Notes
Article history
The research reported in this article of the journal supplement was commissioned and funded by the HTA programme on behalf of NICE as project number 09/98/01. The assessment report began editorial review in June 2010 and was accepted for publication in July 2010. See the HTA programme website for further project information (www.hta.ac.uk). This summary of the ERG report was compiled after the Appraisal Committee’s review. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report. The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2011. This work was produced under the terms of a commissioning contract issued by the Secretary of State for Health. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2011 Queen’s Printer and Controller of HMSO
This paper presents a summary of the evidence review group report into the clinical effectiveness and cost-effectiveness of omalizumab for the treatment of severe persistent asthma in children aged 6–11 years, based upon the evidence submission from Novartis Pharmaceutical UK Ltd to the National Institute for Health and Clinical Excellence (NICE) as part of the single technology appraisal process. The manufacturer’s submission was generally considered to be of good quality. The submission was based primarily on a preplanned subgroup IA-05 EUP (European Union Population) from the IA-05 trial, with outcomes including the number of clinically significant (CS) and clinically significant severe (CSS) exacerbations. Omalizumab therapy was associated with a statistically significant reduction in the rate of CS exacerbations, but the reduction in the rate of CSS exacerbations was not statistically significant. The benefit in terms of CS exacerbations was achieved mainly in patients with more than three exacerbations per year at baseline. The manufacturer found no previous published cost-effectiveness studies of omalizumab in children aged 6–11 years, so their de novo economic evaluation formed the basis of the submitted economic evidence. The economic model was considered appropriate for the decision problem. The results from the model indicated that omalizumab in addition to standard therapy compared with standard therapy alone did not appear cost-effective in either the overall population or a subgroup of patients hospitalised in the year prior to enrolment, with incremental cost-effectiveness ratios of £91,169 and £65,911 per quality-adjusted life-year, respectively. These findings were found to be robust across a wide range of alternative assumptions through one-way sensitivity analyses. The guidance issued by NICE states that omalizumab is not recommended for the treatment of severe persistent allergic asthma in children aged 6–11 years.
Introduction
The National Institute for Health and Clinical Excellence (NICE) is an independent organisation within the NHS, which is responsible for providing national guidance on the treatment and care of people using the NHS in England and Wales. One of responsibilities of NICE is to provide guidance to the NHS on the use of selected new and established health technologies, based on an appraisal of those technologies.
NICE’s single technology appraisal (STA) process is specifically designed for the appraisal of a single product, device or other technology, with a single indication, where most of the relevant evidence lies with one manufacturer or sponsor. 1,2 Typically, it is used for new pharmaceutical products close to launch. The principal evidence for an STA is derived from a submission by the manufacturer/sponsor of the technology. In addition, a report reviewing the evidence submission is submitted by the evidence review group (ERG) – an external organisation independent of the Institute. This paper presents a summary of the ERG report for the STA entitled Omalizumab for the treatment of severe persistent asthma in children aged 6 to 11 years. 3
Description of the underlying health problem
Asthma affects approximately 1.1 million children in the UK,4 and within this group there is a small, but very significant, number of children with severe symptoms in whom asthma control remains poor despite best available therapy. The manufacturer’s submission estimated there to be 307 children in the UK with severe persistent allergic asthma who remain uncontrolled despite best available therapy, and who would meet the criteria for treatment with omalizumab, a recombinant humanised anti-immunoglobulin E (IgE) monoclonal antibody that inhibits the activity of IgE – a key mediator of allergic reactions. These children may receive frequent or maintenance doses of oral corticosteroids (OCSs) together with other controller medications. Children are at risk of serious OCS-related side effects, including growth retardation, osteoporotic fractures, diabetes and cardiovascular events. 5 Clinical guidelines specify that the treatment aim is to control asthma using the lowest possible OCS dose and, if possible, stop OCS treatment completely. 6
Scope of the evidence review group report
The scope for the appraisal specified by NICE was the clinical effectiveness and cost-effectiveness of omalizumab, within its licensed indication, for the treatment of severe persistent allergic asthma in children aged 6–11 years. Omalizumab is licensed as an add-on to existing therapy in patients aged 6–11 years with severe, persistent allergic IgE-mediated asthma whose condition remains uncontrolled despite treatment with high-dose inhaled corticosteroids (ICSs) and long-acting beta-agonist (LABA). This treatment has been appraised previously by NICE for its use in adults. 7
The ERG report presents an assessment of the manufacturer’s (Novartis Pharmaceutical UK Ltd) submission to NICE on the use of omalizumab in addition to standard therapy compared with standard therapy alone. The manufacturer’s submission generally reflected the NICE scope; however, it positions omalizumab as treatment for the most severely affected children who require OCSs [at step 5 of the British Thoracic Society/Scottish Intercollegiate Guidelines Network (BTS/SIGN) guidelines6], i.e. children with more severe asthma than specified in the NICE scope (steps 4 and 5 of the BTS/SIGN guidelines).
The manufacturer’s submission presented evidence for the efficacy of omalizumab based primarily on a preplanned subgroup of children from a single, multinational randomised controlled trial (RCT): the IA-05 trial. 8 The subgroup (European Union Population: IA-05 EUP) comprised those children who received appropriate concomitant medication (high-dose ICS and LABA). (It should be noted that these children were not all in the European Union (EU) but instead received medications in accordance with EU practice.)
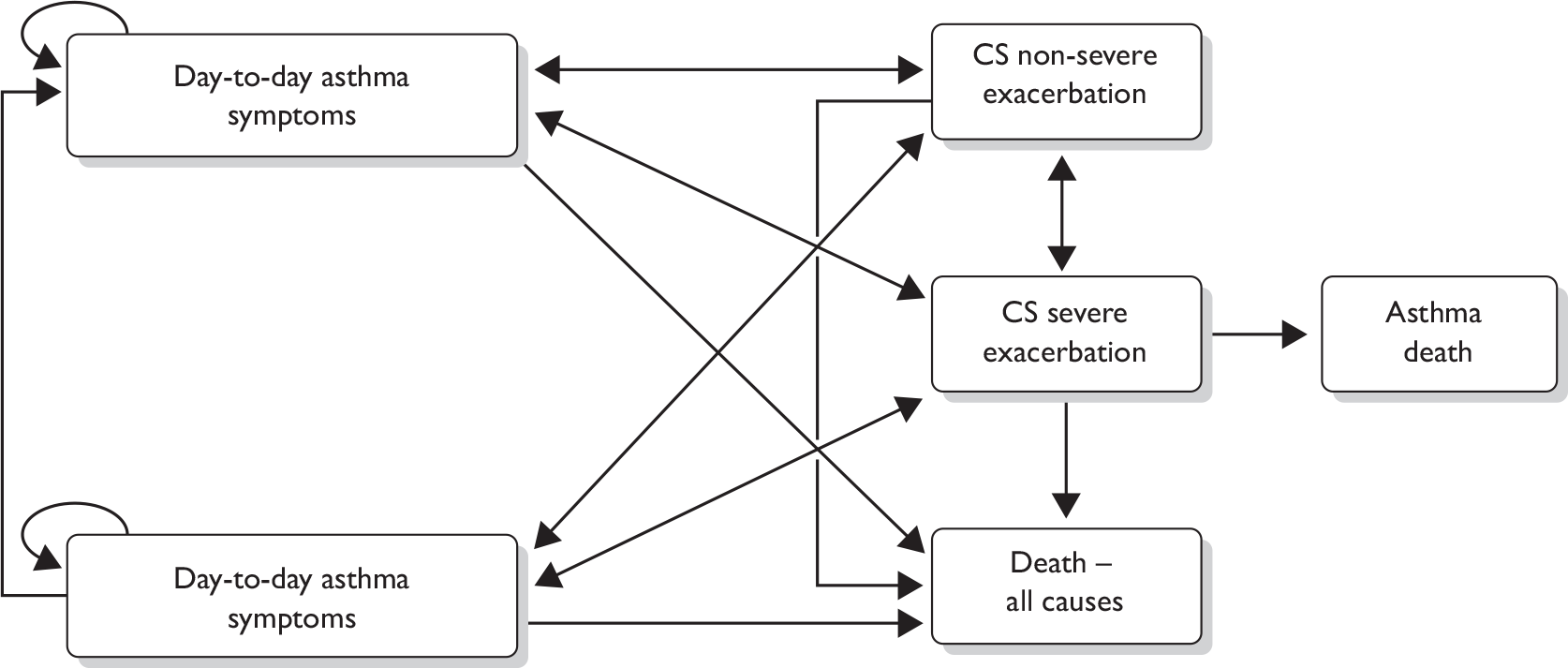
The submission also presented the results of a de novo economic evaluation of the use of omalizumab in addition to standard therapy versus standard therapy alone in the IA-05 EUP patients and in a subgroup of patients who had been hospitalised in the year prior to enrolment. A depiction of the decision-analytic model used in the economic evaluation is shown in Figure 1. The model estimated costs and quality-adjusted life-years (QALYs) from the perspective of the NHS and Personal Social Services, which is consistent with NICE guidelines. 1
FIGURE 1.
Markov model. CS, clinically significant.
Methods
The ERG report comprised a critical review of the evidence for the clinical evidence and cost-effectiveness of the technology based upon the manufacturer’s submission to NICE as part of the STA process. In addition, the ERG modified the manufacturer’s decision-analytic model to examine the impact of altering some of the key assumptions and parameter values.
Results
Summary of submitted clinical evidence
The submission was based primarily on the preplanned subgroup IA-05 EUP from the IA-05 trial, which comprised those children who received appropriate concomitant medication (high-dose ICS and LABA). The primary analysis of efficacy was conducted on a ‘modified’ intention-to-treat population, which excluded participants from trial centres found to be in breach of good clinical practice. Outcomes included the number of clinically significant (CS) exacerbations (defined as those requiring a doubling of the baseline ICS dose and/or treatment with rescue systemic corticosteroids for ≥ 3 days – likely to be managed at home) and clinically significant severe (CSS) exacerbations [defined as requiring treatment with systemic corticosteroids and where the patients had peak expiratory flow or forced expiratory volume of < 60% of their personal best – likely to require hospitalisation]. The ERG noted that the doubling of ICS would not constitute a CS exacerbation in UK clinical practice, and so the numbers classified as CS in the trial may be greater than in clinical practice.
Omalizumab treatment was associated with a statistically significant reduction in the rate of CS exacerbations, but the reduction in the rate of CSS exacerbations did not reach statistical significance (although it should be noted that the trial was not powered to find a difference in CSS exacerbations). The evidence suggests relatively large reductions in the rate of exacerbations with omalizumab compared with placebo, but the absolute reduction in the number of exacerbations is small. However, even small reductions in the number of CS exacerbations can be an important positive outcome for children with severe asthma symptoms. The benefit in terms of CS exacerbations was achieved mainly in children with three or more exacerbations per year at baseline (Table 1).
| Sample size | Initial 24-week fixed-steroid period | 52-week treatment period | ||||
|---|---|---|---|---|---|---|
| Om. | Pl. | Om. | Pl. | Om. | Pl. | |
| Full EUP mITT population | 159 | 76 | 0.42 | 0.63 | 0.73 | 1.44 |
| 0.662a (95% CI 0.441 to 0.995), p = 0.047 | 0.504a (95% CI 0.350 to 0.725), p < 0.001 | |||||
| Two or more exacerbations at baseline | 63 | 31 | 0.45 | 0.29 | 0.71 | 0.67 |
| 1.562a (95% CI 0.662 to 3.684), p = 0.309 | 1.061a (95% CI 0.594 to 1.895), p = 0.842 | |||||
| Three or more exacerbations at baseline | 96 | 45 | 0.37 | 0.78 | 0.69 | 1.78 |
| 0.481a (95% CI 0.305 to 0.758), p = 0.002 | 0.388a (95% CI 0.254 to 0.592), p < 0.001 | |||||
Symptom-free days and nights, primary outcomes required in the NICE scope, were not assessed in the included trial. Mean-change-in-symptom scores were presented as surrogate measures and showed no statistically significant difference between omalizumab and placebo. There were no statistically significant differences in the health-related quality of life (QoL) between omalizumab and placebo, assessed using the standardised Paediatric Quality of Life Questionnaire.
Omalizumab use has been demonstrated to have only numerically small and clinically/statistically insignificant reductions in ICS use. There is no good evidence of a reduction in OCS being achieved with the use of omalizumab.
The adverse effect profile of omalizumab looks favourable but, as with any new drug, particularly one used in children, the long-term adverse effects are uncertain.
Summary of submitted cost-effectiveness evidence
No previously published cost-effectiveness studies of omalizumab in children aged 6–11 years with severe persistent allergic asthma were identified by the manufacturer. Therefore, the manufacturer’s de novo economic evaluation forms the basis of the submitted economic evidence. Omalizumab in addition to standard therapy was compared with standard therapy alone in children with severe persistent allergic asthma, and in a subgroup of patients from the IA-05 EUP study who had been hospitalised in the year before enrolment. The data used to populate the model were largely drawn from the IA-05 EUP study. As no deaths were observed in the study, evidence on asthma-related mortality was drawn from Watson et al. 9 Health-related QoL scores were also not available from the trial so have been drawn from other sources. 10–12 The key effectiveness and mortality data used in the model are presented in Table 2.
| Treatment effectiveness | |||
|---|---|---|---|
| Parameter | Omalizumaba | Standard therapy alone | Source |
| Exacerbation rate per patient for initial 24-week period | 1.363 | 1.939 | IA-05 EUP study |
| Percentage of exacerbations for initial 24-week period that were severe | 23.0 | 23.5 | |
| Proportion of omalizumab patients who respond at 16 weeks (%) | 74.2 | N/A | |
| Exacerbation rate post 24 weeks | 0.519 per year per patient | 2.028 per year per patient | |
| Percentage of exacerbations post 24 weeks that were severe | 27.3 | 22.9 | |
| Asthma-related death by age group | |||
| Age group (years) | Rate of death per CSS (%) | Source | |
| 0–11 | 0.097 | Watson et al.9 | |
| 12–16 | 0.319 | ||
| 17–44 | 0.383 | ||
| 45+ | 2.478 | ||
The economic evaluation was based on a Markov model. The results from the model indicated that omalizumab did not appear to be cost-effective in either the overall population or the subgroup of previously hospitalised patients. The incremental cost-effectiveness ratio (ICER) of £91,169 per QALY (or £65,911 per QALY in the subgroup) is well above the normally accepted NICE threshold of £20,000–30,000 per QALY. 1 These results are presented in Table 3. These findings were found to be robust across a wide range of alternative assumptions through one-way sensitivity analyses.
| Per patient | Total costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | Incremental cost per QALY (£) |
|---|---|---|---|---|---|
| Base case | |||||
| Standard therapy | 39,151 | 16.0793 | |||
| Standard therapy + omalizumab | 94,774 | 16.6894 | 55,623 | 0.6101 | 91,169 |
| Hospitalisation subgroup | |||||
| Standard therapy | 41,333 | 14.36 | |||
| Standard therapy + omalizumab | 82,222 | 14.98 | 40,890 | 0.62 | 65,911 |
The main driver of cost-effectiveness is the reduction in asthma-related mortality associated with the reduced number and frequency of CSS exacerbations. A shorter treatment duration also had a marked effect on cost-effectiveness, increasing the ICER notably (reducing treatment duration from 10 years in the base case to 2 years increased the ICER to £684,665 per QALY).
Commentary on the robustness of submitted evidence
Strengths
The review of clinical effectiveness was considered by the ERG to be thorough. Despite only one RCT8 being eligible for the review, the quality of the included RCT was considered good. The authors made attempts to supplement the data from this trial using other relevant sources and by undertaking a non-systematic survey of UK specialist paediatric respiratory centres.
In general, the ERG considered the economic submission to be of good quality, meeting the requirements of the NICE reference case. The structure of the Markov model was considered appropriate for the decision problem, and many of the key uncertainties were explored through one-way sensitivity analyses.
Weaknesses
The appraisal was based on a small subgroup of children from a single study, many of whom appeared not to be receiving optimal treatment owing to the high rate of exacerbations per year at baseline. The average number of children recruited to each of the 87 trial centres in seven countries was seven for the whole population, and three for the EUP subgroup. This has implications for quality and consistency of application of the trial protocol. There were breaches in good clinical practice at three centres, resulting in recruitment being stopped and children from two centres being excluded from the analysis of efficacy. However, given the rarity of the condition, the need to recruit over such large numbers of trial centres seems unavoidable.
The ERG identified a number of potential weaknesses relating to the economic submission. These included (1) the use of response to omalizumab assessed at 52 weeks rather than 16 weeks as specified in the licence and clinical guidelines; (2) the assumption that exacerbation rates observed in the IA-05 EUP study will remain constant over a child’s lifetime; (3) the non-systematic approach to identifying evidence for the mortality rates associated with exacerbations; (4) uncertainty around costs was omitted from the probabilistic sensitivity analysis; (5) exacerbation costs were not differentiated according to severity; and (6) treatment with omalizumab is assumed to last for 10 years (the clinical adviser to the ERG felt that, in practice, treatment duration could be closer to 1 or 2 years).
The ERG has not been able to explore the robustness of the model results to all of these weaknesses/uncertainties. However, the ERG did explore the main drivers of the cost-effectiveness results and found that the mortality rate associated with CSS exacerbations would have to be significantly higher (> 3% instead of 0.097% as in the model base case) for the ICER to reduce to around £30,000 per QALY.
Areas of uncertainty
From a clinical perspective, the main areas of uncertainty are (1) whether there is any benefit of omalizumab on CSS exacerbations (that would require hospitalisation in clinical practice) or emergency visits; (2) the relative efficacy and safety of omalizumab compared with OCS in children at step 5 of the BTS/SIGN guidelines; and (3) the longer-term safety of omalizumab in a paediatric population.
The cost-effectiveness of omalizumab remains subject to a number of areas of uncertainty in terms of informing current NHS practice. These uncertainties include (1) whether the response to treatment measured at 52 weeks is a reasonable proxy to response at 16 weeks; (2) after 16 weeks, exacerbation rates in the model were determined by comparing the rates observed in omalizumab responders with those in the standard therapy group (the appropriateness of this comparison is questionable as it excludes non-responders entirely); (3) the manufacturer’s assumption that exacerbation rates remain constant over time does not account for patients undergoing adolescence, which can have an impact on the severity of their asthma; (4) the manufacturer’s use of a single observational study for mortality 9 without conducting a systematic search to identify mortality rates; (5) the failure to differentiate CS and CSS exacerbations in terms of cost; (5) the estimates for health-related QoL utilised in the model come from studies in adults7,12 and make use of a mapping algorithm;11 and (6) a potentially relevant subgroup of patients with three or more exacerbations per year was not considered in the cost-effectiveness analysis.
Conclusions
The benefit of omalizumab in children with severe persistent asthma appears to be limited to a reduction in CS exacerbations, with no clear evidence of improvement in day-to-day symptoms. The definition used by the manufacturer for CS exacerbation (worsening of asthma symptoms requiring doubling of the baseline ICS dose and/or treatment with systemic corticosteroids for ≥ 3 days), means that most of these exacerbations would not require hospital admission. No statistically significant benefit of omalizumab on CSS exacerbations (that would require hospitalisation in clinical practice) or in emergency visits or hospitalisations has been demonstrated.
The benefit of omalizumab appears to be in children experiencing frequent (three or more) exacerbations per year at baseline. An apparent increase in the benefit of omalizumab in terms of a reduction in CS exacerbations over time appears to be primarily due to an increase in the exacerbation rate in the placebo group, most likely due to below-optimal treatment and a gradual deterioration in asthma control of children receiving placebo.
The available evidence indicates that omalizumab may be an efficacious alternative to OCS in children with more severe asthma who are not being optimally treated with OCS. Research into the management of the most severely affected children with asthma is warranted, directly comparing the efficacy of these two agents and investigating the OCS-sparing potential of omalizumab.
The main driver of cost-effectiveness is the reduction in asthma-related mortality that is associated with the reduced number and frequency of CSS exacerbations. However, as the absolute reduction in the number of exacerbations is low, and the level of asthma-related mortality in children is also low, the absolute gain in QALYs associated with the use of omalizumab therapy is also low, whereas the additional cost of treatment is high. Although the evidence for the rate of mortality due to CSS exacerbations was not identified in a systematic way, the true rate is unlikely to differ substantially from the values explored in the cost-effectiveness model. The cost per QALY gained with omalizumab was estimated to be far higher than £30,000 in both the overall population of children with severe asthma and in the more severe subgroup of children hospitalised in the previous year owing to asthma exacerbations. The cost per QALY gained with omalizumab remained > £30,000 even under the most favourable scenario analyses, suggesting that the health gains offered by omalizumab in a paediatric population with severe asthma are not sufficient to justify the additional cost of treatment.
Acknowledgements
The ERG would like to thank Dr James Paton, the Royal Hospital for Sick Children, Glasgow, for providing clinical advice and commenting on drafts of the report. We would also like to thank Jonathan Minton for his assistance and comments on an early draft of the report.
Disclaimers
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Department of Health.
Summary of NICE guidance issued as a result of the STA
The guidance issued by NICE in October 2010 states:
Omalizumab is not recommended for the treatment of severe persistent allergic asthma in children aged 6–11 years.
Children currently receiving omalizumab for the treatment of severe persistent allergic asthma should have the option to continue treatment until it is considered appropriate to stop. This decision should be made jointly by the clinician and the child and/or the child’s parents or carers.
Key references
- National Institute for Health and Clinical Excellence (NICE) . Guide to the Methods of Technology Appraisal 2008.
- National Institute for Health and Clinical Excellence (NICE) . Single Technology Appraisal (STA). Manufacturer submission of evidence. Xolair® (Omalizumab) for Severe Persistent Allergic Asthma in Children Aged 6–≪ 12 Years 2009.
- Walker S, Burch J, McKenna C, Wright K, Griffin S, Woolacott N. Omalizumab for the treatment of severe persistent asthma in children aged 6 to 11 years. London: NICE; 2010.
- Asthma UK . For Journalists: Key Facts & Statistics 2010. www.asthma.org.uk/news_media/media_resources/for_journalists_key.html (accessed 27 March 2010).
- Manson S, Brown R, Cerulli A, Fernandez Vidaurre C. The cumulative burden of oral corticosteroid side effects and the economic implications of steroid use. Respir Med 2009;103:975-94.
- Scottish Intercollegiate Guidelines Network, British Thoracic Society (SIGN/BTS) . British Guideline on the Management of Asthma; A National Clinical Guideline 2009.
- Jones J, Shepherd J, Hartwell D, Harris P, Cooper K, Takeda A, et al. Omalizumab for the treatment of severe persistent allergic asthma. Health Technol Assess 2009;13:31-40.
- Lanier B, Bridges T, Kulus M, Taylor AF, Berhane I, Vidaurre CF. Omalizumab for the treatment of exacerbations in children with inadequately controlled allergic (IgE-mediated) asthma. J Allergy Clin Immunol 2009;124:1210-16.
- Watson L, Turk F, James P, Holgate ST. Factors associated with mortality after an asthma admission: a national United Kingdom database analysis. Respir Med 2007;101:1659-64.
- Humbert M, Beasley R, Ayres J, Slavin R, Hebert J, Bousquet J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy 2005;60:309-16.
- Tsuchiya A, Brazier J, McColl E, Parkin D. Deriving preference-based single indices from non-preference-based condition-specific instruments: converting AQLQ into EQ-5D indices. Sheffield Health Economics Group, Discussion Paper Series. Sheffield: ScHARR, University of Sheffield; 2002.
- Lloyd A, Price D, Brown R. The impact of asthma exacerbations on health-related quality of life in moderate to severe asthma patients in the UK. Prim Care Respir J 2007;16:22-7.