writer and Consultant Pharmacist, Rossendale
-
Professor Collette Clifford, Professor of Nursing and Head of Research, The Medical School, University of Birmingham
-
Professor Barry Cookson, Director, Laboratory of Hospital Infection, Public Health Laboratory Service, London
-
Dr Carl Counsell, Clinical Senior Lecturer in Neurology, University of Aberdeen
-
Professor Howard Cuckle, Professor of Reproductive Epidemiology, Department of Paediatrics, Obstetrics & Gynaecology, University of Leeds
-
Professor Carol Dezateux, Professor of Paediatric Epidemiology, Institute of Child Health, London
-
Mr John Dunning, Consultant Cardiothoracic Surgeon, Papworth Hospital NHS Trust, Cambridge
-
Mr Jonothan Earnshaw, Consultant Vascular Surgeon, Gloucestershire Royal Hospital, Gloucester
-
Professor Martin Eccles, Professor of Clinical Effectiveness, Centre for Health Services Research, University of Newcastle upon Tyne
-
Professor Pam Enderby, Dean of Faculty of Medicine, Institute of General Practice and Primary Care, University of Sheffield
-
Professor Gene Feder, Professor of Primary Care Research & Development, Centre for Health Sciences, Barts and The London School of Medicine and Dentistry
-
Mr Leonard R Fenwick, Chief Executive, Freeman Hospital, Newcastle upon Tyne
-
Mrs Gillian Fletcher, Antenatal Teacher and Tutor and President, National Childbirth Trust, Henfield
-
Professor Jayne Franklyn, Professor of Medicine, University of Birmingham
-
Mr Tam Fry, Honorary Chairman, Child Growth Foundation, London
-
Professor Fiona Gilbert, Consultant Radiologist and NCRN Member, University of Aberdeen
-
Professor Paul Gregg, Professor of Orthopaedic Surgical Science, South Tees Hospital NHS Trust
-
Bec Hanley, Co-director, TwoCan Associates, West Sussex
-
Dr Maryann L Hardy, Senior Lecturer, University of Bradford
-
Mrs Sharon Hart, Healthcare Management Consultant, Reading
-
Professor Robert E Hawkins, CRC Professor and Director of Medical Oncology, Christie CRC Research Centre, Christie Hospital NHS Trust, Manchester
-
Professor Richard Hobbs, Head of Department of Primary Care & General Practice, University of Birmingham
-
Professor Alan Horwich, Dean and Section Chairman, The Institute of Cancer Research, London
-
Professor Allen Hutchinson, Director of Public Health and Deputy Dean of ScHARR, University of Sheffield
-
Professor Peter Jones, Professor of Psychiatry, University of Cambridge, Cambridge
-
Professor Stan Kaye, Cancer Research UK Professor of Medical Oncology, Royal Marsden Hospital and Institute of Cancer Research, Surrey
-
Dr Duncan Keeley, General Practitioner (Dr Burch & Ptnrs), The Health Centre, Thame
-
Dr Donna Lamping, Research Degrees Programme Director and Reader in Psychology, Health Services Research Unit, London School of Hygiene and Tropical Medicine, London
-
Professor James Lindesay, Professor of Psychiatry for the Elderly, University of Leicester
-
Professor Julian Little, Professor of Human Genome Epidemiology, University of Ottawa
-
Professor Alistaire McGuire, Professor of Health Economics, London School of Economics
-
Professor Neill McIntosh, Edward Clark Professor of Child Life and Health, University of Edinburgh
-
Professor Rajan Madhok, Consultant in Public Health, South Manchester Primary Care Trust
-
Professor Sir Alexander Markham, Director, Molecular Medicine Unit, St James’s University Hospital, Leeds
-
Dr Peter Moore, Freelance Science Writer, Ashtead
-
Dr Andrew Mortimore, Public Health Director, Southampton City Primary Care Trust
-
Dr Sue Moss, Associate Director, Cancer Screening Evaluation Unit, Institute of Cancer Research, Sutton
-
Professor Miranda Mugford, Professor of Health Economics and Group Co-ordinator, University of East Anglia
-
Professor Jim Neilson, Head of School of Reproductive & Developmental Medicine and Professor of Obstetrics and Gynaecology, University of Liverpool
-
Mrs Julietta Patnick, Director, NHS Cancer Screening Programmes, Sheffield
-
Professor Robert Peveler, Professor of Liaison Psychiatry, Royal South Hants Hospital, Southampton
-
Professor Chris Price, Director of Clinical Research, Bayer Diagnostics Europe, Stoke Poges
-
Professor William Rosenberg, Professor of Hepatology and Consultant Physician, University of Southampton
-
Professor Peter Sandercock, Professor of Medical Neurology, Department of Clinical Neurosciences, University of Edinburgh
-
Dr Philip Shackley, Senior Lecturer in Health Economics, Sheffield Vascular Institute, University of Sheffield
-
Dr Eamonn Sheridan, Consultant in Clinical Genetics, St James’s University Hospital, Leeds
-
Dr Margaret Somerville, Director of Public Health Learning, Peninsula Medical School, University of Plymouth
-
Professor Sarah Stewart-Brown, Professor of Public Health, Division of Health in the Community, University of Warwick, Coventry
-
Dr Nick Summerton, GP Appraiser and Codirector, Research Network, Yorkshire Clinical Consultant, Primary Care and Public Health, University of Oxford
-
Professor Ala Szczepura, Professor of Health Service Research, Centre for Health Services Studies, University of Warwick, Coventry
-
Dr Ross Taylor, Senior Lecturer, University of Aberdeen
-
Dr Richard Tiner, Medical Director, Medical Department, Association of the British Pharmaceutical Industry
-
Mrs Joan Webster, Consumer Member, Southern Derbyshire Community Health Council
-
Professor Martin Whittle, Clinical Co-director, National Co-ordinating Centre for Women’s and Children’s Health, Lymington