Notes
Article history
The research reported in this issue of the journal was commissioned by the HTA programme as project number 09/83/01. The contractual start date was in June 2010. The draft report began editorial review in April 2011 and was accepted for publication in July 2011. As the funder, by devising a commissioning brief, the HTA programme specified the research question and study design. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2012. This work was produced by Picot et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2012 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Description of underlying health problem
The World Health Organization (WHO) describes nutrition as ‘the intake of food, considered in relation to the body’s dietary needs’ and good nutrition is a key determinant of health. 1 When food intake is not in balance with the body’s dietary needs, malnutrition occurs. The term malnutrition encompasses both undernutrition and overnutrition (obesity) and is, therefore, an ambiguous term if the direction of the dietary imbalance is not clarified. In this report, we have not sought to alter the terms used in the published literature, much of which uses ‘malnutrition’ rather than ‘undernutrition’. Therefore, the reader should note that in this report, whenever the term malnutrition is used, it is always to describe undernutrition and never overnutrition (obesity).
Undernutrition makes a major contribution to the global disease burden and more than one-third of child deaths worldwide are attributed to undernutrition. 2 Common causes of undernutrition are as follows. 3,4
-
Inadequate quantity of food: food shortages may be acute (sudden/sharp) or chronic (long-lasting) and arise as a result of poverty, natural disaster (e.g. flood or drought) or conflicts, which may lead to the displacement of people from their homes and disruption of food supplies.
-
Inadequate quality of food: people may not have access to the variety of foods that will provide all the necessary vitamins and minerals in their diet. People may also lack the knowledge needed to make sound choices about the food they eat or provide to their children.
-
Infections: these may reduce appetite, increase energy and nutrient utilisation (e.g. to fight infection) and limit the ability to absorb or retain nutrients (e.g. as a consequence of diarrhoea and/or intestinal parasites).
Consequences of undernutrition
Different terms are used in the literature to reflect the different causes of undernutrition and/or clinical characteristics. When undernutrition is due to the absence of a specific nutrient (micronutrient deficiency), the consequence may be a particular nutritional disorder (e.g. goitre due to a lack of iodine, scurvy due to lack of vitamin C, xerophthalmia due to a lack of vitamin A or anaemia due to a lack of iron). 3 When both protein and energy are lacking from the diet, the term protein–energy malnutrition (PEM) has been commonly applied (but this ‘causal name’ now tends to be avoided because protein and energy deficits are likely to be accompanied by deficiencies of other nutrients). Malnutrition in children is described as chronic when it lasts for a long time (i.e. at least months) and this is strongly associated with shorter adult height (stunting), less schooling, reduced economic activity and, for women, lower birth weight in the next generation. 5 In contrast, the term acute malnutrition is applied to describe the consequences of a sudden/sharp period of food shortage, and this is associated with loss of body fat and wasting of skeletal muscle. 4 A lack of dietary energy and one or more micronutrient deficiencies frequently occur concurrently within the same individual.
Undernutrition/malnutrition can be classified as mild, moderate or severe based on anthropometry (measurement of the size, weight and proportions of the human body), biochemistry and clinical assessment (described in Severe acute malnutrition – classifications and definitions). The focus of this report is on severe acute malnutrition (SAM) in infants and children. Forms of SAM include kwashiorkor [characteristics include oedema which may be mild (bipedal) or severe (generalised), often associated with skin desquamation and hair changes6,7], marasmus (characteristics include emaciated appearance) and marasmic kwashiorkor (which has a combination of features).
The consequences of SAM have been most evident when emergency situations are widely portrayed in the media and responded to by international aid efforts. It should be remembered, however, that such high-profile emergency situations focusing on those with SAM represent only a fraction of the problem. Although food shortages may affect several sections of society, the majority of the undernourished often go unnoticed because they are the most destitute, vulnerable and marginalised people. 8 ‘Endemic’ undernutrition is common, however, throughout much of Africa and parts of Asia, where exposure to pathogens and recurrent cycles of infection compound the problems of nutritional and food security. It is also now known that all degrees of underweight, even in the mild-to-moderate range, carry an increased risk of mortality. 3,9
Infants and children and undernutrition
Although the consequences of undernutrition can be felt by all people, those in the early stages of life (including during the fetal period) are particularly affected. Infants and children are most vulnerable to the effects of undernutrition during the period of their most rapid physical growth and development, which predominantly takes place during the first 2 years of life. They are particularly vulnerable at this time because of the extra nutritional requirements for growth and development. In addition, infants and children have smaller bodily reserves than an adult so undernutrition has a more rapid effect. Infants and children are additionally vulnerable because they are dependent on others to provide and prepare foods, and even to be fed. Inadequate nutrition may lead to impairment of both body function (e.g. of organs) and structure (e.g. brain development). Interventions implemented from pregnancy to 2 years of age can counteract the effects of undernutrition. However, if undernutrition is not halted in the first 2 years of life, irreversible damage may be caused. 5 Children suffering from SAM often have a history of undernutrition and social deprivation and, if they survive, face long-term consequences for their future health and economic well-being. 10
The initial consequences of a deficit in energy obtained from food in mild-to-moderate undernutrition place a child on a continuum of risk leading to a lack of activity (energy conservation) and a decrease in growth rate (weight and height). An energy deficit is often combined with specific nutrient deficiencies, for example of protein, iron or zinc, and this combined deficiency also limits growth. Children who are undernourished are less able to withstand infections, and repeated infections also contribute to reduced growth. When undernutrition becomes severe, the consequences are more drastic and wide-ranging. A range of physical and metabolic changes occur as the body tries to conserve energy and preserve essential functions for as long as possible in a process known as reductive adaptation. 11
Severe acute malnutrition – classifications and definitions
Severe acute malnutrition in children has been defined and/or classified in several ways. Although ‘standard and accepted’ methods have been established by United Nations agencies, particularly WHO, the Food and Agriculture Organization and the United Nations Children’s Fund (UNICEF),12 developments have continued and alternative approaches have emerged to address specific limitations or new evidence. Despite common elements, many of the definitions and classifications differ in the specific criteria and thresholds used. As a result, differing groups of children may be identified as having SAM depending on which of the various definitions and classifications is used, influencing any assessment of interventions to treat SAM. To allow an appropriate comparison of different interventions, it is important to have an understanding of the terminology, definitions and classifications used by studies and, as a consequence, the people treated. This section provides a brief outline of some of the main definitions and classifications that have been used to identify SAM in infants and children aged < 5 years. It is not a comprehensive listing and does not endeavour to provide a complete history or critical assessment of all the different definitions and classifications. It will focus on, and outline, those that are used in the primary studies that are included in the subsequent systematic review of interventions to treat severely malnourished children.
Currently, WHO and UNICEF recommend three key criteria for diagnosing SAM among children aged from 6 months to 5 years. First, a child’s weight relative to his or her height, known as weight-for-height (W/H), is considered to be an important measure of nutritional status and useful in identifying SAM. Using the WHO child growth standards published in 2006,13,14 a cut-off of < 3 standard deviations (SDs) from the median value (also described as a z-score of ≤ –3) is thought to provide an appropriate threshold for diagnosing marasmus among children aged from 6 months to 5 years. This acute form of severe malnutrition is characterised by severe wasting and an elevated risk of death, but therapeutic diets with limited known risks or negative consequences are effective. 12,15 Second, WHO and UNICEF have recommended the use of the mid-upper arm circumference (MUAC) as an independent indicator of severe wasting and SAM. It is a useful measure within community settings or during emergency situations, when measuring the weight and height of children may prove difficult. MUAC is easy and inexpensive to measure and does not require a chart to calculate. Importantly, it has been shown to perform at least as well as measures of W/H for identifying children with SAM. 16 Children aged from 6 months to 5 years are considered to have SAM if they have a MUAC of < 115 mm. Third, the presence of clinical signs of bilateral oedema of nutritional origin provides evidence of SAM (i.e. oedematous malnutrition or kwashiorkor),15 despite the possibility of other weight-related measures remaining above specified thresholds. The three criteria have been endorsed by several other international organisations (e.g. the International Union of Nutritional Sciences and the International Pediatric Association) and adopted by over 90 countries. 12 For infants aged < 6 months of age, WHO currently recommends the use of the same W/H threshold compared with the WHO child growth standards for that age group and the presence of clinical signs of bilateral oedema of nutritional origin. It does not recommend the use of the MUAC. 17
Although the three criteria recommended by WHO and UNICEF are recognised internationally for defining SAM in children aged from 6 months to 5 years, other growth references, thresholds and approaches have been used. The current WHO growth standards were published in 2006,13 replacing the growth reference developed by the US National Center for Health Statistics (NCHS) and employed from 1977. 18,19 Although the NCHS growth reference has been criticised,20 it has been used extensively as part of national programmes and for research. 21 The thresholds for severe wasting using W/H and MUAC measurements have also changed. Previously, severe wasting was defined for a child aged from 6 months to 5 years as a W/H < 70% of the median on the NCHS growth reference or a MUAC < 110 mm. The change from the use of the NCHS growth reference to the WHO growth standards and the different thresholds used have resulted in an increase in the sensitivity of the measures for identifying cases of SAM while maintaining specificity. As a consequence, the number of children identified as having SAM has increased markedly, with developing countries noting a two- to fourfold increase in cases. 12,22
The use of W/H as a measure for diagnosing SAM has increasingly replaced the use of earlier measures based on a child’s weight-for-age (W/A), which is now seen as an inappropriate measure. The W/A measurement does not differentiate children who are wasted from those who have reduced linear growth (i.e. stunted) and, as a result, is unable to distinguish past nutritional history from current nutritional status. 15,23 As a consequence, the W/A measurement is more appropriate for identifying chronic malnutrition and W/H acute malnutrition. Measures based on age-related standards also incur the difficulty that in many communities a child’s age is often unknown. 24 Despite this, different thresholds have been adopted by the various earlier classifications measuring W/A, affecting the population included. 23,25
Several different classifications have developed during the last 50 years, which have used the different anthropometric measures and clinical characteristics to help identify children with acute malnutrition and to diagnose the type and severity of the condition. Although many are similar to those currently adopted by the WHO and UNICEF, differences are evident in the specific criteria and thresholds used. Gómez and colleagues26 developed a classification (the Gómez classification) which identifies three degrees of malnutrition based on W/A according to the Boston (or Harvard) reference for the weight of a normal child (i.e. 50th percentile or median) (Table 1). 25 Children < 60% W/A were classified as having grade III or severe malnutrition (i.e. children with marasmus).
| Per cent of reference W/A (%) | Interpretation |
|---|---|
| 90–110 | Normal |
| 75–89 | Grade I: mild malnutrition |
| 60–74 | Grade II: moderate malnutrition |
| < 60 | Grade III: severe malnutrition |
Classifications have incorporated clinical features to identify different types of severe malnutrition. The Gómez classification was adapted to incorporate the presence of oedema, such that all children with oedema were classified as having third-degree malnutrition or severe malnutrition irrespective of their weight (i.e. kwashiorkor or marasmic kwashiorkor). 23,27 The Wellcome working party23,28 developed a very similar classification (the Wellcome classification) based on the child’s W/A and the presence of oedema (Table 2). It identifies four groups with malnutrition. Children with a W/A < 60% of the Boston reference were diagnosed as having marasmus if oedema was absent and marasmic kwashiorkor if oedema was present. Children with a W/A between 60% and 80% of the Boston reference and oedema were diagnosed as having kwashiorkor. The fourth group with a W/A between 60% and 80% of the Boston reference but no oedema were classified as being undernourished. Children identified as having marasmus, marasmic kwashiorkor or kwashiorkor are considered as having SAM.
| W/A (% of referencea) | Oedema | |
|---|---|---|
| Present | Absent | |
| 60–80 | Kwashiorkor | Undernourished |
| < 60 | Marasmic kwashiorkor | Marasmus |
Waterlow and colleagues29 suggested that it would be beneficial to consider both W/H and height-for-age (H/A) as a basis for assessing the occurrence of SAM (Table 3). Children with severe malnutrition were characterised by a W/H of < 70% or a H/A of < 85% of the reference standard.
| Classification of malnutrition | Per cent W/H (wasting) | Per cent H/A (stunting) |
|---|---|---|
| Normal | > 90 | > 95 |
| Mild | 80–90 | 90–95 |
| Moderate | 70–80 | 85–90 |
| Severe | < 70 | < 85 |
The Indian Academy of Pediatrics (IAP) developed a classification of PEM based on a child’s W/A compared with the Boston reference for a normal child. Children with a W/A from 51% to 60% and < 50% of that expected were classified as having grades III and IV malnutrition, respectively. Both groups were considered to have severe malnutrition. In 2007, the IAP revised their classification and now recommends a W/H/weight-for-length (W/L) < 70% or < 3 SDs of the NCHS median and/or visible severe wasting and/or bipedal oedema. Also, it suggests that MUAC criteria may also be used for identifying severe wasting. 30
Different terminology has developed to refer to infants and children with severe wasting and oedema, including kwashiorkor and marasmus, protein deficiency, PEM, severe malnutrition and SAM. In this report we predominantly use the term SAM in infants and young children. However, when describing individual studies we have not altered the terms used by the authors of those studies.
Epidemiology
Malnutrition (severe or otherwise) is a preventable cause of considerable morbidity and mortality among children. It is a significant contributing factor in approximately half of the 10 million deaths seen annually in children aged < 5 years worldwide. 31,32 Malnutrition is highly prevalent in low-income and middle-income countries – predominantly in Africa and Asia, and to a lesser degree, Latin America – with only 1% of deaths in children < 5 years occurring outside these regions. 3
Severe wasting (W/H z-score < –3), a defining feature of severe malnutrition, is thought to affect around 3.5% of the world’s children (Table 4). 3 Estimates suggest that in developing countries some 19 million children < 5 years old are severely wasted. 3 In 2004, there were approximately 310,000 deaths attributed to severe wasting among children < 5 years old in Africa, Asia and Latin America. 3 The prevalence of severe wasting among children aged < 5 years appears highest in the areas of south-central Asia (5.7%; 10.3 million children) and in middle Africa (5.0%; 1 million children) (see Table 4). Data from 19 surveys carried out in south Asia, Africa and Latin America between 1998 and 2005 by the Demographic and Health Surveys Programme show that the prevalence of severe wasting was higher at younger ages and declined by 24 months of age. 33 This trend may be linked to the initiation of weaning in infants, whereby breastfeeding no longer supplies all the nutritional and energy requirements and there is a lack of suitable or accessible complementary (weaning) foods.
| Regions | Percentage severely wasted (95% CI) | Number severely wasted in millions (95% CI) |
|---|---|---|
| Africa | 3.9 (2.2 to 5.7) | 5.6 (3.0 to 8.0) |
| Eastern | 3.6 (1.5 to 8.4) | 1.8 (0.7 to 4.1) |
| Middle | 5.0 (2.0 to 12.0) | 1.0 (0.4 to 2.4) |
| Northern | 3.3 (1.2 to 8.9) | 0.7 (0.3 to 2.0) |
| Southern | 2.7 (1.0 to 6.8) | 0.2 (0.06 to 0.4) |
| Western | 4.3 (1.8 to 9.6) | 1.9 (0.8 to 4.3) |
| Asia | 3.7 (1.2 to 6.2) | 13.3 (4.4 to 22.3) |
| Eastern | 0.7 (0.3 to 1.6) | 0.7 (0.3 to 1.6) |
| South-central | 5.7 (2.4 to 12.8) | 10.3 (4.4 to 23.3) |
| South-eastern | 3.6 (1.4 to 8.8) | 2.0 (0.8 to 4.9) |
| Western | 1.6 (0.4 to 5.8) | 0.4 (0.1 to 1.5) |
| Latin America | 0.6 (0.2 to 1.0) | 0.3 (0.1 to 1.5) |
| Caribbean | 1.0 (0.4 to 2.5) | 0.03 (0.01 to 0.9) |
| Central America | 0.6 (0.2 to 1.7) | 0.1 (0.04 to 0.3) |
| South America | 0.6 (0.2 to 1.6) | 0.2 (0.07 to 0.6) |
| All developing countries | 3.5 (1.8 to 5.1) | 19.3 (10.0 to 28.6) |
Human immunodeficiency virus infection and severe acute malnutrition
An estimated 2.1 million children in the world are living with the human immunodeficiency virus (HIV) and 90% of them live in sub-Saharan Africa. 34 The nutritional status of these children can be impaired by HIV infection from early in life. 35 A systematic review and meta-analysis of HIV prevalence and mortality among children treated for SAM in sub-Saharan Africa included 17 studies (4891 children), and found that the average prevalence of HIV infection was 29.2%. 36 Children with HIV and SAM were significantly more likely to die than those children who were HIV sero-negative (HIV–ve). 36
Current service provision
Management of disease
The development of SAM can occur rapidly, and is observed commonly in emergency situations, especially if children are already experiencing mild or moderate undernutrition. Many parts of the developing world that are vulnerable to undernutrition also have a high prevalence of diarrhoeal diseases, pneumonia and HIV infection. Therefore, SAM often occurs in association with other underlying problems (e.g. infection, dehydration), which in combination can result in differences in clinical presentation that complicate diagnosis and management. Early identification and treatment is needed, but the urgency of the situation may not always be recognised, and failure to take notice of SAM in a sick child may result in management that reduces the likelihood of survival.
In the 1990s, one in four severely malnourished children died during treatment; however, mortality rates varied between centres from 5% to 50%, a variation that was mainly due to differences in treatment practices. 37 The centres where mortality was low followed a basic set of principles that implemented treatment in stages and addressed clinical problems in a considered order. 37 To try and improve identification and treatment of SAM, WHO introduced guidance in 1999 that provided a 10-step ordered approach through three phases. 10 The guideline takes into account the profound physiological and metabolic changes (reductive adaptation) that have taken place in severely malnourished children, which means that they have to be fed, rehydrated and managed differently from well nourished children. 37 If intensive feeding is started too soon, before metabolic and electrolyte imbalances have been corrected, the child may deteriorate and die (refeeding or recovery syndrome). The WHO 1999 guidelines have been further developed in subsequent WHO publications for the management and inpatient treatment of children with malnutrition. 37,38
The WHO 10-step approach10 to the management of SAM is presented in Table 5. There are three phases to treatment: initial treatment, rehabilitation and follow-up. In the first phase, initial treatment, the focus is on stabilising the child’s condition by careful refeeding and identifying and treating any life-threatening problems (steps 1–7: treating/preventing hypoglycaemia, hypothermia and dehydration, correcting electrolyte imbalance, treating infection, correcting micronutrient deficiencies and giving small frequent feeds of F75 formula, by nasogastric tube if necessary). This first phase usually takes place in a hospital or residential care facility and in most cases will last from 2 to 7 days, by which point the child’s appetite should have improved.
| Activity | Initial treatment | Rehabilitation | Follow-up | |
|---|---|---|---|---|
| Days 1–2 | Days 3–7 | Weeks 2–6 | Weeks 7–26 | |
| Treat or prevent | ||||
| 1. Hypoglycaemia | → | |||
| 2. Hypothermia | → | |||
| 3. Dehydration | → | |||
| 4. Correct electrolyte imbalance | → | |||
| 5. Treat infection | → | |||
| 6. Correct micronutrient deficiencies |
Without iron → |
With iron → |
||
| 7. Begin feeding | → | |||
| 8. Increase feeding to recover lost weight (‘catch-up growth’) | → | |||
| 9. Stimulate emotional and sensorial development | → | |||
| 10. Prepare for discharge | → | |||
The second phase (rehabilitation phase) involves increasing the energy and nutrient content of the feeds (transition from F75 formula to F100 formula) to recover lost weight. Most older children (e.g. those > 2 years of age) can start to receive solid food in this phase. In these guidelines, the use of ready-to-use therapeutic food (RUTF) is not discussed; only local foods are mentioned. At the same time, play interventions to stimulate the child’s emotional and physical development are implemented; these can include different types of play with children individually and in small groups. At this time the child’s carer should also receive training so that he or she understands what causes undernutrition and to prevent a recurrence. Carers should also know how to treat or obtain treatment for common ailments (e.g. diarrhoea, intestinal parasites). The 1999 manual10 and the 2005 guidelines37 indicate that a child can be considered for discharge once his or her W/H has reached –1 SD (90%) of the median WHO reference values, but early discharge can be considered if a carer is able and willing to look after the child and, if possible, a health worker is available to make a visit to the family home (see below). The more recent (2009) statement12 recommends that discharge is based on a percentage weight gain (after loss of oedema) of 15% in most instances, but this can be adjusted up to 20% weight gain depending on the local situation. The third phase begins after discharge and focuses on following up the child and their family at home, and providing support in order to prevent relapse and ensure the continued physical, mental and emotional development of the child.
Treatment of SAM during the rehabilitation phase (steps 8–10) in those aged > 6 months and without medical complications can take place at home within the community, instead of as an inpatient as described above. Other alternatives to inpatient care include residential rehabilitation centres for children and their carers and day-stay rehabilitation centres. 39 Community-based management of SAM is increasingly used in emergency settings and the same approach can be used in non-emergency situations, in which children can be initially assessed and carers counselled. Treatment involves using RUTF, which is a complete food source, nutritionally equivalent to F100 formula, high in energy and protein, containing the appropriate levels of electrolytes, vitamins, minerals and other nutrients. 40 RUTFs are not water based and require no preparation by the child’s carer. It has been suggested that about 80% of children with SAM (i.e. those who do not have medical complications41) who are actively identified in the community could be treated at home using RUTF supported by health workers. 40 Community-based care with RUTF and home-based therapy with locally developed and produced therapeutic diets have, on occasions, resulted in recovery rates of > 90%. 42 However, the evidence to support this community approach, in both the non-emergency setting and during the rehabilitation phase after hospitalisation, has not been fully established.
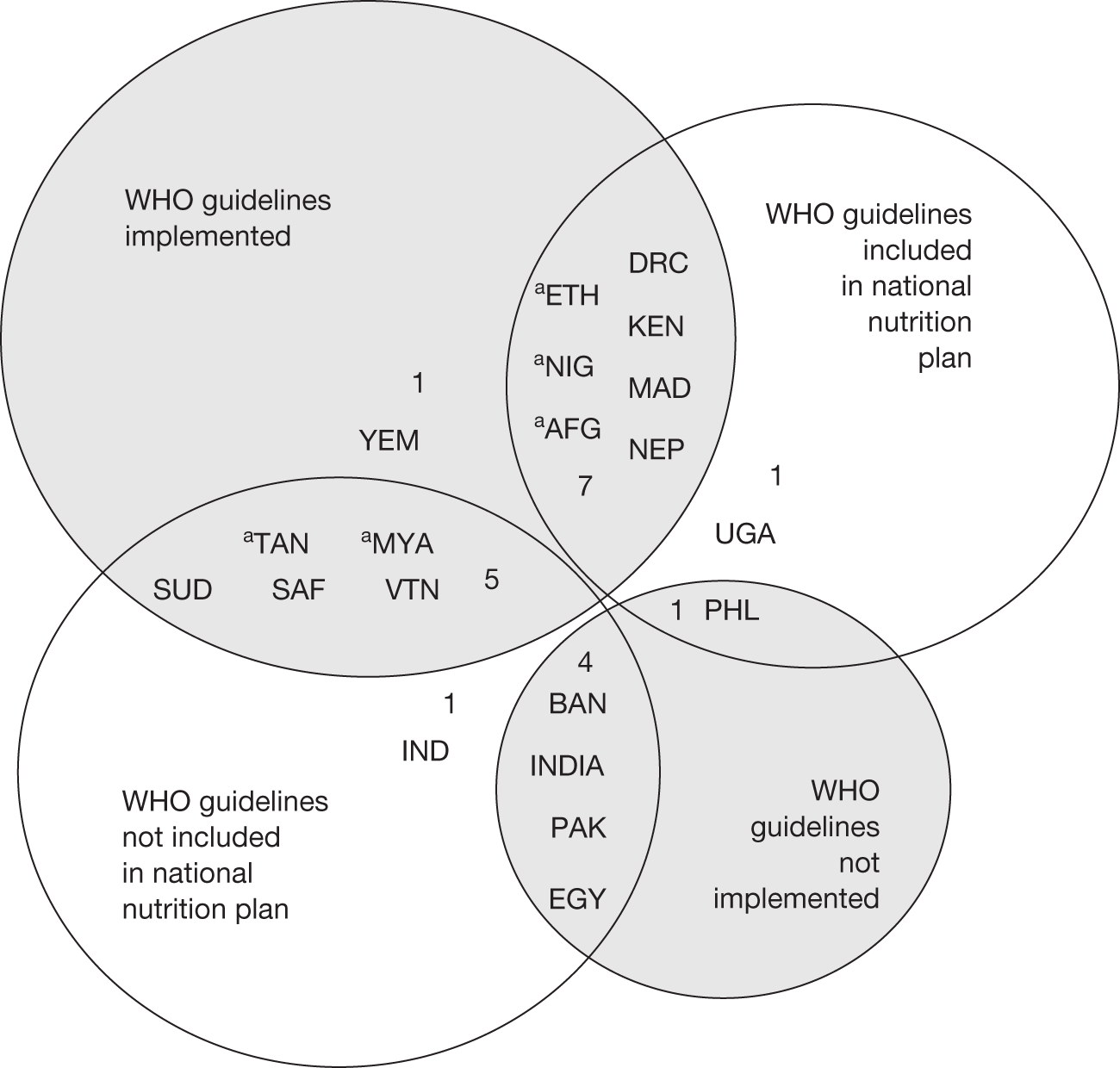
It is recognised that case fatality rates are likely to vary between countries and hospitals. Effective implementation of the WHOs guidance should reduce mortality from SAM to < 5%, a case fatality rate that is considered good in the 2003 guidelines for the inpatient treatment of severely malnourished children. 37 Case fatality rates of 5–10% are considered moderate. However, implementation of the WHO guidance in the 20 countries which are home to 80% of the world’s undernourished children is variable. Five countries (25%) report that they have implemented it nationwide, eight countries (40%) report they have implemented it in selected districts only, data are not reported for two countries (10%) and the remaining five countries (25%) have not implemented the WHO guidelines. 43 Less than half of the 20 countries include the WHO guidance in their national nutrition strategies (Figure 1). As a result of this, and many other factors, mortality from SAM in many areas remains unacceptably high.
FIGURE 1.
Use of the WHO guidelines around the world. 43. aCountry reports that they have implemented WHO guidelines nationwide. Key: Africa: DRC, Democratic Republic of Congo; ETH, Ethiopia; KEN, Kenya; MAD, Madagascar; NIG, Nigeria; SAF, South Africa; SUD, Sudan; UGA, Uganda; TAN, Tanzania. The Middle East: EGY, Egypt; YEM, Yemen. Asia: AFG, Afghanistan; BAN, Bangladesh; INDIA, India; MYA, Myanmar; NEP, Nepal; PAK, Pakistan. Western Pacific: IND, Indonesia; PHL, Philippines; VTN, Vietnam.
Overall aims and objectives of assessment
The project will evaluate the effectiveness of interventions to treat infants and children aged < 5 years who have SAM. It aims to systematically review the evidence assessing the effectiveness of programmes and/or guidelines that have been developed and implemented, as well as the individual components or steps that have been used to treat or manage severely malnourished children. In doing so, it will aim to examine the context in which the interventions are provided to assess the effects of factors such as the setting (e.g. hospital, community, emergency) or different comorbidities (e.g. HIV infection) on their effectiveness. Possible constraints to implementation of the interventions for treating severely malnourished children will be discussed. Finally, it will identify any recommendations for future research.
Chapter 2 Methods for the Delphi process and systematic review of clinical effectiveness
The a priori methods for conducting the Delphi process and for systematically reviewing the evidence of clinical effectiveness are described in the research protocol (see Appendix 1), which was subject to peer review and sent to our expert advisory group for comments. None of the comments we received identified specific problems with the methods of the review, which has been undertaken following the general principles recommended in the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement (see Appendix 2). The methods outlined in the protocol are briefly summarised below.
Delphi process
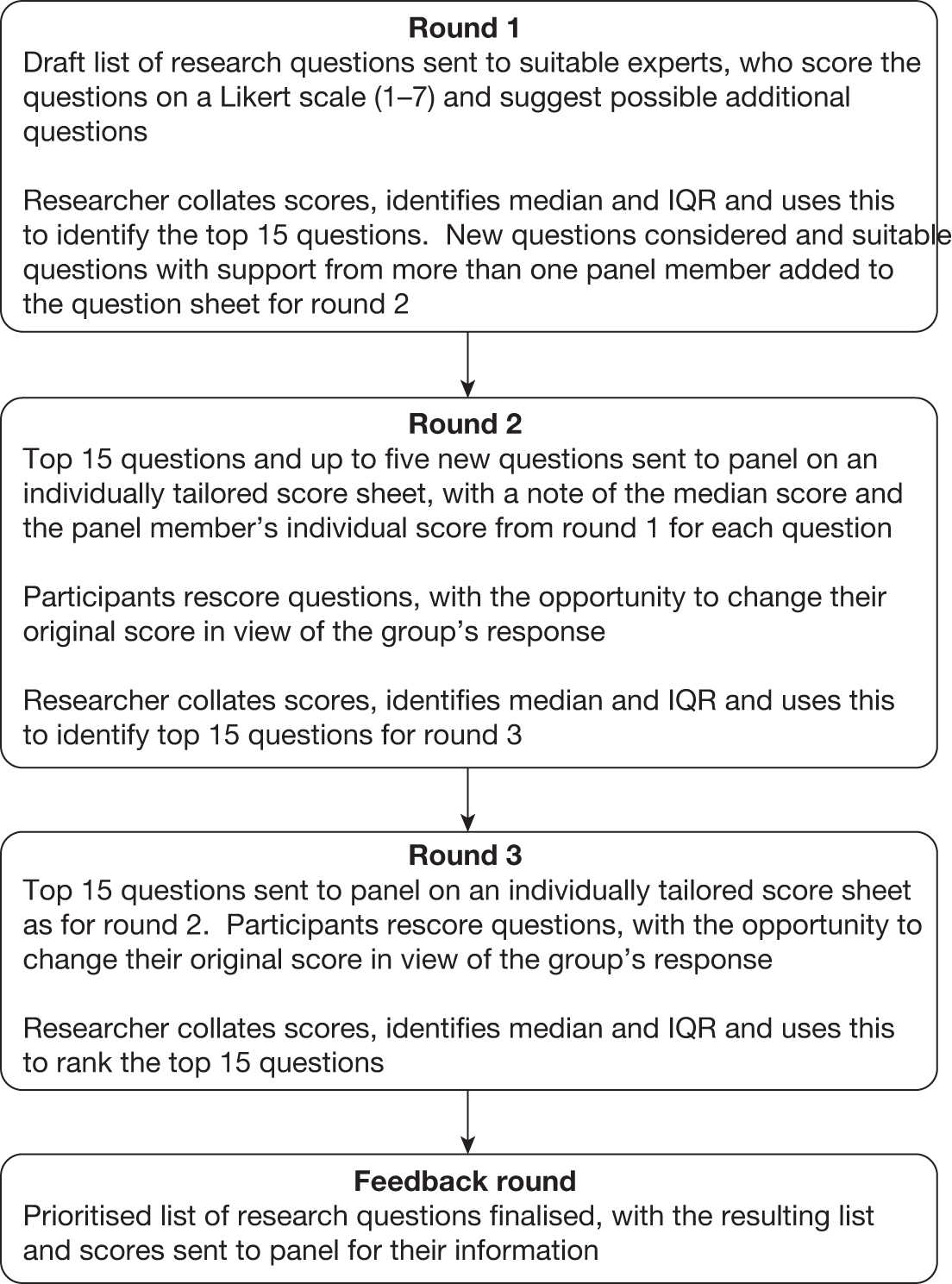
The initial scope of this project had a series of possible research questions relating to the WHO’s 10-step plan, with additional questions being suggested by experts who reviewed the protocol. A Delphi process was used to ensure that appropriate questions were identified and in order to gain an understanding of the priority order of the research questions. The Delphi method is an anonymised, iterative consensus method which follows a series of rounds as described in Figure 2.
FIGURE 2.
Flow chart of Delphi process for severe malnutrition project. IQR, interquartile range.
Identification of studies
The search strategies, which were designed to identify studies reporting clinical effectiveness, were developed and tested by an experienced information specialist.
The following databases were searched for published studies to November/December 2010, unless otherwise stated: MEDLINE (1950 onwards), MEDLINE In-Process & Other Non-Indexed Citations (MEIP), EMBASE (1980 onwards), CAB Abstracts Ovid (this contains a specific database: Nutrition Abstracts and Reviews, searched to December 2009, subscription subsequently withdrawn), Bioline, Centre for Reviews and Dissemination (CRD) [Database of Abstracts of Reviews of Effects (DARE), Health Technology Assessment (HTA) database and NHS Economic Evaluation Database (NHS EED)], The Cochrane Library [Cochrane Reviews, Cochrane Other Reviews, Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Technology Assessment] and EconLit EBSCO. To identify ongoing research, the following databases were searched to December 2010: UK Clinical Research Network (UKCRN), Current Controlled trials.com, WHO International Clinical Trials Registry Platform (ICTRP), clinicaltrials.gov, Australian New Zealand Clinical Trials Register (ANZCTR), Clinical Trials Registry – India (CTRI). Although searches were not restricted by language, only full texts of English-language articles were retrieved for the study selection process. Bibliographies of included articles and grey literature sources were also searched. Our expert advisory group was asked to identify additional published and unpublished references. Further details, including search dates for each database, grey literature sources and an example search strategy, can be found in Appendix 3.
Inclusion and exclusion criteria and process for screening studies
Study design
-
Randomised controlled trials (RCTs), controlled clinical trials (CCTs), cohort with control (prospective and retrospective studies) and case–control studies were eligible for inclusion. Following consultation with the expert advisory group, studies published before 1970 were excluded. It was felt that changes in the diagnostic criteria used to identify SAM and developments in the interventions assessed, rendered any evidence published before 1970 of limited relevance to current and future practice.
-
Where evidence from different types of study design was identified, only those with the most rigorous designs based on the hierarchy of evidence were included.
-
Studies without a comparator group (e.g. before-and-after studies) or those with a comparator group that was not defined in the same way as the experimental group (e.g. a control group of healthy individuals or moderately malnourished children) were excluded.
Intervention(s)
-
Any intervention for treating SAM (either an entire treatment plan or any individual treatment step).
Comparator(s)
-
Any alternative treatment strategy.
-
Comparators could include no intervention and placebos.
Population
-
Infants and children < 5 years old with SAM.
With several different definitions and classifications of SAM having been developed and used, it was considered important to use those in the systematic review that were considered to be either ‘standard and accepted’, key for use within different geographical regions, population groups or settings, or that were thought to incorporate important developments. In doing so, it was important to select those that limited the possible variation in the children studied, allowing appropriate comparison of the interventions assessed. Following consultation with the expert advisory group, the following set of criteria were selected for use in the systematic review.
-
The WHO and UNICEF criteria of a W/H < –3 SDs from the median value using the WHO growth standards published in 200613,14 or < –3 SDs or < 70% of the median value using the NCHS child growth reference standards published in 1977. 18,19
-
A MUAC measurement of < 115 mm.
-
Diagnosis of severe malnutrition with clinical signs of oedema of nutritional origin.
-
Diagnosis of kwashiorkor or marasmic kwashiorkor or where anthropometric measures and/or clinical characteristics have been stated to allow their diagnosis against recognised classifications, specifically:
-
– The Wellcome classification with children defined as having kwashiorkor (60–80% expected body weight plus oedema) and marasmic kwashiorkor (< 60% expected body weight with oedema present),23,28 and
-
– The Save the Children criteria44 for case definition of kwashiorkor (bilateral oedema of nutritional origin and a W/H ≤ –2 SDs from the median value using the WHO growth standard) and marasmic kwashiorkor (bilateral oedema of nutritional origin and a W/H < –2 SDs from the median value using the WHO growth standard).
-
-
Diagnosis of marasmus where anthropometric measures have been stated to allow their diagnosis against other recognised classifications (i.e. in addition to the WHO and UNICEF criteria), specifically:
-
– The Wellcome classification with children defined as having marasmus (< 60% expected body weight with no oedema present).
-
– The Gómez classification,25,26 which defines severe or third-degree malnutrition as a percentage expected W/A of < 60%.
-
– The IAP 1972 definition45 of grade III (51–60% expected W/A) or grade IV PEM (≤ 50% expected W/A).
-
Outcomes
-
Studies were included providing they reported on the primary outcome measures for this review of mortality or weight gain (these outcomes did not have to be the primary outcomes of the study). Other outcomes reported by studies could also be included, providing mortality or weight gain was reported.
-
Outcome measures obtained after any length of follow-up were eligible for inclusion.
Studies were selected for inclusion in the systematic review of clinical effectiveness using a two-stage process. Literature search results (titles and abstracts) were screened independently by two reviewers to identify all the citations that might meet the inclusion criteria. Full manuscripts of selected citations were then retrieved and assessed by one reviewer against the inclusion/exclusion criteria and checked independently by a second reviewer. Discrepancies were resolved by discussion, with the involvement of a third reviewer when necessary.
Mapping the evidence to the prioritised research questions
A ‘map’ of the evidence base was created by categorising each study according to which one of the research questions, prioritised by the Delphi process, it primarily addressed. Inevitably, some of the studies mapped to the questions identified in the Delphi study examined specific sub-questions. These were grouped together within the systematic review under broader topics, allowing comparison of common themes. Each study was mapped to the prioritised research questions by one reviewer and the decision was checked independently by a second reviewer. After the available evidence had been mapped against each research question, the final decision on how many questions would be addressed by the systematic review was taken, based on the extent of the evidence and the resources available for the research.
Data extraction strategy
Data were extracted from the included studies that mapped to prioritised research questions included in the systematic review. Data were extracted by one reviewer using a standardised form and checked for accuracy by a second reviewer. Discrepancies in the extracted data were resolved by discussion, with involvement of a third reviewer when necessary. Data were not extracted from included studies that mapped to questions that were not assessed in the systematic review or from studies that did not map to any question.
Quality assessment strategy
It was anticipated that the evidence base would include studies of different methodological designs. Therefore, a quality assessment tool was chosen which could be used to assess the methodological quality of a range of study types. 46 Details of the tool and scoring system are presented in Appendix 4. Study quality was assessed by one reviewer using a standardised form and checked by a second reviewer. Disagreements were resolved by discussion and, if necessary, by arbitration involving a third reviewer. Included studies that mapped to questions that were not assessed in the systematic review and studies that did not map to any question were not quality assessed.
Method of data synthesis
The methods of data synthesis were determined by the nature of the studies identified through searches and included in the review. Studies were synthesised through a narrative review with tabulation of results of included studies. Meta-analysis was not possible because of the heterogeneous nature of studies identified, including differences in the interventions (e.g. dose and duration of treatment) and the outcomes (e.g. units, time points and measures) assessed.
Chapter 3 Results of the Delphi process
Leading international experts in the field of malnutrition were identified during the time the protocol was being developed for this review. Invitations to participate in the Delphi process were sent to 28 individuals, with a view to balancing input from academics, people working in the field (i.e. in institutions or treatment centres closely linked with the population group), governmental departments, charities and non-governmental organisations (NGOs) and WHO. Table 6 shows the number of people in each area who responded at each stage of the process. Given the nature of this work, some people could be classified as working in two or more areas (e.g. academics who also worked in the field on training courses).
| Stage | Area(s) of work of panel membersa | Total number of individuals | ||||
|---|---|---|---|---|---|---|
| Academic | Field | Government | NGO or charity | WHO | ||
| Initial invite | 10 | 5 | 4 | 7 | 5 | 28 |
| Agreed to participate | 6 | 4 | 1 | 3 | 2 | 16b |
| Completed round 1 | 4 | 3 | 1 | 3 | 2 | 11 |
| Completed round 2 | 6 | 4 | 1 | 3 | 2 | 14 |
| Completed round 3 | 6 | 4 | 1 | 2 | 2 | 13 |
Round 1
For round 1 of the Delphi study, 14 people who had expressed an interest in contributing to the project were sent the question sheet described in Appendix 5. Of these, 11 people returned completed question sheets, seven of whom also contributed additional questions to be considered for inclusion in round 2. The original 18 questions were ranked according to median score, followed by the upper and the lower interquartile range (IQR) limits. The top 15 questions were retained and the three questions which received the lowest scores were removed. The ranked list is shown in Appendix 5.
Development of question sheet and scoring in round 2 and round 3
The 15 retained questions from round 1 were refined either by rewording or by adding sub-questions, and four new questions were added in response to comments received by the Delphi panel members. Full details of the questions presented to the panel in round 2 are available in Appendix 5.
The question sheet for round 2 was sent to 16 people, 14 of whom replied. In this round, each question sheet was individually tailored to show each panel member his or her own scores from round 1 and the overall median score for each question. Participants rescored the questions and were able to take the opportunity to change their score in view of the overall median score from the Delphi group. Once again, the results were used to rank the questions according to median score, followed by the upper and lower IQR limits, and the top 15 questions were retained. Full details, including an additional analysis to assess whether or not rankings were affected by the responses from two people who had not taken part in round 1, are provided in Appendix 5.
For round 3, the same score sheet used for round 2 was sent to 16 people. Thirteen people returned a completed score sheet, one of whom had not returned a score sheet for rounds 2 or 1. The median and IQR limits calculated for all 13 respondents’ scores, and an additional analysis to assess the impact of the scores received from the person who had not contributed to the previous rounds, are provided in Appendix 5.
The final prioritised list of research questions resulting from the Delphi process is shown in Table 7. This list of questions formed the basis for the systematic review.
| Rank: round 3 | Question number | Question |
|---|---|---|
| 1 = | 19 | What methods are effective for treating SAM among infants < 6 months old? |
| 1 = | 20 | How should management of HIV-infected children with SAM differ from those who are severely malnourished but HIV–ve? |
| 1 = | 21 | Which form of i.v. fluid administration is most effective for treating shock? |
| 1 = | 22 | What are the best treatments for children with SAM who have diarrhoea? |
| 5 = | 7 | What methods are effective in treating infection? |
| 5 = | 18 | What factors affect sustainability of programmes, long-term survival and readmission rates? |
| 7 = | 15 | What is the clinical effectiveness of management strategies for treating children with comorbidities such as tuberculosis and Helicobacter pylori? (other than HIV and diarrhoea, which are considered in Q20 and Q22) |
| 7 = | 17 | What factors limit full implementation of treatment programmes? |
| 9 | 14 | What is the clinical effectiveness of interventions in different settings (e.g. hospital, community, emergency)? |
| 10 | 8 | Which methods for correcting micronutrient deficiencies are effective? |
| 11 | 1 | What is the overall effectiveness of current programmes/guidance (e.g. the WHO 10-step plan)? |
| 12 = | 5 | What methods for treating dehydration are effective? |
| 12 = | 9 | What are the most effective methods for feeding during the initial stages of treatment? |
| 12 = | 10 | Which methods are effective in the rehabilitation phase? |
| 15 | 11 | What is the effectiveness of different methods for increasing appetite and food intake to recover lost weight and aid catch-up growth? |
Chapter 4 Assessment of clinical effectiveness
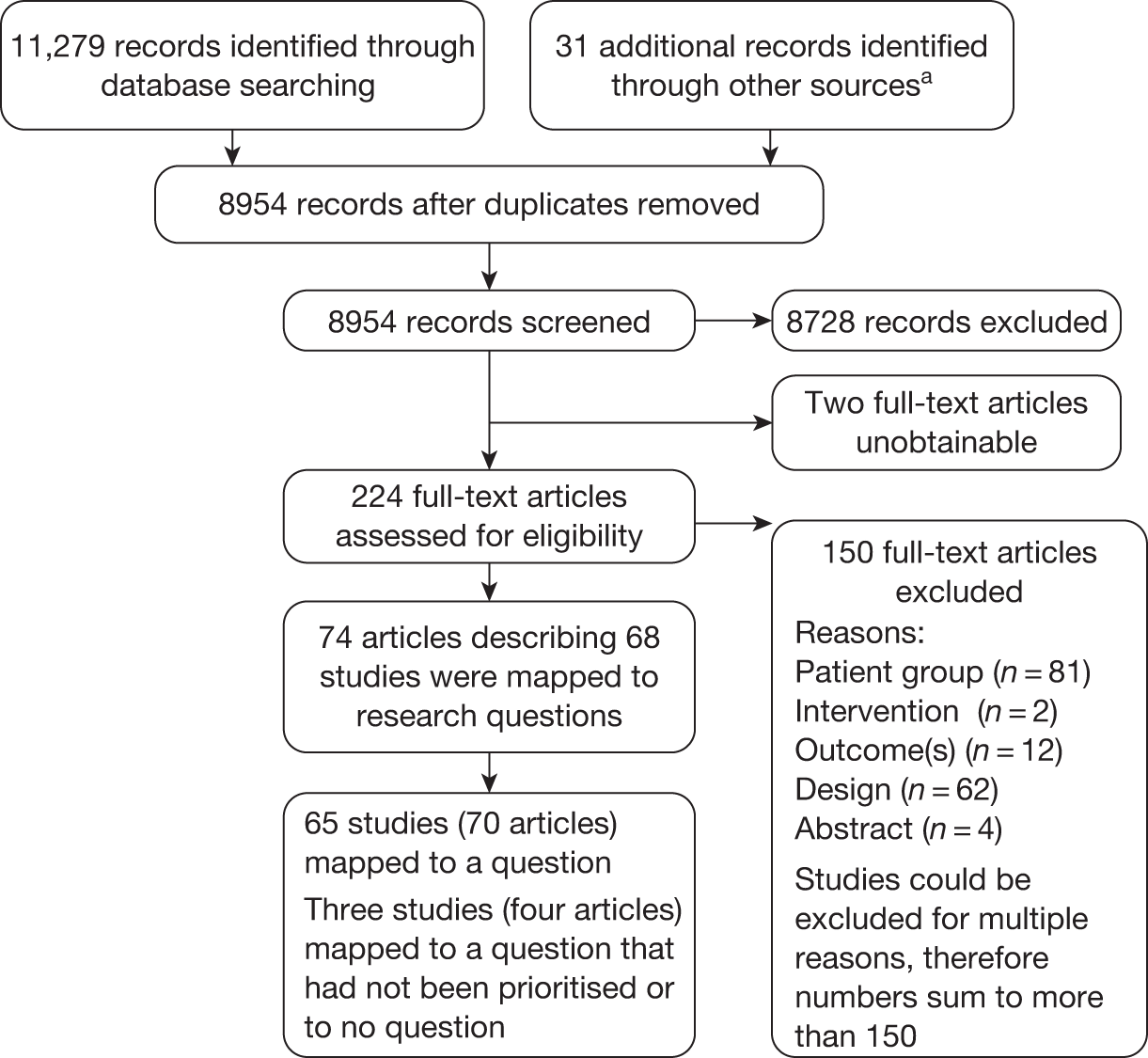
Titles and, where available, abstracts of a total of 8954 records were screened and full copies of the 224 references were retrieved (because of resource limitations only references in English were selected for retrieval). After inspection of the retrieved references, 150 were excluded (see Appendix 6): 81 because they did not focus on the patient group of interest, two because the intervention was not relevant, 12 because they did not report the necessary outcomes, 62 were because of their design and four because they were abstracts containing insufficient information to judge study quality, methodology and results (references could be excluded for more than one reason). Seventy-four retrieved references/full papers describing 68 studies met the inclusion criteria of the review. The total number of records assessed at each stage of the systematic review screening process is shown in the flow chart of Figure 3.
FIGURE 3.
Reference retrieval flow chart.
a, For example, bibliographies of included studies and grey literature identified by the advisory group.
As set out in the protocol for this review, the prioritised list of research questions that resulted from the Delphi process formed the basis for this systematic review. Each of the 68 studies that met the general review inclusion criteria was therefore mapped against the list of prioritised questions to provide an overview of the extent of the available evidence (Table 8).
| Question (rank) | RCT | CCT | PCA + C | PCA + HC | RetroCA + C | Case-control | Other | Unclear |
|---|---|---|---|---|---|---|---|---|
| What methods are effective for treating SAM among infants < 6 months old? ( = first)a | ||||||||
| How should management of HIV-infected children with SAM differ from those who are severely malnourished but HIV–ve? ( = first) | ||||||||
| Which form of i.v. fluid administration is the most effective for treating shock? ( = first) | 1 | |||||||
| What are the best treatments for children with SAM who have diarrhoea? ( = first) | 8 | |||||||
| What methods are effective in treating infection? ( = fifth) | 1 | 1 | ||||||
| What factors affect sustainability of programmes, long-term survival and readmission rates? ( = fifth) | ||||||||
| What is the clinical effectiveness of management strategies for treating children with comorbidities such as TB and H. pylori? ( = seventh) | ||||||||
| What factors limit full implementation of treatment programmes? ( = seventh) | ||||||||
| What is the clinical effectiveness of interventions in different settings? (ninth) | 4 | 1 | 1 | |||||
| Which methods for correcting micronutrient deficiencies are effective? (10th) | 3 | 10 | 1 | |||||
| What is the overall effectiveness of current programmes/guidance? (11th) b | 1 | 4 | ||||||
| What methods for treating dehydration are effective? ( = 12th) b | 1 | |||||||
| What are the most effective methods for feeding during the initial stages of treatment? ( = 12th) b | 1 | 1 | 1 | |||||
| Which methods are effective in the rehabilitation phase? ( = 12th) b | 8 | 13 | 1 | 1 | 2 | 1 | ||
| What is the effectiveness of different methods for increasing appetite and food intake to recover lost weight and aid catch-up growth? (15th) b |
The available evidence mapped against 9 of the 15 prioritised questions. For one other question (Q19), no studies focused on the topic of interest; however, very limited evidence was available in two other studies. These 10 questions in which evidence was included were as follows:
-
What methods are effective for treating SAM among infants < 6 months old? (Q19, limited information only)
-
Which form of intravenous (i.v.) fluid administration is the most effective for treating shock? (Q21)
-
What are the best treatments for children with SAM who have diarrhoea? (Q22)
-
What methods are effective in treating infection? (Q7)
-
What is the clinical effectiveness of interventions in different settings (e.g. hospital, community, emergency)? (Q14)
-
Which methods for correcting micronutrient deficiencies are effective? (Q8)
-
What is the overall effectiveness of current programmes/guidance (e.g. the WHO 10-step plan)? (Q1)
-
What methods for treating dehydration are effective? (Q5)
-
What are the most effective methods for feeding during the initial stages of treatment? (Q9)
-
Which methods are effective in the rehabilitation phase? (Q10)
No evidence was found to inform the remaining five questions:
-
How should management of HIV-infected children with SAM differ from those who are severely malnourished but HIV–ve? (Q20)
-
What factors affect sustainability of programmes, long-term survival and readmission rates? (Q18)
-
What is the clinical effectiveness of management strategies for treating children with comorbidities such as tuberculosis (TB) and Helicobacter pylori? (other than HIV infection and diarrhoea, which are considered in Q20 and Q22) (Q15)
-
What factors limit full implementation of treatment programmes (e.g. insufficient training, cultural difficulties and funding limitations)? (Q17)
-
What is the effectiveness of different methods for increasing appetite and food intake to recover lost weight and aid catch-up growth? (Q11)
After the available evidence had been mapped against each research question, the final decision on how many questions would be addressed was taken, based on the extent of the evidence and the resources available for the research. It was decided that project resources were available to review the evidence for the first six questions for which any evidence was available.
-
What methods are effective for treating SAM among infants < 6 months old? (limited information only)
-
Which form of i.v. fluid administration is most effective for treating shock?
-
What are the best treatments for children with SAM who have diarrhoea?
-
What methods are effective in treating infection?
-
What is the clinical effectiveness of interventions in different settings (e.g. hospital, community, emergency)?
-
Which methods for correcting micronutrient deficiencies are effective?
For each question, evidence was included from studies with the most rigorous designs based on the hierarchy of evidence. For all but one question, this meant that only RCTs and CCTs were included. The exception was Q7 (What methods are effective in treating infection?), where a RCT and a retrospective cohort study with control were the only two studies that addressed this question, but each one focused on a different aspect of this topic.
The evidence is presented in the remainder of this chapter with each of the six questions reviewed being considered in a separate section. The evidence is presented in the remainder of the chapter, with each of the six questions reviewed.
What methods are effective for treating severe acute malnutrition among infants < 6 months old? (Q19, rank 1 = )
No research focusing on treating SAM of infants < 6 months old was identified. The majority of studies excluded this age group, and most of those which allowed for the inclusion of this age group did not report on outcomes for this subgroup. Two studies47,48 were identified that did include infants < 6 months of age within their study populations and provided some outcome information for this subgroup; however, the information available was very limited (see Appendix 7). Although data were extracted, no formal quality assessment was undertaken. The findings are presented to illustrate the nature of the studies and should be interpreted with caution.
Nu Shwe’s retrospective cohort study with control47 described outcomes at a children’s hospital in Myanmar before and after the introduction of the WHO’s guidelines for SAM. In the year before the introduction of the WHO guidelines (1999), 11.4% of children were < 6 months of age, but this proportion fell in subsequent years to 10.7% in 2000 and to 6.4% in 2001. No baseline data were presented for the group of children < 6 months of age; thus, the comparability of the cohorts in each year is unknown. The only outcome reported for the group of interest is proportional mortality (the number of deaths for each age group expressed as a percentage of all the deaths), but a statistical comparison between the control year, 1999, and the years 2000 and 2001, when the WHO guidelines were in use, is not reported (control year 1999: cases 11.4%, proportional mortality 12%; WHO year 2000: cases 10.7%, proportional mortality 9.1%; WHO year 2001: cases 6.5%, proportional mortality 12.5%). The author comments that the introduction of exclusive breast feeding programmes may have reduced SAM in children < 6 months of age and may also have contributed to the lower proportional mortality in the < 12 months age group in comparison with other age groups. Nu Shwe47 states that, comparatively, the proportional mortality in the age groups < 6 months and 6–12 months (9–24%) was lower than in the 13–24 months and > 24 months age groups (20–50%).
Hossain and colleagues48 described a prospective cohort study with concurrent control in Bangladesh, which compared a locally adapted protocol for treatment of SAM with the WHO protocol. They included children in the age range 2–59 months, but the number of children enrolled who were aged < 6 months is not reported and no baseline characteristics are provided for this subgroup of children; therefore, the comparability of the groups with regard to children aged < 6 months is unknown. The only outcome reported for the group of interest is weight gain. There was no statistically significant difference in weight gain for the < 6 months age group between the treatment arms [mean ± SD weight gain: Institute of Child and Mother Health (ICMH) protocol 17.5 ± 7.5 g/kg/day vs the WHO protocol 11.6 ± 6.8 g/kg/day; p = 0.21]. The mortality rate overall in each group was 6.7%, but mortality was not reported on separately for children aged < 6 months.
Which form of intravenous fluid administration is most effective for treating shock? (Q21, rank 1 = )
Quantity and quality of research available: shock
One RCT was included that investigated the efficacy of fluid resuscitation solutions for treating hypovolaemic shock in children with SAM. 49 The key characteristics of the trial can be seen in Table 9, with further details in Appendix 8. The trial was a phase II safety and efficacy RCT conducted in a district hospital in Kenya, and funded by a global charity.
| Study details and target population | Intervention | Comparator |
|---|---|---|
|
Akech et al. 201049 Design: phase II RCT Location: Kenya Length of follow-up: 24 hours’ follow-up for primary outcome; up to 48 hours and thereafter for in-hospital survival No. enrolled: 61 Target population: children aged > 6 months with any of: W/H z-score < –3 or W/H < 70%,c MUAC < 11.0 cm, or oedema involving at least both feet (kwashiorkor) and with hypovolaemic shock |
RLa Ageb (IQR): 16 (6) months Sex F : M, %: 41 : 59 Mean W/H z-score ± SD: –3.9 ± 1.0 Mean MUAC ± SD: 10.0 ± 1.9 cm W/A: NR Met WHO SAM shock criteria,%: 79 Severe dehydrating shock,%: 72 Presumptive septic shock,%: 28 |
WHO fluid resuscitation regimen (HSD/5D) Ageb (IQR): 15 (14) months Sex F : M, %: 42 : 58 Mean W/H z-score ± SD: –3.4 ± 1.3 Mean MUAC ± SD: 10.4 ± 1.4 cm W/A: NR Met WHO SAM shock criteria,%: 69 Severe dehydrating shock,%: 73 Presumptive septic shock,%: 27 |
Severe acute malnutrition was defined in this RCT as any of W/H z-score < –3 or W/H < 70% of reference median, a MUAC measurement of < 11.0 cm, or oedema involving at least both feet (kwashiorkor). Participants were also required to have evidence of shock and were categorised as having either severe dehydration/shock (shock and severe dehydrating diarrhoea defined as ≥ 6 watery stools/day) or presumptive septic shock (non-diarrhoeal shock). The trial predominantly evaluated Ringer’s lactate isotonic fluid (RL) compared with a standard WHO hypotonic fluid solution [half-strength Darrow’s in 5% dextrose (HSD/5D)]. Children with severe dehydrating diarrhoea/shock randomly received RL or HSD/5D, whereas those with presumptive septic shock were randomised to RL, HSD/5D or 4.5% human albumin solution (HAS); although limited data were subsequently reported for the HAS group, owing to small study numbers (n = 6). HSD/5D was given according to the WHO recommendation in a maximum of two boluses of 15 ml/kg over 2 hours, whereas the RL group received 10 ml/kg over 30 minutes (up to a maximum of 40 ml/kg where necessary). HAS was administered in the same dosage as for RL. Follow-up was at 8 and 24 hours for the primary outcome, although the children were followed up intensively for up to 48 hours and thereafter for in-hospital mortality.
Other interventions that all participants received included standard WHO management of SAM comprising treatment of hypoglycaemia, antibiotics and oral rehydration solution (ORS) [rehydration solution for malnutrition (ReSoMal)] for those with dehydrating diarrhoea, and maintenance i.v. dextrose fluids up until tolerance of oral feeds was established.
The trial49 was relatively small with 61 participants, although with few data reported on the six children receiving HAS, this number was reduced to 55 for reported baseline characteristics and most outcomes. Children allocated to the RL and HSD/5D treatment groups were around 15 months of age (though it is not clear from the publication whether this is the mean or median), with a slightly higher proportion being boys (58–59%). The mean W/H z-score at baseline ranged from –3.4 to –3.9 and the mean MUAC was approximately 10 cm. Approximately two-thirds of participants had severe wasting, about 40% were HIV sero-positive (HIV+ve) and around 75% fulfilled the strict WHO definition of advanced shock for severely malnourished children. Of the total included population, approximately twice as many children had severe dehydration/shock as had presumptive septic shock, although within the RL and HSD/5D treatment groups there were approximately an even number of children with each type of shock.
The study was limited to children > 6 months of age with SAM and evidence of shock. The clinical shock criteria were defined and included measures such as a capillary refill time (CRT) > 2 seconds, weak pulse volume and deep ‘acidotic’ breathing, among others (see Appendix 8). Children were excluded if they had known congenital heart disease, severe anaemia, clinical features of pulmonary oedema or raised intracranial pressure. The primary outcome was stipulated as resolution of features of shock, defined as the absence of all of severe tachycardia (heart rate > 160 beats/minute), CRT > 2 seconds or oliguria (urine output < 1 ml/kg/hour) at 8 and 24 hours post treatment. Secondary outcomes included the incidence of adverse events and mortality. Improvements in the W/H z-score or other measures of weight gain were not reported outcomes.
Summary of quality assessment
The methodological rigour of the trial by Akech and colleagues49 was rated moderate overall (Table 10). The trial was potentially at risk of selection bias, because not all of the eligible children who were selected actually participated in the trial either for clinical reasons or because consent was declined. The study was a RCT and an adequate method (use of sealed envelopes) was used for randomisation to treatment groups, resulting in a strong rating for study design. Baseline characteristics and disease severity indices were reported to be balanced across the three fluid intervention arms (although data were not presented for the HAS arm because of small numbers), also leading to a strong rating. However, neither the participants nor the care providers were blinded to treatment and no details were reported regarding the outcome assessors, leading to a higher risk of detection bias and thus a weak rating. For data collection methods, the trial was rated as moderate as it used valid criteria for measuring shock, but it was not possible to judge whether or not these criteria were reliable. There were no dropouts or withdrawals from the trial, only losses because of deaths, and all surviving children completed the study, indicating a low risk of attrition bias. The intervention integrity of the trial was strong as all the participants were deemed likely to have received his or her allocated intervention without any cross-contamination. Appropriate statistical methods were employed in the data analysis and the authors report that all analyses were performed using the intention-to-treat (ITT) principle, although outcomes were presented for all survivors (those who died were not included), rather than for all those randomised. However, the area under curves (AUCs) were calculated in order to compensate for the confounding effect of mortality and, hence, missing observations, leading to biases in the highest risk group and resulting imbalance within the survivors. It should also be noted that the trial was prematurely terminated because of the high overall mortality and inadequate correction of shock in all study arms after an interim review of safety data and consultation with the external safety monitors. As a result, the study did not recruit the required sample size and was therefore underpowered.
| Study | Selection bias | Study design (description) | Confounders | Blinding | Data collection methods | Withdrawals and dropouts | Intervention integrity | Analysis | Global ratinga | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Per cent who received allocation | Consistency measured? | Unintended intervention likely? | Unit of allocation | Unit of analysis | Appropriate statistical methods? | ITT analysis? | ||||||||
| Akech et al. 201049 | M |
S (RCT) |
S | W | M | S | 80–100 | Yes | No | Patient | Patient | Yes | Yes | M |
Assessment of effectiveness: shock
Mortality
Overall mortality was high, with 51% (31/61) of children not surviving. Of these deaths, 39% (12/31) occurred within 24 hours of recruitment,49 whereas 52% (16/31) of fatalities occurred within 48 hours of enrolment (Professor Kathryn Maitland, Imperial College London, 2011, personal communication). There was no statistically significant difference in mortality rates between the three treatment groups (p = 0.62), nor between children who received RL versus HSD/5D (p = 0.34) (Table 11). On Kaplan–Meier survival analysis, there was no significant difference in time to death when any of the intervention fluids were used for resuscitation (log-rank test combined, p = 0.42).
| Study | Treatment arms | p-value | ||
|---|---|---|---|---|
| Akech et al. 201049 | RL (n = 29) | HSD/5D (n = 26) | 4.5% albumin (HAS) (n = 6) | |
| In-hospital mortality, n/N (%) | 13/29 (45) | 15/26 (58) | 3/6 (50) |
0.62a 0.34b |
Mortality rates within a number of subgroups were also reported by Akech and colleagues,49 although not all were presented as comparisons between fluid resuscitation treatment groups. In those with severe diarrhoeal shock, mortality was higher in the standard HSD/5D group than in the RL group {13/19 (68%) vs 9/22 (43%), respectively; p = 0.11 [note: there is a possible error reported in the publication, RL should be 9/21 (43%)]}, although the opposite trend was observed for those with presumptive (non-diarrhoeal) shock [2/7 (29%) vs 4/8 (50%), respectively; p = 0.61 (note: there is a possible error reported in the publication for presumptive shock for HSD/5D)], but neither difference reached statistical significance. Children who fulfilled the WHO malnutrition shock definition at admission were at a statistically significant increased risk of death [risk ratio (RR) 2.0, 95% confidence interval (CI) 0.92 to 4.36; p = 0.05] compared with those who did not fulfil this definition, irrespective of allocated intervention. Similarly, kwashiorkor was associated with an increased risk of death irrespective of treatment arm [odds ratio (OR) 2.2, 95% CI 0.7 to 10.1; p = 0.14], though this was not statistically significant. Mortality in children who were HIV+ve was similar to those that among who were HIV–ve (42% vs 45%, respectively; p-value not reported) and infection with HIV did not significantly increase the risk of death (OR 1.18, 95% CI 0.38 to 3.72; p = 0.76).
Weight gain and anthropometry
Weight gain and anthropometry outcomes were not reported by the Akech and colleagues’ trial49 because of the focus of the study (i.e. the trial was designed to look at emergency management of shock rather than nutritional rehabilitation).
Resolution of shock
The proportion of children in whom shock persisted after fluid resuscitation treatment was considerable, but was not significantly different between RL and HSD/5D at either 8 or 24 hours (Table 12). The authors report that a larger decline in the proportion with shock was observed in children who received RL than in those who received HSD/5D, particularly in the diarrhoeal group, but the differences were not significant at any time point (data not shown).
Oliguria
Adequate urinary output was used as a gold standard for successful fluid resuscitation, with oliguria (the production of an abnormally small volume of urine) being a marker of persistent, severe shock. The incidence of oliguria was significantly higher in children receiving the standard WHO HSD/5D solution than in those receiving RL at 8 hours (reported by the authors as p = 0.02 in the table, but p = 0.05 in the text). This trend was also evident at 24 hours, but was no longer statistically significant (p = 0.16) (Table 13).
| Study | Treatment arms | p-value | |
|---|---|---|---|
| Akech et al. 201049 | RL (n = 29) | HSD/5D (n = 26) | |
| Oliguria (< 1 ml/kg/hour), n/N (%): | |||
| 8 hours | 3/25 (12)a | 9/22 (41)b | 0.02c |
| 24 hours | 6/25 (24)d | 8/18 (44)e | 0.16 |
In an additional analysis, the median AUC for the hourly urine output was significantly lower in HSD/5D participants (51 ml/kg/hour, IQR 36–116) than in RL participants (101 ml/kg/hour, IQR 63–141; Kruskal–Wallis chi-squared = 4.6; p = 0.03) (data not shown).
Tachycardia
Persistent tachycardia is an index of unresolved shock and was defined as a heart rate of > 160 beats/minute. Children who received the standard WHO HSD/5D solution had a higher incidence of tachycardia (and hence unresolved shock) compared with those who received the RL solution, becoming statistically significant at 24 hours (p = 0.04) (Table 14).
| Study | Treatment arms | p-value | |
|---|---|---|---|
| Akech et al. 201049 | RL (n = 29) | HSD/5D (n = 26) | |
| Tachycardia, n/N (%): | |||
| 8 hours | 4/25 (16) | 6/22 (27) | 0.34 |
| 24 hours | 4/25 (16) | 8/14 (57)a | 0.04 |
In the additional analysis, median AUC of heart rates were similar for both treatments (Kruskal–Wallis chi-squared = 0.3; p = 0.59).
Adverse events
Although the incidence of adverse events was not presented, Akech and colleagues49 did report that no child developed clinical features of pulmonary oedema or allergic reaction (to HAS) during the course of study observation. In addition, no diuretics were required or prescribed during the trial and there were no differences in the mean sodium concentration at admission (133 ± 11 mmol/l vs 134 ± 10 mmol/l, respectively; p = 0.81), 8 hours (134 ± 10 mmol/l vs 139 ± 10 mmol/l, respectively; p = 0.09) or 24 hours (138 ± 9 mmol/l vs 140 ± 9 mmol/l, respectively; p = 0.47) between those who received HSD/5D and RL implying that children did not exhibit the problem of either water or sodium retention.
Other outcomes
Additional outcomes such as severe tachypnoea (rapid breathing of > 60 breaths/minute), creatinine levels and resolution of base deficit (acidosis) were also reported in the trial publication, but have not been presented here. Further details are available in the data extraction forms in Appendix 8.
Summary
-
Only one trial49 was identified that evaluated the efficacy of fluid resuscitation solutions for the treatment of children with SAM and hypovolaemic shock. The trial was relatively small and was rated as having a moderate methodological quality overall. It should be noted that the study was underpowered because of premature termination of the trial because of safety issues (i.e. high overall mortality and inadequate correction of shock in both arms) and the results should therefore be interpreted with caution.
-
The overall mortality rate in the trial was high (> 45%), with no statistically significant differences between treatment groups nor any difference in the time to death between treatment arms. There was an inadequate correction of shock that persisted after fluid resuscitation treatment in both the standard WHO HSD/5D hypotonic solution and the isotonic RL solution groups (> 50%).
-
The incidence of oliguria (used as a marker of persistent, severe shock) was higher in children receiving HSD/5D hypotonic solution than in those receiving RL, being significant at 8 hours, but not at 24 hours. Similarly, children who received the HSD/5D solution had a higher incidence of tachycardia (denoting unresolved shock) than those in the RL group, becoming statistically significant at 24 hours.
-
The isotonic RL solution was found to be as safe as the currently recommended WHO HSD/5D hypotonic solution with no adverse events reported. However, it should be noted that all the fluid solutions were deemed inadequate by the authors in the correction of shock.
What are the best treatments for children with severe acute malnutrition who have diarrhoea? (Q22, rank 1 = )
Eight trials50–57 were included that investigated the efficacy of treatments for children with SAM who also had diarrhoea. Within this section, similar trials have been grouped together for ease of comparison between studies. The groupings consist of those with acute diarrhoea and treated with ORS (n = 5,50,51,54,55,57 see Quantity and quality of research available: acute diarrhoea and Assessment of effectiveness: acute diarrhoea) and those with persistent diarrhoea and treated with formula and/or solid diets (n = 3,52,53,56 see Quantity and quality of research available: persistent diarrhoea and Assessment of effectiveness: persistent diarrhoea).
Quantity and quality of research available: acute diarrhoea
Five trials50,51,54,55,57 were included that investigated children with acute diarrhoea, defined as diarrhoea lasting < 2, < 3, < 4 or ≤ 10 days. The key characteristics of these RCTs can be seen in Table 15, with further details of the trials in Appendix 9. All the trials were single-centre RCTs carried out in India51,54,55 or Bangladesh. 50,57 One study50 received funding from WHO and one57 was funded jointly by a commercial organisation and an international health research institution. For three studies51,54,55 the primary source of financial support was not stated, although Alam and colleagues51 received funding for materials from a local medical college/university.
| Study details and target population | Intervention | Comparator | ||
|---|---|---|---|---|
|
Alam et al. 200051 Design: double-blind RCT Location: India Length of follow-up: until recovery and discharge n enrolled: 81 SAM (170 total study population) Target population: children with SAM (W/H < 70% of NCHS) and acute diarrhoea (< 4 days duration) with dehydration, and either with non-cholera diarrhoea (3 months –5 years) or with clinical suspicion of cholera (aged > 3 months) |
H-ORS Mean age ± SD: 25.29 ± 2.09 months Sex F : M: NR W/H: NR MUAC: NR Mean W/A ± SD, %: 52.4 ± 1.64 Duration of diarrhoea: NR Frequency of diarrhoea: NR Dehydration status: NR |
Standard WHO-ORS Mean age ± SD: 24.17 ± 2.23 months Sex F : M: NR W/H: NR MUAC: NR Mean W/A ± SD, %: 58.6 ± 1.12 Duration of diarrhoea: NR Frequency of diarrhoea: NR Dehydration status: NR |
||
|
Alam et al. 200350 Design: double-blind RCT Location: Bangladesh Length of follow-up: until diarrhoea resolved No. enrolled: 130 Target population: children aged 3–36 months with SAM (W/H < 70% of NCHS median or with bilateral pedal oedema) and a history of watery diarrhoea for ≤ 10 days |
ReSoMal (ORS for malnourished children) Mean age ± SD: 15 ± 7 months Sex F : M, %: 60 : 40 Mean % expected W/L ± SD: 66 ± 4 Mean W/L z-score ± SD: –3.6 ± 0.6 MUAC: NR Mean % expected W/A ± SD: 50 ± 7 Mean W/A z-score ± SD: –4.7 ± 1 Mean duration of diarrhoea ± SD: 77 ± 62 hours Mean frequency of diarrhoea ± SD: 12.5 ± 5 stools/24 hours Dehydration status,a % ‘none’/’some’: 32/68 |
Standard WHO-ORS Mean age ± SD: 15 ± 6 months Sex F : M, %: 65 : 35 Mean % expected W/L ± SD: 66 ± 3 Mean W/L z-score ± SD: –3.5 ± 0.5 MUAC: NR Mean % expected W/A ± SD: 51 ± 7 Mean W/A z-score ± SD: –4.6 ± 0.7 Mean duration of diarrhoea ± SD: 74 ± 59 hours Mean frequency of diarrhoea ± SD: 14 ± 9 stools/24 hours Dehydration status,a % ‘none’/’some’: 35/65 |
||
|
Alam et al. 200957 Design: RCT Location: Bangladesh Length of follow-up: ORS until cessation of diarrhoea; standard treatment until 80% W/L reached No. enrolled: 175 Target population: children aged 6–60 months with SAM (< 70% of NCHS median or with bipedal oedema) and acute, watery diarrhoea (< 48 hours duration) and cholera |
Glucose-ORS Mean age ± SD: 27.17 ± 12.36 months Sex F : M, %: 45 : 55 Mean % expected W/L ± SD: 68.99 ± 4.92 Mean W/L z-score ± SD: –3.14 ± 1.88 Mean MUAC ± SD: 112.7 ± 9.9 mm Mean % expected W/A ± SD: 54.51 ± 9.50 Mean W/A z-score ± SD: –4.38 ± 68b Mean duration of diarrhoea ± SD: 12.59 ± 8.27 hours Mean frequency of diarrhoea ± SD: 14.36 ± 6.00 stools/24 hours Dehydration status of ‘severe’, n (%): 48 (84) |
Glucose-ORS + ARS Mean age ± SD: 28.36 ± 13.42 months Sex F : M, %: 58 : 42 Mean % expected W/L ± SD: 69.01 ± 5.27 Mean W/L z-score ± SD: –2.76 ± 46b Mean MUAC ± SD: 113.6 ± 9.7 mm Mean % expected W/A ± SD: 53.42 ± 6.86 Mean W/A z-score ± SD: –4.31 ± 0.63 Mean duration of diarrhoea ± SD: 13.07 ± 9.11 hours Mean frequency of diarrhoea ± SD: 14.02 ± 6.09 stools/24 hours Dehydration status of ‘severe’, n (%): 49 (83) |
Rice-ORS Mean age ± SD: 27.33 ± 11.97 months Sex F : M, %: 55 : 45 Mean % expected W/L ± SD: 67.54 ± 6.19 Mean W/L z-score ± SD: –3.38 ± 0.60 Mean MUAC ± SD: 111.9 ± 10.8 mm Mean % expected W/A ± SD: 53.16 ± 7.94 Mean W/A z-score ± SD: –4.39 ± 0.71 Mean duration of diarrhoea ± SD: 10.98 ± 5.73 hours Mean frequency of diarrhoea ± SD: 14.55 ± 7.16 stools/24 hours Dehydration status of ‘severe’, n (%): 49 (84) |
|
|
Dutta et al. 200055 Design: double-blind RCT Location: India Length of follow-up: treatment until diarrhoea ceased or up to day 5; 30-day follow-up No. enrolled: 80 Target population: male children aged 3–24 months with acute watery diarrhoea for ≤ 72 hours, clinical signs and symptoms of ‘some’ dehydration, and W/A < 80% Harvard standard |
Elemental zinc 40 mg/day, (as syrup of zinc sulphate, 177 mg/day) administered in three divided doses + standard ORS Mean age ± SD: 10.4 ± 5.4 months Sex F : M, %: 0 : 100 W/H: NR Mean MUAC ± SD: 10.3 ± 1.3 W/A < 70% expected, n (%): 38 (87) Mean duration of diarrhoea ± SD: 33.4 ± 11.5 hours Mean frequency of diarrhoea ± SD: 13.8 ± 3.8 per 24 hours Dehydration status of ‘some’, %: 100 |
Placebo syrup + standard ORS Mean age ± SD: 11.0 ± 4.9 months Sex F : M, %: 0 : 100 W/H: NR Mean MUAC ± SD: 10.5 ± 1.0 W/A < 70% expected, n (%): 30 (83) Mean duration of diarrhoea ± SD: 38.3 ± 10.3 hours Mean frequency of diarrhoea ± SD: 13.3 ± 3.9 per 24 hours Dehydration status of ‘some’, %: 100 |
||
|
Dutta et al. 200154 Design: double-blind RCT Location: India Length of follow-up: until diarrhoea ceased or for up to 5 days No. enrolled: 64 Target population: male children aged 6–48 months with SAM (< 60% of Harvard standard W/A without oedema), marasmic, history of watery diarrhoea for ≤ 72 hours and clinical signs and symptoms of ‘some’ dehydration |
Hypo-osmolar ORS Mean age ± SD: 17.3 ± 9.7 months Sex F : M, %: 0 : 100 W/H: NR MUAC: NR W/A < 60% expected, n (%): 30 (94) Mean duration of diarrhoea ± SD: 21.3 ± 8.2 days Mean frequency of diarrhoea ± SD: 15 ± 3 stools/day Dehydration status of ‘some’, %: 100 |
Standard WHO/UNICEF-ORS Mean age ± SD: 22.5 ± 15.6 months Sex F : M, %: 0 : 100 W/H: NR MUAC: NR W/A < 60% expected, n (%): 31 (97) Mean duration of diarrhoea ± SD: 22 ± 8.0 days Mean frequency of diarrhoea ± SD: 13 ± 4 stools/day Dehydration status of ‘some’, %: 100 |
||
Severe acute malnutrition was defined similarly in three trials, being W/H < 70% of the NCHS median,51 and either W/L < 70% of the NCHS median or with bilateral pedal oedema. 50,57 Dutta and colleagues54 defined SAM as being < 60% of the Harvard standard W/A without oedema. In the fifth trial, Dutta and colleagues55 included different grades of malnourished children and used the IAP 1972 classification system. 45 They did not specifically define SAM.
Two trials51,54 evaluated a hypo-osmolar oral rehydration solution (H-ORS) (containing lower concentrations of sodium, chlorine and glucose), and one trial50 evaluated a modified ORS, termed ReSoMal (containing lower concentrations of sodium, chlorine and citrate, and higher concentrations of potassium and glucose, as well as including other selected minerals). The comparator in these three studies was a standard WHO-ORS. In the fourth trial,55 all participants received a standard WHO-ORS initially with either a zinc-supplemented syrup or a placebo syrup [this study is included in this section rather than in Which methods for correcting micronutrient deficiencies are effective? (Q8, rank 10) because the focus of the study was on treatment of diarrhoea]. The fifth trial57 evaluated three types of ORS, which differed only by the addition of glucose, glucose plus amylase-resistant starch (ARS) or rice powder. In four trials,51,54,55,57 the ORS was given over a period of 4–6 hours, whereas in the ReSoMal trial50 the ORS was given more slowly, over a period of 12–14 hours, with all continuing to receive the ORS thereafter, if necessary, until diarrhoea stopped. None of the studies specifically stated the intended total duration of ORS treatment, although it appeared to be until diarrhoea ceased,50,51,54,55,57 with two studies suggesting that if diarrhoea had not ceased treatment continued for up to 5 days. 54,55 The additional treatment with zinc or placebo syrup in the Dutta and colleagues trial55 was continued after discharge until the bottle was finished. None of the studies reported any follow-up beyond the treatment period with the exception of Dutta and colleagues55 who reported outcome data at a follow-up of 30 days.
The trials varied in the other interventions that were offered to participants. Three trials51,55,57 gave i.v. rehydration to participants, where needed, in addition to the ORS. Three trials50,51,57 treated infections with antibiotics, one study54 specifically stated that no drug therapy was given and one55 did not report either way. Most of the studies51,54,55,57 permitted breastfeeding and children were also given solid food where appropriate, with children in the Dutta and colleagues trial54 also given water ad libitum and formula or animal milk. Two trials reported that all children received the standardised treatment for SAM according to either WHO50 or International Centre for Diarrhoeal Disease Research (ICDDR)57 guidelines. It is not clear whether or not this is the case in the other trials, although it is possible that the two H-ORS studies51,54 used the WHO-ORS as their control intervention.
All the trials took place in an inpatient setting, recruiting children from diarrhoea treatment centres50,51,57 or hospital. 54,55 The studies were relatively small, ranging from 64 participants in the Dutta and colleagues trial54 to 175 participants in the Alam and colleagues trial. 57 Although the Alam and colleagues trial51 included 170 children in total, only 81 of these had SAM, with results reported separately for this group. This trial reported baseline characteristics for the whole study population, with only age and W/A reported in the subgroup with SAM. The five trials included children aged from 3 months to 5 years, although most were toddlers, with the average age being around 1–2 years. In one study,57 around half the participants were boys, in another50 approximately two-thirds were boys, whereas in both trials by Dutta and colleagues54,55 all the included children were boys (for the purposes of ease of collection of urine and stools separately). The last study51 did not report the proportions of males and females.
For three trials,50,51,57 the mean W/A (as a percentage of the NCHS median) at admission ranged from 50% to 59%, for one trial54 about 95% of children were < 60% Harvard standard W/A and for the fifth trial55 around 85% of participants were < 70% Harvard standard W/A. Two studies50,57 reported baseline z-scores, with a mean W/A z-score ranging from –4.3 to –4.7 and a mean W/L z-score ranging from –2.8 to –3.6. The mean duration of diarrhoea before admission was very different in the four trials that reported it, ranging from a mean of around 13 hours57 to 75 hours. 50 Dutta and colleagues54 reported a mean of 22 days despite an inclusion criterion of acute diarrhoea for ≤ 72 hours. In three trials, some50,51 or all57 of the children had diarrhoea with cholera.
All five studies had similar inclusion criteria with children required to have SAM, acute, watery diarrhoea for < 48 hours,57 ≤ 72 hours,54,55 < 4 days51 or ≤ 10 days,50 and be within the age range > 3 months and < 5 years. Four trials either required children to have some degree of dehydration51,54,55 or such children were eligible for inclusion. 57 Alam and colleagues51 stipulated that children should be included if aged between 3 months and 5 years with non-cholera diarrhoea or if aged > 3 months with a clinical suspicion of cholera. The two trials by Dutta and colleagues54,55 included only males (for the reasons reported above). Children with severe infections were excluded from all five trials. In addition, some trials also excluded those with invasive,51 bloody50,57 or a previous episode54 of diarrhoea. Other reasons for exclusion included having chronic underlying disease,55 receipt of i.v. fluids50 or antibiotics,54,55 convulsions,51 being exclusively breastfed54,55 or having signs of kwashiorkor. 54
Only two trials specified their primary outcomes. Alam and colleagues57 specified stool output, whereas Alam and colleagues50 specified the proportion of children developing overhydration and with correction of basal hypokalaemia. The other three trials did not specifically report what their primary outcomes were, but the main outcomes presented were similar and included weight gain, duration and volume of diarrhoea, ORS intake and electrolyte concentrations in addition to fluid54,57 or energy intake,51 time to recovery,51,54,55,57 urine output51,57 and requirement for i.v. fluids. 51,57 None of the trials reported W/H or W/A z-scores. Further details on all the outcomes reported in the trials can be seen in the data extractions in Appendix 9.
Summary of quality assessment
The methodological quality and the quality of reporting of the five included trials did not vary greatly. Two trials50,51 were rated strong overall, with the other three trials being rated moderate54,55,57 (Table 16).
| Study | Selection bias | Study design (description) | Confounders | Blinding | Data collection methods | Withdrawals and dropouts | Intervention integrity | Analysis | Global ratingc | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Per cent who received allocation | Consistency measured? | Unintended intervention likely? | Unit of allocation | Unit of analysis | Appropriate statistical methods? | ITT analysis? | ||||||||
| Alam et al. 200051 | S | S (RCT) | S | M | M | S | 80–100 | Yes | No | Patient | Patient | Yes | No | S |
| Alam et al. 200350 | M | S (RCT) | S | S | M | S | 80–100 | Yes | No | Patient | Patient | Yes | Yes | S |
| Alam et al. 200957 | S | S (RCT) | S | W | M | S | 80–100 | Yes | No | Patient | Patient | Yes | No | M |
| Dutta et al. 200055 | W | S (RCT) | S | S | M | S,a Wb | 80–100 | Yes | No | Patient | Patient | Yes | Yes | M |
| Dutta et al. 200154 | W | S (RCT) | S | S | M | S | 80–100 | Yes | No | Patient | Patient | Yes | Yes | M |
Selection bias varied between the studies, with three trials50,54,55 being at potential risk of selection bias. For all of these trials, it was unclear what proportion of selected individuals agreed to participate in the trials before they were randomised. In addition, the included children in both trials by Dutta and colleagues54,55 were considered to be only somewhat likely to be representative of the target population, leading to a higher risk of selection bias. Conversely, the study design of all five trials was strong, with all being RCTs and using an adequate method to generate random allocations. Hence, trial arms within all the studies were balanced with respect to baseline characteristics and confounders, leading to a strong rating. All but one57 trial employed a double-blind method, reporting that the interventions looked identical to participants. Alam and colleagues57 reported that treatments could not be blinded to those involved in the study because of visible differences in the three ORS solutions. Furthermore, neither study by Alam and colleagues51,57 reported sufficient details on the blinding of outcome assessors and they were therefore rated as moderate51 and weak57 as this could lead to detection bias. For data collection methods, all five trials were rated as moderate as they included valid data collection tools, but it was not possible to judge if these tools were reliable.
Sources of attrition bias in clinical trials include losses of participants to follow-up, unequal dropout rates between interventions, selective reporting of outcomes (missing outcomes) and failure to explain why participants are missing (e.g. whether or not they are missing at random). All five trials were rated as strong for withdrawals and dropouts, though they varied in their level of reporting. One trial50 provided both the number and reasons for any losses and had 80–100% of participants completing the study, indicating a low risk of attrition bias. Three trials51,54,57 either did not report any information on dropouts or only reported numbers (without reasons), but had most or all the participants completing the study. Consequently, these were rated as strong as the outcomes can be considered to be reasonably reliable and reflect the study population. In the trial by Dutta and colleagues,55 two contrasting ratings were allocated because all participants completed the acute phase of the study up to the point of recovery (rated strong), but over half the participants were not included in the 30-day follow-up assessments and neither the number nor reasons for the dropouts were reported by the authors (rated weak). The intervention integrity of all five trials was strong, as all the participants were deemed likely to have received their allocated intervention without any cross-contamination. All five trials used appropriate statistical methods in their analysis, although two51,57 did not perform an ITT analysis. For Alam and colleagues,51 this was presumably because the children with SAM were only a subgroup of the total study population. It should be pointed out that all five studies excluded children with severe infections, and as this is not uncommon in hospitalised children with SAM (Professor Kathryn Maitland, Imperial College, London, 2011, personal communication), the results of the studies may not be generalisable to most children with SAM and acute diarrhoea.
Assessment of effectiveness: acute diarrhoea
Mortality
The two studies by Alam and colleagues50,57 were the only trials to report mortality, with no deaths in any treatment group (Table 17). The other trials did not report this outcome, although in the third Alam and colleagues trial51 it is assumed there were no deaths as the children who were not discharged (after having recovered) were accounted for as dropouts. In both trials by Dutta and colleagues,54,55 it remains unclear whether the few children who did not recover within the 5 days of hospitalisation were lost to follow-up or died as no details were reported.
Weight gain
Most of the trials51,54,55,57 reported weight gain as an outcome measure, with two finding significant differences between treatment groups (Table 18). Dutta and colleagues54 found that children receiving the standard WHO/UNICEF-ORS had at discharge gained significantly more weight (p = 0.001) than those receiving the H-ORS (or on day 5 if they did not recover during this period). However, in the Alam and colleagues trial51 weight gain was similar in the H-ORS and WHO-ORS treatment groups. Alam and colleagues57 reported that children receiving the rice-ORS had significantly greater weight gain at 72 hours than those receiving either of the glucose-ORS treatments (p = 0.05). There was no statistically significant benefit on weight gain from a zinc supplement compared with placebo either at the time of recovery or at 30 days follow-up in the trial by Dutta and colleagues. 55 Alam and colleagues50 did not provide any numerical data on weight gain, but stated that weight gain before discharge was similar between the groups.
| Study | Treatment arms | p-value | |||
|---|---|---|---|---|---|
| Alam et al. 200051 | H-ORS (n = 41) | WHO-ORS (n = 40) | |||
| Mean percentage weight gain ± SDa | 4.54 ± 1.79 | 4.45 ± 2.18 | NS | ||
| Dutta et al. 200154 | H-ORS (n = 32) | WHO/UNICEF-ORS (n = 32) | |||
| Mean percentage weight gain ± SDb | 4.3 ± 1.2 | 5.4 ± 1.3 | 0.001 | ||
| Alam et al. 200957 | Glucose-ORS (n = 58) | Glucose-ORS + ARS (n = 59) | Rice-ORS (n = 58) | ||
| Mean percentage weight gain at 72 hours | 11 | 9.7 | 13 | 0.05 | |
| Dutta et al. 200055 | Zinc + ORS (n = 44) | Placebo + ORS (n = 36) | |||
| Mean percentage weight gain ± SDb | 3.9 ± 4.1 | 3.2 ± 2.9 | 0.41 | ||
| Mean percentage weight gain ± SD on 30th dayc | 2.6 ± 3.3 (n = 18) | 2.9 ± 3.7 (n = 16) | 0.88 | ||
Duration of diarrhoea
The length of time that diarrhoea persisted in treated children was reported in four51,54,55,57 of the five trials and can be seen in Table 19. The two trials51,54 evaluating a hypo-osmolar-ORS found similar results. The duration of diarrhoea was statistically significantly shorter in children who received the H-ORS than in those who received the standard WHO/UNICEF-ORS (41.5 vs 66.4 hours, respectively; p = 0.001). 54 In the Alam and colleagues trial,51 the duration of diarrhoea was reported separately for a rehydration phase and maintenance phase (as well as overall duration), though the timescale for these phases was not defined. The difference between treatment groups followed the same pattern and was statistically significant during the maintenance phase in favour of H-ORS (95% CI 0.46 to 0.88; p = 0.007), but was no longer significant when the phases were combined as overall duration. Supplementation with zinc was favourable compared with placebo with a mean difference in duration of diarrhoea of approximately 30 hours (p = 0.0001),55 whereas in another study,57 although the median duration of diarrhoea was lower in the rice-ORS group than in the glucose-ORS or glucose-ORS + ARS groups, this did not reach statistical significance.
| Study | Treatment arms | p-value | |||
|---|---|---|---|---|---|
| Alam et al. 200051 | H-ORS (n = 41) | WHO-ORS (n = 40) | |||
| Mean duration of diarrhoea ± SD, hours | |||||
| Rehydration phase | 10.95 ± 2.23 | 11.72 ± 2.26 | NS | ||
| Maintenance phasea | 10.45 ± 2.09 | 16.36 ± 2.01 | 0.007 (95% CI 0.46 to 0.88) | ||
| Overall | 24.35 ± 1.57 | 30.12 ± 1.69 | NS | ||
| Dutta et al. 200154 | H-ORS (n = 32) | WHO/UNICEF-ORS (n = 32) | |||
| Mean duration of diarrhoea ± SD, hours | 41.5 ± 25.1 | 66.4 ± 32.3 | 0.001 | ||
| Alam et al. 200957 | Glucose-ORS (n = 58) | Glucose-ORS + ARS (n = 59) | Rice-ORS (n = 58) | ||
| Median duration of diarrhoea (95% CI), hours | 72 (62 to 82) | 60 (50 to 70) | 54 (44 to 54) | 0.530 | |
| Dutta et al. 200055 | Zinc + ORS (n = 44) | Placebo + ORS (n = 36) | |||
| Mean duration of diarrhoea ± SD, hours | 70.4 ± 10.0 | 103.4 ± 17.1 | 0.0001 | ||
Frequency of diarrhoea
The frequency of diarrhoea was reported by three trials,51,54,57 although differences in the way this outcome was reported make direct comparisons between trials difficult. Alam and colleagues51 reported the number of stools in a 4-hour period, whereas the other two trials54,57 reported stool output (g/kg and ml/kg, respectively) in several 24-hour periods and also at recovery54 (Table 20). Despite differences in the reporting, for both studies evaluating H-ORS,51,54 the mean frequency of stool output was significantly less in the children receiving H-ORS than in those receiving standard WHO-ORS at all time points. For the third trial, by Alam and colleagues,57 the cumulative mean stool output of children receiving rice-ORS was statistically significantly lower than among children receiving glucose-ORS at 24 hours (32% mean reduction, 95% CI 44% to 174%; p = 0.004), and this statistical difference was maintained at 48 and 72 hours. Compared with the study by Dutta and colleagues,54 data for stool output per kg of body weight were markedly higher in the trial by Alam and colleagues,57 but the reason for this is unclear.
| Study | Treatment arms | p-value | |||
|---|---|---|---|---|---|
| Alam et al. 200051 | H-ORS (n = 41) | WHO-ORS (n = 40) | |||
| Mean frequency of diarrhoea ± SD, stools/4 hours | |||||
| Rehydration phase | 4.27 ± 2.029 | 5.86 ± 1.73 | 0.32a (95% CI 0.55 to 0.97) | ||
| Maintenance phaseb | 1.72 ± 1.92 | 2.45 ± 2.17 | 0.035 (95% CI 0.51 to 0.97) | ||
| Overall | 3.39 ± 1.80 | 4.70 ± 1.68 | 0.011 (95% CI 0.56 to 0.93) | ||
| Dutta et al. 200154 | H-ORS (n = 32) | WHO/UNICEF-ORS (n = 32) | |||
| Mean frequency of stool output ± SD, g/kg | |||||
| 0–24 hours | 73.4 ± 23.1 | 105.9 ± 44.6 | 0.001 | ||
| 24–48 hours | 34.9 ± 13.5 | 87.5 ± 66.5 | 0.001 | ||
| 48–72 hours | 28.4 ± 18.0 | 90.4 ± 67.7 | 0.01 | ||
| At recovery | 52.3 ± 21.3 | 96.6 ± 42.8 | 0.0001 | ||
| Alam et al. 200957 | Glucose-ORS (n = 58) | Glucose-ORS + ARS (n = 59) | Rice-ORS (n = 58) | ||
| Stool output, ml/kgc,d | |||||
| At 24 hours | 355 | 309 | 236 | 0.004, difference 109 (95% CI 44 to 174), 32% reductione | |
| At 48 hours | 600 | 518 | 382 | 0.007, difference 213 (95% CI 79 to 346), 37% reductione | |
| At 72 hours | 735 | 645 | 475 | 0.018, difference 242 (95% CI 73 to 412), 36% reductione | |
Recovery
Two54,55 of the five trials specifically reported recovery (proportion of children who recovered within 5 days) as an outcome (Table 21), with one of these54 also reporting median survival time to recovery. Recovery was defined as the passage of a normal stool or no stool for the last 18 hours,55 or was assumed to be when diarrhoea had ceased (two formed stools passed or no stool for 12 hours). 54 A further two trials50,57 reported outcomes that inferred recovery in the children. Alam and colleagues57 reported the time taken to attain an oedema-free W/L of 80% of the NCHS median, whereas Alam and colleagues50 reported the number of children who were adequately rehydrated at 12 hours.
| Study | Treatment arms | p-value | |||
|---|---|---|---|---|---|
| Dutta et al. 200055 | Zinc + ORS (n = 44) | Placebo + ORS (n = 36) | |||
| Recovered within 5 days, n (%) | 44 (100) | 32 (89) | 0.04 | ||
| Dutta et al. 200154 | H-ORS (n = 32) | WHO/UNICEF-ORS (n = 32) | |||
| Recovered within 5 days, n (%) | 32 (100) | 29 (91) | > 0.05 | ||
| Median survival time to recovery, hours | 36 | 53 | 0.001 | ||
| Alam et al. 200957 | Glucose-ORS (n = 58) | Glucose-ORS + ARS (n = 59) | Rice-ORS (n = 58) | ||
| Mean days to attain 80% of median W/L ± SD | 7.14 ± 2.26 | 7.12 ± 2.2 | 7.2 ± 3.78 | 0.99 | |
| Alam et al. 200350 | ReSoMal (n = 65) | WHO-ORS (n = 65) | |||
| Adequately rehydrated at 12 hours, n/N (%) | 45/59 (76) | 51/63 (81) | 0.68; OR 0.16 (95% CI 0.29 to 1.96) | ||
Dutta and colleagues55 found a small but significant (p = 0.04) difference between treatment groups with all children supplemented with zinc recovering within 5 days of hospitalisation, compared with 89% of children receiving placebo. Dutta and colleagues54 also reported a high recovery rate, with all but three children (all in WHO-ORS group) having recovered within 5 days of treatment, but the difference between treatment groups was not significant. However, children treated with H-ORS recovered significantly quicker than those treated with the WHO-ORS (36 vs 53 hours, respectively; p = 0.001).
In the Alam and colleagues57 trial, it took around 7 days for children to attain an oedema-free W/L of 80%, being similar regardless of the type of ORS (p = 0.99).
In the Alam and colleagues trial,50 most of the children in both treatment arms were adequately rehydrated at 12 hours, with no statistically significant differences between groups.
Consumption of oral rehydration solution
Most of the trials51,54,55,57 measured how much ORS was consumed by the children, either as the total amount consumed (litres)51,55 or as ml/kg of body weight54,57 (Table 22). In two trials,51,54 children receiving H-ORS needed to consume less rehydration solution than those receiving the standard WHO-ORS, although this reached statistical significance in only one of the trials (p = 0.0001). 54 The other two studies also found significant differences in favour of the intervention groups. Dutta and colleagues55 reported a lower ORS consumption in children supplemented with zinc than in those supplemented with placebo (p = 0.0001). Alam and colleagues57 found that children receiving rice-ORS had a significantly lower ORS intake at 18 hours compared with those receiving glucose-ORS (see Appendix 9). This difference was maintained at each 6-hourly interval thereafter until 72 hours, when there was a 38% reduction in intake (p = 0.012).
| Study | Treatment arms | p-value | |||
|---|---|---|---|---|---|
| Alam et al. 200051 | H-ORS (n = 41) | WHO-ORS (n = 40) | |||
| Mean ORS consumed ± SD, l | |||||
| Rehydration phase | 1.45 ± 0.002 | 1.55 ± 0.002 | NS | ||
| Maintenance phasea | 0.69 ± 0.005 | 0.74 ± 0.01 | NS | ||
| Overall | 2.74 ± 0.0017 | 3.32 ± 0.0017 | NS | ||
| Dutta et al. 200154 | H-ORS (n = 32) | WHO/UNICEF-ORS (n = 32) | |||
| Mean ORS intake ± SD, ml/kg | |||||
| 0–24 hours | 109.7 ± 32.2 | 184.5 ± 53.7 | 0.0001 | ||
| 24–48 hours | 73.4 ± 22.7 | 151.2 ± 81.3 | 0.0001 | ||
| 48–72 hours | 54.9 ± 28.3 | 151.5 ± 65.0 | 0.001 | ||
| Mean ORS intake at recovery ± SD, g/kg/day | 111.5 ± 39.4 | 168.9 ± 52.4 | 0.0001 | ||
| Alam et al. 200957 | Glucose-ORS (n = 58) | Glucose-ORS + ARS (n = 59) | Rice-ORS (n = 58) | ||
| Mean ORS intake at 72 hours,b ml/kg | 710 | 620 | 450 | 0.012, 38% reductionc | |
| Dutta et al. 200055 | Zinc + ORS (n = 44) | Placebo + ORS (n = 36) | |||
| Mean ORS consumed ± SD, l | 2.5 ± 1.0 | 3.6 ± 0.8 | 0.0001 | ||
Adverse effects
Adverse effects were not reported in any detail by the included studies. Two trials51,55 did not report any safety issues, whereas two trials54,57 reported that no children developed symptoms of overhydration. Alam and colleagues50 report that prevention of overhydration is the primary theoretical advantage of ReSoMal. Overhydration was defined as a weight gain > 5% after correction of dehydration at any time during the study period with any of the following signs: periorbital oedema/puffy face, increased heart rate (> 160/minute) or increased respiration (> 60/minute). Although there appeared to be a lower occurrence of over-rehydration in those children who received ReSoMal than in those receiving WHO-ORS, numbers were small and this was not supported statistically (Table 23). Alam and colleagues50 also looked in detail at serum electrolytes and, thus, the incidence of hypo- and hyperkalaemia and hypo- and hypernatraemia (these outcomes have not been reported here as they are not main outcomes of interest to this review, but data are available in Appendix 9). However, it is worth noting that three children in the ReSoMal group developed severe hyponatraemia (low serum sodium) by 24 hours, with one child having a resulting convulsion, which the authors highlight as a safety concern that may limit the use of ReSoMal in its current formulation.
| Study | Treatment arms | p-value | |
|---|---|---|---|
| Alam et al. 200350 | ReSoMal (n = 65) | WHO-ORS (n = 65) | |
| Overhydration, n/N (%) | 3/65 (5) | 8/65 (12) | 0.20; OR 0.3 (95% CI 0.1 to 1.5) |
Other outcomes
Additional outcomes, such as caloric or fluid (water, milk) intake, other fluid losses (e.g. urine, vomit) and correction of hypokalaemia, were also reported by some studies, but have not been presented here. Further details are available in the data extraction forms in Appendix 9.
Summary
-
Five trials evaluated the treatment of children with acute diarrhoea with various types of ORS, including a H-ORS,51,54 a modified WHO-ORS (ReSoMal),50 an ORS containing either glucose, glucose plus ARS or rice powder,57 and supplementation with zinc. 55 The trials were all of strong or moderate methodological quality.
-
There were no deaths in the two trials that reported mortality,50,57 and it is assumed that there were no deaths in a third trial,51 as all children who did not recover were accounted for as dropouts.
-
Compared with the standard WHO-ORS, children receiving the H-ORS had a significantly shorter duration and lower frequency of diarrhoea, consumed less ORS and had a quicker time to recovery (one trial54).
-
There appeared to be no benefit from H-ORS with respect to weight gain compared with WHO-ORS.
-
Supplementation with 40 mg elemental zinc (as zinc syrup) in addition to a standard WHO-ORS resulted in a significantly shorter duration of diarrhoea, a better recovery rate and a lower ORS intake, but no difference compared with placebo in terms of weight gain. 55
-
Rice-ORS appeared to be more favourable than glucose-ORS in treating children with cholera diarrhoea. The rice-ORS groups had significantly better weight gain, a lower frequency and duration of diarrhoea and consumed less ORS. 57
-
ReSoMal did not appear to show any advantage over a standard WHO-ORS in rehydrating severely malnourished children with acute diarrhoea, although it was beneficial in correcting potassium depletion.
-
Adverse effects were not generally reported by the trials, although ReSoMal50 may result in symptomatic severe hyponatraemia and seizures in some patients.
Quantity and quality of research available: persistent diarrhoea
Three included trials, reported in four publications,52,53,56,58 pertained to children with persistent diarrhoea. Persistent diarrhoea was defined as diarrhoea lasting ≥ 14 days (Table 24).
| Study details and target population | Intervention | Comparator | ||
|---|---|---|---|---|
|
Amadi et al. 2005,52 Amadi 200258 Design: RCT Location: Zambia Length of follow-up: 4 weeks No. enrolled: 200 Target population: children aged 6–24 months with malnutrition and persistent diarrhoea (≥ 14 days duration) meeting the Wellcome classification for malnutrition (W/A and H/A) 54% of the population are HIV+ve |
Neocate amino acid-based elemental infant formula (and components of the WHO guidelines for management of persistent diarrhoea and malnutrition) Selected baseline characteristics Mean age (range): 18 (13–22) months Sex F : M, %: 55 : 45 W/H: NR Median MUAC (IQR): 11 (10–12) cm Median W/A z-score (IQR): –4.1 (–4.8 to –3.6) Duration of diarrhoea: ≥ 14 days Frequency of diarrhoea: NR Degree dehydration: NR |
Skimmed milk diet, followed by soy-based porridge from week 2 (plus components of the WHO guidelines for management of persistent diarrhoea and malnutrition) Selected baseline characteristics Mean age (range): 17 (14–20) months Sex F : M, %: 51 : 49 W/H: NR Median MUAC (IQR): 11 (10–12.2) cm Median W/A z-score (IQR): –4.0 (–4.6 to –3.4) Duration of diarrhoea: ≥ 14 days Frequency of diarrhoea: NR Degree dehydration: NR |
||
|
Bhutta et al. 199453 Design: RCT Location: Pakistan Length of follow-up: presumed to be 14 days No. enrolled: 51 Target population: male children aged 6–36 months with persistent diarrhoea (≥ 2 weeks) and with severe PEM, i.e. W/A ≤ 80th centile of the median NCHS standard (i.e. Gómez grade II and III malnutrition) |
Full-strength soy formulation (given orally or by nasogastric tube if necessary) Selected baseline characteristics Mean age ± SD: 16 ± 8.6 months Sex F : M, %: 0 : 100a Mean % expected W/L ± SD: 88.4 ± 4.3 Mean MUACb ± SD: 9.9 ± 1.3 cm Mean W/A z-score ± SD: –4.41 ± 0.6 Mean duration of diarrhoea ± SD: 75.0 ± 77.0 days Mean frequency of diarrhoea ± SD: 8.2 ± 2.7 stools/day Degree dehydration: NR |
Half-strength buffalo milk with KY (given orally or by nasogastric tube if necessary) Selected baseline characteristics Mean age ± SD: 13.8 ± 5.8 months Sex F : M, %: 0 : 100a Mean % expected W/L ± SD: 89.5 ± 4.3 Mean MUACb ± SD: 10.6 ± 1.7 cm Mean W/A z-score ± SD: –3.91 ± 0.9 Mean duration of diarrhoea ± SD: 150.0 ± 117.0 days Mean frequency of diarrhoea ± SD: 8.1 ± 2.7 stools/day Degree dehydration: NR |
||
|
Nurko et al. 199756 Design: RCT Location: Mexico Length of follow-up: until full concentration of diet achieved (9 days if no intolerance) plus an additional 7 days No. enrolled: 60 (56 randomised) Target population: children aged 3–36 months with third-degree malnutrition of the marasmic type defined by the Gómez criteria (W/A < 60% of the NCHS 50th percentile) and persistent diarrhoea (≥ 3 loose stools for ≥ 14 days) |
Intervention 1: local chicken-based diet Selected baseline characteristics Mean age ± SD: 6.7 ± 3.7 months Sex F : M, %: 47 : 53a W/H: NR MUAC: NR Mean % expected W/A ± SD: 50.8 ± 7.4 Mean W/A z-score ± SD: –4.2 ± 1.0 Mean duration of diarrhoea ± SD: 36.6 ± 3.9 days Frequency of diarrhoea output first 24 hours ± SD: 41.6 ± 12.1 ml/kg/day Dehydration status of severe, n (%): 4 (21.1) |
Intervention 2: soy-based diet (Nursoy) (both in gradually increasing amounts) Selected baseline characteristics Mean age ± SD: 5.6 ± 4.0 months Sex F : M, %: 42 : 58a W/H: NR MUAC: NR Mean % expected W/A ± SD: 51.0 ± 7.5 Mean W/A z-score ± SD: –3.9 ± 0.7 Mean duration of diarrhoea ± SD: 48.7 ± 5.1 days Frequency of diarrhoea output first 24 hours ± SD: 45.8 ± 13.6 ml/kg/day Dehydration status of severe, n (%): 5 (26.3) |
Elemental diet: standard Vivonex (in gradually increasing amounts) Selected baseline characteristics Mean age ± SD: 6.9 ± 5.3 months Sex F : M, %: 50 : 50a W/H: NR MUAC: NR Mean % expected W/A ± SD: 52.9 ± 7.5 Mean W/A z-score ± SD: –4.0 ± 1.0 Mean duration of diarrhoea ± SD: 41.8 ± 4.0 days Frequency of diarrhoea output first 24 hours ± SD: 52.3 ± 19.6 ml/kg/day Dehydration status of severe, n (%): 6 (33.3) |
|
All three trials were single-centre RCTs, of which one was a single-blind52,58 and one a double-blind trial. 56 The third trial provided no details about blinding. 53 The trials were set in Mexico,56 Pakistan53 and Zambia,52,58 and all received external funding. One trial was funded by a grant from a commercial organisation, with one of the authors also receiving support from a global charity. 52,58 Of the two remaining trials, one was part-funded56 and one fully-funded53 by a US academic institution, by means of a cooperative agreement with a US Government department. The part-funded trial received a further grant from another US Government department. 56
All three trials evaluated varying diets, including soy as either an intervention53,56 or as a comparator (milk followed by a soy porridge). 52,58 Bhutta and colleagues53 evaluated a full-strength soy diet against a half-strength buffalo milk diet with khitchri (rice-lentils) and yoghurt (KY), with diets given in gradually increasing amounts over 14 days. The trial by Nurko and colleagues56 compared three intervention strategies – a local chicken-based diet, a soy-based (Nursoy®; Wyeth Laboratories, Philadelphia, PA, USA) diet and an elemental diet (Vivonex® standard; Norwich Eaton Ltd, Surrey, UK) – all provided at gradually increasing concentrations by nasogastric tube for around 16 days if the diet was tolerated. The third trial by Amadi and colleagues,52,58 compared an amino acid-based infant formula (Neocate®, SHS International Ltd, Liverpool, UK), without cow’s milk, soy and cereal antigen, with a standard skimmed milk diet (followed by soy-based porridge from week 2) for 4 weeks. One of the trials followed the WHO guidelines for the treatment of persistent diarrhoea and malnutrition52,58 and one the WHO/UNICEF guidelines for hydration (standard glucose–electrolyte i.v. solution). 56 In addition, two of the trials provided antibiotic treatment as needed52,56,58 and one provided micronutrient supplements. 52,58
All trials took place in the hospital inpatient setting. Sample sizes were small for two of the trials, 5153 and 5656 children, whereas the third RCT included 200 children. 52,58 The age of children included ranged from 3 to 36 months. Two of the trials had fairly similar ratios of boys and girls in their trial arms,52,56,58 whereas the remaining trial consisted of boys only (to facilitate separate quantitative collections of urine and faeces). 53
Definitions for SAM varied, with Amadi and colleagues52,58 using the Wellcome classification for severe malnutrition (W/A and H/A). The remaining two trials used the NCHS growth reference, with W/A ≤ 80th centile of the median NCHS standard (i.e. Gómez grades II and III malnutrition) described as severe PEM,53 and W/A < 60% of the NCHS 50th percentile for W/A described as third-degree malnutrition of the marasmic type by the Gómez criteria. 56 One trial reported W/Ls at baseline. 53 W/A z-scores were similar across the three trials, ranging from –3.953,56 to –4.41. 53 All three trials excluded exclusively breastfed children. 52,53,56,58 Other exclusion criteria were chronic illnesses,56 neurological or serious systemic disorders52,58 and children with kwashiorkor and the presence of intercurrent infections. 53 The children in the Amadi and colleagues52,58 trial had a high prevalence of intestinal infection, and around half were HIV+ve. In the trial by Nurko and colleagues,56 64% of the sample had associated conditions (e.g. pneumonia, sepsis, infections) on admission.
There were large differences in the baseline duration of diarrhoea between the trials, reported as around 36.6–48.7 days in one trial,56 an average of 75–150 days in another trial,53 but as ≥ 14 days in the remaining trial. 52,58
Trials assessed outcomes of weight gain and some measures of diarrhoea, but only one trial specified these as primary outcomes in addition to mortality. 52,58 Other outcomes included treatment success/failure, nutritional recovery and nitrogen balance,56 as well as developmental milestones achieved, activity and play, and laboratory indicators of severity of illness. 52,58 For further details on reported outcome measures see Appendix 9.
Summary of quality assessment
Two of the included trials were rated overall as ‘weak’ for their methodological quality and quality of reporting (Table 25),52,53,58 with the third being rated overall as ‘strong’. 56
| Study | Selection bias | Study design (description) | Confounders | Blinding | Data collection methods | Withdrawals and dropouts | Intervention integrity | Analysis | Global ratinga | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Per cent who received allocation | Consistency measured? | Unintended intervention likely? | Unit of allocation | Unit of analysis | Appropriate statistical methods? | ITT analysis? | ||||||||
| Amadi et al. 2005,52 200258 | M | S (RCT) | S | W | W | S | 80–100 | Yes | No | Patient | Patient | Yes | No | W |
| Bhutta et al. 199453 | W | S (RCT) | S | W | M | M | 60–79 | Yes | No | Patient | Patient | Yes | ? | W |
| Nurko et al. 199756 | S | S (RCT) | S | S | M | M | 80–100 | Yes | Yes | Patient | Patient | Yes | No | S |
Trials were rated as moderate,52,58 weak53 or strong56 for selection bias. A moderate rating indicates that the selected individuals are at least somewhat likely to be representative of the target population and at least 60% of selected individuals participated in the trial. A weak rating indicates that participants may not be representative of the target population, or that the selection method and/or levels of participation were not described. The two trials with moderate and weak ratings were at potential risk of selection bias. 52,53,58 All three trials were rated as strong for their study design (RCTs).
There were no important differences in baseline characteristics between the trial arms, and without potentially confounding variables all three trials were rated as strong. For blinding, only one trial employed a double-blind method and was therefore rated as strong. 56 Of the other two trials, both were rated as weak, with one employing a single-blind method52,58 and the other reporting no details. 53 It is recognised that blinding of children is not always possible because of the nature of the intervention. This could lead to bias in either the care provided (performance bias) or how the outcomes were assessed (measurement or detection bias), or both. Not blinding children/parents to the research question could lead to reporting bias. Although it may be problematic in some circumstances to blind children/parents to the intervention, the potential bias it can introduce needs to be kept in mind when interpreting the results.
For data collection methods, two trials were rated as moderate. 53,56 Although both trials included valid data collection tools, it was not possible to judge if these tools were reliably employed. The remaining trial was rated as weak, as it was not possible to assess if the data collection tools were either valid or shown to be reliable. 52,58 One trial52,58 provided both numbers and reasons for withdrawals and dropouts, and with a follow-up rate of ≥ 80% received a strong rating. Of the remaining two trials,53,56 both had lower follow-up rates (60–79%) and one provided inadequate information by giving reasons for withdrawal, but not numbers for each group. 53 There was a possible risk of attrition bias in both these trials and they both received an overall rating of moderate for withdrawals and dropouts. For the section of the tool capturing intervention integrity, two trials52,56,58 reported that > 80% of the participants received the intervention, and in the third53 60–79% received the intervention. The consistency of the intervention was measured by all three trials, using weight gain as the measure, and there appeared to be no contamination of the interventions (i.e. all children received the allocated diet only). All trials used patients as the unit of allocation and analysis for statistical analysis of the results, and were judged to use appropriate methods of statistical analysis for the research question. Two of the trials did not perform an ITT analysis,52,56,58 and it was not possible to determine how missing data were dealt with in the analysis in the third trial. 53
Assessment of effectiveness: persistent diarrhoea
Mortality
Only Amadi and colleagues52,58 reported mortality as an outcome (Table 26). Although mortality was highest in the Neocate group (22/100), the difference was not statistically significant (see Table 26). The highest number of deaths for the combined treatment groups (43%) occurred in the second treatment week (week 1 = 31%, week 3 = 26% and week 4 = 10%). Irrespective of treatment arm, death was more likely to occur in children with marasmic kwashiorkor (34.9%; p = 0.004), or cryptosporidiosis (no data reported) and in children identified as HIV+ve (24% compared with 11% of HIV–ve children; p = 0.04). Although mortality was not formally identified as an outcome in the trial by Nurko and colleagues,56 the authors reported that five children died during the trial and how many deaths occurred in each group (see Table 26). However, the causes of death, intestinal pneumatosis (n = 2), central line-associated sepsis (n = 2) or bacterial sepsis (n = 1), were reported only for the whole trial population and not by group. Bhutta and colleagues53 also did not specify mortality as an outcome; however, it can be assumed that there were no deaths, because all children either completed the treatment or were accounted for as dropouts.
Weight gain
Measures of weight gain were employed by all three trials; however, only one trial reported weight gain relative to initial weight (the benefit of relative weight gain measures is that any effects because of starting differences in body weight are removed). In the trial by Amadi and colleagues,52,58 feeding with Neocate was associated with a 41% better gain in weight from nadir compared with the skimmed milk/soy-based diet, and the difference was statistically significant (p = 0.002) (Table 27). In the trial by Bhutta and colleagues,53 weight gain was higher for the intervention diet of soy than for KY milk, but reached statistical significance only at the end of treatment (i.e. week 2; p < 0.02). Conversely, mean daily weight gain was higher in the KY milk group, but the difference between groups was not statistically significant. It should be noted that there was also a reported weight loss in two children in the soy group (10%) and seven (37%) in the KY milk group (p = not statistically significant). Nurko and colleagues56 reported statistically significant weight gains for all three diets used in their trial for their comparison of weight change from admission versus at the end of the protocol and from admission versus discharge. However, no statistically significant differences between the three treatment arms were reported.
| Study | Treatment arms | p-value | |||
|---|---|---|---|---|---|
| Amadi et al. 2005,52, Amadi et al. 200258 | Neocate | Standard skimmed milk/soy-based diet | |||
| Median weight gain (IQR), kg | n = 79 | n = 78 | |||
| From admission | 1.10 (0.55–1.55) | 0.75 (0.2–1.3) | 0.006 | ||
| From nadir | 1.7 (1.2–2.0) | 1.2 (0.6–1.7) | 0.002 | ||
| Bhutta et al. 199453 | Soy (n = 21) | KY milk (n = 19) | |||
| Mean weight change ± SD, g/kg/daya | |||||
| Week 1 | 7.1 ± 11.3 | 3.1 ± 12.1 | NS | ||
| Week 2 | 11.6 ± 10.0 | 4.3 ± 7.2 | < 0.02 | ||
| Mean weight change ± SD, g/kg/day | 3.7 ± 5.9 | 7.9 ± 9.7 | NS | ||
| Percentage (n/N) of participants who lost weight | 10 (2/21) | 37 (7/19) | NS | ||
| Nurko et al. 199756 | Chicken (n = 15) | Nursoy (n = 13) | Vivonex (n = 13) | ||
| Mean weight ± SD, g | |||||
| At admission | 3572 ± 823 | 3270 ± 1167 | 3764 ± 1575 | NR | |
| At end of protocol | 3736 ± 870b | 3495 ± 1172b | 3940 ± 1599b | ||
| At time of discharge | 4133 ± 1160c | 3797 ± 1128c | 4225 ± 1706c | ||
Anthropometry
Two of the studies reported anthropometry outcomes as well as weight gain. Amadi and colleagues52,58 reported that increases in z-scores of W/A and W/H were statistically significantly higher from admission (W/A, p = 0.018; W/H, p < 0.001) and from nadir (W/A, p = 0.002; W/H, p < 0.001) for the Neocate group, with results mirrored in HIV+ve (W/A, p = 0.007; W/H, p < 0.001) and HIV–ve (W/A, p = 0.01; W/H, p = 0.009) subgroups (Table 28). In the trial by Bhutta and colleagues,53 increases in W/A z-score during the study were significantly greater in the soy group (p < 0.001) than in the KY milk group (p = not statistically significant), but no statistical comparison between the groups was reported. Bhutta and colleagues53 did report a statistical comparison between the groups for improvement in mid-arm circumference, which was significantly higher in the soy intervention group (1.0 cm vs 0.1 cm; p < 0.001).
| Study | Treatment arms | p-value | |
|---|---|---|---|
| Amadi et al. 2005,52, Amadi et al. 200258 | Neocate | Standard skimmed milk/soy-based diet | |
| Median increase in W/A z-score (IQR)a | n = 79 | n = 78 | |
| From admission | 0.83 (0.35–1.22) | 0.43 (0–0.9) | 0.018 |
| From nadir | 1.23 (0.89–1.57) | 0.87 (0.47–1.25) | 0.002 |
| Median increase in W/H z-score (IQR)a | n = 79 | n = 78 | |
| From admission | 1.28 (0.52–1.88) | 0.56 (0–1.15) | < 0.001 |
| From nadir | 1.77 (1.30–2.26)a | 1.23 (0.59–1.70) | < 0.001 |
| Median increase in z-score (IQR) from nadir in HIV+ve childrena | n = 38 | n = 40 | |
| W/A | 1.2 (0.8–1.5) | 0.70 (0.4–1.2) | 0.007 |
| W/H | 1.8 (1.1–2.3) | 0.8 (0.4–1.6) | < 0.001 |
| Median increase in z-score (IQR) from nadir in HIV–ve childrena | n = 41 | n = 38 | |
| W/A | 1.29 (0.98–1.57) | 0.95 (0.5–1.45) | 0.01 |
| W/H | 1.82 (1.47–2.38) | 1.43 (0.81–1.86) | 0.009 |
| Bhutta et al. 199453 | Soy (n = 21) | KY milk (n = 19) | |
| Mean improvement in W/A z-score ± SDb | From –4.4 ± 0.6 to –3.6 ± 0.6; p < 0.001 | From –3.9 ± 0.9 to –3.6 ± 1.0; p = NS | NR |
| Mean improvement in MUACc ± SD, cmb | 1.0 ± 0.1 | 0.1 ± 0.05 | < 0.001 |
Diarrhoea
Diarrhoea output was quantified either by collecting urine separately from stools (using adhesive urine bags and pre-weighed nappies/diapers)53 or by the use of metabolic beds/cots for separation of stool from urine. 56 There were no statistically significant differences in any of the measures of diarrhoea between treatment arms in the two trials53,56 that reported these outcomes (Table 29). In addition, Amadi and colleagues,52,58 who presented no numerical data, stated that there were no differences in either stool number or frequency.
| Study | Treatment arms | p-value | |||
|---|---|---|---|---|---|
| Bhutta et al. 199453 | Soy (n = 21) | KY milk (n = 19) | |||
| Mean stool volume ± SD, g/kg/daya | |||||
| Week 1 | 68.8 ± 43.1 | 60.9 ± 40.6 | NS | ||
| Week 2 | 36.2 ± 23.2 | 63.9 ± 61.8 | NS | ||
| Overall | 58 ± 33 | 62 ± 49 | NS | ||
| Mean stool frequency ± SD, n/daya | |||||
| Week 1 | 7.0 ± 3.1 | 6.6 ± 4.4 | NS | ||
| Week 2 | 4.0 ± 2.4 | 5.5 ± 3.8 | NS | ||
| Overall | 6 ± 3 | 6 ± 4 | NS | ||
| Nurko et al. 199756 | Chicken (n = 15) | Nursoy (n = 13) | Vivonex (n = 13) | ||
| Diarrhoea status | |||||
| Mean total stool output/kg/day ± SD | 19.1 ± 7.5 | 18.5 ± 6.6 | 18.8 ± 9.2 | NS | |
| Mean stools/kg/day ± SD | 3.2 ± 1.2 | 2.5 ± 0.7 | 3.4 ± 1.3 | NR | |
| Mean day of cessation ± SD | 6.9 ± 4.7 | 3.9 ± 3 | 8 ± 5.1 | NS | |
Oral rehydration solution intake
Only Bhutta and colleagues53 reported ORS intake, which was significantly reduced by week 2 in the soy intervention arm compared with the KY milk diet (p < 0.05); however, differences in time to recovery were not statistically significant between the two diets (Table 30).
| Study | Treatment arms | p-value | |
|---|---|---|---|
| Bhutta et al. 199453 | Soy (n = 21) | KY milk (n = 19) | |
| Mean ORS intake ± SD, ml/kg/daya | |||
| Week 1 | 33.9 ± 41.0 | 37.9 ± 46.2 | NS |
| Week 2 | 1.7 ± 3.6 | 29.2 ± 58.1 | < 0.05 |
| Mean time to recovery ± SD, days | 6 ± 4 | 5 ± 3 | NS |
Calorie intake
Surprisingly, Amadi and colleagues52,58 reported that intake of calories (per kg per day) as liquid feeds, was statistically significantly higher at all time points for the control group (p < 0.0001). However, it should be noted that in addition to the liquid feed based on skimmed milk, the control group also received soy-based porridge from the beginning of the second week. In contrast, Bhutta and colleagues53 found caloric intake (per kg per day) to be only significantly higher for the soy-based intervention arm than for the KY milk arm at the end of week 1 (p < 0.02), and although this remained higher, it was no longer statistically significant at the end of week 2. Caloric intake in the trial by Nurko and colleagues56 was similar in all three diet groups (Table 31).
| Study | Treatment arms | p-value | |||
|---|---|---|---|---|---|
| Amadi et al. 2005,52, Amadi et al. 200258 | Neocate (n = 100) | Standard skimmed milk/soy diet (n = 100) | |||
| Median (IQR)a intake, kcal/kg/day | |||||
| Week 1 | 116 (86–143), n = 95 | 167 (130–214), n = 97 | < 0.0001 | ||
| Week 2 | 168 (135–203), n = 85 | 258 (210–301), n = 93 | < 0.0001 | ||
| Week 3 | 184 (166–206), n = 75 | 283 (229–337), n = 85 | < 0.0001 | ||
| Week 4 | 187 (163–210), n = 70 | 269 (214–305), n = 79 | < 0.0001 | ||
| Bhutta et al. 199453 | Soy (n = 21) | KY milk (n = 19) | |||
| Mean caloric intake ± SD, kcal/kg/dayb | |||||
| Week 1 | 140.1 ± 33.4 | 115.1 ± 25.1 | < 0.02 | ||
| Week 2 | 157.1 ± 72.3 | 151.6 ± 32.3 | NS | ||
| Overall | 154.2 ± 36.8 | 132.8 ± 27.6 | NS | ||
| Nurko et al. 199756 | Chicken (n = 15) | Nursoy (n = 13) | Vivonex (n = 13) | ||
| Mean number of total calories, /kg/day ± SD after full diet tolerated | 116.0 ± 9.6 | 111.3 ± 9.1 | 115.2 ± 8.3 | NS | |
Treatment success/failure
Although clinical failure appeared to be lower in the soy-based diet arm than in the KY milk arm (no p-value reported), Bhutta and colleagues53 reported no statistical difference between treatment arms. Nurko and colleagues56 reported that there was no statistically significant differences between the three diets in terms of successful outcome (Table 32), with nutritional recovery and treatment failures appearing similar between the groups (p-values not reported). However, across the whole trial population (i.e. analysis not per treatment group), significant differences between treatment success and failure (p < 0.05) were associated with albumin and sodium concentration at admission, as well as the incidence of associated infections. Treatment failures were associated with formula intolerance (Table 33). Of the 15 treatment failures that occurred (see Table 32), 10 were successfully managed. One of the failures in the Nursoy group was because of allergy to the formula. The other five children who failed treatment died (see Table 26).
| Study | Treatment arms | p-value | |||
|---|---|---|---|---|---|
| aBhutta et al. 199453 | Soy (n = 21) | KY milk (n = 19) | |||
| Clinical failures, n | 2 | 7 | NR | ||
| aNurko et al. 199756 | Chicken (n = 15) | Nursoy (n = 13) | Vivonex (n = 13) | ||
| Successful outcome, n (%) | 15 (78.9) | 13 (68.4) | 13 (72.2) | NS | |
| Nutritional recovery, n (%) | 13 (86.6) | 12 (85) | 10 (77) | NR | |
| Treatment failure, n | 4 | 6 | 5 | NR | |
| Study | Treatment arms | p-value | ||
|---|---|---|---|---|
| Nurko et al. 199756 | Chicken (n = 15) | Nursoy (n = 13) | Vivonex (n = 13) | |
| Some formula intolerance, n (%) | 9 (47.4) | 11 (57.9) | 14 (77.8) | NS |
| Intestinal pneumatosis, n | 1 | 1 | 2 | NR |
Safety outcomes
Only the study by Nurko and colleagues56 reported on safety, but there was no statistically significant difference in formula intolerance between the treatment arms (see Table 33). Of those children with formula intolerance, 15 were treatment failures (see Table 32) and four had intestinal pneumatosis (two of those with intestinal pneumatosis died; see Table 26).
Additional outcomes
Additional reported outcomes, such as protein ingested after full diet tolerance or time from diet start to failure, were reported in some studies, but have not been presented here. Further details can be seen in the data extraction forms in Appendix 9.
Summary
-
Three trials52,53,56,58 evaluated the treatment of children with persistent diarrhoea, with each trial comparing different diets. The overall methodological quality was rated as weak for two trials52,53,58 and strong for one trial. 56
-
Although all three trials employed a hospital inpatient setting, making diet intake easier to control and regulate, all three trials were judged to be open to a potential risk of bias in a number of areas and results should therefore be treated with caution.
-
There were no significant differences in mortality rates between the diets employed in the two trials reporting mortality. 52,56,58
-
None of the diets in the three included trials52,53,56,58 pertaining to children with persistent diarrhoea produced statistically significant improvements in measures of diarrhoea.
-
The majority of diets appeared to be effective in increasing weight, with two out of three trials reporting better results for the diet used in the intervention arm. In the trial by Amadi and colleagues,52,58 Neocate produced greater weight gain over a 4-week period than the standard skimmed milk/soy-based diet, which was reflected by increases in W/A and W/H z-scores, as well as weight increases in both HIV+ve and HIV–ve subgroups. The full-strength soy diet in the trial by Bhutta and colleagues53 also produced better weight gain over a 2-week period than the half-strength buffalo milk diet with rice-lentils and yoghurt given to the control group. This was again reflected by increases in W/A z-scores. In contrast, the three diets of chicken, Nursoy and Vivonex (control) employed by Nurko and colleagues56 were found to be equally effective for weight gain.
What methods are effective in treating infection? (Q7, rank 5 = )
The overarching question for this section included within it broader issues regarding antibiotic therapy (examples of these are available in Appendix 5). No study addressed the overarching question directly, but two studies59,60 were included that investigated different aspects of antibiotic therapy in children with severe malnutrition. As these addressed different questions they are presented in separate sections. Dubray and colleagues59 studied the relative effectiveness of two broad-spectrum antibiotics prescribed systematically to all participants (regardless of confirmation of infection) (see Quantity and quality of research available: different antibiotics in the inpatient setting), whereas Trehan and colleagues60 sought to determine whether or not including amoxicillin in the home-based treatment of uncomplicated severe malnutrition with RUTF led to better recovery rates than treatment with RUTF alone (see Quantity and quality of research available: antibiotic use in the outpatient setting).
Quantity and quality of research available: different antibiotics in the inpatient setting
This question was addressed by one RCT59 that met the inclusion criteria for this review. The key characteristics of this RCT are presented in Table 34, and the full data extraction form in Appendix 10 provides further details.
| Study details and target population | Intervention | Comparator |
|---|---|---|
|
Dubray et al. 200859 Design: RCT Location: TFC in Sudan Length of follow-up: not clearly stated, but appears to be until exit from TFC No. enrolled: 460 Target population: severely malnourished children with weight ≥ 5 kg and height > 65 cm and ≤ 109.9 cm (usually corresponding to age 6–59 months); displaced population |
Ceftriaxone (75 mg/kg/day) administered via i.m. injection once daily for 2 days Selected baseline characteristics Mean age ± SD: 17 ± 7 months Sex F : M, %: 48 : 52 W/H < 70% of median,a n (%): 169 (74.1) MUAC < 110 mm,b n (%): 36 (15.8) W/A: NR Fever (≥ 37.5°C), n (%): 70 (30.7) Moderate dehydration, n (%): 33 (14.5) Paracheck positive,c n (%): 4 (1.9) |
Amoxicillin (80 mg/kg/day) administered orally twice daily over 5 days Selected baseline characteristics Mean age ± SD: 18 ± 8 months Sex F : M, %: 45 : 55 W/H < 70% of median,a n (%): 166 (72.1) MUAC < 110 mm,b n (%): 36 (15.7) W/A: NR Fever (≥ 37.5°C), n (%): 67 (29.1) Moderate dehydration, n (%): 23 (10.1) Paracheck positive,c n (%): 2 (0.9) |
Dubray and colleagues59 conducted a randomised, unblinded superiority controlled trial to compare two antibiotic regimens in a therapeutic feeding centre (TFC) in Sudan. This was a single-centre trial funded by an international humanitarian medical aid organisation. Systemic broad-spectrum antibiotic therapy was provided on admission to all participants (with or without any signs of clinical infection), with the aim of improving the outcomes of SAM (reduce mortality and improve nutritional response to feeding). Four hundred and sixty children with SAM were randomly allocated to either ceftriaxone (the intervention, n = 230) or amoxicillin (the comparator, n = 230). Children were eligible to participate if they presented with a W/H < 70% of the reference median [NCHS/Center for Disease Control (CDC) 1977 growth reference curves18] and/or bilateral oedema and/or MUAC < 110 mm. In addition, eligible children had to weigh at least 5 kg and have a height within the range of > 65 cm to ≤ 109.9 cm. Children whose parents refused permission to participate were excluded from the study, as were children who had undertaken treatment with any of the study drugs or had been admitted to any health facility for SAM in the 7 days before admission, children with known hypersensitivity to amoxicillin or ceftriaxone, children whom the physician decided to treat using a different antimicrobial drug on admission and children with acute otitis media (AOM) or severe complications diagnosed on admission.
All participants received the same nutritional rehabilitation and care (further details in Appendix 10). The intervention group received a once-daily intramuscular (i.m.) injection of ceftriaxone at a dose of 75 mg/kg/day for 2 days, whereas the comparator group was given oral amoxicillin (80 mg/kg/day) twice daily over 5 days. When necessary, a second antibiotic (ceftriaxone, chloramphenicol, cotrimoxazole, amoxicillin or metronidazole) was administered as per the TFC protocol.
Dubray and colleagues59 reported that baseline characteristics did not differ significantly between groups. The mean age of the participants was approximately 17 months and just over half of the trial participants were male. More than 70% of the participants had W/H < 70% of the median, 15% had a MUAC measurement of < 110 mm and at least 10% had bilateral oedema. Though there was no diagnostic confirmation of infection, approximately 30% of the participants had fever (≥ 37.5 °C), 1–2% tested positive for malaria, more than 17% presented with an abnormal respiratory rate and at least 10% were moderately dehydrated.
The reported primary outcome was the proportion of children with a weight gain increase of at least 10 g/kg/day calculated over a 14-day period starting on the first day of weight gain after admission. Additionally, the authors considered secondary outcomes such as the recovery rate of discharged children, overall case fatality ratios (CFRs), defaulter rate, referral (to another medical facility) rate and the occurrence of adverse events.
Summary of quality assessment
Although 230 participants were randomly allocated to the ceftriaxone group, two of these were secondarily excluded; thus, only 228 were included in the analyses. The authors state that an ITT analysis was conducted, given that all children who had received at least one dose of the study drug were included. However, because of the post-randomisation exclusion of two participants, this was judged not to be a full ITT analysis during quality assessment.
Dubray and colleagues’ study59 was rated moderate in terms of its overall methodological quality, as shown in Table 35. More than 80% of the selected individuals, who are very likely to be representative of the target population, participated in the RCT. The use of a computer-generated block randomisation method and sealed envelopes for allocation was appropriate. Additionally, there were no important baseline differences between groups and the number and reasons for withdrawals and dropouts were reported per group. Therefore, this study was considered strong regarding the selection bias, study design, confounders and the withdrawals and dropouts components of quality assessment. Despite using valid data collection tools, the reliability of the tools is not reported, and, hence, the study strength on data collection methods was rated moderate. For the blinding component, the study was judged to have weak methodological strength because neither outcome assessors nor participants were blinded. Considering that the consistency of the intervention was measured, that 60–79% of the participants received the allocated intervention and that they are not likely to have received an unintended intervention, the intervention integrity is considered to have been ensured. Furthermore, the analysis performed was found to be appropriate for the study design, despite the shortcomings of the ITT analysis.
| Study | Selection bias | Study design (description) | Confounders | Blinding | Data collection methods | Withdrawals and dropouts | Intervention integrity | Analysis | Global ratinga | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Per cent who received allocation | Consistency measured? | Unintended intervention likely? | Unit of allocation | Unit of analysis | Appropriate statistical methods? | ITT analysis? | ||||||||
| Dubray et al. 200859 | S | S (RCT) | S | W | M | S | 60–79 | Yes | No | Patient | Patient | Yes | Nob | M |
Assessment of effectiveness: different antibiotics in the inpatient setting
Mortality
Dubray and colleagues59 reported several mortality-related secondary outcomes, based on an analysis that excluded two participants who had been randomised, but who did not receive any treatment. As can be seen in Table 36, fewer deaths occurred in the ceftriaxone group, not only within 14 days after admission but also during the whole follow-up period to discharge from the TFC. However, the difference in total deaths during follow-up was not statistically significant (p = 0.62) and no p-value was reported for the former. The 13 deaths that occurred during the first 14 days were because of septic shock (n = 5), lower respiratory tract infections (n = 3), fluid overload (n = 4) and severe dehydration (n = 1).
| Study | Treatment arms | p-value | |
|---|---|---|---|
| Dubray et al. 200859 | Ceftriaxone (n = 228) | Amoxicillin (n = 230) | |
| Deaths within 14 days after admission,a n (%) | 5 (2.2) | 8 (3.5) | NR |
| Total deaths during follow-up,b n (%) | 7 (3.1) | 9 (3.9) | 0.62 |
| Overall CFR | 3.5% (16 deaths in 458 participants) | ||
Weight gain
Table 37 presents the primary outcomes on weight gain from the Dubray and colleagues59 study, which were success rate and mean overall weight gain, as well as a secondary outcome of weight gain at exit from TFC. The reported success rate is defined as a weight gain ≥ 10 g/kg/day by day 14 or discharge before 14 days of weight gain because the TFC exit criteria were met (maintained W/H ≥ 85% for 7 consecutive days). Mean overall weight gain was calculated 14 days after the first weight gain. The groups showed similar results and no statistically significant differences were found between groups for any of the outcomes.
| Study | Treatment arms | p-value | |
|---|---|---|---|
| Dubray et al. 200859 | Ceftriaxone (n = 228) | Amoxicillin (n = 230) | |
| Success rate, n (%) | 127 (55.7) | 123 (53.5) | 0.63, difference: 2.2% (95% CI –6.9% to 11.3%) |
| Mean overall weight gain (95% CI), g/kg/day | 11.4 (10.5 to 12.2) | 11.2 (10.2 to 11.9) | 0.69 |
| Mean weight gain at exit from TFC (95% CI), g/kg/day | 10.2 (9.7 to 10.7) | 10.2 (9.4 to 11.0) | 0.50 |
Length of stay and reasons for exit from the therapeutic feeding centre
As shown in Table 38, the authors reported a slightly shorter length of stay for the ceftriaxone group, but the difference from the amoxicillin control group was not statistically significant. No statistically significant differences were found on the reasons for exit either.
| Study | Treatment arms | p-value | |
|---|---|---|---|
| Dubray et al. 200859 | Ceftriaxone (n = 228) | Amoxicillin (n = 230) | |
| Mean length of stay (95% CI), days | 31.4 (29.4 to 33.3) | 33.5 (31.5 to 35.5) | 0.07 |
| Reasons for exit from TFC, n (%) | |||
| Recovered | 170 (74.6) | 161 (70) | 0.27 |
| Defaulted | 43 (18.9) | 39 (17.0) | 0.59 |
| Referred | 2 (0.9) | 4 (1.7) | 0.68 |
Infection-related deaths and adverse events
Dubray and colleagues59 reported the number of infection-related deaths per type of infection and adverse effects attributed to antibiotics (Table 39). A statistically significant lower rate of adverse events was found in the ceftriaxone group (p = 0.05).
| Study | Treatment arms | p-value | |
|---|---|---|---|
| Dubray et al. 200859 | Ceftriaxone (n = 228) | Amoxicillin (n = 230) | |
| Infection-related deaths after 14 days from admission, n (day after admission)a | |||
| Meningoencephalitis syndrome | 1 (26th) | 0 | NR |
| Severe respiratory infection | 0 | 1 (30th) | NR |
| Pulmonary TB | 1 (50th) | 0 | NR |
| Adverse events, n (%)b | 2 (0.88) | 8 (3.5) | 0.05 |
| Vomiting | 1 | 1 | NR |
| Diarrhoea | 1 | 6 | NR |
| Facial oedema (allergic reaction) | 0 | 1 | NR |
Summary
-
One RCT59 that compared ceftriaxone (i.m.) with oral amoxicillin met the inclusion criteria of the review for this question. The RCT’s methodological quality was summarised as moderate, mainly owing to the fact that blinding of the outcome assessors or the participants was not carried out.
-
Mortality was a secondary outcome of the RCT. Dubray and colleagues59 did not find a statistically significant difference in mortality between the ceftriaxone and amoxicillin groups. Similarly, no statistically significant differences in the number of recovered patients, weight gain, length of stay or reasons for exit from the TFC were found either.
-
A statistically significant lower rate of adverse events was found in participants receiving ceftriaxone (p = 0.05) than in those receiving amoxicillin.
-
No data on resolution of existing infections, development of new infections, relapse or development of antibiotic resistance outcomes were reported.
-
The criteria used to define SAM were broadly in line with current WHO criteria, hence, results are likely to be generalisable to the SAM populations identified by WHO criteria. However, the generalisability to settings where HIV prevalence is high and where children may be receiving long-term cotrimoxazole prophylaxis is uncertain.
-
More than 25% of children in each group received a second antimicrobial treatment (ceftriaxone, chloramphenicol, cotrimoxazole, amoxicillin or metronidazole, in accordance with TFC treatment protocols), which may have reduced the evidence of difference between groups.
-
The study site was chosen because the working conditions were satisfactory, the centre adhered to international standards of nutritional rehabilitation programmes and the political situation was stable. Centres with poorer operational conditions might not be able to reach the same level of care, which might adversely affect outcomes.
Quantity and quality of research available: antibiotic use in the outpatient setting
The key characteristics of the single retrospective cohort study investigating this question are presented in Table 40, and the full data extraction form in Appendix 10 provides further details.
| Study details and Target population | Intervention cohort | Comparator cohort |
|---|---|---|
|
Trehan et al. 201060 Design: retrospective cohort with control Location: home-based treatment in Malawi Length of follow-up: between 4 and 12 weeks No. enrolled: 2453 Target population: severely malnourished children aged 6–59 months with W/H z-score ≤ –3 and/or the presence of bilateral pitting oedema (WHO 1999)10 |
Amoxicillin (60 mg/kg/day) for 7 days + RUTF (175 kcal/kg/day) Selected baseline characteristics Mean age ± SD: 25.5 ± 11.7 months Sex F : M, %: 49.4 : 50.6 Mean W/H z-score ± SD: –1.99 ± 1.26 MUAC: NR Mean W/A z-score ± SD: –3.51 ± 1.20 |
RUTF (175 kcal/kg/day) Selected baseline characteristics Mean age ± SD: 22.3 ± 10.6 months Sex F : M, %: 50.4 : 49.6 Mean W/H z-score ± SD: –1.91 ± 1.45 MUAC: NR Mean W/A z-score ± SD: –3.05 ± 1.36 |
Trehan and colleagues60 conducted a retrospective analysis of outcomes from two cohorts of children in Malawi to determine whether or not including amoxicillin in the home-based treatment of uncomplicated SAM with RUTF led to better recovery rates than treatment with RUTF alone. The study was funded by a US government department. The data were obtained for the same time period from two different feeding projects, one operating in one district of Malawi, the other operating in two other districts of Malawi (the number of feeding centres in each district was not reported). Data from anonymised records of 2453 children who had qualified for outpatient treatment of SAM were included: 1955 children in one cohort had received RUTF alone and 498 children in the second cohort received amoxicillin in addition to RUTF. SAM was defined as W/H z-score ≤ –3 and/or the presence of bilateral pitting oedema. To be eligible for outpatient treatment, children in both cohorts needed to have uncomplicated SAM and a good appetite. Children with poor appetite, altered mental status, compromised perfusion or respiratory distress or who were being transferred from inpatient to outpatient therapy were excluded.
The intervention cohort received a 7-day supply of amoxicillin, equivalent to approximately 60 mg/kg/day, and RUTF to provide 175 kcal/kg/day. Children in the comparison cohort (who met the same criteria for outpatient treatment described above), received the same RUTF provision, but did not receive any antibiotics. In both cohorts RUTF was given until children reached a W/H z-score ≥ –2 with no peripheral oedema for a minimum of 4 weeks and a maximum of 12 weeks. Caregivers of the children in both cohorts were educated about the child’s illness and instructed on optimal feeding practices. They were also referred to local health providers with any concerns about other acute illnesses.
The primary outcome was the nutritional recovery rate, with recovery defined as W/H z-score ≥ –2 and no peripheral oedema. Secondary outcomes were survival, W/H z-scores, W/A z-scores, H/A z-scores and presence of oedema.
Summary of quality assessment
Trehan and colleagues’ retrospective analysis of two cohorts60 was rated moderate in terms of its overall methodological quality (Table 41). The study was judged to be at a low risk of selection bias (rated strong for selection bias), but because this was a cohort with control study a moderate rating was applied for study design. Although there were some important differences between the cohorts prior to the intervention, the study authors indicated that these were taken into account in the analysis, which enabled the confounders section of the quality assessment to be judged strong. Although study participants were not aware of the research question, the outcome assessors knew what treatment participants had received, which led to a moderate rating for the blinding section. The data collection methods were rated weak because tools were not reported to be either valid or reliable. The final section contributing to the global rating, withdrawals and dropouts, was rated strong because 80–100% of participants completed the study, so the risk of bias owing to missing data was considered to be low. The intervention integrity is difficult to determine because the consistency of the intervention did not appear to have been measured, and it was not possible to tell whether or not any unintended intervention could have occurred in either cohort. There was also some uncertainty regarding the analysis of the data. The study was powered to detect a difference of at least 5% in the recovery rate.
| Study | Selection bias | Study design (description) | Confounders | Blinding | Data collection methods | Withdrawals and dropouts | Intervention integrity | Analysis | Global ratinga | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Per cent who received allocation | Consistency measured? | Unintended intervention likely? | Unit of allocation | Unit of analysis | Appropriate statistical methods? | ITT analysis? | ||||||||
| Trehan et al. 201060 | S | M (CA) | S | M | W | S | 80–100 | No | ? | Organisation/institution | Patient | ? | ? | M |
Assessment of effectiveness: antibiotic use in the outpatient setting
Mortality
Mortality was one of the secondary outcomes of the Trehan and colleagues study. 60 The total number of deaths was reported at 4 weeks and at 12 weeks for the overall number of participants in each cohort, but also reported separately for those with and without oedema at baseline (Table 42). The rates of death at both time points were described as similar for each group.
| Study | Cohorts | p-value | |
|---|---|---|---|
| Trehan et al. 201060 | Amoxicillin + RUTF (n = 498) | RUTF (n = 1955) | |
| Deaths at 4 weeks follow-up, n (%) | |||
| Overall | 10 (2.0) | 26 (1.3) | NR |
| With oedema | 8 (2.1) | 16 (1.0) | NR |
| Without oedema | 2 (1.8) | 10 (2.6) | NR |
| Deaths at 12 weeks follow-up, n (%) | |||
| Overall | 13 (2.6) | 34 (1.7) | NR |
| With oedema | 10 (2.6) | 19 (1.2) | NR |
| Without oedema | 3 (2.7) | 15 (3.9) | NR |
Recovery
Recovery was defined as a W/H z-score ≥ –2 and no peripheral oedema (Table 43). Those who remained alive but did not meet the criteria for recovery were classed as remaining malnourished, and those who missed two follow-up visits were categorised as defaulters. At the 4-week follow-up, a greater proportion of children in the RUTF-only cohort had recovered in comparison with the cohort receiving amoxicillin and RUTF (70.8% vs 39.8%; no p-value reported). A statistically significant difference (p < 0.001) in favour of the RUTF-only cohort was reported for the subgroups of children with and without oedema at baseline. In the subgroup of children who recovered after 4 weeks, the W/H z-score was significantly higher in the RUTF cohort than in the RUTF plus amoxicillin cohort (–0.37 vs –0.75; p < 0.0001).
| Study | Cohorts (4-week follow-up) | p-value | Cohorts (12-week follow-up) | p-value | ||
|---|---|---|---|---|---|---|
| Trehan et al. 201060 | Amoxicillin + RUTF (n = 498) | RUTF (n = 1955) | Amoxicillin + RUTF (n = 498) | RUTF (n = 1955) | ||
| Recovered, n (%) | ||||||
| Overall | 198 (39.8) | 1385 (70.8) | NR | 417 (83.7) | 1673 (85.6) | NR |
| With oedema | 170 (43.8) | 1206 (76.6) | p < 0.001 | 336 (86.6) | 1385 (88.0) | NR |
| Without oedema | 28 (25.5) | 179 (47.0) | p < 0.001 | 81 (73.6) | 288 (75.6) | NR |
| Remained malnourished, n (%) | ||||||
| Overall | 264 (53.0) | 423 (21.6) | NR | 29 (5.8) | 66 (3.4) | NR |
| With oedema | 191 (49.2) | 254 (16.1) | NR | 13 (3.4) | 36 (2.3) | NR |
| Without oedema | 73 (66.4) | 169 (44.4) | NR | 16 (14.5) | 30 (7.9) | NR |
| Defaulted, n (%) | ||||||
| Overall | 26 (5.2) | 121 (6.2) | NR | 39 (7.8) | 182 (9.3) | NR |
| With oedema | 19 (4.9) | 98 (6.2) | NR | 29 (7.5) | 134 (8.5) | NR |
| Without oedema | 7 (6.4) | 23 (6.0) | NR | 10 (9.1) | 48 (12.6) | NR |
At the 12-week follow-up, the overall proportion who had recovered in each cohort was described as similar (no p-value reported). Rates of defaulting were described as similar in the two cohorts at 4 and 12 weeks (no p-values reported). Therefore, the proportions of children classed as remaining malnourished were as expected, with a greater proportion remaining malnourished at the 4-week follow-up in the intervention cohort receiving amoxicillin, but more similar proportions from each cohort were malnourished by the 12-week follow-up (no p-values reported for the between group comparison at either time point).
Other outcomes
A regression analysis was conducted to compare recovery rates, while controlling for differences in baseline characteristics between the cohorts. The model based on outcomes at 4 weeks showed that age (older children) and W/H z-score (higher W/H z-score) at baseline were predictive of recovery (p < 0.001 for both), whereas receipt of amoxicillin was correlated with failure to recover at 4 weeks (OR 0.22; p < 0.001). However, the 12-week follow-up regression analysis demonstrated that none of the baseline factors considered were predictive of recovery. The W/A z-score, H/A z-score and presence of oedema were not correlated with recovery in either the 4- or 12-week analysis. Full results are available in Appendix 10.
Summary
-
One cohort study60 of moderate methodological quality compared amoxicillin plus RUTF with RUTF only for the treatment of uncomplicated SAM in children.
-
Mortality rates were < 5% and similar in both cohorts.
-
The primary outcome of the study was recovery rate (W/H z-score ≥ –2 and no peripheral oedema), which appeared substantially greater at 4 weeks in the cohort of children who did not receive amoxicillin. However, by 12 weeks the proportion of children in each cohort who had recovered was described as similar.
-
The provision of a 7-day course of amoxicillin did not improve recovery rates from uncomplicated SAM in children in Malawi in this cohort when compared with the outcomes of a cohort who did not receive amoxicillin.
What is the clinical effectiveness of interventions in different settings (e.g. hospital, community, emergency)? (Q14, rank 9)
Quantity and quality of research available: settings
Four trials62–65 that investigated the clinical effectiveness of interventions in different settings were included. All the studies were CCTs. The key characteristics of these CCTs are presented in Table 44 and Appendix 11 provides further details. These trials were conducted in Niger62 (100 participants), Malawi63 (1178 participants overall, 645 as subgroup with SAM), Jamaica64 (81 participants) and Bangladesh65 (573 participants). Two trials were single-centre64,65 and two were multicentre trials. 62,63 Heikens and colleagues64 received funding from the government of the Netherlands, Chapko and colleagues62 were partially funded by a US governmental education fellowship, Khanum and colleagues65 were supported by a UK charity and the UK government and Ciliberto and colleagues63 received funding from the United Nations, a US charity and hospital foundation, a UK humanitarian organisation and the US government.
| Study details and target population | Intervention | Comparator | ||
|---|---|---|---|---|
|
Chapko et al. 199462 Design: CCTa Location: Niger Length of follow-up: 6 months No. enrolled: 100 Target population: malnourished children ready for discharge after treatment for acute conditions with W/H < –2 SD of NCHS median or a diagnosis of kwashiorkor |
Ambulatory rehabilitation |
Total Study Population Mean age: NR Age range: 5–28 months Sex F : M, %: 46 : 54 Median W/H z-score: –3.16 W/H z-score < –3, %: 59 MUAC: NR W/A: NR |
Hospital rehabilitation | |
|
Ciliberto et al. 200563 Design: CCT Location: Malawi Length of follow-up: 6 months No. enrolled: 1178 (with 645 in the SAM subgroup) Target population: children aged 10–60 months with malnutrition (W/H < –2 SD of NCHS median, mild oedema, or both) and presenting a good appetite. Separate outcomes for the subgroup with SAM (W/H < –3 SD or oedema) |
Home rehabilitation with a 2-week supply of RUFT Total trial population Mean age ± SD: 23 ± 10 months Sex F : M, %: 47 :53 Mean W/H z-score ± SD: –2.2 ± 0.8 Mean MUAC ± SD: 11.6 ± 1.4 cm Mean W/A z-score ± SD: –3.5 ± 1.0 For the SAM subgroup (n = 532) Mean W/H z-score ± SD: –2.5 ± 1.0b |
Hospital rehabilitation or at home using additional cereal–legume supplement Total trial population Mean age ± SD: 24 ± 12 months Sex F : M, %: 47 : 53 Mean W/H z-score ± SD: –2.5 ± 0.9 Mean MUAC ± SD: 11.6 ± 1.5 cm Mean W/A z-score ± SD: –3.7 ± 1.0 For the SAM subgroup (n = 113): Mean W/H z-score ± SD: –2.5 1.1b |
||
|
Heikens et al. 199464 Design: CCTa Location: Jamaica Length of follow-up: 36 months post-admission No. enrolled: 81 Target population: children aged 3–36 months judged to require hospital admission based on W/A < 80% of NCHS median, oedema, anorexia, dermatosis or hair condition symptomatic of kwashiorkor and the need for parenteral antibiotic therapy |
Home rehabilitation supported by CHAs (following initial short stay in hospital) with weekly supply of high-energy supplement and standard care for 3 months + standard care only for a further 3 months Mean age ± SE: 10.8 ± 1.1 months Sex F : M: NR Mean % expected (NCHS) W/H ± SE: 81.6 ± 1.5 MUAC: NR Mean % expected (NCHS) W/A ± SE: 57.9 ± 1.7 |
Hospital rehabilitation (long stay) with high-energy diet until discharge followed by standard care at home for 6 months Mean age ± SE: 11.7 ± 0.9 months Sex F : M: NR Mean % expected (NCHS) W/H ± SE: 80.6 ± 1.7 MUAC: NR Mean % expected (NCHS) W/A ± SE: 60.3 ± 1.7 |
||
|
Khanum et al. 199465 Design: CCT Location: Bangladesh Length of follow-up: to attainment of 80% W/H, plus a further 12 months for those reaching 80% W/H No. enrolled: 573 Target population: children aged from 12–60 months with W/H < 60% of NCHS median, and/or oedema |
Home rehabilitation (following first 7 days in day-care facility) – visited weekly for 1 month and twice monthly from then on until reaching 80% W/H Mean age ± SD: 28 ± 13 months Sex F : M: NR Mean % expected (NCHS including oedema) W/H ± SD: 70 ± 7 MUAC: NR Mean % expected (NCHS including oedema) W/A ± SD: 51 ± 9 |
Ambulatory rehabilitation – children attended day-care facility with their mothers every day except Friday until 80% W/H reached Mean age ± SD: 26 ± 13 months Sex F : M: NR Mean % expected (NCHS including oedema) W/H ± SD: 70 ± 8 MUAC: NR Mean % expected (NCHS including oedema) W/A ± SD: 50 ± 10 |
Hospital rehabilitation – children admitted with their mothers and resident until reaching 80% W/H Mean age ± SD: 25 ± 13 months Sex F : M: NR Mean % expected (NCHS including oedema) W/H ± SD: 67 ± 7 MUAC: NR Mean % expected (NCHS including oedema) W/A ± SD: 48 ± 9 |
|
Chapko and colleagues62 compared inpatient with daily ambulatory rehabilitation, and two of the trials63,64 investigated hospital- and home-based rehabilitation (differing, however, in the level of support provided). These three trials62–64 evaluated alternative settings for the rehabilitation phase of treatment for malnourished children, after an initial phase of hospital care common to both treatment arms. In contrast, the fourth trial, by Khanum and colleagues,65 had three trial arms to compare inpatient care with daily ambulatory care for both the initial and the rehabilitation phases of treatment for children with SAM, and with home rehabilitation (after daily ambulatory care during the initial phase of treatment).
Although hospital care was one of the investigated settings in all of the included trials, the inpatient care provided differed among the trials, for instance not only were different diet formulas and number of meals administered, but staff teams also varied in their composition. Similarly, the home-based care involved in the three trials63–65 that investigated home-based rehabilitation also differed. Ciliberto and colleagues63 studied home-based care provided by caretakers and involved two weekly clinic visits at which RUTF supplies were given, whereas Heikens and colleagues’64 home-based treatment was supported by community health aides (CHAs) who were trained to offer standard health-service care. The frequency of care provided by CHAs, reported in an earlier publication that did not meet the inclusion criteria for this review,66 was unclear. Khanum and colleagues65 studied home-based care with no food supplements, and trained home visitors made home visits weekly for 1 month, then fortnightly. Trials also differed in the duration of the interventions and the length of follow-up.
Two studies62,63 included both moderately and severely malnourished children using similar criteria. Chapko and colleagues62 included children with a W/H z-score < –2 SD or kwashiorkor (not further defined) and Ciliberto and colleagues63 included children with a W/H z-score < –2 SD or mild oedema (< 0.5 cm of pitting oedema on the dorsum of the foot). However, both these studies were eligible for inclusion in this review. In the study by Chapko and colleagues,62 this was because the median W/H z-score was –3.38, with 70% of children having a z-score < –3, whereas in the study by Ciliberto and colleagues63 separate outcome data were presented for a subgroup of children with SAM (W/H z-score < –3 or presence of oedema). Heikens and colleagues64 also included both moderately and severely malnourished children judged to require hospital admission using the admission criteria of W/A < 80% of the NCHS median, oedema, anorexia, dermatosis or hair condition symptomatic of kwashiorkor and the need for treatment with parenteral antibiotics. Baseline status was described according to the Gómez,26 Wellcome67 and Waterlow classifications,29 which enabled the study to be included because the mean baseline W/A was ≤ 60% of the NCHS median. Khanum and colleagues65 included only children with SAM and used W/H < 60% of NCHS median and/or oedema as their admission criterion.
Reporting of exclusion criteria varied, with Chapko and colleagues62 not reporting exclusion criteria at all. Ciliberto and colleagues63 excluded children < 10 months of age and/or with severe oedema, systemic infection or anorexia. Heikens and colleagues64 excluded children with congenital abnormalities and/or siblings in the present study or in the authors’ community study. Khanum and colleagues’65 reasons for excluding children from entry to the study were conditions that might require > 7 days of medical supervision (see Appendix 11), age < 12 months or > 60 months and children having TB or a congenital or metabolic disorder, children whose homes were > 10 km from the unit were also excluded.
Chapko and colleagues,62 Khanum and colleagues65 and Heikens and colleagues64 did not specifically identify their primary outcomes, but their main outcomes included mortality,62,65 utilisation62 (in terms of hospital and ambulatory days), days to reach oedema-free 80% W/H65 and time to discharge,64 W/H,62,64 W/A,62,64 H/A64 treatment completion65 and weight gain. 65 Ciliberto and colleagues63 stated that their primary outcomes were successful recovery (W/H > –2 SD while remaining free of oedema), relapse or death.
Summary of quality assessment
As summarised in Table 45, the overall methodological quality for two of the trials62,63 was found to be moderate, while for the remaining two trials it was found to be weak. 64,65
| Study | Selection bias | Study design (description) | Confounders | Blinding | Data collection methods | Withdrawals and dropouts | Intervention integrity | Analysis | Global ratinga | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Per cent who received allocation | Consistency measured? | Unintended intervention likely? | Unit of allocation | Unit of analysis | Appropriate statistical methods? | ITT analysis? | ||||||||
| Chapko et al. 199462 | M | S (CCT)b | S | W | M | S | ? | No | Yes | Patient | Patient | Yes | No | M |
| Ciliberto et al. 200563 | M | S (CCT) | S | W | M | S | 80–100 | No | ? | Organisation/institution | Patient | ? | Yes | M |
| Heikens et al. 199464 | M | S (CCT)b | S | W | W | S | 80–100 | ? | ? | Patient | Patient | Yes | No | W |
| Khanum et al. 199465 | M | S (CCT) | S | W | W/Sc | M/Wc | 60–79 | Yes | ? | Patient | Patient | ? | No | W |
All four trials were judged to be at moderate risk of selection bias because, although 80–100% of the selected individuals participated in each of the four trials, their selected participants were judged as only somewhat likely to be representative of the target population. Two of the trials62,64 stated that children were randomly allocated to groups; however, during quality assessment they were judged to be CCTs (in accordance with the instructions on the use of the quality assessment tool, see Appendix 4) because the method of randomisation was not described. Nevertheless, the quality assessment tool still led to the trials being rated strong in terms of study design. Ciliberto and colleagues’ study,63 was the only trial with important differences between groups at baseline (including differences in W/H, details in Appendix 11), but as 80–100% of the relevant confounders were controlled for in the analysis, all trials were rated strong with respect to confounders.
All trials showed weak methodological quality on blinding, as the outcome assessor was not blinded in three of the studies62,64,65 (Ciliberto and colleagues63 is unclear on this matter) nor were the participants blinded in any of them. The studies by Chapko and colleagues62 and Ciliberto and colleagues63 were found to be moderate regarding data collection methods, as their tools were valid, but their reliability was not reported. The Heikens and colleagues64 study was rated weak for data collection methods because information on the validity and reliability of the methods used was not reported. Khanum and colleagues65 used valid and reliable methods for the second follow-up after an additional 12 months, and hence, their study was rated as strong; however, the validity of the tools of the initial study could not be determined and their reliability was not reported, so this initial study was classified as weak. Three of the studies had 80–100% of their participants completing the study; hence, they were rated strong on withdrawals and dropouts, in spite of the fact that only Heikens and colleagues64 reported on both the numbers and reasons for missing data.
Studies vary widely in terms of the integrity of intervention. Chapko and colleagues62 reported that some children did not receive the assigned care; in particular, 11% of those assigned to ambulatory treatment received hospital rehabilitation at the insistence of their mothers, but it was not clear whether or not any of the children assigned to hospital care did not attend. Khanum and colleagues65 reported that 60–79% of their participants received their allocated intervention, and the other two trials63,64 reported 80–100%. Consistency was reported to have been measured by one trial65 and not measured by two trials;62,63 one trial was not clear on the matter. 64 Contamination or co-intervention was likely to have occurred in Chapko and colleagues62 trial, whereas the other three studies63–65 are not clear on this aspect.
The infant/child was the unit of allocation in three trials,62,64,65 whereas allocation was established per rehabilitation centre by Ciliberto and colleagues,63 whose trial had a stepped-wedge design. The unit of analysis in all four included trials was the infant/child. Overall, statistical methods were found to be appropriate for the design of two of the included studies,62,64 but it was unclear if they were appropriate for the other two studies. 63,65 Out of the four studies, only Ciliberto and colleagues63 conducted an ITT analysis.
Assessment of effectiveness: settings
Mortality
Although mortality was a primary outcome of the Chapko and colleagues62 study and the Ciliberto and colleagues63 study, Khanum and colleagues65 reported it as a secondary outcome. None of the studies reported statistically significant differences in mortality between the different settings (Table 46). Chapko and colleagues62 reported a higher proportion of deaths in children in hospital than in the ambulatory setting. Ciliberto and colleagues63 reported only a 2.5% (95% CI –0.8% to 6.8%) difference in mortality between the groups, whereas Khanum and colleagues65 reported that mortality was low and did not differ between the groups (no p-value reported). Heikens and colleagues64 did not specify mortality as an outcome, but reported deaths among data on children lost to follow-up, and consequently no p-value was reported.
| Study | Treatment arms | p-value | |||
|---|---|---|---|---|---|
| Chapko et al. 199462 | Ambulatory (n = 47) | Hospital (n = 53) | |||
| Deaths,a % | 33 | 41 | 0.172 | ||
| Ciliberto et al. 200563 (SAM subgroup) | Home (n = 532) | Hospital (n = 113) | Difference (95% CI) | ||
| Deaths, n (%) | 20 (3.7) | 7 (6.2) | 2.5% (–0.8% to 6.8%) | ||
| Heikens et al. 199464 | Home with support (short stay) (n = 39) | Hospital (long stay) (n = 40) | p-value | ||
| Deaths, n (%)b | 2 (5.1) | 1 (2.5) | NR | ||
| Khanum et al. 199465 | Home (n = 173) | Ambulatory (n = 200) | Hospital (n = 200) | p-value | |
| Mortality at initial study,c n (%) | 6 (3.5) | 10 (5.0) | 7 (3.5) | NR | |
| Mortality at 12 months further follow-up, n/N (%)d | 2/130 (1.5) | 2/134 (1.5) | 6/173 (3.4) | NR | |
The authors of the included studies63–65 accounted for the number of deaths that occurred in both the initial and the rehabilitation phases, apart from Chapko and colleagues,62 who reported deaths in the rehabilitation period only. This study had the highest proportion of deaths.
Weight gain
One study, that by Chapko and colleagues,62 did not include weight gain as an outcome. Khanum and colleagues65 reported the mean weight gain from admission to 80% W/H as a primary outcome and weight gain after an additional 12 months’ follow-up as a secondary outcome (Table 47). A statistically significant difference in the primary outcome was found (p < 0.001), with inpatient care resulting in a greater daily mean weight gain from admission to the point at which participants reached 80% W/H than either home care or day care. However, after an additional 12 months of follow-up for all participants who reached 80% W/H, no significant differences in weight gain were apparent. Heikens and colleagues64 stated either that the rates of weight gain were similar or that there was no difference between the groups at the different treatment stages, but it is not clear whether or not any formal statistical testing was conducted and no p-values were reported. The exception was the average final rate of weight gain before discharge of 6–7 g/kg/day. The authors stated that this was maintained over a longer period for the long-stay group, but presented no data. Ciliberto and colleagues63 reported a non-statistically significant difference in the rate of weight gain for children at home compared with hospital during the first 4 weeks of the study. However, Ciliberto and colleagues63 performed a multivariate regression analysis, which showed that the overall rate of weight gain was 1.4 (95% CI 1.1 to 1.7) times as great among the severely malnourished children in those subject to home-based therapy than in those who received standard therapy at the rehabilitation unit.
| Study | Treatment arms | p-value | |||
|---|---|---|---|---|---|
| Ciliberto et al. 2005,63 (SAM subgroup) | Home (n = 532) | Hospital (n = 113) | Difference (95% CI) | ||
| Mean rate of weight gain during first 4 weeks ± SD, g/kg/day | 3.7 ± 4.3 | 3.0 ± 8.8 | 0.7 (–0.4 to 1.8) | ||
| Heikens et al. 199464 | Home with support (short stay) (n = 39) | Hospital (long stay) (n = 40) | p-value | ||
| Rate of weight gain, g/kg/day | NR | ||||
| Range on first 14 days | –8 to 24a | –8 to 24a | |||
| Third and fourth weeks | 12.1 | 10.4 | NR | ||
| Fifth week onwards | 6 to 7 | 6 to 7 | NR | ||
| 3-months post discharge (range) | 1.05 (–4 to 7) | 1.13 (–4 to 7) | NR | ||
| 6-months post discharge | ∼ 0.85 | ∼ 0.85 | NR | ||
| Khanum et al. 199465 | Home (n = 173) | Ambulatory (n = 200) | Hospital (n = 200) | p-value | |
| Mean weight gain from admission to 80% W/H, g/kg/day | 4 | 6 | 11 | < 0.001 | |
| Mean weight gain ± SD at 12 months of follow-up, kg | 2.47 ± 1.13 (n = 106) | 2.39 ± 0.98 (n = 111) | 2.15 ± 1.12 (n = 118) | NS | |
Anthropometry
Chapko and colleagues62 reported W/H data in line graphs separately for those who died and for those who survived. Within both groups, there was no significant difference between the ambulatory-based and hospital-based groups (no p-value reported). Heikens and colleagues64 reported measures of W/H, W/A and H/A at discharge, after 6 months’ home care (end of intervention) and then at 6-month intervals to 36 months post admission (groups altered in size at later time points; see Appendix 11). At discharge, the hospital (long-stay) group had a better W/H z-score than the home (short-stay) group and the difference between the groups was statistically significant [mean z-score ± standard error (SE): long-stay group –0.49 ± 0.11 vs –1.17 ± 0.16 in the short-stay group; p = 0.001]64 (Table 48). However, 6 and 12 months later, the difference between the groups was no longer statistically significant (p-values 0.105 and < 0.1, respectively), and the difference between the groups narrowed further at 18 months and at later time points (no p-values reported). Ciliberto and colleagues63 found a statistically significantly higher proportion of patients with W/H > –2 SD after 8 weeks of treatment in the group under home-based therapy than in the inpatient group.
| Study | Treatment arms | p-value | |
|---|---|---|---|
| Ciliberto et al. 200563 (SAM subgroup) | Home (n = 532) | Hospital (n = 113) | Difference (95% CI) |
| W/H > –2 SD after 8 weeks, n (%) | 382 (72) | 55 (49) | 21% (10% to 32%) |
| Mean rate of MUAC gain during first 4 weeks ± SD, mm/day | 0.42 ± 0.71 | 0.28 ± 0.44 | 0.14 (0.04 to 0.24) |
| Heikens et al. 199464 | Home with support (short stay) (n = 39) | Hospital (long stay) (n = 40) | p-value |
| NCHS z-scores, mean ± SE | |||
| W/H | |||
| Dischargea | –1.17 ± 0.16 | –0.49 ± 0.11 | 0.001 |
| 6 months | –0.80 ± 0.16 | –0.46 ± 0.14 | 0.105 |
| 12 monthsb | –1.00 ± 0.40 | –0.60 ± 0.30 | < 0.1 |
| 18 monthsb | –0.95 ± 0.30 | –0.75 ± 0.30 | NR |
| 24 monthsb | –0.95 ± 0.35 | –0.75 ± 0.30 | NR |
| 30 monthsb | –0.70 ± 0.30 | –0.80 ± 0.30 | NR |
| 36 monthsb | –0.65 ± 0.35 | –0.55 ± 0.30 | NR |
| W/A | |||
| Dischargea | –3.38 ± 0.16 | –2.49 ± 0.12 | 0.001 |
| 6 months | –2.45 ± 0.15 | –1.81 ± 0.16 | 0.006 |
| 12 monthsb | –2.3 ± 0.45 | –1.55 ± 0.30 | < 0.001 |
| 18 monthsb | –2.05 ± 0.40 | –1.40 ± 0.30 | < 0.001 |
| 24 monthsb | –1.90 ± 0.35 | –1.20 ± 0.30 | < 0.01 |
| 30 monthsb | –1.45 ± 0.30 | –1.20 ± 0.30 | NS |
| 36 monthsb | –1.30 ± 0.25 | –1.25 ± 0.45 | NS |
| H/A | |||
| Dischargea | –3.52 ± 0.22 | –3.02 ± 0.18 | 0.086 |
| 6 months | –2.82 ± 0.18 | –2.38 ± 0.17 | 0.059 |
| 12 monthsb | –2.60 ± 0.60 | –1.80 ± 0.35 | < 0.05 |
| 18 monthsb | –2.20 ± 0.45 | –1.10 ± 0.40 | < 0.001 |
| 24 monthsb | –1.85 ± 0.50 | –0.95 ± 0.40 | < 0.01 |
| 30 monthsb | –1.40 ± 0.40 | –0.80 ± 0.40 | < 0.05 |
| 36 monthsb | –1.20 ± 0.40 | –0.95 ± 0.40 | NR |
Weight-for-age and H/A outcomes were reported only by Heikens and colleagues. 64 Statistically significantly higher z-scores were reported for the long-stay group up to 24 months (W/A) or 30 months (H/A), but not thereafter (see Table 48 and Appendix 11).
Only Ciliberto and colleagues63 reported MUAC gain, finding a statistically significantly higher rate of MUAC gain during the first 4 weeks in the group under home-based therapy compared with the inpatient group.
Completion of treatment
Khanum and colleagues65 reported a significantly longer period of time to achieve 80% W/H in the group treated at home than in the group receiving hospital or ambulatory care (Table 49). The hospital (long-stay) group in the Heikens and colleagues64 study was considered to have completed treatment and was discharged when 95–100% W/H was reached (a mean ± SE of 39.45 ± 2.35 days post admission), but these data were not presented for the group that received care at home.
Relapse
Ciliberto and colleagues63 presented the composite outcome of children who relapsed or died (Table 50). It is presumed (although not explicitly stated) that this outcome incorporates the deaths already reported in Table 46, indicating, therefore, that 12 children (10.6%) in the hospital group relapsed, in comparison with 33 (6.2%) in the home group. A multivariate regression analysis was conducted to control for a range of covariates, and this indicated a statistically significantly lower probability of relapse or death [0.5 times (95% CI 0.3 times to 0.7 times)] in the subgroup of SAM children who received home-based therapy with RUTF compared with those receiving standard care at hospital. Khanum and colleagues65 reported on those who were readmitted during the 12-month follow-up. Children were readmitted if they relapsed (became oedematous or were < 60% W/H) or because of medical emergencies. Overall, there were eight readmissions (1.8% of the 437 children followed up), of which 0.6% occurred because of relapse. Data were not presented separately for relapse in each group, but Khanum and colleagues65 stated that relapse did not differ among the groups.
| Study | Treatment arms | |||
|---|---|---|---|---|
| Ciliberto et al. 2005,63 (SAM subgroup) | Home (n = 532) | Hospital (n = 113) | Difference (95% CI) | |
| Children relapsed or died, n (%) | 53 (10) | 19 (16.8) | 6.8% (0.3% to 24.7%) | |
| Khanum et al. 199465 | Home (n = 173) | Ambulatory (n = 200) | Hospital (n = 200) | p-value |
| Readmitted to unit at the 12-month follow-up, n/N (%)a | 3/130 (2.3) | 2/134 (1.5) | 3/173 (1.7) | NR |
Additional outcomes
Other outcomes such as height gain, oedema loss and prevalence of fever, cough or diarrhoea were reported by some studies, but details have not been presented here. Full details are available in the data extraction forms in Appendix 11.
Summary
-
One moderate-quality CCT was found comparing ambulatory care with hospital care. 62 Two other included CCTs involved home- and hospital-based therapy (one of them was graded moderate,63 whereas the other CCT’s methodological quality was considered weak64), and another methodologically weak CCT65 compared the three settings.
-
Only one trial63 undertook an ITT analysis. There is the possibility, therefore, that the results of the remaining trials62,64,65 are at a higher risk of bias and, consequently, the intervention effect may not have been accurately captured.
-
None of the included studies reported a significant difference in mortality between groups.
-
Conflicting results were obtained for weight gain. No significant differences in weight gain during the first 4 weeks,63 at 12 months of follow-up65 or in the different stages up to 6 months after discharge64 were found between settings. However, a separate multivariate regression analysis in one trial found that overall rate of weight gain was greater among children in the home-based group than in those receiving standard therapy at the rehabilitation unit. 63 In contrast, two studies of weaker quality64,65 reported that inpatient care presented a statistically significantly higher mean weight gain to 80% W/H than home or ambulatory care,65 and the final average rate of weight gain was maintained over a longer period in the inpatient group than in the home-care group. 64
-
There was no significant difference during a 6-month follow-up period in W/H between the ambulatory and hospital-based groups in one study. 62 However, two studies63,64 showed conflicting results for the comparison of hospital and home care. According to Heikens and colleagues,64 the inpatient group showed statistically significant improvement in W/H at discharge compared with the home-based group (supported by CHAs). In contrast, Ciliberto and colleagues’63 home-based group (visiting the rehabilitation centre fortnightly) included a statistically significantly higher proportion of patients with W/H > –2 SD after 8 weeks of treatment than in the inpatient group.
-
Statistically significantly higher W/A and H/A z-scores were found after hospital-based treatment than after home care with support of CHAs. 64 In contrast, another trial reported a statistically significantly higher rate of MUAC gain during the first 4 weeks in the group under home-based therapy than in the inpatient group. 63
-
Most studies defined SAM with criteria similar to those currently used by the WHO or analysed a subgroup that met them. 62,63,65 It is not clear, however, whether or not the participants in Heikens and colleagues’ study64 would meet current WHO criteria.
-
Studies varied in the care provided, even if the same setting is considered. For instance, studies on home rehabilitation63–65 involved different diets, time and frequency of contact with nutritional rehabilitation centres/staff. Additionally, Chapko and colleagues62 point out that nutritional rehabilitation differed between ambulatory centres, and between the hospital and the ambulatory centres. Ciliberto and colleagues63 provide no indication regarding similarities or differences between centres.
Which methods for correcting micronutrient deficiencies are effective? (Q8, rank 10)
Thirteen trials68–82 were included that investigated the efficacy of treatments for correcting micronutrient deficiencies in children with SAM. Any supplements or combinations of supplements were eligible for inclusion, providing other review inclusion criteria (e.g. reported outcomes) were met. Within this section, 10 trials68–79 (12 publications) investigating zinc supplements have been grouped together for ease of comparison between studies (see Quantity and quality of research available: zinc and Assessment of effectiveness: zinc). The remaining three trials,80–82 each focus on different interventions: potassium,80 nicotinic acid81 and nucleotides (NTs)82 (see Quantity and quality of research available: other supplements and Assessment of effectiveness: other supplements).
Quantity and quality of research available: zinc
A summary of the key characteristics of the 10 trials68–79 can be seen in Table 51, with further details of the trials in Appendix 12. Three trials took place in Bangladesh,68,69,73,77 two in India,72,78 and one trial each in Pakistan,79 Kenya,70 Jamaica,71 South Africa74,75 and Chile. 76 Two studies were RCTs,68,69,79 the remaining eight were CCTs70–78 and all were conducted at a single centre. Three studies did not report on how the study was funded,72,73,78 one study was funded by a commercial research grant programme76 and another study funded from a US academic source. 79 The remaining five studies received funding from a combination of a commercial and an academic source (two studies68,69,74,75 with academic sources either in the UK68,69 or in South Africa74,75), or an academic source and a government department (both in Kenya),70 or an academic source and a charity (both based in the UK). 71,77
| Study details and target population | Study arms | |||
|---|---|---|---|---|
|
Design: RCT Location: Bangladesh Length of follow-up: 90 days No. enrolled: 141 Target population: children aged 6–36 months and with W/A < 60% of NCHS median, with nutritional oedema or both |
Elemental zinc (as zinc sulphate in suspension) 1.5 mg/kg body weight administered by syringe for 15 days followed by placebo for 15 days Mean age ± SD: 15.5 ± 8.7 months Sex F : M: NR Mean W/H z-score ± SD: –2.56 ± 0.97 MUAC: NR Mean W/A z-score ± SD: –4.47 ± 0.91 Baseline zinc: NR |
Elemental zinc (as zinc sulphate in suspension) 6.0 mg/kg body weight administered by syringe for 15 days followed by placebo for 15 days Mean age ± SD: 15.0 ± 9.0 months Sex F : M: NR Mean W/H z-score ± SD: –2.73 ± 0.90 MUAC: NR Mean W/A z-score ± SD: –4.56 ± 0.98 Baseline zinc: NR |
Elemental zinc (as zinc sulphate in suspension) 6.0 mg/kg body weight administered by syringe for 30 days Mean age ± SD: 16.3 ± 8.6 months Sex F : M: NR Mean W/H z-score ± SD: –2.71 ± 0.93 MUAC: NR Mean W/A z-score ± SD: –4.66 ± 0.86 Baseline zinc: NR |
|
|
Gatheru et al. 198870 Design: CCT Location: Kenya Length of follow-up: 10 days No. enrolled: 82 Target population: children aged 1–3 years with kwashiorkor (Wellcome classification) |
Elemental zinc (as zinc sulphate solution) 5 mg/kg body weight per day given in three divided doses for the duration of treatment Mean age: NR Sex F : M, %: 52 : 48a W/H: NR MUAC: NR W/A: NR |
No zinc supplement Mean age: NR Sex F : M, %: 42 : 58a W/H: NR MUAC: NR W/A: NR |
||
| Mean baseline serum zinc (range) reported for the whole study population: 6.4 (4.0–12.9) μmol/l | ||||
|
Golden et al. 199271 Design: CCT Location: Jamaica and West Indies Length of follow-up: 6 weeks No. enrolled: 11 Target population: severely wasted boys (Wellcome classification) aged 6–31 months |
High: 10 mg (153 μmol) zinc per kg feed (as zinc acetate) + basic diet Mean age ± SE: 13 ± 4 months Sex F : M, %: 0 : 100 Mean % expected W/L ± SEM: 61 ± 2 MUAC: NR W/A: NR Mean baseline plasma zinc ± SE: 9.9 ± 1.3 μmol |
Moderate: 5 mg (76 μmol) zinc per kg feed (as zinc acetate) + basic diet Mean age ± SE: 15 ± 2 months Sex F : M, %: 0 : 100 Mean% expected W/L ± SEM: 60 ± 4 MUAC: NR W/A: NR Mean baseline plasma zinc ± SE: 11.1 ± 1.4 μmol |
Low: basic diet alone [3.5 mg (54 μmol) zinc per kg feed] with no zinc supplement Mean age ± SE: 18 ± 4 months Sex F : M, %: 0 : 100 Mean % expected W/L ± SEM: 63 ± 2 MUAC: NR W/A: NR Mean baseline plasma zinc ± SE: 9.6 ± 1.9 μmol |
|
|
Hemalatha et al. 199372 Design: CCTb Location: India Length of follow-up: 1 month No. enrolled: 33 Target population: children aged 1–5 years with W/A < 60% of that expected compared with the NCHS |
Elemental zinc, 40 mg each day in capsule form (as zinc sulphate) estimated to be about 6 mg/kg body weight per day for 21 days Mean age: NR Sex F : M: NR W/H: NR MUAC: NR W/A: NR Mean baseline plasma zinc ± SE: 80.4 ± 9.972 μg/dl |
Placebo capsule Mean age: NR Sex F : M: NR W/H: NR MUAC: NR W/A: NR Mean baseline plasma zinc ± SE: 83.6 ± 10.363 μg/dl |
||
|
Khanum et al. 198873 Design: CCT Location: Bangladesh Length of follow-up: 36 days No. enrolled: 60 Target population: children aged from 5 months to 5 years with oedema and/or with ≤ 60% W/H as a percentage of the Harvard reference |
Zinc at 10 mg/kg/day (as zinc sulphate) if body weight < 6 kg, 50 mg per day for those > 6 kg; provided on days 15–36, mode of delivery not specified Mean age: 29 months Sex F : M: states equally represented, but numbers NR Mean % expected W/H ± SEM: 70 ± 1.3 MUAC: NR Mean % expected W/A ± SEM: 50.3 ± 1.61 Mean baseline plasma zinc ± SE: 8.23 ± 0.7 mmol/l |
No zinc supplement Mean age: 29 months Sex F : M: states equally represented, but numbers NR Mean % expected W/H ± SEM: 67 ± 1.3 MUAC: NR Mean % expected W/A ± SEM: 47.6 ± 1.60 Mean baseline plasma zinc ± SE: 7.90 ± 0.7 mmol/l |
||
|
Design: CCTb Location: South Africa Length of follow-up: 90 days (post-discharge) No. enrolled: 300 Target population: children aged 6–60 months with PEM (Wellcome classification) or > 80% of expected W/A with signs and symptoms of kwashiorkor |
Zinc 10 mg/day (as zinc sulphate suspension) administered in drop form from admission to 90 days post-discharge Mean age: NR Sex F : M, %: 51 : 49 W/H: NR Mean MUAC ± SD: 11.8 ± 1.6 cm W/A: mean value NR Mean baseline serum zinc ± SD: 6.23 ± 1.83 μmol/l |
Placebo suspension Mean age: NR Sex F : M, %: 49 : 51 W/H: NR Mean MUAC ± SD: 11.9 ± 1.8 cm W/A: mean value NR Mean baseline serum zinc ± SD: 6.25 ± 1.74 μmol/l |
||
|
Schlesinger et al. 199276 Design: CCT Location: Chile Length of follow-up: 105 days No. enrolled: 39 Target population: marasmic infants < 1 year of age |
Zinc (as zinc chloride) at a concentration of 15 mg/l in infant formula for 105 days Mean age ± SD: 7.05 ± 2.0 months Sex F : M, %: 47 : 53a Mean W/H z-score ± SD: –0.83 ± 0.6 MUAC: NR Mean W/A z-score ± SD: –3.13 ± 0.71 Mean baseline serum zinc ± SD: 19.4 ± 5.5 μmol/l |
Infant formula containing 3.2 mg/l zinc Mean age ± SD: 8.1 ± 3.0 months Sex F : M, %: 50 : 50a Mean W/H z-score ± SD: –1.18 ± 0.81 MUAC: NR Mean W/A z-score ± SD: –3.21 ± 0.87 Mean baseline serum zinc ± SD: 23.4 ± 8.4 μmol/l |
||
|
Simmer et al. 198877 Design: CCT Location: Bangladesh Length of follow-up: 2 weeks No. enrolled: 25 Target population: children aged 1–7 years with nutritional oedema or W/A < 60% and W/H < 70% of local standards (< 42% and < 63% respectively of Western standards) |
Zinc at 10 mg/kg/day (as zinc sulphate) if weight < 5 kg, 50 mg per day for those > 5 kg; for 2 weeks starting at least 3 days after admission (usually after 7 days), mode of delivery not specified Mean age ± SE: 35.3 ± 5 months Sex F : M: NR Mean % expected W/H c ± SE: 70% ± 2 MUAC: NR Mean % expected W/A c ± SE: 46% ± 3 Mean plasma zinc at study entry ± SE: 10.8 ± 0.8 μmol/l |
No zinc supplement Mean age ± SE: 42.8 ± 7.8 months Sex F : M: NR Mean % expected W/H c ± SE: 66% ± 2 MUAC: NR Mean % expected W/A c ± SE: 48% ± 3 Mean plasma zinc at study entry ± SE: 8.6 ± 0.8 μmol/l |
||
|
Vasudevan et al. 199778 Design: CCT Location: India Length of follow-up: 3 months No. enrolled: 72 Target population: children aged 8–24 months with PEM grades III and IV (IAP 1972 criteria45) |
Elemental zinc 6.6 mg in capsule form (equivalent to 20 mg of zinc sulphate) once per day for 3 months Mean age: NR Sex F : M: NR W/H: NR MUAC: NR W/A: NR |
Placebo capsule Mean age: NR Sex F : M: NR W/H: NR MUAC: NR W/A: NR |
||
| Meand baseline serum zinc reported for the whole study population: 98.4 ± 26.1 μg/dl | ||||
|
Bhutta et al. 199979 Design: RCT Location: Pakistan Length of follow-up: 28 days No. enrolled: 87 Target population: children aged 6–36 months with persistent diarrhoea and malnutrition (W/A z-score ≤ 2)e |
Elemental zinc 3 mg/kg /day (as zinc sulphate) in a single daily dose for 28 days, mode of delivery not specified Mean age ± SD: 11.6 ± 5.6 months Sex F : M, %: 16 : 27 Mean W/H z-score ± SD: –3.02 ± 0.90 Mean MUAC ± SD: 11.1 ± 1.5 cm Mean W/A z-score ± SD: –3.47 ± 0.97 Mean baseline serum zinc ± SD: 78.0 ± 32.2 μg/dl |
Placebo for 28 days Mean age ± SD: 13.1 ± 6.2 months Sex F : M, %: 18 : 26 Mean W/H z-score ± SD: –3.13 ± 1.19 Mean MUAC ± SD: 11.6 ± 1.9 cm Mean W/A z-score ± SD: –3.27 ± 1.33 Mean baseline serum zinc ± SD: 70.3 ± 19.0 μg/dl |
||
The age range of the children enrolled in each study varied. In three studies,70,72,77 children ranged in age from a minimum of 1 year to a maximum of either 3 years,70 5 years72 or 7 years77 (this study could be included because the mean age of participants was < 5 years of age). Six studies allowed for the inclusion of children under 1 year in age, with ages ranging from 6 months to about 2.5 years in one study,71 6 months to 3 years in two studies,68,69,79 and in three studies from either 6 months to 5 years,74,75 5 months to 5 years73 or 8 months to 2 years. 78 Only one study focused on children aged < 1 year. 76 The total number of children enrolled and reported on in the trials ranged from 1171 to 30074,75 with most studies reporting on fewer than 100 participants.
Four studies enrolled approximately equal numbers of male and female children,70,73–76 in one study the population contained more male than female children,79 and one study enrolled only male children. 71 In the remaining four studies, the ratio of male to female children was not reported. 68,69,72,77,78
The criteria for identifying SAM included a W/A assessment in 8 of the 10 studies,68–72,74,75,77–79 although only three68,69,79 reported baseline W/A for each intervention arm. In three studies,72,78,79 this was the only criterion used. Hemalatha and colleagues72 included children with a W/A < 60% of that expected in comparison with the NCHS reference median using the Gómez criteria,26 whereas Vasudevan and colleagues78 included children with 51–60% expected W/A (PEM grade III) and ≤ 50% W/A (PEM grade IV), based on the Harvard standard according to the IAP 1972 classification of PEM. 45 The third study, by Bhutta and colleagues,79 was the only one to focus on children with persistent diarrhoea in combination with evidence of malnutrition, defined as a W/A z-score ≤ 2. This study was included in this section, rather than in Quantity and quality of research available: persistent diarrhoea and Assessment of effectiveness: persistent diarrhoea, because the primary outcome was weight gain (whereas diarrhoea-related outcomes were secondary outcomes), and plasma zinc levels were checked before and after supplementation. The Wellcome classification67 was employed by three studies. 70,71,74,75 This categorises W/A as either < 60% or 60–80% of that expected based on the Harvard standard, and combines this with an assessment of whether oedema is present or absent to identify children with kwashiorkor, marasmic kwashiorkor or marasmus (children in the fourth category, undernourished, were not included). One of the studies, that by Makonnen and colleagues,74,75 also included children with W/A > 80% if they had signs and symptoms of kwashiorkor. Two other studies also included an assessment of nutritional oedema in their criteria for assessing SAM. Doherty and colleagues68,69 included children with nutritional oedema, or with W/A < 60% of the NCHS median, or both. Simmer and colleagues77 included children with nutritional oedema, or W/A < 60% and W/H < 70% of local standards (< 42% and < 63% respectively of Western standards, not further defined).
Two studies did not use W/A in their assessment of SAM. Khanum and colleagues73 included children with oedema and/or with ≤ 60% of the W/H expected in comparison with the Harvard reference. Schlesinger and colleagues76 described all the infants in their study as marasmic.
Although the 10 trials68–79 investigating zinc supplements have been grouped together for ease of comparison, the interventions varied in many aspects: when zinc supplementation began, the daily dose, the mode used to administer this and the duration it was provided for. In addition to the summary information presented in Table 51, further details of zinc supplementation can be found in Table 52.
| Study | zinc given where | Supplement start | Variable zinc dose | Constant zinc dose | Comparison | zinc content of dietary therapy |
|---|---|---|---|---|---|---|
| Doherty et al. 199868,69 | Inpatient (15 days) and after discharge | Within a week | (i) 1.5 mg/kg/day for 15 days (ii) 6 mg/kg/day for 15 days (iii) 6 mg/kg/day for 30 days | Compared doses and durations | Inpatient: 0.3 mg zinc per 100 ml liquid dietb | |
| Gatheru et al. 198870 | Inpatient | Not stateda | 5 mg/kg/day for the duration of treatment | No supplement | NR | |
| Golden et al. 199271 | Inpatient | When free of oedema and infection. Able to start high-energy feeds |
High: 10 mg/kg feed + basic diet Moderate: 5 mg/kg feed + basic diet Low: basic diet (3.5 mg) For duration of treatment |
Compared doses | 3.5 mg/kg feed | |
| Hemalatha et al. 199372 | Inpatient | From patient admission | Estimated as equivalent to 6 mg/kg/day | 40 mg/day for 21 days | Placebo | Mean ± SE: 7.3 ± 0.49 mg zinc per day |
| Khanum et al. 198873 | Inpatient | Day 15 | 10 mg/kg/day for 22 days in participants weighing < 6 kg | 50 mg/day for 22 days in participants weighing > 6 kg | No supplement | zinc in individual food items ranged from 1.5 to 7 p.p.m. |
| Makonnen et al. 200374,75 | Inpatient and after discharge | From patient admission | 10 mg/day for 90 days | Placebo | NRb | |
| Schlesinger et al. 199276 | Inpatient | Not stateda | 15 mg/l of feed for 105 days | Feed with zinc content 3.2 mg/l | No other dietary therapy (participants infants) | |
| Simmer et al. 198877 | Inpatient | A minimum of 3 days (usually 7 days) after admission and start of treatment | 10 mg/kg/day for 14 days in participants weighing < 5 kg | 50 mg/day for 14 days in participants weighing > 5 kg | No supplement | Mean 3.7 mg zinc per day, range 2.4–5.3 mg |
| Vasudevan et al. 199778 | Outpatient | From enrolment | 6.6 mg for 3 months | Placebo | NRb | |
| Bhutta et al. 199979 | Inpatient and after discharge | After 24 hours stabilisation phase | 3 mg/kg/day for 28 days | Placebo | Inpatient: < 2.5 mg zinc per 100 g of foodb |
The comparator in four trials was a placebo;72,74,75,78,79 three trials did not provide a placebo to the comparator group,70,73,77 and three trials varied dose and/or duration of treatment between the groups. 68,69,71,76
Only 2 of the 10 studies specified what their primary outcome measures were. 74,75,79 Mortality was reported by two studies,68,69,74,75 weight or weight gain was an outcome in seven studies70–73,77–79 and anthropometric measures were reported by five studies68,69,73–76,79 (including the three that did not report on weight). Eight studies reported on zinc levels,70,72–79 four reported on symptoms70,72,74,75,79 (e.g. diarrhoea, oedema) and seven reported one or more other outcomes (e.g. results of biochemical tests, length of hospital stay). 68,69,71,72,74–77,79
Summary of quality assessment
The trials were assessed against a number of criteria to obtain an overview of their methodological quality. The global rating for methodological quality varied: two trials68,69,79 were rated strong overall, two trials74–76 were rated moderate and six trials were rated weak70–73,77,78 (Table 53).
| Study | Selection bias | Study design (description) | Confounders | Blinding | Data collection methods | Withdrawals and dropouts | Intervention integrity | Analysis appropriate to question? | Global ratinga | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Per cent who received allocation | Consistency measured? | Unintended intervention likely? | Unit of allocation | Unit of analysis | Appropriate statistical methods? | ITT analysis? | ||||||||
| Doherty et al. 199868,69 | M | S (RCT) | S | S | S | S | 80–100 | ? | ? | Patient | Patient | Yes | No | S |
| Gatheru et al. 198870 | W | S (CCT) | W | W | M | M | 80–100 | ? | No | Patient | Patient | Yes | No | W |
| Golden et al. 199271 | W | S (CCT) | S | W | W | S | 80–100 | Yes | No | Patient | Patient | Yes | Yes | W |
| Hemalatha et al. 199372 | W | S (CCT)b | W | S | Wc | W | 80–100 | ? | No | Patient | Patient | ? | No | W |
| Khanum et al. 198873 | M | S (CCT) | S | W | Wc | S | 80–100 | Yes | No | Patient | Patient | Yes | No | W c |
| Makonnen et al. 200374,75 | S | S (CCT)b | W | S | M | S | 80–100 | No | ? | Patient | Patient | Yes | No | M |
| Schlesinger et al. 199276 | W | S (CCT) | S | M | S | S | 80–100 | Yes | No | Patient | Patient | Yes | Yes | M |
| Simmer et al. 198877 | M | S (CCT) | S | W | Wc | S | 80–100 | Yes | No | Patient | Patient | Yes | No | W c |
| Vasudevan et al. 199778 | M | S (CCT) | W | S | Wc | S | 80–100 | Yes | No | Patient | Patient | Yes | No | W c |
| Bhutta et al. 199979 | S | S (RCT) | S | S | M | S | 80–100 | Yes | No | Patient | Patient | Yes | Yes | S |
The assessment of selection bias required a judgement about how likely it was that participants selected to take part in the study were representative of the target population and information about the percentage of selected individuals who did participate. In general, this information was not well reported. Consequently, only two studies74,75,79 were rated ‘strong’ (at low risk of selection bias). Of the remaining studies, four had a moderate rating68,69,73,77,78 and four had a weak rating70–72,76 (the latter judged to be at a high risk of selection bias).
The study design of all 10 trials was strong, with two being RCTs68,69,79 and the remaining eight being CCTs70–78 (in two of these,72,74,75 there was a suggestion of randomisation but no information was provided about the method, hence they were judged to be CCTs). Both of the RCTs68,69,79 and four of the CCTs71,73,76,77 were judged to have trial arms that were balanced with respect to baseline characteristics and confounders, leading to a strong rating. Reporting in the remaining four CCTs70,72,74,75,78 was either insufficient or unclear, so it was not possible to be certain whether or not the trial arms were balanced, which led to a weak rating. Six trials68,69,72,74–76,78,79 were described as double-blind; of these, five used a placebo and were judged strong with respect to blinding. 68,69,72,74,75,78,79 One trial76 was judged moderate because it was not clear whether or not the outcome assessor was blinded to the intervention status of the participants. Four trials,70,71,73,77 that did not use a placebo were all judged weak with respect to blinding.
When judging the methodological quality of data collection methods, the judgement could differ depending on the outcome measure. Therefore, the data collection method judgements reported here are for the primary outcomes of this systematic review (see Appendix 12). In five trials,71–73,77,78 the data collection tool was not described and, therefore, could not be judged as valid or reliable. The remaining trials all used valid data collection tools, but only two trials68,69,76 provided information indicating that the tools were reliable, which allowed data collection to be judged strong. The other three trials,70,74,75,79 were rated as moderate because no information about the reliability of the tools was reported. Most of the studies were rated as methodologically strong for the item on reporting of withdrawals and dropouts, even though six studies70–73,76,78 did not report the number and reasons for withdrawals and dropouts. The strong rating could be applied because the proportion of participants completing these studies lay between 80% and 100%. Only two studies did not gain a strong rating: one70 was judged moderate and one72 was judged weak because < 60% of participants completed the study.
The final two elements of the quality assessment tool, ‘intervention integrity’ and ‘analysis appropriate to question’, did not contribute to the global rating, but nevertheless provided important information about each study. In all studies, 80–100% of participants received their allocated intervention, in six trials the consistency of the intervention was measured,71,73,76–79 and in eight trials70–73,76–79 it was judged unlikely that any unintended intervention had been implemented. All studies allocated individual infants/children to trial arms and in only one study72 was there insufficient detail to determine whether or not appropriate statistical methods had been used. Three studies71,76,79 performed an ITT analysis.
Assessment of effectiveness: zinc
Mortality
Mortality was reported by only two of the studies68,69,74,75 investigating zinc as a supplement (Table 54). Doherty and colleagues68,69 conducted a planned interim analysis of the first 100 participants. Data from the two groups receiving 6 mg/kg zinc were combined and, when compared with the group receiving 1.5 mg/kg zinc, the risk of death in the 90-day study period was significantly greater for those receiving 6 mg/kg (Yates’-corrected chi-squared value of risk of death 4.52, 95% CI for relative risk of 1.09 to 18.8; p = 0.03). This led to the suspension of enrolment to the trial, by which point 141 participants had been recruited. Of the 19 deaths that occurred overall (all three groups combined), 13 occurred during the inpatient phase and 11 of these occurred in one of the two 6 mg/kg zinc groups. The six deaths in the outpatient phase all occurred in children who had received 6 mg/kg zinc as inpatients (intervention group two who subsequently received placebo in the outpatient phase) or were still receiving 6 mg/kg zinc (intervention group 3). The clinician’s impression was that sepsis was the cause of death in most cases. Doherty and colleagues68,69 conducted an analysis of a range of predictive/prognostic factors, but found that none of these factors in conjunction with the higher dose of zinc was predictive for death.
| Study | Treatment arms | p-value | |||
|---|---|---|---|---|---|
| Doherty et al. 199868,69 | Zinc 1.5 mg/kg 15 days, then placebo 15 days (n = 49) | Zinc 6 mg/kg 15 days, then placebo 15 days (n = 49) | Zinc 6 mg/kg 30 days (n = 43) | ||
| Inpatient deaths, n | 2 | 5 | 6 | Combined zinc 6 mg/kg groups vs zinc 1.5 mg/kg; 95% CI for relative risk of death 1.09 to 18.8, p = 0.03 | |
| Outpatient deaths, n | 0 | 3 | 3 | ||
| Makonnen et al. 200374,75 | Zinc 10 mg/day until 90 days post discharge (n = 150) | Placebo (n = 150) | 95% CI for difference | ||
| Deaths during hospitalisation, n (%) | 7 (4.7) | 26 (17.3) | 95% CI 5.5 to 19.5 | ||
| Deaths after readmission, n | 1 | 2 | NR | ||
| Total deaths, n (%)a | 8 (5.3) | 28 (18.7) | NR | ||
Makonnen and colleagues74,75 also recorded the majority of deaths during the initial period of hospitalisation. However, in this trial the authors state that there were significantly more deaths in the control group, who did not receive a zinc supplement, than in the group that did receive a zinc supplement [17.3% mortality in the control vs 4.7% in the zinc group; no p-value given, but a 95% CI of 5.5% to 19.5% was reported (although not clear in the paper, it appears that this is likely to be the 95% CI for the difference between the groups)]. In each group, some participants had to be readmitted after discharge. In the zinc group, two participants were readmitted, one 5 days after discharge, who was subsequently discharged, and a second identified at the 30-day follow-up who subsequently died. In the control group, four participants were readmitted, three after initial discharge, of whom one subsequently died; the fourth was identified at the 30-day follow-up and also subsequently died.
Weight gain
Seven studies reported weight gain as an outcome, although the reported measures varied: overall weight gain, daily or weekly weight gain, grams of weight gained per kg body weight per day and proportion meeting a threshold value of weight gain (Table 55). All the studies except that by Gatheru and colleagues70 reported weight gain relative to initial weight or reported the proportion of participants meeting a threshold that was a measure of relative weight gain. These measures help remove differences due to starting differences in body weight. Three studies,70,73,77 reported at least one statistically significant difference in a weight gain outcome between the groups in favour of zinc supplementation, but four studies71,72,78,79 found no evidence for a statistically significant difference between groups. A meta-analysis was not carried out because it was considered to be inappropriate because of heterogeneity in the dose(s) of zinc provided, differences in the reported outcome measures [units and time point(s) of measurement] and other limitations of the data (missing measure of variance).
| Study | Treatment arms | p-value | |||
|---|---|---|---|---|---|
| Gatheru et al. 198870 | Zinc 5 mg/kg/day during treatment (n = 42) | Control (n = 40) | |||
| Mean total weight gain ± SD, g | n = 31, 531 ± 277 | n = 27, 338a ± 235 | < 0.05 | ||
| Mean weight gain/day, g | 67 | 47.3 | NR | ||
| Golden et al. 199271 | Zinc 10 mg/kg feed + basic diet (n = 3) | Zinc 5 mg/kg feed + basic diet (n = 4) | Zinc 3.5 mg/kg feed (basic diet only) (n = 4) | ||
| Mean weight gain ± SE during first 6 weeks of recovery, g/kg/day | 11.67 ± 1.41 | 11.60 ± 0.95 | 10.10 ± 0.22 | NS | |
| Hemalatha et al. 199372 | Zinc 40 mg/day for 21 days (n = 16) | Placebo (n = 17) | |||
| Mean weight gain ± SE in week 4, g/kg body weight/day | n = 12, 22.6 ± 5.100 | n = 15, 24.5 ± 5.035 | NS | ||
| Khanum et al. 198873 | Zinc 10 mg/kg /day or 50 mg/day if body weight > 6 kg, on days 15–36 (n = 30) | Control (n = 30) | |||
| Mean weekly weight gain ± SE during supplementation,b g/week | Week 1: 580 ± 67.6 | Week 1: 342 ± 86.5 | < 0.05 | ||
| Week 2: 403 ± 41.6 | Week 2: 269 ± 47.1 | < 0.05 | |||
| Week 3: 462 ± 42.4 | Week 3: 374 ± 48.9 | NR | |||
| Participants with mean weight gain rate > 10 g/kg/day, % | 66 | 33 | 0.02 | ||
| Simmer et al. 198877 | Zinc 10 mg/kg/day or 50 mg/day if weight > 5 kg, for 2 weeks (n = 12) | Control (n = 11) | |||
| Mean weight gain ± SE week 2, g/day | 70 ± 20 | 40 ± 10 | NR | ||
| Mean weight gain ± SE week 2, g/kg/day | 8.83 ± 1.56 | 5.09 ± 1.62 | NR; 95% CI 0.88 to – 8.36 | ||
| Achieved an optimal rate of weight gain (> 10 g/kg/day), % | 42 | 9 | < 0.001 | ||
| Vasudevan et al. 199778 | Zinc 6.6 mg/day for 3 months (n = 31) | Placebo (n = 31) | |||
| Meanc rate of weight gain, g/kg/day | 1.4 | 0.98 | > 0.1 | ||
| Bhutta et al. 199979 | Zinc 3 mg/kg/day for 28 days (n = 43) | Placebo (n = 44) | |||
| Mean weight gain during 14 days of inpatient care ± SD,d g/kg/day | 10.3 ± 5.7 | 8.7 ± 6.5 | NS | ||
| Mean weight on day 14 of inpatient care ± SD,d kg | 6.67 ± 1.43 | 7.13 ± 1.42 | 0.27e | ||
| Mean weight gain during 14 days of home-based supplementation ± SD,d g/kg/day | 9.2 ± 46 | 7.6 ± 5.7 | NS | ||
Gatheru and colleagues70 reported a significantly greater total weight gain in the zinc-supplemented group than in the control group (mean ± SD, 531 ± 277 g vs 338 ± 235 g; p < 0.05). However as noted above, the measures used in this study do not take into account starting differences in body weight. Approximately one-quarter of each group did not complete the study, but the reasons for this are not provided and it is not clear whether or not this had any impact on the outcome. Mean daily weight gain was greater in the zinc-supplemented group (67 g/day) than in the control group (47.3 g/day), but no p-value for a statistical comparison of these values was reported.
Khanum and colleagues73 found that during the first 2 weeks of rehabilitation (which was before zinc supplementation began), the rate of weight gain was not significantly different between the groups (see Appendix 12). However, at the start of the third week the intervention group started to receive a zinc supplement and Khanum and colleagues73 found that by the end of that week the group receiving the zinc supplement had gained significantly more weight than the non-supplemented group (mean ± SE: zinc group 580 ± 67.6 g/week, control group 342 ± 86.5 g/week; p < 0.05). This statistically significant effect was maintained in the following week, but by the fifth week of the study (after 3 weeks of zinc supplementation), the difference in weekly weight gain was no longer statistically significant. Khanum and colleagues73 also report that the percentage of participants in the zinc-supplemented group who achieved a mean daily weight gain of > 10 g/kg was statistically significantly greater than in the control group (66% vs 33%; p < 0.02). It is not clear for what time period the mean daily weight gain was calculated.
Simmer and colleagues77 also reported that the percentage of participants in the zinc-supplemented group who achieved a mean daily weight gain of > 10 g/kg was statistically significantly greater than in the control group (42% vs 9%; p < 0.001). However, in this study, although the zinc group gained more weight each week than the control group, statistically significant differences between the groups for mean daily weight gain and mean daily weight gain per kg body weight could not be demonstrated in either week 1 (see Appendix 12) or week 2 (see Table 55).
All four of the studies71,72,78,79 that did not find evidence for a statistically significant difference between groups reported on the rate of weight gain in terms of g/kg/day (the benefit of a relative measure such as g/kg/day is that any effects due to starting differences in body weight are removed). Golden and colleagues71 reported mean weight gain during the first 6 weeks of recovery. Although children in the moderate- and high-zinc groups gained weight more rapidly, the difference between the groups was not significant. Hemalatha and colleagues72 reported on this outcome separately for weeks 1, 2 and 3 (see Appendix 12) where gains appeared similar between groups, and for week 4 (see Table 55) when the difference in outcome was described as not statistically significant (mean ± SE: 22.6 ± 5.100 g/kg/day in the zinc group vs 24.5 ± 5.035 g/kg/day in the control group; no p-value reported). Bhutta and colleagues79 reported g/kg/day weight gained separately for the 14 days of inpatient and for the following 14 days of home-based supplementation. In both cases, although the zinc-supplemented group gained a little more weight than the control group and the differences were not found to be statistically significant (see Table 55; no p-value reported). Bhutta and colleagues also reported the total weight of children at baseline, on day 7 (see Appendix 12) and on day 14 of inpatient therapy, but again there was no significant difference between the groups (mean weight on day 14 in the supplemented group 6.67 ± 1.43 kg vs 7.13 ± 1.42 kg in the placebo group; p = 0.27). Finally, Vasudevan and colleagues78 reported a rate of weight gain in terms of g/kg/day, which was much lower than that of the other studies (zinc-supplemented group 1.4 g/kg/day vs 0.98 g/kg/day in the control group; p > 0.1). This lower value may be because the authors appear to have based their calculation on the initial weight and a single further weight measurement taken after 3 months.
Anthropometry
Five studies reported anthropometry outcomes (Table 56). 68,69,73–76,79 Two of these studies also reported weight gain as a separate measure,73,79 but anthropometry outcomes were the only measures capturing weight gain for the other three studies. 68,69,74–76 One study73 reported a statistically significant difference in anthropometry outcomes between the groups in favour of zinc supplementation, two studies reported mixed results74–76 and two studies found no evidence for a statistically significant difference between groups. 68,69,79 It was considered inappropriate to carry out a meta-analysis of these outcomes because of heterogeneity in the dose(s) of zinc provided and differences in the reported outcome measures.
| Study | Treatment arms | p-value | |||
|---|---|---|---|---|---|
| Doherty et al. 199868,69 | Intervention 1 (I1):zinc 1.5 mg/kg for 15 days, then placebo for 15 days (n = 43) | Intervention 2 (I2): zinc 6 mg/kg for 15 days, then placebo for 15 days (n = 38) | Intervention 3 (I3): zinc 6 mg/kg for 30 days (n = 25) | 95% CI for mean difference | |
| Mean change in W/A z-score ± SD at 90 days | 1.35 ± 0.69 | 1.51 ± 0.65 | 1.45 ± 0.66 |
I2–I1: (–0.27 to 0.52) I3–I2: (–0.47 to 0.38) |
|
| Mean change in W/H z-score ± SD at 90 days | 1.54 ± 0.93 | 1.67 ± 0.78 | 1.62 ± 0.86 |
I2–I1: (–0.14 to 0.46) I3–I2: (–0.39 to 0.27) |
|
| Mean change in H/A z-score ± SD at 90 days | 0.44 ± 0.32 | 0.48 ± 0.38 | 0.49 ± 0.27 |
I2–I1: (–0.11 to 0.2) I3–I2: (–0.17 to 0.18) |
|
| Mean change in MUAC ± SD at 90 days, cm | 1.66 ± 1.40 | 1.98 ± 1.17 | 1.9 ± 1.38 |
I2–I1: (–0.26 to 0.89) I3–I2: (–0.72 to 0.57) |
|
| Khanum et al. 198873 | Zinc 10 mg/kg/day or 50 mg/day if body weight > 6 kg on days 15–36 (n = 30) | Control (n = 30) | p-value | ||
| Mean W/Ha ± SE | |||||
| Day 8 | 76 ± 1.4 | 72 ± 1.0 | < 0.05 | ||
| Day 15 (zinc started) | 80 ± 1.4 | 75 ± 1.1 | < 0.05 | ||
| Day 36 (discharged) | 95 ± 1.2 | 86 ± 1.2 | < 0.001 | ||
| Mean W/Aa ± SE | |||||
| Day 8 | 52.5 ± 1.44 (n = 29) | 49.9 ± 1.44 (n = 28) | NR | ||
| Day 15 (zinc started) | 58.1 ± 1.53 (n = 29) | 52.3 ± 1.60 (n = 28) | < 0.05 | ||
| Day 36 (discharged) | 68.1 ± 1.58 (n = 29) | 59.7 ± 1.77 (n = 28) | < 0.001 | ||
| Discharge W/H, n (%) | |||||
| < 80% | 0 (0) | 5 (16.7) | NR | ||
| 80–90% | 7 (23.3) | 18 (60.0) | NR | ||
| ≥ 90% | 23 (76.6) | 7 (23.3) | < 0.001 | ||
| Makonnen et al. 200374,75 | Zinc 10 mg/day to 90 days post discharge (n = 150) | Placebo (n = 150) | 95% CI for difference | ||
| Discharge W/A, n (%) | n = 139 | n = 120 | |||
| < 60% | 44 (31.7) | 30 (25) | –4.4 to 17.4 | ||
| 60–80% | 78 (56.1) | 74 (61.7) | NR | ||
| > 80% without oedemab | 17 (12.2) | 16 (13.3) | NR | ||
| Discharge MUAC less than fifth percentile, n (%) | 92 (92.9) | 82 (85.4) | –1.3 to 16.1 | ||
| 90-day follow-up W/A, n (%) | n = 138 | n = 116 | |||
| < 60% | 5 (3.6%) | 16 (13.8%) | –17.2 to –3.1 | ||
| 60–80% | 52 (37.7%) | 67 (57.8%) | NR | ||
| > 80% without oedema | 81 (58.7%) | 33 (28.4%) | NRc | ||
| 90-day follow-up MUAC less than fifth percentile, n (%) | 66 (54.1%) | 81 (77.9%) | –35.2 to –11.5 | ||
| Schlesinger et al. 199276 | Zinc 15 mg/l in infant formula for 105 days (n = 19) | Zinc 3.2 mg/l in infant formula for 105 days (n = 20) | p-value | ||
| Mean H/A z-score ± SD at 105 days | –2.64 (0.86) | –2.56 (0.84) | NS | ||
| Mean W/A z-score ± SD at 105 days | –1.66 (0.64) | –1.59 (0.88) | NS | ||
| Mean W/L z-score ± SD at 105 days | 0.42 (0.81) | 0.32 (1.22) | NS | ||
| Proportion with increase in H/A percentile score from admission, n/N (%) | |||||
| 30 days | 11/19 (58) | 4/20 (20) | < 0.002 | ||
| 45 days | 15/19 (79) | 9/20 (45) | < 0.03 | ||
| 60 days | 13/19 (68) | 11/20 (55) | NS | ||
| Bhutta et al. 199979 | Zinc 3 mg/kg/day for 28 days (n = 43) | Placebo (n = 44) | p-value | ||
| Mean MUAC ± SDd on day 14 of inpatient care, cm | 12.0 ± 1.4 | 12.4 ± 1.8 | 0.66e | ||
| Mean increment in MUAC ± SDd during 14 days of inpatient care, cm | 0.3 ± 0.3 | 0.4 ± 0.3 | NS | ||
| Mean increment in MUAC ± SDd after 14 days of home-based supplementation, cm | 0.13 ± 0.28 | 0.19 ± 0.40 | NR | ||
Khanum and colleagues73 reported statistically significant differences in favour of zinc supplementation in measures of W/H, W/A and the percentage of patients reaching or exceeding 90% W/H by discharge. There were no significant differences in W/H and W/A at admission (see Appendix 12), but a significant difference in favour of the zinc group was present in W/H by the beginning of the first week (eighth day) and in W/A by the beginning of the second week of nutritional therapy (15th day). As zinc supplementation only began on day 15, it is not clear what led to the differences emerging at this stage. The differences were then maintained for each of the following weeks during zinc supplementation (see Appendix 12) until discharge on day 36 (at discharge W/H in the zinc group 95 ± 1.2, control 86 ± 1.2, p < 0.001; W/A in the zinc group 68.1 ± 1.58, control 59.7 ± 1.77, p < 0.001). Over 75% of participants were discharged with a W/H of ≥ 90% of that expected in the zinc-supplemented group, whereas < 25% of the control group reached W/H of ≥ 90% of that expected at discharge.
Makonnen and colleagues74,75 found that at discharge from inpatient care (after a mean length of stay of approximately 11 days), there were no significant differences in anthropometry between the groups. However, the zinc group continued to receive supplements until 90 days after discharge, and at the 90-day follow-up the proportion of children with W/A < 60% was described as significantly lower in the zinc group than that of the control group (3.6% vs 13.8%, 95% CI for difference –17.2% to –3.1%; no p-value reported), and the proportion with W/A > 80% and no oedema was greater (58.7% vs 28.4%; no 95% CI for difference or p-value reported). The proportion of zinc-supplemented participants whose MUAC remained below the fifth percentile was also lower than those children in the control group (54.1% vs 77.9%, 95% CI for difference –35.2% to –11.5%; no p-value reported).
Schlesinger and colleagues76 reported outcomes after 105 days of supplementation, but this was the only study in which the participants were all < 1 year of age and so received liquid formula only. No significant difference between the groups was apparent when z-scores for H/A, W/A and W/H were analysed (see Table 56 and Appendix 12). However, there was a statistically significant difference in the proportion of infants in the zinc-supplemented group whose percentile H/A score increased after 30 and 45 days of nutritional rehabilitation, indicating that the zinc group began to grow earlier than the control group [58% (at 30 days) and 79% (at 45 days) of the 15 mg/l zinc group had an increase in their H/A percentile score in relation to their admission score in comparison with only 20% (at 30 days) and 45% (at 45 days) in the 3.2 mg/l zinc group; p < 0.002 and p < 0.03, respectively]. When these data were analysed by sex, the authors found that the effect held for males, but not for females (see Appendix 12). This difference was no longer significant after 60 days of nutritional rehabilitation (see Table 56).
Doherty and colleagues68,69 reported on the changes in W/A, W/H and H/A z-scores and MUAC (see Table 56), as well as knemometry and skinfold thickness (see Appendix 12). There were three groups in this study and two comparisons were made, the first between the 1.5 mg/kg and the 6 mg/kg zinc dose for 15 days and the second between the 6 mg/kg zinc dose for 15 days or for 30 days. The results reported after 90 days of follow-up indicated that no significant differences were reported for either comparison for any of the anthropometric measures reported. Doherty and colleagues68,69 did find that, overall, good catch-up growth was achieved over 90 days, with the average intragroup W/H z-score improved from 1.54 to 1.67 units, and the H/A z-score improved from 0.44 to 0.49 units.
Finally, Bhutta and colleagues79 reported on just the anthropometric measure of MUAC, finding no significant differences in this measure after 14 days of inpatient therapy (mean MUAC: zinc group 12.0 ± 1.4 cm vs 12.4 ± 1.8 cm in the placebo group; p = 0.66), and no significant differences emerged after a further 14 days of home-based supplementation (see Table 56).
Comorbidities
The effect of zinc supplementation on comorbidities was an outcome reported by five studies. 70,72,74–76,79 In three studies,70,72,79 at least some of these comorbidities had been present at baseline and the study authors report on the effect of the intervention in resolving these. In the study76 in which comorbidities were recorded daily for 105 days and the study74,75 reporting outcomes at 90-day follow-up, it is likely that new incidences of comorbidities are captured.
Three studies70,72,74,75 reported oedema as an outcome (Table 57). Gatheru and colleagues70 reported that the duration of oedema ranged between 2 and 18 days for both groups. However, a greater proportion of participants in the zinc group had lost their oedema by day 7 (77% vs 55%), and the mean number of days taken to lose oedema was statistically significantly lower in the zinc group (mean ± SD: zinc group 6.3 ± 4.6 days vs control group 8.1 ± 4.4 days; p < 0.05). In contrast, Hemalatha and colleagues72 found no statistically significant difference in the mean number of days taken to lose oedema (mean ± SE: zinc group 9.0 ± 2.0 days vs control group 15.7 ± 2.7 days). In the final study to report oedema, Makonnen and colleagues74,75 found a trend for the zinc group to recover more quickly during the first 3 weeks of hospitalisation, but the difference between the groups was not statistically significant (see Appendix 12) and by the 90-day follow-up no patient in either group had any oedema.
| Study | Treatment arms | p-value | |
|---|---|---|---|
| Gatheru et al. 198870 | Zinc 5 mg/kg/day during treatment (n = 42) | Control (n = 40) | |
| Oedema duration, range in days | 2–18 (n = 31) | 2–18 (n = 26) | NR |
| Oedema lost by end of day 7, % | 77 (n = 31) | 55 (n = 26) | NR |
| Mean days taken to lose oedema ± SD | 6.3 ± 4.6 (n = 31) | 8.1 ± 4.4 (n = 26) | < 0.05 |
| Mean diarrhoeaa duration ± SD, days | 3.62 ± 2.78 (n = 17) | 10.8 ± 3.4 (n = 22) | < 0.001 |
| Mean anorexiaa duration ± SD, days | 6 ± 3.16 (n = 26) | 10.3 ± 5.01 (n = 22) | < 0.01 |
| Mean days taken for skin lesionsa to heal ± SD | 7.9 ± 3.1 (n = 10) | 11.1 ± 2.1 (n = 9) | < 0.03 |
| Hemalatha et al. 199372 | Zinc 40 mg/day for 21 days (n = 16) | Placebo (n = 17) | |
| Mean days for oedema to disappear ± SE | 9.0 ± 2.035 | 15.7 ± 2.7 | NS |
| Mean duration of morbidity because of infections ± SE, days | 6.3 ± 0.959 | 7.7 ± 1.040 | NRb |
| Makonnen et al. 200374,75 | Zinc 10 mg/day until 90 days post discharge (n = 150) | Placebo (n = 150) | 95% CI for difference |
| Morbidity on follow-up (90 days), n (%) | n = 138, 85–95 days c | n = 116, 83–95 daysc | |
| Oedema | 0 (0) | 0 (0) | –2.0 to 1.2 |
| Diarrhoea | 4 (2.9) | 31 (36.7) | –32.0 to –15.0 |
| Vomiting | 1 (0.7) | 8 (6.9) | –11.2 to –1.2 |
| Skin infection | 1 (0.7) | 8 (6.9) | –11.2 to –1.2 |
| Fever | 4 (2.9) | 12 (10.3) | –13.8 to –1.1 |
| ARI | 4 (2.9) | 45 (38.8) | –44.7 to –26.2 |
| Pallor | 32 (23.2) | 62 (53.4) | –41.3 to –18.4 |
| Schlesinger et al. 199276 | Zinc 15 mg/l in infant formula for 105 days (n = 19) | Zinc 3.2 mg/l in infant formula for 105 days (n = 20) | |
| Otitis media episodes (mean ± SD) during the 105 days rehabilitation | 0.73 ± 0.9 | 1.85 ± 2.3 | 0.05 > p < 0.1, Student’s t-test |
| Average number of acute diarrhoeal episodes | 2 | 0 | Statistically significant difference, p-value NR |
| Bhutta et al. 199979 | Zinc 3 mg/kg/day for 28 days (n = 43) | Placebo (n = 44) | |
| Mean stool frequency ± SDd (n/day) | |||
| Day 1 | 10.2 ± 6.4 | 11.8 ± 7.8 | |
| Day 7 | 5.9 ± 5.6 | 5.2 ± 3.7 | |
| Day 14 | 2.9 ± 1.6 | 3.0 ± 2.2 | 0.52e |
| Decrease in stool frequency (n/day) | 7.4 ± 7.4 | 8.1 ± 8.8 | NS |
| Stool volume (g/kg/day) (males) | |||
| Day 1 | 116.8 ± 103.7 | 141.9 ± 171.6 | |
| Day 7 | 66.7 ± 68.1 | 43.9 ± 40.1 | |
| Day 14 | 24.9 ± 16.2 | 27.8 ± 31.4 | 0.42e |
| Decrease in stool volume (g/kg/day) | 91.1 ± 103.6 | 98.0 ± 187.9 | NS |
Diarrhoea was reported by four studies70,74–76,79 and the results were mixed, with two studies70,74,75 finding statistically significant differences in favour of zinc supplementation, one study76 finding a statistically significant difference in favour of the control group, and one79 finding no statistically significant differences between the groups. Gatheru and colleagues70 reported that the duration of diarrhoea was statistically significantly lower in the zinc group than in the control group (zinc group 3.62 ± 2.78 days vs control group 10.8 ± 3.4 days; p < 0.001). It is not clear whether the participants contributing data to this outcome all had diarrhoea at baseline or whether new episodes of diarrhoea arising during treatment are included. Makonnen and colleagues74,75 also found in favour of zinc supplementation when reporting on the proportion of participants with diarrhoea at the 90-day follow-up. A statistically significant difference was observed, with fewer participants having diarrhoea in the zinc-supplemented group than in the placebo group (zinc group 2.9% vs placebo group 36.7%, 95% CI for difference –32% to –15%). In contrast, Schlesinger and colleagues,76 who analysed infectious episodes using three indices (mean episodes/infant, mean duration of each episode and mean percentage days infected during the 105 days of rehabilitation), found a statistically significant difference in the average number of acute diarrhoeal episodes that favoured the formula with low zinc content (zinc 15 mg/l formula two episodes vs zinc 3.2 mg/l zero episodes; p-value not reported). Schlesinger and colleagues76 stated that each episode lasted 1–2 days and had no impact on nutritional rehabilitation. Finally, Bhutta and colleagues,79 who focused their study on the treatment of children with diarrhoea at baseline, recorded two measures for the 14-day inpatient phase of their study: stool frequency and stool volume. No statistically significant differences were found between the groups for either measure (see Table 57).
The remaining morbidity outcomes reported by the studies (see Table 57) provide mixed results. Two studies70,74,75 reported statistically significant differences in favour of the zinc-supplemented group: Gatheru and colleagues70 for duration of anorexia and time taken for skin lesions to heal and Makonnen and colleagues74,75 for the proportion of participants with vomiting, fever, acute respiratory infections, skin infections or pallor at the 90-day follow-up. One study76 reported that the mean number of otitis media episodes came close to reaching a statistically significant difference between the groups in favour of the group receiving a higher concentration of zinc (zinc 15 mg/l formula 0.73 ± 0.9 vs zinc 3.2 formula 1.85 ± 2.3; p-value between 0.05 and 0.1), but the same authors stated that for number or duration of upper and lower respiratory infection, purulent conjunctivitis, and skin and mucous candidiasis, no differences were observed between groups (see Appendix 12). Finally, for the outcome duration of morbidity because of infections, Hemalatha and colleagues72 state that the groups were comparable, but no p-value is reported.
Adverse effects
Oral exposure to large doses of supplemental zinc is associated with some known adverse events, such as gastrointestinal effects (e.g. nausea) and copper deficiency. 83 Two studies specifically checked for the adverse effects of zinc on plasma copper levels. 72,79 Hemalatha and colleagues72 reported that, although plasma copper levels rose significantly in both groups, the zinc supplementation did not adversely affect plasma copper levels. In contrast, Bhutta and colleagues79 found that serum copper levels fell during zinc supplementation, whereas in the placebo group serum copper levels significantly increased. Both Simmer and colleagues77 and Vasudevan and colleagues78 reported that no adverse effects were noted in the zinc-supplemented groups. Simmer and colleagues77 reported that tube feeding was required for one patient in each group and two patients in each group received a blood transfusion. This study additionally reported on anorexia, which can develop because of zinc deficiency and might have been expected to affect the control group, but there was no difference in calorie or protein intakes between the groups. Two trials68,69,76 reported on comorbidity events which have already been noted in the sections above. Doherty and colleagues68,69 had to suspend enrolment to their trial when the groups receiving 6 mg/kg zinc supplements were found to be at a significantly greater risk of death than the group receiving 1.5 mg/kg zinc supplements. Schlesinger and colleagues76 found that infants receiving formula with a zinc content of 15 mg/l had on average two acute diarrhoeal episodes, whereas infants receiving 3.2 mg/l zinc formula had no such episodes. However, this statistically significant difference in diarrhoeal episodes had no impact on nutritional rehabilitation. The remaining studies did not report adverse events. 70,71,73–75
Zinc status
Eight70,72–79 of the 10 studies reported on zinc status, and the results are briefly summarised here, with details provided in Appendix 12. It should be noted, however, that although serum or plasma zinc may be the best available biomarker at a population level to reflect dietary zinc intake, and although it changes in response to zinc supplementation, it does not necessarily reflect individual zinc status. 84 Five studies70,73–75,78,79 found a statistically significant difference in serum zinc concentrations between the groups, with serum zinc being higher following supplementation than it was in the non-supplemented group. One study found a statistically significant difference in the proportion of participants defined as having a low plasma zinc, which was in favour of the zinc-supplemented group. 76 Two studies72,77 did not report differences between groups, but did report statistically significant increases in plasma zinc in comparison with baseline values in either the zinc group or both study groups. Three studies72,76,77 also reported the difference from baseline in leucocyte zinc. One study72 found that leucocyte zinc was statistically significantly increased from baseline in both groups, one77 reported a statistically significant difference for the zinc-supplemented group only, and one76 found no significant differences in either group.
Other outcomes
Additional outcomes, such as duration of hospital stay, calorie intake, nitrogen intake and results of biochemical assays, were also reported by some studies, but have not been presented here. Further details are available in the data extraction forms in Appendix 12.
Summary
-
Ten trials investigated zinc supplements as part of a treatment regimen for SAM. 68–79 The trials took place in seven countries, and employed different criteria for identifying SAM; in addition, the age range of enrolled children differed and most reported on fewer than 100 participants. One study focused on participants who also had persistent diarrhoea. 79 The interventions also varied in many aspects. More than half of the studies were of weak methodological quality,70–73,77,78 two were of moderate quality74–76 and only two were judged to have strong methodological quality. 68,69,79 Only three trials conducted an ITT analysis of the data. 71,76,79
-
Only two studies68,69,74,75 reported mortality as an outcome. One study68,69 of children aged from 6 to 36 months suspended enrolment to the trial after an interim analysis found a significant risk of death for participants receiving 6 mg/kg/day zinc in comparison with those receiving 1.5 mg/kg/day zinc. In contrast, in the other study74,75 that enrolled children aged from 6 to 60 months, significantly more deaths were reported in the group receiving placebo than in the group receiving a zinc dose of 10 mg/day. One study provided zinc according to body weight68,69 and the other as a fixed dose,74,75 and although neither study reports on the weight of participants, the 10 mg/day dose is likely to be one of the lowest provided whereas the 6 mg/kg/day dose is likely to be one of the highest. It is difficult to know how similar the participants were because the studies do not report on the same anthropometric characteristics at baseline, although the criteria for defining SAM were comparable.
-
Intervention effects on weight were reported directly by seven studies70–73,77–79 (e.g. as absolute gains in weight or rate of weight gain) or as anthropometric measures by five studies68,69,73–76,79 (e.g. W/H, W/A), with two of these studies reporting weight both directly and by anthropometry. The results for weight and anthropometry outcomes are conflicting, with three studies70,73,77 reporting statistically significant effects in favour of zinc supplementation, two studies reporting mixed results74–76 (in favour of zinc or no significant differences between the groups) and five studies68,69,71,72,78,79 finding no significant differences between the groups. None of the studies reported a statistically significant effect in favour of the comparator/control group for a weight-related outcome.
-
Five studies70,72,74–76,79 reported the effect of zinc supplementation on comorbidities, either those present at baseline or also including new incidences of comorbidities. Again, the results present a mixed picture. One study70 found that zinc significantly reduced the duration of four comorbidities present at baseline, whereas another study74,75 reported a statistically significant beneficial effect of zinc at the 90-day follow-up for six of the seven comorbidities assessed. In contrast, two other studies found no statistically significant effects on diarrhoea (one study79) or oedema (one study72), and a third study76 stated that there was a statistically significant difference in the average number of acute episodes of diarrhoea that favoured the control group (i.e. lower dose zinc).
-
Four studies70,71,73–75 did not report on adverse events and two77,78 reported that no adverse events occurred because of zinc supplementation. Two studies specifically reported the impact of zinc supplementation on plasma copper because exposure to large doses of supplemental zinc is known to cause copper deficiency. In one study72 plasma copper levels rose in both groups, whereas in the other study79 copper levels significantly increased in the placebo group but fell in the group receiving zinc, and the study authors suggest that it may be more appropriate to provide a mix of micronutrients as a supplement rather than zinc alone. As already noted above, statistically significant differences in mortality were reported by one study67,68 in which more deaths occurred in the 6 mg/kg/day zinc-supplemented group, and in another study more episodes of acute diarrhoea75 occurred in the zinc group, although in this latter case this was stated not to have affected nutritional rehabilitation.
Quantity and quality of research available: other supplements
There were three trials examining supplements other than zinc: one evaluating potassium,80 one evaluating nicotinic acid81 and one evaluating NTs82 (Table 58). All three trials were conducted in single centres. One trial was a double-blind RCT,82 whereas the other two trials were judged to be CCTs during quality assessment (see Table 59). 80,81 The trials were set in India,81 Malawi80 and Mexico,82 with none reporting any external funding.
| Study details and target population | Study arms | |
|---|---|---|
|
Manary and Brewster 199780 Design: CCTa Location: Malawi Length of follow-up: unclear No. enrolled: 116 Target population: children aged < 3 years with kwashiorkor (all had oedema) |
High potassium: 3 mmol/kg potassium above the standard. Total potassium dose of 7.7 mmol/kg/day for the first 7 days of therapy Selected baseline characteristicsb Mean age ± SD: 29.3 ± 14 months Sex F : M: NR Mean W/H z-score ± SD: –2.04 ± 1.20 MUAC: NR W/A: NR Baseline potassium: NR |
Standard potassium: 3.2 mmol/kg/day of potassium. Total potassium dose of 4.7 mmol/kg/day for the first 7 days of therapy Selected baseline characteristicsb Mean age ± SD: 27.9 ± 15 months Sex F : M: NR Mean W/H z-score ± SD: –2.40 ± 1.13 MUAC: NR W/A: NR Baseline potassium: NR |
|
Philip et al. 198281 Design: CCT Location: India Length of follow-up: 1 month No. enrolled: 80 Target population: children aged 0–4 years with marasmus |
Nicotinic acid, 25 mg/kg/day (three divided doses) for 1 month Selected baseline characteristics Mean age: NR Sex F : M: NR W/H: NR MUAC: NR W/A: NR Baseline nicotinic acid: NR |
No nicotinic acid supplement Selected baseline characteristics Mean age: NR Sex F : M: NR W/H: NR MUAC: NR W/A: NR Baseline nicotinic acid: NR |
|
Vásquez-Garibay et al. 200582 Design: RCT Location: Mexico Length of follow-up: 4 weeks No. enrolled: 25 Target population: children aged 3–18 months W/A or W/H < –3 SD from the median NCHS/WHO 1996 standard |
Milk-based formula with NT Selected baseline characteristicsb Mean age ± SD: 7.6 ± 4.6 months Sex F : M, %: 27 : 73c Mean W/H z-score ± SD: –2.80 ± 0.73 Mean MUACd ± SD: 7.9 ± 1.1 cm W/A: NR |
Formula of the same energy density, but no NTs Selected baseline characteristicsb Mean age ± SD: 8.1 ± 3.2 months Sex F : M, %: 44 : 56c Mean W/H z-score ± SD: –2.99 ± 0.74 Mean MUACd ± SD: 7.6 ± 1.0 cm W/A: NR |
| Study | Selection bias | Study design (description) | Confounders | Blinding | Data collection methods | Withdrawals and dropouts | Intervention integrity | Analysis appropriate to question? | Global ratinga | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Per cent who received allocation | Consistency measured? | Unintended intervention likely? | Unit of allocation | Unit of analysis | Appropriate statistical methods? | ITT analysis? | ||||||||
| Manary and Brewster 199780 | S | S (CCTb) | S | S | W | S | 80–100 | No | No | Patient | Patient | Yes | No | M |
| Philip et al. 198281 | W | S (CCT) | W | W | W | S | ? | ? | No | Patient | Patient | Yes | Yes | W |
| Vásquez-Garibay et al. 200582 | S | S (RCT) | W | W | M | S | 80–100 | Yes | No | Patient | Patient | Yes | No | W |
There was no consistency in the type of supplement compared by the three trials. Manary and Brewster80 evaluated a high dose of potassium (total dose 7.7 mmol/kg/day) versus a standard dose (total dose 4.7 mmol/kg/day) for the first 7 days of therapy. Philip and colleagues81 compared the addition of three doses of nicotinic acid per day (25 mg/kg /day) versus none for 1 month. Vásquez-Garibay and colleagues82 compared a milk-based formula with added NTs versus a formula of the same energy density, but without the addition of NTs. All trials took place in the hospital inpatient setting.
The sample sizes were small, varying from 2582 to 116 children. 80 Mean age ranged from 7.6 to 8.1 months in one trial82 and from 27.9 to 29.3 months in another. 80 However, both these trials reported baseline characteristics for children completing the trial only. Philip and colleagues81 did not provide any baseline characteristics, but included children aged from 0 to 4 years. Sex was reported by only one trial,82 with almost double the number of boys as girls.
Only one trial, by Vásquez-Garibay and colleagues,82 provided a definition for severe malnutrition, this being W/A or W/H of < –3 SD from the median NCHS reference. Manary and Brewster80 included children with kwashiorkor, all of whom had oedema, whereas Philip and colleagues81 included children fulfilling what they called ‘the standard criteria’ for marasmus.
Baseline mean W/H z-scores suggested a greater severity of malnutrition in the children in the trial by Vásquez-Garibay and colleagues82 than in those in the trial by Manary and Brewster. 80 However, it appears that the W/H z-scores in the latter study were calculated before loss of oedema (z-scores would be expected to be lower, indicating more severe malnutrition after loss of oedema). Also, in the trial by Vásquez-Garibay and colleagues,82 mean baseline arm circumference (assumed to be MUAC) appears to be very low, falling well below the threshold for SAM. Without baseline characteristics, it is unclear if the severity of malnutrition was comparable with the population in the trial by Philip and colleagues with that in the other two trials. 81
Summary of quality assessment
Two of the included trials were rated overall as weak for their methodological quality and quality of reporting (Table 59),81,82 with the third being rated overall as moderate. 80
For selection bias, two trials were rated as strong,80,82 whereas the study of Philip and colleagues81 was rated as weak and therefore at potential risk of selection bias. A strong rating indicates that the selected individuals are likely to be representative of the target population and ≥ 80% of selected individuals participated in the trial. A weak rating indicates that participants may not be representative of the target population, or that the selection method and/or levels of participation were unclear/not described. All three trials were rated as strong for their study design. Two of the trials were described as RCTs80,82 and one as a CCT;81 however, the trial by Manary and Brewster80 was judged to be a CCT, as it provided no details of the randomisation method/procedure.
Only one trial had no important differences in baseline characteristics between the trial arms, and without potentially confounding variables was rated as strong. 80 The remaining two trials81,82 were rated as weak. Philip and colleagues81 only reported age for baseline characteristics and it is, therefore, unclear if there were any confounding variables, whereas Vásquez-Garibay and colleagues82 acknowledged some differences between the treatment arms. It is unclear if these differences were between or within treatment arms. For blinding, only one trial employed a double-blind method and was therefore rated as strong. 80 The two remaining trials provided no details and were therefore rated as weak. 81,82 Both trials could therefore be at risk of bias in either the care provided (performance bias) or how the outcomes were assessed (measurement or detection bias) or both. Not blinding children/parents to the research question could lead to reporting bias. It may not always be possible to blind children/parents to the intervention, but the potential bias needs to be kept in mind when interpreting the results.
Two trials were rated as weak80,81 and one as moderate82 for their data collection methods. Only the trial by Vásquez-Garibay and colleagues82 used valid data collection tools, but it was not possible to judge if these tools were reliably employed. For the trials rated as weak, it was not possible to assess if either the data collection tools were valid or reliable. 80,81 All three trials were rated as strong for withdrawals and dropouts, providing both numbers and reasons, as well as having ≥ 80% of participants completing the study. For intervention integrity, there was no information in one trial on either the percentage of participants who had received the intervention or if the consistency of the intervention had been measured. 81 In the other two trials,80,82 ≥ 80% of participants received the allocated intervention, but only one of these measured the consistency of the intervention. 82 There appeared to be no contamination of the interventions (i.e. all children received the allocated intervention only) in any of the three trials. All trials used the infant/child as the unit of allocation and for statistical analysis of the results, and were judged to use appropriate methods of statistical analysis for the research question. However, only one of the trials reported on how missing data were dealt with in the analysis (i.e. ITT). 81
Assessment of effectiveness: other supplements
Mortality
Only Manary and Brewster80 reported mortality as an outcome (Table 60). There were a total of 34 (34%) deaths in hospital during the trial, 14 out of 48 deaths in the high-dose potassium intervention group compared with 20 out of 51 in the standard potassium control group. However, although the case fatality rate reduced by 33% in the intervention group, the difference between the groups was not statistically significant (p = 0.40). Twenty-one of the 34 deaths were early deaths (within 5 days) and 13 were late deaths (after 5 days). However, 11 children (intervention n = 3, control n = 8) were taken from hospital after completing the 7-day trial before discharge, resolution of oedema and clinical improvement. Only the percentage of late deaths was statistically significant after adjusting it to include three children (all from the control group) who had left hospital and who were not expected to have survived at home, with a lower number of deaths in the high-dose potassium intervention group (8%) than in the standard potassium control group (32%; p = 0.02) [OR 5.3 (95% CI 1.2 to 31.0)]. The five children whose deaths between days 9 and 13 were classified as unexpected all had persisting diarrhoea.
| Study | Treatment arms | p-value | |
|---|---|---|---|
| Manary and Brewster 199780 | High potassium (n = 48) | Standard potassium (n = 51) | |
| Deaths in hospital, n/N (%) | 14/48 (29.2) | 20/51 (39.2) | 0.40 |
| Deaths during first 48 hours, n | 6 | 6 | NR |
| Death during days 3–5, n | 5 | 4 | NR |
| Late deaths, n | 3 | 10 | NR |
| Adjusted late deaths,a n/N (%) | 3/37 (8.1) | 13/41 (31.7)b | 0.02 |
| Causes of late death | |||
| Sepsis, n | 3 | 3 | NR |
| Anaemia, n | 2 | NR | |
| Unexpected,c n | 5 | NR | |
Weight gain
Two out of the three trials reported weight gain, but only one trial81 reported weight gain relative to initial weight (thus removing effects owing to the starting differences in body weight). 81,82 The trial by Manary and Brewster80 only reported weight loss, although it is not particularly clear if this was because of resolution of oedema.
Philip and colleagues81 calculated separately for each week, with both groups showing maximum gain during week 2, followed by week 3, with the lowest gain in weeks 1 and 4 (no data reported). For both groups, the rate of weight gain was slightly higher in those children with a greater initial weight deficit. Mean weight gain in 1 month was statistically significantly higher in the intervention group with added nicotinic acid (p = 0.001; Table 61). In the trial by Vásquez-Garibay and colleagues,82 mean weight gain per day was similar between groups regardless of the addition of NT (no p-value reported). Typical weight gain was said to be five times higher than that of normal infants aged around 8 months.
| Study | Treatment arms | p-value | |
|---|---|---|---|
| Philip et al. 198281 | Nicotinic acid (n = 40) | Standard diet (n = 40) | |
| Mean weight gain in 1 month ± SD, g/kg | 231.05 ± 20.05 | 171.81 ± 22.01 | 0.001a |
| Vásquez-Garibay et al. 200582 | Added NT (n = 11) | No added NT (n = 9) | |
| Mean weight gain ± SD, g/day | 67 ± 15 | 69 ± 12 | NR |
Anthropometric measures
Only Vásquez-Garibay and colleagues82 reported on this outcome. The authors state that there was a significant improvement in W/A in both groups from the first week regardless of the addition of NTs, but presented no data. The same trend was reported for W/L (Table 62), but only p-values for within-group differences were reported. The pace of linear growth was said to be double that of normal infants aged around 8 months.
| Study | Treatment arms | p-value | |
|---|---|---|---|
| Vásquez-Garibay et al. 200582 | Added NTs (n = 11) | No added NTs (n = 9) | a |
| Mean W/L z-score ± SD | |||
| Week 1 | –2.80 ± 0.73 | –2.99 ± 0.74 | |
| Week 4 | –0.64 ± 0.66b | –0.94 ± 0.47b | |
| Mean initial BMI ± SD | 11.0 ± 0.9 | 10.6 ± 1.0 | 0.33 |
| Mean fourth week BMI ± SD | 15.1 ± 1.0 | 14.5 ± 1.0 | 0.23 |
| Mean skin fold ± SD, mm | |||
| Triceps,b initial week | 3.8 ± 1.0 | 2.8 ± 0.6 | 0.031 |
| Triceps, fourth week | 9.2 ± 2.6 | 8.5 ± 1.6 | 0.517 |
| Subscapular,c initial week | 2.9 ± 0.7 | 2.4 ± 0.6 | 0.076 |
| Subscapular, fourth week | 8.1 ± 2.7 | 6.4 ± 1.1 | 0.112 |
| Subcostal,c initial week | 2.3 ± 0.5 | 1.8 ± 0.3 | 0.045 |
| Subcosta, fourth week | 5.5 ± 1.9 | 4.0 ± 0.6 | 0.004 |
| Suprailiac,c initial week | 2.2 ± 0.5 | 1.7 ± 0.3 | 0.020 |
| Suprailiac, fourth week | 5.7 ± 2.5 | 4.2 ± 0.6 | 0.114 |
Vásquez-Garibay and colleagues82 also included total upper-arm area, upper-arm muscle area, upper-arm fat area and the arm fat index as outcomes, but differences were not significant between groups at week 4 regardless of the addition of NTs, although there were statistically significant within-group differences (see Appendix 12).
Additional outcomes
Manary and Brewster80 reported weight loss as one of their clinical outcomes (although not explicitly stated, this is presumed to be reported as an indication of the resolution of oedema following the start of treatment). There were no statistically significant differences between the treatment arms by day 7 (p = 0.36) or after discharge (p = 0.61) regardless of the added potassium (Table 63), nor were there any significant differences between treatment arms in the number of days children stayed in hospital (p = 0.21). However, the high-potassium intervention group suffered significantly fewer presumed septic episodes (3 vs 18) [OR 8.9 (95% CI 2.2 to 50.9)], respiratory symptoms and new skin ulcerations than the standard-potassium control group, as illustrated in Table 64.
| Study | Treatment arms | p-value | |
|---|---|---|---|
| Manary and Brewster 199780 | High potassium (n = 37)a | Standard potassium (n = 41)a | |
| Mean percentage weight loss by day 7 ± SD | 5.6 ± 8.0 | 4.0 ± 7.2 | 0.36 |
| Mean percentage weight loss by discharge ± SD | 4.9 ± 9.1 | 3.8 ± 10.3 | 0.61 |
| Mean length of hospital stay ± SD, days | 11.6 ± 0.9 | 13.2 ± 4.9 | 0.21 |
| Left before discharge (after day 7), n/N (%) | 3/37 (8) | 8/41 (19.5) | 0.15 |
| Study | Treatment arms | p-value | |
|---|---|---|---|
| Manary and Brewster 199780 | High potassium (n = 37)a | Standard potassium (n = 41)a | |
| Clinical sepsis (days 2–7), n (%) | 0 | 9 (22) | 0.01 |
| Clinical sepsis (days 8–24), n (%) | 3 (9) | 9 (22) | 0.05 |
| New skin ulcers (cases), n (%) | 4 (11) | 13 (33) | 0.05 |
| Mean days of cough ± SD | 2.3 ± 2.6 | 3.9 ± 2.7 | 0.01 |
| Dyspnoea (cases), n (%) | 1 (3) | 10 (24.4) | 0.01 |
| Mean days of irritability ± SD | 3.4 ± 1.7 | 3.7 ± 2.1 | 0.47 |
| Mean days of diarrhoea ± SD | 0.9 ± 2.5 | 1.5 ± 1.7 | 0.14 |
| Mean days with oedema (grade 2+ or grade 3+) ± SD | 2.7 ± 2.2 | 2.7 ± 2.1 | 0.99 |
Mean urea concentration and mean alkaline phosphatase were statistically significantly lower for the intervention with added NTs (p = 0.009 and p = 0.041, respectively). 82 Treatment arms were combined for initial versus final outcome comparisons of white blood cell count, creatinine, glucose, calcium and phosphorus levels, with all showing significant improvements for the whole group apart from changes in white blood cell count. 85 Philip and colleagues81 included an outcome for calories consumed for 1 g gain in weight. Although this was lower in the intervention group (14.2 vs 19.3), which had the added nicotinic acid; no p-value was reported.
Summary
-
Three trials investigated supplements other than zinc as part of a treatment regimen for SAM. Each trial examined a different supplement either potassium,80 nicotinic acid81 or NTs. 82 The trials took place in different countries and enrolled participants meeting different criteria. Two of the studies81,82 were of weak methodological quality; the third80 was of moderate quality. Only one trial81 conducted an ITT analysis of the data.
-
Only the study80 providing high-dose potassium to the intervention group reported mortality as an outcome. This trial found no difference in early deaths, but found a statistically significant benefit for the intervention group receiving high-dose potassium for late deaths, which meant that there was a 33% reduction in the case fatality rate overall in this group.
-
Intervention effects on weight were reported directly by two studies as mean weight gained per kg of body weight81 or mean weight gained per day. 82 One of these studies82 also reported weight gain in terms of body mass index (BMI), alongside other anthropometric measures. Participants receiving nicotinic acid supplementation81 gained statistically significantly more weight per kg in 1 month than participants on the standard diet. In the other study,82 there was no difference in daily weight gain between groups receiving milk-based formula either with or without added NTs, and reporting of anthropometry outcomes was unclear so it was not possible to determine which (if any) p-values indicated a statistically significant difference between the groups.
-
One study80 reported on comorbidities, finding that the high-potassium intervention group suffered significantly fewer presumed septic episodes, respiratory symptoms and new skin ulcerations, whereas there was no statistical difference between the groups for irritability, diarrhoea and oedema.
Ongoing studies
The search for ongoing studies identified 41 records. Of these, 10 appear (from the limited details available) to meet the inclusion criteria for the review. These 10 studies seem to map to questions 7 (two studies), 8 (one study), 10 (four studies), 14 (two studies) and 22 (one study, which may also map to Q8). Summary details of these ongoing studies are presented in Appendix 13.
Chapter 5 Discussion
Statement of principal findings
The aim of this project was to evaluate the effectiveness of interventions to treat infants and children aged < 5 years who have SAM. The initial scope of the project was therefore extremely broad. It covered a series of possible research questions that related to the effectiveness of programmes and/or guidelines that have been developed (e.g. the WHO 10-step plan), as well as covering each discrete step or individual components that have been used to treat or manage severely malnourished children. In addition, factors that might affect the effectiveness of interventions (e.g. setting, presence of comorbidities such as HIV infection) and constraints to the implementation of interventions could also have been examined.
Delphi process
It would not have been possible to systematically review every aspect of the evidence relating to the treatment of children < 5 years of age with SAM during the course of this project. Therefore, a Delphi process was used to gain an understanding of the priority order of the research questions (findings reported in Chapter 3 and Appendix 5), so that the systematic review could focus on the areas identified as being of the highest priority by a panel of leading international experts in the field of malnutrition.
Through an iterative process, the panel of experts (containing academics, people working in the field, government departments, charities, NGOs and WHO) scored 18 questions developed from the WHO 10-step plan for the management of SAM, and added four new questions. After three rounds of the Delphi process, the expert panel had reached a consensus, identifying and ranking 15 priority questions for consideration for the systematic review. All aspects were overseen by an independent chairperson appointed by the National Institute for Health Research (NIHR) HTA programme.
The priority areas identified tended to focus on the most effective approaches to managing specific subgroups (e.g. children infected with HIV, infants < 6 months old) or associated conditions/comorbidities (e.g. shock, diarrhoea, infection, TB and H. pylori). Factors affecting the delivery of the intervention or programme (e.g. factors affecting implementation and sustainability of interventions, effect of different settings) were also considered a priority. All of these priority areas potentially spanned the entire treatment pathway. Areas considered lower priorities and excluded from the final list, tended to focus on individual steps during the initial phase of treatment, particularly for hypothermia, hypoglycaemia and for correction of electrolyte imbalance. Also excluded were discharge criteria, methods for emotional and sensorial development, and strategies for different geographical locations.
Systematic review
The 69 studies that met the inclusion criteria of the review were mapped against the final list of research questions that had been prioritised by the panel of experts who took part in the Delphi process. No evidence was found to inform 5 of the 15 prioritised questions.
The questions for which no evidence was identified included (1) strategies for the management of specific subgroups of children, those infected with HIV or children with other comorbidities such as TB and H. pylori infection; (2) the overarching issues of programme sustainability, long-term survival and readmission rates, and factors limiting full implementation of treatment programmes; and (3) methods for increasing appetite and food intake to recover weight and aid catch-up growth during rehabilitation and follow-up.
Evidence was found that mapped against the remaining 10 prioritised questions; however, for one question only very limited evidence was available.
Project resources were available to review the evidence for the first six questions for which there was any evidence available. These six questions were among those ranked in the top 10 of the 15 prioritised questions and each question is considered separately below.
What methods are effective for treating severe acute malnutrition among infants < 6 months old?
No research focused on treating SAM in infants < 6 months old. However, two cohort studies included this age group within their study populations (without reporting baseline data separately for this age group), and also provided a very limited quantity of separate outcome information. No formal quality assessment was undertaken.
In one cohort study,47 infants < 6 months old made up ≤ 10% of those admitted with SAM in three consecutive years. Outcomes before and after the introduction of the WHO guidelines for treatment of SAM were compared, but the only outcome commented on for the age group of interest was mortality. Mortality was reported as a proportion of overall admissions in a given year and compared with the proportional mortality of other age groups. In the < 12 months age group, proportional mortality was lower than in the other age groups. No statistical comparison was reported.
The second study,48 a prospective cohort study, compared a locally adapted protocol for treatment of SAM with the WHO protocol. The proportion of the study cohort aged < 6 months was not reported. Weight gain was the only outcome reported for the subgroup of children aged < 6 months and there was no statistically significant difference in weight gain between the groups.
The finding of a lack of evidence for this age group is in agreement with other reports. Most recently, the Management of Acute Malnutrition in Infants (MAMI) project,17 which focused on SAM in the context of emergency situations, overviewed 37 guidelines for the treatment of malnutrition. This project also included an analysis of data sets from 12 countries, which indicated that there is a higher rate of mortality in infants aged < 6 months. However, the only guideline86 reviewed that had a specific focus on infants < 6 months old acknowledges that there is little published evidence available on which to base recommendations for treatment in this age group.
In summary, no good-quality evidence or adequately reported studies assessed treatments for SAM in infants < 6 months old. As this was one of the highest ranked questions in the Delphi study, more research is needed to fill this gap in the evidence base.
Which form of intravenous fluid administration is most effective for treating shock?
The second of the four questions, which were prioritised equal first in the Delphi study was also informed by limited evidence as only one RCT of moderate methodological quality was identified that mapped to this question. This RCT49 compared the efficacy of three fluid resuscitation solutions for treating hypovolaemic shock in children with SAM. The three solutions were RL, a standard WHO hypotonic fluid solution (HSD/5D) and HAS. The solutions were either provided according to the WHO recommendation (HSD/5D) or given at similar volumes and rates, but administered to a different schedule (RL and HAS). Participants had severe dehydrating diarrhoea/shock or presumptive septic shock (only the latter were eligible to receive HAS), and the principal comparison was between RL and HSD/5D because few participants received HAS. Other aspects of management in all groups followed the WHO guidelines.
Mortality was high (just over 50%) and there was no statistically significant difference in mortality rates between the treatment groups. Other outcomes related to shock (e.g. resolution of shock, oliguria and tachycardia) indicated inadequate correction of shock in both groups, although the isotonic RL fluid was associated with modest improvements and was found to be as safe as hypotonic HSD/5D solution.
This study found that neither the hypotonic HSD/5D nor the isotonic RL resuscitation fluids were effective in reducing mortality or adequately treating shock after 48 hours of treatment. The high priority given to this question in the Delphi study coupled, with the potential to improve survival if shock can be adequately treated, indicates that further research examining resuscitation regimens for shock is needed.
What are the best treatments for children with severe acute malnutrition who have diarrhoea?
This third question that had been prioritised equal first in the Delphi study was addressed by eight studies. Just over half of these studies focused on treating children with acute diarrhoea and SAM (five RCTs of strong or moderate methodological quality50,51,54,55,57), whereas the remainder focused on children with persistent diarrhoea and SAM (three RCTs of strong or weak methodological quality52,53,56).
Four50,51,54,57 of the five trials treating children with acute diarrhoea and SAM compared different ORSs. Of these, two trials compared H-ORS with the standard WHO-ORS. 51,54 Although the trials differed in some respects, the findings were broadly similar and overall favour the use of H-ORS. One trial compared ReSoMal with standard WHO-ORS. 50 There were no deaths in either group; however, one child in the ReSoMal group had a convulsion because of severe hyponatraemia. For the two outcomes of adequate rehydration at 12 hours and over-rehydration, there was no statistically significant difference between groups. The fourth trial to compare ORSs had three arms: glucose ORS, a glucose ORS plus ARS and a rice-based ORS. 57 The rice-based ORS was more beneficial at 72 hours in promoting weight gain and reducing diarrhoeal output than the glucose-based ORS. In all groups, recovery to 80% W/H took about 7 days.
The fifth trial55 investigated standard WHO-ORS with zinc syrup added to therapy compared with WHO-ORS and placebo. This trial reported statistically significant differences in favour of the zinc-supplemented group for three outcomes, but this did not have an impact on weight gain, which was not significantly different between the groups. No safety issues related to the addition of zinc to therapy were reported.
In summary, children with acute diarrhoea benefited from the use of H-ORS compared with the standard WHO-ORS on measures of frequency, duration and recovery from diarrhoea, and consumption of ORS. In contrast, weight gain was significantly higher in those receiving WHO-ORS (one study). 57 WHO-ORS was not significantly different from ReSoMal for adequacy of hydration or mortality, although ReSoMal may pose safety concerns. A rice-based ORS was more beneficial at 72 hours in promoting weight gain and reducing diarrhoeal output than the glucose-based ORSs, whereas the addition of zinc to a WHO-ORS had a favourable impact on some outcomes. It is not clear how generalisable the results of these studies are, given that all five trials50,51,54,55,57 excluded children with severe infections and all took place either in India or in Bangladesh.
Each of the three trials that focused on participants with persistent diarrhoea52,53,56 compared different dietary treatments. One diet in each trial was either entirely53,56 or predominantly52 soy-based and was compared with an elemental diet in two of the trials (either Neocate52 or Vivonex56), or a local KY-based diet in one trial. 53 One of the trials comparing an elemental diet with a soy-based diet had a third arm in which participants received a chicken-based diet. 56
One trial52 reported that the elemental Neocate diet led to statistically significant greater increases in both weight gain and the anthropometric measures of W/A and W/H in comparison with a diet based on skimmed milk and soy. This trial did not report on measures of diarrhoea. In contrast, the three-arm trial56 reported that there were no differences between the elemental Vivonex diet, a soy-based and a chicken-based diet for outcomes of mortality, weight gain, frequency of diarrhoea and recovery, caloric intake and success.
The trial of a soy-based versus a KY-based diet53 reported that weight gain was greater, and statistically significantly so, by the end of the second week in the soy-based group when compared with the KY-based group, although overall recovery was similar.
Studies comparing different diets for children with persistent diarrhoea had conflicting findings, and only two of the three studies reported on diarrhoea outcomes. A comparison in one study of an elemental diet with a skimmed milk and soy-based diet showed significant improvements in anthropometric measures in the group receiving the elemental diet. However, two other studies found either no difference between an elemental diet, a soy-based and a chicken-based diet (for outcomes of mortality, weight gain, frequency of diarrhoea and recovery) or found a significant benefit (for anthropometric measures and ORS consumption) from a soy-based diet compared with a KY-based diet.
What methods are effective in treating infection?
One RCT59 and one retrospective cohort study with control60 investigated the use of antibiotic therapy in children with SAM, but neither focused on treating diagnosed infection. Nevertheless, these studies are included because they provide information about use of antibiotic therapy in the patient group of interest. Both studies were assessed to be of moderate methodological quality.
The RCT59 took place during inpatient therapy for SAM and compared two different systemic broad-spectrum antibiotic regimens. The majority of deaths occurred within 14 days of admission. There were very few infection-related deaths 14 days or more after admission in either group (a total of three deaths in 458 participants overall), and no statistically significant differences between the groups for outcomes relating to weight gain, success rate and length of hospital stay. A statistically significant greater proportion of adverse events occurred with orally administered amoxicillin than with i.m. injection of ceftriaxone.
The retrospective cohort study60 focused on children with uncomplicated SAM receiving home-based treatment with RUTF. One cohort attended malnutrition treatment clinics where antibiotics were not routinely provided, whereas the clinics that the other cohort attended provided amoxicillin routinely for 7 days. After analysing data from each cohort, the number of deaths was described as similar (although no p-value was reported). Overall recovery, however, was substantially greater at 4 weeks in the cohort that received RUTF alone (statistically significantly so in the subgroups with and without oedema, but p-value not reported for group as a whole). By 12 weeks, recovery rates in the two cohorts were described as similar. Trehan and colleagues60 considered that antibiotic-associated diarrhoea and disruption of the intestinal biome could be biologically plausible reasons for the delayed recovery in the cohort receiving antibiotics. However, the study did not report on diarrhoea as an outcome and one of the expert advisory group for this project felt it was unlikely that antibiotic-associated diarrhoea alone could have accounted for such a large difference.
Retrospective observational evidence indicates that the systematic addition of a broad-spectrum antibiotic to the RUTF home-based treatment may have a statistically significant detrimental effect on the recovery of children with uncomplicated SAM during the first 4 weeks, although no effect was shown on mortality. 60 A RCT comparing administration of i.m. ceftriaxone with orally administered amoxicillin, found no difference in effects on outcomes, apart from ceftriaxone being associated with fewer adverse events. 59
What is the clinical effectiveness of interventions in different settings (e.g. hospital, community, emergency)?
Four included studies62–65 of moderate or weak methodological quality investigated the clinical effectiveness of treating malnutrition in different settings. All trials included the inpatient hospital setting, but the type of inpatient care provided varied. Three trials62–64 evaluated alternative settings for the rehabilitation phase of treatment after the same initial treatment for all participants as inpatients. The fourth trial, which had three arms,65 compared inpatient care during both the initial and rehabilitation phases of SAM treatment with either daily ambulatory care for initial and rehabilitation phases or daily ambulatory care for the initial phase followed by rehabilitation in the home setting.
Drawing comparisons between the included studies is difficult because of the numerous differences between them. These include differences in inpatient care (different diets, formulas, staffing), differences in home-based care (different follow-up arrangements, dietary provision) and differences in lengths of intervention and follow-up.
Two studies62,65 included a comparison between inpatient care and daily ambulatory care. Mortality appeared similar between the different settings, with no significant differences. Changes in weight were less clear. One study,65 reported statistically significant differences in favour of hospital inpatient treatment for greater daily weight gain and shorter treatment time to achieve 80% W/H. However, after 12 months, differences in weight gain were no longer statistically significant. The other trial62 that compared inpatient treatment with daily ambulatory care, found that there was no significant difference between the groups in W/H changes, although some patients did not receive the care they were assigned.
Three studies included a comparison between inpatient care and home-based care. 63–65 In all three trials, mortality appears similar between the groups and the proportion of children who died in each study was comparable. Although one study65 found a statistically significant benefit in weight gain for the inpatient group,63,64 the other two studies reported no significant difference between inpatient care and home-based care. The anthropometry outcomes reported by two studies64,65 also statistically significantly favoured inpatient care initially, but at > 12 months’ follow-up this benefit had disappeared. In contrast, a third study63 found a statistically significantly greater improvement anthropometry outcomes in the group treated at home than in those treated as inpatients.
Different comparisons of inpatient, ambulatory and home-based care varied considerably in the nature of the intervention provided. It appears that children receiving inpatient care do as well as, if not better than, those receiving care in the ambulatory or home setting on anthropometric measures and response time to treatment. Longer-term follow-up shows limited differences between the different settings.
Which methods for correcting micronutrient deficiencies are effective?
Evidence was found for treating children with SAM and providing zinc supplements (10 trials68–79), supplementary potassium (one study80), nicotinic acid (one study81) or NTs (one study82). Although the methodological quality of the studies was found to vary, the majority were assessed as either moderate (n = 3) or weak (n = 8). In general, micronutrient deficiencies were assumed to be present and most studies did not test for specified micronutrient deficiencies. None of the studies examined a mix of micronutrients such as those currently recommended in the WHO guidelines for the treatment of SAM, and no studies investigating vitamin A met the inclusion criteria for this systematic review.
In the trials of supplementary zinc, provision varied in a variety of aspects which made it difficult to compare zinc doses across the studies. The comparator was either a placebo (four trials 72,74,75,78,79), no zinc supplement (three trials70,73,77) or a different dose and/or duration of zinc (three trials68,69,71,76).
Mortality was explicitly reported in just two of the studies. 68,69,74,75 One study74,75 reported significantly fewer deaths in the group receiving zinc. Although difficult to compare doses of zinc between studies, if the children in the study are assumed, as an example, to weigh between 3 kg and 12 kg, the provided dose of 10 mg/day would be one of the lowest. In contrast, the other study68,69 to report mortality was halted early when a significant risk of death was identified for participants receiving 6 mg/kg/day zinc in comparison with those children receiving 1.5 mg/kg/day. If the same example is used, with children assumed to weigh between 3 kg and 12 kg, the 6 mg/kg/day dose would be one of the highest provided. Although zinc dose may be one explanation for the difference in findings on mortality reported by these two studies, other factors, such as characteristics of the participants or other aspects of care, may also be important.
All the studies assessing zinc supplements reported the effects of the intervention on weight gain or anthropometry outcomes (with two studies reporting on both types of outcome). 68–79 The three studies that reported findings in favour of zinc were all studies in which a zinc supplement was compared with no supplement, and they provided zinc at doses which were probably higher than those in most other studies. 70,73,77 Although the higher doses of zinc used in these studies may account for the positive effects of zinc reported, it is also worth bearing in mind that these three studies were all rated as methodologically weak with regard to blinding, and received a summary quality rating of weak. 70,73,77 Of the remaining seven studies, five68,69,71,72,78,79 found no significant differences between the groups (four of which received an overall quality rating of strong) and two74–76 (both with an overall quality rating of medium) reported a mixture of results, some in favour of the zinc group and others indicating no significant difference between the groups.
The pattern of results reported for comorbidity outcomes and adverse events (if trials included these outcomes) followed the same pattern, as noted above, for weight gain and anthropometry. Of the five trials reporting on comorbidities,70,72,74–76,79 two found72,79 no significant differences between the groups (both also reported no significant differences for weight gain/anthropometry), two74–76 reported mixed results (alongside mixed results for weight gain/anthropometry) and one70 trial reported a statistically significantly benefit from zinc supplementation. In this last trial, the significant effects from zinc were on time taken to lose oedema and for skin lesions to heal, duration of diarrhoea and anorexia, and on weight gain. One study68,69 reported on the serious adverse event of increased mortality in the study group receiving 6 mg/kg/day zinc in comparison with those receiving 1.5 mg/kg/day. In the remaining nine studies,70–79 either adverse events were not reported, no adverse events were noted or the differences between groups were reported not to have had an adverse impact on rehabilitation.
Although studies assessing the effects of supplementary zinc were heterogeneous, those considered of a higher methodological quality showed no significant benefit from the addition of zinc supplementation. If there is a benefit to be obtained from zinc supplementation, the included evidence is insufficient to determine which dose of zinc might represent the optimal balance between maximising benefits and minimising any harms.
The final three trials80–82 each provided evidence on the use of a different dietary supplement and consequently they are considered separately. 80–82
Providing a dose of potassium80 in the first 7 days of therapy that was 3 mmol/kg above the standard dose led to statistically significant fewer late deaths in an adjusted analysis, but the overall number of deaths in hospital did not differ between the groups. There were also no significant differences in length of hospital stay or weight loss, and the number of days with irritability, diarrhoea or oedema did not differ between the groups. The higher potassium treatment group did, however, have statistically significantly fewer episodes of sepsis, fewer new skin ulcers, fewer days of cough and fewer cases of dyspnoea.
The trial81 reporting on the addition of nicotinic acid to dietary therapy found a statistically significant benefit on mean weight gain in 1 month in favour of the group receiving the supplement, and for each gain of 1 g in weight fewer calories had to be consumed by the group receiving nicotinic acid (no p-value reported for the latter outcome). No other outcomes were reported.
Weight gain and increases in W/L and BMI were similar regardless of whether a milk-based formula supplemented with NTs82 was provided or a formula of the same energy density without added NTs. Reporting of statistical test outcomes was unclear for mean skinfold thickness measures, so it was not certain which statistically significant differences related to within-group differences and which to between-group differences.
Evidence on other micronutrients was limited, with significant benefits from the addition of potassium (i.e. reducing late deaths, sepsis, skin ulcers, coughs and dyspnoea) and nicotinic acid (i.e. weight gain), but no benefit from the addition of NTs.
Strengths and limitations of the assessment
The review has the following strengths:
-
This technology assessment report was conducted independent of vested interest. It was undertaken following the standard methodology and principles for conducting a systematic review. The methods were set out a priori in the research protocol, in which the inclusion criteria, the quality criteria, the data extraction process and the methods applied in the different stages of the review were defined. The research protocol was informed by an advisory group before the project started. The advisory group also reviewed and commented on the final report.
-
A Delphi study was carried out with an international panel of experts in order to identify and prioritise the research questions for the review.
-
The evidence on the effectiveness of interventions to treat severely malnourished children brought together in this report was critically appraised, and the results are presented in a consistent and transparent manner.
In contrast, the review also has certain limitations:
-
There was a lack of evidence for some questions. Also for those questions where there was some evidence, this did not always address the questions that the Delphi panel had identified as being of particular interest (see Appendix 5). For example, the little information available on treating infants < 6 months did not address the question of the most effective therapeutic milk for this age group. Similarly, although one study49 mapped to the question on i.v. fluid administration for treating shock, no studies addressed the question of the feasibility of blood transfusions for shock.
-
Some studies provided limited details of their methods, making quality assessment difficult. Consequently, 12 included studies52,53,58,64,65,70–73,77,78,81,82 were judged to be of weak methodological quality and this may have been partly related to their publication date. Of the 16 included studies that were published before the year 2000,57,62,64,65,68–73,76–81,95 10 were judged to be of weak methodological quality. 53,64,65,70–73,77,78,81 In contrast, among the 12 included studies published in 2000 or later,49–52,54,55,57–60,63,74,75,82 only two52,58,82 were judged to be methodologically weak. Inevitably the nature of clinical trials, in terms of their methods and their reporting in publications, has changed significantly particularly in the last 10–15 years, for example in response to the Consolidated Standards of Reporting Trials (CONSORT) statement, which was first published in 1996. 87
-
The length of follow-up in the majority of the studies was either for the duration of the intervention or to the point of discharge or recovery. A minority of studies followed up the children after discharge, with periods ranging from 14 days to 30 months. This means that there are very few data on readmission or relapse rates. In some studies, the length of the follow-up period was not clear or not reported.
-
A wide range of outcome measures were reported and the units of measurement for some outcome measures varied. This limited our ability to compare outcomes between studies. Not all studies reported on mortality, and the majority of trials offered no details on adverse events; however, it was unclear whether or not the absence of reporting was because there had been no deaths or adverse events. It is possible that deaths and adverse events may have been under-reported by some studies.
-
Variations between studies, for example in the participants recruited, interventions, settings and outcome measures, meant that meta-analysis was inappropriate and therefore not undertaken.
-
Project resources were available to review the evidence for the first six questions for which any evidence was available. This meant that four questions for which evidence was available were not reported on in detail. However, these were considered low priorities by the expert Delphi panel. These questions:
-
– What is the overall effectiveness of current programmes/guidance, e.g. the WHO 10-step plan?
-
– What methods for treating dehydration are effective?
-
– What are the most effective methods for feeding during the initial stages of treatment?
-
– Which methods are effective in the rehabilitation phase?
-
-
Data were not extracted from studies that did not map to any of the research questions considered in the Delphi process or from studies which mapped to questions that were not systematically reviewed. Although all studies were briefly checked to identify whether or not they contained data of interest to the questions considered, it is possible that some relevant evidence may have been missed.
-
Full texts of non-English-language articles identified by the searches were not retrieved because of resource limitations. Again, this means that it is possible that some relevant evidence may have been missed.
Uncertainties
-
The generalisability of the findings in this review is unclear. The results of a multicentre study may be more likely to be generalisable,88 but the majority of the included studies (26 of 30) were conducted in single centres, so there is uncertainty about their generalisability to other centres (e.g. in other countries). Similarly, there is uncertainty regarding the transferability of results from a study conducted, for example, in children aged from 6 months to 2 years to a population of children aged from 4 to 5 years. This particularly holds true where there is variability in the definition of SAM between studies and the use of different classification systems. Also, some interventions may not be generalisable to other populations or settings because of cultural difficulties, available resources or funding limitations (e.g. for additional training of medical staff and/or caregivers). Finally, as far as is known, the included studies were conducted in hospitals, nutritional rehabilitation units or the community [under normal (non-emergency) operating conditions for the particular location]. Therefore, the generalisability to major emergency settings that may present additional logistical and operational challenges, for example during conflict or widespread famine situations, is not known.
-
No trial evidence was found that specifically evaluated the management of severely malnourished children who were also HIV+ve in terms of how their treatment may differ from children who were HIV–ve. Just 549,50,52,74,80 of the 30 studies that were data extracted reported that children who were HIV+ve formed part of the total study population. One study56 excluded children who were HIV+ve. Two of the studies49,52 that were data extracted, and whose study populations included children who were HIV+ve, provide some information regarding outcomes in HIV–ve versus HIV+ve children. One study52 reported that, although mortality was higher in HIV+ve children than in HIV–ve children, the benefits of the intervention (an elemental diet) were observed in both HIV–ve and HIV+ve children. The second study49 reported that mortality was similar in HIV+ve and HIV–ve children and HIV infection did not significantly increase the risk of death. Finally, one further study,89 which was not reported on in detail (and not data extracted), reported that, although home-based therapy with RUTF led to better outcomes than home-based therapy with traditional foods, the proportion of HIV+ve children reaching 100% W/H was smaller (56% vs 84% for HIV–ve children) and their recovery time was longer (86 days vs 35 days). These data suggest that it may be possible to extend the outcomes of the included studies to a HIV+ve population. However, the benefits may not be as great and, furthermore, the interaction with other factors such as antiretroviral therapy (ART) is not known.
-
There may have been an additional cost associated with some interventions (e.g. provision of elemental formula feed or dietary supplements). In some trials additional resources (e.g. community health workers and day-care facilities) may have been needed to implement the interventions being investigated. Additional costs and/or the need for additional resources could be a limiting factor in sustaining the implementation of an effective intervention beyond the trial period, or in trying to implement an intervention in additional locations. Little is known about how sustainable nutritional programmes would be in any particular country given such factors as the occurrence of severe droughts or other natural disasters, civil conflicts, government unrest, etc.
-
No studies were identified that focused on identifying the factors limiting full implementation of treatment programmes, but some factors mentioned in the studies reviewed for other questions included:
-
– difficulty in obtaining (due to availability and/or cost) commercial supplies of formula, vitamin mix or other dietary supplement
-
– insufficient staff to provide monitoring as frequently as indicated or to provide individualised doses of micronutrients to patients
-
– caretakers’ resistance to aspects of treatment
-
– caretakers did not have sufficient time and/or resources to implement intervention fully. This applied to studies in the inpatient, day-care and community settings.
-
-
As few studies continued to follow up children after recovery, there are uncertainties about the impact of the interventions on the longer-term survival and morbidity of children with SAM.
-
The treatment of children with SAM can be considered a complex intervention (an intervention with several interacting components). Management based on the WHO guideline for treatment of SAM is generally accepted to have improved survival, but it is not clear whether all the facets of management have been optimised. As already noted, the priority areas identified by the Delphi process tended to focus on particular aspects of management. However, in studies focusing on one facet of management, which varied between trial arms, children were also receiving other treatment during the trial period. Often this was as part of an overall management strategy, such as that set out in the WHO guideline, but just over half of the included studies were published in 1999 or earlier and so were undertaken before the 1999 WHO guidelines10 were published. It is uncertain how the other aspects of management may have impacted on the primary outcomes of the systematic review (mortality and weight gain), particularly in the earlier studies, in which overall management may have been inadequate. For example, it is not known whether an intervention effect may have been obscured or enhanced if one or more other aspects of treatment were not optimal. Similarly, when comparing different trials, it is not known what proportion of any difference in findings from two different trials could be due to differences between interventions or to more general differences in the overall management of SAM.
Other relevant factors
-
The included studies were published between 1982 and 2010 and, in general, employed the criteria for SAM in place at the time the research took place. However, as illustrated in the background section (see Chapter 1), ‘standard criteria’ have been revised more than once during the past decades. Our aim was to limit the variation in the children included in the different studies to allow comparison of the interventions assessed. However, it has to be acknowledged that, despite the care we have taken, there may be some differences, and some of the children enrolled in the included studies might not meet the current WHO criteria for SAM.
-
There is an absence of evidence, limited evidence or a lack of good-quality evidence for several of the questions that were prioritised by the Delphi process. Some questions relate to aspects of management that are included in the WHO guidelines for the treatment of SAM. In particular, treatment of children who are HIV+ve, emergency treatment of shock, treatment of diarrhoea, infections, comorbidities such as TB and correction of micronutrient deficiencies. More good quality research is needed to inform guidelines such as those produced by the WHO.
-
About half of the included studies calculated the sample size needed to achieve sufficient power for their study objective. However, not all of these trials achieved sufficient statistical power for the outcomes of interest to this review. In one trial sample size was calculated for a cost outcome, and in another study sample size was calculated for the whole trial population, of which children with SAM were a subgroup. Recruitment to three trials was suspended because of adverse outcomes, and in one study the outcomes were not as had been anticipated during the calculation of sample size, which led to the study being underpowered. Finally, in one study exclusion of participants after recruitment meant that sample size fell below the desired level.
Chapter 6 Conclusions
Implications for service provision
An international panel of experts reached a consensus when asked to identify priority areas for consideration in this systematic review of interventions to treat severely malnourished infants and children. However, this systematic review found that evidence was either lacking or was limited for many of the prioritised research questions that were systematically reviewed in depth. Therefore, although it has not been possible to draw firm conclusions from the evidence that can inform current service provision, a number of areas where research is needed have been identified.
Suggested research priorities
In many countries, current service provision for the treatment of SAM in infants and children is based on the WHO 10-step approach. 10,37,38 This is a complex intervention and there is scope to improve outcomes, including survival, if all the necessary steps can be implemented optimally. It should be recognised, however, that there are many potential difficulties when undertaking research into the effectiveness of treatments for SAM in children. Some research questions may be relatively more straightforward to investigate by a RCT than others (e.g. provision of a micronutrient supplement during the rehabilitation phase of treatment in comparison with i.v. fluid administration for shock during the initial phase of treatment), and there may be fewer practical difficulties in certain settings than in others (e.g. a teaching hospital in comparison with a rural feeding centre or a community setting in comparison with a refugee camp). Thus, there may be a tension between the relative ease of conducting high-quality research and how generalisable the outcome will be to the majority of locations where children with SAM receive treatment. Further research will need to take account of these concerns in developing pragmatic studies that are appropriately designed, rigorously conducted and accurately reported. Although experimental studies are preferred, it is recognised that opportunities may be limited given the nature of the area under study and the availability of funding. As such, well-conducted quasi-experimental and observational studies may provide important evidence.
One approach to research in this area may be to divide the WHO 10-step plan into packages of care (care bundles), so that different care bundles can be compared with one another. It will be necessary for such studies to report in detail on the package of care provided. This should help researchers and policy-makers in the future to make comparisons between studies, and to judge what contribution the differences in overall management may have made to the differences in outcomes between studies.
A difficulty encountered when reviewing and interpreting the evidence was the variation in the criteria used to define SAM. Future trials should include children identified using the current WHO criteria, and ideally should involve more than one centre to generate results with better generalisability to other locations and to aid comparison between different trials.
One of the top-ranked questions focused on i.v. fluid administration for the treatment of shock, which is a cause of high mortality in children with SAM. The only evidence to inform this question was a RCT49 that found that neither of the interventions investigated was effective in reducing mortality or adequately correcting shock. Further prospective RCTs of i.v. resuscitation regimens for shock are therefore needed. 49 Any RCT should be informed by an initial pilot study and should include measures of cardiac dysfunction and haemodynamic response to fluid expansion.
Furthermore, there is a need to optimise management in the specific subgroups identified among the top-ranked priorities for consideration, but for which evidence is lacking, i.e. infants aged < 6 months old, and infants and children with SAM who are HIV+ve. These priority areas are broader than a single research question and, therefore, there may be value in conducting further research to determine what the priority research question(s) are for each priority area. For instance, included on the scoring sheet during rounds 2 and 3 of the Delphi study alongside the overarching question ‘How should management of HIV-infected children with SAM differ from those who are severely malnourished but HIV–ve?’ were four example research questions: (1) how do fluid and electrolyte needs differ?; (2) how effective is zinc in the treatment of HIV+ve children?; (3) what is the most effective use of antibiotics for these patients?; and (4) what is the most effective stage of malnutrition treatment at which to start treatment with ARTs?
The use of antibiotics is another area where more research is needed because the topic was ranked highly, and yet little research was found that met the inclusion criteria of the review. This is another area where there is scope for further prioritisation of potential research questions.
Finally, additional research could be conducted on many other aspects of the management of SAM in children < 5 years, including the areas not mentioned above, which were also prioritised in the Delphi study, but for which little or no research was identified [e.g. the use of multivitamin supplements, optimum dose of vitamin A, or management strategies for children with TB or other comorbidities (other than HIV, covered above)]. It should also be remembered that although some research areas were considered a greater priority than others, there is also scope for research into questions of a lower priority that were not included among the questions systematically reviewed in depth for this study (e.g. overall effectiveness of the current WHO 10-step plan, clinical effectiveness of monitoring for and treating hypoglycaemia and hypothermia, or emotional stimulation through play).
Acknowledgements
We would like to thank members of our advisory group who provided expert advice and comments on the protocol and/or a draft of this report.
Professor Ann Ashworth, Emeritus Professor of Community Nutrition, London School of Hygiene and Tropical Medicine, London, UK.
Dr Bruce Cogill, PhD, Chief, Nutrition Division, Office of Health, Infectious Diseases and Nutrition, Bureau for Global Health, US Agency for International Development.
Professor Kathryn Maitland, Paediatric Tropical Infectious Diseases and Critical Care, Imperial College, London. Based at KEMRI Wellcome Trust Programme, Kilifi, Kenya.
Dr Nigel Rollins, HIV Advisor, Newborn and Child Health and Development, Department of Child Adolescent Health and Development, WHO, Geneva, Switzerland.
Professor Harshpal Singh Sachdev, Senior Consultant Paediatrics and Clinical Epidemiology, Sitaram Bhartia Institute of Science and Research, New Delhi, India.
Mrs Zita Weise Prinzo, Nutrition in the Life Course Unit, Department of Nutrition for Health and Development, WHO, Geneva, Switzerland.
Declared competing interests of advisory group
Professor Ann Ashworth has received payment for manuscript preparation for the WHO, has received travel and accommodation expenses from WHO and helped (unpaid) to develop the WHO guidelines and the WHO training course to improve the management of severe malnutrition (in 1999, 2000 and 2003, respectively). Professor Kathryn Maitland has had travel and/or accommodation expenses reimbursed by the Paediatric Critical Care Society, the British Association for Parenteral and Enteral Nutrition, Médecins Sans Frontières, WHO and the Wellcome Trust. The other members of the advisory group, Dr Bruce Cogill, Dr Nigel Rollins, Professor Harshpal Singh Sachdev and Mrs Zita Weise Prinzo, have no competing interests to declare.
We would also like to thank those who agreed to and took part in the Delphi Study:
Dr Tahmeed Ahmed, Head of Nutrition Programme, ICDDR, Bangladesh, Bangladesh.
Dr Beatrice Amadi, Consultant Paediatrician, Department of Paediatrics and Child Health, University Teaching Hospital, Lusaka, Zambia.
Professor Andrew Argent, Associate Professor, School of Child and Adolescent Health, University of Cape Town and Medical Director, Paediatric Intensive Care, Red Cross War Memorial Children’s Hospital, Cape Town, South Africa.
Professor Ann Ashworth, Emeritus Professor of Community Nutrition, London School of Hygiene and Tropical Medicine, London, UK.
Dr Paluka Bahwere, International Advisor, Valid International, Oxford, UK.
Professor Zulfiqar Bhutta, Department of Paediatrics and Child Health, Aga Khan University, Karachi, Pakistan.
Dr Eunyong Chung, Division of Nutrition, US Agency for International Development, Global Health, Office of Health, Infectious Diseases and Nutrition, Nutrition Division, Washington, DC, USA.
Dr Bruce Cogill, PhD, Chief, Nutrition Division, Office of Health, Infectious Diseases and Nutrition, Bureau for Global Health, US Agency for International Development, Washington, DC, USA.
Professor Michael Golden, County Donegal, Ireland.
Dr Abel Hailu, MD, Valid International, Oxford, UK.
Professor Kathryn Maitland, Paediatric Tropical Infectious Diseases and Critical Care, Imperial College, London. Based at KEMRI Wellcome Trust Programme, Kilifi, Kenya.
Dr Nigel Rollins, HIV Advisor, Newborn and Child Health and Development, Department of Child Adolescent Health and Development, WHO, Geneva, Switzerland.
Professor Harshpal Singh Sachdev, Senior Consultant Paediatrics and Clinical Epidemiology, Sitaram Bhartia Institute of Science and Research, New Delhi, India.
Professor Andrew Tomkins, UCL Centre of International Health and Development (CIHD), Institute of Child Health, London, UK.
Mrs Zita Weise Prinzo, Nutrition in the Life Course Unit, Department of Nutrition for Health and Development, WHO, Geneva, Switzerland.
Dr Marzella Wüstefeld, United Nations System Standing Committee on Nutrition, c/o WHO, Geneva, Switzerland.
We are also grateful to Professor James Raftery, chair of the NIHR Evaluation, Trials and Studies Coordinating Centre (NETSCC) and Director of The Wessex Institute, University of Southampton, Southampton, UK, who was appointed the independent chairperson for the Delphi process by the NIHR HTA programme; Professor Alan A Jackson, Professor of Human Nutrition, School of Medicine, University of Southampton, Southampton, UK, and Dr Penelope Nestel, PhD, Reg PHN, FHEA, Programme Director, MSc Public Health Nutrition, School of Medicine, University of Southampton, Southampton, UK, who advised on the protocol; Ms Karen Welch, Information Scientist, Southampton Health Technology Assessments Centre (SHTAC), University of Southampton, Southampton, UK, for developing the search strategy, and running the literature searches; Dr Jill Colquitt, Senior Research Fellow, SHTAC, University of Southampton, Southampton, UK, who acted as the internal editor by reviewing a draft of the report; and Dr Pamela Fergusson, Academy for Educational Development, Toronto, ON, Canada who reviewed a draft of the report.
Contribution of authors
J Picot, research fellow, developed the research protocol, drafted the background section, assessed studies for inclusion, mapped the evidence base to prioritised research questions, extracted data from and quality assessed included studies, synthesised evidence, drafted and edited the final report, and project managed the study.
D Hartwell, research fellow, developed the research protocol, drafted the background section, assessed studies for inclusion, mapped the evidence base to prioritised research questions, extracted data from and quality assessed included studies, synthesised evidence, and drafted and edited the final report.
P Harris, research fellow, developed the research protocol, assessed studies for inclusion, mapped the evidence base to prioritised research questions, extracted data from and quality assessed included studies, synthesised evidence, and drafted and edited the final report.
D Mendes, research fellow, developed the research protocol, assessed studies for inclusion, mapped the evidence base to prioritised research questions, extracted data from and quality assessed included studies, synthesised evidence, and drafted and edited the final report.
AJ Clegg, Professor/Director of SHTAC, developed the research protocol, assisted in the development of the search strategies, drafted the background section, conducted and analysed the results of the Delphi study, assessed studies for inclusion, mapped the evidence base to prioritised research questions, extracted data from and quality assessed included studies, synthesised evidence, drafted and edited the final report, and was the principal investigator and acted as guarantor.
A Takeda, senior research fellow, developed the research protocol, assisted in the development of the search strategies, drafted the background section, conducted and analysed the results of the Delphi study, assessed studies for inclusion, mapped the evidence base to prioritised research questions, and drafted and edited the final report.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- World Health Organization . Nutrition n.d. www.who.int/topics/nutrition/en/index.html (accessed 7 December 2010).
- World Health Organization . 10 Facts on Nutrition n.d. www.who.int/features/factfiles/nutrition/en/index.html (accessed 7 December 2010).
- Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 2008;371:243-60.
- Shetty P. Malnutrition and undernutrition. Medicine 2003;31:18-22.
- Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter I, et al. Maternal and child undernutrition 2. Maternal and child undernutrition: consequences for adults health and human capital. Lancet 2008;371:23-40.
- Trowell HC, Davies JN, Kwashiorkor I. Nutritional background history, distribution, and incidence. Br Med J 1952;2:796-8.
- Jackson AA, Warrell DA, Cox TM, Firth JD, Benz EJ. Oxford textbook of medicine. Oxford: Oxford University Press; 2003.
- Sheeran J. The challenge of hunger. Lancet 2008;371:180-1.
- Pelletier DL, Frongillo EA, Schroeder DG, Habicht JP. The effects of malnutrition on child mortality in developing countries. Bull World Health Organ 1995;73:443-8.
- World Health Organization . Management of Severe Malnutrition: A Manual for Physicians and Other Senior Health Workers 1999.
- World Health Organization . Serious Childhood Problems in Countries With Limited Resources 2004.
- World Health Organization and United Nations Children’s Fund . WHO Child Growth Standards and the Identification of Severe Acute Malnutrition in Infants and Children. A Joint Statement by the World Health Organization and the United Nations Children’s Fund 2009.
- World Health Organization Multicentre Growth Reference Study Group . WHO Child Growth Standards: Length Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development 2006.
- de Onis M, Garza C, Onyango AW, Martorell R. WHO child growth standards. Acta Paediatr 2006;450:1-101.
- Collins S, Dent N, Binns P, Bahwere P, Sadler K, Hallam A. Management of severe acute malnutrition in children. Lancet 2006;368:1992-2000.
- Berkley J, Mwangi I, Griffiths K, Ahmed I, Mithwani S, English M, et al. Assessment of severe malnutrition among hospitalized children in rural Kenya: comparison of weight for height and mid upper arm circumference. JAMA 2005;294:591-7.
- Kerac M, McGrath M, Grijalva-Eternod C, Bizouerne C, Saxton J, Bailey H, et al. Management of Acute Malnutrition in Infants (MAMI) project. Inter-agency Standing Committee, Action Contre La Faim (France), Emergency Nutritional Network (UK), UCL Centre for International Health and Development (UK); 2010.
- Hamill P, Drizd T, Johnson C, Reed R, Roche AF, Moore W. Physical growth: National Center for Health Statistics percentiles. Am J Clin Nutr 1979;32:607-29.
- Dibley MJ, Goldsby JB, Staehling NW, Trowbridge FL. Development of normalized curves for the international growth reference: historical and technical considerations. Am J Clin Nutr 1987;46:736-48.
- de Onis M, Yip R. The WHO growth chart: historical considerations and current scientific issues. Bibliotheca Nutritio Et Dieta 1996;53:74-89.
- de Onis M, Wijnhoven TMA, Onyango AW. Worldwide practices in child growth monitoring. J Paediatr 2004;144:461-5.
- de Onis M, Onyango AW, Borghi E, Garza C, Yang H. Comparison of the World Health Organization (WHO) child growth standards and the National Center for Health Statistics/WHO international growth reference: implications for child health programmes. Public Health Nutr 2006;9:942-7.
- Waterlow JC. Classification and definition of protein-calorie malnutrition. BMJ 1972;3:566-9.
- Jelliffe DB, Jelliffe EFP. Age-independent anthropometry. Am J Clin Nutr 1971;24:1377-9.
- Stuart HC, Stevenson SS, Nelson WE. Textbook of pediatrics. Philadelphia, PA: Saunders; 1959.
- Gómez F, Galvan RR, Frenk S, Munoz JC, Chavez R, Vazquez J. Mortality in second and third degree malnutrition. J Trop Pediatr 1956;2:77-83.
- Bengoa JM. Classification of infantile malnutrition. WHO Chronicle 1970;24:552-6.
- Dugdale AE. An age-independent anthropemetric index of nutritional status. Am J Clin Nutr 1971;24:174-6.
- Waterlow JC, Buzina R, Keller W, Lane JM, Nichaman MZ, Tanner JM. The presentation and use of height and weight data for comparing the nutritional status of groups of children under the age of 10 years. Bull World Health Organ 1977;55:489-98.
- Bhatnagar S, Lodha R, Choudhury P, Sachdev HPS, Shah N, Narayan S, et al. Guidelines on hospital based management of severely malnourished children (adapted from WHO guidelines). Indian Pediatr 2007;44:443-61.
- Caulfield LE, de Onis M, Blossner M, Black RE. Undernutrition as an underlying cause of child deaths associated with diarrhea, pneumonia, malaria, and measles. Am J Clin Nutr 2004;80:193-8.
- Black R, Morris S, Bryce J. Where and why are 10 million children dying every year?. Lancet 2003;361:2226-34.
- Measure DHS. Demographic and Health Surveys n.d. www.measuredhs.com/aboutdhs/ (accessed 7 December 2010).
- World Health Organization, Department of Child and Adolescent Health and Development (CAH) and HIV/AIDS . WHO Recommendations on the Management of Diarrhoea and Pneumonia in HIV-Infected Infants and Children: Integrated Management of Childhood Illness (IMCI) 2010.
- Rollins N. Guidelines for an integrated approach to the nutritional care of HIV-infected children (6 months–14 years). Geneva: World Health Organization; 2009.
- Fergusson P, Tomkins A. HIV prevalence and mortality among children undergoing treatment for severe acute malnutrition in sub-Saharan Africa: a systematic review and meta-analysis. Trans R Soc Trop Med Hyg 2009;103:541-8.
- Ashworth A, Khanum S, Jackson A, Schofield C. Guidelines for the inpatient treatment of severely malnourished children. Geneva: World Health Organization; 2003.
- World Health Organization, Department of Child and Adolescent Health and Development . Management of the Child With a Serious Infection or Severe Malnutrition – Guidelines for Care at the First-Referral Level in Developing Countries 2000.
- Connelly A, Hill AA. Should the rehabilitation phase of treatment for children with severe malnutrition (marasmus or kwashiorkor) take place within communities or as inpatients?. International Child Health Review Collaboration; 2008.
- World Health Organization, World Food Programme, United Nations System Standing Committee on Nutrition and the United Nations Children’s Fund . Community-Based Management of Severe Acute Malnutrition 2007.
- Collins S, Sadler K, Dent N, Khara T, Guerrero S, Myatt M, et al. Key issues in the success of community-based management of severe malnutrition. Food Nutr Bull 2006;27:S49-S82.
- Manary MJ, Ndkeha MJ, Ashorn P, Maleta K, Briend A. Home based therapy for severe malnutrition with ready-to-use food. Arch Dis Child 2004;89:557-61.
- Bryce J, Coitinho D, Darnton-Hill I, Pelletier D, Pinstrup-Andersen P. Maternal and child undernutrition 4. maternal and child undernutrition: effective action at national level. Lancet 2008;371:65-81.
- Save the Children . Acute Malnutrition Summary Sheet n.d. www.savethechildren.org/atf/cf/%7B9def2ebe-10ae-432c-9bd0-df91d2eba74a%7D/Acute-Malnutrition-Summary-Sheet.pdf (accessed 9 March 2011).
- Nutritional Subcommittee of Indian Academy of Pediatrics . Report of the Convener. Indian J Pediatr 1972;9.
- Thomas BH, Ciliska D, Dobbins M, Micucci S. A process for systematically reviewing the literature: providing the research evidence for public health nursing interventions. Worldviews Evid Based Nurs 2004;1:176-84.
- Nu Shwe T. Logical and Logistic Aspects of Management of a Child With Severe Malnutrition and Serious Infection in Hospital Settings: What Is New, Challenges and Way Forward. Nutrition Goals for Asia-Vision 2020 n.d.
- Hossain MM, Hassan MQ, Rahman MH, Kabir AR, Hannan AH, Rahman AK. Hospital management of severely malnourished children: comparison of locally adapted protocol with WHO protocol. Indian Pediatr 2009;46:213-7.
- Akech S, Karisa J, Nakamya P, Boga M, Maitland K. Phase II trial of isotonic fluid resuscitation in Kenyan children with severe malnutrition and hypovolaemia. BMC Pediatr 2010;10.
- Alam NH, Hamadani JD, Dewan N, Fuchs GJ. Efficacy and safety of a modified oral rehydration solution (ReSoMaL) in the treatment of severely malnourished children with watery diarrhea. J Pediatr 2003;143:614-19.
- Alam S, Afzal K, Maheshwari M, Shukla I. Controlled trial of hypo-osmalar versus World Health Organization oral rehydration solution. Indian Pediatr 2000;37:952-60.
- Amadi B, Mwiya M, Chomba E, Thomson M, Chintu C, Kelly P, et al. Improved nutritional recovery on an elemental diet in Zambian children with persistent diarrhoea and malnutrition. J Trop Pediatr 2005;51:5-10.
- Bhutta ZA, Molla AM, Issani Z, Badruddin S, Hendricks K, Snyder JD. Nutrient absorption and weight gain in persistent diarrhea: comparison of a traditional rice-lentil/yogurt/milk diet with soy formula. J Pediatr Gastroenterol Nutr 1994;18:45-52.
- Dutta P, Mitra U, Manna B, Niyogi SK, Roy K, Mondal C, et al. Double blind, randomised controlled clinical trial of hypo-osmolar oral rehydration salt solution in dehydrating acute diarrhoea in severely malnourished (marasmic) children. Arch Dis Child 2001;84:237-40.
- Dutta P, Mitra U, Datta A, Niyogi SK, Dutta S, Manna B, et al. Impact of zinc supplementation in malnourished children with acute watery diarrhoea. J Trop Pediatr 2000;46:259-63.
- Nurko S, Garcia-Aranda JA, Fishbein E, Perez-Zuniga MI. Successful use of a chicken-based diet for the treatment of severely malnourished children with persistent diarrhea: a prospective, randomized study. J Pediatr 1997;131:405-12.
- Alam NH, Islam S, Sattar S, Monira S, Desjeux JF. Safety of rapid intravenous rehydration and comparative efficacy of 3 oral rehydration solutions in the treatment of severely malnourished children with dehydrating cholera. J Pediatr Gastroenterol Nutr 2009;48:318-27.
- Amadi B. Role of food antigen elimination in treating children with persistent diarrhea and malnutrition in Zambia. J Pediatr Gastroenterol Nutr 2002;34:S54-S56.
- Dubray C, Ibrahim SA, Abdelmutalib M, Guerin PJ, Dantoine F, Belanger F, et al. Treatment of severe malnutrition with 2-day intramuscular ceftriaxone vs 5-day amoxicillin. Ann Trop Paediatr 2008;28:13-22.
- Trehan I, Amthor RE, Maleta K, Manary MJ. Evaluation of the routine use of amoxicillin as part of the home-based treatment of severe acute malnutrition. Trop Med Int Health 2010;15:1022-8.
- World Health Organization . A Growth Chart for International Use in Maternal and Child Health Care. Guidelines for Primary Health Care Personnel 1978.
- Chapko MK, Prual A, Gamatie Y, Maazou AA. Randomized clinical trial comparing hospital to ambulatory rehabilitation of malnourished children in Niger. J Trop Pediatr 1994;40:225-30.
- Ciliberto MA, Sandige H, Ndekha MJ, Ashorn P, Briend A, Ciliberto HM, et al. Comparison of home-based therapy with ready-to-use therapeutic food with standard therapy in the treatment of malnourished Malawian children: a controlled, clinical effectiveness trial. Am J Clin Nutr 2005;81:864-70.
- Heikens GT, Schofield WN, Dawson SM, Waterlow JC. Long-stay versus short-stay hospital treatment of children suffering from severe protein-energy malnutrition. Eur J Clin Nutr 1994;48:873-82.
- Khanum S, Ashworth A, Huttly SR. Controlled trial of three approaches to the treatment of severe malnutrition. Lancet 1994;344:1728-32.
- Heikens GT, Schofield WN, Dawson S, Grantham-McGregor S. The Kingston project. I. growth of malnourished children during rehabilitation in the community, given a high energy supplement. Eur J Clin Nutr 1989;43:145-60.
- Anon . Classification of infantile malnutrition. Lancet 1970;296:302-3.
- Doherty CP, Sarkar MA, Shakur MS, Ling SC, Elton RA, Cutting WA. Zinc and rehabilitation from severe protein-energy malnutrition: higher-dose regimens are associated with increased mortality. Am J Clin Nutr 1998;68:742-8.
- Doherty CP, Crofton PM, Sarkar MA, Shakur MS, Wade JC, Kelnar CJ, et al. Malnutrition, zinc supplementation and catch-up growth: changes in insulin-like growth factor I, its binding proteins, bone formation and collagen turnover. Clin Endocrinol 2002;57:391-9.
- Gatheru Z, Kinoti S, Alwar J, Mwita M. Serum zinc levels in children with kwashiorkor aged one to three years at Kenyatta National Hospital and the effect of zinc supplementation during recovery. East Afr Med J 1988;65:670-9.
- Golden BE, Golden MH. Effect of zinc on lean tissue synthesis during recovery from malnutrition. Eur J Clin Nutr 1992;46:697-706.
- Hemalatha P, Bhaskaram P, Khan MM. Role of zinc supplementation in the rehabilitation of severely malnourished children. Eur J Clin Nutr 1993;47:395-9.
- Khanum S, Alam AN, Anwar I, Akbar AM, Mujibur RM. Effect of zinc supplementation on the dietary intake and weight gain of Bangladeshi children recovering from protein–energy malnutrition. Eur J Clin Nutr 1988;42:709-14.
- Makonnen B, Venter A, Joubert G. A randomized controlled study of the impact of dietary zinc supplementation in the management of children with protein-energy malnutrition in Lesotho. I: mortality and morbidity. J Trop Pediatr 2003;49:340-52.
- Makonnen B, Venter A, Joubert G. A randomized controlled study of the impact of dietary zinc supplementation in the management of children with protein-energy malnutrition in Lesotho. II: special investigations. J Trop Pediatr 2003;49:353-60.
- Schlesinger L, Arevalo M, Arredondo S, Diaz M, Lonnerdal B, Stekel A. Effect of a zinc-fortified formula on immunocompetence and growth of malnourished infants. Am J Clin Nutr 1992;56:491-8.
- Simmer K, Khanum S, Carlsson L, Thompson RP. Nutritional rehabilitation in Bangladesh – the importance of zinc. Am J Clin Nutr 1988;47:1036-40.
- Vasudevan A, Shendurnikar N, Kotecha PV. Zinc supplementation in severe malnutrition. Indian Pediatr 1997;34:236-8.
- Bhutta ZA, Nizami SQ, Isani Z. Zinc supplementation in malnourished children with persistent diarrhea in Pakistan. Pediatrics 1999;103.
- Manary MJ, Brewster DR. Potassium supplementation in kwashiorkor. J Pediatr Gastroenterol Nutr 1997;24:194-201.
- Philip L, Suguna Bai NS, Sathy S, Soman CR. Effect of addition of nicotinic acid to the diet on the rate of weight gain of marasmic children. Indian Pediatr 1982;19:775-7.
- Vásquez-Garibay E, Mendez-Estrada C, Romero–velarde E, Vizmanos B, Campollo-Rivas O. Impact of nucleotides and nutritional support on growth and body composition of severely malnourished infants. Nutr Res 2005;25:727-36.
- Plum LM, Rink L, Haase H. The essential toxin: impact of zinc on human health. Int J Environ Res Public Health 2010;7:1342-65.
- de Benoist B, Darnton-Hill I, Davidsson L, Fontaine O, Hotz C. Conclusions of the joint WHO/UNICEF/IAEA/IZiNCG interagency meeting on zinc status indicators. Food Nutr Bull 2007;28:S480-S484.
- Darwin R, Harahap M, Tjakradinata D. Skin changes in protein calorie deficiency malnutrition. Int J Dermatol 1970;9:247-52.
- Interagency collaboration report by Emergency Nutrition Network (ENN); International Baby Food Action Network (IBFAN); Fondation Terres des hommes; CARE USA; Action Contre la Faim; UNICEF; United Nations High Commissioner for Refugees (UNHCR); and the World Health Organization . Infant Feeding in Emergencies: Module 2 Version 1.1 for Health and Nutrition Workers in Emergency Situations 2007. www.who.int/nutrition/publications/emergencies/ife_module2/en/index.html.
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA 1996;276:637-9.
- Evans D. Hierarchy of evidence: a framework for ranking evidence evaluating healthcare interventions. J Clin Nurs 2003;12:77-84.
- Ndekha MJ, Manary MJ, Ashorn P, Briend A. Home-based therapy with ready-to-use therapeutic food is of benefit to malnourished, HIV-infected Malawian children. Acta Paediatr 2005;94:222-5.
- NHS Centre for Reviews and Dissemination . Undertaking Systematic Reviews of Research on Effectiveness: CRD Guidelines for Those Carrying Out or Commissioning Reviews 2001.
- Spitzer WO, Lawrence V, Dales R, Hill G, Archer MC, Clarck P. Links between passive smoking and disease: a best evidence synthesis. Clin Invest Med 1990;13:17-42.
- World Health Organization . Manual for Laboratory Investigation of Acute Enteric Infection. Programme for Control of Diarrhoeal Diseases. CDD 83.3 1983.
- Ellerstein NS, Ostrov BE. Growth patterns in children hospitalised because of caloric deprivation failure to thrive. Am J Dis Child 1985;139:164-6.
- World Health Organization Working Group . Use and interpretation of anthropometric indicators of nutritional status. Bull WHO 1986;64:929-41.
- Winer BJ. Statistical principles in experimental design. New York, NY: McGraw-Hill; 1981.
- Picou D, Alleyne GAO, . Malnutrition and gastroenteritis in children: a manual for hospital management. Kingston, Jamaica: Caribbean Food and Nutrition Institute; 1975.
- Waterlow JC. Protein–energy malnutrition. Arnold: Sevenoaks; 1992.
- World Health Organization . The Treatment and Management of Severe Protein-Energy Malnutrition 1981.
- Ashworth A, Khanum S. Cost-effective treatment for severely malnourished children: what is the best approach?. Health Policy Plan 1997;12:115-21.
- Khanum S, Ashworth A, Huttly SR. Growth, morbidity, and mortality of children in Dhaka after treatment for severe malnutrition: a prospective study. Am J Clin Nutr 1998;67:940-5.
- Waterlow JC, Beaton GH, Bengoa JM. Nutrition in preventive medicine. Geneva: World Health Organization; 1976.
- Wellcome Trust Working Party . The classification of infantile malnutrition. Lancet 1970;ii:302-3.
- Fernandes EJ, Kahn HL. Clinical method for atomic absorption spectroscopy. Clin Chem Newsl 1971;3:24-8.
- Frisancho AR. Anthropometric standards for the assessment of growth and nutritional status. Ann Arbor, MI: The University of Michigan Press; 1990.
- McLaren DS, Pellett DI, Reed WWC. A simple scoring system of classifying the severe forms of protein-calorie malnutrition of early childhood. Lancet 1967;289:533-5.
- Taha TE, Canner JK, Wange A, Chiphangwi JD, Liamba NG, Miotti PG, et al. Research on human immunodeficiency virus (HIV) in Malawi: the John Hopkins University-Ministry of Health (JHU-MOH) project. Malawi Med J 1994;10:6-11.
- Dunn DT, Newell ML, Ades AE, Peckham CS. Risk of human immunodeficiency virus type 1 transmission through breastfeeding. Lancet 1992;340:585-8.
Appendix 1 Protocol methods
Systematic review
The systematic review will be undertaken in accordance with guidance from the Centre for Reviews and Dissemination (University of York). 90
Delphi study to specify the research question
A Delphi method will be used to help identify and prioritise the key research questions that should be addressed by the systematic review. Through an iterative process a panel of experts will have the opportunity to identify questions and then reach a consensus about which they consider most important. There will be three-rounds in the Delphi process. In the first round a set of questions identified in the development of the research protocol will be sent to the panel members. They will be asked to score these on the basis of their importance, adding any additional questions. Responses from the panel members will be analysed and the questions prioritised on the basis of the median score [plus upper quartile (UQ) and lower quartile (LQ)]. A subset of the questions that receive the highest median scores will go forward to the subsequent rounds. Any additional questions suggested by the panel members will be assessed to see if they are already encompassed within the original list. Up to five new questions may be included in the second round, with selection based on the relevance to the scope of the review and the frequency with which they are identified by the panel members. For the second and third rounds, panel members will see the median score for each question from the previous round and decide whether or not they wish to revise their original score (i.e. whether they wish to move closer to the group consensus or maintain their original score for the question). In addition, they will be asked to score any new questions introduced as part of the first round. At the conclusion of the third round the panel members will be sent a list of the research questions in priority order for information. The prioritised list will form the basis from which the research questions to be addressed by the systematic review will be identified, with the final decision on how many questions will be addressed based on the extent of the evidence and the resources available for the research. Conduct of the Delphi process will be overseen by an independent Chair appointed by NIHR HTA programme.
Literature search
Literature will be identified from several sources including electronic databases, bibliographies of articles and consultation with experts in the area. A comprehensive database of relevant published and unpublished articles will be constructed using the Reference Manager software package.
The searches carried out will include:
-
General health and biomedical databases: MEDLINE; EMBASE; PubMed (previous 6 months); The Cochrane Library.
-
Specialist electronic databases: DARE; The Cochrane Library; Health Technology Assessment Database (HTA); NHS EED; EconLit; Specialist databases as appropriate.
-
Contact with individual experts and those with an interest in the field.
-
Checking of reference lists.
-
Research in Progress: UKCRN.
All databases will be searched from inception to the current date. In the first instance searches will be conducted in all languages with non-English-language articles set to one side in a separate foreign-language reference database. The primary focus will be English-language articles but the need to include non-English articles will be considered in the light of what is found and within the constraints of available time for translation.
Study inclusion
Studies will be selected for inclusion through a two-stage process using the predefined and explicit criteria. The full literature search results will be screened independently by two reviewers to identify all citations that may meet the inclusion criteria. Full manuscripts of all selected citations will be retrieved and assessed by two reviewers against the inclusion criteria. Studies published as abstracts or conference presentations will only be included if sufficient details are presented to allow an appraisal of the methodology and the assessment of results to be undertaken. Any disagreements over study inclusion will be resolved by consensus or if necessary by arbitration by a third reviewer.
The planned inclusion/exclusion criteria for the systematic review are shown in Table 65.
| Participants |
Children < 5 years old with SM (marasmus or kwashiorkor) [such as WHO definition is the presence of severe wasting (< 70% weight for height/length, or < –3 SD) and/or oedema affecting both feet or clinical signs of SM, or a MUAC < 110 mm] |
| Interventions | Any intervention programme, in full or in part, to treat severely malnourished children (such as WHO guidelines and its 10 steps dealing with: hypoglycaemia; hypothermia; dehydration, electrolyte imbalance; infection; micronutrient deficiencies; cautious initial feeding; increased formula feeding; sensory stimulation; preparation of carers for discharge and follow-up). Interventions to be assessed will be prioritised by an expert panel through a Delphi process (see Chapters 2 and 3) |
| Outcome measures |
Primary outcome measures are mortality and rate of weight gain (Secondary outcomes specific to individual steps in any programme, such as WHO 10 steps; progression from initial phase to rehabilitation; catch-up growth; relapse rates) |
| Setting | Inpatient; community; emergency |
| Design |
Pre and post intervention studies for treatment programmes (such as WHO protocol) RCTs, CCTs, cohort with control, case–control, before and after intervention studies for individual steps of any protocol Where evidence from different types of study design is identified, only those with the most rigorous designs will be included |
Data extraction
The extraction of studies’ findings will be conducted by two reviewers using a pre-designed and piloted data extraction form to avoid any errors. Any disagreements between reviewers will be resolved by consensus or if necessary by arbitration by a third reviewer.
Quality assessment
The methodological quality of included studies will be assessed using formal tools specific to the design of the study and focusing on possible sources of bias. Quality assessment of RCTs will be conducted using criteria developed by the CRD (University of York)90 and observational studies will be assessed using criteria such as those developed by CRD (University of York),90 Spitzer. 91 Decisions about the quality assessment tool used will be made following selection of the evidence. Study quality will be assessed by two reviewers. Any disagreements between reviewers will be resolved by consensus or if necessary by arbitration involving a third reviewer.
Data synthesis
The methods of data synthesis will be determined by the nature of the studies identified through searches and included in the review. Studies will be synthesized through a narrative review with tabulation of results of included studies. Where possible the results from individual studies will be synthesized through meta-analysis, with sources of heterogeneity of results investigated by subgroup analyses if applicable. The specific methods for meta-analysis and for the detection and investigation of heterogeneity will depend upon the summary measure selected.
Appendix 2 The Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist
| Section/topic | Item | Checklist item | Reported on page number(s) |
|---|---|---|---|
| Title | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis or both | i, iii |
| Abstract | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number |
Abstract iii–iv Executive summary ix–xiii |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known | 1–9 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to PICOS | 8 |
| Methods | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g. web address), and, if available, provide registration information including registration number | 11, Appendix 1 |
| Eligibility criteria | 6 | Specify study characteristics (e.g. PICOS, length of follow-up) and report characteristics (e.g. years considered, language, publication status) used as criteria for eligibility, giving rationale | 12–14 |
| Information sources | 7 | Describe all information sources (e.g. databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched | 11, Appendix 3 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated | Appendix 3 |
| Study selection | 9 | State the process for selecting studies (i.e. screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis) | 12–14 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g. piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators | 14 |
| Data items | 11 | List and define all variables for which data were sought (e.g. PICOS, funding sources) and any assumptions and simplifications made | 12–14, Appendices 7–12 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level) and how this information is to be used in any data synthesis | 14, Appendix 4 |
| Summary measures | 13 | State the principal summary measures (e.g. RR, difference in means) | N/A, narrative synthesis |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g. I2) for each meta-analysis | N/A, narrative synthesis |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g. publication bias, selective reporting within studies) | 14, Appendix 4 |
| Additional analyses | 16 | Describe methods of additional analyses (e.g. sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified | N/A, narrative synthesis |
| Results | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram | 17, 19 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g. study size, PICOS, follow-up period) and provide the citations | 19–20, 20–22, 26–29, 36–39, 45–47, 50–51, 54–57, 63–67, 81–82, Appendices 7–12 |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see Item 12) | 23, 31, 40,48, 53, 58, 70, 83, Appendices 7–12 |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (1) simple summary data for each intervention group and (2) effect estimates and CIs, ideally with a forest plot | 20, 22–25, 30–36, 39–45, 47–50, 51–54, 59–63, 69–79, 84–87, Appendices 7–12 |
| Synthesis of results | 21 | Present results of each meta-analysis done, including CIs and measures of consistency | N/A |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15) | 23, 31, 40, 48, 53, 58, 70, 83 |
| Additional analysis | 23 | Give results of additional analyses, if done [e.g. sensitivity or subgroup analyses, meta-regression (see Item 16)] | N/A |
| Discussion | |||
| Summary of evidence | 24 | Summarise the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g. health-care providers, users and policy-makers) | 89–95, 99 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g. risk of bias), and at review level (e.g. incomplete retrieval of identified research, reporting bias) | 95–98 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research | 89–95, 99, 100 |
| Funding | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g. supply of data); role of funders for the systematic review | iv |
Appendix 3 Search dates, example search strategy and grey literature sources
| Database searched | Date of most recent search |
|---|---|
| MEDLINE (1950 onwards) | 9 November 2010 |
| MEIP | 9 November 2010 |
| EMBASE (1980 onwards) | 9 November 2010 |
| CAB Abstracts Ovid (this contains a specific database: Nutrition Abstracts, searched to December 2009, subscription subsequently withdrawn) | 15 December 2009 |
| Bioline | 7 December 2010 |
| CRD (DARE, HTA and NHS EED) | 3 November 2010 |
| The Cochrane Library (Cochrane Reviews, Cochrane Other Reviews, CENTRAL and Cochrane Technology Assessment) | 9 November 2010 |
| EconLit EBSCO | 9 November 2010 |
| Databases searched for ongoing research | |
| UKCRN | 7 December 2010 |
| Current Controlled trials.com | 7 December 2010 |
| WHO ICTRP | 8 December 2010 |
| clinicaltrials.gov | 8 December 2010 |
| ANZCTR | 8 December 2010 |
| CTRI | 8 December 2010 |
As an example, the MEDLINE Ovid (1950–2009) search strategy is shown in Box 1. In this initial search on 15 December 2009, 5067 records were identified and additional records were added following the most recent search on 9 November 2010. This search strategy was adapted for other databases.
-
(acute adj2 malnutrition).ti,ab. (267)
-
(severe adj2 malnutrition).ti,ab. (1334)
-
(chronic adj2 malnutrition).ti,ab. (588)
-
“severe acute malnutrition”.ti,ab. (43)
-
“severe malnutrition”.ti,ab. (1136)
-
“acute malnutrition”.ti,ab. (190)
-
“chronic malnutrition”.ti,ab. (344)
-
“severe chronic malnutrition”.ti,ab. (12)
-
“chronic severe malnutrition”.ti,ab. (8)
-
“acute severe malnutrition”.ti,ab. (6)
-
Protein-Energy Malnutrition/or Malnutrition/or Kwashiorkor/ (11,425)
-
(kwashiorkor or marasmus).ti,ab. (1811)
-
(undernutrition adj2 severe).ti,ab. (145)
-
(undernutrition adj2 chronic).ti,ab. (187)
-
(undernutrition adj2 acute).ti,ab. (39)
-
undernutrition.ti. and lancet.so. (26)
-
(severe* adj2 malnourish*).ti,ab. (851)
-
(chronic* adj2 malnourish*).ti,ab. (112)
-
(acute* adj2 malnourish*).ti,ab. (42)
-
or/1–19 (13,940)
-
limit 20 to (“all infant (birth to 23 months)” or “newborn infant (birth to 1 month)” or “infant (1 to 23 months)” or “preschool child (2 to 5 years)”) (5067)
Numbers in brackets denote number of references identified at each stage.
In addition to the bibliographic databases searched, information was also sought from sources of grey literature (Box 2).
CARE www.care.org
Save the Children www.savethechildren.org.uk
Médicins Sans Frontières www.msf.org.uk/ and http://fieldresearch.msf.org/msf/
Action against Hunger www.actionagainsthunger.org/
Aberdeen University www.abdn.ac.uk/medical/unicefprotect/
World for World Organization www.worldforworld.org/index.asp
UN Economic and Social Council www.un.org/en/ecosoc/
Friends of the World Food Programme www.friendsofwfp.org/
Project Concern www.projectconcern.org/
One International www.one.org/international/
World Vision www.worldvision.org.uk/
Department for International Development www.dfid.gov.uk/
UNICEF www.unicef.org/
UNICEF Innocenti Research Centre www.unicef-irc.org/
Valid International www.validinternational.org/
Concern Worldwide www.concern.net/
International Red Cross/Red Crescent www.ifrc.org/
Appendix 4 Quality assessment
The quality assessment tool of Thomas et al. 46 was chosen at the outset of this study because it can be used to assess the methodological quality of a range of study types. This tool is used by the Effective Public Health Practice Project (www.ephpp.ca) because it was developed for use in any public health topic area.
It should be noted that in section B, ‘Study design’, the tool asks the reviewer to note whether the method of randomisation is described, and if so whether the method of randomisation is appropriate (e.g. sequentially numbered, sealed, opaque envelopes). If the answer to either of these questions is no, then the study is scored as a CCT.
An amendment to the tool’s assessment of global study quality was made. The criterion for a global moderate rating in the original tool was ‘fewer than four strong ratings and one weak rating’. Some studies were found to have exactly four strong ratings and one weak rating and it was agreed that these should receive a global rating of ‘moderate’. Similarly, one study had five strong ratings and one weak rating, and reviewers agreed that it should be rated ‘moderate’ overall. Therefore, the global assessments of study quality were:
-
strong = four strong ratings with no weak ratings
-
moderate = one weak rating (altered from the original of fewer than four strong ratings and one weak rating)
-
weak = two or more weak ratings.
The quality assessment part of the data extraction sheet form is shown in Table 67, followed by the guidance that was provided to researchers on scoring each of the sections A–H (guidance based on the quality assessment tool dictionary).
| A. Selection bias | |||||
|---|---|---|---|---|---|
| 1. Are the individuals selected to participate in the study likely to be representative of the target population? | Very likely | Somewhat likely | Not likely | Cannot tell | |
| 2. What percentage of selected individuals participated? | 80–100% | 60–79% | < 60% | N/A | Cannot tell |
| Summary of selection bias (Methodological strength of study) |
Strong | Moderate | Weak | ||
| B. Study design | |||||
|
1. What was the study design? (Please tick appropriate and specify design if categorise as ‘Other’) |
RCT | ||||
| CCT | |||||
| Cohort analytic (two group pre + post) | |||||
| Case–control | |||||
| Cohort [one group pre + post (before and after)] | |||||
| Interrupted time series | |||||
| Other – specify | |||||
| Cannot Tell | |||||
| 2. Was the study described as randomised? | Yes | No | |||
| If answer to no. 2 is ‘No’, complete summary then go to section C, Confounders. If answer is ‘yes’, answer no. 3 and no. 4 below, before completing summary for this section | |||||
| 3. If answer was yes, was the method of randomisation described? | Yes | No | |||
| 4. If answer was yes, was the method appropriate? | Yes | No | |||
| Summary of study design (Methodological strength of study) |
Strong | Moderate | Weak | ||
| C. Confounders | |||||
| 1. Were there important differences between groups prior to the intervention? | Yes | No | Cannot tell | ||
| 2. If yes, indicate the percentage of relevant confounders that were controlled [either in the design (e.g. by stratification or matching) or in the analysis]? | 80–100% | 60–79% | < 60% | Cannot tell | |
| Summary of confounders (Methodological strength of study) |
Strong | Moderate | Weak | ||
| D. Blinding | |||||
| 1. Was the outcome assessor aware of the intervention or exposure status of participants? | Yes | No | Cannot tell | ||
| 2. Were the study participants aware of the research question? | Yes | No | Cannot tell | ||
| Summary of blinding (Methodological strength of study) |
Strong | Moderate | Weak | ||
| E. Data collection methods | |||||
| 1. Were data collection tools shown to be valid? | Yes | No | Cannot tell | ||
| 2. Were data collection tools shown to be reliable? | Yes | No | Cannot tell | ||
| Summary of data collection (Methodological strength of study) |
Strong | Moderate | Weak | ||
| F. Withdrawals and dropouts | |||||
| 1. Were withdrawals and dropouts reported in terms of numbers and reasons per group? | Yes | No | Cannot tell | ||
| 2. Indicate the percentage of participants completing the study (If the percentage differs by groups, record the lowest) | 80–100% | 60–79% | < 60% | Cannot tell | |
| Summary of withdrawals and dropouts (Methodological strength of study) |
Strong | Moderate | Weak | ||
| G. Intervention integrity | |||||
| 1. What percentage of participants received the allocated intervention or exposure of interest? | 80–100% | 60–79% | < 60% | Cannot tell | |
| 2. Was the consistency of the intervention measured? | Yes | No | Cannot tell | ||
| 3. Is it likely that subjects received an unintended intervention (contamination or co-intervention) that may influence the results? | Yes | No | Cannot tell | ||
| H. Analysis | |||||
| 1. Indicate the unit of allocation | Community | Organisation/institution | Practice/office | Provider | Patient |
| 2. Indicate the unit of analysis | Community | Organisation/institution | Practice/office | Provider | Patient |
| 3. Are the statistical methods appropriate for the study design? | Yes | No | Cannot tell | ||
| 4. Is the analysis performed by intervention allocation status (i.e. ITT) rather than actual intervention received? | Yes | No | Cannot tell | ||
|
Global rating for studya (Overall methodological strength of study – based on sections A–F) |
Strong | Moderate | Weak | ||
Guidance for use of the quality assessment tool – severe malnutrition project
A. Selection bias
Use the answers to question 1 (QI) and question 2 (Q2) to rate the selection bias section as strong, moderate or weak. Use the table below as a guide.
| Q2. What percentage of selected individuals agreed to participate? | Q1. Are the individuals selected to participate in the study likely to be representative of the target population? | |||
|---|---|---|---|---|
| Very likely | Somewhat likely | Not likely | Cannot tell | |
| 80–100% | Strong | Moderate | Weak | Weak |
| 60–79% | Moderate | Moderate | Weak | Weak |
| < 60% | Weak | Weak | Weak | Weak |
| N/A | ||||
| Cannot tell | Weak | Weak | Weak | Weak |
B. Study design
| Study design | Methodological quality |
|---|---|
| RCT | Strong |
| CCT | Strong |
| Cohort analytic (two group pre + post) | Moderate |
| Case–control | Moderate |
| Cohort [one group pre + post (before and after)] | Moderate |
| Interrupted time series | Moderate |
| Other – specify | Weak |
| Cannot tell | Weak |
If the study design is not described by the study, the reviewer should try to categorise it according to the descriptions listed in the quality assessment tool dictionary. In such a case, mark the study design as ‘Other’, and specify which study type you think it is (it may be a study type which is not listed in the dictionary), and note that this is the reviewer’s assessment not the author’s (e.g. Other – cohort analytic, reviewer’s opinion).
The tool states that ‘weak’ will be assigned to studies that did not state the method used, so use the ‘weak’ rating in these cases.
C. Confounders
| Potential confounders (Thomas et al. 200446) | Examples |
|---|---|
| Race | |
| Sex | |
| Marital status/family | Number of siblings, birth order |
| Age | |
| SES (income or class) | |
| Education | Parental education |
| Health status | Proportion with additional health issues, e.g. HIV infection, TB, diarrhoea, etc. |
| Pre-intervention score on outcome measure | Severity/type of malnutrition, oedema (affects weight, can be corrected for) |
| Project specific confounders | Staff involved (same staff feeding each group?) |
| Breast feeding |
Note that this is not a complete list.
C. Confounders continued
| Q2. If yes, indicate the percentage of relevant confounders that were controlled [either in the design (e.g. by stratification or matching) or in the analysis] | Q1. Were there important differences between groups prior to the intervention? | ||
|---|---|---|---|
| Yes | No | Cannot tell | |
| 80–100% | Strong | Strong | |
| 60–79% | Moderate | ||
| < 60% | Weak | ||
| Cannot tell | Weak | Weak | |
D. Blinding
Unless explicitly stated otherwise, assume that outcome assessors and participants are aware of intervention/question.
| 2. Were the study participants aware of the research question? | 1. Was the outcome assessor aware of the intervention or exposure status of participants? | ||
|---|---|---|---|
| Yes | No | Cannot tell | |
| Yes | Weak | Moderate | Weak |
| No | Moderate | Strong | Moderate |
| Cannot tell | Weak | Weak | |
E. Data collection methods
Consult quality assessment tool dictionary which lists types of data sources which may have been used.
| 2. Were data collection tools shown to be reliable? | 1. Were data collection tools shown to be valid? | ||
|---|---|---|---|
| Yes | No | Cannot tell | |
| Yes | Strong | Weak | |
| No | Moderate | Weak | Weak |
| Cannot tell | Moderate | Weak | Weak |
F. Withdrawals and dropouts
| 2. Indicate the percentage of participants completing the study (if the percentage differs by groups, record the lowest) | |
|---|---|
| 80–100% | Strong |
| 60–79% | Moderate |
| < 60% | Weak |
| Cannot tell | Weak |
G. Intervention integrity
H. Analysis appropriate to question
Q1 and Q2 – the unit of analysis may be different to the unit of allocation.
Q3 – if there is no statistical analysis, answer ‘no’.
Appendix 5 Delphi study
Round 1: question sheet
The question sheet for the first round of the Delphi study was based on the WHO 10-step plan,10 with one question on its overall effectiveness and 10 further questions on the individual aspects. The remaining questions were suggested by experts who reviewed the draft protocol for the project. The round 1 question sheet and the accompanying instructions received by members of the Delphi panel are shown in Table 68.
| Research questions relating to the treatment of severe malnutrition in children < 5 years old | Importance | |||||||
|---|---|---|---|---|---|---|---|---|
| Low | High | |||||||
| 1 | What is the effectiveness of current programmes/guidance (e.g. the WHO 10-step plan)? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 2 | Which strategies are effective during the initial phase of treatment? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 3 | What approaches are effective for treating hypoglycaemia? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 4 | Which of the different strategies for treating hypothermia are effective (e.g. the ‘kangaroo technique’)? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 5 | What methods for treating dehydration are effective (e.g. oral rehydration with ReSoMal)? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 6 | What is the effectiveness of different strategies for correcting the electrolyte imbalance (e.g. magnesium and potassium supplements)? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 7 | What strategies are effective in treating infection (e.g. broad-spectrum antibiotics?) | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 8 | Which of the different approaches for correcting micronutrient deficiencies are effective (e.g. initial doses of vitamin A, daily doses of a multivitamin, folic acid, zinc and copper)? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 9 | What are the most effective strategies for beginning feeding (e.g. particular fortified milk formulas or special foods)? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 10 | Which approaches are effective in the rehabilitation phase? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 11 | What is the effectiveness of different strategies for increased feeding to recover lost weight and aid catch-up growth? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 12 | What approach should be taken to the emotional stimulation and sensorial development? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 13 | What is the most effective approach to preparing for discharge from inpatient care? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 14 | What is the clinical effectiveness of interventions in different settings (e.g. hospital, community, emergency)? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 15 | What is the clinical effectiveness of management strategies for treating children with comorbidities (e.g. HIV)? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 16 | Should the different strategies/approaches to treatment differ among subgroups (e.g. age, settings, geographical locations)? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 17 | What confounding factors limit full implementation of treatment programmes (e.g. insufficient training, cultural difficulties, funding limitations)? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 18 | What factors affect sustainability of programmes (e.g. beyond initial disaster relief strategies or implementation of new protocols in hospitals)? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
Round 1: results
The top 15 questions were identified based on their median ranking (Table 69). It was not necessary to take the UQ and LQ limits into consideration for identifying the top 15 questions in this round, as there was a clear difference between the median values for question 13 (ranked 15th, median = 5) and question 2 (ranked 16th, median = 4.5). The lowest ranking questions, questions 2, 3 and 4 (marked in italics) were removed for round 2.
| Rank | Question number | Question | LQ | Median | UQ |
|---|---|---|---|---|---|
| 1 | 15 | What is the clinical effectiveness of management strategies for treating children with comorbidities? | 6 | 7 | 7 |
| 2 | 18 | What factors affect sustainability of programmes? | 4 | 7 | 7 |
| 3 | 7 | What strategies are effective in treating infection? | 5.5 | 6 | 7 |
| 4 = | 14 | What is the clinical effectiveness of interventions in different settings? | 5 | 6 | 7 |
| 4 = | 17 | What confounding factors limit full implementation of treatment programmes? | 5 | 6 | 7 |
| 6 | 5 | What methods for treating dehydration are effective? | 4.5 | 6 | 6.5 |
| 7 | 1 | What is the effectiveness of current programmes/guidance (e.g. the WHO 10-step plan)? | 5.25 | 6 | 6 |
| 8 | 11 | What is the effectiveness of different strategies for increased feeding? | 4 | 6 | 6 |
| 9 | 9 | What are the most effective strategies for beginning feeding? | 4 | 5.5 | 7 |
| 10 | 10 | Which approaches are effective in the rehabilitation phase? | 4.25 | 5.5 | 6 |
| 11 | 16 | Should the different strategies/approaches to treatment differ among subgroups? | 5 | 5 | 6.5 |
| 12 | 6 | What is the effectiveness of different strategies for correcting the electrolyte imbalance? | 4.5 | 5 | 6 |
| 13 | 8 | Which of the different approaches for correcting micronutrient deficiencies are effective? | 4 | 5 | 6 |
| 14 = | 12 | What approach should be taken to the emotional stimulation and sensorial development? | 3.5 | 5 | 6 |
| 14 = | 13 | What is the most effective approach to preparing for discharge from inpatient care? | 3.5 | 5 | 6 |
| 16 | 2 | Which strategies are effective during the initial phase of treatment? | 3.25 | 4.5 | 6 |
| 17 | 4 | Which of the different strategies for treating hypothermia are effective? | 1.5 | 3 | 4 |
| 18 | 3 | What approaches are effective for treating hypoglycaemia? | 2 | 3 | 3.5 |
Round 2: question sheet
The contributions and comments received by the seven panel members who took part in round 1, were tabulated and categorised into those that related broadly to existing questions and those that were new. These were used to refine the existing questions retained from round 1, by either rewording or adding sub-questions, and four additional questions were added (the new questions, reworded questions and sub-questions can be seen in Table 70). The new questions (numbers 19–22), reflected the most frequently made observations and were essentially a refinement of the wider points alluded to in the first version of the questions. These are discussed below.
| Rank | Question number | Question | Score in round 1 | Score in round 2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Round 2 | Round 1 | LQ | Median | UQ | LQ | Median | UQ | ||
| 1 | New | 20 |
How should management of HIV-infected children with severe malnutrition differ from those who are severely malnourished but HIV–ve? (a) How do fluid and electrolyte needs differ? (b) How effective is zinc in the treatment of HIV+ve children? (c) What is the most effective use of antibiotics for these patients? (d) What is the most effective stage of malnutrition treatment at which to start treatment with ARTs? |
New | New | New | 6.25 | 7 | 7 |
| 2 = | New | 19 |
What methods are effective for treating SAM among infants < 6 months old? (a) Which is the most effective therapeutic milk for initial feeding of infants < 6 months with severe malnutrition? |
New | New | New | 6 | 7 | 7 |
| 2 = | New | 21 |
Which form of i.v. fluid administration is the most effective for treating shock? (a) Are blood transfusions feasible/practical during treatment of shock? |
New | New | New | 6 | 7 | 7 |
| 2 = | New | 22 |
What are the best treatments for children with SAM who have diarrhoea? (a) What is the most effective approach to the management of primary and secondary diarrhoea? (b) What is the most appropriate therapeutic food for children with diarrhoea? (c) What is the best approach to rehydration for children with diarrhoea? |
New | New | New | 6 | 7 | 7 |
| 5 = | 3 | 7 |
What methods are effective in treating infection? (a) What is the effectiveness of presumptive treatment of infections with broad-spectrum antibiotics for all children being treated for SAM? (b) What are the best first- and second-line antibiotic choices? (c) What is the effectiveness of selective antibiotic prescription compared with systematic antibiotic prescription? |
5.5 | 6 | 7 | 6 | 6 | 7 |
| 5 = | 4 = | 14 |
What is the clinical effectiveness of interventions in different settings (e.g. hospital, community, emergency)? (a) What is the effectiveness of RUTF used in the community setting compared with fortification of other family foods? |
5 | 6 | 7 | 6 | 6 | 7 |
| 5 = | 1 | 15 | What is the clinical effectiveness of management strategies for treating children with comorbidities such as TB and H. pylori? (other than HIV and diarrhoea, which are considered in questions 20 and 22) | 6 | 7 | 7 | 6 | 6 | 7 |
| 8 | 2 | 18 | What factors affect sustainability of programmes, long-term survival and readmission rates? | 4 | 7 | 7 | 5 | 6 | 7 |
| 9 | 6 | 5 |
What methods for treating dehydration are effective? (a) What is the most effective oral rehydration fluid for the treatment of dehydration in severely malnourished children? (b) Should priority be given to preventing dehydration or avoiding the risk of fluid overload? (c) What is the effectiveness of i.v. rehydration? |
4.5 | 6 | 6.5 | 5.25 | 6 | 6.75 |
| 10 | 13 | 8 |
Which methods for correcting micronutrient deficiencies are effective? (a) What is the effectiveness of daily low dose of vitamin A (e.g. in therapeutic milk) compared with large vitamin A dose (e.g. in supplements)? (b) Are there any subgroups that should be considered separately (e.g. children with measles)? (c) What is the optimum timing of administration of vitamin A? (d) What is the role of iron in the different stages of treatment of severe malnutrition? |
4 | 5 | 6 | 5 | 6 | 6.75 |
| 11 | 7 | 1 | What is the overall effectiveness of current programmes/guidance (e.g. the WHO 10-step plan)? | 5.25 | 6 | 6 | 6 | 6 | 6 |
| 12 = | 10 | 10 |
Which methods are effective in the rehabilitation phase? (a) What is the relative effectiveness of different formulations of RTUFs in the treatment of children with SAM? (b) What are the most appropriate methods for transition of children once weight is gained? |
4.25 | 5.5 | 6 | 5 | 6 | 6 |
| 12 = | 4 = | 17 | What factors limit full implementation of treatment programmes (e.g. insufficient training, cultural difficulties and funding limitations)? | 5 | 6 | 7 | 5 | 6 | 6 |
| 14 = | 9 | 9 |
What are the most effective methods for feeding during the initial stages of treatment? (a) Which methods of feeding are best? (b) What is the contribution of milk-based ingredients in treatment products? (c) What is the effectiveness of different formulas and food types? |
4 | 5.5 | 7 | 4 | 6 | 6 |
| 14 = | 8 | 11 | What is the effectiveness of different methods for increasing appetite and food intake to recover lost weight and aid catch-up growth? | 4 | 6 | 6 | 4 | 6 | 6 |
| 16 | 12 | 6 |
What is the effectiveness of different methods for correcting electrolyte imbalances? (a) What are the optimum levels of potassium, phosphorous, protein, sulphur amino acids and other key components? |
4.5 | 5 | 6 | 5 | 5.5 | 6 |
| 17 | 14 = | 13 | What level of weight gain and other indicators are effective and feasible for safe discharge of children being treated for SAM? | 3.5 | 5 | 6 | 5 | 5 | 6 |
| 18 | 14 = | 12 | What methods are effective for emotional stimulation and sensorial development? | 3.5 | 5 | 6 | 4 | 5 | 6 |
| 19 | 11 | 16 | Should treatments differ depending on geographical locations? | 5 | 5 | 6.5 | 5 | 5 | 5 |
Question 19 is a new question about the treatment of infants < 6 months old. This is related to original question 16 [‘Should the different strategies/approaches to treatment differ among subgroups (e.g. age, settings, geographical locations)?’]. Several panel members commented that the most important subgroup would be children < 6 months old, so this was taken out of question 16 and included as a separate question. Question 16 was then rewritten for round 2 and was restricted to asking about treatment in different geographical locations.
Question 20 is a newly defined question about the treatment of severely malnourished children who are also HIV+ve. Panel members commented that this was the most important aspect of original question 15 [‘What is the clinical effectiveness of management strategies for treating with comorbidities, (e.g. HIV)?’]. It has therefore been moved into a separate question and question 15 has been reworded to include less severe comorbidities.
The treatment of shock was not explicitly stated in the questions scored in round 1 of the Delphi process. It forms part of the initial phase of treatment in the WHO’s 10-step plan, so would have come into consideration under question 2 ‘Which strategies are effective during the initial phase of treatment?’. However, three of the seven panel members who commented on the questions mentioned that a question on the treatment of shock, particularly regarding specific i.v. fluids, would be appropriate. This has therefore been included as question 21.
The treatment of diarrhoea was specifically raised as a question by two panel members, and commented on by two others under two of the existing questions (5 and 15). Question 5 (‘What methods for treating dehydration are effective?’) would include an important part of treatment for children with diarrhoea, but also covers more general aspects of rehydration of children with SAM. Similarly, diarrhoea could be considered to be a comorbidity that could be included in question 15. However, given the importance of this comorbidity for children with SAM, it was decided to move it into a new question and reword the existing question 15 to exclude diarrhoea treatment.
The question sheet for round 2 was sent to all 14 of the original panel members who had expressed an interest in the project, plus two additional people. One of the extra people was suggested as a replacement by an expert unable to contribute to the project, and the second was someone contacted for round 1 who did not initially reply. It was decided to include these people in order to get as full a participation response as possible, with a view to carrying out separate analysis with and without those who contributed to round 1.
Panel members were sent an individually tailored question sheet that showed the panel’s median score and their own score for the top 15 questions from round 1, and the four new questions. They were asked to score each of the 19 questions for their overall importance, using the proposed sub-questions as a guide, but not scoring these sub-questions individually.
Round 2: results
For round 2, 14 people replied, including one of the experts who joined at this round and did not contribute to round 1. The ranking of the questions after round 2 is shown in Table 70. The table also includes the questions’ ranks, medians, LQs and UQs from round 1.
The 19 questions scored for round 2 were sorted according to their median, UQ and LQ scores. The top 15 questions for round 3 were identified, and there was a clear cut-off between the median scores for questions 9 and 11, ranked 14th with a median score of 6, and question 6, ranked 16th with a median score of 5.5.
The new questions for round 2 were scored most highly by the panel, achieving the top four places in the ranking. This reflects the importance the panel places on these research questions, both their individual comments during round 1 and their overall scores as a panel. Changes to the ranked order of other questions are generally within 4 points between rounds, i.e. if 4 points are added to the round 1 rank to simulate the addition of four popular questions, the ranked order remains broadly similar for many of the questions. However, larger than expected differences are apparent for some questions. For example, question 8 (‘Which of the different approaches for correcting micronutrient deficiencies are effective?’) actually increased in rank from 13th place in round 1 to 10th place in round 2. This may be because of the sub-questions used to clarify this question, which were expanded considerably from that in the previous round. Question 17, regarding limitations to the full implementation of programmes, dropped from being fourth equal to being 12th equal. It is not clear whether this was because of any particular factor or just because of changes in scores. The scoring is quite close, so small differences in median or lower/upper IQR limits can have quite a large difference in ranking order. Finally, question 16 dropped from 11th place to 19th place. This is probably because of a change to the wording of the question since the first round. In round 1 this included all subgroups including different age groups, whereas in round 2 the treatment of children aged < 6 months is considered in a separate question and question 16 refers only to different geographical locations.
The 14 respondents in round 2 included one panel member who joined the process after round 1 had been completed, and two panel members who received score sheets for round 1 but did not complete them. For comparison purposes, further analysis was undertaken to assess whether or not the ‘new’ members’ scores affected the resulting ranking (Table 71, sub-questions not shown).
| Rank (n = 14) | Rank (n = 11) | Question number | Questiona | Scores (n = 11) | Scores (n = 14) | ||||
|---|---|---|---|---|---|---|---|---|---|
| LQ | Median | UQ | LQ | Median | UQ | ||||
| 1 | 1 | 20 | How should management of HIV-infected children with SAM differ from those who are severely malnourished but HIV–ve? | 7 | 7 | 7 | 6.25 | 7 | 7 |
| 2 = | 2 = | 19 | What methods are effective for treating SAM among infants < 6 months old? | 6 | 7 | 7 | 6 | 7 | 7 |
| 2 = | 2 = | 21 | Which form of i.v. fluid administration is most effective for treating shock? | 6 | 7 | 7 | 6 | 7 | 7 |
| 2 = | 2 = | 22 | What are the best treatments for children with SAM who have diarrhoea? | 6 | 7 | 7 | 6 | 7 | 7 |
| 5 = | 6 | 7 | What methods are effective in treating infection? | 6 | 6 | 7 | 6 | 6 | 7 |
| 5 = | 8 | 14 | What is the clinical effectiveness of interventions in different settings (e.g. hospital, community, emergency)? | 5.25 | 6 | 6.75 | 6 | 6 | 7 |
| 5 = | 7 | 15 | What is the clinical effectiveness of management strategies for treating children with comorbidities such as TB and H. pylori? (other than HIV and diarrhoea, which are considered in questions 20 and 22) | 5.5 | 6 | 7 | 6 | 6 | 7 |
| 8 | 5 | 18 | What factors affect sustainability of programmes, long-term survival and readmission rates? | 5.5 | 7 | 7 | 5 | 6 | 7 |
| 9 | 10 | 5 | What methods for treating dehydration are effective? | 5.5 | 6 | 6.5 | 5.25 | 6 | 6.75 |
| 10 | 14 | 8 | Which methods for correcting micronutrient deficiencies are effective? | 4.5 | 5 | 6.5 | 5 | 6 | 6.75 |
| 11 | 11 | 1 | What is the overall effectiveness of current programmes/guidance (e.g. the WHO 10-step plan)? | 5.25 | 6 | 6 | 6 | 6 | 6 |
| 12 = | 15 = | 10 | Which methods are effective in the rehabilitation phase? | 5 | 5 | 6 | 5 | 6 | 6 |
| 12 = | 9 | 17 | What factors limit full implementation of treatment programmes (e.g. insufficient training, cultural difficulties and funding limitations)? | 5 | 6 | 6.75 | 5 | 6 | 6 |
| 14 = | 13 | 9 | What are the most effective methods for feeding during the initial stages of treatment? | 4 | 6 | 6 | 4 | 6 | 6 |
| 14 = | 12 | 11 | What is the effectiveness of different methods for increasing appetite and food intake to recover lost weight and aid catch-up growth? | 4.25 | 6 | 6 | 4 | 6 | 6 |
| 16 | 15 = | 6 | What is the effectiveness of different methods for correcting electrolyte imbalances? | 5 | 5 | 6 | 5 | 5.5 | 6 |
| 17 | 17 | 13 | What level of weight gain and other indicators are effective and feasible for the safe discharge of children being treated for SAM? | 4.5 | 5 | 6 | 5 | 5 | 6 |
| 18 | 18 | 12 | What methods are effective for emotional stimulation and sensorial development? | 4 | 5 | 5.75 | 4 | 5 | 6 |
| 19 | 19 | 16 | Should treatments differ depending on geographical locations? | 5 | 5 | 5 | 5 | 5 | 5 |
Comparison of rankings with and without panel members who did not contribute to round 1 (see Table 71) shows that most questions have similar rankings regardless of the panel’s composition. However, questions 8, 10 and 17 show considerable differences depending whether or not the additional contributors’ scores are used. Questions 8 and 17 are also those that showed the biggest change between round 1 and round 2. It therefore seems possible that the additional panel members’ scores for these questions have affected their position in the ranking in round 2 compared with round 1.
Round 3: question sheet
For round 3, the same score sheet was used as for round 2, with the top 15 questions retained and an update of the median scores for each question provided.
Round 3: results
Thirteen people completed score sheets for round 3, one of whom had not returned a score sheet for round 1 or round 2. The median and IQR limits were calculated for all 13 respondents’ scores, and these are shown in Table 72.
| Rank | Question number | Questiona | Round 3 | |||
|---|---|---|---|---|---|---|
| Round 3 | Round 2 | LQ | Median | UQ | ||
| 1 = | 1 | 20 | How should management of HIV-infected children with SAM differ from those who are severely malnourished but HIV–ve? | 6 | 7 | 7 |
| 1 = | 2 = | 19 | What methods are effective for treating SAM among infants < 6 months old? | 6 | 7 | 7 |
| 1 = | 2 = | 21 | Which form of i.v. fluid administration is most effective for treating shock? | 6 | 7 | 7 |
| 1 = | 2 = | 22 | What are the best treatments for children with SAM who have diarrhoea? | 6 | 7 | 7 |
| 5 = | 5 = | 7 | What methods are effective in treating infection? | 6 | 6 | 7 |
| 5 = | 8 | 18 | What factors affect sustainability of programmes, long-term survival and readmission rates? | 6 | 6 | 7 |
| 7 = | 5 = | 15 | What is the clinical effectiveness of management strategies for treating children with comorbidities such as TB and H. pylori? (other than HIV and diarrhoea, which are considered in questions 20 and 22) | 5 | 6 | 7 |
| 7 = | 12 = | 17 | What factors limit full implementation of treatment programmes? | 5 | 6 | 7 |
| 9 | 5 = | 14 | What is the clinical effectiveness of interventions in different settings (e.g. hospital, community, emergency)? | 5.75 | 6 | 6.25 |
| 10 | 10 | 8 | Which methods for correcting micronutrient deficiencies are effective? | 6 | 6 | 6 |
| 11 | 11 | 1 | What is the overall effectiveness of current programmes/guidance (e.g. the WHO 10-step plan)? | 5.75 | 6 | 6 |
| 12 = | 12 = | 10 | Which methods are effective in the rehabilitation phase? | 5 | 6 | 6 |
| 12 = | 9 | 5 | What methods for treating dehydration are effective? | 5 | 6 | 6 |
| 12 = | 14 = | 9 | What are the most effective methods for feeding during the initial stages of treatment? | 5 | 6 | 6 |
| 15 | 14 = | 11 | What is the effectiveness of different methods for increasing appetite and food intake to recover lost weight and aid catch-up growth? | 4 | 5 | 6 |
Although there were some changes to positions in the list, the median scores are so close that a small difference can have a big effect on ranked order. The ‘new’ questions added for the second round, based on panel members’ suggestions, remained the most highly scored, with median values of 7 points. All questions, with the exception of question 11, had a median score of 6.
The ranked order remained broadly similar for most questions. Question 18 increased from being ranked eighth to being ranked fifth equal, but this only reflected an increase in the LQ score from 5 to 6. Question 5 dropped from ninth place to 12th equal, owing to a decrease of 0.75 in the UQ value and of 0.25 in the LQ score. Question 17 increased from being 12th equal in the round 2 ranking to being seventh equal in round 3. This was because of a 1-point increase in the UQ limit, from 6 to 7. Question 14 decreased from being fifth equal to ninth place, because of a decrease of 0.25 in the LQ limit and of 0.75 in the UQ limit.
As there was one panel member who contributed to round 3 but not to round 1 or round 2, the analysis was repeated without their scores (Table 73). The ranked order of questions is very similar with and without this set of scores. However, question 5 increases from 12th equal when all 13 members are included to ninth place when this score sheet is removed from the analysis. This places it back in the same order that it was ranked in for round 2 (see Table 72), i.e. it is possible that the introduction of the 13th panel member’s scores may have moved it from the existing panel’s consensus. Similarly, the difference of 0.25 in the UQ and LQ limits for question 14 could partially explain the decrease in rank of this question. Questions 18 and 17, which showed a change in ranked order between rounds 2 and 3, were not affected by the additional panel member’s score.
| Rank | Question number | Questiona | Round 3 (n = 13) | Round 3 (n = 12) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Round 3 (n = 12) | Round 2 (n = 13) | LQ | Median | UQ | LQ | Median | UQ | ||
| 1 = | 1 = | 20 | How should management of HIV-infected children with SAM differ from those who are severely malnourished but HIV–ve? | 6 | 7 | 7 | 6 | 7 | 7 |
| 1 = | 1 = | 19 | What methods are effective for treating SAM among infants < 6 months old? | 6 | 7 | 7 | 6 | 7 | 7 |
| 1 = | 1 = | 21 | Which form of i.v. fluid administration is most effective for treating shock? | 6 | 7 | 7 | 6 | 7 | 7 |
| 1 = | 1 = | 22 | What are the best treatments for children with SAM who have diarrhoea? | 6 | 7 | 7 | 6 | 7 | 7 |
| 5 = | 5 = | 7 | What methods are effective in treating infection? | 6 | 6 | 7 | 6 | 6 | 7 |
| 11 = | 9 | 14 | What is the clinical effectiveness of interventions in different settings (e.g. hospital, community, emergency)? | 5.75 | 6 | 6.25 | 5.5 | 6 | 6 |
| 7 = | 7 = | 15 | What is the clinical effectiveness of management strategies for treating children with comorbidities such as TB and H. pylori? (other than HIV and diarrhoea, which are considered in questions 20 and 22) | 5 | 6 | 7 | 5 | 6 | 7 |
| 5 = | 5 = | 18 | What factors affect sustainability of programmes, long-term survival and readmission rates? | 6 | 6 | 7 | 6 | 6 | 7 |
| 9 | 12 = | 5 | What methods for treating dehydration are effective? | 5 | 6 | 6 | 5.75 | 6 | 6.25 |
| 10 | 10 | 8 | Which methods for correcting micronutrient deficiencies are effective? | 6 | 6 | 6 | 5.75 | 6 | 6 |
| 11 = | 11 | 1 | What is the overall effectiveness of current programmes/guidance (e.g. the WHO 10-step plan)? | 5.75 | 6 | 6 | 5.5 | 6 | 6 |
| 13 | 12 = | 10 | Which methods are effective in the rehabilitation phase? | 5 | 6 | 6 | 5 | 6 | 6 |
| 7 = | 7 = | 17 | What factors limit full implementation of treatment programmes (e.g. insufficient training, cultural difficulties and funding limitations)? | 5 | 6 | 7 | 5 | 6 | 7 |
| 14 | 12 = | 9 | What are the most effective methods for feeding during the initial stages of treatment? | 5 | 6 | 6 | 4.75 | 6 | 6 |
| 15 | 15 | 11 | What is the effectiveness of different methods for increasing appetite and food intake to recover lost weight and aid catch-up growth? | 4 | 5 | 6 | 4 | 5 | 6 |
Appendix 6 Table of excluded studies
| Reference | Exclusion reason |
|---|---|
| Abdelrazik N, Al-Haggar M, Al-Marsafawy H, bdel-Hadi H, Al-Baz R, Mostafa A-H. Impact of long-term oral iron supplementation in breast-fed infants. Indian J Pediatr 2007;74:739–45. | PG |
| Abiodun PO. Use of soya-beans for the dietary prevention and management of malnutrition in Nigeria. Acta Paediatr Scand 1991;80:175–82. | DES |
| Aboud FE, Shafique S, Akhter S. A responsive feeding intervention increases children’s self-feeding and maternal responsiveness but not weight gain. J Nutr 2009;139:1738–43. | PG |
| Afolabi OA, Ojofeitimi EO, Oke OL. Chemical and clinical evaluation of groundnut-maize gruel mixture (‘Epa-Ogi’) in the amelioration of protein energy malnutrition in the developing countries. Nutr Rep Int 1988;38:621–8. | PG |
| Agarwal DK, Pandey CM, Agarwal KN. Vitamin A administration and preschool child mortality. Nutr Res 1995;15:669–80. | PG |
| Ahmed T, Islam MM, Nahar B, Azam MA, Salam MA, Ashworth A, et al. Home-based nutritional rehabilitation of severely-malnourished children recovering from diarrhoea and other acute illnesses. International Centre for Diarrhoeal Diseases, Bangladesh (ICDDR, B) 10th Annual Scientific Conference, 11–13 June 2002, Bangladesh. | Abstract |
| Alderman H, Ndiaye B, Linnemayr S, Ka A, Rokx C, Dieng K, et al. Effectiveness of a community-based intervention to improve nutrition in young children in Senegal: a difference in difference analysis. Public Health Nutr 2009;12:667–73. | PG |
| Arifeen SE, Hoque DME, Tasnima A, Muntasirur R, Hoque ME, Khadija B, et al. Effect of the integrated management of childhood illness strategy on childhood mortality and nutrition in a rural area in Bangladesh: a cluster randomised trial. Lancet 2009;374:393–403. | PG |
| Arora NK, Anand NK, Bhan MK, Jailkhani B, Aggarwal A, Meenu R, et al. Nutrient absorption from a fat-enriched diet in young malnourished children: a randomized controlled trial. Acta Paediatr 1998;87:143–8. | PG |
| Ashraf H, Ahmed T, Hossain MI, Alam NH, Mahmud R, Kamal SM, et al. Day-care management of children with severe malnutrition in an urban health clinic in Dhaka, Bangladesh. J Trop Pediatr 2007;53:171–8. | DES |
| Ashworth A, Chopra M, McCoy D, Sanders D, Jackson D, Karaolis N, et al. WHO guidelines for management of severe malnutrition in rural South African hospitals: effect on case fatality and the influence of operational factors. Lancet 2004;363:1110–15. | PG |
| Ashworth A. Efficacy and effectiveness of community-based treatment of severe malnutrition. Food Nutr Bull 2006;27:S24–S48. | DES |
| Awasthi S, Peto R, Pande VK, Fletcher RH, Read S, Bundy DAP. Effects of deworming on malnourished preschool children in India: an open-labelled, cluster-randomized trial. PLoS Negl Trop Dis 2008;2:e223.doi:10.1371/journal.pntd.0000223. | PG |
| Bachmann MO. Cost effectiveness of community-based therapeutic care for children with severe acute malnutrition in Zambia: decision tree model. Cost Eff Resour Alloc 2009;7:2. | OUT, DES |
| Badaloo A, Reid M, Forrester T, Heird WC, Jahoor F. Cysteine supplementation improves the erythrocyte glutathione synthesis rate in children with severe edematous malnutrition. Am J Clin Nutr 2002;76:646–52. | INT |
| Ba KI. Teaching better nutrition by domiciliary management of cases of protein calorie malnutrition in rural areas (a longitudinal study of clinical and economical aspects). J Trop Pediatr Environ Child Health 1972;18:307–12. | DES |
| Barker D, Younger N, MooSang M, McKenzie CA. HIV serostatus and recovery from severe childhood malnutrition. A retrospective matched case–control study. West Indian Med J 2004;53:89–94. | PG |
| Basu S, Paul DK, Ganguly S, Chatterjee M, Chandra PK. Efficacy of high-dose Lactobacillus rhamnosus GG in controlling acute watery diarrhea in Indian children: a randomized controlled trial. J Clin Gastroenterol 2009;43:208–13. | PG |
| Beaudry-Darisme MICH, Latham MC. Nutrition rehabilitation centers – an evaluation of their performance. J Trop Pediatr 1973;19:299–332. | PG |
| Becker K, Pons-Kuhnemann J, Fechner A, Funk M, Gromer S, Gross HJ, et al. Effects of antioxidants on glutathione levels and clinical recovery from the malnutrition syndrome kwashiorkor – a pilot study. Redox Rep 2005;10:215–26. | PG |
| Beghin I, de-Mello AV, Costa T, Monteiro E, Lucena A, Varela R. Assessment of biological value of a new corn-soy-wheat noodle through recuperation of Brazilian malnourished children. Am J Clin Nutr 1973;26:246–58. | PG |
| Beghin ID, Viteri FE. Nutritional rehabilitation centres: an evaluation of their performance. J Trop Pediatr 1973;19:403–16. | DES |
| Behrens RH, Tomkins AM, Roy SK. Zinc supplementation during diarrhoea, a fortification against malnutrition? Lancet 1990;336:442–3. | PG |
| Bernal C, Velasquez C, Alcaraz G, Botero J. Treatment of severe malnutrition in children: experience in implementing the World Health Organization guidelines in Turbo, Colombia. J Pediatr Gastroenterol Nutr 2008;46:322–8. | DES |
| Bhandari N, Bahl R, Nayyar B, Khokhar P, Rohde JE, Bhan MK. Food supplementation with encouragement to feed it to infants from 4 to 12 months of age has a small impact on weight gain. J Nutr 2001;131:1946–51. | PG |
| Bhatnagar S, Singh KD, Sazawal S, Saxena SK, Bhan MK. Efficacy of milk versus yogurt offered as part of a mixed diet in acute noncholera diarrhea among malnourished children. J Pediatr 1998;132:999–1003. | PG |
| Bhattacharyya AK. Studies on kwashiorkor and marasmus in Calcutta (1957–74): III. therapeutic and follow-up studies. Indian Pediatr 1975;12:1125–33. | DES |
| Bhutta ZA, Ahmed T, Black RE, Cousens S, Dewey K, Giugliani E, et al. Maternal and child undernutrition 3. What works? Interventions for maternal and child undernutrition and survival. Lancet 2008;371:417–40. | DES |
| Bita J, Najjar SS, Asfour RY. Diphenoxylate hydrochloride therapy in the diarrhoea of malnourished infants. Arch Dis Child 1970;45:190–2. | OUT |
| Brewster DR, Manary MJ, Graham SM. Case management of kwashiorkor: an intervention project at seven nutrition rehabilitation centres in Malawi. Eur J Clin Nutr 1997;51:139–47. | PG |
| Brewster DR. Critical appraisal of the management of severe malnutrition: 3. Complications. J Paediatr Child Health 2006;42:583–93. | DES |
| Briend A, Golden MH. Treatment of severe child malnutrition in refugee camps. Eur J Clin Nutr 1993;47:750–4. | DES |
| Briend A, Lacsala R, Prudhon C, Mounier B, Grellety Y, Golden MH. Ready-to-use therapeutic food for treatment of marasmus. Lancet 1999;353:1767–8. | OUT |
| Brown LV, Zeitlin MF, Peterson KE, Chowdhury AM, Rogers BL, Weld LH, et al. Evaluation of the impact of weaning food messages on infant feeding practices and child growth in rural Bangladesh. Am J Clin Nutr 1992;56:994–1003. | PG |
| Brown RC, Brown JE, Teeter RA. Evaluation of a nutrition center program in rural Africa. J Trop Pediatr 1980;26:37–41. | PG |
| Castillo-Duran C, Fisberg M, Valenzuela A, Egana JI, Uauy R. Controlled trial of copper supplementation during the recovery from marasmus. Am J Clin Nutr 1983;37:898–903. | PG |
| Castillo-Duran C, Heresi G, Fisberg M, Uauy R. Controlled trial of zinc supplementation during recovery from malnutrition: effects on growth and immune function. Am J Clin Nutr 1987;45:602–8. | PG |
| Castillo-Duran C, Uauy R. Copper deficiency impairs growth of infants recovering from malnutrition. Am J Clin Nutr 1988;47:710–14. | PG |
| Castillo-Duran C, Uauy R. Zinc supplementation saves the lives of children living in poverty. Pediatrics 2001;108:1366. | DES |
| Castillo DC, Fisberg M, Egana JI, Uauy R. Controlled trial of copper supplementation during the recovery of marasmus. Pediatr Res 1981;15:177. | PG |
| Chaiken MS, Deconinck H, Degefie T. The promise of a community-based approach to managing severe malnutrition: a case study from Ethiopia. Food Nutr Bull 2006;27:95–104. | DES |
| Chatterjee A, Mahalanabis D, Jalan KN, Maitra TK, Agarwal SK, Dutta B, et al. Oral rehydration in infantile diarrhoea. Controlled trial of a low sodium glucose electrolyte solution. Arch Dis Child 1978;53:284–9. | DES |
| Chinkhumba J, Tomkins A, Banda T, Mkangama C, Fergusson P. The impact of HIV on mortality during in-patient rehabilitation of severely malnourished children in Malawi. Tans R Soc Trop Med Hyg 2008;102:639–44. | DES |
| Ciliberto M, Manary M, Ndekha M, Briend A, Ashorn P. Home-based therapy for oedematous malnutrition with ready-to-use therapeutic food. Acta Paediatr 2006;95:1012–15. | DES |
| Collins S, Sadler K. Outpatient care for severely malnourished children in emergency relief programmes: a retrospective cohort study. Lancet 2002;360:1824–30. | DES |
| Cooper E, Headden G, Lawrance C. Caribbean children, thriving and failing, in and out of hospital. J Trop Pediatr 1980;26:232–8. | PG |
| Cutting WA, Cutting MM. Experience with a nutrition rehabilitation unit in the management of protein calorie malnutrition in a rural hospital. Indian Pediatr 1975;12:99–100. | PG |
| das Neves J, Martins PA, Sesso R, Sawaya AL. Malnourished children treated in day-hospital or outpatient clinics exhibit linear catch-up and normal body composition. J Nutr 2006;136:648–55. | PG |
| de Portela ML, Zeni S, Piazza N, Rio ME. Calcium balance in infants recovering from undernutrition. Nutr Rep Int 1982;26:1045–51. | PG |
| Deen JL, Funk M, Guevara VC, Saloojee H, Doe JY, Palmer A, et al. Implementation of WHO guidelines on management of severe malnutrition in hospitals in Africa. Bull World Health Organ 2003;81:237–43. | DES |
| Devadas RP, Chandrasekhar U, Bhooma N. Nutritional outcomes of a rural diet supplemented with low cost locally available foods. 5. impact on pre-schoolers followed over a period of four and a half years. Indian J Nutr Diet 1984;21:153–64. | PG |
| Dewan P, Kaur I, Chattopadhya D, Faridi MMA, Agarwal KN. A pilot study on the effects of curd (dahi) & leaf protein concentrate in children with protein energy malnutrition (PEM). Indian J Med Res 2007;126:199–203. | PG |
| Diop EHI, Dossou NI, Ndour MM, Briend A, Wade S. Comparison of the efficacy of a solid ready-to-use food and a liquid, milk-based diet for the rehabilitation of severely malnourished children: a randomized trial. The Am J Clin Nutr 2003;78:302–7. | PG |
| Diop EI, Dossou NI, Briend A, Yaya MA, Ndour MM, Wade S. O0111 Home rehabilitation of severe malnutrition using locally produced and imported solid ready to use foods (RTUF) after 1 week inpatient care. J Pediatr Gastroenterol Nutr 2004;39:S50–1. | Abstract |
| Donnen P, Dramaix M, Brasseur D, Bitwe R, Vertongen F, Hennart P. Randomized placebo-controlled clinical trial of the effect of a single high dose or daily low doses of vitamin A on the morbidity of hospitalized, malnourished children. Am J Clin Nutr 1998;68:1254–60. | PG |
| Donnen P, Sylla A, Dramaix M, Sall G, Kuakuvi N, Hennart P. Effect of daily low dose of vitamin A compared with single high dose on morbidity and mortality of hospitalized mainly malnourished children in Senegal: a randomized controlled clinical trial. Eur J Clin Nutr 2007;61:1393–9. | PG |
| Eichenberger JR, Hadorn B, Schmidt BJ. A semi-elemental diet with low osmolarity and high content of hydrolyzed lactalbumin in the treatment of acute diarrhea in malnourished children. Arq Gastroenterol 1984;21:130–5. | PG |
| Elizabeth KE, Sathy N. The role of developmental stimulation in nutritional rehabilitation. Indian Pediatr 1997;34:681–95. | PG |
| Elizabeth KE, Sreedevi P, Narayanan SN. Outcome of nutritional rehabilitation with and without zinc supplementation. Indian Pediatr 2000;37:650–5. | PG |
| Falbo AR, Alves JG, Batista FM, de Fatima Costa CM, Cabral-Filho JE. Decline in hospital mortality rate after the use of the World Health Organization protocol for management of severe malnutrition. Trop Doct 2009;39:71–2. | DES |
| Fergusson P, Chinkhumba J, Grijalva-Eternod C, Banda T, Mkangama C, Tomkins A. Nutritional recovery in HIV-infected and HIV-uninfected children with severe acute malnutrition. Arch Dis Child 2009;94:512–16. | DES |
| Fronczak N, Amin S, Laston SL, Baqui AH. An evaluation of community-based nutrition rehabilitation centers. Urban FP/MCH Working paper No. 10. Bangladesh: International Centre for Diarrhoeal Disease Research; 1993. | DES |
| Gaboulaud V, Dan-Bouzoua N, Brasher C, Fedida G, Gergonne B, Brown V. Could nutritional rehabilitation at home complement or replace centre-based therapeutic feeding programmes for severe malnutrition? J Trop Pediatr 2007;53:49–51. | DES |
| Gardner JM, Powell CA, Baker HH, Walker SP, Cole TJ, Grantham-McGregor SM. Zinc supplementation and psychosocial stimulation: effects on the development of undernourished Jamaican children. Am J Clin Nutr 2005;82:399–405. | PG |
| Godard C, Bustos M, Munoz M, Nussle D. Value of a chicken-based formula for refeeding of children with protracted diarrhea and malnutrition in a developing country. J Pediatr Gastroenterol Nutr 1989;9:473–80. | PG |
| Golden MH, Golden BE. Effect of zinc supplementation on the dietary intake, rate of weight gain, and energy cost of tissue deposition in children recovering from severe malnutrition. Am J Clin Nutr 1981;34:900–8. | DES |
| Gopalan C, Swaninathan MC, Kumari VK, Rao DH, Vijayaraghavan K. Effect of calorie supplementation on growth of undernourished children. Am J Clin Nutr 1973;26:563–6. | PG |
| Graham GG, Baertl JM, Cordano A, Morales E. Lactose-free, medium-chain triglyceride formulas in severe malnutrition. Am J Dis Child 1973;126:330–5. | DES |
| Grantham-McGregor S, Stewart M, Powell C, Schofield WN. Effect of stimulation on mental development of malnourished child. Lancet 1979;314:200–1. | OUT |
| Grantham-McGregor SM, Powell C, Stewart M, Schofield WN. Longitudinal study of growth and development of young Jamaican children recovering from severe protein-energy malnutrition. Dev Med Child Neurol 1982;24:321–31. | DES |
| Gueri M, Andrews N, Fox K, Jutsum P, St HD. A supplementary feeding programme for the management of severe and moderate malnutrition outside hospital. J Trop Pediatr 1985;31:101–8. | PG, OUT |
| Hamadani JD, Huda SN, Khatun F, Grantham-McGregor SM. Psychosocial stimulation improves the development of undernourished children in rural Bangladesh. J Nutr 2006;136:2645–52. | PG |
| Heikens GT, Schofield WN, Dawson S, Grantham-McGregor S. The kingston project. I. Growth of malnourished children during rehabilitation in the community, given a high energy supplement. Eur J Clin Nutr 1989;43:145–160. | PG |
| Heikens GT, Schofield WN, Christie CDC, Gernay J, Dawson S. The kingston project. III. The effects of high energy supplement and metronidazole on malnourished children rehabilitated in the community: morbidity and growth. Eur J Clin Nutr 1993;47:174–91. | PG |
| Heikens GT, Schofield WN, Dawson S. The kingston project. II. The effects of high energy supplement and metronidazole on malnourished children rehabilitated in the community: anthropometry. Eur J Clin Nutr 1993;47:160–73. | PG |
| Hone NM, Fermor JK. High-energy feeding for protein-calorie malnutrition. Results in a rural hospital in Zambia. Trop Doct 1987;17:179–81. | DES |
| Hossain MI, Wahed MA, Ahmed S. Increased food intake after the addition of amylase-rich flour to supplementary food for malnourished children in rural communities of Bangladesh. Food Nutr Bull 2005;26:323–9. | PG |
| Husaini YK, Sulaeman Z, Basuki SM, Karyadi D, Matulessy P, Samsudin. Outpatient rehabilitation of severe protein energy malnutrition (PEM). Food Nutr Bull 1986;8:55–9. | DES |
| Intengan CL, Dayrit CS, Pesigan JS, Cawaling T, Zalamea I. Structured lipid of coconut and corn oils vs. soybean oil in the rehabilitation of malnourished children – a field study. Philippine J Intern Med 1992;30:159–64. | PG |
| Jansen AA, Verkley MTB. Ambulatory home nutrition rehabilitation in rural Kenya. J Trop Pediatr 1986;32:258–62. | PG, DES |
| Kapil U, Tandon M, Priyali P, Deepika N. Nutrient intake and consumption of supplementary nutrition by severely malnourished children in two ICDS projects in Rajasthan state. Indian Pediatr 1999;36:799–802. | INT |
| Khan AM, Ahmed T, Alam NH, Chowdhury AK, Fuchs GJ. Extended-interval gentamicin administration in malnourished children. J Trop Pediatr 2006;52:179–84. | PG |
| Khatun UH, Malek MA, Black RE, Sarkar NR, Wahed MA, Fuchs G, et al. A randomized controlled clinical trial of zinc, vitamin A or both in undernourished children with persistent diarrhea in Bangladesh. Acta Paediatr 2001;90:376–80. | PG |
| Kukuruzovic RH, Brewster DR. Milk formulas in acute gastroenteritis and malnutrition: a randomized trial. J Paediatr Child Health 2002;38:571–7. | PG |
| Kumar S, Bhawani L. Managing child malnutrition in a drought affected district of Rajasthan – a case study. Indian J Public Health 2005;49:198–206. | DES |
| Kumari S, Mehra R, Bhargava U, Narang PL, Lall UB. Implications of nutrition education versus food supplementation in pre-school children. Indian Pediatr 1985;22:221–4. | PG |
| Lechtig A. Early malnutrition, growth, and development. In Gracey M, Falkner F, editors. Nutritional needs and assessment of normal growth. New York, NY: Raven Press; 1985. pp. 185–219. | DES |
| Lima AAM, Brito LFB, Ribeiro HB, Martins MCV, Lustosa AP, Rocha EM, et al. Intestinal barrier function and weight gain in malnourished children taking glutamine supplemented enteral formula. J Pediatr Gastroenterol Nutr 2005;40:28–35. | PG |
| Linneman Z, Matilsky D, Ndekha M, Manary MJ, Maleta K, Manary MJ. A large-scale operational study of home-based therapy with ready-to-use therapeutic food in childhood malnutrition in Malawi. Matern Child Nutri 2007;3:206–15. | DES |
| MacIntyre UE, Bac M, Walker ARP. Rehabilitation from protein-energy malnutrition: a study on its effectiveness. South Afr J Epidemiol Infect 1992;7:14–19. | PG, DES |
| MacIntyre UE, Glatthaar II, Kuzwayo PM, Bac M. Protein energy malnutrition – results of rehabilitation. S Afr Med J 1994;84:7–9. | PG, DES |
| MacLean WC, Jr, Graham GG. The effect of energy intake on nitrogen content of weight gained by recovering malnourished infants. Am J Clin Nutr 1980;33:903–9. | DES |
| Madhavi V, Rao NP, Mathur YC, Reddi YR. Brief communication: processed soya flour as substitute for dry skim milk in the “protein packet” in the treatment of protein calorie malnutrition. Indian Pediatr 1973;10:191–2. | DES |
| Maffei HV, Padula NN, Annicchino GP, Ferrari GF, Goldberg TB. Nutritional management and weight changes during hospitalization of Brazilian infants with diarrhoea: primary reliance on oral feeding or continuous nasogastric drip with locally made, modulated minced chicken formula. J Trop Pediatr 1990;36:240–6. | PG |
| Maitland K. Joint BAPEN and Nutrition Society Symposium on ‘Feeding size 0: the science of starvation’. Severe malnutrition: therapeutic challenges and treatment of hypovolaemic shock. Proc Nutri Soc 2009;68:274–80. | DES |
| Manary MJ, Ndkeha MJ, Ashorn P, Maleta K, Briend A. Home based therapy for severe malnutrition with ready-to-use food. Arch Dis Child 2004;89:557–61. | PG |
| Marin L, Gunoz H, Sokucu S, Saner G, Aperia A, Neyzi O, et al. Oral rehydration therapy in malnourished infants with infectious diarrhoea. Acta Paediatr Scand 1986;75:477–82. | PG |
| Mason JB, Hay RW, Leresche J. Treatment of severe malnutrition in relief. Lancet 1974;i:332–5. | PG, DES |
| McDonald K, Grantham-McGregor S, Chang S. Social stimulation of the severely malnourished child: a home training programme. Indian J Pediatr 1989;56:97–103. | PG |
| Morales E, Craig LD, MacLean WC, Jr. Dietary management of malnourished children with a new enteral feeding. J Am Diet Assoc 1991;91:1233–8. | DES |
| Nagpal A, Aneja S. Oral rehydration therapy in severely malnourished children with diarrheal dehydration. Indian J Pediatr 1992;59:313–19. | DES |
| Navarro-Colorado C, McKenny P. Home based rehabilitation in severe malnutrition vs inpatient care in a post-emergency setting. A randomised clinical trial in Sierra Leone. Inter-Agency Workshop, Emergency Nutrition Network, Dublin, 8–10 October 2003. | Abstract |
| Nichols BL, Alvarado J, Hazlewood CF, Viteri F. Magnesium supplementation in protein-calorie malnutrition. Am J Clin Nutr 1978;31:176–88. | PG |
| Ojofeitimi EO, Afolabi OA, Fapojuwo OO. The use of black-eyed-cowpeas-maize gruel mixture, ‘Ewa-ogi’, in the treatment and prevention of infantile protein malnutrition. Nutr Rep Int 1984;30:841–52. | PG |
| Olanrewaju DM, Olusanya O, Oluwole FA. Clinical trial of Pap-salt solution in the treatment of dehydrated children. Nig J Paediatr 1993;20:1–5. | PG |
| Parakh A, Dubey AP, Gahlot N, Rajeshwari K. Efficacy of modified WHO feeding protocol for management of severe malnutrition in children: a pilot study from a teaching hospital in New Delhi, India. Asia Pac J Clin Nutr 2008;17:608–11. | DES |
| Pecoul B, Soutif C, Hounkpevi M, Ducos M. Efficacy of a therapeutic feeding centre evaluated during hospitalization and a follow-up period, Tahoua, Niger, 1987–1988. Ann Trop Paediatr 1992;12:47–54. | PG |
| Polat TB, Uysalol M, Cetinkaya F. Efficacy of zinc supplementation on the severity and duration of diarrhea in malnourished Turkish children. Pediatr Int 2003;45:555–9. | PG, OUT |
| Pot J, Barker M, Brown RS. Comparison of half-cream powdered milk and full-cream acidified powdered milk as a diet for marasmus. S Afr Med J 1970;44:740–1. | PG |
| Puoane T, Sanders D, Chopra M, Ashworth A, Strasser S, McCoy D, et al. Evaluating the clinical management of severely malnourished children – a study of two rural district hospitals. S Afr Med J 2001;91:137–41. | DES |
| Rahman MM, Islam MA, Mahalanabis D, Biswas E, Majid N, Wahed MA. Intake from an energy-dense porridge liquefied by amylase of germinated wheat: a controlled trial in severely malnourished children during convalescence from diarrhoea. Eur J Clin Nutr 1994;48:46–53. | OUT |
| Rao GP, Metta VC. Fish flour in Kwashiorkor. Indian J Pediatr 1970;7:397–401. | DES |
| Rao GP, Ramchandran P. Experience with cotton-seed protein in the therapy of kwashiorkar. Indian J Pediatr 1971;8:380–4. | PG |
| Rao PT, Khan AM, Anjaiah K. Comparative trial of Liv 52 and Orabolin in marasmus. Indian J Pediatr 1972;39:227–30. | PG |
| Ravelomanana N, Razafindrakoto O, Rakotoarimanana DR, Briend A, Desjeux JF, Mary JY. Risk factors for fatal diarrhoea among dehydrated malnourished children in a Madagascar hospital. Eur J Clin Nutr 1995;49:91–7. | DES |
| Razafindrakoto O, Ravelomanana N, Randriamiharisoa F, Rasoarivao V, Ramialimanana V, Rakotoarimanana DR, et al. Rice versus glucose oral rehydration solution (ORS) in severely malnourished children with acute diarrhoea. A controlled clinical trial. Gastroenterology 1991;99:A490. | Abstract |
| Razafindrakoto O, Ravelomanana N, Rasolofo A, Rakotoarimanana RD, Gourgue P, Coquin P, et al. Goat’s milk as a substitute for cow’s milk in undernourished children: A randomized double-blind clinical trial. Pediatrics 1994;94:65–9. | PG |
| Reddy V, Bhaskaram P. Treatment of severe protein energy malnutrition. Indian J Pediatr 1982;19:243–8. | DES |
| Reyes MA, McMurray DN, Watson RR. The influence of renutrition on biochemical and hematological parameters and morbidity in severely malnourished children. Nutr Rep Int 1980;21:63–76. | DES |
| Roode H, Prinsloo JG, Kruger H, Freier E. Effects of an acidified and a non-acidified milk formula on diarrhoea, body mass and serum albumin levels of kwashiorkor patients. S Afr Med J 1972;46:1134–6. | PG |
| Rothman D, Habte D, Latham M. The effect of lactose on diarrhoea in the treatment of kwashiorkor. J Trop Pediatr 1980;26:193–7. | OUT |
| Roy SK, Alam AN, Majid N, Khan AM, Hamadani J, Shome GP. Persistent diarrhoea: a preliminary report on clinical features and dietary therapy in Bangladeshi children. J Trop Pediatr 1989;35:55–9. | PG |
| Roy SK, Tomkins AM, Akramuzzaman SM, Behrens RH, Haider R, Mahalanabis D, et al. Randomised controlled trial of zinc supplementation in malnourished Bangladeshi children with acute diarrhoea. Arch Dis Child 1997;77:196–200. | PG |
| Roy SK, Tomkins AM, Mahalanabis D, Akramuzzaman SM, Haider R, Behrens RH, et al. Impact of zinc supplementation on persistent diarrhoea in malnourished Bangladeshi children. Acta Paediatr 1998;87:1235–9. | PG |
| Roy SK, Tomkins AM, Haider R, Behren RH, Akramuzzaman SM, Mahalanabis D, et al. Impact of zinc supplementation on subsequent growth and morbidity in Bangladeshi children with acute diarrhoea. Eur J Clin Nutr 1999;53:529–34. | PG |
| Sadler K, Myatt M, Feleke T, Collins S. A comparison of the programme coverage of two therapeutic feeding interventions implemented in neighbouring districts of Malawi. Public Health Nutr 2007;10:907–13. | OUT |
| Sadler K, Kerac M, Collins S, Khengere H, Nesbitt A. Improving the management of severe acute malnutrition in an area of high HIV prevalence. J Trop Pediatr 2008;54:364–9. | DES |
| Samadi AR, Wahed MA, Islam MR, Ahmed SM. Consequences of hyponatraemia and hypernatraemia in children with acute diarrhoea in Bangladesh. Br Med J 1983;286:671–3. | PG |
| Sguassero Y, de OM, Carroli G. Community-based supplementary feeding for promoting the growth of young children in developing countries. Cochrane Database Syst Rev 2005;4:CD005039. | PG |
| Sharifi J, Ghavami F, Nowruzi Z. Treatment of severe diarrhoeal dehydration in hospital and home by oral fluids. J Trop Med Hyg 1987;90:19–24. | PG, DES |
| Smith FR, Goodman DS, Arroyave G, Viteri F. Serum vitamin A, retinol-binding protein, and prealbumin concentrations in protein-calorie malnutrition. II. Treatment including supplemental vitamin A. Am J Clin Nutr 1973;26:982–7. | OUT |
| Smith FR, Suskind R, Thanangkul O, Leitzmann C, Goodman DS, Olson RE. Plasma vitamin A, retinol-binding protein and prealbumin concentrations in protein-calorie malnutrition. III. Response to varying dietary treatments. Am J Clin Nutr 1975;28:732–8. | OUT |
| Smith IF. Rehabilitation of protein-energy metabolism and hormonal status in Nigerian children. Nutr Rep Int 1981;24:603–13. | DES |
| Smith IF, Taiwo O, Golden MH. Plant protein rehabilitation diets and iron supplementation of the protein-energy malnourished child. Eur J Clin Nutr 1989;43:763–8. | DES |
| Stanton BF, Phillips N, Clemens JD, Wroot B, Gafur Z, Fleischman J, et al. An urban nutrition education and rehabilitation centre: a description of the programme and change in nutritional status of children who were enrolled. Trop Geogr Med 1987;39:287–95. | DES |
| Stefanak MA, Jarjoura D. Weight gain in supervised and take-home feeding programmes in Chad. J Trop Pediatr 1989;35:214–17. | PG |
| Sullivan PB, Mascie-Taylor CG, Lunn PG, Northrop-Clewes CA, Neale G. The treatment of persistent diarrhoea and malnutrition: long-term effects of in-patient rehabilitation. Acta Paediatr Scand 1991;80:1025–30. | DES |
| Tandon BN. Management of severely malnourished children by village workers in integrated child development services in India. J Trop Pediatr 1984;30:274–9. | DES |
| Thakwalakwa C, Phuka J, Flax V, Maleta K, Ashorn P. Prevention and treatment of childhood malnutrition in rural Malawi: Lungwena nutrition studies. Malawi Med J 2009;21:116–19. | PG, DES |
| Tolboom JJ. Management of severe malnutrition and diarrhea. J Pediatr Gastroenterol Nutr 2000;30:346–8. | DES |
| Torun B, Solomons NW, Caballero B, Flores-Huerta S, Orozco G, Pineda O. The effect of dietary lactose on the early recovery from protein-energy malnutrition. II. Indices of nutrient absorption. Am J Clin Nutr 1984;40:601–10. | OUT |
| van Roosmalen-Wiebenga MW, Kusin JA, de WC. Nutrition rehabilitation in hospital – a waste of time and money? Evaluation of nutrition rehabilitation in a rural district hospital in southwest Tanzania. I. Short-term results. J Trop Pediatr 1986;32:240–3. | DES |
| van Roosmalen-Wiebenga MW, Kusin JA, de WC. Nutrition rehabilitation in hospital – a waste of time and money? Evaluation of nutrition rehabilitation in a rural district hospital in South-west Tanzania. II. Long-term results. J Trop Pediatr 1987;33:24–8. | DES |
| Vasquez-Garibay E, Campollo-Rivas O, Romero–Velarde E, Mendez-Estrada C, Garcia-Iglesias T, vizo-Mora JG, et al. Effect of renutrition on natural and cell-mediated immune response in infants with severe malnutrition. J Pediatr Gastroenterol Nutr 2002;34:296–301. | DES |
| Verkley MTB, Jansen AAJ. A mixed ambulatory-home nutrition rehabilitation programme in a rural area in Kenya. East Afr Med J 1983;60:15–21. | PG |
| Waterlow J. Treatment of children with malnutrition and diarrhoea. Lancet 1999;354:1142. | DES |
| Webb RE, Fougere W, Papillon Y. An evaluation of the educational benefits of nutritional rehabilitation centres as measured by the nutritional status of siblings. J Trop Pediatr Environ Child Health 1975;21:7–10. | PG, DES |
| Weisstaub G, Medina M, Pizarro F, Araya M. Copper, iron, and zinc status in children with moderate and severe acute malnutrition recovered following WHO protocols. Biol Trace Elem Res 2008;124:1–11. | PG, DES |
| Wheeler EF. Changes in anthropometric measurements of children recovering from protein-energy malnutrition. Proc Nutr Soc 1975;34:35A–36A. | DES |
| Winslow C, Lechtig A, Newman B, Klein RE. Impact of a feeding program on preschool children with severe malnutrition. Arch Latinoam Nutr 1980;30:273–8. | DES |
Appendix 7 Question 19: data extraction tables
Shortened data extractions were prepared to obtain information for question 19, ‘What methods are effective for treating SAM among infants < 6 months old?’. Only two studies presented information separately for this age group; however, neither study focused on this age group. No quality assessment was undertaken for either study.
Nu Shwe 200347
Data extraction table
| Reference and design | Intervention | Participants | Outcome measures |
|---|---|---|---|
|
Author: Nu Shwe47 Year: 2003 Country: Myanmar (Burma) Study design: cohort with historic control Setting: secondary care Number of centres: one Funding: NR |
Intervention: the WHO guidelines for management of SAM (with two modifications – all assumed to have hypoglycaemia and given 10% sucrose on admission, monitoring of pulse and respiration every 30 minutes instead of every 10 minutes) Control: standard management of SAM prior to introduction of the WHO guidelines. No details provided Other interventions used: critical-care pathway introduced in late 2001 |
Definition of SAM: W/H or W/L < 70% of the NCHS/WHO reference and/or symmetrical oedema of the feet Number of participants: control year 1999 = 157, of which 18 (11.4%) were < 6 months of age; WHO year 2000 = 196, of which 21 (10.7%) were < 6 months of age; WHO year 2001 = 186, of which 12 (6.4%) were < 6 months of age; WHO year 2002 January to August = 117, of which six (7.7%) were < 6 months of age Sample attrition/dropout: NR Sample crossovers: not applicable Inclusion criteria: W/H or W/L < 70% of the NCHS/WHO reference and/or symmetrical oedema of the feet Exclusion criteria: NR General characteristics of participants: severely malnourished children admitted to Yangon Children’s Hospital |
Primary outcomes: not stated Outcomes: outcomes reported include mortality, duration of hospital stay, readmissions, time taken for recovery. The only outcome reported separately for the < 6 months age group was proportional mortality Method of assessing outcomes: not stated Adverse symptoms: not stated Length of follow-up: not stated Recruitment dates: January 2000 to August 2002 |
| Characteristics of participants | |||
| Characteristic | WHO year 2000 (n = 196) | WHO year 2001 (n = 186) | Control year 1999 (n = 157) |
| Age mean, months (range) | 29 (39 days–12 years) | 28 (2 months–12 years) | 25 (39 days–11 years) |
| Children with oedema, n | 12 | 12 | 15 |
| Children with skin lesions, n (%) | 34 (21.7) | ||
| Comments: characteristics are available only for the whole group, they are not available separately for the group of infants aged < 6 months. Only age, number of children with oedema and with skin lesions have been data extracted. Data on children with hypothermia, hypoglycaemia, mean weight and mean length have not been data extracted. Data extracted only for full years (not the partial year 2002) | |||
| Results | |||
| Primary outcomes | WHO year 2000 (n = 21) | WHO year 2001 (n = 12) | Control year 1999 (n = 18) |
| Proportional mortality for < 6 month age group (%) | Cases: 10.7 | Cases: 6.5 | Cases: 11.4 |
| Deaths: 9.1 | Deaths: 12.5 | Deaths: 12 | |
|
Comments: only results for the 0–6 month age group have been data extracted as these may inform question 19. The overall results have not been data extracted because they relate to question 1, which was not ranked in the top 10 questions by the Delphi process The paper states that, comparatively, the proportional mortality in the age groups < 6 months and 6–12 months was lower than in the 13–24 months and > 24 months age groups (9–24% vs 20–50%). The author also comments that overall SAM in children < 6 months of age had significantly reduced due to implementation of exclusive breastfeeding programmes in hospital, clinic and community. The lower proportional mortality observed in the < 12 months age groups may also be due to the impact of breastfeeding Safety: NR HIV: NR |
|||
| Barriers to implementation | |||
|
Some barriers reported relating to the overall study and implementation of the WHO guidelines, but no barriers specifically relating to children < 6 months of age were reported. Aspects that may have affected this age group include difficulty obtaining ready-made combined mineral–vitamin mix, impracticality of monitoring pulse and respiration every 10 minutes (staff did their best to monitor every 30 minutes) and blood glucose could not be tested in every child, so all children were assumed to have hypoglycaemia and given 10% sucrose solution on admission Other barriers reported related to the critical-care pathway, but as the details of this are not clear these have not been extracted |
|||
| Methodological comments | |||
|
Allocation to treatment groups: not applicable as this was a cohort study with retrospective control Blinding: not explicitly stated, but presume none Comparability of treatment groups: comparability of the 0–6 month age group in each trial arm unknown as data not provided. Baseline characteristics of the participants for each year are broadly comparable although with some changes (e.g. number of children under 6 months admitted occurring over time) Method of data analysis: NR Sample size/power calculation: NR Attrition/dropout: NR |
|||
| General comments | |||
|
Generalisability: difficult to assess. The numbers of children aged < 6 months were small and there was a lack of data presented separately for them Outcome measures: only one outcome measure, proportional mortality, reported for the 0–6 month age group Intercentre variability: not applicable, but there may have been variations between years Conflict of interest: NR |
|||
Hossain et al. 200948
Data extraction table
| Reference and design | Intervention | Participants | Outcome measures | |
|---|---|---|---|---|
|
Author: Hossain et al. 48 Year: 2009 Country: Bangladesh Study design: prospective cohort with concurrent control Setting: secondary care Number of centres: two Funding: ICMH |
Intervention: ICMH protocol for management of SAM with no phasing Control: WHO protocol with two phases of management of SAM; the ICMH and WHO protocols are outlined separately below Other interventions used: none |
Definition of SAM: W/H < 70% of the expected NCHS/WHO references with or without bilateral pitting oedema Number of participants: 60 (number aged < 6 months NR), 30 in each group Sample attrition/dropout: reported for whole group only Sample crossovers: NR Inclusion criteria: SAM children aged 2–59 months with W/H < 70% of the expected (NCHS/WHO references) with or without bilateral pitting oedema Exclusion criteria: children with major congenital abnormalities or disabilities and having feeding difficulty General characteristics of participants: in addition to having SAM, all belonged to urban and periurban areas of Dhaka |
Primary outcomes: not explicitly stated but presumed to be weight gain (in gram per kg per day) as the sample size calculation was based on this Other outcomes: improved appetite, disappearance of oedema, improvement of other associated medical conditions, time taken for gaining target weight, mortality rate Method of assessing outcomes: target weight-W/H reaching 1 SD (90%) of NCHS/WHO median reference values Adverse symptoms: NR Length of follow-up: not explicitly stated, appears to be to discharge Recruitment dates: June to December 2003 |
|
| Characteristics of participants | ||||
| Characteristic | ICMH intervention (n = 30) | WHO control (n = 30) | p-value | |
| Age (months), mean ± SD | 17.90 ± 14.17 | 18.33 ± 13.76 | 0.90 | |
| Sex ratio, F : M | 1 : 1 | 1 : 1 | ||
| Nutritional status | ||||
| Marasmus, n (%) | 20 (66.8) | 20 (66.8) | NR | |
| Marasmic kwashiorkor, n (%) | 5 (16.7) | 4 (13.3) | 0.9 | |
| Kwashiorkor, n (%) | 5 (16.7) | 6 (20) | NR | |
| Comments: characteristics are only available for the whole group, they are not available for the group of infants aged < 6 months. Only age, sex ratio and nutritional status have been data extracted. Data on parents’ education, profession and income has not been data extracted | ||||
| Results | ||||
| Primary outcomes | ICMH intervention | WHO control | p-value | |
| Weight gain for 0–6 month age group, mean ± SD g/kg/day | 17.5 ± 7.5 (n unknown) | 11.6 ± 6.8 (n unknown) | 0.21 | |
| Comments: only results for the 0–6 month age group have been data extracted as these may inform question 19. The overall results have not been data extracted because they relate to question 1, which was not ranked in the top 10 questions by the Delphi process | ||||
| Safety: NR for 0–6 month age group | ||||
| HIV: NR | ||||
| Barriers to implementation | ||||
| Copper not available in the local market, so this could not be used in the provision of minerals and trace elements | ||||
| Methodological comments | ||||
|
Allocation to treatment groups: children at one hospital were managed with the WHO protocol, children at the other hospital were managed with the ICMH protocol. No information regarding allocation of each hospital to which protocol Blinding: not stated Comparability of treatment groups: comparability of the 0–6 month age group in each trial arm unknown as data not provided. Baseline characteristics of the complete trial arms are comparable Method of data analysis: data for appetite, weight, oedema and other clinical parameters were collected daily through a structured questionnaire and checked manually at collection period and prior to entry into Microsoft Access (Microsoft Corporation, Redmond, WA, USA) and subsequently SPSS/PC+ for analysis. Student’s t-test was used for comparing continuous variables and the chi-squared test was used for comparing the mortality rate Sample size/power calculation: a sample size for equivalence was calculated assuming that the mean time taken for targeted weight gain is 25 days in each group with a SD of 6 days. Minimum acceptable difference in the two groups was set at 4.5 days with alpha error of 0.05 and power 80%. Study unlikely to be powered for infants aged < 6 months and the number of such infants recruited is NR Attrition/dropout: NR separately for the 0–6 month age group. Overall, this did not differ between the groups: two children in each group died, two children were discharged on request in the WHO group, three in the ICMH group and one child absconded in the WHO group |
||||
| General comments | ||||
|
Generalisability: difficult to assess generalisability because the numbers of children aged 0–6 months are not known Outcome measures: only one outcome measure, weight gain, reported for the 0–6 month age group Intercentre variability: two centres, but each was applying a different protocol. Unclear how differences between the two centres, other than the different protocols, might have influenced the results Conflict of interest: no competing interests are stated by the report authors |
||||
| ICMH protocol | WHO protocol | |||
| Management | No phasing | Divided into two phases: initial and rehabilitation phase as per WHO 1999 guidelines.10 Reference provided but no details; those below obtained from original WHO paper10 | ||
| Identification of life-threatening problems, and management of unconsciousness, convulsion, hypothermia and hypoglycaemia done according to the WHO protocol for both groups | ||||
| Correction of electrolyte imbalance and micronutrients deficiencies | Locally available minerals and trace elements as below | Added to F75 and F100 formula at concentrations noted below | ||
| Potassium | Potassium chloride 5 mmol/kg/day |
F75: 3.6 mmol per 100 ml F100: 5.9 mmol per 100 ml |
||
| Magnesium | Magnesium sulphate 10 mg/kg/day |
F75: 0.43 mmol per 100 ml F100: 0.73 mmol per 100 ml |
||
| Sodium | NR |
F75: 0.6 mmol per 100 ml F100: 1.9 mmol per 100 ml |
||
| Zinc | Zinc sulphate 2 mg/kg/day |
F75: 2.0 mg per 100 ml F100: 2.3 mg per 100 ml |
||
| Folic acid | 2.5 mg/day | 5 mg of folic acid on day 1 and then 1 mg per day thereafter. Folic acid also present in vitamin mix 0.35 mg per litre of liquid diet | ||
| Multivitamins | 0.6 ml/day orally (composition per 0.6 ml of multivitamin: vitamin D1, 200 IU, thiamine 1 mg, riboflavin 1 mg, pyridoxine 1 mg, panthenol 2 mg, nicotinamide 5 mg and vitamin C 60 mg) | Added to liquid diet in all phases of treatment [per litre of liquid diet: thiamine 0.7 mg, riboflavin 2.0 mg, nicotinic acid 10 mg, pyridoxine 0.7 mg, cyanocobalamin (vitamin B12) 1 μg, vitamin C 100 mg pantothenic acid 3 mg, biotin 0.1 mg, retinol (vitamin A) 1.5 mg, calciferol (vitamin D) 30 μg, vitamin E 22 mg and vitamin K 40 μg] | ||
| Copper | Not available in the local market for use |
F75: 0.25 mg per 100 ml F100: 0.25 mg per 100 ml |
||
| Iron | Supplementation (6 mg/kg/day) was started on the 15th day | Iron should never be given during the initial phase of treatment. During the rehabilitation phase, children with moderate or severe anaemia were given elemental iron orally, 3 mg/kg per day in two divided doses, up to a maximum of 60 mg daily, for 3 months | ||
| Severe anaemia | Blood transfusion given (with or without heart failure) | Blood transfusion given | ||
| Vitamin A supplement | Every child |
For all children, given orally < 6 months of age 50,000 IU 6–12 months of age 100,000 IU > 12 months of age 200,000 IU For those with clinical signs of vitamin A deficiency dose as above given on the first 2 days, followed by a third dose at least 2 weeks later |
||
| Antibiotics | As recommended by WHO for both groups | |||
| Feeds | Made using whole cow’s milk, sugar, soya oil and water to provide 100 kcal in 100 ml/kg/day administered every 2 hours during day and night. If the child wanted more than the prescribed diet, extra family food was given ad libitum and breastfeeding was encouraged | Two formula diets, F75 and F100, are used made from dried skimmed milk, sugar, cereal flour, vegetable oil, mineral and vitamin mixes. F75 (75 kcal th or 315 kJ/100 ml), is used during the initial phase of treatment, whereas F100 (100 kcal th or 420 kJ/100 ml) is used during the rehabilitation phase, after the appetite has returned | ||
| Play therapy, nutrition education and discharge criteria | Similar to those for children in the WHO group | |||
Appendix 8 Question 21: data extraction tables
Akech et al. 201049
Data extraction table
| Reference and design | Intervention | Participants | Outcome measures | ||
|---|---|---|---|---|---|
|
Author: Akech et al. 49 Year: 2010 Country: Kenya Study design: RCT (phase II) Setting: inpatient (district hospital) Number of centres: one Funding: the Wellcome Trust (Sponsor: Oxford University) |
Intervention: RL (see below for dosages) Control: WHO fluid resuscitation regimen (HSD/5D) (see below for dosages) Third treatment arm: 4.5% HAS for those with non-diarrhoeal shock Children with severe dehydrating diarrhoea/shock were randomised to RL or HSD/5D; children with presumptive septic shock (non-diarrhoeal shock) were randomised to RL, HSD/5D or HAS. Paper only reports mortality and safety outcomes for HAS group owing to small numbers (n = 6) Other interventions used: all children treated according to WHO guidelines – hypoglycaemia treated ORS (ReSoMaL) given where appropriate, all received antibiotics, early nasogastric feeding withheld but maintenance i.v. dextrose fluids given until stabilised, intestinal ileus excluded and tolerance of oral feeds established (see below for further details) |
Definition of SAM: any of:Number of participants: 86 assessed for eligibility, 61 enrolled and randomised (RL n = 29, HSD/5D n = 26, HAS n = 6) Sample attrition/dropout: no withdrawals/dropouts; 31/61 (51%) deaths Sample crossovers: none Inclusion criteria: aged > 6 months with SAM, with evidence of shock. Shock criteria included children with more than one of the following: CRT > 2 seconds, lower limb temperature gradient, weak pulse volume, deep ‘acidotic’ or ‘Kussmaul’ breathing, creatinine > 80 µmol/l, or depressed conscious state [prostration (inability to sit up if aged > 8 months)] if present after correction of hypoglycaemia Exclusion criteria: severe anaemia (Hb ≤ 5 g/dl), pulmonary oedema (defined as clinical evidence of presence of fine crepitations in both lung fields plus oxygen saturations < 90% in air), raised intracranial pressure, known congenital heart disease General characteristics of participants: SAM children aged > 6 months with hypovolaemic shock secondary to either dehydrating diarrhoea or sepsis, 42% HIV+ve |
Primary outcomes: resolution of features of shock [including tachycardia and oliguria (production of abnormally small volume of urine)] at 8 and 24 hours Secondary outcomes:Method of assessing outcomes: resolution of shock defined as the absence of all of: severe tachycardia (heart rate > 160 beats/minute), CRT > 2 seconds or oliguria (urine output < 1ml/kg/hour). Dehydrating diarrhoea defined as ≥ 6 watery stools per day MUAC measured with a cloth (non-stretchable) measuring tape; weight with an electronic scale (Soehnle model 7300; CMS Instruments, UK) and length using a measuring board of standard design Temperature gradient defined as cooler extremities to warmer core, and was assessed by running the back of the palm of the hand up the lower limb. Radial pulse was used to assess pulse volume. Oxygen saturation continuously measured using a multichannel Siemens® monitor. Blood pressure and urine output monitored hourly and then every 4 hours after 8 hours Adherence to protocol validated by an internal, but independent monitoring team Adverse symptoms: respiratory distress, pulmonary oedema, allergic reaction (to HAS) Length of follow-up: outcomes at 24 hours; reports that children were followed up intensively up to 48 hours and thereafter for in-hospital survival Recruitment dates: November 2006 to May 2008 (recruitment discontinued early) |
||
| Characteristics of participants: | |||||
| Characteristic | RL (n = 29) | WHO fluid HSD/5D (n = 26) | p-value | ||
| Severe dehydration/shock,a n (%) | 21 (72) | 19 (73) | NR | ||
| Presumptive shock,b n (%) | 8 (28) | 7 (27) | NR | ||
| Male, n (%) | 17 (59) | 15 (58) | 0.94 | ||
| Age, months (IQR)c | 16 (6) | 15 (14) | 0.41 | ||
| MUAC cm, mean ± SD | 10.0 (1.9) | 10.4 (1.4) | 0.43 | ||
| W/H z-score, mean ± SD | –3.9 (1.0) | –3.4 (1.3) | 0.18 | ||
| Severe wasting, n (%) | 21 (72) | 14 (54) | 0.15 | ||
| Kwashiorkor, n (%) | 4 (14) | 8 (31) | 0.19 | ||
| HIV+ve,d n (%) | 14 (48) | 9 (35) | 0.65 | ||
| WHO shock criteria, n (%) | 23 (79) | 18 (69) | 0.39 | ||
| Tachypnoea (> 60 breaths/minute), mean ± SD | 13 (45) | 16 (62) | 0.22 | ||
| Severe tachycardia (> 160 beats/minute), mean ± SD | 8 (28) | 11 (42) | 0.25 | ||
| Hydration, n (%) | |||||
| Reduced skin turgor | 16 (55) | 8 (31) | 0.07 | ||
| Sunken eyes | 19 (66) | 11 (42) | 0.08 | ||
Comments: baseline characteristics data not presented in the paper for HAS group owing to small numbers, though described as similar to other participants with sepsis
|
|||||
| Results | |||||
| Primary outcomes | RL (n = 29) | WHO fluid HSD/5D (n = 26) | p-value | ||
| Number with shock, n/N (%) | |||||
| 8 hours | 14/25 (56) | 15/22 (68) | 0.39 | ||
| 24 hours | 14/25 (56)e | 14/18 (78) | 0.14 | ||
| Oliguria (< 1 ml/kg/hour), n/N (%) | |||||
| 8 hours | 3/25 (12)f | 9/22 (41)g | 0.02h | ||
| 24 hours | 6/25 (24)i | 8/18 (44)j | 0.16 | ||
| Tachycardia (> 160 beats/minute), n/N (%) | |||||
| 8 hours | 4/25 (16) | 6/22 (27) | 0.34 | ||
| 24 hours | 4/25 (16) | 8/14 (44) | 0.04 | ||
Comments: there appear to be discrepancies in the paper between data presented in tables and data presented in figures, all estimated by reviewer (see table footnotes for details)
|
|||||
| Secondary outcomes | RL (n = 29) | WHO fluid HSD/5D (n = 26) | 4.5% albumin (HAS) (n = 6) | p-value | |
| In-hospital mortality, n/N (%) | 13/29 (45) | 15/26 (58) | 3/6 (50) |
0.62k 0.34l |
|
| Tachypnoea (> 60 breaths/minute), n/N (%): | |||||
| 8 hours | 2/25 (8) | 7/22 (32) | NR | 0.04 | |
| 24 hours | 3/25 (12) | 7/18 (39) | NR | 0.04 | |
Comments:
|
|||||
Safety:
|
|||||
HIV:
|
|||||
| Barriers to implementation | |||||
| Participant recruitment was discontinued early after an interim review of the safety data and thus the study was underpowered | |||||
| Methodological comments | |||||
|
Allocation to treatment groups: children were randomly assigned in two batches (1) those with severe dehydration/shock randomised to WHO HSD/5D or RL; and (2) those with presumptive (non-severe diarrhoea) shock randomised to WHO HSD/5D, RL or HAS. Random allocation was assigned by use of sealed cards. No further details were reported Blinding: reports that study interventions were not masked (thus patients and care providers were not blinded). No details on blinding of outcome assessors Comparability of treatment groups: no statistically significant differences between RL and HSD/5D treatment groups (p-values reported). Paper reports that baseline characteristics and disease severity indices were similar across the fluid intervention arms. Also, characteristics and haemodynamic responses in the six HAS individuals were similar to the other participants in the presumptive sepsis shock group who were randomised to HAS/5D and RL treatments (data were not presented because of small numbers) Method of data analysis: the null hypothesis was that there is no difference in the safety profile or effect on physiological parameters of shock when using any of the three fluids for resuscitation. Dichotomous and categorical variables were created from continuous variables. Derived variables were created from clinical factors defined by guidelines as indicating a definitive need for urgent therapeutic intervention and for lab variables. Means and SDs were calculated for continuous variables using Student t-tests. Non-normally distributed data were compared using Sign-rank test and Kruskal–Wallis. Proportions were compared using chi-squared and Fisher’s exact tests as appropriate. Kaplan–Meier survival analysis was also used to compare time-to-event (death). AUCs were calculated for serial measurements and their medians compared using Wilcoxon rank-sum and Kruskal–Wallis tests. AUC was employed to compensate for confounding effect of early mortality, hence, missing observations, leading to biases in the highest risk group and resulting in imbalance within the survivors. Reports that all analyses were ITT; outcomes were reported for all those who survived Sample size/power calculation: the study aimed to recruit 90 children: 45 RL, 45 HSD/5D and 20 HAS (reviewer note: numbers add to 110 not 90) to provide sufficient information on haemodynamic response and adverse events to the two fluid management regimes to understand the potential efficacy rather than for comparison. A specific sample size calculation was not presented. The numbers were not achieved as recruitment was discontinued after an interim review of safety data, and therefore the study was underpowered Attrition/dropout: numbers and reasons reported. No dropouts/withdrawals and 31 deaths (15 HSD/5D, 13 RL, 3 HAS) |
|||||
| General comments | |||||
|
Generalisability: likely that most of the children would meet the current WHO criteria (W/H z-score < –3 SD). Population were largely infants (median age 15 months) with SAM and features of shock (75% had advanced shock as defined by WHO), severe or non-severe diarrhoea, and 42% were HIV+ve Outcome measures: outcomes appropriate for study objectives; weight gain NR Intercentre variability: N/A Conflict of interest: no competing interests declared. All authors were associated with the Wellcome Trust Research Programme, but states that the funders had no role in the research or in the preparation of the manuscript |
|||||
| WHO fluid resuscitation regimen HSD/5D | RL or albumin (HAS) resuscitation | ||||
|
|
||||
|
|||||
| Standard WHO management of SAM | |||||
In all other respects, children were treated according to WHO guidelines
|
|||||
Quality assessment for primary studies (modified for severe malnutrition)
| A. Selection bias | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1. Are the individuals selected to participate in the study likely to be representative of the target population? | Very likely | Somewhat likely | Not likely | Cannot tell | |||||
| ✓ | |||||||||
| 2. What percentage of selected individuals participated? | 80–100% | 60–79% | < 60% | N/A | Cannot tell | ||||
| ✓ | |||||||||
| Summary of selection bias | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| B. Study design | |||||||||
| 1. What was the study design? | RCT | ✓ | |||||||
| (Please tick appropriate and specify design if categorise as ‘Other’) | CCT | ||||||||
| Cohort analytic (two group pre + post) | |||||||||
| Case–control | |||||||||
| Cohort [one group pre + post (before and after)] | |||||||||
| Interrupted time series | |||||||||
| Other – specify | |||||||||
| Cannot Tell | |||||||||
| 2. Was the study described as randomised? | Yes | No | |||||||
| ✓ | |||||||||
| If answer to no. 2 is ‘no’ complete summary then go to section C. Confounders. If answer is ‘yes’, answer no. 3 and no. 4 below, before completing summary for this section | |||||||||
| 3. If answer was yes, was the method of randomisation described? | Yes | No | |||||||
| ✓ | |||||||||
| 4. If answer was yes, was the method appropriate? | Yes | No | |||||||
| ✓ | |||||||||
| Summary of study design | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| C. Confounders | |||||||||
| 1. Were there important differences between groups prior to the intervention? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. If yes, indicate the percentage of relevant confounders that were controlled [either in the design (e.g. by stratification or matching) or in the analysis]? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| Summary of confounders | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| D. Blinding | |||||||||
| 1. Was the outcome assessor aware of the intervention or exposure status of participants? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were the study participants aware of the research question? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| Summary of blinding | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| E. Data collection methods | |||||||||
| 1. Were data collection tools shown to be valid? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were data collection tools shown to be reliable? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| Summary of data collection | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| F. Withdrawals and dropouts | |||||||||
| 1. Were withdrawals and dropouts reported in terms of numbers and reasons per group? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Indicate the percentage of participants completing the study (If the percentage differs by groups, record the lowest) | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
| Summary of withdrawals and dropouts | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| G. Intervention integrity | |||||||||
| 1. What percentage of participants received the allocated intervention or exposure of interest? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
| 2. Was the consistency of the intervention measured? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 3. Is it likely that subjects received an unintended intervention (contamination or co-intervention) that may influence the results? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| H. Analysis | |||||||||
| 1. Indicate the unit of allocation | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 2. Indicate the unit of analysis | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 3. Are the statistical methods appropriate for the study design? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 4. Is the analysis performed by intervention allocation status (i.e. ITT) rather than actual intervention received? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| Global rating for studya | Strong | Moderate | Weak | ||||||
| (Overall methodological strength of study – based on sections A–F) | ✓ | ||||||||
Appendix 9 Question 22: data extraction tables
Alam et al. 200051
Data extraction table
| Reference and design | Intervention | Participants | Outcome measures | ||
|---|---|---|---|---|---|
|
Author: Alam et al. 51 Year: 2000 Country: India Study design: double-blind RCT Setting: inpatient (diarrhoea training and treatment unit) Number of centres: one Funding: Department of Pediatrics, Jawaharlal Nehru Medical College, Aligarh Muslim University, Aligarh (material and preparation of ORS) |
Intervention: H-ORS (see end of table for details) Control: standard WHO-ORS 75 ml/kg of ORS to be taken in 4 hours for both groups following study inclusion (five sachets formulated in 1 litre of water) (see end of table for details) Other interventions used: if severely dehydrated, 50 ml/kg of i.v. RL in first hour prior to study inclusion A single dose of doxycycline (8 mg/kg ) was administered to all with clinical suspicion of cholera or positive stool for motile organisms and dose was repeated if the child vomited within half an hour of taking the drug Indications for i.v. fluids were severe dehydration, persistent vomiting (> 3 hours) and persistent dehydration at the end of 4 hours of oral rehydration therapy. 75 ml/kg of RL were given in the next 3 hours and then the child was put back on the study ORS Khichri, Dalia, curds and banana feeds were offered once hydration improved breastfeeding was continued throughout |
Definition of SAM: W/H < 70%, assessed as per the NCHS, but W/A (not height) is reported in the results Total: n = 170 (88 H-ORS, 82 WHO-ORS) Number of SAM participants: H-ORS n = 41/88 (47%), cholera n = 19/35, non-cholera n = 69/135; WHO-ORS n = 40/82 (49%), cholera n = 16/35, non-cholera n = 66/135 Total sample attrition/dropout: n = 19/170 (11%) ; dropouts n = 11; removed n = 8, treatment failures put on WHO–ORS Sample crossovers: none Inclusion criteria: children with acute (< 4 days duration) diarrhoea with dehydration and > 3 months of age with clinical suspicion of cholera aged 3 months – 5 years with non-cholera diarrhoea Exclusion criteria: children with clinical evidence of systemic infection, encephalopathy, electrolyte imbalance, convulsions or invasive diarrhoea General characteristics of participants: children aged from 3 months to 5 years with cholera and acute non-cholera diarrhoea |
Primary outcomes: not specifically reported Outcomes:Method of assessing outcomes: timescale for rehydration and maintenance phases not defined Intake output records and assessment of dehydration measured four hourly Nutritional status assessed as per NCHS Recovery and discharge criteria: non-cholera diarrhoea – three consecutive semi-formed stools or no stools for 12 hours; cholera – no dehydration for 8 hours or no stools for 6 hours Stool: frequency recorded by mother (tally marking). Motile organisms and stool culture was completed for all. Culture was collected on sterile rectal swab and stored in ‘Careyblair’s media’ and plated within 12 hours Urine: output collected for boys during initial 24 hours Weight: taken at admission, end of rehydration and discharge Serum sodium: estimation was done at 24 hours Adverse symptoms: NR Length of follow-up: none reported, but appears to be until recovery (see definition above) Recruitment dates: only states that authors enrolled until August 1998 |
||
| Comments: H-ORS treatment failures were transferred to WHO-ORS. Treatment failure definition: dehydration > 72 hours, diarrhoea > 7 days, consumption of ORS > 8 litres in < 5 years age group, or > 10 litters in > 5 years age group and needing i.v. fluids > 150 ml/kg. Children leaving study prior to recovery were considered treatment failures, if they had dehydration and or frequency of stools | |||||
| Characteristics of participants | |||||
| Characteristic SAM only | H-ORS (n = 41) | WHO-ORS (n = 40) | p-value | ||
| Mean age, month (SD) | 25.29 (2.09) | 24.17 (2.23) | NR | ||
| Mean W/A, % (SD) | 52.4 (1.64) | 58.6 (1.12) | NR | ||
|
Comments: total sample only. There were no significant differences in the two groups at admission for mean duration (95% CI 11.9 to 20.5; p = 0.6) and frequency (95% CI 1.1 to 1.4; p = 0.79) of diarrhoea, whereas the per cent of children with vomiting (OR 1.06, 95% CI 0.43 to 2.31), with some (OR 0.89, 95% CI 0.71 to 1.12) or severe (OR-1.61, 95% CI 0.59 to 4.33) dehydration and those receiving ORS (OR 0.74, 95% CI 0.24 to 2.22) at admission, were comparable. NR for SAM CI for children with vomiting was reported as 96%. This is assumed to be an error, as all other CIs were reported as 95% |
|||||
| Results | |||||
| Outcomes, mean (SD) | H-ORS (n = 41) | WHO-ORS (n = 40) | p-value (95% CI) | ||
| Weight gain (%) | 4.54 (1.79) | 4.45 (2.18) | Not significantly different (p-value NR) | ||
| Caloric intake (kcal/kg/day) | 42.72 (1.66) | 39.73 (2.03) | Not significantly different (p-value NR) | ||
| Rehydration frequency (stools/4 hours) | 4.27 (2.029) | 5.86 (1.73) | p = 0.32a,b (0.55 to 0.97) | ||
| Rehydration ORS consumed (litres) | 1.45 (0.002) | 1.55 (0.002) | Not significantly different (p-value NR) | ||
| Rehydration duration (hours) | 10.95 (2.23) | 11.72 (2.26) | Not significantly different (p-value NR) | ||
| Maintenance frequency (stools/4 hours)c | 1.72 (1.92) | 2.45 (2.17) | p = 0.035a (0.51 to 0.97) | ||
| Maintenance-ORS consumed (litres)c | 0.69 (0.005) | 0.74 (0.01) | Not significantly different (p-value NR) | ||
| Maintenance duration (hours)c | 10.45 (2.09) | 16.36 (2.01) | p = 0.007a (0.46 to 0.88) | ||
| Overall frequency (stool/4 hours) | 3.39 (1.80) | 4.70 (1.68) | p = 0.011a (0.56 to 0.93) | ||
| Overall ORS consumed (litres) | 2.74 (0.0017) | 3.32 (0.0017) | Not significantly different (p-value NR) | ||
| Overall duration (hours) | 24.35 (1.57) | 30.12 (1.69) | Not significantly different (p-value NR) | ||
| Serum sodium (mEq/l) | 134.89 (1.03) | 137.03 (1.03) | Not significantly different (p-value NR) | ||
| Urine output (boys) (ml/kg/hour)d | 55.79 (1.65) | 55.73 (1.89) | Not significantly different (p-value NR) | ||
| i.v. fluids (ml/kg)e | 121.23 (1.81) | 70.73 (1.51) | Not significantly different (p-value NR) | ||
|
Other (total sample): treatment failure n = 12/170 (7%); H-ORS n = 3/88, WHO-ORS 9/82 (OR 0.28, 95% CI 0.07 to 1.1) Discharged n = 151 (two children recovered after rehydration phase) The paper also reported results for H-ORS vs WHO-ORS in total cases and for H-ORS vs WHO-ORS in non-cholera diarrhoea. These were not data extracted. However, the significant results for the SAM subgroup were in the same direction as the results for H-ORS vs WHO-ORS in total cases Safety: NR HIV: not applicable |
|||||
| Barriers to implementation | |||||
| None reported | |||||
| Methodological comments | |||||
|
Allocation to treatment groups: cases were serially allotted the study ORS packet Blinding: states double-blind trial; packets of sachets were reported to be identical. No details reported on blinding of outcome assessors Comparability of treatment groups: characteristics in whole group were reported to be compatible at admission (p-value or OR plus 95% CI given). Only age and W/A reported for SAM group and not significantly different (p-value not given) Method of data analysis: analyses of different parameters were conducted in the re-hydration phase, in the maintenance phase, for overall combined data, for children split into cholera/non-cholera and repeated for children with W/H < 70% (but W/A reported in tables) and breast fed/non-breastfed children < 2 years old using SPSS (Version 7.5; SPSS Inc., Chicago, IL, USA). Variables with skewed distribution were log transformed and two-tailed Student’s t-test used to compare the groups. Chi-squared tests were used to correlate the qualitative variables. For treatment failures/dropouts, the data that were collected during their stay in the study was included in the analysis. Only data for SAM (W/A < 70%) was extracted, with reference made to direction of whole group results Sample size/power calculation: the study was planned to detect a 30% difference in the frequency and duration of diarrhoea of the two ORS. It was calculated that 82 children were needed per group to detect this difference with a power of 90% and a significance level of 5%. Previous data (frequency of 4.11 ± 2.67 stools/4 hours and duration of diarrhoea 36 ± 20.0 8 hours) from the Diarrhoea Treatment and Training Unit of 70 non-cholera children treated on WHO-ORS was used to determine the sample size. SAM is a subgroup (less than half of the total sample) and analysis is unlikely to be powered Attrition/dropout for total sample: numbers reported, but details omitted. Four cases had a frequency of 310 (meaning unclear) in last 24 hours and were considered treatment failure, six cases required more than the pre-determined volume of ORS, one case had dehydration phase of > 72 hours, one case needed > 150 ml/kg i.v. fluids and 12 cases (7%) of treatment failures on H-ORS were moved to WHO-ORS |
|||||
| General comments | |||||
|
Generalisability: SAM defined using a NCHS criteria of < 70% W/H; however, only W/A is reported in all tables. It is unclear whether or not the participants are severely malnourished as per WHO criteria (< 70% W/H), although in SAM group mean W/A is well below the 70% benchmark (~ 55%). SAM subgroup represents less than half of the total sample (47% H-ORS; 49% WHO-ORS) and children around 2 years of age with dehydration and with/without cholera diarrhoea Outcome measures: appear to be suitable and appropriate Intercentre variability: not applicable, one centre only Conflict of interest: none |
|||||
| Details of intervention and control | |||||
| WHO-ORS and H-ORS packets prepared in the departmental research lab | |||||
| H-ORS | WHO-ORS | ||||
| Component, g | |||||
| NaCl | 2.6 | 3.5 | |||
| KCl | 1.5 | 1.5 | |||
| Trisodium citrate | 2.9 | 2.9 | |||
| Glucose | 13.5 | 20 | |||
| Concentration of, mmol/l | |||||
| Sodium | 75 | 90 | |||
| Potassium | 20 | 20 | |||
| Chloride | 65 | 80 | |||
| Citrate | 10 | 10 | |||
| Glucose | 75 | 111 | |||
| Osmolarity, mosmol/l: | 245 | 311 | |||
Quality assessment for primary studies (modified for severe malnutrition)
| A. Selection bias | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1. Are the individuals selected to participate in the study likely to be representative of the target population? | Very likely | Somewhat likely | Not likely | Cannot tell | |||||
| ✓ | |||||||||
| 2. What percentage of selected individuals participated? | 80–100% | 60–79% | < 60% | N/A | Cannot tell | ||||
| ✓ | |||||||||
| Summary of selection bias | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| B. Study design | |||||||||
| 1. What was the study design? | RCT | ✓ | |||||||
| (Please tick appropriate and specify design if categorise as ‘Other’) | CCT | ||||||||
| Cohort analytic (two group pre + post) | |||||||||
| Case–control | |||||||||
| Cohort [one group pre + post (before and after)] | |||||||||
| Interrupted time series | |||||||||
| Other – specify | |||||||||
| Cannot Tell | |||||||||
| 2. Was the study described as randomised? | Yes | No | |||||||
| ✓ | |||||||||
| If answer to no. 2 is ‘no’ complete summary then go to section C. Confounders. If answer is ‘yes’, answer no. 3 and no. 4 below, before completing summary for this section | |||||||||
| 3. If answer was yes, was the method of randomisation described? | Yes | No | |||||||
| ✓ | |||||||||
| 4. If answer was yes, was the method appropriate? | Yes | No | |||||||
| ✓ | |||||||||
| Summary of study design | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| C. Confounders | |||||||||
| 1. Were there important differences between groups prior to the intervention? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. If yes, indicate the percentage of relevant confounders that were controlled [either in the design (e.g. by stratification or matching) or in the analysis]? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| Summary of confounders | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| D. Blinding | |||||||||
| 1. Was the outcome assessor aware of the intervention or exposure status of participants? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were the study participants aware of the research question? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| Summary of blinding | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| E. Data collection methods | |||||||||
| 1. Were data collection tools shown to be valid? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were data collection tools shown to be reliable? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| Summary of data collection | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| F. Withdrawals and dropouts | |||||||||
| 1. Were withdrawals and dropouts reported in terms of numbers and reasons per group? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Indicate the percentage of participants completing the study (If the percentage differs by groups, record the lowest) | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
| Summary of withdrawals and dropouts | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| G. Intervention integrity | |||||||||
| 1. What percentage of participants received the allocated intervention or exposure of interest? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
| 2. Was the consistency of the intervention measured? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 3. Is it likely that subjects received an unintended intervention (contamination or co-intervention) that may influence the results? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| H. Analysis | |||||||||
| 1. Indicate the unit of allocation | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 2. Indicate the unit of analysis | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 3. Are the statistical methods appropriate for the study design? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 4. Is the analysis performed by intervention allocation status (i.e. ITT) rather than actual intervention received? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| Global rating for studya | Strong | Moderate | Weak | ||||||
| (Overall methodological strength of study – based on sections A–F) | ✓ | ||||||||
Alam et al. 200350
Data extraction table
| Reference and design | Intervention | Participants | Outcome measures | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Author: Alam et al. 50 Year: 2003 Country: Bangladesh Study design: double-blind RCT Setting: inpatient [Clinical Research and Service Centre of International Centre for Diarrhoea, Disease Research (ICDDR), Bangladesh: Centre for Health and Population Research] Number of centres: one Funding: grant from WHO (no. C6/181/377) |
Intervention: oral ReSoMaL (see end of table for details) Control: standard WHO-ORS (see end of table for details) Fluid deficit was corrected with 10 ml/kg/hour of the assigned ORS given over the first 2 hours, followed by 5 ml/kg/hour over a period of 10–12 hours until the deficit was corrected (dehydration was categorised according to the modified WHO guidelines). Ongoing stool losses were corrected with 5–10 ml/kg after each watery or loose stool. In patients with high purging rates, fluid intake was adjusted according to the ongoing stool output. ORS therapy was continued until diarrhoea ceased Other interventions used: pneumonia cases received i.m. or i.v. ceftriaxone 75 mg/kg/day once daily for 5 days and gentamicin 5 mg/kg/day in two divided doses. Other infections, complications, nutritional therapy or aspects of case management were provided consistent with the WHO guidelines All children were treated following the protocol of the WHO manual for the standardised treatment of SAM children and received acute and rehabilitation phase treatment until discharged. Children remained in the study until diarrhoea resolved, with subsequent transfer to a nutritional rehabilitation unit or home-based nutritional follow-up programme of the Clinical Research and Service Centre |
Definition of SAM: W/L < 70% of the NCHS median or with bilateral pedal oedema Number of participants: n = 130 (ReSoMaL n = 65; WHO-ORS n = 65) Sample attrition/dropout: n = 12. ReSoMaL: n = 7 (three severe dehydration requiring i.v.’s, one symptomatic hypokalaemia, one severe hyperkalaemia; one severe pneumonia and one symptomatic hyponatraemia with seizure). WHO-ORS: n = 5 (one symptomatic hypokalaemia, one severe dehydration, one severe pneumonia and two parental withdrawal) Sample crossovers: none reported Children requiring i.v. fluid therapy for severe dehydration, septic shock or convulsion, children with concomitant illness requiring more intensive care, cases with severe hyperkalaemia (serum potassium ≥ 6.0 mmol/l), cases with severe hypokalaemia (serum potassium ≤ 1.5 mmol/l with or without symptoms or < 2.5 mmol/l with symptoms) and cases with severe hyponatraemia (serum sodium < 120 mmol/l with symptoms or < 115 mmol/l with or without symptoms) were withdrawn from the study Inclusion criteria:Exclusion criteria:General characteristics of participants: children aged 6–26 months with history of watery diarrhoea, and with or without cholera |
Primary outcomes: number of children developing over-hydration and number of children with correction of basal hypokalaemia after 24 and 48 hours of treatment Secondary outcome: number of children remaining hyponatraemic at 24 and 48 hours of treatment Method of assessing outcomes:If clinically indicated, urine for microscopy and culture and chest radiographAdverse symptoms: hyponatraemia Length of follow-up: none reported, but states all children remained in the study until diarrhoea resolved Recruitment dates: February 1998 to January 2000 |
|||||||||||||
| Characteristics of participants | ||||||||||||||||
| Characteristic | ReSoMaL (n = 65) | WHO-ORS (n = 65) | p-value | |||||||||||||
| Mean age, months (SD) | 15 (7) | 15 (6) | NR | |||||||||||||
| Sex, n (M : F) | 39 : 26 | 42 : 23 | NR | |||||||||||||
| Mean body weight, kg (SD) | 5.22 (0.92) | 5.26 (0.95) | NR | |||||||||||||
| Mean W/A % of NCHS median (SD) | 50 (7) | 51 (7) | NR | |||||||||||||
| Mean WAZ (SD) | –4.7 (1) | –4.6 (0.7) | NR | |||||||||||||
| Mean W/L % of NCHS median (SD) | 66 (4) | 66 (3) | NR | |||||||||||||
| Mean WLZ (SD) | –3.6 (0.6) | –3.5 (0.5) | NR | |||||||||||||
| Breastfed, n (yes : no) | 45 : 21 | 47 : 17 | NR | |||||||||||||
| Mean duration of diarrhoea before admission, hours (SD) | 77 (62) | 74 (59) | NR | |||||||||||||
| Mean number of stools in 24 hours before admission (SD) | 12.5 (5) | 14 (9) | NR | |||||||||||||
| Dehydration status, n (none : some) | 21 : 45 | 23 : 42 | NR | |||||||||||||
| Oedema present, n (%) | 15/65 (23) | 14/65 (22) | NR | |||||||||||||
| Stool pathogen, n (%) | NR | |||||||||||||||
| Vibrio cholerae | 18/65 (28) | 19/65 (29) | ||||||||||||||
| Shigella | 5/65 (8) | 2/65 (3) | ||||||||||||||
| Salmonella | 2/65 (3) | 0/65 | ||||||||||||||
| Other Vibrio | 3/65 (5) | 5/65 (8) | ||||||||||||||
| Rotavirus | 10/65 (15) | 12/65 (18) | ||||||||||||||
| Results | ||||||||||||||||
| Primary outcomes | ReSoMaL (n = 65) | WHO-ORS (n = 65) | p-value; OR (95% CI) | |||||||||||||
| Children adequately rehydrated at 12 hours, n/N (%) | 45/59 (76) | 51/63 (81) | p = 0.68; OR 0.16 (95% CI 0.29 to 1.96) | |||||||||||||
| Overhydration, n/N (%) | 3/65 (5) | 8/65 (12) | p = 0.20; OR 0.3 (95% CI 0.1 to 1.5) | |||||||||||||
| Basal hypokalaemia (potassium < 3.5 mmol/l), n/N (%) | 39/65 (60) | 44/65 (68) | p = 0.47; OR 0.7 (95% CI 0.3 to 1.6) | |||||||||||||
| Hypokalaemia corrected at 24 hours, n/N (%) | 14/38 (36) | 2/44 (5) | p = 0.0006; OR 12.3 (95% CI 2.4 to 117) | |||||||||||||
| Hypokalaemia corrected at 48 hours, n/N (%) | 18/38 (47) | 7/44 (16) | p = 0.004; OR 1.5 (95% CI 1.5 to 15.6) | |||||||||||||
| Secondary outcomes | ReSoMaL (n = 65) | WHO-ORS (n = 65) | p-value; OR (95% CI) | |||||||||||||
| Mean serum potassium, mmol/l (SD) | ||||||||||||||||
| 0 hours | 3.03 (1) | 3.3 (1) | p = 0.7; OR < 0.08 (–0.3 to 0.4)a | |||||||||||||
| 24 hours | 4.0 (1) | 3.2 (0.7) | p = 0.01; OR (0.49 to 1.1)a,b | |||||||||||||
| 48 hours | 4.6 (0.8) | 3.4 (0.8) | p = 0.01; OR 1.2 (0.3 to 1.0)a | |||||||||||||
| Hyponatraemia (serum sodium < 130 mmol/l), n/N (%) | ||||||||||||||||
| 0 hours | 25/65 (38) | 19/65 (29) | p = 0.35; OR 1.5 (0.7 to 3.4) | |||||||||||||
| 24 hours | 24/62 (39) | 15/64 (23) | p = 0.9; OR 2.1 (0.9 to 4.8) | |||||||||||||
| 48 hours | 17/59 (29) | 6/60 (10) | p = 0.017; OR 3.6 (1.2 to 12.2) | |||||||||||||
| Severe hyponatraemia (serum sodium ≤ 120 mmol/l), n/N (%) | ||||||||||||||||
| 0 hours | 0/65 | 1/65 (2) | p = 1.0; OR 0 (0 to 39) | |||||||||||||
| 24 hours | 3/62 (5) | 1/64 (2) | p = 0.36; OR 3.2 (0.3 to 171) | |||||||||||||
| 48 hours | 0 | 0 | ||||||||||||||
| Mean serum sodium, mmol/l (SD) | ||||||||||||||||
| 0 hours | 132.1 (6) | 132.9 (8) | p = 0.51; OR –0.8 (–3.3 to 1.7)a | |||||||||||||
| 24 hours | 130.5 (6) | 133.3 (6) | p = 0.01; OR–2.8 (–4.9 to 0.7)a | |||||||||||||
| 48 hours | 132.1 (4) | 134.5 (4) | p = 0.001; OR–2.4 (–3.9 to–1.0)a | |||||||||||||
| Comments: three new cases of severe hyponatraemia developed in the ReSoMaL group. Although not explicitly stated, presumably no new case developed in the WHO-ORS group. Stool output, urine output, ORS intake, water intake, calorie intake from supplemented food and duration of diarrhoea and weight gain before discharge reported similar between groups, but no data shown | ||||||||||||||||
| Other: hyponatraemia (serum < 130 mmol) | Non-cholera diarrhoea | Cholera diarrhoea | ||||||||||||||
| ReSoMaL (n = 47) | Standard (n = 46) | p-value; OR (95% CI) | ReSoMaL (n = 18) | Standard n = 19 | p-value: OR (95% CI) | |||||||||||
| 0 hours, n/N (%) | 13/47 (28) | 11/46 (24) | NS; OR 1.2 (0.4 to 3.5) | 12/18 (67) | 9/19 (47) | NS; OR 2.2 (0.5 to 10) | ||||||||||
| 24 hours, n/N (%) | 11/47 (23) | 7/46 (15) | NS; OR 1.7 (0.5 to 5.8) | 13/18 (72) | 8/19 (42) | NS; OR 3.6 (0.8 to 18) | ||||||||||
| 48 hours, n/N (%) | 7/47 (15) | 4/46 (9) | NS; OR 1.84 (0.4 to 8.2) | 10/11 (56) | 2/19 (11) | NS; OR 10.63 (1.6 to 92.1) | ||||||||||
|
Safety: the child in the ReSoMal group who was withdrawn owing to hyponatraemia with associated seizure was reported as having had a high purging rate (18 g/kg/hour) during the first 24-hour period Convulsions: n = 1 ReSoMaL (case did not have cholera). The study authors believed that the occurrence of the convulsion in the ReSoMal group should limit the use of ReSoMal in its current formulation in severely malnourished children with diarrhoea Death: n = 0 HIV: reported |
||||||||||||||||
| Barriers to implementation | ||||||||||||||||
| NR | ||||||||||||||||
| Methodological comments | ||||||||||||||||
|
Allocation to treatment groups: cases were allocated using serially numbered, sealed envelopes supplied to the pharmacist of ICDDR Blinding: states double-blind controlled study, with children assigned on enrolment thorough randomisation list (prepared by the WHO) following a permuted table of variable length. Pharmacist prepared the ORS in a clean bottle marked only with the child’s name and study number according to list inside the serially numbered envelopes. The ORS solutions were reported to look identical and a code in the form of A and B was provided to the investigators for analysis. The group identity was disclosed for preparation of the final report, after preparation of data analysis tables Comparability of treatment groups: states that baseline clinical characteristics such as age, body weight, W/A, W/L, breastfeeding status, oedematous state and dehydration status were comparable between the groups (p-values NR) Method of data analysis: Student’s t-test for comparison between groups of continuous variables for non-continuous variables; the chi-squared test/Fisher’s exact test, using SPSS/PC+. For withdrawals, data collected until the time of withdrawal were included in the analysis Sample size/power calculation: based on an expected reduction of persistence of hypokalaemia from 33% with standard WHO-ORS to 12% with ReSoMaL. Sample size was calculated to be 65 in each group (5% level of significance, 80% power and 10% dropout). Authors state that no reliable data exist on the development of overhydration quantified objectively. A sample size of 52 in each group was estimated, assuming a 20% difference in the development of overhydration between the groups (25% of WHO-ORS group and 5% of ReSoMaL group considered to develop overhydration, with a 5% level of significance, 80% power and 10% dropout). A subgroup analysis for hyponatraemia excluded children with cholera and is unlikely to be powered Attrition/dropout: numbers and reasons reported. Children withdrawn from the study were followed and final outcome recorded |
||||||||||||||||
| General comments | ||||||||||||||||
|
Generalisability: SAM defined using criteria of < 70% of the NCHS median W/L, which is in agreement with the WHO criteria for SAM Outcome measures: appear to be suitable and appropriate. Outcomes are defined where necessary Intercentre variability: not applicable, one centre only Conflict of interest: NR, but staff from WHO reviewed protocol and supplied the ReSoMaL |
||||||||||||||||
| Details of intervention and control | ||||||||||||||||
| Composition of ReSoMaL and standard ORS | ||||||||||||||||
| ReSoMaL | Standard ORS | |||||||||||||||
| Concentration of, mmol/l | ||||||||||||||||
| Sodium | 45 | 90 | ||||||||||||||
| Potassium | 40 | 20 | ||||||||||||||
| Chloride | 76 | 80 | ||||||||||||||
| Citrate | 7 | 10 | ||||||||||||||
| Glucose | 125 | 111 | ||||||||||||||
| Magnesium | 6 | |||||||||||||||
| Concentration of, μmol/l | ||||||||||||||||
| Zinc | 300 | |||||||||||||||
| Copper | 45 | |||||||||||||||
| Osmolarity, mosmol/l | 300 | 311 | ||||||||||||||
Quality assessment for primary studies (modified for severe malnutrition)
| A. Selection bias | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1. Are the individuals selected to participate in the study likely to be representative of the target population? | Very likely | Somewhat likely | Not likely | Cannot tell | |||||
| ✓ | |||||||||
| 2. What percentage of selected individuals participated? | 80–100% | 60–79% | < 60% | N/A | Cannot tell | ||||
| ✓ | |||||||||
| Summary of selection bias | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| B. Study design | |||||||||
| 1. What was the study design? | RCT | ✓ | |||||||
| (Please tick appropriate and specify design if categorise as ‘Other’) | CCT | ||||||||
| Cohort analytic (two group pre + post) | |||||||||
| Case–control | |||||||||
| Cohort [one group pre + post (before and after)] | |||||||||
| Interrupted time series | |||||||||
| Other – specify | |||||||||
| Cannot Tell | |||||||||
| 2. Was the study described as randomised? | Yes | No | |||||||
| ✓ | |||||||||
| If answer to no. 2 is ‘no’ complete summary then go to section C. Confounders. If answer is ‘yes’, answer no. 3 and no. 4 below, before completing summary for this section | |||||||||
| 3. If answer was yes, was the method of randomisation described? | Yes | No | |||||||
| ✓ | |||||||||
| 4. If answer was yes, was the method appropriate? | Yes | No | |||||||
| ✓ | |||||||||
| Summary of study design | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| C. Confounders | |||||||||
| 1. Were there important differences between groups prior to the intervention? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. If yes, indicate the percentage of relevant confounders that were controlled [either in the design (e.g. by stratification or matching) or in the analysis]? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| Summary of confounders | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| D. Blinding | |||||||||
| 1. Was the outcome assessor aware of the intervention or exposure status of participants? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were the study participants aware of the research question? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| Summary of blinding | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| E. Data collection methods | |||||||||
| 1. Were data collection tools shown to be valid? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were data collection tools shown to be reliable? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| Summary of data collection | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| F. Withdrawals and dropouts | |||||||||
| 1. Were withdrawals and dropouts reported in terms of numbers and reasons per group? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Indicate the percentage of participants completing the study (If the percentage differs by groups, record the lowest) | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
| Summary of withdrawals and dropouts | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| G. Intervention integrity | |||||||||
| 1. What percentage of participants received the allocated intervention or exposure of interest? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
| 2. Was the consistency of the intervention measured? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 3. Is it likely that subjects received an unintended intervention (contamination or co-intervention) that may influence the results? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| H. Analysis | |||||||||
| 1. Indicate the unit of allocation | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 2. Indicate the unit of analysis | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 3. Are the statistical methods appropriate for the study design? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 4. Is the analysis performed by intervention allocation status (i.e. ITT) rather than actual intervention received? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| Global rating for studya | Strong | Moderate | Weak | ||||||
| (Overall methodological strength of study – based on sections A–F) | ✓ | ||||||||
Alam et al. 200957
Data extraction table
| Reference and design | Intervention | Participants | Outcome measures | ||
|---|---|---|---|---|---|
|
Author: Alam et al. 57 Year: 2009 Country: Bangladesh Study design: RCT Setting: inpatient (Dhaka hospital of the ICDDR followed by nutrition rehabilitation unit) Number of centres: one Funding: Nestlé Foundation and ICDDR, Bangladesh |
Intervention 1: glucose-ORS Intervention 2: glucose-ORS + ARS Intervention 3: rice-ORS ORS had the same salt composition, but different substrates (see table at end for further details) Children with some dehydration were randomised to receive the assigned ORS within 1 hour, and those with severe dehydration within 6 hours of admission after i.v. rehydration. ORS given on hospital ward and continued until cessation of diarrhoea (acute phase) Other interventions used: i.v rehydration of severe dehydration, antibiotics where appropriate, erythromycin for cholera, vitamin A, folic acid and other multivitamin supplements, glucose solution for hypoglycaemic children, breastfeeding continued ad libitum, supplementary feeding with F100 diet, semi-solid food for older children. Further details at end of paper |
Definition of SAM: W/L < 70% of NCHS median or with bipedal oedema Number of participants: 316 screened, 175 randomised (glucose-ORS n = 58, glucose-ORS + ARS n = 59, rice-ORS n = 58) Sample attrition/dropout: 170 (97%) completed acute phase (five withdrew consent: one glucose-ORS, three glucose-ORS + ARS, one rice-ORS). 137 (78%) completed convalescent phase (42 glucose-ORS, 50 glucose-ORS + ARS, 45 rice-ORS) – reasons not given for convalescent dropouts Sample crossovers: none reported Inclusion criteria: SAM children of either sex, aged 6–60 months, acute watery diarrhoea < 48 hours duration and stool dark-field microscopy demonstrating presence of cholera. Those with hypoglycaemia, hypothermia, hyponatraemia, dehydration and other associated-infections were also eligible Exclusion criteria: dysentery (blood in stool), severe infections (severe pneumonia, clinical sepsis, meningitis) General characteristics of participants: SAM children aged 6–60 months with acute watery diarrhoea and cholera |
Primary outcomes:Secondary outcomes:Method of assessing outcomes: study eligibility confirmed by physical examination, blood, stool and urine samples ‘Some dehydration’ defined as presence of ≥ 2 signs or symptoms (irritable/less active,* sunken eyes, dry mucosa, thirst, reduced skin turgor*) with at least one sign marked by* ‘Severe dehydration’ defined as the presence of signs of ‘some dehydration’ plus at least one key sign (lethargy/coma,* inability to drink, but not refusal to drink,* uncountable/absent radial pulse*) Therapeutic failure defined as continuation of diarrhoea beyond seventh day of randomisation Unscheduled i.v. therapy defined as requirement of i.v. fluid any time after randomisation owing to appearance of signs of severe dehydration, excessive vomiting preventing adequate ORS intake or dehydration signs lasting > 6 hours Hypokalaemia = serum potassium < 3.5 mmol/l; severe hypokalaemia = serum potassium < 1.5 mmol/l; hyperkalaemia = serum potassium > 6.0 mmol/l; hyponatraemia = serum sodium < 130 mmol/l; severe hyponatraemia = serum sodium 115 mmol/l; hypernatraemia = serum sodium > 150 mmol/l Acute illness = diarrhoea phase; convalescent phase = after resolution of diarrhoea and until oedema-free W/L of 80% attained Children weighed on admission and placed on a cholera cot. Paediatric urine collector used to collect stools and urine separately. Body weight and weight of stools and supplemented foods weighed on an electronic scale (Sartorius) with gram precision. All intakes (ORS, water, i.v. fluids and foods) and outputs (stool, urine and vomit) measured for each 6-hour period in acute phase. Vital signs and dehydration and signs of overhydration monitored every 6 hours Duration of diarrhoea calculated from time of randomisation to last watery stool Adverse symptoms: none reported Length of follow-up: ORS continued until cessation of diarrhoea (the last watery stool is followed by ≥ 2 soft/formed stools or no stool for 12 hours). After discharge, children followed-up at home weekly for at least 6 weeks (these data are not presented in this paper) Standard treatment lasted through a convalescent phase until 80% W/L reached Recruitment dates: July 2001 to December 2004 |
||
| Characteristics of participants | |||||
| Characteristic | Glucose-ORS (n = 58) | Glucose-ORS + ARS (n = 59) | Rice-ORS (n = 58) | p-value | |
| Age, months | 27.17 ± 12.36 | 28.36 ± 13.42 | 27.33 ± 11.97 | 0.858 | |
| Sex M : F | 26 : 32 | 34 : 25 | 32 : 26 | 0.357 | |
| Weight, kg | 6.90 ± 1.32 | 7.09 ± 1.52 | 6.78 ± 1.43 | 0.513 | |
| Length, cm | 76.84 ± 7.11 | 77.34 ± 8.31 | 76.54 ± 8.15 | NR | |
| W/A (% of NCHS median) | 54.51 ± 9.50 | 53.42 ± 6.86 | 53.16 ± 7.94 | 0.645 | |
| W/L (% of NCHS median) | 68.99 ± 4.92 | 69.01 ± 5.27 | 67.54 ± 6.19 | 0.257 | |
| WAZ | –4.38 ± 68a | –4.31 ± 0.63 | –4.39 ± 0.71 | 0.793 | |
| WLZ | –3.14 ± 1.88 | –2.76 ± 46a | –3.38 ± 0.60 | 0.185 | |
| MUAC, mm | 112.7 ± 9.9 | 113.6 ± 9.7 | 111.9 ± 10.8 | 0.678 | |
| MUAC with < 110 mm, n (%) | 19 (33) | 18 (31) | 23 (39) | 0.70 | |
| Diarrhoea duration before admission, hours | 12.59 ± 8.27 | 13.07 ± 9.11 | 10.98 ± 5.73 | 0.326 | |
| Stools in last 24 hours before admission, n | 14.36 ± 6.00 | 14.02 ± 6.09 | 14.55 ± 7.16 | 0.901 | |
| Vomiting duration before admission, hours | 11.29 ± 8.01 | 11.31 ± 8.28 | 10.16 ± 4.7 | 0.613 | |
| Vomiting in last 24 hours, n | 10.12 ± 6.93 | 11.83 ± 8.03b | 12.28 ± 7.67c | 0.271 | |
| Breastfed at illness onset, n (%) | 32 (55) | 18 (31) | 29 (50) | 0.018 | |
| Severe dehydration at admission, n (%) | 48 (84) | 49 (83) | 49 (84) | 0.971 | |
| Pedal oedema, n (%) | 47 (81) | 48 (81) | 40 (69) | 0.193 | |
| Hypothermia, n (%)d | 12 (20) | 7 (12) | 11 (19) | 0.405 | |
| Have received i.v. fluids, n (%) | 50 (86) | 50 (86) | 49 (84) | 0.961 | |
| Hyponatraemia, n (%) | 10 (17) | 14 (24) | 14 (24) | 0.599 | |
| Hypokalaemia, n (%) | 23 (40) | 14 (24) | 20 (34) | 0.170 | |
| Hypoglycaemia, n (%) | 2 (4) | 4 (7) | 8 (14) | 0.161 | |
|
Comments: data are mean ±SD unless stated otherwise Baseline characteristics were comparable between the three groups, except for breastfeeding; paper reports this was less frequent in glucose-ORS group, but data indicate lower frequency in the glucose-ORS + ARS group. 147/175 (84%) were clinically assessed to have severe dehydration in agreement with their mean weight gain of 11.4% (95% CI 10.4 to 12.5) at resolution of diarrhoea. Approximately one-third were acutely malnourished as indicated by MUAC < 110 mm; other risks of death included pedal oedema (77%) and hypothermia (17%). Hypernatraemia or severe hyponatraemia was not observed in any child. The paper reports other baseline characteristics including sociodemographic characteristics, serum concentrations of electrolytes and Hb, but these have not been extracted |
|||||
| Results | |||||
| Primary outcomes | Glucose-ORS (n = 58) | Glucose-ORS + ARS (n = 59) | Rice-ORS (n = 58) | p-value (95% CI) | |
| Stool output, ml/kg | |||||
| At 24 hours | 355 | 309 | 236 | 0.004, difference 109 (44 to 174), 32% reductione | |
| At 48 hours | 600 | 518 | 382 | 0.007, difference 213 (79 to 346), 37% reductione | |
| At 72 hours | 735 | 645 | 475 | 0.018, difference 242 (73 to 412), 36% reductione | |
| Comments: Data are mean per cent of initial body weight. The 72-hour results (and 24- and 48-hours results for stool output) reported here for individual study groups are estimated by reviewer from bar charts. SE presented, but not data extracted. The paper presents results for every 6-hourly period up to 72 hours, but these have not been data extracted. Statistical difference was entirely contributed by the rice-ORS group. The trend towards reduction of stool output in glucose-ORS + ARS group vs glucose-ORS group was NS | |||||
| Secondary outcomes | Glucose ORS (n = 58) | Glucose-ORS + ARS (n = 59) | Rice-ORS (n = 58) | p-value | |
| Weight gain at 72 hours, % initial weight | 11 | 9.7 | 13 | 0.05 | |
| Median diarrhoea duration, hours (95% CI) | 72 (62 to 82) | 60 (50 to 70) | 54 (44 to 54) | 0.530 | |
| Days to attain 80% of median W/L, mean ± SD | 7.14 ± 2.26 | 7.12 ± 2.2 | 7.2 ± 3.78 | 0.99 | |
| Vomit output at 72 hours, ml/kg | 30 | 37 | 33 | NR/NS | |
| Urine output at 72 hours, ml/kg | 184 | 186 | 177 | NR/NS | |
| ORS intake at 72 hours, ml/kg | 710 | 620 | 450 | 0.012, 38% reductionf | |
| Water intake at 72 hours, ml/kg | 215 | 230 | 260 | 0.03g | |
| Milk formula intake at 72 hours, ml/kg | 329 | 333 | 346 | NR/NS | |
| Required unscheduled i.v. therapy, n (%) | 10/56 (18) | 11/59 (19) | 6/57 (11) | 0.858 | |
| Therapeutic failure, n (%) | 2 (3.6) | 1 (1.8) | 2 (3.6) | 0.785 | |
| Deaths, n | 0 | 0 | 0 | NR | |
| Comments: outcomes reporting results at 72 hours are estimated by reviewer from bar charts. The paper presents results for every 6-hourly period up to 72 hours, but these have not been data extracted. Significant differences in weight gain, ORS intake and water intake were entirely accounted for by the rice-ORS group, according to the least significant difference post hoc analysis. Overall mean weight gain at 72 hours was 114 g/kg (95% CI 103 to 124 g/kg). Overall mean duration of diarrhoea was 66 hours (95% CI 62 to 71 hours) with an overall median duration of 60 hours (95% CI 54 to 66 hours). Diarrhoea duration compared by log-rank test, df = 2, log-rank = 1.27. A survival plot for recovery from diarrhoea after inclusion was also presented for 167 children, but has not been data extracted. No statistical difference was observed between groups (log-rank = 1.27, df = 2, statistical value = 0.53) | |||||
| Safety: during the acute phase of treatment, no children developed features of overhydration, cardiac failure, hypoglycaemia, severe hypo- or hyperkalaemia or severe hypo- or hypernatraemia | |||||
| HIV: none reported | |||||
| Barriers to implementation | |||||
| None reported | |||||
| Methodological comments | |||||
|
Allocation to treatment groups: randomisation using consecutive sealed envelopes. A statistician not involved in the study prepared the randomisation list and sequentially numbered sealed envelopes containing a slip of paper identifying the allocated ORS. The list was retained by the hospital pharmacist who prepared the ORS in bottles marked with the patient’s name and study number Blinding: states that treatment could not be blinded to the people involved in the study (assume this refers to patients and care providers alike) because of visible differences in the ORS solutions. No details regarding blinding of outcome assessors Comparability of treatment groups: baseline characteristics were comparable between the three groups (p-values reported), except for breastfeeding – paper reports this was less frequent in glucose-ORS group, but data indicate lower frequency in the glucose-ORS + ARS group Method of data analysis: not ITT analysis. The five children withdrawn from the study by their parents were not included in the analysis. States that the baseline characteristics of these children did not differ from the remainder of the included children. Fewer children were analysed at the end of the convalescent phase than the acute phase. Baseline characteristics and outcomes were compared using one-way ANOVA followed by a post hoc least significant difference test, and a non-parametric Kruskal–Wallis test was used for the continuous variables. Chi-squared test used for comparison of categorical variables and Fisher’s exact test was applied when appropriate. Kaplan–Meier survival analysis was performed for comparing diarrhoea duration Sample size/power calculation: Authors state that sample size was not calculated to detect a significant difference in death rates owing to ethical and statistical reasons (requirement of huge sample size). Instead, sample size was calculated to detect a 30% reduction in stool output in first 24 hours of treatment with either glucose-ORS + ARS or rice-ORS. This level of stool output reduction was based on results of an unpublished pilot study in similar children having mean stool weight (± SD) of 158 g (± 95) and of published data in adults. This required a sample size of 63 per group for a two-sided alpha-level of < 0.05 and a beta-level of 0.2 Attrition/dropout: numbers and reasons reported for acute phase, but only numbers given for convalescent phase. 170 (97%) completed acute phase (five withdrew: one glucose-ORS, three glucose-ORS + ARS and one rice-ORS). 137 (78%) completed convalescent phase (42 glucose-ORS, 50 glucose-ORS + ARS, and 45 rice-ORS) |
|||||
| General comments | |||||
|
Generalisability: likely that most of the children would meet the current WHO criteria (W/L < 70%, W/L z-score < –3 SD) given a mean of 68% and –3.09, respectively. Age ranged from 6 to 60 months but mean age 27 months, therefore, it is likely to be representative of infants and toddlers. All had cholera and some had comorbidities (e.g. electrolyte disturbances) Outcome measures: outcomes were appropriate Intercentre variability: N/A Conflict of interest: funded by Nestlé Foundation and ICDDR. No conflicts of interest reported |
|||||
| Standard management | |||||
After randomisation, all children were treated as per the standard ICDDRB protocol for management of severely malnourished children
|
|||||
| Rehydration | |||||
|
|||||
| Composition of ORS (differed only in glucose, ARS and rice composition) | |||||
|
|||||
| Ingredient | Glucose-ORS | Glucose ORS + ARS | Rice-ORS | ||
| Glucose, mmol/l | 90 | 90 | 0 | ||
| Rice powder, g/l | 0 | 0 | 50 | ||
| Amylase-resistant starch, g/l | 0 | 50 | 0 | ||
| Sodium, mmol/l | 75 | 75 | 75 | ||
| Potassium, mmol/l | 40 | 40 | 40 | ||
| Chloride, mmol/l | 87 | 87 | 87 | ||
| Citrate, mmol/l | 10 | 10 | 10 | ||
| Magnesium, mmol/l | 3 | 3 | 3 | ||
| Zinc, µmol/l | 300 | 300 | 300 | ||
| Copper, µmol/l | 45 | 45 | 45 | ||
| Calculated osmolarity, mosmol/l | 305 | 305 | 215 | ||
Quality assessment for primary studies (modified for severe malnutrition)
| A. Selection bias | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1. Are the individuals selected to participate in the study likely to be representative of the target population? | Very likely | Somewhat likely | Not likely | Cannot tell | |||||
| ✓ | |||||||||
| 2. What percentage of selected individuals participated? | 80–100% | 60–79% | < 60% | N/A | Cannot tell | ||||
| ✓ of those randomised | |||||||||
| Summary of selection bias | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| B. Study design | |||||||||
| 1. What was the study design? | RCT | ✓ | |||||||
| (Please tick appropriate and specify design if categorise as ‘Other’) | CCT | ||||||||
| Cohort analytic (two group pre + post) | |||||||||
| Case–control | |||||||||
| Cohort [one group pre + post (before and after)] | |||||||||
| Interrupted time series | |||||||||
| Other – specify | |||||||||
| Cannot Tell | |||||||||
| 2. Was the study described as randomised? | Yes | No | |||||||
| ✓ | |||||||||
| If answer to no. 2 is ‘no’ complete summary then go to section C. Confounders. If answer is ‘yes’, answer no. 3 and no. 4 below, before completing summary for this section | |||||||||
| 3. If answer was yes, was the method of randomisation described? | Yes | No | |||||||
| ✓ | |||||||||
| 4. If answer was yes, was the method appropriate? | Yes | No | |||||||
| ✓ | |||||||||
| Summary of study design | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| C. Confounders | |||||||||
| 1. Were there important differences between groups prior to the intervention? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. If yes, indicate the percentage of relevant confounders that were controlled [either in the design (e.g. by stratification or matching) or in the analysis]? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| Summary of confounders | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| D. Blinding | |||||||||
| 1. Was the outcome assessor aware of the intervention or exposure status of participants? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were the study participants aware of the research question? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| Summary of blinding | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| E. Data collection methods | |||||||||
| 1. Were data collection tools shown to be valid? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were data collection tools shown to be reliable? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| Summary of data collection | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| F. Withdrawals and dropouts | |||||||||
| 1. Were withdrawals and dropouts reported in terms of numbers and reasons per group? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Indicate the percentage of participants completing the study (If the percentage differs by groups, record the lowest) | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
| Summary of withdrawals and dropouts | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| G. Intervention integrity | |||||||||
| 1. What percentage of participants received the allocated intervention or exposure of interest? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
| 2. Was the consistency of the intervention measured? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 3. Is it likely that subjects received an unintended intervention (contamination or co-intervention) that may influence the results? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| H. Analysis | |||||||||
| 1. Indicate the unit of allocation | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 2. Indicate the unit of analysis | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 3. Are the statistical methods appropriate for the study design? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 4. Is the analysis performed by intervention allocation status (i.e. ITT) rather than actual intervention received? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| Global rating for studya | Strong | Moderate | Weak | ||||||
| (Overall methodological strength of study – based on sections A–F) | ✓ | ||||||||
Dutta et al. 200055
Data extraction table
| Reference and design | Intervention | Participants | Outcome measures | |
|---|---|---|---|---|
|
Author: Dutta et al. 55 Year: 2000 Country: India Study design: double-blind, RCT Setting: inpatient (hospital) + community after discharge and until follow-up Number of centres: one Funding: not stated |
Intervention: zinc-supplemented syrup (177 mg/day in three divided doses, 40 mg elemental zinc/day) Control: placebo syrup Other interventions used: all children received standard ORS initially plus standard feeding regimen (see end of table for details) |
Definition of SAM: not specifically stated. Uses the IAP W/A classification system, though results are reported for all children (not separately by grade). Mean baseline MUAC is < 11 cm Number of participants: n = 80 (zinc: n = 44, control n = 36) Sample attrition/dropout: unclear (see Methodological comments on page 177) Sample crossovers: none Inclusion criteria: male children, aged 3–24 months, < 80% Harvard standard W/A, history of watery diarrhoea (more than four times within previous 24 hours) for ≤ 72 hours and clinical signs and symptoms of ‘some’ dehydration (e.g. sunken eyes, reduced skin elasticity, rapid pulse, dry mouth and thirst) Exclusion criteria: history of treatment with antibiotics, other systemic infections (e.g. septicaemia, meningitis, pneumonia, urinary tract infection, otitis media), chronic underlying diseases (TB, liver diseases), need for intensive care (i.e. life-support system, blood transfusion or total parenteral nutrition), exclusively breastfed General characteristics of participants: malnourished male children, aged 3–24 months, with acute dehydrating diarrhoea; majority have SAM (grade III or IV) |
Primary outcomes: not specifically stated as primary, but appear to be:Recovery defined as passage of normal stool or no stool for last 18 hours Secondary outcomes:Method of assessing outcomes: weighed unclothed at same time every day using scales with a sensitivity of 20 g; nutritional status assessed using IAP classification; degree of dehydration assessed by the WHO criteria; stool samples collected in sterile MacCartney’s bottles for detection of enteropathogens using ‘standard methods’92 Stool losses measured on pre-weighed disposable diapers; urine separated from stools using urine collection bags; vomitus weighed on pre-weighed gauze pads. All intake and output measured and recorded every 8 hours until diarrhoea stopped, withdrawal from study, or up to day 5 if child did not fulfil criteria of recovery Adverse symptoms: NR Length of follow-up: treatment until diarrhoea ceased or up to day 5. Additional follow-up up to 30 days (including up to 5 days hospitalisation) Recruitment dates: June 1997 to May 1998 |
|
| Characteristics of participants | ||||
| Characteristic | Zinc syrup (n = 44) | Placebo syrup (n = 36) | p-value | |
| Mean age ± SD, months | 10.4 ± 5.4 | 11.0 ± 4.9 | ||
| Mean body weight ± SD, kg | 5.5 ± 1.6 | 5.8 ± 1.5 | ||
| Mean height ± SD, cm | 65.5 ± 8.4 | 67.5 ± 6.9 | ||
| Mean MUAC ± SD, cm | 10.3 ± 1.3 | 10.5 ± 1.0 | ||
| Nutritional status, n W/A (%) | ||||
| Grade I ≥ 80% of median | – | – | ||
| Grade II 70% < 80% of median | 6 (13) | 6 (17) | ||
| Grade III 60% < 70% of median | 10 (23) | 11 (30) | ||
| Grade IV < 60% of median | 28 (64) | 19 (53) | ||
| Diarrhoea before admission ± SD | ||||
| Mean duration, hours | 33.4 ± 11.5 | 38.3 ± 10.3 | ||
| Frequency/24 hours | 13.8 ± 3.8 | 13.3 ± 3.9 | ||
| Degree of dehydration | Some | Some | ||
| Enteropathogens, n (%) | ||||
| Single pathogen | 34 (77) | 23 (64) | ||
| Mixed pathogens | 7 (16) | 9 (25) | ||
| No pathogen | 3 (7) | 4 (11) | ||
| Comments: the study reports n (%) for specific single and mixed pathogens, but these have been summed by reviewer. Pathogens identified were: single pathogens – enteropathogenic E. coli, enteroaggregative E. coli, Salmonella typhimurium, Shigella flexneri, Shigella sonnei, V. cholera O1, Clostridium difficile, rotavirus, V. cholera non-O1 non-O139; mixed pathogens – EPEC + S. typhimurium, EPEC + rotavirus, EPEC + S. flexneri, rotavirus + S. flexneri, rotavirus + S. typhyimurium. No p-values were reported | ||||
| Results | ||||
| Primary outcomes | Zinc syrup (n = 44) | Placebo syrup (n = 36) | p-value | |
| Patients recovered, n (%)a | 44 (100) | 32 (89) | 0.04 | |
| Mean recovery ± SD, hourb | 70.4 ± 10.0 | 103.4 ± 17.1 | 0.0001 | |
| Total liquid stool output, kg | 1.5 ± 0.7 | 2.4 ± 0.7 | 0.0001 | |
| Total liquid, ml (liquid food + water) | 867.0 ± 466.1 | 1354.7 ± 675.6 | 0.0001 | |
| Consumption of total ORS, litres | 2.5 ± 1.0 | 3.6 ± 0.8 | 0.0001 | |
| Comments: assumed that total stool output, total liquid and consumption of ORS were calculated to recovery or up to day 5 | ||||
| Secondary outcomes | Zinc syrup (n = 44) | Placebo syrup (n = 36) | p-value | |
| Per cent weight gain on recovery (% admission weight) ± SD | 3.9 ± 4.1 | 3.2 ± 2.9 | 0.41 | |
| Per cent weight gain on 30th day (% recovery weight) ± SD | 2.6 ± 3.3c | 2.9 ± 3.7d | 0.88 | |
| Per cent gain in mid-arm circumference on 30th day (% recovery MAC) ± SD | 5.2 ± 3.4c | 3.4 ± 2.3d | 0.08 | |
| Per cent gain in height on 30th day (% recovery height) ± SD | 1.1 ± 0.9c | 0.6 ± 0.5d | 0.06 | |
|
Comments: in subgroup analysis of different nutritional status, the duration of diarrhoea, stool output, consumption of ORS and other fluids were significantly less in the zinc-supplemented group than in the placebo group (numerical data not presented in the paper) Safety: NR HIV: NR |
||||
| Barriers to implementation | ||||
| NR | ||||
| Methodological comments | ||||
|
Allocation to treatment groups: randomised using a random numbers table and patients were allocated a specific-numbered bottle of either zinc or placebo syrup Blinding: double blind. The taste, colour and consistency of the zinc and placebo syrups were identical, as were the bottles that were numbered. The person who made the randomisation was not associated with the study. The serial code numbers were kept in a sealed envelope with a senior officer who identified the groups after the study completion Comparability of treatment groups: paper states that groups were comparable for baseline characteristics, although no p-values were reported. Note that the zinc status of the participants was not assessed, so it is not known whether children were zinc deficient or whether or not this was comparable between the groups Method of data analysis: appears to be ITT analysis for primary outcomes and also weight gain at recovery. The other secondary outcomes were analysed on a proportion of patients. Comparability of the study and control groups according to patient characteristics, and differences in proportion of cured patients in the two groups, were determined using chi-squared tests. Means of outcome variables of the two groups were compared by applying Student’s t-test Sample size/power calculation: NR Attrition/dropout: does not specifically report any dropouts, but outcomes at 30-days follow-up are only presented for 18 and 16 patients in the zinc and placebo groups, respectively. Thus, can possibly assume 26 and 20 patients, respectively, dropped out/withdrew by this time point |
||||
| General comments | ||||
|
Generalisability: young infants (aged 3–24 months) and males only. Definition of SAM is not provided and it is unclear whether or not the children would meet the current WHO criteria as only 59% (47/80) of population are < 60% Harvard standard W/A, but the majority have a MUAC < 11 cm Outcome measures: outcomes were appropriate, although mortality was not a specified outcome (no deaths reported) Intercentre variability: N/A Conflict of interest: funding not stated. Greenco Biologicals (Pvt) Ltd prepared the zinc syrup and placebo syrup |
||||
| All children received standard ORS solution (mmol/l: sodium, 90; potassium, 20; citrate, 10; chloride, 80; glucose, 111) at the rate of 75–100 ml/kg body weight for first 4–6 hours of admission for correction of initial dehydration. If not achieved, the same solution was repeated for another 4–6 hours. When all the signs and symptoms of dehydration disappeared, ORS solution was given as maintenance therapy in amounts matching stool volume and loss in vomitus. However, more fluid was given if the child wanted it and if there were clinical indications. If any patient developed severe dehydration during the follow-up period, he received i.v. infusion of RL according to WHO guidelines | ||||
| Zinc-supplemented syrup | Placebo syrup | |||
| 177 mg/day in three divided doses, 40 mg elemental zinc/day. Each 5 ml of zinc syrup contained 59 mg of zinc sulphate | Identical in taste, consistency and colour to the zinc syrup | |||
|
Immediately after rehydration, feeding was resumed in both groups. Breastfeeding was allowed as wanted. Non-breastfed children received half-strength milk for the first 24 hours, and the strength gradually increased until discharge. Older children were offered the standard hospital diet of rice, lentils and fish (cereal/vegetable diet) appropriate for their age At the time of discharge, all the children were advised to continue the assigned bottle of syrup until it was finished. Mothers were advised to give at least one extra meal or liquid feed per day during the recovery period |
||||
Quality assessment for primary studies (modified for severe malnutrition)
| A. Selection bias | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1. Are the individuals selected to participate in the study likely to be representative of the target population? | Very likely | Somewhat likely | Not likely | Cannot tell | |||||
| ✓ | |||||||||
| 2. What percentage of selected individuals participated? | 80–100% | 60–79% | < 60% | N/A | Cannot tell | ||||
| ✓ | |||||||||
| Summary of selection bias | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| B. Study design | |||||||||
| 1. What was the study design? | RCT | ✓ | |||||||
| (Please tick appropriate and specify design if categorise as ‘Other’) | CCT | ||||||||
| Cohort analytic (two group pre + post) | |||||||||
| Case–control | |||||||||
| Cohort [one group pre + post (before and after)] | |||||||||
| Interrupted time series | |||||||||
| Other – specify | |||||||||
| Cannot Tell | |||||||||
| 2. Was the study described as randomised? | Yes | No | |||||||
| ✓ | |||||||||
| If answer to no. 2 is ‘no’ complete summary then go to section C. Confounders. If answer is ‘yes’, answer no. 3 and no. 4 below, before completing summary for this section | |||||||||
| 3. If answer was yes, was the method of randomisation described? | Yes | No | |||||||
| ✓ | |||||||||
| 4. If answer was yes, was the method appropriate? | Yes | No | |||||||
| ✓ | |||||||||
| Summary of study design | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| C. Confounders | |||||||||
| 1. Were there important differences between groups prior to the intervention? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. If yes, indicate the percentage of relevant confounders that were controlled [either in the design (e.g. by stratification or matching) or in the analysis]? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| Summary of confounders | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| D. Blinding | |||||||||
| 1. Was the outcome assessor aware of the intervention or exposure status of participants? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were the study participants aware of the research question? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| Summary of blinding | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| E. Data collection methods | |||||||||
| 1. Were data collection tools shown to be valid? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were data collection tools shown to be reliable? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| Summary of data collection | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| F. Withdrawals and dropouts | |||||||||
| 1. Were withdrawals and dropouts reported in terms of numbers and reasons per group? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Indicate the percentage of participants completing the study (If the percentage differs by groups, record the lowest) | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓a (primary outcomes) | ✓a (secondary outcomes) | ||||||||
| Summary of withdrawals and dropouts | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓b | ✓a | |||||||
| G. Intervention integrity | |||||||||
| 1. What percentage of participants received the allocated intervention or exposure of interest? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
| 2. Was the consistency of the intervention measured? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 3. Is it likely that subjects received an unintended intervention (contamination or co-intervention) that may influence the results? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| H. Analysis | |||||||||
| 1. Indicate the unit of allocation | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 2. Indicate the unit of analysis | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 3. Are the statistical methods appropriate for the study design? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 4. Is the analysis performed by intervention allocation status (i.e. ITT) rather than actual intervention received? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| Global rating for studyb | Strong | Moderate | Weak | ||||||
| (Overall methodological strength of study – based on sections A–F) | ✓ | ||||||||
Dutta et al. 200154
Data extraction table
| Reference and design | Intervention | Participants | Outcome measures | ||
|---|---|---|---|---|---|
|
Author: Dutta et al. 54 Year: 2001 Country: India Study design: double-blind, RCT Setting: inpatient (hospital) Number of centres: one Funding: not stated |
Intervention: H-ORS (224 mmol/l) Control: standard WHO/UNICEF ORS (311 mmol/l) All children were rehydrated orally within 4–6 hours using the assigned ORS solution. It was then given to replace continuing losses (liquid stool and vomitus) until diarrhoea stopped (two formed stools passed, or no stool for 12 hours) or for up to 5 days if diarrhoea persisted. Children, other than those who were very ill, were discharged on recovery Other interventions used: all children were allowed to drink water ad libitum, breastfeeding and formula/animal milk were permitted, older children received the normal diet, which they were used to before the illness. No drug therapy was given Composition of ORS at end of table |
Definition of SAM: < 60% Harvard standard W/A (without oedema) Number of participants: n = 64 (H-ORS n = 32, standard ORS n = 32) Sample attrition/dropout: appears none (though NR) Sample crossovers: none Inclusion criteria: male children, aged 6–48 months, < 60% Harvard standard W/A without oedema, marasmic, history of watery diarrhoea (three or more loose, watery stools/day) for ≤ 72 hours and clinical signs and symptoms of ‘some’ dehydration (e.g. thirst or eagerness to drink, sunken eyes, dry mouth and tongue and loss of skin elasticity) Exclusion criteria: history of another episode of diarrhoea 1 month prior to onset of present illness, receipt of antibiotics or ORT during this episode of diarrhoea, obvious parenteral infection (septicaemia, meningitis, pneumonia, urinary tract infection), need for special medical care (i.e. life-support system, blood transfusion or total parenteral nutrition), exclusively breastfed, obvious signs of kwashiorkor General characteristics of participants: severely malnourished, marasmic, male children, aged 6–48 months, with dehydrating acute watery diarrhoea |
Primary outcomes: not specifically stated Outcomes:Recovery not specifically defined, but assume is until diarrhoea stopped (two formed stools passed or no stool for 12 hours) Method of assessing outcomes: weighed unclothed at same time each day on a balance of 10 g precision; nutritional status assessed using IAP classification; stool samples examined using ‘standard techniques’92 for characterisation of bacterial isolates; detection of enteropathogens using microscopic examination (trophozoites and cysts of Entamoeba histolytica and Giardia lamblia), enzyme linked immunosorbent assay (ELISA) and polyacrylamide gel elecrophoresis (rotavirus) Serum Sodium and potassium estimated from blood samples Stool losses measured on pre-weighed disposable diapers; urine separated from stools using urine collection bags; vomitus weighed on pre-weighed gauze pads; measurement units sensitive to 1 g or 1 ml. Intake and output measured and recorded 8 hourly until diarrhoea stopped or for up to 5 days if it persisted Adverse symptoms: NR Length of follow-up: not specifically stated but treated until diarrhoea stopped or for up to 5 days Recruitment dates: July 1997 to August 1999 |
||
| Characteristics of participants: | |||||
| Characteristic | H-ORS (n-32) | Standard ORS (n = 32) | p-value | ||
| Age, months | 17.3 (9.7) | 22.5 (15.6) | |||
| Weight on admission, kg | 5.7 (1.7) | 5.8 (1.6) | |||
| W/A, n (%) | |||||
| 60–69% | 2 (6) | 1 (3) | |||
| < 60% | 30 (94) | 31 (97) | |||
| Duration of diarrhoea before admission, daysa | 21.3 (8.2) | 22 (8.0) | |||
| Stool frequency/day | 15 (3) | 13 (4) | |||
| Vomiting, n (%) | 8 (25) | 9 (28) | |||
| Degree of dehydration: | |||||
| ‘Some’ dehydration, n (%) | 32 (100) | 32 (100) | |||
| Serum sodium, mmol/l | 130.0 (3.3) | 129.7 (3.1) | |||
| Serum potassium, mmol/l | 3.1 (0.3) | 3.1 (0.3) | |||
| Per cent weight loss | 6.1 (2.2) | 6.3 (2.1) | |||
| Enteropathogens, n (%) | |||||
| Single pathogen | 24 (75) | 26 (81) | |||
| Mixed pathogens | 5 (16) | 4 (13) | |||
| No pathogens | 3 (9) | 2 (6) | |||
| Comments: results are expressed as mean (SD) unless otherwise stated. The study reports n (%) for specific single and mixed pathogens, but these have been summed by reviewer. Pathogens identified were: enteropathogenic E. coli, rotavirus, Vibrio cholerae, Shigella flexneri, Salmonella typhimurium, Giardia lamblia, Aeromonus sp., Klebsiella. No p-values were reported | |||||
| Results | |||||
| Outcomes | H-ORS (n = 32) | Standard ORS (n = 32) | p-value | ||
| Patients recovered within 5 days, n (%) | 32 (100) | 29 (91) | > 0.05 | ||
| Median survival time to recovery, hours | 36 | 53 | 0.001 | ||
| Duration of diarrhoea after initiation of therapy, hours | 41.5 (25.1) | 66.4 (32.3) | 0.001 | ||
| Stool output | |||||
| 0–24 hours, g/kg | 73.4 (23.1) | 105.9 (44.6) | 0.001 | ||
| 24–48 hours, g/kg | 34.9 (13.5) | 87.5 (66.5) | 0.001 | ||
| 48–72 hours, g/kg | 28.4 (18.0) | 90.4 (67.7) | 0.01 | ||
| At recovery, g/kg/day | 52.3 (21.3) | 96.6 (42.8) | 0.0001 | ||
| ORS intake | |||||
| 0–24 hours, ml/kg | 109.7 (32.2) | 184.5 (53.7) | 0.0001 | ||
| 24–48 hours, ml/kg | 73.4 (22.7) | 151.2 (81.3) | 0.0001 | ||
| 48–72 hours, ml/kg | 54.9 (28.3) | 151.5 (65.0) | 0.001 | ||
| At recovery, ml/kg/day | 111.5 (39.4) | 168.9 (52.4) | 0.0001 | ||
| Fluid intake (ORS + water + liquid food), ml/kg/day | 214.6 (61.2) | 278.3 (99.3) | 0.003 | ||
| Per cent of weight gainb (% of admission weight) | 4.3 (1.2) | 5.4 (1.3) | 0.001 | ||
Comments: results are expressed as mean (SD) unless otherwise stated]
|
|||||
| Barriers to implementation | |||||
| NR | |||||
| Methodological comments | |||||
|
Allocation to treatment groups: a computer-generated randomisation table was used to allocate the different ORS packets. An individual not associated with the study provided the ORS packets Blinding: double blind. The packets of ORS were similar in appearance and packaged in identical sachets. The randomisation table was held by an individual not associated with the study. Decoding was performed at the end of the study Comparability of treatment groups: groups appear similar for baseline characteristics, although the mean age of children in the H-ORS group was slightly lower. The study reports characteristics are comparable although no p-values were reported Method of data analysis: appears to be ITT analysis. Groups were compared using the chi-squared test. Means of the outcome variables of the two groups (time-specific stool output, intake of ORS, total fluid intake, weight gain or loss and electrolyte concentrations on recovery) were compared using the Student’s t-test. The difference in proportions of cured patients between the two groups was examined using the chi-squared test. Recovery time of patients in the two groups was calculated using a survival analysis technique in accordance with the Kaplan–Meyer method Sample size/power calculation: NR Attrition/dropout: none reported, but may have occurred as study reports intake and output measuring took place but stopped if child was withdrawn from study |
|||||
| General comments | |||||
|
Generalisability: vast majority of children were SAM (61/64, 95%) based on W/A criteria (defined here as < 60% Harvard standard W/A); young children (aged 6–48 months), males only. As W/H and W/L is NR it is uncertain whether or not the study group meet the current WHO criteria. However, as they are described as marasmic, it is likely that they would Outcome measures: outcomes appear appropriate, although mortality was not a specified outcome (no deaths are reported) Intercentre variability: N/A Conflict of interest: NR |
|||||
| Composition of ORS | H-ORS | Standard ORS recommended by WHO/UNICEF | |||
| Sodium, mmol/l | 60 | 90 | |||
| Potassium, mmol/l | 20 | 20 | |||
| Chloride, mmol/l | 50 | 80 | |||
| Glucose, mmol/l | 84 | 111 | |||
| Citrate, mmol/l | 10 | 10 | |||
| Made by dissolving the following in one litre of water | |||||
| NaCl, g | 1.75 | 3.5 | |||
| KCl, g | 1.5 | 1.5 | |||
| Trisodium citrate dehydrate, g | 2.9 | 2.9 | |||
| Glucose, g | 15 | 20 | |||
| Resulting osmolarity | 224 | 311 | |||
| Ten 1-litre packets were provided for each child | |||||
Quality assessment for primary studies (modified for severe malnutrition)
| A. Selection bias | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1. Are the individuals selected to participate in the study likely to be representative of the target population? | Very likely | Somewhat likely | Not likely | Cannot tell | |||||
| ✓ | |||||||||
| 2. What percentage of selected individuals participated? | 80–100% | 60–79% | < 60% | N/A | Cannot tell | ||||
| ✓ | |||||||||
| Summary of selection bias | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| B. Study design | |||||||||
| 1. What was the study design? | RCT | ✓ | |||||||
| (Please tick appropriate and specify design if categorise as ‘Other’) | CCT | ||||||||
| Cohort analytic (two group pre + post) | |||||||||
| Case–control | |||||||||
| Cohort [one group pre + post (before and after)] | |||||||||
| Interrupted time series | |||||||||
| Other – specify | |||||||||
| Cannot Tell | |||||||||
| 2. Was the study described as randomised? | Yes | No | |||||||
| ✓ | |||||||||
| If answer to no. 2 is ‘no’ complete summary then go to section C. Confounders. If answer is ‘yes’, answer no. 3 and no. 4 below, before completing summary for this section | |||||||||
| 3. If answer was yes, was the method of randomisation described? | Yes | No | |||||||
| ✓ | |||||||||
| 4. If answer was yes, was the method appropriate? | Yes | No | |||||||
| ✓ | |||||||||
| Summary of study design | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| C. Confounders | |||||||||
| 1. Were there important differences between groups prior to the intervention? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. If yes, indicate the percentage of relevant confounders that were controlled [either in the design (e.g. by stratification or matching) or in the analysis]? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| Summary of confounders | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| D. Blinding | |||||||||
| 1. Was the outcome assessor aware of the intervention or exposure status of participants? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were the study participants aware of the research question? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| Summary of blinding | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| E. Data collection methods | |||||||||
| 1. Were data collection tools shown to be valid? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were data collection tools shown to be reliable? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| Summary of data collection | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| F. Withdrawals and dropouts | |||||||||
| 1. Were withdrawals and dropouts reported in terms of numbers and reasons per group? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Indicate the percentage of participants completing the study (If the percentage differs by groups, record the lowest) | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
| Summary of withdrawals and dropouts | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| G. Intervention integrity | |||||||||
| 1. What percentage of participants received the allocated intervention or exposure of interest? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
| 2. Was the consistency of the intervention measured? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 3. Is it likely that subjects received an unintended intervention (contamination or co-intervention) that may influence the results? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| H. Analysis | |||||||||
| 1. Indicate the unit of allocation | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 2. Indicate the unit of analysis | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 3. Are the statistical methods appropriate for the study design? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 4. Is the analysis performed by intervention allocation status (i.e. ITT) rather than actual intervention received? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| Global rating for studya | Strong | Moderate | Weak | ||||||
| (Overall methodological strength of study – based on sections A–F) | ✓ | ||||||||
Amadi et al. 200552
Data extraction table
| Reference and design | Intervention | Participants | Outcome measures | |
|---|---|---|---|---|
|
Author: Amadi et al. 52 and Amadi 200258 Year: 2005 Country: Zambia Study design: single-blind RCT Setting: inpatient [malnutrition ward in university teaching hospital (UTH)] Number of centres: one Funding: grant received from SHS International Ltd (Scientific Hospital Supplies); one author is supported by the Wellcome Trust |
Intervention: Neocate amino acid-based elemental infant formula feed that excluded cow milk, soy and cereal antigens, 4 weeks (see end of table for details) Control: standard nutritional rehabilitation therapy for persistent diarrhoea and malnutrition using a skimmed milk/soy-based diet, 4 weeks (see end of table for details) Other interventions used: The UTH followed the WHO guidelines for management of persistent diarrhoea and malnutrition. All children received ORT with i.v. fluids given only when strictly indicated. All received oral micronutrient supplements and broad-spectrum antibiotics according to clinical condition Some children treated for TB on clinical grounds, usually after failure to respond to antibiotic therapy for pneumonia Children were tested for HIV infection and given full pre- and post-test counselling where indicated |
Definition of SAM: used Wellcome classification to define malnutrition. States children had SAM and baseline WAZs were –4 Number of participants: n = 200 (Neocate n = 100, control: n = 100) Sample attrition/dropout: 45/200 (22.5%): n = 24 (12%) Neocate (22 died, two withdrawn); n = 21 (10.5%) control (17 died, four withdrawn) Overall, 39 died and of the six withdrawn, three were discharged prematurely owing to a cholera outbreak (NR by group) and three withdrew (mothers needed at home) Sample crossovers: none Inclusion criteria: children aged 6–24 months with malnutrition and persistent diarrhoea (≥ 14 days duration) Exclusion criteria: children with features of measles, chickenpox, neurological disorder (e.g. cerebral palsy), serious systemic disorder or being exclusively breastfed General characteristics of participants: children with persistent diarrhoea and malnutrition, aged 6–24 months, 54% HIV+ve |
Primary outcomes:Secondary outcomes:Method of assessing outcomes: all feeds, fluid balance and stools passed were documented daily Weight recorded three times per week Lactose intolerance tested using Clinitest (Bayer Corporation, Pittsburgh, PA, USA). Blood sugar monitored during feeds and treated appropriately. All initial investigations repeated at the end of 4 weeks (except chest radiography and HIV testing) Adverse symptoms: none reported Length of follow-up: 4 weeks Recruitment dates: April 1998 to June 2000 |
|
| Characteristics of participants | ||||
| Characteristic | Neocate (n = 100) | Control (n = 100) | p-value | |
| Sex, M : F | 49 : 51 | 45 : 55 | 0.64 | |
| Age, months | 17 (14–20) | 18 (13–22) | 0.31 | |
| Diagnosis: | ||||
| Underweight | 10 | 9 | ||
| Marasmus | 21 | 24 | ||
| Kwashiorkor | 44 | 49 | ||
| Marasmic kwash | 25 | 18 | 0.65 | |
| HIV infecteda | 51 | 54 | 0.86 | |
| Fever | 24 | 34 | 0.15 | |
| TB | ||||
| Definite | 13 | 14 | 0.98 | |
| Probable | 15 | 21 | 0.35 | |
| Chest radiograph | ||||
| Normal | 16 | 12 | ||
| Abnormal | 69 | 83 | 0.35 | |
| Intestinal infection | ||||
| C. parvum | 28 | 23 | 0.54 | |
| Salmonella sps. | 23b | 13 | 0.10 | |
| Giardia intestinalis | 6 | 5 | 0.99 | |
| Shigella spp. | 2 | 2 | 0.69 | |
| Ascaris | 3 | 7 | NR | |
| Hookworm | 1 | 2 | NR | |
| WAZ | –4.0 (–4.6 to –3.4) | –4.1 (–4.8 to –3.6) | 0.38 | |
| HAZ | –2.9 (–3.6 to –2.1) | –3.0 (–3.6 to –2.1) | 0.40 | |
| MUAC, cm | 11 (10–12.2) | 11 (10–12) | 0.55 | |
| Haemoglobin concentration, g/dl | 9.3 (8.3–10.1) | 9.0 (8.3–10.0) | 0.28 | |
| Serum albumin concentration, g/dl | 28 (23–31) | 29 (24–34) | 0.41 | |
|
Comments: results with brackets are median (IQR) Text states 106 participants were HIV+ve, although tables suggests 105 participants |
||||
| Results | ||||
| Primary outcomes | Neocate | Control | p-value | |
| Weight gain, kg | n = 79 | n = 78 | ||
| From admission | 1.10 (0.55–1.55) | 0.75 (0.2–1.3) | 0.006 | |
| From nadir | 1.7 (1.2–2.0) | 1.2 (0.6–1.7) | 0.002 | |
| Increase in WAZ | n = 79 | n = 78 | ||
| From admission | 0.83 (0.35–1.22) | 0.43 (0–0.9) | 0.018 | |
| From nadir | 1.23 (0.89–1.57) | 0.87 (0.47–1.25) | 0.002 | |
| Increase in WHZ | n = 79 | n = 78 | ||
| From admission | 1.28 (0.52–1.88) | 0.56 (0–1.15) | < 0.001 | |
| From nadir | 1.77 (1.30–2.26)c | 1.23 (0.59–1.70) | < 0.001 | |
| Increase in z-score from nadir in HIV+ve children | n = 38 | n = 40 | ||
| W/A | 1.2 (0.8–1.5) | 0.70 (0.4–1.2) | 0.007 | |
| W/H | 1.8 (1.1–2.3) | 0.8 (0.4–1.6) | < 0.001 | |
| Increase in z-score from nadir in HIV–ve children | n = 41 | n = 38 | ||
| W/A | 1.29 (0.98–1.57) | 0.95 (0.5–1.45) | 0.01 | |
| W/H | 1.82 (1.47–2.38) | 1.43 (0.81–1.86) | 0.009 | |
| Mortality (over 4 weeks) | 22% (22/100) | 17% (17/100) | 0.48 | |
| Mortality by nutritional status, n (%) | ||||
| Underweight | 2 (10.5) | |||
| Marasmus | 12 (26.7) | |||
| Kwashiorkor | 10 (10.8) | |||
| Marasmic kwashiorkor | 15 (34.9) | 0.004 | ||
|
Comments: data are presented as median (IQR) Neocate was associated with a 41% better gain in weight Diarrhoea, assessed as total number of stools passed over each time period, was not different in the two groups over the 28-days follow-up, nor was there any difference in stool frequency between the groups in the fourth week of follow-up (numerical data not presented in paper) Similar numbers in each group were tested for malabsorption of reducing sugars and there was no significant difference in positive tests between the groups (numerical data presented, but not extracted) Overall deaths = 19.5% (39/200), of which 31% was in week 1, 43% in week 2, 26% in week 3 and 10% in week 4. Amadi et al. 52 reports that death was more likely in children with marasmus, and children with cryptosporidiosis (data NR). However, Amadi58 reports data and shows death was more likely in marasmic kwashiorkor Mortality was lower in HIV–ve children than in HIV+ve children (11% vs 24%, respectively), irrespective of nutritional regimen There was significant correlation between mortality and severity of initial diagnosis of nutritional status, and being HIV+ve, but these results were only reported for the overall study group, not by trial arm Secondary outcomes – achievements of developmental milestones, activity and play and laboratory indicators of severity of illness (haemoglobin and albumin concentrations) – were reported, but have not been extracted here |
||||
| Other outcomes | Neocate (n = 100) | Control (n = 100) | p-value | |
| Week 1 Intake, kcal/kg/day | 116 (86–143), n = 95 | 167 (130–214), n = 97 | < 0.0001 | |
| Week 2 Intake, kcal/kg/day | 168 (135–203), n = 85 | 258 (210–301), n = 93 | < 0.0001 | |
| Week 3 Intake, kcal/kg/day | 184 (166–206), n = 75 | 283 (229–337), n = 85 | < 0.0001 | |
| Week 4 Intake, kcal/kg/day | 187 (163–210), n = 70 | 269 (214–305), n = 79 | < 0.0001 | |
|
Comments: presentation of data believed to be median (IQR), but this is not explicitly state Intake of calories (per kg per day), as liquid feeds for each of the 4 weeks of the study in the control group, were statistically significantly higher (p < 0.0001). Note, in addition to the liquid feed (based on skimmed milk) intake in the control group, soy-based porridge was also given, beginning in week 2 Safety: NR HIV: the Neocate diet benefit was seen in both HIV+ve and HIV–ve patients The statistically significant improvement in weight gain was not only true for the Neocate group as a whole, but also for HIV+ve (p = 0.007) and HIV–ve (p = 0.01) children Death was statistically significantly more likely (p = 0.04) in HIV+ve children (23.6%, n = 25) vs HIV–ve children (11.1%, n = 10) |
||||
| Barriers to implementation | ||||
| Study authors did not believe an elemental feed such as Neocate should be adopted because of the expense. Fifty-one per cent (284/548) of eligible children were not randomised because no bed was available on the day when judged to be eligible. Rate of recruitment had to be limited as the number of eligible patients exceeded the capacity of the nursing staff and laboratory technicians to carry out the full range of study procedures and investigations. A cholera outbreak also temporarily interrupted the study leading to premature discharge of three patients | ||||
| Methodological comments | ||||
|
Allocation to treatment groups: randomisation using consecutive sealed envelopes. The randomisation code was blocked so as to equalise active and placebo for every 20 patients Blinding: single-blind (patients) study because of preparation and administration of feeds; apart from feeds, care was identical in all other respects. Study was double blind up until randomisation and single blind thereafter (care providers and outcome assessors not blinded). However, control group children were given porridge from week 2, thus, participants may have been aware, although knowledge of group assignment was not likely to affect outcomes as these were objective measures Comparability of treatment groups: paper states that groups were well-matched; groups were not significantly different (p-values reported) Method of data analysis: not ITT analysis. Comparison of categorical variables used chi-squared or Fisher’s exact tests, and of continuous variables used the Kruskal–Wallis test Sample size/power calculation: NR Attrition/dropout: numbers and reasons reported. 45/200 (22.5%): n = 24 (12%) Neocate; n = 21 (10.5%) control. Of the 45 participants, 39 died (n = 22, Neocate; n = 17, control), three withdrew and three discharged prematurely because of cholera outbreak (n = 2, Neocate; n = 4, control) |
||||
| General comments | ||||
|
Generalisability: likely that most of the children would meet the current WHO criteria of MUAC < 115 mm. Population was a subsection of children with SAM admitted to the unit (not all eligible children owing to limitation on resources), young infants ( aged 6–24 months), approximately half were HIV+ve. Participants had a high prevalence of intestinal infection, respiratory and systemic infectious disease. The study was designed to investigate a feed for treatment of SAM in children with persistent diarrhoea, it is not clear whether or not this feed would be a suitable treatment for children with SAM, but who do not have persistent diarrhoea. None of those enrolled in the study were > 2 years of age so it is not clear whether or not the results of the study would hold in children aged ≥ 2 years Outcome measures: outcomes appropriate although no numerical data for diarrhoea reported Intercentre variability: N/A Conflict of interest: a grant was received from SHS International Ltd (Scientific Hospital Supplies); the corresponding author is supported by the Wellcome Trust |
||||
| Neocate infant formula feed | Standard nutritional rehabilitation therapy | |||
|
Amino acid-based elemental feed (Neocate) + routine care Complete infant formula feed based on amino acids, maltodextrin and a combination of safflower oil, refined coconut oil and soya oil, with a calorific value of 70 kcal/100 ml. The vitamin and mineral composition reflects that of breastmilk |
Standard therapy as per hospital protocol + routine care Complete feed: mixture of skimmed milk, sugar and vegetable oil given as a liquid feed (100 kcal/100 ml). At beginning of week 2, children given a soya-based, high-energy protein supplement in porridge form providing 400 kcal/100 ml, beginning at 100 ml/day and increasing to 200–300 ml/day |
|||
| Liquid feeds given at 3-hour intervals (2 hours for weaker children) using cup and spoon or via nasogastric tube if necessary. Feeds were introduced gradually, beginning at 80 kcal/kg/day to avoid the refeeding syndrome. If diarrhoea worsened or reappeared, stools were tested for presence of reducing substances to detect lactose intolerance | ||||
| If lactose intolerance test was positive (≥ 1%), feeds were diluted to half strength and gradually reintroduced to full strength | If lactose intolerance test was positive (≥ 1%), skimmed milk was withdrawn and replaced by a commercial fermented milk | |||
| Components of the WHO guidelines for management of persistent diarrhoea employed at UTH: | ||||
| Emphasis on oral/NG rehydration | If i.v. fluids are necessary (for severe dehydration and shock), they are given for shorter periods of 4–6 hours, with close monitoring and a change to the oral route as soon as improvement is noted | |||
| Vitamin, zinc, copper | Vitamins and mineral supplements given when available | |||
| Multivitamin | ||||
| Folic acid | ||||
| Potassium | ||||
| Antibiotics | Often necessary because these children have severe infections (e.g. septicaemia, pneumonia) | |||
| Antimalarials | Given because malaria is endemic in Zambia | |||
Quality assessment for primary studies (modified for severe malnutrition)
| A. Selection bias | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1. Are the individuals selected to participate in the study likely to be representative of the target population? | Very likely | Somewhat likely | Not likely | Cannot tell | |||||
| ✓ | |||||||||
| 2. What percentage of selected individuals participated? | 80–100% | 60–79% | < 60% | N/A | Cannot tell | ||||
| ✓ | |||||||||
| Summary of selection bias | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| B. Study design | |||||||||
| 1. What was the study design? | RCT | ✓ | |||||||
| (Please tick appropriate and specify design if categorise as ‘Other’) | CCT | ||||||||
| Cohort analytic (two group pre + post) | |||||||||
| Case–control | |||||||||
| Cohort [one group pre + post (before and after)] | |||||||||
| Interrupted time series | |||||||||
| Other – specify | |||||||||
| Cannot Tell | |||||||||
| 2. Was the study described as randomised? | Yes | No | |||||||
| ✓ | |||||||||
| If answer to no. 2 is ‘no’ complete summary then go to section C. Confounders. If answer is ‘yes’, answer no. 3 and no. 4 below, before completing summary for this section | |||||||||
| 3. If answer was yes, was the method of randomisation described? | Yes | No | |||||||
| ✓ | |||||||||
| 4. If answer was yes, was the method appropriate? | Yes | No | |||||||
| ✓ | |||||||||
| Summary of study design | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| C. Confounders | |||||||||
| 1. Were there important differences between groups prior to the intervention? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. If yes, indicate the percentage of relevant confounders that were controlled (either in the design (e.g. by stratification or matching) or in the analysis)? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| Summary of confounders | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| D. Blinding | |||||||||
| 1. Was the outcome assessor aware of the intervention or exposure status of participants? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were the study participants aware of the research question? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| Summary of blinding | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| E. Data collection methods | |||||||||
| 1. Were data collection tools shown to be valid? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were data collection tools shown to be reliable? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| Summary of data collection | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| F. Withdrawals and dropouts | |||||||||
| 1. Were withdrawals and dropouts reported in terms of numbers and reasons per group? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Indicate the percentage of participants completing the study (If the percentage differs by groups, record the lowest) | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
| Summary of withdrawals and dropouts | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| G. Intervention integrity | |||||||||
| 1. What percentage of participants received the allocated intervention or exposure of interest? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
| 2. Was the consistency of the intervention measured? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 3. Is it likely that subjects received an unintended intervention (contamination or co-intervention) that may influence the results? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| H. Analysis | |||||||||
| 1. Indicate the unit of allocation | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 2. Indicate the unit of analysis | Community | Organisation/institution | Practice/office | Provider | ✓ | ||||
| 3. Are the statistical methods appropriate for the study design? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 4. Is the analysis performed by intervention allocation status (i.e. ITT) rather than actual intervention received? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| Global rating for studya | Strong | Moderate | Weak | ||||||
| (Overall methodological strength of study – based on sections A–F) | ✓ | ||||||||
Bhutta et al. 199453
Data extraction table
| Reference and design | Intervention | Participants | Outcome measures | ||||
|---|---|---|---|---|---|---|---|
|
Author: Bhutta et al. 53 Year: 1994 Country: Pakistan Study design: RCT Setting: inpatient (Gastroenterology-Nutrition Research Ward at the Aga Khan University Hospital, Karachi, Pakistan) Number of centres: one Funding: provided by the Applied Diarrhoeal Disease Research Project at Harvard University via a co-operative agreement with the US Agency for International Development |
Intervention (soy group): soy formulation (full strength) Control (KY milk group): half-strength buffalo milk with KY Details of diet composition provided at end of table Both diets were provided for 14 days and given in gradually increasing amounts. Day 1 at least 50 kcal/kg/day, increasing by 25 kcal/kg/day to provide a minimum of 100 kcal/kg/day by day 3. Diets were given by nasogastric tube if children were unable to take the stipulated amount orally Other interventions used: NR |
Definition of SAM: W/A ≤ 80th centile of the median NCHS standard, i.e. Gómez grades II and III malnutrition Number of participants: 51 (soy group, n = 25; KY milk group, n = 26) Sample attrition/dropout: after randomisation, 11 participants were subsequently excluded (four from the soy group and seven from the KY milk group); one for pneumonia, four for development of septicaemia, four for hyperpyrexia ≥ 39 °C, or withdrawal by the parents prior to completion of study protocol (two, one in each group) Sample crossovers: NR Inclusion criteria: male children, aged 6–36 months, with persistent diarrhoea (diarrhoea lasting ≥ 2 weeks), and with severe PEM Exclusion criteria: breastfed infants, presence of intercurrent infections, ileus and bloody diarrhoea. Children with kwashiorkor (clinical oedema and/or serum albumin ≤ 20 g/l) excluded because weight gain difficult to interpret in these children In addition, children admitted for the duration of the study were examined twice daily and excluded from the study if they developed a significant intercurrent illness (pneumonia, pyrexia ≥ 39 °C, persistent vomiting, or clinical signs of septicaemia) General characteristics of participants: economically disadvantaged children in Karachi (mean z-score W/A –4.2, SD 0.8) |
Primary outcomes: stool output and weight gain (not explicitly stated, but assumed primary outcomes as used for sample size calculation) Secondary outcomes: not explicitly stated Method of assessing outcomes: vital signs, food and fluid intake, and stool, urine and emesis output were accurately recorded. Adhesive urine bags were used to collect urine separately from stools. Stool volume was measured by weighing pre-weighed diapers on electronic scales accurate to ± 2g (Tanita Inc., Amsterdam, the Netherlands). Daily nude weight obtained prior to morning feed on a double-beam balance accurate to ± 20 g (Detecto, Webb City, MO, USA). Length measured on an infant stadiometer, mid-arm circumference measured using fibreglass tape Growth quotient comparing actual daily weight gain with expected weight gain for age calculated using the method of Ellerstein and Ostrov93 Clinical failure defined as weight loss for ≥ 3 days after meeting the minimum caloric target of 100 kcal/kg/day or persistence of diarrhoea with inability to maintain hydration orally Cessation of diarrhoea defined as passage of semisolid stool, a reduction of stool frequency to ≤ 3/day or a stool volume < 30 g/kg/day A range of laboratory investigations were carried out on stools daily, and metabolic balance studies on days 4–6 and 12–14 of dietary therapy on every third patient admitted (details not data extracted) Adverse symptoms: NR Length of follow-up: not explicitly stated, presumed to be 14 days Recruitment dates: NR |
||||
| Characteristics of participants | |||||||
| Characteristic, all (mean ± SD) | Soy group (n = 25) | KY milk group (n = 26) | p-value | ||||
| Age, months | 16.0 ± 8.6 | 13.8 ± 5.8 | NS | ||||
| Weight, kg | 5.8 ± 1.1 | 6.1 ± 1.1 | NS | ||||
| W/L (%) | 88.4 ± 4.3 | 89.5 ± 4.3 | NS | ||||
| L/A (%) | 71.1 ± 7.6 | 74.5 ± 9.1 | NS | ||||
| z-score W/A | –4.41 ± 0.6 | –3.91 ± 0.9 | NS | ||||
| Mid-arm circumference, cm | 9.9 ± 1.3 | 10.6 ± 1.7 | NS | ||||
| Total protein, g/dl | 5.6 ± 0.9 | 6.1 ± 0.9 | NS | ||||
| Albumin, g/dl | 3.4 ± 0.7 | 3.8 ± 0.7 | NS | ||||
| Haemoglobin, g/dl | 9.5 ± 1.3 | 8.7 ± 1.4 | NS | ||||
| History | |||||||
| Duration of diarrhoea, days | 75.0 ± 77.0 | 150.0 ± 117.0 | NS | ||||
| Stool frequency, n/day | 8.2 ± 2.7 | 8.1 ± 2.7 | NS | ||||
| Observations in first 24 hours | |||||||
| Stool volume, g/kg/day | 69.8 ± 51.9 | 62.3 ± 42.1 | NS | ||||
| Stool frequency, n/day | 7.4 ± 4.7 | 7.1 ± 4.5 | NS | ||||
| ORS intake, ml/kg/day | 47.0 ± 84.5 | 52.8 ± 77.3 | NS | ||||
| Urine volume, ml/kg/day | 38.4 ± 21.3 | 30.0 ± 20.8 | NS | ||||
| Comments: median (range) duration of diarrhoea in the soy group was 180 (15–300) days and in the KY milk group 150 (15–270) days. Two patients in the soy group had pathogens in their stools (one entropathogenic E. coli, one S. paratyphi a), and one patient in the KY milk group had a parasitic infection (G. lamblia). Further information from laboratory investigation of stool samples not data extracted | |||||||
| Results | |||||||
| Primary outcomes | Soy group (n = 21) | KY milk group (n = 19) | p-value | ||||
| Stool volume, g/kg/day | |||||||
| Week one | 68.8 ± 43.1 | 60.9 ± 40.6 | NS | ||||
| Week two | 36.2 ± 23.2 | 63.9 ± 61.8 | NS | ||||
| Overall | 58 ± 33 | 62 ± 49 | NS | ||||
| Weight change, g/kg/day | |||||||
| Week one | 7.1 ± 11.3 | 3.1 ± 12.1 | NS | ||||
| Week two | 11.6 ± 10.0 | 4.3 ± 7.2 | < 0.02 | ||||
| Mean daily weight change, g/kg/day | 3.7 ± 5.9 | 7.9 ± 9.7 | NS | ||||
|
Comments: not explicitly stated but presume data are mean ± SD In the soy group, 10% (2/21) lost weight, in the KY milk group 37% (7/19) lost weight (p = NS) |
|||||||
| Secondary outcomes | Soy group (n = 21) | KY milk group (n = 19) | p-value | ||||
| Caloric intake, kcal/kg/day | |||||||
| Week one | 140.1 ± 33.4 | 115.1 ± 25.1 | < 0.02 | ||||
| Week two | 157.1 ± 72.3 | 151.6 ± 32.3 | NS | ||||
| Overall | 154.2 ± 36.8 | 132.8 ± 27.6 | NS | ||||
| Stool frequency, n/day: | |||||||
| Week one | 7.0 ± 3.1 | 6.6 ± 4.4 | NS | ||||
| Week two | 4.0 ± 2.4 | 5.5 ± 3.8 | NS | ||||
| Overall | 6 ± 3 | 6 ± 4 | NS | ||||
| ORS intake, ml/kg/day: | |||||||
| Week one | 33.9 ± 41.0 | 37.9 ± 46.2 | NS | ||||
| Week two | 1.7 ± 3.6 | 29.2 ± 58.1 | < 0.05 | ||||
| Time to recovery | 6 ± 4 | 5 ± 3 | NS | ||||
| Growth quotient over 14 days | 13.6 ± 13.2 | 7.5 ± 6.9 | NS | ||||
| Improvement in MUAC, cm | 1.0 ± 0.1 | 0.1 ± 0.05 | < 0.001 | ||||
| Clinical failures | 2 | 7 | NR | ||||
|
Comments: not explicitly stated, but presume data are mean ± SD Overall the soy group consumed nearly 15% more calories than the KY milk group, but the difference was NS. Only two children in each group required nasogastric feeding. The improvement in WAZ was significantly greater in the soy group (z-score from –4.4 ± 0.6 to –3.6 ± 0.6; p < 0.001) than in the KY milk group (z-score from –3.9 ± 0.9 to –3.6 ± 1.0; p = NS). Daily urine output and serum sodium levels after 48 hours of therapy were described as similar between the groups, but no numerical data presented Data on normalisation of serum bicarbonate for a subgroup of children not data extracted Data from two nutritional balance studies performed on a subgroup of children not data extracted. Details on the clinical failures not data extracted |
|||||||
| Safety: NR | |||||||
| HIV: NR | |||||||
| Barriers to implementation | |||||||
| NR | |||||||
| Methodological comments | |||||||
|
Allocation to treatment groups: block randomisation process using sealed envelopes Blinding: not described, presume that study was not blinded Comparability of treatment groups: described as similar with regard to age, degree of malnutrition and severity of diarrhoea prior to presentation. Stool volume, frequency, ORS intake and serum electrolytes in both groups were also described as comparable in the first 24 hours after the initiation of dietary therapy Method of data analysis: data were analysed for differences between means using a two-tailed Student’s t-test. Differences in proportions were assessed by chi-squared analysis Sample size/power calculation: estimated that using an alpha-level of 0.05 and a power of 80%, 40 children in each group would be needed to demonstrate a 25% difference in stool output or weight gain between the study groups. However, the mid-term evaluation of the study identified more failures and significantly poorer weight gain the children receiving the KY and buffalo milk diet, and the study was therefore concluded with a total of 51 children randomised (and 11 of these were subsequently excluded) Attrition/dropout: overall number excluded from the study after randomisation given, and deducible for each group from results tables. Reasons given for the population overall, but not by study group (except in stating that one from each group was withdrawn from the study by the parents prior to completion of the study protocol) |
|||||||
| General comments | |||||||
|
Generalisability: children initially identified as potentially eligible from the outpatient and emergency services of a large Government hospital in Karachi. Only male children enrolled (to allow for collection of urine and faeces separately), but it is likely that the results would be generalisable to girls. To be eligible children had to be ≤ 3 years, therefore, the results may not be generalisable to children aged 4–5 years old. The study ward had a research nurse and medical officer in constant attendance, this level of supervision may not be possible in all settings Outcome measures: primary outcomes were not explicitly identified. Methods for assessing outcomes were reported, and definitions for outcome measures such as treatment failure were provided Intercentre variability: not applicable Conflict of interest: NR |
|||||||
| Composition of buffalo milk + KY diet compared with the soy formula diet (based on feeding a 10 kg child at 120 kcal/kg/day) | |||||||
| KY milk | Soy formula | ||||||
| Khitchri | Yoghurt | Buffalo milk (half strength) | Total | ||||
| Volume, ml | 376 | 260 | 1025 | 1661 | 1790 | ||
| Calories, kcal | 444 | 156 | 600 | 1200 | 1200 | ||
| Carbohydrate, g | 71.4 | 10.4 | 25.6 | 107.4 | 118.2 | ||
| Protein, g | 12.4 | 8.1 | 22.6 | 43.1 | 35.8 | ||
| Fat, g | 11.7 | 10.4 | 45.1 | 67.2 | 64.5 | ||
| Other details |
Mixed amounts of khitchri and yoghurt were provided at a 3 : 1 ratio and approximately 50–60% of the daily caloric intake was provided by buffalo milk Khitchri (60 g rice, 30 g lentils, 10 g dry weight cottonseed oil and 1 g salt) prepared in bulk by cooking lentils in water with rice and oil added subsequently until a homogeneous consistency achieved. Aliquots frozen and distributed under supervision of a clinical nutritionist Yoghurt and buffalo milk obtained regularly from a single commercial source. Lactose content of yoghurt 3.0 g/dl, and of half-strength buffalo milk 2.5 g/dl |
One hundred grams of powder consisted of soy protein (15.5 g), glucose polymers (50 g), a fat blend (28 g) of equal amounts of corn oil and coconut oil, and the recommended dietary allowance of vitamins and minerals. Also fortified with l-methionine, taurine, and l-carnitine, and had an osmolality of 200 mOsm/kg | |||||
Quality assessment for primary studies (modified for severe malnutrition)
| A. Selection bias | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1. Are the individuals selected to participate in the study likely to be representative of the target population? | Very likely | Somewhat likely | Not likely | Cannot tell | |||||
| ✓ | |||||||||
| 2. What percentage of selected individuals participated? | 80–100% | 60–79% | < 60% | N/A | Cannot tell | ||||
| ✓ | |||||||||
| Summary of selection bias | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| B. Study design | |||||||||
| 1. What was the study design? | RCT | ✓ | |||||||
| (Please tick appropriate and specify design if categorise as ‘Other’) | CCT | ||||||||
| Cohort analytic (two group pre + post) | |||||||||
| Case–control | |||||||||
| Cohort [one group pre + post (before and after)] | |||||||||
| Interrupted time series | |||||||||
| Other – specify | |||||||||
| Cannot Tell | |||||||||
| 2. Was the study described as randomised? | Yes | No | |||||||
| ✓ | |||||||||
| If answer to no. 2 is ‘no’ complete summary then go to section C. Confounders. If answer is ‘yes’, answer no. 3 and no. 4 below, before completing summary for this section | |||||||||
| 3. If answer was yes, was the method of randomisation described? | Yes | No | |||||||
| ✓ | |||||||||
| 4. If answer was yes, was the method appropriate? | Yes | No | |||||||
| ✓ | |||||||||
| Summary of study design | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| C. Confounders | |||||||||
| 1. Were there important differences between groups prior to the intervention? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. If yes, indicate the percentage of relevant confounders that were controlled [either in the design (e.g. by stratification or matching) or in the analysis]? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| Summary of confounders | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| D. Blinding | |||||||||
| 1. Was the outcome assessor aware of the intervention or exposure status of participants? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were the study participants aware of the research question? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| Summary of blinding | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| E. Data collection methods | |||||||||
| 1. Were data collection tools shown to be valid? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were data collection tools shown to be reliable? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| Summary of data collection | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| F. Withdrawals and dropouts | |||||||||
| 1. Were withdrawals and dropouts reported in terms of numbers and reasons per group? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Indicate the percentage of participants completing the study (If the percentage differs by groups, record the lowest) | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
| Summary of withdrawals and dropouts | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| G. Intervention integrity | |||||||||
| 1. What percentage of participants received the allocated intervention or exposure of interest? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
| 2. Was the consistency of the intervention measured? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 3. Is it likely that subjects received an unintended intervention (contamination or co-intervention) that may influence the results? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| H. Analysis | |||||||||
| 1. Indicate the unit of allocation | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 2. Indicate the unit of analysis | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 3. Are the statistical methods appropriate for the study design? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 4. Is the analysis performed by intervention allocation status (i.e. ITT) rather than actual intervention received? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| Global rating for studya | Strong | Moderate | Weak | ||||||
| (Overall methodological strength of study – based on sections A–F) | ✓ | ||||||||
Nurko et al. 199756
| Reference and design | Intervention | Participants | Outcome measures | |||||
|---|---|---|---|---|---|---|---|---|
|
Author: Nurko et al. 56 Year: 1997 Country: Mexico Study design: double-blind RCT Setting: inpatient (Hospital Infantil de Mexico Federico Gómez, Mexico City) Number of centres: one Funding: part-funded by Applied Diarrhoeal Disease Research Project at Harvard University, by means of a co-operative agreement with the US Agency for International Development and in part by a National Institutes of Health grant (T32-DK 07703) |
Intervention 1: local chicken-based diet Intervention 2: soy diet – Nursoy (Wyeth Laboratories) Control: elemental diet – Vivonex Standard (Norwich Eaton) See end of table for details Diets differed in macronutrient composition and started at the lowest concentration (150 ml/kg/day) via a nasogastric tube and concentrations were advanced every 48 hours after initial overnight fast and hydration. Full concentration was achieved by the ninth day if no intolerance occurred, otherwise the concentration was either: maintained if there were 2% or 3% positive reducing substances (before or after hydrolysis) or if there was an increase in stool output of > 50% (> 20 ml/kg); or decreased if Clinitest results showed 4% or there was an increase of ≥ 75% in stool output (> 20 ml/kg). Cases received 7 days of the maximum diet concentration, followed by whole cows milk administered half-strength (10 ml/kg) and advanced to full strength if tolerated. Milk-tolerant cases continued with lactose-containing formula or whole milk, depending on age (no further details reported). If lactose-intolerant (i.e. return of liquid stools with pH < 5 and > 2% reducing substances in the stool, a milk-free diet was instituted Other interventions used: cases were hydrated on admission following WHO/UNICEF guidelines (standard glucose-electrolyte i.v. solution). When the maximum concentration of the diet was achieved, daily supplementation with 1 mg folic acid, 1 ml multivitamin (Poly-Vi-Sol), and 6 mg/kg elemental iron was added. Suspected systemic infections were treated with broad-spectrum i.v.-administered antibiotics. Otitis media, urinary tract infections and pneumonia were treated with appropriate antibiotics, and dysentery with trimethoprim–sulfamethoxazole and children infected with G. lamblia with metronidazole |
Definition of SAM: third-degree malnutrition of the marasmatic type as defined by the Gómez criteria. W/A < 60% of the NCHS 50th percentile Number of participants: n = 56 (enrolled, n = 60; chicken, n = 19; Nursoy, n = 19; Vivonex, n = 18) Sample attrition/dropout: n = 15 (27%) treatment failures (Chicken, n = 4; Nursoy, n = 6; Vivonex, n = 5). Of these five died (Chicken, n = 2; Nursoy, n = 1; Vivonex, n = 2) and 10 successfully managed and discharged home Sample crossovers: none, although 15 treatment failures (see Sample attrition/dropout) changed diets (chicken and Nursoy for Vivonex; parental nutrition then enteral Vivonex for those originally on Vivonex diet). However, these children discharged home and were not counted as crossovers per se Inclusion criteria: children aged 3–36 months with third-degree malnutrition of the marasmatic type and persistent diarrhoea (defined as three or more loose stools for ≥ 14 days) Exclusion criteria:General characteristics of participants: children aged 3–36 months with SAM and PD Associated conditions on admission: 64% (non-gastrointestinal infection 50%, gastrointestinal infection 14.3%) |
Primary outcomes: not specifically reported Outcomes: diarrhoea status, weight, nitrogen balance, nutritional recovery, treatment success and failure Method of assessing outcomes: all measurements were obtained by trained nutritionists and their accuracy was validated before start of study. All intake/output was recorded, nasogastric tube was inserted by trained nursing staffDefinitions:Adverse symptoms: diet intolerance and intestinal pneumatosis Length of follow-up: NR, approximately 9 days if no intolerance to diet + addition 7 days Recruitment dates: NR |
|||||
| Characteristics of participants | ||||||||
| Characteristic | Chicken (n = 19) | Nursoy (n = 19) | Vivonex (n = 18) | Total (n = 56) | ||||
| Age, months (SD) | 6.7 (3.7) | 5.6 (4.0) | 6.9 (5.3) | 6.4 (4.4) | ||||
| Sex, n (M : F) | 10 : 9 | 11 : 8 | 9 : 9 | 30 : 26 | ||||
| Initial weight, g (SD) | 3647.3 (884.4) | 3575.3 (1397.1) | 3589.8 (1393.5) | 3604.1 (1232) | ||||
| Per cent W/A (% NCHS) (SD) | 50.8 (7.4) | 51.0 (7.5) | 52.9 (7.5) | 51.4 (7.2) | ||||
| Weight z-score (SD) | –4.2 (1.0) | –3.9 (0.7) | –4.0 (1.2) | –4.0 (1.0) | ||||
| Diarrhoea duration, days (SD) | 36.6 (3.9) | 48.7 (5.1) | 41.8 (4.0) | 42.4 (4.4) | ||||
| Severe dehydration, n (%) | 4 (21.1) | 5 (26.3) | 6 (33.3) | 15 (26.8) | ||||
| Faecal output, ml/kg/day (SD) – first 24 hours | 41.6 (12.1) | 45.8 (13.6) | 52.3 (19.6) | 46.4 (15.1) | ||||
| Laboratory tests, (SD) | ||||||||
| Sodium, mmol/l | 135.3 (7.8) | 138.3 (6.9) | 137.6 (6.1) | 137.1 (6.9) | ||||
| Potassium, mmol/l | 3.9 (0.8) | 4.2 (1.0) | 4.3 (0.9) | 4.1 (0.9) | ||||
| Bicarbonate, mEq/l | 15.5 (3.4) | 15.1 (3.8) | 16.5 (5.3) | 15.7 (4.2) | ||||
| Blood urea nitrogen, mg/dl | 22.0 (8.6) | 24.8 (14.9) | 29.2 (14.6) | 24.9 (13.1) | ||||
| Albumin, g/dl | 3.0 (0.6) | 3.3 (0.6) | 3.2 (0.6) | 3.2 (0.6) | ||||
| D-xylose, mg/dl | 22.1 (7.8) | 19.0 (10.5) | 25.6 (13.9) | 22.2 (11.0) | ||||
| Associated conditions on admission, n (%) | ||||||||
| None | 6 (31.6) | 9 (47.4) | 5 (27.8) | 20 (35.7) | ||||
| Sepsis | 5 (26.3) | 7 (36.8) | 5 (27.8) | 17 (30.4) | ||||
| Urinary tract infection | 2 (10.5) | 3 (15.8) | 2 (11.1) | 7 (12.5) | ||||
| Pneumonia | 2 (10.5) | 0 | 2 (11.1) | 4 (7.1) | ||||
| + stool culture | 2 Shigella (10.5) | 0 | 2 Shigella, 1 Salmonella (16.6) | 5 (8.9) | ||||
| + stool ova and parasites | 1 G. lamblia (5.2) | 0 | 1 Cryptosporidium (5.5) | 2 (3.6) | ||||
| + stool culture + ova and parasites | 1 Salmonella and Cryptosporidium (5.2) | 0 | 0 | 1 (1.7) | ||||
|
Comments: results are mean (± SD) unless otherwise stated No significant differences between groups (p-values NR) |
||||||||
| Results | ||||||||
| Outcomes | Chicken (n = 15) | Nursoy (n = 13) | Vivonex (n = 13) | p-value | ||||
| Diarrhoea status, (SD) | ||||||||
| Mean total stool output/kg/day | 19.1 (7.5) | 18.5 (6.6) | 18.8 (9.2) | NS | ||||
| Mean stools/day (SD) | 3.2 (1.2) | 2.5 (0.7) | 3.4 (1.3) | |||||
| Day of cessation (SD) | 6.9 (4.7) | 3.9 (3) | 8 (5.1) | NS | ||||
| Weight, g (SD) | ||||||||
| At admission | 3572 (823) | 3270 (1167) | 3764 (1575) | |||||
| At end of protocol | 3736 (870)b | 3495 (1172)b | 3940 (1599)b | |||||
| At time of discharge | 4133 (1160)c | 3797 (1128)c | 4225 (1706)c | |||||
| Mean number of total calories/kg/day after full diet tolerated (SD) | 116.0 (9.6) | 111.3 (9.1) | 115.2 (8.3) | NS | ||||
| Protein/kg/day ingested after full diet tolerated, g (SD) | 3.5 (0.4) | 3.4 (0.3) | 2.4 (0.2) | < 0.05 | ||||
| Nitrogen balance, mg/kg /day (SD) | 358.2 (13)d | 291.4 (111.6) | 226.6 (61.2) | |||||
| Per cent absorption | 86.0 (10.8) | 85.9 (8.5) | 89.5 (5.4) | |||||
| Per cent retention | 60.7 (19.3) | 50.9 (16.8) | 59.3 (14.0) | |||||
| Biological value | 69.7 (17.3) | 58.7 (16.8) | 66.1 (14.4) | |||||
| Nutritional recovery, n (%) | 13 (86.6) | 12 (85) | 10 (77) | |||||
| Successful outcome, n (%) | 15 (78.9) | 13 (68.4) | 13 (72.2) | NS | ||||
| Safety, n (%)e | ||||||||
| Some formula intolerance | 9 (47.4) | 11 (57.9) | 14 (77.8) | NS | ||||
| Treatment failure, n | 4 | 6 | 5 | |||||
| Mean time from diet start to failure, hours (SD) | 97.5 (99.9) | 98.5 (99.9) | 60.6 (45.7) | NS | ||||
| Intestinal pneumatosis, n | 1 | 1 | 2 | |||||
| Death, n | 2 | 1 | 2 | NS | ||||
|
Comments: Chicken group had a significantly higher nitrogen balance (p < 0.02) and states tendency towards a higher number of nutritional recoveries (NS), but no p-value reported Results per serum albumin and d-xylose concentration, electrolyte abnormalities and results for milk tolerance tests were also reported, but not data extracted Treatment success all: n = 41 (73.2%) Formula intolerance all: n = 34/56 (61%), of which transient formula intolerance n = 19/34 (56%) Treatment failure all: 15 (44%) Mean time from diet start to failure all: 85.6 (72 hours); one treatment failure (Nursoy) was because of allergy to the formula, 10 treatment failures were successfully managed: Mean stay (SD): 50 (30) days Death all: n = 5 (8.9%) because of intestinal pneumatosis (n = 2), central line-associated sepsis (n = 2), bacterial sepsis (K. pneumoniae) (n = 1) Sodium concentration < 130 mmol/l (RR 3.07, 95% CI 1.41 to 6.64) and presence of associated infections (RR 3.61, 95% CI 1.1 to 14.42), particularly Crytosporidium (RR 4.15, 95% CI 1.53 to 6.9), were associated with treatment failure Significant differences (p < 0.05) between treatment success and failure associated with albumin (3.2 vs 2.9 g/dl), sodium concentration (138.4 vs 133.5 mmol/l) and the incidence of associated infections (56.1 vs 86.7%). There were additional differences in stool output on the second day (20.9 vs 47.4 ml/kg) and third day (16.7 vs 54.0). Differences in serum albumin and d-xylose concentration, electrolyte abnormalities and results for milk tolerance tests were also reported, but not data extracted Intestinal pneumatosis all: 7.14% |
||||||||
| HIV: N/A | ||||||||
| Barriers to implementation | ||||||||
| None reported | ||||||||
| Methodological comments | ||||||||
|
Allocation to treatment groups: cases randomly assigned to treatment using a table of random numbers Blinding: only the nutritionist who prepared the formula was aware of assignment group. Investigators, nurses and residents remained masked to the type of diet. Aluminium foil was used to cover the formula bag and tubing. Code was broken for treatment failures and diet changed Comparability of treatment groups: states no significant differences between groups (no p-values reported), but Nursoy group was slightly younger and had higher percentage of children without associated conditions or infections with parasites on admission Method of data analysis: descriptive analyses were used to define the presenting characteristics. To test differences between the groups, multivariate and repeated-measures analyses of variance were used. The data were transformed if they were not normally distributed (no further details reported). Duration of the diarrhoea was compared using survival analysis and chi-squared tests used for categorical variables. For small cells, the Fisher’s exact test was used (no definition of small cells was given). Statistical analysis were performed using SPSS/PC and Epi-Info software (version 5.01; Centers for Disease Control and Prevention, Atlanta, GA, USA), with significance assumed when p < 0.05 Sample size/power calculation: it was calculated that a sample size of 20 children per group would be needed assuming a power of 0.80, an alpha of 0.05 and a difference of 30% in the duration of diarrhoea (no further details reported). A separate analysis was performed to confirm successful separation of stool and urine in girls for all the variables associated with the stool collection at the end of the study. As no differences between sexes were found (data not shown), all data were pooled, however, the analysis is unlikely to be powered Attrition/dropout: numbers and reasons per treatment group reported |
||||||||
| General comments | ||||||||
|
Generalisability: SAM defined using the Gómez criteria of W/A < 60% of the NCHS’s 50th percentile would appear to meet the WHO criteria for SAM. To be eligible children had to hospitalised in a children’s hospital in Mexico, be aged 3–36 months with third-degree malnutrition of the marasmatic type and persistent diarrhoea. The results may not be generalisable to younger or older children Outcome measures: appear to be suitable and appropriate. Primary outcomes were not explicitly identified, but methods for assessing outcomes were reported and definitions for outcome measures such as treatment failure were provided Intercentre variability: N/A, one centre only Conflict of interest: none reported |
||||||||
| Composition of diets at maximum concentration | Chicken | Nursoy | Vivonex | |||||
| Total calories, kcal/dl | 85.6 | 82.0 | 84.87 | |||||
| Protein, g/dl | 1.7 | 2.5 | 2.6 | |||||
| Carbohydrate, g/dl | 19.5 | 8.3 | 10.7 | |||||
| Fat, g/dl | 0.1 | 4.3 | 3.5 | |||||
| Sodium, mEq/dlf | 1.7 | 1.3 | 1.6 | |||||
| Potassium, mEq/dlg | 2.5 | 2.3 | 2.2 | |||||
| Calcium, mg/dl | 47 | 72 | 47 | |||||
| Phosphorus, mg/dl | 47 | 50 | 47 | |||||
| Magnesium, mg/dl | 19 | 8 | 18 | |||||
| Zinc, mg/dl | 0.78 | 0.65 | 0.11 | |||||
| Osmolarity, mOsm/l | 420 | 292 | 292 | |||||
| Percentage of total calories | ||||||||
| Protein | 7.94 | 12.1 | 12.2 | |||||
| Carbohydrate | 90.9 | 40.4 | 50.5 | |||||
| Fat | 1.12 | 47.1 | 37.2 | |||||
| Total calories/total protein per dayh | 128.4/2.6 | 123.0/3.8 | 127.4/3.9 | |||||
| Diet was designed with the use of food composition tables: 8 g boiled comminuted chicken breast; 3 ml vegetable cooking oil; 10.5 g table sugar. Components were blended and minerals added: 5 ml calcium gluconate (10% solution, PISA); 2.7 ml of dibasic sodium phosphate (PISA); 1.7 ml of magnesium sulphate (10% solution, PISA). Boiled water was added to achieve the total volume required (150 ml/kg per day) | Soy formula contained soy protein, coconut, safflower and soy oils, sucrose, minerals and vitamins |
Vivonex contains crystalline amino acids, glucose and glucose oligosaccharides, a small amount of highly purified safflower oil, electrolytes, minerals, micronutrients and vitamins Starting at 150 ml/kal per day in a concentration that provides 47.8 kcal/dl (12.5% weight/volume) and advancing slowly by 2.5% per day to a maximum concentration of 85.6 kcal/dl (22.5% weight/volume) |
||||||
| After the milk challenge, all cases restarted a complete age-appropriate, complex-balanced diet, continued until discharge. All diets were prepared in the paediatric nutrition kitchen of the hospital under the supervision of a trained nutritionist | ||||||||
Quality assessment for primary studies (modified for severe malnutrition)
| A. Selection bias | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1. Are the individuals selected to participate in the study likely to be representative of the target population? | Very likely | Somewhat likely | Not likely | Cannot tell | |||||
| ✓ | |||||||||
| 2. What percentage of selected individuals participated? | 80–100% | 60–79% | < 60% | N/A | Cannot tell | ||||
| ✓ | |||||||||
| Summary of selection bias | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| B. Study design | |||||||||
| 1. What was the study design? | RCT | ✓ | |||||||
| (Please tick appropriate and specify design if categorise as ‘Other’) | CCT | ||||||||
| Cohort analytic (two group pre + post) | |||||||||
| Case–control | |||||||||
| Cohort [one group pre + post (before and after)] | |||||||||
| Interrupted time series | |||||||||
| Other – specify | |||||||||
| Cannot Tell | |||||||||
| 2. Was the study described as randomised? | Yes | No | |||||||
| ✓ | |||||||||
| If answer to no. 2 is ‘no’ complete summary then go to section C. Confounders. If answer is ‘yes’, answer no. 3 and no. 4 below, before completing summary for this section | |||||||||
| 3. If answer was yes, was the method of randomisation described? | Yes | No | |||||||
| ✓ | |||||||||
| 4. If answer was yes, was the method appropriate? | Yes | No | |||||||
| ✓ | |||||||||
| Summary of study design | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| C. Confounders | |||||||||
| 1. Were there important differences between groups prior to the intervention? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. If yes, indicate the percentage of relevant confounders that were controlled [either in the design (e.g. by stratification or matching) or in the analysis]? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| Summary of confounders | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| D. Blinding | |||||||||
| 1. Was the outcome assessor aware of the intervention or exposure status of participants? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were the study participants aware of the research question? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| Summary of blinding | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| E. Data collection methods | |||||||||
| 1. Were data collection tools shown to be valid? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were data collection tools shown to be reliable? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| Summary of data collection | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| F. Withdrawals and dropouts | |||||||||
| 1. Were withdrawals and dropouts reported in terms of numbers and reasons per group? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Indicate the percentage of participants completing the study (If the percentage differs by groups, record the lowest) | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
| Summary of withdrawals and dropouts | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| G. Intervention integrity | |||||||||
| 1. What percentage of participants received the allocated intervention or exposure of interest? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
| 2. Was the consistency of the intervention measured? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 3. Is it likely that subjects received an unintended intervention (contamination or co-intervention) that may influence the results? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| H. Analysis | |||||||||
| 1. Indicate the unit of allocation | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 2. Indicate the unit of analysis | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 3. Are the statistical methods appropriate for the study design? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 4. Is the analysis performed by intervention allocation status (i.e. ITT) rather than actual intervention received? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| Global rating for studya | Strong | Moderate | Weak | ||||||
| (Overall methodological strength of study – based on sections A–F) | ✓ | ||||||||
Appendix 10 Question 7: data extraction tables
Dubray et al. 200859
Data extraction table
| Reference and design | Intervention | Participants | Outcome measures | ||
|---|---|---|---|---|---|
|
Author: Dubray et al. 59 Year: 2008 Country: Sudan Study design: randomised, unblinded, superiority-controlled trial Setting: inpatient TFC Number of centres: one Funding: Médecins Sans Frontières |
Intervention: once daily i.m. injection of 75 mg/kg body weight/day of ceftriaxone for 2 days Control: twice daily oral amoxicillin (80 mg/kg/day) over 5 days (tablets or syrup) Other interventions used: when necessary, a second antimicrobial treatment was administered (as per the TFC protocols): ceftriaxone, chloramphenicol, cotrimoxazole, amoxicillin or metronidazole All participants received the same nutritional rehabilitation in three phases of increasing caloric intake:Standard treatment at the TFC could also include: vitamin A, mebendazole, folic acid and iron supplementation (the latter given 2 weeks after admission) Dehydration treated according to guidelines for SAM using rehydration solution salts (ReSoMal, Nutriset) Vaccinations were completed according to the Sudanese national immunisation schedule |
Definition of SAM:Number of participants: n = 460 randomised (458 in ITT analysis) [intervention ceftriaxone n = 230 (but two secondarily excluded so only 228 in ITT analysis), per protocol analysis n = 140, control amoxicillin n = 230 (ITT), n = 141 (PP)] Sample attrition/dropout: ceftriaxone intervention had 21 defaulters, one rescue treatment and 66 owing to concomitant treatment; amoxicillin control had 12 defaulters, 8 rescue treatment, 62 concomitant treatment and 7 both rescue and concomitant treatment Sample crossovers: NR Inclusion criteria: severely malnourished, weight ≥ 5 kg, 65 cm < height ≤ 109.9 cm (usually corresponding to age 6–59 months) Exclusion criteria: parents who refused permission to participate; treatment with any of the study drugs in the 7 days before admission; admission in the last 7 days to any health facility for SAM; known hypersensitivity to amoxicillin or ceftriaxone; decision by the physician to use a different antimicrobial drug on admission; AOM or severe complications [ongoing vomiting; severe infections; respiratory distress and shock (hypovolaemic or septic); history of a convulsion or impaired consciousness in the 24 hours preceding admission] diagnosed on admission General characteristics of participants: comprised internally displaced population mainly from southern Sudan |
Primary outcomes:Secondary outcomes:Definitions:Method of assessing outcomes:Length of follow-up: not clearly stated, but appears to be to exit from TFC Recruitment dates: January 2002 to September 2003 |
||
| Characteristics of participants | |||||
| Characteristic | Intervention, ceftriaxone (n = 228) | Control, amoxicillin (n = 230) | p-value | ||
| ITT analysis a | n (%) | n (%) | |||
| Age (months) | |||||
| Mean (SD) | 17 (7) | 18 (8) | NR | ||
| Median (IQR) | 16 (12–20) | 18 (12–23) | NR | ||
| Male | 119 (52.2%) | 127 (55.2%) | NR | ||
| W/H % < 70%b | 169 (74.1%) | 166 (72.1%) | NR | ||
| Bilateral oedema | 23 (10.1%) | 28 (12.2%) | NR | ||
| MUAC < 110 mmc | 36 (15.8%) | 36 (15.7%) | NR | ||
| Feverd | 70 (30.7%) | 67 (29.1%) | NR | ||
| Abnormal respiratory ratee | 41 (18.0%) | 40 (17.4%) | NR | ||
| Moderate dehydration | 33 (14.5%) | 23 (10.1%) | NR | ||
| Paracheck positive | 4 (1.9%) | 2 (0.9%) | NR | ||
| Hb < 8 g/dl | 37 (16.4%) | 41 (18.1%) | NR | ||
| Comments: baseline characteristics for the per protocol groups were also reported, but have not been data extracted | |||||
| Results | |||||
| Primary outcomes | Intervention, ceftriaxone (n = 228) | Control, amoxicillin (n = 230) | p-value | ||
| ITT analysis | n (%) | n (%) | |||
| Success ratef | 127 (55.7) | 123 (53.5) | 0.63 | ||
| Difference | 2.2% (95% CI –6.9 to 11.3) | ||||
| Mean overall weight gain (g/kg/day) | 11.4 (95% CI 10.5 to 12.2) | 11.2 (95% CI 10.2 to 11.9) | 0.69 | ||
|
Comments: subgroup analyses of success rate and weight gain according to admission criteria (W/H per cent < 70%, bilateral oedema or MUAC < 110 mm) and age (6–23 months and 24–59 months) are presented, but have not been data extracted. It is not stated whether or not these subgroups were pre-specified and the study may not have been powered for these subgroup analyses A per protocol analysis (and subgroup analyses by baseline characteristics and age) of the primary outcome was also reported, but has not been data extracted The median time from admission to first weight gain was 1 day in both groups (p = 0.33). Median time spent in phase one of treatment was 5 days in the amoxicillin group and 4 days in the ceftriaxone group (p = 0.4) |
|||||
| Secondary outcomes | Intervention: ceftriaxone (n = 228) | Control: amoxicillin (n = 230) | p-value | ||
| ITT analysis | n (%) | n (%) | |||
| Deaths within 14 days after admissiong | 5 (2.2) | 8 (3.5) | NR | ||
| Total deaths during follow-uph | 7 (3.1) | 9 (3.9) | 0.62 | ||
| Overall CFR | 3.5% (16 deaths in 458 participants) | ||||
| Infection-related deaths after 14 days from admissioni | |||||
| Meningoencephalitis syndrome | 1 (26th day after admission) | 0 | NR | ||
| Severe respiratory infection | 0 | 1 (30th day after admission) | NR | ||
| Pulmonary TB | 1 (50th day after admission) | 0 | NR | ||
| Recovered | 170 (74.6) | 161 (70) | 0.27 | ||
| Defaulted | 43 (18.9) | 39 (17.0) | 0.59 | ||
| Referred | 2 (0.9) | 4 (1.7) | 0.68 | ||
| Weight gain at exit (g/kg/day) | 10.2 (9.7–10.7) | 10.2 (9.4–11.0) | 0.50 | ||
| Length of stayj (days) | 31.4 (29.4–33.3) | 33.5 (31.5–35.5) | 0.07 | ||
| Adverse eventsg | 2 (0.88) | 8 (3.5) | 0.05 | ||
| Vomiting | 1 | 1 | NR | ||
| Diarrhoea | 1 | 6 | NR | ||
| Facial oedema (allergic reaction) | 0 | 1 | NR | ||
| Safety: neither infection at injection site nor post-injection local pain was reported by the guardians or medical staff in the ceftriaxone group | |||||
| HIV: NR | |||||
| Barriers to implementation | |||||
| None reported | |||||
| Methodological comments | |||||
|
Antibiotic policy: the administration of systemic broad-spectrum antibiotic therapy on admission aimed at improving the outcomes of SAM (reduce mortality and improve nutritional response to feeding) Intervention administered to all participants (with or without infection) |
|||||
| Reported limitations | |||||
|
(i) The primary outcome (mean daily weight gain) was measured from the first day of weight gain. When weight began to increase, children might have already recovered from infections and therefore the primary outcome might no longer have depended on antibiotic treatment. However, the delay between admission and first weight gain (median time = 1 day) did not differ between the two treatment groups either (p = 0.33) (ii) More than 25% of children in each group received a second antimicrobial treatment (ceftriaxone, chloramphenicol, cotrimoxazole, amoxicillin or metronidazole). Prescriptions were in accordance with the TFC treatment protocols. Where bacteriological analyses are not available (culture and drug susceptibility), the presence or nature of infection cannot be verified (iii) Centre-acquired infections are a frequent source of complications (iv) Staff members might therefore be overcautious and overprescribe antibiotics when they suspect severe bacterial infections, which could attenuate any difference in the ITT analysis. In such a context, results of the per protocol analyses do not reflect the actual situation in the TFC where treatment for complications associated with SAM requires frequent adjustment (v) In 14 patients, amoxicillin was interrupted and replaced by ceftriaxone, in the majority because of respiratory infection, septic shock and allergy. In the absence of blinding, it is not unlikely that this stemmed from a lack of trust in amoxicillin and this switch might have contributed to the reduced difference in the ITT analysis Allocation to treatment groups: a computer-generated randomisation list of a 20-patient block (10 in each treatment group) was drawn by a statistician. A research assistant allocated the next available number to each child on entry to the trial and each number corresponded to a sealed envelope containing the allocated treatment. A nurse administered the treatment under the supervision by the research assistant Blinding: medical staff and patients’ guardians were not blinded to the allocated treatment |
|||||
| Comparability of treatment groups | |||||
| The distribution of baseline sociodemographic, anthropometric and clinical characteristics did not differ significantly between the groups. In both groups, the median time from admission to first weight gain was 1 day (p = 0.33). The median time spent in phase I was 5 days in the amoxicillin group and 4 days in the ceftriaxone group (p = 0.7) | |||||
| Method of data analysis | |||||
|
ITT analysis: included children who had received at least one dose of the study drug, therefore, not a true ITT (because of post-randomisation exclusion of two children) Differences in distributions between groups in the distribution of the baseline characteristics on admission and for secondary outcomes were tested using the chi-squared or Fisher’s exact test for categorical variables. For means and 95% CIs, the Student’s t-test (continuous variables, normal distribution) or Mann–Whitney non-parametric test (continuous variables, distribution not normal) was used Per protocol analysis: excluded from the denominator were children who defaulted before the primary outcome was measurable, children in whom the trial drug failed and had to be replaced by another antimicrobial drug (rescue treatment) and/or children who received one or more additional antimicrobial drug(s) (concomitant treatment) before they reached 14 days of weight gain (ceftriaxone, chloramphenicol, cotrimoxazole, amoxicillin or metronidazole). Results from the per protocol analysis have not been extracted |
|||||
| Sample size/power calculation | |||||
|
The objective of the study was to discover whether or not the intervention improved success in weight gain by at least 10%. Given a success rate of 80% in children receiving amoxicillin and 90% in those receiving ceftriaxone, and with a power of 80% and a one-sided significance level of 5%, the required sample size was calculated to be 177 children per group (a total of 354). The sample size was increased by 10% to adjust for losses to follow-up and for children who died or left the TFC before 14 days of weight gain because of default or referral to other sites (no primary outcome calculable). The final sample included 230 children in each group Attrition/dropout: of the 430 children who met the eligibility criteria, 230 were randomised to each treatment group. However, in the ITT analysis, only 228 participants were assigned to the ceftriaxone group, as one of the allocated children was withdrawn by the mother before the first injection and because another allocated child was secondarily diagnosed with AOM Twenty-four children in the amoxicillin group and 30 in the ceftriaxone group left the TFC before 14 days of weight gain because they had recovered, died, defaulted or were referred to other sites Treatment interruption was significantly more common in the amoxicillin group (17/230, 7.4%) than in the ceftriaxone group (1/228, 0.4%; p < 0.001). There were no significant differences in the administration of an additional treatment before 14 days of weight gain |
|||||
| General comments | |||||
|
Generalisability: the study site was chosen because the working conditions were satisfactory, the centre adhered to international standards of nutritional rehabilitation programmes, and the political situation was stable. Therefore, its results might not be applicable to centres with poorer operational conditions All children admitted to the centre meeting the SAM criteria were enrolled. The criteria used to define SAM were broadly in line with current WHO criteria Outcome measures: methods used for measuring anthropometric variables were given and definitions for outcomes such as ‘success’ were provided The primary outcome measures, needed to indicate how interventions impact mortality and nutritional response to feeding, were reported (mortality and weight gain). Additional outcomes of interest, such as time to recover (length of stay) and adverse effects associated to antibiotics, were reported as well However, no data on resolution of existing infections, development of new infections, relapse or development of antibiotic resistance outcomes seem to have been collected or reported. Only fatal infections were enumerated but without clearly identifying the treatment group in which they occurred Intercentre variability: not applicable Conflict of interest: no potential conflict of interest were reported or identified |
|||||
Quality assessment for primary studies (modified for severe malnutrition)
| A. Selection bias | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1. Are the individuals selected to participate in the study likely to be representative of the target population? | Very likely | Somewhat likely | Not likely | Cannot tell | |||||
| ✓ | |||||||||
| 2. What percentage of selected individuals participated? | 80–100% | 60–79% | < 60% | N/A | Cannot tell | ||||
| ✓ | |||||||||
| Summary of selection bias | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| B. Study design | |||||||||
| 1. What was the study design? | RCT | ✓ | |||||||
| (Please tick appropriate and specify design if categorise as ‘Other’) | CCT | ||||||||
| Cohort analytic (two group pre + post) | |||||||||
| Case–control | |||||||||
| Cohort [one group pre + post (before and after)] | |||||||||
| Interrupted time series | |||||||||
| Other – specify | |||||||||
| Cannot Tell | |||||||||
| 2. Was the study described as randomised? | Yes | No | |||||||
| ✓ | |||||||||
| If answer to no. 2 is ‘no’ complete summary then go to section C. Confounders. If answer is ‘yes’, answer no. 3 and no. 4 below, before completing summary for this section | |||||||||
| 3. If answer was yes, was the method of randomisation described? | Yes | No | |||||||
| ✓ | |||||||||
| 4. If answer was yes, was the method appropriate? | Yes | No | |||||||
| ✓ | |||||||||
| Summary of study design | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| C. Confounders | |||||||||
| 1. Were there important differences between groups prior to the intervention? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. If yes, indicate the percentage of relevant confounders that were controlled [either in the design (e.g. by stratification or matching) or in the analysis]? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| Summary of confounders | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| D. Blinding | |||||||||
| 1. Was the outcome assessor aware of the intervention or exposure status of participants? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were the study participants aware of the research question? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| Summary of blinding | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| E. Data collection methods | |||||||||
| 1. Were data collection tools shown to be valid? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were data collection tools shown to be reliable? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| Summary of data collection | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| F. Withdrawals and dropouts | |||||||||
| 1. Were withdrawals and dropouts reported in terms of numbers and reasons per group? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Indicate the percentage of participants completing the study (If the percentage differs by groups, record the lowest) | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
| Summary of withdrawals and dropouts | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| G. Intervention integrity | |||||||||
| 1. What percentage of participants received the allocated intervention or exposure of interest? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
| 2. Was the consistency of the intervention measured? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 3. Is it likely that subjects received an unintended intervention (contamination or co-intervention) that may influence the results? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| H. Analysis | |||||||||
| 1. Indicate the unit of allocation | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 2. Indicate the unit of analysis | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 3. Are the statistical methods appropriate for the study design? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 4. Is the analysis performed by intervention allocation status (i.e. ITT) rather than actual intervention received? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| Global rating for studya | Strong | Moderate | Weak | ||||||
| (Overall methodological strength of study – based on sections A–F) | ✓ | ||||||||
Trehan et al. 201060
Data extraction table
| Reference and design | Intervention | Participants | Outcome measures | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
Author: Trehan et al. 60 Year: 2010 Country: Malawi Study design: retrospective cohort with control Setting: home based Number of centres: NR. Two different feeding projects, one operating in one district of Malawi, the other operating in two districts of Malawi Funding: USA National Institutes of Health National Research Service Award (T32 HD049338) |
Intervention: amoxicillin (60 mg/kg/day, 7 day supply) + RUTF (175 kcal/kg/day) Control: RUTF (175 kcal/kg/day) Treatments with RUTF were given until child had a WHZ ≥ –2 and no peripheral oedema and for a minimum of 4 weeks and maximum of 12 weeks Other interventions used: none specified, although caretakers were referred to local health providers with any concerns about other acute illness. Caregivers educated about child’s illness and instructed on optimal feeding practices |
Definition of SAM: WHZ ≤ –3 and or presence of bilateral pitting oedema Number of participants: n = 2453 (amoxicillin + RUTF n = 498, RUTF n = 1955) Sample attrition/dropout: defaulters at 4 weeks amoxicillin n = 26 (5.2%), RUTF n = 121 (6.2%). Defaulters at 12 weeks amoxicillin n = 39 (7.8%), RUTF n = 182 (9.3%) Sample crossovers: none Inclusion criteria: children aged 6–59 months, uncomplicated SAM, with good appetite, qualified for outpatient treatment, attending two clinics between 2003–5 Exclusion criteria: children with poor appetite, altered mental status, compromised perfusion, respiratory distress or who were being transferred from inpatient to outpatient therapy were excluded General characteristics of participants: children aged 6–59 months with a SAM from rural subsistence farming villages in Malawi |
Primary outcomes: nutritional recovery rate (WHZ > –2 without oedema) Secondary outcomes: survival, WHZ, WAZ, HAZ and presence of oedema Method of assessing outcomes: data collected on presentation at the clinic by nurses and trained health professionals. Length, weight and MUAC measured and pedal oedema assessed by pressing thumb on dorsa of both feet for 5 seconds and noting visible pitting. Children assessed every 1–2 weeks. If children missed two follow-up visits they were categorised as defaulters Adverse symptoms: not stated Length of follow-up: between 4 and 12 weeks Recruitment dates: 2003–5 |
||||||
| Characteristics of participants | |||||||||
| Characteristic | Amoxicillin + RUTF (n = 498) | RUTF (n = 1955) | p-value | ||||||
| Oedema | 388 (77.9%) | 1574 (80.5%) | NS | ||||||
| Age (months) | |||||||||
| Overall | 25.5 ± 11.7 | 22.3 ± 10.6 | < 0.0001 | ||||||
| With oedema | 27.3 ± 12.0 | 23.3 ± 10.8 | < 0.0001 | ||||||
| Without oedema | 19.1 ± 7.9 | 18.0 ±9 0 | NS | ||||||
| Sex [female n (%)] | |||||||||
| Overall | 246 (49.4) | 986 (50.4) | NS | ||||||
| With oedema | 195 (50.3) | 849 (53.9) | NS | ||||||
| Without oedema | 51 (46.4) | 138 (36.2) | NS | ||||||
| WHZ | |||||||||
| Overall | –1.99 ± 1.26 | –1.91 ± 1.45 | NS | ||||||
| With oedema | –1.62 ± 1.15 | –1.49 ± 1.25 | NS | ||||||
| Without oedema | –3.28 ± 0.67 | –3.64 ± 0.77 | NS | ||||||
| HAZ | |||||||||
| Overall | –3.41 ± 1.45 | –3.18 ± 1.68 | 0.0059 | ||||||
| With oedema | –3.33 ± 1.44 | –3.06 ± 1.64 | 0.0026 | ||||||
| Without oedema | –3.67 ± 1.47 | –3.69 ± 1.74 | NS | ||||||
| WAZ | |||||||||
| Overall | –3.51 ± 1.20 | –3.05 ± 1.36 | < 0.0001 | ||||||
| With oedema | –3.19 ± 1.10 | –2.72 ± 1.23 | < 0.0001 | ||||||
| Without oedema | –4.63 ± 0.82 | –4.41 ± 1.00 | 0.0380 | ||||||
| Comments: p > 0.05, values for WHZ, HAZ and WAZ are mean ± SD | |||||||||
| Results | |||||||||
| Primary outcomes | 4 weeks Amoxicillin + RUTF |
RUTF | p-value | 12 weeks Amoxicillin + RUTF |
RUTF | p-value | |||
| Recovered, n (%) | |||||||||
| Overall | 198 (39.8) | 1385 (70.8) | NR | 417 (83.7) | 1673 (85.6) | NR | |||
| With oedema | 170 (43.8) | 1206 (76.6) | < 0.001 | 336 (86.6) | 1385 (88.0) | NR | |||
| Without oedema | 28 (25.5) | 179 (47.0) | < 0.001 | 81 (73.6) | 288 (75.6) | NR | |||
| Remained malnourished, n (%) | |||||||||
| Overall | 264 (53.0) | 423 (21.6) | NR | 29 (5.8) | 66 (3.4) | NR | |||
| With oedema | 191 (49.2) | 254 (16.1) | NR | 13 (3.4) | 36 (2.3) | NR | |||
| Without oedema | 73 (66.4) | 169 (44.4) | NR | 16 (14.5) | 30 (7.9) | NR | |||
| Died, n (%) | |||||||||
| Overall | 10 (2.0) | 26 (1.3) | NR | 13 (2.6) | 34 (1.7) | NR | |||
| With oedema | 8 (2.1) | 16 (1.0) | NR | 10 (2.6) | 19 (1.2) | NR | |||
| Without oedema | 2 (1.8) | 10 (2.6) | NR | 3 (2.7) | 15 (3.9) | NR | |||
| Defaulted, n (%) | |||||||||
| Overall | 26 (5.2) | 121 (6.2) | NR | 39 (7.8) | 182 (9.3) | NR | |||
| With oedema | 19 (4.9) | 98 (6.2) | NR | 29 (7.5) | 134 (8.5) | NR | |||
| Without oedema | 7 (6.4) | 23 (6.0) | NR | 10 (9.1) | 48 (12.6) | NR | |||
| Comments: at 12 weeks, the overall proportion who recovered in each group was described as similar. Rates of death and defaulting were described as similar between the two groups at 4 and 12 weeks | |||||||||
| Secondary outcomes | |||||||||
| Regression analysis Exploratory variable |
4 weeksa Exp(β)§ (95% CI) |
p-value | Up to 12 weeksb Exp(β) (95% CI) |
p-value | |||||
| Age (months) | 1.02 (1.01 to 1.03) | < 0.001 | 1.01 (1.00 to 1.02) | NS | |||||
| WHZ | 1.72 (1.30 to 2.28) | < 0.001 | 1.30 (0.93 to 1.82) | NS | |||||
| WAZ | 0.83 (0.55 to 1.25) | NS | 1.15 (0.70 to 1.90) | NS | |||||
| HAZ | 1.22 (0.97 to 1.53) | NS | 1.05 (0.79 to 1.38) | NS | |||||
| Presence of oedema | 1.29 (0.99 to 1.69) | NS | 1.08 (0.79 to 1.48) | NS | |||||
| Received amoxicillin | 0.22 (0.17 to 0.28) | < 0.001 | 0.90 (0.65 to 1.25) | NS | |||||
|
Comments: in the subgroup of children who recovered after 4 weeks the WHZ was significantly higher in the RUTF group than those in the Amoxicillin + RUTF group (–0.37 vs –0.75; p < 0.0001) § the exponentiated β coefficient corresponds to change in odds resulting from a unit change in the predictor variable with all other variables are held constant. Values > 1 indicate that as the predictor variable increases, the odds of recovery increases. Values < 1 indicate that as the predictor variable increases, the odds of recovery decreases p > 0.05 Seven cases had incomplete information and were omitted from the model. It is not clear if defaulters were also omitted from the model |
|||||||||
| Safety: NR | |||||||||
| HIV: NR for the study cohorts, although authors note that HIV infection rates differed in the district using amoxicillin (7% inferred from mortality rate) to that in districts using RUTF (rates expected to be) 15% and 16.5% | |||||||||
| Barriers to implementation | |||||||||
| NR | |||||||||
| Methodological comments | |||||||||
|
Allocation to treatment groups: non-random allocation Blinding: not applicable Comparability of treatment groups: children receiving amoxicillin + RUTF were older, more stunted (lower HAZ) and more underweight (lower WAZ) Method of data analysis: continuous variables – mean and SD; dichotomous variables – number and per cent. WAZ, HAZ and WHZ were calculated using the US NCHS/WHO International Growth Reference standards (NCHS 1977). Enrolment and recovery characteristics were compared using Student’s t-test for continuous parameters and Fisher’s exact test for dichotomous parameters. Length measurements were converted to height measurements for children > 2 years by subtracting 0.5 cm from length measurements over 85 cm. Recovery rates were compared using logistic regression modelling while controlling for baseline variables Sample size/power calculation: a sample of 400 children per group was calculated to detect a difference of at least 5% on the recovery rate Attrition/dropout: results show that 39 children (7.8%) receiving amoxicillin + RUTF and 182 children (9.3%) receiving RUTF only defaulted from the study by 12 weeks |
|||||||||
| General comments | |||||||||
|
Generalisability: it was felt that as most patients had kwashiorkor and mild oedema, that the results were not generalisable to those with marasmus Outcome measures: yes Intercentre variability: differences in study populations were examined. Centre differences within each feeding programme are not specifically mentioned, but assumed to be minimal. Differences between feeding programmes in addition to use of antibiotics are discussed Conflict of interest: study funded by USA National Institutes of Health National Research Service Award (T32 HD049338). No other competing interests |
|||||||||
Quality assessment for primary studies (modified for severe malnutrition)
| A. Selection bias | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1. Are the individuals selected to participate in the study likely to be representative of the target population? | Very likely | Somewhat likely | Not likely | Cannot tell | |||||
| ✓ | |||||||||
| 2. What percentage of selected individuals participated? | 80–100% | 60–79% | < 60% | N/A | Cannot tell | ||||
| ✓ | |||||||||
| Summary of selection bias | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| B. Study design | |||||||||
| 1. What was the study design? | RCT | ||||||||
| (Please tick appropriate and specify design if categorise as ‘Other’) | CCT | ||||||||
| Cohort analytic (two group pre + post) | ✓ | ||||||||
| Case–control | |||||||||
| Cohort [one group pre + post (before and after)] | |||||||||
| Interrupted time series | |||||||||
| Other – specify | |||||||||
| Cannot Tell | |||||||||
| 2. Was the study described as randomised? | Yes | No | |||||||
| ✓ | |||||||||
| If answer to no. 2 is ‘no’ complete summary then go to section C. Confounders. If answer is ‘yes’, answer no. 3 and no. 4 below, before completing summary for this section | |||||||||
| 3. If answer was yes, was the method of randomisation described? | Yes | No | |||||||
| 4. If answer was yes, was the method appropriate? | Yes | No | |||||||
| Summary of study design | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| C. Confounders | |||||||||
| 1. Were there important differences between groups prior to the intervention? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. If yes, indicate the percentage of relevant confounders that were controlled [either in the design (e.g. by stratification or matching) or in the analysis]? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
| Summary of confounders | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| D. Blinding | |||||||||
| 1. Was the outcome assessor aware of the intervention or exposure status of participants? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were the study participants aware of the research question? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| Summary of blinding | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| E. Data collection methods | |||||||||
| 1. Were data collection tools shown to be valid? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were data collection tools shown to be reliable? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| Summary of data collection | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| F. Withdrawals and dropouts | |||||||||
| 1. Were withdrawals and dropouts reported in terms of numbers and reasons per group? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Indicate the percentage of participants completing the study (If the percentage differs by groups, record the lowest) | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
| Summary of withdrawals and dropouts | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| G. Intervention integrity | |||||||||
| 1. What percentage of participants received the allocated intervention or exposure of interest? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
| 2. Was the consistency of the intervention measured? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 3. Is it likely that subjects received an unintended intervention (contamination or co-intervention) that may influence the results? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| H. Analysis | |||||||||
| 1. Indicate the unit of allocation | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 2. Indicate the unit of analysis | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 3. Are the statistical methods appropriate for the study design? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 4. Is the analysis performed by intervention allocation status (i.e. ITT) rather than actual intervention received? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| Global rating for studya | Strong | Moderate | Weak | ||||||
| (Overall methodological strength of study – based on sections A–F) | ✓ | ||||||||
Appendix 11 Question 14: data extraction tables
Chapko et al. 199462
Data extraction table
| Reference and design | Intervention | Participants | Outcome measures | ||
|---|---|---|---|---|---|
|
Author: Chapko et al. 62 Year: 1994 Country: Niger Study design: CCT Setting: inpatient and outpatient Number of centres: 12 (1 inpatient and 11 outpatient) Funding: partly supported by a Fulbright Fellowship to Dr Chapko |
Intervention: home based, with daily ambulatory rehabilitation at 1 of 11 centres distributed around Niamey (capital of Niger) see end of table for details) Control: hospital-based rehabilitation (special 20-bed section reserved for malnourished children in the National Hospital, Niamey) see end of table for details) Other interventions used: none reported |
Definition of SAM: defined according to WHO (1986),94 i.e. children with W/H between –2 and –3 SD = moderate acute malnutrition, < –3 SD = severe wasting; H/A between –2 and –3 SD = moderate chronic malnutrition or stunting, < –3 SD = severe stunting Number of participants: n = 100 (home based n = 47, hospital based: n = 53) Sample attrition/dropout: n = 14 (14%; four during first 15 days of follow-up, two between days 15 and 30, four between days 30 and 60, two between days 60 and 90 and two between days 90 and 180 Sample crossovers: none Inclusion criteria: discharge from paediatric service of the hospital (occurred when conditions such as diarrhoea, dehydration, bronchitis or other acute conditions were resolved) W/H < –2 SD or diagnosis of kwashiorkor residence within Niamey, mother agreed to child’s randomisation to either hospital or ambulatory rehabilitation Exclusion criteria: none reported General characteristics of participants: children with SAM discharged from hospital after treatment for conditions such as diarrhoea, dehydration, bronchitis or other acute conditions, but still W/H –2 SD or diagnosis of kwashiorkor and resident within the capital city of Niger |
Primary outcomes: not specifically reported Outcomes:Method of assessing outcomes: details of general condition, symptoms and diagnosis at entry to paediatric service (i.e. initial inpatient treatment prior to discharge and entry to trial), length of stay and anthropometric measures at entry and discharge were abstracted from medical records Anthropometric assessment of child and mother was through interview by research personnel at discharge from paediatric service. Information obtained from mother: child’s age and sex, mother’s age and education, and feeding practices Follow-up anthropometric assessment of child [weight (kg), height (cm), age (months) and sex] and brief interviews with mother were obtained by research personnel in child’s home or in hospital at 15, 30, 60, 90 and 180 days post-discharge from paediatric service A computer program available from the Centers of Disease Control, Atlanta, GA, USA was used to calculate WHZ and HAZ expressed as SD were used to report W/H and H/A Details of cost of care calculations were reported, but not extracted Adverse symptoms: none reported Length of follow-up: 6 months (15, 30, 60, 90 and 180 days) Recruitment dates: March 1990 to April 1991 |
||
| Characteristics of participants | |||||
| Characteristic | All (NR separately for each group) | ||||
| Age range, months | 5 –28 | ||||
| Age, months % | |||||
| 5–6 | 6 | ||||
| 7–12 | 45 | ||||
| 13–18 | 29 | ||||
| 19–24 | 17 | ||||
| > 24 | 3 | ||||
| Sex, M : F % | 54 : 46 | ||||
| Median W/H SD | –3.16 | ||||
| W/H between –2 and –3 SD % | 33 | ||||
| W/H < –3 SD % | 59 | ||||
| Marasmus % | 89 | ||||
| Kwashiorkor % | 9 | ||||
| Mixed % | 2 | ||||
| Median age of mothers, years (range) | 26 (18–52) | ||||
| Still nursing % | 58 | ||||
| Comments: at entry to the hospital’s paediatrics service (initial inpatient treatment prior to discharge and randomisation into the trial), median W/H was –3.38 SD, median H/A –2.22 SD, 76% with marasmus, 14% with kwashiorkor and 10% mixed. Length of hospitalisation prior to nutritional rehabilitation was a median of 7 (range 1–43) days. Details of condition and presenting symptoms prior to randomisation were reported, but not data extracted. W/H between –2 and –3 SD was reported as 33% and W/H < –3 SD as 59%, leaving 8% unaccounted for. It is unclear if this was because details for the 8% were missing or if the 8% did not fit into the two categories | |||||
| Results | |||||
| Outcomes | Home based (n = 47) | Hospital based (n = 53) | p-value | ||
| Deatha | 33% | 41% | 0.172 | ||
| Hospital, mean daysb | 2.2 | 12.9 | < 0.001 | ||
| Ambulatory, mean daysb | 11.9 | 5.6 | < 0.01 | ||
|
Comments: data on location of care indicated that some patients did not receive the assigned care, for example 11% of those assigned to ambulatory treatment received hospital rehabilitation at the insistence of their mothers No significant differences of H/A at follow-up between treatment arms (no data or p-value reported) A figure in the paper presented the comparison of W/H between the home-based and hospital-based groups. Data for those who died was presented separately to the data for those who survived. The paper reports that within both the group that survived and the group that died, there was no significant difference between the home-based and hospital-based groups in W/H (no p-value reported) Comparison of W/H were also made between children who survived, those lost to follow-up and those who died, and an analysis of W/A > 6 months of follow-up in children who survived, but these were not compared between study groups, data not extracted Results of utilisation and cost were also reported. None of these results were data extracted |
|||||
| Safety: NR | |||||
| HIV: NR | |||||
| Barriers to implementation | |||||
|
States that there are indications that some children assigned to hospital or ambulatory rehabilitation did not receive the assigned care. Of those assigned to ambulatory rehabilitation, 11% received hospital rehabilitation at the insistence of their mothers Also states that no extra resources were allocated to either setting or that the findings might have been different if more resources were available for the programmes |
|||||
| Methodological comments | |||||
|
Allocation to treatment groups: states children randomised to either hospital or ambulatory setting after discharge from paediatric service (no details of procedure) Blinding: none reported Comparability of treatment groups: states that groups were compared on variables of age, sex, currently nursing, W/H, H/A, diagnosis, length of hospitalisation, mother’s age and education prior to randomisation. No significant differences were found between the groups with or without dropouts (no data per treatment group or p-value reported) Method of data analysis: comparisons on variables at or prior to randomisation of the two groups was performed using chi-squared for nominal or ordinal variables and Student’s t-tests for continuous variables including utilisation and cost. For anthropometric outcomes, analysis of covariance was used, with the anthropometric assessment at entry into study as covariate. Survival analysis was used to compare mortality in the two groups. Main analyses do not appear to be ITT. In addition, three sensitivity analyses were performed, but details have not been extracted because results were NR in detail for survival as they were not substantially different to the main results. These included an ITT analysis of mortality Sample size/power calculation: none reported. A number of subgroup analysis in W/H at the different assessment points were conduced (survivors, deceased and dropouts), but it is unclear if the study was powered for these kind of analysis Attrition/dropout: total number and timing of loss reported. Reasons for dropout or numbers per treatment group not given, but states that equal numbers were lost between groups and that there were no significant differences between the two groups in timing of loss to follow-up (no data or p-value reported) |
|||||
| General comments | |||||
|
Generalisability: the study was designed to compare nutritional rehabilitation in two different settings (ambulatory vs hospital based), as they occur in a developing country. However, nutritional rehabilitation differed between ambulatory centres and between the hospital and the ambulatory centres. It is unclear if one meal in an ambulatory centre is sufficient for the treatment of SAM and how generalisable the results are to other settings. The majority of children in the study sample were aged 7–12 months (45%), followed by those aged 13–18 months (29%). It is unclear whether or not the results of the study would hold in children of other age groups Outcome measures: no primary outcome was defined, but outcomes appear to be suitable and appropriate Intercentre variability: unclear how many children were assigned to individual ambulatory centres. Differences in centres appear to have not been accounted for in the analysis Conflict of interest: none reported, but study partly supported by funding from a Fulbright Fellowship to Dr Chapko |
|||||
| Rehabilitation details | |||||
| Hospital-based rehabilitation | Home-based rehabilitation | ||||
| Three daily meals prepared by staff and mothers in a common kitchen. Provision of formal and informal educational sessions each day. Full-time staff of the hospital rehabilitation programme included a nurse, social worker and janitor, with 20% of a physician’s time, who made morning rounds. After discharge, children returned home and may have attended an ambulatory rehabilitation centre | One or two daily meals. Mother and child attended the centre early in the morning, preparing a meal with food partially provided by the centre and partially by the mother. Depending on the centre, children left at the end of the morning or stayed for a midday meal and then left. A typical morning included some form of education for the mother. Centres had variable staffing levels, typically one to three full-time nurses and/or social workers, plus one centre had a full or part-time physician as part of the staff | ||||
Quality assessment for primary studies (modified for severe malnutrition)
| A. Selection bias | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1. Are the individuals selected to participate in the study likely to be representative of the target population? | Very likely | Somewhat likely | Not likely | Cannot tell | |||||
| ✓ | |||||||||
| 2. What percentage of selected individuals participated? | 80–100% | 60–79% | < 60% | N/A | Cannot tell | ||||
| ✓ | |||||||||
| Summary of selection bias | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| B. Study design | |||||||||
| 1. What was the study design? | RCT | ||||||||
| (Please tick appropriate and specify design if categorise as ‘Other’) | CCT | ✓ | |||||||
| Cohort analytic (two group pre + post) | |||||||||
| Case–control | |||||||||
| Cohort [one group pre + post (before and after)] | |||||||||
| Interrupted time series | |||||||||
| Other – specify | |||||||||
| Cannot Tell | |||||||||
| 2. Was the study described as randomised? | Yes | No | |||||||
| ✓ | |||||||||
| If answer to no. 2 is ‘no’ complete summary then go to section C. Confounders. If answer is ‘yes’, answer no. 3 and no. 4 below, before completing summary for this section | |||||||||
| 3. If answer was yes, was the method of randomisation described? | Yes | No | |||||||
| ✓ | |||||||||
| 4. If answer was yes, was the method appropriate? | Yes | No | |||||||
| Summary of study design | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| C. Confounders | |||||||||
| 1. Were there important differences between groups prior to the intervention? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. If yes, indicate the percentage of relevant confounders that were controlled [either in the design (e.g. by stratification or matching) or in the analysis]? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| Summary of confounders | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| D. Blinding | |||||||||
| 1. Was the outcome assessor aware of the intervention or exposure status of participants? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were the study participants aware of the research question? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| Summary of blinding | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| E. Data collection methods | |||||||||
| 1. Were data collection tools shown to be valid? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were data collection tools shown to be reliable? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| Summary of data collection | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| F. Withdrawals and dropouts | |||||||||
| 1. Were withdrawals and dropouts reported in terms of numbers and reasons per group? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Indicate the percentage of participants completing the study (If the percentage differs by groups, record the lowest) | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
| Summary of withdrawals and dropouts | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| G. Intervention integrity | |||||||||
| 1. What percentage of participants received the allocated intervention or exposure of interest? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
| 2. Was the consistency of the intervention measured? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 3. Is it likely that subjects received an unintended intervention (contamination or co-intervention) that may influence the results? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| H. Analysis | |||||||||
| 1. Indicate the unit of allocation | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 2. Indicate the unit of analysis | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 3. Are the statistical methods appropriate for the study design? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 4. Is the analysis performed by intervention allocation status (i.e. ITT) rather than actual intervention received? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| Global rating for studya | Strong | Moderate | Weak | ||||||
| (Overall methodological strength of study – based on sections A–F) | ✓ | ||||||||
Ciliberto et al. 200563
Data extraction table
| Reference and design | Intervention | Participants | Outcome measures | |
|---|---|---|---|---|
|
Author: Ciliberto et al. 63 Year: 2005 Country: Malawi Study design: CCT Setting: home or inpatient Number of centres: seven Funding: Doris Duke Clinical Scholars Program; St Louis Children’s Hospital Foundation; the World Food Programme; and Valid International (unclear if this is funding for authors, study, or both). Publication enabled by support to the Food and Nutrition Technical Assistance (FANTA) Project by the Office of Foreign Disaster Assistance of the Bureau for Democracy, Conflict and Humanitarian Assistance, and the Office of Health, Infectious Diseases and Nutrition of the Bureau for Global Health at the US Agency for International Development, under terms of a co-operative agreement awarded to the Acadmy for Educational Development |
Intervention: home-based therapy for the second phase of treatment for childhood malnutrition. A 2-week supply of RUTF was provided at clinic visits based on the weight of the child at that visit Control: standard therapy for the second phase of treatment for childhood malnutrition based on WHO guidelines provided at nutritional rehabilitation units (NRUs) providing inpatient care. Children either received feeds in hospital or received additional cereal–legume supplement for use at home Study participation lasted 8 weeks in both groups after which all children were discharged If children reached a WHZ > 0 (based on admission height); clinically relapsed (recurrence of oedema or systemic infection requiring admission to NRU); or died, they were discharged from the study before week 8 Details of interventions in separate table which follows Other interventions used: None reported |
Definition of SAM: a WAZ < – 2, mild oedema (< 0.5 cm of pitting oedema on the dorsum of the foot), or both (subgroup identified using WHO criteria of either WHZ < –3 or oedema) Number of participants: n = 1178 [home-based therapy, n = 992 (separate results for n = 532 meeting WHO criteria for SAM); standard therapy, n = 186 (separate results for n = 113 meeting WHO criteria for SAM)] Sample attrition/dropout: home-based therapy: 35/992 did not attend follow-up ever, 63 did not complete 8 weeks of follow-up. Standard therapy: 6/186 did not attend follow-up ever, 9 did not complete 8 weeks of follow-up. Attrition from subgroup with SAM NR Sample crossovers: stepped wedge design meant that NRUs switched over from standard to home therapy during the course of the trial. It does not appear that any individuals switched over, although it is possible that if children who had received standard therapy did not recover and were referred back to the health centre for further evaluation they may subsequently have been offered home-based therapy if the centre had crossed over by then Inclusion criteria: age 10–60 months; attending one of seven NRUs (inpatients and children brought from surrounding community); WHZ < – 2, mild oedema (< 0.5 cm of pitting oedema on the dorsum of the foot), or both; a good appetite (determined by observing child eat test dose of 30 g RUTF and by questioning carer) Exclusion criteria: children < 10 months of age. Children with severe oedema (> 0.5 cm of pitting oedema on the dorsum of the foot); evidence of systemic infection or anorexia (but after phase one treatment at the NRU most such children became eligible for enrolment and did join the study of phase two treatment) General characteristics of participants: children aged 10–60 months with moderate or SAM |
Primary outcomes: successful recovery, relapse or death Secondary outcomes: Rates of growth in:Length and number of days of:Method of assessing outcomes: all follow-up data were collected in the same manner for children receiving standard therapy and home-based therapy with RUTF Carers and children returned to the clinic for reassessment every 2 weeks when weight, length and MUAC were measured. Weight gain and growth in MUAC were determined by calculating the change per day during the first 4 weeks of the study. The growth in stature rate was calculated as change in height per day over 8 weeks Carers for both groups were asked about the number of days of fever, cough and diarrhoea experienced by the child in the previous fortnight. Follow-up for assessing morbidity was limited to 2 weeks because many children receiving RUTF recovered before 8 weeks Active case finding began 3-weeks after a child’s last follow-up visit for children failing to attend for follow-up. The aim was to determine whether or not the child had died or relapsed. Reported child deaths were considered to be a consequence of malnutrition Recovery defined as reaching WHZ > –2 while remaining free of oedema, relapse or death Rate of relapse was assessed by asking all children reaching WHZ > –2 to return for follow-up anthropometric measurements after 6 months Adverse symptoms: NR Length of follow-up: 6 months Recruitment dates: December 2002 to June 2003 |
|
| Characteristics of participants: note that demographics were provided for the whole group, except for WHZ and oedema which were provided separately for the severely malnourished group | ||||
| Characteristic | Home-based therapy with RUTF (n = 992) | Standard therapy (n = 186) | p-value | |
| Male % (n) | 53 (526) | 53 (98) | NR | |
| Age, mean months ± SD | 23 ± 10 | 24 ± 12 | NR | |
| Oedema, % (n) | 44 (434) | 46 (86) | NR | |
| Weight, mean kg ± SD | 7.7 ± 1.7 | 7.6 ± 1.9 | NR | |
| Length, mean cm ± SD | 74.8 ± 6.6 | 75.0 ± 7.6 | NR | |
| W/A, mean z-score ± SD | –3.5 ± 1.0 | –3.7 ± 1.0 | NR | |
| H/A, mean z-score ± SD | –3.0 ± 1.5 | –3.2 ± 1.6 | NR | |
| W/H, mean z-score ± SD | –2.2 ± 0.8 | –2.5 ± 0.9 | < 0.05 | |
| MUAC, mean cm ± SD | 11.6 ± 1.4 | 11.6 ± 1.5 | NR | |
| Children still breastfeeding, % (n) | 52 (505) | 58 (72) | NR | |
| Age when breastfeeding stopped, mean months ± SD | 21 ± 7 | 21 ± 8 | NR | |
| Mother alive, % (n) | 98 (905) | 94 (164) | NR | |
| Father alive, % (n) | 93 (842) | 92 (158) | NR | |
| Clean water source, % (n/N) | 83 (812)a | 82 (133/162) | NR | |
| Grass used as roofing material, % (n/N) | 88 (863)a | 90 (137/153) | NR | |
| Subgroup: children with oedema or WHZ < –3 | Home-based therapy with RUTF (n = 532) | Standard therapy (n = 113) | p-value | |
| W/H, mean z-score ± SD | –2.5 ± 1.0 | –2.5 ± 1.1 | NR | |
| Oedema, % (n) | 81 (437) | 78 (87) | NR | |
| Results | ||||
| Primary outcome for subgroup with SAM | Home-based therapy with RUTF (n = 532) | Standard therapy (n = 113) | Difference (95% CI) | |
| Successful recovery (reaching WHZ > –2) after 8 weeks of therapy, % (n) | 72 (382) | 49 (55) | 21 (10 to 32) | |
| Children relapsed or died, % (n) | 10 (53) | 16.8 (19) | 6.8 (0.3 to 24.7) | |
| Children who died, % (n) | 3.7 (20) | 6.2 (7) | 2.5 (–0.8 to 6.8) | |
|
Comments: the subgroup of children with SAM who received home-based therapy with RUTF were 2.0 (95% CI 1.7 to 2.3) times as likely to recover as those receiving standard care (covariates of age, sex, oedema, recent inpatient admission in a NRU, month of admission and WHZ on admission controlled for in the multivariate regression analysis) Results also provided for the whole population, but these have not been data extracted |
||||
| Secondary outcomes for subgroup with SAM | Home-based therapy with RUTF (n = 532) | Standard therapy (n = 113) | Difference (95% CI) | |
| Rate of weight gain during first 4 weeks, mean g/kg/day ± SD | 3.7 ± 4.3 | 3.0 ± 8.8 | 0.7 (–0.4 to 1.8) | |
| Rate of height gain during first 8 weeks, mean mm/day ± SD | 0.2 ± 0.33 | 0.04 ± 0.35 | 0.16 (0.09 to 0.23) | |
| Rate of MUAC gain during first 4 weeks, mean mm/day ± SD | 0.42 ± 0.71 | 0.28 ± 0.44 | 0.14 (0.04 to 0.24) | |
|
Comments: the subgroup of children with SAM who received home-based therapy with RUTF were 0.5 times (95% CI 0.3 to 0.7) as likely to die or relapse as those receiving standard care (covariates of age, sex, oedema, recent inpatient admission in a NRU, month of admission and WHZ on admission controlled for in the multivariate regression analysis). The rate of weight gain was 1.4 (95% CI 1.1 to 1.7) times as great among the severely malnourished children in the home-based therapy group than the standard therapy group Results also provided for the whole population, but these have not been data extracted |
||||
| Secondary outcomes for subgroup with SAM | Home-based therapy with RUTF (n = 532) | Standard therapy (n = 113) | p-value | |
| Prevalence of fever during first 14 days, mean days ± SD | 1.0 ± 2 | 1.8 ± 3.3 | < 0.001 | |
| Prevalence of cough during first 14 days, mean days ± SD | 0.8 ± 2.4 | 1.8 ± 3.6 | < 0.001 | |
| Prevalence of diarrhoea during first 14 days, mean days ± SD | 0.7 ± 1.7 | 1.3 ± 2.7 | < 0.001 | |
| Comments: results also provided for the whole population, but these have not been data extracted | ||||
| Safety: states that no adverse reactions to RUTF were observed | ||||
| HIV: children who participated for 8 weeks but who did not recover were referred to the health centre for further medical evaluation where presumably some of the children received a HIV diagnosis. No indication of HIV prevalence provided | ||||
| Barriers to implementation | ||||
| Poor outcomes with standard therapy may in part be because of the time and resources required from the caretaker to comply with standard therapy. The caretaker must leave the home and stay with the child in the NRU, and then on returning home prepare cereal porridges seven times a day over an open fire in a rural setting. Findings suggest that in this operational setting, practical constraints and challenges were important limitations in the standard treatment | ||||
| Methodological comments | ||||
|
Allocation to treatment groups: stepped wedge design (intervention rolled-out sequentially to NRUs over a number of time periods – only one NRU offered home therapy at the start, other NRUs switch over to offer home therapy at the rate of two NRUs after the first 3 weeks, and one NRU every 3 weeks therafter). Randomisation was not possible owing to resource constraints and cultural beliefs. The stepped wedge design meant that although children receiving standard therapy were enrolled throughout the study, they were present in fewer numbers. The stepped wedge design was used to control bias that might be introduced by seasonal variations in the severity or type of childhood malnutrition in the pre-harvest (December to April) season when most cases of childhood malnutrition occur. As a RCT could not be conducted, the authors followed the Transparent Reporting of Evaluations with Nonrandomized Designs (TREND) statement for reporting of non-randomised clinical trials Blinding: not explicitly stated, but because of the nature of the study design it is unlikely that this was a blinded study Comparability of treatment groups: most children in the home-based therapy group (645/992, 65%) did not receive treatment in a NRU before enrollment, whereas all those in the standard-therapy group began their treatment in a NRU. For those in the home-based therapy group who did begin treatment in a NRU (n = 347), their average stay was 11 ± 9 days, whereas those in the standard group were hospitalised for 22 ± 14 days (difference between groups: 11 days 95% CI 8 to 14 days; no p-value reported). For the groups as a whole, WHZ was significantly different between the two groups (less severe in the home-based group), the paper authors speculate this may have been because when mothers knew the NRU was offering home-based therapy they were more willing to present with a moderately malnourished child than when standard inpatient care was offered. For the subgroup of severely malnourished children WHZ appears comparable between the groups although this is not commented on Method of data analysis: ITT analysis was used. Outcomes were determined for the entire group of participants (those meeting criteria for treatment in Malawi) and also for those children that met the WHO criteria for SAM (oedema or a WHZ < –3). Comparisons for outcomes were made by calculating the differences and 95% CI of the differences between standard therapy and home therapy with RUTF. Linear and logistic regression modelling were used to account for the effect of covariates on the comparisons (using SPSS). Time-event analysis was used to compare rates of reaching a WHZ > –2 over the 8-week study duration. A p < 0.05 was considered to be statistically significant. To compare the case fatality rate of home-based therapy with RUTF to international standards, an estimate of the predicted case fatality rate was made by using a published method (referenced) and this was compared with the actual case fatality rate Sample size/power calculation: because the period of time in which children could be enrolled to standard therapy was much shorter than that for home-based therapy it was anticipated that about 80% of participants would receive home-based therapy and only about 20% standard therapy. A sample size of 1030 children would have provided 95% confidence and 80% power to detect a minimum of a 10% absolute increase in recovery rate, and a 7% absolute decrease in mortality rate, assuming a 1 : 4 allocation of children to standard and home-based groups, and a 70% recovery rate and a 15% mortality rate in the standard group Attrition/dropout: numbers reported for whole group, but no reasons given. NR separately for the subgroup with SAM. The proportion of dropouts in each group was described as similar by the study authors (9.8% home-based group, 8.1% standard group). The authors also state that loss to follow-up was unlikely to be a significant cause of bias in the primary outcome because the differences between the two groups were so great Other: the authors noted that implementation of the interventions was not checked. No observations were made to confirm that mothers fed their children the RUTF, nor that standard therapy was being rigorously administered |
||||
| General comments | ||||
|
Generalisability: children had to have a good appetite to be included so might not be generalisable to those with poor/no appetite. Children met the anthropometric criteria for admission which were those used in Malawi, not those given in WHO guidelines. As children < 10 months were excluded, the study may not be generalisable to this age group. However, the intervention may not be appropriate for this age group anyway because the reasons for this exclusion were (1) few children of this age range were treated at NRUs, and (2) concern that RUTF consumption might interfere with breastfeeding Outcome measures: outcomes appear appropriate Intercentre variability: participating NRUs were in both mission and public facilities in small towns and rural areas of southern Malawi. No indication is given regarding the similarity or differences between the NRUs Conflict of interest: states that none of the authors had a conflict of interest related to the study. Additionally the development and implementation of the study and the data analyses were conducted entirely independently of the study sponsors. Study sponsors had no role in interpretation of data or in preparation of the published paper |
||||
| Home therapy: RUTF was produced as a co-operative effort by the study team and Tambala Foods (Blantyre, Malawi). It was packed in plastic jars containing 260 g without an airtight seal. The amount in each jar was approximately the amount consumed by the malnourished child in 1 day. Typically, children ate the RUTF directly from the jar, without diluting it or mixing it with other foods | ||||
| Peanut butter | 25% | |||
| Sugar | 28% | |||
| Full-cream milk | 30% | |||
| Vegetable oil | 15% | |||
| Imported vitamin and mineral supplement (CMV; Nutriset) | 1.4% | |||
| Energy content | 733 kJ/kg/day (175 kcal/kg/day) | |||
| Protein content | 5.3 g protein/kg/day | |||
| Micronutrient content | Identical to that of F100 before dilution and in accordance with WHO recommendations for catch-up growth | |||
| Inpatient therapy: children fed F100 while inpatients. On discharge from the hospital, the malnourished children received a generous supply of a supplemental blended flour (50 kg, composition below) to be consumed seven times a day. Because this maize–soy flour blend was familiar to mothers as an everyday food, they were expected to prepare it for their children as they would their staple food (i.e. usually consumed as a soft–solid dough) | ||||
| Maize flour | 80% | |||
| Soy flour | 20% | |||
| Vitamins and minerals | According to standard specifications of the World Food Programme | |||
Quality assessment for primary studies (modified for severe malnutrition)
| A. Selection bias | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1. Are the individuals selected to participate in the study likely to be representative of the target population? | Very likely | Somewhat likely | Not likely | Cannot tell | |||||
| ✓ | |||||||||
| 2. What percentage of selected individuals participated? | 80–100% | 60–79% | < 60% | N/A | Cannot tell | ||||
| ✓ | |||||||||
|
Summary of selection bias (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| B. Study design | |||||||||
|
1. What was the study design? (Please tick appropriate and specify design if categorise as ‘Other’) |
RCT | ||||||||
| CCT | ✓ | ||||||||
| Cohort analytic (two group pre + post) | |||||||||
| Case–control | |||||||||
| Cohort [one group pre + post (before and after)] | |||||||||
| Interrupted time series | |||||||||
| Other – specify | |||||||||
| Cannot Tell | |||||||||
| 2. Was the study described as randomised? | Yes | No | |||||||
| ✓ | |||||||||
| If answer to no. 2 is ‘no’ complete summary then go to section C. Confounders. If answer is ‘yes’, answer no. 3 and no. 4 below, before completing summary for this section | |||||||||
| 3. If answer was yes, was the method of randomisation described? | Yes | No | |||||||
| 4. If answer was yes, was the method appropriate? | Yes | No | |||||||
|
Summary of study design (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| C. Confounders | |||||||||
| 1. Were there important differences between groups prior to the intervention? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. If yes, indicate the percentage of relevant confounders that were controlled [either in the design (e.g. by stratification or matching) or in the analysis]? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
|
Summary of confounders (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| D. Blinding | |||||||||
| 1. Was the outcome assessor aware of the intervention or exposure status of participants? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were the study participants aware of the research question? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
|
Summary of blinding (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| E. Data collection methods | |||||||||
| 1. Were data collection tools shown to be valid? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were data collection tools shown to be reliable? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
|
Summary of data collection (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| F. Withdrawals and dropouts | |||||||||
| 1. Were withdrawals and dropouts reported in terms of numbers and reasons per group? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Indicate the percentage of participants completing the study (If the percentage differs by groups, record the lowest) | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
|
Summary of withdrawals and dropouts (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| G. Intervention integrity | |||||||||
| 1. What percentage of participants received the allocated intervention or exposure of interest? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
| 2. Was the consistency of the intervention measured? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 3. Is it likely that subjects received an unintended intervention (contamination or co-intervention) that may influence the results? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| H. Analysis | |||||||||
| 1. Indicate the unit of allocation | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 2. Indicate the unit of analysis | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 3. Are the statistical methods appropriate for the study design? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 4. Is the analysis performed by intervention allocation status (i.e. ITT) rather than actual intervention received? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
|
Global rating for studya (Overall methodological strength of study – based on sections A–F) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
Heikens et al. 199464
Data extraction table
| Reference and design | Intervention | Participants | Outcome measures | |
|---|---|---|---|---|
|
Author: Heikens et al. 64 Year: 1994 Country: Jamaica Study design: CCT Setting: inpatient (university hospital) and community Number of centres: one Funding: fully funded by Ministry of Development Cooperation, the Netherlands, with co-operation of Ministry of Health, Kingston, Jamaica |
Intervention: long stay. Hospital care with high-energy diet (until wasting corrected) + standard health service care at home for 6 months Control: short stay. Home care with high-energy supplement and standard health service care for 3 months + standard health service care for 3 months (Further details of interventions given at end of table) Other interventions used: all children received initial treatment of malnutrition and concurrent illnesses before being randomised. Specific therapy for infections and parasites was instituted if deemed necessary |
Definition of SAM: reports nutritional status according to Gómez, Wellcome and Waterlow classifications, but this showed inconsistencies with only one-third to a half of the study population classified as SAM. However, states children did have SAM and mean baseline W/A expressed as per cent of NCHS reference value was ≤ 60% Number of participants: N = 81 (long stay, n = 40; short stay, n = 39) Sample attrition/dropout: not clear, but appears to be 14% (11/79); n = 5 long stay (one died, four lost for other reasons) and n = 6 short stay (two died and four lost for other reasons) (see Attrition section in Methodological comments on page 228) Sample crossovers: none reported Inclusion criteria: all children referred from public health clinic and judged to require hospital admission based on W/A < 80%, oedema, anorexia, dermatosis or hair condition symptomatic of kwashiorkor, the need for treatment with parenteral antibiotics Exclusion criteria: known congenital abnormality, sibling in present study or in authors’ community study General characteristics of participants: severely malnourished children, aged 3–36 months referred from 40 public health clinics in low income areas of the city |
Primary outcomes: longer-term anthropometric status was focus of paper, though not specifically stated as primary outcomes per se. z-scores (W/A, L/A, W/L) at 12, 18, 24, 30 and 36 months Secondary outcomes: anthropometric status at discharge and after 6 months home care:Method of assessing outcomes: clinical assessments were made at admission, during hospital treatment and monthly throughout the home-care period of 6 months. Anthropometric measurements were made at baseline and 6-monthly intervals. No methods reported Adverse symptoms: NR Length of follow-up: 36 months post-admission Recruitment dates: March 1985 to May 1987, with follow-up measurement until November 1990 |
|
| Characteristics of participants | ||||
| Characteristic | Long stay (n = 40) | Short stay (n = 39) | p-value | |
| Age, months | 11.7 (0.9) | 10.8 (1.1) | NR | |
| Birthweight, kg | 3.0 (0.2) | 2.8 (0.2) | NR | |
| Weight, kg | 5.6 (0.2) | 5.3 (0.3) | NR | |
| Length, cm | 65.0 (6.1) | 63.5 (8.9) | NR | |
| W/Aa | 60.3 (1.7) | 57.9 (1.7) | NR | |
| L/Aa | 88.1 (0.8) | 87.1 (0.8) | NR | |
| W/La | 80.6 (1.7) | 81.6 (1.5) | NR | |
| BMI (weight/height2) | 13.2 (1.5) | 12.8 (1.3) | NR | |
| Number of siblings | 3.2 (0.3) | 3.1 (0.3) | NR | |
| Birth rank | 2.9 (0.3) | 2.8 (0.3) | NR | |
| Mother’s age, years | 27.6 (1.7) | 23.7 (1.0)b | NR | |
| Mother’s height, m | 1.6 (0.06) | 1.6 (0.05) | NR | |
| Diarrhoeac | 21.4 (4.6) | 20.7 (3.8) | NR | |
| Feverc | 13.2 (3.7) | 11.4 (3.2) | NR | |
| Coughc | 22.9 (4.7) | 20.7 (4.6) | NR | |
| Coldc | 18.6 (4.8) | 14.9 (4.2) | NR | |
| Comments: all data are mean (SE). The groups did not differ significantly on any measure (p-values NR) | ||||
| Results | ||||
| Primary outcomes: NCHS z-scoresd | Long stay (n = 40) | Short stay (n = 39) | p-value | |
| W/A | ||||
| Admission | –3.55 (0.30) | –3.70 (0.35) | ||
| Discharge | –2.50 (0.25) | –3.35 (0.30) | < 0.001 | |
| 12 months | –1.55 (0.30) | –2.30 (0.45) | < 0.001 | |
| 18 months | –1.40 (0.30) | –2.05 (0.40) | < 0.001 | |
| 24 months | –1.20 (0.30) | –1.90 (0.35) | < 0.01 | |
| 30 months | –1.20 (0.30) | –1.45 (0.30) | ||
| 36 months | –1.25 (0.45) | –1.30 (0.25) | ||
| L/A | ||||
| Admission | –3.20 (0.40) | –3.35 (0.45) | ||
| Discharge | –2.95 (0.40) | –3.30 (0.50) | < 0.1 | |
| 12 months | –1.80 (0.35) | –2.60 (0.60) | < 0.05 | |
| 18 months | –1.10 (0.40) | –2.20 (0.45) | < 0.001 | |
| 24 months | –0.95 (0.40) | –1.85 (0.50) | < 0.01 | |
| 30 months | –0.80 (0.40) | –1.40 (0.40) | < 0.05 | |
| 36 months | –0.95 (0.40) | –1.20 (0.40) | ||
| W/L | ||||
| Admission | –1.95 (0.35) | –1.85 (0.30) | ||
| Discharge | –0.45 (0.20) | –1.20 (0.35) | < 0.001 | |
| 12 months | –0.60 (0.30) | –1.00 (0.40) | < 0.1 | |
| 18 months | –0.75 (0.30) | –0.95 (0.30) | ||
| 24 months | –0.75 (0.30) | –0.95 (0.35) | ||
| 30 months | –0.80 (0.30) | –0.70 (0.30) | ||
| 36 months | –0.55 (0.30) | –0.65 (0.35) | ||
|
Comments: the paper reports the data in the form of bar charts showing group means and SE. The data here are all estimated to nearest 0.05 by the reviewer from bar charts using Engauge Digitiser version 4.1 (http://digitizer.sourceforge.net; Copyright Mark Mitchell 2002). Cross-sectional data (n at 12, 18, 24, 30 and 36 months: long stay = 37, 35, 35, 31 or 28 months; short stay = 28, 35, 30 or 26 months). Owing to reduced sample size and a change in group constitution as the long-term study progressed, the stability of the findings was tested in longitudinal analyses, adjusting for baseline differences but are not extracted here Two-tailed post-analysis of covariance tests established that the group differences in length were significant at p < 0.02, p < 0.0001, p < 0.005 and p < 0.06 at 12, 18, 24 and 30 months post-admission, respectively Similar comparisons for weight were p < 0.003, p < 0.01, p < 0.033 and p < 0.19 at 12, 18, 24 and 30 months post-admission, respectively. The effect was greater earlier, but was lost sooner than for length The groups did not differ significantly at 36 months on either measure During the first 14 days in hospital, weight velocities were similar between groups (range –8 to 24 g/kg/day). During the following 2 weeks, children remaining in hospital gained rapidly (10.4 vs 12.1 g/kg/day for long- and short-stay, respectively), settling to 6/7 g/kg/day average thereafter for children still in hospital, with no difference between groups at any treatment stage except the final velocity of 6/7 g/kg/day average was maintained over a longer period for the long-stay group. By 3 months post-discharge, velocities were similar at 1.13 vs 1.05 g/kg/day, respectively (range –4 to 7 g/kg/day). After a further 3 months, average velocity was ~ 0.85 g/kg/day for both groups |
||||
| Secondary outcomes | Long stay (n = 40) | Short stay (n = 39) | p-valuee | |
| Discharge | ||||
| Days post-admission | 39.45 (2.35) | 17.99 (1.43) | 0.001 | |
| z-scores | ||||
| W/A | –2.49 (0.12) | –3.38 (0.16) | 0.001 | |
| L/A | –3.02 (0.18) | –3.52 (0.22) | 0.086 | |
| W/L | –0.49 (0.11) | –1.17 (0.16) | 0.001 | |
| After 6 months home care | ||||
| Days post-admission | 218.09 (2.56) | 195.29 (1.91) | 0.001 | |
| z-scores | ||||
| W/A | –1.81 (0.16) | –2.45 (0.15) | 0.006 | |
| L/A | –2.38 (0.17) | –2.82 (0.18) | 0.059 | |
| W/L | –0.46 (0.14) | –0.80 (0.16) | 0.105 | |
| Comments: data are mean (SE) | ||||
| Safety: none reported other than one child in each group died from severe electrolyte disturbance during the first week after admission | ||||
| HIV: NR | ||||
| Barriers to implementation | ||||
| A hurricane during the follow-up period accounted for some missing data owing to being unable to trace the children in the immediate aftermath and industrial action closed the hospital wards causing early discharge for some | ||||
| Methodological comments | ||||
|
Allocation to treatment groups: just states random allocation made, no further details Blinding: not possible because of nature of interventions Comparability of treatment groups: reports that there were no significant differences between groups for any baseline characteristics or clinical findings presented in table nor for any morbidity indicator recorded, but not presented in table (p-values not presented). Mother’s age (27.6 years long stay vs 23.7 years short stay) approached significance at p < 0.05 Method of data analysis: not much detail reported. Not ITT analysis. Groups were compared by analysis of variance and covariance. Repeated measures analyses of covariance using a maximum likelihood method were made on NCHS z-scores at 12, 18, 24, 30 and 36 months. Reports all test assumptions were met. 95 Initial data screening used SPSS and final analysis BMDP Statistical Software programs. Eight children had missing data for between one and three test points as a result of hurricane Gilbert (the values were equally distributed across the groups). Missing data (for primary outcomes) were mostly because of subjects lost at a particular test point, rather than lost altogether. Although 79 cases contributed data to the analyses, only 44 had data for all five test sessions (12, 18, 24, 30 and 36 months) Sample size/power calculation: NR Attrition/dropout: total = 11 (long stay, n = 5; short stay, n = 6). Reasons given: failed to respond to treatment and died from severe electrolyte disturbance in first week after admission (one long stay and one short stay); died during follow-up for reasons unconnected with nutrition or infection (accidental aspiration) (one short stay); dropped from study after admission because of cardiac defect (one short stay); remained in hospital longer than intended because of home difficulties (one short stay); migrated at 24 months post-admission (one long stay); discharged early because industrial action closed hospital wards (one long stay); and lost because of lack/withdrawal of parental consent (two long stay and two short stay) |
||||
| General comments | ||||
|
Generalisability: all children with SAM referred from public health clinics in low income urban areas, aged 3–36 months. Unclear whether or not most would meet the WHO criteria [no, if based on NCHS reference value of < 70% W/L (baseline mean is 81–82%), yes if based on NCHS reference value of < 60% W/A (baseline mean is 58–60%)] Outcome measures: outcomes were appropriate although presentation of graphs required estimation of data points Intercentre variability: N/A Conflict of interest: fully funded by Ministry of Development Cooperation, the Netherlands, with co-operation of Ministry of Health, Kingston, Jamaica. No conflicts of interest reported |
||||
| After hospital admission, initial treatment of malnutrition and other concurrent illnesses was undertaken following established Tropical Metabolism Research Unit (university hospital) procedures.96–98 When the children had lost oedema, could tolerate 5-hourly feeds, gained weight on 3 successive days by at least 5 g/kg/day and no longer needed hospital treatment of concurrent illness or infection, they were randomised to long- or short-stay treatment | ||||
| Long stay, hospital care | Short stay, home care | |||
|
|
|||
| Follow-up continued for the remainder of the 3-year period after treatment ceased where there was no intervention other than 6-monthly anthropometric measurements | ||||
| Standard health service community care comprised training of CHAs, monitoring of CHAs and home-feeding, weighing and bacteriological testing of returned supplement containers, provision of multivitamins and folic acid, outpatient treatment of minor illnesses and infections, nutritional advice on breastfeeding and weaning following Ministry of Health guidelines (refs cited) | ||||
Quality assessment for primary studies (modified for severe malnutrition)
| A. Selection bias | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1. Are the individuals selected to participate in the study likely to be representative of the target population? | Very likely | Somewhat likely | Not likely | Cannot tell | |||||
| ✓ | |||||||||
| 2. What percentage of selected individuals participated? | 80–100% | 60–79% | < 60% | N/A | Cannot tell | ||||
| ✓ | |||||||||
|
Summary of selection bias (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| B. Study design | |||||||||
|
1. What was the study design? (Please tick appropriate and specify design if categorise as ‘Other’) |
RCT | ||||||||
| CCT | ✓ | ||||||||
| Cohort analytic (two group pre + post) | |||||||||
| Case–control | |||||||||
| Cohort [one group pre + post (before and after)] | |||||||||
| Interrupted time series | |||||||||
| Other – specify | |||||||||
| Cannot tell | |||||||||
| 2. Was the study described as randomised? | Yes | No | |||||||
| ✓ | |||||||||
| If answer to no. 2 is ‘no’ complete summary then go to section C. Confounders. If answer is ‘yes’, answer no. 3 and no. 4 below, before completing summary for this section | |||||||||
| 3. If answer was yes, was the method of randomisation described? | Yes | No | |||||||
| ✓ | |||||||||
| 4. If answer was yes, was the method appropriate? | Yes | No | |||||||
|
Summary of study design (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| C. Confounders | |||||||||
| 1. Were there important differences between groups prior to the intervention? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. If yes, indicate the percentage of relevant confounders that were controlled [either in the design (e.g. by stratification or matching) or in the analysis]? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
|
Summary of confounders (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| D. Blinding | |||||||||
| 1. Was the outcome assessor aware of the intervention or exposure status of participants? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were the study participants aware of the research question? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
|
Summary of blinding (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| E. Data collection methods | |||||||||
| 1. Were data collection tools shown to be valid? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were data collection tools shown to be reliable? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
|
Summary of data collection (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| F. Withdrawals and dropouts | |||||||||
| 1. Were withdrawals and dropouts reported in terms of numbers and reasons per group? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Indicate the percentage of participants completing the study (If the percentage differs by groups, record the lowest) | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
|
Summary of withdrawals and dropouts (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| G. Intervention integrity | |||||||||
| 1. What percentage of participants received the allocated intervention or exposure of interest? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
| 2. Was the consistency of the intervention measured? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 3. Is it likely that subjects received an unintended intervention (contamination or co-intervention) that may influence the results? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| H. Analysis | |||||||||
| 1. Indicate the unit of allocation | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 2. Indicate the unit of analysis | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 3. Are the statistical methods appropriate for the study design? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 4. Is the analysis performed by intervention allocation status (i.e. ITT) rather than actual intervention received? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
|
Global rating for studya (Overall methodological strength of study – based on sections A–F) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
Khanum et al. 199465
Data extraction table
| Reference and design | Intervention | Participants | Outcome measures | ||||
|---|---|---|---|---|---|---|---|
|
Author: Khanum et al. 65 Year: 1994 Linked papers: Ashworth and Khanum 199799 and Khanum et al. 1998100 Country: Bangladesh Study design: controlled trial Setting: inpatient, day care, or at home depending on group allocation (additional details at end of table) Number of centres: one Funding: Save the Children Fund, UK and Overseas Development Agency UK |
Intervention 1: inpatient. Children admitted with their mothers and resident until reaching 80% W/H Intervention 2: day care. Children attended with their mothers 0800 to 1700 hours every day except Friday until 80% W/H reached. Mothers permitted to bring another young sibling Intervention 3: care at home. 7 days treatment in day-care facility (or up to 9 days if poor appetite or poor clinical condition persisting). Then home where visited weekly for 1 month, then twice monthly until reaching 80% W/H. Weekly visits continued if children not oedema-free at 1 month Details of diet and nutrition/education interventions provided at end of table Other interventions used: all children received an initial clinical examination including chest radiograph, blood tests, urine and stool tests, laryngeal and wound swabs (full details not extracted), all received a broad-spectrum antibiotic (details at end of table). Xerophthalmia treated following WHO guidelines. Immunisations (diphtheria–pertussis–tetanus, BCG, measles) All breastfed patients continued to receive breast milk |
Definition of SAM: W/H < 60% of NCHS median, and/or oedema. Wellcome classification also used Number of participants: N = 573 [inpatient n = 200, completed n = 173 (86.5%); day care n = 200, completed n = 134 (67%); at home n = 173, completed n = 130 (75.1%)] All 437 children completing the trial entered the 12-month follow-up. At entry to follow-up all had reached 80% W/H Sample attrition/dropout: late exclusion (owing to TB or given blood): inpatients, n = 18 (9%); day care, n = 22 (11%); at home, n = 30 (17.4%). Deaths: inpatients, n = 7 (3.5%); day care, n = 10 (5%); at home, n = 6 (3.5%) Discontinued allotted group: inpatients, n = 2 (1%); day care, n = 34 (17%); at home, n = 7 (4%) Eligible children whose parents later requested treatment in a different group to that assigned were dropped from the trial Attrition from 12-month follow-up:Sample crossovers: none Inclusion criteria: W/H < 60% of the NCHS median, and/or oedema Exclusion criteria: conditions requiring > 7 days medical supervision: packed-cell volume < 20% necessitating blood transfusion, critical illness (e.g. meningitis, encephalitis or other cerebral lesion, haemolytic anaemia), children < 12 months in age, TB or congenital or metabolic disorders, home more than 10 km from unit, age > 60 months General characteristics of participants: W/H < 60% of NCHS median. Ninety per cent of children come from urban slums [brought by family (60%) or referred from other hospitals] |
Primary outcomes: not stated in initial paper. 65 Focus of one linked study92 was on costs, and on morbidity, growth relapse and mortality in the paper reporting 12-month follow-up94 Secondary outcomes:Method of assessing outcomes: completion of treatment – attaining 80% oedema-free W/H (NCHS median as reference). If this was achieved in the home group when visits were fortnightly, interpolation was used to calculate to the nearest week when this occurred Weight measured daily for inpatients and day care. Home group measured weekly for the first month then fortnightly Height measured weekly for inpatients and day care. Home group measured as for weight Structured questionnaire used at every visit to the home group to assess compliance with recommendations for meal frequency, quantities and types of food offered, and amounts consumed Cost data were noted (details not extracted) During 12-month follow-up: children visited at home every 2 weeks by one of eight specially trained field workers. Mothers asked to recall whether child was well or had specific morbidity signs (diarrhoea, vomiting, cough, fever, eye infection, ear infection, passing worms). Mothers recorded presence of morbidity for each day using a pictorial calendar. Study staff recorded morbidity on a pre-coded form during fortnightly interviews with mothers. Children were also examined for infection by the fieldworker and presence of illness recorded Children referred to outpatient department if major illness suspected. Outpatient records of children referred or attending independently were linked to derive total attendances per child during the year Weight: recorded monthly using electronic scales calibrated daily Length/height: measured monthly to nearest 0.1 cm (using standard technique with a locally made board), mean of two values taken (difference of < 0.5 cm between measurements considered acceptable) Relapse definition: child has become oedematous or < 60% W/H Deaths: fieldworkers interviewed mother about cause and place of death Adverse symptoms: NR Length of follow-up: to attainment of 80% W/H, and for those reaching 80% W/H a further 12 months Recruitment dates: December 1990 to November 1991 |
||||
| Characteristics of participants | |||||||
| Characteristic | Inpatients (n = 200) | Day care (n = 200) | At home (n = 173) | ||||
| Mean age, months (SD) | 25 (13) | 26 (13) | 28 (13) | ||||
| Mean W/H (% of NCHS median) including oedema (SD) | 67 (7) | 70 (8) | 70 (7) | ||||
| Mean W/A (% of NCHS median) including oedema (SD) | 48 (9) | 50 (10) | 51 (9) | ||||
| Mean packed cell volume, % (SD) | 28 (3) | 29 (3) | 29 (3) | ||||
| Mean total protein, g (SD) | 4 (1) | 4 (1) | 4 (1) | ||||
| Xerophthalmia (%) | 45 | 46 | 40 | ||||
| Angular stomatitis (%) | 32 | 27 | 26 | ||||
| Infections % | |||||||
| Diarrhoea with dehydration | 58 | 60 | 60 | ||||
| History of measles in last 3 months | 57 | 58 | 52 | ||||
| Upper respiratory infection | 35 | 31 | 31 | ||||
| Lower respiratory tract infection | 19 | 16 | 18 | ||||
| Upper and lower respiratory infection | 18 | 22 | 20 | ||||
| Skin infection | 33 | 30 | 28 | ||||
| Urinary tract infection | 10 | 17 | 17 | ||||
| Middle ear infection (otitis media) | 14 | 11 | 14 | ||||
| Septicaemia (diagnosed clinically) | 7 | 9 | 7 | ||||
| Intestinal parasites (%) | |||||||
| Entamoeba histolytica | 24 | 29 | 26 | ||||
| Ascaris lumbricoides | 24 | 25 | 25 | ||||
| Trichuris trichiura | 19 | 23 | 25 | ||||
| Characteristic at start of 12-month follow-up | Inpatients (n = 118) | Day care (n = 111) | At home (n = 106) | ||||
| Age, mean in months | 26 | 27 | 31a | ||||
| Weight kg, mean ± SD | 7.73 ± 1.81 | 7.46 ± 1.89 | 7.83 ± 2.00 | ||||
| Height cm, mean ± SD | 73.3 ± 8.1 | 72.4 ± 8.4 | 74.4 ± 9.7 | ||||
| Comments: 60% of children had three or more infections in addition to SAM. According to Wellcome classification, of the 437 children who completed the study, 83% were marasmic kwashiorkor and 15% kwashiorkor (98% oedematous overall) | |||||||
| Results | |||||||
| Outcomes of initial study, until children attained 80% W/H | Inpatients (n = 200) | Day care (n = 200) | At home (n = 173) | ||||
| Mortality n/N (%) | 7/200 (3.5) | 10/200 (5.0) | 6/173 (3.5) | ||||
| Rate of oedema loss, median | 11 days | 13 days | 19 days (significantly longer than the other two groups, median test p < 0.001) | ||||
| Mean weight gainb from admission to 80% W/H, g/kg body weight/day | 11 | 6 | 4 | ||||
| Days to achieve 80% oedema-free W/H, median | 18 | 23 | 35 (significantly longer than the other two groups, median test p < 0.001) | ||||
|
Comments: 70% of deaths occurred within 48 hours of admission. Causes of death were respiratory infection n = 15, diarrhoea with dehydration n = 7 and traffic accident n = 1. Those who died had poorer nutritional status on admission than survivors (mean W/H 64%, mean W/A 47%) Costs data not extracted |
|||||||
| Outcomes at 12-month follow-up of those who had attained 80% weight initially | Inpatients (n = 118) | Day care (n = 111) | At home (n = 106) | ||||
| Readmitted to unit, % (n)c | 1.7 (3) | 1.5 (2) | 2.3 (3) | ||||
| Died, % (n)c | 3.4 (6) | 1.5 (2) | 1.5 (2) | ||||
| Weight gain (kg), mean ± SD | 2.15±1.12 | 2.39 ± 0.98 | 2.47 ± 1.13 | ||||
| Height gain (cm) mean ± SD | 6.4 ± 2.6 | 7.2 ± 2.3 | 7.3 ± 2.3 | ||||
| Diarrhoea – percentage of time reported, mean ± SD | 9.5 ± 10.6 | 9.3 ± 10.0 | 7.4 ± 9.1 | ||||
| Diarrhoea episodes, n, mean ± SD | 7.3 ± 6.8 | 7.1 ± 6.1 | 5.7 ± 5.5 | ||||
| Diarrhoea episode duration, days mean ± SD | 4.9 ± 2.0 | 4.8 ± 2.2 | 4.6 ± 1.5 | ||||
| Fever (no diarrhoea, no cough) – percentage of time reported, mean ± SD | 10.7 ± 7.1 | 10.1 ± 10.4 | 7.3 ± 7.3d | ||||
| Cough (no diarrhoea, no fever) – percentage of time reported, mean ± SD | 25.0 ± 16.6 | 25.0 ± 15.2 | 15.0 ± 10.2d | ||||
| Fever and cough – percentage of time reported mean ± SD | 12.6 ± 15.2 | 12.6 ± 15.0 | 7.5 ± 10.0d | ||||
|
Comments: during the 12-month follow-up, emergency readmission (1.2%), relapses (0.6%) and mortality (2.3%) were low and did not differ among the three treatment groups There were no significant differences in weight gain or height gain between the groups (p-values NR). Gains in weight improved children’s mean W/H from 80% at the start of follow-up to 91% of the NCHS median at the end of the year (from z-score –1.60 to –0.92). Weight gains greater in the first semester of follow-up (presumed to be 6 months) than the second (results presented in figure for the whole group and not data extracted). Stated improvement not restricted to the youngest children but no data presented H/A did not change during the year (small positive gain for children ≥ 48 months at start of follow-up, slight negative change for those aged < 48 months) Diarrhoea was experienced by 92% of children during the year, and cough with fever by 96%. Cough and fever were less frequently reported for children in the at home group (p < 0.03) No difference in morbidity found by field worker examination was found among the groups. Outpatient attendance was high (data presented for whole group only and not extracted) and paper states there was no difference between the groups Effect of morbidity on growth reported, but not data extracted (NR by treatment group) |
|||||||
| Safety: deaths were comparable between groups indicating that for the population selected, at home treatment could be an alternative strategy to inpatient care. Paper indicates that although difference in time to recovery were marked, once children reached 80% W/H no group was significantly disadvantaged during the following 12 months | |||||||
| HIV: NR | |||||||
| Barriers to implementation | |||||||
|
Day care was an unpopular option, only 4% of parents indicated they would have chosen this option if they had been offered a free choice, and of those children who discontinued in their assigned group, 79% were in the day-care group. Full-time commitment was needed by a family member for 1 month on average and the sick child had to be transported to and from the facility in busy traffic each day In the at-home group, there was difficulty in preparing salt-free meals (foods for child largely derived from family foods), addition of oil to milk (to increase energy content) was deemed unacceptable by some families, 16% could not achieve recommended meal frequency and 12% could not achieve recommended meal quantity Inpatient care had high institutional costs. Although mothers were expected to be resident day and night, in practice other members were allowed to substitute and this option was more acceptable to families than day care |
|||||||
| Methodological comments | |||||||
|
Allocation to treatment groups: sequential allocation by daily rotation such that recruitment to each group occurred every third day. The initial sequence was randomly determined. From the description provided in the paper approximately equal numbers in each group might be expected, however, this is not the case (n = 200, n = 200, n = 173). The reasons for this are not clear. After registration in the outpatients’ section, mothers proceeded to the unit where a doctor explained the planned treatment and asked consent Blinding: neither mothers, nor the admission officers, were aware of which treatment was available on a particular day Comparability of treatment groups: states groups were similar in age, nutritional status, complications, socioeconomic background, and late exclusions and deaths (although see comment below about discontinuation in day-care group) Method of data analysis: NR for initial-follow-up. For long-term follow-up, data were subjected to range and consistency checks. Data analysed using SPSS/PC+ (version 4) and the Anthro software package (Centers for Disease Control and Prevention, Atlanta, GA, USA) used to obtain anthropometric indexes. ANOVA and chi-squared tests used to test for statistical significance. p < 0.05 accepted as significant. Children expected to receive 24 morbidity visits during 12 months of follow-up. Children with < 18 visits (75%) were excluded from the analysis. An appropriate adjustment was made for those with 18–23 visits to yield morbidity measures for 1 year. Children with ≥ 18 morbidity visits had also completed all 12 of the expected anthropometric measurements Sample size/power calculation: the aim of the study was to identify the most cost-effective method of treatment. Consequently sample size was estimated on the basis of mean (SD) costs of treatment for inpatients and day care. A minimum of 100 children per group was considered sufficient to detect a 15–20% reduction in cost for treatment in the at-home group (90% power, 5% significance level) Attrition/dropout: reported with reasons for each group, however, there is a small discrepancy between one paper65 and the second paper. 92 The discontinuation rate was significantly higher in the day-care group than in the other two groups (p < 0.01). Also reported with reasons for each group for the 12-month follow-up. 94 Losses and intermittent follow-up were more common for children who had been inpatients leading to a lower completion rate compared with the other groups (p = 0.003). Data not shown in paper, but states when groups were combined there were no significant differences for a wide range of anthropometric variables between those who completed follow-up (n = 335), those excluded from analysis for incomplete data (n = 47), and those lost without trace (n = 33) |
|||||||
| General comments | |||||||
|
Generalisability: not generalisable to critically ill children who were excluded (because > 7 days inpatient care needed), and also not generalisable to children < 1 year in age who were also excluded because the mortality risk for domiciliary care was unknown. Likely that the children would meet the current WHO criteria for SAM. Contact during months 6–12 of follow-up was twice as frequent as the usual post-discharge service and all follow-up in the year after discharge took place at home (usual service contact at outpatients), this was likely to have resulted in greater contact with unit staff than would normally occur. The long-term results may therefore not be achievable when long-term follow-up is less frequent, and/or occurs only in outpatient clinics Outcome measures: a primary clinical outcome was not defined because the focus was on costs. Clinical outcome measures that were reported seem appropriate Intercentre variability: not applicable Conflict of interest: no statement made. An author on one paper65 was supported by the UK Overseas Development Administration. Study received funding from Save the Children |
|||||||
| Inpatients | Day care | Care at home | |||||
| Setting and staffing |
|
|
Team of eight specially trained home visitors | ||||
| Broad-spectrum antibiotic on admission: | i.m. injection for first 3 days | Oral delivery (10-day course) | Oral delivery (10-day course) | ||||
|
|||||||
|
Oral delivery thereafter | ||||||
| Comments: provision of broad-spectrum antibiotics adjusted appropriately once results of laboratory investigations became known | |||||||
| Per day week 1 | |||||||
|
Modified milk (75 kcal and 1.5 g protein/100 ml) Anorexic patients fed milk by nasogastric tube (removed for patients going home at night) |
80–100 ml/kg 2-hourly |
80–100 ml/kg 2-hourly between 0800 and 1700 hours Parents advised to give two further milk feeds at home (note, care at home group children were in day care for week 1) On Friday (no day care), parents advised to give at least four cups of milk |
|||||
| Rice-based salt-free meals | Four |
Three between 0800 and 1700 hours Parents advised to give one further meal at home On Friday (no day care), parents advised to give at least four rice-based meals |
|||||
| Per day week 2 onwards | |||||||
|
High-energy milk (100 kcal and 3 g protein/100 ml) (omitted for children aged > 24 months) |
Four feeds (120–150 ml/kg/day) |
Three feeds between 0800 and 1700 hours Mothers advised to give one feed at home |
Mothers provided with a 180 ml cup and asked to give three to four milk feeds | ||||
| Rice-based salt-free meals (recommended for day- and home-care groups: rice pudding, rice with dhal, rice with pumpkin, dhal or potato, oil, and if affordable meat or fish) | Three feeds (four if > 24 months) |
Three feeds (four if > 24 months) between 0800 and 1700 hours Mothers advised to give two meals at home |
Mothers provided with a bowl (capacity 340 g food when full) and asked to feed three rice-based meals (four if > 24 months) | ||||
| Snacks | Two feeds | Two feeds between 0800 and 1700 hours | Asked to provide two feeds | ||||
|
Comments: all mothers/caretakers received 20 minutes of structured instruction each day of their stay on topics relevant to infant feeding, disease prevention and family planning. They also received 20 minutes of practical guidance everyday except Friday. The day-care group had a longer recovery time and therefore received slightly more days of instruction. The at-home group attended the sessions, but only during the initial week of inpatient care. Mothers/caregivers of the day-care and at-home group received additional instruction on what to feed their children at home, how much (quantities to be served in the bowl and cup provided), and how often (meal frequency). This included a practical exercise in which the caregiver prepared a family meal, keeping in mind the special needs of the malnourished child. Additional instruction was required because after the first week children in the at-home group were entirely dependent on home-prepared meals for their rehabilitation, and the day-care group were also expected to receive extra meals at home and all meals on Fridays. Home visitors continued to provide guidance to the at-home group during visits that lasted about 1 hour. Visitors were trained to examine the child for oedema, dehydration, fever, rapid breathing, and throat and ear infection, and to refer child to the unit for consultation if necessary The diets provided described above provided the energy and protein indicated below, with additional dietary supplements also being provided as listed. A cautious approach to feeding was followed in the first week, the emphasis on small but frequent feeds so that the reduced capacity of the malnourished children to absorb and utilise nutrients was not exceeded. Thereafter, the dietary regimen changed to provide high intakes of energy and nutrients to enable rapid ‘catch-up’ growth |
|||||||
| Week 1 – all groups | Week 2 onwards – inpatients and day-care group | Week 2 onwards – at-home group | |||||
| Energy, kcal/kg/day | 100–200 | 150–200 | 150–200 | ||||
| Protein, kcal/kg/day | 2–3 | 3–4 | 3–4 | ||||
| Potassium chloride, mmol/kg/day | 5–6 | 5–6e | |||||
| Magnesium sulphate, mmol/kg/day | 0.5–1.0 | 0.5–1.0e | |||||
| Riboflavin, mg/day | 5 | 5 | |||||
| Folic acid, mg/day | 5 | 5 | |||||
| Ferrous sulphate, mg/kg/day | 4 | 4 | |||||
| Multivitamin drops | Yes | Yes | Yes | ||||
| Vitamin A IU (day 1 onlyf) | 200,000 | ||||||
Quality assessment for primary studies (modified for severe malnutrition)
| A. Selection bias | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1. Are the individuals selected to participate in the study likely to be representative of the target population? | Very likely | Somewhat likely | Not likely | Cannot tell | |||||
| ✓ | |||||||||
| 2. What percentage of selected individuals participated? | 80–100% | 60–79% | < 60% | N/A | Cannot tell | ||||
| ✓ | |||||||||
|
Summary of selection bias (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| B. Study design | |||||||||
|
1. What was the study design? (Please tick appropriate and specify design if categorise as ‘Other’) |
RCT | ||||||||
| CCT | ✓ | ||||||||
| Cohort analytic (two group pre + post) | |||||||||
| Case–control | |||||||||
| Cohort [one group pre + post (before and after)] | |||||||||
| Interrupted time series | |||||||||
| Other – specify | |||||||||
| Cannot Tell | |||||||||
| 2. Was the study described as randomised? | Yes | No | note: mentioned random determination of sequence, but title of paper is controlled trial | ||||||
| ✓ | |||||||||
| If answer to no. 2 is ‘no’ complete summary then go to section C. Confounders. If answer is ‘yes’, answer no. 3 and no. 4 below, before completing summary for this section | |||||||||
| 3. If answer was yes, was the method of randomisation described? | Yes | No | |||||||
| 4. If answer was yes, was the method appropriate? | Yes | No | |||||||
|
Summary of study design (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| C. Confounders | |||||||||
| 1. Were there important differences between groups prior to the intervention? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. If yes, indicate the percentage of relevant confounders that were controlled [either in the design (e.g. by stratification or matching) or in the analysis]? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
|
Summary of confounders (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| D. Blinding | |||||||||
| 1. Was the outcome assessor aware of the intervention or exposure status of participants? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were the study participants aware of the research question? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
|
Summary of blinding (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| E. Data collection methods | |||||||||
| 1. Were data collection tools shown to be valid? | Yes | No | Cannot tell | ||||||
| ✓ 12-month follow-up | ✓ initial study | ||||||||
| 2. Were data collection tools shown to be reliable? | Yes | No | Cannot tell | ||||||
| ✓ 12-month follow-up | ✓ initial study | ||||||||
|
Summary of data collection (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ 12-month follow-up | ✓ initial study | ||||||||
| F. Withdrawals and dropouts | |||||||||
| 1. Were withdrawals and dropouts reported in terms of numbers and reasons per group? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Indicate the percentage of participants completing the study (If the percentage differs by groups, record the lowest) | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ initial study | ✓ 12-month follow-up | ||||||||
|
Summary of withdrawals and dropouts (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ initial study | ✓ 12-month follow-up | ||||||||
| G. Intervention integrity | |||||||||
| 1. What percentage of participants received the allocated intervention or exposure of interest? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
| 2. Was the consistency of the intervention measured? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 3. Is it likely that subjects received an unintended intervention (contamination or co-intervention) that may influence the results? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| H. Analysis | |||||||||
| 1. Indicate the unit of allocation | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 2. Indicate the unit of analysis | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 3. Are the statistical methods appropriate for the study design? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 4. Is the analysis performed by intervention allocation status (i.e. ITT) rather than actual intervention received? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
|
Global rating for studya (Overall methodological strength of study – based on sections A–F) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
Appendix 12 Question 8: data extraction tables
Doherty et al. 199868
Data extraction table
| Reference and design | Intervention | Participants | Outcome measures | ||||
|---|---|---|---|---|---|---|---|
|
Author: Doherty et al. 68 Year: 1998 Linked paper: Doherty et al69 2002 Country: Bangladesh Study design: double-blind RCT Setting: secondary care Number of centres: one Funding: Nestlé UK and the Department of Child Life and Health, University of Edinburgh. Ciba-Geigy, Bangladesh, provided zinc suspensions |
Intervention one: 1.5 mg zinc/kg body weight for 15 days followed by placebo for 15 days Intervention two: 6.0 mg zinc/kg body weight for 15 days followed by placebo for 15 days Intervention three: 6.0 mg zinc/kg body weight for 30 days Elemental zinc was provided as zinc sulphate in all groups. Mothers were instructed how to administer the supplements using labelled syringes, which they continued to use at home up to day 30 Other interventions used: on recruitment, all were treated identically with broad-spectrum antibiotics, diarrhoea and skin sepsis was treated if present. All received a liquid diet with gradually increasing energy and protein according to malnutrition type, vitamin A and a daily multivitamin supplement. Full details in separate table. Days 1–15 involved intensive inpatient nutritional rehabilitation and health education (no details of the latter provided). Subjects discharged on day 15 if clinically fit and followed as outpatients |
Definition of SAM: not explicitly stated, but presumed the same as the inclusion criteria, i.e. W/A < 60% of NCHS median for age, had nutritional oedema, or both Number of participants: N = 141 [intervention one (1.5 mg zinc/placebo) n = 49; intervention two (6 mg zinc/placebo) n = 49; intervention three (6 mg zinc/6 mg zinc) n = 43] Sample attrition/dropout: 106 (75%) completed; n = 16 (11%) dropouts (six because caregiver discharged them, 10 lost to follow-up); 19 (13.5%) deaths. Dropouts by group: 1.5 mg zinc/placebo n = 4; 6.0 mg zinc/placebo: n = 3; 6.0 mg zinc/6.0 mg zinc n = 9 Sample crossovers: none Inclusion criteria: aged 6–36 months and were W/A < 60% of NCHS median for age, had nutritional oedema, or both. Clinically stabilised within 1 week of admission and able to tolerate oral nutritional rehabilitation. Caregivers agreed that their child would remain in hospital for a further 15 days, and be followed up for a total of 90 days Exclusion criteria: strong suspicion of underlying TB (contact history and history of prolonged temperature elevation or cough) General characteristics of participants: severely malnourished children living within 2-hour travelling distance of hospital. 57% were aged < 1 year and average WHZ was –2.66 |
Primary outcomes: not explicitly stated Outcomes included: mortality (during inpatient and outpatient phases) and changes in anthropometric variables (z-scores, knemometry, skinfold thickness, MUAC) The linked paper69 reports on insulin-like growth factor-1, its binding proteins, bone formation and collagen turnover. No further information relating to these outcomes has been data extracted Method of assessing outcomes: weight and length were measured by a team of four nurses and four nutritionists who had received an 8-week training course Two observers undertook all of the knemometry (distance between knee and heel), skinfold and MUAC measurements after an 8-week training period. Five knemometric readings were taken at each assessment and the mean was accepted unless the SD was > 1 mm All staff involved in anthropometric data gathering were subject to regular, unscheduled, formal assessments of measurement technique Weight – electronic scale, graduations to 20 g Length – rollameter with graduations to 1 mm. All measurements taken with child supine Skin-fold thickness – calipers graduated to 0.2 mm MUAC – standard non-stretch tape measure with graduations to 1 mm All anthropometric variables were based on NCHS medians During in patient phase: body weight recorded daily, knemometry on alternate days, all other anthropometric variables on days 1, 8 and 15 During follow-up: all nutritional measurements recorded together in the morning Adverse symptoms: none reported Length of follow-up: during inpatient phase (15 days), and subsequently as outpatients on days 21, 30, 45, 60, 75 and 90 Recruitment dates: November 1995 to November 1996 |
||||
| Characteristics of participants: | |||||||
| Characteristic | Intervention one (1.5 mg zinc/placebo) (n = 49) | Intervention two (6 mg zinc/placebo) (n = 49) | Intervention three (6 mg zinc/6 mg zinc) (n = 43) | ||||
| Age, months | 15.5 ± 8.7 | 15.0 ± 9.0 | 16.3 ± 8.6 | ||||
| WAZ | –4.47 ± 0.91 | –4.56 ± 0.98 | –4.66 ± 0.86 | ||||
| WHZ | –2.56 ± 0.97 | –2.73 ± 0.90 | –2.71 ± 0.93 | ||||
| HAZ | –3.89 ± 1.3 | –3.79 ± 1.4 | –3.98 ± 1.45 | ||||
| Malnutrition, n | |||||||
| Marasmus | 29 | 27 | 26 | ||||
| Marasmic kwashiorkor | 15 | 14 | 11 | ||||
| Kwashiorkor | 5 | 7 | 6 | ||||
| Time from admission to recruitment, days | 2.5 ± 1.5 | 3.5 ± 2.2 | 2.7 ± 1.8 | ||||
| Lower leg length, cm | 17.08 ± 2.30 | 16.91 ± 2.23 | 17.31 ± 2.24 | ||||
|
Comments: data presented are mean ± SD unless otherwise stated 57% of participants were < 1 year of age. Participants were both severely wasted and severely stunted |
|||||||
| Results | |||||||
| Outcomes | Intervention one (1.5 mg zinc/placebo) (n = 49) | Intervention two (6 mg zinc/placebo) (n = 49) | Intervention three (6 mg zinc/6 mg zinc) (n = 43) | ||||
| Inpatient death, n | 2 | 5 | 6 | ||||
| Outpatient death, n | 0 | 3 | 3 | ||||
| Self-discharge or loss to follow-up, n | 4 | 3 | 9 | ||||
| Comments: there were more deaths in the groups receiving 6.0 mg zinc/kg as inpatients. This trend was identified at the interim analysis of the first 100 subjects and enrolment was suspended after 141 recruits. When supplementation regimens two and three were combined, the risk of death was significant (p = 0.03) with exposure to 6.0 mg zinc/kg as compared with 1.5 mg zinc/kg initially (Yates-corrected chi-squared value of risk of death at RR 4.52, 95% CI 1.09 to 18.8). Clinician’s impression was that cause of death was sepsis in most cases, and 13 of the 18 deaths occurred when children were inpatients. The paper presents an analysis looking for possible predictors/prognostic factors for death, but none of the factors considered (age, degree of wasting and stunting, severity of initial illness, type of malnutrition) were found to predict death in association with exposure to the higher initial dose of zinc (data not extracted here) | |||||||
| Change in anthropometric outcomes over 90 days | Intervention one (1.5 mg zinc/placebo) (n = 43) | Intervention two (6 mg zinc/placebo) (n = 38) | Intervention three (6 mg zinc/6 mg zinc) (n = 25) | 95% CI for mean difference | |||
| WAZ | 1.35 ± 0.69 | 1.51 ± 0.65 | 1.45 ± 0.66 |
Intervention two–intervention one: (–0.27 to 0.52) Intervention three–intervention two: (–0.47 to 0.38) |
|||
| WHZ | 1.54 ± 0.93 | 1.67 ± 0.78 | 1.62 ± 0.86 |
Intervention two–intervention one: (–0.14 to 0.46) Intervention three–intervention two: (–0.39 to 0.27) |
|||
| HAZ | 0.44 ± 0.32 | 0.48 ± 0.38 | 0.49 ± 0.27 |
Intervention two–intervention one: (–0.11 to 0.2) Intervention three–intervention two: (–0.17 to 0.18) |
|||
| Lower leg length change (knemometry), cm | 1.04 ± 0.48 | 1.03 ± 0.49 | 1.03 ± 0.33 |
Intervention two–intervention one: (–0.23 to 0.2) Intervention three–intervention two: (–0.22 to 0.22) |
|||
| Skinfold thickness, mm | 3.06 ± 1.94 | 3.63 ± 1.87 | 3.61 ± 1.86 |
Intervention two–intervention one: (–0.29 to 1.43) Intervention three–intervention two: (–0.97 to 0.94) |
|||
| MUAC, cm | 1.66 ± 1.40 | 1.98 ± 1.17 | 1.9 ± 1.38 |
Intervention two–intervention one: (–0.26 to 0.89) Intervention three–intervention two: (–0.72 to 0.57) |
|||
|
Comments: all values mean ± SD. No significant differences in change of any anthropometric variable between regimens Good catch-up growth was achieved over 90 days with the average intragroup WHZ improved from 1.54 to 1.67 units, and the HAZ improved from 0.44 to 0.49 units. Lower leg length grew on average 1.03–1.04 cm in 90 days (data presented in figures, but not extracted) |
|||||||
| Safety: in discussion the authors speculate that the detrimental effect of zinc seen in their study may have been because most children had intercurrent infections when micronutrient supplementation was started early in the treatment regimen. Other trials of zinc supplementation have administered zinc at a later stage of rehabilitation, a point when ongoing sepsis is much less likely, although this is unlikely to be representative of practice in most nutritional rehabilitation units | |||||||
| HIV: NR | |||||||
| Barriers to implementation | |||||||
| A general difficulty in this setting is the pressure on caregivers to leave hospital as quickly as possible. This is presumably why in this study, caregivers were required to consent to their child remaining in hospital for 15 days. Nevertheless, some caregivers still discharged their child early before completion of treatment | |||||||
| Methodological comments | |||||||
|
Allocation to treatment groups: an independent observer performed stratified randomisation into three zinc supplementation regimens. Variable length blocks within six strata generated by age (< 13 months and 13–36 months) and type of malnutrition (as defined by Wellcome classification: marasmus; marasmic kwashiorkor and kwashiorkor) were used Blinding: double-blind study. The zinc sulphate and placebo suspensions were indistinguishable and both were formulated and provided by Ciba-Geigy, Bangladesh. Bottles were identical and labelled sequentially from one to 300. On recruitment to the study, two bottle numbers were provided by the independent observer and the corresponding bottles were then selected for that patient [labelled as Bottle A for days 1–15 (either 1.5 or 6.0 mg zinc/kg), and Bottle B for days 16–30 (either 6.0 mg zinc/kg or placebo)] Comparability of treatment groups: states that baseline characteristics were similar between the groups (no p-values reported). Also, numbers of children with kwashiorkor (10–15%), marasmic kwashiorkor (25–30%) and marasmus (55–60%) were equally distributed between the groups Method of data analysis: not ITT analysis. Epi-Info (version 6) was used for data recording and generation of z-scores. All anthropometric data were entered twice with a validation performed between the two entry records and against the hard copy of the data at the end of the data-gathering period. Differences between groups were compared by using Student’s t-tests or one-way analysis of variance for quantitative variables with approximately normal distributions. Mann–Whitney or Kruskal–Wallis tests were used for ordinal variables, long-rank test for length of breastfeeding, and chi-squared tests for categorised variables, with Yates’ correction used for 2 × 2 tables. For outcomes after discharge, the three treatment groups were treated as ordinal, and trends were tested by using Pearson or Spearman correlations as appropriate. Analysis of covariance was used to test differences in quantitative outcomes between groups after adjustment for other factors. An interim analysis of growth and mortality was planned after the first 100 subjects had been studied. When this took place, a trend for more inpatient deaths was observed in the groups receiving 6 mg zinc/kg and recruitment was suspended Sample size/power calculation: sample size was calculated with a requirement for 90% power at the 5% level for 11 anthropometric and biochemical outcome variables, and a sample size of 60 was chosen, which was at the upper end of the calculated sample sizes. Although not explicitly stated, it appears that 60 should have been the sample size for each group; however, recruitment was suspended when 141 children had been enrolled, therefore the overall sample size of 180 was not reached. The authors of the paper do not comment on this Attrition/dropout: reported for each group with reasons provided for the whole sample (not by group). A follow-up worker visited each dwelling at least twice after a subject defaulted from follow-up. All defaulters could not be found |
|||||||
| General comments | |||||||
|
Generalisability: no children < 6 months or > 36 months were included. It is not clear what proportion of the children would have met the current WHO criteria for SAM based on W/H (average initial z-score –2.66), and baseline data on MUAC were not presented. However, the majority of the sample were classified as having marasmus, which may suggest most participants would meet current criteria for SAM Outcome measures: appropriate outcome measures were reported, together with information about data collection and methods for ensuring data quality Intercentre variability: not applicable Conflict of interest: NR |
|||||||
| Standardised clinical management protocol: for all participants | |||||||
| For all if not already receiving them | Broad-spectrum antibiotics, usually ampicillin and gentamicin | ||||||
| For those with a history of invasive diarrhoea | Nalidixic acid or mecillinam | ||||||
| For those with skin sepsis | Cloxacillin | ||||||
| Liquid dietary regimen according to type of malnutrition and whether diarrhoea present or not, and number of days since recruitment | |||||||
| Per 100ml | No diarrhoea | Diarrhoea present | |||||
| Type | Dried skim-milk based | Rice based | |||||
| Energy | 264 kJ | 259 kJ | |||||
| Protein | 2.2 g | 1.1 g | |||||
| Zinc | 0.3 mg | 0.3 mg | |||||
| Volume delivered every 2 hours (by nasogastric tube initially until appetite improved and child able to take full volume offered by mouth) |
Oedematous malnutrition: 80 ml/kg/day Non-oedematous malnutrition 120 ml/kg/day With incremental steps up to 200 ml/kg/day during the inpatient stay of each child |
||||||
| Breastfeeding was encouraged and solid food was offered ad libitum (no details of solid food provided) | |||||||
| For those aged > 1year | Vitamin A at admission 200,000 IU retinyl palmitate (60,000 µg retinol equivalent) | ||||||
| For those aged < 1 year | Vitamin A at admission 100,000 IU retinyl palmitate (30,000 µg retinol equivalent) | ||||||
| For all those recruited | Daily multivitamin supplement: 3000 IU vitamin A; 30 mg vitamin C; 600 IU vitamin D; 0.96 mg thiamine; 0.6 mg riboflavin; 0.6 mg pyridoxine; 0.6 mg nicotinamide | ||||||
| If blood film taken on day 30 of the trial indicated iron deficiency anaemia | Iron supplementation (no details of dose provided) | ||||||
Quality assessment for primary studies (modified for severe malnutrition)
| A. Selection bias | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1. Are the individuals selected to participate in the study likely to be representative of the target population? | Very likely | Somewhat likely | Not likely | Cannot tell | |||||
| ✓ | |||||||||
| 2. What percentage of selected individuals participated? | 80–100% | 60–79% | < 60% | N/A | Cannot tell | ||||
| ✓ | |||||||||
|
Summary of selection bias (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| B. Study design | |||||||||
|
1. What was the study design? (Please tick appropriate and specify design if categorise as ‘Other’) |
RCT | ✓ | |||||||
| CCT | |||||||||
| Cohort analytic (two group pre + post) | |||||||||
| Case–control | |||||||||
| Cohort [one group pre + post (before and after)] | |||||||||
| Interrupted time series | |||||||||
| Other – specify | |||||||||
| Cannot Tell | |||||||||
| 2. Was the study described as randomised? | Yes | No | |||||||
| ✓ | |||||||||
| If answer to no. 2 is ‘no’ complete summary then go to section C. Confounders. If answer is ‘yes’, answer no. 3 and no. 4 below, before completing summary for this section | |||||||||
| 3. If answer was yes, was the method of randomisation described? | Yes | No | |||||||
| ✓ | |||||||||
| 4. If answer was yes, was the method appropriate? | Yes | No | |||||||
| ✓ | |||||||||
|
Summary of study design (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| C. Confounders | |||||||||
| 1. Were there important differences between groups prior to the intervention? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. If yes, indicate the percentage of relevant confounders that were controlled [either in the design (e.g. by stratification or matching) or in the analysis]? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
|
Summary of confounders (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| D. Blinding | |||||||||
| 1. Was the outcome assessor aware of the intervention or exposure status of participants? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were the study participants aware of the research question? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
|
Summary of blinding (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| E. Data collection methods | |||||||||
| 1. Were data collection tools shown to be valid? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were data collection tools shown to be reliable? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
|
Summary of data collection (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| F. Withdrawals and dropouts | |||||||||
| 1. Were withdrawals and dropouts reported in terms of numbers and reasons per group? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Indicate the percentage of participants completing the study (If the percentage differs by groups, record the lowest) | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
|
Summary of withdrawals and dropouts (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| G. Intervention integrity | |||||||||
| 1. What percentage of participants received the allocated intervention or exposure of interest? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
| 2. Was the consistency of the intervention measured? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 3. Is it likely that subjects received an unintended intervention (contamination or co-intervention) that may influence the results? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| H. Analysis | |||||||||
| 1. Indicate the unit of allocation | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 2. Indicate the unit of analysis | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 3. Are the statistical methods appropriate for the study design? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 4. Is the analysis performed by intervention allocation status (i.e. ITT) rather than actual intervention received? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
|
Global rating for studya (Overall methodological strength of study – based on sections A–F) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
Gatheru et al. 198870
Data extraction table
| Reference and design | Intervention | Participants | Outcome measures |
|---|---|---|---|
|
Author: Gatheru et al. 70 Year: 1988 Country: Kenya Study design: CCT Setting: inpatient Number of centres: one Funding: partly supported by the Kenya Medical Research Institute and the Ministry of Health |
Intervention: zinc supplement of 5 mg elemental zinc/kg body weight/day given in three divided doses Control: no zinc The study also included a third group of children without kwashiorkor who are NR on here Other interventions used: both groups managed with high protein diet, motherly care and warmth. Breastfeeding continued if it was occurring. Antibiotics given if infection suspected or confirmed |
Definition of SAM: kwashiorkor as defined by Wellcome classification Number of participants: N = 82 (zinc group, n = 42; control group, n = 40) Sample attrition/dropout: 24 participants did not complete the study, 11 in the zinc group and 13 in the control group Sample crossovers: none Inclusion criteria: diagnosis of kwashiorkor (Wellcome classification), aged 1–3 years Exclusion criteria: transfusions required, serious disease-like TB or measles present, sickle cell disease, absconded before clinical cure, and if death occurred before completion of study General characteristics of participants: patients aged 1–3 years with kwashiorkor |
Primary outcomes: not specifically stated Outcomes included:Method of assessing outcomes: weights recorded using the Toledo machine model 1361 Sentinel (Toledo, OH, USA) on admission and daily thereafter until discharge Serum zinc determined for admission (or latest on second day) and again on 10th day of treatment from a clotted blood sample by the atomic absorption spectroscopy method. One senior technician made all measurements Signs and symptoms were obtained at admission and daily by the author. Diarrhoea was noted if a patient passed more than three loose stools in 24 hours. Anorexia was noted if the child showed no interest or will to eat or drink the feeds given. Improvement in anorexia was marked by willingness to feed. Skin ulcerations included raw, wet, oozy lesions regardless of the presence of scalding and/or skin dyspigmentation. Healing of lesions was noted as drying up and return of normal colour Discharge criteria: oedema had subsided, diarrhoea had stopped, weight gain on three consecutive readings Adverse symptoms: NR Length of follow-up: 10 days Recruitment dates: presumably the same as the period of study which was March to September 1985 |
| Characteristics of participants | |||
| Characteristic | Zinc (n = 42) | Control (n = 40) | p-value |
| Weight, mean kg | 8.2 | 7.8 | NR |
| Weight 6–10 kg, n | 37 | 38 | NR |
| Weight > 10 kg, n | 5 | 2 | NR |
| Serum zinc, mean (SD) μmol/l | 6.4 (1.36) | 6.4 (1.36) | NR |
| Sex, M : F, n | 20 : 22 | 23 : 17 | NR |
| Age 12–14 months, n | 35 | 35 | NR |
| Age 25–36 months, n | 7 | 5 | NR |
|
Comments: the majority (70/82, 85.4%) of the participants were < 2 years of age The mean (range) serum zinc of the whole group of kwashiorkor patients was 6.4 μmol/l (4.0–12.9 μmol/l), this was statistically significantly lower (p < 0.05) to serum zinc values obtained from a group of children without kwashiorkor |
|||
| Results | |||
| Outcomes | Zinc | Control | p-value |
| Total weight gain,a mean (SD) g | 531 (277) | 338b (235) | < 0.05 |
| Daily weight gain, mean g | 67 | 47.3 | NR |
| Serum zincc after 10 days of treatment, mean change from baseline μmol/l | 0.62 | –0.06 | < 0.05 |
| Diarrhoead duration, mean days (SD) | 3.62 (2.78) | 10.8 (3.4) | < 0.001 |
| Anorexiad duration, mean days (SD) | 6 (3.16) | 10.3 (5.01) | < 0.01 |
| Oedemae duration, range in days | 2–18 | 2–18 | NR |
| Oedemae lost by end of day 7, % | 77 | 55 | NR |
| Days taken to lose oedemae, mean (SD) | 6.3 (4.6) | 8.1 (4.4) | < 0.05 |
| Days taken for skin lesionsd to heal, mean (SD) | 7.9 (3.1) | 11.1 (2.1) | < 0.03 |
| Duration of hospital stay, mean days | 15.9 | 16.9 | > 0.05, NS |
| Safety: NR | |||
| HIV: NR | |||
| Barriers to implementation | |||
| NR | |||
| Methodological comments | |||
|
Allocation to treatment groups: children assigned to two groups in alternating order at the time of admission Blinding: not blinded, although not explicitly stated. Paper refers to a need for a study using a double-blind design Comparability of treatment groups: a limited amount of information was provided about the treatment groups at baseline and this was not commented on by the study authors except to note that both groups contained about equal numbers of males and females Method of data analysis: to test the significance of differences observed in the results the conditional test for the mean using chi-squared approximation was used. A value of p < 0.05 was accepted as significant. The analysis was not by ITT Sample size/power calculation: none reported Attrition/dropout: numbers reported for each group, but no reasons given |
|||
| General comments | |||
|
Generalisability: results likely to be applicable to other patients of this age (1–3 years) with kwashiorkor. The authors do not comment on whether the results could be extrapolated to different ages or patients with different forms of malnutrition (e.g. marasmus) Outcome measures: appear appropriate. Mortality was not noted as an outcome, but as the paper states that those dying before completion of the study were excluded, it is presumed that some participants may have died Intercentre variability: not applicable Conflict of interest: not statement made |
|||
Quality assessment for primary studies (modified for severe malnutrition)
| A. Selection bias | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1. Are the individuals selected to participate in the study likely to be representative of the target population? | Very likely | Somewhat likely | Not likely | Cannot tell | |||||
| ✓ | |||||||||
| 2. What percentage of selected individuals participated? | 80–100% | 60–79% | < 60% | N/A | Cannot tell | ||||
| ✓ | |||||||||
|
Summary of selection bias (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| B. Study design | |||||||||
|
1. What was the study design? (Please tick appropriate and specify design if categorise as ‘Other’) |
RCT | ||||||||
| CCT | ✓ | ||||||||
| Cohort analytic (two group pre + post) | |||||||||
| Case–control | |||||||||
| Cohort [one group pre + post (before and after)] | |||||||||
| Interrupted time series | |||||||||
| Other – specify | |||||||||
| Cannot Tell | |||||||||
| 2. Was the study described as randomised? | Yes | No | |||||||
| ✓ | |||||||||
| If answer to no. 2 is ‘no’ complete summary then go to section C. Confounders. If answer is ‘yes’, answer no. 3 and no. 4 below, before completing summary for this section | |||||||||
| 3. If answer was yes, was the method of randomisation described? | Yes | No | |||||||
| 4. If answer was yes, was the method appropriate? | Yes | No | |||||||
|
Summary of study design (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| C. Confounders | |||||||||
| 1. Were there important differences between groups prior to the intervention? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. If yes, indicate the percentage of relevant confounders that were controlled [either in the design (e.g. by stratification or matching) or in the analysis]? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
|
Summary of confounders (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| D. Blinding | |||||||||
| 1. Was the outcome assessor aware of the intervention or exposure status of participants? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were the study participants aware of the research question? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
|
Summary of blinding (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| E. Data collection methods | |||||||||
| 1. Were data collection tools shown to be valid? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were data collection tools shown to be reliable? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
|
Summary of data collection (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| F. Withdrawals and dropouts | |||||||||
| 1. Were withdrawals and dropouts reported in terms of numbers and reasons per group? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Indicate the percentage of participants completing the study (If the percentage differs by groups, record the lowest) | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
|
Summary of withdrawals and dropouts (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| G. Intervention integrity | |||||||||
| 1. What percentage of participants received the allocated intervention or exposure of interest? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
| 2. Was the consistency of the intervention measured? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 3. Is it likely that subjects received an unintended intervention (contamination or co-intervention) that may influence the results? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| H. Analysis | |||||||||
| 1. Indicate the unit of allocation | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 2. Indicate the unit of analysis | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 3. Are the statistical methods appropriate for the study design? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 4. Is the analysis performed by intervention allocation status (i.e. ITT) rather than actual intervention received? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
|
Global rating for studya (Overall methodological strength of study – based on sections A–F) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
Golden and Golden 199271
Data extraction table
| Reference and design | Intervention | Participants | Outcome measures | ||
|---|---|---|---|---|---|
|
Author: Golden and Golden71 Year: 1992 Country: not clearly stated but appears to be Jamaica, West Indies Study design: CCT Setting: inpatient Number of centres: one Funding: not explicitly stated but appears to be Medical Research Council and the Wellcome Trust |
Moderate Zinc: basic diet supplemented with 76 μmol zinc/kg feed (equivalent to 5 mg zinc) High Zinc: basic diet supplemented with 153 μmol zinc/kg feed (equivalent to 10 mg zinc) Low Zinc: received basic diet throughout recovery (equivalent to 3.5 mg zinc) Zinc supplement a solution of zinc acetate containing 15.3 μmol (1 mg) zinc/ml Other interventions used: prior to selection children had been treated with antibiotics and antihelminthics as appropriate. They had been fed according to a standard protocol (details at end of table) After selection all received a high-energy soy-based formula (details at end of table) |
Definition of SAM: Wellcome criteria Number of participants: N = 11 (moderate zinc, n = 4; high zinc, n = 3; low zinc, n = 4) Sample attrition/dropout: NR Sample crossovers: NR Inclusion criteria: within 2 weeks of admission, free of oedema and signs of systemic infection, ready to commence high-energy feeds Exclusion criteria: NR General characteristics of participants: all boys |
Primary outcomes: no primary outcome explicitly stated Outcomes included:Method of assessing outcomes: daily dietary intakes calculated from the sum of the weight of formula taken at the eight daily feeds Body weights measured to nearest gram at 0800 hours each day. Minimum weight was taken as 0% recovery, 100% recovery was defined as the weight of a reference child (NCHS) of same length as the patient at the time of minimum weight measurement Metabolic balance studies were performed, but details of these not data extracted Adverse symptoms: not explicitly reported (although diarrhoea occurred during 9 of 32 balance experiments) Length of follow-up: not stated, but outcomes reported here are for 6-week follow-up Recruitment dates: NR |
||
| Characteristics of participants | |||||
| Characteristic | Low zinc (n = 4) | Moderate zinc (n = 4) | High zinc (n = 3) | p-value | |
| Age, months | 18 ± 4 | 15 ± 2 | 13 ± 4 | NR | |
| Plasma zinc, μmol | 9.6 ± 1.9 | 11.1 ± 1.4 | 9.9 ± 1.3 | NR | |
| Weight, kg | 4.9 ± 0.3 | 5.1 ± 0.4 | 4.9 ± 0.8 | NR | |
| Length, cm | 67 ± 1 | 70 ± 2 | 68 ± 4 | NR | |
| L/A % | 82 ± 3 | 88 ± 3 | 90 ± 1 | NR | |
| W/L % | 63 ± 2 | 60 ± 4 | 61 ± 2 | NR | |
|
Comments: baseline data reported as mean ± SEM Overall age range 6–31 months (median 15 months). Before selection to the trial, nine children had marasmic kwashiorkor and two had marasmus L/A % and W/L % are per cent of NCHS reference values |
|||||
| Results | |||||
| Outcomes during first 6 weeks of recovery | Low zinc (n = 4) | Moderate zinc (n = 4) | High zinc (n = 3) | p-value | |
| Energy intake, kJ/kg/day | 705 ± 18 | 730 ± 26 | 701 ± 35 | NR but states not significantly different for either measure | |
| Nitrogen intake mmol/kg/day | 41 ± 3 | 42 ± 4 | 42 ± 3 | ||
| Rate of weight gain, g/kg/day | 10.10 ± 0.22 | 11.60 ± 0.95 | 11.67 ± 1.41 | No significant difference, p-value NR | |
| Energy cost of tissue deposition, kJ/g | 29.3 ± 2.6 | 24.8 ± 1.7 | 25.0 ± 0.6 | NR | |
|
Comments: values are mean ± SEM Although zinc-supplemented children gained weight faster, difference with low-zinc group was NS. Energy cost of tissue deposition (ECTD) values higher in the low-zinc group, no p-value reported and states will be published separately Outcomes from metabolic balance studies not data extracted |
|||||
| Safety: NR | |||||
| HIV: NR | |||||
| Barriers to implementation | |||||
| NR | |||||
| Methodological comments | |||||
|
Allocation to treatment groups: selected within 2 weeks of admission. Consecutive children assigned first to moderate-zinc group, then to low-zinc group, then to high-zinc group Blinding: NR Comparability of treatment groups: states that at selection there were no significant anthropometric differences among the groups, and plasma zinc was also not different among the zinc groups Method of data analysis: data were analysed using the statistical routines in Systat (Systat Software Inc., Evanston, IL, USA). ANOVA with post-analysis contrasts and repeated measures analysis of variance were used to assess differences in results. Statistical significance was assumed at the 5% level. The results were presented as means ± SEM, and in some cases as individual values Sample size/power calculation: none reported Attrition/dropout: NR, appears to be none |
|||||
| General comments | |||||
|
Generalisability: participants were all boys (presumably to facilitate separate collection of urine and faeces during metabolic balance experiments); however, there does not seem to be any reason why the results would not hold for girls also Outcome measures: appear appropriate, but the method of obtaining weights and lengths was NR Intercentre variability: not applicable Conflict of interest: no statement made. Funding appears to come from the Medical Research Council and the Wellcome Trust |
|||||
| Initial feeding protocol (before selection into trial) | Cow’s milk diet:
|
||||
| High-energy feeding protocol (after entry into trial) |
Sobee, Mead Johnson diet (Mead Johnson and Company, Evansville, IN, USA):Contents per kg feed:Fed by cup 3-hourly, to appetite (notes that this usually increased rapidly) Potassium, magnesium and vitamin supplements continued as previous dosage Ferrous sulphate commenced 0.4 mmol/child/day |
||||
Quality assessment for primary studies (modified for severe malnutrition)
| A. Selection bias | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1. Are the individuals selected to participate in the study likely to be representative of the target population? | Very likely | Somewhat likely | Not likely | Cannot tell | |||||
| ✓ | |||||||||
| 2. What percentage of selected individuals participated? | 80–100% | 60–79% | < 60% | N/A | Cannot tell | ||||
| ✓ | |||||||||
|
Summary of selection bias (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| B. Study design | |||||||||
|
1. What was the study design? (Please tick appropriate and specify design if categorise as ‘Other’) |
RCT | ||||||||
| CCT | ✓ | ||||||||
| Cohort analytic (two group pre + post) | |||||||||
| Case–control | |||||||||
| Cohort [one group pre + post (before and after)] | |||||||||
| Interrupted time series | |||||||||
| Other – specify | |||||||||
| Cannot Tell | |||||||||
| 2. Was the study described as randomised? | Yes | No | |||||||
| ✓ | |||||||||
| If answer to no. 2 is ‘no’ complete summary then go to section C. Confounders. If answer is ‘yes’, answer no. 3 and no. 4 below, before completing summary for this section | |||||||||
| 3. If answer was yes, was the method of randomisation described? | Yes | No | |||||||
| 4. If answer was yes, was the method appropriate? | Yes | No | |||||||
|
Summary of study design (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| C. Confounders | |||||||||
| 1. Were there important differences between groups prior to the intervention? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. If yes, indicate the percentage of relevant confounders that were controlled [either in the design (e.g. by stratification or matching) or in the analysis]? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
|
Summary of confounders (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| D. Blinding | |||||||||
| 1. Was the outcome assessor aware of the intervention or exposure status of participants? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were the study participants aware of the research question? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
|
Summary of blinding (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| E. Data collection methods | |||||||||
| 1. Were data collection tools shown to be valid? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were data collection tools shown to be reliable? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
|
Summary of data collection (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| F. Withdrawals and dropouts | |||||||||
| 1. Were withdrawals and dropouts reported in terms of numbers and reasons per group? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Indicate the percentage of participants completing the study (If the percentage differs by groups, record the lowest) | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
|
Summary of withdrawals and dropouts (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| G. Intervention integrity | |||||||||
| 1. What percentage of participants received the allocated intervention or exposure of interest? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
| 2. Was the consistency of the intervention measured? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 3. Is it likely that subjects received an unintended intervention (contamination or co-intervention) that may influence the results? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| H. Analysis | |||||||||
| 1. Indicate the unit of allocation | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 2. Indicate the unit of analysis | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 3. Are the statistical methods appropriate for the study design? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 4. Is the analysis performed by intervention allocation status (i.e. ITT) rather than actual intervention received? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
|
Global rating for studya (Overall methodological strength of study – based on sections A–F) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
Hemalatha et al. 199372
Data extraction table
| Reference and design | Intervention | Participants | Outcome measures | |
|---|---|---|---|---|
|
Author: Hemalatha et al. 72 Year: 1993 Country: India Study design: CCT (after quality assessment) Setting: inpatient Number of centres: one Funding: NR |
Intervention: zinc as zinc sulphate (zincSO4) in a capsule (40 mg elemental zinc per capsule). Single dose each day. Estimated to be about 6 mg/kg body weight/day Control: placebo capsule, one each day zinc and placebo administered from admission for 21 days Other interventions used: all children received a cereal-based diet and dairy milk provided ad libitum. Details in separate table at end IM injection of vitamin A 100,000 IU |
Definition of SAM: [v]Gómez classification with W/A < 60% of that expected (NCHS standard). Those with loss of subcutaneous fat and with muscle wasting (marasus), those with oedema with wasting (marasmic kwashiorkor) Number of participants: N = 33 (zinc n = 16, placebo n = 17) Sample attrition/dropout: NR (but there is missing data) Sample crossovers: none Inclusion criteria: children hospitalised for rehabilitation from severe PEM Exclusion criteria: clinical evidence of any infection General characteristics of participants: children aged 1–5 years in hospital with SAM |
Primary outcomes: none specifically reported Outcomes included:Method of assessing outcomes:Adverse symptoms: NR Length of follow-up: 1 month Recruitment dates: August 1990 to August 1991 |
|
| Characteristics of participants | ||||
| Characteristic | Zinc (n = 16) | Placebo (n = 17) | p-value | |
| Age (years) | ||||
| 1–2 | 6 | NR | ||
| 2–5 | 27 | |||
| Marasmic kwashiorkor, n and mean weight (SD) | n = 7, 7.5 kg (0.56) | n = 7, 7.3 kg (0.49) | NR | |
| Marasmic. n and mean weight (SD) | n = 9, 6.7 kg (0.56) | n = 10, 7.2 kg (0.38) | NR | |
| Leucocyte zinc μg/1010 cells, n and mean weight (SD) | n = 12, 46.9 (5.490) | n = 10, 45.7 (4.409) | NR | |
| Plasma zinc μg/dl, n and mean weight (SD) | n = 13, 80.4 (9.972) | n = 12, 83.6 (10.363) | NR, but stated they were comparable at baseline | |
| Plasma copper μg/dl, n and mean weight (SD) | n = 13, 112.1 (9.487) | n = 12, 99.1 (15.346) | NR, but stated they were comparable at baseline | |
| Comments: initial zinc and copper status of the zinc group and placebo group described as comparable, and statistically significantly lower (p < 0.001) than levels in healthy children (not data extracted). Few details about baseline characteristics presented | ||||
| Results | ||||
| Primary outcomes | Zinc (n = 16) | Placebo (n = 17) | p-value | |
| Leucocyte zinc μg/1010 cells, n and mean (SE) | n = 12,a 107.2 (13.224) | n = 10, 70.9 (8.414) | NR | |
| Leucocyte zinc, change from baselineb μg/1010 cells |
n = 12a 60.3 p < 0.001 |
n = 10 25.2 p < 0.025 |
||
| Plasma zinc, μg/dl, mean (SE) | n = 13, 107.5 (11.822) | n = 12, 68.2 (7.031) | NR | |
| Plasma zinc, change from baseline,b μg/dl |
n = 13 27.1 p < 0.01 |
n = 12 –15.4 p = NS |
||
| Plasma copper μg/dl, n and mean (SE) |
n = 13 145.3 (8.621) |
n = 12 144.8 (13.258) |
NR | |
| Plasma copper, change from baseline,b μg/dl |
n = 13 33.2 p < 0.01 |
n = 12 45.7 p = 0.025 |
||
| Days for oedema to disappear, mean (SE) | 9.0 (2.035) | 15.7 (2.7) | NS | |
| Duration of morbidity, days, mean (SE) | 6.3 (0.959) | 7.7 (1.040) | c | |
| Weight gain g/kg body weight/day, n and mean weight (SE) in: | ||||
| Week one | n = 16, 22.2 (8.365) | n = 16, 31.1 (9.629) | NR | |
| Week two | n = 15, 25.1 (5.892) | n = 17, 23.7 (7.494) | NR | |
| Week three | n = 14, 23.1 (4.945) | n = 16, 22.3 (6.155) | NR | |
| Week four | n = 12, 22.6 (5.100) | n = 15, 24.5 (5.035) | NS | |
|
Comments: data on haemoglobin and albumin levels are presented (again no between-group comparison), but have not been data extracted. Data on average energy intake in the two groups is provided separately for each of weeks 1 to 4 but these have not been data extracted Overall reports that zinc supplementation did not have any additional benefit on the clinical or biochemical responses measured |
||||
| Safety: NR other than a statement that the zinc supplements as given in the study were not found to adversely affect plasma copper levels | ||||
| HIV: NR | ||||
| Barriers to implementation | ||||
| NR | ||||
| Methodological comments | ||||
|
Allocation to treatment groups: no details provided. Only states that zinc capsule or placebo was randomly administered Blinding: capsules coded in a laboratory by a person not connected with the study. After analysing clinical findings and completing the biochemical estimations, data were decoded and results analysed Comparability of treatment groups: initial zinc and copper status described as comparable in the two groups, but statistically significantly lower (p < 0.001) than healthy children (based on data from 34 health children with normal nutritional status tested as part of the study). Few baseline characteristics presented Method of data analysis: states that as the results were similar in marasmic and marasmic kwashiorkor children, the findings were pooled for each group. Similarly, results for boys and girls were combined because no significant sex-related differences were observed. t-tests used to compare between groups for outcomes of body weight gain and energy intake, paired t-tests used to compare before and after outcomes within groups for some outcomes. No other information provided Sample size/power calculation: NR Attrition/dropout: NR. However, it is clear from the information provided about numbers of participants contributing data to the different outcomes that there is missing data. For data derived from blood samples (leucocyte zinc, plasma zinc, plasma copper), data is missing because only 25 (of the 33) participants allowed a second blood sample to be taken at 4 weeks. For other outcomes, e.g. duration of morbidity, weight gain, no explanation for missing data is provided |
||||
| General comments | ||||
|
Generalisability: results likely to be generalisable to children > 1 year in age with PEM, providing they do not have infection Outcome measures: appear appropriate but, in general, between group comparisons have not been reported Intercentre variability: not applicable Conflict of interest: none reported |
||||
| Rehabilitation diet | ||||
| Energy/day | 700 kJ (8–10% derived from protein) | |||
| Protein/kg body weight/day | 3–4 g | |||
| Multivitamin | One tablet | |||
| Ferrous sulphate | 20 mg elemental iron in one capsule | |||
| Dietary analysis showed mean dietary zinc values of 7.3 ± 0.49 mg/1 day’s diet. Although not explicitly stated it is assumed that this was the dietary content received by all participants, with those in the zinc group receiving additional zinc via the supplement | ||||
Quality assessment for primary studies (modified for severe malnutrition)
| A. Selection bias | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1. Are the individuals selected to participate in the study likely to be representative of the target population? | Very likely | Somewhat likely | Not likely | Cannot tell | |||||
| ✓ | |||||||||
| 2. What percentage of selected individuals participated? | 80–100% | 60–79% | < 60% | N/A | Cannot tell | ||||
| ✓ | |||||||||
|
Summary of selection bias (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| B. Study design | |||||||||
|
1. What was the study design? (Please tick appropriate and specify design if categorise as ‘Other’) |
RCT | ||||||||
| CCT | ✓ | ||||||||
| Cohort analytic (two group pre + post) | |||||||||
| Case–control | |||||||||
| Cohort [one group pre + post (before and after)] | |||||||||
| Interrupted time series | |||||||||
| Other – specify | |||||||||
| Cannot Tell | |||||||||
| 2. Was the study described as randomised? | Yes | No | |||||||
| ✓ | |||||||||
| If answer to no. 2 is ‘no’ complete summary then go to section C. Confounders. If answer is ‘yes’, answer no. 3 and no. 4 below, before completing summary for this section | |||||||||
| 3. If answer was yes, was the method of randomisation described? | Yes | No | |||||||
| ✓ | |||||||||
| 4. If answer was yes, was the method appropriate? | Yes | No | |||||||
|
Summary of study design (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| C. Confounders | |||||||||
| 1. Were there important differences between groups prior to the intervention? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. If yes, indicate the percentage of relevant confounders that were controlled [either in the design (e.g. by stratification or matching) or in the analysis]? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
|
Summary of confounders (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| D. Blinding | |||||||||
| 1. Was the outcome assessor aware of the intervention or exposure status of participants? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were the study participants aware of the research question? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
|
Summary of blinding (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| E. Data collection methods | |||||||||
| 1. Were data collection tools shown to be valid? | Yes | No | Cannot tell | ||||||
| ✓ – zinc | ✓ – weight | ||||||||
| 2. Were data collection tools shown to be reliable? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
|
Summary of data collection (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ – zinc | ✓ – weight | ||||||||
| F. Withdrawals and dropouts | |||||||||
| 1. Were withdrawals and dropouts reported in terms of numbers and reasons per group? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Indicate the percentage of participants completing the study (If the percentage differs by groups, record the lowest) | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
|
Summary of withdrawals and dropouts (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| G. Intervention integrity | |||||||||
| 1. What percentage of participants received the allocated intervention or exposure of interest? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
| 2. Was the consistency of the intervention measured? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 3. Is it likely that subjects received an unintended intervention (contamination or co-intervention) that may influence the results? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| H. Analysis | |||||||||
| 1. Indicate the unit of allocation | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 2. Indicate the unit of analysis | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 3. Are the statistical methods appropriate for the study design? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 4. Is the analysis performed by intervention allocation status (i.e. ITT) rather than actual intervention received? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
|
Global rating for studya (Overall methodological strength of study – based on sections A–F) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
Khanum et al. 198873
Data extraction table
| Reviewer: DM | Date: 6 September 2010 | Version: 2 | Checked by: DH |
|---|---|---|---|
| Reference and design | Intervention | Participants | Outcome measures |
|
Author: Khanum et al. 73 Year: 1988 Country: Bangladesh Study design: CCT Setting: inpatient (Children’s Nutrition Unit) Number of centres: one Funding: NR |
Intervention: zinc supplement [10 mg zinc/kg/day as zinc sulphate (zincSO4) for those weighing < 6 kg; 50 mg daily for those > 6 kg] given on the 15th hospital day for 3 weeks Control: standard care (no zinc supplement) Other interventions used: all children received milk feeds, rice-based solid foods ad libitum up to four times/day, and vitamins and iron supplementation (see end of table for further details) Infections had been treated before the administration of the intervention (15th hospital day) |
Definition of SAM: Waterlow 1976. 101 All children with oedema and all those, with or without oedema, who were ≤ 60% W/H Number of participants: N = 60 (zinc supplemented, n = 30; control, n = 30) Sample attrition/dropout: NR Sample crossovers: NR Inclusion criteria: SAM children who had been admitted to the Children’s Nutrition Unit Exclusion criteria: NR General characteristics of participants:The prevalence of infections such as diarrhoea (80%), pneumonia (56%), and of other nutrient deficiencies such as xerophthalmia (76%) and anaemia (50%) was similar in both groups |
Primary outcomes: not specifically stated Outcomes:Method of assessing outcomes: nutritional status was assessed by W/A (Harvard standard) for < 1 year, and by W/H (Stuart and Stevenson 195925) and presence or absence of oedema for > 1 year One ml of venous blood was drawn for measurement of plasma zinc and albumin on admission, on the 15th hospital day, and on discharge (36th hospital day) Plasma zinc concentration was estimated by atomic absortion spectrophotometry Weight, height and mid-arm circumference were measured on admission. Body weight was recorded at the same time each day, initially each morning, then weekly, by the same person. Height was measured weekly Dietary intakes were measured by weighting each plate of food and leftovers; any vomitus was recorded for each feed and the total daily intake calculated. The energy value of samples of the diet was estimated by bomb calorimetry, and energy intake was calculated for each week as the average intake/day divided by the average weight of the child during that week Adverse symptoms: NR Length of follow-up: 5 weeks total study time (2 weeks lead in, 3 weeks of treatment; no additional follow-up after treatment ceased) Recruitment dates: NR |
| Characteristics of participants | |||
| Characteristic | Zinc supplemented (n = 30) | Control (non-supplemented) (n = 30) | p-value |
| Age (months) | |||
| 5 –12 | 4 | 2 | NR |
| 12–24 | 6 | 8 | NR |
| 24–36 | 8 | 8 | NR |
| 36–48 | 6 | 8 | NR |
| > 48 | 6 | 4 | NR |
| Kwashiorkor, n (%)a | 13 (43) | 9 (30) | NR, NS |
| Results | |||
| Outcomes | Zinc supplemented (n = 30) | Control (non-supplemented) (n = 30) | p-value |
| Plasma zinc concentration (mmol/l)b | |||
| On admission day | 8.23 ± 0.7 | 7.90 ± 0.7 | NR |
| 15th day (zinc started) | 7.88 ± 0.7 | 8.07 ± 0.5 | NR |
| 36th day (discharged) | 18.53 ± 1.5 | 10.56 ± 0.9 | < 0.001 |
| Weekly weight gain (g/week) | |||
| First week | 600 ± 99.9 | 468 ± 81.7 | NR |
| Second week | 521 ± 75.4 | 330 ± 65.9 | NR |
| Third week (zinc started) | 580 ± 67.6 | 342 ± 86.5 | < 0.05 |
| Fourth week | 403 ± 41.6 | 269 ± 47.1 | < 0.05 |
| Fifth week | 462 ± 42.4 | 374 ± 48.9 | NR |
| Mean weight gain rate > 10 g/kg/day | 66% | 33% | 0.02 |
| W/Hc | |||
| On admission day | 70 ± 1.3 | 67 ± 1.3 | NR |
| Eighth day | 76 ± 1.4 | 72 ± 1.0 | < 0.05 |
| 15th day (zinc started) | 80 ± 1.4 | 75 ± 1.1 | < 0.05 |
| 22nd day | 87 ± 1.2 | 79 ± 1.3 | < 0.001 |
| 29th day | 91 ± 1.4 | 82 ± 1.4 | < 0.001 |
| 36th day (discharged) | 95 ± 1.2 | 86 ± 1.2 | < 0.001 |
| W/Ac | (n = 29) | (n = 28) | |
| On admission day | 50.3 ± 1.61 | 47.6 ± 1.60 | NR |
| Eighth day | 52.5 ± 1.44 | 49.9 ± 1.44 | NR |
| 15th day (zinc started) | 58.1 ± 1.53 | 52.3 ± 1.60 | < 0.05 |
| 22nd day | 62.0 ± 1.57 | 55.2 ± 1.75 | < 0.01 |
| 29th day | 64.8 ± 1.58 | 57.1 ± 1.85 | < 0.01 |
| 36th day (discharged) | 68.1 ± 1.58 | 59.7 ± 1.77 | < 0.001 |
| Per cent of patients with W/H according to the Harvard standard on discharge (36th day), n (%) | (n = 30) | (n = 30) | |
| < 80 | 0 (0) | 5 (16.7) | NR |
| 80–90 | 7 (23.3) | 18 (60.0) | NR |
| ≥ 90 | 23 (76.6) | 7 (23.3) | < 0.001 |
|
Comments: results were reported as mean ± sem Reports no significant difference in energy intake between groups during the total treatment period. The authors also report that weight gain was the same in both sexes; an increase in appetite following zinc supplementation was not observed, and supplemental zinc did not increase energy intake (both groups had a mean energy intake of 200 kcal/kg/day) |
|||
| Safety: NR | |||
| HIV: NR | |||
| Barriers to implementation | |||
| NR | |||
| Methodological comments | |||
|
Allocation to treatment groups: children were randomly selected during recovery at the Children’s Nutrition Unit and were alternately allocated to the treatment or the control group Blinding: NR. Assumed patients, care providers nor outcome assessors were blinded Comparability of treatment groups: the supplemented group contained more cases of kwashiorkor (13 out of 30) compared with the unsupplemented controls (9 out of 30), but the difference was not significant. The age distributions, the prevalence of infections and the H/A on admission was similar in both groups (p-values NR) Method of data analysis: Student’s t-test and chi-squared test were used for statistical interpretation of data. A p-value of < 0.05 was accepted as significant. ITT analysis for all outcomes except W/A Sample size/power calculation: NR Attrition/dropout: NR, but appear to be none |
|||
| General comments | |||
|
Generalisability: the authors refer to the paper by Waterlow (1976) to define SAM. 101 However, it is not clear which were the criteria considered. According to the reported W/H on admission data, on average, participants just meet the WHO criteria (W/H < 70%). All children were diagnosed either kwashiorkor or marasmic kwashiorkor. Participants also met the Gómez severe third-degree malnutrition on admission (W/A < 60%) The age range was 5–60 months, although the majority of participants were 12–48 months A subsection of the population admitted to the Children’s Nutrition Unit was randomly selected during recovery from SAM Outcome measures: the outcome measures were appropriate. However, the impact of the intervention on mortality nor its adverse effects were reported Intercentre variability: not applicable Conflict of interest: NR |
|||
| Recovery diets | |||
|
Aimed to achieve a calorie intake of 100–120 kcal/kg/day in the first week, and thereafter 150–200 kcal/kg/day with approximately 2.5 g protein/kg/day; consisted of dried skimmed milk reconstituted with oil and sugar (100 kcal/100 ml), initially given 2-hourly day and night. Given 90–100 ml/kg/day during the first week and increased gradually to 120–250 ml/kg/day in four to six feeds a day Solid cooked meals were offered from the first week; some children refused it initially, but within a few days solid diets were taken |
|||
| Solid diets | |||
|
Rice pudding or Suji (68 kcal/100 g) at 0800 hours; rice + vegetable + meat (beef) mixture (100 kcal/100 g) at 1200 hours; rolls or chapatti (60 kcal/100 g) at 1500 hours and rice + dal (100 kcal/100 g) at 1800 hours All children received supplements of vitamins (Pharmavit), oral iron [4 mg Fe/kg/day as iron sulphate (FeSO4)] and vitamin A capsules (100,000–200,000 IU) The zinc content of individual food items ranged from 1.5–7 p.p.m. |
|||
| A. Selection bias | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1. Are the individuals selected to participate in the study likely to be representative of the target population? | Very likely | Somewhat likely | Not likely | Cannot tell | |||||
| ✓ | |||||||||
| 2. What percentage of selected individuals participated? | 80–100% | 60–79% | < 60% | N/A | Cannot tell | ||||
| ✓ | |||||||||
|
Summary of selection bias (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| B. Study design | |||||||||
|
1. What was the study design? (Please tick appropriate and specify design if categorise as ‘Other’) |
RCT | ||||||||
| CCT | ✓ | ||||||||
| Cohort analytic (two group pre + post) | |||||||||
| Case–control | |||||||||
| Cohort [one group pre + post (before and after)] | |||||||||
| Interrupted time series | |||||||||
| Other – specify | |||||||||
| Cannot Tell | |||||||||
| 2. Was the study described as randomised? | Yes | No | |||||||
| ✓ | |||||||||
| If answer to no. 2 is ‘no’ complete summary then go to section C. Confounders. If answer is ‘yes’, answer no. 3 and no. 4 below, before completing summary for this section | |||||||||
| 3. If answer was yes, was the method of randomisation described? | Yes | No | |||||||
| 4. If answer was yes, was the method appropriate? | Yes | No | |||||||
|
Summary of study design (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| C. Confounders | |||||||||
| 1. Were there important differences between groups prior to the intervention? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. If yes, indicate the percentage of relevant confounders that were controlled [either in the design (e.g. by stratification or matching) or in the analysis]? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
|
Summary of confounders (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| D. Blinding | |||||||||
| 1. Was the outcome assessor aware of the intervention or exposure status of participants? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were the study participants aware of the research question? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
|
Summary of blinding (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| E. Data collection methods | |||||||||
| 1. Were data collection tools shown to be valid? | Yes | No | Cannot tell | a | |||||
| ✓ | |||||||||
| 2. Were data collection tools shown to be reliable? | Yes | No | Cannot tell | ||||||
| ✓ | ✓ | ||||||||
|
Summary of data collection (Methodological strength of study) |
Strong | Moderate | Weak | b | |||||
| ✓ | ✓ | ||||||||
| F. Withdrawals and dropouts | |||||||||
| 1. Were withdrawals and dropouts reported in terms of numbers and reasons per group? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Indicate the percentage of participants completing the study (If the percentage differs by groups, record the lowest) | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
|
Summary of withdrawals and dropouts (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| G. Intervention integrity | |||||||||
| 1. What percentage of participants received the allocated intervention or exposure of interest? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
| 2. Was the consistency of the intervention measured? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 3. Is it likely that subjects received an unintended intervention (contamination or co-intervention) that may influence the results? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| H. Analysis | |||||||||
| 1. Indicate the unit of allocation | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 2. Indicate the unit of analysis | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 3. Are the statistical methods appropriate for the study design? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 4. Is the analysis performed by intervention allocation status (i.e. ITT) rather than actual intervention received? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
|
Global rating for studyc (Overall methodological strength of study – based on sections A–F) |
Strong | Moderate | Weak | d | |||||
| ✓ | ✓ | ||||||||
Makonnen et al. 200374,75
Data extraction table
| Reference and design | Intervention | Participants | Outcome measures |
|---|---|---|---|
|
Author: Makonnen et al. 74 Linked paper: Makonnen et al. 75 (paper excluded on outcomes) Year: 2003 Country: South Africa Study design: described as prospective, double-blinded RCT, but judged as CCT in quality assessment Setting: inpatient and community Number of centres: one Funding: Central Research Fund of the University of Free State and Nestlé, South Africa |
Intervention: standard management with zinc supplementation [10 mg/d of zinc as zinc sulphate (zincSO4) suspension given in drop form from first day of admission] Control: standard management with placebo Other interventions used: all children received initial management to treat hypoglycaemia and hypothermia, dehydration, electrolyte imbalance, septic shock, infections and any other problems, including vitamin deficiencies and anaemia Both groups received the standard treatment regimen: formula diet or continued breastfeeding Health education was given to mothers and carers when child was ready for discharge (Further details are at the end of the table) |
Definition of SAM: PEM as defined by the Wellcome Trust Working Party102 (see Generalisability for further details) Number of participants: N = 300 (zinc supplemented, n = 150; control, n = 150) Sample attrition/dropout: total 46/300 (15%) did not complete follow-up three (90 days) Zinc group: 12/150 (8%), of which eight died Control group: 34/150 (23%), of which 28 died Sample crossovers: NR Inclusion criteria: PEM as defined by the Wellcome classification; aged 6–60 months, > 80% of expected W/A with signs and symptoms of kwashiorkor Exclusion criteria: severe congenital abnormalities, other medical conditions such as congenital heart disease, Down’s syndrome, cerebral palsy, or refusal to participate in the study General characteristics of participants: aged 6–60 months. Approximately half the population had HIV, > 25% suspected to have TB, ≈ 40–50% had diarrhoea, vomiting and fever |
Primary outcomes: mortality, morbidity (including infections), length of hospital stay, anthropometry and biochemical assays (such as serum zinc levels reported in linked paper75) Secondary outcomes: weight gain and other clinical assessments (including oedema, diarrhoea, fever and other infections) Definitions: criteria for discharge from hospital:Method of assessing outcomes: all data collection and physical examinations were done by the same trained medical officer and anthropometric data were collected by the same nurses Weight was recorded on admission daily using a UNICEF scale to the nearest 100 g, with the child naked or minimum clothing and preferably taken at the same time of the day with the same scale Length was recorded for 6–18 months of age using a firm horizontal board with a fixed vertical headpiece and a sliding vertical foot apiece. In older children, height was taken in a standing position The mid-arm circumference for all age groups was measured (in cm) with a non-stretchable tape measure, with the arms hanging loosely to the side. The measure was passed around the circumference of the arm at the same horizontal level as for the measurement of triceps skin-fold thickness A clinical examination and blood tests were done on admission. Venous blood was obtained under fasting conditions for measurement of serum zinc by atomic absorption spectrometry using Fernandez and Kahn’s method. 103 HIV test using enzyme-linked immunosorbent assay (ELISA), TB test using Mantoux read at 48 hours Follow-up assessments done at 30, 60 and 90 days post-discharge Adverse symptoms: NR Length of follow-up: mean hospital stay was 11–12 days and follow-up for 3 months post-discharge Recruitment dates: from 1 January 1999 |
| Characteristics of participants | |||
| Characteristic | Zinc supplemented (n = 150) | Control (non-zinc supplemented) (n = 150) | p-value |
| Male, % | 48.7 | 50.7 | NR |
| Aged 12–23 months, % | 41 | 52 | NR |
| Morbidity, n (%) | |||
| Poor appetite | 89 (59.3) | 70 (46.7) | NR |
| Swelling of body | 95 (63.3) | 78 (52.0) | NR |
| Diarrhoea | 72 (48.0) | 67 (44.7) | NR |
| Vomiting | 77 (51.3) | 83 (55.3) | NR |
| Cough | 55 (36.7) | 57 (38.0) | NR |
| Fever | 82 (54.7) | 59 (39.3) | NR |
| Loss of weight | 118 (78.7) | 114 (76.0) | NR |
| Oral lesions | 125 (83.3) | 121 (80.7) | NR |
| Per cent of expected W/A on admission, n (%) | |||
| < 60% | 56 (37.3) | 54 (36.0) | NR |
| 60–80% | 81 (54.0) | 77 (51.3) | NR |
| > 80% with oedemaa | 12 (8) | 18 (12) | NR |
| > 80% without oedema | 1 (0.7) | 1 (0.7) | NR |
| Mid-arm circumference lower than fifth percentile, n (%) | 96 (90.6) | 105 (87.5) | NR |
| Weight on admission, mean ± SD | 7.2 ± 2.0 | 7.5 ± 2.4 | NR |
| Height on admission, mean ± SD | 72.2 ± 8.2 | 72.7 ± 8.6 | NR |
| Mid-arm circumference, mean ± SD | 11.8 ± 1.6 | 11.9 ± 1.8 | NR |
| HIV+ve, % | 44.7 | 52 | NR |
| Serum zinc (μmol/l), mean ± SDb | 6.23 ± 1.83 | 6.25 ± 1.74 | NR; 95% CI for difference –0.43 to – 0.39 |
|
Comments: the percentage of children with weight > 80% of expected weight on admission was 8.7% in the zinc group and 12.7% in the control group. These differences were not statistically significant. More than 98% of participants in both groups with PEM were admitted for the first time. The majority were < 2 years of age The number and percentage of participants from rural areas, orphans and breastfed for ≥ 12 months, as well as the past medical history of subjects and controls on admission were reported, but have not been data extracted |
|||
| Results | |||
| Primary outcomes | Zinc supplemented (n = 150) | Control (non-zinc supplemented) (n = 150) | Difference (95% CI) |
| Discharged after hospitalisation, % | 92.7 | 80c | NR |
| Death after hospitalisation, n (%) | 7 (4.7) | 26 (17.3) | NRd |
| Death after readmission, n | 1 | 2 | NR |
| Total deaths, n (%)e | 8 (5.3) | 28 (18.7) | NR |
| Morbidity on follow-up (90 days), n (%) | n = 138, 85–95 daysf | n = 116, 83–95 daysf | 95% CI for difference |
| Diarrhoea | 4 (2.9) | 31 (36.7) | –32 to –15.0 |
| Vomiting | 1 (0.7) | 8 (6.9) | –11.2 to –1.2 |
| Fever | 4 (2.9) | 12 (10.3) | –13.8 to –1.1 |
| Oedema | 0 (0) | 0 (0) | –2.0 to 1.2 |
| Acute respiratory infections | 4 (2.9) | 45 (38.8) | –44.7 to –26.2 |
| Skin infection | 1 (0.7) | 8 (6.9) | –11.2 to –1.2 |
| Pallor | 32 (23.2) | 62 (53.4) | –41.3 to –18.4 |
| Anthropometry on discharge | n = 139 | n = 120 | |
| W/A, n (%) | |||
| < 60% | 44 (31.7) | 30 (25) | –4.4 to 17.4 |
| 60–80% | 78 (56.1) | 74 (61.7) | NR |
| > 80% without oedemab | 17 (12.2) | 16 (13.3) | NR |
| Mid-arm circumference percentiles lower than fifth percentile, n (%) | 92 (92.9) | 82 (85.4) | –1.3 to 16.1 |
| Anthropometry on follow-up (90 days) | n = 138 | n = 116 | |
| W/A, n (%) | |||
| < 60% | 5 (3.6%) | 16 (13.8) | –17.2 to –3.1 |
| 60–80% | 52 (37.7%) | 67 (57.8) | NR |
| > 80% without oedema | 81 (58.7%) | 33 (28.4) | NRs |
| Mid-arm circumference percentiles lower than fifth percentile, n (%) | 66 (54.1) | 81 (77.9) | –35.2 to –11.5 |
| Length of hospital stay, mean ± SD | 10.9 ± 3.9 | 11.7 ± 5.9 | NR; not statistically significant |
| Serum zinc at 90 days follow-up (μmol/l), mean ± SDh | 10.13 ± 2.93 | 7.84 ± 1.72 | 95% CI for difference 1.68 to 2.90 |
|
Comments: p-values were NR Data were presented for morbidities during the first 3 weeks of hospitalisation (no morbidity, poor appetite, oedema, diarrhoea, vomiting, cough, fever, weight loss, and oral lesions). The paper reports a general trend for the zinc-supplemented group to recover more rapidly, though it is not true for all symptoms, nor were there any statistically significant differences over the first 3 weeks Data also presented for morbidities at 30-day and 60-day follow-up, but these have not been data extracted Although length was measured at discharge and every follow-up visit, these results were clearly inaccurate and therefore omitted Results for biochemical assays (additional primary outcomes) were reported on linked paper,75 from which only serum zinc at 90 days has been extracted Gastroenteritis was an important diagnosis in both groups, but showed regression during hospitalisation (78% in both groups in first week, 30.4% and 37.4% in zinc and control groups in second week, respectively) The authors mention further monitoring and evaluation being carried out for secondary outcomes, but results for these were NR |
|||
| Safety: NR | |||
|
HIV: six out of seven (85.7%) children in the zinc group and 15 out of 26 (57.7%) children in the control group, who died in the hospital before discharge, were diagnosed to be HIV+ve. All of them had clinical evidence of HIV-related disease. According to the authors, these data suggest that even if the contribution to the death rate caused by possible HIV disease is eliminated, significantly more children in the control group died during hospitalisation than in the supplemented group TB: TB distribution and related findings for both groups were very similar and would not have been a confounding variable for differences in outcome |
|||
| Barriers to implementation | |||
| NR | |||
| Methodological comments | |||
|
Allocation to treatment groups: children were randomly assigned to one of two treatment regimens. Randomisation was stratified by sex, age and percentage of expected W/A. If children were > 80% of expected weight, but had all the clinical features of PEM (kwashiorkor), they were randomised according to the list for 60–80%. No details of the randomisation method used were provided Blinding: double-blinded study. For the non-zinc group, placebo was presented in a similar bottle and colour with similar taste and smell, so that medical personnel and parents could not differentiate between the zinc sulphate and placebo. No details on whether or not outcome assessors were aware of groups Comparability of treatment groups: the demography of the subjects and controls was similar. Reports that the zinc group might have had a more severe disease profile on admission as more children in this group presented with a history of oedema and fever (table 2), but opposite is shown in table 3. The distribution of symptoms, anthropometry and past medical history was quite similar and comparable in both groups. No p-values were reported Method of data analysis: an ITT analysis was not performed. The two groups were compared with respect to the outcome measures using 95% CIs for the differences in percentages or means. Characteristics were summarised per group by frequencies and percentages (for categorical variables) and means, SDs, medians, minima and maxima (for numerical variables). Anthropometric analyses were done using Epi-Info. Arm circumferences were categorised into percentiles according to tables provided by Frisancho. 104 All other analyses were done using Statistical Analysis System software (SAS Institute Inc, Cary, NC). Duration of breastfeeding was analysed using survival analysis. To compare children within a treatment group who survived with those who died, 95% CI for differences in medians were calculated, because of small group size and skewed distribution Sample size/power calculation: the decision to include 150 children in each group was derived after analysis of the data of a pilot study, which included 60 children with PEM and 60 similar children in the control group Attrition/dropout: in the zinc group, 150 children were entered, of which four (2.7%) absconded and seven (4.7%) died before discharge. One child was readmitted after 5 days discharge from hospital and therefore not assessed at follow-up one (30 days), but the four children who absconded did attend the first follow-up visit. Therefore, 142 supplemented children were assessed at follow-up one. One of these was readmitted at follow-up one and subsequently died, leaving 141 at follow-up two (60 days). At follow-up three (90 days), three children could not be traced and 138 were assessed. In the control group, of the 150 children that entered, four (2.7%) absconded and 26 (17.3%) died before discharge. Three children were readmitted, of which one died and two were discharged. At follow-up one, 121 children were assessed. The four children who absconded did attend the first follow-up visit. One child was readmitted and died. One did not turn up for assessment. One hundred and nineteen children were assessed at follow-up two. At follow-up three, three children could not be traced and 116 were assessed In the two groups, the percentage of children who absconded was similar (2.7%). These eight children were all traced, attended the first follow-up, and it was decided to keep them in the study and their data analysed with the rest |
|||
| General comments: | |||
|
Generalisability: malnutrition is defined according to the Wellcome classification as a reduction in the expected body weight < 80% (of the Boston 50th percentile). Between 60% and 80% of expected weight is underweight in the absence of oedema, and kwashiorkor if oedema is present; < 60% of expected weight is marasmus in the absence of oedema, and marasmic kwashiorkor if oedema is present. It is not clear whether or not participants meet the WHO criteria for SAM. The majority of participants had 60–80% W/A on admission using the Wellcome classification, and the majority had MUAC lower than fifth percentile Outcome measures: outcome measures such as W/A, mortality and morbidity were appropriate. However, outcomes as weight gain and adverse effects of the intervention were NR Intercentre variability: not applicable Conflict of interest: NR |
|||
|
Initial treatment began with admission to the hospital and lasted for about 7 days. Its principal aims were to treat or prevent hypoglycaemia, hypothermia, dehydration and electrolyte imbalance; treat septic shock; start feeding the child; treat infections; identify and treat any other problems, including vitamin deficiencies and manage severe anaemia and heart failure RL (20 ml/kg/hour) was given intravenously for severe dehydration or septic shock Most dehydrated children of both groups received ORS through a nasogastric tube. Children were reassessed every hour and rehydration stopped when the child was clinically rehydrated. ORS was continued until diarrhoea stopped or decreased significantly Standard treatment regimen: the treatment of the intervention and control groups was identical, with the exception of the addition of zinc in the management of the supplemented group. To avoid overloading of the intestine, liver and kidneys, small frequent amounts of food were given (50–100 ml every 4 hours). Children who were unable or unwilling to eat were fed by nasogastric tube as a temporary measure. Patients who did not require other emergency treatment (especially for dehydration or septic shock) were given formula diet (Disco-dried skimmed milk-sugar-oil mixture (DSM): 80 g DSM + 60 g oil + 50 g sugar + water up to 1000 ml) or continued breastfeeding in both the study and control group The rehabilitation phase began at about the second week of admission and lasted around 6 weeks. A child entered the rehabilitation phase when his/her appetite returned. The principal aims during this phase were to encourage the child to eat healthily, stimulate physical and emotional development and prepare the mother or caregivers to continue caring for the child after discharge Health education was given to mothers and carers on nutrition, care (e.g. feeding and nutrition), how to recognise the symptoms and signs of illness, when to seek medical assistance, home treatment for diarrhoea, fever and acute respiratory infections Children were followed up at the hospital at 30, 60 and 90 days after discharge. The aims of this stage were to increase feeding appropriately, monitor weight gain and mid-arm circumference, monitor the physical well-being and mental and emotional development of the child and determine their serum zinc levels |
|||
Quality assessment for primary studies (modified for severe malnutrition)
| A. Selection bias | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1. Are the individuals selected to participate in the study likely to be representative of the target population? | Very likely | Somewhat likely | Not likely | Cannot tell | |||||
| ✓ | |||||||||
| 2. What percentage of selected individuals participated? | 80–100% | 60–79% | < 60% | N/A | Cannot tell | ||||
| ✓ | |||||||||
|
Summary of selection bias (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| B. Study design | |||||||||
|
1. What was the study design? (Please tick appropriate and specify design if categorise as ‘Other’) |
RCT | ||||||||
| CCT | ✓ | ||||||||
| Cohort analytic (two group pre + post) | |||||||||
| Case–control | |||||||||
| Cohort [one group pre + post (before and after)] | |||||||||
| Interrupted time series | |||||||||
| Other – specify | |||||||||
| Cannot Tell | |||||||||
| 2. Was the study described as randomised? | Yes | No | |||||||
| ✓ | |||||||||
| If answer to no. 2 is ‘no’ complete summary then go to section C. Confounders. If answer is ‘yes’, answer no. 3 and no. 4 below, before completing summary for this section | |||||||||
| 3. If answer was yes, was the method of randomisation described? | Yes | No | |||||||
| ✓ | |||||||||
| 4. If answer was yes, was the method appropriate? | Yes | No | |||||||
|
Summary of study design (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| C. Confounders | |||||||||
| 1. Were there important differences between groups prior to the intervention? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. If yes, indicate the percentage of relevant confounders that were controlled [either in the design (e.g. by stratification or matching) or in the analysis]? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
|
Summary of confounders (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| D. Blinding | |||||||||
| 1. Was the outcome assessor aware of the intervention or exposure status of participants? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were the study participants aware of the research question? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
|
Summary of blinding (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| E. Data collection methods | |||||||||
| 1. Were data collection tools shown to be valid? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were data collection tools shown to be reliable? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
|
Summary of data collection (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| F. Withdrawals and dropouts | |||||||||
| 1. Were withdrawals and dropouts reported in terms of numbers and reasons per group? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Indicate the percentage of participants completing the study (If the percentage differs by groups, record the lowest) | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
|
Summary of withdrawals and dropouts (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| G. Intervention integrity | |||||||||
| 1. What percentage of participants received the allocated intervention or exposure of interest? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
| 2. Was the consistency of the intervention measured? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 3. Is it likely that subjects received an unintended intervention (contamination or co-intervention) that may influence the results? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| H. Analysis | |||||||||
| 1. Indicate the unit of allocation | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 2. Indicate the unit of analysis | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 3. Are the statistical methods appropriate for the study design? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 4. Is the analysis performed by intervention allocation status (i.e. ITT) rather than actual intervention received? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
|
Global rating for studya (Overall methodological strength of study – based on sections A–F) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
Schlesinger et al. 199276
Data extraction table
| Reference and design | Intervention | Participants | Outcome measures |
|---|---|---|---|
|
Author: Schlesinger et al. 76 Year: 1992 Country: Chile Study design: double-blind CCT Setting: inpatient, tertiary care (closed nutritional recovery centre) Number of centres: one Funding: Nestlé Nutrition Research Grant Programme |
Intervention: zinc-supplemented formula (zinc 15 mg/l), ad libitum, for 105 days Control: standard infant formula (zinc 3.2 mg/l), ad libitum, for 105 days Both formulas based on full-fat powered cow’s milk fortified with vitamins and minerals as per standard infant formula (Nestlé, Switzerland) except for iron and zinc (see end of table for further details). The formulas differed only in zinc content, which was 3.2 mg/l in the standard formula. No other energy-containing supplements were given to either group Other interventions used: none reported |
Definition of SAM: not specifically stated, but mean NCHS WAZ were < 3 SD on admission Number of participants: N = 39 (zinc supplemented, n = 19; control, n = 20) Sample attrition/dropout: none reported Sample crossovers: none Inclusion criteria: only reports marasmic infants with SAM Exclusion criteria: NR General characteristics of participants: SAM infants (< 1 year) |
Primary outcomes: not specifically stated Outcomes were:Method of assessing outcomes: anthropometric measurements performed by a registered nurse on admission and at 15-day intervals. Intake was determined by weighing each bottle before and after feeding. Nude weights obtained before first morning feed with an infant scale (Condor, Santiago, Chile) with a 5-g precision, calibrated at regular intervals. Lengths to nearest 0.1 cm determined by standard procedures with a portable infantometer. Weight and length measurements assessed using NCHS growth percentile curves. z-scores calculated with the PCTL9Z Anthropometry Subroutine (US Centre for Health Promotion and Education, National Centre for Disease Control, Atlanta, GA, USA) Plasma and polymorphonuclear leucocyte zinc concentrations determined using atomic absorption spectrophotometry. Polymorphonuclear leucocytes were isolated by dextran sedimentation and Ficoll Hypaque-gradient centrifugation. Iron nutrition assessed on admission and after 60 and 105 days by haemoglobin with the cyanomethemoglobin method (Coulter Counter ZBI, Fl, USA) and by serum ferritin with a radioimmunoassay (Travenol, Massachusetts). Serum copper concentrations determined by atomic absorption spectrophotometry on admission and after 30, 60 and 105 days Detailed methodology is reported for assessment of the immunological profile but is not extracted here Signs and symptoms of morbidity were recorded daily on a chart by the attending physician. Every infectious episode was analysed using: mean episodes/infant, mean duration days of each episode/infant, and mean per cent of infected days in the 105 days: [(number days with infection/number observed days) × 100] Adverse symptoms:Length of follow-up: nothing further than the 105 days of nutritional rehabilitation treatment Recruitment dates: NR |
| Characteristics of participants | |||
| Characteristic | Zinc-supplemented formula (n = 19) | Control formula (n = 20) | p-value |
| Sex, M : F | 10 : 9 | 10 : 10 | NS |
| Age, months | 7.05 (2.0) | 8.1 (3.0) | NS |
| WAZ on admission | –3.13 (0.71) | –3.21 (0.87) | NS |
| Birth weight, g | 2886 (307) | 3040 (268) | NS |
| Plasma zinc μmol/l, mean ± SD | 19.4 ± 5.5 (n = 18) | 23.4 ± 8.4 (n = 17) | NS |
| Serum copper μmol/l, mean ± SD | 19.5± 7.0 (n = 18) | 20.1 ± 7.4 (n = 17) | NS |
| Intakes/kg/day | |||
| Energy, kJ | 674 (105) | 682 (80) | NS |
| Protein, g | 4.4 (0.7) | 4.5 (0.5) | NS |
| Zinc, mg | 1.9 (0.3) | 0.35 (0.04) | < 0.01a |
| Iron, mg | 1.9 (0.3) | 1.9 (0.2) | NS |
| Copper, mg | 0.04 (0.007) | 0.04 (0.005) | NS |
| Comments: values are mean (± SD) unless otherwise stated | |||
| Outcomes | Zinc-supplemented formula (n = 19) | Control formula (n = 20) | p-value |
| z-scores, mean (± SD) | |||
| H/A | |||
| On admission | –3.27 (0.93) | –3.19 (1.34) | NS |
| 30 days | –3.02 (0.89) | –3.06 (1.04) | NS |
| 60 days | –2.73 (0.95) | –2.78 (1.12) | NS |
| 105 days | –2.64 (0.86) | –2.56 (0.84) | NS |
| W/A | |||
| On admission | –3.13 (0.71) | –3.21 (0.87) | NS |
| 30 days | –2.32 (0.62) | –2.36 (0.74) | NS |
| 60 days | –2.04 (0.1) | –1.95 (0.91) | NS |
| 105 days | –1.66 (0.64) | –1.59 (0.88) | NS |
| W/H | |||
| On admission | –0.83 (0.6) | –1.18 (0.81) | NS |
| 30 days | –0.07 (0.75) | –0.02 (1.15) | NS |
| 60 days | 0.12 (0.84) | 0.17 (1.27) | NS |
| 105 days | 0.42 (0.81) | 0.32 (1.22) | NS |
| Increase in L/A percentile score in relation to admission,% (n/N) | |||
| 30 days | 58 (11/19) | 20 (4/20)b | < 0.002 |
| 45 days | 79 (15/19) | 45 (9/20)b | < 0.03 |
| Plasma zinc μmol/l, mean ± SD 105 days | 18.6 ± 4.3 (n = 18) | 18.0 ± 5.8 (n = 17) | NS |
| Serum copper μmol/l, mean ± SD 105 days | 24.4 ± 4.4 (n = 18) | 22.8 ± 4.6 (n = 17) | NS |
|
Comments: plasma zinc and serum copper concentrations at 30 and 60 days have not been data extracted. There were no significant differences between the groups Nutritional status: Other outcomes (micronutrients, immune function): |
|||
Safety:
|
|||
| HIV: NR | |||
| Barriers to implementation | |||
| NR | |||
| Methodological comments | |||
|
Allocation to treatment groups: not a randomised study. No details regarding allocation of treatments Blinding: states double blind, but no further details are given as to how blinding was ensured in the patients and care providers (formula was provided in bottles). No details whether or not outcome assessors were blinded Comparability of treatment groups: few baseline characteristics were presented; reports there were no significant differences between groups nor between males and females (though no p-values reported) Method of data analysis: appears to be ITT analysis for z-scores (full number of patients allocated to each treatment group were analysed). The Statistical Analysis System software (SAS Institute Inc., Cary, NC, USA) was used. The paired and non-paired t-test, Cochrane Mantel–Hanzel test, Fisher’s exact probability test and stepwise logistic regression were used in the analysis of data. Significance was determined at p < 0.05 Sample size/power calculation: NR Attrition/dropout: none reported |
|||
| General comments | |||
|
Generalisability: likely that most of the children would meet the current WHO criteria (mean WAZ < –3 SD). Unclear whether the children admitted to the tertiary centre were all those with SAM or a subsection. In addition, the mean age was 7–8 months on admission and, therefore, would not be generalisable to all children < 5 years Outcome measures: outcomes were appropriate although mortality data not specifically reported (even though it appears to be zero) Intercentre variability: N/A Conflict of interest: funded by The Nestlé Nutrition Research Grant Program; no conflicts of interest are apparent |
|||
| Composition of formula (per gram of powder) | |||
| Fat 0.26 g, protein 0.26 g, vitamin A 15.2 IU, cholecalciferol 3 IU, vitamin E 0.06 IU, vitamin C 1.5 mg, folic acid 0.45 µg, thiamine 3 µg, niacin 0.038 mg, vitamin B6 3 µg, biotin 0.11 mg, pantothenate 0.023 mg, riboflavin 4.5 µg, vitamin B12 0.011 µg, vitamin K 0.42 µg, choline 0.38 µg, inositol 0.23 mg, iodine 0.38 µg, copper 3.5 µg, iron 0.15 mg (as ferrous sulphate) and zinc 0.15 mg (as zinc chloride). Formula was prepared at 10% dilution | |||
Quality assessment for primary studies (modified for severe malnutrition)
| A. Selection bias | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1. Are the individuals selected to participate in the study likely to be representative of the target population? | Very likely | Somewhat likely | Not likely | Cannot tell | |||||
| ✓ | |||||||||
| 2. What percentage of selected individuals participated? | 80–100% | 60–79% | < 60% | N/A | Cannot tell | ||||
| ✓ | |||||||||
|
Summary of selection bias (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| B. Study design | |||||||||
|
1. What was the study design? (Please tick appropriate and specify design if categorise as ‘Other’) |
RCT | ||||||||
| CCT | ✓ | ||||||||
| Cohort analytic (two group pre + post) | |||||||||
| Case–control | |||||||||
| Cohort [one group pre + post (before and after)] | |||||||||
| Interrupted time series | |||||||||
| Other – specify | |||||||||
| Cannot Tell | |||||||||
| 2. Was the study described as randomised? | Yes | No | |||||||
| ✓ | |||||||||
| If answer to no. 2 is ‘no’ complete summary then go to section C. Confounders. If answer is ‘yes’, answer no. 3 and no. 4 below, before completing summary for this section | |||||||||
| 3. If answer was yes, was the method of randomisation described? | Yes | No | |||||||
| 4. If answer was yes, was the method appropriate? | Yes | No | |||||||
|
Summary of study design (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| C. Confounders | |||||||||
| 1. Were there important differences between groups prior to the intervention? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. If yes, indicate the percentage of relevant confounders that were controlled [either in the design (e.g. by stratification or matching) or in the analysis]? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
|
Summary of confounders (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| D. Blinding | |||||||||
| 1. Was the outcome assessor aware of the intervention or exposure status of participants? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were the study participants aware of the research question? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
|
Summary of blinding (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| E. Data collection methods | |||||||||
| 1. Were data collection tools shown to be valid? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were data collection tools shown to be reliable? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
|
Summary of data collection (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| F. Withdrawals and dropouts | |||||||||
| 1. Were withdrawals and dropouts reported in terms of numbers and reasons per group? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Indicate the percentage of participants completing the study (If the percentage differs by groups, record the lowest) | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
|
Summary of withdrawals and dropouts (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| G. Intervention integrity | |||||||||
| 1. What percentage of participants received the allocated intervention or exposure of interest? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
| 2. Was the consistency of the intervention measured? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 3. Is it likely that subjects received an unintended intervention (contamination or co-intervention) that may influence the results? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| H. Analysis | |||||||||
| 1. Indicate the unit of allocation | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 2. Indicate the unit of analysis | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 3. Are the statistical methods appropriate for the study design? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 4. Is the analysis performed by intervention allocation status (i.e. ITT) rather than actual intervention received? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
|
Global rating for studya (Overall methodological strength of study – based on sections A–F) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
Simmer et al. 198877
Data extraction table
| Reference and design | Intervention | Participants | Outcome measures |
|---|---|---|---|
|
Author: Simmer et al. 77 Year: 1988 Country: Bangladesh Study design: CCT Setting: inpatient (Children’s Nutrition Unit) Number of centres: one Funding: Save the Children Fund (UK); Heinz Fellowship of the British Paediatric Association |
Intervention: zinc supplement [50 mg of zinc as zinc sulphate (zincSO4) daily or 10 mg/kg daily if weight < 5 kg] for 2 weeks Control: standard care (no zinc supplement) Third group: well-nourished children (no details extracted here) Children were allocated to the two groups after ≥ 3 days and usually after 7 days Other interventions used: participants were fed milk every 2 hours (80–120 ml/kg/day increasing to 250 ml/kg/day). Weaning food was also given, consisting of rice, dal (pulses) and vegetables. Meat and bananas were often included, oil was added when more calories were required and an egg was added when serum proteins were low. A full diet (three cooked meals and four milk feeds a day) was usually tolerated by the third day. Additional vitamin A (100,000–200,000 IU/day) and ferrous sulphate (4–6 mg/kg/day) were routinely given A play area with volunteer therapists provided some psychological stimulation for the children Associated diseases and complications of nutritional rehabilitation, such as hypothermia, hypoglycaemia, and fluid overload, were treated promptly TB: diagnosed and treated if at least two of the following criteria were met: history of contact, gradual wasting, fever and cough for 1 month, failure to gain weight despite adequate caloric intake, painless enlargement of cervical nodes or pneumonia that failed to respond to antibiotics |
Definition of SAM: not specifically defined; the nutritional diagnosis was based on McLaren’s criteria. 105 The Children’s Nutrition Unit is specifically for children with third-degree malnutrition, defined as nutritional oedema or W/A < 60% and W/H < 70% of local standards (or < 42% and 63%, respectively, of Western standards) Number of participants: N = 25 (zinc group, n = 13; control group, n = 12) Sample attrition/dropout: one patient was excluded from each group Sample crossovers: none reported Inclusion criteria:Exclusion criteria: not stated General characteristics of participants: |
Primary outcomes: not specifically stated, but appears to be levels of zinc (plasma + polymorphonuclear) and plasma protein Secondary outcomes: not stated, but appears to be vitamins A and E, ferritin, weight gain, calorie intake and protein intake Method of assessing outcomes: weight and height measured on admission Blood was collected at the beginning and, when possible, at the end of the study period for measurement of polymorphonuclear zinc and plasma levels of zinc, vitamins A and E, and ferritin Zinc concentration was measured by flame atomic absorption spectrophotometry. Vitamin A and vitamin E were measured by high-performance liquid chromatography and ferritin levels by an 125I immunoradiometric assay (Details on blood collection and preparation for analysis are given by the authors, but have not been extracted) Protein and calorie intake were calculated daily by the dietitians at Children’s Nutrition Unit; the quantity and type of food was recorded and duplicate food samples were collected from seven children aged 24–48 months and ashed for zinc concentration measurement by atomic absorption spectrophotometry Discharge: usually within 3 weeks if 75–80% W/H (Western standards), haemoglobin > 100 g/l and total serum proteins > 65 g/l. Children with TB were admitted for 6 weeks to ensure adequate drug therapy Adverse symptoms: medical and nursing staff were aware of the possibility of side effects in the children receiving zinc supplements. Protein and calorie intake of both groups were monitored to study anorexia as a potential adverse effect of zinc supplementation Length of follow-up: 2 weeks for outcomes although hospital stay was usually 3 weeks (6 weeks for children with TB) Recruitment dates: NR |
| Characteristics of participants | |||
| Characteristic | Zinc supplemented (n = 12) | Control (non-supplemented) (n = 11) | p-value |
| Age, months (range) | 35.3 ± 5 (12–96) | 42.8 ± 7.8 (12–96) | NR |
| Weight, kg | 6.7 ± 0.6 | 7.9 ± 1.1 | NR |
| W/A, % | 46 ± 3 | 48 ± 3 | NR |
| W/H, % | 70 ± 2 | 66 ± 2 | NR |
| Height, cm | 76 ± 3 | 80 ± 5 | NR |
| H/A, % | 80 ± 2 | 78 ± 4 | NR |
| Nutritional diagnosis (McLaren’s criteria), n | |||
| Marasmus | 1 | 1 | NR |
| Kwashiorkor | 5 | 3 | NR |
| Marasmic kwashiorkor | 6 | 7 | NR |
|
Comments: results are reported as mean ± SE unless otherwise stated No statistically significant differences between groups were reported Whole group mean age = 38.9 ± 4.6 months, mean weight = 7.3 ± 0.6 kg, mean W/A = 47.1 ± 2.3%, mean W/H = 68.1 ± 1.8% Birth order, number of living siblings and family income per month and per capita per day are reported, but have not been extracted |
|||
| Results | |||
| Primary outcomes | Zinc supplemented (n = 12) | Control (non-supplemented) (n = 11) | p-value |
| Polymorphonuclear zinc, mmol/1010 polymorphonuclear | |||
| On admission | – | – | NR |
| On entry to study | 1.75 ± 0.11 | 2.05 ± 0.18 | NR |
| On conclusion of study | 2.59 ± 0.25a | 1.60 ± 0.23 | NR |
| Plasma zinc, μmol/l | |||
| On admission | – | – | NR |
| On entry to study | 10.8 ± 0.8 | 8.6 ± 0.8 | NR |
| On conclusion of study | 14.6 ± 0.9b | 12.3 ± 0.9c | NR |
| Plasma protein, g/dl | |||
| On admission | 5.3 ± 0.3 | 5.1 ± 0.2 | NR |
| On entry to study | 6.6 ± 0.4 | 6.6 ± 0.3 | NR |
| On conclusion of study | 7.6 ± 0.2(8)d | 7.8 ± 0.1(7)c | NR |
|
Comments: packed cell volume, ferritin, vitamin A and vitamin E levels were reported but have not been extracted Overall, plasma zinc and protein levels were weakly correlated (r = 0.56, p < 0.01); in the non-supplemented children the correlation between plasma zinc and protein levels was stronger (r = 0.73, p < 0.001) Anthropometric characteristics and the results on plasma zinc, polymorphonuclear zinc, plasma vitamins A and E levels of a non-malnourished, non-supplemented control group were reported, but have not been extracted |
|||
| Secondary outcomes | Zinc supplemented (n = 12) | Control (non-supplemented) (n = 11) | p-value |
| Mean weight gain, g/day | |||
| Week one | 35 | 32 | NR |
| Week two | 70 ± 20 | 40 ± 10 | NR |
| Weight gain, g/kg/day | |||
| Week one | 4.6 ± 1.9 | 4.9 ± 3.3 | NR/NS |
| Week two | 8.83 ± 1.56 | 5.09 ± 1.62 | NR; 95% CI 0.88 to 8.36 |
| Calorie intake, kcal/kg/day | |||
| Week one | 161 ± 8 | 156 ± 8 | NR/NS |
| Week two | 180 ± 9 | 169 ± 9 | NR/NS |
| Protein intake, g/kg/day | |||
| Week one | 4.7 ± 0.2 | 4.6 ± 0.3 | NR/NS |
| Week two | 5.3 ± 0.2 | 4.9 ± 0.3 | NR/NS |
| Per cent who achieved an optimal rate of weight gain (at Children’s Nutrition Unit, > 10 g/kg/day) | 42 | 9 | < 0.001 |
| Comments: the mean unsupplemented dietary zinc intake of the malnourished children was 3.7 (range 2.4–5.3) mg/d. The zinc contents of individual foods were reported but not extracted | |||
|
Safety: taking into consideration anorexia as a common feature of severe experimental zinc deficiency in animals, there was no significant difference in the intake of the two groups Tube feeding was required for a few days for one patient in each group. Two patients in each group had a blood transfusion |
|||
| HIV: NR | |||
| Barriers to implementation | |||
| NR | |||
| Methodological comments | |||
|
Participants were randomly selected by the nursing sisters of Children’s Nutrition Unit. During nutritional rehabilitation, the mean supplemented dietary in take of zinc was only 3.7 mg/d, which is < 40% the recommended daily allowance. A daily dose of 50 mg probably is unnecessarily large, but did not cause any side effects Allocation to treatment groups: participants were alternately allocated to groups for a 2-week period Blinding: no details reported. Would assume no blinding of children, investigators nor outcome assessors Comparability of treatment groups: reports no differences in baseline characteristics (no p-values reported). The incidence of TB, pneumonia and vitamin A deficiency was also similar in both groups Method of data analysis: all data were expressed as mean ± SE and were analysed by unpaired Student’s t-test. Not ITT analysis Sample size/power calculation: NR Attrition/dropout: one patient was excluded from the zinc group owing to being transferred to the children’s hospital with a provisional diagnosis of typhoid fever. One patient was excluded from the control group because two doses (100 mg) of zinc had been accidentally given |
|||
| General comments | |||
|
Generalisability: the criteria used to define SAM (McLaren’s criteria: < 75% W/H and W/A), differ from the current WHO criteria; however, the average W/H is < 70%. The age inclusion range of 1–7 years differs from SHTAC’s protocol (< 5-year-old children), though the mean age was 39 months. It is not clear whether or not these results can be extrapolated to the general population, as the random selection of participants was not detailed by the authors. Many children had comorbidities, such as TB, pneumonia and vitamin A deficiency Outcome measures: appropriate, though some key outcomes, such as mortality rate, morbidity, and time to recover were NR Intercentre variability: not applicable Conflict of interest: NR |
|||
Quality assessment for primary studies (modified for severe malnutrition)
| A. Selection bias | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1. Are the individuals selected to participate in the study likely to be representative of the target population? | Very likely | Somewhat likely | Not likely | Cannot tell | |||||
| ✓ | |||||||||
| 2. What percentage of selected individuals participated? | 80–100% | 60–79% | < 60% | N/A | Cannot tell | ||||
| ✓ | |||||||||
|
Summary of selection bias (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| B. Study design | |||||||||
|
1. What was the study design? (Please tick appropriate and specify design if categorise as ‘Other’) |
RCT | ||||||||
| CCT | ✓ | ||||||||
| Cohort analytic (two group pre + post) | |||||||||
| Case–control | |||||||||
| Cohort [one group pre + post (before and after)] | |||||||||
| Interrupted time series | |||||||||
| Other – specify | |||||||||
| Cannot Tell | |||||||||
| 2. Was the study described as randomised? | Yes | No | |||||||
| ✓ | |||||||||
| If answer to no. 2 is ‘no’ complete summary then go to section C. Confounders. If answer is ‘yes’, answer no. 3 and no. 4 below, before completing summary for this section | |||||||||
| 3. If answer was yes, was the method of randomisation described? | Yes | No | |||||||
| 4. If answer was yes, was the method appropriate? | Yes | No | |||||||
|
Summary of study design (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| C. Confounders | |||||||||
| 1. Were there important differences between groups prior to the intervention? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. If yes, indicate the percentage of relevant confounders that were controlled [either in the design (e.g. by stratification or matching) or in the analysis]? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
|
Summary of confounders (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| D. Blinding | |||||||||
| 1. Was the outcome assessor aware of the intervention or exposure status of participants? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were the study participants aware of the research question? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
|
Summary of blinding (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| E. Data collection methods | |||||||||
| 1. Were data collection tools shown to be valid? | Yes | No | Cannot tell | a | |||||
| ✓ | ✓ | ||||||||
| 2. Were data collection tools shown to be reliable? | Yes | No | Cannot tell | a | |||||
| ✓ | ✓ | ||||||||
|
Summary of data collection (Methodological strength of study) |
Strong | Moderate | Weak | b | |||||
| ✓ | ✓ | ||||||||
| F. Withdrawals and dropouts | |||||||||
| 1. Were withdrawals and dropouts reported in terms of numbers and reasons per group? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Indicate the percentage of participants completing the study (If the percentage differs by groups, record the lowest) | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
|
Summary of withdrawals and dropouts (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| G. Intervention integrity | |||||||||
| 1. What percentage of participants received the allocated intervention or exposure of interest? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
| 2. Was the consistency of the intervention measured? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 3. Is it likely that subjects received an unintended intervention (contamination or co-intervention) that may influence the results? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| H. Analysis | |||||||||
| 1. Indicate the unit of allocation | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 2. Indicate the unit of analysis | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 3. Are the statistical methods appropriate for the study design? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 4. Is the analysis performed by intervention allocation status (i.e. ITT) rather than actual intervention received? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
|
Global rating for studyc (Overall methodological strength of study – based on sections A–F) |
Strong | Moderate | Weak | ||||||
| ✓ (zinc status) | ✓ (weight) | ||||||||
Vasudevan et al. 199778
Data extraction table
| Reference and design | Intervention | Participants | Outcome measures |
|---|---|---|---|
|
Author: Vasudevan et al. 78 Year: 1997 Country: India Study design: double-blind placebo-controlled trial Setting: outpatient (Division of Department of Paediatric Medical College) Number of centres: one Funding: not stated |
Intervention: zinc-supplemented group received 6.6 mg of elemental zinc, equivalent to 20 mg of zinc sulphate, once daily Control: placebo was provided in similar looking capsules to zinc supplement Other comparator group: normal, healthy children, not malnourished or ill, who were siblings or volunteers were analysed for serum zinc to determine the normal range (outcomes NR) Other interventions used: nutritional counselling to parents, dietary intake adjusted to 100–120 calories/kg/day by instructing the mother |
Definition of SAM: protein energy malnutrition grades III and IV using IAP criteria Number of participants: 72 children recruited, 62 children completed designated follow-up period (31 per group) Sample attrition/dropout: 10 children (five per group) Sample crossovers: not stated Inclusion criteria: aged 8–24 months, suffering from protein–energy malnutrition grades III and IV Exclusion criteria: children with other concurrent causes of malnutrition by history, physical examination and investigations General characteristics of participants: none stated other than inclusion criteria |
Outcomes: weight of the child; serum zinc. Primary and secondary outcomes were not defined Method of assessing outcomes: serum zinc analysis by calorimetric methods using a kit obtained from Randox Laboratories (UK). Weight of the child and serum zinc was assessed at baseline and at 3-months follow-up. Serum zinc was assessed at end of 3 months, allowing 6 days after the last dose of zinc prior to analysis Adverse symptoms: none stated Length of follow-up: 3 months Recruitment dates: none reported |
| Characteristics of participants | |||
| Characteristic | Zinc supplemented (n = 31) | Placebo (n = 31) | p-value |
| Mean serum zinc levels | 98.4 ± 26.1 µg/dl | ||
| Comments: mean serum zinc levels for healthy group 154.4 ± 24 µg/dl significantly different to malnourished children 98.4 ± 26.1 µg/dl (p < 0.001) | |||
| Results | |||
| Outcomes | Zinc supplemented (n = 31) | Placebo (n = 31) | p-value |
| Change in zinc levels (µg/dl) (before-and-after study) | + 51.3 | + 16.4 | < 0.001 |
| Rate of weight gain (g/kg/day) | 1.4 | 0.98 | > 0.1 |
| Comments: states that none of the children with zinc supplementation developed any related side effects | |||
| Safety: none stated | |||
| HIV: none stated | |||
| Barriers to implementation | |||
| None stated | |||
| Methodological comments | |||
|
Allocation to treatment groups: not stated Blinding: double blind Comparability of treatment groups: matched for age (within 3 months), sex, W/A, socioeconomic status, ethnic background (data NR) Method of data analysis: t-tests (paired and Student’s) Sample size/power calculation: not stated Attrition/dropout: 10 children (five per group) did not complete the designated follow-up. Reasons for dropout were NR |
|||
| General comments | |||
|
Generalisability: limited details are provided about the group and so it is only possible to indicate that the study is relevant to children aged 8–24 months with PEM Outcome measures: suitable outcomes were reported Intercentre variability: not relevant Conflict of interest: none stated |
|||
Quality assessment for primary studies (modified for severe malnutrition)
| A. Selection bias | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1. Are the individuals selected to participate in the study likely to be representative of the target population? | Very likely | Somewhat likely | Not likely | Cannot tell | |||||
| ✓ | |||||||||
| 2. What percentage of selected individuals participated? | 80–100% | 60–79% | < 60% | N/A | Cannot tell | ||||
| ✓ | |||||||||
|
Summary of selection bias (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| B. Study design | |||||||||
|
1. What was the study design? (Please tick appropriate and specify design if categorise as ‘Other’) |
RCT | ||||||||
| CCT | ✓ | ||||||||
| Cohort analytic (two group pre + post) | |||||||||
| Case–control | |||||||||
| Cohort [one group pre + post (before and after)] | |||||||||
| Interrupted time series | |||||||||
| Other – specify | |||||||||
| Cannot Tell | |||||||||
| 2. RWas the study described as randomised? | Yes | No | |||||||
| ✓ | |||||||||
| If answer to no. 2 is ‘no’ complete summary then go to section C. Confounders. If answer is ‘yes’, answer no. 3 and no. 4 below, before completing summary for this section | |||||||||
| 3. If answer was yes, was the method of randomisation described? | Yes | No | |||||||
| 4. If answer was yes, was the method appropriate? | Yes | No | |||||||
|
Summary of study design (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| C. Confounders | |||||||||
| 1. Were there important differences between groups prior to the intervention? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. If yes, indicate the percentage of relevant confounders that were controlled [either in the design (e.g. by stratification or matching) or in the analysis]? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
|
Summary of confounders (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| D. Blinding | |||||||||
| 1. Was the outcome assessor aware of the intervention or exposure status of participants? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were the study participants aware of the research question? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
|
Summary of blinding (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| E. Data collection methods | |||||||||
| 1. Were data collection tools shown to be valid? | Yes | No | Cannot tell | ||||||
| ✓ – zinc | ✓ – weight | ||||||||
| 2. Were data collection tools shown to be reliable? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
|
Summary of data collection (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ – zinc | ✓ – weight | ||||||||
| F. Withdrawals and dropouts | |||||||||
| 1. Were withdrawals and dropouts reported in terms of numbers and reasons per group? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Indicate the percentage of participants completing the study (If the percentage differs by groups, record the lowest) | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
|
Summary of withdrawals and dropouts (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| G. Intervention integrity | |||||||||
| 1. What percentage of participants received the allocated intervention or exposure of interest? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
| 2. Was the consistency of the intervention measured? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 3. Is it likely that subjects received an unintended intervention (contamination or co-intervention) that may influence the results? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| H. Analysis | |||||||||
| 1. Indicate the unit of allocation | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 2. Indicate the unit of analysis | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 3. Are the statistical methods appropriate for the study design? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 4. Is the analysis performed by intervention allocation status (i.e. ITT) rather than actual intervention received? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
|
Global rating for studya (Overall methodological strength of study – based on sections A–F) |
Strong | Moderate | Weak | ||||||
| ✓ – zinc | ✓ – weight | ||||||||
Bhutta et al. 199979
Data extraction table
| Reference and design | Intervention | Participants | Outcome measures |
|---|---|---|---|
|
Author: Bhutta et al. 79 Year: 1999 Country: Pakistan Study design: double-blind RCT Setting: Nutrition Research ward at the National Institute of Child Health Number of centres: one Funding: the Applied Diarrhoeal Disease Research Program of the Harvard Institute for International Development via an agreement with the US Agency for International Development |
Intervention: zinc supplementation (3 mg/kg/day of elemental zinc sulphate, single daily dose) during 14 days of inpatient dietary therapy and continued for 14 days at home (with home available diets) after discharge Control: placebo during 14 days of inpatient dietary therapy and continued for 14 days at home (with home available diets) after discharge Other interventions used: applied to all children: stabilisation period of 24 hours during which i.v. and oral rehydration fluids were administered as necessary and antibiotic therapy for concomitant non-enteric infections was initiated. Stool output quantified and and any coexisting dehydration or electrolyte imbalance corrected Dietary therapy with rice-lentil KY diet, supplemented with vitamins initiated and continued under supervision for 14 days. Diet administered ad libitum, in gradually increasing amounts, to provide at least 100 kcal/kg/day by day 4 of therapy Details of the diet below Breastfeeding continued as required Degree of dehydration, body temperature, vital signs and clinical status recorded twice daily or more frequently as clinically indicated. In cases of suspected septicaemia a blood culture was obtained before initiation of broad-spectrum antibiotics (usually i.v. ampicillin and gentamicin, or i.v. ceftriaxone in suspected typhoidal salmonellosis). Suspected bacterial lower respiratory infections evaluated by chest radiography and treated according to the standard WHO guidelines |
Definition of SAM: not defined, although children were shown to meet W/A and MUAC criteria Number of participants: N = 87 (intervention, n = 43; control, n = 44) Sample attrition/dropout: 10 participants did not complete the inpatient part of the study and did not take supplements at home. Zinc group: two discharged prematurely, two because of concomitant infection precluding full enteral feeds, and one because of development of recurrent dehydration. Control: two discharged prematurely, three because of concomitant infection precluding full enteral feeds Sample crossovers: not applicable Inclusion criteria: children aged 6–36 months with persistent diarrhoea (four or more unformed stools per day continuously for at least 14 days) and malnutrition (WAZ ≤ 2) Exclusion criteria:General characteristics of participants: children aged 6–36 months with persistent diarrhoea and evidence of malnutrition |
Primary outcome: overall weight gain by day 14 of inpatient therapy Secondary outcomes (> 14 days inpatient therapy):Method of assessing outcomes: unclothed weight obtained prior to feed at admission, and daily, on a double-beam balance sensitive up to 10 g. Length measured on an infant stadiometer, occipito-frontal, mid-arm, and mid-thigh circumferences measured using paper tape. Anthropomorphic measures repeated at days 7, 14 and 28 Laboratory measurements were undertaken at baseline, 7 and 14 days Daily amounts of food consumed estimated by weighing left-over food. Breastfed amount estimated by immediate test weighing Accurate records of stool, vomitus and urinary output were maintained by quantifying stool output separately from urine by means of adhesive bags. For females, only stool frequency and character were recorded after 72 hours of therapy (because of high rates of urine–stool admixture) A range of laboratory investigations were carried out at baseline, day 7 and day 14 on stools and blood (details not data extracted).Intestinal permeability was also assessed. Children were considered zinc deficient based on plasma zinc levels < 60 μg/dl (9.18 μmol/l) Time to weight gain: time taken to achieve weight gain for three or more days consecutively after achieving a caloric intake of 100 kcal/kg/day Time to diarrhoeal recovery: time taken to achieve a reduction in stool volume to < 30 g/kg/day in males, stool frequency less than four per day in both, and achievement of a semisoft stool consistency Compliance with therapy: assessed by estimation of remaining supplement volume at return appointment Adverse symptoms: NR Length of follow-up: 28 days Recruitment dates: July 1993 to September 1995 |
| Characteristics of participants | |||
| Characteristic | Intervention (zinc) (n = 43) | Control (placebo) (n = 44) | p-value |
| Sex (M : F) | 27 : 16 | 26 : 18 | NS |
| Age, months | 11.6 ± 5.6 | 13.1 ± 6.2 | NS |
| WAZ | –3.47 ± 0.97 | –3.27 ± 1.33 | NS |
| HAZ | –1.68 ± 1.14 | –1.44 ± 1.34 | NS |
| WHZ | –3.02 ± 0.90 | –3.13 ± 1.19 | NS |
| Mid-arm circumference, cm | 11.1 ± 1.5 | 11.6 ± 1.9 | NS |
| Total protein, g/l | 55.0 ± 9.2 | 56.8 ± 8.9 | NS |
| Serum albumin, g/l | 33.7 ± 7.8 | 33.5 ± 6.5 | NS |
| Serum prealbumin, mg/l | 93.8 ± 40.2 | 77.4 ± 35.0 | NS |
| Haemoglobin, g/l | 92.3 ± 18.2 | 91.6 ± 19.0 | NS |
| Haematocrit, % | 29.9 ± 4.3 | 29.8 ± 4.9 | NS |
| C-reactive protein, mg/l | 32.9 ± 42.5 | 41.4 ± 67.6 | NS |
| Plasma zinc, μg/dl | 78.0 ± 32.2 | 70.3 ± 19.0 | NS |
| Plasma copper, μg/dl | 67.4 ± 34.2 | 64.1 ± 19.2 | NS |
| Duration of diarrhoea 14–30 days | 33 (77%) | 32 (73%) | NS |
| > 30 days | 10 (23%) | 12 (27%) | |
| Stool at admission, n (%) | |||
| Watery | 32 (74) | 28 (64) | NS |
| Bloody | 3 (7) | 2 (5) | |
| Mucoid | 3 (7) | 6 (14) | |
| Mixed | 5 (12) | 8 (18) | |
| Stool volume n (%)a | |||
| < 40 g/kg/day | 13 (30) | 9 (20) | NS |
| 40–70 g/kg/day | 10 (23) | 17 (39) | |
| > 70 g/kg/day | 20 (47) | 18 (41) | |
| Stool frequency n (%)a | |||
| 1–5 per day | 10 (23) | 8 (18) | NS |
| 6–10 per day | 14 (33) | 15 (34) | |
| > 10 per day | 19 (44) | 21 (48) | |
| Degree of dehydration at admission n (%) | |||
| None | 23 (53) | 29 (66) | NS |
| Mild | 16 (37) | 11 (25) | |
| Moderate | 2 (5) | 2 (5) | |
| Severe | 2 (5) | 2 (5) | |
| Comments: at baseline, overall, 25 children (29%) had plasma zinc levels < 60 μg/dl (9.18 μmol/l) and were therefore considered zinc deficient. Stool pathogens: enteropathogenic E. coli and Campylobacter jejuni in two each, S. paratyphi and Aeromonas hydrophilia in two children in the zinc group, and V. cholerae ogawa in one child in the placebo group. Degree of dehydration at admission similar in both groups, amounts of i.v. fluids (not data extracted) and ORS (not data extracted) consumed during initial stabilisation were comparable | |||
| Results | |||
| Primary outcomes | Intervention (zinc) | Control (placebo) | p-value |
| Overall weight increment, g/kg/day | 10.3 ± 5.7 | 8.7 ± 6.5 | NS |
| Weight, kg | |||
| Day 1 | 6.08 ± 1.32 | 6.33 ± 1.56 | |
| Day 7 | 6.27 ± 1.29 | 6.84 ± 1.41 | |
| Day 14 | 6.67 ± 1.43 | 7.13 ± 1.42 | 0.27b |
| Comments: text indicates that rate of weight gain was slow in children with evidence of systemic infection requiring antibiotics, but numerical data are not presented. These patients were distributed equally between the two groups | |||
| Secondary outcomes | Intervention (zinc), mean ± SD (n = 43) | Control (placebo), mean ± SD (n = 44) | p-value |
| Plasma zinc, μg/dlc | |||
| Day 1 | 78.0 ± 32.2 | 70.3 ± 19.0 | |
| Day 7 | 100 ± 48 | 64 ± 20 | |
| Day 14 | 112 ± 64 | 68 ± 20 | 0.03d |
| Caloric intake, kcal/kg/day | |||
| Day 1 | 83.1 ± 37.5 | 80.2 ± 28.6 | |
| Day 7 | 129.6 ± 39.6 | 123.8 ± 36.9 | |
| Day 14 | 130.7 ± 46.6 | 121.1 ± 49.7 | 0.79b |
| Overall increment in caloric intake, kcal/kg/day | 39.9 ± 46.5 | 40.0 ± 51.3 | NS |
| Stool frequency, n/day | |||
| Day 1 | 10.2 ± 6.4 | 11.8 ± 7.8 | |
| Day 7 | 5.9 ± 5.6 | 5.2 ± 3.7 | |
| Day 14 | 2.9 ± 1.6 | 3.0 ± 2.2 | 0.52b |
| Decrease in stool frequency, n/day | 7.4 ± 7.4 | 8.1 ± 8.8 | NS |
| Stool volume, g/kg/day (males) | |||
| Day 1 | 116.8 ± 103.7 | 141.9 ± 171.6 | |
| Day 7 | 66.7 ± 68.1 | 43.9 ± 40.1 | |
| Day 14 | 24.9 ± 16.2 | 27.8 ± 31.4 | 0.42b |
| Decrease in stool volume (g/kg/day) | 91.1 ± 103.6 | 98.0 ± 187.9 | NS |
| Mid-arm circumference (cm) | |||
| Day 1 | 11.4 ± 1.5 | 11.5 ± 1.9 | |
| Day 7 | 11.7 ± 1.4 | 12.0 ± 1.8 | |
| Day 14 | 12.0 ± 1.4 | 12.4 ± 1.8 | 0.66b |
| Overall increment in mid-arm circumference | 0.3 ± 0.3 | 0.4 ± 0.3 | NS |
| Weight gain during the 14 days of ambulatory home based supplementation, g/kg/day | 9.2 ± 46 | 7.6 ± 5.7 | NS |
| Increment in mid-arm circumference after 14 days of ambulatory home based supplementation | 0.13 ± 0.28 | 0.19 ± 0.40 | NR |
|
Comments: data from Kaplan–Meier plots of time-to-diarrhoeal-recovery and time-to-weight-gain have not been data extracted. Although children in the zinc group had a faster initial reduction in stool output (log-rank test for time to 30% reduction in stool output; p < 0.03) there was no significant difference between the groups for the time take for a 50% reduction in stool output (p = 0.24). The overall time taken for diarrhoeal recovery (p = 0.713) and weight gain (p = 0.397) were comparable The authors performed subgroup analyses on outcomes for the subgroup with low plasma zinc levels at admission (not data extracted), and for the subgroup of stunted children (HAZ < –2) (data not presented in paper). There were no significant differences, but the authors acknowledge that their study had insufficient power to detect significant differences in these subgroups Data on the lactulose: rhamnose ratio, and the sequential breath hydrogen excretion values were not extracted |
|||
|
Safety: no child had a relapse of diarrhoea and the morbidity patterns were comparable during the 14-day period of home supplementation and follow-up The authors point out that care is needed when supplementing with single nutrients as some may interfere with the absorption of others. In particular, significant interaction of zinc absorption with copper and iron has been described. Data on plasma copper have not been data extracted from a line figure. A significant trend in reduction of serum copper was seen in the zinc group, whereas values significantly increased in the placebo group by the end of the second week of therapy. Numerical values (as well as the line graph) are provided in the paper, but it is not clear what these correspond to as they do not appear to match expected values on the graph for plasma copper at day 14 |
|||
| HIV: NR | |||
| Barriers to implementation | |||
| NR | |||
| Methodological comments | |||
|
Zinc dose: the authors note that zinc could have been provided at a fixed daily dose for ease of administration. However, they gave 3 mg/kg/day of elemental zinc in an attempt to evaluate a level of zinc intake that provided almost twice the recommended daily allowance. In addition, this level could also have been emulated from dietary sources subsequently. The dose was also believed to be sufficient for replenishment of plasma zinc levels Allocation to treatment groups: block randomisation. The randomisation code, maintained by the Pharmacy Department at the Aga Khan University Hospital was not available to the investigators until the end of the study. The pharmacy department were unaware of the identity of enrolled patients Blinding: described as double blind Comparability of treatment groups: described as closely comparable for all admission clinical, nutritional, and laboratory parameters. Also comparable for the duration and severity of diarrhoea, as assessed by history as well as during the period of stabilisation. An equal number of children in both groups revealed stool pathogens on cultures Method of data analysis: A mid-term analysis of morbidity and mortality among the participants was conducted independently by consultants from Applied Diarrhoeal Disease Research Program, and the study was allowed to proceed to conclusion. Final analysis was on an ITT basis, irrespective of length of stay in the study. Differences between groups evaluated for categorical data by chi-squared analysis or Fisher’s exact test as appropriate. Differences for continuous data compared by two-tailed Student’s t-test. Sequential data for primary and secondary outcomes at baseline, day 7 and 14 evaluated by analysis of variance for repeated measures, evaluating the interaction of time trend and treatment effect. Time to event data for the two groups compared by survival analysis using the log-rank test. A subgroup analysis was conducted for the subgroup of children considered zinc deficient. Significance was set at 5% Sample size/power calculation: reported and reference provided for the formula used. The formula used was for analysis of longitudinal continuous data, and the calculation was based on the known pattern and rate of weight gain (5 ± 3 g/kg/day) in comparably malnourished children with persistent diarrhoea receiving the same KY-based diet. It was estimated that to achieve at least a 30% difference in weight gain after 14 days of therapy, with 80% power and a type 1 error of 0.05, 40 participants would be needed in each group. However, the authors note that although overall weight gain exceeded their initial estimates, the SDs were wide, which led to the possibility that the study had insufficient power to elucidate smaller put potentially significant differences in stool output or weight gain. The authors estimated the final power of the study to detect a 25% difference in rates of weight gain was < 60% Attrition/dropout: numbers overall and by trial group were provided with reasons |
|||
| General comments | |||
|
Generalisability: a doctor and nurse in constant attendance on the ward, this level of supervision might not be possible in all settings. As children with kwashiorkor or symptoms suggestive of vitamin A or zinc deficiency were excluded from this study, the results may not be applicable to these groups Outcome measures: a primary outcome measure was stated although this outcome was subsequently presented among other results. Outcomes were listed and defined where necessary. Outcome data were presented as mean ± SD Intercentre variability: not applicable Conflict of interest: not stated |
|||
| Dietary therapy | |||
| Khitchri (60 g rice, 30 g lentils, 10 g dry weight cottonseed oil and 1 g salt) prepared on site daily. Fresh live yoghurt obtained from a single source. Zinc content estimated to be < 2.5 mg zinc per 100 g. Vitamin mixture (1.5 the daily recommended doses): vitamin A (4500 units, 1.35 mg), vitamin D (600 units, 15 μg), vitamin B1 (2.2 mg), vitamin B2 (1.8 mg), vitamin B6 (1.5 mg), vitamin B12 (4.5 μg), nicotinamide (15 mg), vitamin C (75 mg) | |||
Quality assessment for primary studies (modified for severe malnutrition)
| A. Selection bias | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1. Are the individuals selected to participate in the study likely to be representative of the target population? | Very likely | Somewhat likely | Not likely | Cannot tell | |||||
| ✓ | |||||||||
| 2. What percentage of selected individuals participated? | 80–100% | 60–79% | < 60% | N/A | Cannot tell | ||||
| ✓ | |||||||||
|
Summary of selection bias (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| B. Study design | |||||||||
|
1. What was the study design? (Please tick appropriate and specify design if categorise as ‘Other’) |
RCT | ✓ | |||||||
| CCT | |||||||||
| Cohort analytic (two group pre + post) | |||||||||
| Case–control | |||||||||
| Cohort [one group pre + post (before and after)] | |||||||||
| Interrupted time series | |||||||||
| Other – specify | |||||||||
| Cannot Tell | |||||||||
| 2. Was the study described as randomised? | Yes | No | |||||||
| ✓ | |||||||||
| If answer to no. 2 is ‘no’ complete summary then go to section C. Confounders. If answer is ‘yes’, answer no. 3 and no. 4 below, before completing summary for this section | |||||||||
| 3. If answer was yes, was the method of randomisation described? | Yes | No | |||||||
| ✓ | |||||||||
| 4. If answer was yes, was the method appropriate? | Yes | No | |||||||
| ✓ | |||||||||
|
Summary of study design (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| C. Confounders | |||||||||
| 1. Were there important differences between groups prior to the intervention? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. If yes, indicate the percentage of relevant confounders that were controlled [either in the design (e.g. by stratification or matching) or in the analysis]? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
|
Summary of confounders (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| D. Blinding | |||||||||
| 1. Was the outcome assessor aware of the intervention or exposure status of participants? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were the study participants aware of the research question? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| Summary of blinding (Methodological strength of study) | Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| E. Data collection methods | |||||||||
| 1. Were data collection tools shown to be valid? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were data collection tools shown to be reliable? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
|
Summary of data collection (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| F. Withdrawals and dropouts | |||||||||
| 1. Were withdrawals and dropouts reported in terms of numbers and reasons per group? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Indicate the percentage of participants completing the study (If the percentage differs by groups, record the lowest) | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
|
Summary of withdrawals and dropouts (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| G. Intervention integrity | |||||||||
| 1. What percentage of participants received the allocated intervention or exposure of interest? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
| 2. Was the consistency of the intervention measured? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 3. Is it likely that subjects received an unintended intervention (contamination or co-intervention) that may influence the results? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| H. Analysis | |||||||||
| 1. Indicate the unit of allocation | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 2. Indicate the unit of analysis | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 3. Are the statistical methods appropriate for the study design? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 4. Is the analysis performed by intervention allocation status (i.e. ITT) rather than actual intervention received? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
|
Global rating for studya (Overall methodological strength of study – based on sections A–F) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
Manary and Brewster 199780
Data extraction table
| Reference and design | Intervention | Participants | Outcome measures | |
|---|---|---|---|---|
|
Author: Manary and Brewster80 Year: 1997 Country: Malawi Study design: double-blind RCT (judged as CCT in quality assessment) Setting: inpatient (hospital-based NRU) Number of centres: one Funding: none reported |
Intervention: high potassium supplementation (additional 3 mmol/kg potassium above the standard supplement given in corn syrup as a medication, total potassium dose of 7.7 mmol/kg/day in phase one of diet, i.e. first 7 days) Control: standard potassium supplementation (3.2 mmol/kg/day of potassium plus placebo of corn syrup given as a medication, total potassium dose of 4.7 mmol/kg/day in phase one of diet, i.e. first 7 days) Other interventions used: initial routine medications were cotrimoxazole, albendazole, magnesium (2.8 mmol/kg/day), zinc (40 mg daily as lactate) and multivitamins. Oral rehydration solution and i.v. fluid were used cautiously to avoid excess sodium and fluid loads. Standard regime of mild feeds (see end of table) |
Definition of SAM: only described as children with kwashiorkor Number of participants: N = 116 (intervention, n = 55; control, n = 61) Sample attrition/dropout: n = 17 were excluded because they absconded before completion of the 7-day potassium supplement or placebo (intervention n = 7, control n = 10) Sample crossovers: none reported Inclusion criteria: all children admitted with kwashiorkor to the NRU Exclusion criteria: children with oedema owing to renal disease or malarial anaemia General characteristics of participants: rural children < 3 years of age admitted to hospital with kwashiorkor, with or without diarrhoea or HIV infection, but excluding oedema owing to renal disease or malarial anaemia |
Primary outcomes: NR Outcomes: deaths, clinical sepsis, skin ulcers, per cent weight loss, cough, dyspnoea, duration of hospital stay, irritability, diarrhoea and oedema Method of assessing outcomes: daily weight taken plus examination for oedema, fever, respiratory signs, oral ulcers, skin ulcers and irritability Number of days for: cough, duration of hospital stay, irritability, diarrhoea and 2+ or 3+ oedema Number of cases for: dyspnoea Per cent weight loss: assessed by day 7 and by discharge Clinical sepsis: days 2–7 and days 8–24. Diagnosis based on fever, shock without dehydration, dyspnoea or an abrupt change in mental status or general condition (no microbiological investigations to confirm diagnosis) Pedal oedema was graded on a 0–3 scale (1+ = < 0.5 cm of pitting oedema of the dorsum of the foot; 3+ = gross oedema of shins and eyelids) Deaths: defined as early if it occurred in the first 5 days; defined as late if it occurred after at least 5 days of NRU treatment; defined as unexpected if there were no clinical indications of a life-threatening complication Adverse symptoms: mothers were asked daily for 7 days if child was irritable, anorexic, able to finish the feeds, had diarrhoea, vomiting, a cough or respiratory distress Charts of seriously ill children taken home against medical advice were reviewed blindly if they had received ≥ 7 days of treatment, to decide whether or not they were likely to have died at home and these children were then added to the late deaths Length of follow-up: unclear Recruitment dates: 10 February 1995 to 16 March 1995 |
|
| Characteristics of participants | ||||
| Characteristic | Intervention (n = 48) | Control (n = 51) | p-value | |
| Mean age, months (SD) | 29.3 (14) | 27.9 (15) | 0.62 | |
| Wasting (%) | > –1 | 9 (19) | 8 (17) | NR |
| W/H (SD) | –2 | 15 (32) | 6 (12) | 0.91 |
| z-scores (SD) | –3 | 12 (26) | 20 (42) | NR |
| Oedema free (SD) | < –3 | 11 (23) | 14 (29) | NR |
| Mean (SD) | –2.04 (1.20) | –2.40 (1.13) | 0.13 | |
| Stunting (%) | > –2 | 11 (23) | 5 (10) | NR |
| H/A (SD) | –3 | 10 (21) | 13 (27) | 0.92 |
| (z-scores) (SD) | –4 | 11 (23) | 15 (31) | NR |
| < –4 | 15 (32) | 15 (31) | NR | |
| Mean (SD) | –3.01 (1.73) | –3.44 (1.25) | 0.16 | |
| Oedema on admission (%) | ||||
| 1+ | 9 (19) | 9 (18) | NR | |
| 2+ | 13 (27) | 16 (31) | 0.90 | |
| 3+ | 26 (54) | 26 (51) | NR | |
| Rash (%) | ||||
| Nil | 16 (33) | 22 (43) | NR | |
| Mild | 16 (33) | 15 (29) | 0.90 | |
| Moderate | 13 (27) | 9 (18) | NR | |
| Severe | 3 (6) | 5 (10) | NR | |
| Cough (%) | 24 (50) | 34 (67) | 0.14 | |
| Clinical sepsis (%)a | 4 (8) | 7 (14) | 0.59 | |
| Fever > 38.0 °C (%) | 7 (15) | 11 (22) | 0.52 | |
| Haematocrit, mean % (SD) | 31 (7) | 30 (10) | 0.49 | |
| Diarrhoea, n (%) | 16 (33) | 19 (37) | 0.84 | |
| Mean days of diarrhoea before admission (SD) | 4.2 (3.1) | 4.6 (4.0) | 0.56 | |
| Severe anorexia (%) | 12 (25) | 14 (27) | 0.96 | |
| Irritability (%) | 40 (83) | 42 (82) | 0.89 | |
| Skin ulcers (%) | 18 (37) | 19 (37) | 0.86 | |
|
Comments: clinical signs and symptoms on admission for whole sample: fever (39%), cough (53%), shortness of breath (12%), sore mouth (28%), oral thrush (24%), hair changes (58%), hepatomegaly of > 2 cm below the costal margin (28%) and splenomegaly (10%) Baseline characteristics only provided for those followed up |
||||
| Results | ||||
| Outcomes | Intervention (n = 37)b | Control (n = 41)b | p-value | |
| Late death (%)c | 3 (8) | 13 (32) | 0.02 | |
| Left before discharge (after day 7) (%) | 3 (8) | 8 (19.5) | 0.15 | |
| Clinical sepsis (days 2–7) (%) | 0 (0) | 9 (22) | 0.01 | |
| Clinical sepsis (days 8–24) (%) | 3 (9) | 9 (22) | 0.05 | |
| New skin ulcers, number of cases (%) | 4 (11) | 13 (33) | 0.05 | |
| Weight loss by day 7, % (SD) | 5.6 (8.0) | 4.0 (7.2) | 0.36 | |
| Weight loss by discharge, % (SD) | 4.9 (9.1) | 3.8 (10.3) | 0.61 | |
| Cough, number of days (SD) | 2.3 (2.6) | 3.9 (2.7) | 0.01 | |
| Dyspnoea, number of cases (%) | 1 (3) | 10 (24.4) | 0.01 | |
| Hospital stay, number of days (SD) | 11.6 (0.9) | 13.2 (4.9) | 0.21 | |
| Irritability, number of days (SD) | 3.4 (1.7) | 3.7 (2.1) | 0.47 | |
| Diarrhoea, number of days (SD) | 0.9 (2.5) | 1.5 (1.7) | 0.14 | |
| Oedema 2+ or 3+, number of days (SD) | 2.7 (2.2) | 2.7 (2.1) | 0.99 | |
| Number of deaths in hospital (%) | 14 (29.2)d | 20 (39.2)d | 0.40 | |
| Number of death in | ||||
| First 48 hours | 6 | 6 | NR | |
| Days 3–5 | 5 | 4 | NR | |
| Late deaths | 3 | 10 | NR | |
| Adjusted late deaths (%) | 3/37 (8.1) | 13/41 (31.7) | 0.02e | |
| Causes of late death | ||||
| Sepsis | 3 | 3 | NR | |
| Anaemia | 2 | NR | ||
| Unexpected | 5f | NR | ||
|
Comments: case-fatality rate was reduced by 33% in the intervention group (13/48) compared with the control group (21/51). Note, possible error in n/N, as all other information suggests 14/48 and 20/51 deaths The intervention group had significantly fewer presumed septic episodes (3 vs 18) [OR 8.9 (95% CI 2.2 to 50.9)] respiratory symptoms and new skin ulcerations than controls |
||||
| Safety: none stated | ||||
| HIV: no enzyme-linked immunosorbent assays for HIV infection were conducted because of a refusal of consent. Paper reported prevalence figures from unpublished 1993–4 data (n = 519) as 6% for kwashiorkor patients and 17% for marasmic kwashiorkor patients. Also states that > 30% of Blantyre mothers are infected, and the transmission rate (by PCR) at birth is 27%106 with presumably an additional 14% infected via breast milk;107 therefore, expected prevalence rates for infants are around 12% before ceasing breastfeeding | ||||
| Barriers to implementation | ||||
|
Lack of skilled management of individual cases, owing to a variety of constraints which are not readily remediable, were responsible for a case-fatality rate of 34% for kwashiorkor. Authors state that they are attempting the rate through feasible changes in management. Nasogastric tube feeding was used infrequently because of resistance from mothers, reducing the potassium intake in anorexic children It is suggested that the blanket recommendation of a supplement of 4 mmol/kg is insufficient for phase one and might well be too much for the rapid growth phase when added to the diet. Authors state that although individualising doses of micronutrients as a medication has merits, the constraints at NRU make adding them to the diet a much more convenient option when nursing care is limited Authors recommend that results can not be extrapolated to this setting, as there are regional differences in the prevalence of potassium depletion in kwashiorkor, which may be related to the mineral content of weaning diets and that additional losses of potassium can occur in stool with diarrhoea (present on admission to the NRU in this study in 33–37% of cases) |
||||
| Methodological comments | ||||
|
Allocation to treatment groups: described as randomised, but no details provided Blinding: described as double-blind, placebo-controlled trial. Investigators, health workers and mothers unaware of child’s allocation group Comparability of treatment groups: no significant differences between treatment arms (all p-values reported) Method of data analysis: dichotomous parameters were evaluated as ORs with 95% CI with Fisher’s exact test and Yates’ corrected p-values. Continuous parameters were evaluated using Student’s t-test (Epi Info version 6) Sample size/power calculation: none reported Attrition/dropout: numbers and reasons reported. Discontinuation rates appears to be similar between the two groups |
||||
| General comments | ||||
|
Generalisability: not generalisable to children with oedema because of renal disease or malarial anaemia who were excluded and also not generalisable to older children. Not all the children may have met the current WHO criteria for SAM, as the sample included children with kwashiorkor categorised as W/H –2 Outcome measures: no primary outcome defined. Outcome measures appear suitable and appropriate Intercentre variability: not applicable Conflict of interest: none reported |
||||
| Diet for all admissions (phase one and phase two) | ||||
|
Phase 1: dried skimmed milk, sugar, vegetable oil and water containing 278 kJ (66 kcal) and 1.0 g of protein per 100 ml. Daily intake per kilogram of body weight was approximately 332 kJ (79 kcal), 1.2 g of protein and 1.5 mmol of potassium. Once oedema, appetite and mental status had improved, children advanced to a phase two diet (generally in the second week of treatment, after completion of the potassium supplement or placebo) Phase 2: four feeds of high-energy milk 477 kJ (114 kcal) and 4.1 g of protein per 100 ml, as well as two feeds of a local weaning porridge of maize, soya, sugar and oil consisting of 468 kJ (112 kcal) and 3.3 g of protein per 100 ml. Daily intake of 150 mmol/kg/day: 712 kJ (170 kcal), 5.8 g of protein and 7.6 mol of potassium/kg/day. The higher protein intake in phase one was necessitated by the use of a milk-oil-sugar premix for both phases The protein and energy densities of these diets were similar to those recommended by Waterlow97 of 336 kJ (80 kcal) and 0.7 g of protein/kg/day in phase one and 735 kJ (175 kcal) and 5.75 g of protein/kg/day in phase two. States that the potassium treatment doses for children in both groups were within the ranges of those recommended for SAM in the scientific literature |
||||
Quality assessment for primary studies (modified for severe malnutrition)
| A. Selection bias | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1. Are the individuals selected to participate in the study likely to be representative of the target population? | Very likely | Somewhat likely | Not likely | Cannot tell | |||||
| ✓ | |||||||||
| 2. What percentage of selected individuals participated? | 80–100% | 60–79% | < 60% | N/A | Cannot tell | ||||
| ✓ | |||||||||
| Summary of selection bias | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| B. Study design | |||||||||
| 1. What was the study design? | RCT | ||||||||
| (Please tick appropriate and specify design if categorise as ‘Other’) | CCT | ✓ | |||||||
| Cohort analytic (two group pre + post) | |||||||||
| Case–control | |||||||||
| Cohort [one group pre + post (before and after)] | |||||||||
| Interrupted time series | |||||||||
| Other – specify | |||||||||
| Cannot Tell | |||||||||
| 2. Was the study described as randomised? | Yes | No | |||||||
| ✓ | |||||||||
| If answer to no. 2 is ‘no’ complete summary then go to section C. Confounders. If answer is ‘yes’, answer no. 3 and no. 4 below, before completing summary for this section | |||||||||
| 3. If answer was yes, was the method of randomisation described? | Yes | No | |||||||
| ✓ | |||||||||
| 4. If answer was yes, was the method appropriate? | Yes | No | |||||||
| Summary of study design | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| C. Confounders | |||||||||
| 1. Were there important differences between groups prior to the intervention? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. If yes, indicate the percentage of relevant confounders that were controlled [either in the design (e.g. by stratification or matching) or in the analysis]? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| Summary of confounders | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| D. Blinding | |||||||||
| 1. Was the outcome assessor aware of the intervention or exposure status of participants? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were the study participants aware of the research question? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| Summary of blinding | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| E. Data collection methods | |||||||||
| 1. Were data collection tools shown to be valid? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were data collection tools shown to be reliable? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| Summary of data collection | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| F. Withdrawals and dropouts | |||||||||
| 1. Were withdrawals and dropouts reported in terms of numbers and reasons per group? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Indicate the percentage of participants completing the study (If the percentage differs by groups, record the lowest) | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
| Summary of withdrawals and dropouts | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| G. Intervention integrity | |||||||||
| 1. What percentage of participants received the allocated intervention or exposure of interest? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
| 2. Was the consistency of the intervention measured? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 3. Is it likely that subjects received an unintended intervention (contamination or co-intervention) that may influence the results? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| H. Analysis | |||||||||
| 1. Indicate the unit of allocation | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 2. Indicate the unit of analysis | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 3. Are the statistical methods appropriate for the study design? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 4. Is the analysis performed by intervention allocation status (i.e. ITT) rather than actual intervention received? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| Global rating for studya | Strong | Moderate | Weak | ||||||
| (Overall methodological strength of study – based on sections A–F) | ✓ | ||||||||
Philip et al. 198281
Data extraction table
| Reference and design | Intervention | Participants | Outcome measures |
|---|---|---|---|
|
Author: Philip et al. 81 Year: 1982 Country: India Study design: CCT Setting: inpatient Number of centres: one Funding: NR |
Intervention: standard diet + nicotinic acid, 25 mg/kg/day (three divided doses) for 1 month Control: standard diet for 1 month Standard diet contained 4 g protein and 200 kcal obtained from K Mix two (supplied by UNICEF), tapioca, sugar, gingelly oil and rice (no further details reported) Other interventions used: none reported |
Definition of SAM: no specific reference made to SAM, only those ‘fulfilling the standard criteria for marasmas’ (no further details reported) Number of participants: N = 80 (nicotinic acid, n = 40; control, n = 40) Sample attrition/dropout: none reported Sample crossovers: none reported Inclusion criteria: standard criteria for marasmus (no reference or details provided) Exclusion criteria: none reported. General characteristics of participants: marasmic children aged 0–4 years |
Primary outcomes: weight gain Secondary outcomes: calorie consumption Method of assessing outcomes: weight was recorded every morning before being given the standard diet. The calculated amount of food was given five times daily at 0700, 1000, 1300, 1600 and 2100 hours in divided quantities for 1 month. No further details Adverse symptoms: none reported Length of follow-up: none beyond the 1 month treatment period Recruitment dates: 1974–6 |
| Characteristics of participants | |||
| Characteristic | Nicotinic acid (n = 40) | Standard diet (n = 40) | p-value |
| Age (years), n (%) | |||
| 0–1 | 10 (25) | 7 (17.5) | NR |
| 1–2 | 22 (55)a | 23 (57.5) | NR |
| 2–3 | 7 (17.5) | 8 (20) | NR |
| 3–4 | 1 (2.5) | 2 (5) | NR |
| Comments: no difference in sex distribution was noted. No other baseline characteristics were reported by the authors | |||
| Results | |||
| Primary outcomes | Nicotinic acid (n = 40) | Standard diet (n = 40) | p-value |
| Weight gain in 1 month, g/kg | 231.05 (20.05) | 171.81 (22.01) | 0.001b |
|
Comments: results are reported as mean (SD) When weight gain was calculated separately for each week, both groups showed maximum gain during week 2, followed by week 3, with the lowest gain in weeks 1 and 4 (no data reported) For both groups, the rate of weight gain was slightly higher in those children with a greater initial weight deficit |
|||
| Secondary outcomes | Nicotinic acid (n = 40) | Standard diet (n = 40) | p-value |
| Calories consumed for 1 g gain in weight | 14.2 | 19.3 | NR |
| Safety: none of the children experienced any remarkable side effects of nicotinic acid | |||
| HIV: NR | |||
| Barriers to implementation | |||
| NR | |||
| Methodological comments | |||
|
Allocation to treatment groups: not randomised. No details on allocation Blinding: NR. No details on how nicotinic acid administered and thus blinding of patients, care providers and outcome assessors is unknown Comparability of treatment groups: age is the only baseline characteristic reported; the distribution of the age ranges from 0–4 years was similar between the two groups, but no comment or p-value was reported. Authors noted that there was no difference in sex distribution (no data or p-value) Method of data analysis: ITT analysis as data at end of study period is for all 80 subjects. No further details reported Sample size/power calculation: NR Attrition/dropout: none reported. Data at 1 month is for all included subjects so assume no dropouts |
|||
| General comments | |||
|
Generalisability: unable to tell whether or not the included children would meet the current WHO criteria as no specific definition of SAM was given; majority of children < 2 years. Unable to compare these children to the general SAM population as no baseline characteristics were given and reporting is limited Outcome measures: primary outcome of weight gain was appropriate although mortality was NR Intercentre variability: N/A Conflict of interest: no details on funding nor any conflicts of interest were reported |
|||
Quality assessment for primary studies (modified for severe malnutrition)
| A. Selection bias | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1. Are the individuals selected to participate in the study likely to be representative of the target population? | Very likely | Somewhat likely | Not likely | Cannot tell | |||||
| ✓ | |||||||||
| 2. What percentage of selected individuals participated? | 80–100% | 60–79% | < 60% | N/A | Cannot tell | ||||
| ✓ | |||||||||
| Summary of selection bias | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| B. Study design | |||||||||
| 1. What was the study design? | RCT | ||||||||
| (Please tick appropriate and specify design if categorise as ‘Other’) | CCT | ✓ | |||||||
| Cohort analytic (two group pre + post) | |||||||||
| Case–control | |||||||||
| Cohort [one group pre + post (before and after)] | |||||||||
| Interrupted time series | |||||||||
| Other – specify | |||||||||
| Cannot Tell | |||||||||
| 2. Was the study described as randomised? | Yes | No | |||||||
| ✓ | |||||||||
| If answer to no. 2 is ‘no’ complete summary then go to section C. Confounders. If answer is ‘yes’, answer no. 3 and no. 4 below, before completing summary for this section | |||||||||
| 3. If answer was yes, was the method of randomisation described? | Yes | No | |||||||
| 4. If answer was yes, was the method appropriate? | Yes | No | |||||||
| Summary of study design | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| C. Confounders | |||||||||
| 1. Were there important differences between groups prior to the intervention? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. If yes, indicate the percentage of relevant confounders that were controlled [either in the design (e.g. by stratification or matching) or in the analysis]? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| Summary of confounders | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| D. Blinding | |||||||||
| 1. Was the outcome assessor aware of the intervention or exposure status of participants? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were the study participants aware of the research question? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| Summary of blinding | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| E. Data collection methods | |||||||||
| 1. Were data collection tools shown to be valid? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were data collection tools shown to be reliable? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| Summary of data collection | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| F. Withdrawals and dropouts | |||||||||
| 1. Were withdrawals and dropouts reported in terms of numbers and reasons per group? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Indicate the percentage of participants completing the study (If the percentage differs by groups, record the lowest) | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
| Summary of withdrawals and dropouts | Strong | Moderate | Weak | ||||||
| (Methodological strength of study) | ✓ | ||||||||
| G. Intervention integrity | |||||||||
| 1. What percentage of participants received the allocated intervention or exposure of interest? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
| 2. Was the consistency of the intervention measured? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 3. Is it likely that subjects received an unintended intervention (contamination or co-intervention) that may influence the results? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| H. Analysis | |||||||||
| 1. Indicate the unit of allocation | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 2. Indicate the unit of analysis | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 3. Are the statistical methods appropriate for the study design? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 4. Is the analysis performed by intervention allocation status (i.e. ITT) rather than actual intervention received? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| Global rating for studya | Strong | Moderate | Weak | ||||||
| (Overall methodological strength of study – based on sections A–F) | ✓ | ||||||||
Vásquez-Garibay 200582
Data extraction table
| Reference and design | Intervention | Participants | Outcome measures | ||||
|---|---|---|---|---|---|---|---|
|
Author: Vásquez-Garibay82 Year: 2005 Country: Mexico Study design: RCT Setting: inpatient (Unit of Studies of Infantile Nutrition, Metabolic ward, Unit of Studies of Infantile Nutrition, Civil Hospital of Guadalajara) Number of centres: one Funding: none reported |
Intervention: added NT (NT+) (SMA; Wyeth de México, SA de CV, Mexico): a milk-based formula with NT and corn syrup added to increase energy density to 3.35 kJ/ml (casein-dominant formula) (see end of table for details) Control: no added NT (NT–) (S26; Wyeth de México, SA de CV, Mexico): similar formula with the same energy density, but no added NT (whey-dominant formula) (see end of table for details) Feeding was through a nasogastric tube with infant formula (3.35 kJ/ml) for 2 weeks and ad libitum for a further 2 weeks Other interventions used: parasites found in faeces were treated prior to acceptance into study |
Definition of SAM: W/A or W/H < –3 SD from the median using the NCHS/WHO reference Number of participants: N = 25 (NT+, n = 12; NT–, n = 13) Sample attrition/dropout : n = 5 NT+: n = 1 (excluded owing to a non-determined liver disease) NT–: n = 4 (excluded owing to fever syndrome n = 1, emetic syndrome n = 1, poor nutritional progress and a positive HIV test n = 2) Sample crossovers: none reported Inclusion criteria:Exclusion criteria:General characteristics of participants: Infants aged 3–18 months with severe PEM and who are free of infection and moderate or severe diarrhoea |
Primary outcomes: not specifically reported Outcomes: weight, length, head circumference, arm circumference, triceps, subscapular, subcostal and suprailiac skin fold thickness Method of assessing outcomes: specialised personnel took care of the infants for the duration of the study. Two observers carried out the measurements Anthropometric measurements were taken at start of study and once a week for 4 weeks. Blood samples were obtained by antecubital venopuncture at the start of study (at 0700 hours prior to first feed), after 2 weeks and at end of study (see end of table for details) Weight: taken in a calibrated scale without clothes (Bame model 440, Mexico; with a minimum of 5 g). Before and after each bottle feed, bottles were weighed on a triple-beam balance (Ohaus, Florhand Park, New Jersey) Length: measured on infant-measuring board (read to the nearest 0.1 cm) Age and measurements of length and weight, W/A, L/A and W/L, were calculated and expressed as z-scores. Head circumference, arm circumference, and triceps, subscapular, subcostal and suprailiac skin fold thickness were determined with a Lange Skinfold Caliper (Cambridge Scientific Industries, Inc, Cambridge, Maryland) Definitions:Adverse symptoms: none reported Length of follow-up: 4 weeks Recruitment dates: March 1996 to February 1999 |
||||
| Characteristics of participants | |||||||
| Characteristic | NT+ (n = 11) | NT– (n = 9) | p-value | ||||
| Mean birth weight, g (SD) | 2975 (387) | 3021 (369) | 0.81 | ||||
| Mean age, days (SD) | 228 (138) | 242 (173) | 0.84 | ||||
| Mean age, months (SD) | 7.6 (4.6) | 8.1 (3.2) | NR | ||||
| Sex, M : F | 8 : 3 | 5 : 4 | NR | ||||
| Mean weight, g (SD) | 4246 (1403) | 3955 (1250) | 0.87 | ||||
| Mean length, cm (SD) | 61.1 (8.0) | 60.2 (7.7) | 0.95 | ||||
| Mean head circumference, cm (SD) | 39.7 (3.4) | 38.9 (2.1) | 0.85 | ||||
| Mean arm circumference, cm (SD) | 7.9 (1.1) | 7.6 (1.0) | 0.44 | ||||
| Mean triceps, mm (SD)a | 3.8 (1.0) | 2.8 (0.6) | 0.031 | ||||
| Mean subscapular, mm (SD)a | 2.9 (0.7) | 2.4 (0.6) | 0.076 | ||||
| Mean subcostal, mm (SD)a | 2.3 (0.5) | 1.8 (0.3) | 0.045 | ||||
| Mean suprailiac, mm (SD)a | 2.2 (0.5) | 1.7 (0.3) | 0.020 | ||||
| Mean total upper arm area, mm2 (SD) | 512 (139) | 463 (114) | 0.54 | ||||
| Mean upper arm muscle area, mm2 (SD) | 369 (89) | 361 (84) | 0.82 | ||||
| Mean upper arm fat area, mm2 (SD) | 143 (53) | 101 (33) | 0.003 | ||||
| Mean arm fat index, % (SD) | 27 (4) | 22 (2) | 0.005 | ||||
| Mean BMI (SD) | 11.0 (0.9) | 10.6 (1.0) | 0.33 | ||||
| W/H mean z-score (SD) | –2.80 (0.73) | –2.99 ± 0.74 | 0.001 | ||||
| Results | |||||||
| Outcomes: indicatorb | NT+ (n = 11) | NT– (n = 9) | p-valuec | ||||
| Mean skin fold, mm (SD) | |||||||
| Triceps | |||||||
| Initial | 3.8 (1.0) | 2.8 (0.6) | 0.031 | ||||
| Fourth week | 9.2 (2.6) | 8.5 (1.6) | 0.517 | ||||
| Subscapular | |||||||
| Initial | 2.9 (0.7) | 2.4 (0.6) | 0.076 | ||||
| Fourth week | 8.1 (2.7) | 6.4 (1.1) | 0.112 | ||||
| Subcostal | |||||||
| Initial | 2.3 (0.5) | 1.8 (0.3) | 0.045 | ||||
| Fourth week | 5.5 (1.9) | 4.0 (0.6) | 0.004 | ||||
| Suprailiac | |||||||
| Initial | 2.2 (0.5) | 1.7 (0.3) | 0.02 | ||||
| Fourth week | 5.7 (2.5) | 4.2 (0.6) | 0.114 | ||||
| Body composition, mean (SD) | |||||||
| Total upper arm area, mm2 | |||||||
| Initial | 512 (139) | 463 (114) | 0.54 | ||||
| Fourth week | 960 (199) | 903 (148) | 0.49 | ||||
| Upper arm muscle area, mm2 | |||||||
| Initial | 369 (89) | 361 (84) | 0.82 | ||||
| Fourth week | 571 (73) | 508 (112) | 0.83 | ||||
| Upper arm fat area, mm2 | |||||||
| Initial | 143 (54) | 101 (33) | 0.003 | ||||
| Fourth week | 443 (154) | 395 (84) | 0.42 | ||||
| Arm fat index, % | |||||||
| Initial | 27 (4) | 22 (2) | 0.005 | ||||
| Fourth week | 45 (8) | 44 (7) | 0.76 | ||||
| BMI, kg/m2 | |||||||
| Initial | 11.0 (0.9) | 10.6 (1.0) | 0.33 | ||||
| Fourth week | 15.1 (1.0) | 14.5 (1.0) | 0.23 | ||||
| Mean weight gain, g/day (SD) | 67 (15) | 69 (12) | |||||
| W/H mean z-score (SD), fourth week | –0.64 (0.66) | –0.94 (0.47) | 0.001 | ||||
|
Comments: both NT+ and NT– showed significant improvement in W/A and W/L indices from the first week; however, p-values were reported for within group differences only. Mean weight gain was similar between groups (no p-value reported) Paper talks of W/A and W/L, but only outcomes for W/L and L/A are provided (not W/A) Typical weight gain was five times higher than that of normal infants aged around 8 months and the pace of linear growth was doubled |
|||||||
| Other outcomes | NT+ (n = 11) | NT– (n = 9) | p-value | ||||
| Mean urea concentration, mg/l (SD) | 136 (36) | 214 (66) | 0.009 | ||||
| Mean alkaline phosphatase, U/l (SD) | 152 (77) | 218 (46) | 0.041 | ||||
| Comments: both groups were integrated for initial vs final outcome comparison of creatinine, glucose, calcium and phosphorus levels, showing significant improvements in each for the whole group. The same was true for haemoglobin levels and mean corpuscular volume. There were no significant changes in white blood cell count | |||||||
| Safety: NR | |||||||
| HIV: although not specifically part of the exclusion criteria, two infants with positive HIV tests were excluded | |||||||
| Barriers to implementation | |||||||
| None reported | |||||||
| Methodological comments | |||||||
|
Allocation to treatment groups: random assignment into two groups following an arbitrary schedule precisely. When one patient was eliminated, another one was included, receiving the formula that corresponded to the next number in the random sample Blinding: none reported Comparability of treatment groups: baseline age, weight and length were similar, although fat stores were slightly higher in the NT+ group. However, apart from significant differences in skin fold, there were also significant baseline differences in upper-arm muscle area, upper-arm fat area and arm fat index between the groups Method of data analysis: paired Student’s t-tests for the analysis of all initial vs weekly anthropometric indicators (including initial vs final means of the biochemical and haematological indicators). Non-paired Student t-tests were used to compare the anthropometric, biochemical and haematologic mean indicators of group NT+ vs group NT– at different stages in the study. Dbase-IV (Microsoft Corporation, Redmond, WA, USA), Epi Info 6.04 and SPSS/PC programmes were used for capturing, processing and analysing data. Null hypothesis was rejected with a p-value of ≤ 0.05 Sample size/power calculation: sample size calculated at 12 for each group (calculations reported). Authors state that the sample size was large enough to compare both groups, considering they had similar means and SDs in most of the anthropometric indicators at the end of the study. However, after exclusions, number of participants was below the sample size needed Attrition/dropout: number of exclusions and reasons reported |
|||||||
| General comments | |||||||
|
Generalisability: only to full-term infants with normal birth weight, with primary and severe PEM aged 3–18 months were included. Generalisability might therefore not extent to older children or to children with below birth weight. Definition of SAM meets the WHO criteria Outcome measures: outcomes appear appropriate Intercentre variability: N/A, one centre only Conflict of interest: none reported |
|||||||
| Milk-based infant formulasd | NT+ (SMA) | NT– (S26) | |||||
| Nutrients (per litre) | |||||||
| Energy, kJ | 2845 | 2800 | |||||
| Fat, g | 36 | 33.9 | |||||
| Linoleate, g | – | 7.99 | |||||
| Protein, g | 15 | 14.9 | |||||
| Carbohydrate, g | 72 | 75.9 | |||||
| Mineral salts (ashes), g | 2.5 | 2.0 | |||||
| Sodium, mg | 150 | 156 | |||||
| Potassium, mg | 560 | 659 | |||||
| Chloride, mg | 380 | 429.5 | |||||
| Calcium, mg | 420 | 419.5 | |||||
| Phosphorus, mg | 280 | 210 | |||||
| Vitamin A, IU | 2000 | 1998 | |||||
| Vitamin D, IU | 400 | 400 | |||||
| Vitamin E, IU | 19 | 17.9 | |||||
| Vitamin K, μg | 55 | 54.9 | |||||
| Vitamin C, mg | 55 | 53.9 | |||||
| Thiamin B1, μg | 670 | 400 | |||||
| Riboflavin B2, μg | 1000 | 899 | |||||
| Niacin, μg | 5000 | 4995 | |||||
| Vitamin B6, μg | 420 | 499.5 | |||||
| Folic acid, μg | 50 | 59.9 | |||||
| Pantothenic acid, μg | 2100 | 2992 | |||||
| Vitamin B12, μg | 1.3 | 1.3 | |||||
| Biotin, μg | 15 | 14.6 | |||||
| Choline, mg | 100 | 49.9 | |||||
| Magnesium, mg | 35 | 40 | |||||
| Iron, mg | 12 | 8 | |||||
| Iodine, μg | 60 | 33 | |||||
| Copper, μg | 470 | 413 | |||||
| Zinc, mg | 5 | 5 | |||||
| Manganese, μg | 100 | 46.9 | |||||
|
Commercially available formulas with NT (in milligrams per liter) cytidine monophosphate (16.5), uridine monophosphate (5.0), adenosine monophosphate (4.0), guanosine monophosphate (2.0) and inosine monophosphate (2.0) (SMA; 2845 kJ/L); and without NT (S26; 2800 kJ/L). Both formulas, belonging to the same batch, had a similar nutritional content and were within the accepted range for infant formula. The formula was placed in a feeding bag of 500 ml (Pisa; Guadalajara, Jalisco, Mexico), then introduced into a feeding tube (D-731 o 732; Desvar de Mexico, Sociedad Anónima, Mexico) and administered to infants by continuous infusion pump (Braun, Germany) From day 1: daily oral vitamins (vitamin A 5000 IU, vitamin D 1000 IU, vitamin C 50 mg, thiamin 1 mg, riboflavin 0.8 mg, niacin 6 mg and folic acid 0.5 mg) During the first 5 days: energy intake = 670 kJ/kg/day, protein intake 3.2 g/kg/day After day 5: depending on the new weight (kilograms), the energy and protein intake was adjusted to 837 kJ/kg/day and 4 g/kg/day, respectively From day 6: elemental iron 3 mg/kg daily Start of third week: infants were fed ad libitum by bottle. The total amount of formula, protein and energy intake was calculated daily. The formula included all the water, energy, proteins and other nutrients required. No other foods were offered during the 4-week nutritional period (infants were started with complementary foods before being discharged) Laboratory tests: blood samples at start for total proteins, serum albumin, calcium, phosphorus, magnesium, alkaline phosphatase, urea, creatinine, glucose, sodium, potassium, chloride and haemoglobin, as well as urine analysis. The calcium, phosphorus, magnesium and total protein determinations were done by the final point colorimetric method (RA-1000 Technicon; Bayer Diagnostic, Tarrytown, NY); alkaline phosphatase, by an enzymatic method of zero order and a C-405 filter; and haemoglobin, by a modified haemiglobincyanide method (CELL-DYN 3500R; Abbott Laboratories, Diagnostics Division, North Chicago, IL, USA) |
|||||||
Quality assessment for primary studies (modified for severe malnutrition)
| A. Selection bias | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1. Are the individuals selected to participate in the study likely to be representative of the target population? | Very likely | Somewhat likely | Not likely | Cannot tell | |||||
| ✓ | |||||||||
| 2. What percentage of selected individuals participated? | 80–100% | 60–79% | < 60% | N/A | Cannot tell | ||||
| ✓ | |||||||||
|
Summary of selection bias (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| B. Study design | |||||||||
|
1. What was the study design? (Please tick appropriate and specify design if categorise as ‘Other’) |
RCT | ✓ | |||||||
| CCT | |||||||||
| Cohort analytic (two group pre + post) | |||||||||
| Case–control | |||||||||
| Cohort [one group pre + post (before and after)] | |||||||||
| Interrupted time series | |||||||||
| Other – specify | |||||||||
| Cannot Tell | |||||||||
| 2. Was the study described as randomised? | Yes | No | |||||||
| ✓ | |||||||||
| If answer to no. 2 is ‘no’ complete summary then go to section C. Confounders. If answer is ‘yes’, answer no. 3 and no. 4 below, before completing summary for this section | |||||||||
| 3. If answer was yes, was the method of randomisation described? | Yes | No | |||||||
| ✓ | |||||||||
| 4. If answer was yes, was the method appropriate? | Yes | No | |||||||
| ✓ | |||||||||
|
Summary of study design (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| C. Confounders | |||||||||
| 1. Were there important differences between groups prior to the intervention? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. If yes, indicate the percentage of relevant confounders that were controlled [either in the design (e.g. by stratification or matching) or in the analysis]? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
|
Summary of confounders (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| D. Blinding | |||||||||
| 1. Was the outcome assessor aware of the intervention or exposure status of participants? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were the study participants aware of the research question? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
|
Summary of blinding (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| E. Data collection methods | |||||||||
| 1. Were data collection tools shown to be valid? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Were data collection tools shown to be reliable? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
|
Summary of data collection (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| F. Withdrawals and dropouts | |||||||||
| 1. Were withdrawals and dropouts reported in terms of numbers and reasons per group? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 2. Indicate the percentage of participants completing the study (If the percentage differs by groups, record the lowest) | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
|
Summary of withdrawals and dropouts (Methodological strength of study) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
| G. Intervention integrity | |||||||||
| 1. What percentage of participants received the allocated intervention or exposure of interest? | 80–100% | 60–79% | < 60% | Cannot tell | |||||
| ✓ | |||||||||
| 2. Was the consistency of the intervention measured? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 3. Is it likely that subjects received an unintended intervention (contamination or co-intervention) that may influence the results? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| H. Analysis | |||||||||
| 1. Indicate the unit of allocation | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 2. Indicate the unit of analysis | Community | Organisation/institution | Practice/office | Provider | Patient | ||||
| ✓ | |||||||||
| 3. Are the statistical methods appropriate for the study design? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
| 4. Is the analysis performed by intervention allocation status (i.e. ITT) rather than actual intervention received? | Yes | No | Cannot tell | ||||||
| ✓ | |||||||||
|
Global rating for studya (Overall methodological strength of study – based on sections A–F) |
Strong | Moderate | Weak | ||||||
| ✓ | |||||||||
Appendix 13 Ongoing studies
| Study title/link to record | Location | Estimated enrolment | Intervention | Comparator | Design | Outcomesa | Start to end date |
|---|---|---|---|---|---|---|---|
| Status of trial as ongoing | |||||||
|
Growth and body composition in acute severe malnutrition (SAM) in Indian children: effect of vitamin B12 supplementation in a double-blind randomised controlled pilot study URL: www.controlled-trials.com/ISRCTN67437725 Mapping: Q8 |
India | n = 100, 6–36 months | Multiple micronutrients with vitamin B12 | Multiple micronutrients without vitamin B12 | RCT, double blind | Anthropometry | 1 July 2010 to 30 June 2011 |
|
Randomised, placebo-controlled trial of cotrimoxazole prophylaxis among HIV-uninfected children with severe malnutrition URL: http://clinicaltrials.gov/show/NCT00934492 Mapping: Q7 |
Kenya | n = 1600, 2 months to 5 years | Cotrimoxazole | Placebo | RCT, double blind | Mortality, growth | November 2009 to December 2013 |
|
Randomised, double-blind, placebo-controlled trial to evaluate the effectiveness of oral antibiotics in the community-based treatment of severe acute malnutrition in Malawian children URL: http://clinicaltrials.gov/show/NCT01000298 Mapping: Q7 |
Malawi | n = 2700, 6 months to 5 years |
Intervention 1: amoxicillin + RUTF Intervention 2: cefdinir + RUTF |
Placebo + RUTF | RCT, double blind | Weight gain, nutritional recovery | December 2009 to January 2011 |
|
Comparison of the efficacy of a ready-to-use therapeutic food with a milk-based diet in the rehabilitation of severely malnourished Ugandan children URL: http://clinicaltrials.gov/show/NCT00131417 Mapping: Q10 |
Uganda | n = 128, 6–59 months | RUTF | Standard liquid milk-based diet – high-energy milk | RCT, open label | Weight gain, anthropometry, mortality | October 2004 to February 2005 |
|
Randomised, controlled clinical trial of day-care based and hospitalised management of severe and very severe pneumonia, with severe malnutrition, with/without associated comorbidities in children URL: http://clinicaltrials.gov/show/NCT00968370 Mapping: Q14 |
Bangladesh | n = 440, 2–59 months | Day-care clinic | Hospital management | RCT, open label | Mortality | November 2008 to October 2011 |
| Status of trial as completed | |||||||
|
High/low dose vitamin A in diarrhoea/acute lower respiratory infections (ALRI) in severe protein energy malnutrition (PEM) URL: http://clinicaltrials.gov/show/NCT00388921 Mapping: Q22 + Q8 |
Bangladesh | n = 260, 6–59 months | Low-dose, daily vitamin A | Initial mega-dose followed by low-dose, daily vitamin A | RCT, double blind | Weight gain, anthropometry, resolution of diarrhoea, mortality | October 2005 (end date NR) |
|
Efficacy of community-based follow-up, food supplementation and psychosocial stimulation in the home-management of young, severely malnourished Bangladeshi children: a randomised intervention trial URL: http://clinicaltrials.gov/show/NCT01157741 Mapping: Q14 |
Bangladesh | n = 507, 6–24 months |
Intervention 1: standard treatment with community-based follow-up (C-C) Intervention 2: C-C + supplementary food (SF) Intervention 3: C-C + psychosocial stimulation (PS) Intervention 4: C-C + SF + PS |
Standard treatment with hospital-based follow-up (H-C) | RCT, open label | Weight gain | October 2003 to June 2008 |
|
Randomised, double-blind, controlled clinical effectiveness trial comparing 10% milk RUTF with 25% milk RUTF in the treatment of severe acute malnutrition in rural Malawian children URL: www.controlled-trials.com/ISRCTN54186063 Mapping: Q10 |
Malawi | n = 1800, 12–60 months | 10% milk RUTFb | 25% milk RUTFb | RCT, double blind | Anthropometry, gains in weight, height and MUAC, mortality | 6 January 2008 to 5 January 2009 |
|
Acceptability, effectiveness and cost-effectiveness of soya maize sorghum-based RUTF in treating severe acute malnutrition in children under five in Lusaka, Zambia URL: www.controlled-trials.com/ISRCTN62376241 Mapping: Q10 |
Zambia | n = 1654,c 6–59 months | Soya, maize and sorghum-based RUTF | Peanut-based RUTF | Crossover study followed by open-label RCT + cost-effectiveness analysis | Weight gain, anthropometry, diarrhoea morbidity, mortality | 16 June 2008 to 3 August 2009 |
|
Community-based, cluster randomised trial of RUTF in malnourished children < 5 years of aged URL: http://ctri.nic.in/Clinicaltrials/ViewTrial.jsp?trialno=419 Mapping: Q10 |
India | n = 120, 18–60 months | RUTF | Fortified milk supplement | RCT, open label but outcome assessor blinded | Anthropometry | 21 January 2008 (end date NR but study lasted 3 months) |
List of abbreviations
- ANZCTR
- Australian New Zealand Clinical Trials Register
- AOM
- acute otitis media
- ARS
- amylase-resistant starch
- ART
- antiretroviral therapy
- AUC
- area under the curve
- BMI
- body mass index
- CCT
- controlled clinical trial (non-randomised)
- CDC
- Centers for Disease Control
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CFR
- case fatality ratio
- CHA
- community health aide
- CI
- confidence interval
- CRD
- Centre for Reviews and Dissemination
- CRT
- capillary refill time
- CTRI
- Clinical Trials Registry – India
- DARE
- Database of Abstracts of Reviews of Effects
- H/A
- height-for-age
- HAS
- human albumin solution
- HIV
- human immunodeficiency virus
- HIV–ve
- human immunodeficiency virus sero-negative
- HIV+ve
- human immunodeficiency virus sero-positive
- H-ORS
- hypo-osmolar oral rehydration solution
- HSD/5D
- half-strength Darrow’s in 5% dextrose
- HTA
- health technology assessment
- IAP
- Indian Academy of Pediatrics
- ICDDR
- International Centre for Diarrhoeal Disease Research
- ICMH
- Institute of Child and Mother Health
- ICTRP
- WHO International Clinical Trials Registry Platform
- i.m.
- intramuscular
- IQR
- interquartile range
- ITT
- intention to treat
- i.v.
- intravenous
- KY
- khitchri and yoghurt
- LQ
- lower quartile
- MEIP
- MEDLINE In-Process & Other Non-Indexed Citations
- MUAC
- mid-upper arm circumference
- NCHS
- National Center for Health Statistics
- NGO
- non-governmental organisation
- NHS EED
- Economic Evaluation Database
- NIHR
- National Institute for Health Research
- NT
- nucleotide
- OR
- odds ratio
- ORS
- oral rehydration solution
- PEM
- protein–energy malnutrition
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RCT
- randomised controlled trial
- ReSoMal
- rehydration solution for malnutrition
- RL
- Ringer’s lactate isotonic fluid
- RR
- risk ratio
- RUTF
- ready-to-use therapeutic food
- SAM
- severe acute malnutrition
- SD
- standard deviation
- SE
- standard error
- SHTAC
- Southampton Health Technology Assessment Centre
- TB
- tuberculosis
- TFC
- therapeutic feeding centre
- UKCRN
- UK Clinical Research Network
- UNICEF
- United Nations Children’s Fund
- UQ
- upper quartile
- W/A
- weight-for-age
- W/H
- weight-for-height
- W/L
- weight-for-length
- WHO
- World Health Organization
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Department of Public Health and Epidemiology, University of Birmingham
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, University of Liverpool
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Judith Bliss, Director of ICR-Clinical Trials and Statistics Unit, The Institute of Cancer Research
-
Professor Peter Brocklehurst, Professor of Women’s Health, Institute for Women’s Health, University College London
-
Professor David Fitzmaurice, Professor of Primary Care Research, Department of Primary Care Clinical Sciences, University of Birmingham
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Angela Harden, Professor of Community and Family Health, Institute for Health and Human Development, University of East London
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford
-
Dr Joanne Lord, Reader, Health Economics Research Group, Brunel University
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor Dion Morton, Professor of Surgery, Academic Department of Surgery, University of Birmingham
-
Professor Gail Mountain, Professor of Health Services Research, Rehabilitation and Assistive Technologies Group, University of Sheffield
-
Professor Irwin Nazareth, Professor of Primary Care and Head of Department, Department of Primary Care and Population Sciences, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norrie, Chair in Clinical Trials and Biostatistics, Robertson Centre for Biostatistics, University of Glasgow
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Peter Tyrer, Professor of Community Psychiatry, Centre for Mental Health, Imperial College London
-
Professor Martin Underwood, Professor of Primary Care Research, Warwick Medical School, University of Warwick
-
Professor Caroline Watkins, Professor of Stroke and Older People’s Care, Chair of UK Forum for Stroke Training, Stroke Practice Research Unit, University of Central Lancashire
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Tom Foulks, Medical Research Council
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Diana Baralle, Consultant and Senior Lecturer in Clinical Genetics, University of Southampton
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Public contributor
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Nicola Lennard, Senior Medical Officer, MHRA
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Public contributor
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Public contributor
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Matthew Thompson, Senior Clinical Scientist and GP, Department of Primary Health Care, University of Oxford
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Joanna Jenkinson, Board Secretary, Neurosciences and Mental Health Board (NMHB), Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Public contributor
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire County Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Mrs Jean Thurston, Public contributor
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Public contributor
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Public contributor
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Public contributor
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Public contributor
-
Mr Sankaran Chandra Sekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Public contributor
-
Mr Seumas Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Dr Matthew Wilson, Consultant Anaesthetist, Sheffield Teaching Hospitals NHS Foundation Trust
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan, Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Public contributor
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer, Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Public contributor
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Public contributor
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Public contributor
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health