Notes
Article history
The research reported in this issue of the journal was commissioned by the HTA programme as project number 06/79/01. The contractual start date was in March 2008. The draft report began editorial review in July 2010 and was accepted for publication in February 2011. As the funder, by devising a commissioning brief, the HTA programme specified the research question and study design. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2011. This work was produced by Lewis et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2011 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Research is needed to identify the most clinically effective and cost-effective management strategies for sciatica. Many treatment modalities for sciatica have been evaluated in placebo-controlled trials (or usual care used as the comparator), and the evidence relating to the direct comparison of numerous treatment modalities is missing. Previous systematic reviews have found evidence for the clinical effectiveness of invasive treatments such as epidural steroid injection (ESI), chemonucleolysis and lumbar discectomy, but found insufficient evidence to advise bed rest, keeping active, analgesia, intramuscular steroid injection or traction. None of the reviews made indirect comparisons across separate trials or examined cost-effectiveness. Previous economic evaluations that have been conducted vary quite considerably, and their value is limited to the perspective and setting for which they were undertaken. We undertook a systematic review of the clinical effectiveness and cost-effectiveness of the different management strategies for sciatica, which tries to address some of these issues. We have also developed a decision-analytic model to assess the cost-effectiveness of different treatment modalities from the UK NHS perspective.
Chapter 2 Research objectives
-
To undertake a systematic review of the clinical effectiveness and cost-effectiveness of different management strategies for sciatica.
-
To synthesise the results using meta-analyses and a mixed treatment comparison (MTC) method.
-
To construct an appropriate decision-analytic model to estimate costs per quality-adjusted life-year (QALY) gained for each treatment strategy.
Chapter 3 Background
Definition of sciatica
Sciatica is a symptom defined as unilateral, well-localised leg pain with a sharp, shooting or burning quality that approximates to the dermatomal distribution of the sciatic nerve down the posterior lateral aspect of the leg, and normally radiates to the foot or ankle. It is often associated with numbness or paraesthesia in the same distribution. 1,2 The symptom of sciatica is used by clinicians in different ways. Some refer to any leg pain referred from the back as sciatica, others prefer to restrict its use to pain originating from the lumbar nerve root. Some authors prefer to use the term ‘lumbar nerve root pain’ to distinguish it from referred leg pain. 3
Epidemiology of sciatica
The lack of clarity in the definition of sciatica persists in the epidemiological literature. In the UK, the prevalence of ‘sciatica suggesting a herniated lumbar disc’ has been reported as 3.1% in men and 1.3% in women. 4 However, like most surveys, this study did not use strict criteria to diagnose sciatica. A large population survey in Finland which did found a lifetime prevalence of 5.3% in men and 3.7% in women. 5 Sciatica accounts for < 5% of the cases of lower back pain presenting to primary care. 3 Some cohort studies have found that most cases resolve spontaneously, with 30% of patients experiencing persistent troublesome symptoms at 1 year, 20% out of work and 5–15% requiring surgery. 6,7 However, another cohort found that 55% still had symptoms of sciatica 2 years later, and 53% after 4 years (which included 25% who had recovered after 2 years, but had relapsed again by 4 years). 8 As the sciatica becomes more chronic (> 12 weeks), or with recurrent episodes, it becomes less responsive to treatment. 9 Effective treatment for patients with acute or subacute sciatica is therefore important in order to prevent patients developing a more chronic condition that is resistant to treatment and likely to incur high health-care and socioeconomic costs. The cost of sciatica to society in the Netherlands in 1991 was estimated at US$128M for hospital care, US$730M for absenteeism and US$708M for disablement. 10
Pathological mechanism
Sciatica caused by lumbar nerve root pain usually arises from a prolapsed intervertebral disc, but also from spinal stenosis, or surgical scarring as well as other aetiologies such as trauma and tumours. 6 It was initially thought to occur predominantly as a result of compression of the nerve root,11 leading to neural ischaemia, oedema (which would, in turn, lead to chronic inflammation), scarring and perineural fibrosis. However, it is now known that symptoms can occur in the absence of direct nerve root compression, possibly as a result of release of proinflammatory factors from the damaged disc. Pain occurs because of chronic, repetitive firing of the inflamed nerve root. 12,13 Referred leg pain occurs because pain fibres from paraspinal structures and from the leg converge on interneurons in the spinal cord and brain, so that nociceptive input from painful paraspinal tissues is perceived as leg pain.
Clinical diagnosis
It has been claimed that nerve root pain can be distinguished from referred leg pain because it is unilateral, radiates below the knee, results in leg pain that is worse than the back pain, can be aggravated by coughing or sneezing and has a segmental distribution. Important clinical signs include provocation tests for dural irritation, such as a limited straight leg raise (SLR) reproducing the leg pain, and compromised nerve root function leading to reduced power, sensation or reflexes in one nerve root. 3 A systematic review of the diagnostic value of history and physical examination in nerve root pain found that pain distribution was the only useful item in the history. The SLR test was the only sensitive sign in the physical examination, but had poor specificity; the crossed SLR test was the only specific sign, but had poor sensitivity. 14 However, another review found that there was no standard SLR procedure, no consensus on interpretation of results, no evidence of intra- and inter-observer reliability and its predictive value in lumbar intervertebral disc surgery was unknown. 15
Treatments
A variety of surgical and non-surgical treatments have been used to treat sciatica and have been the subject of previous systematic reviews, the findings of which are summarised below. However, none of the reviews examined the cost-effectiveness of the various treatment modalities.
Bed rest and advice to stay active
Most cases resolve spontaneously and, traditionally, bed rest has been advised. A Cochrane systematic review of bed rest16 found that there was high-quality evidence of little or no difference in pain or functional status between bed rest and staying active; moderate-quality evidence of little or no difference in pain intensity between bed rest and physiotherapy, but small improvements in functional status with physiotherapy; and moderate-quality evidence of little or no difference in pain intensity or functional status between 2–3 and 7 days’ bed rest. A Cochrane systematic review of advice to keep active reviewed the same trials comparing bed rest with activity and came to the same conclusions. Although there is no evidence to advise bed rest for sciatica, there is also very little evidence of any benefit of keeping active. 16
Analgesia
Most patients will obtain analgesic medication either on prescription or purchased ‘over the counter’ from their pharmacist. A systematic review of the conservative treatment for sciatica identified three randomised controlled trials (RCTs) that compared non-steroidal anti-inflammatory drugs (NSAIDs) with a placebo tablet and found no evidence of efficacy. 17
Intramuscular steroids
Part of the mechanism of production of nerve root pain is the release of proinflammatory factors from damaged discs, so administration of intramuscular corticosteroid steroid injections to reduce inflammation of the nerve root has a theoretical basis. The systematic review of conservative treatment for sciatica identified two RCTs comparing steroid injections with a placebo injection and found no evidence of efficacy. 17
Traction
Traction is used relatively frequently to treat sciatica in North America, but less frequently in the UK, Ireland and the Netherlands. 18,19 A Cochrane systematic review found strong evidence that there was no significant difference between either continuous or intermittent traction versus placebo, sham or other treatments. 20
Epidural steroids
Introduction of corticosteroids into the epidural space is a commonly used treatment for lumbar nerve root pain, with the rationale of reducing nerve root inflammation. It was performed on 47,665 occasions in the NHS in England in 2005–6. 21 Systematic reviews of ESIs have reached conflicting conclusions with regard to their efficacy compared with placebo and their effectiveness compared with other treatments. 17,22–24
Spinal manipulation
The systematic review of conservative treatment for sciatica identified two RCTs of spinal manipulation. One found that manipulation was more effective than placebo, and another found no difference compared with manual traction, exercises or corset. 17
Chemonucleolysis
Chemonucleolysis is a technique that is now rarely used. It attempts to decrease the volume of a disc herniation by reducing the amount of material contained within the nucleus pulposus by injecting the enzyme chymopapain. A systematic review of lumbar discectomy and percutaneous treatments identified three RCTs that compared chymopapain with placebo injection, and reported that symptom relief was greater in the group that received chymopapain. 25
Lumbar discectomy
Between 5% and 15% of patients with lumbar nerve root pain are treated with surgery,6,7 usually involving a lumbar discectomy. In 2005–6, 8683 lumbar discectomies were performed in the NHS in England. 21 A Cochrane systematic review of surgery for lumbar disc prolapse26 found 40 RCTs and two quasi-randomised controlled trials (Q-RCTs), but only four RCTs comparing discectomy with conservative management, which suggested a temporary benefit in clinical outcomes at 1 year, but no difference at longer-term follow-up. Meta-analyses showed that surgical discectomy produced better clinical outcomes than chemonucleolysis, which was better than placebo. The review concluded that there was considerable evidence of the clinical effectiveness of discectomy for carefully selected patients with sciatica caused by lumbar disc prolapse that fails to resolve with conservative management. Serious complications from lumbar disc surgery are uncommon, with one study25 reporting a mortality rate of 0.3% an infection rate of 3% and 4% requiring an intraoperative transfusion. Surgery failed to relieve symptoms in 10–20% of the cases. 25
Other treatments
A number of other treatments that have not been included in previous systematic reviews, for example complementary therapies such as acupuncture, will be included in this review.
Pattern of treatments
Overall, there is no close correlation between symptom severity and pathology in sciatica. Increasing distance between onset and effective treatment has an unfavourable influence on symptoms and disability. Although there is reason to suppose that a stepped-care approach to sciatica could be helpful, the application of the various available treatments depends more on availability, clinician preference and socioeconomic variables than on patient needs. In practice, some patients will recover under an analgesic cocktail while on a waiting list, some will be offered surgery as a first-line intervention, and yet others will receive a combination of treatments in no particular order. With few exceptions, it would appear that the patients receiving differing treatments are clinically indistinguishable.
Chapter 4 Evidence synthesis: methods
Methods for reviewing clinical effectiveness and cost-effectiveness
The review was undertaken according to the methodology reported in the Centre for Reviews and Dissemination (CRD) report Undertaking systematic reviews of research on effectiveness: CRD’s guidance for those carrying out or commissioning reviews27 and the Cochrane handbook for systematic reviews of interventions. 28 Studies examining clinical effectiveness and those evaluating cost-effectiveness were reviewed separately. (The review protocol is presented in the appendices.)
Literature search
The following databases were searched for published, semi-published and grey literature. Full details of the search strategies are reported in Appendix 1. Initial searches took place in June 2008 and were then updated in December 2009, with databases searched from inception to the date of the search:
-
MEDLINE
-
MEDLINE In-Process & Other Non-Indexed Citations
-
OLDMEDLINE
-
EMBASE
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL)
-
Allied and Complimentary Medicine Database (AMED)
-
British Nursing Index
-
Health Management Information Consortium (HMIC)
-
PsychINFO
-
Inspec
-
Cochrane Central Register of Controlled Trials (CENTRAL)
-
Database of Abstracts of Reviews of Effects (DARE)
-
Cochrane Database of Systematic Reviews (CDSR)
-
Health Technology Assessment (HTA) Database
-
NHS Economic Evaluation Database (NHS EED)
-
System for Information on Grey Literature In Europe (SIGLE)
-
Science Citation Index
-
Social Science Citation Index (SSCI)
-
Index to Scientific & Technical Proceedings (ISTP)
-
Physiotherapy Evidence Database (PEDro)
-
BIOSIS
-
National Research Register (NRR)
-
National Institute for Health’s ClinicalTrials.gov database
-
CenterWatch Clinical Trials Listing Service
-
Current Controlled Trials (CCT)
-
World Health Organization’s (WHO) International Clinical Trials Registry Platform (ICTRP) this collects weekly data from:
-
– Australian New Zealand Clinical Trials Registry
-
– ClinicalTrials.gov
-
– International Standard Randomised Controlled Trial Number Register (ISRCTN) and monthly data from:
-
– Chinese Clinical Trial Registry
-
– Clinical Trials Registry – India
-
– German Clinical Trials Register
-
– Iranian Registry of Clinical Trials
-
– Japan Primary Registries Network
-
– Sri Lanka Clinical Trials Registry
-
– The Netherlands National Trial Register
-
-
Australian New Zealand Clinical Trials Registry
-
Clinical Trials Search.
The bibliographies of previous systematic reviews and included studies were screened to identify further relevant studies.
Management of references
The results of the searches were entered onto the reference management software E[sc]ndnote[/sc] (Thomson Reuters, CA, USA) and duplicate records removed. Articles written in a language other than English were translated whenever possible. Multiple publications arising from the same study were identified, grouped together and represented by a single reference.
Inclusion and exclusion of studies
Selection criteria
Study design
Studies using any of the following study designs were considered for inclusion: RCTs, Q-RCTs, non-RCTs, cohort studies (with concurrent or historical controls), case–control studies, before and after studies and full economic evaluations as defined by Drummond et al. 29 and The Cochrane handbook. 28
Patient population
Studies involving adults with sciatica or lumbar nerve root pain diagnosed clinically or confirmed by imaging were eligible. The essential clinical criterion was leg pain worse than back pain. Other clinical criteria which support the diagnosis include unilateral leg pain, pain radiation below the knee, pain aggravated by coughs/sneezes, segmental distribution of pain, pain induced by provocation tests (e.g. impaired SLR) and reduced power, sensation or reflexes in one nerve root. Studies that included participants with low back pain were included only if the findings for patients with sciatica were reported separately; studies in which the results were not reported separately for sciatica were excluded. Studies of sciatica caused by specific conditions such as spinal stenosis or discogenic pain were only included if it was documented that leg pain was worse than back pain. If imaging was used it had to demonstrate evidence of nerve root irritation. Studies of sciatica caused by a tumour were excluded.
Interventions
Any intervention or comparator used to treat sciatica was included. Treatments were categorised using the system reported in Table 1. Inactive control represents placebo or sham treatment used within the study setting and could include sham traction or placebo epidural.
| Level 1 | Level 2 | Level 3 | |
|---|---|---|---|
| Invasiveness | Treatment category | Category codea | Type of treatment |
| Inactive control | Inactive control | A |
Placebo Sham treatment No treatment |
| Non-invasive | Usual/conventional care | B |
Usual care Conventional care Non-surgical treatment GP care |
| Invasive – surgical | Disc surgery | C |
Discectomy Microdiscectomy Automated percutaneous discectomy Nucleoplasty Laser discectomy Disc sequestrectomy Laminectomy Surgical decompression |
| Invasive – non-surgical | Epidural/intradiscal injections (includes spinal nerve block) | D |
Caudal epidural Segmental epidural Intradiscal injections Facet joints injections Intraforaminal injections Spinal nerve root block |
| Invasive – non-surgical | Chemonucleolysis | E |
Chymopapain Collagenase Ozone |
| Non-invasive | Non-opioids | F |
Oral, i.v. or intramuscular Steroids COX-2 inhibitors NSAIDs Paracetamol Muscle relaxants Neuropathic pain treatment |
| Invasive – surgical | Intraoperative interventions | G | |
| Non-invasive | Traction | H | Mechanical traction |
| Non-invasive | Manipulation | I |
Manipulation Chiropractic Osteopathic McKenzie |
| Non-invasive | Alternative | J |
Acupuncture Feldenkrais Muscle energy Reiki therapy Energy work Magnets |
| Non-invasive | Active PT/exercise therapy | K |
Flexibility Strengthening Conditioning Stabilisation |
| Non-invasive | Passive PT | L |
Ultrasound/phonophoresis Iontophoresis Heat/ice Massage Therapeutic touch Interferential Electrical stimulation techniques (TENS/PENS) Laser |
| Non-invasive | Biological agents | M | Anti-TNFs (and other antibody related interventions) |
| Non-invasive | Activity restriction | N | Bed rest |
| Non-invasive | Opioids | O | Oral, i.v. or intramuscular opioids |
| Non-invasive | Education/advice | P |
Back school Home exercise instruction Coping skills training Vocational counselling Activities of daily living (ALD) |
| Invasive + non-invasive | Mixed treatments | Q | Combination of different physical therapies and advice, etc. |
| Invasive – non-surgical | Others | R |
Peripheral nerve block Spinal cord stimulation (level 2, code Q) Radiofrequency lesioning (level 2, code S) |
Outcome measures
All relevant patient-based outcome measures such as pain, disability, functional status, adverse effects, health status, quality of life (QoL), analgesic use, operation rates, health utility, return to work, health-service use and costs were considered for inclusion in the review. Biochemical outcomes and biomechanical measurements (e.g. change in disc space) were excluded. Although all relevant outcome measures were extracted, because of the high volume of studies and time constraints, only those covered by the following important patient-centred outcome9 domains were included in the analysis of clinical effectiveness: global effect, pain intensity, condition-specific outcome measures (CSOMs) (Table 2) and adverse event data. This means that the outcomes health status, QoL, analgesic use, operation rates, health utility, return to work, health-service use and costs have not been analysed in the clinical effectiveness section of the review.
| Measure | Interpretation |
|---|---|
| Global effect | |
| MacNab criteria | Excellent, good, fair, poor |
| Global perceived effect (GPE) | Complete recovery to vastly worse |
| Patient perceived overall improvement | Various ordinal or dichotomous scales |
| Physician perceived overall improvement | Various ordinal or dichotomous scales |
| Proportion of patients below a threshold on a specific scale | |
| Proportion of patients free of pain | |
| Sciatica bothersomeness | Higher score indicates greater bothersomeness |
| Pain intensity outcomes | |
| Visual analogue scale (VAS) | Higher score indicates greater pain |
| Bergquist-Ullman and Larson, pain index (B-U&LPI) | Higher score indicates greater pain |
| Numerical rating scale (NRS) | Higher score indicates greater pain |
| Likert scale | Higher score indicates greater pain |
| Low back pain rating scale (LBRS) (pain subscale) | Higher score indicates greater pain |
| McGill Pain Questionnaire (subscales: VAS, present pain inventory) | Higher score indicates greater pain |
| Japanese Orthopaedic Association (JOA) score (pain subscale) | Lower score indicates greater pain |
| Roland–Morris annotated thermometer | Higher score indicates greater pain |
| Von Korff pain intensity | Higher score indicates greater pain |
| Pain diagram | Higher score indicates greater pain |
| CSOMs | |
| Roland–Morris Disability Questionnaire (RMDQ) (including modified versions) | Higher score indicates greater disability |
| Revised RMDQ | Lower score indicates greater disability |
| Oswestry Disability Index (ODI, also referred to as Oswestry Low Back Pain Disability Questionnaire) [including modified versions, e.g. Modified Oswestry Disability Index (MODEMS)] | Higher score indicates greater disability |
| Japanese Orthopaedic Association (JOA) score | Lower score indicates greater disability |
| Low back outcome score (LBOS) | Lower score indicates greater disability |
| Dallas Pain Questionnaire (subscales: daily activities, work and leisure activities, anxiety-depression and sociability) | Higher score indicates greater disability |
| Low back pain rating scale (LBRS) (subscales: pain, activity of daily living and physical function) | Higher score indicates greater disability |
| North American Spine Society (NASS) instrument score (subscales: neurogenic symptoms score and pain and disability score) | Lower score indicates greater disability |
| Symptom scoring system | Higher score indicates greater disability |
| Waddell Disability Index | Higher score indicates greater disability |
| Sciatica index | Higher score indicates greater disability |
| Funktionsfragebogen Hannover (FFbH) | Lower score indicates greater disability |
| Core Outcome Measures Index (COMI) | Higher score indicates greater disability |
| Quebec Back Pain Disability Scale (QDS) | Higher score indicates greater disability |
Assessing relevancy of included studies
Two reviewers independently screened the titles and abstracts identified by the electronic searches for relevance. Potentially relevant studies were ordered and assessed for inclusion, using the criteria reported above, by two independent reviewers. Disagreements during both stages were resolved by discussion or if necessary taken to a third reviewer.
Data extraction
Data were extracted using predefined forms developed on a Microsoft A[sc]ccess[/sc] database (Microsoft Corporation, Redmond, WA, USA). Separate forms were used for clinical effectiveness and cost-effectiveness studies. Data were extracted by one reviewer and checked for accuracy, against the original paper, by a second independent reviewer. Any disagreements were resolved by discussion or by a third reviewer if necessary.
Data extracted for clinical effectiveness studies included study location and setting, description of study population (including method of diagnosis and previous treatment), type of intervention and control used, how allocation was performed, outcome measures used and results (such as final means, change scores and proportions) with sufficient information, such as standard errors (SEs), significance levels and confidence intervals (CIs), in order to estimate missing standard deviations (SDs) wherever possible. When necessary, the results and the measures of dispersion were approximated from figures in the reports. Data for both continuous and binary outcomes were extracted based on the number of patients included in the analysis. Where possible, reported findings based on intention-to-treat (ITT) analysis were used. However, we did not recalculate findings based on the ITT principle, e.g. using worst- or best-case scenario for missing variables, as we believed we would be unlikely to have data on crossovers. For studies in which arm-level data were not available, but the mean difference between arms and associated SE had been reported, these were extracted and used in the synthesis instead. Additionally, if studies reported the mean difference between arms adjusted for baseline values, e.g. using analyses of covariates (ANCOVA), these were also extracted.
Data extraction for cost-effectiveness studies included the following: type of economic evaluation, specific details about the interventions being compared, study population, time period, measures of effectiveness, direct costs (medical and non-medical), productivity costs, resource use, currency, results and details of any decision modelling and sensitivity analysis.
Quality assessment
Quality assessment was undertaken by two independent reviewers with differences being resolved by consensus or by a third reviewer if necessary. Data relating to quality assessment were recorded in an Access database.
For clinical effectiveness studies, the quality of both trials and observational studies was assessed using the same checklist based on the one used by the ‘Back Review Group’ of the Cochrane Collaboration for RCTs30 and the one developed by the Hamilton Effective Public Health Practice Project (EPHPP) team for quantitative studies (which includes both comparative observational studies and RCTs). 31 The checklist is presented in Appendix 2. The criteria cover selection bias and confounding, detection bias, performance bias and attrition bias. Criteria relating to external validity have also been added.
The quality of the economic evaluations was assessed according to an updated version of the checklist developed by Drummond et al. 29 (see Appendix 2). The checklist reflects the criteria for economic evaluation detailed in the methodological guidance developed by the National Institute for Health and Clinical Excellence (NICE). For studies based on decision models, the critical appraisal was based on the checklist developed by Weinstein et al. 32 (see Appendix 2).
Methods of analysis/synthesis
Treatments were categorised according to the system reported in Table 1. Pair-wise (standard) meta-analyses were initially conducted followed by MTC analysis. These were based on the three main outcome domains: global improvement (including absence of pain), reduction in pain intensity (measured using a continuous scale) and improvement in function based on a composite CSOM. Where feasible, the data were analysed according to chronicity of sciatica (acute ≤ 3 months; chronic > 3 months). The global effect was synthesised as binary data, pain intensity and the composite CSOM as continuous data.
Missing study-level outcome data, where feasible, were dealt with by deriving/imputing replacement values. Where mean values were unavailable but the medians were reported, the latter were used instead (i.e. medians were assumed to be equal to means). Where possible, SDs were estimated from SEs, 95% CIs or p-values, using methods reported in The Cochrane handbook,33 and for median values,using the interquartile range (IQR). If SDs for baseline values were available, then these were substituted for missing SDs. Finally, for studies that did not report sufficient data to derive the SDs, these were imputed using the weighted mean,34 which was calculated separately for each intervention category.
Global effect (including the absence of pain)
When this outcome was reported in an ordinal format, this was converted into binary data (e.g. improved, not improved, absence of pain, presence of pain). For studies that used ordinal scales, where little improvement (or similar terms) was a central category or grouped with unchanged, the data for patients in this group were classified as not improved. Where both treatment success and failure were reported, treatment success was used. Where treatment failure was reported on its own, the data were converted to treatment success. Where studies reported both overall improvement (sometimes based on a number of scales) and improvement in pain (categorical data), the data on overall improvement were used. For studies that reported both physician- and patient-perceived global effect, the data for patients’ perceived effect were used, as this is considered to be the most useful; if the study reported only physician’s assessment, then this was used.
Pain intensity (based on a continuous scale)
Most of the studies reporting pain intensity used a visual analogue scale (VAS) to measure pain, with a mixture of both final mean and change scores reported. Studies were pooled using weighted mean difference (WMD). Studies that measured pain intensity on a similar continuous scale were also included, with the data converted to a scale of 0–100. Other types of pain measures were excluded as their inclusion would have necessitated using standardised mean differences (SMDs), where both final and change scores could not be used. Multiple and different locations of the pain were assessed across the studies. We included a pain assessment from only one site from each study using the following preference hierarchy: leg pain (preferred), then overall pain, and then back pain.
Condition-specific outcome measures
The included studies used a number of different scales to measure condition-specific functional status. The Roland–Morris Disability Questionnaire (RMDQ)35 and the Oswestry Disability Index (ODI)36 are the most widely used CSOMs for sciatica studies,37 and an expert panel has recommended the use of either in lower back pain research. 35 The RMDQ was designed, and is more widely used, in primary care settings; the ODI was designed, and is more widely used, in secondary care. Both show some evidence of criterion and construct validity. The RMDQ is the more frequently cited and is more responsive than the ODI, which in turn has better test–retest reliability. 36 The RMDQ has undergone Rasch analysis to examine item separation, which found that all but four of the items contributed to a single underlying construct, but several items in the middle of the disability hierarchy were too similar and there were insufficient items at the upper and lower extremes. 38 The ODI has not undergone Rasch analysis, but like the RMDQ shows evidence of ceiling and floor effects. There are also different versions of the ODI following its adaptation by different groups. 39
To enable synthesis, the data were combined using a SMD. We had initially intended using change scores. In order to impute change from baseline SDs for studies that report only baseline and final means, it is necessary to include an estimate of the correlation between baseline and follow-up values for individuals. This entails estimating the correlation coefficient from (other) studies in the synthesis that reported SDs for baseline, final and change from baseline. 40 However, when doing this we found the average correlation to be ≤ 0.5 for most treatment categories, which means that there is little advantage over using final means. Some studies report findings for more than one CSOM scale, but results from only one scale from each study were used in the analyses, based on the following preference hierarchy: RMDQ,41 ODI,42 Quebec Back Pain Disability Scale (QDS), others.
Standard pair-wise meta-analyses
Data were analysed according to three follow-up periods: short (≤ 6 weeks), medium (6 weeks to 6 months) and long (> 6 months). Where studies reported findings for multiple follow-up intervals within a single follow-up period, the data relating to the duration closest to the upper limit were used.
Results are presented in structured tables and forest plots, grouped according to the treatment category being evaluated (see Table 1). Studies were pooled using the random effects model43 in Stata (StataCorp LP, College Station, TX, USA), with between-study heterogeneity examined using I2 and chi-squared statistics. [There were insufficient studies to use individual treatments (level 3) as separate meta-analyses.]
Although studies comparing different interventions that fell into the same category were included in the review, their findings are not reported here, e.g. studies comparing different types of surgery or different types of epidural injections.
Mixed treatment comparison meta-analyses
Prior to performing the MTC we checked whether or not the included studies formed a closed network using level 2 treatment categorisations (see Table 1) [there were insufficient data to use individual (level 3) treatments as nodes]. Studies evaluating mixed treatments (or combination therapy) were excluded, because of the uncertainty regarding the extent of interaction between the combined interventions. For the MTC, only one time point was considered, with the findings from individual studies closest to 6 months’ follow-up used in the analyses. Analyses were conducted for global effect, pain intensity and CSOMs, for all study designs and after excluding observational studies and non-RCTs.
The analyses were performed by the Multi-parameter Evidence Synthesis Research Group in the Bayesian framework and the modelling computed with Markov chain Monte Carlo stimulation methods using Winbugs (MRC Biostatistics Unit, Cambridge, UK). The codes that were used are presented in the Appendix 3 and are based on those used elsewhere. 44 An inactive control was used as the reference treatment. In all cases, an initial burn-in of at least 50,000 stimulations was discarded and all the results presented are based on a further sample of at least 50,000 stimulations. Convergence was assessed using the Brooks–Gelman–Rubin diagnostic tool in Winbugs and the inspection of the auto-correlation and history plots. The model fit was checked by the global goodness of fit statistic, residual deviance. If the model is an adequate fit, it is expected that the residual deviance would be roughly equal to the number of unconditional data points.
The main parameters of interest in an MTC are the estimates of effects of treatments B, C, D, etc. relative to a baseline ‘treatment’ A (which is considered as a ‘nuisance’ variable). In our review, ‘usual care’ was a treatment category that we were interested in, and we therefore considered inactive control to be the most appropriate ‘baseline’ comparator. We also included treatment categories such as non-opioids, which could similarly be used as a baseline comparator if considering the use of usual care.
Analysis of covariates
Where 10 or more studies were included in the pair-wise meta-analyses described in Chapter 6, it had been our intention to evaluate the effect of study-level covariates (e.g. symptom duration used and study quality criteria such as adequate randomisation procedure, adequate allocation concealment, > 80% followed up and blind outcome assessment) on between-study heterogeneity using metaregression, but only one comparison (disc surgery vs chemonucleolysis for global effect at long-term follow-up) included sufficient studies. The possible effect of covariates such as study design, study quality and duration of symptoms on pooled results has been discussed when summarising the findings.
Publication bias
For all comparisons for which there were more than eight studies, funnel plots together with associated statistical tests were used to assess the potential publication bias.
Economic evaluations
Given the nature and lack of homogeneity between included economic evaluations, we performed a narrative review of the included studies and made overall conclusions. Details of each published economic evaluation, together with a critical appraisal of its quality, are presented in structured tables with a narrative summary. Where appropriate and where the data permitted, indications of the uncertainty underlying the estimation of the differential cost and effects of the alternative treatment options were summarised.
Economic model
The methods and results of the economic model are reported separately in Chapter 9.
Chapter 5 Results of searches
The electronic searches identified 33,560 references and a further 30 references were identified by hand searching. Of these, 777 references were ordered and, after collating multiple publications, 270 studies that met the inclusion criteria were identified. These included 12 economic evaluations performed as part of the clinical effectiveness studies, but reported separately.
A flow diagram showing the number of references identified, retrieved and included in the review is presented in Figure 1.
FIGURE 1.
Flow diagram showing the number of references identified, documents/studies retrieved for assessment and included in the review.
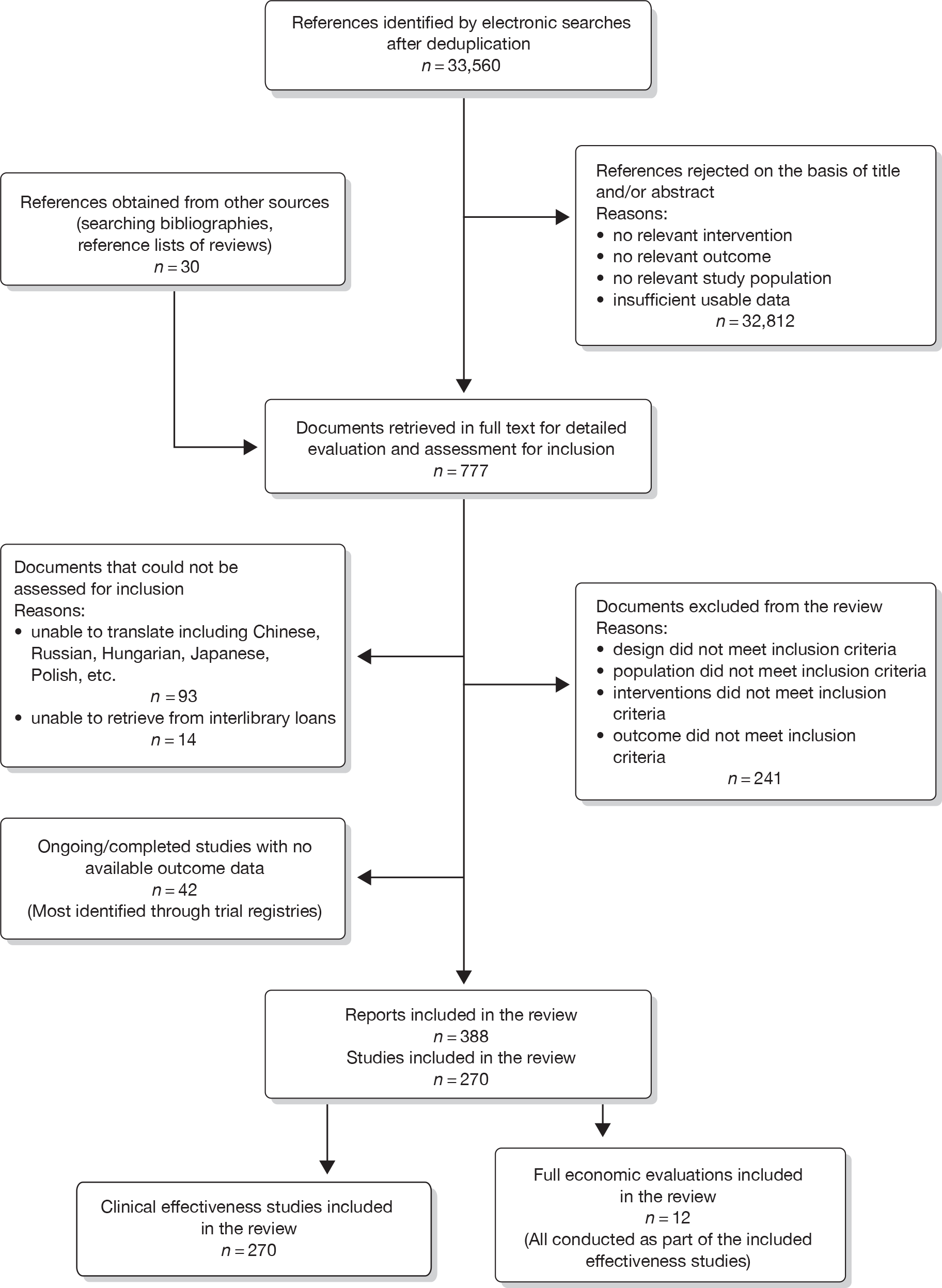
Forty-two ongoing or unpublished studies were identified while searching trial registries and are summarised in Appendix 4.
Seventeen (18%) out of 96 studies that reported data on CSOMs used more than one condition-specific outcome scale, five (5%) of which reported data on both RMDQ and ODI.
Chapter 6 Review of clinical effectiveness: results
The results of clinical effectiveness are presented for each intervention category separately, according to the order that interventions are listed in Table 1. Findings relating to usual care and inactive control are not reported separately (only as comparators for other interventions). Studies that evaluated mixed treatments are also not reported separately. Studies that compared interventions that fell under the same treatment category were included in the review as a whole, but their findings are not presented here. However, information on the type of interventions that they examined is presented (see Chapter 4, Standard pair-wise meta-analysis).
The results are presented for overall recovery (global effect), pain intensity and back-specific functional status (CSOMs) at short-, medium- and long-term follow-up. The findings for any adverse effects that occurred during the study (overall follow-up) are also reported.
Details of the quality assessment of individual studies are presented in Appendix 5.
Disc surgery (including intraoperative interventions)
Intraoperative interventions have been considered as a separate intervention category to disc surgery in the MTC and are therefore treated the same here. Intraoperative interventions are supplemental procedures undertaken during surgery, such as the application of steroids or free fat grafts.
Description of disc surgery studies
Summary of interventions
A total of 97 studies evaluated disc surgery for sciatica. 45–141 Sixty-three of these studies compared disc surgery with an alternative type of intervention (including intraoperative). 45–107 The type of interventions being compared are listed in Table 3a. One of theses studies,46 which compared disc surgery with chemonucleolysis, did not include useable comparative data and reported only descriptive results for change from baseline for each group separately. One further study61 did not report any data on global effect, pain intensity or CSOMs.
| ID no. | Author, year | Study design | Treatment description | Control description |
|---|---|---|---|---|
| Disc surgery vs chemonucleolysis | ||||
| 884 | Alexander, 1989103 | CCS | Disc surgery (removal of protruding disc fragment only + free fat graft) | Chemonucleolysis with chymopapain (2000 U) |
| 43 | van Alphen, 198947 | RCT | Discectomy with emptying of disc space | Chemonucleolysis with chymopapain (4000 U) |
| 441 | Bonafe, 199375 (French language) | CCS | Percutaneous automated nucleotomy | Chemonucleolysis with chymopapain (4000 U) |
| 183 | Bouillet, 198361 | CCS | Conventional lumbar disc surgery | Chemonucleolysis with chymopapain injections |
| 453 | Brown, 198976 | CCS | Disc surgery | Chemonucleolysis with chymopapain |
| 453 | Brown, 198976 | CCS | Disc surgery | Collagenase chemonucleolysis |
| 454 | Buric, 200577 | Non-RCT | Standard microdiscectomy | Chemonucleolysis with ozone–oxygen mixture |
| 166 | Crawshaw, 198460 | RCT | Disc surgery | Chemonucleolysis with chymopapain (4000 U) |
| 48 | Dabezies, 197851 | CCS | Laminectomy with or without fusion | Chemonucleolysis with chymopapain (2 ml) |
| 471 | Dei-Anang, 199079 (German language) | CCS | Percutaneous nucleotomy | Chemonucleolysis with chymopapain (4000 U) or collagenase (600 U) |
| 727 | Ejeskar, 198396 | RCT | Discectomy with unilateral laminotomy and removal of disc hernia only | Chemonucleolysis with chymopapain (400 IU) |
| 132 | Hoogmartens, 197656 | HCS | Discectomy | Chemonucleolysis with chymopapain |
| 44 | Javid, 199548 | CCS | Partial hemilaminectomy using magnification and fat graft | Chemonucleolysis with chymopapain (3000 IU) |
| 35 | Krugluger, 200046 | RCT | Automated percutaneous discectomy | Chemonucleolysis with chymodiactin (4000 U) |
| 117 | Lagarrigue, 199154 (French language) | CCS | Discectomy with minimal bony resection | Chemonucleolysis with chymopapain (2000–5000 U) |
| 129 | Lavignolle, 198755 (French language) | RCT |
Microscopic discectomy Unilateral limited interlaminar |
Chemonucleolysis with chymopapain (4000 U) |
| 889 | Lee, 1996104 (German language) | CCS | Automated percutaneous lumbar discectomy | Chemonucleolysis with chymopapain |
| 889 | Lee, 1996104 (German language) | CCS | Percutaneous manual and laser discectomy | Chemonucleolysis with chymopapain |
| 593 | Muralikuttan, 199285 | RCT | Standard discectomy with fenestration, disc space cleared | Chemonucleolysis with chymopapain (2000 U) |
| 47 | Norton, 198650 | CCS | Conventional surgical discectomy | Chemonucleolysis with chymopapain |
| 45 | Postacchini, 198749 | Non-RCT | Disc excision using unilateral laminotomy | Chemonucleolysis with chymopapain (2 ml) |
| 617 | Revel, 199388 | RCT | Automated percutaneous lumbar discectomy | Chemonucleolysis |
| 641 | Steffen, 199990 (German language) | RCT | Laser disc decompression | Chemonucleolysis with chymodiactin (2 ml) |
| 49 | Stula, 199052 (German language) | RCT | Conventional disc surgery | Chemonucleolysis with chymopapain (500 U) |
| 61 | Tregonning, 199153 | CCS | Fenestration or partial laminectomy removing extruded disc material | Chemonucleolysis with chymopapain |
| 893 | Watters,1988105 | Non-RCT | Microdiscectomy with free fat graft over exposed dura | Chemonucleolysis with chymopapain (4000 U) |
| 160 | Watts, 197559 | CCS | Discectomy with laminotomy and foraminotomy | Chemonucleolysis with chymopapain (4 mg) |
| 672 | Weinstein, 198692 | CCS | Discectomy | Chemonucleolysis with chymopapain |
| 150 | Zeiger, 198758 | CCS | Microdiscectomy with intraoperative injection into intervertebral space with steroid 125 mg methylprednisolone + morphine 4 mg used to reduce postoperative pain and morbidity | Chemonucleolysis with chymodiactin (2.5 ml) |
| Disc surgery vs epidural/intradiscal injection | ||||
| 725 | Buttermann, 200495 | RCT | Discectomy | Epidural injection of steroid betamethasone 10–15 mg up to three injections |
| Disc surgery vs exercise therapy | ||||
| 300 | Osterman, 200668 | RCT | Microdiscectomy and exercise therapy | Exercise therapy |
| Disc surgery vs intraoperative interventions | ||||
| 268 | Aminmansour, 200664 | Q-RCT | Discectomy with fenestration + distilled water injection | Discectomy with fenestration + 40 mg intravenous dexamethasone |
| 268 | Aminmansour, 200664 | Q-RCT | Discectomy with fenestration + distilled water injection | Discectomy with fenestration + 80 mg intravenous dexamethasone |
| 436 | Bernsmann, 200174 | RCT | Microdiscectomy with partial hemi-laminectomy, but no free fat graft | Microdiscectomy with partial hemi-laminectomy and free fat graft |
| 470 | Debi, 200278 | RCT | Lumbar discectomy with saline applied to exposed nerve route on a collagen sponge | Lumbar discectomy with steroid methylprednisolone 80 mg applied to exposed nerve route on a collagen sponge |
| 492 | Gerszten, 200381 | RCT | Sham irradiation prior to repeat surgical decompression (control group) | Irradiation prior to repeat surgical decompression (treatment group) |
| 497 | Glasser, 199382 | RCT | Microdiscectomy with partial hemilaminectomy and emptying of disc space only (group 3) | Microdiscectomy with partial hemilaminectomy, emptying of disc space and intraoperative steroid methylprednisolone 490 mg + local anaesthetic 30 ml bupivacaine (group 1) |
| 497 | Glasser, 199382 | RCT | Microdiscectomy with partial hemilaminectomy and emptying of disc space only (group 3) | Microdiscectomy with partial hemilaminectomy, emptying of disc space and intraoperative local anaesthetic 30 ml bupivacaine (group 2) |
| 520 | Jensen, 199683 | RCT | Flavectomy, partial laminectomy without free fat transplantation (group B) | Flavectomy, partial laminectomy with free fat transplantation (group A) |
| 909 | Jirarattanaphochai, 2007106 | RCT | Disc surgery + saline administered to nerve root + intramuscularly (placebo group) | Disc surgery + corticosteroid administration (80 mg of methylprednisolone sodium succinate) to nerve root + bupivacaine (30 ml 0.375%) intramuscularly (steroid group) |
| 400 | Kim, 200373 | RCT | Discectomy without Oxiplex®/SP Gel (FzioMed, CA, USA) | Discectomy with anti-adhesion barrier Oxiplex®/SP Gel |
| 551 | Langmayr, 199584 | RCT | Microdiscectomy plus intrathecal saline injection (placebo group) | Microdiscectomy with intrathecal steroid injection betamethasone (2 ml) (steroid group) |
| 366 | Lavyne, 199270 | Q-RCT | Microdiscectomy followed with epidural irrigation of saline | Microdiscectomy followed with epidural irrigation of steroid methylprednisolone 40 mg |
| 276 | Lundin, 200366 | RCT | Discectomy + saline (control group) | Discectomy + intramuscular, intravenous and fat graft soaked in steroids methylprednisolone 490 mg |
| 270 | MacKay, 199565 | RCT | Standard hemilaminotomy, limited discectomy, dura left uncovered | Standard hemilaminotomy, limited discectomy, dura covered with free fat graft |
| 270 | MacKay, 199565 | RCT | Standard hemilaminotomy, limited discectomy, dura left uncovered | Standard hemilaminotomy, limited discectomy, dura covered with gelfoam interposion membrane |
| 854 | Rasmussen, 2008101 | RCT | Patients received disc surgery only | Local application of 40 mg methylprednisolone following disc excision |
| 618 | Richter, 200189 | RCT | Microdiscectomy unilateral interlaminar without applying any gel | Microdiscectomy unilateral interlaminar with the application of ADCON-L gel (Gliatech Inc., OH, USA) |
| 856 | Ronnberg, 2008102 | RCT | Partial discectomy with no gel applied prior to closure of the wound | Partial discectomy and ADCON-L gel applied around the nerve root, thecal sac and posterior longitudinal ligament |
| 316 | Cengiz, 200769 | RCT | Disc surgery + no adhesion barrier | Disc surgery + anti-adhesion barrier ADCON-L |
| 316 | Cengiz, 200769 | RCT | Disc surgery + no adhesion barrier | Disc surgery + anti-adhesion barrier Healon GV |
| 915 | de Tribolet, 1998107 | RCT | Decompression of the affected nerve root. Type of surgery: laminectomy 4, laminotomy 25, hemilaminectomy 53, hemilaminotomy 58, foraminotomy 1. Incision was closed in a routine fashion. No gel applied | Decompression of the affected nerve root. Type of surgery: laminectomy 2, laminotomy 22, hemilaminectomy 49, hemilaminotomy 55, foraminotomy 0. Before closure 3–5 g of ADCON-L gel applied to nerve root |
| Disc surgery vs mixed treatments | ||||
| 734 | Hoogland, 200697 | Q-RCT | Endoscopic discectomy |
(Surgery + chemonucleolysis) Endoscopic discectomy and chemonucleolysis with chymopapain (1000 U) |
| 379 | Prestar, 199571 (German language) | RCT | Discectomy without preoperative, intraoperative or postoperative steroid |
(Surgery + non-opioids) Discectomy with preoperative, intraoperative and postoperative steroid dexamethasone 4–40 mg for 7 days |
| 705 | Starkweather, 200693 | RCT | Microdiscectomy and placebo medication |
(Surgery + non-opioids) Microdiscectomy and antidepressant medication – amitriptyline 75 mg for 7 days prior |
| 705 | Starkweather, 200693 | Non-RCT |
(An additional non-randomised group) Microdiscectomy with no intervention |
(Surgery + non-opioids) Microdiscectomy and antidepressant medication – amitriptyline 75 mg for 7 days prior |
| 263 | Wang, 200063 | RCT | Placebo acupuncture before and after surgery |
(Surgery + alternative) Classical acupuncture before or after surgery |
| Disc surgery vs non-opioids | ||||
| 475 | Dubourg, 200280 | CCS | Disc surgery (operative group) (various surgical techniques) | Non-operative intervention group. Some received steroids |
| 144 | Rossi, 199357 (Italian language) | Non-RCT | Percutaneous discectomy (groups Ia and IIa) | Oral dexamethasone 8 mg for 9 days, naproxen 500–1000 mg for 5 days (group Ib) |
| 144 | Rossi, 199357 (Italian language) | Non-RCT | Microdiscectomy (group 2b) | Oral dexamethasone 8 mg for 9 days, naproxen 500–1000 mg for 5 days (group Ib) |
| Disc surgery vs others | ||||
| 600 | North, 200586 | RCT | Re-operation with laminectomy, discectomy with our without fusion | Spinal cord stimulation group |
| Disc surgery vs usual/conventional care | ||||
| 716 | Alaranta, 199094 | CCS | Discectomy with partial laminectomy | Conservative treatment |
| 386 | Atlas, 199672 | CCS | Surgery most had open discectomy | Various non-surgical treatments |
| 772 | Hansson, 2007100 | CCS | Surgical treatment | Conservative non-surgical treatment. No further details |
| 294 | Koranda, 199567 (Czech language) | Q-RCT | Disc surgery | Conservative therapy |
| 606 | Peul, 200787 | RCT | Microdiscectomy | Conventional care control |
| 211 | Shvartzman, 199262 | HCS | Standard lumbar discectomy | Physical therapy at a local rehabilitation centre. No further details |
| 2 | Thomas, 200745 | CCS | Lumbar microdiscectomy with hemilaminotomy | Non-operative multidisciplinary care, no injections |
| 664 | Weber, 198391 | RCT | Discectomy | Bed rest, physiotherapy, analgesia |
| 750 | Weinstein, 200698 | CCS | Open or microdiscectomy (group S) | Non-operative treatment (usual care) |
| 751 | Weinstein, 200699 | RCT | Standard open or microdiscectomy (group S) | Non-operative treatment (usual care) |
Thirty-eight studies compared different types of disc surgery64,65,69,82,108–141 and five compared different intraoperative interventions64,65,69,82,141 (four of these studies were three-arm studies that also compared intraoperative interventions with disc surgery64,65,69,82). The types of surgical procedures being compared are listed in Table 3b, but the findings of these studies are not considered any further than this.
| ID no. | Author, year | Study design | Intervention type | Treatment description | Control type | Control description |
|---|---|---|---|---|---|---|
| Bilateral vs unilateral | ||||||
| 21 | Barlocher, 2000108 | CCS | Unilateral (microscope) | Unilateral fenestration with microdiscectomy | Bilateral (microscope) | Bilateral fenestration with microdiscectomy |
| 502 | Hagen, 1977128 | CCS | Bilateral | Discectomy with bilateral laminectomy and emptying of disc space (group1) | Unilateral | Discectomy with unilateral laminectomy and emptying of disc space (group 2) |
| Day case vs inpatient | ||||||
| 219 | Gonzalez-Castro, 2002117 | Q-RCT | Day-case | Conventional discectomy (fenestration) day-case surgery – disc space cleared, no microscope | Inpatient | Conventional discectomy (fenestration) inpatient stay – disc space cleared, no microscope |
| Disc surgery + fusion vs disc surgery alone | ||||||
| 66 | Takeshima, 2000109 | HCS | Disc surgery + fusion | Disc excision with posterolateral fusion (fusion group) | Disc surgery alone | Disc excision without fusion (non-fusion group) |
| 653 | Tria, 1987136 | HCS | Disc surgery + fusion | Laminectomy combined with spinal fusion | Disc surgery alone | Simple laminectomy |
| 673 | White, 1987138 | Non-RCT | Disc surgery + fusion | Discectomy with laminectomy plus fusion with internal fixation | Disc surgery alone | Simple laminectomy with no fusion |
| Discectomy + endplate curettage vs disc surgery alone | ||||||
| 430 | Balderston, 1991124 | CCS | Discectomy + endplate curettage | Lumbar discectomy combined with vertebral endplate curettage | Discectomy alone | Lumbar discectomy with laminectomy, but no endplate curettage |
| Endoscopic discectomy vs endoscopic discectomy | ||||||
| 680 | Yang, 2005140 | HCS | Endoscopic discectomy (without laser) | Automated percutaneous lumbar discectomy | Endoscopic discectomy (laser decompression) | Percutaneous laser disc decompression |
| 164 | Righesso, 2007114 | RCT | Open discectomy (no microscope) | Open discectomy using magnification | Endoscopic discectomy (microscope) | Microendoscopic discectomy |
| 402 | Ruetten, 2008121 | Q-RCT | Open discectomy (microscope) | Conventional microsurgical discectomy | Endoscopic discectomy (no microscope) | Full endoscopic interlaminar or transforaminal discectomy |
| 403 | Ryang, 2008122 | RCT | Open discectomy (microscope) | Standard open microdiscectomy | Endoscopic discectomy (microscope) | Minimal access trocar microdiscectomy |
| 651 | Toyone, 2004135 | Non-RCT | Open discectomy (no microscope) | Standard open microdiscectomy with removal of herniated material only | Endoscopic discectomy (microscope) | Microendoscopic discectomy |
| Endoscopic discectomy vs open discectomy | ||||||
| 460 | Chatterjee, 1995127 | RCT | Endoscopic discectomy | Automated percutaneous lumbar discectomy | Open discectomy | Microdiscectomy |
| 536 | Kim, 2007130 | CCS | Endoscopic discectomy (no microscope) | Targeted percutaneous transforaminal endoscopic discectomy | Open discectomy (no microscope) | Microscopic discectomy |
| 582 | Mayer, 1993131 | RCT | Endoscopic discectomy (no microscope) | Percutaneous endoscopic discectomy | Open discectomy (no microscope) | Microdiscectomy |
| 632 | Schizas, 2005132 | Non-RCT | Endoscopic discectomy (no microscope) | Microendoscopic discectomy | Open discectomy (no microscope) | Microdiscectomy |
| 327 | Shin, 2008119 | RCT | Endoscopic discectomy (microscope) | Microendoscopic discectomy with partial hemilaminectomy | Open discectomy (microscope) | Microscopic discectomy with partial hemilaminectomy |
| 409 | Wu, 2006123 | CCS | Endoscopic discectomy (microscope) | Microendoscopic discectomy | Open discectomy (no microscope) | Standard open posterior lumbar discectomy |
| 459 | Zhang, 2007126 | Non-RCT | Endoscopic discectomy (microscope) | Microendoscopic discectomy | Open discectomy (no microscope) | Open lumbar discectomy |
| Extensive disc surgery vs limited disc surgery | ||||||
| 391 | Carragee, 2006120 | HCS | Open discectomy | Subtotal discectomy with removal of extruded fragments and emptying of disc space | Limited discectomy | Limited discectomy with removal of extruded fragments only |
| 525 | Kahanovitz, 1989129 | CCS | Extensive disc surgery (microscope) | Microdiscectomy (with an operating microscope) | Limited disc surgery (no microscope) | Limited unilateral discectomy without magnification |
| 643 | Striffeler, 1991133 | CCS | Limited discectomy (microscope) | Conservative microdiscectomy with removal of prolapsed disc, disc space irrigated | Extensive discectomy (microscope) | Standard microdiscectomy with emptying of disc space |
| 647 | Thome, 2005134 | RCT | Extensive discectomy (microscope) | Microdiscectomy with emptying of disc space | Limited discectomy (microscope) | Sequestrectomy with removal of herniated material only |
| Laser discectomy vs open discectomy | ||||||
| 116 | Lee, 2006111 | CCS |
Endoscopic discectomy (no microscope) Laser decompression |
Percutaneous endoscopic lumbar discectomy |
Open dicectomy (microscope) No laser |
Open lumbar microdiscectomy with partial hemilaminectomy |
| 165 | Tassi, 2006115 | HCS | Laser decompression | Percutaneous laser disc decompression | (Microscope) | Standard surgical microdiscectomy |
| Ligamentum flavum preservation vs ligamentum flavum excision | ||||||
| 69 | Aydin, 2002110 | HCS | Ligamentum flavum preservation (microscope) | Microdiscectomy with preservation of ligamentum flavum (group 1) | Ligamentum flavum excision (microscope) | Standard microdiscectomy with fenestration, foraminotomy, partial or total excision of ligamentum flavum (group 2) |
| Microscope vs no microscope | ||||||
| 432 | Barrios, 1990125 | CCS | Microscope | Standard discectomy with partial hemilaminectomy | No microscope | Microdiscectomy |
| 167 | Katayama, 2006116 | RCT | Microscope | Microdiscectomy without laminectomy, disc space emptied (group B) | No microscope | Macrodiscectomy with partial laminectomy, no microscope, disc space emptied (group A) |
| 143 | Kho, 1986113 (German language) | HCS | Microscope | Microdiscectomy | No microscope | Lumbar discectomy without microscope |
| 126 | Lagarrigue, 1994112 (French language) | RCT | Microscope | Microscopic lumbar discectomy | No microscope | Normal lumbar discectomy (without microscope) |
| 232 | Tullberg, 1993118 | RCT | Microscope | Microscopic surgery (micro-group) – disc space cleared | No microscope | Standard macrodiscectomy (without microscope) – disc space cleared |
| 654 | Tureyen, 2003137 | RCT | Microscope | Microdiscectomy with emptying of disc space (group A) | No microscope | Macrodiscectomy with laminectomy and emptying of disc space, no microscope (group B) |
| 674 | Wilson, 1981139 | HCS | Microscope | Microdiscectomy with evacuation of disc space, but no curettage of end plates | No microscope | Standard open discectomy with evacuation of disc space, but no curettage of end plates |
One further study142 compared disc surgery plus epidural (mixed treatments) with conventional care given while waiting for surgery. However, the study only reported health-care utilisation and employment-related outcomes.
Summary of study participants for disc surgery
Summary data for included participants are presented in Table 4. The number of participants included in the 61 studies that reported outcome data for global effect, pain or CSOMs ranged from 10 to 2749 (median 103). A similar number of studies included patients with chronic sciatica, or either chronic or acute sciatica, or did not report this information. Four studies62,68,80,87 included patients with acute sciatica, with a mean duration of symptoms ranging from 25.7 days80 to 68.5 days. 68 Four studies54,67,69,83 included some patients with spinal stenosis and 1068,74,83,95,97,98,99,101,103,107 included patients with sequestered or extruded discs. The diagnosis of sciatica, or the presence of herniated disc, was confirmed by imaging in 52 (85%) studies. Six studies49,66,74,92,95,105 included patients who had sciatica for the first time and seven studies50,57,63,72,80,81,83,86 included only patients with recurrent sciatica. The remaining studies included patients with either first-episode or recurrent sciatica, or did not report this information. The majority of studies (n = 40) included patients who had received previous treatment for their current episode of sciatica. Ten studies45,56,59,63,71,80,81,86,88,95 included patients who had received previous disc surgery and 32 studies included patients who had not.
| ID no. | Author, year | Study design | No. of patients | Mean age (years) | No. of men (%) | Symptom duration | Type of sciatica | Confirmed by imaging? | Recurrent episode | Included patients with stenosis? a | Included patients with sequestered disc (or extruded)?a | Any previous treatment for sciatica? | Any previous back surgery for sciatica? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disc surgery vs chemonucleolysis | |||||||||||||
| 884 | Alexander, 1989103 | CCS | 100 | 33.5 (range 18–65) | 90 (90) | Mean 5.5 months | Nerve root pain | Yes | NR | No | Yes | Yes | No |
| 43 | van Alphen, 198947 | RCT | 151 | 34 (range 18–45) | 99 (66) | 55% < 6 months; 45% > 6 months | Nerve root pain | Yes | NR | No | No | Yes | No |
| 441 | Bonafe, 199375 (French language) | CCS | 40 | 46 (range 27–68) | 28 (70) | Mean 3 months (range several days to 15 months) | Nerve root pain | Yes | NR | No | No | Yes | NR |
| 183 | Bouillet, 198361 | CCS | 2749 | NR | NR | Ranged from weeks to months | Nerve root pain | Yes | NR | No | No | Yes | NR |
| 453 | Brown, 198976 | CCS | 85 | 37.6 | 59 (69) | At least 3 months | Nerve root pain | Yes | NR | No | No | Yes | No |
| 454 | Buric, 200577 | Non-RCT | 45 | 45 (SD 14.2; range 19–77) | 23 (51) | Mean 203.9 days (SD 129.6; range 21 to > 365 days) | Nerve root pain | Yes | NR | No | No | Yes | No |
| 166 | Crawshaw, 198460 | RCT | 52 | 37 | NR | NR | Nerve root pain | Yes | NR | No | No | Yes | No |
| 48 | Dabezies, 197851 | CCS | 200 | 39 | 132 (66) | NR | Nerve root pain and referred pain | No | Recurrent and first episode | No | No | Yes | NR |
| 471 | Dei-Anang, 199079 (German language) | CCS | 201 | NR | NR | NR | Nerve root pain | NR | NR | No | No | NR | NR |
| 727 | Ejeskar, 198396 | RCT | 29 | 39.3 | 21 (72) | Mean 4.5 months (SD 3 months) | Nerve root pain | Yes | NR | No | No | NR | No |
| 132 | Hoogmartens, 197656 | HCS | 97 | 35.5 | 48 (49) | 25–35 months | Nerve root pain | NR | Recurrent and first episode | No | No | Yes | Yes |
| 44 | Javid, 199548 | CCS | 200 | 39 (range 17–81) | 134 (67) | Mean 7.2 months | Nerve root pain | Yes | NR | No | No | Yes | No |
| 35 | Krugluger, 200046 | RCT | 22 | 40 (range 24–60) | 16 (73) | Mean 7 months | Nerve root pain | Yes | NR | No | No | Yes | NR |
| 117 | Lagarrigue, 199154 (French language) | CCS | 1085 | 42 (range 14–83) | 682 (63) | Mean 13.4 months | Nerve root pain | No | NR | Yes | No | Yes | NR |
| 129 | Lavignolle, 198755 (French language) | RCT | 358 | 41 (SD 12.03) | 225 (63) | NR | Nerve root pain | NR | NR | No | No | NR | NR |
| 889 | Lee, 1996104 (German language) | CCS | 300 | 50% < 30; 25% > 40 | 213 (71) | NR | Nerve root pain | Yes | NR | No | No | Yes | NR |
| 593 | Muralikuttan, 199285 | RCT | 92 | 35 (range 19–60) | 55 (60) | Mean 24 weeks | Nerve root pain | Yes | NR | No | No | Yes | NR |
| 47 | Norton, 198650 | CCS | 105 | 40 (range 20–67) | 86 (82) | Mean 18.5 months (range 5 days–128 months) | Nerve root pain | Yes | Recurrent | No | No | Yes | No |
| 45 | Postacchini, 198749 | Non-RCT | 161 | NR | NR | Mean 8.75 months (range 1.2–36.0 months) | Nerve root pain and referred pain | Yes | First episode | No | No | Yes | NR |
| 617 | Revel, 199388 | RCT | 165 | 39 (SD 9; range 21–65) | 96 (68) | NR | Nerve root pain | Yes | NR | No | No | Yes | Yes |
| 641 | Steffen, 199990 (German language) | RCT | 69 | NR | NR | 10.6 months | Nerve root pain | Yes | NR | No | No | Yes | NR |
| 49 | Stula, 199052 (German language) | RCT | 69 | Range 22–54 | 57 (83) | < 1 year | Nerve root pain | Yes | Recurrent and first episode | No | No | Yes | No |
| 61 | Tregonning, 199153 | CCS | 268 | 40.4 (range 20–65) | 135 (68) | NR | Nerve root pain | Yes | NR | No | No | Yes | No |
| 893 | Watters,1988105 | Non-RCT | 100 | 36.5 | 59 (59) | Mean 13 weeks | Nerve root pain | Yes | First episode | No | No | NR | NR |
| 160 | Watts, 197559 | CCS | 274 | Range 24–62 | 55 (55) | NR | Nerve root pain and referred pain | Yes | Recurrent and first episode | No | No | Yes | Yes |
| 672 | Weinstein, 198692 | CCS | 159 | 41 (range 28–57) | 64 (41) | Minimum period of 3 months | Nerve root pain | Yes | First episode | No | No | Yes | No |
| 150 | Zeiger, 198758 | CCS | 126 | NR | NR | 4 weeks or more | Nerve root pain | Yes | NR | No | No | Yes | No |
| Disc surgery vs epidural | |||||||||||||
| 725 | Buttermann, 200495 | RCT | 100 | 40.5 (SD 12) | Mean 3.55 months (SD 2.75 months) | Nerve root pain | Yes | First episode | No | Yes | Yes | Yes | |
| Disc surgery vs exercise therapy | |||||||||||||
| 300 | Osterman, 200668 | RCT | 57 | 38 (SD 7) | 34 (61) | Mean 68.5 days (SD 27 days) | Nerve root pain | Yes | Recurrent and first episode | No | Yes | NR | No |
| Disc surgery vs intraoperative interventions | |||||||||||||
| 268 | Aminmansour, 200664 | Q-RCT | 61 | 38.5 (SD 10.4) | 35 (57) | NR | Nerve root pain | Yes | NR | No | No | NR | NR |
| 436 | Bernsmann, 200174 | RCT | 200 | 43 (range 22–75) | 97 (52) | NR | Nerve root pain | Yes | First episode | No | Yes | NR | NR |
| 470 | Debi, 200278 | RCT | 70 | 41 (range 18–60) | 43 (70) | Mean 56 days (range 12–135 days) | Nerve root pain | NR | NR | No | No | Yes | No |
| 492 | Gerszten, 200381 | RCT | 10 | 42 | 5 (50) | Mean 3.5 years (range 1.5–10.0 years) | Nerve root pain | Yes | Recurrent | No | No | Yes | Yes |
| 497 | Glasser, 199382 | RCT | 32 | 46.1 (SD 3.66) | Within 6 months | Nerve root pain | Yes | NR | No | No | Yes | No | |
| 520 | Jensen, 199683 | RCT | 118 | Median 46 (range 19–75) | 53 (45) | NR | Nerve root pain | Yes | Recurrent | No central stenosis but some had lateral stenosis | Yes | NR | No |
| 909 | Jirarattanaphochai, 2007106 | RCT | 103 | 52 (SD 11; range 21–79) | 48 (47) | NR | Nerve root pain | NR | NR | No | No | NR | NR |
| 400 | Kim, 200373 | RCT | 35 | 43.5 (range 28–65) | 11 (31) | NR | Nerve root pain | Yes | NR | No | No | NR | No |
| 551 | Langmayr, 199584 | RCT | 26 | 42 | 20 (77) | Median 35 days (range 14–150 days) | Nerve root pain | Yes | NR | No | No | Yes | No |
| 366 | Lavyne, 199270 | Q-RCT | 84 | 40 (range 17–70) | 57 (73) | Few days to several months | Nerve root pain | Yes | NR | No | No | Yes | NR |
| 276 | Lundin, 200366 | RCT | 80 | 41.7 | 44 (55) | Mean 4.5 months | Nerve root pain | Yes | First episode | No | No | NR | No |
| 270 | MacKay, 199565 | RCT | 190 | 39 (range 14–79) | 106 (69) | NR | Nerve root pain | Yes | NR | No | No | Yes | NR |
| 854 | Rasmussen, 2008101 | RCT | 200 | 42.5 (range 18–66) | 122 (61) | NR | Nerve root pain | Yes | NR | No | Yes | Yes | NR |
| 618 | Richter, 200189 | RCT | 398 | 43 (range 30–65) | 221 (62) | NR | Nerve root pain | Yes | NR | No | No | NR | No |
| 856 | Ronnberg, 2008102 | RCT | 128 | 39 (range 18–66) | 68 (53) | NR | Nerve root pain | Yes | NR | No | No | Yes | No |
| 316 | Cengiz, 200769 | RCT | 60 | 44.2 (SD 10.2) | 35 (58) | Mean 12.3 years (SD 9.2 years) | Nerve root pain | Yes | Recurrent and first episode | Yes | No | NR | No |
| 915 | de Tribolet, 1998107 | RCT | 298 | 39.1 (SD 9.5) | 167 (56) | Not stated | Nerve root pain | Yes | Recurrent and first episode | No | Yes extruded and sequestered discs | Yes | No |
| Disc surgery vs mixed treatments | |||||||||||||
| 734 | Hoogland, 200697 | Q-RCT | 280 | 40.5 (range 18–60) | 186 (66) | NR | Nerve root pain | Yes | NR | No | Yes | Yes | No |
| 379 | Prestar, 199571 (German language) | RCT | 100 | 44.7 (range 26–76) | NR | NR | Nerve root pain | Yes | NR | No | No | Yes | Yes |
| 705 | Starkweather, 200693 | RCT | 70 | 45.5 (SD 11; range 21–65) | 40 (57) | 61% < 1 year; 39% > 1 year | Nerve root pain | Yes | NR | No | No | NR | NR |
| 263 | Wang, 200063 | RCT | 145 | 21–80 | 78 (59) | At least 6 months | Nerve root pain | Yes | Recurrent | No | No | Yes | Yes |
| Disc surgery vs non-opioids | |||||||||||||
| 475 | Dubourg, 200280 | CCS | 67 | 48.8 (SD 13.9; range 28–81) | 42 (63) | Mean 25.7 days (SD 28.7 days) | Nerve root pain | Yes | Recurrent and first episode | No | No | NR | Yes |
| 144 | Rossi, 199357 (Italian language) | Non-RCT | 40 | 42.5 (SD 10.5; range 20–65) | NR | < 6 months | Nerve root pain | Yes | Recurrent | No | No | NR | NR |
| Disc surgery vs others | |||||||||||||
| 600 | North, 200586 | RCT | 60 | 50.2 (SD 13.3; range 26–76) | 30 (50) | NR | Nerve root pain | Yes | Recurrent | No | No | Yes | Yes |
| Disc surgery vs usual/conventional care | |||||||||||||
| 716 | Alaranta, 199094 | CCS | 357 (322 partial rhizography) | 39.6 | 179 (50) | Mean 3.6 months | Nerve root pain | No | NR | No | No extrusion | NR | No |
| 386 | Atlas, 199672 | CCS | 507 | 42 (range 18–85) | 322 (64) | 41% < 1 year; 1–24% 5 years; 35% > 5 years | Nerve root pain | No | Recurrent | No | No | Yes | No |
| 772 | Hansson, 2007100 | CCS | 184 | 43 (range 22–59) | 87 (47) | 20% < 1 week; 39% 1 week to 1 year; 42% > 1 year | Nerve root pain | Yes | NR | No | No | NR | No |
| 294 | Koranda, 199567 (Czech language) | Q-RCT | 100 | NR | NR | NR | Nerve root pain | Yes | Recurrent and first episode | Yes | No | Yes | NR |
| 211 | Shvartzman, 199262 | HCS | 55 | 42.3 (SD 11.1; range 23–59) | 55 (100) | Patients presented with acute episode of sciatica, actual duration NR | Nerve root pain | Yes | NR | No | No | Yes | No |
| 2 | Thomas, 200745 | CCS | 623 | 42.9 | 291 (59) | Mean 191.5 days (SD 195 days) | Nerve root pain | Yes | Recurrent and first episode | No | No | NR | Yes |
| 664 | Weber, 198391 | RCT | 126 | 41 (range 25–55) | 68 (54) | At least 14 days | Nerve root pain | Yes | NR | No | No | NR | No |
| 751 | Weinstein, 200699 | RCT | 501 | 42 (SD 11.6) | 278 (59) | 79% < 6 months | Nerve root pain | Yes | Recurrent and first episode | No | Yes | Yes | No |
| 606 | Peul, 200787 | RCT | 283 | 42.6 (SD 9.8) | 186 (66) | Mean 9.5 weeks (range 6–12 weeks) | Nerve root pain | Yes | NR | No | No | NR | No |
| 750 | Weinstein, 200698 | CCS | 743 | 41.4 (SD 11.2) | 406 (56) | 77% < 6 months | Nerve root pain | Yes | Recurrent and first episode | No | Yes | Yes | No |
Summary of study design and quality for disc surgery studies
Summary information on study details are presented in Table 5. The full results of the quality assessment are presented in the Appendix 5. Just over half (33/62, 53%) of the disc surgery studies were RCTs, of which only two87,99 were good quality overall (comparing disc surgery with usual care). Four RCTs68,73,89,99 had used both adequate randomisation and allocation concealment (comparators included exercise therapy, intraoperative interventions and usual care). A further eight studies81,85–88,101,106,107 used adequate randomisation, but not allocation concealment (although two studies87,106 used sealed envelopes), and one study69 used adequate allocation concealment, but not randomisation. Two studies91,93 used sealed envelopes, but gave no further details on method of randomisation. Three studies45,47,87 had strong external validity.
| ID no. | Author, year | Study size | Overall follow-up | Study design | Adequate randomisation? | Allocation concealment? | Follow-up (%) | Blind outcome assessment? | Overall quality rating | Overall external validity rating |
|---|---|---|---|---|---|---|---|---|---|---|
| Disc surgery vs chemonucleolysis | ||||||||||
| 884 | Alexander, 1989103 | 100 | Mean 14 months; range 6–35 months | CCS | No | No | 80–100 | Unclear | Weak | Weak |
| 43 | van Alphen, 198947 | 151 | 12 months | RCT | Partial | Unclear | 80–100 | No | Moderate | Strong |
| 441 | Bonafe, 199375 (French language) | 40 | Mean 15 months; range 3–36 months | CCS | No | No | 80–100 | Unclear | Weak | Weak |
| 453 | Brown, 198976 | 85 | 3 months | CCS | No | No | 80–100 | Yes | Weak | Weak |
| 454 | Buric, 200577 | 45 | 18 months | Non-RCT | No | No | 80–100 | NA | Weak | Weak |
| 166 | Crawshaw, 198460 | 52 | 1 year | RCT | Unclear | Unclear | 80–100 | Unclear | Weak | Moderate |
| 48 | Dabezies, 197851 | 200 | 2 years | CCS | No | No | Can’t tell | No | Weak | Moderate |
| 471 | Dei-Anang, 199079 (German language) | 201 | 1 year | CCS | No | No | NA | Unclear | Weak | Weak |
| 727 | Ejeskar, 198396 | 29 | 1 year | RCT | Unclear | Unclear | 80–100 | Unclear | Weak | Moderate |
| 132 | Hoogmartens, 197656 | 97 | 58 months for discectomy and 38 months for chemonucleolysis | HCS | No | No | NA | NA | Weak | Moderate |
| 44 | Javid, 199548 | 200 | 1 year | CCS | No | No | 80–100 | No | Weak | Moderate |
| 35 | Krugluger, 200046 | 22 | 2 years | RCT | Unclear | Unclear | 80–100 | Unclear | Weak | Weak |
| 117 | Lagarrigue, 199154 (French language) | 1085 | Mean 17.2 months; range 12–4 months | CCS | No | No | 80–100 | Unclear | Weak | Moderate |
| 129 | Lavignolle, 198755 (French language) | 358 | Mean 25 months for surgery and 22 months for chemonucleolysis | RCT | Unclear | Unclear | 80–100 | Unclear | Weak | Weak |
| 889 | Lee, 1996104 (German language) | 300 | 1 year | CCS | No | No | Can’t tell | Unclear | Weak | Weak |
| 593 | Muralikuttan, 199285 | 92 | 1 year | RCT | Yes | Unclear | 80–100 | Unclear | Moderate | Moderate |
| 47 | Norton, 198650 | 105 | At least 1 year | CCS | No | No | NA | Unclear | Weak | Weak |
| 45 | Postacchini, 198749 | 161 | Mean 2.9 years; range 20–38 months in chemonucleolysis. Mean 2.8 years; range 21–42 months in surgery | Non-RCT | No | No | 80–100 | No | Weak | Moderate |
| 617 | Revel, 199388 | 165 | 1 year | RCT | Yes | Unclear | 80–100 | Unclear | Moderate | Weak |
| 641 | Steffen, 199990 (German language) | 69 | 1 year | RCT | Unclear | Unclear | 80–100 | Yes | Weak | Weak |
| 49 | Stula, 199052 (German language) | 69 | Postoperative | RCT | Unclear | Unclear | 80–100 | Unclear | Weak | Weak |
| 61 | Tregonning, 199153 | 268 | 10 years | CCS | No | No | 80–100 | No | Weak | Moderate |
| 893 | Watters,1988105 | 100 | 3 years | Non-RCT | No | No | 80–100 | No | Weak | Weak |
| 160 | Watts, 197559 | 274 | 2 years | CCS | No | No | 80–100 | Unclear | Weak | Weak |
| 672 | Weinstein, 198692 | 159 | Mean 10.3 years; range 10.0–13.5 years | CCS | No | No | 80–100 | NA | Weak | Weak |
| 150 | Zeiger, 198758 | 126 | Range 6–46 months; average time from treatment to follow-up 18 months | CCS | No | No | NA | Yes | Weak | Weak |
| Disc surgery vs epidural | ||||||||||
| 725 | Buttermann, 200495 | 100 | 2–3 years | RCT | Unclear | Unclear | 80–100 | No | Moderate | Moderate |
| Disc surgery vs exercise therapy | ||||||||||
| 300 | Osterman, 200668 | 57 | 2 years | RCT | Yes | Yes | 80–100 | NA | Moderate | Weak |
| Disc surgery vs intraoperative interventions | ||||||||||
| 268 | Aminmansour, 200664 | 61 | 2 months | Q-RCT | No | No | 80–100 | Yes | Weak | Moderate |
| 436 | Bernsmann, 200174 | 200 | Median of 24.2 months after surgery | RCT | Unclear | Unclear | 80–100 | Yes | Moderate | Weak |
| 470 | Debi, 200278 | 70 | 1 year | RCT | Unclear | Partial | 80–100 | No | Weak | Weak |
| 492 | Gerszten, 200381 | 10 | 1 year | RCT | Yes | Unclear | 80–100 | NA | Moderate | Weak |
| 497 | Glasser, 199382 | 32 | 1 month | RCT | Unclear | Unclear | 80–100 | Unclear | Weak | Weak |
| 520 | Jensen, 199683 | 118 | Median 376 days; range 276–501 days | RCT | Unclear | Unclear | 80–100 | Yes | Moderate | Moderate |
| 909 | Jirarattanaphochai, 2007106 | 103 | 3 months | RCT | Yes | Partial | 80–100 | Yes | Moderate | Moderate |
| 400 | Kim, 200373 | 35 | 6 months | RCT | Yes | Yes | 80–100 | NA | Moderate | Weak |
| 551 | Langmayr, 199584 | 26 | 6 months | RCT | Unclear | Unclear | 80–100 | Yes | Moderate | Moderate |
| 366 | Lavyne, 199270 | 84 | 6 weeks | Q-RCT | No | No | 80–100 | Unclear | Weak | Weak |
| 276 | Lundin, 200366 | 80 | 2 years | RCT | Unclear | Unclear | 80–100 | Yes | Moderate | Moderate |
| 270 | MacKay, 199565 | 190 | 1 year | RCT | Unclear | Unclear | 80–100 | Unclear | Weak | Weak |
| 854 | Rasmussen, 2008101 | 200 | 2 years | RCT | Yes | Unclear | 80–100 | Yes | Moderate | Weak |
| 618 | Richter, 200189 | 398 | 6 months | RCT | Yes | Yes | 80–100 | Yes | Moderate | Weak |
| 856 | Ronnberg, 2008102 | 128 | 2 years | RCT | Unclear | Partial | 80–100 | Yes | Weak | Weak |
| 316 | Cengiz, 200769 | 60 | 12 months | RCT | Unclear | Yes | 80–100 | Unclear | Moderate | Weak |
| 915 | de Tribolet, 1998107 | 298 | 6 months | RCT | Yes | Unclear | 80–100 | Yes | Moderate | Moderate |
| Disc surgery vs mixed treatments | ||||||||||
| 734 | Hoogland, 200697 | 280 | 2 years | Q-RCT | No | No | 80–100 | Unclear | Weak | Weak |
| 379 | Prestar, 199571 (German language) | 100 | 1 year | RCT | Unclear | Unclear | 60–79 | Unclear | Weak | Moderate |
| 705 | Starkweather, 200693 | 70 | 6 weeks | RCT | Unclear | Partial | 80–100 | Unclear | Weak | Weak |
| 263 | Wang, 200063 | 145 | 3 days | RCT | Unclear | Unclear | 80–100 | Unclear | Moderate | Moderate |
| Disc surgery vs non-opioids | ||||||||||
| 475 | Dubourg, 200280 | 67 | 6 months | CCS | No | No | 80–100 | No | Weak | Weak |
| 144 | Rossi, 199357 (Italian language) | 40 | 6 months | Non-RCT | Unclear | Unclear | 80–100 | Yes | Weak | Weak |
| Disc surgery vs others | ||||||||||
| 600 | North, 200586 | 60 | 2 years | RCT | Yes | Partial | 60–79 | No | Weak | Moderate |
| Disc surgery vs usual/conventional care | ||||||||||
| 716 | Alaranta, 199094 | 357 (322 with partial rhizography) | 1 year | CCS | No | No | 80–100 | No | Weak | Moderate |
| 386 | Atlas, 199672 | 507 | 10 years | CCS | No | No | 60–79 | NA | Moderate | Moderate |
| 772 | Hansson, 2007100 | 184 | 2 years | CCS | No | No | 80–100 | NA | Weak | Moderate |
| 294 | Koranda, 199567 (Czech language) | 100 | 3 months | Q-RCT | No | No | 80–100 | Unclear | Weak | Moderate |
| 606 | Peul, 200787 | 283 | 1 year (main follow-up visits at 8 weeks, 6 months and 1 year). 2 years’ data reported later | RCT | Yes | Partial | 80–100 | NA | Strong | Strong |
| 211 | Shvartzman, 199262 | 55 | 2 years | HCS | No | No | NA | NA | Weak | Weak |
| 2 | Thomas, 200745 | 623 | 12 months | CCS | No | No | 80–100 | NA | Moderate | Strong |
| 664 | Weber, 198391 | 126 | 10 years | RCT | Unclear | Partial | 60–79 | No | Weak | Moderate |
| 751 | Weinstein, 200699 | 501 | 2 years | RCT | Yes | Yes | 80–100 | NA | Strong | Weak |
| 750 | Weinstein, 200698 | 743 | 2 years | CCS | No | No | 80–100 | NA | Moderate | Weak |
Disc surgery results at short-term follow-up (≤ 6 weeks)
Global effect at short-term follow-up
The results for the global effect at short-term follow-up are presented in Table 6 and the accompanying forest plot (Figure 2). Disc surgery was compared with exercise therapy, chemonucleolysis (which is not widely used in the UK NHS) and intraoperative interventions. Most studies included patients with chronic sciatica.
| ID no. | Author, year | Chronicity | Study design | Follow-up | Outcome measure | Perspective | Intervention | Control | OR (95% CI) | Comments | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n) | Outcome (n) | Withdrawal rate | Total (n) | Outcome (n) | Withdrawal rate | |||||||||
| Disc surgery vs chemonucleolysis | ||||||||||||||
| 471 | Dei-Anang, 199079 (German language) | NR | CCS | 42 days | Reported absence of pain | Patient | 100 | 72 | 0 | 101 | 79 | 0 | 0.72 (0.38 to 1.36) | Data inferred from graphs, presented as percentages |
| 44 | Javid, 199548 | C | CCS | 6 weeks | Successful outcome: good or excellent (vs unsuccessful: slight or no improvement) | Patient | 100 | 92 | 0 | 100 | 82 | 0 | 2.52 (1.04 to 6.11) | |
| 889 |
Lee, 1996104 (German language) (i)a (APLD) |
NR | CCS | 6 weeks | Disappearance of back pain | 100 | 16 | ? | 100 | 16 | ? | 1.00 (0.47 to 2.13) | Number randomised not stated, 300 included in analysis Excluded 29% chemonucleolysis and 14% surgery | |
| 889 |
Lee, 1996104 (German language) (ii)a (PELD) |
NR | CCS | 6 weeks | Disappearance of back pain | 100 | 29 | ? | 100 | 16 | ? | 2.14 (4.08 to 4.26) | Number randomised not stated, 300 included in analysis Excluded 29% chemonucleolysis and 29% surgery | |
| 45 | Postacchini, 198749 | A + C | Non-RCT | 1 month | Satisfactory outcome: good or excellent (vs unsuccessful: fair or poor) | 84 | 52 | 0.03 | 72 | 39 | 0.03 | 1.38 (0.73 to 2.61) | Data inferred from graphs. Five patients lost to follow-up were excluded. Patients who had surgery in chemonucleolysis group regarded as failure | |
| 49 | Stula, 199052 (German language) | C | RCT | Postoperative | Successful outcome: good (vs unsuccessful: unsatisfactory) | Physician | 44 | 40 | 0.76 | 25 | 22 | 0.43 | 1.36 (0.28 to 6.65) | Nineteen patients in chemonucleolysis group received surgery and analysed as surgery group |
| 672 | Weinstein, 198692 | C | CCS | < 6 weeks | Recovered within 2–6 weeks or immediate (vs no recovery, > 12 weeks or 6–12 weeks) | 63 | 39 | 0.11 | 85 | 61 | 0.03 | 0.64 (0.32 to 1.28) | Data presented as percentages | |
| Disc surgery vs exercise therapy | ||||||||||||||
| 300 | Osterman, 200668 | A | RCT | 6 weeks | Reported full recovery | Patient | 28 | 5 | 0.03 | 28 | 0 | 0 | 13.34 (0.70 to 253.89) | |
| Disc surgery vs intraoperative interventions | ||||||||||||||
| 497 | Glasser, 199382 (i)b | C | RCT | 1 month | Radicular pain: complete relief (vs partial or no relief) | 7 | 5 | 0.3 | 9 | 8 | 0.25 | 0.31 (0.02 to 4.41) | ||
| 497 | Glasser, 199382 (ii)b | C | RCT | 1 month | Radicular pain: complete relief (vs partial or no relief) | 7 | 5 | 0.3 | 7 | 6 | 0.3 | 0.42 (0.03 to 6.06) | ||
| 379 | Prestar, 199571 (German language) | NR | RCT | At discharge | Success: pain free, slight lumbar pain, slight radicular pain (vs failure: radicular pain considerably improved, complaint unchanged) | 50 | 41 | 0.0 | 50 | 41 | 0.0 | 1.00 (0.36 to 2.77) | ||
FIGURE 2.
Summary of the findings of the global effect at short-term follow-up (≤ 6 weeks) for studies comparing disc surgery with alternative interventions. CCS, concurrent cohort study; PT, physical therapy. Note: weights are from random effects analysis.
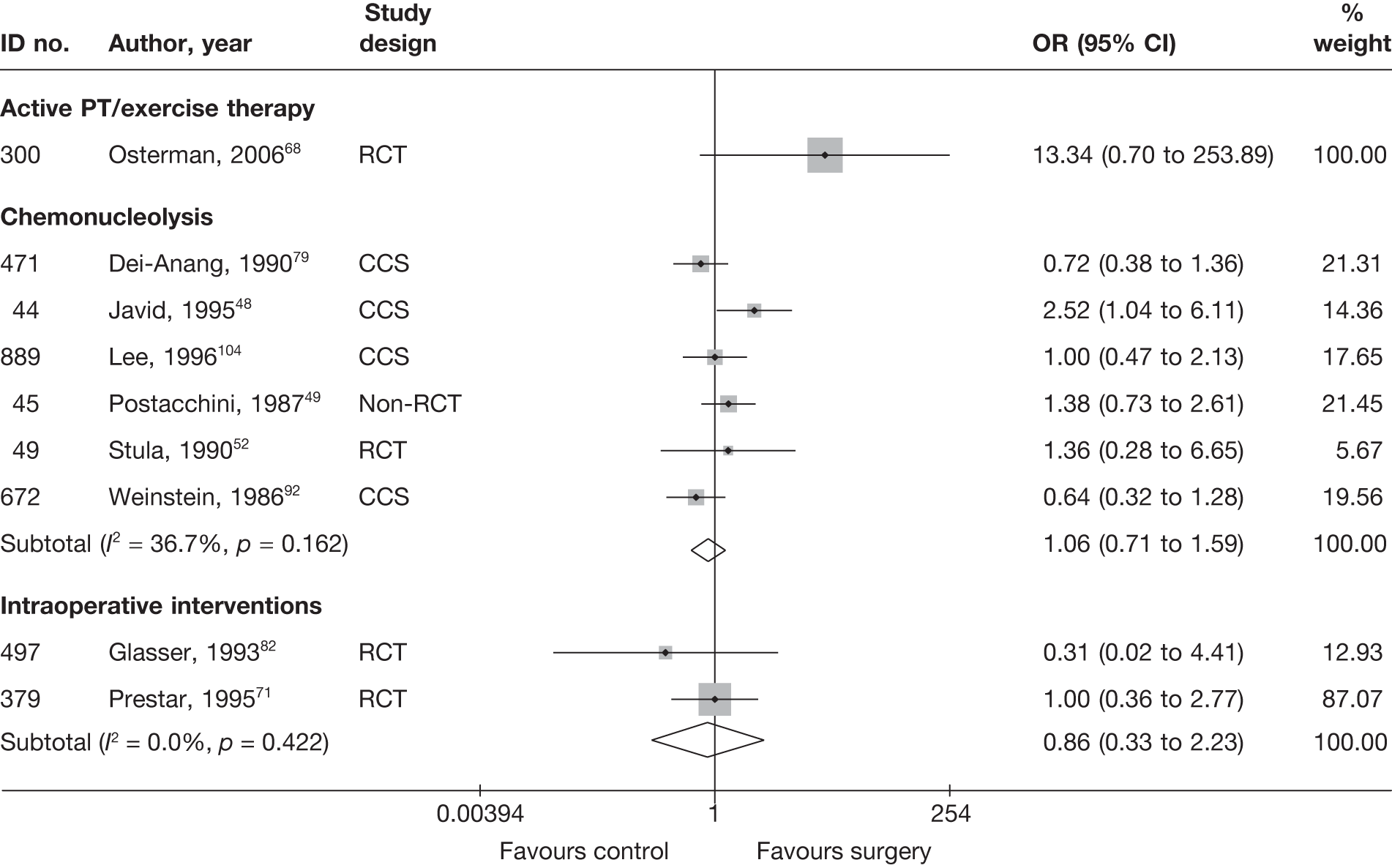
One well-conducted RCT68 compared disc surgery plus exercise therapy with exercise therapy alone for patients with acute sciatica owing to an intervertebral disc extrusion or sequester. Disc surgery plus exercise therapy was found to be superior to exercise therapy alone, but the findings were not statistically significant, probably owing to a lack of power as a result of the analysis of a small sample size (n = 57).
Six studies48,49,52,79,92,104 compared disc surgery with chemonucleolysis, for which there was no overall difference between the groups. Only one of these studies was an RCT,52 which was poorly reported with method of randomisation and allocation concealment not stated. Forty-four patients were randomised to each group, but 19 in the chemonucleolysis group received surgery and were analysed as surgery group patients. The results and methods of the remaining studies were also poorly reported.
Two RCTs71,82 compared surgery with intraoperative interventions and found no overall statistically significant difference.
Pain intensity at short-term follow-up
The results for pain intensity at short-term follow-up are presented in Table 7 and the accompanying forest plot (Figure 3). Disc surgery was compared with usual care, intraoperative interventions, exercise therapy, mixed treatments and chemonucleolysis. Most studies included patients with chronic sciatica.
| ID no. | Author, year | Chronicity | Study design | Follow-up | Location | Scale (range)a | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)b | Comment/conversionc | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |||||||||
| Disc surgery vs chemonucleolysis | ||||||||||||||||
| 453 | Brown, 198976 (i)d (chymopapain) | C | CCS | 6 weeks | Leg | VAS (0–100) | 19 | 51 | 70 | 60 | 3 (20.87) | 22 (25.48) | –19.00 (–30.70 to –7.30) | SD imputed from weighted average | ||
| 453 | Brown, 198976 (ii)d (collagenase) | C | CCS | 6 weeks | Leg | VAS (0–100) | 19 | 15 | 70 | 58 | 3 (20.87) | 46 (25.48) | –43.00 (–58.95 to –27.05) | SD imputed from weighted average | ||
| 593 | Muralikuttan, 199285 | A + C | RCT | 6 weeks | Leg | VAS (0–100) | 46 | 46 | 72 | 64 | 19 (20.87) | 19 (25.48) | 0.0 (–3.52 to 9.52) | SD imputed from weighted average | ||
| 617 | Revel, 199388 | NR | RCT | 1 month | Leg | VAS (0–100) | 62 | 68 | 68.1 (21.6) | 63.4 (24.6) | 39.4 (32.28) | 28.3 (27.21) | 11.10 (0.79 to 21.41) | SD estimated from SE ITT not used Dropouts: 24/165 (15%), group allocation not stated plus further 11/141 (8%); intervention = 7/69, control = 4/72 | ||
| Disc surgery vs exercise therapy | ||||||||||||||||
| 300 | Osterman, 200668 | A | RCT | 6 weeks | Leg | VAS (0–100) | 28 | 28 | 61 (20) | 57 (21) | 12 (20) | 25 (27) | –13.00 (–25.45 to –0.55) | |||
| Disc surgery vs intraoperative interventions | ||||||||||||||||
| 470 | Debi, 200278 | A + C | RCT | 14 days | Leg | VAS (0–10) | 35 | 26 | 71 | 58 | 22 (20.87) | 18 (37.61) | 4.00 (–12.03 to 20.03) |
SD imputed from weighted average Data inferred from graphs |
||
| 909 | Jirarattanaphochai, 2007106 | NR | RCT | 1 month | Leg | NRS (0–10) | 52 | 51 | 80 | 80 | 0 (20.87) | 0 (37.61) |
0.00 (–11.78 to 11.78) |
Median used as mean SD imputed from weighted average ITT using LOCF Dropouts 2/52 (4%): intraoperative 1/51, surgery 2/52 |
||
| 400 | Kim, 200373 | NR | RCT | 30 days | Leg | Composite score (0–100) | 12 | 23 | 65.8 (16.7) | 57.8 (18.4) | 25 (28.2) | 13.2 (18.8) | –40.8 (30) | –44.60 (29.7) |
3.80 (–17.07 to 24.67) |
Pain scale 1–6 (also taking into account when patients had pain); six scores per patient combined into a single score (0–100) Dropouts 2/35 (6%): intervention = 1/23, control = 1/12 |
| 551 | Langmayr, 199584 | A + C | RCT | 8 days | Overall | VAS (0–100) | 12 | 12 | 55 (11.54) | 54 (21.27) | 10 (13.86) | 5 (4.5) |
5.00 (–3.24 to 13.24) Repeated measures analysis: between subjects – use of steroids p = 0.014; within subjects – time preoperative to 8 days postoperative p < 0.001; interaction between time and steroids p = 0.04 |
Data imputed from graph SD estimated from SE Small sample size ITT not used Dropouts 8%: intervention = 1/13, control = 1/13 |
||
| 276 | Lundin, 200366 | C | RCT | 6 weeks | Overall | VAS (0–100) | 42 | 38 | 48 | 54 | 14 (20.87) | 9 (37.61) | 5.00 (–8.52 to 18.52) | SD imputed from weighted average Mean inferred from graphs | ||
| 618 | Richter, 200189 | NR | RCT | 1 month | Leg | VAS (0–10) | 142 | 147 | 75 (14.8) | 78 (14.8) | 20 (22.2) | 22 (22) | –2.00 (–7.10 to 3.10) | SD estimated from IQR Dropouts 109 (27%): intervention = 57/199, control = 52/199 | ||
| Disc surgery vs mixed treatments | ||||||||||||||||
| 705 | Starkweather, 200693 (surgery + non-opioids) | C | RCT | 6 weeks | Overall | VAS (0–100) | 20 | 10 | 70 (13.42) | 66 (18.97) | 21 (26.83) | 6 (6.32) | 15.00 (2.61 to 27.39) | Data extracted from graphs. SD derived from SE | ||
| 263 | Wang, 200063 (surgery + alternative) | C | RCT | 3 days | Leg | VAS (0–10) | 32 | 32 | 75.9 (23.2) | 71.5 (25.5) | 72.4 (23.8) | 29.8 (17.5) | 42.60 (32.36 to 52.84) |
SD calculated from SE eSubgroup analysis of 64/145 (44%) patients who were given preoperative acupuncture ITT not used 13/145 (9%) dropped out, group allocation not stated (intervention = 32/67, control = 32/65) |
||
| Disc surgery vs usual care | ||||||||||||||||
| 606 | Peul, 200787 | A | RCT | 2 weeks | Leg | VAS (0–100) | 140 | 141 | 67.2 (27.7) | 64.4 (21.2) | 28.5 (22.56) | 44.2 (22.64) |
–15.70 (–20.98 to –10.42) Repeated measures analysis, difference between groups: 15.7 (95% CI 11.7 to 19.7) |
SD estimated from SE ITT based on LOCF used, but two patients lost to follow-up early on excluded (intervention = 1, control = 1) | ||
FIGURE 3.
Summary of the findings of pain intensity at short-term follow-up (≤ 6 weeks) for trials and observational studies comparing disc surgery with alternative interventions. PT, physical therapy. Note: weights are from random effects analysis.
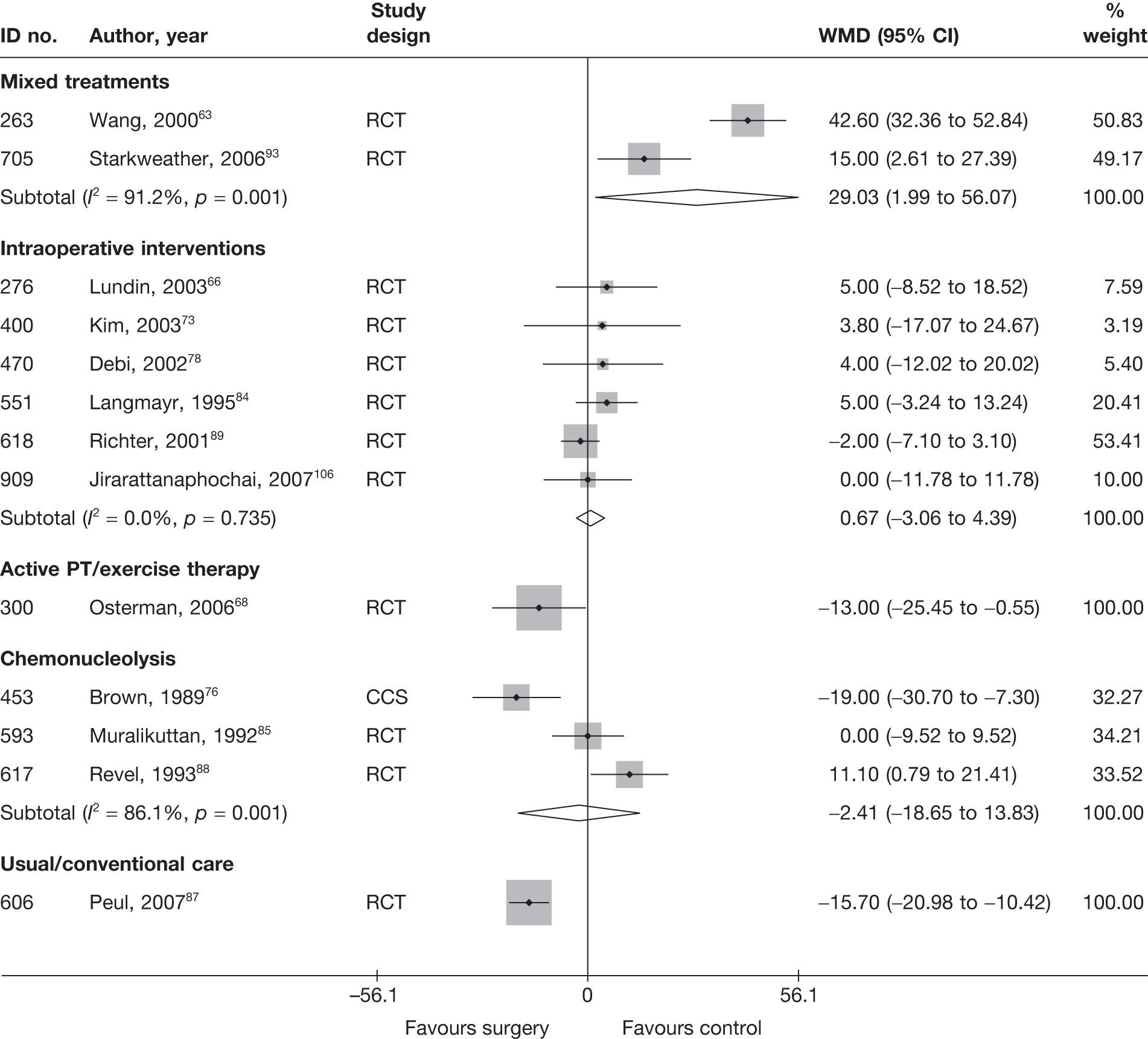
One study based in the Netherlands87 compared early surgical intervention with usual care in patients with severe sciatica for 6–12 weeks. The study was well conducted with good external validity. Patients in the disc surgery group experienced a significantly greater reduction in pain intensity than those who received conventional care (WMD –15.70; 95% CI –20.98 to –10.42). Conventional care included rehabilitation at home supervised by a physiotherapist using a standardised exercise protocol, advice to resume work as soon as possible, pain medication and conservative treatment provided by general practitioners (GPs) (or neurologist where necessary). Microdiscectomy was offered if sciatica persisted for more than 6 months after randomisation. Patients with increasing leg pain not responsive to medication or progressive neurological deficits were offered surgery sooner.
As with global effect, one well-conducted RCT68 found disc surgery plus exercise therapy to be superior to exercise therapy alone for acute sciatica due to an intervertebral disc extrusion or sequestration, but the findings were not statistically significant.
Two studies,63,93 compared disc surgery with mixed treatments: acupuncture plus surgery63 and disc surgery plus non-opioids. 93 Both found that the added intervention was significantly more effective than disc surgery alone for chronic sciatica. Both were poorly reported RCTs. For one study,63 patients in the intervention group were divided into two non-random groups, with half receiving preoperative acupuncture and the other half postoperative acupuncture. The results were reported separately for preoperative and postoperative patients; thus, only those who had preoperative acupuncture are included in the meta-analysis.
Six RCTs66,73,78,84,89,106 compared surgery with intraoperative interventions and found no overall significant difference between treatment groups. Two studies78,84 included patients with either chronic or acute sciatica and one66 included patients who had had sciatica for longer than 3 months; the chronicity of sciatica was not reported in three studies. 73,89,106 Three studies73,89,106 were of moderate to good quality, with adequate randomisation in all three and allocation concealment in two. 73,89
Three studies compared disc surgery with chemonucleolysis: two were RCTs85,88 and one was a concurrent cohort study (CCS). 76 Overall, there was no statistically significant difference between the intervention groups. However, the results were heterogeneous, with the CCS favouring disc surgery and one of the RCTs88 showing statistically significant findings in favour of chemonucleolysis. One study76 included patients who had not received previous disc surgery, whereas the other88 included patients who had had previous surgery and also included a high proportion of men.
Condition-specific outcome measures at short-term follow-up
The results for CSOMs at short-term follow-up are presented in Table 8 and the accompanying forest plot (Figure 4). Disc surgery was compared with usual care, exercise therapy, intraoperative interventions and chemonucleolysis. Most studies included patients with chronic sciatica.
| ID no. | Author, year | Chronicity | Study design | Follow-up | Scale (range)a | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)b | Comment/conversionc | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | ||||||||
| Disc surgery vs chemonucleolysis | |||||||||||||||
| 593 | Muralikuttan, 199285 | A + C | RCT | 6 weeks | Part of Waddell Disability Index | 46 | 46 | 6.7 | 6.2 | 2.8 (1.21) | 3.5 (1.21) | 3.9 | –2.7 | –0.58 (–1.00 to –0.16) | SD for final means calculated from p-values (Mann–Whitney U-test); most outcomes showed skewed distribution |
| 617 | Revel, 199388 | NR | RCT | 1 month | Waddell Disability Index and Main Scale | 62 | 69 | 6 (2.55) | 4.9 (2.49) | 1.5 (3.15) | 1.5 (1.21) | –1.05 | –3.4 | 0.00 (–0.34 to 0.34) | SD derived from SE Dropouts: 24/165 (15%), group allocation not stated plus further 7/141 (5%); intervention = 7/69, control = 3/72 |
| Disc surgery vs exercise therapy | |||||||||||||||
| 300 | Osterman, 200668 | A + C | RCT | 6 weeks | ODI | 28 | 28 | 39 (15) | 39 (14) | 16 (16) | 22 (16) | –23 | –17 | –0.38 (–0.90 to 0.15) | ITT (LOCF), but one patient who did not meet inclusion criteria was excluded from analysis |
| Disc surgery vs intraoperative interventions | |||||||||||||||
| 400 | Kim, 200373 | NR | RCT | 30 days | Composite scale | 12 | 23 | 52.3 (22.7) | 46.9 (21.3) | 32.8 (22.2) | 19.7 (20.5) | –19.4 (25.2) | –27.2 (26) | 0.62 (–0.09 to 1.34) | |
| 366 | Lavyne, 199270 | A + C | Q-RCT | 6 weeks | Scale based on analgesic use, functional status, hospital stay and return to work interval (max score 8) | 36 | 42 | 7.44 (0.13) | 7.3 (0.12) | 1.12 (0.64 to 1.60) | ITT not used Dropouts 6 (7%): intervention = 0/42, control = 6/42 | ||||
| 618 | Richter, 200189 | NR | RCT | 1 month | FFbH-R | 177 | 180 | 50 | 49 | 27.3 (22.48) | 28.7 (22.48) | –0.06 (–0.27 to 0.15) |
SD calculated from weighted average SD for FFbH-R from long-term follow-up disc surgery studies ITT not used Dropouts 109 (27%): intervention = 52/199, control = 57/199 |
||
| Disc surgery vs usual/conventional care | |||||||||||||||
| 606 | Peul, 200787 | A | RCT | 2 weeks | RMDQ | 140 | 141 | 16.5 (4.4) | 16.3 (3.9) | 14.4 (5.92) | 13 (5.96) | –2.1 | –3.3 |
0.24 (0.00 to 0.47) –1.6 (95% CI –2.8 to –0.3), repeated-measures analysis of variance based on final means |
SD derived from SE |
FIGURE 4.
Summary of the findings of CSOMs at short-term follow-up (≤ 6 weeks) for trials comparing disc surgery with alternative interventions (grouped by comparator then ordered by author). PT, physical therapy. Note: weights are from random effects analysis.
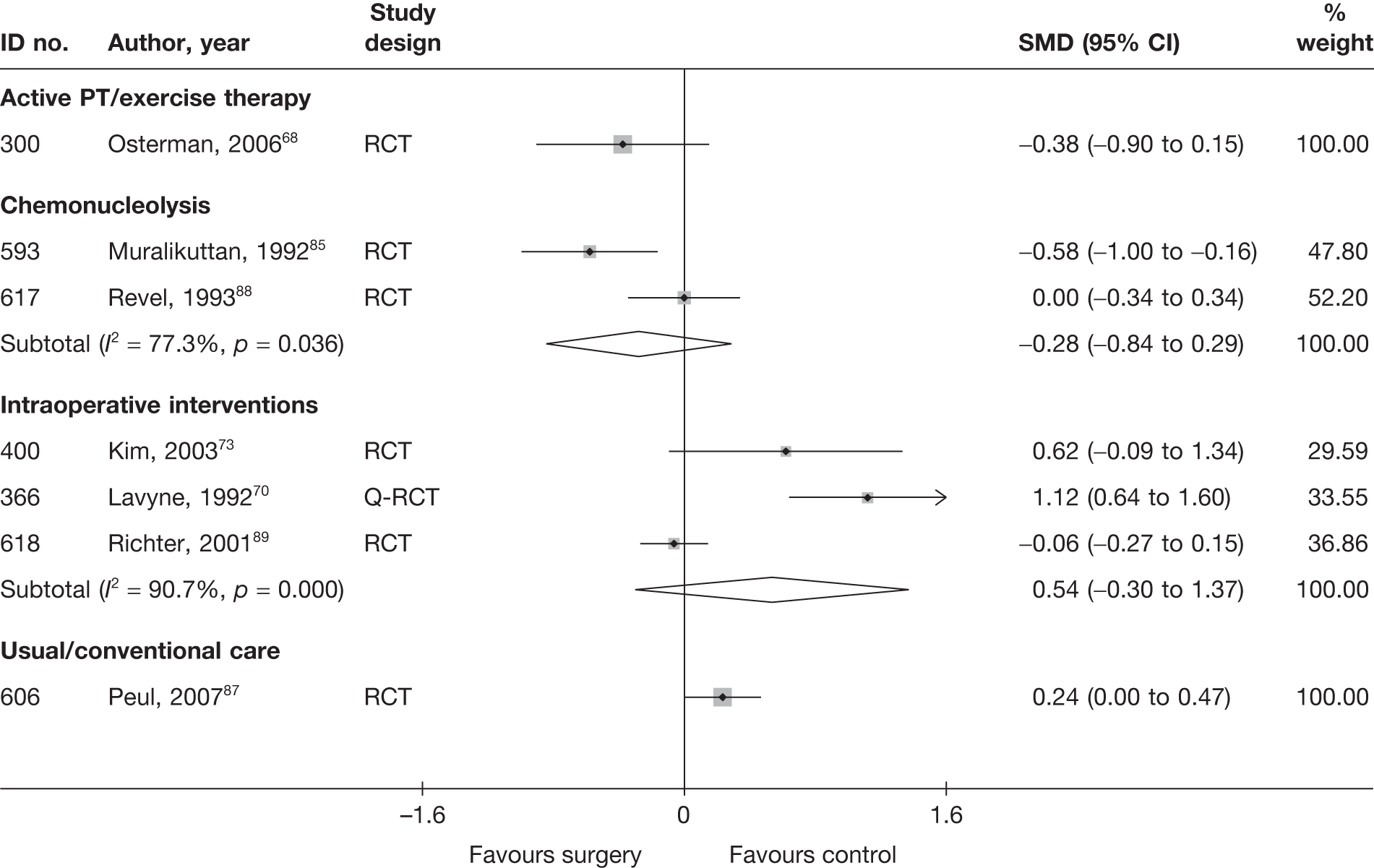
One well-conducted RCT87 compared early surgical intervention with conservative care in patients with severe sciatica for 6–12 weeks. Conservative care included exercise, pain medication and conservative treatment by their GP (or neurologist where necessary). Functional improvement was marginally, but statistically significantly, higher in patients in the conservative or usual care group than in those who received early surgery at 2 weeks. The findings reported by the authors based on repeated-measures analyses showed that patients in the control group had a greater improvement in functional status at 2 weeks (difference between groups for mean RMDQ: –1.6; 95% CI –2.8 to –0.3), which then reversed to show a greater improvement among patients treated with surgery at 8 weeks (difference between groups for mean RMDQ 3.1; 95% CI 1.7 to 4.3). Mean scores plotted over time showed that the curves crossed at 4 weeks.
One well-conducted RCT68 found disc surgery plus exercise therapy to be superior to exercise therapy alone for acute sciatica due to an intervertebral disc extrusion or sequester, but the findings were not statistically significant.
Three studies70,73,89 compared disc surgery with intraoperative interventions, for which the overall findings showed a greater improvement in functional status associated with disc surgery at 4–6 weeks, but the difference between the treatment groups was not statistically significant. The findings were heterogeneous. One study70 included patients with either chronic or acute sciatica, but the chronicity of sciatica was not reported in the remaining two studies. 73,89 Two studies73,89 were RCTs of moderate quality with adequate randomisation and allocation concealment, and the remaining study was a Q-RCT. 70
Two moderate quality RCTs85,88 compared disc surgery with chemonucleolysis. Pooled analysis showed a non-statistically significant difference between the intervention groups in favour of disc surgery.
Disc surgery results at medium-term follow-up (> 6 weeks to ≤ 6 months)
Global effect at medium-term follow-up
The results for the global effect at medium-term follow-up are presented in Table 9 and the accompanying forest plot (Figure 5). Disc surgery was compared with usual care, non-opioids and chemonucleolysis. One further study68 compared disc surgery plus exercise therapy with exercise therapy alone for patients with acute sciatica due to an intervertebral disc extrusion or sequestered disc. Duration of follow-up ranged from 2 to 3 months.
| ID no. | Author, year | Chronicity | Study design | Follow-up | Outcome measure | Perspective | Intervention | Control | OR (95% CI)a | Comments | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n) | Outcome (n) | Withdrawal rate | Total (n) | Outcome (n) | Withdrawal rate | |||||||||
| Disc surgery vs chemonucleolysis | ||||||||||||||
| 453 | Brown, 198976 (i)b (chymopapain) | C | CCS | 3 months | Overall improvement: excellent or good (vs fair, poor or failed) | 19 | 16 | 0 | 51 | 26 | 0 | 5.13 (1.33 to 19.78) | Data reported as percentages | |
| 453 | Brown, 198976 (ii)b (collagenase) | C | CCS | 3 months | Overall improvement: excellent or good (vs fair, poor or failed) | 19 | 16 | 0 | 15 | 9 | 0 | 3.56 (0.71 to 17.76) | Data reported as percentages | |
| 44 | Javid, 199548 | C | CCS | 6 months | Successful outcome: good or excellent (vs slight or no improvement) | Patient | 100 | 85 | 0 | 100 | 88 | 0 | 0.77 (0.34 to 1.75) | |
| 117 | Lagarrigue, 199154 (French language) | C | CCS | 2 months | MacNab criteria: excellent or good (vs mediocre, failure) | Patient and physician | 751 | 675 | 0 | 334 | 238 | 0 | 3.58 (2.56 to 5.01) | Data reported as percentages |
| 889 | Lee, 1996104 (German language) (i)c (APLD) | NR | CCS | 2 months | Disappearance of back pain | 100 | 35 | ? | 100 | 29 | ? | 1.32 (1.73 to 2.39) |
Number randomised not stated, 300 included in analysis Excluded: chemonucleolysis 29%, surgery 14% |
|
| 889 | Lee, 1996104 (German language) (ii)c (PELD) | NR | CCS | 2 months | Disappearance of back pain | 100 | 8 | ? | 100 | 29 | ? | 0.21 (0.09 to 0.49) |
Number randomised not stated, 300 included in analysis Excluded: chemonucleolysis 29%, surgery 29% |
|
| 45 | Postacchini, 198749 | A + C | Non-RCT | 3 months | Successful outcome: excellent or good (vs fair or poor) | 84 | 65 | 0.03 | 72 | 51 | 0.03 | 1.41 (0.69 to 2.90) | Data inferred from graphs. Five lost to follow-up were excluded. Patients who had surgery in chemonucleolysis group regarded as failure | |
| 617 | Revel, 199388 | NR | RCT | 6 months | Treatment success: good or very good (vs none or moderate) | Patient | 69 | 30 | ? | 72 | 44 | ? | 0.49 (0.25 to 0.96) | ITT not used. 24/165 patients dropped out at beginning, group allocation not stated |
| 893 | Watters,1988105 | A + C | Non-RCT | Mean 46 days | Success of surgical results: excellent or good (vs fair or poor) | Physician | 50 | 44 | 0.0 | 50 | 32 | 0.0 | 4.13 (1.47 to 11.56) | Data reported as percentages |
| 672 | Weinstein, 198692 | C | CCS | 3–6 months | Recovered within 6–12 weeks, 2–6 weeks or immediate (vs no recovery or > 12 weeks) | 63 | 53 | 0.11 | 85 | 71 | 0.03 | 1.05 (0.43 to 2.53) | Data reported as percentages | |
| Disc surgery vs exercise therapy | ||||||||||||||
| 300 | Osterman, 200668 | A | RCT | 6 months | Reported full recovery | Patient | 28 | 5 | 0.03 | 28 | 4 | 0 | 1.30 (0.31 to 5.47) | |
| Disc surgery vs non-opioids | ||||||||||||||
| 475 | Dubourg, 200280 | A | CCS | 6 months | Recovery improvement (vs failure) according to change in VAS and muscle strength | 32 | 25 | 0.18 | 25 | 24 | 0.11 | 0.15 (0.02 to 1.30) | ||
| 144 | Rossi, 199357 (Italian language) | C | Non-RCT | 6 months | Patient | ? | 68% | ? | ? | 55% | ? | Data reported as percentages; 40 patients included, but not stated how many were in each group. The study included three intervention groups, but all surgery patients (two groups) were compared with conservative treatment | ||
| Disc surgery vs usual care | ||||||||||||||
| 294 | Koranda, 199567 (Czech language) | C | Q-RCT | 3 months | Effective results: excellent, very good, good (vs satisfactory, poor or worse) | Patient | 54 | 42 | 0.0 | 46 | 27 | 0.0 | 2.46 (1.03 to 5.88) | Duration of follow-up not clear; both groups had 3 months’ conservative treatment then one group received surgery. Patients in control group who required surgery were classified as treatment failure. 28 patients in surgery group did not receive surgery as they got better during the conservative therapy period |
| 606 | Peul, 200787 | A | RCT | 26 weeks | Satisfaction with recovery: ‘complete’ or ‘nearly recovery complete’ on a seven-point Likert scale (other 5 scores = unsatisfactory recovery) | Patient | 140 | 108 | 0.01 | 141 | 100 | 0.01 |
1.38 (0.81 to 2.37) Repeated measurements analysis adjusting for baseline values: 6.6% (95% CI –3.7% to 17.0%) |
Data presented as percentages. ITT using LOCF reported for mean Likert score |
| 750 | Weinstein, 200698 (a) | A + C | CCS | 3 months | Satisfaction with current symptoms: very/somewhat satisfied | Patient | 198 | Change: 54% (SE 3.5) | 0.19 | 211 | Change: 43% (SE 3.4) | 0.18 | Treatment effect 11.3% (95% CI 1.6% to 20.9%) | Only mean percentage change and difference between groups reported. 19/222 patients who chose to be in non-operative group received surgery and 44/521 who chose to be in surgery group did not have surgery. Analysis based on treatment received not initial group allocation |
| 751 | Weinstein, 200699 (b) | A + C | RCT | 3 months | Satisfaction with current symptoms: very/somewhat satisfied | Patient | 466 | Change: 68% (SE 2.3) | 0.11 | 190 | Change: 29% (SE 3.7) | 0.14 | Treatment effect 38.7% (95% CI 30.0% to 47.7%) |
Only mean percentage change and difference between groups reported 472/501 included in ITT analysis using LOCF and longitudinal regression models Crossovers: intervention 117/232 (50%), control 71/240 (30%) |
FIGURE 5.
Summary of the findings of global effect at medium-term follow-up (> 6 weeks to ≤ 6months) for studies comparing disc surgery with alternative interventions. PT, physical therapy. Note: weights are from random effects analysis.
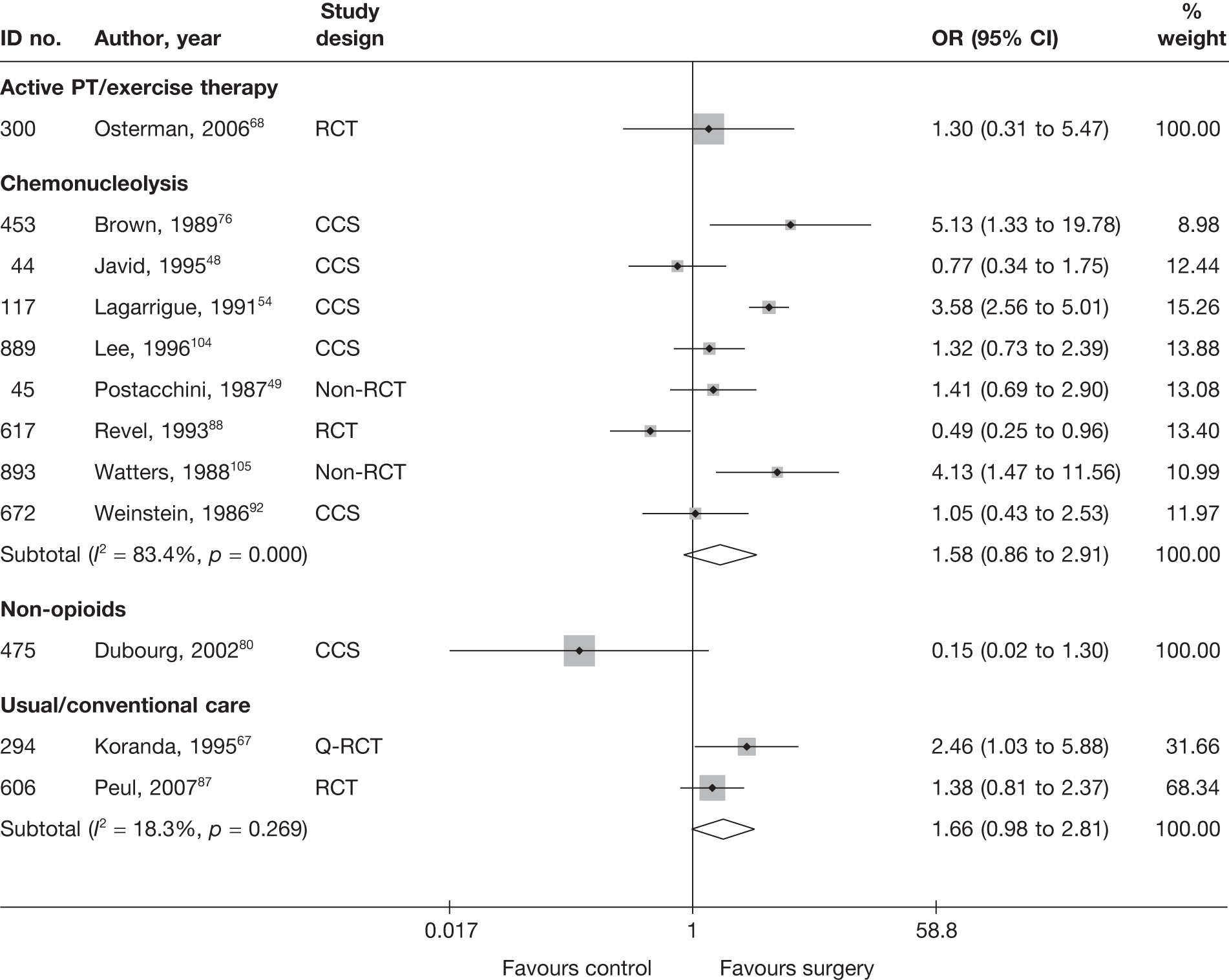
Four studies67,87,98,99 showed that disc surgery was superior to conservative treatment or usual care, but the meta-analysis of two studies67,87 was not statistically significant. One was a well-conducted RCT87 that included patients with acute sciatica and the other was a poorly reported and conducted Q-RCT67 that included patients with chronic sciatica. The remaining two studies98,99 could not be included in the meta-analysis because they only reported the percentage change and difference between groups. One was an RCT [the Spine Patient Outcomes Research Trial (SPORT)]99 and the other a parallel observational cohort study. Both included patients with acute or chronic sciatica. The RCT was well conducted and rated strong for external validity, but recruitment rates were poor and may have been affected by the fact that all patients had already tried non-operative treatment for 6 weeks. Adherence to treatment protocols was also low, with 71/240 (30%) patients in the usual care group having had surgery at 3 months (44 patients at 6 weeks) and only 115/232 (50%) patients in the surgery group having undergone surgery during the same interval (74 patients at 6 weeks). The analyses in both studies were adjusted for a number of covariates including missing data. Both studies reported statistically significant findings in favour of disc surgery.
According to a well-conducted RCT,68 there was no real difference between disc surgery plus exercise therapy and exercise therapy alone in terms of reported full recovery at 6 months in patients with acute sciatica.
One poorly reported CCS80 found non-opioids to be more effective than disc surgery for recovery or improvement in patients with acute sciatica, but the findings were not statically significant. A second poorly conducted study57 found that more patients in the surgery group were satisfied with cure than those in the non-opioids group, but results were only reported as percentages without stating how many patients were in each group.
Eight studies48,49,54,76,88,92,104,105 compared disc surgery with chemonucleolysis, for which there was no overall difference between the groups. Only one of these studies was an RCT,88 of moderate quality, which found chemonucleolysis more effective than disc surgery. However, the withdrawal rate in the surgery group (at least 41%) was much greater than that of the chemonucleolysis group (at least 19%), with dropouts being given a poor outcome in the analysis. The duration, or chronicity of sciatica was not stated. The results and methods of the remaining studies were generally poorly reported. The funnel plot (Figure 6), for publication and other biases, does not appear to show asymmetry, but does not include many studies and demonstrates a lack of large studies.
FIGURE 6.
Funnel plot with pseudo 95% CIs for studies comparing disc surgery with chemonucleolysis at medium-term follow-up (> 6 weeks to ≤ 6months)
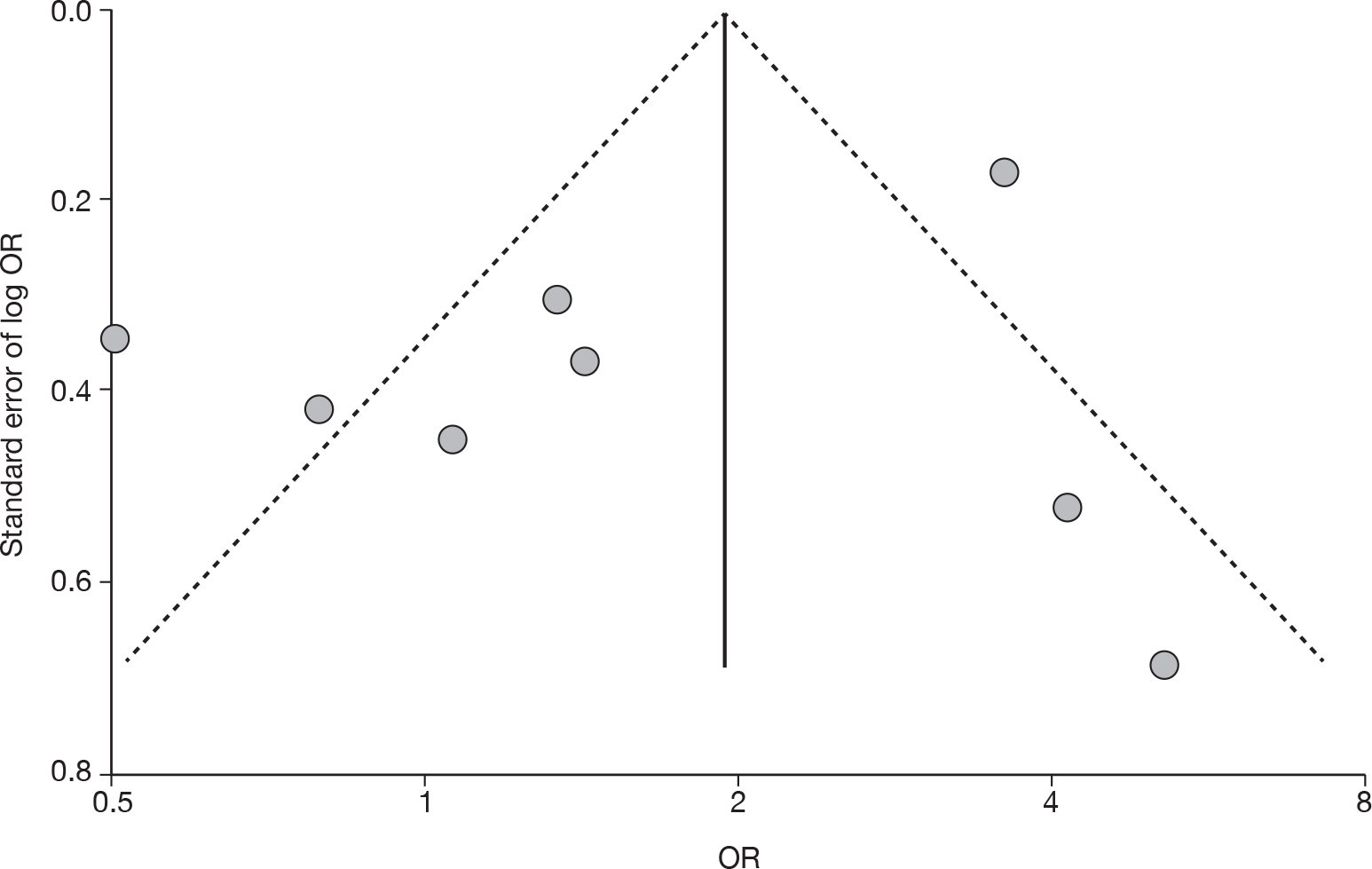
Pain intensity at medium-term follow-up
The results for pain intensity at medium-term follow-up are presented in Table 10 and the accompanying forest plot (Figure 7). Disc surgery was compared with usual care, non-opioids, exercise therapy, epidurals, chemonucleolysis and intraoperative interventions.
| ID no. | Author, year | Chronicity | Study design | Follow-up | Location | Scale (range)a | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)b | Comment/conversionc | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |||||||||
| Disc surgery vs chemonucleolysis | ||||||||||||||||
| 453 | Brown, 198976 (i)d (chymopapain) | C | CCS | 12 weeks | Leg | VAS (0–100) | 19 | 51 | 70 | 60 | 4 (24.43) | 14 (23.76) | –10.0 (–22.77 to 2.77) | SD imputed from weighted average | ||
| 453 | Brown, 198976 (ii)d (collagenase) | C | CCS | 12 weeks | Leg | VAS (0–100) | 19 | 15 | 70 | 58 | 4 (24.43) | 22 (23.76) | –18.0 (–34.29 to –1.71) | SD imputed from weighted average | ||
| 593 | Muralikuttan, 199285 | A + C | RCT | 3 months | Leg | VAS (0–100) | 46 | 46 | 72 | 64 | 14 (24.43) | 20 (23.76) | –6.00 (–15.85 to 3.85) | SD imputed from weighted average Most outcomes showed skewed distribution | ||
| 617 | Revel, 199388 | NR | RCT | 6 months | Leg | VAS (0–100) | 69 | 72 | 68.1 (21.6) | 63.4 (24.61) | 35.6 (34.89) | 17.6 (23.76) | 18.00 (8.11 to 27.89) |
SD estimated from SE 24 patients excluded from analysis, group allocation not stated |
||
| Disc surgery vs epidural/intradiscal injection | ||||||||||||||||
| 725 | Buttermann, 200495 | C | RCT | 4–6 months | Leg | VAS (0–10) | Significant less pain experienced by surgery group at 1–3 months’ and 4–6 months’ follow-up: p < 0.0001 and p = 0.03 respectively, Student’s t-test | No data reported | ||||||||
| Disc surgery vs exercise therapy | ||||||||||||||||
| 300 | Osterman, 200668 | A | RCT | 6 months | Leg | VAS (0–100) | 28 | 28 | 61 (20) | 57 (21) | 9 (20) | 18 (29) | –9.00 (–22.05 to 4.05) | |||
| Disc surgery vs intraoperative interventions | ||||||||||||||||
| 268 | Aminmansour, 200664 (i)e (40 mg dexamethasone) | NR | Q-RCT | 2 months | Leg | VAS (0–10) | 22 | 19 | 55.5 (14.3) | 54.2 (15) | 28.2 (26.7) | 11.6 (12.4) | 16.60 (4.13 to 29.07) | |||
| 268 | Aminmansour, 200664 (ii)e (80 mg dexamethasone) | NR | Q-RCT | 2 months | Leg | VAS (0–10) | 22 | 20 | 55.5 (14.3) | 53 (13.4) | 28.2 (26.7) | 11.5 (14.4) | 16.70 (3.88 to 29.52) | |||
| Disc surgery vs non-opioids | ||||||||||||||||
| 475 | Dubourg, 200280 | A | CCS | 6 months | Overall | VAS (0–100) | 36 | 28 | 52.2 (28.5) | 47.7 (34) | 13.2 (18.8) | 14.8 (20.6) | –1.60 (–11.39 to 8.19) | Dropouts 7/67 (10%): intervention 4/39, control 3/28 | ||
| 909 | Jirarattanaphochai, 2007106 | NR | RCT | 3 months | Leg | NRS (0–10) | 52 | 51 | 80 | 80 | 0 (24.43) | 0 (19.98) | 4.80 | 0.0 (–8.61 to 8.61) |
Median used as mean SD imputed from weighted average ITT using LOCF Dropouts 2/52 (4%): intraoperative 1/51, surgery 2/52 |
|
| 400 | Kim, 200373 | NR | RCT | 6 months | Leg | Composite scale (0–100) | 11 | 22 | 65.8 (16.7) | 57.8 (18.4) | 20.6 (29.4) | 15.8 (16) | –44.2 (32.5) | –40.9 (27.8) | 4.80 (–13.82 to 23.42) |
Pain scale 1–6 (also taking into account when patients had pain); six scores per patient combined into a single score (0–100) Change scores used for the meta-analysis. Dropouts 2/35 (6%): intervention 1/23, control 1/12 |
| 551 | Langmayr, 199584 | A + C | RCT | 6 months | Overall | VAS (0–100) | 12 | 12 | 55 (11.54) | 54 (21.27) | 5 (24.43) | 4 (19.98) |
1.00 (–16.86 to 18.86) Repeated-measures analysis: between subjects – use of steroids p = 0.014; within subjects – time preoperative to 8 days postoperative p < 0.001; interaction between time and steroids p = 0.04 |
SD imputed from weighted average Small sample size Dropouts 8%: intervention 1/13, control 1/13 | ||
| 276 | Lundin, 200366 | C | RCT | 26 weeks | Overall | VAS (0–100) | 42 | 38 | 48 | 54 | 10 (24.43) | 8 (19.98) | 2.00 (–7.74 to 11.74) | SD imputed from weighted average Mean inferred from graphs | ||
| 854 | Rasmussen, 2008101 | NR | RCT | 2 months | Leg | Composite NRS (0–30) | 100 | 100 | 68.3 | 70 | 43.3 (24.43) | 23.3 (19.98) | 20.0 (13.81 to 26.19) | Median used to represent mean SD imputed from weighted average Three separate pain measures using NRS (0–10) combined: pain now, worst, and average pain in the last 2 weeks, for back and leg pain separately | ||
| 618 | Richter, 200189 | NR | RCT | 6 months | Leg | VAS (0–10) | 176 | 180 | 75 (14.8) | 78 (14.8) | 20 (25.9) | 23 (29.6) | –3.00 (–8.77 to 2.77) |
SD estimated from IQR ITT not used Dropouts 42 (11%): intervention 23/199, control 19/199 |
||
| Disc surgery vs usual care | ||||||||||||||||
| 606 | Peul, 200787 | A | RCT | 26 weeks | Leg | VAS (0–100) | 140 | 141 | 67.2 (27.7) | 64.4 (21.2) | 8.4 (22.56) | 14.5 (22.64) |
–6.10 (–11.38 to –0.82) Repeated measures analysis, difference between groups: 6.1 (95% CI 2.2 to 10.0) |
SD estimated from SE ITT based on LOCF used, but two patients lost to follow-up early on excluded (intervention = 1, control = 1) |
||
FIGURE 7.
Summary of the findings of pain at medium-term follow-up (> 6 weeks to ≤ 6 months) for studies comparing disc surgery with alternative interventions. PT, physical therapy. Note: weights are from random effects analysis.
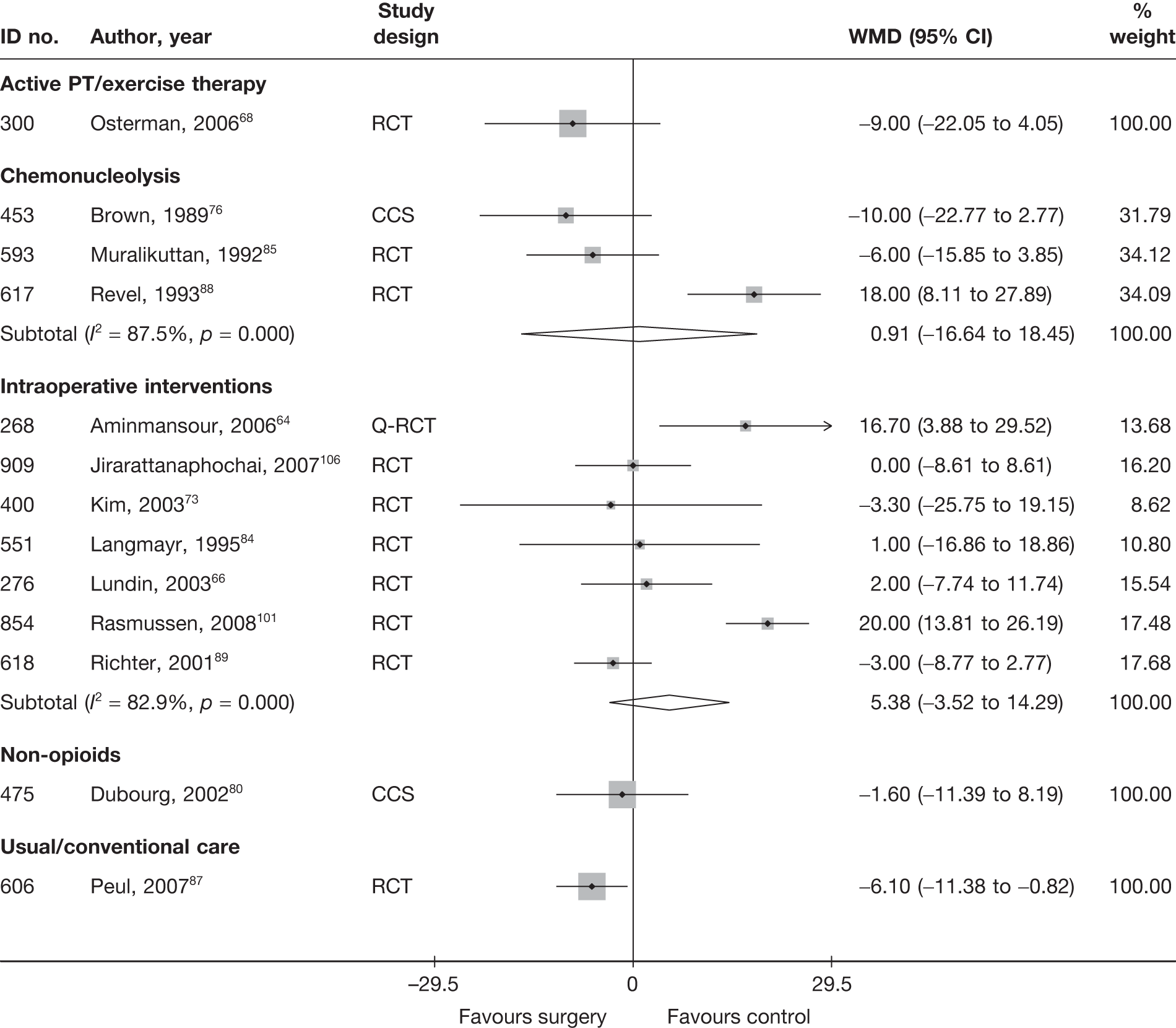
One well-conducted RCT87 showed that early surgical intervention, compared with usual care, resulted in a statistically significantly greater reduction in pain intensity in patients with severe sciatica for 6–12 weeks. However, the size of the effect, or reduction in pain, at 6 months was less than that at 2 weeks (WMD –6.10; 95% CI –11.38 to –0.82).
One poorly reported CCS80 found no important difference between disc surgery and non-opioids in reduction in pain intensity at 6 months.
As with the global effect, one well-conducted RCT68 found non-statistically significant findings in favour of disc surgery plus exercise therapy, compared with exercise therapy alone, in patients with acute sciatica at 6 months’ follow-up.
One poorly reported RCT95 compared the use of epidurals with disc surgery in patients with chronic sciatica [mean 3.55 months, standard deviation (SD) 2.75 months], and found that patients in the disc surgery group experienced significant less leg pain at 1–3 months’ and 4–6 months’ follow-up than those in the control group (p < 0.0001 and p = 0.03 respectively; Student’s t-test). The methods of randomisation and allocation concealment were not reported.
Six RCTs66,73,84,89,101,106 and one Q-RCT64 compared surgery with intraoperative interventions and found no overall statistically significant difference between treatment groups. The results were heterogeneous, with two studies64,101 reporting statistically significant findings in favour of intraoperative interventions. One study84 included patients with acute and chronic sciatica (median symptom duration 35 days, range 14–150 days) and one66 included patients with chronic sciatica (mean 4.5 months); duration of symptoms was not stated in the remaining studies. Duration of follow-up ranged from 2 months to 6 months. Four studies73,89,101,106 were of moderate to good quality, with adequate randomisation in all four and allocation concealment in two. 73,89
As with pain at short-term follow-up, these studies compared disc surgery with chemonucleolysis; two were RCTs85,88 and one was a CCS. 76 Overall, there was no statistically significant difference between the intervention groups, but again the results were heterogeneous, with one study88 showing statistically significant findings in favour of chemonucleolysis. This study included patients who had had previous surgery and also included a high proportion of men.
Condition-specific outcome measures at medium-term follow-up
The results for CSOMs at medium-term follow-up are presented in Table 11 and the accompanying forest plot (Figure 8). Disc surgery was compared with usual care, exercise therapy, epidural, intraoperative interventions and chemonucleolysis.
| ID no. | Author, year | Chronicity | Study design | Follow-up | Scale (range)a | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)b | Comment/conversionc | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | ||||||||
| Disc surgery vs chemonucleolysis | |||||||||||||||
| 727 | Ejeskar, 198396 | A + C | RCT | 6 months | Composite score | 14 | 15 | 9.71 (4.79) | 9.27 (6.62) | 0.08 (–0.65 to 0.80) | |||||
| 593 | Muralikuttan, 199285 | A + C | RCT | 3 months | Part of Waddell Disability index | 46 | 46 | 6.7 | 6.2 | 2.3 (1.28) | 3 (1.28) | –4.4 | –3.2 | –0.55 (–0.96 to –0.13) | SD for final means calculated from p-values (Mann–Whitney U-test); most outcomes showed skewed distribution |
| 617 | Revel, 199388 | NR | RCT | 6 months | Waddell Disability Index and Main Scale | 69 | 72 | 6 (3.9) | 4.9 (2.55) | 3.4 (3.32) | 2.3 (4.65) | –2.6 | –2.6 | 0.27 (–0.06 to 0.60) |
SD calculated from SE Dropouts 24/165 (15%): group allocation not stated |
| Disc surgery vs epidural | |||||||||||||||
| 725 | Buttermann, 200495 | A + C | RCT | 1–3 months | ODI | 50 | 50 | Significantly greater decrease in disability in discectomy group compared with epidural; p < 0.015, Student’s t-test | |||||||
| Disc surgery vs exercise therapy | |||||||||||||||
| 300 | Osterman, 200668 | A | RCT | 6 months | ODI | 28 | 28 | 39 (15) | 39 (14) | 8 (12) | 12 (15) | –31 | –27 | –0.29 (–0.82 to 0.23) | ITT used LOCF, but one patient that did not meet inclusion criteria excluded from analysis |
| Disc surgery vs intraoperative interventions | |||||||||||||||
| 909 | Jirarattanaphochai, 2007106 | NR | RCT | 3 months | ODI | 52 | 51 | 49 (16) | 54 (15) | Repeated measures of analysis of variance using generalised estimating equation models: –0.52 (95% CI –3.91 to 2.87), p = 0.763 |
ITT used LOCF Dropouts 3/103 (3%): intervention 2/52, control 1/51 |
||||
| 400 | Kim, 200373 | NR | RCT | 6 months | Composite scale | 11 | 22 | 52.3 (22.7) | 46.9 (21.3) | 19.4 (23.3) | 17.6 (19.8) | –30.4 (25.8) | –28.1 (21.7) | 0.09 (–0.64 to 0.81) | |
| 618 | Richter, 200189 | NR | RCT | 6 months | FFbH-R | 177 | 180 | 50 | 49 | 20 (22.48) | 21.5 (22.48) | –0.07 (–0.27 to 0.14) |
SD calculated from weighted average SD for FFbH-R from long-term follow-up disc surgery studies ITT not used Dropouts 42 (11%): intervention 19/199, control 23/199 |
||
| 915 | de Tribolet, 1998107 | NR | RCT | 6 months | 128 | 128 | 1.58 (0.99) | 1.24 (1.02) | 0.34 (0.09 to 0.59) | ||||||
| Disc surgery vs usual/conventional care | |||||||||||||||
| 386 | Atlas, 199672 | C | CCS | 6 months | Modified RMDQ | 236 | 181 | 17.8 (4) | 13.6 (5.9) | 7 (4) | 9.7 (5.9) | –10.8 | –3.9 | Data inferred from graphs. No SDs reported. Baseline SD taken from 10-year follow-up data (see Condition-specific outcome measures at long-term follow-up), but this does not relate to same number of patients. Same SDs used for final means | |
| 606 | Peul, 200787 | A | RCT | 26 weeks | RMDQ | 140 | 141 | 16.5 (4.4) | 16.3 (3.9) | 4 (5.94) | 4.8 (5.96) | –12.5 | –11.5 | –0.13 (–0.37 to 0.10) | ITT using LOCF, but two patients lost to follow-up early on were not included in analysis; Number randomised: intervention 141, control 142, baseline data based on all patients (sensitivity analysis showed no difference between ITT and non-ITT) |
| 751 | Weinstein, 200699 | A + C | RCT | 3 months | MODEMS version of ODI | 198 | 211 | 47.5 (21.4) | 46.3 (20.6) | 21.5 (21.4) | 25 (20.6) | –26 (23.92) | –21.3 (23.24) | Adjusted difference between groups based on change scores: –4.7 (95% CI –9.3 to –0.2) |
Baseline SD used for final mean ITT using LOCF and longitudinal mixed model controlling for covariates associated with missing values, but only included 472/501 patients with baseline data Dropouts: intervention 47/245 (19%), control 45/256 (18%) Crossovers: intervention 117/232 (50%), control 71/240 (30%) |
| 750 | Weinstein, 200698 | A + C | CCS | 3 months | MODEMS version of ODI | 466 | 190 | 56.7 (18.9) | 35.9 (20.1) | 20.6 (18.9) | 15 (20.1) | –36.1 (18.78) | –20.9 (20.68) | Adjusted difference between groups based on change scores: –15.2 (95% CI –18.5 to –11.8) |
Baseline SD used for final mean Dropouts 87/743 (12%): intervention 55/521, control 32/222. 19/222 patients who chose to be in the non-operative group received surgery and 44/521 who chose to be in the surgery group did not have surgery Analysis based on treatment received, not initial group allocation |
FIGURE 8.
Summary of the findings of CSOMs at medium-term follow-up (> 6 weeks to ≤ 6months) for studies comparing disc surgery with alternative interventions. PT, physical therapy. Note: weights are from random effects analysis.
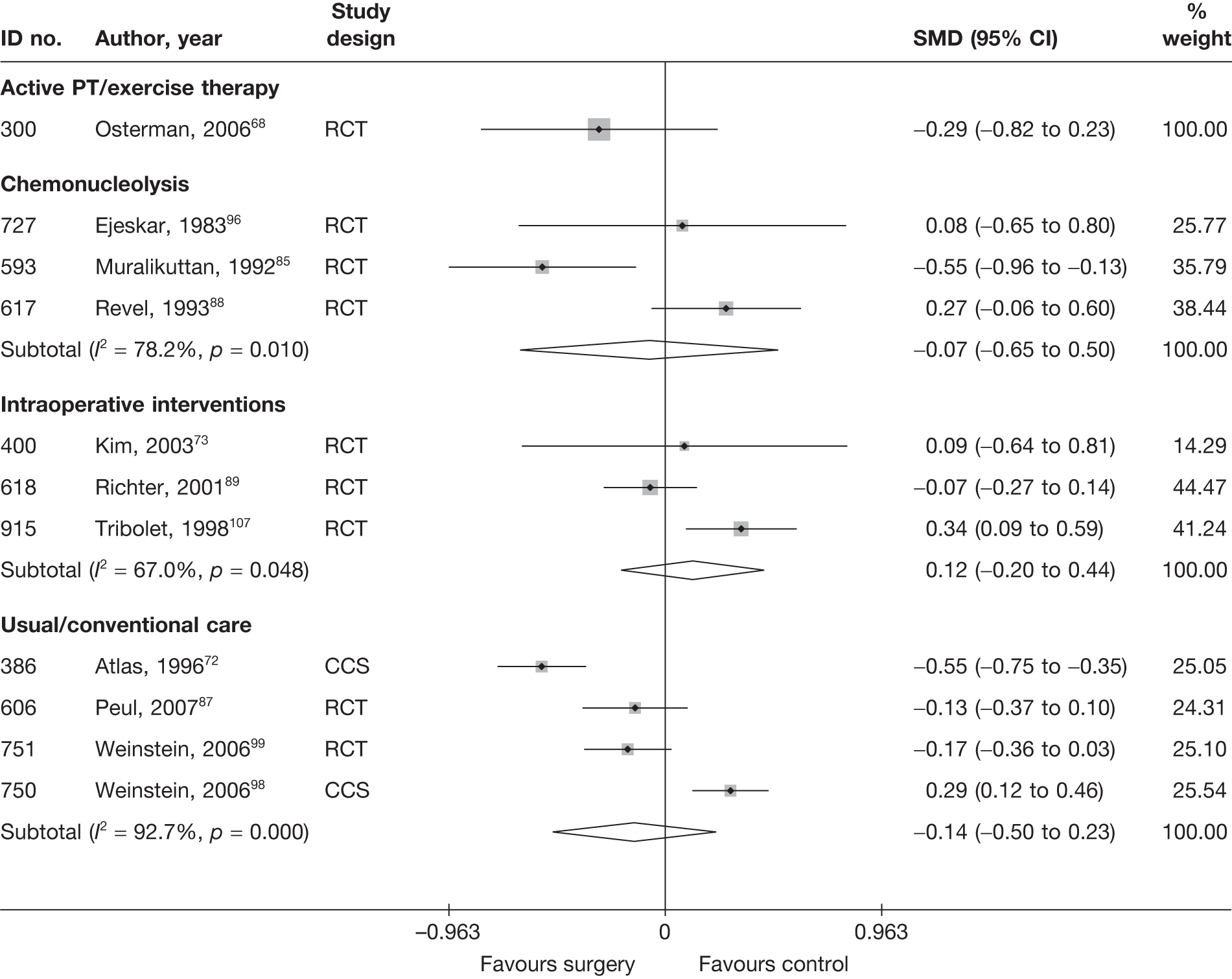
Four studies72,87,98,99 compared disc surgery with usual care, for which the pooled findings showed no statistically significant difference between the intervention groups at 3–6 months. However, the findings were very heterogeneous, with one CCS reporting statistically significant findings in favour of surgery and another CCS reporting statistically significant findings in favour of usual care. Pooled analysis of the two well-conducted RCTs showed marginally statistically significant findings in favour of surgery (SMD –0.15; 95% CI –0.30 to –0.00; the findings were homogeneous I2 = 0%, p = 0.84).
One well-conducted RCT68 found non-statistically significant findings in favour of disc surgery plus exercise therapy compared with exercise therapy alone in patients with acute sciatica at 6 months’ follow-up.
One poorly reported RCT95 compared the use of epidurals with disc surgery in patients with chronic sciatica. The methods of randomisation and allocation concealment were not stated and insufficient data were reported to estimate the mean difference between the intervention groups. The authors reported that there was a significantly greater decrease in disability in the discectomy group than in the epidural group at the 1–3 month follow-up interval (p < 0.015, Student’s t-test).
Four moderate RCTs73,89,106,107 compared disc surgery with intraoperative interventions. Pooled analysis for three RCTs73,89,107 showed no overall statistically significant difference between treatment groups at 6 months. The fourth RCT106 did not report arm-level data, but also found no statistically significant difference between the intervention groups (at 3 months), based on repeated measures of analysis of variance using generalised estimating equation models (difference between groups –0.52, 95% CI –3.91 to 2.87, favouring intraoperative group; p = 0.763).
Three RCTs85,88,96 compared disc surgery with chemonucleolysis, for which pooled analyses showed no important difference between the intervention groups at 3–6 months. However, the findings were heterogeneous.
Results at long-term follow-up (> 6 months)
Global effect at long-term follow-up
The results for the global effect at long-term follow-up are presented in Table 12 and the accompanying forest plot (Figure 9). Disc surgery was compared with usual care, active physical therapy (PT), intraoperative interventions, mixed treatments, chemonucleolysis and spinal cord stimulation (others). Duration of follow-up ranged from 1 year to 10 years. Most studies included patients with chronic sciatica or a mixture of chronic and acute symptoms.
| ID no. | Author, year | Chronicity | Study design | Follow-up | Outcome measure | Perspective | Intervention | Control | OR (95% CI)a | Comments | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n) | Outcome (n) | Withdrawal rate | Total (n) | Outcome (n) | Withdrawal rate | |||||||||
| Disc surgery vs chemonucleolysis | ||||||||||||||
| 884 | Alexander, 1989103 | C | CCS | Mean 14 (range 6–35) months | Satisfactory clinical outcome (vs unsatisfactory results) | Physician | 49 | 39 | 0 | 51 | 40 | 0 | 1.07 (0.41 to 2.81) | Follow-up differed in each group: surgery mean 12 months (range 6–24 months), chemonucleolysis mean 16 months (range 6–35 months) |
| 43 | van Alphen, 198947 | C | RCT | 12 months | Satisfied with final result of treatment: yes or largely (vs barely or no) | Patient | 77 | 61 | 0.01 | 73 | 53 | 0 | 1.44 (0.68 to 3.06) | |
| 441 | Bonafe, 199375 (French language) | A + C | CCS | 1 year | Overall treatment success using modified MacNab criteria: excellent or good (vs satisfactory or worse) | 20 | 11 | 0 | 20 | 16 | 0 | 0.31 (0.07 to 1.25) | ||
| 166 | Crawshaw, 198460 | NR | RCT | 1 year | Overall outcome: excellent or good (vs poor) | 26 | 23 | 0 | 24 | 11 | 0 | 9.06 (2.13 to 38.49) | ||
| 48 | Dabezies, 197851 | NR | CCS | 2 years | Treatment outcome: excellent or good (vs unimproved) | Patient | 100 | 63 | 0 | 100 | 71 | 0 | 0.70 (0.38 to 1.26) | |
| 132 | Hoogmartens, 197656 | C | HCS | Mean 49 months | Satisfactory result for radicular pain: excellent or good (vs fair or poor) | 53 | 37 | 0 | 44 | 24 | 0 | 1.93 (0.84 to 4.44) |
Data inferred from percentages Follow-up differed for the two groups: surgery mean 58 months, chemonucleolysis mean 38 months |
|
| 44 | Javid, 199548 | C | CCS | 1 year | Successful outcome: good or excellent (vs slight or no improvement) | Patient | 100 | 82 | 0 | 100 | 87 | 0 | 0.68 (0.31 to 1.48) | |
| 129 | Lavignolle, 198755 (French language) | NR | RCT | Mean: surgery 24 months, chemonucleolysis 2 months | Overall success: MacNab type scores: good or medium (vs mediocre or bad) | 182 | 150 | 0 | 176 | 141 | 0 | 1.16 (0.68 to 1.98) | ||
| 889 | Lee, 1996104 (German language) (i)b (APLD) | NR | CCS | 1 year | Results of treatment: very good or good; (vs moderate or bad) | Patient | 100 | 48 | ? | 100 | 55 | ? | 1.74 (0.98 to 3.09) |
Number randomised not stated, 300 included in analysis Excluded: chemonucleolysis 29%, surgery 29% |
| 889 | Lee, 1996104 (German language) (ii)b (PELD) | NR | CCS | 1 year | Results of treatment: very good or good; (vs moderate or bad) | Patient | 100 | 68 | ? | 100 | 55 | ? | 0.76 (0.43 to 1.32) |
Number randomised not stated, 300 included in analysis Excluded: chemonucleolysis 29%, surgery 14% |
| 593 | Muralikuttan, 199285 | A + C | RCT | 1 year | Completely pain free (vs residual back pain only or residual back and referred pain) | 46 | 14 | 0 | 46 | 8 | 0 | 2.08 (0.77 to 5.58) |
Reported as percentages One patient crossed over to surgery |
|
| 47 | Norton, 198650 | A + C | CCS | ≥ 1 year | Treatment success: satisfactory (vs unsatisfactory) based on patient and physician report | Patient + physician | 44 | 26 | 0 | 61 | 17 | 0 | 3.74 (1.64 to 8.50) | |
| 45 | Postacchini, 198749 | A + C | Non-RCT | > 20 months | Treatment success: excellent or good (vs fair or poor) | Patient + physician | 84 | 70 | 0.03 | 72 | 54 | 0.03 | 1.67 (0.76 to 3.65) |
Data inferred from graphs Five lost to follow-up were excluded |
| 617 | Revel, 199388 | NR | RCT | 1 year | Overall success rate | Patient | 69 | 25 | > 0.41 | 72 | 48 | > 0.19 | 0.28 (0.14 to 0.57) |
High dropout rate 24/165 excluded patients dropped out at beginning, group allocation not stated A further 30% dropped out (surgery 28/69; chemonucleolysis 14/72), but included in analysis (given poor outcome) |
| 641 | Steffen, 199990 (German language) | C | RCT | 1 year | MacNab criteria: good or very good (vs satisfactory or poor) | 36 | 11 | 0 | 33 | 17 | 0 | 0.41 (0.15 to 1.11) | Reported as percentages only | |
| 61 | Tregonning, 199153 | C | CCS | 10 years | MacNab criteria: excellent or good (vs fair or poor) | 91 | 51 | 0.13 | 145 | 47 | 0.12 | 2.66 (1.55 to 4.56) | ||
| 160 | Watts, 197559 | C | CCS | 2 years | Overall outcome: successful (vs failure) | 174 | 134 | 0 | 100 | 59 | 0 | 2.33 (1.37 to 3.96) | ||
| 672 | Weinstein, 198692 | C | CCS | > 1 year | Recovered within > 12 weeks, 6–12 weeks, 2–6 weeks or immediate (vs no recovery) | 63 | 56 | 0.11 | 88 | 77 | 0.03 | 0.83 (0.28 to 2.43) | ||
| 150 | Zeiger, 198758 | A + C | CCS | Mean 18 months (range 6–46 months) | Current level of discomfort: pain free or improvement (vs no better or worse) | Patient | 81 | 72 | 0 | 45 | 27 | 0 | 5.33 (2.14 to 13.31) | Results included seven surgery patients who had had reoperation; five with good results |
| Disc surgery vs exercise therapy | ||||||||||||||
| 300 | Osterman, 200668 | A | RCT | 2 years | Full recovery | Patient | 28 | 7 | 0.03 | 28 | 5 | 0 | 1.53 (0.42 to 5.58) | |
| Disc surgery vs intraoperative interventions | ||||||||||||||
| 436 | Bernsmann, 200174 | NR | RCT | Median 24.2 months | Permanently free of complaints or permanent improvement (vs initially free of complaints then just improvement, same complaints, initially improvement then same complaints, initially improvement then worse or no effect) | Patient | 94 | 70 | 0.06 | 92 | 77 | 0.08 | 0.57 (0.28 to 1.17) | |
| 492 | Gerszten, 200381 | C | RCT | 1 year | Pain free or improvement (vs no improvement). Improvement = increases of ≥ 7 points on SF-36 | 5 | 3 | 0 | 5 | 5 | 0 | 0.13 (0.00 to 3.52) | ||
| 520 | Jensen, 199683 | NR | RCT | 1 year | Overall assessment: very satisfied or satisfied little discomfort (vs acceptable some discomfort, unchanged or aggravated) | Patient | 49 | 36 | ? | 50 | 37 | ? | 0.97 (0.40 to 2.38) | 19/118 (16%) dropped out; group allocation not stated |
| 270 | MacKay, 199565 (i)c (gelfoam) | C | RCT | 1 year | Overall outcome: excellent or good (vs fair or poor) | 50 | 40 | ? | 54 | 46 | ? | 0.70 (0.25 to 1.93) | 36/190 excluded from analysis, group allocation not stated (three intervention groups) | |
| 270 | MacKay, 199565 (ii)c (free fat graft) | C | RCT | 1 year | Overall outcome: excellent or good (vs fair or poor) | 50 | 40 | ? | 50 | 42 | ? | 1.14 (0.25 to 1.93) | 36/190 excluded from analysis, group allocation not stated (three intervention groups) | |
| 856 | Ronnberg, 2008102 | NR | RCT | 24 months | MacNab criteria: excellent or good (vs fair or poor) | Physician | 48 | 30 | ? | 60 | 41 | ? | 0.77 (0.44 to 2.94) |
Interim analysis based on first 40/61 patients (65%) to complete 12 months’ follow-up (group allocation of remainder not stated) Dropouts at 6 months 10/61 (16%): intervention 5/20, control 5/20 All included in ITT analysis |
| Disc surgery vs other | ||||||||||||||
| 600 | North, 200586 (spinal cord stimulation) | C | RCT | 2 years | Success: ≥ 50% pain relief and patient satisfaction with treatment rated as success (vs failure) | Patient + physician | 26 | 9 | 0.13 | 24 | 9 | 0.2 | 0.88 (0.28 to 2.80) | |
| Disc surgery vs usual care | ||||||||||||||
| 386 | Atlas, 199672 | C | CCS | 10 years | Improvement in predominant symptom: completely gone, much better or better (vs not improved or worse) | Patient | 207 | 143 | 0.25 | 175 | 107 | 0.25 | 1.42 (0.93 to 2.17) | |
| 606 | Peul, 200787 | A | RCT | 52 weeks | Satisfaction with recovery: ‘complete’ or ‘nearly recovery complete’ on seven-point Likert scale (other 5 scores = unsatisfactory recovery) | Patient | 130 | 106 | 0.08 | 130 | 103 | 0.08 |
1.16 (0.63 to 2.14) Repeated measurements analysis adjusting for baseline values: 2.4% (95% CI: –7.2% to 12.0%) |
Data presented as percentages ITT using LOCF reported for mean Likert score |
| 211 | Shvartzman, 199262 | A | HCS | 2 years | Results categorised as good (vs satisfactory; poor) using composite scale based on functional (work-related) and perceptual (subjective-opinion) criteria | Patient | 25 | 16 | 0 | 30 | 14 | 0 | 2.03 (0.69 to 6.02) | |
| 664 | Weber, 198391 | NR | RCT | 10 years | Overall outcome: good or fair (vs poor or bad) | Physician | 55 | 34 | 0.08 | 66 | 27 | 0 | 2.34 (1.12 to 4.87) |
17 from control group received surgery, but analysed according to randomised group Seven patients registered as permanently incapacitated were categorised as ‘fair’ in final analysis |
| 750 | Weinstein, 200698 (a) | A + C | CCS | 2 years | Satisfaction with current symptoms: very/somewhat satisfied | Patient | 456 | Change: 72% (SE 2.2) | 0.12 | 165 | Change: 49% (SE 4.3) | 0.26 | Adjusted treatment effect 22.4% (95% CI 12.8% to 32.0%) |
Only mean percentage change and difference between groups reported 48/222 patients who chose to be in non-operative group received surgery and 40/521 who chose to be in surgery group did not have surgery Analysis based on treatment received not initial group allocation Sensitivity analyses used to determine the impact of missing data |
| 751 | Weinstein, 200699 (b) | A + C | RCT | 2 years | Satisfaction with current symptoms: very/somewhat satisfied | 186 | Change: 68% (SE 3.4) | 0.24 | 187 | Change: 64% (SE 3.5) | 0.37 | Adjusted treatment effect 4.0% (95% CI –5.6% to 13.5%) |
Only mean percentage change and difference between groups reported ITT included 472/501 using LOCF and longitudinal mixed model controlling for covariates associated with missed visits Crossovers: intervention 92/232 (40%), control 107/240 (45%) |
|
| Disc surgery vs mixed treatments | ||||||||||||||
| 734 | Hoogland, 200697 (discectomy + chemonucleolysis) | C | Q-RCT | 2 years | Satisfaction with results classified as excellent or good (vs fair or not satisfied) | Patient | 119 | 101 | 0.16 | 116 | 108 | 0.16 | 0.42 (0.17 to 1.00) | Reported as percentages only |
| 379 | Prestar, 199571 (German language) (discectomy + non-opioids) | NR | RCT | 1 year | Treatment success: very good or good (vs inadequate or poor). | 34 | 9 | 0.32 | 34 | 13 | 0.32 | 0.58 (0.21 to 1.63) | ||
FIGURE 9.
Summary of the findings of the global effect at long-term follow-up (> 6 months) for studies comparing disc surgery with alternative interventions. HCS, historical cohort study; PT, physical therapy. Note: weights are from random effects analysis.
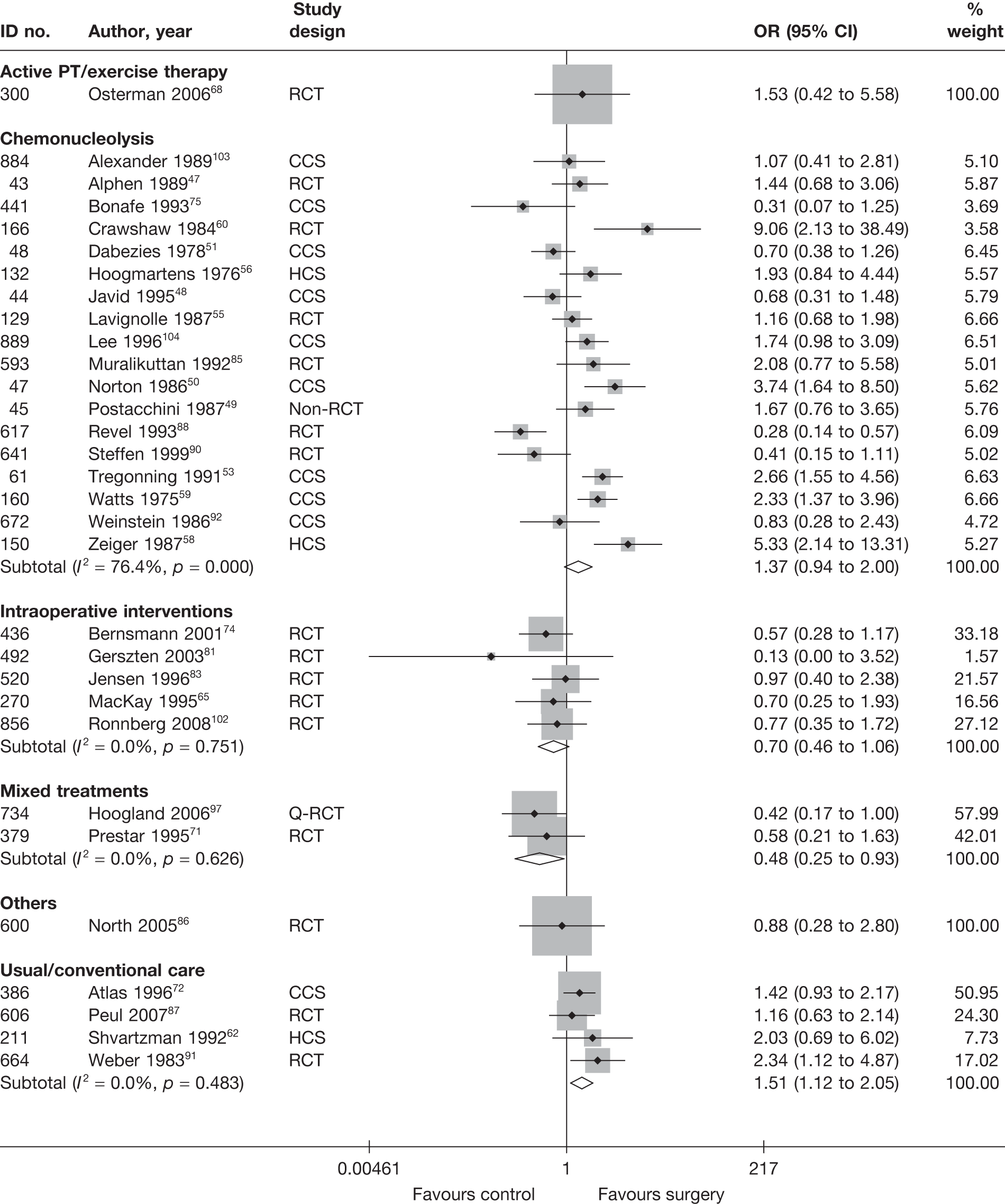
Six studies62,72,87,91,98,99 compared disc surgery with usual care; the overall findings for four62,72,87,91 included in the meta-analysis showed a statistically significant difference in favour of surgery. Two were RCTs, for which the duration of follow-up ranged from 1 year to 10 years. 87,91 Only one RCT,91 which included patients with chronic sciatica, reported statistically significant findings. The overall quality rating for this study was poor, with the method of randomisation not stated and allocation concealment considered partial. The study was published in 1983 and surgical techniques are likely to have changed since then. The remaining RCT87 was published in 2007. It was a well-conducted study that included patients with acute sciatica. Two further studies98,99 could not be included in the meta-analysis because they reported only the percentage change and difference between groups. One was a well-conducted RCT (SPORT)99 and the other a parallel observational cohort study. 98 Both included patients with acute or chronic sciatica. The analyses in both studies were adjusted for a number of covariates including missing data. The treatment effect was much smaller in the RCT99 than in the CCS98 and the findings were not statistically significant. However, adherence to treatment protocols was low in the RCT, with 107/240 (45%) patients in the usual care group having surgery after 2 years and only 140/232 (60%) patients in the surgery group receiving surgery during the same 2-year period.
According to a well-conducted RCT,68 there was no real difference between disc surgery plus exercise therapy and exercise therapy alone in terms of reported full recovery at 2 years in patients with acute sciatica.
Intraoperative interventions were found to be superior to disc surgery alone in five RCTs,65,74,81,83,102 but the overall findings were not statistically significant. One study81 reported a large effect size, but had a very wide CI owing to a small sample size (n = 10).
Two studies71,97 compared disc surgery with mixed treatments: chemonucleolysis plus surgery97 and disc surgery plus non-opioids. 71 Both found non-statistically significant findings in favour of the combined interventions. One was a Q-RCT97 and the other a poor-quality and poorly reported RCT,71 for which the method of randomisation and allocation concealment were unclear. The withdrawal rate in this study was also high (32% in both intervention groups).
Eighteen studies47,48–51,53,55,56,58–60,75,85,88,90,92,103,104 compared disc surgery and chemonucleolysis, for which the findings were very heterogeneous, giving a pooled result that was borderline statistically significant in favour of surgery. There was a mixture of study designs. The duration of follow-up ranged from 1 year to 10 years and duration of sciatica varied between studies. If only the six RCTs47,55,60,85,88,90 were considered, the findings were still heterogeneous, although most reported findings in favour of disc surgery [pooled analysis: odds ratio (OR) 1.12; 95% CI 0.51 to 2.49]. One moderate-quality RCT88 found chemonucleolysis to be more effective than disc surgery, but the study had a high withdrawal rate in the surgery group (at least 41%) compared with chemonucleolysis (at least 19%), with dropouts being given a poor outcome in the analysis. The funnel plot (Figure 10), for publication and other biases, does not appear to show asymmetry, but does indicate a lack of large studies.
FIGURE 10.
Funnel plot with pseudo 95% CIs for studies comparing disc surgery with chemonucleolysis at long-term follow-up (> 6 months).
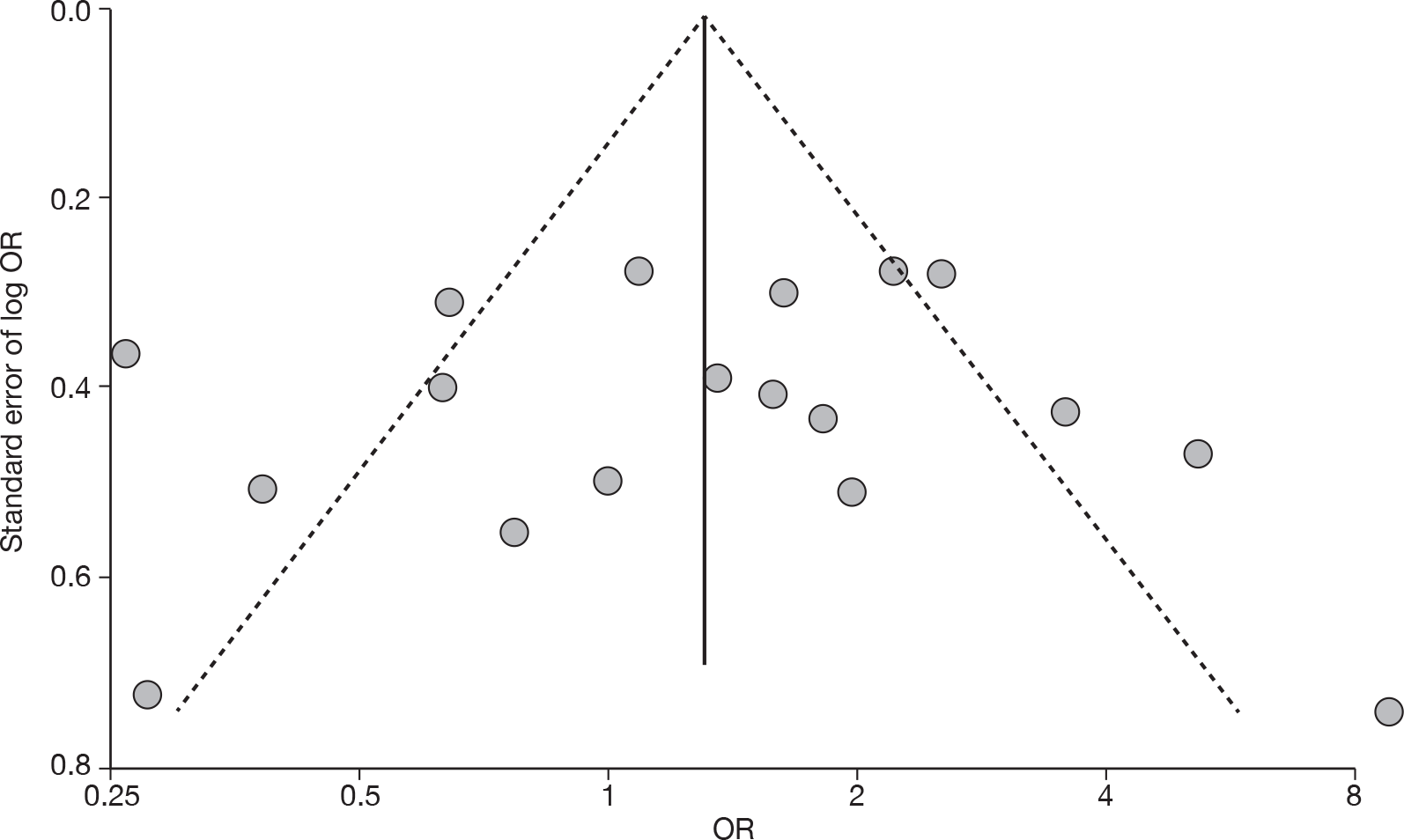
According to one RCT,86 there was no important difference between repeat disc surgery and spinal cord stimulation (others) in terms of treatment success for chronic sciatica following previous disc surgery.
Pain intensity at long-term follow-up
The results for pain intensity at long-term follow-up are presented in Table 13 and the accompanying forest plot (Figure 11). Disc surgery was compared with usual care, exercise therapy, epidural, intraoperative interventions, chemonucleolysis and mixed treatments.
| ID no. | Author, year | Chronicity | Study design | Follow-up | Location | Scale (range)a | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)b | Comment/conversionc | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |||||||||
| Disc surgery vs chemonucleolysis | ||||||||||||||||
| 454 | Buric, 200577 | A + C | Non-RCT | 18 months | Overall | VAS (0–10) | 15 | 30 | 61 (31) | 53 (22) | 20 (13) | 22 (13) | –41 | –40 | 7.0 (–1.72 to 15.72) | Two patients crossed over to surgery, classed as treatment failures |
| 593 | Muralikuttan, 199285 | A + C | RCT | 3 months | Leg | VAS (0–100) | 46 | 46 | 72 | 64 | 14 (24.43) | 20 (23.76) | –2.00 (–10.49 to 6.49) |
SD imputed from weighted average Most outcomes showed skewed distribution |
||
| Disc surgery vs epidural | ||||||||||||||||
| 725 | Buttermann, 200495 | A + C | RCT | 2–3 years | Back | VAS (0–10) | No significant differences between groups, Student’s t-test (p-value not given) | No summary estimates reported | ||||||||
| Disc surgery vs exercise therapy | ||||||||||||||||
| 300 | Osterman, 200668 | A | RCT | 2 years | Leg | VAS (0–100) | 28 | 28 | 61 (20) | 57 (21) | 6 (11) | 15 (24) | –9.00 (–18.78 to 0.78) |
ITT using LOCF Dropouts: surgery 2/29, exercise 4/28 |
||
| Disc surgery vs intraoperative interventions | ||||||||||||||||
| 470 | Debi, 200278 | A + C | RCT | 1 year | Leg | VAS (0–10) | 35 | 26 | 71 | 58 | 13 (20.31) | 13 (8.68) | 0.0 (–7.51 to 7.51) |
SD imputed from weighted average Mean inferred from graphs Dropouts 9/70 (13%): intervention 9/35, control 0/35 |
||
| 276 | Lundin, 200366 | C | RCT | 104 weeks | Overall | VAS (0–100) | 42 | 38 | 48 | 54 | 14 (20.31) | 8 (8.68) | 6.00 (–0.73 to 12.73) |
SD imputed from weighted average Mean inferred from graphs |
||
| 854 | Rasmussen, 2008101 | NR | RCT | 2 years | Leg | Composite NRS (0–30) | 100 | 100 | 68.3 | 70 | 33.33 (20.31) | 16.7 (8.68) | 5.54 (1.21 to 9.87) |
Median used to represent mean SD imputed from weighted average Three separate pain measures using NRS (0–10) combined: pain now, worst, and average pain in the last 2 weeks, for back and leg pain separately ITT using LOCF. Dropouts 2/200 (1%): group allocation not stated |
||
| 316 | Cengiz, 200769 (i)c (anti–adhesion barrier ADCON-L) | C | RCT | 12 months | Overall | VAS (0–10) | 18 | 21 | 100 (0.0) | 92.8 (10.5) | 46.6 (12.3) | 44.7 (9.8) | 1.90 (–5.16 to 8.96) | |||
| 316 | Cengiz, 200769 (ii)d (anti-adhesion barrier Healon GV) | C | RCT | 12 months | Overall | VAS (0–10) | 18 | 21 | 100 (0.0) | 97.1 (9.5) | 46.6 (12.3) | 48 (7.4) | –1.40 (–7.90 to 5.10) | |||
| Disc surgery vs usual care | ||||||||||||||||
| 716 | Alaranta, 199094 | A + C | CCS | 12 months | B-U&LPI (0–30) | 235 | 122 |
Surgery group had greatest decrease in pain indices Pain index: surgery vs control p < 0.001; surgery vs control not significant, Student’s t-test |
Patients in control group had no disc herniation on rhizography or did not meet criteria for surgery Data presented in unusable graphical form |
|||||||
| 772 | Hansson, 2007100 | A + C | CCS | 2 years | Overall | Von%%Korff – pain scale (0–10) | 92 | 92 | 71 | 70 | 45 | 59 | –26 (34) | –11 (73.3) | –15 (–31.51 to 1.51) | SD for change score derived from p-value of t-test (individual group) converted to 0–100 |
| 606 | Peul, 200787 | A | RCT | 104 weeks | Leg | VAS (0–100) | 130 | 130 | 67.2 (27.7) | 64.4 (21.2) | 11 (21.66) | 9 (21.66) |
2.0 (–3.27 to 7.27) Repeated measures analysis, difference between groups: –2.0 (95% CI –6.0 to 2.0) |
Final SD based on SE Dropouts 23 (8%): intervention 11/141, control 12/142 ITT not done because no difference between ITT and non-ITT at 1-year follow-up |
||
| Disc surgery vs mixed treatments | ||||||||||||||||
| 734 | Hoogland, 200697 (surgery + chemonucleolysis) | C | Q-RCT | 2 years | Leg | VAS (0–10) | 119 | 116 | 80.5 | 82.2 | 20.2 (20.31) | 18.5 (21.22) | 1.70 (–3.61 to 7.01) |
SD imputed from weighted average ITT not used Dropouts 45 (16%): intervention 23/142, control 22/138 |
||
FIGURE 11.
Summary of the findings of pain intensity at long-term follow-up studies (> 6 months) comparing disc surgery with alternative interventions. Note: weights are from random effects analysis.
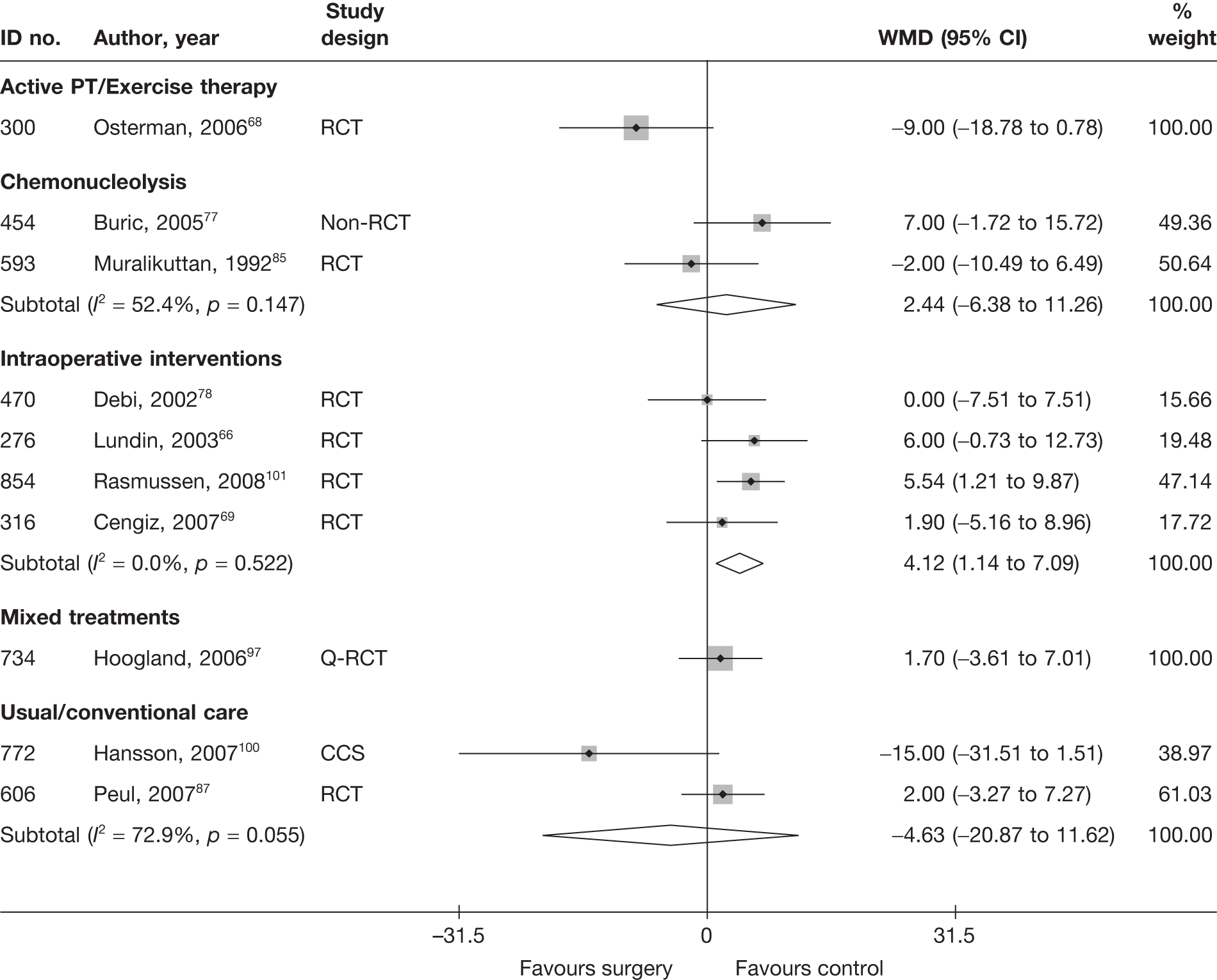
Three studies87,94,100 compared disc surgery with usual care. One well-conducted RCT87 included patients with severe sciatica for 6–12 weeks. The study did not find any important differences between the interventions groups for pain intensity at 104 weeks. The other two studies were CCSs that included patients with acute and chronic sciatica. Neither study used VAS as their pain scale. Only one study94 found statistically significant findings in favour of surgery, but the data were reported in an unusable graphical format and could not be included in the meta-analysis. The study was poorly reported in general and had obvious selection bias, with patients in the comparator group including those with no disc herniation on rhizography or who were not eligible for disc surgery.
As with the global effect, one well-conducted RCT68 found non-statistically significant findings in favour of disc surgery plus exercise therapy compared with exercise therapy alone in patients with acute sciatica at 2 years’ follow-up.
One poorly reported study95 compared the use of epidurals with disc surgery in patients with chronic sciatica [mean 3.55 months (SD 2.75 months)], and found no statistically significant difference between the intervention groups for back pain intensity at follow-up intervals of 7–12 months, 1–2 years or 2–3 years (Student’s t-test). Results of leg pain were not reported beyond 6 months.
The pooled analysis of four RCTs66,69,78,101 found a statistically significant improvement following intraoperative interventions compared with disc surgery alone. One study78 included patients with acute and chronic sciatica (mean symptom duration 56 days, range 12–135 days), two studies66,69 included patients with chronic sciatica, and duration of symptoms was not stated in the remaining study. 101 Duration of follow-up ranged from 1 year to 2 years. Overall study quality was moderate66,69,101 or poor. 78
Two studies77,85 compared disc surgery with chemonucleolysis: one was an RCT85 and the other a non-RCT. 77 Overall, there was no statistically significant difference between the intervention groups.
A Q-RCT97 evaluated the use of chemonucleolysis plus surgery versus surgery alone in patients with chromic sciatica. There was no statistically significant difference between the intervention groups.
Condition-specific outcome measures at long-term follow-up
The results for CSOMs at long-term follow-up are presented in Table 14 and the accompanying forest plot (Figure 12). Disc surgery was compared with usual care, exercise therapy, intraoperative interventions and chemonucleolysis.
| ID no. | Author, year | Chronicity | Study design | Follow-up | Scale | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)a | Comment/conversionb | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | ||||||||
| Disc surgery vs chemonucleolysis | |||||||||||||||
| 454 | Buric, 200577 | A + C | Non-RCT | 18 months | RMDQ | 15 | 30 | 12.4 (4.3) | 9.1 (3.5) | 2.1 (1.9) | 2.2 (3.2) | –10.3 | –6.9 | –0.04 (–0.66 to 0.58) | ITT used but method not stated Dropouts: two, considered as treatment failure |
| 727 | Ejeskar, 198396 | A + C | RCT | 12 months | Composite score | 14 | 15 | 8.79 (6.02) | 9.4 (6.88) | –0.08 (–0.3 to 0.21) | |||||
| 593 | Muralikuttan, 199285 | A + C | RCT | 1 year | Part of the Waddell Disability Index | 46 | 46 | 6.7 | 6.2 | 2.8 (1.21) | 2.6 (1.21) | –3.9 | –3.6 | 0.17 (–0.24 to 0.57) |
SD for final means calculated from p-values (Mann–Whitney U-test); most outcomes showed skewed distribution ITT not used, but all patients included in analysis except one for psychological outcomes |
| 672 | Weinstein, 198692 | C | CCS | Mean 10.3 years | Composite score | 71 | 85 | Results of MANOVA showed no significant relationship between pain outcome measures and treatment type [Wilks’ criterion: F(6,54) = 1.18, p < 0.34] |
Pain + disability measured in six different scales Actual data not presented Dropouts: 3/159 (2%) (chemonucleolysis group) |
||||||
| Disc surgery vs exercise therapy | |||||||||||||||
| 300 | Osterman, 200668 | A | RCT | 2 years | ODI | 28 | 28 | 39 (15) | 39 (14) | 6 (9) | 11 (16) | –33 | –28 | –0.39 (–0.91 to 0.14) | ITT using LOCF, but one patient who did not meet inclusion criteria excluded from analysis |
| Disc surgery vs intraoperative interventions | |||||||||||||||
| 436 | Bernsmann, 200174 | NR | RCT | Median 24.2 months | FFbH-R | 94 | 92 | 4.64 (22.48) | 5.15 (22.48) | 0.02 (–0.31 to 0.26) |
Final SD imputed from weighted mean SDs of FFbH from other studies of disc surgery long-term follow-up ITT not used Dropouts 14 (7%): intervention 8/100, control 6/100 |
||||
| 492 | Gerszten, 200381 | C | RCT | 1 year | ODI | 5 | 5 | 31.4 (5.5) | 32.6 (7.8) | 21.2 (8.8) | 20.4 (10.6) | 0.08 (–1.16 to 1.32) | |||
| 520 | Jensen, 199683 | NR | RCT | 1 year | LBPRS | 49 | 50 | 57.0 | 54.5 | 23.0 (10.85) | 23.5 (10.85) | –0.05 (–0.44 to 0.35) |
Median used for mean, final SD imputed from weighted mean of SDs of LBRS for post-operative interventions ITT not used Dropouts 19/118 (16%): group allocation not stated |
||
| 316 | Cengiz, 200769 | C | RCT | 12 months | ODI | 18 | 21 | 16.66 (12.5) | 19.66 (9.59) | –0.27 (–0.90 to 0.36) | |||||
| Disc surgery vs usual/conventional care | |||||||||||||||
| 386 | Atlas, 199672 | C | CCS | 10 years | Modified RMDQ | 188 | 152 | 17.7 (4) | 13.5 (5.9) | 6 (7) | 7.6 (7) | –11.7 (7.2) | –5.8 (7.6) |
–0.23 (–0.44 to –0.–1) Difference between groups for change score p < 0.001 using multiple linear regression models that control for baseline score |
Number of patients included in analysis was unclear |
| 772 | Hansson, 2007100 | A + C | CCS | 2 years | FFbH-R | 92 | 92 | 47 | 58 | 35 (13.09) | 36 (13.09) | 18 | 6 | –0.08 (–0.37 to 0.21) | Final SD imputed from weighted means of FFbH-R for usual care |
| 606 | Peul, 200787 | A | RCT | 2 years | RMDQ | 130 | 130 | 16.5 (4.4) | 16.3 (3.9) | 3.1 (5.7) | 2.6 (5.7) | –13.4 | –13.7 |
0.09 (–0.16 to 0.33) Adjusted mean difference 0.5 (95% CI –0.8 to 1.8), repeated-measures analysis of variance; difference between groups based on AUC also reported |
SDs calculated from SE ITT not used because sensitivity analysis showed no difference between ITT and non-ITT at 1-year follow-up; 23 (8%) patients lost to follow-up; no randomised intervention 141, control 142 |
| 2 | Thomas, 200745 | C | CCS | Intervention: 6 months; control: 12 months | NASS Lumbar Spine Q subscale – pain and disability | 333 | 164 | 21.4 (10) | 29 (10) | 58.3 (10) | 57.7 (10) | 20.2 | 13.3 |
0.06 (–0.13 to 0.25) Adjusted mean difference 3.46 (95% CI 0.17 to 6.75) p = 0.04 |
ITT used (method of dealing with missing values not reported) Dropouts 126 (20%): intervention 84/417, control 42/206 |
| 750 | Weinstein, 200698 | A + C | CCS | 2 years | MODEMS version of ODI | 456 | 165 | 56.7 (18.9) | 35.9 (20.1) | 19.1 (18.9) | 11.7 (20.1) | –37.6 (18.15) | –24.2 (21.84) |
0.38 (0.21 to 0.56) Adjusted mean difference –13.4 (95% CI –17.0 to –9.7); n = 620/743 |
Final score calculated from change score No final SD so baseline SD used, adjusted difference between groups based on change scores Missed visits adjusted for in analysis. 48/222 patients who chose to be in non-operative group received surgery and 40/521 who chose to be in surgery group did not have surgery Analysis based on treatment received not initial group allocation |
| 751 | Weinstein, 200699 | A + C | RCT | 2 years | MODEMS version of ODI | 186 | 187 | 47.5 (21.4) | 46.3 (20.6) | 16.1 (21.4) | 17.6 (20.6) | –31.4 | –28.7 |
–0.07 (–0.27 to 0.13) Adjusted mean difference –2.7 (95% CI –7.4 to 1.9); n = 472/501 |
Final score calculated from change score No final SD so baseline SD used, adjusted difference between groups based on change scores ITT analysis included 472/501 patients using LOCF (longitudinal mixed model controlling for covariates associated with missing values) Dropouts: intraoperative 59/245 (24%), chemonucleolysis 69/256 (27%) Crossovers: intervention 92/232 (40%), control 107/240 (45%) |
FIGURE 12.
Summary of the findings of CSOMs at long-term follow-up (> 6 months) for studies comparing disc surgery with alternative interventions. Note: weights are from random effects analysis.
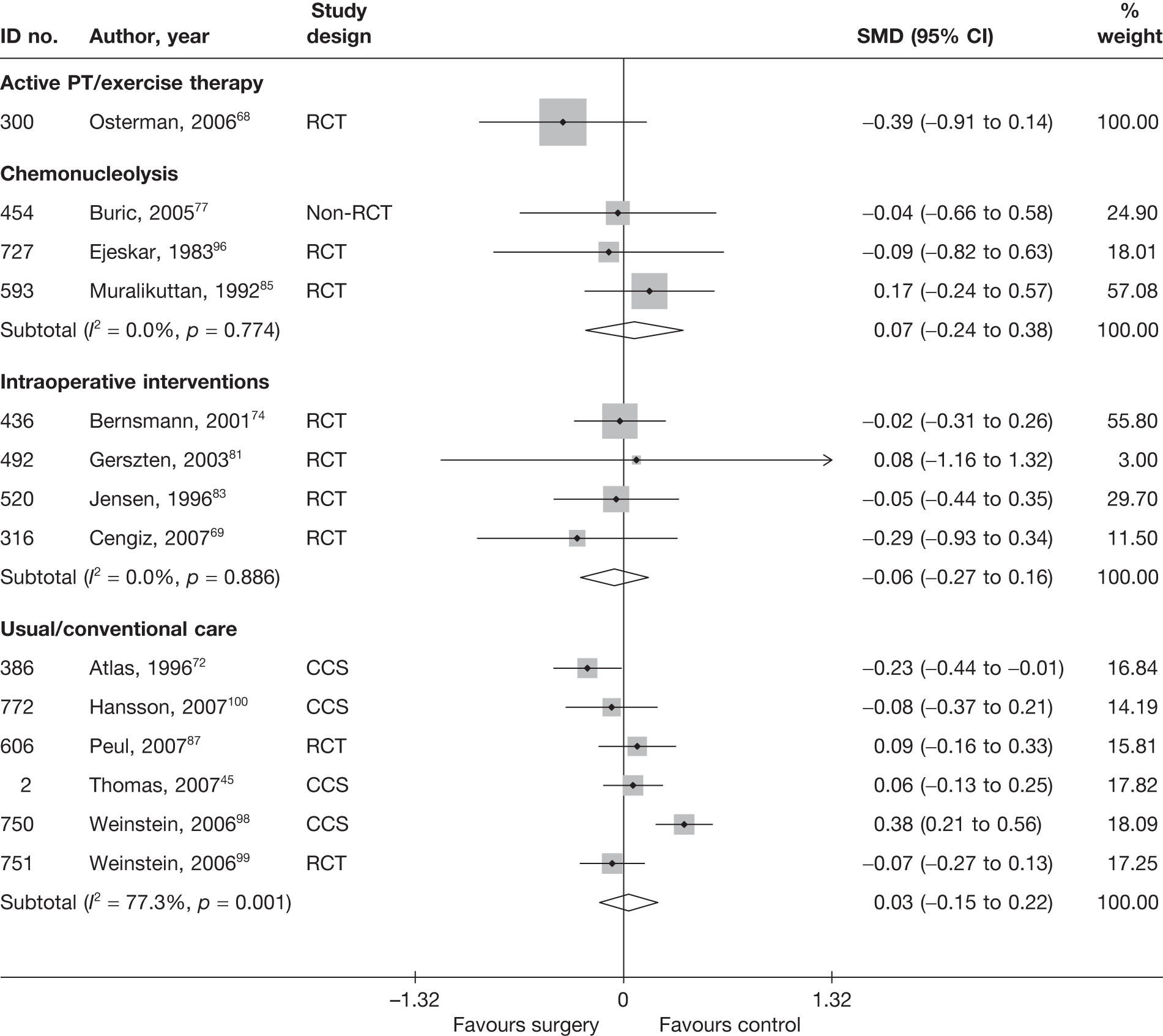
Six studies45,72,87,98–100 compared disc surgery with usual care, for which the pooled findings showed no statistically significant difference between the intervention groups at 1 year to 10 years45,72 (median 2 years). Two studies87,99 were well-conducted RCTs and the remaining four45,72,98,100 were CCSs. Pooled analysis of the RCTs also showed no important differences between the intervention groups (SMD –0.01; 95% CI –0.16 to 0.15).
One well-conducted RCT68 found non-statistically significant findings in favour of disc surgery plus exercise therapy compared with exercise therapy alone in patients with acute sciatica at 2 years’ follow-up.
The pooled analysis of four RCTs69,74,81,83 showed no important difference between disc surgery and intraoperative interventions for CSOMs at 1 year’s69,81,83 follow-up or a median of 2 years’ follow-up. 74
Four studies77,85,92,96 compared disc surgery and chemonucleolysis: two were RCTs,85,96 one was a non-RCT77 and one was a CCS. 92 The CCS92 reported insufficient data to be included in the meta-analysis. The results of six pain and disability outcome measures were analysed in a one-way multivariate analysis of variance (MANOVA), the results of which showed no significant relationship between pain outcome measures and treatment type (Wilks’ criterion F(6,54) = 1.18; p < 0.34). Pooled analysis of the remaining three studies77,85,96 showed no statistically significant difference between the intervention groups.
Analysis of adverse effects for disc surgery
Adverse events were very poorly reported in most studies. Table 15 and Figure 13 present the overall number of any adverse event that occurred.
| ID no. | Author, year | Study design | No. of events in intervention group | No. of participants in intervention group | No. of events in control group | No. of participants in control group | OR (95% CI) |
|---|---|---|---|---|---|---|---|
| Disc surgery vs chemonucleolysis | |||||||
| 884 | Alexander, 1989103 | CCS | 8 | 49 | 8 | 51 | 1.05 (0.36 to 3.06) |
| 43 | van Alphen, 198947 | RCT | 3 | 78 | 3 | 73 | 0.93 (0.18 to 4.78) |
| 441 | Bonafe, 199375 | CCS | 1 | 20 | 10 | 20 | 0.05 (0.01 to 0.47) |
| 183 | Bouillet, 198361 | CCS | 91 | 613 | 152 | 2136 | 2.28 (1.72 to 3.00) |
| 453 | Brown, 198976 (chemopapain) | CCS | NR | NR | NR | NR | |
| 453 | Brown, 198976 (collagenase) | CCS | NR | NR | NR | NR | |
| 454 | Buric, 200577 | Non-RCT | NR | NR | NR | NR | |
| 166 | Crawshaw, 198460 | RCT | 0 | 27 | 1 | 25 | 0.30 (0.01 to 7.63) |
| 48 | Dabezies, 197851 | CCS | 0 | 100 | 2 | 100 | 0.20 (0.01 to 4.14) |
| 471 | Dei-Anang, 199079 (German language) | CCS | NR | NR | NR | NR | |
| 727 | Ejeskar, 198396 | RCT | 1 | 14 | 1 | 15 | 1.08 (0.06 to 19.05) |
| 132 | Hoogmartens, 197656 | HCS | 19 | 53 | 3 | 44 | 7.64 (2.08 to 28.02) |
| 44 | Javid, 199548 | CCS | 4 | 100 | 6 | 100 | 0.65 (0.18 to 2.39) |
| 35 | Krugluger, 200046 | RCT | 1 | 10 | 5 | 12 | 0.16 (0.01 to 1.65) |
| 117 | Lagarrigue, 199154 (French language) | CCS | 30 | 751 | 5 | 334 | 2.74 (1.05 to 7.12) |
| 129 | Lavignolle, 198755 (French language) | RCT | 7 | 182 | 7 | 176 | 0.97 (0.33 to 2.81) |
| 889 | Lee, 1996104 (APLD) | CCS | 3 | 100 | 73 | 100 | 0.01 (0.00 to 0.04) |
| 889 | Lee, 1996104 (PELD) | CCS | 4 | 100 | 73 | 100 | 0.02 (0.01 to 0.05) |
| 593 | Muralikuttan, 199285 | RCT | 0 | 46 | 1 | 46 | 0.33 (0.01 to 8.22) |
| 47 | Norton, 198650 | CCS | 2 | 44 | 12 | 61 | 0.19 (0.04 to 0.92) |
| 45 | Postacchini, 198749 | Non-RCT | 20 | 84 | 2 | 72 | 10.94 (2.46 to 48.65) |
| 617 | Revel, 199388 | RCT | 15 | 69 | 35 | 72 | 0.29 (0.14 to 0.61) |
| 641 | Steffen, 199990 | RCT | NR | NR | NR | NR | |
| 49 | Stula, 199052 (German language) | RCT | NR | NR | NR | NR | |
| 61 | Tregonning, 199153 | CCS | 4 | 145 | 5 | 91 | 0.49 (0.13 to 1.87) |
| 893 | Watters,1988105 | Non-RCT | 1 | 50 | 2 | 50 | 0.49 (0.04 to 5.58) |
| 160 | Watts, 197559 | CCS | 2 | 174 | 3 | 100 | 0.38 (0.06 to 2.29) |
| 672 | Weinstein, 198692 | CCS | NR | NR | NR | NR | |
| 150 | Zeiger, 198758 | CCS | 5 | 81 | 16 | 45 | 0.12 (0.04 to 0.36) |
| Disc surgery vs epidural/intradiscal injection | |||||||
| 725 | Buttermann, 200495 | RCT | 7 | 77 | 5 | 50 | 0.90 (0.27 to 3.01) |
| Disc surgery vs active PT/exercise therapy | |||||||
| 300 | Osterman, 200668 | RCT | 1 | 28 | 0 | 28 | 3.11 (0.12 to 79.64) |
| Disc surgery vs intraoperative interventions | |||||||
| 268 | Aminmansour, 200664 (control = 40 mg) | Q-RCT | 1 | 22 | 0 | 19 | 3.46 (0.13 to 89.95) |
| 268 | Aminmansour, 200664 (control = 80 mg) | Q-RCT | 1 | 22 | 0 | 20 | 2.72 (0.10 to 70.79) |
| 436 | Bernsmann, 200174 | RCT | 0 | 94 | 0 | 92 | |
| 470 | Debi, 200278 | RCT | 0 | 26 | 0 | 35 | |
| 492 | Gerszten, 200381 | RCT | 1 | 5 | 1 | 5 | 1.00 (0.05 to 22.18) |
| 497 | Glasser, 199382 (control = LA) | RCT | NR | NR | NR | NR | |
| 497 | Glasser, 199382 (control = steroid + LA) | RCT | NR | NR | NR | NR | |
| 520 | Jensen, 199683 | RCT | NR | NR | NR | NR | |
| 909 | Jirarattanaphochai, 2007106 | RCT | 2 | 51 | 1 | 52 | 2.08 (0.18 to 23.70) |
| 400 | Kim, 200373 | RCT | NR | NR | NR | NR | |
| 551 | Langmayr, 199584 | RCT | NR | NR | NR | NR | |
| 366 | Lavyne, 199270 | Q-RCT | 0 | 42 | 0 | 42 | |
| 276 | Lundin, 200366 | RCT | 1 | 42 | 0 | 38 | 2.78 (0.11 to 70.39) |
| 270 | MacKay, 199565 (control = free fat graft) | RCT | NR | NR | NR | NR | |
| 270 | MacKay, 199565 (control = gelfoam membrane) | RCT | NR | NR | NR | NR | |
| 379 | Prestar, 199571 (German language) | RCT | 6 | 34 | 0 | 34 | 15.74 (0.85, 291.46) |
| 854 | Rasmussen, 2008101 | RCT | NR | NR | NR | NR | |
| 618 | Richter, 200189 | RCT | 3 | 177 | 3 | 180 | 1.02 (0.20 to 5.11) |
| 856 | Ronnberg, 2008102 | RCT | NR | NR | NR | NR | |
| 316 | Cengiz, 200769 (control = Adcon-L) | RCT | 1 | 18 | 0 | 21 | 3.69 (0.14 to 96.22) |
| 316 | Cengiz, 200769 (control = Healon GV) | RCT | 1 | 18 | 0 | 21 | 3.69 (0.14 to 96.22) |
| 705 | Starkweather, 200693 | RCT | NR | NR | NR | NR | |
| 915 | de Tribolet, 1998107 | RCT | 81 | 141 | 65 | 128 | 1.31 (0.81 to 2.12) |
| Disc surgery vs mixed treatments | |||||||
| 734 | Hoogland, 200697 | Q-RCT | 3 | 119 | 2 | 116 | 1.47 (0.24 to 8.99) |
| 600 | North, 200586 | RCT | 0 | 26 | 1 | 19 | 0.23 (0.01 to 6.03) |
| 263 | Wang, 200063 | RCT | NR | NR | NR | NR | |
| Disc surgery vs non-opioids | |||||||
| 475 | Dubourg, 200280 | CCS | 1 | 39 | 0 | 28 | 2.22 (0.09 to 56.54) |
| 144 | Rossi, 199357 (surgery = microdiscectomy) | Non-RCT | 0 | NR | 1 | NR | |
| 144 | Rossi, 199357 (surgery = percutaneous discectomy) | Non-RCT | 0 | NR | 1 | NR | |
| Disc surgery vs usual/conventional care | |||||||
| 716 | Alaranta, 199094 | CCS | NR | NR | NR | NR | |
| 386 | Atlas, 199672 | CCS | 16 | 275 | 0 | 232 | 29.57 (1.76 to 495.56) |
| 772 | Hansson, 2007100 | CCS | NR | NR | NR | NR | |
| 294 | Koranda, 199567 | Q-RCT | NR | NR | NR | NR | |
| 606 | Peul, 200787 | RCT | NR | NR | NR | NR | |
| 211 | Shvartzman, 199262 | HCS | NR | NR | NR | NR | |
| 2 | Thomas, 200745 | CCS | NR | NR | NR | NR | |
| 664 | Weber, 198391 | RCT | NR | NR | NR | NR | |
| 750 | Weinstein, 200698 | CCS | 2 | 538 | 0 | 216 | 2.02 (0.10 to 42.20) |
| 751 | Weinstein, 200699 | RCT | 24 | 232 | 0 | 240 | 56.52 (3.42 to 935.13) |
FIGURE 13.
Summary of the findings of any adverse effect for studies comparing disc surgery with alternative interventions. Note: weights are from random effects analysis.
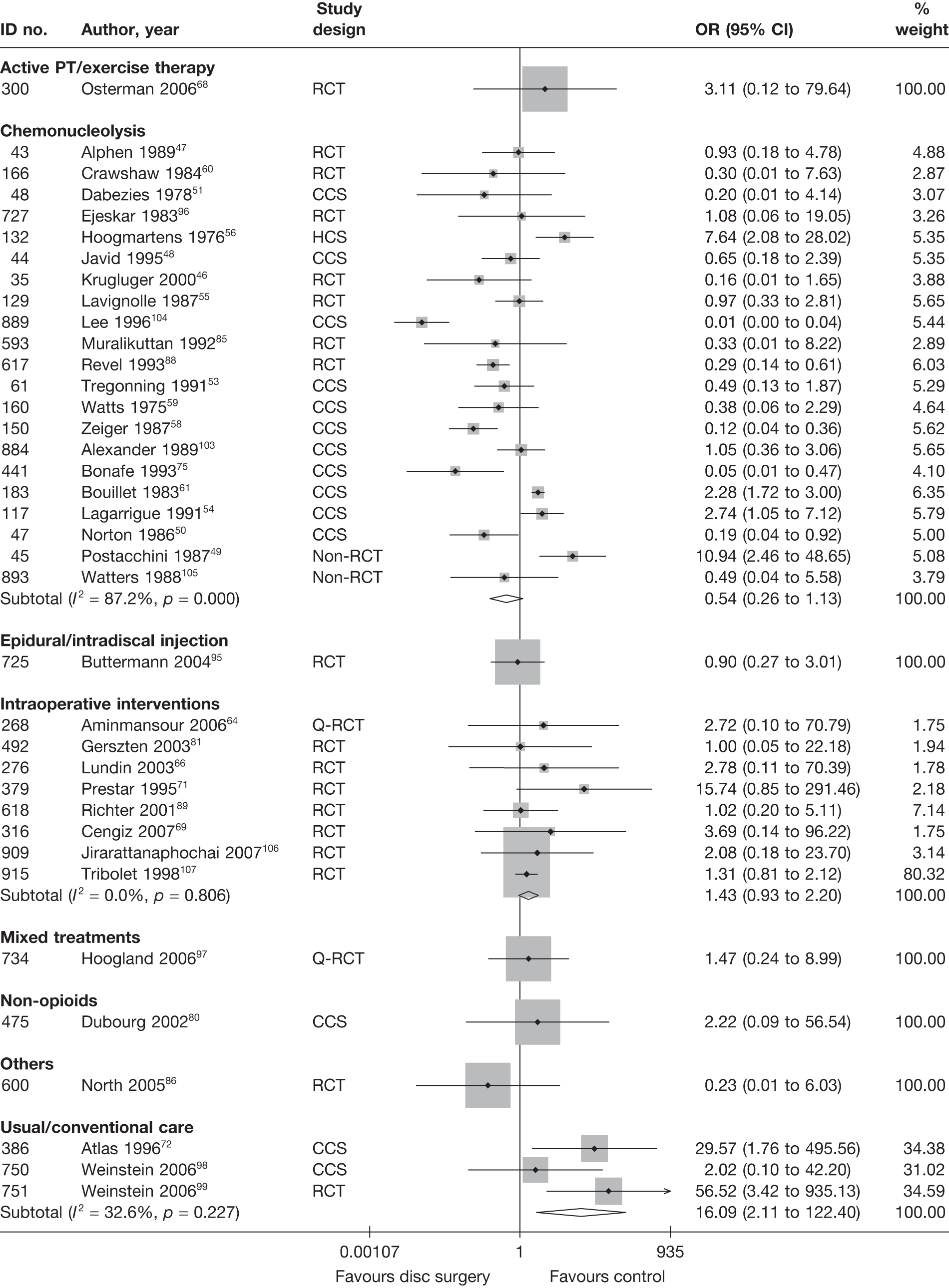
There was a statistically significant greater number of adverse effects with disc surgery compared with usual care. Overall there was no statistically significant difference in the number of adverse effects following disc surgery compared with: epidural and exercise therapy, chemonucleolysis, epidural, intraoperative interventions, mixed treatments, non-opioids or others.
SUMMARY OF OVERALL FINDINGS FOR DISC SURGERY COMPARED WITH ALTERNATIVE INTERVENTIONS
Most disc surgery studies included patients with chronic sciatica or both acute and chronic sciatica. Four studies62,68,80,87 included acute sciatica, for which the comparator included exercise therapy,68 non-opioids80 and usual care. 62,87 Just over half of the disc surgery studies were RCTs. There were only a small number of good-quality studies, two of which compared disc surgery with usual care (Table 16).
| Control category | No. of studies (arms) | Sample size range (median) | Proportion of studies that were RCTs(%) | Proportion of studies that were deemed good quality (%) | Proportion of studies that only included acute sciatica (%) | Proportion of studies that included patients with nerve root pain(%) | Proportion of studies that reported diagnosis confirmed by imaging (%) | Proportion of studies that included patients with stenosis (%) | Proportion of studies that included patients with extruded/sequestered discs (%) | Proportion of studies that only included patients with first episode (%) | Proportion of studies that included patients who had received previous treatment (%) | Proportion of studies that included patients who had received previous surgery (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disc surgery vs chemonucleolysis | 27 (29) | 29–1085 (126) | 8/27 (30) | 0/27 (0) | 0/27 (0) | 27/27 (100) | 22/27 (81) | 1/27 (4) | 1/27 (4) | 3/27 (11) | 22/27 (81) | 3/27 (11) |
| Disc surgery vs epidural/Intradiscal injection | 1 (1) | 100 (100) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 1/1 (100) | 1/1 (100) | 0/1 (0) | 1/1 (100) | 1/1 (100) | 1/1 (100) | 1/1 (100) |
| Disc surgery vs exercise therapy | 1 (1) | 57 (57) | 1/1 (100) | 0/1 (0) | 1/1 (100) | 1/1 (100) | 1/1 (100) | 0/1 (0) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 0/1 (0) |
| Disc surgery vs intraoprative interventions | 17 (17) | 10–398 (84) | 15/17 (88) | 0/17 (0) | 0/17 (0) | 17/17 (100) | 15/17 (88) | 1/17 (6) | 4/17 (24) | 2/17 (12) | 9/17 (53) | 1/17 (6) |
| Disc surgery vs mixed treatments | 4 (5) | 70–280 (123) | 3/4 (75) | 0/4 (0) | 0/4 (0) | 4/4 (100) | 4/4 (100) | 0/4 (0) | 1/4 (24) | 0/4 (0) | 3/4 (75) | 2/4 (50) |
| Disc surgery vs non-opioids | 2 (3) | 40–67 (54) | 0/2 (0) | 0/2 (0) | 1/2 (50) | 2/2 (100) | 2/2 (100) | 0/2 (0) | 0/2 (0) | 0/2 (0) | 0/2 (0) | 1/2 (50) |
| Disc surgery vs others | 1 (1) | 60 (60) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 1/1 (100) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 1/1 (100) | 1/1 (100) |
| Disc surgery vs usual/conventional care | 10 (10) | 55–743 (320) | 3/10 (30) | 2/10 (20) | 2/10 (20) | 10/10 (100) | 8/10 (80) | 1/10 (10) | 2/10 (20) | 0/10 (0) | 5/10 (50) | 1/10 (10) |
| Total (for disc surgery studies) | 62 (65) | 10–1085 (105) | 32/62 (52) | 2/62 (3) | 4/62 (6) | 62/62 (100) | 53/62 (85) | 3/62 (5) | 10/62 (16) | 6/62 (10) | 41/62 (62) | 10/62 (16) |
One well-conducted RCT87 found that early disc surgery resulted in a statistically significant improvement in pain at short- and medium-term follow-up compared with usual care, with a greater reduction at short-term follow-up. The same RCT found that functional status after disc surgery was significantly worse than usual care for the first 4 weeks, but significantly better after 4 weeks. However, there was no statistically significant difference between the treatment groups at medium-term follow-up. Pooled data from two RCTs67,87 showed a small improvement, which was not statistically significant, in favour of surgery for the global effect at medium-term follow-up. One further RCT99 (that could not be included in the meta-analysis) showed a small but statistically significant effect in favour of surgery for satisfaction with symptoms. Pooled data showed disc surgery to be better than usual care for the global effect at long-term follow-up [two RCTs,87,91 one CCS,72 one historical cohort study (HCS)62]. There were no statistically significant differences between intervention groups for pain intensity87,100 or CSOMs at long-term follow-up. 45,72,87,98–100 The number of adverse effects was statistically significantly higher following disc surgery than after usual care (one RCT,99 two CCSs72,98).
Disc surgery was significantly better than epidural at reducing pain intensity at medium-term follow-up but not at long-term follow-up (one poor-quality RCT95). There was no statistically significant difference between the intervention groups for adverse effects.
There was no statistically significant difference between disc surgery and non-opioids for global effect (one non-RCT,57 one CCS80) and pain intensity (one CCS80) at medium-term follow-up, or for adverse effects, according to two poor-quality studies. 57,80 Disc surgery in combination with non-opioids led to a greater reduction in pain intensity than disc surgery alone at short-term follow-up (one poor-quality RCT93), but there was no statistically significant difference between similar comparisons at long-term follow-up for global effect (one poor-quality RCT71).
There was no statistically significant difference between disc surgery plus exercise therapy and exercise therapy alone in terms of reported full recovery, pain intensity or functional status at short-, medium- or long-term follow-up in patients with acute sciatica (one small, well-conducted RCT68). There was also no significant difference between the intervention groups in terms of adverse effects.
One poorly reported RCT63 (moderate quality) found that disc surgery in combination with acupuncture led to a greater reduction in pain intensity than disc surgery alone at short-term follow-up.
Intraoperative interventions led to a greater reduction in pain intensity at long-term follow-up than did disc surgery alone (four moderate- to poor-quality RCTs66,69,78,101). However, there was no statistically significant difference between the intervention groups for global effect (at short-71,82 and long-term65,74,81,83,102 follow-up), pain intensity (at short-66,73,78,84,89,106 and medium-term64,66,73,84,89,101,106 follow-up), CSOMs (at short-70,73,89 and medium-term73,89,107 follow-up) and adverse effects (according to a number of studies, ranging from good to poor quality64,66,69,71,81,89,106,107).
Pooled analysis of 18 studies47–51,53,55,56,58–60,75,85,88,90,92,103,104 showed marginally statistically significant findings in favour of disc surgery, compared with chemonucleolysis, for the global effect at long-term follow-up (see Figure 9). However, there was no statistically significant difference between the intervention groups for the global effect at short-48,49,52,79,92,104 and medium-term48,49,54,76,88,92,104,105 follow-up; pain intensity at short-,76,85,88 medium-76,85,88 and long-term77,85 follow-up; CSOMs at short-,85,88 medium-85,88,96 and long-term77,85,96 follow-up; or adverse effects46–51,53–56,58–61,75,85,88,96,103–105 (according to a number of studies, ranging from good to poor quality). There was no statistically significant difference between disc surgery in combination with chemonucleolysis and disc surgery alone, at long-term follow-up, for global effect, pain, or for adverse effects (one poor-quality Q-RCT97).
There was no statistically significant difference between repeat disc surgery and spinal cord stimulation for the global effect at long-term follow-up or adverse effects of patients with chronic sciatica following previous disc surgery (one RCT86).
Epidural/intradiscal injection
This category includes the use of epidural (injection into the epidural space) or intradiscal (injection into disc) injection of steroid and/or local anaesthetic in various combinations, as well as spinal nerve block using local anaesthetic. Studies that evaluate the use of an alternative class of medication via epidural or intradiscal injection have been classified according to the medication used. The use of a peripheral nerve block is not included in this section.
Description of epidural/intradiscal injection studies
Summary of interventions
Sixty-three studies evaluated the use of epidural/intradiscal injection for sciatica95,143–204 (eight studies had more than two treatment arms146,149,161,163,167,169,183,197), of which 3595,143–176 compared epidural/intradiscal injection with alternative interventions; the type of interventions being compared are listed in Table 17a. Five of these did not report usable data for pain, global or CSOMs,146,161,164,169,172 but three146,161,169 provided data on adverse effects. (Two studies161,169 were pilot studies that reported only baseline data for main outcome measures and follow-up data for adverse effects and cost.)
| ID no. | Author, year | Study design | Treatment description | Control description |
|---|---|---|---|---|
| Epidural vs activity restriction | ||||
| 140 | Coomes, 1961145 | Non-RCT | Sacral epidural injection local anaesthetic 50–60 ml procaine | Bed rest at home on fracture-boards |
| Epidural vs alternative/non-traditional | ||||
| 667 | Wehling, 1997167 (German language) | CCS | Nerve root blockade with local anaesthetic 5 ml mepivacaine twice a week for 5 weeks | Acupuncture and herbal medication |
| 667 | Wehling, 1997167 (German language) | CCS | Nerve root blockade with steroid triamcinolone 20 mg + local anaesthetic 5 ml mepivacaine twice a week for 5 weeks | Acupuncture and herbal medication |
| Epidural vs biological agents | ||||
| 321 | Becker, 2007149 | RCT | Epidural injection of steroid triamcinolone 10 mg + local anaesthetic 1 ml (group 2) | Epidural injection of autologous conditioned serum (group 1) |
| 321 | Becker, 2007149 | RCT | Epidural injection of steroid triamcinolone 5 mg + local anaesthetic 1 ml (group 3) | Epidural injection of autologous conditioned serum (group 1) |
| Epidural vs chemonucleolysis | ||||
| 720 | Bontoux, 1990168 (French language) | RCT | Intradiscal injection of triamcinolone 70 mg | Chemonucleolysis with chymopapain 4000 U |
| 447 | Bourgeois, 1988160 (French language) | RCT | Intradiscal injection of triamcinolone 80 mg | Chemonucleolysis with chymopapain 4000 U |
| 729 | Gallucci, 2007170 | RCT | Intraforaminal and intradiscal injections of steroid triamcinolone 80 mg + local anaesthetic 2–4 ml ropivacaine (group A) | Intraforaminal and intradiscal injections of steroid triamcinolone 80 mg + local anaesthetic 2–4 ml ropivacaine plus ozone–oxygen (group B) |
| 50 | Graham, 1976144 | Non-RCT | Intradiscal hydrocortisone injection (dose not stated) | Chemonucleolysis with chymopapain (dose not stated) |
| Epidural vs disc surgery | ||||
| 725 | Buttermann, 200495 | RCT | Epidural injection of steroid betamethasone 10–15 mg up to three injections | Discectomy |
| Epidural vs education/advice | ||||
| 722 | Bronfort, 2004169 | RCT | Three ESIs over 12 weeks | Self-care education |
| Epidural vs inactive control | ||||
| 203 | Bush, 1991147 | RCT | Caudal epidural injection of steroid (80 mg of triamcinolone acetonide) + local anaesthetic (0.5% procaine hydrochloride) | Caudal injection of 25 ml normal saline |
| 350 | Carette, 1997152 | RCT | Epidural injection of steroid methylprednisolone 80 mg, 1–3 injections | Normal saline epidural injections |
| 383 | Dilke, 1973157 | RCT | Lumbar epidural injection of steroid methylprednisolone 80 mg | Injection of saline into interspinous ligament |
| 512 | Helliwell, 1985162 | RCT | Epidural injection of steroid methylprednisolone 80 mg (EDI) | Interspinous saline injections (control) |
| 739 | Karppinen, 2001171 | RCT | Periradicular injection of steroid methylprednisolone 40 mg + local anaesthetic bupivacaine | Periradicular saline injection |
| 539 | Klenerman, 1984163 | RCT | Epidural injection of steroid methylprednisolone 80 mg | Epidural injection of saline |
| 539 | Klenerman, 1984163 | RCT | Epidural injection of local anaesthetic 20 ml bupivacaine | Epidural injection of saline |
| 905 | Mathews, 1987176 | RCT |
Caudal epidural injection Injections of 20 ml of 0.125% bupivacaine and 2 ml (80 mg) methylprednisolone acetate given at fortnightly intervals, up to three times as needed |
Control injection Injection of 2 ml lidocaine over the sacral hiatus or into a tender spot |
| 778 | Price, 2005173 | RCT | Epidural injection of steroid triamcinolone 80 mg and local anaesthetic 10 ml bupivacaine | Saline injection into interspinous ligament (placebo) |
| 620 | Ridley, 1988165 | RCT | Epidural injection of steroid methylprednisolone 80 mg | Saline injection into interspinous ligament (placebo) |
| 240 | Snoek, 1977148 | RCT | Epidural injection of steroid methylprednisolone 80 mg | Epidural injection of saline |
| 406 | Vad, 2002158 | RCT | Transforaminal epidural steroid injections with betamethasone 9 mg and 1.5 ml xylocaine, 1–3 injections | Trigger-point saline injections epidural steroid injections, 1–2 injections |
| 351 | Valat, 2003153 | RCT | Three interlaminar epidural injections of steroid methylprednisolone 50 mg at two day intervals | Three interlaminar epidural injections of saline at 2-day intervals |
| 175 | Yates, 1978146 | RCT (crossover) | Caudal epidural injections of steroid | Caudal epidural injections of saline |
| 175 | Yates, 1978146 | RCT (crossover) | Caudal epidural injections of local anaesthetic | Caudal epidural injections of saline |
| 175 | Yates, 1978146 | RCT (crossover) | Caudal epidural injections of steroid + local anaesthetic | Caudal epidural injections of saline |
| Epidural vs manipulation | ||||
| 451 | Bronfort, 2000161 | RCT | Epidural injection of steroid injections, 1–3 injections | Chiropractic spinal manipulation |
| 722 | Bronfort, 2004169 | RCT | Three epidural steroid injections over 12 weeks | Chiropractic spinal manipulation |
| Epidural vs mixed treatment | ||||
| 439 | Blonna, 2004159 (Italian language) | RCT | Epidural steroid + local anaesthetic injections (4 mg betamethasone + 3 ml ropovicaine 0.2%) |
(Epidural + non-opioids) Epidural steroid + local anaesthetic injections (4 mg betamethasone + 3 ml ropovicaine 0.2%) and oral gabapentin (Neurontin®, Pfizer) (up to 900 mg daily) |
| 348 | Pirbudak, 2003150 | RCT | Epidural injection of steroid betamethasone 14 mg and local anaesthetic bupivacaine + oral placebo for 9 months |
(Epidural + non-opioids) Epidural injection of steroid betamethasone 14 mg and local anaesthetic bupivacaine + oral amitriptyline 10 mg daily for 9 months |
| Epidural vs non-opioids | ||||
| 451 | Bronfort, 2000161 | RCT | Epidural injection of steroid injections, 1–3 injections | Paracetamol, NSAIDs, activity modification |
| 20 | Dincer, 2007143 | RCT | Caudal epidural injection 40 mg methylprednisolone acetate, 8 mg dexamethasone phosphate, 7 ml of 2% prilocaine | Oral diclofenac 75 mg for 14 days (NSAID) |
| 771 | Lafuma, 1997172 | RCT | Epidural steroid (125 mg prednisolone) injections at admission | Usual care (rest + NSAIDs) without epidural injections during hospital admission |
| 362 | Wilson-MacDonald, 2005156 | RCT | Epidural injection of steroid methylprednisolone 80 mg and local anaesthetic 8 ml bupivacaine | Intramuscular injections of steroid methylprednisolone 80 mg and local anaesthetic 8 ml bupivacaine |
| 846 | Murata, 2009175 | RCT | L2 nerve block using steroid (3.3 mg dexamethasone sodium phosphate) and local anaesthetic (2 ml of 1% lidocaine) | Injection of steroid (3.3 mg dexamethasone sodium phosphate) and local anaesthetic (7 ml of 1% lidocaine) in the back muscles of L2 area (control block) |
| Epidural vs passive PT | ||||
| 9 | Veihelmann, 2006155 | RCT | Epidural injection via epidural catheter (neuroplasty) of steroid triamcinolone 40 mg and ropivacaine | Conservative physiotherapy |
| Epidural vs usual/conventional care | ||||
| 349 | Buchner, 2000151 | RCT | Three epidural injections of steroid methylprednisolone 100 mg and 10 ml bupivacaine plus conservative therapy and graded rehabilitation | Conservative therapy and graded rehabilitation without epidural injections |
| 828 | Laiq, 2009174 | Q-RCT | Epidural steroid (80 mg methylprednisolone) + local anaesthetic (3 ml of 2% plain xylocaine) + 3 ml normal saline (steroid group) | Bed rest, NSAIDs, muscle relaxants and opioids (Conservative group) |
| 581 | Matyjek, 1986164 (Polish language) | CCS | Caudal epidural injection. Seven doses of hydrocortisone acetate 0.025 g and a final injection of methylprednisolone 0.04 g | Control group treated by various other methods which were not stated |
| 358 | Popiolek, 1991154 (Polish language) | Non-RCT | Epidural injection of steroid and local anaesthetic. Injected with separate syringes of 5 ml of 0.5% bupivacaine then 40 mg methylprednisolone (n = 15) or 40 mg triamcinolone (n = 15). Repeated after 14 days if necessary | No epidural injection |
| Mixed treatment incorporating epidural vs mixed treatment without epidural | ||||
| 644 | Styczynski, 1997166 (Polish language) | Non-RCT | Epidural, traction and therapeutic exercises | Traction and therapeutic exercises |
Thirty studies149,167,177–204 compared different types (in terms of content) of epidural/intradiscal injections, 10 studies181,183–185,187,193,194,197,200,202 (two studies had more than two treatment arms183,197) compared different modes of administering epidural/intradiscal injections and 20 studies149,167,177–180,182,186,188–192,195,196,198,199,203,204,207 compared the use of different epidural/intradiscal injections. Details of the interventions are summarised in Table 17b, but the findings of these studies are not considered any further here.
| ID no. | Author, year | Study design | Treatment category | Treatment description | Control category | Control description |
|---|---|---|---|---|---|---|
| Comparison of different modes of administration | ||||||
| 326 | Acherman, 2007183 | RCT | Epidural/intradiscal injection | Intralaminar epidural injections of steroid triamcinolone 40 mg | Epidural/intradiscal injection | Caudal epidural injections of steroid triamcinolone 40 mg |
| 326 | Acherman, 2007183 | RCT | Epidural/intradiscal injection | Transforaminal epidural injection of steroid triamcinolone 40 mg | Epidural/intradiscal injection | Caudal epidural injections of steroid triamcinolone 40 mg |
| 389 | Candido, 2008187 | RCT | Epidural/intradiscal injection | Epidural steroid injection (80 mg prednisolone with lidocaine) using parasagittal interlaminar approach | Epidural/intradiscal injection | ESIs (80 mg prednisolone with lidocaine) using transforaminal approach |
| 302 | Jeong, 2007181 | RCT | Epidural/intradiscal injection | Transforaminal epidural steroid injection (ganglionic group) | Epidural/intradiscal injection | Transforaminal epidural steroid injection (preganglionic group) |
| 328 | Kolsi, 2000184 | RCT | Epidural/intradiscal injection | Nerve root injections of steroid cortivazol 3.75 mg + local anaesthetic 2 ml lidocaine | Epidural/intradiscal injection | Interspinous epidural injection of steroid cortivazol 3.75 mg + local anaesthetic 2 ml lidocaine |
| 556 | Lee, 2006193 | HCS | Epidural/intradiscal injection | Preganglionic epidural injection of steroid triamcinolone 40 mg and 0.5 ml bupivacaine (preganglionic) | Epidural/intradiscal injection | Interlaminar or caudal epidural injection of steroid triamcinolone 40 mg and 0.5 ml bupivacaine (conventional) |
| 830 | Lee, 2009197 | CCS | Epidural/intradiscal injection | Translaminar epidural steroid (40 mg triamcinolone) and local anaesthetic (8 ml of 0.5% lidocaine) injection | Epidural/intradiscal injection | Caudal epidural steroid (40 mg triamcinolone) and local anaesthetic (15 ml of 0.5% lidocaine) injection |
| 830 | Lee, 2009197 | CCS | Epidural/intradiscal injection | Translaminar epidural steroid (40 mg triamcinolone) and local anaesthetic (8 ml of 0.5% lidocaine) injection | Epidural/intradiscal injection | Transforaminal epidural steroid (40 mg triamcinolone) and local anaesthetic (2 ml of 0.5% lidocaine) injection; small volume group |
| 830 | Lee, 2009197 | CCS | Epidural/Intradiscal injection | Translaminar epidural steroid (40 mg triamcinolone) and local anaesthetic (8 ml of 0.5% lidocaine) injection | Epidural/intradiscal injection | Transforaminal epidural steroid (40 mg triamcinolone) and local anaesthetic (2 ml of 0.5% lidocaine) injection; large volume group |
| 842 | Mendoza-Lattes200 | CCS | Epidural/intradiscal injection | Caudal epidural steroid (either 2 ml of 80 mg methylprednisolone or 3 ml of 18 mg betamethasone) injection | Epidural/intradiscal injection | Transforaminal epidural injection of steroid [methylprednisolone (40 mg/ml) or betamethasone (6 mg/ml)] and local anaesthetic (1.5–2.0 cc 1 : 1 solution of bupivacaine 0.25%) injections |
| 630 | Schaufele, 2006194 | CCS | Epidural/intradiscal injection | Interlaminar epidural injection of steroid methylprednisolone 80 mg + 3 ml lidocaine | Epidural/intradiscal injection | Transforaminal epidural injection of steroid methylprednisolone 80 mg + 2 ml lidocaine |
| 330 | Thomas, 2003185 | RCT | Epidural/intradiscal injection | Interspinous epidural injection of steroid dexamethasone 5 mg | Epidural/intradiscal injection | Transforaminal epidural injection of steroid dexamethasone 5 mg |
| 895 | Winnie, 1972202 | RCT | Epidural/intradiscal injection | Epidural corticosteroid (80 mg of methylprednisolone). Average of 2.1 injections | Epidural/intradiscal injection | Intrathecal corticosteroid (80 mg of methylprednisolone). Average of 2.1 injections |
| Comparison of different type of epidurals (content) | ||||||
| 896 | Anwar, 2005203 | RCT | Epidural/intradiscal injection | Caudal epidural steroid injection with triamcinolone (40 mg) and local anaesthetic (5 ml of 1% lignocaine) | Epidural/intradiscal injection | Caudal epidural steroid injection with methylprednisolone (40 mg) and local anaesthetic (5 ml of 1% lignocaine) |
| 321 | Becker, 2007149 | RCT | Epidural/intradiscal injection | Epidural injection of steroid triamcinolone 10 mg + local anaesthetic 1 ml (group 2) | Epidural/intradiscal injection | Epidural injection of steroid triamcinolone 5 mg + local anaesthetic 1 ml (group 3) |
| 141 | Beliveau, 1971177 | Q-RCT | Epidural/intradiscal injection | Epidural injection of steroid 80 mg methylprednisolone + local anaesthetic 40 ml procaine | Epidural/intradiscal injection | Epidural injection of 42 ml procaine |
| 437 | Blankenbaker, 2005189 | HCS | Epidural/intradiscal injection | Selective lumbar nerve root block with triamcinolone 40 mg | Epidural/intradiscal injection | Selective lumbar nerve root block with betamethasone 6 mg |
| 450 | Breivik, 1976190 | RCT | Epidural/intradiscal injection | Epidural steroid + local anaesthetic injections (80 mg depot methylprednisolone + 20 ml bupivacaine 0.25%) | Epidural/intradiscal injection | Epidural bupivacaine injections 20 ml |
| 803 | Cocelli, 2009195 | RCT | Epidural/intradiscal injection | Epidural injection of betamethasone (10 mg) and bupivacaine (0.125% in 20 ml), 1–3 injections (group 1) | Epidural/intradiscal injection | Epidural injection of triamcinolone (80 mg) and bupivacaine (0.125% in 20 ml), 1–3 injections (group 2) |
| 413 | Cuckler, 1985188 | RCT | Epidural/intradiscal injection | Epidural injection of steroid methylprednisolone 80 mg and local anaesthetic 5 ml procaine | Epidural/intradiscal injection | Epidural injection of saline and local anaesthetic 5 ml procaine |
| 149 | Dashfield, 2005178 | RCT | Epidural/intradiscal injection | Targeted injection during spinal endoscopy of steroid 40 mg triamcinolone + 10 ml lidocaine | Epidural/intradiscal injection | Caudal epidural injection of steroid 40 mg triamcinolone + local anaesthetic 10 ml lidocaine |
| 483 | Faraj, 2006191 | RCT | Epidural/intradiscal injection | Nerve root infiltration using steroid + local anaesthetic (40 mg + 0.5 ml of 0.5% bupivacaine) with the aid of nerve stimulator | Epidural/intradiscal injection | Nerve root infiltration using steroid + local anaesthetic (40 mg + 0.5 ml bupivacaine 0.5%) without the aid of nerve stimulator |
| 500 | Gronemeyer, 1995192 (German language) | RCT | Epidural/intradiscal injection | Epidural injection of steroid triamcinolone 40 mg. 2–11 treatments over 3–8 weeks | Epidural/intradiscal injection | Epidural injection of steroid triamcinolone 10 mg. 2–11 treatments over 3–8 weeks |
| 814 | Hagihara, 2009196 | Q-RCT | Epidural/intradiscal injection | Selective nerve root block with steroid (4 mg in 1 ml betamethasone) and local anaesthetic (2 ml of lidocaine hydrochloride) | Epidural/intradiscal injection | Selective nerve root block of local anaesthetic only (3 ml of lidocaine hydrochloride) |
| 838 | Manchikanti, 2008198 | RCT | Epidural/intradiscal injection | Caudal epidural steroid (either 6 mg of betamethasone or 40 mg of methylprednisolone) and local anaesthetic (9 ml of 0.5% lidocaine) injections (steroid group) | Epidural/intradiscal injection | Caudal epidural local anaesthetic (10 ml of lidocaine 0.5%) injections (local anaesthetic group) |
| 908 | Manchikanti, 2009204 | RCT | Epidural/intradiscal injection | Caudal epidural steroid (either 6 mg of betamethasone or 40 mg of methylprednisolone) and local anaesthetic (9 ml of 0.5% lidocaine) injections (steroid group) | Epidural/intradiscal injection | Caudal epidural injections of local anaesthetic (0.5% lidocaine 9 ml) (local anaesthetic group) |
| 839 | Manchikanti, 2009199 | RCT | Epidural/intradiscal injection | Caudal epidural steroid (either 6 mg of betamethasone or 40 mg of methylprednisolone) and local anaesthetic (9 ml of 0.5% lidocaine) injections (steroid group) | Epidural/intradiscal injection | Caudal epidural local anaesthetic (10 ml of lidocaine 0.5%) injections (local anaesthetic group) |
| 318 | Ng, 2005182 | RCT | Epidural/intradiscal injection | Periradicular injection of steroid methylprednisolone 40 mg + local anaesthetic 2 ml bupivacaine | Epidural/intradiscal injection | Periradicular injection of local anaesthetic 2 ml bupivacaine |
| 176 | Owlia, 2007179 | RCT | Epidural/intradiscal injection | Epidural injection of 80 mg methylprednisolone acetate (80 mg steroid group) | Epidural/intradiscal injection | Epidural injection of 40 mg methylprednisolone acetate (40 mg steroid group) |
| 273 | Riew, 2000180 | RCT | Epidural/intradiscal injection | Nerve root injection of steroid betamethasone 6 mg + local anaesthetic 1 ml bupivacaine up to four injections | Epidural/intradiscal injection | Nerve root injection of local anaesthetic 1 ml bupivacaine up to four injections |
| 365 | Rogers, 1992186 | RCT | Epidural/intradiscal injection | Epidural injection of steroid methylprednisolone 80 mg and local anaesthetic 14 ml lidocaine | Epidural/intradiscal injection | Epidural injection of local anaesthetic 14 ml lidocaine |
| 866 | Tafazal, 2009201 | RCT | Epidural/intradiscal injection | Periradicular infiltration of steroid (40 mg methylprednisolone) and bupivacaine (2 ml of 0.25%) injection | Epidural/intradiscal injection | Periradicular infiltration bupivacaine (2 ml of 0.25%) |
| 667 | Wehling, 1997167 (German language) | CCS | Epidural/intradiscal injection | Nerve root blockade with steroid triamcinolone 20 mg + local anaesthetic 5 ml mepivacaine, twice a week for 5 weeks | Epidural/intradiscal injection | Nerve root blockade with local anaesthetic 5 ml mepivacaine, twice a week for 5 weeks |
Two further studies142,166 evaluated mixed treatments which included epidural. One study166 compared the use of epidural plus traction and exercise therapy with traction and exercise therapy without epidural.
One further study142 compared disc surgery plus epidural (mixed treatments) with conventional care given while waiting for surgery. However, the study reported only health-care utilisation and employment-related outcomes.
Summary of study participants for epidural/intradiscal injections
Summary data for included participants are presented in Table 18. The number of participants included in the 28 studies that reported outcome data for global, pain or CSOMs ranged from 23 to 278 (median 74). Most epidural studies included patients with either acute or chronic sciatica. Only two studies145,176 included patients with acute sciatica (one epidural vs activity restriction and one epidural vs inactive control), with a mean of 34 days145 or a median 4 weeks176 for symptom duration of the current episode. One study94 only included patients with the first episode of sciatica (epidural vs disc surgery) and one study154 only included patients with recurrent symptoms (epidural vs usual care). The remaining studies included first and recurrent episodes or more usually did not report this information. Fifteen studies included patients who had received previous treatment for their current episode of sciatica; this information was not stated for the remaining studies. Two studies included patients who had previously received an epidural for their current episode, but this information was not reported for most studies. Three studies94,156,169 included patients who had had previous disc surgery, one of which169 did not report data on global effect, pain or CSOMs. (One study95 compared the use of epidural with disc surgery.) Two studies156,166 (comparator included non-opioids) included some patients with spinal stenosis and one study94 (epidural vs disc surgery) included patients with sequestered discs.
| ID no. | Author, year | Study design | No. of patients | Age (years) | No. of men (%) | Symptom duration | Type of sciatica | Confirmed by imaging? | Recurrent episode | Included patients with stenosis?a | Included patients with sequestered disc (or extruded)?a | Any previous treatment for sciatica? | Any previous back surgery for sciatica? | Any previous epidural? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epidural vs activity restriction | ||||||||||||||
| 140 | Coomes, 1961145 | Non-RCT | 40 | Mean 43 (range 16–70) | 26 (65) | Mean 34 days | Nerve root pain | No | NR | No | No | Yes | NR | NR |
| Epidural vs alternative/non-traditional | ||||||||||||||
| 667 | Wehling, 1997167 (German language) | CCS | 278 | NR | NR | At least 3 months | Nerve root pain and referred pain | No | NR | No | No | NR | NR | Yes |
| Epidural vs biological agents | ||||||||||||||
| 321 | Becker, 2007149 | RCT | 90 | Mean 53.9 (range 29–81) | 52 (62) | At least 6 weeks | Nerve root pain | Yes | NR | No | No | NR | NR | No epidural in last 3 months |
| Epidural vs chemonucleolysis | ||||||||||||||
| 720 | Bontoux, 1990168 (French language) | RCT | 80 | Mean 40 | 50 (63) | At least 2 months; > 6 months 34% | Nerve root pain | Yes | NR | No | No | Yes | NR | Yes |
| 447 | Bourgeois, 1988160 (French language) | RCT | 60 | Mean 37 (range 26–62) | 40 (67) | Mean 178 (range 50–700) days | Nerve root pain | Yes | Recurrent and first episode | No | No | Yes | NR | Yes |
| 729 | Gallucci, 2007170 | RCT | 159 | Mean 41.5 (range 18–71) | 86 (54) | Mean 15 weeks | Nerve root pain | Yes | NR | No | No | Yes | ||
| 50 | Graham, 1976144 | Non-RCT | 40 (23 with sciatica) | Mean 42 Sciatica patients: mean 41 (range 24–66) | 25 (63). Sciatica patients: 13 (57) | Mean back pain or sciatica for whole group 5.35 years. Sciatica patients median 1 year (range 12 weeks–25 years) | Nerve root pain and referred pain | Yes | NR | No | No | Yes | NR | NR |
| Epidural vs disc surgery | ||||||||||||||
| 725 | Buttermann, 200495 | RCT | 100 | Mean 40.5 (SD 12) | Mean 3.55 months (SD 2.75 months) | Nerve root pain | Yes | First episode | No | Yes | Yes | Yes | NR | |
| Epidural vs education/advice | ||||||||||||||
| 722 | Bronfort, 2004169 | RCT | 32 | Mean 49.0 (SD 9.1) | 18 (56) | 1–3 months 19%, 4–6 months 6%, 7–12 months 9%, > 12 months 66% | Nerve root pain and referred pain | No | Recurrent and first episode | No | No | NR | Yes | NR |
| Epidural vs inactive control | ||||||||||||||
| 203 | Bush, 1991147 | RCT | 23 | Mean 37.8 (range 23–71) | 15 (65) | Mean 4.7 months (range 1–13 months) | Nerve root pain | No | NR | No | No | NR | NR | NR |
| 350 | Carette, 1997152 | RCT | 158 | Mean 39.8 (SD 10.2) | 103 (65) | Median 13 weeks | Nerve root pain | Yes | Recurrent and first episode | No | No | NR | No | No epidural in last year |
| 383 | Dilke, 1973157 | RCT | 100 | Mean 40.4 (range 18–75) | 55 (56) | 1–4 weeks 10%, 4 weeks–3 months 27%, 3–6 months 33%, 6–12 months 17%, 1–2 years 10%, > 2 years 2% | Nerve root pain | No | Recurrent and first episode | No | No | NR | No | NR |
| 512 | Helliwell, 1985162 | RCT | 39 | Mean 46 (range 20–69) | 9 (23) | Mean 10.7 months (range 2.5–48 months) | Nerve root pain | No | NR | No | No | NR | No | NR |
| 739 | Karppinen, 2001171 | RCT | 160 | Mean 43.8 (SD 13) | 115 (72) | 2.5 months (SD 1.5 months) | Nerve root pain | Yes | Recurrent and first episode | No | No | NR | No | No |
| 539 | Klenerman, 1984163 | RCT | 74 | NR | NR | < 6 months | Nerve root pain | No | NR | No | No | NR | No | NR |
| 905 | Mathews, 1987176 | RCT | 57 | Median 40 (range 18–59) | 43 (75) | Median 4 weeks (range 3 days–3 months) | Nerve root pain | No | NR | NR | NR | NR | NR | NR |
| 778 | Price, 2005173 | RCT | 228 | Mean 43.5 (SD 12) | 121 (53) | < 4 months 37%, 4–18 months 63% | Nerve root pain | No | Recurrent and first episode | No | No | Yes | No | No |
| 620 | Ridley, 1988165 | RCT | 39 | Mean 39 (SD 10) | 15 (43) | Mean 8.2 months (SD 6.8 months) | Nerve root pain | No | Recurrent and first episode | No | No | NR | No | None for current episode |
| 240 | Snoek, 1977148 | RCT | 51 | Mean 45 (range 26–67) | 26 (51) | Mean 11.2 weeks (range 12 days–36 weeks) | Nerve root pain | Yes | NR | No | No | NR | No | NR |
| 406 | Vad, 2002158 | RCT | 50 | Mean 41.5 | NR | > 6 weeks | Nerve root pain | Yes | NR | No | No | Yes | No | No |
| 351 | Valat, 2003153 | RCT | 85 | Mean 41 (SD 10.4) | 46 (54) | > 15 days and < 180 days | Nerve root pain and refereed pain | No | Recurrent and first episode | No | No | NR | No | No spinal injection in last month |
| 175 | Yates, 1978146 | RCT | 20 | NR | NR | NR | Nerve root pain and referred pain | No | NR | No | No | NR | NR | NR |
| Epidural vs mixed treatment | ||||||||||||||
| 439 | Blonna, 2004159 (Italian language) | RCT | 50 | Mean 61 (SD 15) | NR | Mean 84 days (SD 48 days) | Nerve root pain | Yes | NR | Yes | NR | Yes | No | No |
| 348 | Pirbudak, 2003150 | RCT | 92 | Mean 49 (SD 12.1) | 30 (33) | Median 16.5 months (range 6–48 months) | Nerve root pain | Yes | NR | No | No | Yes | No | No epidural in last year |
| Epidural vs non-opioids | ||||||||||||||
| 451 | Bronfort, 2000161 | RCT | 20 | Mean 44.5 (SD 10.6) | 12 (60) | ≤ 3 weeks n = 6, 4–12 weeks n = 14 | Nerve root pain and refereed pain | No | NR | No | No | Yes | No | NR |
| 20 | Dincer, 2007143 | RCT | 64 | Mean 28 (SD 5) | 46 (72) | 1–12 months | Nerve root pain and refereed pain | Yes | NR | No | No | NR | No | NR |
| 771 | Lafuma, 1997172 | RCT | 108 | Mean 42.1 (SD 10.6) | 66 (61) | Mean 56 days (range 1–854 days) | Nerve root pain | NR | Recurrent and first episode | No | NR | Yes | NR | NR |
| 362 | Wilson-MacDonald, 2005156 | RCT | 93 | Mean 49 (range 23–79) | 37 (40) | > 6 weeks, exact duration NR | Nerve root pain | Yes | NR | Yes | No | Yes | Yes | Partial (seven patients had previous epidural) |
| 846 | Murata, 2009175 | RCT | 246 (136 radicular pain) | Mean 68 (SD 12, range 27–90) | 90 (37) | Median 31 months (SD 52 months) | Nerve root pain | No | NR | NR | NR | Yes | No | No |
| Epidural vs passive PT | ||||||||||||||
| 359 | Veihelmann, 2006155 | RCT | 99 | Mean 44.5 (SD 24) | 45 (45) | NR | Nerve root pain | Yes | NR | No | No | Yes | NR | NR |
| Epidural vs usual/conventional care | ||||||||||||||
| 349 | Buchner, 2000151 | RCT | 36 | Mean 34.3 (range 20–50) | 23 (64) | Median 8 weeks (range 1–150 weeks) | Nerve root pain | Yes | NR | No | No | NR | No | NR |
| 828 | Laiq, 2009174 | Q-RCT | 52 | Mean 40.5 (SD 2.3) | 32 (62) | > 2 weeks | Nerve root pain | Yes | NR | No | NR | NR | NR | No |
| 581 | Matyjek, 1986164 (Polish language) | CCS | 629 | NR | NR | NR | Nerve root pain | No | NR | NR | NR | NR | NR | NR |
| 358 | Popiolek, 1991154 (Polish language) | Non-RCT | 60 | Mean 41.3 (range 27–63) | 39 (65) | Mean 1.95 months | Nerve root pain | Yes | Recurrent | NR | NR | NR | NR | NR |
| Mixed treatment incorporating epidural vs mixed treatment without epidural | ||||||||||||||
| 644 | Styczynski, 1997166 (Polish language) | Non-RCT | 103 | Range 27–85 | 57 (55) | Mean 4 weeks one group; 5 months | Nerve root pain | Yes | NR | Yes | NR | NR | No | NR |
Summary of study design and quality for epidural/intradiscal injection studies
Summary information on study details is presented in Table 19, excluding studies146,161,164,169,172 that did not report outcome data for global effect, pain intensity or CSOMs. Most included epidural studies were RCTs (24/29, 83%); however, the proportion that were deemed good quality was very low (4/29, 14%), all of which compared epidural with inactive control. Although 10 studies149,152,153,156,160,163,165,168,171,173 used and adequate method for generating a random number sequence, eight of these used sealed envelopes to conceal allocation, which is a partially adequate method. Only one study had good external validity. 171
| ID no. | Author, year | Study size | Overall follow-up | Study design | Adequate randomisation? | Allocation concealment? | Follow-up (%) | Blind outcome assessment? | Overall quality rating | Overall external validity rating |
|---|---|---|---|---|---|---|---|---|---|---|
| Epidural vs activity restriction | ||||||||||
| 140 | Coomes, 1961145 | 40 | 9 weeks | Non-RCT | No | No | 80–100 | No | Weak | Weak |
| Epidural vs alternative/non-traditional | ||||||||||
| 667 | Wehling, 1997167 (German language) | 278 | 5 weeks | CCS | No | No | 80–100 | No | Weak | Weak |
| Epidural vs biological agents | ||||||||||
| 321 | Becker, 2007149 | 90 | 22 weeks | RCT | Yes | Partial | 80–100 | Yes | Moderate | Weak |
| Epidural vs chemonucleolysis | ||||||||||
| 720 | Bontoux, 1990168 (French language) | 80 | 3 months | RCT | Yes | Unclear | 80–100 | Yes | Moderate | Weak |
| 447 | Bourgeois, 1988160 (French language) | 60 | 6 months | RCT | Yes | Partial | 80–100 | Yes | Moderate | Weak |
| 729 | Gallucci, 2007170 | 159 | 6 months | RCT | Unclear | Unclear | 80–100 | Yes | Moderate | Weak |
| 50 | Graham, 1976144 | 40 (23 with sciatica) | 2 years | Non-RCT | No | No | 80–100 | Yes | Weak | Weak |
| Epidural vs disc surgery | ||||||||||
| 725 | Buttermann, 200495 | 100 | 2–3 years | RCT | Unclear | Unclear | 80–100 | No | Moderate | Moderate |
| Epidural vs education/advice | ||||||||||
| 722 | Bronfort, 2004169 | 32 | 52 weeks | RCT | Unclear | Partial | 80–100 | Unclear | Weak | Weak |
| Epidural vs inactive control | ||||||||||
| 203 | Bush, 1991147 | 23 | 1 year | RCT | Unclear | Unclear | 60–79 | Yes | Moderate | Weak |
| 350 | Carette, 1997152 | 158 | 3 months | RCT | Yes | Partial | 60–79 | Yes | Strong | Moderate |
| 383 | Dilke, 1973157 | 100 | 3 months | RCT | Unclear | Unclear | 60–79 | Yes | Moderate | Weak |
| 512 | Helliwell, 1985162 | 39 | 3 months | RCT | Unclear | Unclear | 80–100 | Unclear | Moderate | Weak |
| 739 | Karppinen, 2001171 | 160 | 1 year | RCT | Yes | Partial | 80–100 | Yes | Strong | Strong |
| 539 | Klenerman, 1984163 | 74 | 2 months | RCT | Yes | Partial | 80–100 | Yes | Weak | Weak |
| 905 | Mathews,1987176 | 57 | 12 months | RCT | Partial | Unclear | 60–79 | Yes | Moderate | Moderate |
| 778 | Price, 2005173 | 228 | 12 months | RCT | Yes | Partial | 80–100 | Yes | Strong | Moderate |
| 620 | Ridley, 1988165 | 39 | 6 months | RCT | Yes | Unclear | 80–100 | Yes | Moderate | Weak |
| 240 | Snoek, 1977148 | 51 | Ranged from 8 to 20 months | RCT | Unclear | Unclear | 80–100 | Yes | Weak | Weak |
| 406 | Vad, 2002158 | 50 | Mean 16 months (range 12–21 months) | RCT | Unclear | Unclear | 80–100 | Yes | Moderate | Weak |
| 351 | Valat, 2003153 | 85 | 35 days | RCT | Yes | Partial | 80–100 | Yes | Strong | Weak |
| 175 | Yates, 1978146 | 20 | 1 month | RCT | Unclear | Unclear | Cannot tell | Unclear | Weak | Weak |
| Epidural vs mixed treatment | ||||||||||
| 439 | Blonna, 2004159 (Italian language) | 50 | 60 days | RCT | Unclear | Partial | 80–100 | Unclear | Weak | Moderate |
| 348 | Pirbudak, 2003150 | 92 | 9 months | RCT | Partial | No | 80–100 | Yes | Moderate | Weak |
| Epidural vs non-opioids | ||||||||||
| 451 | Bronfort, 2000161 | 20 | 12 weeks | RCT | Unclear | Partial | 80–100 | NA | Moderate | Weak |
| 20 | Dincer, 2007143 | 64 | 3 months, assessment at day 15, first month and third month | RCT | Unclear | Unclear | 80–100 | Yes | Moderate | Moderate |
| 771 | Lafuma, 1997172 | 108 | 3 months | RCT | Unclear | Unclear | 80–100 | No | Weak | Weak |
| 362 | Wilson-MacDonald, 2005156 | 93 | 35 days | RCT | Yes | Partial | 80–100 | Unclear | Moderate | Moderate |
| 846 | Murata, 2009175 | 246 (136 radicular pain) | 7 days | RCT | Unclear | Partial | 80–100 | Unclear | Weak | Weak |
| Epidural vs passive PT | ||||||||||
| 359 | Veihelmann, 2006155 | 99 | 12 months | RCT | Partial | Yes | < 60 | Yes | Moderate | Weak |
| Epidural vs usual/conventional care | ||||||||||
| 349 | Buchner, 2000151 | 36 | 6 months | RCT | Partial | Partial | 80–100 | Unclear | Moderate | Weak |
| 828 | Laiq, 2009174 | 52 | 6 months | Q-RCT | No | No | 80–100 | No | Weak | Weak |
| 581 | Matyjek, 1986164 (Polish language) | 629 | Not stated | CCS | No | No | 80–100 | No | Weak | Weak |
| 358 | Popiolek, 1991154 (Polish language) | 60 | 21 days | Non-RCT | Unclear | Unclear | 80–100 | Unclear | Weak | Weak |
| Mixed treatment incorporating epidural vs mixed treatment without epidural | ||||||||||
| 644 | Styczynski, 1997166 (Polish language) | 103 | 10 days | Non-RCT | No | No | 80–100 | No | Weak | Weak |
Epidural/intradiscal injection results at short-term follow-up (≤ 6 weeks)
Global effect at short-term follow-up
The results for the global effect at short-term follow-up are presented in Table 20 and the accompanying forest plot (Figure 14). Epidural/intradiscal injections were compared with inactive control, usual care and chemonucleolysis (not widely used in the UK NHS). One study176 included only patients with acute sciatica, and the remaining studies included patients with either acute or chronic sciatica. The duration of follow-up ranged from 24 hours148 to 6 weeks. 173
| ID no. | Author, year | Chronicity | Study design | Follow-up | Outcome measure | Perspective | Intervention | Control | OR (95% CI) | Comments | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n) | Outcome (n) | Withdrawal rate | Total (n) | Outcome (n) | Withdrawal rate | |||||||||
| Epidural vs chemonucleolysis | ||||||||||||||
| 729 | Gallucci, 2007170 | A + C | RCT | 2 weeks | Treatment success: ODI ≤ 20% | 82 | 72 | 0 | 77 | 69 | 0 | 0.83 (0.31 to 2.24) | ||
| Epidural vs inactive control | ||||||||||||||
| 350 | Carette, 1997152 | A + C | RCT | 3 weeks | Marked or very marked improvement | 75 | 42 | 0.04 | 78 | 44 | 0.03 | 1.15 (0.58 to 2.27) | Data reported as percentages. ITT reported for study using LOCF, but data missing for three patients for global outcome; not stated how missing data handled for binary outcomes | |
| 905 | Mathews, 1987176 | A | RCT | 1 month | Recovered: pain score of 5 or 6 (vs not recovered: scores of 1–4) | 21 | 14 | 0.09 | 32 | 18 | 0.06 | 1.56 (0.50 to 4.89) | Number of dropouts reported were different to the number missing from the analysis | |
| 778 | Price, 2005173 | A + C | RCT | 6 weeks | Global improvement: 75% improvement in ODI | 120 | 20 | 0 | 108 | 16 | 0 | 1.15 (0.56 to 2.35) | ||
| 620 | Ridley, 1988165 | A + C | RCT | 2 weeks | Reported some improvement | Patient | 19 | 17 | 0.10 | 16 | 3 | 2 | 36.83 (5.35 to 253.62) | |
| 240 | Snoek, 1977148 | A + C | RCT | 24 hours | Improvement in radiating pain | 27 | 7 | 0 | 24 | 3 | 0 | 2.45 (0.56 to 10.81) | ||
| 351 | Valat, 2003153 | A + C | RCT | 35 days | Overall success | Patient | 43 | 21 | 0 | 42 | 20 | 0 | 1.05 (0.45 to 2.46) | |
| Epidural vs usual/conventional care | ||||||||||||||
| 358 | Popiolek, 1991154 (Polish language) | A + C | Non-RCT | 21 days | Overall improvement: large improvement or moderate improvement (vs no improvement) | 30 | 28 | 0 | 30 | 8 | 0 | 38.50 (7.42 to 199.87) | ||
FIGURE 14.
Summary of the findings of the global effect at short-term follow-up (≤ 6 weeks) for studies comparing epidural/interdiscal injection with alternative interventions. Note: weights are from random effects analysis.
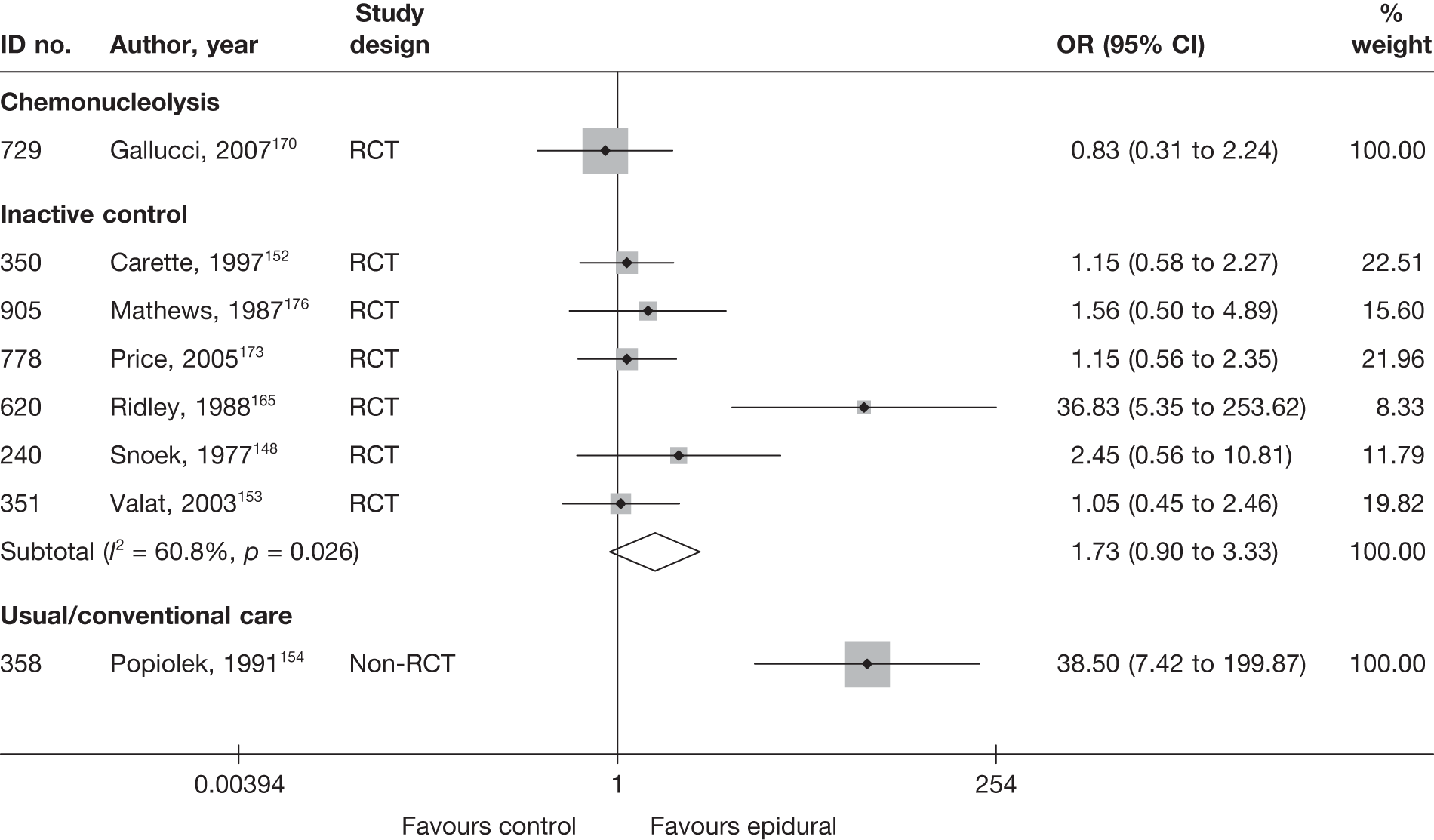
Six RCTs148,152,153,165,173,176 compared epidural injections with inactive control; the overall findings were found to be in favour of epidural, but were not statistically significant. Three RCTs152,153,173 were good quality. The study that had the largest effect size in favour of epidural injections,165 and the only study to have statistically significant results, was of poor quality.
One poorly reported non-RCT154 found that epidural injections were much better than usual care, in terms of the global effect at 21 days, in patients who had had sciatica for a mean of 2 months.
One moderate-quality RCT170 found no statistically significant difference between intraforaminal and intradiscal injections of steroid plus local anaesthetic (categorised as epidural) compared with intraforaminal and intradiscal injections of steroid, local anaesthetic and ozone–oxygen (categorised as chemonucleolysis). The study included patients with both acute and chronic sciatica, with a mean duration of symptoms of 15 weeks.
Pain intensity at short-term follow-up
The results for pain intensity at short-term follow-up are presented in Table 21 and the accompanying forest plot (Figure 15). Epidural injections/nerve block were compared with inactive control, usual care, non-opioids, alternative therapy and mixed treatments. Three studies150,167,175 included patients with chronic sciatica, one study174 did not report the duration of symptoms, and the remaining studies included patients with either acute or chronic sciatica. The duration of follow-up ranged from post treatment to 6 weeks. 158,173
| ID no. | Author, year | Chronicity | Study design | Follow-up | Location | Scale | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)a | Comment/conversionb | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |||||||||
| Epidural vs alternative | ||||||||||||||||
| 667 | Wehling, 1997167 (i)c (German language) (steroid + local anaesthetic) | C | CCS | 5 weeks | Overall | Percentage improvement (0–100) | 26 | 230 | –66 (24) | –62 (28) | –4.0 (–18.18 to 10.18) | Results reported as percentage improvement (100% improvement = no pain; 0% pain reduction = pain the same as before treatment) | ||||
| 667 | Wehling, 1997167 (ii)c (German language) | C | CCS | 5 weeks | Overall | Percentage improvement (0–100) | 26 | 230 | –48 (24) | –62 (28) | 14.0 (–2.84 to 30.84) | Results reported as percentage improvement (100% improvement = no pain; 0% pain reduction = pain the same as before treatment) | ||||
| Epidural vs inactive control | ||||||||||||||||
| 203 | Bush, 1991147 | A + C | RCT | 4 weeks | Overall | VAS (0–100) | 12 | 11 | 38.5 | 49.2 | 16.0 (22.48) | 45.0 (23.67) | –29.00 (–50.71 to –7.29) |
SD imputed from weighted average Dropouts 22%: intervention 1/12, control 4/11 ITT analysis based on LOCF |
||
| 350 | Carette, 1997152 | A + C | RCT | 3 weeks | Overall | VAS (0–100) | 77 | 79 | 65.6 (21.6) | 61.5 (21.4) | 44.9 | 49.1 | –21 (29.2) | –12.4 (27.3) | –8.60 (–17.48 to 0.28) | |
| 512 | Helliwell, 1985162 | C | RCT | 1 month | Overall | VAS (0–10) | 20 | 19 | –27.0 (21.0) | –7 (14) | –20.00 (–31.15 to –8.85) | Summary statistics derived from graphs | ||||
| 739 | Karppinen, 2001171 | A + C | RCT | 4 weeks | Leg | VAS (0–100) | 80 | 80 | 71.0 (18) | 75.2 (19) | 36.9 (35.66) | 43.9 (35.66) |
–2.80 (–13.76 to 8.16) Multivariate analysis (adjusted change from baseline): 2.3 (95% CI –8.7 to 13.4) |
SDs (and SEs) for change estimated from 95% CI of difference between treatment groups Two patients lost to follow-up from steroid group |
||
| 778 | Price, 2005173 | A + C | RCT | 6 weeks | Leg | VAS (0–100) | 120 | 108 | 52 (23) | –15 (32) | –15 (32) | 0.00 (8.32 to 8.32) | ||||
| 620 | Ridley, 1988165 | A + C | RCT | 2 weeks | Overall | VAS (0–100) | 19 | 16 | –46 | 0 | –46 | Only median percentage improvement and range reported | ||||
| 406 | Vad, 2002158 | A + C | Non-RCT | Post-treatment | Overall | VAS (0–10) | 25 | 25 | 88 (14) | 94 (14) | 16 (8) | 36 (11) | –2.70 (–12.52 to 7.12) | |||
| 351 | Valat, 2003153 | A + C | RCT | 35 days | Overall | VAS (0–100) | 43 | 42 | 57.5 (16.3) | 58 (16.6) | 22.1 (20.1) | 24.8 (25.7) | –10.73 (–18.47 to –2.99) | |||
| Epidural vs mixed treatments | ||||||||||||||||
| 439 | Blonna, 2004159 (Italian language) | A + C | RCT | 14 days | Overall | VAS (0–10) | 24 | 26 | 80.4 (10.0) | 83.5 (12.6) | 34.3 (22.48) | 35.6 (22.86) | –1.30 (–22.07 to 19.47) |
SD imputed from weighted average ITT using LOCF, dropouts 3 (6%): intervention 3/26, control 0/24 |
||
| 348 | Pirbudak, 2003150 | C | RCT | 6 weeks | Overall | VAS (0–10) | 46 | 46 | 84.0 (17.0) | 78.1 (40.0) | 40 | 11.0 | –44.0 (22.0) | –49.0 (10.0) | 5.00 (–1.98 to 11.98) | |
| Epidural vs non-opioids | ||||||||||||||||
| 20 | Dincer, 2007143 | A + C | RCT | 1 month | Overall | VAS (0–10) | 34 | 30 | 69 (10) | 68 (10) | 32 (11) | 44 (13) | –12.00 (–17.94 to –6.06) | |||
| 846 | Murata, 2009175 | C | RCT | 7 days | Leg | VAS (0–100) | 69 | 65 | 43 (22.48) | 67 (22.86) | –24.00 (–31.63 to –16.37) |
SD imputed from weighted average Subgroup analysis based on 136/246 (55%) with radicular pain; intervention 71/122, control 65/124. Dropouts: 8/246 (3%); no further details |
||||
| 362 | Wilson-MacDonald, 2005156 | NR | RCT | 35 days | Overall | Oxford pain chart | Significant difference in pain relief between groups, in favour of epidural (p < 0.004, test not stated) |
Summary statistics not reported Dropouts 14/93 (15%): group allocation not stated |
||||||||
| Epidural vs usual/conventional care | ||||||||||||||||
| 349 | Buchner, 2000151 | A + C | RCT | 2 weeks | Overall | VAS (0–100) | 17 | 19 | 84.4 | 81 | 30.8 (12.47) | 37.1 (12.47) | –6.30 (–14.46 to 1.86) |
2-week data used instead of 6-weeks because p-value for one-sided t-test available to calculate SD Dropouts 9/31 (29%): intervention 4/16, control 5/15 |
||
| 828 | Laiq, 2009174 | NR | Q-RCT | 1 month | Overall | VAS (0–10) | 25 | 25 | 20 (15) | 45 (14.8) | –15.64 (–33.96 to 2.69) | Dropouts 2/52 (4%): intervention 1/26, control 1/26 | ||||
| Mixed treatment incorporating epidural vs mixed treatment without epidural | ||||||||||||||||
| 644 | Styczynski, 1997166 (Polish language) | A + C | Non-RCT | 10 days | Overall | VAS (1–100) | 58 | 45 | 100 | 100 | 39.8 | 53.8 | 14 |
Pain scale used was not stated SD not reported and no statistical analysis undertaken |
||
FIGURE 15.
Summary of the findings of pain intensity at short-term follow-up (≤ 6 weeks) for studies comparing epidural/intradiscal injections with alternative interventions. Note: weights are from random effects analysis.
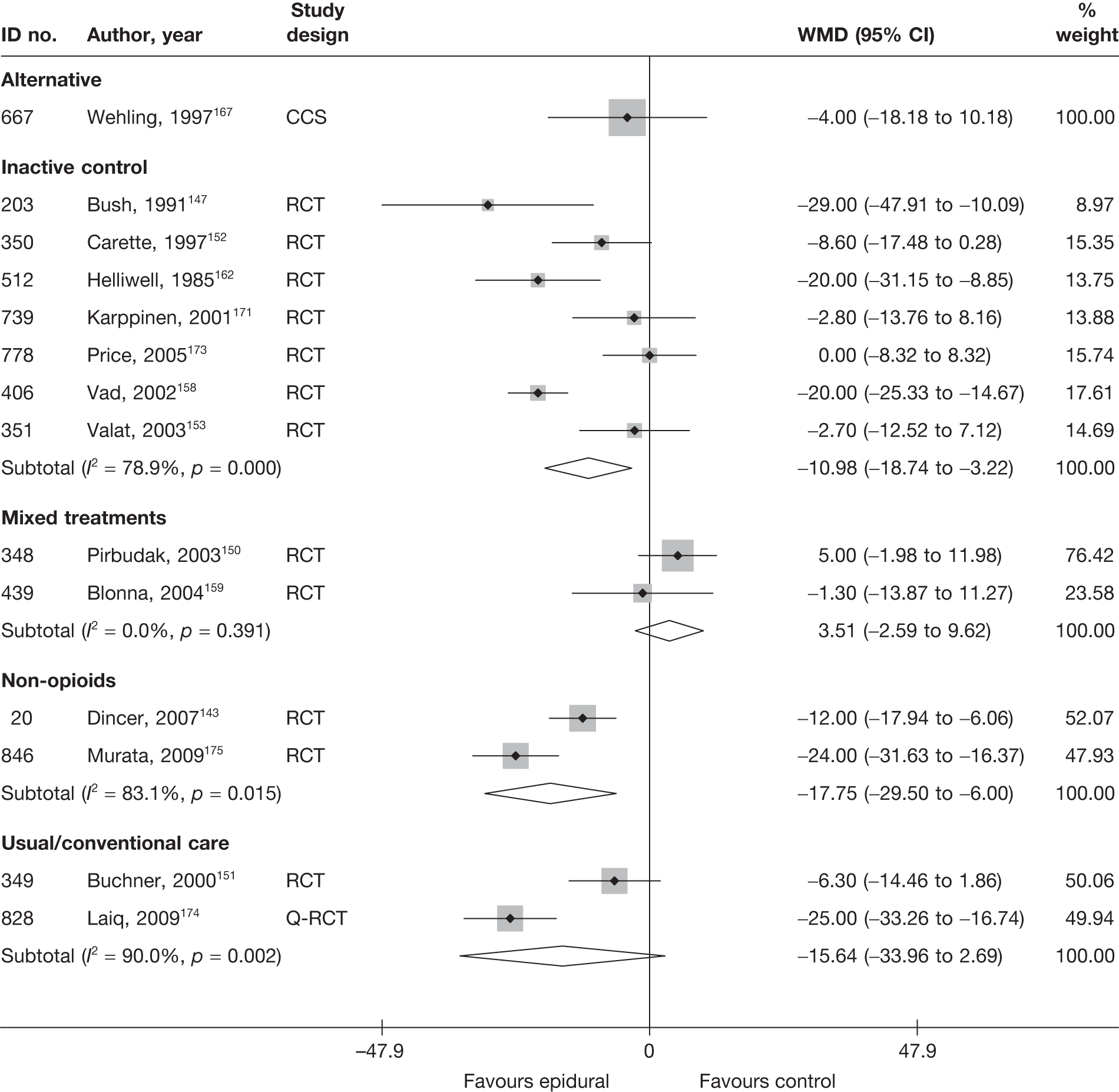
The overall findings from seven RCTs147,152,153,158,162,171,173 found a statistically significant reduction in pain intensity for epidural injections compared with inactive control. Four of these RCTs152,153,171,173 were good quality; three were moderate quality. One study171 was also considered as having good external validity, whereas four147,153,158,162 of the seven were rated as poor. One further RCT165 found epidural injection to be superior to inactive control, but reported data only for median percentage improvement.
One moderate-quality RCT151 and one Q-RCT174 compared epidural injections with usual care. The Q-RCT174 reported a statistically significant improvement in favour of epidural injection; the RCT151 reported a smaller improvement which was not statistically significant. When the results were combined in a meta-analysis, there was no statistically significant difference.
Epidural injections were found to be significantly better than non-opioids at reducing pain at 1 week to 1 month, according to two poorly reported RCTs of weak to moderate quality. 143,175 One further poorly reported RCT,156 of moderate quality, found epidural to be significantly better than non-opioids for pain relief at 35 days (p < 0.004, statistical test not stated), but did not report any summary statistics.
Two RCTs150,159 compared the use of epidural injection with epidural injection plus non-opioids (mixed treatments) at 2–6 weeks, and found no overall benefit. One RCT150 was of moderate quality, and included blinding of participants, clinicians and outcome assessors. Patients were randomly assigned to the two groups by one of the authors by drawing sealed envelopes from a box. The second RCT159 was poorly reported and of poor quality. The SDs for this study were not reported and have been imputed using the weighted mean.
One CCS167 found no important difference between nerve root block and acupuncture plus herbal medicine for pain relief at 5 weeks in patients with chronic sciatica.
Condition-specific outcome measures at short-term follow-up
The results for CSOMs at short-term follow-up are presented in Table 22 and the accompanying forest plot (Figure 16). Epidural injections were compared with inactive control, usual care, biological agents and mixed treatments. One study150 included patients with chronic sciatica, and the remaining studies included patients with either acute or chronic symptoms. The duration of follow-up ranged from post treatment to 6 weeks. 149–151,158,173
| ID no. | Author, year | Chronicity | Study design | Follow-up | Scale | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)a | Comment/conversionb | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | ||||||||
| Epidural vs biological agents | |||||||||||||||
| 321 | Becker, 2007149 (i)c (5 mg) | A + C | RCT | 6 weeks | ODI | 27 | 32 | 20.6 (8.1) | 22.0 (8.3) | 12.1 (9.0) | 13.8 (9.8) | –0.18 (–0.69 to 0.33) | |||
| 321 | Becker, 2007149 (ii)c (10 mg) | A + C | RCT | 6 weeks | ODI | 25 | 32 | 19.4 (9.9) | 22.0 (8.3) | 11.0 (9.5) | 13.8 (9.8) | –0.29 (–0.82 to 0.24) | |||
| Epidural vs inactive control | |||||||||||||||
| 350 | Carette, 1997152 | A + C | RCT | 3 weeks | Modified ODI | 77 | 80 | 49.6 (15.7) | 50 (15.5) | 41.6 (15.7) | 44.5 (15.5) | –8 (15.3) | –5.5 (14.3) | –0.19 (–0.50 to 0.13) | Final SD missing, so baseline SD used ITT using LOCF: one dropout excluded Analysis of variance results not reported |
| 739 | Karppinen, 2001171 | A + C | RCT | 4 weeks | ODI | 80 | 80 | 42.9 (16) | 43.5 (15) | 26.8 (16) | 29.1 (15) | –16.1 (18.88) | –14.4 (18.88) |
–0.15 (–0.46 to 0.16) Adjusted change from baseline –0.4 (95% CI –7.0 to 6.2) |
Final SD missing, so baseline SD used |
| 778 | Price, 2005173 | A + C | RCT | 6 weeks | ODI | 120 | 108 | 44 (15) | 45 (18) | 31 (15) | 35 (18) | –13 (17) | –10 (18) | –0.24 (–0.50 to 0.02) |
Final mean calculated from change scores Baseline SD used ITT using LOCF |
| 406 | Vad, 2002158 | A + C | RCT | Post-treatment | RMDQ | 25 | 25 | 8.8 (1.2) | 9.6 (1.3) | 0.9 (1.6) | 4.7 (2.1) | 13.3 | 8.7 | –2.04 (–2.72 to –1.35) | |
| 351 | Valat, 2003153 | A + C | RCT | 35 days | RMDQ | 43 | 42 | 15.1 (4.7) | 14.2 (4.2) | 8.5 (5.4) | 9.1 (5.4) | –6.6 | –5.1 | –0.11 (–0.54 to 0.31) | ITT using LOCF Dropouts 22/85 (26%): intervention 9/43, control 13/42 |
| Epidural vs non-opioids | |||||||||||||||
| 20 | Dincer, 2007143 | A + C | RCT | 1 month | ODI | 34 | 30 | 35.8 (6.7) | 34.4 (6.7) | 17 (7.3) | 22.2 (8.6) | –18.8 | –12.2 | –0.66 (–1.16 to –0.15) | |
| Epidural vs mixed treatments | |||||||||||||||
| 348 | Pirbudak, 2003150 | C | RCT | 6 weeks | ODI | 46 | 46 | 49.6 (15.5) | 50.2 (15.2) | 32 (15.5) | 28 (15.2) | –17.6 (20.5) | –21.8 (24.5) | 0.26 (–0.15 to 0.67) | Final SD missing, so baseline SD used |
| Epidural vs usual/conventional care | |||||||||||||||
| 349 | Buchner, 2000151 | A + C | RCT | 6 weeks | Hannover Functional Ability | 17 | 19 | 38.5 | 39.9 | 36.3 (6.01) | 42.5 (6.01) | –1.03 (–1.73 to –0.33) | 2-week data used instead of 6-week data because p-value for one-sided t-test available to calculate SD | ||
FIGURE 16.
Summary of the findings of CSOMs at short-term follow-up (≤ 6 weeks) for studies comparing epidural/intradiscal injections with alternative interventions. Note: weights are from random effects analysis.
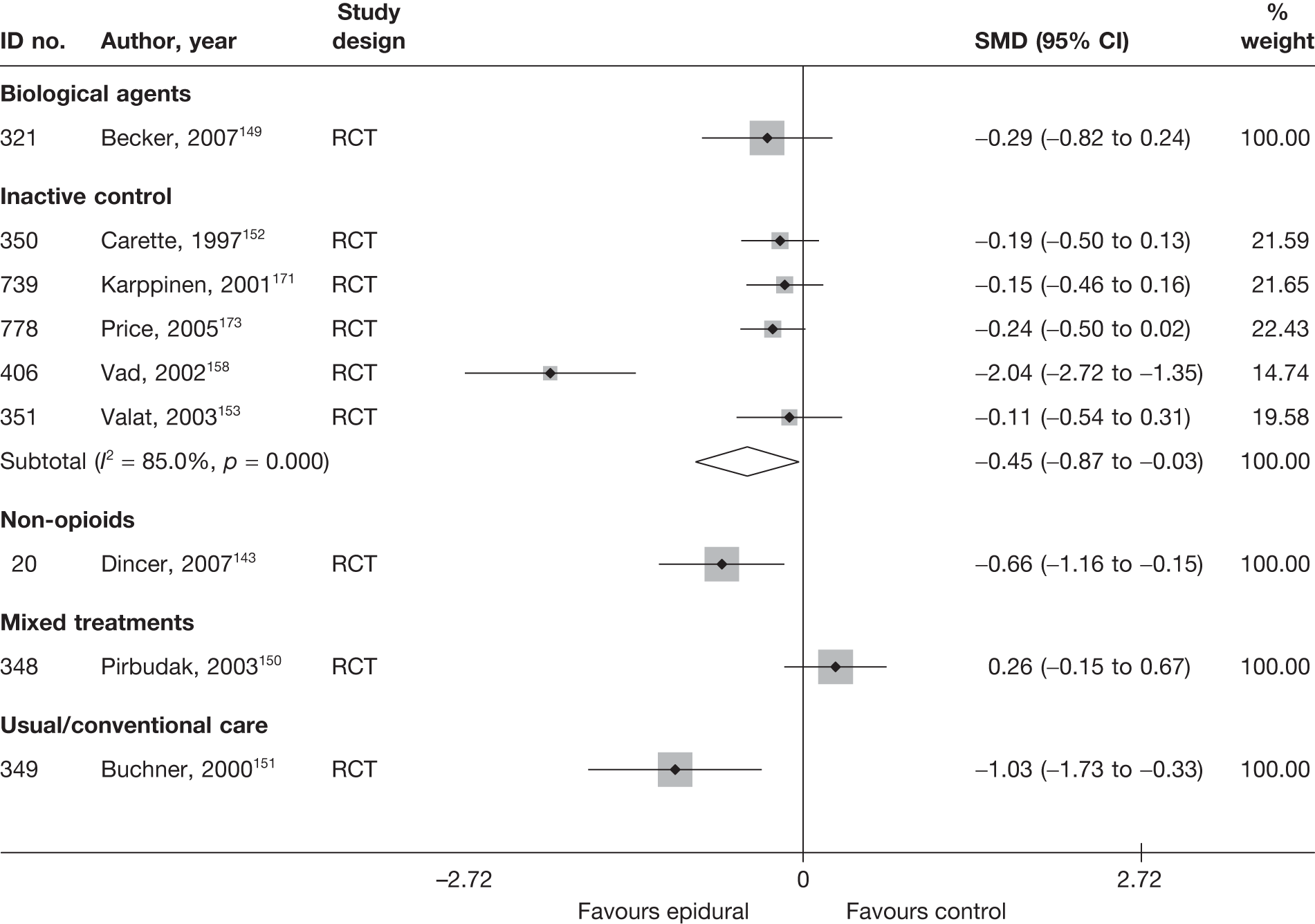
The overall findings from five RCTs152,153,158,171,173 showed epidural injections to be significantly better than inactive control for improving function. The findings were heterogeneous, with one poor-quality RCT158 reporting a large effect size in favour of epidural injection. The quality of the remaining RCTs152,153,171,173 was good, and pooled analysis showed a significant difference in favour of epidural (SMD –0.19; 95% CI –0.34 to –0.03).
One moderate-quality RCT151 found epidural to be significantly better than usual care for improving functional status at 6 weeks’ follow-up.
One moderate-quality RCT143 found epidural to be significantly better than non-opioids for improving functional status at 4 weeks’ follow-up. The methods of randomisation and allocation concealment were not stated.
One moderate-quality RCT150 found no statistically significant difference between epidural injection in combination with non-opioids (mixed treatments) and epidural injection alone for improving functional status for patients with chronic sciatica at 6 weeks’ follow-up.
One moderate-quality RCT149 compared epidural using two different dosages of steroid with an epidural injection of autologous conditioned serum (biological agent). There was no statistically significant difference between either dose of epidural steroid and the biological agent at 6 weeks.
One poorly conducted non-RCT,166 reported a greater decrease in pain intensity for patients treated with epidural, traction and exercise therapy than those treated with traction and exercise therapy without epidural.
Epidural/intradiscal injections results at medium-term follow-up (> 6 weeks to ≤ 6 months)
Global effect at medium-term follow-up
The results for the global effect at medium-term follow-up are presented in Table 23 and the accompanying forest plot (Figure 17). Epidural/intradiscal/nerve block injections were compared with inactive intervention, usual care, activity restriction, non-opioids, passive PT and chemonucleolysis. One study145 included only patients with acute sciatica, whereas five studies155,160,162,168,175 included only patients with chronic symptoms. The remaining studies included patients with either acute or chronic sciatica, or did not state the duration of symptoms. 174 The duration of follow-up ranged from 2 months163,175 to 6 months. 151,155,160,170,174
| ID no. | Author, year | Chronicity | Study design | Follow-up | Outcome measure | Perspective | Intervention | Control | OR (95% CI)a | Comments | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n) | Outcome (n) | Withdrawal rate | Total (n) | Outcome (n) | Withdrawal rate | |||||||||
| Epidural vs activity restriction | ||||||||||||||
| 140 | Coomes, 1961145 | A | Non-RCT | 9 weeks | Neurological state: completely relieved or improved (vs not changed or worse) | Physician | 20 | 12 | 0 | 20 | 5 | 0 | 4.50 (1.17 to 17.37) | |
| Epidural vs chemonucleolysis | ||||||||||||||
| 720 |
Bontoux, 1990168 (French language) |
C | RCT | 3 months | Overall improvement: very good or good (vs mediocre or bad; other cases) | 40 | 27 | 0 | 40 | 26 | 0 | 1.12 (0.44 to 2.83) | ||
| 447 |
Bourgeois, 1988160 (French language) |
C | RCT | 6 months | Overall pain relief: very good or good (vs failure) | 30 | 16 | 0 | 30 | 20 | 0 | 0.57 (0.20 to 1.62) | ||
| 729 | Gallucci, 2007170 | A + C | RCT | 6 months | Treatment success: ODI ≤ 20% | 77 | 36 | 0 | 82 | 61 | 0 | 3.31 (1.70 to 6.45) | ||
| Epidural vs inactive control | ||||||||||||||
| 350 | Carette, 1997152 | A + C | RCT | 3 weeks | Marked or very marked improvement | 77 | 25 | 0.01 | 78 | 23 | 0.03 |
0.98 (0.52 to 1.86) Treatment effect –0.4% (95% CI –16.5% to 15.7%); not clear if adjusted for baseline values |
Data reported as percentages ITT reported for study using LOCF, but data missing for five patients for global outcome; not stated how missing data handled for binary outcomes |
|
| 383 | Dilke, 1973157 | A + C | RCT | 3 months | Pain: not severe or none (vs severe and unknown) | Patient | 44 | 40 | 0.14 | 38 | 28 | 0.21 | 3.57 (1.02 to 12.54) | |
| 512 | Helliwell, 1985162 | C | RCT | 3 months | Definitive improvement | Patient | 20 | 14 | 0 | 19 | 5 | 0 | 6.53 (1.61 to 26.47) | |
| 539 | Klenerman, 1984163 (i)b (steroid) | A + C | RCT | 2 months | Treatment success based on overall pain (VAS) and physical examination: not failed, i.e. improved or cured (vs failed) | Physician | 19 | 15 | ? | 16 | 11 | ? | 1.70 (0.37 to 7.85) | Number randomised unclear |
| 539 | Klenerman, 1984163 (ii)b (anaestheticd) | A + C | RCT | 2 months | Treatment success based on overall pain (VAS) and physical examination: not failed, i.e. improved or cured (vs failed) | Physician | 16 | 11 | ? | 16 | 11 | ? | 1.00 (0.22 to 4.46) | Number randomised unclear |
| 778 | Price, 2005173 | A + C | RCT | 12 weeks | Global improvement: ≥ 75% improvement in ODI | 120 | 22 | 0 | 108 | 26 | 0 | 0.71 (0.37 to 1.34) |
Data inferred from graphs reporting percentages ITT using LOCF |
|
| Epidural vs non-opioids | ||||||||||||||
| 846 | Murata, 2009175 | C | RCT | 24 weeks | Adequate recovery from leg pain | 71 | 11 | ? | 65 | 5 | ? | 2.20 (0.72 to 6.72) |
Subgroup analysis of 136/246 (55%) patients with radicular pain: intervention 71/122, control 65/124 8/246 patients dropped out, group allocation or radicular pain not stated |
|
| Epidural vs passive PT | ||||||||||||||
| 359 | Veihelmann, 2006155 | C | RCT | 6 months | Gerbershagen score (chronification index), GHS I (vs GHS II, III) | 46 | 31 | 0.02 | 27 | 8 | 0.48 | 4.91 (1.75 to 13.76) | ||
| Epidural vs usual/conventional care | ||||||||||||||
| 349 | Buchner, 2000151 | A + C | RCT | 6 months | Overall assessment: very good or good based on VAS, SLR and functional status | 17 | 15 | 0 | 19 | 14 | 0 | 2.68 (0.45 to 16.11) | ITT used | |
| 828 | Laiq, 2009174 | NR | Q-RCT | 6 months | Successfully treated: ≥ 50% reduction in pain using VAS | 25 | 21 | 0.04 | 25 | 19 | 0.04 | 1.66 (0.41 to 6.78) | Findings reported in terms of treatment failure | |
FIGURE 17.
Summary of the findings of the global effect at medium-term follow-up (> 6 weeks to ≤ 6 months) for studies comparing epidural/intradiscal injections with alternative interventions. Note: weights are from random effects analysis.
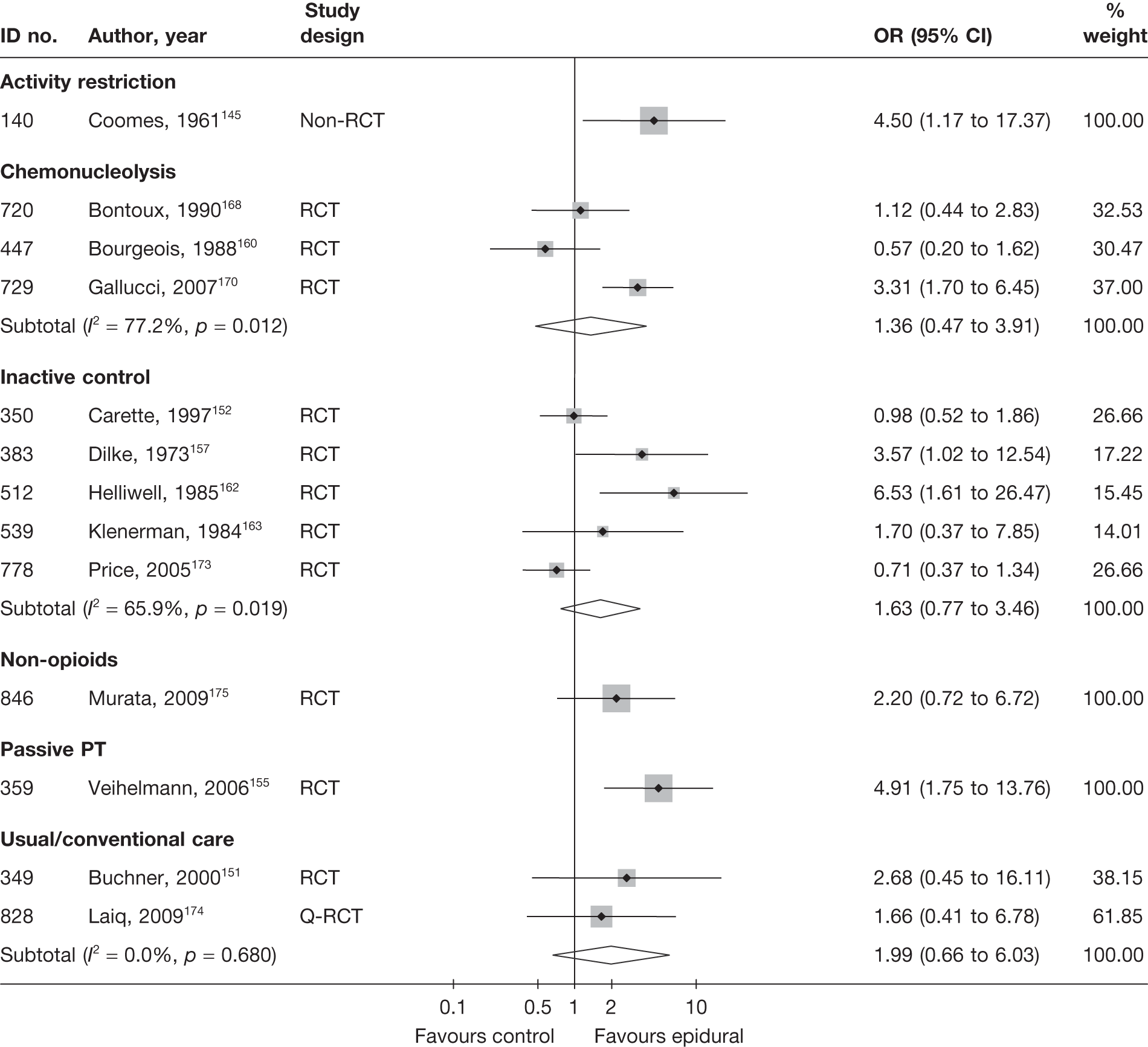
Five RCTs152,157,162,163,173 compared epidural injections with inactive control; the overall findings were in favour of epidural at 2–3 months, but the difference was not statistically significant. Two of these RCTs152,173 were good quality and two157,162 were of moderate quality.
Two moderate- or poor-quality RCTs151,174 compared epidural injection with usual care; the overall finding was in favour of epidural at 6 months, but the difference was not statistically significantt.
Epidural injection was found to be significantly better than activity restriction for overall improvement in neurological state for patients with acute sciatica (mean duration of symptoms 34 days) at 9 weeks. But these findings are based on a poor-quality non-RCT,145 which also had poor external validity.
One poor-quality RCT175 reported non-statistically significant findings in favour of epidural, compared with non-opioids, for adequate recovery from leg pain at 24 weeks. The findings were based on a subgroup analysis of 136/246 (55%) patients with radicular pain.
One moderate-quality RCT155 found epidural injections to be significantly better than passive PT in terms of the number for patients with Gerbershagen pain chronicity score I (vs II or III; pain staging system) at 6 months. However, the withdrawal rate was very high in the control group (48%) compared with the intervention group (2%). Patients in the control group had the choice to cross over to the epidural group after 3 months of unsatisfactory treatment with PT. These patients were then excluded from analysis (n = 12/52).
Two moderate-quality RCTs160,168 compared intradiscal injection with chemonucleolysis using chymopapain for chronic sciatica, and one poorly reported but moderate-quality RCT170 compared intraforaminal/intradiscal injections of steroid plus local anaesthetic (epidural) with intraforaminal/intradiscal injections of steroid, local anaesthetic and ozone–oxygen (chemonucleolysis). The first RCTs160,168 found no statistically significant difference between the intervention groups, while the third RCT170 found statistically significant findings in favour of the epidural group for patients who had had symptoms for a mean of 15 weeks.
Pain intensity at medium-term follow-up
The results for pain intensity at medium-term follow-up are presented in Table 24 and the accompanying forest plot (Figure 18). Epidural injections were compared with inactive control, usual care, passive PT, mixed treatments, disc surgery and biological agents. Three studies150,155,162 included only patients with chronic sciatica, one study174 did not report the duration of symptoms, and the remaining studies included patients with either acute or chronic sciatica. The duration of follow-up ranged from 60 days159 to 6 months. 150,151,155,171,174
| ID no. | Author, year | Chronicity | Study design | Follow-up | Location | Scale (range)a | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)b | Comment/conversionc | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |||||||||
| Epidural vs biological agents | ||||||||||||||||
| 321 | Becker, 2007149 (i)d (5 mg) | A + C | RCT | 22 weeks | Overall | VAS (0–100) | 27 | 32 | 82 | 78 | –13.5 (95% CI –27.4 to 0.4); repeated measures analysis of variance | Summary statistics not reported | ||||
| 321 | Becker, 2007149 (ii)c (10 mg) | A + C | RCT | 22 weeks | Overall | VAS (0–100) | 24 | 32 | 85 | 78 | –9.3 (95% CI –23.5 to 4.9); repeated measures analysis of variance |
Summary statistics not reported One patient in epidural group dropped out |
||||
| Epidural vs disc surgery | ||||||||||||||||
| 725 | Buttermann, 200495 | A + C | RCT | 4–6 months | Leg | VAS (0–10) | 50 | 50 | Statistically significant greater pain experienced by epidural group (p < 0.03, Student’s t-test) | Summary statistics not reported | ||||||
| Epidural vs inactive control | ||||||||||||||||
| 350 | Carette, 1997152 | A + C | RCT | 3 months | Overall | VAS (0–100) | 77 | 79 | 65.6 (21.6) | 61.5 (21.4) | 38.9 | 39.5 | –26.5 (36) | –22.5 (34.4) | –4.00 (–15.05 to 7.05) | ITT analysis used |
| 512 | Helliwell, 1985162 | C | RCT | 3 months | Overall | VAS (0–100) | 20 | 19 | –27 (21) | –4 (21) | –23.00 (–36.19 to –9.81) | |||||
| 739 | Karppinen, 2001171 | A + C | RCT | 6 months | Leg | VAS (0–100) | 78 | 80 | 71 (18) | 75.2 (19) | 30.7 (33.99) | 21.6 (33.99) |
13.30 (2.78 to 23.82) Multivariate analysis (adjusted change from baseline): –16.2 (95% CI –26.8 to –5.6) |
SDs (and SEs) for change estimated from 95% CI of difference between treatment groups ITT not used Two patients lost to follow-up from steroid group |
||
| 778 | Price, 2005173 | A + C | RCT | 12 weeks | Leg | VAS (0–100) | 120 | 108 | 52 (23) | 56 (22) | –13 (33) | –18 (33) | 5.00 (–3.58 to 13.58) | |||
| Epidural vs passive PT | ||||||||||||||||
| 359 | Veihelmann, 2006155 | C | RCT | 6 months | Leg | VAS (0–10) | 46 | 27 | 72 (135.6) | 67 (103.9) | 23 (142.4) | 58 (114.3) | –35.00 (–94.60 to 24.60) |
SD derived from SE 26 (26%) dropped out: intervention 1/47, control 25/52 |
||
| Epidural vs mixed treatments | ||||||||||||||||
| 439 | Blonna, 2004159 (Italian language) | A + C | RCT | 60 days | Overall | VAS (0–10) | 24 | 26 | 80.4 (10.0) | 83.5 (12.6) | 16.9 (12.8) | 10.2 (18.0) | 6.70 (–1.91 to 15.31) |
SD imputed from weighted average from non-opioids for intervention ITT using LOCF Dropouts: intervention 3/26, control 0/24 |
||
| 348 | Pirbudak, 2003150 | C | RCT | 6 months | Overall | VAS (0–10) | 46 | 46 | 84 (17) | 78.1 (40.0) | 42 | 8.0 | –42.0 (17.0) | –70.0 (5.0) | 28.00 (22.88 to 33.12) | |
| Epidural vs usual/conventional care | ||||||||||||||||
| 349 | Buchner, 2000151 | A + C | RCT | 6 months | Overall | VAS (0–100) | 17 | 19 | 84.4 | 81 | 32.9 (20.35) | 39.2 (20.35) | –6.30 (–19.62 to 7.02) | One-sided t-test (comparing final means) used to calculate SD | ||
| 828 | Laiq, 2009174 | NR | Q-RCT | 6 months | Overall | VAS (0–10) | 25 | 25 | 60 (14.5) | 65 (13) | –5.00 (–12.63 to 2.63) |
ITT not used Dropouts 2/52 (4%): intervention 1/26, control 1/26 |
||||
FIGURE 18.
Summary of the findings of pain at medium-term follow-up (> 6 weeks to ≤ 6 months) for studies comparing epidural/intradiscal injections with alternative interventions. Note: weights are from random effects analysis.
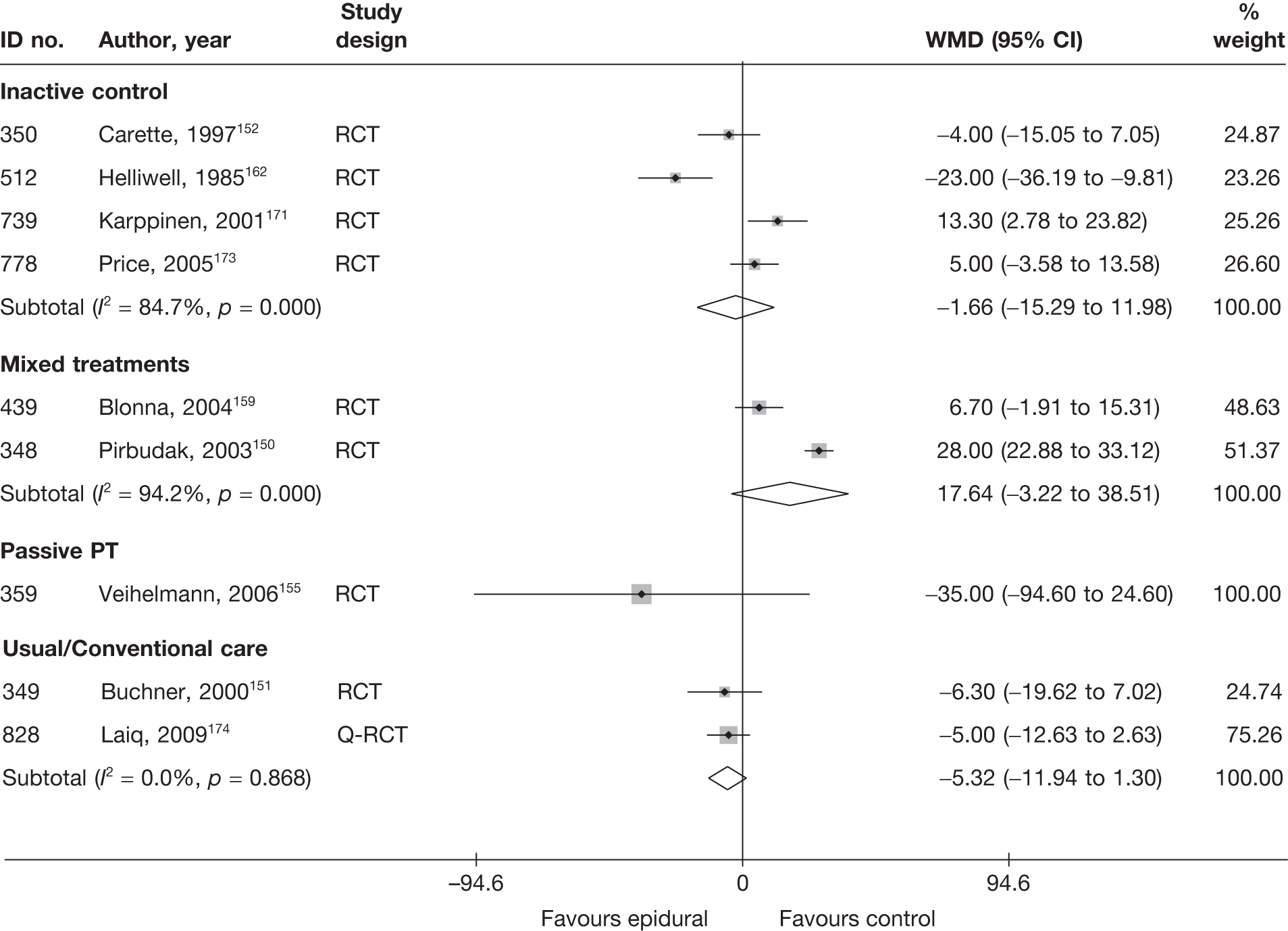
Four RCTs152,162,171,173 compared epidural injections with inactive control, for which pooled analyses showed no important difference between the groups at 3152,162,173 and 6171 months.However, the findings were heterogeneous. The overall quality for three trials152,171,173 was good. The fourth study162 was small (n = 39), poorly reported and of moderate quality, and, unlike the remaining studies, found statistically significant findings in favour of epidural. One RCT171 also reported findings based on ANCOVA, adjusted for baseline values, which favoured inactive control for leg pain at 3 months (–12.2; 95% CI –23.5 to –1.0, p = 0.003; negative values indicate a negative effect). The same analyses showed no statistically significant difference between the groups at 12 months.
Two studies151,174 compared epidural injections with usual care; the overall findings at 6 months were in favour of epidural, but were not statistically significant. One was a moderate-quality RCT and the other a Q-RCT.
One moderate-quality RCT155 reported a non-statistically significant reduction in pain intensity at 6 months in favour of epidural, compared with passive PT. The withdrawal rate was much higher in the control group (48%) than in the intervention group (2%). Patients in the control group had the choice to cross over to the epidural group after 3 months of unsatisfactory treatment with PT. These patients were then excluded from the analysis (n = 12/52).
Two RCTs150,159 compared the use of epidural injection with epidural injection plus non-opioids (mixed treatments) at 2 months159 or 6 months. 150 Overall, there was a non-statistically significant finding in favour of the mixed treatments. A much greater (and statistically significant) reduction in pain was achieved by the better-quality RCT150 than by the poor-quality and poorly reported study. 159
One poorly reported RCT95 of moderate quality compared epidural with disc surgery. The method of randomisation and allocation concealment were not reported. The level of leg pain experienced by the epidural group was significantly more than that of the disc surgery group at 4–6 months’ follow-up (p = 0.03, Student’s t-test). No summary statistics were reported and, therefore, the study is not presented in Figure 18.
One moderate-quality RCT149 compared two types of epidural (containing local anaesthetic plus triamcinolone at a dose of either 5 mg or 10 mg) with biological agents (epidural injection of autologous conditioned serum). Insufficient data were reported to include the study in Figure 18. Pair-wise analysis showed a non-statistically significant difference in favour of the biological agent for pain reduction at 22 weeks.
Condition-specific outcome measures at medium-term follow-up
The results for CSOMs at medium-term follow-up are presented in Table 25 and the accompanying forest plot (Figure 19). Epidural injections were compared with inactive control, usual care, non-opioids, passive PT, biological agents and mixed treatments. Two studies150,155 only included patients with chronic sciatica, and the remaining studies143,149,151,152,171,173 included patients with either acute or chronic sciatica. The duration of follow-up ranged from 395 to 6 months. 150,151,155,171
| ID no. | Author, year | Chronicity | Study design | Follow-up | Scale | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)a | Comment/conversionb | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | ||||||||
| Epidural vs biological agents | |||||||||||||||
| 321 | Becker, 2007149 (i)c (5 mg) | A + C | RCT | 22 weeks | ODI | 27 | 32 | 20.6 (8.1) | 22.0 (8.3) | 11.1 (7.1) | 11.7 (9.2) | –0.07 (–0.58 to 0.044) |
Dropouts 7 (8%): Number originally randomised to each group not stated |
||
| 321 | Becker, 2007149 (ii)c (10 mg) | A + C | RCT | 22 weeks | ODI | 25 | 32 | 19.4 (9.9) | 22.0 (8.3) | 11.0 (9.5) | 11.7 (9.2) | –0.08 (–0.60 to 0.45) |
Dropouts 7 (8%): Number originally randomised to each group not stated |
||
| Epidural vs disc surgery | |||||||||||||||
| 725 | Buttermann, 200495 | A + C | RCT | 1–3 months | ODI | There was a significantly greater decreasing in disability in the discectomy group compared with the epidural group at the 1–3 month follow-up interval; p < 0.015, Student’s t-test | |||||||||
| Epidural vs inactive control | |||||||||||||||
| 350 | Carette, 1997152 | A + C | RCT | 3 months | Modified ODI | 77 | 79 | 49.6 (15.7) | 50 (15.5) | 32.2 (15.7) | 34.6 (15.5) | –17.3 (20.6) | –15.4 (25.5) | –0.15 (–0.47 to 0.16) |
Final SD missing so baseline SD used ITT used LOCF Two patient dropouts excluded Analysis of variance, but results not reported |
| 739 | Karppinen, 2001171 | A + C | RCT | 6 months | ODI | 78 | 80 | 42.9 (16) | 43.5 (15) | 18.9 (16) | 15.8 (15) | –24 (21.0) | –27.7 (21.0) |
0.20 (–0.11 to 0.51) Adjusted change from baseline –5.9 (95% CI –12.4 to 7.0) |
Final SD missing, so baseline SD used ITT not used; two patients lost to follow-up from steroid group |
| 778 | Price, 2005173 | A + C | RCT | 12 weeks | ODI | 120 | 108 | 44 (15) | 45 (18) | 32 (15) | 27 (18) | –12 (19) | –12 (21) | 0.30 (0.04 to 0.56) |
Final score calculated from change score Final SD missing so baseline SD used ITT used LOCF |
| Epidural vs non-opioids | |||||||||||||||
| 20 | Dincer, 2007143 | A + C | RCT | 3 months | ODI | 34 | 30 | 35.8 (6.7) | 28.4 (5.4) | 16.2 (9.4) | 20.3 (10.1) | –19.6 | –8.1 | –0.42 (–0.92 to 0.08) | |
| Epidural vs mixed treatments | |||||||||||||||
| 348 | Pirbudak, 2003150 | C | RCT | 6 months | ODI | 46 | 46 | 49.6 (15.5) | 50.2 (15.2) | 45 (15.5) | 25 (15.2) | –7.6 (15.3) | –13.2 (15.5) | 1.30 (0.85 to 1.75) | |
| Epidural vs passive PT | |||||||||||||||
| 359 | Veihelmann, 2006155 | C | RCT | 6 months | ODI | 46 | 27 | 23.1 | 21.4 | 10.8 (50.19) | 22.5 (55.58) | –0.22 (–0.70 to 0.25) |
SD based on weighted average Dropouts 26 (26%): intervention 1/47, control 25/52 |
||
| Epidural vs usual/conventional care | |||||||||||||||
| 349 | Buchner, 2000151 | A + C | RCT | 6 months | Hannover Functional Ability (0–100) | 17 | 17 | 38.5 | 39.9 | 38.2 (13.09) | 42.8 (13.09) | –0.35 (–1.03 to 0.33) |
SD calculated from SE Dropouts 26 (26%): intervention 1/47, control 25/52 |
||
FIGURE 19.
Summary of the findings of CSOMs at medium-term follow-up (> 6 weeks to ≤ 6 months) for studies comparing epidural/intradiscal injections with alternative interventions. Note: weights are from random effects analysis.
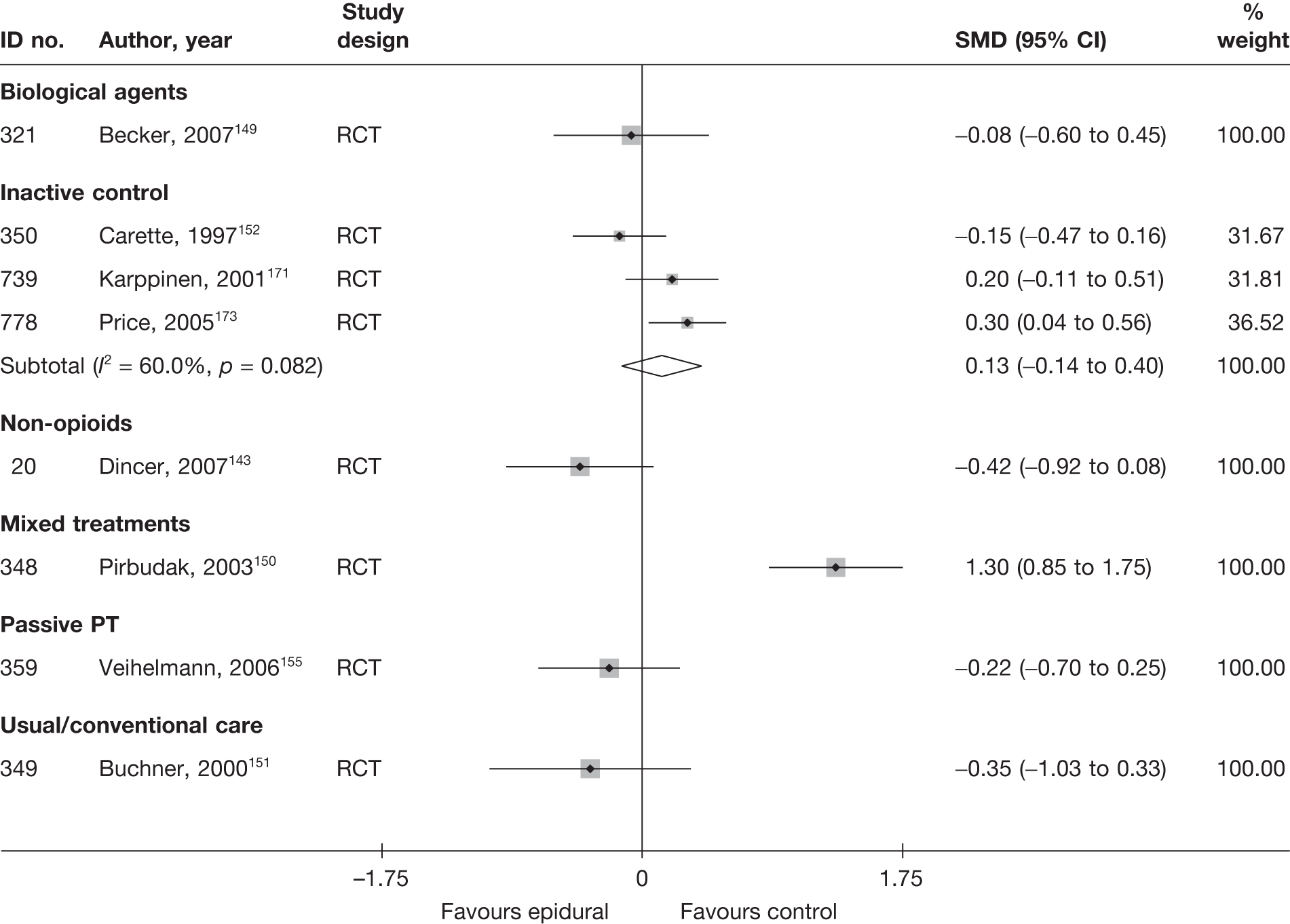
There was no overall statistically significant difference between epidural and inactive control for improving functional status, according to three good-quality RCTs. 152,171,173 The duration of follow-up ranged from 3 months152,173 to 6 months. 171 All three studies included patients with either acute or chronic sciatica.
One moderate-quality RCT151 reported non-statistically significant findings in favour of epidural compared with usual care for improving functional status at 6 months’ follow-up.
One moderate-quality RCT143 reported non-statistically significant findings in favour of epidural compared with non-opioids for improving functional status at 3 months’ follow-up. The methods of randomisation and allocation concealment were not stated.
One moderate-quality RCT150 found epidural used in combination with non-opioids (mixed treatments) to be significantly better than epidural used alone for improving functional status at 6 months’ follow-up. The study included patients with duration of symptoms ranging from 1 month to 12 months.
There was no statistically significant difference between epidural and passive PT in terms of improvement in functional status for chronic sciatica at 6 months. This was according to one moderate-quality study155 with a differential dropout rate in favour of epidural.
There was no important difference between epidural using either a low- or high-dose steroid and biological agents, in terms of functional status at 22 weeks. This was according to one moderate-quality RCT149 that included patients with chronic or acute sciatica.
One poorly reported RCT95 of moderate quality compared epidural with disc surgery. The method of randomisation and allocation concealment were not reported. There was a significantly greater decreasing in disability in the discectomy group compared with the epidural group at the 1–3 month follow-up interval (p < 0.015, Student’s t-test). No summary statistics were reported and, therefore, the study is not presented in Figure 19.
Results at long-term follow-up for epidural/intradiscal injections (> 6 months)
Global effect at long-term follow-up
The results for the global effect at long-term follow-up are presented in Table 26 and the accompanying forest plot (Figure 20). Epidural/intradiscal injections were compared with inactive control, passive PT and chemonucleolysis. One study158 only included patients with acute sciatica, two studies144,155 only included patients with chronic sciatica and the remaining two studies158,173 included patients with either acute or chronic sciatica. The duration of follow-up ranged from 1 year155,173 to 2 years. 144
| ID no. | Author, year | Chronicity | Study design | Follow-up | Outcome measure | Perspective | Intervention | Control | OR (95% CI) | Comments | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n) | Outcome (n) | Withdrawal rate | Total (n) | Outcome (n) | Withdrawal rate | |||||||||
| Epidural vs chemonucleolysis | ||||||||||||||
| 50 | Graham, 1976144 | C | Non-RCT | 2 years | Perceived effect: good (vs fair or unimproved) | Physician | 13 | 2 | 0 | 10 | 6 | 0 | 0.12 (0.02 to 0.87) | Only sciatica patients included here (23/40) |
| Epidural vs inactive control | ||||||||||||||
| 778 | Price, 2005173 | A + C | RCT | 52 weeks | Global improvement: ≥ 75% improvement in ODI | 120 | 39 | 0 | 108 | 32 | 0 | 1.14 (0.65 to 2.01) |
Data inferred from graphs reporting percentages ITT using LOCF |
|
| 406 | Vad, 2002158 | A + C | Non-RCT | Mean 1.4 years (range 12–21 months) | Successful outcome: patient satisfaction (score of 2 or 3), improvement on the RMDQ (≥ 5), and pain reduction (≥ 50%) | Patient + physician | 25 | 21 | 0 | 23 | 11 | 0.09 | 5.73 (1.49 to 22.01) | |
| Epidural vs passive PT | ||||||||||||||
| 359 | Veihelmann, 2006155 | C | RCT | 12 months | Gerbershagen score (chronification index), GHS I (vs GHS II, III) | 46 | 30 | 0.02 | 27 | 7 | 0.48 | 2.52 (1.87 to 15.36) |
Almost half of patients in control group missing ITT not used |
|
FIGURE 20.
Summary of the findings of global effect at long-term follow-up (> 6 months) for studies comparing epidural/intradiscal injections with alternative interventions. Note: weights are from random effects analysis.
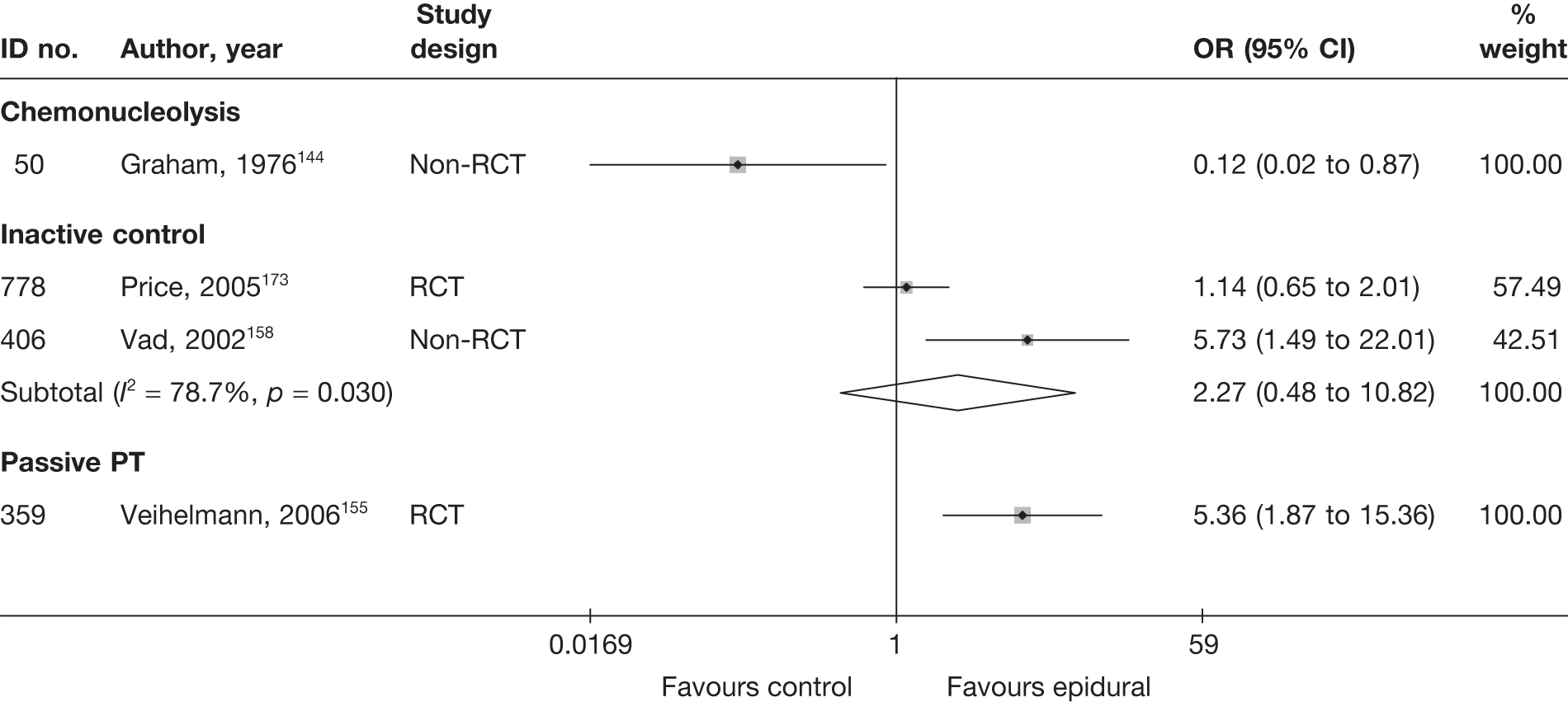
Two studies158,173 compared epidural injections with inactive control in patients with either acute or chronic sciatica, for which there was a non-statistically significant overall findings in favour of epidural. One study was a good-quality RCT,173 whereas the other was a poorly reported non-RCT. 158
As with medium-term follow-up, one RCT,155 of moderate quality, found epidural injections to be significantly better than passive PT at 12 months. However, the withdrawal rate was very high in the control group (48%) compared with the intervention group (2%). Patients in the PT group were able to cross over to an epidural injection after 3 months of unsatisfactory treatment, but were then excluded from the analysis (n = 12/52).
One poorly reported non-RCT144 found chemonucleolysis to be significantly more effective than epidural injection in terms of overall recovery according to the physician, for patients with chronic sciatica at 2 years. All patients had been treated by the author. The findings were based on a subgroup of included patients with sciatica, for whom symptom duration ranged from 12 weeks to 25 years (median 1 year). All patients had already tried various treatments for at least 3 months.
Pain intensity at long-term follow-up
The results for pain intensity at long-term follow-up are presented in Table 27 and the accompanying forest plot (Figure 21). Epidural injections were compared with inactive control, passive PT, mixed treatments and disc surgery. Two studies150,155 included patients with chronic sciatica and the remaining studies included patients with either acute or chronic sciatica. The duration of follow-up ranged from 9 months150 to 2–3 years. 95
| ID no. | Author, year | Chronicity | Study design | Follow-up | Location | Scale (range)a | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)b | Comment/conversionc | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |||||||||
| Epidural vs disc surgery | ||||||||||||||||
| 725 | Buttermann, 200495 | A + C | RCT | 2–3 years | Back | VAS (0–10) | 50 | 50 | There were no significant differences between intervention groups (Student’s t-test) |
Summary statistics not reported Dropouts 4/100 (4%): intervention 3/50, control 1/50 |
||||||
| Epidural vs inactive control | ||||||||||||||||
| 203 | Bush, 1991147 | C | RCT | 52 weeks | Overall | VAS (0–100) | 12 | 11 | 38.5 | 49.2 | 14.2 (15.94) | 29.6 (23.67) | –15.40 (–32.04 to 1.24) |
SD imputed from weighted average Dropouts 22%: intervention 1/12, control 4/11 ITT analysis based on LOCF |
||
| 739 | Karppinen, 2001171 | A + C | RCT | 12 months | Leg | VAS (0–100) | 78 | 80 | 71 (18) | 75.2 (19) | 23.9 (17.15) | 24.2 (17.15) |
3.90 (–6.37 to 14.17) Multivariate analysis (adjusted change from baseline) –5.3 (95% CI –15.7 to 5.0) |
SDs (and SEs) for change estimated from 95% CI of difference between treatment groups Two patients lost to follow-up from steroid group ITT not used |
||
| 778 | Price, 2005173 | A + C | RCT | 52 weeks | Leg | VAS (0–100) | 120 | 108 | 52 (23) | 56 (22) | –17 (36) | –20 (34) | 3.00 (–6.09 to 12.09) | |||
| Epidural vs passive PT | ||||||||||||||||
| 359 | Veihelmann, 2006155 | C | RCT | 12 months | Leg | VAS (0–10) | 46 | 27 | 72 (135.6) | 67 (103.92) | 28 (189.91) | 59 (119.51) | –31.00 (–102.02 to 40.02) |
SD estimated from SE Dropouts 26%: intervention 1/47, control 25/52 Almost half of control group dropped out |
||
| Epidural vs mixed treatments | ||||||||||||||||
| 348 | Pirbudak, 2003150 | C | RCT | 9 months | Overall | VAS (0–10) | 46 | 46 | 84 (17) | 78.1 (40.0) | 75 | 16 | –9 (18) | –62.0 (8.0) | 53.00 (47.31 to 58.69) | |
FIGURE 21.
Summary of the findings of pain intensity at long-term follow-up (> 6 months) for studies comparing epidural/intradiscal injections with alternative interventions. Note: weights are from random effects analysis.
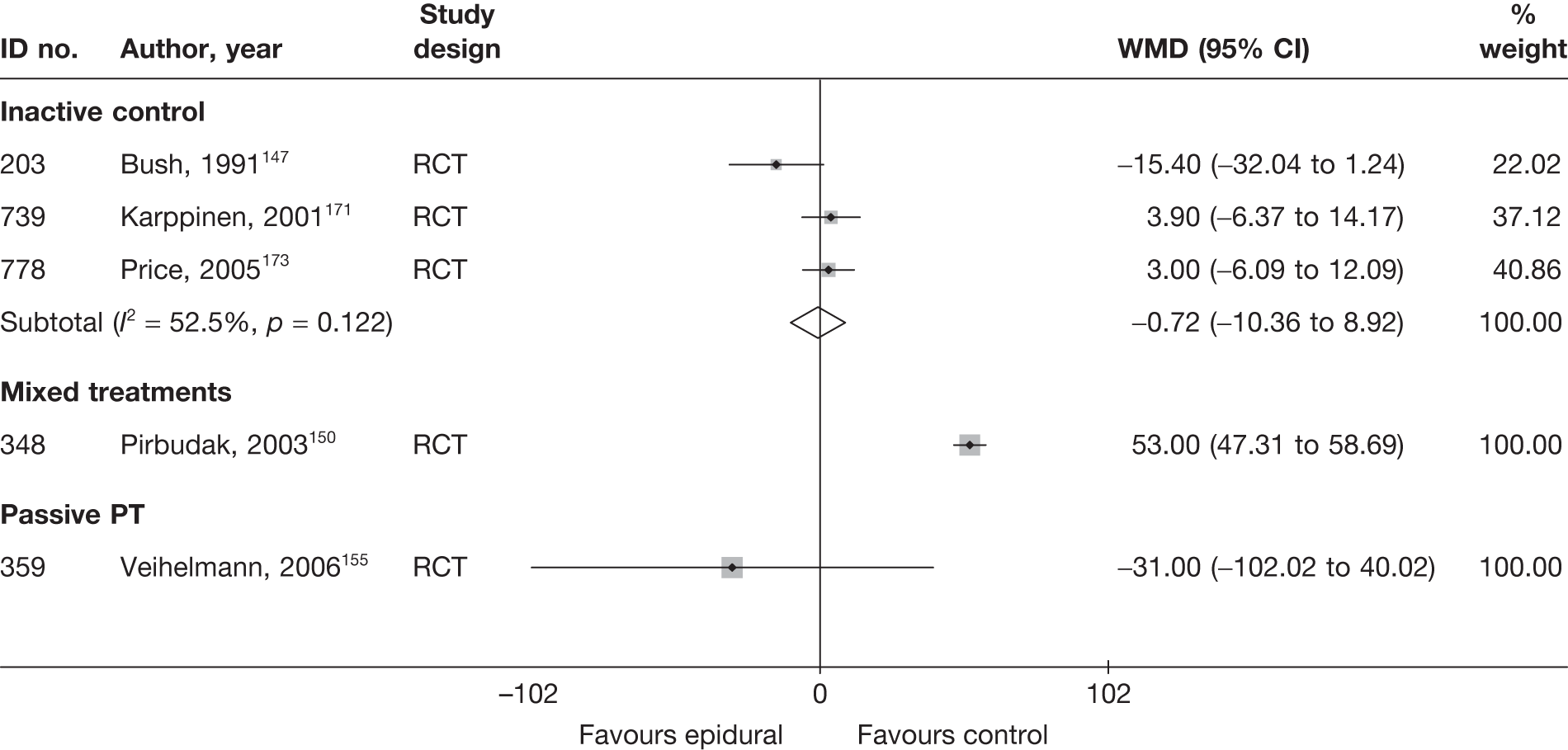
Three RCTs147,171,173 compared epidural injections with inactive control, for which pooled analyses showed no important difference between the groups at 12 months. The overall quality of two trials171,173 was good. The third study147 was small (n = 23), poorly reported and of moderate quality. The method of randomisation and allocation concealment were not stated, but the study included blind outcome assessment. SDs for final mean were not reported, so were imputed using the weighted mean. Unlike the remaining studies, the WMD for this study was statistically significant in favour of epidural. One of the RCTs171 also reported findings based on ANCOVA, adjusted for baseline values, which favoured inactive control for leg pain at 6 months (–16.2; 95% CI –26.8 to –5.6, p = 0.003; negative values indicate a negative effect). The same analysis showed no statistically significant difference between the groups at 12 months.
One moderate-quality RCT155 found epidural injection to be significantly better than passive PT in terms of pain reduction in chronic sciatica at 12 months. The withdrawal rate was much higher in the control group (48%) than the intervention group (2%).
One moderate-quality RCT150 found epidural injection in combination with non-opioids (mixed treatments) to be significantly better than epidural injection alone in terms of pain reduction in chronic sciatica at 9 months’ follow-up.
One poorly reported RCT94 of moderate quality, compared epidural injection with disc surgery. The method of randomisation and allocation concealment were not reported. There were no significant differences between the epidural injection and disc surgery groups at 2–3 years follow-up for low back pain (Student’s t-test). No summary statistics were reported and, therefore, the study is not presented in Figure 21.
Condition-specific outcome measures at long-term follow-up
The results for CSOMs at long-term follow-up are presented in Table 28 and the accompanying forest plot (Figure 22). Epidural injections were compared with inactive control, passive PT and mixed treatments. Two studies150,155 included patients with chronic sciatica and the remaining studies included patients with either acute or chronic sciatica. The duration of follow-up ranged from 9 months150 to 12 months. 155,171,173
| ID no. | Author, year | Chronicity | Study design | Follow-up | Scale | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)a | Comment/conversionb | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | ||||||||
| Epidural vs inactive control | |||||||||||||||
| 739 | Karppinen, 2001171 | A + C | RCT | 12 months | ODI | 78 | 80 | 42.9 (16) | 43.5 (15) | 15.9 (16) | 16.3 (15) | –27 (21.16) | –27.2 (21.16) |
–0.3 (–0.34 to 0.29) Adjusted change from baseline –0.4 (95% CI –7.0 to 6.2) |
No final SD so baseline SD used Two patients lost to follow-up from steroid group |
| 778 | Price, 2005173 | A + C | RCT | 52 weeks | ODI | 120 | 108 | 44 (15) | 45 (18) | 28 (15) | 27 (18) | –16 (23) | –14 (24) | 0.06 (–0.20 to 0.32) |
Final score calculated from change score No final SD, so baseline SD used ITT used LOCF |
| Epidural vs mixed treatment | |||||||||||||||
| 348 | Pirbudak, 2003150 | C | RCT | 9 months | ODI | 46 | 46 | 49.6 (15.5) | 50.2 (15.2) | 46 (15.5) | 26 (15.2) | –7.6 (15.3) | –13.2 (15.5) | 1.30 (0.85 to 1.75) | No final SD, so baseline SD used |
| Epidural vs passive PT | |||||||||||||||
| 359 | Veihelmann, 2006155 | C | RCT | 12 months | ODI | 46 | 27 | 23.1 | 21.4 | 11.6 (13.04) | 21.6 (13.04) | –0.77 (–1.26 to –0.28) |
Final SD imputed from weighted mean of SDs of ODI for epidural Dropouts 26 (26%): intervention 1/47, control 25/52 |
||
FIGURE 22.
Summary of the findings of CSOMs at long-term follow-up (> 6 months) for studies comparing epidural/intradiscal injections with alternative interventions. Note: weights are from random effects analysis.
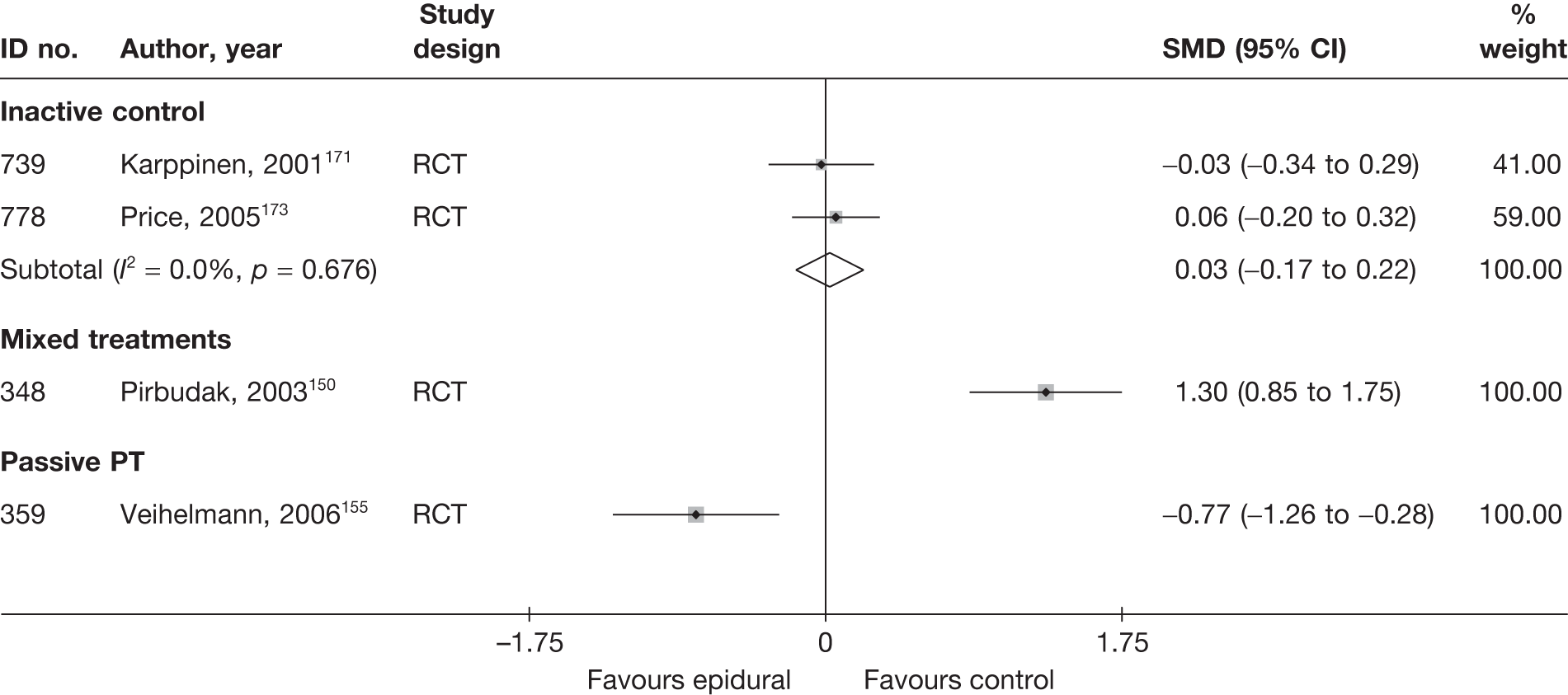
Two good-quality RCTs171,173 compared epidural injections with inactive control; the pooled analyses showed no statistically significant difference between the groups at 12 months.
One moderate-quality RCT155 found epidural injections to be significantly better than passive PT for improving functional status for patients with chronic sciatica at 12 months. However, the withdrawal rate was much higher in the control group (48%) than in the intervention group (2%).
One moderate-quality RCT150 found epidural injection in combination with non-opioids (mixed treatments) to be significantly better than epidural injection alone for improving functional status in patients with chronic sciatica at 9 months’ follow-up.
Analysis of adverse effects for epidural/intradiscal injections
The results for the occurrence of any reported adverse effects are presented in Table 29 and the accompanying forest plot (Figure 23). The incidence of adverse effects were significantly greater for epidural injections compared with either education/advice, passive PT or usual care. Overall there was no statistically significant difference in the number of adverse effects when comparing epidural injections with either activity restriction, biological agents, chemonucleolysis, disc surgery, manipulation, mixed treatments, non-opioids or inactive control.
| ID no. | Author, year | Study design | No. of events in intervention group | No. of participants in intervention group | No. of events in control group | No. of participants in control group | OR (95% CI) |
|---|---|---|---|---|---|---|---|
| Epidural vs activity restriction | |||||||
| 140 | Coomes, 1961145 | Non-RCT | 1 | 20 | 0 | 20 | 3.15 (0.12 to 82.16) |
| Epidural vs alternative | |||||||
| 667 | Wehling, 1997167 (epidural = steroid + LA) | CCS | NR | NR | NR | NR | |
| 667 | Wehling, 1997167 (epidural = LA) | CCS | NR | NR | NR | NR | |
| Epidural vs biological agents | |||||||
| 321 | Becker, 2007149 (epidural = 10 mg steroid) | RCT | 1 | 27 | 1 | 32 | 1.19 (0.07 to 20.01) |
| 321 | Becker, 2007149 (epidural = 5 mg steroid) | RCT | 1 | 25 | 1 | 32 | 1.29 (0.08 to 21.73) |
| Epidural vs chemonucleolysis | |||||||
| 720 | Bontoux, 1990168 | RCT | NR | NR | NR | NR | |
| 447 | Bourgeois, 1988160 | RCT | 0 | 30 | 3 | 30 | 0.13 (0.01 to 2.61) |
| 729 | Gallucci, 2007170 | RCT | 0 | 82 | 0 | 77 | |
| 50 | Graham, 1976144 | Non-RCT | NR | NR | NR | NR | |
| Epidural vs disc surgery | |||||||
| 725 | Buttermann, 200495 | RCT | 5 | 50 | 7 | 77 | 1.11 (0.33 to 3.72) |
| Epidural vs education/advice | |||||||
| 722 | Bronfort, 2004169 | RCT | 10 | 10 | 0 | 10 | 441.00 (7.98 to 24,372.70) |
| Epidural vs inactive control | |||||||
| 203 | Bush, 1991147 | RCT | 1 | 12 | 0 | 11 | 3.00 (0.11 to 81.61) |
| 350 | Carette, 1997152 | RCT | 22 | 77 | 17 | 79 | 1.46 (0.70 to 3.03) |
| 383 | Dilke, 1973157 | RCT | 6 | 51 | 0 | 48 | 13.86 (0.76 to 253.00) |
| 512 | Helliwell, 1985162 | RCT | 0 | 20 | 0 | 19 | |
| 739 | Karppinen, 2001171 | RCT | 1 | 80 | 0 | 80 | 3.04 (0.12 to 75.69) |
| 539 | Klenerman, 1984163 (epidural = LA) | RCT | 0 | 16 | 0 | 16 | |
| 539 | Klenerman, 1984163 (epidural = steroid) | RCT | 1 | 19 | 0 | 16 | 2.68 (0.10 to 70.31) |
| 905 | Matthews, 1987176 | RCT | NR | NR | NR | NR | |
| 778 | Price, 2005173 | RCT | 12 | 120 | 11 | 108 | 0.98 (0.41 to 2.32) |
| 620 | Ridley, 1988165 | RCT | 2 | 21 | 0 | 18 | 4.74 (0.21 to 106.00) |
| 240 | Snoek, 1977148 | RCT | 0 | 27 | 0 | 24 | |
| 406 | Vad, 2002158 | Non-RCT | 0 | 25 | 0 | 25 | |
| 351 | Valat, 2003153 | RCT | 2 | 42 | 3 | 42 | 0.65 (0.10 to 4.10) |
| 175 | Yates, 1978146 (epidural = LA) | RCT (crossover) | 0 | 20 | 0 | 20 | |
| 175 | Yates, 1978146 (epidural = steroid) | RCT (crossover) | 0 | 20 | 0 | 20 | |
| 175 | Yates, 1978146 (epidural = steroid + LA) | RCT (crossover) | 0 | 20 | 0 | 20 | |
| Epidural vs manipulation | |||||||
| 451 | Bronfort, 2000161 | RCT | 6 | 6 | 3 | 7 | 16.71 (0.68 to 409.09) |
| 722 | Bronfort, 2004169 | RCT | 10 | 10 | 6 | 11 | 17.77 (0.84 to 377.00) |
| Epidural vs mixed treatment | |||||||
| 439 | Blonna, 2004159 | RCT | 0 | 24 | 3 | 26 | 0.14 (0.01 to 2.80) |
| 348 | Pirbudak, 2003150 | RCT | 0 | 46 | 0 | 46 | |
| Epidural vs non-opioids | |||||||
| 451 | Bronfort, 2000161 | RCT | 6 | 6 | 4 | 6 | 7.22 (0.28 to 189.19) |
| 20 | Dincer, 2007143 | RCT | 2 | 34 | 0 | 30 | 4.69 (0.22 to 102.00) |
| 771 | Lafuma, 1997172 | RCT | NR | NR | NR | NR | |
| 362 | Wilson-MacDonald, 2005156 | RCT | NR | NR | NR | NR | |
| 846 | Murata, 2009175 | RCT | NR | NR | NR | NR | |
| Epidural vs passive PT | |||||||
| 359 | Veihelmann, 2006155 | RCT | 16 | 46 | 0 | 39 | 42.74 (2.47 to 741.00) |
| Epidural vs usual care | |||||||
| 349 | Buchner, 2000151 | RCT | NR | NR | NR | NR | |
| 828 | Laiq, 2009174 | Q-RCT | 8 | 52 | 0 | 52 | 24.77 (1.34 to 458.00) |
| 581 | Matyjek, 1986164 | CCS | NR | NR | NR | NR | |
| 358 | Popiolek, 1991154 | Non-RCT | NR | NR | NR | NR | |
| Mixed treatments including epidural vs mixed treatments without epidural | |||||||
| 913 | Saberski, 2000142 | RCT | NR | NR | NR | NR | |
| 644 | Styczynski, 1997166 | Non-RCT | NR | NR | NR | NR | |
FIGURE 23.
Summary of the findings of any adverse effect for studies comparing epidural/intradiscal injections with alternative interventions (grouped by comparator then ordered by author). Note: weights are from random effects analysis.
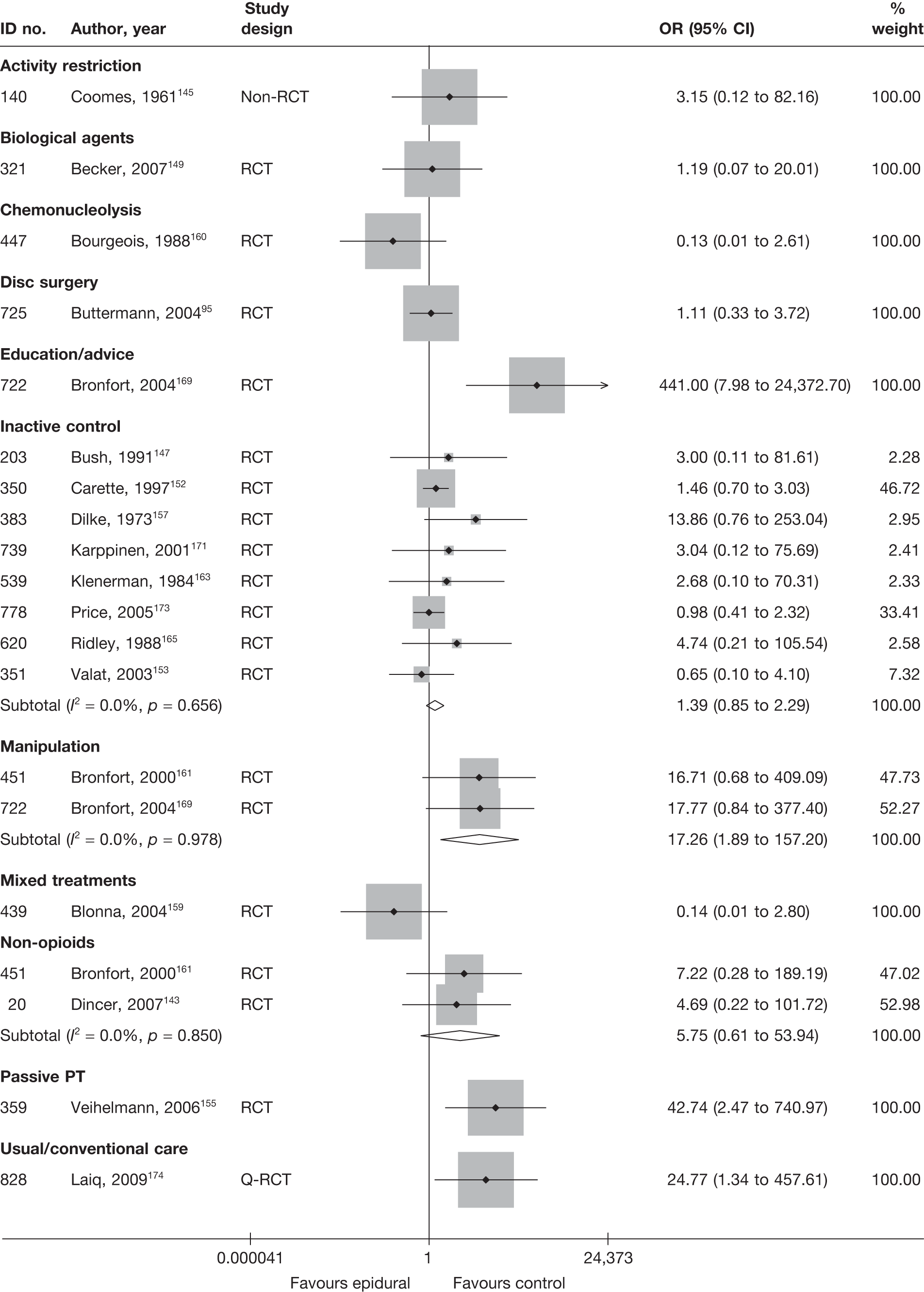
SUMMARY OF OVERALL FINDINGS FOR EPIDURAL/INTRADISCAL INJECTIONS COMPARED WITH ALTERNATIVE INTERVENTIONS
Most epidural injection studies included patients with chronic sciatica or both acute and chronic sciatica. One study included acute sciatica. 145 Less than half of the studies were RCTs. Apart from studies comparing epidural with inactive control, the quality of studies was poor (Table 30).
| Control category | No. of studies (arms) | Sample size range (median) | Proportion of studies that were RCTs (%) | Proportion of studies that were deemed good quality (%) | Proportion of studies that only included acute sciatica (%) | Proportion of studies that included patients with nerve root pain (%) | Proportion of studies that reported diagnosis confirmed by imaging (%) | Proportion of studies that included patients with stenosis (%) | Proportion of studies that included patients with extruded/sequestered discs (%) | Proportion of studies that only included patients with first episode (%) | Proportion of studies that included patients who had received previous treatment (%) | Proportion of studies that included patients who had received previous surgery (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epidural vs activity restriction | 1 (1) | 40 (40) | 0/1 (0) | 0/1 (0) | 1/1 (100) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 1/1 (100) | 0/1 (0) |
| Epidural vs alternative/non-traditional | 1 (2) | 278 (278) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) |
| Epidural vs biological agents | 1 (1) | 90 (90) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 1/1 (100) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) |
| Epidural vs chemonucleolysis | 4 (4) | 40–159 (70) | 3/4 (75) | 0/4 (0) | 0/4 (0) | 4/4 (100) | 4/4 (100) | 0/4 (0) | 0/4 (0) | 0/4 (0) | 4/4 (100) | 0/4 (0) |
| Epidural vs disc surgery | 1 (1) | 100 (100) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 1/1 (100) | 1/1 (100) | 0/1 (0) | 1/1 (100) | 1/1 (100) | 1/1 (100) | 1/1 (100) |
| Epidural vs inactive control | 12 (13) | 23–288 (67) | 12/12 (100) | 4/12 (33) | 1/12 (8) | 12/12 (100) | 4/12 (33) | 0/12 (0) | 0/12 (0) | 0/12 (0) | 2/12 (17) | 0/12 (0) |
| Epidural vs mixed treatment | 2 (2) | 50–92 (71) | 2/2 (100) | 0/2 (0) | 0/2 (0) | 2/2 (100) | 2/2 (100) | 1/2 (50) | 0/2 (0) | 0/2 (0) | 2/2 (100) | 0/2 (0) |
| Epidural vs non-opioids | 3 (3) | 64–246 (93) | 3/3 (100) | 0/3 (0) | 0/3 (0) | 3/3 (100) | 2/3 (67) | 1/3 (33) | 0/3 (0) | 0/3 (0) | 2/3 (67) | 1/3 (33) |
| Epidural vs passive PT | 1 (1) | 99 (99) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 1/1 (100) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 1/1 (100) | 0/1 (0) |
| Epidural vs usual/conventional care | 3 (3) | 36–60 (52) | 1/3 (33) | 0/3 (0) | 0/3 (0) | 3/3 (100) | 3/3 (100) | 0/3 (0) | 0/3 (0) | 0/3 (0) | 0/3 (0) | 0/3 (0) |
| Total (results for epidural studies) | 29 (31) | 23–278 (74) | 24/29 (83) | 4/29 (14) | 2/29 (7) | 29/29 (100) | 18/29 (62) | 2/29 (7) | 1/29 (3) | 1/29 (3) | 13/29 (45) | 2/29 (7) |
Meta-analysis of the mainly good-quality RCTs (up to seven studies) showed epidural injections to be significantly better than the inactive control at short-term follow-up for reducing pain147,152,153,158,162,171,173 and improving functional status. 152,153,158,171,173 However, there was no statistically significant difference between intervention groups for the global effect. 148,152,153,165,173,176 Furthermore, there was no statistically significant difference between epidural injection and inactive control for global effect,152,157,162,163,173 pain intensity152,162,171,173 or CSOMs152,171,173 at medium-term follow-up or global effect,158,173 pain intensity147,171,173 or CSOMs171,173 at long-term follow-up, or in terms of the number of adverse effects. 146–148,152,153,157,158,162,163,165,171,173 A similar pattern was found for epidural injection compared with usual care. There was a statistically significant difference in favour of epidural for overall recovery (one non-RCT154) and functional status (one RCT151) at short-term follow-up, but not for pain intensity (one RCT,151 one Q-RCT174). There were no statistically significant difference between epidural injection and usual care at medium-term follow-up for global effect,151,174 pain intensity151,174 or CSOMs. 151 However, usual care was associated with significantly fewer adverse effects than epidural injection (one Q-RCT174).
Epidural injections were found to be better than non-opioids for reducing pain and improving functional status at short-term follow-up according to three poorly reported RCTs. 143,156,175 There was no statistically significant difference between epidural and non-opioids for global effect (one RCT175) or CSOMs (one RCT143) at medium-term follow-up or adverse effects (two RCTs143,161). One poorly reported RCT found that epidural injection in combination with non-opioids was better than epidural injection alone for reducing pain and improving functional status at long-term follow-up. 150 However, there was no statistically significant difference between the intervention groups at short- and medium-term follow-up for pain (two poorly reported RCTs150,159) and CSOMs (RCT150) or in terms of the number of adverse effects. 150,159
Chemonucleolysis using chymopapain was found to be better than epidural injection for the global effect at long-term follow-up (one poor-quality non-RCT144). There was no statistically significant difference between epidural injection and chemonucleolysis for the global effect at short-term (one poorly reported RCT170 using ozone–oxygen) or medium-term follow-up (three RCTs;160,168,170 one RCT170 used ozone–oxygen). There was no statistically significant difference in the number of adverse effects experienced with epidural than with chemonucleolysis (one RCT160).
Statistically significant findings in favour of epidural injection were found when compared with passive PT for global effect (at medium-155 and long-term155 follow-up) and activity restriction for global effect (medium-term follow-up145), but these findings were reported by a single RCT155 or non-RCT. 145 Disc surgery was found to be significantly better than epidural injection at reducing pain intensity at medium-term follow-up, but not at long-term follow-up (one poor-quality RCT95). There was also no statistically significant difference in pain intensity between epidural injection and acupuncture (CCS167 at short-term follow-up) and biological agents (poorly reported RCT149 at medium-term follow-up).
Chemonucleolysis
Description of chemonucleolysis studies
Summary of interventions
Forty studies evaluated chemonucleolysis for sciatica,46–56,58–61,75–77,79,85,88,90,92,96,103–105,144,160,168,170,205–213 3746–56,58–61,75–77,79,85,88,90,92,96,103–105,144,160,168,170,205–210 of which compared chemonucleolysis with alternative interventions. The type of interventions evaluated by these latter studies are listed in Table 31a. One of these studies,46 which compared disc surgery with chemonucleolysis, did not include comparative data and reported only descriptive results for change from baseline for each group. 46 One further study61 did not report any global effect, pain intensity or CSOM data. 61
| ID no. | Author, year | Study design | Chemonucleolysis description | Control description |
|---|---|---|---|---|
| Chemonucleolysis vs disc surgery | ||||
| 884 | Alexander, 1989103 | CCS | Chymopapain chemonucleolysis (2000 U) | Disc surgery (removal of protruding disc fragment only + free fat graft) |
| 43 | van Alphen, 198947 | RCT | Chemonucleolysis with 4000 U chymopapain | Discectomy with emptying of disc space |
| 441 | Bonafe, 199375 (French language) | CCS | Nucleolysis using chymopapain (4000 U) | Percutaneous automated nucleotomy |
| 183 | Bouillet, 198361 | CCS | Chemonucleolysis by chymopapain injections | Conventional lumbar disc surgery |
| 453 | Brown, 198976 | CCS | Chemonucleolysis with chymopapain | Disc surgery |
| 453 | Brown, 198976 | CCS | Collagenase chemonucleolysis | Disc surgery |
| 454 | Buric, 200577 | Non-RCT | Chemonucleolysis with ozone–oxygen mixture | Standard microdiscectomy |
| 166 | Crawshaw, 198460 | RCT | Chemonucleolysis with 4000 U chymopapain | Disc surgery |
| 48 | Dabezies, 197851 | CCS | Chemonucleolysis using 2 ml chymopapain | Laminectomy with or without fusion |
| 471 | Dei-Anang, 199079 (German language) | CCS | Chemonucleolysis with 4000 U chymopapain or 600 units collagenase | Percutaneous nucleotomy |
| 727 | Ejeskar, 198396 | RCT | Chemonucleolysis with chymopapain 400 IU | Discectomy with unilateral laminotomy and removal of disc hernia only |
| 132 | Hoogmartens, 197656 | HCS | Chymopapain chemonucleolysis | Discectomy |
| 44 | Javid, 199548 | CCS | Chemonucleolysis with 3000 IU chymopapain | Partial hemilaminectomy using magnification and fat graft |
| 35 | Krugluger, 200046 | RCT | Chemonucleolysis using 4000 U chymodiactin | Automated percutaneous discectomy |
| 117 | Lagarrigue, 199154 (French language) | CCS | Chemonucleolysis with 2000–5000 U chymopapain | Discectomy with minimal bony resection |
| 129 | Lavignolle, 198755 (French language) | RCT | Chemonucleolysis with 4000 U chymopapain | Microscopic discectomy. Unilateral limited interlaminar |
| 889 | Lee, 1996104 (German language) | CCS | Chemonucleolysis with chymopapain | Percutaneous manual and laser discectomy |
| 889 |
Lee, 1996104 (German language) |
CCS | Chemonucleolysis with chymopapain | Automated percutaneous lumbar discectomy |
| 593 | Muralikuttan, 199285 | RCT | Chemonucleolysis with chymopapain 2000 U | Standard discectomy with fenestration, disc space cleared |
| 47 | Norton, 198650 | CCS | Chymopapain chemonucleolysis | Conventional surgical discectomy |
| 45 | Postacchini, 198749 | Non-RCT | 2 ml chymopapain chemonucleolysis | Disc excision using unilateral laminotomy |
| 617 | Revel, 199388 | RCT | Chemonucleolysis | Automated percutaneous lumbar discectomy |
| 641 | Steffen, 199990 (German language) | RCT | Chemonucleolysis with 2 ml chymodiactin | Laser disc decompression |
| 49 |
Stula, 199052 (German language) |
RCT | Chemonucleolysis with 500 U chymopapain | Conventional disc surgery |
| 61 | Tregonning, 199153 | CCS | Chemonucleolysis with chymopapain | Fenestration or partial laminectomy removing extruded disc material |
| 893 | Watters,1988105 | Non-RCT | Chemonucleolysis using chymopapain (4000 U) | Microdiscectomy with free fat graft over exposed dura |
| 160 | Watts, 197559 | CCS | Chemonucleolysis with chymopapain 4 mg | Discectomy with laminotomy and foraminotomy |
| 672 | Weinstein, 198692 | CCS | Chemonucleolysis with chymopapain | Discectomy |
| 150 | Zeiger, 198758 | CCS | Chemonucleolysis with 2.5 ml chymodiactin | Microdiscectomy with intraoperative injection into intervertebral space with steroid 125 mg methylprednisolone + morphine 4 mg used to reduce postoperative pain and morbidity |
| Chemonucleolysis vs epidural | ||||
| 720 | Bontoux, 1990168 (French language) | RCT | Chemonucleolysis with chymopapain 4000 U | Intradiscal injection of triamcinolone 70 mg |
| 447 | Bourgeois, 1988160 (French language) | RCT | Chemonucleolysis with chymopapain 4000 U | Intradiscal injection of triamcinolone 80 mg |
| 729 | Gallucci, 2007170 | RCT | Intraforaminal and intradiscal injections of steroid triamcinolone 80 mg + local anaesthetic 2–4 ml ropivacaine plus ozone–oxygen (group B) | Intraforaminal and intradiscal injections of steroid triamcinolone 80 mg + local anaesthetic 2–4 ml ropivacaine (group A) |
| 50 | Graham, 1976144 | Non-RCT | Chemonucleolysis with chymopapain (dose not stated) | Intradiscal hydrocortisone injection (dose not stated) |
| Chemonucleolysis vs inactive control | ||||
| 726 | Dabezies, 1988209 | RCT | Chemonucleolysis using 8 mg chymopapain | Placebo injections |
| 244 | Feldman, 1986207 (French language) | RCT | Chemonucleolysis with 4000 U chymopapain | Intradiscal injection of distilled water |
| 55 | Gogan, 1992205 | RCT | Chemonucleolysis with 8 mg chymopapain | Intradiscal injection of normal saline 2 ml |
| 738 | Javid, 1983210 | RCT | Chymopapain injections of 3.0 ml (3000 U/1.5 ml) | Placebo group (3 ml of sterile pyrogen-free saline solution) |
| 236 | Schwetschenau, 1976206 | RCT | Chemonucleolysis by 4 mg chymopapain | Intradiscal injection of inactive control (placebo group) |
| Chemonucleolysis vs manipulation | ||||
| 723 | Burton, 2000208 | RCT | Chemonucleolysis with 400 U chymopapain | Osteopathic spinal manipulation for up to 12 weeks |
Three studies compared different types of chemonucleolysis211–213 and one study213 included three intervention arms.The types of chemonucleolysis being compared are listed in Table 31b, but the findings of these studies are not considered any further than this.
| ID no. | Author, year | Study design | Chemonucleolysis description | Control description |
|---|---|---|---|---|
| Chemonucleolysis vs chemonucleolysis | ||||
| 435 | Benoist, 1993212 | RCT | Chemonucleolysis using low-dose chymopapain 2000 U | Chemonucleolysis using standard-dose chymopapain 4000 U |
| 453 | Brown, 198976 | CCS | Chemonucleolysis with chymopapain | Collagenase chemonucleolysis |
| 511 | Hedtmann, 1987213 | Q-RCT | Chemonucleolysis with collagenase 600 ABC U (high dose) | Chemonucleolysis with chymopapain 400 ABC U |
| 511 | Hedtmann, 1987213 | Q-RCT | Chemonucleolysis with collagenase 400 ABC U (low dose) | Chemonucleolysis with chymopapain 400 ABC U |
| 407 | Wittenberg, 2001211 | RCT | Chemonucleolysis with 4000 IU chymopapain | Chemonucleolysis with 400 ABC U collagenase |
Summary of study participants for chemonucleolysis
The summary data for included participants are presented in Table 32. The number of participants included in the 36 studies that reported outcome data for global effect, pain or CSOMs ranged from 22 to 1085 participants (median 100 participants). A similar number of studies included patients with chronic sciatica or included patients with either chronic or acute sciatica. One study (comparing chemonucleolysis with disc surgery),103 included some patients with spinal stenosis and none included patients with sequestered or extruded discs. The diagnosis of sciatica, or the presence of herniated disc, was confirmed by imaging in 31 (84%) studies. Two studies49,105 compared the use of chemonucleolysis with disc surgery in only patients who had sciatica for the first time, and one study50 compared the same intervention in patients who had recurrent sciatica. The remaining studies included a mixture of patients with either first episode or recurrent sciatica or, more usually, did not report this information. The majority of studies included patients who had received previous treatment for their current episode of sciatica, with this information not being stated in the remaining studies. Three studies56,59,88 that compared chemonucleolysis with disc surgery, included patients who had received previous disc surgery.
| ID no. | Author, year | Study design | No. of patients | Age (years) | No. of men (%) | Symptom duration | Type of sciatica | Confirmed by imaging? | Recurrent episode | Included patients with stenosis?a | Included patients with sequestered disc (or extruded)?a | Any previous treatment for sciatica? | Any previous back surgery for sciatica? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemonucleolysis vs disc surgery | |||||||||||||
| 884 | Alexander, 1989103 | CCS | 100 | Mean 33.5 (range 18–65) | 90 (90) | Mean 5.5 months | Nerve root pain | Yes | NR | No | Yes | Yes | No |
| 43 | van Alphen, 198947 | RCT | 151 | Mean 34 (range 18–45) | 99 (66) | < 6 months 55%; > 6 months 45% | Nerve root pain | Yes | NR | No | No | Yes | No |
| 441 | Bonafe, 199375 (French language) | CCS | 40 | Mean 46 (range 27–68) | 28 (70) | Mean 3 months (range several days to 15 months) | Nerve root pain | Yes | NR | No | NR | Yes | NR |
| 183 | Bouillet, 198361 | CCS | 2749 | NR | NR | Range (weeks to months) | Nerve root pain | Yes | NR | No | NR | Yes | NR |
| 453 | Brown, 198976 | CCS | 85 | Mean 37.6 | 59 (69) | At least 3 months | Nerve root pain | Yes | NR | No | No | Yes | No |
| 454 | Buric, 200577 | Non-RCT | 45 | Mean 45 (SD 14.2, range 19–77) | 23 (51) | Mean 203.9 days (SD 129.6, range 21 to > 365 days) | Nerve root pain | Yes | NR | No | No | Yes | No |
| 166 | Crawshaw, 198460 | RCT | 52 | Mean 37 | NR | NR | Nerve root pain | Yes | NR | No | No | Yes | No |
| 48 | Dabezies, 197851 | CCS | 200 | Mean 39 | 132 (66) | NR | Nerve root pain and referred pain | Clinical | Recurrent and first episode | No | No | Yes | NR |
| 471 | Dei-Anang, 199079 (German language) | CCS | 201 | NR | NR | NR | Nerve root pain | NR | NR | No | No | NR | NR |
| 727 | Ejeskar, 198396 | RCT | 29 | Mean 39.3 | 21 (72) | Mean 4.5 months (SD 3 months) | Nerve root pain | Yes | NR | No | No | NR | No |
| 132 | Hoogmartens, 197656 | HCS | 97 | Mean 35.5 | 48 (49) | 25–35 months | Nerve root pain | NR | Recurrent and first episode | No | No | Yes | Yes |
| 44 | Javid, 199548 | CCS | 200 | Mean 39 (range 17–81) | 134 (67) | Mean 7.2 months | Nerve root pain | Yes | NR | No | No | Yes | No |
| 35 | Krugluger, 200046 | RCT | 22 | Mean 40 (range 24–60) | 16 (73) | Mean 7 months | Nerve root pain | Yes | NR | No | No | Yes | NR |
| 117 | Lagarrigue, 1991 (French language)54 | CCS | 1085 | Mean 42 (range 14–83) | 682 (63) | Mean 13.4 months | Nerve root pain | Clinical | NR | Yes | No extrusion | Yes | NR |
| 129 | Lavignolle, 198755 (French language) | RCT | 358 | Mean 41 (SD 12.03) | 225 (63) | NR | Nerve root pain | NR | NR | No | No | NR | NR |
| 889 | Lee, 1996104 (German language) | CCS | 300 | <30 50%; > 40 25% | 213 (71) | NR | Nerve root pain | Yes | NR | No | No | Yes | NR |
| 593 | Muralikuttan, 199285 | RCT | 92 | Mean 35 (range 19–60) | 55 (60) | Mean 24 weeks | Nerve root pain | Yes | NR | No | No | Yes | NR |
| 47 | Norton, 198650 | CCS | 105 | Mean 40 (range 20–67) | 86 (82) | Mean 18.5 months (range 5 days–128 months) | Nerve root pain | Yes | Recurrent | NR | NR | Yes | No |
| 45 | Postacchini, 198749 | Non-RCT | 161 | NR | NR | Mean 8.75 months (range 1.2–36.0 months) | Nerve root pain and referred pain | Yes | First episode | No | No | Yes | No |
| 617 | Revel, 199388 | RCT | 165 | Mean 39 (SD 9, range 21–65) | 96 (68) | NR | Nerve root pain | Yes | NR | No | No | Yes | Yes |
| 641 | Steffen, 199990 (German language) | RCT | 69 | NR | NR | 10.6 months | Nerve root pain | Yes | NR | No | No | Yes | No |
| 49 | Stula, 199052 (German language) | RCT | 69 | Range 22–54 | 57 (83) | < 1 year | Nerve root pain | Yes | Recurrent and first episode | No | No | Yes | No |
| 61 | Tregonning, 199153 | CCS | 268 | Mean 40.4 (range 20–65) | 135 (68) | NR | Nerve root pain | Yes | NR | No | No | Yes | No |
| 893 | Watters,1988105 | Non-RCT | 100 | Mean 36.5 | 59 (59) | Mean 13 weeks | Nerve root pain | Yes | First episode | No | NR | NR | NR |
| 160 | Watts, 197559 | CCS | 274 | Range 24–62 | 55 (55) | NR | Nerve root pain and referred pain | Yes | Recurrent and first episode | No | Unclear | Yes | Yes |
| 672 | Weinstein, 198692 | CCS | 159 | Mean 41 (range 28–57) | 64 (41) | Minimum period of 3 months | Nerve root pain | Yes | First episode | No | No | Yes | No |
| 150 | Zeiger, 198758 | CCS | 126 | NR | NR | ≥ 4 weeks | Nerve root pain | Yes | NR | No | No | Yes | No |
| Chemonucleolysis vs epidural | |||||||||||||
| 720 | Bontoux, 1990 (French language)168 | RCT | 80 | Mean 40 | 50 (63) | At least 2 months, > 6 months 34% | Nerve root pain | Yes | NR | No | No | Yes | NR |
| 447 | Bourgeois, 1988160 (French Language) | RCT | 60 | Mean 37 (range 26–62) | 40 (67) | Mean 178 (range 50–700) days | Nerve root pain | Yes | Recurrent and first episode | No | No | Yes | NR |
| 729 | Gallucci, 2007170 | RCT | 159 | Mean 41.5 (range 18–71) | 86 (54) | Mean 15 weeks | Nerve root pain | Yes | NR | No | No | Yes | No |
| 50 | Graham, 1976144 | Non-RCT | 40 (23 with sciatica) |
Mean 42 Sciatica patients: mean 41 (range 24–66) |
25 (63) Sciatica patients: 13 (57%) |
Mean whole group 5.35 years Sciatica patients median 1 year (range 12 weeks–25 years) |
Nerve root pain and referred pain | Yes | NR | No | No | Yes | NR |
| Chemonucleolysis vs inactive control | |||||||||||||
| 726 | Dabezies, 1988209 | RCT | 173 | NR | NR | NR | Nerve root pain | Yes | Recurrent and first episode | No | NR | Yes | No |
| 244 | Feldman, 1986207 (French language) | RCT | 39 | Mean 42.5 (range 21–77) | 19 (49) | Mean 6.6 months (range 1–18 months) | Nerve root pain | Yes | Recurrent and first episode | No | No | Yes | NR |
| 55 | Gogan, 1992205 | RCT | 60 | Mean 37 (range 19–69) | 39 (65) | < 6 weeks 10%, 6 weeks to 6 month 75%, > 6 months 15% | Nerve root pain | Yes | NR | No | No | Yes | NR |
| 738 | Javid, 1983210 | RCT | 108 | NR | NR | Mean 26 weeks | Nerve root pain | Yes | NR | No | NR | Yes | No |
| 236 | Schwetschenau, 1976206 | RCT | 66 | Mean 36.2 (SE 1.9) | 44 (67) | Mean 11.6 weeks (SE 1.9 weeks) | Nerve root pain | Yes | NR | No | NR | Yes | No |
| Chemonucleolysis vs manipulation | |||||||||||||
| 723 | Burton, 2000208 | RCT | 40 | Mean 41.9 (SD 10.6) | 19 (48) | Mean 31 weeks (SD 35 weeks) | Nerve root pain | Yes | Recurrent and first episode | No | No | NR | No |
Summary of study design and quality for chemonucleolysis studies
Summary information on study details are presented in Table 33. Fewer than half (17/36, 47%) of chemonucleolysis studies were RCTs, and only one of these206 was good quality (comparator was inactive control). Eleven studies47,85,88,160,168,170,205,207–210 were of moderate quality. One study206 used both adequate randomisation and allocation concealment (comparator included inactive control). A further five studies85,88,160,205,210 used adequate randomisation, but not allocation concealment (although two studies160,210 used sealed envelopes), and one study69 used adequate allocation concealment but not randomisation. One multicentre study209 reported that separate randomisation sequences were provided for each participating institute, but gave no details on how these sequences were generated. One study47 had strong external validity (comparator included inactive control).
| ID no. | Author, year | Study size | Overall follow-up | Study design | Adequate randomisation? | Allocation concealment? | Follow-up (%) | Blind outcome assessment? | Overall quality rating | Overall external validity rating |
|---|---|---|---|---|---|---|---|---|---|---|
| Chemonucleolysis vs disc surgery | ||||||||||
| 884 | Alexander, 1989103 | 100 | Mean 14 (range 6–35) months | CCS | No | No | 80–100 | Unclear | Weak | Weak |
| 43 | van Alphen, 198947 | 151 | 12 months | RCT | Partial | Unclear | 80–100 | No | Moderate | Strong |
| 441 | Bonafe, 199375 (French language) | 40 | Mean 15 (range 3–36) months | CCS | No | No | 80–100 | Unclear | Weak | Weak |
| 183 | Bouillet, 198361 | 2749 | NR | CCS | No | No | NA | No | Weak | Moderate |
| 453 | Brown, 198976 | 85 | 3 months | CCS | No | No | 80–100 | Yes | Weak | Weak |
| 454 | Buric, 200577 | 45 | 18 months | Non-RCT | No | No | 80–100 | NA | Weak | Weak |
| 166 | Crawshaw, 198460 | 52 | 1 year | RCT | Unclear | Unclear | 80–100 | Unclear | Weak | Moderate |
| 48 | Dabezies, 197851 | 200 | 2 years | CCS | No | No | Cannot tell | No | Weak | Moderate |
| 471 | Dei-Anang, 199079 (German language) | 201 | 1 year | CCS | No | No | NA | Unclear | Weak | Weak |
| 727 | Ejeskar, 198396 | 29 | 1 year | RCT | Unclear | Unclear | 80–100 | Unclear | Weak | Moderate |
| 132 | Hoogmartens, 197656 | 97 | 58 months for discectomy and 38 months for chemonucleolysis | HCS | No | No | NA | NA | Weak | Moderate |
| 44 | Javid, 199548 | 200 | 1 year | CCS | No | No | 80–100 | No | Weak | Moderate |
| 35 | Krugluger, 200046 | 22 | 2 years | RCT | Unclear | Unclear | 80–100 | Unclear | Weak | Weak |
| 117 | Lagarrigue, 199154 (French language) | 1085 | Mean 17.2 (range 12–84) months | CCS | No | No | 80–100 | Unclear | Weak | Moderate |
| 129 | Lavignolle, 198755 (French language) | 358 | Mean 25 months for surgery and 22 months for chemonucleolysis | RCT | Unclear | Unclear | 80–100 | Unclear | Weak | Weak |
| 889 | Lee, 1996104 (German language) | 300 | 1 year | CCS | No | No | Cannot tell | Unclear | Weak | Weak |
| 593 | Muralikuttan, 199285 | 92 | 1 year | RCT | Yes | Unclear | 80–100 | Unclear | Moderate | Moderate |
| 47 | Norton, 198650 | 105 | At least 1 year | CCS | No | No | NA | Unclear | Weak | Weak |
| 45 | Postacchini, 198749 | 161 |
Mean 2.9 years (range 20–38 months) in chemonucleolysis group Mean 2.8 years (range 21–42 months in surgery) group |
Non-RCT | No | No | 80–100 | No | Weak | Moderate |
| 617 | Revel, 199388 | 165 | 1 year | RCT | Yes | Unclear | 80–100 | Unclear | Moderate | Weak |
| 641 | Steffen, 199990 (German language) | 69 | 1 year | RCT | Unclear | Unclear | 80–100 | Yes | Weak | Weak |
| 49 | Stula, 199052 (German language) | 69 | Postoperative | RCT | Unclear | Unclear | 80–100 | Unclear | Weak | Weak |
| 61 | Tregonning, 199153 | 268 | 10 years | CCS | No | No | 80–100 | No | Weak | Moderate |
| 893 | Watters,1988105 | 100 | 3 years | Non-RCT | No | No | 80–100 | No | Weak | Weak |
| 160 | Watts, 197559 | 274 | 2 years | CCS | No | No | 80–100 | Unclear | Weak | Weak |
| 672 | Weinstein, 198692 | 159 | Mean 10.3 (range 10.0–13.5) years | CCS | No | No | 80–100 | NA | Weak | Weak |
| 150 | Zeiger, 198758 | 126 | Range 6–46 months, with an average time from treatment procedure to follow-up evaluation of 18 months | CCS | No | No | NA | Yes | Weak | Weak |
| Chemonucleolysis vs epidural | ||||||||||
| 720 | Bontoux, 1990168 (French language) | 80 | 3 months | RCT | Yes | Unclear | 80–100 | Yes | Moderate | Weak |
| 447 | Bourgeois, 1988160 (French language) | 60 | 6 months | RCT | Yes | Partial | 80–100 | Yes | Moderate | Weak |
| 729 | Gallucci, 2007170 | 159 | 6 months | RCT | Unclear | Unclear | 80–100 | Yes | Moderate | Weak |
| 50 | Graham, 1976144 | 40 (23 with sciatica) | 2 years | Non-RCT | No | No | 80–100 | Yes | Weak | Weak |
| Chemonucleolysis vs inactive control | ||||||||||
| 726 | Dabezies, 1988209 | 173 | 6 months | RCT | Partial | Yes | 60–79 | Yes | Moderate | Weak |
| 244 | Feldman, 1986207 (French language) | 39 | 3 months | RCT | Unclear | Unclear | 80–100 | Unclear | Moderate | Moderate |
| 55 | Gogan, 1992205 | 60 | 10 Years | RCT | Yes | Unclear | 80–100 | Yes | Moderate | Moderate |
| 738 | Javid, 1983210 | 108 | 6 months | RCT | Yes | Partial | 80–100 | Yes | Moderate | Weak |
| 236 | Schwetschenau, 1976206 | 66 | 1 year | RCT | Yes | Yes | 80–100 | Yes | Strong | Moderate |
| Chemonucleolysis vs manipulation | ||||||||||
| 723 | Burton, 2000208 | 40 | 12 months | RCT | No | No | 60–79 | Yes | Moderate | Weak |
| Chemonucleolysis vs mixed treatments | ||||||||||
| 534 | Khoromi, 2007214 | 55 | 36 weeks | RCT (crossover) | Yes | Yes | < 60 | Yes | Moderate | Strong |
Chemonucleolysis results at short-term follow-up (≤ 6 weeks)
Global effect at short-term follow-up
The results for the global effect at short-term follow-up are presented in Table 34 and the accompanying forest plot (Figure 24). Chemonucleolysis was compared with inactive control, disc surgery and epidural. Five studies46,48,52,92,205 included only patients with chronic sciatica, four studies49,170,206,207 included patients with either acute or chronic sciatica and the remaining studies did not report the duration of symptoms. The duration of follow-up ranged from 72 hours206 to 6 weeks. 46,48,79,104,205,209
| ID no. | Author, year | Chronicity | Study design | Follow-up | Outcome measure | Perspective | Intervention | Control | OR (95% CI) | Comments | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n) | Outcome (n) | Withdrawal rate | Total (n) | Outcome (n) | Withdrawal rate | |||||||||
| Chemonucleolysis vs disc surgery | ||||||||||||||
| 471 | Dei-Anang, 199079 (German language) | NR | CCS | 42 days | Reported absence of pain | Patient | 101 | 79 | 0 | 100 | 72 | 0 | 1.40 (0.73 to 2.66) | Data inferred from percentages reported in graphs |
| 44 | Javid, 199548 | C | CCS | 6 weeks | Successful outcome: good or excellent (vs slight or no improvement) | Patient | 100 | 82 | 0 | 100 | 92 | 0 | 0.40 (0.16 to 0.96) | |
| 889 | Lee, 1996104 (German language) (i)a (APLD) | NR | CCS | 6 weeks | Disappearance of back pain | 100 | 16 | ? | 100 | 16 | ? | 1.00 (0.47 to 2.13) |
Number randomised not stated, 300 included in analysis Excluded: chemonucleolysis 29%, surgery 14% |
|
| 889 | Lee, 1996104 (German language) (ii)a (PELD) | NR | CCS | 6 weeks | Disappearance of back pain | 100 | 16 | ? | 100 | 29 | ? | 0.47 (0.23 to 0.93) |
Number randomised not stated, 300 included in analysis Excluded: chemonucleolysis 29%, surgery 29% |
|
| 45 | Postacchini, 198749 | A + C | Non-RCT | 1 month | Treatment success: excellent or good (vs fair or poor) | 72 | 39 | 0.03 | 84 | 52 | 0.03 | 0.40 (0.16 to 0.96) |
Data inferred from graphs Five lost to follow-up were excluded Patients in chemonucleolysis group who had surgery regarded as failure |
|
| 49 | Stula, 199052 (German language) | C | RCT | Postoperative | Therapeutic success: good (vs unsatisfactory) | Physician | 25 | 22 | 0.43 | 44 | 40 | 0.76 | 0.73 (0.38 to 1.38) | Per protocol analysis with 19 crossed over to surgery |
| 672 | Weinstein, 198692 | C | CCS | < 6 weeks | Recovered within 2–6 weeks or immediate (vs no recovery, 6–12 weeks recovery or > 12 weeks recovery) | Patient | 88 | 61 | 0.04 | 71 | 39 | 0.13 | 1.56 (0.78 to 3.13) | |
| Chemonucleolysis vs epidural/intradiscal injection | ||||||||||||||
| 729 | Gallucci, 2007170 | A + C | RCT | 2 weeks | Treatment success: ODI ≤ 20% | 82 | 72 | 0 | 77 | 69 | 0 | 1.20 (0.45 to 3.21 | ||
| Chemonucleolysis vs inactive control | ||||||||||||||
| 726 | Dabezies, 1988209 | NR | RCT | 6 weeks | Treatment success: pain free or moderate improvement (vs unimproved or worse) | 77 | 56 | 0.11 | 81 | 42 | 0.06 | 2.48 (1.27 to 4.81) | ||
| 244 |
Feldman, 1986207 (French language) |
A + C | RCT | 1 month | Favourable results – based on VAS pain assessment: very good or good (vs mediocre, bad or failures) | Patient | 20 | 11 | 0 | 19 | 5 | 0 | 3.42 (0.89 to 13.18) | |
| 55 | Gogan, 1992205 | C | RCT | 6 weeks | Treatment success (yes or no) | Patient | 30 | 22 | 0 | 30 | 11 | 0 | 4.45 (1.58 to 14.25) | Data inferred from graphs |
| 236 | Schwetschenau, 1976206 | A + C | RCT | 72 hours | Symptom improvement: excellent or good (vs fair) | 31 | 8 | 0. | 35 | 13 | 0 | 0.59 (0.20 to 1.69) | ||
FIGURE 24.
Summary of the findings of the global effect at short-term follow-up (≤ 6 weeks) for studies comparing chemonucleolysis with alternative interventions. Note: weights are from random effects analysis.
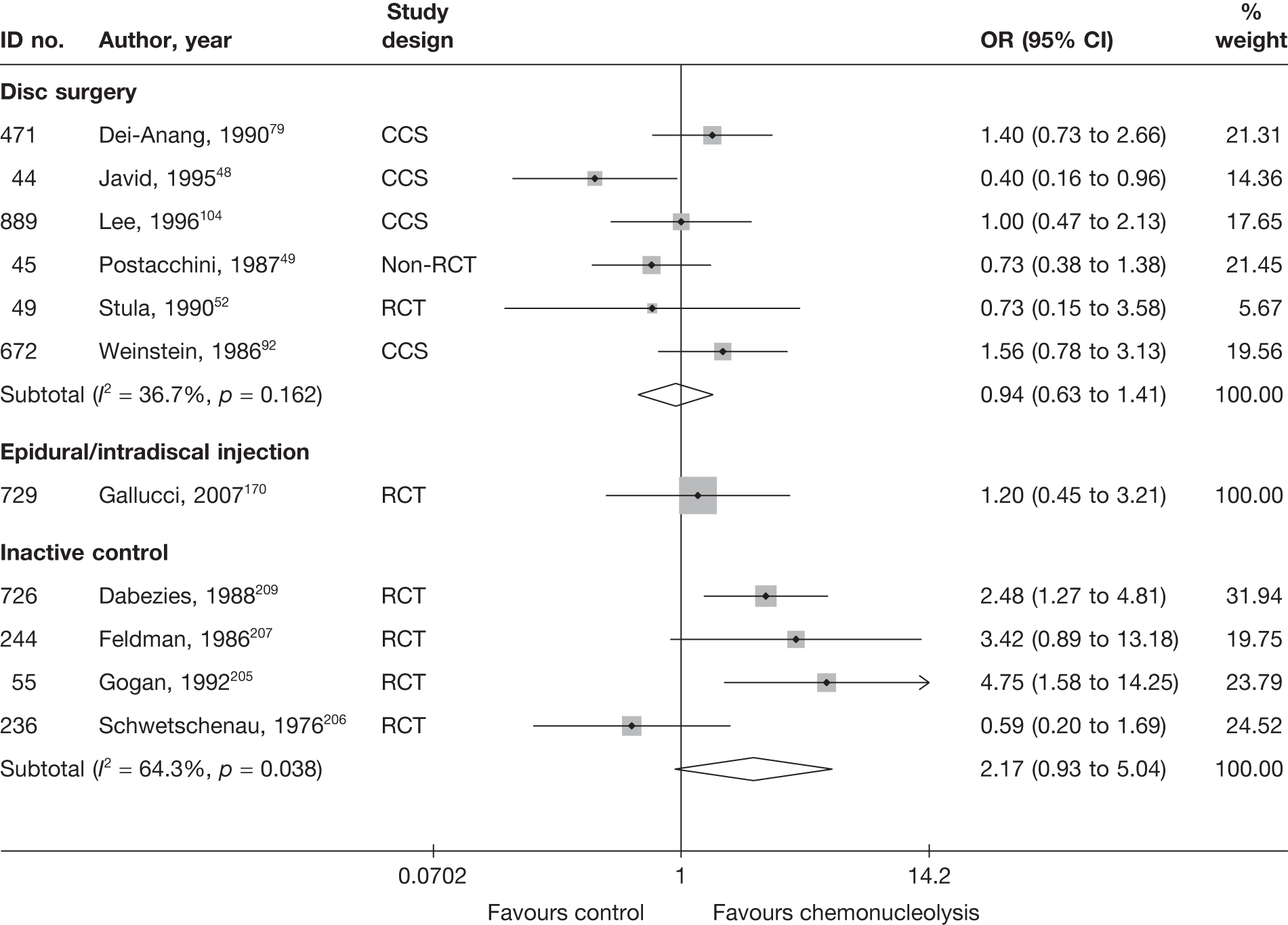
Chemonucleolysis was compared with an inactive control in four RCTs,205–207,209 for which the pooled analysis showed a non-statistically significant difference in favour of the chemonucleolysis group. One RCT206 was good quality and the remaining three were of moderate quality, with most using adequate randomisation. Unlike the remaining RCTs, this study206 reported non-statistically significant findings in favour of the inactive control.
Six studies48,49,52,79,92,104 compared chemonucleolysis with disc surgery, for which there was no overall statistically significant difference between the groups. Only one of these studies was a RCT,52 which was poorly reported with method of randomisation and allocation concealment not stated. Nineteen patients in the chemonucleolysis group crossed over to receive surgery and were analysed accordingly. The results and methods of the remaining studies were also poorly reported.
One poorly reported RCT,170 of moderate quality, compared intraforaminal and intradiscal injections of steroid, local anaesthetic and ozone–oxygen (categorised as chemonucleolysis) with intraforaminal and intradiscal injections of steroid plus local anaesthetic (epidural), for which there was no overall difference between the groups. The study included patients with mainly acute sciatica (mean duration of symptoms of 15 weeks).
Pain intensity at short-term follow-up
The results for pain intensity at short-term follow-up are presented in Table 35 and the accompanying forest plot (Figure 25). Chemonucleolysis was compared with inactive control, disc surgery and manipulation. One study76 included patients with chronic sciatica, three studies85,207,208 included patients with either acute or chronic sciatica, and the remaining study88 did not report the duration of symptoms. The duration of follow-up ranged from 4 weeks88,207 to 6 weeks. 76,85,208
| ID no. | Author, year | Chronicity | Study design | Follow-up | Location | Scale (range)a | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)b | Comment/conversionc | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |||||||||
| Chemonucleolysis vs disc surgery | ||||||||||||||||
| 453 | Brown, 198976 (i)d (chymopapain) | C | CCS | 6 weeks | Leg | VAS (0–100) | 51 | 19 | 60 | 70 | 22 (25.48) | 3 (20.87) | 19.00 (7.30 to 30.70) | SD imputed from weighted average | ||
| 453 | Brown, 198976 (ii)d (collagenase) | C | CCS | 6 weeks | Leg | VAS (0–100) | 15 | 19 | 58 | 70 | 46 (25.48) | 3 (20.87) | 43.00 (27.05 to 58.95) | SD imputed from weighted average | ||
| 593 | Muralikuttan, 199285 | A + C | RCT | 6 weeks | Leg | VAS (0–100) | 46 | 46 | 6 | 72 | 19 (25.48) | 19 (20.87) | 0.00 (–9.52 to 9.52) | SD imputed from weighted average (one study) | ||
| 617 | Revel, 199388 | NR | RCT | 1 month | Leg | VAS (0–100) | 68 | 62 | 63.4 (24.6) | 68.1 (21.6) | 28.3 (27.21) | 39.4 (32.28) | –11.10 (–21.41 to –0.79) |
SD derived from SE Dropouts 24/165 (15%): intervention 4/72, control 7/69 A further 24 patients were also excluded from the analysis, group allocation not stated |
||
| Chemonucleolysis vs inactive control | ||||||||||||||||
| 244 | Feldman, 1986207 (French language) | A + C | RCT | 28 days | Leg | VAS (0–100) | 20 | 19 | 64.0 | 54.1 | 30.3 (25.48) | 40.2 (23.67) | –9.90 (–25.33 to 5.53) | SD imputed from weighted average (one study) | ||
| Chemonucleolysis vs manipulation | ||||||||||||||||
| 723 | Burton, 2000208 | A + C | RCT | 6 weeks | Leg | Annotated thermometer (0–6) | 18 | 19 | 60.8 (26.5) | 66.7 (14.7) | 45.3 (17.0) | 44.7 (26.7) | 0.63 (–13.72 to 14.98) | Missing data: intervention 2/20, control 1/20 | ||
FIGURE 25.
Summary of the findings of pain intensity at short-term follow-up (≤ 6 weeks) for studies comparing chemonucleolysis with alternative interventions. Note: weights are from random effects analysis.
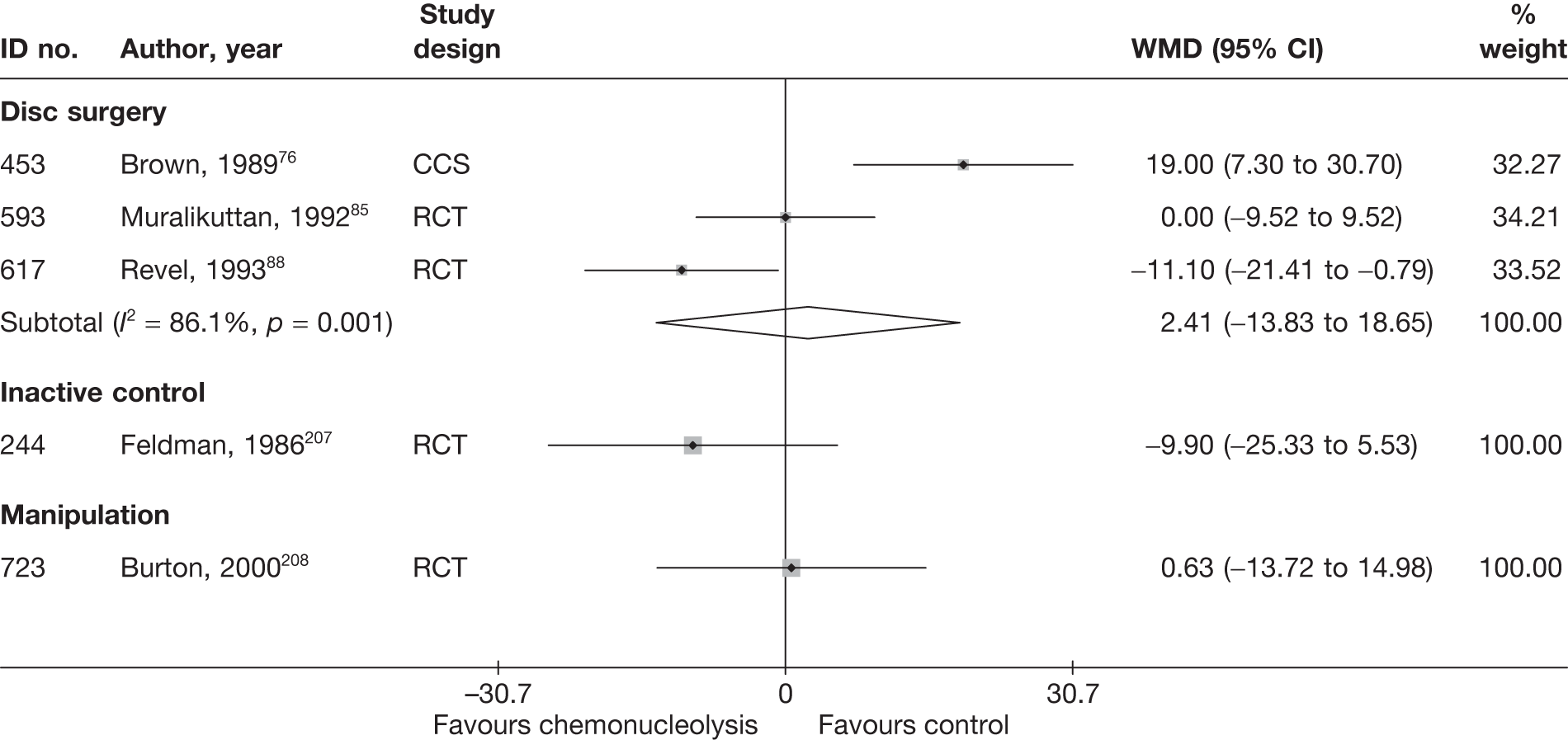
One poorly reported RCT,207 of moderate quality, showed non-statistically significant findings in favour of chemonucleolysis compared with inactive control, for reduction in leg pain at 28 days.
Three studies76,85,88 compared chemonucleolysis with disc surgery, for which there was no overall statistically significant difference between the intervention groups. However, the results were heterogeneous. One CCS76 reported findings in favour of disc surgery and one RCT88 reported findings in favour of chemonucleolysis, whereas the remaining RCT85 reported no statistically significant difference between the interventions. One study76 included patients who had not received previous disc surgery, whereas the other88 included patients who had had previous surgery and also had a high proportion of men.
According to one RCT,208 there was no important difference between chemonucleolysis and osteopathic manipulation at 6 weeks in terms of pain reduction. However, although the randomisation sequence was generated by computer and treatment allocated using envelopes, some patients were not randomised according to the predetermined order because of administrative problems.
Condition-specific outcome measures at short-term follow-up
The results for CSOMs at short-term follow-up are presented in Table 36 and the accompanying forest plot (Figure 26). Chemonucleolysis was compared with disc surgery and manipulation. Two studies46,92 included patients with chronic sciatica, two studies85,208 included patients with either acute or chronic symptoms, and the remaining study88 did not report this information. The duration of follow-up ranged from 1 month88 to 6 weeks. 46,85,208
| ID no. | Author, year | Chronicity | Study design | Follow-up | Scale | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)a | Comment/conversionb | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | ||||||||
| Chemonucleolysis vs disc surgery | |||||||||||||||
| 593 | Muralikuttan, 199285 | A + C | RCT | 6 weeks | Part of Waddell Disability Index | 46 | 46 | 6.2 | 6.7 | 3.5 (1.21) | 2.8 (1.21) | –2.7 | 3.9 | 0.58 (0.16 to 1.00) |
SD imputed from weighted average Most outcomes showed skewed distribution |
| 617 | Revel, 199388 | NR | RCT | 1 month | Waddell Disability Index and Main Scale | 69 | 62 | 4.9 (2.49) | 6 (2.55) | 1.5 (2.55) | 1.5 (3.15) | –3.4 | –1.05 | –0.00 (–0.34 to 0.34) |
Final SDs derived from SEs 24 patients were excluded from analysis, group allocation not stated, plus further 10/141 (7%): intervention 3/72, control 7/69 |
| Chemonucleolysis vs manipulation | |||||||||||||||
| 723 | Burton, 2000208 | A + C | RCT | 6 weeks | RMDQ | 18 | 19 | 11.95 (5.83) | 11.9 (5.48) | 11 (5.69) | 7.79 (6.65) | –0.95 | –4.11 | ||
FIGURE 26.
Summary of the findings of CSOMs at short-term follow-up (≤ 6 weeks) for studies comparing chemonucleolysis with alternative interventions. Note: weights are from random effects analysis.
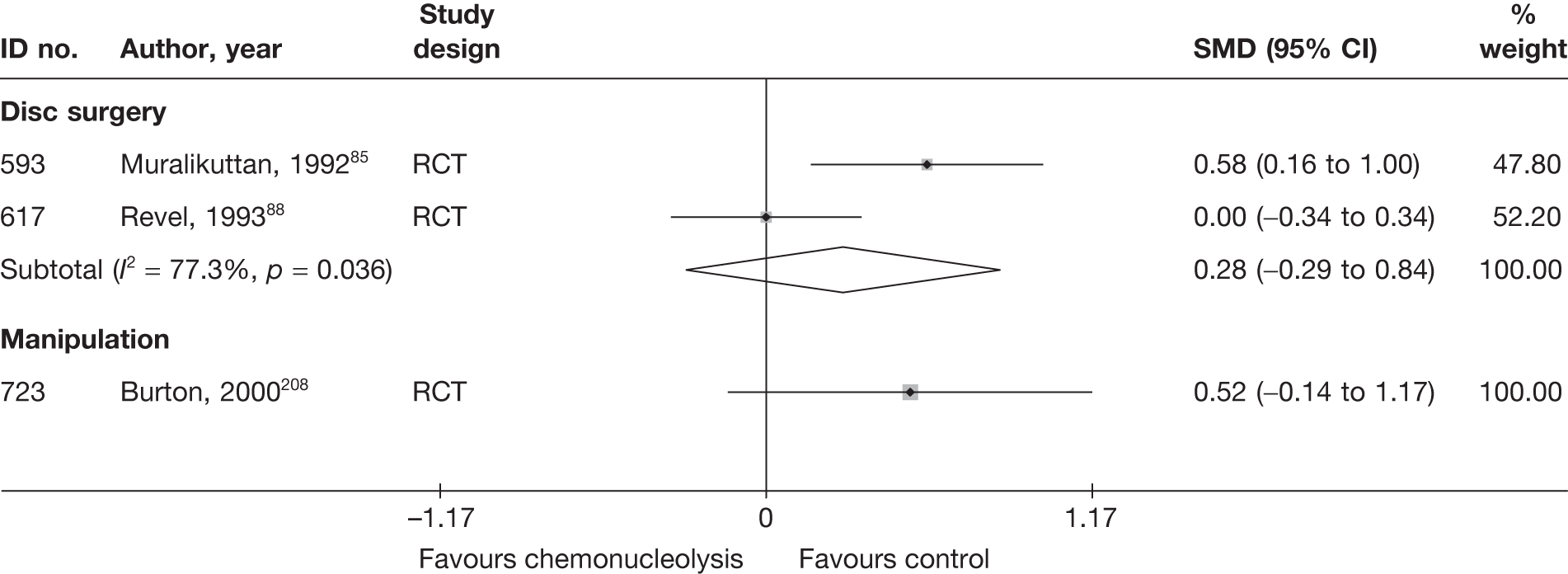
Two studies compared chemonucleolysis with disc surgery; one was an RCT85 and one was a non-RCT. 77 Overall, there was a non-statistically significant difference between the intervention groups in favour of disc surgery.
One moderate-quality RCT208 showed a non-statistically significant improvement in function in favour of manipulation, compared with chemonucleolysis, at 6 weeks. The study experienced problems with the randomisation process.
Chemonucleolysis results at medium-term follow-up (> 6 weeks to ≤ 6 months)
Global effect at medium-term follow-up
The results for the global effect at medium-term follow-up are presented in Table 37 and the accompanying forest plot (Figure 27). Chemonucleolysis was compared with inactive intervention, disc surgery, and epidural. Eight studies48,54,76,92,160,168,205,210 only included patients with chronic symptoms. The remaining studies included patients with either acute or chronic sciatica49,105,170,206,207 or did not state the duration of symptoms. 88,104,209 The duration of follow-up ranged from 2 to 6 months, or mean 13105 to 23 weeks. 206
| ID no. | Author, year | Chronicity | Study design | Follow-up | Outcome measure | Perspective | Intervention | Control | OR (95% CI) | Comments | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n) | Outcome (n) | Withdrawal rate | Total (n) | Outcome (n) | Withdrawal rate | |||||||||
| Chemonucleolysis vs disc surgery | ||||||||||||||
| 453 | Brown, 198976 (i)a (chymopapain) | C | CCS | 3 months | Final outcome: excellent or good (vs fair, poor or failed) | NR | 51 | 26 | 0 | 19 | 16 | 0 | 0.19 (0.05 to 0.75) | Data reported as percentages |
| 453 | Brown, 198976 (ii)a (collagenase) | C | CCS | 3 months | Final outcome: excellent or good (vs fair, poor or failed) | NR | 15 | 9 | 0 | 19 | 16 | 0 | 0.28 (0.06 to 1.41) | Data reported as percentages |
| 44 | Javid, 199548 | C | CCS | 6 months | Successful outcome: good or excellent (vs slight or no improvement) | Patient | 100 | 88 | 0 | 100 | 85 | 0 | 1.29 (0.57 to 2.93) | |
| 117 | Lagarrigue, 199154 (French language) | C | CCS | 2 months | MacNab criteria: excellent or good (vs mediocre or failure) | Patient + physician | 334 | 238 | 0 | 751 | 675 | 0 | 0.28 (0.20 to 0.39) | Data reported as percentages |
| 889 | Lee, 1996104 (German language) (i)b (APLD) | NR | CCS | 2 months | Disappearance of back pain | 100 | ? | 29 | 100 | 35 | ? | 0.76 (0.42 to 1.38) |
Number randomised not stated, 300 included in analysis Excluded: chemonucleolysis 29%, surgery 14% |
|
| 889 | Lee, 1996104 (German language) (ii)b (PELD) | NR | CCS | 2 months | Disappearance of back pain | 100 | ? | 29 | 100 | 8 | ? | 4.70 (2.02 to 10.90) |
Number randomised not stated, 300 included in analysis Excluded: chemonucleolysis 29%, surgery 29% |
|
| 45 | Postacchini, 198749 | A + C | Non-RCT | 3 months | Treatment success: excellent or good (vs fair or poor) | 72 | 51 | 0.03 | 84 | 65 | 0.03 | 0.71 (0.35 to 1.46) |
Data inferred from graphs Five lost to follow-up were excluded Patients who had surgery in chemonucleolysis group regarded as failure |
|
| 617 | Revel, 199388 | NR | RCT | 6 months | Treatment success categorised as: very good or good (vs none or moderate) | Patient | 72 | 44 | ? | 69 | 30 | ? | 2.04 (1.04 to 4.00) | ITT not used. 24/165 (15%) patients excluded from analysis, group allocation not stated |
| 893 | Watters,1988105 | A + C | Non-RCT | Mean 46 days | Success of surgical results: excellent or good (vs fair or poor) | Physician | 50 | 32 | 0 | 50 | 44 | 0 | 0.24 (0.09 to 0.68) | Reported as percentages only |
| 672 | Weinstein, 198692 | C | CCS | 3–6 months | Recovered within 2–6 weeks, 6–12 weeks or immediate (vs no recovery, > 12 weeks) | Patient | 85 | 71 | 0.03 | 63 | 53 | 0.11 | 0.96 (0.39 to 2.32) | Data reported as percentages |
| Chemonucleolysis vs epidural/intradiscal injection | ||||||||||||||
| 720 | Bontoux, 1990168 (French language) | C | RCT | 3 months | Overall improvement: very good or good (vs mediocre or bad) | 40 | 26 | 0 | 40 | 27 | 0 | 0.89 (0.35 to 2.26) | ||
| 447 | Bourgeois, 1988160 (French language) | C | RCT | 6 months | Overall pain relief: very good or good (vs failure) | 30 | 20 | 0 | 30 | 16 | 0 | 1.75 (0.62 to 4.97) | ||
| 729 | Gallucci, 2007170 | A + C | RCT | 6 months | Treatment success: ODI ≤ 20% | 82 | 61 | 0 | 77 | 36 | 0 | 0.30 (0.15 to 0.59) | ||
| Chemonucleolysis vs inactive control | ||||||||||||||
| 726 | Dabezies, 1988209 | NR | RCT | 6 months | Treatment success: pain free or moderate improvement (vs unimproved or worse) | 62 | 44 | 0.29 | 74 | 33 | 0.14 | 3.04 (1.49 to 6.21) | ||
| 244 | Feldman, 1986207 (French language) | A + C | RCT | 3 months | Favourable results – based on VAS pain assessment: very good or good (vs mediocre, bad or failures) | 20 | 13 | 0 | 19 | 8 | 0 | 2.55 (0.70 to 9.31) | ||
| 55 | Gogan, 1992205 | C | RCT | 6 months | Treatment success (yes or no) | Patient | 30 | 24 | 0 | 30 | 17 | 0 | 3.06 (0.97 to 9.66) | Data inferred from graphs |
| 738 | Javid, 1983210 | C | RCT | 6 months | Success (vs failure) | 55 | 40 | 0 | 53 | 22 | 0 | 3.76 (1.68 to 8.42) | ||
| 236 | Schwetschenau, 1976206 | A + C | RCT | Mean 23 weeks | Symptom improvement: excellent or good (vs fair) | 31 | 9 | 0 | 35 | 11 | 0 | 0.89 (0.31 to 2.56) | ||
FIGURE 27.
Summary of the findings of the global effect at medium-term follow-up (> 6 weeks to ≤ 6 months) for studies comparing chemonucleolysis with alternative interventions. Note: weights are from random effects analysis.
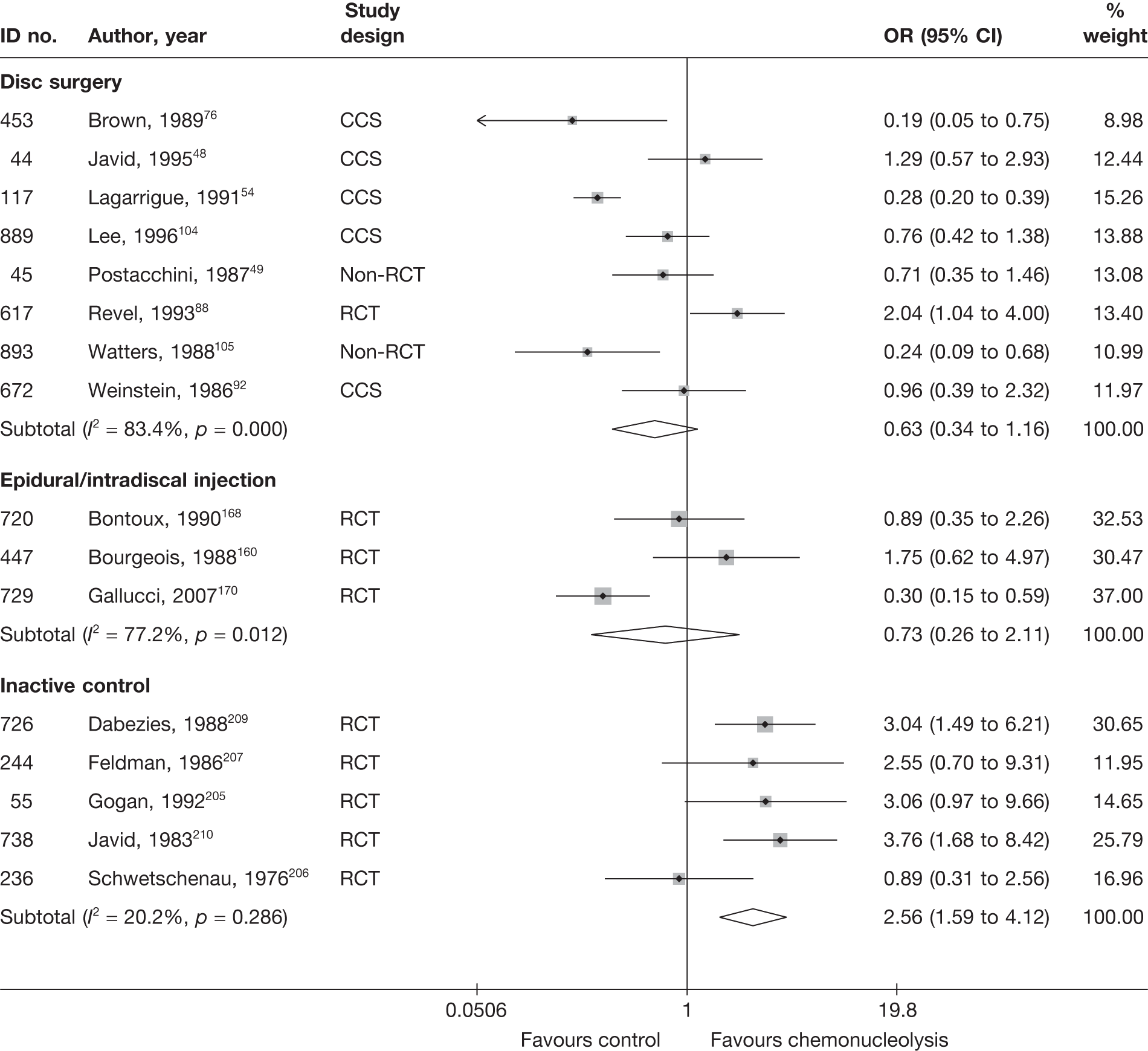
Pooled analysis of five RCTs205–210 showed chemonucleolysis to be significantly better than inactive control for overall recovery at 3–6 months205,207,209,210 or mean 23 weeks. 206
Eight studies48,49,54,76,88,92,104,105 compared chemonucleolysis and disc surgery, for which there was no overall difference between the groups. One moderate-quality RCT88 found chemonucleolysis to be more effective than disc surgery. However, the withdrawal rate in the surgery group (at least 41%) was much greater than that in the chemonucleolysis group (at least 19%), with dropouts being given a poor outcome in the analysis. The remaining studies were observational or non-RCTs, the results and methods of which were generally poorly reported.
Three RCTs160,168,170 compared chemonucleolysis with epidural, two of which used chymopapain160,168 and one170 used injections of steroid, local anaesthetic, and ozone–oxygen. The first two RCTs found no important difference between the intervention groups for chronic sciatica, whereas the third RCT170 found statistically significant findings in favour of the epidural group for patients who had had symptoms for a mean of 15 weeks. However, the study was poorly reported (with method of randomisation not stated) and of moderate quality. The first two studies were also of moderate quality overall, but used an adequate method of randomisation.
Pain intensity at medium-term follow-up
The results for pain intensity at medium-term follow-up are presented in Table 38 and the accompanying forest plot (Figure 28). Chemonucleolysis was compared with inactive control and disc surgery. One study76 only included patients with chronic sciatica, one study88 did not report the duration of symptoms and the remaining studies207,85 included patients with either acute or chronic sciatica. The duration of follow-up ranged from 60 days159 to 6 months. 150,151,155,171,174
| ID no. | Author, year | Chronicity | Study design | Follow-up | Location | Scale (range)a | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)b | Comment/conversionc | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |||||||||
| Chemonucleolysis vs disc surgery | ||||||||||||||||
| 453 | Brown, 198976 (i)d (chymopapain) | C | CCS | 12 weeks | Leg | VAS (0–100) | 51 | 19 | 60 | 70 | 14 (23.76) | 4 (24.43) | 10.00 (–2.77 to 22.77) | SD imputed from weighted average | ||
| 453 | Brown, 198976 (ii)d (collagenase) | C | CCS | 12 weeks | Leg | VAS (0–100) | 15 | 19 | 58 | 70 | 22 (23.76) | 4 (24.43) | 18.00 (1.71 to 34.29) | SD imputed from weighted average | ||
| 593 | Muralikuttan, 199285 | A + C | RCT | 3 months | Leg | VAS (0–100) | 46 | 46 | 64 | 72 | 20 (23.76) | 14 (24.43) |
6.00 (–3.85 to 15.85) Statistically significant difference between groups, p < 0.05, Mann–Whitney U-test |
SD imputed from weighted average Most outcomes showed skewed distribution |
||
| 617 | Revel, 199388 | NR | RCT | 6 months | Leg | VAS (0–100) | 72 | 69 | 63.4 (24.61) | 68.1 (21.6) | 17.6 (23.76) | 35.6 (34.89) | –18.00 (–27.89 to –8.11) |
SDs derived from SEs 24 patients were excluded from the analysis, group allocation not stated |
||
| Chemonucleolysis vs inactive control | ||||||||||||||||
| 244 | Feldman, 1986207 (French language) | A + C | RCT | 90 days | Leg | VAS (0–100) | 14 | 10 | 64.0 | 54.1 | 8.7 (23.76) | 14.1 (30.1) | –5.40 (–27.83 to 17.03) |
SD imputed from weighted average Missing data: intervention 6/20, control 9/19 |
||
FIGURE 28.
Summary of the findings of pain at medium-term follow-up (> 6 weeks to ≤ 6 months) for studies comparing chemonucleolysis with alternative interventions. Note: weights are from random effects analysis.
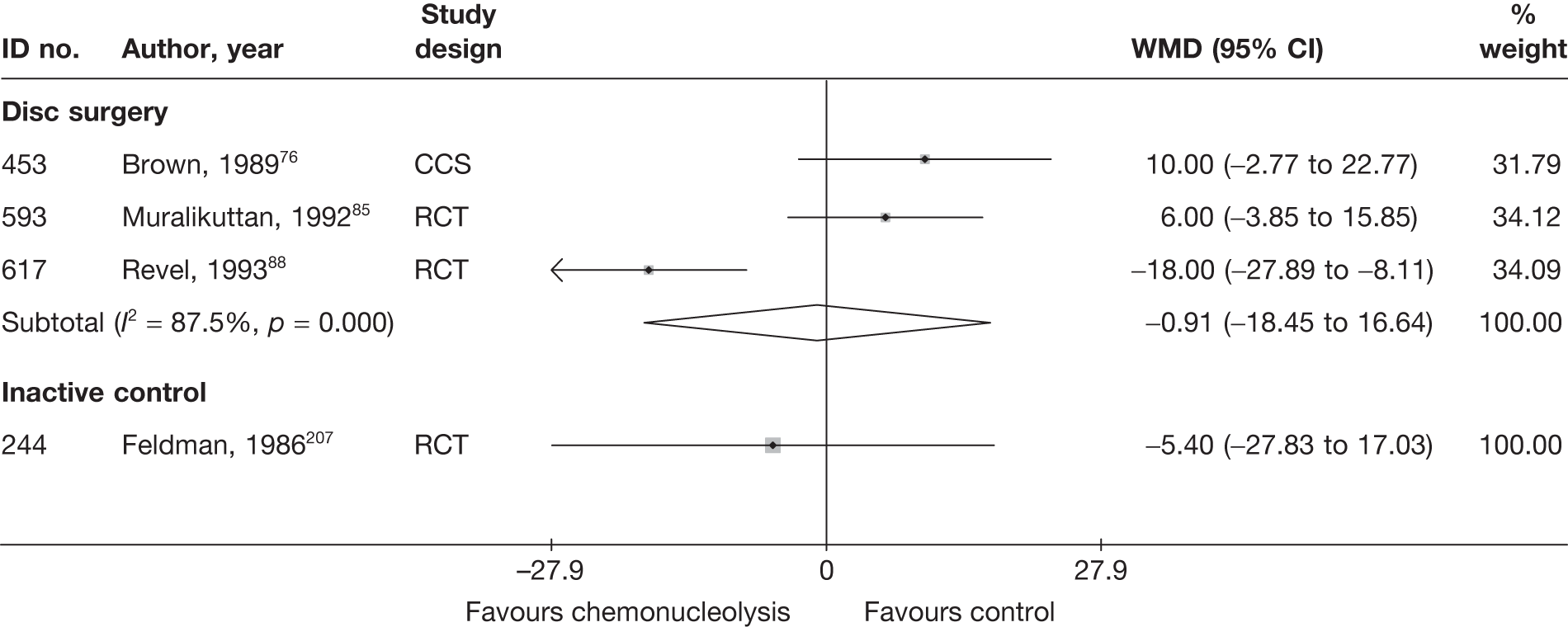
One small, poorly reported RCT of moderate quality, showed a non-statistically significant findings in favour of chemonucleolysis, compared with inactive control, at 90 days. The number of dropouts for the study was quite high, and more patients were lost to follow-up in the control group (47%) than in the intervention group (30%).
Three studies76,85,88 compared chemonucleolysis with disc surgery; two were RCTs85,88 and one was a CCS. 76 Overall, there was no statistically significant difference between the intervention groups, but the results were heterogeneous, with one RCT88 showing statistically significant findings in favour of chemonucleolysis. This study included patients who had had previous surgery and also included a high proportion of men.
Condition-specific outcome measures at medium-term follow-up
The results for the CSOMs at medium-term follow-up are presented in Table 39 and the accompanying forest plot (Figure 29). Chemonucleolysis was compared with disc surgery.
| ID no. | Author, year | Chronicity | Study design | Follow-up | Scale | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)a | Comment/conversionb | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | ||||||||
| Chemonucleolysis vs disc surgery | |||||||||||||||
| 727 | Ejeskar, 198396 | A + C | RCT | 6 months | Composite score | 15 | 14 | 9.27 (6.62) | 9.71 (4.79) | –0.08 (–0.80 to 0.65) | |||||
| 593 | Muralikuttan, 199285 | A + C | RCT | 3 months | Part of Waddell Disability Index | 46 | 46 | 6.2 | 6.7 | 3 (1.28) | 2.3 (1.28) | –3.2 | –4.4 | 0.55 (0.13 to 0.96) | SD for final means calculated from p-values (Mann–Whitney U-test); most outcomes showed skewed distribution |
| 617 | Revel, 199388 | NR | RCT | 6 months | Waddell Disability Index and Main Scale | 72 | 69 | 4.9 (2.55) | 6 (3.9) | 2.3 (4.65) | 3.4 (3.32) | –2.6 | –2.6 | –0.27 (–0.60 to 0.06) |
SD calculated from SE Dropouts 24/165 (15%): group allocation not stated |
FIGURE 29.
Summary of the findings of CSOMs at medium-term follow-up (> 6 weeks to ≤ 6 months) for studies comparing chemonucleolysis with alternative interventions. Note: weights are from random effects analysis.
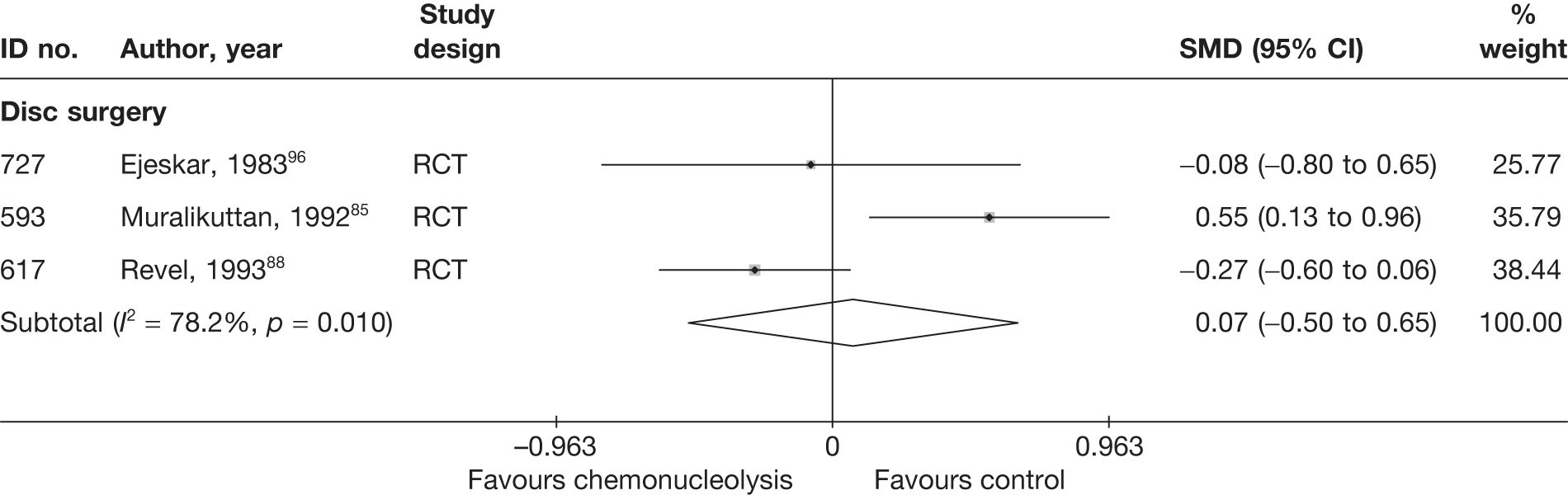
Three RCTs85,88,96 compared chemonucleolysis with disc surgery; the pooled analysis showed no statistically significant difference between the intervention groups at 3–6 months. However, the findings were heterogeneous.
Results at long-term follow-up (> 6 months)
Global effect at long-term follow-up
The results for the global effect at long-term follow-up are presented in Table 40 and the accompanying forest plot (Figure 30). Chemonucleolysis was compared with inactive control, disc surgery and epidural. Ten studies47,48,53,56,59,90,92,103,144,205 included patients with chronic sciatica and six studies included patients with either acute or chronic sciatica,49,50,58,75,85,206 although the remaining five studies did not report this information. 51,55,60,88,104 The duration of follow-up ranged from < 1 year92 to 10 years. 53,205
| ID no. | Author, year | Chronicity | Study design | Follow-up | Outcome measure | Perspective | Intervention | Control | OR (95% CI) | Comments | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n) | Outcome (n) | Withdrawal rate | Total (n) | Outcome (n) | Withdrawal rate | |||||||||
| Chemonucleolysis vs disc surgery | ||||||||||||||
| 884 | Alexander, 1989103 | C | CCS | Mean 14 (range 6–35) months | Satisfactory clinical outcome (vs unsatisfactory results) | Physician | 51 | 40 | 0 | 49 | 39 | 0 | 0.93 (0.36 to 2.44) | Follow-up differed in each groups: chemonucleolysis mean 16 (range 6–35) months, surgery mean 12 (range 6–24) months |
| 43 | van Alphen, 198947 | C | RCT | 12 months | Satisfaction with final result of treatment: yes or largely; (vs barely or no) | Patient | 73 | 53 | 0 | 77 | 61 | 1 | 0.70 (0.33 to 1.48) | |
| 441 | Bonafe, 199375 (French language) | A + C | CCS | 1 year | Overall treatment success using modified MacNab criteria: excellent or good (vs satisfactory or worse) | 20 | 16 | 0 | 20 | 11 | 0 | 3.27 (0.80 to 13.35) | ||
| 166 | Crawshaw, 198460 | NR | RCT | 1 year | Overall outcome: excellent or good (vs poor) | 24 | 11 | 0. 04 | 26 | 23 | 0.04 | 0.11 (0.03 to 0.47) | ||
| 48 | Dabezies, 197851 | NR | CCS | 2 years | Results categorised as excellent or good (vs unimproved) | Patient | 100 | 71 | 0 | 100 | 63 | 0 | 1.44 (0.79 to 2.60) | |
| 132 | Hoogmartens, 197656 | C | HCS | Mean 49 months | Satisfactory result for amount of radicular pain: excellent or good (vs fair or poor) | 44 | 24 | 0 | 53 | 37 | 0 | 0.52 (0.23 to 1.20) |
Data inferred from percentages Follow-up differed for the two groups: surgery mean 58 months, chemonucleolysis mean 38 months |
|
| 44 | Javid, 199548 | C | CCS | 1 year | Success categorised as: good or excellent (vs slight or no improvement) | Patient | 100 | 87 | 0 | 100 | 82 | 0 | 1.47 (0.68 to 3.19) | |
| 129 | Lavignolle, 198755(French language) | NR | RCT | Mean: surgery 25 months; chemonucleolysis 22 months | Overall success using MacNab type score: good or medium (vs mediocre or bad) | Patient | 176 | 141 | 0 | 182 | 150 | 0 | 0.86 (0.51 to 1.46) | |
| 889 | Lee, 1996104 (German language) (i)a (APLD) | NR | CCS | 1 year | Results of treatment: very good or good; (vs moderate or bad) | Patient | 100 | 55 | ? | 100 | 48 | ? | 1.32 (0.76 to 2.31) |
Number randomised not stated, 300 included in analysis Excluded: chemonucleolysis 29%, surgery 29% |
| 889 | Lee, 1996104 (German language) (ii)a (PELD) | NR | CCS | 1 year | Results of treatment: very good or good (vs moderate or bad) | Patient | 100 | 55 | ? | 100 | 68 | ? | 0.58 (0.32 to 1.02) |
Number randomised not stated, 300 included in analysis Excluded: chemonucleolysis 29%, surgery 14% |
| 593 | Muralikuttan, 199285 | A + C | RCT | 1 year | Completely pain free (vs residual back pain only, residual back and referred pain) | 46 | 8 | 0 | 46 | 14 | 0 | 0.48 (0.18 to 1.29) |
Reported as percentages One patient crossed over to surgery |
|
| 47 | Norton, 198650 | A + C | CCS | ≥ 1 year | Treatment success: satisfactory (vs unsatisfactory) based on patient and physician report |
Patient + physician |
61 | 17 | 0 | 44 | 26 | 0 | 0.27 (0.12 to 0.61) | |
| 45 | Postacchini, 198749 | A + C | Non-RCT | > 20 months | Treatment success: excellent or good (vs fair or poor) |
Patient + physician |
72 | 54 | 0.03 | 84 | 70 | 0.03 | 0.60 (0.27 to 1.31) |
Data inferred from graphs Five lost to follow-up were excluded |
| 617 | Revel, 199388 | NR | RCT | 1 year | Treatment success | Patient | 58 | 48 | ? | 41 | 25 | ? | 3.52 (1.76 to 7.04) |
24/165 (15%) patients dropped out at beginning, group allocation not stated A further 30% dropped out (surgery: 28/69; chemonucleolysis 14/72), but included in analysis (given poor outcome) |
| 641 | Steffen, 199990 (German language) | C | RCT | 1 year | MacNab criteria: good or very good (vs satisfactory or poor) | 33 | 17 | 0 | 36 | 11 | 0 | 2.41 (0.90 to 6.46) | Reported as percentages only | |
| 61 | Tregonning, 199153 | C | CCS | 10 years | MacNab criteria: excellent or good (vs fair or poor) | 145 | 47 | 0.12 | 91 | 51 | 0.13 | 0.38 (0.22 to 0.65) | ||
| 160 | Watts, 197559 | C | CCS | 2 years | Overall outcome: success (vs failure) | 100 | 59 | 0 | 174 | 134 | 0 | 0.43 (0.25 to 0.73) | ||
| 672 | Weinstein, 198692 | C | CCS | > 1 year | Recovered within 2–6 weeks, 6–12 weeks, > 12 weeks or immediate (vs no recovery) | Patient | 88 | 77 | 3 | 71 | 56 | 8 | 1.20 (0.41 to 3.51) | |
| 150 | Zeiger, 198758 | A + C | CCS | Mean 18 (range 6–46) months | Current level of discomfort: pain free or improvement (vs no better or worse) | 45 | 27 | 0 | 81 | 72 | 0 | 0.19 (0.08 to 0.47) | Results included seven surgery patients who had had reoperation; five with good results | |
| Chemonucleolysis vs epidural/intradiscal injection | ||||||||||||||
| 50 | Graham, 1976144 | C | Non-RCT | 2 years | Results categorised as good (vs fair or unimproved) | Physician | 10 sciatica patients | 6 | 0 | 13 | 2 | 0 | 8.25 (1.15 to 59.00) | |
| Chemonucleolysis vs inactive control | ||||||||||||||
| 55 | Gogan, 1992205 | C | RCT | 10 years | Treatment success (yes or no) | Patient | 30 | 24 | 0 | 26 | 9 | 4 | 7.56 (2.26 to 25.22) | Data inferred from graphs |
| 236 | Schwetschenau, 1976206 | A + C | RCT | 1 year | Symptom improvement: excellent or good (vs fair) | 31 | 9 | 0 | 35 | 13 | 0 | 2.24 (0.22 to 23.30) | ||
FIGURE 30.
Summary of the findings of the global effect at long-term follow-up (> 6 months) for studies comparing chemonucleolysis with alternative interventions. Note: weights are from random effects analysis.
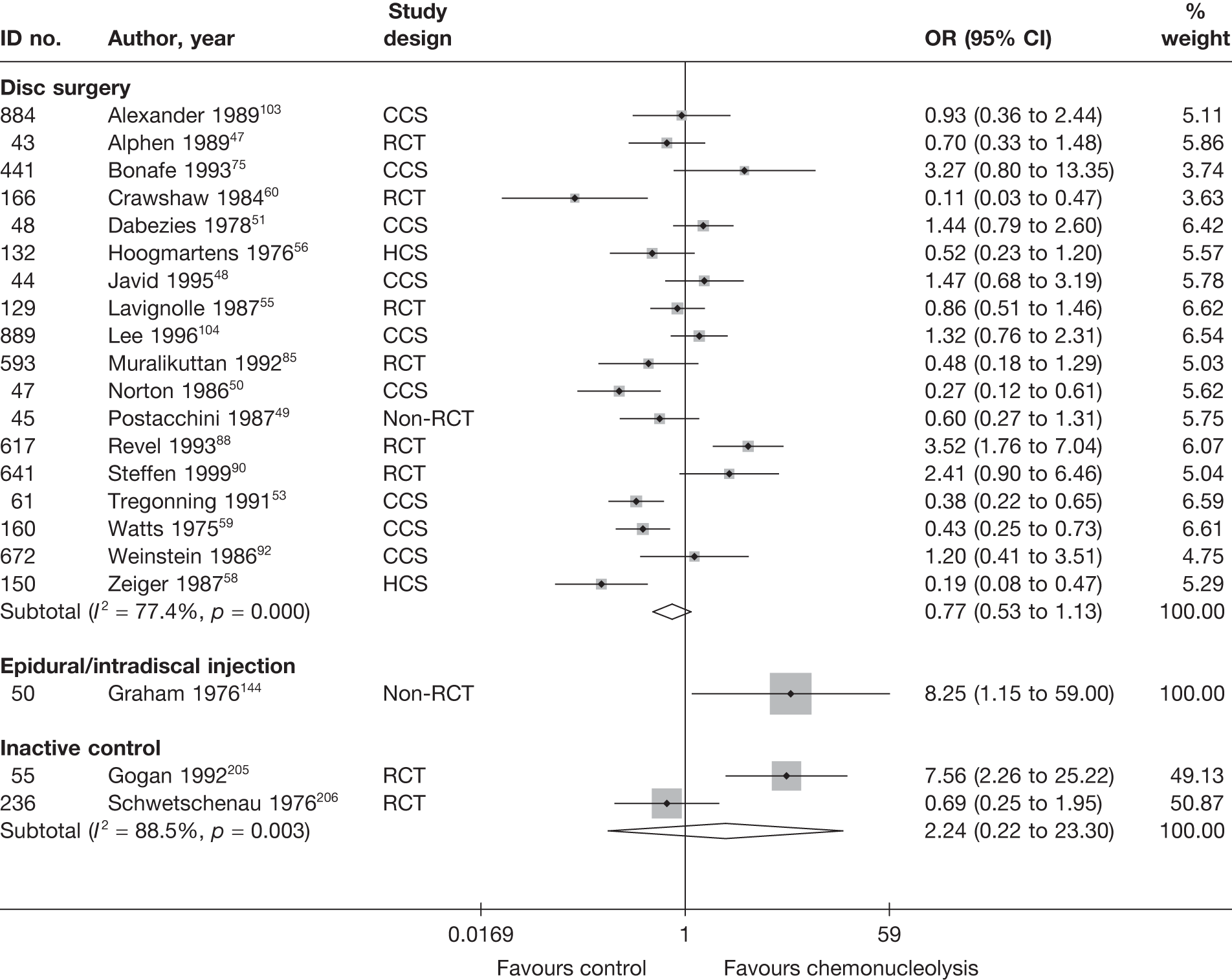
Two RCTs, which were good to moderate quality,205,206 compared chemonucleolysis with inactive control. Pooled analysis showed no statistically significant difference between the intervention groups, but there was some degree of heterogeneity between the studies. The duration of follow-up ranged from 1 year206 to 10 years. 205 The mean duration of symptoms was 11.6 weeks in one study,206 whereas in the second study205 75% of participants had symptoms for between 6 weeks and 6 months and a further 15% had symptoms for > 6 months. The second study205 reported statistically significant better outcomes in patients treated with chemonucleolysis than in those who received inactive control.
Eighteen studies47–51,53,55,56,58–60,75,85,88,90,92,103,104 compared chemonucleolysis with disc surgery, the findings of which were very heterogeneous. The pooled result were borderline statistically significant in favour of surgery. There was a mixture of study designs. The duration of follow-up ranged from 1 year to 10 years and duration of sciatica varied between studies. Even when considering the six RCTs on their own,47,55,60,85,88,90 the findings were still heterogeneous, although most reported findings in favour of disc surgery (pooled analysis: OR 1.12; 95% CI 0.51 to 2.49). One moderate-quality RCT88 found chemonucleolysis to be more effective than disc surgery. But the study had a high withdrawal rate in the surgery group (at least 41%), compared with chemonucleolysis (at least 19%), with dropouts being given a poor outcome in the analysis.
One poorly reported non-RCT144 found chemonucleolysis to be significantly better than epidural in terms of overall recovery, according to the physician, among patients with chronic sciatica at 2 years. All patients had been treated by the author. The study included patients with long-term back pain or sciatica, and these findings are based on a subgroup of patients with sciatica (23/40), among whom symptom duration ranged from 12 weeks to 25 years (median 1 year). All patients had already tried various treatments for at least 3 months.
Pain intensity at long-term follow-up
The results for pain intensity at long-term follow-up are presented in Table 41 and the accompanying forest plot (Figure 31). Chemonucleolysis was compared with disc surgery and manipulation. Three studies77,85,208 included patients with either acute or chronic symptoms. The duration of follow-up ranged from 1285,208 to 18 months. 77
| ID no. | Author, year | Chronicity | Study design | Follow-up | Location | Scale (range)a | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)b | Comment/conversionc | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |||||||||
| Chemonucleolysis vs disc surgery | ||||||||||||||||
| 454 | Buric, 200577 | A + C | Non-RCT | 18 months | Overall | VAS (0–10) | 30 | 15 | 53 (22) | 61 (31) | 13 (16) | 20 (13) | –40.0 | –41 | –7.00 (–15.7 to 1.72) | Two patients crossed over to surgery, classed as treatment failures |
| 593 | Muralikuttan, 199285 | A + C | RCT | 1 year | Leg | VAS (0–100) | 46 | 46 | 64 | 72 | 18 (21.22) | 16 (20.31) | 2.00 (–6.49 to 10.49) | SD imputed from weighted average Most outcomes showed skewed distribution | ||
| Chemonucleolysis vs manipulation | ||||||||||||||||
| 723 | Burton, 2000208 | A + C | RCT | 12 months | Leg | Annotated thermometer (0–6) | 15 | 15 | 60.8 (26.5) | 66.7 (14.2) | 37.8 (29.2) | 35.5 (32) | 2.30 (–19.62 to 24.22) | Missing data: intervention 5/20, control 5/20 | ||
FIGURE 31.
Summary of the findings of pain intensity at long-term follow-up (> 6 months) for studies comparing chemonucleolysis with alternative interventions. Note: weights are from random effects analysis.
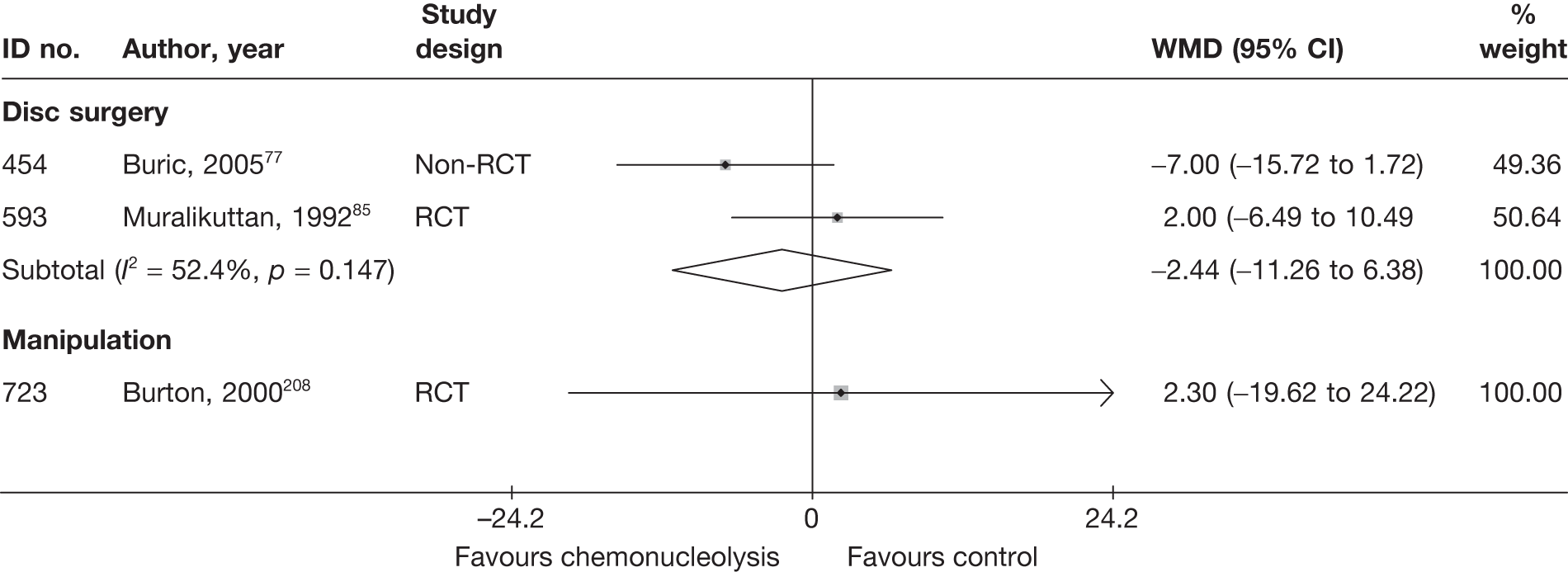
Two studies compared chemonucleolysis with disc surgery; one was a moderate-quality RCT85 and one was a non-RCT. 77 Overall, there was a non-statistically significant difference between the intervention groups, in favour of chemonucleolysis.
One moderate-quality RCT208 showed a non-statistically significant reduction in pain intensity in favour of manipulation, compared with chemonucleolysis, at 12 months. As previously stated the study experienced problems with the randomisation process.
Condition-specific outcome measures at long-term follow-up
The results for pain intensity at long-term follow-up are presented in Table 42 and the accompanying forest plot (Figure 32). Chemonucleolysis was compared with disc surgery and manipulation. Three studies77,85,208 included patients with either acute or chronic symptoms. The duration of follow-up ranged from 1285,208 to 18 months. 77
| ID no. | Author, year | Chronicity | Study design | Follow-up | Scale | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)a | Comment/conversionb | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | ||||||||
| Chemonucleolysis vs disc surgery | |||||||||||||||
| 454 | Buric, 200577 | A + C | Non-RCT | 18 months | RMDQ | 30 | 15 | 9.1 (3.5) | 12.4 (4.3) | 2.2 (3.2) | 2.1 (1.9) | –6.9 | –10.3 | 0.04 (–0.58 to 0.66) |
ITT used but method not stated Dropouts: two, considered as treatment failure |
| 727 | Ejeskar, 198396 | A + C | Non-RCT | 12 months | Composite score | 15 | 14 | 9.4 (6.88) | 8.79 (6.02) | –0.07 (–0.38 to 0.24) | |||||
| 593 | Muralikuttan, 199285 | A + C | RCT | 1 year | Part of Waddell Disability Index | 46 | 46 | 6.2 | 6.7 | 2.6 (1.21) | 2.8 (1.21) | –3.6 | –3.9 | –0.17 (–0.57 to 0.24) |
SD for final means calculated from p-values (Mann–Whitney U-test); most outcomes showed skewed distribution ITT not used, but all patients included in analysis except one for psychological outcomes |
| 672 | Weinstein, 198692 | C | CCS | Mean 10.3 years | Composite score | 81 | 71 | – | Results of MANOVA showed no significant relationship between pain outcome measures and treatment type, Wilks’ criterion F(6, 54) = 1.18, p < 0.34 |
Pain + disability measured on six different scales Actual data not presented Dropouts 3/159 (2%): (chemonucleolysis group) |
|||||
| Chemonucleolysis vs manipulation | |||||||||||||||
| 723 | Burton, 2000208 | A + C | CCS | 12 months | RMDQ | 15 | 15 | 11.95 (5.83) | 11.9 (5.48) | 7.27 (6.65) | 5.87 (5.96) | –4.68 | –6.03 | 0.22 (–0.50 to 0.94) | |
FIGURE 32.
Summary of the findings of CSOMs at long-term follow-up (> 6 months) for studies comparing chemonucleolysis with alternative interventions. Note: weights are from random effects analysis.
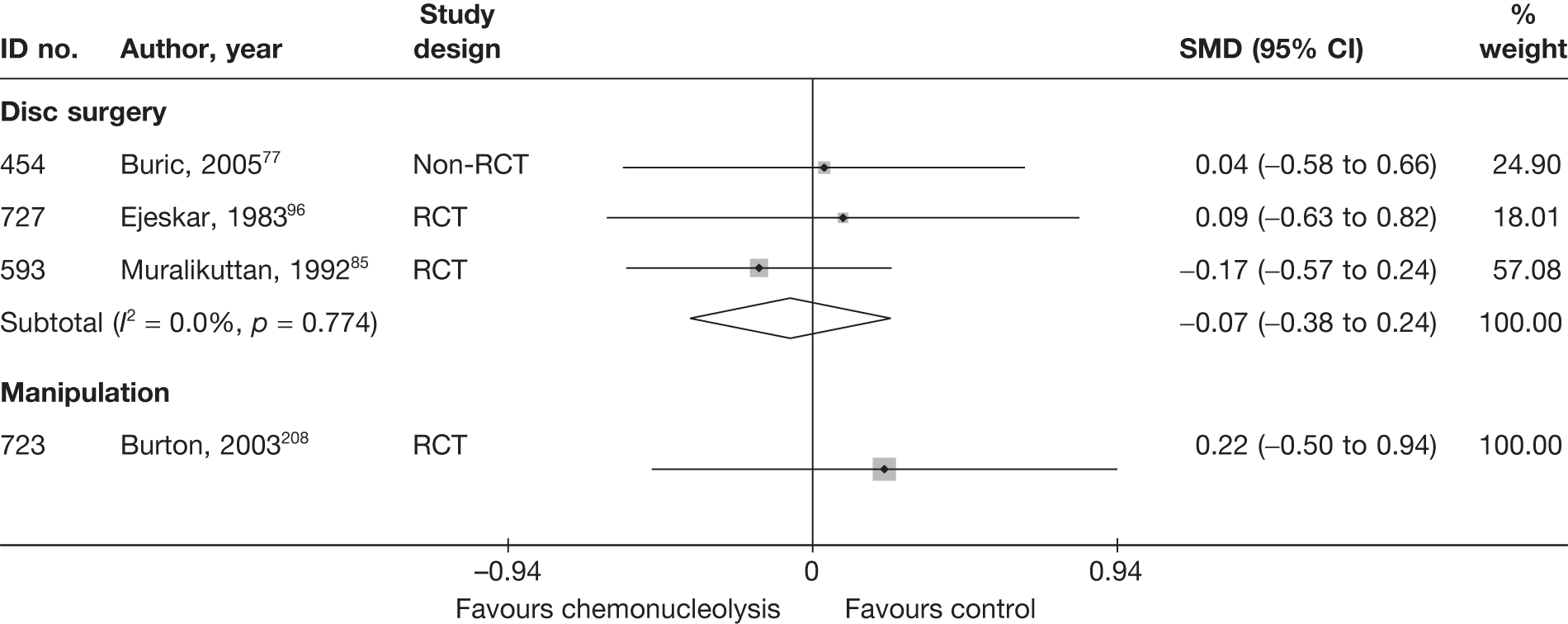
Four studies77,85,92,96 compared chemonucleolysis with disc surgery. Pooled analysis of three weak-to-moderate quality studies77,85,96 showed a non-statistically significant difference between the intervention groups in favour of chemonucleolysis. One CCS88 reported insufficient data to be included in the meta-analysis. The study followed patients for a mean of 10.3 years. The results of six pain and disability outcome measures were analysed in a one-way MANOVA, the results of which showed no significant relationship between pain outcome measures and treatment type (Wilks’ criterion F(6,54) = 1.18; p < 0.34).
One moderate-quality RCT208 showed a non-statistically significant reduction in functional status in favour of manipulation, compared with chemonucleolysis, at 12 months. As previously stated, the study experienced problems with the randomisation process.
Analysis of adverse effects for chemonucleolysis
The results for the occurrence of any reported adverse effects are presented in Table 43 and the accompanying forest plot (Figure 33).
| ID no. | Author, year | Study design | No. of events in intervention group | No. of participants in intervention group | No. of events in control group | No. of participants in control group | OR (95% CI) |
|---|---|---|---|---|---|---|---|
| Chemonucleolysis vs disc surgery | |||||||
| 884 | Alexander, 1989103 | CCS | 8 | 51 | 8 | 49 | 1.64 (0.50 to 5.40) |
| 43 | van Alphen, 198947 | RCT | 3 | 73 | 3 | 78 | 1.07 (0.21 to 4.57) |
| 441 | Bonafe, 199375 | CCS | 0 | 20 | 1 | 20 | 0.32 (0.01 to 8.26) |
| 183 | Bouillet, 198361 | CCS | 152 | 2136 | 91 | 613 | 0.44 (0.33 to 0.58) |
| 453 | Brown, 198976 (chymopapain) | CCS | NR | NR | NR | NR | |
| 453 | Brown, 198976(collagenase) | CCS | NR | NR | NR | NR | |
| 454 | Buric, 200577 | Non-RCT | NR | NR | NR | NR | |
| 166 | Crawshaw, 198460 | RCT | 1 | 25 | 0 | 27 | 3.37 (0.13 to 86.55) |
| 48 | Dabezies, 197851 | CCS | 2 | 100 | 0 | 100 | 5.10 (0.24 to 107.62) |
| 471 | Dei-Anang, 199079 | CCS | NR | NR | NR | NR | |
| 727 | Ejeskar, 198396 | RCT | 1 | 15 | 1 | 14 | 0.93 (0.05 to 16.42) |
| 132 | Hoogmartens, 197656 | HCS | 3 | 44 | 19 | 53 | 0.13 (0.04 to 0.48) |
| 44 | Javid, 199548 | CCS | 4 | 100 | 6 | 100 | 0.65 (0.18 to 2.39) |
| 35 | Krugluger, 200046 | RCT | 5 | 12 | 1 | 10 | 6.43 (0.60 to 68.31) |
| 117 | Lagarrigue, 199154 | CCS | 5 | 334 | 30 | 751 | 0.37 (0.14 to 0.95) |
| 129 | Lavignolle, 198755 | RCT | 7 | 176 | 7 | 182 | 1.04 (0.36 to 3.02) |
| 889 | Lee, 1996104 (control = APLD) | CCS | 73 | 100 | 3 | 100 | 87.42 (25.53 to 299.34) |
| 889 | Lee, 1996104 (control = PELD) | CCS | 73 | 100 | 4 | 100 | 64.89 (21.75 to 193.63) |
| 593 | Muralikuttan, 199285 | RCT | 1 | 46 | 0 | 46 | 3.07 (0.12 to 77.24) |
| 47 | Norton, 198650 | CCS | 12 | 61 | 2 | 44 | 5.14 (1.09 to 24.29) |
| 45 | Postacchini, 198749 | Non-RCT | 2 | 72 | 0 | 84 | 5.99 (0.28 to 126.89) |
| 617 | Revel, 199388 | RCT | 35 | 72 | 15 | 69 | 3.41 (1.63 to 7.10) |
| 641 | Steffen, 199990 | RCT | NR | NR | NR | NR | |
| 49 | Stula, 199052 | RCT | NR | NR | NR | NR | |
| 61 | Tregonning, 199153 | CCS | 4 | 145 | 5 | 91 | 0.49 (0.13 to 1.87) |
| 893 | Watters,1988105 | Non-RCT | 2 | 50 | 1 | 50 | 2.04 (0.18 to 23.27) |
| 160 | Watts, 197559 | CCS | 3 | 100 | 32 | 174 | 0.14 (0.04 to 0.46) |
| 672 | Weinstein, 198692 | CCS | NR | NR | NR | NR | |
| 150 | Zeiger, 198758 | CCS | 16 | 45 | 5 | 81 | 8.39 (2.82 to 24.98) |
| Chemonucleolysis vs epidural | |||||||
| 447 | Bourgeois, 1988160 | RCT | 3 | 30 | 30 | 30 | 0.00 (0.00 to 0.04) |
| 720 | Bontoux, 1990168 | RCT | NR | NR | NR | NR | |
| 729 | Gallucci, 2007170 | RCT | 0 | 82 | 0 | 77 | |
| 50 | Graham, 1976144 | Non-RCT | NR | NR | NR | NR | |
| Chemonucleolysis vs inactive control | |||||||
| 726 | Dabezies, 1988209 | RCT | 14 | 87 | 1 | 86 | 16.3 (2.09 to 126.97) |
| 244 | Feldman, 1986207 | RCT | 0 | 14 | 2 | 10 | 0.12 (0.01 to 2.74) |
| 55 | Gogan, 1992205 | RCT | 2 | 30 | 2 | 26 | 2.07 (0.18 to 24.15) |
| 738 | Javid, 1983210 | RCT | 28 | 55 | 7 | 53 | 6.81 (2.62 to 17.71) |
| 236 | Schwetschenau, 1976206 | RCT | 0 | 31 | 0 | 35 | |
| Chemonucleolysis vs manipulation | |||||||
| 723 | Burton, 2000208 | RCT | 4 | 15 | 5 | 15 | 0.73 (0.15 to 3.49) |
FIGURE 33.
Summary of the findings of any adverse effect for studies comparing chemonucleolysis with alternative interventions. Note: weights are from random effects analysis.
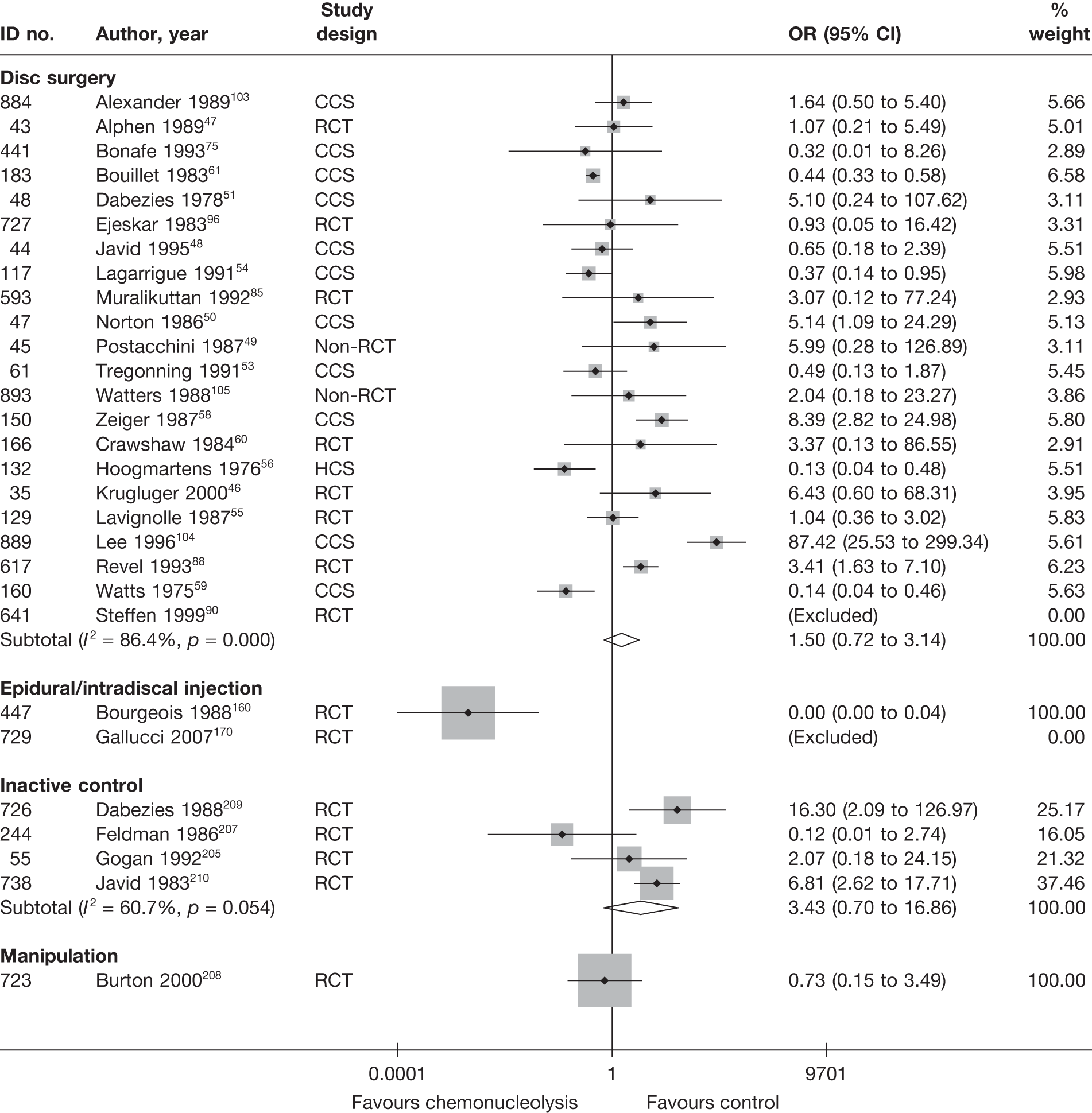
The number of adverse effects were significantly less with chemonucleolysis compared with epidural injection. Pooled analyses showed no statistically significant differences between the intervention groups in the number of adverse effects when comparing chemonucleolysis with disc surgery, manipulation or inactive control.
Serious adverse effects (as considered by the review team) reported by patients receiving chemonucleolysis included nerve root injury, dural defect with subsequent leakage of cerebrospinal fluid, phlebitis, disc space infection, discitis, pulmonary embolus and deep-vein thrombosis plus pulmonary embolism. 47,48,51,56,205 However, these were experienced by only one or two participants within each study (that compared chemonucleolysis with another types of treatment). One study211 that compared two types of chemonucleolysis (with 5 years’ follow-up data) reported slightly higher levels of serious adverse effects. When combining data from both treatment arms (n = 50), 12 participants experienced severe pain and 11 experienced neurological deficit.
SUMMARY OF OVERALL FINDINGS FOR CHEMONUCLEOLYSIS COMPARED WITH ALTERNATIVE INTERVENTIONS
Most of the chemonucleolysis studies included patients with chronic sciatica or both acute and chronic sciatica. Almost half (47%) of the studies were RCTs. One study was deemed to be of good quality (comparator was inactive control206) and 12 studies47,85,88,160,168,170,205,207–210,214 (36%) were of moderate quality, most of which compared chemonucleolysis with an inactive control or epidural. One study had good external validity (comparator was disc surgery47) (Table 44).
| Control category | No. of studies (arms) | Sample size range (median) | Proportion of studies that were RCTs (%) | Proportion of studies that were deemed good quality (%) | Proportion of studies that only included acute sciatica (%) | Proportion of studies that included patients with nerve root pain (%) | Proportion of studies that reported diagnosis confirmed by imaging (%) | Proportion of studies that included patients with stenosis (%) | Proportion of studies that included patients with extruded/sequestered discs (%) | Proportion of studies that only included patients with first episode (%) | Proportion of studies that included patients who had received previous treatment (%) | Proportion of studies that included patients who had received previous surgery (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemonucleolysis vs disc surgery | 26 (29) | 29–1085 (116) | 8/26 (31) | 0/26 (0) | 0/26 (0) | 26/26 (100) | 21/26 (81) | 1/26 (4) | 1/26 (4) | 3/26 (12) | 21/26 (81) | 3/26 (12) |
| Chemonucleolysis vs epidural/intradiscal injection | 4 (4) | 40–159 (70) | 3/4 (75) | 0/4 (0) | 0/4 (0) | 4/4 (100) | 4/4 (100) | 0/4 (0) | 0/4 (0) | 0/4 (0) | 4/4 (100) | 0/4 (0) |
| Chemonucleolysis vs inactive control | 5 (5) | 39–173 (66) | 5/5 (100) | 1/5 (20) | 0/5 (0) | 5/5 (100) | 5/5 (100) | 0/5 (0) | 0/5 (0) | 0/5 (0) | 5/5 (100) | 0/5 (0) |
| Chemonucleolysis vs manipulation | 1 (1) | 40 (40) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 1/1 (100) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) |
| Total (for chemonucleolysis results) | 36 (39) | 29–1085 (100) | 17/36 (47) | 1/36 (3) | 0/36 (0) | 36/36 (100) | 31/36 (86) | 1/36 (3) | 1/36 (3) | 3/36 (8) | 30/36 (83) | 3/36 (8) |
Meta-analysis of five RCTs205–207,209,210 deemed to be moderately or well conducted showed chemonucleolysis to be significantly better than the inactive control, in terms of improved global effect, at medium-term follow-up. However, there was no significant difference between the intervention groups in terms of global effect (four RCTs205–207,209) or pain intensity (one small RCT207) at short-term follow-up; in terms of pain intensity at medium term (one small RCT with fairly high dropout rate207); global effect (two good- to moderate-quality RCTs205,206) at long-term follow-up for; or for overall adverse effects. 205,207,209,210
Pooled analysis of 18 studies47–51,53,55,56,58–60,75,85,88,90,92,103,104 showed marginally statistically significant findings in favour of disc surgery, compared with chemonucleolysis, for the global effect at long-term follow-up (see Figure 30). However, there was no statistically significant difference between the intervention groups for the global effect at short-48,49,52,79,92,104 and medium-term48,49,54,76,88,92,104,105 follow-up; pain intensity at short-,76,85,88 medium-76,85,88 and long-term77,85 follow-up; CSOMs at short-,85,88 medium-85,88,96 and long-term77,85,96 follow-up; or adverse effects46–51,53–56,58–61,75,85,88,96,103–105 (according to a number of studies, ranging from good to poor quality). There was no statistically significant difference between disc surgery in combination with chemonucleolysis and disc surgery alone, at long-term follow-up, for global effect, pain, or for adverse effects (one poor-quality Q-RCT97).
Chemonucleolysis using steroid plus ozone–oxygen was found to be better than epidural for overall recovery at short-term follow-up (one poorly reported RCT170) and chemonucleolysis using chymopapain better than epidural at long-term follow-up (one poor-quality non-RCT144). There was no statistically significant difference between epidural and chemonucleolysis for overall recovery at medium-term follow-up (three RCTs,160,168,170 one of which used ozone–oxygen170). There were more adverse effects experienced with epidural injections than with chemonucleolysis (one RCT160).
There was no statistically significant difference between chemonucleolysis and osteopathic manipulation, in terms of pain intensity and functional status, at short- or medium-term follow-up (one RCT208).
Non-opioids
Description of non-opioids studies
Summary of interventions
Thirty-six studies evaluated the use of non-opioids for sciatica,6,57,80,143,156,161,172,175,214–241 25 of which compared non-opioids with alternative interventions. 6,57,80,143,156,162,173,176,214–230 (Two studies were reported in a single publication;223 studies 696 and 99999.) Seven studies included more than two arms. 57,166,214,215,223,227,229 The types of intervention being evaluated by the studies are presented in Table 45a. Three studies161,172,226 did not report any pain, global or CSOM data. 161,172,226
| ID no. | Author, year | Study design | Treatment description | Control description |
|---|---|---|---|---|
| Non-opioids vs alternative/non-traditional | ||||
| 801 | Chen, 2009215 | RCT | Western medicine – oral nimesolide (NSAIDs) 2 g daily for 10 days (WMG) | Warming acupuncture by burning moxa daily for 10 days (WAG) |
| 801 | Chen, 2009215 | RCT | Western medicine – oral nimesolide (NSAIDs) 2 g daily for 10 days (WMG) | Anisodamine (2 mg) point injections into acupoints daily for 10 days (PIG) |
| Non-opioids vs biological agents | ||||
| 323 | Genevay, 2004216 | HCS | Three intravenous injections of methylprednisolone 250 mg | Three subcutaneous injections of etanercept (Enbrel®, Wyeth Pharmaceuticals) 25 mg (anti-TNF-α) |
| Non-opioids vs disc surgery | ||||
| 475 | Dubourg, 200280 | CCS | Non-operative intervention group. Some received steroids | Disc surgery (operative group) (various surgical techniques) |
| 144 | Rossi, 199357 (Italian language) | RCT | Oral dexamethasone 8 mg for 9 days, naproxen 500–1000 mg for 5 days (group Ib) | Percutaneous discectomy (groups Ia and IIa) |
| 144 | Rossi, 199357 (Italian language) | RCT | Oral dexamethasone 8 mg for 9 days, naproxen 500–1000 mg for 5 days (group Ib) | Microdiscectomy (group IIb) |
| Non-opioids vs epidural/intradiscal injection | ||||
| 451 | Bronfort, 2000161 | RCT | Paracetamol, NSAIDs, activity modification | Epidural injection of steroid injections, 1–3 injections |
| 20 | Dincer, 2007143 | RCT | Oral diclofenac 75 mg for 14 days (NSAID) | Caudal epidural injection 40 mg methylprednisolone acetate, 8 mg dexamethasone phosphate, 7 ml of 2% prilocaine |
| 771 | Lafuma, 1997172 | RCT | Usual care (rest + NSAIDs) without epidural injections during hospital admission | Epidural steroid (125 mg prednisolone) injections at admission |
| 362 | Wilson-MacDonald, 2005156 | RCT | Intramuscular injections of steroid methylprednisolone 80 mg and local anaesthetic 8 ml bupivacaine | Epidural injection of steroid methylprednisolone 80 mg and local anaesthetic 8 ml bupivacaine |
| 846 | Murata, 2009175 | RCT | Injection of steroid (3.3 mg dexamethasone sodium phosphate) and local anaesthetic (7 ml 1% lidocaine) in the back muscles of L2 area (control block) | L2 nerve block using steroid (3.3 mg dexamethasone sodium phosphate) and local anaesthetic (2 ml of 1% lidocaine) |
| Non-opioids vs inactive control | ||||
| 696 | Dreiser, 2001223 | RCT | Oral meloxicam (NSAID) 7.5 mg for 7 days (M I) | Oral placebo for 7 days |
| 696 | Dreiser, 2001223 | RCT | Oral meloxicam (NSAID) 15 mg for 7 days (M II) | Oral placebo for 7 days |
| 334 | El-Zahaar, 1995221 | RCT | Intravenous injections of colchicine 1 mg twice weekly for 3 weeks | Intravenous injections of saline twice weekly for 3 weeks |
| 728 | Finckh, 2006224 | RCT | Intravenous steroid methylprednisolone 500 mg | Intravenous saline infusion (placebo) |
| 62 | Gibson, 1975217 | Non-RCT | Chymoral tablets (proteolytic enzymes) for 7 days | Placebo tablets for 7 days |
| 97 | Goldie, 1968218 | RCT | Oral indomethacin 75 mg daily | Oral placebo |
| 732 | Grevsten, 1975225 | RCT | Phenylbutazone (NSAID) 300–600 mg for 15 days | Intramuscular and oral placebo |
| 312 | Hedeboe, 1982220 | RCT | Intramuscular injection dexamethasone (8–64 mg) for 7 days | Intramuscular injection of saline |
| 816 | Herrmann, 2009227 | RCT | Lornoxicam 8 mg | Placebo |
| 816 | Herrmann, 2009227 | RCT | Diclofenac 50 mg | Placebo |
| 817 | Holve, 2008228 | Q-RCT | Steroid oral tablets (prednisolone decreasing dose from 60 mg to 20 mg every 3 days) + standard medical + PT | Placebo tablets + standard medical + PT |
| 736 | Jacobs, 1968226 | Q-RCT | Oral indomethacin (NSAID) 75–100 mg for 7 days | Oral placebo for 7 days |
| 534 | Khoromi 2007214 | RCT (crossover) | Oral nortriptyline (Allegron®, King Pharmaceuticals) plus inert placebo (up to 100 mg/day for 7.5 weeks) | Oral benztropine (active placebo) plus inert placebo (0.25–1 mg/day for 8.5 weeks) |
| 611 | Porsman, 1979222 | RCT | Intramuscular dexamethasone 8–64 mg for 7 days | Intramuscular saline for 7 days (placebo) |
| 665 | Weber, 19936 | RCT | Oral pirixicam (NSAID) 20–40 mg for 14 days | Oral placebo for 14 days |
| 297 | Yildirim, 2003219 | RCT | Oral gabapentin 900–3600 mg for 2 months | Oral placebo for 2 months |
| Non-opioids vs manipulation | ||||
| 451 | Bronfort, 2000161 | RCT | Paracetamol, NSAIDs, activity modification | Chiropractic spinal manipulation |
| Non-opioids vs mixed treatment | ||||
| 534 | Khoromi 2007214 | RCT (crossover) | Oral nortriptyline plus inert placebo (up to 100 mg/day for 7.5 weeks) | (Opioids + non-opioids). Morphine plus nortriptyline (oral morphine up to 90 mg/day for 8.5 weeks; oral nortriptyline up to 100 mg/day for 7.5 weeks) |
| Non-opioids vs opioids | ||||
| 534 | Khoromi 2007214 | RCT (crossover) | Oral nortriptyline plus inert placebo (up to 100 mg/day for 7 weeks) | Sustained-release morphine (oral) plus inert placebo (up to 90 mg/day for 7 weeks) |
| 368 | Kwasucki, 2002229 (Polish language) | RCT | Fluvoxamine (10 mg oral) | Tramadol (100 mg intramuscular injection) |
| 368 | Kwasucki, 2002229 (Polish language) | RCT | Imipramine (25 mg oral) | Tramadol (100 mg intramuscular injection) |
| 547 | Kwasucki, 1993230 (Polish language) | RCT | Dexamethasone. First and second days 24 mg (16 mg at 7 am, 8 mg at 7 pm); third day 8 mg twice daily; fourth and fifth days 4 mg twice daily; sixth and seventh days 4 mg once daily | Tramadol. First 5 days 100 mg twice daily; sixth and seventh days 100 mg once daily |
Fifteen studies compared different types of non-opioids223,227,229,231–241 (seven of which were three-arm studies57,215,223,227,229 and two studies of which were reported in a single publication223). The types of non-opioids being compared are presented in Table 45b but the findings are not considered further.
| ID no. | Author, year | Study design | Treatment description | Control description |
|---|---|---|---|---|
| 238 | Andersen, 1978235 | RCT | Oral proquazone (NSAID) | Oral naproxen (NSAID) |
| 122 | Blazek, 1986232 | RCT | Oral proquazone | Oral diclofenac |
| 159 | Borms, 1988234 | RCT | Intramuscular tiaprofenic acid | Intramuscular ketoprofen |
| 721 | Braun, 1982238 (German language) | RCT | Intramuscular injection of ketoprofen | Intramuscular injection of corticosteroid containing antirheumatic combination preparation (sodium phenylbutazone, dexamethasone, lidocaine, cyanocobalamin) |
| 136 | Desnuelle, 198656 (French language) | RCT | Intramuscular indomethacin injections | Intramuscular diclofenac injections |
| 696 | Dreiser, 2001223 | RCT | Oral meloxicam (NSAID) 7.5 mg for 7 days (M I) | Oral meloxicam (NSAID) 15 mg for 7 days (M II) |
| 9999 | Dreiser, 2001223 | RCT | NSAID (low-dose meloxicam, M I) | Traditional NSAID (diclofenac) |
| 9999 | Dreiser, 2001223 | RCT | NSAID (high-dose meloxicam, M II) | Traditional NSAID (diclofenac) |
| 810 | Friedman, 2008239 | RCT | Steroid intramuscular injection (160 mg of methylprednisolone acetate) + oral naproxen + oral oxycodone/acetaminophen | Placebo intramuscular injection + oral naproxen + oral oxycodone/acetaminophen |
| 816 | Herrmann, 2009227 | RCT | Lornoxicam 8 mg | Diclofenac 50 mg |
| 527 | Kanayama, 2005237 | RCT | 5-HT2A receptor inhibitor. Sarpogrelate hydroxychloride 300 mg orally for 2 weeks | NSAID. Sodium diclofenac 75 mg orally for 2 weeks |
| 368 | Kwasucki, 2002229 (Polish language) | RCT | Fluvoxamine (10 mg oral) | Imipramine (25 mg oral) |
| 841 | Memeo, 2008240 | Q-RCT | Acetyl-l-carnitine 1180 mg/day | Thiotic acid 600 mg/day |
| 109 | Schuermans, 1988231 | RCT | Intramuscular tiaprofenic acid | Intramuscular alclofenac |
| 241 | Stevanovic, 1986236 | RCT | Intramuscular injection of tenoxicam | Intramuscular injection of piroxicam |
| 871 | Toroudi, 2009241 | RCT | 500 mg of oral ibuprofen prescribed three times a day for 9 days | 400 mg of oral mesalamine prescribed three times a day for 9 days |
Summary of study participants for non-opioids
Summary data for included participants are presented in Table 46. The number of participants included in the 22 studies that reported outcome data for global effect, pain or CSOMs ranged from 10 to 532 participants (median 65 participants). Nine studies (41%) included patients with acute sciatica and six studies (27%) included patients with chronic sciatica, whereas the majority of the remaining studies included patients with either acute or chronic sciatica (one study did not report this information). Two studies (one in which the comparator was epidural156 and one in which the comparator was opioids229) included some patients with spinal stenosis and none included patients with sequestered or extruded discs. The diagnosis of sciatica, or the presence of herniated disc, was confirmed by imaging in eight studies (38%). One study57 compared the use of non-opioids with disc surgery in patients who had recurrent sciatica. The remaining studies included a mixture of patients with either first-episode or recurrent sciatica or, more likely, did not report this information. One study (comparator was inactive control)6 included patients who had not received any previous treatment for their current episode of sciatica. Eleven studies (50%) included patients who had received previous treatment for their current episode of sciatica and this information was not stated in the remaining studies. Two studies that compared non-opioids with disc surgery80 or epidural156 included patients who had received previous disc surgery.
| ID no. | Author, year | Study design | No. of patients | Age (years) | No. of men (%) | Symptom duration | Type of sciatica | Confirmed by imaging? | Recurrent episode | Included patients with stenosis?a | Included patients with sequestered disc (or extruded)?a | Any previous treatment for sciatica? | Any previous back surgery for sciatica? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-opioids vs alternative/non-traditional | |||||||||||||
| 801 | Chen, 2009215 | RCT | 90 | Mean 34.5 (SD 7.7) | 63 (70) | Mean 5.3 years (SD 4.14 years) | Nerve root pain | No | NR | No | No | NR | NR |
| Non-opioids vs biological agents | |||||||||||||
| 323 | Genevay, 2004216 | HCS | 10 | Mean 47.3 (SD 13.3, range 1 to > 18) | 10 (50) | Mean 3.2 weeks (SD 3.7 weeks) | Nerve root pain | No | NR | No | No | NR | NR |
| Non-opioids vs disc surgery | |||||||||||||
| 475 | Dubourg, 200280 | CCS | 67 | Mean 48.8 (SD 13.9, range 28–81) | 42 (63) | Mean 25.7 days (SD 28.7 days) | Nerve root pain | Yes | Recurrent and first episode | No | No | NR | Yes |
| 144 | Rossi, 199357 (Italian language) | RCT | 40 | Mean 42.5 (SD 10.5, range 20–65) | NR | < 6 months | Nerve root pain | Yes | Recurrent | No | No | NR | NR |
| Non-opioids vs epidural/intradiscal injection | |||||||||||||
| 451 | Bronfort, 2000161 | RCT | 20 | Mean 44.5 (SD 10.6) | 12 (60) | ≤ 3 weeks n = 6; 4–12 weeks n = 14 | Nerve root pain and refereed pain | No | NR | No | No | Yes | No |
| 20 | Dincer, 2007143 | RCT | 64 | Mean 28 (SD 5) | 46 (72) | 1–12 months | Nerve root pain and refereed pain | Yes | NR | No | No | NR | No |
| 771 | Lafuma, 1997172 | RCT | 108 | Mean 42.1 (SD 10.6) | 66 (61) | Mean 56 days (range 1–854 days) | Nerve root pain | NR | Recurrent and first episode | No | No | Yes | NR |
| 362 | Wilson-MacDonald, 2005156 | RCT | 93 | Mean 49 (range 23–79) | 37 (40) | > 6 weeks, exact duration NR | Nerve root pain | Yes | NR | Yes | No | Some had | Yes |
| 846 | Murata, 2009175 | RCT | 246 (136 radicular pain) | Mean 68 (SD 12, range 27–90) | 90 (37) | Median 31 months (SD 52 months) | Nerve root pain | No | NR | No | No | Yes | No |
| Non-opioids vs inactive control | |||||||||||||
| 696 | Dreiser, 2001223 | RCT | 532 | Mean 47 years | 234 (44) | 93% within 3 days | Nerve root pain and referred pain | No | Recurrent and first episode | No | No | NR | No |
| 334 | El-Zahaar, 1995221 | RCT | 100 | Mean 38.7 (range 26–58) | NR | NR | Nerve root pain and referred pain | Yes | Recurrent and first episode | No | No | Yes | NR |
| 728 | Finckh, 2006224 | RCT | 65 | Mean 47.2 (SD 15.2) | 29(48) | Median 15 days | Nerve root pain | Yes | Recurrent and first episode | No | No | NR | No |
| 62 | Gibson, 1975217 | Non-RCT | 93 | Mean 40 (range 19–67) | 55 (59) | < 1–6 months | Nerve root pain and referred pain | No | NR | No | No | NR | No |
| 97 | Goldie, 1968218 | RCT | 50 | Range 15–65 | 26 (52) | 1 week 34%; 2 weeks 56%; 3 weeks 10% | Nerve root pain and referred pain | No | NR | No | No | NR | NR |
| 732 | Grevsten, 1975225 | RCT | 36 | Range 23–62 | 17 (47) | Days to years | Nerve root pain and referred pain | No | Recurrent and first episode | No | No | NR | NR |
| 312 | Hedeboe, 1982220 | RCT | 39 | Mean 41.8 (range 24–63) | 25 (64) | < 2 weeks 36%, 2–8 weeks 28%, > 2 months 36% | Nerve root pain and referred pain | No | NR | No | No | Yes | NR |
| 816 | Herrmann, 2009227 | RCT | 171 | Mean 50.2 (SD12.6) | 76 (44) | < 72 hours | Nerve root pain | No | Recurrent and first episode | No | No | No | NR |
| 817 | Holve, 2008228 | Q-RCT | 29 | Mean 43.7 | 17 (59) | < 1 week | Nerve root pain | No | First episode | No | No | NR | NR |
| 736 | Jacobs, 1968226 | Q-RCT | 110 (50 sciatica) | NR | NR | Inclusion criteria acute and chronic | Nerve root pain | No | NR | No | No | NR | NR |
| 534 | Khoromi, 2007214 | RCT (cross–over) | 55 | Median 53 (range 19–65) | 30 (55) | Median 5 years (range 0.3–37.0 years) | Nerve root pain | Yes | Recurrent and first episode | NR | NR | Yes | NR |
| 611 | Porsman, 1979222 | RCT | 52 | Mean 44.8 (range 21–67) | 33 (67) | Range few days–6 months | Nerve root pain | No | Recurrent and first episode | No | No | Yes | NR |
| 665 | Weber, 19936 | RCT | 214 | Mean 48 | NR | Recruited at onset sciatica | Nerve root pain | No | NR | No | No | No | NR |
| 297 | Yildirim, 2003219 | RCT | 50 | Mean 39.3 (SD 8.2, range 25–60) | 18 (36) | Mean 68.5 months (SD 60.2, range 3–240 months) | Nerve root pain | Yes | NR | No | No | Yes | No |
| Non-opioids vs manipulation | |||||||||||||
| 451 | Bronfort, 2000161 | RCT | 20 | Mean 44.5 (SD 10.6) | 12 (60) | ≤ 3 weeks n = 6; 4–12 weeks n = 14 | Nerve root pain and refereed pain | No | Not reported | No | No | Yes | No |
| Non-opioids vs mixed treatment | |||||||||||||
| 534 | Khoromi, 2007214 | RCT (cross–over) | 55 | Median 53 (range 19–65) | 30 (55) | Median 5 years (range 0.3–37.0 years) | Nerve root pain | Yes | Recurrent and first episode | NR | NR | Yes | NR |
| Non-opioids vs opioids | |||||||||||||
| 534 | Khoromi, 2007214 | RCT (cross–over) | 55 | Median 53 (range 19–65) | 30 (55) | Median 5 years (range 0.3–37.0 years) | Nerve root pain | Yes | Recurrent and first episode | NR | NR | Yes | NR |
| 368 | Kwasucki, 2002229 (Polish language) | RCT | 70 | Mean 42.8 (range 23–68) | 51 (73) | Range 1 week–8 months | Nerve root pain | Yes | Recurrent and first episode | Yes | No | Yes | NR |
| 547 | Kwasucki, 1993230 (Polish language) | RCT | 43 | Mean 43.2 (range 27–69) | 37 (86) | Mean 6.3 weeks (range 1 week–8 months) | Nerve root pain and referred pain | No | Recurrent and first episode | No | No | NR | NR |
Summary of study quality for non-opioids studies
Summary information on study details is presented in Table 47. Most of the non-opioid studies were RCTs (17/21, 81%), but none was good quality. Ten studies6,143,161,214,218,220,223,224,227,228 were of moderate quality, most of which compared non-opioids with inactive control. Two of these studies214,227 used adequate methods for random sequence generation and allocation concealment (comparators included inactive control, opioids and mixed treatment). A further two studies156,224 used adequate randomisation, but not allocation concealment, although both used sealed envelopes. Two studies218,222 used adequate allocation concealment, but the method of randomisation was unclear. Only one study214 had strong external validity, although it had a high attrition rate.
| ID no. | Author, year | Study size | Overall follow-up | Study design | Adequate randomisation? | Allocation concealment? | Follow-up (%) | Blind outcome assessment? | Overall quality rating | Overall external validity rating |
|---|---|---|---|---|---|---|---|---|---|---|
| Non-opioids vs alternative/non-traditional | ||||||||||
| 801 | Chen, 2009215 | 90 | 1 year | RCT | Unclear | Unclear | 80–100 | Unclear | Weak | Moderate |
| Non-opioids vs biological agents | ||||||||||
| 323 | Genevay, 2004216 | 10 | 6 weeks | HCS | No | No | 80–100 | No | Weak | Moderate |
| Non-opioids vs disc surgery | ||||||||||
| 144 | Rossi, 199357 (Italian language) | 40 | 6 months | RCT | Unclear | Unclear | 80–100 | Yes | Weak | Weak |
| 475 | Dubourg, 200280 | 67 | 6 months | CCS | No | No | 80–100 | No | Weak | Weak |
| Non-opioids vs epidural/intradiscal injection | ||||||||||
| 451 | Bronfort, 2000161 | 20 | 12 weeks | RCT | Unclear | Partial | 80–100 | NA | Moderate | Weak |
| 20 | Dincer, 2007143 | 64 | 3 months Assessment at day 15, first month and third month | RCT | Unclear | Unclear | 80–100 | Yes | Moderate | Moderate |
| 771 | Lafuma, 1997172 | 108 | 3 months | RCT | Unclear | Unclear | 80–100 | No | Weak | Weak |
| 362 | Wilson-MacDonald, 2005156 | 93 | 35 days | RCT | Yes | Partial | 80–100 | Unclear | Moderate | Weak |
| 846 | Murata, 2009175 | 246 (136 RP) | 7 days | RCT | Unclear | Partial | 80–100 | Unclear | Weak | Weak |
| Non-opioids vs inactive control | ||||||||||
| 696 | Dreiser, 2001223 | 532 | 7 days | RCT | Unclear | Unclear | 80–100 | Yes | Moderate | Weak |
| 334 | El-Zahaar, 1995221 | 100 | 3 weeks | RCT | Unclear | Unclear | 80–100 | Yes | Weak | Weak |
| 728 | Finckh, 2006224 | 65 | 30 days | RCT | Yes | Partial | 80–100 | Yes | Moderate | Weak |
| 62 | Gibson, 1975217 | 93 | 3 months | Non-RCT | No | No | 80–100 | Unclear | Weak | Weak |
| 97 | Goldie, 1968218 | 50 | 14 days | RCT | Unclear | Yes | 80–100 | Yes | Moderate | Weak |
| 732 | Grevsten, 1975225 | 36 | 2 weeks | RCT | Unclear | Unclear | 80–100 | Yes | Weak | Weak |
| 312 | Hedeboe, 1982220 | 39 | 3 months | RCT | Partial | Partial | 80–100 | Unclear | Moderate | Moderate |
| 816 | Herrmann, 2009227 | 171 | 5 days | RCT | Yes | Yes | 80–100 | Unclear | Moderate | Weak |
| 817 | Holve, 2008228 | 29 | 6 months | Q-RCT | No | Partial | 80–100 | Yes | Moderate | Weak |
| 736 | Jacobs, 1968226 | 110 (50 NRP, 60 BP) | 1 week | Q-RCT | No | Unclear | 80–100 | Yes | Weak | Weak |
| 534 | Khoromi, 2007214 | 55 | 36 weeks | RCT (cross–over) | Yes | Yes | < 60 | Yes | Moderate | Strong |
| 611 | Porsman, 1979222 | 52 | 9 days | RCT | Unclear | Yes | 80–100 | Yes | Weak | Moderate |
| 665 | Weber, 19936 | 214 | 4 weeks | RCT | Unclear | Unclear | 80–100 | No | Moderate | Weak |
| 297 | Yildirim, 2003219 | 50 | 2 months | RCT | Unclear | Unclear | 80–100 | Unclear | Weak | Weak |
| Non-opioids vs manipulation | ||||||||||
| 451 | Bronfort, 2000161 | 20 | 12 weeks | RCT | Unclear | Partial | 80–100 | NA | Moderate | Weak |
| Non-opioids vs mixed treatment | ||||||||||
| 534 | Khoromi, 2007214 | 55 | 36 weeks | RCT (cross–over) | Yes | Yes | < 60 | Yes | Moderate | Strong |
| Non-opioids vs opioids | ||||||||||
| 534 | Khoromi, 2007214 | 55 | 36 weeks | RCT (cross–over) | Yes | Yes | < 60 | Yes | Moderate | Strong |
| 368 | Kwasucki, 2002229 (Polish language) | 70 | 19 days | RCT | Unclear | Unclear | 80–100 | Unclear | Weak | Weak |
| 547 | Kwasucki, 1993230 (Polish language) | 43 | 2 weeks | RCT | Unclear | Unclear | 80–100 | Unclear | Weak | Weak |
Non-opioids results at short-term follow-up (≤ 6 weeks)
Global effect at short-term follow-up
The results for the global effect at short-term follow-up are presented in Table 48 and the accompanying forest plot (Figure 34). Non-opioids were compared with inactive control and opioids. One study221 included only patients with chronic sciatica, five studies218,220,223,224,227 included only patients with acute sciatica and the remainder included patients with either acute or chronic sciatica. The duration of follow-up ranged from 1 day224 to 19 days. 230
| ID no. | Author, year | Chronicity | Study design | Follow-up | Outcome measure | Perspective | Intervention | Control | OR (95% CI) | Comments | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n) | Outcome (n) | Withdrawal rate | Total (n) | Outcome (n) | Withdrawal rate | |||||||||
| Non-opioid vs inactive control | ||||||||||||||
| 696 | Dreiser, 2001223 (i)a (7.5 mg) | A | RCT | 7 days | Global efficacy: good or satisfactory (vs not satisfactory or bad) | Patient | 171 | 130 | 0 | 180 | 117 | 0 | 1.71 (1.07 to 2.72) |
Data inferred from graphs reporting percentages ITT using worst-case analysis |
| 696 | Dreiser, 2001223 (ii)a (15 mg) | A | RCT | 7 days | Global efficacy: good or satisfactory (vs not satisfactory or bad) | Patient | 181 | 138 | 0 | 180 | 117 | 0 | 1.73 (1.09 to 2.74) |
Data inferred from graphs reporting percentages ITT using worst-case analysis |
| 334 | El-Zahaar, 1995221 | C | RCT | 3 weeks | Number of patients with pain improvement for sciatica, low back pain and sciatica, and low back pain | 49 | 46 | 0.02 | 48 | 2 | 0.04 | 352.00 (56.27 to 2210.11) | ||
| 728 | Finckh, 2006224 | A | RCT | 1 day | Responders: decrease in VAS > 20 mm | 31 | 15 | ? | 29 | 8 | ? | 2.46 (0.84 to 7.22) |
Dropouts 5/65 (8%): group allocation not stated ITT where missing values assumed to be missing at random and imputed using longitudinal regression model |
|
| 62 | Gibson, 1975217 | A + C | Non-RCT | 7 days | Fully recovered | Physician | 45 | 7 | 0.02 | 44 | 10 | 0.06 | 0.63 (0.21 to 1.83) | |
| 97 | Goldie, 1968218 | A | RCT | 14 days | Relief from pain: complete (vs fair or none) | Patient | 25 | 14 | 0 | 25 | 16 | 0 | 0.72 (0.23 to 2.23) | |
| 732 | Grevsten, 1975225 | A + C | RCT | 2 weeks | Overall improvement (vs uncertain or not improved) | 18 | 15 | 0 | 18 | 8 | 0 | 6.25 (1.33 to 29.43) | ||
| 312 | Hedeboe, 1982220 | A | RCT | 9 days | Overall pain improvement: better (vs unchanged or worst) | Patient | 19 | 13 | 0 | 20 | 7 | 0 | 4.02 (1.06 to 15.28) | |
| 816 | Herrmann, 2009227 (i)b (lornoxicam) | A | RCT | 5 days | Overall assessment of efficacy and tolerability: very good or good (vs fair or poor) | Patient | 57 | 38 | 0 | 57 | 32 | 0 | 1.56 (0.73 to 3.34) | ITT reported; seven patients dropped out: lornoxicam 4/57, diclofenac 2/57, placebo 1/57 |
| 816 | Herrmann, 2009227 (ii)b (diclofenac) | A | RCT | 5 days | Overall assessment of efficacy and tolerability: very good or good (vs fair or poor) | Patient | 57 | 42 | 0 | 57 | 32 | 0 | 2.19 (0.99 to 4.81) | ITT reported; seven patients dropped out: lornoxicam 4/57, diclofenac 2/57, placebo 1/57 |
| 611 | Porsman, 1979222 | A + C | RCT | 9 days | Treatment effective (vs not effective) | 25 | 13 | 0.07 | 24 | 14 | 0.04 | 0.77 (0.25 to 2.39) | ||
| Non-opioid vs opioids | ||||||||||||||
| 547 | Kwasucki, 1993230 (Polish language) | A + C | RCT | 2 weeks | Improvement in pain: cessation of symptoms or clear improvement (vs no improvement or mild improvement) | 21 | 16 | 0 | 22 | 8 | 0 | 5.60 (1.48 to 21.13) | Data extracted from histograms of raw pain scores | |
| 368 | Kwasucki, 2002229 (Polish language) (i)c (fluvoksamine) | A + C | RCT | 19 days (end of treatment) | Overall improvement: complete relief or improvement (vs no improvement) | Patient | 24 | 18 | 0 | 22 | 17 | 0 | 0.88 (0.23 to 3.44) | |
| 368 | Kwasucki, 2002229 (Polish language) (ii)c (imipramine) | A + C | RCT | 19 days (end of treatment) | Overall improvement: complete relief or improvement (vs no improvement) | Patient | 24 | 06 | 0 | 22 | 17 | 0 | 0.59 (0.16 to 2.18) | |
FIGURE 34.
Summary of the findings of the global effect at short-term follow-up (≤ 6 weeks) for studies comparing non-opioids with alternative interventions. Note: weights are from random effects analysis.
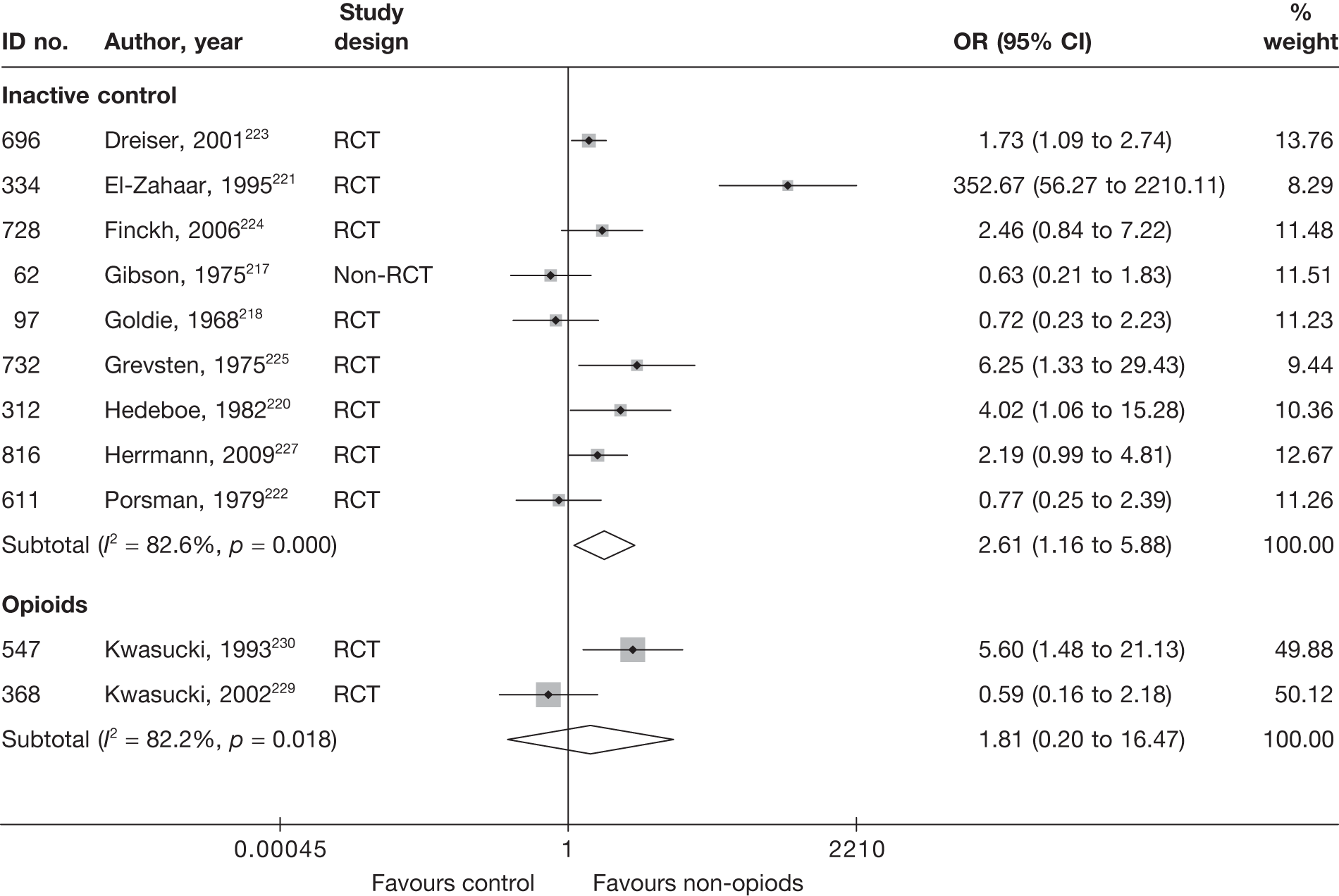
Pooled analysis of nine studies217,218,220–226 showed non-opioids to be significantly better than inactive control at 1 day224 to 21 days. 221 Eight studies were RCTs and one was a non-RCT. There was much heterogeneity between studies (I2 = 82.6%), with one RCT, which evaluated the use of intravenous injections of colchicine for patients with chronic sciatica, having a larger effect size than the other studies. Excluding this study reduced the effect size to an OR of 1.63 (95% CI 1.03 to 2.59) and improved homogeneity (I2 = 44.3%); follow-up ranged from 1 day224 to 14 days. 218,225
Non-opioids were compared with opioids in two RCTs;229,230 the pooled analysis showed a non-statistically significant difference in favour of non-opioids. Both studies were poorly reported and conducted. Follow-up ranged from 14229 to 19 days. 230
Pain intensity at short-term follow-up
The results for pain intensity at short-term follow-up are presented in Table 49 and the accompanying forest plot (Figure 35). Non-opioids were compared with inactive control, opioids, epidural, alternative therapy and biological agents. Five studies216,223,224,227,228 included only patients with acute sciatica, three175,215,219 included only patients with chronic sciatica, one156 did not report the duration of symptoms and the remaining studies included patients with acute or chronic sciatica. The duration of follow-up ranged from 8 hours227 to 36 days. 215
| ID no. | Author, year | Chronicity | Study design | Follow-up | Location | Scale (range)a | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)b | Comment/conversionc | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |||||||||
| Non-opioids vs alternative | ||||||||||||||||
| 801 | Chen, 2009215 (i)d (WAG) | C | RCT | 36 days (end of treatment) | Leg | Not stated | 30 | 30 | 1.42 (0.37) | 1.56 (0.35) | 2.42 (0.33) | 5.74 (0.25) | –3.32 (–3.47 to –3.17) |
Outcome = improvement in clinical symptoms (scale and range not stated) Reported separately for: sciatica, lumbago, aggravated pain on coughing, aggravated pain on sneezing, aggravated pain on defaecation |
||
| 801 | Chen, 2009215 (ii)d (PIG) | C | RCT | 36 days (end of treatment) | Leg | Not stated | 30 | 30 | 1.42 (0.37) | 1.75 (0.32) | 2.42 (0.33) | 2.75 (0.32) | –0.33 (–0.49 to –0.17) |
Outcome = improvement in clinical symptoms (scale and range not stated) Reported separately for: sciatica, lumbago, aggravated pain on coughing, aggravated pain on sneezing, aggravated pain on defecation |
||
| Non-opioids vs biological agents | ||||||||||||||||
| 323 | Genevay, 2004216 | A | HCS | 6 weeks | Leg | VAS (0–100) | 10 | 10 | 75.1 (14.2) | 74.4 (12.9) | 52.9 (25.1) | 12.4 (13.2) | 40.50 (22.92 to 58.08) | |||
| Non-opioids vs epidural/intradiscal injection | ||||||||||||||||
| 20 | Dincer, 2007143 | A + C | RCT | 1 month | Overall | VAS (0–100) | 30 | 34 | 68 (10) | 69 (10) | 44 (13) | 32 (11) | 12.00 (6.06 to 17.94) | |||
| 846 | Murata, 2009175 | C | RCT | 7 days | Leg | VAS (0–100) | 65 | 71 | 74 | 69 | 67 (22.86) | 43 (22.48) | 24.00 (16.37 to 31.63) |
SD imputed from weighted average Subgroup analysis based on 136/246 (55%) with radicular pain: intervention 71/122, control 65/124 Dropouts 8/246 (3%): no further details |
||
| 362 | Wilson-MacDonald, 2005156 | NR | RCT | 35 days | Overall | Oxford pain chart | 36 | 36 | There was a significant difference in pain relief between the two groups with the epidural group being better (p < 0.004) |
No data other than p-values presented Dropouts 23%: non-opioids 12/44, epidural 8/44 |
||||||
| Non-opioids vs inactive control | ||||||||||||||||
| 696 | Dreiser, 2001223 | A | RCT | 7 days | Overall | VAS (0–100) | 171 | 180 | 75.6 (11.4) | 76 (10.7) | –46 (26.15) | –40 (26.83) | –6.00 (–11.54 to –0.46) |
SD estimated from SE ITT using LOCF Dropouts 32/532 (6%): low dose 6/171, placebo 12/180 |
||
| 696 | Dreiser, 2001223 | A | RCT | 7 days | Overall | VAS (0–100) | 181 | 180 | 75.4 (10.6) | 76 (10.7) | –45 (26.91) | –40 (26.83) | –5.00 (–10.54 to 0.54) |
SD estimated from SE ITT using LOCF Dropouts 32/532 (6%): high dose 14/181, placebo 12/180 |
||
| 728 | Finckh, 2006224 | A | RCT | 30 days | Leg | VAS (0–100) | 31 | 29 | 67.1 (22.1) | 63.3 (20.7) | 36.1 (22.1) | 45.3 (20.7) | –31 | –18 |
–9.20 (–20.03 to 1.63) Significantly greater pain reduction in steroid group during the first 3 days (p = 0.04), but both groups similar after 3 days (p = 0.22); linear spline regression model |
Final mean calculated using change score and baseline SD used Dropouts 5/65 (8%): group allocation not stated ITT where missing values assumed to be missing at random and imputed using longitudinal regression model |
| 816 | Herrmann, 2009227 | A | RCT | 8 hours | Overall | VAS (0–100) | 57 | 57 | 84.9 (7.5) | 83.2 (7.0) | 62.9 (22.86) | 69.5 (23.67) | –22.0 | –13.7 | –6.60 (–15.14 to 1.94) |
Final mean derived from change scores and SD imputed from weighted average Treatment administered over 4 days (with an optional 5 days), but PID was measured at day 1 and therefore only evaluates the effectiveness of the loading dose Mean PID using VAS (0–100) compared with baseline |
| 816 | Herrmann, 2009227 | A | RCT | 8 hours | Overall | VAS (0–100) | 57 | 57 | 83.9 (6.7) | 83.2 (7.0) | 59.8 (22.86) | 69.5 (23.67) | –24.1 | –13.7 | –9.70 (–18.24 to –1.16) |
Mean PID using VAS (0–100) compared with baseline Final mean derived from change scores and SD imputed from weighted average Treatment administered over 4 days (with an optional 5 days), but PID was measured at day 1 and therefore only evaluates the effectiveness of the loading dose |
| 817 | Holve, 2008228 | A | Q-RCT | 4 weeks | Overall | RMDQ subscale (0–5) | 13 | 14 | 76 | 62 | 32 (22.86) | 32 (23.67) | –24.1 | –13.7 | 0.00 (–17.55 to 17.55) |
SD imputed from weighted average RMDQ (scored on a pain thermometer range 0–5) ITT not used Dropouts 2/29 (7%): intervention 2/15, control 0/14 |
| 297 | Yildirim, 2003219 | C | RCT | 1 month | Overall | Pain severity (0–3) | 23 | 20 | 53.3 (31.3) | 56.0 (22.3) | 24.3 (25.0) | 49.0 (23.0) | –24.70 (–39.05 to –10.35) |
ITT not used Dropouts 7 (14%): intervention 2/25, control 5/25 |
||
| Non-opioids vs opioids | ||||||||||||||||
| 547 | Kwasucki, 1993230 (Polish language) | A + C | RCT | 2 weeks | Overall | NRS (0–4) | 21 | 22 | 77.5 (12.5) | 77.5 (15) | 27.5 (17.5) | 50.0 (22.5) | –22.50 (–34.52 to –10.48) | |||
| 368 | Kwasucki, 2002229 (Polish language) (i)e (fluvoxamine) | A + C | RCT | 19 days | Overall | NRS (0–4) | 24 | 22 | 67.5 (15) | 70.0 (17.5) | 30 (20) | 50.0 (25) | –20.00 (–33.16 to –6.84) | Data derived from histograms of pain scores | ||
| 368 | Kwasucki, 2002229 (Polish language) (ii)e (imipramine) | A + C | RCT | 19 days | Overall | NRS (0–4) | 24 | 22 | 75 (25) | 70.0 (17.5) | 37.5 (25) | 50.0 (25) | –12.50 (–26.96 to 1.96) | Data derived from histograms of pain scores | ||
FIGURE 35.
Summary of the findings of pain intensity at short-term follow-up (≤ 6 weeks) for studies comparing non-opioids with alternative interventions. Note: weights are from random effects analysis.
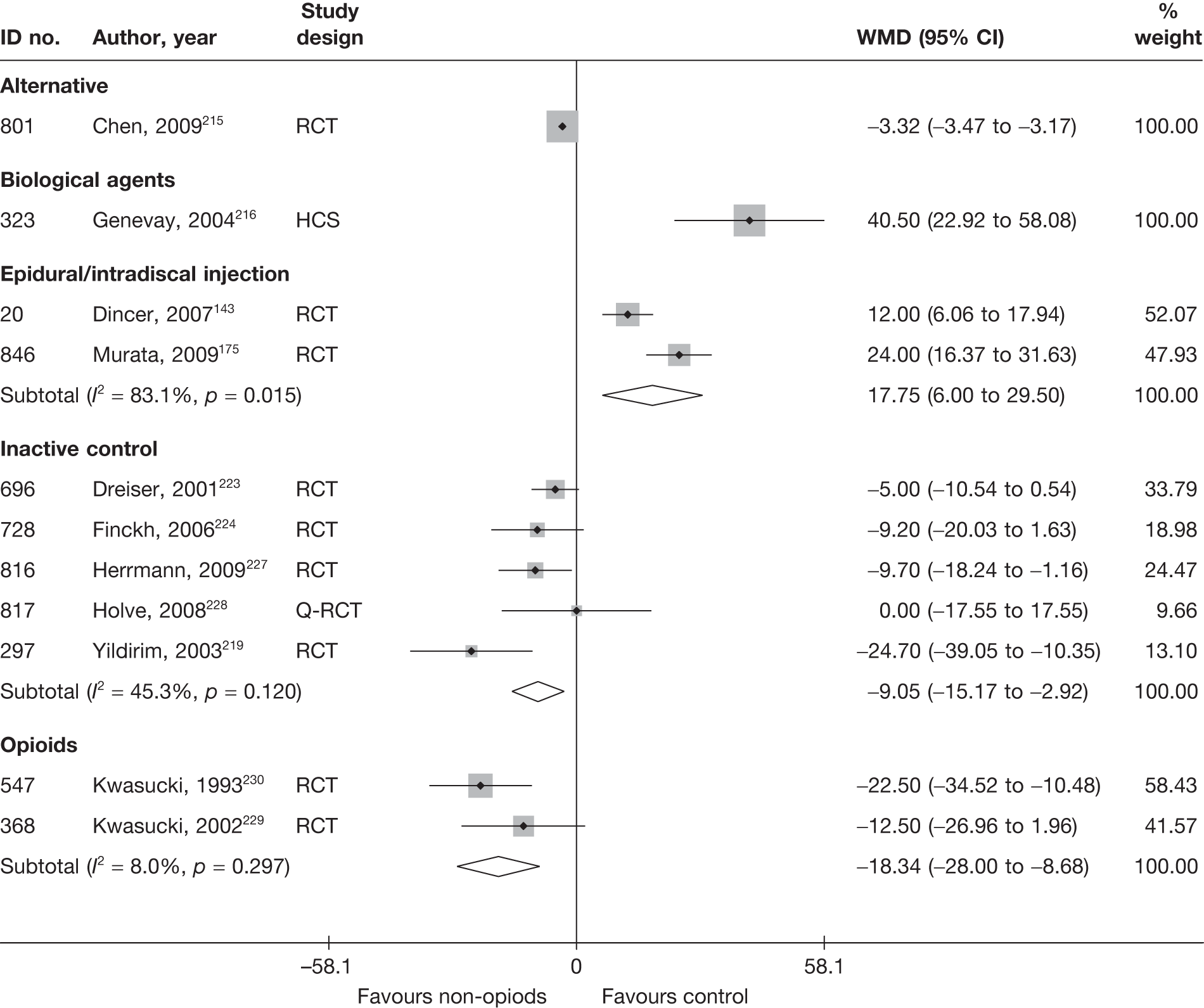
The overall findings from five studies219,223,224,227,228 showed non-opioids to be significantly better than inactive control for reducing pain. Four studies included patients with acute sciatica, and one poorly reported and poorly conducted RCT219 included patients with chronic sciatica (evaluating the use of oral gabapentin). As with the global effect, excluding the study with chronic sciatica improved homogeneity (I2 = 0%), giving a pooled WMD for four studies of –6.45 (95% CI –10.60 to –2.30). Three of the four studies were moderate-quality RCTs;223,224,227 the remaining study228 was a Q-RCT.
Pooled analysis from two RCTs229,230 showed non-opioids to be significantly better than opioids for reducing pain. Both studies were poorly reported and conducted. Follow-up ranged from 14229 to 19 days. 230
According to two RCTs,143,175 non-opioids were significantly less effective than epidural at reducing pain at 1 week175 to 1 month. 143 Both were poorly reported and of weak to moderate quality. One further poorly reported RCT156 of moderate quality also found non-opioids to be statistically significantly less effective than epidural for pain relief at 35 days (p < 0.004; statistical test not stated), but did not report any summary statistics.
One poorly reported and poorly conducted RCT215 found non-opioids to be significantly better than warming acupuncture (alternative therapy) for reducing pain in patients with chronic sciatica at the end of a 35-day treatment period.
A small HCS (323, n = 20) found biological agents to be significantly better than non-opioids for reducing pain intensity in patients with acute severe sciatica.
Condition-specific outcome measures at short-term follow-up
The results for CSOMs at short-term follow-up are presented in Table 50 and the accompanying forest plot (Figure 36). Epidural injections were compared with inactive control, epidural injections and biological agents. Three studies6,216,228 included only patients with acute sciatica and the remaining study143 included patients with either acute or chronic symptoms. The duration of follow-up ranged from 46,143,228 to 6 weeks. 216
| ID no. | Author, year | Chronicity | Study design | Follow-up | Scale | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)a | Comment/conversionb | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | ||||||||
| Non-opioids vs biological agents | |||||||||||||||
| 323 | Genevay, 2004216 | A | HCS | 6 weeks | RMDQ | 10 | 10 | 15.5 (2.9) | 17.8 (3.3) | 11.1 (4.6) | 5.8 (5.5) | 1.05 (0.10 to 1.99) | |||
| Non-opioids vs epidural/intradiscal injection | |||||||||||||||
| 20 | Dincer, 2007143 | A + C | RCT | 1 month | ODI | 30 | 34 | 34.4 (6.7) | 35.8 (6.7) | 22.2 (8.6) | 17 (7.3) | –12.2 | –18.8 | 0.66 (0.15 to 1.16) | |
| Non-opioids vs inactive control | |||||||||||||||
| 817 | Holve, 2008228 | A | Q-RCT | 4 weeks | RMDQ | 13 | 14 | 16 | 16 | 8 (4.6) | 9.2 (4.47) | –0.26 (–1.02 to 0.49) |
Final SD imputed from weighted mean of SDs from other studies ITT not used Dropouts 2/29 (7%): intervention 2/15, control 0/14 |
||
| 665 | Weber, 19936 | A | RCT | 4 weeks | Disability, Roland’s Functional Test (0–17) | 120 | 94 | 55 (14) | 54 (12) | 22 (14) | 16 (14) | –33 | –38 | 0.43 (0.16 to 0.70) | |
FIGURE 36.
Summary of the findings of CSOMs at short-term follow-up (≤ 6 weeks) for studies comparing non-opioids with alternative interventions. Note: weights are from random effects analysis.
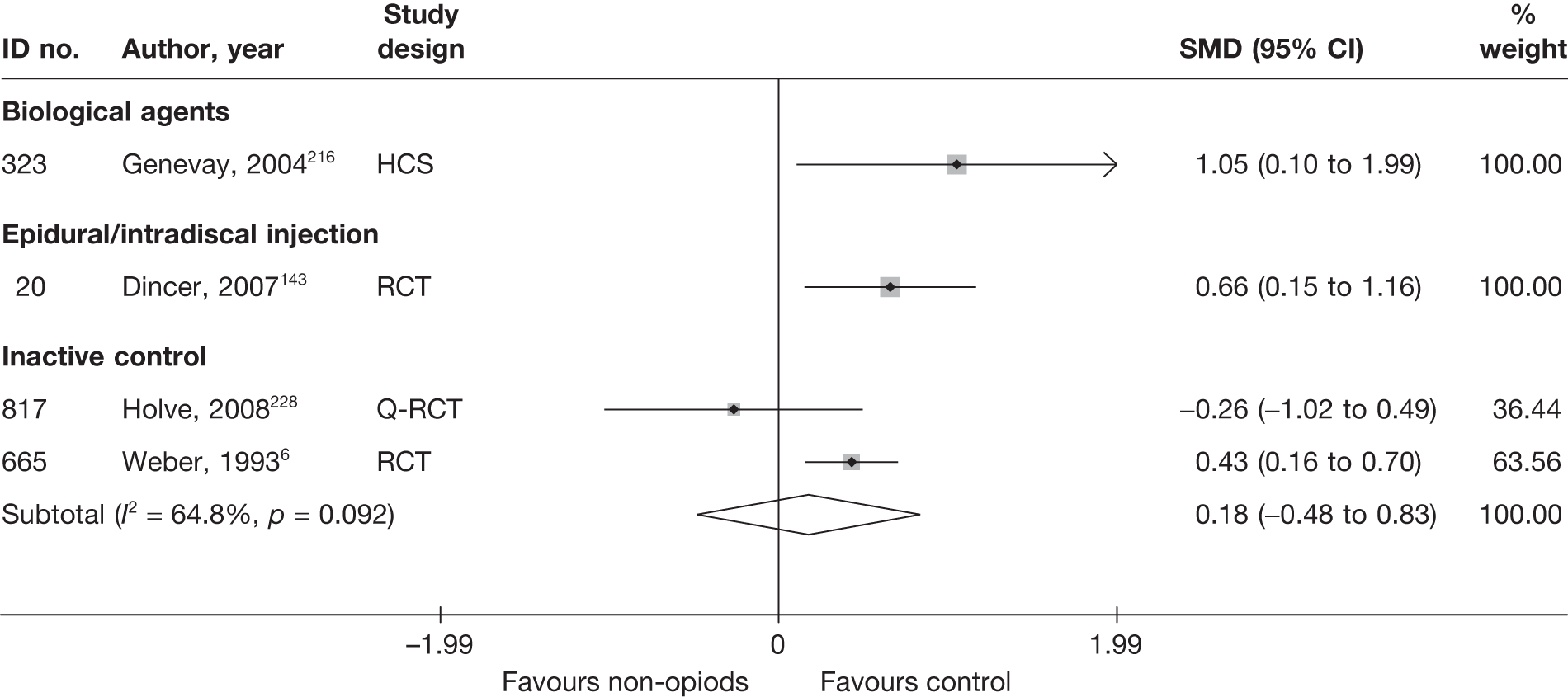
Two studies6,228 compared non-opioids with inactive control; there was an overall non-statistically significant finding in favour of inactive control at 4 weeks. One was a moderate-quality RCT6 that did not report the methods of randomisation and allocation concealment and the other was a Q-RCT. 228
One moderate-quality RCT143 found epidural to be significantly better than non-opioids for improving functional status in patients with acute or chronic sciatica. The methods of randomisation and allocation concealment were not stated.
A small (n = 20) historical cohort study216 found biological agents to be significantly better than non-opioids for improving functional status in patients with acute severe sciatica at 6 weeks.
Non-opioid results at medium-term follow-up (> 6 weeks to ≤ 6 months)
Global effect at medium-term follow-up
The results for the global effect at medium-term follow-up are presented in Table 51 and the accompanying forest plot (Figure 37). Non-opioids were compared with inactive intervention, epidural injections, disc surgery, opioids and mixed treatment. Two studies220,80 included only patients with acute sciatica and the remaining three studies175,214,220 included only patients with chronic sciatica. The duration of follow-up ranged from 9 weeks214 to 6 months. 57,175
| ID no. | Author, year | Chronicity | Study design | Follow-up | Outcome measure | Perspective | Intervention | Control | OR (95% CI) | Comments | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n) | Outcome (n) | Withdrawal rate | Total (n) | Outcome (n) | Withdrawal rate | |||||||||
| Non-opioids vs disc surgery | ||||||||||||||
| 144 | Rossi, 199357 (Italian language) | C | Non-RCT | 6 months | Reduction of pain | Patient | ? | 68 | ? | 55 |
Study included three comparative groups, but the two surgical groups were combined for the analysis of global effect Total number of participants was 40, but number in each group not stated and results reported only as percentages, therefore could not include in the meta-analysis |
|||
| 475 | Dubourg, 200280 | A | CCS | 6 months | Recovery improvement (vs failure) according to change in VAS and muscle strength | 25 | 24 | 0.11 | 32 | 25 | 0.18 | 6.72 (0.77 to 58.79) | ||
| Non-opioids vs epidural/intradiscal injection | ||||||||||||||
| 846 | Murata, 2009175 | C | RCT | 24 weeks | Satisfactory clinical outcome (vs unsatisfactory results) | Physician | 65 | 5 | ? | 71 | 11 | ? | 0.45 (0.15 to 1.39) |
Subgroup analysis of 136/246 (55%) patients with radicular pain: intervention 71/122, control 65/124 Eight patients dropped out, group allocation or radicular pain not stated |
| Non-opioids vs inactive control | ||||||||||||||
| 312 | Hedeboe, 1982220 | A | RCT | 3 months | Overall pain improvement: better (vs unchanged or worst) | Patient | 19 | 6 | 0 | 20 | 5 | 0 | 1.38 (0.34 to 5.62) | |
| 534 | Khoromi, 2007214 | C | RCT (crossover) | 9 weeks (end of treatment) | Global pain relief (GPR): worse pain or no pain relief | 33 | 11 | 0.40 | 32 | 13 | 0.42 | 1.26 (0.45 to 3.51) | Pain reported only for 28/50 patients (56%) who completed study (all treatments) | |
| Non-opioids vs mixed treatment | ||||||||||||||
| 534 | Khoromi, 2007214 (opioids + non-opioids) | C | RCT (crossover) | 9 weeks (end of treatment) | Global pain relief (GPR): worse pain or no pain relief | 28 | 18 | 0.49 | 32 | 13 | 0.42 | 0.35 (0.12 to 1.01) | Pain reported only for 28/50 patients (56%) who completed study (all treatments) | |
| Non-opioids vs opioids | ||||||||||||||
| 534 | Khoromi, 2007214 | C | RCT (crossover) | 9 weeks (end of treatment) | Global pain relief (GPR): worse pain or no pain relief | 31 | 12 | 0.44 | 32 | 13 | 0.42 | 0.92 (0.34 to 2.53) | Pain reported only for 28/50 patients (56%) who completed study (all treatments) | |
FIGURE 37.
Summary of the findings of the global effect at medium-term follow-up (> 6 weeks to ≤ 6 months) for studies comparing non-opioids with alternative interventions. Note: weights are from random effects analysis.
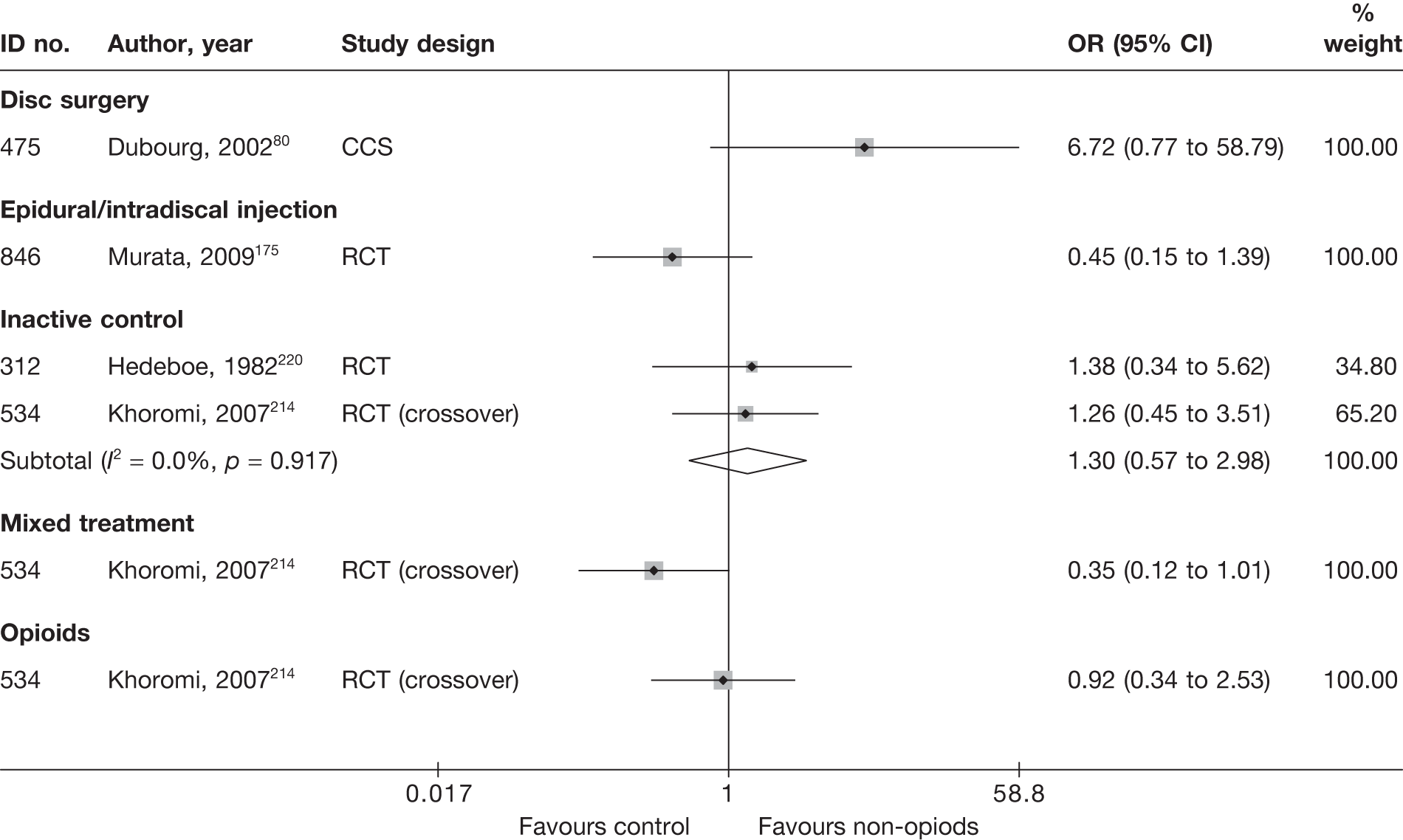
Two moderate-quality RCTs214,220 compared non-opioids with inactive control; there was a non-statistically significant finding in favour of non-opioids, for both acute and chronic sciatica. One study214 was a four-arm crossover RCT with a high dropout rate; only 44% of patients who completed the study were included in the analysis.
One poor-quality RCT175 reported non-statistically significant findings in favour of epidural, compared with non-opioids, for adequate recovery from leg pain at 24 weeks. The findings were based on a subgroup analysis of 136/246 (55%) patients with chronic radicular pain.
Two studies compared disc surgery with non-opioids. One poorly reported CCS80 found non-opioids to be more effective than disc surgery for recovery or improvement in patients with acute sciatica, but the findings were not statistically significant. A second poorly conducted study57 found that more patients in the surgery group (68%) than in the non-opioids group (55%) were satisfied with cure, but the findings were reported only as percentages, and the number of patients in each treatment group was not stated. The study was essentially two studies that were very poorly reported, and which included the comparison of two surgical procedures (percutaneous discectomy and microdiscectomy) with medical treatment. Patients (n = 40) were initially divided into two groups according to the type of disc herniation they had, with patients in one group randomised to one of two surgical procedures; the other group does not appear to have been randomised.
A moderate-quality crossover RCT214 compared non-opioids with opioids or a combination of both opioids and non-opioids (mixed treatments). There was no statistically significant difference between non-opioids and opioids, but combination therapy (mixed treatments) resulted in marginally statistically significant better outcomes than non-opioids used alone. Only 28 patients (44%) who completed the study were included in the analysis.
Pain intensity at medium-term follow-up
The results for pain intensity at medium-term follow-up are presented in Table 52 and the accompanying forest plot (Figure 38). Non-opioids were compared with inactive control and disc surgery, opioids and mixed treatments. Two studies80,228 included patients with acute sciatica and two studies214,219 included patients with chronic sciatica. The duration of follow-up ranged from 2219 to 6 months. 80,228
| ID no. | Author, year | Chronicity | Study design | Follow-up | Location | Scale (range)a | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)b | Comment/conversionc | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |||||||||
| Non-opioids vs disc surgery | ||||||||||||||||
| 475 | Dubourg, 200280 | A | CCS | 6 months | Overall | VAS (0–100) | 28 | 36 | 47.7 (34) | 52.2 (28.5) | 14.8 (20.6) | 13.2 (18.8) | 1.60 (–8.19 to 11.39) | Dropouts 7/67 (10%): intervention 4/39, control 3/28 | ||
| Non-opioids vs inactive control | ||||||||||||||||
| 817 | Holve, 2008228 | A | Q-RCT | 6 months | Overall | RMDQ subscale (0–5) | 13 | 14 | 76 | 62 | 8 (22.76) | 32 (30.1) | –24.00 (–44.04 to –3.96) |
SD imputed from weighted average ITT not used Dropouts 2/29 (7%): intervention 2/15, control 0/14 |
||
| 297 | Yildirim, 2003219 | C | RCT | 2 months | Overall | NRS (0–3) | 23 | 20 | 53.33 (31.33) | 56 (22.33) | 18.67 (19.33) | 45.33 (19.67) | –26.66 (–38.35 to –14.97) | Dropouts: intervention 2/25, control 5/25 | ||
| 534 | Khoromi, 2007214 | C | RCT (crossover) | 9 weeks (end of treatment) | Leg | NRS (0–10) | 28 | 28 | 49 (24.3) | 49 (24.3) | 30.0 (27) | 37.0 (27) | –7.00 (–21.14 to 5.38) | Pain reported only for 28/50 patients (56%) who completed study (all treatments) | ||
| Non-opioids vs mixed treatment | ||||||||||||||||
| 534 | Khoromi, 2007214 | C | RCT (crossover) | 9 weeks (end of treatment) | Leg | NRS (0–10) | 28 | 28 | 49 (24.3) | 49 (24.3) | 30.0 (27) | 38.0 (24) | –8.00 (–21.38 to 5.38) | Pain reported only for 28/50 patients (56%) who completed study (all treatments) | ||
| Non-opioids vs opioids | ||||||||||||||||
| 534 | Khoromi, 2007214 | C | RCT (crossover) | 9 weeks (end of treatment) | Leg | NRS (0–10) | 28 | 28 | 49 (24.3) | 49 (24.3) | 30.0 (27) | 34 (28) | –4.00 (–18.41 to 10.41) | Pain reported only for 28/50 patients (56%) who completed study (all treatments) | ||
FIGURE 38.
Summary of the findings of pain at medium-term follow-up (> 6 weeks to ≤ 6 months) for studies comparing non-opioids with alternative interventions. Note: weights are from random effects analysis.
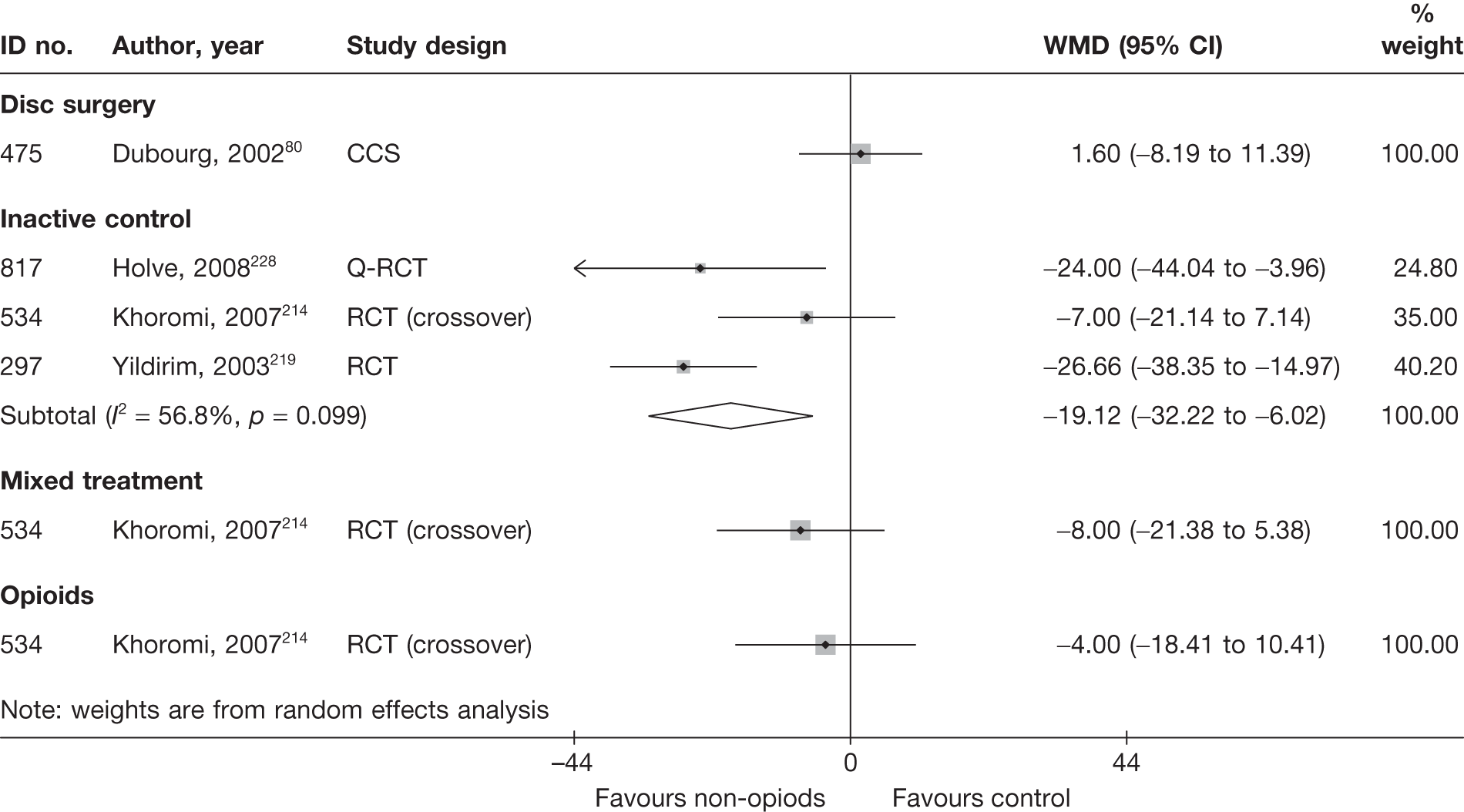
Pooled analysis from three studies214,219,228 showed non-opioids to be significantly better than the inactive control for reducing the overall pain of acute228 or chronic214,219 sciatica. One was a four-arm crossover RCT, one was a Q-RCT228 and the other a poor-quality RCT. 219 Follow-up ranged from 2219 to 6 months. 228 Two studies were of moderate quality. 214,228
One poorly reported CCS80 found no important difference between non-opioids and disc surgery for reducing pain intensity of acute sciatica at 6 months.
One moderate-quality crossover RCT214 compared non-opioids with opioids or a combination of both opioids and non-opioids (mixed treatments) for reducing pain intensity at 9 weeks. There was a non-statistically significant difference between the intervention groups in favour of non-opioids for both comparators. Only 28 patients (44%) who completed the study were included in the analysis.
Condition-specific outcome measures at medium-term follow-up
The results for CSOMs at medium-term follow-up are presented in Table 53 and the accompanying forest plot (Figure 39). Non-opioids were compared with the inactive control, and epidural injections, opioids and mixed treatments. One study228 included patients with acute sciatica and two studies143,214 included patients with either acute or chronic sciatica. The duration of follow-up ranged from 9 weeks214 to 6 months. 228
| ID No. | Author, year | Chronicity | Study design | Follow-up | Scale | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)a | Comment/conversionb | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | ||||||||
| Non-opioids vs epidural/intradiscal injection | |||||||||||||||
| 20 | Dincer, 2007143 | A + C | RCT | 3 months | ODI | 30 | 34 | 28.4 (5.4) | 35.8 (6.7) | 20.3 (10.1) | 16.2 (9.4) | –8.1 | –19.6 | 0.42 (–0.08 to 0.92) |
Final SD imputed from weighted mean of SDs for RMDQ at short-term follow-up Dropouts 2/29 (7%): intervention 2/15, control 0/14 |
| Non-opioids vs inactive control | |||||||||||||||
| 817 | Holve, 2008228 | A | Q-RCT | 6 months | RMDQ | 13 | 14 | 16 | 16 | 1.1 (4.6) | 2.1 (4.47) | –0.22 (–0.98 to 0.54) | |||
| 534 | Khoromi, 2007214 | C | RCT (crossover) | 9 weeks (end of treatment) | ODI | 28 | 28 | 30 (15) | 30 (15) | 27.5 (16.7) | 30.5 (15.9) | 0.18 (–0.71 to 0.34) | Pain reported only for 28/50 patients (56%) who completed study (all treatments) | ||
| Non-opioids vs mixed treatment | |||||||||||||||
| 534 | Khoromi, 2007214 | C | RCT (crossover) | 9-weeks (end of treatment) | ODI | 28 | 28 | 30 (15) | 30 (15) | 27.5 (16.7) | 27.4 (15.4) | 0.01 (–0.52 to 0.53) | Pain reported only for 28/50 patients (56%) who completed study (all treatments) | ||
| Non-opioids vs opioids | |||||||||||||||
| 534 | Khoromi, 2007214 | C | RCT (crossover) | 9 weeks (end of treatment) | ODI | 28 | 28 | 30 (15) | 30 (15) | 27.5 (16.7) | 25.7 (16.5) | 0.11 (–0.42 to 0.63) | Pain reported only for 28/50 patients (56%) who completed study (all treatments) | ||
FIGURE 39.
Summary of the findings of CSOMs at medium-term follow-up (> 6 weeks to ≤ 6 months) for studies comparing non-opioids with alternative interventions. Note: weights are from random effects analysis.
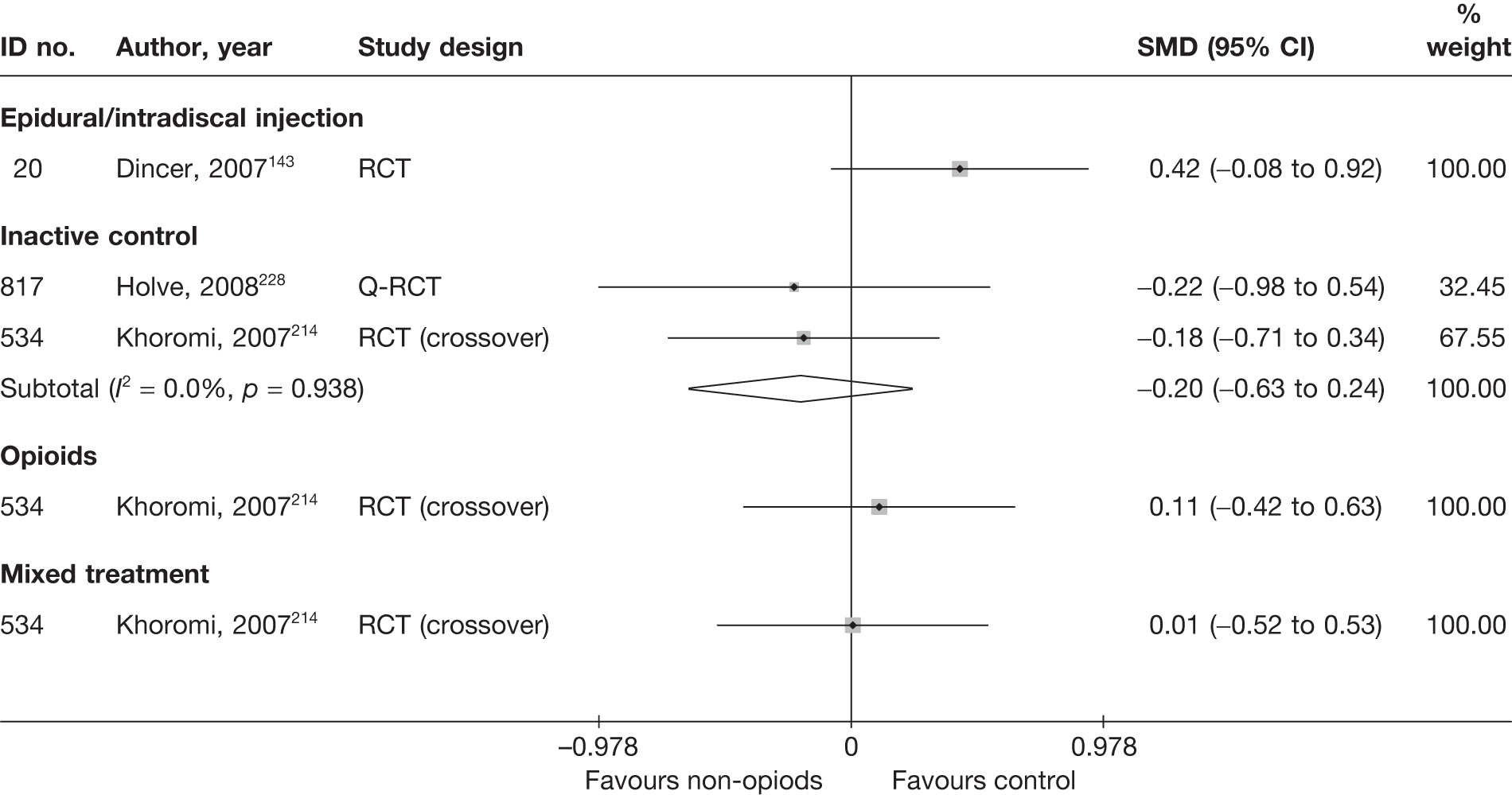
Pooled analysis of two studies showed a non-statistically significant finding in favour of non-opioids at 2214–6228 months, when compared with inactive control. One study was a moderate-quality, four-arm crossover RCT214 with adequate randomisation and allocation concealment but only 44% of patients were included in the analysis. The second study was a Q-RCT. 228 Patients were sequentially entered into the study by the pharmacy department, with odd-numbered patients given prednisone and even-numbered patients given the placebo. The principal investigator and research nurse were blind to the specific group allocation and to the methods used to make that assignment.
One moderate-quality RCT143 reported non-statistically significant findings in favour of epidural compared with non-opioids for improving functional status at 3 months’ follow-up. The methods of randomisation and allocation concealment were not stated.
A moderate-quality, crossover RCT214 compared non-opioids with opioids or a combination of opioids and non-opioids (mixed treatments). There was no statistically significant difference between the intervention groups for either comparison.
Results at long-term follow-up (> 6 months)
Global effect at long-term follow-up
The results for the global effect at long-term follow-up are presented in Table 54 and the accompanying forest plot (Figure 40).
| ID no. | Author, year | Chronicity | Study design | Follow-up | Outcome measure | Perspective | Intervention | Control | OR (95% CI) | Comments | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n) | Outcome (n) | Withdrawal rate | Total (n) | Outcome (n) | Withdrawal rate | |||||||||
| Non-opioid vs alternative | ||||||||||||||
| 801 | Chen, 2009215 (i)a (WAG) | C | RCT | 1 year | Success: cured or improved (vs no improvement) | Patient | 30 | 22 | 0 | 30 | 27 | 0 | 0.31 (0.07 to 1.29) |
Data inferred from graphs reporting percentages ITT using worst-case analysis (with non-opioids as the control group) |
| 801 | Chen, 2009215 (ii)a (PIG) | C | RCT | 1 year | Success: cured or improved (vs no improvement) | Patient | 30 | 22 | 0 | 30 | 19 | 0 | 1.59 (0.53 to 4.77) |
Data inferred from graphs reporting percentages ITT using worst-case analysis (with non-opioids as the control group) |
FIGURE 40.
Summary of the findings of the global effect at long-term follow-up (> 6 months) for studies comparing non-opioids with alternative interventions. Note: weights are from random effects analysis.
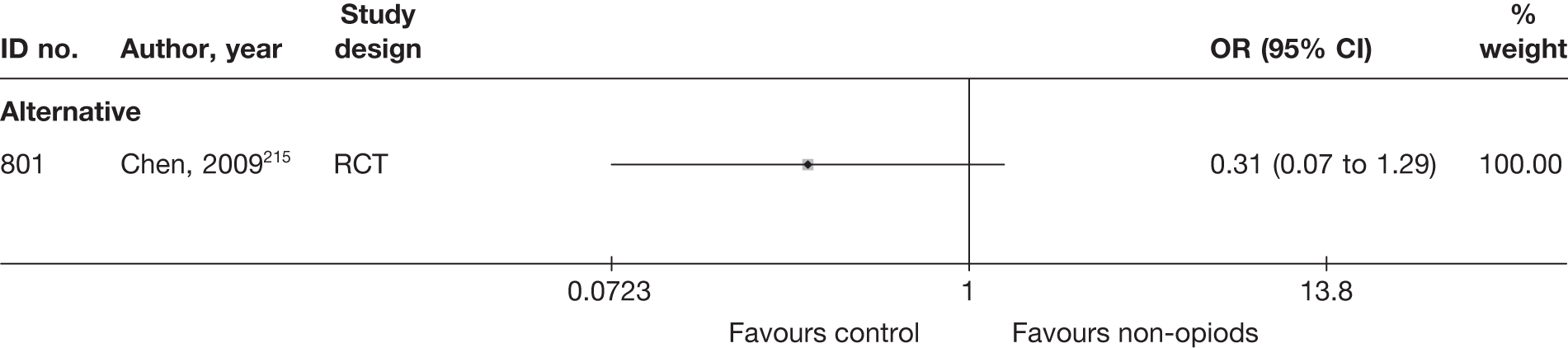
One study215 compared the overall success of the use of non-opioids or warming acupuncture in patients with chronic sciatic at 1 year’s follow-up. The study was a poorly conducted RCT and found a non-statistically significant difference between the intervention groups, in favour of acupuncture.
Pain intensity at long-term follow-up
No study reported long-term outcome in terms of pain intensity.
Condition-specific outcome measures at long-term follow-up
No study reported long-term outcome in terms of CSOMs.
Analysis of adverse effects for non-opioids
The results for the occurrence of any reported adverse effects are presented in Table 55 and the accompanying forest plot (Figure 41).
| ID no. | Author, year | Study design | No. of events in intervention group | No. of participants in intervention group | No. of events in control group | No. of participants in control group | OR (95% CI) |
|---|---|---|---|---|---|---|---|
| Non-opioids vs alternative treatment | |||||||
| 801 | Chen, 2009215 (warming acupuncture) | RCT | NR | NR | NR | NR | |
| 801 | Chen, 2009215 [anisodamine (2 mg) point injections] | RCT | NR | NR | NR | NR | |
| Non-opioids vs biological agent | |||||||
| 323 | Genevay, 2004216 | HCS | NR | NR | NR | NR | |
| Non-opioids vs disc surgery | |||||||
| 475 | Dubourg, 200280 | CCS | 0 | 28 | 1 | 39 | 0.45 (0.02 to 11.46) |
| 144 | Rossi, 199357 (microdiscectomy) | RCT | 1 | NR | 0 | NR | |
| 144 | Rossi, 199357 (percutaneous discectomy) | RCT | 1 | NR | 0 | NR | |
| Non-opioids vs epidural | |||||||
| 451 | Bronfort, 2000161 | RCT | 4 | 6 | 6 | 6 | 0.14 (0.01 to 3.63) |
| 20 | Dincer, 2007143 | RCT | 0 | 30 | 2 | 34 | 0.21 (0.01 to 4.62) |
| 771 | Lafuma, 1997172 | RCT | NR | NR | NR | NR | |
| 362 | Wilson-MacDonald, 2005156 | RCT | NR | NR | NR | NR | |
| 846 | Murata, 2009175 | RCT | NR | NR | NR | NR | |
| Non-opioids vs inactive control | |||||||
| 696 | Dreiser, 2001223 (low dose) | RCT | 10 | 171 | 9 | 180 | 1.18 (0.47 to 2.98) |
| 696 | Dreiser, 2001223 (high dose) | RCT | 13 | 181 | 9 | 180 | 1.47 (0.61 to 3.53) |
| 334 | El-Zahaar, 1995221 | RCT | 3 | 50 | 0 | 50 | 7.44 (0.37 to 148.00) |
| 728 | Finckh, 2006224 | RCT | 3 | 31 | 0 | 29 | 7.25 (0.36 to 147.00) |
| 62 | Gibson, 1975217 | Non-RCT | NR | NR | NR | NR | |
| 97 | Goldie, 1968218 | RCT | 8 | 25 | 5 | 25 | 1.88 (0.52 to 6.84) |
| 732 | Grevsten, 1975225 | RCT | 3 | 18 | 4 | 18 | 0.70 (0.13 to 3.70) |
| 312 | Hedeboe, 1982220 | RCT | 6 | 19 | 1 | 20 | 8.77 (0.94 to 81.70) |
| 816 | Herrmann, 2009227 | RCT | 11 | 57 | 7 | 57 | 1.71 (0.61 to 4.78) |
| 816 | Herrmann, 2009227 (diclofenac) | RCT | 6 | 57 | 7 | 57 | 0.84 (0.26 to 2.68) |
| 817 | Holve, 2008228 | Q-RCT | 0 | 15 | 0 | 14 | |
| 736 | Jacobs, 1968226 | Q-RCT | 28 | 55 | 20 | 55 | 1.81 (0.85 to 3.89) |
| 534 | Khoromi, 2007214 | RCT (crossover) | 37 | 55 | 28 | 55 | 1.98 (0.92 to 4.29) |
| 611 | Porsman, 1979222 | RCT | 1 | 25 | 1 | 24 | 0.96 (0.06 to 16.24) |
| 665 | Weber, 19936 | RCT | 22 | 120 | 13 | 94 | 1.4 (0.66 to 2.95) |
| 297 | Yildirim, 2003219 | RCT | 2 | 23 | 0 | 20 | 4.77 (0.22 to 105.00) |
| Non-opioids vs manipulation | |||||||
| 451 | Bronfort, 2000161 | RCT | 4 | 6 | 3 | 7 | 2.67 (0.28 to 25.64) |
| Non-opioids vs opioids | |||||||
| 534 | Khoromi, 2007214 |
RCT (crossover) |
37 | 55 | 51 | 55 | 0.16 (0.05 to 0.52) |
| 368 | Kwasucki, 2002229 (fluvoxamine) | RCT | 2 | 24 | 1 | 22 | 1.91 (0.16 to 22.66) |
| 368 | Kwasucki, 2002229 (imipramine) | RCT | 12 | 24 | 1 | 22 | 21.00 (2.42 to 182.00) |
| 547 | Kwasucki, 1993230 | RCT | NR | NR | NR | NR | NR |
| Non-opioids vs mixed treatment | |||||||
| 534 | Khoromi, 2007214 | RCT (crossover) | 37 | 55 | 49 | 55 | 0.25 (0.09 to 0.70) |
FIGURE 41.
Summary of the findings of any adverse effect for studies comparing non-opioids with alternative interventions. Note: weights are from random effects analysis.
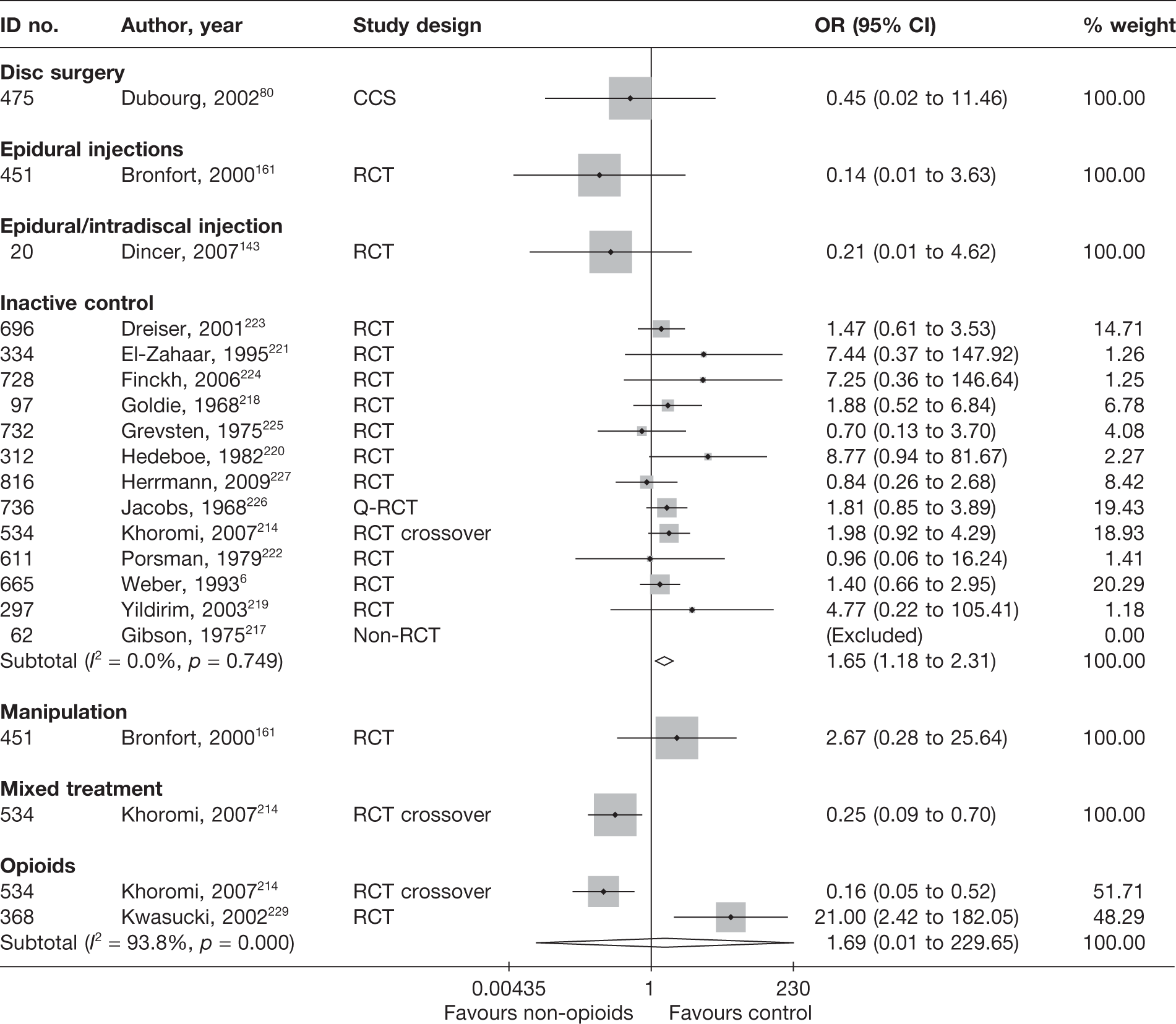
The incidence of adverse effects associated with non-opioids was statistically significantly greater than the incidence of adverse events associated with inactive control and significantly lower than the incidence of adverse events associated with mixed treatments (opioids plus non-opioids). Pooled analyses showed no statistically significant differences between the intervention groups for the number of adverse effects when comparing non-opioids with disc surgery, epidural, mixed treatments (morphine plus nortriptyline) or opioids.
SUMMARY OF OVERALL FINDINGS FOR NON-OPIOIDS COMPARED WITH ALTERNATIVE INTERVENTIONS
Almost half (9/22,6,80,216,218,220,223,224,227,228 41%) of the non-opioid studies included patients with acute sciatica; 27% (6/2257,175,214,215,219,221) included patients with chronic sciatica. Most of the non-opioid studies (77%) were RCTs. None of the studies was deemed good quality overall; although two214,227 included adequate randomisation and allocation concealment, one214 of these studies had a high dropout rate. Both compared non-opioids with inactive control (one also included comparisons with opioids and mixed treatments214) (Table 56).
| Control category | No. of studies (arms) | Sample size range (median) | Proportion of studies that were RCTs (%) | Proportion of studies that were deemed good quality (%) | Proportion of studies that only included acute sciatica (%) | Proportion of studies that included patients with nerve root pain (%) | Proportion of studies that reported diagnosis confirmed by imaging (%) | Proportion of studies that included patients with stenosis (%) | Proportion of studies that included patients with extruded/sequestered discs (%) | Proportion of studies that only included patients with first episode (%) | Proportion of studies that included patients who had received previous treatment (%) | Proportion of studies that included patients who had received previous surgery (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-opioids vs alternative/non-traditional | 1 (2) | 90 (90) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) |
| Non-opioids vs biological agents | 1 (1) | 10 (10) | 0/1 (0) | 0/1 (0) | 1/1 (100) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) |
| Non-opioids vs disc surgery | 2 (3) | 40–67 (54) | 1/2 (50) | 0/2 (0) | 0/2 (0) | 2/2 (100) | 2/2 (100) | 0/2 (0) | 0/2 (0) | 0/2 (0) | 0/2 (0) | 1/2 (50) |
| Non-opioids vs intradiscal injection | 3 (3) | 64–246 (93) | 3/3 (100) | 0/3 (0) | 0/3 (0) | 3/3 (100) | 2/3 (67) | 1/3 (33) | 0/3 (0) | 0/3 (0) | 1/3 (33) | 1/3 (33) |
| Non-opioids vs inactive control | 13 (14) | 29–532 (55) | 10/13 (77) | 1/13 (8) | 8/13 (62) | 13/13 (100) | 4/13 (31) | 0/13 (0) | 0/13 (0) | 1/13 (8) | 5/13 (38) | 0/13 (0) |
| Non-opioids vs mixed treatment | 1 (1) | 55 (55) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 1/1 (100) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 1/1 (100) | 0/1 (0) |
| Non-opioids vs opioids | 3 (4) | 43–70 (55) | 2/3 (67) | 0/3 (0) | 0/3 (0) | 3/3 (100) | 2/3 (67) | 1/3 (33) | 0/3 (0) | 0/3 (0) | 2/3 (67) | 0/3 (0) |
| Total (for non-opioid studies)a | 22 (29) | 10–532 (38) | 17/22 (77) | 0/22 (0) | 9/22 (41) | 22/22 (100) | 9/22 (41) | 2/22 (9) | 0/22 (0) | 1/22 (5) | 8/22 (36) | 2/22 (9) |
Non-opioids resulted in a statistically significant greater proportion of patients who recovered at short term follow-up than inactive control (eight RCTs218,220–225,227 and one non-RCT217). Non-opioids were also significantly better than inactive control for reducing pain intensity of acute (three RCTs223,224,227 and one Q-RCT,228 all moderate quality) and chronic sciatica (one poor-quality RCT219) at short-term follow-up. However, there were no statistically significant difference between the intervention groups in terms of functional status (one RCT6 and one Q-RCT228) during the same follow-up period. Non-opioids were significantly better than inactive control for reducing pain intensity of acute (one moderate-quality Q-RCT228) and chronic sciatica (one poor-quality RCT219 and one moderate-quality crossover RCT214) at medium-term follow-up. There was no statistically significant difference between the intervention groups in terms of the proportion of patients who recovered (two moderate-quality RCTs214,220) or functional status (one moderate-quality Q-RCT228 and one moderate-quality crossover RCT214) at medium-term follow-up. Non-opioids resulted in significantly more adverse effects than inactive control. 6,214,217–227
There was no statistically significant difference between non-opioids and disc surgery for global effect (one non-RCT57 and one CCS80) and pain intensity (one CCS80) at medium-term follow-up or for adverse effects, according to two poor-quality studies. 57,80
Non-opioids were less effective than epidural for reducing pain143,156,175 and improving functional status143 at short-term follow-up according to three poorly reported RCTs. There was no statistically significant difference between non-opioids and epidural for functional status at medium-term follow-up (RCT143).
Non-opioids were found to be statistically significantly better than opioids for reducing pain intensity at short-term follow-up,229,230 but there was no significant difference between the intervention groups for global effect229,230 or adverse effects (two poor-quality RCTs214,229).
One poor-quality RCT215 found non-opioids to be significantly better than warming acupuncture for reducing pain intensity of chronic sciatica at short-term follow-up, but there was no significant difference between the intervention groups for the global effect at long-term follow-up.
One small historical CCS216 found biological agents to be to be significantly better than non-opioids for reducing pain intensity and functional status at short-term follow-up.
Traction
Description of traction studies
Summary of interventions
Twelve studies evaluated traction for sciatica. 176,242–252 Ten of these studies compared traction to an alternative intervention (three were multiple-arm studies). 176,242–250 One further study compared mixed treatment that included traction, with mixed treatments or with other comparators without traction (Table 57a). 253–256
| ID no. | Author, year | Study design | Treatment description | Control description |
|---|---|---|---|---|
| Traction vs activity restriction | ||||
| 222 | Moret, 1998243 | RCT | Bed rest and traction (vertical traction using patient weight), 180 minutes daily for 1–2 weeks | Bed rest |
| Traction vs exercise therapy | ||||
| 2 | Ljunggren, 1992242 | RCT | Manual traction | Isometric exercises |
| Traction vs inactive control | ||||
| 553 | Larsson, 1980246 | RCT | Auto-traction, three treatments | Inactive corset |
| 579 | Mathews, 1975247 | RCT | Traction (full traction) 5 days per week for 3 weeks | Sham traction (minimal traction) 5 days per week for 3 weeks |
| 206 | Pal, 1986244 | RCT | Weighted traction: continuous lumbar traction of 5.5–8.2 kg according to body weight | Sham traction: continuous lumbar traction of 1.4–1.8 kg according to body weight |
| 299 | Rattanatharn, 2004245 | RCT |
Traction three times per week Traction force of 35–50% of the body weight performed intermittently |
Sham traction three times per week Traction force of < 20% of body weight performed intermittently |
| 746 | Reust, 1988248 (French language) | RCT | Normal traction (50 kg) | Placebo traction (5 kg) |
| 746 | Reust, 1988248 (French language) | RCT | Light traction (15 kg) | Placebo traction (5 kg) |
| Traction vs passive PT | ||||
| 9059 | Mathews, 1987176 | RCT | Lumbar traction of at least 45 kg, but sufficient to relieve pain sustained for 30 minutes | Control treatment. Infrared heat treatment to the low back area at 60 cm for 15 minutes, three times per week |
| 148 | Unlu, 2008249 | RCT | Lumbar traction | Ultrasound treatment |
| 148 | Unlu, 2008249 | RCT | Lumbar traction | Low-power laser |
| Traction vs usual/conventional care | ||||
| 77 | Styczynski, 1991250 (Polish language) | Non-RCT | Antigravitational traction. Up to 15 treatments, mean 12.3 |
Conservative treatment without traction Up to 15 treatment sessions, mean 12.0 |
| 77 | Styczynski, 1991257 (Polish language) | Non-RCT | Chair traction. Up to 15 treatments, mean 11.7 |
Conservative treatment without traction Up to 15 treatment sessions, mean 12.0 |
| 77 | Styczynski, 1991257 (Polish language) | Non-RCT | Pulse traction. Up to 15 treatments, mean 11.3 |
Conservative treatment without traction Up to 15 treatment sessions, mean 12.0 |
| Mixed treatment including traction vs mixed treatment without traction | ||||
| 301 | Harte, 2007254 | RCT | Traction and/or manual therapy, exercise and/or advice to stay active | Manual therapy, exercise and/or advice to stay active |
Three studies compared different types of traction (Table 57b). 248,251,252
Summary of study participants for traction
Summary data for included participants are presented in Table 58. The number of participants included in the 10 studies that reported outcome data for global, pain or CSOMs ranged from 16 to 157 (median 60 participants). Five studies176,243,245,246,249 (45%) included patients with acute sciatica, one study242 (9%) included patients with chronic sciatica, one study247 included patients with either acute or chronic sciatica and the remaining three studies244,248,250 did not report this information. None of the studies included patients with spinal stenosis or sequestered or extruded discs. The diagnosis of sciatica, or the presence of herniated disc, was confirmed by imaging in four studies (40%). Two studies243,246 included a mixture of patients with either recurrent or first episode of sciatica, whereas the remaining studies did not report this information. Two studies (one in which the comparator was activity restriction243 and one in which the comparator was inactive control247) included patients who had already received previous treatment for their current episode of sciatica. This information was not stated for the remaining studies. One study,243 which compared traction with activity restriction, included patients who had received previous disc surgery.
| ID no. | Author, year | Study design | No. of patients | Age (years) | No. of men (%) | Symptom duration | Type of sciatica | Confirmed by imaging? | Recurrent episode | Included patients with stenosis?a | Included patients with sequestered disc (or extruded)?a | Any previous treatment for sciatica? | Any previous back surgery for sciatica? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Traction vs activity restriction | |||||||||||||
| 222 | Moret, 1998243 | RCT | 16 | Mean 41.9 (SD 8.7) | 12 (75) | Acute symptoms 50% | Nerve root pain | No | Recurrent and first episode | No | NR | Yes | Yes |
| Traction vs exercise therapy | |||||||||||||
| 570 | Ljunggren, 1992242 | RCT | 50 | Mean 41.6 (range 19–62) | 27 (54) | Mean 5 months | Nerve root pain | Yes | NR | No | No | NR | No |
| Traction vs inactive control | |||||||||||||
| 553 | Larsson, 1980246 | RCT | 84 | Mean 37 (range 20–55) | 51 (62) | Mean 6.7 weeks (range 2–14 weeks) | Nerve root pain | No | Recurrent and first episode | No | No | NR | NR |
| 579 | Mathews, 1975247 | RCT | 27 | Range 20–60 | NR | Mean 13 weeks | Nerve root pain and refereed pain | No | NR | No | No | Yes | NR |
| 206 | Pal, 1986244 | RCT | 41 | Mean 39 | 23 (59) | Median 49 days | Nerve root pain and referred pain | NR | NR | No | No | NR | NR |
| 299 | Rattanatharn, 2004245 | RCT | 120 | Mean 37.3 | 47 (46) | < 3 months | Nerve root pain | No | NR | No | No | Yes | No |
| 746 | Reust, 1988248 (French language) | RCT | 60 | Mean 50.8 (SD 12.5) | 35 (58) | NR | Nerve root pain and referred pain | No | NR | No | No | NR | NR |
| Traction vs passive PT | |||||||||||||
| 9059 | Mathews, 1987176 | RCT | 143 | Median 40 (range 20–60) | 80 (56) | Median 3.5 weeks (range 0 days–3 months) | Nerve root pain | No | NR | NR | NR | NR | NR |
| 148 | Unlu, 2008249 | RCT | 60 | Mean 44.5 (range 20–60) | 18 (30) | < 3 months | Nerve root pain | Yes | NR | No | No | NR | No |
| Traction vs usual/conventional care | |||||||||||||
| 77 | Styczynski, 1991250 (Polish language) | Non-RCT | 157 | Range 18–67 | 84 (54) | NR | Nerve root pain | Yes | NR | NR | NR | NR | NR |
| Mixed treatment including traction vs mixed treatment without traction | |||||||||||||
| 301 | Harte, 2007254 | RCT | 64 | Mean 41.1 (SD 9.8) | 28 (44) | Median 47.5 days (range 2–671 days) | Nerve root pain | No | Recurrent and first episode | No | No | Yes | Yes |
Summary of study quality for traction studies
Summary information on study details are presented in Table 59. Most of the traction studies were RCTs (9/10, 90%), but none was deemed to be good quality overall. Seven studies176,242,243,245,246,248,249 were of moderate quality. Three studies243,245,248 used adequate randomisation, but not allocation concealment, although two243,245 used sealed envelopes. One study243 had strong external validity.
| ID no. | Author, year | Study size | Overall follow-up | Study design | Adequate randomisation? | Allocation concealment? | Follow-up (%) | Blind outcome assessment? | Overall quality rating | Overall external validity rating |
|---|---|---|---|---|---|---|---|---|---|---|
| Traction vs activity restriction | ||||||||||
| 222 | Moret, 1998243 | 16 | 3 weeks | RCT | Yes | Partial | 80–100 | No | Moderate | Strong |
| Traction vs active PT/exercise therapy | ||||||||||
| 570 | Ljunggren, 1992242 | 50 | 1 week | RCT | Unclear | Unclear | 80–100 | Yes | Moderate | Weak |
| Traction vs inactive control | ||||||||||
| 553 | Larsson, 1980246 | 84 | 3 months | RCT | Unclear | Unclear | 80–100 | Unclear | Moderate | Weak |
| 579 | Mathews, 1975247 | 27 | 3 months | RCT | Unclear | Unclear | Cannot tell | Unclear | Weak | Weak |
| 206 | Pal, 1986244 | 41 | 2 years | RCT | Unclear | Unclear | 80–100 | Yes | Weak | Weak |
| 299 | Rattanatharn, 2004245 | 120 | 4 weeks | RCT | Yes | Partial | 60–79 | NA | Moderate | Weak |
| 746 | Reust, 1988248 (French language) | 60 | 12 days | RCT | Yes | Unclear | < 60 | Yes | Moderate | Weak |
| Traction vs passive PT | ||||||||||
| 9059 | Mathews, 1987176 | 143 | 12 months | RCT | Partial | Unclear | < 60 | Yes | Moderate | Moderate |
| 148 | Unlu, 2008249 | 60 | 3 months | RCT | Unclear | Unclear | 80–100 | Yes | Moderate | Weak |
| Traction vs usual/conventional care | ||||||||||
| 77 | Styczynski, 1991250 (Polish language) | 157 | After treatment | Non-RCT | No | No | 80–100 | Unclear | Weak | Weak |
| Mixed treatment including traction vs mixed treatment without traction | ||||||||||
| 301 | Harte, 2007254 | 30 | 6 months | RCT | Yes | Partial | 60–79 | Yes | Moderate | Strong |
Traction results at short-term follow-up (≤ 6 weeks)
Global effect at short-term follow-up
The results for the global effect at short-term follow-up are presented in Table 60 and the accompanying forest plot (Figure 42). Traction was compared with inactive control, usual care/conservative treatment, activity restriction, exercise therapy and passive PT. Only one study242 included patients with chronic sciatica; four studies176,243,245,246 included patients with acute sciatica and the remaining study250 did not report this information. The duration of follow-up ranged from 1 week242 to 4 weeks. 245 Three further studies254–256 combined the use of mixed treatments that incorporated traction with an alternative treatment.
| ID no. | Author, year | Chronicity | Study design | Follow-up | Outcome measure | Perspective | Intervention | Control | OR (95% CI) | Comments | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n) | Outcome (n) | Withdrawal rate | Total (n) | Outcome (n) | Withdrawal rate | |||||||||
| Traction vs activity restriction | ||||||||||||||
| 222 | Moret, 1998243 | A | RCT | 3 weeks | Leg pain: recovered or strongly improved (vs little improved, no change, little worse, much worse or worse than ever) | Patient | 8 | 4 | 0 | 8 | 4 | 0 | 1.00 (0.14 to 7.10) | |
| Traction vs exercise therapy | ||||||||||||||
| 570 | Ljunggren, 1992242 | C | RCT | 1 week | Global evaluation: symptom-free or satisfactory improvement (vs unsatisfactory improvement or unchanged) | Physician | 24 | 10 | 0 | 26 | 10 | 0 | 1.14 (0.37 to 3.55) | |
| Traction vs inactive control | ||||||||||||||
| 553 | Larsson, 1980246 | A | RCT | 3 weeks | Completely recovered: free from back or leg pain (vs partially recovered 1 = no leg pain, partially recovered 2 = no back pain or no recovery) | 41 | 7 | 0.05 | 41 | 3 | 0 | 2.61 (0.62 to 10.89) | ||
| 299 | Rattanatharn, 2004245 | A | RCT | 4 weeks | Global improvement: complete recovery or much improved (vs little improved/unchanged or little/much worse) | Patient | 54 | 38 | 0.10 | 48 | 34 | 0.20 | 0.98 (0.42 to 2.30) | |
| Traction vs passive PT | ||||||||||||||
| 9059 | Mathews, 1987176 | A | RCT | 2 weeks | Recovered: pain score of 5 or 6 (vs not recovered = scores of 1–4) | 77 | 40 | 0.07 | 54 | 27 | 0.10 | 1.08 (0.54 to 2.17) | Number of dropouts reported was different from the number missing from the analysis | |
| Traction vs usual/conventional care | ||||||||||||||
| 77 | Styczynski, 1991250 (i) (antigravity)a | NR | Non-RCT | 3 weeks | Overall improvement | 38 | 26 | 0.10 | 29 | 17 | 0.03 | 1.53 (0.56 to 4.19) | ||
| 77 | Styczynski, 1991250 (ii) (chair)a | NR | Non-RCT | 3 weeks | Overall improvement | 41 | 28 | 0.05 | 29 | 17 | 0.03 | 1.52 (0.57 to 4.09) | ||
| 77 | Styczynski, 1991250 (iii) (pulse)a | NR | Non-RCT | 3 weeks | Overall improvement | 41 | 28 | 0.02 | 29 | 17 | 0.03 | 1.52 (0.57 to 4.09) | ||
| Mixed treatment including traction vs mixed treatment without traction | ||||||||||||||
| 301 | Harte, 2007254 | A | RCT | Post-treatment | Median percentage overall improvement | Patient | 16 | Median 90% (IQR 24) | 0.13 | 4 | Median 90% (IQR 22.5) LT | 0.14 |
ITT not used for dichotomous outcome Percentage improvement reported, not number of patients who improved |
|
FIGURE 42.
Summary of the findings of the global effect at short-term follow-up (≤ 6 weeks) for studies comparing traction with alternative interventions. Note: weights are from random effects analysis.
Pooled analysis of two moderate-quality RCTs245,246 showed non-statistically significant difference in favour of traction, compared with inactive control, for overall recovery from acute sciatica at 3 weeks246 to 4 weeks. 245
One poorly reported non-RCT250 found a non-statistically significant difference in favour of pulse traction, compared with conservative treatment without traction, for overall improvement at 3 weeks. All patients were in bed for at least 18 hours a day in a position taking the strain off with legs bent at hips and knees for 3 weeks, and undertaking isometric exercises to strengthen muscles around the spine, hips, abdomen and limbs.
One small (n = 16), moderate-quality RCT243 found no statistically significant difference between vertical traction using patient weight plus bed rest and bed rest alone (activity restriction) in terms of the proportion of patients with improvement in leg pain for acute sciatica at 3 weeks. Twelve patients (75%) were hospitalised.
One RCT242 found no statistically significant difference between manual traction and isometric exercise (active PT) for overall improvement of chronic sciatica at 1 week. All patients were hospital inpatients and used crutches and elastic lumbar supports for any necessary out-of-bed activities. The study was of moderate quality, but the method of randomisation and allocation concealment was not stated.
One moderate-quality RCT176 found no important difference between traction and infrared heat treatment (passive PT) for overall recovery from acute sciatica at 2 weeks. Patients were also given paracetamol to take when necessary and offered a corset. All patients attended a special outpatients clinic.
One small, moderate-quality, pilot RCT254 reported the same median percentage improvement, as perceived by the patient, for mixed treatment (manual therapy, exercise and advice) with or without traction.
Pain intensity at short-term follow-up
The results for pain intensity at short-term follow-up are presented in Table 61 and the accompanying forest plot (Figure 43). Traction was compared with inactive control, activity restriction and passive PT. Two studies243,249 included patients with acute sciatica; the remaining two studies244,248 did not report the duration of symptoms. The duration of follow-up ranged from 12 days248 to 3 weeks. 243,244 Three further studies253–255 compared the use of mixed treatments that incorporated traction with alternative interventions.
| ID no. | Author, year | Chronicity | Study design | Follow-up | Location | Scale (range)a | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)b | Comment/conversionc | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |||||||||
| Traction vs passive PT | ||||||||||||||||
| 148 | Unlu, 2008249 (i)d (ultrasound) | A | RCT | 1 month | Leg | VAS (0–100) | 20 | 20 | 59.6 (15.4) | 56 (15.3) | 21.8 (15.4) | 26.8 (18.36) | –5.00 (–15.58 to 5.58) | |||
| 148 | Unlu, 2008249 (ii)d (laser) | A | RCT | 1 month | Leg | VAS (0–100) | 20 | 20 | 59.6 (15.4) | 53.1 (25.9) | 21.8 (15.4) | 25.6 (21.1) | –3.80 (–15.25 to 7.65) | |||
| Traction vs inactive control | ||||||||||||||||
| 206 | Pal, 1986244 | NR | RCT | 3 weeks | Overall | VAS (0–100) | 24 | 15 | 50 | 50 | 5 | 3 | 2.00 (–11.68 to 15.68) | Median used for mean; SD imputed from weighted average | ||
| 746 | Reust, 1988248 (i)e (French language) (50 kg) | NR | RCT | 12 days | Overall | VAS (0–100) | 18 | 20 | 75.28 (23.85) | 61.5 (23.63) | 33.61 (29.55) | 30.25 (26.23) | 3.36 (–14.49 to 21.21) |
ITT using LOCF Dropouts: intervention 3/18, control (placebo) 2/31 |
||
| 746 | Reust, 1988248 (ii)e (French language) (15 kg) | NR | RCT | 12 days | Overall | VAS (0–100) | 22 | 20 | 67.27 (23.74) | 61.5 (23.63) | 30.68 (26.83) | 30.25 (26.23) | –0.43 (–15.63 to 16.49) |
ITT using LOCF Dropouts: intervention 3/22, control (placebo) 2/31 |
||
| Traction vs activity restriction | ||||||||||||||||
| 222 | Moret, 1998243 | A | RCT | 3 weeks | Leg | NRS (0–10) | 8 | 8 | 74 (12.0) | 73 (10.0) | 44 (12) | 63 (10) | –30 | –10 | –19.00 (–29.82 to –8.18) | Final mean calculated from change score and baseline SD used |
| Mixed treatment including traction vs mixed treatment without traction | ||||||||||||||||
| 301 | Harte, 2007254 | A | RCT | Post treatment | Overall | McGill | 16 | 14 | 20.5 (6.67) | 29 (14.81) | 4 (11.33) | 12 (12.22) | 17.5 (9.48) | 15.5 (19.11) | –8.00 (–16.47 to 0.47) |
Small sample sizes Mean and SDs derived from median and IQR values ITT use LOCF Dropouts 3/30 (10%): intervention 2/16, control 1/14 |
FIGURE 43.
Summary of the findings of pain intensity at short-term follow-up (≤ 6 weeks) for studies comparing traction with alternative interventions. Note: weights are from random effects analysis.
Two RCTs247,248 compared the use of traction with inactive control; the pooled analysis showed a non-statistically significant difference in favour of inactive control for overall pain at 2 weeks248 to 3 weeks. 244 The quality of the studies was poor in one case244 and moderate in the other. 248 Only one of these used an adequate method of randomisation. 248 The method of randomisation was not stated in the second study and allocation concealment was not reported for either study. Inactive treatment included sham traction in both studies (1.4–1.8 kg according to body weight244 or 20% of body weight248).
One small (n = 16), moderate-quality RCT243 found vertical traction plus bed rest to be significantly better than bed rest alone (activity restriction) for reducing leg pain in patients with acute sciatica at 3 weeks. Twelve patients (75%) were hospitalised.
One moderate-quality RCT249 compared the use of standard motorised traction with ultrasound (passive PT); there was an overall non-statistically significant finding in favour of ultrasound, for acute sciatica at 1 month. The method of randomisation and allocation concealment was not reported.
One small, moderate-quality pilot RCT254 that compared manual exercise therapy, exercise and advice found that the traction combination resulted in a non-statistically significantly greater reduction in overall pain intensity that the control intervention.
Condition-specific outcome measures at short-term follow-up
The results for CSOMs at short-term follow-up are presented in Table 62 and the accompanying forest plot (Figure 44). Traction was compared with inactive control, activity restriction and passive PT. All three studies243,245,249 included patients with acute sciatica. The duration of follow-up ranged from 12 days248 to 3 weeks. 243,244 Two further studies254,255 compared the use of mixed treatments that incorporated traction with alternative treatments.
| ID no. | Author, year | Chronicity | Study design | Follow-up | Scale | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)a | Comment/conversionb | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | ||||||||
| Traction vs activity restriction | |||||||||||||||
| 222 | Moret, 1998243 | A | RCT | 3 weeks | RMDQ | 8 | 8 | 18.1 (1.8) | 18.5 (2.1) | 14.5 (3.87) | 17.1 (6.2) | –3.6 | –1.4 | –0.50 (–1.50 to 0.49) | Final mean calculated from change scores, final SD imputed from weighted mean of SDs from other studies |
| Traction vs inactive control | |||||||||||||||
| 299 | Rattanatharn, 2004245 | A | RCT | 4 weeks | ODI | 54 | 48 | 47.97 (15.32) | 40.61 (13.94) | 22.72 (18.61) | 21.36 (17.27) | –25.25 (16.68) | –19.25 (15.9) |
ANCOVA for change scores showed no statistically significant difference, p = 0.301 0.08 (–0.31 to 0.46) |
ITT not reported, no dropouts |
| Traction vs passive PT | |||||||||||||||
| 148 | Unlu, 2008249 (i)c | A | RCT | 1 month | RMDQ | 20 | 20 | 14.2 (4.3) | 12.5 (5) | 8.5 (3.5) | 7.3 (4.3) | –5.7 | –5.2 | 0.31 (–0.32 to 0.93) | |
| 148 | Unlu, 2008249 (ii)c | A | RCT | 1 month | RMDQ | 20 | 20 | 14.2 (4.3) | 13.4 (4.5) | 8.5 (3.5) | 8.2 (6) | –5.7 | –5.2 | 0.06 (–0.56 to 0.68) | |
| Mixed treatment incorporating traction vs mixed treatment without traction | |||||||||||||||
| 301 | Harte, 2007254 | A | RCT | Post-treatment | RMDQ | 16 | 14 | 10 (3.33) | 11.5 (6.3) | 4 (4.3) | 4 (7.63) | –4.5 (5.41) | –3 (5.93) | 0.0 (–0.72 to 0.72) |
Medians used for means and SDs calculated from IQRs Small sample sizes – likely to be skewed ITT using LOCF |
FIGURE 44.
Summary of the findings of CSOMs at short-term follow-up (≤ 6 weeks) for studies comparing traction with alternative interventions. Note: weights are from random effects analysis.
One RCT,245 of moderate quality, compared traction with inactive control and found a non-statistically significant difference, in favour of inactive control, in improved function in patients with acute sciatica at 4 weeks.
One small RCT,243 of moderate quality, compared traction plus bed rest with bed rest alone (activity restriction) and found a non-statistically significant difference, in favour of traction, for improved function in patients with acute sciatica at 3 weeks.
One moderate-quality RCT249 compared traction with ultrasound (passive PT); at 1 month, there was an overall non-statistically significant finding in favour of ultrasound for the treatment of acute sciatica. The methods of randomisation and allocation concealment were not reported.
One small, moderate-quality study254 found no important difference between traction or no traction, with manual exercise therapy, exercise and advice for acute sciatica at treatment completion.
Traction results at medium-term follow-up (> 6 weeks to ≤ 6 months)
Global effect at medium-term follow-up
The results for the global effect at medium-term follow-up are presented in Table 63 and the accompanying forest plot (Figure 45).
| ID no. | Author, year | Chronicity | Study design | Follow-up | Outcome measure | Perspective | Intervention | Control | OR (95% CI) | Comments | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n) | Outcome (n) | Withdrawal rate | Total (n) | Outcome (n) | Withdrawal rate | ||||||||||||
| Traction vs inactive control | |||||||||||||||||
| 553 | Larsson, 1980246 | A | RCT | 3 months | Symptom free (vs persisting symptoms) | 40 | 19 | 0.07 | 41 | 17 | 0 | 1.28 (0.53 to 3.07) | |||||
FIGURE 45.
Summary of the findings of the global effect at medium-term follow-up (> 6 weeks to ≤ 6 months) for studies comparing traction with alternative interventions. Note: weights are from random effects analysis.
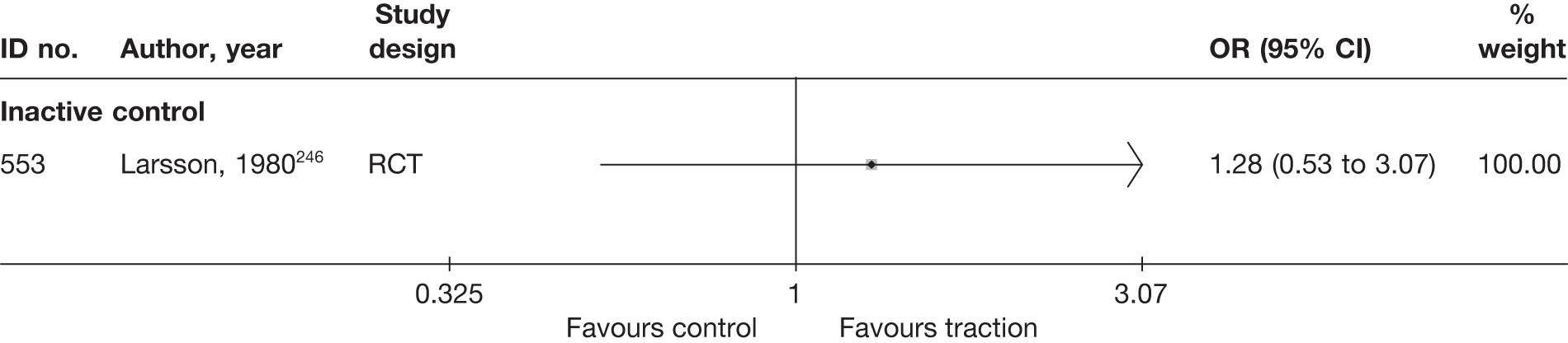
One moderate-quality RCT246 compared the use of auto-traction with inactive control (inactive corset) in terms of the proportion of patients with acute sciatica who were symptom free at 3 months’ follow-up. The methods of randomisation and allocation concealment used were not reported. There was a non-statistically significant difference between the groups in favour of inactive control. Most patients were treated as outpatients [20/84 (24%) were hospitalised] and patients in both groups were were supplied with a corset and advised to rest.
Pain intensity at medium-term follow-up
The results for pain intensity at medium-term follow-up are presented in Table 64 and the accompanying forest plot (Figure 46). Traction was compared with inactive control and passive PT. One further study254 compared the use of mixed treatments that incorporated traction with mixed treatments without traction for acute sciatica.
| ID no. | Author, year | Chronicity | Study design | Follow-up | Location | Scale (range)a | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)b | Comment/conversionc | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |||||||||
| Traction vs passive PT | ||||||||||||||||
| 148 | Unlu, 2008249 (i)d (ultrasound) | A | RCT | 3 months | Leg | VAS (0–100) | 20 | 20 | 59.6 (15.4) | 56 (15.3) | 29.5 (16.7) | 25.2 (13.9) | 4.30 (–5.22 to 13.82) | |||
| 148 | Unlu, 2008249 (ii)d (laser) | A | RCT | 3 months | Leg | VAS (0–100) | 20 | 20 | 59.6 (15.4) | 53.1 (25.9) | 29.5 (16.7) | 23.6 (17.7) | 5.90 (–4.76 to 16.56) | |||
| Traction vs inactive control | ||||||||||||||||
| 579 | Mathews, 1975247 | A + C | RCT | 3 months | Overall | Improvement (0–100) | 13 | 14 | 28.80 | 18.90 |
Average percentage improvement in pain since starting treatment All patients asked to judge by what percentage pain had changed assuming the level on entry to the trial to be 100% |
|||||
| Mixed treatment incorporating traction vs mixed treatment without traction | ||||||||||||||||
| 301 | Harte, 2007254 | A | RCT | 6 months | Overall | McGill | 16 | 14 | 20.5 (6.67) | 29 (14.8) | 10 (15.9) | 6.5 (15.56) | 15.5 (12.81) | 16.5 (22.81) | 3.50 (–7.54 to 14.54) | Mean and SDs derived from median and IQR values |
FIGURE 46.
Summary of the findings of pain at medium-term follow-up (> 6 weeks to ≤ 6 months) for studies comparing traction with alternative interventions. Note: weights are from random effects analysis.
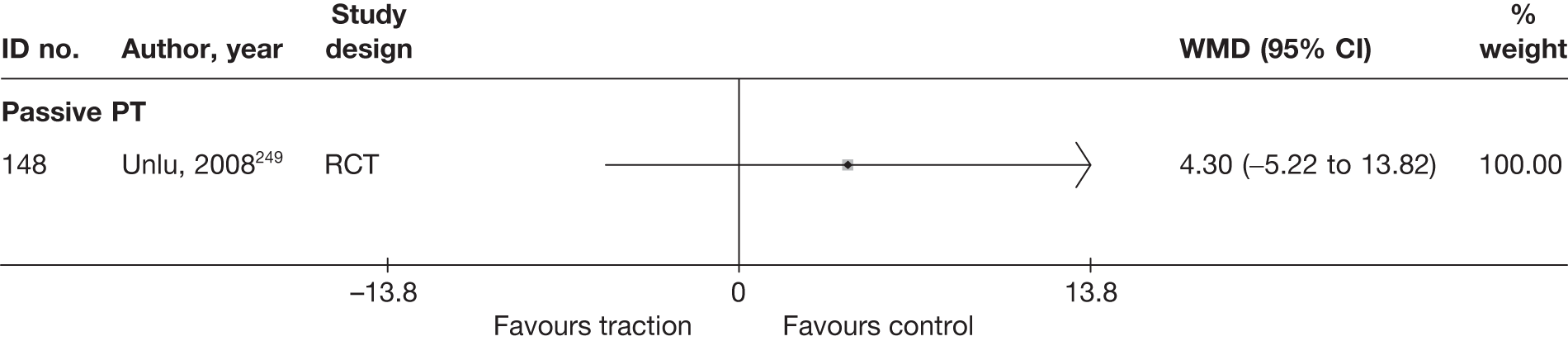
One small (n = 27), poor-quality and poorly reported RCT247 compared traction with inactive control (sham traction using a maximum force of 9 kg). The study was published in 1975 and carried out by single physiotherapist. Patients were asked to judge by what percentage their pain had changed, assuming the level of pain at baseline to be 100%. The average improvement at 6 weeks was 28.8% in the traction group compared with 18.9% in the control group.
One moderate-quality RCT249 compared traction with ultrasound (passive PT); at 3 months, there was a non-statistically significant improvement in acute sciatica, in favour of ultrasound. The methods of randomisation and allocation concealment were not reported.
One moderate-quality pilot study254 compared the use of motorised lumbar traction combined with manual therapy, exercise and/or advice to stay active compared with manual therapy, exercise and/or advice to stay active without traction. There was no statistically significant difference between the intervention groups at 6 months’ follow-up, but this may be due to the small sample size (n = 30).
Condition-specific outcome measures at medium-term follow-up
The results for CSOMs at medium-term follow-up are presented in Table 65 and the accompanying forest plot (Figure 47). Traction was compared with passive PT for acute sciatica. One further study254 compared the use of mixed treatments that incorporated traction with mixed treatments without traction for acute sciatica.
| ID no. | Author, year | Chronicity | Study design | Follow-up | Scale | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)a | Comment/conversionb | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | ||||||||
| Traction (ultrasound) vs passive PT | |||||||||||||||
| 148 | Unlu, 2008249 (i)c (ultrasound) | A | RCT | 3 months | RMDQ | 20 | 20 | 14.2 (4.3) | 12.5 (5) | 8.9 (4) | 6.7 (4.5) | –5.3 | –5.8 | 0.52 (–0.11 to 1.15) | |
| 148 | Unlu, 2008249 (ii)c (laser) | A | RCT | 3 months | RMDQ | 20 | 20 | 14.2 (4.3) | 13.4 (4.5) | 8.9 (4) | 8.6 (6) | –5.3 | –4.8 | 0.06 (–0.56 to 0.68) | |
| Mixed treatment incorporating manipulation vs mixed treatment without manipulation | |||||||||||||||
| 301 | Harte, 2007254 | A | RCT | 6 months | RMDQ | 16 | 14 | 10 (3.33) | 11.5 (6.3) | 4.5 (11.33) | 11.5 (6.3) | –4 (9.11) | –3 (9.11) | –0.75 (–1.49 to –0.01) |
IQR used to calculate SD, but small sample sizes – likely to be skewed ITT used LOCF |
FIGURE 47.
Summary of the findings of CSOMs at medium-term follow-up (> 6 weeks to ≤ 6 months) for studies comparing traction with alternative interventions. Note: weights are from random effects analysis.
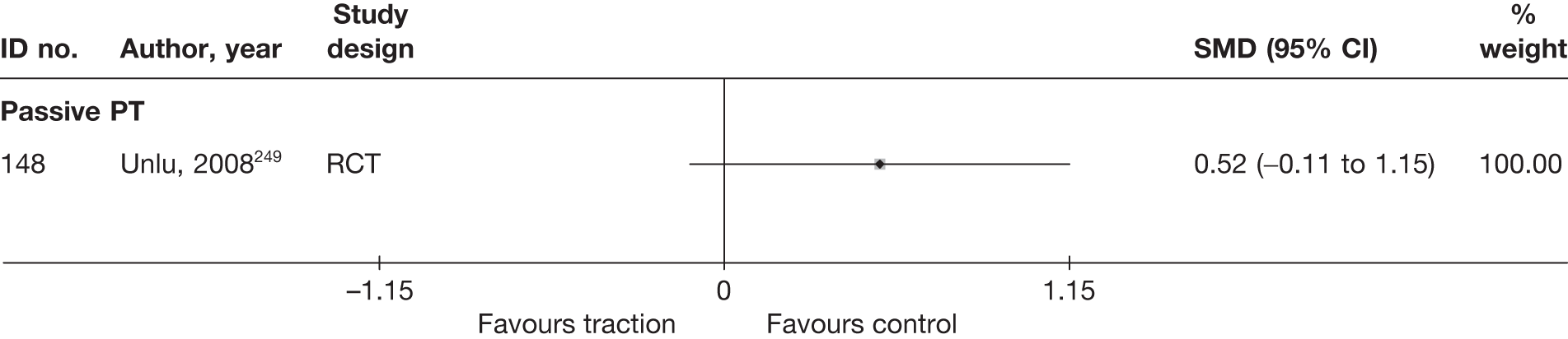
One moderate-quality RCT249 compared traction with ultrasound (passive PT); at 3 months, there was an overall non-statistically significant improvement in acute sciatica, in favour of ultrasound.
One moderate-quality pilot study254 compared the use of motorised lumbar traction combined with manual therapy, exercise and/or advice to stay active with manual therapy, exercise and/or advice to stay active without traction. Improvement in functional status at 6 months’ follow-up was marginally higher in the traction group and the difference was statistically significant.
Results at long-term follow-up (> 6 months)
No long-term outcomes were reported for traction.
Analysis of adverse effects for traction
The results for the occurrence of any reported adverse effects are presented in Table 66 and the accompanying forest plot (Figure 48).
| ID no. | Author, year | Study design | No. of events in intervention group | No. of participants in intervention group | No. of events in control group | No. of participants in control group | OR (95% CI) |
|---|---|---|---|---|---|---|---|
| Traction vs active PT/exercise therapy | |||||||
| 570 | Ljunggren, 1992242 | RCT | 8 | 24 | 8 | 26 | 1.13 (0.34 to 3.69) |
| Traction vs activity restriction | |||||||
| 222 | Moret, 1998243 | RCT | 6 | 8 | 0 | 8 | 44.2 (1.8 to 1088.0) |
| Traction vs inactive control | |||||||
| 206 | Pal, 1986244 | RCT | NR | NR | NR | NR | |
| 299 | Rattanatharn, 2004245 | RCT | 4 | 54 | 2 | 48 | 1.84 (0.32 to 10.52) |
| 553 | Larsson, 1980246 | RCT | NR | NR | NR | NR | |
| 579 | Mathews, 1975247 | RCT | NR | NR | NR | NR | |
| 746 | Reust, 1988248 (French language) | RCT | NR | NR | NR | NR | |
| 746 | Reust, 1988248 (French language) | RCT | NR | NR | NR | NR | |
| Traction vs passive PT | |||||||
| 148 | Unlu, 2008249 | RCT | NR | NR | NR | NR | |
| 148 | Unlu, 2008249 | RCT | NR | NR | NR | NR | |
| Traction vs usual care | |||||||
| 77 | Styczynski, 1991250 | Non-RCT | 7 | 38 | 1 | 29 | 6.32 (0.73 to 54.64) |
| Mixed treatment including traction vs mixed treatment without traction | |||||||
| 301 | Harte, 2007254 | RCT | NR | NR | NR | NR | |
FIGURE 48.
Summary of the findings of any adverse effect for studies comparing traction with alternative interventions. Note: weights are from random effects analysis.
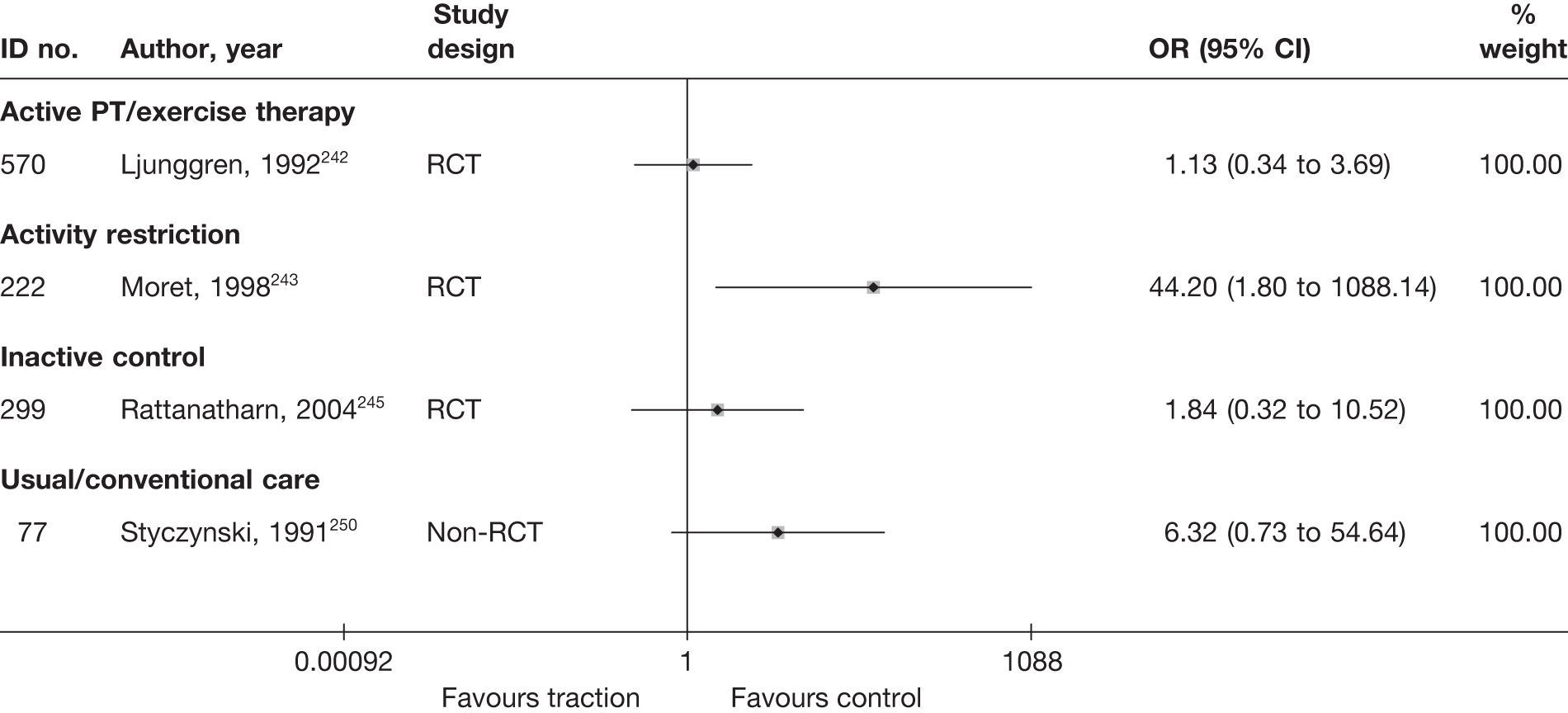
The number of adverse effects associated with traction was significantly greater than the number associated with activity restriction. Pooled analyses showed no statistically significant differences for the number of adverse effects when comparing traction with inactive control, usual care or exercise therapy.
SUMMARY OF OVERALL FINDINGS FOR TRACTION COMPARED WITH ALTERNATIVE INTERVENTIONS
Half (5/10,176,243,245,246,249 50%) of the traction studies included patients with acute sciatica; 10% (1/10242) included patients with chronic sciatica. Most of the traction studies (90%) were RCTs,176,242–249 but none was of a good quality (Table 67). One small, moderate-quality, pilot study evaluated mixed treatment (manual therapy, exercise and advice) with and without traction for patients with acute sciatica. 254
| Control category | No. of studies (arms) | Sample size range (median) | Proportion of studies that were RCTs (%) | Proportion of studies that were deemed good quality (%) | Proportion of studies that only included acute sciatica (%) | Proportion of studies that included patients with nerve root pain (%) | Proportion of studies that reported diagnosis confirmed by imaging (%) | Proportion of studies that included patients with stenosis (%) | Proportion of studies that included patients with extruded/sequestered discs (%) | Proportion of studies that only included patients with first episode (%) | Proportion of studies that included patients who had received previous treatment (%) | Proportion of studies that included patients who had received previous surgery (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Traction vs activity restriction | 1 (1) | 16 (16) | 1/1 (100) | 0/1 (0) | 1/1 (100) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 1/1 (100) | 1/1 (100) |
| Traction vs exercise therapy | 1 (1) | 50 (50) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 1/1 (100) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) |
| Traction vs inactive control | 5 (6) | 27–120 (60) | 5/5 (100) | 0/5 (0) | 2/5 (40) | 5/5 (100) | 0/5 (0) | 0/5 (0) | 0/5 (0) | 0/5 (0) | 2/5 (40) | 0/5 (0) |
| Traction vs passive PT | 2 (3) | 60–143 (102) | 2/2 (100) | 0/2 (0) | 2/2 (100) | 2/2 (100) | 1/2 (50) | 0/2 (0) | 0/2 (0) | 0/2 (0) | 0/2 (0) | 0/2 (0) |
| Traction vs usual/conventional care | 1 (3) | 157 (157) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 1/1 (100) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) |
| Total (for traction studies) | 10 (14) | 16–157 (60) | 9/10 (90) | 0/10 (0) | 5/10 (50) | 10/10 (100) | 3/10 (30) | 0/10 (0) | 0/10 (0) | 0/10 (0) | 3/10 (30) | 1/10 (10) |
There was no statistically significant difference between traction and inactive control for the treatment of acute sciatica in terms of the global effect (two moderate-quality RCTs245,246), reduction in pain intensity (two moderate-quality RCTs244,248) and improvement in functional status (one moderate-quality RCT245) at short-term follow-up, or in terms of the global effect at medium-term follow-up (one moderate-quality RCT246), or in adverse effects. 245
One poorly reported non-RCT250 found no statistically significant difference between traction and usual care in terms of the global effect at short-term follow-up or for adverse effects.
One small RCT243 (moderate quality) found traction plus bed rest to be significantly better than bed rest alone (activity restriction) for reducing leg pain in patients with acute sciatica at short-term follow-up. Patients who received traction experienced significantly more adverse effects than those in the control group. There was no statistically significant difference between the treatment groups for global effect and CSOMs at short-term follow-up (one small, moderate-quality RCT243).
There was no statistically significant difference between traction and exercise therapy for the treatment of chronic sciatica in terms of the global effect at short-term follow-up or for adverse effects, according to one moderate-quality RCT. 242
According to two moderate-quality RCTs,176,249 there were no statistically significant difference between traction and passive PT for the treatment of acute sciatica in terms of global effect,176 reduction in pain intensity249 and improvement in functional status249 at short-term follow-up, global effect249 and functional status249 at medium-term follow-up, or adverse effects. 249 There were no important differences between mixed treatments with or without traction for overall improvement, pain intensity or functional status at the end of the treatment (pilot RCT254).
Manipulation
Description of manipulation studies
Summary of interventions
Four studies compared spinal manipulation with an alternative type of intervention for sciatica. 169,208,258,259 Summary data of the interventions used are presented in Table 68. One RCT258 compared chiropractic spinal manipulation with sham manipulation. One RCT208 compared osteopathic spinal manipulation with chemonucleolysis. Two three-armed pilot RCTs compared chiropractic spinal manipulation with epidural corticosteroid injections, and also with either self-care education169 or paracetamol, NSAIDs and activity modification. 161 Neither of these pilot RCTs reported outcomes at follow-up apart from adverse effects and cost data. One further non-RCT compared massage, traction and spinal manipulation (mixed treatment) with digital stimulation of acupuncture points and traction. 260
| ID no. | Author, year | Study design | Treatment description | Control description |
|---|---|---|---|---|
| Manipulation vs chemonucleolysis | ||||
| 723 | Burton, 2000208 | RCT | Osteopathic spinal manipulation for up to 12 weeks | Chemonucleolysis with 400 U chymopapain |
| Manipulation vs education/advice | ||||
| 722 | Bronfort, 2004169 | RCT | Chiropractic spinal manipulation | Self-care education |
| Manipulation vs epidural | ||||
| 451 | Bronfort, 2000161 | RCT | Chiropractic spinal manipulation | Epidural corticosteroid injection (one to three times) |
| 722 | Bronfort, 2004169 | RCT | Chiropractic spinal manipulation | Epidural corticosteroid injection (three times) |
| Manipulation vs inactive control | ||||
| 52 | Santilli, 2006258 | RCT | Chiropractic manipulation up to 20 sessions | Sham manipulation up to 20 sessions |
| Manipulation vs non-opioids | ||||
| 451 | Bronfort, 2000161 | RCT | Chiropractic spinal manipulation | Paracetamol, NSAIDs, activity modification |
| Mixed treatment including manipulation vs mixed treatment without manipulation | ||||
| 687 | Zhang, 2005260 | Non-RCT | Massage, traction and spinal manipulation | Digital stimulation of acupuncture points and traction |
Summary of study participants for manipulation
Summary data on the included participants are presented in Table 69. The two RCTs comparing manipulation with alternative interventions that reported follow-up results included 142 participants with mean ages between 42 and 43 years (48–63% men): one with acute symptom duration and one with chronic symptoms. One study included patients with recurrent episodes. Sciatica was confirmed by imaging in both. There were no patients with spinal stenosis or previous back surgery or sequestered discs.
| ID no. | Author, year | Study design | No. of patients | Age (years) | No. of men (%) | Symptom duration | Type of sciatica | Confirmed by imaging? | Recurrent episode | Included patients with stenosis?a | Included patients with sequestered disc (or extruded)?a | Any previous treatment for sciatica? | Any previous back surgery for sciatica? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Manipulation vs chemonucleolysis | |||||||||||||
| 723 | Burton, 2000208 | RCT | 40 | Mean 41.9 (SD 10.6) | 19 (48) | Mean 31 (SD 35) weeks | Nerve root pain | Yes | Recurrent and first episode | No | No | NR | No |
| Manipulation vs education/advice | |||||||||||||
| 722 | Bronfort, 2004169 | RCT | 32 | Mean 49.0 (SD 9.1) | 18 (56) | 1–3 months 19%; 4–6 months 6%; 7–12 months 9%; > 12 months 66% | Nerve root pain and referred pain | No | Recurrent and first episode | No | No | NR | No previous spinal fusion |
| Manipulation vs epidural | |||||||||||||
| 451 | Bronfort, 2000161 | RCT | 20 | Mean 44.5 (SD 10.6) | 12 (60) | ≤ 3 weeks n = 6; 4–12 weeks n = 14 | Nerve root pain and referred pain | No | NR | No | No | Yes | No |
| 722 | Bronfort, 2004169 | RCT | 32 | Mean 49.0 (SD 9.1) | 18 (56) | 1–3 months 19%; 4–6 months 6%; 7–12 months 9%; > 12 months 66% | Nerve root pain and referred pain | No | Recurrent and first episode | No | No | NR | No previous spinal fusion |
| Manipulation vs inactive control | |||||||||||||
| 52 | Santilli, 2006258 | RCT | 102 | Mean 43.1 (range 19–63) | 64 (63) | < 10 days | Nerve root pain and referred pain | Yes | NR | No | No | NR | No |
| Manipulation vs non-opioids | |||||||||||||
| 451 | Bronfort, 2000161 | RCT | 20 | Mean 44.5 (SD 10.6) | 12 (60) | ≤ 3 weeks n = 6; 4–12 weeks n = 14 | Nerve root pain and referred pain | No | NR | No | No | Yes | No |
| Mixed treatment including manipulation vs mixed treatment without manipulation | |||||||||||||
| 687 | Zhang, 2005260 | Non-RCT | 210 | Mean 41.8 | 112 (53) | NR | Nerve root pain | Yes | NR | No | No | NR | NR |
Summary of study quality for manipulation studies
Study details are summarised in Table 70. All of the studies comparing manipulation with alternative interventions were RCTs and one was of good quality,258 which was the only RCT with an adequate method of random number generation, a secure method of allocation concealment and good external validity.
| ID no. | Author, year | Study size | Overall follow-up | Study design | Adequate randomisation? | Allocation concealment? | Follow-up (%) | Blind outcome assessment? | Overall quality rating | Overall external validity rating |
|---|---|---|---|---|---|---|---|---|---|---|
| Manipulation vs chemonucleolysis | ||||||||||
| 723 | Burton, 2000208 | 40 | 12 months | RCT | No | No | 60–79 | Yes | Moderate | Weak |
| Manipulation vs education/advice | ||||||||||
| 722 | Bronfort, 2004169 | 32 | 52 weeks | RCT | Unclear | Partial | 80–100 | Unclear | Weak | Weak |
| Manipulation vs epidural | ||||||||||
| 451 | Bronfort, 2000161 | 20 | 12 weeks | RCT | Unclear | Partial | 80–100 | NA | Moderate | Weak |
| 722 | Bronfort, 2004169 | 32 | 52 weeks | RCT | Unclear | Partial | 80–100 | Unclear | Weak | Weak |
| Manipulation vs inactive control | ||||||||||
| 52 | Santilli, 2006258 | 102 | 6 months | RCT | Yes | Yes | 80–100 | Yes | Strong | Strong |
| Manipulation vs non-opioids | ||||||||||
| 451 | Bronfort, 2000161 | 20 | 12 weeks | RCT | Unclear | Partial | 80–100 | NA | Moderate | Weak |
| Mixed treatment including spinal manipulation vs mixed treatment without | ||||||||||
| 687 | Zhang, 2005260 | 210 | 1 day | Non-RCT | No | No | 80–100 | Unclear | Weak | Weak |
Manipulation results at short-term follow-up (≤ 6 weeks)
Global effect at short-term follow-up
The results for the global effect at short-term follow-up are presented in Table 71 and the accompanying forest plot (Figure 49). There was no significant difference in the global effect in one good-quality RCT comparing chiropractic spinal manipulation with sham manipulation. 258 There was a significant improvement in global effect in one poor-quality non-RCT of massage, traction and spinal manipulation compared with digital stimulation of acupuncture points and traction. 260
| ID no. | Author, year | Chronicity | Study design | Follow-up | Outcome measure | Perspective | Intervention | Control | OR (95% CI) | Comments | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n) | Outcome (n) | Withdrawal rate | Total (n) | Outcome (n) | Withdrawal rate | |||||||||
| Manipulation vs inactive control | ||||||||||||||
| 52 | Santilli, 2006258 | A | RCT | 30 days | Becoming pain free – radiating leg pain | 53 | 12 | 0 | 49 | 6 | 0 | 2.10 (0.72 to 6.11) | Number randomised used as denominators by authors (two dropped out and four discontinued treatment) | |
| Mixed treatment including spinal manipulation vs mixed treatment without | ||||||||||||||
| 687 | Zhang, 2005260 | C | Non-RCT | 1 day | Remarkable effect on pain, SLR and analgesia score | 108 | 56 | 0 | 102 | 35 | 0 | 0.40 (0.20 to 0.78) | ||
FIGURE 49.
Summary of the findings of the global effect short-term follow-up (≤ 6 weeks) for studies comparing manipulation with alternative interventions. Note: weights are from random effects analysis.
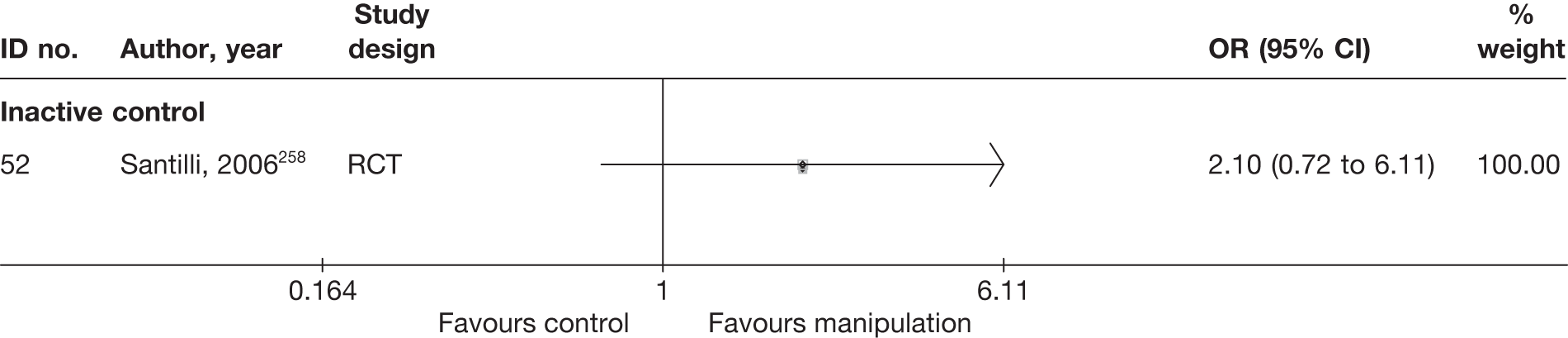
Pain intensity at short-term follow-up
The results for pain intensity at short-term follow-up are presented in Table 72 and the accompanying forest plot (Figure 50). There was no significant difference in pain intensity in one moderate-quality RCT comparing osteopathic spinal manipulation with chemonucleolysis. 208
| ID no. | Author, year | Chronicity | Study design | Follow-up | Location | Scale (range)a | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)b | Comment/conversionc | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |||||||||
| Manipulation vs chemonucleolysis | ||||||||||||||||
| 723 | Burton, 2000208 | A + C | RCT | 6 weeks | Leg | RMDQ annotated thermometer (0–6) | 19 | 18 | 66.67 (14.17) | 60.83 (26.5) | 44.67 (26.7) | 45.3 (17) | –0.63 (–14.98 to 13.72) | 3/40 (8%) dropped out: intervention 1/20, control 2/20 | ||
FIGURE 50.
Summary of the findings of pain intensity at short-term follow-up (≤ 6 weeks) for studies comparing manipulation with alternative interventions. Note: weights are from random effects analysis.
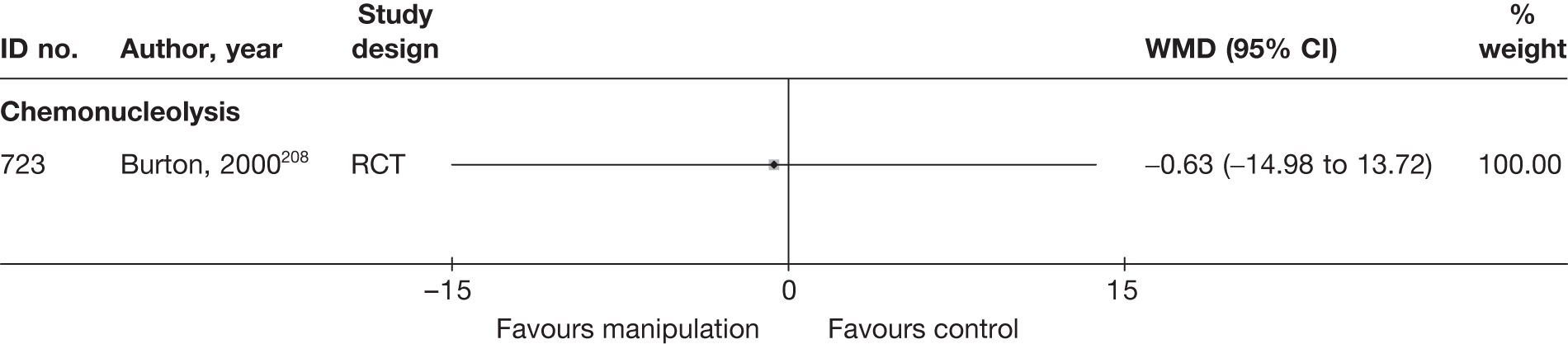
Condition-specific outcome measures at short-term follow-up
The results for CSOMs at short-term follow-up are presented in Table 73 and the accompanying forest plot (Figure 51). There was no significant difference in CSOMs in one moderate-quality RCT comparing osteopathic spinal manipulation with chemonucleolysis. 208
| ID no. | Author, year | Chronicity | Study design | Follow-up | Scale | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)a | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |||||||
| Manipulation vs chemonucleolysis | ||||||||||||||
| 723 | Burton, 2000208 | A + C | RCT | 6 weeks | RMDQ | 19 | 18 | 11.9 (5.48) | 11.95 (5.83) | 7.79 (6.65) | 11 (5.69) | –4.11 | –0.95 | –0.52 (–1.17 to 0.14) |
FIGURE 51.
Summary of the findings of CSOMs at short-term follow-up (≤ 6 weeks) for studies comparing manipulation with alternative interventions. Note: weights are from random effects analysis.
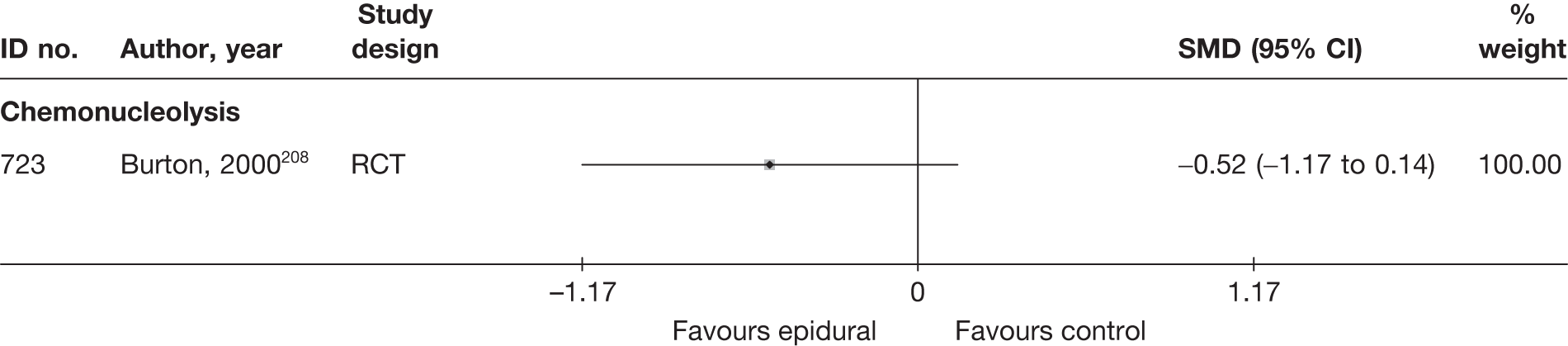
Manipulation results at medium-term follow-up (> 6 weeks to ≤ 6 months)
Global effect at medium-term follow-up
The results for the global effect at medium-term follow-up are presented in Table 74 and the accompanying forest plot (Figure 52). There was significant improvement in global effect in one good-quality RCT comparing chiropractic spinal manipulation with sham manipulation. 258
| ID no. | Author, year | Chronicity | Study design | Follow-up | Outcome measure | Perspective | Intervention | Control | OR (95% CI)a | Comments | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n) | Outcome (n) | Withdrawal rate | Total (n) | Outcome (n) | Withdrawal rate | |||||||||
| Manipulation vs inactive control | ||||||||||||||
| 52 | Santilli, 2006258 | A | RCT | 180 days | Becoming pain free – radiating leg pain | 53 | 29 | 0 | 49 | 10 | 0 | 4.71 (1.95 to 11.37) | Number randomised used as denominators by authors (two dropped out and four discontinued treatment) | |
FIGURE 52.
Summary of the findings of the global effect at medium-term follow-up (> 6 weeks to ≤ 6 months) for studies comparing manipulation with alternative interventions. Note: weights are from random effects analysis.
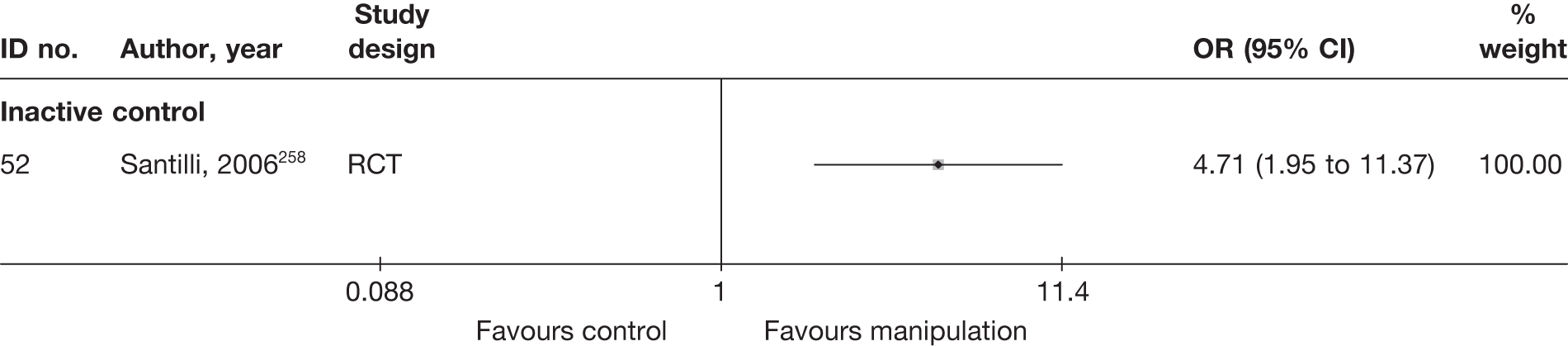
Pain intensity at medium-term follow-up
No study reported medium-term outcomes for pain intensity.
Condition-specific outcome measures at medium-term follow-up
No study reported medium-term outcomes for CSOMs.
Results at long-term follow-up (> 6 months)
Global effect at long-term follow-up
No study reported long-term outcomes for the global effect.
Pain intensity at long-term follow-up
The results for pain intensity at long-term follow-up are presented in Table 75 and the accompanying forest plot (Figure 53). There was no significant difference in pain intensity in one moderate-quality RCT comparing osteopathic spinal manipulation with chemonucleolysis. 208
| ID no. | Author, year | Chronicity | Study design | Follow-up | Location | Scale (range)a | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)b | Comment/conversionc | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |||||||||
| Manipulation vs chemonucleolysis | ||||||||||||||||
| 723 | Burton, 2000208 | A + C | RCT | 12 months | Leg | RMDQ annotated thermometer (0–6) | 15 | 15 | 66.67 (14.17) | 60.83 (26.5) | 35.5 (32) | 37.8 (29.2) | –2.30 (–24.22 to 19.62) | 10/40 (25%) dropped out: intervention 5/20, control 5/20 | ||
FIGURE 53.
Summary of the findings of pain intensity at long-term follow-up (> 6 months) for studies comparing manipulation with alternative interventions. Note: weights are from random effects analysis.
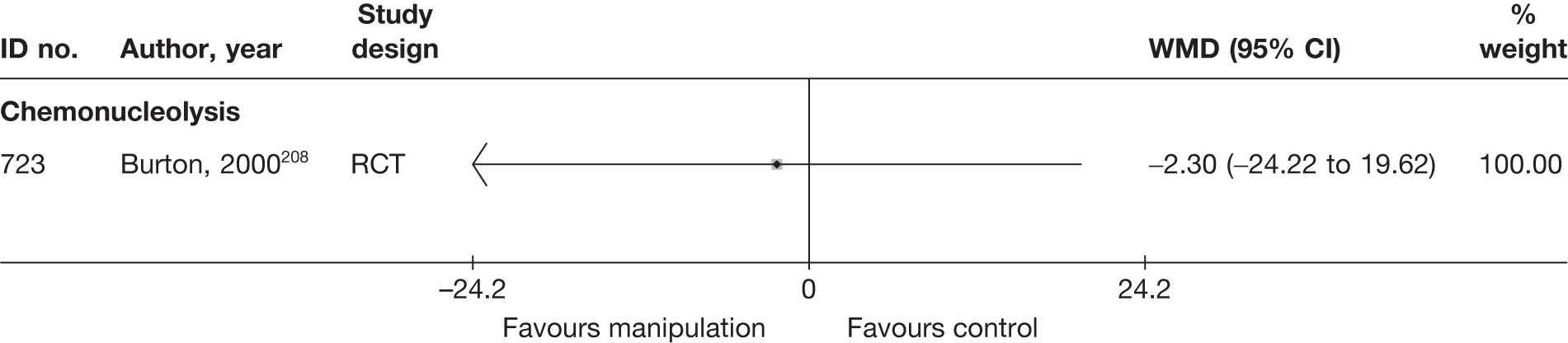
Condition-specific outcome measures at long-term
The results for CSOMs at long-term follow-up are presented in Table 76 and the accompanying forest plot (Figure 54). There was no significant difference in CSOMs in one moderate-quality RCT comparing osteopathic spinal manipulation with chemonucleolysis. 208
| ID no. | Author, year | Chronicity | Study design | Follow-up | Scale | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)a | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |||||||
| Manipulation vs chemonucleolysis | ||||||||||||||
| 723 | Burton, 2000208 | A + C | RCT | 6 weeks | RMDQ | 15 | 15 | 5.87 (5.96) | 7.27 (6.65) | 7.79 (6.65) | 11 (5.69) | –0.22 (–0.94 to 0.50) | ||
FIGURE 54.
Summary of the findings of CSOMs at long-term follow-up (> 6 months) for studies comparing manipulation with alternative interventions. Note: weights are from random effects analysis.
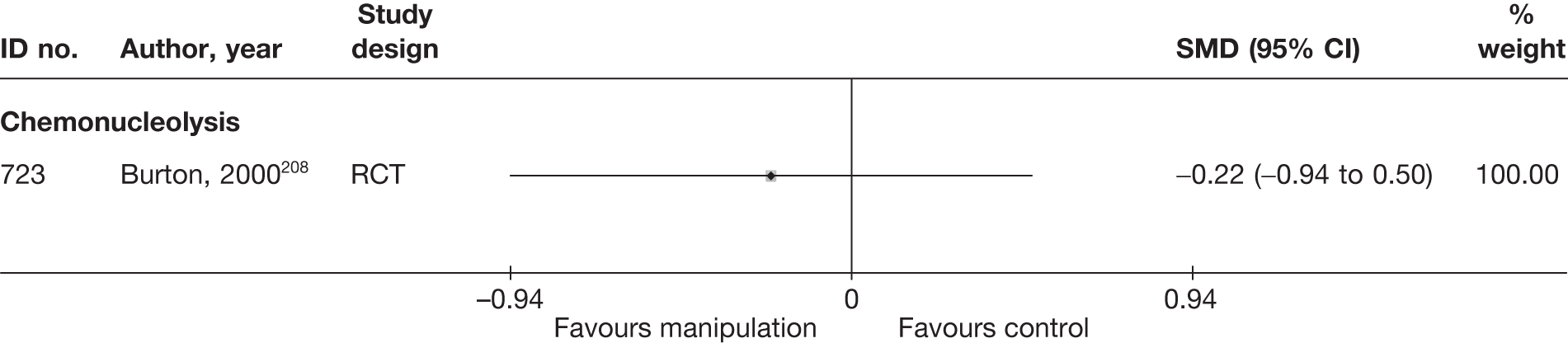
Analysis of adverse effects for spinal manipulation
The total number of adverse effects is presented in Table 77 and the accompanying forest plot (Figure 55). Significantly more adverse effects were associated with manipulation than with self-care education,169 but there was no significant difference compared with inactive control,258 epidural injections169 or chemonucleolysis. 208
| ID no. | Author, year | Study design | No. of events in intervention group | No. of participants in intervention group | No. of events in control group | No. of participants in control group | OR (95% CI) |
|---|---|---|---|---|---|---|---|
| Manipulation vs chemonucleolysis | |||||||
| 723 | Burton, 2000208 | RCT | 5 | 15 | 4 | 15 | 1.38 (0.29 to 6.60) |
| Manipulation vs education/advice | |||||||
| 722 | Bronfort, 2004169 | RCT | 6 | 11 | 0 | 10 | 24.82 (1.17 to 527.00) |
| Manipulation vs epidural injection | |||||||
| 451 | Bronfort, 2000208 | RCT | 3 | 7 | 6 | 6 | 0.60 (0.00 to 1.46) |
| 722 | Bronfort, 2004169 | RCT | 6 | 11 | 10 | 10 | 0.06 (0.00 to 1.20) |
| Manipulation vs inactive control | |||||||
| 52 | Santilli, 2006258 | RCT | 0 | 53 | 0 | 49 | |
| Manipulation vs non-opioids | |||||||
| 451 | Bronfort, 2000208 | RCT | 3 | 7 | 4 | 6 | 0.38 (0.04 to 3.61) |
| Mixed treatment including spinal manipulation vs mixed treatment without | |||||||
| 687 | Zhang, 2005260 | Non-RCT | NR | NR | NR | NR | |
FIGURE 55.
Summary of the findings of any adverse effect for studies comparing spinal manipulation with alternative interventions. Note: weights are from random effects analysis.
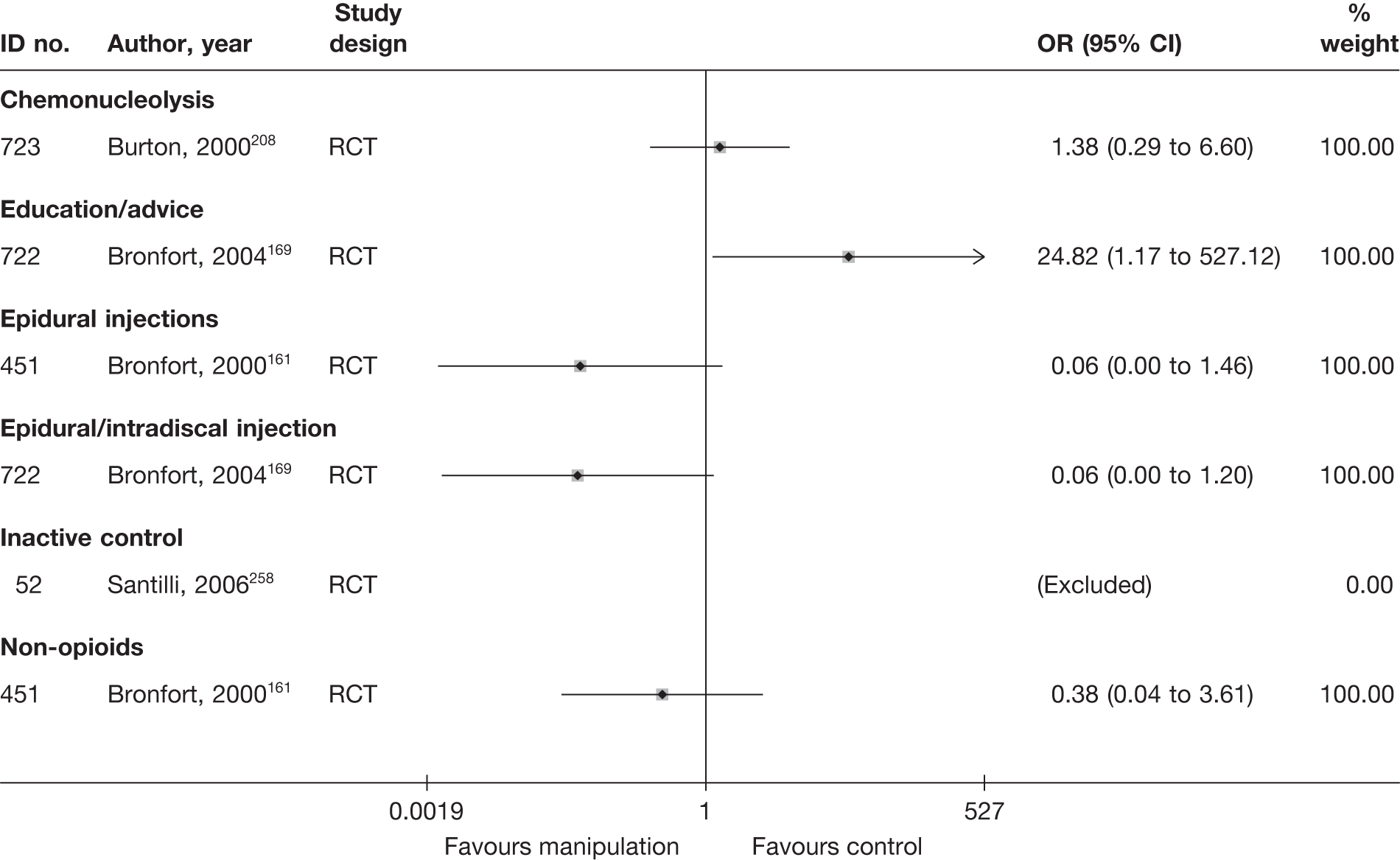
SUMMARY OF OVERALL FINDINGS FOR MANIPULATION COMPARED WITH ALTERNATIVE INTERVENTIONS
Two RCTs208,258 compared the use of manipulation with other interventions, one of which restricted inclusion to patients with acute sciatica (Table 78).
| Control category | No. of studies (arms) | Sample size range (median) | Proportion of studies that were RCTs (%) | Proportion of studies that were deemed good quality (%) | Proportion of studies that only included acute sciatica (%) | Proportion of studies that included patients with nerve root pain (%) | Proportion of studies that reported diagnosis confirmed by imaging (%) | Proportion of studies that included patients with stenosis (%) | Proportion of studies that included patients with extruded/sequestered discs (%) | Proportion of studies that only included patients with first episode (%) | Proportion of studies that included patients who had received previous treatment (%) | Proportion of studies that included patients who had received previous surgery (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Manipulation vs chemonucleolysis | 1 (1) | 40 (40) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 1/1 (100) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) |
| Manipulation vs inactive control | 1 (1) | 102 (102) | 1/1 (100) | 1/1 (100) | 1/1 (100) | 1/1 (100) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) |
| Total (for manipulation studies) | 2 (2) | 40–102 (71) | 2/2 (100) | 1/2 (50) | 1/2 (50) | 2/2 (100) | 2/2 (100) | 0/2 (0) | 0/2 (0) | 0/2 (0) | 0/2 (0) | 0/2 (0) |
There was a statistically significant improvement in medium-term (but not short-term) global effect in a good-quality RCT258 of chiropractic manipulation compared with sham manipulation. There was no significant difference in short- or long-term pain intensity, or in short-term CSOMs, in a moderate-quality RCT208 comparing osteopathic manipulation with chemonucleolysis.
Alternative therapies
Description of alternative therapy studies
Summary of interventions
Five studies evaluated alternative therapies for sciatica. 167,215,261–263 Three of these studies compared alternative therapy with an alternative intervention. 167,215,261 The types of interventions being compared are presented in Table 79a. One RCT compared acupuncture in correct acupuncture points with acupuncture in non-acupuncture points. One three-armed RCT215 compared warming acupuncture by burning moxa with injections of an herbal preparation anisodamine, and with an oral NSAID nimesolide. One three-armed CCS167 compared acupuncture and herbal medication with epidural injection of corticosteroid and local anaesthetic, and with epidural injection of local anaesthetic.
| ID no. | Author, year | Study design | Treatment description | Control description |
|---|---|---|---|---|
| Alternative vs epidural | ||||
| 667 | Wehling, 1997167 (German language) | CCS | Acupuncture and herbal medication | Nerve root blockade with local anaesthetic 5 ml mepivacaine twice a week for 5 weeks |
| 667 | Wehling, 1997167 (German language) | CCS | Acupuncture and herbal medication | Nerve root blockade with steroid triamcinolone 20 mg + local anaesthetic 5 ml mepivacaine twice a week for 5 weeks |
| Alternative vs inactive control | ||||
| 476 | Duplan, 1983261 (French language) | RCT | Acupuncture | Placebo (same acupuncture procedure but in non-acupuncture points) |
| Alternative vs non-opioids | ||||
| 801 | Chen, 2009215 | RCT | Warming acupuncture by burning moxa daily for 10 days (WAG) | Western medicine – oral nimesolide (NSAIDs) 2 g daily for 10 days (WMG) |
| 801 | Chen, 2009215 | RCT | Anisodamine (2 mg) point injections into acupoints daily for 10 days (PIG) | Western medicine – oral nimesolide (NSAIDs) 2 g daily for 10 days (WMG) |
Two studies compared different types of alternative therapy. 262,263 The types of alternative therapy compared are listed in Table 79b, but the findings of these studies are not considered any further.
| ID no. | Author, year | Study design | Treatment description | Control description |
|---|---|---|---|---|
| 533 | Khoromi, 2007262 | RCT (crossover) | Use of 200-g magnets in belts | Use of 50-g magnets in belts |
| 72 | Zhi, 1995263 | Non-RCT | Scalp acupuncture combined with single body acupoint using scalp needles | Body acupuncture alone using stainless steel needles |
Summary of study participants for alternative therapy
Summary data on the included participants are presented in Table 80. The three studies that compared alternative therapies with comparator treatments included 398 participants with mean ages between 35 and 40 years (70% men): two with acute symptom duration and one with chronic symptoms. Recurrent episodes were not reported. Sciatica was not confirmed by imaging in any of the studies. There were no patients with spinal stenosis or previous back surgery or sequestered discs.
| ID no. | Author, year | Study design | No. of patients | Age (years) | No. of men (%) | Symptom duration | Type of sciatica | Confirmed by imaging | Recurrent episode | Included patients with stenosis?a | Included patients with sequestered disc (or extruded)?a | Any previous treatment for sciatica? | Any previous back surgery for sciatica? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alternative vs epidural/intradiscal injection | |||||||||||||
| 667 | Wehling, 1997167 (German language) | CCS | 278 | NR | NR | ≤ 3 months | Nerve root pain and referred pain | Clinical | NR | No | No | NR | NR |
| Alternative vs inactive control | |||||||||||||
| 476 | Duplan, 1983261 (French language) | RCT | 30 | Mean 40 (SD 10) | 21 (70) | Mean 34 days (SD 15 days) | Nerve root pain and referred pain | Clinical | NR | No | No | Yes | No |
| Alternative vs non-opioids | |||||||||||||
| 801 | Chen, 2009215 | RCT | 90 | Mean 34.5 (SD 7.7) | 63 (70) | Mean 5.3 years (SD 4.14 years) | Nerve root pain | Clinical | NR | NR | NR | NR | NR |
Summary of study quality for alternative therapy studies
Study details are summarised in Table 81. Two of the studies were RCTs215,261 and none was of good quality. Neither an adequate method of random number generation nor a secure method of allocation concealment was recorded. No studies had good external validity.
| ID no. | Author, year | Study size | Overall follow-up | Study design | Adequate randomisation? | Allocation concealment? | Follow-up (%) | Blind outcome assessment? | Overall quality rating | Overall external validity rating |
|---|---|---|---|---|---|---|---|---|---|---|
| Alternative vs epidural/intradiscal injection | ||||||||||
| 667 | Wehling, 1997167 (German language) | 278 | 5 weeks | CCS | No | No | 80–100 | No | Weak | Weak |
| Alternative vs inactive control | ||||||||||
| 476 | Duplan, 1983261 (French language) | 30 | 5 days | RCT | Unclear | Unclear | 80–100 | Yes | Moderate | Moderate |
| Alternative vs non-opioids | ||||||||||
| 801 | Chen, 2009215 | 90 | 1 year | RCT | Unclear | Unclear | 80–100 | Unclear | Weak | Moderate |
Alternative therapy results at short-term follow-up (≤ 6 weeks)
Global effect at short-term follow-up
No study reported short-term outcomes for global effect.
Pain intensity at short-term follow-up
The results for pain intensity at short-term follow-up are presented in Table 82 and the accompanying forest plot (Figure 56). There was a significant improvement in pain intensity in a moderate-quality RCT of true acupuncture compared with needling non-acupuncture points261 and in a poor-quality RCT of oral NSAID compared with warming acupuncture by burning moxa. 215 There was no significant difference in pain intensity in a poor-quality CCS of acupuncture and herbal medication compared with epidural injection. 264
| ID no. | Author, year | Chronicity | Study design | Follow-up | Location | Scale (range)a | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)b | Comment/conversionc | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |||||||||
| Alternative vs epidural/intradiscal injections | ||||||||||||||||
| 667 | Wehling, 1997167 (German language) (i)d (steroid + LA) | C | CCS | 5 weeks | VAS (0–100) | 230 | 26 | –62 (28) | –66 (24) | 4.0 (10.18 to 18.18) | Results reported as percentage improvement (100% improvement = no pain; 0% pain reduction = pain the same as before treatment) | |||||
| 667 | Wehling, 1997167 (German language) (ii)d (LA) | C | CCS | 5 weeks | VAS (0–100) | 230 | 22 | –62 (28) | –48 (31) | –14.00 (–27.45 to –0.55) | Results reported as percentage improvement (100% improvement = no pain; 0% pain reduction = pain the same as before treatment) | |||||
| Alternative vs inactive control | ||||||||||||||||
| 476 | Duplan, 1983261 (French language) | A | RCT | 5 days | Overall | VAS (0–100) | 15 | 15 | 48 | 45 | 19 (21.51) | 44 (23.67) | –25.00 (–41.19 to –8.81) |
Mean percentage VAS score SD imputed from weighted average Dropouts not stated |
||
| Alternative vs non-opioids | ||||||||||||||||
| 801 | Chen, 2009215 (i)e (WAG) | C | RCT | 36 days (end of treatment) | Leg | Not stated | 30 | 30 | 1.56 (0.35) | 1.42 (0.37) | 5.74 (0.25) | 2.42 (0.33) | 3.32 (3.17 to 3.47) |
Outcome = improvement in clinical symptoms (scale and range not stated) Reported separately for: sciatica, lumbago, aggravated pain on coughing, aggravated pain on sneezing and aggravated pain on defecation |
||
| 801 | Chen, 2009215 (ii)e (PIG) | C | RCT | 36 days (end of treatment) | Leg | Not stated | 30 | 30 | 1.75 (0.32) | 1.42 (0.37) | 2.75 (0.32) | 2.42 (0.33) | 0.33 (0.17 to 0.49) |
Outcome = improvement in clinical symptoms (scale and range not stated) Reported separately for: sciatica, lumbago, aggravated pain on coughing, aggravated pain on sneezing and aggravated pain on defecation |
||
FIGURE 56.
Summary of the findings of pain intensity at short-term follow-up (≤ 6 weeks) for studies comparing alternative therapies with alternative interventions. Note: weights are from random effects analysis.
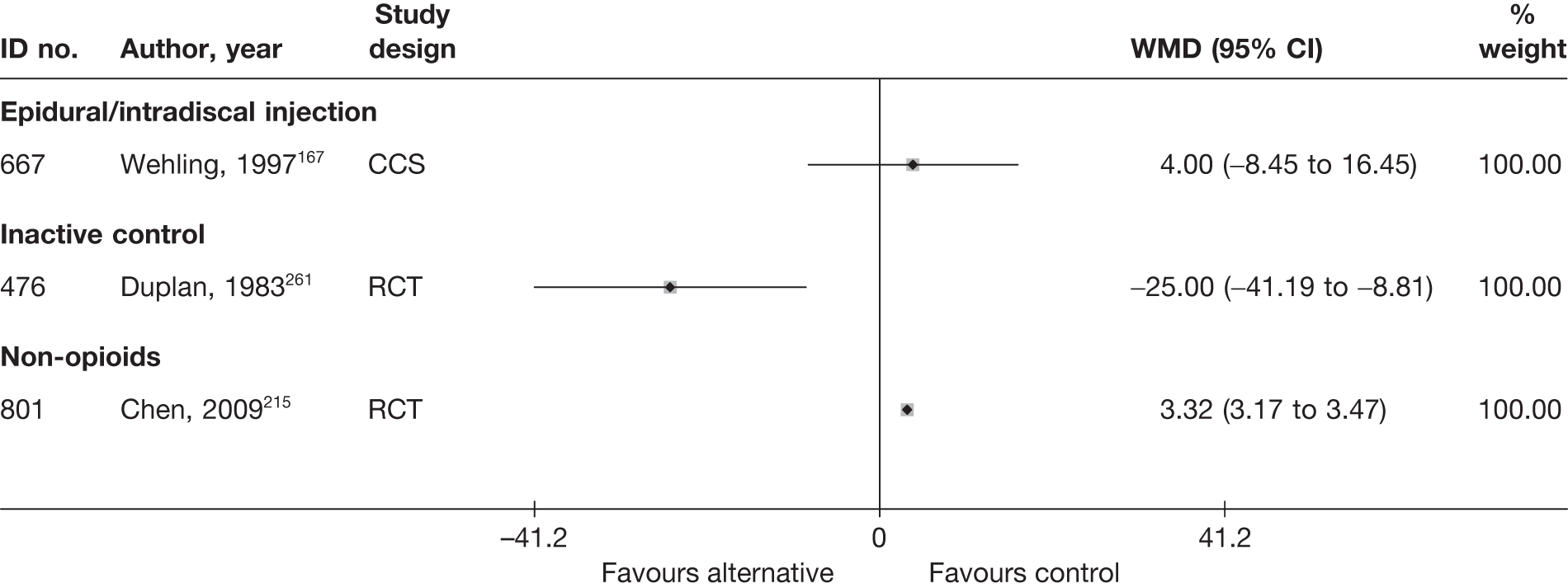
Condition-specific outcome measures at short-term follow-up
No study reported short-term CSOMs.
Alternative therapy results at medium-term follow-up (> 6 weeks to ≤ 6 months)
No study reported medium-term outcomes for global effect, pain intensity or CSOMs.
Alternative therapy results at long-term follow-up (> 6 months)
Global effect at long-term follow-up
The results for the global effect at long-term follow-up are presented in Table 83 and the accompanying forest plot (Figure 57). There was no significant difference in the global effect in one poor-quality RCT comparing warming acupuncture by burning moxa, or injections of an herbal preparation anisodamine, with an oral NSAID. 215
| ID no. | Author, year | Chronicity | Study design | Follow-up | Outcome measure | Perspective | Intervention | Control | OR (95% CI) | Comments | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n) | Outcome (n) | Withdrawal rate | Total (n) | Outcome (n) | Withdrawal rate | |||||||||
| Alternative vs non-opioids | ||||||||||||||
| 801 | Chen, 2009215 (i)a (WAG) | C | RCT | 1 year | Success: cured or improved (vs no improvement) | Patient | 30 | 27 | 0 | 30 | 22 | 0 | 3.27 (0.77 to 13.83) |
Data inferred from graphs reporting percentages ITT using worst-case analysis (with non-opioids as the control group) |
| 801 | Chen, 2009215 (ii)a (PIG) | C | RCT | 1 year | Success: cured or improved (vs no improvement) | Patient | 30 | 19 | 0 | 30 | 22 | 0 | 0.63 (0.21 to 1.88) |
Data inferred from graphs reporting percentages ITT using worst-case analysis (with non-opioids as the control group) |
FIGURE 57.
Summary of the findings of the global effect at long-term follow-up (> 6 months) for studies comparing alternative therapies with alternative interventions. Note: weights are from random effects analysis.
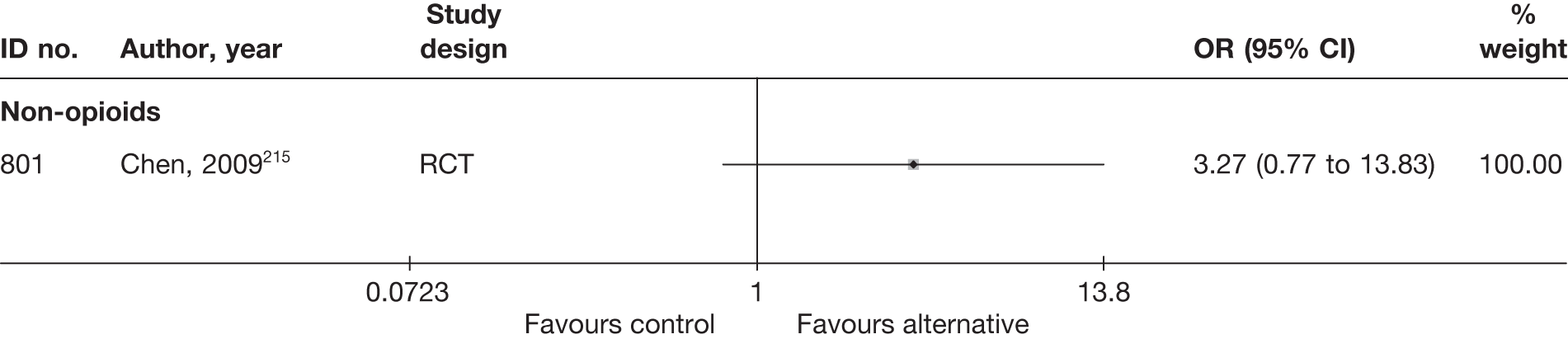
Pain intensity at long-term follow-up
No study reported long-term outcomes for pain intensity.
Condition-specific outcome measures at long-term follow-up
No study reported short-term CSOMs.
Analysis of adverse effects for alternative therapies
No adverse effects were reported in any of the studies (Table 84).
| ID no. | Author, year | Study design | No. of events in intervention group | No. of participants in intervention group | No. of events in control group | No. of participants in control group |
|---|---|---|---|---|---|---|
| Alternative vs epidural injections | ||||||
| 667 | Wehling, 1997167 | RCT | NR | NR | NR | NR |
| 667 | Wehling, 1997167 | RCT | NR | NR | NR | NR |
| Alternative vs inactive control | ||||||
| 476 | Duplan, 1983261 | RCT | NR | NR | NR | NR |
| Alternative vs non-opioid | ||||||
| 801 | Chen, 2009215 | RCT | NR | NR | NR | NR |
| 801 | Chen, 2009215 | RCT | NR | NR | NR | NR |
SUMMARY OF OVERALL FINDINGS FOR ALTERNATIVE INTERVENTIONS COMPARED WITH COMPARATOR INTERVENTIONS
Three studies,167,215,261 two of which were RCTs,215,261 compared the use of acupuncture with other interventions (Table 85).
| Control category | No. of studies (arms) | Sample size range (median) | Proportion of studies that were RCTs (%) | Proportion of studies that were deemed good quality (%) | Proportion of studies that only included acute sciatica (%) | Proportion of studies that included patients with nerve root pain (%) | Proportion of studies that reported diagnosis confirmed by imaging (%) | Proportion of studies that included patients with stenosis (%) | Proportion of studies that included patients with extruded/sequestered discs (%) | Proportion of studies that only included patients with first episode (%) | Proportion of studies that included patients who had received previous treatment (%) | Proportion of studies that included patients who had received previous surgery (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alternative vs epidural/intradiscal injection | 1 (2) | 278 (278) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 1/1 (100) | 0/1 (0) |
0/1 (0) |
0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) |
| Alternative vs inactive control | 1 (1) | 30 (30) | 1/1 (100) | 0/1 (0) | 1/1 (100) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 1/1 (100) | 0/1 (0) |
| Alternative vs non-opioids | 1 (2) | 90 (90) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) |
| Total (for alternative therapies) | 3 (4) | 30–278 (90) | 2/3 (67) | 0/3 (0) | 1/3 (33) | 3/3 (100) | 0/3 (0) | 0/3 (0) | 0/3 (0) | 0/3 (0) | 1/3 (33) | 0/3 (0) |
There was a significant improvement in pain intensity in a moderate-quality RCT of true acupuncture compared with needling non-acupuncture points,261 but pain intensity was significantly worse in another poor-quality RCT215 comparing warming acupuncture by burning moxa, or injecting a herbal preparation into acupuncture points, with an oral NSAID. There was no significant difference in pain intensity in a poor-quality CCS167 of acupuncture and herbal medication compared with epidural injection.
Active physical therapy/exercise therapy
Description of exercise therapy studies
Summary of interventions
Six studies compared active physical/exercise therapy with an alternative type of intervention for sciatica. 68,242,255,256,264,265 Summary data of the interventions used are presented in Table 86. One crossover RCT265 compared a 4-week course of lumbar-stabilising exercise with no exercise. One three-arm RCT256 compared massage, hot packs and exercise with hot packs and rest or with pelvic traction and strengthening exercises. One RCT68 compared exercise therapy alone with disc surgery plus exercise therapy. One RCT255 compared an extension-orientated treatment including exercise, mobilisation and education with lumbar traction plus the extension-orientated treatment approach. One RCT242 compared isometric exercises with manual traction. One RCT266 compared physiotherapy plus GP care with GP care alone.
| ID no. | Author, year | Study design | Treatment description | Control description |
|---|---|---|---|---|
| Exercise therapy vs activity restriction | ||||
| 564 | Lidstrom, 1970256 | RCT | Massage, hot packs and exercise (conventional treatment) | Hot packs and rest (control group) |
| Exercise therapy vs disc surgery | ||||
| 300 | Osterman, 200668 | RCT | Exercise therapy (conservative treatment) | Microdiscectomy and exercise therapy (surgery) |
| Exercise therapy vs inactive control | ||||
| 429 | Bakhtiary, 2005265 | RCT (crossover) |
4 weeks of lumbar-stabilising exercise followed by a 4 weeks of no exercise (group A) Only 4-week outcomes used |
4 weeks of no exercise followed by 4 weeks of lumbar-stabilising exercise (group B) Only 4-week outcomes used |
| Exercise therapy vs mixed treatment | ||||
| 395 | Fritz, 2007255 | RCT | Extension-oriented treatment approach (exercises, mobilisation and education) only | Traction and extension-oriented treatment approach |
| 564 | Lidstrom, 1970256 | RCT | Hot packs, massage, mobilising exercise and strengthening exercises | Traction and strengthening exercises |
| Exercise therapy vs traction | ||||
| 570 | Ljunggren, 1992242 | RCT | Isometric exercises | Manual traction |
| Exercise therapy vs usual/conventional care | ||||
| 742 | Luijsterburg, 2008264 | RCT | General practitioner care plus PT | General practitioner care |
Summary of study participants for active physical therapy/exercise therapy
Summary data on the included participants are presented in Table 87. The six trials included 305 participants with mean ages between 32 and 42 years; between 44% and 61% were men; and, three with acute and chronic symptom duration and three with chronic symptoms. Two RCTs included participants with first and recurrent episodes of sciatica, but this was not reported in the remainder. Sciatica was confirmed by imaging in three trials. There were no patients with spinal stenosis, or previous back surgery, and one RCT included patients with sequestered discs.
| ID no. | Author, year | Study design | No. of patients | Age (years) | No. of men (%) | Symptom duration | Type of sciatica | Confirmed by imaging? | Recurrent episode | Included patients with stenosis?a | Included patients with sequestered disc (or extruded)?a | Any previous treatment for sciatica? | Any previous back surgery for sciatica? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exercise therapy vs activity restriction | |||||||||||||
| 564 | Lidstrom, 1970256 | RCT | 62 | Range 21–61 | 29 (47) | > 1 year 52% | Nerve root pain and referred pain | No | NR | No | No | NR | NR |
| Exercise therapy vs disc surgery | |||||||||||||
| 300 | Osterman, 200668 | RCT | 57 | Mean 38 (SD 7) | 34 (61) | Mean 68.5 days (SD 27 days) | Nerve root pain | Yes | Recurrent and First episode | No | Yes | NR | No |
| Exercise therapy vs inactive control | |||||||||||||
| 429 | Bakhtiary, 2005265 | RCT (crossover) | 60 | Mean 32 (SD 5.79) | Not reported | Mean 3.95 months (SD 1.30 months) | NR | Yes | NR | No | No | NR | NR |
| Exercise therapy vs mixed treatment | |||||||||||||
| 395 | Fritz, 2007255 | RCT | 64 | Mean 41.1 (SD 9.8; range 18–60) | 28 (44) | Median 47.5 days (range 2–761 days) | Nerve root pain | No | 49 (77%) had prior history of low back pain | No | No | NR | No |
| 564 | Lidstrom, 1970256 | RCT | 62 | Range 21–61 | 29 (47) | > 1 year 52% | Nerve root pain and referred pain | No | NR | No | No | NR | NR |
| Exercise therapy vs traction | |||||||||||||
| 570 | Ljunggren, 1992242 | RCT | 50 | Mean 41.6 (range 19–62) | 27 (54) | Mean 5 months | Nerve root pain | Yes | NR | No | No | NR | No |
| Exercise therapy vs usual/conventional care | |||||||||||||
| 742 | Luijsterburg, 2008264 | RCT | 135 | Mean 43 (SD 11) | 70 (52) | > 6 weeks | Nerve root pain | No | NR | No | No | NR | No |
Summary of study quality for active physical therapy/exercise therapy
Study details are summarised in Table 88. All of the studies were RCTs and one was of good quality. 266 Four had an adequate method of random number generation and two documented a secure method of allocation concealment. One study had good external validity. 266
| ID no. | Author, year | Study size | Overall follow-up | Study design | Adequate randomisation? | Allocation concealment? | Follow-up (%) | Blind outcome assessment? | Overall quality rating | Overall external validity rating |
|---|---|---|---|---|---|---|---|---|---|---|
| Exercise therapy vs activity restriction | ||||||||||
| 564 | Lidstrom, 1970256 | 62 | 1 month | RCT | Unclear | Unclear | 80–100 | Unclear | Weak | Weak |
| Exercise therapy vs disc surgery | ||||||||||
| 300 | Osterman, 200668 | 57 | 2 years | RCT | Yes | Yes | 80–100 | NA | Moderate | Weak |
| Exercise therapy vs inactive control | ||||||||||
| 429 | Bakhtiary, 2005265 | 60 | 8 weeks | RCT | Yes | Partial | 80–100 | Yes | Moderate | Weak |
| Exercise therapy vs mixed treatments | ||||||||||
| 395 | Fritz, 2007255 | 64 | 6 weeks | RCT | Yes | Partial | 80–100 | Partial | Moderate | Weak |
| 564 | Lidstrom, 1970256 | 62 | 1 month | RCT | Unclear | Unclear | 80–100 | Unclear | Weak | Weak |
| Exercise therapy vs traction | ||||||||||
| 570 | Ljunggren, 1992242 | 50 | 1 week | RCT | Unclear | Unclear | 80–100 | Yes | Moderate | Weak |
| Exercise therapy vs usual/conventional care | ||||||||||
| 742 | Luijsterburg, 2008264 | 135 | 12 months | RCT | Yes | Yes | 80–100 | NA | Strong | Strong |
Active physical therapy/exercise therapy results at short-term follow-up (≤ 6 weeks)
Global effect at short-term follow-up
The results for the global effect at short-term follow-up are presented in Table 89 and the accompanying forest plot (Figure 58). There was no significant difference in global effect in a moderate-quality RCT comparing isometric exercises with manual traction;242 a moderate-quality RCT comparing exercise therapy alone with disc surgery plus exercise therapy;68 a poor-quality RCT comparing massage, hot packs and exercise with hot packs and rest;256 a moderate-quality RCT comparing exercise, mobilisation and education with extension-orientated approach and traction;255 and a good-quality RCT comparing general practitioner care and PT with GP care. 266 In a poor-quality RCT, there was a significant improvement in global effect with pelvic traction and strengthening exercises compared with massage, hot packs and exercise. 256
| ID no. | Author, year | Chronicity | Study design | Follow-up | Outcome measure | Perspective | Intervention | Control | OR (95% CI) | Comments | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n) | Outcome (n) | Withdrawal rate | Total (n) | Outcome (n) | Withdrawal rate | |||||||||
| Exercise therapy vs activity restriction | ||||||||||||||
| 564 | Lidstrom, 1970256 (ii)a (Rest) | A + C | RCT | 1 month | Noticeable improvement (vs no change or worse) | Patient | 21 | 10 | 0 | 21 | 14 | 0 | 0.45 (0.13 to 1.58) | |
| Exercise therapy vs disc surgery | ||||||||||||||
| 300 | Osterman, 200668 | A | RCT | 6 weeks | Full recovery | Patient | 28 | 0 | 0 | 28 | 5 | 0.03 | 0.07 (0.00 to 1.43) | |
| Exercise therapy vs mixed treatments | ||||||||||||||
| 395 | Fritz, 2007255 | A | RCT | 6 weeks | Improved: Likert-type scale rating > 2 (scale range –7 to + 7: worsened < –2; unchanged –2 to + 2) | Patient | 33 | 21 | 0 | 31 | 21 | 0 | 0.83 (0.30 to 2.34) | ITT using LOCF Dropouts 13%: intervention 3/33, control 5/31 |
| 564 | Lidstrom, 1970256 (i)a (traction + excercise) | A + C | RCT | 1 month | Noticeable improvement (vs no change or worse) | Patient | 21 | 10 | 0 | 20 | 18 | 0 | 0.10 (0.02 to 0.55) | |
| Exercise therapy vs traction | ||||||||||||||
| 570 | Ljunggren, 1992242 | C | RCT | 1 week | Global evaluation: symptom-free or satisfactory improvement (vs unsatisfactory improvement or unchanged) | Patient | 26 | 10 | 0 | 24 | 1 | 0 | 0.88 (0.28 to 2.72) | |
| Exercise therapy vs usual/conventional care | ||||||||||||||
| 742 | Luijsterburg, 2008264 | A | RCT | 6 weeks | Improved (on seven-point Likert scale): ‘completely recovered’ and ‘much improved’ (vs ‘slightly improved’, ‘not changed’, ‘slightly worsened’, ‘much worsened’ and ‘worse than ever’) | Patient | 67 | 38 | 0 | 68 | 30 | 0 | 1.66 (0.84 to 3.28) | ITT using LOCF Dropouts 4%: intervention 2/67, control 4/68 |
FIGURE 58.
Summary of the findings of the global effect at short-term follow-up (≤ 6 weeks) for studies comparing exercise therapy with alternative interventions. Note: weights are from random effects analysis.
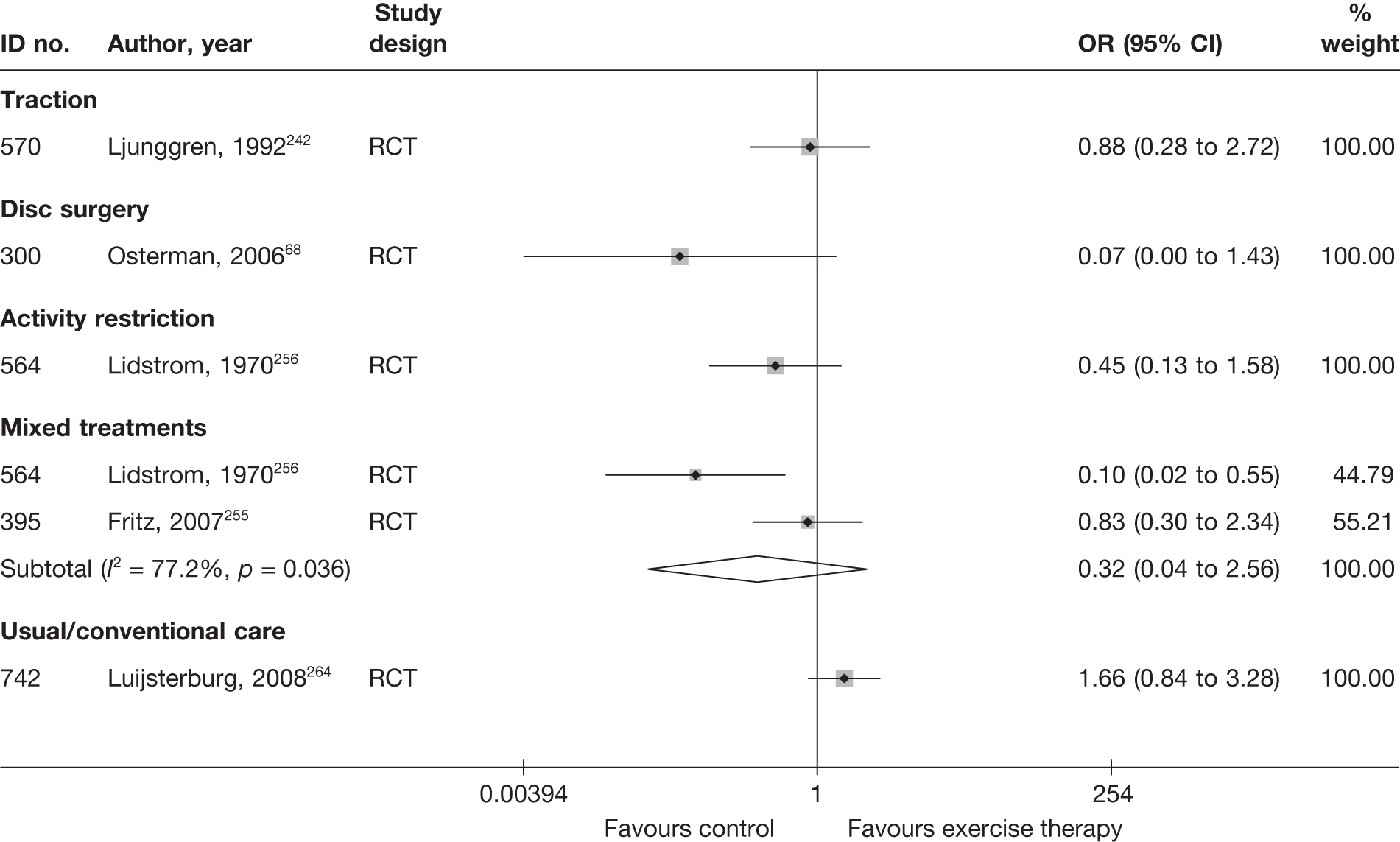
Pain intensity at short-term follow-up
The results for pain intensity at short-term follow-up are presented in Table 90 and the accompanying forest plot (Figure 59). There was a significant improvement in pain intensity in a moderate-quality crossover RCT of exercise therapy compared with inactive control,265 and in a moderate-quality RCT of disc surgery plus exercise therapy compared with exercise therapy alone. 68 In a good-quality RCT there was no significant difference in pain intensity with GP care and PT compared with GP care alone,266 or in a moderate-quality RCT of exercise, mobilisation and education compared with extension-orientated approach and traction. 255
| ID no. | Author, year | Chronicity | Study design | Follow-up | Location | Scale (range)a | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)b | Comment/conversionc | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |||||||||
| Exercise therapy vs disc surgery | ||||||||||||||||
| 300 | Osterman, 200668 | A | RCT | 6 weeks | Leg | VAS (0–100) | 28 | 28 | 57 (21) | 61 (20) | 25 (27) | 12 (20) | 13.00 (0.55 to 25.45) | Dropouts 2%: surgery 1/29 (did not meet inclusion criteria, excluded from analysis), exercise 0/28 | ||
| Exercise therapy vs inactive control | ||||||||||||||||
| 429 | Bakhtiary, 2005265 | A + C | RCT (crossover) | 4 weeks | Overall | VAS (0–10) | 30 | 30 | 42.9 (9) | 45 (11) | –32 (14.7) | –5 (11.7) |
–27.00 (–33.72 to –20.28) Mean difference –2.7 (–3.5 to –1.9), p < 0.0001 (two-way ANOVA) |
ITT, method not stated Dropouts 1%: intervention 3/30, control 3/30 This was a crossover study, where all patients received LSE or no exercise; however, the authors compared the outcomes of group A (LSE followed by no exercise) vs group B (no exercise followed by LSE) not LSE vs no exercise |
||
| Exercise therapy vs mixed treatments | ||||||||||||||||
| 395 | Fritz, 2007255 | A | RCT | 6 weeks | Overall | NRS (0–10) | 33 | 31 | 53.0 (15.0) | 50.0 (18.0) | 30.0 (24.0) | 32.0 (25.0) |
–2.00 (–14.02 to 10.02) Adjusted mean difference, ANCOVA –0.17 (95% CI –1.4 to 1.1) |
ITT using LOCF Dropouts 13%: intervention 3/33, control 5/31 |
||
| Exercise therapy vs usual/conventional care | ||||||||||||||||
| 742 | Luijsterburg, 2008264 | A | RCT | 6 weeks | Leg | NRS (0–10) | 67 | 68 | 63 (22) | 63 (22) | –30 (27) | –33 (28) |
3.00 (–6.28 to 12.28) Unadjusted mean difference 3 (95% CI –6 to 12) |
ITT using LOCF Dropouts 4%: intervention 2/67, control 4/68 |
||
FIGURE 59.
Summary of the findings of pain intensity at short-term follow-up (≤ 6 weeks) for studies comparing exercise therapy with alternative interventions. Note: weights are from random effects analysis.
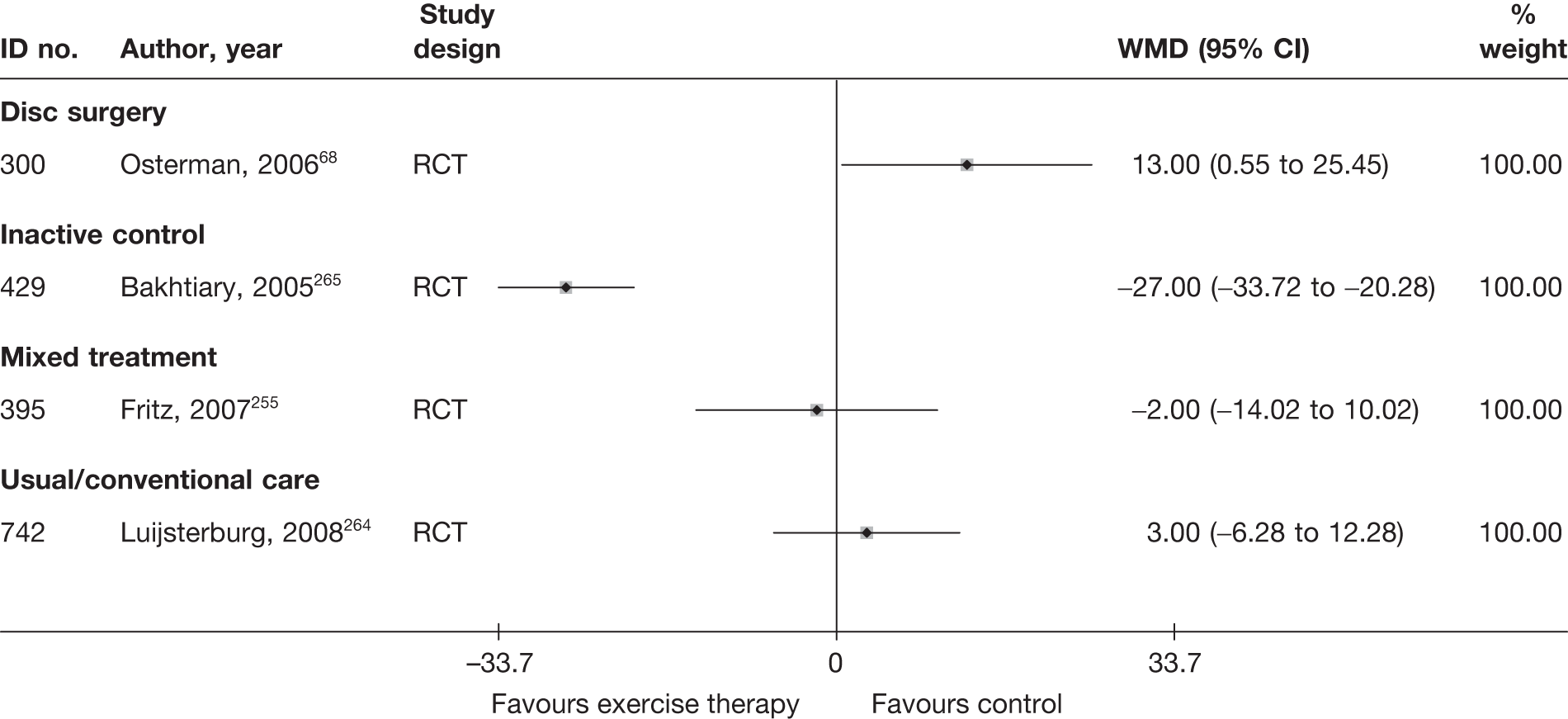
Condition-specific outcomes at short-term follow-up
The results for CSOMs at short-term follow-up are presented in Table 91 and the accompanying forest plot (Figure 60). There was no significant difference in CSOMs in a moderate-quality RCT of exercise therapy alone compared with disc surgery plus exercise therapy68 or in a moderate-quality RCT of exercise, mobilisation and education compared with extension-orientated approach and traction. 255 There was a marginal statistically significant improvement in a good-quality RCT of pain intensity for GP care alone compared with PT and GP care. 266
| ID no. | Author, year | Chronicity | Study design | Follow-up | Scale | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)a | Comment/conversionb | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | ||||||||
| Active PT/exercise therapy vs disc surgery | |||||||||||||||
| 300 | Osterman, 200668 | A | RCT | 6 weeks | ODI | 28 | 28 | 39 (14) | 39 (15) | 22 (16) | 16 (16) | –17 | –23 | 0.38 (–0.15 to 0.90) | ITT used LOCF, but one patient that did not meet inclusion criteria excluded from analysis |
| Active PT/exercise therapy vs mixed treatment | |||||||||||||||
| 395 | Fritz, 2007255 | A | RCT | 6 weeks | Modified ODI | 33 | 31 | 41.5 (10.7) | 46.1 (14.9) | 25.6 (19.9) | 28.3 (19.3) |
–0.14 (–0.63 to 0.35) Adjusted mean difference, ANCOVA: 1.8 (95% CI –6.4 to 10.1) |
ITT used LOCF Dropouts 13%: intervention 3/33, control 5/31 |
||
| Active PT/exercise therapy vs usual/conventional care | |||||||||||||||
| 742 | Luijsterburg, 2008264 | A | RCT | 6 weeks | RMDQ | 67 | 68 | 15.9 (4.1) | 15.4 (5) | 10.6 (4.1) | 8.8 (6.1) | –5.3 (7) | –6.6 (6.1) | 0.35 (0.01 to 0.69) | Final mean calculated from change score, final SD missing so baseline SD used |
FIGURE 60.
Summary of the findings of CSOMs at short-term follow-up (≤ 6 weeks) for studies comparing exercise therapy with alternative interventions. Note: weights are from random effects analysis.
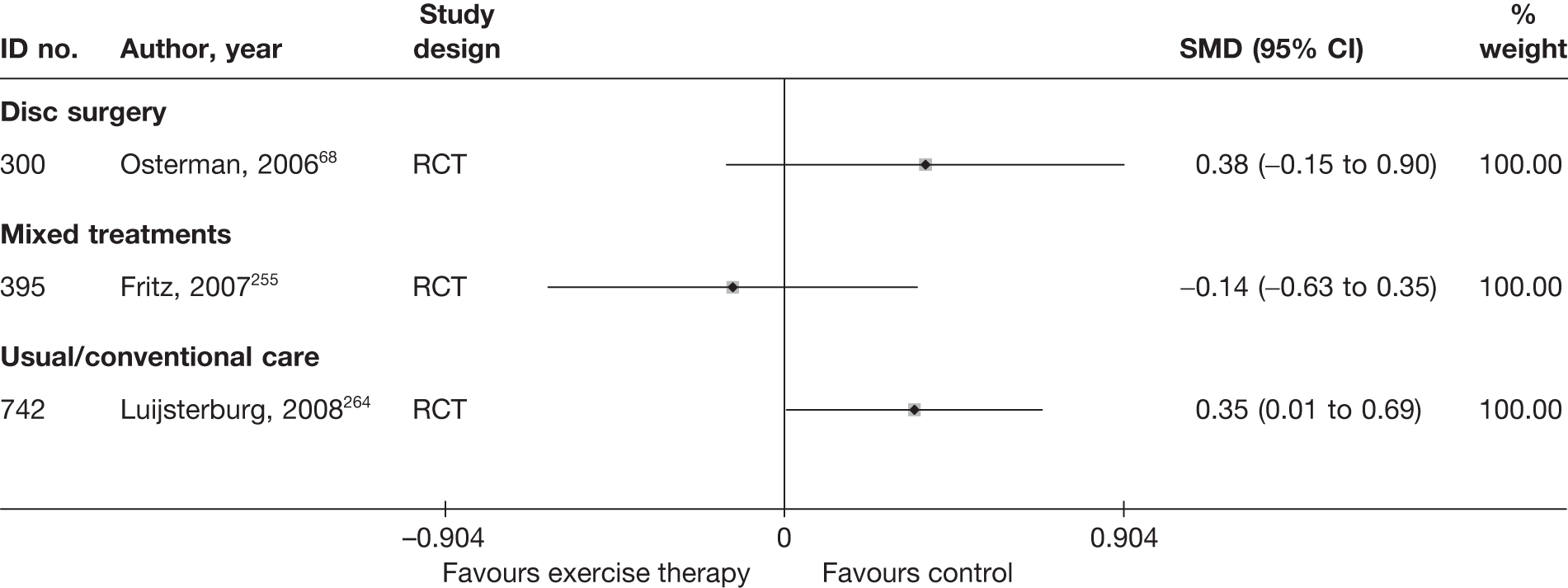
Active physical therapy/exercise therapy results at medium-term follow-up (> 6 weeks to ≤ 6 months)
Global effect at medium-term follow-up
The results for the global effect at medium-term follow-up are presented in Table 92 and the accompanying forest plot (Figure 61). In a moderate-quality RCT there was no significant difference in global effect with exercise therapy alone compared with disc surgery plus exercise therapy,68 or in a good-quality RCT of GP care and PT compared with GP care alone. 266
| ID no. | Author, year | Chronicity | Study design | Follow-up | Outcome measure | Perspective | Intervention | Control | OR (95% CI) | Comments | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n) | Outcome (n) | Withdrawal rate | Total (n) | Outcome (n) | Withdrawal rate | |||||||||
| Exercise therapy vs disc surgery | ||||||||||||||
| 300 | Osterman, 200668 | A | RCT | 6 months | Full recovery | Patient | 28 | 4 | 0 | 28 | 5 | 0 | 0.77 (0.18 to 3.22) |
ITT using LOCF Dropouts 12%: surgery 3/29 (one patient did not meet inclusion criteria, excluded from analysis), exercise 4/28 |
| Exercise therapy vs usual/conventional care | ||||||||||||||
| 742 | Luijsterburg, 2008264 | A | RCT | 12 weeks | Improved (seven-point Likert scale): ‘completely recovered’ and ‘much improved’ (vs ‘slightly improved’, ‘not changed’, ‘slightly worsened’, ‘much worsened’ and ‘worse than ever’) | Patient | 67 | 47 | 0 | 68 | 42 | 0 | 1.45 (0.71 to 2.98) | ITT using LOCF Dropouts 7%: intervention 3/67, control 6/68 |
FIGURE 61.
Summary of the findings of the global effect at medium-term follow-up (> 6 weeks to ≤ 6 months) for studies comparing exercise therapy with alternative interventions. Note: weights are from random effects analysis.
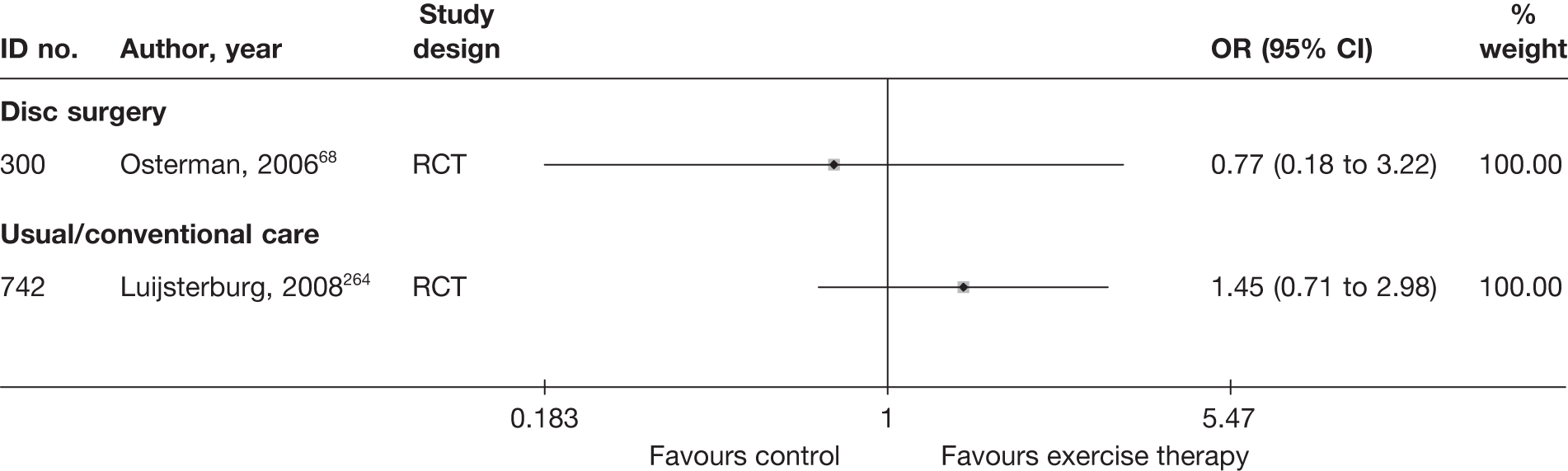
Pain intensity at medium-term follow-up
The results for pain intensity at medium-term follow-up are presented in Table 93 and the accompanying forest plot (Figure 62). There was no significant difference in pain intensity in a moderate-quality RCT of exercise therapy alone compared with disc surgery plus exercise therapy68 or in a good-quality RCT of GP care and PT compared with GP care alone. 266
| ID no. | Author, year | Chronicity | Study design | Follow-up | Location | Scale (range)a | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI) | Comment/conversionc | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |||||||||
| Exercise therapy vs disc surgery | ||||||||||||||||
| 300 | Osterman, 200668 | A | RCT | 6 months | Leg | VAS (0–100) | 28 | 28 | 57 (21) | 61 (20) | 18 (29) | 9 (20) | 9.00 (–4.05 to 22.05) |
ITT using LOCF Dropouts 12%: surgery 3/29 (one patient did not meet inclusion criteria, excluded from analysis), exercise 4/28 |
||
| Exercise therapy vs usual/conventional care | ||||||||||||||||
| 742 | Luijsterburg, 2008264 | A | RCT | 12 weeks | Leg | NRS (0–10) | 67 | 68 | 63 (22) | 63 (22) | –39 (28) | –37 (31) |
–2.00 (–11.96 to 7.96) Mean difference –0.2 (95% CI –1.2 to 0.8) |
ITT using LOCF Dropouts 7%: intervention 3/67, control 6/68 |
||
FIGURE 62.
Summary of the findings of pain intensity at medium-term follow-up (> 6 weeks to ≤ 6 months) for studies comparing exercise therapy with alternative interventions. Note: weights are from random effects analysis.
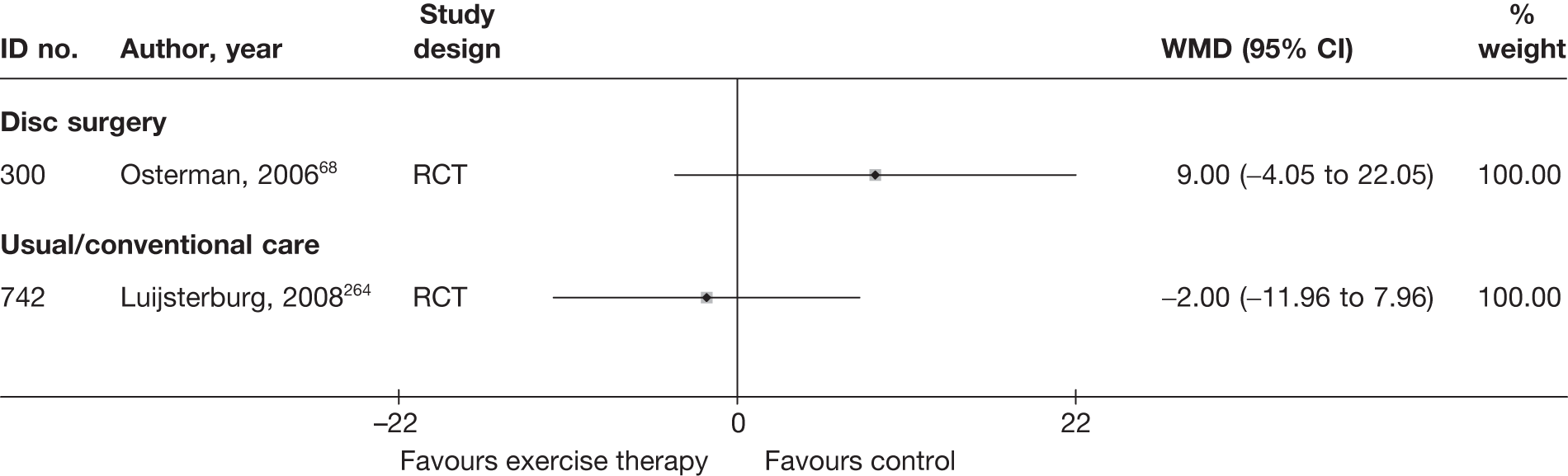
Condition-specific outcome measures at medium-term follow-up
The results for CSOMs at medium-term follow-up are presented in Table 94 and the accompanying forest plot (Figure 63). There was no significant difference in CSOMs in a moderate-quality RCT of exercise therapy alone compared with disc surgery plus exercise therapy68 or in a good-quality RCT of GP care and PT compared with GP care alone. 266
| ID no. | Author, year | Chronicity | Study design | Follow-up | Scale | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)a | Comment/conversionb | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | ||||||||
| Exercise therapy vs disc surgery | |||||||||||||||
| 300 | Osterman, 200668 | A | RCT | 6 months | ODI | 28 | 28 | 39 (14) | 39 (15) | 12 (15) | 8 (12) | –27 | –31 | 0.29 (–0.23 to 0.82) | ITT used LOCF, but one patient that did not meet inclusion criteria excluded from analysis |
| Exercise therapy vs usual/conventional care | |||||||||||||||
| 742 | Luijsterburg, 2008264 | A | RCT | 12 weeks | RMDQ | 67 | 68 | 15.9 (4.1) | 15.4 (5) | 8.2 (4.10) | 6.9 (5) | –7.7 (7.3) | –8.5 (6.7) | 0.28 (–0.05 to 0.62) |
Baseline SD used for final mean SD ITT used LOCF |
FIGURE 63.
Summary of the findings of CSOMs at medium-term follow-up (> 6 weeks to ≤ 6 months) for studies comparing exercise therapy with alternative interventions. Note: weights are from random effects analysis.
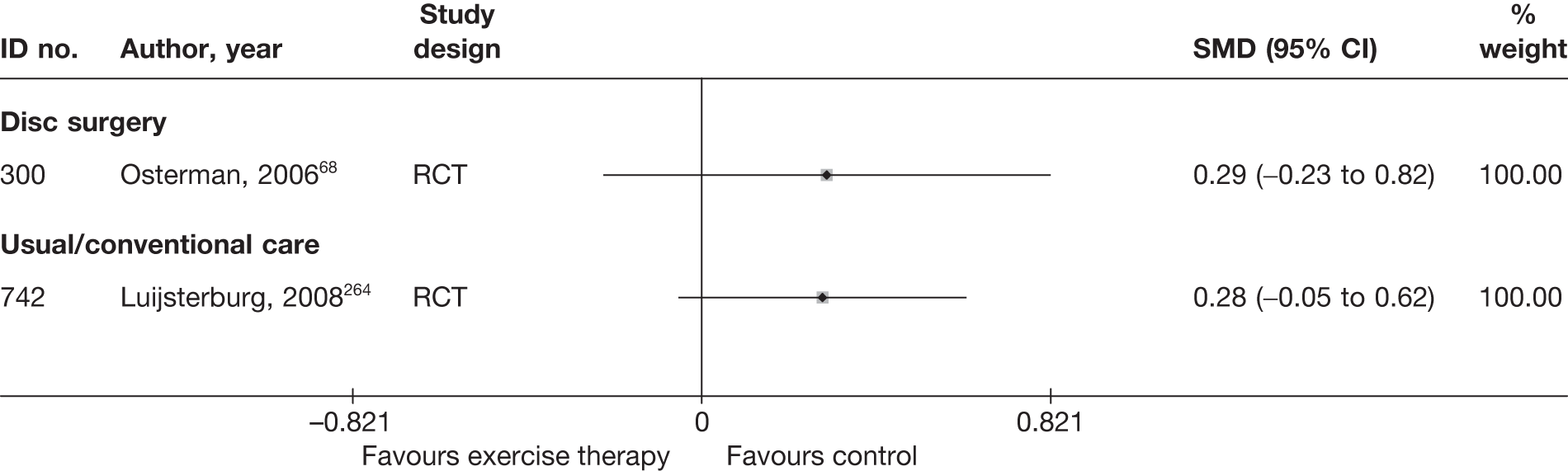
Active physical therapy results at long-term follow-up (> 6 months)
Global effect at long-term follow-up
The results for the global effect at long-term follow-up are presented in Table 95 and the accompanying forest plot (Figure 64). There was no significant difference in CSOMs in a moderate-quality RCT of exercise therapy alone compared with disc surgery plus exercise therapy. 68 There was a significant improvement for the global effect in a good-quality RCT of GP care and PT compared with GP care alone. 266
| ID no. | Author, year | Chronicity | Study design | Follow-up | Outcome measure | Perspective | Intervention | Control | OR (95% CI) | Comments | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n) | Outcome (n) | Withdrawal rate | Total (n) | Outcome (n) | Withdrawal rate | |||||||||
| Exercise therapy vs disc surgery | ||||||||||||||
| 300 | Osterman, 200668 | A | RCT | 2 years | Full recovery | Patient | 28 | 5 | 0 | 28 | 7 | 0.03 | 0.66 (0.18 to 2.37) |
ITT using LOCF Dropouts 12%: surgery 3/29 (one patient did not meet inclusion criteria, excluded from analysis), exercise 4/28 |
| Exercise therapy vs usual/conventional care | ||||||||||||||
| 742 | Luijsterburg, 2008264 | A | RCT | 52 weeks | Improved (seven-point Likert scale): ‘completely recovered’ or ‘much improved’ (vs ‘slightly improved’, ‘not changed’, ‘slightly worsened’, ‘much worsened’ or ‘worse than ever’) | Patient | 67 | 53 | 0 | 68 | 38 | 0 | 2.99 (1.40 to 6.38) |
ITT using LOCF Dropouts 13%: intervention 7/67, control 11/68 |
FIGURE 64.
Summary of the findings of the global effect at long-term follow-up (> 6 months) for studies comparing exercise therapy to alternative interventions. Note: weights are from random effects analysis.
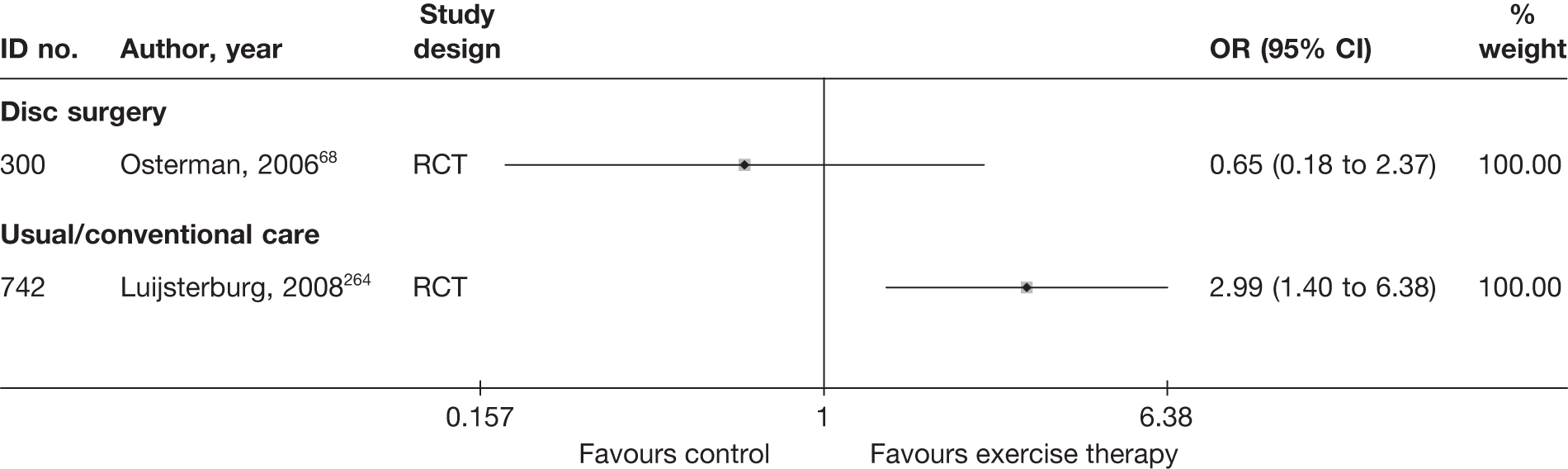
Pain intensity at long-term follow-up
The results for pain intensity at long-term follow-up are presented in Table 96 and the accompanying forest plot (Figure 65). There was no significant difference in pain intensity with exercise therapy alone compared with disc surgery plus exercise therapy68 or with GP care and PT compared with GP care alone. 266
| ID no. | Author, year | Chronicity | Study design | Follow-up | Location | Scale (range)a | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)b | Comment/conversionc | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |||||||||
| Exercise therapy vs disc surgery | ||||||||||||||||
| 300 | Osterman, 200668 | A | RCT | 2 years | Leg | VAS (0–100) | 28 | 28 | 57 (21) | 61 (20) | 15 (24) | 6 (11) | 9.00 (–0.78 to 18.78) |
ITT using LOCF Dropouts 12%: surgery 4/29 (one patient did not meet inclusion criteria, excluded from analysis), exercise 4/28 |
||
| Exercise therapy vs usual/conventional care | ||||||||||||||||
| 742 | Luijsterburg, 2008264 | A | RCT | 52 weeks | Leg | NRS (0–10) | 67 | 68 | 63 (22) | 63 (22) | –44 (27) | –37 (27) |
–7.00 (–16.11 to 2.11) Mean difference –0.7 (95% CI –1.7 to 0.2) |
ITT using LOCF Dropouts 13%: intervention 7/67, control 11/68 |
||
FIGURE 65.
Summary of the findings of pain intensity at long-term follow-up (> 6 months) for studies comparing exercise therapy with alternative interventions. Note: weights are from random effects analysis.
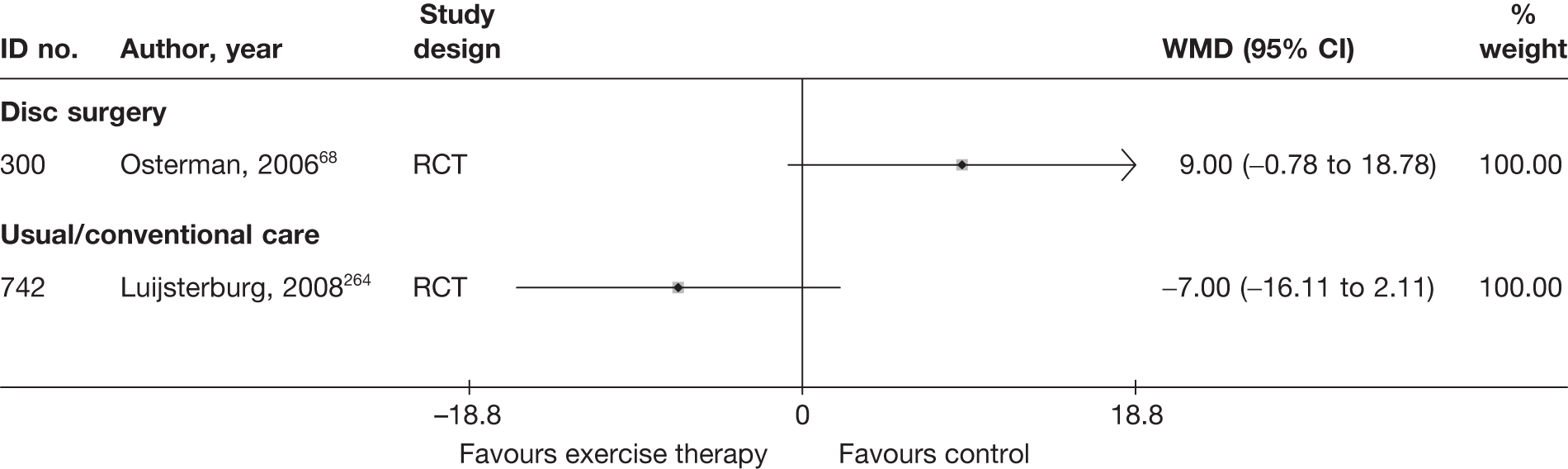
Condition-specific outcome measures at long-term follow-up
The results for CSOMs at long-term follow-up are presented in Table 97 and the accompanying forest plot (Figure 66). There was no significant difference in CSOMs in a moderate-quality RCT of exercise therapy alone compared with disc surgery plus exercise therapy,68 or in a good-quality RCT of GP care and PT compared with GP care alone. 266
| ID no. | Author, year | Chronicity | Study design | Follow-up | Scale | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)a | Comment/conversionb | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | ||||||||
| Exercise therapy vs disc surgery | |||||||||||||||
| 300 | Osterman, 200668 | A | RCT | 2 years | ODI | 28 | 28 | 39 (14) | 39 (15) | 11 (16) | 6 (9) | –28 | –33 | 0.39 (–0.14 to 0.91) | ITT used LOCF, but one patient that did not meet inclusion criteria excluded from analysis |
| Exercise therapy vs usual/conventional care | |||||||||||||||
| 742 | Luijsterburg, 2008264 | A | RCT | 52 weeks | RMDQ | 67 | 68 | 15.9 (4.1) | 15.4 (5) | 5.9 (4.1) | 6.3 (5) | –10 (6.5) | –9.1 (6.1) | 0.09 (–0.42 to 0.25) |
Final score calculated from change score No final SD, so baseline SD used ITT used LOCF |
FIGURE 66.
Summary of the findings of CSOMs at long-term follow-up (> 6 months) for studies comparing exercise therapy with alternative interventions. Note: weights are from random effects analysis.
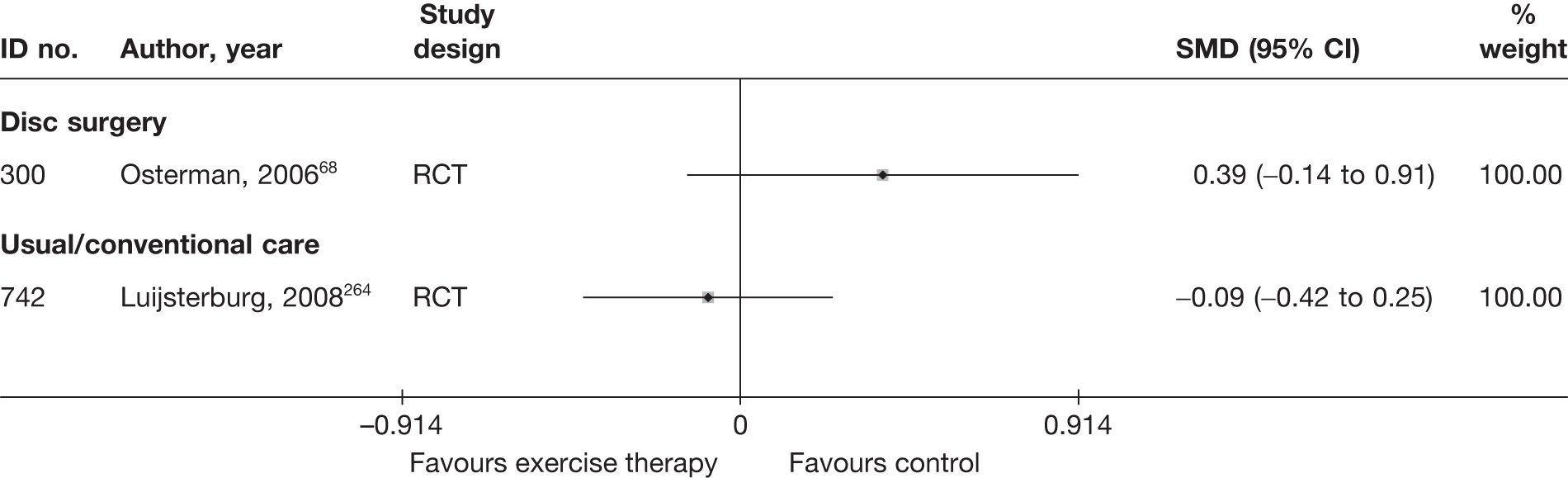
Adverse effects
The total number of adverse effects is presented in Table 98 and the accompanying forest plot (Figure 67). There was no significant difference between exercise therapy and disc surgery with exercise therapy,68 or between isometric exercises and manual traction. 242
| ID no. | Author, year | Study design | No. of events in intervention group | No. of participants in intervention group | No. of events in control group | No. of participants in control group | OR (95% CI) |
|---|---|---|---|---|---|---|---|
| Exercise therapy vs activity restriction | |||||||
| 429 | Bakhtiary, 2005265 | RCT (crossover) | NR | NR | NR | NR | |
| 564 | Lidstrom, 1970256 | RCT | NR | NR | NR | NR | |
| Exercise therapy vs activity restriction | |||||||
| 713 | Hofstee, 2002267 | RCT | 0 | 83 | 2 | 84 | 5.00 (0.24 to 100.00) |
| Exercise therapy vs disc surgery | |||||||
| 300 | Osterman, 200668 | RCT | 0 | 28 | 1 | 28 | 0.32 (0.01 to 8.24) |
| Exercise therapy vs mixed treatment | |||||||
| 564 | Lidstrom, 1970256 | RCT | NR | NR | NR | NR | |
| Exercise therapy vs traction | |||||||
| 570 | Ljunggren, 1992242 | RCT | 8 | 26 | 8 | 24 | 0.89 (0.27 to 2.92) |
| Exercise therapy vs usual care | |||||||
| 742 | Luijsterburg, 2008264 | RCT | NR | NR | NR | NR | |
FIGURE 67.
Summary of the findings of any adverse effect for studies comparing active PT with alternative interventions. Note: weights are from random effects analysis.
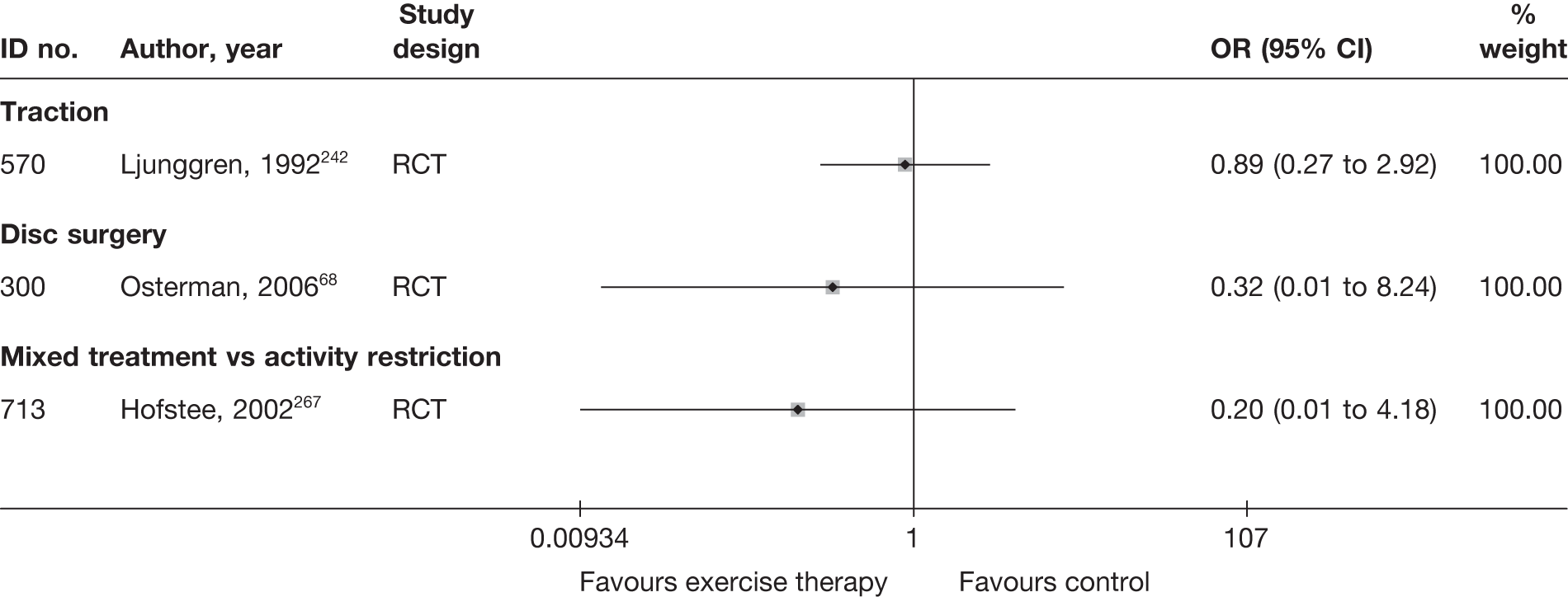
SUMMARY OF OVERALL FINDINGS FOR ACTIVE PHYSICAL/EXERCISE THERAPY COMPARED WITH ALTERNATIVE INTERVENTIONS
Six RCTs,68,242,255,256,264,265 one of which was a crossover trial,265 compared the use of active physical therapy with other interventions (Table 99).
| Control category | No. of studies (arms) | Sample size range (median) | Proportion of studies that were RCTs (%) | Proportion of studies that were deemed good quality (%) | Proportion of studies that only included acute sciatica (%) | Proportion of studies that included patients with nerve root pain (%) | Proportion of studies that reported diagnosis confirmed by imaging (%) | Proportion of studies that included patients with stenosis (%) | Proportion of studies that included patients with extruded/ sequestered discs (%) | Proportion of studies that only included patients with first episode (%) | Proportion of studies that included patients who had received previous treatment (%) | Proportion of studies that included patients who had received previous surgery (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exercise therapy vs activity restriction | 1 (1) | 62 (62) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) |
| Exercise therapy vs disc surgery | 1 (1) | 57 (57) | 1/1 (100) | 0/1 (0) | 1/1 (100) | 1/1 (100) | 1/1 (100) | 0/1 (0) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 0/1 (0) |
| Exercise therapy vs inactive control | 1 (1) | 60 (60) | 1/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) |
| Exercise therapy vs mixed treatment | 1 (1) | 62 (63) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) |
| Exercise therapy vs traction | 1 (1) | 50 (50) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 1/1 (100) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) |
| Exercise therapy vs usual/conventional care | 1 (1) | 135 (135) | 1/1 (100) | 1/1 (100) | 1/1 (100) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) |
| Total (for exercise therapy studies) | (6) (7) | 50–135 (62) | 6/6 (100) | 1/6 (17) | 3/6 (50) | 5/6 (83) | 3/6 (50) | 0/6 (0) | 1/6 (17) | 0/6 (0) | 0/6 (0) | 0/6 (0) |
One moderate-quality crossover RCT265 found that lumbar-stabilising exercises, compared with no exercise, resulted in a significant improvement in pain intensity in the short term. However, in another poor-quality RCT,256 massage, hot packs and exercise resulted in no significant difference in short-term global effect compared with hot packs and rest. In this same RCT, short-term global effect of massage, hot packs and exercise were worse than those of pelvic traction and strengthening exercises, but two other moderate-quality RCTs242,255 found no significant difference in short-term global effect between isometric exercises and traction, and no significant difference in short-term global effect, pain intensity or CSOMs between an extension-orientated treatment approach consisting of exercise, mobilisation and exercises and the extension-orientated treatment approach plus traction. In one good-quality RCT,266 PT plus GP care, compared with GP care alone, resulted in significantly worse short-term CSOMs and significantly better long-term global effect, but there was no significant difference at other follow-up periods or in pain intensity at any of the three follow-up periods. In one moderate-quality RCT,68 short-term pain intensity was significantly worse in the group that received exercise therapy than in the group treated with exercise therapy plus microdiscectomy, but there was no significant difference in pain intensity at medium- and long-term follow-up, or in the global effect or CSOMs at any of the three follow-up periods.
Passive physical therapy
Description of passive physical therapy studies
Summary of interventions
Six studies compared passive PT with an alternative type of intervention for sciatica. 155,176,249,253,268,269 Summary data of the interventions used are presented in Table 100a. Two of these studies also included more than two arms and both compared different types of passive PT (Table 100b). 249,268 One three-armed crossover RCT268 compared transcutaneous electrical nerve stimulation (TENS) with percutaneous electrical nerve stimulation (PENS) and with sham PENS. One three-armed RCT249 compared ultrasound treatment with a low-power laser and with lumbar traction. One RCT253 compared a PT programme (consisting of hot packs, ultrasound and diadynamic electric currents) with the PT programme and traction. One RCT176 compared infrared heat treatment with lumbar traction. One RCT155 compared conservative physiotherapy (no further details given) with epidural steroid and local anaesthetic injection. One non-RCT269 compared physiotherapy (no further details given) with ESI and active or passive PT.
| ID no. | Author, year | Study design | Treatment description | Control description |
|---|---|---|---|---|
| Passive PT vs epidural/intradiscal injection | ||||
| 359 | Veihelmann, 2006155 | RCT | Conservative physiotherapy | Epidural injection via epidural catheter (neuroplasty) of steroid triamcinolone 40 mg and ropivacaine |
| Passive PT vs inactive control | ||||
| 496 | Ghoname, 1999268 | RCT (crossover) | PENS | Sham PENS |
| 496 | Ghoname, 1999268 | RCT (crossover) | TENS | Sham PENS |
| Passive PT vs mixed treatment | ||||
| 354 | Bokonjic, 1975269 (German language) | Non-RCT | Physiotherapy alone | Three epidural injection of steroid dexa-neurobion every 4 days + active or passive PT |
| 266 | Ozturk, 2006253 (traction vs passive PT) | RCT | PT programme (control group) | Traction and PT programme (traction group) |
| Passive PT vs traction | ||||
| 9059 | Mathews, 1987176 | RCT | Infrared heat treatment | Lumbar traction |
| 148 | Unlu, 2008249 | RCT | Ultrasound treatment | Lumbar traction |
| 148 | Unlu, 2008249 | RCT | Low-power laser | Lumbar traction |
Summary of study participants in passive physical therapy studies
Summary data on the included participants are presented in Table 101. The six trials included 468 participants with mean ages between 31 and 46 years (30–60% men): one with acute symptom duration, three with chronic symptoms and two that did not report length of symptoms. One non-RCT included participants with first and recurrent episodes of sciatica, but this was not reported in the remainder. Sciatica was confirmed by imaging in five trials. There were no patients with spinal stenosis or sequestered discs and previous back surgery was excluded in two trials.
| ID no. | Author, year | Study design | No. of patients | Age (years) | No. of men (%) | Symptom duration | Type of sciatica | Confirmed by imaging? | Recurrent episode | Included patients with stenosis?a | Included patients with sequestered disc (or extruded)?a | Any previous treatment for sciatica? | Any previous back surgery for sciatica? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Passive PT vs epidural/intradiscal injection | |||||||||||||
| 359 | Veihelmann, 2006155 | RCT | 99 | Mean 44.5 (SD 24) | 45 (45) | NR | Nerve root pain | Yes | NR | No | No | Yes | Yes |
| Passive PT vs inactive control | |||||||||||||
| 496 | Ghoname, 1999268 | RCT (crossover) | 64 | Mean 43 (range ± 19) | 30 (47) | Mean 21 months (SD 9; range 6–28 months) | Nerve root pain and referred pain | Yes | NR | No | No | Yes | Yes |
| Passive PT vs mixed treatments | |||||||||||||
| 354 | Bokonjic, 1975269 (German language) | Non-RCT | 56 | Mean 31.1 | 23 (64) | NR | Nerve root pain and referred pain | Yes | Recurrent and first episode | No | No | NR | NR |
| 266 | Ozturk, 2005253 | RCT | 46 | Mean 46.2 (SD 10.2); range 16–70) | 22 (48) | Inclusion criteria ≥ 6 months | Nerve root pain | Yes | NR | No | No | NR | No |
| Passive PT vs traction | |||||||||||||
| 9059 | Mathews, 1987176 | RCT | 143 | Median 40 (range 20–60) | 80 (56) | Median 3.5 weeks (range 0 days–3 months) | Nerve root pain | No | NR | No | No | NR | NR |
| 148 | Unlu, 2008249 | RCT | 60 | Mean 44.5 (range 20–60) | 18 (30) | > 3 months | Nerve root pain | Yes | NR | No | No | NR | No |
Summary of study quality for passive physical therapy
Study details are summarised in Table 102. Five of the studies were RCTs (5/6, 83%) and none was of good quality. None had an adequate method of random number generation and only one documented a secure method of allocation concealment. 155 No studies had good external validity.
| ID no. | Author, year | Study size | Overall follow-up | Study design | Adequate randomisation? | Allocation concealment? | Follow-up (%) | Blind outcome assessment? | Overall quality rating | Overall external validity rating |
|---|---|---|---|---|---|---|---|---|---|---|
| Passive PT vs epidural/intradiscal injection | ||||||||||
| 359 | Veihelmann, 2006155 | 99 | 12 months | RCT | Partial | Yes | < 60 | Yes | Moderate | Weak |
| Passive PT vs inactive control | ||||||||||
| 496 | Ghoname, 1999268 | 64 | 11 weeks | RCT | Unclear | Unclear | Can’t tell | NA | Weak | Weak |
| Passive PT vs mixed treatments | ||||||||||
| 354 | Bokonjic, 1975269 (German language) | 56 | 12 days | Non-RCT | No | No | 80–100 | Unclear | Weak | Weak |
| 266 | Ozturk, 2006253 | 46 | 2 weeks | RCT | Unclear | Unclear | 80–100 | Unclear | Weak | Weak |
| Passive PT vs traction | ||||||||||
| 9059 | Mathews, 1987176 | 143 | 12 months | RCT | Partial | Unclear | < 60 | Yes | Moderate | Moderate |
| 148 | Unlu, 2008249 | 60 | 3 months | RCT | Unclear | Unclear | 80–100 | Yes | Moderate | Weak |
Passive physical therapy results at short-term follow-up (≤ 6 weeks)
Global effect at short-term follow-up
The results for the global effect at short-term follow-up are presented in Table 103 and the accompanying forest plot (Figure 68). There was a significant improvement in the global effect in the TENS or PENS group compared with inactive control in one poor-quality crossover RCT. 268 However, one poor-quality non-RCT found a significant improvement in the global effect when ESI was combined with active or passive PT compared with physiotherapy alone. 269 There was no significant difference in the global effect in one moderate-quality RCT comparing heat treatment with traction. 176
| ID no. | Author, year | Chronicity | Study design | Follow-up | Outcome measure | Perspective | Intervention | Control | OR (95% CI) | Comments | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n) | Outcome (n) | Withdrawal rate | Total (n) | Outcome (n) | Withdrawal rate | |||||||||
| Passive PT vs inactive control | ||||||||||||||
| 496 | Ghoname, 1999268 (i)a (PENS) | A + C | RCT (crossover) | 72 hours | ‘Improved sense of well being’ selected out of four subheadings (asked about treatment preference in crossover trial) | Patient | 64 | 42 | 0 | 64 | 5 | 0 | 22.53 (7.89 to 64.28) | |
| 496 | Ghoname, 1999268 (ii)a (TENS) | A + C | RCT (crossover) | 72 hours | ‘Improved sense of well being’ selected out of four subheadings (asked about treatment preference in crossover trial) | Patient | 64 | 17 | 0 | 64 | 5 | 0 | 4.27 (1.47 to 12.42) | |
| Passive PT vs mixed treatments | ||||||||||||||
| 354 | Bokonjic, 1975269 (German language) | NR | Non-RCT | 12 days | Improved = excellent or good (vs no change = moderate or poor) | 20 | 4 | 0 | 34 | 17 | 0.06 | 0.25 (0.07 to 0.90) | ||
| Passive PT vs traction | ||||||||||||||
| 9059 | Mathews, 1987176 | A | RCT | 2 weeks | Number of patients recovered (percentage). Pain score of 5 or 6 represented definite improvement and designated ‘recovered’, scores of 1–4 designated ‘not recovered’ | 54 | 27 | 0.10 | 77 | 40 | 0.07 | 0.93 (0.46 to 1.86) | Number of dropouts reported were different to the number missing from the analysis | |
FIGURE 68.
Summary of the findings of the global effect at short-term follow-up (≤ 6 weeks) for studies comparing passive PT with alternative interventions. Note: weights are from random effects analysis.
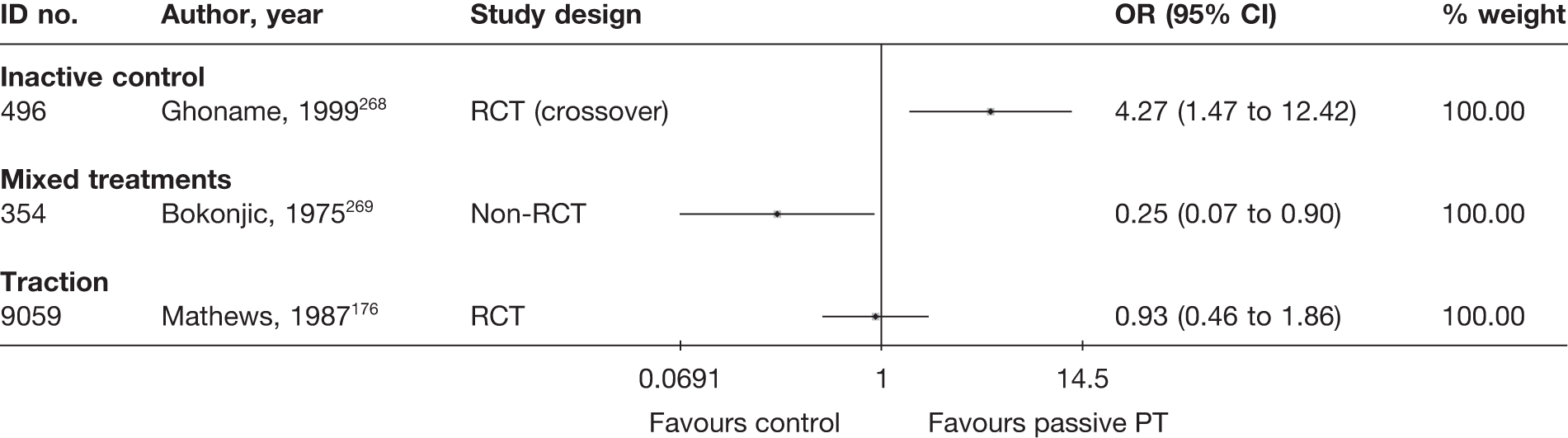
Pain intensity at short-term follow-up
The results for pain intensity at short-term follow-up are presented in Table 104 and the accompanying forest plot (Figure 69). There was a significant improvement in pain intensity in the groups receiving TENS or PENS compared with inactive control in one poor-quality non-RCT. 268 There was no significant difference in pain intensity in two moderate- or poor-quality RCTs comparing ultrasound or laser with traction249 or unspecified PT with PT and traction. 253
| ID no. | Author, year | Chronicity | Study design | Follow-up | Location | Scale (range)a | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)b | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | ||||||||
| Passive PT vs inactive control | |||||||||||||||
| 496 | Ghoname, 1999268 (i)c (PENS) | A + C | RCT (crossover) | 72 hours | VAS (0–10) | 64 | 64 | 72 (18) | 66 (19) | 41 (14) | 61 (19) | –20.00 (–25.78 to –14.22) | |||
| 496 | Ghoname, 1999268 (ii)c (TENS) | A + C | RCT (crossover) | 72 hours | Leg | VAS (0–10) | 28 | 28 | 70 (19) | 66 (19) | 54 (19) | 61 (19) | –7.00 (–13.58 to –0.42) | ||
| Passive PT vs traction | |||||||||||||||
| 148 | Unlu, 2008249 (i)d (ultrasound) | A | RCT | 1 month | Leg | VAS (0–100) | 20 | 20 | 56.0 (15.3) | 59.6 (15.4) | 26.8 (18.6) | 21.8 (15.4) | 5.00 (–5.58 to 15.58) | ||
| 148 | Unlu, 2008249 (ii)d (laser) | A | RCT | 1 month | Leg | VAS (0–100) | 20 | 20 | 53.1 (25.9) | 59.6 (15.4) | 25.6 (21.1) | 21.8 (15.4) | 3.80 (–7.65 to 15.25) | ||
| Passive PT vs mixed treatments | |||||||||||||||
| 266 | Ozturk, 2006253 (traction + PT vs PT) | NR | RCT | 15 days | Overall | VAS (0–10) | 22 | 24 | 68 (11) | 63 (14) | 36 (27) | 24 (17) | 12.00 (–1.17 to 25.17) | ||
FIGURE 69.
Summary of the findings of pain intensity at short-term follow-up (≤ 6 weeks) for studies comparing passive PT with alternative interventions. Note: weights are from random effects analysis.
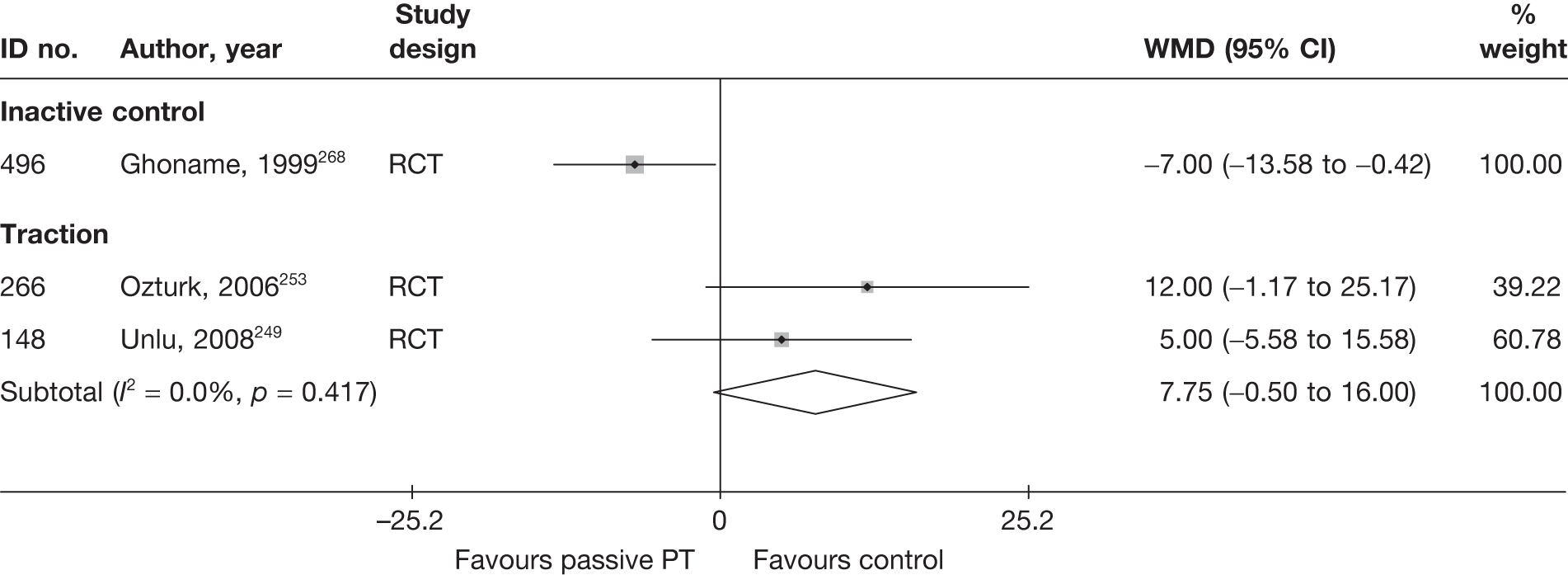
Condition-specific outcome measures at short-term follow-up
The results for CSOMs at short-term follow-up are presented in Table 105 and the accompanying forest plot (Figure 70). There was no significant difference in CSOMs in one moderate-quality RCT comparing ultrasound or laser with traction. 249
| ID no. | Author, year | Chronicity | Study design | Follow-up | Scale | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)a | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |||||||
| Passive PT vs traction | ||||||||||||||
| 148 | Unlu, 2008249 (i)b (ultrasound) | A | RCT | 1 month | RMDQ | 20 | 20 | 13.4 (4.5) | 14.2 (4.3) | 8.2 (6) | 8.5 (3.5) | –5.2 | –5.7 | –0.06 (–0.68 to 0.56) |
| 148 | Unlu, 2008249 (ii)b (laser) | A | RCT | 1 month | RMDQ | 20 | 20 | 12.5 (5) | 14.2 (4.3) | 7.3 (4.3) | 8.5 (3.5) | –5.2 | –5.7 | –0.31 (–0.93 to 0.32) |
FIGURE 70.
Summary of the findings of CSOMs at short-term follow-up (≤ 6 weeks) for studies comparing passive PT with alternative interventions. Note: weights are from random effects analysis.
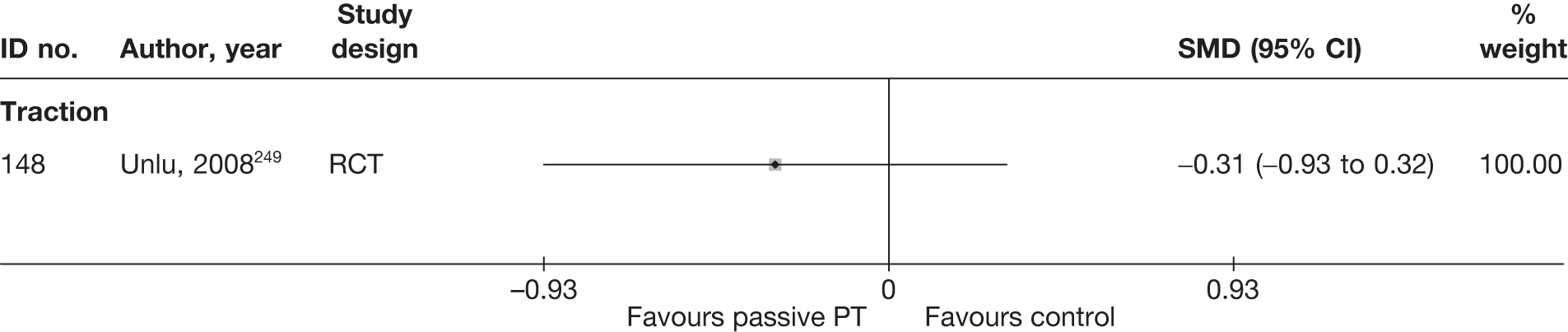
Passive physical therapy results at medium-term follow-up (> 6 weeks to ≤ 6 months)
Global effect at medium-term follow-up
The results for the global effect at medium-term follow-up are presented in Table 106 and the accompanying forest plot (Figure 71). In one moderate-quality RCT there was a significant improvement in global effect in a group receiving epidural steroids compared with conservative physiotherapy. 155
| ID no. | Author, year | Chronicity | Study design | Follow-up | Outcome measure | Perspective | Intervention | Control | OR (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n) | Outcome (n) | Withdrawal rate | Total (n) | Outcome (n) | Withdrawal rate | ||||||||
| Passive PT vs epidural/intradiscal injection | |||||||||||||
| 359 | Veihelmann, 2006155 | C | RCT | 6 months | Gerbershagen score (Chronification Index), GHS I (vs GHS II, III) | 27 | 8 | 0.48 | 46 | 31 | 0.02 | 0.19 (0.07 to 0.54) | |
FIGURE 71.
Summary of the findings of the global effect at medium-term follow-up (> 6 weeks to ≤ 6 months) for studies comparing passive therapy with alternative interventions. Note: weights are from random effects analysis.
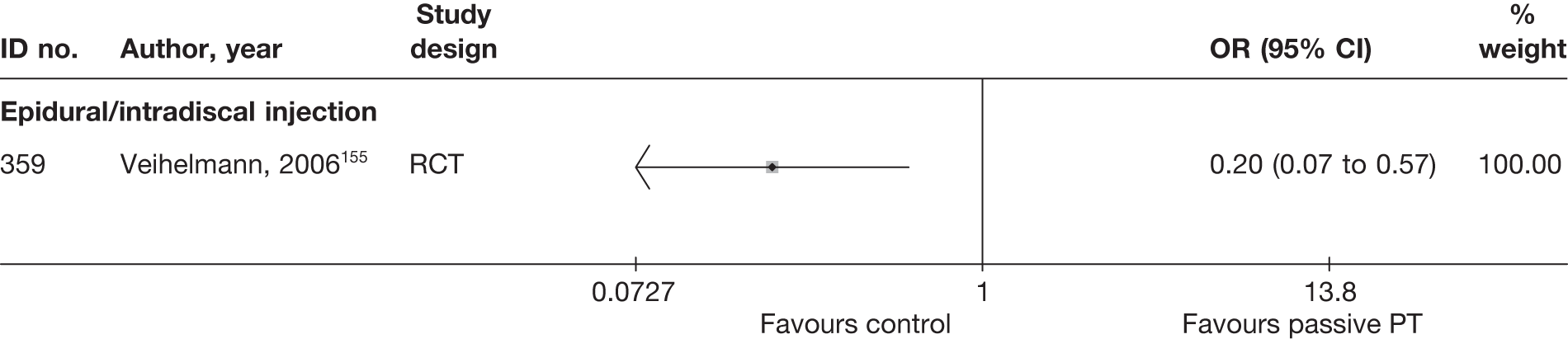
Pain intensity at medium-term follow-up
The results for pain intensity at medium-term follow-up are presented in Table 107 and the accompanying forest plot (Figure 72). There was no significant difference in pain intensity in one moderate-quality RCT comparing ultrasound or laser with traction249 or in another moderate-quality RCT that compared epidural steroids with conservative physiotherapy. 155
| ID no. | Author, year | Chronicity | Study design | Follow-up | Location | Scale (range)a | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)b | Comment/conversionc | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |||||||||
| Passive PT vs epidural | ||||||||||||||||
| 359 | Veihelmann, 2006155 | C | RCT | 6 months | Leg | VAS (0–10) | 27 | 46 | 67 (103.9) | 72 (135.6) | 58 (114.3) | 23 (142.4) | 35.00 (–24.60 to 94.60) | SD derived from SE | ||
| Passive PT vs traction | ||||||||||||||||
| 148 | Unlu, 2008249 (i)d (ultrasound) | A | RCT | 3 months | Leg | VAS (0–100) | 20 | 20 | 56.0 (15.3) | 59.6 (15.4) | 25.2 (13.9) | 29.5 (16.7) | –4.30 (–13.82 to 5.22) | |||
| 148 | Unlu, 2008249 (ii)d (laser) | A | RCT | 3 months | Leg | VAS (0–100) | 20 | 20 | 53.1 (25.9) | 59.6 (15.4) | 23.6 (17.7) | 29.5 (16.7) | –5.90 (–16.56 to 4.76) | |||
FIGURE 72.
Summary of the findings of pain intensity at medium-term follow-up (> 6 weeks to ≤ 6 months) for studies comparing passive PT with alternative interventions. Note: weights are from random effects analysis.
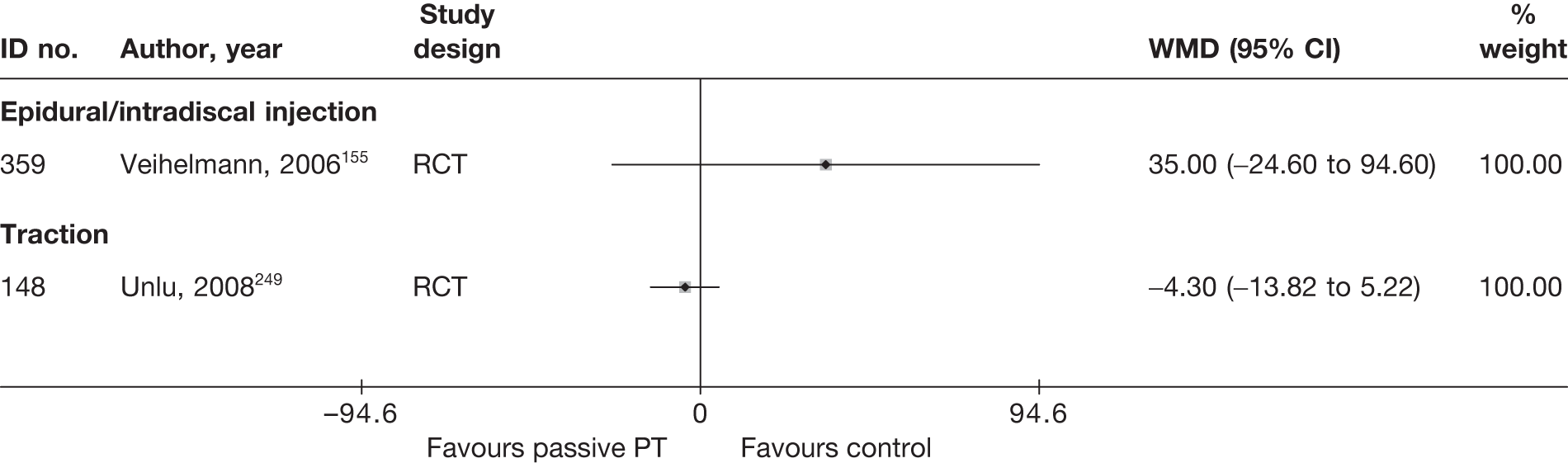
Condition-specific outcome measures at medium-term follow-up
The results for CSOMs at medium-term follow-up are presented in Table 108 and the accompanying forest plot (Figure 73). There was no significant difference in CSOMs in one moderate-quality RCT comparing ultrasound or laser with traction,249 or in another moderate-quality RCT that compared epidural steroids with conservative physiotherapy. 155
| ID no. | Author, year | Chronicity | Study design | Follow-up | Scale | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)a | Comment/conversionb | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | ||||||||
| Passive PT vs epidural | |||||||||||||||
| 359 | Veihelmann, 2006155 | C | RCT | 6 months | ODI | 27 | 46 | 23.1 | 21.4 | 22.5 (46.25) | 10.8 (50.19) | –0.22 (–0.70 to 0.25) |
SD based on weighted average Dropouts 26 (26%): control (epidural) 1/47, intervention (PT) 25/52 |
||
| Passive PT vs traction | |||||||||||||||
| 148 | Unlu, 2008249 (i)c (laser) | A | RCT | 3 months | RMDQ | 20 | 20 | 13.4 (4.5) | 14.2 (4.3) | 8.6 (6) | 8.94 (4) | –4.8 | –5.3 | –0.06 (–0.68 to 0.56) | |
| 148 | Unlu, 2008249 (ii)c (ultrasound) | A | RCT | 3 months | RMDQ | 20 | 20 | 12.5 (5) | 14.2 (4.3) | 6.7 (4.5) | 8.9 (4) | –5.8 | –5.3 | –0.52 (–1.15 to 0.11) | |
FIGURE 73.
Summary of the findings of CSOMs at medium-term follow-up (> 6 weeks to ≤ 6 months) for studies comparing passive PT with alternative interventions. Note: weights are from random effects analysis.
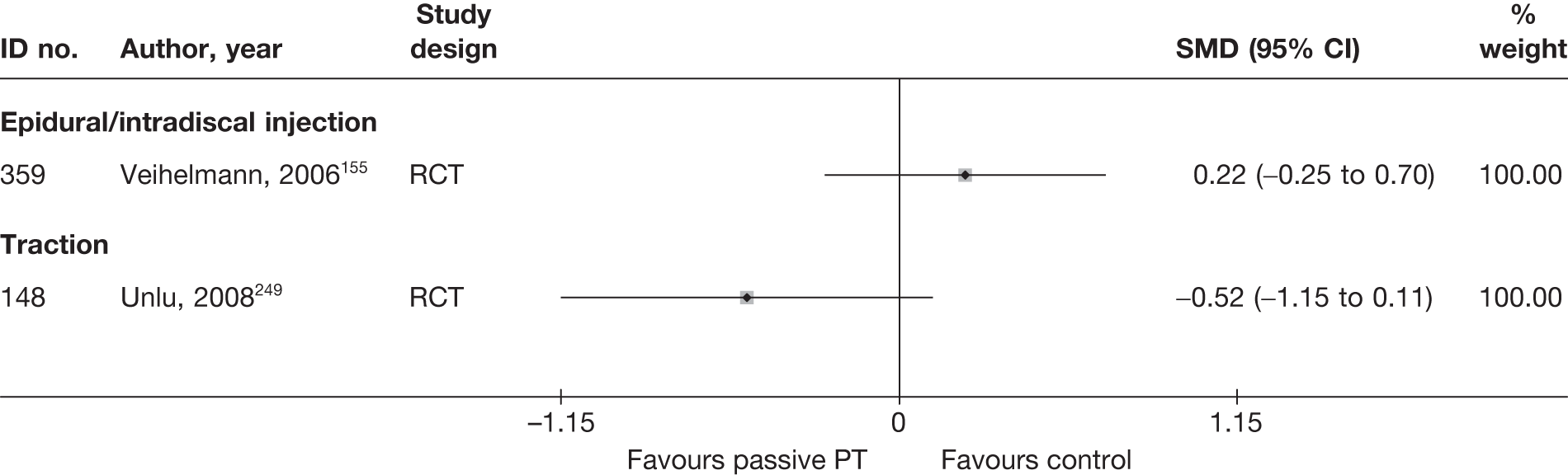
Passive physical therapy results at long-term follow-up (> 6 months)
Global effect at long-term follow-up
The results for the global effect at long-term follow-up are presented in Table 109 and the accompanying forest plot (Figure 74). In one moderate-quality RCT, there was a significant improvement in global effect in a group receiving epidural steroids compared with a group receiving conservative physiotherapy. 155
| ID no. | Author, year | Chronicity | Study design | Follow-up | Outcome measure | Perspective | Intervention | Control | OR (95% CI) | Comments | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n) | Out come (n) | Withdrawal rate | Total (n) | Outcome (n) | Withdrawal rate | |||||||||
| Passive PT vs epidural/intradiscal injection | ||||||||||||||
| 359 | Veihelmann, 2006155 | C | RCT | 12 months | Gerbershagen score (Chronification Index), GHS I (vs GHS II, III) | – | 27 | 7 | 0.48 | 46 | 30 | 0.02 | 0.20 (0.07 to 0.57) | Twelve patients moved over to epidural group and excluded from analysis |
FIGURE 74.
Summary of the findings of the global effect at long-term (> 6 months) follow-up for studies comparing passive PT with alternative interventions. Note: weights are from random effects analysis.
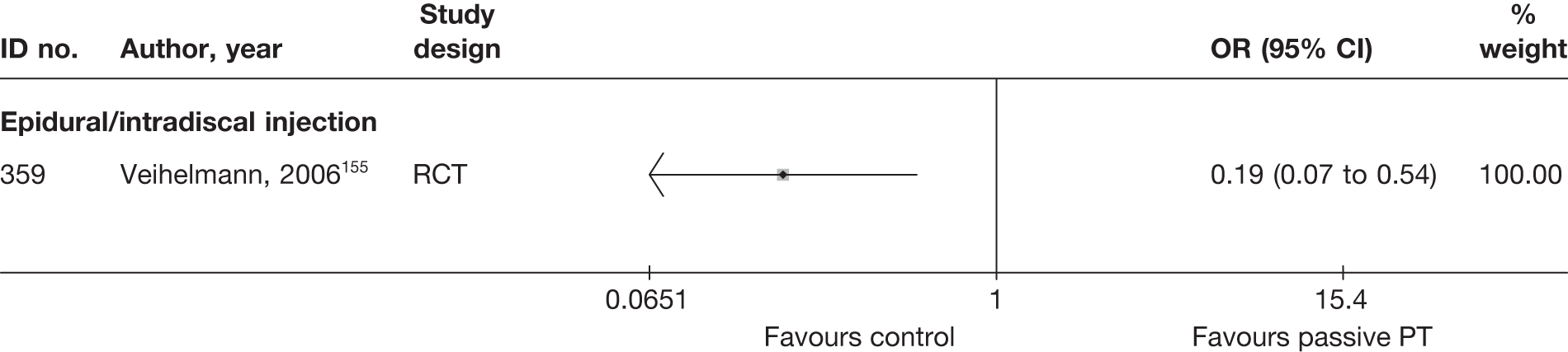
Pain intensity at long-term follow-up
The results for pain intensity at long-term follow-up are presented in Table 110 and the accompanying forest plot (Figure 75). There was no significant difference in pain intensity in one moderate-quality RCT that compared conservative physiotherapy with epidural steroids. 155
| ID no. | Author, year | Chronicity | Study design | Follow-up | Location | Scale (range)a | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)b | Comment/conversionc | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |||||||||
| Passive PT vs epidural/intradiscal injection | ||||||||||||||||
| 359 | Veihelmann, 2006155 | C | RCT | 12 months | Leg | VAS (0–10) | 27 | 46 | 67 (103.9) | 72 (135.6) | 59 (119.51) | 28 (189.91) | 35.00 (–24.60 to 94.60) |
SD derived from SE Dropouts 26/99 (26%): intervention 22/52 (48%), control (epidural) 1/47 (2%) Twelve patients in PT group moved over to epidural and excluded from analysis |
||
FIGURE 75.
Summary of the findings of pain intensity at long-term follow-up (> 6 months) for studies comparing exercise therapy with alternative interventions. Note: weights are from random effects analysis.
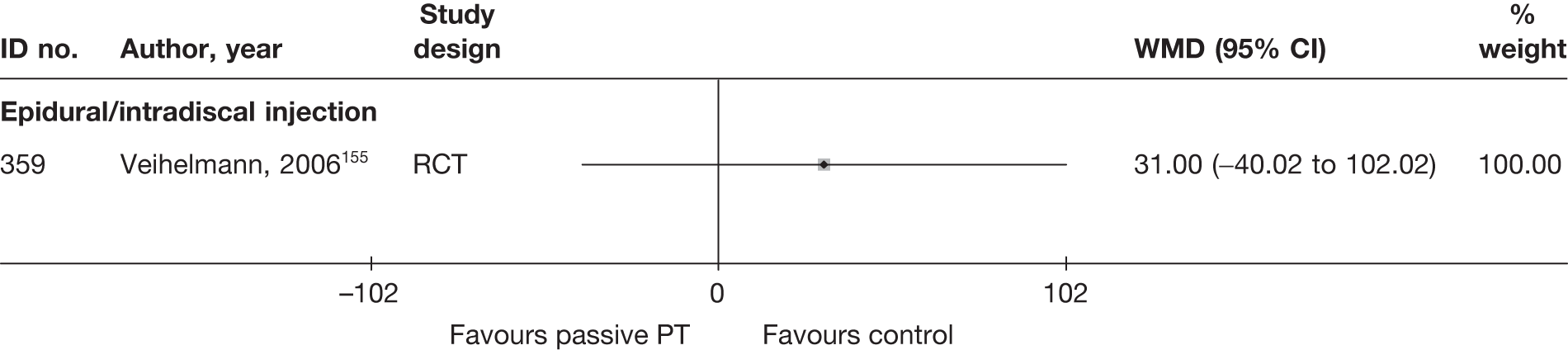
Condition-specific outcome measures at long-term follow-up
The results for CSOMs at long-term follow-up are presented in Table 111 and the accompanying forest plot (Figure 76). In one moderate-quality RCT, there was a significant improvement in CSOMs in a group receiving epidural steroids compared with conservative physiotherapy. 155
| ID no. | Author, year | Chronicity | Study design | Follow-up | Scale | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)a | Comment/conversionb | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | ||||||||
| Passive PT vs epidural/intradiscal injection | |||||||||||||||
| 359 | Veihelmann, 2006155 | C | RCT | 12 months | ODI | 27 | 46 | 23.1 | 21.4 | 21.6 (54.2) | 11.6 (67.82) | –0.77 (–1.26 to –0.28) |
SD based on weighted average Dropouts 26/99 (26%): intervention 22/52 (48%), control (epidural) 1/47 (2%) Twelve patients in PT group moved over to epidural and excluded from analysis |
||
FIGURE 76.
Summary of the findings of CSOMs at long-term follow-up (> 6 months) for studies comparing exercise therapy with alternative interventions. Note: weights are from random effects analysis.
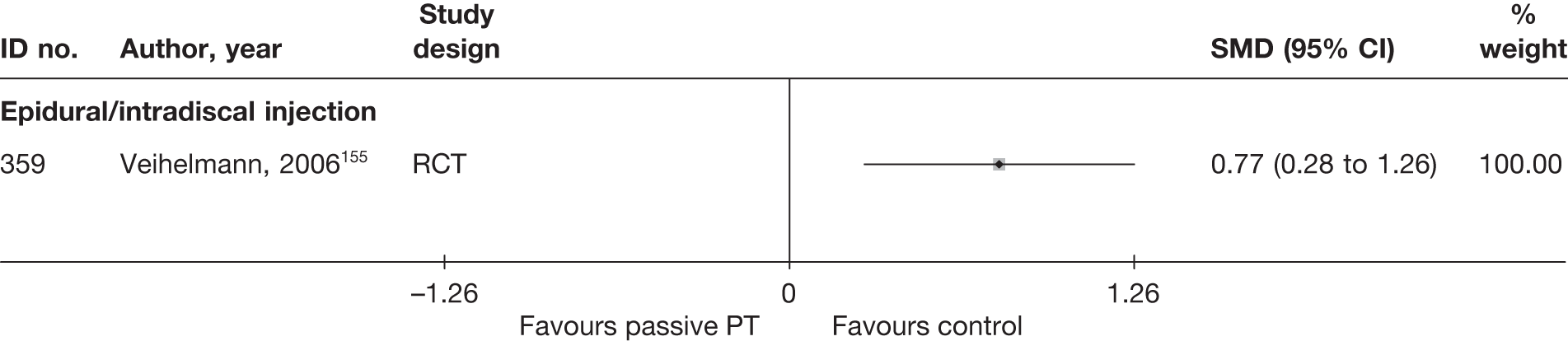
Adverse effects
The total number of adverse effects is presented in Table 112 and the accompanying forest plot (Figure 77). Adverse effects were reported in only one RCT, which found significantly more adverse events in the group receiving epidural steroids than in the group receiving conservative physiotherapy. 155
| ID no. | Author, year | Study design | No. of events in intervention group | No. of participants in intervention group | No. of events in control group | No. of participants in control group | OR (95% CI) |
|---|---|---|---|---|---|---|---|
| Passive PT vs epidural | |||||||
| 359 | Veihelmann, 2006155 | RCT | 0 | 39 | 16 | 46 | 0.02 (0.00 to 0.40) |
| Passive PT vs inactive control | |||||||
| 496 | Ghoname, 1999268 (PENS) | RCT | NR | NR | NR | NR | |
| 496 | Ghoname, 1999268 (TENS) | RCT | NR | NR | NR | NR | |
| Passive PT vs mixed treatment | |||||||
| 354 | Bokonjic, 1975269 | Non-RCT | NR | NR | NR | NR | |
| 266 | Ozturk, 2006253 (traction vs passive PT) | RCT | NR | NR | NR | NR | |
| Passive PT vs traction | |||||||
| 9059 | Mathews, 1987176 | RCT | NR | NR | NR | NR | |
| 148 | Unlu, 2008249 (laser) | RCT | NR | NR | NR | NR | |
| 148 | Unlu, 2008249 (ultrasound) | RCT | NR | NR | NR | NR | |
FIGURE 77.
Summary of the findings of any adverse effect for studies comparing passive PT with alternative treatment. Note: weights are from random effects analysis.
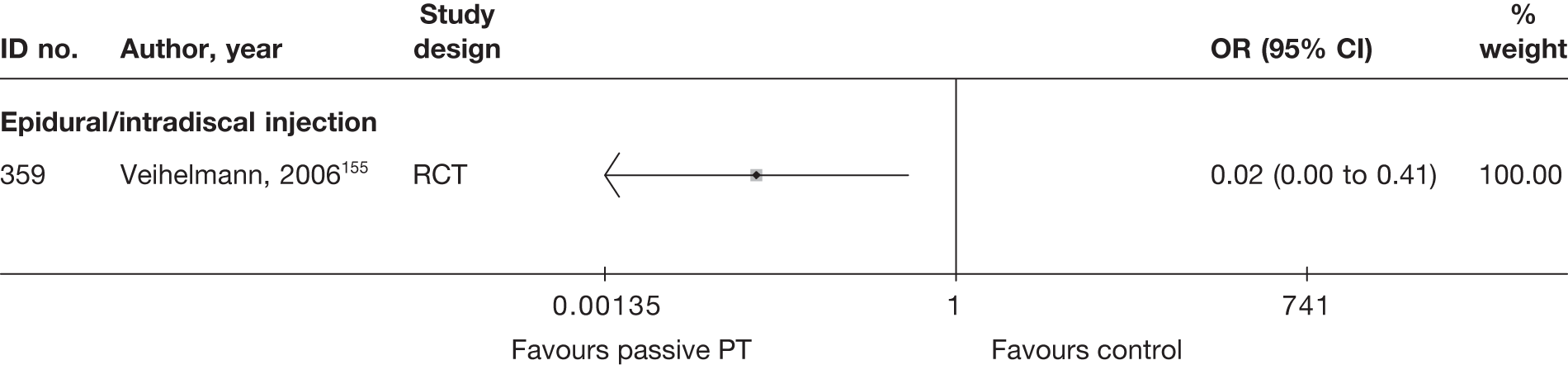
SUMMARY OF OVERALL FINDINGS FOR PASSIVE PHYSICAL THERAPY COMPARED WITH ALTERNATIVE INTERVENTIONS
Six studies, five of which were RCTs155,176,249,253,269 (one was a crossover trial268), compared the use of passive physical therapy with other interventions. Two RCTs176,249 restricted inclusion to patients with acute sciatica (Table 113).
| Control category | No. of studies (arms) | Sample size range (median) | Proportion of studies that were RCTs (%) | Proportion of studies that were deemed good quality (%) | Proportion of studies that only included acute sciatica (%) | Proportion of studies that included patients with nerve root pain (%) | Proportion of studies that reported diagnosis confirmed by imaging (%) | Proportion of studies that included patients with stenosis (%) | Proportion of studies that included patients with extruded/sequestered discs (%) | Proportion of studies that only included patients with first episode (%) | Proportion of studies that included patients who had received previous treatment (%) | Proportion of studies that included patients who had received previous surgery (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Passive PT vs epidural/intradiscal injection | 1 (1) | 99 (99) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 1/1 (100) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 1/1 (100) | 1/1 (100) |
| Passive PT vs inactive control | 1 (2) | 64 (64) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 1/1 (100) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 1/1 (100) | 1/1 (100) |
| Passive PT vs mixed treatment | 2 (2) | 46–56 (51) | 1/2 (50) | 0/2 (0) | 0/2 (0) | 2/2 (100) | 2/2 (100) | 0/2 (0) | 0/2 (0) | 0/2 (0) | 0/2 (0) | 0/2 (0) |
| Passive PT vs traction | 2 (2) | 60–143 (102) | 2/2 (100) | 0/2 (0) | 2/2 (100) | 2/2 (100) | 1/2 (50) | 0/2 (0) | 0/2 (0) | 0/2 (0) | 0/2 (0) | 0/2 (0) |
| Total (for passive PT studies) | 6 (6) | 46–143 (62) | 5/6 (83) | 0/6 (0) | 2/6 (33) | 6/6 (100) | 5/6 (83) | 0/6 (0) | 0/6 (0) | 0/6 (0) | 2/6 (33) | 2/6 (33) |
In one poor-quality crossover RCT268 there was a significant improvement in global effect and pain intensity in the short term with TENS or PENS compared with inactive control. There was no significant difference in terms of global effect, pain intensity or CSOMs at short-, medium- or long-term follow-up in three moderate- or poor-quality RCTs176,249,253 that compared heat, ultrasound, laser or an unspecified PT programme with traction. Physiotherapy programmes were less effective than epidural corticosteroid injections in terms of short-term global effect in one poor-quality non-RCT269 and in terms of medium- and long-term global effect, pain intensity and CSOMs in one moderate-quality RCT. 155 Adverse effects were less common with physiotherapy than with epidural injection of corticosteroid in this latter RCT.
Biological agents
Biological agents are derived from living material and have a highly complex chemical structure. They are being used increasingly in rheumatological practice to control inflammatory disease. Tumour necrosis factor-alpha (TNF-α) is one of the proinflammatory factors released from prolapsed intervertebral discs that is responsible for inflammation of the affected nerve root in sciatica and may be amenable to treatment by these biological therapies. Biological agents that inhibit TNF-α include etanercept, infliximab (Remicade®, Schering-Plough Ltd) and adalimumab (Humira®, Abbott).
Description of biological agents studies
Summary of interventions
Five studies evaluated biological agents for sciatica. 149,216,270–272 Four of these studies compared biological agents with an alternative type of intervention. 149,216,270,271 Summary data of the interventions used are presented in Table 114a. Two RCTs,149,271 one non-RCT270 and one HCS216 compared biological agents with alternative treatments. One RCT270 and one non-RCT271 compared intravenous infusions of infliximab with placebo injections of saline. One RCT149 compared epidural injections of autologous conditioned serum, rich in anti-inflammatory cytokines, with epidural injections of corticosteroid and local anaesthetic. One CCS216 compared subcutaneous injections of etanercept with intravenous injections of corticosteroid.
| ID no. | Author, year | Study design | Treatment description | Control description |
|---|---|---|---|---|
| Biological agents vs epidural/intradiscal injection | ||||
| 321 | Becker, 2007149 | RCT | Epidural injection of autologous conditioned serum (group 1) | Epidural injection of steroid triamcinolone 5 mg + local anaesthetic 1 ml (group 3) |
| 321 | Becker, 2007149 | RCT | Epidural injection of autologous conditioned serum (group 1) | Epidural injection of steroid triamcinolone 10 mg + local anaesthetic 1 ml (group 2) |
| Biological agents vs inactive control | ||||
| 398 | Karppinen, 2003270 | Non-RCT | Intravenous infusion of infliximab 3 mg/kg (anti-TNF-α) | Periradicular saline injection |
| 741 | Korhonen, 2005271 | RCT | Intravenous infliximab 5 mg/kg | Intravenous saline (placebo) |
| Biological agents vs non-opioids | ||||
| 323 | Genevay, 2004216 | HCS | Three subcutaneous injections of etanercept 25 mg (anti-TNF-α) | Three intravenous injection of methylprednisolone 250 mg |
One three-armed study compared different doses of the same biological agent with each other. 272 The doses and biological agent being compared are presented in Table 114b, but this study is not considered any further.
| ID no. | Author, year | Study design | Treatment description | Control description |
|---|---|---|---|---|
| 804 | Cohen, 2009272 | RCT | Transforaminal epidural injections of etanercept (4 mg) | Transforaminal epidural injections of etanercept (2 mg) |
| 804 | Cohen, 2009272 | RCT | Transforaminal epidural injections of etanercept (6 mg) | Transforaminal epidural injections of etanercept (2 mg) |
Summary of study participants in biological agent studies
Summary data on the included participants are presented in Table 115. The four studies that compared biological agents with alternative treatments included 213 participants with mean ages between 39 and 54 years (50–80% men), all with acute symptom duration. One non-RCT included only participants with the first episode of sciatica, one RCT also included recurrent symptoms, but symptom duration was not reported in two studies. Sciatica was confirmed by imaging in three trials. There were no patients with spinal stenosis or sequestered discs and previous back surgery was excluded in two trials. 270,271
| ID no. | Author, year | Study design | No. of patients | Age (years) | No. of men (%) | Symptom duration | Type of sciatica | Confirmed by imaging | Recurrent episode | Included patients with stenosis?a | Included patients with sequestered disc (or extruded)?a | Any previous treatment for sciatica? | Any previous back surgery for sciatica? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biological agents vs epidural/intradiscal injection | |||||||||||||
| 321 | Becker, 2007149 | RCT | 90 | Mean 53.9 (range 29–81) | 52 (62) | At least 6 weeks | Nerve root pain | Yes | NR | No | No | NR | NR |
| Biological agents vs inactive control | |||||||||||||
| 398 | Karppinen, 2003270 | Non-RCT | 72 | TNF-α group: mean 38.5 | TNF-α group: 8 (80) | TNF-α group: mean 7.2 weeks (range 2–12 weeks); no data for saline group | Nerve root pain | Yes | First episode | No | No | NR | No |
| 741 | Korhonen, 2005271 | RCT | 41 | Mean 40.7 (SD 8.4) | 24 (60) | Median 61 days (range 20–102 days) | Nerve root pain | Yes | Recurrent and first episode | No | No | Yes | No |
| Biological agents vs non-opioids | |||||||||||||
| 323 | Genevay, 2004216 | HCS | 10 | Mean 47.3 (SD 13.3, range > 18) | 10 (50) | Mean 3.2 weeks (SD 3.7 weeks) | Nerve root pain | No | NR | No | No | NR | NR |
Summary of study quality for biological agents
Study details are summarised in Table 116. Half of the studies were RCTs (2/4, 50%) and none was of good quality. Only two had an adequate method of random number generation,149,271 and none documented a secure method of allocation concealment. No studies had good external validity.
| ID no. | Author, year | Study size | Overall follow-up | Study design | Adequate randomisation? | Allocation concealment? | Follow-up (%) | Blind outcome assessment? | Overall quality rating | Overall external validity rating |
|---|---|---|---|---|---|---|---|---|---|---|
| Biological agents vs epidural/intradiscal injection | ||||||||||
| 321 | Becker, 2007149 | 90 | 22 weeks | RCT | Yes | Partial | 80–100 | Yes | Moderate | Weak |
| Biological agents vs inactive control | ||||||||||
| 398 | Karppinen, 2003270 | 72 | 3 months | Non-RCT | No | No | Cannot tell | No | Weak | Weak |
| 741 | Korhonen, 2005271 | 41 | 1 year | RCT | Yes | Unclear | 80–100 | Unclear | Moderate | Weak |
| Biological agents vs non-opioids | ||||||||||
| 323 | Genevay, 2004216 | 10 | 6 weeks | HCS | No | No | 80–100 | No | Weak | Moderate |
Biological agent results at short-term follow-up (≤ 6 weeks)
Global effect at short-term follow-up
No studies reported global effect data at short-term follow-up.
Pain intensity at short-term follow-up
The results for pain intensity at short-term follow-up are presented in Table 117 and the accompanying forest plot (Figure 78). There was a significant improvement in pain intensity in the infliximab group compared with the inactive control group in one poor-quality non-RCT,270 and also in the etanercept group compared with the intravenous corticosteroid injection group in a poor-quality HCS. 216
| ID no. | Author, year | Chronicity | Study design | Follow-up | Location | Scale (range)a | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)b | Comment/conversionc | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |||||||||
| Biological agents vs inactive control | ||||||||||||||||
| 398 | Karppinen, 2003270 | A | Non-RCT | 1 month | Leg | VAS (0–100) | 10 | 62 | 80 (18) | 76 (19) | 18 (19) | 47 (32) | –62 | –29 | –40.50 (–43.22 to –14.78) | Change scores presented as percentages |
| Biological agents vs non-opioids | ||||||||||||||||
| 323 | Genevay, 2004216 | A | HCS | 6 weeks | Leg | VAS (0–100) | 10 | 10 | 74.4 (12.9) | 75.1 (14.2) | 12.4 (13.2) | 52.9 (25.1) | –40.50 (–58.08 to –22.92) | |||
FIGURE 78.
Summary of the findings of pain intensity at short-term follow-up (≤ 6 weeks) for studies comparing biological agents with alternative interventions. Note: weights are from random effects analysis.
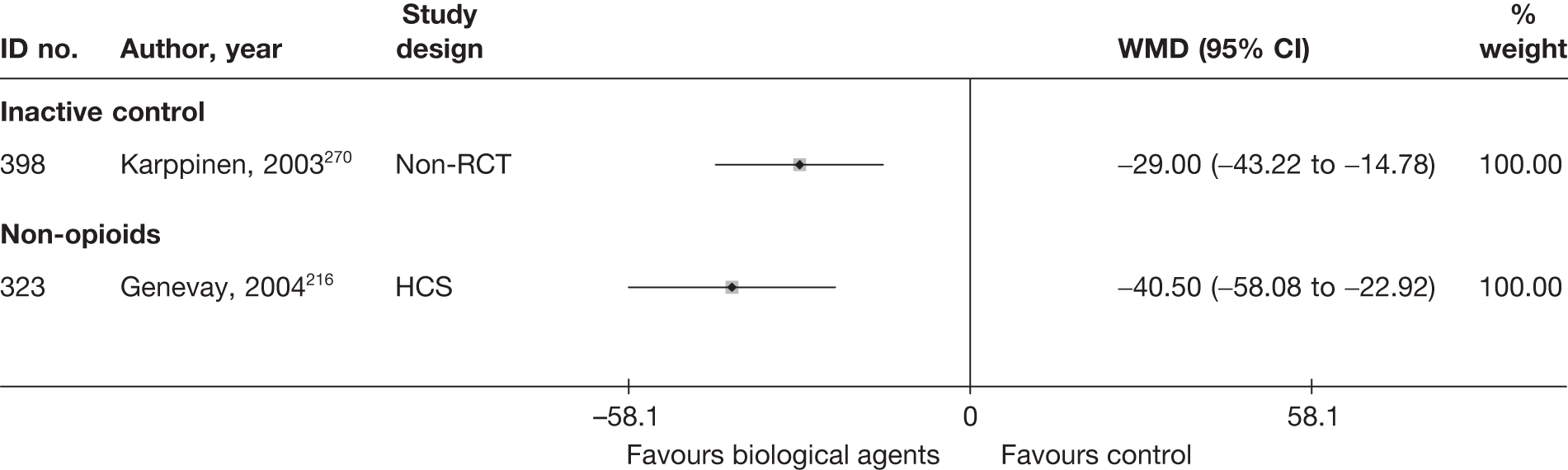
Condition-specific outcome measures at short-term follow-up
The results for CSOMs at short-term follow-up are presented in Table 118 and the accompanying forest plot (Figure 79). There was a significant improvement in CSOMs with infliximab compared with placebo injection in one poor-quality non-RCT,270 and also with etanercept compared with intravenous corticosteroid in one poor-quality HCS. 216 There was no significant difference in CSOMs in the group receiving an epidural injection of autologous conditioned serum compared with epidural injection of corticosteroid and local anaesthetic in one moderate-quality RCT. 149
| ID no. | Author, year | Chronicity | Study design | Follow-up | Scale | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)a | Comment/conversionb | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | ||||||||
| Biological agents vs epidural | |||||||||||||||
| 321 | Becker, 2007149 (i)c (5 mg) | A + C | RCT | 6 weeks | ODI | 32 | 27 | 22.0 (8.3) | 20.6 (8.1) | 13.8 (9.8) | 12.1 (9.0) | 0.18 (–0.33 to 0.69) |
ITT not used Dropouts 6 (7%); number originally randomised to each group not stated |
||
| 321 | Becker, 2007149 (ii)c (10 mg) | A + C | RCT | 6 weeks | ODI | 32 | 25 | 22.0 (8.3) | 19.4 (9.9) | 13.8 (9.8) | 11.0 (9.5) | 0.29 (–0.24 to 0.82) |
ITT not used Dropouts 6 (7%); number originally randomised to each group not stated |
||
| Biological agents vs inactive control | |||||||||||||||
| 398 | Karppinen, 2003270 | A | Non-RCT | 1 month | ODI | 10 | 62 | 43 (21) | 44 (15) | 15 (9) | 30 (17) | –28 | –14 | –0.93 (–1.61 to –0.24) |
Percentage change scores from baseline and adjusted difference between groups Percentage change converted |
| Biological agents vs non-opioids | |||||||||||||||
| 323 | Genevay, 2004216 | A | HCS | 6 weeks | RMDQ | 10 | 10 | 17.8 (3.3) | 15.5 (2.9) | 5.8 (5.5) | 11.1 (4.6) | –1.05 (–1.99 to –0.10) | |||
FIGURE 79.
Summary of the findings of CSOMs at short-term follow-up (≤ 6 weeks) for studies comparing biological agents with alternative interventions. Note: weights are from random effects analysis.
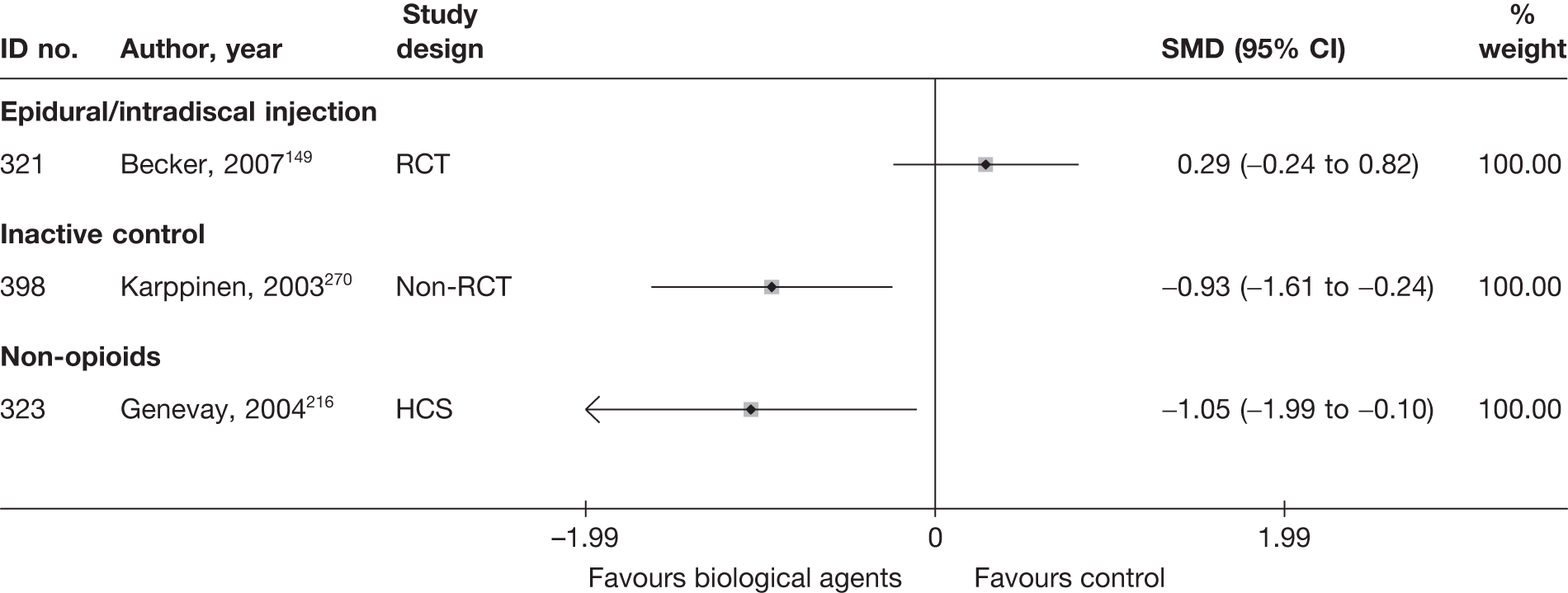
Biological agents results at medium-term follow-up (> 6 weeks to ≤ 6 months)
Global effect at medium-term follow-up
No studies reported global effect data at long-term follow-up.
Pain intensity at medium-term follow-up
The results for pain intensity at medium-term follow-up are presented in Table 119 and the accompanying forest plot (Figure 80). There was a significant improvement in pain intensity in one poor-quality non-RCT of infliximab compared with placebo injection,270 but not in another moderate-quality RCT,271 and there was no significant difference when these results were combined in a meta-analysis. There was no significant difference in pain intensity in a group receiving an epidural injection of autologous conditioned serum compared with epidural injection of corticosteroid and local anaesthetic in one moderate-quality RCT. 149
| ID no. | Author, year | Chronicity | Study design | Follow-up | Location | Scale (range) | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)a | Comment/conversionb | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |||||||||
| Biological agents vs epidural | ||||||||||||||||
| 321 | cBecker, 2007149 (5 mg) | A + C | RCT | 22 weeks | Overall | VAS (0–100) | 32 | 24 | 78 | 85 | Adjusted mean difference between groups (i) and (ii): –9.3 (95% CI –23.5 to 4.9) | Seven participants (8%) dropped out; number originally randomised to each group not stated | ||||
| 321 | cBecker, 2007149(10 mg) | A + C | RCT | 22 weeks | Overall | VAS (0–100) | 32 | 27 | 78 | 82 | Adjusted mean difference between groups (i) and (iii): –13.5 (95% CI –27.4 to 0.4); repeated measures analysis of variance | Seven participants (8%) dropped out; number originally randomised to each group not stated | ||||
| Biological agents vs inactive control | ||||||||||||||||
| 398 | Karppinen, 2003270 | A | Non-RCT | 3 months | Leg | VAS (0–100) | 10 | 62 | 80 (18) | 76 (19) | 10 (16) | 37 (35) | –70 | –39 | –27.00 (–40.20 to –13.80) | Change scores presented as percentages |
| 741 | Korhonen, 2005271 | A + C | RCT | 12 weeks | Leg | VAS (0–100) | 21 | 19 | 73 | 73 | 30 (15.31) | 23 (30.1) | –43 | –50 | 7.00 (–8.04 to 22.04) |
Median reported for baseline, reduction in pain and range Median used form means and final score derived form change SD imputed from weighted average |
FIGURE 80.
Summary of the findings of pain intensity at medium-term follow-up (> 6 weeks to ≤ 6 months) for studies comparing biological agents with alternative interventions. Note: weights are from random effects analysis.
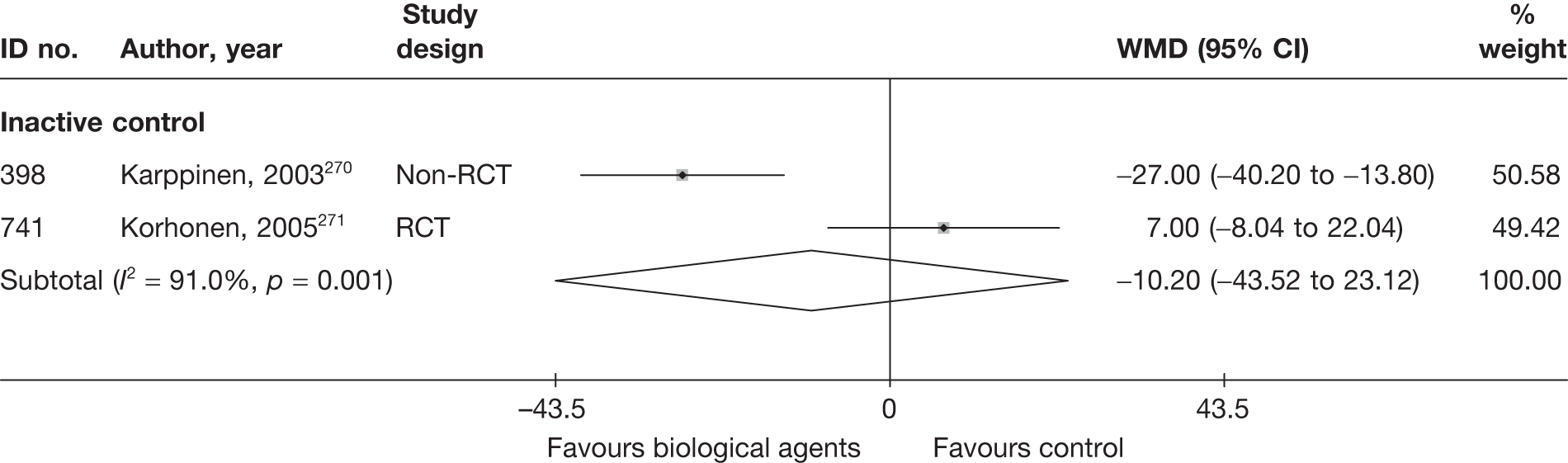
Condition-specific outcome measures at medium-term follow-up
The results for CSOMs at medium-term follow-up are presented in Table 120 and the accompanying forest plot (Figure 81). There was a significant improvement in CSOMs in one poor-quality non-RCT of infliximab compared with placebo injection. 270 There was no significant difference in CSOMs in a group receiving an epidural injection of autologous conditioned serum compared with epidural injection of corticosteroid and local anaesthetic in one moderate-quality RCT. 149
| ID no. | Author, year | Chronicity | Study design | Follow-up | Scale | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)a | Comment/conversionb | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | ||||||||
| Biological agents vs epidural | |||||||||||||||
| 321 | Becker, 2007149 (i)c (5 mg) | A + C | RCT | 6 weeks | ODI | 32 | 27 | 22.0 (8.3) | 11.1 (7.1) | 11.7 (9.2) | 11.1 (7.1) | 0.07 (–0.44 to 0.58) |
ITT not used Dropouts: 7 (8%) Number originally randomised to each group not stated |
||
| 321 | Becker, 2007149 (ii)c (10 mg) | A + C | RCT | 6 weeks | ODI | 32 | 25 | 22.0 (8.3) | 11.0 (9.5) | 11.7 (9.2) | 11.0 (9.5) | 0.08 (–0.45 to 0.60) |
ITT not used Dropouts: 7 (8%) Number originally randomised to each group not stated |
||
| Biological agents vs inactive control | |||||||||||||||
| 398 | Karppinen, 2003270 | A | Non-RCT | 1 month | ODI | 10 | 62 | 43 (21) | 24 (20) | 7 (6) | 24 (20) | –36 | –20 |
–0.90 (–1.59 to –0.22) Adjusted mean difference 13% (95% CI 4 to 22); ANOVA (poor-quality study) |
Percentage change scores from baseline and adjusted difference between groups – not based on summary score (repeated ANCOVA) |
| 741 | Korhonen, 2005271 | A + C | RCT | 12 weeks | ODI (%) | 21 | 19 |
Only medians reported; p-values reported based on Mann–Whitney U-test or Fisher’s exact-test ITT not used but all patients included in analysis except one who did not meet including criteria |
|||||||
FIGURE 81.
Summary of the findings of CSOMs at medium-term follow-up (> 6 weeks to ≤ 6 months) for studies comparing biological agents with alternative interventions. Note: weights are from random effects analysis.
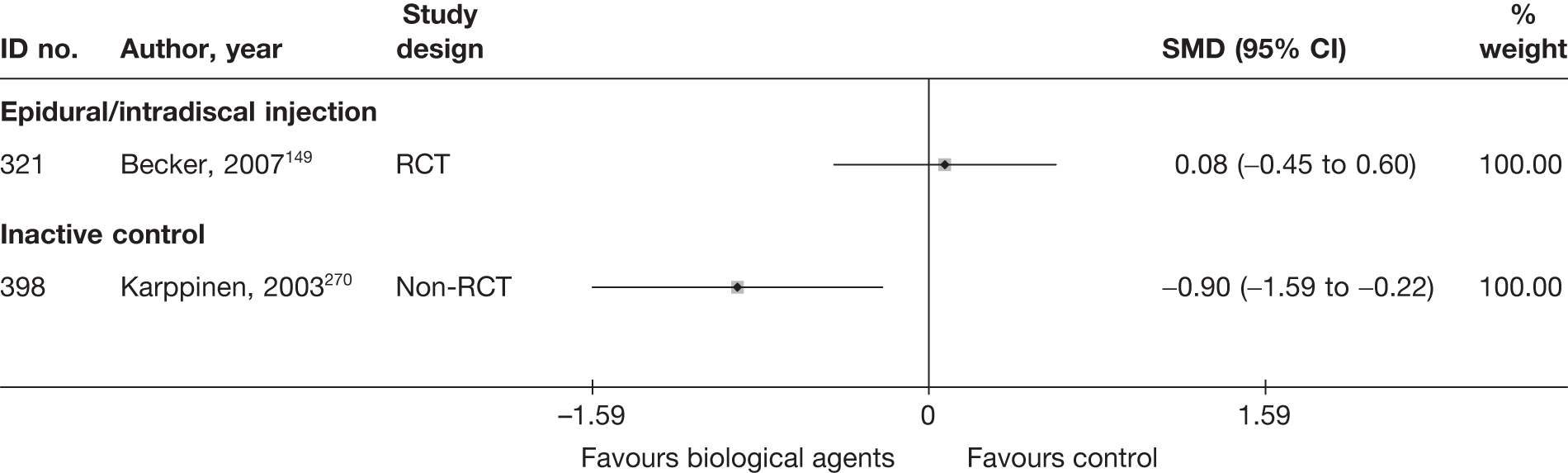
Biological agent results at long-term follow-up (> 6 months)
Global effect at long-term follow-up
No studies reported the global effect data at long-term follow-up.
Pain intensity at long-term follow-up
The results for pain intensity at long-term follow-up are presented in Table 121 and the accompanying forest plot (Figure 82). There was no significant difference in pain intensity in one moderate-quality RCT of infliximab compared with placebo injection. 271
| ID no. | Author, year | Chronicity | Study design | Follow-up | Location | Scale (range)a | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)b | Comment/conversionc | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |||||||||
| Biological agents vs inactive control | ||||||||||||||||
| 741 | Korhonen, 2005271 | A + C | RCT | 12 weeks | Leg | VAS (0–100) | 21 | 19 | 73 | 73 | 23 (15.31) | 12 (23.67) | –43 | –50 |
11.00 (–1.50 to 23.50) |
Median reported for baseline, reduction in pain and range Median used form means and final score derived form change SD imputed from weighted average Dropouts 1/41 (2%): group allocation not stated |
FIGURE 82.
Summary of the findings of pain intensity at long-term follow-up (> 6 months) for studies comparing biological agents with alternative interventions. Note: weights are from random effects analysis.
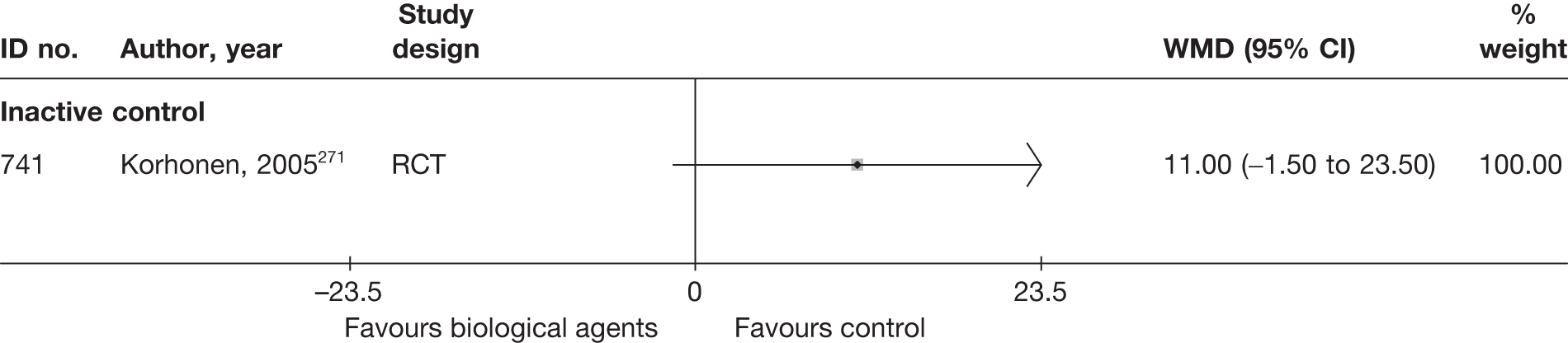
Condition-specific outcome measures at long-term follow-up
The results for CSOMs at long-term follow-up are presented in Table 122 and the accompanying forest plot (Figure 83). There was no significant difference in CSOMs in one moderate-quality RCT of infliximab compared with placebo injection. 271
| ID no. | Author, year | Chronicity | Study design | Follow-up | Scale | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)a | Comment/conversionb | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | ||||||||
| Biological agents vs inactive control | |||||||||||||||
| 741 | Korhonen, 2005271 | A + C | RCT | 12 months | ODI (%) | 21 | 19 | 45 | 48 | 9 (10.71) | 10 (20) | –28 | –23 | –0.06 (–0.68 to 0.56) |
Only median reported – used as mean p-values reported based on Mann–Whitney U-test or Fisher’s exact test Final SD imputed from WMD of SDs for ODI medium-term follow-up ITT not used, but all patients included in analysis except one who did not meet inclusion criteria |
FIGURE 83.
Summary of the findings of CSOMs at long-term follow-up (> 6 months) for studies comparing biological agents with alternative interventions. Note: weights are from random effects analysis.
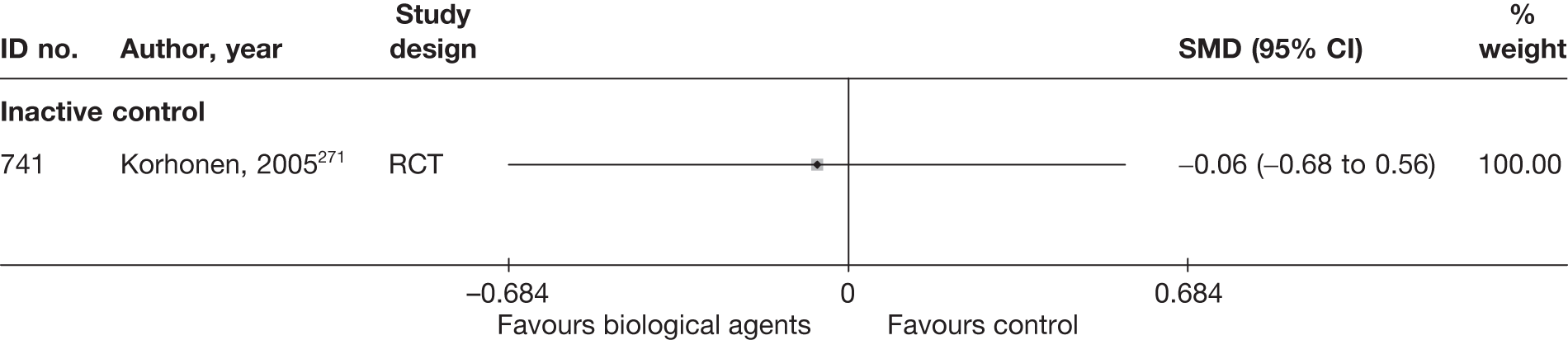
Adverse effects
The total number of adverse effects are presented in Table 123 and the accompanying forest plot (Figure 84). There was no significant difference in the number of adverse events between infliximab and placebo in two RCTs,270,271 or between epidural injections of autologous conditioned serum compared with corticosteroid and local anaesthetic in one RCT. 149
| ID no. | Author, year | Study design | No. of events in intervention group | No. of participants in intervention group | No. of events in control group | No. of participants in control group | OR (95% CI) |
|---|---|---|---|---|---|---|---|
| Biological agent vs epidural | |||||||
| 321 | Becker, 2007149 (i)a (5 mg) | RCT | 1 | 32 | 1 | 27 | 0.84 (0.05 to 14.08) |
| 321 | Becker, 2007149 (ii)a (10 mg) | RCT | 1 | 32 | 1 | 25 | 0.77 (0.05 to 13.02) |
| Biological agent vs inactive control | |||||||
| 398 | Karppinen, 2003270 | Non-RCT | 0 | 10 | 0 | 62 | |
| 741 | Korhonen, 2005271 | RCT | 0 | 21 | 0 | 19 | |
| Biological agent vs non-opioids | |||||||
| 323 | Genevay, 2004216 | HCS | NR | NR | NR | NR | |
FIGURE 84.
Summary of the findings of any adverse effects for studies comparing biological agents with alternative interventions. Note: weights are from random effects analysis.

SUMMARY OF OVERALL FINDINGS FOR BIOLOGICAL AGENT COMPARED WITH ALTERNATIVE INTERVENTIONS
Four studies,149,216,270,271 three of which were RCTs,149,216,271 compared the use of biological agents with other interventions (Table 124).
| Control category | No. of studies (arms) | Sample size range (median) | Proportion of studies that were RCTs (%) | Proportion of studies that were deemed good quality (%) | Proportion of studies that only included acute sciatica (%) | Proportion of studies that included patients with nerve root pain (%) | Proportion of studies that reported diagnosis confirmed by imaging (%) | Proportion of studies that included patients with stenosis (%) | Proportion of studies that included patients with extruded/sequestered discs (%) | Proportion of studies that only included patients with first episode (%) | Proportion of studies that included patients who had received previous treatment (%) | Proportion of studies that included patients who had received previous surgery (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biological agents vs epidural/intradiscal injection | 1 (2) | 90 (90) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 1/1 (100) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) |
| Biological agents vs inactive control | 2 (2) | 41–72 (57) | 1/2 (50) | 0/2 (0) | 1/2 (50) | 2/2 (100) | 2/2 (100) | 0/2 (0) | 0/2 (0) | 1/2 (50) | 1/2 (50) | 0/2 (0) |
| Biological agents vs non-opioids | 1 (1) | 10 (10) | 0/1 (0) | 0/1 (0) | 1/1 (100) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) |
| Total (for biological agent studies) | 4 (5) | 10–90 (57) | 2/4 (50) | 0/4 (0) | 2/4 (50) | 4/4 (100) | 3/4 (75) | 0/4 (0) | 0/4 (0) | 1/4 (25) | 1/4 (25) | 0/4 (0) |
There was conflicting evidence for the efficacy of intravenous infliximab as one poor-quality non-RCT found significant improvement in global effect and pain intensity at short- and medium-term follow-up,270 but one moderate-quality RCT did not. 271 A poor-quality HCS found significant improvement in short-term pain intensity and CSOMs with etanercept compared with intravenous corticosteroids. 216 There was no significant difference in pain intensity or CSOMs in the short or medium term with epidural injection of autologous conditioned serum compared with epidural injection of corticosteroid and local anaesthetic in one moderate-quality RCT. 149 There was no difference in the number of adverse effects.
Activity restriction
Description of activity restriction studies
Summary of interventions
Five studies compared passive PT with an alternative type of intervention. 14,145,243,256,267 Summary data of the interventions used are presented in Table 125. Two RCTs compared bed rest for 1 or 2 weeks with advice to keep active,14 or continue activities of daily living. 267 This last RCT267 was a three-arm study which also compared bed rest with twice weekly hospital physiotherapy for at least 4 weeks, consisting of segmental mobilisation, exercises and hydrotherapy. Another three-arm RCT256 compared rest and hot packs with hot packs, massage, mobilising and isotonic strengthening exercise, and also with intermittent pelvic traction and isometric strengthening exercises. Another RCT243 compared bed rest at home with bed rest and vertical traction. A non-RCT145 compared 1–2 weeks of bed rest with a sacral epidural injection of local anaesthetic.
| ID no. | Author, year | Study design | Treatment description | Control description |
|---|---|---|---|---|
| Activity restriction vs exercise therapy | ||||
| 564 | Lidstrom, 1970256 | RCT | Rest | Massage + mobilising and strengthening exercises |
| Activity restriction vs education/advice | ||||
| 713 | Hofstee, 2002267 | RCT | Bed rest | Advised to continue activities of daily living |
| 658 | Vroomen, 199914 | RCT | Bed rest | Advice to keep active |
| Activity restriction vs epidural/intradiscal injection | ||||
| 140 | Coomes, 1961145 | Non-RCT | Bed rest at home on fracture boards | Sacral epidural injection local anaesthetic 50–60 ml procaine |
| Activity restriction vs mixed treatment | ||||
| 713 | Hofstee, 2002267 | RCT | Bed rest | Hospital physiotherapy: segmental mobilisation + exercises + hydrotherapy |
| 564 | Lidstrom, 1970256 | RCT | Rest | Traction + strengthening exercises |
| Activity restriction vs traction | ||||
| 222 | Moret, 1998243 | RCT | Bed rest | Bed rest and traction (vertical traction using patient weight), 180 minutes daily for 1–2 weeks |
Summary of study participants in activity restriction studies
Summary data on the included participants are presented in Table 126. The five studies included 551 participants with mean ages between 39 and 46 years (47–76% men). Symptom duration was acute in two studies, chronic in one and a mixture of acute and chronic in the other. Three studies included patients with recurrent symptoms, and not recorded in two. Sciatica was confirmed by imaging in one RCT. 267 There were no patients with spinal stenosis or sequestered discs, and previous back surgery was excluded in one RCT. 14
| ID no. | Author, year | Study design | No. of patients | Age (years) | No. of men (%) | Symptom duration | Type of sciatica | Confirmed by imaging? | Recurrent episode | Included patients with stenosis?a | Included patients with sequestered disc (or extruded)?a | Any previous treatment for sciatica? | Any previous back surgery for sciatica? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Activity restriction vs education/advice | |||||||||||||
| 713 | Hofstee, 2002267 | RCT | 250 | Mean 39 (SD 10) | 150 (60) | Mean 2 weeks | Nerve root pain and referred pain | Yes | Recurrent | No | No | NR | Yes |
| 658 | Vroomen, 199914 | RCT | 183 | Mean 46 (SD 12) | 103 (56) | Median 16 days | Nerve root pain | No | Recurrent and first episode | No | No | NR | No |
| Activity restriction vs epidural | |||||||||||||
| 140 | Coomes, 1961145 | Non-RCT | 40 | Mean 43 (range 16–70) | 26 (65) | Mean of 34 days | Nerve root pain | No | NR | No | No | Yes | NR |
| Activity restriction vs exercise therapy | |||||||||||||
| 564 | Lidstrom, 1970256 | RCT | 62 | Range 21–61 | 29 (47) | > 1 year 52% | Nerve root pain and referred pain | No | NR | No | No | NR | NR |
| Activity restriction vs mixed treatments | |||||||||||||
| 713 | Hofstee, 2002267 | RCT | 250 | Mean 39 (SD 10) | 150 (60) | Mean 2 weeks | Nerve root pain and referred pain | Yes | Recurrent | No | No | NR | Yes |
| 564 | Lidstrom, 1970256 | RCT | 62 | Range 21–61 | 29 (47) | > 1 year 52% | Nerve root pain and referred pain | No | NR | No | No | NR | NR |
| Activity restriction vs traction | |||||||||||||
| 222 | Moret, 1998243 | RCT | 16 | Mean 41.9 (SD 8.7) | 12 (75) | Acute symptoms 50% | Nerve root pain | No | Recurrent and first episode | No | NR | Yes | Yes |
Summary of study quality for activity restriction studies
Study details are summarised in Table 127. Most studies were RCTs (4/5, 80%); however, the proportion that were of good quality was low (1/5, 20%). Only three had an adequate method of random number generation14,243,267 and none documented a secure method of allocation concealment. Two studies had good external validity. 14,243
| ID no. | Author, year | Study size | Overall follow-up | Study design | Adequate randomisation? | Allocation concealment? | Follow-up (%) | Blind outcome assessment? | Overall quality rating | Overall external validity rating |
|---|---|---|---|---|---|---|---|---|---|---|
| Activity restriction vs education/advice | ||||||||||
| 713 | Hofstee, 2002267 | 250 | 6 months | RCT | Yes | No | 80–100 | No | Moderate | Moderate |
| 658 | Vroomen, 199914 | 183 | 12 weeks | RCT | Yes | No | 80–100 | Yes | Moderate | Strong |
| Activity restriction vs epidural/intradiscal injection | ||||||||||
| 140 | Coomes, 1961145 | 40 | 9 weeks | Non-RCT | No | No | 80–100 | No | Weak | Weak |
| Activity restriction vs exercise therapy | ||||||||||
| 564 | Lidstrom, 1970256 | 62 | 1 month | RCT | Unclear | Unclear | 80–100 | Unclear | Weak | Weak |
| Activity restriction vs mixed treatments | ||||||||||
| 713 | Hofstee, 2002267 | 250 | 6 months | RCT | Yes | No | 80–100 | No | Moderate | Moderate |
| 564 | Lidstrom, 1970256 | 62 | 1 month | RCT | Unclear | Unclear | 80–100 | Unclear | Weak | Weak |
| Activity restriction vs traction | ||||||||||
| 222 | Moret, 1998243 | 16 | 3 weeks | RCT | Yes | Partial | 80–100 | No | Moderate | Strong |
Activity restriction results at short-term follow-up (≤ 6 weeks)
Global effect at short-term follow-up
The results for the global effect at short-term follow-up are presented in Table 128 and the accompanying forest plot (Figure 85). There was no significant difference between bed rest and advice to keep active in two RCTs. 14,267 There was no significant difference between bed rest and mobilisation with exercises carried out in a hospital physiotherapy department,267 between rest and spinal manipulation with exercises, pelvic traction and exercises,137 or between bed rest and bed rest with vertical traction. 243
| ID no. | Author, year | Chronicity | Study design | Follow-up | Outcome measure | Perspective | Intervention | Control | OR (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n) | Outcome (n) | Withdrawal rate | Total (n) | Outcome (n) | Withdrawal rate | ||||||||
| Activity restriction vs education/advice | |||||||||||||
| 713 | Hofstee, 2002267 | A | RCT | 1 month | Treatment failure. Opposite extracted | Physician | 84 | 79 | 0.00 | 83 | 77 | 0.00 | 1.23 (0.36 to 4.20) |
| 658 | Vroomen, 199914 | A | RCT | 2 weeks | Assessment of improvement | Patient | 92 | 64 | 0.00 | 91 | 59 | 0.00 | 1.24 (0.67 to 2.30) |
| Activity restriction vs exercise therapy | |||||||||||||
| 564 | Lidstrom, 1970256 | A + C | RCT | 1 month | Patient’s ability to function socially was a decisive factor for both evaluations (no change or worse) | Patient | 21 | 14 | 0.00 | 21 | 10 | 0.00 | 2.20 (0.63 to 7.66) |
| Activity restriction vs mixed treatments | |||||||||||||
| 713 | Hofstee, 2002267 | A | RCT | 1 month | Treatment failure. Opposite extracted | Physician | 84 | 79 | 0.00 | 83 | 81 | 0.00 | 0.39(0.07 to 2.07) |
| 564 | Lidstrom, 1970256 | A + C | RCT | 1 month | Patient’s ability to function socially was a decisive factor for both evaluations (no change or worse) | Patient | 21 | 14 | 0.00 | 20 | 18 | 0.00 | 0.22 (0.04 to 1.24) |
| Activity restriction vs traction | |||||||||||||
| 222 | Moret, 1998243 | A | RCT | 3 weeks | Leg pain: recovered or strongly improved (vs little improved, no change, little worse, much worse or worse than ever) | Patient | 8 | 4 | 0.00 | 8 | 4 | 0.00 | 1.00 (0.14 to 7.10) |
FIGURE 85.
Summary of the findings of the global effect at short-term follow-up (≤ 6 weeks) for studies comparing activity restriction with alternative interventions. Note: weights are from random effects analysis.
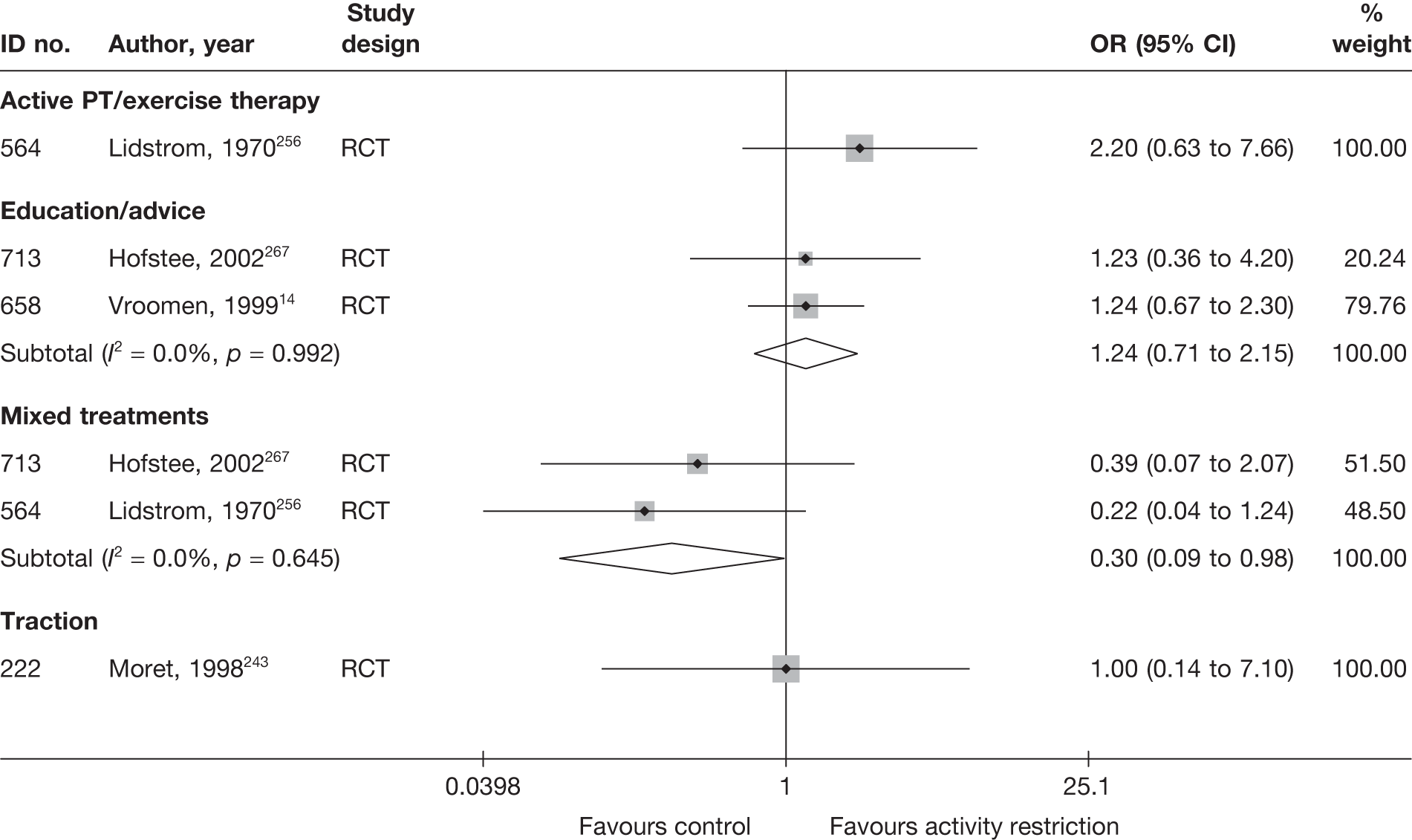
Pain intensity at short-term follow-up
The results for pain intensity at short-term follow-up are presented in Table 129 and the accompanying forest plot (Figure 86). There was a significant improvement in pain intensity in the bed rest group compared with advice to keep active in one RCT,14 but no significant difference in another RCT,267 and none when these results were combined in a meta-analysis. There was no significant difference in pain intensity between bed rest and mobilisation with exercises carried out in a hospital physiotherapy department. 267 There was a significant improvement in pain intensity in the bed rest with vertical traction group compared with the group treated with bed rest alone. 243
| ID no. | Author, year | Chronicity | Study design | Follow-up | Location | Scale (range)a | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)b | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | ||||||||
| Activity restriction vs education/advice | |||||||||||||||
| 713 | Hofstee, 2002267 | A | RCT | 1 month | Leg | VAS (0–100) | 82 | 83 | 65.5 (18.5) | 60.7 (21.4) | –25.9 (29.16) | –23.4 (29.16) | –2.50 (–11.40 to 6.40) | ||
| 658 | Vroomen, 199914 | A | RCT | 2 weeks | Leg | VAS (0–100) | 92 | 91 | 62 (22) | 68 (21) | 36 (28) | 44 (27) | –8.00 (–15.97 to –0.03) | ||
| Activity restriction vs mixed treatment | |||||||||||||||
| 713 | Hofstee, 2002267 (manipulation + exercise therapy | A | RCT | 1 month | Leg | VAS (0–100) | 82 | 80 | 65.5 (18.5) | 60.9 (20.1) | –25.9 (29.16) | –24.2 (29.31) | –1.70 (–10.70 to 7.30) | ||
| Activity restriction vs traction | |||||||||||||||
| 222 | Moret, 1998243 | A | RCT | 3 weeks | Leg | NRS (0–10) | 8 | 8 | 73 (10.0) | 74 (12.0) | 63 (10) | 44 (12) | –10.0 | –30.0 | 19.00 (8.18 to 29.82) |
FIGURE 86.
Summary of the findings of pain intensity at short-term follow-up (≤ 6 weeks) for studies comparing activity restriction with alternative interventions. Note: weights are from random effects analysis.
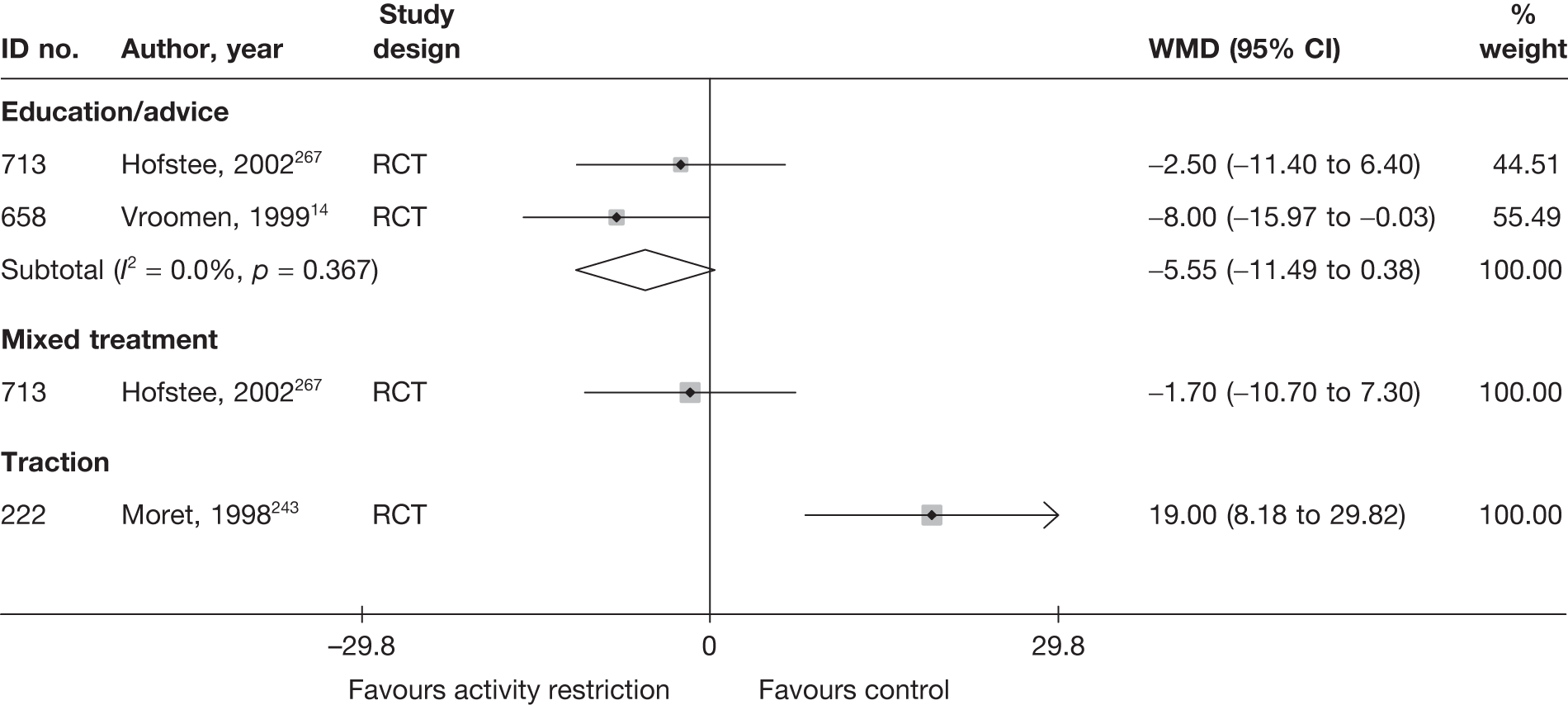
Condition-specific outcome measures at short-term follow-up
The results for CSOMs at short-term follow-up are presented in Table 130 and the accompanying forest plot (Figure 87). There was a significant improvement in CSOMs with advice to keep active compared with bed rest when the two RCTs were combined in a meta-analysis. 14,267 There was a significant improvement in CSOMs in the group receiving mobilisation with exercises carried out in a hospital physiotherapy department compared with the bed rest group in one RCT. 267 There was no significant difference in CSOMs in the bed rest with vertical traction group compared with the group treated with bed rest alone. 243
| ID no. | Author, year | Chronicity | Study design | Follow-up | Scale | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)a | Comment/conversionb | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | ||||||||
| Activity restriction vs education/advice | |||||||||||||||
| 713 | Hofstee, 2002267 | A | RCT | 1 month | QDS | 82 | 83 | 58.6 (14.6) | 57.4 (16.3) | 47.2 (14.6) | 41.2 (16.3) | –11.4 (18.84) | –16.2 (18.84) | 0.39 (0.08 to 0.70) |
Final means calculated from change scores Distribution at follow-up reported to be skewed ITT used (incorporating treatment compliance and dropouts), but dropouts excluded the results reported Dropouts: intervention 2/84, control 0/83 |
| 658 | Vroomen, 199914 | A | RCT | 3 weeks | Revised RMDQ | 92 | 91 | 5.5 (3.9) | 5.2 (3.8) | 14.8 (6.2) | 13.8 (6.3) | –2.7 | –4.0 |
0.16 (–0.13 to 0.45) Adjusted mean difference –1.6 (95% CI –3.7 to 0.4) |
ITT used For baseline and mean, high score = good outcome; sign of change score altered so that negative indicates improvement Adjusted difference between groups not based on change scores |
| Activity restriction vs traction | |||||||||||||||
| 222 | Moret, 1998243 | A | RCT | 3 weeks | RMDQ | 8 | 8 | 18.5 (2.1) | 18.1 (1.8) | 17.1 (6.2) | 14.5 (3.87) | –1.4 | –3.6 | 0.50 (1.50 to –0.49) | Final mean based on change score with SD imputed from weighted average |
| Activity restriction vs mixed treatment | |||||||||||||||
| 713 | Hofstee, 2002267 | A | RCT | 1 month | QDS | 82 | 80 | 58.6 (14.6) | 56 (17.6) | 47.2 (14.6) | 40.3 (14.6) | –11.4 (18.84) | –45.7 (18.89) | 0.47 (0.16 to 0.78) |
Final means calculated from change scores Distribution at follow-up reported to be skewed ITT used (incorporating treatment compliance and dropouts), but dropouts excluded in the results reported Dropouts: intervention 2/84, control 3/83 |
FIGURE 87.
Summary of the findings of CSOMs at short-term follow-up (≤ 6 weeks) for studies comparing activity restriction with alternative interventions. Note: weights are from random effects analysis.
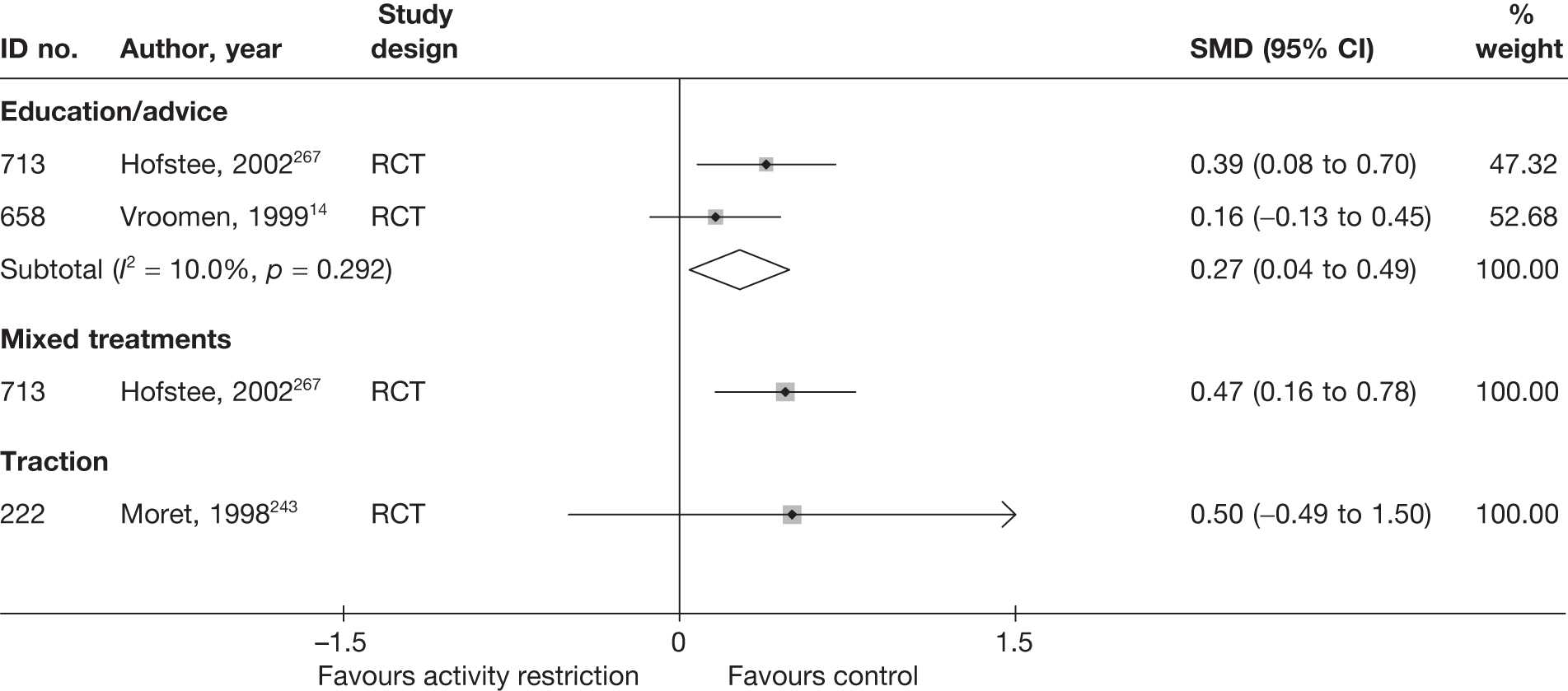
Activity restriction results at long-term follow-up
Global effect at medium-term follow-up
The results for the global effect at medium-term follow-up are presented in Table 131 and the accompanying forest plot (Figure 88). There was no significant difference between bed rest and advice to keep active in two RCTs. 14,267 There was no significant difference in one RCT between bed rest and mobilisation with exercises carried out in a hospital physiotherapy department. 267 There was a significant improvement in global effect for epidural injections compared with bed rest in one RCT. 145
| ID no. | Author, year | Chronicity | Study design | Follow-up | Outcome measure | Perspective | Intervention | Control | OR (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n) | Outcome (n) | Withdrawal rate | Total (n) | Outcome (n) | Withdrawal rate | ||||||||
| Activity restriction vs education/advice | |||||||||||||
| 713 | Hofstee, 2002267 | A | RCT | 6 months | Treatment failure. Opposite extracted | Physician | 84 | 63 | 0.00 | 83 | 69 | 0.00 | 0.16 (0.29 to 1.30) |
| 658 | Vroomen, 199914 | A | RCT | 12 weeks | Assessment of improvement | Patient | 92 | 80 | 0.00 | 91 | 79 | 0.00 | 1.01 (0.43 to 2.39) |
| Activity restriction vs epidural/intradiscal injection | |||||||||||||
| 140 | Coomes, 1961145 | A | Non-RCT | 9 weeks | Neurological state: completely relieved or improved (vs not changed or worse) | Physician | 20 | 5 | 0.00 | 20 | 12 | 0.00 | 0.22 (0.06 to 0.86) |
| Activity restriction vs mixed treatments | |||||||||||||
| 713 | Hofstee, 2002267 | A | RCT | 6 months | Treatment failure. Opposite extracted | Physician | 84 | 63 | 0.00 | 83 | 64 | 0.00 | 0.89 (0.44 to 1.81) |
FIGURE 88.
Summary of the findings of the global effect at medium-term follow-up (> 6 weeks to ≤ 6 months) for studies comparing activity restriction with alternative interventions. Note: weights are from random effects analysis.
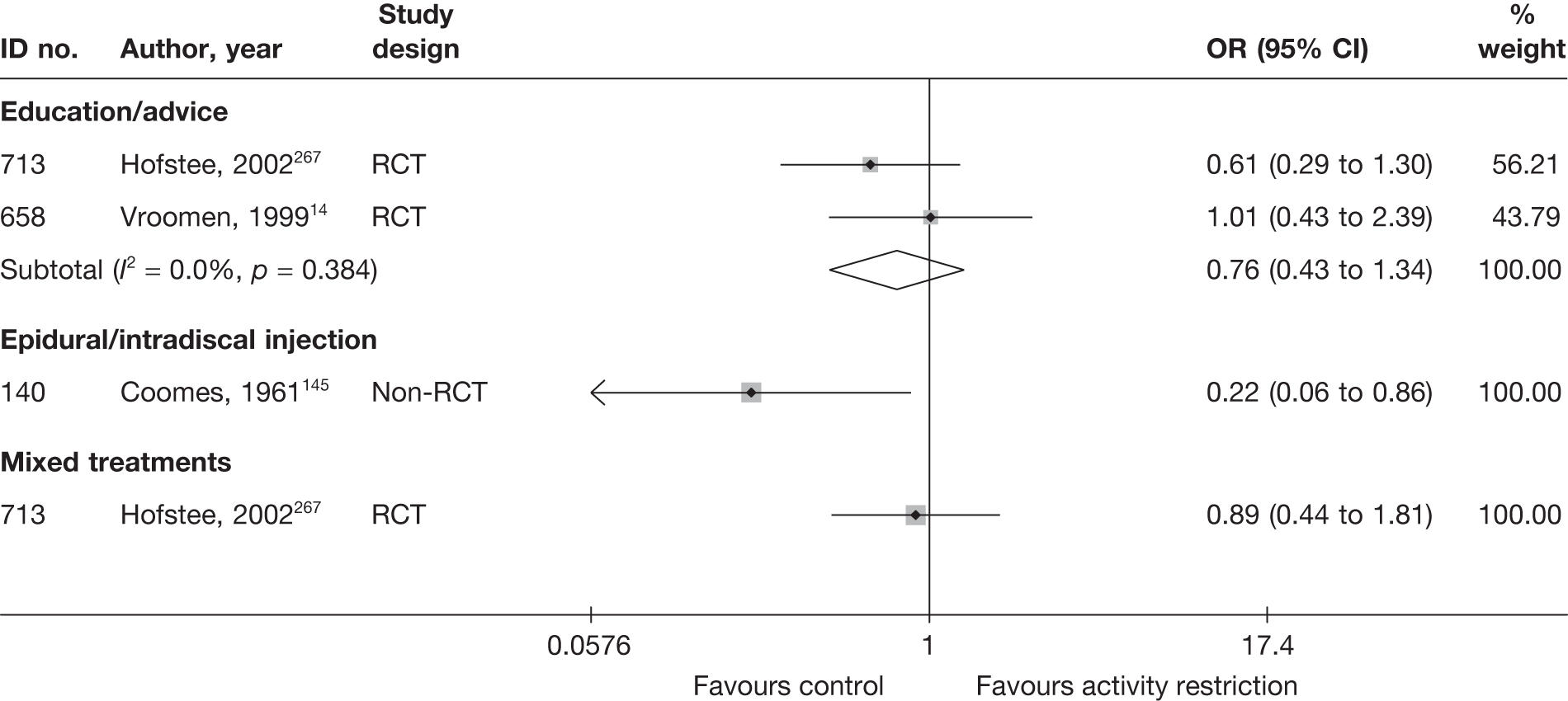
Pain intensity at medium-term follow-up
The results for pain intensity at medium-term follow-up are presented in Table 132 and the accompanying forest plot (Figure 89). There was no significant difference between bed rest and advice to keep active in two RCTs. 14,267 There was no significant difference in one RCT between bed rest and mobilisation with exercises carried out in a hospital physiotherapy department. 267
| ID no. | Author, year | Chronicity | Study design | Follow-up | Location | Scale (range)a | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)b | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | ||||||||
| Activity restriction vs education/advice | |||||||||||||||
| 713 | Hofstee, 2002267 | A | RCT | 6 months | Leg | VAS (0–100) | 78 | 75 | 65.5 (18.5) | 60.7 (21.4) | –48.2 (27.92) | –47.8 (30.45) | –0.40 (–9.67 to 8.87) | ||
| 658 | Vroomen, 199914 | A | RCT | 12 weeks | Leg | VAS (0–100) | 92 | 91 | 62 (22) | 68 (21) | 16 (26) | 14 (24) | 2.00 (–5.25 to 9.25) | ||
| Activity restriction vs mixed treatment | |||||||||||||||
| 713 | Hofstee, 2002267 | A | RCT | 6 months | Leg | VAS (0–100) | 78 | 72 | 65.5 (18.5) | 60.9 (20.1) | –48.2 (27.92) | –46.8 (27.83) | –1.40 (–10.33 to 7.53) | ||
FIGURE 89.
Summary of the findings of pain intensity at medium-term follow-up (> 6 weeks to ≤ 6 months) for studies comparing activity restriction with alternative interventions. Note: weights are from random effects analysis.
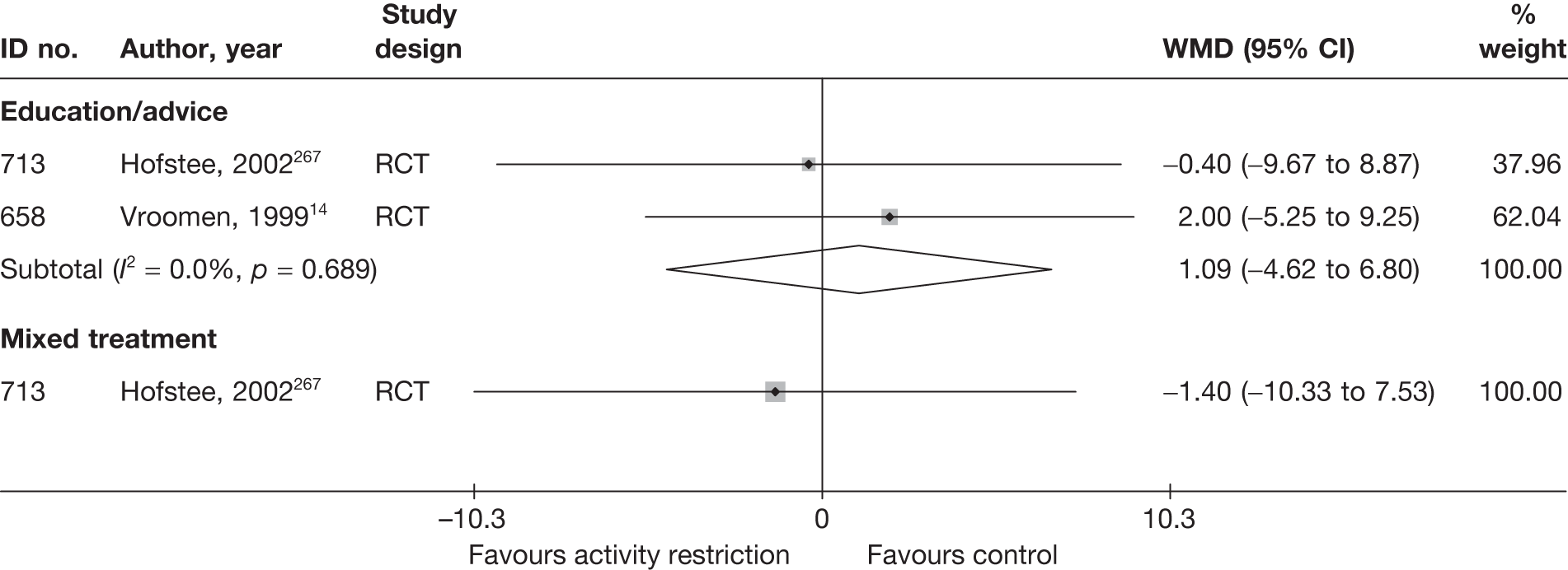
Condition-specific outcome measures at medium-term follow-up
The results for CSOMs at medium-term follow-up are presented in Table 133 and the accompanying forest plot (Figure 90). There was no significant difference between bed rest and advice to keep active in two RCTs. 14,267 There was no significant difference in one RCT between bed rest and mobilisation with exercises carried out in a hospital physiotherapy department. 267
| ID no. | Author, year | Chronicity | Study design | Follow-up | Scale | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)a | Comment/conversionb | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | ||||||||
| Activity restriction vs education/advice | |||||||||||||||
| 713 | Hofstee, 2002267 | A | RCT | 6 months | QDS | 78 | 75 | 58.6 (14.6) | 57.4 (16.3) | 25.9 (14.6) | 22 (16.3) | –32.7 (23.66) | –35.4 (23.66) | 0.25 (–0.07 to 0.57) | |
| 658 | Vroomen, 199914 | A | RCT | 12 weeks | Revised RMDQ | 92 | 91 | 5.5 (3.9) | 5.2 (3.8) | 7.8 (7) | 7.3 (7) | –9.7 | –10.5 |
0.07 (–0.22 to 0.36) Adjusted mean difference –0.5 (95% CI –2.6 to 1.6) |
ITT used For baseline and mean, high score = good outcome; sign of change score altered so that negative indicates improvement in our analysis |
| Activity restriction vs mixed treatment | |||||||||||||||
| 713 | Hofstee, 2002267 | A | RCT | 6 months | QDS | 78 | 75 | 58.6 (14.6) | 56 (17.6) | 25.9 (14.6) | 21.4 (17.6) | –32.7 (23.66) | –34.6 (23.9) | 0.28 (–0.04 to 0.60) | |
FIGURE 90.
Summary of the findings of CSOMs at medium-term follow-up (> 6 weeks to ≤ 6 months) for studies comparing activity restriction with alternative interventions. Note: weights are from random effects analysis.
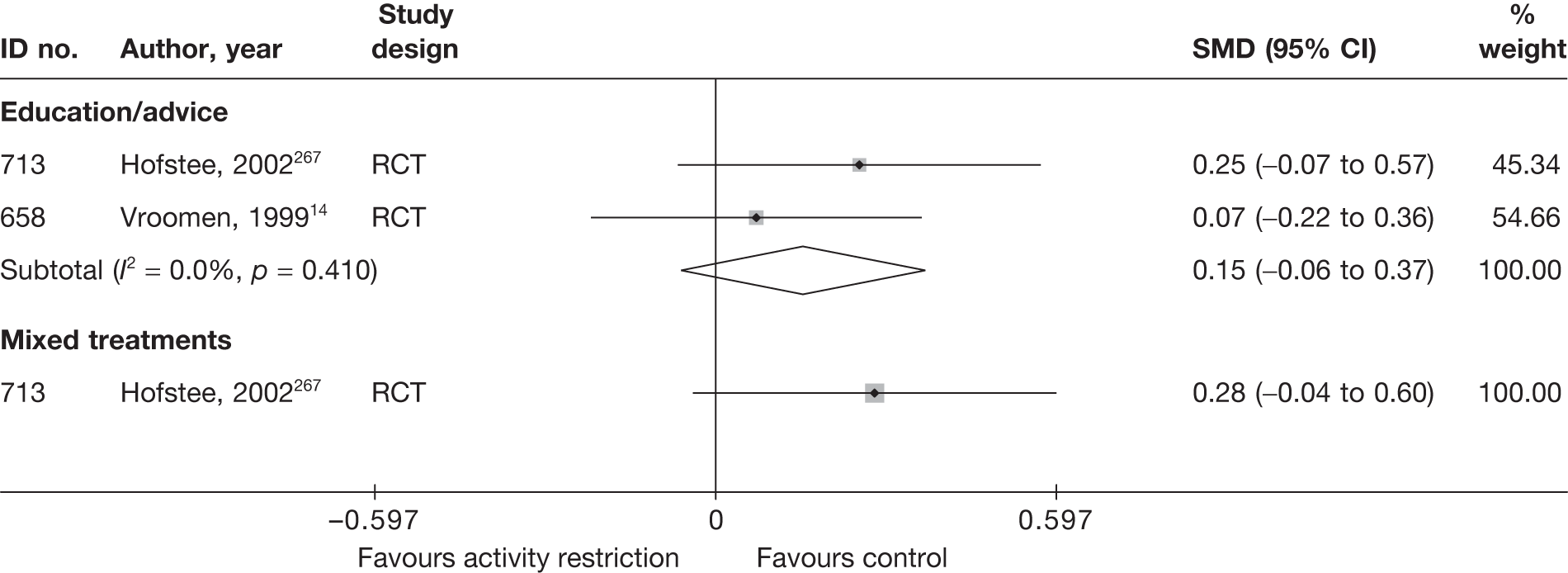
Activity restriction results at long-term follow-up (> 6 months)
No long-term outcomes were reported for global effect, pain intensity or CSOMs.
Adverse effects
The total number of adverse effects are presented in Table 134 and the accompanying forest plot (Figure 91). There was no significant difference between bed rest and advice to keep active in two RCTs,14,267 or between bed rest and epidural injection. 145 However, there were significantly fewer adverse effects in the bed rest group compared with the traction group in one RCT. 243
| ID no. | Author, year | Study design | No. of events in intervention group | No. of participants in intervention group | No. of events in control group | No. of participants in control group | OR (95% CI) |
|---|---|---|---|---|---|---|---|
| Activity restriction vs education/advice | |||||||
| 658 | Vroomen, 199914 | RCT | 2 | 92 | 4 | 91 | |
| 713 | Hofstee, 2002267 | RCT | 2 | 84 | 0 | 83 | 0.20 (0.01 to 4.18) |
| Activity restriction vs epidural | |||||||
| 140 | Coomes, 1961145 | Non-RCT | 0 | 20 | 1 | 20 | 0.32 (0.01 to 8.33) |
| Activity restriction vs exercise therapy | |||||||
| 564 | Lidstrom, 1970256 | RCT | NR | NR | NR | NR | |
| Activity restriction vs mixed treatment | |||||||
| 564 | Lidstrom, 1970256 | RCT | NR | NR | NR | NR | |
| 713 | Hofstee, 2002267 | RCT | 2 | 84 | 0 | 83 | 0.20 (0.01 to 4.18) |
| Activity restriction vs traction | |||||||
| 222 | Moret, 1998243 | RCT | 0 | 8 | 6 | 8 | 0.02 (0.00 to 0.56) |
FIGURE 91.
Summary of the findings of any adverse effect for studies comparing activity restriction with alternative interventions. Note: weights are from random effects analysis.
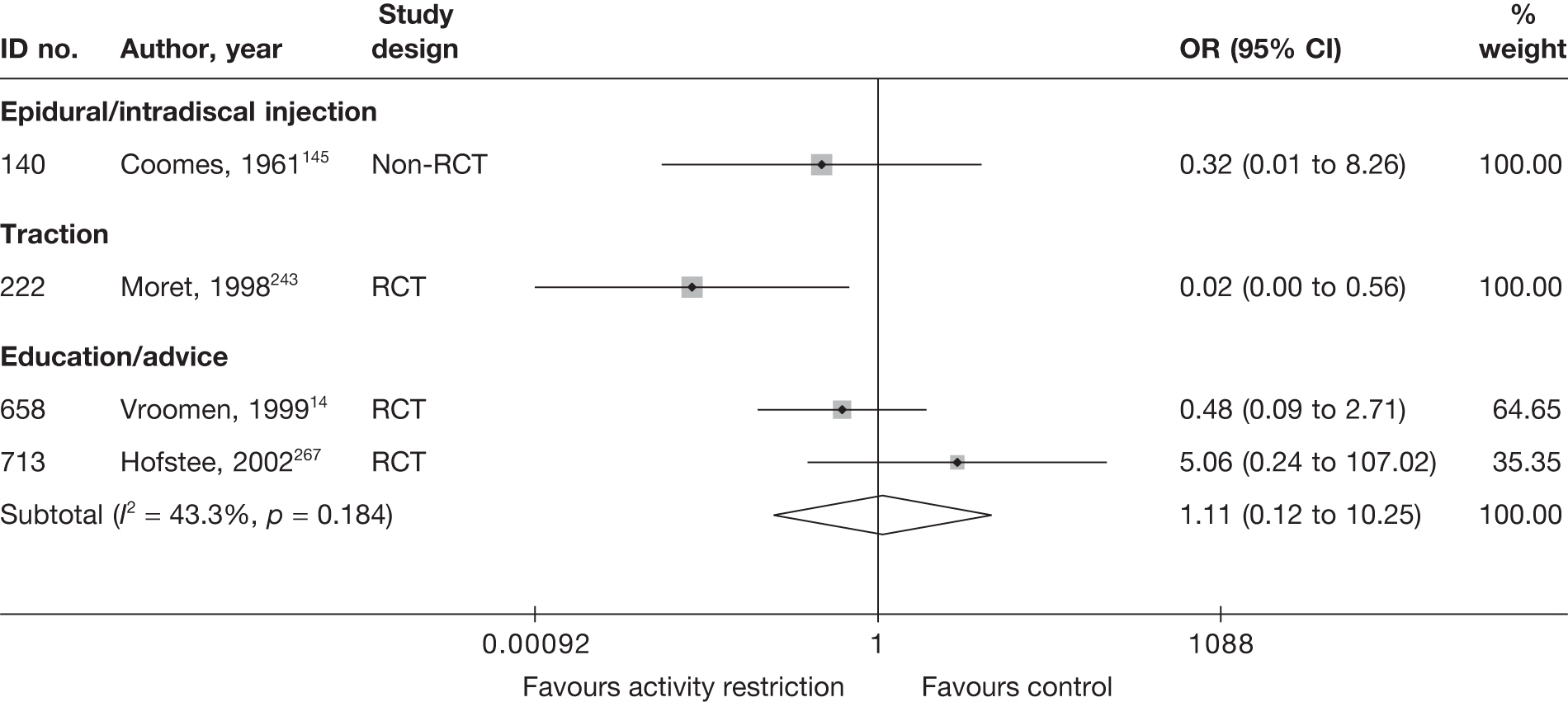
SUMMARY OF OVERALL FINDINGS FOR ACTIVITY RESTRICTION COMPARED WITH ALTERNATIVE INTERVENTIONS
Five studies,14,145,243,256,267 four of which were RCTs,14,243,256,267 compared the use of activity restriction with other interventions. Four RCTs restricted inclusion to patients with acute sciatica (Table 135). 14,243,256,267
| Control category | No. of studies (arms) | Sample size range (median) | Proportion of studies that were RCTs (%) | Proportion of studies that were deemed good quality (%) | Proportion of studies that only included acute sciatica (%) | Proportion of studies that included patients with nerve root pain (%) | Proportion of studies that reported diagnosis confirmed by imaging (%) | Proportion of studies that included patients with stenosis (%) | Proportion of studies that included patients with extruded/sequestered discs (%) | Proportion of studies that only included patients with first episode (%) | Proportion of studies that included patients who had received previous treatment (%) | Proportion of studies that included patients who had received previous surgery (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Activity restriction vs education/advice | 2 (2) | 183–250 (217) | 2/2 (100) | 0/2 (0) | 2/2 (100) | 2/2 (100) | 1/2 (50) | 0/2 (0) | 0/2 (0) | 0/2 (0) | 0/2 (0) | 1/2 (50) |
| Activity restriction vs epidural/intradiscal injection | 1 (1) | 40 (40) | 0/1 (0) | 0/1 (0) | 1/1 (100) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 1/1 (100) | 0/1 (0) |
| Activity restriction vs exercise therapy | 1 (1) | 62 (62) | 1/1 (100) | 0/1 (0) | 1/1 (100) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) |
| Activity restriction vs mixed treatment | 2 (2) | 183–250 (217) | 2/2 (100) | 0/2 (0) | 1/2 (50) | 2/2 (100) | 1/2 (50) | 0/2 (0) | 0/2 (0) | 0/2 (0) | 0/2 (0) | 1/2 (50) |
| Activity restriction vs traction | 1 (1) | 16 (16) | 1/1 (100) | 1/1 (100) | 1/1 (100) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 1/1 (100) | 1/1 (100) |
| Total (for activity restriction studies) a | 5 (7) | 16–250 (62) | 4/5 (80) | 1/5 (20) | 4/5 (80) | 5/5 (100) | 1/5 (20) | 0/5 (0) | 0/5 (0) | 0/5 (0) | 2/5 (40) | 2/5 (40) |
There was no significant difference between bed rest and advice to keep active, or between bed rest and mobilisation with exercises carried out in a hospital physiotherapy department, in terms of global effect or pain intensity at short- and medium-term follow-up. However, CSOMs at short-term follow-up were significantly better in the active groups, although there was no significant difference at medium-term follow-up. There was no significant difference between rest and spinal manipulation with exercises, or between pelvic traction and exercises, in terms of global effect or pain intensity at short-term follow-up. Nor was there a significant difference between bed rest and bed rest with vertical traction, in terms of short-term global effect or CSOMs, but there was a significant reduction in pain intensity in the short term in the traction group. There was a significant improvement in medium-term global effect following epidural injections compared with bed rest, with a significantly greater number of adverse effects (Table 135).
Opioids
Description of opioid studies
Summary of interventions
Three studies compared opioids with alternative types of intervention for sciatica. 214,229,230 Summary data of the interventions used are presented in Table 136. One three-arm RCT229 compared 10-day courses of intramuscular injections of a moderate-strength opioid tramadol with two oral antidepressants: imipramine or fluvoxamine. One RCT230 compared a 7-day course of oral tramadol with a tapering dose of the oral corticosteroid dexamethasone. The third was a four-arm crossover trial214 comparing 7-week courses of a potent opioid (morphine), an antidepressant (nortriptyline), a combination of morphine and nortriptyline and a placebo (benztropine).
| ID no. | Author, year | Study design | Treatment description | Control description |
|---|---|---|---|---|
| Opioids vs inactive control | ||||
| 534 | Khoromi, 2007214 | RCT (crossover) | Sustained-release morphine plus inert placebo (oral, ≤ 90 mg/day for 8.5 weeks) | Benztropine (active placebo) plus inert placebo (oral, 0.25–1.00 mg/day for 8.5 weeks) |
| Opioids vs mixed treatments (opioids and non-opioids) | ||||
| 534 | Khoromi, 2007214 | RCT (crossover) | Sustained-release morphine plus inert placebo (oral, ≤ 90 mg/day for 8.5 weeks) | Morphine plus nortriptyline (oral morphine, ≤ 90 mg/day for 8.5 weeks; oral nortriptyline, ≤ 100 mg/day for 7.5 weeks) |
| Opioids vs non-opioids | ||||
| 534 | Khoromi, 2007214 | RCT (crossover) | Sustained-release morphine plus inert placebo (oral, ≤ 90 mg/day for 8.5 weeks) | Nortriptyline plus inert placebo (oral, ≤ 100 mg/day for 7.5 weeks) |
| 547 | Kwasucki, 1993230 (Polish language) | RCT | Tramadol. First 5 days of 100 mg twice daily; sixth and seventh days 100 mg once daily | Dexamethasone. First and second days 24 mg (16 mg at 7am, 8 mg at 7pm); third day 8 mg twice daily; fourth and fifth days 4 mg twice daily; sixth and seventh days 4 mg once daily |
| 368 | Kwasucki, 2002229 (Polish language) | RCT | Tramadol (100 mg intramuscular injection) | Fluvoxamine (10 mg oral) |
| 368 | Kwasucki, 2002229 (Polish language) | RCT | Tramadol (100 mg intramuscular injection) | Imipramine (25 mg oral) |
Summary of study participants in opioid studies
The three RCTs214,229,230 included 168 participants with mean ages ranging from 43 to 53 years, a majority of men, acute and chronic symptom duration and all included recurrent episodes. Sciatica was confirmed by imaging in two out of three studies. One RCT included patients with spinal stenosis. Previous back surgery was either excluded or not reported (Table 137).
| ID no. | Author, year | Study design | No. of patients | Age (years) | No. of men (%) | Symptom duration | Type of sciatica | Confirmed by imaging? | Recurrent episode | Included patients with stenosis?a | Included patients with sequestered disc (or extruded)?a | Any previous treatment for sciatica? | Any previous back surgery for sciatica? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Opioids vs inactive control | |||||||||||||
| 534 | Khoromi, 2007214 | RCT (crossover) | 55 | Median 53 (range 19–65) | 30 (55) | Median 5 years (range 0.3–37 years) | Nerve root pain | Yes | Recurrent and first episode | No | No | Yes | No |
| Opioids vs mixed treatments (opioids and non-opioids) | |||||||||||||
| 534 | Khoromi, 2007214 | RCT (crossover) | 55 | Median 53 (range 19–65) | 30 (55) | Median 5 years (range 0.3–37 years) | Nerve root pain | Yes | Recurrent and first episode | No | No | Yes | No |
| Opioids vs non-opioids | |||||||||||||
| 534 | Khoromi, 2007214 | RCT (crossover) | 55 | Median 53 (range 19–65) | 30 (55) | Median 5 years (range 0.3–37 years) | Nerve root pain | Yes | Recurrent and first episode | No | No | Yes | No |
| 368 | Kwasucki, 2002229 (Polish language) | RCT | 70 | Mean 42.8 (range 23–68) | 51 (73) | Range 1 week–8 months | Nerve root pain | Yes | Recurrent and first episode | Yes | No | Yes | No |
| 547 | Kwasucki, 1993230 (Polish language) | RCT | 43 | Mean 43.2 (range 27–69) | 37 (86) | Mean 6.3 weeks (range 1 week–8 months) | Nerve root pain and referred pain | No | Recurrent and first episode | No | No | NR | NR |
Summary of study quality for opioid studies
Study details are summarised in Table 138. The full results of the quality assessment are presented in the appendices. None of the RCTs was of good quality, but one214 had an adequate method of random number generation, a secure method of allocation concealment and good external validity.
| ID no. | Author, year | Study size | Overall follow-up | Study design | Adequate randomisation? | Allocation concealment? | Follow-up (%) | Blind outcome assessment? | Overall quality rating | Overall external validity rating |
|---|---|---|---|---|---|---|---|---|---|---|
| Opioids vs inactive control | ||||||||||
| 534 | Khoromi, 2007214 | 55 | 36 weeks | RCT (crossover) | Yes | Yes | < 60 | Yes | Moderate | Strong |
| Opioids vs mixed treatment (opioids and non-opioids) | ||||||||||
| 534 | Khoromi, 2007214 | 55 | 36 weeks | RCT (crossover) | Yes | Yes | < 60 | Yes | Moderate | Strong |
| Opioids vs non-opioids | ||||||||||
| 534 | Khoromi, 2007214 | 55 | 36 weeks | RCT (crossover) | Yes | Yes | < 60 | Yes | Moderate | Strong |
| 368 | Kwasucki, 2002229 (Polish language) | 70 | 19 days | RCT | Unclear | Unclear | 80–100 | Unclear | Weak | Weak |
| 547 | Kwasucki, 1993230 (Polish language) | 43 | 2 weeks | RCT | Unclear | Unclear | 80–100 | Unclear | Wear | Weak |
Opioid results at short-term follow-up (≤ 6 weeks)
Global effect at short-term follow-up
The results for the global effect at short-term follow-up are presented in Table 139 and the accompanying forest plot (Figure 92). Short courses of opioids were compared with short courses of antidepressants or oral corticosteroids. One poor-quality RCT229 found that a course of intramuscular injections of tramadol was not significantly different from oral antidepressants, and one poor-quality RCT230 found that oral tramadol was significantly worse than a course of oral corticosteroid.
| ID no. | Author, year | Chronicity | Study design | Follow-up | Outcome measure | Perspective | Intervention | Control | OR (95% CI) | Comments | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n) | Outcome (n) | Withdrawal rate | Total (n) | Outcome (n) | Withdrawal rate | |||||||||
| Opioids vs non-opioids | ||||||||||||||
| 547 | Kwasucki, 1993230 (Polish language) | A + C | RCT | 2 weeks | Improvement in pain: cessation of symptoms or clear improvement (vs no improvement or mild improvement) | 22 | 8 | 0.00 | 21 | 16 | 0.00 | 22.50 (10.48 to 34.52) | Data extracted from histograms of raw pain scores | |
| 368 | Kwasucki, 2002229 (Polish language) (i)a (fluvoxamine) | A + C | RCT | 19 days (end of treatment) | Overall improvement: complete relief or improvement (vs no improvement) | Patient | 22 | 17 | 0.00 | 24 | 18 | 0.00 | 20.00 (6.84 to 33.16) | |
| 368 | Kwasucki, 2002229 (Polish language) (ii)a (imipramine) | A + C | RCT | 19 days (end of treatment) | Overall improvement: complete relief or improvement (vs no improvement) | Patient | 22 | 17 | 0.00 | 24 | 16 | 0.00 | 21.36 (12.49 to 30.24) | |
FIGURE 92.
Summary of the findings of the global effect at short-term follow-up (≤ 6 weeks) for studies comparing opioids with alternative interventions. Note: weights are from random effects analysis.
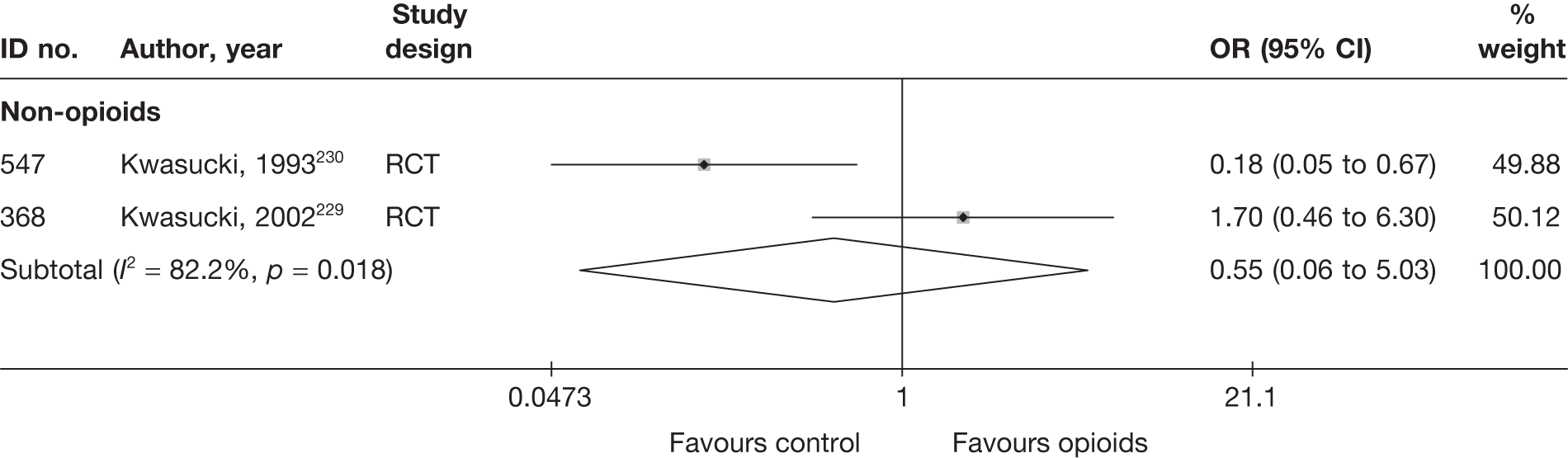
Pain intensity at short-term follow-up
The results for pain intensity at short-term follow-up are presented in Table 140 and the accompanying forest plot (Figure 93). Short courses of opioids were compared with short courses of antidepressants or oral corticosteroids. One poor-quality RCT229 found that a course of intramuscular injections of tramadol was not significantly different from oral antidepressants, and one moderate-quality RCT230 found that oral tramadol was significantly worse than a course of oral corticosteroid.
| ID no. | Author, year | Chronicity | Study design | Follow-up | Location | Scale (range)a | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)b | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | ||||||||
| Opioids vs non-opioids | |||||||||||||||
| 547 | Kwasucki, 1993230 (Polish language) | A + C | RCT | 2 weeks | Overall | NRS (0–4) | 22 | 21 | 77.5 (15) | 77.5 (12.5) | 50.0 (22.5) | 27.5 (17.5) | 22.50 (10.48 to 34.52) | ||
| 368 | Kwasucki, 2002229 (Polish language) (i)c (fluvoxamine) | A + C | RCT | 19 days | Overall | NRS (0–4) | 22 | 24 | 70 (17.5) | 67.5 (15) | 50.0 (25.0) | 30 (20) | 20.00 (6.84 to 33.16) | ||
| 368 | Kwasucki, 2002229 (Polish language) (ii)c (imipramine) | A + C | RCT | 19 days | Overall | NRS (0–4) | 22 | 24 | 70 (17.5) | 75 (25) | 50.0 (25.0) | 37.5 (25.0) | 12.50 (–1.96 to 26.96) | ||
FIGURE 93.
Summary of the findings of pain intensity at short-term follow-up (≤ 6 weeks) for studies comparing opioids with alternative interventions. Note: weights are from random effects analysis.
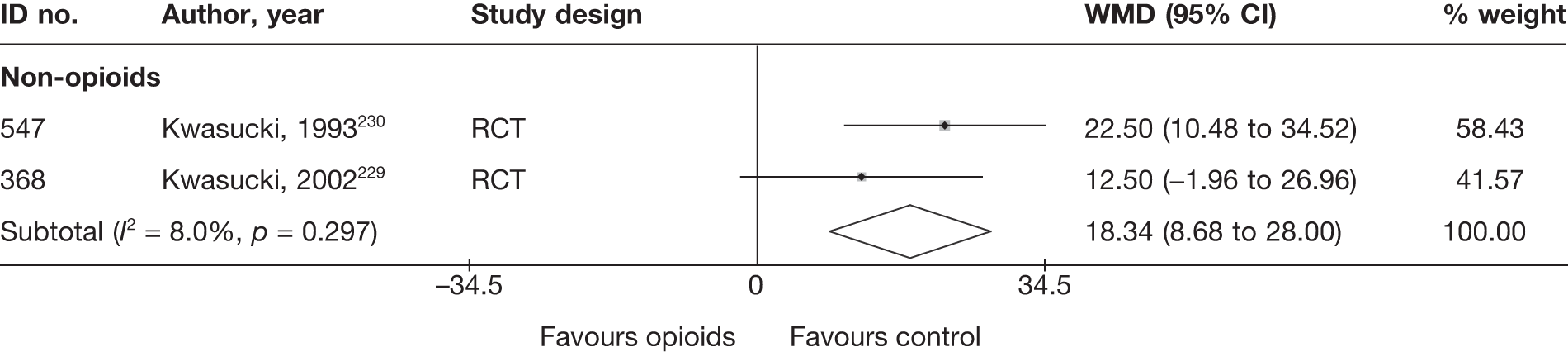
Condition-specific outcome measures at short-term follow-up
There were no CSOMs at short-term follow-up.
Opioid results at medium-term follow-up (> 6 weeks to ≤ 6 months)
Global effect at medium-term follow-up
The results for the global effect at medium-term follow-up are presented in Table 141 and the accompanying forest plot (Figure 94). One moderate-quality, four-arm crossover RCT214 found that a 7-week course of oral morphine had similar effects to 7-week courses of oral nortriptyline, a combination of morphine and nortriptyline or a placebo (benztropine).
| ID no. | Author, year | Chronicity | Study design | Follow-up | Outcome measure | Perspective | Intervention | Control | OR (95% CI) | Comments | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n) | Outcome (n) | Withdrawal rate | Total (n) | Outcome (n) | Withdrawal rate | |||||||||
| Opioids vs inactive control | ||||||||||||||
| 534 | Khoromi, 2007214 | C | RCT (crossover) | 9 weeks (end of treatment) | Global pain relief (GPR): worse pain, no pain relief | 32 | 13 | 0.42 | 33 | 11 | 0.40 | 1.37 (0.50 to 3.76) | Pain reported only for 28/50 patients (56%) who completed study (all treatments) | |
| Opioids vs non-opioids | ||||||||||||||
| 534 | Khoromi, 2007214 | C | RCT (crossover) | 9 weeks (end of treatment) | Global pain relief (GPR): worse pain, no pain relief | 32 | 13 | 0.42 | 31 | 12 | 0.44 | 1.08 (0.39 to 2.97) | Pain reported only for 28/50 patients (56%) who completed study (all treatments) | |
| Opioids vs mixed treatment (opioids and non-opioids) | ||||||||||||||
| 534 | Khoromi, 2007214 (opioids + non-opioids) | C | RCT (crossover) | 9 weeks (end of treatment) | Global pain relief (GPR): worse pain, no pain relief | 32 | 13 | 0.42 | 28 | 18 | 0.49 | 0.38 (0.13 to 1.08) | Pain reported only for 28/50 patients (56%) who completed study (all treatments) | |
FIGURE 94.
Summary of the findings of the global effect at medium-term follow-up (> 6 weeks to ≤ 6 months) for studies comparing opioids with alternative interventions. Note: weights are from random effects analysis.
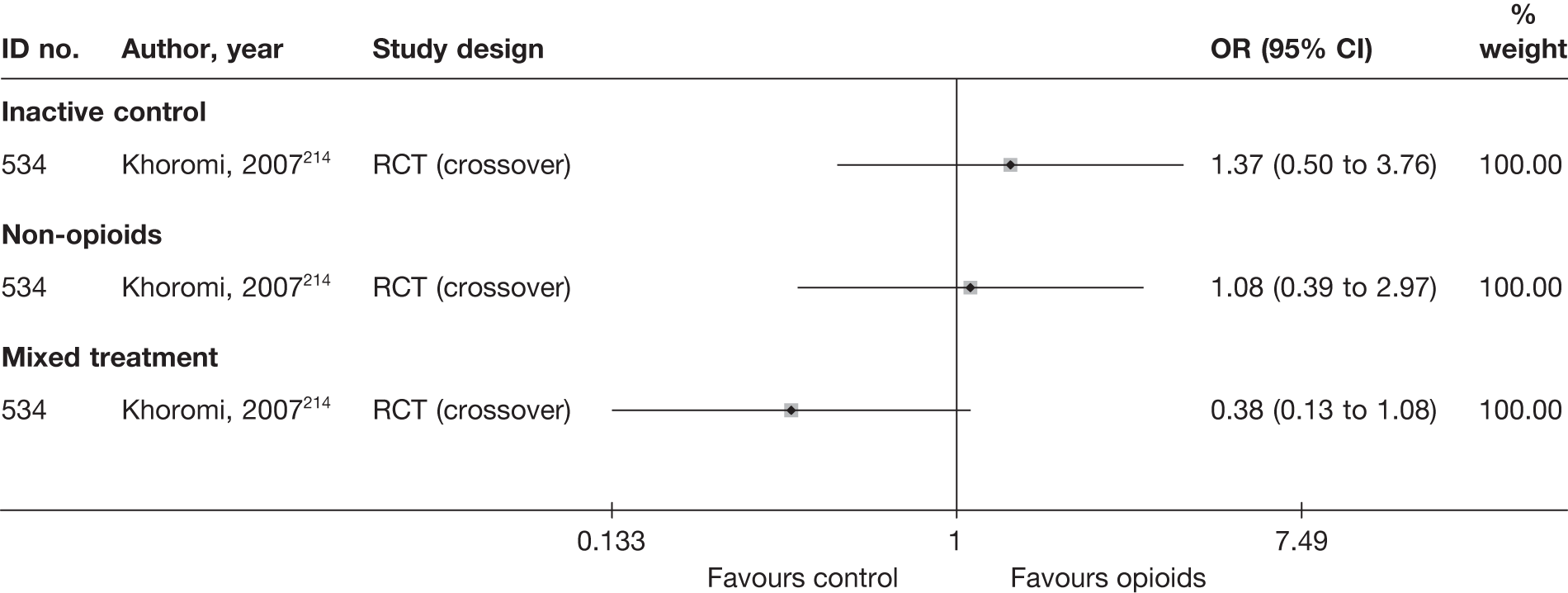
Pain intensity at medium-term follow-up
The results for pain intensity at medium-term follow-up are presented in Table 142 and the accompanying forest plot (Figure 95). One moderate-quality, four-arm crossover RCT214 found that a 7-week course of oral morphine had similar effects to 7-week courses of oral nortriptyline, a combination of morphine and nortriptyline or a placebo (benztropine).
| ID no. | Author, year | Chronicity | Study design | Follow-up | Location | Scale (range)a | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)b | Comment/conversionc | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |||||||||
| Opioids vs inactive control | ||||||||||||||||
| 534 | Khoromi, 2007214 | C | RCT (crossover) | 9 weeks (end of treatment) | Leg | NRS (0–10) | 28 | 28 | 49 (24.3) | 49 (24.3) | 34 (28) | 37.0 (27) | –3.00 (–17.41 to 11.41) | Pain reported only for 28/50 patients (56%) who completed study (all treatments) | ||
| Opioids vs non-opioid | ||||||||||||||||
| 534 | Khoromi, 2007214 | C | RCT (crossover) | 9 weeks (end of treatment) | Leg | NRS (0–10) | 28 | 28 | 49 (24.3) | 49 (24.3) | 34 (28) | 30.0 (27) | 4.00 (–10.41 to 18.41) | Pain reported only for 28/50 patients (56%) who completed study (all treatments) | ||
| Opioids vs mixed treatment (opioids and non-opioids) | ||||||||||||||||
| 534 | Khoromi, 2007214 | C | RCT (crossover) | 9 weeks (end of treatment) | Leg | NRS (0–10) | 28 | 28 | 49 (24.3) | 49 (24.3) | 34 (28) | 38.0 (24) | –4.00 (–17.66 to 9.66) | Pain reported only for 28/50 patients (56%) who completed study (all treatments) | ||
FIGURE 95.
Summary of the findings of pain intensity at medium-term follow-up (> 6 weeks to ≤ 6 months) for studies comparing opioids with alternative interventions. Note: weights are from random effects analysis.
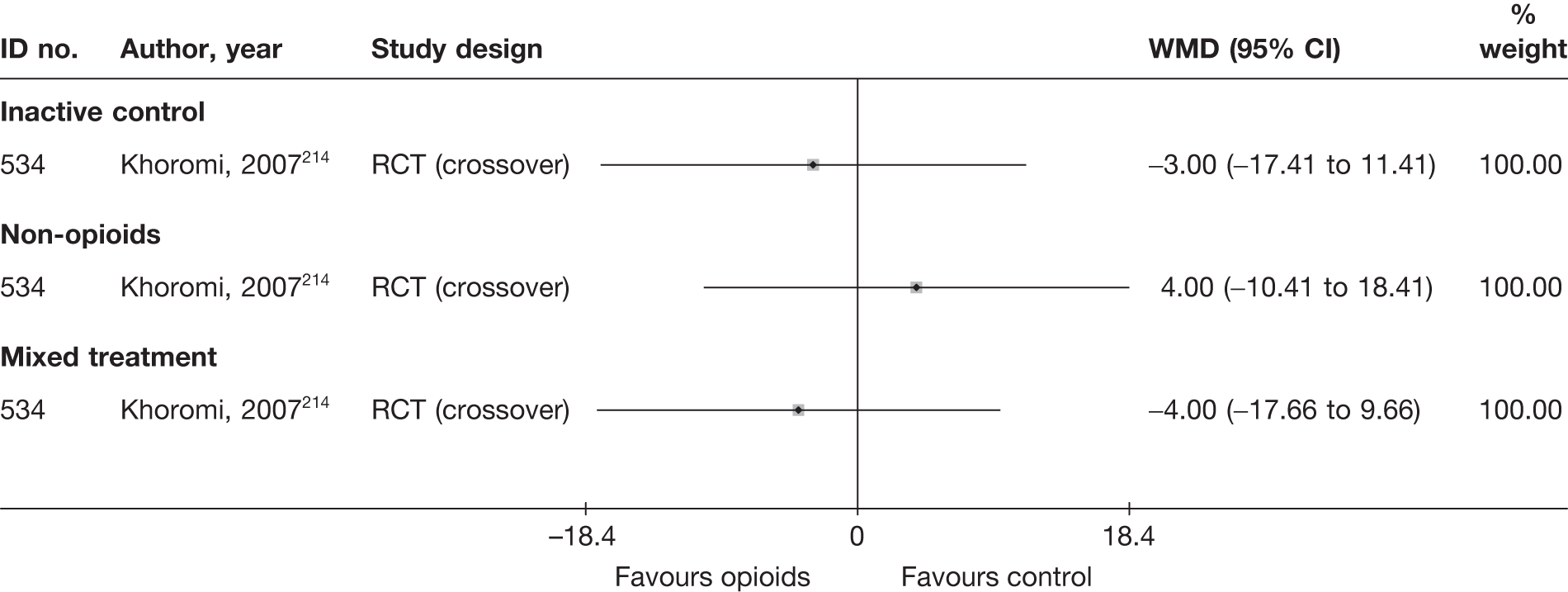
Condition-specific outcome measures at medium-term follow-up
The results for CSOMs at medium-term follow-up are presented in Table 143 and the accompanying forest plot (Figure 96). One moderate-quality, four-arm crossover RCT214 found that a 7-week course of oral morphine had similar effects to 7-week courses of oral nortriptyline, a combination of morphine and nortriptyline or a placebo (benztropine).
| ID no. | Author, year | Chronicity | Study design | Follow-up | Scale | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)a | Comment/conversionb | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | ||||||||
| Opioids vs inactive control | |||||||||||||||
| 534 | Khoromi, 2007214 | C | RCT (crossover) | 9 weeks (end of treatment) | NRS (0–10) | 28 | 28 | 30 (15) | 30 (15) | 27.5 (16.7) | 30.5 (15.9) | –0.18 (–0.71 to 0.35) | Pain reported only for 28/50 patients (56%) who completed study (all treatments) | ||
| Opioids vs non-opioid | |||||||||||||||
| 534 | Khoromi, 2007214 | C | RCT (crossover) | 9 weeks (end of treatment) | NRS (0–10) | 28 | 28 | 30 (15) | 30 (15) | 25.7 (16.5) | 27.5 (16.7) | 0.01 (–0.52 to 0.53) | Pain reported only for 28/50 patients (56%) who completed study (all treatments) | ||
| Opioids vs mixed treatment (opioids and non-opioids) | |||||||||||||||
| 534 | Khoromi, 2007214 | C | RCT (crossover) | 9 weeks (end of treatment) | NRS (0–10) | 28 | 28 | 30 (15) | 30 (15) | 27.5 (16.7) | 27.4 (15.4) | –0.11 (–0.63 to 0.42) | Pain reported only for 28/50 patients (56%) who completed study (all treatments) | ||
FIGURE 96.
Summary of the findings of CSOMs at medium-term follow-up (> 6 weeks to ≤ 6 months) for studies comparing opioids with alternative interventions. Note: weights are from random effects analysis.
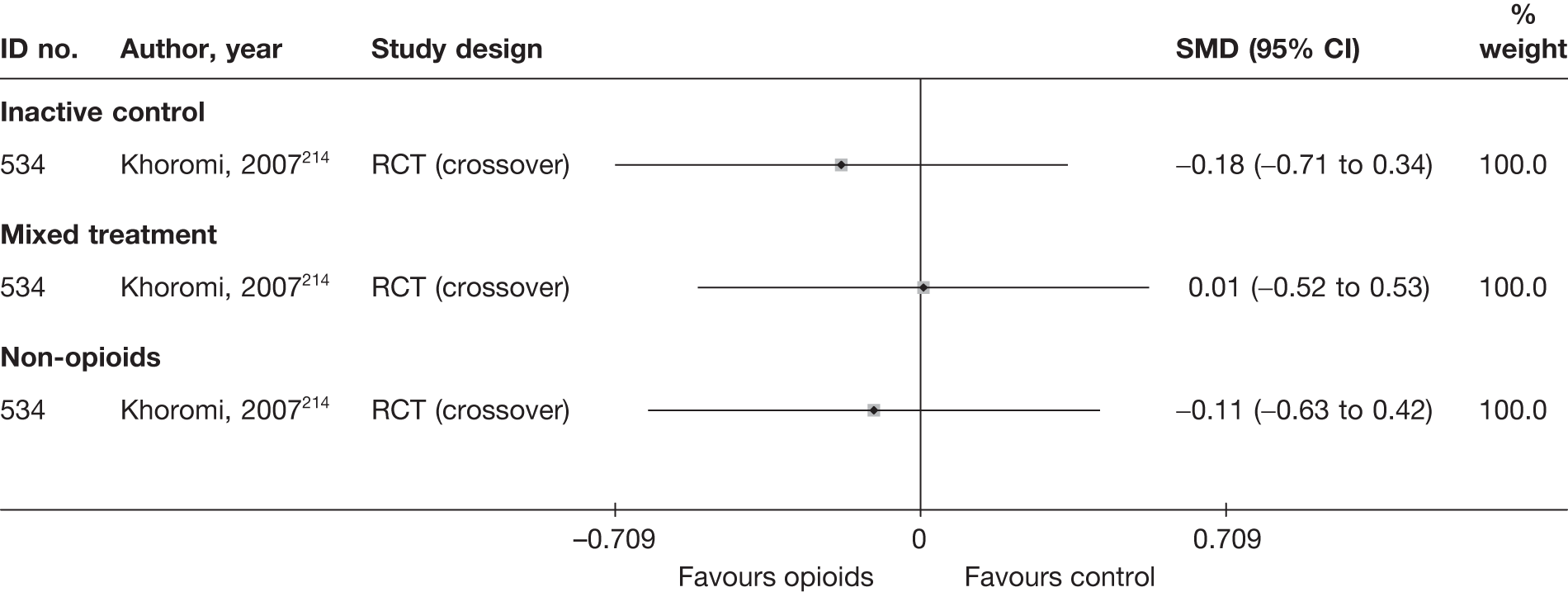
Opioid results at long-term follow-up (> 6 months)
No studies with long-term global effect, pain intensity or CSOMs were identified.
Adverse effects
Adverse effects were very poorly reported in most studies. Table 144 and the accompanying forest plot (Figure 97) present the overall number of any adverse event that occurred. More detailed description of these are presented in the appendices. There was evidence from one RCT214 that opioids had more adverse effects than placebo, but there was conflicting evidence from two RCTs229,214 about the number of adverse effects associated with placebo compared with antidepressants.
| ID no. | Author, year | Study design | No. of events in intervention group | No. of participants in intervention group | No. of events in control group | No. of participants in control group | OR (95% CI) |
|---|---|---|---|---|---|---|---|
| Opioids vs inactive control | |||||||
| 534 | Khoromi, 2007214 | RCT (crossover) | 51 | 55 | 28 | 55 | 12.29 (3.91 to 38.7) |
| Opioids vs mixed treatment (opioids and non-opioids) | |||||||
| 534 | Khoromi, 2007214 | RCT (crossover) | 51 | 55 | 49 | 55 | 1.56 (0.42 to 5.87) |
| Opioids vs non-opioids | |||||||
| 534 | Khoromi, 2007214 | RCT (crossover) | 51 | 55 | 37 | 55 | 6.20 (1.94 to 19.85) |
| 547 | Kwasucki, 1993230 | RCT | NR | NR | NR | NR | |
| 368 | Kwasucki, 2002229(fluvoxamine) | RCT | 1 | 22 | 2 | 24 | 0.52 (0.04 to 6.22) |
| 368 | Kwasucki, 2002229(imipramine) | RCT | 1 | 22 | 12 | 24 | 0.05 (0.01 to 0.41) |
FIGURE 97.
Summary of the findings of any adverse effect for studies comparing opioids with alternative interventions. Note: weights are from random effects analysis.
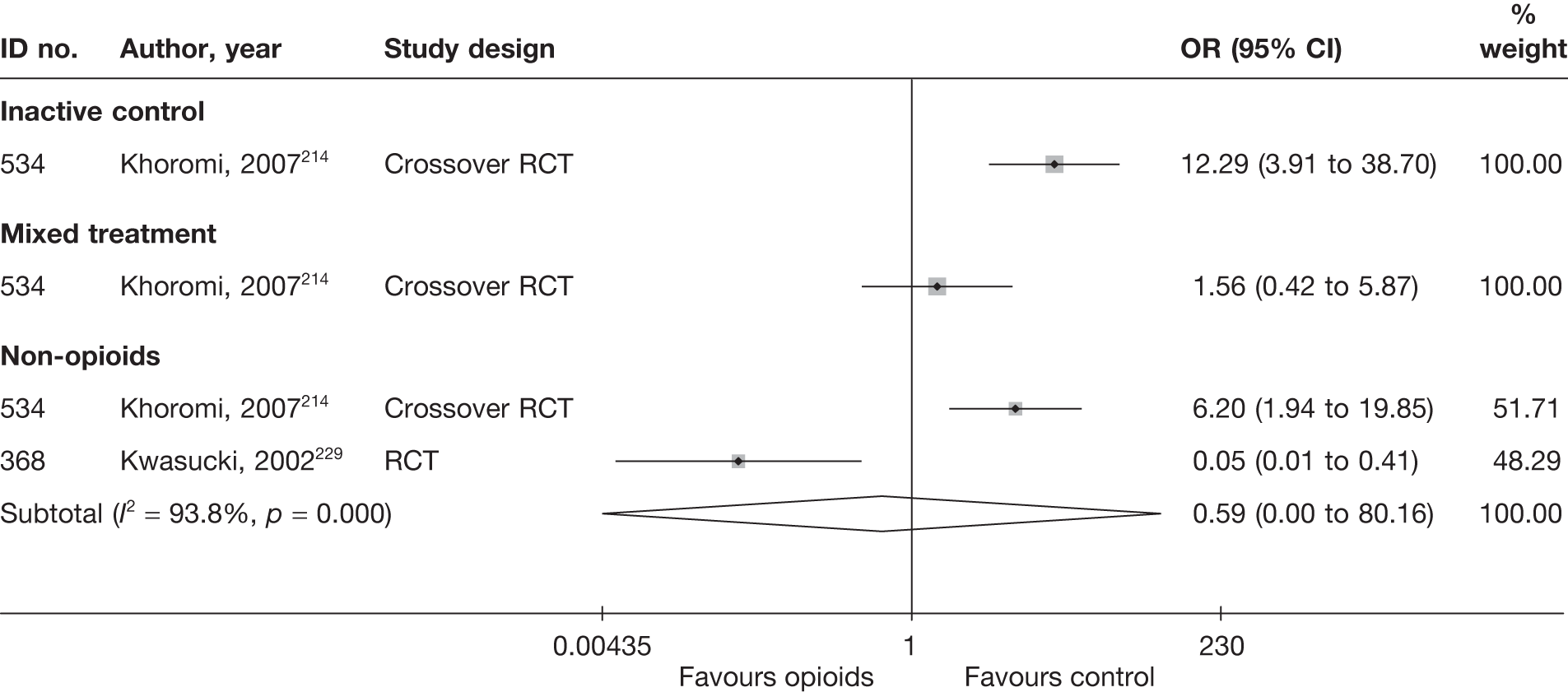
SUMMARY OF OVERALL FINDINGS FOR OPIOIDS COMPARED WITH ALTERNATIVE INTERVENTIONS
Three RCTs compared the use of opioids with other interventions (Table 145). 214,229,230
| Control category | No. of studies (arms) | Sample size range (median) | Proportion of studies that were RCTs (%) | Proportion of studies that were deemed good quality (%) | Proportion of studies that only included acute sciatica (%) | Proportion of studies that included patients with nerve root pain (%) | Proportion of studies that reported diagnosis confirmed by imaging (%) | Proportion of studies that included patients with stenosis (%) | Proportion of studies that included patients with extruded/sequestered discs (%) | Proportion of studies that only included patients with first episode (%) | Proportion of studies that included patients who had received previous treatment (%) | Proportion of studies that included patients who had received previous surgery (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Opioids vs inactive control | 1 (1) | 55 (55) | 1/1 (100) | 1/1 (100) | 0/1 (0) | 1/1 (100) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 1/1 (100) | 0/1 (0) |
| Opioids vs mixed treatment | 1 (1) | 55 (55) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 1/1 (100) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 1/1 (100) | 0/1 (0) |
| Opioids vs non-opioids | 3 (4) | 43–70 (55) | 2/3 (67) | 1/3 (33) | 0/3 (0) | 3/3 (100) | 2/3 (67) | 1/3 (33) | 0/3 (0) | 0/3 (0) | 2/3 (67) | 0/3 (0) |
| Total (for opioid studies) a | 3 (6) | 43–70 (55) | 3/3 (100) | 1/3 (33) | 0/3 (0) | 3/3 (100) | 2/3 (67) | 1/3 (33) | 0/3 (0) | 0/3 (0) | 2/3 (67) | 0/3 (0) |
At short-term follow-up opioids were more efficacious than placebo in one moderate-quality crossover RCT214 in terms of global effect, but not pain intensity. There was no significant difference in effectiveness compared with antidepressants in terms of the global effect or pain intensity at short-term and medium-term follow-up in two moderate- or poor-quality RCTs. 229,214 Opioids were significantly less effective than a course of corticosteroids in one moderate-quality RCT. 230 Opioids had more adverse effects than placebo in one RCT, but there was conflicting evidence from two RCTs about the number of adverse effects associated with placebo compared with antidepressants.
Education/advice
Description of education/advice studies
Summary of interventions
Three studies compared educational interventions or advice with alternative treatments. 14,169,267 Summary data for the interventions are presented in Table 146. One RCT14 compared advice to keep active with bed rest for 2 weeks. One three-arm RCT267 compared bed rest for 7 days with advice to continue activities of daily living, or with hospital physiotherapy twice weekly for at least 4 weeks. Another three-arm pilot study169 compared two 60-minute educational sessions about postural advice and an educational booklet with a course of chiropractic spinal manipulation or three epidural injections of corticosteroid. This pilot RCT169 did not compare outcome measures between groups.
| ID no. | Author, year | Study design | Treatment description | Control description |
|---|---|---|---|---|
| Education/advice vs activity restriction | ||||
| 713 | Hofstee, 2002267 | RCT | Advised to continue activities of daily living (ADL) | Bed rest (BR) |
| 658 | Vroomen, 199914 | RCT | Advice to keep active | Bed rest |
| Education/advice vs epidural/intradiscal injection | ||||
| 722 | Bronfort, 2004169 | RCT | Self-care education | Three ESIs over 12 weeks |
| Education/advice vs manipulation | ||||
| 722 | Bronfort, 2004169 | RCT | Self-care education | Chiropractic spinal manipulation |
| Education/advice vs mixed treatments | ||||
| 713 | Hofstee, 2002267 | RCT | Advised to continue activities of daily living (ADL) | Hospital physiotherapy (Ph) – manipulation + exercises |
Summary of study participants in education/advice studies
The two RCTs that compared outcomes included 433 participants with mean ages between 39 and 46 years, mostly men, with acute symptom duration, and including recurrent symptoms. Sciatica was confirmed by imaging in one RCT. 267 There were no patients with spinal stenosis or sequestered discs and previous back surgery was excluded in one RCT (Table 147). 14
| ID no. | Author, year | Study design | No. of patients | Age | No. of men (%) | Symptom duration | Type of sciatica | Confirmed by imaging? | Recurrent episode | Included patients with stenosis?a | Included patients with sequestered disc (or extruded)?a | Any previous treatment for sciatica? | Any previous back surgery for sciatica? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Education/advice vs activity restriction | |||||||||||||
| 713 | Hofstee, 2002267 | RCT | 250 | Mean 39 (SD 10) | 150 (60) | Mean 2 weeks | Nerve root pain and referred pain | Yes | Recurrent | No | No | NR | Yes |
| 658 | Vroomen, 199914 | RCT | 183 | Mean 46 (SD 12) | 103 (56) | Median 16 days | Nerve root pain | No | Recurrent and first episode | No | No | NR | No |
| Education/advice vs epidural/intradiscal injection | |||||||||||||
| 722 | Bronfort, 2004169 | RCT | 32 | Mean 49.0 (SD 9.1) | 18 (56) | 1–3 months 19%, 4–6 months 6%, 7–12 months 9%, > 12 months 66% | Nerve root pain and referred pain | No | Recurrent and first episode | No | No | NR | No |
| Education/advice vs manipulation | |||||||||||||
| 722 | Bronfort, 2004169 | RCT | 32 | Mean 49.0 (SD 9.1) | 18 (56) | 1–3 months 19%, 4–6 months 6%, 7–12 months 9%, > 12 months 66% | Nerve root pain and referred pain | No | Recurrent and first episode | No | No | NR | No |
| Education/advice vs mixed treatments | |||||||||||||
| 713 | Hofstee, 2002267 | RCT | 250 | Mean 39 (SD 10) | 150 (60) | Mean 2 weeks | Nerve root pain and referred pain | Yes | Recurrent | No | No | NR | Yes |
Summary of study quality for education/advice studies
Study details are summarised in Table 148. The full results of the quality assessment are presented in the appendices. All of the studies were RCTs and one was of good quality. 14 Two had used an adequate method of random number generation,14,267 but none had a secure method of allocation concealment, and only one had good external validity. 14
| ID no. | Author, year | Study size | Overall follow-up | Study design | Adequate randomisation? | Allocation concealment? | Follow-up (%) | Blind outcome assessment? | Overall quality rating | Overall external validity rating |
|---|---|---|---|---|---|---|---|---|---|---|
| Education/advice vs activity restriction | ||||||||||
| 658 | Vroomen, 199914 | 183 | 12 weeks | RCT | Yes | No | 80–100 | Yes | Moderate | Strong |
| 713 | Hofstee, 2002267 | 250 | 6 months | RCT | Yes | No | 80–100 | No | Moderate | Moderate |
| Education/advice vs epidural/intradiscal injection | ||||||||||
| 722 | Bronfort, 2004169 | 32 | 52 weeks | RCT | Unclear | Partial | 80–100 | Unclear | Weak | Weak |
| Education/advice vs manipulation | ||||||||||
| 722 | Bronfort, 2004169 | 32 | 52 weeks | RCT | Unclear | Partial | 80–100 | Unclear | Weak | Weak |
| Education/advice vs mixed treatments | ||||||||||
| 713 | Hofstee, 2002267 | 250 | 6 months | RCT | Yes | No | 80–100 | No | Moderate | Moderate |
Education/advice results at short-term follow-up (≤ 6 weeks)
Global effect at short-term follow-up
The results for the global effect at short-term follow-up are presented in Table 149 and the accompanying forest plot (Figure 98). There was no significant difference between advice to keep active and bed rest in two moderate- or good-quality RCTs. 14,267 There was no significant difference between advice to keep active and mobilisation with exercises carried out in a hospital physiotherapy department in one RCT. 267
| ID no. | Author, year | Chronicity | Study design | Follow-up | Outcome measure | Perspective | Intervention | Control | OR (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n) | Outcome (n) | Withdrawal rate | Total (n) | Outcome (n) | Withdrawal rate | ||||||||
| Education/advice vs activity restriction | |||||||||||||
| 713 | Hofstee, 2002267 | A | RCT | 1 month | Treatment failure Opposite extracted | Physician | 83 | 77 | 0.00 | 84 | 79 | 0.00 | 0.81 (0.24 to 2.77) |
| 658 | Vroomen, 199914 | A | RCT | 2 weeks | Assessment of improvement | Patient | 91 | 59 | 0.00 | 92 | 64 | 0.00 | 0.81 (0.43 to 1.50) |
| Education/advice vs mixed treatments | |||||||||||||
| 713 | Hofstee, 2002267 | A | RCT | 1 month | Treatment failure Opposite extracted | Physician | 83 | 77 | 0.00 | 83 | 81 | 0.00 | 0.32 (0.06 to 1.62) |
FIGURE 98.
Summary of the findings of the global effect at short-term follow-up (≤ 6 weeks) for studies comparing education/advice with alternative interventions. Note: weights are from random effects analysis.
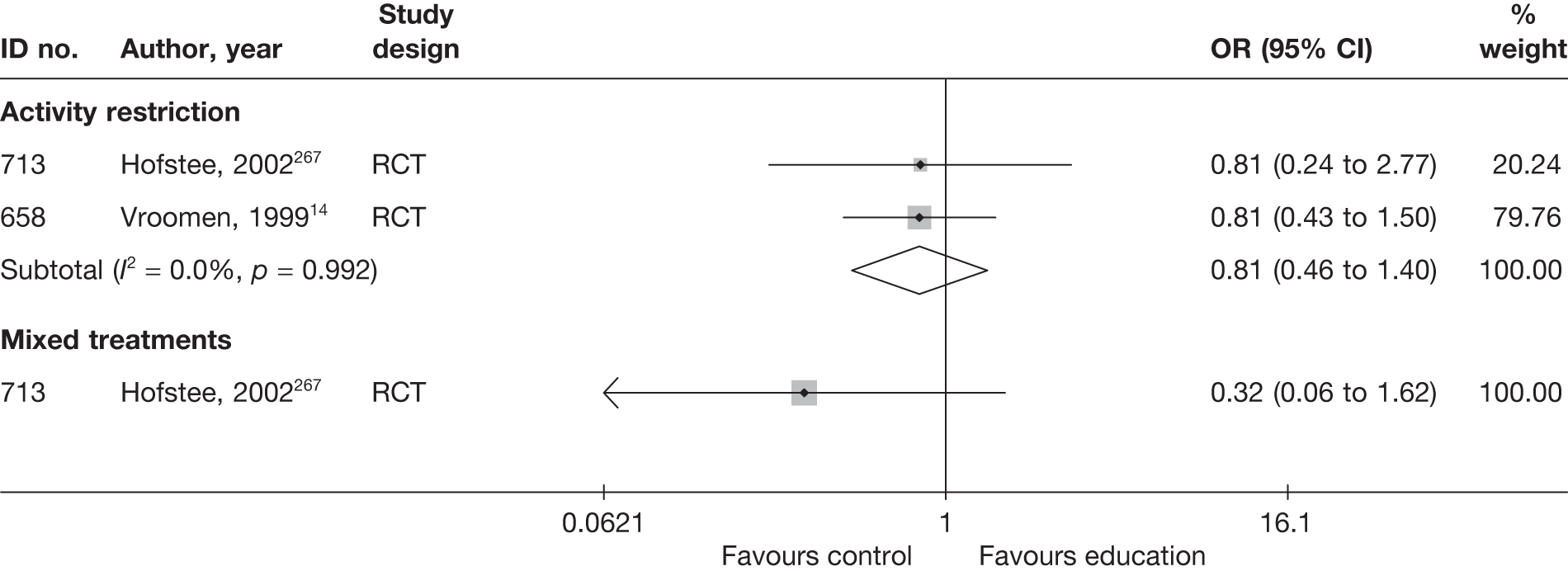
Pain intensity at short-term follow-up
The results for pain intensity at short-term follow-up are presented in Table 150 and the accompanying forest plot (Figure 99). There was no significant difference between advice to keep active and bed rest in two good- or moderate-quality RCTs. 14,267 There was no significant difference between advice to keep active and mobilisation with exercises carried out in a hospital physiotherapy department in one moderate-quality RCT. 267
| ID no. | Author, year | Chronicity | Study design | Follow-up | Location | Scale (range)a | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)b | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | ||||||||
| Education/advice vs activity restriction | |||||||||||||||
| 713 | Hofstee, 2002267 | A | RCT | 1 month | Leg | VAS (0–100) | 83 | 82 | 60.7 (21.4) | 65.5 (18.5) | –23.4 (29.16) | –25.9 (29.16) | 2.50 (–6.40 to 11.40) | ||
| 658 | Vroomen, 199914 | A | RCT | 2 weeks | Leg | VAS (0–100) | 91 | 92 | 68 (21) | 62 (22) | 44 (27) | 36 (28) | 8.00 (0.03 to 15.97) | ||
| Education/advice vs mixed treatment | |||||||||||||||
| 713 | Hofstee, 2002267 | A | RCT | 1 month | Leg | VAS (0–100) | 83 | 80 | 60.7 (21.4) | 60.9 (20.1) | –23.4 (29.31) | –24.2 (29.31) | 0.80 (–8.20 to 9.80) | ||
FIGURE 99.
Summary of the findings of pain intensity at short-term follow-up (≤ 6 weeks) for studies comparing education/advice with alternative interventions. Note: weights are from random effects analysis.
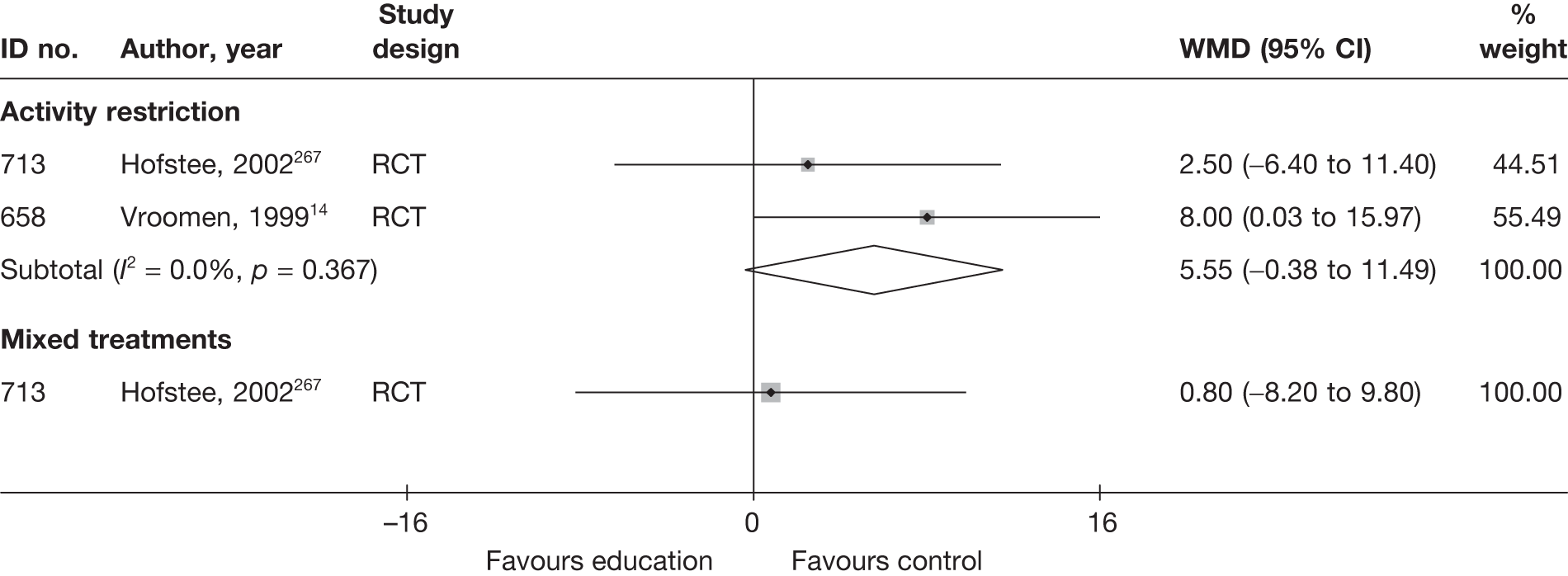
Condition-specific outcome measures at short-term follow-up
The results for CSOMs at short-term follow-up are presented in Table 151 and the accompanying forest plot (Figure 100). There was no significant difference between advice to keep active and bed rest in two good- or moderate-quality RCTs. 14,267 There was no significant difference between advice to keep active and mobilisation with exercises carried out in a hospital physiotherapy department in one moderate-quality RCT. 267
| ID no. | Author, year | Chronicity | Study design | Follow-up | Scale | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)a | Comment/conversionb | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | ||||||||
| Education/advice vs activity restriction | |||||||||||||||
| 713 | Hofstee, 2002267 | A | RCT | 1 month | QDS | 83 | 82 | 57.4 (16.3) | 58.6 (14.6) | 41.2 (16.3) | 41.2 (16.3) | –16.2 (18.84) | –16.2 (18.84) | 0.00 (–0.3 to 0.31) |
Final means calculated from change scores Distribution at follow-up reported to be skewed ITT analyses reported (incorporating treatment compliance and dropouts), but dropouts excluded the results reported Number randomised: BR 84, Ph 83, ADL (control) 83 |
| 658 | Vroomen, 199914 | A | RCT | 3 weeks | Revised RMDQ | 91 | 92 | 5.2 (3.8) | 5.5 (3.9) | 9.2 (6.3) | 9.2 (6.3) | –4 | –4 |
–0.16 (–0.43 to 0.13) Adjusted mean difference 1.6 (95% CI –0.4 to 3.7) |
ITT used For baseline and mean, high score = good outcome; sign of change score altered so that negative indicates improvement Adjusted difference between groups not based change scores |
| Education/advice vs mixed treatments | |||||||||||||||
| 713 | Hofstee, 2002267 | A | RCT | 1 month | QDS | 83 | 80 | 57.4 (16.3) | 56 (17.6) | 41.2 (16.3) | 41.2 (16.3) | –16.2 (18.89) | –16.2 (18.89) | 0.00 (–0.31 to 0.31) |
Final means calculated from change scores Distribution at follow-up reported to be skewed ITT analyses reported (incorporating treatment compliance and dropouts), but dropouts excluded the results reported Number randomised: BR 84, Ph 83, ADL (control) 83 |
FIGURE 100.
Summary of the findings of CSOMs at short-term follow-up (≤ 6 weeks) for studies comparing education/advice with alternative interventions. Note: weights are from random effects analysis.
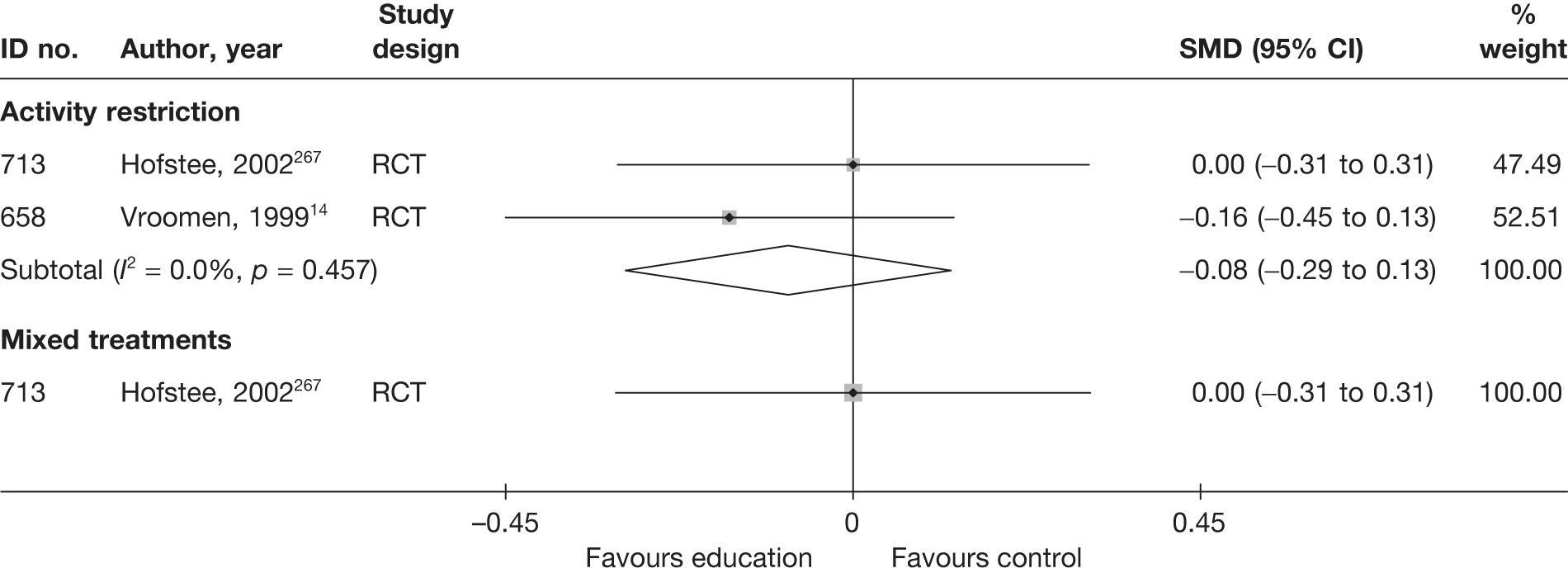
Education/advice results at medium-term follow-up (> 6 weeks to ≤ 6 months)
Global effect at medium-term follow-up
The results for the global effect at medium-term follow-up are presented in Table 152 and the accompanying forest plot (Figure 101). There was no significant difference between advice to keep active and bed rest in two good- or moderate-quality RCTs. 14,267 There was no significant difference between advice to keep active and mobilisation with exercises carried out in a hospital physiotherapy department in one moderate-quality RCT. 267
| ID no. | Author, year | Chronicity | Study design | Follow-up | Outcome measure | Perspective | Intervention | Control | OR (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n) | Outcome (n) | Withdrawal rate | Total (n) | Outcome (n) | Withdrawal rate | ||||||||
| Education/advice vs activity restriction | |||||||||||||
| 713 | Hofstee, 2002267 | A | RCT | 6 months | Treatment failure. Opposite extracted | Physician | 83 | 69 | 0.00 | 84 | 63 | 0.00 | 1.46 (0.77 to 3.50) |
| 658 | Vroomen, 199914 | A | RCT | 12 weeks | Assessment of improvement | Patient | 91 | 79 | 0.00 | 92 | 80 | 0.00 | 0.99 (0.42 to 2.33) |
| Education/advice vs mixed treatments | |||||||||||||
| 713 | Hofstee, 2002267 | A | RCT | 6 months | Treatment failure. Opposite extracted | Physician | 83 | 69 | 0.00 | 83 | 64 | 0.00 | 1.46 (0.68 to 3.16) |
FIGURE 101.
Summary of the findings of the global effect at medium-term follow-up (> 6 weeks to ≤ 6 months) for studies comparing education/advice with alternative interventions. Note: weights are from random effects analysis.
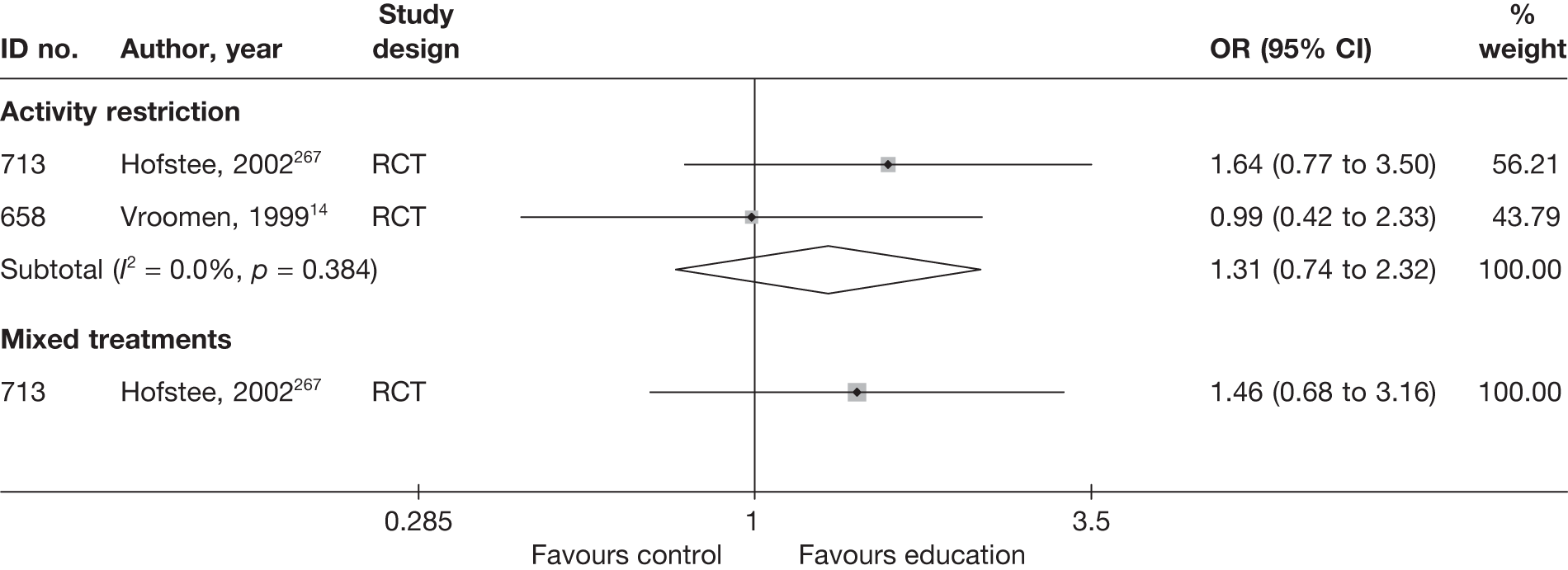
Pain intensity at medium-term follow-up
The results for pain intensity at medium-term follow-up are presented in Table 153 and the accompanying forest plot (Figure 102). There was no significant difference between advice to keep active and bed rest in two good- or moderate-quality RCTs. 14,267 There was no significant difference between advice to keep active and mobilisation with exercises carried out in a hospital physiotherapy department in one moderate-quality RCT. 267
| ID no. | Author, year | Chronicity | Study design | Follow-up | Location | Scale (range)a | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)b | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | ||||||||
| Education/advice vs activity restriction | |||||||||||||||
| 713 | Hofstee, 2002267 | A | RCT | 6 months | Leg | VAS (0–100) | 75 | 78 | 60.7 (21.4) | 65.5 (18.5) | –47.8 (30.45) | –48.2 (27.92) | 0.40 (–8.87 to 9.67) | ||
| 658 | Vroomen, 199914 | A | RCT | 12 weeks | Leg | VAS (0–100) | 91 | 92 | 68 (21) | 62 (22) | 14 (24) | 16 (26) | –2.00 (–9.25 to 5.25) | ||
| Education/advice vs mixed treatment | |||||||||||||||
| 713 | Hofstee, 2002267 | A | RCT | 6 months | Leg | VAS (0–100) | 75 | 72 | 60.7 (21.4) | 60.9 (20.1) | –47.8 (29.99) | –46.8 (27.83) | –1.00 (–10.35 to 8.35) | ||
FIGURE 102.
Summary of the findings of pain intensity at medium-term follow-up (> 6 weeks to ≤ 6 months) for studies comparing education/advice with alternative interventions. Note: weights are from random effects analysis.
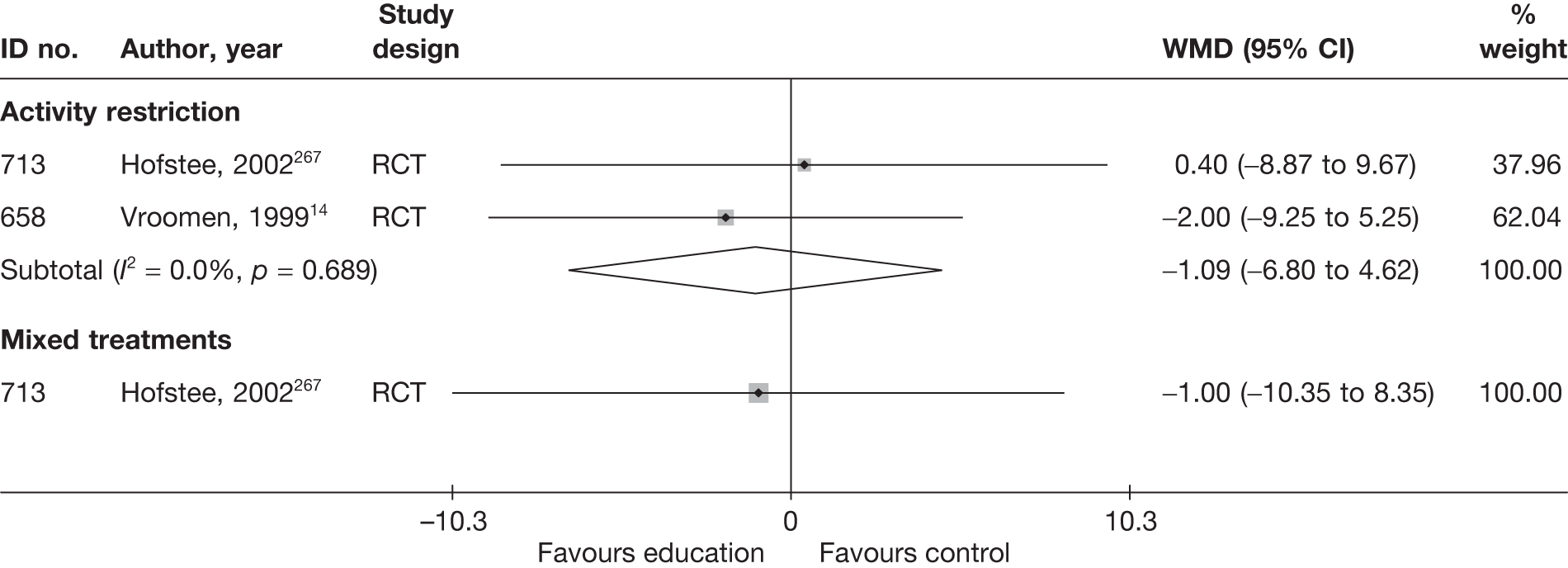
Condition-specific outcome measures at medium-term follow-up
The results for CSOMs at medium-term follow-up are presented in Table 154 and the accompanying forest plot (Figure 103). There was no significant difference between advice to keep active and bed rest in two good- or moderate-quality RCTs. 14,267 There was no significant difference between advice to keep active and mobilisation with exercises carried out in a hospital physiotherapy department in one moderate-quality RCT. 267
| ID no. | Author, year | Chronicity | Study design | Follow-up | Scale | Total (n) | Baseline mean (SD) | Final mean (SD) | Change scores (SD) | Mean difference (95% CI)a | Comment/conversionb | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | ||||||||
| Education/advice vs activity restriction | |||||||||||||||
| 713 | Hofstee, 2002267 | A | RCT | 2 months | QDS | 75 | 78 | 57.4 (16.3) | 58.6 (14.6) | 22 (16.3) | 25.9 (14.6) | –35.4 (23.66) | –32.7 (23.66) | –0.25 (–0.57 to 0.07) |
Final means calculated from change scores Distribution at follow-up reported to be skewed ITT analyses reported (incorporating treatment compliance and dropouts), but dropouts excluded the results reported Number randomised: BR 84, Ph 83, ADL (control) 83 |
| 658 | Vroomen, 199914 | A | RCT | 12 weeks | Revised RMDQ | 91 | 92 | 5.2 (3.8) | 5.5 (3.9) | 7.3 (7) | 7.8 (7) | –10.5 | –9.7 |
–0.07 (–0.36 to 0.22) Adjusted mean difference 0.5 (95% CI –1.6 to 2.6) |
For baseline and mean, high score = good outcome; sign of change score altered so that negative indicates improvement ITT used, method not stated |
| Education/advice vs mixed treatment | |||||||||||||||
| 713 | Hofstee, 2002267 | A | RCT | 2 months | QDS | 75 | 75 | 57.4 (16.3) | 56 (17.6) | 22 (16.3) | 21.4 (17.6) | –35.4 (23.9) | –34.6 (23.9) | 0.04 (0.28 to 0.36) |
Final means calculated from change scores Distribution at follow-up reported to be skewed ITT analyses reported (incorporating treatment compliance and dropouts), but dropouts excluded the results reported Number randomised: BR 84, Ph 83, ADL (control) 83 |
FIGURE 103.
Summary of the findings of CSOMs at medium-term (> 6 weeks to ≤ 6 months) follow-up for studies comparing education/advice with alternative interventions. Note: weights are from random effects analysis.
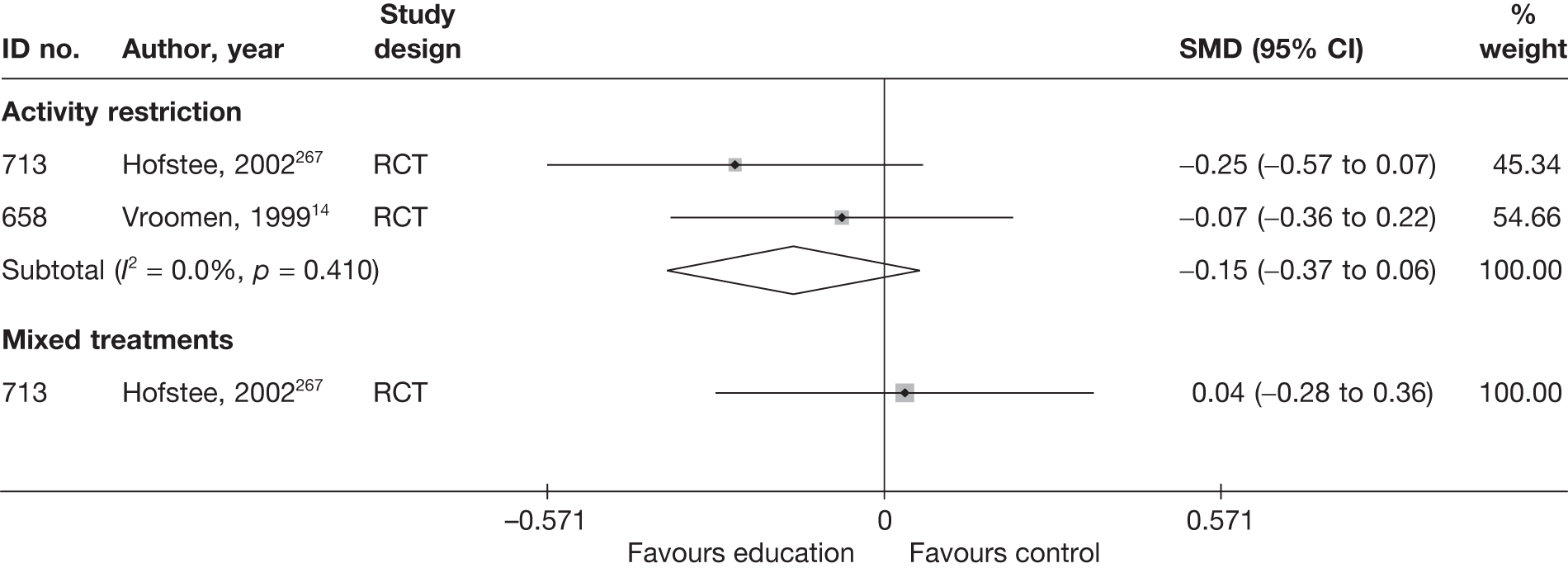
Education/advice at long-term follow-up (> 6 months)
No long-term outcomes were reported for global effect, pain intensity or CSOMs.
Adverse effects
Adverse effects were very poorly reported in most studies. Table 155 and the accompanying forest plot (Figure 104) present the overall number of any adverse event that occurred. More detailed descriptions of these are presented in the appendices. Education or advice interventions were associated with significantly fewer adverse events, in single RCTs, than epidural injections or spinal manipulation. There was no significant difference between the number of adverse events associated with education or advice compared with activity restriction in two RCTs.
| ID no. | Author, year | Study design | No. of events in intervention group | No. of participants in intervention group | No. of events in control group | No. of participants in control group | OR (95% CI) |
|---|---|---|---|---|---|---|---|
| Education/advice vs activity restriction | |||||||
| 713 | Hofstee, 2002267 | RCT | 0 | 83 | 2 | 84 | 0.20 (0.00 to 4.18) |
| 658 | Vroomen, 199914 | RCT | 4 | 91 | 2 | 92 | 2.07 (0.37 to 11.59) |
| Education/advice vs epidural | |||||||
| 722 | Bronfort, 2004169 | RCT | 0 | 10 | 10 | 10 | 0.00 (0.00 to 0.13) |
| Education/advice vs manipulation | |||||||
| 722 | Bronfort, 2004169 | RCT | 0 | 10 | 6 | 11 | 0.04 (0.00 to 0.86) |
| Education/advice vs mixed treatment | |||||||
| 713 | Hofstee, 2002267 | RCT | 0 | 83 | 0 | 83 | |
FIGURE 104.
Summary of the findings of any adverse effect for studies comparing education/advice with alternative interventions. Note: weights are from random effects analysis.
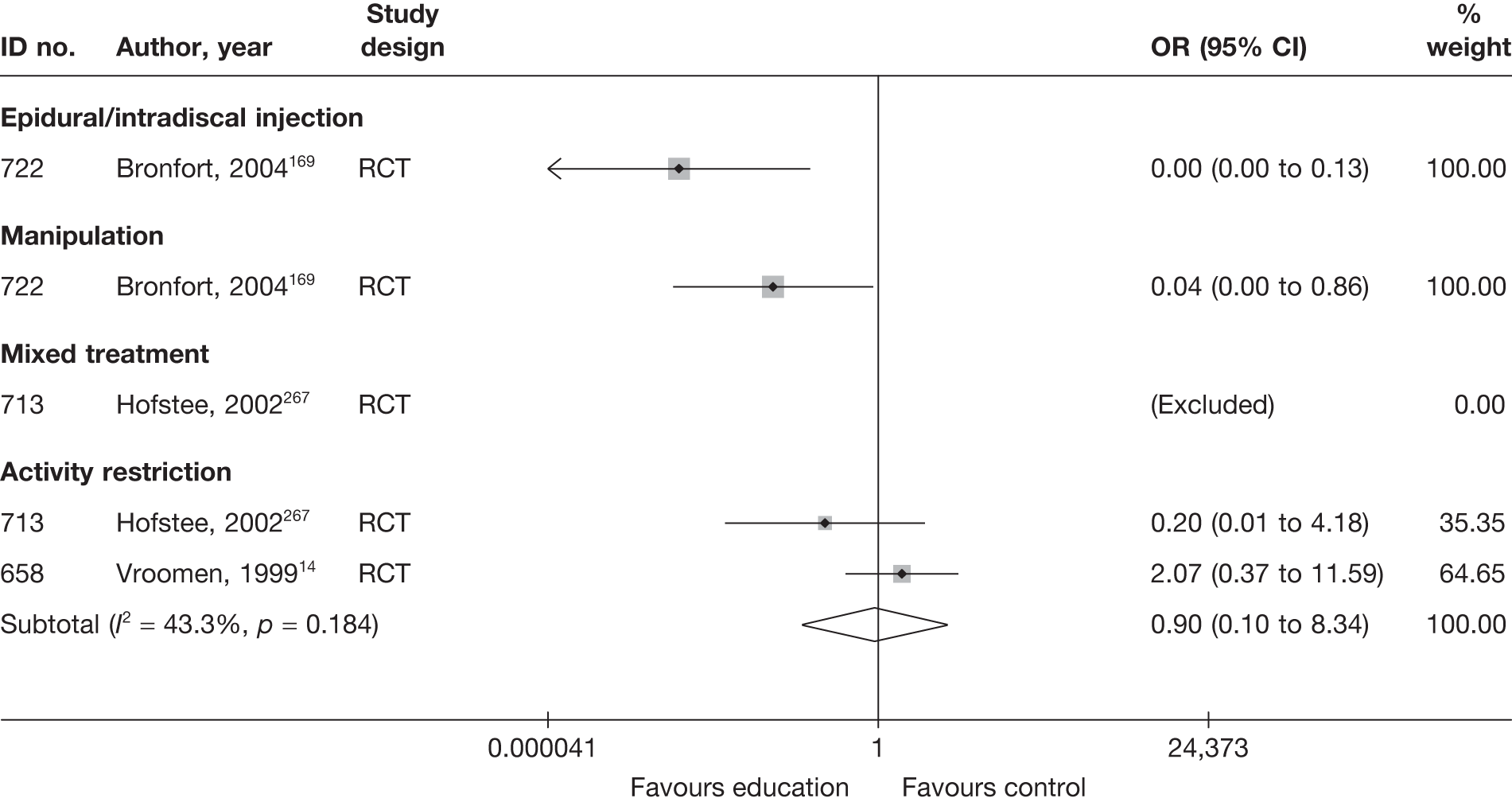
SUMMARY OF OVERALL FINDINGS FOR EDUCATION/ADVICE COMPARED WITH ALTERNATIVE INTERVENTIONS
Two moderate- or good-quality RCTs compared the use of opioids with other interventions (Table 156). 14,267
| Control category | No. of studies (arms) | Sample size range (median) | Proportion of studies that were RCTs (%) | Proportion of studies that were deemed good quality (%) | Proportion of studies that only included acute sciatica (%) | Proportion of studies that included patients with nerve root pain (%) | Proportion of studies that reported diagnosis confirmed by imaging (%) | Proportion of studies that included patients with stenosis (%) | Proportion of studies that included patients with extruded/sequestered discs (%) | Proportion of studies that only included patients with first episode (%) | Proportion of studies that included patients who had received previous treatment (%) | Proportion of studies that included patients who had received previous surgery (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Education/advice vs activity restriction | 2 (2) | 183–250 (217) | 2/2 (100) | 0/2 (0) | 2/2 (100) | 2/2 (100) | 1/2 (50) | 0/2 (0) | 0/2 (0) | 0/2 (0) | 0/2 (0) | 1/2 (50) |
| Education/advice vs mixed treatment | 1 (1) | 250 (250) | 1/1 (100) | 0/1 (0) | 1/1 (100) | 1/1 (100) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 1/1 (100) |
| Total (for education/advice studies) a | 2 (3) | 183–250 (217) | 2/2 (100) | 0/2 (0) | 2/2 (100) | 2/2 (100) | 1/2 (50) | 0/2 (0) | 0/2 (0) | 0/2 (0) | 0/2 (0) | 1/2 (50) |
In two moderate- or good-quality RCTs there was no significant difference between advice to keep active and bed rest, in terms of the global effect, pain intensity and CSOMs at short- and medium-term follow-up, in two good- or moderate-quality RCTs. 14,267 There was no significant difference between advice to keep active and mobilisation with exercises carried out in a hospital physiotherapy department in terms of the global effect, pain intensity and CSOMs at short- and medium-term follow-up in a moderate-quality RCT. 267
Chapter 7 Mixed treatment comparisons: results
Description of mixed treatment comparison models
The network for studies reporting the outcome global effect is presented in Figure 105. In total, six MTC analyses were conducted for the three types of outcome (global effect, pain intensity and CSOMs) for all study designs and for RCTs and Q-RCTs only. The network diagrams for pain intensity and CSOMs are presented in Appendix 6, as well as the network diagram for global effect that only includes RCTs and Q-RCTs.
FIGURE 105.
Mixed treatment comparison network for global effect, including all studies.
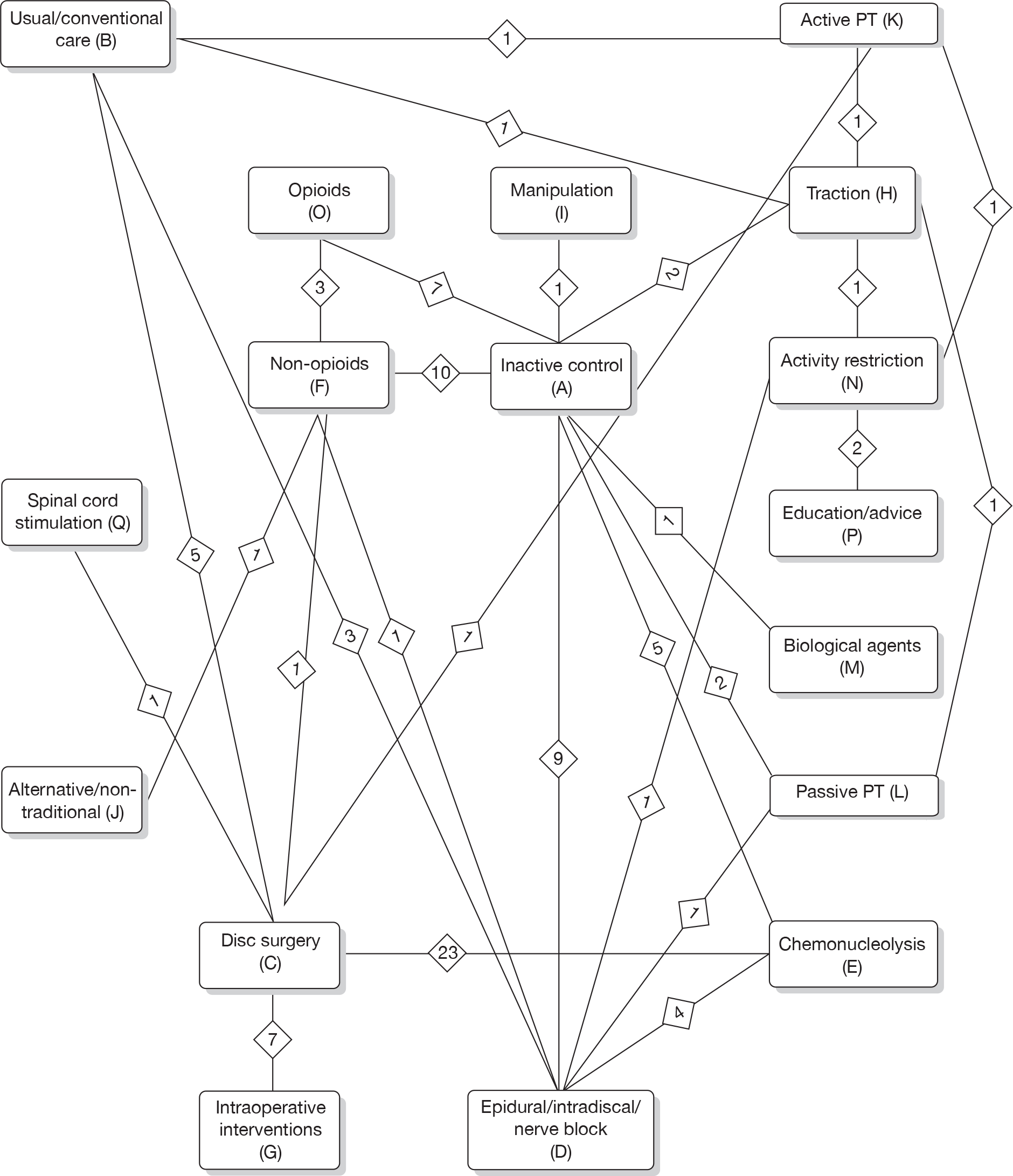
The MTC analyses rely on the key assumption that the relative treatment effect of one treatment versus another is the same across the entire set of studies. 273,274 We used a random effects model, which means that we assumed that the common distribution of effects is the same across all sets of studies. A further assumption that was made in the analyses was that the relative efficacy of different treatments is the same at different stages in the care pathway.
Convergence was assessed using the Gelman–Rubin statistic (R) monitored over iteration–time. (R = B/W, where B represents the within-chain variability and W the between-chain variability.) Convergence occurred at around 6000–8000 iterations for all three outcome measures (global effect, pain intensity and CSOMs), as demonstrated in the random selection of plots presented in Appendix 7. The auto-correlation and history plots also showed good convergence. The goodness of fit of the models to the data, measured by the residual deviance, was found to be high (data presented in Appendix 8).
The results of the evaluation of between-study heterogeneity, presented in Appendix 8, showed a moderate-to-high level of statistical heterogeneity for many of the pair-wise comparisons, as well as across all studies as a whole.
The mean pain scores (scale 0–100) at baseline for each treatment category, according to the studies included in the MTC, were fairly similar and are presented in Table 157. With the exception of biological agents, most ranged from 60 to 69.
| Treatment category | No. of studies (no. of RCTs/Q-RCTs) | Mean baseline pain |
|---|---|---|
| Alternative/non-traditional | Not reported | |
| Intraoperative interventions | 7 (7) | 59.8 |
| Active PT/exercise therapy | 2 (2) | 60.0 |
| Chemonucleolysis | 5 (3) | 60.2 |
| Education/advice | 1 (1) | 60.7 |
| Inactive control | 18 (17) | 63.3 |
| Opioids | 2 (2) | 63.3 |
| Non-opioids | 12 (10) | 64.4 |
| Usual/conventional care | 4 (3) | 65.8 |
| Activity restriction | 3 (3) | 66.8 |
| Epidural/intradiscal injection | 11 (11) | 67.6 |
| Traction | 4 (4) | 68.0 |
| Passive PT | 3 (3) | 68.3 |
| Disc surgery | 15 (11) | 68.7 |
| Biological agents | 2 (1) | 76.5 |
The MTC method enables us to estimate the probability that each treatment category is best (or most effective), the findings of which are presented in Tables 159–164, along with the summary effect estimates for comparisons of each intervention category with inactive control. The credible intervals (or the CIs presented in Figures 106–111) provide an indication of the uncertainty surrounding the effect sizes, which needs to be taken into account. For example, for global effect the estimates of the medians for biological agents and alternative therapy are associated with a great deal of uncertainty. Although they had the highest probability of being the best interventions, their 95% credible intervals were very wide and included unity, so were not statistically significant. Although the estimates of the median effect size for disc surgery and epidural injections were smaller, the 95% credible intervals were narrower and their findings were statistically significant (the direction of benefit in the forest plot is different for pain and CSOM is different from the direction for global effect).
The indirect comparison, as part of the MTC analysis, provides a full set of comparisons for all treatment groups. The summary estimates of effect (with 95% credible intervals) for each treatment comparison in the network for the analysis of global effect, which included all study designs is presented in Table 158. The results of each treatment comparison in the MTC analyses for all the networks are also presented in Appendix 9. The MTC findings can be directly compared with summaries of the pair-wise meta-analysis (with 95% CIs) derived from Stata, which are also presented in the same matrices (top right-hand corner). For example, when considering all study types, pair-wise data from nine studies show epidural to be significantly better than the inactive control for global effect (OR 2.58; 95% CI 1.25 to 5.29), and the indirect data show a similar result (OR 3.10; 95% credible interval 1.79 to 5.46). An example of where there is no direct comparison of interventions is that between disc surgery and epidural injections for global effect, but the indirect comparison shows a non-statistically significant finding in favour of surgery (OR 1.11; 95% credible interval 0.55 to 2.25).
| A | n = 9, 2.58 (1.3 to 5.3) | n = 5, 2.56 (1.6 to 4.1) | n = 10, 2.16 (1.1 to 4.5) | n = 2 to 1.11 (0.6 to 2.1) | n = 1, 4.71 (2.0 to 11.4) | n = 2, 1.57 (0.2 to 11.3) | n = 1, 10.0 (0.7 to 167) | n = 1, 1.37 (0.5 to 3.8) | ||||||||
| 0.83 (0.4 to 1.9) | B | n = 5, 2.60 (1.6 to 4.3) | n = 3, 5.46 (0.8 to 38.5) | n = 1, 1.53 (0.6 to 4.2) | n = 1, 1.45 (0.7 to 3.0) | |||||||||||
| 2.78 (1.4 to 5.6) | 3.37 (1.7 to 6.8) | C | n = 23, 0.65 (0.5 to 0.9) | n = 1, 6.72 (0.8 to 58.8) | n = 7, 1.49 (1.0 to 2.2) | n = 1, 0.77 (0.2 to 3.2) | n = 1, 1.13 (0.4 to 3.6) | |||||||||
| 3.10 (1.8 to 5.5) | 3.75 (1.7 to 8.4) | 1.11 (0.6 to 2.3) | D | n = 4, 1.13 (0.4 to 3.6) | n = 1, 0.45 (0.2 to 1.4) | n = 1, 0.20 (0.1 to 0.6) | n = 1, 0.22 (0.1 to 0.9) | |||||||||
| 2.00 (1.1 to 3.8) | 2.42 (1.2 to 5.1) | 0.72 (0.5 to 1.1) | 0.65 (0.3 to 1.2) | E | ||||||||||||
| 2.55 (1.4 to 4.7) | 3.09 (1.2 to 8.4) | 0.92 (0.4 to 2.2) | 0.82 (0.4 to 1.8) | 1.27 (0.6 to 2.9) | F | n = 1, 3.27 (0.8 to 13.8) | n = 2, 0.55 (0.1 to 5.0) | |||||||||
| 4.73 (1.6 to 14.0) | 5.72 (2.0 to 16.8) | 1.70 (0.8 to 3.9) | 1.52 (0.5 to 4.5) | 2.35 (1.0 to 5.8) | 1.85 (0.6 to 6.1) | G | ||||||||||
| 1.20 (0.5 to 3.1) | 1.46 (0.5 to 4.2) | 0.44 (0.2 to 1.2) | 0.39 (0.1 to 1.1) | 0.60 (0.2 to 1.7) | 0.47 (0.2 to 1.4) | 0.26 (0.1 to 1.0) | H | n = 1, 0.88 (0.3 to 2.7) | n = 1, 0.93 (0.5 to 1.9) | n = 1, 1.00 (0.1 to 7.0) | ||||||
| 4.88 (0.7 to 33.2) | 5.91 (0.7 to 47.1) | 1.76 (0.2 to 13.4) | 1.57 (0.2 to 11.4) | 2.45 (0.3 to 18.3) | 1.91 (0.3 to 14.0) | 1.03 (0.1 to 9.1) | 4.06 (0.5 to 33.8) | I | ||||||||
| 9.32 (1.0 to 104.5) | 11.27 (1.0 to 144.5) | 3.35 (0.3 to 40.9) | 2.99 (0.3 to 35.6) | 4.64 (0.4 to 56.2) | 3.65 (0.4 to 38.0) | 1.98 (0.2 to 27.5) | 7.73 (0.7 to 102) | 1.91 (0.1 to 41.7) | J | |||||||
| 1.10 (0.3 to 3.8) | 1.33 (0.4 to 4.4) | 0.40 (0.1 to 1.3) | 0.35 (0.1 to 1.2) | 0.55 (0.2 to 1.9) | 0.43 (0.1 to 1.6) | 0.23 (0.1 to 1.0) | 0.90 (0.3 to 3.2) | 0.22 (0.0 to 2.2) | 0.12 (0.0 to 1.6) | K | n = 1, 2.2 (0.6 to 7.7) | |||||
| 1.14 (0.4 to 3.2) | 1.38 (0.4 to 4.7) | 0.41 (0.1 to 1.3) | 0.37 (0.1 to 1.1) | 0.57 (0.2 to 1.8) | 0.45 (0.1 to 1.4) | 0.24 (0.1 to 1.0) | 0.94 (0.3 to 3.1) | 0.23 (0.0 to 2.0) | 0.13 (0.0 to 1.5) | 1.04 (0.2 to 4.7) | L | |||||
| 15.77 (0.6 to 1002) | 19.26 (0.7 to 1357) | 5.68 (0.2 to 396) | 5.10 (0.2 to 335) | 7.90 (0.3 to 545) | 6.19 (0.2 to 409) | 3.38 (0.1 to 249) | 13.2 (0.4 to 943) | 3.36 (0.1 to 306) | 1.75 (0.0 to 180) | 14.6 (0.4 to 1085) | 14.03 (0.5 to 974) | M | ||||
| 1.28 (0.3 to 5.5) | 1.54 (0.3 to 7.1) | 0.46 (0.1 to 2.0) | 0.41 (0.1 to 1.7) | 0.64 (0.1 to 2.8) | 0.50 (0.1 to 2.4) | 0.27 (0.1 to 1.5) | 1.05 (0.2 to 4.7) | 0.26 (0.0 to 2.9) | 0.14 (0.0 to 2.0) | 1.16 (0.3 to 5.1) | 1.12 (0.2 to 6.0) | 0.08 (0.0 to 2.9) | N | n = 2, 1.32 (0.8 to 2.3) | ||
| 1.60 (0.5 to 5.4) | 1.95 (0.5 to 8.4) | 0.58 (0.2 to 2.3) | 0.52 (0.1 to 1.9) | 0.80 (0.2 to 3.1) | 0.63 (0.2 to 2.0) | 0.34 (0.1 to 1.7) | 1.33 (0.3 to 6.2) | 0.33 (0.0 to 3.3) | 0.17 (0.0 to 2.1) | 1.46 (0.3 to 8.3) | 1.41 (0.3 to 6.8) | 0.10 (0.0 to 3.3) | 1.26 (0.2 to 8.7) | O | ||
| 1.63 (0.2 to 12.1) | 1.98 (0.3 to 14.7) | 0.59 (0.1 to 4.3) | 0.53 (0.1 to 3.7) | 0.81 (0.1 to 6.0) | 0.64 (0.1 to 5.0) | 0.34 (0.0 to 3.0) | 1.35 (0.2 to 10.0) | 0.33 (0.0 to 5.3) | 0.17 (0.0 to 3.5) | 1.48 (0.2 to 10.9) | 1.43 (0.2 to 12.5) | 0.10 (0.0 to 4.9) | 1.28 (0.3 to 4.9) | 1.02 (0.1 to 10.2) | P | |
| 3.19 (0.4 to 27.6) | 3.84 (0.4 to 33.7) | 1.14 (0.2 to 8.9) | 1.03 (0.1 to 8.9) | 1.59 (0.2 to 12.8) | 1.25 (0.1 to 11.6) | 0.67 (0.1 to 5.9) | 2.66 (0.3 to 26.0) | 0.65 (0.0 to 12.2) | 0.34 (0.0 to 8.0) | 2.90 (0.3 to 30.8) | 2.80 (0.3 to 28.9) | 0.19 (0.0 to 10.1) | 2.54 (0.2 to 31.9) | 2.0 (0.2 to 23.5) | 1.97 (0.1 to 34.8) | Q |
The results of the mixed treatment comparison of each intervention category with inactive control
Comparisons of the findings of the pair-wise meta-analysis for each intervention category with inactive control are presented in Tables 159–164 and Figures 106–111. When these direct comparisons are compared with those obtained from the MTC analysis, it can be seen that there is a broad agreement for the global effect, but there are more discrepancies for pain intensity and for CSOMs. These discrepancies are greatest for comparisons for which there is very little direct evidence, such as biological agents versus inactive control (one study271).
| Treatment category | Code | Probability of being ‘best’ (mean) | Median OR (95% credible interval) | Results of standard pair-wise meta-analysis | |
|---|---|---|---|---|---|
| No. of studies | ORs (95% CI) | ||||
| Biological agents | M | 0.5062 | 15.77 (0.61 to 1002.00) | 1 | 10.00 (0.65 to 100.00) |
| Alternative/non-traditional | J | 0.2764 | 9.32 (0.95 to 104.50) | ||
| Manipulation | I | 0.0990 | 4.88 (0.73 to 33.20) | 1 | 4.76 (11.11 to 1.96) |
| Spinal cord stimulation | Q | 0.0604 | 3.19 (0.36 to 27.57) | ||
| Intraoperative interventions | G | 0.0389 | 4.72 (1.61 to 13.99) | ||
| Education/advice | P | 0.0142 | 1.63 (0.22 to 12.05) | ||
| Opioids | O | 0.0018 | 1.60 (0.48 to 5.41) | 1 | 1.37 (0.50 to 3.70) |
| Epidural/nerve block | D | 0.0017 | 3.09 (1.79 to 5.46) | 9 | 2.63 (1.27 to 5.56) |
| Usual care | B | 0.0000 | 0.83 (0.35 to 1.91) | ||
| Chemonucleolysis | E | 0.0000 | 2.00 (1.05 to 3.82) | 5 | 2.56 (1.59 to 4.17) |
| Activity restriction | N | 7.2 × 10–4 | 1.28 (0.29 to 5.51) | ||
| Non-opioids | F | 4.4 × 10–4 | 2.55 (1.42 to 4.65) | 10 | 2.71 (1.05 to 4.55) |
| Disc surgery | C | 2.4 × 10–4 | 2.78 (1.37 to 5.59) | ||
| Active PT | K | 1.4 × 10–4 | 1.09 (0.32 to 3.78) | ||
| Passive PT | L | 1.0 × 10–4 | 1.14 (0.41 to 3.17) | 2 | 1.56 (0.22 to 11.11) |
| Traction | H | 4.0 × 10–5 | 1.20 (0.47 to 3.07) | 2 | 1.11 (0.60 to 2.04) |
| Inactive control | A | 0.0000 | |||
| Treatment category | Code | Probability of being ‘best’ (mean) | Median OR (95% credible interval) | Results of standard meta-analysis | |
|---|---|---|---|---|---|
| No. of studies | ORs (95% CI) | ||||
| Biological agents | M | 0.4847 | 16.04 (0.60 to 1138.00) | 1 | 10.00 (0.65 to 100.00) |
| Intraoperative interventions | G | 0.3930 | 4.99 (1.50 to 17.47) | ||
| Alternative/non-traditional | J | 0.2568 | 9.25 (0.90 to 107.70) | ||
| Manipulation | I | 0.0882 | 4.90 (0.70 to 34.48) | 1 | 4.76 (1.96 to 11.11) |
| Education/advice | P | 0.0593 | 3.12 (0.29 to 34.36) | ||
| Spinal cord stimulation | Q | 0.0582 | 3.30 (0.34 to 32.70) | ||
| Activity restriction | N | 0.00944 | 2.43 (0.35 to 17.52) | ||
| Epidural/nerve block | D | 0.00164 | 3.14 (1.77 to 5.65) | 9 | 2.63 (1.27 to 5.56) |
| Opioids | O | 0.00112 | 1.62 (0.46 to 5.66) | 1 | 1.37 (0.50 to 3.70) |
| Traction | H | 1.0 × 10–4 | 1.36 (0.47 to 3.94) | 2 | 1.12 (0.60 to 2.04) |
| Non-opioids | F | 2.6 × 10–4 | 2.59 (1.37 to 4.96) | 9 | 2.56 (1.16 to 5.26) |
| Disc surgery | C | 3.0 × 10–4 | 2.94 (1.18 to 7.49) | ||
| Usual care | B | 4.0 × 10–5 | 1.14 (0.38 to 3.46) | ||
| Active PT | K | 4.2 × 10–4 | 1.46 (0.38 to 5.75) | ||
| Chemonucleolysis | E | 6.0 × 10–5 | 2.38 (1.19 to 4.81) | 5 | 2.56 (1.59 to 4.17) |
| Passive PT | L | 6.0 × 10–6 | 1.19 (0.42 to 3.42) | 2 | 1.56 (0.22 to 11.11) |
| Inactive control | A | 0.0000 | |||
| Treatment category | Code | Probability of being ‘best’ (mean) | Median of the posterior (95% credible interval) | Results of standard meta-analysis | |
|---|---|---|---|---|---|
| No. of studies | WMD (95% CI) | ||||
| Alternative/non-traditional | J | 0.4397 | –26.08 (–46.65 to –6.06) | 1 | –25.00 (–41.75 to –8.24) |
| Biological agents | M | 0.2344 | –21.80 (–35.95 to –7.95) | 2 | −9.91 (−43.23 to 23.41) |
| Manipulation | I | 0.1474 | –11.72 (–44.97 to 21.59) | ||
| Intraoperative interventions | G | 0.0688 | –14.88 (–34.05 to 4.02) | ||
| Chemonucleolysis | E | 0.01566 | –11.24 (–29.76 to 7.20) | 1 | –5.40 (–23.66 to 12.86) |
| Active PT | K | 0.014 | –3.04 (–27.35 to 20.94) | ||
| Education/advice | P | 0.0083 | 17.04 (–20.80 to 54.62) | ||
| Traction | H | 0.00716 | –1.21 (–22.07 to 20.04) | 1 | 3.36 (–14.49 to 21.21) |
| Passive PT | L | 0.0039 | –0.40 (–19.33 to 19.00) | 1 | –7.00 (–13.58 to –0.42) |
| Epidural/nerve block | D | 0.00306 | –12.85 (–20.91 to –5.14) | 8 | –12.31 (–23.90 to –0.72) |
| Radiofrequency lesioning | S | 0.00222 | 12.94 (–13.38 to 39.01) | 1 | 13.00 (2.04 to 23.96) |
| Activity restriction | N | 0.0015 | 18.00 (–15.57 to 51.16) | ||
| Disc surgery | C | 0.0011 | –9.78 (–26.51 to 6.81) | ||
| Usual care | B | 7.2 ✗ 10–4 | –3.184 (–19.45 to 13.18) | ||
| Non-opioids | F | 8 ✗ 10–5 | –4.07 (–13.57 to 5.11) | 5 | –10.70 (–21.21 to −0.19) |
| Opioids | O | 6 ✗ 10–5 | 9.34 (–9.15 to 27.40) | ||
| Inactive control | A | 0.0 | |||
| Treatment category | Code | Probability of being ‘best’ (mean) | Median of the posterior (95% credible interval) | Results of standard meta-analysis | |
|---|---|---|---|---|---|
| No. of studies | WMD (95% CI) | ||||
| Alternative/non-traditional | J | 0.4945 | –24.89 (–55.67 to 5.35) | 1 | –25.00 (–41.75 to −8.24) |
| Manipulation | I | 0.1859 | –12.79 (–50.28 to 24.55) | ||
| Intraoperative interventions | G | 0.1016 | –13.94 (–39.47 to 11.56) | ||
| Biological agents | M | 0.07186 | –11.18 (–30.77 to 8.83) | 1 | 7.00 (–5.25 to 19.25) |
| Chemonucleolysis | E | 0.04438 | –12.28 (–35.85 to 11.38) | 1 | –5.40 (–23.66 to 12.89) |
| Epidural/nerve block | D | 0.02446 | –12.66 (–21.47 to –4.11) | 8 | –12.31 (–23.90 to –0.72) |
| Active PT | K | 0.02244 | –3.39 (–30.69 to 23.94) | ||
| Traction | H | 0.01374 | –1.32 (–23.17 to 20.91) | 1 | 3.36 (–14.49 to 21.21) |
| Education/advice | P | 0.0115 | 16.62 (–22.42 to 26.93) | ||
| Passive PT | L | 0.00792 | –0.23 (–20.29 to 20.33) | 1 | –7.00 (–13.58 to −0.42) |
| Disc surgery | C | 0.00516 | –8.87 (–32.27 to 14.47) | ||
| Usual care | B | 0.00464 | –4.45 (–23.49 to 14.63) | ||
| Radiofrequency lesioning | S | 0.00408 | 13.01 (–14.41 to 40.77) | 1 | 13.00 (2.04 to 23.96) |
| Non-opioids | F | 0.00408 | –5.84 (–16.65 to 4.47) | 5 | –10.70 (–20.21 to –0.19) |
| Activity restriction | N | 0.0025 | 17.44 (–16.86 to 52.78) | ||
| Opioids | O | 0.00122 | 7.41 (–12.54 to 26.94) | ||
| Inactive control | A | 0.0 | |||
| Treatment category | Code | Probability of being ‘best’ (mean) | Median SMD (95% credible interval) | Results of standard meta-analysis | |
|---|---|---|---|---|---|
| No. of studies | SMD (95% CI) | ||||
| Activity restriction | N | 0.3223 | –0.82 (–2.58 to 0.74) | ||
| Biological agents | M | 0.2393 | –0.67 (–1.27 to –0.08) | 3 | –0.90 (–1.52 to –0.18) |
| Education/advice | P | 0.1741 | –0.66 (–2.59 to 1.00) | ||
| Passive PT | L | 0.1186 | –0.47 (–1.36 to 0.43) | ||
| Intraoperative interventions | G | 0.05489 | –0.06 (–1.38 to 1.29) | ||
| Active PT | K | 0.0393 | 0.18 (–1.26 to 1.61) | ||
| Traction | H | 0.03458 | –0.35 (–1.21 to 0.46) | 1 | 0.08 (–0.31 to 0.47) |
| Chemonucleolysis | E | 0.00496 | 0.38 (–0.99 to 1.80) | ||
| Usual care | B | 0.00365 | 0.16 (–1.07 to 1.42) | ||
| Disc surgery | C | 0.00341 | 0.10 (–1.17 to 1.39) | ||
| Epidural/nerve block | D | 0.00324 | –0.16 (–0.53 to 0.20) | 5 | 0.34 (–0.81 to 0.13) |
| Non-opioids | F | 0.00162 | 0.08 (–0.48 to 0.66) | 2 | 0.30 (–0.14 to 0.74) |
| Inactive control | A | 9.0 ✗ 105 | |||
| Treatment category | Code | Probability of being ‘best’ (mean) | Median SMD (95% credible interval) | Results of standard meta-analysis | |
|---|---|---|---|---|---|
| No. of studies | SMD (95% CI) | ||||
| Activity restriction | N | 0.3562 | –0.75 (–2.47 to 1.03) | ||
| Education/advice | P | 0.1825 | –0.61 (–2.40 to 1.31) | ||
| Biological agents | M | 0.1786 | –0.41 (–1.18 to 0.37) | 2 | –1.07 (–2.64 to 0.50) |
| Passive PT | L | 0.1285 | –0.34 (–1.26 to 0.57) | ||
| Intraoperative interventions | G | 0.05476 | 0.15 (–1.29 to 1.58) | ||
| Traction | H | 0.05209 | –0.30 (–1.15 to 0.54) | 1 | 0.08 (–0.31 to 0.47) |
| Active PT | K | 0.01826 | 0.39 (–1.05 to 1.87) | ||
| Non-opioids | F | 0.01192 | 0.08 (–0.49 to 0.66) | 2 | 0.30 (–0.141 to 0.74) |
| Usual care | B | 0.00661 | 0.35 (–0.94 to 1.62) | ||
| Chemonucleolysis | E | 0.00341 | 0.62 (–0.86 to 2.13) | ||
| Disc surgery | C | 0.00326 | 0.29 (–1.07 to 1.70) | ||
| Epidural/nerve block | D | 0.00165 | 0.04 (–0.35 to 0.43) | 4 | –0.03 (–0.18 to 0.13) |
| Inactive control | A | 0.00222 | |||
FIGURE 106.
Odds ratios for the global effect of the different treatment categories for all studies compared with the inactive control from the MTC analysis. Note: weights are from random effects analysis.
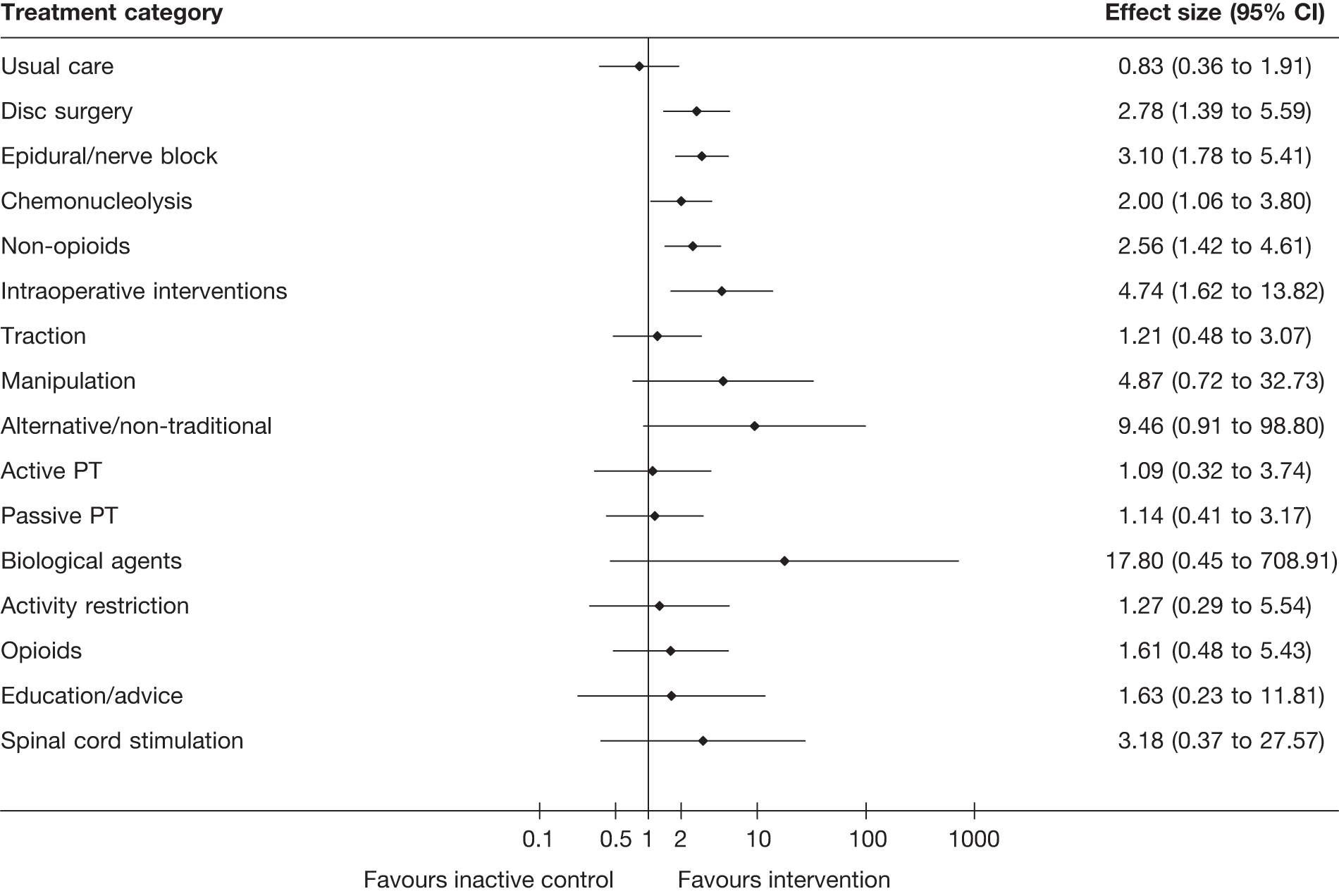
FIGURE 107.
Odds ratios for the global effect of the different treatment categories for RCTs and Q-RCTs compared with the inactive control from the MTC analysis. Note: weights are from random effects analysis.
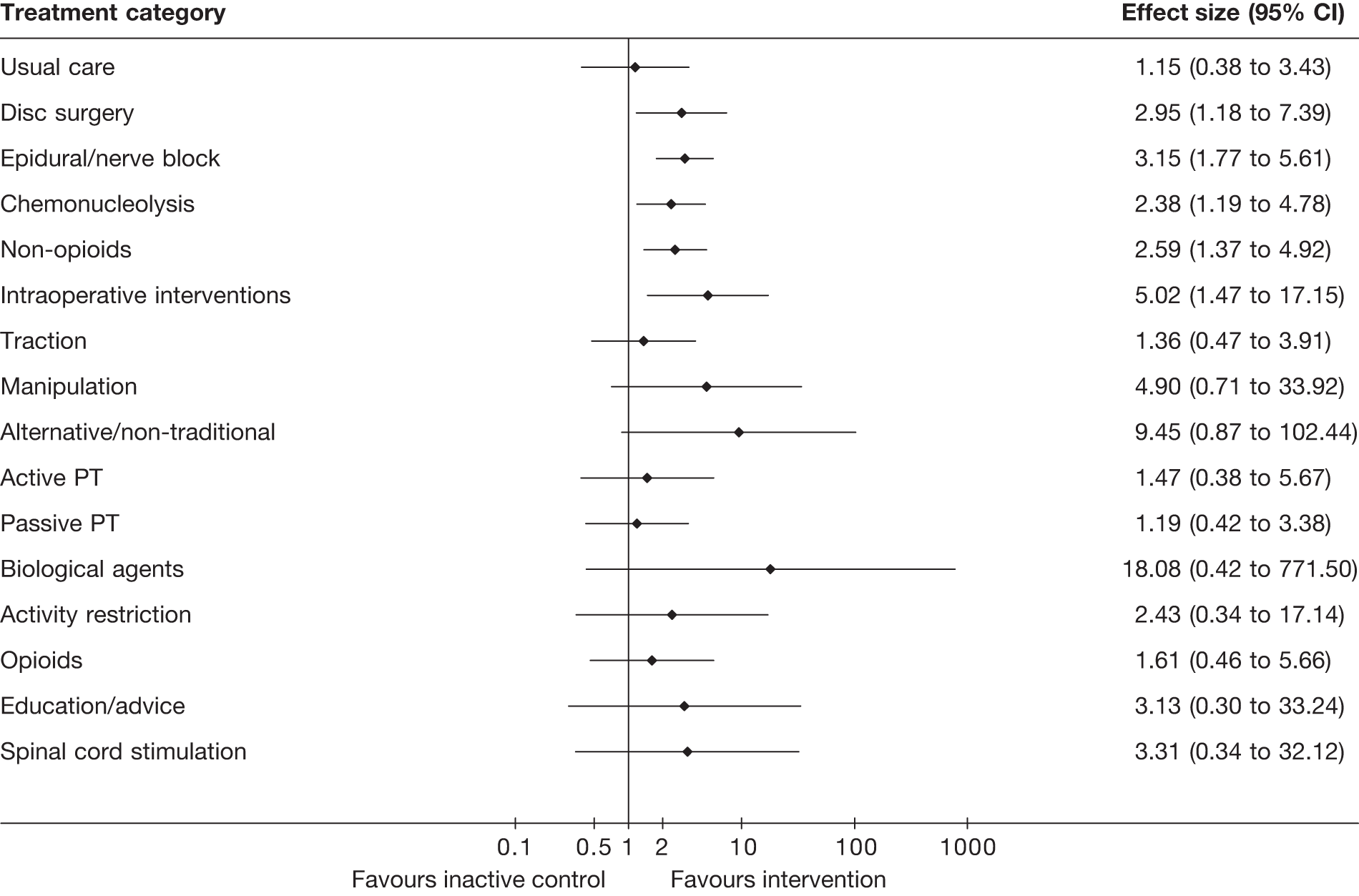
FIGURE 108.
Weighted mean difference for pain intensity of the different treatment categories for all studies compared with the inactive control from the MTC analysis. Note: weights are from random effects analysis.
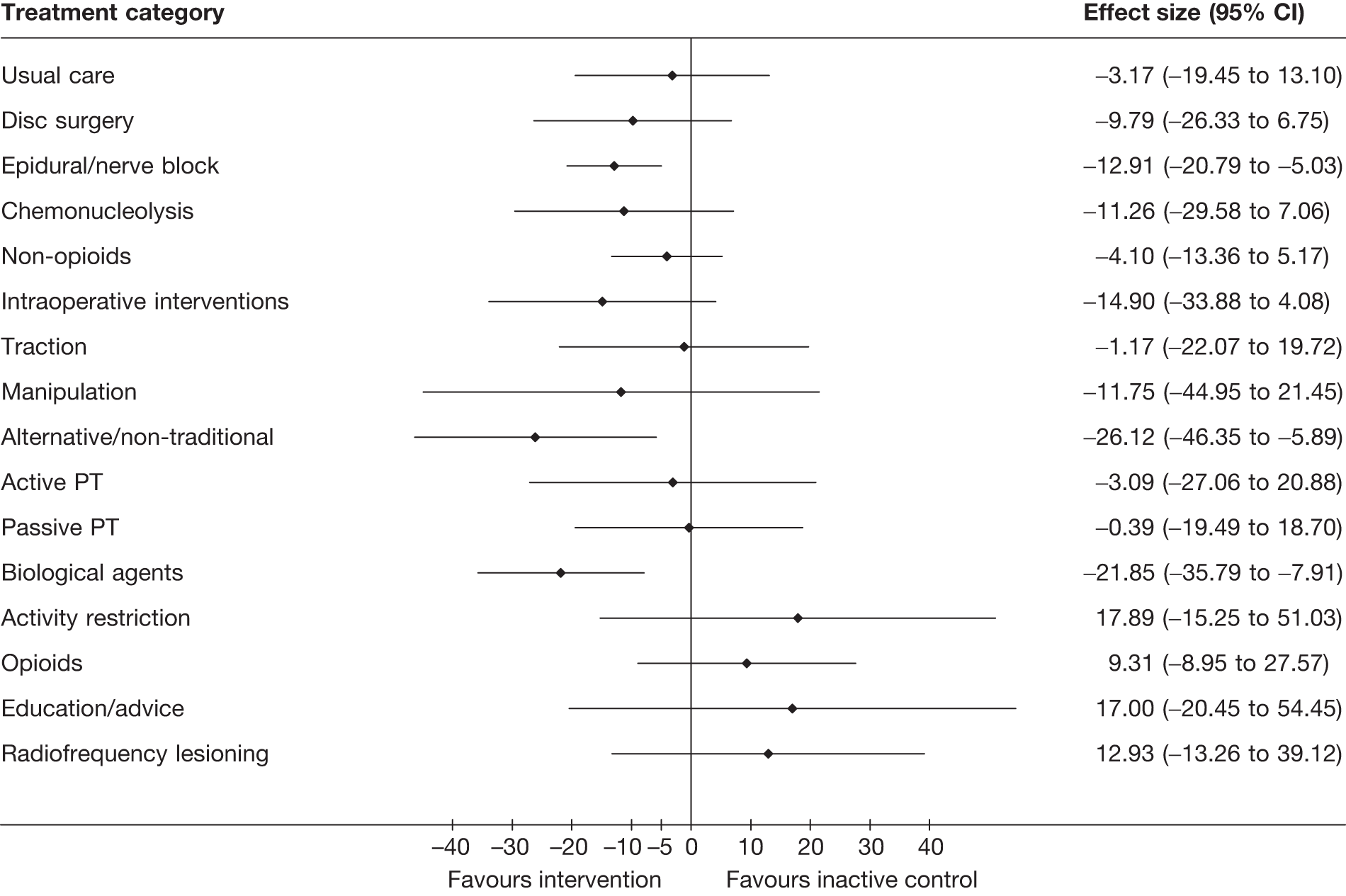
FIGURE 109.
Weighted mean difference for pain intensity of the different treatment categories for RCTs and Q-RCTs compared with the inactive control from the MTC analysis. Note: weights are from random effects analysis.
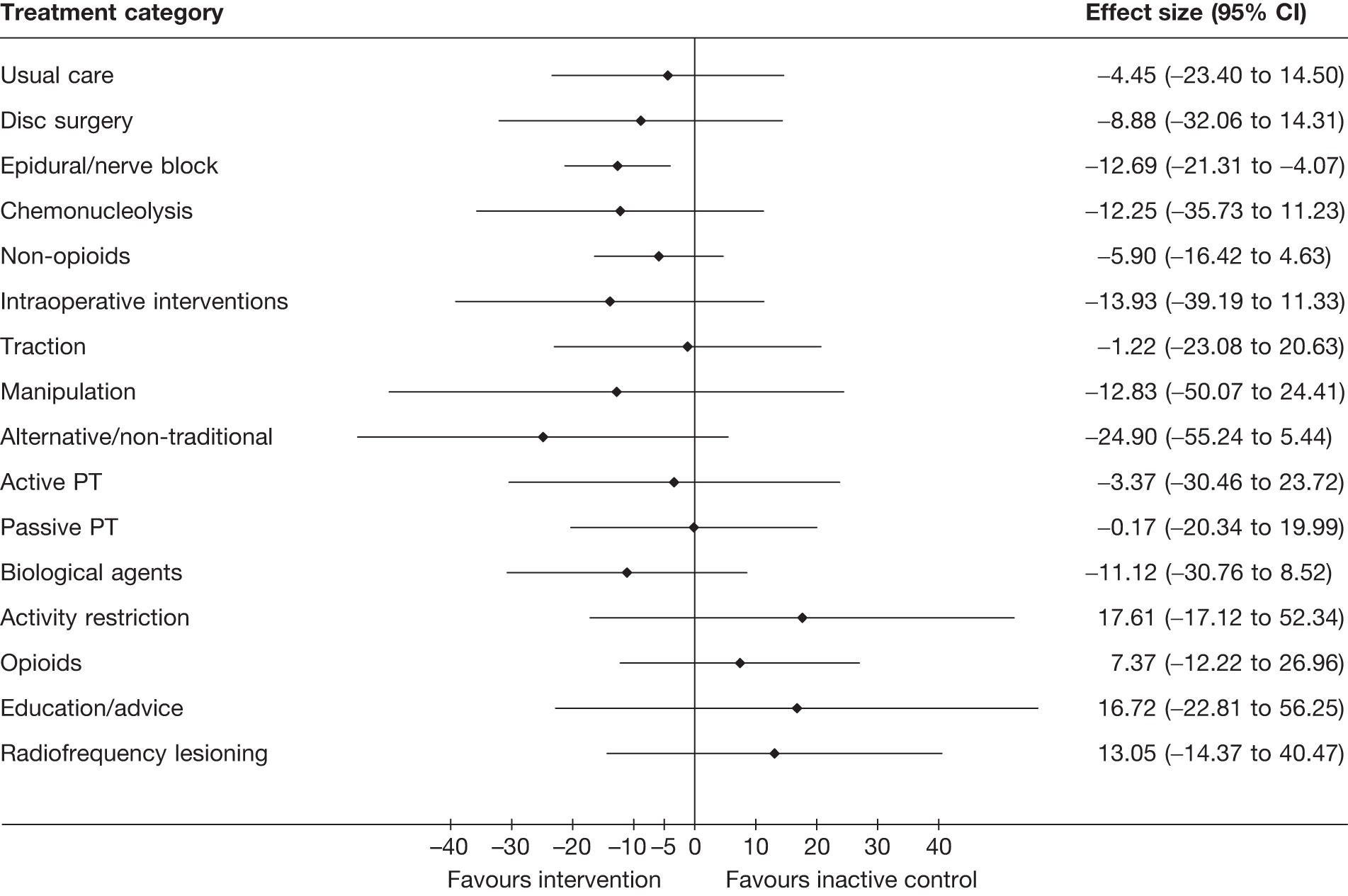
FIGURE 110.
Standardised mean difference for CSOMs of the different treatment categories for all studies compared with the inactive control from the MTC analysis. Note: weights are from random effects analysis.
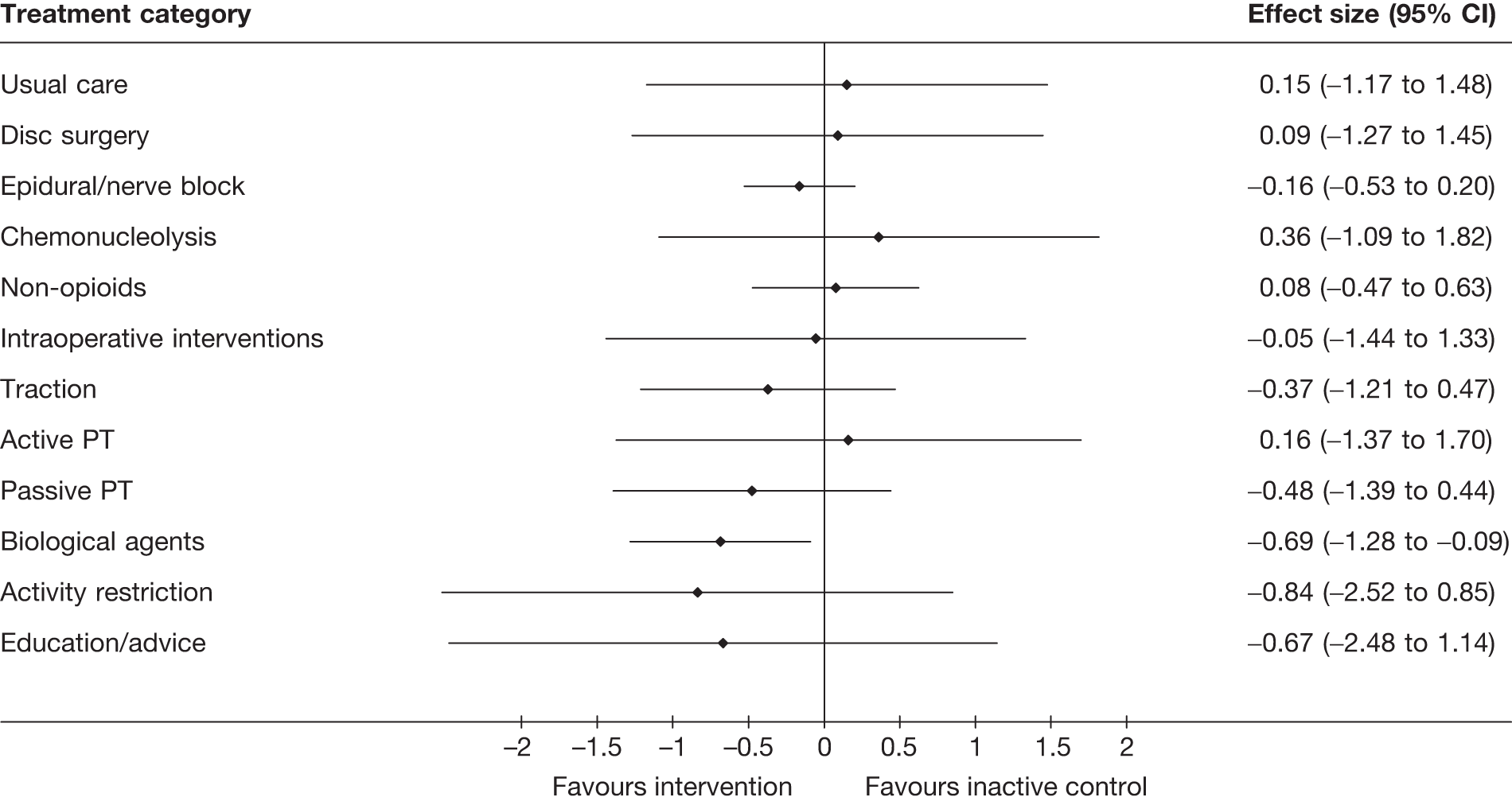
FIGURE 111.
Standardised mean difference for CSOMs of the different treatment categories for RCTs and Q-RCTs compared with the inactive control from the MTC analysis. Note: weights are from random effects analysis.
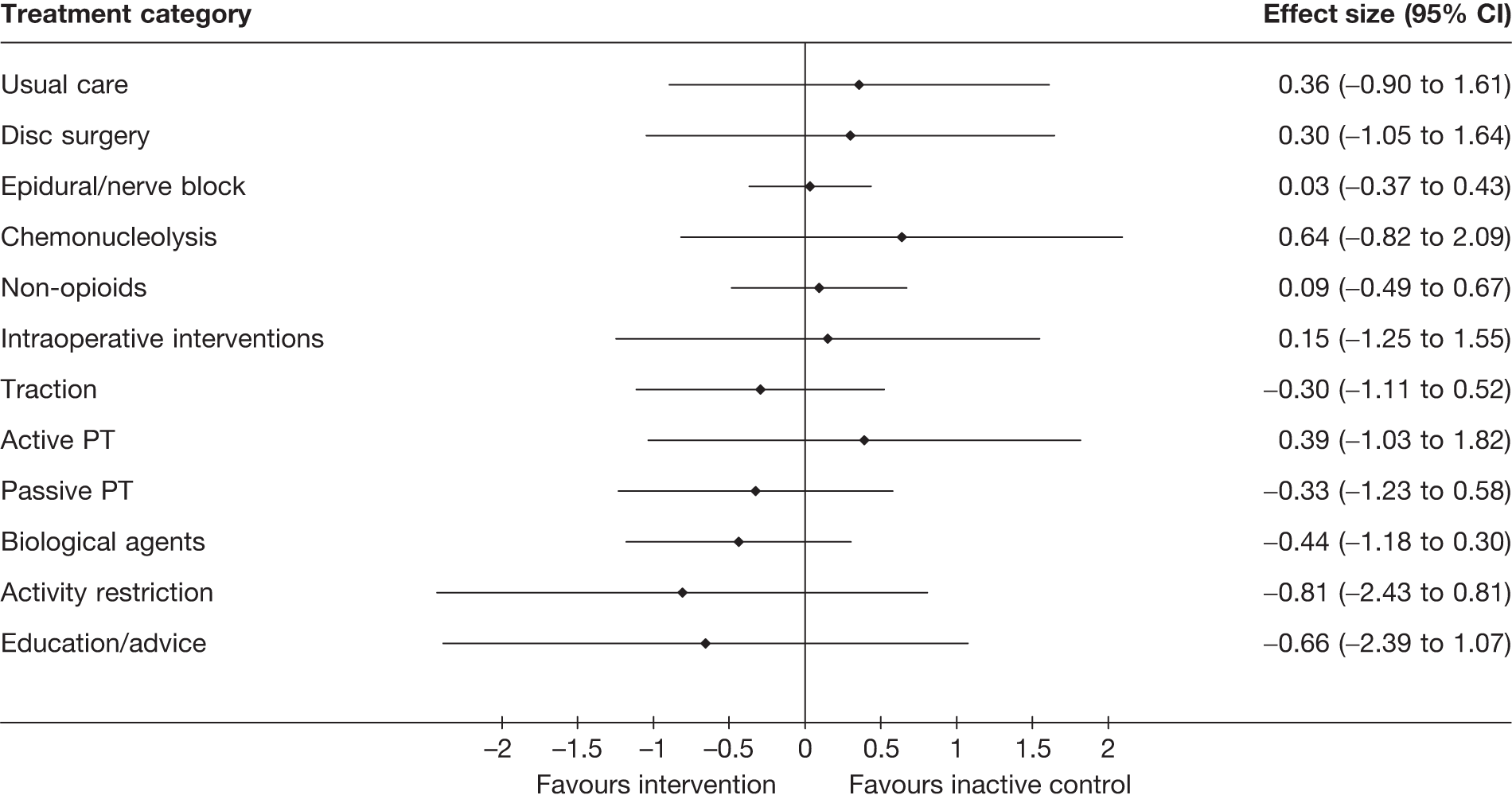
For global effect, interventions that resulted in a statistically significant improvement compared with inactive control were, in order of effect size, intraoperative interventions, epidural injections, disc surgery, non-opioids and chemonucleolysis. For pain intensity these included alternative, biological agents and epidural. Opioids were found to be significantly less effective than inactive control for reducing pain. For CSOMs, biological agents resulted in statistically significant improvement compared with inactive control. When the analyses were limited to RCTs/Q-RCTs, the only interventions that remained significantly better than inactive control were intraoperative interventions, epidural injections, disc surgery and non-opioids for global effect and epidural for pain intensity.
Results when observational studies were excluded were broadly similar.
Results of the mixed treatment comparison comparing all interventions that formed a connected network
The following is a summary of the remaining results without the inactive control, for global effect, pain intensity or CSOMs, according to whether or not there was a statistically significant difference between the intervention groups.
For disc surgery, the MTC analysis that included all study types showed a significant improvement in global effect when compared with usual care (OR 3.4, 95% credible interval 1.7 to 6.8). Following intra-operative intervention there was also significant improvement in the global effect for the comparison with usual care (OR 5.7, 95% credible interval 2.0 to 16.8). These comparisons remained statistically significant when the observational studies were excluded from the MTC analyses.
For epidural injection, the MTC analysis that included all study types found a significant improvement in global effect for the comparison with usual care (OR 3.8, 95% credible interval 1.7 to 8.4), and for pain intensity when compared with opioid medication (WMD –22.2, 95% credible interval –3.3 to –41.1). When observational studies were excluded from the MTC analysis there was no longer a significant difference for either of these outcomes.
For chemonucleolysis, the MTC analysis that included all study types found a significant improvement in the global effect compared with usual care (OR 2.4, 95% credible interval 1.2 to 5.1). When observational studies were excluded from the MTC analysis these findings were no longer significant.
For non-opioid medication, the MTC analysis that included all study types found a significant improvement in the global effect compared with usual care (OR 3.1, 95% credible interval 1.2 to 8.4). There was a significantly worse result in pain intensity compared with alternative therapy (mainly acupuncture) (WMD 22.1, 95% credible interval 0.1 to 43.8) or biological agents (OR 17.8, 95% credible interval 2.5 to 33.0). When observational studies were excluded from the MTC analysis these findings were no longer significant.
For alternative therapies (mainly acupuncture), the MTC analysis that included all study types found a significant improvement in pain intensity compared with activity restriction (WMD –44.1, 95% credible interval –82.9 to –4.9), opioids (WMD –35.5, 95% credible interval –62.3 to –8.3), non-opioid medication (WMD –22.1, 95% credible interval –43.8 to –0.1), or education/advice (WMD –44.2, 95% credible interval –85.5 to –0.2). When observational studies were excluded from the MTC analysis these findings were no longer significant.
For passive PT, the MTC analysis that included all study types found a significantly worse result in pain intensity for the comparison with biological agents (WMD 21.3, 95% credible interval 1.9 to 45.5). This finding was no longer a significant when observational studies were excluded from the MTC analysis.
For biological agents, the MTC analysis that included all study types found a significant improvement in pain intensity compared with activity restriction (WMD –39.7, 95% credible interval –75.8 to –3.6), opioids (WMD –31.2, 95% credible interval –53.0 to –9.2), non-opioid medication (WMD –17.8, 95% credible interval –2.46 to –33.0), or passive PT (WMD –21.3, 95% credible interval –45.5 to –1.9), and CSOMs compared with non-opioid medication (SMD –0.8, 95% credible interval –1.5 to –0.0). When observational studies were excluded from the MTC analysis these findings were no longer significant.
For activity restriction, the MTC analysis that included all study types found a significantly worse result in pain intensity compared with biological agents (WMD 39.7, 95% credible interval 3.6 to 75.8) or alternative therapies (WMD 44.1, 95% credible interval 4.9 to 82.9). When observational studies were excluded from the MTC analysis these findings were no longer significant.
For opioid medication, the MTC analysis that included all study types found a significantly worse result in terms of pain intensity compared with epidural injections (WMD 22.2, 95% credible interval 3.3 to 41.1), alternative therapy (mainly acupuncture) (WMD 35.5, 95% credible interval 8.3 to 62.3) or biological agents (WMD 31.2, 95% credible interval 9.2 to 53.0). When observational studies were excluded from the MTC analysis these findings were no longer significant.
For education/advice, the MTC analysis that included all study types found a significantly worse result in terms of pain intensity compared with alternative therapy (WMD 43.2, 95% credible interval 0.2 to 85.5). This finding was no longer significant when observational studies were excluded from the MTC analysis.
Chapter 8 Review of existing economic evaluations: results
Introduction
It was anticipated that the existing evidence relating to the cost-effectiveness of treatments would have a number of limitations that would make it insufficient to inform decision-making regarding the most appropriate management strategy for patients with sciatica. The findings from this review, alongside the review of clinical effectiveness, are intended to assist in informing the basis for the economic model.
Summary of results
Twelve studies were reviewed, data extracted and appraised. 62,100,173,275–283 A brief summary of these studies is presented in Table 164. A full summary is presented in Appendix 10. Studies evaluated the cost-effectiveness of single interventions for the treatment of sciatica (i.e. pair-wise comparisons) rather than mixed treatment effects. There was significant variation in the quality of studies presented as economic evaluations.
The majority of studies (9/12) were conducted primarily from a health-care or payer perspective. Several studies considered employment-related losses related to work days lost owing to sciatica; with three studies conducted from a societal perspective. The studies covered a diverse range of population settings, with some variation in age range and gender within the studies. Most studies considered a relatively short time horizon. One of the limitations of all studies was the lack of data relating to the longer-term outcome of sciatica. There was little distinction made in most studies between acute and chronic sciatica.
With the exception of one earlier study which employed a decision tree to represent potential pathways, all studies were based on individual patient data derived from RCTs and observational studies. As the majority of identified studies focused on intermediate or surgical interventions, resource utilisation and costs were commonly evaluated with respect to secondary care contacts and associated resource usage. Only one study focused specifically on primary care. Outcomes varied across studies, but the majority considered a global outcome and condition-specific or health-related quality of life (HRQoL). The measures used varied considerably from instruments designed specifically for the study to the use of established generic measures.
Of considerable importance to the review was the quality and robustness of the cost-effectiveness analysis (CEA). Only five studies were considered as full economic evaluations, i.e. reported incremental cost-effectiveness ratios (ICERs), when reviewed against established guidelines. 29,31 The other seven studies reported costs per adjusted outcome,62 unsuccessful outcome,281 cost per response276 or costs per extra success,275 with no ICERs presented. One study, published 16 years previously,277 reported a decision-analytic model to compare chemonucleolysis with surgical disctectomy. Again, this study did not present ICERs.
Economic evaluation conducted alongside trials, modelling studies and analyses of administrative databases were included if they compared two or more treatments, and considered both costs and consequences (including cost-effectiveness, cost–utility, cost–benefit and cost-consequences analysis). Some comparative studies included in the effectiveness section of the review also reported cost data, but the data on costs and consequences were not combined. Although not conforming to a full economic evaluation under our definition, two studies warrant specific attention as providing useful information on the cost–utility of interventions for sciatica.
Hansson and Hansson100 undertook a cost–utility analysis (CUA) of 92 individuals who underwent surgery for lumbar disc herniation in a cohort of 1822 individuals aged between 18 and 59 years and selected consecutively in five regions of Sweden between 1994 and 1995. All participants had been off work for at least 28 days as a result of either low back pain or neck problems. The intervention was surgery with conservative treatment as the comparator. Outcome measures were HRQoL using European Quality of Life-5 Dimensions (EQ-5D); functional restrictions because of back problems using the Hannover Activities of Daily Living questionnaire; and pain experienced during the previous 6 months using the Von Korff pain scale. Medical costs for back pain were estimated (appointments, admission, examination and treatment) over a 2-year study period. Cost of work absenteeism was also estimated. A 5% discount rate and an assumed annual increase in productivity of 1.5% were used to convert future years’ production loss to present values. Costs of illness, HRQoL and cost–utility (presented as difference in utility between 28 days and 2 years) were used as the gain in QALY.
The findings showed that the total cost of surgical treatment of lumbar disc herniation during a 2-year period was lower than the cost of non-surgical treatment. The direct cost of surgery was much higher than the direct cost of non-surgical treatment, whereas the indirect cost was lower. Lower indirect costs were the effect of lower rates of recurrence of work absence episodes and permanent disability benefits. Surgery reduced pain and improved back function and HRQoL to a greater extent than non-surgical treatments. The effects on HRQoL in combination with lower costs for surgery resulted in a better cost–utility for surgical treatment. The authors concluded that surgery for lumbar disc herniation is quite cost-effective.
Patients were drawn from a cohort study100 with explicit selection criteria in place, although the well-reported difficulties of selecting appropriate controls was acknowledged. The EQ-5D was used with utility values derived from a time trade-off (TTO) method, although a UK (rather than Swedish) population was used. Resource costs appear limited and methods to collect cost information were not fully described. Discounting was applied, but not at comparable NHS rates. Costs of illness were reported based on mean costs over 2 years (no CIs were presented). Cost per QALY were then calculated by calculating the difference between 28 days and 2 years. It is not clear why baseline values were not used. In addition, no ICERs were presented to explore QALY gain/loss over a longer time period. No sensitivity analysis was presented, with the authors stating that the Swedish cohort had a lower frequency of disc surgery within the starting 3 months than other national cohorts.
Manca et al. 280 reported HRQoL, resource consumption and costs of spinal cord stimulation compared with conventional medical management in 100 patients aged ≥ 18 years participating in the PROCESS (prospective, randomised controlled multicentre study of patients with failed back surgery syndrome) trial. Conservative medical management included oral medications, nerve blocks, epidural corticosteroids, physical and psychological rehabilitative therapy, or chiropractic care. HRQoL using the Short Form questionnaire-36 items (SF-36) and EQ-5D was measured at baseline and 3 months and 6 months after initiation of treatment. Unit costs were calculated using UK and Canadian figures. Health resource-data were prospective and collected over a comprehensive range of resources. Because of the time line, discounting was not performed.
The 6-month mean total costs were significantly higher (£15,081) in the spinal cord stimulation group than in the conservative management group (£3573), with a statistically significant adjusted differential mean cost of £11,373. However, the gain in HRQoL with spinal cord stimulation over the same period was considerably greater in this group, with a mean EQ-5D score difference of 0.25 (p < 0.001) and 0.21 (p < 0.001), respectively, at 3 and 6 months after adjustment for baseline characteristics. The authors concluded that the addition of spinal cord stimulation to conservative medical management in patients resulted in higher costs to health-care systems, but generated important improvements in patients EQ-5D over the same period.
Resource data were collected in detail and unit costs were undertaken using Canadian and UK figures, although patient resource data were derived from eight countries participating in the study. However, analysis of ‘country effect’ suggested that the differences in the total cost for UK and Canada did not appear to be statistically significantly different from the trial overall mean. The study did not take into account the patients’ perspective in the economic evaluation. EQ-5D data were collected and utilities were derived from a UK sample. The analysis of cost and HRQoL were presented separately. The limited follow-up period was the main limitation of this study and the authors acknowledged that a full CEA would need to consider how costs and HRQoL difference developed beyond the 6-month period.
With significant heterogeneity across these studies, it was difficult for any reliable conclusions from the results to be drawn from the existing economic evaluation evidence base.
A summary of the main issues identified include:
-
studies were undertaken across different countries
-
variability in the population settings across studies
-
lack of information on the clinical management pathways with many studies not indicating the previous treatment strategies or the timing of the intervention since diagnosis (e.g. patients who received conservative management for longer periods may be less likely to receive surgery which could lead to differences in costs and QALYs)
-
different perspectives were adopted (a significant limitation; of particular relevance for this review was the lack of a NHS and personal social services perspective in the studies)
-
unclear distinctions between acute and chronic sciatica
-
different comparators were used across studies
-
usual care was often poorly defined and variable across studies
-
short time horizons for studies with little consideration for the longer-term outcomes of sciatica
-
lack of discounting
-
the difficulty in blinding patients in the RCTs reported (patients’ preferences for treatment may have influenced the reported utilities and costs)
-
different approaches to measuring resource utilisation and unit costs
-
different outcome measures used across studies
-
limited data (particularly in earlier studies) of preference-based valuations
-
lack of information on the overall duration of symptoms and how these varied across different patient groups and treatments in order to adjust for these durations in any estimation of QALYs
-
the potential for crossover between interventions and additional co-interventions (e.g. owing to recurring or worsening symptoms/relapse/complications over time) has been overlooked in the majority of economic evaluations
-
variability in the CEA presented, with nearly 60% of studies not presenting an ICER
-
lack of sensitivity analysis in these evaluations (where sensitivity analysis is performed, there was considerable variability in the parameters used for changing the base-case analysis).
A recognised limitation in reporting this review is the relevance of these studies and data to current decision-making in the UK NHS. However, even with the significant heterogeneity precluding any formal comparison or conclusions from the results, the ICER estimates reported in Table 165 suggest marked differences between treatments. The approaches, assumptions and results of these five studies are reviewed in detail to identify possible key differences and issues in order to assist in the development of the new model. Five studies were reviewed. One study compared PT with GP care,278 one study compared an intermediate intervention (ESI with placebo)173 and three studies compared surgery with conservative treatment,283 usual care282 or chemonucleolysis. 279
| No. | Study | Country | Perspective | Source | Intervention and comparator(s) | Outcomes | ICER | |
|---|---|---|---|---|---|---|---|---|
| Intervention | Control | |||||||
| 1 | Dullerud, 1999275 | Norway | Health provider | Prospective cohort | Surgical macrodiscectomy | Nucleotomy | Marginal cost per extra success choosing surgery as primary outcome | |
| 2 | Hansson, 2007100 | Sweden | Societal | Prospective cohort | Disc surgery | Conservative treatment | Cost per QALY | |
| 3 | Karppinen, 2001276 | Finland | Health provider | RCT | Methylprednisolone-bupivacaine | Saline | Cost per response | |
| 4 | Launois, 1994277 | France | Health provider | Published studies + prospective survey | Chemonucleolysis | Surgical discectomy | Cost per QALY | |
| 5 | Luijsterburg, 2007278 | Netherlands | Societal | RCT | PT + GP care | GP care | Cost per global perceived effect gain |
Direct costs: €837 (95% CI –€732 to €3186) Total costs: €6224 (95% CI –€10,419 to €27,551) |
| 6 | Malter, 1996279 | USA | Health purchaser perspective | RCT, published studies | Lumbar discectomy | Conservative management | Cost per QALY |
Non discounted: US$29,200 5% discounted: US$33,900 Based on HMO data: US$12,000 |
| 7 | Manca, 2008280 | Canada, UK and Europe | Health-care provider (Canada and UK) | RCT | Spinal cord stimulation + non surgical conservative medical management | Non-surgical conservative medical management | Costs and HRQoL outcomes considered separately | |
| 8 | Price, 2005173 | UK | Health provider and purchaser (NHS) | RCT | Epidural steroid (ESI) + local anaesthetic | Normal saline (placebo) | Cost per QALY |
Provider: £44,701 Purchaser: £354,171 If only one ESI Provider: £25,745 Purchaser: £167,145 |
| 9 | Shvartzman, 199262 | USA | Health payer (insurance) | Retrospective chart review | Surgery | Conservative treatment | Cost per adjusted outcome | |
| 10 | Stevenson, 1995281 | UK | Health provider | RCT | Automated percutaneous disctectomy | Microdistectomy | Costs per successful outcome | |
| 11 | Tosteson, 2008282 | USA | Societal | RCT + observational cohort | Standard open aminectomy/laminectomy with removal of herniation + examination of involved nerve route | Non-operative (usual care decided by physician and patient) | Cost per QALY |
US$69,404 (95% CI US$49,523 to US$94,999) using general adult surgery costs US$34,355 (95% CI US$20,419 to US$52,512) using Medicare costs |
| 12 | van den Hout, 2008283 | Netherlands | Health-care and societal perspective | RCT | 6 months of prolonged conservative care | Early surgery | Cost per QALY |
Health-care perspective: €41,000 (95% CI €14,000 to €430,000) Societal: –€12 (95% CI –€4029 to €4006) |
Review of full economic evaluations
Primary care
Luijsterburg et al.
Luijsterburg et al. 278 undertook an economic evaluation as part of an RCT with 112 GPs in Rotterdam. One hundred and thirty-five patients aged between 18 and 65 years with duration of symptoms of < 6 weeks were randomised to PT and GP care compared with GP care alone. PT consisted of exercise therapy with information and advice provided by physical therapists. Passive therapies were not allowed. GP care was defined as care according to GP clinical guidelines and included information, advice and, if necessary, prescribed analgesia. A societal perspective was taken to the economic evaluation.
Source of effectiveness data
The primary outcome measure was global perceived effect (GPE) measured on a seven-point scale, dichotomised to improved and much improved versus not improved. GPE was rated as the percentage of patients who reported improvement. The EQ-5D was a secondary outcome measure that measured health utilities in order to calculate QALYs. Outcome measures and costs were assessed at baseline and at 3, 6, 12 and 52 weeks. Longer time horizons were not examined and discounting was not applied.
Source of cost data
Direct health-care costs included the costs of PT, GP care, medication, additional visits to other health-care providers and hospitalisations. Prices were obtained from Dutch guidelines284 or from the Professional Association. 285 The currency was euros (€), but the year was not reported. Indirect costs outside the health-care system included the costs of production losses caused by absence from work. Costs for paid work were calculated by using the friction cost approach (period 154 days) based on the overall mean income of the Dutch population.
Summary of cost-effectiveness analysis
Analysis was undertaken using the ITT principle. Difference in resource utilisation between the two groups was assessed using non-parametric methods because of the skewed nature of the cost data. For the CEA, GPE and EQ-5D were used to calculate benefits. Utilities derived from the EQ-5D allowed a CUA to be performed, although this was not reported. ICERs were constructed and CIs were calculated using Fieller’s methods using bootstrapping methods with the construction of cost-effectiveness acceptability curves. No sensitivity analysis was undertaken because it was claimed that most variations in cost or health effects were included in the bootstrap estimates of the ICER.
Summary of the findings
Total costs (direct and indirect) at 3, 6, 12 and 52 weeks consisted mainly of production losses with significant differences between groups for PT visits in favour of the control groups. Total direct costs were also significantly different at the four follow-up time points in favour of the control group. At baseline and 6 and 12 weeks, the mean utility score was higher in the control group (0.41, 0.70 and 0.73 compared with 0.39, 0.34 and 0.65), but the difference was statistically significant only at 6 weeks. At 52 weeks, the utility in the intervention group was higher (0.76 compared with 0.73).
The ICERs were: for direct costs €837 (95% CI –€732 to €3186) per improved patient gained and for total costs €6224 (95% CI €10,419 to €27,551) per patient improvement gained. The ICERs and CIs estimated by bootstrap and Fieller’s methods were similar. The cost-effectiveness acceptability curve constructed for direct costs showed, for a threshold of €600 per patient improved, an ICER acceptable with 35% certainty and, for a threshold of €1200 per patient improved, an ICER acceptable with 69% certainty. For total costs, the curve showed, for a threshold of €4000 per patient improved, an ICER acceptable at 37%, and for a threshold of €12,000 per patient improved, an ICER acceptable at 68%.
The authors concluded that treatment of patients with lumbar radicular syndrome (LRS) with PT and GP care was not more cost-effective than GP care alone.
Critique of Luijsterburg et al.
The study research question was justified because there was a lack of knowledge concerning the cost-effectiveness of PT in sciatica. The economic evaluation has been conducted alongside a RCT which appeared to have good internal validity.
However, some clear issues were identified. The data collection methods used to collect resource utilisation and cost data were not well explained and the reliability of this information could be questioned. For example, the authors recognised that some aspects that may have affected absence from work and productivity costs (e.g. waiting times) were ignore . The authors conceded that future studies should pay more attention to analysing the effect of these factors on absence from work and costs. Costs were cumulative so recall bias from patients may have occurred, but the authors did state that differences between the groups would be minimised by the randomisation process. The authors did not clarify why only a 1-year time horizon was considered, apart from the implicit reason of length of follow-up for the RCT. The collection of outcome measures was also highlighted as a possible limitation, with the EQ-5D criticised as not being sensitive enough to capture the health effects of the additional PT, but no information was given about how benefits were valued. A CUA was not undertaken as there was no effect on QoL between the two groups with higher costs for the intervention group, and in the case of no effect the authors suggested that interventions with the lowest cost were the preferred option. However, despite no significant differences reported, the authors could have estimated an ICER based on best information available, and this highlights the continued criticism that few studies are adequately powered to detect a difference in QoL outcomes.
The issue of uncertainty around the ICER was assessed using the bootstrap method. However, although this allowed CIs to be estimated, and reliability confirmed by comparison with the results of the parametric Fieller’s method, it did not allow changes in the base-case assumptions to be explicitly examined (e.g. to take into account increased waiting time).
Surgery
Malter and Weinstein
Malter et al. 286 undertook a review of published studies and estimated the cost-effectiveness of lumbar discectomy for herniated intervertral disc. The study was of 126 patients randomly assigned to medical or surgical treatment for radicular pain unresponsive to conservative therapy and was supplemented by data from a second trial to account for early surgery. Estimates of effectiveness were derived from a survey of 42 surgeons. This US-based study took the perspective of the health payer.
Source of effectiveness data
Effectiveness was defined as the number of QALYs gained with surgical treatment versus medical treatment. The comparator was chemonucleolysis. To determine effectiveness, results from the two trials were adjusted by QoL values obtained in a separate study of 83 subjects reporting an episode of severe back pain. A TTO utility measure was administered to estimate QoL. Mean TTO values were calculated and self-assessed outcomes reported in the trials were weighted by corresponding QoL values. For discectomy, a 2-week postoperative period was included in the base-case model. Benefits were discounted by an annual 5% rate.
Source of resource utilisation and costs
Rates of service utilisations were obtained, from a commercially available database, using data from 2175 patients diagnosed with a herniated disc. Demographic details of these patients were reported as similar to the trial participants. From this database, patients operated within 6 weeks of treatment were defined as surgically treated. Those patients who never underwent surgery and those operated on after 6 weeks were categorised as medically treated. Operation costs for medical patients requiring late surgery were counted as costs of initially choosing medical treatment. Direct costs were not discounted. Direct costs reflected costs for all services related to disc herniation (patient visits, diagnostic tests, procedures and hospitalisations). The quantity–cost boundary adopted was that of the hospital. The estimation of quantities and costs was based on actual data. Costs and rates of service utilisation were derived from MEDSTAT (January 1987–December 1989) and data on 78 patients diagnosed at a health maintenance organisation (HMO). Costs were adjusted to 1993 prices using the medical component of the Consumer Price Index and presented in US dollars ($). A 10-year time horizon was undertaken.
Summary of cost-effectiveness analysis
A model-based cost-effectiveness analysis was undertaken. Sensitivity analyses were conducted on efficacy (± 25%), QoL (± 50%) and costs. Additional estimates were obtained from a survey of spine surgeons, who were presented with case scenarios and asked to estimate the probabilities of excellent to poor outcome after surgical or medical treatment. However, these estimates were not reported, but were available on request from the authors. Additional cost estimates were undertaken from 78 patients diagnosed at a HMO. The authors stated that these were designed to estimate the true resource cost and may have reflected the actual costs more accurately than those used in the base-case analysis.
Summary of the findings
Patients treated with surgical discectomy or chemonucleolysis experienced faster improvement than patients treated medically. The probability of a good outcome varied between 0.36 and 0.56 after medical treatment and between 0.64 and 0.70 after discectomy. For a poor outcome, the probability varied between 0.06 and 0.20 after medical treatment and between 0.07 and 0.14 after discectomy. QoL values associated with a good outcome were 0.95, with a fair outcome 0.77, with a poor outcome 0.62 and with a bad outcome 0.5.
During the 10 years after surgery the average surgical patient experienced 8.7 QALYs whereas the average medical patient experienced 8.27 QALYs, with the difference of 0.43 representing the non-discounted improvement in QALYs associated with surgery. Total costs for the 18-month period beginning 6 months before diagnosis, were $17,020 for the surgical group compared with $4470 for the medical group. The non-discounted cost-effectiveness ratio of surgical over medical therapy was $29,200 per QALY. The discounted cost-effectiveness was $33,900 per QALY. Cost-effectiveness of discectomy remained < $100,000 as long as surgery produced an incremental quality-adjusted benefit of at least 0.125 years. The authors concluded that, for carefully selected patients with herniated discs, surgical discectomy was a cost-effective treatment with favourable cost-effectiveness results obtained from its effect on QoL coupled with moderate costs.
Critique of Malter and Weinstein
There are key limitations of Malter and Weinstein’s study which limit its relevance to current practice. It is a US study, involving a comparator not currently available to the UK NHS. In addition, the effectiveness data were from the 1970s and 1980s; improvements in surgical management may be important, so caution would be needed if attempting to generalise these findings to current management.
Although not reported in accordance with accepted current guidelines, the paper reasonably reported the economic evaluation undertaken. One possible issue was the robustness of the review undertaken, with effectiveness estimates derived from a qualitative synthesis. Effectiveness data were collected from different subjects, combined, then the estimation of benefits was modelled. The reporting of this process was limited; however, the TTO method used to derive the measure of benefits appears to be appropriate.
All costs relevant to the perspective adopted appeared to have been included in the analysis. The authors were unable to assess costs incurred more than 1-year after diagnosis from the MEDSTAT database. A sensitivity analysis was conducted on prices, but not on costs. The authors did make appropriate comparisons of their findings with those from other studies at the time of publication.
van den Hout et al.
van den Hout et al. 283 examined the cost-effectiveness of early surgery compared with 6 months of prolonged conservative care, for patients aged 18–65 years with sciatica for 6–12 weeks because of lumbar disc herniation. Economic evaluation was conducted alongside a RCT.
Source of effectiveness data
The source of clinical effectiveness data was a RCT undertaken in nine hospitals in the Netherlands. 87 Two hundred and eighty-three patients were randomised with 142 patients (mean age 43 ± 10 years; 68% men). Patients were followed up in the trial for 12 months. A CUA was undertaken from the perspectives of the health-care system and society.
Source of resource utilisation and cost data
Costs included the costs of hospital stay, visits to health-care professionals, home care, paid domestic help, informal care, drugs and aids, out-of-pocket expenses as a result of the disc hernia (e.g. swimming) and hours of absenteeism from work. Resource-use data were collected using patient-completed diaries and collected at several time-points over the study period. Nine per cent of patients who did not return resource diaries were equally distributed across the two comparator groups and less likely to have undergone surgery. Correction for selected non-response was made by multiple imputation of data on costs from patients in the same group with same surgical status who returned diaries. This did not substantially change the results compared with excluding these patients. For patients who did return cost diaries, the diaries covered 97%, 91%, 83% and 84% at 3, 6, 9 and 12 months respectively. For periods that were not covered, data were imputed from the closest available diary from the same patient.
Hospital costs were obtained following diagnosis using treatment prices available from 75 different centres, excluding the two highest and two lowest prices. Other health-care costs were based on Dutch standard prices. The costs of absenteeism were valued using the human capital approach. All costs were presented in euros and at 2008 Dutch consumer index prices. As a 1-year time horizon was used, costs were not discounted.
Summary of cost-effectiveness analysis
Utilities were obtained from the same patients participating in the RCT, through the administration of the EQ-5D (US and UK), the SF-6D (derived from the SF-36) and the VAS. Utilities were derived at several time points from baselines to 52 weeks after randomisation. Missing data were present in 4%, 5% and 5% of the EQ-5D, SF-36 and VAS, respectively, and inputted using the rounded average within the same randomisation group at the same time. QALYs were derived, using the area under the curve (AUC) method, for each separate quarter of the year after randomisation and during the entire year as the summary benefit measure.
Uncertainty was addressed by calculating CIs around the cost–utility ratios. Cost-effective acceptability curves were presented. Sensitivity analysis was carried out on the different utility measures and on the included cost categories using a health-care or societal perspective.
Summary of the findings
Over 12 months, the differences in QALYs and all four utility measures during all four quarters were consistently more favourable after early surgery. The differences in QALYs reported according to the utility measure used were UK EQ-5D 0.044 (95% CI 0.0005 to 0.083), US EQ-5D 0.032 (95% CI 0.005 to 0.059), SF-6D 0.024 (95% CI 0.003 to 0.046) and VAS 0.032 (95% CI –0.003 to 0.066).
From the perspective of the health-care system, total health-care costs remained significantly higher than the costs of prolonged conservative care, with a difference in costs of €1819 (95% CI €842 to €2790) per patient. Total societal costs were –€12 (95% CI –€4029 to €4006): slightly in favour of early surgery. The probability that early surgery is cost-effective compared with conservative care varies with willingness to pay. From a societal perspective it was 76% at €40,000 per QALY and was 87% at €80,000 per QALY. Smaller differences were seen with other utility measures.
From the health-care perspective, according to the UK EQ-5D and US EQ-5D, the incremental cost per QALY gained with early surgery was estimated at €41,000 (95% CI €14,000 to €430,000) and €57,000 (95% CI €19,000 to €436,000), respectively.
The authors concluded that faster recovery from sciatica makes early surgery more cost-effective than prolonged conservative care. The estimated differences in health-care costs were acceptable and were compensated for by the difference in absenteeism from work. For a ‘willingness-to-pay’ ceiling ratio of €40,000 or more per QALY, early surgery need not be withheld for economic reasons.
Critique of van den Hout et al.
The source of economic data, methodology and interpretation of findings from this study were generally of good quality in this well-presented paper.
The economic evaluation was performed alongside a RCT, so selection bias was unlikely with comparable clinical, demographic and economic characteristics at baseline. The comparators were well defined and justified on the basis that prolonged conservative care is often advocated with no evidence available on the optimal timing of disc surgery.
There were clear inclusion criteria, robust power calculation and analysis undertaken using ITT principles. The internal validity of the study underpinning the economic evaluation was good. One of the strengths of the paper was the considered approach taken to the instruments used to derive utilities. In the absence of a condition-specific measure of health utility, three different generic instruments were used to measure patient preferences, which were compared in a sensitivity analysis.
Costs were considered within the two perspectives. Although there are inherent difficulties associated with the collection of resource data using patient diaries, adherence was high and, where necessary, appropriate analysis was undertaken to account for missing data. A detailed breakdown of costs was presented in the paper including sources of data, price year and statistical analysis. A limitation of the paper, which was clearly acknowledged by the authors, was the considerable variation depending on the method used for assigning costs.
Cost and benefits were appropriately analysed using an ICER. These were clearly presented. Uncertainty was addressed by calculating CIs; however, these were extremely wide. The authors did caution about the limitation of this study owing to the particular characteristics of the Dutch health-care system, citing a high rate of surgery, quicker waiting times and legislation which protects employees resulting in higher absenteeism, but not necessarily lower productivity.
Other limitations acknowledged were the 1-year time horizon for the study; a longer time horizon would have reduced statistical power and the clinical evaluation showed no differences after year 1. Another limitation was that patients were inevitably aware of the randomised group they were in; their reported utilities and costs may have been influenced by their preference for treatment. A final limitation identified was that 40% of patients randomised to receive prolonged conservative care underwent disc surgery at some time, although this was similar to other reported studies. The authors stated that this was an expected clinical consequence, as the study compared two different management strategies and that persistent or increasing symptoms that caused some patients to cross over should be part of the economic evaluation.
Tosteson et al.
Tosteson et al. 282 reported a cost-effectiveness analysis based on data derived from the pooled analysis of the SPORT randomised and observational cohorts, based in the USA. The interventions compared were standard open laminectomy, laminectomy with removal of herniation and examination of the involved nerve root, and non-operative treatment, defined as usual care chosen individually by patients and physicians. Participants were aged ≥ 18 years, diagnosed with herniated intervertebral disc and confirmed as surgical candidates with a symptom history of at least 6 weeks.
Source of effectiveness data
Cost-effectiveness analysis was based on data from 1191 participants, including 775 who underwent surgery and 416 who were treated non-operatively for the entire follow-up period of 2 years. Clinical effectiveness was evaluated using QALYs at baseline, 6 weeks and 3, 6, 12 and 24 months. Health-utility values were obtained using the EQ-5D with US scoring. Time-weighted sums of EQ-5D values, adjusted to the overall mean baseline health-state value, provided the estimate of QALYs for each treatment group. CEA was based on the perspective of the health insurer and society.
At baseline, differences in patient demographic and clinical status were noted. Surgical patients were significantly younger, more likely to work full-time and to receive or be in receipt of social security compensation. Clinically, surgical patients were more likely to have L5–S1 (lumbar segment 5 to sacral segment 1) herniation, worse bodily pain, physical function, mental health and ODI and EQ-5D scores compared with non-operative patients.
Source of resource utilisation and cost data
Costs were collected on health-care costs (visits to health-care professionals, diagnostic tests, other health-care services, medications and surgery, including repeat surgery costs). Other costs included lost productivity, measured as missed work, unpaid caregiving time and missed housekeeping. Resource-use data were collected at each follow-up visit for health-care costs. A nurse-administered survey collected detail on medication usage. Recall time for self-reports of resource utilisation and time away from work/usual activities were 6 weeks for the 6-week and 3-month visits. For all other times a 1-month recall was used. Participants were provided with a diary to assist in tracking resource utilisation and missed work/housekeeping days.
Direct medical costs were estimated by multiplying patient-reported medical resource use by unit costs for each cost component. These were presented in the paper. Unit costs for office visits, hospitalisation, diagnostic test and procedures are based on 2004 Medicare national allowable payment amounts and medication prices on 2004 Red Book prices. 287 Costs were adjusted for inflation, expressed in 2004 US dollars with a 3% annual discount rate used in the analysis of costs and QALYs. The differences in surgical costs were considered in terms of the procedure performed and the cost of intraoperative complications, which determined their diagnostic-related group (DRG). This was handled in the following manner: (1) a cost approximating the value paid by non-Medicare insurers was estimated to be 70% of the mean amount billed to Medicare in 2004; and (2) the observed 2004 Medicare mean total DRG price was used to reflect hospital-related surgery costs population aged > 65 years. Surgeons’ costs were based on 2004 Medicare amounts; anaesthesiology costs were estimated using operative time with a fixed amount added if an intraoperative complications occurred. For non-spine-related hospitalisations, costs were based on the DRG and priced using mean observed Medicare prices in 2004 for each admission.
Loss of productivity costs due to spine-related problems were calculated by recording missed days of work (for those employed) and missed homemaking days. Use of unpaid caregivers (including spousal care given) were obtained and costs were estimated using the standard human capital approach; for work days lost this was estimated by multiplying change in hours worked by the gross of tax wage rate on self-reported wages at study entry. For homemaking and caregiving these were valued using the average wage plus non-health benefits for individuals aged ≤ 35 years.
Summary of cost-effectiveness
Owing to the high rates of non-adherence in the original randomised and observational cohorts, the two cohorts were combined and analysed according to treatment received using regression modelling of longitudinal data via generalised estimating equations. Separate models were fitted for EQ-5D and 30-day cost rates; measured at 6 weeks and 3, 6, 12 and 24 months. Cost rates were based on reported utilisation rates at each time period taking into account the recall period used.
Outcomes were assigned to the surgical group with follow-up times measured from the surgery date. To take into account the windows for scheduled visits and crossover, the actual time of the outcome assessment varied. This was included as adjusting variables in the longitudinal variables. To adjust for potential confounding baselines, variables associated with missing data or treatment received were included as covariates.
Based on the adjusted mean differences in EQ-5D from the longitudinal regression, an AUC/time-weighted average was undertaken to estimate QALY differences between surgical and non-operative costs, adjusted to a common baseline value. ICER CIs were estimated using bootstrapping methods. Sensitivity analyses were undertaken to consider the impact of limiting costs included in the analysis to direct medical cost or direct medical costs plus costs of work loss for those employed.
Summary of the findings
Mean health scores improved over time for both groups of patients. Total mean discounted QALYs were 1.64 (95% CI 1.62 to 1.67) for surgical patients and 1.44 (95% CI 1.40 to 1.47) for non-operative patients, a difference of 0.21 (95% CI 0.16 to 0.25).
Ninety-six per cent of surgical procedures were back and neck without complications (DRG 500) with a mean cost of $12,754 (95% CI $12,740 to $12,760). Three per cent had complications (DRG 499) with mean costs estimated at $19,063 (95% CI $18,960 to $19,160). Repeat surgery occurred in 6.8% of surgical patients with a mean cost of $28,019 (95% CI $19,950 to $26,730).* Total mean costs were $27,273 (95% CI $26,009 to $28,644) for surgical patients and $13,135 (95% CI $11,244 to $14,902) for non-operative patients. Total direct costs were $20,237 (95% CI $19,314 to $21,160) for surgery and $5804 (95% CI $4639 to $6969) for non-operative patients. Total loss of productivity costs were $7089 (95% CI $6155 to $8022) for surgical patients and $7399 (95% CI $6221 to $8577) for non-operative costs. Over the 2-year period, indirect costs contented for 26% of costs for surgical patients and 57% of non-operative patients. The distribution of non-surgical direct costs was similar across both groups. Both types of cost were highest following the first 6 weeks among those undergoing surgery. Mean indirect costs for non-operative patients were higher over time than for surgically treated patients.
When all costs were considered, the cost per QALY gained for surgical treatment relative to non-operative care in the general population was $69,403 (95% CI $4923 to $94,999). For those aged ≥ 65 years, the cost per QALY gained decreased to $34,355 (95% CI $20,419 to $25,512).* Limiting costs to direct costs alone for general population ($72,181, 95% CI $56,473 to $92,394) and Medicare ($37,285, 95% CI $28,364 to $48,993) or direct costs with lost work days (general population $77,300, 95% CI $60,009 to $99,544) or Medicare ($42,111, 95% CI $30,976 to $56,284) had little change. This also had little impact on the ICER, which was estimated at $33,176 (95% CI $18,348 to $54,157) under Medicare pricing.
The authors concluded that surgery for intervertabral disc herniation was moderately cost-effective over 2 years, but expressed caution about the different values for surgery according to the method used for assigning surgical costs.
*There was obviously an error in the published paper for the figures, but no erratum could be found; therefore, we do not know whether it is the mean estimate or the CI that is correct.
Critique of Tosteson et al.
The approach and interpretation of the data and findings in the paper appeared to be of good quality. Efforts were made by the authors to capture the different resource costs associated with different surgery, and also indirect costs. The justifications for taking into account the high non-adherence rates and the variations encountered during follow-up (e.g. missed visits, delaying surgery, timing of assessment and confounding variables) were well explained.
The rationale for the study is based upon critiquing the findings from Malter and Weinstein’s study. 286 In this paper, the comparators could be better described. The type of surgical technique was not controlled for. There is also little description of what constituted non-operative care beyond ‘usual care chosen individually by patients and physicians’.
The data were derived from two cohorts of patients: randomised and observational. The demographics of the cohorts showed significant differences. Although these were considered in the analysis, there was little interpretation beyond a descriptive analysis of these differences. Possible reasons for the decision to have surgery (e.g. surgical patients were younger, less likely to be working full-time or to be receiving or have applied for compensation, and generally had worse clinical signs and symptoms) may have resulted in worse outcomes, which in turn influenced QALYs.
The authors considered resource usage. However, the limitations of using patients’ self-reporting of resource use are referred to. The paper mentions the data collection approaches to obtain patient-reported data, but provides little information on how reliable or valid the data were. Recall bias is a potential concern, and the authors attempted to minimise this by limiting the recall window to 6 weeks after early visits and 1 month after annual visits. The authors expressed reasonable confidence that chronic problems were captured as they incurred ongoing costs, and that large costs including hospitalisation and repeat surgery were not limited by the recall period. However, some acute costs could have been missed and the small but important biases when reporting indirect costs may be a factor to take into account. However, it would seem likely this bias was applicable to both groups. The authors considered better ways of capturing resource costs, e.g. linking with electronic billing records, but this would have been likely to have biased cost ascertainment with near-complete capture of surgery compared with non-operative care.
Epidural steroids
Price et al.
Price et al. 173 undertook a multicentre, double-blinded RCT of ESIs versus placebo in 228 patients with clinically diagnosed unilateral sciatica aged between 18 and 70 years who had duration of symptoms between 4 weeks and 18 months. The justification for the study was that, although 45,938 ESIs were performed in the NHS in 2002–3, there was a lack of evidence of their benefit, with safety and cost-effectiveness not previously evaluated.
Source of effectiveness data
The intervention was up to three ESIs compared with normal saline. The primary outcome was the ODI with measures of pain, physical and psychological function collected alongside objective measures of sciatic root irritation, neurological deficit and procedural side effects. QoL was determined using the SF-36.
Source of resource utilisation and cost data
A pilot was undertaken to inform the data collection method. Resource-use data were collected using an instrument completed by all clinical staff which recorded their time spent on patient consultation, aiding the patient before or after the consultation, the time associated with patient administration for all patients presenting with sciatica not included in the trial, pathology tests and imaging. Data were collected across all three centres during July–October 2000. Costs of initial radiology and pathology, if not already performed by the referring centre, were included. Analgesic costs were examined and assumed not to differ between the two groups, so were not considered in the economic analysis.
Cost data were used to calculate a cost per patient for treating sciatica with epidural injections from the perspective of health provider and purchaser. An average cost per patient was based on two management practices. Under each management practice it was assumed that patients had an initial consultation and follow-up. Owing to the short time horizon when costs and benefits were incurred, discounting was not performed.
Summary of cost-effectiveness analysis
Cost-effectiveness was undertaken from the perspective of the health provider and purchaser (NHS).
QALYS were derived from SF-6D health-utility scores using SF-36 raw data by the Brazier et al. 288 technique. CUA was undertaken using the standard gamble (SG) method to derive incremental cost per QALY ratios for managing a patient with an ESI. Sensitivity analysis was undertaken to explore how cost estimates changed, given the assumptions that underlay resources, resource-base costs were relaxed. Sensitivity analysis was not undertaken for purchaser costs.
Summary of the findings
The study found ESIs conferred a short-term benefit only. The resource savings could be substantial even with a modest change to treatment. For example (from the purchasers’ perspective), the saving from moving from an assumed model of current pragmatic practice (maximum of three ESIs) to a patient management strategy suggested by the trial (one ESI) would represent a saving of £16,505,700 in the sector.
The estimated average cost per patient treated from the provider’s perspective was £265.30 per patient for the trial protocol and £152.80 per patient assuming a management strategy based on trial costs. Using NHS recharge cost from the purchaser’s perspective, the estimated average cost was £2102 per patient to deliver treatment based on the trial protocol and £992 per patient for one epidural injection, based on the trial results.
The incremental analysis is shown in Table 166.
| Perspective | Trial protocol (up to three ESIs) | Strategy based on trial results (one ESI) |
|---|---|---|
| Provider | ||
| Incremental cost (£) | 265.30 | 152.80 |
| Incremental QALY | 0.0059350 | 25,745.68 |
| Cost per benefit gain (£) | 44,701.11 | |
| Purchaser | ||
| Incremental cost (£) | 2102 | 992 |
| Cost per benefit gain (£) | 354,171.65 | 167,144.76 |
To obtain an improvement at 3 weeks in one patient based on the trial protocol is £16,816–23,963 [depending on number needed to treat (NNT) assumed (8–11.4)], or one epidural to improvement in one patient at 3 weeks is £936–11,306.
In the sensitivity analysis, relaxation of the base-case assumptions of labour time, using the maximum recorded time for nurses and clinicians, more than doubled the average patient cost under each management strategy. Changing from day case to overnight stay also increased average patient costs. Assuming that QALYs remain unchanged, the effect would be to increase the cost–utility ratio further. The authors concluded that although ESIs are relatively safe, they confer only transient benefits in symptoms and self-reported function in a small group of patients with sciatica at substantial costs. ESIs failed the QALY threshold recommended by NICE and do not represent good value for money if NICE recommendations are followed.
Critique of Price et al.
Reporting of the economic evaluation conforms to accepted guidelines and is presented in detail. The authors recognised the limitations of the pragmatic study design and attempted to overcome this through their recruitment strategy. The intention was to compare epidural corticosteroid injections with placebo. The duration of symptoms varied from 4 weeks to 18 months, with patients who had previous back surgery excluded. There was a clear acknowledgement that the intention was to consider only patients who presented with sciatica at the point of referral to secondary care, and for the economic analysis a standard package of care was assumed. Costs associated with this package were not considered, as it was assumed that these would be incurred regardless of whether or not the patient received an epidural. Costs of health-service utilisation after week 52 were not included as no significant difference was found. There was a variability in resource usage across the three centres, reflecting the persistent limitation of a lack of clinical consensus in the management of sciatica.
The perspective taken in the economic evaluation was clearly defined and resource data appeared to have been systematically collected across the three centres. Direct costs were appropriately collected based on the perspective chosen. Indirect costs were not obtained, as it was argued that inclusion of indirect costs could overstate potential costs savings and that such savings were not relevant to resource allocation decisions. The authors clearly stated that resource data did not reflect resources expended in the trial per se, but represented the costs to normal practice.
Where differences occurred, these have been highlighted in the study. One of the most notable differences was the difference in clinicians’ and nurses’ time across the three centres, which probably reflected differences in practice and culture rather than marked differences in the quality of patient care. Although the justification of staff costs were made explicit, several resource costs appeared to have been generalised across several categories.
Cost–utility analysis was clearly presented. SF-6D scores were derived from the SF-36 using an established technique with SG scores calculated, assuming the trial protocol of three injections. The authors note the variability in the number in each sample, so average SG score were derived for patients with observations for all visits up to week 12 to correct for possible sample bias. One of the possible issues was the lack of sensitivity of this generic measure to detect small but important changes that may have affected the findings of limited changes in QoL. QALYs were derived and benefits were appropriately analysed using an incremental analysis.
Cost per QALY gained to the provider using a patient management strategy administering only one epidural injection. These results assumed that gain in QALY calculated would approximate that under a patient management strategy based on the trial results (one ESI). This was not considered an unreasonable assumption by the authors as change in SG score after week 3 was lower in the active group than the placebo group. However, only 21 patients received one injection to confirm this from the clinical data. Costs derived using NNT recognised the fact that ESI was compared with placebo and may therefore increase NNT and subsequent costs.
Sensitivity analysis was appropriately carried out to take into account how costs would change if base-case assumptions were relaxed. These examined changes in variation of clinical labour practices and resource use. The base-case assumption had implied that patients would be treated as day cases, so this assumption was changed. However, in practice this was felt to be too extreme, as in reality there was more likely to be a mix of day-case care and inpatient stay. In both cases, the cost increases. Assuming that QALYs remained unchanged, the effect of this would be to increase the cost–utility ratio further.
As noted by the authors, indirect costs and return to work were not considered. This was justified in terms of the recognised difficulties in using such an outcome measure owing to its definition and collection in a population of mixed age, gender and socioeconomic groups, and that there are many risk factors associated with chronic work disability apart from the level of pain. The study clearly acknowledges that the UK NHS charges differ from the actual resource used. In addition, some strategies for sciatica can be purchased from the private sector. Although these are not true resource costs (in terms of a UK NHS perspective), these may still have an opportunity cost attached. Such costs are substantial for a short period of pain relief. The lack of an individual perspective might limit the interpretation of findings, as a small chance of short-term pain relief (1 in 8 to 1 in 11) based on NNT might be welcomed by some patients. As would be expected, these findings cannot be translated into private clinical practice.
Summary
Although some economic evaluations identified in the systematic review were of reasonable to good quality, they were not able to fully address our research question. Although individual studies raised a number of important issues, it was difficult to draw meaningful conclusions across these studies because of their heterogeneity. Although there was some indication of favourable benefit such as with disc surgery, robust findings could not be reliably drawn.Although an evidence base is emerging, there remains a lack of well-designed economic evaluations. The majority of evaluations were undertaken in conjunction with clinical trials, with a lack of published decisions models. There was considerable variation with each of the studies to the management of patients with sciatica, thus limiting the lessons that can be drawn from current evidence in order to understand the relative cost-effectiveness of current management strategies that reflect current practice. Of particular note is the relevance of these studies to the UK NHS setting.
Chapter 9 Economic evaluation
Introduction
The aim of the economic evaluation was to determine the relative cost-effectiveness of the treatment regimens for managing patients with sciatica. The existing evidence relating to the cost-effectiveness of treatments had a number of limitations, which made it insufficient to inform decision-making regarding the most appropriate management strategy for patients with sciatica. The majority of evaluations were undertaken in conjunction with clinical trials with a lack of published decisions models. There was considerable variation with each of the studies to the management of patients with sciatica, thus limiting the lessons that can be drawn from current evidence in order to understand the relative cost-effectiveness of current management strategies that reflect current practice. Hence, it was necessary to construct a decision-analytic model to address a number of these issues more formally. The model provided a framework for the synthesis of data from the clinical effectiveness, economic reviews and other relevant sources. It was developed to estimate costs from the perspective of the UK NHS289,290 and health outcomes in terms of successful treatment and utility gain for all the relevant treatment strategies.
Development of the economic model
The limitations associated with the economic evaluation studies reviewed resulted in a decision-analytic model being developed to estimate the relative cost-effectiveness of management strategies for patients with sciatica. The heterogeneous nature of the condition, the lack of recognised guidelines for the management of patients with sciatica and considerable variation within practice all made it extremely difficult to develop a model that reflected current practice. Further, the considerable levels of uncertainty surrounding the outcomes from the MTCs restricted the development of a probabilistic model and, therefore, a deterministic model structure was constructed based on information from some of the studies reviewed, the findings from the review of effectiveness and MTCs undertaken, published sources of unit costs and expert opinion from clinicians and other health-care professionals. The decision tree model, highlighted in Figure 112, was used to estimate the expected costs and number of successful treatments over a 12-month period. The perspective employed was that of the UK NHS and out-of-pocket expenditures on over-the-counter (OTC) medications and alternative therapies, for example, have not been included. This has important ramifications as it is assumed that ultimate treatment failures will resort to alternative therapies outside the conventional health-care system, at zero cost to the NHS.
FIGURE 112.
Decision tree.
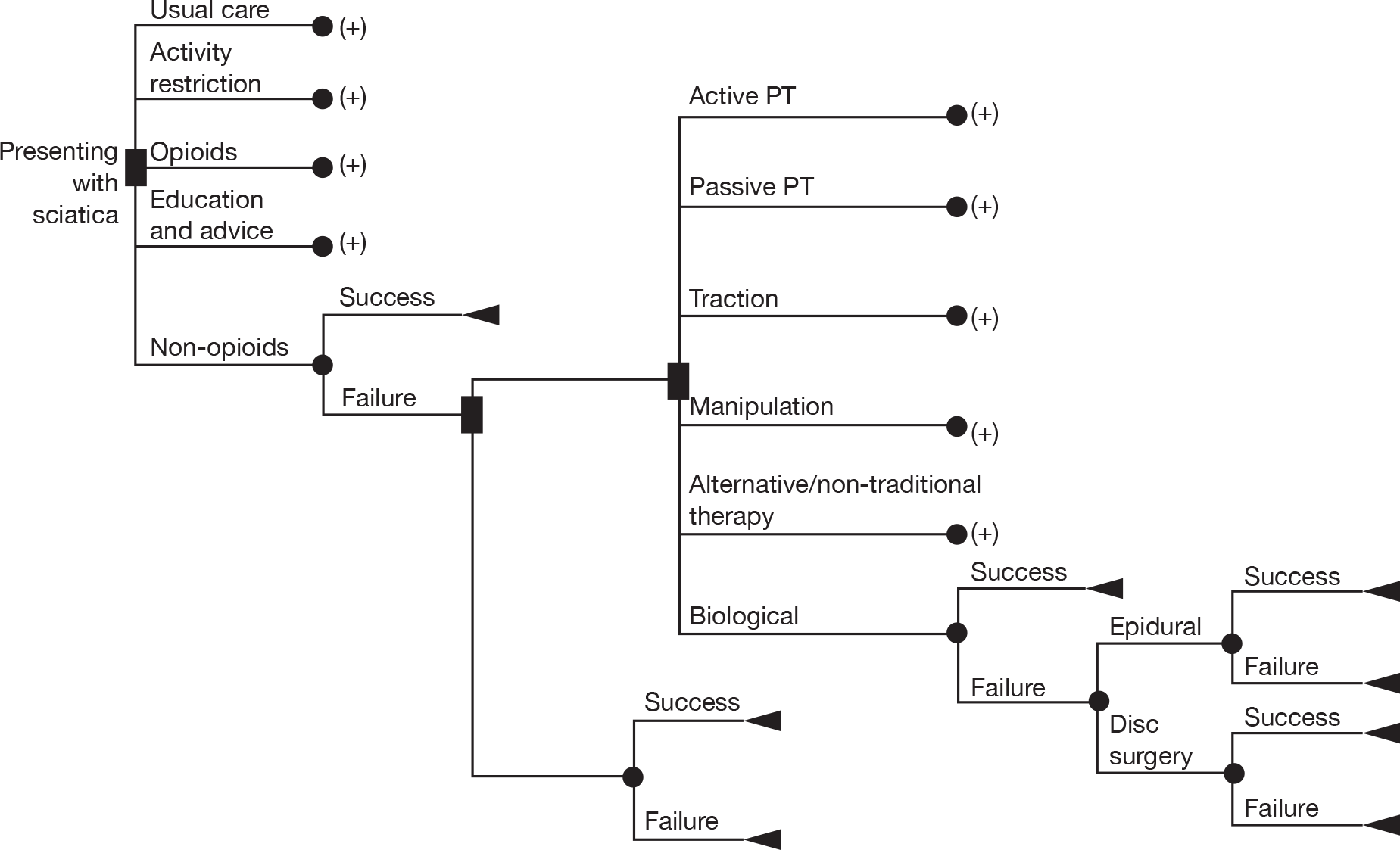
The number of appropriate and relevant health states was informed by the results of the service provider survey (see Chapter 10, Summary of economic evaluation), the literature review and from advice within the research team. The cost of managing patients within each state was reflected in the model, although it was not envisaged that patient progression will be seamless, or indeed linear and uni-directional. The structure of the model will reflect this and the probability of movement between health states will be based on the evidence from the literature review, including the distribution around the point estimates. In addition, a sensitivity analysis was used to assess the impact of ‘changes’ in the variable estimates, and identify potential areas for future research.
Telephone survey of service providers
A panel of service providers known to the advisory group members were contacted by telephone to determine their usual clinical practice, the usual treatment pathways and whether or not they use a stepped-care approach. This information was used to inform which sequence of treatments to include in the economic model.
Recruitment and access for the telephone survey was undertaken between June 2009 and September 2009. Three local health boards in Wales and six primary care trusts and hospital trusts in England were contacted. As required under the Research Governance Frameworks for England and Wales, permission was sought from each relevant research and development department prior to seeking and recruiting a range of service providers (e.g. spinal surgeons, physiotherapists, service commissioners). The response rate was poor from England, with only three contacts established, predominantly because of difficulty in locating the suitable person with research governance responsibility (e.g. web-based contacts out of date, lack of clarity of specific research governance procedures in primary care trusts). Of these three, two primary care trusts request evidence of NHS Ethical Committee review, despite confirmation from Cardiff University Research Governance Officer that this was deemed audit/service evaluation.
Preliminary informal interviews were conducted with four service providers. However, these generated wide disparities in services (e.g. whether or not an intermediate care service was provided) and interventions offered (e.g. biologicals were not licensed for use and so would not be considered), resulting in difficulty in using individual service providers to contextualise a generic ‘sequence of treatments’ in relation to the findings emerging from the systematic review for the purposes of developing the structure for the economic model base case. On review of these difficulties, the economic team felt that the provider survey would be better placed once the MTC analysis was completed in order to ‘validate’ the interventions/care approaches drawn from the review findings. However, owing to time constraints, these initial interviews were used along with input from the steering group (clinicians on the review team) to build up a staged treatment approach through the assumption of patient progression through primary, intermediate and specialist care.
Previously conducted systematic reviews were used to generate a list of potential treatments for sciatica. During the telephone interviews, clinicians were asked initially what treatments (including combination and sequence of treatments) they usually use, and, afterwards, if prominent treatments identified from previous reviews were not mentioned, they were asked if they have ever considered using these.
Model description
The model was constructed on the assumption that patients presenting with sciatica would be managed through one of three pathways, with alternative treatments within each of the pathways. The first pathway would involve management within primary care and revolve around what might be termed usual care, with use of analgesics and other medications if considered appropriate, to attempt to secure symptom resolution. The treatments included within this pathway therefore include:
-
usual care
-
education/advice
-
activity restriction
-
non-opioids
-
opioids.
The second pathway would involve a stepped approach and include the use of intermediate treatments – offered in addition to the initial treatments provided within primary care – and provided in secondary care outpatients by multidisciplinary teams including physiotherapists, musculoskeletal physicians, etc. The treatments here include:
-
manipulation
-
traction
-
passive PT
-
active PT
-
alternative treatments
-
biological agents
followed by more invasive treatment (epidural followed by disc surgery if there was no symptom resolution).
The third pathway would involve immediate referral for surgery to alleviate symptoms.
There does not appear to be any data to determine the proportion of patients managed through each pathway and therefore the treatment pathways represent the decision choices available for GPs and their patients on presentation. Each of the pathways and the treatment variations available within them were compared with ‘inactive control’, which, according to the findings from the MTC, had a non-zero probability of symptom resolution, but was assumed to cost £0 in the baseline model.
The decision tree model comprised the three treatment pathways: initial treatments, initial treatments followed by intermediate treatments and invasive treatments, and initial treatments followed by disc surgery. The treatment options available within each of the pathways are shown in Table 167.
| Pathways | Treatments |
|---|---|
| Initial treatments | Inactive control |
| Usual care | |
| Education/advice | |
| Activity restriction | |
| Alternative/non-traditional | |
| Non-opioids | |
| Opioids | |
| Biological agents | |
| Intraoperative interventions | |
| Spinal cord stimulation | |
| Intermediate treatments | Manipulation |
| Traction | |
| Passive PT | |
| Active PT | |
| Surgery | Epidural/nerve block |
| Disc surgery |
The focus was on the binary outcomes used in the global effect measure from the MTC, representing successful or unsuccessful symptom resolution and with results expressed as incremental cost per patient with symptoms successfully resolved. Analysis also included utility gain associated with symptom resolution, with results expressed as incremental cost per utility gain (over a 12-month period). The heterogeneity of duration effect and not evidence of relapse and recurrence, made it difficult to extend the analysis beyond this time period, with the assumption made that the utility gained following successful treatment would continue for this period.
Dealing with uncertainty
A series of one-way sensitivity analyses were used to address uncertainty in the model. The baseline estimates were based around the best-case scenarios identified for cost and then adjusted to reflect what was regarded as worst-case scenarios. Similarly, the probabilities of success were those determined from the Winbugs output from the MTC in the baseline model and then adjusted to assess the impact on baseline findings. The utility values for symptoms and symptom remission were also adjusted to determine impact on baseline findings.
Data sources
The probabilities of success for each treatment were derived from the Winbugs output from the MTC. The Winbugs output provides a summary output of the posterior distributions of the relevant parameters. The probability of success is the median value of the posterior distribution of the global effect measure.
The probabilities of success are shown in Table 168.
| Pathways | Treatments | Probability of success | Probability of failure |
|---|---|---|---|
| Inactive control | 0.3828 | 0.6172 | |
| Initial treatments | Usual care | 0.3393 | 0.6607 |
| Education/advice | 0.5025 | 0.4975 | |
| Activity restriction | 0.4411 | 0.5589 | |
| Non-opioids | 0.6129 | 0.3871 | |
| Opioids | 0.4985 | 0.5015 | |
| Intermediate treatments | Alternative/non-traditional treatments | 0.8523 | 0.1477 |
| Biological agents | 0.9074 | 0.0926 | |
| Manipulation | 0.7518 | 0.2482 | |
| Traction | 0.4277 | 0.5723 | |
| Passive PT | 0.4147 | 0.5853 | |
| Active PT | 0.4043 | 0.5957 | |
| Invasive therapies | Epidural/nerve block | 0.6577 | 0.3423 |
| Disc surgery | 0.6330 | 0.3670 | |
| Intraoperative interventions | 0.7454 | 0.2546 | |
| Spinal cord stimulation | 0.6643 | 0.3357 |
The costs associated with managing patients with sciatica were based on clinical opinion and derived from published cost sources (and based on 2008–9 prices), as shown in Table 169.
| Description | Unit cost (£) | Cost (£) | Source of data | ||
|---|---|---|---|---|---|
| Primary care | |||||
| GP consultation for all patients (within 6 weeks) | 35 | Average two consultations (varies between one and three) £70 | Curtis, 2009291 | ||
| GP consultation for patients referred to intermediate care/surgery (± 6 weeks) | 35 | Referral usually triggered after three consultation £105 | Curtis, 2009291 | ||
| GP contact following discharge from intermediate care/surgery | 35 | Typically one follow-up to GP for post-operative analgesia/sick note | Curtis, 2009291 | ||
| Other primary HP contact (surgery patients only) | 10 | Typically one intervention to remove suture by practice nurse | Curtis, 2009291 | ||
| Drugs | Description | Dose | Cost (£) | Continuing therapy | Source of data |
| Prescriptions | |||||
| Paracetamol and/or ibuprofen | Likely to be OTC and patient self-management for all patients, but GP would start as initial/continuing therapy in first 6 weeks | Paracetamol: dosage 4 g per 24 hours at 6 week prescription = approximately 336 tablets | £3.57 (based on 16 tablets = £0.17) | 1 week cost £0.60 | BNF No. 59292 |
| Ibuprofen: dosage 1600 mg per 24 hours at 6 week prescription = approximately 168 tables (if 400 mg tablets) | £3.74 (based on 84 400 mg tablets = £1.87) | 1 week cost £0.62 | |||
| Mild opioids (codeine phosphate) | Prescribed if initial analgesia is not working | 240 mg per 24 hours at 6 weeks = 168 tablets (if 60 mg tablets) | 6-week prescription = £11.88 (28 60 mg tablets = £1.98) | £1.98 | BNF No. 59292 |
| If added in at second visit – 4 weeks prescription | 4 weeks = £7.92 | ||||
| Other NSAIDs (naproxen) | Prescribed if initial analgesia is not working and/or with mild opioid | 1250 mg per 24 hours at 6 weeks = 210 tablets | 6 weeks = £10.65 (based on 250 mg 28 tablets) | £1.78 | BNF No. 59292 |
| 4 weeks = 140 tablets | 4 weeks = £7.10 | ||||
| Strong opioids (morphine) – considered only after no success with mild opioids/combinations with NSAIDs | Often in combination with co-analgesic amitriptyline or gabapentin | £9.61 (MST 30 mg day) for 2 weeks | £4.81 | BNF No. 59292 | |
| £1.04 (25 mg per day) for 2 weeks | £0.52 | ||||
| £7.88 for 2 weeks (based on titrating dose from 900 mg towards maximum dose) | £5.52 (based on maximum dose of 3.6 g as maintenance) | ||||
| Diazepam | For muscle spasm | 6 mg per 24 hours but p.r.n. | £1.96 | BNF No. 59292 | |
| Intervention | Description | Cost (£) | Source of data | ||
| Intermediate care | |||||
| Initial consultation | First attendance consultant led (110N) | 124 (94–147) – skill mix can vary | NHS reference costs 2008–2009293 | ||
| First physiotherapy contact (650A) | 55 (53–53) | NHS reference costs 2008–2009293 | |||
| MRI | RA027b– one area post contrast | 195 (142–239) | NHS reference costs 2008–2009293 | ||
| Pathology | Haematology | 3 (2–4) | NHS reference costs 2008–2009293 | ||
| Biochemistry | 1 (1–2) | ||||
| Follow-up | Consultant led (110N) | 86 (64–99) | NHS reference costs 2008–2009293 | ||
| Follow-up physiotherapy | 19 (19–19) | NHS reference costs 2008–2009293 | |||
| Biological therapies | Unlicensed for use in patients with sciatica in the NHS. Therefore, assumed similar dosage and duration in line with documented indications for other spinal conditions such as ankylosing spondylitis | BNF No. 59;292 NHS reference costs 2008–2009293 | |||
| For adalimumab, it was assumed to be a 12-week course with subcutaneous injection by a practice nurse | 1647 | ||||
| For influximab (worst case), it was assumed to be an i.v. administration in an outpatient setting with prophylactic antihistamine | 2219 | ||||
| Epidural steroids | Outpatient Intermediate pain procedure (ABO5Z) | 190 (125–205) – up to 3 | NHS reference costs 2008–2009293 | ||
| Procedure | Cost (£) | Source of data | |||
| Surgery | |||||
| Day-case extradural spinal minor (1) without CC (HCO6c) | 980 (570–954) | NHS reference costs 2008–2009293 | |||
| Inpatient extradural spinal minor (1) without CC (HCO6c), average stay 1.9 days | 1657 (1956–2314) | NHS reference costs 2008–2009293 | |||
| Inpatient extradural spinal minor (2) without CC (HCO6c), average stay 3.33 days | 2858 (1699–3184) | NHS reference costs 2008–2009293 | |||
| Follow-up consultant-led appointment | 86 (64–99) | NHS reference costs 2008–2009293 | |||
Drug treatments were costed according to British National Formulary (BNF)292 list prices at the time and calculated based on the dosage and durations in line with documented indications for use. Where required, it was assumed that dosage was based on an adult male of 65 kg. It was also assumed that paracetamol and ibuprofen were OTC medication, NSAIDs and opioids would be prescribed as slow-release tablets. Where multiple products were available, the least expense option was assumed.
It was assumed that each prescription required a GP consultation and that analgesics would be prescribed in accordance with the WHO analgesic ladder; therefore, a stepped approach would be taken to analgesia prescription and consultations would be separate. For non-opioid analgesia, two GP consultations were assumed with three consultations for opioid analgesia. Unit costs of GP consultations were taken from Curtis. 291 The base-case analysis assumed that analgesics were prescribed separately. NSAIDs and opioids were costed based on single treatment for base-case analysis and multiple analgesics in the sensitivity analysis.
Intermediate care interventions reflected treatments provided in secondary care outpatient settings and included non-traditional and alternative therapies. Unit costs were taken from published NHS reference costs 2008–2009. 293 It was assumed that an initial consultant assessment would be undertaken with one follow-up, with routine pathology and haematology blood tests and magnetic resonance imaging (MRI) (one area post contrast) performed for diagnosis. Passive and physical active therapies, manipulation and traction were assumed to be a physiotherapist-administered interventions. Biological therapies are unlicensed for use in patients with sciatica in the NHS. Therefore, we assumed a similar dosage and duration in line with documented indications for other spinal conditions such as ankylosing spondylitis. For, the base-case analysis, it was assumed that a 12-week course of adalimumab would be prescribed for subcutaneous injection by a practice nurse. The sensitivity analysis assumed an intravenous (i.v.) administration of infliximab in an outpatient setting with prophylactic antihistamine.
Intraoperative interventions are extra interventions during disc surgery (e.g. introduction of steroid around exposed nerve root, fat graft covering nerve root, exposed nerve root covered with a gel or membrane to reduce fibrosis, etc.) and are not routinely carried out in the UK NHS and have therefore been excluded. Spinal cord stimulation involves implantation of an electrode and is used only if disc surgery has failed and has therefore also been excluded from the model.
Epidural steroids were assumed to be a consultant outpatient intervention, with one treatment being used in the base-case and three treatments in the sensitivity analysis. Surgical unit costs were taken from NHS reference costs 2008–2009. 293 It was assumed that an initial consultant assessment would be undertaken with one follow-up, with routine pathology and haematology blood tests and MRI (one area post contrast) performed for diagnosis. A follow-up consultant appointment was assumed with one GP follow-up and practice nurse intervention for removal of sutures. Surgery was costed on inpatient extradural spinal minor, (1) with an average length of stay of 1.9 days for base-case and inpatient extradural spinal minor and (2) with an average length of stay of 3.33 days for sensitivity analysis.
The resultant costs are shown in Table 170.
| Treatments | Base case (£) | Sensitivity analysis (£) |
|---|---|---|
| Initial treatments | ||
| Inactive control | 0.00 | 0.00 |
| Usual care | 73.74 | 80.68 |
| Education/advice | 81.00 | 81.00 |
| Activity restriction | 70.00 | 70.00 |
| Alternative/non-traditional | 70.00 | 70.00 |
| Chemonucleolysis | Not included | Not included |
| Non-opioids | 122.23 | 129.33 |
| Opioids | 130.26 | 152.71 |
| Biological agents | 1646.74 | 3467.24 |
| Intraoperative interventions (not routine) | 1462.74 | 2218.71 |
| Spinal cord stimulation | 1462.74 | 2218.71 |
| Intermediate treatments | ||
| Manipulation | 349.00 | 578.00 |
| Traction | 349.00 | 578.00 |
| Passive PT | 349.00 | 578.00 |
| Active PT | 349.00 | 578.00 |
| Surgery | ||
| Epidural/nerve block | 602.76 | 990.28 |
| Disc surgery | 1433.66 | 3794.71 |
The utility values used in the model for symptoms and symptom resolution were derived from the review of studies. However, the lack of specific utility values for sciatica symptoms pre-intervention and following symptom resolution was problematic. The baseline values were derived from those in van den Hout et al. ,283 where the utility value at point of randomisation was 0.37 and the best value obtained was 0.83. The values were adjusted within the sensitivity analysis to compensate for the lack of consensus within the literature.
Cost-effectiveness results
The purpose of the cost-effectiveness assessment was to determine whether or not the additional costs required to increase likelihood of success, over and above usual care, can be regarded as representing value for money. The comparator chosen for this analysis was that of ‘inactive control’, which counterintuitively is more effective than usual care. Similarly, ultimate failures were assumed to have zero cost to NHS, although the extent to which this is reflected in practice is subject to some debate.
A series of 100 + independent scenarios were considered in which each initial treatment was considered in relation to inactive control; combined with each intermediate treatment followed by epidural/nerve block and then disc surgery; or following an initial treatment, patients were immediately referred for disc surgery. The number of successful outcomes of each treatment regime was combined with the utility of success (0.83) and failure (0.37) to give a total utility measure for each treatment regime. It was assumed that there was no reduction in utility for previous unsuccessful interventions, so a successful outcome was deemed to have utility 0.83 in baseline, regardless of how many interventions were required to achieve success.
The model demonstrated that none of the treatment regimes resulted in 100% success. In terms of initial treatments to alleviate symptoms and wait for symptom resolution, the most successful regime in the first treatment pathway was non-opioids, with a probability of success of 0.613, with treatment being unsuccessful in 39 of every 100 patients treated. When the second treatment pathway was considered, the most successful strategy was non-opioids, followed by biological agents, followed by epidural/nerve block and disc surgery, with a probability of success of 0.996, that is treatment was unsuccessful in three out of every 1000 patients treated.
A conventional approach to examining the cost-effectiveness of the treatment regimes was employed. Firstly, it was determined whether or not any of the regimes was dominated by others with both lower costs and greater probability of success and, secondly, whether or not any of the treatments were subject to extended dominance, with a more expensive treatment regime strategy having a lower ICE than the less expensive regime. This process generated the ‘efficiency frontier’ of increasingly more costly and more effective regimes for the management of patients with sciatica.
Table 171 highlights the mean cost, probability of success and 12-month utility gain for all possible treatment strategies.
| Treatments | Mean cost (£) | No. of successes | Utility gain |
|---|---|---|---|
| Inactive control | 0 | 383 | 176 |
| Usual care | 73,740 | 383 | 156 |
| Usual care and active PT | 304,324 | 606 | 279 |
| Usual care and passive PT | 304,324 | 613 | 282 |
| Usual care and traction | 304,324 | 622 | 286 |
| Usual care and manipulation | 304,324 | 836 | 385 |
| Usual care and alternative/non-traditional treatments | 304,324 | 902 | 415 |
| Usual care and biological agents | 1,161,741 | 939 | 432 |
| Usual care and active PT and epidural | 541,558 | 865 | 398 |
| Usual care and passive PT and epidural | 537,416 | 868 | 399 |
| Usual care and traction and epidural | 532,239 | 871 | 400 |
| Usual care and manipulation and epidural | 403,168 | 944 | 434 |
| Usual care and alternative/non-traditional treatments and epidural | 363,145 | 967 | 445 |
| Usual care and biological agents and epidural | 1,198,618 | 979 | 450 |
| Usual care and active PT and epidural and disc surgery | 738,621 | 951 | 437 |
| Usual care and passive PT and epidural and disc surgery | 731,039 | 951 | 438 |
| Usual care and traction and epidural and surgery | 721,562 | 952 | 438 |
| Usual care and manipulation and epidural and surgery | 485,275 | 979 | 451 |
| Usual care and alternative/non-traditional treatments and epidural and surgery | 412,005 | 988 | 454 |
| Usual care and biological agents and epidural and surgery | 1,229,251 | 992 | 456 |
| Usual care and disc surgery | 1,040,172 | 758 | 348 |
| Activity restriction | 70,000 | 441 | 203 |
| Activity restriction and active PT | 265,056 | 667 | 307 |
| Activity restriction and passive PT | 265,056 | 673 | 310 |
| Activity restriction and traction | 265,056 | 680 | 313 |
| Activity restriction and manipulation | 265,056 | 861 | 396 |
| Activity restriction and alternative/non-traditional treatments | 265,056 | 917 | 422 |
| Activity restriction and biological agents | 990,363 | 948 | 436 |
| Activity restriction and active PT and epidural | 465,737 | 886 | 408 |
| Activity restriction and passive PT and epidural | 462,233 | 888 | 408 |
| Activity restriction and traction and epidural | 457,854 | 891 | 410 |
| Activity restriction and manipulation and epidural | 348,670 | 953 | 438 |
| Activity restriction and alternative/non-traditional treatments and epidural | 314,814 | 972 | 447 |
| Activity restriction and biological agents and epidural | 1,021,558 | 982 | 452 |
| Activity restriction and active PT and epidural and disc surgery | 632,437 | 958 | 441 |
| Activity restriction and passive PT and epidural and disc surgery | 626,023 | 959 | 441 |
| Activity restriction and traction and epidural and surgery | 618,006 | 960 | 442 |
| Activity restriction and manipulation and epidural and surgery | 418,126 | 983 | 452 |
| Activity restriction and alternative/non-traditional treatments and epidural and surgery | 356,146 | 990 | 455 |
| Activity restriction and biological agents and epidural and surgery | 1,047,471 | 993 | 457 |
| Activity restriction and disc surgery | 887,525 | 795 | 366 |
| Opioids | 130,260 | 499 | 229 |
| Opioids and active PT | 305,284 | 701 | 323 |
| Opioids and passive PT | 305,284 | 706 | 325 |
| Opioids and traction | 305,284 | 713 | 328 |
| Opioids and manipulation | 305,284 | 876 | 403 |
| Opioids and alternative/non-traditional treatments | 305,284 | 926 | 426 |
| Opioids and biological agents | 956,100 | 954 | 439 |
| Opioids and active PT and epidural | 485,354 | 898 | 413 |
| Opioids and passive PT and epidural | 482,210 | 900 | 414 |
| Opioids and traction and epidural | 478,281 | 902 | 415 |
| Opioids and manipulation and epidural | 380,310 | 957 | 440 |
| Opioids and alternative/non-traditional treatments and epidural | 349,931 | 975 | 448 |
| Opioids and biological agents and epidural | 984,092 | 984 | 453 |
| Opioids and active PT and epidural and disc surgery | 634,934 | 962 | 443 |
| Opioids and passive PT and epidural and disc surgery | 629,179 | 963 | 443 |
| Opioids and traction and epidural and surgery | 621,985 | 964 | 443 |
| Opioids and manipulation and epidural and surgery | 442,633 | 984 | 453 |
| Opioids and alternative/non-traditional treatments and epidural and surgery | 387,018 | 991 | 456 |
| Opioids and biological agents and epidural and surgery | 1,007,343 | 994 | 457 |
| Opioids and disc surgery | 863,824 | 816 | 375 |
| Education and advice | 81,000 | 503 | 231 |
| Education and advice and active PT | 254,628 | 704 | 324 |
| Education and advice and passive PT | 254,628 | 709 | 326 |
| Education and advice and traction | 254,628 | 715 | 329 |
| Education and advice and manipulation | 254,628 | 877 | 403 |
| Education and advice and alternative/non-traditional treatments | 254,628 | 927 | 426 |
| Education and advice and biological agents | 900,253 | 954 | 439 |
| Education and advice and active PT and epidural | 433,262 | 899 | 413 |
| Education and advice and passive PT and epidural | 430,143 | 900 | 414 |
| Education and advice and traction and epidural | 426,245 | 903 | 415 |
| Education and advice and manipulation and epidural | 329,056 | 958 | 441 |
| Education and advice and alternative/non-traditional treatments and epidural | 298,919 | 975 | 448 |
| Education and advice and biological agents and epidural | 928,021 | 984 | 453 |
| Education and advice and active PT and epidural and disc surgery | 581,649 | 963 | 443 |
| Education and advice and passive PT and epidural and disc surgery | 575,939 | 963 | 443 |
| Education and advice and traction and epidural and surgery | 568,803 | 964 | 444 |
| Education and advice and manipulation and epidural and surgery | 390,882 | 984 | 453 |
| Education and advice and alternative/non-traditional treatments and epidural and surgery | 335,710 | 991 | 456 |
| Education and advice and biological agents and epidural and surgery | 951,088 | 994 | 457 |
| Education and advice and disc surgery | 808,713 | 817 | 376 |
| Non-opioids | 122,230 | 613 | 282 |
| Non-opioids and active PT | 257,328 | 769 | 354 |
| Non-opioids and passive PT | 257,328 | 773 | 356 |
| Non-opioids and traction | 257,328 | 778 | 358 |
| Non-opioids and manipulation | 257,328 | 904 | 416 |
| Non-opioids and alternative/non-traditional treatments | 257,328 | 943 | 434 |
| Non-opioids and biological agents | 759,683 | 964 | 444 |
| Non-opioids and active PT and epidural | 396,322 | 921 | 424 |
| Non-opioids and passive PT and epidural | 393,895 | 922 | 424 |
| Non-opioids and traction and epidural | 390,862 | 924 | 425 |
| Non-opioids and manipulation and epidural | 315,240 | 967 | 445 |
| Non-opioids and alternative/non-traditional treatments and epidural | 291,791 | 980 | 451 |
| Non-opioids and biological agents and epidural | 781,289 | 988 | 454 |
| Non-opioids and active PT and epidural and disc surgery | 594,629 | 915 | 421 |
| Non-opioids and passive PT and epidural and disc surgery | 588,740 | 917 | 422 |
| Non-opioids and traction and epidural and surgery | 581,379 | 919 | 423 |
| Non-opioids and manipulation and epidural and surgery | 397,865 | 965 | 444 |
| Non-opioids and alternative/non-traditional treatments and epidural and surgery | 340,960 | 979 | 450 |
| Non-opioids and biological agents and epidural and surgery | 812,116 | 987 | 454 |
| Non-opioids and disc surgery | 688,457 | 858 | 395 |
The majority of treatment strategies were excluded on the grounds of strict dominance (the next regime was both more effective and less costly) or extended dominance (a regime has an ICER that is higher than the next more effective regime). The regimes that represent the efficiency frontier are those based on non-opioids and are highlighted in Table 172.
| Treatment | Cost (£) | Probability of success | Utility gain | Incremental cost (£) | Incremental success | ICER | Incremental utility gain | ICER |
|---|---|---|---|---|---|---|---|---|
| Inactive control | 0 | 383 | 176 | |||||
| Non-opioids and alternative/non-traditional treatments | 257,328 | 943 | 434 | 257,328 | 560 | 459 | 258 | 999 |
| Non-opioids, alternative/non-traditional treatments and epidural | 291,791 | 980 | 451 | 34,463 | 38 | 916 | 17 | 1992 |
| Non-opioids, alternative/non-traditional treatments, epidural and disc surgery | 320,418 | 993 | 457 | 28,627 | 12 | 2311 | 6 | 5023 |
| Non-opioids, biological therapies, epidural and disc surgery | 799,237 | 995 | 458 | 478,819 | 3 | 178,700 | 1.23 | 388,478 |
In terms of net benefit, four of the five strategies would be regarded as cost-effective if the ceiling ratio for an additional unit of utility gain over a 12-month period was < £5100, and if the ceiling ratio for each additional success was < £2500.
Sensitivity analysis
The use of the highest cost estimates results in a similar overall picture and, although the cost per QALY estimates are higher, the stepped approaches based on non-opioids remain the most cost-effective strategies, as shown in Table 173.
| Treatment | Cost (£) | Utility gain | Success | Incremental cost (£) | Incremental success | ICER | Incremental utility | ICER |
|---|---|---|---|---|---|---|---|---|
| Inactive control | 0 | 176 | 383 | |||||
| Non-opioids | 129,330 | 282 | 613 | 129,330 | 230 | 562 | 106 | 1222 |
| Non-opioids and alternative/non-traditional treatments | 353,074 | 434 | 943 | 223,744 | 330 | 678 | 152 | 1474 |
| Non-opioids and alternative/non-traditional treatments and epidural | 409,693 | 451 | 980 | 56,619 | 38 | 1506 | 17 | 3273 |
| Non-opioids and alternative/non-traditional treatments and epidural and surgery | 483,959 | 457 | 993 | 74,266 | 12 | 5995 | 6 | 13,032 |
| Non-opioids and biological agents and epidural and surgery | 1,553,556 | 458 | 995 | 1,069,598 | 3 | 399,184 | 1 | 867,791 |
When the highest cost scenarios are employed, four of the five strategies are cost-effective if the ceiling ratio for an additional success is < £6000 and < £13,100 for an additional unit of utility gain.
In order for the third pathway – immediate referral for surgery – to feature on the efficiency frontier, the costs associated with the treatment regimen following initial treatment with non-opioids, would have to fall by 49% or the likelihood of success would have to increase by 10 percentage points to 0.95.
Adjusting utility values and probability of success had limited effect on baseline findings, and would need to be increased outside the bounds of probability to affect the basic premise that stepped approaches are more cost-effective than direct referral for surgery following initial treatments (as the differential in effectiveness for disc surgery is not sufficient to offset the differential in cost from conducting the procedure).
Discussion
The economic model has demonstrated that stepped approaches based on initial treatment with non-opioids represent the most cost-effective regimens for the treatment of sciatica. The treatment regimes that constituted the efficiency frontier were inactive control; non-opioids followed by alternative/non-traditional treatments; non-opioids followed by alternative/non-traditional treatments followed by epidural; non-opioids followed by alternative/non-traditional treatments followed by epidural followed by disc surgery; and non-opioids followed by biological therapies followed by epidural and followed by disc surgery (although this last regime would not be regarded as cost-effective when measured in terms of current cost-effectiveness thresholds). Further, the extent of potential net benefit from these treatment strategies would have relatively minor impact on NHS budgets and, when a broader societal perspective is employed, the extent of such net benefits is likely to be considerably more.
The extent to which changes in parameter estimates affect baseline findings is small, with improbable reductions in cost and improvements in success rates required to suggest that direct referral to disc surgery represents a cost-effective approach to managing patients with sciatica.
However, there are a number of limitations associated with the analysis. Firstly, the nature of the evidence has meant that the time perspective is limited to an assumed 12-month duration, with no evidence available to inform the inclusion of relapse and recurrence within the model. The perspective of the NHS does not enable issues relating to work and productivity and the preferences of patients for symptom resolution and treatment duration. Further work is needed to establish patient preferences relating to time taken to achieve success and the implications of failure after a series of treatments.
Secondly, the assumption regarding ultimate failure having a zero cost to the NHS is contentious, but again lack of data and consensus has meant that it has not been possible to provide a counterview. It is highly likely that patients will resort to alternative therapies, but outside the conventional health-care system.
Thirdly, it is acknowledged that the nature of the specified model is simplistic and fails to account fully for structural and parameter uncertainty and distributions. Further work is required to consider the implications of different modelling approaches in determining the relative cost-effectiveness of treatment regimens relating to managing patients with sciatica. However, the extent to which the findings from this study are likely to change would require a dramatic change in the evidence base surrounding the range of treatments available for use within patients. The choice of the global effect as the indicator of success can also be viewed as a limitation, although it again would probably not have changed the nature of the findings significantly.
Conclusion
The stepped approaches to managing sciatica based on an initial treatment with non-opioids, represent the most cost-effective regimens relative to direct referral to disc surgery, with positive net benefits emerging if the acceptable ceiling ratio for an additional unit of success was < £2500 with base-case costs and < £6000 if higher costs were applied to the model. The strategy of referring patients who fail initial treatments directly to disc surgery is unlikely to be cost-effective, with highly improbable reductions in cost and/or rates of success being required to elevate these regimens to the efficiency frontier. However, these findings remain tentative, and more research is required to develop the evidence base to inform more structurally appropriate economic models and to determine patient preferences regarding treatment durations and extent of invasive treatments that would be acceptable.
Chapter 10 Discussion
Summary of clinical effectiveness review
Description of studies
The number of studies evaluating each treatment category ranged from two (manipulation and education/advice) to 62 (disc surgery), with median sample sizes ranging from 55 (opioids) to 217 (education/advice). The proportion of studies that were RCTs also varied between treatment categories, with the lowest being for disc surgery (51%), anti-inflammatory biological agents (50%) and chemonucleolysis (47%).
In practice, the term sciatica is used by some clinicians for any leg pain referred from the back, whereas others prefer to restrict its use to pain originating from lumbar nerve root irritation, usually associated with disc herniation/prolapse. Most studies included patients with nerve root pain; although some included patients with referred pain, only one study of exercise therapy specifically included such patients. The presence of disc herniation was confirmed by imaging in a greater proportion of studies evaluating invasive treatments such as disc surgery (86%), epidural injections (62%) and chemonucleolysis (86%) than in studies evaluating less invasive interventions such as non-opioids (41%), traction (30%), alternative therapies (0%), exercise therapy (50%), activity restriction (20%) and education/advice (50%). The severity of herniation also varied slightly for disc surgery studies, with the proportion of studies that specifically included some patients with sequestered or extruded disc being higher (16%) than for other intervention categories. However, 17% of exercise therapy studies also included patients with sequestered or extruded discs, but the proportion of exercise therapy studies and other intervention categories will have been influenced by the small number of included studies (chemonucleolysis was 3% and all others 0%). The proportion of studies that limited inclusion to patients with acute sciatica (with the duration of symptoms being < 3 months) was much lower for invasive interventions such as surgery (6%), epidurals (7%) and chemonucleolysis (0%) than for less invasive interventions such as education (100%), activity restriction (80%), traction (50%) and exercise therapy (50%); surprisingly, this information was not reported for many studies. Five treatment categories included a small number of studies that restricted inclusion to patients experiencing their first episode (disc surgery 10%, epidural injections 3%, chemonucleolysis 8%, non-opioids 5% and biological agents 25%). The proportion of studies that included patients who had received previous treatment was higher for studies of invasive treatments such as disc surgery (65%), epidural injections (45%) and chemonucleolysis (83%) than for studies of less invasive interventions such as manipulation (0%), exercise therapy (0%) and traction (30%). However, the portion was also fairly high for opioids (67%) and activity restriction (40%) and low for biological agents (25%).
Summary of the findings comparing different interventions
An overall summary of the results for pair-wise analyses is presented in Table 174 and for the MTC analyses in Table 175. The following discussion is based upon whether or not there is a statistically significant difference between the intervention groups in the direct comparison of all study types and the MTC for randomised and Q-RCTs. For the MTC analyses, only one follow-up interval (closest to 6 months) was considered. The treatment categories are compared in a set order and, once a comparison has been made, it is not discussed again, e.g. disc surgery versus epidural injections medication is discussed only in the first paragraph and the comparison of epidural injections versus disc surgery is not repeated later. The term ‘significantly’ is used here in its statistical sense, not as a indication of effect size.
| Comparison | Short-term follow-up | Medium-term follow-up | Long-term follow-up | Adverse effects | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Global effect | Pain intensity | CSOMs | Global effect | Pain intensity | CSOMs | Global effect | Pain intensity | CSOMs | ||
| Disc surgery vs usual care | + | + | + | <> | + | <> | <> | – | ||
| Disc surgery vs epidural | + | <> | <> | |||||||
| Disc surgery vs non-opioids | <> | <> | <> | |||||||
| Disc surgery vs disc surgery and non-opioids | – | <> | ||||||||
| Disc surgery plus exercise therapy vs exercise therapy | <> | + | <> | <> | <> | <> | <> | <> | <> | <> |
| Disc surgery vs disc surgery and acupuncture | – | |||||||||
| Disc surgery vs intraoperative interventions | <> | <> | <> | <> | <> | <> | – | <> | <> | |
| Disc surgery vs chemonucleolysis | <> | <> | <> | <> | <> | <> | + (marginal) | <> | <> | <> |
| Disc surgery vs disc surgery and chemonucleolysis | <> | <> | <> | |||||||
| Epidural vs inactive control | <> | + | + | <> | <> | <> | <> | <> | <> | <> |
| Epidural vs usual care | + | <> | + | <> | <> | <> | – | |||
| Epidural vs non-opioids | + | + | <> | <> | <> | |||||
| Epidural vs epidural and non-opioids | <> | <> | <> | – | – | – | <> | |||
| Epidural vs chemonucleolysis | <> | <> | – | – | ||||||
| Epidural vs passive PT | + | <> | <> | + | <> | + | – | |||
| Epidural vs activity restriction | + | <> | ||||||||
| Epidural vs acupuncture | <> | <> | ||||||||
| Epidural vs biological agents | <> | <> | <> | |||||||
| Physiotherapy vs physiotherapy and epidural | – | |||||||||
| Chemonucleolysis vs inactive control | <> | <> | + | <> | <> | <> | ||||
| Chemonucleolysis vs manipulation | <> | <> | <> | <> | <> | |||||
| Non-opioids vs inactive control | + | + | <> | <> | + | <> | – | |||
| Non-opioids vs opioids | <> | + | <> | |||||||
| Non-opioids vs acupuncture | + | <> | ||||||||
| Non-opioids vs biological agents | – | – | ||||||||
| Traction vs inactive control | <> | <> | <> | <> | <> | <> | ||||
| Traction vs usual care | <> | <> | ||||||||
| Traction vs exercise therapy | <> | |||||||||
| Traction vs passive PT | <> | <> | <> | <> | <> | <> | ||||
| Exercise therapy vs exercise therapy and traction | <> | <> | <> | <> | <> | |||||
| Passive PT vs passive PT and traction | <> | |||||||||
| Activity restriction vs activity restriction and traction | <> | – | <> | + | ||||||
| Manipulation vs inactive control | <> | + | ||||||||
| Alternative interventions vs inactive control | + | |||||||||
| Exercise therapy vs activity restriction | <> | |||||||||
| Exercise therapy vs usual care | <> | – | <> | <> | <> | + | <> | <> | ||
| Exercise therapy vs inactive control | + | |||||||||
| Activity restriction vs manipulation and exercise therapy | <> | – | <> | <> | <> | |||||
| Passive PT vs inactive control | + | + | ||||||||
| Biological agents vs inactive control | + | + | <> | + | <> | <> | ||||
| Activity restriction vs education/advice | <> | <> | – | <> | <> | <> | <> | |||
| Opioids vs inactive control | <> | <> | <> | – | ||||||
| Opioids vs opioids and non-opioids | <> | <> | <> | |||||||
| Comparison (intervention vs control)a | Global effect all studies (OR) | Pain intensity all studies (WMD) | CSOMs all studies (SMD) | Global effect RCTs/Q-RCTs (OR) | Pain intensity RCTs/Q-RCTs (WMD) | CSOMs RCTs/Q-RCTs (SMD) |
|---|---|---|---|---|---|---|
| Disc surgery vs inactive control | 2.78 | –9.78 | 0.10 | 2.94 | –8.87 | 0.29 |
| Disc surgery vs usual care | 3.37 | –6.64 | –0.06 | 2.57 | –4.43 | –0.06 |
| Chemonucleolysis vs disc surgery | 0.72 | –1.44 | 0.27 | 0.81 | –3.37 | 0.34 |
| Non-opioids vs disc surgery | 0.92 | 5.71 | –0.00 | 0.88 | 3.05 | –0.20 |
| Intraoperative interventions vs disc surgery | 1.70 | –5.11 | –0.14 | 1.7 | –5.07 | –0.15 |
| Traction vs disc surgery | 0.44 | 8.52 | –0.47 | 0.46 | 7.57 | –0.58 |
| Manipulation vs disc surgery | 1.76 | –1.94 | 1.67 | –3.95 | ||
| Alternative/non-traditional vs disc surgery | 3.35 | –16.36 | 3.16 | –15.95 | ||
| Active PT vs disc surgery | 0.40 | 6.64 | 0.08 | 0.50 | 5.55 | 0.09 |
| Passive PT vs disc surgery | 0.41 | 9.34 | –0.58 | 0.41 | 8.71 | –0.62 |
| Biological agents vs disc surgery | 5.68 | –12.09 | –0.78 | 5.48 | –2.32 | –0.71 |
| Activity restriction vs disc surgery | 0.46 | 27.68 | –0.96 | 0.83 | 26.41 | –1.10 |
| Opioids vs disc surgery | 0.58 | 19.12 | 0.55 | 16.33 | ||
| Education/advice vs disc surgery | 0.59 | 26.84 | –0.78 | 1.07 | 25.51 | –0.96 |
| Intraoperative interventions vs inactive control | 4.73 | –14.88 | –0.04 | 4.99 | –13.94 | 0.13 |
| Intraoperative interventions vs usual care | 5.72 | –11.75 | –0.21 | 4.36 | –9.51 | –0.21 |
| Intraoperative interventions vs epidural | 1.52 | –2.01 | 0.14 | 1.59 | –1.27 | 0.10 |
| Intraoperative interventions vs chemonucleolysis | 2.36 | –3.66 | –0.42 | 2.10 | –1.65 | –0.49 |
| Intraoperative interventions vs non-opioids | 1.85 | –10.81 | –0.13 | 1.93 | –8.16 | 0.05 |
| Traction vs intraoperative interventions | 0.26 | 13.62 | –0.31 | 0.27 | 12.71 | –0.44 |
| Manipulation vs intraoperative interventions | 1.03 | 3.19 | 0.98 | 1.12 | ||
| Alternative/non-traditional vs intraoperative interventions | 1.98 | –11.27 | 1.85 | –10.90 | ||
| Active PT vs intraoperative interventions | 0.23 | 11.75 | 0.22 | 0.29 | 10.61 | 0.24 |
| Passive PT vs intraoperative interventions | 0.24 | 14.42 | –0.43 | 0.24 | 13.75 | –0.47 |
| Biological agents vs intraoperative interventions | 3.38 | –6.99 | –0.64 | 3.24 | 2.74 | –0.57 |
| Activity restriction vs intraoperative interventions | 0.27 | 32.82 | –0.81 | 0.49 | 31.41 | –0.95 |
| Opioids vs intraoperative interventions | 0.34 | 24.23 | 0.32 | 21.36 | ||
| Education/advice vs intraoperative interventions | 0.34 | 31.95 | –0.62 | 0.63 | 30.61 | –0.81 |
| Epidural vs inactive control | 3.10 | –12.85 | –0.16 | 3.14 | –12.66 | 0.03 |
| Epidural vs usual care | 3.75 | –9.71 | –0.34 | 2.74 | –8.19 | –0.32 |
| Epidural vs disc surgery | 1.11 | –3.10 | –0.28 | 1.07 | –3.78 | –0.26 |
| Chemonucleolysis vs epidural | 0.65 | 1.65 | 0.55 | 0.76 | –0.40 | 0.60 |
| Non-opioids vs epidural | 0.82 | 8.78 | 0.24 | 0.82 | 6.80 | 0.06 |
| Traction vs epidural | 0.39 | 11.68 | –0.21 | 0.43 | 11.36 | –0.33 |
| Manipulation vs epidural | 1.57 | 1.11 | 1.56 | –0.17 | ||
| Alternative/non-traditional vs epidural | 2.99 | –13.28 | 2.95 | –12.20 | ||
| Active PT vs epidural | 0.35 | 9.84 | 0.33 | 0.47 | 9.29 | 0.36 |
| Passive PT vs epidural | 0.37 | 12.48 | –0.31 | 0.38 | 1240 | –0.36 |
| Biological agents vs epidural | 5.10 | –8.93 | –0.51 | 5.11 | 1.41 | –0.48 |
| Activity restriction vs epidural | 0.41 | 30.90 | –0.70 | 0.77 | 30.08 | –0.84 |
| Opioids vs epidural | 0.52 | 22.21 | 0.52 | 20.08 | ||
| Education/advice vs epidural | 0.53 | 29.97 | –0.50 | 0.99 | 29.19 | –0.70 |
| Chemonucleolysis vs inactive control | 2.00 | –11.24 | 0.37 | 2.38 | –12.28 | 0.63 |
| Chemonucleolysis vs usual care | 2.42 | –8.02 | 0.21 | 2.07 | –7.86 | 0.28 |
| Non-opioids vs chemonucleolysis | 1.27 | 7.15 | –0.29 | 1.09 | 6.46 | –0.54 |
| Traction vs chemonucleolysis | 0.60 | 10.03 | –0.74 | 0.57 | 11.06 | –0.92 |
| Manipulation vs chemonucleolysis | 2.45 | –0.48 | 2.05 | –0.62 | ||
| Alternative/non-traditional vs chemonucleolysis | 4.64 | –14.89 | 3.89 | –12.57 | ||
| Active PT vs chemonucleolysis | 0.55 | 8.17 | –0.20 | 0.62 | 8.94 | –0.25 |
| Passive PT vs chemonucleolysis | 0.57 | 10.76 | –0.85 | 0.50 | 12.09 | –0.98 |
| Biological agents vs chemonucleolysis | 7.90 | –10.68 | –1.05 | 6.76 | 1.17 | –1.05 |
| Activity restriction vs chemonucleolysis | 0.64 | 29.21 | –1.24 | 1.03 | 29.69 | –1.45 |
| Opioids vs chemonucleolysis | 0.80 | 20.55 | 0.68 | 19.73 | ||
| Education/advice vs chemonucleolysis | 0.81 | 28.35 | –1.06 | 1.32 | 28.78 | –1.30 |
| Non-opioids vs inactive control | 2.55 | –4.07 | 0.08 | 2.59 | –5.84 | 0.09 |
| Non-opioids vs usual care | 3.09 | 0.92 | –0.08 | 2.26 | –1.36 | –0.26 |
| Traction vs non-opioids | 0.47 | –2.87 | –0.46 | 0.52 | 4.58 | –0.39 |
| Manipulation vs non-opioids | 1.91 | –7.56 | 1.89 | –6.98 | ||
| Alternative/non-traditional vs non-opioids | 3.65 | –22.05 | 3.59 | –18.97 | ||
| Active PT vs non-opioids | 0.43 | –0.96 | 0.08 | 0.57 | 2.45 | 0.29 |
| Passive PT vs non-opioids | 0.45 | 3.66 | –0.56 | 0.46 | 5.61 | –0.42 |
| Biological agents vs non-opioids | 6.19 | –17.79 | –0.76 | 6.20 | –5.35 | –0.53 |
| Activity restriction vs non-opioids | 0.50 | 22.05 | –0.93 | 0.93 | 23.29 | –0.89 |
| Opioids vs non-opioids | 0.63 | 13.41 | 0.62 | 13.27 | ||
| Education/advice vs non-opioids | 0.64 | 21.13 | –0.76 | 1.20 | 22.42 | –0.74 |
| Traction vs inactive control | 1.20 | –1.21 | –0.37 | 1.36 | –1.32 | –0.29 |
| Traction vs usual care | 1.46 | 1.90 | –0.53 | 1.19 | 3.29 | –0.65 |
| Manipulation vs traction | 4.06 | –10.48 | 3.62 | –11.54 | ||
| Alternative/non-traditional vs traction | 7.73 | –24.96 | 6.84 | –23.70 | ||
| Active PT vs traction | 0.90 | –1.85 | 0.54 | 1.07 | –2.14 | 0.69 |
| Passive PT vs traction | 0.94 | 0.75 | –0.10 | 0.87 | 1.03 | –0.03 |
| Biological agents vs traction | 13.2 | –20.58 | –0.31 | 11.77 | –9.85 | –0.15 |
| Activity restriction vs traction | 1.05 | 19.08 | –0.46 | 1.78 | 18.75 | –0.52 |
| Opioids vs traction | 1.33 | 10.51 | 1.18 | 8.77 | ||
| Education/advice vs traction | 1.35 | 18.20 | –0.30 | 2.30 | 17.96 | –0.37 |
| Manipulation vs inactive control | 4.88 | –11.72 | 4.90 | –12.79 | ||
| Manipulation vs usual care | 5.91 | –8.58 | 4.31 | –8.49 | ||
| Alternative/non-traditional vs manipulation | 1.91 | –14.41 | 1.92 | –11.97 | ||
| Active PT vs manipulation | 0.22 | 8.57 | 0.30 | 9.55 | ||
| Passive PT vs manipulation | 0.23 | 11.19 | 0.24 | 12.56 | ||
| Biological agents vs manipulation | 3.36 | –10.19 | 3.32 | 1.69 | ||
| Activity restriction vs manipulation | 0.26 | 29.50 | 0.50 | 30.31 | ||
| Opioids vs manipulation | 0.33 | 20.95 | 0.33 | 20.29 | ||
| Education/advice vs manipulation | 0.33 | 28.75 | 0.64 | 29.32 | ||
| Alternative/non-traditional vs inactive control | 9.32 | –26.08 | 9.25 | –24.89 | ||
| Alternative/non-traditional vs usual care | 11.27 | –23.00 | 8.15 | –20.33 | ||
| Active PT vs alternative/non-traditional | 0.12 | 23.14 | 0.16 | 21.63 | ||
| Passive PT vs alternative/non-traditional | 0.13 | 25.67 | 0.13 | 24.73 | ||
| Biological agents vs alternative/non-traditional | 1.75 | 4.24 | 1.76 | 13.73 | ||
| Activity restriction vs alternative/non-traditional | 0.14 | 44.08 | 0.26 | 42.63 | ||
| Opioids vs alternative/non-traditional | 0.17 | 35.48 | 0.17 | 32.34 | ||
| Education/advice vs alternative/non-traditional | 0.17 | 43.22 | 0.33 | 41.57 | ||
| Active PT vs inactive control | 1.10 | –3.04 | 0.17 | 1.46 | –3.39 | 0.39 |
| Active PT vs usual care | 1.33 | 0.08 | 0.02 | 1.28 | 1.01 | 0.03 |
| Passive PT vs active PT | 1.04 | 2.59 | –0.66 | 0.81 | 3.26 | –0.72 |
| Biological agents vs active PT | 14.6 | –18.76 | –0.83 | 11.04 | –7.82 | –0.83 |
| Activity restriction vs active PT | 1.16 | 21.10 | –1.02 | 1.65 | 20.99 | –1.21 |
| Opioids vs active PT | 1.46 | 12.51 | 1.10 | 10.79 | ||
| Education/advice vs active PT | 1.48 | 20.21 | –0.85 | 2.14 | 20.11 | –1.06 |
| Passive PT vs inactive control | 1.14 | –0.40 | –0.47 | 1.19 | –0.23 | –0.32 |
| Passive PT vs usual care | 1.38 | –2.72 | –0.64 | 1.04 | 4.29 | –0.69 |
| Biological agents vs passive PT | 14.0 | –21.31 | –0.20 | 13.54 | –10.83 | –0.12 |
| Activity restriction vs passive PT | 1.12 | 18.47 | –0.37 | 2.04 | 17.77 | –0.47 |
| Opioids vs passive PT | 1.41 | 9.82 | 1.35 | 7.69 | ||
| Education/advice vs passive PT | 1.43 | 17.60 | –0.19 | 2.62 | 16.82 | –0.32 |
| Biological agents vs inactive control | 15.77 | –21.80 | –0.68 | 16.04 | –11.18 | –0.44 |
| Biological agents vs usual care | 19.26 | –18.67 | –0.85 | 14.11 | –6.66 | –0.79 |
| Activity restriction vs biological agents | 0.08 | 39.74 | –0.18 | 0.15 | 28.68 | –0.36 |
| Opioids vs biological agents | 0.10 | 31.20 | 0.10 | 18.63 | ||
| Education/advice vs biological agents | 0.10 | 38.94 | –0.01 | 0.19 | 27.7 0 | –0.22 |
| Activity restriction vs inactive control | 1.28 | 18.00 | –0.84 | 2.43 | 17.44 | –0.80 |
| Activity restriction vs usual care | 1.54 | 21.18 | –1.03 | 2.14 | 21.96 | –1.18 |
| Opioids vs activity restriction | 1.26 | –8.58 | 0.67 | –10.05 | ||
| Education/advice vs activity restriction | 1.28 | –0.88 | 0.17 | 1.29 | –0.86 | 0.15 |
| Opioids vs inactive control | 1.60 | 9.34 | 1.62 | 7.41 | ||
| Opioids vs usual care | 1.95 | 12.60 | 1.41 | 11.92 | ||
| Education/advice vs opioids | 1.02 | 7.72 | 1.94 | 9.18 | ||
| Education/advice vs inactive control | 1.63 | 17.04 | –0.66 | 3.12 | 16.62 | –0.65 |
| Education/advice vs usual care | 1.98 | 20.22 | –0.83 | 2.73 | 21.04 | –1.02 |
Disc surgery was found to be significantly better than usual care for reducing pain at short- and medium-term follow-up and improving back-specific function at short-term follow-up (according to one good-quality RCT). It was also found to be significantly better than conventional care in terms of overall improvement at long-term follow-up, but this finding is based on the meta-analysis of four studies, only one of which was a good-quality RCT that found no statistical difference between the groups. Two further studies that could not be included in the meta-analysis also reported on this outcome; one was a good-quality RCT that also found no significant difference between the intervention groups. Overall, disc surgery was associated with significantly more adverse effects than usual care. One poor-quality RCT reported that disc surgery was significantly better than epidural injection for reducing pain at medium-term follow-up. Intraoperative interventions (mainly involving application of corticosteroids to the affected nerve root) were better than conventional disc surgery in reducing pain at long-term follow-up (three medium-quality RCTs and one poor-quality RCT), but there was no difference for other outcome measures at any follow-up interval. Disc surgery was marginally but significantly better than chemonucleolysis in effecting global improvement at long-term follow-up, based on a meta-analysis of 18 RCTs, but, again, there was no difference for other outcome measures. One moderate-quality RCT found disc surgery plus exercise therapy to be marginally but significantly better than disc surgery alone for improving pain at short-term follow-up. According to one poor-quality RCT, disc surgery used in combination with non-opioids was also found to be significantly better than disc surgery alone for reducing pain at short-term follow-up. In the MTC analyses of disc surgery, there was a significant improvement in global effect in favour of disc surgery when compared with inactive control or usual care. There was a significantly worse result for pain intensity following disc surgery compared with disc surgery combined with intraoperative interventions. In the MTC analyses of intraoperative intervention, there was a significant improvement in global effect compared with inactive control or usual care.
Epidural injection was found to be significantly better than inactive control for reducing pain (four good- and three medium-quality RCTs) and improving back-related function (four good- and one poor-quality RCT) at short-term follow-up, but was also associated with a greater number of adverse effects. Epidural injection was superior to usual care in terms of global effect and condition-specific function at short-term follow-up, but these findings were based on one non-RCT and one moderate-quality RCT, respectively. Epidural injection was associated with more adverse effects than usual care. Epidural injection was better than non-opioids for reducing pain (two medium- and one poor-quality RCT) and improving back-related function (one medium-quality RCT) at short-term follow-up. In one medium-quality RCT, the addition of non-opioids to epidural injection resulted in significantly better outcomes for condition-specific function at medium- and long-term follow-up and greater pain reduction at long-term follow-up than epidural injection alone. In one medium-quality RCT, epidural injection was superior to passive PT for overall improvement at medium- and long-term follow-up, but not for reducing pain at long-term follow-up. One non-RCT found epidural injection to be significantly better than activity restriction in terms of overall improvement. One non-RCT found chemonucleolysis to be better than epidural injection for global effect at long-term follow-up. Epidural used in combination with physiotherapy was better than physiotherapy alone for overall improvement at short-term follow-up, according to one non-RCT. There was no significant difference between epidural injection and acupuncture or biological agents. In the MTC analyses of epidural injections, there was a significant improvement in pain intensity when compared with inactive control or opioid medication. There was also a significant improvement in global effect when compared with inactive control or usual care.
Chemonucleolysis was superior to inactive control for overall improvement at medium-term follow-up (one medium-quality RCT, one poor-quality RCT and one Q-RCT), but not for any other outcomes at short- or medium-term intervals. There was no significant difference between chemonucleolysis and manipulation (one medium-quality RCT). In the MTC analyses of chemonucleolysis, there was a significant improvement in global effect compared with inactive control or usual care.
Non-opioid medication were better than inactive control for reducing pain at short-term follow-up (three medium-quality RCTs, one poor-quality RCT and one Q-RCT) and medium-term follow-up (one medium-quality RCT, one poor-quality RCT and one Q-RCT), but there were no difference between the interventions for other short- and medium-term outcome measures. Non-opioids, which included tricyclic antidepressants for treating neurogenic pain, were significantly superior to opioids for reducing pain at short-term follow-up, but there was no significant difference between the intervention groups for overall improvement; according to two poor-quality RCTs. Non-opioids were significantly better than acupuncture for reducing pain at short-term follow-up (one poor-quality RCT). Although a small, poor-quality HCS found biological agents to be better than non-opioids for reducing pain and improving condition-specific function at short-term follow-up, non-opioids resulted in significantly greater adverse effects than inactive control. In the MTC analyses of non-opioids, there was a significant improvement in the global effect when compared with the inactive control or usual care.
Traction was compared with the following treatment categories (mainly by one or two medium-quality RCTs) for which there were no significant findings: inactive control, usual care, exercise therapy, passive PT. According to two medium- and one poor-quality RCT, there was also no significant difference between traction used in combination with exercise therapy and exercise therapy used alone for most short-to-medium term outcomes. One medium-quality RCT found traction plus activity restriction to be significantly better than activity restriction alone for reducing pain, but there was no difference between the groups in terms of overall improvement and CSOMs at short-term follow-up. Activity restriction plus traction was associated with more adverse effects than traction alone. The MTC analyses found no significant findings.
Spinal manipulation was superior to inactive control for overall improvement at medium-term follow-up, but not short-term follow-up, according to one good-quality RCT. The MTC analysis of spinal manipulation, found no significant findings.
One moderate-quality RCT found alternative therapy (acupuncture) to be better than inactive control for the reduction of pain intensity at short-term follow-up. No other outcomes were evaluated. In the MTC analysis of alternative therapy, there was a significant improvement in pain intensity compared with inactive control, usual care, activity restriction, opioids, medication, or education/advice.
According to one medium-term crossover RCT, active PT/exercise therapy was better than inactive control for reducing pain at short-term follow-up. Exercise therapy was marginally significantly worse than usual care for condition-specific function at short-term follow-up, but significantly better in terms of overall improvement at long-term follow-up, according to one-good quality RCT. There was no significant difference for other outcomes. Exercise therapy was compared with activity restriction for the global effect at short-term follow-up by one poor-quality RCT, for which there was no significant difference between the interventions. According to a moderate-quality RCT, physiotherapy including exercise and passive PT was significantly better than activity restriction for improving function at short-term follow-up. The MTC analysis of active PT/exercise therapy found no significant findings.
Passive PT was significantly better than inactive control in terms of overall improvement and pain reduction at short-term follow-up, according to one poor-quality crossover RCT. One non-RCT found passive PT in combination with epidural to be significantly better than passive PT alone in terms of overall improvement at short-term follow-up. In the MTC analysis of passive PT, there a significantly worse result in pain intensity compared with biological agents.
According to one non-RCT, anti-inflammatory biological agents were significantly better than inactive control for reducing pain at short-term follow-up and improving condition-specific function at short- and medium-term follow-up. However, there was no significant difference in terms of pain intensity at medium-term follow-up (one medium-quality RCT and one non-RCT) or condition-specific function at long-term follow-up (one medium-quality RCT). In the MTC analysis of biological agents, there was a significant improvement in pain intensity compared with inactive control, activity restriction, or opioids.
Activity restriction was less effective than advice to stay active in terms of CSOMs at short-term follow-up, but there was no difference between the intervention groups for other outcome measures at short- and medium-term follow-up, according to two moderate-quality RCTs. In the MTC analysis of activity restriction trials, there was a significantly worse result in pain intensity from activity restriction compared with usual care.
There was no significant difference between opioids medication and inactive control (one medium-quality RCT) or a combination of opioids and non-opioids (one medium-quality crossover RCT) in terms of global pain or CSOMs at medium-term follow-up. However, opioids were associated with more adverse effects than inactive control. In the MTC analysis of opioids, there was a significantly worse result in terms of pain intensity from opioids compared with inactive control or usual care.
Summary of cost-effectiveness review
The full economic evaluations identified in the systematic review were of reasonable to good quality, but were not able to fully address our research question. Although individual studies raised a number of important issues, it was difficult to draw meaningful conclusions across these studies because of their heterogeneity. Although there was some indication of favourable benefit, such as with disc surgery, robust findings could not be reliably drawn. While an evidence base is emerging, there remains a lack of well-designed economic evaluations. Of particular note are the lack of published decision models and the relevance of these studies to the UK NHS setting.
Summary of economic evaluation
Description of economic evaluation
A decision-analytic model from the perspective of the UK NHS was constructed on the assumption that patients presenting with sciatica would be managed through one of three pathways, with alternative treatments within each of the pathways. The first pathway would involve management within primary care and revolve around what might be termed usual care, with the use of analgesics and other medications if considered appropriate, to attempt to secure symptom resolution. The second pathway would involve a stepped approach and include the use of intermediate treatments – offered in addition to the initial treatments provided within primary care – and provided in secondary care outpatients by multidisciplinary teams including physiotherapists, musculoskeletal physicians, etc. (the principle is one of ramping up the level of intervention if there is no timely symptom resolution following simpler, less invasive interventions). The third pathway would involve immediate referral for surgery to alleviate symptoms.
Each of the pathways and the treatment variations available were compared with ‘inactive control’, which, according to the findings from the MTC, has a non-zero probability of symptom resolution, but has been assumed to cost £0 in the baseline model.
A series of 100 independent scenarios were considered, with the utilities associated with success used to generate a utility score for each treatment regime and combined with costs to determine relative incremental cost/QALY ratios. Similarly, costs were combined with likelihood of success to generate ICERs.
A number of sensitivity analyses were conducted on the baseline findings.
Results of economic evaluation
The initial treatment of non-opioids followed by biological agents and epidural then disc surgery for those who have failed is the most effective strategy and has an incremental cost per QALY of £4500 compared with the option of not providing surgery. The strategy of referring patients who fail initial treatments directly to disc surgery is dominated by the stepped treatment pathway, with referral for surgery being the most expensive strategy and generally less effective than the stepped approaches. The stepped approaches remain the more cost-effective options even when the use of biological agents or alternative therapies is not included, as the differential in effectiveness for disc surgery is not sufficient to offset the differential in cost from conducting the procedure. For referral directly to disc surgery to be the cost-effective strategy the success rate for disc surgery would need to be 40% higher or the costs of surgery 30% lower.
All of the treatment strategies are within the cost per QALY threshold considered to represent value for money of £20,000–30,000 relative to inactive control. However, a number would be excluded on the grounds of being dominated by a more effective and less costly strategy. The issue of which strategy is the most cost-effective is therefore far from conclusive, and more research is required to determine patient preferences regarding treatment durations and extent of invasive treatments that would be acceptable.
Comparison with previous systematic reviews
Previous systematic reviews of sciatica have examined individual treatments or have considered non-surgical or surgical management strategies separately. Where multiple interventions have been included, they have been analysed either using a narrative synthesis or with pair-wise meta-analyses using direct comparisons of individual treatments. Indirect comparisons have not been attempted and this is the first review to use a MTC method. Previous reviews of non-surgical treatments have found either no evidence of effectiveness16,17 or conflicting evidence,294,295 or have reached different conclusions concerning the effectiveness of ESIs. 17,23,24,294 The Cochrane systematic review of surgical management has also made direct comparisons using pair-wise meta-analyses, particularly in comparison with chemonucleolysis,26 but because of study heterogeneity was unable to combine the results of four RCTs comparing discectomy with non-surgical treatment and concluded that the results suggested only a temporary benefit of disc surgery at 1-year follow-up. This review, however, justified the effectiveness of discectomy by using an indirect comparison of chemonucleolysis with placebo and chemonucleolysis with disc surgery. Chemonucleolysis was more effective than placebo and discectomy more effective than chemonucleolysis; therefore, disc surgery was superior to placebo. In our review, the same RCTs comparing chymopapain with placebo and chymopapain with surgery, were identified. Five additional RCTs, one non-RCT, 13 CCSs and one HCS comparing chymopapain with disc surgery were identified. In the MTC analysis it was possible to make a more robust comparison of disc surgery compared with placebo. The OR in terms of global effect was 2.8 (95% credible interval 1.4 to 5.6) in favour of disc surgery. The WMD in pain intensity was –9.8 (95% credible interval –26.5 to 6.8) in favour of disc surgery. The SMD in CSOMs was 0.1 (95% credible interval –1.4 to 1.5) in favour of disc surgery. Thus, disc surgery was significantly better than placebo in terms of the global effect but not pain intensity and CSOMs.
Assumptions, limitations and uncertainties
One of the strengths of this review is the extensive literature searches that were undertaken to identify published, unpublished and grey literature. Where possible, non-English language reports were translated; however, we were unable to translate a number of studies published in Chinese, which may have affected the overall findings relating to alternative therapy, particularly acupuncture. Forty-two ongoing (or not yet reported) studies were identified, the findings of which may influence our conclusions: 26 compared different treatment categories including surgery versus usual care (n = 1), surgery versus mixed treatments (n = 1), epidural versus inactive control (n = 7), epidural versus usual care (n = 1), epidural versus other (n = 1), opioids versus inactive control (n = 2), alternative versus mixed treatments (n = 1), active PT versus mixed treatments (n = 1), biological agents versus inactive control (n = 4) and others versus inactive control (n = 1).
Our review represents an attempt to answer the question ‘Which treatment should I use for sciatica?’ In order for the findings of the review to be relevant to the full spectrum of patients who suffer from sciatica, we tried to be as inclusive as possible. Observational studies and non-RCTs were included, as they are likely to have better external validity than RCTs296,297 and thus provide more generalisable findings. For example, participants keen to have surgery may have been less likely to accept randomisation to either surgery or usual care. Furthermore, some interventions may not have been evaluated by RCTs. The inclusion of observational studies and non-RCTs means that these interventions would not be excluded owing to lack of RCTs or, alternatively, lead to an increase in the precision of the overall findings for interventions evaluated by only a limited number of RCTs.
However, the RCT is widely regarded as the design of choice when assessing the effectiveness of health-care interventions298 and we acknowledge the controversy over the inclusion of non-randomised evidence. The observed effect of an intervention may not necessarily be due to the therapeutic intervention itself, it could be due to confounding factors such as the natural course of sciatica (including variability of the disease status or the influence of different prognostic factors), extraneous factors (such as lifestyle, the use of other medication and placebo effect) and information errors (such as incorrect assessment or reporting of the outcome measure). A well-conducted RCT would provide an unbiased estimate of effect by ensuring the comparator groups are the same for these factors and only differ in terms of the intervention given. Observational studies, on the other hand, are likely to be affected by selection bias and confounding and may therefore yield estimates of association that deviate from the true underlying relationship beyond the play of chance. 299 However, not all RCTs are well conducted, and they are generally smaller than observational studies. It is therefore unclear whether or not a poorly conducted RCT provides a better estimate of the treatment effect than a large, well-conducted observational study. When summarising the findings of the pair-wise analyses in our review, priority was given to RCTs, and the quality of the studies noted.
Poor reporting and variation in the way data were analysed in the included studies meant that imputation or substitution of missing data was necessary in order for the meta-analyses to be as inclusive as possible (increasing precision of the findings). Omitting studies with missing SDs may induce bias in the summary effect estimate,300 and Furukawa et al. 33 have shown that it is safe to borrow SDs from other studies. Where SDs were missing and could not be estimated from the published data, we imputed them using a weighted mean SD. 33,300 This is based on the assumption that the variance is similar between studies and that the data are not skewed. 28 Ideally, the impact of this assumption would be assessed using sensitivity analysis. However, this was not possible in the time frame available and will be done at a later date. This will include comparing the pooled mean differences of studies that have reported SDs against the pooled estimate of the same studies based on imputed SDs to see if they converge. 33 Further sensitivity analyses are also needed to assess the impact of substituting mean values with medians.
Our review explored the use of MTC synthesis methodology274 to simultaneously compare all treatment modalities for sciatica, by providing estimates for all possible pair-wise comparisons, based on both the direct and indirect evidence. One of the main assumptions underpinning these methods is that included studies represent a coherent body of data whose relative treatment effects are effectively identical or at least exchangeable throughout. 301 Comparing two treatments indirectly, but in very different populations, is likely to produce misleading results if the treatments interact with population characteristics. 302 Our review included a diverse set of studies with a number of potential sources of heterogeneity, including the diagnostic criteria used, type and extent of herniation, severity of sciatica, duration of symptoms, previous treatment, mode of administration and dosages of treatments, study design, study quality, outcome measures and duration of follow-up. These characteristics especially varied between invasive and non-invasive treatments. The MTC methods can be used to show the degree of inconsistency in the evidence base. 301 Although we have used informal methods for comparing estimated effects from the (direct pair-wise) meta-analyses and the MTC analysis, more formal methods to assess coherence and consistency of the evidenced network using deviance information criteria302 and related statistics are yet to be made.
Sciatica is a condition where, in clinical practice, a sequential stepped-care approach using different treatment modalities is considered useful, usually starting with non-invasive treatments and progressing to more invasive treatments if symptoms persist. However, primary studies tended to examine individual treatments in isolation and the clinical effectiveness of treatment strategies in our review were also compared on an equal basis, irrespective of their position in the care pathway. Owing to the novel and speculative use of MTC methods and the breadth of our review, covering such a broad condition with a large number of possible treatments, we did not incorporate a stepped-care approach in the MTC analyses. The optimum sequence of treatment modalities and what sequence is best for which patients are therefore not known. However, we plan to undertake further analyses to develop these methods, in order to derive comparative estimates of the effectiveness of the different interventions, conditional on the administration of previous interventions. Multiple treatments may also be administered sequentially in the hope of producing additive effects using combined therapy; therefore, the additive and interaction effects of multiple interventions also need to be explored.
When a stepped-care approach is used, the characteristics of the patient will vary in different parts of the clinical pathway. This means that the prognosis or baseline risk of the study population is likely to differ (inconsistently) for different interventions. For example, disc surgery is usually offered to patients who have failed conservative treatment, which means that patients receiving surgery will differ in terms of the type, severity and duration of symptoms compared with those receiving conservative treatment. This trend was reflected in the included studies, with the method and criteria used for diagnosing sciatica (and therefore the patient population) differing according to the invasiveness of the treatment, which was likely to have affected the findings of the MTC analysis. This inconsistency is also present when making informal comparisons between treatment categories in the pair-wise meta-analyses. We plan to further explore this effect as part of the proposed analysis of sequential treatments.
Different countries appear to have a different preference for various treatment modalities, as well as the use of co-interventions. When simultaneously comparing treatment modalities for sciatica, it is important to note that the use of inactive control, usual care and co-interventions is likely to vary across treatment categories and between studies. There is also likely to be a placebo effect occurring with inactive control, which appears to vary according to the type of intervention being used, e.g. sham traction or placebo acupuncture. This is likely to account for why inactive control was shown to be more effective than usual care for global effect (but not for pain intensity) in the MTC analyses, although these findings were not statistically significant.
Implications for further research
The MTC analyses (for all studies and RCTs/Q-RCTs) showed alternative therapy and biological agents to be promising interventions for reducing pain intensity. However, only one non-RCT270 and one moderate-quality RCT271 compared biological agents with inactive control, and one moderate-quality RCT261 compared acupuncture with inactive control; two studies261,270 reported statistically significant findings in favour of the intervention. One small HCS found biological agents to be more effective than non-opioids and one poor-quality RCT found non-opioids to be more effective than acupuncture. Further research is needed on the use of alternative therapy and biological agents compared with interventions that are currently being used in practice, such as non-opioids and epidural injections. Four ongoing RCTs have been identified comparing the biological agent anti-TNF-α with placebo.
Interestingly, the MTC analyses showed opioids to be significantly less effective than inactive control for reducing pain intensity. In the pair-wise analysis, two small, poor-quality RCTs229,230 found non-opioids to be significantly more effective than opioids at reducing pain at short-term follow-up, and one medium-quality crossover RCT214 found no statistically significant difference between opioids and inactive control for global pain and CSOMs at medium-term follow-up. Further research is needed to provide more evidence for the use of opioids and drugs used to treat neurogenic nerve pain, such as tricyclic antidepressants and gabapentin, for the treatment of sciatica. Two ongoing RCTs have been identified, one comparing opioids and the tricyclic antidepressant nortriptyline with placebo and the other comparing anticonvulsant pregabalin (Lyrica®, Pfizer) with placebo (see Appendix 4).
There were more studies evaluating invasive interventions, such as surgery, epidural and chemonucleolysis than there were studies evaluating non-invasive interventions, such as education/advice, alterative therapies, manipulation and opioids. More research is needed for non-invasive treatments such as manipulation and exercise therapy. Further research is also needed to compare invasive treatments such as epidural and surgery, which was only evaluated by one poor-quality RCT.
Further research is needed to evaluate exactly which intervention within each treatment category is most effective and whether or not this differs for any subgroup of patients. We have identified a number of studies that compared treatments within the same treatment category (e.g. microdicectomy vs open discectomy), the findings of which are not presented here, but would help answer these questions.
Further research is needed to determine patient preferences regarding treatment durations and extent of invasive treatments that would be acceptable.
Further work to consider implications of ultimate treatment failure and loss of utility is also needed.
Mixed treatment comparison methods include indirect comparisons which are made without breaking within-study comparison and, hence, fully respect the randomised structure of the evidence. 303 Further research is needed to explore the potential effect of including observational and non-RCTs in MTC analyses. More sophisticated methods, such as the confidence profile method297 or using Bayesian statistics,296 could also be explored as a means of incorporating information relating to the differences in study design or internal and external validity in the meta-analyses.
Chapter 11 Conclusions
The review findings provide support for the effectiveness of currently used invasive treatments for treating sciatica, such as disc surgery and epidural corticosteroid injections; however, these were also associated with more adverse effects than usual care. They also provide support for the effectiveness of non-opioid medication for reducing pain in sciatica. Chemonucleolysis was also effective for reducing pain, but is no longer used in the UK NHS. With the exception of non-opioids, there were only a few studies evaluating each of the non-invasive treatment categories. The findings of these studies do not provide support for the effectiveness of opioid analgesia, which is widely used in this patient group. The mixed treatment analyses and limited pair-wise analyses suggest that less frequently used treatments such as acupuncture and experimental treatments such as anti-inflammatory biological agents may be effective. There was also a limited evidence base showing that spinal manipulation and exercise therapy may be effective. The findings do not support the use of activity restriction or traction.
The MTC method enabled both the simultaneous comparison of all treatment categories and the comparison of treatments that had not been directly compared in RCTs or observational studies. However, encouraging results for the interventions (e.g. biological agents) from a small number of poor-quality studies need to be treated with caution. Sciatica is generally treated using a stepped-care approach, starting with non-invasive treatments, such as non-opioid medication, and progressing, if necessary, to more invasive treatments, such as epidural injections or surgery. This means that the population of patients treated with non-invasive treatments in the MTC analyses is likely to differ from that treated with invasive treatments, which may have affected the MTC findings. However, the findings of the pair-wise and MTC analyses were broadly similar.
In terms of cost-effectiveness, the argument for stepped approaches based on an initial treatment with non-opioids, relative to direct referral for surgery, was apparent and, although there are a number of limitations associated with the economic model, this finding was shown to be relatively robust.
Further RCTs with concurrent economic evaluation are needed to evaluate the use of biological agents and acupuncture compared with interventions that are currently being used in practice, such as non-opioids and epidural injections. Four RCTs comparing biological agents with placebo that are in progress, have been identified from searches of trial registries (see Appendix 4). Further research is also needed comparing the use of opioids with drugs used to treat neurogenic nerve pain or other treatments currently used in practice. One RCT of oral morphine compared with nortriptyline or placebo was identified from the trial registries (see Appendix 4). Further work is also needed to develop alternative economic modelling approaches to assess the relative cost-effectiveness of treatment regimes in these proposed trials.
Acknowledgements
The review team would like to thank the following people for their help during the review process: Ralph Hendry, Nicola Hulley, Daniel Hodgson, Eleanor Pasterfield, Thomas France and Jacob Neal for editing and developing summary tables for the final report; Annie Williams for helping with the analyses of adverse effects; Ian Braithwaite (Consultant Surgeon) for providing clinical input at various stages of the review; and, John Belcher for statistical advice and proposal development.
Contribution of authors
Ruth Lewis (Lecturer) was co-principal investigator and lead reviewer responsible for writing the protocol and clinical effectiveness section of the review, involved in the study selection, data extraction and validity assessment, conducted the conventional pair-wise and MTC analyses and jointly co-ordinated the final report.
Nefyn Williams (Clinical Senior Lecturer and GP) was co-principal investigator with overall responsibility for the project, was involved in the study selection, data extraction and validity assessment and contributed to the analyses as well as the protocol and report writing.
Hosam Matar (Research Associate) was involved in the study selection, data extraction and validity assessment.
Nafees Din (Research Associate) carried out the literature searches and was involved in the study selection, data extraction and validity assessment.
Deb Fitzsimmons (Senior Lecturer) was responsible for conducting and writing the review of economic evaluations, conducting the service provider survey, was involved in the economic model and contributed to the protocol writing.
Ceri Phillips (Professor of Health Economics) was responsible for the development of the economic model and writing the cost-effectiveness section and contributed to the protocol writing.
Mari Jones (Postdoctoral Research Fellow) was involved in the development of the economic model.
Alex Sutton (Professor of Medical Statistics) was involved in and oversaw all aspects of the clinical effectiveness analyses, provided input at all stages and contributed to the protocol and report writing.
Kim Burton (Director of the Spinal Research Unit, University of Huddersfield) provided clinical input at various stages of the review, contributed to the analyses of adverse effects and the discussion and commented on the draft report.
Sadia Nafees (Research Assistant) was involved in the study selection and contributed to the report writing.
Maggie Hendry (Research Fellow) provided input at various stages of the review and commented on the draft report.
Ian Rickard (Patient Representative) provided input at various stages of the review and commented on the draft report.
Rob Chakraverty (Sports Physician) provided clinical input at various stages of the review and commented on the draft report.
Clare Wilkinson (Professor of General Practice and GP) provided input at various stages of the review and commented on the draft report.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- Deyo RA, Rainville J, Kent DL. What can the history and physical examination tell us about low back pain?. JAMA 1992;268:760-5.
- Clinical Standards Advisory Group . Back Pain 1994.
- Waddell G. The back pain revolution. Edinburgh: Churchill Livingstone; 1998.
- Lawrence J. Rheumatism in populations. London: Heinemann; 1977.
- Heliovaara M, Impivaara O, Sievers K, Melkas T, Knekt P, Korpi J. Lumbar disc syndromein Finland. J Epidemiol Community Health 1987;41:251-8.
- Weber H, Holme I, Amlie E. The natural course of acute sciatica with nerve root symptoms in a double-blind placebo-controlled trial evaluating the effect of piroxicam. Spine 1993;18:1433-8.
- Bush K, Cowan N, Katz DE, Gishen P. The natural history of sciatica associated with disc pathology. A prospective study with clinical and independent radiologic follow-up. Spine 1992;17:1205-12.
- Tubach F, Beaute J, Leclerc A. Natural history and prognostic indicators of sciatica. J Clin Epidemiol 2004;57:174-9.
- Furlan AD, Pennick V, Bombardier C, Van Tulder MW. 2009 updated methods guidelines for systematic reviews in the Cochrane Back Review Group. Spine 2009;34:1929-41.
- van Tulder MW, Koes BW, Bouter LM. A cost-of-illness study of back pain in the Netherlands. Pain 1995;62:233-40.
- Mixer WJ, Barr JS. Rupture of the intervertebral disc with involvement of the spinal canal. N Engl J Med 1934;211:210-15.
- Revdivik B, Brown M, Lundborg G. Pathoanatomy and pathophysiology of nerve root compression. Spine 1984;9:7-15.
- Goupille P, Jayson MI, Valat JP, Freemont AJ. The role of inflammation in disc herniation-associated radiculopathy. Semin Arthritis Rheum 1998;28:60-71.
- Vroomen PC, de Krom MC, Wilmink JT, Kester AD, Knottnerus JA. Lack of effectiveness of bed rest for sciatica. N Engl J Med 1999;340:418-23.
- Rebain R, Baxter DG, McDonough S. A systematic review of the passive straight leg raising test as a diagnostic aid for low back pain. Spine 2002;27:E388-E395.
- Hagen KB, Hilde G, Jamtvedt G, Winnem M. Best rest for acute low back pain and sciatica. Cochrane Database Syst Rev 2000;2.
- Vroomen PC, de Krom MC, Slofstra PD, Knottnerus JA. Conservative treatment of sciatica: a systematic review. J Spinal Disord 2000;13:463-9.
- Li LC, Bombardier C. Physical therapy management of low back pain: an exploratory survey of therapist approaches. Phys Ther 2001;81:1018-28.
- Harte AA, Gracey JH, Baxter GD. Current use of lumbar traction in the management of low back pain: results of a survey of physiotherapists in the United Kingdom. Arch Phys Med Rehabil 2005;86:1164-9.
- Clarke JA, van Tulder MW, Blomberg SEI, de Vet HCW, van der Heijden GJMG, Bronfort G. Traction for low back pain with or without sciatica. Cochrane Database Syst Rev 2005;4.
- NHS The information centre for health and social care . Hospital Episode Statistics (admitted Patient care), England 2006–7 2007. www.ic.nhs.uk/statistics-and-data-collections/hospital-care/hospital-activity-hospital-episode-statistics--hes/hospital-episode-statistics-admitted-patient-care-england-2006-07 (accessed 29 April 2007).
- Luijsterburg PA, Verhagen AP, Ostelo RW, van Os TA, Peul WC, Koes BW. Effectiveness of conservative treatments for the lumbosacral radicular syndrome: a systematic review. Eur Spine J 2007;7:881-99.
- Boswell MV, Hansen HC, Trescot AM, Hirsch JA. Epidural steroids in the management of chronic spinal pain and radiculopathy. Pain Physician 2003;6:319-34.
- Abdi S, Datta S, Trescot AM, Schultz DM, Adlaka R, Atluri SL, et al. Epidural steroids in the management of chronic spinal pain: a systematic review. Pain Physician 2007;10:185-212.
- Stevens CD, Dubois RW, Larequi-Lauber T, Vader JP. Efficacy of lumbar discectomy and percutaneous treatments for lumbar disc herniation. Soz Praventivmed 1997;42:367-79.
- Gibson JNA, Waddell G. Surgical interventions for lumbar disc prolapse. Cochrane Database Syst Rev 2007;2.
- Undertaking systematic reviews of research on effectiveness: CRD’s guidance for those carrying out or commissioning reviews. York: CRD; 2001.
- Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. Chichester: John Wiley & Sons; 2009.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2005.
- van Tulder MW, Furlan A, Bombardier C, Bouter LM. Editorial board of the Cochrane Collaboration Back Review Group. Updated method guidelines for systematic reviews in the Cochrane Collaboration Back Review Group. Spine 2003;28:1290-9.
- Ministry of Health and Long-Term Care, City of Hamilton Public Services . Effective Public Health Practice Project n.d. www.myhamilton.ca/myhamilton/CityandGovernment/HealthandSocialServices/Research/EPHPP (accessed 12 May 2008).
- Weinstein J, O’Brien B, Hornberger J, Jackson J, Johannesson MCM. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR task force on good research practices-modeling studies. Value Health 2003;6:9-17.
- Higgins JPT, Deeks JJ, Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. Chichester: John Wiley & Sons; 2008.
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. J Clin Epidemiol 2006;59:7-10.
- Kopec JA, Esdaile JM. Functional disability scales for back pain. Spine 1995;20:1943-9.
- Deyo RA, Battie M, Beurskens AJHM, Bombardier C, Croft P, Koes B, et al. Outcome measures for low back pain research: a proposal for standardized use. Spine 1998;23:2003-13.
- Davidson M, Keating JL. A comparison of five low back disability questionnaires: reliability and responsiveness. Phys Ther 2002;82:8-24.
- Garratt AM. Rasch analysis of generic and specific measuresof health outcome in low back pain. Spine 2003;26:71-7.
- Fairbank JCT. Use and abuse of the oswesrty disability index. Spine 2007;32:2787-9.
- Higgins JPT, Deeks JJ, Altman DG, Higgins JPT, Deeks JJ, Altman DG. Cochrane handbook for systematic reviews of interventions. Chichester: John Wiley & Sons Ltd; 2008.
- Roland M, Morris R. A study of the natural history of back pain. Part I: development of a reliable and sensitive measure of disability in low-back pain. Spine 1983;8:141-4.
- Fairbank JC, Couper J, Davies JB, O’Brien JP. The oswestry low back pain disability questionnaire. Physiotherapy 1980;66:271-3.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Clin Trials 1986;7:177-88.
- Multi-parameter Evidence Synthesis Research Group . Mixed Treatment Comparisons 2009. www.bris.ac.uk/cobm/research/mpes/mtc.html (accessed 15 March 2010).
- Thomas KC, Fisher CG, Boyd M, Bishop P, Wing P, Dvorak MF. Outcome evaluation of surgical and nonsurgical management of lumbar disc protrusion causing radiculopathy. Spine 2007;32:1414-22.
- Krugluger J, Knahr K. Chemonucleolysis and automated percutaneous discectomy – a prospective randomized comparison. Int Orthop 2000;24:167-9.
- van Alphen HA, Braakman R, Bezemer PD, Broere G, Berfelo MW. Chemonucleolysis versus discectomy: a randomized multicenter trial. J Neurosurg 1989;70:869-75.
- Javid MJ. Chemonucleolysis versus laminectomy – a cohort comparison of effectiveness and charges. Spine 1995;20:2016-22.
- Postacchini F, Lami R, Massobrio M. Chemonucleolysis versus surgery in lumbar disc herniations: correlation of the results to preoperative clinical pattern and size of the herniation. Spine 1987;12:87-96.
- Norton WL. Chemonucleolysis versus surgical discectomy. Comparison of costs and results in workers’ compensation claimants. Spine 1986;11:440-3.
- Dabezies EJ, Brunet M. Chemonucleolysis vs laminectomy. Orthopedics 1978;1:26-9.
- Stula D. Chemonucleolysis with chymopapain in intervertebral-disk hernia – a randomized comparative-study of patients following surgery. Neurochirurgie 1990;33:169-72.
- Tregonning GD, Transfeldt EE, McCulloch JA, Macnab I, Nachemson A. Chymopapain versus conventional surgery for lumbar disc herniation. 10-year results of treatment. J Bone Joint Surg Br 1991;73:481-6.
- Lagarrigue J, Lazorthes Y, Verdie JC, Richaud J. Comparative results of surgery and nucleolysis in 1085 cases of herniated lumbar disc. Neurochirurgie 1991;37:96-105.
- Lavignolle B, Vital JM, Baulny D, Grenier F, Castagnera L. Comparative study of surgery and chemonucleolysis in the treatment of sciatica caused by a herniated disk. Acta Orthop Belg 1987;53:244-9.
- Hoogmartens M, Van Loon L, Demedts D, Desmet L. Comparative study of the results obtained with discectomy and chemonucleolysis. Acta Orthop Belg 1976;42:134-8.
- Rossi D, Munari L, Ubbiali A, Palumbo D, Fornari M, Lucarelli G, et al. Comparison between percutaneous discectomy according to Onik, microdiscectomy and conservative treatment. Neuroradiology 1993;6:445-52.
- Zeiger HE. Comparison of chemonucleolysis and microsurgical discectomy for the treatment of herniated lumbar disc. Spine 1987;12:796-9.
- Watts C, Hutchison G, Stern J, Clark K. Comparison of intervertebral disc disease treatment by chymopapain injection and open surgery. J Neurosurg 1975;42:397-400.
- Crawshaw C, Frazer AM, Merriam WF, Mulholland RC, Webb JK. A comparison of surgery and chemonucleolysis in the treatment of sciatica. A prospective randomized trial. Spine 1984;9:195-8.
- Bouillet R. Complications of discal hernia therapy. Comparative study regarding surgical therapy and nucleolysis by chymopapain. Acta Orthop Belg 1983;49:48-77.
- Shvartzman L, Weingarten E, Sherry H, Levin S, Persaud A. Cost-effectiveness analysis of extended conservative therapy versus surgical intervention in the management of herniated lumbar intervertebral disc. Spine 1992;17:176-82.
- Wang RR, Tronnier V. Effect of acupuncture on pain management in patients before and after lumbar disc protrusion surgery – a randomized control study. Am J Chin Med 2000;28:25-33.
- Aminmansour B, Khalili HA, Ahmadi J, Nourian M. Effect of high-dose intravenous dexamethasone on postlumbar discectomy pain. Spine 2006;31:2415-7.
- MacKay MA, Fischgrund JS, Herkowitz HN, Kurz LT, Hecht B, Schwartz M. The effect of interposition membrane on the outcome of lumbar laminectomy and diskectomy. Spine 1995;20:1793-6.
- Lundin A, Magnuson A, Axelsson K, Kogler H, Samuelsson L. The effect of perioperative corticosteroids on the outcome of microscopic lumbar disc surgery. Eur Spine J 2003;12:625-30.
- Koranda I, Sefrna F. Effectiveness of conservative and surgical treatment of lumboischiadic syndrome. Cas Lek Cesk 1995;134:474-7.
- Osterman H, Seitsalo S, Karppinen J, Malmivaara A. Effectiveness of microdiscectomy for lumbar disc herniation: a randomized controlled trial with 2 years of follow-up. Spine 2006;31:2409-14.
- Cengiz SL, Baysefer A. Efficacy of Adcon-L gel or Healon-GV in epidural fibrosis after lumbar microdiscectomy. Neurosciences 2007;12:109-13.
- Lavyne MH, Bilsky MH. Epidural steroids, postoperative morbidity, and recovery in patients undergoing microsurgical lumbar discectomy. J Neurosurg 1992;77:90-5.
- Prestar FJ, Jollenbeck B. Experience with intra- and perioperative high-dosage steroids in microneursurgical revision operations on lumbar discs. Schmerz 1995;9:78-83.
- Atlas SJ, Deyo RA, Keller RB, Chapin AM, Patrick DL, Long JM, et al. The Maine Lumbar Spine Study. 1-year outcomes of surgical and nonsurgical management of sciatica. Spine 1996;21:1777-86.
- Kim KD, Wang JC, Robertson DP, Brodke DS, Olson EM, Duberg AC, et al. Reduction of radiculopathy and pain with Oxiplex/SP gel after laminectomy, laminotomy, and discectomy: a pilot clinical study. Spine 2003;28:1080-7.
- Bernsmann K, Kramer J, Ziozios I, Wehmeier J, Wiese M. Lumbar micro disc surgery with and without autologous fat graft – a prospective randomized trial evaluated with reference to clinical and social factors. Arch Orthop Trauma Surg 2001;121:476-80.
- Bonafe A, Tremoulet M, Sabatier J, Boetto S, Docco A, Richardi G, et al. Foraminal and lateroforaminal herniated disks – midterm results of percutaneous treatment with chemonucleolysis or nucleotomy. Neurochirurgie 1993;39:110-15.
- Brown MD, Tompkins JS. Pain response post-chemonucleolysis or disc excision. Spine 1989;14:321-6.
- Buric J. Ozone chemyonucleolysis vs microdiscectomy. Prospective controlled study with 18 months follow-up. Rivista Italiana Di Ossigeno-Ozonoterapia 2005;4:49-54.
- Debi R, Halperin N, Mirovsky Y. Local application of steroids following lumbar discectomy. J Spinal Disord Tech 2002;15:273-6.
- Dei-Anang K, Weigand H, Mader U. Is the percutaneous discectomy an alternative to chemonucleolysis. Radiologe 1990;30:70-4.
- Dubourg G, Rozenberg S, Fautrel B, Valls-Bellec I, Bissery A, Lang T, et al. A pilot study on the recovery from paresis after lumbar disc herniation. Spine 2002;27:1426-31.
- Gerszten PC, Moossy JJ, Flickinger JC, Welch WC. Low-dose radiotherapy for the inhibition of peridural fibrosis after reexploratory nerve root decompression for postlaminectomy syndrome. J Neurosurg 2003;99:271-7.
- Glasser RS, Knego RS, Delashaw JB, Fessler RG. The perioperative use of corticosteroids and bupivacaine in the management of lumbar disc disease. J Neurosurg 1993;78:383-7.
- Jensen TT, Asmussen K, Berg-Hansen EM, Lauritsen B, Manniche C, Vinterberg H, et al. First-time operation for lumbar disc herniation with or without free fat transplantation. Prospective triple-blind randomized study with reference to clinical factors and enhanced computed tomographic scan 1 year after operation. Spine 1996;21:1072-6.
- Langmayr JJ, Obwegeser AA, Schwarz AB, Laimer I, Ulmer H, Ortler M. Intrathecals to reduce pain after lumbar disc surgery – a double-blind, placebo-controlled prospective-study. Pain 1995;62:357-61.
- Muralikuttan KP, Hamilton A, Kernohan WG, Mollan RA, Adair IV. A prospective randomized trial of chemonucleolysis and conventional disc surgery in single level lumbar disc herniation. Spine 1992;17:381-7.
- North RB, Kidd DH, Farrokhi F, Piantadosi SA. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trial. Neurosurgery 2005;56:98-106.
- Peul WC, Van Houwelingen HC, van den Hout WB, Brand R, Eekhof JAH, Tans JTJ, et al. Surgery versus prolonged conservative treatment for sciatica. N Engl J Med 2007;356:2245-56.
- Revel M, Payan C, Vallee C, Laredo JD, Lassale B, Roux C, et al. Automated percutaneous lumbar discectomy versus chemonucleolysis in the treatment of sciatica – a randomized multicenter trial. Spine 1993;18:1-7.
- Richter HP, Kast E, Tomczak R, Besenfelder W, Gaus W. Results of applying ADCON-L gel after lumbar discectomy: the German ADCON-L study. J Neurosurg 2001;95:179-89.
- Steffen R. Laser discotomy/chemonucleolysis ini the therapy of displaced lumbal intervertebral disc – prospective randomised comparison. Hefte Zur Der Unfallchirurg 1999;271:215-21.
- Weber H. Lumbar disc herniation. A controlled, prospective study with ten years of observation. Spine 1983;8:131-40.
- Weinstein J, Spratt KF, Lehmann T, McNeill T, Hejna W. Lumbar disc herniation. A comparison of the results of chemonucleolysis and open discectomy after ten years. J Bone Joint Surg Am 1986;68:43-54.
- Starkweather A, Witek-Janusek L, Nockels R. The impact of psychological and immune factors in sciatic pain: a randomized controlled trial among herniated disc patients. Nurs Health SCI 2006;23.
- Alaranta H, Hurme M, Einola S, Falck B, Kallio V, Knuts LR, et al. A prospective study of patients with sciatica. A comparison between conservatively treated patients and patients who have undergone operation, part II: results after one year follow-up. Spine 1990;15:1345-9.
- Buttermann GR. Treatment of lumbar disc herniation: epidural steroid injection compared with discectomy: s prospective, randomized study. J Bone Joint Surg Am 2004;86:670-9.
- Ejeskar A, Nachemson A, Herberts P, Lysell E, Andersson G, Irstam L, et al. Surgery versus chemonucleolysis for herniated lumbar discs. A prospective study with random assignment. Clin Orthop Relat Res 1983;174:236-42.
- Hoogland T, Schubert M, Miklitz B, Ramirez A. Transforaminal posterolateral endoscopic discectomy with or without the combination of a low-dose chymopapain: a prospective randomized study in 280 consecutive cases. Spine 2006;31:E890-7.
- Weinstein JN, Lurie JD, Tosteson TD, Skinner JS, Hanscom B, Tosteson ANA, et al. Surgical vs nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT) observational cohort. JAMA 2006;296:2451-9.
- Weinstein JN, Tosteson TD, Lurie JD, Tosteson ANA, Hanscom B, Skinner JS, et al. Surgical vs nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT): a randomized trial. JAMA 2006;296:2441-50.
- Hansson E, Hansson T. The cost-utility of lumbar disc herniation surgery. Eur Spine J 2007;16:329-37.
- Rasmussen S, Krum-Moller DS, Lauridsen LR, Jensen SEH, Mandoe H, Gerlif C, et al. Epidural steroid following discectomy for herniated lumbar disc reduces neurological impairment and enhances recovery: a randomized study with two-year follow-up. Spine 2008;33:2028-33.
- Ronnberg K, Lind B, Zoega B, Gadeholt-Gothlin G, Halldin K, Gellerstedt M, et al. Peridural scar and its relation to clinical outcome: a randomised study on surgically treated lumbar disc herniation patients. Eur Spine J 2008;17:1714-20.
- Alexander AH, Burkus JK, Mitchell JB, Ayers WV. Chymopapain chemonucleolysis versus surgical discectomy in a military population. Clin Orthop Relat Res 1989:158-65.
- Lee SH, Lee SJ, Park KH, Lee IM, Sung KH, Kim JS, et al. Comparison of percutaneous manual and endoscopic laser diskectomy with chemonucleolysis and automated nucleotomy. Orthopade 1996;25:49-55.
- Watters WC, Mirkovic S, Boss J. Treatment of the isolated lumbar intervertebral disc herniation: microdiscectomy versus chemonucleolysis. Spine 1988;13:360-2.
- Jirarattanaphochai K, Jung S, Thienthong S, Krisanaprakornkit W, Sumananont C. Peridural methylprednisolone and wound infiltration with bupivacaine for postoperative pain control after posterior lumbar spine surgery – a randomized double-blinded placebo-controlled trial. Spine 2007;32:609-16.
- de Tribolet N, Porchet F, Lutz TW, Gratzl O, Brotchi J, van Alphen HA, et al. Clinical assessment of a novel antiadhesion barrier gel: prospective, randomized, multicenter, clinical trial of ADCON-L to inhibit postoperative peridural fibrosis and related symptoms after lumbar discectomy. Am J Orthop 1998;27:111-20.
- Barlocher CB, Krauss JK, Seiler RW. Central lumbar disc herniation. Acta Neurochir 2000;142:1369-74.
- Takeshima T, Kambara K, Miyata S, Ueda Y, Tamai S. Clinical and radiographic evaluation of disc excision for lumbar disc herniation with and without posterolateral fusion. Spine 2000;25:450-6.
- Aydn Y, Ziyal IM, Duman H, Turkmen CS, Basak M, Ahin Y. Clinical and radiological results of lumbar microdiskectomy technique with preserving of ligamentum flavum comparing to the standard microdiskectomy technique. Surg Neurol 2002;57:5-13.
- Lee SH, Chung SE, Ahn Y, Kim TH, Park JY, Shin SW. Comparative radiologic evaluation of percutaneous endoscopic lumbar discectomy and open microdiscectomy: a matched cohort analysis. Mt Sinai J Med 2006;73:795-801.
- Lagarrigue J, Chaynes P. Comparative study of lumbar discectomy with or without microscope. A prospective analyse of 80 cases. Neurochirurgie 1994;40:116-20.
- Kho HC, Steudel WI. Comparison between lumbar microdiscectomy and standard discectomy in lumbar-disk herniations – a retrospective analysis of 267 cases. Neurochirurgie 1986;29:181-5.
- Righesso O, Falavigna A, Avanzi O. Comparison of open discectomy with microendoscopic discectomy in lumbar disc herniations: results of a randomized controlled trial. Neurosurgery 2007;61:545-9.
- Tassi GP. Comparison of results of 500 microdiscectomies and 500 percutaneous laser disc decompression procedures for lumbar disc herniation. Photomed Laser Surg 2006;24:694-7.
- Katayama Y, Matsuyama Y, Yoshihara H, Sakai Y, Nakamura H, Nakashima S, et al. Comparison of surgical outcomes between macro discectomy and micro discectomy for lumbar disc herniation: a prospective randomized study with surgery performed by the same spine surgeon. J Spinal Disord Tech 2006;19:344-7.
- Gonzalez-Castro A, Shetty A, Nagendar K, Greenough CG. Day-case conventional discectomy: a randomised controlled trial. Eur Spine J 2002;11:67-70.
- Tullberg T, Isacson J, Weidenhielm L. Does microscopic removal of lumbar disc herniation lead to better results than the standard procedure? Results of a one-year randomized study. Spine 1993;18:24-7.
- Shin DA, Kim KN, Shin HC, Yoon DH. The efficacy of microendoscopic discectomy in reducing iatrogenic muscle injury. J Neurosurg 2008;8:39-43.
- Carragee EJ, Spinnickie AO, Alamin TF, Paragioudakis S. A prospective controlled study of limited versus subtotal posterior discectomy: short-term outcomes in patients with herniated lumbar intervertebral discs and large posterior anular defect. Spine 2006;31:653-7.
- Ruetten S, Komp M, Merk H, Godolias G. Full-endoscopic interlaminar and transforaminal lumbar discectomy versus conventional microsurgical technique – a prospective, randomized, controlled study. Spine 2008;33:931-9.
- Ryang Y-M, Oertel MF, Mayfrank L, Gilsbach JM, Rohde V. Standard open microdiscectomy versus minimal access trocar microdiscectomy: results of a prospective randomized study. Neurosurgery 2008;62:174-81.
- Wu X, Zhuang S, Mao Z, Chen H. Microendoscopic discectomy for lumbar disc herniation: surgical technique and outcome in 873 consecutive cases. Spine 2006;31:2689-94.
- Balderston RA, Gilyard GG, Jones AA, Wiesel SW, Spengler DM, Bigos SJ, et al. The treatment of lumbar disc herniation: simple fragment excision versus disc space curettage. J Spinal Disord 1991;4:22-5.
- Barrios C, Ahmed M, Arrotegui J, Bjornsson A, Gillstrom P. Microsurgery versus standard removal of the herniated lumbar disc. A 3-year comparison in 150 cases. Acta Orthop 1990;61:399-403.
- Chao Z, Yue Z, Tong-wei C, Jian W, Yong H, Yong P. Microendoscopic discectomy, a less traumatic procedure for lumbar disk herniation. Chin J Traumatol 2007;10:311-4.
- Chatterjee S, Foy PM, Findlay GF. Report of a controlled clinical trial comparing automated percutaneous lumbar discectomy and microdiscectomy in the treatment of contained lumbar disc herniation. Spine 1995;20:734-8.
- Hagen R, Engesaeter LB. Unilateral and bilateral partial laminectomy in lumbar disc prolapse. A follow-up study of 156 patients. Acta Orthop 1977;48:41-6.
- Kahanovitz N, Viola K, Muculloch J. Limited surgical discectomy and microdiscectomy. A clinical comparison. Spine 1989;14:79-81.
- Kim M-J, Lee S-H, Jung E-S, Son B-G, Choi E-S, Shin J-H, et al. Targeted percutaneous transforaminal endoscopic diskectomy in 295 patients: comparison with results of microscopic diskectomy. Surg Neurol 2007;68:623-31.
- Mayer HM, Brock M. Percutaneous endoscopic discectomy – surgical technique and preliminary-results compared to microsurgical discectomy. J Neurosurg 1993;78:216-25.
- Schizas C, Tsiridis E, Saksena J. Microendoscopic discectomy compared with standard microsurgical discectomy for treatment of uncontained or large contained disc herniations. Neurosurgery 2005;57:357-60.
- Striffeler H, Groger U, Reulen HJ. “Standard” microsurgical lumbar discectomy vs. “conservative” microsurgical discectomy. A preliminary study. Acta Neurochir 1991;112:62-4.
- Thome C, Barth M, Scharf J, Schmiedek P. Outcome after lumbar sequestrectomy compared with microdiscectomy: a prospective randomized study. J Neurosurg Spine 2005;2:271-8.
- Toyone T, Tanaka T, Kato D, Kaneyama R. Low-back pain following surgery for lumbar disc herniation. A prospective study. J Bone Joint Surg 2004;86:893-6.
- Tria AJ, Williams JM, Harwood D, Zawadsky JP. Laminectomy with and without spinal fusion. Clin Orthop Relat Res 1987:134-7.
- Tureyen K. One-level one-sided lumbar disc surgery with and without microscopic assistance: 1-year outcome in 114 consecutive patients. J Neurosurg 2003;99:247-50.
- White AH, von Rogov P, Zucherman J, Heiden D. Lumbar laminectomy for herniated disc: a prospective controlled comparison with internal fixation fusion. Spine 1987;12:305-7.
- Wilson DH, Harbaugh R. Microsurgical and standard removal of the protruded lumbar disc: a comparative study. Neurosurgery 1981;8:422-7.
- Yang J, Du F, Zhao DQ, Zheng YB, Li JG, Shao YG. Two different intervention measures in recovery of lumbar function of patients who underwent lumbar discectomy. Chin J Clin Rehabil 2005;9:268-9.
- Mastronardi L, Pappagallo M, Puzzilli F, Tatta C. Efficacy of the morphine-Adcon-L compound in the management of postoperative pain after lumbar microdiscectomy. Neurosurgery 2002;50:518-24.
- Saberski LR. A retrospective analysis of spinal canal endoscopy and laminectomy outcomes data. Pain Physician 2000;3:193-6.
- Dincer U, Kiralp MZ, Cakar E, Yasar E, Dursan H. Caudal epidural injection versus non-steroidal anti-inflammatory drugs in the treatment of low back pain accompanied with radicular pain. Joint Bone Spine 2007;74:467-71.
- Graham CE. Chemonucleolysis: a double blind study comparing chemonucleolysis with intra discal hydrocortisone: in the treatment of backache and sciatica. Clin Orthop Relat Res 1976:179-92.
- Coomes EN. Comparison between epidural anaesthesia and bed rest in sciatica. Br Med J 1961;1:20-4.
- Yates DW. A comparison of the types of epidural injection commonly used in the treatment of low back pain and sciatica. Rheumatol Rehabil 1978;17:181-6.
- Bush K, Hillier S. A controlled study of caudal epidural injections of triamcinolone plus procaine for the management of intractable sciatica. Spine 1991;16:572-5.
- Snoek W, Weber H, Jorgensen B. Double-blind evaluation of extradural methyl prednisolone for herniated lumbar discs. Acta Orthop 1977;48:635-41.
- Becker C, Heidersdorf S, Drewlo S, de Rodriguez SZ, Kramer J, Willburger RE. Efficacy of epidural perineural injections with autologous conditioned serum for lumbar radicular compression: an investigator-initiated, prospective, double-blind, reference-controlled study. Spine 2007;32:1803-8.
- Pirbudak L, Karakurum G, Oner U, Gulec A, Karadasli H. Epidural corticosteroid injection and amitriptyline for the treatment of chronic low back pain associated with radiculopathy. Pain Clinic 2003;15:247-53.
- Buchner M, Zeifang F, Brocai DR, Schiltenwolf M. Epidural corticosteroid injection in the conservative management of sciatica. Clin Orthop Relat Res 2000;375:149-56.
- Carette S, Leclaire R, Marcoux S, Morin F, Blaise GA, St Pierre A, et al. Epidural corticosteroid injections for sciatica due to herniated nucleus pulposus. N Engl J Med 1997;336:1634-40.
- Valat JP, Giraudeau B, Rozenberg S, Goupille P, Bourgeois P, Micheau-Beaugendre V, et al. Epidural corticosteroid injections for sciatica: a randomised, double blind, controlled clinical trial. Ann Rheum Dis 2003;62:639-43.
- Popiolek A, Domanik A, Mazurkiewicz G. Epidural injections of steroids in the treatment of patients with chronic sciatica in discopathy. Neurol Neurochir Pol 1991;25:640-6.
- Veihelmann A, Devens C, Trouillier H, Birkenmaier C, Gerdesmeyer L, Refior HJ. Epidural neuroplasty versus physiotherapy to relieve pain in patients with sciatica: a prospective randomized blinded clinical trial. J Orthop Sci 2006;11:365-9.
- Wilson-MacDonald J, Burt G, Griffin D, Glynn C. Epidural steroid injection for nerve root compression – a randomized, controlled trial. J Bone Joint Surg Bm 2005;87:352-5.
- Dilke TF, Burry HC, Grahame R. Extradural corticosteroid injection in management of lumbar nerve root compression. Br Med J 1973;2:635-7.
- Vad VB, Bhat AL, Lutz GE, Cammisa F. Transforaminal epidural steroid injections in lumbosacral radiculopathy: a prospective randomized study. Spine 2002;27:11-6.
- Blonna D, Calvi V, Collo G, Marmotti A, Castoldi F. Gabapentin in the conservative treatment of radiculopathy. Minerva Ortopedica E Traumatologica 2004;55:15-22.
- Bourgeois P, Benoist M, Palazzo E, Belmatoug N, Folinais D, Frot B, et al. Multicenter, randomized and double-blind study of triamcinolone hexacetonide versus chymopapain in the treatment of lumbar sciatica. Preliminary results at six months. Rev Rhum Mal Osteo Articulaires 1988:767-9.
- Bronfort G, Evans RL, Anderson AV, Schellhas KP, Garvey TA, Marks RA, et al. Nonoperative treatments for sciatica: a pilot study for a randomized clinical trial. J Manipulative Physiol Ther 2000;23:536-44.
- Helliwell M, Robertson JC, Ellis RM. Outpatient treatment of low pack pain and sciatica by a single extradural corticosteroid injection. Br J Clin Pract 1985;39:228-31.
- Klenerman L, Greenwood R, Davenport HT, White DC, Peskett S. Lumbar epidural injections in the treatment of sciatica. Rheumatology 1984;23:35-8.
- Matyjek J, Lubinski I. Treatment of sciatica with injection of hydrocortisone into the sacral hiatus. Neurol Neurochir Pol 1986;20:218-21.
- Ridley MG, Kingsley GH, Gibson T, Grahame R. Outpatient lumbar epidural corticosteroid injection in the management of sciatica. Rheumatology 1988;27:295-9.
- Styczynski T, Parnarauskiene R, Zarski S, Kaubrys G, Klezys V, Krzeminska-Dabrowska I, et al. The assessment of therapeutic effectiveness of paravertebral blockades in patients with painful radicular syndromes in discopathy and in lumbar spine spondylosis. Neurol Neurochir Pol 1997;31:939-49.
- Wehling P, Reinecke J. Acupuncture together with cytokine depressing herbs in comparison to injection therapy with steroids in sciatic pain. Schmerz 1997;11:180-4.
- Bontoux D, Alcalay M, Debiais F, Garrouste O, Ingrand P, Azais I, et al. Treatment of lumbar-disk herniation with intradiscal injection of chymopapain or triamcinolone hexacetonid – a comparative-study of 80 cases. Revue Du Rhumatisme 1990;57:327-31.
- Bronfort G, Evans RL, Maiers M, Anderson AV. Spinal manipulation, epidural injections, and self-care for sciatica: a pilot study for a randomized clinical trial. J Manipulative Physiol Ther 2004;27:503-8.
- Gallucci M, Limbucci N, Zugaro L, Barile A, Stavroulis E, Ricci A, et al. Sciatica: treatment with intradiscal and intraforaminal injections of steroid and oxygen-ozone versus steroid only. Radiology 2007;242:907-13.
- Karppinen J, Malmivaara A, Kurunlahti M, Kyllonen E, Pienimaki T, Nieminen P, et al. Periradicular infiltration for sciatica – a randomized controlled trial. Spine 2001;26:1059-67.
- Lafuma A, Bouvenot G, Cohen C, Eschwege E, Fagnani F, Vignon E. A pragmatic cost-effectiveness study of routine epidural corticosteroid injections for lumbosciatic syndrome requiring inhospital management. Revue Du Rhumatisme 1997;64:549-55.
- Price C, Arden N, Coglan L, Rogers P. Cost-effectiveness and safety of epidural steroids in the management of sciatica. Health Technol Assess 2005;9.
- Laiq N, Khan MN, Iqbal MJ, Khan S. Comparison of epidural steroid injections with conservative management in patients with lumbar radiculopathy. J Coll Physicians Surg Pak 2009;19:539-43.
- Murata Y, Kato Y, Miyamoto K, Takahashi K. Clinical study of low back pain and radicular pain pathways by using l2 spinal nerve root infiltration: a randomized, controlled, clinical trial. Spine 2009;34:2008-13.
- Mathews JA, Mills SB, Jenkins VM, Grimes SM, Morkel MJ, Mathews W, et al. Back pain and sciatica: controlled trials of manipulation, traction, sclerosant and epidural injections. Br J Rheumatol 1987;26:416-23.
- Beliveau P. A comparison between epidural anaesthesia with and without corticosteroid in the treatment of sciatica. Rheumatol Phys Med 1971;11:40-3.
- Dashfield AK, Taylor MB, Cleaver JS, Farrow D. Comparison of caudal steroid epidural with targeted steroid placement during spinal endoscopy for chronic sciatica: a prospective, randomized, double-blind trial. Br J Anaesth 2005;94:514-9.
- Owlia MB, Salimzadeh A, Alishiri G, Haghighi A. Comparison of two doses of corticosteroid in epidural steroid injection for lumbar radicular pain. Singapore Med J 2007;48:241-5.
- Riew KD, Yin Y, Gilula L, Bridwell KH, Lenke LG, Lauryssen C, et al. The effect of nerve-root injections on the need for operative treatment of lumbar radicular pain. A prospective, randomized, controlled, double-blind study. J Bone Joint Surg Am 2000;82:1589-93.
- Jeong HS, Lee JW, Kim SH, Myung JS, Kim JH, Kang HS. Effectiveness of transforaminal epidural steroid injection by using a preganglionic approach: a prospective randomized controlled study. Radiology 2007;245:584-90.
- Ng L, Chaudhary N, Sell P. The efficacy of corticosteroids in periradicular infiltration for chronic radicular pain: a randomized, double-blind, controlled trial. Spine 2005;30:857-62.
- Ackerman WE, Ahmad M. The efficacy of lumbar epidural steroid injections in patients with lumbar disc herniations. Anesth Analg 2007;104:1217-22.
- Kolsi I, Delecrin J, Berthelot JM, Thomas L, Prost A, Maugars Y. Efficacy of nerve root versus interspinous injections of glucocorticoids in the treatment of disk-related sciatica. A pilot, prospective, randomized, double-blind study. Joint Bone Spine 2000;67:113-18.
- Thomas E, Cyteval C, Abiad L, Picot MC, Taourel P, Blotman F. Efficacy of transforaminal versus interspinous corticosteroid injectionin discal radiculalgia – a prospective, randomised, double-blind study. Clin Rheumatol 2003;22:299-304.
- Rogers P, Nash T, Schiller D, Norman J. Epidural steroids for sciatica. Pain Clinic 1992;5:67-72.
- Candido KD, Raghavendra MS, Chinthagada M, Badiee S, Trepashko DW. A prospective evaluation of iodinated contrast flow patterns with fluoroscopically guided lumbar epidural steroid injections: the lateral parasagittal interlaminar epidural approach versus the transforaminal epidural approach. Anesth Analg 2008;106:638-44.
- Cuckler JM, Bernini PA, Wiesel SW, Booth RE, Rothman RH, Pickens GT. The use of epidural steroids in the treatment of lumbar radicular pain. A prospective, randomized, double-blind study. J Bone Joint Surg 1985;67:63-6.
- Blankenbaker DG, De Smet AA, Stanczak JD, Fine JP. Lumbar radiculopathy: treatment with selective lumbar nerve blocks – comparison of effectiveness of triamcinolone and betamethasone injectable suspensions. Radiology 2005;237:738-41.
- Breivik H, Hesla PE, Molnar I, Lind B. Treatment of chronic low back pain and sciatica: comparison of caudal epidural injections of bupivacaine and methylprednisolone with bupivacaine followed by saline. Adv Pain Res Ther 1976;1:927-32.
- Faraj AA, Mulholland RC. The value of nerve root infiltration for leg pain when used with a nerve stimulator. Eur Spine J 2006;15:1495-9.
- Gronemeyer D, Seibel R, Schindler O, Schattauer K, Lange S, Schmidt A. Microinvasive, CT-controlled periradicular therapy in treatment of chronic intervertebral disk-induced functional disorders. Wien Med Wochenschr 1995;145:129-39.
- Lee JW, Kim SH, Choi J-Y, Yeom J-S, Kim K-J, Chung S-K, et al. Transforaminal epidural steroid injection for lumbosacral radiculopathy: preganglionic versus conventional approach. Korean J Radiol 2006;7:139-44.
- Schaufele MK, Hatch L, Jones W. Interlaminar versus transforaminal epidural injections for the treatment of symptomatic lumbar intervertebral disc herniations 414. Pain Physician 2006;9:361-6.
- Cocelli LP, Karakurum G, Cebesoy O, Karadasli H, Oner U. Clinical comparison of effectiveness of epidural triamcinolone and betamethasone in discal radiculalgia: a prospective, randomized study. J Muscoskel Pain 2009;17:281-6.
- Hagihara Y, Ogata S, Hirayama H, Koyama T, Watanabe E. Why use steroids in lumbar selective nerve root block? – A randomized control study. Chiba Med 2009;85:71-6.
- Lee JH, Moon J, Lee SH. Comparison of effectiveness according to different approaches of epidural steroid injection in lumbosacral herniated disk and spinal stenosis. BMR 2009;22:83-9.
- Manchikanti L, Singh V, Cash KA, Pampati V, Damron KS, Boswell MV. Preliminary results of a randomized, equivalence trial of fluoroscopic caudal epidural injections in managing chronic low back pain: part 2 – disc herniation and radiculitis. Pain Physician 2008;11:801-15.
- Manchikanti L, Singh V, Cash KA, Pampati V, Datta S. Preliminary results of a randomized, equivalence trial of fluoroscopic caudal epidural injections in managing chronic low back pain: part 3 – post surgery syndrome. Pain Physician 2008;11:817-31.
- Mendoza-Lattes S, Weiss A, Found E, Zimmerman B, Gao Y. Comparable effectiveness of caudal vs. trans-foraminal epidural steroid injections. Iowa Orthop J 2009;29:91-6.
- Tafazal S, Ng L, Chaudhary N, Sell P. Corticosteroids in peri-radicular infiltration for radicular pain: a randomised double blind controlled trial. One year results and subgroup analysis. Eur Spine J 2009;18:1220-5.
- Winnie AP, Hartman JT, Meyers HL, Jr, Ramamurthy S, Barangan V. Pain clinic. II. Intradural and extradural corticosteroids for sciatica. Anesth Analg 1972;51:990-1003.
- Anwar A, Zaidah I, Rozita R. Prospective randomised single blind study of epidural steroid injection comparing triamcinalone acetonide with methylprednisolone acetate. Int J Rheumatol 2005;8:51-3.
- Manchikanti L, Cash KA, McManus CD, Pampati V, Abdi S. Preliminary results of a randomized, equivalence trial of fluoroscopic caudal epidural injections in managing chronic low back pain: part 4 – spinal stenosis. Pain Physician 2008;11:833-48.
- Gogan WJ, Fraser RD. Chymopapain – a 10-year, double-blind-study. Spine 1992;17:388-94.
- Schwetschenau PR, Ramirez A, Johnston J. Double blind evaluation of intradiscal chymopapain for herniated lumbar discs; early results. J Neurosurg 1976;45:622-7.
- Feldman J, Menkes CJ, Pallardy G, Chevrot A, Horreard P, Zenny JC, et al. Double-blind study of the treatment of disc lumbosciatica by chemonucleolysis. Revue Du Rhumatisme Et Des Maladies Osteo-Articulaires 1986;53:147-52.
- Burton AK, Tillotson KM, Cleary J. Single-blind randomised controlled trial of chemonucleolysis and manipulation in the treatment of symptomatic lumbar disc herniation. Eur Spine J 2000;9:202-7.
- Dabezies EJ, Langford K, Morris J, Shields CB, Wilkinson HA. Safety and efficacy of chymopapain (discase) in the treatment of sciatica due to a herniated nucleus pulposus. Results of a randomized, double-blind study. Spine 1988;13:561-5.
- Javid MJ, Nordby EJ, Ford LT, Hejna WJ, Whisler WW, Burton C, et al. Safety and efficacy of chymopapain (chymodiactin) in herniated nucleus pulposus with sciatica. Results of a randomized, double-blind study. JAMA 1983;249:2489-94.
- Wittenberg RH, Oppel S, Rubenthaler FA, Steffen R. Five-year results from chemonucleolysis with chymopapain or collagenase – a prospective randomized study. Spine 2001;26:1835-41.
- Benoist M, Bonneville JF, Lassale B, Runge M, Gillard C, Vazquez-Suarez J, et al. A randomized, double-blind study to compare low-dose with standard-dose chymopapain in the treatment of herniated lumbar intervertebral discs. Spine 1993;18:28-34.
- Hedtmann A, Steffen R, Kramer J. Prospective comparative study of intradiscal high-dose and low-dose collagenase versus chymopapain. Spine 1987;12:388-92.
- Khoromi S, Cui L, Nackers L, Max MB. Morphine, nortriptyline and their combination vs. placebo in patients with chronic lumbar root pain. Pain 2007;130:66-75.
- Chen M-R, Wang P, Cheng G, Guo X, Wei G-W, Cheng X-H. The warming acupuncture for treatment of sciatica in 30 cases. J Tradit Chin Med 2009;29:50-3.
- Genevay S, Stingelin S, Gabay C. Efficacy of etanercept in the treatment of acute, severe sciatica: a pilot study. Ann Rheum Dis 2004;63:1120-3.
- Gibson T, Dilke TF, Grahame R. Chymoral in the treatment of lumbar disc prolapse. Rheumatol Rehabil 1975;14:186-90.
- Goldie I. A clinical trial with indomethacin (indomee (R)) in low back pain and sciatica. Acta Orthop 1968;39:117-28.
- Yildirim K, Sisecioglu M, Karatay S, Erdal A, Levent A, Ugur M, et al. The effectiveness of gabapentin in patients with chronic radiculopathy. Pain Clinic 2003;15:213-18.
- Hedeboe J, Buhl M, Ramsing P. Effects of using dexamethasone and placebo in the treatment of prolapsed lumbar disc. Acta Neurol Scand 1982;65:6-10.
- El-Zahaar MS. The Egyptian experience in the use of colchicine in lumbar disc disease. J Neurol Orthop Med Surg 1995;16:147-52.
- Porsman O, Friis H. Prolapsed lumbar disc treated with intramuscularly administered dexamethasonephosphate. A prospectively planned, double-blind, controlled clinical trial in 52 patients. Scand J Rheumatol 1979;8:142-4.
- Dreiser RL, Le Parc JM, Velicitat P, Lleu PL. Oral meloxicam is effective in acute sciatica: two randomised, double-blind trials versus placebo or diclofenac. Inflamm Res 2001;50:S17-23.
- Finckh A, Zufferey P, Schurch M-A, Balague F, Waldburger M, So AKL. Short-term efficacy of intravenous pulse glucocorticoids in acute discogenic sciatica. A randomized controlled trial. Spine 2006;31:377-81.
- Grevsten S, Johansson H. Phenylbutazone in treatment of acute lumbago-sciatica. Z Rheumatol 1975;34:444-7.
- Jacobs JH, Grayson MF. Trial of an anti-inflammatory agent (indomethacin) in low back pain with and without radicular involvement. Br Med J 1968;3:158-60.
- Herrmann WA, Geertsen MS. Efficacy and safety of lornoxicam compared with placebo and diclofenac in acute sciatica/lumbo-sciatica: an analysis from a randomised, double-blind, multicentre, parallel-group study. Int J Clin Pract 2009;63:1613-21.
- Holve RL, Barkan H. Oral steroids in initial treatment of acute sciatica. J Am Board Fam Med 2008;21:469-74.
- Kwasucki J, Stepien A, Maksymiuk G, Olbrych-Karpinska B. Evaluation of analgesic action of fluvoxamine compared with efficacy of imipramine and tramadol for treatment of sciatica – open trial. Wiad Lek 2002;55:42-50.
- Kwasucki J, Olbrych K, Ska B, Stepie A, Dec S, Maksymiuk G. Assessment of dexamethasone effectiveness in the treatment of ischalgia. Neurol Neurochir Pol 1993;27:515-22.
- Schuermans Y, Rauis A. Comparative clinical trial of two injectable NSAIDs, tiaprofenic acid and alclofenac, in acute sciatica. Drugs 1988;35:83-5.
- Blazek M, Keszthelyi B, Varhelyi M, Korosi O. Comparative study of Biarison and Voltaren in acute lumbar pain and lumbo-ischialgia. Therapia Hungarica 1986;34:163-6.
- Desnuelle C, Acquaviva PC. Comparative trial of injectable Indocid 50 mg against injectable diclofenac in lumbosciatica (single-blind, multicenter, randomized trial). Sem-Hop 1986;62:2197-201.
- Borms T. Comparison of injectable formulations of tiaprofenic acid and ketoprofen in acute lumbar sciatica. Single-blind randomised trial. Drugs 1988;35:85-7.
- Andersen RB, Halskov O. A double-blind clinical comparison of proquazone and naproxen in the treatment of patients with symptoms of lumbar nerve root compression syndrome. Scand J Rheumatol 1978:18-20.
- Stevanovic L. Double-blind parallel clinical trial with tenoxicam vs. piroxicam in patients suffering from acute lumbalgia and subacute lumboischialgia. Aktuelle Rheumatologie 1986;11:196-203.
- Kanayama M, Hashimoto T, Shigenobu K, Oha F, Yamane S. New treatment of lumbar disc herniation involving 5-hydroxytryptamine2A receptor inhibitor: a randomized controlled trial. J Neurosurg Spine 2005;2:441-6.
- Braun H, Huberty R. Therapy of lumbar sciatica. A comparative clinical study of a corticoid-free monosubstance and a corticoid–containing combination drug. Medizinische Welt 1982;33:490-1.
- Friedman BW, Esses D, Solorzano C, Choi HK, Cole M, Davitt M, et al. A randomized placebo-controlled trial of single-dose IM corticosteroid for radicular low back pain. Spine 2008;33:E624-9.
- Memeo A, Loiero M. Thioctic acid and acetyl-L-carnitine in the treatment of sciatic pain caused by a herniated disc: a randomized, double-blind, comparative study. Clin Drug Investig 2008;28:495-500.
- Toroudi HP, Borghei Razavi M, Razavi HB, Tabatabayi RM, Tabar YT, Yahyavi ST, et al. Comparison of the analgesic effect of ibuprofen with mesalamine after discectomy surgery in patients with lumbar disc herniation: a double-blind randomized controlled trial. Neurol India 2009;57:305-9.
- Ljunggren AE, Walker L, Weber H, Amundsen T. Manual traction versus isometric exercises in patients with herniated intervertebral lumbar discs. Physiother Theory Pract 1992;8:207-13.
- Moret NC, van der Stap M, Hagmeijer R, Molenaar A, Koes BW. Design and feasibility of a randomized clinical trial to evaluate the effect of vertical traction in patients with a lumbar radicular syndrome. Man Ther 1998;3:203-11.
- Pal B, Mangion P, Hossain MA, Diffey BL. A controlled trial of continuous lumbar traction in the treatment of back pain and sciatica. Rheumatology 1986;25:181-3.
- Rattanatharn R, Sanjaroensuttikul N, Anadirekkul P, Chaivisate R, Wannasetta W. Effectiveness of lumbar traction with routine conservative treatment in acute herniated disc syndrome. J Med Assoc Thai 2004;87:S272-7.
- Larsson U, Choler U, Lidstrom A, Lind G, Nachemson A, Nilsson B, et al. Auto traction for treatment of lumbago sciatica a multicenter controlled investigation. Acta Orthop 1980;51:791-8.
- Mathews JA, Hickling J. Lumbar traction: a double-blind controlled study for sciatica. Rheumatol Rehabil 1975;14:222-5.
- Reust P, Chantraine A, Vischer TL. Treatment of lumbar sciatica with or without neurological deficit using mechanical traction. A double-blind study. Swiss Med Wkly 1998;118:271-4.
- Unlu Z, Tasci S, Tarhan S, Pabuscu Y, Islak S. Comparison of 3 physical therapy modalities for acute pain in lumbar disc herniation measured by clinical evaluation and magnetic resonance imaging. J Manipulative Physiol Ther 2008;31:191-8.
- Styczynski T, Zarski S, Drozdowska Z, Krzeminska-Dabrowska I, Lesiak A, Pirog A, et al. Clinical evaluation of the effectiveness of traction treatment of patients with herniation of lumbar intervertebral discs. Reumatologia 1991;29:275-81.
- Guvenol K, Tuzun C, Peker O, Goktay Y. A comparison of inverted spinal traction and conventional traction in the treatment of lumbar disc herniations. Physiother Theory Pract 2000;16:151-60.
- Ljunggren AE, Weber H, Larsen S. Autotraction vs. manual traction in patients with prolapsed lumbar intervertebral discs. Scand J Rehabil Med 1984;16:117-24.
- Ozturk B, Gunduz OH, Ozoran K, Bostanoglu S. Effect of continuous lumbar traction on the size of herniated disc material in lumbar disc herniation. Rheumatol Int 2006;26:622-6.
- Harte AA, Baxter GD, Gracey JH. The effectiveness of motorised lumbar traction in the management of LBP with lumbo sacral nerve root involvement: a feasibility study. BMC Musculoskelet Disord 2007;8.
- Fritz JM, Lindsay W, Matheson JW, Brennan GP, Hunter SJ, Moffit SD, et al. Is there a subgroup of patients with low back pain likely to benefit from mechanical traction? Results of a randomized clinical trial and subgrouping analysis. Spine 2007;32:E793-800.
- Lidstrom A, Zachrisson M. Physical therapy on low back pain and sciatica. An attempt at evaluation. Scand J Rehabil Med 1970;2:37-42.
- Nykvist F, Knuts LR, Alaranta H, Hurme M, Torma T, Ronnemaa T, et al. Clinical, social, and psychological factors and outcome in a 5-year follow-up study of 276 patients hospitalized because of suspected lumbar disc herniation. Int Disabil Stud 1990;12:107-12.
- Santilli V, Beghi E, Finucci S. Chiropractic manipulation in the treatment of acute back pain and sciatica with disc protrusion: a randomized double-blind clinical trial of active and simulated spinal manipulations. Spine 2006;6:131-7.
- Brotchi J, Pirotte B, De Witte O, Levivier M. Prevention of epidural fibrosis in a prospective series of 100 primary lumbo-sacral discectomy patients: follow-up and assessment at re-operation. Neurol Res 1999;21:S47-50.
- Zhang SQ, Wang Z, Wang HL. Analgesia of “three points and five methods” therapy on radiculalgia in protrusion of lumbar intervertebral disc. Chin J Clin Rehabil 2005;9:226-7.
- Duplan B, Cabanel G, Piton JL, Grauer JL, Phelip X. Acupuncture and sciatica in the acute phase. Double-blind study of 30 cases. Semaine Des Hopitaux 1983;59:3109-14.
- Khoromi S, Blackman MR, Kingman A, Patsalides A, Matheny LA, Adams S, et al. Low intensity permanent magnets in the treatment of chronic lumbar radicular pain. J Pain Symptom Manage 2007;34:434-45.
- Zhi L, Jing S. Clinical comparison between scalp acupuncture combined with a single body acupoint and body acupuncture alone for the treatment of sciatica. AJA 1995;23:305-7.
- Luijsterburg PAJ, Verhagen AP, Ostelo RWJG, van den Hoogen HJMM, Peul WC, Avezaat CJJ, et al. Physical therapy plus general practitioners’ care versus general practitioners’ care alone for sciatica: a randomised clinical trial with a 12-month follow-up. Eur Spine J 2008;17:509-17.
- Bakhtiary AH, Safavi-Farokhi Z, Rezasoltani A. Lumbar stabilizing exercises improve activities of daily living in patients with lumbar disc herniation. BMR 2005;18:55-60.
- Jaffe G. Double-blind, betweenpatient comparison of alclofenac (prinalgin) and indomethacin in treatment of low back pain and sciatica. Curr Med Res Opin 1974;2:424-9.
- Hofstee DJ, Gijtenbeek JMM, Hoogland PH, van Houwelingen HC, Kloet A, Lotters F, et al. Westeinde sciatica trial: randomized controlled study of bed rest and physiotherapy for acute sciatica. J Neurosurg 2002;96:45-9.
- Ghoname EA, White PF, Ahmed HE, Hamza MA, Craig WF, Noe CE. Percutaneous electrical nerve stimulation: an alternative to TENS in the management of sciatica. Pain 1999;83:193-9.
- Bokonjic R. Epidural infiltration of dexa-neurobion for treatment of intervertebral disk hernia. Medizinische Welt 1975;26:302-5.
- Karppinen J, Korhonen T, Malmivaara A, Paimela L, Kyllonen E, Lindgren K-A, et al. Tumor necrosis factor-alpha monoclonal antibody, infliximab, used to manage severe sciatica. Spine 2003;28:750-3.
- Korhonen T, Karppinen J, Paimela L, Malmivaara A, Lindgren KA, Jarvinen S, et al. The treatment of disc herniation-induced sciatica with infliximab – Results of a randomized, controlled, 3-month follow-up study. Spine 2005;30:2724-8.
- Cohen S, Bogduk N, Dragovich A, Buckenmaier C, Griffith S, Kurihara C, et al. Randomized, double-blind, placebo-controlled, dose–response, and preclinical safety study of transforaminal epidural etanercept for the treatment of sciatica. Anesthesiology 1116;110:1116-26.
- Caldwell DM, Ades AE, Higgins JPT. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 2005;331:897-900.
- Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 2004;23:3105-24.
- Dullerud R, Lie H, Magnaes B. Cost-effectiveness of percutaneous automated lumbar nucleotomy – comparison with traditional macro-procedure discectomy. Intervent Neuroradiol 1999;5:35-41.
- Karppinen J, Ohinmaa A, Malmivaara A, Kurunlahti M, Kyllonen E, Pienimaki T, et al. Cost effectiveness of periradicular infiltration for sciatica – subgroup analysis of a randomized controlled trial. Spine 2001;26:2587-95.
- Launois R, Henry B, Marty JR, Gersberg M, Lassale C, Benoist M, et al. Chemonucleolysis versus surgical discectomy for sciatica secondary to lumbar disc herniation. A cost and quality-of-life evaluation. Pharmacoeconomics 1994;6:453-63.
- Luijsterburg PAJ, Lamers LM, Verhagen AP, Ostelo RWJG, van den Hoogen HJMM, Peul WC, et al. Cost-effectiveness of physical therapy and general practitioner care for sciatica. Spine 2007;32:1942-8.
- Malter AD, Larson EB, Urban N, Deyo RA. Cost-effectiveness of lumbar discectomy for the treatment of herniated intervertebral disc. Spine 1996;21:1048-54.
- Manca A, Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, et al. Quality of life, resource consumption and costs of spinal cord stimulation versus conventional medical management in neuropathic pain patients with failed back surgery syndrome (PROCESS trial). Eur J Pain 2008;12:1047-58.
- Stevenson RC, McCabe CJ, Findlay AM. An economic evaluation of a clinical trial to compare automated percutaneous lumbar discectomy with microdiscectomy in the treatment of contained lumbar disc herniation. Spine 1995:739-42.
- Tosteson ANA, Skinner JS, Tosteson TD, Lurie JD, Andersson GB, Berven S, et al. The cost effectiveness of surgical versus nonoperative treatment for lumbar disc herniation over two years: evidence from the Spine Patient Outcomes Research Trial (SPORT). Spine 2008;33:2108-15.
- van den Hout WB, Peul WC, Koes BW, Brand R, Kievit J, Thomeer RTWM. Prolonged conservative care versus early surgery in patients with sciatica from lumbar disc herniation: cost utility analysis alongside a randomised controlled trial. BMJ 2008;336:1351-4.
- Oostenbrink JB, Bouwmans CAM, Koopmanschap MA, Rutten FFH. Handbook for cost studies, methods and guidelines for economic evaluation in health Care] [Dutch. Netherlands: Health Care Insurance Council; 2004.
- Health Council of the Netherlands . Management of the Lumbosacral Radicular Syndrome (sciatica) 1999.
- Malter AD, Larson EB, Nicole ScD, Deyo RA. Cost-effectiveness of lumbar discectomy for the treatment of herniated intervertebral disc. Spine 1996;21:1048-54.
- Anonymous . RBRVS Fee Schedule: A Plain-English Guide 2004.
- Brazier JE, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ 2002;21:271-92.
- Curtis LJ, Pennock M. Social assistance, lone parents and health: what do we know, where do we go?. Can J Public Health 2006;97:S4-11.
- Department of Health . NHS Reference Costs 2005–06 n.d. http://webarchive.nationalarchives.gov.uk/+/dh.gov.uk/en/publicationsandstatistics/publications/publicationspolicyandguidance/dh_062884 (accessed 29 January 2007).
- Curtis L. Unit costs of health and social care. PSSRU: University of Kent; 2009.
- British Medical Association and Royal Pharmaceutical Society of Great Britain . British National Formulary 2010.
- Department of Health . NHS Reference Costs 2008–2009 n.d. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_111591 (accessed 29 January 2009).
- Luijsterburg PAJ, Verhagen AP, Ostelo RWJG, van Os TAG, Peul WC, Koes BW. Effectiveness of conservative treatments for the lumbosacral radicular syndrome: a systematic review. Eur Spine J 2007;16:881-99.
- Clarke JA, van Tulder MW, Blomberg SEI, de Vet HCW, van der Heijden GJM, Bronfort G, et al. Traction for low-back pain with or without sciatica. Cochrane Database Syst Rev 2007.
- Turner RM, Spiegelhalter DJ, Smith GCS, Thompson SG. Bias modelling in evidence synthesis. J R Stat Soc Ser A Stat Soc 2009;172:21-47.
- Eddy DM, Hasselblad V, Shachter R. An introduction to bayesian methods for meta-analysis: the confidence profile method. Med Decis Making 1990;10:15-23.
- Deeks JJ, Dinnes J, D’Amico R, Sowden AJ, Sakarovitch C, Song F, et al. Evaluating non-randomised intervention studies. Health Technol Assess 2003;7.
- Egger M, Smith GD, Schneider M, Egger M, Smith GD. Systematic reviews in health care. Meta-analysis in context. London: Wiley-Blackwell; 2001.
- Wiebe N, Vandermeer B, Platt RW, Klassen TP, Moher D, Barrowman NJ. A systematic review identifies a lack of standardization in methods for handling missing variance data. J Clin Epidemiol 2006;59:342-53.
- Caldwell DM, Welton NJ, Ades AE. Mixed treatment comparison analysis provides internally coherent treatment effect estimates based on overviews of reviews and can reveal inconsistency. J Clin Epidemiol 2010;63:875-82.
- Spiegelhalter DJ, Best NG, Carlin BP. Bayesian measures of model complexity and fit. R Stat Soc (B) 2002;64:583-616.
- Welton NJ, Caldwell DM, Adamopoulos E, Vedhara K. Mixed treatment comparison meta-analysis of complex interventions: psychological interventions in coronary heart disease. Am J Epidemiol 2009;169:1158-65.
Appendix 1 Search strategies
MEDLINE (OVID) 1950 to week 1 June 2008
Searched on 16 June 2008. Search updated on 4 December 2009.
-
Sciatica/
-
(ischialg$or sciatic$).ti,ab.
-
((lumb$or sacra$or spin$) adj5 radicul$).ti,ab.
-
((sciatic nerve or lumbar nerve or spinal nerve or sacral nerve) adj5 (irritation or inflammat$or pain or neuropath$or dysfunction$or compressio$or injur$or traum$)).ti,ab
-
Intervertebral Disk Displacement/
-
((intervertebral disk or intervertebral disc or lumbar disc or lumbar disk or lumbosacral disc or lumbosacral disk or lumbo-sacral disc or lumbo-sacral disk) adj5 (hernia$or slip$or prolapse or degeneration or fusion or sclerosis or rupture or distortion or fracture or displacement)).ti,ab.
-
((lumbosacral nerve root or lumbo-sacral nerve root or lumbar nerve root) adj5 (irritat$or inflammat$or pain$or neuropath$or dysfunction$or compressio$or injur$or traum$)).ti,ab.
-
((refer$or radiat$) adj5 (back or leg or foot)).ti,ab.
-
or/1-8
-
(treatment$or therap$or manag$or surg$or modalit$or intervention$).ti,ab.
-
Bed rest/
-
(bed rest$or activ$or exercise$or education$or instruction$or advice$).ti,ab.
-
Physical Therapy Modalities/
-
((heat or hot or thermal or infra?red or ultrasound or ultrasonic or short-wave or physio$or physical or exercise) adj5 (therap$or treatm$)).ti,ab.
-
Transcutaneous Electric Nerve Stimulation/
-
(transcutaneous electric nerve stimulation or TENS).ti,ab.
-
Complementary Therapies/
-
Exp Musculoskeletal Manipulations/
-
Exp Acupuncture Therapy/
-
((spina$or chiropract$or osteopath$or physi$or homeopath$or acupunctur$or musculo?skeletal or myofunctional) adj5 (massage or manipulat$or therap$or treatment$)).ti,ab.
-
Homeopathy/
-
homeopathy.ti,ab.
-
Herbal Medicine/
-
herbal medicine.ti,ab.
-
Orthotic Devices/
-
(braces or slings or splints or corset).ti,ab.
-
Traction/
-
traction.ti,ab.
-
Drug Therapy/
-
Exp Analgesics/
-
Anti-Inflammatory Agents, Non-Steroidal/
-
((non-steroidal anti inflammatory or non-steroidal anti-inflammatory or non?narcotic or narcotic or opioid$or opiate$) adj5 (drug$or analges$)).ti,ab.
-
(paracetamol or acetaminophen).ti,ab.
-
(ibuprofen or aceclofenac or acemetacin or celecoxib or dexketoprofen or diclofenac sodium or etodolac or etoricoxib or fenbufen or fenoprofen or flurbiprofen or indometacin or indomethacin or ketoprofen or mefenamic acid or meloxicam or nabumetone or naproxen or piroxicam or sulindac or tenoxicam or tiaprofenic acid or azapropazone or biarison or acetaminophen or nimesulide or oxyphenbutazone or azapropazone or felbinac or alclofenac or nimesulid or etofenama or loxoprofen or phenylbutazone or valdecoxib or lornoxicam or etoricoxib).ti,ab.
-
(buprenorphine or butorphanol or codeine or dextromoramide or dextropropoxyphene or dihydromorphine or diphenoxylate or etorphine or fentanyl or heroin or hydrocodone or hydromorphone or levorphanol or meperidine or meptazinol or methadone or methadyl acetate or morphine or nalbuphine or oxycodone or oxymorphone or pentazocine or phenazocine or phenoperidine or pirinitramide or promedol or sufentanil or tilidine or tramadol).ti,ab.
-
Epidural Analgesia/
-
Epidural Injections/
-
((intramuscular or intravenous or peri?neural$or epidura$or inject$) adj5 (cortico?steroid$or steroid$or ana?lgesic$or chymopapain)).ti,ab.
-
(dexamethasone or hydrocortisone or prednisolone or methylprednisolone or prednisone or methylprednisone or triamcinolone).ti,ab.
-
Orthopedic Procedures/
-
Intervertebral Disk Chemolysis/
-
((disc or disk) adj5 (chemolysis or chemonucleolysis)).ti,ab.
-
Vertebroplasty/
-
Diskectomy/
-
Neurosurgical Procedures/
-
Laminectomy/
-
Rhizotomy/
-
(discectomy or diskectomy or microdiscectomy or microdiskectomy or rhizotomy or sequestrectomy or vertebroplasty or nucleoplasty or laminectomy).ti,ab.
-
Surgical Decompression/
-
surgical decompression.ti,ab.
-
or/11-50
-
9 and 51
-
limit 52 to humans
OLDMEDLINE (OVID) 1947–67
Searched with updated searches on 4 December 2009.
-
Sciatica/
-
(ischialg$or sciatic$).ti,ab.
-
((lumb$or sacra$or spin$) adj5 radicul$).ti,ab.
-
((sciatic nerve or lumbar nerve or spinal nerve or sacral nerve) adj5 (irritation or inflammat$or pain or neuropath$or dysfunction$or compressio$or injur$or traum$)).ti,ab
-
Intervertebral Disk Displacement/
-
((intervertebral disk or intervertebral disc or lumbar disc or lumbar disk or lumbosacral disc or lumbosacral disk or lumbo-sacral disc or lumbo-sacral disk) adj5 (hernia$or slip$or prolapse or degeneration or fusion or sclerosis or rupture or distortion or fracture or displacement)).ti,ab.
-
((lumbosacral nerve root or lumbo-sacral nerve root or lumbar nerve root) adj5 (irritat$or inflammat$or pain$or neuropath$or dysfunction$or compressio$or injur$or traum$)).ti,ab.
-
((refer$or radiat$) adj5 (back or leg or foot)).ti,ab.
-
or/1-8
-
(treatment$or therap$or manag$or surg$or modalit$or intervention$).ti,ab.
-
Bed rest/
-
(bed rest$or activ$or exercise$or education$or instruction$or advice$).ti,ab.
-
Physical Therapy Modalities/
-
((heat or hot or thermal or infra?red or ultrasound or ultrasonic or short-wave or physio$or physical or exercise) adj5 (therap$or treatm$)).ti,ab.
-
Transcutaneous Electric Nerve Stimulation/
-
(transcutaneous electric nerve stimulation or TENS).ti,ab.
-
Complementary Therapies/
-
Exp Musculoskeletal Manipulations/
-
Exp Acupuncture Therapy/
-
((spina$or chiropract$or osteopath$or physi$or homeopath$or acupunctur$or musculo?skeletal or myofunctional) adj5 (massage or manipulat$or therap$or treatment$)).ti,ab.
-
Homeopathy/
-
homeopathy.ti,ab.
-
Herbal Medicine/
-
herbal medicine.ti,ab.
-
Orthotic Devices/
-
(braces or slings or splints or corset).ti,ab.
-
Traction/
-
traction.ti,ab.
-
Drug Therapy/
-
Exp Analgesics/
-
Anti-Inflammatory Agents, Non-Steroidal/
-
((non-steroidal anti inflammatory or non-steroidal anti-inflammatory or non?narcotic or narcotic or opioid$or opiate$) adj5 (drug$or analges$)).ti,ab.
-
(paracetamol or acetaminophen).ti,ab.
-
(ibuprofen or aceclofenac or acemetacin or celecoxib or dexketoprofen or diclofenac sodium or etodolac or etoricoxib or fenbufen or fenoprofen or flurbiprofen or indometacin or indomethacin or ketoprofen or mefenamic acid or meloxicam or nabumetone or naproxen or piroxicam or sulindac or tenoxicam or tiaprofenic acid or azapropazone or biarison or acetaminophen or nimesulide or oxyphenbutazone or azapropazone or felbinac or alclofenac or nimesulid or etofenama or loxoprofen or phenylbutazone or valdecoxib or lornoxicam or etoricoxib).ti,ab.
-
(buprenorphine or butorphanol or codeine or dextromoramide or dextropropoxyphene or dihydromorphine or diphenoxylate or etorphine or fentanyl or heroin or hydrocodone or hydromorphone or levorphanol or meperidine or meptazinol or methadone or methadyl acetate or morphine or nalbuphine or oxycodone or oxymorphone or pentazocine or phenazocine or phenoperidine or pirinitramide or promedol or sufentanil or tilidine or tramadol).ti,ab.
-
Epidural Analgesia/
-
Epidural Injections/
-
((intramuscular or intravenous or peri?neural$or epidura$or inject$) adj5 (cortico?steroid$or steroid$or ana?lgesic$or chymopapain)).ti,ab.
-
(dexamethasone or hydrocortisone or prednisolone or methylprednisolone or prednisone or methylprednisone or triamcinolone).ti,ab.
-
Orthopedic Procedures/
-
Intervertebral Disk Chemolysis/
-
((disc or disk) adj5 (chemolysis or chemonucleolysis)).ti,ab.
-
Vertebroplasty/
-
Diskectomy/
-
Neurosurgical Procedures/
-
Laminectomy/
-
Rhizotomy/
-
(discectomy or diskectomy or microdiscectomy or microdiskectomy or rhizotomy or sequestrectomy or vertebroplasty or nucleoplasty or laminectomy).ti,ab.
-
Surgical Decompression/
-
surgical decompression.ti,ab.
-
or/11-50
-
9 and 51
-
limit 52 to humans
MEDLINE(R) In-Process & Other Non-Indexed Citations
Searched on 12 June 2008. Search updated on 4 December 2009.
-
(ischialg$or sciatic$).ti,ab.
-
((lumb$or sacra$or spin$) adj5 radicul$).ti,ab.
-
((sciatic nerve or lumbar nerve or spinal nerve or sacral nerve) adj5 (irritation or inflammat$or pain or neuropath$or dysfunction$or compressio$or injur$or traum$)).ti,ab
-
((lumbar disc or lumbar disk or lumbosacral disc or lumbosacral disk or lumbo-sacral disc or lumbo-sacral disk or intervertebral disk or intervertebral disc) adj5 (herni$or slip$or prolapse or degeneration or fusion or sclerosis or rupture or distortion or fracture or displacement)).ti,ab.
-
((lumbosacral nerve root or lumbo-sacral nerve root or lumbar nerve root) adj5 (irritat$or inflammat$or pain$or neuropath$or dysfunction$or compressio$or injur$or traum$)).ti,ab.
-
((back or leg or foot) adj5 (refer$or radiat$)).ti,ab.
-
or/1-6
-
(treatment$or therap$or manag$or surg$or modalit$or intervention$).ti,ab
-
(bed rest$or activ$or exercise$or education$or instruction$or advice$).ti,ab.
-
((heat or hot or thermal or infra?red or ultrasound or ultrasonic or short-wave or physio$or physical or exercise) adj5 (therap$or treatm$)).ti,ab.
-
(transcutaneous electric nerve stimulation or TENS).ti,ab.
-
Complementary Therapies/
-
((spina$or chiropract$or osteopath$or physi$or homeopath$or acupunctur$or musculo?skeletal or myofunctional) adj5 (massage or manipulat$or therap$or treatment$)).ti,ab.
-
homeopathy.ti,ab.
-
herbal medicine.ti,ab.
-
braces or slings or corset.ti,ab.
-
traction.ti,ab.
-
Exp Analgesics/
-
Anti-Inflammatory Agents, Non-Steroidal/
-
((non-steroidal anti inflammatory or non-steroidal anti-inflammatory or non?narcotic or narcotic or opioid$or opiate$) adj5 (drug$or analges$)).ti,ab.
-
(paracetamol or acetaminophen).ti,ab.
-
(ibuprofen or aceclofenac or acemetacin or celecoxib or dexketoprofen or diclofenac sodium or etodolac or etoricoxib or fenbufen or fenoprofen or flurbiprofen or indometacin or indomethacin or ketoprofen or mefenamic acid or meloxicam or nabumetone or naproxen or piroxicam or sulindac or tenoxicam or tiaprofenic acid or azapropazone or biarison or acetaminophen or nimesulide or oxyphenbutazone or azapropazone or felbinac or alclofenac or nimesulid or etofenama or loxoprofen or phenylbutazone or valdecoxib or lornoxicam or etoricoxib).ti,ab.
-
(buprenorphine or butorphanol or codeine or dextromoramide or dextropropoxyphene or dihydromorphine or diphenoxylate or etorphine or fentanyl or heroin or hydrocodone or hydromorphone or levorphanol or meperidine or meptazinol or methadone or methadyl acetate or morphine or nalbuphine or oxycodone or oxymorphone or pentazocine or phenazocine or phenoperidine or pirinitramide or promedol or sufentanil or tilidine or tramadol).ti,ab.
-
((intramuscular or intravenous or peri?neural$or epidura$or inject$) adj5 (cortico?steroid$or steroid$or ana?lgesic$or chymopapain)).ti,ab.
-
(dexamethasone or hydrocortisone or prednisolone or methylprednisolone or prednisone or methylprednisone or triamcinolone).ti,ab.
-
((disc or disk) adj5 (chemolysis or chemonucleolysis)).ti,ab.
-
(discectomy or diskectomy or microdiscectomy or microdiskectomy or rhizotomy or sequestrectomy or vertebroplasty or nucleoplasty or laminectomy).ti,ab.
-
surgical decompression.ti,ab.
-
or/8-28
-
7 and 29
EMBASE 1980 to week 23 June 2008
Searched on 12 June 2008. Search updated on 4 December 2009.
-
Ischialgia/
-
(ischialgia$or sciatic$).ti,ab.
-
((lumb$or sacra$or spin$) adj5 radicul$).ti,ab.
-
((sciatic nerve or lumbar nerve or spinal nerve or sacral nerve) adj5 (irritation or inflammat$or pain or neuropath$or dysfunction$or compressio$or injur$or traum$)).ti,ab
-
Exp Intervertebral Disk Hernia/
-
((intervertebral disk or intervertebral disc or lumbar disc or lumbar disk or lumbosacral disc or lumbosacral disk or lumbo-sacral disc or lumbo-sacral disk) adj5 (hernia$or slip$or prolapse or degeneration or fusion or sclerosis or rupture or distortion or fracture or displacement)).ti,ab.
-
((lumbosacral nerve root or lumbo-sacral nerve root or lumbar nerve root) adj5 (irritat$or inflammat$or pain$or neuropath$or dysfunction$or compressio$or injur$or traum$)).ti,ab.
-
((back or leg or foot) adj5 (refer$or radiat$)).ti,ab.
-
or/1-8
-
(treatment$or therap$or manag$or surg$or modalit$or intervention$).ti,ab
-
Conservative Treatment/
-
Bed Rest/
-
(bed rest$or activ$or exercise$or education$or instruction$or advice$).ti,ab.
-
Exp Traction Therapy/
-
traction.ti,ab.
-
Physical Medicine/
-
Physiotherapy/
-
Microwave Therapy/
-
Ultrasound Therapy/
-
((heat or hot or thermal or infra?red or ultrasound or ultrasonic or short-wave or microwave$or physio$or physical or exercise or daitherm$) adj5 (therap$or treatm$)).ti,ab.
-
Transcutaneous Nerve Stimulation/
-
(transcutaneous electric nerve stimulation or TENS).ti,ab.
-
Alternative Medicine/
-
Exp Manipulative Medicine/
-
Exp Acupuncture/
-
((spina$or chiropract$or osteopath$or physi$or homeopath$or acupunctur$or musculo?skeletal or myofunctional) adj5 (massage or manipulat$or therap$or treatment$)).ti,ab.
-
Homeopathy/
-
homeopathy.ti,ab.
-
Traditional Medicine/
-
Herbal Medicine/
-
herbal medicine.ti,ab.
-
Orthopedic Equipment/
-
Orthotics/
-
Corset/
-
(braces or slings or corset).ti,ab.
-
Analgesia/
-
Exp Analgesic Agent/
-
Nonsteroid Antiinflammatory Agent/
-
((non-steroidal anti inflammatory or non-steroidal anti-inflammatory or non?narcotic or narcotic or opioid$or opiate$) adj5 (drug$or analges$)).ti,ab.
-
(paracetamol or acetaminophen).ti,ab.
-
(ibuprofen or aceclofenac or acemetacin or celecoxib or dexketoprofen or diclofenac sodium or etodolac or etoricoxib or fenbufen or fenoprofen or flurbiprofen or indometacin or indomethacin or ketoprofen or mefenamic acid or meloxicam or nabumetone or naproxen or piroxicam or sulindac or tenoxicam or tiaprofenic acid or azapropazone or biarison or acetaminophen or nimesulide or oxyphenbutazone or azapropazone or felbinac or alclofenac or nimesulid or etofenama or loxoprofen or phenylbutazone or valdecoxib or lornoxicam or etoricoxib).ti,ab.
-
(buprenorphine or butorphanol or codeine or dextromoramide or dextropropoxyphene or dihydromorphine or diphenoxylate or etorphine or fentanyl or heroin or hydrocodone or hydromorphone or levorphanol or meperidine or meptazinol or methadone or methadyl acetate or morphine or nalbuphine or oxycodone or oxymorphone or pentazocine or phenazocine or phenoperidine or pirinitramide or promedol or sufentanil or tilidine or tramadol).ti,ab.
-
Epidural Drug Administration/
-
((intramuscular or intravenous or peri?neural$or epidura$or inject$) adj5 (cortico?steroid$or steroid$or ana?lgesic$or chymopapain)).ti,ab.
-
(dexamethasone or hydrocortisone or prednisolone or methylprednisolone or prednisone or methylprednisone or triamcinolone).ti,ab.
-
Orthopedic Surgery/
-
Chemonucleolysis/
-
((disc or disk) adj5 (chemolysis or chemonucleolysis)).ti,ab.
-
Percutaneous Vertebroplasty/
-
Spinal Cord Surgery/
-
Intervertebral Diskectomy/
-
Laminectomy/
-
Rhizotomy/
-
Laminoplasty/
-
(discectomy or diskectomy or microdiscectomy or microdiskectomy or rhizotomy or sequestrectomy or vertebroplasty or nucleoplasty or laminectomy or laminoplasty).ti,ab.
-
Nerve Decompression/
-
Spinal Cord Decompression/
-
((nerve or spinal cord) adj5 decompression).ti,ab.
-
or/10-58
-
9 and 59
-
limit 60 to humans
EMBASE 1974–9
Searched on 12 June 2008.
-
Ischialgia/
-
(ischialgia$or sciatic$).ti,ab.
-
((lumb$or sacra$or spin$) adj5 radicul$).ti,ab.
-
((sciatic nerve or lumbar nerve or spinal nerve or sacral nerve) adj5 (irritation or inflammat$or pain or neuropath$or dysfunction$or compressio$or injur$or traum$)).ti,ab
-
Exp Intervertebral Disk Hernia/
-
((intervertebral disk or intervertebral disc or lumbar disc or lumbar disk or lumbosacral disc or lumbosacral disk or lumbo-sacral disc or lumbo-sacral disk) adj5 (hernia$or slip$or prolapse or degeneration or fusion or sclerosis or rupture or distortion or fracture or displacement)).ti,ab.
-
((lumbosacral nerve root or lumbo-sacral nerve root or lumbar nerve root) adj5 (irritat$or inflammat$or pain$or neuropath$or dysfunction$or compressio$or injur$or traum$)).ti,ab.
-
((back or leg or foot) adj5 (refer$or radiat$)).ti,ab.
-
or/1-8
-
(treatment$or therap$or manag$or surg$or modalit$or intervention$).ti,ab
-
Conservative Treatment/
-
Bed Rest/
-
(bed rest$or activ$or exercise$or education$or instruction$or advice$).ti,ab.
-
Exp Traction Therapy/
-
traction.ti,ab.
-
Physical Medicine/
-
Physiotherapy/
-
Microwave Therapy/
-
Ultrasound Therapy/
-
((heat or hot or thermal or infra?red or ultrasound or ultrasonic or short-wave or microwave$or physio$or physical or exercise or daitherm$) adj5 (therap$or treatm$)).ti,ab.
-
Transcutaneous Nerve Stimulation/
-
(transcutaneous electric nerve stimulation or TENS).ti,ab.
-
Alternative Medicine/
-
Exp Manipulative Medicine/
-
Exp Acupuncture/
-
((spina$or chiropract$or osteopath$or physi$or homeopath$or acupunctur$or musculo?skeletal or myofunctional) adj5 (massage or manipulat$or therap$or treatment$)).ti,ab.
-
Homeopathy/
-
homeopathy.ti,ab.
-
Exp Traditional Medicine/
-
Orthotics/
-
Corset/
-
(braces or slings or corset).ti,ab.
-
Analgesia/
-
Exp Analgesic Agent/
-
Nonsteroid Antiinflammatory Agent/
-
((non-steroidal anti inflammatory or non-steroidal anti-inflammatory or non?narcotic or narcotic or opioid$or opiate$) adj5 (drug$or analges$)).ti,ab.
-
(paracetamol or acetaminophen).ti,ab.
-
(ibuprofen or aceclofenac or acemetacin or celecoxib or dexketoprofen or diclofenac sodium or etodolac or etoricoxib or fenbufen or fenoprofen or flurbiprofen or indometacin or indomethacin or ketoprofen or mefenamic acid or meloxicam or nabumetone or naproxen or piroxicam or sulindac or tenoxicam or tiaprofenic acid or azapropazone or biarison or acetaminophen or nimesulide or oxyphenbutazone or azapropazone or felbinac or alclofenac or nimesulid or etofenama or loxoprofen or phenylbutazone or valdecoxib or lornoxicam or etoricoxib).ti,ab.
-
(buprenorphine or butorphanol or codeine or dextromoramide or dextropropoxyphene or dihydromorphine or diphenoxylate or etorphine or fentanyl or heroin or hydrocodone or hydromorphone or levorphanol or meperidine or meptazinol or methadone or methadyl acetate or morphine or nalbuphine or oxycodone or oxymorphone or pentazocine or phenazocine or phenoperidine or pirinitramide or promedol or sufentanil or tilidine or tramadol).ti,ab.
-
Epidural Drug Administration/
-
((intramuscular or intravenous or peri?neural$or epidura$or inject$) adj5 (cortico?steroid$or steroid$or ana?lgesic$or chymopapain)).ti,ab.
-
(dexamethasone or hydrocortisone or prednisolone or methylprednisolone or prednisone or methylprednisone or triamcinolone).ti,ab.
-
Orthopedic Surgery/
-
((disc or disk) adj5 (chemolysis or chemonucleolysis)).ti,ab.
-
Exp Spine Surgery/
-
Exp Spinal Cord Surgery/
-
(discectomy or diskectomy or microdiscectomy or microdiskectomy or rhizotomy or sequestrectomy or vertebroplasty or nucleoplasty or laminectomy or laminoplasty).ti,ab.
-
((nerve or spinal cord) adj5 decompression).ti,ab.
-
or/10-48
-
9 and 49
EMBASE 1947–73
Searched on 12 June 2008. Search updated on 4 December 2009 for EMBASE Classic 1947–79.
-
Ischialgia/
-
(ischialgia$or sciatic$).ti,ab.
-
((lumb$or sacra$or spin$) adj5 radicul$).ti,ab.
-
((sciatic nerve or lumbar nerve or spinal nerve or sacral nerve) adj5 (irritation or inflammat$or pain or neuropath$or dysfunction$or compressio$or injur$or traum$)).ti,ab
-
Exp Intervertebral Disk Hernia/
-
((intervertebral disk or intervertebral disc or lumbar disc or lumbar disk or lumbosacral disc or lumbosacral disk or lumbo-sacral disc or lumbo-sacral disk) adj5 (hernia$or slip$or prolapse or degeneration or fusion or sclerosis or rupture or distortion or fracture or displacement)).ti,ab.
-
((lumbosacral nerve root or lumbo-sacral nerve root or lumbar nerve root) adj5 (irritat$or inflammat$or pain$or neuropath$or dysfunction$or compressio$or injur$or traum$)).ti,ab.
-
((back or leg or foot) adj5 (refer$or radiat$)).ti,ab.
-
or/1-8
-
(treatment$or therap$or manag$or surg$or modalit$or intervention$).ti,ab
-
Conservative Treatment/
-
Bed Rest/
-
(bed rest$or activ$or exercise$or education$or instruction$or advice$).ti,ab.
-
Exp Traction Therapy/
-
traction.ti,ab.
-
Physical Medicine/
-
Physiotherapy/
-
Microwave Therapy/
-
Ultrasound Therapy/
-
((heat or hot or thermal or infra?red or ultrasound or ultrasonic or short-wave or microwave$or physio$or physical or exercise or daitherm$) adj5 (therap$or treatm$)).ti,ab.
-
Electrostimulation/
-
(transcutaneous electric nerve stimulation or electro?stimulation or TENS).ti,ab.
-
Alternative Medicine/
-
Exp Manipulative Medicine/
-
Exp Acupuncture/
-
((spina$or chiropract$or osteopath$or physi$or homeopath$or acupunctur$or musculo?skeletal or myofunctional) adj5 (massage or manipulat$or therap$or treatment$)).ti,ab.
-
Homeopathy/
-
homeopathy.ti,ab.
-
Traditional Medicine/
-
Herbal Medicine/
-
herbal medicine.ti,ab.
-
Orthopedic Equipment/
-
Orthotics/
-
Corset/
-
(braces or slings or corset).ti,ab.
-
Analgesia/
-
Exp Analgesic Agent/
-
Nonsteroid Antiinflammatory Agent/
-
((non-steroidal anti inflammatory or non-steroidal anti-inflammatory or non?narcotic or narcotic or opioid$or opiate$) adj5 (drug$or analges$)).ti,ab.
-
(paracetamol or acetaminophen).ti,ab.
-
(ibuprofen or aceclofenac or acemetacin or celecoxib or dexketoprofen or diclofenac sodium or etodolac or etoricoxib or fenbufen or fenoprofen or flurbiprofen or indometacin or indomethacin or ketoprofen or mefenamic acid or meloxicam or nabumetone or naproxen or piroxicam or sulindac or tenoxicam or tiaprofenic acid or azapropazone or biarison or acetaminophen or nimesulide or oxyphenbutazone or azapropazone or felbinac or alclofenac or nimesulid or etofenama or loxoprofen or phenylbutazone or valdecoxib or lornoxicam or etoricoxib).ti,ab.
-
(buprenorphine or butorphanol or codeine or dextromoramide or dextropropoxyphene or dihydromorphine or diphenoxylate or etorphine or fentanyl or heroin or hydrocodone or hydromorphone or levorphanol or meperidine or meptazinol or methadone or methadyl acetate or morphine or nalbuphine or oxycodone or oxymorphone or pentazocine or phenazocine or phenoperidine or pirinitramide or promedol or sufentanil or tilidine or tramadol).ti,ab.
-
Epidural Drug Administration/
-
((intramuscular or intravenous or peri?neural$or epidura$or inject$) adj5 (cortico?steroid$or steroid$or ana?lgesic$or chymopapain)).ti,ab.
-
(dexamethasone or hydrocortisone or prednisolone or methylprednisolone or prednisone or methylprednisone or triamcinolone).ti,ab.
-
Orthopedic Surgery/
-
Chemonucleolysis/
-
((disc or disk) adj5 (chemolysis or chemonucleolysis)).ti,ab.
-
Percutaneous Vertebroplasty/
-
Exp Spinal Cord Surgery/
-
(discectomy or diskectomy or microdiscectomy or microdiskectomy or rhizotomy or sequestrectomy or vertebroplasty or nucleoplasty or laminectomy or laminoplasty).ti,ab.
-
Nerve Decompression/
-
Spinal Cord Decompression/
-
((nerve or spinal cord) adj5 decompression).ti,ab.
-
or/10-54
-
9 and 55
-
limit 57 to humans
Cumulative Index to Nursing and Allied Health Literature 1982 to week 2 June 2008
Searched on 15 June 2008.
-
Sciatica/
-
(ischialg$or sciatic$).ti,ab.
-
((lumb$or sacra$or spin$) adj5 radicul$).ti,ab.
-
((sciatic nerve or lumbar nerve or spinal nerve or sacral nerve) adj5 (irritation or inflammat$or pain or neuropath$or dysfunction$or compressio$or injur$or traum$)).ti,ab
-
Intervertebral Disk Displacement/
-
((intervertebral disk or intervertebral disc or lumbar disc or lumbar disk or lumbosacral disc or lumbosacral disk or lumbo-sacral disc or lumbo-sacral disk) adj5 (hernia$or slip$or prolapse or degeneration or fusion or sclerosis or rupture or distortion or fracture or displacement)).ti,ab.
-
((lumbosacral nerve root or lumbo-sacral nerve root or lumbar nerve root) adj5 (irritat$or inflammat$or pain$or neuropath$or dysfunction$or compressio$or injur$or traum$)).ti,ab.
-
((back or leg or foot) adj5 (refer$or radiat$)).ti,ab.
-
or/1-8
-
(treatment$or therap$or manag$or surg$or modalit$or intervention$).ti,ab.
-
Bed Rest/
-
(bed rest$or activ$or exercise$or education$or instruction$or advice$).ti,ab.
-
Physical Therapy/
-
((heat or hot or thermal or infra?red or ultrasound or ultrasonic or short-wave or physio$or physical or exercise or message) adj5 (therap$or treatm$)).ti,ab.
-
Transcutaneous Electric Nerve Stimulation/
-
(transcutaneous electric nerve stimulation or TENS).ti,ab.
-
Alternative Therapies/
-
Exp Manual Therapy/
-
Exp Acupuncture/
-
Homeopathy/
-
((spina$or chiropract$or osteopath$or physi$or homeopath$or acupunctur$or musculo?skeletal or myofunctional) adj5 (massage or manipulat$or therap$or treatment$)).ti,ab.
-
Exp Medicine, Herbal/
-
herbal medicine.ti,ab.
-
Exp Orthoses/
-
(braces or slings or splints or corset).ti,ab.
-
Drug Therapy/
-
Exp Analgesics/
-
Anti-Inflammatory Agents, Non-Steroidal/
-
((non-steroidal anti inflammatory or non-steroidal anti-inflammatory or non?narcotic or narcotic or opioid$or opiate$) adj5 (drug$or analges$)).ti,ab.
-
(paracetamol or acetaminophen).ti,ab.
-
(ibuprofen or aceclofenac or acemetacin or celecoxib or dexketoprofen or diclofenac sodium or etodolac or etoricoxib or fenbufen or fenoprofen or flurbiprofen or indometacin or indomethacin or ketoprofen or mefenamic acid or meloxicam or nabumetone or naproxen or piroxicam or sulindac or tenoxicam or tiaprofenic acid or azapropazone or biarison or acetaminophen or nimesulide or oxyphenbutazone or azapropazone or felbinac or alclofenac or nimesulid or etofenama or loxoprofen or phenylbutazone or valdecoxib or lornoxicam or etoricoxib).ti,ab.
-
(buprenorphine or butorphanol or codeine or dextromoramide or dextropropoxyphene or dihydromorphine or diphenoxylate or etorphine or fentanyl or heroin or hydrocodone or hydromorphone or levorphanol or meperidine or meptazinol or methadone or methadyl acetate or morphine or nalbuphine or oxycodone or oxymorphone or pentazocine or phenazocine or phenoperidine or pirinitramide or promedol or sufentanil or tilidine or tramadol).ti,ab.
-
Epidural Analgesia/
-
Epidural Injections/
-
((intramuscular or intravenous or peri?neural$or epidura$or inject$) adj5 (cortico?steroid$or steroid$or ana?lgesic$or chymopapain)).ti,ab.
-
(dexamethasone or hydrocortisone or prednisolone or methylprednisolone or prednisone or methylprednisone or triamcinolone).ti,ab.
-
Intervertebral Disk Chemolysis/
-
((disc or disk) adj5 (chemolysis or chemonucleolysis)).ti,ab.
-
Orthopedic Surgery/
-
Traction/
-
traction.ti,ab.
-
Diskectomy/
-
Neurosurgery/
-
Laminectomy/
-
Rhizotomy/
-
(discectomy or diskectomy or microdiscectomy or microdiskectomy or rhizotomy or sequestrectomy or vertebroplasty or nucleoplasty or laminectomy).ti,ab.
-
Surgical Decompression/
-
surgical decompression.ti,ab.
-
or/10-48
-
9 and 49
Allied and Complimentary Medicine Database 1985 to June 2008
Searched on 12 June 2008. Search updated on 4 December 2009.
-
Sciatica/
-
(ischialg$or sciatic$).ti,ab.
-
((lumb$or sacra$or spin$) adj5 radicul$).ti,ab.
-
((sciatic nerve or lumbar nerve or spinal nerve or sacral nerve) adj5 (irritation or inflammat$or pain or neuropath$or dysfunction$or compressio$or injur$or traum$)).ti,ab
-
Intervertebral Disk Displacement/
-
((intervertebral disk or intervertebral disc or lumbar disc or lumbar disk or lumbosacral disc or lumbosacral disk or lumbo-sacral disc or lumbo-sacral disk) adj5 (hernia$or slip$or prolapse or degeneration or fusion or sclerosis or rupture or distortion or fracture or displacement)).ti,ab.
-
((lumbosacral nerve root or lumbo-sacral nerve root or lumbar nerve root) adj5 (irritat$or inflammat$or pain$or neuropath$or dysfunction$or compressio$or injur$or traum$)).ti,ab.
-
((back or leg or foot) adj5 (refer$or radiat$)).ti,ab.
-
or/1-8
-
(treatment$or therap$or manag$or surg$or modalit$or intervention$).ti,ab.
-
Bed Rest/
-
(bed rest$or activ$or exercise$or education$or instruction$or advice$).ti,ab.
-
Exp Physical Therapy Modalities/
-
Traction/
-
traction.ti,ab.
-
Transcutaneous Electric Nerve Stimulation/
-
(transcutaneous electric nerve stimulation or TENS).ti,ab.
-
((Heat or hot or thermal or infra?red or ultrasound or ultrasonic or Short-Wave or physio$or physical or exercise) adj5 (therap$or treatm$)).ti,ab.
-
Complementary Therapies/
-
Exp Acupuncture Therapy/
-
Homeopathy/
-
((spina$or chiropract$or osteopath$or physi$or homeopath$or acupunctur$or musculo?skeletal or myofunctional) adj5 (massage or manipulat$or therap$or treatment$)).ti,ab.
-
Herbal Drugs/
-
(herbal medicin$or drug$).ti,ab.
-
Orthotic Devices/
-
(braces or slings or corset).ti,ab.
-
Drug Therapy/
-
Analgesics/
-
Anti-Inflammatory Agents, Non-Steroidal/
-
((non-steroidal anti inflammatory or non-steroidal anti-inflammatory or non?narcotic or narcotic or opioid$or opiate$) adj5 (drug$or analges$)).ti,ab.
-
(paracetamol or acetaminophen).ti,ab.
-
(ibuprofen or aceclofenac or acemetacin or celecoxib or dexketoprofen or diclofenac sodium or etodolac or etoricoxib or fenbufen or fenoprofen or flurbiprofen or indometacin or indomethacin or ketoprofen or mefenamic acid or meloxicam or nabumetone or naproxen or piroxicam or sulindac or tenoxicam or tiaprofenic acid or azapropazone or biarison or acetaminophen or nimesulide or oxyphenbutazone or azapropazone or felbinac or alclofenac or nimesulid or etofenama or loxoprofen or phenylbutazone or valdecoxib or lornoxicam or etoricoxib).ti,ab.
-
Analgesics, Opioid/
-
(buprenorphine or butorphanol or codeine or dextromoramide or dextropropoxyphene or dihydromorphine or diphenoxylate or etorphine or fentanyl or heroin or hydrocodone or hydromorphone or levorphanol or meperidine or meptazinol or methadone or methadyl acetate or morphine or nalbuphine or oxycodone or oxymorphone or pentazocine or phenazocine or phenoperidine or pirinitramide or promedol or sufentanil or tilidine or tramadol).ti,ab.
-
Analgesia Epidural/
-
Drug Administration Routes/
-
Injections/
-
((intramuscular or intravenous or peri?neural$or epidura$or inject$) adj5 (cortico?steroid$or steroid$or ana?lgesic$or chymopapain)).ti,ab.
-
(dexamethasone or hydrocortisone or prednisolone or methylprednisolone or prednisone or methylprednisone or triamcinolone).ti,ab.
-
Surgery Operative/
-
((disc or disk) adj5 (chemolysis or chemonucleolysis)).ti,ab.
-
Laminectomy/
-
(discectomy or diskectomy or microdiscectomy or microdiskectomy or rhizotomy or sequestrectomy or vertebroplasty or nucleoplasty or laminectomy).ti,ab.
-
surgical decompression.ti,ab.
-
or/10-44
-
9 and 45
British Nursing Index and Archive 1985 to June 2008
Searched on 12 June 2008. Search updated on 4 December 2009.
-
(ischialg$or sciatic$).ti,ab.
-
((lumb$or sacra$or spin$) adj5 radicul$).ti,ab.
-
((sciatic nerve or lumbar nerve or spinal nerve or sacral nerve) adj5 (irritation or inflammat$or pain or neuropath$or dysfunction$or compressio$or injur$or traum$)).ti,ab.
-
((intervertebral disk or intervertebral disc or lumbar disc or lumbar disk or lumbosacral disc or lumbosacral disk or lumbo-sacral disc or lumbo-sacral disk) adj5 (hernia$or slip$or prolapse or degeneration or fusion or sclerosis or rupture or distortion or fracture or displacement)).ti,ab.
-
((lumbosacral nerve root or lumbo-sacral nerve root or lumbar nerve root) adj5 (irritat$or inflammat$or pain$or neuropath$or dysfunction$or compressio$or injur$or traum$)).ti,ab.
-
((back or leg or foot) adj5 (refer$or radiat$)).ti,ab.
-
or/1-6
-
(treatment$or therap$or manag$or surg$or modalit$or intervention$).ti,ab
-
(bed rest$or activ$or exercise$or education$or instruction$or advice$).ti,ab.
-
((heat or hot or thermal or infra?red or ultrasound or ultrasonic or short-wave or physio$or physical or exercise) adj5 (therap$or treatm$)).ti,ab.
-
(transcutaneous electric nerve stimulation or TENS).ti,ab.
-
((spina$or chiropract$or osteopath$or physi$or homeopath$or acupunctur$or musculo?skeletal or myofunctional) adj5 (massage or manipulat$or therap$or treatment$)).ti,ab.
-
homeopathy.ti,ab.
-
herbal medicine.ti,ab.
-
braces or slings or corset.ti,ab.
-
traction.ti,ab.
-
(paracetamol or acetaminophen).ti,ab.
-
((non-steroidal anti inflammatory or non-steroidal anti-inflammatory or non?narcotic or narcotic or opioid$or opiate$or NSAID$) adj5 (drug$or analges$)).ti,ab.
-
(paracetamol or acetaminophen).ti,ab.
-
(ibuprofen or aceclofenac or acemetacin or celecoxib or dexketoprofen or diclofenac sodium or etodolac or etoricoxib or fenbufen or fenoprofen or flurbiprofen or indometacin or indomethacin or ketoprofen or mefenamic acid or meloxicam or nabumetone or naproxen or piroxicam or sulindac or tenoxicam or tiaprofenic acid or azapropazone or biarison or acetaminophen or nimesulide or oxyphenbutazone or azapropazone or felbinac or alclofenac or nimesulid or etofenama or loxoprofen or phenylbutazone or valdecoxib or lornoxicam or etoricoxib).ti,ab.
-
(buprenorphine or butorphanol or codeine or dextromoramide or dextropropoxyphene or dihydromorphine or diphenoxylate or etorphine or fentanyl or heroin or hydrocodone or hydromorphone or levorphanol or meperidine or meptazinol or methadone or methadyl acetate or morphine or nalbuphine or oxycodone or oxymorphone or pentazocine or phenazocine or phenoperidine or pirinitramide or promedol or sufentanil or tilidine or tramadol).ti,ab.
-
((intramuscular or intravenous or peri?neural$or epidura$or inject$) adj5 (cortico?steroid$or steroid$or ana?lgesic$or Chymopapain)).ti,ab.
-
(dexamethasone or hydrocortisone or prednisolone or methylprednisolone or prednisone or methylprednisone or triamcinolone).ti,ab.
-
((disc or disk) adj5 (chemolysis or chemonucleolysis)).ti,ab.
-
(discectomy or diskectomy or microdiscectomy or microdiskectomy or rhizotomy or sequestrectomy or vertebroplasty or nucleoplasty or laminectomy).ti,ab.
-
surgical decompression.ti,ab.
-
or/8-26
-
7 and 27
Health Management Information Consortium May 2008
Searched on 12 June 2008. Search updated on 4 December 2009.
-
Sciatica/
-
(ischialg$or sciatic$).ti,ab.
-
((intervertebral disk or intervertebral disc or lumbar disc or lumbar disk or lumbosacral disc or lumbosacral disk or lumbo-sacral disc or lumbo-sacral disk) adj5 (hernia$or slip$or prolapse or degeneration or fusion or sclerosis or rupture or distortion or fracture or displacement)).ti,ab.
-
((back or leg or foot) adj5 (refer$or radiat$)).ti,ab.
-
or/1-4
-
(treatment$or therap$or manag$or surg$or modalit$or intervention$).ti,ab.
-
(bed rest$or activ$or exercise$or education$or instruction$or advice$).ti,ab.
-
((heat or hot or thermal or infra?red or ultrasound or ultrasonic or short-wave or physio$or physical or exercise) adj5 (therap$or treatm$)).ti,ab.
-
(transcutaneous electric nerve stimulation or TENS).ti,ab.
-
((spina$or chiropract$or osteopath$or physi$or homeopath$or acupunctur$or musculo?skeletal or myofunctional) adj5 (massage or manipulat$or therap$or treatment$)).ti,ab.
-
Homeopathy/
-
homeopathy.ti,ab.
-
herbal medicine.ti,ab.
-
Orthotic Devices/
-
(braces or slings or splints or corset).ti,ab.
-
Traction/
-
traction.ti,ab.
-
Drug Therapy/
-
Exp Analgesics/
-
((non-steroidal anti inflammatory or non-steroidal anti-inflammatory or non?narcotic or narcotic or opioid$or opiate$) adj5 (drug$or analges$)).ti,ab.
-
(paracetamol or acetaminophen).ti,ab.
-
(ibuprofen or aceclofenac or acemetacin or celecoxib or dexketoprofen or diclofenac sodium or etodolac or etoricoxib or fenbufen or fenoprofen or flurbiprofen or indometacin or indomethacin or ketoprofen or mefenamic acid or meloxicam or nabumetone or naproxen or piroxicam or sulindac or tenoxicam or tiaprofenic acid or azapropazone or biarison or acetaminophen or nimesulide or oxyphenbutazone or azapropazone or felbinac or alclofenac or nimesulid or etofenama or loxoprofen or phenylbutazone or valdecoxib or lornoxicam or etoricoxib).ti,ab.
-
(buprenorphine or butorphanol or codeine or dextromoramide or dextropropoxyphene or dihydromorphine or diphenoxylate or etorphine or fentanyl or heroin or hydrocodone or hydromorphone or levorphanol or meperidine or meptazinol or methadone or methadyl acetate or morphine or nalbuphine or oxycodone or oxymorphone or pentazocine or phenazocine or phenoperidine or pirinitramide or promedol or sufentanil or tilidine or tramadol).ti,ab.
-
((intramuscular or intravenous or peri?neural$or epidura$or inject$) adj5 (cortico?steroid$or steroid$or ana?lgesic$or chymopapain)).ti,ab.
-
(dexamethasone or hydrocortisone or prednisolone or methylprednisolone or prednisone or methylprednisone or triamcinolone).ti,ab.
-
((disc or disk) adj5 (chemolysis or chemonucleolysis)).ti,ab.
-
(discectomy or diskectomy or microdiscectomy or microdiskectomy or rhizotomy or sequestrectomy or vertebroplasty or nucleoplasty or laminectomy).ti,ab.
-
Exp Surgery/
-
or/6-28
-
5 and 29
PsycINFO 1806 to week 2 June 2008
Searched on 12 June 2008. Search updated on 4 December 2009.
-
(ischialg$or sciatic$).ti,ab.
-
((lumb$or sacra$or spin$) adj5 radicul$).ti,ab.
-
((sciatic nerve or lumbar nerve or spinal nerve or sacral nerve) adj5 (irritation or inflammat$or pain or neuropath$or dysfunction$or compressio$or injur$or traum$)).ti,ab
-
((intervertebral disk or intervertebral disc or lumbar disc or lumbar disk or lumbosacral disc or lumbosacral disk or lumbo-sacral disc or lumbo-sacral disk) adj5 (hernia$or slip$or prolapse or degeneration or fusion or sclerosis or rupture or distortion or fracture or displacement)).ti,ab.
-
((lumbosacral nerve root or lumbo-sacral nerve root or lumbar nerve root) adj5 (irritat$or inflammat$or pain$or neuropath$or dysfunction$or compressio$or injur$or traum$)).ti,ab.
-
((back or leg or foot) adj5 (refer$or radiat$)).ti,ab.
-
or/1-6
-
(treatment$or therap$or manag$or surg$or modalit$or intervention$).ti,ab.
-
(bed rest$or activ$or exercise$or education$or instruction$or advice$).ti,ab.
-
Exp Physical Treatment Methods/
-
((heat or hot or thermal or infra?red or ultrasound or ultrasonic or short-wave or physio$or physical or exercise) adj5 (therap$or treatm$)).ti,ab.
-
Electrical Stimulation/
-
(transcutaneous electric nerve stimulation or TENS).ti,ab.
-
Exp Alternative Medicine/
-
((spina$or chiropract$or osteopath$or physi$or homeopath$or acupunctur$or musculo?skeletal or myofunctional or herbal$) adj5 (massage or manipulat$or therap$or treatment$)).ti,ab.
-
(braces or slings or splints or corset).ti,ab.
-
traction.ti,ab.
-
Drug Therapy/
-
Exp Analgesic Drugs/
-
((non-steroidal anti inflammatory or non-steroidal anti-inflammatory or non?narcotic or narcotic or opioid$or opiate$) adj5 (drug$or analges$)).ti,ab.
-
(paracetamol or acetaminophen).ti,ab.
-
(ibuprofen or aceclofenac or acemetacin or celecoxib or dexketoprofen or diclofenac sodium or etodolac or etoricoxib or fenbufen or fenoprofen or flurbiprofen or indometacin or indomethacin or ketoprofen or mefenamic acid or meloxicam or nabumetone or naproxen or piroxicam or sulindac or tenoxicam or tiaprofenic acid or azapropazone or biarison or acetaminophen or nimesulide or oxyphenbutazone or azapropazone or felbinac or alclofenac or nimesulid or etofenama or loxoprofen or phenylbutazone or valdecoxib or lornoxicam or etoricoxib).ti,ab.
-
Exp Opiates/
-
(buprenorphine or butorphanol or codeine or dextromoramide or dextropropoxyphene or dihydromorphine or diphenoxylate or etorphine or fentanyl or heroin or hydrocodone or hydromorphone or levorphanol or meperidine or meptazinol or methadone or methadyl acetate or morphine or nalbuphine or oxycodone or oxymorphone or pentazocine or phenazocine or phenoperidine or pirinitramide or promedol or sufentanil or tilidine or tramadol).ti,ab.
-
Drug Administration Methods/
-
((intramuscular or intravenous or peri?neural$or epidura$or inject$) adj5 (cortico?steroid$or steroid$or ana?lgesic$or chymopapain)).ti,ab.
-
(dexamethasone or hydrocortisone or prednisolone or methylprednisolone or prednisone or methylprednisone or triamcinolone).ti,ab.
-
Surgery/
-
(surgery or surgical treatment).ti,ab.
-
((disc or disk) adj5 (chemolysis or chemonucleolysis)).ti,ab.
-
Neurosurgery/
-
(neuro?surgery or neuro?surgical treatment).ti,ab.
-
(discectomy or diskectomy or microdiscectomy or microdiskectomy or rhizotomy or sequestrectomy or vertebroplasty or nucleoplasty or laminectomy).ti,ab.
-
surgical decompression.ti,ab.
-
or/8-34
-
7 and 35
Inspec 1969 to week 22 2008
Searched on 9 June 2008. Search updated on 4 December 2009.
-
(ischialg$or sciatic$).ti,ab.
-
((lumb$or sacra$or spin$) adj5 radicul$).ti,ab.
-
((sciatic nerve or lumbar nerve or spinal nerve or sacral nerve) adj5 (irritation or inflammat$or pain or neuropath$or dysfunction$or compressio$or injur$or traum$)).ti,ab
-
((intervertebral disk or intervertebral disc or lumbar disc or lumbar disk or lumbosacral disc or lumbosacral disk or lumbo-sacral disc or lumbo-sacral disk) adj5 (hernia$or slip$or prolapse or degeneration or fusion or sclerosis or rupture or distortion or fracture or displacement)).ti,ab.
-
((lumbosacral nerve root or lumbo-sacral nerve root or lumbar nerve root) adj5 (irritat$or inflammat$or pain$or neuropath$or dysfunction$or compressio$or injur$or traum$)).ti,ab.
-
((back or leg or foot) adj5 (refer$or radiat$)).ti,ab.
-
or/1-6
-
(treatment$or therap$or manag$or surg$or modalit$or intervention$).ti,ab.
-
(bed rest$or activ$or exercise$or education$or instruction$or advice$).ti,ab.
-
((heat or hot or thermal or infra?red or ultrasound or ultrasonic or short-wave or physio$or physical or exercise) adj5 (therap$or treatm$)).ti,ab.
-
(transcutaneous electric nerve stimulation or TENS).ti,ab.
-
((spina$or chiropract$or osteopath$or physi$or homeopath$or acupunctur$or musculo?skeletal or myofunctional) adj5 (massage or manipulat$or therap$or treatment$)).ti,ab.
-
homeopathy.ti,ab.
-
Orthotic/
-
(braces or slings or splints or corset).ti,ab.
-
Traction/
-
traction.ti,ab.
-
Drugs/
-
((non-steroidal anti inflammatory or non-steroidal anti-inflammatory or non?narcotic or narcotic or opioid$or opiate$) adj5 (drug$or analges$)).ti,ab.
-
(paracetamol or acetaminophen).ti,ab.
-
(ibuprofen or aceclofenac or acemetacin or celecoxib or dexketoprofen or diclofenac sodium or etodolac or etoricoxib or fenbufen or fenoprofen or flurbiprofen or indometacin or indomethacin or ketoprofen or mefenamic acid or meloxicam or nabumetone or naproxen or piroxicam or sulindac or tenoxicam or tiaprofenic acid or azapropazone or biarison or acetaminophen or nimesulide or oxyphenbutazone or azapropazone or felbinac or alclofenac or nimesulid or etofenama or loxoprofen or phenylbutazone or valdecoxib or lornoxicam or etoricoxib).ti,ab.
-
(buprenorphine or butorphanol or codeine or dextromoramide or dextropropoxyphene or dihydromorphine or diphenoxylate or etorphine or fentanyl or heroin or hydrocodone or hydromorphone or levorphanol or meperidine or meptazinol or methadone or methadyl acetate or morphine or nalbuphine or oxycodone or oxymorphone or pentazocine or phenazocine or phenoperidine or pirinitramide or promedol or sufentanil or tilidine or tramadol).ti,ab.
-
((intramuscular or intravenous or peri?neural$or epidura$or inject$) adj5 (cortico?steroid$or steroid$or ana?lgesic$or chymopapain)).ti,ab.
-
(dexamethasone or hydrocortisone or prednisolone or methylprednisolone or prednisone or methylprednisone or triamcinolone).ti,ab.
-
Exp Orthopedics/
-
((disc or disk) adj5 (chemolysis or chemonucleolysis)).ti,ab.
-
(discectomy or diskectomy or microdiscectomy or microdiskectomy or rhizotomy or sequestrectomy or vertebroplasty or nucleoplasty or laminectomy).ti,ab.
-
surgical decompression.ti,ab.
-
or/8-28
-
7 and 29
The Cochrane Library (all databases) week 2 June 2008
Searched on 15 June 2008. Search updated on 4 December 2009.
-
Sciatica/
-
(ischialg* or sciatic*)
-
((lumb* or sacra* or spin*) near (radicul*))
-
((sciatic nerve* or lumbar nerve* or spinal nerve* or sacral nerve*) near (irritation* or inflammation* or pain* or neuropath* or dysfunction* or compressio* or injur* or traum*))
-
Intervertebral Disk Displacement/
-
((intervertebral disk) near (hernia* or slip* or displac*))
-
((lumbar disc* or lumbar disk* or lumbosacral disc* or lumbosacral disk* or lumbo-sacral disc* or lumbo-sacral disk*) near (hernia* or slip* or prolapse* or degeneration* or fusion* or sclerosis* or rupture* or distortion* or fracture*))
-
((lumbosacral nerve root* or lumbo-sacral nerve root* or lumbar nerve root*) near (irritat* or inflammat* or pain* or neuropath* or dysfunction* or compressio* or injur* or traum*))
-
((refer* or radiat*) near (back or leg or foot))
-
(#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9)
-
(treatment* or therap* or manag* or surg* or modalit*)
-
Bed Rest/
-
(bed rest* or active* or exercise* or education* or instruction* or advice*)
-
Physical Therapy Modalities/
-
Transcutaneous Electric Nerve Stimulation/
-
Complementary Therapies/
-
Musculoskeletal Manipulations/
-
Acupuncture/
-
Chiropractic/
-
Osteopathic Medicine/
-
Massage/
-
Traction/
-
Herbal Medicine/
-
Short-Wave Therapy/
-
Homeopathy/
-
Holistic Health/
-
((physio* or physic* or exercis* or heat* or hot* or thermal* or infra?red* or ultrasound* or ultrasonic* or short-wave* or complement* or holistic* or spina* or chiropract* or osteopath* or homeopath* or acupunctur* or musculo?skeletal or myofunctional) near (massage* or manipulate* or therap* or treatment* or medicin*))
-
Orthotic Devices/
-
(braces or slings or splints or corset)
-
Drug Therapy/
-
Analgesia/
-
Non-Narcotic Analgesics/
-
(paracetamol or acetaminophen)
-
Non-Steroidal Anti-Inflammatory Agents/
-
((non?steroidal anti?inflammatory or non?narcotic or opioid* or opiate*) near (drug* or analges*))
-
(ibuprofen or aceclofenac or acemetacin or celecoxib or dexketoprofen or diclofenac sodium or etodolac or etoricoxib or fenbufen or fenoprofen or flurbiprofen or indometacin or indomethacin or ketoprofen or mefenamic acid or meloxicam or nabumetone or naproxen or piroxicam or sulindac or tenoxicam or tiaprofenic acid or azapropazone or biarison or acetaminophen or nimesulide or oxyphenbutazone or azapropazone or felbinac or alclofenac or nimesulid or etofenama or loxoprofen or phenylbutazone or valdecoxib or lornoxicam or etoricoxib)
-
Opioid Analgesics/
-
Narcotics Analgesics/
-
(buprenorphine or butorphanol or codeine or dextromoramide or dextropropoxyphene or dihydromorphine or diphenoxylate or etorphine or fentanyl or heroin or hydrocodone or hydromorphone or levorphanol or meperidine or meptazinol or methadone or methadyl acetate or morphine or nalbuphine or oxycodone or oxymorphone or pentazocine or phenazocine or phenoperidine or pirinitramide or promedol or sufentanil or tilidine or tramadol)
-
Epidural Analgesia/
-
Epidural Injections/
-
((intramuscular or intravenous or peri?neural or epidural* or inject*) near (cortico?steroid* or steroid* or ana?lgesic* or chymopapain))
-
(dexamethasone or hydrocortisone or prednisolone or methylprednisolone or prednisone or methylprednisone or triamcinolone)
-
Orthopedic Procedures/
-
Traction/
-
Intervertebral Disk Chemolysis/
-
((disc or disk) near (chemolysis or chemonucleolysis))
-
Vertebroplasty/
-
Diskectomy/
-
Neurosurgical Procedures/
-
Laminectomy/
-
Rhizotomy/
-
(discectomy or diskectomy or microdiscectomy or microdiskectomy or rhizotomy or sequestrectomy or vertebroplasty or nucleoplasty or laminectomy)
-
(surgical decompression)
-
(#11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54)
-
#10 and #55
System for Information on Grey Literature in Europe 1980 to March 2005
Searched on 16 June 2008. Search updated on 4 December 2009.
-
(ischialg* or sciatic*)
-
(lumb* or sacra* or spin*) and (radicul*)
-
(sciatic nerve* or lumbar nerve* or spinal nerve* or sacral nerve*) and (irritation* or inflammation* or pain* or neuropath* or dysfunction* or compressio* or injur* or traum*)
-
(intervertebral disk or intervertebral disc or lumbar disc* or lumbar disk* or lumbosacral disc* or lumbosacral disk* or lumbo-sacral disc* or lumbo-sacral disk*) and (hernia* or slip* or prolapse* or degeneration* or fusion* or sclerosis* or rupture* or distortion* or fracture*)
-
(refer* or radiat*) and (back or leg or foot)
-
#5 OR #4 OR #3 OR #2 OR #1
-
(treatment* or therap* or manag* or surg* or modalit*)
-
(bed rest* or activ* or exercise* or education* or instruction* or advice*)
-
(physio* or physical* or exercis* or heat* or hot* or thermal* or infra?red* or ultrasound* or ultrasonic* or short-wave* or exercise*) and (therap* or treatm* or modalit*)
-
(nerve or electric) and (stimulation)
-
(complement* or holistic* or spina* or chiropract* or osteopath* or physic* or homeopath* or acupunctur* or musculo?skeletal or myofunctional) and (massage* or manipulate* or therap* or treatment* or medicin*)
-
(brace* or sling* or splint* or corset*)
-
(non?steroidal anti?inflammatory or non?narcotic or opioid* or opiate*) and (drug* or analges* or agent*)
-
(paracetamol or acetaminophen)
-
(ibuprofen or aceclofenac or acemetacin or celecoxib or dexketoprofen or diclofenac sodium or etodolac or etoricoxib or fenbufen or fenoprofen or flurbiprofen or indometacin or indomethacin or ketoprofen or mefenamic acid or meloxicam or nabumetone or naproxen or piroxicam or sulindac or tenoxicam or tiaprofenic acid or azapropazone or biarison or acetaminophen or nimesulide or oxyphenbutazone or azapropazone or felbinac or alclofenac or nimesulid or etofenama or loxoprofen or phenylbutazone or valdecoxib or lornoxicam or etoricoxib)
-
(buprenorphine or butorphanol or codeine or dextromoramide or dextropropoxyphene or dihydromorphine or diphenoxylate or etorphine or fentanyl or heroin or hydrocodone or hydromorphone or levorphanol or meperidine or meptazinol or methadone or methadyl acetate or morphine or nalbuphine or oxycodone or oxymorphone or pentazocine or phenazocine or phenoperidine or pirinitramide or promedol or sufentanil or tilidine or tramadol)
-
(epidural) and (analges* or injection*)
-
(dexamethasone or hydrocortisone or prednisolone or methylprednisolone or prednisone or methylprednisone or triamcinolone)
-
(traction or stretch or weights)
-
(chemolysis or chemonucleolysis)
-
(discectomy or diskectomy or microdiscectomy or microdiskectomy or rhizotomy or sequestrectomy or vertebroplasty or nucleoplasty or laminectomy)
-
surgical decompression*
-
#22 OR #21 OR #20 OR #19 OR #18 OR #17 OR #16 OR #15 OR #14 OR #13 OR #12 OR #11 OR #10 OR #9 OR #8
-
#6 and #23
Web of Knowledge [all databases: Science Citation Index-Expanded and Social Sciences Citation Index, BIOSIS Previews (with Human Studies Restriction) and ISI Proceedings]. All time span to 16 June 2008
Searched on 16 June 2008. Search updated on 4 December 2009.
-
TS = (sciatic* or ischialg*)
-
TS = ((lumb* or sacra* or spin*) SAME (radicul*))
-
TS = ((sciatic nerve* or lumbar nerve* or spinal nerve* or sacral nerve*) SAME (irritation* or inflammation* or pain* or neuropath* or dysfunction* or compressio* or injur* or traum*))
-
TS = ((intervertebral disk or intervertebral disc or lumbar disc or lumbar disk or lumbosacral disc or lumbosacral disk or lumbo-sacral disc or lumbo-sacral disk) SAME (hernia* or slip* or prolaps* or degenerat* or fusion* or sclerosis* or rupture* or distortion* or fracture*))
-
TS = ((lumbosacral nerve root* or lumbo-sacral nerve root* or lumbar nerve root*) SAME (irritat* or inflammat* or pain* or neuropath* or dysfunction* or compressio* or injur* or traum*))
-
TS = ((refer* or radiat*) SAME (back or leg or foot))
-
#6 OR #5 OR #4 OR #3 OR #2 OR #1
-
TS = (bed rest* or activ* or exercise* or education* or instruction* or advice*)
-
TS = (trans?cutaneous electric nerve stimulation*)
-
TS = ((complement* or holistic* or herbal* or spina* or chiropract* or massage* or osteopath* or homeopath* or acupunctur* or musculo?skeletal or myofunctional or physio* or physical* or exercis* or heat* or hot* or thermal* or infra?red* or ultrasound* or ultrasonic* or short-wave*) SAME (therap* or treatm* or modalit* or manag* or manipulate* or medicin*))
-
TS = (orthotic device* or brace* or sling* or splint* or corset*)
-
TS = analgesia*
-
TS = ((non-steroidal anti inflammatory or non-steroidal anti-inflammatory or non?narcotic or opioid* or opiate*) SAME (drug* or analges* or agent*))
-
TS = (paracetamol or acetaminophen)
-
TS = (ibuprofen or aceclofenac or acemetacin or celecoxib or dexketoprofen or diclofenac sodium or etodolac or etoricoxib or fenbufen or fenoprofen or flurbiprofen or indometacin or indomethacin or ketoprofen or mefenamic acid or meloxicam or nabumetone or naproxen or piroxicam or sulindac or tenoxicam or tiaprofenic acid or azapropazone or biarison or acetaminophen or nimesulide or oxyphenbutazone or azapropazone or felbinac or alclofenac or nimesulid or etofenama or loxoprofen or phenylbutazone or valdecoxib or lornoxicam or etoricoxib)
-
TS = (buprenorphine or butorphanol or codeine or dextromoramide or dextropropoxyphene or dihydromorphine or diphenoxylate or etorphine or fentanyl or heroin or hydrocodone or hydromorphone or levorphanol or meperidine or meptazinol or methadone or methadyl acetate or morphine or nalbuphine or oxycodone or oxymorphone or pentazocine or phenazocine or phenoperidine or pirinitramide or promedol or sufentanil or tilidine or tramadol)
-
TS = ((epidural) SAME (analges* or injection*))
-
TS = (dexamethasone or hydrocortisone or prednisolone or methylprednisolone or prednisone or methylprednisone or triamcinolone)
-
TS = (orthopedic* or traction*)
-
TS = ((disc or disk) SAME (chemolysis or chemonucleolysis))
-
TS = (discectomy or diskectomy or microdiscectomy or microdiskectomy or rhizotomy or sequestrectomy or vertebroplasty or nucleoplasty or laminectomy)
-
TS = ((neuro* or surg*) SAME (decompression))
-
#22 OR #21 OR #20 OR #19 OR #18 OR #17 OR #16 OR #15 OR #14 OR #13 OR #12 OR #11 OR #10 OR #9 OR #8
-
#23 AND #7
Appendix 2 Quality assessment checklist
Quality assessment for effectiveness studies
Controlled trials and observational studies will be assessed using the following criteria, which are based on the checklist reported by van Tulder et al. 30 and a checklist developed by the EPHPP team. 31
The definition for selection bias used by EPHPP relates to the study sample not being representative of the target population. However, here we have used the term selection bias in relation to the systematic difference between the comparison groups at baseline.
External validity
External validity will be assessed according to the context of the study, within the country of origin.
Are the individuals selected to participate in the study likely to be representative of the target population?
In order to receive a YES, authors must have done everything reasonably possible to ensure that the target population is represented. The study will be scored PARTIAL if participants might not be representative, e.g. if they were referred from a specific source (a single GP practice, clinic, etc.) within a target population, even if it is in a systematic manner; or, included the patients of a single clinician/surgeon. The study will be scored NO if patients are self-referred (e.g. private clinic) or attending a clinic where care is provided by health-care professionals that are still training (e.g. physiotherapy or chiropractic clinics at a college/university). Clinics held at university hospitals or within tertiary care settings that also provide a service to the local community, i.e. receive routine referrals, will be scored PARTIAL.
What percentage of selected individuals agreed to participate?
The percentage of subjects in the control and intervention groups that agreed to participate in the study before they were assigned to intervention or control groups (80–100%, 60–79% and < 60%). This item will be graded as not applicable (NA) if the study was directed at a group of people in a specific geographical area, city, etc.
Were the staff, places and facilities where the patients were treated, representative of the treatment the majority of patients receive?
The reviewer will determine if this is adequate or if enough information is given in order to score YES. The study will be scored PARTIAL if the study only includes care provided by a single clinician/surgeon.
Selection bias and confounders
Study design
Studies will be categorised using the taxonomy reported by Deeks et al. 298 (which has been adapted from CRD report 427).
Was the method of randomisation adequate?
The method of random allocation is adequate, if the randomisation sequence allows each study participant to have an equal chance of receiving each intervention, e.g. computer-generated random numbers and random number tables. The method of random allocation is deemed inadequate (and scored NO), if it is not entirely transparent, e.g. the method of randomisation is described as alternation, case record numbers, dates of birth, day of the week. Studies that use serially numbered envelopes with no further information about how the random number sequence was generated, will be scored as UNCLEAR. Studies that just use the term ‘randomisation’ or ‘random allocation’ will be scored as UNCLEAR. Non-randomised studies would score NO.
Was the treatment allocation concealed?
In order to receive a YES, the person recruiting and assessing the eligibility of participants should have no information or influence on assignment of the intervention and they should not be able to predict allocation. Ideally, allocation should be remote or secure from all clinicians. Examples of adequate approaches include centralised or pharmacy-controlled randomisation and on-site computer-based systems with randomisation sequence that is not readable until allocation. The reviewer will score studies that use serially numbered identical containers, serially or sequentially numbered envelopes, or opaque sealed envelopes as PARTIAL. Examples of inadequate approaches include alteration, case record numbers, week days and open random number lists. Observational studies would score NO.
Indicate the percentage of relevant prognostic factors that were measured in both groups prior to the intervention
Relevant prognostic factors relate to demographic factors, socioeconomic factors, duration and severity of sciatica, psychological factors, previous treatments, past medical history, physical factors (e.g. SLR test) and value of main outcomes (80–100%, 60–79% and < 60%).
Were the groups similar at baseline for relevant prognostic factors?
The reviewer will determine if this is adequate, or if enough information is given, in order to score YES.
Were all participants recruited from same population (or appropriate alternative)?
The reviewer will determine if this is adequate, or if enough information is given, in order to score YES.
Were participants in both groups recruited over the same time (or similar point in their disease/illness/treatment?)
The reviewer will determine if this is adequate, or if enough information is given, in order to score YES.
Was an ANCOVA or similar method used to allow for possible baseline imbalance?
In order to receive a YES, the study should use a method of analysis that controls for possible baseline imbalance between groups. If differences between groups for important confounders have been controlled for in the design (stratification or matching), then the study should also be marked as YES. If randomisation was used, then studies must report that groups are balanced at baseline to score YES. If < 60% of important prognostic factors are reported, then the study is scored NO?
Were co-interventions avoided or similar?
In order to score YES, co-interventions should either be avoided in the trial design or similar between the intervention and control groups.
Detection bias
Accuracy of data collection tool.
(a) Were tools shown to be valid?
The item will receive a YES, if the tools are known or have been shown to measure what they were intended to measure and NO, if there was no attempt to show that the tools measured what they were intended to measure. Tools that are unreferenced are unlikely to been validated. Where the primary outcomes are reported, these are the outcome measures which will be used to assess this criterion.
(b) Were tools shown to be reliable?
The item will receive a YES, if the tools are known or have been shown to be consistent and accurate in measuring the outcome of interest (e.g. test–retest, Cronbach’s alpha, inter-rater reliability) and NO, if there was no attempt to show that the tools were consistent and accurate in measuring the outcome of interest. Tools that are unreferenced are unlikely to been tested for reliability.
Was the timing of outcome assessment in all groups similar?
In order to score YES, the timing of outcome assessment should be identical for all intervention groups and for all important outcomes assessments.
Were the outcome assessors blinded to the intervention or exposure status of participants?
The study will be scored YES if the assessors were described as blinded to which participants were in the control and intervention groups and NO, if the assessors were able to determine what group the participants were in. The study will be scored NA if the data were self-reported and collected by way of a survey, questionnaire or interview.
Were data analysts blinded to participants groups?
The study will be scored YES, if the analysts were described as being blind to which participants were in the control and intervention groups and NO, if the analysts were able to determine which group the participants were in.
(Studies that fail this last criterion will only receive a ‘moderate’ or ‘weak’ for performance bias.)
Performance bias
Were the participants blinded to the intervention?
The reviewer will need to determine if this is adequate or if enough information about the blinding is given in order to score YES. Studies marked as ‘double blind’ with no further information will be marked as PARTIAL.
Were the physicians blinded to participants groups?
The reviewer will need to determine if this is adequate or if enough information about the blinding is given in order to score YES. Studies marked as ‘double blind’ with no further information will be marked as PARTIAL.
Were there any attempts to test the efficacy of blinding procedures?
The reviewer will need to determine if this is adequate, or if enough information is given, in order to score YES.
Attrition bias
Were the characteristics of dropouts similar to those who remained in the study?
The reviewer will need to determine if this is adequate, or if enough information is given, in order to score YES. Studies with ≤ 5% dropouts will receive a YES.
Was there a differential dropout rate between the groups?
The reviewer will need to determine if this is adequate, or if enough information is given, in order to score YES.
Indicate the percentage of participants completing the study (if the percentage differs by groups, record the lowest)
The number of participants who were included in the study, but did not complete the observation period or were not included in the analysis, must be described and reasons given. If the percentage of withdrawals and dropouts does not exceed 20% for short-term follow-up (< 3 months) or medium-term follow-up (3–11 months) and 30% for long-term follow-up (≥ 12 months) and does not lead to substantial bias, a YES is scored (80–100%, 60–79% and < 60%). (Note these percentages are arbitrary, not supported by literature.)
Is the analysis performed according to intervention allocation status rather than actual intervention received?
The reviewer will need to determine if this is adequate, or if enough information is given, in order to score YES.
Did the analysis include all allocated patients irrespective of non-compliance?
The reviewer will need to determine if this is adequate, or if enough information is given, in order to score YES. Studies with ≤ 5% dropouts will receive a YES.
Items will be graded as either YES (+), NO (–), PARTIAL (±), UNCLEAR (or not enough information or not stated) and NA.
Quality assessment of economic evaluations
Economic evaluations
Cost-effectiveness studies were assessed using the following criteria, which is an updated version of the checklist developed by Drummond et al. 29
Study question
-
1. Costs and effects are examined.
-
2. Alternatives are compared.
-
3. The viewpoint(s)/perspective of the analysis is clearly stated (e.g. NHS, society).
Selection of alternatives
-
4. All relevant alternatives are compared (including ‘do nothing’ if applicable).
-
5. The alternatives being compared are clearly described (who did what, to whom, where and how often).
-
6. The rationale for choosing the alternative programmes or interventions compared is stated.
Form of evaluation
-
7. The choice of form of economic evaluation is justified in relation to the questions addressed.
-
8. If a cost minimisation design is chosen, equivalent outcomes are adequately demonstrated.
Effectiveness data
-
9. The source(s) of effectiveness estimates are stated (e.g. single study, selection of studies, systematic review, expert opinion).
-
10. Effectiveness data from an RCT or review of RCTs.
-
11. Potential biases identified (especially if data are not from an RCT).
-
12. Details of the method of synthesis or meta-analysis of estimates are given (if based on an overview of a number of effectiveness studies).
Costs
-
13. All the important and relevant resource use is included.
-
14. All the important and relevant resource use is measured accurately (with methodology).
-
15. Appropriate unit costs are estimated (with methodology).
-
16. Unit costs are reported separately from resource-use data.
-
17. Productivity costs are treated separately from other costs.
-
18. The year and country to which unit costs apply is stated with appropriate adjustments for inflation and/or currency conversion.
Benefit measurement and evaluation
-
19. The primary outcome measure(s) for the economic evaluation are clearly stated (cases detected, life-years, QALYs, etc.).
-
20. Methods to value health states and other benefits are stated (e.g. TTO).
-
21. Details of the individuals from whom valuations were obtained are given (patients/members of the public/health-care professionals).
Decision modelling
-
22. Details of any decision model used are given (e.g. decision tree, Markov model).
-
23. The choice of model used and the key input parameters on which it is based are adequately detailed and justified.
-
24. All model outputs are described adequately.
Discounting
-
25. A discount rate is used for both costs and benefits.
-
26. The discount rates accord with NHS guidelines (3.5% for costs and benefits and adjusted to 0% and 6% in sensitivity analysis).
Allowance for uncertainty
Stochastic analysis of patient-level data
-
27. Details of statistical tests and CIs are given for stochastic data.
-
28. Uncertainty around cost-effectiveness is expressed (e.g. CIs around ICER, cost-effectiveness acceptability curves).
-
29. Sensitivity analysis is used to assess uncertainty in non-stochastic variables (e.g. unit costs, discount rates) and analytic decisions (e.g. methods to handle missing data).
Stochastic analysis of decision models
-
30. All appropriate input parameters are included with uncertainty.
-
31. Second-order uncertainty (uncertainty in means) is included rather than first-order uncertainty (uncertainty between patients).
-
32. Probability distributions are adequately detailed and appropriate.
-
33. Sensitivity analysis is used to assess uncertainty in non-stochastic variables (e.g. unit costs, discount rates) and analytic decisions (e.g. methods to handle missing data).
Deterministic analysis
-
34. The approach to sensitivity analysis is given (e.g. univariate, threshold analysis).
-
35. The choice of variables for sensitivity analysis is justified.
-
36. The ranges over which the variables are varied are stated.
Presentation of results
-
37. Incremental analysis is reported using appropriate decision rules.
-
38. Major outcomes are presented in a disaggregated as well as an aggregated form.
-
39. Applicable to the NHS setting.
Items will be graded as either YES (+ item adequately addressed), NO (– item not adequately addressed), PARTIAL (±), UNCLEAR (or not enough information or not stated) and NA.
Studies that incorporated a decision-analytic model were further evaluated using the following criteria based on work of Weinstein et al. 31
Decision context
-
40. Is there a full description of the decision question, its context and the process by which this was identified?
-
41. Do the model structure and parameters adequately represent the key decision options and perspective?
-
42. Do the treatment options cover those of immediate interest to the decision-maker?
-
43. Are there additional treatment options likely to be of interest in other decision and clinical contexts?
-
44. Is the model structure easily adaptable to include future developments?
Health states and clinical outcomes
-
45. Does the model structure fit (appropriate and relevant) with the clinical theory of the disease process?
-
46. Does the model appropriately capture the full impact and cost of treatments?
-
47. Does the model appropriately represent the patient population(s) of concern?
-
48. How has heterogeneity been included in the model?
-
49. Were appropriate methods used to include patients’ treatment and disease history and effects on event rates?
-
50. Does the model clearly list and justify structural assumptions, and likely impacts on outcomes?
-
51. How were structural aspects tested by the modeller (e.g. clinical opinion, literature review, clinical guidelines)?
-
52. Was the modelling methodology fully justified (e.g. Markov, decision tree, discrete stimulation)?
Transparency
-
53. Is the model structure transparent (structure, parameters and values)?
-
54. Is the physical model fully accessible to a non-modelling audience?
Timing
-
55. Are the time horizons appropriate, given the disease, treatments and decision context (1 year, 10 years, lifetime)?
-
56. Are the model’s cycle times appropriate to the disease and treatments of interest?
-
57. Have appropriate methods been used to extrapolate data over extended time horizons?
Data values
-
58. Is there a full description of a thorough review process identifying data values?
-
59. Are the sources of data values fully described and appropriate?
-
60. Are there clear criteria for data inclusion/exclusion?
-
61. Are there appropriately documented value ranges for data parameters for sensitivity analysis?
-
62. Is there clear identification of areas in the model populated with clinical opinion? Is the approach appropriate?
Data preparation
-
63. Are there full details on data preparation to generate parameter values (e.g. meta-analysis, relative risk rates, estimation of utility, calculation of transition rates)?
-
64. Were transition rates correctly calculated from interval data?
-
65. Were survival data appropriately extrapolated/modelled (e.g. Weibull, exponential)?
-
66. Are sensitivity analyses adequately handled and classified (e.g. probabilistic, one way, multiway)?
Data incorporation
-
67. Are data units, time intervals and patient characteristics consistent?
-
68. Was uncertainty adequately incorporated in the model using appropriate sensitivity structures and analyses?
Internal validation
-
69. Was there a thorough and adequate quality control/error checking test plan?
-
70. Was the model replicated and compared using alternative software?
-
71. Was there a clinical face value reality check? How was this conducted (e.g. internal review, expert review)?
-
72. Was the model shown to accurately replicate data used in model construction?
Cross-model validation
-
73. Was the model directly compared and contrasted with existing models in the same disease area?
-
74. Were differences between models appropriately discussed, categorised and acted on?
External validation
-
75. Was the model validated against independent data?
-
76. Were data suitable in terms of its context for comparison (patient group, treatments, timelines, outcomes)?
-
77. Which interim outputs were matched?
Items will be graded as either YES (+ item adequately addressed), NO (– item not adequately addressed), PARTIAL (±), UNCLEAR (or not enough information or not stated) and NA.
Appendix 3 Winbugs code used for the mixed treatment comparison analyses
Global effect
Pain
CSOMs
Appendix 4 Ongoing or unpublished studies identified from search of trial registries
| No. | Trial number/trial registry | Author/contact and funding body | Aims/objectives | Study design | Study population/target sample size (n) | Intervention | Comparison | Outcomes | Recruitment status/estimated completion date |
|---|---|---|---|---|---|---|---|---|---|
| 1 | ACTRN12606000456550/Australian New Zealand Clinical Trials Registry |
Guy Ludbrook Metabolic Pharmaceuticals Ltd |
To examine the efficacy of ACV1 [(conotoxin VC1.1) neuronal nicotinic receptor antagonist] to relieve sciatic pain | RCT (crossover) | Patients with moderate-to-severe spontaneous neuropathic pain radiating down both buttocks or legs for ≥ 3 months (n = 40) | Subcutaneous injections of 0.4 mg/kg ACV1 once daily for 7 days | Placebo | Safety and tolerability of ACV1 | Completed |
| 2 | ACTRN12608000401358/Australian New Zealand Clinical Trials Registry |
Nikolai Bogduk Charities/societies/foundations |
To test the efficacy of transforaminal epidural injection for relieving pain and restoring function | RCT | Potential patients identified by neurosurgeons who have radicular pain and disc herniation demonstrated on computerised tomography (CT) or MRI (n = 240) | Transforaminal ESI under fluoroscopic guidance | Placebo | Percentage relief of pain. Restoration of function | Open to recruitment |
| 3 | ACTRN12609000205235/Australian New Zealand Clinical Trials Registry |
Nicholas Taylor LifeCare Health (a division of Health Networks Australia Ltd) |
To compare the outcomes and adverse events of two different physiotherapy treatment approaches | RCT | Patients with clinical and radiological confirmation of lumbar disc herniation with associated radiculopathy for a duration of 6 weeks to 6 months n = 150) | Ten sessions of physiotherapy functional restoration | Two sessions of physiotherapy advice over a 10-week period |
Back-specific function and leg pain intensity QoL: rate and nature of adverse events Participant satisfaction |
Open to recruitment |
| 4 | ACTRN12609000207213/Australian New Zealand Clinical Trials Registry |
Guy Ludbrook Bioassets Development Corporation CMAX – a division of IDT Australia Limited |
To evaluate the safety of three different doses of etanercept versus placebo when administered epidurally | RCT | Healthy males or females with primary diagnosis of sciatica of between 6 weeks’ and 26 week’s duration (n = 40) | Three dose levels of etanercept (0.5 mg, 2.5 mg or 12.5 mg), to be administered twice, 2 weeks apart via the epidural route | 2 ml of normal saline, administered twice, 2 weeks apart, via the epidural route | Pain reduction | Open to recruitment |
| 5 | ACTRN12609000334202/Australian New Zealand Clinical Trials Registry |
Jon Ford LifeCare Health (a division of Health Networks Australia Ltd) |
To compare the outcomes and adverse events of two different physiotherapy treatment approaches | RCT | Patients with low back pain with or without leg pain with a 6 weeks to 6 months duration (n = 150) | Ten 30-minute sessions of physiotherapy over a 10-week period | Two 30-minute sessions of physiotherapy advice over a 10-week period |
Back-specific function and leg pain intensity QoL: rate and nature of adverse events Participant satisfaction |
Open to recruitment |
| 6 | ACTRN12609000343202/Australian New Zealand Clinical Trials Registry |
Jon Ford LifeCare Health (a division of Health Networks Australia Ltd) |
To compare the outcomes and adverse events of two different physiotherapy treatment approaches | RCT | People with reducible low back pain (with or without associated leg pain) with a directional preference for extension with or without a lateral component, for duration of 6 weeks to 6 months (n = 124) | Ten sessions of specific physiotherapy management over 10 weeks, involving 30-minute sessions | Two sessions of physiotherapy advice over a 10-week period |
Back-specific function and leg pain intensity QoL: rate and nature of adverse events Participant satisfaction |
Open to recruitment |
| 7 | ACTRN12609000412235/Australian New Zealand Clinical Trials Registry |
Megan Davidson LifeCare Health (a division of Health Networks Australia Ltd) |
To compare the outcomes and adverse events of two different physiotherapy treatment approaches | RCT | Patients with lumbar non-reducible discogenic lower back pain with or without associated leg pain, pins and needles, or numbness for a duration of 6 weeks to 6 months (n = 150) | Ten sessions (30 minutes) of physiotherapy functional restoration over a 10-week period | Two sessions (30 minutes) of physiotherapy advice over a 10-week period |
Back-specific function and leg pain intensity QoL: rate and nature of adverse events Participant satisfaction |
Open to recruitment |
| 8 | ACTRN12609000834257/Australian New Zealand Clinical Trials Registry |
Jon Ford LifeCare Health (a division of Health Networks Australia Ltd) |
To compare the outcomes and adverse events of two different physiotherapy treatment approaches | RCT | People with low back pain with or without sciatica for duration of 6 weeks to 6 months (n = 200) | Ten sessions (each session 30 minutes, one-on-one) of specific physiotherapy over a 10-week period | Two sessions (each session 30 minutes, one-on-one) of physiotherapy advice over a 10-week period |
Back-specific function and leg pain intensity QoL: rate and nature of adverse events Participant satisfaction |
Open to recruitment |
| 9 | ChiCTR-TRC-09000604/Chinese Clinical Trials Registry |
Rixin Chen Affiliated hospital of Jiangxi University of Traditional Chinese Medicines |
Not stated | RCT | n = 456 | Heat-sensitive point suspension moxibustion |
Non-heat-sensitive point suspension moxibustion Western medicine |
Pain reduction | Recruiting |
| 10 | ChiCTR-TRC-09000695/Chinese Clinical Trials Registry |
Chan, Simon Kin Cheong Department of Anaesthesia and Intensive Care, Chinese University of Hong Kong |
Not stated | RCT | Adult patients interested in participating with sciatica and radiological evidence of disc degeneration (n = 100) | Pulsed radiofrequency treatment for a duration of 6 weeks | Spinal nerve root steroid injection | Pain reduction | Not yet recruiting |
| 11 | DRKS00000092/German Clinical Trials Register |
Christian-Andreas Müller Institutional budget, no external funding |
To evaluate the influence of the microsurgical versus minimal invasive (metrix) approaches on clinical results following lumbar disc herniation surgery | RCT | Patients with mono-segmental radicular compression syndrome due to lumbar disc herniation, indicated to be treated surgically (n = 250) | Conventional approach for lumbar disc herniation surgery Intervention | Minimal invasive (metrix) approach for lumbar disc herniation surgery | Reduction of postoperative low back and sciatic pain | Recruiting planned |
| 12 | IRCT138809251766N2/IRCT |
Karim Nasseri Vice chancellor for research, Kurdistan University of Medical Sciences |
To compare the effect of ESI on pain relief | RCT | Patients with history of intervertebral disc herniation with or without spinal stenosis confirmed with MRI (n = 50) | ESI | Placebo | Pain relief | Complete |
| 13 | ISRCTN12574253/ISRCTN |
Trond Iversen Health North RHF (Regional Health Authority, Norway) |
To test the efficacy of epidural sacral injection with triamcinolone for relieving radiculopathy pain | RCT | Patients with sciatic pain > 12 weeks and radiculopathy L3-S1 (n = 240) | Epidural sacral injection with triamcinolone | Epidural sacral injection saline | ODI | Completed/not recruiting |
| 14 | ISRCTN14206374/ISRCTN |
Bart Depreitere Medtronic B.V. and Stryker Nederland |
To test the hypothesis that transmuscular surgical approach causes less damage to postoperative posture control and early postoperative physiotherapy improves short- and long-term posture control | RCT | One level disc herniation, either L4-L5 or L5–S1, explaining symptoms and representing operative indication (n = 100) |
Transmuscular surgical approach Early postoperative physiotherapy |
Classic paramedian approach ‘conservative’ treatment | Muscle control (postural balance test and sit-to-stand test), assessed in the laboratory | Completed/not recruiting |
| 15 | ISRCTN94584126 |
Maziar Badii WorkSafeBC – Workers Compensation Board of British Columbia Research Secretariat |
To test the hypothesis that fluoroscopically guided transforaminal ESI (TFESI) into the immediate vicinity of the affected nerve root is associated with (1) improvement in pain and functional status and (2) reduction in rate of progression to surgery | RCT | Patients with pain in a single lower extremity below the level of the knee of < 18 weeks duration (n = 88) | 1.0 cc Celestone (Bayer Shering Parma, Berlin, Germany) (40 mg/ml) plus 1.0 cc of 0.5% bupivicaine (treatment) | 1.0 cc sterile saline plus 1.0 cc of 0.5% bupivicaine (control) | Leg pain relief Modified RMDQ | Completed/not recruiting |
| 16 | NCT00009672/ClinicalTrials.gov | National Institute of Dental and Craniofacial Research (NIDCR) | To test the effectiveness of two drugs – nortriptyline and MS contin (a type of morphine) – to treat pain caused by lumbar radiculopathy or sciatica | RCT | Patients between 18 and 65 years of age who have had sciatica pain daily for ≥ 3 months (n = 80) | Nortriptyline morphine nortriptyline plus morphine | Active placebo, benztropine. An inert placebo | Daily overall pain relief | Completed/December 2006 |
| 17 | NCT00107055/ClinicalTrials.gov |
Randall Moreadith Renovis Inc. |
To gain initial safety and efficacy data on the experimental agent REN-1654 in patients with pain that radiates down the leg(s), and is typical of sciatica (lumbosacral radiculopathy) | RCT | Subjects with leg pain radiating to or below the knee in a dermatomal pattern, diagnosed as being due to sciatica or lumbar or lumbosacral radiculopathy, the onset of which occurred 2–12 weeks prior to initiation of study treatment (n = 72) | REN-1654 | Placebo | Leg pain | Active, not recruiting |
| 18 | NCT00159705/ClinicalTrials.gov |
PfizerCT.gov call centre Pfizer |
To test the efficacy and safety of pregabalin in the treatment of neuropathic pain associated with lumbo-sacral radiculopathy | RCT | Patient having pain consistent with a diagnosis of chronic lumbosacral radiculopathy due to spinal stenosis or disc herniation and present for ≥ 3 months (n = 276) | Pregabalin | Placebo | Pain measurement | Completed |
| 19 | NCT00163553/ClinicalTrials.gov |
Dean Cowie Austin Health |
To test the hypothesis that epidural pethidine is an effective form of pain relief following lumbar spinal surgery, resulting in significantly lower usage of concomitantly administered (intravenous) patient-controlled analgesia (PCA) pethidine | RCT | Adults ≥ 18 years, undergoing lumbar spinal surgery (n = 60) | Epidural pethidine | Placebo | Cumulative 24-hour pethidine consumption | Recruiting |
| 20 | NCT00220935/ClinicalTrials.gov |
John Triano Texas Back Institute |
To evaluate the results of using the Orthotrac Pneumatic Vest vs an EZ form brace in patients with radiating leg pain from disc bulge/protrusion/herniation | RCT | Patients aged 21–55 years with no history of prior surgery or spine surgery in previous 6 months, having symptoms for > 4 weeks and MRI confirmed disc bulge/protrusion/herniation (HNP) site consistent with symptoms with no neurological deficit (n = 150) | Orthotrac Pneumatic Vest | EZ form brace | VAS, ODI and SF-36 | Completed |
| 21 | NCT00300898/ClinicalTrials.gov |
Leonardo Kapural Cleveland Clinic |
To learn which of three minimally invasive procedures [nucleoplasty, percutaneous decompression and intervertebral electrothermal disc decompression (IDET)] is the most effective for treatment of contained lumbar disc herniation | RCT | Patients with a history of concordant radicular leg pain unresponsive to conservative treatment for > 3 months; leg pain must be greater than back pain; contained disc herniation as evidenced by MRI; and no evidence of psychological issues by exam or history (n = 72) |
Nucleoplasty Percutaneous decompression Intervertebral electrothermal disc decompression (IDET) |
Behavioural: conservative treatment with oral medications, PT, ESIs | Pain intensity using VAS | Not yet recruiting |
| 22 | NCT00384007/ClinicalTrials.gov | Pinnacle Pain Medicine/HydroCision Inc. | To compare a standard surgical procedure, open surgical microdiscectomy, used primarily to relieve leg pain and repair disc herniation with a newer surgical procedure, hydrodiscectomy with Spinejet® | RCT | Patients aged 18–75 years who have had posterolateral single lumbar contained disc herniation, any level, up to one-third of spinal canal sagittal diameter, concordant radicular pain ± back pain, and MRI confirmed nerve root contact/compression; failed trial of at least one NSAID within 6 months; failed at least 2 weeks’ PT within 6 months; failed at least two ESIs, no less than 2 weeks apart within 6 months; and, initial or recurrent episode of radiculitis (n = 58) | Hydrodiscectomy with Spinejet | Open surgical microdiscectomy | Pain reduction | Completed |
| 23 | NCT00385086/ClinicalTrials.gov |
Francois Rannou Assistance Publique – Hôpitaux de Paris |
To test the efficacy of TNF-α inhibition in sciatica with postoperative lumbar spinal fibrosis | RCT | Patients > 18 years old who had postdiscectomy sciatica; pain with VAS > 40 mm and impossibility to have their usual activity; surgical discectomy (< 2 years and > 6 months); no pain of > 1 month and < 1 year after the discectomy; MRI with gadolinium injection of < 6 months and done > 6 months after the discectomy; presence of spinal fibrosis on MRI (hyposignal in T1 enhanced by gadolinium and hypersignal in T2); and failure of epidural injection treatment (n = 40) | TNF blocker | Placebo | Sciatica pain | Recruiting |
| 24 | NCT00444405/ClinicalTrials.gov |
Alan Scarrow, Layla Stanek, Pete Miles St. John’s Health System |
To compare patients who underwent decompression/discectomy with pedicle screw fusion to patients who received decompression/discectomy without fusion | Observational | Male or female patients, 18–75 years old who had recurrent lumbar disc herniation by MRI or CT with history of decompression at the same level in the past; recurrent symptomatic history (with or without back pain) with radicular leg pain that improved following the first surgery; flexion and extension radiography that demonstrate an absence of sponylolisthesis or spondylolisthesis with < 3 mm of movement (n = 50) | Repeat lumbar disc decompression/discectomy | Repeat decompression/discectomy with pedicle screw fusion | Patient-reported pain, physical function and satisfaction | Recruiting |
| 25 | NCT00470509/ClinicalTrials.gov |
Stéphane Genevay University Hospital, Geneva |
To determine whether adalimumab (a TNF-α inhibitor) is effective in the treatment of severe and acute sciatica | RCT | Male or female patients, ≥ 18 years old who had episode of radicular pain in one lower limb for < 12 weeks; medical evaluation requiring hospitalisation because of pain or functional handicap; a characteristic leg pain in the L3, L4, L5 or S1 territories; a confirmed herniated disc on usual imaging techniques (CT scan or MRI) in the vicinity of the clinically involved nerve root (n = 61) | Adalimumab | Placebo | Leg pain using VAS | Completed |
| 26 | NCT00516009/ClinicalTrials.gov |
Radi Shahien Ziv Hospital |
To evaluate the efficacy of intravenous dexamethasone for acute disc herniation-induced sciatica | RCT | Patients aged ≥ 18 years or presented with acute radicular pain for < 6 weeks (n = 40) | Intravenous dexamethasone | Placebo | Pain | Recruiting |
| 27 | NCT00573807/ClinicalTrials.gov |
Ken Nedd GAAD Medical Research Institute Inc. |
To determine the use of far infrared radiation for the treatment of back pain and sciatica | Observational | Persons with lower back pain and sciatica (n = 2) | Radiation: far infrared radiation (5–20 µm wavelength) | Not stated | Sciatica pain | Active, not recruiting |
| 28 | NCT00634946/ClinicalTrials.gov | Seikagaku Corporation | To determine whether or not SI-6603 is effective in the treatment of lumbar disc herniation | RCT | Patients: with lumbar disc herniation (L4-L5 or L5–S1) as assessed by MRI and clinical symptoms corresponding to position of the impaired nerve root; assessed as positive in the SLR test; with sciatica in either lower leg; with no improvement from pharmacotherapy or concomitant treatment with drug and nerve block (n = 195) | SI-6603 [(condoliase) chondroitinase] | Placebo | Changes in leg pain from baseline | Active, not recruiting |
| 29 | NCT00668434/ClinicalTrials.gov |
Harley Goldberg, Andrew Avins, William Firtch National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) |
To determine the effectiveness of the steroid prednisone in decreasing pain and improving function in people with sciatica | RCT | Patients with complaints of low back pain and functionally incapacitating leg pain extending below the knee with a nerve root distribution; nerve root tension signs with or without neurologic abnormalities, fitting the level of the radiculopathy; score of at least 20 on the modified ODI; MRI confirmation of herniated lumbar disc consistent with the signs and symptoms (n = 220) | Oral prednisone | Placebo | Changes in physical functioning and pain | Recruiting |
| 30 | NCT00681447/ClinicalTrials.gov |
Laxmaiah Manchikanti Pain Management Center of Paducah |
To demonstrate clinically significant improvements in patients undergoing lumbar interlaminar epidurals. Improvement will be assessed in relation to the clinical outcome measures of pain and function and to evaluate and compare the adverse event profile in all patients | RCT | Subjects of ≥ 18 years of age with a history of chronic, function-limiting chronic low back pain ≥ 6 months in duration and who have not had recent surgical procedures within the last 3 months (n = 180) | Lumbar interlaminar epidural | Lumbar interlaminar epidural | Pain improvement | Enrolling by invitation |
| 31 | NCT00733096/ClinicalTrials.gov |
Steven Cohen, Connie Kurihara Johns Hopkins University |
To determine the efficacy of transforaminal epidural corticosteroids and transforaminal epidural etanercept, in patients with lumbosacral radiculopathy | RCT | Patients who are 19–70 years old with chronic low back pain of radicular origin of > 4 weeks’, but < 6 months’ duration; leg pain greater than back pain; failure of conservative therapy to include physical and pharmacotherapy; and, MRI evidence of a lateral or paracentral herniated disc corresponding to the patient’s radicular symptoms (n = 78) | Etanercept methylprednisolone | Normal saline | Numerical rating pain score | Recruiting |
| 32 | NCT00749996/ClinicalTrials.gov |
Ferdinand Krappel Medtronic Spinal and Biologics |
To assess the short- and long-term effectiveness and patient perception of benefit with the use of a DIAM™ Spinal Stabilization System in the treatment of complex disc disease at a single level from L2 to L5 | RCT | n = 288 | Single-level herniectomy followed by placement of the DIAM™ Spinal Stabilization System | Single-level herniectomy | Back-pain score on VAS | Recruiting |
| 33 | NCT00894972/ClinicalTrials.gov |
Julie Fritz University of Utah |
To determine the most effective PT programme for individuals who have undergone lumbar discectomy | RCT | Patients 18–60 years old who had a diagnosis of lumbar disc herniation based on imaging (MRI or CT scan) of the lumbar spine with concurring clinical examination findings (based on the judgment of the attending neurosurgeon) and are appropriate surgical candidate based on the opinion of the attending spine surgeon, and scheduled for single-level lumbar discectomy (open or microdiscectomy) (n = 70) | General plus specific strengthening rehabilitation following lumbar disc surgery (discectomy) | General strengthening rehabilitation following lumbar disc surgery (discectomy) | Modified ODI | Recruiting |
| 34 | NCT00934284/ClinicalTrials.gov |
Julie Fritz University of Utah |
To determine if participation in PT in conjunction with a selective nerve root block in the lumbar spine is more effective than just receiving the injection alone for patients with low back and leg pain from a disc herniation (sciatica) | Patients with MRI evidence of disc herniation in the lumbar spine consistent with clinical presentation; and pain and/or paresthesia in the lumbar spine and a distribution extending distal to the gluteal fold within 24 hours of enrolment (n = 44) | Injection plus PT | Injection only | Modified ODI, Global Rating of Change | Completed | |
| 35 | NCT00942227/ClinicalTrials.gov |
Julie Fritz Intermountain Health Care Inc., University of Utah |
To determine the effectiveness of adding mechanical traction to standard PT treatments for patients with low back pain | RCT | Patients aged at ≥ 18 years and < 60 years with a chief complaint of pain and/or paresthesia in the lumbar spine with a distribution of symptoms that has extended distal to the gluteal fold on at least one lower extremity within the past 24 hours based on the patient’s self-report and ODI score of at least 20% (n = 120) | Mechanical traction plus standard PT | Standard PT alone | ODI, Global Rating of Change | Recruiting |
| 36 | NCT00961766/ClinicalTrials.gov | Biogen Idec | To evaluate the safety, tolerability and pharmacokinetics of BG00010 after intravenous administration to sciatica subjects | RCT | Patients aged 18–70 years, with a diagnosis of unilateral sciatica, determined by the investigator. Sciatica symptoms must be present for ≥ 6 weeks prior to the screening visit (n = 56) | BG00010 [(Neublastin) glial cell line-derived neurotrophic factor] | Placebo |
Clinical laboratory values, antibodies, pharmacokinetics and adverse events Subject assessments (Likert scale, VAS, quantitative sensation testing and intraepidermal nerve fibre density) |
Recruiting |
| 37 | NCT01041391/ClinicalTrials.gov |
William Lavelle State University of New York – Upstate Medical University |
To examine outcome measurements on patients who undergo surgery to removed a damage lumbar spine disc vs those that chose not to have surgery | Observational model: case–control | Patients 18–90 years of age with a diagnosis of lumbar disc herniation (n = 200) | Surgical treatment for lumbar disc herniation | Non-surgical treatment for lumbar disc herniation | SF-36, VAS and ODI | Recruiting |
| 38 | NCT01052571/ClinicalTrials.gov |
Laxmaiah Manchikanti, Trisha Burks Pain Management Center of Paducah |
To evaluate differences in outcomes in patients receiving steroids compared with those patients randomised to the local anaesthetic group who did not receive steroids and to evaluate and compare the adverse event profile in all patients | RCT | Patients who are 18 years of age with disc herniation or radiculitis and with a history of chronic function-limiting low back and lower extremity pain of at ≥ 6 months' duration (n = 120) | Lumbar transforaminal epidural injections | Lumbar transforaminal epidural injections | Numeric rating scale, ODI, duration of significant pain relief | Not yet recruiting |
| 39 | NCT01073995/ClinicalTrials.gov |
Neil Manson, Melissa McKeon Saint John Regional Hospital |
To find alternative treatments for lumbar disc herniations other than surgery | RCT | Patients aged 18–65 years diagnosed with lower extremity radiculopathy (sciatica) secondary to a lumbar disc herniation who had failed non-operative measures, medication, modification of daily activities and physiotherapy (n = 100) | Kenalog and sensorcaine | Placebo | Surgical avoidance | Recruiting/March 2014 |
| 40 | NCT01110057/ClinicalTrials.gov |
GlaxoSmithKline GSK Clinical Trials |
To evaluate the safety and efficacy of the p38 kinase inhibitor, GW856553, in subjects with neuropathic pain from lumbosacral radiculopathy | RCT | Subjects aged 18–80 years inclusive with a diagnosis of neuropathic pain due to lumbosacral radiculopathy (n = 142) | GW856553 [(losmapimod) P38 kinase inhibitor] | Placebo | Pain intensity | Recruiting/August 2010 |
| 41 | NCT01117870/ClinicalTrials.gov |
Harsha Shanthanna, Philip Chan McMaster University |
To assess the efficacy of pulsed radiofrequency for chronic lumbar radicular pain and to assess whether a larger scale clinical study with the same methods can be used | RCT | Patients > 18 years of age with chronic lumbar radiculopathy for at least ≥ 4 months, with concordant findings on either MRI and CT scan and a VAS score of at least 60/100 at presentation (n = 32) | Pulsed radio-frequency | Placebo |
Feasibility of doing a larger scale trial Improvement in ODI Decrease in the analgesic medications used |
Not yet recruiting/August 2011 |
| 42 | NTR342/Nederlands Trial Register |
A. Spijker-Huiges University Medical Center Groningen (UMCG), Department of General Practice |
To test the hypothesis that adding segmental steroid injections to usual care in the treatment of acute lumbosacral radicular syndrome will reduce pain and fasten recovery in general practice | RCT | Patients aged between 18 and 60 years who underwent usual medical care for lumbosacral radicular syndrome with insufficient response in 1–2 weeks of treatment (n = 80) | Care as usual, combined with one or two segmental epidural corticosteroid injections | Care as usual | Pain in back and/or leg, while walking, standing, lying down and night pain using a numerical rating scale (0–10) | Pending |
Appendix 5 Quality assessment of included clinical effectiveness studies
Quality assessment of included clinical effectiveness studies
| Type of bias | Abbreviation | Quality assessment question |
|---|---|---|
| External validity | EV1 | Are the individuals selected to participate in the study likely to be representative of the target population? |
| EV2 | What percentage of selected individuals agreed to participate? | |
| EV3 | Were staff, places and facilities where the patients were treated, representative of the treatment the majority of patients recorded? | |
| EVR | Overall external validity rating | |
| Selection bias | SB1 | Study design |
| SB2 | Was the method of randomisation adequate? | |
| SB3 | Was the treatment allocation concealed? | |
| SB4 | Indicate the percentage of relevant prognostic factors that were measured in both groups prior to intervention | |
| SB5 | Were the groups similar at baseline for relevant prognostic factors? | |
| SB6 | Were all participants recruited form same population? | |
| SB7 | Were participants in both groups recruited over the same time (or similar point in their disease/illness/treatment)? | |
| SB8 | Was an ANCOVA or similar method used to allow for possible baseline imbalance? | |
| SB9 | Were co-interventions avoided or similar? | |
| SBR | Selection bias confounded | |
| Detection bias | DB1a | Accuracy of data collection tool: (a) were tools shown to be valid? |
| DB1b | Accuracy of data collection tool: (b) were tools shown to be reliable? | |
| DB2 | Was the timing of outcome assessment in all groups similar? | |
| DB3 | Were the outcome assessors blinded to the intervention or exposure status of participants? | |
| DB4 | Were data analysts blinded to participant groups? | |
| DBR | Detection bias rating | |
| Performance bias | PB1 | Were the participants blinded to the intervention? |
| PB2 | Were the physicians blind to the participants groups? | |
| PB3 | Were there any attempts to test the efficiency of blinding the procedures? | |
| PBR | Overall performance bias rating | |
| Attrition bias | AB1 | Were the characteristics of dropouts similar to those who remained in the study? |
| AB1a | Was there a different dropout rate between the groups? | |
| AB2 | Indicate the percentage of participates completing the study (if the percentage differs by groups, record the lowest) | |
| AB3 | Is the analysis performed according to intervention allocation status rather than actual intervention received? | |
| AB4 | Did the analysis include all the allocated patients irrespective of non-compliance? | |
| ABR | Overall attrition bias rating | |
| Global rating | GR | Global rating |
Disc surgery (including intraoperative interventions)
| Study | ID no. | EV1 | EV2 (%) | EV3 | EVR | SB1 | SB2 | SB3 | SB4 (%) | SB5 | SB6 | SB7 | SB8 | SB9 | SBR | DB1a | DB1b | DB2 | DB3 | DB4 | DBR | PB1 | PB2 | PB3 | PBR | AB1 | AB1a | AB2 (%) | AB3 | AB4 | ABR | GR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disc surgery vs active PT/exercise therapy | ||||||||||||||||||||||||||||||||
| Osterman, 200668 | 300 | ? | NR | ± | W | RCT | + | + | 80–100 | + | + | + | + | ± | S | + | + | + | NA | ? | M | – | – | NA | W | ? | – | 80–100 | + | + | S | M |
| Disc surgery vs chemonucleolysis | ||||||||||||||||||||||||||||||||
| Krugluger, 200046 | 35 | ? | NR | ? | W | RCT | ? | ? | < 60 | ? | + | + | ? | ? | W | + | + | + | ? | ? | M | – | – | NA | W | + | – | 80–100 | ? | ? | W | W |
| van Alphen, 198947 | 43 | + | 60–79 | + | S | RCT | ± | ? | < 60 | ? | + | + | - | ? | M | – | – | + | – | ? | W | – | – | NA | W | + | – | 80–100 | + | + | S | M |
| Javid, 199548 | 44 | ± | NR | ± | M | CCS | – | – | 60–79 | ± | – | + | – | ? | W | – | – | + | – | ? | W | – | – | NA | W | + | – | 80–100 | + | + | S | W |
| Postacchini, 198649 | 45 | ± | NR | ± | M | Non-RCT | – | – | < 60 | ? | – | ± | – | – | W | – | – | + | – | ? | W | – | – | NA | W | ? | – | 80–100 | – | – | W | W |
| Norton, 198650 | 47 | – | NR | ± | W | CCS | – | – | < 60 | – | – | + | – | ? | W | – | – | ? | ? | ? | W | – | – | NA | W | NA | NA | NA | NA | NA | NA | W |
| Dabezies, 197851 | 48 | ± | NR | ± | M | CCS | – | – | < 60 | ? | – | ? | – | ? | W | – | – | + | – | – | W | – | – | NA | W | ? | ? | Cannot tell | + | ? | W | W |
| Stula, 199052 (German language) | 49 | ? | NR | ± | W | RCT | ? | ? | < 60 | ? | + | + | – | ? | M | – | – | ? | ? | ? | W | – | – | NA | W | + | – | 80–100 | – | + | W | W |
| Tregonning, 199153 | 61 | ± | NA | ± | M | HCS | – | – | < 60 | ? | – | ± | – | ? | W | + | + | + | – | ? | M | – | – | NA | W | ? | – | 80–100 | + | – | M | W |
| Lagarrigue, 199154 (French language) | 117 | ± | 60–79 | ± | M | CCS | – | – | < 60 | – | – | + | – | – | W | + | + | ± | ? | ? | W | – | – | NA | W | + | – | 80–100 | + | + | S | W |
| Lavignolle, 198755 (French language) | 129 | ? | NR | ± | W | RCT | ? | ? | < 60 | ? | + | + | – | ? | W | ± | ± | ± | ? | ? | W | – | – | NA | W | + | – | 80–100 | + | + | S | W |
| Hoogmartens, 197656 | 132 | ± | NR | ± | M | HCS | – | – | < 60 | ? | – | – | – | ? | W | – | – | – | NA | ? | W | – | – | NA | W | NA | NA | NA | NA | NA | NA | W |
| Zeiger, 198758 | 150 | ? | NR | ? | W | CCS | – | – | < 60 | ? | ? | ? | ? | ? | W | – | – | + | + | ? | W | – | – | – | W | NA | ? | NA | + | ? | W | W |
| Watts, 197559 | 160 | ? | NR | ± | W | CCS | – | – | < 60 | ? | – | + | – | ? | W | – | – | + | ? | ? | W | – | – | NA | W | + | – | 80–100 | + | + | S | W |
| Crawshaw, 198460 | 166 | ± | NR | ± | M | RCT | ? | ? | < 60 | ? | + | + | – | ? | M | – | – | + | ? | ? | W | – | – | NA | W | + | – | 80–100 | + | + | S | W |
| Bouillet, 198361 | 183 | ± | NA | + | M | CCS | – | – | < 60 | – | – | – | – | ? | W | – | – | – | – | – | W | – | – | NA | W | NA | NA | NA | NA | NA | NA | W |
| Bonafe, 199375 (French language) | 441 | ? | NR | ± | W | CCS | – | – | < 60 | – | ? | ? | – | ? | W | + | + | + | ? | ? | W | – | – | NA | W | + | – | 80–100 | + | + | S | W |
| Brown, 198976 | 453 | ? | NR | ± | W | CCS | – | – | < 60 | ? | – | + | + | ? | W | + | + | + | + | ? | M | – | – | NA | W | ± | + | 80–100 | – | + | W | W |
| Buric, 200577 | 454 | ? | NR | + | W | Non-RCT | – | – | < 60 | – | + | + | – | ? | W | + | + | + | NA | – | M | – | – | NA | W | ? | 80–100 | + | + | S | W | |
| Dei-Anang, 199079 (German language) | 471 | ? | NR | ± | W | CCS | – | – | < 60 | ? | – | – | – | ? | W | – | – | + | ? | ? | W | – | – | NA | W | NA | NA | NA | NA | NA | NA | W |
| Muralikuttan, 199285 | 593 | ± | 60–79 | + | M | RCT | + | ? | 80–100 | + | + | + | NA | ? | M | + | + | + | ? | ? | M | – | – | NA | W | ? | 80–100 | + | + | M | M | |
| Revel, 199388 | 617 | ? | NR | ± | W | RCT | + | ? | 60–79 | ± | + | + | – | ? | M | + | + | + | ? | ? | M | – | – | NA | W | – | + | 80–100 | + | – | W | M |
| Steffen, 199990 (German language) | 641 | ? | NR | ± | W | RCT | ? | ? | < 60 | ? | + | + | – | ? | M | + | + | + | + | ? | M | – | – | NA | W | + | – | 80–100 | + | + | S | W |
| Weinstein, 198692 | 672 | – | 60–79 | – | W | CCS | – | – | < 60 | – | – | ? | – | ? | W | + | – | + | NA | ? | W | – | – | NA | W | + | 80–100 | + | – | W | W | |
| Ejeskar, 198396 | 727 | ± | NR | ± | M | RCT | ? | ? | < 60 | ? | + | + | – | ? | M | – | – | + | ? | ? | W | – | – | NA | W | + | – | 80–100 | + | + | S | W |
| Alexander, 1989103 | 884 | ± | NR | – | W | CCS | – | – | < 60 | ? | – | + | – | ? | W | + | + | ± | ? | ? | W | NA | NA | NA | W | + | – | 80–100 | + | + | S | W |
| Lee, 1996104 (German language) | 889 | ? | NR | ± | W | CCS | – | – | < 60 | ? | – | + | – | ? | W | – | – | + | ? | ? | W | – | – | NA | W | ? | ? | Cannot tell | + | ? | W | W |
| Watters,1988105 | 893 | ± | NR | ± | W | Non-RCT | – | – | < 60 | ? | – | – | – | ? | W | – | – | – | – | ? | W | – | – | NA | W | + | – | 80–100 | + | + | S | W |
| Disc surgery vs epidural/intradiscal injection | ||||||||||||||||||||||||||||||||
| Buttermann, 200495 | 725 | ± | 80–100 | ± | M | RCT | ? | ? | 60–79 | ± | + | + | - | - | M | + | + | ? | – | – | W | – | – | NA | W | + | 80–100 | – | ? | W | M | |
| Disc surgery vs intraoperative interventions | ||||||||||||||||||||||||||||||||
| Aminmansour, 200664 | 268 | ± | NR | ± | M | Q-RCT | – | – | < 60 | ? | + | + | – | ? | W | + | + | + | + | + | S | + | + | ? | M | + | – | 80–100 | + | + | S | W |
| MacKay, 199565 | 270 | ? | NR | ± | W | RCT | ? | ? | < 60 | ? | + | + | - | ? | M | – | – | + | ? | ? | W | ? | – | ? | W | ? | ? | 80–100 | + | – | W | W |
| Lundin, 200366 | 276 | ± | 80–100 | ± | M | RCT | ? | ? | < 60 | ? | + | + | – | ? | M | + | + | + | + | ? | M | + | – | ? | M | + | – | 80–100 | + | + | S | M |
| Cengiz, 200769 | 316 | ? | NR | ± | W | RCT | ? | + | < 60 | ? | + | + | + | ? | M | + | + | + | ? | ? | W | ? | – | NA | W | + | – | 80–100 | + | + | S | M |
| Lavyne, 199270 | 366 | ± | NR | ± | W | Q-RCT | – | – | < 60 | ? | + | + | - | + | W | – | – | + | ? | ? | W | + | – | – | M | – | + | 80–100 | + | + | M | W |
| Kim, 200373 | 400 | ? | NR | ± | W | RCT | + | + | < 60 | – | + | + | + | ? | M | + | + | + | NA | ? | M | + | – | – | M | + | + | 80–100 | + | + | S | M |
| Bernsmann, 200174 | 436 | ? | NR | ± | W | RCT | ? | ? | < 60 | ? | + | + | - | ? | M | + | + | + | + | ? | M | + | – | ? | M | ? | ? | 80–100 | + | – | W | M |
| Debi, 200278 | 470 | ? | NR | ± | W | RCT | ? | ± | < 60 | ? | + | + | ? | ± | M | ± | ± | + | – | – | W | ? | – | – | W | ? | 80–100 | + | – | W | W | |
| Gerszten, 200381 | 492 | ? | NR | ± | W | RCT | + | ? | < 60 | ? | + | + | – | ? | M | + | + | + | NA | ? | M | + | + | ? | M | + | – | 80–100 | + | + | S | M |
| Glasser, 199382 | 497 | ? | NR | ± | W | RCT | ? | ? | < 60 | ? | + | + | – | ? | M | – | – | + | ? | ? | W | + | – | – | M | ? | – | 60–79 | + | – | W | W |
| Jensen, 199683 | 520 | + | 80–100 | ± | M | RCT | ? | ? | < 60 | ? | + | + | – | ? | M | + | + | + | + | ? | M | + | – | ? | M | ? | ? | 80–100 | + | – | M | M |
| Langmayr, 199584 | 551 | ± | < 60 | ± | M | RCT | ? | ? | < 60 | ? | + | + | ? | ? | M | ± | ± | + | + | ? | M | + | + | ? | M | ? | 80–100 | + | – | M | M | |
| Richter, 200189 | 618 | ? | NR | + | W | RCT | + | + | < 60 | ? | + | + | – | ? | M | + | + | + | + | ? | M | + | – | ? | M | ? | – | 80–100 | + | – | M | M |
| Rasmussen, 2008101 | 854 | ? | NR | ± | W | RCT | + | ? | < 60 | ? | + | + | – | ? | M | + | + | + | + | ? | M | + | – | ? | M | + | – | 80–100 | + | + | S | M |
| Ronnberg, 2008102 | 856 | ? | NR | ± | W | RCT | ? | ± | < 60 | ? | + | + | – | ? | M | + | + | + | + | ? | M | ? | – | ? | W | ? | ? | 80–100 | + | – | M | W |
| Jirarattanaphochai, 2007106 | 909 | + | 80–100 | ± | M | RCT | + | ± | < 60 | ? | + | + | – | ? | M | + | + | + | + | ? | M | + | + | ? | M | + | – | 80–100 | + | + | S | M |
| Tribolet, 1998107 | 915 | ± | NR | + | M | RCT | + | ? | < 60 | ? | + | + | – | ? | M | + | + | + | + | ? | M | + | – | ? | M | ? | + | 80–100 | + | – | M | M |
| Disc surgery vs mixed treatments | ||||||||||||||||||||||||||||||||
| Wang, 200063 | 263 | ± | NR | ± | M | RCT | ? | ? | < 60 | ? | + | + | – | + | M | + | + | + | ? | ? | M | + | – | ? | M | – | ? | 80–100 | + | – | W | M |
| Hoogland, 200697 | 734 | ? | NR | ± | W | Q-RCT | – | – | < 60 | ? | + | + | – | ? | W | + | + | + | ? | ? | M | ? | – | NA | W | ? | + | 80–100 | + | – | M | W |
| Prestar, 199571 (German language) | 379 | ± | NR | ± | M | RCT | ? | ? | < 60 | ? | + | + | – | ? | M | – | – | + | ? | ? | W | ? | – | ? | W | ? | – | 60–79 | + | – | W | W |
| Starkweather, 200693 | 705 | ? | NR | ± | W | RCT | ? | ± | 60–79 | + | – | + | – | ? | M | + | + | + | ? | ? | W | – | – | NA | W | ? | + | 80–100 | + | – | W | W |
| North, 200586 | 600 | + | 60–79 | ± | M | RCT | + | ± | < 60 | ? | + | + | – | ? | M | – | – | + | – | ? | W | – | – | NA | W | – | + | 60–79 | – | – | W | W |
| Disc surgery vs non-opioids | ||||||||||||||||||||||||||||||||
| Rossi, 199357 (Italian language) | 144 | ? | NR | ± | W | RCT | ? | ? | < 60 | ? | + | + | – | ? | M | – | – | + | + | ? | M | – | – | NA | M | + | – | 80–100 | ? | ? | W | W |
| Dubourg, 200280 | 475 | ? | NR | + | W | CCS | – | – | 60–79 | ± | – | + | – | ? | W | + | + | + | – | ? | W | – | – | NA | W | ? | ? | 80–100 | + | – | W | W |
| Disc surgery vs usual/conventional care | ||||||||||||||||||||||||||||||||
| Thomas, 200745 | 2 | + | 60–79 | ± | S | Non-RCT | – | – | 80–100 | – | – | + | + | ? | W | + | + | – | NA | ? | M | – | – | NA | W | + | – | 80–100 | + | – | M | M |
| Shvartzman, 199262 | 211 | – | NR | – | W | HCS | – | – | < 60 | ? | – | + | – | ? | W | – | – | + | NA | ? | W | – | – | NA | W | NA | NA | NA | NA | NA | NA | W |
| Koranda, 199567 (Czech language) | 294 | ± | NR | ± | M | Q-RCT | – | – | < 60 | ? | – | + | – | ? | W | – | – | + | ? | ? | W | – | – | NA | W | + | – | 80–100 | – | + | M | W |
| Atlas, 199672 | 386 | + | NR | + | M | CCS | – | – | 80–100 | – | + | + | + | ? | M | + | + | + | NA | ? | M | – | – | NA | W | – | 60–79 | – | – | M | M | |
| Weber, 198391 | 664 | ± | NR | ± | M | RCT | ? | ± | < 60 | ? | + | + | – | ? | M | – | – | + | – | ? | W | – | – | NA | W | – | + | 60–79 | + | – | W | W |
| Alaranta, 199094 | 716 | ± | 80–100 | ± | M | CCS | – | – | 60–79 | – | – | + | – | ? | W | + | + | ? | – | – | W | – | – | NA | W | ? | – | 80–100 | – | – | W | W |
| Weinstein, 200699 | 751 | – | < 60 | + | W | RCT | + | + | 60–79 | + | + | + | + | – | S | + | + | + | NA | ? | M | – | – | NA | W | ? | + | 80–100 | + | – | M | S |
| Hansson, 2007100 | 772 | ± | < 60 | + | M | CCS | – | – | 60–79 | + | ± | + | – | – | W | + | + | + | NA | ? | W | – | – | NA | W | + | – | 80–100 | + | + | S | W |
| Peul, 200787 | 606 | + | 80–100 | + | S | RCT | + | ± | 80–100 | + | + | + | + | ? | S | + | + | + | NA | ? | M | – | – | NA | W | + | 80–100 | + | + | S | S | |
| Weinstein, 200698 | 750 | – | < 60 | + | W | CCS | – | – | 60–79 | – | + | + | + | – | M | + | + | + | NA | ? | M | – | – | NA | W | + | + | 80–100 | + | – | M | M |
Epidural/intradiscal injection
| Study | ID no. | EV1 | EV2 (%) | EV3 | EVR | SB1 | SB2 | SB3 | SB4 (%) | SB5 | SB6 | SB7 | SB8 | SB9 | SBR | DB1a | DB1b | DB2 | DB3 | DB4 | DBR | PB1 | PB2 | PB3 | PBR | AB1 | AB1a | AB2 (%) | AB3 | AB4 | ABR | GR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epidural vs activity restriction | ||||||||||||||||||||||||||||||||
| Coomes, 1961145 | 140 | ? | NR | ± | W | Non-RCT | – | – | < 60 | ± | + | + | – | ? | W | – | – | + | – | – | W | – | – | NA | W | + | 80–100 | + | + | S | W | |
| Epidural vs alternative/non-traditional | ||||||||||||||||||||||||||||||||
| Wehling, 1997167 (German language) | 667 | ? | NR | ± | W | CCS | – | – | < 60 | ? | – | + | – | ? | W | – | – | + | – | ? | W | – | – | NA | W | + | – | 80–100 | + | + | S | W |
| Epidural vs biological agents | ||||||||||||||||||||||||||||||||
| Becker, 2007149 | 321 | ± | NR | ± | W | RCT | + | ± | < 60 | ? | + | + | + | + | M | + | + | + | + | ? | M | + | – | – | M | ? | – | 80–100 | + | – | M | M |
| Epidural vs chemonucleolysis | ||||||||||||||||||||||||||||||||
| Bontoux, 1990168 (French language) | 720 | ? | NR | ± | W | RCT | + | ? | < 60 | ? | + | + | – | ? | M | – | – | + | + | ? | M | + | – | ? | M | + | – | 80–100 | + | + | S | M |
| Bourgeois, 1988160 (French language) | 447 | ? | NR | + | W | RCT | + | ± | < 60 | ? | + | + | – | ? | M | – | – | + | + | ? | W | + | + | ? | M | + | – | 80–100 | + | + | S | M |
| Gallucci, 2007170 | 729 | ? | NR | ± | W | RCT | ? | ? | < 60 | ? | + | + | ? | ? | M | ± | ± | + | + | ? | M | + | – | – | M | + | 80–100 | + | + | S | M | |
| Graham, 1976144 | 50 | – | NR | – | W | Non–RCT | – | – | < 60 | – | ? | ? | – | ? | W | – | – | + | + | ? | M | + | – | – | M | NA | 80–100 | + | + | S | W | |
| Epidural vs disc surgery | ||||||||||||||||||||||||||||||||
| Buttermann, 200495 | 725 | ± | 80–100 | ± | M | RCT | ? | ? | 60–79 | ± | + | + | – | – | M | + | + | ? | – | – | W | – | – | NA | W | + | 80–100 | – | ? | W | M | |
| Epidural vs education/advice | ||||||||||||||||||||||||||||||||
| Bronfort, 2004169 | 722 | ? | < 60 | ± | W | RCT | ? | ± | 60–79 | + | + | + | – | ? | M | + | + | + | ? | ? | W | – | – | NA | W | ? | ? | 80–100 | NA | – | W | W |
| Epidural vs inactive control | ||||||||||||||||||||||||||||||||
| Bush, 1991147 | 203 | ? | NR | ± | W | RCT | ? | ? | < 60 | ? | + | + | – | ± | M | + | + | + | + | ? | M | + | – | NA | M | – | + | 60–79 | + | + | W | M |
| Carette, 1997152 | 350 | ? | NR | + | M | RCT | + | ± | 80–100 | + | + | + | + | + | S | + | + | + | + | ? | M | + | + | + | S | – | + | 60–79 | + | + | M | S |
| Dilke, 1973157 | 383 | ? | NR | ± | W | RCT | ? | ? | < 60 | ? | + | + | ± | ? | M | – | – | + | + | ? | M | + | + | – | M | – | 60–79 | + | – | W | M | |
| Helliwell, 1985162 | 512 | ? | NR | ± | W | RCT | ? | ? | < 60 | ? | + | + | – | + | M | + | + | + | ? | ? | M | + | – | – | M | + | – | 80–100 | + | + | S | M |
| Karppinen, 2001171 | 739 | + | 80–100 | ± | S | RCT | + | ± | 80–100 | ± | + | + | + | – | S | + | + | + | + | ? | M | + | + | + | S | + | 80–100 | + | + | S | S | |
| Klenerman, 1984163 | 539 | ? | NR | ± | W | RCT | + | ± | < 60 | ? | + | + | ? | + | M | ± | ± | + | + | – | M | ? | ? | ? | W | – | 80–100 | – | – | W | W | |
| Mathews, 1987176 | 905 | ± | NR | ± | M | RCT | ± | ? | < 60 | ? | + | + | – | + | M | ± | ± | + | + | ? | M | + | – | ? | M | ? | + | 60–79 | + | – | W | M |
| Price, 2005173 | 778 | ? | NR | + | M | RCT | + | ± | 80–100 | + | + | + | NA | – | S | + | + | + | + | ? | M | + | ± | ± | S | ? | 80–100 | + | + | S | S | |
| Ridley, 1988165 | 620 | ? | NR | ± | W | RCT | + | ? | < 60 | ? | + | + | ? | ? | M | ± | ± | + | + | ? | M | + | – | – | M | ? | 80–100 | + | – | M | M | |
| Snoek, 1977148 | 240 | ? | NR | ± | W | RCT | ? | ? | < 60 | ? | + | + | – | ± | M | – | – | + | + | ? | M | + | – | – | M | + | – | 80–100 | + | + | S | W |
| Vad, 2002158 | 406 | ± | NR | ± | W | Non–RCT | – | – | < 60 | ? | + | + | – | ± | W | + | + | + | + | ? | M | – | – | NA | W | ? | + | 80–100 | + | – | M | M |
| Valat, 2003153 | 351 | ? | NR | ± | W | RCT | + | ± | 80–100 | + | + | + | NA | + | S | + | + | + | + | ? | M | + | – | – | M | ? | 80–100 | + | + | S | S | |
| Yates, 1978146 | 175 | ? | NR | ± | W | RCT (crossover) | ? | ? | < 60 | ? | + | + | – | ? | W | – | – | + | ? | ? | W | + | – | NA | M | ? | ? | Can’t tell | + | ? | W | W |
| Epidural vs manipulation | ||||||||||||||||||||||||||||||||
| Bronfort, 2004169 | 722 | ? | < 60 | ± | W | RCT | ? | ± | 60–79 | + | + | + | – | ? | M | + | + | + | ? | ? | W | – | – | NA | W | ? | ? | 80–100 | NA | – | W | W |
| Bronfort, 2000161 | 451 | – | < 60 | ± | W | RCT | ? | ± | 60–79 | – | + | + | NA | ? | M | + | + | + | NA | ? | M | – | – | NA | W | ? | 80–100 | + | – | M | M | |
| Epidural vs mixed treatment | ||||||||||||||||||||||||||||||||
| Styczynski, 1997166 (Polish language) | 644 | ? | NR | ± | W | Non-RCT | – | – | < 60 | ? | ? | ? | – | ? | W | + | + | + | ? | ? | W | – | – | NA | W | + | – | 80–100 | + | + | S | W |
| Blonna, 2004159 (Italian language) | 439 | ± | NR | ± | M | RCT | ? | ± | < 60 | – | + | + | – | ? | M | + | + | ? | ? | ? | W | – | – | NA | W | + | – | 80–100 | + | + | S | W |
| Pirbudak, 2003150 | 348 | ? | NR | ± | W | RCT | ± | ± | < 60 | ? | + | + | – | ? | M | + | + | + | + | ? | M | + | + | – | M | + | – | 80–100 | + | + | S | M |
| Epidural vs non-opioids | ||||||||||||||||||||||||||||||||
| Bronfort, 2000161 | 451 | – | < 60 | ± | W | RCT | ? | ± | 60–79 | – | + | + | NA | ? | M | + | + | + | NA | ? | M | – | – | NA | W | ? | 80–100 | + | – | M | M | |
| Dincer, 2007143 | 20 | + | NR | ± | M | RCT | ? | ? | 60–79 | + | + | + | NA | + | M | + | + | + | + | – | M | – | – | – | W | + | 80–100 | + | + | S | M | |
| Lafuma, 1997172 | 771 | ? | NR | + | W | RCT | ? | ? | 60–79 | + | + | + | – | + | M | + | + | + | – | ? | W | – | – | NA | W | + | – | 80–100 | + | + | S | W |
| Wilson-MacDonald, 2005156 | 362 | ? | NR | ± | W | RCT | + | ± | 60–79 | ± | + | + | – | ? | M | + | + | ? | ? | – | M | + | – | – | M | ? | 80–100 | + | – | M | M | |
| Murata, 2009175 | 846 | ? | NR | + | W | RCT | ? | ± | < 60 | ? | + | + | – | + | M | + | + | + | ? | ? | W | ? | – | ? | W | + | ? | 80–100 | + | ? | M | W |
| Epidural vs passive PT | ||||||||||||||||||||||||||||||||
| Veihelmann, 2006155 | 359 | ? | NR | ± | W | RCT | ± | + | < 60 | ? | + | + | – | ? | M | + | + | + | + | ? | M | – | – | NA | W | – | + | < 60 | + | – | W | M |
| Epidural vs usual/conventional care | ||||||||||||||||||||||||||||||||
| Buchner, 2000151 | 349 | ? | NR | ± | W | RCT | ± | ± | 60–79 | + | + | + | NA | ? | M | + | + | + | ? | ? | M | – | – | NA | W | + | 80–100 | + | + | S | M | |
| Laiq, 2009174 | 828 | ? | NR | ± | W | Q-RCT | – | – | < 60 | ? | ± | + | – | ? | W | + | + | + | – | ? | W | – | – | NA | W | + | – | 80–100 | + | – | M | W |
| Matyjek, 1986 (Polish language)164 | 581 | ? | NR | ± | W | CCS | – | – | < 60 | ? | ? | ? | – | ? | W | – | – | ? | ? | ? | W | – | – | NA | W | NA | NA | NA | NA | NA | NA | W |
| Popiolek, 1991 (Polish language)154 | 358 | ? | NR | ± | W | Non-RCT | ? | ? | 60–79 | – | + | + | – | – | W | – | – | + | ? | ? | W | – | – | NA | W | + | – | 80–100 | + | + | S | W |
Chemonucleolysis
| Study | ID no. | EV1 | EV2 (%) | EV3 | EVR | SB1 | SB2 | SB3 | SB4 (%) | SB5 | SB6 | SB7 | SB8 | SB9 | SBR | DB1a | DB1b | DB2 | DB3 | DB4 | DBR | PB1 | PB2 | PB3 | PBR | AB1 | AB1a | AB2 (%) | AB3 | AB4 | ABR | GR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemonucleolysis vs disc surgery | ||||||||||||||||||||||||||||||||
| Krugluger, 200046 | 35 | ? | NR | ? | W | RCT | ? | ? | < 60 | ? | + | + | ? | ? | W | + | + | + | ? | ? | M | – | – | NA | W | + | – | 80–100 | ? | ? | W | W |
| van Alphen, 198947 | 43 | + | 60–79 | + | S | RCT | ± | ? | < 60 | ? | + | + | – | ? | M | – | – | + | – | ? | W | – | – | NA | W | + | – | 80–100 | + | + | S | M |
| Javid, 199548 | 44 | ± | NR | ± | M | CCS | – | – | 60–79 | ± | – | + | – | ? | W | – | – | + | – | ? | W | – | – | NA | W | + | – | 80–100 | + | + | S | W |
| Postacchini, 198649 | 45 | ± | NR | ± | M | Non-RCT | – | – | < 60 | ? | – | ± | – | – | W | – | – | + | – | ? | W | – | – | NA | W | ? | – | 80–100 | – | – | W | W |
| Norton, 198650 | 47 | – | NR | ± | W | CCS | – | – | < 60 | – | – | + | – | ? | W | – | – | ? | ? | ? | W | – | – | NA | W | NA | NA | NA | NA | NA | NA | W |
| Dabezies, 197851 | 48 | ± | NR | ± | M | CCS | – | – | < 60 | ? | – | ? | – | ? | W | – | – | + | – | – | W | – | – | NA | W | ? | ? | Cannot tell | + | ? | W | W |
| Stula, 199052 (German language) | 49 | ? | NR | ± | W | RCT | ? | ? | < 60 | ? | + | + | – | ? | M | – | – | ? | ? | ? | W | – | – | NA | W | + | – | 80–100 | – | + | W | W |
| Tregonning, 199153 | 61 | ± | NA | ± | M | HCS | – | – | < 60 | ? | – | ± | – | ? | W | + | + | + | – | ? | M | – | – | NA | W | ? | – | 80–100 | + | – | M | W |
| Lagarrigue, 199154 (French language) | 117 | ± | 60–79 | ± | M | CCS | – | – | < 60 | – | – | + | – | – | W | + | + | ± | ? | ? | W | – | – | NA | W | + | – | 80–100 | + | + | S | W |
| Lavignolle, 198755 (French language) | 129 | ? | NR | ± | W | RCT | ? | ? | < 60 | ? | + | + | – | ? | W | ± | ± | ± | ? | ? | W | – | – | NA | W | + | – | 80–100 | + | + | S | W |
| Hoogmartens, 197656 | 132 | ± | NR | ± | M | HCS | – | – | < 60 | ? | – | – | – | ? | W | – | – | – | NA | ? | W | – | – | NA | W | NA | NA | NA | NA | NA | NA | W |
| Zeiger, 198758 | 150 | ? | NR | ? | W | CCS | – | – | < 60 | ? | ? | ? | ? | ? | W | – | – | + | + | ? | W | – | – | – | W | NA | ? | NA | + | ? | W | W |
| Watts, 197559 | 160 | ? | NR | ± | W | CCS | – | – | < 60 | ? | – | + | – | ? | W | – | – | + | ? | ? | W | – | – | NA | W | + | – | 80–100 | + | + | S | W |
| Crawshaw, 198460 | 166 | ± | NR | ± | M | RCT | ? | ? | < 60 | ? | + | + | – | ? | M | – | – | + | ? | ? | W | – | – | NA | W | + | – | 80–100 | + | + | S | W |
| Bouillet, 198361 | 183 | ± | NA | + | M | CCS | – | – | < 60 | – | – | – | – | ? | W | – | – | – | – | – | W | – | – | NA | W | NA | NA | NA | NA | NA | NA | W |
| Bonafe, 199375 (French language) | 441 | ? | NR | ± | W | CCS | – | – | < 60 | – | ? | ? | – | ? | W | + | + | + | ? | ? | W | – | – | NA | W | + | – | 80–100 | + | + | S | W |
| Brown, 198976 | 453 | ? | NR | ± | W | CCS | – | – | < 60 | ? | – | + | + | ? | W | + | + | + | + | ? | M | – | – | NA | W | ± | + | 80–100 | – | + | W | W |
| Buric, 200577 | 454 | ? | NR | + | W | Non-RCT | – | – | < 60 | – | + | + | – | ? | W | + | + | + | NA | – | M | – | – | NA | W | ? | 80–100 | + | + | S | W | |
| Dei–Anang, 199079 (German language) | 471 | ? | NR | ± | W | CCS | – | – | < 60 | ? | – | – | – | ? | W | – | – | + | ? | ? | W | – | – | NA | W | NA | NA | NA | NA | NA | NA | W |
| Muralikuttan, 199285 | 593 | ± | 60–79 | + | M | RCT | + | ? | 80–100 | + | + | + | NA | ? | M | + | + | + | ? | ? | M | – | – | NA | W | ? | 80–100 | + | + | M | M | |
| Revel, 199388 | 617 | ? | NR | ± | W | RCT | + | ? | 60–79 | ± | + | + | – | ? | M | + | + | + | ? | ? | M | – | – | NA | W | – | + | 80–100 | + | – | W | M |
| Steffen, 199990 (German language) | 641 | ? | NR | ± | W | RCT | ? | ? | < 60 | ? | + | + | – | ? | M | + | + | + | + | ? | M | – | – | NA | W | + | – | 80–100 | + | + | S | W |
| Weinstein, 198692 | 672 | – | 60–79 | – | W | CCS | – | – | < 60 | – | – | ? | – | ? | W | + | – | + | NA | ? | W | – | – | NA | W | + | 80–100 | + | – | W | W | |
| Ejeskar, 198396 | 727 | ± | NR | ± | M | RCT | ? | ? | < 60 | ? | + | + | – | ? | M | – | – | + | ? | ? | W | – | – | NA | W | + | – | 80–100 | + | + | S | W |
| Alexander, 1989103 | 884 | ± | NR | – | W | CCS | – | – | < 60 | ? | – | + | – | ? | W | + | + | ± | ? | ? | W | NA | NA | NA | W | + | – | 80–100 | + | + | S | W |
| Lee, 1996104 (German language) | 889 | ? | NR | ± | W | CCS | – | – | < 60 | ? | – | + | – | ? | W | – | – | + | ? | ? | W | – | – | NA | W | ? | ? | Cannot tell | + | ? | W | W |
| Watters, 1988105 | 893 | ± | NR | ± | W | Non-RCT | – | – | < 60 | ? | – | – | – | ? | W | – | – | – | – | ? | W | – | – | NA | W | + | – | 80–100 | + | + | S | W |
| Chemonucleolysis vs epidural/intradiscal injection | ||||||||||||||||||||||||||||||||
| Graham, 1976144 | 50 | – | NR | – | W | Non-RCT | – | – | < 60 | – | ? | ? | – | ? | W | – | – | + | + | ? | M | + | – | – | M | NA | 80–100 | + | + | S | W | |
| Bourgeois, 1988160 (French language) | 447 | ? | NR | + | W | RCT | + | ± | < 60 | ? | + | + | – | ? | M | – | – | + | + | ? | W | + | + | ? | M | + | – | 80–100 | + | + | S | M |
| Bontoux, 1990168 (French language) | 720 | ? | NR | ± | W | RCT | + | ? | < 60 | ? | + | + | – | ? | M | – | – | + | + | ? | M | + | – | ? | M | + | – | 80–100 | + | + | S | M |
| Gallucci, 2007170 | 729 | ? | NR | ± | W | RCT | ? | ? | < 60 | ? | + | + | ? | ? | M | ± | ± | + | + | ? | M | + | – | – | M | + | 80–100 | + | + | S | M | |
| Chemonucleolysis vs inactive control | ||||||||||||||||||||||||||||||||
| Gogan, 1992205 | 55 | ± | NR | + | M | RCT | + | ? | 60–79 | ± | + | + | – | ? | M | + | + | + | + | ? | M | ? | – | – | W | ? | ± | 80–100 | + | – | M | M |
| Schwetschenau, 1976206 | 236 | ± | < 60 | – | M | RCT | + | + | 60–79 | ± | + | + | – | ? | M | – | – | + | + | ? | M | + | + | ? | M | + | – | 80–100 | + | + | S | S |
| Feldman, 1986207 (French language) | 244 | ± | NR | ± | M | RCT | ? | ? | 80–100 | ± | + | + | – | ? | M | + | + | + | ? | ? | M | + | ? | – | M | ? | ? | 80–100 | + | + | M | M |
| Dabezies, 1988209 | 726 | ? | NR | + | W | RCT | ± | + | < 60 | ? | + | + | – | ? | M | – | – | + | + | ? | M | + | + | ? | M | ? | + | 60–79 | + | – | W | M |
| Javid, 1983210 | 738 | ? | NR | + | W | RCT | + | ± | < 60 | ? | + | + | – | ? | M | – | – | + | + | ± | M | + | + | ? | M | + | – | 80–100 | + | – | S | M |
| Chemonucleolysis vs manipulation | ||||||||||||||||||||||||||||||||
| Burton, 2000208 | 723 | ? | NR | + | W | RCT | – | – | 80–100 | + | + | + | NA | ? | M | + | + | + | + | + | S | – | – | NA | W | ± | 60–79 | + | – | M | M | |
| Chemonucleolysis vs mixed treatments | ||||||||||||||||||||||||||||||||
| Khoromi, 2007214 | 534 | ± | 80–100 | + | S | RCT (crossover) | + | + | 80–100 | + | + | + | + | + | S | + | + | + | + | ? | M | + | + | + | S | ? | + | < 60 | + | – | W | M |
Non-opioids
| Study | ID no. | EV1 | EV2 (%) | EV3 | EVR | SB1 | SB2 | SB3 | SB4 (%) | SB5 | SB6 | SB7 | SB8 | SB9 | SBR | DB1a | DB1b | DB2 | DB3 | DB4 | DBR | PB1 | PB2 | PB3 | PBR | AB1 | AB1a | AB2 (%) | AB3 | AB4 | ABR | GR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-opioids vs alternative/non-traditional | ||||||||||||||||||||||||||||||||
| Chen, 2009215 | 801 | ± | NR | ± | M | RCT | ? | ? | < 60 | ? | + | + | – | ? | M | – | – | + | ? | ? | W | – | – | NA | W | + | – | 80–100 | + | + | S | W |
| Non-opioids vs biological agents | ||||||||||||||||||||||||||||||||
| Genevay, 2004216 | 323 | ± | 80–100 | ± | M | HCS | – | – | 60–79 | ± | – | – | – | ? | W | + | + | + | – | ? | W | – | – | – | W | + | – | 80–100 | + | + | S | W |
| Non-opioids vs disc surgery | ||||||||||||||||||||||||||||||||
| Rossi, 199357 (Italian language) | 144 | ? | NR | ± | W | RCT | ? | ? | < 60 | ? | + | + | – | ? | M | – | – | + | + | ? | M | – | – | NA | M | + | – | 80–100 | ? | ? | W | W |
| Dubourg, 200280 | 475 | ? | NR | + | W | CCS | – | – | 60–79 | ± | – | + | – | ? | W | + | + | + | – | ? | W | – | – | NA | W | ? | ? | 80–100 | + | – | W | W |
| Non-opioids vs epidural/intradiscal injection | ||||||||||||||||||||||||||||||||
| Dincer, 2007143 | 20 | + | NR | ± | M | RCT | ? | ? | 60–79 | + | + | + | NA | + | M | + | + | + | + | – | M | – | – | – | W | + | 80–100 | + | + | S | M | |
| Wilson-MacDonald, 2005156 | 362 | ? | NR | ± | W | RCT | + | ± | 60–79 | ± | + | + | – | ? | M | + | + | ? | ? | – | M | + | – | – | M | ? | 80–100 | + | – | M | M | |
| Bronfort, 2000161 | 451 | – | < 60 | ± | W | RCT | ? | ± | 60–79 | – | + | + | NA | ? | M | + | + | + | NA | ? | M | – | – | NA | W | ? | 80–100 | + | – | M | M | |
| Lafuma, 1997172 | 771 | ? | NR | + | W | RCT | ? | ? | 60–79 | + | + | + | – | + | M | + | + | + | – | ? | W | – | – | NA | W | + | – | 80–100 | + | + | S | W |
| Murata, 2009175 | 846 | ? | NR | + | W | RCT | ? | ± | < 60 | ? | + | + | – | + | M | + | + | + | ? | ? | W | ? | – | ? | W | + | ? | 80–100 | + | ? | M | W |
| Non-opioids vs inactive control | ||||||||||||||||||||||||||||||||
| Gibson, 1975217 | 62 | ? | NR | ± | W | Non-RCT | – | – | < 60 | ? | + | + | ? | ± | W | – | – | + | ? | ? | W | + | + | – | M | ? | 80–100 | ? | ? | M | W | |
| Goldie, 1968218 | 97 | ? | NR | ? | W | RCT | ? | + | < 60 | ? | + | + | – | + | M | – | – | + | + | ? | W | + | + | – | M | + | – | 80–100 | + | + | S | M |
| Yildirim, 2003219 | 297 | ? | NR | ± | W | RCT | ? | ? | < 60 | ? | + | + | – | + | M | – | – | + | ? | ? | W | + | ? | ? | M | – | + | 80–100 | + | – | W | W |
| Hedeboe, 1982220 | 312 | ± | 80–100 | ± | M | RCT | ± | ± | 60–79 | + | + | + | – | ? | M | – | – | + | + | ? | W | + | + | – | M | + | – | 80–100 | – | + | M | M |
| El-Zahaar, 1995221 | 334 | ? | NR | ? | W | RCT | ? | ? | < 60 | ? | + | + | – | ? | M | – | – | + | + | ? | W | + | ? | – | M | + | – | 80–100 | + | + | S | W |
| Khoromi, 2007214 | 534 | ± | 80–100 | + | S | RCT (crossover) | + | + | 80–100 | + | + | + | + | + | S | + | + | + | + | ? | M | + | + | + | S | ? | + | < 60 | + | – | W | M |
| Porsman, 1979222 | 611 | ± | NR | + | M | RCT | ? | + | < 60 | ? | + | + | – | + | M | – | – | + | + | + | M | + | + | ? | M | ? | – | 80–100 | + | – | M | W |
| Weber, 19936 | 665 | ? | NR | ? | W | RCT | ? | ? | < 60 | ? | + | + | – | + | M | + | + | + | – | ? | W | + | + | – | M | + | – | 80–100 | + | + | S | M |
| Dreiser, 2001223 | 696 | ? | NR | ? | W | RCT | ? | ? | 60–79 | + | + | + | + | ± | M | + | + | + | + | ? | M | + | + | – | S | + | – | 80–100 | + | + | S | M |
| Finckh, 2006224 | 728 | ? | NR | ± | W | RCT | + | ± | 60–79 | + | + | + | + | + | S | + | + | + | + | ? | M | + | + | ? | M | ? | – | 80–100 | + | + | M | M |
| Grevsten, 1975225 | 732 | ? | NR | ± | W | RCT | ? | ? | < 60 | ? | + | + | – | + | M | – | – | + | + | ? | W | + | + | – | M | + | + | 80–100 | + | + | S | W |
| Jacobs, 1968226 | 736 | ? | NR | + | W | Q-RCT | – | ? | < 60 | ? | + | + | ? | + | W | – | – | + | + | – | M | + | – | ? | M | – | 80–100 | + | – | W | W | |
| Herrmann, 2009227 | 816 | ? | NR | + | W | RCT | + | + | 60–79 | + | + | + | – | ? | S | + | + | + | ? | ? | M | + | + | ? | M | + | – | 80–100 | + | + | S | M |
| Holve, 200828 | 817 | ? | NR | ± | W | Q-RCT | – | ± | < 60 | ? | + | + | – | ? | W | + | + | + | + | ? | M | + | + | ? | M | ? | + | 80–100 | + | – | M | M |
| Non-opioids vs manipulation | ||||||||||||||||||||||||||||||||
| Bronfort, 2000161 | 451 | – | < 60 | ± | W | RCT | ? | ± | 60–79 | – | + | + | NA | ? | M | + | + | + | NA | ? | M | – | – | NA | W | ? | 80–100 | + | – | M | M | |
| Non-opioids vs mixed treatments | ||||||||||||||||||||||||||||||||
| Khoromi, 2007214 | 534 | ± | 80–100 | + | S | RCT (crossover) | + | + | 80–100 | + | + | + | + | + | S | + | + | + | + | ? | M | + | + | + | S | ? | + | < 60 | + | – | W | M |
| Non-opioids vs opioids | ||||||||||||||||||||||||||||||||
| Kwasucki, 2002229 (Polish language) | 368 | ? | NR | ± | W | RCT | ? | ? | < 60 | ? | + | + | – | + | M | ? | ? | + | ? | ? | W | ? | ? | ? | W | + | – | 80–100 | + | + | S | W |
| Kwasucki, 1993230 (Polish language) | 547 | ? | NR | ± | W | RCT | ? | ? | < 60 | ? | + | + | – | ? | M | ± | ± | + | ? | ? | W | ? | ? | ? | W | + | – | 80–100 | + | + | S | W |
Traction
| Study | ID no. | EV1 | EV2 (%) | EV3 | EVR | SB1 | SB2 | SB3 | SB4 (%) | SB5 | SB6 | SB7 | SB8 | SB9 | SBR | DB1a | DB1b | DB2 | DB3 | DB4 | DBR | PB1 | PB2 | PB3 | PBR | AB1 | AB1a | AB2 (%) | AB3 | AB4 | ABR | GR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Traction vs active PT/exercise therapy | ||||||||||||||||||||||||||||||||
| Ljunggren, 1992242 | 570 | ? | NR | ± | W | RCT | ? | ? | 60–79 | + | + | + | – | + | M | + | + | + | + | ? | M | – | – | NA | W | + | – | 80–100 | + | + | S | M |
| Traction vs activity restriction | ||||||||||||||||||||||||||||||||
| Moret, 1998243 | 222 | + | 80–100 | ± | S | RCT | + | ± | 60–79 | ± | + | + | – | + | M | + | + | + | – | ? | M | – | – | NA | W | + | – | 80–100 | + | + | S | M |
| Traction vs inactive control | ||||||||||||||||||||||||||||||||
| Pal, 1986244 | 206 | ? | NR | ? | W | RCT | ? | ? | < 60 | ± | + | + | – | ? | W | ± | ± | + | + | – | M | + | – | – | M | ? | 80–100 | + | – | M | W | |
| Rattanatharn, 2004245 | 299 | ? | NR | + | W | RCT | + | ± | 60–79 | – | + | + | + | + | M | + | + | + | NA | ? | M | + | – | – | M | ? | + | 60–79 | + | – | W | M |
| Larsson, 1980246 | 553 | ? | NR | + | W | RCT | ? | ? | 60–79 | ± | + | + | – | + | M | – | – | + | ? | ? | W | – | – | NA | W | + | + | 80–100 | + | – | M | M |
| Mathews, 1975247 | 579 | ? | NR | ± | W | RCT | ? | ? | < 60 | ± | + | + | – | ? | M | – | – | + | ? | – | W | + | – | – | M | ? | Cannot tell | + | ? | W | W | |
| Reust, 1988248 (French language) | 746 | ? | NR | ± | W | RCT | + | ? | < 60 | ? | + | + | – | + | M | + | + | + | + | ? | M | + | – | ? | M | + | + | < 60 | + | + | W | M |
| Traction vs passive PT | ||||||||||||||||||||||||||||||||
| Styczynski, 1991250 (Polish language) | 77 | ? | NR | ± | W | Non-RCT | – | – | < 60 | ? | + | + | – | + | W | – | – | + | ? | ? | W | – | – | NA | W | ? | + | 80–100 | + | – | M | W |
| Unlu, 2008249 | 148 | ? | NR | ± | W | RCT | ? | ? | 60–79 | + | + | + | NA | + | M | + | + | + | + | ? | M | – | – | NA | W | + | 80–100 | + | + | S | M | |
| Mixed treatments (including traction) vs active PT/exercise therapy | ||||||||||||||||||||||||||||||||
| Lidstrom, 1970256 | 564 | ? | NR | ± | W | RCT | ? | ? | < 60 | ? | + | + | – | ? | M | – | – | + | ? | ? | W | – | – | NA | W | + | – | 80–100 | + | + | S | W |
| Mixed treatments (including traction) vs activity restriction | ||||||||||||||||||||||||||||||||
| Lidstrom, 1970256 | 564 | ? | NR | ± | W | RCT | ? | ? | < 60 | ? | + | + | – | ? | M | – | – | + | ? | ? | W | – | – | NA | W | + | – | 80–100 | + | + | S | W |
Manipulation
| Study | ID no. | EV1 | EV2 (%) | EV3 | EVR | SB1 | SB2 | SB3 | SB4 (%) | SB5 | SB6 | SB7 | SB8 | SB9 | SBR | DB1a | DB1b | DB2 | DB3 | DB4 | DBR | PB1 | PB2 | PB3 | PBR | AB1 | AB1a | AB2 (%) | AB3 | AB4 | ABR | GR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Manipulation vs chemonucleolysis | ||||||||||||||||||||||||||||||||
| Burton, 2000208 | 723 | ? | NR | + | W | RCT | – | – | 80–100 | + | + | + | NA | ? | M | + | + | + | + | + | S | – | – | NA | W | ± | 60–79 | + | – | M | M | |
| Manipulation vs education/advice | ||||||||||||||||||||||||||||||||
| Bronfort, 2004169 | 722 | ? | < 60 | ± | W | RCT | ? | ± | 60–79 | + | + | + | – | ? | M | + | + | + | ? | ? | W | – | – | NA | W | ? | ? | 80–100 | NA | – | W | W |
| Manipulation vs epidural | ||||||||||||||||||||||||||||||||
| Bronfort, 2000161 | 451 | – | < 60 | ± | W | RCT | ? | ± | 60–79 | – | + | + | NA | ? | M | + | + | + | NA | ? | M | – | – | NA | W | ? | 80–100 | + | – | M | M | |
| Bronfort, 2004169 | 722 | ? | < 60 | ± | W | RCT | ? | ± | 60–79 | + | + | + | – | ? | M | + | + | + | ? | ? | W | – | – | NA | W | ? | ? | 80–100 | NA | – | W | W |
| Manipulation vs inactive control | ||||||||||||||||||||||||||||||||
| Santilli, 2006258 | 52 | + | 80–100 | + | S | RCT | + | + | < 60 | + | + | + | NA | ? | S | ? | ± | + | + | ? | S | + | – | – | M | + | 80–100 | + | – | S | S | |
| Manipulation vs non-opioids | ||||||||||||||||||||||||||||||||
| Bronfort, 2000161 | 451 | – | < 60 | ± | W | RCT | ? | ± | 60–79 | – | + | + | NA | ? | M | + | + | + | NA | ? | M | – | – | NA | W | ? | 80–100 | + | – | M | M | |
Alternative therapies
| Study | ID no. | EV1 | EV2 (%) | EV3 | EVR | SB1 | SB2 | SB3 | SB4 (%) | SB5 | SB6 | SB7 | SB8 | SB9 | SBR | DB1a | DB1b | DB2 | DB3 | DB4 | DBR | PB1 | PB2 | PB3 | PBR | AB1 | AB1a | AB2 (%) | AB3 | AB4 | ABR | GR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alternative vs epidural/intradiscal injection | ||||||||||||||||||||||||||||||||
| Wehling, 1997167 (German language) | 667 | ? | NR | ± | W | CCS | – | – | < 60 | ? | – | + | – | ? | W | – | – | + | – | ? | W | – | – | NA | W | + | – | 80–100 | + | + | S | W |
| Alternative vs inactive control | ||||||||||||||||||||||||||||||||
| Duplan, 1983261 (French language) | 476 | ± | 80–100 | ± | M | RCT | ? | ? | < 60 | ? | + | + | – | + | M | + | + | + | + | ? | M | + | – | ? | M | – | + | 80–100 | + | ? | M | M |
| Alternative vs non-opioids | ||||||||||||||||||||||||||||||||
| Chen, 2009215 | 801 | ± | NR | ± | M | RCT | ? | ? | < 60 | ? | + | + | – | ? | M | – | – | + | ? | ? | W | – | – | NA | W | + | – | 80–100 | + | + | S | W |
Active physical therapy/exercise therapy
| Study | ID no. | EV1 | EV2 (%) | EV3 | EVR | SB1 | SB2 | SB3 | SB4 (%) | SB5 | SB6 | SB7 | SB8 | SB9 | SBR | DB1a | DB1b | DB2 | DB3 | DB4 | DBR | PB1 | PB2 | PB3 | PBR | AB1 | AB1a | AB2 (%) | AB3 | AB4 | ABR | GR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exercise therapy vs activity restriction | ||||||||||||||||||||||||||||||||
| Lidstrom, 1970256 | 564 | ? | NR | W | RCT | ? | ? | < 60 | ? | + | + | – | ? | M | – | – | + | ? | ? | W | – | – | NA | W | + | – | 80–100 | + | + | S | W | |
| Exercise therapy vs disc surgery | ||||||||||||||||||||||||||||||||
| Osterman, 200668 | 300 | ? | NR | ± | W | RCT | + | + | 80–100 | + | + | + | + | ± | S | + | + | + | NA | ? | M | – | – | NA | W | ? | – | 80–100 | + | + | S | M |
| Exercise therapy vs inactive control | ||||||||||||||||||||||||||||||||
| Gerszten, 200381 | 492 | ? | NR | ± | W | RCT | + | ? | < 60 | ? | + | + | – | ? | M | + | + | + | NA | ? | M | + | + | ? | M | + | – | 80–100 | + | + | S | M |
| Exercise therapy vs mixed treatments | ||||||||||||||||||||||||||||||||
| Fritz, 2007255 | 395 | ? | NR | + | W | RCT | + | ± | 80–100 | + | + | + | + | + | S | + | + | + | ± | ? | M | – | – | NA | W | ± | + | 80–100 | + | + | M | M |
| Lidstrom, 1970256 | 564 | ? | NR | ± | W | RCT | ? | ? | < 60 | ? | + | + | – | ? | M | – | – | + | ? | ? | W | – | – | NA | W | + | – | 80–100 | + | + | S | W |
| Exercise therapy vs traction | ||||||||||||||||||||||||||||||||
| Ljunggren, 1992242 | 570 | ? | NR | ± | W | RCT | ? | ? | 60–79 | + | + | + | – | + | M | + | + | + | + | ? | M | – | – | NA | W | + | – | 80–100 | + | + | S | M |
| Exercise therapy vs usual/conventional care | ||||||||||||||||||||||||||||||||
| Luijsterburg, 2008264 | 742 | + | 80–100 | + | S | RCT | + | + | 80–100 | + | + | + | NA | + | S | + | + | + | NA | ? | M | – | – | NA | W | ? | 80–100 | + | + | M | S | |
Passive physical therapy
| Study | ID no. | EV1 | EV2 | EV3 | EVR | SB1 | SB2 | SB3 | SB4 | SB5 | SB6 | SB7 | SB8 | SB9 | SBR | DB1a | DB1b | DB2 | DB3 | DB4 | DBR | PB1 | PB2 | PB3 | PBR | AB1 | AB1a | AB2 (%) | AB3 | AB4 | ABR | GR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Passive PT vs epidural/intradiscal injection | ||||||||||||||||||||||||||||||||
| Veihelmann, 2006155 | 359 | ? | NR | ± | W | RCT | ± | + | < 60 | ? | + | + | – | ? | M | + | + | + | + | ? | M | – | – | NA | W | – | + | < 60 | + | – | W | M |
| Passive PT vs inactive control | ||||||||||||||||||||||||||||||||
| Ghoname, 1999268 | 496 | ? | NR | ? | W | RCT (crossover) | ? | ? | < 60 | ± | + | + | ? | ? | M | + | + | + | NA | ? | M | – | – | NA | W | ? | Cannot tell | + | ? | W | W | |
| Passive PT vs mixed treatments | ||||||||||||||||||||||||||||||||
| Bokonjic, 1975269 (German language) | 354 | ? | NR | ± | W | Non-RCT | – | – | < 60 | ? | ? | + | – | ? | W | – | – | + | ? | ? | W | – | – | NA | W | + | – | 80–100 | + | + | S | W |
| Passive PT vs traction | ||||||||||||||||||||||||||||||||
| Unlu, 2008249 | 148 | ? | NR | ± | W | RCT | ? | ? | 60–79 | + | + | + | NA | + | M | + | + | + | + | ? | M | – | – | NA | W | + | 80–100 | + | + | S | M | |
| Ozturk, 2006253 | 266 | ? | NR | ± | W | RCT | ? | ? | < 60 | – | + | + | – | ? | W | ± | ± | + | ? | ? | W | – | – | NA | W | + | 80–100 | + | + | S | W | |
Biological agents
| Study | ID no. | EV1 | EV2 (%) | EV3 | EVR | SB1 | SB2 | SB3 | SB4 (%) | SB5 | SB6 | SB7 | SB8 | SB9 | SBR | DB1a | DB1b | DB2 | DB3 | DB4 | DBR | PB1 | PB2 | PB3 | PBR | AB1 | AB1a | AB2 (%) | AB3 | AB4 | ABR | GR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biological agents vs epidural/Intradiscal injection | ||||||||||||||||||||||||||||||||
| Becker, 2007149 | 321 | ± | NR | ± | W | RCT | + | ± | < 60 | ? | + | + | + | + | M | + | + | + | + | ? | M | + | – | – | M | ? | – | 80–100 | + | – | M | M |
| Biological agents vs inactive control | ||||||||||||||||||||||||||||||||
| Karppinen, 2003270 | 398 | ? | NR | ± | W | Non-RCT | – | – | < 60 | ? | – | – | + | ? | W | + | + | – | – | ? | M | – | – | NA | W | ? | ? | Cannot tell | + | ? | W | W |
| Korhonen, 2005271 | 741 | ? | NR | ± | W | RCT | + | ? | 60–79 | + | + | + | + | ? | S | + | + | + | ? | ? | M | + | ? | ? | M | + | – | 80–100 | + | + | S | M |
| Biological agents vs non-opioids | ||||||||||||||||||||||||||||||||
| Genevay, 2004216 | 323 | ± | 80–100 | ± | M | HCS | – | – | 60–79 | ± | – | – | – | ? | W | + | + | + | – | ? | W | – | – | – | W | + | – | 80–100 | + | + | S | W |
Activity restriction
| Study | ID no. | EV1 | EV2 (%) | EV3 | EVR | SB1 | SB2 | SB3 | SB4 (%) | SB5 | SB6 | SB7 | SB8 | SB9 | SBR | DB1a | DB1b | DB2 | DB3 | DB4 | DBR | PB1 | PB2 | PB3 | PBR | AB1 | AB1a | AB2 (%) | AB3 | AB4 | ABR | GR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Activity restriction vs exercise therapy | ||||||||||||||||||||||||||||||||
| Lidstrom, 1970256 | 564 | ? | NR | ± | W | RCT | ? | ? | < 60 | ? | + | + | – | ? | M | – | – | + | ? | ? | W | – | – | NA | W | + | – | 80–100 | + | + | S | W |
| Activity restriction vs education/advice | ||||||||||||||||||||||||||||||||
| Hofstee, 2002267 | 713 | ± | 80–100 | + | M | RCT | + | – | 60–79 | + | + | + | + | ± | M | + | + | + | – | ? | W | – | – | NA | W | ? | – | 80–100 | + | + | M | M |
| Vroomen, 199914 | 658 | + | 80–100 | + | S | RCT | + | – | 60–79 | ± | + | + | + | + | M | + | + | + | + | + | S | – | – | NA | W | + | 80–100 | + | + | S | M | |
| Activity restriction vs epidural/intradiscal injection | ||||||||||||||||||||||||||||||||
| Coomes, 1961145 | 140 | ? | NR | ± | W | Non-RCT | – | – | < 60 | ± | + | + | – | ? | W | – | – | + | – | – | W | – | – | NA | W | + | 80–100 | + | + | S | W | |
| Activity restriction vs mixed treatments | ||||||||||||||||||||||||||||||||
| Hofstee, 2002267 | 713 | ± | 80–100 | + | M | RCT | + | – | 60–79 | + | + | + | + | ± | M | + | + | + | – | ? | W | – | – | NA | W | ? | – | 80–100 | + | + | M | M |
| Lidstrom, 1970256 | 564 | ? | NR | ± | W | RCT | ? | ? | < 60 | ? | + | + | – | ? | M | – | – | + | ? | ? | W | – | – | NA | W | + | – | 80–100 | + | + | S | W |
| Activity restriction vs traction | ||||||||||||||||||||||||||||||||
| Moret, 1998243 | 222 | + | 80–100 | ± | S | RCT | + | ± | 60–79 | ± | + | + | – | + | M | + | + | + | – | ? | M | – | – | NA | W | + | – | 80–100 | + | + | S | M |
Opioids
| Study | IID no. | EV1 | EV2 (%) | EV3 | EVR | SB1 | SB2 | SB3 | SB4 (%) | SB5 | SB6 | SB7 | SB8 | SB9 | SBR | DB1a | DB1b | DB2 | DB3 | DB4 | DBR | PB1 | PB2 | PB3 | PBR | AB1 | AB1a | AB2 (%) | AB3 | AB4 | ABR | GR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Opioids vs non-opioids | ||||||||||||||||||||||||||||||||
| Kwasucki, 2002229 (Polish language) | 368 | ? | NR | ± | W | RCT | ? | ? | < 60 | ? | + | + | – | + | M | ? | ? | + | ? | ? | W | ? | ? | ? | W | + | – | 80–100 | + | + | S | W |
| Kwasucki, 1993230 (Polish language) | 547 | ? | NR | ± | W | RCT | ? | ? | < 60 | ? | + | + | – | ? | M | ± | ± | + | ? | ? | W | ? | ? | ? | W | + | – | 80–100 | + | + | S | W |
| Khoromi, 2007214 | 534 | ± | 80–100 | + | S | RCT (crossover) | + | + | 80–100 | + | + | + | + | + | S | + | + | + | + | ? | M | + | + | + | S | ? | + | < 60 | + | – | W | M |
| Opioids vs mixed treatments | ||||||||||||||||||||||||||||||||
| Khoromi, 2007214 | 534 | ± | 80–100 | + | S | RCT (crossover) | + | + | 80–100 | + | + | + | + | + | S | + | + | + | + | ? | M | + | + | + | S | ? | + | < 60 | + | – | W | M |
Education/advice
| Study | ID no. | EV1 | EV2 (%) | EV3 | EVR | SB1 | SB2 | SB3 | SB4 (%) | SB5 | SB6 | SB7 | SB8 | SB9 | SBR | DB1a | DB1b | DB2 | DB3 | DB4 | DBR | PB1 | PB2 | PB3 | PBR | AB1 | AB1a | AB2 (%) | AB3 | AB4 | ABR | GR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Education/advice vs activity restriction | ||||||||||||||||||||||||||||||||
| Vroomen, 199914 | 658 | + | 80–100 | + | S | RCT | + | – | 60–79 | ± | + | + | + | + | M | + | + | + | + | + | S | – | – | NA | W | + | 80–100 | + | + | S | M | |
| Hofstee, 2002267 | 713 | ± | 80–100 | + | M | RCT | + | – | 60–79 | + | + | + | + | ± | M | + | + | + | – | ? | W | – | – | NA | W | ? | – | 80–100 | + | + | M | M |
| Education/advice vs epidural/intradiscal injection | ||||||||||||||||||||||||||||||||
| Bronfort, 2004169 | 722 | ? | < 60 | ± | W | RCT | ? | ± | 60–79 | + | + | + | – | ? | M | + | + | + | ? | ? | W | – | – | NA | W | ? | ? | 80–100 | NA | – | W | W |
| Education advice vs manipulation | ||||||||||||||||||||||||||||||||
| Bronfort, 2004169 | 722 | ? | < 60 | ± | W | RCT | ? | ± | 60–79 | + | + | + | – | ? | M | + | + | + | ? | ? | W | – | – | NA | W | ? | ? | 80–100 | NA | – | W | W |
| Education advice vs mixed treatments | ||||||||||||||||||||||||||||||||
| Hofstee, 2002267 | 713 | ± | 80–100 | + | M | RCT | + | – | 60–79 | + | + | + | + | ± | M | + | + | + | – | ? | W | – | – | NA | W | ? | – | 80–100 | + | + | M | M |
Appendix 6 Network diagrams
Global effect mixed treatment comparison network for randomised controlled trials and quasi-randomised controlled trials
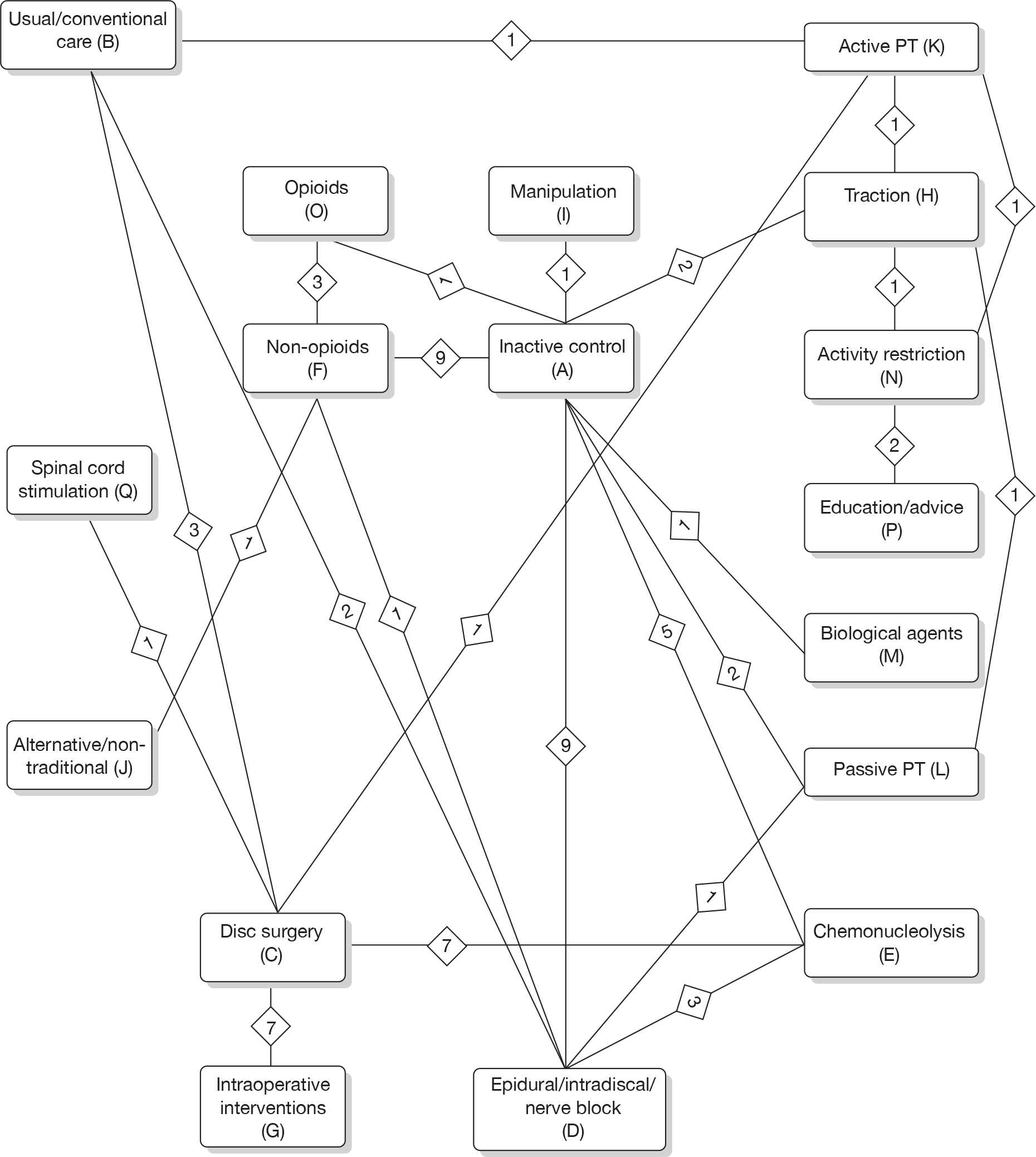
Pain mixed treatment comparison network for all study designs
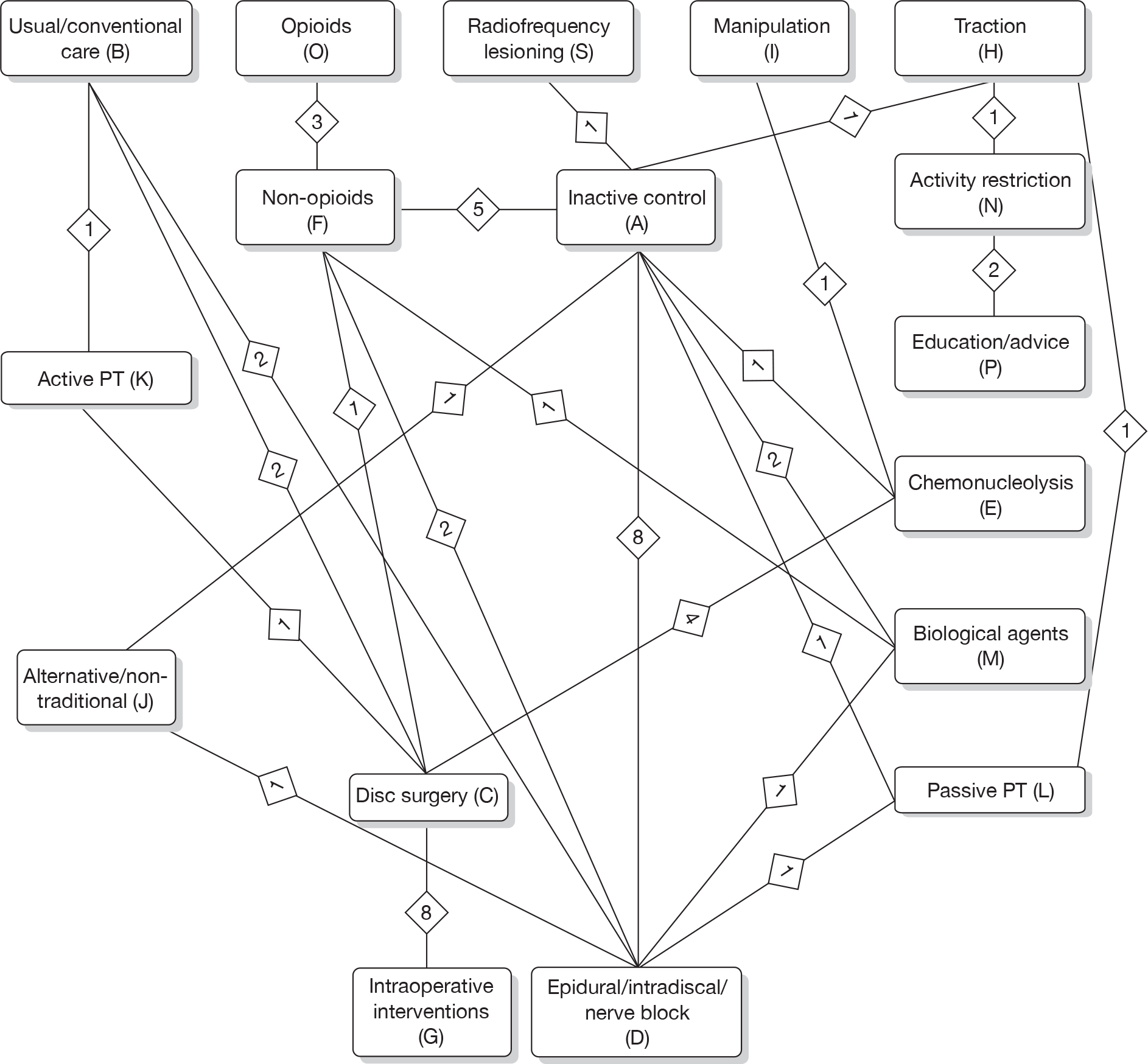
Pain mixed treatment comparison network for randomised controlled trials and quasi-randomised controlled trials
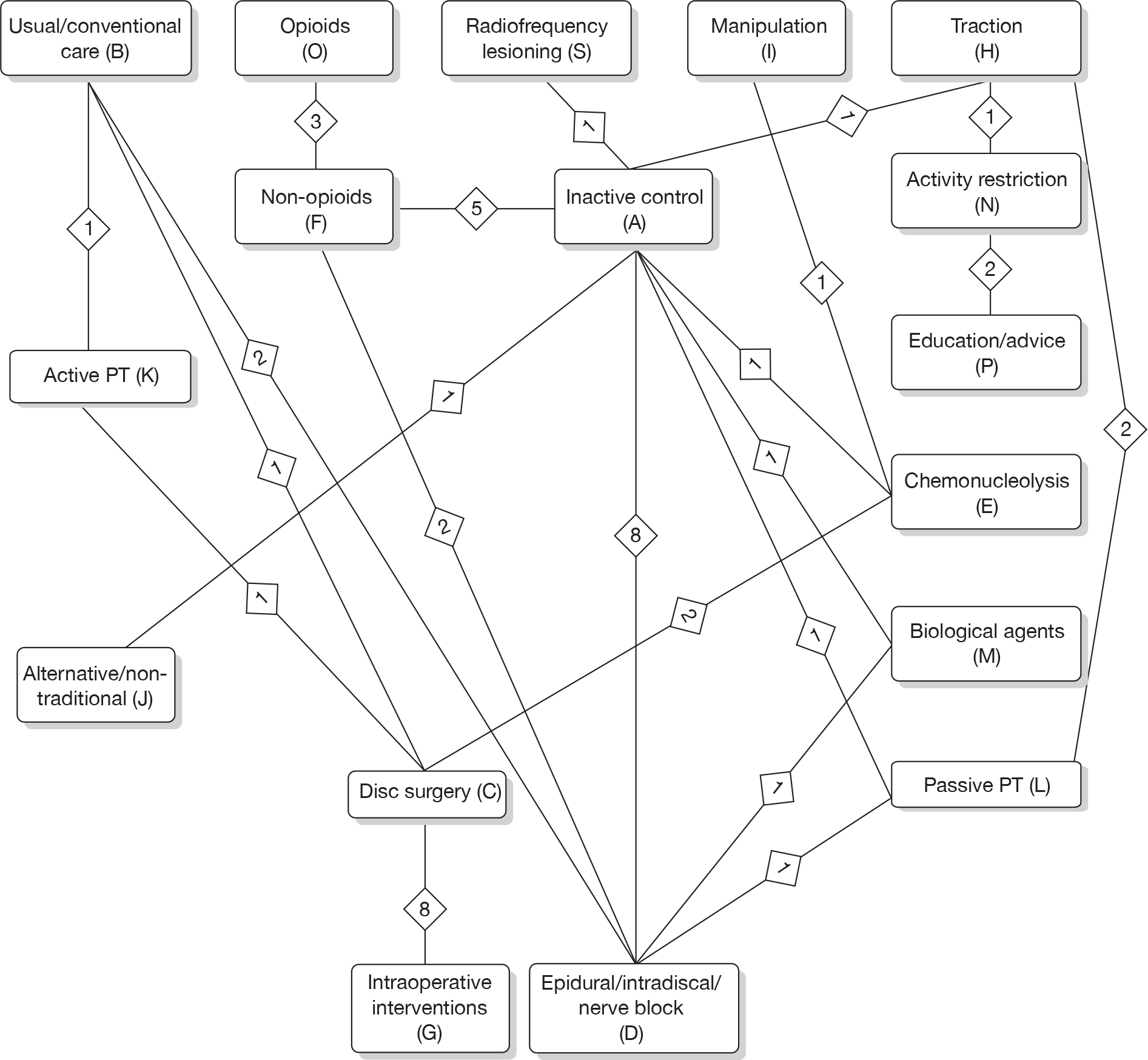
Condition-specific outcome measure mixed treatment comparison network for all study designs
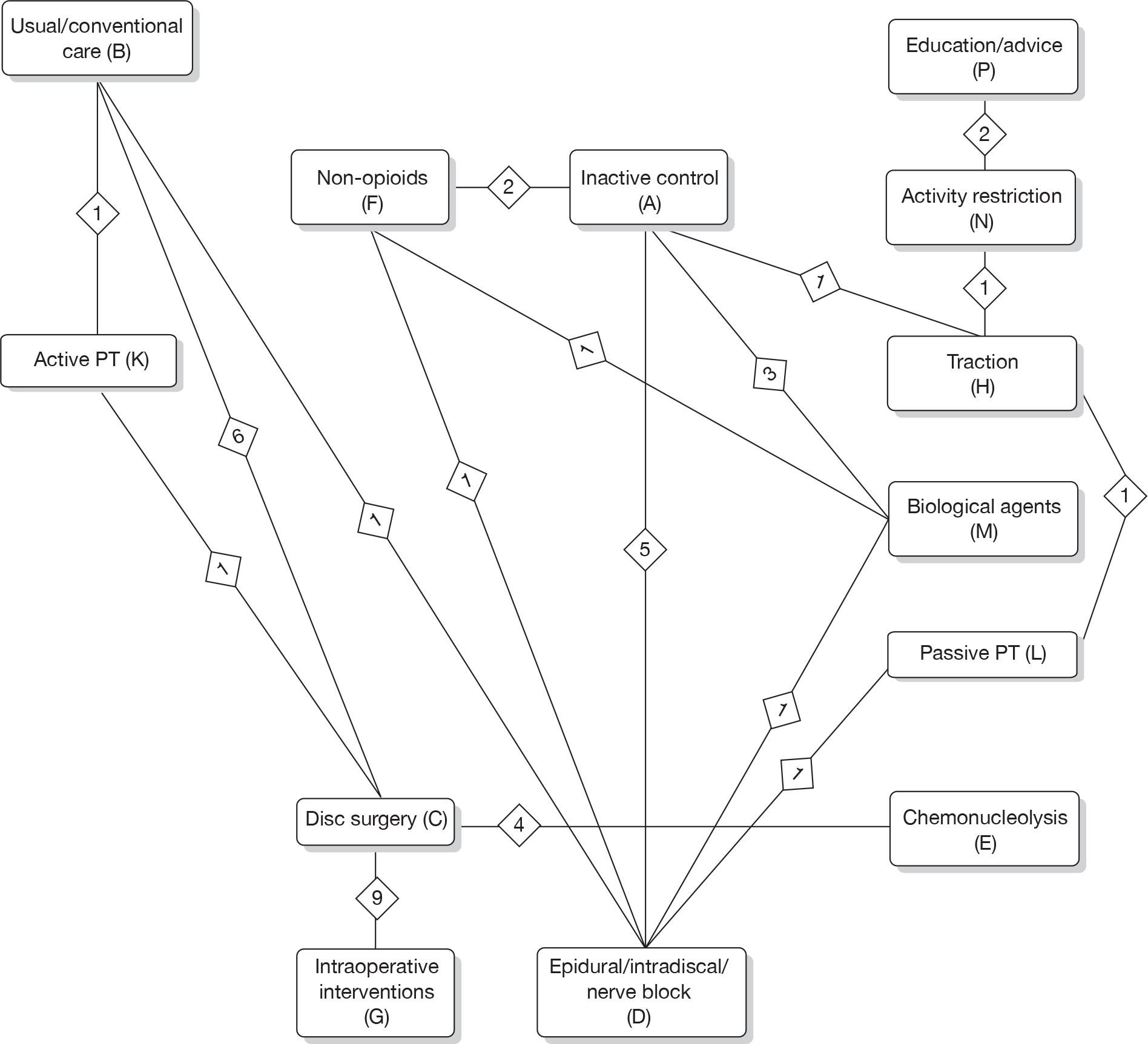
Condition-specific outcome measure mixed treatment comparison network for randomised controlled trials and quasi-randomised controlled trials
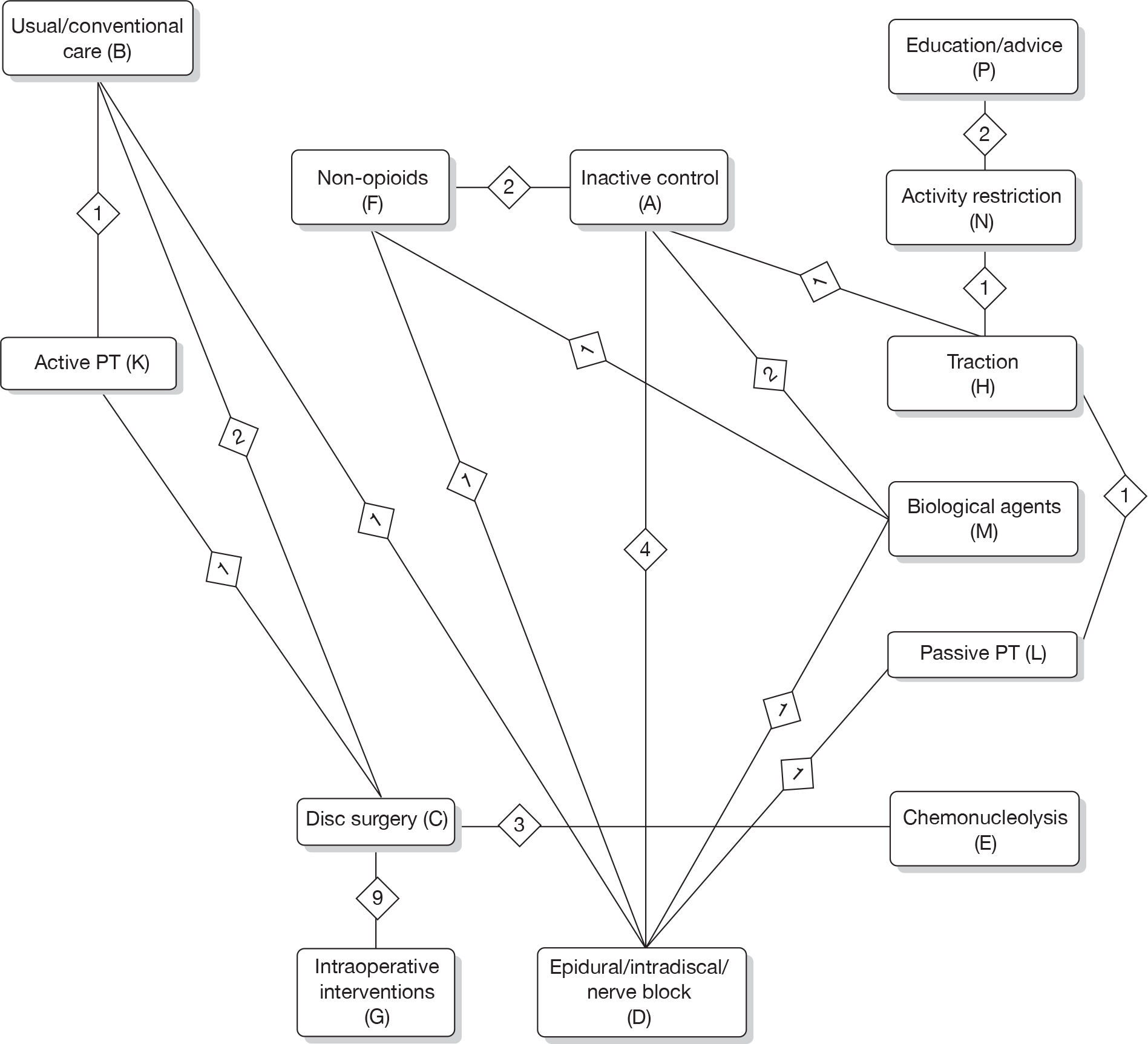
Appendix 7 Winbugs plots for the Gelman–Rubin statistic
Here are a selection of Brook–Gelman–Rubin diagnostic plots that demonstrate that convergence was achieved at around 6–8000 iterations for all three outcome measures included in the MTC analyses [global effect (Figures 113–115), pain intensity (Figures 116–118) and CSOMs (Figures 119–121)]. The black line represents R, the dark grey line represents B (pooled) and the light grey line represents W (average). R = B/W, where B is the within-chain variability and W the between-chain variability. It is important not only that R has converged around 1, but also that B and W have converged to stability.
FIGURE 113.
Diagnostic plot for the parameter T (risk of improving) for treatment 11 (K – active PT).
FIGURE 114.
Diagnostic plot for the parameter d (log OR) for treatment 5 (E – chemonucleolysis) compared with inactive control.
FIGURE 115.
Diagnostic plot for the parameter OR of treatment 7 (G – intraoperative interventions) compared with inactive control.
FIGURE 116.
Diagnostic plot for the parameter SMD (mean difference) for the comparison of treatment 7 (G – intraoperative interventions) with treatment 13 (M – biological agents).
FIGURE 117.
Diagnostic plot for the parameter SMD (mean difference) for the comparison of treatment 14 (N – activity restriction) with treatment 15 (O – opioids).
FIGURE 118.
Diagnostic plot for the parameter SMD (mean difference) for the comparison of treatment 9 (I – manipulation) with treatment 16 (P – education/advice).
FIGURE 119.
Diagnostic plot for the parameter d (mean difference) for the comparison of treatment 6 (F – non-opioids) with inactive control.
FIGURE 120.
Diagnostic plot for the parameter sd.d (between-study deviation).
FIGURE 121.
Diagnostic plot for the parameter SMD (mean difference) for the comparison of treatment 2 (B – usual care) with treatment 3 (C – disc surgery).
Appendix 8 Heterogeneity and model fit for the mixed treatment comparison analyses
Model fit
| Model | No. of data points | Posterior mean deviance |
|---|---|---|
| Global effect including all study designs | 178 | 186 |
| Global effect including RCTs and Q-RCTs | 130 | 135 |
| Pain intensity including all study designs | 108 | 109 |
| Pain intensity including RCTs and Q-RCTs | 94 | 94 |
| CSOMs including all study designs | 82 | 41 |
| CSOMs including RCTs and Q-RCTs | 66 | 28 |
Assessment of heterogeneity
Global effect
For the MTC analyses of global effect that included all study designs, the median between-trial variance (tau-squared) observed in the posterior distributions was 0.72 (95% credible interval 0.44 to 1.18). However, this does not give an indication of the between-study heterogeneity within each intervention comparison. This information has been derived from the standard, pair-wise meta-analyses shown in Table 177. There was high-to-moderate heterogeneity between studies for the following comparators: epidural versus inactive control, non-opioids versus inactive control, passive PT versus inactive control, disc surgery versus usual care, epidural versus usual care, chemonucleolysis versus disc surgery, chemonucleolysis versus epidural and opioids versus non-opioids.
| Treatment comparators (no. of studies) | Chi-squared statistic | Degrees of freedom | p-value | I 2 (%)a | τ2 |
|---|---|---|---|---|---|
| DA (9) | 26.58 | 8 | 0.001 | 69.9 | 0.7921 |
| EA (5) | 5.01 | 4 | 0.286 | 20.2 | 0.0604 |
| FA (10) | 45.44 | 9 | 0.000 | 80.2 | 1.0142 |
| HA (2) | 0.18 | 1 | 0.669 | 0.0 | 0.0000 |
| IA (1) | 0.00 | 0 | 0.0000 | ||
| LA (2) | 6.64 | 1 | 0.010 | 84.9 | 1.7336 |
| MA (1) | 0.00 | 0 | 1.7336 | ||
| OA (1) | 0.00 | 0 | 1.7336 | ||
| CB (5) | 10.15 | 4 | 0.038 | 60.6 | 0.1806 |
| DB (3) | 8.82 | 2 | 0.012 | 77.3 | 2.2995 |
| HB (1) | 0.00 | 0 | 2.2995 | ||
| KB (1) | 0.00 | 0 | 2.2995 | ||
| EC (23) | 94.63 | 22 | 0.000 | 76.8 | 0.4426 |
| FC (1) | 0.00 | 0 | 0.4426 | ||
| GC (7) | 2.32 | 6 | 0.888 | 0.0 | 0.0000 |
| KC(1) | 0.00 | 0 | 0.0000 | ||
| QC (1) | 0.00 | 0 | 0.0000 | ||
| ED (4) | 15.34 | 3 | 0.002 | 80.4 | 1.0747 |
| FD (1) | 0.00 | 0 | 1.0747 | ||
| LD (1) | 0.00 | 0 | 1.0747 | ||
| ND (1) | 0.00 | 0 | 1.0747 | ||
| JF (1) | 0.00 | 0 | 1.0747 | ||
| OF (2) | 5.61 | 1 | 0.018 | 82.2 | 2.0863 |
| KH (1) | 0.00 | 0 | 2.0863 | ||
| LH (1) | 0.00 | 0 | 2.0863 | ||
| NH (1) | 0.00 | 0 | 2.0863 | ||
| NK (1) | 0.00 | 0 | 2.0863 | ||
| PN (2) | 0.76 | 1 | 0.384 | 0.0 | 0.0000 |
| Overall | 412.18 | 89 | 0.000 | 78.4 | 0.7237 |
For the MTC analyses of global effect that included only the RCTs and Q-RCTs, the median between-trial variance (tau-squared) observed in the posterior distributions was 0.74 (95% credible interval 0.40 to 1.34). The between-study heterogeneity for each intervention comparison, derived from the standard, pair-wise meta-analyses, is shown in Table 178. There was high-to-moderate heterogeneity between studies for the following comparators: epidural versus inactive control, non-opioids versus inactive control, passive PT versus inactive control, disc surgery versus usual care, chemonucleolysis versus disc surgery, chemonucleolysis versus epidural and opioids versus non-opioids.
| Treatment comparators (no. of studies) | Chi-squared statistic | Degrees of freedom | p-value | I 2 (%)a | τ2 |
|---|---|---|---|---|---|
| DA (9) | 26.58 | 8 | 0.001 | 69.9 | 0.7921 |
| EA (5) | 5.01 | 4 | 0.286 | 20.2 | 0.0604 |
| FA (9) | 41.56 | 8 | 0.000 | 80.8 | 1.0459 |
| HA (2) | 0.18 | 1 | 0.669 | 0.0 | 0.0000 |
| IA (1) | 0.00 | 0 | 0.0000 | ||
| LA (2) | 6.64 | 1 | 0.010 | 84.9 | 1.7336 |
| MA (1) | 0.00 | 0 | 1.7336 | ||
| OA (1) | 0.00 | 0 | 1.7336 | ||
| CB (3) | 8.96 | 2 | 0.011 | 77.7 | 0.4647 |
| DB (2) | 0.17 | 1 | 0.680 | 0.0 | 0.0000 |
| KB (1) | 0.00 | 0 | 0.0000 | ||
| EC (7) | 19.69 | 6 | 0.003 | 69.5 | 0.4323 |
| GC (7) | 2.32 | 6 | 0.888 | 0.0 | 0.0000 |
| KC (1) | 0.00 | 0 | 0.0000 | ||
| QC (1) | 0.00 | 0 | 0.0000 | ||
| ED (3) | 8.79 | 2 | 0.012 | 77.2 | 0.6664 |
| FD (1) | 0.00 | 0 | 0.6664 | ||
| LD (1) | 0.00 | 0 | 0.6664 | ||
| JF (1) | 0.00 | 0 | 0.6664 | ||
| OF (2) | 5.61 | 1 | 0.018 | 82.2 | 2.0863 |
| KH (1) | 0.00 | 0 | 2.0863 | ||
| LH (1) | 0.00 | 0 | 2.0863 | ||
| NH (1) | 0.00 | 0 | 2.0863 | ||
| NK (1) | 0.00 | 0 | 2.0863 | ||
| PN (2) | 0.76 | 1 | 0.384 | 0.0 | 0.0000 |
| Overall | 199.22 | 65 | 0.000 | 67.4 | 0.4844 |
Pain intensity
For the MTC analyses of pain intensity that included all study designs, the median between-trial variance (tau-squared) observed in the posterior distributions was 140.40 (95% credible interval 77.86 to 261.50). The between-study heterogeneity for each intervention comparison, derived from the standard, pair-wise meta-analyses, is shown in Table 179. There was high-to-moderate heterogeneity between studies for the following comparators: epidural versus inactive control, chemonucleolysis versus disc surgery, non-opioids versus inactive control, non-opioids versus epidural, intraoperative interventions versus disc surgery, traction versus passive PT and biological agents versus inactive control.
| Treatment comparator (no. of studies) | Chi-squared statistic | Degrees of freedom | p-value | I 2 (%)a | τ2 |
|---|---|---|---|---|---|
| CB (2) | 1.02 | 1 | 0.313 | 1.8 | 0.7251 |
| DA (8) | 69.78 | 7 | 0.000 | 90.0 | 239.5391 |
| DB (2) | 0.03 | 1 | 0.868 | 0.0 | 0.0000 |
| DL (1) | 0.00 | 0 | 0.0000 | ||
| EA (1) | 0.00 | 0 | 0.0000 | ||
| EC (4) | 16.99 | 3 | 0.001 | 82.3 | 122.3658 |
| FA (5) | 36.88 | 4 | 0.000 | 89.2 | 118.1834 |
| FC (1) | 0.00 | 0 | 0.0000 | ||
| FD (2) | 7.92 | 1 | 0.005 | 87.4 | 62.9147 |
| GC (8) | 29.82 | 7 | 0.000 | 76.5 | 72.3876 |
| HA (1) | 0.00 | 0 | 0.0000 | ||
| HL (2) | 4.28 | 1 | 0.038 | 76.7 | 122.8115 |
| IE (1) | 0.00 | 0 | 0.0000 | ||
| JA (1) | 0.00 | 0 | 0.0000 | ||
| JD (1) | 0.00 | 0 | 0.0000 | ||
| KB (1) | 0.00 | 0 | 0.0000 | ||
| KC (1) | 0.00 | 0 | 0.0000 | ||
| LA (1) | 0.00 | 0 | 0.0000 | ||
| MA (2) | 13.69 | 1 | 0.000 | 92.7 | 535.7751 |
| MF (1) | 0.00 | 0 | 0.0000 | ||
| NH (1) | 0.00 | 0 | 0.0000 | ||
| OF (3) | 3.81 | 2 | 0.149 | 47.5 | 43.5168 |
| PN (2) | 0.16 | 1 | 0.689 | 0.0 | 0.0000 |
| SA (1) | 0.00 | 0 | 0.0000 |
For the MTC analyses of pain intensity that included only the RCTs and Q-RCTs, the median between-trial variance (tau-squared) observed in the posterior distributions was 155.90 (95% credible interval 82.98 to 306.20). The between-study heterogeneity for each intervention comparison, derived from the standard pair-wise meta-analyses, is shown in Table 180. There was high-to-moderate heterogeneity between studies for the following comparators: epidural versus inactive control, non-opioids versus inactive control, chemonucleolysis versus disc surgery, intraoperative interventions versus disc surgery, non-opioids versus epidural and traction versus passive PT.
| Treatment comparator (no. of studies) | Chi-squared statistic | Degrees of freedom | p-value | I 2 (%)a | τ2 |
|---|---|---|---|---|---|
| DA (8) | 69.78 | 7 | 0.000 | 90.0 | 239.5391 |
| EA (1) | 0.00 | 0 | 0.0000 | ||
| FA (5) | 36.88 | 4 | 0.000 | 89.2 | 118.1834 |
| HA (1) | 0.00 | 0 | 0.0000 | ||
| JA (1) | 0.00 | 0 | 0.0000 | ||
| LA (1) | 0.00 | 0 | 0.0000 | ||
| MA (1) | 0.00 | 0 | 0.0000 | ||
| SA (1) | 0.00 | 0 | 0.0000 | ||
| CB (1) | 0.00 | 0 | 0.0000 | ||
| DB (2) | 0.03 | 1 | 0.868 | 0.0 | 0.0000 |
| KB (1) | 0.00 | 0 | 0.0000 | ||
| EC (2) | 11.73 | 1 | 0.001 | 91.5 | 263.4570 |
| GC (8) | 29.82 | 7 | 0.000 | 76.5 | 72.3876 |
| KC (1) | 0.00 | 0 | 0.0000 | ||
| FD (2) | 7.92 | 1 | 0.005 | 87.4 | 62.9147 |
| IE (1) | 0.00 | 0 | 0.0000 | ||
| OF (3) | 3.81 | 2 | 0.149 | 47.5 | 43.5168 |
| NH (1) | 0.00 | 0 | 0.0000 | ||
| DL (1) | 0.00 | 0 | 0.0000 | ||
| HL (2) | 4.28 | 1 | 0.038 | 76.7 | 122.8115 |
| PN (2) | 0.16 | 1 | 0.689 | 0.0 | 0.0000 |
Condition-specific outcome measures
For the MTC analyses of CSOMs that included all study designs, the median between-trial variance (tau-squared) observed in the posterior distributions was 0.02 (95% credible interval 4.84 × 105 to 0.18). The between-study heterogeneity for each intervention comparison, derived from the standard, pair-wise meta-analyses, is shown in Table 181. There was high-to-moderate heterogeneity between studies for the following comparators: epidural versus inactive control, disc surgery versus usual care and intraoperative interventions versus disc surgery.
| Treatment comparator (no. of studies) | Chi-squared statistic | Degrees of freedom | p-value | I 2 (%)a | τ2 |
|---|---|---|---|---|---|
| DA (5) | 34.06 | 4 | 0.000 | 88.3 | 0.2445 |
| FA (2) | 1.56 | 1 | 0.212 | 35.9 | 0.0469 |
| HA (1) | 0.00 | 0 | 0.0000 | ||
| MA (3) | 3.75 | 2 | 0.153 | 46.7 | 0.1602 |
| CB (6) | 41.21 | 5 | 0.000 | 87.9 | 0.0790 |
| DB (1) | 0.00 | 0 | 0.0000 | ||
| KB (1) | 0.00 | 0 | 0.0000 | ||
| EC (4) | 2.44 | 3 | 0.486 | 0.0 | 0.0000 |
| GC (8) | 26.04 | 7 | 0.000 | 73.1 | 0.0946 |
| KC (1) | 0.00 | 0 | 0.0000 | ||
| FD (1) | 0.00 | 0 | 0.0000 | ||
| LD (1) | 0.00 | 0 | 0.0000 | ||
| MD (1) | 0.00 | 0 | 0.0000 | ||
| MF (1) | 0.00 | 0 | 0.0000 | ||
| LH (1) | 0.00 | 0 | 0.0000 | ||
| NH (1) | 0.00 | 0 | 0.0000 | ||
| PN (2) | 0.68 | 1 | 0.410 | 0.0 | 0.0000 |
For the MTC analyses of CSOMs that included only the RCTs and Q-RCTs, the median between-trial variance (tau-squared) observed in the posterior distributions was 0.01 (95% credible interval 2.89 × 105 to 0.17). The between-study heterogeneity for each intervention comparison, derived from the standard, pair-wise meta-analyses, is shown in Table 182. There was high-to-moderate heterogeneity between studies for the following comparators: biological agents versus inactive control, chemonucleolysis versus disc surgery and intraoperative intervention versus disc surgery.
| Treatment comparator (no. of studies) | Chi-squared statistic | Degrees of freedom | p-value | I 2 (%)a | τ2 |
|---|---|---|---|---|---|
| DA (4) | 2.86 | 3 | 0.413 | 0.0 | 0.0000 |
| FA (2) | 1.56 | 1 | 0.212 | 35.9 | 0.0469 |
| HA (1) | 0.00 | 0 | 0.0000 | ||
| MA (2) | 3.42 | 1 | 0.064 | 70.7 | 0.9498 |
| CB (2) | 0.04 | 1 | 0.835 | 0.0 | 0.0000 |
| DB (1) | 0.00 | 0 | 0.0000 | ||
| KB (1) | 0.00 | 0 | 0.0000 | ||
| EC (4) | 9.17 | 3 | 0.027 | 67.3 | 0.1253 |
| GC (8) | 26.04 | 7 | 0.000 | 73.1 | 0.0946 |
| KC (1) | 0.00 | 0 | 0.0000 | ||
| FD (1) | 0.00 | 0 | 0.0000 | ||
| LD (1) | 0.00 | 0 | 0.0000 | ||
| MD (1) | 0.00 | 0 | 0.0000 | ||
| LH (1) | 0.00 | 0 | 0.0000 | ||
| NH (1) | 0.00 | 0 | 0.0000 | ||
| PN (2) | 0.68 | 1 | 0.410 | 0.0 | 0.0000 |
Appendix 9 Results of the mixed treatment comparison analyses
| Treatment category | Treatment code | Treatment code | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | ||
| Inactive control | A |
N = 9 2.58 (1.25 to 5.29) |
N = 5, 2.56 (1.59 to 4.12) |
N = 10 (n = 9) 2.16 (1.05 to 4.46) |
N = 2 1.11 (0.60 to 2.05) |
N = 1 4.71 (1.95 to 11.37) |
N = 2 1.57 (0.22 to 11.33) |
N = 1 10.0 (0.65 to 166.6) |
N = 1 1.37 (0.50 to 3.76) |
|||||||||
| Usual care | B | 0.83 (0.35 to 1.91) |
N = 5 (n = 3) 2.60 (1.59 to 4.25) |
N = 3 (n = 2) 5.46 (0.77 to 38.50) |
N = 1 (n = 0) 1.53 (0.56 to 4.19) |
N = 1 1.45 (0.71 to 2.98) |
||||||||||||
| Disc surgery | C | 2.78 (1.37 to 5.59) | 3.37 (1.70 to 6.76) |
N = 23 (n = 7) 0.65 (0.47 to 0.89) |
N = 1, (n = 0) 6.72 (0.77 to 58.79) |
N = 7 1.49 (1.03 to 2.15) |
N = 1 0.77 (0.18 to 3.22) |
N = 1 1.13 (0.36 to 3.60) | ||||||||||
| Epidural/nerve Block | D | 3.10 (1.79 to 5.46) | 3.75 (1.69 to 8.42) | 1.11 (0.55 to 2.25) |
N = 4 (n = 3) 1.13 (0.35 to 3.61) |
N = 1 0.45 (0.15 to 1.39) |
N = 1 0.20 (0.07 to 0.57) |
N = 1 (n = 0) 0.22 (0.06 to 0.86) |
||||||||||
| Chemonucleolysis | E | 2.00 (1.05 to 3.82) | 2.42 (1.16 to 5.12) | 0.72 (0.49 to 1.06) | 0.65 (0.34 to 1.24) | |||||||||||||
| Non-opioids | F | 2.55 (1.42 to 4.65) | 3.09 (1.16 to 8.39) | 0.92 (0.39 to 2.2) | 0.82 (0.38 to 1.78) | 1.27 (0.56 to 2.94) |
N = 1 3.27 (0.77 to 13.83) |
N = 2 0.55 (0.06 to 5.03) |
||||||||||
| Intraoperative interventions | G | 4.73 (1.61 to 13.99) | 5.72 (1.97 to 16.75) | 1.70 (0.76 to 3.86) | 1.52 (0.52 to 4.49) | 2.35 (0.96 to 5.82) | 1.85 (0.57 to 6.06) | |||||||||||
| Traction | H | 1.20 (0.47 to 3.08) | 1.46 (0.51 to 4.20) | 0.44 (0.15 to 1.23) | 0.39 (0.14 to 1.05) | 0.60 (0.21 to 1.68) | 0.47 (0.16 to 1.40) | 0.26 (0.07 to 0.95) |
N = 1 0.88 (0.28 to 2.72) |
N = 1 0.93 (0.46 to 1.86) |
N = 1, 1.00 (0.14 to 7.01) | |||||||
| Manipulation | I | 4.88 (0.73 to 33.2) | 5.91 (0.72 to 47.07) | 1.76 (0.23 to 13.38) | 1.57 (0.21 to 11.36) | 2.45 (0.32 to 18.31) | 1.91 (0.26 to 14.0) | 1.03 (0.11 to 9.12) | 4.06 (0.47 to 33.75) | |||||||||
| Alternative/non-traditional | J | 9.32 (0.95 to 104.5) | 11.27 (0.99 to 144.5) | 3.35 (0.31 to 40.88) | 2.99 (0.29 to 35.58) | 4.64 (0.43 to 56.23) | 3.65 (0.39 to 38.01) | 1.98 (0.16 to 27.49) | 7.73 (0.65 to 102.4) | 1.91 (0.10 to 41.68) | ||||||||
| Active PT | K | 1.10 (0.32 to 3.78) | 1.33 (0.40 to 4.35) | 0.40 (0.12 to 1.30) | 0.35 (0.10 to 1.20) | 0.55 (0.16 to 1.85) | 0.43 (0.11 to 1.63) | 0.23 (0.05 to 0.99) | 0.90 (0.26 to 3.16) | 0.22 (0.02 to 2.24) | 0.12 (0.01 to 1.57) |
N = 1 2.20 (0.63 to 7.66) |
||||||
| Passive PT | L | 1.14 (0.41 to 3.17) | 1.38 (0.40 to 4.72) | 0.41 (0.13 to 1.33) | 0.37 (0.12 to 1.07)a | 0.57 (0.18 to 1.79) | 0.45 (0.14 to 1.43) | 0.24 (0.06 to 1.01) | 0.94 (0.29 to 3.07) | 0.23 (0.03 to 2.03) | 0.13 (0.01 to 1.50) | 1.04 (0.23 to 4.73) | ||||||
| Biological agents | M | 15.77 (0.61 to 1002.00) | 19.26 (0.66 to 1357.00) | 5.68 (0.21 to 396.40) | 5.10 (0.18 to 335.30) | 7.90 (0.29 to 544.60) | 6.19 (0.23 to 408.60) | 3.38 (0.11 to 248.70) | 13.20 (0.44 to 942.70) | 3.36 (0.07 to 306.30) | 1.75 (0.03 to 179.20) | 14.6 (0.44 to 1085.00) | 14.03 (0.45 to 973.70) | |||||
| Activity restriction | N | 1.278 (0.29 to 5.51) | 1.54 (0.33 to 7.08) | 0.46 (0.10 to 2.03) | 0.41 (0.09 to 1.71) | 0.64 (0.14 to 2.80) | 0.50 (0.10 to 2.36) | 0.27 (0.05 to 1.49) | 1.05 (0.24 to 4.7) | 0.26 (0.02 to 2.9) | 0.14 (0.01 to 2.03) | 1.16 (0.26 to 5.07) | 1.12 (0.20 to 5.98) | 0.08 (0.00 to 2.89) |
N = 2 1.32 (0.75 to 2.32) |
|||
| Opioids | O | 1.60 (0.48 to 5.41) | 1.95 (0.45 to 8.36) | 0.58 (0.15 to 2.27) | 0.52 (0.14 to 1.92) | 0.80 (0.21 to 3.07) | 0.63 (0.20 to 1.96) | 0.34 (0.07 to 1.67) | 1.33 (0.29 to 6.19) | 0.33 (0.03 to 3.27) | 0.17 (0.01 to 2.10) | 1.46 (0.27 to 8.33) | 1.41 (0.29 to 6.83) | 0.10 (0.00 to 3.27) | 1.26 (0.19 to 8.66) | |||
| Education/advice | P | 1.63 (0.22 to 12.05) | 1.98 (0.26 to 14.69) | 0.59 (0.08 to 4.34) | 0.53 (0.07 to 3.72) | 0.81 (0.11 to 6.00) | 0.64 (0.08 to 5.0) | 0.34 (0.04 to 2.99) | 1.35 (0.18 to 9.99) | 0.33 (0.02 to 5.3) | 0.17 (0.01 to 3.49) | 1.48 (0.20 to 10.85) | 1.43 (0.17 to 12.47 | 0.10 (0.00 to 4.86) | 1.28 (0.34 to 4.88) | 1.02 (0.10 to 10.18) | ||
| Spinal cord stimulation | Q | 3.19 (0.36 to 27.57) | 3.84 (0.44 to 33.72) | 1.14 (0.15 to 8.89) | 1.03 (0.12 to 8.91) | 1.59 (0.20 to 12.84) | 1.25 (0.13 to 11.55) | 0.67 (0.07 to 5.93) | 2.66 (0.26 to 25.95) | 0.65 (0.04 to 12.15) | 0.34 (0.01 to 8.00) | 2.90 (0.27 to 30.79) | 2.80 (0.26 to 28.88 | 0.19 (0.00 to 10.13) | 2.54 (0.20 to 31.92) | 2.0 (0.16 to 23.5) | 1.97 (0.11 to 34.79) | |
| Treatment category | Treatment code | Treatment code | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | ||
| Inactive control | A |
N = 9 2.58 (1.25 to 5.29) |
N = 5 2.56 (1.59 to 4.12) |
N = 9 2.50 (1.16 to 5.42) |
N = 2 1.11 (0.60 to 2.05) |
N = 1 4.71 (1.95 to 11.37) |
N = 2 1.57 (0.22 to 11.33) |
N = 1 10.00 (0.65 to 166.60) |
N = 1 1.37 (0.50 to 3.76) |
|||||||||
| Usual care | B | 1.14 (0.39 to 3.46) |
N = 3 2.62 (1.09 to 6.31) |
N = 2 1.99 (0.66 to 6.03) |
N = 1 1.46 (0.71 to 2.98) |
|||||||||||||
| Disc surgery | C | 2.94 (1.18 to 7.49) | 2.57 (1.03 to 6.42) |
N = 7 0.83 (0.45 to 1.52) |
N = 7 1.49 (1.03 to 2.15) |
N = 1 0.77 (0.18 to 3.22) |
N = 1 1.13 (0.36 to 3.60) |
|||||||||||
| Epidural/nerve block | D | 3.14 (1.77 to 5.65) | 2.74 (0.94 to 8.02) | 1.07 (0.42 to 2.69) |
N = 3 0.74 (0.26 to 2.11) |
N = 1 0.46 (0.15 to 1.39) |
N = 1 0.20 (0.07 to 0.57) |
|||||||||||
| Chemonucleolysis | E | 2.38 (1.20 to 4.81) | 2.07 (0.75 to 5.75) | 0.81 (0.40 to 1.61) | 0.76 (0.37 to 1.57) | |||||||||||||
| Non-opioids | F | 2.59 (1.37 to 4.95) | 2.26 (0.64 to 7.88) | 0.88 (0.29 to 2.66) | 0.82 (0.36 to 1.89) | 1.09 (0.43 to 2.80) |
N = 1 3.27 (0.77 to 13.83) |
N = 20.55 (0.06 to 5.03) | ||||||||||
| Intraoperative interventions | G | 4.99 (1.50 to 17.47) | 4.36 (1.29 to 14.94) | 1.7 (0.76 to 3.88) | 1.59 (0.48 to 5.53) | 2.10 (0.72 to 6.25) | 1.93 (0.49 to 7.82) | |||||||||||
| Traction | H | 1.36 (0.48 to 3.95) | 1.19 (0.30 to 4.69) | 0.46 (0.13 to 1.67) | 0.43 (0.13 to 1.37) | 0.57 (0.17 to 1.91) | 0.52 (0.15 to 1.80) | 0.27 (0.06 to 1.23) |
N = 1 0.88 (0.28 to 2.72) |
N = 1 0.93 (0.46 to 1.86) |
N = 1 1.00 (0.14 to 7.10) |
|||||||
| Manipulation | I | 4.90 (0.70 to 34.48) | 4.31 (0.45 to 38.85) | 1.67 (0.20 to 14.14) | 1.56 (0.20 to 11.7) | 2.05 (0.26 to 16.04) | 1.89 (0.24 to 14.64) | 0.98 (0.10 to 9.52) | 3.62 (0.39 to 32.82) | |||||||||
| Alternative/non-traditional | J | 9.25 (0.90 to 107.70) | 8.15 (0.62 to 116.60) | 3.16 (0.25 to 43.04) | 2.95 (0.27 to 36.25) | 3.89 (0.34 to 49.11) | 3.59 (0.38 to 38.59) | 1.85 (0.13 to 28.39) | 6.84 (0.53 to 96.63) | 1.92 (0.09 to 42.55) | ||||||||
| Active PT | K | 1.46 (0.38 to 5.76) | 1.28 (0.34 to 4.69) | 0.50 (0.13 to 1.84) | 0.47 (0.12 to 1.85) | 0.62 (0.16 to 2.38) | 0.57 (0.13 to 2.5) | 0.29 (0.06 to 1.35) | 1.07 (0.28 to 4.26) | 0.30 (0.03 to 3.22) | 0.16 (0.01 to 2.35) |
N = 1 2.20 (0.63 to 7.66) |
||||||
| Passive PT | L | 1.19 (0.42 to 3.42) | 1.04 (0.25 to 4.5) | 0.41 (0.11 to 1.53) | 0.38 (0.12 to 1.15) | 0.50 (0.15 to 1.70) | 0.46 (0.14 to 1.55) | 0.24 (0.05 to 1.12) | 0.87 (0.25 to 3.09) | 0.24 (0.03 to 2.17) | 0.13 (0.01 to 1.64) | 0.81 (0.17 to 4.05) | ||||||
| Biological agents | M | 16.04 (0.60 to 1138.00) | 14.11 (0.43 to 1128.00) | 5.48 (0.18 to 419.60) | 5.11 (0.18 to 369.00) | 6.76 (0.23 to 505.40) | 6.20 (0.21 to 448.50) | 3.24 (0.09 to 252.80) | 11.77 (0.36 to 959.10) | 3.32 (0.07 to 353.20) | 1.76 (0.03 to 215.70) | 11.04 (0.31 to 946.50) | 13.54 (0.42 to 1081.00) | |||||
| Activity restriction | N | 2.43 (0.35 to 17.52) | 2.14 (0.28 to 15.85) | 0.83 (0.11 to 6.06) | 0.77 (0.11 to 5.68) | 1.03 (0.14 to 7.39) | 0.93 (0.12 to 7.33) | 0.49 (0.06 to 4.10) | 1.78 (0.28 to 11.55) | 0.50 (0.03 to 7.64) | 0.26 (0.01 to 5.66) | 1.65 (0.29 to 9.68) | 2.04 (0.25 to 16.92) | 0.15 (0.00 to 7.27) |
N = 2 1.32 (0.75 to 2.32) |
|||
| Opioids | O | 1.62 (0.46 to 5.67) | 1.41 (0.27 to 7.42) | 0.55 (0.12 to 2.60) | 0.52 (0.13 to 2.01) | 0.68 (0.16 to 2.84) | 0.62 (0.20 to 1.98) | 0.32 (0.06 to 1.81) | 1.18 (0.23 to 6.16) | 0.33 (0.03 to 3.33) | 0.17 (0.01 to 2.18) | 1.10 (0.17 to 6.93) | 1.35 (0.27 to 6.93) | 0.10 (0.00 to 3.52) | 0.67 (0.06 to 6.78) | |||
| Education/advice | P | 3.12 (0.29 to 34.36) | 2.73 (0.24 to 30.54) | 1.07 (0.10 to 11.63) | 0.99 (0.09 to 10.91) | 1.32 (0.12 to 14.57) | 1.20 (0.11 to 14.2) | 0.63 (0.05 to 7.85) | 2.30 (0.23 to 23.04) | 0.64 (0.03 to 14.12) | 0.33 (0.01 to 9.53) | 2.14 (0.23 to 19.50) | 2.62 (0.22, to 32.80) | 0.19 (0.00 to 11.79) | 1.29 (0.33 to 4.95) | 1.94 (0.13 to 28.94) | ||
| Spinal cord stimulation | Q | 3.30 (0.34 to 32.73) | 2.88 (0.30 to 27.71) | 1.13 (0.14 to 9.05) | 1.05 (0.11 to 10.34) | 1.39 (0.16 to 12.78) | 1.27 (0.12 to 13.36) | 0.66 (0.07 to 6.12) | 2.43 (0.21 to 28.78) | 0.67 (0.03 to 13.70) | 0.35 (0.01 to 9.65) | 2.26 (0.19 to 26.77) | 2.79 (0.23 to 33.22) | 0.20 (0.00 to 12.04) | 1.35 (0.08 to 25.48) | 2.04 (0.16 to 27.35) |
1.05 (0.04 to 26.90) |
|
| Treatment category | Treatment code | Treatment code | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | S | ||
| Inactive control | A |
N = 8 –12.31 (–23.90 to –0.72) |
N = 1 –5.40 (–23.66, to 12.86) |
N = 5 –10.70 (–21.21 to –0.19) |
N = 1 3.36 (–14.49 to 21.21) |
N = 1 –25.00 (–41.75 to –8.24) |
N = 1 –7.00 (–13.58 to –0.42) |
N = 2 (n = 1) −9.00 (−43.23 to 23.41) |
N = 1 13.00 (2.04 to 23.96) |
|||||||||
| Usual care | B | –3.184 (–19.45 to 13.18) |
N = 2 (n = 1) –7.00 (–12.25 to –1.74) |
N = 2 –5.32 (–11.94 to 1.30) |
N = 1 –2.00 (–11.96 to 7.96) |
|||||||||||||
| Disc surgery | C | –9.78 (–26.51 to 6.81) | –6.64 (–21.11 to 7.76) |
N = 4 (n = 2) –2.59 (–14.58 to 9.40) |
N = 1 (n = 0) 1.60 (–8.20 to 11.40) |
N = 8 –5.17 (–12.21 to 1.88) |
N = 1 9.00 (–4.05 to 22.05) |
|||||||||||
| Epidural/nerve block | D | –12.85 (–20.91 to –5.14) | –9.71 (–25.14 to 5.62) | –3.10 (–19.68 to 13.43) |
N = 2 18.01 (6.25 to 29.77) |
N = 1 (n = 0) –14.00 (–27.45 to –0.55) |
N = 1 35.00 (–24.60 to 94.60) |
|||||||||||
| Chemonucleolysis | E | –11.24 (–29.76 to 7.20) | –8.02 (–26.15 to 9.77) | –1.44 (–13.82 to 10.64) | 1.65 (–17.04 to 20.15) |
N = 1 –0.63 (–14.98 to 13.72) |
||||||||||||
| Non-opioids | F | –4.07 (–13.57 to 5.11) | 0.92 (–18.10 to 16.17) | 5.71 (–11.21 to 22.62) | 8.78 (–1.55 to 19.24) | 7.15 (–11.81 to 26.3) |
N = 1 (n = 0) –40.50 (–58.08 to –22.92) |
N = 3 13.60 (2.78 to 24.42) |
||||||||||
| Intraoperative interventions | G | –14.88 (–34.05 to 4.02) | –11.75 (–28.87 to 5.38) | –5.11 (–14.41 to 4.20) | –2.01 (–20.98 to 17.09) | –3.66 (–19.02 to 11.79) | –10.81 (–30.10 to 8.48) | |||||||||||
| Traction | H | –1.21 (–22.07 to 20.04) | 1.90 (–24.28 to 28.84) | 8.52 (–17.94 to 35.63) | 11.68 (–10.39 to 34.32) | 10.03 (–17.58 to 38.21) | –2.87 (–20.02 to 2.87) | 13.62 (–14.54 to 42.31) |
N = 2 2.61 (–14.91 to 20.14) |
N = 1 19.00 (8.18 to 29.82) |
||||||||
| Manipulation | I | –11.72 (–44.97 to 21.59) | –8.58 (–41.76 to 24.46) | –1.94 (–32.62 to 28.27) | 1.11 (–32.16 to 34.64) | –0.48 (–28.48 to 27.31) | –7.56 (–41.24 to 26.05) | 3.19 (–28.63 to 34.92) | –10.48 (–50.41 to 28.56) | |||||||||
| Alternative/non-traditional | J | –26.08 (–46.65 to -6.06) | –23.00 (–48.02 to 2.38) | –16.36 (–41.91 to 9.29) | –13.28 (–33.37 to 7.09) | –14.89 (–41.69 to 12.17) | –22.05 (–43.82 to –0.11) | –11.27 (–38.44 to 16.18) | –24.96 (–54.12 to 4.16) | –14.41 (–53.23 to 24.17) | ||||||||
| Active PT | K | –3.04 (–27.35 to 20.94) | 0.08 (–19.84 to 20.16) | 6.64 (–13.61 to 27.10) | 9.84 (–13.97 to 33.74) | 8.17 (–15.08 to 31.54) | –0.96 (–23.41 to 25.50) | 11.75 (–10.45 to 34.39) | –1.85 (–34.27 to 29.92) | 8.57 (–27.25 to 45.28) | 23.14 (–8.13 to 53.85) | |||||||
| Passive PT | L | –0.40 (–19.33 to 19.0) | –2.72 (–22.17 to 28.03) | 9.34 (–15.66 to 35.04) | 12.48 (–7.67 to 33.38) | 10.76 (–15.62 to 37.78) | 3.66 (–17.32 to 25.3) | 14.42 (–12.47 to 41.62) | 0.75 (–16.02 to 17.69) | 11.19 (–26.92 to 50.11) | 25.67 (–1.88 to 53.54) | 2.59 (–27.72 to 33.73) | ||||||
| Biological agents | M | –21.80 (–35.95 to –7.95) | –18.67 (–39.17 to 1.87) | –12.09 (–32.85 to 8.74) | –8.93 (–23.51 to 5.59) | –10.68 (–32.97 to 11.88) | –17.79 (–32.99 to –2.46) | –6.99 (–29.96 to 15.99) | –20.58 (–46.05 to 4.22) | –10.19 (–45.66 to 25.66) | 4.24 (–19.93 to 28.40) | –18.76 (–45.93 to 8.62) | –21.31 (–45.47 to –1.87) | |||||
| Activity restriction | N | 18.00 (–15.57 to 51.16) | 21.18 (–16.40 to 58.23) | 27.68 (–9.54 to 65.22) | 30.90 (–3.38 to 64.72) | 29.21 (–9.02 to 67.35) | 22.05 (–12.57 to 56.55) | 32.82 (–5.63 to 71.33) | 19.08 (–7.01 to 45.22) | 29.50 (–17.36 to 77.28) | 44.08 (4.85 to 82.93) | 21.10 (–20.36 to 62.3) | 18.47 (–13.19 to 49.04) | 39.74 (3.59 to 75.82) |
N = 2 –1.09 (–6.80 to 4.62) |
|||
| Opioids | O | 9.34 (–9.15 to 27.40) | 12.60 (–11.16 to 35.67) | 19.12 (–4.13 to 42.57) | 22.21 (3.32 to 41.09) | 20.55 (–4.29 to 45.29) | 13.41 (–2.61 to 29.23) | 24.23 (–0.61 to 49.21) | 10.51 (–17.88 to 38.26) | 20.95 (–16.01 to 58.50) | 35.48 (8.30 to 62.33) | 12.51 (–16.93 to 41.65) | 9.82 (–17.09 to 36.06) | 31.20 (9.17 to 52.98) | –8.58 (–46.97 to 29.51) | |||
| Education/advice | P | 17.04 (–20.8 to 54.62) | 20.22 (–21.16 to 61.27) | 26.84 (–14.40 to 68.38) | 29.97 (–8.72 to 68.38) | 28.35 (–13.73 to 70.55) | 21.13 (–17.79 to 60.14) | 31.95 (–10.38 to 74.44) | 18.20 (–13.40 to 49.86) | 28.75 (–21.32 to 79.48) | 43.22 (0.19 to 85.54) | 20.21 (–25.13 to 64.84) | 17.60 (–18.48 to 52.95) | 38.94 (–1.36 to 79.35) | –0.88 (–18.75 to 17.16) | 7.72 (–34.07 to 49.88) | ||
| Radiofrequency lesioning | S | 12.94 (–13.38 to 39.01) | 16.26 (–14.79 to 47.04) | 22.77 (–8.36 to 53.87) | 25.8 (–1.58 to 53.22) | 24.19 (–7.81 to 56.38) | 16.99 (–10.81 to 44.92) | 27.92 (–4.76 to 60.22) | 14.1 (–19.73 to 47.59) | 24.65 (–17.6 to 67.26) | 39.09 (6.11 to 72.26) | 16.13 (–19.84 to 51.7) | 13.41 (–19.5 to 45.84) | 34.71 (4.92 to 64.35) | –4.96 (–47.48 to 37.82) | 3.61 (–28.33 to 35.48) | –4.06 (–50.05 to 42.13) | |
| Treatment category | Treatment code | Treatment code | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | S | ||
| Inactive control | A |
N = 8 –12.31 (–23.90 to –0.72) |
N = 1 –5.40 (–23.66 to 12.89) |
N = 5 –10.70 (–12.21 to –0.19) |
N = 1 3.36 (–14.49 to 21.21) |
N = 1 –25.00 (–41.75 to –8.24) |
N = 1 –7.00 (–13.58 to –0.42) |
N = 1 7.00 (–5.25 to 19.25) |
N = 1 13.00 (2.04 to 23.96) |
|||||||||
| Usual care | B | –4.45 (–23.49 to 14.63) |
N = 1 –6.10 (–11.39 to –0.82) |
N = 2 –5.32 (–11.94 to 1.30) |
N = 1 –2.00 (–11.96 to 7.96) |
|||||||||||||
| Disc surgery | C | –8.87 (–32.27 to 14.47) | –4.43 (–23.65 to 14.85) |
N = 2 –5.96 (–29.45 to 17.56) |
N = 8 –5.17 (–12.21 to 1.88) |
N = 1 9.00 (–4.05 to 22.05) |
||||||||||||
| Epidural/nerve block | D | –12.66 (–21.47 to –4.11) | –8.19 (–26.07 to 9.35) | –3.78 (–26.92 to 19.12) |
N = 2 18.01 (6.25 to 29.77) |
N = 1 35.00 (–24.60 to 94.60) |
||||||||||||
| Chemonucleolysis | E | –12.28 (–35.85 to 11.38) | –7.856 (–30.88 to 15.18) | –3.37 (–20.87 to 13.95) | –0.40 (–23.17 to 24.50) |
N = 1 –0.63 (–14.98, 13.72) |
||||||||||||
| Non-opioids | F | –5.84 (–16.65 to 4.47) | –1.36 (–22.58 to 19.27) | 3.05 (–22.19 to 27.94) | 6.80 (–5.20 to 18.71) | 6.46 (–19.41 to 31.55) |
N = 3 13.60 (2.78 to 24.42) |
|||||||||||
| Intraoperative interventions | G | –13.94 (–39.47 to 11.56) | –9.51 (–31.10 to 12.22) | –5.07 (–14.80 to 4.87) | –1.27 (–26.35 to 24.08) | –1.65 (–21.78 to 18.34) | –8.16 (–34.85 to 19.42) | |||||||||||
| Traction | H | –1.32 (–23.17 to 20.91) | 3.29 (–25.71 to 32.27) | 7.57 (–24.35 to 39.72) | 11.36 (–11.75 to 35.12) | 11.06 (–20.95 to 43.07) | 4.58 (–19.35 to 29.3) | 12.71 (–20.73 to 46.25) |
N = 2 2.61 (–14.91 to 20.14) |
N = 1 19.00 (8.18 to 29.82) |
||||||||
| Manipulation | I | –12.79 (–50.28 to 24.55) | –8.49 (–45.20 to 28.75) | –3.95 (–38.07 to 30.14) | –0.17 (–37.29 to 37.62) | –0.62 (–29.80 to 28.80) | –6.98 (–45.24 to 31.74) | 1.12 (–34.16 to 36.58) | –11.54 (–54.97 to 31.98) | |||||||||
| Alternative/non-traditional | J | –24.89 (–55.67 to 5.35) | –20.33 (–56.61 to 15.17) | –15.95 (–54.37 to 21.99) | –12.2 (–43.99 to 19.26) | –12.57 (–51.22 to 25.59) | –18.97 (–51.52 to 13.03) | –10.9 (–50.82 to 28.28) | –23.7 (–61.56 to 13.97) | –11.97 (–60.19 to 36.22) | ||||||||
| Active PT | K | –3.39 (–30.69 to 23.94) | 1.01 (–20.55 to 22.90) | 5.55 (–16.71 to 27.55) | 9.29 (–17.11 to 36.10) | 8.94 (–17.82 to 35.50) | 2.45 (–25.97 to 31.34) | 10.61 (–13.71 to 34.65) | –2.14 (–36.92 to 32.78) | 9.55 (–29.95 to 48.78) | 21.63 (–19.22 to 62.57) | |||||||
| Passive PT | L | –0.23 (–20.29 to 20.33) | 4.29 (–23.39 to 32.18) | 8.71 (–21.98 to 39.56) | 12.40 (–8.92 to 34.41) | 12.09 (–19.06 to 43.11) | 5.61 (–16.74 to 28.82) | 13.75 (–18.31 to 46.44) | 1.03 (–16.63 to 18.91) | 12.56 (–29.58 to 55.39) | 24.73 (–11.97 to 61.69) | 3.26 (–30.42 to 37.25) | ||||||
| Biological agents | M | –11.18 (–30.77 to 8.83) | –6.66 (–32.67 to 19.50) |
–2.32 (–31.48, 27.46) |
1.41 (–17.69 to 21.50) | 1.17 (–28.76 to 31.35) | –5.35 (–26.77 to 17.03) | 2.74 (–28.24 to 34.48) | –9.85 (–39.52 to 19.26) | 1.69 (–40.18 to 43.76) | 13.73 (–22.52 to 50.22) | –7.82 (–40.38 to 25.16) | –10.83 (–39.24 to 16.76) | |||||
| Activity restriction | N | 17.44 (–16.86 to 52.78) | 21.96 (–17.44 to 62.08) | 26.41 (–15.19 to 68.47) | 30.08 (–4.92 to 66.52) | 29.67 (–11.96 to 72.55) | 23.29 (–12.20 to 60.40) | 31.41 (–11.49 to 75.09) | 18.75 (–8.49 to 46.19) | 30.31 (–20.81 to 81.71) | 42.63 (–3.83 to 89.39) | 20.99 (–23.27 to 65.66) | 17.77 (–14.51 to 50.31) | 28.65 (–10.75 to 69.12) |
N = 2 –1.09 (–6.80 to 4.62) |
|||
| Opioids | O | 7.41 (–12.54 to 26.94) | 11.92 (–15.08 to 38.42) | 16.33 (–14.11 to 46.03) | 20.08 (–0.43 to 40.68) | 19.73 (–11.03 to 49.76) | 13.27 (–3.28 to 29.78) | 21.36 (–10.68 to 52.76) | 8.77 (–21.05 to 37.81) | 20.29 (–21.91 to 62.08) | 32.34 (–4.08 to 68.75) | 10.79 (–22.61 to 43.95) | 7.69 (–21.01 to 35.46) | 18.63 (–9.02 to 45.64) | –10.05 (–50.65 to 29.11) | |||
| Education/advice | P | 16.62 (–22.42 to 26.93) | 21.04 (–22.72 to 65.52) | 25.51 (–20.19 to 71.75) | 29.19 (–10.49 to 70.51) | 28.78 (–16.75 to 75.11) | 22.42 (–17.72 to 64.59) | 30.61 (–16.38 to 77.81) | 17.96 (–15.05 to 51.26) | 29.32 (–24.71 to 84.19) | 41.57 (–8.33 to 92.29) | 20.11 (–28.17 to 68.22) | 16.82 (–20.41 to 54.37) | 27.70 (–15.97 to 72.22) | –0.86 (–20.06 to 18.29) | 9.18 (–34.25 to 54.14) | ||
| Radiofrequency lesioning | S | 13.01 (–14.41 to 40.77) | 17.36 (–15.83 to 51.35) | 21.8 (–14.29 to 58.17) | 25.62 (–2.79 to 55.11) | 25.34 (–10.94 to 61.79) | 18.78 (–10.35 to 48.87) | 26.90 (–10.50 to 64.68) | 14.25 (–21.2 to 49.57) | 25.92 (–20.62 to 71.92) | 37.86 (–2.80 to 79.10) | 16.39 (–22.72 to 55.21) | 13.23 (–20.77 to 47.23) | 24.14 (–9.99 to 58.32) | –4.55 (–48.84 to 39.46) | 5.57 (–27.90 to 39.77) | –3.47 (–52.37 to 44.44) | |
| Treatment category | Treatment code | Treatment code | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | K | L | M | N | P | ||
| Inactive control | A |
N = 5 (n = 4) –0.34 (–0.81 to 0.13) |
N = 2 0.30 (–0.14 to 0.74) |
N = 1 0.08 (–0.31 to 0.47) |
N = 3 (n = 2) –0.85 (–1.52 to –0.18) |
|||||||||
| Usual care | B | 0.17 (–1.31 to 1.45) |
N = 6 (n = 2) –0.09 (–0.33 to 0.15) |
N = 1 –0.35 (–1.03 to 0.33) |
a |
N = 1 0.28 (–0.06 to 0.62) |
||||||||
| Disc surgery | C | 0.10 (–1.42 to 1.45) | –0.06 (–0.35 to 0.23) |
N = 4 (n = 3) 0.30 (0.08 to 0.53) |
N = 8 –0.16 (–0.43 to 0.11) |
N = 1 –0.29 (–0.82 to 0.23) |
||||||||
| Epidural/nerve Block | D | –0.16 (–0.53 to 0.19) | –0.34 (–1.56 to 1.09) | –0.28 (–1.55 to 1.20) |
N = 1 –0.42 (–0.92 to 0.08) |
N = 1 –0.83 (–1.32 to –0.33) |
N = 1 0.07 (–0.44 to 0.59) |
|||||||
| Chemonucleolysis | E | 0.37 (–1.23 to 1.81) | 0.21 (–0.38 to 0.80) | 0.27 (–0.24 to 0.78) | 0.55 (–1.02 to 1.91) | |||||||||
| Non-opioids | F | 0.08 (–0.49 to 0.61) | –0.08 (–1.46 to 1.37) | –0.00 (–1.45 to 1.45) | 0.24 (–0.37 to 0.82) | –0.29 (–1.81 to 1.27) |
N = 1 (n = 0) –1.05 (–1.99 to –0.11) |
|||||||
| Intraoperative interventions | G | –0.04 (–1.51 to 1.36) | –0.21 (–0.64 to 0.23) | –0.14 (–0.48 to 0.18) | 0.14 (–1.35 to 1.44) | –0.42 (–1.03 to 0.20) | –0.13 (–1.61 to 1.34) | |||||||
| Traction | H | –0.37 (–1.25 to 0.44) | –0.53 (–2.00 to 1.16) | –0.47 (–1.98 to 1.23) | –0.21 (–1.05 to 0.64) | –0.74 (–2.35 to 1.06) | –0.46 (–1.41 to 0.57) | –0.31 (–1.89 to 1.34) |
N = 1 0.52 (–0.11 to 1.15) |
N = 1, –0.46 (–1.45 to 0.54) |
||||
| Active PT | K | 0.17 (–1.45 to 1.65) | 0.02 (–0.73 to 0.72) | 0.08 (–0.67 to 0.79) | 0.33 (–1.25 to 1.76) | –0.20 (–1.09 to 0.69) | 0.08 (–1.56 to 1.66) | 0.22 (–0.59 to 1.02) | 0.54 (–1.23 to 2.18) | |||||
| Passive PT | L | –0.47 (–1.39 to 0.45) | –0.64 (–2.16 to 1.02) | –0.58 (–2.13 to 1.10) | –0.31 (–1.19 to 0.60) | –0.85 (–2.46 to 0.99) | –0.56 (–1.57 to 0.54) | –0.43 (–2.01 to 1.26) | –0.10 (–1.02 to 0.77) | –0.66 (–2.32 to 1.16) | ||||
| Biological agents | M | –0.68 (–1.29 to –0.10) | –0.85 (–2.22 to 0.60) | –0.78 (–2.20 to 0.67) | –0.51 (–1.19 to 0.09) | –1.05 (–2.57 to 0.51) | –0.76 (–1.50 to –0.03) | –0.64 (–2.07 to 0.83) | –0.31 (–1.31 to 0.66) | –0.83 (–2.40 to 0.87) | –0.20 (–1.33 to 0.86) | |||
| Activity restriction | N | –0.84 (–2.49 to 0.90) | –1.03 (–3.14 to 1.28) | –0.96 (–3.14 to 1.36) | –0.70 (–2.36 to 1.12) | –1.24 (–3.45 to 1.14) | –0.93 (–2.67 to 0.92) | –0.81 (–3.02 to 1.54) | –0.46 (–1.94 to 1.06) | –1.02 (–3.32 to 1.44) | –0.37 (–2.10 to 1.42) | –0.18 (–1.87 to 1.70) |
N = 2 0.15 (–0.06 to 0.37) |
|
| Education/advice | P | –0.66 (–2.47 to 1.24) | –0.83 (–3.08 to 1.58) | –0.78 (–3.08 to 1.65) | –0.50 (–2.35 to 1.41) | –1.06 (–3.40 to 1.41) | –0.76 (–2.62 to 1.24) | –0.62 (–2.94 to 1.82) | –0.30 (–1.93 to 1.40) | –0.85 (–3.26 to 1.72) | –0.19 (–2.06 to 1.69) | –0.01 (–1.84 to 1.98) | 0.17 (–0.46 to 0.79) | |
| Treatment category | Treatment code | Treatment code | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | K | L | M | N | P | ||
| Inactive control | A |
N = 4 –0.03 (–0.18 to 0.13) |
N = 2 0.30 (–0.14 to 0.74) |
N = 1 0.08 (–0.31 to 0.47) |
N = 2 –1.07 (–2.64 to 0.50) |
|||||||||
| Usual care | B | 0.34 (–0.90 to 1.62) |
N = 2 –0.15 (–0.30 to 0.00) |
N = 1 –0.35 (–1.03 to 0.33) |
N = 1 0.28 (–0.06 to 0.62) |
|||||||||
| Disc surgery | C | 0.29 (–1.00 to 1.67) | –0.06 (–0.54 to 0.44) |
N = 3 0.06 (–0.37 to 0.50) |
N = 8 –0.16 (–0.43 to 0.11) |
N = 1 0.29 (–0.23 to 0.82) |
||||||||
| Epidural/nerve Block | D | 0.03 (–0.37 to 0.43) | –0.32 (–1.52 to 0.84) | –0.26 (–1.58 to 0.98) |
N = 1 0.42 (–0.08 to 0.92) |
N = 1 0.83 (0.33 to 1.32) |
N = 1 0.07 (–0.44 to 0.59) |
|||||||
| Chemonucleolysis | E | 0.63 (–0.81 to 2.12) | 0.28 (–0.47 to 1.02) | 0.34 (–0.24 to 0.91) | 0.60 (–0.78 to 2.03) | |||||||||
| Non-opioids | F | 0.09 (–0.50 to 0.66) | –0.26 (–1.61 to 1.09) | –0.20 (–1.67 to 1.22) | 0.06 (–0.57 to 0.69) | –0.54 (–2.10 to 1.02) | ||||||||
| Intraoperative interventions | G | 0.13 (–1.17 to 1.57) | –0.21 (–0.79 to 0.39) | –0.15 (–0.49 to 0.18) | 0.10 (–1.16 to 1.49) | –0.49 (–1.14 to 0.17) | 0.05 (–1.39 to 1.56) | |||||||
| Traction | H | –0.29 (–1.12 to 0.50) | –0.65 (–2.12 to 0.83) | –0.58 (–2.17 to 0.93) | –0.33 (–1.18 to 0.51) | –0.92 (–2.61 to 0.72) | –0.39 (–1.39 to 0.61) | –0.44 (–2.06 to 1.11) |
N = 1 –0.52 (–1.15 to 0.11) |
N = 1 0.46 (–0.54 to 1.45) |
||||
| Active PT | K | 0.39 (–1.01 to 1.80) | 0.03 (–0.68 to 0.75) | 0.09 (–0.66 to 0.83) | 0.36 (–0.99 to 1.72) | –0.25 (–1.20 to 0.70) | 0.29 (–1.19 to 1.81) | 0.24 (–0.59 to 1.06) | 0.69 (–0.92 to 2.28) | |||||
| Passive PT | L | –0.32 (–1.21 to 0.59) | –0.69 (–2.15 to 0.78) | –0.62 (–2.19 to 0.91) | –0.36 (–1.22 to 0.51) | –0.98 (–2.62 to 0.65) | –0.42 (–1.43 to 0.64) | –0.47 (–2.09 to 1.07) | –0.03 (–0.93 to 0.86) | –0.72 (–2.33 to 0.94) | ||||
| Biological agents | M | –0.44 (–1.19 to 0.30) | –0.79 (–2.21 to 0.57) | –0.71 (–2.24 to 0.70) | –0.48 (–1.22 to 0.28) | –1.05 (–2.69 to 0.48) | –0.53 (–1.47 to 0.40) | –0.57 (–2.16 to 0.89) | –0.15 (–1.23 to 0.96) | –0.83 (–2.38 to 0.69) | –0.12 (–1.25 to 1.04) | |||
| Activity restriction | N | –0.80 (–2.46 to 0.79) | –1.18 (–3.17 to 0.83) | –1.10 (–3.16 to 0.93) | –0.84 (–2.48 to 0.78) | –1.45 (–3.54 to 0.66) | –0.89 (–2.62 to 0.80) | –0.95 (–3.04 to 1.11) | –0.52 (–1.92 to 0.91) | –1.21 (–3.30 to 0.88) | –0.47 (–2.13 to 1.18) | –0.36 (–2.15 to 1.35) |
N = 2 –0.15 (–0.37 to 0.06) |
|
| Education/advice | P | –0.65 (–2.40 to 1.04) | –1.02 (–3.10 to 1.04) | –0.96 (–3.11 to 1.13) | –0.70 (–2.44 to 1.03) | –1.30 (–3.49 to 0.91) | –0.74 (–2.58 to 1.05) | –0.81 (–2.98 to 1.33) | –0.37 (–1.89 to 1.19) | –1.06 (–3.24 to 1.11) | –0.32 (–2.11 to 1.45) | –0.22 (–2.09 to 1.61) | 0.15 (–0.44 to 0.76) | |
Appendix 10 Full summary of economic evaluations
| Study details | Source of data | Method for estimation of benefits/costs | Results/statistical analysis | Recommendations |
|---|---|---|---|---|
|
Dullerud, 1999 275 Type of economic evaluation: cost-effectiveness analysis Currency/country: $/USA Cost year: not stated Perspective: provider – Norwegian Ministry of Health and Social Affairs Study population: two cohorts of 68 patients with herniated discs treated with traditional macro-procedure operations compared with 90 patients receiving nucleotomy followed up for one year Interventions: percutaneous automated lumbar nuclectomy Comparator: macro-procedure discectomy |
Source of effectiveness data: single study Source of cost data: combination of study data and literature sources |
Link between cost and effectiveness data: retrospective/disconnected Clinical outcome measures and method of evaluation: a clinical overall score (COS) was derived from pain intensity, physical examination, functional status according to ODI and consumption of analgesics. COS was defined as the weighted sum of these four subsets with a maximum score of 1000 (worst conceivable status) and 0 being best attainable treatment outcome. Comparison of 1-year outcome was used in the study Direct costs: operation costs, use of X-ray laboratory and other equipment (not specified), nucleotomy equipment Productivity costs: not stated Resource use: radiologist and radiographer fee, use of X-ray laboratory and other equipment Discounting: not presented Modelling: not presented |
Synthesis of costs and benefits: the costs of primary and secondary treatment were calculated by (i) costs per successfully treated patient using COS as a dichotomous variable and (ii) costs relative to the reduction of COS as a continuous variable. Marginal costs per extra success obtained by one of the other alternatives compared with the other were also calculated Statistical analysis: no incremental analysis (ICER) undertaken Sensitivity analysis: not presented Main results: Cost Average cost per success in nucleotomy group is 36% that of surgery Cost per point reduction of COS in nucleotomy group is 40% reduction of surgical costs Marginal costs Average cost per surgical patient – US$6389 Average cost per nucelotomy patient – US$2272 Marginal cost per extra success choosing surgery as primary treatment – US$205,850 Primary treatment only Average cost per success per surgical patient US$7850 Average cost per success per nucelotomy patient US$2012 |
Author’s conclusions/implication for practice: despite higher success rates in surgical discectomy than automated percutaneous nucleotomy, nucleotomy is significantly more cost-effective both in terms of primary cure and when secondary treatment is included Nucleotomy as a mini-invasive procedure with low complication rate and the potential of a quick recovery is more cost-effective than traditional surgical treatment for lumbar disc herniation |
|
Hansson, 2007 100 Type of economic evaluation: CUA Currency/country: $/USA Cost year: 1994–5 Perspective: societal Study population: 92 individuals who underwent surgery for lumbar disc herniation in a cohort of 1822 individuals, aged between 18–59 years, sick-listed at least 28 days owing to either low back pain or neck problems, selected consecutively in five regions of Sweden between 1994 and 1995 Interventions (including comparator): surgery because of lumbar disc herniation. Surgery group were individually matched to individuals treated conservatively for the same type of symptoms. Matching controlled for gender, age, diagnoses, pain distribution, pain intensity and the presence of sciatica |
Source of effectiveness data: single study Source of cost data: not stated Source of cost data: single study |
Link between cost and effectiveness data: prospective/concurrent Clinical outcome measures and method of evaluation: HRQoL using EQ-5D; functional restrictions due to back problems using the Hannover Activities of Daily Living questionnaire; pain experienced during past 6 months using the Von Korff pain scale Direct costs: primarily medical costs for then-current pack pain were estimated (appointments, admission, examination and treatment) over 2-year study period Productivity costs: cost of work absenteeism due to back pain estimated by multiplying total number of sick days listed by cost per time unit (monthly salary + employee payroll taxes converted to a daily cost). For those granted permanent disability during the study period, production loss calculated up until aged 65 years. A 5% discount rated and assumed annual increase in productivity of 1.5% was used to convert future years production loss to present values Resource use: resource use not specified Discounting: a 5% discount rate and an assumed annual increase in productivity of 1.5% were used to convert future years production loss to present values Modelling: not presented |
Synthesis of costs and benefits: Costs of illness, HRQoL and cost–utility (QALY). Difference in utility between 28 days and 2 years used as the gain in QALY Statistical analysis: not presented. No incremental analysis (ICER) undertaken Sensitivity analysis: not performed Main results: Cost of illness: mean direct costs Surgical – US$10,311 Medical – US$2068 Indirect costs Surgical – US$32,807 Medical – US$42,570 Total costs Surgical – US$43,118 Medical – US$44,638 Direct costs accounted for 24% of the total costs for the surgical group and 5% for the nonsurgical group Cost–utility Surgical – cost US$43,119; median QALY 0.363 Non-surgical – cost US$44,638; median QALY 0.036 Difference – cost US$1520; median QALY 0.327 Cost per QALY – US$4648 |
Author’s conclusions/implications for practice: total cost for surgical treatment of lumbar disc herniation during a 2-year period lower than non-surgical treatment. Direct cost of surgery was much higher than for non-surgical treatment, whereas the indirect cost was lower. Lower indirect costs were the effect of lower rates of reoccurrence of sick-listing episodes and permanent disability benefits. Surgery improved pain, back function and HRQoL to a greater extent than non-surgical treatments. The effects on HRQoL in combination with lower costs for surgery resulted in a better cost–utility for surgery Surgery for lumbar disc herniation is quite cost-effective |
|
Karppinen, 2001 171 Type of economic evaluation: cost-effectiveness analysis Currency/country: $/USA Cost year: 1998 Perspective: provider Study population: 160 Patients with unilateral sciatica (pain radiating dermatology from the back to below the knee) that had lasted 1–6 months, subgrouped on MRI as herniation or extrusion Intervention: periradicular infiltration with methylprednisolone 40 mg/ml bupivacaine 5 mg/ml Comparator: periradicular infiltration with 0.9% of a sodium chloride solution |
Source of effectiveness data: single study Source of cost data: data from actual source Source of cost data: single study – data gathered from study questionnaires and medical records |
Link between cost and effectiveness data: prospective/concurrent Clinical outcome measures and method of evaluation: leg pain (VAS); straight leg raising and lumbar flexion (modified Schober's measure); pain distribution; MRI findings of disc morphology Direct costs: medicine costs; additional treatments. Visits to physiotherapists, osteopaths and physicians, back operations Productivity costs: days of sick leave Resource use: health service usage, home help Discounting: not reported Modelling: not presented |
Statistical analysis: AUC analysis (adjusted for symptom duration). No ICERs presented Sensitivity analysis: not performed Main results: Cost Mean cumulative costs of periradicular infiltration per one responder Subgroup analysis Contained herniations vs extrusions Savings in home care (4 weeks) US$200 per patient (95% CI US$46 to US$355; p = 0.013) Total costs (6 months) $1795 (95% CI US$1069 to US$2521; p < 0.001) Therapy visits (4 weeks) significantly less $182 (95% CI US$79 to US$285; p = 0.001) Contained herniations cost $1266 more per patient to obtain one painless patient in the saline group. For extrusions, the steroid treatment was more expensive: $4445 per painless patient |
Author’s conclusions/implications for practice: no significant differences observed between the treatment in terms of symptomatic disc level or ITT analysis. When analysed according to MRI classification, no significant differences seen at 3 months, but steroid option was significant at 1 year. Methylprednisolone 40 mg/ml bupivacaine 5 mg/ml is cost-effective for contained herniations, producing a saving of US$12,600 per responder |
|
Launois, 1994 277 Type of economic evaluation: cost-effectiveness analysis Currency/country: Francs/France Cost year: 1990 Perspective: health-care provider and patient Study population: 146 patients who had undergone chemonucleolysis or surgery 2–3 months before data collection Intervention: chemonucleolysis Comparator: surgery (discectomy) |
Source of effectiveness data: single study Source of cost data: combination of both Source of cost data: single study |
Link between cost and effectiveness data: retrospective/disconnected Clinical outcome measures and method of evaluation: clinician rating of the patients’ condition at 3 months was undertaken using the Rosser–Watts classification of illness state. In addition, Dallas Pain Questionnaire and Health Measurement Questionnaire used to assess self-rated functioning Direct costs: direct medical costs were subdivided into: hospitalisation costs, physician services and drug costs. Best estimate of hospital true cost was obtained using the hotel approximation method for evaluating public hospitals. This assumes that hotel costs are evenly distributed over all inpatient days regardless of reason for admission. Costs included items such as administration, housekeeping, maintenance and general equipments, averaged over all in patient days for 1 year. Physician services included the cost of salaried physicians and nurses, pharmacy, laboratory and radiology use, equipment and supplies. Specific expenses directly related to the two procedures performed in the operation room were calculated using ‘component enumeration method’ involving two steps: (1) measurement of personnel time and (2) disposable equipment consumed in each intervention and assignment of monetary cost for resources consumed. Outpatient costs were estimated on the basis of prescriptions made at discharge including pharmaceutical and physiotherapy expenses and costs of follow-up medical treatments. Costs of medical services and drugs were based on French Relative Value Scale and retail prices, respectively Productivity costs: not collected Resource use: resource use considered as part of direct costs Discounting: no discounting referred to in method, but results give discounted QALYs in results Modelling: a decision model as designed to schematise events according to physician choice or dictated by natural course of events. The tree involved two major branches: surgical treatment and chemonucleolysis. For each treatment short-term (1 year) and long-term (2–7 years) events were set. Distribution of events over time were made according to two rules: (1) deteriorations appearing within a period of time were assumed to occur midway though it; and (2), reoperations because of recurrent pain were assumed to take place after 3 months (i.e. 0.25 years) of failed medical treatment. For a cohort of 100 patients, the number of years or year fractions of good or bad health were cumulated at 7 years. In a given year and for 100 patients, the potential years of life are equal to 100 patient-years of which x are in good health and 100 patient-years of which x are in poor health |
Synthesis of costs and benefits: Statistical analysis: Krukall–Wallis Test was used to compare Rosser–Watts QoL coefficients on clinical outcomes and Pearson correlation coefficients were determined between four Dallas scores and Rosser–Watts QoL coefficients. Incremental cost–utility ratio was calculated by dividing the difference in utility between the two treatments giving the extra gain per QALY per extra French franc (FF) spent through switching from one treatment to another No ICERs presented Sensitivity analysis: not performed Main results: Cost Total cost was FF15,400 for discectomy, FF800 for chemonucleolysis Additional discounted cost per patient of discectomy compared with chemonucleolysis was FF9126 Additional discounted benefits at 7 years associated with chemonucleolysis was 0.142 years of life (52 days) per patient QoL Disectomy patient scores 65 patient-years in those with initial success (87%). Of these, 60 patient-years owing to initial success sustained at 7 years and 5 patient years owing to successful reoperation Individual probability of a patient being in good health at year 7 was 0.84 following chemotherapy vs 0.65 following disctectomy In discectomy group with initial success, 44.24 potential life years were lost bacause of a reduction in QoL vs 27.36 in chemonucleolysis cohort |
Author’s conclusions/implications for practice: although surgical discetomy may produce slightly better immediate clinical results than chemonucleolysis with chymopapain, evaluation of costs and benefit up to 7 years after intervention, identified additional benefit in factor of chemonucleolysis, owing mostly to results from post-chymopapain surgery offering a second chance to these patients |
|
Luijsterburg, 2007 278 Type of economic evaluation: cost-effectiveness analysis Currency/country: Euro (€) Cost year: 2005 Perspective: societal Study population: 135 patients with acute lumbosacral radicular syndrome (sciatica) from participating GPs (112) in the Rotterdam and surrounding area who were participating in an RCT to evaluate the effectiveness of PT and GP care Interventions: PT + information (GP care) Comparator: GP care |
Source of effectiveness data: single study Source of cost data: single study |
Link between cost and effectiveness data: prospective/concurrent Clinical outcome measures and method of evaluation: the primary outcome measure was GPE rated on seven-point scale from 1 (completely recovered) to 7 (vastly worsened); dichotomised as improved vs not improved. GPE rated as percentage of patients that reported to be improved. This was considered a disease-specific outcome. The secondary outcome was QoL, measured by the EQ-5D (considered the generic measure of health) Direct costs: direct health-care costs were costs of PT, GP care, medication and additional visits to other health-care providers. Direct (non-health-care) costs were costs of devices, out-of-pocket expenses and costs of help in housekeeping Productivity costs: costs of production losses caused by absence from work Resource use: differences in resource utilisation were assessed between the two study arms; costs were calculated by multiplication of each unit of resource use by its unit price Discounting: not reported Modelling: not performed |
Synthesis of costs and benefits: global perceived effect, HRQoL (EQ-5D) Statistical analysis: the ICER on total costs and direct costs only, between both study arms, was constructed. CIs calculated via parametric (Fieller’s method) and non-parametric (bootstrapping methods), and presented using a cost-effectiveness acceptability curve Sensitivity analysis: variations in cost or health effect were included in the bootstrapped estimated of the ICER Main results Cost Costs (direct and indirect) shown at 3, 6, 12 and 52 weeks. Consisted of mainly production losses Significant differences between groups on costs for PT in favour of control group at all time points Total direct and indirect costs statistically significant in favour of control group at all time points Cost-effectiveness ICER for direct costs €837 (95% CI –€732 to €3186) per improved patient gained ICER for total costs €6224 (95% CI –€10,419 to €27,551) per improved patient gained Cost-effectiveness acceptability curve Direct costs Threshold of €600 per patient improved, ICER acceptable with 35% certainty Threshold of €1200 per patient improved, ICER acceptable with 69% certainty Total costs Threshold of €4000 per patient improved, ICER acceptable at 37% Threshold of €12,000 per patient improved, ICER acceptable at 68% |
Author’s conclusions/implications for practice: the treatment of patients with LRS with PT and GP care is not more cost-effective than GP care alone |
|
Malter, 1996 279 Type of economic evaluation: cost-effectiveness analysis Currency/country: $/USA Cost year: 1993 Perspective: health payers (health insurer) Study population: patients who had herniated lumbar discs unresponsive to conservative therapy, who undergo lumbar discectomy. Data was derived from two RCTs: (1) 126 patients receiving medical vs surgical treatment for radicular pain unresponsive to conservative therapy; and (2) 106 patients randomly assigned to receive chemonucleolysis or placebo. An ongoing cohort study of medical treatment vs discectomy was used to examine the effect of replacing the chemonucleolysis data as this may be less effective than discectomy |
Source of effectiveness data: review/synthesis Source of cost data: combination of both Source of cost data: review/synthesis |
Link between cost and effectiveness data: retrospective/disconnected Clinical outcome measures and method of evaluation: analysis was based on the proportions of medical and surgical patients with self-reported good, fair, poor or bad outcomes at 1-, 4- and 10-year follow-up Direct costs: costs were identified from MEDSTAT a commercially available database which included patients < 65 years from all 50 US stages by commercial insurers, Blue Cross/Shield and self-insured businesses. Claims were reported as charges reimbursed that were used as proxies for costs. All claims submitted by 1.3 million individuals from January 1987 to December 1989 were available for analysis Productivity costs: not presented Resource use: Rates service utilisation determined from commercially available database Cost data handled appropriately: discounting was at 5% per year Modelling: base-case model. The mean TTO value was calculated for each level of recovery and self-assessed outcomes reported in the RCTs were weighted |
Synthesis of costs and benefits: cost-effectiveness was defined as the non-discounted incremental cost of surgical vs medical treatment per QALY gained Statistical analysis: cost and effects were determined from data from different sources Sensitivity analysis: estimates for cost, efficacy and QoL were varied simultaneously. Efficacy varied from < 25% to 25% more than the main estimate based on the consistency of base-case and secondary analysis. For estimates of changes in QoL associated with symptom resolution, a broader range (± 50%) was used to account for the uncertainty. Incremental costs associated with surgery were varied from the insurance-based estimate to the HMO estimate Main results: the probability of a good outcome varied between 0.36 and 0.56 after medical treatment and between 0.64 and 0.70 after discectomy. For a poor outcome, the probability varied between 0.06 and 0.20 after medical treatment and between 0.07 and 0.14 after discectomy QoL values associated with a good outcome were 0.95, with a fair outcome 0.77, with a poor outcome 0.62 and with a bad outcome 0.50 During the 10 years after surgery, the average surgical patient experienced 8.7 QALYs whereas the average medical patient experienced 8.27 QALYs, with the difference of 0.43 representing the non-discounted improvement in QALYs associated with surgery Total costs for the 18-month interval beginning 6 months before diagnosis were US$17,020 for the surgical group compared with US$4470 for the medical group. The non-discounted cost-effectiveness ratio of surgical over medical therapy was US$29,200 per QALY Cost-effectiveness of discectomy remained < US$100,000 as long as surgery produced an incremental quality-adjusted benefit of at least 0.125 years |
Author’s conclusions/implications for practice: for carefully selected patients with herniated discs, surgical discectomy is a cost-effective treatment. Discectomy’s favourable cost-effectiveness results from its substantial effect on QoL and moderate cost |
|
Manca, 2008 280 Type of economic evaluation: cost-analysis Currency/country: £/UK Cost year: 2005–6 Perspective: health-care provider Study population: 100 patients participating in the PROCESS (Prospective, randomised controlled multicentre study of patients with failed back surgery syndrome) trial. Patients ≥ 18 years suffering from predominant neuropathic pain of radicular origin in the legs with or without associated less severe back pain were included Interventions: spinal cord stimulation (SCS) Comparator: conservative medical management (CMM) |
Source of effectiveness data: single study Source of cost data: literature and published sources Source of cost data: single study |
Link between cost and effectiveness data: prospective/concurrent Clinical outcome measures and method of evaluation: HRQoL using the SF-36 and EQ-5D measured at baseline, 3 months and 6 months after initiation of treatment Direct costs: unit costs were calculated using UK and Canadian figures. The total cost of each element of resource consumption was estimated by obtaining country-specific unit costs from published sources and literature. Equipment and consumables were costed using manufacturer list prices and drug prices from each national drug formulary. Inpatient stay were estimated using fully allocated cost figures whereas non-drug therapies were costed using published tariffs and literature Productivity costs: not recorded Resource use: health-resource data were prospective collected for each patient using case report forms. Resource data were collected on preimplant screening and implant (time of theatre, use of hardware, length of hospital stay and type of ward); use of medications; non-drug therapy; complications (SCS and non SCS complications including those related to CMM were recorded). In addition, SCS-specific health-care utilisations such as additional surgery, initial hospitalisation and readmission to overcome complications and non-invasive tests were collected Where necessary, cost figures were up-rated for inflation using the national health-care specific price indexes. Total costs for resource use were calculated by multiplying resource use by the relevant national average cost. Canadian dollars and UK sterling are presented as these were the two largest recruiters to the trial. Euros are reported by converting the Canadian figures with the euro exchange rate at the time of writing the paper. Use of Canadian prices (one currency only) for conversion into euros was undertaken because of the differences in prices between the two countries, converting total costs in UK sterling and Canadian dollars would lead to different total cost estimates Discounting: not performed Modelling: not performed |
Synthesis of costs and benefits: costs and HRQoL outcomes are presented separately Statistical analysis: not presented Sensitivity analysis: not performed Main results The 6-month mean total costs in the SCS group were significantly higher (£15,081) than the CMM group (£3573), with a statistically significant adjusted differential mean cost of £11,373. However, the gain in HRQoL with SCS over the same period was considerably greater in this group with a mean EQ-5D score difference of 0.25 (p < 0.001) and 0.21 (p < 0.001), respectively at 3 months and 6 months after adjustment for baseline characteristics |
Author’s conclusions/implications for practice: addition of SCS to CMM in patients resulted in higher costs to health-care systems, but generated important improvements in patients EQ-5D over the same period |
|
Price, 2005 173 Type of economic evaluation: CUA Currency/country: £/UK Cost year: 2002–3 Perspective: health-care provider and purchaser Study population: 228 patients listed for ESIs with clinically diagnosed unilateral sciatica aged between 18 and 70 years, who had a duration of symptoms between 4 weeks and 18 months. Part of a pragmatic prospective multicentre RCT with a 12-month follow-up Interventions (including comparator): up to three injections of epidural steroid and local anaesthetic into the interspinous ligament Injections of normal saline into the interspinous ligament |
Source of effectiveness data: single study Source of cost data: data from actual source Source of cost data: single study |
Link between cost and effectiveness data: prospective/concurrent Clinical outcome measures and method of evaluation: functional outcome (ODI functional outcome); health status (SF-36); pain (VAS and McGill questionnaire); psychological functioning [Hospital Anxiety and Depression Scale (HADS) score]; analgesic intake; physical function (using standardised objective tests); objective measures of sciatic root irritation and neurological deficit, procedural side effects Direct costs: NHS recharge costs calculated to identify costs from the purchaser perspective (Southampton Trust). Average prices charged to patients were based on total costs of service (including overheads) Productivity costs: Resource use Real resource costs calculated to identify costs from the provider perspective. A pilot was done to inform method of data collection. Resource-use data were collected through the use of a self-completion record on which all clinical staff recorded resources they used inclusive of their own time. Specifically time in patient consultation, aiding the patient before or after the consultation and time associated with patient administration for all patients presenting for the sciatica not included in the trial. Pathology and radiology use was collected. Data were collected across all three centres during July–October 2000 Cost data were used to calculate a cost per patient for treating sciatica with epidural injections from the perspective of health-care provider and purchaser. An average cost per patient was based on two management practices. Under each management practice it was assumed that patients had an initial consultation and follow-up. Owing to the short time horizon of when costs and benefits were incurred, discounting was not performed |
Synthesis of costs and benefits: QALYs were derived from SF-6D health utility scored using SF-36 raw data using the Brazier et al. 288 technique Statistical analysis: CUA was undertaken using SG to derive incremental cost per QALY for managing a patient with an ESI Sensitivity analysis: sensitivity analysis was undertaken to explore how cost estimates change, given the assumptions that underlay resources resource base costs are relaxed. Sensitivity analysis was not undertaken for purchaser costs |
Author’s conclusions/implications for practice: although ESI are relative safe, they confer only transient benefits in symptoms and self-reported function in a small group of patients with sciatica at substantial costs. ESI’s failed the QALY threshold recommended by NICE and do not represent good value for money if NICE recommendations are followed The study did not find a place for ESI in early stages of the disease. ESI’s only conferred a short-term benefit only and no predictors of response. They did not defer surgery and short-term repeat injections made no difference. The resource savings could be substantial even with a modest change to treatment. For example (from the purchaser’s perspective), the saving from moving from an assumed model of current pragmatic practice (maximum of three ESI’s) to a patient management strategy suggested by the trial (one ESI) would represent a saving of £16,505,700 in the sector |
|
Shvartzman, 1992 62 Type of economic evaluation: CUA Currency/country: $/USA Cost year: 1989 Perspective: purchaser Study population: 55 white male truckers who presented with an acute episode of sciatica between 1985–9, who after 3 months of conservative therapy were categorised as equivocal to undergo surgical or medical treatments based on study eligibility criteria Interventions (including comparator): surgery (n = 25); conservative treatment after initial rehabilitation (n = 30) |
Source of effectiveness data: single study Source of cost data: single study |
Link between cost and effectiveness data: retrospective/disconnected Clinical outcome measures and method of evaluation: functional (work-related) and perceptual (subjective opinion) criteria based on the Mayo Clinic System. Each patient was graded in all outcomes; therefore, total points accumulated reflected the overall outcome. A scoring system was devised (good 0–1, satisfactory 2–3 and poor ≥ 4). Reference made to comparing outcomes to a typical patient in the literature, but no reference given Direct costs: medical costs for surgery: hospital costs, surgeon’s fee, anaesthesia, radiology, office visits and follow-up rehabilitation. Medical costs for conservative treatment: office visits, radiology and physical rehabilitation Productivity costs: not considered Discounting not reported Modelling: not reported |
Synthesis of costs and benefits: utility scores were derived from treatment outcomes and ‘quality adjusted’ with a modified utility scale. Utility scores were multiplied by corresponding number of outcomes to make them quality adjusted Statistical analysis: cost-effectiveness was measured by net health-care costs/net health-care effectiveness = sum of all costs for all patients in both modalities/1 × (number of good outcomes) + 0.7 × (number of satisfactory outcomes) + 0.15 × (number of poor outcomes) Sensitivity analysis: not specified Main results: average medical compensation and costs given per patient following date of injury (1985–9) Total costs Surgical US$56,054 Conservative US$53,638 Average 5-year medical costs Surgical US$26,643 Conservative US$16,572 Average 5-year cost-effectiveness calculated US$64,700/adjusted outcome for surgical group US$60,700/adjusted outcome for conservative group |
Author’s conclusions/implications for practice: cost of effectiveness for both treatment modalities was US$63,000 ± US$2000 adjusted outcomes The authors state that patients who do not respond to an initial 3-month trial of PT, should not be managed aggressively if they have not deteriorated. The option to undergo surgery or conservative treatment should remain with the patient |
|
Stevenson, 1995 281 Type of economic evaluation: cost-minimisation Currency/country: £/UK Cost year: 1992 Perspective: provider Study population: 71 patients participating in an RCT of automised percutaneous lumbar discetomy (APLD) vs initial microsdiscetomy (IMD) in the treatment of contained lumbar herniation Intervention: patients treated by APLD Comparator: patients treated with IMD only |
Source of effectiveness data: single study Source of cost data: data from actual source Source of cost data: single study |
Link between cost and effectiveness data: prospective/concurrent Clinical outcome measures and method of evaluation: outcomes were assessed by two clinicians (one masked to treatment method) on a four point scale: 1 = poor, 2 = fair, 3 = good and 4 = excellent Direct costs: comprehensive costing schedules prepared from observation of the two surgical procedures and postoperative care. Included time spent by staff of different grades, drugs, disposables and capital equipment. Capital consumption and hospital overhead expenditures allowed Productivity costs: employment status, income in and out of work, compensation claims Resource use: medical and social service usage, private expenses were also collected Discounting: not reported Modelling: not reported |
Statistical analysis: not reported Sensitivity analysis: not reported Main results: IMD £1506 APLD £752 Total cost APLD £71,812 (average of £2317 per patient) Total cost IMD £62,665 (average of £31,567 per patient) Cost per outcome IMD – 32 successful outcomes, total cost £62,665 APLD – 22 successful outcomes, total cost £71,812 Average cost per IMD successful outcome (£1958) was 60% of average cost per APLD successful outcome £3264 |
Author’s conclusions/implications for practice: within the restrictions imposed by the dataset, automated percutaneous lumbar discetomy was less cost-effective than microdiscetomy If conclusion is replicated, it has strong resource implications for al health systems where cost-effectiveness and cost containment is a matter for concern |
|
Tosteson, 2008 282 Type of economic evaluation: cost-effectiveness analysis Currency/country: $/USA Cost year: 2004 Perspective: health payer (Medicare) and societal Study population: men and women, aged ≤ 18 years and diagnosed with intervertabral disc herniation. All had symptoms for at least 6 weeks Interventions (including comparator): standard open laminectomy/laminectomy with removal of herniation and examination of the involved nerve route Non-operative usual care chosen individually by patients and physicians |
Source of effectiveness data: pooled data from an RCT and observational cohorts from SPORT study Source of cost data: data from actual source Source of cost data: single study |
Link between cost and effectiveness data: prospective/concurrent Direct costs: direct costs at each time point were measured by self-reported instances of medical resource use × unit costs for each cost component. Unit costs for office visits, hospitalisations, diagnostic tests and procedures were based on 2004 Medicare national allowance payment amounts. Medication prices were based on 2004 Red Book prices. 287 Surgery costs depended on procedure performed and complications, these determined the diagnostic related group. Associated costs were assigned in two ways. First a cost paid by non-Medicare insurers was estimated at 70% of mean amount billed to Medicare in 2004. Second, the observed 2004 Medicare mean total diagnosis-related group price was used to reflect hospital-related costs for those ≥ 65 years of age. Surgeon costs were based on 2004 Medicare allowable amounts, using the resource-based relative value scale and anaesthesiology costs were estimated using operative time with a fixed amount of time added for postacute care. For non-spine surgical hospitalisations, costs were based on the diagnosis-related group and priced using mean observed Medicare 2004 prices for each admission Productivity costs: productivity losses due to spine-related problems (e.g. missed work days for those employed outside the home and missed home working days for those who reported housekeeping as their primary activity) were recorded. Use of unpaid care giving (including spousal care giving) was obtained. Costs were estimated using the standard human capital approach. Costs for missed days of housekeeping and unpaid care givers were valued based on average wages plus non-health benefits for individuals aged ≥ 35 years. Resource use: resource data included health-care visits (surgeons, chiropractors, other physicians, physical therapies, acupuncturists or other health-care providers); spine-related diagnostic tests; injections; devices (braces, canes, walkers, shoe inserts, etc.) emergency room visits; rehabilitation or nursing home days; paid caregiver; medications; and surgery Discounting: all costs were adjusted for inflation. A 3% annualised discount rate was used in the analysis of both costs and QALYs Modelling: to complete the CEA, the two cohorts were combined and analysed according to treatment received using regression models for longitudinal data via generalised estimating equations. Separate models for fit for the EQ-5D and 30-day cost rates were measured. Mean costs over each interval were summed to provide estimates of total mean costs for each treatment group. The treatment indicator was a time dependant covariate allowing for variable surgery time. This would have the effect of incorporating the non-operative experience of patients who postponed surgery beyond 3 months from enrolment. Because of the allowable windows for scheduled visits and crossover, the actual time for outcome assessment varied – this was included as an adjusting variable in the regression, to adjust for potential confounding baseline variables associated with missing data or treatment were included as covariates |
Synthesis of costs and benefits: HRQoL using EQ-5D to generate QALYs Statistical analysis: AUC analysis was formed to estimate difference in QALYs between the surgical and non-operative treatments, adjusted to a common baseline value. For costs, the adjusted mean 30-day difference in rates at 6 weeks, 3, 6, 12 and 24 months, was used to form a mean difference in total costs over 2 years by multiplying each adjusted rate by corresponding time interval between visits and summing to obtain total costs in each treatment group. To estimate and ICER CI, bootstrap methods were used from 100 samples taken, with replacement from the original sample with the individual as the unit of observation Sensitivity analysis: sensitivity analysis was undertaken to assess the impact of assumption and analytical approach to cost-effectiveness results. This included the impact of limiting costs to direct medical costs or direct medical costs, plus costs of work loss for those employed in the workforce. Main results: Total mean discounted QALYs were 1.64 (95% CI 1.62 to 1.67) for surgical patients and 1.44 (95% CI 1.4 to 1.47) for non-operative patients; a difference of 0.21 (95% CI 0.16 to 0.25) Total mean costs were US$27,273 (95% CI US$26,009 to US$28,644) for surgical patients and US$13,135 (95% CI US$11,244 to US$14,902) for non-operative patients. Total direct costs were US$20,237 (95% CI US$19,314 to US$21,160) for surgery and US$5804 (95% CI US$4639 to US$6969) for non-operative patients Total loss of productivity costs were US$7089 (95% CI US$6155 to US$8022) for surgical patients and US$7399 (95% CI $6221 to US$8577) for non-operative costs The cost per QALY gained for surgical treatment relative to non-operative care in the general population was US$69,403 (95% CI US$4923 to US$94,999) For those aged ≥ 65 years, the cost per QALY gained decreased to US$34,355 (95% CI US$20,419 to US$25,512) Limiting costs to direct costs alone for general population (US$72,181, 95% CI US$56,473 to US$92,394) and Medicare (US$37,285, 95% CI US$28,364 to US$48,993) or direct costs with lost work days (general population US$77,300, 95% CI US$60,009 to US$99,544) or Medicare (US$42,111, 95% CI US$30,976 to US$56,284) had little change This also had little impact on the ICER which was estimated at US$33,176 (95% CI US$18,348 to US$54,157) under Medicare pricing |
Author’s conclusions/implications for practice: surgery for intervertabral disc herniation was moderately cost-effective when evaluated over 2 years. The estimated economic value of surgery varied considerably according to the method used for assigning surgical costs Surgical treatment of herniated disc represents a reasonably cost-effective health-care intervention when compared with other common health-care interventions |
|
van den Hout, 2008 283 Type of economic evaluation: CUA Currency/country: Euro (€) Cost year: 2008 Perspective: societal perspective, but sensitivity analysis considered societal and health-care perspective Study population: 283 patients aged 18–65 years enrolled in a multicentred randomised trial who had sciatica for 6–12 weeks caused by lumbar disc herniation Interventions (including comparator): early surgery vs 6 months of usual conservative care |
Source of effectiveness data: single study Source of cost data: data from actual source Source of cost data: single study |
Link between cost and effectiveness data: prospective/concurrent Direct costs: cost diaries completed by patients were used to report hospital admissions, visits (by health-care professional), home care, paid domestic help, informal care. Out of pocket expenses. Prices were obtained from 75 Dutch hospitals; with the two highest and lowest excluded. A cost structure was applied by converting average price E2357 admission to hospital plus E390 per bed day. Average stay was 3.7 days and adding cost of related specialist visits resulted in average cost per hospital stay equal to average diagnosis-treatment price. For other health-care costs, Dutch standard prices were used Productivity costs: hours of absenteeism from work – this was valued according the human capital method at standard cost Resource use: included in direct costs Discounting: discounting not applied as only 1-year follow-up Modelling: base-case CUA compared societal cost at 1 year with QALYs at 1 year based on UK EQ-5D. No further time horizons considered |
Synthesis of costs and benefits: QALYs derived from patient-reported QoL, collected using the EQ-5D and SF-36 (from which SF-6D utilities were calculated) Statistical analysis: followed ITT principle. Cost-effectiveness acceptability curves were produced. CIs for cost–utility ratios were calculated Sensitivity analysis: sensitivity analysis carried out only on use of different utility measures (UK EQ-5D, US EQ-5D, SF-6D or VAS and on the included cost categories (societal or health-care perspective) Main results: the differences in QALYs reported according to the utility measure used were: UK EQ-5D 0.044 (95% CI 0.0005 to 0.083); US EQ-5D 0.032 (95% CI 0.005 to 0.059); SF-6D 0.024 (95% CI 0.003 to 0.0046) and VAS 0.032 (95% CI –0.003 to 0.066) From the perspective of the health-care system, total health-care costs remained significantly higher than prolonged conservative care, with a difference in costs of €1819 (95% CI €842 to €2790) per patient Total societal costs were –€12 (95% CI –€4029 to €4006) slightly in favour of early surgery The probability that early surgery is cost-effective compared with conservative care varies with willingness to pay From a societal perspective it was 76% at €40,000 per QALY and was 87% at €80,000 per QALY From the health-care perspective, according to the UK EQ-5D and US EQ-5D, the incremental cost per QALY gained with early surgery was estimated at €41,000 (95% CI €14,000 to €430,000) and €57,000 (95% CI €19,000 to €436,000), respectively |
Author’s conclusions/implications for practice: faster recovery from sciatica makes early surgery more cost-effective than prolonged conservative care. The estimated differences in health-care costs were acceptable and were compensated for by the difference in absenteeism from work. For a ‘willingness to pay’ ceiling ratio of €40,000 or more per QALY, early surgery need not be withheld for economic reasons |
Appendix 11 Study protocol
The effectiveness and cost-effectiveness of management strategies for sciatica: systematic review and economic model
Introduction
Research is needed to identify the most clinical effective and cost-effective management strategies for sciatica. Many treatment modalities for sciatica have been evaluated in placebo controlled trials (or usual care used as the comparator) and the evidence relating to the direct comparison of numerous treatment modalities are missing. In addition, in clinical practice a sequential stepped care approach, using different treatment modalities is considered useful. However, primary studies have tended to examine individual treatments given in isolation, rather than sequential, stepwise treatment provision. The optimum sequence of treatment modalities and what sequence is best for which patients are therefore not known. In order to evaluate this, comparative estimates of the effectiveness of the different interventions, conditional on the administration of previous interventions, is required. Multiple treatments may also be administered sequentially in the hope of additive effects in combined therapy; therefore, the additive and interaction effects of multiple interventions also needs to be explored. Previous systematic reviews have found evidence for the effectiveness of invasive treatments such as ESI, chemonucleolysis and lumbar discectomy, but found insufficient evidence to advise bed rest, keeping active, analgesia, intramuscular steroid injection or traction. None of the reviews made indirect comparisons across separate trials or examined cost-effectiveness. Previous economic evaluations that have been conducted vary quite considerably, and their value is limited to the perspective and setting for which they were undertaken. We therefore plan to undertake a systematic review of the clinical effectiveness and cost-effectiveness of different management strategies for sciatica, which tries to address some of these issues. We will also develop a decision-analytic model to assess the cost-effectiveness of different treatment modalities from the UK perspective.
Research objectives
-
To undertake a systematic review of the clinical effectiveness and cost-effectiveness of different management strategies for sciatica.
-
To synthesise the results using meta-analyses and a MTC method.
-
To construct an appropriate probabilistic decision-analytic model to estimate costs per QALY gained for each treatment strategy.
-
To make recommendations for clinical practice and commissioning in the UK NHS.
Background
Definition of sciatica
Sciatica is a symptom defined as unilateral, well-localised leg pain, with a sharp, shooting or burning quality that approximates to the dermatomal distribution of the sciatic nerve down the posterior lateral aspect of the leg (and normally radiates to the foot or ankle). It is often associated with numbness or paraesthesia in the same distribution. 1,2 The symptom of sciatica is used by clinicians in different ways. Some refer to any leg pain referred from the back as sciatica; others prefer to restrict its use to pain originating from the lumbar nerve root. Some authors prefer to use the term ‘lumbar nerve root pain’ to distinguish it from referred leg pain. 3
Epidemiology of sciatica
The lack of clarity in the definition of sciatica persists in the epidemiological literature. In a UK study, the prevalence of ‘sciatica suggesting a herniated lumbar disc’ was reported as 3.1% in men and 1.3% in women. 4 However, like most surveys, this study did not use strict criteria to diagnose sciatica. A large population survey in Finland which did found a lifetime prevalence of 5.3% in men and 3.7% in women. 5 Sciatica accounts for < 5% of the cases of low back pain presenting to primary care. 3 Some cohort studies have found that most cases resolve spontaneously, with 30% of patients continuing to experience troublesome symptoms at 1 year, 20% out of work and 5–15% requiring surgery. 6,7 However, another cohort found that 55% still had symptoms of sciatica 2 years later and 53% after 4 years (which included 25% who had recovered after 2 years but had relapsed again by 4 years). 8 The cost of sciatica to society in the Netherlands in 1991 was estimated at US$128M for hospital care, US$730M for absenteeism and US$708M for disablement. 9
Pathological mechanism
Sciatica caused by lumbar nerve root pain usually arises from a prolapsed intervertebral disc, but also from spinal stenosis or surgical scarring. 7 It was initially thought to occur predominantly as a result of compression of the nerve root,10 leading to neural ischaemia, oedema (which would in turn lead to chronic inflammation), scarring and perineural fibrosis. However, it is now known that symptoms can occur in the absence of direct nerve root compression, possibly as a result of release of proinflammatory factors from the damaged disc. Pain occurs because of chronic, repetitive firing of the inflamed nerve root. 11,12 Referred leg pain occurs because pain fibres from paraspinal structures and from the leg converge on interneurons in the spinal cord and brain, so that nociceptive input from painful paraspinal tissues is perceived as leg pain.
Clinical diagnosis
It has been claimed that nerve root pain can be distinguished from referred leg pain because it is unilateral, radiates below the knee, results in leg pain that is worse than back pain, can be aggravated by coughing or sneezing and has a segmental distribution. Important clinical signs include provocation tests for dural irritation, such as a limited SLR reproducing the leg pain, and compromised nerve root function leading to reduced power, sensation or reflexes in one nerve root. 3 A systematic review of the diagnostic value of history and physical examination in nerve root pain found that pain distribution was the only useful item in the history. The SLR test was the only sensitive sign in the physical examination, but had poor specificity; the crossed SLR test was the only specific sign, but had poor sensitivity. 13 However, another review found that there was no standard SLR procedure, no consensus on interpretation of results and no evidence of intra- and inter-observer reliability, and its predictive value in lumbar intervertebral disc surgery was unknown. 14
Treatments
A variety of surgical and non-surgical treatments have been used to treat sciatica and have been the subject of previous systematic reviews, the findings of which are summarised below. However, none of the reviews examined the cost-effectiveness of the various treatment modalities.
Two sets of guidelines on the management of sciatica from 1994 recommend initial conservative management with advice, reassurance and analgesia if there is no major or progressive motor weakness, and urgent referral for specialist assessment and investigation if symptoms are not resolving satisfactorily after 6 weeks. 2 If strong physiological evidence of a specific nerve root dysfunction with intervertebral disc herniation is confirmed at the corresponding level and side by findings on an imaging study, surgical options can be discussed. Standard discectomy or microdiscectomy is the surgical treatment of choice. 15 More recent guidelines have concentrated on non-specific low back pain and have not discussed the management of sciatica. This review will inform the development of up-to-date management recommendations by other groups.
Bed rest and advice to stay active
Most cases resolve spontaneously, and traditionally bed rest has been advised. A Cochrane systematic review of bed rest16 found high-quality evidence of little or no difference in pain or functional status between bed rest and staying active; moderate-quality evidence of little or no difference in pain intensity between bed rest and physiotherapy, but small improvements in functional status with physiotherapy; and moderate-quality evidence of little or no difference in pain intensity or functional status between 2–3 and 7 days’ bed rest. A Cochrane systematic review of advice to keep active reviewed the same trials comparing bed rest with activity and came to the same conclusions. Although there is no evidence to advise bed rest for sciatica, there is also very little evidence of benefit for advice to keep active. 17
Analgesia
Most patients will obtain analgesic medication either on prescription or ‘over the counter’ from their pharmacist. A systematic review of conservative treatment for sciatica identified three RCTs that compared NSAIDs with a placebo tablet and found no evidence of efficacy. 18
Intramuscular steroids
Part of the mechanism for producing nerve root pain is by release of proinflammatory factors from damaged discs, so administration of intramuscular corticosteroid steroid injections to reduce inflammation of the nerve root has a theoretical basis. The systematic review of conservative treatment for sciatica identified two RCTs comparing steroid injections with a placebo injection and found no evidence of efficacy. 18
Traction
Traction is used relatively frequently to treat sciatica in North America, but less frequently in the UK, Eire and the Netherlands. 19,20 A Cochrane systematic review found strong evidence that there was no significant difference between either continuous or intermittent traction versus placebo, sham or mixed treatments. 21
Epidural steroids
Introduction of corticosteroids into the epidural space is a commonly used treatment for lumbar nerve root pain, with the rationale of reducing nerve root inflammation. It was performed on 47,665 occasions in the NHS in England in 2005–6. 22 A systematic review of ESIs compared with saline, local anaesthetic injection or dry needling reported that six RCTs found epidural steroids to be better than a control treatment whereas six RCTs found them to be no better or worse. The methodological quality of these RCTs was criticised. 23 A further systematic review that examined selective nerve root blocks, excluding epidurals given by the caudal route, found five RCTs (one of high quality) providing moderate evidence that nerve root blocks were more effective than a local anaesthetic or saline injection. 24 Since then, an RCT funded by the HTA25 found that ESI resulted in a small, transient improvement in function and pain, compared with placebo, 3 weeks after injection, but no relative improvement after 6 weeks and 1 year. This RCT also performed a health economic analysis; none of the cost per QALY estimates was below the implied NICE ceiling of £30,000 per QALY gain.
Spinal manipulation
The systematic review of conservative treatment for sciatica identified two RCTs of spinal manipulation. One found that manipulation was more effective than placebo and another found no difference when compared with manual traction, exercises or corset. 18
Chemonucleolysis
Chemonucleolysis is a technique that attempts to decrease the volume of a disc herniation by reducing the amount of material contained within the nucleus pulposus by injecting the enzyme chymopapain. A systematic review of lumbar discectomy and percutaneous treatments found three RCTs that compared chymopapain with placebo injection found greater symptom relief in the group that received chymopapain. 26
Lumbar discectomy
Between 5% and 15% of patients with lumbar nerve root pain are treated with surgery,6,7 usually involving a lumbar discectomy. In the NHS in England in 2005–6, 8683 lumbar discectomies were performed. 22 A Cochrane systematic review of surgery for lumbar disc prolapse27 found 26 RCTs, but only one compared discectomy with conservative management. Meta-analyses have shown that surgical discectomy produces better clinical outcomes than chemonucleolysis, which is better than placebo. Three RCTs found no difference in clinical outcomes between microdiscectomy and standard discectomy, but in three other studies both microdiscectomy and standard discectomyproduced better results than percutaneous discectomy. The review concluded that there was considerable evidence of the clinical effectiveness of discectomy for carefully selected patients with sciatica caused by lumbar disc prolapse that fails to resolve with conservative management. Serious complications from lumbar disc surgery are uncommon, with a mortality rate of 0.3%, and infection rate of 3.0% and 4.0% requiring an intraoperative transfusion. Surgery fails to relieve symptoms in 10–20% of cases. 26
Other treatments
There are a number of other treatments that have not been included in previous systematic reviews, for example complementary therapies such as acupuncture. These will be included in the proposed review.
Pattern of treatments
Overall, there is no close correlation between symptom severity and pathology in sciatica. Increasing distance between onset and effective treatment has an unfavourable influence on symptoms and disability. While there is reason to suppose that a stepped care approach to sciatica could be helpful, the application of the various available treatments depends more on availability, clinician preference and socioeconomic variables than patient needs. In practice, some patients will recover under an analgesic cocktail while on a waiting list, some will be offered surgery as a first line intervention and yet others will receive a combination of treatments in no particular order. With few exceptions, it would appear that the patients attending differing treatment approaches are clinically indistinguishable. This set of issues will be central to the proposed review and synthesis.
Sources of heterogeneity in studies of sciatica
We anticipate that the review will find a diverse set of studies. Some of the potential sources of heterogeneity includes the following.
Diagnostic heterogeneity
As discussed in the introduction, sciatica is a symptom rather than a strict pathological label. Many of the studies will include patients with referred leg pain as well as nerve root pain. Stricter diagnostic criteria including findings from imaging studies are used more in surgical compared with non-surgical trials. Similarly, when nerve root pain is responsible, causes other than prolapsed intervertebral discs are more likely to be included trials that do not use imaging findings as inclusion criteria.
Treatment group heterogeneity
Different treatments are likely to include different patient groups because of the diagnostic heterogeneity discussed above. Treatments that are further up the gradient of invasiveness (e.g. disc surgery), are more likely to be used in patient populations with fewer cases of referred leg pain, longer duration of symptoms, greater degree of disability and psychosocial morbidity, particularly if patients are receiving treatments in a sequential manner.
Heterogeneity of co-interventions
Co-interventions vary between trials testing the same intervention as well as between different interventions. For example, postoperative management after lumbar disc surgery is inconsistent with regard to postoperative restrictions, reactivation and return to work. 28
Heterogeneity of health-care provision
There is wide variation in the management of sciatica between countries in terms of the use of primary care,3 the rate of disc surgery29 and social security provision. 30
Heterogeneity of outcome measures
The relative importance of the various outcomes (e.g. pain, disability, work status, costs) varies across groups of stakeholders (patients, clinicians, providers) and can change over time. For example, during the initial stages the patient may value pain relief, but with time functional status may become more important. This will be considered during both review and synthesis.
Systematic review method
The review will follow the methodology reported in CRD report Undertaking systematic reviews of research on effectiveness: CRD’s guidance for those carrying out or commissioning reviews. 31 Studies examining effectiveness and those evaluating cost-effectiveness will be reviewed separately.
Literature search
The following databases will be searched for published, semi-published and grey literature:
-
MEDLINE
-
MEDLINE In-Process & Other Non-Indexed Citations
-
EMBASE
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL)
-
PsychINFO
-
Allied and Complimentary Database (AMED)
-
Health Management Information Consortium (HMIC)
-
British Nursing Index
-
BIOSIS
-
Cochrane Central Register of Controlled Trials (CENTRAL)
-
Database of Abstracts of Reviews of Effects (DARE)
-
Cochrane Database of Systematic Reviews (CDSR)
-
Health Technology Assessment (HTA) Database
-
NHS Economic Evaluation database (NHS EED)
-
Science Citation Index
-
Social Science Citation Index (SSCI)
-
Index to Scientific & Technical Proceedings (ISTP)
-
System for Information on Grey Literature In Europe (SIGLE)
-
Inspec
-
Physiotherapy Evidence Database (PEDro).
The search strategy for MEDLINE (via OVID) is presented in Appendix 1 and will be translated for use on other databases. No language or date restrictions will be used.
The following trial registries will be searched to identify any further completed or ongoing trials:
-
National Research Register (NRR)
-
National Institute for Health’s ClinicalTrials.gov database
-
CenterWatch Clinical Trials Listing Service
-
Current Controlled Trials (CCT).
The following conference proceedings will be searched by hand where feasible (pending availability) for the last 5 years:
-
EuroSpine
-
International Society for the Study of Spine
-
BritSpine
-
North American Spine Society.
The journal Spine will also be searched by hand for the last 5 years.
Reference lists of previous systematic reviews and included studies will be screened and citation tracking undertaken where feasible.
The results of the searches will be entered onto the reference management software, Endnote. Articles written in a language other than English will be translated whenever possible. Multiple publication of the same study will be identified, grouped together and represented by a single reference.
Methods of study selection
Planned inclusion/exclusion criteria
| Criteria | Clinical effectiveness | Cost-effectiveness |
|---|---|---|
| Study design | RCTs and non-RCTs, as well as controlled observational studies | Economic evaluations conducted alongside trials, modelling studies and analyses of administrative databases will be included if they compare two or more treatments and consider both costs and consequences (including cost-effectiveness, cost–utility, cost–benefit and cost-consequences analysis). Cost-analysis undertaken as part of a comparative study, where data on both costs and consequences are reported, but not combined will also be included |
| Patient population | Adults with sciatica or lumbar nerve root pain diagnosed clinically or confirmed by imaging. The essential clinical criterion is leg pain worse than back pain. Other clinical criteria that support the diagnosis include: unilateral leg pain; pain radiation below the knee; aggravated by cough/sneeze; segmental distribution; provocation tests (e.g. impaired SLR); reduced power, sensation or reflexes in one nerve root. Studies that include participants with low back pain will be included if the findings for patients with sciatica are reported separately; studies where the results are not reported separately for sciatica, will be excluded. Studies of specific conditions such as spinal stenosis or discogenic pain will only be included if it is documented that leg pain is worse than back pain. If imaging has been used it must demonstrate evidence of nerve root irritation | |
| Interventions | Any | |
| Comparators | Any placebo, manual, medical, or surgical treatment for sciatica | |
| Outcomes | Any relevant patient-based outcome measure such as pain, disability, functional status, adverse effects, health status, QoL, analgesic use, operation rates, health utility, return to work, health service use and costs. Biochemical outcomes and biomechanical measurements (e.g. change in disc space) will be excluded | Any outcome measure |
Assessing relevancy of included studies
Two reviewers will independently screen the titles and abstracts identified by the electronic searches for relevancy. Potentially relevant studies will be ordered and assessed for inclusion, using the criteria reported above, by two independent reviewers. Disagreements during both stages will be resolved by discussion or if necessary taken to a third reviewer.
Further literature needed to inform the economic model
As well as searching for clinical effectiveness and cost-effectiveness studies, we will systematically search for epidemiological studies and case series with long-term follow-up data that will inform the economic model. We will also search for studies that identify the type of treatment strategies being used in practice, report prevalence data, provide information on the probability of moving to different states, give estimates of duration in different states, report information on utilities or identify the type of outcome measures that are of importance to patients, clinicians or policy makers. The model will also use resource data from the NHS including costs, tariffs and unit costs, available from national sources.
Quality assessment
Quality assessment will be undertaken by two independent reviewers with differences being resolved by consensus or by a third reviewer if necessary. Data relating to quality assessment will be inputted onto a Microsoft Access database.
Effectiveness studies
The quality of included trials and observational studies will be assessed using a checklist based on the one used by the ‘Back Review Group’ of the Cochrane Collaboration for RCTs32 and the one developed by the Hamilton Effective Public Health Practice Project (EPHPP) Team for quantitative studies (which includes both comparative observational studies and RCTs). 33 The checklist is presented in Appendix 2. The criteria cover selection bias and confounding, detection bias, performance bias and attrition bias. Criteria relating to external validity have also been added.
The quality checklist will be used to describe the overall quality of individual studies and the likelihood of bias, and will not be used to calculate an overall quality score. Alternatively the robustness of the quality assessment will be assessed using sensitivity analyses, which will examine the influence of the following individual criteria: randomisation, concealment of allocation, blinding of outcome assessment and loss to follow-up ≤ 80%.
Economic evaluations
The quality of the cost-effectiveness studies will be assessed according to an updated version of the checklist developed by Drummond et al. (see Appendix 2). 34 The checklist reflects the criteria for economic evaluation detailed in the methodological guidance developed by NICE. For studies based on decision models, the critical appraisal will be based on the checklist developed by Weinstein et al. (see Appendix 2). 35
Data extraction
Data will be extracted using predefined forms developed on a Microsoft Access database. Separate forms will be used for clinical effectiveness studies and cost-effectiveness studies; these will be piloted on a small selection of relevant studies in advance and adjusted if necessary. Multiple publications of the same study will be identified and collated. Data will be extracted by one reviewer and checked against the original paper by a second independent reviewer. Any disagreements will be resolved by discussion, or by a third reviewer if necessary.
Data extraction for effectiveness studies
Study location and setting, description of study population (including method of diagnosis and previous treatment), type of intervention and control used, how allocation was performed, outcome measures used and results with sufficient information (such as proportions, means, SDs, SEs, significance levels, CIs, NNTs) to estimate effect sizes wherever possible.
Data extraction for cost-effectiveness studies
Type of economic evaluation, specific details about the interventions being compared, study population, time period, measures of effectiveness, direct costs (medical and non-medical), productivity costs, resource use, currency, results and details of any decision modelling and sensitivity analysis.
Methods of analysis/synthesis
Effectiveness studies
The findings will initially be subdivided according to the different treatment modalities. The results of data extraction and quality assessment will be presented in structured tables and also as a narrative summary. Ongoing studies will be reported separately and the potential impact of their findings will be discussed.
Meta-analysis and metaregression
This will be conducted for each treatment comparison for which there are compatible multiple studies and each outcome measure (including separate analyses for short- and long-term follow-up). Random effects will be included in the modelling36 when between-study heterogeneity is present as ascertained by examining (chi-squared and I-squared) statistics. 37 The results of these will be presented using forest plots, subgrouping results by study design. In an attempt to explain any between-study heterogeneity, metaregression will be conducted. 38 This will examine: the influence of characteristics of study design (year, location, randomisation, concealment of allocation, blinding of outcome assessment, > 80% follow-up); patient characteristics (mean age, gender proportion); diagnostic heterogeneity (inclusion criteria including physical examination or imaging findings); symptom duration; level of disability and psychosocial morbidity (from baseline measures of health status); failed previous treatment; and use of co-interventions. In addition, a sensitivity analysis excluding any non-randomised studies from the analysis will be conducted to assess the influence of the lower quality evidence on the conclusions. For all comparisons for which there are more than five studies, funnel plots together with associated tests,39,40 will be considered to assess the potential for publication bias.
Mixed treatment comparison
Since it is anticipated that not all treatment comparisons of interest will have been evaluated in controlled studies, we will then synthesise all RCTs that form a closed network,41 using a MTC synthesis methodology. 42 This allows the estimation of all treatment comparisons of interest, without breaking within-study comparisons and hence randomisation where it exists. 43 Particular care will be taken to ensure treatment regimens are comparable in studies used for the direct and indirect estimation within the model. Informal comparisons between the estimated effects from the individual (direct-comparison) meta-analyses and the MTC model will be made, and more formal assessments of the coherence and consistency of the evidence network will be made using deviance information criteria and related statistics,44 as calculated by the Bayesian Winbugs software. Important covariates identified from the metaregression analyses that explain between-study heterogeneity will be included in the MTC model. Novel modelling will also be developed and used to acknowledge issues relating to sequential intervention effects and other specific issues relating to sciatica treatment. The MTC model will then be further extended by including non-trial data for those comparisons for which there is no available data from RCTs. Information on study quality will be incorporated to take into account the use of data from imperfect sources. 45 It is anticipated that the MTC modelling approach will give estimates of the parameters required for the economic decision model.
Economic evaluations
Details of each published economic evaluation, together with a critical appraisal of its quality, will be presented in structured tables and narrative summary. Where appropriate and where the data presented permit, indications of the uncertainty underlying the estimation of the differential cost and effects of the alternative treatment options will be summarised.
Other parameters for the economic evaluation
Previous experience of conducting meta-analyses and associated cost-effectiveness modelling, indicates that outcome measures that are of most clinical relevance and interest, are not necessarily the outcomes that are most compatible and relevant to the economic model. Therefore, early in the project, those carrying out the evidence synthesis will liaise closely with the decision modellers to ensure syntheses that are required for the decision model are conducted.
In addition to the syntheses to estimate clinical effectiveness, further syntheses may be desirable to estimate other parameters in the economic decision model. 46
Cost-effectiveness modelling
It is likely that the existing evidence relating to the cost-effectiveness of treatments, will have a number of limitations that make it insufficient to inform decision-making regarding the most appropriate management strategy for patients with sciatica. Thus, it will be necessary to construct an appropriate probabilistic, decision-analytic model to address a number of these issues more formally. This model will provide a framework for the synthesis of data from the clinical effectiveness, economic reviews and other relevant sources. It will be developed to estimate costs from the perspective of the UK NHS and personal social services47,48 and health outcomes in terms of QALYs gained for the range of relevant treatment strategies. (If the findings of the literature review indicate that patients value different outcomes to those of policy makers and clinicians, then the model will be developed using two iterations, including one from the patient perspective.) The number of appropriate and relevant health states will be informed by the results of the service provider survey (see Telephone survey of service providers), the literature review and from advice within the research team. The cost of managing patients within each state will be reflected in the model, while it is not envisaged that patient progression will be seamless, or indeed linear and uni-directional. The structure of the model will reflect this and the probability of movement between health states will be based on the evidence from the literature review, including the distribution around the point estimates. In addition, a sensitivity analysis will be used to assess the impact of ‘changes’ in the variable estimates and identify potential areas for future research. A probabilistic sensitivity analysis will assess the extent to which any one particular strategy is likely to be within the bounds of what is considered to be cost-effective.
The model will incorporate a range of time horizons. It is proposed that a probabilistic model be constructed to ensure that uncertainty can be appropriately characterised depending on the range of comparators included in the analysis. 49 Given that mean costs and QALYs gained will therefore be estimated with uncertainty, the outputs from the simulations will be used to generate cost-effectiveness acceptability curves for the alternative analyses. These curves detail the probability that each intervention is cost-effective over a range of potential maximum values that the health service is prepared to pay for an additional QALY. 50 The budgetary impact (again from a NHS perspective), will also be assessed as part of the health economic evaluation.
The findings of the model will be contrasted with other economic evaluations identified by the review, which will also be used to test the inputs and assumptions made in our model.
Telephone survey of service providers
Approximately 30 service providers, known to the advisory group members, will be contacted by telephone to determine their usual clinical practice, the usual treatment pathways and if they use a stepped care approach. This information will be used to inform which sequence of treatments to include in the economic model.
Previously conducted systematic reviews will be used to generate a list of potential treatments for sciatica. During the telephone interviews, clinicians will be asked initially what treatments (including combination and sequence of treatments) they usually use, and afterwards, if prominent treatments identified from previous reviews are not mentioned, they will be asked if they have ever considered using these.
Recommendations for practice and research
Recommendations for practice
We will make recommendations for practice based on what is feasible within the UK NHS setting. The importance of sequential therapies and a stepped care approach will also be considered, with recommendations being made about possible optimum care pathways. We will make comparisons between clinical resolution and return to work.
Recommendations for further research
The overall findings of the review will be used to make recommendations for further research, including details (such as optimal comparator treatments) of the types of trials that would make important contributions to the existing evidence base. 51 The modelling will inform future research recommendations using ‘value of information’ methods, which equate the cost of further research to the cost of improved decision making that could be done as a result of having the further information. In particular we will use ‘expected value of perfect information’ to estimate the benefit of having perfect information on all parameters in the model, in order to give an upper bound on the payoff of further information. Additionally, the findings of the quality assessment of the existing comparative studies evaluating treatment effectiveness, will be used to make recommendations about how to improve conduct of such studies in the future.
The findings of previous systematic reviews, which are based on standard meta-analyses, will be compared with ours to see if using the data from observational studies and the additional modelling work results in different conclusions being made.
Project management
Study management
A study management group will be formed, it will be responsible for overseeing the progress of the study throughout all of its phases and will meet regularly (every 1–2 months). The day-to-day management of the study will be co-ordinated through the study co-ordination centre (Wrexham). The reviewing team (Wrexham) and the team conducting the economic evaluation (Swansea) will meet as and when is required by teleconference. A steering committee will be held every 3–6 months. Data monitoring and quality assurance will be overseen by the steering group.
Steering group
The review team as a whole will form a steering group, which will meet every 3–6 months. The role of the steering group will be to ensure that the study is conducted to a rigorous standard and to make any necessary strategic decisions. Members of the group will also be responsible for approving the protocol and ensuring that the study adheres to it; provide information support (such as answering methodological and clinical queries) to those conducting the review or economic analysis; identify relevant studies that the literature searches may have missed; assist with the analysis and interpretation of the findings; and approve of the final report and any subsequent publications.
Service users
The review team (steering group) includes a number of service users, which includes clinicians working in the field and a patient representative. The clinicians include general practitioners (NW, CW), osteopaths (NW, KB), a spinal surgeon (IB) and a musculoskeletal physician (RC). The patient representative will be IR who has undergone spinal surgery. A second patient representative who has not undergone surgery will be recruited with the help of the Clinical Research Collaboration Cymru Involving People /Cynnwys Pobl, patient, service user and carer network. Service user representatives will be able to help us ensure the appropriateness of the research question (and inclusion/exclusion criteria), provide input on the type of data to be extracted from primary studies and provide input on the interpretation of the findings.
Dissemination
A final report will be submitted to the funding body. Papers will be submitted to high-quality journals and presented at national and international conferences.
References
- Deyo RA, Rainville J, Kent DL. What can the history and physical examination tell us about low back pain?. JAMA 1992;268:760-5.
- Clinical Standards Advisory Group . Back Pain 1994.
- Waddell G. The back pain revolution. Edinburgh: Churchill Livingstone; 1998.
- Lawrence JS. Rheumatism in populations. London: Heinemann; 1977.
- Heliovaara M, Impivaara O, Sievers K, Melkas T, Knekt P, Korpi J, et al. Lumbar disc syndromein. J Epidemiol Community Health 1987;41:251-8.
- Bush K, Cowan N, Katz DE, Gishen P. The natural history of sciatica associated with disc pathology; a prospective study with clinical and independent radiological follow-up. Spine 1992;18:1433-8.
- Weber H, Holme I, Arnlie E. The natural course of acute sciatica with nerve root symptoms in a double blind placebo controlled trial evaluating piroxicam. Spine 1993;18:1433-8.
- Tubach F, Beaute J, Leclerc A. Natural history and prognostic indicators of sciatica. J Clin Epidemiol 2004;57:174-9.
- van Tulder MW, Koes BW, Bouter LM. A cost of illness study of back pain in the Netherlands. Pain 1995;62:233-40.
- Mixer WJ, Barr JS. Rupture of the intervertebral disc with involvement of the spinal canal. N Engl J Med 1934;211:210-15.
- Revdivik B, Brown M, Lundborg G. Pathoanatomy and pathophysiology of nerve root compression. Spine 1984;9:7-15.
- Goupille P, Jayson MI, Valat JP, Freemont AJ. The role of inflammation in disc herniationassociated radiculopathy. Semin Arthritis Rheum 1998;28:60-71.
- Vroomen PCAJ, de Krom MCTFM, Knotternus JA. Diagnostic value of history and physical examination in patients suspected o sciatica due to disc herniation: a systematic review. J Neurol 1999;246:899-906.
- Rebain R, Baxter DG, McDonough S. A systematic review of the passive straight leg raising test as a diagnostic aid for low back pain. Spine 2002;27:E388-E395.
- Agency for Health Care Policy and Research . Management Guidelines for Acute Low Back Pain 1994.
- Hagen KB, Hilde G, Jamtvedt G, Winnem M. Best rest for acute low back pain and sciatica. Cochrane Database Syst Rev 2000;2.
- Hilde G, Hagen KB, Jamtvedt G, Winnem M. Advice to stay active as a single treatment for acute low back pain and sciatica. Cochrane Database Syst Rev 2004;2.
- Vroomen PCAJ, de Krom MCTFM, Slofstra PD, Knotternus JA. Conservative treatment of sciatica: a systematis revies. J Spinal Disord 2000;13:463-9.
- Li LC, Bombardier C. Physical therapy management of low back pain: an exploratory survey of therapist approaches. Phys Ther 2001;81:1018-28.
- Harte AA, Gracey JH, Baxter GD. Current use of lumbar traction in the management of low back pain: results of a survey of physiotherapists in the United Kingdom. Arch Phys Med Rehabil 2005;86:1164-9.
- Clarke JA, van Tulder MW, Blomberg SEI, de Vet HCW, van der Heijden GJMG, Brinfort G. Traction for low back pain with or without sciatica. Cochrane Database Syst Rev 2005;4.
- Hospital Episode Statistics 2007. URL: www.hesonline.nhs.uk (accessed 25 January 2007).
- Koes BW, Scholten RJPM, Mens JMA, Bouter LM. Efficacy of epidural steroid injections for low back pain and sciatica: a systematic review of randomized control trials. Pain 1995;63:279-88.
- DePalma MJ, Bhagava A, Slipman CW. A critical appraisal of the evidence for selective nerve root injection in the treatment of lumbosacral radiculopathy. Arch Phys Med Rehabil 2005;86:1477-83.
- Price C, Arden N, Coglan L, Rogers P. Cost-effectiveness and safety of epidural steroids in the management of sciatica. Health Technol Assess 2005;9.
- Stevens CD, Dubois RW, Larequi-Lauber T, Vader J-P. Efficacy of lumbar discectomy and percutaneous treatments for lumbar disc herniation. Soz Praventivmed 1997;42:367-79.
- Gibson JNA, Grant IC, Waddell G. The Cochrane review of surgery for lumbar disc prolapse and degenerative lumbar spondylosis. Spine 1999;24:1820-32.
- McGregor AHB, Burton AK, Sell P, Waddell G. The development of an evidence based patient booklet for patients undergoing lumbar discectomy and un-instrumented decompression. Eur Spine J 2007;16:339-46.
- Cherkin DC, Deyo RA, Loeser JD, Bush T, Waddell G. An international comparison of back surgery rates. Spine 1994;19:1201-6.
- Waddell GN, Nachemson AL, Jonsson E. Neck and back pain. Philadelphia, PA: Lippincott Williams & Wilkins; 2000.
- NHS Centre for Reviews and Dissemination (CRD) . Undertaking Systematic Reviews of Research on Effectiveness: CRD’s Guidance for Those Carrying Out or Commissioning Reviews 2001.
- van Tulder MW, Furlan A, Bombardier C, Bouter LM. Editorial Board of the Cochrane Collaboration Back Review Group . Updated method guidelines for systematic reviews in the Cochrane collaboration back review group. Spine 2003;28:1290-9.
- Effective Public Health Practice Project n.d. URL: www.myhamilton.ca/myhamilton/CityandGovernment/HealthandSocialServices/Research/EPHPP (accessed 12 May 2008).
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2005.
- Weinstein MC, O’Brien B, Hornberger J, Jackson J, Johannesson M, McCabe C. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR task force on good research practices-modeling studies. Value Health 2003;6:9-17.
- Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F. Methods for meta-analysis in medical researched. London: John Wiley; 2000.
- Higgins JPT, Thompson SG, Deeks JD, Altman DG. Education and debate: measuring inconsistency in meta-analysis. BMJ 3003;327:557-60.
- Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med 1999;18:2693-708.
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34.
- Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA 2006;295:676-80.
- Lumley T. Network meta-analysis for indirect treatment comparisons. Stat Med 2002;21:2313-24.
- Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 2004;23:3105-24.
- Caldwell DM, Ades AE, Higgins JPT. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 2005;331:897-900.
- Spiegelhalter DJ, Best NG, Carlin BR, van der Linde A. Bayesian measures of model complexity and fit. J R Stat Soc Ser B Stat Soc 2002;64:583-616.
- Turner RM, Spiegelhalter DJ, Smith GCS, Thompson SG. Bias modelling in evidence synthesis. J R Stat Soc Ser A Stat Soc 2009;172:21-47.
- Cooper NJ, Sutton AJ, Abrams KR, Turner D, Waioloo A. Comprehensive decision analytical modelling in economic evaluation: a Bayesian approach. Health Economics 2004;13:203-26.
- Curtis L, Netten A. Unit Costs of Health and Social Care 2006. URL: www.pssru.ac.uk/pdf/uc/uc2006/uc2006.pdf (accessed 29 January 2007).
- Department of Health . NHS Reference Costs 2005–06 n.d. URL: www.dh.gov.uk/assetRoot/04/14/14/30/04141430.xls (accessed 29 January 2007).
- Briggs AH, Drummond MF, McGuire A. Economic evaluation in health care: merging theory with practice. Oxford: Oxford University Press; 2001.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Economics 2001;10:779-87.
- Sutton AJ, Cooper NJ, Jones DR, Lambert PC, Thompson JR, Abrams KR. Evidence-based sample size calculations based upon updated meta-analysis. Stat Med 2007;26:2479-500.
- Deeks JJ, Dinnes J, D’Amico R, Sowden AJ, Sakarovitch C, Song F, et al. Evaluating nonrandomised intervetnion studies. Health Technol Assess 2003;7.
List of abbreviations
- ANCOVA
- analysis of covariance
- AUC
- area under the curve
- CCS
- concurrent cohort study
- CEA
- cost-effectiveness analysis
- CI
- confidence interval
- CSOM
- condition-specific outcome measure
- CUA
- cost–utility analysis
- DRG
- diagnostic-related group
- EPHPP
- Effective Public Health Practice Project
- EQ-5D
- European Quality of Life-5 Dimensions
- ESI
- epidural steroid injection
- GP
- general practitioner
- GPE
- global perceived effect
- HCS
- historical cohort study
- HMO
- health maintenance organisation
- HRQoL
- health-related quality of life
- ICER
- incremental cost-effectiveness ratio (e.g. incremental cost per QALY gained)
- IQR
- interquartile range
- ITT
- intention to treat
- LRS
- lumbar radicular syndrome
- MANOVA
- multivariate analysis of variance
- MRI
- magnetic resonance imaging
- MTC
- mixed treatment comparison
- NICE
- National Institute for Health and Clinical Excellence
- NNT
- number needed to treat
- NSAID
- non-steroidal anti-inflammatory drug
- ODI
- Oswestry Disability Index
- OR
- odds ratio
- OTC
- over the counter
- PENS
- percutaneous electrical nerve stimulation
- PT
- physical therapy
- QALY
- quality-adjusted life-year
- QDS
- Quebec Back Pain Disability Scale
- QoL
- quality of life
- Q-RCT
- quasi-randomised controlled trial
- RCT
- randomised controlled trial
- RMDQ
- Roland–Morris Disability Questionnaire
- SD
- standard deviation
- SE
- standard error
- SF-36
- Short Form questionnaire-36 items
- SG
- standard gamble
- SLR
- straight leg raise
- SMD
- standardised mean difference
- SPORT
- Spine Patient Outcomes Research Trial
- TENS
- transcutaneous electrical nerve stimulation
- TNF-α
- tumour necrosis factor-alpha
- TTO
- time trade-off
- VAS
- visual analogue scale
- WHO
- World Health Organization
- WMD
- weighted mean difference
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Department of Public Health and Epidemiology, University of Birmingham
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, University of Liverpool
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Peter Brocklehurst, Professor of Women’s Health, Institute for Women’s Health, University College London
-
Professor Jenny Donovan, Professor of Social Medicine, University of Bristol
-
Professor Jonathan Green, Professor and Acting Head of Department, Child and Adolescent Psychiatry, University of Manchester Medical School
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Freddie Hamdy, Professor of Urology, Head of Nuffield Department of Surgery, University of Oxford
-
Professor Allan House, Professor of Liaison Psychiatry, University of Leeds
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor Irwin Nazareth, Professor of Primary Care and Head of Department, Department of Primary Care and Population Sciences, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norrie, Chair in Clinical Trials and Biostatistics, Robertson Centre for Biostatistics, University of Glasgow
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Martin Underwood, Professor of Primary Care Research, Warwick Medical School, University of Warwick
-
Professor Marion Walker, Professor in Stroke Rehabilitation, Associate Director UK Stroke Research Network, University of Nottingham
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Tom Foulks, Medical Research Council
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Diana Baralle, Consultant and Senior Lecturer in Clinical Genetics, University of Southampton
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Public contributor
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Nicola Lennard, Senior Medical Officer, MHRA
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Public contributor
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Public contributor
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Matthew Thompson, Senior Clinical Scientist and GP, Department of Primary Health Care, University of Oxford
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Joanna Jenkinson, Board Secretary, Neurosciences and Mental Health Board (NMHB), Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Public contributor
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire County Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Mrs Jean Thurston, Public contributor
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Public contributor
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Public contributor
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Public contributor
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Public contributor
-
Mr Sankaran Chandra Sekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Public contributor
-
Mr Seumas Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Dr Matthew Wilson, Consultant Anaesthetist, Sheffield Teaching Hospitals NHS Foundation Trust
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan, Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Public contributor
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer, Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Public contributor
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Public contributor
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Public contributor
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Expert Advisory Network
-
Professor Douglas Altman, Professor of Statistics in Medicine, Centre for Statistics in Medicine, University of Oxford
-
Professor John Bond, Professor of Social Gerontology & Health Services Research, University of Newcastle upon Tyne
-
Professor Andrew Bradbury, Professor of Vascular Surgery, Solihull Hospital, Birmingham
-
Mr Shaun Brogan, Chief Executive, Ridgeway Primary Care Group, Aylesbury
-
Mrs Stella Burnside OBE, Chief Executive, Regulation and Improvement Authority, Belfast
-
Ms Tracy Bury, Project Manager, World Confederation of Physical Therapy, London
-
Professor Iain T Cameron, Professor of Obstetrics and Gynaecology and Head of the School of Medicine, University of Southampton
-
Professor Bruce Campbell, Consultant Vascular & General Surgeon, Royal Devon & Exeter Hospital, Wonford
-
Dr Christine Clark, Medical Writer and Consultant Pharmacist, Rossendale
-
Professor Collette Clifford, Professor of Nursing and Head of Research, The Medical School, University of Birmingham
-
Professor Barry Cookson, Director, Laboratory of Hospital Infection, Public Health Laboratory Service, London
-
Dr Carl Counsell, Clinical Senior Lecturer in Neurology, University of Aberdeen
-
Professor Howard Cuckle, Professor of Reproductive Epidemiology, Department of Paediatrics, Obstetrics & Gynaecology, University of Leeds
-
Professor Carol Dezateux, Professor of Paediatric Epidemiology, Institute of Child Health, London
-
Mr John Dunning, Consultant Cardiothoracic Surgeon, Papworth Hospital NHS Trust, Cambridge
-
Mr Jonothan Earnshaw, Consultant Vascular Surgeon, Gloucestershire Royal Hospital, Gloucester
-
Professor Martin Eccles, Professor of Clinical Effectiveness, Centre for Health Services Research, University of Newcastle upon Tyne
-
Professor Pam Enderby, Dean of Faculty of Medicine, Institute of General Practice and Primary Care, University of Sheffield
-
Professor Gene Feder, Professor of Primary Care Research & Development, Centre for Health Sciences, Barts and The London School of Medicine and Dentistry
-
Mr Leonard R Fenwick, Chief Executive, Freeman Hospital, Newcastle upon Tyne
-
Mrs Gillian Fletcher, Antenatal Teacher and Tutor and President, National Childbirth Trust, Henfield
-
Professor Jayne Franklyn, Professor of Medicine, University of Birmingham
-
Mr Tam Fry, Honorary Chairman, Child Growth Foundation, London
-
Professor Fiona Gilbert, Consultant Radiologist and NCRN Member, University of Aberdeen
-
Professor Paul Gregg, Professor of Orthopaedic Surgical Science, South Tees Hospital NHS Trust
-
Bec Hanley, Co-director, TwoCan Associates, West Sussex
-
Dr Maryann L Hardy, Senior Lecturer, University of Bradford
-
Mrs Sharon Hart, Healthcare Management Consultant, Reading
-
Professor Robert E Hawkins, CRC Professor and Director of Medical Oncology, Christie CRC Research Centre, Christie Hospital NHS Trust, Manchester
-
Professor Richard Hobbs, Head of Department of Primary Care & General Practice, University of Birmingham
-
Professor Alan Horwich, Dean and Section Chairman, The Institute of Cancer Research, London
-
Professor Allen Hutchinson, Director of Public Health and Deputy Dean of ScHARR, University of Sheffield
-
Professor Peter Jones, Professor of Psychiatry, University of Cambridge, Cambridge
-
Professor Stan Kaye, Cancer Research UK Professor of Medical Oncology, Royal Marsden Hospital and Institute of Cancer Research, Surrey
-
Dr Duncan Keeley, General Practitioner (Dr Burch & Ptnrs), The Health Centre, Thame
-
Dr Donna Lamping, Research Degrees Programme Director and Reader in Psychology, Health Services Research Unit, London School of Hygiene and Tropical Medicine, London
-
Professor James Lindesay, Professor of Psychiatry for the Elderly, University of Leicester
-
Professor Julian Little, Professor of Human Genome Epidemiology, University of Ottawa
-
Professor Alistaire McGuire, Professor of Health Economics, London School of Economics
-
Professor Neill McIntosh, Edward Clark Professor of Child Life and Health, University of Edinburgh
-
Professor Rajan Madhok, Consultant in Public Health, South Manchester Primary Care Trust
-
Professor Sir Alexander Markham, Director, Molecular Medicine Unit, St James’s University Hospital, Leeds
-
Dr Peter Moore, Freelance Science Writer, Ashtead
-
Dr Andrew Mortimore, Public Health Director, Southampton City Primary Care Trust
-
Dr Sue Moss, Associate Director, Cancer Screening Evaluation Unit, Institute of Cancer Research, Sutton
-
Professor Miranda Mugford, Professor of Health Economics and Group Co-ordinator, University of East Anglia
-
Professor Jim Neilson, Head of School of Reproductive & Developmental Medicine and Professor of Obstetrics and Gynaecology, University of Liverpool
-
Mrs Julietta Patnick, Director, NHS Cancer Screening Programmes, Sheffield
-
Professor Robert Peveler, Professor of Liaison Psychiatry, Royal South Hants Hospital, Southampton
-
Professor Chris Price, Director of Clinical Research, Bayer Diagnostics Europe, Stoke Poges
-
Professor William Rosenberg, Professor of Hepatology and Consultant Physician, University of Southampton
-
Professor Peter Sandercock, Professor of Medical Neurology, Department of Clinical Neurosciences, University of Edinburgh
-
Dr Philip Shackley, Senior Lecturer in Health Economics, Sheffield Vascular Institute, University of Sheffield
-
Dr Eamonn Sheridan, Consultant in Clinical Genetics, St James’s University Hospital, Leeds
-
Dr Margaret Somerville, Director of Public Health Learning, Peninsula Medical School, University of Plymouth
-
Professor Sarah Stewart-Brown, Professor of Public Health, Division of Health in the Community, University of Warwick, Coventry
-
Dr Nick Summerton, GP Appraiser and Codirector, Research Network, Yorkshire Clinical Consultant, Primary Care and Public Health, University of Oxford
-
Professor Ala Szczepura, Professor of Health Service Research, Centre for Health Services Studies, University of Warwick, Coventry
-
Dr Ross Taylor, Senior Lecturer, University of Aberdeen
-
Dr Richard Tiner, Medical Director, Medical Department, Association of the British Pharmaceutical Industry
-
Mrs Joan Webster, Consumer Member, Southern Derbyshire Community Health Council
-
Professor Martin Whittle, Clinical Co-director, National Co-ordinating Centre for Women’s and Children’s Health, Lymington