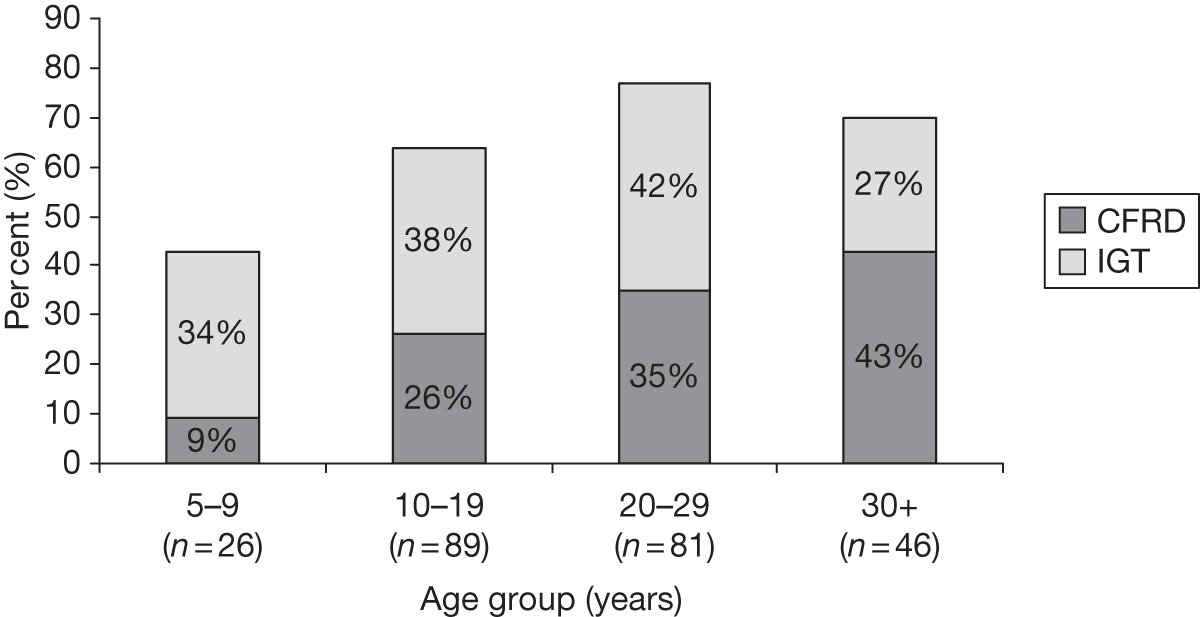Notes
Article history
The research reported in this issue of the journal was commissioned by the HTA programme as project number 07/45/05. The contractual start date was in July 2008. The draft report began editorial review in October 2011 and was accepted for publication in February 2012. As the funder, by devising a commissioning brief, the HTA programme specified the research question and study design.The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2012. This work was produced by Waugh et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2012 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Cystic fibrosis
Cystic fibrosis (CF) is a disease that was first described in 1936 by Guido Fanconi. 1 It is an autosomal recessive disease that can present at any age, but is more commonly diagnosed in early childhood. 2,3 Screening for CF is offered to all babies in Scotland, England, Wales and Northern Ireland. A systematic population antenatal screening is not recommended in the UK but this is currently under review. 4
The defective gene causes faulty transport of sodium chloride in the body, leading to thick viscous secretions, mainly affecting the lungs and the digestive system. 5 CF affects the lungs, pancreas, liver and intestines, and the process involved eventually leads to multisystem organ failure. According to the Cystic Fibrosis Trust, there are over 8500 people in the UK with CF, the severity of which varies from person to person and changes throughout their life. 6 For example, a person with CF may initially have a good quality of life (QoL), where little physiotherapy is required and they are able to play sports, but then recurrent chest infections can lead to deterioration in respiratory function.
There have been major advances in management of, and outcomes from, CF over recent decades. Littlewood has provided a valuable history of the disease, noting that in the course of a professional lifetime, CF has changed from being regarded as almost always fatal in early childhood to a disease in which the aim now is ‘striving to maintain the affected person in the best possible condition to reach adulthood with minimal respiratory and nutritional damage’ (J Littlewood, Cystic Fibrosis Trust, 2010, personal communication; comment was previously in a historical account on the Cystic Fibrosis Trust website).
Epidemiology
The prevalence and distribution of the gene varies among ethnic groups,5 with Caucasians having a higher probability of carrying the abnormal gene. 7 Table 1 shows the incidence of CF in various populations.
| Country/regions | Incidence per live births |
|---|---|
| Scotland8 | 1/1984 |
| Ireland5 | 1/1700 |
| Brittany5 | 1/1700 |
| Australia5 | 1/3500 |
| Finland5 | 1/25,000 to 1/40,000 |
| Estonia5 | 1/7750 |
| UK9 | 1/2415 |
| USA5 | 1/2000–1/4000 |
| African Americans5 | 1/17,000 |
| South America5 | 1/9000 |
| China10 | Very rare |
The incidence in the Caucasian population is approximately 1 : 2500–4000,5 with a carrier frequency of 1 in 25 live births. 7 Ashkenazi Jews and non-Hispanic Caucasians also have a carrier rate of 1 in 25 live births, which is higher than the carrier rate in other ethnic groups;11 Hispanic Americans have a carrier rate of approximately 1 in 46, African Americans have a carrier rate of 1 in 62, and for Asian Americans the carrier rate is 1 in 90. 11 There are quite large variations in incidence within Europe, ranging from a high of 1 in 1353 births in Ireland to 1 in 25,000 in Finland. 12
Within countries, there are sometimes populations or areas of much higher incidence, such as:13
-
North Brittany – 1 in 377 births
-
The Amish in the USA – 1 in 569 births
-
Saguenay–Lac-Saint-Jean, Quebec – 1 in 902 births.
The incidence rate in the UK is 1 in 2500 live births. 8
Genetics
A gene defect occurs on chromosome 7, which affects the production of a protein called cystic fibrosis transmembrane conductance regulator (CFTR). This dysfunctional chloride channel affects the water and electrolyte composition of secretions from various places including the pancreatic ducts and airways. This leads to an accumulation of thick viscous secretions7 and eventually destruction of the affected organs. 8
Many genes can cause CF. They are grouped into five classes, as follows:14
-
class I defective protein production; few or no functioning CFTR chloride channels
-
class II defective processing, so that CFTR does not reach the surface membrane where it normally functions
-
class III defective regulation, but it does reach its site of action
-
class IV defective conductance – CFTR is in the right place, but the channel fails to conduct properly
-
class V reduced amounts of functional CTFR protein.
The less functioning CFTR there is, the more severe the phenotype. Classes I–III are associated with more severe disease and higher mortality. Class II is by far the most common type in the UK.
The commonest mutation is delta F508 (ΔF508). There are international variations in the frequency of mutations which can affect the severity of CF and the prevalence of cystic fibrosis-related diabetes (CFRD). For example, in the Netherlands, the second commonest mutation is A445E, which is associated with milder disease. 3
There are over 1000 relevant mutations, some of which cause mild disease.
Pathology
The build-up of viscous secretions in the lungs means that patients are prone to repeated infections by organisms such as Staphylococcus aureus, Haemophilus influenzae and Pseudomonas aeruginosa. 5 Owing to the stasis of the secretions, bacterial clearance is reduced and inflammatory lung damage ensues. 5 Once severe lung disease is established, lung transplantation is required and if this cannot be carried out, respiratory failure occurs, which eventually leads to death.
The effect on the pancreas causes deficiency of digestive enzymes, leading to malabsorption of undigested foods and undernutrition. Although the primary defect is of exocrine secretion, the islet cells that are initially preserved may become damaged with time, thereby leading to a decrease in insulin and glucagon secretion. Other recognised problems include hepatic cirrhosis and infertility in males.
Management
Management is complex and includes daily bronchial drainage by physiotherapy, nebulised bronchodilators and mucolytics, chronic suppressive antibiotics if infected, anti-inflammatory therapy, nutritional support (such as pancreatic enzymes and vitamin supplements), and frequent monitoring of pulmonary function and microbial carriage. 15
Treatment imposes a significant burden on most people with CF. This burden may include getting up at 6.30 am every day so that physiotherapy can be carried out before going to school, ingesting enzymes after consuming any amount of food (e.g. a biscuit), and more physiotherapy in the evenings before going to bed. 6 Treatment is generally tailored to the individual but the constant ingestion of medication and the rigid treatment schedule removes the spontaneity and pleasure of life in general.
The burden has been quantified by Sawicki et al. 16 in the Project on Adult Care in CF (PAC-CF) carried out in 10 centres in the USA. The median number of daily therapies was seven, and an average of 108 minutes a day was spent on treatment. Common medications were pancreatic enzymes (taken by 85%), β-agonist bronchodilators (65%), anti-reflex agents (50%), DNase (49%) and azithromycin (47%). Ninety-three per cent were on at least one nebulised medication.
Prognosis
In 1938, Andersen17 was the first person to give a comprehensive description of CF. Over 70% of the 49 patients examined in her study died before their first birthday. In the mid-1950s, few children with CF would live to attend elementary school. 18 Dodge et al. 19 reported that over the period 1947–2003, the average per cent surviving by age were 97% to age 10 years, 90% to age 20 year, 63% to age 30 years and 45% to age 40 years.
However, median survival has been steadily improving. In the UK, median survival was 38.8 years in 2008;20 43.8% of those on the register were aged 20 years or over. In the USA, the median predicted survival in 2007 was 37.4 years. 18 One feature associated with this is the improvement in lung function, with the proportion of 18-year-olds with good lung function [forced expiratory volume in 1 second (FEV1) > 70% predicted] increasing from around 32% in 1985 to near 70% in 2008. 18 Most people with CF die of lung disease.
The improvement has not applied at all ages. Kulich et al. ,21 using US Cystic Fibrosis Foundation Patient Registry data on 31,012 patients with 5234 deaths from 1985 to 1999 (17% of the cohort), reported that mortality had fallen by 61% in the age range 2–5 years, by 70% in the range 6–10 years and by 45% in the range 11–15 years. 21 Females had poorer survival. There was little improvement in the over-20s but, as the authors note, this may have been because some who would have died before reaching 20 years were now surviving past it, but not for very long. In the UK, Lewis et al. 22 also noted an increase in survival only up to the age of 20 years.
In the UK, Dodge et al. 23 reported that CF was no longer an important cause of death in children. With better treatment now available, it is estimated that a child born with CF in 2000 would live to approximately 50 years of age. 19
As a result, an increasing proportion of people with CF are adults. In the USA in 1990, about 30% of the patients in the US Cystic Fibrosis Foundation Patient Registry were 18 years or older; in 2008, that figure had reached 46%. 18 One consequence of this is that many women with CF are living to have children of their own. A UK survey by Edenborough et al. 24 reported 48 live births from 72 pregnancies, with almost half of the births being premature. However, a French study reported 64 live births from 75 pregnancies, with only 18% being premature. 25 Gestational diabetes is common, with McMullen et al. 26 reporting a baseline diabetes prevalence of 9%, rising to 21% during pregnancy, in a group of women whose age ranged from 15 to 38 years (median 24 years). McMullen et al. 26 did note that the high prevalence seen in pregnancy might reflect the more thorough screening during pregnancy.
In the UK, the 2008 Cystic Fibrosis Trust Annual Data Report, using a slightly different age breakdown, showed that 43.8% of people with CF were aged 20 years or over. 20 In Canada, similar improvements have been reported, with (rounded) median survival being 24 years in 1982, and 29, 34, 33 and 37 years in 1987, 1992, 1997 and 2002, respectively, reaching 48 years in 2007. 27
The severity of CF can be assessed by the Shwachman clinical score (SS), which allocates points for general activity, physical examination, nutritional status and radiographic findings with a score out of 100, with severe disease having a score of < 40. 28
Most deaths are due to lung damage. 29
Cystic fibrosis-related diabetes
Diabetes mellitus was first described as a complication of CF in 1955. 30 The incidence of diabetes is related to the duration of CF, and with the significantly improved survival into adulthood, more patients are living long enough to develop diabetes. Thus, a higher proportion of patients with CF will develop diabetes than would have done in the past.
Epidemiology
The prevalence of CFRD increases with age and occurs in up to 40% of patients with CF by the fourth decade of life. 31 The risk factors for developing CFRD are increasing age, genetic factors, pancreatic insufficiency, pulmonary infections, corticosteroid therapy and supplemental nutrition. 1 The median age at onset of CFRD is 20 years, and females tend to develop this disease at a younger age than their male counterparts. 1
In one study of 448 patients with CF, the median age at onset of CFRD was reported as approximately 20 years (18.7 years for females and 21 years for males). 32 The prevalence of CFRD has been variably reported and increases with age owing to the natural progression of impaired glucose metabolism. Lanng et al. 33 reported a CFRD prevalence of 1%, 30%, and 75% in those under 10, at 20 and at 30 years of age, respectively. 33 In a recent UK-based prospective study, Adler et al. 34 reported the incidence of CFRD as 3.4% per year. The definition of diabetes that was used included physician diagnosis, a 2-hour post glucose load blood glucose (BG) concentration of > 11.1 mmol/l or treatment with insulin or oral hypoglycaemic agents (OHAs).
Rosenecker et al. 35 reported that CFRD was more common in females, with, for example, prevalence in the age range of 21–25 years being 6% in males and 17% in females.
Although the aetiology of this is unknown, it may be due to the earlier onset of puberty in girls. 7 There is also a greater prevalence of CFRD in females. 36 Figure 1 shows the prevalence of CFRD and impaired glucose tolerance (IGT) for both sexes in various age groups. 37 Here, it can be seen that in the over-30s, > 40% have diabetes and nearly 30% have IGT.
The UK Cystic Fibrosis database7 reported that 39% of those > 10 years and who had been tested were diabetic. For the over-30-year-olds it was 59%; 47% of the over-10s had not been tested. In the 15-year-olds, 9% had diabetes and another 8% were classed as glucose intolerant.
The Cystic Fibrosis Foundation (CFF) 2008 annual data report18 showed that in the USA the prevalence of CFRD reached a plateau in the 35- to 44-year age range, with about 32% having CFRD. This may imply that screening for diabetes could stop after the age of 40 years, because those who are going to develop diabetes will have done so by then.
A more recent update from the USA from Moran et al. ,38 based on the Minnesota data, reported that CFRD was present in 2% of children (< 10 years), 19% of adolescents (11–17 years) and 40–50% of adults. The younger patients tended to have CFRD without fasting hyperglycaemia (FH), but with age the proportion with FH rose to about half in the 30–39 years age group and about two-thirds in the over-40s (estimated from graph). A higher proportion of women than men in the 30–39 years’ age range had CFRD: about 60% versus 40%.
In Australia, Rana39 reported that the incidence of reported CFRD in the under-18-year age group had risen from 0.6 per 106 in 2000 to 6.7 per 106 in 2008, although this may be due to better detection, as 53% were diagnosed by oral glucose tolerance test (OGTT) in 2007–8 compared with 5% in earlier years.
Mackie et al. 1 stated that in the UK the prevalence of CFRD has risen from 3–10% in 1969 to 14–30% in the early 1990s, based on differing screening methods.
Droumaguet et al. 40 in Paris reported a prevalence of 36% among 243 adults with CF, but their cohort was somewhat unusual in having a mean age at diagnosis of CF of 21.5 years. The mean age at onset of CFRD was 27 years (range 18–60 years).
In Denmark, Lanng et al. 33 demonstrated a prevalence of 24% for all ages, rising to 34% in those aged 10 years and above. In the USA, Moran et al. (2009)38 reported an overall prevalence of 33%, with the highest prevalence of just under 50% in the 30- to 39-year age group (from graph, figure 1a).
In Canada, only 21% had developed CFRD by age of 35 years and over and the prevalence had reached a plateau after the age of 25 years. 27
Table 2 shows the prevalence of CFRD at different age groups in various different countries.
| Country | Under-12s | Adolescents | Young adults | Adults 30 years and over |
|---|---|---|---|---|
| UK41 | 0% for ≤ 9 years | 5% for 10–19 years | 10% for 20–29 years | 16% |
| Denmark33 | 34% for 10–19 years | 53% for ≥ 20 years | ||
| USA38 | 2% for < 11years | 19% for 11–17 years | 40% for 18–29 years | 45–50% |
| Mid-Europe35 | 1% < 11 years | 8% for 11–20 years | 12% 21–25 years | 15% for ≥ 26 years |
| The Netherlands3 | 22% for 10–17 years | 36% for 18–30 years | 50% for ≥ 31 years | |
| Canada 2007 registry27 | 1% < 11 years | 5% 11–17 years | 14% 18–24 years | 20% 25–34 years, 21.5% ≥ 35 years |
Genetics
The risk of CFRD varies among the five classes of CF. Unfortunately, the risk is highest in the commonest classes, II and III, with 22% of these adults being diabetic, compared with < 2% in classes IV and V. 14 In the UK, Adler et al. ,42 using UK CF Registry data on a large cohort, found that the incidence of CFRD was 3.5% a year, and was highest in those with CFTR class I and II mutations. About 80% of UK patients have class II mutations.
The ΔF508 mutation appears to increase the risk of CFRD, whereas the N1303K mutation may reduce the risk. 43,44 In populations with low prevalence of ΔF508, such as in Brazil, CFRD is less common. 45
There appears to be a small subgroup with adult onset and a milder form of CF, with a low prevalence of CFRD. Gilljam et al. 46 in Toronto reported 7% of their adult patients to be in this group. 46
The risk of CFRD may be increased if there is a family history of type 2 diabetes mellitus (T2DM), possibly because a gene linked to T2DM increases the risk and lowers the age of onset of CFRD. 47
Pathology
Endocrine function
In CF, the abnormal function of CFTR leads to the production of viscous secretions and this causes obstructive damage to the pancreas. 7 Fibrosis and fatty infiltration of the pancreatic exocrine glands occur and disrupt the islet architecture. Many, but not all, of the islet cells are destroyed and this leads to a progressive loss of endocrine cells,7,15 the main cause of CFRD. 48 Whole islets are destroyed, unlike the β-cell-specific obliteration seen in type 1 diabetes mellitus (T1DM),49 leading to the damage of α-cells, β-cells and pancreatic polypeptide-producing cells. This leads to a reduction in glucagon, insulin and pancreatic polypeptide secretions, respectively. 7 By the time of diagnosis, there has been a loss of 50% of β-cell mass, similar to that seen in T2DM. 50 In addition, amyloid deposits are found within the β-cells. However, it is not clear if the amyloid accumulates during the disease process or even if it contributes to β-cell dysfunction. 36
Cystic fibrosis-related diabetes is described more fully in Chapter 2.
The precise mechanism of CFRD is unclear. 1 CFRD is characterised by an insulin deficiency7 owing to the loss of insulin-producing β-cells. 31 Couce et al. 50 state that there is approximately a 50% loss in β-cell mass, which is similar to that seen in patients with T2DM. This occurs after fibrosis and fatty infiltration of the pancreas. This leads to destruction of the pancreatic islet architecture. 31
Insulin resistance has also been reported,51 especially at times of infection and inflammation, but the main problem is a progressive fall in β-cell capacity. 48,49 This leads to a progressive impairment of insulin production.
Hyperglycaemia may first be seen only at time of metabolic stress, such as lung infections, but is later seen as postprandial hyperglycaemia (PPH) [initially only immediately after meals, so that plasma glucose (PG) may be normal by the time of a 2-hour OGTT test], progressing to IGT then to CFRD without FH, and then to CFRD with FH. Schwarzenberger et al. 52 reported that most of their patients (a large cohort of 775) without FH progressed to it over time.
Lung function in diabetes mellitus
As previously mentioned, CF affects the lungs, where the build-up of viscous secretions is not only difficult to expel from the body, but also leaves the person prone to various chest infections. In addition, diabetes also affects the lungs. Although the effects are not widely recognised, owing to any abnormalities being slight and subclinical, in a person with CF these changes could have a greater impact. 53 This is discussed in Chapter 2.
Management
Patients with CFRD have the same problems with malabsorption and malnutrition as all other patients with CF do and so their dietary requirements are essentially unchanged. 7 In addition, as CFRD is due to insulin deficiency, management with insulin is standard practice. Increasingly, centres treating patients with CF administer insulin early in an attempt to influence body mass index (BMI) and pulmonary function. 54 Insulin treatment is used more liberally in Europe, but in the USA it has been mainly used in patients with FH, although guidelines did permit usage in those without FH at the clinician’s discretion. 1 Treatment options are reviewed in Chapter 3.
As mentioned previously, patients with CF have the daily chore of complying with a relatively rigid schedule, which includes a long list of therapies. If CFRD develops, extra medical therapies and regular health checks are added to the existing burden of self-management. Patients with CFRD need to regularly monitor his or her BG levels, regularly administer insulin and undergo various screening tests for diabetic complications. Furthermore, patients with CFRD need to deal with temporary disturbances of glucose regulation during bouts of illness, when more frequent BG tests need to be carried out55 because control of BG levels is harder. 56 As one CFRD patient mentioned, ‘You cannot just go out and do what you want, when you want, you’ve got to think hard and plan it a bit better. It’s inconvenient.’56
Prognosis
The life expectancy of patients with CF is fortunately improving; the median survival age for a child born in 2000 is approximately 50 years. 19 However, patients with CFRD have poorer nutritional status and worse lung function than patients with CF, which leads to a higher mortality rate. 36 In 1988, a retrospective study of 448 patients with CF living and deceased showed that < 25% of patients with CFRD reached the age of 30 years compared with nearly 60% of patients with CF. 32 Age at onset is lower in females than in their male counterparts. Females also have a reduced life expectancy. It is not clear whether or not these two facts are connected. Milla et al. 57 found that the median age of survival was 30.7 years for females with CFRD, and for males it was 47.4 years. It must be noted that this difference in age survival may be due to CFRD or it may arise from other factors (e.g. pregnancy can cause a rapid decline in lung function, a trait seen in both CF patients and patients with CFRD). Miller et al. 58 reported that patients with CFRD were more likely to have a decline in FEV1 than patients with only CF, and that this affected women especially, suggesting that women were more severely affected by CFRD than men.
Srivastava et al. 59 from London also reported that CFRD reduced survival; 25% of patients with CFRD died by the age of 26 years compared with 31 years for those without diabetes. With respect to the patients with CFRD, females had a 50% mortality rate at 29 years, whereas males had the same mortality rate at the age of 37 years. These figures were for the cohort born 1970–91. This may be related to reports that lung function was worse in women than men. 60
Kampfert et al. 61 in Germany and Austria also noted that the outlook was poorer for women. Among 1334 patients, the prevalence of CFRD at the age of 18 years was 12.5% in women and 4% in men.
However, the most recent mortality data from the USA show no difference between men and women. 38 This was different from the previous report from the same centre by Milla et al. in 2005. 55 They also found a marked decline in mortality in people with CFRD in both sexes. The authors note that CFRD treatment has become much more vigorous than in the past.
Chamnan et al. 62 carried out a retrospective cohort study to determine mortality rates, estimate the risk increase associated with diabetes, and calculate the population attributable fraction (PAF) for mortality associated with diabetes. Their cohort included 8029 people aged 0–65 years, registered on the UK Cystic Fibrosis Registry from 1996 to 2005, of whom 5892 had data for mortality rate follow-up, with 4234 complete data for analysis of risk factors for mortality; 393 subjects died during follow-up. Of the 696 with CFRD, 141 died.
For CF in general, crude annual mortality was 2.2% per annum. Mortality increased with age, but for those with CFRD peaked in the 20- to 29-year age range. 62 The risk of death was higher among females than males, with age-adjusted mortality rates of 2.0 [95% confidence interval (CI) 1.8 to 2.4 age-adjusted mortality rate] and 1.6 (95% CI 1.4 to 1.9 age-adjusted mortality rate), respectively. Those with CFRD had much higher age-adjusted mortality rates at 4.2 (95% CI 3.4 to 5.1 age-adjusted mortality rate) per 100 person-years than those with CF alone: 1.5 (95% CI 1.3 to 1.7 age-adjusted mortality rate per 100 person-years). The higher diabetic mortality was seen in all ages.
Chamnan et al. 63 estimated that the PAF for diabetes was 14% (95% CI 8% to 19%), i.e. that 14% of all deaths in people with CFRD are due to diabetes. They make the striking point that standardised mortality rates show that the CF population in the UK, with a median age of 13 years, has a mortality rate similar to that of 70- to 74-year-olds in the general population of England and Wales.
Finkelstein et al. 32 in 1988 reported that < 25% of patients with CFRD survived (then) to the age of 30 years compared with 60% of those with CF without diabetes.
The excess mortality has been reported to be much worse in females than males. Milla et al. 57 reported that median survival was 35.6 years in those with CFRD and 47 years in those with CF without diabetes. However, the median survival in females with CFRD was 30.7 years and in males 47.4 years. Miller et al. 58 also reported higher mortality in women with CFRD than in those with CF alone, and that the decline in lung function over time was more marked in females.
Recent work from the UK has shown that there is a link between hyperglycaemia and mortality. Adler et al. ,64 using UK Cystic Fibrosis Registry data, found that patients with CFRD who died had higher glycated haemoglobin (HbA1c) levels (7.3%) than those who did not (6.7%). Around 60% of deaths were due to respiratory disease, and those who died had a much lower FEV1 than those who did not (33% vs 54% of predicted).
Survival in patients with CF whose FEV1 has fallen below 30% of expected used to be poor, with half surviving for < 2 years. However, George et al. 65 from the Brompton group reported survival of 2-year cohorts: 1990–1 to 2002–3. Median survival improved from 1.2 years in the 1990–1 cohort to 5.3 years in the 2002–3 cohort. The improvement in survival started in the 1994–5 cohort, and reached a plateau after the 1996–7 one, and coincided with the introduction of nebulised human DNase. The proportions with CFRD changed little. In univariate analysis, the presence of CFRD increased mortality by about 80% (our calculations – the figure in the published paper looks wrong).
Complications
Microvascular complications (e.g. retinopathy, neuropathy and nephropathy) occur in patients with CFRD. 66,67 Yung et al. ,63 albeit in a small study, reported a prevalence of retinopathy among patients with CFRD who had been diagnosed for 5 years or more of 16% (5 out of 31 patients) and among those who had been diagnosed for 10 years or more of 23% (3 out of 13 patients). The prevalence of nephropathy was between 3% and 16% and of peripheral neuropathy between 5% and 21%. 68 One problem is that microalbuminuria is common in patients with CF without diabetes and so is not a reliable marker for diabetic nephropathy. 69 The microvascular complications appear to occur only in those patients with CFRD with FH. 52
Macrovascular complications have been rare. 68 It is thought that this is because patients with CFRD do not live with diabetes for long enough for macrovascular complications to occur. Indeed, at least one authority has stated that no patient with CF has so far died of atherosclerotic cardiovascular disease. 70 A study from London reported retinopathy, but also that no macrovascular complications were found. 31
Georgiopoulou et al. 71 may have provided much of the explanation. In their study of metabolic aspects of CF, they noted that total and low-density lipoprotein cholesterol were low (total cholesterol 3.5 mmol/l, low-density lipoprotein 1.27 mmol/l), but that high-density lipoprotein cholesterol was near normal. They also reported low BMI (21 kg/m2), and lowish systolic blood pressure (116 mmHg) and diastolic blood pressure (74 mmHg).
However, as more patients with CFRD progress into the fifth and sixth decades of his or her lives, this may become more common. Rhodes et al. 72 from Toronto have reported that adult patients with CF do develop dyslipidaemia, but mainly those with pancreatic sufficiency. Those with CFRD did not have more dyslipidaemias.
In children, CFRD is associated with reduced growth rates, both in the 2 years before and after diagnosis. 73
Terminology
In this review, the following categories of glucose status will be used.
-
Normal glucose tolerance (NGT) requires both fasting plasma glucose (FPG) of < 5.6 mmol/l and 2-hour OGTT level of < 7.8 mmol/l, 2 hours after a 75-g glucose load.
-
Diabetes is defined as FPG level of > 7.0 mmol/l and/or 2-hour OGTT level of > 11.1 mmol/l, except that the diagnosis must be confirmed – a single glucose level is not enough. Some studies from the USA subdivide diabetes into ‘with FH’ or ‘without FH’. This is partly a question of stage of disease, with diabetes manifesting itself first mainly as PPH.
-
IGT is based on a 2-hour OGTT level of between 7.8 and 11.1 mmol/l.
-
Impaired fasting glucose (IFG) means a FPG level of between 6.1 and 6.9 mmol/l, as used by the World Health Organization (WHO). 74 The American Diabetes Association (ADA) defines it at a lower threshold of 5.6 mmol/l. The WHO system does not give a name to those with a FPG level of 5.6–6.0 mmol/l, who are above normal but under the IFG threshold.
-
PPH. There are patients in whom PG after a meal is abnormally high for the first hour or so, but returns to normal by 2 hours. The term ‘lag storage’ has been used in the past. Data from the Royal Hospital for Sick Children in Glasgow show that many patients with CF have high PG levels at 30, 60 and 90 minutes but normal fasting and 2-hour levels. Some of these results are into the range for random BG at which diabetes would be diagnosed. 75
The WHO criteria for diabetes are based on the risk of harms such as retinopathy (although the existence of a clear threshold for retinopathy risk has been challenged in recent years, with retinopathy reported in IGT). 76 It may be that the threshold for harm in CF, such as bacterial growth, may have a different threshold and that we need a new definition of CFRD. This is discussed further in Chapter 2.
It is usually assumed that people who develop CFRD go through the above stages in sequence, but several studies have shown that there can be regression as well as progression in the early stages. Carpenter et al. 77 repeated OGTTs in 94 adolescents and found that 50% (8 out of 16) who had IGT reverted to NGT. The other half progressed to CFRD. Thorsteinsson et al. 78 had similar results, with 58% of those with IGT reverting to NGT at the next annual OGTT. Other studies have reported similar results, with very variable glucose tolerance over time79 or reversion from IGT to NGT. 33
Decision analysis
Screening for CFRD is necessary because the onset can be insidious, and because it can cause harm before diagnosis. The first question for this review is therefore how best to screen for CFRD – which tests, starting when and how often?
A survey in the USA by Allen et al. 80 found a wide range of screening practices and tests, with random PG the most common, followed by HbA1c, and urinary glucose. 80 Very few used the OGTT. Most guidelines recommend an annual OGTT but it appears that, owing to the cost, inconvenience and unpleasantness of that test, the guidelines are largely ignored in practice. A similar survey in the UK by Mohan et al. 81 also found that there was variation in screening methods. Only 30% used the recommended (by a working group of the UK Cystic Fibrosis Trust) method of the combination of the OGTT and serial glucose monitoring, with another 49% using the OGTT alone. Other tests used (usually in combinations) included HbA1c, FPG, random PG, and glycosuria. However, the survey reported the policies used, but not the proportions of patients screened according to the local policies.
As mentioned, most guidelines regard the OGTT as the ‘gold standard’, but it is often not used in practice. It is therefore necessary to consider:
-
Could other tests such as HbA1c, continuous blood glucose monitoring (CBGM) or home serial capillary BG profiles could be used? Even tests not as sensitive (perhaps such as HbA1c) might still detect more cases in practice owing to better compliance. A test that is 100% sensitive but which has only 50% acceptance will detect 50% of cases; one that has a sensitivity of 80% and an acceptance of 80% will detect 64% of cases.
-
Could a combinations of tests might give better overall results, for example if screening was undertaken in two or more stages? For example, would it be helpful to test HbA1c in the first instance, with patients divided into three groups, as follows?
-
– HbA1c-negative for diabetes. The cut-off value might be under 5.7%, as recommended by the Expert Working Group on the diagnosis of diabetes,82 but this would need to be reviewed in the context of CFRD. Anaemia is common in adults with CF (43% in a study by Von Drygalski and Biller83) and any reduction in red-cell life would give misleadingly good HbA1c results. Anaemia was much less common in children, so HbA1c might be useful for screening for them, but not for adults.
-
– HbA1c diagnostic for diabetes (perhaps 6.5%).
-
– Intermediate HbA1c (say 5.7 to < 6.5%) followed by OGTT.
-
A sequence with HbA1c or random PG first might allow many patients to avoid OGTT.
In T2DM, HbA1c level is influenced in the early stages more by non-FPG than FPG. 84 Whether or not it would be sensitive enough to pick up isolated PPH (without IGT) remains to be examined. The sensitivity would depend on the threshold at which patients were referred for OGTT.
Continuous BG monitoring is carried out by inserting a disposable glucose monitor under the skin, connected to a meter worn externally. A chemical reaction generates a current that is proportional to the level of glucose in the tissues. Strictly speaking it is interstitial tissue glucose that is monitored. A review by the Australia and New Zealand Horizon Scanning Network (ANZHSN) noted that CBGM systems seemed to be better at detecting hyperglycaemia than hypoglycaemia, a problem that would not be relevant to its use in screening for CFRD. All of the trials reported in the ANZHSN review were in people with diabetes; no use in screening was found. 85
Home BG involves testing with sticks and meters over the course of a day. This is called blood glucose profiling (BGP).
Again, as with OGTT, these could be used on all patients or only on those shown likely to have CFRD or IGT after a preliminary screen with, for example, HbA1c or a casual PG.
In addition to diabetes, two other conditions may cause harm. The first is IGT, which, as mentioned above, can be associated with microvascular disease. 76 IGT is also associated with a reduction in lung function [FEV1 and forced vital capacity (FVC)]. 86
The second is PPH because it has been suggested that this alone may lead to end products of glycation, which may cause irreversible damage. Gerich87 notes that isolated PPH, with normal FPG and normal HbA1c, is associated with an increase in vascular disease, although he was referring to 2-hour PG. Hanefeld et al. 88 reported that glycaemic excursions were associated with carotid intimal thickening in non-diabetic subjects. Hence, it is important to know if isolated PPH can affect lung function. If we should be concerned with IGT, or even just PPH, then that has implications for the choice of screening test. FPG would not be satisfactory.
The second question for this review is therefore whether or not we should be screening for a wider range of hyperglycaemia than diabetes? It would only be worth doing that if treatment of that level of hyperglycaemia was shown to improve outcomes.
Chapter 2 Defining cystic fibrosis-related diabetes
Cystic fibrosis itself was described as a discrete clinical entity only in the late 1930s17 and impaired glucose metabolism in CF was not described until 1955. 89 Although a number of similarities to T1DM and T2DM are recognised, the impaired glucose metabolism associated with CF is a distinct clinical entity90,91 with a different aetiology, mode of onset, clinical course and outcome.
To detect, manage and prevent cystic fibrosis-related impaired glucose tolerance (CFRIGT), it is necessary to define its onset, severity, progression and impact. A number of questions are raised:
-
How are impaired glucose metabolism and diabetes mellitus currently defined and classified?
-
How are CFRIGT and CFRD currently defined and classified?
-
How do CFRIGT and CFRD differ from other forms of impaired glucose metabolism and diabetes?
-
What glucose level should be used to define CFRIGT?
How are impaired glucose tolerance and diabetes mellitus currently defined and classified?
There are several forms of diabetes, but most patients have T1DM or T2DM. A classification system based on aetiology, and not clinical features, was proposed by the WHO in the mid-1980s. 92
The WHO has defined the term ‘diabetes mellitus’ as ‘a metabolic disorder of multiple aetiology characterised by chronic hyperglycaemia with disturbances of carbohydrate, fat and protein metabolism resulting from defects in insulin secretion, insulin action, or both’. Diabetes resulting from autoimmune destruction of the insulin-producing β-cells of the pancreas causes an absolute insulin deficiency, known as T1DM. T2DM is probably multifactorial, with relative insulin deficiency or loss of sensitivity to insulin considered the major causal factors.
Under the WHO aetiological classification system, diabetes associated with CF is known as ‘cystic fibrosis-related diabetes’ or ‘CFRD’, and is listed in the category of ‘Other specific types (of diabetes)’, and further subclassified within ‘Diseases of the exocrine pancreas’.
The impaired glucose metabolism associated with CF has some similar, and some quite different, features compared with T1DM and T2DM. These similarities and differences are summarised in Table 3, taken from a recent review by Laguna et al. 93 Of particular note is the insidious and intermittent nature of its presentation, along with evidence for both insulin deficiency, which is almost always incomplete, and sometimes insulin resistance,94 which varies with nutrition, infective status and medication.
| CFRD | T1DM | T2DM | |
|---|---|---|---|
| Prevalence in population (%) | 35 | 0.2 | 11 |
| Peak age of onset | 20–24 years | Childhood, adolescence | Mid- to late adulthood |
| Usual body habitus | Normal to underweight | Normal | Obese |
| Insulin deficiency | Severe but not complete | Complete | Partial, variable |
| Insulin resistance | Usually modest, waxes and wanes with infection | Usually modest | Severe |
| Autoimmune aetiology | No | Yes | No |
| Ketones | Rare | Yes | Rare |
| HbA1c | Unpredictable relation to mean BG | Related to mean BG | Related to mean BG |
| Usual treatment | Insulin | Insulin | Oral agents, insulin |
| Microvascular complications | Yes | Yes | Yes |
| Macrovascular complications | No | Yes | Yes |
| Metabolic syndrome features | No | No | Yes |
| Cause of death | Lung disease | Cardiovascular | Cardiovascular |
Marshall et al. 36 listed some of the other differences between CFRD and the more common types of diabetes, including the following:
-
nutritional status – often poor in CF
-
infection (acute and chronic)
-
catabolism and increased energy expenditure
-
glucagon deficiency
-
malabsorption
-
abnormal intestinal transit time
-
hepatic dysfunction
-
increased work of breathing.
When does diabetes start?
All forms of diabetes are characterised by hyperglycaemia, but determining the threshold above which a BG result should be considered abnormal proves more difficult.
To standardise the terminology used when referring to disorders of glucose metabolism, both the WHO92- and the National Diabetes Data Group (NDDG)95- based diagnostic criteria and classification of hyperglycaemic states on the results of a standardised 75-g OGTT. They recommended that a FPG level of ≥ 7.8 mmol/l and a FPG level of ≥ 11.1 mmol/l 2 hours after a standardised glucose load be considered diagnostic of diabetes mellitus.
The WHO and NDDG documents also introduced the concept of IGT, which referred to a state with BG results 2 hours after an OGTT higher than the upper limit of normal but below the threshold for diabetes mellitus itself. IGT was recognised as a stage in the progression from normal to impaired glucose metabolism and distinct from the diagnosis of diabetes. It is known to indicate increased risk of developing diabetes at a later stage, although not all people with IGT progress to T2DM. 96
These standardised diagnostic criteria for diabetes mellitus and impaired glucose metabolism were based on two main sources of data:
-
cross-sectional studies that derived thresholds above which complications that are specific to diabetes occurred
-
bimodal distribution of BG excursion noted in certain populations with a high prevalence of diabetes (e.g. Pima Indians). 97
Retinopathy was the diabetes-related complication used to define these thresholds, in three populations. 98 Initially, PG was used but, more recently, HbA1c has also been recommended, and an equivalent threshold for diabetes has been identified. 82 In those populations with bimodal distribution of BG, the point above which the higher group of results were recorded was also used to define recommended diagnostic limits.
Had we defined diabetes on the basis of macrovascular disease, a lower threshold would have been chosen but that approach was not used because, unlike retinopathy, macrovascular disease is not unique to diabetes but only increased by it.
With improved understanding of the aetiology of IGT, the criteria for diagnosing diabetes mellitus were further modified by the ADA in 1997,98 and the WHO adopted similar criteria the following year. 74 The currently accepted ADA–WHO diagnostic criteria for diagnosing diabetes mellitus are outlined below. The main difference is the reduction in the FPG.
World Health Organization–American Diabetes Association criteria for the diagnosis of diabetes mellitus
-
Symptoms/signs of diabetes + random PG level of ≥ 11.1 mmol/l.
-
FPG level of ≥ 7.0 mmol/l.
-
PG 2-hour post 75-g glucose load OGTT level of ≥ 11.1 mmol/l.
Hyperglycaemia determined by any of these methods requires confirmation on a subsequent day by any of the methods.
Impaired fasting glucose and IGT are both associated with an increased risk of subsequently developing diabetes and cardiovascular disease but do not have the same association with microvascular disease (such as retinopathy) as does diabetes mellitus itself. (Although this has been challenged in recent years. 76)
In summary, the diagnosis of T1DM and T2DM is based on BG thresholds derived from epidemiological data which show that those with a FPG of ≥ 7.0 mmol/l or a PG ≥ 11.1 mmol/l 2 hours after a 75-g glucose load OGTT have a greater risk of retinopathy.
How are cystic fibrosis-related impaired glucose tolerance and cystic fibrosis-related diabetes currently defined and classified?
Applying the WHO–ADA diagnostic system to CFRIGT is problematic.
Diseases should be defined by the harm they do. The most critical organ in CF is the lung and, given the evidence that diabetes can harm the lung, there is a case for defining CFRD by the threshold at which lung damage (‘pulmonopathy’) occurs, rather than retinopathy. Retinopathy was in the past rarely diagnosed in those with CF owing to the poor longevity of patients. Although patients with CF are now living longer and microvascular complications are described,52,67 the significant morbidity and mortality associated with CFRD (e.g. deteriorating pulmonary function) usually occurs before retinopathy develops.
Brodsky et al. 99 carried out OGTTs in 101 patients and found that isolated 1-hour hyperglycaemia (i.e. with normal 2-hour levels) was associated with reduced FEV1, although numbers were few, with only nine patients in this group.
It may therefore be argued that hyperglycaemia thresholds based on the specific features of pulmonary function decline would be of greater relevance to those with CF than any based on the statistics for developing microvascular disease. Diagnostic criteria based on lung function therefore need to be developed in order to decide on the level of PG that should be the cut-off in a screening programme.
Diabetes can affect the lung in different ways by:
-
increasing infections
-
reducing gas diffusion
-
increasing the stiffness of the lung and increasing the effort of breathing.
The last two of these are seen in all forms of diabetes, but are normally not noticed. However, in CF, when lung function may be seriously impaired, the normally marginal effect of diabetic pulmonopathy may be more important.
The current accepted diagnostic criteria for CF-related diabetes are based on a consensus conference held in 1998,100 which included experts in CF, diabetes and nutrition. Diagnostic glucose thresholds were defined as follows and patients are categorised depending on their glucose tolerance (Table 4).
| Abbreviation | FPG | Two-hour post 75 g glucose load | |
|---|---|---|---|
| CF patients with NGT | NGT | < 7.0 mmol/l | < 7.8 mmol/l |
| CF patients with IGT | IGT | < 7.0 mmol/l | 7.8–11.0 mmol/l |
| CFRD without FH | CFRD – FH | < 7.0 mmol/l | ≥ 11.1 mmol/l |
| CFRD with FH | CFRD + FH | ≥ 7.0 mmol/l | OGTT not necessary |
Biochemical thresholds for glucose in cystic fibrosis-related diabetes
-
Two-hour OGTT glucose of ≥ 11.1 mmol/l.
-
Fasting BG of ≥ 7 mmol/l on two or more occasions.
-
Fasting BG of ≥ 7 mmol/l plus casual BG level of ≥ 11.1 mmol/l.
-
Casual BG levels of ≥ 11.1 mmol/l with symptoms* on two or more occasions.
(*Symptoms include polydipsia, polyuria, weight loss, inability to gain weight despite nutritional interventions, poor growth, poor progression of puberty, unexplained chronic decline in pulmonary function.)
More recently, it has been suggested that there should be a fifth class of CFRD, namely CF associated with intermittent diabetes, defined as temporary diabetes occurring during period of infections or steroid treatment followed by a reversion to NGT. 68
Surprisingly, Frohnert et al. 103 have reported that IFG is not associated with reduced survival or progression to diabetes.
The effect of impaired glucose metabolism in cystic fibrosis
As described in Chapter 1, pancreatic histology in those with CFRD shows fibrosis, fatty infiltration and disorganisation of islets. This disruption is largely due to the viscous pancreatic secretions in CF, which causes obstruction of pancreatic ducts. 104 Destruction of insulin-producing β-cells leads to a decline in insulin release. However, poor correlation between the extent of pancreatic fibrosis and islet cell damage has been reported, as well as little correlation between the degree of insulinopenia and OGTT results. 94
The evidence for the impact of diabetes mellitus on the clinical status of those with CF is conflicting, with some reporting steady clinical decline, whereas others do not.
The distinction between diabetes with and without FH has been specific to CFRD because of its importance in the prognosis and/or treatment indications, because until recently only those with CFRD with FH have been treated, as it was thought that only those with FH would develop complications. 70 However, it is now accepted that treatment with insulin is also beneficial at the CFRD – FH-negative stage. 101
Significant clinical deterioration may occur some years before the patient develops the consistently high BG results of overt diabetes. Finkelstein et al. ,32 in a retrospective analysis of 448 patients with CF, noted deterioration in general clinical score [National Institutes of Health (NIH) score] 2 years before the formal diagnosis of diabetes was made.
In T1DM and T2DM, good BG control has been shown to be associated with much lower incidences of retinopathy, nephropathy and neuropathy. 90–92,105–107
Effect on the lungs
Subjects with diabetes mellitus have been shown to have higher morbidity and mortality from pulmonary infection than those with normal BG. 108–111 A review by Ardigo et al. 112 concluded that although the effect on lung function might be quite small (a reduction of 8%, related to vessel wall thickness, leading to stiffness and impaired gas exchange), this would be enough to cause problems when lung function was threatened by other comorbidities. They also noted the poorer outcomes in pneumonia in people with diabetes.
Niranjan et al. 113 found that patients with T1DM demonstrated significant impairments in lung volume and maximal O2 uptake, compared with control subjects without diabetes, but that these could be reduced by improved glycaemic control [in this case, using continuous subcutaneous insulin infusion (CSII)]. 113
Chance et al. 114 found that gas exchange was impaired in T2DM, and that the reduction was associated with microvascular disease and with elevated levels of HbA1c. They assumed that the lung damage was probably due to microvascular disease affecting the very extensive pulmonary capillary bed, but wondered if abnormal connective tissue metabolism could also lead to stiffness. Weynand et al. ,115 in a small series of six deceased diabetics and six non-diabetic control subjects, found that diabetes causes thickening of the pulmonary basal lamina. In a subset of the Fremantle Diabetes Study patients with T2DM, Davis et al. 116 found that FVC fell over time, by about 1% a year, lung function started to decline before diabetes was diagnosed, and there was an association between impaired lung function and mortality, with a 12% increase in all-cause mortality for every 10% reduction in FEV1.
Black et al. 53 reviewed evidence on the effects of diabetes on the lung for a Health Technology Assessment (HTA) review of inhaled insulin and noted:
-
There is a loss of lung elasticity and recoil in diabetes and a greater rate of decline in lung function with age compared with non-diabetic subjects. As a result, the lungs become stiffer and harder to inflate and deflate. This is reflected in reductions in FEV1 and FVC.
-
The diffusion capacity is slightly reduced. This is measured by the diffusion of carbon monoxide from the alveoli, across the epithelium and into the blood. The diffusion capacity is probably reduced owing to thickening in the alveolar epithelium and the pulmonary capillary basal lamina. Changes have been seen in arterioles and capillaries of the lung, which are similar to those in the diabetic kidney, although less marked.
There are several mechanisms by which elevation in airways secretion glucose concentration might be related to increased frequency and severity of pulmonary infection. 117 The air spaces are lined with a thin layer of fluid which normally contains little or no glucose,118 but the level can be increased by both hyperglycaemia and inflammation, both of which occur in CF. The presence of glucose encourages the proliferation of colonising and infective microorganisms. It may also foster virulence. 117 Increased glycosylation of both immune proteins and epithelial cells might further impair local defences. 118 Optimising glycaemic control, and so maintenance of normal or near-normal concentration of glucose in airways secretions, could be a significant factor protecting patients with CF from intercurrent and chronic microbial infection.
Deterioration in pulmonary function is now well reported in those with CFRD. 15,119,120 Adler et al. 121 noted reductions in FEV1 and FVC in both CFRD and CFIGT. It is concerning that this decline is seen from at least 2–4 years before diabetes is diagnosed using the standard OGTT. 32,122
In non-diabetic adults, lower FVC and FEV1 were associated with higher fasting glucose,123,124 and with hyperinsulinaemia and estimated insulin resistance. 125–127
McKeever et al. 86 used data from the National Health and Nutrition Examination Survey (NHANES) to examine the effect of hyperglycaemia below diabetes levels. They found a correlation between 2-hour OGTT glucose in the IGT range and reduced FEV1 and FVC. This association was seen also if the HbA1c level was raised, but there was no clear link with FPG.
Decline in pulmonary function, even before the classical definition of diabetes mellitus has been achieved, was reported by Schaedel et al. 128 from Sweden. They followed up 343 patients with CF (out of a prevalent total of 475 for all of Sweden), who all had at least two sets of pulmonary function tests (PFTs), and examined the effects on lung function of genotype, gender, pancreatic exocrine sufficiency, Pseudomonas colonisation, diabetes and liver disease. There was a faster decline in PFTs in those with diabetes, but this was seen only in the over-15-year-olds. One problem with interpretation was the close link between diabetes and pancreatic insufficiency – all of those with diabetes had pancreatic insufficiency. This raises the possibility that the mechanism is via undernutrition, leading to poor lung function.
Milla et al. ,15 from Minnesota, reviewing the previous studies, noted that a number of studies suggested a cause-and-effect relationship between insulin deficiency and decline in health. However, most of these were retrospective, making it difficult to decide whether glucose intolerance accelerated the decline or whether the sickest patients were more likely to get diabetes. Therefore, they carried out a prospective study of 152 patients who did not have CFRD with FH, divided into three groups by OGTT:
-
NGT – 45%
-
IGT – 39%
-
CFRD without FH – 16%.
Over the 4-year follow-up period, lung function declined in those with IGT and CFRD without fasting hyperglycaemia (CFRD – no FH), but not in those with baseline NGT. Interestingly, there was an association between baseline insulin production and lung function decline, with the highest decline in those with the lowest quartile of baseline insulin. However, insulin levels did not correlate with the glucose groups. This suggests a direct relationship between insulin and lung function, rather than it all being related to PG. Milla et al. 15 speculate that this may be related to the catabolic effect of insulin deficiency.
Lanng et al. 122 reported that FEV1 and FVC were reduced (by 20% and 10%, respectively) 6 years prior to the diagnosis of CFRD. Koch et al. ,14 from the European Epidemiologic Cystic Fibrosis Registry, also noted that FEV1 was reduced in those patients with CFRD compared with those with CF alone. Brown et al. 129 found a reduction in lung function prior to diabetes only in females.
Studies of the effect of insulin show that the decline in lung function is halted after insulin is started. Drummond et al. 130 reported a steady decline in the 5 years before insulin was started, and a plateau afterwards, and recommend treatment at the IGT stage.
Glucose is not usually detectable from the airways secretions of those with normal BG, but is found in such fluids in those with hyperglycaemia. Wood et al. 131 determined the BG threshold at which glucose became detectable in nasal secretions by raising BG concentrations in 12 healthy human volunteers (using either a 20% dextrose intravenous infusion or a 75-g oral glucose load) and then measuring nasal glucose concentrations with modified glucose oxidase strips. An airway glucose threshold of 6.7–9.7 mmol/l was identified (n = 12). Nasal glucose was never as high as BG and fell in parallel.
The presence of such a threshold, along with the concentration of BG being constantly higher than that of nasal secretions, was said to suggest that an active glucose transport system in the airway epithelium maintained low glucose concentrations in normal subjects. As BG was detected in the nasal secretions of usually normoglycaemic individuals who had BG raised with an insulin infusion or measured oral glucose load, it was postulated that people with hyperglycaemia would daily experience prolonged periods of glucose in their airways secretions. So a short peak of hyperglycaemia after meals might cause longer periods of high glucose levels in the fluid lining the airways.
Brennan et al. have carried out a number of studies examining the relationship between BG and airway glucose. Having noted that the presence of glucose in airway secretions was associated with increased infection in people intubated in intensive care, they hypothesised that a similar effect might be seen in CFRD. In a 2005 study,132 they studied breath condensates in groups of healthy volunteers (n = 23), people with CF with (n = 10) and without (n = 10) CFRD, and people with diabetes but not CF (n = 17). Glucose levels in breath condensates were low in the healthy volunteers, but raised in the other groups. However, the levels were higher in those with CF than in those with just diabetes, leading Brennan et al. 132 to conclude that the airway glucose was raised by both hyperglycaemia and inflammation. The highest levels were seen in those with CFRD.
In a study published in 2007, Brennan et al. 133 compared BG and airway secretion glucose (using nasal secretions), but added studies of the growth rates of S. aureus and P. aeruginosa. They found that glucose was present in airway secretions in 85% of cases when BG levels were > 8 mmol/l, but in only 19% (but none with high airway glucose) when it was < 8 mmol/l. It was also higher (0.5–3.0 mmol/l) in the former than the latter (0.5–1.0 mmol/l). People with CFRD had PG levels of > 8 mmol/l for 45% of the day compared with 6% in people with CF but NGT, and 1% in healthy volunteers. S. aureus growth increased once glucose concentration reached 0.5 mmol/l, and P. aeruginosa growth increased at 1–4 mmol/l.
The relationship between PG and airway glucose in bronchial secretions was similar to that seen in the intensive care unit study,118 in which glucose was found in 70% when PG level was ≥ 8 mmol/l but in only 16% when it was < 8 mmol/l.
Other effects of insulin deficiency
One can speculate on the number of ways in which insulinopenia, before causing overt symptoms of hyperglycaemia, might be detrimental to patients with CF (increased protein catabolism, intermittent glycosuria, altered immune function).
There is also a strong association of respiratory function with overall nutritional status. Insulin is a growth factor and its use is associated with stabilisation of weight loss and possibly even weight gain. 134,135 Milla et al. ,15 in the prospective Minnesota study, referred to above, noted a direct link between lung function decline and insulin levels, possibly via loss of the anabolic effect.
Yet another possible mechanism is through anaemia. Von Drygalski and Biller83 noted that anaemia became more prevalent as people with CF aged – from 12% in the under-16-year-olds to 58% in the over-40-year-olds – and that it was associated with poorer pulmonary function. FEV1 was 52% of that expected in those with anaemia, and 83% in those without. However, this may be another example of correlation rather than cause but it remains a highly relevant finding, as oxygen carriage will be diminished.
Conclusions
-
As the organ most at risk in CF is the lung, and as hyperglycaemia appears to adversely affect lung function, we should probably define CFRD and CFRIGT according to the level of PG at which pulmonopathy develops.
-
The adverse effects of raised BG include stiffening of the lungs, impaired gas diffusion, and promotion of colonisation and infection.
-
The level at which harm is done is well below the threshold for the usual definition of diabetes. Harm starts at or below a PG level of 8 mmol/l.
-
The implication is that we should be screening, and intervening, at IGT stage (2-hour OGTT level of 7.8 mmol/l).
-
It may be that insulin deficiency and consequent catabolism play a part, and it is possible that early PPH (i.e. high PG level at intermediate time points, but normal by 2 hours) could be used as an indication that insulin should be considered.
The current evidence on treatment is considered in the next chapter.
Chapter 3 Treatment of hyperglycaemia in cystic fibrosis
Introduction
The usual practice in health technology assessment of treatments is to rely on high-quality evidence from randomised controlled trials (RCTs). This is also the approach used by the Cochrane Collaboration, which is why the Cochrane review by Onady et al. 136 (which is discussed below) concluded that no recommendation could be made from the current evidence base.
If there are no RCTs addressing a treatment issue then there are two options. We can follow the RCT-only route and say that there are no acceptable data or we can try to make the most of what there is, including results from lower grades of evidence such as case series, but adding caveats and highlighting uncertainties.
In some situations, where the natural history is certain, for example if a disease has consequences that are predictable and inevitable, a case series may provide sufficient evidence.
The inclusion of lower-grade evidence may be more admissible if the purpose of the technology assessment report is to identify the research needs, rather than to provide evidence to underpin national policy, as in a review for the National Institute for Health and Clinical Excellence (NICE) or the National Screening Committee (NSC). The HTA programme for the National Institute for Health Research always wants some evidence of efficacy before it will commission a trial of an intervention. Case series may be sufficient to provide justification for a trial, but not for policy (although some NICE decisions on new drugs have been based on case series – the first appraisal of imatinib for chronic myeloid leukaemia being one example137).
It is not uncommon for HTA reports to exclude studies with small numbers. We have not adopted that approach in this chapter: one study has only four patients138 and another has only three. 139 We have excluded single-case reports. The study with only four patients is one of very few that address a key question (is it worthwhile to treat PPH that has not reached the IGT level) and has hence been included. A study of that size looking at an issue for which there are other larger studies might not have been included.
In summary, the evidence base is sparse and to glean as much as we can from it we have widened the range of study designs and size beyond what is normally acceptable.
Identification of treatment studies
Our intention was to identify all of the trials and other studies of treatment of hyperglycaemia in CF, to data extract the good-quality ones, and, if appropriate, to carry out a meta-analysis. A highly sensitive search strategy was run in order to identify all aspects of patients with CF with diabetes and hyperglycaemia, including treatment, screening and diagnosis.
The databases searched were MEDLINE (1950 to May 2008), EMBASE (1980 to 2008 Week 20), Web of Science databases (1970 to May 2008), ISI Proceedings (1990 to May 2008) and Cochrane Central Register of Controlled Trials (Issue 2, 2008). Auto-alerts were run in Ovid MEDLINE and EMBASE from May 2008 to December 2010. No restrictions were placed on language and several papers were translated. Full details of the search strategies are shown in Appendix 1.
Reference lists of included studies and relevant review articles were scanned.
The internet was searched for grey literature, publications and reports, including websites of the Cystic Fibrosis Trust UK and similar organisations worldwide.
The meeting abstracts of Diabetes UK, ADA, the European Association for the Study of Diabetes (EASD), the European Cystic Fibrosis Society, the Annual North American Cystic Fibrosis Conference, and the International Society for Pediatric and Adolescent Diabetes (ISPAD) were searched up until 2010.
Research in progress was searched on ClinicalTrials.gov, Controlled-trials.com and the UK Clinical Research Network.
Full details are shown in Appendix 1, Figure 5.
We started from the position that insulin treatment is beneficial in CFRD (compared with no glucose-lowering treatment), so most interest was in the following four questions:
-
Are oral agents, such as sulfonylureas or meglitinide analogues, useful?
-
Are any treatments beneficial at lesser stages of hyperglycaemia, such as IGT or PPH?
-
How big a difference does insulin treatment make, not just to glycaemic control, but also to lung function and other morbidities that are specifically associated with CF?
-
Which form(s) of insulin is/are best?
We use the term ‘PPH’ here to refer to the lag storage state, with glucose elevated after meals, including at the intermediate time points in the OGTT (30, 60 and 90 minutes) but normal by 2 hours, hence excluding IGT. This creates two problems. First, most studies use the reduced OGTT with only fasting and 2-hour glucoses measured. Second, most of the literature on PPH refers to hyperglycaemia 2 hours after a meal.
It is believed that PPH is a risk factor for macrovascular disease, even when levels of HbA1c and FPG are normal. 87 The DECODE (Diabetes Epidemiology: Collaborative Analysis of Diagnostic Criteria in Europe) study140 found that there was a relative risk for heart disease (compared with people with normal glucose levels) of 1.5 for men and 1.6 for women with IGT, whereas there was little increase in risk for those with only IFG. However, macrovascular risk is not currently a problem in CFRD.
Unfortunately, the quantity and quality of evidence were disappointing. There are very few randomised trials, and only one RCT141 that addresses the question of whether or not treatment of CFRD IGT is beneficial (although it included only those with ‘severe IGT’). Some studies (9 out of 27) were available only as abstracts. Some of these abstracts appeared several years ago, making it unlikely that all will be followed by full publications.
A Cochrane review published in 2005136 looked at the use of insulin and other oral agents for managing CFRD and examined the evidence that these agents have a beneficial impact on lung function and weight when used on patients with CF. The authors did a thorough search of relevant databases to find studies that compared different insulin regimens with each other and with regimens of oral diabetic medications. The results and outcome measures to be used were glycaemic control, pulmonary function, nutritional status and mortality, together with the prevalence of CFRD complications and its therapeutic management. Twenty references to 14 studies were identified by searches, but none was deemed eligible for inclusion in the review, as none was a RCT. The authors concluded that no firm conclusions can be made about the optimal management method for controlling glucose metabolism in CFRD, and identified the need for a multicentre RCT examining both the efficacy of insulin or oral agents and their possible adverse effects in managing CFRD. An update in 2009 found little change. 136
A survey was conducted recently by Mohan et al. 81 looking at the management of CFRD in the UK. A questionnaire survey regarding screening, diagnosis, treatment and monitoring of CFRD was sent to all 45 recognised UK CF centres (19 adults, 22 paediatric and 4 joint, with > 50 patients), asking about clinical practice and the extent to which this adhered to the recommendations published by the UK CF Trust Diabetes Working Group in 2004. Completed questionnaires were returned by 37 centres (82%). The overall prevalence of CFRD at these centres was 18%; 6% in paediatric (126 of 2083 patients), 28% in adult (659 of 2340), and 18% in joint centres (174 of 955), respectively, which suggests that they were representative of the UK estimated 10–15% prevalence of CFRD in all people with CF.
Insulin was the preferred treatment of choice in all but one centre. Oral glucose-lowering drugs were little used. Twenty-one centres (57%) reported that they would never use them and the remainder considered them only in the early stage of disease, when patients could not cope with insulin treatment or when glucose intolerance was induced by treatment with steroids. Oral glucose-lowering drugs were even less popular in paediatric centres than in adult centres [used in 4/17 (23.5%) vs 9/16 (56%); p < 0.05 – as reported by authors, but our calculations give Fisher’s exact test p = 0.08]. Twenty-six (70%) centres would consider short-term insulin when faced with hyperglycaemia (≥ 11.1 mmol/l) in patients admitted for pulmonary exacerbation and arrange outpatient investigation during clinical stability. No centres imposed any significant dietary restrictions, but 18 (49%) advised against sugary drinks.
Studies of treatment of cystic fibrosis-related diabetes
The studies that follow are in chronological order of publication. Appendix 2 tabulates the key features of the 27 studies discussed below.
Culler 1994
Culler et al. 142 looked at the use of glipizide in patients with CF with IGT. Treatment was not randomised and numbers were few – six patients aged from 12 to 25 years, with elevated BG level 2 hours after oral administration of 1.75 g/kg of glucose and normal fasting BG. It was a case series with no control group and it is not clear how these patients were selected for treatment. The size of the clinic population was not given, so the proportion, and hence representativeness, cannot be assessed.
Results were measured before treatment, and again at 3 and 6 months (for height, weight and BMI) after treatment. Outcome measures used were HbA1c level, 24-hour urine glucose level, insulin sensitivity, first-phase insulin response (FPIR), and changes in growth assessed as height, weight and BMI. Results showed significant improvements in HbA1c level, 24-hour urine glucose level and FPIR, but not in insulin sensitivity or weight gain. Three months after glipizide administration, the mean FPIR was raised by 60% (from 287 to 459 pmol/l; p < 0.05), although it was just below the lowest range of normal FPIR (466 pmol/l); glycosuria and HbA1c both decreased significantly in all patients from 57.5 g/day to 23.2 g/day (p < 0.01) and 6.3% to 5.8% (p < 0.05) over the same period, respectively. The insulin sensitivity values in these subjects were within normal range before treatment and an increase in four of the six patients was observed, although it was not statistically significant in the group as a whole. No changes were found in either weight or BMIs at 3 and 6 months after treatment. Apart from occasional mild symptomatic hypoglycaemia, no other adverse effects were described. The authors suggested that ‘glipizide can be used in the treatment of patients with CF with IGT, especially if a patient has elevated postprandial glucose levels but normal fasting BG levels; and if persistent hyperglycaemia or significant elevation of HbA1c occurs, then insulin therapy should be instituted’.
The small numbers and short duration, and the lack of a control group, reduce the value of the study. It shows that glipizide can be effective in the short term, but, ideally, we would have a RCT against other agents, such as a meglitinide analogue or a short-acting insulin.
Lanng 1994
Lanng et al. 134 studied the effect of insulin therapy in patients with CF. Treatment was not randomised and numbers were few: 18 patients aged from 3 to 28 years, from a total clinic population of 240 patients with CF, of whom 41 patients with CFRD had received insulin therapy for at least 2 years. Under half (18 of these with at least 2 years of follow-up on insulin) took part in the study. They had a comparison group of 18 non-diabetic patients with CF who were matched with age, sex and presence of chronic lung infection at the time of diagnosis of diabetes in the diabetic patients.
Data on body weight, BMI, FEV1, FVC, microscopy, and culture of sputum and precipitins against different bacteria were collected 6 years before and 2 years after the onset of insulin therapy. For data on lung function, only those from patients > 6 years of age were included.
Results before and after insulin were similar to other studies: a decline in BMI, FEV1 and FVC in the months leading up to the start of insulin therapy (e.g. BMI: patients with CFRD 16.9 ± 0.7 kg/m2 vs control subjects 19.2 ± 0.6 kg/m2). At the time at onset of insulin therapy, the patients with CFRD differed significantly to non-diabetic control subjects in BMI, FEV1 and FVC, but not body weight. After 2 years on insulin therapy the diabetic and non-diabetic groups had similar body weight and BMI. Also the per cent differences in FEV1 and FVC between the two groups were similar to those found 6 years before insulin therapy.
The study also collected data on lung infections and carriage of organisms. Chronic P. aeruginosa lung infection was present in (diabetic patients vs control subjects) 14 patients compared with 13 at entry, 15 compared with 15 at onset of treatment, and 15 compared with 17 at the end of the study. Precipitins against P. aeruginosa increased in both groups, with no difference between levels at any time during the study. The number of weeks of intravenous anti-Pseudomonas treatment did not differ between the groups before and after insulin treatment. This seems disappointing.
However, the per cent of sputum examinations positive for H. influenzae and Streptococcus pneumoniae decreased significantly (from 11.6% to 7.1% and from 2.4% to 0.3%, respectively) after insulin therapy; these were unchanged in the control subjects; parameters of lung infections with P. aeruginosa and S. aureus remained unchanged.
The authors conclude that insulin improves lung function after the insidious decline resulting from the pre-diabetic condition in patients with CF and recommended its commencement when diagnosis of CFRD is made.
Bertele-Harms 1996 (abstract only)
Bertele-Harms and Harms143 studied the effect of glibenclamide in patients with CFRD. Treatment was not randomised and numbers were small. Twenty patients were selected from an original 26 patients with CFRD, aged from 12.8 to 26.5 years when CFRD became manifest, with fasting glucose level > 140 mg/dl, marked glycosuria, dehydration and elevated HbA1c level. The size of the clinic population was not given, so the proportion, and hence representativeness, cannot be assessed. There was no control group and patients were selected based on the availability of data on HbA1c.
Results were obtained before and after treatment over a period of 15 years. The initial mean HbA1c value at onset of CFRD was 5.34% (3.6–7.8%) (normal range 4.2–6.3%). All patients improved on glibenclamide; for instance, glycosuria disappeared in 85% after 6–8 weeks of treatment and the HbA1c value returned to normal range in 65% of patients, although it remained elevated (6.4–7.5%) in 20% patients. The other 15% (three) patients, who had the most elevated initial HbA1c values, were switched to insulin after a mean of 8 months owing to insufficient response with the sulfonylurea. Further increases in sulfonylurea doses were ineffective. The mean duration of glibenclamide effectiveness was 2.4 years (range 0.6–5.5 years), but patients considered afterwards that delaying insulin treatment had been worthwhile.
Kentrup 1999
Kentrup et al. 144 studied the efficacy and safety of acarbose in patients with CF with IGT, in a double-blind, randomised crossover trial. There were 12 patients, all inpatients for treatment of Pseudomonas infection, aged from 8 to 22 years. Patients were selected based on their BG response being abnormal after a standard test meal.
The trial lasted only 14 days. Patients were randomised to either acarbose or placebo for 5 days (day 4–8), then had a day of washout (day 9) before receiving the other drug for another 5 days (days 10–14). On day 2 (before the start of study medication) and on the last day of both study periods (days 8 and 14), a standardised nutritional load (a carbohydrate content of 1.75 g/kg body weight) was given, and blood samples were taken before and at 30, 60, 90, 120 and 180 minutes after the test load. A baseline measurement was also taken after a 10-hour overnight fast in resting patients. There were significant reductions in PG, insulin and C-peptide with acarbose treatment compared with baseline values. This was also true with acarbose treatment compared with placebo in mean BG level (6.12 ± 0.82 mmol/l vs 7.54 ± 1.42 mmol/l; p < 0.05) and mean peak BG level (8.01 ± 1.79 mmol/l vs 11.56 ± 2.65 mmol/l; p < 0.01).
Gastrointestinal disturbances, such as diarrhoea, flatulence, loss of appetite, nausea and abdominal cramps, were recorded in 67% of patients during therapy with acarbose. The authors concluded that acarbose has a beneficial therapeutic effect on glucose tolerance in patients with CF, but its side effects are likely to prevent patients from accepting it as a long-term therapy.
Moran 2001
Moran et al. 145 studied the use of preprandial insulin and repaglinide in patients with CFRD without FH. There were seven patients aged 24 ± 5 years. The size of the clinic population was not clearly given, so the proportion, and hence representativeness, cannot be assessed. Seven healthy, non-athletic normal control patients, matched for age, sex and BMI, were recruited by poster advertisement, acting as a reference to the outcome measures. Patients were studied on three separate occasions over a 1- to 2-month period, receiving a test meal in the morning after a night fasting with treatment in a random order: (1) no preprandial diabetes medication (baseline meal); (2) insulin lispro, 10 minutes preprandial; and (3) repaglinide, 10 minutes preprandial. Control subjects received the test meal under the same conditions on a single occasion with no preprandial medication.
Plasma glucose and insulin levels were recorded at the beginning of the meal and after the meal at 20-minute intervals for 5 hours.
After the test meal without medication, postprandial glucose excursion was elevated, with a peak glucose level of 228 ± 30 mg/dl (12.6 mmol/l) at 74 ± 7 minutes after the beginning of meal. The peak insulin levels (53 ± 11 µU/ml) were delayed and blunted at 117 ± 11 minutes post meal.
After the meal with preprandial lispro, there was a significant decrease in the peak glucose level (172 ± 9 mg/dl; p = 0.0004), the 2-hour glucose area under the curve (AUC) (p = 0.001) and the 5-hour glucose AUC (p < 0.0001) compared with the untreated baseline meal. However, glucose excursion was not completely controlled.
After the meal with preprandial repaglinide, the 5-hour glucose AUC was significantly less than baseline (p = 0.03), but there were no differences seen in the 2-hour glucose AUC (p = 0.39) or the peak glucose level (p = 0.17).
Comparing insulin lispro with repaglinide in CFRD, insulin lispro seemed to be better than repaglinide on postprandial glucose excursion, with significant differences observed between the two drugs in the peak glucose level (172 ± 9 vs 208 ± 18 mg/dl; p = 0.02), the 2-hour glucose AUC (p = 0.02) and the 5-hour glucose AUC (p = 0.01). Curiously, neither drug, at the doses used in the study, significantly changed the peak insulin level or the 2-hour insulin AUC compared with baseline. Four episodes of hypoglycaemia (glucose level 48–54 mg/dl, 2.7–3.0 mmol/l) occurred in patients with CF during the study: one after the test meal without medication, two after administration of insulin lispro and one after administration of repaglinide. Hence, both lispro and repaglinide reduced PPH, but insulin was more effective. The authors commented that although based on standard practice recommendations, the doses of insulin and repaglinide seemed to be too low (as instanced by the non-significant difference in peak insulin levels); therefore, higher doses of these drugs may have had greater therapeutic effect.
Nousia-Arvanitakis 2001
Nouisa-Arvanitakis et al. 54 studied the effect of biphasic (rapid and intermediate) insulin on nutrition, lung function and clinical status in a small case series of six patients, aged 15–22 years, who developed CFRD in a 5-year follow-up of 30 patients with CF, and were thought to require insulin treatment. A control group of non-diabetic patients with CF, matched with the diabetic group for age, sex, pubertal stage, BMI, FEV1 and SS at the onset of the study, was selected for the comparison of FPIR, BMI, FEV1 and SS (maximum score of 100) among non-diabetic patients and patients with CF.
The outcome measures used were BMI, FEV1, SS, intravenous glucose tolerance test and FPIR, at time of diagnosis of CFRD and 6 months after starting insulin. There was significant improvement in BMI (16.36 ± 1.34 kg/m2 vs 19.07 ± 1.06 kg/m2; p = 0.0018), FEV1 (50.66 ± 6.68 l vs 70.83 ± 5.40 l; p = 0.0062) and SS (66.00 ± 3.84 vs 84.50 ± 4.41; p = 0.0006) in all six patients following insulin treatment. A significant difference was found in FPIR (p < 0.0001), BMI (p = 0.0003), FEV1 (p = 0.0071) and SS (p = 0.0009) when comparing the six patients with CFRD to the control subjects at the time of diagnosis of diabetes mellitus.
A positive correlation between FPIR and BMI was detected in the 30 patients with CF (Pearson’s correlation coefficient r = 0.759). The authors considered that there was an association between insulin hyposecretion and an overall deterioration in the clinical status of the patients with CF involving nutrition, lung function and clinical scores, which were improved significantly after the institution of insulin. They believed it is important to identify patients with CF who are at risk of developing diabetes, so that early insulin therapy can be given.
Rolon 2001
Rolon et al. 146 assessed the impact of hyperglycaemia preceding diabetes (pre-diabetes) on nutritional status and respiratory function in patients with CF, and to describe the clinical characteristics of CFRD at the start of insulin treatment, insulin regimens and effects of insulin therapy. Of a total of 220 patients receiving follow-up at their clinic, 21 (aged 10–21 years) had insulin-treated diabetes mellitus, with no lung transplantation or immunosuppressive therapy. Of these patients, 14 were selected based on the completeness of clinical data. There were 14 non-diabetic patients matched for age, sex and chronic lung infection by P. aeruginosa. They had normal fasting glucose and normal OGTT.
Results were reported 5 years before and after insulin treatment. Outcome measures used were BMI, BMI z-score, FVC, FEV1, insulin regimen, mean insulin dosage, hypoglycaemic events and mean HbA1c value. However, these data were available for 12 patients during the first year, eight during the second year and seven during the third, fourth and fifth years, as seven died during the study period. Hence, only seven patients had 5 years of follow-up at the time of study. Insulin treatment was started either on the basis of symptoms of hyperglycaemia (n = 7) or on the basis of the presence of diabetes mellitus diagnosed by a systematic screening and a nutritional status (n = 7). Results showed no differences in BMI z-score between the CFRD patients and the non-diabetic control subjects during the 4 years prior to insulin treatment but it was significantly lower in the cases (–1.66 ± 1.5 vs –0.3 ± 0.95; p = 0.03) 6 months prior to the treatment; so were BMI (15.9 ± 1.8 kg/m2 vs 17.3 ± 1.3 kg/m2; p = 0.04) and lung function (FVC 52% ± 20% vs 79% ± 20%; p = 0.01; FEV1 37% ± 19% vs 72% ± 23%; p = 0.01).
After insulin treatment was started, respiratory function improved and the BMI returned to normal (compared with the French population) within 2 years. A decreased rate of FVC decline was seen in five of the seven patients 5 years post insulin (p = 0.1) and FEV1 improved in all seven patients after the start of treatment (p = 0.02). The mean insulin dose increased from 0.62 units/kg/day during the first year to 1.25 units/kg/day during the fifth year.
Mean HbA1c value was 8.8% at the start of treatment, fell to 6.6% during the first year of insulin treatment, but rose to 7.8% during the fifth year. Two episodes of severe hypoglycaemia (symptomatic and BG level of < 50 mg/dl) were reported over the total follow-up of 42 patient-years (4.8 episodes per 100 patient-years). Mild hypoglycaemia (BG level of < 60 mg/dl) occurred with a frequency of 10.3 episodes per patient-year. It was concluded that the clinical status of pre-diabetic patients with CF deteriorates before the start of insulin therapy, and that insulin treatment improves anabolism and provides good glycaemic control with few hypoglycaemic events in patients with CFRD with or without FH.
Rosenecker 2001
Rosenecker et al. 120 compared the effects of insulin with glibenclamide on patients with CFRD. Patients were not randomly allocated to either treatment, so, in effect, this study present data from two case series. There were 45 patients, with 34 on insulin (mean age of 24.0 ± 4.7 years) and 11 on glibenclamide (mean age of 27.7 ± 5.4 years). Five centres participated in this study but the size of the centre populations was not given, so the proportion, and hence representativeness, cannot be assessed. There was no control group. Patients who had been seen regularly in one of the centres and had received a prior CFRD diagnosis of at least 1 year were included.
Results were obtained by questionnaire surveys. Outcome measures used were FEV1, FVC, weight for height and SS. Of the 34 insulin-treated patients, 13 had been treated initially with glibenclamide, which failed after a mean time interval of 18.2 ± 14.5 months. The diagnosis of CFRD was earlier in the insulin-treated group than in the sulfonylurea group (16.4 ± 3.6 years vs 24.2 ± 4.8 years; p < 0.001) and the durations of treatment with insulin and glibenclamide were 7.6 ± 4.6 years and 3.5 ± 2.0 years, respectively. At the start of the study, the mean HbA1c levels and mean BG values in the insulin-treated group were 8.3 ± 2.8% and 11.8 ± 8.0 mmol/l, respectively; in the glibenclamide group the levels were 7.0 ± 1.1% and 7.9 ± 4.3 mmol/l, respectively. At the end of the study, no significant differences were found between the two groups in the most recent FEV1, FVC, SS or BMI measurements. There were no severe hypoglycaemic events in patients treated with insulin or glibenclamide. The authors concluded that ‘CFRD can be treated orally with glibenclamide in some patients with CF, at least in a subgroup with a late onset of diabetes. FEV1, FVC, SS and BMI were maintained equally well by both treatments’. However, there are problems with comparisons between treatments from a non-randomised study.
Dobson 2002
Dobson et al. 138 studied the effect of insulin on lung function and weight in four patients aged 15–23 years with long-standing CF, who had weight loss and deteriorating lung function without a clear cause, and had high random glucose values but normal OGTT. The paper mentions that some of these high results were postprandial, implying that they would fall into our non-IGT PPH group.
Weight and spirometry (FEV1/FVC) were recorded before and 3 months after insulin treatment. Insulin treatment was accompanied by increases in both weight and spirometry in all four patients.
The authors concluded that insulin can result in a significant clinical improvement in patients with CF with normal OGTT results or HbA1c value, although they all had multiple random blood glucose (RBG) levels above 11.1 mmol/l. They also commented that the benefit seen in these patients was unlikely to result from improved glucose concentrations, as HbA1c values were normal before insulin treatment and not altered significantly by it. However, the improvement might have been only in PPH. They proposed that in patients with CF clinically significant insulin deficiency may precede the development of diabetes as defined by OGTT.
This study is very small, but its value may be in the implication that treatment is worthwhile even at the isolated PPH stage. The four patients gained weight, with increases ranging from 0.7–5.7 kg, on 6–12 units of insulin daily.
Ballmann 2003 (abstract only)
Ballmann et al. 147 studied the use of glibenclamide in patients with CFRD. Treatment was not randomised and numbers were small: 19 patients aged [mean ± standard deviation (SD)] 13.7 ± 3.7 years (out of a total of 41 patients with CFRD) were initially treated with glibenclamide and had completed follow-up for 2 years. Six patients changed to insulin (group 1) after 14–24 months owing to hyperglycaemia in all, systemic steroids in one and nocturnal percutaneous endoscopic gastrostomy feeding in three patients; the rest remained on glibenclamide after 2 years (group 2). The mean time until starting insulin treatment was 4.5 years in those treated initially with glibenclamide. There was no control group and it is not clear how these patients were selected for treatment.
Results at the start of glibenclamide treatment were given for both groups. Final results for group 1 were when they changed to insulin, and for group 2 were after 2 years of glibenclamide treatment. Outcome measures used were nutritional status (BMI z-score), lung function [per cent predicted forced expiratory volume in 1 second (%FEV1)] and metabolic control (HbA1c), as shown in Table 5. The authors commented that ‘more than 68% of those on glibenclamide were in a stable clinical condition (BMI z-score and %FEV1) and good metabolic control after 2 years’.
| Group 1 initially | Group 1 at starting insulin | Group 2 initially | Group 2 after 2 years | |
|---|---|---|---|---|
| HbA1c% | 5.7 ± 0.5 | 8.2 ± 0.4 | 5.2 ± 0.6 | 5.5 ± 0.8 |
| %FEV1 | 68 ± 22 | 58 ± 23 | 76 ± 26 | 73 ± 29 |
| BMI z-score | –1.7 ± 1.2 | –2.1 ± 1.5 | –0.8 ± 1.0 | –0.9 ± 1.0 |
So those who remained on glibenclamide appeared to do well. The authors did emphasise the need for controlled randomised prospective studies comparing insulin with oral antidiabetic treatment of CFRD.
Boyle 2004 (abstract only)
Boyle et al. 148 studied the effects of early insulin treatment in 30 patients with CF, who were drawn from a clinic with 155 patients. The average age of the included patients was 26.9 years: 13 had CFRD, 10 had IGT and 7 had NGT. Treatment was not randomised. There was no control group and patients were selected because they were insulin treated.
Outcome measures used were weight gain and FEV1 changes, before and after a year of insulin treatment. Weight (% change year before, then year after) seemed to improve in all patients: pre-insulin –3.14% versus 0.59% post insulin (CFRD), 1.1% versus 1.56% (IGT) and –2.1% versus 0.45% (NGT). The results for FEV1 were: –0.28% versus –1.47% (CFRD), –7.08% versus +1.46% (IGT) and –2.68% versus +1.47% (NGT). However, statistical significance of the results is unclear, as p-values were not given. The authors concluded that insulin treatment prior to the development of diabetes appears to have positive effect on lung function and body weight.
Franzese 2005
This study149 was reported only in a letter. Franzese et al. 149 studied the use of glargine in patients with CFRD. Treatment was not randomised and numbers were small: eight patients aged from 10 to 29 years, four with chronic CFRD who were treated with rapid insulin in the previous 1–3 years (group A) and another four patients with intermittent CFRD requiring insulin only during infections (group B). The size of the clinic population was not given, so the proportion, and hence representativeness, cannot be assessed. There was a control group (non-glargine treated) comprising six patients (aged 14–18 years) with intermittent CFRD. It is not clear how these patients were selected.
Results were before and 6 months after glargine treatment (plus preprandial rapid insulin in group A). The outcome measures used were BMI, FEV1, HbA1c and the number of lung infections. There was a significant decrease in the number of lung infections in both group A and group B, from 3.75 ± 0.5 to 1.75 ± 0.9 (p < 0.01) and from 2.75 ± 0.5 to 1.25 ± 0.5 (p < 0.001), respectively; no change was seen in the control group (3.3 ± 1.2 vs 3.1 ± 0.4). There were no positive changes in HbA1c value or BMI, and no hypoglycaemic events were recorded. This was attributed to the short period of observation. The conclusion was that basal insulin may play a role in reducing the number of lung infections in both overt CFRD and pre-patients with CFRD.
Minicucci 2005 (abstract only)
Minicucci et al. 150 looked at the efficacy and safety of insulin glargine in a case series of 12 CFRD and three CF IGT patients aged from 14 to 34 years: six had CFRD treated with insulin (group A); six appeared to have CFRD but without FH, diagnosed on the basis of OGTT (group B), and three had CFRIGT (group C). Group A had been on insulin regular or rapid analogue before meals. It is not clear whether they were also on neutral protamine Hagedorn (NPH) but the abstract says that glargine took the place of intermediate insulin, which implies that the short-acting insulin was continued. Neither group B nor group C had ever been treated with insulin. The size of the clinic population was not given, so the proportion, and hence representativeness, cannot be assessed. There was no control group and it is not clear how these patients were selected for treatment.
Results were collected at the start of, and 3 months after, glargine treatment. Outcome measures used were HbA1c value, BMI, frequency of hypoglycaemia, and compliance with the therapy. The results showed no significant difference in HbA1c value in any group (group A: 9.6% vs 9.2% – no difference, indicating that glargine had similar effects to NPH; group B: 7% vs 7.47%; group C: 6.76% vs 6.74%). The failure to reduce HbA1c value in groups B and C is odd; the authors suggest this could be due to small numbers and short follow-up. BMI changed little (group A: 21 vs 21.5 kg/m2; group B: 16.68 vs 17.2 kg/m2; group C: 18.17 vs 18.55 kg/m2) in all groups. The frequency of hypoglycaemia did not change in group A. No hypoglycaemia events were observed in groups B and C. The authors state that glargine seemed to be safe and well accepted. A larger multicentre study in Italy is under way, but there is no mention of a RCT.
Bizzarri 2006
Bizzarri et al. 151 studied the effects of insulin glargine in patients with CFRIGT. Treatment was not randomised and numbers were small: six patients aged from 9.2 to 27.8 years, who were identified with normal fasting glucose and IGT (FPG < 110 mg/dl and 2-hour PG 140–199 mg/dl) out of a total of 113 patients with CF. There was no control group.
Results were before and after glargine treatment over median follow-up of 1.4 years (range 1.0–1.8 years). Outcome measures used were HbA1c value, BMI z-score, FEV1 and number of hospitalisations for clinical exacerbation. There were significant improvements in both median BMI z-scores (– 0.95 vs – 0.5; p = 0.026) and median FEV1 (72.7% vs 76.7%; p = 0.027). No significant difference was observed in the median HbA1c value (5.9% vs 6.1%; p = 0.496) or the median number of hospitalisations for clinical exacerbation (1.95 patients/year vs 2.0 patients/year; p = 0.715). Hypoglycaemia was not a problem. The authors concluded that ‘early insulin glargine is well tolerated and safe, and that it seemed to slow down the deterioration of the clinical status (particularly nutritional condition and lung function) seen in the years before treatment in some patients’.
Drummond 2006 (abstract only)
Another study152 from the Glasgow group, as in the Boyle abstract, which may include some of the same patients, gives data for 5 years before and after insulin treatment.
Drummond et al. 152 studied the effect of insulin treatment in patients with CF. Treatment was not randomised. The study included 54 patients aged from 16 to 52 years (mean age 27.6 years), not all of whom had CFRD: some had IGT and some had NGT, but numbers were not given. The size of the clinic population was not given, so the proportion, and hence representativeness, cannot be assessed. There was no control group and it is not clear how these patients were selected for treatment.
The outcome measures used were lung function and weight gain, 5 years before and 5 years after insulin initiation. FEV1 declined from 2.6 ± 0.14 l to 1.78 ± 0.12 l (p < 0.001) 5 years prior to insulin treatment and the mean 5-year post-insulin FEV1 was 1.74 ± 0.20 l (p = 0.15). So decline seemed to be arrested after insulin initiation. But when stratified according to the OGTT at initiation, the rate of FEV1 decline in patients with IGT changed significantly from 0.51 ± 0.31 l pre-insulin to 0.04 ± 0.12 l post insulin (p = 0.02); changes were not significant in those with NGT (p = 0.86) or with CFRD (p = 0.70). Numbers of patients are not given. Weight increased significantly with insulin therapy from 53.08 ± 1.53 kg to 56.22 ± 2.08 kg (p = 0.05). The authors concluded that insulin therapy reduced the decline in lung function in patients with CF and recommended its commencement at the IGT stage.
Hardy 2006 (abstract only)
Hardy et al. 153 reported the effect of insulin treatment on growth and lung function in children with CF with abnormal OGTT but normal fasting glucose. Treatment was not randomised and numbers were small: 27 children (age not given), with 14 on insulin glargine (group A) owing to clinical deterioration and 13 not given insulin (group B). The size of the clinic population was not given, so the proportion, and hence representativeness, cannot be assessed. There was a control group of 55 patients with CF with normal OGTT. It is not clear how these patients were selected.
Height, weight, BMI and best %FEV1 were measured 12 months before and after either treatment with glargine (group A) or without (group B). Group A had higher 2-hour PG levels (11.9 vs 9.5 mmol/l; p = 0.01), lower BMI and a significant decline in weight in the preceding 12 months (p = 0.02) compared with group B, so the two groups were not matched. Compared with the control subjects, groups A and B had lower height (p = 0.03), FEV1 (p < 0.001) and FEV1 12 months before treatment (group A p = 0.03, group B p = 0.01). FEV1 declined significantly (> 5%) before treatment in eight patients from group A but improved in six of these eight after insulin treatment. FEV1 also declined in seven patients from group B, but improved in five of these seven patients without insulin treatment. It was concluded that glargine arrested the progressive decline in lung function in patients with more severe undernutrition and hyperglycaemia, but it also improved in patients who were not given insulin (group B), suggesting that spontaneous improvement also occurs.
McGinnity 2006 (abstract only)
McGinnity et al. 154 examined the effect of once-daily long-acting insulin (detemir or glargine) in a case series of five patients with CFRD aged from 11 to 18 years. The size of the clinic population was not given, so the proportion, and hence representativeness, cannot be assessed. It is not clear how patients were selected. All patients had received treatment with once-daily long-acting human insulin analogues for more than 12 months prior to the study, so were presumed to have reached a stable state after titration against blood glucose monitoring. Insulin had been started because of CFRD, PPH, weight loss or declining lung function; the implication is that it was started earlier rather than later.
Blood glucose was measured over a 3-day period using a subcutaneous continuous glucose monitor. BG levels in the five patients were within normal limits 65%, 93%, 94%, 96% and 99% of the time. Mean glucose levels (range) were 7.6 mmol/l (2.2–17.2 mmol/l), 6.6 mmol/l (3.8–13.7 mmol/l), 5.3 mmol/l (2.2–9.0 mmol/l), 5.9 mmol/l (3.4–12.9 mmol/l), 6.4 mmol/l (4.1–11.8 mmol/l). Hyperglycaemia seems to have been commonest round midday. Symptomatic hypoglycaemia did not occur, which seems odd, given the low end of two of the ranges. The authors conclude that these preliminary data indicate that good control is achievable with the early use of long-acting insulins for CFRD. CBGM was well tolerated. HbA1c value was not reported.
Onady 2006
Onady et al. 155 compared the effects of insulin, sulfonylurea, metformin and thiazolidinedione in patients with CFRD. 155 However, treatment was not randomised and numbers were small: 20 patients aged from 13 to 49 years, with eight initially chosen to be on insulin, five on sulfonylureas, four on metformin and three on thiazolidinediones. (There are uncertainties over the numbers in the study. Twenty-four patients were originally diagnosed with CFRD during the 10-year span but four were excluded: three after lung transplantation and one on combination diabetic therapy during the study period. Hence, a total of 20 patients with CFRD over the 10-year period remained for the prospective study. However, the tables of results show a total of 25 patients in both the baseline and final results.)
The size of the clinic population was not given, so the proportion included, and hence representativeness, cannot be assessed. Patients chose their treatment based on risk and benefit information provided for treatment options. Baseline variables varied among groups, for example with HbA1c value ranging from 7.2% on sulfonylurea to 9.5% on insulin.
Follow-up was for 10 years. There were no statistically significant differences in overall glycaemic control, changes in weight, liver function testing and FEV1 between oral agents and insulin. All patients tolerated the initial therapy and none had to change treatment because of side effects. Four patients with inadequate HbA1c control, discontinued insulin and switched to oral agents. No adverse effects from oral agents during the study were reported. Mortality was highest among patients in the sulfonylurea group (60%), followed by the insulin group (37%), with no deaths from the biguanide and thiazolidinedione treatment groups, but these differences were not statistically significant (p = 0.062). Sixty patients with CF had been followed in the centre during the 10-year period, with a mortality rate of 23% observed in those without diabetes and 38% in those with diabetes. One patient who was on a thiazolidinedione had been identified with diabetic nephropathy 18 months after the diagnosis of CFRD (a surprisingly short duration). There were no reports of abnormal urine microalbumin measures or retinal examinations indicating microvascular disease in these patients. The authors concluded that OHAs were effective and safe in treating selected patients with CFRD, and may provide an alternative for patients reluctant to use insulin. However, the groups showed baseline differences, treatment was not allocated randomly and no firm conclusions can be reached.
Drummond 2007 (abstract only)
This is another abstract from the Glasgow group,156 with data on the incidence, awareness and apparent symptoms of hypoglycaemia experienced by patients with CF who were receiving insulin. Drummond et al. 156 retrospectively estimated the frequency of hypoglycaemia and the associated symptoms experienced in insulin-treated patients. Treatment was not randomised and numbers were small: 24 patients with a mean age of 30.7 ± 9.1 years. Patients had been on insulin treatment for 7.3 years ± 6.4 years and HbA1c value was 6.56 ± 1.13%.
The frequency of mild hypoglycaemia over a period of 6 months was recorded, along with details of usual symptoms and awareness of hypoglycaemia. Hypoglycaemic events were reported in 13 patients who experienced between one and four episodes: six (25%) had five or more and five patients experienced no hypoglycaemic events (21%). Sweating, hunger, warmness, confusion and trembling were the most common symptoms. Seventy-five of the insulin-treated patients had hypoglycaemic unawareness. So, hypoglycaemia episodes and hypoglycaemic unawareness were common among patients with CF. The frequency may relate in part to the loss of glucagon-producing α-cells.
Sulli 2007
Sulli et al. 139 described three case reports of insulin pump therapy in patients with CFRD over 2 years of CSII treatment. The patients were two males (aged 5.5 and 21 years) and a female (aged 28 years). All patients were receiving multiple daily injections [(MDIs): four insulin injections per day] in the year prior to CSII use.
During the CSII treatment, all patients experienced a reduction in their annual mean level of HbA1c. This reduction was more or less steady over the 2 years. At the end of the 2-year period, there were significant reductions (1.7%, 2.7% and 1.2%, respectively) in HbA1c levels compared with baseline values.
Also, during the CSII treatment, the annual mean level of BMI increased and the insulin requirements decreased. None of the patients experienced episodes of diabetic ketoacidosis (DKA) or hypoglycaemia during the CSII treatment. Only two episodes of lipohypertrophy and a slight local cutaneous inflammation were reported.
Grover 2008
Grover et al. 157 looked at the effect of glargine versus NPH in patients with CFRD with FH. They carried out a randomised, non-blinded, crossover study but the numbers were small: 19 patients aged 34 ± 8 years. All were clinically well and receiving a single dose of bedtime NPH insulin plus rapid-acting insulin before meals. The size of the clinic population was not given so the proportion, and hence representativeness, cannot be assessed. Twenty patients with CFRD with FH were recruited and one dropped out.
Patients received 12 weeks’ therapy with bedtime NPH (plus rapid-acting insulin) or bedtime glargine (plus rapid-acting insulin); nine patients received NPH first and the rest received glargine first. Before each study period there was a 1-month insulin adjustment period. Outcome measure used were BG control (HbA1c, FPG, 2-hour postprandial glucose) and weight change (weight, fat mass, lean mass). There was a significantly greater reduction in FPG with glargine therapy (p = 0.03) but no changes in HbA1c value and postprandial PG level. More weight gain in patients on glargine was observed, but this did not achieve statistical significance (p = 0.07). No differences in adverse events and QoL were seen between the groups. There were no serious hypoglycaemic episodes, but minor hypoglycaemic episodes occurred with both treatments (NPH: 5 ± 1 times per participant; glargine: 6 ± 1 times; p = 0.3). After the study, all 19 patients chose to continue glargine therapy as they believed that daytime BG levels seemed more consistent and some were less worried about night-time hypoglycaemia.
It was commented that glargine was a newer agent and patients’ perception could had been influenced by the health-care team. Variability in glucose levels appeared to be similar between the groups, as the within-patient SD of fasting glucose levels (NPH 50 ± 10 mg/dl, glargine 40 ± 6 mg/dl; p = 0.18) and 2-hour postprandial glucose levels (NPH 91 ± 7 mg/dl, glargine 83 ± 6 mg/dl; p = 0.28) were not significantly different.
The conclusion was that ‘long-term studies are needed to determine the metabolic and nutritional impact of glargine in CFRD, but the initial data suggested that it is a promising therapy’. The trial was sponsored by the manufacturer of glargine.
Mohan 2008
Mohan et al. 158 looked at the long-term impact of insulin therapy in 42 patients with CFRD aged from 16 to 39 years. There was a total of 215 patients in their unit, of whom 65 (30.2%) had CFRD. There was no control group in the study. Forty-two out of the 65 patients were selected based on the completeness of data required for the study, hence possible selection bias.
Results were 5 years before and 3 years after insulin therapy. Outcome measures included FEV1, FVC, BMI and the number of pulmonary exacerbations requiring hospital admissions. At 3 months following institution of insulin therapy, there was significant improvement compared with baseline in mean FEV1 (51.6% vs 58.2%; p < 0.0001), mean FVC (66.4% vs 75.5%; p < 0.0001) and mean BMI (19.5 vs 20.5 kg/m2; p < 0.0001). This improvement over baseline was maintained at 1 year for FEV1 (mean 55.1%; p < 0.002), 2 years for FVC (mean 72.1%; p < 0.01) and at 3 years for BMI (mean 20.43 kg/m2; p < 0.002). However, the mean rate of FEV1 decline from 3 months to 3 years after treatment was comparable with that of the pre-treatment period (–3.2% vs –3.1% per year; p = 0.77); and the mean post-insulin FEV1 value returned to baseline at 34 months. The annual rate of FVC decline was also similar to the pre-insulin values during the same period (–2.6% vs –2.5% per year; p = 0.96). There was no difference in the number of hospital admissions for pulmonary exacerbations before and after insulin treatment (1.8 vs 2.1 per year; p = 0.19). HbA1c values were available in 32 patients, but no significant change was found at the end of the follow-up period (mean 6.8% vs 6.7%). Seventeen of the 32 had elevated values (mean 8.1%) at diagnosis that improved significantly during the 3 years following insulin treatment (mean HbA1c value: first year, 6.9%, p = 0.004; second year, 7.1%, p = 0.04; third year, 7.0%, p = 0.02).
The conclusion was that insulin treatment is associated with temporary improvement in lung function and BMI in symptomatic patients with CFRD, with FEV1 decline delayed by an average of 34 months.
Hardin 2009
A before-and-after study by Hardin et al. 159 evaluated the safety, efficacy and metabolic benefits of CSII via an insulin pump in nine patients with CFRD over 6 months.
To be eligible for inclusion, patients had to be between 18 and 32 years old and to be treated with a minimum of three subcutaneous injections per day, based on a basal bolus regimen, for a minimum of 6 months, and be recording blood sugar readings at least four times daily. Patients were converted to CSII therapy in a single visit, and asked to report results of self-BG monitoring (measured before all major meals and bed) and a minimum of four postprandial BG levels a week. Baseline measurements were taken of each patient’s HbA1c level, body weight, lean body mass, and whole-body protein turnover (using a stable isotope of leucine).
The mean age of the nine patients (five males and four females) was 27 years. After 6 months of CSII therapy, body weight increased significantly from 55.6 kg (SD 3.5 kg) at baseline to 59.2 kg (SD 3.3 kg) (p = 0.01). HbA1c level decreased from 8.2% (SD 1.9%) to 7.1% (SD 1.5%) (p = 0.05). In addition, there were significant improvements in fasting and postprandial BG levels and lean body mass. Protein catabolism was significantly decreased. No patient had an episode of hypoglycaemia, whereas prior to CSII the patients reported several hypoglycaemic episodes per month. All patients but one wanted to continue pump therapy.
Hence, in this study of patients with CFRD, the use of CSII over 6 months led to improved glycaemic control and safety compared with multiple daily subcutaneous insulin injections. In addition, metabolic benefits were shown.
Mozillo 2009
Mozillo et al. 160 reported preliminary data from a study designed to evaluate the effect of glargine treatment on lung function, BMI, lung infections and HbA1c level in patients with CF with early glucose derangements.
A total of 98 of 220 patients with CF who attended the CF unit at a Department of Pediatrics in Naples were screened for glucose abnormalities on the basis of an OGTT and/or continuous glucose monitoring system (CGMS), and 65 patients had been enrolled in this ongoing open trial. There was no control group. The data of the first 22 patients who completed 12 months of glargine were presented. Their mean age was 12.4 years. Four had abnormal glucose tolerance on a CGMS, nine had IGT, seven had diabetes mellitus without FH and two had diabetes mellitus with FH. After 12 months of glargine therapy there was an 8.8% increase in per cent predicted FEV1 (%FEV1) (p = 0.01) and a 42% decrease in the number of lung infections (p = 0.003). The BMI z-score and HbA1c level did not show any significant difference for the whole group. However, a significant (p = 0.017) improvement was found in those patients (n = 8) with the worst BMI z-scores, i.e. baseline BMI z-score of < –1.
These data suggest that glargine could benefit patients with CF with early glucose derangements. However, RCTs with more patients and longer follow-up are needed to confirm this.
Moran 2009: Cystic Fibrosis-Related Diabetes Therapy trial
The aim of the Cystic Fibrosis-Related Diabetes Therapy (CFRDT) trial141 was to determine whether or not diabetes therapy improves BMI in patients with CFRD without FH (CFRD FH–). The trial was a three-arm multicentre trial comparing preprandial insulin aspart, repaglinide and oral placebo. Patients were randomised to receive insulin aspart 0.5 units per 15 g of dietary carbohydrate, repaglinide 2.0 mg orally or oral placebo three times a day before meals. Ongoing diabetes education was also provided.
Measurements on the patients’ BMI and lung function 12 months prior to the study were retrospectively obtained from chart reviews and then measured prospectively for 12 months after randomisation. BMI was the primary study end point. Measures of DEXA (dual-energy X-ray absorptiometry), NIH prognostic score, Cystic Fibrosis Quality-of-Life questionnaire (CFQoL), 3-day dietary histories, and HbA1c level were measured at baseline and after 1 year in the study.
One hundred adult patients were enrolled: 74 CFRD patients without FH and 26 with severe IGT. ‘CFRD FH–’ was defined as FPG level of < 126 mg/dl (7.0 mmol/l) and a 2-hour glucose level of ≥ 200 mg/dl (11.1 mmol/l) and severe IGT was defined as a glucose level of ≥ 200 mg/dl (11.1 mmol/l) during the OGTT and a 2-hour glucose level of 180–199 mg/dl (10.0–11.1 mmol/l). The mean age of the patients was 27 years (SD 8 years) and mean HbA1c value was 6.0% (SD 0.7%) and the ratio of males to females was 53 : 47.
Results were presented for the 81 patients (61 CFRD FH– and 20 with IGT) who completed the trial. The absolute change in BMI during the study year did not differ significantly between the groups for the CFRD patients without FH. However, in the IGT group, the BMI change was significantly worse for the repaglinide-treated patients than in those on placebo.
The results for the change in BMI for the 12 months prior to the study compared with the change during the study year showed a significant improvement for the CFRD patients without FH on insulin. For the 12 months prior to baseline, the change in BMI was –0.30 kg/m2 (SE 0.21 kg/m2) and after 12 months on insulin the decline was reversed, and there was an increase of 0.39 kg/m2 (SD 0.21 kg/m2) (p = 0.02). The CFRD FH– repaglinide and placebo groups did not show a significant change, i.e. changes in BMI 12 months prior to the study were –0.14 kg/m2 (SD 0.21 kg/m2) and –0.29 kg/m2 (SD 0.25 kg/m2), respectively, and 12 months after the study the changes in BMI were +0.15 kg/m2 (SD 0.21 kg/m2) and –0.02 kg/m2 (SD 0.25 kg/m2).
All study arms for the CFRD patients without FH showed a decline in FVC during the study when compared with 12 months prior to study, and the insulin and repaglinide arms showed a reduction in decline in FEV1. The patients with IGT in the insulin and repaglinide arms showed no significant change in rate of BMI decline compared with the previous year, although, surprisingly, the placebo-treated patients showed a significant improvement (p = 0.02).
After 1 year on therapy, there was no significant change in HbA1c level in any group and no difference in fasting glucose levels within or between groups compared with baseline. During the study year there were no differences in the number of episodes of acute illness between treatment groups or between CFRD patients without FH and IGT patients. Also, NIH and CFQoL scores showed no differences between or within groups during the 1-year treatment period. There were no serious adverse events related to the study medication.
In conclusion, the CFRDT trial showed that preprandial rapid-acting insulin given for 1 year significantly reversed the chronic weight loss in CFRD patients without FH, without any adverse effects. However, it had no significant effect on lung function or acute illnesses.
Hameed 2011
This before-and-after study161 looked at the effect of a single daily dose of insulin detemir on weight change and lung function in six patients with CFRD and 12 with ‘early insulin deficiency’, defined by peak BG level during OGTT but with a 2-hour level of < 11.1 mmol/l. The median age of the patients was 12.5 years, and all but one had exocrine pancreatic insufficiency. Changes in mean weight SD score (WtSDS), mean change in per cent predicted FVC (%FVC) and %FEV1 were measured.
The values at 1 year before treatment versus those after a median of 42 weeks of insulin treatment showed improvements of 0.22 in WtSDS (p = 0.003), 5.3% in %FEV1 (p = 0.004) and 5.8% in FVC (p = 0.024). No episodes of severe hypoglycaemia were reported.
Minicucci 2011 (Pediatric Diabetes 2011 online)
Minicucci et al. 162 reported a randomised controlled study of the effect of insulin glargine in patients with CF with IGT. Patients were selected because BMI was under the 10th percentile or had fallen by one percentile over the previous year, or if there were similar findings for FEV. All were aged > 10 years. The study initially recruited 45 patients but, after dropouts, 34 remained for analysis at 18 months. They were randomised to low-dose insulin glargine, starting with a dose of 0.1 units/kg/day, increasing to 0.15 units/kg/day if no hypoglycaemia occurred. The dose could be increased to 0.2 units/kg/day at the physician’s discretion. The primary end point was BMI, with HbA1c level and FEV being secondary end points. At 18 months, there were no significant differences between the groups in BMI or FEV, but some difference in HbA1c level, with a reduction of 0.11% in the insulin group compared with a rise of 0.26% in the control subjects (p = 0.04). There was a bigger reduction of 0.52% in four patients who had received 0.2 units/kg/day.
The authors suggest that the lack of effect might be due to the low dose of glargine used or to the trial duration being too short.
Can we quantify the utility of insulin treatment?
Insulin treatment is clearly beneficial, but for later estimation of cost-effectiveness it would be useful if we could quantify the utility gain. The quality of the studies is not high, but a RCT of insulin treatment versus no insulin would be unethical.
The benefits include:
-
An improvement in HbA1c value, with the biggest improvement being the 2% (at 1 year) and 1% (at 5 years) in the Rolon et al. study. 146 Other studies found no difference.
-
An increase in weight or BMI, with studies reporting rises of 1–3 points in BMI.
-
Improvements in lung function, such as rises in FEV1, expressed as per cent of expected normal. Rises ranged from 6% to 35%.
-
Reductions in lung infections – only four studies reported this. 134,149,158,160 Two studies149,160 reported reductions of almost half in the frequency of infections, and two134,158 showed little difference.
The studies were often too small and too short, or did not report all outcomes of interest. None reported QoL. Improvements in lung function in patients with compromised respiratory function should improve QoL.
Table 6 summarises the benefits of insulin treatment in patients with CFRD and CF with non-diabetic hyperglycaemia.
| Study ID | Study design | No. of patients | Patients’ characteristics | Age (years) | Treatment | Follow-up (post insulin) | HbA1c and glucose levels | Lung function | Weight or BMI | Adverse or other effects |
|---|---|---|---|---|---|---|---|---|---|---|
| Bizarri 2006151 | Case series, before-and-after study | 6 | Patients with CF with IGT | Median 18.1 (range 9.2–27.8) | Insulin – glargine | 1.4 years (range 1.0–1.8 years) | No change | Significant improvement in median FEV1 (72.7% vs 76.7%; p = 0.027) | Significant improvements in both median BMI z-scores (– 0.95 vs – 0.5; p = 0.026) | No hypoglycaemia, no change in median number of hospitalisations for clinical exacerbation |
| Boyle 2004148 | Before-and-after study (comparison of 1 year before insulin vs 1 year after insulin) | 30 | 13 CFRD,10 IGT, 7 NGT | Mean 26.9 | Insulin | 1 year | FEV1 decline reversed in IGT and NGT patients; FEV1 changes were: –0.28% vs –1.47% (CFRD), –7.08% vs +1.46% (IGT), –2.68% vs +1.47% (NGT) | Weight improved in all patients: –3.14% vs 0.59% (CFRD), 1.1% vs 1.56% (IGT) and –2.1% vs 0.45% (NGT) | ||
| Dobson 2002138 | Case series (comparison of 3 months before insulin and 3 months after insulin) | 4 | Long-standing CF, weight loss, deteriorating lung function, high random glucose values but normal OGTT (non-IGT PPH?) | Range 15–23 | Three with Insulatard® (Novo Nordisk); one with Novorapid® (Novo Nordisk)insulin + Mixtard® (Novo Nordisk) | 3 months | FEV1 increased by 8.3% to 101% (0.11–1.03 l); FVC increased by 4.33% to 70% (0.16–0.89 l) | Weight increased by 2.4% to 13% (0.7 to 5.7 kg) | ||
| Drummond 2006152 | Before-and-after case series (comparison 5 years before and 5 years after insulin) | 54 | CFRD, IGT and NGT (nos. of each not given) | Mean 27.6 (range 16–52) | Insulin | 5 years | Rate of FEV1 decline in patients with IGT changed significantly from 0.51 ± 0.31 pre-insulin to 0.04 ± 0.12 post insulin (p = 0.02); changes not significant in patients with NGT or CFRD | Weight increased significantly with insulin therapy from 53.08 ± 1.53 kg to 56.22 ± 2.08kg (p = 0.05) | ||
| Drummond 2007156 | Case series – retrospective | 24 | Patients with CF on insulin for mean of 7.3 years | 30.7 | Insulin | 6 months |
Five (21%) patients had no hypoglycaemic episodes, 13 had between one and four episodes, six (25%) had five or more Majority (75%) had hypoglycaemic episode unawareness |
|||
| Franzese 2005149 | Before-and-after case series (comparison of 6 months before and 6 months after insulin) | 8 |
Group A: Four patients with chronic CFRD treated with rapid insulin 1–3 years prior to study Group B: Four patients with intermittent CFRD treated with insulin for infection only; plus six control subjects with intermittent CFRD |
Range 10–29 | Insulin – glargine | 6 months | No change in HbA1c value in any group | FEV – no change; lung infections decreased by 50% | No change | No hypoglycaemic events recorded |
| Grover 2008157 | Randomised non-blinded, crossover study | 19 | CFRD with FH | Mean 34 | Glargine vs NPH | 12 weeks | No changes in HbA1c | More weight gain on glargine – but not significant (p = 0.07) |
No difference in adverse events and QoL between the groups No serious hypoglycaemic episodes |
|
| Hameed 2009161 | Before-and-after study (compared with 1 year before treatment) | 8 | Newly diagnosed patients with CFRD | Median 13.5 | Pre-breakfast insulin detemir – median dose of 0.1 units/kg/day | 15 weeks |
%FVC change was +7.8 compared with decline –6.9 (in the year before insulin (p = 0.002) %FEV1 change was +7.3 compared with decline –6.9 in the year before (p = 0.005) |
Mean WtSDS improved by +0.46 compared with decline of 0.34 in the year prior to insulin (p = 0.003) | No episodes of severe hypoglycaemia | |
| Hardin 2009159 | Before-and-after study | 9 | Patients with CFRD who had been treated with at least three subcutaneous injections per day for a minimum of 6 months | Mean 27 | CSII | 6 months | HbA1c level decreased from 8.2% (SD 1.9%) to 7.1% (SD 1.5%), p = 0.05 | Weight increased significantly from 55.6 kg (SD 3.5 kg) at baseline to 59.2 kg (SD 3.3 kg), p = 0.01 | No patient had an episode of hypoglycaemia | |
| Hardy 2006153 | Before-and-after study (comparison of 1 year before vs 1 year after insulin) | 27 | CF children with abnormal OGTT but normal fasting glucose | Not given |
Group A: 14 on insulin glargine (owing to clinical deterioration) Group B: 13 not given insulin Control: 55 patients with CF with normal OGTT |
1 year |
Group A: FEV1 declined significantly (> 5%) before treatment in 8/14 patients, but improved in six of these eight after insulin treatment Group B: FEV1 also declined in 7/13, but improved in five of these seven without insulin treatment |
|||
| Lanng 1994134 | Case–control (6 years before and 2 years after onset of insulin therapy) | 16 patients/16 control subjects | CFRD with at least 2 years of follow-up on insulin; matched with 18 non-diabetic control subjects | 3–28 | Insulin | 2 years |
FEV1 increased from 38% to 44% of normal FVC increased from 61% to 73% of normal No. of patients with chronic P. aeruginosa lung infection remained the same; sputum positive for S. aureus reduced from 19% to 15%; H. influenzae 12% to 7%; S. pneumoniae 2.4% to 0.3% |
Weight increased from 46% to 53% BMI increased from 17% to 19% |
||
| McGinnity 2006154 | Case series | 5 | CFRD – all had received treatment with once-daily long-acting human insulin analogues for > 12 months prior to the study | 11–18 | Long-acting insulin (detemir or glargine) | 3 days | CBGM showed mean glucose levels were (range) 7.6 mmol/l (2.2–17.2 mmol/l), 6.6 mmol/l (3.8–13.7 mmol/l), 5.3 mmol/l (2.2–9.0 mmol/l), 5.9 mmol/l (3.4–12.9 mmol/l), 6.4 mmol/l (4.1–11.8 mmol/l) | FVC increased from 61% to 73% of normal | No symptomatic hypoglycaemia | |
| Minicucci 2005150 | Case series | 15 | Twelve patients with CFRD and three with CF IGT |
Group A: Six had CFRD treated with insulin glargine (had been on insulin regular or rapid analogue before meals) Group B: Six appeared to have CFRD but without FH, diagnosed on the basis of OGTT (insulin naive) Group C: Three had CFRIGT (insulin naive) |
3 months | No change in HbA1c value in any group | Very little change | Frequency of hypoglycaemia did not change in Group A. No hypoglycaemia events were observed in Groups B and C | ||
| Minicucci 2009162 | Randomised controlled study | 34 | Patients with CF with IGT |
Group A: Eighteen received insulin Group B: Sixteen received no insulin |
18 months | HbA1c value improved by 0.11% in the insulin group but increased by 0.26% in the control group |
Improvement in weight and BMI in Group A and worsening in Group B Difference not statistically significant |
FEV1 improved by 6% in Group A and worsened by –2.5% in Group B. Difference between groups not statistically significant | ||
| Mohan 2008158 | Before-and-after study (comparison of 5 years before and 3 years after insulin therapy) | 42 | CFRD | 16–39 | Thirty-one with short-acting insulin with meal; nine with basal insulin plus short-acting insulin; two with intermediate-acting insulin | 3 years | HbA1c value available for only 32 patients. Overall no mean change in HbA1c value from diagnosis (6.8% vs 6.7%). 17/32 had elevated values (mean 8.1%) at diagnosis – improved significantly following 3 years of insulin treatment |
FEV1: Improvement over baseline maintained at 1 year for FEV1 (mean 55.1%, p < 0.002); 2 years for FVC (mean 72.1%, p < 0.01); increased from 52% to 58% predicted by 3 months; returned to baseline by 3 years FVC: Increased from 66 to 76% predicted by 3 months; remained at 71% by 3 years; no change in lung infections |
BMI: No change | No side effects experienced |
| Moran 2001145 | Pharmacodynamic study | 7 | CFRD without FH | Mean 24 | Three study conditions administered in random order on separate mornings: (1) no preprandial diabetes medication, (2) insulin lispro preprandial and (3) repaglinide preprandial | 1 to 2 months | Insulin lispro better than repaglinide on postprandial glucose excursion, significant differences between the two drugs in the peak glucose level (172 ± 9 mg/dl vs 208 ± 18 mg/dl, p = 0.02), the 2-hour glucose AUC (p = 0.02), and the 5-hour glucose AUC (p = 0.01) | Four episodes of hypoglycaemia: one after the test meal without medication, two after insulin lispro and one after repaglinide | ||
| Moran 2009: CFRDT trial141 | Randomised trial | 100 | Seventy-four CFRD FH– patients and 26 with severe IGT | Mean 27 |
Group 1: Insulin aspart Group 2: Repaglinide Group 3: Oral placebo |
1 year | No change in HbA1c value in any group; no difference in fasting glucose levels within or between groups compared with baseline | All study arms for the CFRD FH– patients showed decline in FVC during the study compared with 12 months prior to study; the insulin and repaglinide arms showed a reduction in decline in FEV1 | Significant improvement for the CFRD FH– patients on insulin when comparing change in BMI for the 12 months prior to the study to the change during the study year. No significant change between groups for CFRD FH– patients | |
| Mozillo 2009160 | Ongoing open study – no control group | Sixty-five enrolled – data on first 22 patients | Patients with CF with early glucose derangements Four had abnormal glucose tolerance on CGMS, nine had IGT, seven had diabetes mellitus without FH and two had iabetes mellitus with FH | Mean 12.4 | Insulin – glargine | 1 year | No significant difference in HbA1c value | An 8.8% increase in %FEV1 (p = 0.01); 42% decrease in the number of lung infections (p = 0.003) | No significant difference in BMI z-score | |
| Nousia-Arvanitakis 200154 | Case series | 6 | Patients who developed CFRD in a 5-year follow-up of 30 patients with CF | 15–22 | Insulin – biphasic (rapid and intermediate) (matched to a control group of non-diabetic patients with CF) | 5 years | FEV1 increased significantly from 51% to 71% (p = 0.0062) | BMI increased significantly from 16 to 19 kg/m2 (p = 0.0018) | ||
| Onady 2006155 | Prospective case series | 20 | CFRD | 13–49 |
Eight initially chose to be on insulin, five chose sulfonylureas, four chose metformin, three chose thiazolidinediones Four patients switched from insulin to oral agents owing to inadequate HbA1c control |
10 years | No statistically significant differences in overall glycaemic control between oral agents and insulin | No statistically significant differences in FEV1 between oral agents and insulin | No statistically significant differences in changes in weight between oral agents and insulin | All patients tolerated the initial therapy and none had to change treatment because of side effects |
| Rolon 2001146 | Case–control (compared 5 years before and after treatment) | Fourteen cases (matched to 14 non-diabetic control subjects) | CFRD insulin treated | Mean 14 (10–22) | A mixture of short- and intermediate-acting insulin in 10/12 cases; one with short-acting insulin; one switched from basal–bolus regimen to bolus only | 5 years (only seven patients had 5 years of follow-up at the time of the study as the rest had died) | Reduction of 2% at 1 year (12 patients) and 1% at 5 years (seven patients) |
FEV1: an annualised rate improved by 3–18.5% in all patients (5 years pre- and post insulin, seven patients) FVC: 2.5–15% decrease in the annualised rate of decline was seen in 5/7 patients; 2/7 had 4–4.5% increase in the decline (5 years pre- and post insulin, seven patients) |
BMI z-score increased from –1.7 to –0.24 SD of the reference population (14 patients) | Two episodes of severe hypoglycaemia over the total follow-up of 42 patient-years + mild hypoglycaemia with a frequency of 10.3 episodes/patient-years |
| Rosenecker 2001120 | Two case series | 45 | CFRD | Mean 24 and 27 | Insulin (34) and glibenclamide (11) | 7.6 years (insulin), 3.5 years (glibenclamide) | No significant differences were found between the two groups in the most recent FEV1, FVC readings | No significant differences were found between the two groups in the most recent BMI | No severe hypoglycaemic events occurred in patients treated with insulin or glibenclamide | |
| Sulli 2007139 | Case reports | 3 | CFRD. All had been on MDI treatment (four injections/day) prior to going on a pump | 5, 21, 28 | Insulin pump therapy | 2 years | After 2 years, all three patients had significant reductions (between 1.2% and 1.7%) in HbA1c levels compared with MDI before treatment. Good metabolic control over 2 years with CSII. Insulin requirements decreased during CSII treatment | Annual mean level of BMI increased |
Two episodes of lipohypertrophy and a slight local cutaneous inflammation No DKA or hypoglycaemic episodes |
Overview of review articles of cystic fibrosis-related diabetes treatment
A number of previous reviews have commented on treatment of CFRD. 1,7,63,68,164–168 Most conclude that insulin is the treatment of choice. Some recommended that insulin should be initiated when CFRD is diagnosed. 1,166 Other studies suggested that insulin can also be used temporarily for intermittent hyperglycaemia, as a result of infection, steroid therapy and augmented nutrition. 1,164 However, it was noted that despite data from other populations suggesting that insulin may be beneficial in maintaining euglycaemia during infection, no studies have examined the benefits of such in hospitalised patients with CF. 168 Dobson et al. believed further prospective randomised control trials are required to investigate the benefits of insulin therapy after the diagnosis of CFRD. 49 They also pointed out some drawbacks of insulin therapy, such as compliance problems and the increased risk of hypoglycaemia. O’Riordan et al. 168 in the ISPAD guidelines recommended that ‘the decision to treat should be based on consideration of BG levels and the impact of treatment on the individual’s overall condition’.
Those commenting on the use of sulfonylureas say that these drugs augment insulin secretion by stimulating the sulfonylurea receptor in pancreatic β-cells, enhancing insulin release, and therefore may be useful in some patients with CFRD. 63,68,164 However, this is questioned by De Valk et al. ,165 who argue that the progressive destruction of the β-cells means that these agents have limited value in CFRD, certainly in the longer term. Yung et al. 63 suggested that if patients are asymptomatic and clinically well, a trial of OHAs can be used initially, along with close monitoring of BG profiles, body weight and lung function at least monthly. They can also be used in patients with steroid-induced glucose intolerance and for those who find insulin treatment difficult to cope with. 7,63
Most reviews do not favour the use of sulfonylureas in CFRD, especially when there are concerns about side effects, such as hypoglycaemia, and potential hepatic toxicity in patients with hepatic impairment. 68 The latter may limit dosage below optimal therapeutic levels. More theoretically, there are worries that sulfonylureas could bind to and inhibit CFTR and interfere with new treatments designed to improve CFTR function,68,164 although the clinical importance of such remains unclear. 49 Dobson et al. 49 considered that the risk of hypoglycaemia with sulfonylureas is slight in CF and it would be of even less concern if newer shorter-acting agents were developed.
Generally, it is recommended that sulfonylureas should not be used until further data and side effect profiles are available. 49,166–168
As regards whether or not to start at the IGT stage, most agree there are insufficient data on the management of CFRIGT patients to support guidelines. 68,167,168 Brennan et al. 164 felt that when CFRD without FH or IGT is identified, it is not known whether or not benefits of treatment outweigh the burden of management and at which point treatment should be initiated. Although the UK Cystic Fibrosis Trust7 recommended no treatment for patients with IGT who are asymptomatic, with stable weight, pulmonary function and a normal HbA1c level, others68,167,168 consider the risk of patients progressing to diabetes, such that they should be monitored with an annual OGTT and BG levels should be measured during illnesses. Interestingly, De Valk et al. 165 suggested that nutritional treatment may be sufficient in early stages (IFG and IGT). In the 2008 ISPAD Clinical Practice Consensus,168 insulin treatment was not recommended for patients with IGT unless there were persisting signs of poor growth, inability to maintain weight and unexpected decline in pulmonary function (despite optimisation of other medical management) or the development of overt signs of diabetes. In general, it is agreed that further studies are needed to establish whether or not early management of hyperglycaemia in these people can prevent pulmonary decline and prolong survival. 49,164
Most reviews reported that the choice of insulin should be made flexible, and be tailored to an individual’s eating habits and lifestyle,1,63,164,166–168 especially when taking into account patients’ erratic dietary habits. Although there is a variety of insulins, all with different speeds of onset and duration, there is no evidence to support any specific type of insulin or insulin regime in CFRD. 49 Some studies suggested the use of short-acting insulin, as it provides flexibility, allowing better adjustment of insulin dose for each meal, and additional boluses can be given for snacks or night feeds. 164,167
Mackie et al. 1 believe that the short duration of action of short-acting analogues can be beneficial in adapting to the dietary habits of most patients with CF. For those who have a more regular eating pattern, they recommend a twice-daily insulin regimen, which is sufficient to achieve adequate glycaemic control. 1 Lanng166 reported experience in the use of insulin, starting with NPH insulin as a single dose in the morning or twice daily; later, premixed insulins are often used. If patients wished a more flexible lifestyle, a basal–bolus regimen with injections of soluble insulin before each main meal would be used, combined with NPH insulin at bedtime. Alternatively, insulin pump infusion can also provide an effective basal–bolus therapy. 168 Insulin pump therapy has been successfully used in CFRD, but very low basal rates are usually needed. 167
There have been several recent reviews of the management of CFRD. 30,93,169–171 There is consensus that oral agents are not recommended. There is now agreement that CFRD without FH should be treated. Laguna et al. 93 note that it was previously believed that CFRD patients without FH did not need to start insulin because they were asymptomatic, had minimal HbA1c level elevation, and were not thought to be at risk of diabetic complications. However, they note that recent research has shown that insulin therapy reversed chronic weight loss and raised BMI, and that better nutritional status was associated with improved survival.
There is less consensus about whether to treat IGT or PPH that has returned to normal by 2 hours [called INDET (intermediate hyperglycaemia with normal FPG and 2-hour PG) by Laguna et al. 93]. Laguna et al. 93 note a lack of evidence as to best management. In another review by the same group, Nathan et al. 169 note that some studies have shown improvements in lung function from treating IGT but others have not, but that these studies have been too small to give definite answers. The ISPAD 2009 guidelines say that there is insufficient evidence to make recommendations for patients with IGT or for the group who have normal OGTTs but intermittent hyperglycaemia shown by self-monitoring of BG. 171 Rana et al. 170 say that treatment of IGT is currently not recommended unless there is poor growth, inability to gain weight or unexpected decline in pulmonary function. They call for RCTs of longer duration.
Discussion
Summary of main findings
Use of oral agents
There were seven studies, all small and most of short duration. Five were case series (some of which had, in effect, several case series of different drugs) and two were crossover studies. The case series suggest that sulfonylureas (glipizide and glibenclamide) have some effect, but do not provide sufficient evidence for any firm recommendation.
One randomised crossover study using acarbose was only for 2 weeks’ duration but suggested that side-effects were a problem. The crossover study with repaglinide and insulin suggested that repaglinide had some beneficial effect, but that insulin was better. However, it was very short term.
There are no studies of newer agents, such as the glucagon-like peptide analogues, but, as they often cause nausea, their use in a disease characterised by low BMI might be undesirable.
International guidelines do not recommend any oral agents.
Treatment of impaired glucose tolerance
Five studies148,150–153,162 reported the effects of insulin at the IGT stage (treating the Boyle148 and Drummond152 papers as reporting the same study). Some had very small numbers (i.e. three, six and nine subjects). Two studies (with 54152 and 6151 patients) reported that the decline in lung function was halted or reversed by insulin treatment. One study160 with 13 patients reported a reduction in pulmonary exacerbations. Two studies were inconclusive. 150,162 Only one study162 was a RCT. Most were available only as abstracts with little detail.
So there is insufficient evidence to justify routine treatment with insulin at the IGT stage, but enough to justify a RCT of treatment of IGT with insulin versus waiting until diabetes develops. Outcomes should include lung function, microbial colonisation and BMI, as well as glycaemic control.
Benefits of insulin in cystic fibrosis-related diabetes
Insulin appears beneficial in CFRD and probably at the IGT stage. For cost-effectiveness purposes, we need to quantify the utility gain from insulin treatment, as well as the survival gain. No studies reported QoL by a reliable method, such as European Quality of Life-5 Dimensions (EQ-5D). We need better studies, with larger numbers, with data collected on all important benefits and disbenefits.
Conclusions
The evidence base for treatment of CFRD and lesser degrees of hyperglycaemia is weak. Studies are mostly case series, which are too small and too short.
Research needs:
-
The most important immediate question is when treatment with insulin should start: whether it is better to start at the IGT stage or wait till diabetes develops? It appears that some damage occurs at the IGT stage and a trial of early versus later treatment is indicated. There could be two approaches at that stage: a once-daily basal insulin or short-acting mealtime insulins. The latter would be more troublesome but might be justified on the basis that at the IGT stage most hyperglycaemia is postprandial, with normal fasting glucose.
-
More data are required on the relative merits of glargine, NPH and detemir.
-
We need to know whether immediate PPH, not lasting for as long as 2 hours (so not IGT), is harmful, and whether treatment would be beneficial.
-
In the longer term, we need to find out whether the pancreatic damage can be prevented, and diabetes avoided, or at least delayed.
Chapter 4 Systematic review of screening tests
Terminology
The term ‘screening’ usually refers to the use of a simple but imperfect test, in asymptomatic people, in order to distinguish between those who probably have the condition and those who probably do not. It is usually used in the context of population screening but is also used in the context of screening people with a condition for a complication of it, such as retinopathy screening in diabetes. Screening tests are now being called ‘index tests’ in some research studies.
Those who have positive screening tests go on to a definitive diagnostic test, usually called the reference standard or sometimes ‘gold standard’, in research studies. The diagnostic test is assumed to more accurate and to give a definite diagnosis.
The reference standard test is usually more complex or more expensive; if not, it would be used as a perfect screening test.
Screening terminology includes the following terms, derived from the classic 2 × 2 table, as shown in Table 7.
| Screening test result | Disease status by reference test | ||
|---|---|---|---|
| Have disease | Do not have disease | ||
| Positive | a | b | a + b |
| Negative | c | d | c + d |
| a + c | b + d | Total | |
Sensitivity The per cent of patients with the disease who have positive screening tests. Those with the disease who are screening test-negative are false-negatives. Sensitivity = a/a + c.
Specificity The per cent of people who do not have the disease and who are screening test-negative. Specificity = d/b + d. So if specificity is 90%, 10% of people without the disease are screen-positives but false-positives.
Positive predictive value (PPV) = per cent of those with disease among those with positive screening tests a/a + b.
Negative predictive value (NPV) = per cent of those with a negative test who are true-negatives. It is about how good the screen test is at ruling out disease.
The reliability of a screening test can also be expressed as the per cent of results that are correct: a + d/a + b + c + d.
Background
A survey in the USA by Allen et al. 80 found a wide range of screening practices and tests for the detection of CFRD, with random PG the most common, followed by HbA1c, and urinary glucose. Most guidelines recommend an annual OGTT,172,173 but it appears that, owing to the cost, inconvenience and unpleasantness of the test, the guidelines are largely ignored in practice. 80 Some of the variation in the tests used may relate to differences in the target diagnoses; tests may be perceived as being more or less able to detect different levels of glucose intolerance.
A survey in the UK obtained data from 37 of the 45 recognised centres (based on having ≥ 50 patients with CF). 81 Most centres said that they screened patients annually. Most of the paediatric centres started screening at the of age 10 years, but a few started at the age of 12 years. The UK Cystic Fibrosis Trust recommends that screening should start at the age of 12 years. 7
Six tests were used: the OGTT, random BG, serial glucose monitoring, HbA1c, FPG and glycosuria. It appears that the OGTT is the reduced version (ROGTT), with only fasting and 2-hour glucose levels measured, as recommended by the UK Cystic Fibrosis Trust, but the study does not say whether or not any units used the full OGTT (FOGTT). Serial glucose monitoring is taken to be a series of BG tests done with finger-prick, testing strips and meter; there is no mention of automated CGMSs being used. The commonest method used was the ROGTT, followed by various combinations of OGTT and other tests, such as FPG and HbA1c.
These methods may be the policies of the individual clinics, but what happens in routine care may differ owing to poor compliance. The survey did not provide data on numbers actually screened, and how.
Issues
There is some evidence (see Chapter 3) that treatment may be beneficial not only in diabetes, but also in IGT. There is even a suggestion that treatment of isolated early PPH might be worthwhile, although this is based on very small numbers.
The suggestion of benefit from treating hyperglycaemia at non-diabetic levels would fit with the conclusion from Chapter 2, that adverse effects on the lung may start at PG levels as low as 8 mmol/l.
There are therefore uncertainties about what we should be screening for, with three groups:
-
diabetes, including those without FH
-
IGT
-
PPH with return to normal by 2 hours.
Given the transient nature of PPH, and the scanty evidence on benefit of treatment at that stage, we focus in this review on screening for diabetes, and for both diabetes and IGT.
Our default position is that diabetes and IGT are defined as per the WHO definition, but, as discussed in Chapter 2, this may be inappropriate if lung damage starts at lower levels of hyperglycaemia than retinopathy on which the WHO definition is based.
The screen-positives could potentially benefit in two ways – earlier treatment in those who would have been diagnosed later, after developing symptoms; treatment in those who would never have been diagnosed. We should also consider that some people who are detected and treated would never have developed symptoms and might have died from unrelated causes.
Methods
Criteria for considering studies for this review
Types of studies
Studies of screening tests can be:
-
RCTs of one or more screening tests or strategies versus no/opportunistic screening.
-
Case series, comparing a diagnostic test with an established reference standard. These can be either prospective or retrospective in nature.
-
Case control, where test performance is compared between patients with known disease (i.e. diabetes) and those without the disease of interest; this type of design is known to be significantly more susceptible to bias than the case series design, especially when healthy control patients are included. The artificial selection of patients leads to an unrepresentative case mix.
Owing to the anticipated dearth of studies in the area, searches were for all study designs.
To be included for formal data extraction, studies had to report sufficient data for the construction of a 2 × 2 table.
Participants
Based on the findings of Chapter 1, it was decided that screening for CF-related hyperglycaemia would not start before the age of 10 years, and so studies of adults or children > 10 years were eligible for inclusion.
Reference standards
The test recommended by most consensus statements is the OGTT, often only in its reduced form. We assumed that the gold standard reference test is the FOGTT, but there are reservations about acceptability. However, we expected many studies to use the ROGTT as the reference standard, especially as the definition of diabetes is based on fasting and 2-hour results.
Reference standards for diabetes in CF therefore include:
-
the 75-g (weight-adjusted) FOGTT result, with BG measured fasting and at 30, 60, 90 and 120 minutes
-
the ROGTT, with only fasting and 2-hour measurements.
Ideally, a reference standard should indicate with absolute certainty the disease status of an individual. In reality, this is rarely achieved and less accurate reference standards must be accepted. For example, the ROGTT will miss PPH of the lag storage type, and even the FOGTT may miss hyperglycaemia if that occurs only in the evening. As will be reported later, there are also doubts about the reproducibility of the OGTT, so it is used more as a reference test than a gold standard.
Screening tests
Studies of any test to assess glucose intolerance in patients with CF were eligible for inclusion. These might include:
-
the 50-g glucose challenge test (GCT), with 60-minute glucose level
-
continuous glucose monitoring (CGM)
-
FPG
-
RBG levels
-
HbA1c
-
serial capillary blood glucose profiling
-
fructosamine
-
urine glucose tests
-
combinations of the above, for example a FPG test followed by an OGTT.
Search methods for identification of studies
As previously described in Chapter 3, a highly sensitive search strategy was run, in order to identify all aspects of patients with CF with diabetes and hyperglycaemia, including screening, diagnosis and treatment. Full details of the search strategy are shown in Appendix 1.
Selection of studies
Studies were selected for inclusion in the review in a two-stage process. In the first instance, the literature search results (titles and abstracts) were screened independently by two reviewers to identify all citations that appeared to meet our inclusion criteria as described above. Full manuscripts of all selected citations were obtained. One article in German174 and two in French175–178 were translated into English. Where it was not possible to determine study eligibility from the title and/or abstract, the full manuscript was obtained. Any disagreements over study inclusion were resolved by consensus. It was never necessary to have arbitration by a third reviewer.
Studies were selected at two levels: first, those that yielded sufficient detail for 2 × 2 tables, and, second, other studies that might yield fewer but useful data.
The flow of studies is shown in Appendix 1.
Data extraction and management
For the first few studies, data were extracted independently by three or four reviewers, until we were happy that the predesigned data extraction form was satisfactory; some revisions were made. Information on study participants, study design, tests and reference test details, test performance (2 × 2 contingency tables) and potential sources of bias was extracted.
Assessment of methodological quality
The methodological quality of all included studies was appraised using a modified version of the QUADAS (quality assessment of diagnostic accuracy studies) tool. 179 Ten items were initially included, but items 7a, 7b, 8 and 9 were deemed to be usually not applicable in a situation where results were numerical from a laboratory (and hence not susceptible to observer interpretation), and dichotomised. An 11th item on reporting of definitions of the different hyperglycaemic states was added.
Study quality was assessed by two reviewers. Each item was scored as ‘yes’, ‘no’, ‘unclear’ or ‘not applicable’. Appendix 3 shows the blank quality assurance form.
A summary of the reviews authors’ judgements about the methodological quality item for each included study is shown in Table 8.
| Representative spectrum? | Acceptable reference standard? | Acceptable delay between tests? | Whole sample verified using reference standard? | Same reference standard used? | Reference standard independent of the index test? | Reference standard results blinded? | Index test results blinded? | Same clinical data as used in test results available in practice? | Uninterpretable/intermediate test results reported? | Withdrawals explained? | Definitions of the different hyperglycaemic states given? | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buck 2000174 | Yes | Yes | Yes | Yes | Yes | Yes | n/a | n/a | n/a | ? | ? | Yes |
| De Luca 1991183 | ? | Yes | Yes | Yes | Yes | Yes | n/a | n/a | n/a | n/a | n/a | n/a |
| De Schepper 1991184 | ? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | n/a | ? | ? | Yes |
| Lee 2007185 | ? | Yes | Yes | No | Yes | Yes | n/a | n/a | n/a | n/a | No | Yes |
| Magni 1996186 | No | Yes | Yes | Yes | Yes | Yes | n/a | n/a | n/a | n/a | Yes | Yes |
| Moreau 2008187 | Yes | Yes | Yes | Yes | Yes | Yes | n/a | n/a | n/a | n/a | Yes | Yes |
| Mueller-Brandes 2005205 | Yes | Yes | ? | Yes | Yes | Yes | n/a | n/a | n/a | n/a | No | Yes |
| Robert 1992188 | ? | ? | Yes | No | Yes | No | n/a | n/a | n/a | ? | ? | Yes |
| Yung 1999189 | No | Yes | Yes | Yes | Yes | Yes | n/a | n/a | n/a | n/a | Yes | Yes |
Figure 2, presents a graphical summary of overall quality by showing the per cent of studies that did or did not fulfil each item.
FIGURE 2.
The per cent of studies that fulfilled each quality item.
No summary scores estimating the overall quality of a study were calculated, as their interpretation is potentially misleading. 180
The items of the QUADAS tool and their interpretation are as follows:
-
1. Was the spectrum of patients representative of the patients who will receive the test in practice? The characteristics to be considered here included:
-
– Age – the likelihood of diabetes increases with age, and so if the test was applied to a mainly older population it might appear more accurate. Hence, we looked for a sample of patients that was typical of the population in the centre, either paediatric or adult.
-
– Selection bias, where we looked to see what proportion of the centre’s patient population was included in the study. The greater the proportion, the less the bias. To estimate the proportion, we looked for the total clinic population.
-
– Whether the patients on whom the screening test was being tested, had an over-representation of those with conditions likely to cause fluctuations in BG, such as exacerbations of lung disease. Studies in which all or a significant proportion were suffering from such exacerbations at the time of screening, were excluded.
-
– Whether or not any particularly high-risk (or low-risk) groups were selected for screening.
-
-
2. Is the reference standard likely to correctly classify the target condition?
-
– For the reasons given above, we used the OGTT as the reference standard. Ideally, this would have been the FOGTT but the reduced version correctly classifies the target conditions (diabetes and IGT), as they are defined on the basis of it.
-
-
3. Is the time period between reference standard and index test ≤ 1 month?
-
– The time period between screening and reference testing needs to be short enough to ensure that the presence or absence of the condition does not change between tests. We assumed that a month (mean or median) was short enough, although this does leave some problems with skew. Ideally, we would exclude patients whose interval was much longer but studies did not give sufficient detail. In practice, it is probably more important that patients are in the same condition (e.g. free of infectious exacerbations) at both screening and reference testing.
-
-
4. Did the whole sample or a random selection of the sample receive verification using a reference standard of diagnosis?
-
– The issue here was whether the reference test differed according to the result of the screening test (e.g. if definite positives did not have the reference test but ‘borderline positives’ did).
-
-
5. Did patients receive the same reference standard regardless of the index test result?
-
– The issue here is whether all people having the screening test had the same reference test.
-
-
6. Was the reference standard independent of the index test result (i.e. the index test did not form part of the reference standard)?
-
– When OGTT is the reference standard, this does not apply to screening tests such as CGMSs or HbA1c. The FPG is part of the OGTT, but in practice, the diagnosis of CFRD is based more on the 2-hour level (because FH occurs later than PPH) and so this is not a problem.
-
-
7a. Were the index test results interpreted without knowledge of the results of the reference standard?
-
– Because of the objective nature of the screening and test results, neither this nor the next question were applicable.
-
-
7b. Were the reference standard results interpreted without knowledge of the results of the index test?
-
8. Were the same clinical data available when test results were interpreted as would be available when the test is used in practice?
-
– Again, when the test results are objective and defined in advance, and not open to interpretation, this criterion is not applicable.
-
-
9. Were uninterpretable/intermediate test results reported?
-
– With objective testing, uninterpretable results should not be obtained. However, intermediate ones might arise if the investigators subdivided groups, for example splitting CFRD into those with and without FH, or into normal GT (NGT), IGT and diabetes. Problems would arise if results were described simply as normal or abnormal without defining meanings. Where intermediate results (usually IGT) were given, options included producing a 3 × 3 table, or two 2 × 2 tables, for example one defining abnormal as diabetes and normal as everything else, and the other defining abnormal as IGT + diabetes. Where appropriate, we used the second option, which seems correct given the possibility that treatment should start at the IGT stage.
-
-
10. Were withdrawals from the study explained?
-
– This usually refers to the possibility of bias if only some of the screened people go on to reference testing.
-
-
11. Were definitions of the different hyperglycaemic states given?
-
– This is important given changes in the classification of diabetes and other states, and differences in definitions such as the ADA and WHO definitions of IFG.
-
Data extraction
When data permit, 2 × 2 tables are produced for each study, with sensitivity, specificity, PPV and NPV, and CIs. Some studies report on IGT, and two 2 × 2 tables are produced: one with just diabetes as the target condition, the other with both diabetes and IGT.
Analysis of 2 × 2 tables
Analysis was undertaken using the MedCalc diagnostic test evaluations program, version 11.6.1 (MedCalc Software, Mariakerke, Belgium). 181
Results
Nine studies174,183–189,205 (one in German174) provided sufficient data for 2 × 2 tables with actual numbers, not just per cent, so that CIs could be produced. Full details are given in the data extraction forms in Appendix 4.
Studies are identified hereafter by the name of the first author and year of publication.
Buck 2000
Buck et al. 174,182 carried out their study in two hospitals in Ulm and Hannover, in 102 patients aged between 5 and 33 years, with a median age of 13 years. They compared the results of OGTTs (1.75 g glucose/kg body weight, up to a maximum of 75 g) with FPG and HbA1c levels. Results are shown in Tables 9 and 10.
| Result, % (95% CI) | |
|---|---|
| Sensitivity | 23 (10 to 40) |
| Specificity | 96 (87 to 99) |
| PPV | 73 (39 to 94) |
| NPV | 70 (60 to 79) |
| Diagnostic accuracy | 71 |
| Result, % (95% CI) | |
|---|---|
| Sensitivity | 23 (5 to 54) |
| Specificity | 91 (83 to 96) |
| PPV | 27 (6 to 61) |
| NPV | 89 (81 to 95) |
| Diagnostic accuracy | 82 |
Because the reference ranges for HbA1c level were slightly different in the two centres, results were pooled and reported as being normal or abnormal. The upper limits of normal were 5.0% and 5.7% in the two centres. It is not clear whether or not these limits were used to define screen positivity.
Of the 102 patients, 22% had IGT and 13% had diabetes. None of those with diabetes had experienced symptoms (perhaps because those with symptoms would have been diagnosed without screening), and none had an elevated FPG level. HbA1c level was not a sensitive test for diabetes.
De Luca 1991
This Italian study by De Luca et al. 183 included 39 children and adolescents, in the age range of 5 to 22 years, who had had normal random BG results over the previous year. Their BMIs ranged from 13 to 24 kg/m2. They had HbA1c tests and FOGTTs. Results were given for both diabetes (two patients) and IGT (seven patients); numbers were small. Insulin levels were also measured and noted to be normal when fasting, but delayed after the glucose load, even in some patients with normal OGTTs. The normal range for HbA1c level was 4–6%. The results are shown in Tables 11 and 12.
| Results, % (95% CI) | |
|---|---|
| Sensitivity | 22 (3 to 60) |
| Specificity | 87 (69 to 96) |
| PPV | 33 (5 to 77) |
| NPV | 79 (61 to 91) |
| Diagnostic accuracy | 72 |
| Results, % (95% CI) | |
|---|---|
| Sensitivity | 100 (19 to 100) |
| Specificity | 89 (75 to 97) |
| PPV | 33 (5 to 77) |
| NPV | 100 (89 to 100) |
| Diagnostic accuracy | 90 |
The authors commented, ‘In our experience, HbA1c did not constitute a sensitive and specific screening test for detection of patients with CF with glucose intolerance.’
The results were actually quite good, but with such small numbers, CIs were wide. There was no difference in HbA1c between patients with NGT and those with IGT.
De Schepper 1991
De Schepper et al. 184 from Brussels used HbA1c value of > 7.5% as the screening test in a group of 48 patients aged 2–28 years. All had a normal FPG (< 120 mg/dl) and were clinically stable (which we take to mean absence of acute lung infection). They had FOGTT (but not full reporting of the intermediate results), which was considered abnormal if the 2-hour PG was > 140 mg/dl (7.8 mmol/). This was seen in 15 of the 48 patients. HbA1c level was over 7.5% in 22 patients (46%). It was normal in four patients with glucose intolerance, and 11 patients with normal OGTTs had raised HbA1c level. The results are shown in Table 13.
| Results, % (95% CI) | |
|---|---|
| Sensitivity | 73 (45 to 92) |
| Specificity | 67 (48 to 82) |
| PPV | 50 (28 to 71) |
| NPV | 85 (65 to 96) |
| Diagnostic accuracy | 69 |
Lee 2007
Lee et al. 185 compared both the 50-g non-fasting GCT and HbA1c testing with the 75-g OGTT. Unfortunately, only just over half of those who had the GCT returned for the OGTT, and many did not do so within the intended 1-week period: the mean interval was 35 days but 61% returned within a week, and the mean is skewed by a 264-day outlier. The median was 7 days. The results are shown in Table 14.
| Results, % (95% CI) | ||
|---|---|---|
| 50-g GCT | HbA1c level > 6% | |
| Sensitivity | 100 (66 to 100) | 50 (23 to 77) |
| Specificity | 50 (28 to 72) | 90 (73 to 98) |
| PPV | 45 (23 to 68) | 70 (35 to 93) |
| NPV | 100 (71 to 100) | 79 (62 to 91) |
| Diagnostic accuracy | 65 | 77 |
The 50-g GCT had perfect sensitivity for diabetes and IGT. Most (six out of nine) patients had only IGT. The 11 patients who were OGTT normal but GCT abnormal had elevated 1-hour levels, which had returned to normal in the 2-hour OGTT. Abnormal was defined as PG level of > 7.8 mmol/l, but about half had results of > 11.0 mmol/l. Hence, the GCT appears to be useful for detecting PPH, which might cause alveolar fluid hyperglycaemia. Note also that the GCT was non-fasting, which could improve convenience. The authors conclude that the GCT is useful for reducing the number of OGTTs required, because none of the 35% of patients with normal GCTs had abnormal OGTTs.
It should be noted that information on postprandial glucose levels could also be obtained if the FOGTT was performed, but the GCT has the advantage of not requiring fasting.
Magni 1996
Magni,186 from Italy, compared levels of HbA1c, FPG and PG 2 hours after breakfast. They also used fructosamine and glycosuria tests but found those unhelpful. Glycosuria was present in only two patients, one of whom had a normal OGTT. The recruits comprised 65 inpatients, but all were free of respiratory exacerbations and none was on steroids. The reason for admission is not given, but the implication is that they were admitted for assessment or research purposes. The results are shown in Tables 15 and 16.
| Results, % (95% CI) | |||
|---|---|---|---|
| HbA1c > 5.1% | FPG > 85 mg | Two-hour PG post breakfast | |
| Sensitivity | 60 (36 to 81) | 70 (46 to 88) | 60 (36 to 81) |
| Specificity | 69 (53 to 82) | 64 (49 to 78) | 69 (53 to 82) |
| PPV | 46 (27 to 67) | 47 (28 to 66) | 46 (27 to 67) |
| NPV | 79 (64 to 91) | 83 (66 to 93) | 79 (64 to 91) |
| Diagnostic accuracy | 66 | 66 | 66 |
| Results, % (95% CI) | ||
|---|---|---|
| HbA1c > 5.3% | FPG > 88 mg | |
| Sensitivity | 100 (83 to 100) | 100 (83 to 100) |
| Specificity | 62 (47 to 76) | 56 (40 to 70) |
| PPV | 54 (37 to 71) | 50 (34 to 66) |
| NPV | 100 (88 to 100) | 100 (86 to 100) |
| Diagnostic accuracy | 74 | 69 |
The high sensitivities are not surprising in view of the low threshold because the thresholds were chosen to give complete capture, at a cost of poor specificity. Magni186 concluded that the ROGTT should be used as the screening test.
Moreau 2008
Moreau et al. ,187 from Strasbourg, compared the ROGTT with CGM in 49 patients. CGM involved 288 readings of tissue glucose per day. Four capillary BGs were required each day for calibration, so those could have been used as another screening option. However, no data were given in the paper.
For the OGTT, the standard WHO definitions were used to divide patients into NGT, IGT and diabetes groups. The CGM results were expressed in two main ways. The first was the presence of peaks of PG level of > 200 mg/ml (11.1 mmol/l). The second was quantitative: mean glucose value and AUC.
All patients with diabetes had peaks of > 200 mg/dl at least once after a meal, but so did 36% of patients in the NGT group and 52% in the IGT group. Results are shown in Table 17.
| Results, % (95% CI) | ||
|---|---|---|
| Diabetes mellitus alone | Diabetes mellitus + IGT | |
| Sensitivity | 100 (69 to 100) | 70 (50 to 86) |
| Specificity | 56 (40 to 72) | 64 (41 to 83) |
| PPV | 37 (19 to 57) | 70 (50 to 86) |
| NPV | 100 (84 to 100) | 64 (41 to 83) |
| Diagnostic accuracy | 65 | 67 |
The presence of the peaks in the NGT group may be due simply to some patients having PPH, and so rather than this causing a problem of false-positives it could be regarded as true-positives if it was decided that treatment was justified at that stage.
Mueller-Brandes 2005
In one of the largest studies, Mueller-Brandes et al. 205 used data from OGTTs in 1128 patients to assess the value of FPG alone. The FPG was at two levels, using the old and new ADA definitions for elevated glucose: ≥ 6.1 mmol/l and ≥ 5.6 mmol/l, respectively (Table 18). The authors’ main question was whether in patients with FPG levels of < 5.6 mmol/l, OGTTs were unnecessary. In effect, the reference standard was the 2-hour PG, not the whole OGTT.
| Test | Reference test | Sensitivity, % | Specificity, % |
|---|---|---|---|
| Old ADA criteria for IFG – according to Mueller-Brandes187 | Diabetes or IGT vs NGT | 65 | 94 |
| New ADA criteria for IFG – according to Mueller-Brandes187 | Diabetes or IGT vs NGT | 82 | 70 |
Sensitivity and specificity were reported but no CIs were given, and it was necessary to read some figures from the graph to construct a 2 × 2 table, so what follows may not be very precise (Table 19).
| Diabetes or IGT vs NGT: results, % (95% CI) | ||
|---|---|---|
| Old ADA criteria for IFG (according to our calculations reconstructing a 2 × 2 table) | New ADA criteria for IFG (according to our calculations reconstructing a 2 × 2 table | |
| Sensitivity | 65 (55 to 75) | 82 (73 to 89) |
| Specificity | 91 (89 to 93) | 68 (65 to 71) |
| PPV | 41 (34 to 49) | 20 (16 to 24) |
| NPV | 96 (95 to 97) | 97 (96 to 99) |
| Diagnostic accuracy | 89 | 69 |
So, using the new 5.6 mmol/l threshold improves sensitivity but reduces specificity. However, even using the new ADA threshold for IFG, 18% of patients with diabetic OGTTs would have been missed. The authors conclude that FPG is unsatisfactory for screening for CFRD.
Mueller-Brandes et al. 205 note that the OGTT is not a gold standard because of its poor reproducibility. They note the need for a confirmatory test but report that only 47% of those with a positive OGTT (34 out of 73 patients) had this confirmed by a repeat OGTT.
Robert 1992
Robert et al. ,188 from Paris, studied both FPG (> 6 mmol/l) and HbA1c (> 5.6%) levels as screening tests, with the FOGTT as the reference test, in a paediatric clinic. The mean age of the 49 patients was only 11 years, but the range was 2 to 21 years. The diagnosis of diabetes was based only on 2-hour levels of 11 mmol/l or above. Results are shown in Table 20.
| Results, % (95% CI) | ||
|---|---|---|
| HbA1c > 5.6% | FPG > 6 mmol/l | |
| Sensitivity | 63 (38 to 84) | 15 (3 to 38) |
| Specificity | 79 (59 to 92) | 97 (82 to 99) |
| PPV | 67 (41 to 87) | 75 (20 to 96) |
| NPV | 76 (56 to 90) | 62 (47 to 76) |
| Diagnostic accuracy | 72 | 63 |
Of 10 patients with glucose intolerance, seven were under the age of 10 years, with two aged 5 years.
Yung 1999
Yung et al. 189 investigated five screening tests and nine combinations of them, in 91 adult (> 16 years) patients attending the Royal Brompton Hospital CF clinic, London, UK, as shown in Table 21.
| Screening test | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) |
|---|---|---|
| RBG (> 11 mmol/l) | 33 (7 to 60) | 97 (94 to 100) |
| HbA1c (> 6.1%) | 83 (62 to 100) | 89 (82 to 96) |
| Symptoms: hyperglycaemia and/or unexplained weight loss | 58 (30 to 86) | 87 (80 to 95) |
| Glycosuria | 17 (0 to 38) | 97 (94 to 100) |
| Fasting BG (> 7.7 mmol/l) | 25 (1 to 50) | 100 |
|
HbA1c > 6.1%, glycosuria Symptoms: hyperglycaemia and/or unexplained weight loss, RBG > 11 mmol/l |
92 (76 to 100) | 79 (70 to 88) |
|
HbA1c > 6.1%, glycosuria Symptoms: hyperglycaemia and/or unexplained weight loss, RBG > 8.5 mmol/l |
92 (76 to 100) | 74 (65 to 84) |
|
HbA1c > 6.1%, glycosuria Symptoms: hyperglycaemia and/or unexplained weight loss, RBG > 6.0 mmol/l |
92 (76 to 100) | 65 (54 to 75) |
|
HbA1c> 6.1% Symptoms: hyperglycaemia and/or unexplained weight loss, RBG > 11.0 mmol/l |
92 (76 to 100) | 79 (70 to 88) |
|
HbA1c > 6.1% Symptoms: hyperglycaemia and/or unexplained weight loss, RBG > 8.5 mmol/l |
92 (76 to 100) | 75 (65 to 84) |
|
HbA1c > 6.1% Symptoms: hyperglycaemia and/or unexplained weight loss, RBG > 6.0 mmol/l |
92 (76 to 100) | 65 (54 to 75) |
| HbA1c > 6.1%, RBG > 11.0 mmol/l | 83 (62 to 100) | 86 (78 to 94) |
| HbA1c > 6.1%, RBG > 8.5 mmol/l | 83 (62 to 100) | 84 (75 to 92) |
| HbA1c > 6.1%, RBG > 6.0 mmol/l | 92 (76 to 100) | 70 (59 to 80) |
Based on the above findings, Yung et al. 189 advocated a selective approach to screening, but because of their fairly small numbers, with only 12 patients with diabetes, they advocated larger studies.
Other studies
A number of studies did not provide enough data for a 2 × 2 table but, nonetheless, provided some useful information.
Craigie et al. in the Royal Hospital for Sick Children in Glasgow have used BGP in children with CF, and have data (partly reported in conference abstract,75 partly unpublished) showing that home glucose profiling is more acceptable than the annual OGTT (so far, 100% acceptance of profiles vs 50% acceptance of OGTT) and had a number of advantages, including:
-
It reflects ‘real-life’ situations, such as activities and meals.
-
The technique is widely available and understood by all diabetes services.
-
It does not require hospital attendance once the technique is taught.
-
It is relatively inexpensive, for example compared with CGMSs.
-
It is readily accepted by patients.
-
It can be used to directly demonstrate the relationships between specific foods and BG.
-
It provides multiple readings over a 24-hour period.
But, there are also some disadvantages:
-
Waking is necessary to do overnight testing.
-
There is not the same 24-hour profile as obtained with CGMSs.
-
Capillary BG may be 10–15% higher than venous BG.
-
The expense of the meter and testing strips.
-
The need for repeated skin pricks.
One of the issues has been over the age at which to start screening. The Scottish Intercollegiate Guidelines Network (SIGN) guideline on diabetes (SIGN 116)190 recommends screening from the age of 10 years, as does the ISPAD guideline. 171 However, a study from Naples by De Simone et al. ,191 admittedly in only 22 patients, and available in abstract only, reported that 17% of patients below the age of 10 years had glucose intolerance. In a larger study, Ode et al. 173 from Minnesota reported that 39 of 94 children aged 6–9 years had abnormal glucose tolerance (defined as either IGT or INDET). None had diabetes, but during 5 years of follow-up, CFRD developed in 42% of those with abnormal glucose tolerance at baseline and 3% of those with NGT.
The study by Dobson192,193 was carried out in two stages. First, FOGTTs were undertaken in 20 patients (originally 21, but one dropped out because of venepuncture problems). Five had IGT and were excluded from the next stage, which was a comparison with CGMSs. So the remaining 15 subjects all had NGT. They also had an equal number of control subjects without CF.
HbA1c, FPG and 2-hour PG levels were similar among the patients with CF with NGT and the comparison group, but those with CF had higher 30-, 60- and 90-minute PGs. Their mean CGMS level was also higher, by 14%. Five of the CF group had peak CGMS readings of > 11.1 mmol/l, compared with one of the non-CF group.
The value of this study comes from the demonstration that the CGMSs can detect PPH, whereas HbA1c level and the OGTT do not. If we link that with the (admittedly scanty) evidence from the pilot of treating at the PPH stage,138 the message may be that either CGMSs or intermediate levels after an OGTT could be the best test if we are to treat at PPH stage.
Franzese et al. 194 also examined the use of CGMSs, this time in further investigation of PPH. Eighty-seven patients aged > 10 years had OGTTs, and 27 had at least one abnormal intermediate (30, 60 or 90 minutes, but details not given) level of > 7.6 mmol/l. Only this group, and five younger children who had experienced high glucose levels while on steroid treatment, had CGMSs. So, this was a study examining CGMSs only in subjects with previous PPH, rather than a screening study in a representative sample of patients with CF.
NGT, IGT and DM were defined by the 2-hour level of CGMS positivity by any value over a 72-hour period. The CGMS results classified more patients as having glucose intolerance than the ROGTT (Table 22).
| ROGTT | CGMS | |
|---|---|---|
| Diabetic | 7% | 20% |
| IGT | 10% | 8% |
| NGT | 15% | 4% |
However, it is likely that a FOGTT would have given similar results, as diabetes in the CGMSs is based on any one elevated glucose value over 72 hours. So this study does not show that CGMSs are superior to FOGTT.
Another small study of CGMS by Jefferies et al. ,195 from Toronto, used CGMSs in a group of 19 adolescents who had all had at least one previous BG level of > 7 mmol/l (not clear whether blood or plasma). All of seven patients who were diabetic on OGTT 2-hour level were also diabetic by the CGMSs (> 11.1 mmol/l). The results for IGT were unclear: two of the six patients who had IGT by OGTT had NGT by CGMSs, and three of the seven patients who had IGT by CGMSs had NGT on OGTT.
O’Riordan et al. ,196–198 from Dublin, in a series of abstracts with increasing numbers, compare CGMSs and OGTT (FOGTT, because there is mention of five time points). HbA1c level was also measured. They assert that neither HbA1c nor OGTT are sensitive and advocate the use of CGMSs, but give insufficient details for 2 × 2 tables.
Middleton and Bishop199 (abstract only), from Sydney, reported that 17 of about 25 patients with abnormal OGTT, had normal HbA1c levels. They also repeated OGTTs 1–2 years later and noted regression to NGT in some (numbers not given).
Solomon et al. ,200 from Toronto, also compared the results of the ROGTT with HbA1c and FPG in 10- to 18-year-olds, finding both insensitive. Of those with normal FPG levels, 17% had IGT, and 4% had CFRD. All of those with CFRD had pancreatic insufficiency, and there was an association with more severe classes of mutations. However, as the authors say, there is as yet no evidence that specific mutations predict CFRD. ΔF508 has been incriminated.
Thorsteinsson et al. 78 (abstract only) provided insufficient data for assessing screening tests, but reported some useful natural history. The authors’ key points were:
-
At diagnosis of diabetes mellitus by annual OGTTs, FPG and HbA1c levels were raised in only 16% and 16%.
-
Presence of IGT increased risk of later diabetes (odds ratio 5.6).
-
But in 58% of IGTs, next OGTT was normal, so OGTT is far from a gold standard.
The debate on the use of glycated haemoglobin
Iron deficiency is common in CF, and may be associated with higher HbA1c levels in people with T1DM. 176 (Conversely, increased red cell turnover may be associated with reduced HbA1c level, and if present in CF could give a misleading indicator of glycaemia control.)
A small study from Texas by Hardin et al. 201 (abstract only) divided nine patients with CF and previously detected IGT into those with good pulmonary function (FEV1 and FVC 82–92% predicted) and those with poor (FEV1 and FVC 32–48% predicted). Red blood cell turnover was faster in those with poor function, which led them to conclude that HbA1c level was not suitable as a screening test for CFRD.
Brennan et al. 175 state that only about 10% of HbA1c comes from red blood cells surviving 80–120 days, suggesting that glycation is not linear, and that increased turnover would not necessarily affect the usefulness of HbA1c.
Allen202 notes the poor PPV of HbA1c (as reported by Lanng et al. 33), advocating caution in its use as a screening tool in CF, and calling for a large trial.
Garagorri et al. ,203 from Zaragoza in Spain, screened 28 patients with CF using HbA1c and FOGTTs. The authors say that the results of the OGTT were classified as per the WHO criteria. In total, 12 or 13 (the numbers are not entirely clear) had IGT or diabetes. HbA1c level was no different between the groups, suggesting that it was not sensitive enough to use as a screening test for IGT.
Holl et al. 182 (letter only), from Hannover, also advised against the use of HbA1c level as a screening test, reporting a sensitivity of only 31% in 13 patients diagnosed with CFRD on the basis of 2-hour PG level of > 200 mg/dl. Insufficient data were given to derive sensitivity.
Monitoring of glycaemic control in existing cystic fibrosis-related diabetes
Al-Aloul et al. 204 (abstract only) examined the relative value of preprandial and postprandial PG in patients with known CFRD, being considered for insulin treatment. It is not clear how many patients their results were based on – the abstract says initially 11 but then mentions details for six. The main conclusion was that neither FPG nor HbA1c level was abnormal in most patients, but that the postprandial level usually was. No details are given of how the CFRD was diagnosed.
Brennan et al. 175,176 set out to assess the value of HbA1c level in monitoring diabetic control in CFRD, and to compare its usefulness with monitoring in T1DM. They used CGMSs to determine mean PG. They compared the results in 20 people with CF, 10 of who had CFRD, with previous results from people with T1DM. They did not assess the value of HbA1c in screening for or diagnosis of CFRD.
They concluded that HbA1c level was a reliable guide to glycaemia in CFRD, the relationship between HbA1c level and mean BG level being similar to that in T1DM.
Discussion
Is there an identifiable subgroup in which screening is not required? Is it possible to say that if people with CF do not have hyperglycaemia by, say, the age of 30 years, they will never get it? That implies that pancreatic damage ceases to progress. This is probably unlikely but there are clearly some people in whom CF is much less serious, although that might just mean they get complications such as diabetes much later in life?
The screening parameter relevant to this (hypothetical) subgroup would be NPV, which can be used to ‘rule out’ conditions.
Would combinations of tests give better results? Or provide a more cost-effective strategy, for example if a simple test could reduce the need for OGTTs in some patients, with only those with intermediate results going on to OGTTs.
One issue to be considered is acceptability. A strategy that is 90% sensitive and 90% specific but has 50% compliance, would detect 90 × 50 = 45% of true-positives. Specificity will always be 100% for the non-compliant (those who do not take the test can never be false-positives) so specificity will be 95%. If the most accurate test has lower acceptability to patients, other less sensitive tests with better compliance might in practice detect more cases.
Conclusion
There is good evidence on tests that appear unsatisfactory, including HbA1c and FPG levels. There is less evidence on CGMSs, but it appears useful and may be especially so for detecting hyperglycaemia, which happens only at certain times of day. However, the diagnosis of diabetes is not based on elevations during CGM.
There is very little evidence on the 50-g GCT, but it may be the best test if the aim is to detect PPH. It can be given to non-fasting patients. However, as one of the (anonymous) referees pointed out, if the FOGTT is carried out, the intermediate values such as the 1-hour PG will also identify patients with PPH, and would have the advantage of linkage with the fasting and 2-hour values.
Meanwhile, guidelines continue to recommend screening for CFRD using the 75-g OGTT. 172
Table 23 provides a summary of CFRD diagnostics studies.
| Study | Screening test | OGTT reference test cut-offs | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) |
|---|---|---|---|---|
| Buck 2000, Germany174 | HbA1c > 5.7% (Ulm) or > 5.0% Hannover | Diabetes + IGT vs NGT | 22.9 (10.5 to 40.0) | 95.5 (87.5 to 99) |
| Diabetes vs IGT + NGT | 23.1 (5.3 to 53.8) | 91.0 (83.1 to 96.0) | ||
| De Luca 1991, Italy183 | HbA1c > 6% | Diabetes + IGT vs NGT | 100 (19.3 to 100) | 89 (74.6 to 96.9) |
| HbA1c > 6% | Diabetes vs IGT+ NGT | 22.2 (3.5 to 59.9) | 86.7 (69.3 to 96.2) | |
| De Schepper 1991, Belgium184 | HbA1c > 7.5% | Diabetes + IGT vs NGT | 73.3 (44.9 to 92.1) | 66.7 (48.2 to 82.0) |
| Lee 2007, Canada185 | GCT > 7.8 mmol/l | OGTT ≥ 7.8 mmol/l (diabetes + IGT vs NGT) | 100 (66.2 to 100) | 50 (28.3 to 71.8) |
| HbA1c > 6.0% | OGTT ≥ 7.8 mmol/l (diabetes + IGT vs NGT) | 50 (23.1 to 76.9) | 89.7 (72.6 to 97.7) | |
| Magni 1996, Italy186 | HbA1c > 5.1% | Diabetes + IGT vs NGT | 60 (36.1 to 80.8) | 68.9 (53.54 to 81.8) |
| Fasting glycaemia > 85 mg% | Diabetes + IGT vs NGT | 70 (45.7 to 88.0) | 64.4 (48.8 to 78.1) | |
| 120 minutes after meal glycaemia > 84 mg% | Diabetes + IGT vs NGT | 60 (36.1 to 80.8) | 68.9 (53.4 to 81.8) | |
| HbA1c > 5.3% | Diabetes vs IGT + NGT | 100 (83.0 to 100) | 62.2 (46.5 to 76.2) | |
| Fasting glycaemia > 88 mg% | Diabetes vs IGT + NGT | 100 (83.0 to 100) | 55.6 (40.0 to 70.4) | |
| Moreau 2008, France187 | CGMS | Diabetes vs IGT + NGT | 100 (69.0 to 100) | 56.4 (39.6 to 72.2) |
| Diabetes + IGT vs NGT | 70.4 (49.8 to 86.2) | 63.6 (40.7 to 82.8) | ||
| Mueller-Brandes 2005, Germany205 | Old ADA criteria for IFG – according to Mueller-Brandes | Diabetes + IGT vs NGT | 65 | 94 |
| Old ADA criteria for IFG – according to our calculations reconstructing a 2 × 2 table | Diabetes + IGT vs NGT | 65.3 (55.2 to 74.6) | 90.9 (88.9 to 92.6) | |
| New ADA criteria for IFG – according to Mueller-Brandes | Diabetes + IGT vs NGT | 82 | 70 | |
| New ADA criteria for IFG – according to our calculations reconstructing a 2 × 2 table | Diabetes + IGT vs NGT | 82 (73.3 to 89.1) | 68 (65.0 to 70.8) | |
| Robert 1992, France188 | Fasting glycaemia, WHO criteria (> 6 mmol/l) | Diabetes + IGT vs NGT | 15 (3.4 to 37.9) | 96.6 (82.1 to 99.4) |
| HbA1c% > 5.6% | Diabetes + IGT vs NGT | 63.1 (38.4 to 83.7) | 78.6 (59.0 to 91.7) | |
| Yung 1999, UK189 | Random BG (> 11 mmol/l) | Diabetes vs IGT + NGT | 33 (7 to 60) | 97 (94 to 100) |
| HbA1c (> 6.1%) | Diabetes vs IGT + NGT | 83 (62 to 100) | 89 (82 to 96) | |
| Symptoms: hyperglycaemia and/or unexplained weight loss | Diabetes vs IGT + NGT | 58 (30 to 86) | 87 (80 to 95) | |
| Glycosuria | Diabetes vs IGT + NGT | 17 (0 to 38) | 97 (94 to 100) | |
| Fasting BG (> 7.7 mmol/l) | Diabetes vs IGT + NGT | 25 (1 to 50) | 100 | |
|
HbA1c > 6.1%, glycosuria Symptoms: hyperglycaemia and/or unexplained weight loss, RBG > 11 mmol/l |
Diabetes vs IGT + NGT | 92 (76 to 100) | 79 (70 to 88) | |
|
HbA1c > 6.1%, glycosuria Symptoms: hyperglycaemia and/or unexplained weight loss, RBG > 8.5 mmol/l |
Diabetes vs IGT + NGT | 92 (76 to 100) | 74 (65 to 84) | |
|
HbA1c > 6.1%, glycosuria Symptoms: hyperglycaemia and/or unexplained weight loss, RBG > 6.0 mmol/l |
Diabetes vs IGT + NGT | 92 (76 to 100) | 65 (54 to 75) | |
|
HbA1c > 6.1% Symptoms: hyperglycaemia and/or unexplained weight loss, RBG > 11.0 mmol/l |
Diabetes vs IGT + NGT | 92 (76 to 100) | 79 (70 to 88) | |
|
HbA1c > 6.1% Symptoms: hyperglycaemia and/or unexplained weight loss, RBG > 8.5 mmol/l |
Diabetes vs IGT + NGT | 92 (76 to 100) | 75 (65 to 84) | |
|
HbA1c > 6.1% Symptoms: hyperglycaemia and/or unexplained weight loss, RBG > 6.0 mmol/l |
Diabetes vs IGT + NGT | 92 (76 to 100) | 65 (54 to 75) | |
| HbA1c > 6.1%, RBG > 11.0 mmol/l | Diabetes vs IGT + NGT | 83 (62 to 100) | 86 (78 to 94) | |
| HbA1c > 6.1%, RBG > 8.5 mmol/l | Diabetes vs IGT + NGT | 83 (62 to 100) | 84 (75 to 92) | |
| HbA1c > 6.1%, RBG > 6.0 mmol/l | Diabetes vs IGT + NGT | 92 (76 to 100) | 70 (59 to 80) |
A table of excluded studies, with reasons for exclusion, is shown in Appendix 5.
Chapter 5 Health economics
Cost-effectiveness analysis is not possible at present due to lack of data, and so the purpose of this chapter is to consider modelling approaches, and to identify the data required.
Modelling approach
The model might follow 1000 children with CF, initially aged 10 years. This is based on guideline recommendations.
Arm 1 – Natural history/no screening
The base arm would be ‘natural history arm’ or NHA. There would be no screening, so there would be three groups of patients:
-
those who do not develop diabetes
-
those who do become diabetic but never have symptoms and are never diagnosed, so die earlier than they would have done had they been diagnosed and treated; note that some might die from unrelated causes and would not benefit from screening
-
those who do develop symptoms, are diagnosed and treated, and live longer because of that.
The data required to populate Arm 1 are:
-
1a What proportions of patients with CF develop diabetes at each age? Given that the natural history may be poorer in female patients, modelling should be done separately by gender.
-
1b How many would be diagnosed because of symptoms? And hence how many would never be diagnosed without screening?
-
1c How much longer do people with CFRD live once the diabetes is treated after diagnosis by symptoms? The fact that people with CFRD have shorter lifespans than those without may not be entirely due to the diabetes. It may be that more severe CF leads not only to diabetes, but also shortens life in other ways.
-
1d What is the best treatment? Because the cause of the diabetes is loss of β-cells in the pancreas, and insulin sensitivity is normal, insulin is the standard treatment. But does that mean basal insulin, or mealtime boluses, or both, or CSII? The extra cost of CSII may be justified by improvements in QoL.
Figure 3 shows the outline of the model. The model shows the course the disease would take if a person were left untreated, unless diagnosed later owing to presenting symptoms. Without screening, people with CF could either become hyperglycaemic with symptoms and be diagnosed, could become hyperglycaemic without symptoms and remain undiagnosed, or could remain free of hyperglycaemia for the rest of their lives. There are different levels of hyperglycaemia:
-
PPH – for the purposes of this review, we define this as hyperglycaemia after meals, at 30, 60 or 90 minutes, but where BG level is normal by 2 hours: PG level of > 11.0 mmol/l at 30, 60 and 90 minutes, but is < 7.8 mmol/l by 2 hours.
-
IGT, where hyperglycaemia after meals has not returned to normal: FPG level of < 7.0 mmol/l and 2-hour PG level of ≥ 7.8 and < 11.1 mmol/l (WHO definition).
-
Diabetes mellitus.
FIGURE 3.
No-screening model.
In both of the first two levels, we assume that some would progress to the next level and others would not. Thus, the ones who develop IGT and are undiagnosed may either become diabetic or they may live with IGT until they die. Some of those patients that become diabetic will show symptoms and some will not. Those that do not show symptoms may live with diabetes, undiagnosed and untreated, till they die. Those that do show symptoms will be treated until they die.
Screening
Then we would have some screening arms. In each of these arms, we would need to model both longevity and QoL, to derive quality-adjusted life-years (QALYs).
Arm 2
Arm 2 would be the current screening default, the OGTT. This is usually only FPG and 2-hour PG, rather than the FOGTT. The baseline would be annual screening from the age of 10 years, but different thresholds could be examined. The key question might be when the benefits of treating detecting and treating those with diabetes are enough to justify the costs of screening, both in terms of monetary cost and inconvenience to those who do not have diabetes. Screening itself would have a disutility though this is transient.
Data required:
-
2a How much longer do patients with diabetes live when it is detected by screening? Life-years gained.
-
2b How good would their QoL in the added years be?
-
2c Hence QALYs gained. Would some patients not live longer, but have better QoL after diabetes was treated? Some QALYs might be gained from QoL alone?
-
2d What is cost of screening all patients once a year with OGTT? The cost will decline each year because those patients with diabetes will not need screened next year.
-
2e What is the sensitivity of the test – would OGTT miss some patients? Although if screening is annual, they might only be missed for 1 year.
-
2f What is the specificity of the test – would some patients be wrongly diagnosed with diabetes and treated inappropriately? (They would then probably get hypoglycaemia and be rapidly recognised as wrongly diagnosed, and have treatment stopped, so no long-term harm?)
-
2g So far, we have not taken compliance into account. OGTTs are not popular, so not all patients would attend. Modelling has to take that into account, by adding a ‘screening-declined’ arm. It would start by assuming that those who decline screening have same outcomes as the NHA 1, but in practice, people who decline screening may have other health behaviours that make their outcomes poorer, so that might need a sensitivity analysis. So the screening arms all have two branches – those who accept and those who decline. The screening declined branch does not incur screening costs.
Compliance is important. A less sensitive but more acceptable test may result in more cases of CFRD being diagnosed. Oversimplifying:
-
OGTT 100% sensitive, but 50% acceptance identifies 50% of CFRD
-
HbA1c 80% sensitive, but 80% acceptance identifies 64% of CFRD.
We could then look at costs and benefits and consider whether or not screening with annual OGTTs is cost-effective.
Figure 4 shows the outline for modelling the screening arms.
FIGURE 4.
Screening arms.
Screening model
In this part of the model, it is assumed that patients with CF are offered screening, although they may not all accept. The acceptance rate may vary among the screening tests. Based on the evidence in earlier chapters, it is assumed that those patients who do not develop diabetes live longer than those who do. In addition, it is possible that patients who never develop PPH live longer than those who do, who live longer than those that develop IGT, who live longer than patients that develop diabetes. It is assumed that progression to diabetes is through PPH then IGT then diabetes (initially without FH and later with). It is assumed that screening and rescreening will take place every year from the 10th birthday onwards. With more data it may be possible to identify some patients whose risk is lower and who may be screened less often.
At the start of the model, screening is offered from 10 years of age. People who have a negative result are offered rescreening each year. Those who declined in the past are also offered screening. The negative results may contain both true- and false-negatives. From this screening point, people may have hyperglycaemia, of varying degrees, or they may not. If they never develop hyperglycaemia, when they die, it will be with CF alone.
If they do develop hyperglycaemia, they are treated. For simplicity, it is assumed that once a hyperglycaemic state is reached, regression to normoglycaemia does not occur, except for when transient hyperglycaemia is seen during acute infective exacerbations. As with the previous case, if the patients with PPH test negative for an IGT screen then they are rescreened the following year. If these patients remain stable and do not regress or progress, when they die, it will be with CF and PPH.
If the patients do develop IGT then they would be treated, if considered necessary. The key missing data here are whether or not earlier treatment (i.e. before diabetes has developed) is beneficial, as discussed in Chapters 2 and 3. The patients are monitored for progression rather than screened; they may remain stable with IGT, or they may progress and develop diabetes. Again, as with the previous case, if the patients test negative for diabetes, they are rechecked again the following year. If these patients remain stable and do not regress or progress, when they die, it will be with CF and IGT. If the patients that have tested negative are in fact false-negatives and if they were not screened again, or do not accept screening, they may at some stage develop symptoms and will be diagnosed and treated, or they may remain asymptomatic, undiagnosed, yet suffering harm. If the patients that have tested positive are true-positives and have developed diabetes, they are treated with insulin. These patients with CFRD do not regress and they do not progress to any other stage.
Other screening tests
We know that OGTTs are unpopular. Other screening options worthy of trialling are the 50-g non-fasting GCT (with dose adjusted for age or body weight) and CGM, so there should be an arm for each of those:
-
Arm 3 CGM
-
Arm 4 GCT
-
Arm 5 serial glucose profile undertaken at home: say 6–8 per day for 2 days.
Data requirements would be similar to the OGTT arm. All screen-positive patients would require a confirmatory second test, as asymptomatic diabetes should not be diagnosed on one abnormal glucose result.
It might also be worth having combination testing; for example, a first stage screen to reduce the number requiring OGTT.
What are we screening for?
In the present state of knowledge, it appears that screening for both CFRD and IGT would be worthwhile. We can hypothesise, based on Chapter 2, that it might be worth intervening as soon as patients start having episodes when glucose exceeds 8 mmol/l, but we have few data to support that at present.
Hence, the most important screening question at present is what we should be screening for.
Other problems with modelling
Survival with cystic fibrosis
Survival has been improving over recent decades, and we do not know how long those currently in the screening age band (assumed to be 10–30 years) will live for. We could use recent estimates from CF registries, and check these against the trends over time data from Dodge et al. ,19 by extrapolating from the current survival lines for those diagnosed in recent decades.
Most papers on survival give mean age at death, but have a mixture of ages. We need data by decade of birth in order to estimate further improvements in survival.
The effect of cystic fibrosis-related diabetes
The figure of 11 years as being the loss of life-years owing to CFRD, based on the data from Milla et al. ,57 could be used as the default, with other figures used in sensitivity analyses. However, we need three figures for loss of years:
-
those with diabetes detected by screening and treated at early stage
-
those with diabetes diagnosed and treated when they developed symptoms
-
those with undiagnosed diabetes – by definition data will not be available, but the 11-year figure could be used; however, this may be an overestimate, as the absence of symptoms may imply lower glucose levels.
Koch et al. 14 found that patients with CFRD have a median survival age of 24 years compared with 34 years in non-diabetic control subjects with CF.
Several groups have reported a decline in clinical status occurs in patients with CF-related hyperglycaemia before the diagnosis of CFRD is made. 15,54,122,146,206–208 This may occur for several years before the diagnosis is made.
Overall annual incidence of cystic fibrosis-related diabetes
The overall annual incidence of CFRD was 3.5%209 but it will vary with age and one issue is when the incidence plateaus sufficiently for screening to stop, if indeed it does.
Quality-of-life studies in cystic fibrosis and cystic fibrosis-related diabetes
Health-related QoL has been described as ‘a multi-dimensional construct comprised of several domains as reported by the patient (e.g. physical, social and psychological functioning, respiratory symptoms, treatment burden and body image)’. 210
To populate a model of CF and CFRD that allows us to evaluate the clinical effectiveness and cost-effectiveness of screening for CFRD, we ideally need:
-
Data on QoL over the lifetime of CF in patients who do not develop CFRD. We would expect a decline in QoL over the decades.
-
Similar data on those who develop CFRD but in whom it is not diagnosed, in whom we might expect a steeper decline in QoL.
-
Data on the QoL in those who are diagnosed and treated after developing symptoms. We might expect a diminution in QoL after onset but an improvement after treatment.
-
Similar data on those in whom CFRD was detected by screening. We might expect much less, or no, diminution in QoL before diagnosis, because the onset might be insidious, and by definition they would have no or few symptoms. However, they might feel better after starting treatment.
-
To assess the effect of treatment with insulin in patients with CFRD, and with lesser degrees of hyperglycaemia. For example, given that there is evidence that suggests that treatment with insulin may be of benefit at the IGT stage, we need to be able to quantify the effects on QoL.
One expectation might be that the development of CFRD would reduce the QoL, partly owing to symptoms or impaired performance, partly owing to the need for yet another treatment. A second might be that, in terms of QoL, the net reduction would be less in those detected early by screening, unless of course the disutility from insulin treatment was greater than the benefit from it, given that they are symptom free. The disutility will include that from injections, hypoglycaemic episodes and self-testing of BG level.
Ideally, we would have such data also for those with IGT, who would not include all the groups above, having no diagnosis via diabetes symptoms. However, they may still get benefit from treatment with insulin, and again there would be a trade-off between feeling better and the disutilities of insulin treatment.
We need both a sensitive measure of QoL that could pick up changes of value to people with CFRD, but also a generic measure of QoL from which we can derive a utility score for cost-effectiveness estimations.
We also need a measure that takes account of the fact that people with respiratory impairment may adjust their lifestyles accordingly.
Quittner211 reviewed the available instruments in 1998, dividing them into three main types:
-
Utility measures that provide a single value, with ‘1’ representing perfect health and ‘0’ representing death. They are used to generate QALYs and hence cost per QALY for assessing the cost-effectiveness of different treatments. Examples include the EQ-5D.
-
Health profiles, which generate scores for a number of domains of everyday living, such as energy, emotional state, physical functioning, etc. They can be applied to any disease state and hence may not be sensitive enough to detect disease-specific changes. Examples include the Short Form questionnaire-36 items (SF-36).
-
Disease-specific measures, designed to capture information on the symptoms and areas of functioning associated with specific diseases. They may therefore be more sensitive than health profiles, but cannot be converted to a generic utility measure. The main one discussed by Quittner is the Cystic Fibrosis Questionnaire (CFQ).
The CFQ consists of a suite of age-banded questionnaires, which include five generic domains (physical symptoms, role functioning, psychological/emotional functioning, energy and social functioning).
Studies with data on cystic fibrosis-related diabetes
Tierney et al. 212 in Manchester compared QoL and experiences with hypoglycaemia in people with CFRD (treated with insulin) and T1DM. They noted that while there are studies in CF, there is a lack of studies in CFRD. Questionnaires were sent to 295 T1DM and 145 patients with CFRD. Instruments used included the Edinburgh Hypoglycaemia Scale (EHS) and the Diabetes Quality-of-Life (DQoL) measure. They noted that the DQoL had not been validated in CFRD.
Clinical data on HbA1c level, BMI and lung function (for patients with CFRD) were obtained from case notes.
The response rates were low: 52 (36%) patients with CFRD and 60 (20%) with T1DM. Of these, 20 patients with CFRD and 43 patients with T1DM completed diaries for hypoglycaemic episodes, giving return rates from the whole populations of 14% and 15%. The mean CFRD age was 30 years, and about half had CRFD for over 6 years.
Almost all patients had experienced at least one hypoglycaemic episode, but only 20% of the CFRD group had experienced hypoglycaemia with loss of consciousness, compared with 40% of the T1DM group. There was not much difference in hypoglycaemic episode symptoms, but the T1DM group reported slightly more neuroglycopenic symptoms.
Quality of life was better for the CFRD group than for the T1DM group: DQoL score 74 versus 66, respectively (a lower score is worse). This may relate to the hypoglycaemic episode scores on the EHS, which correlate with DQoL.
Reduced pulmonary function (FEV1) correlated negatively with DQoL.
Overall, the findings suggest that diabetes has less of a negative effect on QoL in CFRD than in T1DM, but the low response rates and inevitable bias should be taken into account. In addition, the authors suggest that, to people with CF, CFRD is just one more life-diminishing factor, and they retain some β-cell function, unlike those with T1DM. The CFRD group had fewer problems with hypoglycaemia than the T1DM group. They were less worried about the long-term complications of diabetes, perhaps because they had too many other problems to worry about, and perhaps because long-term diabetic complications have been less of a problem in CFRD and so receive less attention in clinics. This will change with increasing longevity.
There are around 20 studies of QoL in CF which do not mention CFRD. Brief details are given in Appendix 6. They fall into two main groups. First, there are those that use tools specific to CF, including the CFQ (five studies) and the CFQoL (one study). Second, there are those that use generic instruments, including:
-
Child Health Questionnaire (CHQ) (five studies)
-
Nottingham Health Profile (one study)
-
Quality of Well-Being Questionnaire (three studies)
-
EQ-5D (one study)
-
Sickness Impact Profile (one study)
-
Chronic Respiratory Disease Questionnaire (one study)
-
Questions of Life Satisfaction Questionnaire (two studies)
-
SF-36 (one study).
Chapter 6 Discussion
Statement of principal findings
‘In slightly less than 70 years, cystic fibrosis has moved from a little known genetic condition, usually fatal in infancy and early childhood, to a complex multisystem disorder which now affects as many adults as children’ (J Littlewood, Cystic Fibrosis Trust, 2007, personal communication; this quotation was formerly on the Cystic Fibrosis Trust website).
-
CFRD is a common complication of CF. The proportion of people with CF who have CFRD increases with age, and because survival in CF has improved markedly over time, the prevalence of CFRD has increased.
-
Diabetes has been defined by WHO and other bodies based on the level of BG above which the risk of diabetic retinopathy occurs. However, in CF, the key organ is the lung and we should define CFRD, or CF-related hyperglycaemia, according to when harm to the lungs takes place.
-
This harm could take at least three forms: stiffening of the lungs, increasing the work of breathing; impaired gas diffusion; and promotion of microbial colonisation and infections.
-
Harm appears to occur at BG levels well below the threshold for the usual definition of diabetes, probably around 8 mmol/l.
-
The implication is that we should be screening for IGT (2-hour OGTT > 7.8 mmol/l) and intervening at that stage.
-
The only recommended treatment for controlling hyperglycaemia is insulin.
-
The current recommendation for screening test is for annual OGTTs from the age of 10 or 12 years. The OGTT is far from being a gold standard, it is time-consuming and not popular with patients, and, in practice, is often not undertaken.
-
Most of the evidence on simpler tests is on FPG and HbA1c levels. Neither appears sensitive enough.
-
There is some evidence that CGMSs and serial profiles may be more useful.
-
There is very little evidence on the 50-g GCT, but it appears promising, and worthy of further research.
How sensitive do we need screening to be?
It looks as if the most sensitive test of hyperglycaemia in CF may be the immediate (about 60-minute) postprandial PG. However, there is no evidence (or very little – just the four-patient Exeter study138) that treatment at that stage is beneficial.
So we would not currently start treatment until the IGT stage, except in trials. This suggests that screening should be for IGT, and that the added sensitivity of the 1-hour glucose is unnecessary – we should be looking for more prolonged elevation. However, trials of treatment at the PPH stage appear worthwhile, with a key outcome being microbial colonisation of the lung.
Question: What time of day to test?
The usual approach to the OGTT is an overnight fast then morning testing. That may be less reliable in CF because:
-
Fasting may be a problem in people who are otherwise encouraged to eat regularly and who have difficulty ensuring adequate calorie intake on many days.
-
β-Cell function may be better in the morning and wane as the day goes on.
-
Patients may take their largest meal in the evening, so that may be when sustained hyperglycaemia is most likely. If we want to go for evening glucose levels, the GCT might be an option because it does not require fasting.
-
Patients may be relatively anorexic in the morning.
Question: Does isolated postprandial hyperglycaemia do harm?
It might do harm in two ways: first, the usual hyperglycaemic harm by structural means, such as on the alveolar basement membrane, which would be proportional to both height and duration of elevation; but, second, by increasing the risk of infection/colonisation in the lungs. Does short-duration PPH increase the risk of colonisation? Most patients with CF with elevated glucoses on serial profiles (none yet diabetic) have Pseudomonas colonisation (Craigie, Royal Hospital for Sick Children, Glasgow, 2006; unpublished Glasgow data).
If isolated PPH leads to pulmonopathy, then the aim of treatment would be to try to avoid PG going above 8 mmol/l or at least to minimise the time periods when it exceeds that level. For detecting such elevations, the CGMSs may be much more effective than occasional OGTTs. Hameed et al. 213 compared OGTT and CGMSs in a group of children and related PG levels to weight, FEV1 and FVC. They found that CGM time > 7.8 mmol/l for 4.5% or more of the day detected declining WtSDS with 89% sensitivity and 70% specificity, and was a better predictor than the 2-hour OGTT level, partly because in most patients the peak PG level occurred long before the 2-hour time. They concluded that elevated 2-hour PG was a late event. This paper provides further support for the hypothesis that the critical feature in CF-related hyperglycaemia is progressive insulin deficiency that manifests itself first as weight loss and impaired lung function, well before the ROGTT is abnormal. This hyperglycaemia may be episodic, may appear only at certain times of day, such as the evening, and may best be detected by CGM.
How would we treat isolated PPH? Options include low-dose, prandial short-acting analogues or a simpler regimen of once-daily premixed, such as Mixtard (before the evening meal, especially in those having enteral feeding overnight). The idea of giving a once-daily, long-acting basal insulin to ‘rest’ the pancreas is probably illogical because the first insulin secretion problem is loss of first-phase response due to pancreatic unresponsiveness, which would not be affected by resting.
Question: At what age should screening start?
Most guidelines recommend that screening should start at the age of 10 years. However, a recent study reported that 17% of children of < 10 years had abnormal glucose levels, although only two had diabetes as defined by WHO. 190
Question: Might there be an age at which screening could be reduced?
Would it be safe to reduce the frequency of screening if people have not developed diabetes by, say, the age of 25 years? It may be that patients with stable lung function and weight do not need annual screening, but that an assessment of glucose metabolism should be considered in any patient in whom there is clinical deterioration.
Question: Does screening for cystic fibrosis-related diabetes and impaired glucose tolerance meet the criteria of the National Screening Committee?
Screening for CFRD does not fall within the remit of the NSC, but the criteria may provide a useful framework. 214
1. The condition should be an important health problem.
Cystic fibrosis-related diabetes is important for two main reasons. First, it has become more common owing to improved survival in people with CF – more are living long enough to develop CFRD. Second, it reduces survival – people with CFRD do not live as long as those with CF alone.
2. The epidemiology and natural history of the condition, including development from latent to declared disease, should be adequately understood and there should be a detectable risk factor, disease marker, latent period or early symptomatic stage.
In the early stages, CFRD is asymptomatic even although BG level is rising high enough to cause damage, especially to the lungs. As explained earlier in this report, the definition of CFRD may need to be different from that for T1DM and T2DM.
3. All the cost-effective primary prevention interventions should have been implemented as far as practicable.
There are no known ways of preventing CFRD, which occurs because of progressive pancreatic damage.
4. If the carriers of a mutation are identified as a result of screening the natural history of people with this status should be understood, including the psychological implications.
Not applicable.
5. There should be a simple, safe, precise and validated screening test.
There are simple, safe and precise tests for measuring BG levels.
6. The distribution of test values in the target population should be known and a suitable cut-off level defined and agreed.
The distribution of BG in the CF population is well known. However, the optimum cut-off is not known. As discussed earlier, it may be that the definition of CFRD should be based on when pulmonopathy first starts, and on when treatment with insulin is worthwhile.
7. The test should be acceptable to the population.
The evidence on this is mixed. HbA1c, as a simple non-fasting blood test, is likely to be acceptable. Glucose profiles and CGMSs appear to be acceptable in research studies. The OGTT does not appear popular with patients, but we are aware that some clinics have a full day of annual assessment with the OGTT part of this. However, other evidence suggests that compliance with the annual OGTT is low.
8. There should be an agreed policy on the further diagnostic investigation of individuals with a positive test result and on the choices available to those individuals.
The current consensus is that if a simple screening test is positive, patients should have an OGTT, although this is sometimes the full version with five measurements (0, 30, 60, 90 and 120 minutes) and at other times is the reduced version.
9. If the test is for mutations, the criteria used to select the subset of mutations to be covered by screening, if all possible mutations are not being tested, should be clearly set out.
Not applicable.
10. There should be an effective treatment or intervention for patients identified through early detection, with evidence of early treatment leading to better outcomes than late treatment.
There is an effective treatment, and some evidence that earlier treatment improves outcomes. However. there is uncertainty about how early it should be (see Research needs).
11. There should be agreed evidence-based policies covering which individuals should be offered treatment and the appropriate treatment to be offered.
At present, there is agreement that patients should be treated at the stage of CFRD without FH. We do not know whether or not insulin treatment at earlier stages would be worthwhile.
12. Clinical management of the condition and patient outcomes should be optimised in all health-care providers prior to participation in a screening programme.
We do not have full details on clinical management but in the UK there are national guidelines on the management of CF, which will be updated in the near future.
13. There should be evidence from high-quality RCTs that the screening programme is effective in reducing mortality or morbidity. Where screening is aimed solely at providing information to allow the person being screened to make an ‘informed choice’ (e.g. Down syndrome, CF carrier screening), there must be evidence from high-quality trials that the test accurately measures risk. The information that is provided about the test and its outcome must be of value and readily understood by the individual being screened.
Not yet met because there are no RCTs of screening versus no screening. However, the main need is for a RCT of treatment at different stages, without which we are uncertain what we should be screening for – CFRD, IGT or PPH.
14. There should be evidence that the complete screening programme (test, diagnostic procedures, treatment/intervention) is clinically, socially and ethically acceptable to health professionals and the public.
There is a lack of evidence on these aspects, but we have no reason to doubt acceptability.
15. The benefit from the screening programme should outweigh the physical and psychological harm (caused by the test, diagnostic procedures and treatment).
We believe this criterion to be met. There are no significant harms of screening for hyperglycaemia, and the benefits of treatment are known, although, as stated above, the benefits may be applicable to a wider group.
16. The opportunity cost of the screening programme (including testing, diagnosis and treatment, administration, training and quality assurance) should be economically balanced in relation to expenditure on medical care as a whole (i.e. value for money). Assessment against these criteria should have regard to evidence from cost–benefit and/or cost-effectiveness analyses and have regard to the effective use of available resource.
There is a lack of data for this criterion. We need better evidence to feed into economic modelling.
17. All other options for managing the condition should have been considered (e.g. improving treatment, providing other services) to ensure that no more cost-effective intervention could be introduced or current interventions increased within the resources available.
No other options are currently available. We cannot prevent the pancreatic damage, or restore β-cell function once it has been impaired. Effective treatments for diagnosed CFRD are already provided.
18. There should be a plan for managing and monitoring the screening programme and an agreed set of quality assurance standards.
No plan will be available till gaps in evidence have been resolved, and we know what we should be screening for. Polices for screening are part of the UK national guidelines.
19. Adequate staffing and facilities for testing, diagnosis, treatment and programme management should be available prior to the commencement of the screening programme.
This criterion is not yet met, pending resolution of uncertainties about what stage to screen for.
20. Evidence-based information, explaining the consequences of testing, investigation and treatment, should be made available to potential participants to assist them in making an informed choice.
Met for CFRD. Not met for earlier stages of hyperglycaemia.
21. Public pressure for widening the eligibility criteria for reducing the screening interval, and for increasing the sensitivity of the testing process, should be anticipated. Decisions about these parameters should be scientifically justifiable to the public.
Not applicable.
22. If screening is for a mutation, the programme should be acceptable to people identified as carriers and to other family members.
Not applicable.
Conclusions
Screening for CFRD meets the criteria. However, screening for earlier stages of hyperglycaemia does not yet meet all of the criteria. The main problems are with criterion 6 on cut-off levels, criteria 10 and 11 because of uncertainties about treatment threshold, and criterion 13 because of the lack of RCTs.
Research needs
Ongoing studies are listed in Appendix 7. They include:
-
several studies of repaglinide, compared with insulin
-
several case series of detemir or glargine
-
one study assessing the effect of adding metformin to insulin
-
one case series of CSII
-
one study of selective versus universal screening for CFRD
-
two trials of sitagliptin compared with placebo.
As mentioned above, the main problem is uncertainty about when to intervene, and hence what level of hyperglycaemia needs to be detected. Therefore, the highest research priority is for a trial of starting insulin treatment at different stages of hyperglycaemia, starting with PPH, diagnosed by 1-hour GCT, or by CGMSs or serial profiles. Outcomes should include weight and lung function, not just glycaemic control. If our hypothesis that transient hyperglycaemia (BG level of > 8 mmol/l) is harmful to the lung is correct, then treatment at the stage of isolated PPH would be beneficial for lung function. Trials should be of adequate duration, of at least several years. As one of the HTA programme referees noted: ‘… the outcome of BMI can be assessed over 1 year (as shown by the CFRDT trial) but lung function changes related to abnormal glucose tolerance do not become significant until after about 4 years (as shown by the Milla study15) probably because they are occurring on top of baseline lung function deterioration in CF.’
Second, trials of different insulin regimens are required. These could include a once-daily basal insulin, compared with short-acting meal-time insulins alone (especially as in the early stages hyperglycaemia is mainly postprandial) and (perhaps at later stages) CSII. More data are required on the relative merits of NPH, glargine and detemir, particularly in view of the cost differences. Given the considerable treatment burden associated with CF and CFRD, the impact of different regimens, and screening methods, needs to be assessed.
The third need is for a trial of different screening tests. The OGTT could be used as the reference standard, and candidates screening methods include the GCT (dose adjusted for weight), CGMSs and profiles.
More evidence on the relative merits of the 1-hour GCT, the FOGTT, CGMSs and serial profiles is required, especially if the aim is to detect any hyperglycaemia (BG level of > 8 mmol/l). Hameed et al. 213 reported that in children (age range 10–18 years) having OGTTs, the 2-hour PG level was not associated with declining BMI but the 30-minute PG level was. They concluded that hyperglycaemia at 2 hours was a later change than at earlier time points. So if the OGTT is being used, there is a case for using the FOGTT.
In the longer term, we need to find out if pancreatic damage can be prevented, and diabetes avoided or delayed.
The improvement in survival has been marked over the years. Barr et al. 215 reported that the medical age at death has risen from 6 months in 1959–63, to 27 years in 2001–8. However, they also noted that socioeconomic difference in age at death persist, with, at times, a 10-year survival difference (read from Figure 4). The reasons for the difference are not known. The authors suggest that reasons could include passive smoking, poorer nutrition or poorer adherence to treatment in lower socioeconomic groups. However, they also note that CF itself can affect social group, with possibly those worst affected having poorer education and hence more likely to be in lower socioeconomic groups. The sizeable difference in survival needs to be further researched.
Acknowledgements
We thank Professor Ken Stein and Dr Martin Pitt, Peninsula Medical School, and Rodolfo Hernandes, University of Aberdeen, for assistance in the planning stages of this review. We thank Dr C Clar for translating articles in German and French. We thank the Scottish Study Group for the Care of Diabetes in the Young for convening a meeting with expert presentations on CFRD.
Contribution of authors
Leena Pandit drafted Chapter 1, contributed to Chapter 5, and designed a model for cost-effectiveness analysis.
Ian Craigie drafted Chapter 2.
Pamela Royle and Vivien Ho drafted Chapter 3.
Pamela Royle calculated screening parameters and drafted Chapter 4.
Norman Waugh wrote Chapters 5 and 6.
Pamela Royle undertook the literature searching for all chapters.
Pamela Royle and Norman Waugh edited all chapters.
Paul Ewings, Amanda Adler, Chris Sheldon and Peter Helms provided expert advice and commented on drafts.
Disclaimer
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- Mackie AD, Thornton SJ, Edenborough FP. Cystic fibrosis-related diabetes. Diabet Med 2003;20:425-36.
- Ratjen F, Doring G. Cystic fibrosis. Lancet 2003;361:681-9.
- Van den Berg JM, Kouwenberg JM, Heijerman HG. Demographics of glucose metabolism in cystic fibrosis. J Cyst Fibros 2009;8:276-9.
- UK National Screening Committee . The UK NSC Policy on Cystic Fibrosis Screening in Newborn 2010. www.screening.nhs.uk/cysticfibrosis-newborn (accessed 28 July 2010).
- Warrell DA, Cox TM, Firth JD, Benz EJ. Oxford textbook of medicine. Oxford: Oxford University Press; 2005.
- Cystic Fibrosis Trust . Living With Cystic Fibrosis 2010. www.cftrust.org.uk/aboutcf/livingwithcf/ (accessed 2 August 2010).
- UK Cystic Fibrosis Trust Diabetes Working Group . Management of Cystic Fibrosis Related Diabetes Mellitus 2004. www.cftrust.org.uk/aboutcf/publications/consensusdoc/diabetes.pdf.
- Brock DJ, Gilfillan A, Holloway S. The incidence of cystic fibrosis in Scotland calculated from heterozygote frequencies. Clin Genet 1998;53:47-9.
- Dodge JA, Morison S, Lewis PA, Coles EC, Geddes D, Russell G, et al. Incidence, population, and survival of cystic fibrosis in the UK, 1968–95. UK Cystic Fibrosis Survey Management Committee. Arch Dis Child 1997;77:493-6.
- Li N, Pei P, Bu D-f, He B, Wang G-f. A novel CFTR mutation found in a Chinese patient with cystic fibrosis. Chin Med J 2006;119:103-9.
- LabCorp . Genetic Testing for Cystic Fibrosis 2004. www.labcorp.com/pdf/Cystic_Fibrosis_LabCapsule.pdf (accessed 2 August 2010).
- Farrell PM. The prevalence of cystic fibrosis in the European Union. J Cyst Fibros 2008;7:450-3.
- Scotet V, Audrezet M, Ferer C. Incidence of cystic fibrosis worldwide. J Cyst Fibros 2004;3.
- Koch C, Cuppens H, Rainisio M, Madessani U, Harms H, Hodson M, et al. European Epidemiologic Registry of Cystic Fibrosis (ERCF): comparison of major disease manifestations between patients with different classes of mutations. Pediatr Pulmonol 2001;31:1-12.
- Milla CE, Warwick WJ, Moran A. Trends in pulmonary function in patients with cystic fibrosis correlate with the degree of glucose intolerance at baseline. Am J Respir Crit Care Med 2000;162:891-5.
- Sawicki GS, Sellers DE, Robinson WM. High treatment burden in adults with cystic fibrosis: challenges to disease self-management. J Cyst Fibros 2009;8:91-6.
- Andersen DH. Cystic fibrosis of the pancreas and its relation to celiac disease: a clinical and pathological study. Am J Dis Child 1938;56:344-99.
- Cystic Fibrosis Foundation . Patient Registry: Annual Data Report 2008. www.cff.org/LivingWithCF/QualityImprovement/PatientRegistryReport/ (accessed 28 July 2010).
- Dodge JA, Lewis PA, Stanton M, Wilsher J. Cystic fibrosis mortality and survival in the UK: 1947–2003. Eur Respir J 2007;29:522-6.
- UK Cystic Fibrosis Registry . Annual Data Report 2008. www.cftrust.org.uk/aboutcf/publications/cfregistryreports/ (accessed 13 September 2010).
- Kulich M, Rosenfeld M, Goss CH, Wilmott R. Improved survival among young patients with cystic fibrosis. J Pediatr 2003;142:631-6.
- Lewis PA, Morison S, Dodge JA, Geddes D, Coles EC, Russell G, et al. Survival estimates for adults with cystic fibrosis born in the United Kingdom between 1947 and 1967. The UK Cystic Fibrosis Survey Management Committee. Thorax 1999;54:420-2.
- Dodge JA, Lewis PA. Cystic fibrosis is no longer an important cause of childhood death in the UK. Arch Dis Child 2005;90.
- Edenborough FP, Mackenzie WE, Stableforth DE. The outcome of 72 pregnancies in 55 women with cystic fibrosis in the United Kingdom 1977–1996. BJOG 2000;107:254-61.
- Gillet D. Cystic fibrosis and pregnancy. Report from French data (1980–1999). BJOG 2002;109:912-18.
- McMullen AH, Pasta DJ, Frederick PD, Konstan MW, Morgan WJ, Schechter MS, et al. Impact of pregnancy on women with cystic fibrosis. Chest 2006;129:706-11.
- Canadian Cystic Fibrosis Foundation . Canadian Cystic Fibrosis Patient Data Registry Report 2007. www.cysticfibrosis.ca/assets/files/pdf/CPDR_ReportE.pdf (accessed 2 August 2010).
- Shwachman H, Kulczycki LL. Long-term study of one hundred five patients with cystic fibrosis; studies made over a five- to fourteen-year period. AMA J Dis Child 1958;96:6-15.
- Cystic Fibrosis Australia . Cystic Fibrosis in Australia: 11th Annual Report from the Australian Cystic Fibrosis Data Registry 2008. www.cysticfibrosis.org.au/projects/dataregistry/ (accessed 2 August 2010).
- O’Riordan SM, Dattani MT, Hindmarsh PC. Cystic fibrosis-related diabetes in childhood. Horm Res Paediatr 2010;73:15-24.
- Elston C, Fairhurst M, Scott S, Bridges N, Shotliff K, Gyi K. Presence of diabetic complications in an adult cystic fibrosis population. Pediatr Pulmonol 2007.
- Finkelstein SM, Wielinski CL, Elliott GR, Warwick WJ, Barbosa J, Wu SC, et al. Diabetes mellitus associated with cystic fibrosis. J Pediatr 1988;112:373-7.
- Lanng S, Hansen A, Thorsteinsson B, Nerup J, Koch C. Glucose tolerance in patients with cystic fibrosis: 5-year prospective-study. BMJ 1995;311:655-9.
- Adler A, Bilton D, Haworth C, Gunn E, Shine B. Epidemiology of cystic fibrosis-related diabetes – Results from a British Cohort. Pediatr Pulmonol 2007:363-4.
- Rosenecker J, Eichler I, Kuhn L, Harms HK, von der Hardt H. Genetic Determination of Diabetes-Mellitus in Patients with Cystic-Fibrosis. J Pediatr 1995;127:441-3.
- Marshall BC, Butler SM, Stoddard M, Moran AM, Liou TG, Morgan WJ. Epidemiology of cystic fibrosis-related diabetes. J Pediatr 2005;146:681-7.
- Moran A, Doherty L, Wang X, Thomas W. Abnormal glucose metabolism in cystic fibrosis. J Pediatr 1998;133:10-7.
- Moran A, Dunitz J, Nathan B, Saeed A, Holme B, Thomas W. Cystic fibrosis related diabetes: current trends in prevalence, incidence and mortality. Diabetes Care 2009;32:1626-31.
- Rana M. Population-based incidence of CFRD in NSW and ACT, Australia. Diabetes 2010;59.
- Droumaguet C, Elie C, Mosnier-Pudar H, Pesce F, Desmazes-Dufeu N, Hubert D. Risk of diabetes in a cohort of 243 adults with cystic fibrosis. J Cyst Fibros 2005;4.
- Hodson ME. Diabetes mellitus and cystic fibrosis. Bailliere Clin Endocrinol Metabol 1992;6:797-805.
- Adler A, Shine B, Haworth C. Genetic determinants and epidemiology of cystis fibrosis-related diabetes: results from a British cohort of children and adults. Diabetes Care 2008;31:1789-94.
- Cucinotta D, De Luca F, Scoglio R, Lombardo F, Sferlazzas C, Di Benedetto A, et al. Factors affecting diabetes mellitus onset in cystic fibrosis: evidence from a 10-year follow-up study. Acta Paediatr 1999;88:389-93.
- Fischman D, Nookala VK. Cystic fibrosis-related diabetes mellitus: etiology, evaluation, and management. Endocr Pract 2008;14:1169-79.
- Alves C, Lima DS, Cardeal M, Santana A. Low prevalence of glucose intolerance in racially mixed children with cystic fibrosis. Pediatr Diabetes 2010;11:493-7.
- Gilljam M, Ellis L, Corey M, Zielenski J, Durie P, Tullis DE. Clinical manifestations of cystic fibrosis among patients with diagnosis in adulthood. Chest 2004;126:1215-24.
- Blackman SM, Hsu S, Ritter SE, Naughton KM, Wright FA, Drumm ML, et al. A susceptibility gene for type 2 diabetes confers substantial risk for diabetes complicating cystic fibrosis. Diabetologia 2009;52:1858-65.
- Yung B, Noormohamed FH, Kemp M, Hooper J, Lant AF, Hodson ME. Cystic fibrosis-related diabetes: the role of peripheral insulin resistance and beta-cell dysfunction. Diabet Med 2002;19:221-6.
- Dobson L, Sheldon CD, Hattersley AT. Understanding cystic-fibrosis-related diabetes: best thought of as insulin deficiency?. J R Soc Med 2004;97:26-35.
- Couce M, Obrien TD, Moran A, Roche PC, Butler PC. Diabetes mellitus in cystic fibrosis is characterized by islet amyloidosis. J Clin Endocrinol Metabol 1996;81:1267-72.
- Zirbes J, Milla CE. Cystic fibrosis related diabetes. Paediatr Respir Rev 2009;10:118-23.
- Schwarzenberg SJ, Walk D, Thomas W, Milla C, Olsen TW, Moran A, et al. Microvascular complications in cystic fibrosis-related diabetes. Diabetes Care 2007;30:1056-61.
- Black C, Cummins E, Royle P, Philip S, Waugh N. The clinical effectiveness and cost-effectiveness of inhaled insulin in diabetes mellitus: a systematic review and economic evaluation. Health Technol Assess 2007;11.
- Nousia-Arvanitakis S, Galli-Tsinopoulou A, Karamouzis M. Insulin improves clinical status of patients with cystic-fibrosis-related diabetes mellitus. Acta Paediatr 2001;90:515-19.
- Brunzell C, Hardin DS, Moran A, Schindler T, Schissel K. Managing Cystic Fibrosis-Related Diabetes “(CFRD)”: An Instruction Guide for Patients and Families 2009. www.cff.org/LivingWithCF/StayingHealthy/Diet/Diabetes/ (accessed 2 August 2010).
- Tierney S, Deaton C, Webb K, Jones A, Dodd M, McKenna D, et al. Isolation, motivation and balance: living with type 1 or cystic fibrosis-related diabetes. J Clin Nurs 2008;17:235-43.
- Milla CE, Billings J, Moran A. Diabetes is associated with dramatically decreased survival in female but not male subjects with cystic fibrosis. Diabetes Care 2005;28:2141-4.
- Miller RJ, Tildesley HD, Wilcox PG, Zhang H, Kreisman SH. Sex disparities in effects of cystic fibrosis-related diabetes on clinical outcomes: a matched study. Can Respir J 2008;15:291-4.
- Srivastava S, Gyi K, Bilton D, Hodson M. Impact of cystic fibrosis related diabetes on survival in adult patients with cystic fibrosis at a single uk centre. Pediatr Pulmonol 2008;43:442-3.
- Sims EJ, Green MW, Mehta A. Decreased lung function in female but not male subjects with established cystic fibrosis-related diabetes. Diabetes Care 2005;28:1581-7.
- Kampfert C, Holl RW, Marquart A, Ballmann M. Is earlier development of CFRD one possible reason for worse clinical findings in women with CFRD?. J Cyst Fibros 2006;5.
- Chamnan P, Shine BS, Haworth CS, Bilton D, Adler AI. Diabetes as a determinant of mortality in cystic fibrosis. Diabetes Care 2010;33:311-16.
- Yung B, Hodson ME. Diabetes in cystic fibrosis. J R Soc Med 1999;92:35-40.
- Adler AI, Shine B, Haworth C, Leelarathna L, Bilton D. Hyperglycemia and death in cystic fibrosis-related diabetes. Diabetes Care 2011;34.
- George PM, Banya W, Pareek N, Bilton D, Cullinan P, Hodson ME, et al. Improved survival at low lung function in cystic fibrosis: cohort study from 1990 to 2007. BMJ 2011;342.
- Andersen HU, Lanng S, Pressler T, Laugesen CS, Mathiesen ER. Cystic fibrosis-related diabetes: the presence of microvascular diabetes complications. Diabetes Care 2006;29:2660-3.
- Van den Berg JM, Morton AM, Kok SW, Pijl H, Conway SP, Heijerman HG. Microvascular complications in patients with cystic fibrosis-related diabetes (CFRD). J Cyst Fibros 2008;7:515-19.
- Costa M, Potvin S, Berthiaume Y, Gauthier L, Jeanneret A, Lavoie A, et al. Diabetes: a major co-morbidity of cystic fibrosis. Diabetes Metab 2005;31:221-32.
- Dobson L, Stride A, Bingham C, Elworthy S, Sheldon CD, Hattersley AT. Microalbuminuria as a screening tool in cystic fibrosis-related diabetes. Pediatr Pulmonol 2005;39:103-7.
- Moran A. Abnormal glucose tolerance in CF: when should we offer diabetes treatment?. Pediatr Diabetes 2009;10:159-61.
- Georgiopoulou VV, Denker A, Bishop KL, Brown JM, Hirsh B, Wolfenden L, et al. Metabolic abnormalities in adults with cystic fibrosis. Respirology 2010;15:823-9.
- Rhodes B, Nash EF, Tullis E, Pencharz PB, Brotherwood M, Dupuis A, et al. Prevalence of dyslipidemia in adults with cystic fibrosis. J Cyst Fibros 2010;9:24-8.
- Cheung MS, Bridges NA, Prasad SA, Francis J, Carr SB, Suri R, et al. Growth in children with cystic fibrosis-related diabetes. Pediatr Pulmonol 2009;44:1223-5.
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998;15:539-53.
- Wilkinson JD, Craigie IP, Allison G, Gallacher C, Crocker J, Kent S. Investigating suspected CF-related diabetes mellitus utilising serial capillary blood glucose profiling. J Cyst Fibros 2008;7.
- Wong TY, Liew G, Tapp RJ, Schmidt MI, Wang JJ, Mitchell P, et al. Relation between fasting glucose and retinopathy for diagnosis of diabetes: three population-based cross-sectional studies. Lancet 2008;371:736-43.
- Carpenter S, Grasemann H. Evolution of glucose intolerance in adolescent CF patients. J Cyst Fibros 2007;6.
- Thorsteinsson B, Lanng S, Hansen A, Nerup J, Koch C. Glucose tolerance in cystic fibrosis: a 5-year prospective study. Diabetologia 1995;38.
- Sterescu AE, Rhodes B, Jackson R, Dupuis A, Hanna A, Wilson DC, et al. Natural history of glucose intolerance in patients with cystic fibrosis: ten-year prospective observation program. J Pediatr 2010;156:613-17.
- Allen HF, Klingensmith GJ, Gay EC, Hamman RF. Identification and treatment of cystic fibrosis-related diabetes: a survey of current medical practice in the US. Diabetes Care 1998;21:943-8.
- Mohan K, Miller H, Burhan H, Ledson MJ, Walshaw MJ. Management of cystic fibrosis related diabetes: A survey of UK cystic fibrosis centers. Pediatr Pulmonol 2008;43:642-7.
- International Expert Committee . International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009;32:1327-34.
- Von Drygalski A, Biller J. Anemia in cystic fibrosis: incidence, mechanisms, and association with pulmonary function and vitamin deficiency. Nutr Clin Pract 2008;23:557-63.
- Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care 2003;26:881-5.
- Australia and New Zealand Horizon Scanning Network (ANZHSN) . Continuous Glucose Monitoring Devices 2006. http://nzhta.chmeds.ac.nz/publications/finalcgmd.pdf (accessed 2 August 2010).
- McKeever TM, Weston PJ, Hubbard R, Fogarty A. Lung function and glucose metabolism: an analysis of data from the Third National Health and Nutrition Examination Survey. Am J Epidemiol 2005;161:546-56.
- Gerich JE. Clinical significance, pathogenesis, and management of postprandial hyperglycemia. Arch Intern Med 2003;163:1306-16.
- Hanefeld M, Koehler C, Schaper F, Fuecker K, Henkel E, Temelkova-Kurktschiev T. Postprandial plasma glucose is an independent risk factor for increased carotid intima media thickness in non-diabetic individuals. Atherosclerosis 1999;144:229-35.
- Shwachman H, Leubner H, Catzel P. Mucoviscidosis. Adv Pediatr 1955;7:249-323.
- Moran A. Cystic fibrosis-related diabetes: an approach to diagnosis and management. Pediatr Diabetes 2000;1:41-8.
- Sheldon CD, Dobson L, Hodson M, Bush A, Geddes D. Cystic fibrosis. London: Hodder Arnold; 2007.
- WHO Study Group . Diabetes Mellitus: Report of a WHO Study Group 1985. http://whqlibdoc.who.int/trs/WHO_TRS_727.pdf (accessed 2 August 2010).
- Laguna T, Nathan BM, Moran A. Managing diabetes in cystic fibrosis. Diabetes Obes Metabol 2010;12:858-64.
- Hardin DS, Leblanc A, Lukenbaugh S, Seilheimer DK. Insulin resistance is associated with decreased clinical status in cystic fibrosis. J Pediatr 1997;130:948-56.
- National Diabetes Data Group . Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039-57.
- Alberti KG. The clinical implications of impaired glucose tolerance. Diabet Med 1996;13:927-37.
- Rushforth NB, Miller M, Bennett PH. Fasting and two-hour post-load glucose levels for the diagnosis of diabetes. The relationship between glucose levels and complications of diabetes in the Pima Indians. Diabetologia 1979;16:373-9.
- Expert Committee on the Diagnosis and Classification of Diabetes Mellitus . Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183-97.
- Brodsky J, Dougherty S, Makani R, Rubenstein RC, Kelly A. Elevation of 1-hour plasma glucose during oral glucose tolerance testing is associated with worse pulmonary function in cystic fibrosis. Diabetes Care 2011;34:292-5.
- Moran A. Highlights of the February 1998 consensus conference on CFRD. Pediatr Pulmonol 1998;17:104-5.
- Moran A, Becker D, Casella SJ, Gottlieb PA, Kirkman MS, Marshall BC, et al. Epidemiology, pathophysiology, and prognostic implications of cystic fibrosis-related diabetes: a technical review. Diabetes Care 2010;33:2677-83.
- Moran A, Hardin D, Rodman D, Allen HF, Beall RJ, Borowitz D, et al. Diagnosis, screening and management of cystic fibrosis related diabetes mellitus: a consensus conference report. Diabetes Res Clin Pract 1999;45:61-73.
- Frohnert BI, Ode KL, Moran A, Nathan BM, Laguna T, Holme B, et al. Impaired fasting glucose in cystic fibrosis. Diabetes Care 2010;33:2660-4.
- Lohr M, Goertchen P, Nizze H, Gould NS, Gould VE, Oberholzer M, et al. Cystic fibrosis associated islet changes may provide a basis for diabetes. An immunocytochemical and morphometrical study. Virchows Arch A Pathol Anat Histopathol 1989;414:179-85.
- WHO Expert Committee . WHO Expert Committee on Diabetes Mellitus 1980. http://whqlibdoc.who.int/trs/WHO_TRS_646.pdf (accessed 2 August 2010).
- Reichard P, Berglund B, Britz A, Cars I, Nilsson BY, Rosenqvist U. Intensified conventional insulin treatment retards the microvascular complications of insulin-dependent diabetes mellitus (IDDM): the Stockholm Diabetes Intervention Study (SDIS) after 5 years. J Intern Med 1991;230:101-8.
- The Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977-86.
- Bouter KP, Diepersloot RJ, van Romunde LK, Uitslager R, Masurel N, Hoekstra JB, et al. Effect of epidemic influenza on ketoacidosis, pneumonia and death in diabetes mellitus: a hospital register survey of 1976–1979 in the Netherlands. Diabetes Res Clin Pract 1991;12:61-8.
- Fine MJ, Smith MA, Carson CA, Mutha SS, Sankey SS, Weissfeld LA, et al. Prognosis and outcomes of patients with community-acquired pneumonia. A meta-analysis. JAMA 1996;275:134-41.
- Kornum JB, Thomsen RW, Riis A, Lervang HH, Schonheyder HC, Sorensen HT. Diabetes, glycemic control, and risk of hospitalization with pneumonia: a population-based case–control study. Diabetes Care 2008;31:1541-5.
- Koziel H, Koziel MJ. Pulmonary complications of diabetes mellitus. Pneumonia. Infect Dis Clin North Am 1995;9:65-96.
- Ardigo D, Valtuena S, Zavaroni I, Baroni MC, Delsignore R. Pulmonary complications in diabetes mellitus: the role of glycemic control. Curr Drug Targets Inflamm Allergy 2004;3:455-8.
- Niranjan V, McBrayer DG, Ramirez LC, Raskin P, Hsia CC. Glycemic control and cardiopulmonary function in patients with insulin-dependent diabetes mellitus. Am J Med 1997;103:504-13.
- Chance WW, Rhee C, Yilmaz C, Dane DM, Pruneda ML, Raskin P, et al. Diminished alveolar microvascular reserves in type 2 diabetes reflect systemic microangiopathy. Diabetes Care 2008;31:1596-601.
- Weynand B, Jonckheere A, Frans A, Rahier J. Diabetes mellitus induces a thickening of the pulmonary basal lamina. Respiration 1999;66:14-9.
- Davis WA, Knuiman M, Kendall P, Grange V, Davis TM. Glycemic exposure is associated with reduced pulmonary function in type 2 diabetes: the Fremantle Diabetes Study. Diabetes Care 2004;27:752-7.
- Baker EH, Wood DM, Brennan AL, Clark N, Baines DL, Philips BJ. Hyperglycaemia and pulmonary infection. Proc Nutr Soc 2006;65:227-35.
- Philips BJ, Meguer JX, Redman J, Baker EH. Factors determining the appearance of glucose in upper and lower respiratory tract secretions. Intensive Care Med 2003;29:2204-10.
- Koch C, Rainisio M, Madessani U, Harms HK, Hodson ME, Mastella G, et al. Presence of cystic fibrosis-related diabetes mellitus is tightly linked to poor lung function in patients with cystic fibrosis: Data from the European Epidemiologic Registry of Cystic Fibrosis. Pediatr Pulmonol 2001;32:343-50.
- Rosenecker J, Eichler I, Barmeier H, von der Hardt H. Diabetes mellitus and cystic fibrosis: comparison of clinical parameters in patients treated with insulin versus oral glucose-lowering agents. Pediatr Pulmonol 2001;32:351-5.
- Adler AI, Gunn E, Haworth CS, Shine BS, Diana B. Diabetes mellitus and impaired glucose tolerance are associated with poor pulmonary function in adults with cystic fibrosis. Diabetes 2007;56.
- Lanng S, Thorsteinsson B, Nerup J, Koch C. Influence of the development of diabetes mellitus on clinical status in patients with cystic fibrosis. Eur J Pediatr 1992;151:684-7.
- Barrett-Connor E, Frette C. NIDDM, impaired glucose tolerance, and pulmonary function in older adults. The Rancho Bernardo Study. Diabetes Care 1996;19:1441-4.
- Lange P, Groth S, Kastrup J, Mortensen J, Appleyard M, Nyboe J, et al. Diabetes mellitus, plasma glucose and lung function in a cross-sectional population study. Eur Respir J 1989;2:14-9.
- Lawlor DA, Ebrahim S, Smith GD. Associations of measures of lung function with insulin resistance and Type 2 diabetes: findings from the British Women’s Heart and Health Study. Diabetologia 2004;47:195-203.
- Lazarus R, Sparrow D, Weiss ST. Baseline ventilatory function predicts the development of higher levels of fasting insulin and fasting insulin resistance index: the Normative Aging Study. Eur Respir J 1998;12:641-5.
- Yeh HC, Punjabi NM, Wang NY, Pankow JS, Duncan BB, Cox CE, et al. Cross-sectional and prospective study of lung function in adults with type 2 diabetes: the Atherosclerosis Risk in Communities (ARIC) study. Diabetes Care 2008;31:741-6.
- Schaedel C, de Monestsrol I, Hjelte L, Johannesson M, Kornfalt R, Lindblad A, et al. Predictors of deterioration of lung function in cystic fibrosis. Pediatr Pulmonol 2002;33:483-91.
- Brown J, Elston C, Driver R, Hitman GA, Kuitert LM. Decline in lung function and BMI prior to the diagnosis of CFRD and the impact of gender. J Cyst Fibros 2006;5.
- Drummond RS, Love A, Semple K, Bicknell S, Ross E, Small M, et al. Insulin therapy ameliorates loss of lung function in cystic fibrosis and should be commenced when impaired glucose tolerance is present. Diabetes 2006;55.
- Wood DM, Brennan AL, Philips BJ, Baker EH. Effect of hyperglycaemia on glucose concentration of human nasal secretions. Clin Sci 2004;106:527-33.
- Brennan AL, Gyi KM, Clark N, Fisher DA, Wood DM, Baines DL, et al. Detection of increased glucose concentrations in lower airway secretions from people with cystic fibrosis. Thorax 2005;60.
- Brennan AL, Gyi KM, Wood DM, Johnson J, Holliman R, Baines DL, et al. Airway glucose concentrations and effect on growth of respiratory pathogens in cystic fibrosis. J Cyst Fibros 2007;6:101-9.
- Lanng S, Thorsteinsson B, Nerup J, Koch C. Diabetes mellitus in cystic fibrosis: effect of insulin therapy on lung function and infections. Acta Paediatr 1994;83:849-53.
- Rafii M, Chapman K, Stewart C, Kelly E, Hanna A, Wilson DC, et al. Changes in response to insulin and the effects of varying glucose tolerance on whole-body protein metabolism in patients with cystic fibrosis. Am J Clin Nutr 2005;81:421-6.
- Onady GM, Stolfi A. Insulin and oral agents for managing cystic fibrosis-related diabetes. Cochrane Database Syst Rev 2009;3.
- National Institute for Health and Clinical Excellence (NICE) . Imatinib for Chronic Myeloid Leukaemia (TA50) 2002.
- Dobson L, Hattersley AT, Tiley S, Elworthy S, Oades PJ, Sheldon CD. Clinical improvement in cystic fibrosis with early insulin treatment. Arch Dis Child 2002;87:430-1.
- Sulli N, Bertasi S, Zullo S, Shashaj B. Use of continuous subcutaneous insulin infusion in patients with cystic fibrosis related diabetes: three case reports. J Cyst Fibros 2007;6:237-40.
- The DECODE study group . Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. European Diabetes Epidemiology Group. Diabetes Epidemiology: Collaborative Analysis of Diagnostic Criteria in Europe. Lancet 1999;354:617-21.
- Moran A, Pekow P, Grover P, Zorn M, Slovis B, Pilewski J, et al. Insulin therapy to improve BMI in cystic fibrosis related diabetes without fasting hyperglycemia: results of the CFRDT Trial. Diabetes Care 2009;32:1783-8.
- Culler FL, Mckean LP, Buchanan CN, Caplan DB, Meacham LR. Glipizide treatment of patients with cystic-fibrosis and impaired glucose-tolerance. J Pediatr Gastroenterol Nutr 1994;18:375-8.
- Bertele-Harms RM, Harms HK. Sulfonylurea (SU) in the treatment of CF related diabetes mellitus (CFDM). A 15 year experience. Pediatr Pulmonol 1996;22.
- Kentrup H, Bongers H, Spengler M, Kusenbach G, Skopnik H. Efficacy and safety of acarbose in patients with cystic fibrosis and impaired glucose tolerance. Eur J Pediatr 1999;158:455-9.
- Moran A, Phillips J, Milla C. Insulin and glucose excursion following premeal insulin lispro or repaglinide in cystic fibrosis-related diabetes. Diabetes Care 2001;24:1706-10.
- Rolon MA, Benali K, Munck A, Navarro J, Clement A, Tubiana-Rufi N, et al. Cystic fibrosis-related diabetes mellitus: clinical impact of prediabetes and effects of insulin therapy. Acta Paediatr 2001;90:860-7.
- Ballmann M, Mueller-Brandes C. Longitudinal follow-up of clinical and laboratory data in pediatric patients with cystic fibrosis related diabetes mellitus (CFRD) after initial treatment with an oral antidiabetic drug (Glibenclamid). Pediatr Pulmonol 2003;36.
- Boyle BJ, Henderson P, Bicknell S, Small M, Jones GC. Early insulin treatment improves weight and lung function in patients with cystic fibrosis. Diabet Med 2004;21.
- Franzese A, Spagnuolo MI, Sepe A, Valerio G, Mozzillo E, Raia V. Can glargine reduce the number of lung infections in patients with cystic fibrosis-related diabetes?. Diabetes Care 2005;28.
- Minicucci L, De Alessandri A, Casciaro R, Burlando O, Lugani F, Poggi E, et al. Insulin glargine (IG) for therapy of cystic fibrosis related diabetes (CFRD) and impaired glucose tolerance (IGT) in cystic fibrosis (CF) patients (pts). J Cyst Fibros 2005;4.
- Bizzarri C, Lucidi V, Ciampalini P, Bella S, Russo B, Cappa M. Clinical effects of early treatment with insulin glargine in patients with cystic fibrosis and impaired glucose tolerance. J Endocrinol Invest 2006;29:RC1-4.
- Drummond RS, Love A, Semple K, Bicknell S, Ross E, Small M, et al. The favourable effect of insulin upon pulmonary function in patients with cystic fibrosis correlates with the degree of glucose intolerance at baseline. Diabet Med 2006;23.
- Hardy J, Dixon M, Bone M, Vyas J, David TJ, Patel L. Does insulin treatment influence growth and lung function in children with abnormal oral glucose tolerance test (OGTT) but normal fasting glucose?. J Cyst Fibros 2006;5.
- McGinnity T, Harms B, Trevellyan N, Connett GJ. Once daily long acting insulin achieves good glycaemic control in Cystic Fibrosis related diabetes (CFRD). J Cyst Fibros 2006;5.
- Onady GM, Langdon LJ. Insulin versus oral agents in the management of cystic fibrosis related diabetes: a case based study. BMC Endocr Disord 2006;6.
- Drummond RS, Carty D, Small M, Jones GC. The characterisation of hypoglycaemia in insulin treated patients with cystic fibrosis. Diabet Med 2007;24.
- Grover P, Thomas W, Moran A. Glargine versus NPH insulin in cystic fibrosis related diabetes. J Cyst Fibros 2008;7:134-6.
- Mohan K, Israel KL, Miller H, Grainger R, Ledson MJ, Walshaw MJ. Long-term effect of insulin treatment in cystic fibrosis-related diabetes. Respiration 2008;76:181-6.
- Hardin DS, Rice J, Rice M, Rosenblatt R. Use of the insulin pump in treating cystic fibrosis related diabetes. J Cyst Fibros 2009;8:174-8.
- Mozzillo E, Franzese A, Valerio G, Sepe A, De S, I, Mazzarella G, et al. One-year glargine treatment can improve the course of lung disease in children and adolescents with cystic fibrosis and early glucose derangements. Pediatr Diabetes 2009;10:162-7.
- Hameed S, Morton JR, Field PI, Belessis Y, Yoong T, Katz T, et al. Once daily insulin detemir in cystic fibrosis with insulin deficiency [published online ahead of print 14 April 2011]. Arch Dis Child 2011. 10.1136/adc.2010.204636.
- Minicucci L, Casciaro R, De Alessandri A, Haupt M, Caso M, Lucidi V, et al. Efficacy of slow release insulin in patients with cystic fibrosis and glucide intolerance. J Cyst Fibros 2009;8.
- Hameed S, Morton JR, Jaffe A, Field PI, Belessis Y, Yoong T, et al. Once-daily insulin detemir in cystic fibrosis related diabetes (CFRD). Horm Res 2009;72:160-1.
- Brennan AL, Geddes DM, Gyi KM, Baker EH. Clinical importance of cystic fibrosis-related diabetes. J Cyst Fibros 2004;3:209-22.
- de Valk HW, van der Graaf EA. Cystic fibrosis-related diabetes in adults: where can we go from here?. Rev Diabet Stud 2007;4:6-12.
- Lanng S. Glucose intolerance in cystic fibrosis patients. Paediatr Respir Rev 2001;2:253-9.
- Moran A. Diagnosis, screening, and management of cystic fibrosis-related diabetes. Curr Diab Rep 2002;2:111-15.
- O’Riordan SM, Robinson PD, Donaghue KC, Moran A. Management of cystic fibrosis-related diabetes. Pediatr Diabetes 2008;9:338-44.
- Nathan BM, Laguna T, Moran A. Recent trends in cystic fibrosis-related diabetes. Current Opinion Endocrinol Diabetes Obes 2010;17:335-41.
- Rana M, Munns CF, Selvadurai H, Donaghue KC, Craig ME. Cystic fibrosis-related diabetes in children-gaps in the evidence?. Nature Reviews Endocrinology 2010;6:371-8.
- O’Riordan SM, Robinson PD, Donaghue KC, Moran A. Management of cystic fibrosis-related diabetes in children and adolescents. Pediatr Diabetes 2009;10:43-50.
- Moran A, Brunzell C, Cohen RC, Katz M, Marshall BC, Onady G, et al. Clinical care guidelines for cystic fibrosis-related diabetes: a position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care 2010;33:2697-708.
- Ode KL, Frohnert B, Laguna T, Phillips J, Holme B, Regelmann W, et al. Oral glucose tolerance testing in children with cystic fibrosis. Pediatr Diabetes 2010;11:487-92.
- Buck C, Thon A, Wolf A, Kohne E, Holl RW. Diagnosis of diabetes mellitus in cystic fibrosis (CF) – role of blood sugar, HbA(1c) and oral glucose tolerance test [German]. Monatsschr Kinderheilkd 2000;148:698-701.
- Brennan AL, Hodson ME, Geddes DM, Gyi KM, Baker EH. Describing the relationship between HbA1c and mean plasma glucose in people with cystic fibrosis. J Cyst Fibros 2004;3.
- Brennan AL, Gy KM, Wood DM, Hodson ME, Geddes DM, Baker EH. Relationship between glycosylated haernoglobin and mean plasma glucose concentration in cystic fibrosis. J Cyst Fibros 2006;5:27-31.
- Haesebaert J, Bourdy S, Perceval M, Chabloz C, Nove-Josserand R, Reix P, et al. Screening and management of glucose metabolism disorders in cystic fibrosis patients. Practices survey in 4 French reference centers. Arch Pediatr 2009;16:1435-42.
- Khammar A, Stremler N, Dubus JC, Gross G, Sarles J, Reynaud R. Value of continuous glucose monitoring in screening for diabetes in cystic fibrosis. Arch Pediatr 2009;16:1540-6.
- Whiting P, Rutjes AW, Dinnes J, Reitsma J, Bossuyt PM, Kleijnen J. Development and validation of methods for assessing the quality of diagnostic accuracy studies. Health Technol Assess 2004;8.
- Whiting P, Rutjes AW, Dinnes J, Reitsma JB, Bossuyt PM, Kleijnen J. A systematic review finds that diagnostic reviews fail to incorporate quality despite available tools. J Clin Epidemiol 2005;58:1-12.
- MedCalc Software . MedCalc 2011. wwwmedcalc.org/calc/diagnostic_test.php (accessed 22 June 2011).
- Holl RW, Buck C, Babka C, Wolf A, Thon A. HbA1c is not recommended as a screening test for diabetes in cystic fibrosis. Diabetes Care 2000;23.
- De Luca F, Arrigo T, Nibali SC, Sferlazzas C, Gigante A, DiCesare E, et al. Insulin secretion, glycosylated hemoglobin and islet cell antibodies in cystic-fibrosis children and adolescents with different degrees of glucose tolerance. Horm Metab Res 1991;23:495-8.
- De Schepper J, Dab I, Derde MP, Loeb H. Oral glucose tolerance testing in cystic fibrosis: correlations with clinical parameters and glycosylated hemoglobin determinations. Eur J Pediatr 1991;150:403-6.
- Lee KM, Miller RJ, Rosenberg FM, Kreisman SH. Evaluation of glucose tolerance in cystic fibrosis: comparison of 50-g and 75-g tests. J Cyst Fibros 2007;6:274-6.
- Magni A. Screening tests for glucose metabolism abnormalities in cystic fibrosis. Eur J Lab Med 1996;4:6-10.
- Moreau F, Weiller MA, Rosner V, Weiss L, Hasselmann M, Pinget M, et al. Continuous glucose monitoring in cystic fibrosis patients according to the glucose tolerance. Horm Metab Res 2008;40:502-6.
- Robert JJ, Grasset E, de Montalembert M, Chevenne D, Deschamps I, Boitard C, et al. Factors for Glucose-Intolerance in Cystic-Fibrosis. Arch Fr Pediatr 1992;49:17-22.
- Yung B, Kemp M, Hooper J, Hodson ME. Diagnosis of cystic fibrosis related diabetes: a selective approach in performing the oral glucose tolerance test based on a combination of clinical and biochemical criteria. Thorax 1999;54:40-3.
- Scottish Intercollegiate Guidelines Network (SIGN) . Management of Diabetes 2010. wwwsign.ac.uk/guidelines/fulltext/116/index.html.
- De Simone I, Raia V, Sepe A, Valerio G, De Gregorio F, Buono P, et al. Early detection of glucose derangements in children with cystic fibrosis under ten years of age. Pediatr Diabetes 2009;10.
- Dobson L, Sheldon CD, Hattersley AT. Validation of interstitial fluid continuous glucose monitoring in cystic fibrosis. Diabetes Care 2003;26:1940-1.
- Dobson L, Sheldon CD, Hattersley AT. Conventional measures underestimate glycaemia in cystic fibrosis patients. Diabet Med 2004;21:691-6.
- Franzese A, Valerio G, Buono P, Spagnuolo MI, Sepe A, Mozzillo E, et al. Continuous glucose monitoring system in the screening of early glucose derangements in children and adolescents with cystic fibrosis. J Pediatr Endocrinol Metab 2008;21:109-16.
- Jefferies C, Solomon M, Perlman K, Sweezey N, Daneman D. Continuous glucose monitoring in adolescents with cystic fibrosis. J Pediatr 2005;147:396-8.
- O’Riordan S, Hoey H, George S, Costigan C. Can continuous glucose monitoring (CGMS) enhance the detection of CFRD in 167 cystic fibrosis children?. Diabetes 2006;55:A17-18.
- O’Riordan S, George S, Hoey H, Greally P, Costigan C, Canny G. Glucose homeostasis and continuous glucose monitoring in 167 children with cystic fibrosis. Diabet Med 2006;23.
- O’Riordan SMP, Roche EF, George S, Hoey HMV, Costigan C. Continuous glucose monitoring (CGMS) enhances the detection of cystic fibrosis related diabetes (CFRD) in children with cystic fibrosis. Diabetologia 2007;50:S84-5.
- Middleton PG, Bishop J. Oral glucose tolerance testing in CF adults: cross-sectional and longitudinal comparisons. J Cyst Fibros 2006;5.
- Solomon MP, Wilson DC, Corey M, Kalnins D, Zielenski J, Tsui LC, et al. Glucose intolerance in children with cystic fibrosis. J Pediatr 2003;142:128-32.
- Hardin DS, Grilley K, Baron B, Hale KA. Accelerated red blood cell turnover can invalidate the use of haemoglobin A1c as a diagnostic test for cystic fibrosis related diabetes. Pediatr Res 1999;45.
- Allen HF. Potential impact of HbA1c determination on clinical decision making in patients with cystic fibrosis-related diabetes – reply to Hunkert et al. Diabetes Care 1999;22:1009-10.
- Garagorri JM, Rodriguez G, Ros L, Sanchez A. Early detection of impaired glucose tolerance in patients with cystic fibrosis and predisposition factors. J Pediatr Endocrinol Metab 2001;14:53-60.
- Al-Aloul M, Pandya S, Cowperthwaite C, Walshaw MJ, Ledson MJ. Post prandial glucose measurement in the assessment of CF related diabetes mellitus (CFRDM). J Cyst Fibros 2003;2.
- Mueller-Brandes C, Holl RW, Nastoll M, Ballmann M. New criteria for impaired fasting glucose and screening for diabetes in cystic fibrosis. Eur Respir J 2005;25:715-17.
- Goldstein DE, Little RR, Wiedmeyer HM, England JD, Rohlfing CL, Wilke AL. Is glycohemoglobin testing useful in diabetes mellitus? Lessons from the diabetes control and complications trial. Clin Chem 1994;40:1637-40.
- Goldstein DE, Little RR, Lorenz RA, Malone JI, Nathan D, Peterson CM. Tests of glycemia in diabetes. Diabetes Care 1995;18:896-909.
- Tahara Y, Shima K. The response of GHb to stepwise plasma glucose change over time in diabetic patients. Diabetes Care 1993;16:1313-14.
- Adler A, Shine B. Epidemiology of cystic fibrosis-related diabetes; results from a British cohort of children and adults. Diabetologia 2007;50.
- Abbott J. Health-related quality of life measurement in cystic fibrosis: advances and limitations. Chronic Respir Dis 2009;6:31-4.
- Quittner AL. Measurement of quality of life in cystic fibrosis. Curr Opin Pulm Med 1998;4:326-31.
- Tierney S, Webb K, Jones A, Dodd M, McKenna D, Rowe R, et al. Living with cystic fibrosis-related diabetes or type 1 diabetes mellitus: a comparative study exploring health-related quality of life and patients’ reported experiences of hypoglycaemia. Chronic Illness 2008;4:278-88.
- Hameed S, Morton JR, Jaffe A, Field PI, Belessis Y, Yoong T, et al. Early glucose abnormalities in cystic fibrosis are preceded by poor weight gain. Diabetes Care 2010;33:221-6.
- UK National Screening Committee . Programme Appraisal Criteria: Criteria for Appraising the Viability, Effectiveness and Appropriateness of a Screening Programme 2012. www.screening.nhs.uk/criteria (accessed 20 January 2012).
- Barr HL, Britton J, Smyth AR, Fogarty AW. Association between socioeconomic status, sex, and age at death from cystic fibrosis in England and Wales (1959 to 2008): cross sectional study. BMJ 2011;343.
- Allen HF. Glycosylated hemoglobin levels and new diagnostic criteria: should the oral glucose tolerance test be performed as a routine screening test for diabetes in cystic fibrosis patients? Response. Diabetes Care 1998;21:2199-200.
- Bistritzer T. Hemoglobin A and pancreatic beta cell function in cystic fibrosis. Isr J Med Sci 1983;19:600-3.
- Holl RW, Buck C, Cario H, Wolf A, Thon A, Kohne E, et al. Diagnosis of diabetes in cystic fibrosis and thalassemia major. Diabetes Care 1998;21:671-2.
- Huot C, Fortin H, So VC, Gonthier M, Lamarre A. Screening for abnormal blood glucose metabolism in children and adolescents with cystic fibrosis. Diabetes 1997;46.
- Lanng S, Thorsteinsson B, Nerup J, Koch C. Diabetes mellitus in cystic fibrosis: a ten-year prospective study. Diabetologia 2000;43.
- Ledson MJ, Mohan K, Dyce P, Miller H, Walshaw MJ. Comparison of the oral glucose tolerance test and serial glucose monitoring in CF patients. J Cyst Fibros 2007;6.
- Liou TG, Brayshaw S, Brown M, Chatfield B, Jensen M, McDonald C, et al. Improving performance in the detection and management of CFRD in the Mountain West Consortium. Pediatr Pulmonol 2006;41.
- Loo SWH, Matthews WJ, Gabbay KH. Glycosylated hemoglobin in cystic fibrosis: distribution and relationship to glucose intolerance. Pediatr Res 1979;13.
- Mohan K, Miller H, Dyce P, Ledson MJ, Walshaw MJ. Comparison of the oral glucose tolerance test and serial glucose monitoring in cystic fibrosis patients. Thorax 2007;62:A102-3.
- Richmond R, McKenna D, Tierney S, Rowe R, Dodd M, Jones A. Can patients at risk of cystic fibrosis-related diabetes be identified early?. J Cyst Fibros 2008;7.
- Stutchfield PR, O’Halloran S, Teale JD, Isherwood D, Smith CS, Heaf D. Glycosylated haemoglobin and glucose intolerance in cystic fibrosis. Arch Dis Child 1987;62:805-10.
- Verma A, Claridge A, Havelock T, Biesty J, McMenna D, Webb AK. Re-audit of the screening protocol for cystic fibrosis related diabetes (CFRD) in an adult centre. Thorax 2002;57.
- Watson H, Barker H, Henman S, Lyon A, Haworth CS, Bilton D. Three day continuous subcutaneous glucose monitoring (CSGM) in the clinical management of adult patients with CF and diabetes (CFRD). J Cyst Fibros 2007;6.
- Yung B, Kemp M, Hooper J, Hodson M. Random blood glucose alone in the diagnosis of cystic fibrosis related diabetes. Lancet 1997;349.
- Yung B. Should the oral glucose tolerance test be performed as a routine screening test for diabetes in cystic fibrosis patients? Response to Allen et al. and Holl et al. Diabetes Care 1998;21:2199-200.
- Quittner AL, Buu A, Messer MA, Modi AC, Watrous M. Development and validation of The Cystic Fibrosis Questionnaire in the United States: a health-related quality-of-life measure for cystic fibrosis. Chest 2005;128:2347-54.
- Riekert KA, Bartlett SJ, Boyle MP, Krishnan JA, Rand CS. The association between depression, lung function, and health-related quality of life among adults with cystic fibrosis. Chest 2007;132:231-7.
- Urquhart DS, Field B, Bryon M, Jaffe A. Effects of lung function and exercise capacity on quality of life in cystic fibrosis using the UK cystic fibrosis questionnaire. Thorax 2007;62:A103-4.
- Thomas C, Mitchell P, O’Rourke P, Wainwright C. Quality-of-life in children and adolescents with cystic fibrosis managed in both regional outreach and cystic fibrosis center settings in Queensland. J Pediatr 2006;148:508-16.
- Klijn PH, van Stel HF, Quittner AL, van der NJ, Doeleman W, van der Schans CP, et al. Validation of the Dutch cystic fibrosis questionnaire (CFQ) in adolescents and adults. J Cyst Fibros 2004;3:29-36.
- Gee L, Abbott J, Hart A, Conway SP, Etherington C, Webb AK. Associations between clinical variables and quality of life in adults with cystic fibrosis. J Cyst Fibros 2005;4:59-66.
- Powers PM, Gerstle R, Lapey A. Adolescents with cystic fibrosis: family reports of adolescent health-related quality of life and forced expiratory volume in one second. Pediatrics 2001;107.
- Britto MT, Kotagal UR, Hornung RW, Atherton HD, Tsevat J, Wilmott RW. Impact of recent pulmonary exacerbations on quality of life in patients with cystic fibrosis. Chest 2002;121:64-72.
- Sawyer MG. Health-related quality of life of children and adolescents with chronic illness: a two year prospective study. Qual Life Res 2004;13:1309-19.
- Sawyer MG. A two-year prospective study of the health-related quality of life of children with chronic illness – The parents’ perspective. Qual Life Res 2005;14:395-40.
- Ingerski LM, Modi AC, Hood KK, Pai AL, Zeller M, Piazza-Waggoner C, et al. Health-related quality of life across pediatric chronic conditions. J Pediatr 2010;156:639-44.
- Congleton J. Quality of life in adults with cystic fibrosis. Eur Respir Rev 1997;7:74-6.
- Orenstein DM, Nixon PA, Ross EA, Kaplan RM. The quality of well-being in cystic fibrosis. Chest 1989;95:344-7.
- Kotwicki RJ, Condra L, Vermeulen L, Wolf T, Douglas J, Farrell PM. Assessing the quality of life in children with cystic fibrosis. WMJ 2001;100:50-4.
- Suri R, Metcalfe C, Wallis C, Bush A. Assessing the usefulness of outcomes measured in a cystic fibrosis treatment trial. Respir Med 2007;101:254-60.
- Eidt-Koch D, Mittendorf T, Greiner W. Cross-sectional validity of the EQ-5D-Y as a generic health outcome instrument in children and adolescents with cystic fibrosis in Germany. BMC Pediatr 2009;9.
- de Jong W, Kaptein AA, vanderSchans CP, Mannes GPM, vanAalderen WMC, Grevink RG, et al. Quality of life in patients with cystic fibrosis. Pediatr Pulmonol 1997;23:95-100.
- Bradley J, Dempster M, Wallace E, Elborn S. The adaptations of a quality of life questionnaire for routine use in clinical practice: the Chronic Respiratory Disease Questionnaire in cystic fibrosis. Qual Life Res 1999;8:65-71.
- Goldbeck L, Zerrer S, Schmitz TG. Monitoring quality of life in outpatients with cystic fibrosis: feasibility and longitudinal results. J Cyst Fibros 2007;6:171-8.
- Goldbeck L, Schmitz TG. Comparison of three generic questionnaires measuring quality of life in adolescents and adults with cystic fibrosis: the 36-item short form health survey, the quality of life profile for chronic diseases, and the questions on life satisfaction. Qual Life Res 2001;10:23-36.
- Weiner JR, Toy EL, Sacco P, Duh MS. Costs, quality of life and treatment compliance associated with antibiotic therapies in patients with cystic fibrosis: a review of the literature. Exp Opin Pharmacother 2008;9:751-66.
- Cruz I, Marciel KK, Quittner AL, Schechter MS. Anxiety and depression in cystic fibrosis. Semin Respir Crit Care Med 2009;30:569-78.
- Quittner AL, Barker DH, Snell C, Grimley ME, Marciel K, Cruz I. Prevalence and impact of depression in cystic fibrosis. Curr Opin Pulm Med 2008;14:582-8.
Appendix 1 Details of search strategy and PRISMA flow diagram
Ovid MEDLINE In-Process & Other Non-Indexed Citations and Ovid MEDLINE 1950 to May 2008, and Ovid EMBASE, 1980 to 2008 week 20
These databases were searched using the following search strategy:
-
exp Cystic Fibrosis/
-
exp Diabetes Mellitus/
-
(cystic fibrosis or cfrd).tw.
-
(diabet* or glucose or hyperglycaemia or hyperglycaemia or postprandial or post-prandial or insulin or hypoglycemia or hypoglycaemia or IGT or OGTT or CGMS).tw.
-
1 or 3
-
2 or 4
-
5 and 6.
MEDLINE = 1064 retrieved, EMBASE = 1281 retrieved.
Plus, auto-alerts were run in Ovid MEDLINE and EMBASE from May 2008 to December 2010, using the following search strategy:
-
(cystic fibrosis or cfrd).tw.
-
(diabet* or glucose or hyperglycemia or hyperglycaemia or postprandial or post-prandial or insulin or hypoglycemia or hypoglycaemia or IGT or OGTT or CGMS).tw.
-
1 and 2.
Web of Science Databases (Science Citation Index, Social Sciences Citation Index, 1970 – May 2008)
Title = ((cystic fibrosis or CFRD) and (diabet* or glucose or hyperglycemia or hyperglycaemia or postprandial or post-prandial or insulin or hypoglycemia or hypoglycaemia))
342 retrieved.
ISI Proceedings, 1990 to May 2008
Topic = ((cystic fibrosis or CFRD) and (diabet* or glucose or hyperglycaemia or hyperglycemia or glycemia or glycaemia or postprandial or post-prandial or insulin or hypoglycemia or hypoglycaemia))
116 retrieved.
Cochrane Central Register of Controlled Trials, Issue 2, 2008
(cystic fibrosis or CFRD):ti,ab,kw and (diabet* or glucose or hyperglycaemia or hyperglycemia or glycemia or glycaemia or postprandial or post-prandial or insulin or hypoglycemia or hypoglycaemia):ti,ab,kw
42 retrieved.
Meeting abstracts, searched up until 2010
Diabetes UK
ADA
EASD
European Cystic Fibrosis Society
Annual North American Cystic Fibrosis Conference
Annual Meeting of the ISPAD
89 downloaded.
Research in progress: searched in June 2011
ClinicalTrials.gov (http://clinicaltrials.gov/ct2/home)
Controlled-trials.com/http://www.controlled-trials.com/
UK Clinical Research Network (http://public.ukcrn.org.uk/search/)
FIGURE 5.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
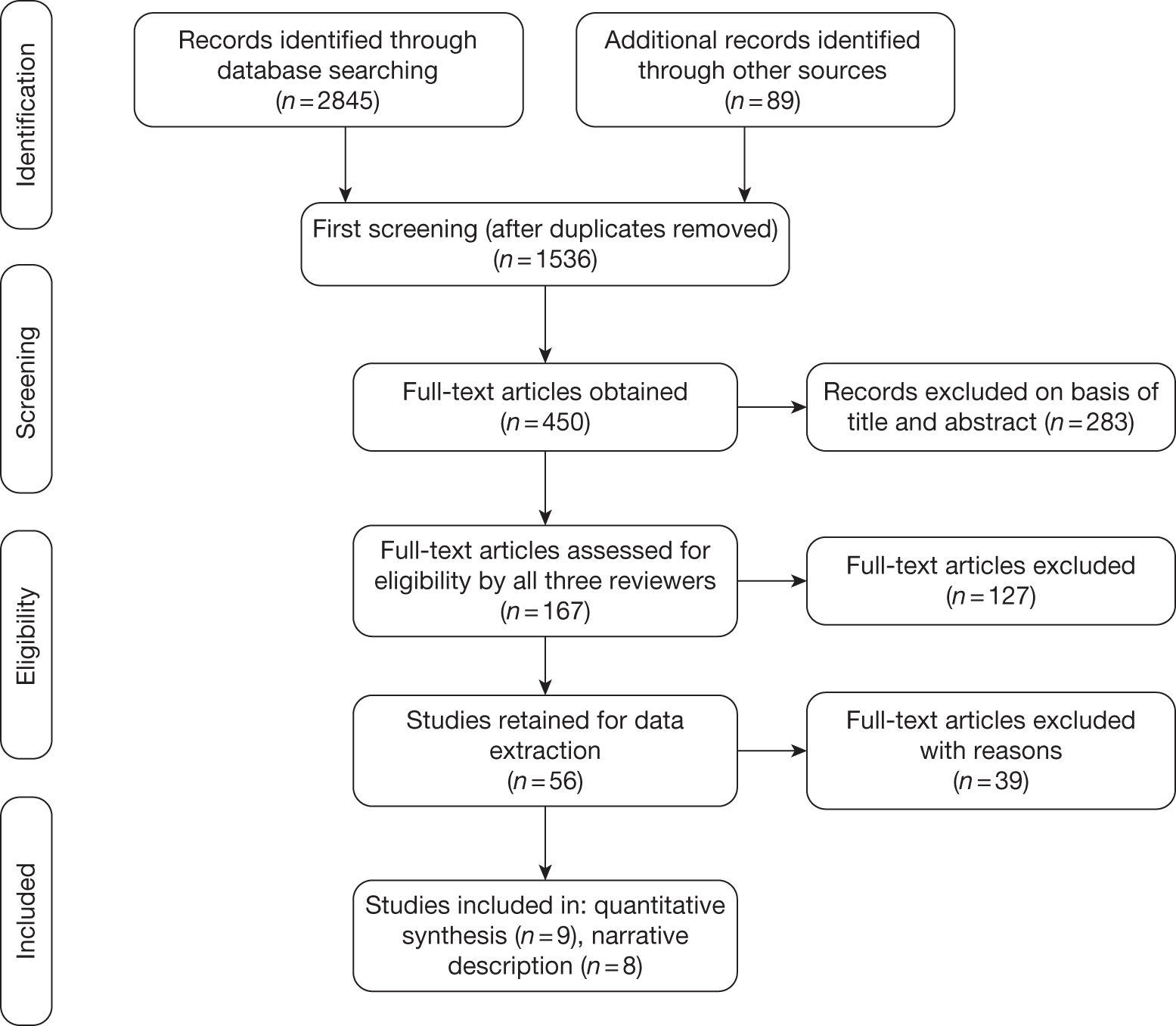
Appendix 2 Studies of treatment of cystic fibrosis-related diabetes and cystic fibrosis with non-diabetic hyperglycaemia
| Study ID | Study design | Type of patients | No. of patients | Age (years) | Treatment | Comparator(s) | Duration | Main outcome measures | Results | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|
| Ballmann 2003147 | Case series: before-and-after study | Patients with CFRD treated with glibenclamide who completed follow-up for 2 years | 19 | Mean 13.7 | Six patients who changed from glibenclamide to insulin | Patients remained on glibenclamide | 2 years | Nutritional status (BMI z-score), lung function (%FEV1) and metabolic control (HbA1c) | The mean time till starting insulin treatment was 4.5 years in those treated initially with glibenclamide | ‘More than 68% of those on glibenclamide were in a stable clinical condition (BMI z-score and %FEV1) and good metabolic control after 2 years’ |
| Bertele-Harms 1996143 | Case series: before-and-after study | CFRD | 20 | 12.8–26.5 | Glibenclamide | 15 years | The effect of glibenclamide in patients with CFRD | The mean duration of glibenclamide effectiveness was 2.4 years (range 0.6–5.5 years) but patients considered afterwards that delaying insulin treatment had been worthwhile | ||
| Bizarri 2006151 | Before-and-after study | Patients with CF with IGT | 6 | 9.2–27.8 | Insulin – glargine | 1.4 years median follow-up | HbA1c, BMI z-score, FEV1 and no. of hospitalisations for clinical exacerbation | There were significant improvements in both median BMI z-scores and median FEV1. No significant difference was observed in the median HbA1c or the median no. of hospitalisations for clinical exacerbation | ‘Early insulin glargine is well tolerated and safe – seemed to slow down the deterioration of the clinical status (particularly nutritional condition and lung function) seen in the years before treatment in some patients’ | |
| Boyle 2004148 | Retrospective comparison: 1 year before and 1 year after insulin treatment | 13 CFRD, 10 IGT, 7 NGT | 30 | Mean 26.9 | Insulin | 1 year | Weight gain and FEV1 changes with early insulin treatment – before and after a year of insulin treatment | Weight seemed to improve in all patients | ||
| Culler 1994142 | Case series | Patients with CF with IGT | 6 | 12–25 | Glipizide | None | 6 months | HbA1c, 24-hour urine glucose, insulin sensitivity, FPIR, changes in growth assessed as height, weight and BMI | Significant improvements in HbA1c, 24-hour urine glucose and FPIR, but not in insulin sensitivity or weight gain | ‘Glipizide can be used in the treatment of patients with CF with IGT, especially if a patient has elevated postprandial glucose levels but normal fasting BG levels; and if persistent hyperglycaemia or significant elevation of HbA1c occurs, then insulin therapy should be instituted’ |
| Dobson 2002138 | Case series | Long-standing CF, weight loss, deteriorating lung function, high random glucose values but normal OGTT (non-IGT PPH group) | 4 | 15–23 | Insulin | Weight and spirometry (FEV1/FVC) | Insulin treatment was accompanied by increases in both weight and spirometry in all four patients | Treatment is worthwhile even at the isolated PPH stage | ||
| Drummond 2006152 | Retrospective comparison: 5 years before and 5 years after insulin | CFRD, IGT and NGT (nos. of each not given) | 54 | Mean 27.6 | Insulin | Lung function and weight gain, 5 years before and 5 years after insulin initiation | FEV1 decline arrested after initiation and weight increased significantly | That insulin therapy improved the loss of lung function in patients with CF and recommended its commencement at the IGT stage (may include those in the Boyle 2004 abstract;146 gives data for 5 years before and after insulin treatment) | ||
| Drummond 2007156 | Case series Retrospective | Patients with CF on insulin for mean 7.3 years | 24 | 30.7 | Insulin | 6 months | Frequency of hypoglycaemia and the associated symptoms experienced in insulin-treated patients | Hypoglycaemic events were reported in 13 patients who experienced between one and four episodes, six patients (25%) had five or more and five patients experienced no hypoglycaemic events (21%). 75% of insulin-treated patients had hypoglycaemic unawareness | Hypoglycaemia episodes and hypoglycaemic episode unawareness were common among patients with CF | |
| Franzese 2005149 | Before-and-after study | Chronic CFRD (× 4); intermittent CFRD (× 4) | 8 | 10–20 | Group A: Four patients with chronic CFRD treated with rapid insulin in the previous 1–3 years |
Group B: Intermittent CFRD requiring insulin only during infections Group C: Control group (non-glargine treated) comprising six patients (aged 14–18 years) with intermittent CFRD |
6 months before and after glargine | BMI, FEV1, HbA1c and the number of lung infections | Significant decrease in the no. of lung infections in both groups A and B; no change was seen in the control group. There were no positive changes in HbA1c or BMI, and no hypoglycaemic events were recorded | Basal insulin may play a role in reducing the number of lung infections in both overt CFRD and pre-patients with CFRD (a follow-up of Franzese 2005147 study?) |
| Grover 2008157 | Randomised, non-blinded, crossover study | Patients with CFRD with FH receiving a single dose of bedtime NPH insulin plus rapid-acting insulin before meals | 19 | Mean 34 | Bedtime glargine (plus rapid insulin) | 24 weeks (12 weeks on each therapy) | HbA1c and weight change | Significantly greater reduction in FPG with glargine therapy (p = 0.03) but no changes in HbA1c and postprandial PG levels. Nineteen patients chose to continue glargine therapy, as they believed that daytime BG levels seemed more consistent and some were less worried about night-time hypoglycaemia | ‘Long-term studies are needed to determine the metabolic and nutritional impact of glargine in CFRD, but the initial data suggested that it is a promising therapy’ | |
| Hameed 2009163 | Before-and-after study | Newly diagnosed patients with CFRD, all with pancreatic insufficiency | 8 | Median 13.5 | Pre-breakfast detemir at median dose 0.1 units/kg/day | 15 weeks | Change in mean WtSDS (WtSDS),%FVC and %FEV1 | Significant changes in WtSDS (p = 0.003), %FVC (p = 0.002) and %FEV1 (p = 0.005) after treatment compared with 1 year prior to treatment | Once-daily detemir was well tolerated and resulted in significant weight gain and improved lung function | |
| Hardin 2009159 | Before-and-after study | Patients with CFRD who had been treated with at least three subcutaneous injections per day for a minimum of 6 months | 9 | Mean 27 | CSII | 6 months | HbA1c, body weight, lean body mass, and whole-body protein turnover | Significant improvements in both fasting and post-prandial BG levels, body weight, HbA1c and lean body mass. Protein catabolism was significantly decreased. No hypoglycaemic episodes, whereas prior to CSII patients reported several hypo glycaemic episodes per month | The use of CSII over 2 years led to improved glycaemic control and safety compared with multiple daily subcutaneous insulin injections. In addition, metabolic benefits were shown | |
| Hardy 2006153 | Before-and-after study | CF children with abnormal OGTT but normal fasting glucose | 27 | Not given | Group 1: insulin (n = 14) (owing to clinical deterioration) |
Group 2: no insulin (n = 13) Group 3: 55 patients with CF with normal OGTT |
1 year before and after | Growth and lung function | FEV1 declined significantly ( > 5%) before treatment in eight patients from group A, but improved in six of these eight after insulin treatment. FEV1 also declined in seven from group B, but improved in five of these seven without insulin treatment | Glargine arrested the progressive decline in lung function in patients with more severe undernutrition and hyperglycaemia, but it also improved in patients not given insulin (group B). However, the results from group B suggest that spontaneous improvement also occurs |
| Kentrup 1999144 | Randomised crossover trial | Patients with CF with IGT | 12 | 8–22 | Acarbose | Placebo | 14 days | There were significant reductions in PG, insulin and C-peptide with acarbose treatment compared with baseline values | Acarbose has a beneficial therapeutic effect on glucose tolerance in patients with CF, but its side effects may prevent patients from accepting it as a long-term therapy | |
| Lanng 1994134 | Case–control study: 6 years before and 2 years after onset of insulin therapy | CFRD with at least 2 years of follow-up on insulin | 18 patients and 18 control subjects | 3–28 | Insulin | Control group (n = 18) non-diabetic patients with CF who were matched with age, sex and presence of chronic lung infection | 6 years before and 2 years after insulin therapy | Body weight, BMI, FEV1, FVC, microscopy and culture of sputum and precipitins | Decline in BMI, FEV1 and FVC in the months leading up to the start of insulin therapy by 2 years, BMI, FEV1 and FVC approached those in the control subjects. The no. of weeks of intravenous anti-Pseudomonas treatment did not differ between the groups before and after insulin treatment | Insulin improves lung function after the insidious decline resulting from the pre-diabetic condition in patients with CF and its commencement when diagnosis of CFRD is made is recommended |
| McGinnity 2006154 | Case series | CFRD: All had received treatment with once-daily long-acting human insulin analogues for more than 12 months prior to the study | 5 | 11–18 | Long-acting insulin (detemir or glargine) | 3 days | BG was measured over a 3-day period using a MiniMed subcutaneous continuous glucose monitor | BG levels, for each of the 5 patients, respectively, were within normal limits 65%, 93%, 94%, 96% and 99% of the time. Mean glucose levels were (range) 7.6 mmol/l (2.2–17.2 mmol/l), 6.6 mmol/l (3.8–13.7 mmol/l), 5.3 mmol/l (2.2–9.0 mmol/l), 5.9 mmol/l (3.4–12.9 mmol/l), 6.4 mmol/l (4.1–11.8 mmol/l). Hyperglycaemia seems to have been commonest round midday. Symptomatic hypoglycaemia did not occur | Preliminary data indicate that good control is achievable with the early use of long-acting insulins for CFRD. CBGM was well tolerated | |
| Minicucci 2005150 | Case series | Twelve CFRD and three CF IGT | 15 | 14–34 | Group A: CFRD treated with insulin glargine (had been on insulin regular or rapid analogue before meals) (n = 6) |
Group B: CFRD FH– patients, diagnosed on the basis of OGTT (n = 6) Group C: CF IGT (insulin naive)(n = 3) |
Results were collected at the start of, and 3 months after, glargine treatment | HbA1c, BMI, frequency of hypoglycaemia and compliance to the therapy | Results showed no significant difference in HbA1c in any group. BMI changed little. Frequency of hypoglycaemia did not change in group A. No hypoglycaemic episodes in groups B and C | Glargine seemed to be safe and well accepted |
| Minicucci 2009162 | Randomised controlled study | Patients with CF with glucose intolerance | 45 | Group A: insulin glargine (0.2 units/kg/day) (n = 23) | Group B: No insulin (n = 22) | 9 months | After the enrolment, 11 patients left the study. The data after 9 months showed a non-statistical improvement in mean BMI, weight, %FEV1 in group A patients (+ 0.3 kg/m2, + 1 kg, + 6%, respectively) compared with a mean worsening in those in group B (–0.1 kg/m2, –0.3 kg, –2.5%, respectively). No adverse effects were reported | |||
| Mohan 2008158 | Before-and-after study | CFRD | 42 | 16–39 | Insulin | 5 years before and 3 years after insulin therapy | Included FEV1, FVC, BMI and the no. of pulmonary exacerbations requiring hospital admissions | Improvement 1 year for FEV1; 2 years for FVC, and 3 years for BMI. FEV1 value returned to baseline at 34 months. Annual rate of FVC decline was also similar to the pre-insulin values during the same period | Insulin treatment is associated with temporary improvement in lung function and BMI in symptomatic patients with CFRD, with FEV1 decline delayed by an average of 34 months | |
| Moran 2001145 | Pharmacodynamic study. Three study conditions administered in random order on separate mornings: (1) no preprandial diabetes medication, (2) insulin lispro preprandial, and (3) repaglinide preprandial | CFRD without FH | 7 | Mean 24 | Group 1: Insulin lispro |
Group 2: Repaglinide Group 3: Matched for age, sex and BMI to seven healthy control subjects |
Three occasions over 1- to 2-month period | PG and insulin levels were recorded at the beginning of the meal and after meal at 20-minute intervals for 5 hours | Insulin lispro seemed to be better than repaglinide on post-prandial glucose excursion. Hence, both lispro and repaglinide reduced PPH but insulin was more effective | |
| Moran 2009 – CFRDT Trial141 | Randomised trial | Seventy-four CFRD FH– patients and 26 with severe IGT | 100 enrolled, results for 81 completed | Mean 27 | Group A: Insulin aspart |
Group B: Repaglinide Group C: oral placebo |
Retrospective measurements 12 months prior to the study; prospective measurements 12 months after randomisation | Whether or not diabetes therapy improves BMI and lung function in CFRD FH– patients | Change in BMI for the 12 months prior to the study compared with the change during the study year showed a significant improvement for the CFRD FH– patients on insulin, but not repaglinide and placebo groups. All study arms for the CFRD FH– patients showed in decline FVC during the study when compared with 12 months prior to study and the insulin and repaglinide arms showed a reduction in decline in FEV1 | Preprandial rapid acting insulin given for 1 year significantly reversed the chronic weight loss in CFRD FH– patients, without any adverse effects. However, it had no significant effect on lung function or acute illnesses |
| Mozillo 2009160 | Preliminary data from an ongoing open study | Patients with CF with early glucose derangements: four had abnormal glucose tolerance on CGMS, nine had IGT, seven had DM without FH and two with DM with FH | 65 enrolled – data on first 22 patients | 12.4 | Insulin glargine | No control group | 1 year | Lung function, BMI, lung infections and HbA1c in patients with CF | 8.8% increase in %FEV1 (p = 0.01) and a 42% decrease in the no. of lung infections (p = 0.003). The BMI z-score and HbA1c did not show any significant difference for the whole group. Significant improvement found in those patients (n = 8) with the worst BMI z-scores | Glargine could benefit patients with CF with early glucose derangements |
| Nousia-Arvanitakis 200154 | Case series | Patients who developed CFRD in a 5-year follow-up of 30 patients with CF | 6 | 15–22 | Insulin: Biphasic (rapid and intermediate) | Control group of non-diabetic patients with CF matched with the diabetic group for age, sex, pubertal stage, BMI, FEV1 and SS | 5 years | BMI, FEV1, SS, intravenous glucose tolerance test and FPIR, at time of diagnosis of CFRD and six months after starting insulin | There was significant improvement in BMI, FEV1, and SS in all six patients following insulin treatment | An association between insulin hyposecretion and an overall deterioration in the clinical status of the patients with CF involving nutrition, lung function and clinical scores improved significantly after the institution of insulin. Important to identify patients with CF at risk of developing diabetes so early insulin therapy can be given |
| Onady 2006155 | Not randomised – patients chose treatment (case series?) | CFRD | 20 | 13–49 |
Group A: Insulin (n = 8) |
Group B: Sulfonylurea (n = 5) Group C: Metformin (n = 4) Group D: Thiazolidinedione (n = 3) |
10 years | No statistically significant differences in overall glycaemic control, changes in weight, liver function testing and FEV1 between oral agents and insulin. Four patients switched from insulin to oral agents owing to inadequate HbA1c control | OHAs were effective and safe in treating selected patients with CFRD and may provide an alternative for patients reluctant to use insulin | |
| Rolon 2001146 | Case–control study: 5 years before and after treatment | CFRD insulin treated | 14 patients and 14 control subjects | Insulin | Fourteen non-diabetic patients matched for age, sex and chronic lung infection by P. aeruginosa | 5 years before and after treatment | Outcome measures used were BMI, BMI z-score, FVC, FEV1, insulin regimen, mean insulin dosage, hypoglycaemic events and mean HbA1c value | Only seven patients had 5 years of follow-up at the time of study. After insulin was started, respiratory function improved and the BMI returned to normal (compared with the French population) within 2 years. A decreased rate of FVC decline was seen in five of the seven patients 5 years post insulin (p = 0.1) and FEV1 improved in all seven patients after the start of treatment (p = 0.02) | Clinical status of pre-diabetic patients with CF deteriorates before the start of insulin therapy; insulin treatment improves anabolism and provides good glycaemic control with few hypoglycaemic events in patients with CFRD with or without FH | |
| Rosenecker 2001120 | Not randomised (two case series) | CFRD | 45 | Mean 24 and 27 | Glibenclamide (n = 11) | Insulin (n = 34) | Insulin 7.6 years, glibenclamide 3.5 years | FEV1, FVC, weight for height and SS | At the end of the study, no significant differences were found between the two groups in the most recent FEV1, FVC, SS or BMI |
‘CFRD can be treated orally with glibenclamide in some patients with CF, at least in a subgroup with a late onset of diabetes. FEV1, FVC, SS and BMI were maintained equally well by both treatments’ No comparison between treatments can be made from a non-randomised study |
| Sulli 2007139 | Case reports | Patients with CFRD on insulin pump therapy. All were receiving MDIs, 4 injections/day in the year prior to CSII use | 3 | 5, 21, 28 | CSII | 2 years of CSII treatment | HbA1c, weight | After 2 years, all three patients had significant reductions (between 1.2% and 1.7%) in HbA1c levels; annual mean level of BMI increased and the insulin requirements decreased. No DKA or hypoglycaemic episodes. Two episodes of lipohypertrophy reported | The use of CSII in patients with CFRD resulted in improvements in both the metabolic controls of diabetes and the nutritional status with no concomitant problems |
Appendix 3 The quality assessment of diagnostic accuracy studies tool to assess the quality of diagnostic accuracy studies
| Item | Yes | No | Unclear | Not applicable | |
|---|---|---|---|---|---|
| 1 | Was the spectrum of patients representative of the patients who will receive the test in practice? | ||||
| 2 | Is the reference standard likely to correctly classify the target condition? | ||||
| 3 | Is the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? | ||||
| 4 | Did the whole sample, or a random selection of the sample, receive verification using a reference standard of diagnosis? | ||||
| 5 | Did patients receive the same reference standard regardless of the index test result? | ||||
| 6 | Was the reference standard independent of the index test (i.e. the index test did not form part of the reference standard)? | ||||
| 7a | Were the reference standard results interpreted without knowledge of the results of the index test? | ||||
| 7b | Were the index test results interpreted without knowledge of the results of the reference standard? | ||||
| 8 | Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | ||||
| 9 | Were uninterpretable/intermediate test results reported? | ||||
| 10 | Were withdrawals from the study explained? | ||||
| 11 | Were definitions of the different hyperglycaemic states given? (diabetes, IGT, lag storage, IFG) | ||||
Appendix 4 Data extractions of diagnostic studies
Buck 2000174,182
| Author, year, country | Buck, 2000, Germany |
| Reference | Monatsschr Kinderh 2000;148:698–701 |
| Aim | To examine FBG HbA1c level and OGTT in the diagnosis of patients with CF during routine care |
Verification of study eligibility
| Study design | Case series |
| Screening test | HbA1c |
| Reference test | OGTT (WHO criteria) |
| Accuracy | 3 × 2 table reduced to a 2 × 2 table |
| Target disorder | Diabetes and IGT |
Study characteristics: population
| Target population | Patients with CF during routine care in two university children’s hospitals in Germany (Ulm, n = 32; Hannover, n = 70) | |
| CF diagnosis | NR | |
| Inclusion criteria | NR | |
| Exclusion criteria | NR | |
| Prior testing | NR | |
| Recruitment procedures | NR | |
| Data collection | Part of routine diagnostic procedures | |
| Participant characteristics | % male | 59 |
| Median age, years | 13 (range 5–33) | |
| Mean BMI, kg/m2 (SD) | NR | |
| Long-term oral steroids | None | |
| Enteral feeding | NR | |
| Established chronic liver disease | NR | |
| Pancreatic insufficiency | NR |
Study characteristics: screening tests
| No. of tests | 1 |
| Tests | HbA1c |
| Description of tests | HbA1c level was determined using high-performance liquid chromatography (Pharmacia, Freiburg, Germany) |
| Setting | University children’s hospital |
| Timing | Same time as OGTT? |
| Cut-offs | Owing to variations in the conditions of carrying out the test, there were differences between the normal range of the method between the children’s hospital in Ulm (HbA1c normal range of 3.5–5.7%) and the children’s hospital in Hannover (HbA1c normal range 3.5–5.0%). To take account of these differences, the HbA1c value of a given patient was classified as normal or pathological with respect to the method of measurement used |
Study characteristics: reference test – oral glucose tolerance test
| Reference test | OGTT |
| Delay from index test | NR – but assume same time as HbA1c? |
| Description | Receive 1.75 g/kg body weight of glucose (maximum 75 g) at 8 am to drink over 3–4 minutes |
| Setting | University children’s hospital |
| Timing | Given at 8 am after 10- to 14-hour fast |
| Cut-offs |
According to WHO criteria NGT if FBG and the 2-hour value were < 140 mg/dl IGT if fasting BG was < 140 mg/dl and the 2-hour value was between 140 and 200 mg/dl Diabetes mellitus if fasting BG was > 140 mg/dl and/or the 2-hour value was > 200 mg/dl |
Study characteristics: outcomes
| Accuracy | 3 × 2 table reduced to two 2 × 2 tables. Calculated sensitivity and specificity values for: diabetes + IGT vs NGT and diabetes vs IGT + NGT |
| Patient acceptability | NR |
| Failure rate of test | NR |
Study results: recruitment
| Original population | (A) | NR |
| Pre-enrolment exclusions | (B) | NR |
| Reasons, e.g. population characteristics | ||
| No. invited to participate (A–B) | (C) | NR |
| Refusal to participate | (D) | NR |
| Reasons, e.g. missing data, etc. | ||
| No. enrolled (C–D) | (E) | NR |
| Post-enrolment exclusions | (F) | NR |
| Reasons, e.g. missing data, etc. | ||
| Analysable data (E–F) | (G) | 102 |
| Completeness of follow-up (G/C × 100%) |
Study results: accuracy
| Screening test | OGTT reference test cut-offs | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|
| HbA1c > 5.7% (Ulm) or > 5.0% Hannover | Diabetes + IGT vs NGT | 22.9 (10.45 to 40.14) | 95.5 (87.45 to 99.02) |
| HbA1c > 5.7% (Ulm) or > 5.0% Hannover | Diabetes vs IGT+ NGT | 23.1 (5.31 to 53.80) | 91.0 (83.05 to 96.03) |
| Test | Diabetes + IGT | NGT | Total |
|---|---|---|---|
| HbA1c-positive | 8 | 3 | 11 |
| HbA1c-negative | 27 | 64 | 91 |
| Total | 35 | 67 | 102 |
| Test | Diabetes | IGT + NGT | Total |
|---|---|---|---|
| HbA1c-positive | 3 | 8 | 11 |
| HbA1c-negative | 10 | 81 | 91 |
| Total | 13 | 89 | 102 |
De Luca 1991183
| Author, year, country | De Luca, 1991, Italy |
| Reference | Horm Metab Res 1991;23:495–8 |
| Aim | To assess the ability of glycosylated haemoglobin assay to discriminate different degrees of glucose tolerance |
Verification of study eligibility
| Study design | Case series |
| Screening test(s) | HbA1c |
| Reference test | Full OGTT |
| Accuracy reported (sensitivity, specificity only; 2 × 2) | 2 × 2 |
| Target disorder | CF Diabetes and CF IGT |
Study characteristics: population
| Target population | Thirty-nine CF children and adolescents attending the CF centre of the University Hospital | |
| CF diagnosis | NR | |
| Inclusion criteria | Negative family history for diabetes mellitus and repeatedly normal glucose values. None had been receiving treatment for β-lactam antibiotics and/or steroids for the last 3 months | |
| Exclusion criteria | Severe liver and/or kidney dysfunction as well as acute infections | |
| Prior testing | Repeatedly normal, i.e. < 115 mg/dl PG found on random assessments during the year | |
| Recruitment procedures | Unclear | |
| Data collection | Prospective | |
| Participant characteristics | % male | Reported as per cent of those who agreed to participate |
| Mean age, years (SD) | 13.6 (4.7), range 5.5–22.2 | |
| Mean BMI, kg/m2 (SD) | 17.7 (2.5), range 13.3–24.2 | |
| Long-term oral steroids | None taken for last 3 months | |
| Enteral feeding | NR | |
| Established chronic liver disease | Exclusion | |
| Pancreatic insufficiency | NR |
Study characteristics: screening tests
| No. of tests | 1 |
| Tests | HbA1c |
| Description of tests | Blood samples were taken at time 0 minutes on OGTT, HbA1c was assessed by high-pressure liquid chromatography with a fully automated instrument |
| Setting | CF centre |
| Timing | Same day as OGTT |
| Cut-offs | HbA1c > 6% (normal range in laboratories was 4–6%) |
Study characteristics: reference test
| Reference test | Full OGTT |
| Delay from index test | None |
| Description | After an overnight fast, patients underwent a standard OGTT (1.75 g/kg body weight, maximum 75 g). PG was determined by means of the glucose oxidase method |
| Setting | CF centre |
| Timing | Blood samples were taken at –10, 0, 30, 60, 90, 120 and 180 minutes after glucose load, for measurement of PG and insulin levels |
| Cut-offs | WHO criteria |
Study characteristics: outcomes
| Accuracy |
Detection of diabetic, impaired and NGT for OGTT and diabetes vs non-diabetes for HbA1c 3 × 2 tables reduced to two 2 × 2 tables: one with diabetes + IGT vs normal and other with diabetes vs IGT + normal |
| Patient acceptability | NR |
| Failure rate of test | NR |
Study results: recruitment
| Original population | (A) | NR |
| Pre-enrolment exclusions | (B) | NR |
| Reasons, e.g. population characteristics | ||
| No. invited to participate (A–B) | (C) | NR |
| Refusal to participate | (D) | NR |
| Reasons, e.g. missing data, etc. | ||
| No. enrolled (C–D) | (E) | NR |
| Post-enrolment exclusions | (F) | NR |
| Reasons, e.g. missing data, etc. | ||
| Analysable data (E–F) | (G) | 39 |
| Completeness of follow-up (G/C × 100%) | NR |
Study results: test acceptability
| Test | NR |
Study results: accuracy
| Screening test | Reference test | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|
| HbA1c > 6% | Diabetes vs IGT + NGT | 100 (19.29 to 100.00) | 89.2 (74.56 to 96.91) |
| HbA1c > 6% | Diabetes vs IGT + NGT | 22.2 (3.47 to 59.94) | 86.7 (69.26 to 96.16) |
Diabetes versus impaired glucose tolerance + normal glucose tolerance
| Test | Disease | No disease | Total |
|---|---|---|---|
| Positive | 2 | 4 | 6 |
| Negative | 0 | 33 | 33 |
| Total | 2 | 37 | 39 |
Diabetes ± impaired glucose tolerance versus normal glucose tolerance
| Test | Disease | No disease | Total |
|---|---|---|---|
| Positive | 2 | 4 | 6 |
| Negative | 7 | 26 | 33 |
| Total | 9 | 30 | 39 |
De Schepper 1991184
| Author, year, country | De Schepper, 1991, Belgium |
| Reference | Eur J Pediatr 1991;150:403–6 |
| Aim | To evaluate the correlation of serial HbA1c determinations with the results of the OGTT |
Verification of study eligibility
| Study design | Case series |
| Screening test | HbA1c |
| Reference test | ROGTT |
| Accuracy reported | 2 × 2 |
| Target disorder | IGT (GI) |
Study characteristics: population
| Target population | Forty-eight patients with CF | |
| CF diagnosis | NR | |
| Inclusion criteria | Normal fasting glycaemia (< 120 mg/dl) and a clinically stable condition at the moment of testing. No other oral medication was taken by the patients in the 2 months preceding the testing | |
| Exclusion criteria | NR | |
| Prior testing | Initial evaluation included an OGTT, HbA1c determination, liver function studies, lung perfusion scintigraphy and ultrasonography of the liver | |
| Recruitment procedures | NR | |
| Data collection | Prospective | |
| Participant characteristics | % male | 48 |
| Mean age, years (SD) | IGT = 15.4 (range 2–29), NGT = 11.7 (range 3–23) | |
| Mean weight index (%)a | IGT = 85.5; NGT = 86.0 | |
| Long-term oral steroids | No oral medication (apart from pancreatic enzyme replacement) was taken by patients in the 2 months preceding testing | |
| Enteral feeding | NR | |
| Established chronic liver disease |
Mean serum transaminase levels normal in both groups; elevated levels in six patients Transaminases (IU) (n < 35 IU) IGT = 20 (range 7–46); NGT 31 (range 7–143) |
|
| Pancreatic insufficiency | All patients showed ultrasound abnormalities of the pancreas and were receiving pancreatic enzyme replacement |
Study characteristics: screening tests
| No. of tests | 1 |
| Tests | HbA1c |
| Description of tests | Determined by iso-electric focusing using a modified commercial kit |
| Setting | NR |
| Timing | Same time |
| Cut-offs | Normal = HbA1c < 7.5% |
Study characteristics: reference test
| Reference test | OGTT |
| Delay from index test | Same time |
| Description | Oral glucose load of 1.75 g/kg body weight (maximum 75 g) was given following an overnight fast. Intra-assay variation is < 9% and inter-assay variation 12% |
| Setting | NR |
| Timing | After an overnight fast and again after 120 minutes following glucose load |
| Cut-offs | Abnormal if glucose concentration at 120 minutes was > 140 mg/dl |
Study characteristics: outcomes
| Accuracy | Detection of IGT (IGT + diabetes) and NGT on OGTT and elevated HbA1c levels (> 7.5%) |
| Patient acceptability | NR |
| Failure rate of test | NR |
Study results: recruitment
| Original population | (A) | NR |
| Pre-enrolment exclusions | (B) | NR |
| Reasons, e.g. population characteristics | ||
| No. invited to participate (A–B) | (C) | NR |
| Refusal to participate | (D) | NR |
| Reasons, e.g. missing data, etc. | ||
| No. enrolled (C–D) | (E) | NR |
| Post-enrolment exclusions | (F) | NR |
| Reasons, e.g. missing data, etc. | ||
| Analysable data (E–F) | (G) | NR |
| Completeness of follow-up (G/C × 100%) |
Study results: test acceptability
| Test | NR |
Study results: accuracy
| Screening test | Reference test | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|
| HbA1c > 7.5% | Diabetes + IGT vs NGT in OGTT | 73.3 (44.91 to 92.05) | 66.7 (48.17 to 82.02) |
| Test | Disease | No disease | Total |
|---|---|---|---|
| Positive | 11 | 11 | 22 |
| Negative | 4 | 22 | 26 |
| Total | 15 | 33 | 48 |
Lee 2007185
| Author, year, country | Lee, 2007, Canada |
| Reference | J Cyst Fibros 2007;6:274–6. |
| Aim | To evaluate the GCT (50 g, 1-hour GCT) as a screen for glucose intolerance in patients with CF |
Verification of study eligibility
| Study design | Case series |
| Screening tests |
|
| Reference test | OGTT |
| Accuracy reported | 2 × 2, sensitivity, specificity |
| Target disorder | IGT and diabetes |
Study characteristics: population
| Target population | Data were obtained from routine blood work performed on patients who attended the adult CF clinic at St Paul’s Hospital, Vancouver, Canada between June 2002 and May 2003 | |
| CF diagnosis | NR | |
| Inclusion criteria | Patients attending the adult CF clinic were eligible | |
| Exclusion criteria | Patients previously diagnosed with CFRD were not tested and transplant patients were followed elsewhere | |
| Prior testing | NR | |
| Recruitment procedures | NR | |
| Data collection | Unclear. Likely to be retrospective – data ‘obtained’ from routine blood work | |
| Participant characteristics | % male | 53 (30/57) |
| Mean age, years (SD) | 32.6 | |
| Mean BMI, kg/m2 (SD) | NR | |
| Long-term oral steroids | NR | |
| Enteral feeding | NR | |
| Established chronic liver disease | NR | |
| Pancreatic insufficiency | NR |
Study characteristics: screening tests
| No. of tests | 2 |
| Tests |
|
| Description of tests |
GCT consisted of a 50-g glucose load administered in a non-fasting state and followed by glucose measurement 1 hour later. Patients were required to stay seated at the laboratory Glucose was measured on serum samples using oxidase reagents and a Vitros 950 analyzer (Ortho-clinical Diagnostics, Rochester, NY, USA) |
| Setting | Cystic fibrosis clinic annual review visit |
| Timing | GCT undertaken during annual review visit or immediately afterwards |
| Cut-offs |
Criteria for a positive test: GCT: > 7.8 mmol/l FBG: ≥ 6.0 mmol/l HbA1c: > 6.0% IFG defined as FBG: ≥ 6.0 mmol/l and < 7.0 mmol/l |
Study characteristics: reference test
| Reference test | OGTT |
| Delay from index test | Aimed to be performed within 1 week of annual review. Only 19/31 (61%) tests were completed within the requested 1-week period. Time delay between tests ranged from 1 to 264 days, median 7 days, mean 35 days |
| Description | After an overnight fast the patient was asked to drink a solution containing 1.75 g/kg body weight (maximum 75 g) of glucose BP dissolved in 250 ml of water within 2–3 minutes |
| Setting | Return visit to cystic fibrosis clinic |
| Timing | Blood samples were taken just before and 2 hours after ingestion of the glucose solution |
| Cut-offs |
Patients’ glucose tolerance status was classified into normal, impaired or diabetic glucose tolerance. Criteria for a positive test result was OGTT ≥ 7.8 mmol/l, i.e. IGT IGT defined as: OGTT = 7.8–11.0 mmol/l CFRD without FH: OGTT ≥ 11.0 mmol/l with FBG < 7.0 mmol/l CFRD with FH: OGTT ≥ 11.0 mmol/l with FBG ≥ 7.0 mmol/l |
Study characteristics: outcomes
| Accuracy |
Detection of IGT Patients with IGT considered to be test- and reference test-positive by study authors |
| Patient acceptability | Not directly assessed, but can be inferred from uptake rate of test |
| Failure rate of test | NR |
Study results: recruitment
| Original population | (A) | NR |
| Pre-enrolment exclusions | (B) | NR |
| Reasons, e.g. population characteristics | ||
| No. invited to participate (A–B) | (C) | NR |
| Refusal to participate | (D) | NR |
| Reasons, e.g. missing data, etc. | ||
| No. enrolled (C–D) | (E) | 57 |
| Post-enrolment exclusions | (F) |
26 (GCT/OGTT comparison) 14 (HbA1c/OGTT comparison) |
| Reasons, e.g. missing data, etc. | Did not complete tests (14 OGTT) | |
| Analysable data (E–F) | (G) | 31 for GCT; 43 for HbA1c |
| Completeness of follow-up (G/C × 100%) | NR |
Study results: test acceptability
| GCT | (23%) 13/57 did not complete GCT but did complete OGTT |
| OGTT | (23%) 13/57 did not complete OGTT but did complete GCT |
| HbA1c | (2%) 1/57 did not complete HbA1c |
Study results: accuracy
| Screening test | Reference test | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|
| GCT > 7.8 mmol/l | OGTT ≥ 7.8 mmol/l | 100 (66.21 to 100.00) | 50.0 (28.25 to 71.75) |
| HbA1c > 6.0% | OGTT ≥ 7.8 mmol/l | 50.0 (23.12 to 76.88) | 89.7 (72.62 to 97.69) |
| Test | Disease: OGTT positive (≥ 7.8 mmol/l) | No disease: OGTT negative | Total |
|---|---|---|---|
| Positive: 50-g non-fasting 1-hour GCT | 9 | 11 | 20 |
| Negative | 0 | 11 | 11 |
| Total | 9 | 22 | 31 |
| Test | Disease: OGTT positive (≥ 7.8 mmol/l) | No disease: OGTT negative | Total |
|---|---|---|---|
| Positive: HbA1c > 6.0% | 7 | 3 | 10 |
| Negative | 7 | 26 | 33 |
| Total | 14 | 29 | 43 |
Magni 1996186
| Author, year, country | Magni, 1996, Italy |
| Reference | Eur J Lab Med 1996;4:6–10 |
| Aim | To identify which test among the simpler and faster ones is able to recognise at an early stage glucose metabolism alteration in CCF, taking OGTT as reference test |
Verification of study eligibility
| Study design | Case series |
| Screening tests | HbA1c, fasting glycaemia, 120-minute glycaemia |
| Reference test | Full OGTT |
| Accuracy reported | 2 × 2 tables |
| Target disorder | Diabetes and glucose intolerance |
Study characteristics: population
| Target population | Sixty-five inpatients admitted to the centre | |
| CF diagnosis | At least two positive sweat tests performed according to Gibson and Cooke | |
| Inclusion criteria | Age > 10 years; clinical remission from possible respiratory exacerbations | |
| Exclusion criteria | Glucose metabolism abnormality previously known; treatment with corticosteroids at time of observation | |
| Prior testing | NR | |
| Recruitment procedures | Randomly chosen following order of admission | |
| Data collection | Prospective | |
| Participant characteristics | % male | 57 |
| Mean age, years (SD) | 17.75 (5.2), range 10–38 | |
| Mean BMI, kg/m2 (SD) | NR | |
| Long-term oral steroids | None | |
| Enteral feeding | NR | |
| Established chronic liver disease | NR | |
| Pancreatic insufficiency (%) | 72 |
Study characteristics: screening tests
| No. of tests | 3 |
| Tests | HbA1c, fasting glycaemia, 120-minute glycaemia |
| Description of tests |
HbA1c by ion exchange chromatography as per cent of the total haemoglobin Glycaemia 120 minutes after breakfast (a standard meal was not used) Fasting glycaemia (as part of OGTT time = 0 minutes) |
| Setting | Same as OGTT? |
| Timing | Same as OGTT? |
| Cut-offs |
HbA1c > 5.3% and 5.1% Fasting glycaemia > 88 mg% and 85 mg% 120-minute glycaemia > 84 mg% |
Study characteristics: reference test
| Reference test | OGTT |
| Delay from index test | Does not specifically say but assume it is within a few days, as subjects were inpatients |
| Description | Test in morning, after at least 10 hours’ fasting: 1.75 g glucose/kg body weight, maximum 75 g |
| Setting | Inpatients admitted to CF centre in Verona |
| Timing | Glucose assay on venous plasma at times 0, 30, 60, 90, 120 and 180 minutes |
| Cut-offs |
Used National Diabetes Data Group Criteria: Glucose intolerance = 120-minute glucose > 140 mg/dl Diabetes = 120-minute glucose > 200 mg/dl |
Study characteristics: outcomes
| Accuracy |
Altered OGTT (normal + IGT) vs diabetes-derived 2 × 2 tables for HbA1c and fasting glycaemia Non-diabetic (normal + IGT) vs diabetic OGTT-derived 2 × 2 tables for HbA1c and fasting glycaemia and 120 minutes postprandial glycaemia |
| Patient acceptability | NR |
| Failure rate of test | NR |
Study results: recruitment
| Original population | (A) | NR |
| Pre-enrolment exclusions | (B) | NR |
| Reasons, e.g. population characteristics | ||
| No. invited to participate (A–B) | (C) | NR |
| Refusal to participate | (D) | NR |
| Reasons, e.g. missing data, etc. | ||
| No. enrolled (C–D) | (E) | NR |
| Post-enrolment exclusions | (F) | NR |
| Reasons, e.g. missing data, etc. | ||
| Analysable data (E–F) | (G) | 65 |
| Completeness of follow-up (G/C × 100%) |
Study results: test acceptability
| Test | NR |
Study results: accuracy
| Test | Reference test | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|
| HbA1c > 5.1% | Diabetes + IGT vs NGT | 60.0 (36.07 to 80.83) | 68.9 (53.35 to 81.82) |
| Fasting glycaemia > 85 mg% | Diabetes + IGT vs NGT | 70.0 (45.73 to 88.03) | 64.4 (48.78 to 78.12) |
| 120 min after meal glycaemia > 84 mg% | Diabetes + IGT vs NGT | 60.0 (36.07 to 80.83) | 68.9 (53.35 to 81.82) |
| HbA1c > 5.3% | Diabetes vs IGT + NGT | 100 (83.01 to 100.00) | 62.2 (46.54 to 76.22) |
| Fasting glycaemia > 88 mg% | Diabetes vs IGT + NGT | 100 (83.01 to 100.00) | 55.6 (40.00 to 70.35) |
| Test | Disease (diabetes + IGT) | No disease (NGT) | Total |
|---|---|---|---|
| Positive (HbA1c > 5.1%) | 12 | 14 | 26 |
| Negative | 8 | 31 | 39 |
| Total | 20 | 45 | 65 |
| Test | Disease (diabetes + IGT) | No disease (NGT) | Total |
|---|---|---|---|
| Positive (fasting glycaemia > 85 mg% | 14 | 16 | 30 |
| Negative | 6 | 29 | 35 |
| Total | 20 | 45 | 65 |
| Test | Disease (diabetes + IGT) | No disease (normal OGTT) | Total |
|---|---|---|---|
| Positive 120-minutes postprandial glycaemia > 84 mg% | 12 | 14 | 26 |
| Negative | 8 | 31 | 39 |
| Total | 20 | 45 | 65 |
| Test | Disease (diabetes) | No disease (normal + IGT) | Total |
|---|---|---|---|
| Positive (HbA1c > 5.1%) | 20 | 20 | 40 |
| Negative | 0 | 25 | 25 |
| Total | 20 | 45 | 65 |
| Test | Disease (diabetes) | No disease (normal + IGT) | Total |
|---|---|---|---|
| Positive | 20 | 20 | 40 |
| Negative | 0 | 25 | 25 |
| Total | 20 | 45 | 65 |
Moreau 2008187
| Reviewers initials | VH, PR |
| Author, year, country | Moreau, 2008, France |
| Reference | Horm Metab Res 2008;40:502–6 |
| Aim |
To evaluate the profile of glucose tolerance in adults with CF with OGTT To compare results with those obtained by continuous subcutaneous glucose monitoring |
Verification of study eligibility
| Study design | Case series |
| Screening test | CGM |
| Reference test | OGTT |
| Accuracy reported | 2 × 2 table |
| Target disorder | Diabetes and IGT |
Study characteristics: population
| Target population | CF patients in pneumology department | |
| CF diagnosis | Based on clinical features and positive CF genotype | |
| Inclusion criteria | CF patients, fasting glucose < 126 mg/dl; aged ≥ 15 years | |
| Exclusion criteria | Taking steroid or any medical conditions, such as pulmonary exacerbation of acute infection or previous history of hyperglycaemia | |
| Prior testing | All patients controlled for a stable lung function and nutritional state without any diet | |
| Recruitment procedures | Consecutively admitted for yearly check-up in pneumology department | |
| Data collection | Prospective, from February 2004 to September 2006 | |
| Participant characteristics | % male | 55.1% |
| Mean age, years (SD) | NGT = 25.7 (7.1); IGT = 19.7 (4.1); diabetes = 19.1 (4.3) | |
| Mean BMI, kg/m2 (SD) | NGT = 20.8 (2.3); IGT = 20.9 (3.2); diabetes = 18.3 (2.1) | |
| Long-term oral steroids | Nil | |
| Enteral feeding | NR | |
| Established chronic liver disease | NR | |
| Exocrine pancreatic insufficiency (%) | NGT = 73, IGT = 92, diabetes = 90 |
Study characteristics: screening tests
| No. of tests | 1 |
| Tests | CGMS over 3 days |
| Description of tests | Medtronic and Sylmar – subcutaneous glucose-sensing device connected by a cable to a pager-sized glucose monitor. Downloaded data on to PC (MiniMed) |
| Setting | At home in ambulatory conditions with usual dietary intake – over a 3-day period |
| Timing | Registered glucose concentration every 10 seconds and stored an average value every 5 minutes. Total 288 data points collected every day (range 40–400 mg/dl) |
| Cut-offs | Glucose AUC expressed as mean per day of area including all glucose values > 140 mg/dl over 3 days and duration of hyperglycaemia period in per cent of daily monitoring for glucose value as > 140 mg/dl during 3-day period |
Study characteristics: reference test
| Reference test | OGTT |
| Delay from index test | CGMS 1 month after OGTT |
| Description | Subjects drank glucose solution with dose of 1.75 g/kg (up to maximum of 75 g) over 2 minutes. BG and C-peptides samples collected 2 hours after glucose load |
| Setting | Pneumology department |
| Timing | Two hour (venous glucose) |
| Cut-offs | WHO criteria: NGT = < 140 mg/dl, IGT = 140 to 200 mg/dl, diabetes > 200 mg/dl |
Study characteristics: outcomes
| Accuracy | Detection of subjects with either NGT, IGT or diabetes; two 2 × 2 tables derived |
| Patient acceptability | NR |
| Failure rate of test | NR |
Study results: recruitment
| Original population | (A) | NR |
| Pre-enrolment exclusions | (B) | NR |
| Reasons, e.g. population characteristics | ||
| No. invited to participate (A–B) | (C) | NR |
| Refusal to participate | (D) | NR |
| Reasons, e.g. missing data, etc. | ||
| No. enrolled (C–D) | (E) | 49 |
| Post-enrolment exclusions | (F) | 0 |
| Reasons, e.g. missing data, etc. | ||
| Analysable data (E–F) | (G) | 49 |
| Completeness of follow-up (G/C × 100%) |
Study results: test acceptability
| Test |
Study results: accuracy
| Test | Reference test | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|
| CGMS | Diabetes vs IGT + NGT | 100 (68.97 to 100.00) | 56.41% (39.62 to 72.18) |
| CGMS | Diabetes + IGT vs NGT | 70.37 (49.82 to 86.21) | 63.64% (40.67 to 82.76) |
Notes
|
Correlation between blood and subcutaneous glucose measurements was good (r = 0.95, p < 0.001) Peak of CGMS glucose reached 182 ± 60 mg in NGT group despite normal glucose profile at OGTT Thirty-eight per cent of CF subjects with normal glucose profile and 52% with IGT at OGTT had pathological glucose excursions Glucose excursions > 200 mg/dl were observed in all patients with CFRD |
| Test | Disease (diabetes) | No disease (IGT + NGT) | Total |
|---|---|---|---|
| Positive (CGMS) | 10 | 17 | 27 |
| Negative | 0 | 22 | 22 |
| Total | 10 | 39 | 49 |
| Test | Disease (diabetes + IGT) | No disease (NGT) | Total |
|---|---|---|---|
| Positive (CGMS) | 19 | 8 | 27 |
| Negative | 8 | 14 | 22 |
| Total | 27 | 22 | 49 |
Mueller-Brandes 2005205
| Reviewers initials | PR |
| Author, year, country | Mueller-Brandes C, 2005, Germany |
| Reference | Eur Respir J 2005;25:715–17 |
| Aim | To determine how many patients with impaired glucose regulation would remain undiagnosed, and therefore untreated when using OGTT only in patients with IFG based on new ADA criteria |
Verification of study eligibility
| Study design | Case series |
| Screening test | FPG |
| Reference test | OGTT |
| Accuracy reported | Sensitivity and specificity |
| Target disorder | Diabetes, IFG |
Study characteristics: population
| Target population | Patients with CF | |
| CF diagnosis | NR | |
| Inclusion criteria | Age ≥ 10 years | |
| Exclusion criteria | NR | |
| Prior testing | NR | |
| Recruitment procedures | Part of annual OGTT screening test (patients who were identified as diabetic by an annual screening programme were asked to take part over a 2-year period in a RCT) | |
| Data collection | Unclear – probably retrospective. Authors evaluated data from an ongoing two-step prospective randomised multicentre study on patients with CF | |
| Participant characteristics | % male | 53 |
| Median age, years | 17.1 | |
| Mean BMI, kg/m2 (SD) | NR | |
| Long-term oral steroids | 82 (7.3%) were on oral corticosteroids | |
| Parenteral feeding | NR | |
| Established chronic liver disease | NR | |
| Pancreatic insufficiency | NR |
Study characteristics: screening tests
| No. of tests | 2 |
| Tests | ADA new and old FPG tests |
| Description of tests |
|
| Setting | Paediatric department – annual OGTT screening |
| Timing | NR |
| Cut-offs |
|
Study characteristics: reference test
| Reference test | OGTT |
| Delay from index test | NR |
| Description | OGTT according to WHO criteria, performed during clinically stable conditions, including no actual changes in corticosteroid dose |
| Setting | Paediatric department, Hannover |
| Timing | NR |
| Cut-offs | According to WHO recommendations |
Study characteristics: outcomes
| Accuracy | Sensitivity and specificity reported (but no CIs), so needed to construct 2 × 2 table. A number of data for 2 × 2 tables in text and a number from graph, so figures not precise |
| Patient acceptability | NR |
| Failure rate of test | NR |
Study results: recruitment
| Original population | (A) | NR |
| Pre-enrolment exclusions | (B) | NR |
| Reasons, e.g. population characteristics | ||
| No. invited to participate (A–B) | (C) | NR |
| Refusal to participate | (D) | NR |
| Reasons, e.g. missing data etc. | ||
| No. enrolled (C–D) | (E) | NR |
| Post-enrolment exclusions | (F) | NR |
| Reasons, e.g. missing data etc. | ||
| Analysable data (E–F) | (G) | 1128 |
| Completeness of follow-up (G/C × 100%) |
Study results: test acceptability
| Test |
Study results: accuracy
| Test | Reference test | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|
| Old ADA criteria for IFG according to Mueller-Brandes | Diabetes or IGT vs NGT | 65 | 94 |
| Old ADA criteria for IFG according to our calculations reconstructing a 2 × 2 table | Diabetes or IGT vs NGT | 65.3 (55.23 to 74.54) | 90.85 (88.92 to 92.54) |
| New ADA criteria for IFG according to Mueller-Brandes | Diabetes or IGT vs NGT | 82 | 70 |
| New ADA criteria for IFG according to our calculations reconstructing a 2 × 2 table | Diabetes or IGT vs NGT | 82.18 (73.30 to 89.08) | 67.90 (65.01 to 70.81) |
Notes
| A number of data read from the graph, did not get exactly the same sensitivity and specificity as reported in paper |
| Test | Disease (diabetes or IGT) | No disease (NGT) | Total |
|---|---|---|---|
| Positive (new ADA criteria for elevated FPG) | 83 | 329 | 412 |
| Negative | 18 | 698 | 716 |
| Total | 101 | 1027 | 1128 |
| Test | Disease (diabetes or IGT) | No disease (NGT) | Total |
|---|---|---|---|
| Positive (old ADA criteria for elevated FPG) | 66 | 94 | 160 |
| Negative | 35 | 933 | 968 |
| Total | 101 | 1027 | 1128 |
Robert 1992188
| Author, year, country | Robert, 1992, France |
| Reference | Robert JJ, Grasset E, de Montalembert M, Chevenne D, Deschamps I, Boitard C, et al. [Factors for glucose-intolerance in cystic fibrosis.] [French] Arch Fr Pediatr 1992;49:17–22 |
| Aim | To study glucose intolerance factors associated with CF |
Verification of study eligibility
| Study design | Case series |
| Screening test | Fasting glycaemia and HbA1c |
| Reference test | OGTT |
| Accuracy reported (sensitivity, specificity only; 2 × 2) | Sensitivity and specificity. Reported as 3 × 3 tables – reduced to 2 × 2 tables |
| Target disorder | Glucose intolerance |
Study characteristics: population
| Target population | Patients with CF treated at the general paediatric unit of the children’s hospital in Paris, France | |
| CF diagnosis | NR | |
| Inclusion criteria | NR | |
| Exclusion criteria | Patients who already had diabetes mellitus | |
| Prior testing | NR | |
| Recruitment procedures | NR | |
| Data collection | NR | |
| Participant characteristics, reported as per cent of those who agreed to participate | % male | 51 |
| Mean age, years (SD) | 10.9 (5.3) | |
| Mean BMI, kg/m2 (SD) | Their mean weight was 1.09 ± 1.06 SD below the mean values for their age | |
| Long-term oral steroids | NR | |
| Enteral feeding | NR | |
| Established chronic liver disease | NR | |
| Pancreatic insufficiency | Yes |
Study characteristics: screening tests
| No. of tests | 2 |
| Tests |
|
| Description of tests | The patients were in a fasting state for HbA1c test (in 47 patients) and OGTT. HbA1c was measured by HPLC (Riamat) |
| Setting | Any biological studies were carried out outside any acute infective attacks in the paediatric endocrinological and diabetological unit at the children’s hospital |
| Timing | Fasting glycaemia = time 0 on OGTT. Assume HbA1c test at same time as OGTT |
| Cut-offs | HBA1c normal values between 4.2 and 5.6% |
Study characteristics: reference test
| Reference test | OGTT |
| Delay from index test | Same time as FPG test. Assume HbA1c done at same time as OGTT |
| Description | Two-hour OGTT: absorption of 1.75 g/kg of glucose, with a maximum of 75 g |
| Setting | Any biological studies were carried out outside any acute infective attacks in the paediatric endocrinological and diabetological unit at the children’s hospital |
| Timing | Samples at 0, 20, 60, and 120 minutes for measuring glycaemia |
| Cut-offs |
NGT (NGT) defined as:IGT (or glucose intolerance) if glycaemia is above these values Diabetes present if it stays above ≥ 11 mmol/l (2 g/l) at 120 minutes |
Study characteristics: outcomes
| Accuracy | 3 × 3 tables condensed to 2 × 2 tables |
| Patient acceptability | NR |
| Failure rate of test | NR |
Study results: recruitment
| Original population | (A) | NA |
| Pre-enrolment exclusions | (B) | NA |
| Reasons, e.g. population characteristics | ||
| No. invited to participate (A–B) | (C) | NA |
| Refusal to participate | (D) | NA |
| Reasons, e.g. missing data, etc. | ||
| No. (C–D) | (E) | NA |
| Post-enrolment exclusions | (F) | NA |
| Reasons, e.g. missing data, etc. | ||
| Analysable data (E–F) | (G) | 49 for FPG and 47 for HbA1c |
| Completeness of follow-up (G/C × 100%) |
Study results: test acceptability
| Test | NR |
Study results: accuracy
| Screening test | OGTT Reference test cut-offs | Selectivity (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|
| Fasting glycaemia | WHO criteria (> 6 mmol/l) | 15.00 (3.38 to 37.92) | 96.55 (82.17 to 99.42) |
| HbA1c% | > 5.6% | 63.16 (38.38 to 83.65) | 78.57 (59.04 to 91.65) |
| Test | Disease (diabetes + IGT) | No disease (NGT) | Total |
|---|---|---|---|
| Fasting glycaemia positive (> 6 mmol/l) | 3 | 1 | 4 |
| Fasting glycaemia negative (≤ 6 mmol/l) | 17 | 28 | 45 |
| Total | 20 | 29 | 49 |
Glycated haemoglobin (note: only 47 patients)
| Test | Disease (diabetes + IGT) | No disease (NGT) | Total |
|---|---|---|---|
| Positive HbA1c (> 5.6%) | 12 | 6 | 18 |
| Negative HbA1c (≤ 5.6%) | 7 | 22 | 29 |
| Total | 19 | 28 | 47 |
Yung 1999189
| Author, year, country | Yung, 1999, UK |
| Reference | Thorax 1999;54:40–3 |
| Aim | To identify a more selective approach in performing OGTT in the diagnosis of CFRD, based on the use of a combination of clinical and biochemical criteria |
Verification of study eligibility
| Study design | Case series |
| Screening tests |
|
| Reference test | OGTT |
| Accuracy reported | Sensitivity, specificity |
| Target disorder | Diabetes, IGT |
Study characteristics: population
| Target population | Adult patients with CF not known to be diabetic who attended the Royal Brompton Hospital Adult Cystic Fibrosis Clinic, London, UK for their annual review between August 1996 and May 1997 | |
| CF diagnosis | Positive sweat tests with typical clinical findings, with or without genotype confirmation | |
| Inclusion criteria | All patients aged 16 years or above attending the CF clinic were eligible | |
| Exclusion criteria | Patients with pulmonary exacerbations requiring oral or intravenous antibiotic therapy, recent (within 6 weeks) increase or change in systemic steroid dosage, recent commencement of enteral feeding, and pregnant patients were excluded | |
| Prior testing | CF diagnosis (sweat tests, clinical assessment, genotype testing) | |
| Recruitment procedures | Not stated; appears to be consecutive patients invited | |
| Data collection | Unclear. Does not state prospective/retrospective | |
| Participant characteristics | % male | 63.7 |
| Mean age, years (SD) | 27 (8) | |
| Mean BMI, kg/m2 (SD) | 21 (2.9) | |
| Long-term oral steroids (%) | 9.8 | |
| Enteral feeding | 3.3 | |
| Established chronic liver disease (%) | 8.8 | |
| Pancreatic insufficiency (%) | 89.0 |
Study characteristics: screening tests
| No. of tests | Five, plus nine different combinations of the five |
| Tests |
RBG HbA1c Symptoms of hyperglycaemia and/or unexplained weight loss Presence of glycosuria FBG |
| Description of tests |
Blood samples for PG were collected in fluoride oxalate tubes and venous PG was determined by an oxygen rate method using a Beckman CX 7 Delta analyser (Beckman Instruments, Brea, CA, USA) Blood samples for the determination of HbA1c were collected in EDTA-containing tubes and HbA1c was determined by an ion capture assay using an Abbott IMX analyser (Abbott Laboratories, Abbott Park, IL, USA) Presence of glycosuria was determined by Multistix (Bayer Diagnostics, Newbury, UK) |
| Setting | Cystic fibrosis clinic annual review |
| Timing | Blood samples and clinical assessment undertaken during same annual review visit. No description of FBG given |
| Cut-offs | Three cut-off values for RBG (6, 8.5 and 11 mmol/l) were chosen. According to WHO criteria, diabetes is ‘likely’ in patients with RBG levels of > 11 mmol/l, ‘unlikely’ if RBG level is ≤ 6 mmol/l; 8.5 mmol/l represents the mid-point of these two values |
Study characteristics: reference test
| Reference test | OGTT |
| Delay from index test | Performed within 1 month of annual review visit |
| Description | After an overnight fast the patient was asked to drink a solution containing 1.75 g/kg body weight (maximum 75 g) of glucose BP dissolved in 250 ml of water within 2–3 minutes |
| Setting | Return visit to cystic fibrosis clinic |
| Timing | Blood samples were taken just before and 2 hours after ingestion of the glucose solution |
| Cut-offs |
Patients’ glucose tolerance status was classified according to WHO criteria into normal, impaired or diabetic glucose tolerance Two-hour venous PG: |
Study characteristics: outcomes
| Accuracy |
Detection of diabetic glucose tolerance Patients with IGT considered to be test and reference test negative by study authors, i.e. 3 × 3 table collapsed into 2 × 2 table |
| Patient acceptability | Refusal to participate |
| Failure rate of test | NR |
Study results: recruitment
| Original population | (A) | 152 |
| Pre-enrolment exclusions | (B) | 30 |
| Reasons, e.g. population characteristics |
Twenty-three known to have diabetes Seven reasons not reported (Further 366 were clinic attenders, but did not attend for annual review during time period of study) |
|
| No. invited to participate (A–B) | (C) | 122 |
| Refusal to participate | (D) | 31 |
| Reasons, e.g. missing data, etc. | Inability to attend owing to work commitments or long distance to travel were ‘usual reasons’ | |
| No. enrolled (C–D) | (E) | 91 |
| Post-enrolment exclusions | (F) | 0 |
| Reasons, e.g. missing data, etc. | ||
| Analysable data (E–F) | (G) | 91 |
| Completeness of follow-up (G/C × 100%) | 74.6% |
Study results: test acceptability
| Test | 31/122 (25%) refused to participate in study. Only general reasons for refusal given – judgement is that they relate to unwillingness to return for OGTT as other tests were part of routine review |
Study results: accuracy (calculations taken directly from paper)
| Screening test | Reference test | Selectivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|
| RBG ( > 11 mmol/l) | Diabetes vs IGT + NGT | 33 (7 to 60) | 97 (94 to 100) |
| HbA1c (> 6.1%) | 83 (62 to 100) | 89 (82, 96) | |
| Symptoms: hyperglycaemia and/or unexplained weight loss | 58 (30 to 86) | 87 (80, 95) | |
| Glycosuria | 17 (0 to 38) | 97 (94 to 100) | |
| Fasting BG (> 7.7 mmol/l) | 25 (1 to 50) | 100 | |
|
HbA1c > 6.1%, glycosuria Symptoms: hyperglycaemia and/or unexplained weight loss, RBG > 11 mmol/l |
92 (76 to 100) | 79 (70 to 88) | |
|
HbA1c > 6.1%, glycosuria Symptoms: hyperglycaemia and/or unexplained weight loss, RBG > 8.5 mmol/l |
92 (76 to 100) | 74 (65 to 84) | |
|
HbA1c > 6.1%, glycosuria Symptoms: hyperglycaemia and/or unexplained weight loss, RBG > 6.0 mmol/l |
92 (76 to 100) | 65 (54 to 75) | |
|
HbA1c > 6.1% Symptoms: hyperglycaemia and/or unexplained weight loss, RBG > 11.0 mmol/l |
92 (76 to 100) | 79 (70 to 88) | |
|
HbA1c> 6.1% Symptoms: hyperglycaemia and/or unexplained weight loss, RBG > 8.5 mmol/l |
92 (76 to 100) | 75 (65 to 84) | |
|
HbA1c > 6.1% Symptoms: hyperglycaemia and/or unexplained weight loss, RBG > 6.0 mmol/l |
92 (76 to 100) | 65 (54 to 75) | |
| HbA1c > 6.1%, RBG > 11.0 mmol/l | 83 (62 to 100) | 86 (78 to 94) | |
| HbA1c > 6.1%, RBG > 8.5 mmol/l | 83 (62 to 100) | 84 (75 to 92) | |
| HbA1c > 6.1%, RBG > 6.0 mmol/l | 92 (76 to 100) | 70 (59 to 80) |
Appendix 5 Reasons for exclusion of screening studies
| References | Reason for exclusion | Described narratively in text |
|---|---|---|
| Al-Aloul 2003 (abstract)204 | This study examined various indicators of glycaemia in patients with confirmed CFRD, who were being considered for insulin treatment. No details are given on how diabetes was confirmed. It was not about screening of people not known to have CFRD. There were only 11 patients. However, it does provide some useful data, including:
|
No |
| Allen 1998 (letter)216 | (Letter in response to Yung.) This correspondence followed the publication of the Allen et al.215 survey of US practice. It has no new data, but provides useful discussion. Useful comments: ‘Before recommending that all adult patients with CF have annual OGTTs, we must have solid evidence that a worthwhile intervention is available to those who have abnormal results’ | No |
| Allen 1999 (letter)202 | Expresses reservations about HbA1c. (Letter in response to Hunkert 1999) | No |
| Bistritzer 1983217 | Early paper on HbA1 (not A1c), so now obsolete | No |
| Brennan 2004 (abstract)175 | Superseded by full paper in 2006 | No |
| Brennan 2006176 | Useful paper, although does not allow a 2 × 2 table. It sets out to assess how good HbA1c is for monitoring diabetic control in CFRD, following the discussions about red cell turnover and iron deficiency anaemia. It used CGMSs to determine mean PG in CFRD and T1DM. Conclusion was that HbA1c is a reliable measure in CFRD. The study did not examine the use of HbA1c in screening for or diagnosis of CFRD. Iron deficiency is common in CF, and iron deficiency may be associated with higher Hba1c in people with T1DM. Conversely, reduced red blood cells survival, if present in CF (Allen says evidence for that is weak), would lower HbA1c. There is a statement in the abstract but not the full paper that says ‘Only about 10% of HbA1c is determined by red blood cells surviving 80–120 days’ | No |
| Craigie (unpublished) and Wilkinson 2008 (abstract)75 | Insufficient data for a 2 × 2 table | Yes |
| Dobson 2003 (letter) and Dobson 2004192,193 | Cannot be used for screening for diabetes or IGT when no diabetics and IGTs excluded. It is about PPH, and they do not really compare CGMSs with FOGTT. They say that five subjects with NGT had raised CGMSs, but not how many also had PPH on OGTT | Yes |
| Dobson 200569 | Microalbuminuria not useful as screening test | No |
| Franzese 2008194 | Has the right data to populate the 2 × 2 table, but spectrum bias because only those with at least one PG > 7.7 in an OGTT were included, so an exclusion | Yes |
| Garagorri 2001203 | Twenty-eight patients with CF had OGTTs. Thirteen had IGT or diabetes. HbA1c was no different between groups, suggesting no use as screening test for IGT. But not a screening study | No |
| Hardin 1999 (abstract)201 | Nine adults with CF plus IGT had HbA1c value ranging from 5.1% to 6.1%. Red cell turnover was higher than normal in four with poor pulmonary function tests | No |
| Holl 1998 (letter)218 | Comparison of FPG and OGTT. Insufficient data for 2 × 2 table | No |
| Holl 2000 (letter)182 | Same series as 1998 study, but this time looking at HbA1c. Only 4 of 13 patients who became diabetic had raised HbA1c. Cannot get 2 × 2 table as cannot get data for cells ‘b’ and ‘d’ | No |
| Huot 1997219 | Insufficient details to be of use | No |
| Jefferies 2005195 | Good data tables but spectrum bias so not to be used. Would imply that FPG was useful. All had had a previous glucose > 7 mmol/l; 19 subjects out of 100 eligibles and all ‘deemed diabetic on OGTT’, but these 19 merely had no level over 7 mmol/l, so could not be classed as diabetic. Really, another pilot of CGMS | Yes |
| Khammar 2009178 | Excluded because of selection bias of patients who were screened by CGMS – 20 selected out of all non-diabetic patients – hence, spectrum bias likely | No |
| Lanng 1995 and 2000 (full paper and abstract)33,220 | Cannot get 2 × 2 data from paper – only PPV and NPV | No |
| Lanng 2001166 | Good review but no new data | No |
| Ledson 2007221 | OGTT vs profiles but only in small number of patients admitted with acute lung exacerbations. Exclusion | No |
| Liou 2006222 | Only measured adherence to guidelines, which recommend OGTT. Adherence was low | No |
| Loo 1979223 | Not used – insufficient data | No |
| Middleton 2006 (abstract)199 | OGTT vs HbA1c. Mixed results. Of 49 non-diabetics: 17 had abnormal OGTT but normal A1c. Follow-up showed that some subjects with abnormal OGTTs were normal 102 years later (nos. not given). 1-hour OGTT > 11 mmol but normal at 2 hours in seven patients. No 2 × 2 data | Yes |
| Mohan 2007 (abstract)224 | Exclusion – subjects had been admitted with pulmonary exacerbation, and most were on steroids | No |
| O’Riordan 2006, 2007 (three abstracts)196–198 | Insufficient data for 2 × 2 table. Nos. do not always match. Test data given in table for 6 months’ follow-up but text uses 12 months. Advocates paired OGTT and CGMS but rationale not clear | Yes |
| Richmond 2008 (abstract)225 | Uses 27-item questionnaire to predict CFRD, vs OGTT. Preliminary data only | No |
| Solomon 2003200 | Ninety-four patients: 10–18 had modified OGTTs (FPG and 2 hour); four had CFRD. No data for 2 × 2 table. HbA1c and FPG insensitive. CFRD was related to more severe pancreatic deficiency | Yes |
| Stutchfield 1987226 | Excluded – too old. Used HbA1 in days before HbA1c (HbA1 = HbA1a + b + c) | No |
| Thorsteinsson 199578 | Insufficient data for assessing screening tests, but useful natural history | Yes |
| Verma 2002 (abstract)227 | Concludes that selective OGTT screening as advocated by Yung (gives reference to J R Soc Med,63 but Yung,189 Thorax 2008, similar) would miss too many patients and that annual screening of all is required | No |
| Watson 2007228 | Exclusion. CGMS used as guide for adjusting insulin treatment, not for screening | No |
| Wilkinson 200875 | Insufficient data for a 2 × 2 table | No |
| Yung 1997229 | Very small study in seven patients noting that some had abnormal BGs at intermediate intervals after OGTT (e.g. 30 and 60 minutes) but that all OGTTs were normal as defined by fasting and 2-hour levels. Recommends that RBG should not be used to diagnose CFRD. In effect results reflect the postprandial, lag storage type of hyperglycaemia | No |
| Yung 1999 (letter)230 | Ninety-one adults (> 16 years), Royal Brompton Hospital, London, UK. Reported that compared with the OGTT, FBG had a sensitivity of 25% and RBG of 33%. This was based on only 12 diabetic patients. No specificity data given. Exclusion – no ‘b’ or ‘d’ values | No |
Appendix 6 Quality-of-life studies not mentioning cystic fibrosis-related diabetes but with cystic fibrosis-specific measures
Cystic Fibrosis Questionnaire
Several studies have used the CFQ. The origins of this are reported by Quittner et al. 231 CFQ is a disease-specific measure (or rather ‘measures’, as there are versions for children, parents and adults).
Riekert et al. 232 examined associations between lung function, depression and QoL in adults with CF, using:
-
FEV divided into good (≥ 70%) and poor (< 70% predicted)
-
depression as reported by Beck Depression Inventory (BDI) score 0–63 (high is bad)
-
CFQ teenagers and adults score 0–100 (high is better).
They had a 57% response rate among 133 eligible adults, mean age 31 years (range 19–65 years) of whom 26% had CFRD. Unfortunately, no results were given separately. The mean FEV was 63% (range 21–116%) of predicted, with 62% < 70%.
Thirty per cent screened positive for depression (BDI score > 10).
The CFQ results were proportional to depression scores and to FEV. There was a clear association between QoL and lung function.
Urquhart et al. 233 used CFQ-UK to assess the effects of lung function and exercise capacity on QoL in 35 children aged 11–15 years. The correlation between QoL and lung function tests was weak, but there was a stronger correlation (r2 = 0.4) with exercise capacity (VO2 peak).
Thomas et al. 234 used CFQ and a generic health status measure [Pediatric Quality of Life Inventory (PedsQL)] to compare outcomes in patients looked after in a city centre clinic and in country areas, but they also reported the association of QoL and lung function. Thirty-three teenagers had results suggesting a decline in lung function with age, and this correlated with QoL as reflected in the CFQ. There was no correlation with lung function in younger children, but they showed very little decline in lung function. The authors attribute the lack of correlation to the good lung function in younger children.
Klijn et al. 235 set out to validate the Dutch version of the CFQ but also provide data comparing results in mild (FEV > 70% predicted, mean was 89%), moderate (FEV 41–70% predicted, mean 56%) and severe disease (FEV < 41% predicted, mean 26%). There were clear differences in CFQ results among these groups but SDs were quite large. The biggest differences were in physical functioning, especially between moderate and severe disease. However, most differences were not statistically significant owing to the degree of scatter about the group means, raising doubts about the sensitivity of the CFQ for reflecting small changes in lung function.
The CFQ has also been validated in the USA by Quittner et al. ,231 who also examined results after dividing patients into three groups by FEV, these groups having similar bands to those in the Dutch validation. Again, there were marked differences among these groups, most marked for physical functioning, role functioning and weight. The authors do not provide data on the statistical significance of differences in CFQ domains.
Cystic Fibrosis Quality of Life
Gee et al. 236 used the CFQoL questionnaire to examine associations between various clinical variables and QoL in 223 patients, and this was one of the few studies to provide any data on the effect of CFRD. Those with CFRD (49 patients) had lower FEV (mean 41% predicted, 25–75 percentile, range 30–59) than those without (mean 55%, range 41–77). The diabetic and non-diabetic groups had similar BMIs (both 20 and 21 kg/m2) and ages (26 and 24 years).
The association between FEV and QoL was weak, and the authors conclude that large differences in FEV would be required before the CFQoL changed significantly.
Studies using generic health-status measures
Child Health Questionnaire
The CHQ covers 10 domains via 75 questions, and is designed to be used by both children and parents. It has a scale of 0 to 100, with high scores being better.
Powers et al. ,237 from Massachusetts, set out to administer the CHQ to 39 adolescent patients, their mothers and fathers. The response rates were 82% for patients and mothers but only 64% for fathers. So final results are based on 24 triads. They found a moderate-to-strong relationship between FEV and QoL but only the correlation coefficient (0.73) is given, not the incremental relationship or the scatter about the regression line.
Britto et al. ,238 from Ohio, also compared QoL with pulmonary function as measured by predicted FEV1 and with exercise capacity, in 63 children aged 5–17 years, using the CHQ. Patients aged > 18 years (48 patients) used the SF-36. Although QoL scores fell with %FEV1, the trend was not statistically significant. Nor was there any association between QoL and the 6-minute walk distance. The strongest determinant of QoL was recent pulmonary exacerbations.
Sawyer,239 from Adelaide, used CHQ in a follow-up study of children aged 10–16 years with diabetes (n = 44), asthma (n = 40) and CF (n = 39), recording results at baseline, 6, 12, 18 and 24 months. This allowed them to compare results of children with those from healthy children, and among the three diseases, and to look at time trends, albeit over a timescale short relative to life-time. They also used disease-specific measures, including the CFQoL. Over time, the physical health scores of the CF children declined from 65 to 56 (described as significant but no p-value given), whereas there was no change in the diabetic children, and those with asthma showed a non-significant improvement (55–60).
The same group240 asked parents to assess their children’s QoL using CHQ and found that children with CF were less healthy than those with diabetes or asthma, and also that the CF children deteriorated. Children scored their QoL better than their parents did.
Another comparison of QoL among different childhood conditions was reported by Ingerski et al. 241 The authors noted that QoL was poorer in children with CF than in healthy children, was about the same as in children with T1DM, but was better than in obese children.
Nottingham Health Profile
Congleton,242 from London, assessed QoL in 240 adults (> 16 years) with CF, using the Nottingham Health Profile, with a small CF supplement. They then compared the results with healthy people (from a community survey) and with people who had other conditions.
The patients with CF had significantly worse scores in energy, pain and social isolation (men) or pain, emotion and sleep (women). Men showed a decline with age compared with the general population, but women did not.
Both men and women reported more problems of daily living than the general population, with five scores of around 30% problem frequency in the men with CF compared with < 10% in the men in the community survey. There were similar, but fewer, marked increases in problems in women.
However, when the CF scores were compared with those from patients with other conditions, CF, surprisingly, came out better than pregnancy and peripheral vascular disease, and about the same as ‘minor non-acute conditions’ (such as varicose veins and hernias).
The Quality of Well-Being scale
The Quality of Well-Being (QWB) scale, which is not specific to any disease, was first validated for use in CF by Orenstein et al. 243 in a mixed group of adults and children. However, Kotwicki and colleages244 found it less useful in children, although they did conclude that some children found the treatment worse than the disease.
Suri et al. 245 used QWB in a treatment trial in a group of children, and also found it had short-comings, including that it was not sensitive to clinically meaningful changes and that it had ‘uncertain applicability to children and adolescents’.
European Quality of Life-5 Dimensions
The EQ-5D, sometimes also called the EuroQol, is a generic measure of health based on the five domains of mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Each domain is scored on three levels, for no problems, some problems or severe problems, but there is also a visual analogue scale (VAS) option with a scale of 0 to 100. EQ-5D is the measure preferred by NICE because it can provide utilities for cost-effectiveness analysis. There is now a version for use in children: the EQ-5D-Y.
Eidt-Koch et al. ,246 from four CF centres in Germany, carried out a study in which they administered both the EQ-5D-Y and the CFQ to 96 patients aged 8–17 years. They found good but not perfect correlations with the CFQ, suggesting that the EQ-5D-Y could be used for assessing changes in utility in young patients with CF. There was higher correlation with the VAS.
Other studies
From the Netherlands, de Jong et al. 247 contribute a small study of 15 patients with CF and a control group. They report pulmonary function, exercise capacity, dyspnoea and QoL using the Sickness Impact Profile (SIP). They found marked effects on physical functioning scores (5.4 vs 0.7), but no significant difference in psychosocial ones (possibly because of numbers, because the scores were 2.65 and 1.04). SIP scores deteriorated as exercise capacity and dyspnoea scores did, but did not correlate with FEV1.
Bradley et al. 248 from Belfast also reported little correlation between QoL and spirometric measures of lung function, this time using the Chronic Respiratory Disease Questionnaire, in a study concerned mainly with amending that tool for use in CF.
Goldbeck et al. ,249 from Munchen, carried out a feasibility study to measure QoL in a CF clinic. (The authors note that the usual way of doing so is to ask ‘How are you?’) They set out to see if sequential measurement of QoL would be feasible, using the Questions on Life Satisfaction [FLZ(M)] questionnaire and doing so in parallel with lung function tests.
They studied 108 patients over 18 months, from an initial population of 148, all aged > 15 years. The interest from our perspective is what most determined QoL. There were correlations of QoL with acute infective exacerbations and colonisation with Pseudomonas. However, neither slow declines in pulmonary function nor FEV affected QoL. QoL was generally quite stable over the 18-month period.
Goldbeck and Schmitz250 compared the SF-36, the FLZ(M) (questions on life satisfaction) questionnaire and a QoL profile for chronic diseases (Quality-of-Life Profile) in 70 adolescents and adults with CF. They included a control group of healthy peers, which gives us data on the impact of CF on QoL. The SF-36 results showed poorer QoL in the CF adolescents on most dimensions, especially general health, physical functioning and vitality. There was little difference in mental health or social role functioning.
Weiner et al. ,251 from Boston, carried out a literature review examining costs, QoL and compliance with treatment. The cost data are useful and are referred to elsewhere. They did not consider CFRD and it is not mentioned. Their main interest was in the use of antibiotics, particularly tobramycin, and the review was funded by Novartis, the manufacturer of tobramycin.
Cruz et al. 252 reviewed the literature on anxiety and depression in CF, concluding that both were more common than in the general population, with anxiety commoner but depression probably more important. However, they also concluded that the body of evidence was based on too many small studies from single centres, using a wide range of instruments. The same group253 had reported that depression was commoner in people with CF and in parents of children with CF.
Appendix 7 Ongoing studies
| Title | Sponsors and collaborators | Aim/hypothesis | Study type | Inclusion criteria | Intervention | Primary outcome | Status and dates |
|---|---|---|---|---|---|---|---|
|
Pilot and Feasibility Study for the Treatment of Pre-Diabetes in Patients With Cystic Fibrosis Study ID: NCT00763412 |
Arbelaez, Ana Maria Washington University School of Medicine NIH Novo Nordisk |
To design a larger, full-scale clinical trial to determine if repaglinide can improve the nutritional status and pulmonary function of adolescents and young adults with CF and pre-diabetes by improving BG control |
Pilot double-blind RCT Estimated enrolment: 40 |
Male or females 12–24 years old; diagnosis of CF by sweat test with exocrine pancreatic insufficiency OGTT with fasting BG < 126 mg/dl and 2 hour: 140–199 mg/dl or > 200 mg/dl Weight stable within 5% for 3 months prior to initiation visit |
1. Placebo comparator: one pill before each meal, 3–4 times a day for 2 years 2. Repaglinide: Experimental repaglinide 0.5 mg before each meal, 3–4 times a day for 2 years |
Feasibility Time frame: Every 3 months for 2 years |
This study is ongoing, but not recruiting participants Start date: November 2006 Estimated study completion date: November 2010 |
|
Increased Gluconeogenesis is One Cause of CFRD Study ID: NCT00082238 |
National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) | To better describe the unique metabolism of people with CF, and to provide a comprehensive evaluation of pathophysiological changes that contribute to the development of CFRD and to wasting, and are part of the applicant’s long-range goal, which is to identify the underlying causes of CF-related diabetes and catabolism so that disease-specific therapies can be developed |
Interventional Study design: Diagnostic, non-randomised, single group assignment, pharmacokinetics/dynamics Study estimated enrolment: 60 |
Ages eligible for study: 18–45 years Genders eligible for study: Both CF with any type of glucose tolerance |
Drug: Ibuprofen |
This study has been completed Study start date: March 2003 |
|
|
Use of Levemir® Improves Metabolic and Clinical Status in CFRD Study ID: NCT00639626 |
Nationwide Children’s Hospital Novo Nordisk |
To find out if Levemir (a long-acting or basal insulin) is safe and effective in treating CFRD |
Interventional Study design: Treatment, non-randomised, open label, historical control, single group assignment, safety/efficacy study Estimated enrolment: 20 |
Ages eligible for study: 16–45 years Genders eligible for study: Both Patients diagnosed with CFRD by OGTT who are medically stable Medical stability will be defined as: No hospital admission for 6 weeks or more before the study No oral or intravenous antibiotics for at least 6 weeks preceding the study (subjects will be allowed to use low doses of inhaled corticosteroids) |
Drug: insulin detemir (rDNA origin) injection | To evaluate the effectiveness of Levemir to improve glycaemic control in patients who have CFRD |
This study is currently recruiting participants Study start date: August 2008 Estimated study completion date: December 2010 Estimated primary completion date: June 2010 (final data collection date for primary outcome measure) |
|
Use of the Insulin Pump in Cystic Fibrosis Patients With IGT or CFRD and in Type 1 Diabetes Patients Study ID: NCT00287456 |
University of Texas Southwestern Medical Center |
We hypothesise use of the insulin pump will improve body weight, lean body mass, whole-body protein turnover, hepatic glucose production, and blood sugar control in patients with CF with IGT or patients with CFRD. We further hypothesise that hepatic glucose production is also elevated in children/adolescents with T1DM and that the insulin pump will result in decreased hepatic glucose production |
Interventional Study design: Treatment, non-randomised, open label, active control, single group assignment, safety/efficacy study Estimated enrolment: 16 |
Ages eligible for study: 12–32 years Genders eligible for study: Both Patients with CF aged 12–32 years IGT or CFRD defined as fasting BG and post-prandial BG equal to: FBG < 126 mg/100 ml and post-prandial BG 151–200, or FBG < 126 mg/100 ml and postprandial BG > 200, or FBG > 126 mg/100 ml and post-prandial BG > 200 T1DM control patients aged 12–32 years |
Device: Insulin pump Drug: Insulin Procedure: OGTT Procedure: Whole-body protein turnover |
Weight gain of lean body mass increased protein synthesis decreased protein breakdown | The recruitment status of this study is unknown |
|
Repaglinide for Adolescents With Cystic Fibrosis-Related Diabetes Study ID: NCT00231192 |
Children’s Hospital of Philadelphia | To test the hypothesis that oral repaglinide is equivalent to insulin in the treatment of new-onset CFRD in adolescents. In addition, successful treatment of CFRD with repaglinide will improve nutritional status, ameliorate declines in pulmonary function, and will not have a negative impact on QoL |
Interventional Study design: Treatment, non-randomised, open label, uncontrolled, parallel assignment, efficacy study Enrolment: 0 |
Ages eligible for study: 12–20 years Genders eligible for study: Both CF, BG concerning for diabetes |
Drugs: Repaglinide and Insulin | BG insulin excursion during OGTT fasting BG 2-hour postprandial BG HbA1C serum fructosamine | This study has been terminated. (Unable to recruit any subjects) |
|
A Multicentre Randomised Trial of Insulin Detemir in Pre-diabetes Associated with Cystic Fibrosis Study ID:ISRCTN71877586 |
Sheffield Children’s NHS Foundation Trust (UK) Dr Neil Wright |
To establish a better methodology for identifying patients with CFRD and to show that early treatment produces clinical benefits |
RCT Target number of participants: 240 |
Any child > 10 years of age with either FPG > 6.1 mmol/l but < 7.0 mmol/l and/or a 2-hour glucose of > 7.8 mmol/l but < 11.1 mmol/l | Insulin detemir 0.2 units per kg of body weight by once-daily subcutaneous injection daily for 1 year | Measurements of β-cell function |
Status of trial: Completed Anticipated start date: 1 October 2006 Anticipated end date: 1 November 2009 |
|
Study on the Efficacy of Slow Release Insulin in Cystic Fibrosis Patients With Glucide Intolerance and Clinical Decay Study ID: NCT00687466 |
Fondazione per la Ricerca sulla Fibrosi Cistica | To evaluate whether the anticipated use of glargine in patients with CF with glucose intolerance may prevent the worsening of nutritional status and pulmonary function |
Randomised, open-label, Phase III, active control, parallel assignment, efficacy study Estimated enrolment: 70 |
10–70 years Ascertained diagnosis of CF Glucide intolerance: Two pathological OGTT (at 2-hour glucose value: > 140 mg% and < 200 mg%) at 2–6 months’ interval between each other At least one of the following conditions: BMI < 10th percentile for age and sex; loss of one BMI percentile class for age and sex in the last year; FEV1 ≤ 80% of predicted FEV1 decrease ≥ 10% in the last year |
Drug: Insulin (glargine) | Nutritional status evaluated as variations of z-score of BMI at recruitment time and at +3, +6, +9, +12, +15, +18 months |
This study is ongoing, but not recruiting participants Study start date: August 2005 Expected completion date: October 2009 |
|
Early Diagnosis of Diabetes Mellitus in Patients With Cystic Fibrosis Study ID: NCT00662714 |
Mukoviszidose Institut gGmbH Novo Nordisk Mucoviscidose-ABCF2 | Is oral therapy with repaglinide equivalent to insulin therapy with three daily injections with respect to BG control, weight and pulmonary function over 2 years in patients with CF and secondary diabetes mellitus? |
Randomised, open-label, active control, parallel assignment, efficacy study Estimated enrolment: 74 |
Inclusion criteria for the screening: Diagnosed CF, age 10 years and older Inclusion criteria for the therapeutic part of the study: Newly diagnosed diabetes mellitus in the screening |
Drug: Repaglinide Drug: Short-acting insulin (Actrapid®, Novo Nordisk Ltd) |
HbA1c |
This study is ongoing, but not recruiting participants Study start date: September 2001 Estimated study completion date: December 2009 |
|
Comparing Two Different Approaches in the Screening of Cystic Fibrosis Related Diabetes (CFRD) Study ID: NCT01091025 |
Imperial College London |
1. To compare the clinical efficiency in the screening for CFRD in the two different methods: (1) a selective approach and (2) an unselected annual OGTT for all patients 2. To compare the cost-effectiveness of the two approaches in the screening for CFRD |
Screening, single-group assignment, single-blind (investigator) Enrolment: 100 |
First 100 consecutive clinically stable patients with CF attending annual review from January 2009 Those ≥ 16 years of age will be eligible for the study |
Patients identified for OGTT based on the selective approach by the two independent reviewers will be compared Patients will form two groups: 1. Those identified as needing OGTT 2. Those on whom they considered it unnecessary |
The results of the two groups will then be compared with the data obtained from OGTT to which the two reviewers were ‘blinded’ |
This study has been completed Study start date: March 2009 Study completion date: January 2010 |
|
Genetic Modifiers of Cystic Fibrosis-Related Diabetes Study ID: NCT01113216 |
Johns Hopkins University | To find the genes and other factors that are responsible for differences among persons with CF. We are particularly interested in the factors that relate to the development of CFRD |
Observational Estimated enrolment: 500 |
Any person with CF and his/her parents | Blood will be drawn from study and will be used to extract DNA and to establish cell lines that we will store as a permanent source of DNA | Identification of genes or other factors that influence the development of CFRD |
This study is currently recruiting participants Study start date: April 2008 Estimated study completion date: April 2012 |
|
Sitagliptin in Cystic Fibrosis-Related Diabetes Study ID: NCT01257464 |
University of British Columbia | To determine whether or not the dipeptidyl peptidase IV inhibitor sitagliptin is effective in the treatment of CFRD. Hypothesis is that sitagliptin will improve meal-stimulated insulin secretion |
Randomised, double-blind, crossover trial Estimated enrolment: 20 |
≥ 19 years of age CFRD with or without fasting hyperglycaemia either untreated or using only pre-prandial repaglinide or pre-prandial bolus insulin therapy |
Sitagliptin, (100 mg orally, one dose) vs placebo |
Insulin release Time frame: 180 minutes (during clamp) |
This study is currently recruiting participants Study start date: September 2010 Estimated study completion date: July 2011 |
|
Prevention of Cystic Fibrosis Diabetes Study ID: NCT00967798 |
Emory University Collaborators: Ohio State University; Merck; FDA Office of Orphan Products Development |
To show that chronic treatment with sitagliptin prevents the conversion to diabetes; results in preservation of β-cell mass and function; reduces airway and systemic measures of oxidative stress and inflammation; and slows the rate of progression of lung disease |
A randomised, double-blind, placebo-controlled study Estimated enrolment: 186 |
Aged ≥ 16 years; diagnosis of CF; clinically stable; high-risk pre-diabetes as defined by high-risk IFG levels of 110–125 mg/dl and/or a 2-hour PG level of 140–199 mg/dl found on an OGTT performed at screening 4 weeks or less before enrolment |
Sitagliptin phosphate (Januvia®, Merck & Co) 100 mg orally once a day for up to 24 months |
Conversion to CFRD Time frame: Every 2 weeks for 2 years |
This study is currently recruiting participants Study start date: May 2010 Estimated study completion date: April 2014 |
|
Cystic Fibrosis – Insulin Deficiency, Early Action (CF-IDEA) Study ID: NCT01100892 |
Sydney Children’s Hospital | Whether starting insulin treatment before the onset of diabetes (earlier than current practice) will improve the health of children with CF by improving body weight and lung function |
Randomised, open-label, Phase III trial Estimated enrolment: 100 |
Patients with CF aged ≥ 8 years; CFID1 or CFID2 (defined as BG-max ≥ 8.2 and BG-120 < 11.1 mmol/l on OGTT performed within the last 6 months, when respiratory function stable as judged by the treating respiratory team, not taking fluoroquinolone antibiotics, and not taking systemic glucocorticoids) | Once-daily insulin detemir vs observation only (no detemir) | Change in WtSDS; change in lung function (FEV1, FVC) |
This study is currently recruiting participants Study start date: December 2010 Estimated study completion date: December 2012 |
Appendix 8 Project description from original grant application
1. Title
Screening for cystic fibrosis related diabetes and impaired glucose regulation, HTA 07/45.
2. Background
2.1. Cystic fibrosis
Cystic fibrosis is an autosomal recessive disease occurring in from about 1 in 1984 children in Scotland,1 to 1 in 2415 in the whole UK,2 to about 1 in 2650 live births in Italy. 3
The gene defect leads to a defect in a protein called cystic fibrosis transmembrane regulator (CFTR), a cAMP-dependent chloride channel. This affects the water and electrolyte composition of secretions in various organs including the pancreatic ducts and airways, leading to viscous tenacious secretions. The viscid lung secretions render the patients very prone to repeated infections. The lung is colonised by atypical bacteria such as pseudomonas, and once severe lung disease is established, death follows from respiratory failure unless lung transplantation can be provided. The intense neutrophil recruitment in the lungs, with associated release of products such as neutrophil elastase, results in further tissue damage with an inflammatory element, which may be treated with anti-inflammatory agents including corticosteroids. An asthma-like component may be seen.
The effect on the pancreas causes deficiency of digestive enzymes, leading to malabsorption of undigested foods and under-nutrition. Although the primary defect is of exocrine secretion, the islet cells which are initially preserved may become damaged with time. Other recognised problems include hepatic cirrhosis, and infertility in males.
The UK Cystic Fibrosis database (http://cystic-fibrosis.org.uk accessed July 2007) has data from specialist centres in the UK (but may not be complete), and recorded 7046 people with CF in 2004. There were 123 deaths in that year with a mean age at death of 27.6 years. 15% of patients were over 30.
2.2. Cystic fibrosis related diabetes (CFRD)
The incidence of diabetes is related to duration of CF, and with the significantly improved survival into adulthood, more are surviving long enough to develop diabetes. Dodge and colleagues (2005)4 reported that CF was no longer an important cause of death in children in the UK. Better treatment means that about half are expected to survive beyond the age of 50. 5 So a higher proportion will develop diabetes than in the past.
The proportion that develops CFRD depends on age at which prevalence is reported, comprehensiveness of screening, genetic factors, and possibly other factors (to be determined from the systematic review). Reports of the prevalence of CFRD have risen from 3–10% in 1969, to 14–30% in the early 1990s (see Mackie 2003 for review). 6 Similar numbers have impaired glucose tolerance (IGT). The average age at onset is 20. In the over-30s, about 40% have diabetes and 30% have IGT. 7
The UK Cystic Fibrosis database (2004) reported that 39% of those over 10 years and who had been tested, were diabetic. For the over 30-year olds it was 59%. 47% of the over 10s had not been tested. In the 15 year olds, 9% had diabetes and another 8% were classed as glucose intolerant.
CFRD is characterised by insulin deficiency,8 with an approximately 50% loss in β-cell mass, which is similar to that seen in type II diabetes mellitus patients. 9 This occurs after fibrosis and fatty infiltration of the pancreas. 8 Many, but not all, of the islets are destroyed. 7 The glucagon and pancreatic polypeptide secretions are also reduced,7 because whole islets are destroyed, unlike the β-cell specific defect seen in type 1 diabetes. 10 Islet amyloid deposits are also within the β-cells. However, it is not clear if the amyloid accumulates during the disease process or if it contributes to β-cell dysfunction. 6
So CFRD does not fit into the definitions of either type 1 or type 2 diabetes. It is more like T2DM in that there are functioning islet cells, but with a reduced total beta cell capacity. However patients are not overweight and are not, at least initially,10 insulin resistant, though those with IGT appear more likely to have insulin resistance, and others may have resistance during infective exacerbations (see Brennan 2004 for review). 11 It also resembles T2DM in that onset can be insidious (hence the putative need for screening). However treatment is usually with insulin because of the reduced beta cell mass, though sulphonylureas have been used. Repaglinide has also been shown to reduce postprandial glucose, though not as effectively as insulin lispro. 12
As people with CF live longer, they may acquire not only diabetes but its complications such as retinopathy and nephropathy. 13–15 However two complications are particularly important. The first is the direct effect of diabetes on the lung. The second is increased growth of some bacteria due to elevated glucose levels in pulmonary tissue and secretions.
Lung function in diabetes mellitus
Diabetes itself can affect the lung. This was reviewed in detail in our technology assessment report on inhaled insulin (to be published in September – full version available on request). In brief:
-
diabetes is associated with loss of lung recoil, and a greater rate of decline in lung function with increasing age than in normal subjects. This makes the lungs a little stiffer to inflate/deflate. The pulmonary function tests which measure the ability to breath out rapidly (forced expiratory volume in one second – FEV – and the volume of air expelled after a deep breath – forced vital capacity or FVC) show some reduction
-
there are changes in small blood vessels, similar to those seen in the kidney but less marked
-
the diffusion capacity, as measured by diffusion of carbon monoxide (DLco), is slightly reduced, probably due to changes in the alveolar epithelium and the pulmonary microvasculature.
In diabetes, pulmonary effects are slight and usually subclinical. However in people with CF, in whom pulmonary function is impaired, the changes due to diabetes itself may have a greater impact. It is also worth noting that some of the microvascular changes considered characteristic of diabetes, may also be seen in IGT. In the Diabetes Prevention Programme, 10% of those with only IGT had retinopathy. 16
Some of the lung changes appear to be related to control, so if treatment improves control, it might have beneficial effects on lung function. Insulin treatment has been reported to improve lung function. 17
Bacterial growth
Brennan and colleagues18 reported that elevated blood glucose levels led to elevated glucose in the airways, and that growth of S. aureus and P. aeruginosa was increased when airway glucose was elevated.
2.3. Terminology
In this proposal, the following categories of glucose status will be used.
-
Normal glucose tolerance. Normal glucose tolerance (NGT) requires both fasting PG of under 5.6 mmol/l, and 2-hour under 7.8 mmol/l 2 hours after a 75 g glucose load.
-
Diabetes is defined as fasting plasma glucose over 7.0 mmol/l and/or 2-hour OGTT level of over 11.1 mmol/l, except that the diagnosis must be confirmed – a single glucose level is not enough.
-
Impaired glucose tolerance (IGT) is based on a 2-hours OGTT level of 7.8 to 11.1 mmol/l.
-
Impaired fasting glucose (IFG) means a fasting PG between 6.1 and 6.9 mmol/l, as used by WHO (Alberti 1998). The American Diabetes Association defines it at a lower threshold of 5.6 mmol/l. The WHO system does not give any name to those with FPGs of 5.6 to 6.0 mmol/l, who are above normal but under the IFG threshold.
-
Postprandial hyperglycaemia (PPG). There are patients in whom PG after a meal is abnormally high for the first hour or so, but returns to normal by 2 hours. The term ‘lag storage’ has been used in the past. Unpublished data from the Royal Hospital for Sick Children in Glasgow (Craigie and colleagues, submitted for publication) show that many patients have high PG levels at 30, 60 and 90 minutes but normal fasting and 2-hour levels. Some of these results are into the range for random blood glucose at which diabetes would be diagnosed.
The WHO criteria for diabetes are based on the risk of harms such as retinopathy. It may be that the threshold for harm in cystic fibrosis, such as bacterial growth, may have a different threshold, and one by-product of this review, or of subsequent primary research, may be to produce a definition of CFRD.
We also need to take into account the occurrence of temporary disturbances of glucose regulation in CF, for example during infectious episodes or steroid treatment.
2.4. Screening for CFRD
Screening for CFRD is necessary because the onset can be insidious, and because it can cause harm before diagnosis. But two other conditions may cause harm. The first is IGT, which as mentioned above, can be associated with microvascular disease. 16 IGT is also associated with a reduction in lung function (FEV and FVC). A survey in the USA by Allen and colleagues20 found a wide range of screening practices and tests, with random PG the most common, followed by HbA1c, and urinary glucose. Very few used the OGTT.
The second is PPG, because it has been suggested that PPG may lead to end-products of glycation, which may cause irreversible damage. Gerich notes that isolated PPG, with normal fasting PG and normal HbA1c, is associated with an increase in vascular disease. 21 Though he was referring to 2-hour PG. Hanefield and colleagues reported that glycaemic excursions were associated with carotid intimal thickening in non-diabetic subjects. 22 The systematic review will examine the evidence for harm in people with CF and isolated PPG. If the review of treatment shows that PPG does harm, and can be effectively treated, it will be included in modelling.
If we should be concerned with PPG, or even just IGT, then that has implications for the choice of screening test. Fasting PG would not be satisfactory. Most guidelines recommend an annual OGTT, but it appears that due to the cost, inconvenience and unpleasantness of that test, that the guidelines are largely ignored in practice.
It is therefore necessary to consider:
-
whether other tests such as HbA1c, continuous blood glucose monitoring or home serial capillary blood glucose profiles could be used. Even tests not as sensitive (perhaps such as HbA1c) might still detect more cases in practice due to better compliance. A test which is 100% sensitive but which has only 50% acceptance will detect 50% of cases; one which has a sensitivity of 80% and an acceptance of 80% will detect 64% of cases.
-
whether combinations of tests might give better overall results, for example if screening was done in two or more stages. Such as by HbA1c in the first instance, with patients divided into three groups:
-
– HbA1c negative for diabetes. The cut-off value might be under 5%, but this would need to be reviewed following the systematic review. Anaemia is common in adults with CF (43% in a study by Drygalski and Biller 2006),23 and any reduction in red cell life would give misleadingly good HbA1c results. Anaemia was much less common in children, so HbA1c might be useful for screening for them, but not for adults.
-
– HbA1c diagnostic for diabetes (perhaps 6.0%).
-
– Intermediate HbA1c (say 5.1–5.9%, depending on literature review findings) followed by OGTT.
-
The sequence with HbA1c or random PG first might allow many patients to avoid OGTT.
In T2DM, HbA1c is influenced in the early stages more by non-fasting PG than fasting PG. 24 Whether it would be sensitive enough to pick up isolated PPG (without IGT) remains to be examined. The sensitivity would depend on the threshold at which patients were referred for OGTT.
Other tests include:
-
Automated serial blood glucose monitoring
-
Home blood glucose testing with sticks and meters – blood glucose profiling (BGP).
Again, as with OGTT, these could be used on all patients, or only on those shown likely to have CFRD or IGT after a preliminary screen with, for example, HbA1c or a casual PG.
Yung and colleagues (1999)25 reported that a combination of abnormal HbA1c and/or abnormal random PG and/or weight loss or symptoms of hyperglycaemia identified 11 out of 12 who had diabetes on OGTT.
Automated blood glucose monitoring is done by inserting a disposable glucose monitor under the skin, connected to a meter worn externally. A chemical reaction generates a current which is proportional to the level of glucose in the tissues. Strictly speaking it is interstitial tissue glucose which is monitored. A review by the Australia and New Zealand Horizon Scanning Network (ANZHSN 2006)26 noted that continuous blood glucose monitoring systems seemed to be better at detecting hyperglycaemia than hypoglycaemia, a problem which would not be relevant to its use in screening for CFRD. All the trials reported in the ANZHSN review were in people with diabetes; no use in screening was found.
Craigie and colleagues in the Royal Hospital for Sick Children in Glasgow have used BGP in children with CF, and have data (submitted for publication) showing that home glucose profiling is more acceptable than the annual OGTT (so far, 100% acceptance of profiles versus 50% acceptance of OGTT) and had a number of advantages, including:
-
It reflects “real-life” situations such as activities and meals
-
The technique is widely available and understood by all diabetes services
-
It does not require hospital attendance, once the technique is taught
-
It is relatively inexpensive, for example compared with CGMS
-
It is readily accepted by patients
-
It can be used to directly demonstrate the relationships between specific foods and blood glucose
-
It provides multiple readings over a 24 hour period.
But there are also some disadvantages:
-
Waking is necessary to do overnight testing
-
There is not the same 24-hour profile as obtained with CGMS
-
Capillary blood glucose may be 10–15% higher than venous BG
-
The expense of the meter and testing strips
-
The need for repeated skin pricks.
Fasting plasma glucose, even using the lowered ADA criteria for IFG, is considered insufficiently sensitive for screening for CFRD. 27
3. Research objectives
There are two questions to be addressed:
-
Does screening for diabetes or lesser disturbances of glucose metabolism in people with cystic fibrosis improve outcomes?
-
If, what is the best screening test or combination of tests?
If the answer to the first question is negative, the second need not be addressed.
4. Methods
4.1. The survey of current practice in the UK
We propose to do this by sending a brief questionnaire out via Cystic Fibrosis Trust network of centres (http://www.cftrust.org.uk/aboutcf/cfcare/ukcfcentres), but would also contact the British Thoracic Society, the British Paediatric Respiratory Society, and the Scottish Cystic Fibrosis Group.
We also propose a survey of the views of people with CF on screening options, and have approached the CF trust, which has indicated willingness in principle to collaborate. The intention would be that we would provide a set of scenarios for the different test strategies combined with a questionnaire which they would send to members. Anonymised replies would come back to us.
4.2. The systematic review of evidence on screening
Modelling
We have produced a pilot clinical model, and three extracts are attached, for illustrative purposes only, considering three broad approaches:
-
The zero option of no screening, included as a baseline for future economic appraisal
-
A one-stage screening approach, using OGTT in this extract
-
A two stage approach, for example, an initial screen with HbA1c followed by OGTT after borderline results.
The model would be further developed as part of the project. Once fully developed, it would allow costing of pathways and some consideration of cost-effectiveness issues. We suspect that data deficits would mean that primary research might be needed before definitive costs per QALY could be produced, but some ranging estimates based on plausible hypotheses could be produced. These would help to indicate the screening strategies most likely to be worth testing in a randomised trial.
The model allows us to consider the data requirement from the systematic review to be produced.
For points indicated by numbers on the model extracts, these are as follow.
-
1. The baseline “natural history” arm is included as the “no screening” option, which provides the baseline against which the cost-effectiveness of other options can be tested. There will be three groups:
-
– Those who do not develop diabetes or IGT in their lifetimes
-
– Those who do become diabetic but are never diagnosed
-
– Those who become diabetic because of symptoms and are diagnosed and treated.
-
The data requirements here include:
-
what proportion of people with CF will develop diabetes and at what ages?
-
of these, how many will develop symptoms of diabetes, and be diagnosed and treated?
-
how long after the onset of diabetes would symptoms occur?
-
how much does treatment of diabetes extend life and improve quality of life, in those developing symptoms?
Important intermediate outcomes are likely to include the rate of lung function development in children, and the rate of decline with adult ageing, and possibly other features such as liver disease.
-
2. Before considering the screening options, we need to review the advantages of diagnosis of CFRD. What is the best treatment? The beta cell loss might imply that the best treatment is insulin, but at the earlier PPG stage, is there a place for sulphonylureas or meglitinide analogues? How successful is treatment, in terms of extending life and quality of life? Does control of PG affect the frequency or severity of pulmonary infections? Would admissions to hospital be reduced?
A systematic review of treatment and outcomes would be required.
-
3. Screening with OGTT. Having clarified the natural history and the benefits of treatment, we would then consider screening options. In line with current guidelines,28 our first screening option would be the OGTT, assumed to be applied annually. 29 Data requirements at point 3 include:
-
– The sensitivity, specificity, negative and positive predictive values (the screening results)?
-
– What is the acceptance rate for OGTT in practice?
-
– At what age should screening start? The answer might be when the benefits of detecting and treating those with diabetes are high enough to justify the costs to NHS and patients of screening. The key determinant would be prevalence by age, followed by the success of treatment.
-
– The costs of screening by annual OGTT. The benefits would be obtained from the systematic review in stage 2 above.
-
This arm of the model needs five initial branches:
-
Screening accepted, CFRD diagnosed, treatment initiated and benefits obtained.
-
Screening accepted, diabetes excluded (for the time being), patient reassured.
-
Screening declined, but symptoms develop and treatment is started, but at a later stage, hence allowing some hyperglycaemic damage to have occurred.
-
Screening declined, diabetes develops but is not diagnosed in patient’s lifetime, which is shortened because of the diabetes.
-
Screening declined, but diabetes does not develop.
The outcomes in the second group would be as for the non-diabetic group in the baseline no screening option. The outcomes in the third and fourth groups might be worse than in the relevant groups in the no-screening option, since declining screening may be associated with other less than optimal health behaviours. The literature review will need to consider this.
However, those who decline OGTT screening might accept other forms of screening such as HbA1c. Additional arms could be added wherein those who decline are offered screening by other means.
One-stage screening with HbA1c, CGMS or glucose profiles would have similar data requirements and arms in the model.
Two-stage screening
We know that the alleged gold standard of the OGTT is not popular with patients, and the results of the American survey show that it is little used. If we conclude from the review above that the OGTT is best, another option is to have two-stage screening with only some patients going on to OGTT.
For example (please see extract from model), HbA1c could be used as the first test, with patients being divided into three groups (the precise thresholds would be determined by the systematic review):
-
HbA1c under 5.0% – classed as not diabetic or IGT
-
HbA1c of 6% of over – assumed to have diabetes or IGT
-
HbA1c > 5 and < 6 – referred for OGTT.
Far fewer patients might need OGTT, and the suspicion of diabetes might give a better acceptance rate. It assumes that HbA1c is not good enough on its own, but that remains to be assessed in the review.
HbA1c could also precede tests such as CGMS and glucose profiling.
The current recommendation is for annual screening but other options should be considered.
Methods of systematic review of clinical effectiveness
Standard HTA methods would be used.
Literature searches would be done of selected bibliographic databases – MEDLINE, EMBASE, The Cochrane Library (reviews, CENTRAL, HTA and DARE). See appendix 1.
Reference lists of relevant studies would be checked.
Searches have been done for research in progress.
Inclusion criteria will be specified in advance and will include:
-
For clinical effectiveness of treatment of CFRD and IGT, randomised controlled trials of any therapies used, compared with a baseline of no treatment (if available).
-
For effectiveness of screening options, studies reporting screening parameters and/or acceptance rates. Ideally, we would find randomised trials of screening but we do not think there are any.
Search products (titles and abstracts in a Reference Manager database) will be screened independently by two reviewers. Any discrepancies will be resolved by discussion, involving a third reviewer if necessary.
Data extraction forms will be developed and piloted. Data will be extracted by one reviewer and checked by a second. (We are aware that double data extraction is recommended by some people but we do not think this is cost-effective because there is usually good agreement between data extractors (Haywood et al 2004) and when discrepancies do occur, they are usually minor (Jones et al 2005). However we would do it if required by the HTA Programme, at extra cost.)
Quality assessment of studies will be done using:
-
For trials, the usual criteria such as security of randomisation, baseline matching of participants, numbers recruited and losses to follow-up, intention to treat analysis, as per CRD report 4.
-
For screening studies, we will use a modification of the QUADAS criteria (see appendix 2).
Methods of analysis
Data will be tabulated and discussed in a narrative review.
If data permit, results will be synthesised in meta-analyses, using Review Manager software. Checks for heterogeneity would be carried out first.
For screening options, receiver operator characteristic (ROC) curves will be produced if sufficient data are available. Areas under the curves (AUC) will be derived to compare the performance of tests.
Outcomes
We will seek data on the following outcomes:
Primary outcomes:
-
Mortality
-
Morbidity such as respiratory disease and complications of diabetes
-
Quality of life
-
And if possible will summarise the above as quality adjusted life years and cost per QALY.
Secondary outcomes will include (if data allow):
-
Measures of nutritional status
-
Measures of lung function
-
Frequency of IV antibiotics
-
Hospital admissions
-
Time spent on physiotherapy.
Test accuracy will be reported but is not classed as an outcome. Other process measure will include cost per test and cost per case found.
Cost-effectiveness: systematic review of existing economic evaluations
Inclusion criteria: We would include studies that evaluate screening for CFRD in general, and individual tests, in terms of both costs and outcomes.
Data extraction: Data would be extracted on the strategies compared, study population, dates, measures and source of effect, costs, price year and currency, results including any sensitivity analyses, and structure and form of analysis (patient-level data or model).
Quality assessment: Existing economic evaluations will be critically appraised using the BMJ guidelines for reviewers of economic evaluations. 30 Any models will be assessed against guidelines for good practice in modelling. 31
Data synthesis: A narrative synthesis will be conducted, presenting data for relevant subgroups where possible. It will also distinguish between studies that relate to a measure of diagnostic performance and those that relate to outcomes such as health gain.
However, our preliminary searches of MEDLINE, EMBASE and the CRD databases including NHS EED, and using Google for costs and economics of CFRD, have found nothing. A rapid search on costs and economics of CF found some studies but from the abstracts, none report the costs for CFRD.
Cost-effectiveness: assessment of cost-effectiveness
A full economic model will be developed. It will identify possible pathways from initial screening to the costs and consequences for those who receive correct and incorrect diagnoses, and of non-diagnosis for those who decline screening, or are not selected for screening (for example according to age thresholds for starting screening).
The economic model will consider short term and long-term costs and consequences. Costs will include those of the test options, and of other health service costs. Potential costs to patients of different screening strategies will be based on estimates from the literature, or from clinical experts, or from the survey of patient views.
The model would have at least six arms – no screening, OGTT, HbA1c, CGMS, glucose profiling, and one or more sequences. Each would have similar terminal branches, and the clinical effectiveness would depend on the proportions going down each branch. If we assume 1000 going down each arm, we can then derive the net QALYs from the proportions. The costs of each can also be derived, including not just those of screening but subsequent treatment and outcomes. The most cost-effective screening option can then be compared with no screening to tell us whether screening is cost-effective.
Discounting of future costs and QALYs would be done using 3.5% for both costs and QALYs.
The results of the model will be presented:
-
Firstly, in terms of a cost-consequence analysis (proportion of patients screened; number of cases detected; cost per case detected)
-
Secondly, as incremental cost-effectiveness ratios, and as cost per QALY if utilities can be derived
-
Thirdly, we will explore uncertainties using sensitivity analyses, for example applying the 95% confidence intervals around the screening parameters and treatment outcomes.
If data permit, the relative cost-effectiveness of different screening intervals will be assessed.
Modelling software
The model we are proposing would require a sophisticated simulation software package. The model would be dynamic, with screening would be carried out at least annually, if not more often. Thus, this requires discrete event simulation (DES) modelling; an event occurs (screening) at specific times and so a model is required that can move the simulation clock to the next time an event occurs. There are many DES simulation packages available to use. One of these is Simul8. It is a user-friendly simulation software that can also be linked to Visual Basic for Applications (VBA), a programming tool used in Excel. In addition, the processes being carried out in the model are very clear visually and are easy to comprehend.
Assessment of the case for screening against the NSC criteria
The National Screening Committee has a set of criteria, developed from those originally drawn up by the WHO. Not all are relevant to screening for CFRD, but appendix 3 (excised for space reasons, but available on request) lists those which are most relevant, and we would assess the case for screening against these in the Discussion of the report.
References
- Brock DJ, Gilfillan A, Holloway S. The incidence of cystic fibrosis in Scotland calculated from heterozygote frequencies. Clinical Genetics 1998;53:47-9.
- Dodge JA, Morison S, Lewis PA, Coles EC, Geddes D, Russell G, et al. Incidence, population, and survival of cystic fibrosis in the UK, 1968–95. UK Cystic Fibrosis Survey Management Committee. Archives of Disease in Childhood 1997;77:493-6.
- Assael BM, Castellani C, Ocampo MB, Iansa P, Callegaro A, Valsecchi MG. Epidemiology and survival analysis of cystic fibrosis in an area of intense neonatal screening over 30 years. American Journal of Epidemiology 2002;156:397-401.
- Dodge JA, Lewis PA. Cystic fibrosis is no longer an important cause of childhood death in the UK. Archives of Disease in Childhood 2005;90.
- Dodge JA, Lewis PA, Stanton M, Wilsher J. Cystic fibrosis mortality and survival in the UK: 1947–2003. European Respiratory Journal 2007;29:522-6.
- Mackie AD, Thornton SJ, Edenborough FP. Cystic fibrosis-related diabetes. Diabetic Medicine 2003;20:425-36.
- Milla CE, Warwick WJ, Moran A. Trends in pulmonary function in patients with cystic fibrosis correlate with the degree of glucose intolerance at baseline. American Journal of Respiratory &Amp; Critical Care Medicine 2000;162:t-5.
- Yung B, Noormohamed FH, Kemp M, Hooper J, Lant AF, Hodson ME. Cystic fibrosis-related diabetes: the role of peripheral insulin resistance and beta-cell dysfunction. Diabetic Medicine 2002;19:221-6.
- Couce M, O’Brien TD, Moran A, Roche PC, Butler PC. Diabetes mellitus in cystic fibrosis is characterized by islet amyloidosis. J Clin Endocrinol Metab 1996;81:1267-72.
- Dobson L, Sheldon CD, Hattersley AT. Understanding cystic-fibrosis-related diabetes: best thought of as insulin deficiency?. [Review] [78 refs]. Journal of the Royal Society of Medicine 2004;97.
- Brennan AL, Geddes DM, Gyi KM, Baker EH. Clinical importance of cystic fibrosis-related diabetes. Journal of Cystic Fibrosis 2004;3:209-22.
- Moran A, Phillips J, Milla C. Insulin and glucose excursion following premeal insulin lispro or repaglinide in cystic fibrosis-related diabetes. Diabetes Care 2001;24:1706-10.
- Andersen HU, Lanng S, Pressler T, Laugesen CS, Mathiesen ER. Cystic fibrosis-related diabetes: the presence of microvascular diabetes complications. Diabetes Care 2006;29:2660-3.
- Dobson L, Stride A, Bingham C, Elworthy S, Sheldon CD, Hattersley AT. Microalbuminuria as a screening tool in cystic fibrosis-related diabetes. Pediatric Pulmonology 2005;39:103-7.
- Lanng S, Thorsteinsson B, Lund-Andersen C, Nerup J, Schiotz PO, Koch C. Diabetes mellitus in Danish cystic fibrosis patients: prevalence and late diabetic complications. Acta Paediatrica 1994;83:72-7.
- Diabetes Prevention Program . The prevalence of retinopathy in impaired glucose tolerance and recent-onset diabetes in the Diabetes Prevention Program. Diabet Med 2007;24:137-44.
- Drummond RS, Love A, Semple K, Bicknell S, Ross E, Small M, et al. The favourable effect of insulin upon pulmonary function in patients with cystic fibrosis correlates with the degree of glucose intolerance at baseline. Diabetic Medicine 2006;23.
- Brennan AL, Gyi KM, Wood DM, Johnson J, Holliman R, Baines DL, et al. Airway glucose concentrations and effect on growth of respiratory pathogens in cystic fibrosis. Journal of Cystic Fibrosis 2007;6:101-9.
- Rolon MA, Benali K, Munck A, Navarro J, Clement A, Tubiana-Rufi N, et al. Cystic fibrosis-related diabetes mellitus: clinical impact of prediabetes and effects of insulin therapy. Acta Paediatrica 2001;90:860-7.
- Allen HF, Gay EC, Klingensmith GJ, Hamman RF. Identification and treatment of cystic fibrosis-related diabetes. A survey of current medical practice in the US. Diabetes Care 1998;21:943-8.
- Gerich JE. Clinical significance, pathogenesis, and management of postprandial hyperglycemia. Arch Intern Med 2003;163:1306-16.
- Hanefeld M, Koehler C, Schaper F, Fuecker K, Henkel E, Temelkova-Kurktschiev T. Postprandial plasma glucose is an independent risk factor for increased carotid intima–media thickness in non-diabetic individuals. Atherosclerosis 1999;144:229-35.
- von Drygalski A, Biller J. Anemia in cystic fibrosis (CF): Prevalence, mechanisms and correlation with pulmonary function. Blood (ASH Annual Meeting Abstracts) 2006;108:414-1.
- Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care 2003;26:881-5.
- Yung B, Kemp M, Hooper J, Hodson ME. Diagnosis of cystic fibrosis related diabetes: a selective approach in performing the oral glucose tolerance test based on a combination of clinical and biochemical criteria. Thorax 1999;54:40-3.
- Australia and New Zealand Horizon Scanning Network . Continuous Glucose Monitoring Devices: Horizon Scanning Report 2006. URL: http://nzhta.chmeds.ac.nz/publications/finalcgmd.pdf (accessed 6 August 2007).
- Mueller-Brandes C, Holl RW, Nastoll M, Ballmann M. New criteria for impaired fasting glucose and screening for diabetes in cystic fibrosis. European Respiratory Journal 2005;25:715-17.
- UK Cystic Fibrosis Trust Diabetes Working Group . Management Of Cystic Fibrosis Related Diabetes Mellitus 2004. URL: http://www.cftrust.org.uk/aboutcf/publications/consensusdoc/diabetes.pdf.
- Scottish Intercollegiate Guidelines Network . Management of Diabetes: SIGN Publication No. 55 2001. URL: http://www.sign.ac.uk/guidelines/fulltext/55/index.html.
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ 1996;313:275-83.
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8:iii-xi.
Appendix 1
CFRD search strategy
Due to the relatively small amount of publications available, a sensitive search would be done to capture all aspects of the CFRD literature.
-
The journal literature will be searched using MEDLINE, EMBASE, the Science Citation Index and all sections of The Cochrane Library (including the Cochrane Database of Systematic Reviews, CENTRAL and the Health Technology Assessment Database). The MEDLINE search strategy below will be used and appropriately adapted for the other databases:
-
(cystic fibrosis adj2 diabetes).tw
-
exp Cystic Fibrosis/
-
exp Diabetes Mellitus/
-
2 and 3
-
1 or 4
-
limit 5 to english language.
-
-
The meeting abstracts of the Annual Meetings of the American Diabetes Association (ADA), the European Association for the Study of Diabetes (EASD), the European Cystic Fibrosis Society (ECFS), the North American Cystic Fibrosis Conference and the Australasian Cystic Fibrosis Conference will be searched for recent literature that has not yet been published in full.
-
Research in progress will be searched for using the National Research Register and Current Controlled Trials
-
The internet will be searched for grey literature publications and reports, including those of the Cystic Fibrosis Trust UK and similar organisations worldwide.
-
Experts in the area will be contacted for unpublished studies.
Appendix 2
DRAFT quality assessment checklist (derived from QUADAS tool)
Study ID: Paper no:
Assessor initials: Date assessed:
| Item | Yes | No | Unclear | |
|---|---|---|---|---|
| 1 | Was the spectrum of patients representative of the patients who will receive the test in practice? | |||
| 2 | Is the reference standard likely to correctly classify the target condition? Our default will be that the OGTT will be used as the reference standard | |||
| 3 | Is the time period between the reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? We will take one month as the maximum interval | |||
| 4 | Did the whole sample or a random selection of the sample receive verification using a reference standard of diagnosis? | |||
| 5 | Did patients receive the same reference standard regardless of the index test result? | |||
| 6 | Was the reference standard independent of the index test (i.e. the index test did not form part of the reference standard)? This would apply if fasting plasma glucose was the index test | |||
| 7 | Were uninterpretable/intermediate/test results reported? This might not be relevant but anaemia and HbA1c might be an example where it is. | |||
| 8 | Were withdrawals from the study explained? | |||
| 9 | If a cut-off value has been used, was it established before the study was started (prespecified cut-off value)? | |||
| 10 | Is it unlikely that the technology of the index test has changed since the study was carried out? | |||
| 11 | Did the study provide a clear definition of what was considered to be a ‘positive’ result? | |||
| 12 | Was treatment withheld until both the index test and reference standard were performed? | |||
| 13 | Were data on test methods reported; for example was HbA1c aligned with DCCT? | |||
| 14 | Were data presented for appropriate subgroups of patients? | |||
| 15 | Was an appropriate sample size included? | |||
List of abbreviations
- %FEV1
- per cent predicted forced expiratory volume in 1 second
- %FVC
- per cent predicted forced vital capacity
- ADA
- American Diabetes Association
- AUC
- area under the curve
- BDI
- Beck Depression Inventory
- BG
- blood glucose
- BGP
- blood glucose profiling
- BMI
- body mass index
- CBGM
- continuous blood glucose monitoring
- CF
- cystic fibrosis
- CFQ
- Cystic Fibrosis Questionnaire
- CFQoL
- Cystic Fibrosis Quality of Life
- CFRD
- cystic fibrosis-related diabetes
- CFRD FH–
- cystic fibrosis-related diabetes without fasting hyperglycaemia
- CFRIGT
- cystic fibrosis-related impaired glucose tolerance
- CFTR
- cystic fibrosis transmembrane conductance regulator
- CGM
- continuous glucose monitoring
- CGMS
- continuous glucose monitoring system
- CHQ
- Child Health Questionnaire
- CI
- confidence interval
- CSII
- continuous subcutaneous insulin infusion
- DECODE
- Diabetes Epidemiology: Collaborative Analysis of Diagnostic Criteria in Europe
- DKA
- diabetic ketoacidosis
- DQoL
- Diabetes Quality-of-Life measure
- EASD
- European Association for the Study of Diabetes
- EHS
- Edinburgh Hypoglycaemia Scale
- EQ-5D
- European Quality of Life-5 Dimensions
- EQ-5D-Y
- EQ-5D version for children and adolescents
- FEV
- forced expiratory volume
- FEV1
- forced expiratory volume in 1 second
- FH
- fasting hyperglycaemia
- FOGTT
- full oral glucose tolerance test
- FPG
- fasting plasma glucose
- FPIR
- first-phase insulin response
- FVC
- forced vital capacity
- GCT
- glucose challenge test
- HbA1c
- glycated haemoglobin
- HTA
- Health Technology Assessment
- IFG
- impaired fasting glucose
- IGT
- impaired glucose tolerance
- INDET
- intermediate hyperglycaemia with normal FPG and 2-hour PG
- ISPAD
- International Society for Pediatric and Adolescent Diabetes
- MDI
- multiple daily injection
- NDDG
- National Diabetes Data Group
- NGT
- normal glucose tolerance
- NHA
- natural history arm
- NHANES
- National Health and Nutrition Examination Survey
- NICE
- National Institute for Health and Clinical Excellence
- NIH
- National Institutes of Health
- NPH
- neutral protamine Hagedorn
- NPV
- negative predictive value
- NSC
- National Screening Committee
- OGTT
- oral glucose tolerance test
- OHA
- oral hypoglycaemic agent
- PAF
- population attributable fraction
- PFT
- pulmonary function test
- PG
- plasma glucose
- PPH
- postprandial hyperglycaemia
- PPV
- positive predictive value
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- QUADAS
- quality assessment of diagnostic accuracy studies
- QWB
- Quality of Well-Being scale
- RBG
- random blood glucose
- RCT
- randomised controlled trial
- ROGTT
- reduced oral glucose tolerance test
- SD
- standard deviation
- SF-36
- Short Form questionnaire-36 items
- SIGN
- Scottish Intercollegiate Guidelines Network
- SIP
- Sickness Impact Profile
- SS
- Shwachman clinical score
- T1DM
- type 1 diabetes mellitus
- T2DM
- type 2 diabetes mellitus
- VAS
- visual analogue scale
- WHO
- World Health Organization
- WtSDS
- weight standard deviation score
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Department of Public Health and Epidemiology, University of Birmingham
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, University of Liverpool
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Judith Bliss, Director of ICR-Clinical Trials and Statistics Unit, The Institute of Cancer Research
-
Professor Peter Brocklehurst, Professor of Women’s Health, Institute for Women’s Health, University College London
-
Professor David Fitzmaurice, Professor of Primary Care Research, Department of Primary Care Clinical Sciences, University of Birmingham
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Angela Harden, Professor of Community and Family Health, Institute for Health and Human Development, University of East London
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford
-
Dr Joanne Lord, Reader, Health Economics Research Group, Brunel University
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor Dion Morton, Professor of Surgery, Academic Department of Surgery, University of Birmingham
-
Professor Gail Mountain, Professor of Health Services Research, Rehabilitation and Assistive Technologies Group, University of Sheffield
-
Professor Irwin Nazareth, Professor of Primary Care and Head of Department, Department of Primary Care and Population Sciences, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norrie, Chair in Clinical Trials and Biostatistics, Robertson Centre for Biostatistics, University of Glasgow
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Peter Tyrer, Professor of Community Psychiatry, Centre for Mental Health, Imperial College London
-
Professor Martin Underwood, Professor of Primary Care Research, Warwick Medical School, University of Warwick
-
Professor Caroline Watkins, Professor of Stroke and Older People’s Care, Chair of UK Forum for Stroke Training, Stroke Practice Research Unit, University of Central Lancashire
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Tom Foulks, Medical Research Council
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Diana Baralle, Consultant and Senior Lecturer in Clinical Genetics, University of Southampton
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Public contributor
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Nicola Lennard, Senior Medical Officer, MHRA
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Public contributor
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Public contributor
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Matthew Thompson, Senior Clinical Scientist and GP, Department of Primary Health Care, University of Oxford
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Joanna Jenkinson, Board Secretary, Neurosciences and Mental Health Board (NMHB), Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Public contributor
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire County Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Mrs Jean Thurston, Public contributor
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Public contributor
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Public contributor
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Public contributor
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Public contributor
-
Mr Sankaran Chandra Sekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Public contributor
-
Mr Seumas Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Dr Matthew Wilson, Consultant Anaesthetist, Sheffield Teaching Hospitals NHS Foundation Trust
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan, Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Public contributor
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer, Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Public contributor
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Public contributor
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Public contributor
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health