Notes
Article history
The research reported in this article of the journal supplement was commissioned and funded by the HTA programme on behalf of NICE as project number 08/230/01. The assessment report began editorial review in October 2010 and was accepted for publication in November 2010. See the HTA programme website for further project information (www.hta.ac.uk). This summary of the ERG report was compiled after the Appraisal Committee’s review. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report. The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
Declared competing interests of authors
NW is a member of the Scottish Study Group for the Care of Diabetes in the Young, whose educational meetings are part sponsored by Novo Nordisk.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2011. This work was produced under the terms of a commissioning contract issued by the Secretary of State for Health. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2011 Queen’s Printer and Controller of HMSO
This paper presents a summary of the evidence review group (ERG) report into the clinical effectiveness and cost-effectiveness of liraglutide in the treatment of type 2 diabetes mellitus, based upon the manufacturer’s submission to the National Institute for Health and Clinical Excellence (NICE) as part of the single technology appraisal process. The manufacturer proposed the use of liraglutide as a second or third drug in patients with type 2 diabetes whose glycaemic control was unsatisfactory with metformin, with or without a second oral glucose-lowering drug. The submission included six manufacturer-sponsored trials that compared the efficacy of liraglutide against other glucose-lowering agents. Not all of the trials were relevant to the decision problem. The most relevant were Liraglutide Effects and Actions in Diabetes 5 (LEAD-5) (liraglutide used as part of triple therapy and compared against insulin glargine) and LEAD-6 [liraglutide in triple therapy compared against another glucagon-like peptide-1 agonist, exenatide]. Five of the six trials were published in full and one was then unpublished. Two doses of liraglutide, 1.2 and 1.8 mg, were used in some trials, but in the two comparisons in triple therapy, against glargine and exenatide, only the 1.8-mg dose was used. Liraglutide in both doses was found to be clinically effective in lowering blood glucose concentration [glycated haemoglobin (HbA1c)], reducing weight (unlike other glucose-lowering agents, such as sulphonylureas, glitazones and insulins, which cause weight gain) and also reducing systolic blood pressure (SBP). Hypoglycaemia was uncommon. The ERG carried out meta-analyses comparing the 1.2- and 1.8-mg doses of liraglutide, which suggested that there was no difference in control of diabetes, and only a slight difference in weight loss, insufficient to justify the extra cost. The cost-effectiveness analysis was carried out using the Center for Outcomes Research model. The health benefit was reported as quality-adjusted life-years (QALYs). The manufacturer estimated the cost-effectiveness to be £15,130 per QALY for liraglutide 1.8 mg compared with glargine, £10,054 per QALY for liraglutide 1.8 mg compared with exenatide, £10,465 per QALY for liraglutide 1.8 mg compared with sitagliptin, and £9851 per QALY for liraglutide 1.2 mg compared with sitagliptin. The ERG conducted additional sensitivity analyses and concluded that the factors that carried most weight were:
-
in the comparison with glargine, the direct utility effects of body mass index (BMI) changes and SBP, with some additional contribution from HbA1c
-
in the comparison with exenatide, HbA1c, with some additional effects from cholesterol and triglycerides
-
in the comparison with sitagliptin, HbA1c and direct utility effects of BMI changes.
The European Medicines Agency has approved liraglutide in dual therapy with other oral glucose-lowering agents. NICE guidance recommends the use of liraglutide 1.2 mg in triple therapy when glycaemic control remains or becomes inadequate with a combination of two oral glucose-lowering drugs. The use of liraglutide 1.2 mg in a dual therapy is indicated only in patients who are intolerant of, or have contraindications to, three oral glucose-lowering drugs. The use of liraglutide 1.8 mg was not approved by NICE. The ERG recommends research into the (currently unlicensed) use of liraglutide in combination with long-acting insulin.
Introduction
The National Institute for Health and Clinical Excellence (NICE) is an independent organisation within the NHS that is responsible for providing national guidance on the treatment and care of people using the NHS in England and Wales. One of the responsibilities of NICE is to provide guidance to the NHS on the use of selected new and established health technologies, based on an appraisal of those technologies.
NICE’s single technology appraisal (STA) process is designed for the appraisal of a single product, device or other technology, with a single indication, where most of the relevant evidence lies with one manufacturer or sponsor. 1 Typically, it is used for new pharmaceutical products close to launch. The principal evidence for an STA is derived from a submission by the manufacturer/sponsor of the technology. In addition, a report reviewing the evidence submission is submitted by the evidence review group (ERG); an external organisation independent of the Institute. This paper presents a summary of the ERG report for the STA entitled Liraglutide for the treatment of type 2 diabetes. 2
Description of the underlying health problem
Type 2 diabetes mellitus is one of the most common chronic metabolic disorders found in both England and Wales. In England, it is estimated that > 2.1 million people have diabetes mellitus and the majority, i.e. about 90% of them, have type 2 diabetes. 3
Type 2 diabetes is treated first with lifestyle measures aiming at weight loss and increased physical activity, but most patients will need drug treatment as well, partly because most do not achieve sufficient weight loss. However, type 2 diabetes is a progressive disease because of loss over time of beta-cell capacity and falling insulin production. Standard therapy in the UK is to add metformin as first drug when lifestyle measures fail, and then to add a sulphonylurea. When dual therapy fails, triple therapy with insulin or a glitazone is next. 4 However, many patients fail to achieve good control on insulin, and weight gain is a common unwanted side effect.
Scope of the decision problem
Liraglutide is a glucagon-like peptide-1 (GLP-1) receptor agonist. Naturally occurring GLP-1 is released by the small intestine in response to food, and has a number of actions, including stimulating insulin release, inhibiting glucagon release, delaying gastric emptying and promoting a feeling of satiety. Liraglutide is taken once daily and has a plasma half-life of approximately 13 hours (compared with that of native GLP-1, 1.5–2.1 minutes). 5 Liraglutide (Victoza®, Novo Nordisk) received marketing authorisation by the European Medicines Agency on 30 June 2009. It was subsequently launched in the UK on 6 July 2009. Liraglutide is licensed for treatment of adults with type 2 diabetes mellitus in combination with (1) metformin or a sulphonylurea in patients with insufficient glycaemic control despite maximal tolerated dose of monotherapy with metformin or sulphonylurea or (2) metformin and a sulphonylurea or metformin and a thiazolidinedione in patients with insufficient glycaemic control despite dual therapy.
The Novo Nordisk submission provided data on the clinical effectiveness of liraglutide as a second- and third-line drug for type 2 diabetes, taken from a suite of trials known as the LEAD (Liraglutide Effects and Actions in Diabetes) trials. Two doses are available in the UK: 1.2 or 1.8 mg once daily. The trials compared liraglutide with glargine and exenatide in triple therapy, and with sitagliptin, rosiglitazone and glimepiride in dual therapy.
The annual costs are £954.84 for the 1.2-mg dose and £1432.26 for the 1.8-mg dose.
Methods
The ERG report comprised a critical review of the evidence for the clinical evidence and cost-effectiveness of the technology based upon the manufacturer’s/sponsor’s submission to NICE. The ERG review was also informed by a Cochrane review6 of the GLP-1 agonists being undertaken by the Diabetes and Health Technology Assessment group at the University of Aberdeen.
The ERG ran searches to identify studies that compared safety and efficacy of liraglutide with other drugs. To compare data and also to resolve some discrepancies, the ERG used the submission, the published papers and the full clinical trial reports of some trials (LEAD-5,7 LEAD-68 and Pratley and colleagues9) provided by the manufacturer.
The Novo Nordisk submission used the Center for Outcomes Research (CORE) model for economic analysis. Although this model is not one of the standard software packages defined by NICE, it was agreed by NICE and the ERG that it would be acceptable because the complexity of economic modelling in diabetes made it sensible to use an existing and tried-and-tested model rather than develop a new one.
The ERG carried out additional sensitivity analyses using the CORE model.
Results
Summary of submitted clinical evidence
Of the six clinical trials8–12 included in the submission report, five were published in full and one was then unpublished. 9 All were sponsored by the manufacturer. The main evidence was from the LEAD phase III randomised controlled trials. All trials were multicentred and had glycated haemoglobin (HbA1c) level as the primary outcome. Secondary outcomes measured included percentage of patients reaching HbA1c level of 7%, percentage of patients reaching HbA1c level of < 6.5%, changes in body weight, body mass index (BMI), fasting plasma glucose (FPG), systolic blood pressure (SBP) and lipids, and numbers of patients experiencing adverse events, such as hypoglycaemia and nausea. Patients aged 18–80 years were included and all trials had a duration of 26 weeks.
All studies analysed data for the intention-to-treat population for subjects who were exposed to at least one dose of the drug and had one postbaseline measurement of the parameter. Each end point was analysed using an analysis of covariance model with treatment, pretreatment and country as fixed effects and baseline as a covariate. Missing data were imputed as last observation carried forward.
One of the recommendations in the NICE guideline is that GLP-1 agonists should be used as a triple therapy only in people whose control is unsatisfactory on a combination of two oral agents, usually metformin and a sulphonylurea. Some people would be unable to tolerate these and might take a glitazone or a gliptin instead. Therefore, on the basis of this guideline, not all LEAD trials were relevant. Therefore, the ERG paid most attention to the studies that compared liraglutide in triple therapy, but studies that used liraglutide in dual therapy were reviewed in case NICE decided to approve it for such use.
The two trials that were most relevant were LEAD-5,7 in which liraglutide 1.8 mg was compared with the long-acting insulin glargine (in combination with metformin and glimepiride), and LEAD-6,8 in which liraglutide 1.8 mg was compared with another GLP-1 agonist, exenatide. Approximately 63% of patients in both arms were on metformin plus a sulphonylurea, with 27.5% on metformin only and ∼9.5% on sulphonylurea only. 8
In LEAD-5,7 liraglutide 1.8 mg daily reduced HbA1c level by 0.24% (p = 0.0015) more than glargine 24 units/day. Liraglutide also resulted in statistically significant reductions in weight (3.4 kg) and SBP (4.51 mmHg) compared to glargine, but no difference in FPG. The ERG wondered if the dose of glargine had been sufficiently titrated, being only 24 units a day at study end.
In LEAD-6,8 liraglutide reduced HbA1c level by 0.33% (p < 0.0001) more than exenatide twice daily. FPG was reduced by 1.01 mmol/l (p < 0.0001) in favour of liraglutide, but weight and SBP showed no significant difference. There was less nausea with liraglutide.
Three trials9–11 examined liraglutide in dual therapy. LEAD-110 compared liraglutide 1.2 and 1.8 mg with rosiglitazone 4 mg daily, added to existing sulphonylurea in both arms. Liraglutide showed a significant improvement in HbA1c level, but no difference in weight and SBP.
LEAD-211 investigated patients who were inadequately controlled on metformin alone, and compared liraglutide 1.2 and 1.8 mg daily with glimepiride (a sulphonylurea) as the second drug. There was no difference in HbA1c level between the drugs, but liraglutide showed a favourable difference in weight of 3.7 kg and SBP of 3.2 mmHg compared with glimepiride.
Pratley and colleagues9 compared the efficacy and safety of liraglutide 1.2 or 1.8 mg once daily with sitagliptin 100 mg once daily. All groups continued on metformin therapy. Compared with sitagliptin, liraglutide 1.2 mg showed a reduction in HbA1c level of 0.34%, a reduction in weight of 1.9 kg and an increase in SBP of 0.39 mmHg.
Because of the significant cost difference between the two doses of liraglutide, the ERG compared the relative benefits between the two in the meta-analyses shown in Figures 1–4. Data used in the meta-analyses come from a fully published paper. 9–12 There were no significant differences in changes in HbA1c, in proportions achieving HbA1c level or in SBP. There was a statistically significant difference in weight, of 0.48 kg, where the clinical significance is doubtful.
FIGURE 1.
Change in HbA1c level (%) from baseline liraglutide 1.2 versus 1.8 mg.
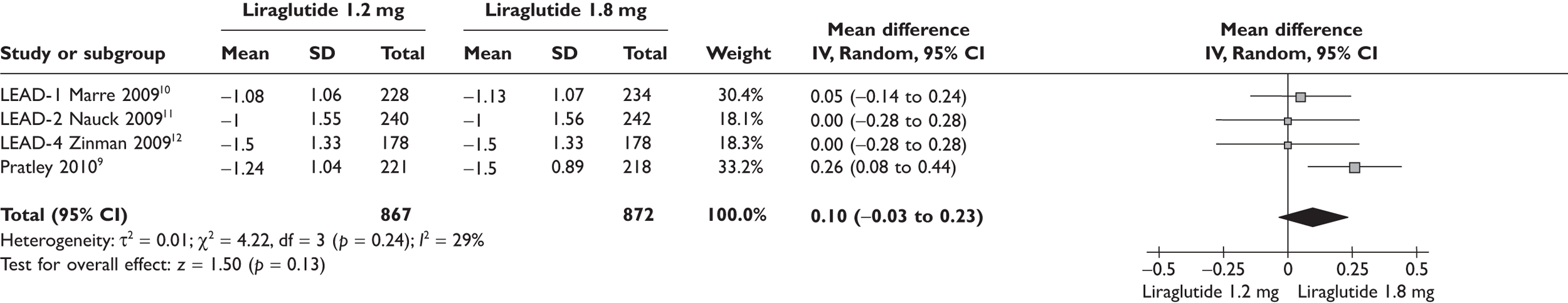
FIGURE 2.
Patients reaching HbA1c level of < 7% liraglutide 1.2 versus 1.8 mg.
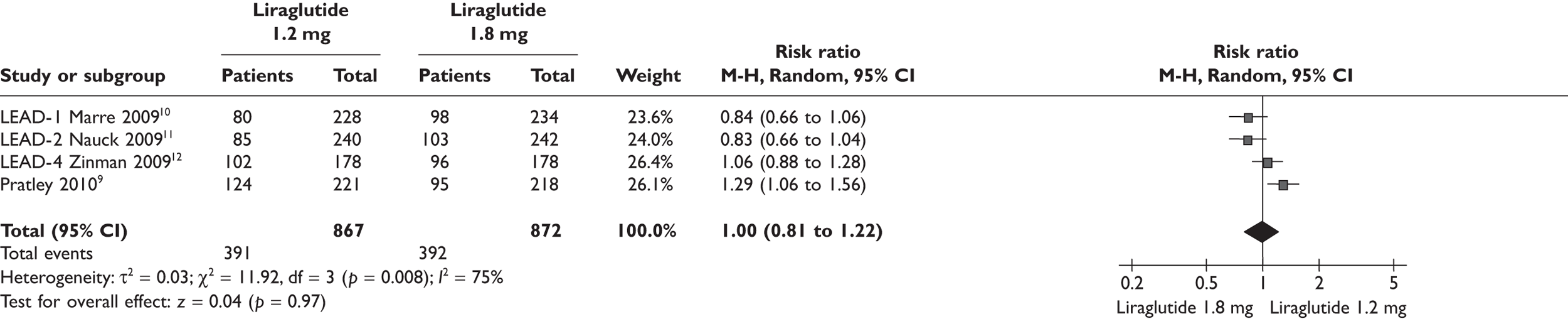
FIGURE 3.
Change in weight (kg) from baseline liraglutide 1.2 versus 1.8 mg.
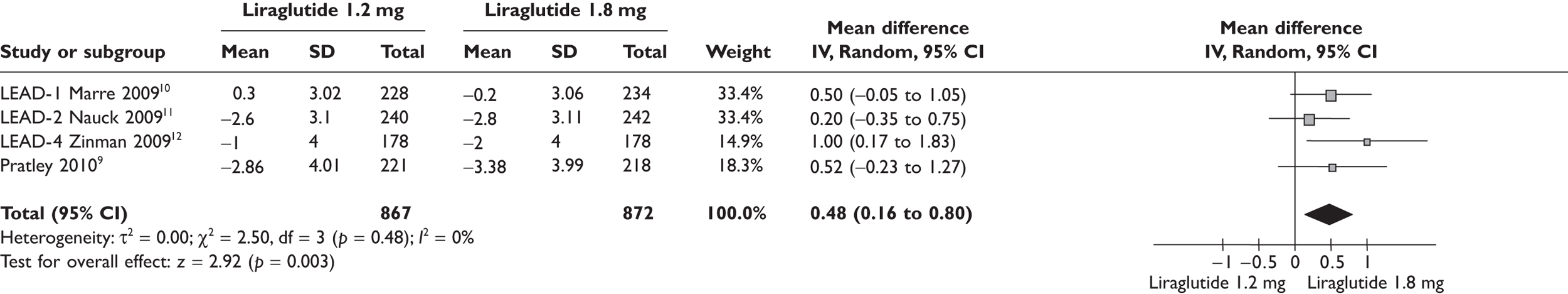
FIGURE 4.
Change in SBP (mmHg) from baseline liraglutide 1.2 versus 1.8 mg.
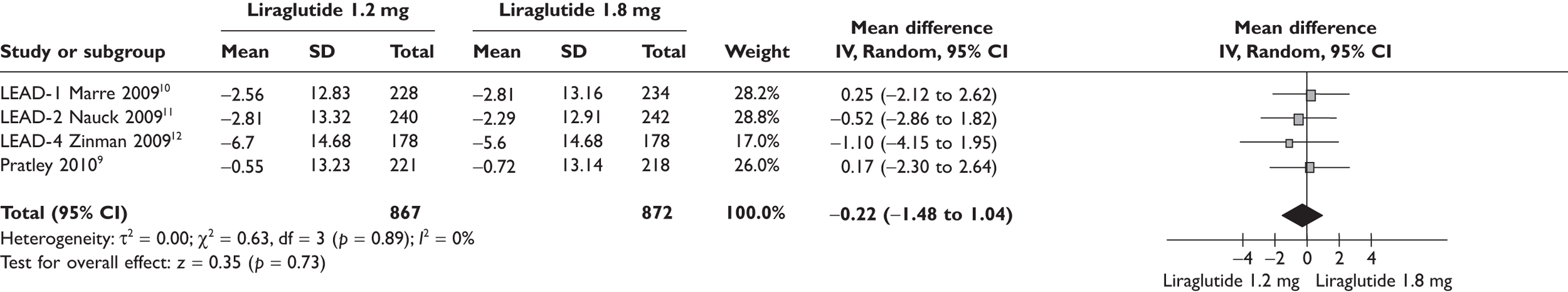
As the trials were of short duration, there was a lack of data on the long-term safety of liraglutide. Concerns have been raised about the risk of pancreatitis with GLP-1 agonists.
The ERG concluded that liraglutide was effective in lowering blood glucose, while avoiding weight gain and hypoglycaemia, and was a useful addition to the therapeutic options available for type 2 diabetes.
Summary of submitted cost-effectiveness evidence
The manufacturer based cost-effectiveness analysis on data from LEAD-57 (liraglutide 1.8 mg vs glargine), LEAD-68 (liraglutide 1.8 mg vs glargine) and a trial by Pratley and colleagues9 (liraglutide 1.2 and 1.8 mg vs sitagliptin). The ERG re-ran the base cases in the CORE model, using the manufacturer’s assumptions, and the results matched with those reported in the submission. The measure of health benefits was quality-adjusted life-years (QALYs). The manufacturer estimated the incremental cost-effectiveness ratios to be £15,130 per QALY for liraglutide 1.8 mg compared with glargine, £10,054 per QALY for liraglutide 1.8 mg compared with exenatide, £10,465 per QALY for liraglutide 1.8 mg compared with sitagliptin and £9851 per QALY for liraglutide 1.2 mg compared with sitagliptin. It was also reported that liraglutide was more cost-effective for patients with higher BMI; however, the cost-effectiveness for patients with lower BMI was not reported.
The ERG conducted additional sensitivity analyses and concluded that the factors that carried most weight were:
-
in the comparison with glargine, the direct utility effects of BMI changes and SBP, with some additional contribution from HbA1c
-
in the comparison with exenatide, HbA1c, with some additional effects from cholesterol and triglycerides
-
in the comparison with sitagliptin, HbA1c and direct utility effects of BMI changes.
Because the trials were of short duration, the costs and outcomes in the CORE model had to be modelled far beyond the duration of the trials.
Commentary on the robustness of submitted evidence
The manufacturer gives an accurate description of type 2 diabetes and of the current treatments available, correctly noting that existing treatments are not wholly satisfactory and that patients often suffer from adverse events, such as hypoglycaemia and weight gain. However, the manufacturer did not report the findings of a trial that compared insulin against an intensive lifestyle intervention in patients poorly controlled by combination oral glucose-lowering agents. Aas and colleagues13 reported that intensive life modification was better than starting insulin. However, the findings of Aas and colleagues13 were not confirmed in the TULIP (Testing the Usefulness of gLargine when Initiated Promptly) study. 14 The latter,14 sponsored by the manufacturer of glargine, reported that adding glargine early in the conventional treatment with oral glucose-lowering drugs and lifestyle interventions resulted in better glycaemic control than intensifying lifestyle interventions.
The LEAD studies are of good quality. The trials were conducted in multiple settings in multiple countries, therefore increasing the generalisability of the results, though only a few patients were from the UK.
NICE recommends neutral protamine Hagedorn (NPH) as the first-choice basal insulin in type 2 diabetes, and none of the liraglutide trials provides a comparison with NPH. This might be justified on the grounds that glargine is now the most commonly used long-acting insulin,15 but NPH is considerably cheaper. The advantages of glargine over NPH in type 2 diabetes are slight. 16
One weakness was the short durations of the trials. We do not have data on how long the GLP-1 agonists will be effective for in this progressive disease. The ERG and the manufacturer assumed a mean duration of use of 5 years.
Conclusions
The Novo Nordisk submission was considered to be of good quality. All of the relevant studies were included. Evidence from the trials shows that liraglutide is a useful addition to options for treating type 2 diabetes, being effective in reducing blood glucose while avoiding hypoglycaemia and weight gain. The ERG did not think the marginal benefits of the 1.8-mg dose over the 1.2-mg dose justified the much higher cost. Data are required on long-term safety of the drug, as are trials against other options in triple therapy. The ERG noted that trials were under way on use in combination with long-acting insulin, a use that seems logical but which is not currently licensed.
Summary of NICE final guidance issued as a result of the STA
1.1 Liraglutide 1.2 mg daily in triple-therapy regimens (in combination with metformin and a sulfonylurea, or metformin and a thiazolidinedione) is recommended as an option for the treatment of people with type 2 diabetes, only if used as described for exenatide in Type 2 diabetes: The Management of Type 2 diabetes (NICE clinical guideline 87), that is, when control of blood glucose remains or becomes inadequate (HbA1c ≥ 7.5%, or other higher level agreed with the individual), and the person has:
-
a BMI of ≥ 35 kg/m2, is of European descent (with appropriate adjustment for other ethnic groups) and has specific psychological or medical problems associated with high body weight, or
-
a BMI of < 35 kg/m2, and therapy with insulin would have significant occupational implications or weight loss would benefit other significant obesity-related comorbidities.
1.2 Treatment with liraglutide 1.2 mg daily in a triple-therapy regimen should only be continued as described for exenatide in Type 2 Diabetes: The Management of Type 2 Diabetes (NICE clinical guideline 87), that is, if a beneficial metabolic response has been shown (defined as a reduction of at least 1 percentage point in HbA1c and a weight loss of at least 3% of initial body weight at 6 months).
1.3 Liraglutide 1.2 mg daily in dual-therapy regimens (in combination with metformin or a sulphonylurea) is recommended as an option for the treatment of people with type 2 diabetes, only if:
-
the person is intolerant of either metformin or a sulphonylurea, or treatment with metformin or a sulphonylurea is contraindicated, and the person is intolerant of thiazolidinediones and dipeptidyl peptidase-4 (DPP-4) inhibitors, or treatment with thiazolidinediones and DPP-4 inhibitors is contraindicated.
1.4 Treatment with liraglutide 1.2 mg daily in a dual-therapy regimen should only be continued if a beneficial metabolic response has been shown (defined as a reduction of at least 1 percentage point in HbA1c at 6 months).
1.5 Liraglutide 1.8 mg daily is not recommended for the treatment of people with type 2 diabetes.
1.6 People with type 2 diabetes currently receiving liraglutide who do not meet the criteria specified in section 1.1 or 1.3, or who are receiving liraglutide 1.8 mg, should have the option to continue their current treatment until they and their clinicians consider it appropriate to stop.
Disclaimers
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Department of Health.
Key references
- National Insitute for Health and Clinical Excellence . Guide to the Single Technology Appraisal (STA) Process 2010. www.nice.org.uk/aboutnice/howwework/devnicetech/developing_nice_single_technology_appraisals.jsp?domedia=1%26mid=912F667C-19B9-E0B5-D43AD56E114A62D9 (accessed 4 October 2010).
- Cummins E, Royle P, Shyangdan D, Waugh N. Evidence Review: Liraglutide for the Treatment of Type 2 Diabetes: A Single Technology Appraisal 2010. www.hta.ac.uk/erg/reports/2157.pdf (accessed 9 September 2010).
- Yorkshire and Humber Public Health Observatory . Diabetes in England 2008. www.yhpho.org.uk/resource/item.aspx?RID=10113 (accessed 4 October 2010).
- National Institute for Health and Clinical Excellence . Type 2 Diabetes: Newer Agents (partial Update of CG66).Clinical Guideline: CG87 2009. http://guidance.nice.org.uk/CG87 (accessed 1 May 2010).
- Vilsboll T. The effects of glucagon-like peptide-1 on the beta cell. Diabetes Obes Metab 2009;11:11-8.
- Shyangdan DS, Royle P, Clar C, Sharma P, Snaith A, Waugh N. Glucagon-like peptide analogues for type 2 diabetes mellitus: a Cochrane review. Cochrane Database Syst Rev 2011.
- Russell-Jones D, Vaag A, Schmitz O, Sethi BK, Lalic N, Antic S, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 Met+SU): a randomised controlled trial. Diabetologia 2009;52:2046-55.
- Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 2009;374:39-47.
- Pratley RE, Nauck M, Bailey T, Montanya E, Cuddihy R, Filetti S, et al. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet 2010;375:1447-56.
- Marre M, Shaw J, Brandle M, Bebakar WMW, Kamaruddin NA, Strand J, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with Type 2 diabetes (LEAD-1 SU). Diabet Med 2009;26:268-78.
- Nauck M, Frid A, Hermansen K, Shah NS, Tankova T, Mitha IH, et al. efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes. The LEAD (Liraglutide Effect and Action in Diabetes)–2 study. Diabetes Care 2009;32:84-90.
- Zinman B, Gerich J, Buse JB, Lewin A, Schwartz S, Raskin P, et al. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD). Diabetes Care 2009;32:1224-30.
- Aas AM, Bergstad I, Thorsby PM, Johannesen O, Solberg M, Birkeland KI. An intensified lifestyle intervention programme may be superior to insulin treatment in poorly controlled Type 2 diabetic patients on oral hypoglycaemic agents: results of a feasibility study. Diabet Med 2005;22:316-22.
- Blickle JF, Hancu N, Piletic M, Profozic V, Shestakova M, Dain MP, et al. Insulin glargine provides greater improvements in glycaemic control vs. intensifying lifestyle management for people with type 2 diabetes treated with OADs and 7–8% A1c levels. The TULIP study. Diabetes Obes Metab 2009;11:379-86.
- Yorkshire and Humber Public Health Observatory . Prescribing for Diabetes in England. An Analysis of Volume, Expenditure and Trends 2009. www.yhpho.org.uk/resource/item.aspx?RID=9711 (accessed 4 October 2010).
- Waugh N, Cummins E, Royle P, Clar C, Marien M, Richter B, et al. Newer agents for blood glucose control in type 2 diabetes: systematic review and economic evaluation. Health Technol Assess 2010;14.