Notes
Article history
The research reported in this issue of the journal was commissioned by the HTA programme as project number 09/108/02. The contractual start date was in September 2010. The draft report began editorial review in March 2011 and was accepted for publication in May 2011. As the funder, by devising a commissioning brief, the HTA programme specified the research question and study design. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2011. This work was produced by Carroll et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2011 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Description of health problem
Hip fracture is a common problem in the population aged ≥ 60 years. The annual rate of hip fracture in women in the UK has been reported to be exponentially distributed and to be 20 per 10,000, 38 per 10,000 and 73 per 10,000 at 65, 70 and 75 years of age, respectively. 1 Only 5% of fractures occur in men and women under the age of 60 years. 2 Owing to increasingly ageing populations, the absolute number of hip fractures is expected to rise. 3–5 Half of all hip fractures are displaced intracapsular fractures, i.e. unstable fractures in which the blood supply to the femoral head may be impaired, affecting the rate of fracture healing. 2,6,7
The treatment for displaced intracapsular fractures is currently determined by the mobility and functional demands of the patient. Individuals with a displaced intracapsular fracture and low pre-fracture mobility, cognitive impairment or low functional demands are generally treated with hemiarthroplasty (HA);2,8,9 as many as 37% of individuals with hip fractures may be cognitively impaired. 10 Other patients with displaced intracapsular fractures, i.e. young patients and very frail elderly patients with limited mobility or cognitive impairment, tend to be treated with internal fixation. 8 However, there is no consensus regarding the optimal treatment for older individuals who are cognitively intact and have high pre-fracture mobility or function: the options are HA or total hip arthroplasty (THA). 8,9,11 The reported rate of THA in the Trent region of the UK for 1991–2004 was 2.3 per 100,000 diagnosed hip fractures. 12 The vast majority of mobile patients with a displaced intracapsular hip fracture are treated by HA rather than by THA. 13
The principal outcomes associated with hip arthroplasty are dislocation, revision rates and resultant quality of life. THA has been associated with higher rates of dislocation, which may be due to the greater degree of mobility permitted. 4,14 It has also been reported that higher rates of dislocation are more likely if the surgical approach is posterolateral rather than anterolateral and if a smaller femoral head is used. 15–17 The incidence or recurrence of dislocation has been found to be significantly related to a reduction in an individual’s quality of life. 18 HA is particularly associated with pain, infection, loosening of the joint and acetabular erosion. 6,19 Postoperative complications such as loosening and acetabular erosion, in particular, can necessitate revision surgery. Revision rates may therefore be higher for HA than for THA.
Current service provision
In the UK, the vast majority of mobile patients with a displaced intracapsular hip fracture are treated by HA rather than by THA. 13 A survey of 223 UK hospitals in 2000 reported that, for active patients, HA was undertaken at 73% of hospitals, THA at 16% and internal fixation at 37% (the proportions exceed 100% as some hospitals reported using more than one method of treatment). Cemented prostheses were used in 74% of arthroplasties for active patients. 11 The actual number of patients receiving only the two interventions for intracapsular hip fracture, and who were without cognitive impairment and were also independently mobile prior to the fracture, is not known. The National Joint Registry does not report these discrete data.
Description of technology under assessment
The technologies under assessment are HA and THA. HA involves replacing the femoral head, whereas THA replaces both the femoral head and the acetabular articular surface. HA may be unipolar (generally used for patients with lower functional demands2) or, more recently, the more mobile bipolar, which aims to reduce acetabular erosion. 6 These prostheses may or may not be cemented into place. 2
Chapter 2 Definition of the decision problem
The purpose of this report is to perform a review of the evidence to determine the clinical effectiveness and cost-effectiveness of THA in comparison with HA.
Decision problem
What is the clinical effectiveness and cost-effectiveness of THA compared with HA?
Overall aims and objectives of assessment
-
To identify, appraise and synthesise relevant studies satisfying the inclusion criteria for an assessment of clinical effectiveness of THA compared with HA.
-
To identify relevant studies satisfying the inclusion criteria for an assessment of cost-effectiveness, and to summarise the available evidence.
-
To construct a mathematical model to estimate the cost-effectiveness of THA with HA.
Chapter 3 Assessment of clinical effectiveness
Methods for reviewing effectiveness
A review of the evidence for clinical effectiveness has been undertaken systematically following the general principles recommended in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. 20 English and non-English-language studies were included and there was no limit by date.
Identification of studies
A comprehensive search was undertaken in October and December 2010 to identify systematically both clinical effectiveness and cost-effectiveness literature comparing THA and HA in patients with fractures of the femoral neck. The search consisted only of combining terms for THA with terms for HA. The MEDLINE search strategy is reported in Appendix 1. The aim of the strategy was to identify all studies that reported on trials comparing THA with HA. No MeSH (medical subject heading) term was used as the only appropriate term, ‘arthroplasty, replacement, hip’ covers both HA and THA. The strategy using the MeSH term therefore retrieved many studies concerning only one of the procedures, e.g. either THA or HA, but few studies covered both, the study design required for the review. This search was developed by the reviewer (CC) and the information specialist (PE).
The following electronic databases and online conference proceedings were searched from inception for published and unpublished research evidence:
-
MEDLINE (Ovid) 1950 to December 2010
-
EMBASE 1980 to December 2010
-
Cumulative Index to Nursing and Allied Health Literature (EBSCO) 1982 to December 2010
-
The Cochrane Library including the Cochrane Systematic Reviews Database, Cochrane Controlled Trials Register, Database of Abstracts of Reviews of Effects, Health Technology Assessment and NHS Economic Evaluation Database 1991 to December 2010
-
Biological Abstracts [via Institute for Scientific Information (ISI) Web of Science] 1969 to December 2010
-
Science Citation Index (via ISI Web of Science) 1900 to December 2010
-
Social Science Citation Index (via ISI Web of Science) 1956 to December 2010
-
Conference Proceedings Citation Index-Science (via ISI Web of Science) 1990 to December 2010
-
UK Clinical Trials Research Network and the National Research Register archive up to December 2010
-
Current Controlled Trials up to December 2010
-
ClinicalTrials.gov up to December 2010.
All citations were imported into Reference Manager Version 12 (Thomson Reuters, CA, USA) software and duplicates were deleted. Titles and abstracts of all unique citations were then double-screened by two reviewers (CC and AS) using the inclusion criteria outlined below. Any discrepancies were resolved by retrieving the full paper. The full papers of all potentially relevant citations were retrieved so that an in-depth assessment concerning inclusion could be made. The reference lists of all included studies and relevant reviews were also screened to identify additional, relevant studies not retrieved by the search of electronic databases.
Inclusion criteria
Population
Patients eligible for hip replacement as a result of intracapsular fracture and who are able to give consent and were independently mobile prior to fracture.
Intervention
Total hip replacement.
Comparator
Hemiarthroplasty.
Settings
Secondary care.
Outcomes
Primary outcomes
-
Dislocation rate.
-
Revision rate: where possible, the data were analysed separately for early revision, i.e. up to 1 year of surgery or revision for the duration of follow-up as a whole. Revision indicates that the original implant was either replaced by a new prosthesis of the same type or changed for a different type, e.g. HA was revised to THA.
-
Non-revision surgery: (further surgical intervention relating to the affected hip, involving anaesthesia that does not involve the revision or removal of implant, e.g. reduction, removal of cement fragments or application of distal trochanteric transfer) where these data are reported separately from revisions. Analysis describes re-operation events relating to the operated hip only.
-
Any surgery: a combined outcome measure to include all forms of surgery, i.e. an intervention on the affected hip requiring anaesthetic. This includes open and closed reduction of dislocations, and revision and non-revision surgery. The aim was to accommodate event data that do not explicitly specify revision or non-revision surgery, but only ‘additional surgery’ or ‘reoperations’.
Secondary outcomes
-
Hip ratings [e.g. Oxford Hip Score (OHS)].
-
Mobility.
-
Mortality.
-
Surgery duration (in minutes).
-
Hypotension during surgery.
-
Operative blood loss (in millilitres).
-
Postoperative blood transfusion (in units).
-
Postoperative complications, e.g. loosening, erosion, wound infection, pneumonia, deep-vein thrombosis (DVT).
-
Length of hospital stay.
-
Health-related quality of life.
-
Resource utilisation.
-
Cost–utility.
Follow-up
There was no minimum duration of follow-up.
Study design
Randomised controlled trials (RCTs) only, as a scoping report for this project (HTA 09/108/01) identified at least seven such trials.
Exclusion criteria
Population
Patients eligible for hip replacement as a result of intracapsular fracture who are cognitively impaired or who were not independently mobile prior to fracture.
Intervention
Internal fixation.
Data extraction strategy
Data were extracted independently from all included studies by one reviewer (CC) using a data extraction form developed for this review and piloted on two studies (see Appendix 2). All data extracted were checked thoroughly by a second reviewer (AS) and any discrepancies were resolved by discussion and reference to the full paper.
Quality assessment strategy
The quality assessment of included RCTs was undertaken using appropriate quality assessment criteria. There is no published surgical RCT checklist, so this review applied surgical-quality assessment criteria outlined in a relevant Cochrane review. 21 These are included in the Appendix 3. Critical appraisal was performed by one reviewer (CC) and checked thoroughly by a second reviewer (AS). Any discrepancies or differences were resolved by discussion and reference to the full paper.
Methods of analysis/synthesis
Meta-analysis of trials was performed using RevMan 5.0 (The Nordic Cochrane Centre, Copenhagen, Denmark). For discrete and numerical outcomes, relative risk (RR; also known as risk ratio) and risk difference (RD) were reported with 95% confidence intervals (CIs). For continuous outcomes, weighted mean differences were calculated using the inverse variance and reported with 95% CIs. The studies were appraised in terms of clinical validity and methodological heterogeneity to determine whether or not statistical pooling of trial data within a meta-analysis was appropriate. Where studies were meta-analysed, the more conservative random-effects model was used to account for clinical and methodological variations between trials. 22 Statistical heterogeneity was described using the I2 statistic, and potential reasons for any heterogeneity were discussed. The level of heterogeneity was defined as low (< 25%), moderate (25–50%) or high (≥ 50%). 23 Only randomised participants for whom a valid outcome had been evaluated and reported were included in the analysis. 24 The denominators used were determined based on the intention-to-treat principle, i.e. follow-up denominators included individuals who had died, unless an outcome (e.g. hip score) required the patient to respond at a specific point in time (e.g. 1 year). Otherwise, individuals lost to follow-up and therefore without a possible evaluated outcome, e.g. missing data, were excluded. Forest plots are presented for all the analyses in which there was more than one relevant study and sufficient data to undertake a meta-analysis. Results for all analyses, including those of single studies, are presented in summary tables. One comparison is analysed and presented: THA versus HA. Separate analyses were performed both for early follow-up (≤ 1 year), where these data were available, and for all follow-up periods, for the outcomes of dislocations, revisions, any surgery and mortality. The possibility of a difference in outcome for surgical approach, cementing of the prosthesis and the use of unipolar or bipolar prosthesis in hemiarthroplasty has been suggested, but not conclusively addressed, by previous research using randomised trial evidence. 19,21,25,26 Subgroup analyses were therefore performed using Altman and Bland’s27 test of interaction, comparing treatment effects between independent subgroups, applying a method for estimating the ratio of two relative risks. The aim was to determine whether or not differences in outcomes between THA and HA were sensitive to the following potentially confounding variables: approach (anterolateral vs posterior); the use of cement; the use of unipolar or bipolar HA prostheses; and study quality. The subgroups were defined by these variables.
Results
Quantity of research available
The search of electronic databases identified 532 unique citations. After screening, 13 citations representing seven published RCTs satisfied all of the inclusion criteria: Dorr et al. ,28 Skinner et al. ,29 Ravikumar and Marsh,30 Baker et al. ,19 Keating et al. ,2,31 Blomfeldt et al. ,32,33 Macaulay et al. 34–36 and Mouzopoulos et al. 37 An eighth RCT, van den Bekerom et al. ,38 was identified by the clinical advisor (SB) shortly before completion of the report. This study had not been published and catalogued in the databases at the time at which the searches were performed. One further potentially relevant study was excluded because it was unclear whether or not it satisfied the population inclusion criteria (it was published as an abstract only), and it did not report any of the primary outcomes. 39 Three ongoing trials were also identified (ISRCTN70736853, NCT00556842 and NCT01109862). No additional relevant papers were identified from reference tracking. Details of the screening and inclusion process are provided in the PRISMA flow chart (Figure 1).
FIGURE 1.
PRISMA flow diagram: clinical effectiveness. a: Multiple publications, including abstracts.
Summary of studies
Eight RCTs were identified that provided data on primary outcomes comparing THA with HA for adults with displaced intracapsular or subcapital hip fracture (Table 1). 2,19,28–36,37,38 The mean age of participants in the included trials ranged from 69 to 82 years, with an overall age range of 41–96 years. At least 68% of participants in each of the trials were women. The number of participants in the eight trials ranged from 40 to 252. Five studies compared THA with cemented19,31,32,38 or uncemented HA29,30 or with a mixture of both types of prosthesis fixture. 28,34 Mouzopoulos et al. 37 did not report whether or not the prosthesis was cemented or uncemented. 37 The surgery reported in the trials by Baker et al. 19 and Blomfeldt et al. 32 was undertaken using the direct lateral approach; the trials reported by Dorr et al. ,28 Skinner et al. ,29 and Ravikumar and Marsh30 used the posterior approach; and the trials reported by Keating et al. ,31 Macaulay et al. 35 and van den Bekerom et al. 38 used a mixture of the two approaches, depending on surgeon’s choice. The approach used was not reported by Mouzopoulos et al. 37 The time from fracture to treatment was reported in only three trials and ranged from within 24 hours of admission29 to within up to 48 hours of trial entry. 19,31 Dorr et al. ,28 Skinner et al. ,29 Baker et al. ,19 Blomfeldt et al. ,32 Macaulay et al. ,35 Mouzopoulos et al. 37 and van den Bekerom et al. 38 all reported follow-up data on a primary outcome for up to 1 year, and Dorr et al. ,28 Ravikumar and Marsh,30 Baker et al. ,19 Keating et al. ,31 Mouzopoulos et al. 37 and van den Bekerom et al. 38 all reported data on these outcomes for follow-up points > 1 year. Some trials reported primary and secondary outcome data for a number of different follow-up periods.
| Study author, date, country | Study design | Inclusion criteria | Exclusion criteria | Intervention (THA) characteristics Population characteristics n 1. Mean age, gender (f/m)a 2. Comorbidities 3. Time from fracture to surgery |
Comparison (HA) characteristics Population characteristics n 1. Mean age, gender (f/m)a 2. Comorbidities 3. Time from fracture to surgery |
|---|---|---|---|---|---|
| Dorr et al.,28 1986, USA | RCT | Displaced femoral hip fractures (Garden grades III and IV);40 ambulatory, oriented to time, place and person | Ambulation and mental status: ambulatory with confusion; non-ambulatory |
Posterolateral approach; size of head = 28 mm (cemented); type of head NR n = 39 1. 69 (51–87) years; gender = 23/16 2. NR 3. NR |
Approach = posterolateral; type of head NR n = 37 CHA (bipolar) n = 13 UHA (bipolar) 1. Mean age (range): CHA = 72 (53–79), UHA = 66 (41–85) gender = 35/15 2. NR 3. NR |
| Skinner et al.,29 1989; Ravikumar and Marsh,30 2000, UK | RCT |
Displaced subcapital femoral neck fracture (Garden grades III and IV);40 age ≥ 65 years (Note: includes unknown number of patients with ‘compromised mental state’: Ravikumar and Marsh,30 p. 794) |
Patients with old fractures, pathological fractures or those suffering from rheumatoid arthritis |
Posterolateral approach; size of head = 32 mm (cemented); Howse II prosthesis bn = 89 (exact numbers not reported for 1-year data) 1. 81 years; gender = 90% women (overall) 2. NR 3. ‘Usually within 24 hours of admission’29 |
Posterolateral approach; size of head = NR; Austin Moore prosthesis bn = 91 UHA (unipolar) (exact numbers not reported for 1-year data) 1. 82 years; gender = 90% women (overall) 2. NR 3. ‘Usually within 24 hours of admission’29 |
| Baker et al.,19 2006, UK | RCT | Displaced fracture of the femoral neck; age > 60 years, a normal Abbreviated Mini Mental Test score,41 the ability to walk ≥ 0.5 miles (0.8 km), the ability to live independently (without reliance on a caregiver), a non-pathological fracture, and a hip with no or minimal osteoarthritic changes | Age < 60 years, medical or physical comorbidities that limited the walking distance to < 0.5 miles (0.8 km), a pre-existing hip abnormality requiring THA, or a pathological fracture secondary to malignant disease |
Lateral approach; size of head = 28 mm (cemented); mean of outer diameter of acetabular component = 44–55 mm n = 40 1. 74.2 (63–86) years; gender = 32/8 2. NR 3. 1.75 days |
Lateral approach; Endo femoral head (Zimmer); cemented n = 41 CHA (unipolar) 1. 75.8 years (range 66–86 years) Gender = 32/9 2. NR 3. 1.95 days |
| Keating et al.,31 2006, UK | RCT | Displaced intracapsular hip fracture; no formal age criteria, but protocol indicated that it was expected to be ≥ 60 years of age; normal cognitive function (a Mini Mental Test score41 of > 6), the ability to be mobile, independent of another person prior to the fracture, and no serious concomitant disease (or other clinical reason for exclusion) | Undisplaced or valgus impacted intracapsular fracture |
Direct lateral and posterior (60 vs 9); size of head NR; Charnley or Exeter head n = 69 (cemented) 1. 75.2 (SD 6) years; gender = 52/17 2. NR 3. Within 48 hours of trial entry |
Approach: direct lateral and posterior (62 vs 7); size of head NR; predominantly Charnley or Exeter head n = 69 (cemented) (bipolar; two receive unipolar prosthesis) 1.75 years (SD 6 years) ; gender = 54/15 2. NR 3. Within 48 hours of trial entry |
| Blomfeldt et al.,32 2007, Sweden | RCT | Acute displaced intracapsular fracture of the femoral neck (Garden grades III and IV)40 following a fall; age 70–90 years; absence of severe cognitive dysfunction, non-institutionalised independent living status and pre-injury independent walking capability with or without aids | Patients with pathological fractures and displaced fractures present for > 48 hours before presentation; patients with rheumatoid arthritis or osteoarthritis |
Lateral (modified Hardinge) approach; size of head ≥ 28 mm (cemented); modular Exeter femoral component n = 60 1. 80.5 years (range 70–90 years); gender = 47/13 2. Ceder A or B (i.e. full health or other illness not affecting rehabilitation): 88% 3. NR |
Lateral approach; size of head ≥ 28 mm; modular Exeter femoral component n = 60 (cemented) (bipolar) 1. 80.7 years (range 70–89 years); gender = 54/6 2. Ceder A or B: 83% 3. NR |
| Macaulay et al.,35 2008, USA | RCT | > 50 years of age; ability for independent ambulation before fracture; displaced fracture of the femoral neck (Garden grades III and IV);40 ability to comprehend or read English or Spanish | Chronic-to-severe dementia (< 23/30 on Folstein MMSE); pathologic fracture; other concomitatant bone fractures requiring surgical repair; pre-existing arthritis of the hip |
Posterolateral or direct lateral (modified Hardinge) approach (surgeon’s choice); size of head, ≥ 28mm; type of head, NR; cement vs ‘press-fit stem’ (surgeon’s choice) bn = 18 1. NR; gender = NR 2. NR 3. NR |
Posterolateral or direct lateral approach (surgeon’s choice); size of head, NR; type of head, NR; bi- vs unipolar (surgeon’s choice: 5 vs 18); cement vs ‘press-fit stem’ (surgeon’s choice) bn = 23 1. NR; gender = NR 2. NR 3. NR |
| Mouzopoulos et al.,37 2008, USA and Germany | RCT | Patients with displaced subcapital hip fracture (Garden grade III or IV)40 after falling down and having treatment in our hospitals from April 1999 to April 2002; (p. 372: aged ≥ 70 years, with good cognitive status and moderate dependency) | Previous hip fracture, history of cancer or Paget’s disease, or rheumatic arthritis |
Approach NR; size of head NR; type of head ‘Plus’; cement: NR n = 37 1. 73 years (5 years) ; gender = 28/9 2. NR 3. 45 ± 7 (hours) |
Approach NR; size of head NR; type of head ‘Merete’; cement: NR n = 34 1. 74 years (4 years) ; gender = 24/10 2. NR 3. 46 ± 2 (hours) |
| van den Bekerom et al.,38 2010, Netherlands | RCT | ≥ 70 years of age; displaced intracapsular fracture of the femoral neck; ability to give informed consent; no metastatic disease; no contraindications to anaesthesia before fracture; ability to understand written Dutch | Inability to fulfil inclusion criteria; advanced radiological osteoarthritis or rheumatoid arthritis in the fractured hip; significant senile dementia; suspected pathological fracture; patients who were bedridden or barely mobile from bed to chair |
Approach was surgeon’s choice (anterolateral/posterolateral = 93/22); size of head, 32 mm; type of head, Weber Rotationsprosthese (Sulzer AG, Winterthur, Switzerland) or Muller Geradschaftprosthese (Proteli AG, Münsingen, Switzerland); cemented n = 115 1. 82.1 years (range 70.1–95.6 years) ; gender = 90/25 2. Cardiovascular (33%), malignancies (5%), pulmonary (16%), neurological (29%), locomotive (27%), diabetes (10%) 3. NR |
Approach was surgeon’s choice (anterolateral/posterolateral = 132/5); size of head, NR; type of head, Weber Rotationsprosthese or Muller Geradschaftprosthese; (cemented) (bipolar) n = 137 1. 80.3 years (range 70.2–93.9 years) ; gender = 115/22 2. Cardiovascular (25%), malignancies (8%), pulmonary (12%), neurological (19%), locomotive (16%), diabetes (14%) 3. NR |
Quality assessment
Randomisation and allocation concealment were considered adequate in the studies by Baker et al. ,19 Blomfeldt et al. ,32 Macaulay et al. ,35 Keating et al. 31 and van den Bekerom et al. 38 (e.g. use of sealed envelopes or a computer-generated randomisation sequence). In the studies by Dorr et al. ,28 Skinner et al. ,29 and Ravikumar and Marsh,30 randomisation was by hospital number only and allocation concealment was not reported. Mouzopoulos et al. 37 reported randomisation to intervention based on selection of every third admission; details of allocation concealment were unreported. All eight RCTs defined inclusion criteria for the study and reported follow-up of at least 1 year (Table 2). Only Dorr et al. 28 did not describe fully or compare intervention groups. van den Bekerom et al. 38 described both groups fully, but did not perform any tests to determine whether or not the differences in terms of cardiovascular, neurological and locomotive comorbidities, the taking of analgesics and pre-operation mobility were statistically significant. Four studies19,31,32,35 clearly conducted intention-to-treat analyses, but this was unclear in the remaining four studies. Baker et al. ,19 Blomfeldt et al. ,32 Mouzopoulos et al. 37 and van den Bekerom et al. 38 also clearly reported comparable care for both intervention groups; this was unclear in the remaining four trials.
| Study | Allocation concealment | Inclusion criteria defined | Intention-to-treat analysis | Intervention groups described and comparable | Surgeons experienced in both operations | Care identical other than intervention | Outcome measures defined | Outcome assessors blind | Follow-up of at least 1 year | Loss to follow-up reported and ≤ 5% |
|---|---|---|---|---|---|---|---|---|---|---|
| Dorr et al.28 | No | Yes | Unclear | Unclear | No | Unclear | Yes | No | Yes | Unclear |
| Skinner et al.,29 Ravikumar and Marsh30 | No | Yes | Unclear | Yes | Unclear | Unclear | Yes | No | Yes | Unclear |
| Baker et al.19 | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | No | Yes | No |
| Keating et al.31 | Unclear | Yes | Yes | Yes | Unclear | Unclear | Yes | No | Yes | Yes |
| Blomfeldt et al.32 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | No |
| Macaulay et al.35 | Yes | Yes | Yes | Yes | Unclear | Unclear | Yes | No | Yes | Yes |
| Mouzopoulos et al.37 | No | Yes | Unclear | Yes | Unclear | Yes | Yes | Yes | Yes | No |
| van den Bekerom et al.38 | Yes | Yes | Unclear | Unclear | Unclear | Yes | Yes | No | Yes | No |
Only Blomfeldt et al. 32 reported that the surgeons involved were experienced in both procedures; Baker et al. 19 reported that the surgery in each trial arm was performed by surgeons with similar levels of training; and Keating et al. 31 reported that more patients were treated by consultants/senior surgeons in the THA group than in the HA group. The relative expertise of the surgeons conducting the two procedures was only reported in two studies. 19,38 Only in one study was it clear that the outcome assessors were blind to the intervention. 37 Keating et al. 31 and Macaulay et al. 35 both reported ≤ 5% loss to follow-up, and Blomfeldt et al. 32 reported a loss to follow-up of 6–8% across arms. The remaining studies all had an attrition rate of ≥ 10% or did not report whether or not any loss to follow-up had occurred.
Summary of effectiveness
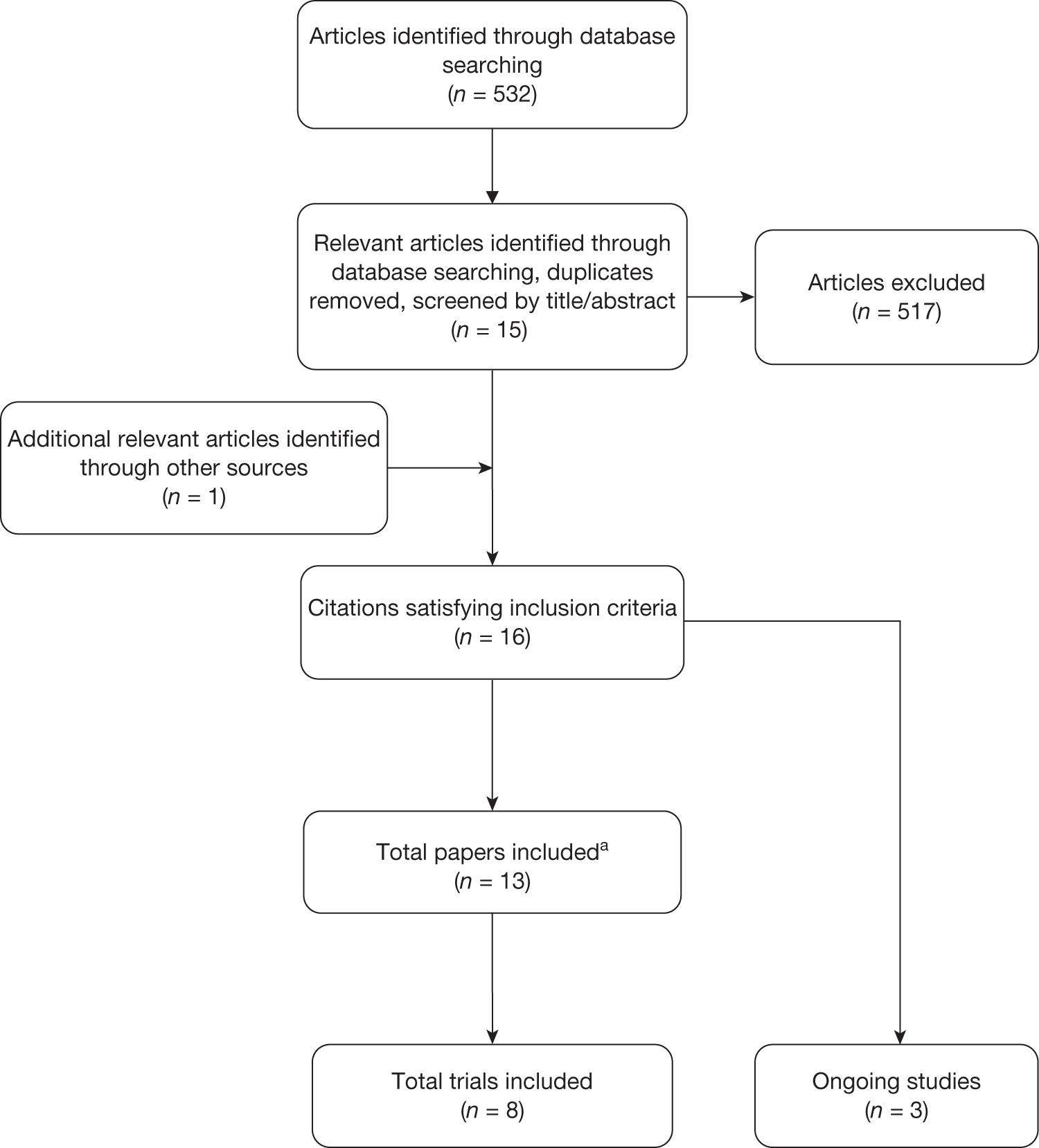
Numbers of patients experiencing dislocations
Six studies19,28,29,32,35,38 (762 analysed participants) compared numbers of patients with dislocations within or up to 1 year post operation. A meta-analysis demonstrated a borderline statistically significant increased risk of dislocation for those receiving THA compared with HA (RR 3.98, 95% CI 0.98 to 16.12, p = 0.05), with a moderate level of statistical heterogeneity (I2 = 46%) (Figure 2 and Table 3). There was a 4% increase in the absolute risk of dislocation for those receiving THA compared with HA (meta-analysed RD 0.04, 95% CI 0.00 to 0.09, p = 0.05, with a high level of statistical heterogeneity (I2 = 59%) (see Table 3). The presence of such heterogeneity may be because of the absence, or very small number, of events in some of the trial arms.
FIGURE 2.
Risk of dislocations within and up to 1 year.
| Included studies | Number of studies | Follow-up | THA vs HA | RR (95% CI) | I2 (%) | RD (95% CI) | I2 (%) |
|---|---|---|---|---|---|---|---|
| Dislocations | |||||||
| Dorr et al.,28 Skinner et al.,29 Baker et al.,19 Blomfeldt et al.,32 Macaulay et al.,35 van den Bekerom et al.38 | 6 | ≤ 1 year | 26/360 vs 10/402 | 3.98 (0.98 to 16.12), p = 0.05 | 46 | 0.04 (0.00 to 0.09), p = 0.05 | 59 |
| Dorr et al.,28 Ravikumar and Marsh,30 Baker et al.,19 Keating et al.,31 Blomfeldt et al.,32 Macaulay et al.,35 van den Bekerom et al.38 | 7 | Up to 13 years | 40/429 vs 16/471 | 2.40 (1.41 to 2.76), p = 0.01 | 13 | 0.05 (0.00 to 0.09), p = 0.03 | 64 |
| Revisions | |||||||
| Skinner et al.,29 Blomfeldt et al.,32 Macaulay et al.,35 Mouzopoulos et al.,37 van den Bekerom et al.38 | 5 | ≤ 1 year | 5/320 vs 15/349 | 0.41 (0.16 to 1.03), p = 0.06 | 0 | –0.02 (–0.06 to 0.02), p = 0.35 | 64 |
| Dorr et al.,28 Ravikumar and Marsh,30 Baker et al.,19 Blomfeldt et al.,32 Macaulay et al.,35 Mouzopoulos et al.,37 van den Bekerom et al.38 | 7 | Up to 13 years | 12/399 vs 42/440 | 0.31 (0.17 to 0.59), p = 0.0003 | 0 | –0.05 (–0.12 to 0.01), p = 0.09 | 80 |
| Any surgery | |||||||
| Skinner et al.,29 Blomfeldt et al.,32 Macaulay et al.,35 Mouzopoulos et al.,37 van den Bekerom et al.38 | 5 | ≤ 1 year | 24/320 vs 22/349 | 1.72 (0.41 to 7.21), p = 0.46 | 56 | 0.01 (–0.04 to 0.07), p = 0.61 | 57 |
| Dorr et al.,28 Ravikumar and Marsh,30 Baker et al.,19 Keating et al.,31 Blomfeldt et al.,32 Macaulay et al.,35 Mouzopoulos et al.,37 van den Bekerom et al.38 | 8 | Up to 13 years | 50/468 vs 54/509 | 1.09 (0.65 to 1.83), p = 0.75 | 33 | 0.01 (–0.04 to 0.05), p = 0.74 | 40 |
| Mortality | |||||||
| Skinner et al.,29 Blomfeldt et al.,32 Keating et al.,31 Mouzopoulos et al.,37 van den Bekerom et al.38 | 5 | ≤ 1 year | 50/376 vs 58/400 | 0.91 (0.65 to 1.29), p = 0.60 | 0 | –0.01 (–0.05 to 0.04), p = 0.75 | 0 |
| Ravikumar and Marsh,30 Baker et al.,19 Keating et al.,31 Blomfeldt et al.,32 Macaulay et al.,35 Mouzopoulos et al.,37 van den Bekerom et al.38 | 7 | Up to 13 years | 176/433 vs 180/464 | 1.03 (0.80 to 1.32), p = 0.81 | 48 | 0.00 (–0.07 to 0.07), p = 1.00 | 52 |
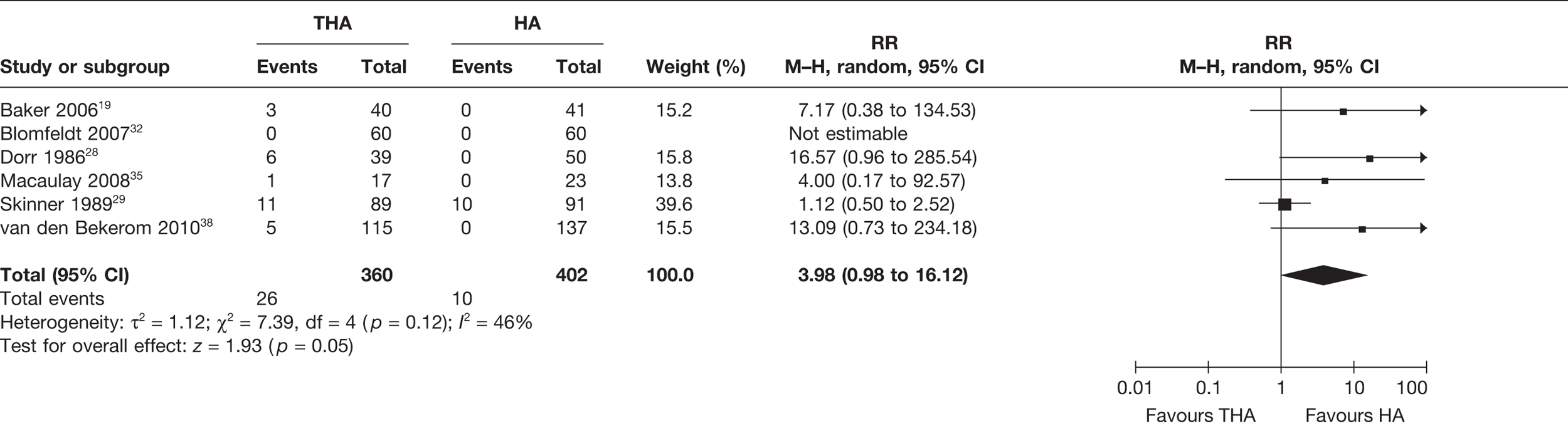
Seven studies19,28,30–32,35,38 (900 analysed participants) compared the number of patients with dislocations for all follow-up periods post operation, up to 13 years. A meta-analysis demonstrated a statistically significant increased risk of dislocation for those receiving THA compared with HA (RR 2.40, 95% CI 1.41 to 2.76, p = 0.01), with a low level of statistical heterogeneity (I2 = 13%) (see Figure 3 and Table 3). The 1-year follow-up data may have also generated a statistically significant difference had the sample been larger. There was a 5% increase in the absolute risk of dislocation for those treated with THA compared with HA (meta-analysed RD 0.05, 95% CI 0.00 to 0.09, p = 0.03), with a high level of statistical heterogeneity (I2 = 64%) (see Table 3).
FIGURE 3.
Risk of dislocations up to 13 years.
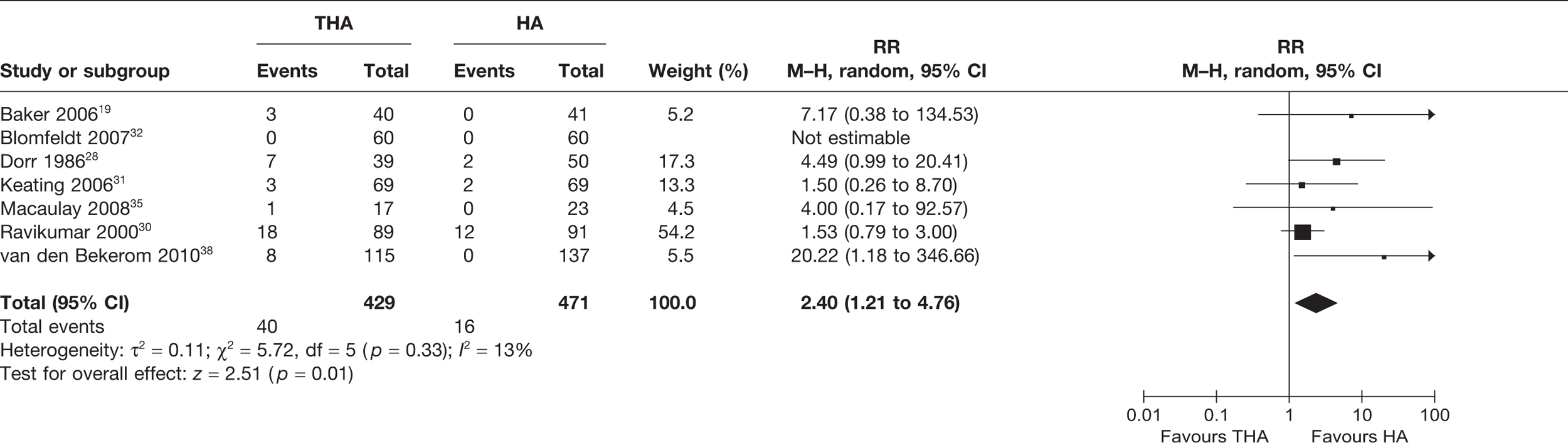
Number of patients experiencing revision surgery or any surgery
Revisions included revisions because of all causes, including dislocations. Five studies29,32,35,38,39 (669 analysed participants) compared the number of patients who experienced revision surgery within or up to 1 year post operation. A meta-analysis demonstrated a statistically non-significant 59% reduced risk of revision for those receiving THA compared with HA (RR 0.41, 95% CI 0.16 to 1.03, p = 0.06), with no statistical heterogeneity (I2 = 0%) (see Figure 4 and Table 3). There was a 2% reduction in the absolute risk of revision for those receiving THA compared with HA (meta-analysed RD –0.02, 95% CI –0.06 to 0.02, p = 0.35), with a high level of statistical heterogeneity (I2 = 64%) (see Table 3).
FIGURE 4.
Risk of revision surgery within and up to 1 year.
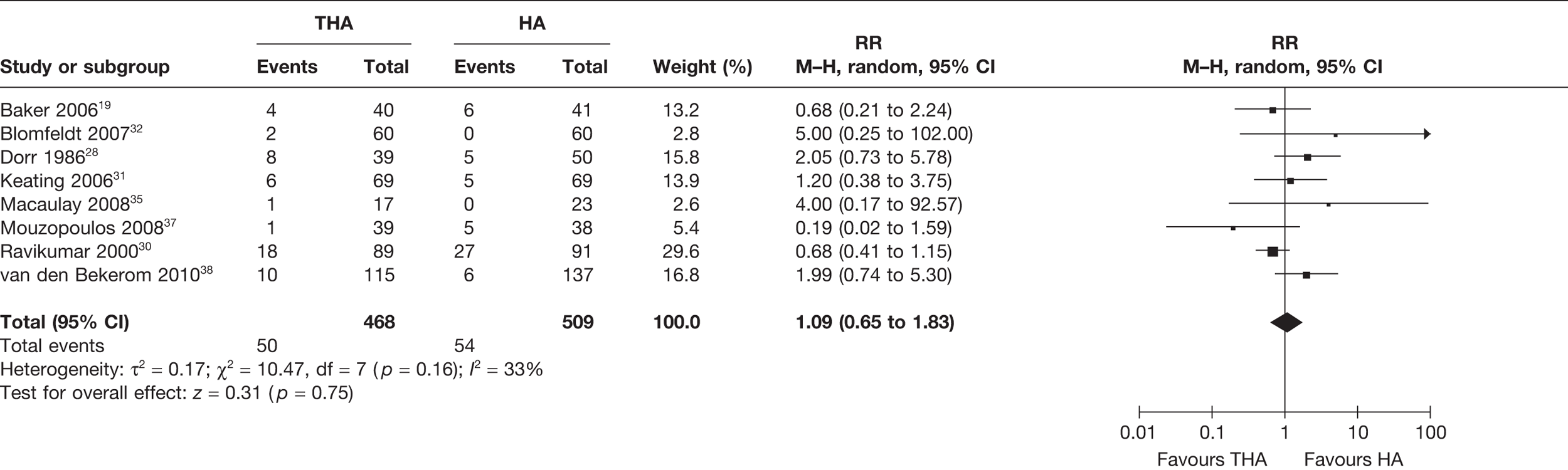
Seven studies19,28,30,32,35,38,39 (839 analysed participants) compared the numbers of patients who experienced revision surgery for all follow-up periods post operation, up to 13 years. A meta-analysis demonstrated a statistically significant 69% reduced risk of revision for those receiving THA compared with HA (RR 0.31, 95% CI 0.17 to 0.59, p = 0.0003), with no statistical heterogeneity (I2 = 0%) (see Figure 5 and Table 3). There was a 5% reduction in the absolute risk of revision for those exposed to THA compared with HA (meta-analysed RD –0.05, 95% CI –0.12 to 0.01, p = 0.09), with a high level of statistical heterogeneity (I2 = 80%) (see Table 3).
FIGURE 5.
Risk of revision surgery up to 13 years.
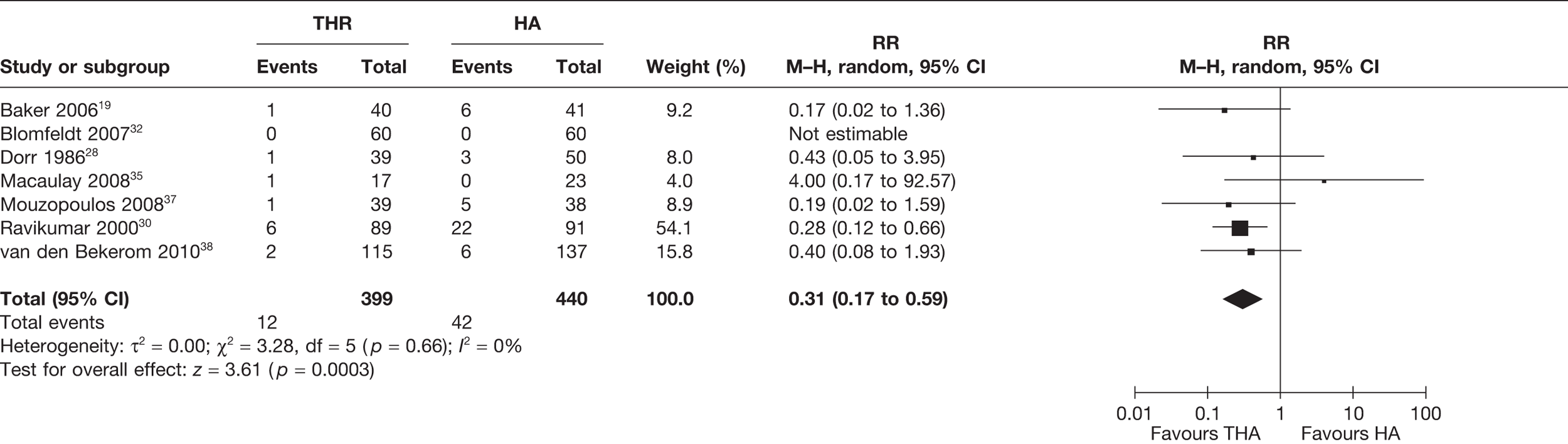
Five studies29,32,35,38,39 (669 analysed participants) compared the number of patients who experienced any form of surgery (including open or closed reduction of a dislocation, revision or surgery for any other cause) within or up to 1 year post operation. A meta-analysis demonstrated a statistically non-significant increased risk of any surgery for those receiving THA compared with HA (RR 1.72, 95% CI 0.41 to 7.21, p = 0.46), with a high level of statistical heterogeneity (I2 = 56%) (see Figure 6 and Table 3). There was a 2% increase in the absolute risk of surgery for those receiving THA compared with HA (meta-analysed RD 0.01, 95% CI –0.04 to 0.07, p = 0.61), with a high level of statistical heterogeneity (I2 = 57%) (see Table 3).
FIGURE 6.
Risk of any surgery within and up to 1 year.
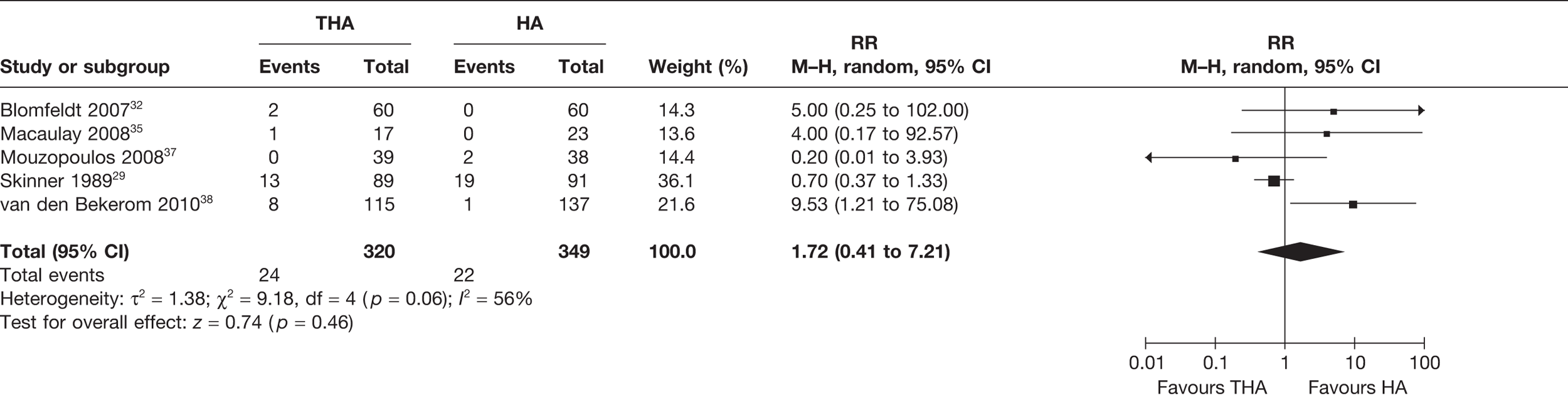
Eight studies19,28,30–32,35,38,39 (977 analysed participants) compared the number of patients who experienced any surgery for all follow-up periods post operation, up to 13 years. A meta-analysis demonstrated a statistically non-significant increased risk of any surgery for those receiving THA compared with HA (RR 1.09, 95% CI 0.65 to 1.83, p = 0.75), with a moderate level of statistical heterogeneity (I2 = 33%) (see Figure 7 and Table 3). There was a 1% increase in the absolute risk of surgery for those receiving THA compared with HA (meta-analysed RD –0.01, 95% CI –0.04 to 0.05, p = 0.74), with a moderate level of statistical heterogeneity (I2 = 40%) (see Table 3).
FIGURE 7.
Risk of any surgery up to 13 years.
This analysis combined outcome data on patients with dislocations, revisions (not including dislocated revisions) and, where reported, other non-revision or dislocation surgery. Baker et al. ,19 Macaulay et al. ,35 Mouzopoulos et al. 37 and van den Bekerom et al. 38 reported only dislocation and/or revision event data, and no data on any other surgery. However, the exclusion of these four studies from the analysis, so that only studies reporting data on all three types of possible surgery were included, does not affect the result: RR 1.14, 95% CI 0.57 to 1.26, p = 0.72.
Hip scores and walking
All eight trials19,28,30–32,35,38,39 reported patient-reported assessments of pain, function and mobility using hip scores. Only Macaulay et al. 35 and Mouzopoulos et al. 37 compared ratings using the same scale of the Harris Hip Score (HHS) at 1 year post operation, permitting meta-analysis (Table 4). However, because of the small number of studies, meta-analysis was not performed. Macaulay et al. 35 reported a non-significant difference in favour of THA using the HHS and pain and function subscales at 1 year, but statistically significant differences in favour of THA at 2 years for pain and function (p < 0.05). Blomfeldt et al. 32 reported a statistically significant (p < 0.001) difference after 1 year in favour of THA compared with HA, and Ravikumar and Marsh30 and Mouzopoulos et al. 37 reported the average HHS to be higher for individuals treated with THA than for those treated with HA (p-values not reported). van den Bekerom et al. 38 also reported higher scores for THA than for HA for both 1 and 5 years, but the differences were not statistically significant. Three studies also reported hip scores using different scales (see Table 4). 19,28,31 Baker et al. 19 reported a statistically significant (p = 0.033) difference after 3 years in favour of THA compared with HA using the OHS. Keating et al. 31 reported a statistically non-significant (p = 0.38) difference after 1 year in favour of THA compared with HA using the Hip Rating Questionnaire, but a statistically significant (p = 0.04) difference after 2 years. Dorr et al. 28 reported two subscales of a modified version of the D’Aubigne/Postel hip score: individuals receiving THA reported less pain and better ambulation than those receiving HA, especially uncemented HA.
| Study | Study duration/follow-up | Primary outcomes (THA vs HA) 1. Number of patients with dislocations 2. Number of patients who had a revision 3. Number of patients who had a non-revision reoperation |
Secondary outcomes Hip ratings (e.g. HHS) (THA vs HA) |
Mobility, n, e.g. walking distance (THA vs HA) | Utility data 1. Quality of life 2. Length of hospital stay 3. Resource utilisation and/or cost–utility |
|---|---|---|---|---|---|
| Dorr et al.28 | 3, 12 and 24–48 months |
1. 2–4 years unless stated: 7/39 (18%) vs 2/50 (4%) (at ‘final follow-up’; six THA dislocations occurred immediately post operation or up to 3 months post operation; it is not reported when remaining dislocations occurred) 2. 2–4 years unless stated: THA 1/39 (3%) for loosening and heterotopic ossification at 3 years vs CHA 2/37 (5%) for heterotopic ossification and dislocation vs UHA 1/13 (8%) for femoral loosening 3. 1 year: THA 1/39 (3%) for recurrent dislocation in first month vs CHA 1/37 (3%) for removal of a cement fragment at 2 weeks |
Modified D’Aubigne/Postel hip score (higher better) 1 year: pain = 5.5 (THA) vs 5.2 (CHA) vs 3.6 (UHA); ambulation = 4.1 (THA) vs 4.2 (CHA) vs 3.0 (UHA) 2 years: pain = 5.5 (THA) vs 5.1 (CHA) vs 3.0 (UHA); ambulation = 5.5 (THA) vs 4.0 (CHA) vs 3.0 (UHA) |
Not walking at final follow-up: 1/39 vs 3/50 |
1. NR 2. ‘There was no difference in the hospital time’, pp. 22–3 3. NR |
|
Skinner et al. 29 Ravikumar and Marsh30 |
1 year, 13 years |
1. a1 year: 11/89 (12%) vs 10/91 (11%) (includes both ‘fit’ and ‘unfit’ patients; the latter were at significantly higher risk, p < 0.05) 13 years: 18/89 (20%), of which five had recurrent dislocations, four of which were revised vs 12/91 (13%) 2. 1 year: 4/89 (4%) for recurrent dislocation vs 12/91 (13%) for loosening, further fracture or ectopic calcification, p < 0.01 13 years: 6/89 (7%) (two for infection and four recurrent dislocations) vs 22/91 (24%) for acetabular erosion, loosening, heterotopic ossification and deep infection Mean time to revision: 27.3 months (THA) vs 22.1 months (HA) 3. a1 year: 13/89 (15%) vs 19/91 (21%)b (reoperations, defined as second anaesthetic, includes reduction of a dislocation and/or a revision) 13 years: 18/89 (20%) vs 27/91 (30%)b (reoperations, defined as second anaesthetic, includes reduction of a dislocation and or a revision) |
HHS (higher better) 1 year: NR Average score among survivors at 13 years: 80 vs 55 Pain at 1 year (% of patients with highest pain score of 3–4, i.e. requiring analgesia): 0% vs 27% Pain at 13 years: 6% vs 45% |
Patients with no loss of mobility at 1 year: 49% vs 30%, p < 0.05 Patients mobile at 1 year: 73% vs 66% At 13 years: 70% vs 53 , p < 0.05 |
1. NR 2. NR 3. NR |
| Baker et al.19 |
3, 12 and 36 months (Data for 3 years from Baker et al. ,19 unless stated; mean follow-up was 39 months) |
1. 30 days post operation only: 3/40 (8%) vs 0/41 (0%) (p = 0.116, Fisher exact test) (1 month only) (no dislocation was revised) 2. 3 years (revisions or ‘planned revisions’): 1/40 (3%) for pain due to femoral subsidence vs 6/41 (15%) for pain owing to acetabular erosion or periprosthetic fracture (p = 0.058, Fisher's exact test) This includes a patient categorised for revision, but who ‘declined additional intervention’, p. 2587 (unclear how many events occurred before 1 year) 3. NR |
OHS (lower better) [mean (range)] THA (n = 36) vs HA (n = 33) 18.8 (12–47) vs 22.3 (12–48) p = 0.033 (Mann–Whitney) 18.8 vs 22.5 (Baker et al. 19) |
Walking distance (km) [mean (range)]: THA (n = 36) vs HA (n = 33): 3.6 (0–40.2) vs 1.9 (0–6.4), p = 0.039 (Student's t-test) 2.23 miles vs 1.09 miles (Baker et al. 42) |
1. SF-36 [mean (range)] THA (n = 36) vs HA (n = 33) Not significant (p = 0.356) Physical: 40.53 (16.2–56.5) vs 38.10 (16–58.8) Mental: 52.00 (24.2–68.4) vs 55.32 (39–66.6) 2. NR 3. NR |
| Keating et al.31 | 12 and 24 months |
1. 2 years: 3/69 (4%) (all led to ‘additional surgery’) vs 2/69 (3%): OR 0.63 (95% CI 0.10 to 3.92), p = 0.62 (unclear how many occurred before 1 year) 2. NR 3. 2 years (‘Additional’ or ‘Further surgery’: ‘any procedure requiring general or regional anaesthesia. This included manipulative reduction of prosthetic dislocations’): 6/69 (9%) for dislocation (n = 3), infection (n = 2) and wound dehiscence (n = 1) vs 5/69 (7%) (reasons not given): OR 0.81 (95% CI 0.25 to 2.65), p = 0.73 (unclear how many events occurred before 1 year) |
Hip Rating Questionnaire (higher better); THA n = 66 vs HA n = 65 1 year: 79.4 (17) vs 76.5 (13) (95% CI −8.00 to 3.09), p = 0.38 2 years: 79.9 (17) vs 73.8 (16) (95% CI −12.53 to −0.37), p = 0.04 |
Walking (note: included in Hip score): THR n = 66 vs HA n = 65 1 year: 19.3 (6) vs 16.9 (5) (95% CI −4.15 to –0.03), p = NR 2 years: 19.3 (6) vs 16.2 (6) (95% CI −4.97 to –0.66), p = NR |
1. EQ-5D at 2 years [mean (SD)]: (THR n = 66 vs HA n = 65): 0.69 (0.32) vs 0.53 (0.36) (95% CI −0.28 to −0.04), p = 0.008 2. 11.5 vs 12.3 days (post operation) 3. Hip-related re-admissions: 7 (10%) vs 8 (12%) |
| Blomfeldt et al.32 | 4 and 12 months (only 12-month data) |
1. 1 year: 0/60 (0%) vs 0/60 (0%) 2. 1 year: 0/60 (1%) vs 0/60 (0%) 3. 1 year: 2/60 (3%) for a peri-prosthetic fracture post fall, fixed internally with a plate, and for a wound revision following infection vs 0/60 (0%) |
HHS (higher better) [mean (range)] (THA n = 56 vs HA n = 55) at 1 year 87.2 (58.6–100.0) vs 79.4 (51.3–99.8), p < 0.001 Pain subscale at 1 year: (THA n = 56 vs HA n = 55) 43.1 (30.0–44.0) vs 39.1 (20.0–44.0), p < 0.001 |
NR |
1. EQ-5D: 0.68 vs 0.63 (p = 0.636) 2. NR 3. NR |
| Macaulay et al.35 | 6, 1235 and 24 months34 |
1. 1 year: 1/17 (5.9%) 5 months post surgery and revised vs 0/23 (0%) 2. 1 year: 1/17 (5.9%) due to dislocation vs 0/23 (0%) 3. NR |
At 1 year HHS (higher better) [mean (SD)] (THA n = 17 vs HA n = 23): 84.2 (± 12) vs 80.6 (± 14.3), –3.6 (95% CI –15.3 to 8.3), p = 0.55 At 1 year WOMAC function subscale (higher better) (mean ± SD): 75.9 ± 19.8 vs 78.7 ± 16.8, p = 0.71 WOMAC pain subscale (higher better) (mean ± SD): 92.5 ± 14.6 vs 88.5 ± 13.6, p = 0.50 At 2 years WOMAC function subscale (higher better) (mean ± SD): 81.8 ± 10.2 vs 65.1 ± 18.1, p = 0.03 WOMAC pain subscale (higher better) (mean ± SD): 94.4 ± 6.8 vs 77.8 ± 20.9, p = 0.05 |
At 6 months: TUG test: 14.2 seconds vs 20.7 seconds At 1 and 2 years: not statistically significant, but TUG indicates that THA patients complete the test about 2 seconds faster than the HA patients At 1 year: walking independently or with a cane 57% vs 41% |
1. At 1 year SF-36 pain subscale (higher better) (mean ± SD): 53.2 ± 10.2 vs 42.4 ± 11.5, p = 0.02 SF-36 mental health subscale (mean ± SD): 55.7 ± 15.8 vs 49.0 ± 12.0, p = 0.25 At 2 years SF-36 pain subscale (higher better) (mean ± SD): 54.8 ± 7.9 vs 44.7 ± 10.5, p = 0.04 SF-36 mental health subscale (mean ± SD): 54.9 ± 9.4 vs 40.9 ± 10.3, p = 0.006 2. NR 3. NR |
| cMouzopoulos et al.37 | 1 year, 4 years |
1. NR 2. 1 year: 0/39 (0%) vs 2/38 (5%) Up to 4 years: 1/39 (3%) vs 5/38 (13%) 3. NR |
HHS (higher better) 1 year (THA n = 37 vs HA n = 34) (mean ± SD): 81.6 ± 4.9 vs 77.8 ± 9.6, p = NR 4 years (THA n = 33 vs HA n = 30) (mean ± SD): 83.7 ± 4.8 vs 79.5 ± 6.5; p = NR Function using Barthel Index (higher better) 1 year (THA n = 33 vs HA n = 30) (mean ± SD): 84.8 ± 14.8 vs 76.8 ± 6.8, p = NR 4 years (THA n = 23 vs HA n = 20) (mean ± SD): 85.3 ± 11.6 vs 79.6 ± 6.3, p = NR |
NR |
1. NR 2. THA (8.3 ± 6.2) vs HA (9.1 ± 3.4) (in days, mean ± SD) (p-value NR) 3. NR |
| van den Bekerom et al.38 | 1 year, 5 years |
1. 1 year: 5/115 (4%) vs 0/137 (0%) 5 years: 8/115 (7%) vs 0/137 (0%) (p = 0.002) Note: dislocations: 3/93 (3%) anterolateral approach vs 5/22 (23%) posterolateral approach for THA; 0/132 (0%) vs 0/5 (0%) for HA 2. 1 year: 0/115 (0%) vs 1/137 (1%) 5 years: 2/115 (2%) vs 6/137 (4%) (p = 0.29) (for loosening, osteoarthritis of acetabulum and low-grade deep infection) 3. NR |
At 1 year [mean (range)] HHS (higher better): (THA n = 115 vs HA n = 137)d: 76 (44–100) vs 73.9 (23–100), p = 0.40 HHS (pain subscale): 40 (20–44) vs 37.5 (10–44), p = NR HHS (function subscale): 20.8 (0–36) vs 20.7 (0–36), p = NR 5 years (mean and range) (THA n = 115 vs HA n = 137)d: 75.2 (45–98) vs 71.9 (33–99), p = 0.22 HHS (pain subscale): 40.1 (20–44) vs 38.6 (10–44), p = NR HHS (function subscale): 20.1 (7–33) vs 18.6 (4–35), p = NR |
NR |
1. NR 2. THA: 18.4 (4–86) vs HA 17.1 (2–89) (days and range) 3. NR |
Six studies also reported additional mobility data (see Table 4). 19,28–31,35 Skinner et al. 29 and Ravikumar and Marsh30 reported significant differences (p < 0.05) in favour of THA in the number of participants walking or mobile at 1 year and 13 years, respectively. Baker et al. 19 reported a statistically significant difference (p = 0.039) in favour of THA for mean walking distance. Dorr et al. ,28 Keating et al. 31 and Macaulay et al. 35 also reported greater degrees of mobility among participants in the THA arms of trials (p-values not reported) for 4, 2 and 1 year(s), respectively.
Mortality
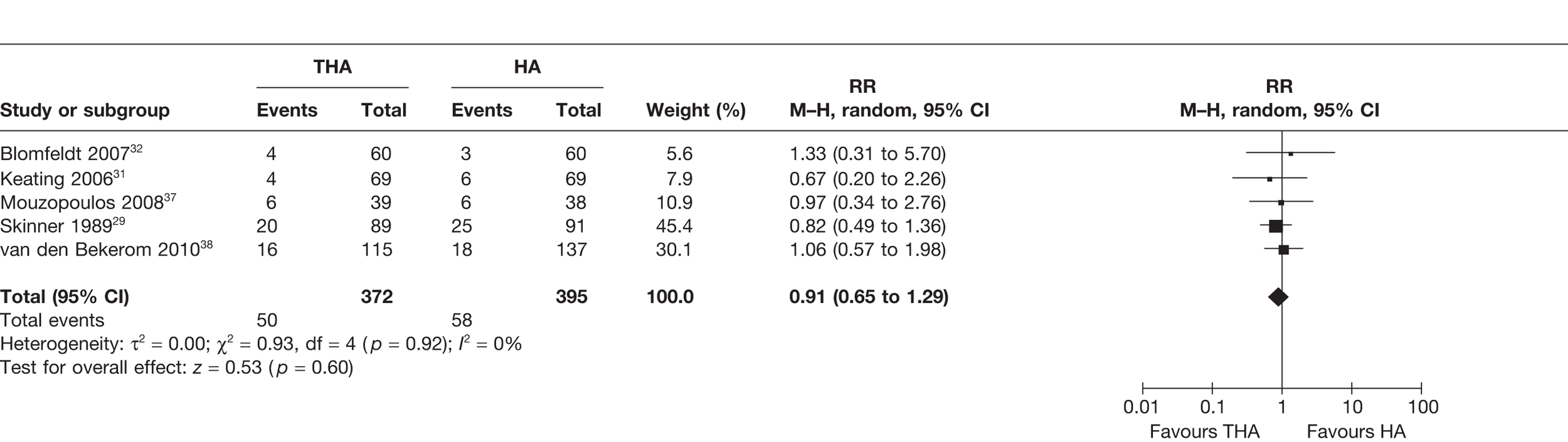
Five studies29,31,32,38,39 (767 analysed participants) compared the number of patients who died within and up to 1 year post operation. A meta-analysis demonstrated a non-statistically significant 9% reduced risk of mortality for those treated with THA compared with HA (RR 0.91, 95% CI 0.65 to 1.29, p = 0.60), with no statistical heterogeneity (I2 = 0%) (see Figure 8 and Table 3). There was a 1% reduction in the absolute risk difference (meta-analysed RD –0.01, 95% CI –0.05 to 0.04, p = 0.75), with no statistical heterogeneity (I2 = 0%) (see Table 3).
FIGURE 8.
Risk of mortality up to 1 year.
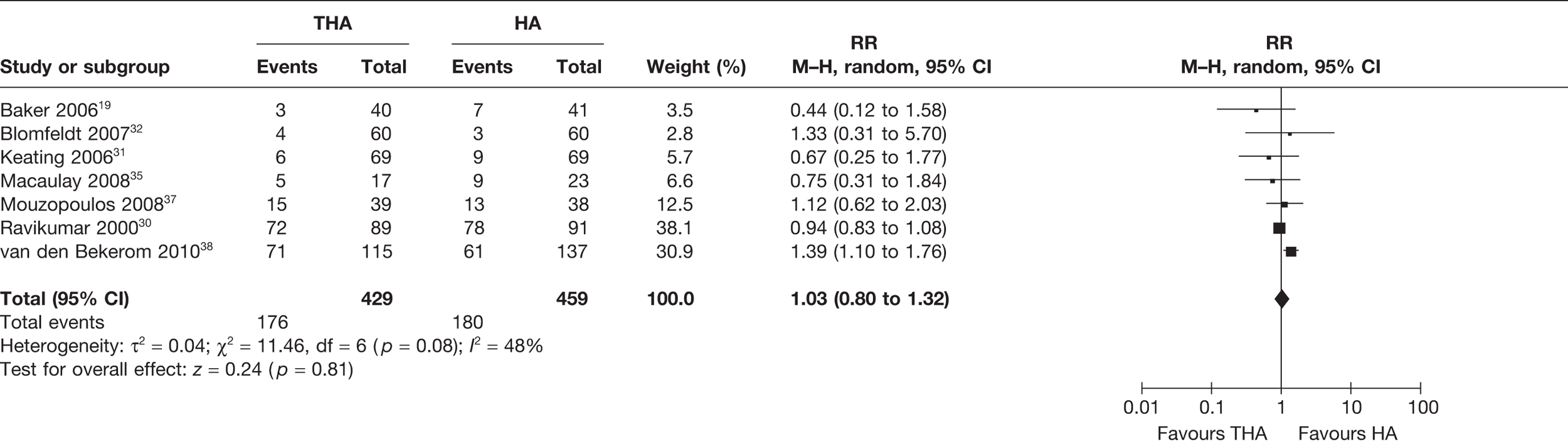
Seven studies19,30–32,35,38,39 (888 analysed participants) compared the number of patients who died for all follow-up periods post operation, up to 13 years. A meta-analysis demonstrated a statistically non-significant 4% increased risk of death for those treated with THA compared with HA (RR 1.03, 95% CI 0.80 to 1.32, p = 0.81), with a moderate level of statistical heterogeneity (I2 = 48%) (see Figure 9 and Table 3). There was no reduction in the absolute risk difference (meta-analysed RD 0.00, 95% CI –0.07 to –0.07, p = 1.00), with a high level of statistical heterogeneity (I2 = 52%) (see Table 3). It is commented that as the time period increases it is expected that the RR of mortality would become nearer to 1 as the patients are elderly and at risk of dying from causes other than those associated with either THA or HA.
FIGURE 9.
Risk of mortality up to 13 years.
Quality of life
Four trials reported scores on utility scales, or subscales, for THA compared with HA. 19,31,32,35 Blomfeldt et al. 32 and Keating et al. 31 both used the European Quality of Life-5 Dimensions (EQ-5D). Blomfeldt et al. 32 reported a slightly higher, statistically non-significant difference in favour of THA at 1 year (0.68 vs 0.63, p = 0.636), whereas Keating et al. 31 reported a statistically significant difference in favour of THA at 2 years (0.69 vs 0.53, p = 0.008). Using the Short Form questionnaire-36 items (SF-36), Macaulay et al. 35 reported a statistically significant difference at 1 year in favour of THA for pain (53.2 vs 42.4, p = 0.02) but not mental health (55.7 vs 49.0, p = 0.25), but statistically significant differences on both subscales at 2 years (54.8 vs 44.7 and 54.9 vs 40.9, respectively, p < 0.05). Baker et al. 19 reported a statistically non-significant difference between the two interventions at 3 years (p = 0.356) on the SF-36.
Peri- and postoperative outcomes and complications
Four studies reported data on surgery duration: Baker et al. 19 and Blomfeldt et al. 32 both reported that THA surgery took significantly longer (p < 0.001) than HA; Keating et al. 31 and van den Bekerom et al. 38 also reported that THA surgery was longer. Blomfeldt et al. 32 and van den Bekerom et al. 38 reported a significantly (p < 0.001) higher rate of intraoperative blood loss for THA surgery than for HA. Keating et al. 31 reported blood transfusions for a significantly (p = 0.02) higher number of patients receiving THA than for those receiving HA, although Blomfeldt et al. 32 reported no such statistically significant difference (p = 0.322) between groups in terms of the mean units of blood transfused.
Baker et al. ,19 Keating et al. 31 and Blomfeldt et al. 32 reported numbers of both peri- and postoperative adverse events or complications in each trial arm (Table 5). The most frequently reported adverse events were pneumonia, pulmonary embolism, DVT, wound infection and urinary tract infection. Rates of DVT were higher in the THA arms and rates of pulmonary embolism were higher in the HA arms; rates of pneumonia, infection and urinary tract infection were similar across arms. None of the studies reported any statistically significant differences between groups. The only significant difference between groups reported for postoperative complications was for the number of patients with radiographic evidence of acetabular erosion at a mean follow-up of 40 months:19 higher rates were reported for HA than for THA (66% vs 0%). Macaulay et al. 35 also reported peri-operative complications and found rates of pneumonia, pulmonary embolism, urinary tract infection and infection to be higher in the HA arm; it was not reported whether or not these differences were statistically significant. Ravikumar and Marsh30 also reported a difference in the proportion of patients with evidence of acetabular erosion or loosening at a follow-up of 13 years: higher rates were reported for HA than for THA (21% vs 0%).
| Study | Mortality (THA vs HA) | Peri-operative outcomes (THA vs HA) Surgery duration (minutes) |
Peri-operative complications (THA vs HA), e.g. hypotension, wound infection, pneumonia, DVT | Intraoperative blood loss (ml) | Blood transfusion (in units), THA vs HA | Postoperative complications, e.g. loosening, erosion |
|---|---|---|---|---|---|---|
| Dorr et al.28 |
‘no difference in mortality between groups...’ p. 23 (Overall n = 7, but event data for each arm NR) |
NR | Overall numbers only: PE (n = 3); DVT (n = 2); acute congestive heart failure (n = 1); acute respiratory failure (n = 1); pneumonia (n = 1); UTI (n = 3); wound haematoma (n = 1); Gram-negative sepsis from cholelithiasis (n = 1) | NR | NR | No infections; no differences between groups |
|
Skinner et al. 29 Ravikumar and Marsh30 |
1 yeara: 20/89 (22%) vs 25/91 (27%) 13 yearsa: 72/89 (81%) vs 78/91 (86%) |
NR | Overall numbers only: PE (n = 2); myocardial infarction (n = 3); peroneal nerve palsy (n = 1); iatrogenic femoral fracture (n = 1)31 | NR | NR |
Acetabular erosion and loosening affected 0% (THA) vs 21% (HA) Overall superficial infection rate: 1.43% at 1 year; 3.3% (THA) vs 7.4% (HA) at 13 years |
| Baker et al.19 |
Approximately 3 years: 3/40 (8%) vs 7/41b (17%) (p = 0.194) None related to the procedure |
Mean (range): 93 (60–135) vs 78 (45–120), p < 0.001 |
Up to 30 days post operation (no difference was significant): THA = 40 vs HA = 41: PE (0 vs 3); DVT (4 vs 0); pneumonia (3 vs 2); wound infection (3 vs 1); UTI (1 vs 0); atrial fibrillation (0 vs 1); haematemesis (0 vs 1); hyponatraemia (1 vs 0) | NR | NR | Only significant reported difference between groups was for radiographic evidence of acetabular erosion at a mean of 40 (range 12–66) months: 0/32 (0%) (THA) vs 21/32 (66%) (HA) |
| Keating et al.31 |
1 year: 4/69 (6%) vs 6/69 (9%) 2 years: 6/69 (9%) vs 9/69 (13%): OR 1.62 (95% CI 0.58 to 4.56) p = 0.36 |
Mean (SD): 82.4 (25) vs 64.3 (15) | THA = 69 vs HA = 69: PE (1 vs 4); DVT (4 vs 0); pneumonia (3 vs 2); wound infection (3 vs 3); myocardial infarction (2 vs 3); septicaemia (1 vs 1) | NR | Numbers who received a transfusion: 23/69 (33%) vs 11/69 (16%), OR 0.38 (95% CI 0.17 to 0.86), p = 0.02 | No differences reported between groups |
| Blomfeldt et al.32 | 1 year: 4/60 (7%) vs 3/60 (5%), p = 0.697 | Mean (range): 102 (70–151) vs 78 43–131), p < 0.001 | THA = 60 vs HA = 60: DVT (0 vs 1); pneumonia (1 vs 0); wound infection (3 vs 2); myocardial infarction (1 vs 1); atrial fibrillation (0 vs 1); congestive heart failure (1 vs 0); decubitus ulcer (1 vs 0) | Mean: 460 (100–1100) vs 320 (50–850), p < 0.001 | Mean: 270 (0–1200) ml vs 200 (0–1200) ml, p = 0.322 | No differences between groups regarding hip or general complications; no signs of erosion or loosening in either group at 12 months |
| Macaulay et al.35 |
2 years: 5/17 (29%) vs 9/23 (39%), p = 0.5334 (mean 34 months; range 29–42 months)c 4/17 (24%) vs 7/23 (30%), p = 0.2035 (mean 19 months; range 3–33 months) |
NR | THA = 17 vs HA = 23: anaemia (4 vs 3); pneumonia (0 vs 3); PE (0 vs 1); UTI (0 vs 3); wound infection (0 vs 1) | NR | NR | NR |
| Mouzopoulos et al.37 |
1 year: 6/39 (15%) vs 6/83 (7%) 4 years: 15/39 (38%) vs 13/38 (34%) |
NR | NR | NR | NR | NR |
| van den Bekerom et al.38 |
1 year: 16/115 (14%) vs 18/137 (13%) (p = 0.86) 5 years: 71/115 (62%) vs 61/137 (45%) (p = 0.09) |
< 60: 10% vs 35% 60–90: 57% vs 53% > 90: 20% vs 12% Unknown: 9% vs 16% |
No differences reported between groups (p = 0.93) in terms of general complications: cardiovascular, urological, neurological, respiratory, gastrointestinal, pressure ulcer, allergic reaction or kidney failure No differences reported between groups (p = 0.36) in terms of local, in-hospital complications, including haematomas, infections, dislocations, wound dehiscence and superior gluteal palsy |
< 500: 61% vs 81% > 500: 22% vs 6% Unknown: 17% vs 14% p < 0.001 (X2 test) |
NR | No differences reported between groups (THA vs HA): loosening of femoral component (1% vs 4%); protrusio acetabuli (1% vs 3%); fissure at the acetabulum (1% vs 2%); heterotopic ossification (15% vs 10%) |
Subgroup analyses
A series of analyses were performed comparing treatment effects for dislocations, revisions, additional surgery and mortality for independent subgroups, defined by studies of different quality (Table 6), the different approach taken by surgeons (anterolateral or posterolateral, Table 7), whether or not cemented or uncemented prostheses were used (Table 8) and whether or not bipolar or unipolar hemiarthroplasty prostheses were used (Table 9). Studies were categorised as being of higher or lower quality based principally on reported methods of randomisation and allocation concealment and the explicit application of intention-to-treat analysis (see Quality assessment and Table 2). On this basis, Baker et al. ,19 Keating et al. ,31 Blomfeldt et al. 32 and Macaulay et al. 35 were categorised as higher quality, and Dorr et al. ,28 Skinner et al. ,29 Ravikumar and Marsh,30 Mouzopoulos et al. 37 and van den Bekerom et al. 38 were categorised as lower-quality studies. Meta-analysis of the lower-quality studies alone found a statistically significant reduced risk of revision for THA compared with HA (p = 0.0004); a similar but statistically non-significant relative risk was found in the analysis of the higher-quality studies. Neither lower- nor higher-quality study subgroups found a statistically significant risk of dislocation, any surgery or mortality. Meta-analysis of the lower-quality studies did find a statistically non-significant increased risk of mortality for THA compared with HA, and analysis of the higher-quality studies found a non-significant reduced risk of mortality for THA. However, despite these differences, there was no statistically significant difference between these study quality subgroups for any of the outcomes assessed (see Table 6).
| Included studies | Number of studies | Variable | THA vs HA, n | RR (95% CI) | I2 (%) | RRR (95% CI) |
|---|---|---|---|---|---|---|
| Dislocations | ||||||
| Baker et al.,19 Keating et al.,31 Blomfeldt et al.,32 Macaulay et al.35 | 4 | Higher quality | 7/182 vs 2/188 | 2.52 (0.65 to 9.82), p = 0.18 | 0 | 0.77 (0.12 to 5.08), p = 0.78 |
| Dorr et al.,28 Ravikumar and Marsh,30 van den Bekerom et al.38 | 3 | Lower quality | 33/243 vs 14/278 | 3.28 (0.88 to 12.16), p = 0.08 | 58 | |
| Revisions | ||||||
| Baker et al.,19 Blomfeldt et al.,32 Macaulay et al.35 | 3 | Higher quality | 2/113 vs 6/119 | 0.66 (0.03 to 13.98), p = 0.79 | 63 | 2.20 (0.09 to 59.12), p = 0.62 |
| Dorr et al.,28 Ravikumar and Marsh,30 Mouzopoulos et al.,37 van den Bekerom et al.38 | 4 | Lower quality | 10/232 vs 36/316 | 0.30 (0.15 to 0.58), p = 0.0004 | 0 | |
| Any surgery | ||||||
| Baker et al.,19 Keating et al.,31 Blomfeldt et al.,32 Macaulay et al.35 | 4 | Higher quality | 13/186 vs 11/193 | 1.12 (0.52 to 2.41), p = 0.78 | 0 | 1.08 (0.32 to 3.62), p = 0.90 |
| Dorr et al.,28 Ravikumar and Marsh,30 Mouzopoulos et al.,37 van den Bekerom et al.38 | 4 | Lower quality | 37/282 vs 43/316 | 1.04 (0.36 to 2.36), p = 0.92 | 63 | |
| Mortality | ||||||
| Baker et al.,19 Keating et al.,31 Blomfeldt et al.,32 Macaulay et al.35 | 4 | Higher quality | 18/186 vs 28/193 | 0.71 (0.41 to 1.23), p = 0.22 | 0 | 0.63 (0.33 to 1.20), p = 0.16 |
| Ravikumar and Marsh,30 Mouzopoulos et al.,37 van den Bekerom et al.38 | 3 | Lower quality | 158/243 vs 152/266 | 1.13 (0.80 to 1.59), p = 0.49 | 80 | |
| Included studies | Number of studies | Variable | THA vs HA, n | RR (95% CI) | I2 (%) | RRR (95% CI) |
|---|---|---|---|---|---|---|
| Dislocations (all follow-up periods) | ||||||
| Baker et al.,19 Blomfeldt et al.,32 van den Bekerom et al.38 | 3 | Direct lateral | 6/189 vs 0/228 | 8.42 (1.05 to 67.38), p = 0.16 | 0 | 3.61 (0.33 to 39.97), p = 0.29 |
| Dorr et al.,28 Skinner et al.,29 van den Bekerom et al.38 | 3 | Posterior | 23/150 vs 12/156 | 2.33 (0.70 to 7.76), p = 0.17 | 48 | |
| Any surgery (all follow-up periods) | ||||||
| Baker et al.,19 Blomfeldt et al.32 | 2 | Direct lateral | 6/100 vs 6/101 | 1.14 (0.20 to 6.47), p = 0.88 | 33 | 1.06 (0.14 to 8.11), p = 0.96 |
| Dorr et al.,28 Ravikumar and Marsh30 | 2 | Posterior | 26/128 vs 32/141 | 1.08 (0.37 to 3.12), p = 0.89 | 71 | |
| Mortality at 1 year | ||||||
| Baker et al.,19 Blomfeldt et al.32 | 2 | Direct lateral | 6/100 vs 11/101 | 0.55 (0.21 to 1.45), p = 0.23 | 0 | 0.67 (0.22 to 2.00), p = 0.47 |
| Skinner et al.29 | 1 | Posterior | 20/89 vs 25/91 | 0.82 (0.49 to 1.36), p = 0.44 | NA | |
| Included studies | Number of studies | Variable | THA vs HA, n | RR (95% CI) | I2 (%) | RRR (95% CI) |
|---|---|---|---|---|---|---|
| Dislocations | ||||||
| Baker et al.,19 Keating et al.,31 Blomfeldt et al.,32 van den Bekerom et al.38 | 4 | Cemented THA vs cemented HA | 14/280 vs 2/302 | 4.39 (0.82 to 23.63), p = 0.08 | 31 | 2.34 (0.39 to 13.91), p = 0.35 |
| Dorr et al.,28 Ravikumar and Marsh,30 Macaulay et al.35 | 3 | Cemented or mixed THA vs uncemented or mixed HA | 26/145 vs 14/164 | 1.88 (1.03 to 3.43), p = 0.04 | 0 | |
| Revisions | ||||||
| Dorr et al.,28 Baker et al.,19 Blomfeldt et al.,32, van den Bekerom et al.38 | 4 | Cemented THA vs cemented HA | 4/250 vs 13/270 | 0.34 (0.11 to 1.05), p = 0.13 | 0 | 0.83 (0.16 to 4.36), p = 0.86 |
| Dorr et al.,28 Ravikumar and Marsh,30 Macaulay et al.35 | 3 | Cemented or mixed THA vs uncemented or mixed HA | 8/145 vs 23/147 | 0.41 (0.12 to 1.37), p = 0.15 | 23 | |
| Any surgery | ||||||
| Dorr et al.,28 Baker et al.,19 Keating et al.,31 Blomfeldt et al.,32 van den Bekerom et al.38 | 5 | Cemented THA vs cemented HA | 21/319 vs 19/339 | 1.15 (0.54 to 2.38), p = 0.71 | 22 | 1.06 (0.30 to 3.03), p = 0.92 |
| Dorr et al.,28 Ravikumar and Marsh,30 Macaulay et al.35 | 3 | Cemented or mixed THA vs uncemented or mixed HA | 27/145 vs 28/127 | 1.08 (0.38 to 3.06), p = 0.89 | 30 | |
| Mortality | ||||||
| Baker et al.,19 Keating et al.,31 Blomfeldt et al.,32 van den Bekerom et al.38 | 4 | Cemented THA vs cemented HA | 83/284 vs 81/307 | 0.91 (0.49 to 1.66), p = 0.75 | 48 | 0.97 (0.52 to 1.81), p = 0.92 |
| Ravikumar and Marsh,30 Macaulay et al.35 | 2 | Cemented or mixed THA vs uncemented or mixed HA | 77/106 vs 87/114 | 0.94 (0.82 to 1.07), p = 0.34 | 0 | |
| Included studies | Number of studies | Variable | THA vs HA, n | RR (95% CI) | I2 (%) | RRR (95% CI) |
|---|---|---|---|---|---|---|
| Dislocations | ||||||
| Dorr et al.,28 aKeating et al.,31 Blomfeldt et al.,32 van den Bekerom et al.38 | 4 | Bipolar | 18/283 vs 4/316 | 3.87 (1.09 to 13.80), p = 0.04 | 25 | 2.28 (0.51 to 10.09), p = 0.28 |
| Ravikumar and Marsh,30 Baker et al.19 | 2 | Unipolar | 21/129 vs 12/132 | 1.70 (0.78 to 3.70), p = 0.18 | 4 | |
| Revisions | ||||||
| Dorr et al.,28 Blomfeldt et al.,32 van den Bekerom et al.38 | 3 | Bipolar | 3/214 vs 9/247 | 0.41 (0.11 to 1.48), p = 0.17 | 0 | 1.58 (0.35 to 7.18), p = 0.56 |
| Ravikumar and Marsh,30 Baker et al.19 | 2 | Unipolar | 7/129 vs 28/132 | 0.26 (0.12 to 0.57), p = 0.0008 | 0 | |
| Mortality | ||||||
| aKeating et al.,31 Blomfeldt et al.,32 van den Bekerom et al.38 | 3 | Bipolar | 81/244 vs 73/266 | 1.30 (0.97 to 1.74), p = 0.08 | 6 | 1.60 (0.77 to 3.35), p = 0.21 |
| Ravikumar and Marsh,30 Baker et al.19 | 2 | Unipolar | 75/129 vs 85/132 | 0.81 (0.41 to 1.58), p = 0.53 | 40 | |
There was no difference in the direction of effect, or any statistically significant difference, between the groups of studies reporting the use of either a direct lateral or a posterior surgical approach for any of the outcomes assessed (see Table 7), or for those subgroups of studies using cemented rather than uncemented or a mix of uncemented and cemented prostheses (see Table 8). When compared with bipolar HA, there was a statistically significant increased risk of dislocation for THA (p = 0.04); this difference was not significant for THA compared with unipolar HA (p = 0.18) (see Table 9). However, in a test of the ratio of RRs (RRR), this difference was found not to be statistically significant. When compared with unipolar HA, there was a statistically significant reduced risk of revision for THA (p = 0.0008); this difference was not significant for THA compared with bipolar HA (p = 0.17). However, again, in a test of the RRR, this difference was found not to be statistically significant. Meta-analysis of the bipolar HA studies found a statistically non-significant increased risk of mortality for THA compared with HA, and analysis of the unipolar studies found a non-significant reduced risk of mortality for THA. Again, despite these differences, there was no statistically significant difference in mortality between subgroups comparing individuals receiving either a unipolar or a bipolar hemiarthroplasty (see Table 9).
Despite the absence of any statistically significant findings in these subgroup analyses, it cannot be excluded that this lack of difference may be owing to small samples in one or more of the groups.
Chapter 4 Assessment of cost-effectiveness
Methods for reviewing cost-effectiveness
A review of the evidence for cost-effectiveness has also been undertaken. The searches performed were as described in Chapter 3, Identification of studies, but slightly different study selection criteria were applied to the results. Studies with either the outcomes of resource utilisation or cost–utility (as listed in Chapter 3, Secondary outcomes) or economic evaluations relating to the population and interventions specified in Chapter 3, Inclusion criteria, were included.
Results
Quantity of research available
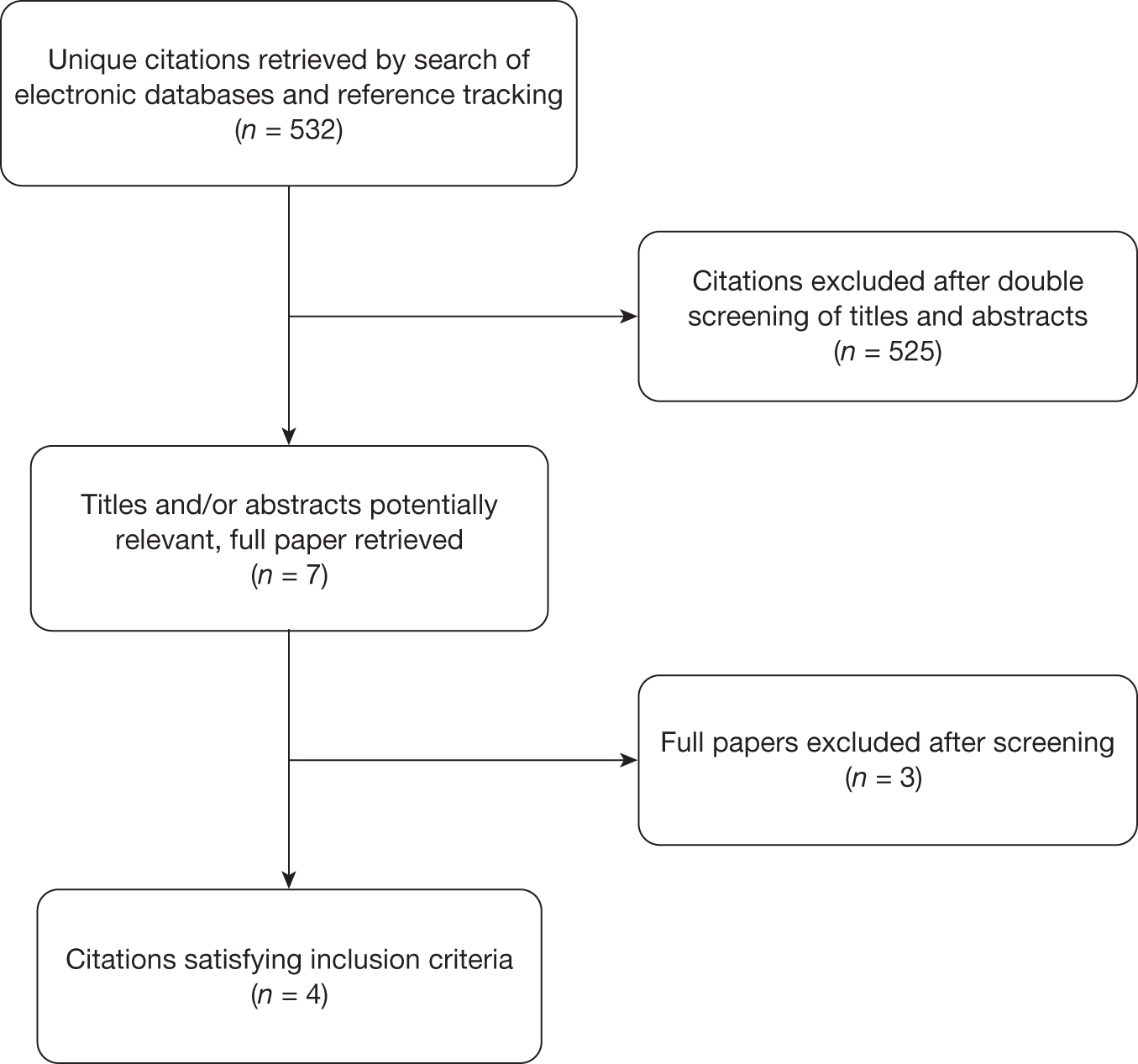
The search of electronic databases identified 532 unique citations. Seven full papers were retrieved to determine whether or not they were relevant to this review. After screening, four studies satisfied the inclusion criteria. 31,44–46 Details of the screening and inclusion process are provided in the PRISMA flow chart (Figure 10). Three studies used mathematical models to perform an economic evaluation,44–46 and one paper31 reported the costs and utilities collected alongside an RCT.
FIGURE 10.
PRISMA flow diagram – cost-effectiveness.
A review of the cost-effectiveness literature
Four papers were identified as having an economic element, although only one took the form of a cost–utility analysis. This paper46 evaluated a patient population with a displaced femoral neck fracture who were elderly and active and treated in an American setting. All costs associated with the surgical procedure and future revisions were included in a Markov model. The conclusion from the model was that THA was the more cost-effective treatment in the patient population with an expected 1.53 quality-adjusted life-years (QALYs) being provided at a cost of US$3000. The cost per QALY ratio of US$1960 would be viewed as extremely cost-effective using standard UK cost-effectiveness thresholds. 47 It was seen that the key driver of this result was the increased utility associated with patients who had undergone THA compared with those that had HA. These data were taken from Keating et al. ,31 with the difference in utility shown to be significant at 24 months (p = 0.008).
The study by Aleem et al. 45 did not include the costs associated with either surgical procedure and focused on the procedure that produced the greatest patient benefit, which implicitly assumes that costs are equal for THA and HA. The derivation of the utilities used within the model was far from ideal as these were derived from asking surgeons and hypothetical patients to rate model outcomes in terms of 0 (death) to 100 (perfect health), rather than using utilities reported directly from patients and with the derived utilities based on public preferences, using a choice-based method, as recommended by the National Institute for Health and Clinical Excellence (NICE). 47 Additionally, median values were used rather than mean values, which is incorrect in economic evaluations. The authors concluded that arthroplasty produces better patient outcomes than internal fixation and that THA had slightly better outcomes than HA.
The analysis of Iorio et al. 44 reported outcomes in terms of the cost per ambulatory patient at 2 years for four procedures (reduction with internal fixation, unipolar HA, bipolar HA and THA). As such, differences in the quality and length of life of patients during this period were ignored and the paper in essence reports a cost minimisation analysis. The authors concluded that THA was the most cost-effective of the four procedures.
Keating et al. 31 reported the costs and utility consequences from an RCT which compared THA and HA. The authors claim that THA is more cost-effective, but do not provide incremental cost-effectiveness ratios. The data contained within this manuscript could be used to calculate an estimate of the likely cost-effectiveness of THA compared with HA at the duration of follow-up (2 years) and at extrapolated time horizons. The authors of this report undertook this using a simple mathematical model that is detailed later (see Chapter 4, The economic evaluation undertaken within this report).
An assessment of Slover et al. ,46 using the Drummond et al. 48 checklist, is contained in Appendix 4. The remaining three papers with economic elements were not assessed as they were considered less appropriate owing to undertaking either a cost minimisation44 or a benefit maximisation45 approach or simply reported data from an RCT. 31
The economic evaluation undertaken within this report
It was deemed that the Iorio et al. 44 and Aleem et al. 45 studies were too limited to inform the decision problem fully. The paper by Slover et al. 46 was a mathematical model of reasonable quality, but was based on a US setting rather than a UK one. The authors of this report decided to perform an economic evaluation based on the Keating et al. 31 RCT as this had high internal validity and was directly applicable to the study population. If the results from this analysis concurred with those from Slover et al. ,46 and to a lesser extent those of Iorio et al. 44 and Aleem et al. ,45 then this would support the conclusions that THA was more cost-effective than HA. The proposed modelling methodology was discussed with the clinical expert, who deemed that this was an acceptable conceptual model. Given the resource constraints, a decision to employ a simplistic model was undertaken.
The Keating et al. 31 RCT is directly relevant to the decision problem as it was conducted in Scotland and compared the two interventions of interest. The data reported contained the utility of patients at 4, 12 and 24 months using the EQ-5D questionnaire and the mean costs associated with each intervention over the 2-year period. The EQ-5D is the utility measure preferred by NICE. 47 Costs were presented in five categories: initial inpatient episode; hip-related admissions; non-hip-related admissions; total hip-related costs; and total costs. Data concerning the characteristics of the Keating et al. 31 RCT are presented in Table 1. Owing to the direct relevance and high internal validity, the authors believed that these data were more appropriate to populate the economic model than the results produced by the meta-analyses undertaken earlier in this report.
An estimate of the cost-effectiveness of THA compared with HA was calculated assuming that the increased costs associated with THA were normally distributed with a mean of £3010 with a standard error (SE) of £2250. This cost differential is given some support by data from the American 2003 National Inpatient Survey reported in Slover et al. 46 that stated that the average hospital charges for THA compared with HA were US$4409 higher. The costs from Keating et al. 31 were inflated from the 2000–1 price year to a 2007–8 price year,49 resulting in a mean increase in costs associated with THA compared with HA of £3937; the SE of this increase was assumed to increase to £2943. It was assumed that all costs were incurred in the first year and that costs would remain constant for both arms for the remainder of the model. This approach has support in research undertaken by Haentjens et al. ,50 which indicated that the type of surgical procedure (THA or HA) was not associated with differential costs in the year following hospital discharge. Given this methodology, costs were not discounted.
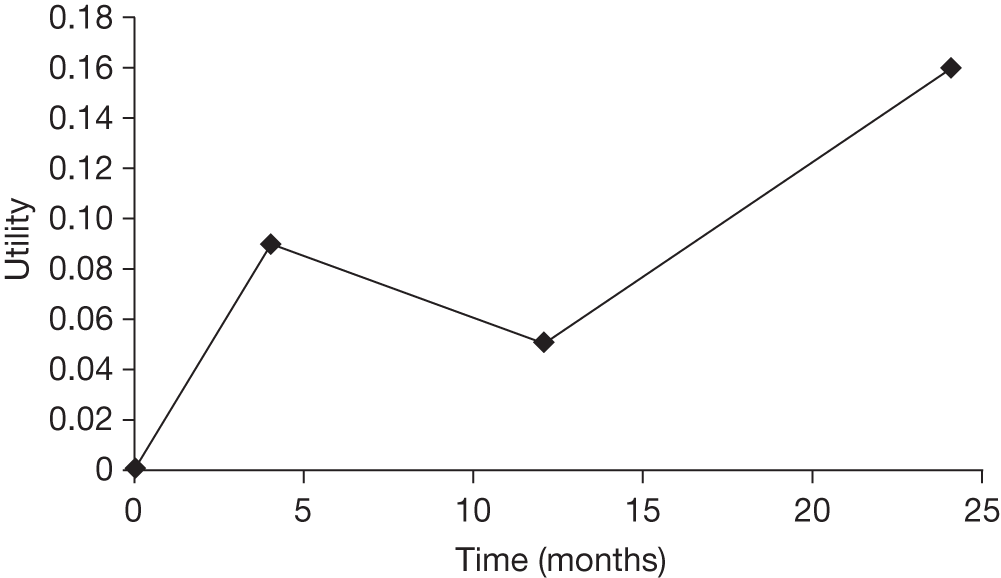
Based on distributions presented in Keating et al. ,31 it was assumed that the EQ-5D increase was 0.09 (SE 0.05), 0.05 (SE 0.05) and 0.16 (SE 0.06) at 4, 12 and 24 months, respectively. It was assumed that there was a linear change from zero to the sampled difference in utility at 4 months, a linear change between the sampled differences at 4 and 12 months and a linear change between the sampled differences at 12 and 24 months. The difference at 24 months was assumed to persist until the end of the modelling horizon. Utilities were discounted at 3.5% per annum as recommended by NICE. 47 In the analyses undertaken, time horizons of 2, 3 and 5 years were assessed as it was believed that the vast majority of patients who were alive at 2 years would survival an additional 3 years.
The incremental cost per QALY of THA was calculated as the incremental cost of THA divided by the incremental QALY. A plot of the modelled utilities is provided in Figure 11 assuming that the midpoint estimates for both THA and HA are correct.
The mortality rates observed within the trial were considered. In the Keating et al. 31 RCT there was a greater proportion of deaths in the HA arm (13%) than in the THA arm (9%), although this was not statistically significant (p = 0.36). These data were pooled to form a risk of mortality in both arms of 11%, and it was assumed that the incremental QALY gain estimated for THA would be reduced by 11% to account for mortality.
FIGURE 11.
The assumed gain in utility associated with THA compared with HA.
In order to preserve consistency between the sampled utility differences when conducting the probabilistic sensitivity analyses, the same random number was used to select from the cumulative distribution function for each time point. This would ensure that if the value sampled for the difference at 4 months was higher than the median; the differences at 12 and 24 months would also be higher than the median value.
For clarity, the parameter values used in the probabilistic sensitivity analyses are given in Table 10.
| Parameter | Distribution used (mean, SE) | Note |
|---|---|---|
| Mean Incremental cost of THA compared with HA | Normal (£3937, £2943) | The Keating et al.31 value at 2 years was inflated to 2007–8 prices. No other costs assumed |
| Mean incremental utility gain of THA compared with HA at: | ||
| 4 months | Normal (0.09, 0.05) | Taken from Keating et al.31 The values were sampled using the same random number. Linear interpolation was assumed. For longer time horizons it was assumed that the utility gain at 24 months remained constant |
| 12 months | Normal (0.05, 0.05) | |
| 24 months | Normal (0.16, 0.06) | |
| Assumed mortality rate over the model horizon | 11% | Data taken from Keating et al.31 value at 2 years. No further mortality was considered |
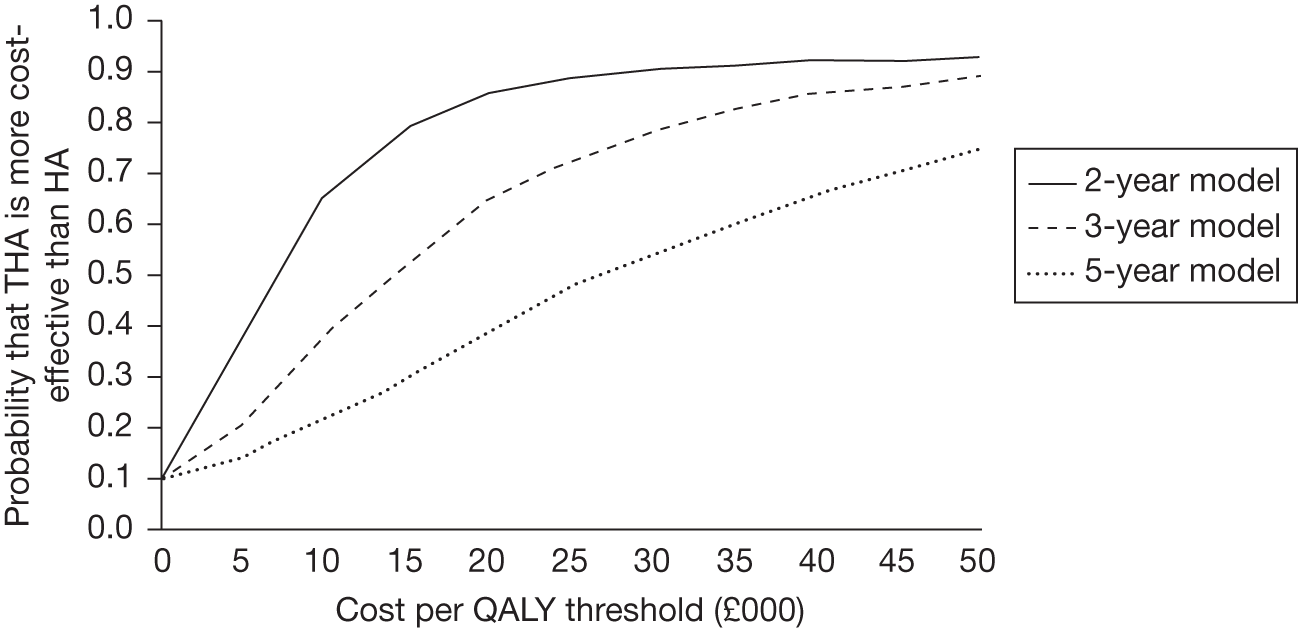
The results from this model are provided in Table 11 and used 1000 Monte Carlo simulations. It is seen that even when the utility benefits are constrained to the 2-year horizon the cost per QALY is < £30,000. When the time horizon is extrapolated to more realistic values, the cost per QALY decreases, reaching a value < £10,000 with a horizon of only 5 years. This value would be seen as cost-effective under current cost-effectiveness thresholds. 47 It is seen that the results produced within our analyses concur with previous authors44–46 in that THA is likely to be more cost-effective than HA.
| Time horizon (years) | Incremental costs (£) | Incremental QALYs | Incremental cost per QALY (£) |
|---|---|---|---|
| 2 | 3989 | 0.147 | 27,023 |
| 3 | 3989 | 0.285 | 16,146 |
| 5 | 3989 | 0.580 | 7952 |
The likelihood of THA being more cost-effective than HA can be displayed on a cost-effectiveness acceptability curve; this is shown in Figure 12. All time horizons are shown simultaneously on this figure for brevity; these are different modelling scenarios rather than competing strategies within one decision problem.
FIGURE 12.
Cost-effectiveness acceptability curves depicting the likelihood that THA is more cost-effective than HA.
Limitations of the analyses
It is commented that longer-term consequences, such as the rates of revision and dislocation, have not been considered in this analysis. Data from studies with a follow-up to 13 years indicate that THA is associated with significantly fewer revisions (RR 0.31, 95% CI 0.17 to 0.59; see Table 3), whereas HA is associated with significantly fewer dislocations (RR 2.40, 95% CI 1.21 to 4.76; see Table 3). The impact of these omissions is likely to be unfavourable to THA as clinical advice indicates that the costs and disutility associated with revisions are far greater than those associated with dislocations. As such, this strengthens the conclusions that THA is more cost-effective than HA.
The effect of ageing on the incremental gain in utility has not been considered. There are no data to indicate whether or not the gain would increase, decrease or remain static as patients age; however, it is expected that the results may be more uncertain than presented.
Exploratory sensitivity analyses
Exploratory sensitivity analyses were undertaken assuming that the increased utility associated with THA compared with HA was equal to the midpoint reported by Blomfeldt et al. ,32 which was 0.05 (0.68 for THA and 0.63 for HA). Although this difference was statistically non-significant, the indication from Keating et al. 31 is that there is a real difference in utility. In this sensitivity analysis, the cost per QALY was £44,997, £30,511 and £18,932 at 2, 3 and 5 years, respectively. These values were was not as favourable to THA as the analyses based on Keating et al. ,31 but they still indicate that THA is likely to be more cost-effective than HA assuming a time horizon of ≥ 5 years using standard UK thresholds. 48
However, the authors prefer the data from Keating et al. 31 as this study has a UK setting, has a slightly larger sample size, has a greater follow-up period and is consistent with the values used for increased costs associated with THA.
The cost data from Keating et al. 31 were inflated to 2007–8 prices, and it is uncertain whether or not the costs originally reported would have risen equally for both THA and HA, although it is likely there would have been some correlation regarding costs, such as inpatient costs that would be incurred in each operation.
Additionally, although there is some support for equal costs after the 2-year period,50 it may be that the different rates of revisions and dislocations have a cost implication.
In order to explore the possibility that these incremental costs may differ from that used in the base case, sensitivity analyses were conducted varying the incremental cost of THA compared with HA (Table 12).
| Assumed incremental cost | Time horizon | ||
|---|---|---|---|
| 2 years | 3 years | 5 years | |
| £0 | Dominates | Dominates | Dominates |
| £2000 | 13,550 | 7008 | 3451 |
| £3937a | 27,023 | 16,146 | 7952 |
| £6000 | 40,659 | 21,023 | 10,354 |
| £8000 | 54,198 | 28,031 | 13,805 |
These sensitivity analyses indicate that even if the incremental cost of THA compared with HA increased to £8000 then it is likely that THA would still be cost-effective provided that the time horizon was ≥ 5 years, given current cost-effectiveness thresholds. 47
The expected value of perfect information.
The expected value of perfect information (EVPI)51 was calculated for the base-case model, assuming that the funders were prepared to pay a cost of £20,000 per QALY gained. 47 Population EVPI provides the maximum that a funder would be prepared to pay to eliminate all uncertainty in the decision problem and thus know with certainty which option was more cost-effective. If the cost of the research required to provide further information is greater than the population EVPI then the research should not be funded.
The estimated EVPI per patient is given in Table 13 using time horizons of 3 and 5 years. At these time points the adoption decision would be THA. Population EVPI is calculated by the number of patients who are assumed to benefit owing to the greater certainty of which procedure is the more cost-effective.
| Time horizon | EVPI per patient |
|---|---|
| 3 years | £1043 |
| 5 years | £548 |
As previously discussed, the omission of the costs and disutilities associated with revisions and dislocations is likely to strengthen the conclusion that THA is more cost-effective than HA. This would reduce the uncertainty in the decision and therefore it is likely that the EVPI is overestimated.
It is seen that the EVPI decreases as the modelling horizon increases. This is due to the greater certainty that THA is more cost-effective than HA when the time horizon is of larger duration. These values, however, are likely to change when trials currently under way report their findings and the evidence base expands.
Chapter 5 Assessment of factors relevant to the NHS and other parties
Total hip arthroplasty appears to be more cost-effective than HA although it is likely that THA will be associated with increased costs in the initial 2-year period. The longer-term costs owing to potentially lower revision rates associated with THA have not been estimated. The capacity and experience of surgeons to perform THA have not been explored and these would need to be addressed at local level were THA to become recommended for active, elderly patients in whom THA is not contraindicated. Most orthopaedic surgeons would agree that THA is a more complex procedure than HA. According to clinical advice, the vast majority of HA cases are performed by a wide range of surgeons including more senior trainees. In contrast, THA procedures for fracture tend, in most units, to be performed by only trained, experienced joint replacement surgeons or under their direct supervision. If there was to be a significant increase in the use of THA for fracture of the femoral neck, there would need to be either a change in practice for these surgeons or extra training for the remainder to become confident in this procedure.
Chapter 6 Discussion
Statement of principal findings
Eight RCTs19,28–30,32,37–39 satisfied the inclusion criteria. The number of participants in all of the trials was 972. Meta-analysis found a near significant increased risk of early dislocation within 1 year for THA compared with HA (RR 3.98, 95% CI 0.98 to 16.12, p = 0.05), but also found a statistically significant increased risk of dislocation for patients treated with THA compared with HA (RR 2.40, 1.41 to 2.76, p = 0.01) for all follow-up periods up to 13 years.
Meta-analyses of five trials29,32,35,38,39 found a statistically non-significant 59% reduced risk of revision within 1 year for THA compared with HA (p = 0.06), but meta-analysis of seven trials19,28,30,32,35,38,39 found a statistically significant 69% reduced risk of revision for patients treated with THA compared with HA (RR 0.31, 0.17 to 0.59, p = 0.0003) for all follow-up periods up to 13 years.
Meta-analyses of five trials29,32,35,38,39 and eight19,28,30–32,35,38,39 trials, respectively, found a statistically non-significant reduced risk of any surgery (dislocation reduction, revisions or other reoperations), both within 1 year and for all follow-up periods, for THA compared with HA (p = 0.46 and 0.75, respectively). Meta-analysis also found a statistically non-significant reduced risk of mortality both within 1 year and for all follow-up periods for THA compared with HA (p = 0.60 and 0.81, respectively). Subgroup analyses found that neither study quality, the surgical approach taken (lateral or posterior), the use of cement, nor the use of unipolar or bipolar prostheses were statistically significant confounding variables affecting any of these outcomes when comparing data on THA and HA from the RCTs identified for this review.
Eight studies19,28–30,32,37–39 reported hip ratings, measuring function, mobility and level of pain experienced by participants post operation for between 6 months’ and 13 years’ follow-up. Five studies30,32,35,37,38 used the HHS, one the OHS, one the Hip Rating Questionnaire and one a modified version of the D’Aubigne/Postel hip score. In all studies, individuals treated with THA reported better scores (i.e. more function and mobility and less pain) than those treated with HA. In four studies19,31,32,35 this difference was reported to be statistically significant (Blomfeldt et al. 32 at 1 year; Keating et al. 31 and Macaulay et al. 35 at 2 years; Baker et al. 19 at 3 years). Clinical advice suggests that the hip scores used were designed and validated with reference to treatment of degenerative disease of the hip. There are no validated scores for the assessment and follow-up of patients with fractured neck of the femur. None of the scoring systems could be used after the fracture and before treatment as the factors involved in the score are not validated for this diagnosis. The best that can be said is that using these scoring systems prospectively after fracture will give a measure of improvement over time. The OHS would be seen as the most relevant as it is a patient-reported measure concerning hip health over the preceding 4 weeks and can be used in follow-up. The HHS may be considered less robust for this population as this includes clinician-led measures of movement and function.
Four trials19,31,32,35 reported utility data using the EQ-5D and SF-36 measures: participants treated with THA reported statistically significantly better scores than those treated with HA in two studies;31,35 the remaining two studies19,32 reported no significant difference between groups.
The only statistically significant differences between groups for peri- and postoperative adverse events or complications reported by any study were higher intraoperative blood loss for THA by two studies;32,38 higher numbers of patients receiving blood transfusion for THA than for HA in one study;31 and higher percentages of patients experiencing acetabular erosion or loosening for HA than for THA in two studies. 19,24
Five relevant reviews and meta-analyses have been published in the last 2 years. 21,26,52–54 Liao et al. 52 identified six of the eight RCTs analysed in the present review (only Mouzopoulous et al. 37 and van den Bekerom et al. 38 were omitted) and reported similar findings: the risk of dislocation was found to be significantly higher in THA than in HA (RR 3.45, 95% CI 1.29 to 9.19, p = 0.01), and the risk of revision was significantly lower (RR 0.28, 95% CI 0.12 to 0.66, p = 0.003); blood loss and surgery duration were significantly higher for THA than for HA; but mobility and pain were better for THA than for HA (p < 0.05). A review by Hopley et al. 26 had similar inclusion criteria, but included non-RCTs also. This review reported no difference between THA and HA in the risk of dislocation, although the ‘tendency’ favouring THA ‘was most pronounced in studies with balanced patient baseline profiles and follow-up intervals of 2 or more years’. 26 THA was also associated with a lower, statistically non-significant risk (p = 0.16) of ‘re-operation’ (this outcome was not defined, but appears to consist of revisions only in some cases and all additional surgery in others) across the RCT and non-RCT included studies. There was also no reported difference between treatments in terms of 1-year mortality, but ‘Notable benefits [of THA] were observed in randomised trials’. 26 Independent subgroup analyses found no statistically significant effect of any confounding variables (including characteristics of study quality and surgeon experience) on dislocation and, for revision rates, significant differences only between cemented versus mixed prostheses, oriented and ambulatory versus mixed populations, and whether or not intention-to-treat analyses had been specified or unspecified. However, these findings were the result of pooling both RCTs and retrospective cohort studies. Despite the authors’ argument that the inclusion of non-experimental studies substantially increased the overall sample size and enhanced the robustness of the resulting estimates, the pooling of data from studies using different study designs is questionable owing to the differing risk of bias inherent in the different designs. 22,55 Previous research in this subject area and population has also demonstrated significant discrepancies between the findings of randomised and non-randomised studies, concluding that the merits of non-randomised studies need to be considered very carefully. 56
A recently updated Cochrane review by Parker et al. 21 with similar inclusion criteria to the present report undertook separate meta-analyses for both uncemented and cemented HA versus THA for the following outcomes of interest to the current review: dislocation rates, minor, major and ‘any’ ‘reoperations’, and mortality. The updated review included only full published data from six19,28,31,32,35,37 of the eight RCTs identified by the present review. The Ravikumar and Marsh30 paper, which reported follow-up data from the Skinner et al. 29 study, was not identified in the original review of 2006 and was also missed in subsequent updates. The updates could not have identified the study by van den Bekerom et al. ,38 but they did still fail to identify the Ravikumar and Marsh30 paper, despite it being referenced in every trial published since 2006 and the authors stating explicitly that the reference lists of all included studies were checked. The uncemented HA analyses contained two trials,28,29 and the cemented HA analyses contained four trials;19,28,31,32 Macaulay et al. 35 and Mouzopoulous et al. 37 were included as mixed populations in the comparisons with THA only. All RR analyses were calculated using a fixed-effects model, which may be a questionable assumption. The review reported that THA had a statistically significantly higher likelihood of dislocation than HA, but found no statistically significant differences in terms of any ‘reoperations’ or mortality. The Parker et al. 21 review also performed analyses of the complications (e.g. infection rates, embolisms and medical complications) reported by the trials and found no significant differences between the two interventions.
A series of analyses by Goh et al. 53 applied the same inclusion criteria as the present review, but identified only four of the eight relevant RCTs. Goh et al. 53 did not combine these trials in a meta-analysis, but reported the difference in the RRs of dislocation or revision between THA and HA, using a fixed-effects model only, only for the individual trials. The findings of four of the eight relevant RCTs were also summarised in a review by Schmidt et al. :54 Dorr et al. ,28 Skinner et al. ,29 Keating et al. 31 and Blomfeldt et al. 32 This was not a systematic review and no formal analysis or meta-analysis was performed.
Three papers were found that reported economic evaluations of THA compared with HA,44–46 with a further paper reporting cost and utility data from an RCT. 31
The data in the RCT were used in exploratory modelling work undertaken by the authors. All modelling exercises concluded that THA was likely to be cost-effective compared with HA.
Strengths and limitations of the assessment
Strengths
-
This review developed a sensitive search strategy combining only terms for THA and HA, rather than limiting the search further by using terms for population, outcomes or study design. This search identified studies satisfying the inclusion criteria for both clinical effectiveness and cost-effectiveness.
-
The search was comprehensive. There were no limitations of language or date (e.g. non-English-language studies have been included), reference tracking was applied to all included studies and an expert was consulted on other potentially relevant studies. Unpublished studies and ongoing trials were also identified. This suggests that the likelihood of publication bias in this review is relatively small.
-
The review process: all titles and abstracts of citations retrieved by the search of electronic databases were screened independently for inclusion and exclusion by two reviewers; and all data extraction and quality assessment of included studies were checked thoroughly by two reviewers and any discrepancies identified and resolved.
-
The impact of possible confounding variables, such as study quality, surgical approach, the use of cement and unipolar of bipolar prostheses, have been tested for.
-
Exploratory modelling has been undertaken that shows that THA is highly likely to be cost-effective compared with HA even when the limitations of the data and methodology are considered. This concurs with previously published economic analyses.
Weaknesses
-
Despite efforts to identify all published and unpublished research satisfying the inclusion criteria, publication bias as a result of the non-publication of trials demonstrating no effect cannot be entirely discounted.
-
The costs and disutilities associated with revisions and dislocations were not included in the economic evaluation undertaken by the authors. However, this omission strengthens the conclusion that THA is more cost-effective than HA.
Uncertainties
-
This review identified eight RCTs with a total of 972 participants in THA and HA treatment arms. The overall sample is therefore not very large.
-
There was moderate or high statistical heterogeneity in some meta-analyses, which therefore increases the risk of uncertainty regarding some of the findings reported in this review.
-
There was some clinical heterogeneity between studies in terms of surgical approach, type and size of prostheses used, outcome measures applied (i.e. revisions, ‘reoperations’, second anaesthetic and hip scores) and length of follow-up, which prevented the pooling of results from all eight RCTs for all outcomes. Some analyses of the primary outcomes therefore combined data from only four or five trials.
-
The relative experience of surgeons in performing the two procedures is also not reported in seven19,28,30–32,35,38 of the eight trials. This is a potential confounding variable and may have affected outcomes.
-
The majority of independent subgroup analyses to test for the potential confounding effect of certain variables included only three or four trials.
-
Revision of THA may present a more complex complication as clinical advice suggests that it is sometimes easier to revise a HA to a THA than it is to revise a THA to a further THA. There may have been greater reluctance to revise THAs, which might explain the smaller incidence of THA revisions within these trials; however, this is not always the case. Each revision must be viewed on its merits and a blanket comment cannot be made.
-
The exploratory analyses did not consider the costs of future revisions or dislocations or of any differential rates of mortality, although, other than for dislocations, there was a trend for THA to have better outcomes. The inclusion of such factors is likely to strengthen the conclusion that THA is more effective than HA, but the reduction in the cost per QALY gained is uncertain.
Other relevant factors
None.
Chapter 7 Conclusions
Implications for service provision
Total hip arthroplasty appears to be more cost-effective than HA, although it is likely that this will be associated with increased costs in the initial 2-year period. The longer-term reduction in costs owing to potentially lower revision rates associated with THA have not been estimated. The capacity and experience of surgeons to perform THA have not been explored and these would need to be addressed at local level were THA to become recommended for active, elderly patients who were not contraindicated for THA.
Suggested research priorities
Eight head-to-head RCTs of THA and HA have currently been published. The EVPI per patient has been estimated, although it is expected that there is limited value in conducting a new trial comparing THA and HA as three such trials are ongoing at the time of writing. The biggest of these trials [Comparing Total Hip Arthroplasty and Hemi-Arthroplasty on Revision Surgery and Quality of Life in Adults With Displaced Hip Fractures (the HEALTH study): NCT00556842] has an estimated enrolment of 306 participants. Some of the published trials may also report more data as they become available. Furthermore, the findings of these trials are generally consistent regarding the relative efficacy and trends of the two interventions in terms of dislocation rates, revision rates, hip scores and quality of life. These findings do not appear to be affected by potential confounding variables identified in the literature (i.e. study quality, surgical approach, the use of cement and unipolar of bipolar hemiarthroplasty prostheses), as far as these data were available and permitted relevant subgroup analysis. However, further studies examining the impact of surgeons’ experience in performing the two procedures (i.e. THA and HA each being performed by surgeons equally experienced in the respective procedures) may offer some more robust evidence on outcomes.
Acknowledgements
Andrea Shippam provided administrative support in preparing and formatting the report.
Contributions of authors
Christopher Carroll acted as the principal investigator for this assessment, and designed, performed and wrote the review; Christopher Carroll and Philippa Evans designed search strategies and Philippa Evans undertook the searches; Alison Scope contributed to the review; and, Matt Stevenson reviewed the economic literature and constructed, populated and interpreted the results from the mathematical model. Simon Buckley provided expert clinical advice.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- Stevenson M, Lloyd-Jones M, Papaioannou D. Vitamin K to prevent fractures in older women: a systematic review and economic evaluation. Health Technol Assess 2009;13.
- Keating J, Grant A, Masson M, Scott NW, Forbes JF. Displaced intracapsular hip fractures in fit, older people: a randomised comparison of reduction and fixation, bipolar hemiarthroplasty and total hip arthroplasty. Health Technol Assess 2005;9.
- Cummings S, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet 2002;359:1761-7.
- Macaulay W, Pagnotto MR, Iorio R, Mont MA, Saleh KJ. Displaced femoral neck fractures in the elderly: Hemiarthroplasty versus total hip arthroplasty. J Am Acad Orthop Surg 2006;14:287-93.
- Riggs B, Melton LJ. The worldwide problem of osteoporosis: insights afforded by epidemiology. Bone 1995;17:505S-511S.
- Parker MJ, Gurusamy K. Disabil Rehabil 2005;27:1045-51.
- Singer B, McLauchlan G, Robinson C, Christie J. Epidemiology of fractures in 15000 adults: the influence of age and gender. J Bone Joint Surg Br 1998;80:243-8.
- Bhandari M, Devereaux PJ, Tornetta P, Swiontkowski MF, Berry DJ, Haidukewych G, et al. Operative management of dsiplaced femoral neck fractures in elderly patients: an international survey. J Bone Joint Surg Am 2005;87:122-30.
- Parker MJ, Pryor GA. Internal fixation or arthroplasty for displaced cervical hip fractures in the elderly: a randomised controlled trial of 208 patients. Acta Orthop Scand 2000;71.
- Heetveld MJ, Rogmark C, Frihagen F, Keating J. Internal fixation versus arthroplasty for displaced femoral neck fractures: What is the evidence?. J Orthop Trauma 2009;23:395-402.
- Crossman PTK. A survey of the treatment of displaced intracapsular femoral neck fractures in the UK. Injury 2002;33:383-6.
- Ibrahim T, Bloch B, Esler CN, Abrams KR, Harper WM. Temporal trends in primary total hip and knee arthroplasty surgery: results from a UK regional joint register, 1991–2004. Ann R Coll Surg Engl 2010;92:231-5.
- Sayana MK, Lakshmanan P, Peehal JP, Wynn-Jones C, Maffuli N. Total hip replacement for acute femoral neck fracture: A survey of National Joint Registries. Acta Orthop Belg 2008;74:54-8.
- Messick K. Gwathmey Arthroplasty in the management of acute femoral neck fractures in the elderly. Semin Arthroplasty 2008;19:283-90.
- Enocson A, Hedbeck CJ, Tidermark J, Pettersson H, Ponzer S, Lapidus LJ. Dislocation of total hip replacement in patients with fractures of the femoral neck. Acta Orthop 2009;80:184-9.
- Leighton RK, Schmidt AH, Collier P, Trask K. Advances in the treatment of intracapsular hip fractures in the elderly. Injury 2007;38:S24-34.
- Berry D, Von Knoch M, Schleck CD, Harmsen WS. Effect of femoral head diameter and operative approach on risk of dislocation after primary total hp arthroplasty. J Bone Joint Surg Am 2005;87:2456-63.
- Enocson A, Pettersson H, Ponzer S, Tornkvist H, Dalen N, Tidermark J. Quality of life after dislocation of hip arthroplasty: a prospective cohort study on 319 patients with femoral neck fractures with a one-year follow-up. Qual Life Res 2009;18.
- Baker RP, Squires B, Gargan MF, Bannister GC. J Bone Joint Surg Am 2006;88:2583-9.
- Moher D, Liberati A Tetzlaff J, Altman DG. The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Int Med 2009;151:264-9.
- Parker MJ, Gurusamy KS, Azegami S. Arthroplasties (with and without bone cement) for proximal femoral fractures in adults. [Update of Cochrane Database Syst Rev 2006;3:CD001706; PMID: 16855974.]. Cochrane Database Syst Rev 2010;6.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. n.d. www.cochrane-handbook.org.
- Higgins J, Thompson S, Deeks JJ, Altman D. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60.
- Montori V, Guyatt G. Intention-to-treat principle. CMAJ 2001;165:1339-41.
- Keene G, Parker M. Hemiarthroplasty of the hip: The anterior or posterior approach? A comparison of surgical approaches. Injury 1993;24:611-13.
- Hopley C, Stengel D, Ekkernkamp A, Wich M. Primary total hip arthroplasty versus hemiarthroplasty for displaced intracapsular hip fractures in older patients: systematic review. BMJ 2010;340.
- Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ 2003;326.
- Dorr LD, Glousman R, Hoy AL, Vanis R, Chandler R. Treatment of femoral neck fractures with total hip replacement versus cemented and noncemented hemiarthroplasty. J Arthroplasty 1986;1:21-8.
- Skinner P, Riley D, Ellery J, Beaumont A, Coumine R, Shafighian B. Displaced subcapital fractures of the femur: a prospective randomized comparison of internal fixation, hemiarthroplasty and total hip replacement. Injury 1989;20:291-3.
- Ravikumar KJ, Marsh G. Internal fixation versus hemiarthroplasty versus total hip arthroplasty for displaced subcapital fractures of femur – 13 year results of a prospective randomised study. Injury 2000;31.
- Keating JF, Grant A, Masson M, Scott NW, Forbes JF. Randomized comparison of reduction and fixation, bipolar hemiarthroplasty, and total hip arthroplasty. Treatment of displaced intracapsular hip fractures in healthy older patients. J Bone Joint Surg Am 2006;88:249-60.
- Blomfeldt R, Törnkvist H, Eriksson K, Söderqvist A, Ponzer S, Tidermark J. A randomised controlled trial comparing bipolar hemiarthroplasty with total hip replacement for displaced intracapsular fractures of the femoral neck in elderly patients. J Bone Joint Surg Br 2007;89:160-5.
- Blomfeldt R, Tornkvist H, Eriksson K, Soderqvist A, Ponzer S, Tidermark J. Bipolar hemiarthroplasty compared with total hip replacement for displaced femoral neck fractures in the elderly. A randomised, controlled trial. J Bone Joint Surg 2009;S1.
- Macaulay W, Nellans KW, Garvin KL, Iorio R, Healy WL, Rosenwasser MP, et al. Prospective randomized clinical trial comparing hemiarthroplasty to total hip arthroplasty in the treatment of displaced femoral neck fractures: winner of the Dorr Award. J Arthroplasty 2008;23:2-8.
- Macaulay W, Nellans K, Iorio R, Garvin KL, Healy W, Rosenwasser MP. Total hip arthroplasty in less painful at 12 months compared with hemiarthroplasty in treatment of displaced femoral neck fracture. HSS J 2008;4.
- Macaulay W, Nellans K, Garvin K, Iorio R, Healy W, Teeny S, et al. Prospective randomized clinical trial comparing hemiarthroplasty to total hip arthroplasty: Functional outcomes in the treatment of displaced femoral neck fractures. Osteoporos Int 2006;17:S238-9.
- Mouzopoulos G, Stamatakos M, Arabatzi H, Vasiliadis G, Batanis G, Tsembeli A, et al. The four-year functional result after a displaced subcapital hip fracture treated with three different surgical options. Int Orthop 2008;32:367-73.
- van den Bekerom P, Hilverdink E, Sierevelt I, Reuling E, Schnater J, Bonke H, et al. A comparison of hemiarthroplasty with total hip replacement for displaced intracapsular fracture of the femoral neck. J Bone Joint Surg Br 2010;92:1422-8.
- Bonke H, Schnater J, Kleijnen J, Raaymakersk E. Hemiarthroplasty or total hip replacement for femoral neck fractures. A preliminary report of a randomized trial. Hefte Unfallchirurg 1999;272:176-7.
- Garden R. Low-Angele Fixation in fractures of the femoral neck. J Bone Joint Surg Am 1961;43(B):647-63.
- Hodkinson H. Evaluation of a mental test score for assessment of mental impairment in the elderly. Age Ageing 1972;1:233-8.
- Baker R, Squires B, Gargan M, Bannister G. A randomised controlled comparison of total hip arthroplasty and hemiarthroplasty in mobile independent patients with displaced intracapsular femoral neck fracture. J Bone Joint Surg Br 2008;90.
- Fergusson D, Aaron S, Guyatt G, Hebert P. Post-randomisation exclusions: the intention to treat principle and excluding patients from analysis. BMJ 2002;25:652-4.
- Iorio R, Healy WL, Lemos DW, Appleby D, Lucchesi CA, Saleh KJ. Displaced femoral neck fractures in the elderly – outcomes and cost effectiveness. Clin Orthop Relat Res 2001;383:229-42.
- Aleem IS, Karanicolas PJ, Bhandari M. Arthroplasty versus internal fixation of femoral neck fractures: a clinical decision analysis. Ortopedia Traumatologia Rehabilitacja 2009;11:233-41.
- Slover J, Hoffman MV, Malchau H, Tosteson ANA, Koval KJ. A Cost-effectiveness analysis of the arthroplasty options for displaced femoral neck fractures in the active, healthy, elderly population. J Arthroplasty 2009;24:854-60.
- National Institute for Health and Clinical Excellence . Guide to the Methods of Technology Appraisals. 2008. www.nice.org.uk/media/B52/A7/TAMethodsGuideUpdatedJune2008.pdf (accessed 3 August 2011).
- Drummond M, Sculpher M, Torrance G, O’Brien B, Stoddart G. Critical assessment of economic evaluation. Oxford: Oxford University Press; 2005.
- Curtis L. Unit costs of health and social care. Personal Social Services Research Unit; 2009.
- Haentjens P, Autier P, Barette M, Boonen S. Belgian Hip Fracture Study Group. Costs of care after hospital discharge among women with femoral neck fracture. Clin Orthop Relat Res 2003;414:250-8.
- Claxton K, Posnett J. An economic approach to clinical trial design and research priority-setting. Health Econ 1996;5:513-24.
- Liao L, Zhao J, Su W, Sha K, Ding X. Meta analysis of total hip arthroplasty versus hemiarthroplasty for displaced femoral neck fractures in elderly patients. Journal of Clinical Rehabilitative Tissue Engineering Research 2010;14:3991-5.
- Goh SK, Samuel M, Su DH, Chan ES, Yeo SJ. Meta-analysis comparing total hip arthroplasty with hemiarthroplasty in the treatment of displaced neck of femur fracture (provisional abstract). J Arthroplasty 2009;24:400-6.
- Schmidt AH, Leighton R, Parvizi J, Sems A, Berry DJ. Optimal arthroplasty for femoral neck fractures: Is total hip arthroplasty the answer?. J Orthop Trauma 2009;23:428-33.
- Sauerland S, Seiler CM. Role of systematic reviews and meta-analysis in evidence-based medicine. World J Surg 2005;29:582-7.
- Bhandari M, Tornetta P, Ellis T, Audige L, Sprague S, Kuo JC, et al. Hierarchy of evidence: differences in results between non-randomized studies and randomized trials in patients with femoral neck fractures. Arch Orthop Trauma Surg 2004;124:10-6.
Appendix 1 Literature search strategies
Example search strategy:
Database: Ovid MEDLINE(R) from 1950 to September Week 3 2010
Search strategy:
-
((large adj femoral adj head adj3 replac$) or (total hip adj3 replac$) or (total hip arthroplasty)).mp.
-
((hemi adj5 arthroplasty) or hemiarthroplasty or hemi?arthroplasty).mp.
-
1 and 2
Appendix 2 Data extraction tables
Characteristics of included studies
| Reference Manager ID | Study reference Author, date, country |
Study design | Inclusion criteria (inclusion criteria for diagnosis) |
Exclusion criteria (including number excluded) |
Intervention group (THA) population characteristics n 1. Age, gender (f/m) 2. Comorbidities 3. Time from fracture to surgery |
Comparison group (HA) and population characteristics n 1. Age, gender (f/m) 2. Comorbidities 3. Time from fracture to surgery |
|---|---|---|---|---|---|---|
Study outcomes
| Reference Manager ID | Study reference Author, date |
Study duration/follow-up | Primary outcomes (THR vs HA) 1. Dislocation rate 2. Revision rate 3. Non-revision operations |
Secondary outcomes (THA vs HA) Mobility, e.g. walking distance |
Hip ratings (e.g. OHS) | Mortality | Quality of life Other outcomes (e.g. pain) |
Resource utilisation Cost–utility |
Complications Descriptions and frequency |
|---|---|---|---|---|---|---|---|---|---|
Appendix 3 Critical appraisal quality assessment criteria for a surgical randomised controlled trial (based on Parker et al.21)
Yes/No/Unclear
(1) Was there clear concealment of allocation? If allocation clearly concealed (e.g. numbered sealed opaque envelopes drawn consecutively). No or unclear if the method of allocation concealment or randomisation was not stated, unclear or if allocation concealment was clearly not concealed such as those using quasi-randomisation (e.g. even or odd date of birth).
(2) Were the inclusion and exclusion criteria clearly defined?
(3) Were the outcomes of participants who withdrew or excluded after allocation described and included in an intention-to-treat analysis? Yes (including if text states that no withdrawals occurred or data are presented clearly showing ‘participant flow’ which allows this to be inferred. Otherwise no.
(4) Were the treatment and control groups adequately described at entry and if so were the groups well matched, or an appropriate covariate adjustment made?
(5) Were the surgeons experienced at both operations prior to commencement of the trial?
(6) Were the care programmes other than the trial options identical?
(7) Were all the outcome measures clearly defined in the text with a definition of any ambiguous terms encountered?
(8) Were the outcome assessors blind to assignment status?
(9) Was the timing of outcome measures appropriate? A minimum of 12 months’ follow-up for all surviving participants with active review of participants at set time periods.
(10) Was loss to follow-up reported and if so were less than five per cent of surviving participants lost to follow-up?
Appendix 4 Quality assessment of the economic evaluation undertaken by Slover et al.46 through use of the Drummond et al.48 checklist
| 1. Was a well-defined question posed in answerable form? | |
| 1.1. Did the study examine both costs and effects of the service(s) or program(s)? | Yes |
| 1.2. Did the study involve a comparison of alternatives? | Yes |
| 1.3. Was a viewpoint for the analysis stated and was the study placed in any particular decision-making context? | The viewpoint although not stated, could be determined as the hospitalisation costs were borne per patient within the USA, with benefits incorporating the longevity and quality of life of the patient. The decision-making context was for a patient aged 65–75 years in whom an arthroplasty (either THA or HA) would be performed |
| 2. Was a comprehensive description of the competing alternatives given (i.e. can you tell who did what to whom, where and how often)? | |
| 2.1. Were there any important alternatives omitted? | No |
| 2.2. Was (should) a do-nothing alternative be considered? | No, displaced femoral neck fractures would always be treated |
| 3. Was the effectiveness of the program or services established? | |
| 3.1a. Was this done through an RCT? | No |
| 3.1b. If so, did the trial protocol reflect what would have happened in regular practice? | Not applicable |
| 3.2. Was the effectiveness established through an overview of clinical studies? | No |
| 3.3. Were observational data or assumptions used to establish effectiveness? If so, what are the potential biases in results? | It was reported that data are lacking. In the base case the survival rates for THA and HA were assumed to be equal. Revision rates were assumed to be identical for THA and HA as was the risk of mortality following a revision. If these assumptions are incorrect then the conclusions presented could change |
| 4. Were all the important and relevant costs and consequences measured accurately in appropriate physical units (e.g. hours of nursing time, number of physician visits, lost work-days, life-years gained)? | |
| 4.1. Was the range wide enough for the research question at hand? | Yes, long-term costs and the utility of the patient were considered |
| 4.2. Did it cover all relevant viewpoints? (Possible viewpoints include the community or social viewpoint, and those of patients and third-party payers. Other viewpoints may also be relevant depending upon the particular analysis) | Yes, the key outcomes were the costs borne by the hospitals and the longer time utility and survival of the patient |
| 4.3. Were the capital costs, as well as operating costs, included? | Unclear. The costing data were taken from the 2003 National Inpatient Survey.a It is not stated whether or not these include capital costs |
| 5. Were costs and consequences measured accurately in appropriate physical units (e.g. hours of nursing time, number of physician visits, lost work-days, life-years gained)? | |
| 5.1. Were any of the identified items omitted from measurement? If so, does this mean that they carried no weight in the subsequent analysis? | The costing data were taken from the 2003 National Inpatient Survey.a It is expected that these would include all relevant costs |
| 5.2. Were there any special circumstances (e.g. joint use of resources) that made measurement difficult? Were these circumstances handled appropriately? | No |
| 6. Were costs and consequences valued credibly? | |
| 6.1. Were the sources of all values clearly identified? (Possible sources include market values, patient or client preferences and views, policy-makers’ views and health professionals’ judgments) | Partly. The costing data were taken from the 2003 National Inpatient Survey.a Longer-term utility data were taken from an RCT. The utility decrement assumed because the procedure was not referenced |
| 6.2. Were market values employed for changes involving resources gained or depleted? | Yes |
| 6.3. Where market values were absent (e.g. volunteer labour), or market values did not reflect actual values (such as clinic space donated at a reduced rate), were adjustments made to approximate market values? | Not applicable |
| 6.4. Was the valuation of consequences appropriate for the question posed (i.e. has the appropriate type or types of analysis – cost-effectiveness, cost–benefit, cost–utility – been selected)? | Yes. A cost–utility approach was deemed reasonable |
| 7. Were costs and consequences adjusted for differential timing? | |
| 7.1. Were costs and consequences that occur in the future ‘discounted’ to their present values? | Yes. Both future costs and future benefits were discounted at 3% per annum |
| 7.2. Was there any justification given for the discount rate used? | Yes. This was in accordance with current US practice |
| 8. Was an incremental analysis of costs and consequences of alternatives performed? | |
| 8.1. Were the additional (incremental) costs generated by one alternative over another compared to the additional effects, benefits, or utilities generated? | Yes |
| 9. Was allowance made for uncertainty in the estimates of costs and consequences? | |
| 9.1 If data on costs and consequences were stochastic (randomly determined sequence of observations), were appropriate statistical analyses performed? | Stochastic analyses were not performed. Threshold analyses were performed to indicate when the conclusion in terms of cost-effectiveness would alter |
| 9.2. If a sensitivity analysis was employed, was justification provided for the range of values (or for key study parameters)? | Threshold analyses were performed. No justification was provided for the ranges analysed, but these appeared appropriate. The threshold analyses varied the utility following THA or HA, the costs of THA and HA and the RR of revision between THA and HA |
| 9.3. Were the study results sensitive to changes in the values (within the assumed range for sensitivity analysis, or within the CI around the ratio of costs to consequences)? | Yes, the results were sensitive to the change in values. Although, in the majority of cases THA was estimated to be more cost-effective than HA assuming a cost per QALY gained threshold of US$50,000 |
| 10. Did the presentation and discussion of study results include all issues of concern to users? | |
| 10.1. Were the conclusions of the analysis based on some overall index or ratio of costs to consequences (e.g. cost-effectiveness ratio)? If so, was the index interpreted intelligently or in a mechanistic fashion? | Yes. A cost per QALY gained ratio was presented. However, from the manuscript it was not possible to indicate why the incremental cost of the THA strategy was not equal to the incremental cost of the THA procedures given that all transition probabilities and future costs were assumed equal. Also, the interpretation of the threshold analyses appears incorrect in the figure legend (for example Figure 3a) |
| 10.2. Were the results compared with those of others who have investigated the same question? If so, were allowances made for potential differences in study methodology? | No |
| 10.3. Did the study discuss the generalizability of the results to other settings and patient/client groups? | No |
| 10.4. Did the study allude to, or take account of, other important factors in the choice or decision under consideration (e.g. distribution of costs and consequences, or relevant ethical issues)? | No |
| 10.5. Did the study allude to, or take account of, other important factors in the choice or decision under consideration (e.g. distribution of costs and consequences, or relevant ethical issues)? | No |
Appendix 5 Hemiarthroplasty and total-hip arthroplasty for treating primary intracapsular fracture of the hip protocol
26 August 2010
1. Title of the project
What is the clinical and cost effectiveness of total hip-arthroplasty compared to hemi hip-arthroplasty?
2. Project lead
The University of Sheffield, School of Health and Related Research (ScHARR)
Dr Christopher Carroll, Research Fellow
ScHARR, University of Sheffield, Regent Court, 30 Regent Street, Sheffield, S1 4DA
Tel: 0114 22 20864
Email: c.carroll@sheffield.ac.uk
3. Plain english summary
Hip fracture is a common problem in the population aged 60 years or more. The annual rate of hip fracture in women in the UK has been reported to be exponentially distributed and to be 20 per 10,000, 38 per 10,000 and 73 per 10,000 at 65, 70 and 75 years of age respectively. 1 Only 5% of fractures occur in men and women under the age of 60 years. 2 Due to increasingly aging populations the absolute number of hip fractures is expected to rise. 3,4,5 Half of all hip fractures are displaced intracapsular fractures, i.e. unstable fractures in which the blood supply to the femoral head may be impaired, affecting the rate of fracture healing. 2,6,7
The treatment for displaced intracapsular fractures is currently determined by the mobility and functional demands of the patient. Individuals with a displaced intracapsular fracture and low pre-fracture mobility, cognitive impairment or low functional demands are generally treated with hemiarthroplasty (HA);2,8,9 as many as 37% of individuals with hip fracture may be cognitively impaired. 10 By contrast, there is no consensus regarding the optimal treatment for individuals who are cognitively intact and with high pre-facture mobility or function: the options are HA or total hip arthroplasty (THA). 8,9,11
The principal outcomes associated with hip arthroplasty are dislocation, revision rates and resultant quality of life. THA is particularly associated with higher rates of dislocation, which may be due to the greater degree of mobility permitted. 4 It has also been reported that rates of dislocation are more likely if the surgical approach is posterolateral rather than anterolateral and if a smaller femoral head is used. 12,13,14 The incidence or recurrence of dislocation has been found to be significantly related to a reduction in an individual’s quality of life. 15 HA is particularly associated with pain, infection, loosening of the joint and acetabular erosion. 6,16 Post-operative complications such as loosening and acetabular erosion, in particular, can necessitate revision surgery. Revision rates may therefore be higher for HA than for THA. In the only head-to-head trial of THA versus HA the quality of life was shown to be significantly higher at 24 months in patients following a THA. 17
4. Decision problem
4.1 Purpose of the decision to be made
The assessment will address the question: What is the clinical and cost effectiveness of total hip-arthroplasty compared to hemi hip-arthroplasty?
4.2 Clear definition of the intervention
Total hip arthroplasty (THA). THA involves replacing both the femoral head and acetabular articular surface. These prostheses may or may not be cemented into place. 2
4.3 Place of the intervention in the treatment pathway(s)
This review will focus on the use of interventions in the treatment of intracapsular hip fractures.
4.4 Relevant comparators
Hemiarthroplasty (HA). HA involves replacing the femoral head and may be unipolar (generally used for patients with lower functional demands2), or, more recently, the more mobile bipolar, which aims to reduce acetabular erosion. 6 These prostheses may or may not be cemented into place. 2
4.5 Population and relevant sub-groups
Patients eligible for hip replacement due to intracapsular fracture, who are able to give consent and are independently mobile prior to fracture.
4.6 Key factors to be addressed
-
Evaluate the clinical and cost-effectiveness of THA versus HA.
-
Evaluate the safety of THA versus HA.
-
Identify any key areas for further research.
5. Report methods for synthesis of evidence of clinical effectiveness
A review of the evidence for clinical effectiveness will be undertaken systematically following the general principles recommended in the PRISMA statement. 18 English and non-English language studies will be included and there will be no limit by date.
5.1 Population
Adult patients eligible for hip replacement due to intracapsular fracture, who are able to give consent and are independently mobile prior to fracture.
5.2 Intervention
THA.
5.3 Comparator
HA.
5.4 Settings
Secondary care.
5.5 Outcomes
5.5.1 Primary outcome
-
Dislocation rate.
-
Revision rate. Where possible, the data will be analysed separately, for early revision, i.e. within 1 year of surgery, or revision for the duration of follow-up as a whole.
-
Non-revision reoperations (re-operations that do not involve revision or removal of implant). Where these data are reported separately from revisions.
5.5.2 Secondary outcomes
-
Hip ratings (e.g. Oxford Hip Score).
-
Mobility.
-
Mortality.
-
Surgery duration (in minutes).
-
Hypotension during surgery.
-
Operative blood loss (in millilitres).
-
Post-operative blood transfusion (in units).
-
Post-operative complications, e.g. loosening, erosion, wound infection, pneumonia, DVT.
-
Length of hospital stay.
-
Health-related Quality of life.
-
Resource utilisation.
-
Cost utility.
5.6 Follow-up
There is to be no minimum duration of follow-up.
5.7 Study design
Randomised Controlled Trials (RCTs) only, as a scoping report for this project identified seven such trials (09/108/01).
5.8 Search strategy
The search strategy will comprise the following main elements:
-
Searching of electronic databases
-
Contact with experts in the field
-
Scrutiny of bibliographies of retrieved papers.
5.8.1 Electronic searches
A comprehensive search will be undertaken to identify systematically both clinical and cost-effectiveness literature comparing THA and HA in patients with fractures of the femoral neck. The search will involve the combining of terms for THA with terms for HA. An example MEDLINE search strategy is reported in Appendix 10.1. The aim of the strategy is to identify all studies that report on trials or studies comparing THA with HA. All searches will be done by an Information Specialist (PE). These searches will update the searches performed for the scoping report (09/108/01).
5.8.2 Databases
The following electronic databases will be searched from inception for published and unpublished research evidence:
-
MEDLINE (Ovid) 1950–
-
EMBASE 1980–
-
CINAHL (EBSCO) 1982–
-
The Cochrane Library including the Cochrane Systematic Reviews Database, Cochrane Controlled Trials Register, DARE, HTA and NHS EED databases 1991–
-
Biological Abstracts (via ISI Web of Science) 1969–
-
Science Citation Index (via ISI Web of Science) 1900–
-
Social Science Citation Index (via ISI Web of Science) 1956–
-
Conference Proceedings Citation Index- Science (CPCI-S)– (via ISI Web of Science) 1990–
-
UK Clinical Trials Research Network (UKCRN) and the National Research Register archive (NRR)
-
Current Controlled Trials
-
Clinical Trials.gov up
All citations will be imported into Reference Manager software and duplicates deleted.
5.9 Inclusion criteria
The inclusion criteria are as reported in 5.1-5.7 above. Titles and abstracts of all unique citations will be screened independently by two reviewers using the inclusion criteria outlined below. Disagreement will be resolved by consensus, or with reference to a third reviewer when necessary. The full papers of all potentially relevant citations will be retrieved so that an in-depth assessment concerning inclusion could be made. Reference-tracking of all included studies and relevant reviews will also be performed to identify additional, relevant studies not retrieved by the search of electronic databases.
5.10 Exclusion criteria
Reviews of primary studies, and non-RCT evidence which otherwise satisfies the criteria, will not be included in the analysis, but will be retained for discussion and identification of additional trials.
5.11 Data extraction strategy
Data will be extracted independently from all studies by two reviewers using a standardised data extraction form (see Appendix 10.2). Discrepancies will be resolved by discussion, and with reference to a third reviewer if necessary. Authors will also be contacted for relevant missing data, as far as time allows.
5.12 Quality assessment strategy
The quality assessment of included RCTs will be undertaken using an appropriate quality assessment criteria. This is no published surgical RCT checklist, so this review will apply surgical quality assessment criteria outlined in a relevant Cochrane review. 21 These are included in Appendix 10.3. Critical appraisal will be performed by two reviewers independently. Discrepancies will be resolved by discussion, with involvement of a third reviewer when necessary.
5.13 Methods of analysis/synthesis
Data will be tabulated and relative risks (RRs) using both fixed and random effects models will be calculated where possible (if they were not published). 19 Included studies will be combined in a meta-analysis if clinical advice suggests that the included trials are sufficiently, clinically homogenous. Two previous reviews comparing THA with HA have clearly applied different criteria when choosing to combine or not combine included trials, but the rationale behind the approaches taken was not reported in either review. 20,21 Clinical advice will therefore dictate whether trials of unipolar and bipolar, or cemented and uncemented HA can be justifiably combined, and whether trials employing different surgical approaches (anterior or posterior) may be meaningfully combined. Statistical heterogeneity between trials will also be tested using the I2 statistic. 19 If clinical advice dictates that the combining of all studies is not appropriate, then sub-group and sensitivity analyses will be performed based on type of prostheses or surgical approach.
6. Report methods for synthesising evidence of cost-effectiveness
A systematic review of the existing literature studying the cost-effectiveness of THA compared to HA will be undertaken. In addition, a new economic model will be developed to compare a treatment strategy which incorporates THA with a strategy that uses HA (i.e. which is the approach most frequently used in current practice).
6.1 Identifying and systematically reviewing published cost effectiveness studies
The search strategy and sources detailed in Section 5 will be used to identify studies of cost effectiveness. The approach described is very sensitive as no study design filters are being used and will retrieve any relevant cost-effectiveness studies. Identified economic literature will be critically appraised and assessed using the Drummond checklist. 22 Existing cost effectiveness analyses will also be used to identify sources of evidence to inform structural modelling assumptions and parameter values for the economic model.
6.2 Development of a health economic model
A de novo economic evaluation will be constructed, with the primary outcome from the model being an estimate of the incremental cost per additional quality adjusted life year (QALY) gained associated with use of echocardiography for newly diagnosed AF patient. The time horizon of our analysis will be a patient’s lifetime in order to reflect the chronic nature of the disease and potential mortality. The perspective will be that of the National Health Services and Personal Social Services. Both costs and QALYs will be discounted at 3.5%. 23
The model structure will be determined in consultation with clinical experts. It will incorporate the costs of each intervention and that of subsequent events that are dependent on the rates of adverse events associated with intervention. The health impacts of these events will also be simulated, which will allow an analysis of whether THA is more cost effective than HA for selected patient groups. Modelling assumptions will be taken from the literature, supplemented by clinical expert opinion where necessary.
Ideally, health related quality of life estimates will be available from the reviewed literature. In the absence of such evidence, the economic model may use indirect evidence on quality of life from alternative sources. Quality of life data will be reviewed and used to generate the quality adjustment weights required for the model. National sources (e.g. NHS reference costs,24 national unit costs25) as well as the reviewed literature will be used to estimate resource use and costs for use in the economic model.
It is anticipated that there may be limited evidence for some of parameters that will be included in the economic model. Therefore the uncertainty around the parameter estimates will be modelled to take account of this. The uncertainty in the central value for each required parameter will be represented by a distribution, enabling probabilistic sensitivity analysis to be undertaken on the model results. This will allow an assessment of the uncertainty to be made, and the results will be interpreted accordingly. Through expected value of perfect information analysis26 and, if resources allow, expected value of partial perfect information analyses27 we will identify whether further research is valuable, and in which areas further research is likely to be particularly valuable.
7. Expertise in this TAR team
TAR Centre
The ScHARR Technology Assessment Group (ScHARR-TAG) undertakes reviews of the effectiveness and cost effectiveness of healthcare interventions for the NHS R&D Health Technology Assessment Programme on behalf of a range of policy makers in a short timescale, including the National Institute for Health and Clinical Excellence. A list of our publications can be found at: http://www.sheffield.ac.uk/scharr/sections/heds/collaborations/scharr-tag/reports.
Much of this work, together with our reviews for the international Cochrane Collaboration, underpins excellence in healthcare worldwide.
Team members’ contributions
Christopher Carroll, Research Fellow, ScHARR: has extensive experience in systematic reviews of health technologies. CC will lead the project and undertake the review of effectiveness. He will co-ordinate the review process, protocol development, abstract assessment for eligibility, quality assessment of trials, data extraction, data entry, data analysis and review development of background information and clinical effectiveness.
Matt Stevenson, Reader in Health Technology Assessment, ScHARR: has extensive experience in performing health technology assessments and has been the lead, or co-author on over ten full Health Technology reports. MS is also a NICE committee member and is the technical director of ScHARR-TAG. MS will construct, operate and interpret the results from the mathematical model.
Alison Scope, Research Associate, ScHARR: has experience in systematic reviews of health technologies. AS will assist CC with the abstract assessment for eligibility, quality assessment of trials, data extraction, data entry and data analysis for the clinical effectiveness review.
Philippa Evans, Systematic Reviews Information Officer, ScHARR: has experience of undertaking literature searches for the ScHARR Technology Assessment Group systematic reviews and other external projects. PE will be involved in developing the search strategy and undertake the electronic literature searches.
Gill Rooney, Project Administrator: will assist in the retrieval of papers and in preparing and formatting the report.
Clinical and expert advisors
Simon Buckley, Consultant, Northern General Hospital, Sheffield: Simon is a consultant surgeon in primary and revision hip and knee arthroplasty. He gained the FRCS (Tr & Orth) qualification in 2000, was previously the Cavendish Hip Fellow in Sheffield, and President of the British Orthopaedic Trainees Association (2001-2002). He is the audit lead for the Orthopaedic department at Sheffield Teaching Hospitals.
8. Competing interests of authors
The authors do not have any competing interests.
The clinical advisor does not have any competing interests
9. Timetable/milestones
| Milestone | |
|---|---|
| Draft protocol | 31 August 2010 |
| Final protocol | 15 September 2010 |
| Start review | 16 September 2009 |
| Progress report | 16 November, October 2010 |
| Assessment report | 31 December 2010 |
10. Appendices
10.1 Draft Medline search strategy
Database: Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) < 1950 to Present >
Search Strategy:
-
((femoral adj head) or total hip replacement).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] (5092)
-
hemiarthroplasty.mp. (1129)
-
(hemi adj5 arthroplasty).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] (103)
-
hemi hip arthroplasty.mp. (1)
-
2 or 3 or 4 (1217)
-
1 and 5 (90)
10.2 Data extraction forms
| Ref Man ID | Study ref Author, date, country |
Study design | Inclusion criteria (incl. criteria for diagnosis) | Exclusion criteria (incl. number excluded) | Intervention group (THA) population characteristics N= 1.Age, gender (f/m) 2.Co-morbidities 3.Time from fracture to surgery |
Comparison group (HA) and population characteristics N= 1.Age, gender (f/m) 2.Co-morbidities 3.Time from fracture to surgery |
|---|---|---|---|---|---|---|
| Ref Man ID | Study ref Author, date |
Study duration/follow-up | Primary outcomes (THA vs HA) 1. Dislocation rate 2. Revision rate |
Secondary outcomes (THA vs HA) Mobility, eg. walking distance |
Hip ratings (eg. Oxford hip score i.e. OHS) | Mortality | Quality of life Other outcomes (eg. pain) |
Resource utilisation Cost utility |
Complications Descriptions and frequency |
|---|---|---|---|---|---|---|---|---|---|
10.3 Critical appraisal quality assessment criteria for a surgical RCT (from Parker et al. 200621)
Though the scores of the individual items may be summed, the principal aim is to gain an overall impression of quality, rather than for quantitative purposes.
-
Was there clear concealment of allocation?Score 3 (and code A) if allocation clearly concealed (e.g. numbered sealed opaque envelopes drawn consecutively). Score 2 (and code B) if there was a possible chance of disclosure before allocation. Score 1 (and code B) if themethod of allocation concealment or randomisation was not stated or was unclear. Score 0 (and code C) if allocation concealment was clearly not concealed such as those using quasirandomisation (e.g. even or odd date of birth).
-
Were the inclusion and exclusion criteria clearly defined? Score 1 if text states type of fracture and which patients were included and excluded. Otherwise score 0.
-
Were the outcomes of participants who withdrew or excluded after allocation described and included in an intention-to-treat analysis? Score 1 if yes or text states that nowithdrawals occurred or data are presented clearly showing ’participant flow’ which allows this to be inferred. Otherwise score 0.
-
Were the treatment and control groups adequately described at entry and if so were the groups well matched, or an appropriate co-variate adjustment made? Score 1 if at least four admission details given (e.g. age, sex, mobility, function score, mental test score) with either no important difference between groups or an appropriate adjustment made. Otherwise score 0.
-
Were the surgeons experienced at both operations prior to commencement of the trial? Score 1 if text states there was an introductory period or all surgeons were experienced in both operations. Otherwise score 0.
-
Were the care programmes other than the trial options identical? Score 1 if text states they were or this can be inferred. Otherwise score 0.
-
Were all the outcome measures clearly defined in the text with a definition of any ambiguous terms encountered?Score 1 if yes. Otherwise score 0.
-
Were the outcome assessors blind to assignment status? Score 1 if assessors of anatomical restoration, pain and function at follow up were blinded to treatment outcome. Otherwise score 0.
-
Was the timing of outcome measures appropriate?A minimum of 12 months follow up for all surviving participants with active review of participants at set time periods. Score 1 if yes.Otherwise score 0.
-
Was loss to follow up reported and if so were less than five per cent of surviving participants lost to follow up? Score 1 if yes. Otherwise score 0.
References
- Stevenson MD, Lloyd Jones M. Vitamin K to prevent fractures in older women: a systematic review and economic evaluation. Health Technology Assessment 2009;13:1-134.
- Keating J, Grant A, Masson M, Scott NW, Forbes JF. Displaced intracapsular hip fractures in fit, older people: a randomised comparison of reduction and fixation, bipolar hemiarthroplasty and total hip arthroplasty. Health Technology Assessment (Winchester, England) 2005;9.
- Cummings S, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet 2002;359:1761-7.
- Macaulay W, Pagnotto MR, Iorio R, Mont MA, Saleh KJ. Displaced femoral neck fractures in the elderly: Hemiarthroplasty versus total hip arthroplasty. Journal of the American Academy of Orthopaedic Surgeons 2006;14:287-93.
- Riggs B, Melton LJ. The worldwide problem of osteoporosis: insights afforded by epidemiology. Bone 1995;17.
- Parker MJ, Gurusamy K. Modern methods of treating hip fractures. Disability and Rehabilitation 2005;27:1045-51.
- Singer B, McLauchlan G, Robinson C, Christie J. Epidemiology of fractures in 15000 adults: the influence of age and gender. Journal of Bone &Amp; Joint Surgery - British Volume 1998;80:243-8.
- Bhandari M, Devereaux PJ, Tornetta P, . Operative management of dsiplaced femoral neck fractures in elderly patients: an international survey. Journal of Bone &Amp; Joint Surgery - American Volume 2005;87:122-30.
- Parker MJ, Pryor GA. Internal fixation or arthroplasty for displaced cervical hip fractures in the elderly: a randomised controlled trial of 208 patients. Acta Orthopaedica Scandinavica 2000;71:440-6.
- Heetveld MJ, Rogmark C, Frihagen F, Keating J. Internal Fixation Versus Arthroplasty for Displaced Femoral Neck Fractures: What is the Evidence?. Journal of Orthopaedic Trauma 2009;23:395-402.
- Crossman PTK. A survey of the treatment of displaced intracapsular femoral neck fractures in the UK. Injury 2002;33:383-6.
- Enocson A, Hedbeck CJ, Tidermark J, Pettersson H, Ponzer S, Lapidus LJ. Dislocation of total hip replacement in patients with fractures of the femoral neck. Acta Orthopaedica 2009;80:184-9.
- Leighton RK, Schmidt AH, Collier P, Trask K. Advances in the treatment of intracapsular hip fractures in the elderly. Injury-International Journal of the Care of the Injured 2007;38:24-3.
- Berry D, Von Knoch M, Schleck CD, Harmsen WS. Effect of femoral head diameter and operative approach on risk of dislocation after primary total hp arthroplasty. Journal of Bone &Amp; Joint Surgery - American Volume 2005;87:2456-63.
- Enocson A, Pettersson H, Ponzer S, Tornkvist H, Dalen N, Tidermark J. Quality of life after dislocation of hip arthroplasty: a prospective cohort study on 319 patients with femoral neck fractures with a one-year follow-up. Quality of Life Research 2009;18:1177-84.
- Baker RP, Squires B, Gargan MF, Bannister G. C. Total hip arthroplasty and hemiarthroplasty in mobile, independent patients with a displaced intracapsular fracture of the femoral neck. A randomized, controlled trial. The Journal of Bone and Joint surgery.American Volume 2006;88:2583-9.
- Keating JF, Grant A, Masson M, Scott NW, Forbes JF. Randomized comparison of reduction and fixation, bipolar hemiarthroplasty, and total hip arthroplasty. Treatment of displaced intracapsular hip fractures in healthy older patients. The Journal of Bone and Joint Surgery. American Volume 2006;88:249-60.
- Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group 2009 Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Annals of Internal Medicine 2009;151.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2. 2010.
- Goh SK, Samuel M, Su DH, Chan ES, Yeo SJ. Meta-analysis comparing total hip arthroplasty with hemiarthroplasty in the treatment of displaced neck of femur fracture (Provisional abstract). Journal of Arthroplasty 2009;24:400-6.
- Parker Martyn J, Gurusamy Kurinchi Selvan. Parker.Martyn.J., Gurusamy.Kurinchi. Selvan.Arthroplaties.for.proximal.femoral.fractures.in adults.Cochrane Database of Systematic Reviews: Reviews 2006.Issue.3. Chichester, UK: John.Wiley.& Sons., Ltd.; 2006.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. ‘Methods for the Economic Evaluation of Health Care programmes’ 2005.
- Guide to the Methods of Technology Appraisals. National Institute for Clinical Excellence. 2008 n.d. URL: http://www.nice.org.uk/media/B52/A7/TAMethodsGuideUpdatedJune2008.pdf.
- Department of Health NHS Reference Costs 2007-08. 2009 n.d.
- Curtis L. Unit Costs of Health and Social Care. 2008 n.d.
- Eckermann S, Willan A. Expected Value of Information and Decision Making in HTA. Health Econ 2007;16:195-209.
- Felli JC, Hazen GB. Sensitivity analysis and the expected value of perfect information. Medical Decision Making 1998;18:95-109.
List of abbreviations
- CI
- confidence interval
- DVT
- deep vein thrombosis
- EQ-5D
- European Quality of Life-5 Dimensions
- EVPI
- expected value of perfect information
- HA
- hemiarthroplasty
- HHS
- Harris Hip Score
- NICE
- National Institute for Health and Clinical Excellence
- OHS
- Oxford Hip Score
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RD
- risk difference
- RR
- relative risk
- RRR
- ratio of relative risks
- SE
- standard error
- SF-36
- Short Form questionnaire-36 items
- THA
- total hip arthroplasty
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Department of Public Health and Epidemiology, University of Birmingham
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, University of Liverpool
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Peter Brocklehurst, Professor of Women’s Health, Institute for Women’s Health, University College London
-
Professor Jenny Donovan, Professor of Social Medicine, University of Bristol
-
Professor Jonathan Green, Professor and Acting Head of Department, Child and Adolescent Psychiatry, University of Manchester Medical School
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Freddie Hamdy, Professor of Urology, Head of Nuffield Department of Surgery, University of Oxford
-
Professor Allan House, Professor of Liaison Psychiatry, University of Leeds
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor Irwin Nazareth, Professor of Primary Care and Head of Department, Department of Primary Care and Population Sciences, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norrie, Chair in Clinical Trials and Biostatistics, Robertson Centre for Biostatistics, University of Glasgow
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Martin Underwood, Professor of Primary Care Research, Warwick Medical School, University of Warwick
-
Professor Marion Walker, Professor in Stroke Rehabilitation, Associate Director UK Stroke Research Network, University of Nottingham
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Tom Foulks, Medical Research Council
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Diana Baralle, Consultant and Senior Lecturer in Clinical Genetics, University of Southampton
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Public contributor
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Nicola Lennard, Senior Medical Officer, MHRA
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Public contributor
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Public contributor
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Matthew Thompson, Senior Clinical Scientist and GP, Department of Primary Health Care, University of Oxford
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Joanna Jenkinson, Board Secretary, Neurosciences and Mental Health Board (NMHB), Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Public contributor
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire County Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Mrs Jean Thurston, Public contributor
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Public contributor
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Public contributor
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Public contributor
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Public contributor
-
Mr Sankaran Chandra Sekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Public contributor
-
Mr Seumas Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Dr Matthew Wilson, Consultant Anaesthetist, Sheffield Teaching Hospitals NHS Foundation Trust
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan, Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Public contributor
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer, Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Public contributor
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Public contributor
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Public contributor
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Expert Advisory Network
-
Professor Douglas Altman, Professor of Statistics in Medicine, Centre for Statistics in Medicine, University of Oxford
-
Professor John Bond, Professor of Social Gerontology & Health Services Research, University of Newcastle upon Tyne
-
Professor Andrew Bradbury, Professor of Vascular Surgery, Solihull Hospital, Birmingham
-
Mr Shaun Brogan, Chief Executive, Ridgeway Primary Care Group, Aylesbury
-
Mrs Stella Burnside OBE, Chief Executive, Regulation and Improvement Authority, Belfast
-
Ms Tracy Bury, Project Manager, World Confederation of Physical Therapy, London
-
Professor Iain T Cameron, Professor of Obstetrics and Gynaecology and Head of the School of Medicine, University of Southampton
-
Professor Bruce Campbell, Consultant Vascular & General Surgeon, Royal Devon & Exeter Hospital, Wonford
-
Dr Christine Clark, Medical Writer and Consultant Pharmacist, Rossendale
-
Professor Collette Clifford, Professor of Nursing and Head of Research, The Medical School, University of Birmingham
-
Professor Barry Cookson, Director, Laboratory of Hospital Infection, Public Health Laboratory Service, London
-
Dr Carl Counsell, Clinical Senior Lecturer in Neurology, University of Aberdeen
-
Professor Howard Cuckle, Professor of Reproductive Epidemiology, Department of Paediatrics, Obstetrics & Gynaecology, University of Leeds
-
Professor Carol Dezateux, Professor of Paediatric Epidemiology, Institute of Child Health, London
-
Mr John Dunning, Consultant Cardiothoracic Surgeon, Papworth Hospital NHS Trust, Cambridge
-
Mr Jonothan Earnshaw, Consultant Vascular Surgeon, Gloucestershire Royal Hospital, Gloucester
-
Professor Martin Eccles, Professor of Clinical Effectiveness, Centre for Health Services Research, University of Newcastle upon Tyne
-
Professor Pam Enderby, Dean of Faculty of Medicine, Institute of General Practice and Primary Care, University of Sheffield
-
Professor Gene Feder, Professor of Primary Care Research & Development, Centre for Health Sciences, Barts and The London School of Medicine and Dentistry
-
Mr Leonard R Fenwick, Chief Executive, Freeman Hospital, Newcastle upon Tyne
-
Mrs Gillian Fletcher, Antenatal Teacher and Tutor and President, National Childbirth Trust, Henfield
-
Professor Jayne Franklyn, Professor of Medicine, University of Birmingham
-
Mr Tam Fry, Honorary Chairman, Child Growth Foundation, London
-
Professor Fiona Gilbert, Consultant Radiologist and NCRN Member, University of Aberdeen
-
Professor Paul Gregg, Professor of Orthopaedic Surgical Science, South Tees Hospital NHS Trust
-
Bec Hanley, Co-director, TwoCan Associates, West Sussex
-
Dr Maryann L Hardy, Senior Lecturer, University of Bradford
-
Mrs Sharon Hart, Healthcare Management Consultant, Reading
-
Professor Robert E Hawkins, CRC Professor and Director of Medical Oncology, Christie CRC Research Centre, Christie Hospital NHS Trust, Manchester
-
Professor Richard Hobbs, Head of Department of Primary Care & General Practice, University of Birmingham
-
Professor Alan Horwich, Dean and Section Chairman, The Institute of Cancer Research, London
-
Professor Allen Hutchinson, Director of Public Health and Deputy Dean of ScHARR, University of Sheffield
-
Professor Peter Jones, Professor of Psychiatry, University of Cambridge, Cambridge
-
Professor Stan Kaye, Cancer Research UK Professor of Medical Oncology, Royal Marsden Hospital and Institute of Cancer Research, Surrey
-
Dr Duncan Keeley, General Practitioner (Dr Burch & Ptnrs), The Health Centre, Thame
-
Dr Donna Lamping, Research Degrees Programme Director and Reader in Psychology, Health Services Research Unit, London School of Hygiene and Tropical Medicine, London
-
Professor James Lindesay, Professor of Psychiatry for the Elderly, University of Leicester
-
Professor Julian Little, Professor of Human Genome Epidemiology, University of Ottawa
-
Professor Alistaire McGuire, Professor of Health Economics, London School of Economics
-
Professor Neill McIntosh, Edward Clark Professor of Child Life and Health, University of Edinburgh
-
Professor Rajan Madhok, Consultant in Public Health, South Manchester Primary Care Trust
-
Professor Sir Alexander Markham, Director, Molecular Medicine Unit, St James’s University Hospital, Leeds
-
Dr Peter Moore, Freelance Science Writer, Ashtead
-
Dr Andrew Mortimore, Public Health Director, Southampton City Primary Care Trust
-
Dr Sue Moss, Associate Director, Cancer Screening Evaluation Unit, Institute of Cancer Research, Sutton
-
Professor Miranda Mugford, Professor of Health Economics and Group Co-ordinator, University of East Anglia
-
Professor Jim Neilson, Head of School of Reproductive & Developmental Medicine and Professor of Obstetrics and Gynaecology, University of Liverpool
-
Mrs Julietta Patnick, Director, NHS Cancer Screening Programmes, Sheffield
-
Professor Robert Peveler, Professor of Liaison Psychiatry, Royal South Hants Hospital, Southampton
-
Professor Chris Price, Director of Clinical Research, Bayer Diagnostics Europe, Stoke Poges
-
Professor William Rosenberg, Professor of Hepatology and Consultant Physician, University of Southampton
-
Professor Peter Sandercock, Professor of Medical Neurology, Department of Clinical Neurosciences, University of Edinburgh
-
Dr Philip Shackley, Senior Lecturer in Health Economics, Sheffield Vascular Institute, University of Sheffield
-
Dr Eamonn Sheridan, Consultant in Clinical Genetics, St James’s University Hospital, Leeds
-
Dr Margaret Somerville, Director of Public Health Learning, Peninsula Medical School, University of Plymouth
-
Professor Sarah Stewart-Brown, Professor of Public Health, Division of Health in the Community, University of Warwick, Coventry
-
Dr Nick Summerton, GP Appraiser and Codirector, Research Network, Yorkshire Clinical Consultant, Primary Care and Public Health, University of Oxford
-
Professor Ala Szczepura, Professor of Health Service Research, Centre for Health Services Studies, University of Warwick, Coventry
-
Dr Ross Taylor, Senior Lecturer, University of Aberdeen
-
Dr Richard Tiner, Medical Director, Medical Department, Association of the British Pharmaceutical Industry
-
Mrs Joan Webster, Consumer Member, Southern Derbyshire Community Health Council
-
Professor Martin Whittle, Clinical Co-director, National Co-ordinating Centre for Women’s and Children’s Health, Lymington