Notes
Article history
The research reported in this issue of the journal was commissioned by the National Coordinating Centre for Research Methodology (NCCRM), and was formally transferred to the HTA programme in April 2007 under the newly established NIHR Methodology Panel. The HTA programme project number is 06/91/10. The contractual start date was in October 2005. The draft report began editorial review in March 2011 and was accepted for publication in August 2011. The commissioning brief was devised by the NCCRM who specified the research question and study design. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Nicky Welton has received honoraria for teaching and consultancy relating to indirect and mixed treatment comparison meta-analyses from Abacus International (application areas unknown to Dr Welton), Pfizer (teaching only), United Biosource (rheumatoid arthritis) and the Canadian Agency for Drugs and Technology in Health (lung cancer and type 2 diabetes). In all cases, Dr Welton was blind to the included treatments. Hayley Jones has received honoraria for consultancy relating to mixed-treatment comparison meta-analysis from Novartis Pharma AG, but worked only with simulated data and was blind to any specific applications.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2012. This work was produced by Savović et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to NETSCC. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2012 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Although meta-analyses of randomised trials offer the best evidence for the evaluation of clinical interventions, they are not immune from bias. 1 Bias may arise because of selective reporting of whole trials or of outcomes within trials,2–5 or because of study design characteristics that compromise internal validity. 6 The design of randomised controlled trials (RCTs) should incorporate characteristics that avoid biases resulting from lack of comparability of the intervention and control groups. For example, concealment of randomised allocation avoids selection bias (differences in the probability of recruitment to the intervention and control groups based on participant characteristics at the time of recruitment), blinding of participants and trial personnel avoids performance bias (differences in aspects of patient management between intervention groups) and blinding of outcome assessors avoids detection bias (differences in outcome measurement).
Empirical evidence about the magnitude and relative importance of the influence of reported study design characteristics on trial results comes from ‘meta-epidemiological’ studies in which collections of meta-analyses are used to study associations between trial characteristics and intervention effect estimates. 7,8 There is evidence from such studies that inadequate allocation concealment and lack of blinding lead, on average, to exaggeration of intervention effect estimates. 9–11 However, the evidence is not consistent: some studies did not find evidence of effects of these characteristics,12–14 whereas others suggested that other study design characteristics were of importance. 9–11,15 Possible reasons for this lack of consistency include differential effects of study design characteristics across different clinical interventions or between different objectively and subjectively assessed outcome measures,16 differences in definitions of characteristics and methods of assessment (some studies relied on assessments reported in contributing systematic reviews9), and chance. 8 The extent of overlap between meta-analyses contributing to the different studies is unclear.
As the effects of study design characteristics tend to be estimated imprecisely within individual meta-analyses,8 large collections of meta-analyses are needed to estimate effects of these characteristics with precision, and to examine variability in these effects according to clinical area or type of outcome measure. It is, therefore, desirable to combine the collections of meta-analyses that were assembled in previous studies so that unified analyses can be conducted. However, these studies assembled their collections of meta-analyses independently, and so the extent of overlap between the meta-analyses and trials included in the different studies is unknown. Identification and resolution of such overlaps is difficult because of the multiplicities inherent in the data structure: trials report results on multiple outcomes and may be included in multiple meta-analyses, while systematic reviews may contain many different meta-analyses. Data are extracted from multiple publications that may describe trials, systematic reviews or both.
By combining data from 10 meta-epidemiological studies into a single database we identified and removed overlaps between trials and meta-analyses, and investigated the consistency of assessments of reported study design characteristics between the different contributing studies. 17 Based on seven contributing studies in which both study design characteristics and results were recorded, we investigated the influence of different study design characteristics on both average intervention effects and between-trial heterogeneity, according to type of intervention and type of outcome measure.
Chapter 2 Development of a combined database for meta-epidemiological research
Methods
Data
Data were combined as part of the BRANDO (Bias in Randomized and Observational studies) project. The authors of 10 meta-epidemiological studies9,11–15,18–21 (Table 1) agreed to contribute their original data to a combined database that would be used to conduct combined analyses and investigate reasons for differences between the results of the original studies. The combined database was created using Microsoft Access™ software (Microsoft Corporation, Redmond, WA, USA). Most authors supplied separate tables containing data on included trials and meta-analyses, allowing linkage between these two levels of data. When such information was not supplied, information from publications of relevant systematic reviews was used to link trials and meta-analyses. 15 Citations (of publications from which the data were extracted) were linked to the data sets using matching via source systematic review, author name and publication year. 12–15 Studies by Sampson et al. 20 and McAuley et al. 18 and the part of the study by Egger et al. 9 that was based on published journal articles [referred to hereafter as Egger (journal)] did not include information on study design characteristics in the included trials, whereas the study by Royle and Milne19 did not include outcome data. These studies cannot contribute to meta-epidemiological analyses, but were retained to contribute to other, descriptive analyses.
| Contributing meta-epidemiological study | Number of contributed meta-analyses (trials) | Clinical areas/types of interventions | Number of meta-analyses (trial results) in final database |
|---|---|---|---|
| Als-Nielsen et al.12,22 | 48 (523) | Various | 46 (506) |
| Balk et al.13 | 26 (276) | Circulatory, paediatrics, infection, surgery | 23 (251) |
| Contopoulos-Ioannidis et al.21 | 16 (133) | Mental health | 11 (94) |
| Egger et al.9 | 165 (1776)a | Various | 121 (1115)a |
| Kjaergard et al.15 | 14 (190) | Various | 8 (72) |
| McAuley et al.18 | 31 (454) | Various | 18 (205)b |
| Pildal et al.14 | 68 (474) | Various | 67 (460) |
| Royle and Milne19 | 29 (541) | Various | 28 (452)c |
| Sampson et al.20 | 24 (257) | Circulatory, digestive, mental health, pregnancy and childbirth | 14 (112)b |
| Schulz et al.11 | 33 (250) | Pregnancy and childbirth | 27 (210) |
Assigning identification numbers and creating a unified data set
To enable automated identification of meta-analyses and trials that occurred in more than one meta-epidemiological study, we labelled each trial and review entry in contributing data sets with a MEDLINE, EMBASE or ISI Web of Science unique publication identifier (ID), in respective order of preference. We first searched MEDLINE (through PubMed™) using bibliographic information for every trial and review included in contributing data sets [e.g. full title, author(s), publication year, journal, volume, pagination] and retrieved their unique MEDLINE identifier (PMID). For references that were not located in MEDLINE, EMBASE was searched (through Ovid™) in the same way and EMBASE-unique identifiers were retrieved. For references that were not located in EMBASE the search was carried out in the ISI Web of Science database, but only a small number of the remaining non-indexed citations were identified in this way. Trial references not indexed in MEDLINE, EMBASE or ISI were cross-checked for duplication with other references using duplicate search facilities in Reference Manager™ bibliographic database software (Thompson Reuters, New York, NY, USA), followed by manual checking. All non-indexed, unique references were assigned a unique identification number derived from their ID number in the Reference Manager database.
We defined in the master database the variables that we wished to combine from the contributing studies. An initial combined data set was then created by mapping each variable in each contributed data set to the predefined variable in the master database that it most closely matched. New variables were then added to the master database to capture contributed data that did not fit the a priori variable definitions.
Identification and removal of duplicate meta-analyses and trials
The unique identifiers of trials included in each meta-analysis in the combined data set were compared with those in every other meta-analysis (regardless of whether or not the meta-analyses assessed the same outcome). Using Intercooled Stata™ version 9 (StataCorp LP, College Station, TX, USA), meta-analyses that contained any trials in common with any other meta-analysis were grouped together. Not all meta-analyses within these sets contained overlapping trials: for example, two meta-analyses with no trials in common might each contain trials in common with a third meta-analysis and these three meta-analyses formed one set.
Meta-analyses that had no overlap with any other meta-analysis in the combined data set were transferred to the final data set. We then considered each set of overlapping meta-analyses in turn. Meta-analyses were excluded from each set until there was minimal overlap between the remaining meta-analyses, according to the following rules:
-
Exclude meta-analyses from the Royle and Milne19 study, which did not include outcome data (entire meta-analysis removed regardless of the extent of overlap).
-
Exclude meta-analyses for which information on study design characteristics was not available in preference to those for which such information was available. If both meta-analyses in a duplicate pair contain no information on study design characteristics, exclude meta-analyses from earlier studies first: in order Egger (journal),9 Sampson et al. ,20 McAuley et al. 18 (entire meta-analysis removed regardless of the extent of overlap).
-
Exclude meta-analyses from the portion of the study by Egger et al. 9 that was based on assessment of study design characteristics in Cochrane reviews in preference to meta-analyses from studies that directly assessed these characteristics.
-
Exclude meta-analyses with fewer assessed study design characteristics in preference to those with more assessed characteristics.
-
Exclude meta-analyses from less recently published systematic reviews in preference to more recently published reviews.
-
Exclude meta-analyses including fewer trials in preference to meta-analyses including more trials.
These rules were used in order of priority, that is, the next rule was applied only if the previous could not yield a decision. We recorded reasons for all decisions to exclude meta-analyses. Pairs of meta-analyses with recorded study design characteristics and results that had only a minimal overlap between them were retained at this stage. Overlap was considered minimal if the number of overlapping trials was no more than 10% of the sum of the numbers of overlapping trials and unique trials from both meta-analyses. We then removed the overlapping trials from the meta-analysis in the duplicate pair that (1) contained more trials or (2) according to the rules 3 to 5 above. If these rules did not yield a clear decision, overlapping trials were removed from one of the meta-analyses at random. At both stages, the choice of meta-analyses or trials to be removed was independent of the assessment of the study design characteristics, and disagreements in these assessments between studies were not considered. Similarly, the decision on removal was also independent of the types of outcome measure assessed in the overlapping meta-analyses.
Some meta-analyses contained multiple results from the same trial, usually because they included multiarm trials in which the same control/comparison group was compared with two different treatment groups. Where appropriate, the two treatment groups were combined. In other cases one of the results was removed at random. We then checked the 2 × 2 results tables from trials in the deduplicated data set against the data in the source review publications. Inconsistencies were clarified with the contributors and corrected where necessary. We recoded the direction of outcome events where necessary so that the coded outcome for each trial corresponded to an adverse (undesirable) event.
Assessing consistency of assessment of reported study design characteristics in different contributing studies
We used trials included in more than one meta-epidemiological study to assess the reliability of the assessment of methodological characteristics between studies. For this analysis we defined such trials as those with the same bibliographic reference occurring in more than one meta-epidemiological study, irrespective of numbers of participants or events, or the type of outcome measure. To assess the inter-rater reliability we compared contributors’ assessments of the following three methodological characteristics: adequacy of the method for generating the random sequence used for allocating participants to treatment groups (sequence generation), adequacy of concealment of treatment allocation from participants and investigators at the point of enrolment into the trial (allocation concealment), and contributors’ assessments of whether or not a trial was double blind or not (blinding). Kappa statistics were calculated for the assessment of sequence generation (inadequate/unclear compared with adequate), allocation concealment (inadequate/unclear compared with adequate) and blinding (not double blind/unclear compared with double blind) of duplicated trials for each pair-wise comparison between contributing meta-epidemiological studies. Only comparisons with at least 10 overlapping trials between any two meta-epidemiological studies were analysed, for each of the three characteristics.
Classification of interventions and outcome measures
We classified the type of experimental intervention, type of comparison intervention and type of outcome measure for each meta-analysis in the final data set. Interventions were classified based on an expanded and modified version of the classification by Moja et al. 23 Comparison interventions were further classified as inactive (e.g. placebo, standard care) or active. When it was not clear which intervention group should be considered experimental and which the comparison, at least two study collaborators made a consensus decision. In cases when a decision could not be reached, such meta-analyses were excluded from analyses of associations between study design characteristics and intervention effect estimates.
We classified outcome measures according to an expanded and modified version of the classification developed by Wood et al. 16 Outcome measures were further grouped as all-cause mortality, other objectively assessed (including pregnancy outcomes and laboratory outcomes), objectively measured but potentially influenced by clinician/patient judgement (e.g. hospital admissions, total dropouts/withdrawals, caesarean section, operative/assisted delivery) and subjectively assessed. When a review reported that different methods of outcome assessment were used in different trials within the same meta-analysis, the meta-analysis was classified according to the most subjective method of outcome assessment. This was the case in 16 meta-analyses. For example, some trials of smoking cessation assessed outcomes using exhaled carbon dioxide or salivary cotinine (classified as objectively assessed), whereas for others it was by patient self-report (classified as subjectively assessed). Classifications of interventions and outcome measures were checked by at least two of the collaborators who were clinicians by training (CG, LLG, BA-N or JP). Classifications of both outcome measures and interventions were based solely on the information provided in the review from which the meta-analysis was extracted. We did not retrieve further information on individual outcome measures from publications of included trials.
Results
Database structure
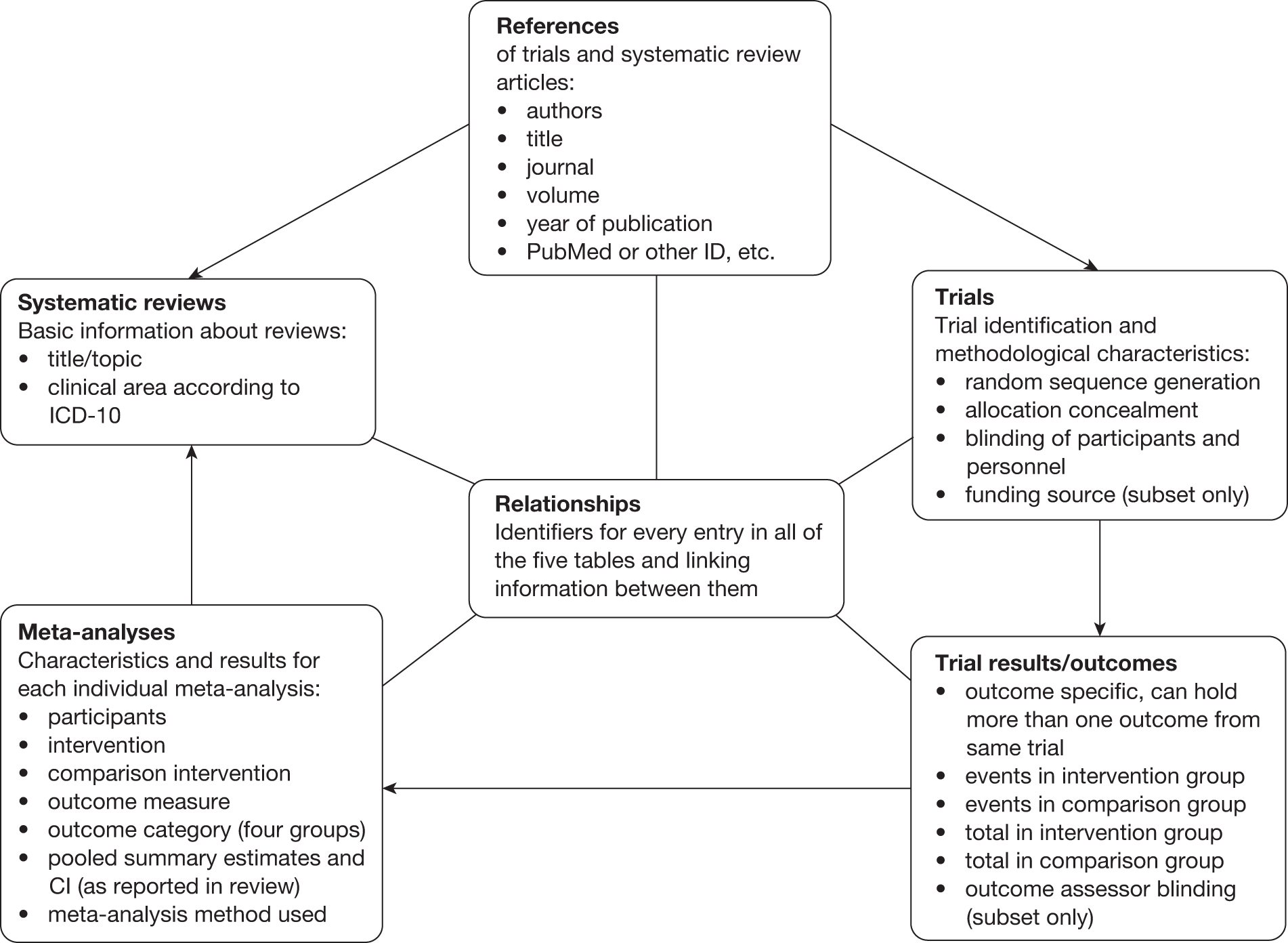
The database design allowed for multiple results from the same trial to be contained in different meta-analyses, multiple meta-analyses in systematic reviews, overlapping meta-analyses between systematic reviews and multiple references to the same trial or review. The final database structure consisted of six tables (Figure 1). The first five tables, References, Trials, Trial Results, Meta-analyses and Systematic Reviews, contained relevant study characteristics. The sixth table, Relationships, contained the identifiers necessary to link information between all the other tables. Each reference entry was linked to its related trial or systematic review, and further links were established between trials, trial results, meta-analyses and systematic reviews in the corresponding database tables. Figure 1 lists core variables included in each table.
FIGURE 1.
Schematic representation of the final database structure. ICD-10, International Classification of Disease, 10th edition. 30
Removing overlaps
Figure 2 depicts the derivation of the final database through removal of overlaps between meta-analyses. The initial combined data set contained 427 systematic reviews with 454 meta-analyses, 4857 trials and 4874 trial results. Of the 454 meta-analyses, 196 contained at least one trial that overlapped with at least one other meta-analysis, among which we identified 71 sets of meta-analyses containing overlapping trials. The size of these sets varied from 2 to 17 meta-analyses (median 2). We removed 91 entire meta-analyses containing 1354 trial results during the deduplication process. Of the 1354 results removed, 844 were duplicates of trials retained in the database, whereas 510 were unique. Of those that were unique, 340 (67%) were from studies that did not record either methodological characteristics or results, thus 170 potentially informative trials were removed during this process. A further 24 individual trial results were removed from 16 additional meta-analyses for which overlap was minimal (see methods). One trial was removed from 10 meta-analyses, two trials from four meta-analyses, and three trials from two meta-analyses each. An additional 19 trial results were removed as a result of overlaps within nine of the meta-analyses (e.g. where there were two comparisons with a common control group). The final database contained 352 systematic reviews contributing 363 meta-analyses, 3474 trials and 3477 trial results.
FIGURE 2.
Flow diagram depicting the deduplication process.
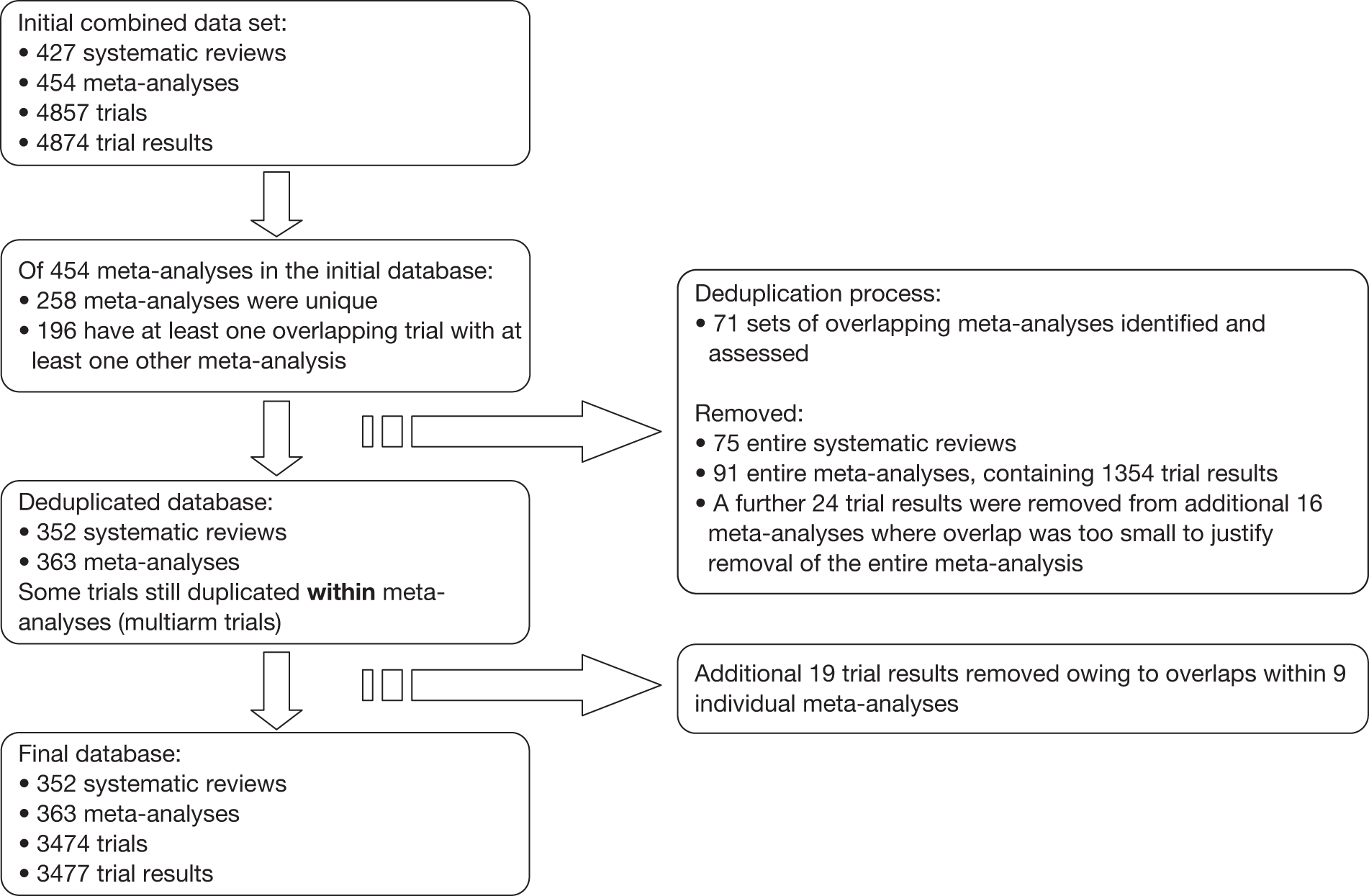
Table 2 shows an example of a set of four meta-analyses containing overlapping trials. Three meta-analyses (1005, 2456 and 2531) were taken from the same Cochrane review,24 whereas 2097 was from a journal review25 with a similar topic. Table 3 summarises the overlap between the trials in each pair of meta-analyses. To remove overlaps, meta-analyses were dropped in the following order: (1) meta-analysis 2097, because it was contributed by the Egger (journal) study,9 which did not include study design characteristics; (2) meta-analysis 2456, because study design characteristics in this study were extracted from a Cochrane review rather than from primary publications. The overlap between the remaining two meta-analyses (1005, from the study by Contopoulos-Ioannidis et al. 21 and 2531 from the study by Kjaergard et al. 15) was then examined in detail (Table 4). Each meta-analysis contained 10 trials, of which eight overlapped. Of the overlapping trials, seven had the same totals per group (different event numbers), and one trial had different data for the control group. These meta-analyses assessed two different outcomes: that in meta-analysis 1005 was ‘No change in positive and negative syndrome scale (data greater than 20%)’, whereas that in meta-analysis 2531 was ‘dropouts’. This explains why there was no exact correspondence in the 2 × 2 data for any trial. Meta-analysis 1005 was retained because the contributing study provided information on one additional study design characteristic (sequence generation).
| Meta-analysis ID | PMID of review | Title of systematic review | Assessed outcome | Meta-epidemiological study |
|---|---|---|---|---|
| 1005 | 10796543 | Risperidone versus typical antipsychotic medication for schizophrenia (Cochrane review–CD000440)24 | No change in positive and negative syndrome scale | Contopoulos-Ioannidis et al.21 |
| 2097 | 9097896 | Risperidone in the treatment of schizophrenia: a meta-analysis of randomized controlled trials (journal review)25 | Clinical improvement | Egger (journal)9 |
| 2456 | 10796543 | Risperidone versus typical antipsychotic medication for schizophrenia (Cochrane review–CD000440)24 | Withdrawals/dropouts | Egger (CDSR)9 |
| 2531 | 10796543 | Risperidone versus typical antipsychotic medication for schizophrenia (Cochrane review–CD000440)24 | Withdrawals/dropouts | Kjaergard et al.15 |
| Meta-analysis 1 | Meta-analysis 2 | Overlaps | Comment | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ID | Contributing study | Unique trials | ID | Contributing study | Unique trials | Exact | Same totals | Different | Within | |
| 1005 | Contopoulos-Ioannidis et al.21 | 3 | 2097 | Egger (journal)9 | 4 | 3 | 2 | 2 | 0 | 2097 removed, no methodology data |
| 1005 | Contopoulos-Ioannidis et al.21 | 3 | 2456 | Egger (CDSR)9 | 5 | 0 | 7 | 0 | 0 | 2456 removed, data from review only |
| 1005 | Contopoulos-Ioannidis et al.21 | 2 | 2531 | Kjaergard et al.15 | 2 | 0 | 7 | 1 | 0 | Detailed assessment needed (see Table 4) |
| 2097 | Egger (journal)9 | 4 | 2456 | Egger (CDSR)9 | 5 | 0 | 5 | 2 | 0 | Both already removed |
| 2097 | Egger (journal)9 | 3 | 2531 | Kjaergard et al.15 | 2 | 0 | 6 | 2 | 0 | 2097 already removed |
| 2456 | Egger (CDSR)9 | 3 | 2531 | Kjaergard et al.15 | 1 | 8 | 0 | 1 | 0 | 2456 already removed |
| Trial IDa | Meta-analysis 1005 (Contopoulos-Ioannidis21) – kept | Meta-analysis 2531 (Kjaergard15) – removed | Trial unique or overlaps? | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| d1 | h1 | n1 | d0 | h0 | n0 | d1 | h1 | n1 | d0 | h0 | n0 | ||
| R_17134 | 65 | 28 | 93 | 77 | 14 | 91 | Unique | ||||||
| R_17135 | 126 | 223 | 349 | 170 | 156 | 326 | Unique | ||||||
| P_1375801 | 15 | 7 | 22 | 17 | 5 | 22 | 1 | 21 | 22 | 5 | 17 | 22 | Overlap (equal totals) |
| P_1381102 | 26 | 27 | 53 | 31 | 22 | 53 | Unique | ||||||
| P_7508675 | 18 | 37 | 55 | 22 | 30 | 52 | 14 | 41 | 55 | 15 | 37 | 52 | Overlap (equal totals) |
| P_7514366 | 139 | 117 | 256 | 46 | 20 | 66 | 122 | 134 | 256 | 38 | 28 | 66 | Overlap (equal totals) |
| P_7542829 | 20 | 28 | 48 | 29 | 21 | 50 | 17 | 31 | 48 | 23 | 27 | 50 | Overlap (equal totals) |
| P_7545060 | 457 | 679 | 1136 | 97 | 129 | 226 | 280 | 856 | 1136 | 63 | 163 | 226 | Overlap (equal totals) |
| P_7683702 | 44 | 48 | 92 | 11 | 10 | 21 | 36 | 56 | 92 | 13 | 8 | 21 | Overlap (equal totals) |
| P_7691017 | 6 | 10 | 16 | 5 | 14 | 19 | 3 | 13 | 16 | 0 | 19 | 19 | Overlap (equal totals) |
| P_7694306 | 0 | 31 | 31 | 3 | 28 | 31 | Unique | ||||||
| P_8834417 | 4 | 17 | 21 | 8 | 12 | 20 | 4 | 17 | 21 | 6b | 4b | 10b | Overlap (different)b |
Reliability of assessment of reported study design characteristics
Eight of the participating meta-epidemiological studies contained sufficient trials (at least 10) in common to contribute to analyses of the reliability of assessment of reported study design characteristics (Table 5). Overall, there was good agreement between assessments carried out in the different contributing studies. For sequence generation (two comparisons), the percentages of studies in which the assessments were in agreement were 81% and 82% and kappa statistics were 0.56 and 0.64. For allocation concealment (12 comparisons), percentage agreement varied between 52% and 100% and kappa statistics between 0.19 and 1.00 (median 0.58). The lowest kappa (0.19) was observed in the comparison between the Egger et al. 9 and Pildal et al. 14 studies: 10 (48%) studies assessed as having adequate concealment of allocation in the Egger study (which used assessments reported by Cochrane review authors) were assessed as having inadequate or unclear concealment of allocation in the Pildal study. Assessments were most reliable for blinding (nine comparisons): percentage agreement ranged from 80% to 100% (in four comparisons), and kappa statistics ranged from 0.55 to 1.00 (median 0.87).
| Study 1 | Study 2 | Kappa | Agreement (%) | No. of trials | n00a | n01a | n10a | n11a |
|---|---|---|---|---|---|---|---|---|
| Sequence generation (inadequate/unclear vs adequate) | ||||||||
| Als-Nielsen et al.12,22 | Pildal et al.14 | 0.64 | 82 | 22 | 11 | 0 | 4 | 7 |
| Schulz et al.11 | 0.56 | 81 | 16 | 10 | 0 | 3 | 3 | |
| Allocation concealment (inadequate/unclear vs adequate) | ||||||||
| Als-Nielsen et al.12,22 | Balk et al.13 | 0.63 | 93 | 14 | 12 | 1 | 0 | 1 |
| Egger et al.9 | 0.78 | 96 | 94 | 82 | 2 | 2 | 8 | |
| Kjaergard et al.15 | 0.48 | 86 | 56 | 43 | 6 | 2 | 5 | |
| Pildal et al.14 | 0.58 | 86 | 22 | 16 | 2 | 1 | 3 | |
| Royle and Milne19 | 0.63 | 85 | 54 | 36 | 8 | 0 | 10 | |
| Schulz et al.11 | 0.76 | 94 | 16 | 13 | 1 | 0 | 2 | |
| Egger et al.9 | Contopoulos-Ioannidis et al.21 | NA | 100 | 14 | 14 | 0 | 0 | 0 |
| Pildal et al.14 | 0.19 | 52 | 21 | 8 | 0 | 10 | 3 | |
| Kjaergard et al.15 | Balk et al.13 | 0.39 | 67 | 15 | 6 | 0 | 5 | 4 |
| Egger et al.9 | 0.58 | 90 | 63 | 52 | 0 | 6 | 5 | |
| Contopoulos-Ioannidis et al.21 | 0.38 | 80 | 10 | 7 | 1 | 1 | 1 | |
| Schulz et al.11 | Egger et al.9 | 1.00 | 100 | 20 | 14 | 0 | 0 | 6 |
| Blinding (not double blind/unclear vs double blind) | ||||||||
| Als-Nielsen et al.12,22 | Egger et al.9 | 0.89 | 95 | 112 | 63 | 4 | 2 | 43 |
| Kjaergard et al.15 | 0.74 | 88 | 56 | 20 | 5 | 2 | 29 | |
| Pildal et al.14 | 1.00 | 100 | 22 | 10 | 0 | 0 | 12 | |
| Schulz et al.11 | NA | 100 | 15 | 15 | 0 | 0 | 0 | |
| Egger et al.9 | Contopoulos-Ioannidis et al.21 | 1.00 | 100 | 17 | 10 | 0 | 0 | 7 |
| Pildal et al.14 | 0.87 | 95 | 19 | 5 | 0 | 1 | 13 | |
| Kjaergard et al.15 | Egger et al.9 | 0.69 | 84 | 77 | 32 | 6 | 6 | 33 |
| Contopoulos-Ioannidis et al.21 | 0.55 | 80 | 10 | 2 | 2 | 0 | 6 | |
| Schulz et al.11 | Egger et al.9 | NA | 100 | 15 | 15 | 0 | 0 | 0 |
Interventions and outcome measures
Outcome measures were initially classified into one of 23 categories. Table 6 shows the numbers of meta-analyses with outcomes in each of these categories, together with the final category grouping. The most common outcome measure was all-cause mortality (64 meta-analyses, 18%), followed by clinician-assessed outcomes (51 meta-analyses, 14%).
| Type of outcome measure | No. of meta-analyses | Outcome groupa |
|---|---|---|
| Adverse events (as adverse effects of the treatment) | 6 | 4 |
| All-cause mortality | 64 | 1 |
| Cause-specific mortality | 2 | 4 |
| Clinician-assessed outcomes (e.g. body mass index, blood pressure, lung function, infant weight) | 51 | Mostly 4 |
| Composite end point including end points other than mortality/major morbidity | 0 | NA |
| Composite end point including mortality and/or major morbidity | 9 | 2, 3 or 4 |
| Global improvement | 4 | 4 |
| Health perceptions (person’s own view of general health) | 0 | 4 |
| Laboratory-reported outcomes (e.g. blood components, tissue analysis, urinalysis) | 29 | Mostly 2 (two 4) |
| Lifestyle outcomes (including diet, exercise, smoking) | 12 | Mostly 4 (one 2) |
| Major morbidity event (including myocardial infarction, stroke, haemorrhage) | 6 | 4 |
| Mental health outcomes (including cognitive function, depression and anxiety scores) | 16 | 4 |
| Other outcomes (not classified elsewhere) | 7 | 2, 3 or 4 |
| Pain (extent of pain a patient is experiencing) | 13 | 4 |
| Perinatal outcomes | 32 | 2 or 3 |
| Pregnancy outcomes | 11 | 2 |
| Quality of life (including ability to perform physical, daily and social activities) | 0 | 4 |
| Radiological outcomes (including radiograph abnormalities, ultrasound, magnetic resonance imaging results) | 12 | 4 |
| Resource use (including cost, hospital stay duration, number of procedures) | 4 | 3 |
| Satisfaction with care (including patient views and clinician assessments) | 0 | 4 |
| Surgical and device-related outcomes | 16 | Mostly 4 (two 3) |
| Symptoms or signs of illness or condition | 35 | 4 |
| Withdrawals/dropouts/compliance | 16 | 3 |
Experimental and comparison interventions were classified as shown in Table 7. The majority of included meta-analyses (242 meta-analyses, 67%) assessed a pharmacological experimental intervention. The most common comparison intervention was placebo or no treatment (225 meta-analyses, 62%), followed by a pharmacological comparison (54 meta-analyses, 15%). Forty-eight meta-analyses (397 trial outcomes) in which it was unclear what was the experimental intervention were excluded from meta-epidemiological analyses.
| Intervention categories | Meta-analyses per categorya | |
|---|---|---|
| Experimental | Comparison | |
| Experimental and comparison interventions | ||
| Diagnostics and screening | 7 | 4 |
| Interventions applying energy source for therapeutic purposes (e.g. ECT/radiotherapy, light therapy, etc.) | 5 | 1 |
| Lifestyle interventions (diet change, exercise, smoking cessation, etc.) | 5 | 1 |
| Medical devices | 15 | 11 |
| Pharmacological | 242 | 54 |
| Physical and manipulative therapy (physiotherapy, chiropractics, etc.) | 2 | 0 |
| Psychosocial (including psychotherapy, counselling, behavioural, advice, guidelines, self-help, etc.) | 16 | 2 |
| Resources and infrastructure/provision of care | 9 | 0 |
| Specialist nutritional interventions and fluid delivery (e.g. parenteral nutrition) | 8 | 6 |
| Surgical interventions or procedures | 26 | 19 |
| Therapies of biological origin (excluding vaccines, including in vitro fertilisation) | 2 | 1 |
| Vaccines | 4 | 1 |
| Other | 7 | 3 |
| Dual intervention (a complex intervention with components that fall in two different categories) | 12 | 0 |
| Multiple intervention (a complex intervention with components that fall in more than two different categories) | 3 | 2 |
| Comparison interventions only | ||
| No treatment | 58 | |
| Placebo | 99 | |
| Placebo or no treatment combined at meta-analysis level | 68 | |
| Standard/usual care | 11 | |
| Standard care and/or placebo and/or no treatment combined at meta-analysis level | 8 | |
Description of the final database
The final data set contained information from 363 meta-analyses containing 3474 trials. Of these, 282 meta-analyses (2572 trials) had 2 × 2 results data available. A total of 186 meta-analyses (1236 trials) had information on randomisation sequence generation, 228 (1840) on allocation concealment and 234 (1970) on blinding. In total, 175 meta-analyses (1171 trials) had information on 2 × 2 results data and these three study design characteristics.
Chapter 3 Influence of reported study design characteristics on average intervention effects and between-trial heterogeneity
Introduction
In this chapter we report the results of analyses of the influence of three reported study design characteristics [inadequate or unclear (compared with adequate) random sequence generation; inadequate or unclear (compared with adequate) allocation concealment; and absent or unclear double blinding (compared with double blinding)] on both average intervention effects and between-trial heterogeneity, according to the type of intervention and type of outcome. We examine whether or not these influences vary with the type of clinical area, intervention, comparison and outcome measure, examine effects of combinations of study design characteristics, estimate-adjusted effects using multivariable models, compare results with those derived using previously used (meta-meta-analytic) methods and explore implications of these findings for downweighting of trials whose study design characteristics are associated with bias in future meta-analyses.
Methods
For this part of the study we removed, from the database described in Chapter 2, data from three meta-epidemiological studies18–20 and one part of the Egger et al. study9 that did not collect data on study design characteristics (87 meta-analyses with 1093 trials). We also removed 36 meta-analyses (300 trials) in which it was not possible to classify one intervention as experimental and the other as control, one meta-analysis (four trials) that had a continuous outcome, 45 trials in which outcome data were missing and 50 trials in which either no or all participants experienced the outcome event.
Categories of intervention (see Table 7) containing fewer than 10 meta-analyses or 50 trials were combined into four types of intervention: pharmacological, surgical, psychosocial and behavioural, and all other interventions. Comparison interventions were classified as inactive (e.g. placebo, no intervention, standard care) or active. Outcome measures were grouped as all-cause mortality, other objectively assessed (including pregnancy outcomes and laboratory outcomes), objectively measured but potentially influenced by clinician/patient judgement (e.g. hospital admissions, total dropouts/withdrawals, caesarean section, operative/assisted delivery) and subjectively assessed (e.g. clinician-assessed outcomes, symptoms and symptom scores, pain, metal health outcomes, cause-specific mortality). Too few meta-analyses were categorised as having ‘other objectively assessed’ and ‘objectively measured but potentially influenced by clinician/patient judgement’ outcome measures to allow separate analyses; these categories were therefore grouped together.
Statistical methods
Intervention effects were modelled as log-odds ratios and outcomes were recoded where necessary so that odds ratios (ORs) < 1 corresponded to beneficial intervention effects. We fitted Bayesian hierarchical bias models using the formulation previously described as ‘Model 3’ by Welton et al. 26 We assumed that the observed number of events in each arm of each trial has a binomial distribution, with the underlying log-odds ratio in trial i in meta-analysis m (LORim) equal to
where Xim = 1 and 0 for trials with and without the reported characteristic. The parameter δim represents the intervention effect in trial i of meta-analysis m. These are assumed to be randomly distributed with variance τm2 within each meta-analysis:
Parameter βim quantifies the potential bias associated with the study design characteristic of interest. We assumed the following model structure:
for trials i with the reported characteristic in each meta-analysis m, and
across meta-analyses.
This allows for three effects of bias. First, mean intervention effects may differ between trials with and without the reported study design characteristic. Estimated mean differences (b0) were exponentiated and are thus reported as ratios of odds ratios (RORs). Second, variation in bias between trials within meta-analyses is quantified by standard deviation κ; κ2 corresponds to the average increase in between-trial heterogeneity in trials with a specified study design characteristic. Third, variation in mean bias between meta-analyses is quantified by between-meta-analysis standard deviation φ. We derived 95% credible intervals (CrI) for each parameter. In presenting results from our primary analyses, we also display the posterior variance of the parameter b0, denoted by V0. Use of this value in downweighting results from trials at high risk of bias in future meta-analyses is discussed below. For the results of our secondary analyses we do not present V0 explicitly, but the posterior uncertainty about b0 is reflected in the CrI for the ROR.
Data management and cleaning prior to analysis, and graphical displays of results, were carried out using Stata Version 11. Bias models were then fitted using WinBUGS Version 1.4 (MRC Biostatistics Unit, Cambridge, UK),27 assuming vague prior distributions for unknown parameters. Preliminary results indicated that estimated variance components κ and φ were sensitive to the prior distributions assumed for these parameters. Sensitivity to priors for variance parameters is a well-known problem in Bayesian hierarchical modelling. 28,29 This motivated a simulation study in which the performance of a range of prior distributions for variance components was compared, assuming typical values from the BRANDO database (Harris et al. , Health Protection Agency, 2010, personal communication). The prior found to give the best overall performance was a modified Inverse Gamma(0.001, 0.001) prior with increased weight on small values. This prior was therefore assumed for each variance parameter in all analyses. For location parameters (overall mean bias, baseline response rates, treatment effects), Normal(0, 1000) priors were assumed.
Meta-analyses can inform estimates of the effect of a study design characteristic only if they contain at least one trial with and one without the characteristic. We refer to such meta-analyses and trials from these meta-analyses as informative. As it is impossible to estimate both τ2 and κ in a meta-analysis with fewer than two studies with and without the study design characteristic of interest, such meta-analyses were prevented from contributing to the estimation of κ by use of the ‘cut’ function in WinBUGS.
We first conducted univariable analyses for each study design characteristic separately using all informative meta-analyses for that characteristic. The primary analysis used dichotomised variables for each characteristic (inadequate/unclear compared with adequate for sequence generation and allocation concealment, and not double blind/unclear compared with double bind). All such analyses were repeated separately for different types of outcome measure (all-cause mortality, other objectively assessed and subjectively assessed). Evidence that effects of bias differed according to the type of outcome was quantified using posterior probabilities that effects for subjective or other objective outcomes were larger than those for mortality outcomes: for example Pr(κsubjective > κmortality). The main univariable analyses were repeated using the meta-meta-analytic approach used in previous analyses8,16 allowing for random effects both within and between meta-analyses.
Further univariable and multivariable analyses were conducted using two data subsets: (1) meta-analyses of trials with information on all three study design characteristics and (2) meta-analyses of trials with information on both allocation concealment and blinding. Subset 2 was used because many studies did not have a recorded bias judgement on sequence generation (see Table 11). We conducted univariable analyses on three composite dichotomous variables: risk of bias due to inadequate/unclear allocation concealment or lack of blinding (using subset 2), any risk of selection bias (inadequate or unclear sequence generation or allocation concealment) and any risk of bias (inadequate or unclear sequence generation or allocation concealment, or not double blind). Analyses of the second two composite variables used subset 1.
Multivariable analyses were based on an extension of ‘Model 3’ of Welton et al. 26 We assumed distinct variance components associated with each study design characteristic. In the main multivariable analyses, we assumed no interactions between the different characteristics. In an additional analysis on subset 2 we allowed for interactions between inadequate allocation concealment and lack of double blinding. Interaction terms were assumed to have the same hierarchical structure as the main effects, again with distinct variance components. The implied average bias in studies with both characteristics was estimated (on the log-odds scale) as the sum of the fitted coefficients representing the average effect of each of the two characteristics and the fitted coefficient representing the average interaction term. A 95% CrI for this sum, accounting for correlations between the three coefficients, was calculated using WinBUGS. These measures were exponentiated in order to express the implied average bias as ROR. For comparison, we also calculated the corresponding implied average bias for the model without interaction terms for subset 2. We repeated all univariable analyses on subsets 1 and 2 to allow comparisons with the results of multivariable analyses.
In additional analyses we estimated separate effects of ‘inadequate’ and ‘unclear’, for each study design characteristic, by fitting models in which these two categories had different average bias (compared with ‘adequate’ trials) and distinct variance components (κ and φ). It was necessary to exclude some of the contributing meta-epidemiological studies from these analyses because their original data coding had not separated unclear from inadequate (see Tables 9 and 11). We conducted separate analyses according to clinical area and type of intervention, and repeated analyses in meta-analyses that were derived from the subset of contributing studies not included in the study of Wood et al. 16 For meta-analyses comparing two active interventions it is not possible to estimate RORs quantifying average bias, or between-meta-analysis heterogeneity in average bias, because there is no clear direction in which bias operates. Instead, we estimated parameters from restricted models to estimate increases in between-trial (within-meta-analysis) heterogeneity in trials with, compared with those without, specified study design characteristics, among meta-analyses containing at least two trials with and without the characteristic of interest.
Welton et al. 26 showed how results from hierarchical bias models can be used to formulate a prior distribution for the bias associated with a study design characteristic in a new fixed-effect meta-analysis that is assumed to be statistically exchangeable with the meta-analyses used to estimate the model parameters. This approach was based on a normal approximation to the distribution of the observed intervention effect yi in each new trial i, which is assumed to have known sampling variance σi2. Assuming known b0, κ, φ and V0, a posteriori use of the empirically based prior distribution leads to results from trials with the reported characteristic being corrected for the estimated average bias across meta-analyses (estimated b0), and the variance of such results being increased from σi2 to (σi2 + κ2 + φ2 + V0). The minimum variance of the estimated intervention effect that can in theory be achieved by an infinitely large trial with the reported characteristic is therefore κ2 + φ2 + V0.
It is of interest to quantify the likely magnitude of increases in variance resulting from application of this bias adjustment to future trial results. To do so, for each trial in the BRANDO data set we calculated the observed log-odds ratio yi and the Woolf estimate of its sampling variance, σi2. We assumed that these represented a typical range of σi2s that may be observed in future trials. For each high/unclear-risk trial result in turn, we calculated the percentage increase in variance that would result from bias adjustment at the trial level, that is:
The calculations used the posterior median values of κ and φ. We summarised these percentage increases in variance by the median and interquartile range (IQR) across trials for each study design characteristic.
Formulae from Welton et al. 26 also allow us to calculate a bias-adjusted summary mean and variance of the intervention effect in a new fixed-effect meta-analysis. Using this approach, results from trials at low risk of bias are assigned the usual inverse variance (1/σi2) weight. For trials at high/unclear risk of bias, the bias-corrected estimated effect size is used, and downweighted according to a function of σi2, κ2, φ2 and V0 (interested readers are referred to page 123 of Welton et al. 26). The resulting bias-adjusted estimate of the summary effect size will therefore have a larger variance than the standard unadjusted meta-analytic summary. The magnitude of this increase in variance will depend on the number of trials and the variances of the intervention effect estimates in the new meta-analysis, and the number of trials classified as high/unclear risk of bias. We assumed that the meta-analyses in the BRANDO database are typical in terms of these characteristics, and calculated the percentage increase in variance of the summary log-odds ratio due to bias adjustment for each meta-analysis in turn. These were summarised by the median and IQR across meta-analyses for each study design characteristic.
Results
Table 8 shows the included meta-epidemiological studies, the sources of their collections of meta-analyses and the study design characteristics that they assessed. For five studies12–15,21 data from each trial report were extracted by two researchers independently; in the study by Pildal et al. 14 the assessors were also blinded to trial results. In the study by Schulz et al. ,11 one researcher, who was blinded to the trial outcome, assessed the reported methodological characteristics of included trials using a detailed classification scheme. The study of Egger et al. 9 was based on quality assessments by the authors of the included Cochrane reviews, which were generally carried out in duplicate by two observers.
| Contributing study | Source of systematic reviews/meta-analyses | Choice of meta-analyses | Study design characteristics examined | No. of meta-analyses (trials) |
|---|---|---|---|---|
| Als-Nielsen et al.12,22 | Randomly selected from The Cochrane Library, Issue 2, 2001 | Binary outcome and ≥ 5 full-paper trials of which at least one had adequate and one inadequate allocation concealment | Sequence generation, allocation concealment, blinding, intention-to-treat analysis, power calculation | 38 (401) |
| Balk et al.13 | From four clinical areas (cardiovascular disease, infectious disease, paediatrics, surgery) identified from previous research database, MEDLINE (1966–2000) and The Cochrane Library, Issue 4, 2000 | Binary outcome, ≥ 6 trials, significant between-study heterogeneity (OR scale) | 27 characteristics including allocation concealment, blinding, intention-to-treat analysis, power calculation, stopping rules, baseline comparability | 20 (229) |
| Contopoulos-Ioannidis et al.21 | Mental health-related interventions identified from the Mental Health Library, 2002 (Issue 1) | At least one large and one small trial | Trial size, method of randomisation, allocation concealment, blinding | 9 (66) |
| Egger et al.9 | Meta-analyses from the Cochrane Database of Systematic Reviews that had performed comprehensive literature searches | Outcome measure reported by the largest number of trials | Publication status, language of publication, publication in MEDLINE-indexed journals, allocation sequence generation (subset), allocation concealment, blinding | 79 (643) |
| Kjaergard et al.15 | In The Cochrane Library, MEDLINE or PubMed with at least one trial with ≥ 1000 patients | Outcome measure described as primary by the review authors or reported by the largest number of trials | Sequence generation, allocation concealment, blinding, description of dropouts and withdrawals | 6 (59) |
| Pildal et al.14 | Random sample of 38 reviews from The Cochrane Library, Issue 2, 2003, and 32 other reviews from PubMed accessed in 2002 | Binary outcome from a meta-analysis presented as the first statistically significant result that supported a conclusion in favour of one of the interventions | Language of publication, sequence generation, allocation concealment, blinding | 56 (370) |
| Schulz et al.11 | Cochrane Pregnancy and Childbirth Group, ≥ 5 trials containing ≥ 25 events in the control group, at least one trial with and without adequate allocation concealment | The most homogeneous group of interventions | Allocation sequence generation, allocation concealment, blinding, reporting of exclusions | 26 (205) |
Table 9 shows the definitions of adequate sequence generation, allocation concealment and blinding used in the seven studies. Definitions of adequate sequence generation and adequate allocation concealment were similar in all seven studies and were based on the definitions originally proposed by Schulz et al. 11 Sequence generation was assessed as adequate, unclear or inadequate in five studies. The study by Kjaergard et al. 15 provided only dichotomised assessments of adequate compared with inadequate or unclear sequence generation, and Balk et al. 13 did not assess adequacy of sequence generation. Allocation concealment was assessed as adequate, unclear or inadequate in all seven studies. Definitions of ‘double blind’ varied between studies and were somewhat stricter in the studies by Schulz et al. 11 and Pildal et al. 14 Trials were categorised as double blind, unclear or not double blind in three studies,9,12,14 with the remaining four11,13,15,21 categorising trials as either double blind or unclear/not double blind.
| Study | Sequence generation | Allocation concealment | Blinding | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Adequate | Unclear | Inadequate | Adequate | Unclear | Inadequate | Double blind | Unclear | Not double blind | |
| Als-Nielsen et al.12,22 | Computer-generated, random number tables, flip of coin, drawing cards or lots, comparable stochastic method | ND | Quasi-randomised (dates, alternation or similar) | Central randomisation (incl. pharmacy controlled), coded identical drug boxes, sealed envelopes, on-site locked computer system or comparable | ND | Open allocation sequence | Described as double blind or at least two key groups (patient/doctor/assessor/analyst) blinded | ND | Single blind or not blinded |
| Balk et al.13 | Did not assess adequacy of sequence generation | Central randomisation; blinded code, coded drug containers; drugs prepared by pharmacy; serially numbered, opaque, sealed envelopes | ND | Random tables, cards, methods using year of birth or registration numbers | Patients and either caregivers or outcome assessors blinded | NA | Any other descriptions not classified as adequate or unclear | ||
| Contopoulos-Ioannidis et al.21 | Computer-generated, random number tables, coin or dice toss, other methods ensuring random order | ND or unclear | Alternation, case records, dates or similar non-random method | Central facility, central pharmacy, with sealed and opaque envelopes | ND or unclear | Any other methods that could not be classified as adequate | Described as double blind, or patients and either outcome assessor or caregiver blinded | NA | Not blinded, single blind, blinding not feasible, ND unclear |
| bEgger et al.9 | Computer-generated, random number tables or other methods that ensure random order | ND | Alternation, case record numbers, date of birth, etc. | Central randomisation; numbered or coded bottles or containers; drugs prepared by pharmacy; serially numbered, opaque, sealed envelopes; other convincing description implying concealment | ND | Alternation, open random number tables, etc. | Described as double blind | ND | Described as open or similar |
| Kjaergard et al.15 | Computer-generated or similar | NA | ND or non-random | Central independent unit, sealed envelopes or similar | NA | ND, open random number table or similar | Described as double blind and used identical placebo or similar | NA | Open (not blind), or described as single blind |
| Pildal et al.14 | Computer-generated sequence, random number tables, drawing lots/envelopes, coin toss | ND or unclear | Alternation, case record numbers, date of birth, etc. | Central randomisation; coded drug containers; drugs prepared by central pharmacy, serially numbered, opaque, sealed envelopes; other convincing description implying concealment | ND or approach not falling into other categories | Obvious which treatment the next patient would be allocated (alternation, case record numbers, dates of birth, etc.) | Described as double blind or patients and caregivers reported as blinded, placebo controlled without indication that treatments distinguishable or investigators unblindeda | ND or unclear | Not blinded, single blind, did not fit the definition of double blinda |
| Schulz et al.11 | Computer random number generator, random number tables, coin tossing, shuffling, other random process, minimisation | ND | Non-random | Central randomisation; numbered or coded bottles or containers; drugs prepared by pharmacy; serially numbered, opaque, sealed envelopes; other convincing description implying concealment | ND or approach not falling into other categories | Alternation or allocation by case record number or date of birth | Participants, caregivers and outcome assessors all described as blinded | NA | Descriptions not consistent with definition of double blind, blinding not feasible, ND or unclear |
Table 10 shows characteristics of the 234 meta-analyses and 1973 trials included in the database analysed. The median year of publication was 2000 for meta-analyses and 1989 for trials, whereas the median sample size was 1264 for meta-analyses and 112 for trials. A total of 57 meta-analyses (24.4%) were concerned with conditions related to pregnancy and childbirth, followed by circulatory system conditions (31, 13.3%) and mental health (26, 11.1%). The majority of experimental interventions were pharmacological (162 meta-analyses, 69.2%), whereas placebo or no treatment was the most common comparison intervention (172, 73.5%). A total of 98 meta-analyses (41.9%) analysed a subjectively assessed outcome, followed by all-cause mortality (44, 18.8%), outcomes that are objectively measured, but potentially influenced by patient/clinician judgement (42, 18.0%) and other objectively assessed outcomes (36, 15.4%); 14 meta-analyses (6.0%) contained trials with both objective and subjective outcome measures (e.g. validated and self-reported smoking cessation).
| Characteristics of meta-analyses and trials | Meta-analyses (n = 234) | Trials (n = 1973) | ||
|---|---|---|---|---|
| n | % | n | % | |
| Contributing meta-epidemiological study | ||||
| Als-Nielsen et al.12,22 | 38 | 16.2 | 401 | 20.3 |
| Balk et al.13 | 20 | 8.6 | 229 | 11.6 |
| Contopoulos-Ioannidis et al.21 | 9 | 3.9 | 66 | 3.4 |
| Egger et al.9 | 79 | 33.8 | 643 | 32.6 |
| Kjaergard et al.15 | 6 | 2.6 | 59 | 3.0 |
| Pildal et al.14 | 56 | 23.9 | 370 | 18.8 |
| Schulz et al.11 | 26 | 11.1 | 205 | 10.4 |
| Clinical area according to ICD-10 chapters30 | ||||
| Pregnancy and childbirth (chapter XV, blocks O) | 57 | 24.4 | 447 | 22.7 |
| Mental and behavioural (chapter V, F) | 26 | 11.1 | 302 | 15.3 |
| Circulatory system (chapter IX, I) | 31 | 13.3 | 277 | 14.0 |
| Digestive system (chapter XI, K) | 17 | 7.3 | 152 | 7.7 |
| Other factorsa (chapter XXI, Z) | 18 | 7.7 | 128 | 6.5 |
| Respiratory system (chapter X, J) | 14 | 6.0 | 125 | 6.3 |
| Other ICD-10 chapters | 70 | 29.9 | 539 | 27.3 |
| Unclassified | 1 | 0.4 | 3 | 0.2 |
| Type of experimental intervention | ||||
| Pharmacological | 162 | 69.2 | 1418 | 71.9 |
| Surgical | 14 | 6.0 | 122 | 6.2 |
| Psychosocial/behavioural/educational | 13 | 5.6 | 121 | 6.1 |
| Other | 45 | 19.2 | 312 | 15.8 |
| Type of comparison intervention | ||||
| Placebo or no treatment | 172 | 73.5 | 1438 | 72.9 |
| Other inactive (‘standard care’) | 16 | 6.8 | 152 | 7.7 |
| Active comparison | 44 | 18.8 | 368 | 18.7 |
| Mixture of active and inactive within meta-analysis | 2 | 0.9 | 15 | 0.8 |
| Type of outcome measure | ||||
| All-cause mortality | 44 | 18.8 | 364 | 18.5 |
| Other objective | 36 | 15.4 | 213 | 10.8 |
| Objectively measured but influenced by judgement | 42 | 18.0 | 407 | 20.6 |
| Subjective | 98 | 41.9 | 809 | 41 |
| Mixture of objective and subjective | 14 | 6.0 | 180 | 9.1 |
| Year of publication of reviewb/trial | ||||
| Median (range) | 2000 (1983–2005) | 1989 (1948–2002) | ||
| IQR | 2000 to 2001 | 1983 to 1994 | ||
| Sample size of meta-analysis/trial | ||||
| Median (range) | 1264 (72–176,733) | 112 (2–82,892) | ||
| IQR | 533 to 2582 | 58 to 267 | ||
Table 11 summarises the characteristics of trials included in analyses. Information on sequence generation was available for 1207 (61.2%) trials included in 186 meta-analyses, of which 112 meta-analyses containing 944 trials were informative. Sequence generation was assessed as unclear in 769 (63.7%) of these trials, although 306 (25.4%) were assessed as having adequate sequence generation. Percentages were similar for trials included in informative meta-analyses.
| Study design characteristic | No. (%) of meta-analyses with information (n = 234) | No. (%) of trials with information (n = 1973) | No. (%) of informative meta-analyses with information | No. (%) of trials with information included in informative meta-analyses |
|---|---|---|---|---|
| Adequate sequence generation | 186 (79.5) | 1207 (61.2) | 112 (47.9) | 944 (47.8) |
| Yes | 306 (25.4) | 248 (26.3) | ||
| Unclear | 769 (63.7) | 598 (63.3) | ||
| No | 101 (8.4) | 67 (7.1) | ||
| No/uncleara | 31 (2.6) | 31 (3.3) | ||
| Adequate allocation concealment | 228 (97.4) | 1796 (91.0) | 146 (62.4) | 1292 (65.5) |
| Yes | 416 (23.2) | 376 (29.1) | ||
| Unclear | 1244 (69.3) | 828 (64.1) | ||
| No | 136 (7.6) | 88 (6.8) | ||
| Double blind | 234 (100.0) | 1970 (99.8) | 104 (44.4) | 1057 (53.6) |
| Yes | 929 (47.2) | 590 (55.8) | ||
| Unclear | 109 (5.5) | 63 (6.0) | ||
| No | 683 (34.7) | 249 (23.6) | ||
| No/uncleara | 249 (12.6) | 155 (14.7) | ||
| Information on both allocation concealment and blinding | 228 (97.4) | 1793 (90.9) | ||
| Information on all three characteristics | 175 (74.8) | 1171 (59.4) |
Information on allocation concealment was available for most meta-analyses (228, 97.4%) and trials (1796, 91.0%), of which 146 meta-analyses containing 1292 trials were informative. In 1244 (69.3%) trials, allocation concealment was assessed as unclear; 416 (23.2%) trials reported sufficient information to be classed as having adequate allocation concealment. The percentage of trials assessed as having adequate allocation concealment was somewhat higher (29.1%) among those included in informative meta-analyses.
Information on double blinding was available for all except three trials. However, only 104 meta-analyses (1057 trials) were informative; 77 meta-analyses contained no trials that were double blind, whereas 53 contained only double-blind trials. A total of 929 (47.2%) trials were classified as double blind, compared with 590 (55.8%) trials in informative meta-analyses.
Information on both allocation concealment and blinding was available in 1793 (90.9%) trials contained in 228 (97.4%) meta-analyses, although information on all three study design characteristics was available in 1171 (59.4%) trials contained in 175 (74.8%) meta-analyses.
Table 12 shows associations between the reported study design characteristics, for all trials combined and separately according to the nature of the outcome measure. Trials reporting adequate sequence generation were more likely to report adequate allocation concealment [OR 3.01, 95% confidence interval (CI) 2.20 to 4.12], but there was little association between adequate sequence generation and double blinding (OR 1.03, 95% CI 0.78 to 1.35). However, adequately concealed trials were more likely to be double blind (OR 3.14, 95% CI 2.49 to 3.96).
| Study characteristic 1 | Study characteristic 2 | No. (%) of trials | |||
|---|---|---|---|---|---|
| All trials | Mortality outcome | Objective outcome | Subjective outcome | ||
| Sequence generation | Allocation concealment | 1171 | 157 | 368 | 646 |
| Adequate | Adequate | 91 (7.8) | 16 (10.2) | 32 (8.7) | 43 (6.7) |
| Adequate | Inadequate/unclear | 182 (15.5) | 25 (15.9) | 64 (17.4) | 93 (14.4) |
| Inadequate/unclear | Adequate | 128 (10.9) | 15 (9.6) | 45 (12.2) | 68 (10.5) |
| Inadequate/unclear | Inadequate/unclear | 770 (65.8) | 101 (64.3) | 227 (61.7) | 442 (68.4) |
| OR (95% CI) | 3.01 (2.20 to 4.12) | 4.31 (1.88 to 9.88) | 2.52 (1.48 to 4.29) | 3.01 (1.93 to 4.68) | |
| Sequence generation | Blinding | 1171 | 157 | 368 | 646 |
| Adequate | Double blind | 127 (10.8) | 21 (13.4) | 44 (12.0) | 62 (9.6) |
| Adequate | Not double blind/unclear | 146 (12.5) | 20 (12.7) | 52 (14.1) | 74 (11.5) |
| Inadequate/unclear | Double blind | 412 (35.2) | 53 (33.8) | 127 (34.5) | 232 (35.9) |
| Inadequate/unclear | Not double blind/unclear | 486 (41.5) | 63 (40.1) | 145 (39.4) | 278 (43.0) |
| OR (95% CI) | 1.03 (0.78 to 1.35) | 1.25 (0.61 to 2.55) | 0.97 (0.61 to 1.54) | 1.00 (0.69 to 1.47) | |
| Allocation concealment | Blinding | 1793 | 328 | 550 | 915 |
| Adequate | Double blind | 283 (15.8) | 65 (19.8) | 93 (16.9) | 125 (13.7) |
| Adequate | Not double blind/unclear | 133 (7.4) | 30 (9.1) | 45 (8.2) | 58 (6.3) |
| Inadequate/unclear | Double blind | 556 (31.0) | 108 (32.9) | 159 (28.9) | 289 (31.6) |
| Inadequate/unclear | Not double blind/unclear | 821 (45.8) | 125 (38.1) | 253 (46.0) | 443 (48.4) |
| OR (95% CI) | 3.14 (2.49 to 3.96) | 2.51 (1.52 to 4.15) | 3.29 (2.19 to 4.94) | 3.30 (2.34 to 4.66) | |
Associations between reported study design characteristics were similar for the different types of outcome measure. Table 13 shows the number of trials assessed with each of the eight possible combinations of the study design characteristics, overall and according to the type of outcome measure. Only 60 (5.1%) trials were assessed as at low risk of bias for all three characteristics, whereas 453 (38.7%) were assessed as at high risk of bias for all three characteristics.
| Study design characteristics | Number of trials | |||||
|---|---|---|---|---|---|---|
| Sequence generation | Allocation concealment | Blinding | All | Mortality | Objective | Subjective |
| Adequate | Adequate | Double blind | 60 | 12 | 22 | 26 |
| Adequate | Adequate | Not double blinda | 31 | 4 | 10 | 17 |
| Adequate | Inadequatea | Double blind | 67 | 9 | 22 | 36 |
| Adequate | Inadequatea | Not double blinda | 115 | 16 | 42 | 57 |
| Inadequatea | Adequate | Double blind | 95 | 9 | 35 | 51 |
| Inadequatea | Adequate | Not double blinda | 33 | 6 | 10 | 17 |
| Inadequatea | Inadequatea | Double blind | 317 | 44 | 92 | 181 |
| Inadequatea | Inadequatea | Not double blinda | 453 | 57 | 135 | 261 |
| Total | 1171 | 157 | 368 | 646 | ||
Influence of reported study design characteristics on intervention effect estimates: univariable analyses of individual characteristics
Figure 3 and Table 14 present results from univariable analyses of the influence of reported study design characteristics on intervention effect estimates, both overall and separately according to type of outcome measure. Compared with Figure 3, Table 14 additionally includes 95% CrIs for the variance parameters κ and φ and displays the numbers of trials, and trials at high risk of bias, included in analyses. Overall, intervention effect estimates were exaggerated by an average of 11% in trials with inadequate or unclear sequence generation (ROR 0.89, 95% CrI 0.82 to 0.96), and between-trial heterogeneity was higher among such trials (κ = 0.16, 95% CrI 0.03 to 0.27). When analyses were stratified according to the type of outcome measure, the average effect of inadequate or unclear sequence generation appeared greatest for subjective outcomes [ROR 0.83, 95% CrI 0.74 to 0.94, posterior probability (PPr) that RORsubjective < RORmortality = 0.73], and the increase in between-trial heterogeneity was also greatest for such outcomes (κ = 0.20, CrI 0.03 to 0.32, PPr that κsubjective > κmortality = 0.78). In contrast, there was little evidence that inadequate or unclear sequence generation was associated with exaggeration of intervention effects for all-cause mortality (ROR 0.89, 95% CrI 0.75 to 1.05) or for other objective outcomes (ROR 0.99, 95% CrI 0.84 to 1.16). For all types of outcome measure there was only limited between-meta-analysis heterogeneity in mean bias (estimated φ between 0.04 and 0.07).
| Study design characteristic and outcome | No. of meta-analyses | No. of trials | Contributing meta-analysesa | Contributing trials | No. (%) of trials at high risk of biasb | ROR | 95% CrI | κ | 95% CrI | φ | 95% CrI | V0 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inadequate or unclear sequence generation (vs adequate) | ||||||||||||
| All | 186 | 1207 | 112 (57) | 944 | 696 (73.7) | 0.89 | 0.82 to 0.96 | 0.16 | 0.03 to 0.27 | 0.04 | 0.01 to 0.14 | 0.002 |
| Mortality | 30 | 163 | 16 (12) | 129 | 92 (71.3) | 0.89 | 0.75 to 1.05 | 0.10 | 0.01 to 0.29 | 0.06 | 0.01 to 0.25 | 0.008 |
| Objective | 66 | 387 | 47 (19) | 328 | 234 (71.3) | 0.99 | 0.84 to 1.16 | 0.09 | 0.01 to 0.34 | 0.07 | 0.01 to 0.25 | 0.007 |
| Subjective | 90 | 657 | 49 (26) | 487 | 370 (76.0) | 0.83 | 0.74 to 0.94 | 0.20 | 0.03 to 0.32 | 0.06 | 0.01 to 0.22 | 0.004 |
| Inadequate or unclear allocation concealment (vs adequate) | ||||||||||||
| All | 228 | 1796 | 146 (80) | 1292 | 916 (70.9) | 0.93 | 0.87 to 0.99 | 0.12 | 0.02 to 0.23 | 0.04 | 0.01 to 0.13 | 0.001 |
| Mortality | 44 | 328 | 32 (15) | 268 | 183 (68.3) | 0.98 | 0.88 to 1.10 | 0.08 | 0.01 to 0.21 | 0.05 | 0.01 to 0.18 | 0.003 |
| Objective | 76 | 551 | 45 (21) | 372 | 253 (68.0) | 0.97 | 0.85 to 1.10 | 0.06 | 0.01 to 0.26 | 0.05 | 0.01 to 0.24 | 0.004 |
| Subjective | 108 | 917 | 69 (44) | 652 | 480 (73.6) | 0.85 | 0.75 to 0.95 | 0.20 | 0.02 to 0.33 | 0.09 | 0.01 to 0.29 | 0.003 |
| Lack of double blinding or unclear double blinding (vs double blind) | ||||||||||||
| All | 234 | 1970 | 104 (66) | 1057 | 467 (44.2) | 0.87 | 0.79 to 0.96 | 0.14 | 0.02 to 0.30 | 0.14 | 0.03 to 0.28 | 0.002 |
| Mortality | 44 | 364 | 25 (14) | 245 | 109 (44.5) | 0.92 | 0.80 to 1.04 | 0.06 | 0.01 to 0.20 | 0.06 | 0.01 to 0.22 | 0.004 |
| Objective | 78 | 619 | 28 (17) | 282 | 120 (42.6) | 0.93 | 0.74 to 1.18 | 0.08 | 0.01 to 0.38 | 0.13 | 0.01 to 0.50 | 0.014 |
| Subjective | 112 | 987 | 51 (35) | 530 | 238 (44.9) | 0.78 | 0.65 to 0.92 | 0.37 | 0.19 to 0.53 | 0.23 | 0.04 to 0.44 | 0.008 |
FIGURE 3.
Estimated RORs and effects on heterogeneity associated with reported study design characteristics, according to the type of outcome measure: univariable analyses based on all available data. Random sequence generation: 944 trials from 112 informative meta-analyses; allocation concealment: 1292 trials from 146 informative meta-analyses; blinding: 1057 trials from 104 informative meta-analyses.
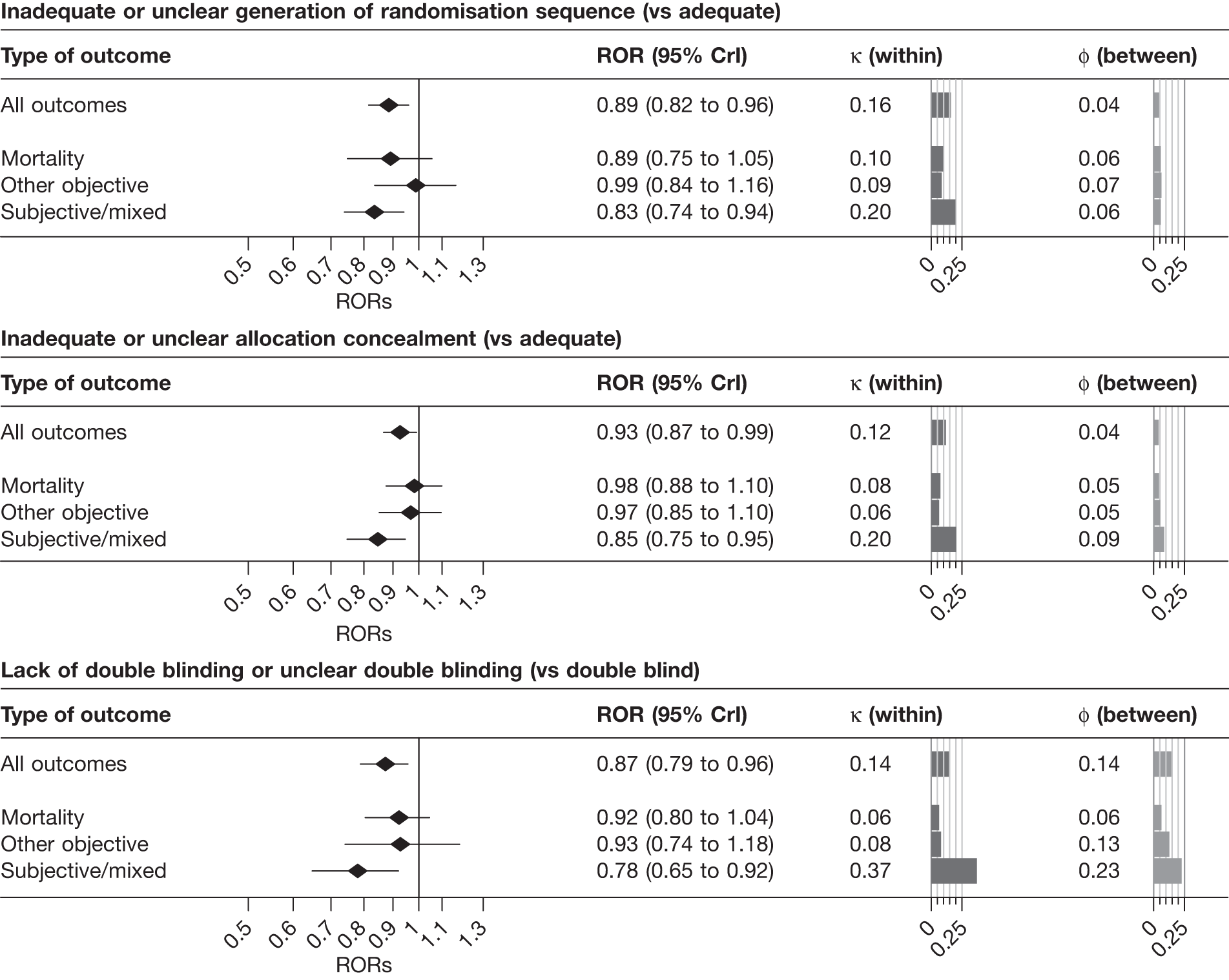
Overall, intervention effect estimates were exaggerated by 7% in trials with inadequate or unclear allocation concealment (ROR 0.93, 95% CrI 0.87 to 0.99), and there was evidence that between-trial heterogeneity was increased for such studies (κ = 0.12, 95% CrI 0.02 to 0.23). The influence of inadequate or unclear allocation concealment appeared greatest among meta-analyses with a subjectively assessed outcome measure (ROR 0.85, 95% CrI 0.75 to 0.95, PPr that RORsubjective < RORmortality = 0.97; κ = 0.20, 95% CrI 0.02 to 0.33, PPr that κsubjective > κmortality = 0.85). In contrast, the average effect of inadequate or unclear allocation concealment was close to the null for meta-analyses with mortality (ROR 0.98, 95% CrI 0.88 to 1.10) and other objective outcomes (ROR 0.97, 95% CrI, 0.85 to 1.10). Estimates of both between-trial and between-meta-analyses heterogeneity in bias were lower for such outcomes than for subjectively assessed outcomes.
Lack of, or unclear, double blinding was associated with an average 13% exaggeration of intervention effects (ROR 0.87, 95% CrI 0.79 to 0.96). There was evidence that between-trial heterogeneity was increased for such studies (κ = 0.14, 95% CrI 0.02 to 0.30), and that average bias varied between meta-analyses (φ = 0.14, 95% CrI 0.03 to 0.28). Average bias (ROR 0.78, 95% CrI 0.65 to 0.92), increased between-trial heterogeneity (κ = 0.37, 95% CrI 0.19 to 0.53) and between-meta-analysis heterogeneity in average bias (φ = 0.23, 95% CrI 0.04 to 0.44) all appeared greatest for meta-analyses assessing subjective outcomes (PPr RORsubjective < RORmortality = 0.94, PPr κsubjective > κmortality = 0.99, PPr φsubjective > φmortality = 0.90). Among meta-analyses with subjectively assessed outcomes, the influence of lack of blinding appeared greater than the influence of inadequate or unclear sequence generation or allocation concealment.
Results from univariable analyses of the influence of inadequate or unclear allocation concealment and lack of double blinding restricted to the 228 meta-analyses (1793 trials) that contained information on both of these characteristics were similar (Table 15) to those presented in Figure 3 and Table 14. We also repeated the univariable analyses in a data set restricted to 175 meta-analyses (1171 trials) that had information on all three study design characteristics (sequence generation, allocation concealment and blinding). The results were similar (Table 16), although the influence of inadequate allocation concealment appeared somewhat greater in analyses restricted to the 88 informative meta-analyses (811 trials) that had information on all three characteristics.
| Study design characteristic and outcome | No. of meta-analyses | No. of trials | Contributing meta-analysesa | Contributing trials | No. (%) of trials at high risk of biasb | ROR | 95% CrI | κ | 95% CrI | φ | 95% CrI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Inadequate or unclear allocation concealment (vs adequate) | |||||||||||
| All | 228 | 1793 | 146 (80) | 1291 | 915 (70.9) | 0.93 | 0.87 to 0.99 | 0.11 | 0.02 to 0.22 | 0.04 | 0.01 to 0.13 |
| Mortality | 44 | 328 | 32 (15) | 268 | 183 (68.3) | 0.98 | 0.88 to 1.10 | 0.08 | 0.01 to 0.21 | 0.05 | 0.01 to 0.18 |
| Objective | 76 | 550 | 45 (21) | 372 | 253 (68.0) | 0.97 | 0.85 to 1.10 | 0.06 | 0.01 to 0.26 | 0.05 | 0.01 to 0.21 |
| Subjective | 108 | 915 | 69 (44) | 651 | 479 (73.6) | 0.84 | 0.75 to 0.94 | 0.20 | 0.03 to 0.33 | 0.09 | 0.01 to 0.30 |
| Lack of double blinding or unclear double blinding (vs double blind) | |||||||||||
| All | 228 | 1793 | 101 (62) | 977 | 432 (44.2) | 0.87 | 0.78 to 0.96 | 0.13 | 0.02 to 0.29 | 0.13 | 0.02 to 0.26 |
| Mortality | 44 | 328 | 25 (13) | 215 | 99 (46.0) | 0.92 | 0.80 to 1.05 | 0.06 | 0.01 to 0.20 | 0.07 | 0.01 to 0.24 |
| Objective | 76 | 550 | 27 (17) | 259 | 108 (41.7) | 0.88 | 0.71 to 1.11 | 0.07 | 0.01 to 0.38 | 0.13 | 0.01 to 0.49 |
| Subjective | 108 | 915 | 49 (32) | 503 | 225 (44.7) | 0.79 | 0.65 to 0.93 | 0.33 | 0.08 to 0.49 | 0.21 | 0.04 to 0.41 |
| Inadequate or unclear allocation concealment or not double blind (vs adequate allocation concealment and double blind) | |||||||||||
| All | 228 | 1793 | 104 (55) | 990 | 731 (73.8) | 0.88 | 0.81 to 0.95 | 0.12 | 0.02 to 0.22 | 0.05 | 0.01 to 0.14 |
| Mortality | 44 | 328 | 25 (13) | 220 | 157 (71.4) | 0.95 | 0.84 to 1.06 | 0.08 | 0.01 to 0.22 | 0.05 | 0.01 to 0.19 |
| Objective | 76 | 550 | 30 (16) | 268 | 191 (71.3) | 0.84 | 0.69 to 1.00 | 0.07 | 0.01 to 0.33 | 0.07 | 0.01 to 0.33 |
| Subjective | 108 | 915 | 49 (26) | 502 | 383 (76.3) | 0.83 | 0.73 to 0.93 | 0.17 | 0.02 to 0.31 | 0.06 | 0.01 to 0.23 |
| Study design characteristic and outcome | No. of meta-analyses | No. of trials | Contributing meta-analysesa | Contributing trials | No. (%) of trials at high risk of biasb | ROR | 95% CrI | κ | 95% CrI | φ | 95% CrI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Inadequate or unclear sequence generation (vs adequate) | |||||||||||
| All | 175 | 1171 | 104 (54) | 911 | 676 (74.2) | 0.88 | 0.81 to 0.96 | 0.17 | 0.03 to 0.27 | 0.04 | 0.01 to 0.15 |
| Mortality | 27 | 157 | 15 (11) | 122 | 88 (72.1) | 0.88 | 0.73 to 1.05 | 0.11 | 0.01 to 0.32 | 0.06 | 0.01 to 0.26 |
| Objective | 62 | 368 | 42 (18) | 310 | 223 (71.9) | 0.99 | 0.84 to 1.18 | 0.08 | 0.01 to 0.32 | 0.07 | 0.01 to 0.27 |
| Subjective | 86 | 646 | 47 (25) | 479 | 365 (76.2) | 0.83 | 0.73 to 0.93 | 0.20 | 0.03 to 0.32 | 0.06 | 0.01 to 0.22 |
| Inadequate or unclear allocation concealment (vs adequate) | |||||||||||
| All | 175 | 1171 | 88 (45) | 811 | 605 (74.6) | 0.88 | 0.80 to 0.96 | 0.13 | 0.02 to 0.26 | 0.05 | 0.01 to 0.18 |
| Mortality | 27 | 157 | 15 (5) | 118 | 89 (75.4) | 0.97 | 0.80 to 1.18 | 0.09 | 0.01 to 0.31 | 0.07 | 0.01 to 0.32 |
| Objective | 62 | 368 | 31 (15) | 257 | 180 (70.0) | 0.93 | 0.80 to 1.09 | 0.06 | 0.01 to 0.24 | 0.06 | 0.01 to 0.26 |
| Subjective | 86 | 646 | 42 (25) | 436 | 336 (77.1) | 0.79 | 0.67 to 0.90 | 0.22 | 0.03 to 0.36 | 0.10 | 0.01 to 0.36 |
| Lack of double blinding or unclear double blinding (vs double blind) | |||||||||||
| All | 175 | 1171 | 60 (36) | 592 | 251 (42.4) | 0.83 | 0.72 to 0.95 | 0.26 | 0.05 to 0.42 | 0.16 | 0.03 to 0.33 |
| Mortality | 27 | 157 | 9 (3) | 74 | 29 (39.2) | 1.06 | 0.82 to 1.44 | 0.11 | 0.01 to 0.47 | 0.08 | 0.01 to 0.41 |
| Objective | 62 | 368 | 17 (9) | 165 | 73 (44.2) | 0.89 | 0.64 to 1.26 | 0.10 | 0.01 to 0.49 | 0.22 | 0.02 to 0.79 |
| Subjective | 86 | 646 | 34 (24) | 353 | 149 (42.2) | 0.73 | 0.57 to 0.89 | 0.33 | 0.09 to 0.50 | 0.22 | 0.05 to 0.42 |
| Inadequate or unclear sequence generation or allocation concealment (vs adequate sequence generation and allocation concealment) | |||||||||||
| All | 175 | 1171 | 53 (22) | 534 | 445 (83.3) | 0.89 | 0.78 to 1.00 | 0.12 | 0.02 to 0.27 | 0.06 | 0.01 to 0.22 |
| Mortality | 27 | 157 | 10 (4) | 79 | 65 (82.3) | 0.94 | 0.74 to 1.15 | 0.08 | 0.01 to 0.29 | 0.08 | 0.01 to 0.38 |
| Objective | 62 | 368 | 19 (10) | 176 | 144 (81.8) | 0.82 | 0.57 to 1.10 | 0.15 | 0.01 to 0.61 | 0.20 | 0.02 to 0.68 |
| Subjective | 86 | 646 | 24 (8) | 279 | 236 (84.6) | 0.85 | 0.70 to 1.01 | 0.15 | 0.01 to 0.32 | 0.08 | 0.01 to 0.34 |
| Inadequate or unclear sequence generation or allocation concealment or not double blind (vs adequate sequence generation and allocation concealment and double blind) | |||||||||||
| All | 175 | 1171 | 37 (14) | 409 | 351 (85.8) | 0.79 | 0.64 to 0.92 | 0.12 | 0.01 to 0.28 | 0.12 | 0.02 to 0.40 |
| Mortality | 27 | 157 | 7 (3) | 65 | 55 (84.6) | 0.94 | 0.72 to 1.19 | 0.09 | 0.01 to 0.32 | 0.08 | 0.01 to 0.38 |
| Objective | 62 | 368 | 14 (6) | 139 | 117 (84.2) | 0.63 | 0.42 to 0.98 | 0.24 | 0.02 to 0.77 | 0.23 | 0.02 to 0.82 |
| Subjective | 86 | 646 | 16 (5) | 205 | 179 (87.3) | 0.71 | 0.52 to 0.89 | 0.12 | 0.01 to 0.31 | 0.16 | 0.02 to 0.55 |
Influence of reported study design characteristics: univariable analyses of combinations of characteristics
We conducted further univariable analyses using variables defined based on combinations of the study design characteristics. Figure 4 and Table 16 show the estimated effects of any risk of selection bias (inadequate or unclear sequence generation or allocation concealment, compared with other trials) on intervention effects and heterogeneity, based on 53 informative meta-analyses containing 534 trials, of which 89 (17%) were assessed as being at low risk of selection bias. Risk of selection bias was associated with an average 11% exaggeration of intervention effect estimates (ROR 0.89, 95% CrI 0.78 to 1.00) and with increased between-trial heterogeneity (κ = 0.12, 95% CrI 0.02 to 0.27). Average effects did not differ substantially according to type of outcome measure. Between-meta-analysis heterogeneity was highest for objective outcomes (φ = 0.20, 95% CrI 0.02 to 0.68).
FIGURE 4.
Estimated RORs and effects on heterogeneity associated with combinations of study design characteristics: univariable analyses according to type of outcome measure. Analyses based on subsets of trials with assessments of all three study design characteristics (upper and middle panels), and of allocation concealment and blinding (lower panel). Upper panel: inadequate or unclear sequence generation or allocation concealment (any risk of selection bias); middle panel: inadequate or unclear sequence generation or allocation concealment or lack of or unclear double blinding (any risk of bias); lower panel: inadequate or unclear allocation concealment or lack of or unclear double blinding.
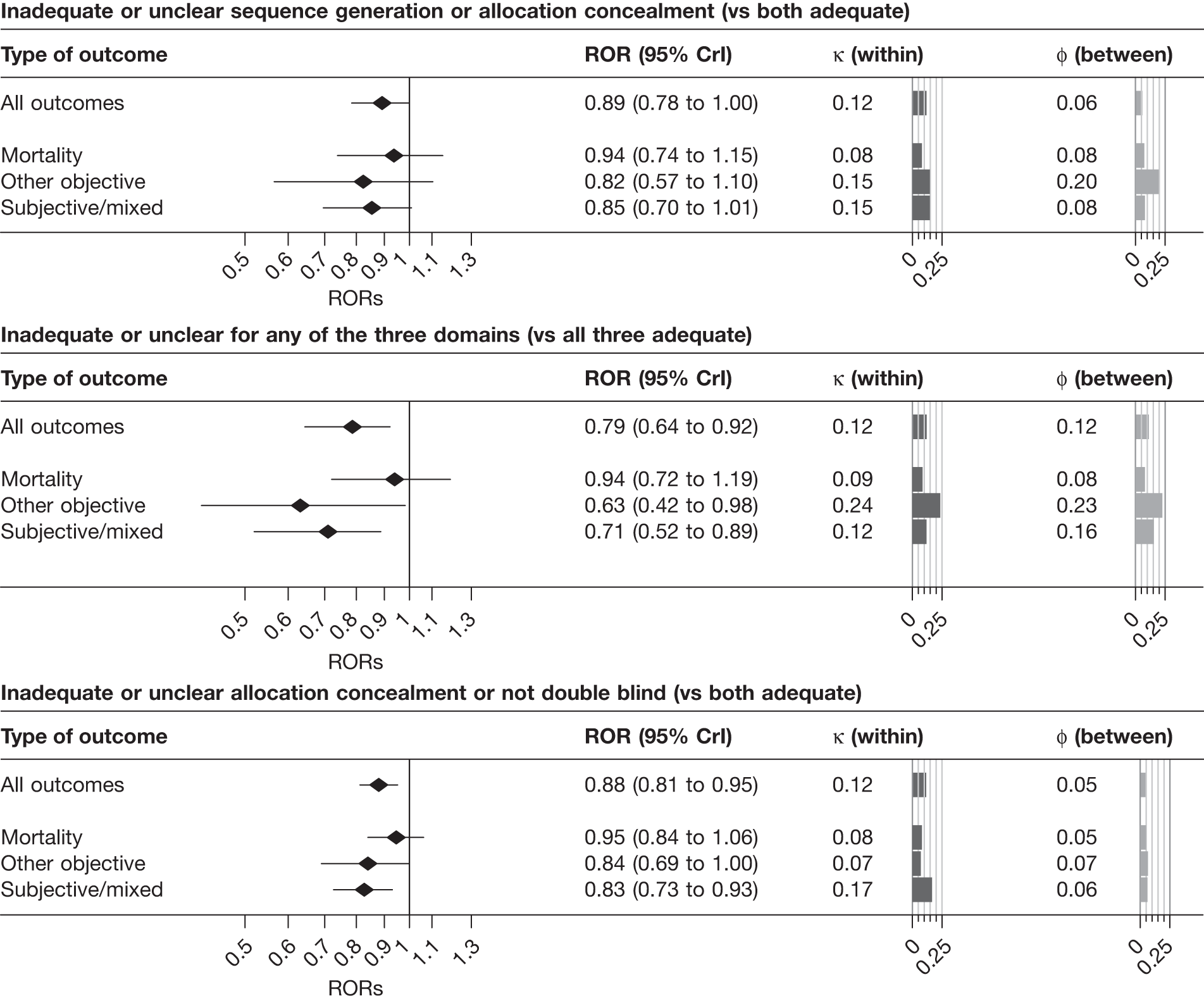
Only 37 informative meta-analyses [409 trials, of which 58 (14%) were assessed as being at low risk of bias] contributed to the analysis of any risk of bias (inadequate or unclear sequence generation or allocation concealment, or lack of or unclear double blinding, compared with all other trials); Figure 4 and Table 16 show estimated effects of any risk of bias from one of the three characteristics (compared with low risk of bias from all three). Any risk of bias was associated with an average 21% exaggeration of intervention effect estimates (ROR 0.79, 95% CrI 0.64 to 0.92). The numbers of informative meta-analyses and trials included in analyses stratified by type of outcome measure were small, but the influence of any risk of bias appeared smallest for all-cause mortality outcomes.
A total of 104 informative meta-analyses [990 trials, of which 259 (26%) were assessed as at low risk of bias] contributed to analyses of the influence of inadequate or unclear allocation concealment or lack of double blinding (compared with adequate allocation concealment and presence of double blinding). Figure 4 and Table 15 show that intervention effects from trials at high risk of bias according to this variable were exaggerated by an average of 12% (ROR 0.88, 95% CrI 0.81 to 0.95). The ROR was closer to 1 (0.95) for all-cause mortality outcomes than for other objective or subjective outcomes (0.84, 95% CrI 0.69 to 1.00 and 0.83, 95% CrI 0.73 to 0.93 respectively). The increase in between-trial heterogeneity appeared greatest for subjective outcomes (κ = 0.17, 95% CrI 0.02 to 0.31).
Univariable analyses: comparison with meta-meta-analytic approach
Table 17 displays results of univariable analyses, based on all available data, conducted using the meta-meta-analytic approach8 employed in previous work. 16 Using this approach, we estimated the ROR and the between-meta-analysis standard deviation φ associated with each study design characteristic. Increases in between-trial variability are not estimated using this approach.
| Study design characteristic and outcome | No. of trials | ROR (95% CI) | φ |
|---|---|---|---|
| Inadequate or unclear sequence generation (vs adequate) | |||
| All | 944 | 0.94 (0.89 to 1.00) | 0.00 |
| Mortality | 129 | 0.91 (0.78 to 1.05) | 0.00 |
| Objective | 328 | 1.01 (0.89 to 1.15) | 0.00 |
| Subjective | 487 | 0.87 (0.79 to 0.96) | 0.08 |
| Inadequate or unclear allocation concealment (vs adequate) | |||
| All | 1292 | 0.93 (0.88 to 0.99) | 0.08 |
| Mortality | 268 | 0.99 (0.90 to 1.09) | 0.00 |
| Objective | 372 | 0.98 (0.88 to 1.09) | 0.00 |
| Subjective | 652 | 0.85 (0.76 to 0.95) | 0.19 |
| Lack of double blinding or unclear double blinding (vs double blind) | |||
| All | 1057 | 0.86 (0.79 to 0.94) | 0.21 |
| Mortality | 245 | 0.90 (0.80 to 1.00) | 0.00 |
| Objective | 282 | 0.97 (0.77 to 1.21) | 0.32 |
| Subjective | 530 | 0.78 (0.67 to 0.90) | 0.27 |
| Inadequate or unclear sequence generation or allocation concealment (vs adequate sequence generation and allocation concealment) | |||
| All | 534 | 0.95 (0.88 to 1.03) | 0.04 |
| Mortality | 79 | 0.97 (0.83 to 1.13) | 0.00 |
| Objective | 176 | 0.91 (0.72 to 1.14) | 0.03 |
| Subjective | 279 | 0.91 (0.80 to 1.03) | 0.10 |
| Inadequate or unclear sequence generation or allocation concealment or not double blind (vs adequate sequence generation and allocation concealment and double blind) | |||
| All | 409 | 0.81 (0.71 to 0.93) | 0.14 |
| Mortality | 65 | 0.96 (0.82 to 1.13) | 0.00 |
| Objective | 139 | 0.68 (0.47 to 0.96) | 0.00 |
| Subjective | 205 | 0.72 (0.57 to 0.91) | 0.25 |
| Inadequate or unclear allocation concealment or not double blind (vs adequate allocation concealment and double blind) | |||
| All | 990 | 0.91 (0.87 to 0.96) | 0.00 |
| Mortality | 220 | 0.92 (0.83 to 1.02) | 0.00 |
| Objective | 268 | 0.86 (0.74 to 1.00) | 0.00 |
| Subjective | 502 | 0.83 (0.75 to 0.93) | 0.13 |
For inadequate or unclear sequence generation, inadequate or unclear allocation concealment and lack of or unclear double blinding, estimated RORs are broadly consistent with those from the hierarchical bias models displayed in Figure 3. For each study design characteristic, the exaggeration of intervention effect estimates is greater for meta-analyses with subjectively assessed outcomes than for meta-analyses with all-cause mortality or other objectively assessed outcomes. Consistent with the small estimated values of φ in the hierarchical models, the between-meta-analysis standard deviation was estimated as zero for each of these characteristics in meta-analyses with all-cause mortality outcomes and also, for sequence generation and allocation concealment, in trials with other objectively assessed outcomes. Between-trial variability in bias was greatest for lack of or unclear double blinding, in meta-analyses with other objective or subjective outcomes. Results from analyses of combined study design characteristics were also broadly consistent with those from the hierarchical bias models.
Influence of reported study design characteristics: multivariable analyses
Table 18 presents results from multivariable analyses of the influence of inadequate or unclear allocation concealment and lack of or unclear double blinding, based on the 169 informative meta-analyses (1456 trials) in which both characteristics were assessed. Results from models without interaction terms are displayed in Figure 5: estimated RORs were similar to, or modestly attenuated compared with, the univariable analyses presented in Table 15. Estimated effects on heterogeneity were also modestly attenuated. Results from the model including interaction terms are displayed in Figure 6. RORs for the interaction between inadequate or unclear allocation concealment and lack of double blinding were close to 1, with wide CIs. Therefore, these results are consistent with the effects of these two characteristics being multiplicative.
| Model, study design characteristic and outcome | ROR | 95% CrI | κ | 95% CrI | φ | 95% CrI |
|---|---|---|---|---|---|---|
| Model without interaction | ||||||
| Inadequate or unclear allocation concealment (vs adequate) | ||||||
| All | 0.93 | 0.87 to 1.00 | 0.08 | 0.01 to 0.20 | 0.05 | 0.01 to 0.14 |
| Mortality | 1.00 | 0.89 to 1.13 | 0.06 | 0.01 to 0.20 | 0.05 | 0.01 to 0.18 |
| Objective | 0.97 | 0.84 to 1.13 | 0.06 | 0.01 to 0.25 | 0.05 | 0.01 to 0.21 |
| Subjective | 0.85 | 0.76 to 0.96 | 0.07 | 0.01 to 0.27 | 0.06 | 0.01 to 0.22 |
| Lack of double blinding or unclear double blinding (vs double blind) | ||||||
| All | 0.88 | 0.79 to 0.97 | 0.12 | 0.02 to 0.28 | 0.13 | 0.03 to 0.28 |
| Mortality | 0.92 | 0.80 to 1.06 | 0.06 | 0.01 to 0.20 | 0.07 | 0.01 to 0.24 |
| Objective | 0.90 | 0.71 to 1.15 | 0.08 | 0.01 to 0.38 | 0.16 | 0.01 to 0.54 |
| Subjective | 0.82 | 0.68 to 0.96 | 0.30 | 0.04 to 0.48 | 0.19 | 0.03 to 0.40 |
| Implied average bias in trials with high risk of bias for both characteristics | ||||||
| All | 0.83 | 0.74 to 0.92 | ||||
| Mortality | 0.92 | 0.78 to 1.09 | ||||
| Objective | 0.87 | 0.68 to 1.12 | ||||
| Subjective | 0.70 | 0.57 to 0.84 | ||||
| Model including interaction terms | ||||||
| Inadequate or unclear allocation concealment (vs adequate), in double-blind trials | ||||||
| All | 0.90 | 0.83 to 0.98 | 0.07 | 0.01 to 0.19 | 0.05 | 0.01 to 0.14 |
| Mortality | 0.98 | 0.84 to 1.13 | 0.06 | 0.01 to 0.20 | 0.05 | 0.01 to 0.19 |
| Objective | 0.86 | 0.70 to 1.02 | 0.06 | 0.01 to 0.24 | 0.05 | 0.01 to 0.21 |
| Subjective | 0.85 | 0.73 to 0.96 | 0.08 | 0.01 to 0.25 | 0.07 | 0.01 to 0.22 |
| Lack of double blinding or unclear double blinding (vs double blind), in adequately concealed trials | ||||||
| All | 0.84 | 0.73 to 0.95 | 0.10 | 0.01 to 0.26 | 0.11 | 0.01 to 0.26 |
| Mortality | 0.87 | 0.69 to 1.06 | 0.06 | 0.01 to 0.20 | 0.06 | 0.01 to 0.24 |
| Objective | 0.72 | 0.52 to 1.01 | 0.08 | 0.01 to 0.38 | 0.15 | 0.01 to 0.56 |
| Subjective | 0.85 | 0.67 to 1.05 | 0.16 | 0.01 to 0.41 | 0.09 | 0.01 to 0.29 |
| Interaction | ||||||
| All | 1.08 | 0.95 to 1.24 | 0.10 | 0.01 to 0.27 | 0.07 | 0.01 to 0.24 |
| Mortality | 1.10 | 0.85 to 1.43 | 0.06 | 0.01 to 0.21 | 0.05 | 0.01 to 0.22 |
| Objective | 1.30 | 1.00 to 1.78 | 0.07 | 0.01 to 0.28 | 0.06 | 0.01 to 0.26 |
| Subjective | 0.95 | 0.74 to 1.21 | 0.20 | 0.01 to 0.43 | 0.23 | 0.02 to 0.46 |
| Implied average bias in trials with high risk of bias for both characteristics | ||||||
| All | 0.82 | 0.73 to 0.91 | ||||
| Mortality | 0.93 | 0.78 to 1.10 | ||||
| Objective | 0.81 | 0.63 to 1.05 | ||||
| Subjective | 0.68 | 0.55 to 0.83 | ||||
FIGURE 5.
Estimated RORs and effects on heterogeneity from multivariable analyses, according to type of outcome measure. Analyses based on trials with assessments of both allocation concealment and blinding. Upper panel, influence of inadequate or unclear versus adequate allocation concealment, adjusted for blinding; lower panel, influence of lack of double blinding or unclear blinding status versus double blinding, adjusted for allocation concealment.
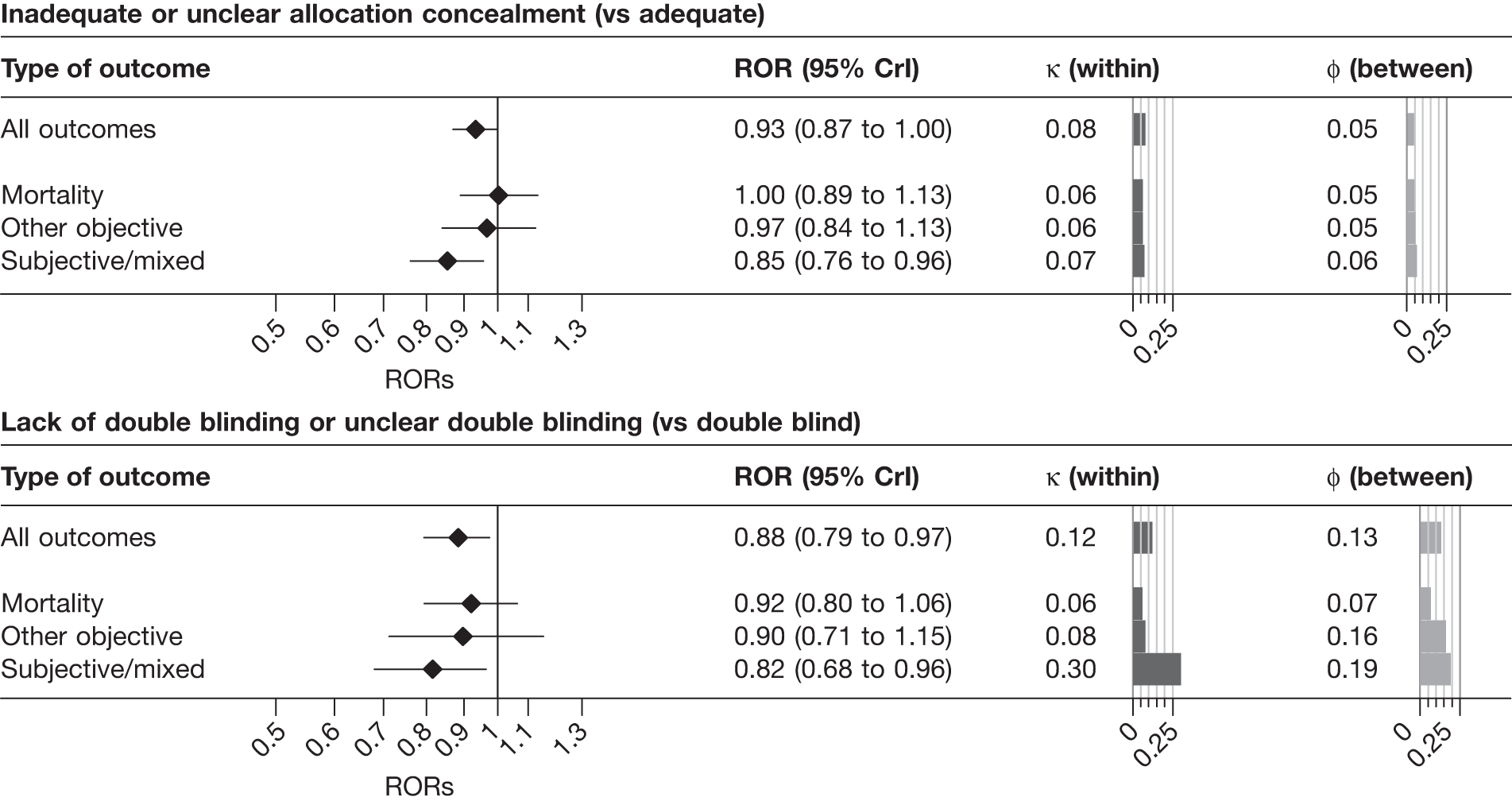
FIGURE 6.
Estimated RORs and effects on heterogeneity from multivariable analyses including interaction terms, according to type of outcome measure. Analyses based on trials with assessments of both allocation concealment and blinding. Upper panel, influence of inadequate or unclear versus adequate allocation concealment, in double-blind trials; middle panel, influence of lack of double blinding or unclear blinding status versus double blinding, in trials with adequate allocation concealment; lower panel, interaction between allocation concealment and blinding.
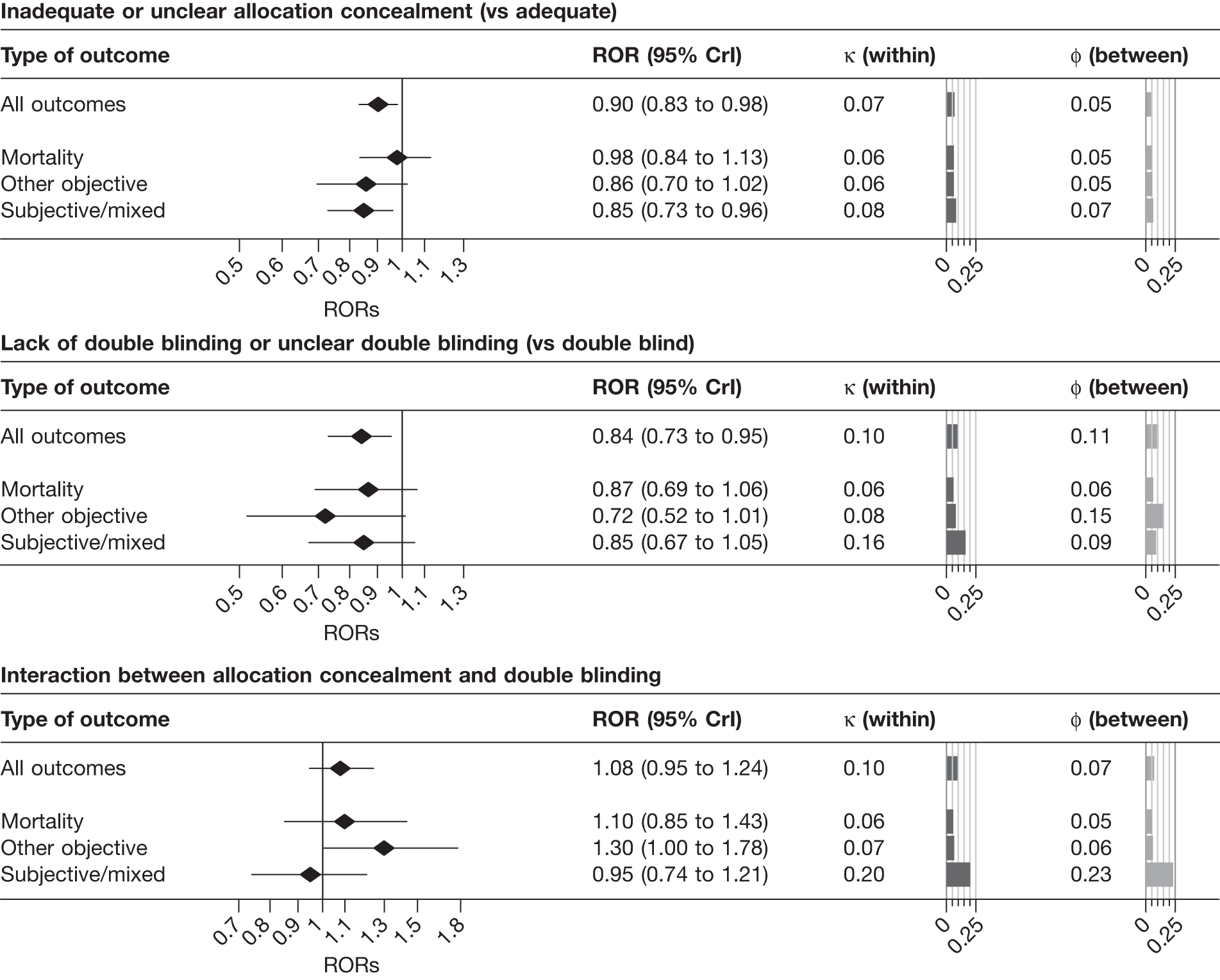
Results from multivariable analyses of the influence of all three study design characteristics are presented in Table 19 and displayed in Figure 7. Estimated RORs for each study design characteristic were of similar magnitudes to those in the univariable analyses presented in Table 16. For inadequate or unclear sequence generation or allocation concealment, estimated increases in between-trial heterogeneity (quantified by κ) were smaller in multivariable analyses than in the corresponding univariable analyses (see Table 16). Estimates of between-meta-analysis variability in average bias changed little compared with univariable analyses.
| Study design characteristic and outcome | ROR | 95% CrI | κ | 95% CrI | φ | 95% CrI |
|---|---|---|---|---|---|---|
| Inadequate or unclear sequence generation (vs adequate) | ||||||
| All | 0.90 | 0.82 to 0.99 | 0.06 | 0.01 to 0.20 | 0.05 | 0.01 to 0.15 |
| Mortality | 0.86 | 0.69 to 1.06 | 0.08 | 0.01 to 0.31 | 0.06 | 0.01 to 0.28 |
| Objective | 1.00 | 0.84 to 1.20 | 0.07 | 0.01 to 0.30 | 0.07 | 0.01 to 0.27 |
| Subjective | 0.88 | 0.76 to 1.00 | 0.05 | 0.01 to 0.21 | 0.06 | 0.01 to 0.24 |
| Inadequate or unclear allocation concealment (vs adequate) | ||||||
| All | 0.89 | 0.81 to 0.99 | 0.06 | 0.01 to 0.19 | 0.05 | 0.01 to 0.18 |
| Mortality | 1.03 | 0.82 to 1.31 | 0.07 | 0.01 to 0.30 | 0.07 | 0.01 to 0.33 |
| Objective | 0.92 | 0.76 to 1.12 | 0.06 | 0.01 to 0.24 | 0.06 | 0.01 to 0.29 |
| Subjective | 0.82 | 0.70 to 0.94 | 0.08 | 0.01 to 0.27 | 0.07 | 0.01 to 0.30 |
| Lack of double blinding or unclear double blinding (vs double blind) | ||||||
| All | 0.86 | 0.73 to 0.98 | 0.20 | 0.02 to 0.39 | 0.17 | 0.03 to 0.32 |
| Mortality | 1.07 | 0.78 to 1.48 | 0.09 | 0.01 to 0.44 | 0.08 | 0.01 to 0.42 |
| Objective | 0.91 | 0.64 to 1.33 | 0.10 | 0.01 to 0.50 | 0.20 | 0.02 to 0.85 |
| Subjective | 0.77 | 0.61 to 0.93 | 0.24 | 0.02 to 0.45 | 0.20 | 0.04 to 0.39 |
FIGURE 7.
Estimated RORs and effects on heterogeneity from multivariable analyses including all three study design characteristics, according to type of outcome measure. Analyses based on trials with assessments of all three study design characteristics. Upper panel, influence of inadequate or unclear versus adequate sequence generation, adjusted for allocation concealment and blinding; middle panel, influence of inadequate or unclear versus adequate allocation concealment, adjusted for sequence generation and blinding; lower panel, influence of lack of double blinding or unclear blinding status versus double blinding, adjusted for sequence generation and allocation concealment.
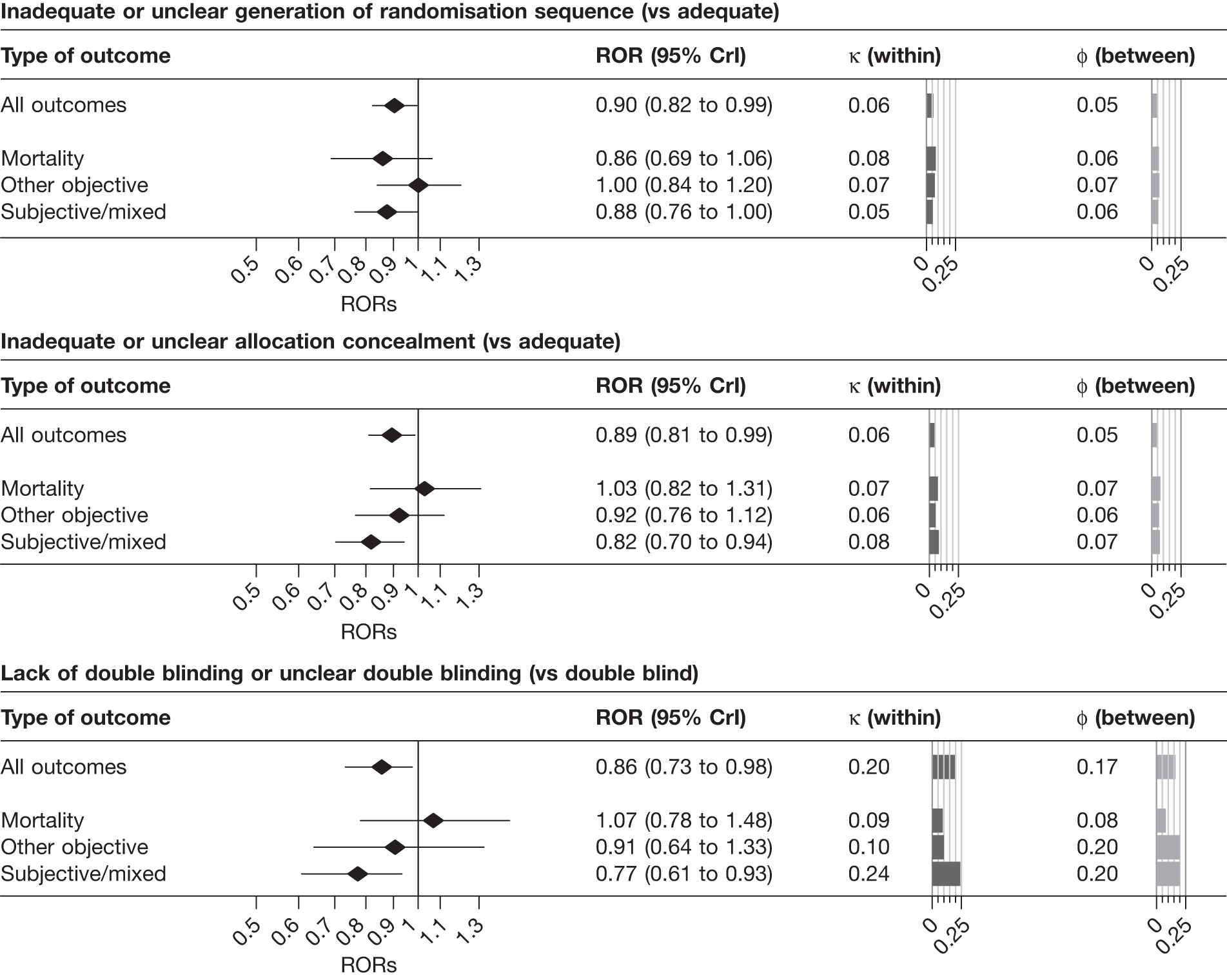
Analyses according to type of intervention and clinical area.
Results from univariable analyses of the influence of the three study design characteristics according to clinical area are shown in Table 20. For pregnancy and childbirth – the clinical area contributing most meta-analyses to the combined data set – RORs were further from 1 than in the analyses of the whole data set displayed in Figure 3 and Table 14, whereas estimates of effects on heterogeneity were broadly consistent with analyses of the whole data set. For the other two clinical areas, RORs were attenuated towards 1. Only small numbers of meta-analyses contributed to estimation of κ, but estimated values of κ and φ were generally smaller for circulatory system meta-analyses than for the other two clinical areas.
| Study design characteristic by clinical area | No. of meta-analyses | No. of trials | Contributing meta-analysesb | Contributing trials | No. (%) of trials at high risk of biasc | ROR (95% CrI) | κ (95% CrI) | φ (95% CrI) |
|---|---|---|---|---|---|---|---|---|
| Pregnancy and childbirth | ||||||||
| Sequence generation | 53 | 298 | 31 (15) | 214 | 148 (69.2) | 0.85 (0.69 to 1.05) | 0.09 (0.01 to 0.33) | 0.10 (0.01 to 0.47) |
| Allocation concealment | 57 | 407 | 44 (26) | 325 | 209 (64.3) | 0.83 (0.70 to 0.97) | 0.17 (0.02 to 0.47) | 0.09 (0.01 to 0.33) |
| Blinding | 57 | 447 | 29 (19) | 249 | 129 (51.8) | 0.78 (0.63 to 0.97) | 0.29 (0.02 to 0.63) | 0.10 (0.01 to 0.42) |
| Mental and behavioural | ||||||||
| Sequence generation | 21 | 207 | 15 (4) | 171 | 138 (80.7) | 0.82 (0.67 to 1.01) | 0.10 (0.01 to 0.29) | 0.09 (0.01 to 0.42) |
| Allocation concealment | 25 | 287 | 8 (5) | 144 | 119 (82.6) | 1.01 (0.77 to 1.40) | 0.20 (0.02 to 0.36) | 0.14 (0.01 to 0.58) |
| Blinding | 26 | 302 | 6 (4) | 145 | 42 (29.0) | 0.91 (0.43 to 1.35) | 0.14 (0.01 to 0.41) | 0.27 (0.02 to 1.17) |
| Circulatory system | ||||||||
| Sequence generation | 18 | 119 | 11 (8) | 100 | 72 (72.0) | 0.90 (0.72 to 1.08) | 0.13 (0.01 to 0.39) | 0.06 (0.01 to 0.33) |
| Allocation concealment | 31 | 257 | 24 (13) | 216 | 148 (68.5) | 0.98 (0.87 to 1.09) | 0.06 (0.01 to 0.19) | 0.04 (0.01 to 0.17) |
| Blinding | 31 | 277 | 20 (12) | 187 | 83 (44.4) | 0.92 (0.79 to 1.04) | 0.06 (0.01 to 0.20) | 0.06 (0.01 to 0.24) |
Table 21 displays results of univariable analyses restricted to pharmacological and surgical interventions. The majority of meta-analyses included in the full data set addressed pharmacological interventions; it was therefore unsurprising that overall results restricted to such interventions were consistent with those from the full data set. For surgical interventions, the influence of inadequate or unclear sequence generation and allocation concealment was estimated from only six and nine meta-analyses respectively: CIs were too wide to allow substantive conclusions to be drawn.
| Bias domain by intervention type | No. of meta-analyses | No. of trials | Contributing meta-analysesb | Contributing trials | No. (%) of trials at high risk of biasc | ROR (95% CrI) | κ (95% CrI) | φ (95% CrI) |
|---|---|---|---|---|---|---|---|---|
| Pharmacological interventions | ||||||||
| Sequence generation | 124 | 869 | 76 (38) | 691 | 519 (75.1) | 0.86 (0.78 to 0.95) | 0.16 (0.03 to 0.27) | 0.05 (0.01 to 0.18) |
| Allocation concealment | 157 | 1301 | 108 (63) | 1012 | 713 (70.5) | 0.88 (0.81 to 0.94) | 0.12 (0.02 to 0.23) | 0.04 (0.01 to 0.14) |
| Blinding | 162 | 1418 | 94 (59) | 987 | 420 (42.6) | 0.87 (0.77 to 0.96) | 0.13 (0.02 to 0.29) | 0.16 (0.03 to 0.31) |
| Surgical interventions | ||||||||
| Sequence generation | 11 | 58 | 6 (5) | 48 | 30 (62.5) | 0.93 (0.47 to 1.79) | 0.29 (0.02 to 1.36) | 0.11 (0.01 to 0.92) |
| Allocation concealment | 14 | 107 | 9 (5) | 66 | 50 (75.8) | 1.41 (0.52 to 2.58) | 0.15 (0.01 to 0.88) | 0.15 (0.01 to 1.94) |
| Blinding | No data | |||||||
Analyses of meta-analyses comparing two active interventions
For meta-analyses comparing two active interventions we estimated increases in between-trial (within-meta-analysis) heterogeneity in trials with, compared with trials without, a study design characteristic of interest (among meta-analyses containing at least two trials with and without the characteristic of interest). Based on eight meta-analyses containing 84 trials, the between-trial standard deviation (corresponding to κ in previous analyses) for trials with inadequate or unclear (compared with adequate) sequence generation was 0.35 (95% CrI 0.02 to 0.88), similar to the estimate for inadequate or unclear (compared with adequate) allocation concealment (0.36, 95% CrI 0.02 to 0.88, based on six meta-analyses containing 52 trials). The estimated increase in heterogeneity was somewhat lower for lack of or unclear (compared with adequate) double blinding (0.24 95% CrI 0.02 to 0.79, based on seven meta-analyses containing 58 trials).
Results after excluding meta-analyses that contributed to the study of Wood et al.16
Data from the three contributing meta-epidemiological studies by Schulz et al. ,11 Kjaergard et al. 15 and Egger et al. 9 were combined in a study previously reported by Wood et al. ,16 which used a meta-meta-analytic approach for statistical analyses. 8 A total of 123 meta-analyses (1066 trials) were contributed by other meta-epidemiological studies. Table 22 shows numbers of contributing meta-analyses and results from univariable analyses of the influence of the three study design characteristics, both overall and separately according to type of outcome measure. Compared with the full data set, estimated RORs tend to be closer to the null, except for the influence of inadequate or unclear (compared with adequate) sequence generation in meta-analyses with subjective outcomes (ROR 0.86, 95% CrI 0.75 to 0.97). However, effects on the between-trial heterogeneity are broadly consistent with those for the main analyses reported in Table 14, with between-trial heterogeneity increased in meta-analyses with subjective outcomes, for all study design characteristics.
| Study design characteristic and outcome | No. of meta-analyses | No. of trials | Contributing meta-analysesa | Contributing trials | No. (%) of trials at high risk of biasb | ROR (95% CrI) | κ (95% CrI) | φ (95% CrI) |
|---|---|---|---|---|---|---|---|---|
| Inadequate or unclear sequence generation (vs adequate) | ||||||||
| All | 103 | 837 | 74 (40) | 690 | 516 (74.8) | 0.91 (0.83 to 1.00) | 0.16 (0.02 to 0.29) | 0.05 (0.01 to 0.17) |
| Mortality | 17 | 100 | 11 (7) | 76 | 57 (75.0) | 0.94 (0.71 to 1.23) | 0.07 (0.01 to 0.37) | 0.08 (0.01 to 0.43) |
| Objective | 31 | 253 | 26 (12) | 221 | 159 (71.9) | 1.03 (0.84 to 1.26) | 0.13 (0.01 to 0.54) | 0.07 (0.01 to 0.29) |
| Subjective | 55 | 484 | 37 (21) | 393 | 300 (76.3) | 0.86 (0.75 to 0.97) | 0.19 (0.02 to 0.32) | 0.06 (0.01 to 0.21) |
| Inadequate or unclear allocation concealment (vs adequate) | ||||||||
| All | 123 | 1066 | 68 (39) | 727 | 551 (75.8) | 0.98 (0.90 to 1.07) | 0.09 (0.01 to 0.22) | 0.04 (0.01 to 0.15) |
| Mortality | 25 | 191 | 16 (8) | 147 | 105 (71.4) | 1.00 (0.86 to 1.14) | 0.07 (0.01 to 0.24) | 0.05 (0.01 to 0.22) |
| Objective | 32 | 261 | 18 (11) | 173 | 124 (71.7) | 0.96 (0.78 to 1.20) | 0.07 (0.01 to 0.30) | 0.08 (0.01 to 0.41) |
| Subjective | 66 | 614 | 34 (20) | 407 | 322 (79.1) | 0.97 (0.85 to 1.11) | 0.16 (0.02 to 0.32) | 0.07 (0.01 to 0.25) |
| Lack of double blinding or unclear double blinding (vs double blind) | ||||||||
| All | 123 | 1063 | 45 (28) | 525 | 237 (45.1) | 0.91 (0.80 to 1.02) | 0.09 (0.01 to 0.28) | 0.14 (0.02 to 0.32) |
| Mortality | 25 | 191 | 12 (7) | 115 | 55 (47.8) | 0.89 (0.74 to 1.06) | 0.06 (0.01 to 0.22) | 0.08 (0.01 to 0.32) |
| Objective | 32 | 260 | 8 (5) | 109 | 47 (43.1) | 0.90 (0.50 to 1.92) | 0.16 (0.01 to 0.87) | 0.38 (0.02 to 1.48) |
| Subjective | 66 | 612 | 25 (16) | 301 | 135 (44.9) | 0.93 (0.74 to 1.10) | 0.22 (0.02 to 0.42) | 0.14 (0.02 to 0.45) |
Univariable three-category analyses
Table 23 presents results from analyses of all trials in which reported methodological characteristics were assessed in three categories (low, unclear or high risk of bias). The number of trials and meta-analyses contributing to each analysis are shown in Table 24. There were no consistent patterns when comparing RORs for unclear (compared with low) and high (compared with low) risk of bias, which provides some support for combining these effects in the main analyses. Consistent with the main analyses, estimated values of κ were greatest for meta-analyses with subjectively assessed outcomes, both for high and unclear (compared with low) risk of bias.
| Study design characteristic and outcome | No. of meta-analyses | No. of trials | ROR (95% CrI) | κ (95% CrI) | φ (95% CrI) | |||
|---|---|---|---|---|---|---|---|---|
| Uncleara | Higha | Uncleara | Higha | Uncleara | Higha | |||
| Sequence generation | ||||||||
| All | 186 | 1176 | 0.90 (0.83 to 0.98) | 0.78 (0.62 to 0.98) | 0.06 (0.01 to 0.16) | 0.49 (0.11 to 0.75) | 0.05 (0.01 to 0.15) | 0.07 (0.01 to 0.34) |
| Mortality | 30 | 137 | 0.88 (0.68 to 1.11) | 1.34 (0.70 to 2.84) | 0.06 (0.01 to 0.20) | 0.08 (0.01 to 0.50) | 0.07 (0.01 to 0.34) | 0.14 (0.01 to 1.41) |
| Objective | 66 | 387 | 1.02 (0.85 to 1.21) | 0.87 (0.57 to 1.29) | 0.05 (0.01 to 0.18) | 0.38 (0.02 to 1.03) | 0.07 (0.01 to 0.28) | 0.11 (0.01 to 0.67) |
| Subjective | 90 | 652 | 0.85 (0.75 to 0.96) | 0.66 (0.46 to 0.90) | 0.11 (0.01 to 0.25) | 0.57 (0.22 to 0.91) | 0.06 (0.01 to 0.20) | 0.09 (0.01 to 0.55) |
| Allocation concealment | ||||||||
| All | 228 | 1796 | 0.92 (0.86 to 0.99) | 0.95 (0.82 to 1.10) | 0.13 (0.02 to 0.22) | 0.21 (0.02 to 0.48) | 0.04 (0.01 to 0.13) | 0.09 (0.01 to 0.32) |
| Mortality | 44 | 328 | 0.98 (0.87 to 1.10) | 1.03 (0.69 to 1.63) | 0.07 (0.01 to 0.19) | 0.19 (0.01 to 1.06) | 0.05 (0.01 to 0.17) | 0.15 (0.01 to 0.70) |
| Objective | 76 | 551 | 0.96 (0.84 to 1.10) | 1.12 (0.78 to 1.65) | 0.06 (0.01 to 0.20) | 0.18 (0.01 to 0.86) | 0.06 (0.01 to 0.25) | 0.17 (0.01 to 0.68) |
| Subjective | 108 | 917 | 0.84 (0.76 to 0.94) | 0.86 (0.71 to 1.03) | 0.22 (0.08 to 0.32) | 0.18 (0.02 to 0.50) | 0.06 (0.01 to 0.22) | 0.08 (0.01 to 0.35) |
| Blinding | ||||||||
| All | 224 | 1721 | 0.90 (0.69 to 1.15) | 0.82 (0.70 to 0.96) | 0.14 (0.01 to 0.44) | 0.11 (0.01 to 0.23) | 0.16 (0.01 to 0.59) | 0.23 (0.06 to 0.42) |
| Mortality | 44 | 304 | 0.86 (0.39 to 1.57) | 0.94 (0.69 to 1.26) | 0.15 (0.01 to 1.04) | 0.06 (0.01 to 0.22) | 0.19 (0.01 to 1.33) | 0.08 (0.01 to 0.41) |
| Objective | 77 | 591 | 1.30 (0.76 to 2.22) | 0.89 (0.67 to 1.25) | 0.08 (0.01 to 0.35) | 0.05 (0.01 to 0.18) | 0.11 (0.01 to 0.74) | 0.16 (0.01 to 0.65) |
| Subjective | 103 | 826 | 0.75 (0.51 to 1.09) | 0.70 (0.53 to 0.89) | 0.26 (0.02 to 0.77) | 0.27 (0.08 to 0.38) | 0.15 (0.01 to 0.70) | 0.33 (0.13 to 0.59) |
| Study design characteristic and outcome | High vs low risk of bias | Unclear vs low risk of bias | ||||
|---|---|---|---|---|---|---|
| Contributing meta-analysesa | Contributing trials | Contributing high-risk trials (%) | Contributing meta-analysesa | Contributing trials | Contributing unclear-risk trials (%) | |
| Sequence generation | ||||||
| All | 48 (17) | 453 | 81 (18) | 100 (57) | 906 | 648 (72) |
| Mortality | 3 (2) | 26 | 7 (27) | 12 (8) | 83 | 59 (71) |
| Objective | 22 (6) | 175 | 32 (18) | 38 (18) | 303 | 204 (67) |
| Subjective | 23 (9) | 252 | 42 (17) | 50 (31) | 520 | 385 (74) |
| Allocation concealment | ||||||
| All | 75 (29) | 716 | 136 (19) | 171 (99) | 1485 | 998 (67) |
| Mortality | 11 (2) | 95 | 17 (18) | 35 (18) | 287 | 187 (65) |
| Objective | 26 (6) | 215 | 38 (18) | 54 (30) | 442 | 293 (66) |
| Subjective | 38 (21) | 406 | 81 (20) | 82 (51) | 756 | 518 (69) |
| Blinding | ||||||
| All | 71 (36) | 725 | 290 (40) | 33 (17) | 286 | 84 (29) |
| Mortality | 13 (5) | 119 | 39 (33) | 5 (3) | 48 | 10 (21) |
| Objective | 20 (12) | 208 | 81 (39) | 9 (5) | 63 | 23 (37) |
| Subjective | 38 (19) | 398 | 170 (43) | 19 (9) | 175 | 51 (29) |
Downweighting potentially biased evidence in future meta-analyses
Table 25 presents implications of the results from the primary univariable analyses (see Table 14) for downweighting of potentially biased evidence in future meta-analyses, based on formulae from Welton et al. 26 Because estimated values of κ and φ were greatest for meta-analyses with subjectively assessed outcomes, the minimum variance of the estimated intervention effect for a trial at high or unclear risk of bias is greatest for such trials. Across all BRANDO trials with inadequate or unclear sequence generation, bias adjustment led to a median 10% (IQR 4% to 23%) increase in trial-level variance. Downweighting based on results specific to type of outcome measure has the greatest effect in trials with subjectively assessed outcomes [median 20% (IQR 8% to 39%) increase in variance]. Results were broadly similar for downweighting based on inadequate or unclear allocation concealment. The median increase in variance for trials with subjectively measured outcomes that were not double blind or unclearly blinded was 63% (IQR 22% to 138%).
| Study design characteristic and outcome | No. of high-risk trials | Minimum variance of trial at high risk of bias (V0 + κ2 + φ2) | Median (IQR) increase in trial-level variance (%) | Median (IQR) increase in variance of summary intervention effect (%) | ||
|---|---|---|---|---|---|---|
| Downweighting all meta-analyses | Downweighting informative meta-analyses | Excluding all trials at high or unclear risk of bias | ||||
| Inadequate or unclear sequence generation (vs adequate) | ||||||
| All | 901 | 0.030 | 10 (4 to 23) | 12 (2 to 32) | 13 (5 to 32) | 217 (87 to 482) |
| Mortality | 116 | 0.020 | 6 (3 to 14) | 11 (1 to 25) | 13 (6 to 36) | 119 (70 to 336) |
| Objective | 273 | 0.019 | 5 (3 to 11) | 8 (1 to 19) | 11 (2 to 32) | 145 (62 to 559) |
| Subjective | 512 | 0.046 | 20 (8 to 39) | 31 (6 to 64) | 31 (11 to 56) | 282 (126 to 482) |
| Inadequate or unclear allocation concealment (vs adequate) | ||||||
| All | 1380 | 0.017 | 5 (2 to 12) | 9 (3 to 23) | 7 (3 to 20) | 150 (49 to 411) |
| Mortality | 233 | 0.011 | 4 (1 to 11) | 8 (3 to 34) | 8 (3 to 19) | 121 (39 to 468) |
| Objective | 413 | 0.011 | 3 (1 to 6) | 9 (3 to 22) | 6 (3 to 13) | 175 (52 to 337) |
| Subjective | 734 | 0.053 | 18 (7 to 40) | 36 (8 to 73) | 27 (7 to 59) | 146 (55 to 411) |
| Lack of double blinding or unclear double blinding (vs double blind) | ||||||
| All | 1041 | 0.044 | 13 (6 to 31) | 16 (0 to 62) | 15 (3 to 48) | 62 (19 to 143) |
| Mortality | 170 | 0.013 | 4 (2 to 10) | 5 (0 to 18) | 5 (0 to 18) | 46 (18 to 101) |
| Objective | 336 | 0.036 | 11 (5 to 24) | 22 (0 to 67) | 16 (3 to 46) | 79 (22 to 202) |
| Subjective | 535 | 0.200 | 63 (22 to 138) | 41 (1 to 175) | 36 (8 to 72) | 62 (19 to 140) |
Downweighting all trials with inadequate or unclear sequence generation led to a median 13% (IQR 5% to 32%) increase in the variance of the summary (meta-analytic) intervention effect estimate among informative meta-analyses in the BRANDO database. This is in contrast to a median increase of 217% (IQR 87% to 482%) that results from completely excluding such trials, because only 26% of trials were assessed to have adequate sequence generation. Bias adjustment for meta-analyses with subjectively assessed outcomes led to a median 31% (IQR 11% to 56%) increase in the variance of the summary intervention effect estimate, which was again small compared with complete exclusion of such trials. Results were broadly similar for the other study design characteristics, although differences between the effects of downweighting and excluding trials at high or unclear risk of bias were smaller for double blinding, because 56% of trials from informative meta-analyses were double blind. Even for subjectively assessed outcomes, excluding trials not assessed as double blind led to a greater loss of precision than retaining but downweighting them [median increase in variance 36% (IQR 8% to 72%) for downweighting compared with median 62% (IQR 19% to 140%) for excluding].
Chapter 4 Discussion
Summary of findings
Using data from 10 empirical (meta-epidemiological) studies, we developed a combined database for meta-epidemiological research. The database structure comprised six tables with defined relationships between them, which reflects the complexity of relationships between reviews, their publications, meta-analyses, trials and their publications, and trial characteristics and results. This database structure allowed us to identify duplicated entries, so that in the final database there were no overlaps between meta-analyses. This database design is potentially relevant to any situation in which collections of meta-analyses are being combined, and may also be of interest to researchers wishing to identify overlaps between two or more previously published meta-analyses.
We estimated the influence of three types of study design characteristic on average intervention effect estimates, and in increasing between-trial, within-meta-analysis, heterogeneity, in 1973 trials (234 meta-analyses) for which information on both study design characteristics and trial results was available. Bias in intervention effect estimates resulting from inadequate or unclear sequence generation, inadequate or unclear allocation concealment or lack of or unclear double blinding varied according to the type of outcome measure assessed. Overall, there was little evidence of bias in trials assessing all-cause mortality or other objectively assessed outcomes. In contrast, inadequate sequence generation, inadequate allocation concealment or lack of blinding were associated with exaggerated estimates of the benefit of interventions in trials reporting subjectively assessed outcomes. The direction and magnitude of bias associated with reported study design characteristics varies between trials and meta-analyses, and with the type of outcome measure. Increases in between-trial heterogeneity associated with study design characteristics were greatest for trials reporting subjectively assessed outcomes. Except for the effect of lack of or unclear double blinding in trials reporting subjectively assessed outcomes, estimates of between-meta-analysis variability in mean bias were small. Analyses of the effects of combined study design characteristics suggested that effects of individual characteristics were less than multiplicative, in that estimated effects of two study design characteristics together were attenuated compared with the combined individual effects. Effects were somewhat attenuated in multivariable analyses.
Strengths and weaknesses
To our knowledge, this study represents the most comprehensive attempt to date to quantify the influence of inadequate or unclear random sequence generation, inadequate or unclear allocation concealment and lack of or unclear double blinding on intervention effect estimates from RCTs. So far as we are aware, no previous study has quantified the effect of reported study design characteristics on between-trial heterogeneity as well as on average intervention effects, although Schulz et al. 11 noted that ORs from inadequately concealed trials appeared more heterogeneous than those from adequately concealed trials. Thanks to the generosity of their investigators, we were able to combine the results of all meta-epidemiological studies of which we were aware at the start of the study, with the exception of a study for which data were no longer available. The size of the data set meant that we were able to quantify the influence of study design characteristics in subgroups defined by types of intervention, comparison and outcome measure.
Combining multiple collections of meta-analyses and removing overlaps is a labour-intensive process. We estimate that for the very large BRANDO study database this process took approximately 24 months’ person time. However, a substantial proportion of this time was devoted to database development and programming, which might be avoided in future studies. Harmonising the definition of trial characteristics across different studies was also time-consuming. Nonetheless, de novo data extraction would have taken substantially longer. It would be difficult to publish results from a combined database in which the extent of overlap between meta-analyses was unknown. However, combining meta-epidemiological studies on different topics would likely avoid the need for a deduplication exercise.
Inadequate reporting of trial methods can severely impede the assessment of trial quality and the risk of bias in trial results. This is a particular problem for the assessment of sequence generation and allocation concealment, which are often not described at all in trial publications. Table 11 shows that, for 64% of trials with assessment of sequence generation and 69% of trials with assessments of allocation concealment, these characteristics were assessed as unclear. In most cases this was because the method was not described at all in the publication. Transparent and standardised reporting of trials is an essential part of clinical research. Many trials included in this data set were published before the publication of the CONSORT (Consolidated Standards of Reporting Trials) recommendations in 1996. 31,32 There is evidence that reporting of trials improved between 2000 and 2006, although it remained below an acceptable level. 33
Our study was based on reported study design characteristics, which need not correspond to how a trial was in fact conducted: well-conducted trials may be reported badly. 34 A study of discrepancies between published reports and the actual conduct of RCTs found that, in more than three-quarters of trials in which sequence generation or allocation concealment were unclear, these characteristics were in fact adequate,35 whereas Soares et al. 36 found that the methodology of 56 trials of radiation therapy in oncology was better than reported. In contrast, Pildal et al. 37 found that most trials with unclear allocation concealment on the basis of the trial publication also have unclear allocation concealment according to their protocol. A recent study of reporting of blinding suggested that, although reporting is often inadequate, it rarely contradicts the methods specified in the trial protocol. 38
The study of Egger et al. 9 relied on assessments of trial quality by the authors of the included Cochrane reviews rather than by methodological experts. Despite the standardised guidelines specified in the Cochrane Handbook39 at the time, evaluations by authors of Cochrane reviews of whether or not a study had adequate allocation concealment may be inconsistent. 37 The effect of trial quality on estimates of intervention effect in this study was, however, in line with previous studies in which quality was assessed by the same observers: one would expect attenuation of effects if assessments in Cochrane reviews were less reliable.
As is common in Bayesian hierarchical modeling,28,29 we found a high degree of sensitivity of estimated variance components to the prior distributions assumed for these parameters. This is of concern because the estimates of κ and φ are of substantial interest, having the potential to drive downweighting of evidence from high or unclear risk trials in future meta-analyses, based on formulae from Welton et al. 26 Lambert et al. 28 have previously demonstrated an upward bias of Monte Carlo Markov Chain estimates of variance components when the true variance is very close to zero. We were, therefore, cautious in interpreting small estimates of heterogeneity parameters, and those for which the lower limit of the CI is close to zero.
We estimated the impact of downweighting trials in a new fixed-effect meta-analysis, based on our estimates of the influence of study design characteristics on intervention effect estimates. In practice, a random-effects model might have been preferable for many of the meta-analyses on which these results are based. The hierarchical bias model used to estimate κ2, φ2 and V0 assumed random intervention effects within each meta-analysis, and can be expected to produce estimates of κ2 and φ2 that are smaller than those estimated from a fixed-treatment-effect model. These results should therefore be interpreted only as an approximate guide to the likely impact of bias adjustment in a new fixed-effect meta-analysis.
The present study in context with other literature
Because different meta-epidemiological studies have reported on similar associations (e.g. the association of inadequate or unclear concealment of allocation with intervention effect estimates), some authors have previously reported meta-analytic estimates combined across different meta-epidemiological studies. 14 The validity of such estimates would be undermined if different meta-epidemiological studies were reporting on the same meta-analyses, but the extent of overlap has until now been unclear. Our study provides some reassurance in that only 69 (21%) meta-analyses (492 trial results, 16%) were contained in more than one meta-epidemiological study that had both recorded trial results and assessed study design characteristics.
The overlap between different meta-epidemiological studies provided an opportunity to examine the reliability of assessments of sequence generation, allocation concealment and double blinding between pairs of contributing studies. Overall, we found good agreement. This is encouraging given our aim of conducting combined analyses, particularly as assessments were carried out completely independently, and because definitions were not completely consistent between the different contributing studies. Double blinding was the characteristic for which there was the highest inter-rater agreement; this may be because the presence of and the methods for blinding tend to be better reported than the methods of sequence generation and concealment. It is relatively easy to identify that a trial has been reported as either ‘double blind’ or ‘open’, whereas no such standard terms exist for the randomisation process. A recent study by Hartling et al. 40 assessed inter-rater agreement between two independent reviewers who applied the Cochrane Collaboration’s risk of bias tool on a convenience sample of 163 published randomised trials. They found moderate to good agreement for sequence generation, allocation concealment and blinding, but in contrast to our study they observed the highest agreement for sequence generation (kappa = 0.74, 95% CI 0.64 to 0.85) and lowest for the assessment of blinding (kappa = 0.35; 95% CI 0.22 to 0.47). This could be because the new Cochrane guidelines41 for risk of bias assessment state that it is inappropriate to pass a judgement of a low risk of bias if the study is merely described as ‘double blind’ without further details, thus rendering the assessment of blinding less straightforward. Accurate measurement of methodological characteristics is essential for the validity of meta-epidemiological studies. Given that the average effects of such characteristics are modest,6,16 non-differential misclassification may dilute or even extinguish them, whereas differential misclassification could create spurious effects.
Despite the large number of meta-epidemiological studies that contributed to the BRANDO study, only the three study design characteristics, sequence generation, allocation concealment and double blinding, were consistently assessed across these studies. The Cochrane Collaboration’s risk of bias tool, which was first published in 200841 and was recently updated,42 includes assessment of the risk of bias due to incomplete outcome data and selective reporting of outcomes. There is recent empirical evidence of bias in the results of RCTs as a result of both attrition43,44 and selective outcome reporting. 2,45,46 It is possible that our results were confounded by the influence of these or other types of bias, which could not be adjusted for. However, estimated effects of the study design characteristics analysed in our study were only modestly attenuated in adjusted analysis. The influence of study design characteristics may also vary in clinical areas that were not well represented in the BRANDO database. 47
Definitions of adequate sequence generation and allocation concealment were relatively consistent between the contributing studies (see Table 9). For allocation concealment, all studies adhered to the definitions originally formulated by Schulz et al. 11 In contrast, definitions used to assess a trial as double blind varied somewhat (see Table 9), with some being more strict11,14 than others: three studies9,12,21 considered a trial double blind merely if the trial report described it as double blind. However, we found that the assessment of double blinding was consistent between the contributing studies, with a median κ statistic of 0.87. 17 The term ‘double blind’ is problematic because at least three distinct groups (trial participants, trial personnel and outcome assessors) can potentially be blinded. Both physicians and textbooks vary in their interpretations and definitions of ‘single’, ‘double’ and ‘triple’ blinding. 48 We hope that future trials will report the blinding status of the different groups involved, as specified in the CONSORT statement,31,32 rather than using the vague term ‘double blind’. 49 The recent update of the Cochrane Collaboration’s risk of bias tool42 separates assessment of the risk of performance bias (adequacy of blinding of participants and personnel) from assessment of the risk of detection bias (adequacy of blinding of outcome assessors).
Implications for research
The database structure that we developed may have wide applicability in evidence synthesis research. It has the potential to ensure data integrity and consistency in situations in which it is necessary to store data from multiple systematic reviews, or to accommodate multiple publications and multiple study designs in large systematic reviews. The database structure can be modified to store information on a variety of study types and additional facilities for duplicate data entry and checking have also been developed for different projects using the basic structure.
We found that a lack of or unclear double blinding was associated with marked exaggeration of intervention effect estimates and increases in between-trial heterogeneity in trials with subjectively assessed outcome measures. This is consistent with there being more prominent placebo effects in such trials. 50,51 It is possible that placebo effects are themselves of clinical utility. It would be of great interest to examine separate effects of blinding of participants and personnel (performance bias) and blinding of outcome assessors (detection bias) once such assessments are available from large collections of trials and meta-analyses.
In contrast to previous studies,9,11 we found the influence of lack of double blinding to be greater than that of inadequate or unclear random sequence generation or allocation concealment. Our finding that the influence of these last two study design characteristics is most marked for trials with subjectively assessed outcomes was unexpected, although it is consistent with the results of Wood et al. 16 for allocation concealment. The purpose of random sequence generation and allocation concealment is to avoid selection bias, whereby knowledge of prognosis at the time of recruitment to a randomised trial influences the intervention group to which the patient is allocated. 52 Such selection bias would be expected to be greatest when it is easy to assess patients’ prognosis at the time they are recruited to a trial, and affect the results of trials with objectively assessed as well as subjectively assessed outcome measures. As shown in Table 12, presence of double blinding was associated with adequate allocation concealment but not with adequate sequence generation. The influence of sequence generation and allocation concealment in trials with subjectively assessed outcomes was little attenuated in multivariable analyses. Therefore, these effects may result at least in part from their association with subsequent flaws in the conduct of trials, in particular with biased outcome assessment, rather than from selection bias. Adequate sequence generation and allocation concealment may also be markers for other strategies for reducing bias, beyond blinding.
Ownership of the BRANDO database remains with the investigators of the contributing studies. However, the steering group would be happy to consider requests for access to the database once the main study results are published.
Recommendations for future research
Tools for assessing risk of bias in results of RCTs41,42 and guidelines for summarising the quality of evidence from systematic reviews53 should account for the findings of this study. In particular, it appears that when study design characteristics associated with bias prevention are not in place, trial results based on subjectively assessed outcome measures are at greatest risk of bias.
Practical and acceptable methods for correcting and downweighting the results of trials at high risk of bias in new meta-analyses should be developed. The trade-off between bias and variation is a familiar problem in statistics: our results suggest that exclusion of trials at high risk of bias leads to much greater decreases in precision than empirically based downweighting of such studies.
The influence of further study design characteristics should be explored in new meta-epidemiological studies based on more recently reported trials than are available in the BRANDO database. These include the influence of incomplete outcome data and selective reporting of outcomes, examining separate effects of blinding of participants and personnel (performance bias) and blinding of outcome assessors (detection bias) and the influence of characteristics specific to particular study designs (e.g. carry-over effects in crossover trials). It would also be of interest to examine clinical areas that are not well represented in the BRANDO data set.
Bayesian models could be extended to deal with misclassification of study design characteristics, based on empirical evidence such as that presented in Table 5.
Our results suggest that, as far as possible, clinical and policy decisions should not be based on trials in which blinding is not feasible and outcome measures are subjectively assessed. Therefore, trials in which blinding is not feasible should focus as far as possible on objectively measured outcomes, and should aim to blind outcome assessors.
Acknowledgements
We thank Pamela Royle and Matthias Egger for providing data and contributing to the initial stages of this project, and Pete Shiarly for his advice on database design and data management. Jelena Savović was partly funded by the Medical Research Council UK, grant number G0701659/1. Data contributed by Als-Nielsen et al. were from a study that was partly funded by the Danish Centre for Evaluation and Health Technology Assessment (DACEHTA).
Contribution of authors
Dr Jelena Savović (Postdoctoral Fellow, Epidemiology and Evidence Synthesis) contributed to the study design, designed the database, combined and harmonised data contributed by co-authors, removed overlapping trials and meta-analyses from the database, extracted additional data from reviews, carried out most of the data cleaning and data management, co-ordinated the study and contacts with all co-authors, carried out descriptive analyses, co-wrote the first draft and contributed to revisions of the manuscript.
Dr Hayley E Jones (Research Fellow, Medical Statistics, Bayesian Modelling and Evidence Synthesis) conducted most analyses, wrote sections of the first draft and contributed to the revisions of the manuscript.
Professor Douglas G Altman (Professor, Statistics in Medicine) played a key role in the design and planning, and in making strategic decisions throughout the project, and contributed to the database design and to harmonising and integration of the contributed data, and to the drafting and editing of the manuscript.
Ross J Harris (Statistician, Medical Statistics, Bayesian Modelling and Evidence Synthesis) carried out data manipulation and identification of overlapping trials, conducted initial analyses, refined the statistical model and contributed to the editing of the manuscript.
Professor Peter Jűni (Professor and Head of Division, Clinical Epidemiology and Biostatistics) provided raw data from his original study, advised on the interpretation and integration of data from his study into the combined database and on extraction and classification of additional data, and contributed to the steering of the project and to the editing of the manuscript.
Dr Julie Pildal (Specialist Registrar, Endocrinology and Research Synthesis) provided raw data from her original study, advised on the interpretation and integration of data from her study into the combined database and on extraction and classification of additional data, and contributed to the steering of the project and to the editing of the manuscript.
Dr Bodil Als-Nielsen (Specialist Registrar, Paediatrics) provided raw data from her original study, advised on the interpretation and integration of data from her study into the combined database and on extraction and classification of additional data, and contributed to the steering of the project and to the editing of the manuscript.
Dr Ethan M Balk (Associate Director, Clinical Evidence Synthesis) provided raw data from his original study, advised on the interpretation and integration of data from his study into the combined database and on extraction and classification of additional data, and contributed to the steering of the project and to the editing of the manuscript.
Dr Christian Gluud (Head of Department) provided raw data from his original study, advised on the interpretation and integration of data from his study into the combined database and on extraction and classification of additional data, and contributed to the steering of the project and to the editing of the manuscript.
Dr Lise Lotte Gluud (Consultant, Internal Medicine) provided raw data from her original study, advised on the interpretation and integration of data from her study into the combined database and on extraction and classification of additional data, and contributed to the steering of the project and to the editing of the manuscript.
Professor John PA Ioannidis (Professor and Director, Medicine, Health Research and Policy) provided raw data from his original study, advised on the interpretation and integration of data from his study into the combined database and on extraction and classification of additional data, and contributed to the steering of the project and to the editing of the manuscript.
Dr Kenneth F Schulz (Distinguished Scientist and Vice-President, Clinical Research Methodology) provided raw data from his original study, advised on the interpretation and integration of data from his study into the combined database and on extraction and classification of additional data, and contributed to the steering of the project and to the editing of the manuscript.
Rebecca Beynon (Research Associate, Evidence Synthesis) participated in study design, data extraction, entering and checking, and contributed to the editing of the manuscript.
Dr Nicky Welton (Senior Lecturer, Medical Statistics and Evidence Synthesis) refined the statistical model and contributed to the design of the statistical analyses, and contributed to the editing of the manuscript.
Dr Lesley Wood (Data Analyst, Evidence Synthesis) contributed to the development of the process of identifying and removing overlapping trials in the database, and to the drafting and editing of the manuscript.
Dr David Moher (Senior Scientist and Associate Professor, Epidemiology) provided raw data from his original study, advised on the interpretation and integration of data from his study into the combined database and on extraction and classification of additional data, and contributed to the steering of the project and to the editing of the manuscript.
Professor Jonathan J Deeks (Professor, Biostatistics) played a key role in the design and planning and in making strategic decisions throughout the project, and contributed to the database design and to harmonising and integration of the contributed data, and to the drafting and editing of the manuscript.
Professor Jonathan AC Sterne (Professor, Medical Statistics and Epidemiology) was the principal investigator, led the design and planning of the study, made strategic decisions throughout the project, supervised research staff working on the project, managed the project budget, developed the software to identify overlapping trials in the database, contributed to the design of the statistical analyses, co-wrote the first draft and contributed to revisions of the manuscript.
Publication
-
Savović J, Harris RJ, Wood L, Beynon R, Altman D, Als-Nielsen B, et al. Development of a combined database for meta-epidemiological research. Res Syn Meth 2010;1:212–25.
-
Savovic J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Ann Intern Med 2012;157:429–38.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- Altman DG, Schulz KF. Statistics notes: concealing treatment allocation in randomised trials. BMJ 2001;323:446-7.
- Chan AW, Hrobjartsson A, Haahr MT, Gotzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. J Am Med Assoc 2004;291:2457-65.
- Dickersin K, Min YI, Meinert CL. Factors influencing publication of research results. Follow-up of applications submitted to two institutional review boards. J Am Med Assoc 1992;267:374-8.
- Hopewell S, Loudon K, Clarke MJ, Oxman AD, Dickersin K. Publication bias in clinical trials due to statistical significance or direction of trial results. Cochrane Database Syst Rev 2009;1.
- Scherer RW, Langenberg P, von EE. Full publication of results initially presented in abstracts. Cochrane Database Syst Rev 2007;2.
- Gluud LL. Bias in clinical intervention research. Am J Epidemiol 2006;163:493-501.
- Naylor CD. Meta-analysis and the meta-epidemiology of clinical research. BMJ 1997;315:617-19.
- Sterne JA, Juni P, Schulz KF, Altman DG, Bartlett C, Egger M. Statistical methods for assessing the influence of study characteristics on treatment effects in ‘meta-epidemiological’ research. Stat Med 2002;21:1513-24.
- Egger M, Juni P, Bartlett C, Holenstein F, Sterne J. How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study. Health Technol Assess 2003;7.
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses?. Lancet 1998;352:609-13.
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. J Am Med Assoc 1995;273:408-12.
- Als-Nielsen B, Chen W, Gluud LL, Siersma V, Hilden J, Gluud C. Are Trial Size and Reported Methodological Quality Associated With Treatment Effects? Observational Study of 523 Randomised Trials n.d.
- Balk EM, Bonis PA, Moskowitz H, Schmid CH, Ioannidis JP, Wang C, et al. Correlation of quality measures with estimates of treatment effect in meta-analyses of randomized controlled trials. J Am Med Assoc 2002;287:2973-82.
- Pildal J, Hrobjartsson A, Jorgensen KJ, Hilden J, Altman DG, Gotzsche PC. Impact of allocation concealment on conclusions drawn from meta-analyses of randomized trials. Int J Epidemiol 2007;36:847-57.
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med 2001;135:982-9.
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ 2008;336:601-5.
- Savovic J, Harris RJ, Wood L, Beynon R, Altman D, Als-Nielsen B, et al. Development of a combined database for meta-epidemiological research. Res Syn Meth 2010;1:212-25.
- McAuley L, Pham B, Tugwell P, Moher D. Does the inclusion on grey literature influence estimates of intervention effectiveness reported in meta-analyses?. Lancet 2000;356:1228-31.
- Royle P, Milne R. Literature searching for randomised controlled trials used in Cochrane reviews: rapid versus exhaustive searches. Int J Technol Assess Health Care 2003;19:1-13.
- Sampson M, Barrowman NJ, Moher D, Klassen TP, Pham B, Platt R, et al. Should meta-analysts search EMBASE in addition to MEDLINE?. J Clin Epidemiol 2003;56:943-55.
- Contopoulos-Ioannidis DG, Gilbody SM, Trikalinos TA, Churchill R, Wahlbeck K, Ioannidis JP. Comparison of large versus smaller randomized trials for mental health-related interventions. Am J Psychiatry 2005;162:578-84.
- Siersma V, Als-Nielsen B, Chen W, Hilden J, Gluud LL, Gluud C. Multivariable modelling for meta-epidemiological assessment of the association between trial quality and treatment effects estimated in randomized clinical trials. Stat Med 2007;26:2745-58.
- Moja LP, Telaro E, D’Amico R, Moschetti I, Coe L, Liberati A. Assessment of methodological quality of primary studies by systematic reviews: results of the metaquality cross sectional study. BMJ 2005;330.
- Kennedy E, Song F, Hunter R, Clarke A, Gilbody S. Risperidone versus typical antipsychotic medication for schizophrenia. Cochrane Database Syst Rev 2000;2.
- Song F. Risperidone in the treatment of schizophrenia: a meta-analysis of randomized controlled trials. J Psychopharmacol 1997;11:65-71.
- Welton NJ, Ades AE, Carlin JB, Altman DG, Sterne JAC. Models for potentially biased evidence in meta-analysis using empirically based priors. J R Stat Soc Ser A Stat Soc 2009;172:119-36.
- Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS – a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput 2000;10:325-37.
- Lambert PC, Sutton AJ, Burton PR, Abrams KR, Jones DR. How vague is vague? A simulation study of the impact of the use of vague prior distributions in MCMC using WinBUGS. Stat Med 2005;24:2401-28.
- Gelman A. Prior distributions for variance parameters in hierarchical models. Bayesian Anal 2006;1:515-33.
- World Health Organization . International Statistical Classification of Diseases and Related Health Problems 10th Revision. Version for 2007 n.d. http://apps.who.int/classifications/apps/icd/icd10online/ (accessed 19 August 2010).
- Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340.
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340.
- Hopewell S, Dutton S, Yu LM, Chan AW, Altman DG. The quality of reports of randomised trials in 2000 and 2006: comparative study of articles indexed in PubMed. BMJ 2010;340.
- Huwiler-Muntener K, Juni P, Junker C, Egger M. Quality of reporting of randomized trials as a measure of methodologic quality. J Am Med Assoc 2002;287:2801-4.
- Hill CL, LaValley MP, Felson DT. Discrepancy between published report and actual conduct of randomized clinical trials. J Clin Epidemiol 2002;55:783-6.
- Soares HP, Daniels S, Kumar A, Clarke M, Scott C, Swann S, et al. Bad reporting does not mean bad methods for randomised trials: observational study of randomised controlled trials performed by the Radiation Therapy Oncology Group. BMJ 2004;328:22-4.
- Pildal J, Chan AW, Hrobjartsson A, Forfang E, Altman DG, Gotzsche PC. Comparison of descriptions of allocation concealment in trial protocols and the published reports: cohort study. BMJ 2005;330.
- Hrobjartsson A, Pildal J, Chan AW, Haahr MT, Altman DG, Gotzsche PC. Reporting on blinding in trial protocols and corresponding publications was often inadequate but rarely contradictory. J Clin Epidemiol 2009;62:967-73.
- Cochrane Reviewers’ Handbook 3.0.2 . The Cochrane Library. The Cochrane Collaboration 1997.
- Hartling L, Ospina M, Liang Y, Dryden DM, Hooton N, Krebs SJ, et al. Risk of bias versus quality assessment of randomised controlled trials: cross sectional study. BMJ 2009;339.
- Higgins JPT, Altman DG, Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. Chichester: John Wiley; 2008.
- Higgins JPT, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343.
- Nuesch E, Trelle S, Reichenbach S, Rutjes AWS, Burgi E, Scherer M, et al. The effects of excluding patients from the analysis in randomised controlled trials: meta-epidemiological study. BMJ 2009;339.
- Vestbo J, Anderson JA, Calverley PMA, Celli B, Ferguson GT, Jenkins C, et al. Bias due to withdrawal in long-term randomised trials in COPD: evidence from the TORCH study. Clin Respir J 2011;5:44-9.
- Dwan K, Altman DG, Arnaiz JA, Bloom J, Chan AW, Cronin E, et al. Systematic review of the empirical evidence of study publication bias and outcome reporting bias. PLoS One 2008;3.
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340.
- Nuesch E, Reichenbach S, Trelle S, Rutjes AWS, Liewald K, Sterchi R, et al. The importance of allocation concealment and patient blinding in osteoarthritis trials: a meta-epidemiologic study. Arthritis Rheum 2009;61:1633-41.
- Devereaux PJ, Manns BJ, Ghali WA, Quan H, Lacchetti C, Montori VM, et al. Physician interpretations and textbook definitions of blinding terminology in randomized controlled trials. J Am Med Assoc 2001;285:2000-3.
- Montori VM, Bhandari M, Devereaux PJ, Manns BJ, Ghali WA, Guyatt GH. In the dark: the reporting of blinding status in randomized controlled trials. J Clin Epidemiol 2002;55:787-90.
- Hrobjartsson A, Gotzsche PC. Is the placebo powerless? An analysis of clinical trials comparing placebo with no treatment. N Engl J Med 2001;344:1594-602.
- Krogsboll LT, Hrobjartsson A, Gotzsche PC. Spontaneous improvement in randomised clinical trials: meta-analysis of three-armed trials comparing no treatment, placebo and active intervention. BMC Med Res Methodol 2009;9.
- Forder PM, Gebski VJ, Keech AC. Allocation concealment and blinding: when ignorance is bliss. Med J Aust 2005;182:87-9.
- GRADE Working Group n.d. www.gradeworkinggroup.org (accessed 15 August 2011).
Appendix 1 Protocol
This HTA report describes the work conducted by the workstream A, as designated in the original funding proposal. The report of the work carried out by the workstream B will be published separately.
I. SUMMARY OF PROPOSAL
TITLE OF PROJECT
Quantification of bias in randomised controlled trials and non-randomised studies: implications for systematic reviews and evidence synthesis
APPLICANTS (NOTE: Section IV should also be completed for ALL applicants)
| Surname(s): | Sterne |
| Forename(s): | Jonathan A C |
| Title: | Dr |
List separately each individual involved in the research project, giving their name, title, and responsibility:
| Name | Jon Deeks |
| Name: | Douglas Altman |
| Name: | Matthias Egger |
| Name: | David Moher |
| Name: | Lise Gluud |
| Name: | John Ioannidis |
| Name: | Kenneth F. Schulz |
| Name: | Pamela Royle |
| Name: | Julie Pildal |
SUMMARY OF RESEARCH
ABSTRACT OF RESEARCH. No more than 200 words covering the following topics: aims of project; research subject group; sample size, type and location; methods of working.
This project aims to examine the importance of different types of bias and other sources of variability in estimates of the effect of health care interventions, in different settings and areas of medicine. An existing database will be extended to combine data from all existing ‘meta-epidemiological’ studies of the effect of methodological quality and other trial characteristics on intervention effect estimates in randomised controlled trials (RCTs). Based on the results of a review conducted by the applicants, we will also assemble a new database that will be used to compare results of RCTs and non-randomised studies (NRS) that estimated the effects of comparable interventions on comparable outcomes. We will use a database of NRS examining the association of diet and physical activity with cancer to quantify differences in the magnitude of associations between types of non-randomised study. Statistical methods developed by the applicants will be extended to quantify differences in intervention effect estimates and the between-meta-analysis variability in these differences. These will lead to further development of guidelines for the conduct and reporting of RCTs and NRS, and to evidence-based methods for combining results of RCTs of varying quality, and RCTs and NRS, in systematic reviews of health care interventions.
II. DETAILS OF PROPOSED RESEARCH
Background
Bias in randomised controlled trials
Randomised controlled trials (RCTs) provide the best evidence in the evaluation of medical interventions. However, the number of patients included in trials is often inadequate and single trials may fail to detect, or exclude with certainty, a modest but medically important difference in the effects of two therapies. 1,2 An examination of clinical trials which reported no statistically significant differences between experimental and control therapy showed that false negative results in health care research are common: for a medically important difference in outcome the probability of missing this effect given the trial size was greater than 20% in 115 (85%) of the 136 trials examined. 1 Similarly, a recent examination of 1941 trials relevant to the treatment of schizophrenia showed that only 58 (3%) studies were large enough to detect a modest but important improvement. 3 Although there is some evidence that the number of patients included in trials published in general health care journals has increased,4 under-powered trials continue to be published in large numbers. 5
Systematic reviews and meta-analyses aim to address this situation. Statistical combination of results from several small trials in meta-analysis will increase the precision of estimated treatment effect, reduce the probability of ‘false negative’ results, and potentially lead to more timely introduction of effective treatments. 6 Systematic reviews and meta-analyses provide the basis for development of sound clinical practice guidelines and, in general, for evidence based health care. 7,8
Systematic reviews and meta-analyses are not immune to bias, however. The majority of published meta-analyses are based on relatively few, small trials. 9,10 Small trials tend to show bigger treatment effects than larger trials, a difference that may be due to publication bias and related reporting biases such as time lag bias or language bias, or bias due to inadequate methodological quality, both of which are more common among small trials. Such bias may distort the results from meta-analyses. 11,12 Bias in randomised controlled trials research has been examined by considering collections of meta-analyses in which component trials are classified according to characteristics such as study quality or publication type. The landmark study by Schulz et al. 11 pioneered this approach, for trials with binary outcomes included in meta-analyses from the Cochrane Pregnancy and Childbirth Database. Since then several other studies,12–19 have examined the influence of different types of biases on the results of systematic reviews and meta-analyses of randomised controlled trials. Such studies, which are based on meta-regression analyses of several meta-analyses, have been termed ‘meta-epidemiological’ studies to distinguish them from standard meta-analyses. 20 The applicants include investigators from all identified studies of this type. 12–19,21–23
These studies have so far identified two dimensions of trial quality that are empirically associated with bias: concealment of the allocation sequence (to prevent selection bias) and blinding (keeping the trial participants, care providers and outcome assessors unaware of which intervention is being administered). 11,12,15 Biased dissemination of the results of trials (publication bias, language bias) may also affect meta-analyses. 24 For none of these factors are the data unequivocal, however:17 more work is needed to increase confidence in the findings. Further, the relative importance of different sources of bias is unclear at present. A recent comparison of results from these studies indicated that trial quality appears to be more important than the reporting and dissemination of results. 25 However, such comparisons are problematic. First, the definitions of allocation concealment, double blinding etc. varied to some extent across studies. Second, there is a degree of overlap between studies in the meta-analyses included. Third, most studies used logistic regression models in which the evidence for interaction between the effects of trial quality and intervention group is examined, after controlling for the interaction between meta-analysis and treatment group. This assumes that the effect of bias is constant across meta-analyses. If this assumption is false then standard errors of estimated differences will be too small. We have proposed new methods that address these problems, and shown that both within and between meta-analysis heterogeneity may be of importance. 20 These methods require further development, in particular to control for confounding effects of different trial characteristics within a modelling framework that allows appropriately for between meta-analysis variability in the effects of trial characteristics. It would also be desirable to extend this work to different effect measures (for example mean difference, hazard ratio, risk ratio) in addition to odds ratios.
Such influences are likely to vary according to clinical context, but none of the existing studies was large enough to examine this. Specific issues apply in some specialties for which we have limited data, for example surgery and vaccination. Furthermore, much of the literature included in the existing studies is rather old, with a minority of studies from the 1990s. It would be interesting to examine whether improved reporting of trials since the CONSORT statement (Consolidated Standards for the Reporting of Randomised Trials)26,27 has changed the impact of poor trial quality on meta-analyses, although inevitably the number of post-CONSORT trials included in published meta-analyses is relatively small. In interpreting trends over time it will be important to realise that CONSORT deals mainly with reporting of trials and may have changed reporting behaviour rather than actual conduct. This could lead to the attenuation of apparent associations of trial quality with intervention effect estimates over time.
More information on the epidemiology of bias in randomised clinical trial research is essential to provide a reliable methodological underpinning for unbiased conduct and appropriate interpretation of systematic reviews, meta-analysis and guidelines. Meta-analyses have sometimes been found to produce misleading results. 28,29 At present, unreliable answers are being generated by small randomised studies of doubtful quality and meta-analyses based on such trials give the illusion of reliability. 30 These trials and meta-analyses are nevertheless often the best available evidence, and as such are used to guide decision making in clinical practice. The collaborative research proposed here brings together the leading investigators in the field, and will represent the most comprehensive study ever done in this area. A number of new research questions will be addressed. The results will have considerable impact on clinical trials research, the practice and interpretation of systematic reviews and meta-analysis, and the delivery of effective health care.
Bias in non-randomised studies
Several scenarios remain under which an RCT may be unnecessary, inappropriate, impossible or inadequate. 31 Examples include the assessment of rare side-effects of treatments, some preventive interventions and policy changes. Furthermore, there must be hundreds of examples of interventions for which RCTs would be possible but have not yet been carried out, leaving the medical and policy community to rely on non-randomised evidence only. There are instances where non-randomised studies have either been sufficient to demonstrate effectiveness, or where they appear to have arrived at results similar to those of RCTs. However, where randomisation is possible, most agree that the RCT should be the preferred method of evaluating effectiveness. 32–34 The risks of relying solely on non-randomised evidence include failing to convince some people of the validity of the result, or successfully convincing others of an incorrect result. 33 It would be of value to have clear estimates of the degree to which non-randomised studies may be biased, and whether the bias is consistent across clinical settings, to assist interpretation of non-randomised evidence.
There is inconsistent use of nomenclature when describing non-randomised studies (NRS), and other taxonomies may apply different definitions to the same study designs. To attempt to avoid the problems of inconsistent terminology, six features can be identified that differentiate between these studies. First, some studies make comparisons between groups, whilst some simply describe outcomes in a single group (e.g. case series). Second, comparative designs differ in the way that participants are allocated to groups, varying from the use of randomisation (RCTs), quasi-randomisation, geographical or temporal factors (comparative cohort studies), the decisions of health care professionals (clinical database cohorts), to the identification of comparison groups with specific outcomes (case–control studies). Third, studies differ in the degree to which they are prospective (and therefore planned) or retrospective, for matters such as the recruitment of participants, collection of baseline data, collection of outcome data and generation of hypotheses. Fourth, the method used to investigate comparability of the groups varies: in RCTs no investigation is necessary (although it is often carried out), in controlled before-and-after designs baseline outcome measurements are used, whilst in cohort and case–control studies investigation of confounders is required. Fifth, studies differ in the level at which the intervention is applied: sometimes it is allocated to individuals, other times to groups or clusters. Finally, some studies are classified as experimental whilst others are observational. In experimental studies the study investigator has some degree of control over the allocation of interventions. Most importantly he/she has control over the allocation of participants to intervention groups either using randomisation of participants, or haphazard allocation by alternation, dates of birth, day of the week or case record numbers. In observational studies, on the other hand, the groups that are compared are generated according to variation in the use of interventions that occurs regardless of the study. When allocation is determined largely by health professionals, the treatment decision is based not only on ‘hard’ data such as age, sex and diagnostic test results, but on ‘soft’ data including type and severity of symptoms, rate of development of the illness, and severity of any co-morbid conditions, which are rarely made explicit. 35 Allocation in non-randomised studies may also be based on factors such as availability of care or geographical location. In non-randomised studies, therefore, there are likely to be systematic differences in the case-mix of patients in the intervention and comparison groups. Furthermore, some participants may not have been eligible for all the treatments being considered in the study.
The degree to which non-random allocation methods are susceptible to selection bias and therefore may produce biased estimates of the effect of treatment is not clearly understood, although it seems likely that the potential for bias will vary between clinical areas. It is reasonable to expect that the comparability of the groups in terms of prognostic factors, and the extent to which prognosis influences both selection for treatment and treatment outcome, will be of particular relevance. For example, in evaluations of childhood vaccines there are few indicators of prognosis that could be used to influence allocation, so randomisation may not be necessary (although there are dangers that allocation in clusters could be confounded by exposure to infectious disease, which means that in practice randomisation is recommended). By contrast, when patient factors could have a strong influence on allocation or where prognosis is strongly linked to outcomes (such as in cancer treatment), then randomisation is likely to be extremely important.
The use of meta-epidemiology has been informally extended from the comparison of design features of RCTs to comparisons between different study designs. A recent HTA report for which one of the applicants was lead author36 identified 8 empirical studies37–44 (7 from the medical field) that compared the results of RCTs with those from non-randomised studies across multiple interventions to estimate the bias removed by randomisation. Between study-design comparisons were noted to be particularly challenging because of the magnitude of potential meta-confounding of study design with differences in participants, interventions, outcomes and other features of study design, and because of the likelihood that any bias would act to inflate the variability of estimates as well as acting in a systematic manner.
The conclusions of the eight comparisons of RCTs and NRS are divergent, and all the comparisons were noted to have methodological weaknesses. There are issues as to whether identification of included studies was likely to be biased, the similarity of participants, comparability of interventions and outcome measures between RCTs and NRS, whether study methodology was similar in all respects other than the allocation mechanism, and the definitions used to assess whether results of RCTs and NRS revealed differences or were similar. The only robust conclusion that can be drawn is that in some circumstances the results of randomised and non-randomised studies differ, but it cannot be proved that differences are not due to other confounding factors. The frequency, size and direction of the biases cannot be judged reliably from the information presented in the literature to date. The current study will contribute to clarifying these issues and inform the interpretation of single NRS and systematic reviews and meta-analyses of NRS. The results will also inform the development of guidelines for the reporting of NRS, the Standards for the Reporting of Observational Studies in Epidemiology (STROBE) initiative, which is led by two of the applicants (Matthias Egger, Douglas Altman) and is supported by the Research Methodology Programme.
Aim
To examine the importance of different types of bias and other sources of variability in estimates of the effect of health care interventions, in different settings and areas of medicine.
Objectives
Overall objectives:
-
To implement and enhance recently developed statistical methods to quantify the implications for systematic reviews of the estimated mean and variance of the bias associated with different study characteristics, before and after controlling for confounding between different sources of bias.
-
To examine:
-
the extent of associations between the different dimensions of methodological quality, publication bias and other reporting biases and the degree of confounding between them
-
the relative importance of different biases and study characteristics in terms of distortion of combined effect estimates
-
the relative importance of different biases and study characteristics as sources of between-study heterogeneity
-
the implications of bias and other sources of between-study heterogeneity for the conduct and analysis of systematic reviews of the effect of health care interventions.
-
-
To inform the development of improved guidelines for the conduct and reporting of randomised controlled trials and non-randomised studies.
Workstream A: Bias and other sources of variability in the results of randomised controlled trials
-
To extend and analyse a combined database of randomised trials included in meta-analyses that have been assessed according to standard methodological criteria. As part of this process, to refine definitions of blinding and intent to treat analysis, taking into account the feasibility of blinding patients, care givers and outcome assessors, and the explicit reporting, or otherwise, of exclusions from the analysis.
-
For comparisons of randomised controlled trials: to estimate the mean and variance of the bias comparing:
-
adequately concealed trials with trials where concealment was inadequate and/or remained unclear
-
trials using different degrees of blinding with open trials, and whether the importance of blinding varies between trials with ‘hard’ outcomes (e.g. mortality) and those with more subjective outcomes such as pain relief or patient satisfaction with the treatment regimen.
-
trials analysed according to the intent to treat principle with other trials
-
mode of generation of the randomisation sequence
-
trials with varying lengths and completeness of follow up
-
trials where the sponsor does and does not have a commercial interest in the results in different fields of medicine, different types of trial and different settings defined by the presence or absence of known prognostic factors. It should be noted that although some of these differences have already been estimated in published studies, there is little or no published information on the between-meta-analysis variation in these differences.
-
Workstream B: Bias and other sources of variability in the results of non-randomised studies, compared to randomised controlled trials
-
To use a standardised assessment procedure to assemble a new database of sets of randomised trials and non-randomised studies that compared the effect of the same intervention on the same outcome.
-
For comparisons of randomised controlled trials with non-randomised studies estimating the same intervention effect: to estimate the mean and variance of the bias comparing:
-
randomised and non-randomised studies
-
different types of non-randomised studies
-
different strategies for dealing with confounding (e.g. logistic regression, propensity score)
-
different settings defined by the presence or absence of known prognostic factors (e.g. trials in oncology versus vaccine trials, or paediatric trials, which tend to be particularly small).
-
-
To inform the development of guidelines for the conduct and reporting of randomised controlled trials and non-randomised studies, and systematic reviews of these studies.
Research methods
The project will involve the construction and analysis of two large databases to be used for empirical research on bias and other sources of variability in estimates of the effect of health care interventions. The first database will contain information on the characteristics and results of randomised controlled trials included in meta-analyses, and will be the responsibility of the research associate to be based in Bristol and directly supervised by Jonathan Sterne (see Workstream A below). The second database will contain information on the characteristics and results of randomised controlled trials and non-randomised studies that evaluated comparable interventions using comparable outcomes, and will be the responsibility of the research associate to be based in Oxford and directly supervised by Jon Deeks (see Workstream B below). Data analyses, using the methods described in the section on data analysis, will be the responsibility of the project statistician, supervised by Jonathan Sterne, Jon Deeks and Douglas Altman.
Although there appears to be symmetry between Workstreams A and B, in reality there are some important differences. In A we will be looking at effect modifiers (methodological and other) within the class of RCTs to explain variation among RCTs. In B we will examine the magnitude, extent of and reasons for variation between RCTs and NRS, and perhaps also within different types of NRS. Several good studies of effect modifiers in RCTs (the studies in Table 1) have already been published, and we propose to build on this work. In contrast, there is (to our knowledge) no published evidence for B. Although there are several studies comparing RCTs and NRS (Table 2), none looked in detail at the characteristics of the RCTs and NRS included in those reviews, and there is little information on the magnitude of effect modification comparing RCTs and NRS or between different designs of NRS. Given that Workstream B starts from a much lower knowledge base than Workstream A and also is more complex than Workstream A, the objectives for B are more limited than for A.
| Study | Main comparison | Clinical topic | No. of meta-analyses | Trials appearing in > 1 component meta- analysis excluded? | No. of trials | Year of trial publication | Source of meta-analyses |
|---|---|---|---|---|---|---|---|
| Schulz et al.11 | Study quality (allocation concealment, randomisation sequence generation method, blinding and exclusion of patients) | Pregnancy & childbirth | 33 | Yes, if several overlapping trials. For minor overlap a single trial was removed | 250 | 1955–1992 | Reviews from Cochrane Pregnancy & Childbirth Group with ≥ 5 trials containing ≥ 25 events in the control group, and at ≥ 1 trial with and one without adequate allocation concealment |
| Moher et al.12 | Study quality (allocation concealment method, randomisation sequence generation method, blinding and exclusion of patients) | Circulatory & digestive, mental health, pregnancy & childbirth | 11 | Yes | 127 | 1960–1995 | Random selection of meta-analyses from large database |
| Kjaergard et al.15 | Large and small trials according to quality (sequence generation, concealment, blinding and exclusion of patients) | Various, including cardiac, childbirth, schizophrenia, smoking cessation | 14 | Yes, overlapping meta-analyses were excluded | 190 | 1960–1998 | Meta-analyses in the Cochrane Library, MEDLINE or PubMed with at least one trial with more than 1000 patients |
| McAuley et al.,13 Moher et al.14 | Grey literature, and trials published in languages other than English | Various, including GI, cardiac, infection, reproduction, circulatory | 41 (grey literature), 18 (language) | Yes | 467 (grey literature), 178 (language) | Min. range 1984–1990 | Random selection of meta-analyses from large database |
| Jüni et al.,16 Egger et al.18 | Grey literature, non-English languages, indexing in MEDLINE, study quality (concealment of allocation and blinding) | Any | 39 to 60 | Yes, trials that appeared in > 1 meta-analysis were counted only once | From 304 (concealment) to 783 (grey literature) | 1955–1998 | Meta-analyses from 8 specified journals, HTA reports, DARE and Cochrane Database of Systematic Reviews that had performed comprehensive literature searches |
| Balk et al.17 | Study quality (including 28 distinct quality measures) | Cardiovascular disease, infectious diseases, pediatrics, surgery | 26 | No overlap | 276 | 1955–2000 | MEDLINE and Cochrane Database of Systematic Reviews; for cardiovascular diseases, database from Lau et al. NEJM 1992 |
| Contopoulos-Ioannidis et al.22 | Study quality (allocation concealment, blinding, sequence generation), also compared large and small trials | Mental health | 16 | For overlapping reviews, retained the latest with most complete information | 133 | 1960–2002 | Cochrane Database of Systematic Reviews, DARE, Mental Health Library (415 reviews screened) |
| Royle23 | Indexing in major bibliographic databases and (a) concealment method and (b) number of patients enrolled | Any | 29 | Meta-analyses not examined for duplicate trials | 541 | Not stated | Cochrane reviews new to 2001 Issue 1 that included at least 1 unindexed trial |
| Pildal (unpublished) | The % conclusions that remain supported if only adequately concealed trials are considered. (Data on blinding & sequence generation are available) | Any | 70 (34 allowed subgroup analysis) | 3 trials re-occurred in two meta-analyses that comprised 6 and 17 each but addressed different outcomes | 499 | Pending, will be available | Recent reviews stating a conclusive preference supported by a statistically significant binary outcome. 32 were from PubMed and 38 from Cochrane Library |
| Sacks | Kunz | Britton | MacLehose | Benson | Concato | Ioannidis | |
|---|---|---|---|---|---|---|---|
| Comparisons | 6 | 23 (11) | 18 | 14 (38 outcomes) | 19 | 5 | 45 |
| Non-randomised study designs included | Historically controlled trials (HCTs) | Quasi-experiments, historically controlled trials (HCTs), patient preference trials | Quasi-experiments, natural experiments and prospective observational studies | Quasi-experimental and observational studies | Observational studies | Case–control and cohort studies | Quasi-experiments, cohort and case–control studies |
| Number of RCTs/NRS | 50/56 | 263/246 | 46/41 | 31/68 | 83/53 | 55/44 | 240/168 |
| Method of identification of comparisons | Primary studies: Personal systematic collection of RCTs and HCTs in specific fields of interest | Secondary studies: Electronic and manual search for studies comparing randomised and non-randomised studies | Primary and secondary studies: Electronic searches for studies comparing randomised and non-randomised groups | Primary and secondary studies: Electronic and manual searches for studies comparing randomised and non-randomised groups | Primary studies: Electronic search of MEDLINE and CCTR for observational studies and matching RCTs | Secondary studies: Meta-analyses published in 5 leading journals that included non-randomised studies | Secondary studies: Meta-analyses located by electronic searches (MEDLINE and Cochrane library) and manual searches |
| Similarity of patients, interventions and outcomes | Same intervention for the same medical condition | 2 controlled for clinical differences in participants and interventions, 6 did so partly, and 7 did not at all. | Same intervention, similar setting, similar control therapy, comparable outcomes measures | Same intervention. Similarity of eligibility, time period, co-morbidity, disease severity and other prognostic factors assessed | Restricted to interventions allocated by physicians to induce comparability | Not assessed | Not assessed |
| Similarity of other study methods | Not assessed | 5 papers judged partly comparable, 10 not comparable on double blinding, complete follow-up and other methodological issues | Not assessed | Blinding of outcome assessed | Not assessed | Analysis according to study quality mentioned in discussion, but no methods or results presented | Not assessed |
| Method of summarising study findings | Vote counting of results classified as positive (either statistically significant or had positive conclusions if no statistical analysis) | No consistent summary: results of randomised and non-randomised groups described using a variety of measures | Results of randomised and non-randomised groups described using risk differences, risk ratios or odds ratios | Risk ratios and risk differences calculated separately for MAs for randomised and non-randomised studies | Calculation of overall meta-analytical results and confidence intervals (odds ratios for binary data, differences in means for continuous) | Calculation of overall meta-analytical results and confidence intervals (risk ratios and odds ratios) | Calculation of fixed and random meta-analytical estimates expressed as odds ratios and log odds ratios |
| Method for comparing results between groups | Comparison of the percentage with positive results | No overall analysis presented | Statistical significance of the difference in effect sizes | Distribution of relative and absolute differences in results reported | Assessment of whether observational point estimate fell within 95% CI for RCTs | No overall analysis presented | Calculation of Z-scores for difference between treatment effects |
| Criteria for comparing variability of results | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed | Dispersion of points calculated without considering differences in sample size | Significance (p < 0.1) of tests of between-study heterogeneity |
Workstream A: Bias and other sources of variability in the results of randomised controlled trials
Identification of relevant studies
Individual patient data (IPD) meta-analyses which involve obtaining individual information or ‘raw data’ on all patients included in each of the trials included in the review have, in a number of cases, produced definitive answers which might not have been obtained in any other way. 45,46 Similarly, in the realm of empirical studies of bias, a collaborative individual trial data (ITD) meta-analysis will overcome the problems mentioned above by eliminating duplicates, standardising definitions and collaboratively developing an appropriate strategy for the analysis of the common database.
We have identified 11 relevant meta-epidemiological studies of randomised trials: 10 are published11–19,23 and 1 is ongoing. 21 The lead investigators of all of these studies (characteristics summarised in Table 1) have agreed to participate in this project. Meta-analyses included in these studies were mainly identified from Cochrane reviews, from existing databases or from searches of medical journals. Although definitions used in published articles differed it was feasible to establish common definitions for dimensions of trial quality.
Assembly of database for empirical research
Data sets from 7 studies11,13,15,16,18,19,23 have been combined to form a comprehensive database of systematic reviews and trials. This contains a common set of variables based on agreed definitions. This work has been done in Bristol as part of an MRC-supported PhD project by Mrs Lesley Wood (who has agreed to collaborate on the project); and has been further supported by a grant from the Swiss National Science Foundation.
The unique PMID identifier from PubMed/MEDLINE was assigned to all published meta-analyses and trials from these datasets whenever such an identifier was available. Where there is no PMID the unique identifier from EMBASE was used if available; if not then a unique database ID was assigned. The common database includes 2948 unique trials in 264 unique meta-analyses of which 174 (59%) were extracted from the Cochrane Database of Systematic Reviews. There was surprisingly little duplication: only 363 trials and 23 meta-analyses appeared in two or more of the studies. The original papers for duplicated trials and meta-analyses have been obtained. However, there were differences in the definition of components of trial quality between meta-epidemiological studies: for example, a paper that described allocation concealment using sealed envelopes without providing further details might or might not be coded as adequately concealed.
We will extend the database to include all the additional studies that have been identified, and aim to continue to maintain and update the database as required for future studies.
Extraction of additional data
We will compile a complete set of the original publications and extract additional information on trials or reviews where current information is inconsistent or incomplete. This process will be informed by recent research and debate on definitions of components of quality such as blinding. 47 For some trial characteristics (e.g. whether published and language of publication) it will be relatively straightforward to add information, while other study characteristics, such as length and completeness of follow up, have not been considered in any of the existing studies and will therefore be more difficult to add. If accumulation of complete data is not cost-effective we will collect information on a subset of trials and/or meta-analyses.
We will identify areas in which the identification and inclusion of additional meta-analyses and trials is required to address our objectives. For example, we may need to identify additional meta-analyses in surgery and of vaccine trials, or to include recently published meta-analyses. We intend to examine at least nine areas in conventional medicine (cardiovascular, oncology, obstetrics and gynaecology, rheumatology, psychiatry, paediatrics, infectious diseases, surgery and vaccine trials). Similarly, we may have to add meta-analyses of trials with certain types of outcome measures or interventions to address some of the objectives. We will adopt a strategic approach and make optimal use of the information available from the existing studies. However, we anticipate that, in addition to collecting additional information on trials and meta-analyses already in the database, we will be able to add approximately 20 meta-analyses and 200 trials to the database.
Investigations
The primary comparison will be between results of RCTs with different characteristics (e.g. components of methodological quality). We will use statistical methods developed by the applicants (see below) to estimate the average effect of the characteristics, the overall variability in these effects and the extent to which such variability is explained by factors such as medical specialty or the presence of known prognostic factors. These analyses will also investigate the extent of confounding between different study characteristics (for example low quality studies may also be less likely to be published). We will quantify the implications of our findings for systematic reviews in which studies of varying quality are located.
Workstream A will be conducted primarily by the research associate based in Bristol, supervised by Jonathan Sterne and Matthias Egger, with additional regular input from Jon Deeks, Douglas Altman and other applicants.
Workstream B: Bias and other sources of variability in the results of non-randomised studies, compared to randomised controlled trials
There are major challenges in investigating differences between the findings of randomised and non-randomised studies. In what follows we outline the issues that we wish to examine, but we realise that it will not be possible to resolve them all. The main impediments are the subjectivity of some of the judgements (e.g. the comparability of interventions in randomised and non-randomised settings) coupled with the quality of the available information in published reports of the primary studies. Thus, the planned analyses will only be carried out if the available data make them possible. By attempting to address these difficult issues we will help to clarify what is possible and what is not possible in this important area of research.
Identification of relevant studies
Empirical studies comparing randomised and non-randomised studies were identified as part of the published HTA report. 36 Seven studies in medical fields were found, evaluating a total of 82 interventions after accounting for duplication between studies (characteristics summarised in Table 2). 37–43 A separate database will be constructed along the same lines as for randomised trials. Here, additional fields will identify the study design (RCT, cohort study, case–control study, etc.) and other characteristics relevant to non-randomised studies. We will search for additional publications that have compared results of RCTs and NRS within multiple meta-analyses, and for other studies that have compared the results of RCTs and NRS. 48 One important and untapped resource for location of such studies is the Evidence-based Practice Center evidence reports (http://www.ahcpr.gov/clinic/epcix.htm). Currently there are 13 EPCs in North America. Their remit is to conduct systematic reviews in a very wide variety of topics. Approximately 90 reviews, which include both randomised and non-randomised studies, have been completed, and these reviews have been able to ‘control’ (ensure comparability of) the intervention between RCTs and NRS.
Assembly of databases for empirical research
Workstream B will produce a similar common database for the meta-analyses of randomised and non-randomised studies included in the 7 existing empirical reviews of bias associated with non-randomisation. However, as the 7 empirical studies each included and assessed different dimensions of study design, execution and comparability of groups, the original publications for the included randomised and non-randomised studies will each be retrieved and reassessed using a standard protocol. In this work we will draw on the suggestions of Ioannidis and Lau. 49
A substantial database containing the results of different types of NRS is being constructed during a systematic review of the relationship of diet and physical activity with bladder, prostate, kidney and skin cancer currently underway in Bristol (principal investigators Jonathan Sterne and George Davey Smith). All published information on associations between any aspect of diet or physical activity is being extracted and will be converted to estimates of the association between these exposures and the specified cancer. We have recorded both the type of NRS and key aspects of study quality (for example in case–control studies whether the control group was randomly sampled from a relevant population). Results of the studies are being transformed into common estimates of dose–response effect, according to an algorithm developed by Jonathan Sterne, Matthias Egger and colleagues. This database, which will contain the results of at least 500 NRS, will be used to investigate differences between the results of NRS according to type of study, methodological quality and extent of control for confounding factors.
Extraction of data
An assessment protocol will be developed and its reliability assessed on a sample of studies. Once consensus on the content and wording of the tool is agreed, approximately 300 RCTs and 300 non-randomised studies will be assessed and included in the database of RCTs and NRS. The database of RCTs will be linked with the Workstream A database to check whether any trials have already been assessed.
One area requiring development and detailed analysis will be assessing the similarity of interventions, populations and outcomes, as these may be major sources of confounding which prevent the impact of non-randomisation per se being evaluated. For example, a RCT that examined the effect of vitamin E supplementation might be considered comparable to a NRS in which the dominant source of vitamin E was supplements, but not to a NRS in which vitamin E was mainly of dietary origin. Another issue that is much less simple than for RCTs is to consider dimensions of methodological quality of NRS. The choice of aspects to consider will be informed by the review of Deeks et al. 36
A second question of interest is whether bias in non-randomised allocation is related to the health care context. The most important aspect is probably the degree to which individual prognoses of study participants is predictable. The magnitude of bias that can be introduced in allocation is limited by the accuracy with which prognoses can be made. In studies of primary prevention interventions, such as infant vaccination programs, little prognostic information is known, whereas in studies in the treatment of cancer detailed prognostic information is available. A method of classifying clinical topics according to the amount of prognostic knowledge the researcher can have will be developed, as far as possible blind to knowledge of the comparisons being studied, and used to stratify analyses comparing randomised and non-randomised allocation methods. (The same classification could also be investigated as a possible explanation for inconsistent results of the impact of methodological aspects of RCTs, such as allocation concealment, in Workstream A.) Additional concerns are the difference between alternative experimental and observational methods of constructing non-randomised control groups and the impact of different case-mix adjustment methods in NRS.
Investigations
The primary comparison will be between average results in RCTs with average results in comparable NRS. We will investigate the extent to which candidate predictors of effects of intervention among NRS (e.g. control of confounding, type of study) influence the comparison. We will need to consider the impact of methodological quality of the RCTs in making this comparison. In addition, we will investigate the possibility of greater heterogeneity among NRS after allowance for effect modifiers. A side-product of these analyses will be insight into possible differences between results from different types of NRS (e.g. case–control vs cohort).
With respect to the assessment of the nature of the interventions, two alternative analyses will be undertaken: (a) a restricted analysis where similarity of confounding factors is demonstrated; (b) an unrestricted analysis where all comparisons are included. This approach will allow (a) estimation of biases directly linked to allocation method, and (b) a more pragmatic assessment of the overall differences in findings between randomised and non-randomised evaluations.
The database on studies of diet and cancer will be used to estimate the association between characteristics of NRS, including type of study, methodological quality and extent of control for confounding factors, in the particular context of the association of diet and physical activity with the development of cancer.
We will quantify the implications of our findings for systematic reviews in which estimates of the effect of health interventions are available from both RCTs and NRS.
Workstream B will be conducted primarily by the research associate based in Oxford. Jon Deeks and Douglas Altman will provide day-to-day supervision, with additional regular input from Jonathan Sterne, Matthias Egger and other applicants.
Statistical methods and data analysis
We first describe statistical methods relevant to the analysis of meta-epidemiological studies. We then state the broad aims of the statistical analyses. Predicted outputs from the analyses appear in a separate section.
Standard logistic regression models
Suppose that we have data from M meta-analyses, containing a total of S studies. To estimate the effect of a binary study characteristic C (for example C = 1 in published trials, 0 in unpublished trials) on estimated treatment effects we fit the model:
where π is the probability that an (adverse) outcome event is observed, It, Itc, {Itmi} and {Isj} are all indicator variables denoting, respectively, the effects of treatment, the treatment–characteristic interaction, the treatment–meta-analysis interactions and study number, and {β}, {γ} and {δ} are the parameters of the logistic regression model. 20 This model allows the probability of the outcome event to vary according to treatment group, trial characteristic and trial, while the interaction terms {Imi} mean that the effect of treatment is estimated separately in each meta-analysis. The estimated effect of the characteristic C on average treatment effects is then given by parameter β2 (treatment–characteristic interaction), which estimates the log of the ratio of treatment odds ratios (ROR) in trials with and without the characteristic. This is assumed to be constant across meta-analyses: violation of this assumption will lead to standard errors that are too small. Only meta-analyses that contain trials with and without the characteristic contribute to this estimate. Corresponding methods using linear regression can be used for meta-analyses in which the outcome is numerical, and mean differences can be converted to corresponding odds ratios to facilitate comparisons across meta-analyses.
Robust standard errors
The assumption that RORs are constant across meta-analyses can be examined via ‘robust’ standard errors, which use the ‘information sandwich’50,51 to estimate standard errors based on the regression residuals. Robust standard errors may also be estimated after allowing for clustering,52 providing that the number of clusters is at least 20. 53
Meta-analytic approaches
If the effect of a trial characteristic varies between meta-analyses then analyses based on the logistic regression approach will underestimate the uncertainty in estimated RORs. An obvious alternative is to estimate the effect of the characteristic using a separate logistic regression in each meta-analysis (i.e. fixed-effect within meta-analyses). Estimated RORs in each meta-analysis can then be combined using meta-analytic methods, using inverse-variance weighting and either fixed-effect or random-effects between meta-analyses. Random-effects analyses use the moment-based variance estimator (τ^2) proposed by DerSimonian and Laird. 54 The fixed-effect assumption may also be violated within meta-analyses. This can be addressed by using random-effects meta-regression to allow for between-trial (within meta-analysis) heterogeneity. 55,56 Meta-regression examines associations between the estimated treatment effect (log OR) in each trial and one or more trial characteristics, allowing appropriately for the precision of the treatment effect via the standard error of the log OR in each trial. For a single meta-analysis, meta-regression estimates the same quantity (the ratio of ORs comparing trials with and without the characteristic) as is estimated using the logistic regression approach. In the absence of within meta-analysis heterogeneity the ratios of ORs estimated using meta-regression and logistic regression will be similar. Estimates of the between meta-analysis variance in the effect of trial characteristics are likely to be of key importance in synthesis of evidence from studies of different types (see ‘Evidence synthesis’ below).
Random effects logistic regression and multilevel modelling
We have experience with all the methods described above20 but have not yet used two promising alternatives: first, the logistic regression approach could be extended to allow for random effects between or within meta-analyses and, second, a multilevel approach could be developed, based on summary treatment effect estimates from each trial. 57 As well as providing estimates of both the effect of trial characteristics and the between meta-analysis variance in these effects, these approaches could have particular advantages in analyses that try to control for confounding effects of other trial characteristics, since the estimated effect of a characteristic in a particular meta-analysis would be shrunk towards the overall mean by an amount depending on the amount of information in the data for that meta-analysis. In analyses using the meta-analytic approach we have seen that the effect of trial characteristics is estimated very imprecisely, which will make it difficult to control for confounding effects. The logistic regression approach discussed above could obviously be used to control for confounding (for example between different components of methodological quality) by including the effects of more than one trial characteristic in the model. However, this assumes that the effect of each characteristic is constant across meta-analyses. An alternative is to estimate effects controlling for confounding factors separately in each meta-analysis, using random-effects meta-regression.
Evidence synthesis using recently developed methodology
Jonathan Sterne (lead applicant) and John Carlin (Professor, University of Melbourne Departments of Paediatrics and Public Health) have recently been developing methods to use the results of meta-epidemiological studies to provide estimates of the effect of interventions based on evidence from studies of differing methodological quality, or different types of study. We now briefly describe this: a paper is currently being prepared for publication.
We wish to combine the results of two types of studies to provide an overall estimate of the effect of a particular intervention. We will denote these two types of studies by H (high quality) and L (low quality). Two examples are that H and L could be RCTs in which randomisation was and was not adequately concealed, or that H could be RCTs while L could be cohort studies.
We will assume that meta-analyses of the effect of the intervention are conducted separately in studies of the two types, leading to estimates β^H and β^L of the intervention effect. For meta-analyses based on binary outcomes, β^H and β^L will generally correspond to the log intervention odds ratios in type H and type L studies respectively. The variances of β^H and β^L will be denoted by σH2 and σL2> respectively. Based on the usual assumption that intervention effect estimates from meta-analysis are asymptotically normally distributed, we will assume that β^H∼N(μ,σH2) (β^H is normally distributed with mean µ and variance σH2). We will interpret µ as the (true) intervention effect in type H studies, where this is assumed to be constant across all studies (the usual ‘fixed effect’ assumption).
We will assume that in type L studies, the intervention effect is estimated with bias δ (in studies with binary outcomes, δ is the log of the ratio of intervention odds ratios comparing type L and type H studies). Therefore, β^L∼N(μ+δ,σL2). If δ were known, then we could obtain a corrected estimate of µ, based on the type L studies, as μ^L+β^L−δ. However, if δ is unknown, as will invariably be the case, the results of the type L studies cannot distinguish µ and δ without further assumptions.
A solution to this problem is to incorporate prior information about the bias δ, using a Bayesian framework. As described earlier, the effect of components of trial quality on intervention effect estimates, and the between meta-analysis variability in such effects, may be estimated in meta-epidemiological studies, using data from collections of meta-analyses. For example, in a recent re-analysis of the data of Schulz et al. 11 the ratio of odds ratios (ROR) comparing studies that were not and were adequately concealed was 0.67 (95% CI 0.57 to 0.78), while the between-meta-analysis variance in the log ROR was 0.065. 20 We propose to formalise evidence about this variability in a prior distribution for δ, and a convenient assumption is a normal distribution: δ∼N(δ0,τ02), where δ0 represents our best a priori estimate of the average bias in studies of type L, while τ02 describes uncertainty around this estimate. The results just quoted could motivate the particular specifications δ0 = loge 0.67 = –0.40 and τ02=0.1, say.
Based on these assumptions, it is straightforward to obtain the posterior distribution of the true intervention effect µ, given the information from both type H and type L studies. In particular, integrating over the prior distribution of δ, we obtain the marginal distribution of β^L∼N(μ+δ0,σL2+τ02).
It follows by standard normal distribution calculations,58 assuming a diffuse (‘noninformative’) prior distribution for µ, that the posterior distribution for µ given the combined data D=(β^H,β^L) is normal with mean and variance
Equations 1 and 2 show that the posterior mean of µ is a weighted average of the estimates of µ from the two types of studies (β^H in the type H studies and β^L−δ0 in the type L studies), with weights [σH2]−1 and [σL2+τ02]−1 in the type H and type L studies respectively. It follows that τ02, the prior variability in the amount of bias, is key in determining the contribution of the type L studies to the posterior mean of µ, since the larger is τ02, the smaller is the weight given to the type L studies. When τ02 is zero (the amount of bias is known with certainty), equations 1 and 2 correspond to a standard inverse-variance weighted meta-analysis of the estimates of µ from the two types of studies. As τ02 tends to infinity, the contribution of the type L studies tends to zero, and equations 1 and 2 simplify to give E(μ)=β^H, Var(μ)=σH2, so that the posterior information about µ corresponds to the information provided by the type H studies. Note also that the information from the type L studies is both corrected for bias and downweighted according to our uncertainty about the magnitude of the bias.
These statistical methods will be used to analyse data from workstreams A and B, during the second 12 months of the project. We will estimate both the mean differences in estimated effect of interventions, and the variation in these differences, according to characteristics of studies and types of studies, before and after controlling for the confounding effects of other study characteristics. This will lead to formal proposals, based on the methods for evidence synthesis described above on how to combine estimates of the effect of interventions from RCTs differing in quality and from different types of study.
Schedule of work
| Task | Month | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1–2 | 3–4 | 5–6 | 7–8 | 9–10 | 11–12 | 13–14 | 15–16 | 17–18 | 19–20 | 21–22 | 23–24 | |
| Workstream A | ||||||||||||
| Obtain data from additional meta-epidemiological studies | • | |||||||||||
| Combine new studies with existing database | • | • | ||||||||||
| Retrieve papers and extract new information on trials | • | • | • | • | • | • | • | • | ||||
| Workstream B | ||||||||||||
| Design, piloting and revision of data extraction forms | • | • | ||||||||||
| Retrieve original publications | • | • | • | • | • | • | ||||||
| Data extraction | • | • | • | • | • | • | • | |||||
| Analysis and reporting | ||||||||||||
| Statistical analysis of data from both workstreams | • | • | • | • | • | • | ||||||
| Preparation of reports to funder and papers for publication | • | • | • | |||||||||
Outputs
Workstream A
-
Updated estimates of the effect of components of trial quality and reporting characteristics on estimated treatment effects in randomised controlled trials, both overall and for the first time for different clinical settings, types of intervention, outcomes and trial designs.
-
Refined definitions of established markers of trial quality and identification of additional markers, including aspects of reporting (reporting of sample size calculations, eligibility criteria, primary and secondary outcomes), the degree of baseline imbalances, the number of centres involved, and the source of funding.
-
An improved understanding of the extent and effect of confounding between dimensions of methodological quality and other trial characteristics, and hence their relative importance in the epidemiology of bias in clinical trial research and the extent to which confounding by different trial characteristics affects estimates of the effect of methodological quality and reporting biases.
-
New estimates of the effect and impact of components of trial quality in different medical specialties, comparing conventional with complementary medicine, and comparing settings defined by the presence or absence of known prognostic factors.
-
New estimates of the effect and impact of components of trial quality in different types of controlled clinical trials, and comparing trials with ‘hard’ and ‘soft’ end points.
Workstream B
-
Estimates of the average difference between estimates of health interventions derived from RCTs and NRS.
-
Estimates of the importance of prognostic information in the difference between randomised and non-randomised studies.
-
Estimates of the difference between alternative experimental and observational methods of constructing non-randomised control groups.
-
Estimates of the impact of case-mix adjustment methods in non-randomised studies.
General
-
All analyses will lead to estimates of the between meta-analysis variation in the effect of study characteristics on intervention effect estimates. These will be used in analyses showing the effect of combining different types of studies in meta-analyses, based on the methods for evidence synthesis described earlier and incorporating prior estimates of both the expected bias and its variance.
-
Based on these results, and on refined methods of characterising trials developed as part of Workstream A, we will produce updated recommendations for the conduct, reporting and appraisal of randomised controlled trials, including revised editions of the CONSORT statement and the continuing development of the STROBE statement. All applicants will contribute to planning, conducting, interpreting and reporting these analyses. We will also aim to contribute to the continuing development of guidelines for the conduct and reporting of systematic reviews and meta-analyses, including the QUORUM statement, particularly to the debate over whether and how to include studies of differing methodological quality and/or different study designs.
-
Ultimately, the project will contribute to improved interpretation of existing evidence about the effect of medical interventions, the improved conduct and reporting of future randomised controlled trials and other evaluations of the effect of medical interventions, and hence to better medical care.
People
The team of applicants comprises the leading investigators in the field. All have extensive experience of conducting randomised trials and meta-analyses. Together, the team has links with clinical researchers in many branches of medicine, and covers a breadth of expertise, including clinical medicine and epidemiology (Egger, Ioannidis, Gluud, Pildal), methodological and reporting issues in randomised trials research (Altman, Schulz, Moher, Egger, Ioannidis), Cochrane methodology and Cochrane databases (Gluud, Ioannidis, Jüni, Pildal) and the statistical issues involved in the analysis of meta-epidemiological studies (Sterne, Altman, Ioannidis, Deeks, Schulz). Several applicants (Moher, Altman, Schulz, Egger, Ioannidis) are members of the CONSORT Group,26,27 the QUOROM Group (Moher, Altman) and the STROBE Group (Egger, Sterne, Altman) and most are affiliated to the Cochrane Collaboration. Three of the applicants (Egger, Moher, Sterne) are co-convenors of the Cochrane Reporting Bias Methods Group and two (Altman, Deeks) are co-convenors of the Cochrane Statistical Methods Group.
Dissemination of research results
We will disseminate our findings in journal articles and at national and international meetings. The final project report will be made available to organisations in the UK and worldwide that fund trials and systematic reviews. In particular, we will meet with representatives of the NHS Health Technology Assessment (HTA) programme and the National Institute for Clinical Excellence (NICE) to discuss the implications of our findings for the research that they commission. We will prepare educational articles and materials, and will make particular efforts to disseminate these in specialist areas for which the research identifies problems with the conduct and reporting of trials. A number of the applicants and collaborators run regular workshops at meetings of the Cochrane Collaboration, and these, together with courses on randomised trials and systematic reviews run in Bristol and Oxford, will incorporate the results of the research as it becomes available. We will also discuss, with other colleagues involved in the Cochrane Collaboration, how the Cochrane Reviewers’ Handbook might be updated in the light of our results. Those applicants involved with the development and updating of the CONSORT statement on the reporting of randomised controlled trials will ensure that this is updated to take account of new empirical evidence on aspects of trial quality which emerges from the study. Perhaps the most powerful influence on the conduct and reporting of trials and systematic reviews is medical journals’ publication policies. Here, CONSORT has direct and indirect influence: direct through the membership of several editors of the most influential journals and indirect through endorsement from International Committee of Medical Journal Editors and Council of Science Editors. Similarly, the findings of this research will feed into updates of and extensions to the QUOROM and STROBE Statements.
References
- Freiman JA, Chalmers TC, Smith H, Kuebler RR, Bailar JC, Mosteller F. Medical uses of statistics. Boston, MA: NEJM Books; 1992.
- Moher D, Dulberg CS, Wells GA. Statistical power, sample size, and their reporting in randomized controlled trials. JAMA 1994;272:122-4.
- Thornley B, Adams C. Content and quality of 2000 controlled trials in schizophrenia over 50 years. BMJ 1998;317:1181-4.
- McDonald S, Westby M, Clarke M, Lefebvre C. and the Cochrane Centres’ Working Group . Number and size of randomised trials reported in general health care journals from 1948 to 1997. Int J Epidemiol 2002;31:125-7.
- Chan AW, Altman DG. A cross-sectional study of randomised controlled trials published in 2000 n.d.
- Egger M, Smith DG, O’Rourke K, Egger M, Smith DG, Altman DG. Systematic reviews in health care: meta-analysis in context. London: BMJ Books; 2001.
- Eccles M, Freemantle N, Mason J, Egger M, Smith GD, Altman DG. Systematic Reviews in Health Care: Meta-Analysis in Context. London: BMJ Books; 2001.
- Gray JAM, Egger M, Smith GD, Altman DG. Systematic reviews in health care: meta-analysis in context. London: BMJ Books; 2001.
- Sterne JAC, Gavaghan DJ, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol 2000;53:1119-29.
- Mallet S, Clarke M. The typical Cochrane review. How many trials? How many participants?. Int J Technol Assess Health Care 2002;18:820-31.
- Schulz KF, Chalmers I, Hayes RJ, Altman D. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408-12.
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses?. Lancet 1998;352:609-13.
- McAuley L, Pham B, Tugwell P, Moher D. Does the inclusion of grey literature influence estimates of intervention effectiveness reported in meta-analyses?. Lancet 2000;356:1228-31.
- Moher D, Pham B, Klassen TP, Schulz KF, Berlin JA, Jadad AR, et al. What contributions do languages other than English make on the results of meta-analyses. J Clin Epidemiol 2000;53:964-72.
- Kjaergard LL, Villumsen J, Gluud C. Reported methodological quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med 2001;135:982-9.
- Jüni P, Holenstein F, Sterne J, Bartlett C, Egger M. Direction and impact of language bias in meta-analyses of controlled trials: empirical study. Int J Epidemiol 2002;31:115-23.
- Balk EM, Bonis PA, Moskowitz H, Schmid CH, Ioannidis JP, Wang C, et al. Correlation of quality measures with estimates of treatment effect in meta-analyses of randomized controlled trials. JAMA 2002;287:2973-82.
- Egger M, Jüni P, Bartlett C, Holenstein F, Sterne J. How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study. Health Technol Assess 2003;7.
- Sampson M, Barrowman NJ, Moher D, Klassen TP, Pham B, Platt R, et al. Should meta-analysts search EMBASE in addition to MEDLINE?. J Clin Epidemiol 2003;56:943-55.
- Sterne JAC, Jüni P, Schulz KF, Altman DG, Bartlett C, Egger M. Statistical methods for assessing the influence of study characteristics on treatment effects in ‘meta-epidemiological’ research. Stat Med 2002;21:1513-24.
- Pildal J, Hróbjartsson A, Jørgensen KJ, Hilden J, Bradburn MJ, Altman DG, et al. How Often Do Positive Conclusions Drawn from Meta-Analyses Remain Substantiated If Only Data from Randomised Trials With Adequate Allocation Concealment Are Considered? 2004.
- Contopoulos-Ioannidis D, Gilbody S, Trikalinos TA, Churcill R, Wahlbeck K, Ioannidis JP. Comparison of large vs. smaller randomized trials for mental health related interventions. Am J Psychiatry 2005;162:578-84.
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. Int J Technol Assess Health Care 2003;19:591-603.
- Egger M, Davey Smith G. Meta-analysis: bias in location and selection of studies. BMJ 1998;316:61-6.
- Egger M, Jüni P, Bartlett C, Holenstein F, Sterne J. How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study. Health Technol Assess 2003;7.
- Altman DG, Schulz KF, Moher D, Egger M, Davidoff F, Elbourne D, et al. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med 2001;134:663-94.
- Moher D, Schulz KF, Altman DG. for the CONSORT Group . The CONSORT statement: revised recommendations for improving the quality of reports of parallel group randomized trials. Lancet 2001;357:1191-4.
- LeLorier J, Grégoire G, Benhaddad A, Lapierre J, Derderian F. Discrepancies between meta-analyses and subsequent large randomized, controlled trials. N Engl J Med 1997;337:536-42.
- Egger M, Dickersin K, Davey Smith G, Egger M, Smith DG, Altman DG. Systematic reviews in health care: meta-analysis in context. London: BMJ Books; 2001.
- Peto R, Baigent C. Trials: the next 50 years. Large scale randomised evidence of moderate benefits. BMJ 1998;317:1170-1.
- Black N. Why we need observational studies to evaluate the effectiveness of health care. BMJ 1996;312:1215-18.
- Abel U, Koch A. The role of randomization in clinical studies: myths and beliefs. J Clin Epidemiol 1999;52:487-97.
- Green SB, Byar DP. Using observational data from registries to compare treatments: the fallacy of omnimetrics. Stat Med 1984;3:361-70.
- Chalmers I. Assembling comparison groups to assess the effects of health care. J R Soc Med 1997;90:379-86.
- Moses LE. Measuring effects without randomized trials? Options, problems, challenges. Med Care 1995;33:AS8-14.
- Deeks JJ, Dinnes J, D’Amico R, Sowden AJ, Sakarovitch C, Song F, et al. Evaluating non-randomised intervention studies. Health Technol Assess 2003;7.
- Britton A, McKee M, Black N, McPherson K, Sanderson C, Bain C. Choosing between randomised and non-randomised studies: a systematic review. Health Technol Assess 1998;2.
- MacLehose RR, Reeves BC, Harvey IM, Sheldon TA, Russell IT, Black AMS. A systematic review of comparisons of effect sizes derived from randomised and non-randomised studies. Health Technol Assess 2000;4.
- Sacks H, Chalmers TC, Smith H. Randomized versus historical controls for clinical trials. Am J Med 1982;72:233-40.
- Kunz R, Vist G, Oxman AD. Randomisation to protect against selection bias in healthcare trials. Oxford: Update Software; 2002.
- Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. N Engl J Med 2000;342:1878-86.
- Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med 2000;342:1887-92.
- Ioannidis JP, Haidich AB, Pappa M, Pantazis N, Kokori SI, Tektonidou MG, et al. Comparison of evidence of treatment effects in randomized and nonrandomized studies. JAMA 2001;286:821-30.
- Wilson DB, Lipsey MW. The role of method in treatment effectiveness research: evidence from meta-analysis. Psychol Methods 2001;6:413-29.
- Stewart LA, Parmar MKB. Meta-analysis of the literature or of individual patient data: is there a difference?. Lancet 1993;341:418-22.
- Horton R. The information wars [see comments]. Lancet 1999;353:164-5.
- Devereaux PJ, Manns BJ, Ghali WA, Quan H, Lacchetti C, Montori VM, et al. Physician interpretations and textbook definitions of blinding terminology in randomized controlled trials. JAMA 2001;285:2000-3.
- Bhandari M, Tornetta P, Ellis T, Audige L, Sprague S, Kuo JC, et al. Hierarchy of evidence: differences in results between non-randomized studies and randomized trials in patients with femoral neck fractures. Arch Orthop Trauma Surg 2004;124:10-6.
- Ioannidis JP, Lau J. Heterogeneity of the baseline risk within patient populations of clinical trials: a proposed evaluation algorithm. Am J Epidemiol 1998;148:1117-26.
- Huber PJ. The Behaviour of Maximum Likelihood Estimates under Non-Standard Conditions 1967:221-33.
- White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica 1980;48:817-30.
- Rogers WH. sg17: regression standard errors in clustered samples. Stata Technical Bulletin 1993;3:88-94.
- Donner A. Some aspects of the design and analysis of cluster randomization trials. Appl Statist 1998;47:95-113.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88.
- Berkey CS, Hoaglin DC, Mosteller F, Colditz GA. A random-effects regression model for meta-analysis. Stat Med 1995;14:395-411.
- Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med 1999;18:2693-708.
- Turner RM, Omar RZ, Yang M, Goldstein H, Thompson SG. A multilevel model framework for meta-analysis of clinical trials with binary outcomes. Stat Med 2000;19:3417-32.
- Gelman A, Carlin JB, Stern HB, Rubin DB. Bayesian data analysis. New York: Chapman and Hall; 2003.
List of abbreviations
- BRANDO
- Bias in Randomised and Observational studies (study acronym)
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CrI
- credible interval
- ID
- unique identifier/identification number in the database
- IQR
- interquartile range
- OR
- odds ratio
- PMID
- unique PubMed™ identifier
- PPr
- posterior probability
- RCT
- randomised controlled (clinical) trial
- ROR
- ratio of odds ratios
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, Department of Pharmacology and Therapeutics, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, Department of Pharmacology and Therapeutics, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Professor of Bio-Statistics, Department of Public Health and Epidemiology, University of Birmingham
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, Department of Pharmacology and Therapeutics, University of Liverpool
-
Professor Zarko Alfirevic, Head of Department for Women’s and Children’s Health, Institute of Translational Medicine, University of Liverpool
-
Professor Judith Bliss, Director of ICR-Clinical Trials and Statistics Unit, The Institute of Cancer Research
-
Professor David Fitzmaurice, Professor of Primary Care Research, Department of Primary Care Clinical Sciences, University of Birmingham
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, Department of Specialist Radiology, University College Hospital, London
-
Professor Angela Harden, Professor of Community and Family Health, Institute for Health and Human Development, University of East London
-
Dr Joanne Lord, Reader, Health Economics Research Group, Brunel University
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor Dion Morton, Professor of Surgery, Academic Department of Surgery, University of Birmingham
-
Professor Gail Mountain, Professor of Health Services Research, Rehabilitation and Assistive Technologies Group, University of Sheffield
-
Professor Irwin Nazareth, Professor of Primary Care and Head of Department, Department of Primary Care and Population Sciences, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norrie, Director, Centre for Healthcare Randomised Trials, Health Services Research Unit, University of Aberdeen
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Peter Tyrer, Professor of Community Psychiatry, Centre for Mental Health, Imperial College London
-
Professor Martin Underwood, Professor of Primary Care Research, Warwick Medical School, University of Warwick
-
Professor Caroline Watkins, Professor of Stroke and Older People’s Care, Chair of UK Forum for Stroke Training, Stroke Practice Research Unit, University of Central Lancashire
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Tom Foulks, Medical Research Council
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Diana Baralle, Consultant and Senior Lecturer in Clinical Genetics, University of Southampton
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Public contributor
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Nicola Lennard, Senior Medical Officer, MHRA
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Public contributor
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Public contributor
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Matthew Thompson, Senior Clinical Scientist and GP, Department of Primary Health Care, University of Oxford
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Joanna Jenkinson, Board Secretary, Neurosciences and Mental Health Board (NMHB), Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Public contributor
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire County Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Mrs Jean Thurston, Public contributor
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Public contributor
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Public contributor
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Public contributor
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Public contributor
-
Mr Sankaran Chandra Sekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Public contributor
-
Mr Seumas Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Dr Matthew Wilson, Consultant Anaesthetist, Sheffield Teaching Hospitals NHS Foundation Trust
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan, Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Public contributor
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer, Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Public contributor
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Public contributor
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Public contributor
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health