Notes
Article history
This issue of the Health Technology Assessment journal series contains a project commissioned by the Methodology research programme (MRP). The Medical Research Council (MRC) is working with NIHR to deliver the single joint health strategy and the MRP was launched in 2008 as part of the delivery model. MRC is lead funding partner for MRP and part of this programme is the joint MRC-NIHR funding panel ‘The Methodology Research Programme Panel’. To strengthen the evidence base for health research, the MRP oversees and implements the evolving strategy for high quality methodological research. In addition to the MRC and NIHR funding partners, the MRP takes into account the needs of other stakeholders including the devolved administrations, industry R&D, and regulatory/advisory agencies and other public bodies. The MRP funds investigator-led and needs-led research proposals from across the UK. In addition to the standard MRC and RCUK terms and conditions, projects commissioned by the MRP are expected to provide a detailed report on the research findings and publish the findings in the HTA monograph series, if supported by NIHR funds.
Declared competing interests of authors
At the time that this research was conducted some of the research team at York and Brunel served on NICE Appraisal Committees (KC, SP, LL and SG), some of whom are also members of the NICE Decision Support Unit (KC, SP). York also provides analysis for the NICE appraisal process as one of a number of Evidence Review Groups.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2012. This work was produced by Claxton et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to NETSCC.
2012 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction and overview
The National Institute for Health and Clinical Excellence (NICE) is increasingly making decisions about health technologies close to licence through the single technology assessment (STA) process. Inevitably these decisions are being made when the evidence base to support these technologies is least mature and when there may be substantial uncertainty surrounding their cost-effectiveness, including their effectiveness and potential for harms. In these circumstances further evidence may be particularly valuable as it would lead to better decisions that improve patient outcome and/or reduce resource costs. However, a decision to approve a technology will often have an impact on the prospects of acquiring further evidence to support its use. This is because, once positive guidance has been issued, the incentives for manufacturers to conduct research are limited. Also, the clinical community is unlikely to regard further randomised controlled trials (RCTs) to be ethical once positive guidance provides access with a funding mandate. Therefore, the decision to approve a technology should account for both the potential benefits of access to a cost-effective technology and the potential costs to future NHS patients in terms of the value of evidence that may be forgone by early adoption. The general issue of balancing the value of evidence about the performance of a technology and the value of access to a technology can be seen as central to a number of policy questions. Establishing the key principles of what assessments are needed for ‘only in research’ (OIR) or ‘approval with research’ (AWR) recommendations, as well as how these assessments should be made, will enable them to be addressed in an explicit and transparent manner.
The Medical Research Council (MRC) and National Institute for Health Research (NIHR) Methodology Research Programme recently funded the Universities of York and Brunel to undertake research to help inform when NICE should recommend the use of health technologies only in the context of an appropriately designed programme of evidence development (see Appendix 5). The NICE Guide to the Methods of Technology Appraisal1 states that ‘the Appraisal Committee may recommend that particular interventions are used within the NHS only in the context of research’. It indicates that four issues should be considered by the Appraisal Committee when recommending further research. These are (1) whether or not the intervention is reasonably likely to benefit patients and the public, (2) how easily the research can be set up or whether or not it is already planned or in progress, (3) how likely the research is to provide further evidence and (4) whether or not the research is good value for money.
The aims of this research are to:
-
establish the key principles of what assessments are needed to inform an OIR or AWR recommendation
-
evaluate previous NICE guidance in which OIR or AWR recommendations were either made or considered, and examine the extent to which the key principles from (1) are evident
-
evaluate a range of alternative options to establish the criteria, additional information and/or analyses that could be made available to help the assessments needed to inform an OIR or AWR recommendation
-
provide a series of final recommendations, with the involvement of key stakeholders, establishing both the key principles and associated criteria that might guide OIR and AWR recommendations, identifying what, if any, additional information or analyses might be included in the NICE technology appraisal process and how such recommendations might be more likely to be implemented through publically funded and sponsored research.
The relevance of this work to NICE has been evaluated through a series of two workshops involving key stakeholders, including members of NICE and its Advisory Committees (including lay members and members of other NICE programmes), patient representatives, manufacturers, and research and NHS commissioners, as well as relevant academics. Establishing the key principles of what assessments are needed to inform OIR or AWR recommendations requires a critical review of a diverse literature on principles and policy and previous NICE guidance (see Chapters 2 and 4); the development of a coherent conceptual framework (see Chapter 3, Key principles and assessments needed and Changes in prices and evidence); and consideration of whether or not such principles conflict with established ethical principles and the social value judgements adopted by NICE (see Chapter 3, Social value judgements and ethical principles).
The first workshop took place in September 2010 and considered four main topics:
-
the relevance to NICE of existing literature
-
whether or not the key principles and assessment that have been identified provide useful guidance on when OIR and AWR might be considered
-
the insights from a detailed review of previous NICE guidance
-
whether or not the proposed methods to inform assessment and criteria to select case studies are suitable.
Relevant summaries of the key issues were provided in the form of briefing documents that covered these four topics. These documents, which formed the basis for the workshop presentations and related group discussions, as well as a summary of feedback and list of participants, are available at www.york.ac.uk/che/research/teehta/workshops/only-in-research-workshop/.
The primary output of this workshop was a set of principles and explicit criteria (a sequence of assessments and decisions) to support OIR and AWR recommendations. This sequence of assessments (an algorithm) can be summarised as a simple 7-point checklist, which was subsequently applied to a series of four case studies. Each case study examines how each of the assessments might be made based on the type of evidence and analysis currently provided in NICE technology appraisals and how these assessments might be better informed with a range of additional information and/or analyses.
The second workshop took place in June 2011 and considered:
-
whether or not the revised algorithm of assessments and the associated checklist has identified the key judgements that need to be made when considering OIR and AWR guidance
-
based on the application of this checklist to the series of case studies, whether or not such assessments can be made based on existing information and analysis provided to NICE and in what circumstances could additional information and/or analysis be useful
-
what implications this more explicit assessment of OIR and AWR might have for policy (e.g. NICE guidance and drug pricing), the process of appraisal (e.g. greater involvement of research commissioners) and methods of appraisal (e.g. should additional information, evidence and analysis be required).
Relevant summaries of the key issues were provided in the form of briefing documents that covered these three topics. These documents, which formed the basis for the workshop presentations and related group discussions, as well as a summary of feedback and list of participants, is available at www.york.ac.uk/che/research/teehta/workshops/only-in-research-workshop/.
The primary output of this workshop was a list of possibilities that NICE might choose to take forward in the next revision of the Guide to the Methods of Technology Appraisal1 and how this might inform the formulation of guidance in the other NICE programmes. It might also suggest how a consideration of uncertainty and the need for evidence might influence value-based pricing and the impact of patient access schemes on OIR and AWR guidance.
The main report is intended to be accessible to a wide audience, providing intuitive explanations of why certain assessments are important and illustrating how they might be informed using examples. Notes have been used extensively, especially in Chapters 3 and 5, to provide explanatory detail without adding undue complexity to the exposition in the main text (see Notes). The main report is accompanied by (1) supporting material for each chapter (see Appendices 1–5), (2) a technical appendix (see Appendix 6), which provides a more formal treatment of why, in principle, each type of assessment is important, (3) full details of the analysis undertaken for each of the four case studies referred to in Chapter 5 of the main report (see Appendices 7–10) and (4) a series of technical notes (see Appendix 11) that deal with some conceptual and analytical details that are common to the case studies reported in Appendices 7–10.
The main report is organised as follows. The results of a critical review of policy, practice and literature in this area are presented in Chapter 2, supported by material in Appendix 1. The key principles and the sequence of assessments needed are presented in Chapter 3, supported by Appendix 2 and critically the technical appendix (see Appendix 6), which provides more formal treatment of the issues. A review of NICE technology appraisal guidance is presented in Chapter 4, supported by material in Appendix 3. The checklist of assessment needed and its application to the four case studies using a range of additional information and analysis is reported in Chapter 5. Chapter 5 is supported by material in Appendix 4 and critically by the full details of the analysis undertaken for each case study, reported in Appendices 7–10, and the series of technical notes in Appendix 11. Finally, Chapter 6 briefly draws together some of the possible implications for policy, process and methods of appraisal, distinguishing those issues directly relevant to the NICE remit and those that might be most relevant to other public bodies and stakeholders. Chapter 6 draws on the feedback provided at the second workshop, which has been summarised and is available online (see above).
Chapter 2 Critical review of policies, practice and literature
Aims and objectives
There is a growing and diverse literature on OIR/AWR2–5 and on when decisions to adopt a technology should be delayed. 6,7 There is also considerable variation in the terminology used across this literature, which reflects the different decision contexts, not all of which are relevant to NICE. There is a need to critically review this literature to distil any common themes and core principles relevant to the NICE context. The main purpose of the review is to help to inform the development of a unifying conceptual framework within which these themes and principles can be located and understood. This, in turn, will enable a consistent and clear terminology to be established which will assist clarity in subsequent policy debates and may provide NICE with useful definitions and terminology that can be used in communicating its guidance, considerations and methods. The specific aims of the review were:
-
to review alternative terminologies and taxonomies used to describe and classify approaches to OIR/AWR and to establish their relevance to the NICE context
-
to identify any common themes and principles discussed in relation to OIR/AWR.
Methods
The existing literature on OIR and AWR is only partly represented in traditionally published papers; much is located in policy and discussion documents. The diversity in these sources was reflected in the range of search strategies employed, covering (1) traditional published literature, (2) grey literature and (3) policy and discussion documents. In addition, relevant interest groups and policy websites were searched, reference lists of previous reviews8 were checked, separate citation searches were performed using key references and discussions were held with our Advisory Group. In reviewing the results of the systematic search and selecting relevant studies for inclusion, a relatively inclusive approach was adopted. Case study examples of AWR/OIR were not searched for explicitly; instead the focus of the searches was to identify relevant methodological papers. Case study examples comprising a significant discussion of methodological issues were, however, included in the review.
A summary of the search strategies are discussed under the relevant subheadings below. Full details of the separate searches are reported in Appendix 1.
Traditional published literature
A traditional systematic search was undertaken in MEDLINE, MEDLINE In-Process & Other Non-Indexed Citations, EMBASE, The Cochrane Library, EconLit and Cumulative Index to Nursing and Allied Health Literature (CINAHL) based on appropriate search terms informed by key publications to identify relevant literature. 8 An iterative approach was used in the development of search terms and strategies incorporating ‘pearl-growing’ techniques through additional citation and reference searching of key publications and discussions with our Advisory Group. Searches were restricted to documents produced from 1999 onwards as the use of OIR and AWR policies is a relatively recent process and so it is not expected that any relevant references will be identified before this date.
To test the performance of the search strategy, records initially identified from the case for support and citations contained within those documents and articles were cross-referenced with the results identified from the searches (capture–recapture method). In doing this it was concluded that the final search strategy identified all of the records it would be likely to identify, that is, those accessible from traditional (non-grey literature) sources.
Grey literature searches
Additional searches of grey literature were also undertaken using a similar approach reported in the review by Stafinski et al. 8 The following grey literature databases were searched: the New York Academy of Medicine Grey Literature Report and the IDEAS database (Department of Economics, University of Connecticut). A similar set of key words as for the review of the traditional published literature was used for the grey literature searches, with some modifications because of the different recording mechanisms operated by the grey literature databases. Searches were again restricted to documents produced from 1999 onwards.
Policy and discussion documents
The third element of the search was based on policy and discussion documents. Although the focus of the search related to UK policy and discussion documents, documents from key international organisations were also included. Relevant international organisations and websites were identified in conjunction with our Advisory Group. A number of policy documents were also identified from those referenced in the case for support and from the reference lists of key papers. 9–12 A number of additional organisation websites were searched for relevant documents. Where possible, web searches were restricted to documents produced from 1999 onwards for consistency with the other search elements.
Other sources
The final element of the search considered a variety of other sources including relevant interest groups [e.g. the Health Technology Assessment international (HTAi) interest subgroup on conditional coverage and evidence development for promising technologies and the Programs for Assessment of Technology in Health (PATH) website containing publications relating to the programme of conditionally funded field evaluations (CFEEs) in Ontario], other reviews,8 references suggested by our Advisory Group and additional searches based on citations of papers identified from earlier stages.
Literature search results
A summary of the search results is provided in the following sections. Full details of the results and a summary of included references are reported in Appendix 1.
Identified references
In total, 59 references were included in the review; 43 of these were journal articles,2–5,8,13–50 11 were policy documents (eight UK and three non-UK)9–12,22,51–56 and five were based on presentation slides or discussion documents. 56–60
Of the 43 journal articles, the majority are academic ‘think pieces’ on issues relating to OIR and AWR with additional responses to previous articles. 5,13–16 Although a number of the journal articles provide a discussion of many of the general issues relating to the use of OIR and AWR,2,3,17–24 a smaller proportion explicitly address the issue of terminology and/or provide a taxonomy of OIR/AWR types (n = 11). The majority of the journal articles discuss specific issues relating to OIR/AWR implementation, most commonly in relation to evidence collection and design (n = 41). These issues are often illustrated in the context of specific case study applications such as the positron emission tomography register or the National Emphysema Treatment Trial (NETT). A relatively large number of these articles also discuss ethical issues and social value judgements relating to the implementation of OIR/AWR policies (n = 20) and issues of investment and reversal costs (n = 21).
The 11 policy documents91–112,22,51–56 identified are from UK and non-UK sources. From the UK, relevant documents are produced by HM Treasury,9 the Department of Health,52 the House of Commons Health Committee,10 NICE and the Agency for Healthcare Research and Quality (AHRQ),51 NICE Citizens Council,11 NHS Scotland,53 the Office of Fair Trading (OFT)12 and the Office of Life Sciences. 54 Outside of the UK, relevant documents identified are all produced by US organisations: the Centers for Medicare & Medicaid Services (CMS),55 the Health Industry Forum56 and the National Health Policy Forum. 22 In many of these policy documents OIR and AWR are typically discussed as part of a wider general health policy theme as opposed to being the central issue under consideration. Only a small number22,51,55 offer any insights into terminology and/or taxonomies relating to OIR/AWR. Over half (n = 7) present general issues of OIR/AWR and specific issues most commonly related to evidence collection. Issues of investment and reversal costs, changing prices and ethical issues/social value judgements are covered to a lesser extent in the policy documents.
The final five references included are presentation slides or discussion documents. 56–60 These are all widely cited in the OIR and AWR literature. None of these references discusses terminology or taxonomies of OIR/AWR types. Instead they again focus on general themes of OIR/AWR, with some discussing specific issues such as evidence collection.
Summary of the key issues for practice and policies for ‘only in research’ and ‘approval with research’ schemes
The following sections discuss the main findings in line with the key objectives of the review.
Review of terminology and taxonomies of ‘only in research’/‘approval with research’ schemes
Multiple definitions of OIR- and AWR-type schemes are reported in the literature. These are commonly provided within a broader consideration of conditional coverage or risk-sharing schemes. Despite the variation in terminology that exists, a number of common themes emerge. Most notably, the use of OIR/AWR is commonly defined as providing an alternative to a binary accept/reject decision for policy-makers in situations in which the technology does not appear to meet the standard criteria for reimbursement, predominantly because of uncertainty surrounding the existing evidence base and when additional data collection could reduce this uncertainty. 21 The emphasis placed on uncertainty and the specific role that the collection/generation of additional evidence plays in reducing existing uncertainty is what distinguishes OIR/AWR schemes from the broader range of conditional coverage or risk-sharing approaches,25 in which the focus is to shift the burden of uncertainty onto another party (usually the manufacturer) rather than to collect information to reduce this uncertainty for future decisions. 4,24
In considering how to appropriately define and categorise OIR/AWR schemes, it is important to consider what particular terms mean in their various contexts. In the context of NICE, OIR is the term used when a recommendation is made to restrict an approval decision to only those patients who subsequently receive the intervention as part of a well-designed programme of research. OIR has been available as a formal policy option since the inception of NICE52 and provides an important additional option to accept and reject decisions. 3
In contrast to an OIR recommendation, the use of AWR does not necessarily limit coverage to those participating in the clinical study or registry. Hence, the distinction between OIR and AWR is primarily the degree of coverage that each confers for reimbursement purposes. Although AWR is not currently a formal policy option available to NICE, it is able to issue specific research recommendations as part of any guidance and can link this to the timing of any reappraisal. Consequently, AWR represents a valid option for consideration. Importantly, both OIR and AWR strategies are distinct from general recommendations for further research made as part of the appraisal process, in which no link to generating evidence as a condition of coverage is made. However, given the current directives and remit of NICE, the research recommendations issued as part of an AWR decision are not a mandatory requirement of approval. As a result, inevitably there exists some uncertainty following an AWR recommendation over whether or not the stated research recommendations will actually be conducted.
Although slightly different definitions and terminology are applied in the broader literature relating to OIR and AWR schemes, they are relatively similar in their meaning. For example, in the USA, the term ‘coverage with evidence development’ (CED) is often used as a catch-all term for OIR- and AWR-type schemes. Sections 1862(a)(1)(A) and (1)(E) of the Social Security Act provide statutory provision for the CMS to issue both OIR- and AWR-type coverage decisions involving the collection of additional evidence in registries or clinical trials, through national coverage determinations (NCDs). CMS describes two related but distinct processes: coverage with appropriate determination (CAD) and coverage with study participation (CSP). 55 As with OIR and AWR, these separate forms of CED are also closely linked to the level of coverage. CMS may issue a CAD to determine that patients receiving the treatment meet the conditions specified in the NCD. As part of this they may request more data. CSP allows coverage of certain items or services for which the evidence is not adequate to support full coverage and for which further data would be of benefit. Coverage may be extended to patients enrolled in a clinical research study. To recommend a CSP the evidence should assure basic safety, there should be high potential to provide significant benefit and there may be significant barriers to conducting clinical trials. Consequently, CSP would fit with OIR as it currently exists in the UK and CAD is closer to an AWR-type scheme.
Another example of the use of CED as a catch-all term for OIR/AWR schemes is the conditionally funded field evaluations (CFFEs) conducted in Ontario, Canada. These are recommended on the basis of a Health Technology Policy Analysis undertaken by the Medical Advisory Secretariat for the Ontario Health Technology Advisory Committee, which may conclude that there is not enough evidence to support uptake and diffusion of the technology. In these circumstances, coverage for a technology is provided conditional upon additional data being collected to specifically address residual uncertainty to better inform evidence-based decision-making. Primarily, the impetus for conducting a CFFE is a lack of evidence on transferability of evidence to a particular jurisdiction and/or its effectiveness or cost-effectiveness within particularly subgroups. 17,20 There are, however, reasons to recommend a CFFE on the basis of any decision uncertainty regarding the quality of evidence, safety data and cost-effectiveness. 20 In practice, coverage is sometimes restricted during the course of the field evaluation to only those patients participating in the study (e.g. the use of positron emission tomography scanners), that is, akin to an OIR recommendation; however, in other instances, hospitals can still purchase technologies and provide services through global budgets during the period of evaluation, thus appearing less restrictive than an OIR recommendation. Although this implicitly suggests that separate coverage schemes are considered when a CFFE is commissioned, there appears to be no formal distinction made within existing policy documents and other published literature between different types of schemes and also no discussion of the specific factors that might influence the degree of coverage. Instead, the type of scheme and degree of coverage appear to be determined on a case-by-case basis.
As well as any commonalities between CED and OIR/AWR schemes, there also appear to be similarities in terms of the challenges faced in successfully undertaking these schemes. In the UK, an OIR scheme can be recommended by NICE when appraising health technologies, but it also has potential in public health, diagnostics and devices. However, following an OIR recommendation, there are no formal arrangements to develop the research study required to reduce uncertainties. 3 NICE does not hold a budget to commission research so unless it is publically funded by research commissioners it will be undertaken only if manufacturers conduct it, with the NHS contributing excess treatment costs. The lack of co-ordination also makes it difficult to ensure an update of the recommendation following production of new evidence. 3 In the USA, although the CMS will cover the costs of a trial or registry associated with a CSP decision, there is currently no Medicare-specific funding mechanism for the additional data collection under CAD and hence there exists similar uncertainty concerning who will be responsible for paying for the additional data collection under CAD.
Although there have been several previous attempts to develop taxonomies,8,21,23,25 none of these has been focused specifically on OIR/AWR schemes and typically these are presented as part of a broader categorisation of conditional coverage and risk-sharing schemes. For example, in the taxonomy developed by Carlson et al. ,25 conditional coverage schemes are divided into CED and conditional treatment continuation schemes. Within CED, two subtypes are presented – OIR and ‘only with research’ (OWR) – with OWR similar to the term AWR used in this report. However, a more detailed consideration of OIR/AWR schemes and the potential for further subtypes within these schemes has not been previously explored in existing taxonomies.
General issues of ‘only in research’/‘approval with research’ schemes
There are many issues that need to be resolved to enable the successful implementation of an OIR or AWR scheme both generally and also within the specific constraints of NICE. 11 Central to this is the need to clarify the objectives of these schemes and the relevant criteria for their use. However, the critical review identified only limited discussion on the specific circumstances under which an OIR or AWR scheme may be an appropriate policy option. 9,10,51 The lack of any clear guidance has led to concerns expressed over ambiguity regarding their use26,57 and that OIR is currently being used as a ‘polite no’ by NICE. 11 Importantly, these concerns are not restricted to the use of OIR/AWR schemes by NICE. The general lack of clarity on the principles and criteria for using these schemes is reflected in many commentators’ views that the development and use of schemes internationally has appeared to be rather ad hoc to date. 57 Such concerns clearly highlight the importance of developing a clear set of principles for the use of OIR/AWR by NICE.
Although the majority of existing policy documents related to NICE are not explicit about the rationale and principles for the use of OIR and AWR in the UK, the report from the NICE Citizens Council11 on the use of OIR does describe the particular circumstances that the Council thought should be taken into account when NICE considers whether or not to issue OIR recommendations. These circumstances include:
-
Whether at least one appropriate, relevant study is:
-
– planned (e.g. the study will definitely start within 6 months of the guidance publication date)
-
– in progress (e.g. recruitment to the study is open and is expected to last at least 1 year beyond the guidance publication date) or
-
– could be established quickly (analysis in Chapter 5 explores the impact of the time taken for research to report).
-
-
Whether or not the question addressed by the study will contribute to reducing the uncertainties identified during the preparation of NICE guidance.
-
Whether or not the research is feasible (in terms of numbers of patients, recruitment, etc.) and is likely to deliver results within an appropriate time period.
-
Whether or not a fully supportive decision would lead to significant irrecoverable fixed costs of implementation (the impact of these types of cost are explored in Chapter 3, Technologies with significant irrecoverable costs and Chapter 5, Point 2: Are there significant irrecoverable costs?).
-
Whether a fully supportive decision, instead of an OIR recommendation, would lead to the termination of research in progress or prevent new research from beginning and thus have a negative impact on future collection of relevant information.
-
Whether or not it is realistic to hope that research can be carried out to the satisfaction of NICE. Factors to be considered include the timeliness of the research, potential number of patients able to participate in research, the pace of the current research and the precise nature of the questions to be answered.
In setting out a clear rationale and set of principles for the use of OIR/AWR, NICE will also be able to work towards identifying which technologies may be suitable for such policies. Ideally they should be those with potential net benefit but also some degree of uncertainty. 4 It has also been argued that these schemes could also be used to ‘fast track’ particular treatments. 26 However, in addition to their role in new and emerging technologies,3 other commentators have also stressed their potential use for established interventions to inform recommendations for increased investment or for disinvestment. 5
In addition to the rationale and principles, there are also numerous practical issues that need to be resolved for the successful use of such policies. The recent lung volume reduction surgery case study highlighted a number of challenges for OIR/AWR, in particular significant opposition from the clinical community, the significant level of funding required, the length of time required to complete data collection and limited access for patients in remote areas. As a result of the multiple sclerosis risk-sharing scheme, the importance of interagency collaboration, achieving consensus on acceptable quality of evidence, external peer review, predefined clinical benefit and determining who pays for treatment was also apparent. There also remain other important challenges, including the need to ensure that research is actually conducted and is fit for purpose, as well as ensuring that the process is undertaken in a legal, ethical and acceptable manner. 51 Another important consideration is that these schemes need to be designed in order to develop appropriate incentives to produce evidence in a timely fashion and strategies need to be put into place to ensure that the research is actually carried out.
Within the UK, the importance of interagency collaboration has been highlighted as a key issue in ensuring the success of OIR/AWR schemes. In particular, the Cooksey report9 recommended that arrangements between the Health Technology Assessment (HTA) programme, the NIHR Service Delivery and Organisation programme and NICE should be formalised so that recommendations for the use of interventions in the context of clinical studies can be operationalised.
Specific issues of ‘only in research’/‘approval with research’
As well as the more general issues that need to be resolved to ensure the effective use of OIR/AWR policy options, there are a number of specific issues that need to be addressed.
Evidence collection
Acquiring appropriate evidence following an OIR or AWR policy is of paramount importance. 25 Without an appropriately designed and conducted study, it is likely that little will be achieved in terms of reducing the uncertainty that led to the use of such policies in the first instance. OIR and AWR policies allow evidence to be generated specifically to inform decisions, a role not intended for traditional regulatory trials. 5 This raises a number of issues and potential challenges related to the design and funding of further research studies. First, there is currently very little in the way of formalised arrangements following an OIR/AWR recommendation in many countries. 18,28 One exception to this are CFFEs conducted in Ontario, which have a specific funding stream (albeit modest) covering the evaluation by PATH and additional monies for the fieldwork itself. However, in many instances this budget is not sufficient to cover the full costs of a CFFE and therefore other avenues must be explored, such as cost-sharing. 17,20
A key issue identified in determining the success of these schemes is the development of working partnerships between stakeholders (clinical community, decision-makers and manufacturers). 3 Related to this is the issue of obtaining funding for OIR/AWR studies and establishing who pays for the research. In relation to NICE, it has been recommended that the relevant study should be either planned or currently in progress, or alternatively that a new study could be established quickly. 11 Without secure funding the research may never be undertaken and thus the uncertainties leading to an OIR/AWR recommendation will remain.
The design of the OIR/AWR study will ultimately determine its success23 and some of the failures of existing schemes have been attributed to inappropriately designed studies. 57 Perhaps the most important consideration emerging from the literature is the issue of which type of study is most appropriate for an OIR/AWR scheme. 51 OIR/AWR research (unlike licensing research) is not confined to RCTs and, depending on the source of uncertainties, other types of evidence may be sufficient. 29 The choice of study is ultimately context specific and related to the source of uncertainty; however, it may also be influenced by factors such as cost and availability of suitable patients, collaborating clinical centres and potential ethical considerations. Good routine data capture mechanisms have a potentially crucial role to play in the feasibility of any scheme18 and the development of health informatics could greatly reduce the cost of evidence. 18,55 Whichever study type is chosen, Tunis and Whicher30 argue that discussion regarding the study design should not take place when the decision is made over who should pay for the study as this imposes restrictions. 30 Clarification is also needed on how the evidence collected as a result of a OIR/AWR policy will be used in an updated coverage decision31 and also how many data are enough to inform subsequent decisions. 29
Important lessons can be learnt from previous studies commissioned as part of an OIR or AWR policy. The multiple sclerosis risk-sharing scheme has been heavily criticised by many. 26 Despite its intention to reduce uncertainty23 associated with the use of the disease-modifying drug therapies beta-interferon and glatiramer acetate in the UK NHS, the exclusion of a control group from the study has meant that it is difficult to determine effectiveness. Similarly, the Implantable Cardioverter-Defibrillator Registry failed to provide the CMS with data required to answer questions regarding patient survival. 56 Another widely cited example, a trial of lung volume reduction surgery (NETT),5,22,27,28,32,33,57,58 is looked on favourably by some32,57 but has also been criticised for taking too long5 and for not addressing the uncertainties relevant for decision-making. A number of implications for future studies were also noted, in particular the importance of interagency collaboration, achieving consensus on acceptable quality of evidence, external peer review, predefined clinical benefit, determining who pays for treatment and establishing longer-term follow-up. 27
Investment and reversal costs
Investment and reversal costs have also been identified as relevant considerations in the existing literature. In particular, NICE needs to determine whether a fully supportive decision (as opposed to OIR) would lead to significant irretrievable costs of implementation and if it would lead to termination of ongoing research or prevent future research. 11 Gafni and Birch14 also highlight the need to consider what an intervention that has been subjected to a CED scheme is displacing before any assessment of potential cost savings through such schemes can be made.
There is also an ongoing challenge of disinvesting in technologies that have previously been approved. 18 Withdrawing coverage is logistically and politically difficult and it is considered more difficult to reverse a ‘yes’ than a ‘no’. 24 Although no clear consensus has emerged on how these costs could be factored into the decision-making process, it has been suggested that these could be based on formal options analysis. 29
Changing prices
Although discounting list prices can be thought of as an example of a risk-sharing agreement,23 depending on how the OIR/AWR system operates, it may also lead the manufacturer to reconsider the pricing of the technology. Allowing prices to change as part of an OIR/AWR scheme also further extends the options available to decision-makers. Evidence generated as part of an OIR/AWR clinical study may also lead to a change in price if NICE believes that there is significant new evidence that will affect a drug’s value. Something similar was observed with the multiple sclerosis risk-sharing scheme. Depending on the results observed, potential adjustments to the price of the drugs will be made at intervals to achieve an agreed cost per quality-adjusted life-year (QALY) of no more than £36,000. 23 The wider coverage associated with the multiple sclerosis risk-sharing scheme meant that it was necessary to have an upfront agreement on price changes following provision of evidence. It is not clear, however, to what extent changing prices will reduce uncertainty regarding the coverage decision.
Ethical issues
The potential ethical issues arising from the use of OIR/AWR schemes is another important theme emerging from the existing literature. For OIR, the issue of compulsory participation is often raised as a concern. Also, because of practical arrangements under OIR, treatments may not be available in all areas, causing geographical inequalities. 34 If a RCT is commissioned following an OIR recommendation, this raises a greater issue in terms of participation than a simple registry. These access issues in relation to an OIR policy linked to a clinical trial can be somewhat remedied by a large-scale geographically diverse trial with broad inclusion criteria. 22
In addition, it has been argued that denying access to a treatment demonstrated to be effective (however uncertain) is unethical. Patient advocacy groups may also be unwilling to accept this policy especially if the treatment is considered to be safe and efficacious. 24 These issues have important implications for both the design and the successful conduct of research and hence are considered in more detail in later sections.
Summary
The critical review identified a number of important themes and principles in relation to the use of OIR/AWR schemes. However, much of the existing literature is relatively discursive and there is a need to provide a set of principles and to establish an analytical framework to help guide and develop appropriate criteria for the use of OIR/AWR schemes by NICE.
Chapter 3 What assessments are needed?
Since an important objective of the NHS is to improve health outcomes across the population it serves, a technology can be regarded as valuable if its approval is expected to increase overall population health. The resources available to the NHS must be regarded as fixed (certainly by NICE) and so it is not sufficient to establish that a technology is more effective (the health benefits compensate for any potential harms) than the alternative interventions available, because approving a more costly technology will displace other health-care activities that would have otherwise generated improvements in health for other patients. 62 Therefore, even if a technology is expected to be more effective, the health gained must be compared with the health expected to be forgone elsewhere as a consequence of additional NHS costs, that is, a cost-effective technology will offer positive net health effects (NHEs). 63–65 A social objective of health improvement and an ethical principle that all health impacts are of equal significance, whether they accrue to those who might benefit from the technology or other NHS patients, is an established starting point for the NICE appraisal process (see Social value judgements and ethical principles). 1
An assessment of expected cost-effectiveness or NHEs relies on evidence about effectiveness, impact on long-term overall health and potential harms, as well as the costs that fall on the NHS budget together with some assessment of what health is likely to be forgone as a consequence (the cost-effectiveness threshold). 66 Such assessments are inevitably uncertain and, without sufficient and good-quality evidence, subsequent decisions about the use of technologies will also be uncertain and there will be a chance that the resources committed by the approval of a new technology may be wasted if the expected positive NHEs are not realised. Equally, rejecting a new technology will risk failing to provide access to a valuable intervention if the NHEs prove to be greater than expected. Therefore, if the social objective is to improve overall health for both current and future patients then the need for and value of additional evidence is an important consideration when making decisions about the use of technologies. 67–69
This is even more critical once it is recognised that the approval of a technology for widespread use might reduce the prospects of conducting the type of research that would provide the evidence needed. 70 In these circumstances there will be a trade-off between the NHEs for current patients from early access to a cost-effective technology and the health benefits for future patients from withholding approval until valuable research has been conducted. A key ethical question arising from this trade-off is whether or not the health impacts for future patients should be considered and regarded as of similar significance to impacts on current patients (see Social value judgements and ethical principles). 24
Because publically funded research also consumes valuable resources that could have been devoted to patient care, or other more valuable research priorities, there are a number of trade-offs that must be made. In making these trade-offs consideration also needs to be given to uncertain events in the near or distant future, which may change the value of the technology and the need for evidence. 71 In addition, implementing a decision to approve a new technology is, in general, not a costless activity and may commit resources that cannot subsequently be recovered if the guidance changes in the future. 6,7,72 For example, there may be costs associated with implementing guidance or training health-care professionals, or other investment costs associated with equipment and facilities. 73,74 The irrecoverable nature of these costs can have particular influence on a decision to approve a technology if new research is likely to report or other events may occur in the future (e.g. the launch of new technologies or changes in the price of existing technologies).
The primary purpose of this chapter is to provide a non-technical exposition of the conceptual framework, developed more formally in Appendix 6, which identifies the key principles and assessments that are needed when considering both approval and research decisions. The first section outlines the key principles and the different types of assessment needed and how each sequence might lead to different categories of guidance. The next section, Changes in prices and evidence, examines how guidance might change if there are changes in the effective price of the technology or evidence. The following section, Social value judgements and ethical principles, highlights the social values and ethical principles associated with OIR and AWR. a Importantly, within this chapter we do not presuppose how the assessments ought to be made because there are a range of different types of additional information, evidence and methods of analysis that might be useful. These alternatives are examined in Chapter 5 where they are more fully explored and evaluated through four case studies.
Key principles and assessments needed
The key principles and assessments fall into four broad areas:
-
expected cost-effectiveness and population NHEs (including benefits, harms and NHS costs)
-
the need for evidence and whether or not the type of research required can be conducted once a technology is approved for widespread use
-
whether or not there are sources of uncertainty that cannot be resolved by research but only over time
-
whether or not there are significant (opportunity) costs that will be committed and cannot be recovered once the technology is approved.
Guidance will depend on the combined effect of all of these assessments because they influence whether the benefits of research are likely to exceed the costs and whether any benefits of early approval are greater than withholding approval until additional research is conducted or other sources of uncertainty are resolved.
This can be complex because these different considerations interact. For example, the effect of irrecoverable costs will depend on the need for additional research and will also influence whether research is worthwhile. The sequence of assessments, decisions and resulting guidance can be represented by a flow chart or algorithm. Although such a representation is an inevitable simplification of the necessary trade-offs it helps to (1) identify how different guidance might be arrived at, (2) indicate the order in which assessments might be made, (3) identify how similar guidance might be arrived at through different combinations of considerations and (4) identify how guidance might change (e.g. following a reduction in price) and when it might be reviewed and decisions reconsidered. The complete algorithm is complex (reported in Appendix 2 for completeness), representing the sequences of assessments and associated decisions, each leading to a particular category and type of guidance. However, the key decision points in the algorithm, reflecting the main assessments and judgements required during appraisal, can be represented as a simple 7-point checklist (see Chapter 5, A checklist of assessment).
Four broad categories of guidance are represented within the algorithm and include ‘approve’, ‘AWR’, ‘OIR’ and ‘reject’. Each of the categories is further subdivided and numbered to indicate the different types of apparently similar guidance that could arise from different considerations. ‘Delay’ is not considered a particularly useful category because NICE always has the opportunity to revise its guidance, that is, a decision to reject can always be revised but it is only with hindsight that reject might appear to be delayed approval. The distinction made between assessment and decision reflects the NICE appraisal process: first, critically evaluate the information, evidence and analysis (an assessment), which can then assist the judgements (decisions) that are required in appraisal when formulating guidance.
Technologies without significant irrecoverable costs
Some element of cost that once committed by approval cannot be subsequently recovered is almost always present. However, the significance of these types of costs depends on their scale relative to expected population NHEs associated with the technology as well as the nature of subsequent events (see Technologies with significant irrecoverable costs and Chapter 5, Point 2: Are there significant irrecoverable costs?). 75 In this section we consider the relatively simple sequence of assessments and decisions which lead to guidance for those technologies that are not judged to have ‘significant’ irrecoverable costs associated with approval.
Technologies expected to be cost-effective
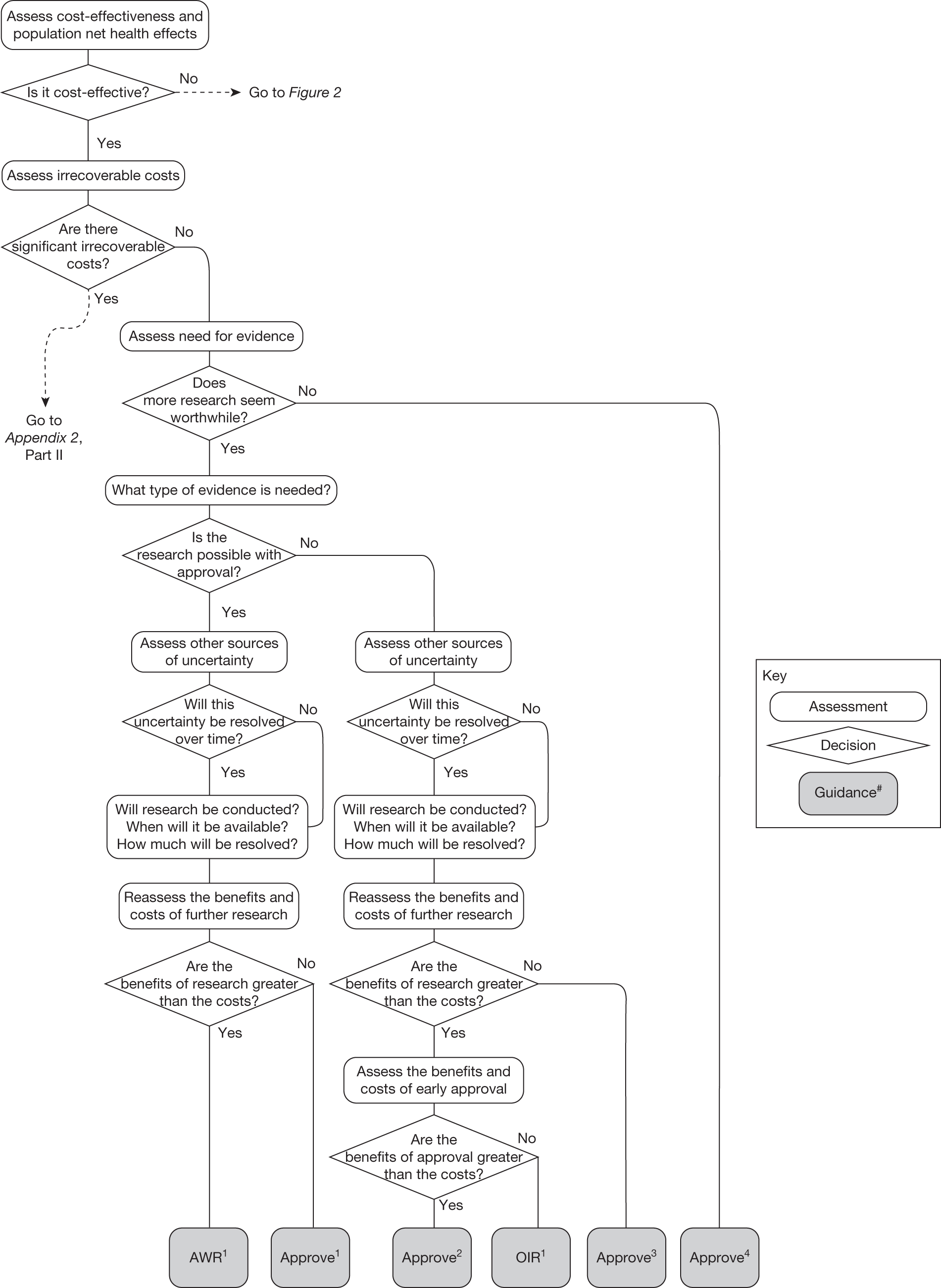
The sequence of assessments and decisions, which ultimately leads to guidance, starts with cost-effectiveness and the expected impact on population NHEs, that is, where existing NICE appraisal currently ends. This is an assessment of expected cost-effectiveness, that is, ‘on average’, based on the balance of the evidence and analyses currently available. It will include an assessment of effectiveness and potential for harms as well as NHS costs (see NICE’s Guide to Methods of Technology Appraisal1). Any assessment may be very uncertain with the scale and consequences of uncertainty assessed subsequently when considering the need for additional evidence. The sequence of assessments and decisions (judgements required) is illustrated in Figure 1. This demonstrates that an assessment of cost-effectiveness is only a first step and does not itself inevitably lead to a particular category of guidance. For example, a technology that might on balance be expected to be cost-effective might nevertheless receive OIR guidance if the additional evidence that is needed cannot be acquired if the technology is approved.
FIGURE 1.
Algorithm for technologies expected to be cost-effective.
Need for evidence
Some initial assessment of the need for further evidence and a decision about whether or not further research might be potentially worthwhile is important because a ‘no’ at this point can avoid further and complex assessments, for example a technology offering substantial and well-evidenced health benefits at modest additional cost is likely to exhibit little uncertainty about whether or not the expected population NHEs are positive. In these circumstances, further research may not even be potentially worthwhile (i.e. the opportunity costs of conducting this research exceed its potential value) and so guidance to approve could be issued on the basis of existing evidence and at the current price of the technology (e.g. ‘Approve4’ in Figure 1). If additional evidence is needed and further research might be worthwhile, then further assessments and decisions are required before guidance can be issued. Critically, some assessment is required of the type of evidence that is needed and whether or not the type of research required to provide it is likely to be conducted if approval is granted. 29
Research is possible with approval
If research is possible with approval, some further assessment of the long-term benefits of research is required, including (1) the likelihood that the type of research needed will be commissioned by research funders or conducted by manufacturers, (2) how long until such research will be commissioned, recruit and successfully report and (3) how much of the uncertainty might be resolved by the type of research that is likely to be undertaken. 70 An assessment of other sources of uncertainty that will resolve only over time is also needed, for example changes in prices or the launch of new technologies. 71 These sources of uncertainty will influence the future benefits of research that could be undertaken as part of AWR. For example, even if the current benefits of research, which might be very likely to be undertaken, are considerable, if the price of the technology is likely to fall significantly before or shortly after the research reports, or if future innovation makes the current technology obsolete (or more effective), then the future benefits, once the research reports, might be very limited. In these circumstances it might be better to approve (rather than use a policy of AWR) and reconsider whether and what type of research is needed at a later date once these uncertainties have resolved. The judgement of whether the long-term benefits of research are likely to exceed its expected costs determines guidance, with ‘AWR1’ and ‘Approve1’ in Figure 1 dependent on ‘yes’ and ‘no’, respectively.
Research is not possible with approval
The type of research needed, for example RCTs, may not be possible once a technology is approved for widespread NHS use (because of ethical concerns, recruitment problems and limited incentives for manufacturers). In these circumstances the expected benefits of approval for current patients must be balanced against the benefits to future patients of withholding approval to allow the research to be conducted. Initially, the same assessment of the long-term value of the type of research that might be conducted if approval is withheld is still required. Similarly, the impact of other sources of uncertainty on the longer-term benefits of research is also needed. If the benefits of research are judged to be less than the costs (i.e. research is not worthwhile anyway), the technology can be approved based on current evidence and prices (‘Approve3’ in Figure 1). However, judging that research is worthwhile at this point is not sufficient for OIR guidance. In addition, an assessment of whether the benefits of early approval (expected population net benefits for current patients) are greater than the opportunity costs (the net benefit of the evidence likely to be forgone for future patients as a consequence of approval) is required. If the expected benefits of early approval are judged to be less than the opportunity costs then OIR guidance would be appropriate (‘OIR1’ in Figure 1). Alternatively, if the expected benefits of early access for current patients are judged to be greater than the opportunity costs for future patients then approval would be appropriate (‘Approve2’ in Figure 1). All of these assessments, including the benefits of early approval and the value of evidence, will change if the effective price of the technology is reduced (see Changes in effective prices).
Technologies not expected to be cost-effective
A technology that is not expected to be cost-effective will, on balance, impose negative population NHEs if it is approved. These negative NHEs can arise because the technology may not be effective, the potential for harm exceeds any benefits and/or the additional NHS costs are not justified by the magnitude of the expected health benefits offered. In these circumstances approval can be ruled out, but which of the other categories of guidance might be appropriate will depend on subsequent assessments and decisions (Figure 2).
FIGURE 2.
Algorithm for technologies not expected to be cost-effective.
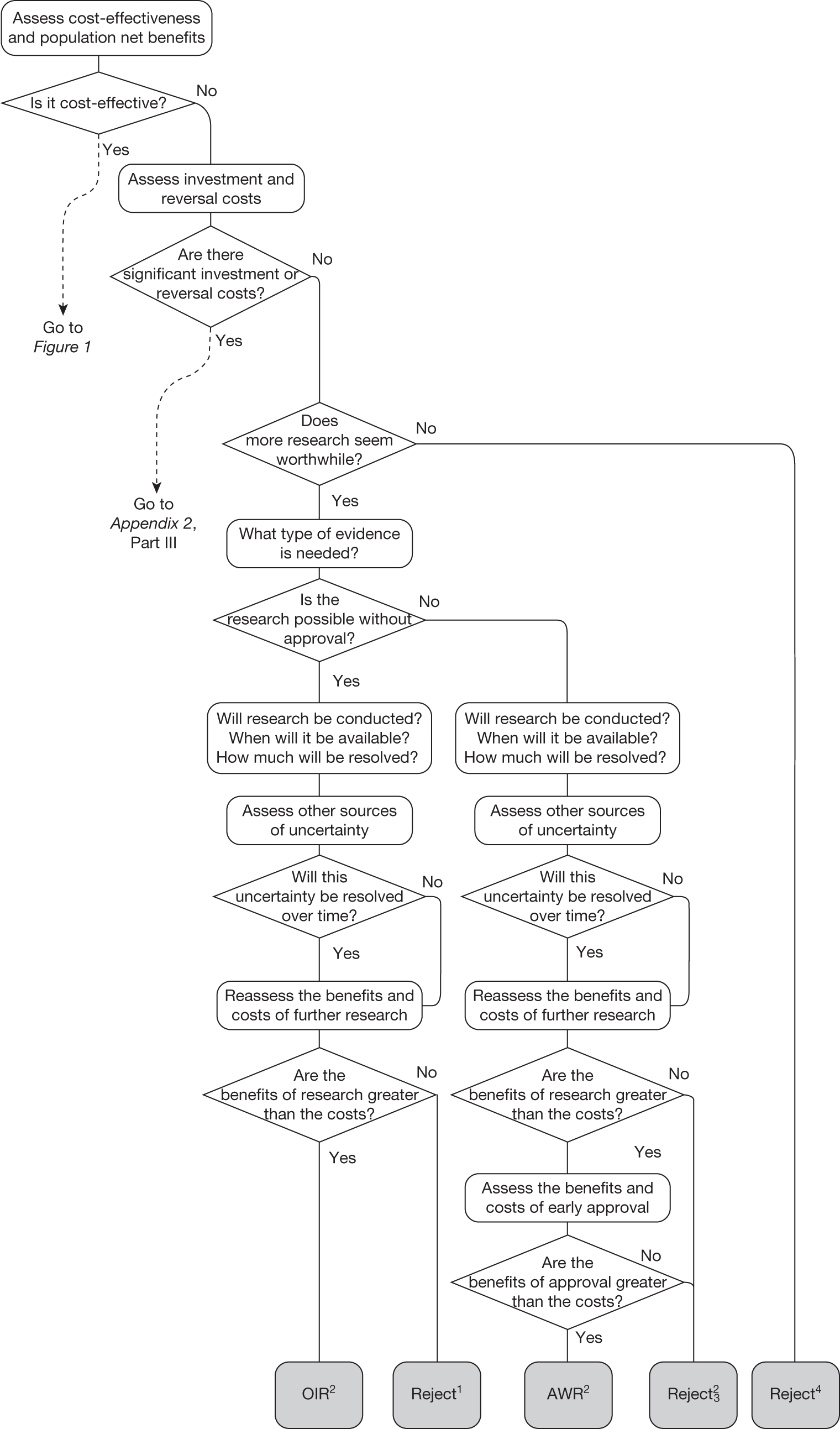
Need for evidence
Any assessment will be uncertain, so it remains possible that a technology that is not expected to be cost-effective on the balance of existing evidence might offer positive NHEs. Therefore, the scale and consequences of this uncertainty must be considered to make an initial assessment of the need for additional evidence and whether additional research might, in principle, be worthwhile. If it is not then the technology can be rejected based on existing evidence and its current price (‘Reject4’ in Figure 2). Alternatively, if further research might be worthwhile then an additional assessment is required of whether the type of evidence and research that is needed can be conducted without approval.
Research is possible without approval
Generally, most types of research (including RCTs) will be possible without approval. Further assessment of the longer-term benefits of the type of research that is likely to be conducted, and when it might report, is required, including the impact of other sources of uncertainty that will resolve over time. If, following this reassessment, the expected benefits of research are judged to exceed the associated costs then OIR would be appropriate (‘OIR2’ in Figure 2). Alternatively, if the costs of research are likely to exceed the longer-term expected benefits then the technology should be rejected at this point (‘Reject1’ in Figure 2).
Research is not possible without approval
In some circumstances it is possible that certain types of evidence might be acquired only, or be more easily acquired (more quickly and at lower cost), once a technology is in widespread use, for example linking surrogates (specific to the technology) to longer-term health outcomes, longer-term and/or rare adverse events, greater understanding of learning and incremental improvements in the use of a technology, or identifying the particular types of patients that might benefit most. 76 In this less common situation, in which the type of research needed is not possible (or is significantly more costly) without approval, the same assessment of the longer-term benefits of research is required. If further research is judged not to be worthwhile following this reassessment, the technology can be rejected (‘Reject2’ in Figure 2). Alternatively, if research is judged worthwhile, an additional assessment of whether the benefits of approval exceed the costs is required. In this case, approval of a cost-ineffective technology would make the research possible, but will impose opportunity costs (negative expected population NHEs). The key question is whether the net benefits of the research exceed these opportunity costs. If they do not then the technology should be rejected even though research, had it been possible without approval, would have been worthwhile (‘Reject3’ in Figure 2). Alternatively, if the net benefits of research more than offset the opportunity costs then AWR would be appropriate even though the technology is expected to be cost-ineffective (‘AWR2’ in Figure 2).
Therefore, AWR guidance for technologies not expected to be cost-effective is certainly possible but is appropriate only in certain circumstances: (1) the type of research needed is not possible without approval, (2) the long-term benefits of the research are likely to exceed the expected costs and (3) the additional net benefits of such research exceed the opportunity costs of approving a cost-ineffective technology. More commonly, research might be possible but more costly without approval. In these circumstances, AWR guidance could be considered only if the additional costs of research without approval exceed the opportunity costs of approving a cost-ineffective technology.
Technologies with significant irrecoverable costs
Irrecoverable costs are those that, once committed, cannot be recovered should guidance be revised at a later date. In most NICE appraisals these are included in the expected (per patient) cost of a technology. However, rarely is their potential additional impact explored when future events, such as research reporting or other sources of uncertainty resolving, might mean that guidance will be revised in the near or distant future. 6,72 These types of cost are commonly thought of as capital expenditure on equipment or facilities which have a life expectancy that extends beyond the current patient population. They might also include the resources required to implement guidance or to train staff to use a new health technology or a period of ‘learning’ during which NHEs are lower. Although these costs are incurred up front, they tend to be included in NICE assessments as if they are paid per patient treated over the lifetime of the equipment or facility. This common assumption will have no effect as long as guidance is certain not to change during this period. However, if it is possible that initial approval might be withdrawn at some point, then, although future patients will no longer use the technology, these upfront costs cannot be recovered (see Issues specific to ‘only in research’ recommendations). Therefore, in these circumstances it would be inappropriate to include these costs as if they were paid per patient treated (see Point 2: Are there significant irrecoverable costs?). The possibility that approve or AWR might be reconsidered after research reports, for example, and the impact that this would have on expected costs need to be considered before committing these types of capital costs, that is, it may be better to withhold approval and avoid commitment of resources until the uncertainty is resolved.
However, irrecoverable costs may be much more common. Even in the absence of capital investment in equipment and facilities, most new technologies offer a ‘risky investment profile’ for each patient treated. Generally they impose initial per patient treatment costs that exceed the immediate health benefits (see Point 1: Is it expected to be cost-effective?). These irrecoverable treatment costs are offset only by cost savings and health benefits in the longer run, that is, initially negative NHEs (losses) are only gradually compensated by later positive ones (gains). Therefore, a technology expected to be cost-effective may be expected to break-even, that is, when accumulated ‘gains’ compensate earlier ‘losses’, after some considerable time. If guidance is likely to change it is possible that initial losses will not be compensated by later gains and the expected additional NHEs will not be realised. 75 This type of investment profile becomes significant (has some influence on a decision to approve) if the decision to treat a presenting patient can be delayed until uncertainty is resolved (e.g. research reports or other events occur) because the commitment of irrecoverable opportunity costs (negative NHEs) can be avoided (see Point 2: Are there significant irrecoverable costs?). In these circumstances, OIR or reject avoids this commitment and preserves the option to approve the technology at a later date when its purchase by the NHS represents a ‘less risky investment’. 75,b
Although aspects of irrecoverable cost are almost always present, their potential significance also depends on their scale relative to expected population NHEs of the technology. Critically, their impact depends on the chance that guidance will be revised in the near or distant future as a result of new evidence becoming available or changes in prices and technologies. The full algorithm becomes more complex (see Figures 27 and 28 in Appendix 2) so here we focus on the key differences from the section on technologies without significant irrecoverable costs.
Technologies expected to be cost-effective
The presence of irrecoverable costs associated with a technology that is expected to be cost-effective will influence guidance and be regarded as ‘significant’ only if there are future events (research reporting or other sources of uncertainty resolving) that might change guidance. For example, if research is possible with approval and is expected to be worthwhile, AWR does not necessarily follow as previously (e.g. see ‘AWR1’ in Figure 1) because the impact of irrecoverable costs must also be considered. Now OIR may be more appropriate than AWR (e.g. the choice between ‘OIR4’ or ‘AWR4’ in Figure 27), even though the research would be possible with approval, because OIR avoids the commitment of irrecoverable costs until the results of research are known. This is especially so when there are also other sources of uncertainty that might resolve while the research is being conducted because they increase the chance that guidance will be revised (e.g. ‘OIR3’ or ‘AWR3’ in Figure 27).
If research is not possible with approval but is expected to be worthwhile, then OIR will be appropriate if the opportunity costs of early approval are judged to exceed the benefits (e.g. ‘OIR6’ rather than ‘Approve9’ in Figure 27). These opportunity costs will now also include the impact of irrecoverable costs when guidance might be changed as well as the value of evidence that will be forgone by early approval. Therefore, irrecoverable costs will tend to make OIR rather than approval more likely, particularly when there are other sources of uncertainty that might resolve while the research is being conducted (e.g. ‘OIR5’ rather than ‘Approve7’ in Figure 27).
If research is not judged worthwhile, approval does not necessarily follow as previously (e.g. ‘Approve1,3,4’ in Figure 26). Now the technology should be approved only if there are no other sources of uncertainty. If there are other sources of uncertainty, then an assessment of the benefits and costs of early approval is needed that takes account of irrecoverable costs and the risk that guidance might change in the future. Therefore, reject rather than approval is possible, even though a technology is expected to be cost-effective, because the decision to commit the irrecoverable costs can be reconsidered once the other sources of uncertainty have resolved (e.g. ‘Reject5,6’ in Figure 27).
Technologies not expected to be cost-effective
The presence of irrecoverable costs for technologies not expected to be cost-effective does not change the categories of guidance, or how they might be arrived at. However, it does mean that reject is more likely to be appropriate than AWR when research is not possible without approval (see ‘AWR5’ in Figure 28). This is because a decision to reject, although it may be revised to approve, generally does not commit irrecoverable costs. Although there may be resources associated with making sure subsequent approval is properly implemented, these costs are properly considered as an irrecoverable cost associated with approval (rather than a reversal cost of reject). There may be circumstances when implementing guidance to reject a technology also requires resources if it has already diffused into clinical practice. If these are significant they should be taken into account in the same way as other irrecoverable costs, tending to make AWR more likely to be appropriate.
Different types of guidance
Each sequence of assessment and decision leads to different categories and ‘types’ of guidance for technologies with differing characteristics, indications and target populations. The different ‘types’ of guidance illustrate how similar guidance might be arrived at in different ways, helping to identify the particular combinations of considerations that might underpin guidance, contributing to the transparency of the appraisal process. The possible categories and types of guidance are summarised in Table 1; the numbers in the body of the table refer to the numbered guidance in Figures 1 and 2 and the algorithm in Appendix 2 (see Figures 26–28).
| Type of guidance | No significant irrecoverable costs | Significant irrecoverable costs | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Research not needed | Research possible with approval | Research not possible with approval | Research not needed | Research possible with approval | Research not possible with approval | |||||
| Benefits > costs | Benefits < costs | Benefits > costs | Benefits < costs | Benefits > costs | Benefits < costs | Benefits > costs | Benefits < costs | |||
| Technologies expected to be cost-effective | ||||||||||
| Approve (12) | 4 | 1 | 2 | 3 | 11, 12 | 5, 6 | 7, 9 | 8, 10 | ||
| AWR (3) | 1 | 3, 4 | ||||||||
| OIR (5) | 1 | 3, 4 | 5, 6 | |||||||
| Reject (3) | 7 | 5 | 6 | |||||||
| Technologies not expected to be cost-effective | ||||||||||
| Approve (0) | ||||||||||
| AWR (2) | 2 | 5 | ||||||||
| OIR (2) | 2 | 7 | ||||||||
| Reject (8) | 4 | 1 | 2 | 3 | 11 | 8 | 9 | 10 | ||
The categories of guidance available to NICE have wider application than is reflected in previous guidance (see Chapter 4). For example, there are five different types of OIR that may be appropriate when a technology is expected to be cost-effective. Indeed, OIR may be appropriate even when research is possible with approval if there are significant irrecoverable costs. AWR can be considered only when research is possible with approval, but reject remains a possibility even for a cost-effective technology if there are irrecoverable costs. Therefore, the full range of categories of guidance (OIR and reject as well as AWR and approve) ought to be considered for technologies that, on the balance of existing evidence and current prices, are expected to be cost-effective.
It is only approval that can be ruled out if a technology is not expected to be cost-effective, that is, cost-effectiveness is necessary but not sufficient for approval but lack of cost-effectiveness is neither necessary nor sufficient for rejection. Although likely to be uncommon, there are circumstances when AWR may be appropriate even when a technology is not expected to be cost-effective (see Technologies without significant irrecoverable costs and Technologies expected to be cost-effective). More commonly the choice of appropriate guidance will be either reject or OIR. Importantly, which category of guidance will be appropriate depends only partly on an assessment of expected cost-effectiveness and hence this assessment should be regarded only as an initial step in formulating guidance. Guidance will depend on a number of other key assessments including (1) the need for evidence, (2) whether or not the type of research required is possible with approval, (3) the expected longer-term benefits and costs of the type of research likely to be conducted, (4) the impact of other sources of uncertainty that will resolve over time and (5) the significance of any irrecoverable costs.
Changes in prices and evidence
The type of guidance that might be appropriate will be influenced by changes in the effective price of the technology, the type of evidence available to support its use and whether or not further research is likely to be undertaken, either by manufacturers or research commissioners, as a result of OIR or AWR guidance.
Changes in effective prices
Any change in the effective price of a technology, either through patient access schemes (which offer some form of discount that reduces NHS costs) or direct price changes (possibly negotiated though a future value-based pricing scheme), will affect key assessments and decisions, leading to different ‘paths’ through the algorithm, consequently changing the category of guidance that would be appropriate (Simon Walker, Centre for Health Economics, University of York, UK, July 2011, personal communication). 4,23 For example, provisional OIR guidance for a technology that is expected to be cost-effective might be revised to approve with a sufficient price reduction because the benefits of early approval will be greater and uncertainty about its cost-effectiveness and therefore the value of additional evidence will tend to be lower (e.g. from ‘OIR1’ to ‘Approve2’ in Figure 1). c Similarly, AWR might be revised to approve if the benefits of early approval now exceed the value of additional evidence (e.g. from ‘AWR1’ to ‘Approve2’ in Figure 1). d
Equally, provisional guidance to reject a technology that is not expected to be cost-effective might be revised to OIR if the reduction in price is not sufficient to make it cost-effective but makes the costs associated with a reject decision more uncertain and hence the value of research worthwhile (e.g. from ‘Reject1’ to ‘OIR2’ in Figure 2). e If the reduction in price is greater and sufficient to make the technology cost-effective, then guidance might be revised to AWR if research remains worthwhile and possible with approval (e.g. from ‘Reject1’ or ‘OIR2’ in Figure 2 to ‘AWR1’ in Figure 1). Clearly, with an even greater reduction in price, it is possible that provisional guidance to reject could be altered to early approval (e.g. ‘Approve1’ in Figure 1). Even if research is not possible with approval, a sufficient reduction in price could also lead to early approval (e.g. from ‘Reject1’ or ‘OIR2’ in Figure 2 to ‘Approve2,3,4’ in Figure 1). f
Therefore, consideration of the effect of price changes on OIR and AWR is needed when assessing the potential impact of patient access schemes and more direct price negotiation through value-based pricing. 65,77–79 It should be noted that, all other things being equal, the presence of significant irrecoverable costs will require greater reductions in effective price to achieve the same revision to a more permissive category of guidance.
Threshold prices and value-based prices
The price at which the technology would just be expected to be cost-effective is commonly regarded as the value-based price for the technology, that is, the maximum price that the NHS can afford to pay without imposing negative health effects. 65,80 This single price describes the threshold for approve/reject decisions and would be the relevant threshold price when (1) OIR or AWR guidance is not available to the decision-maker or there is no uncertainty in cost-effectiveness or (2) the research, if needed, can be conducted with approval and (3) there are no irrecoverable costs. In all other circumstances there are a number of other threshold (value-based) prices. The number and value of these thresholds depends on the characteristics of the technology (the path through the algorithm); however, the threshold prices for approval will always be lower than the single approve/reject price based on expected cost-effectiveness.
For example, for a technology (without significant irrecoverable costs) for which research could be conducted without approval but not with it, there are two threshold prices: (1) the threshold that would move guidance from reject to OIR and (2) the threshold that would move guidance from OIR to approve. The latter will always be lower than the price that would move the same technology from reject to approve if OIR was excluded from consideration. If a technology also imposes significant irrecoverable costs then there may be more threshold prices. For example, when research can be conducted with or without approval there are three thresholds: (1) reject to OIR, (2) OIR to AWR and (3) AWR to approve. Again, (3) will be lower than the approve/reject threshold for the same technology if AWR was excluded from consideration. All other things being equal the presence of irrecoverable costs will tend to reduce the threshold price for approval.
Even in circumstances in which price negotiation becomes possible alongside NICE appraisal, it will be important to retain the OIR and AWR as available categories of guidance for two reasons. First, there is no guarantee that manufacturers will always agree to the lower price threshold that would lead to approval rather than OIR or AWR. Second, and possibly more importantly, there may be many circumstances when there is no effective price reduction that would make approval appropriate. g For example, reject or OIR guidance may still be appropriate even if the effective price of a technology is zero if there is substantial uncertainty about its effectiveness and/or potential for harms.
Incentives for evaluative research
These threshold prices represent the maximum effective price at launch to achieve a particular category of guidance when the results of any subsequent research that might be undertaken are not yet known. This is different to the type of flexible pricing agreements described in the current Pharmaceutical Price Regulation Scheme (PPRS) in which price is revised once the research reports and the results are known, with prices increasing if the evidence suggests that benefits were originally underestimated and reducing if they were overestimated. 81 This means that manufacturers retain an incentive to conduct further evaluative research if they believe that there are additional benefits that could not be evidenced at launch. Publically funded evaluative research, however, will still be required when these incentives are insufficient and especially in those cases in which the original evidence is likely to have overestimated the benefits or underestimated the potential for harm. However, it should be noted that linking effective prices to the results of publically funded research means that the NHS will benefit (realise the value of evidence) only if the results lead to a lower price or more restrictive guidance because the technology is found not to be cost-effective (thus avoiding the losses associated with negative NHEs). Manufacturers will, however, be able to appropriate the value of evidence if research results suggest that NHEs were originally underestimated because prices will increase within the flexible pricing scheme (or a value-based pricing scheme that might replace it). Even under current arrangements this value can be appropriated when the technology is reappraised by NICE (e.g. any patient access scheme could be withdrawn or less restrictive positive guidance issued). Consideration of how the NHS and manufacturers are likely to share the value of evidence might inform whether manufacturers should be expected to conduct the research specified in AWR or OIR guidance. As long as incentive-consistent contractual arrangements can be set in place, that is, those that can be monitored and enforced with credible penalties, it should ensure that any agreed research is conducted in the way intended. Alternatively, manufacturers might be expected to make some contribution to the costs of publically funded research that may ultimately benefit their product (see Chapter 5, Point 7: Are the benefits of approval greater than the costs?).
It is important that policy provides (or at least does not undermine) appropriate incentives for manufacturers to conduct the type of research needed to support NICE guidance at launch. The use of OIR and AWR guidance, as described in the algorithm, could provide clear signals and incentives. For example, the threshold price for reject/OIR and reject/AWR will be higher than that for OIR/approve and AWR/approve. Guidance restricted to OIR also offers very limited NHS volumes and revenue to manufacturers. This provides a strong incentive to ensure that the type of evidence which would require research that cannot be conducted once a technology is approved for NHS use is available and is sufficient at launch (e.g. relative effectiveness and subtle but important differences in side-effect profiles). Therefore, a predictable OIR and AWR policy signals what type of evidence is likely to be most important at an early stage.
The use of OIR and AWR, as described in the algorithm, offers manufacturers a choice to (1) accept OIR and AWR guidance at a higher price, (2) reduce the effective price to achieve approval, when that is possible or (3) conduct the evaluative research at an earlier stage so that cost-effectiveness is not uncertain at launch. Other things being equal, those new technologies that are supported during the NICE appraisal process by more, better-quality and relevant evidence will be more likely to be approved (rather than receiving OIR or AWR guidance) and at higher prices than those that are not, because additional evidence is less likely to be needed. Therefore, greater consideration of OIR and AWR will tend to reward those manufacturers who have invested in good-quality and relevant evidence with earlier approval of their technology. In addition, the effect of price on OIR and AWR recommendations suggests that those technologies supported by better evidence will tend to get approval at higher effective prices, providing an incentive for manufacturers to invest in the type of evidence needed earlier in the development process.
Assessing the prospects of research
When considering OIR or AWR guidance there must be some assessment of (1) the type of research needed to address the key uncertainties, (2) whether or not this will be regarded as ethical and can be undertaken while the technology is approved for use, (3) whether or not it is likely to be a priority for public funding and be commissioned and (4) when it is likely to report.
Although the NICE appraisal process may be well suited to identifying the need for evidence when assessing cost-effectiveness, these other critical assessments are not necessarily ones for which NICE and its Advisory Committees, as currently constituted, have particular expertise, not least because they reflect the decisions of those responsible for research design, prioritisation and commissioning. 3,82 Without sufficient co-ordination between these communities there is a danger that OIR or AWR could be issued when the type of research required would not be regarded as either ethical or feasible or of sufficient priority compared with other competing research needs to be commissioned. Because publically funded research is also budget constrained, it is perfectly possible that research that might be valuable from a wider NHS perspective might nevertheless not be a priority if other more valuable research might be displaced. This might be a particular concern if there is a possibility that the research could be undertaken by the manufacturer rather than displacing other research without proprietary interest. Therefore, a decision of whether OIR or AWR research should be undertaken by the manufacturer or through publically funded research is one that NICE cannot properly take alone.
Although some judgement about how the research community might respond to OIR or AWR recommendations when NICE is formulating guidance is clearly possible, more informed judgements and better decisions might be possible through greater involvement of the research community. For example, a Research Advisory Committee could be constituted that could consider provisional OIR or AWR guidance, making recommendations during the consultation period about the type of research needed and its ethics, feasibility and likely priority before final appraisal and guidance. It might also make recommendations about whether research should be publically funded or undertaken by the manufacturer with appropriate contractual arrangements. There are of course many different ways in which greater co-ordination might be achieved. However, because some of the assessments that NICE must make in formulating OIR or AWR guidance are, in fact, research decisions that fall outside its remit, it would seem sensible to draw on the expertise of those involved in, and responsible for, these types of research decisions to help make these assessments.
Social value judgements and ethical principlesh
This section clarifies and discusses ethical issues arising from OIR and AWR decisions. There are some important preliminary points. First, the question considered here is whether or not OIR and AWR recommendations are consistent with the values and principles that currently underpin standard NICE practices. 1 It is not in the remit of this report to evaluate those underpinning values and principles themselves. In particular, it is assumed here that the health budget is necessarily limited; also that, generally speaking – and although also taking into account issues of need and equity as discussed in the NICE values statements83 – scarce health-care resources ought to be broadly allocated so as to maximise health outcomes of the population as a whole and hence that treatments that benefit one group of patients will be funded at an opportunity cost to other patients. Given these assumptions, the emphasis in this discussion is on new ethical challenges created by OIR and AWR decisions11 as distinct from issues shared with standard NICE recommendations.
This section is in four parts: first, ethical issues raised by both OIR and AWR are discussed; second, ethical issues raised specifically by OIR are addressed; third, ethical issues specific to AWR are considered; and, finally, a summary of the ethical analysis is provided. It is important to have a working definition of OIR and AWR. The defining characteristic of an OIR decision is that a necessary condition of a patient receiving the treatment in question is that he or she participates in the relevant research; typically, NICE recommends that the innovative treatment is available under the NHS only as part of a RCT (although other types of research study might be relevant). The defining characteristic of an AWR decision is that receipt of the new treatment by patients is not conditional on their participation in the relevant research programme; typically, NICE approves the treatment but stipulates that further evidence must be collected, for example on long-term outcomes and adverse events, often in patient registries. This distinction between OIR and AWR is not always clear-cut. For example, an AWR decision limiting coverage to a subpopulation – members of a geographical region, say – whose access to the treatment is explicitly conditional on their contributing to evidence development by submitting their health information to a registry is, on the above definitions, more like an OIR than an AWR decision. Nonetheless, the working definitions of OIR and AWR suggested here suffice for the purposes of this ethical analysis.
Issues common to ‘only in research’ and ‘approval with research’ recommendations
Generally speaking, the benefit of attaching research conditions to NICE recommendations is an improved evidence base for resource allocation decisions in the future. The beneficiaries of the research are members of future populations who will profit from better informed allocation decisions. But achieving this benefit can impose significant opportunity costs on current patients. This is true for some OIR decisions and some AWR decisions.
To be clear about when a research condition does impose an opportunity cost on the present population, and how significant this is, two issues need to be carefully considered. The first is what is meant by present and future populations. For the purposes of this discussion the present population comprises people whose interests are directly affected by a NICE recommendation (e.g. they receive an innovative treatment approved by NICE, or benefit from resources made available because NICE rejects an innovative treatment). Future populations comprise people whose interests are indirectly affected by decisions, in particular by the subsequent research results that improve the evidence base for future NICE judgements. It is important to note that on these definitions some people – specifically patients with a chronic condition who live sufficiently long – will be members of both the present and the future populations. Thus, some individuals in the present population may benefit from the research condition because they will also be members of the future population. This will not be true of all, so the issue of balancing the interests of some individuals in the present population against some individuals in the future population remains.
The second issue is under what circumstances the present population is disadvantaged by the research condition compared with the alternative recommendation that NICE might make. This will depend both on what the alternative recommendation would be and on the level of current evidence about cost-effectiveness for the intervention. Three general types of situation might arise:
-
The evidence concerning the cost-effectiveness of a new treatment compared with standard treatment is genuinely balanced such that the likelihood of a new treatment turning out to be less cost-effective than standard treatment is the same as the likelihood of that new treatment turning out to be more cost-effective than standard treatment. Under these conditions it makes no difference to the present population whether a research-conditional recommendation is made or not. Indeed it makes no difference whether the new intervention is accepted or rejected. Future populations, however, will benefit from the research. Some current patients will be members of both present and future populations and will therefore benefit from research.
-
The balance of evidence as judged by NICE suggests that the new treatment is less cost-effective than standard treatment but NICE wishes strongly to encourage further research, hence it is considering a research-conditional recommendation. Assuming that NICE’s judgements on this issue are generally better than chance then the present population is disadvantaged by a research-conditional recommendation compared with rejection but advantaged by a research-conditional recommendation compared with acceptance. Future populations are always advantaged by effective research.
-
The balance of evidence is that the new treatment is more cost-effective than standard treatment. In this situation the present population is disadvantaged by a research-conditional recommendation compared with acceptance but advantaged by a research-conditional recommendation compared with rejection. Again, future populations will be advantaged by effective research.
There is currently little empirical evidence about whether or not NICE’s judgements about the relative cost-effectiveness of two interventions, in situations (2) and (3) above, are better than chance. If research-conditional recommendations are allowed then it will be important to collect data to examine whether or not further research, on average, confirms NICE’s initial judgement about expected cost-effectiveness. There is some evidence which suggests that relevant authorities do accurately judge when evidence favours neither one intervention nor the other. 84 This provides some grounds for expecting that when NICE judges that the evidence on cost-effectiveness favours an intervention, such a judgement will be correct more often than it is false.
In summary, in judging whether or not it is right to make a research-conditional recommendation there are four key issues to consider:
-
What is the likely effect on the current population of a research-conditional recommendation compared with whichever would be the alternative recommendation?
-
What is the likely benefit to the future population from the research?
-
What proportion of individuals in the present population is also likely to be in the future population?
-
How should we weigh up the disadvantages to individuals in the present population in relation to the advantages to individuals in the future population (some of whom will be in the present population)?
The first three of these considerations are essentially empirical issues and NICE will have to make judgements in the setting of uncertainty. The fourth issue is an ethical one. Do the interests of members of the future population count? If so, how are they to be weighed against the interests of members of the present population? Which set of interests should prevail?
One way of addressing this question is to consider how radical a departure from current NICE values, principles and practices it would be to accord weight to the interests of future populations. Arguably, doing so would not be much of a departure at all. For one thing, taking the interests of future populations into account is consistent with fundamental NICE assumptions about how to make allocation decisions. Specifically, NICE currently considers how the requirements of maximising health gain, need, equity and so on are to be balanced against each other; in doing so, NICE takes the identifiability of patients who benefit from an intervention to be irrelevant. So, the fact that beneficiaries of putting a research condition on approval are unidentifiable because they will exist only in the future does not seem to add anything to considerations that NICE currently recognises and weighs. Similarly, some consideration of future populations is implicit in standard NICE judgements. Consider, for example, new treatments for which evidence is poor but, nonetheless, what evidence there is suggests that they are cost-effective. If NICE took only the interests of the present population into account then these should be funded on the basis of the poor but positive evidence. In fact, such innovations are often rejected to ensure that the evidence attains the standard that NICE requires. The beneficiaries of this decision are not in the present population; what NICE has in mind is future populations of patients who will benefit from allocation decisions made on better evidence.
It would seem, then, that decisions that take into account the interests of future populations are consistent with NICE’s values, principles and practices. Nonetheless, there are some issues that require further thought. Although trading the interests of present and future populations coheres with the general NICE framework, quite how these sets of interests should be weighted will be important. Consider, for example, what might be called the ‘bird in the hand’ argument. Suppose that NICE is balancing the health benefit that will accrue from early approval against the value of further evidence. That the latter is considered greater than the former has to be offset against the fact that the former is more secure than the latter simply because one can be more confident about events in the near future (in this case that patients will enjoy health benefits from early approval) than about events in the further future (in this case that the research will report and improve the evidence base for allocation decisions). At this point, the discussion of the ethics of research-conditional judgements segues into practical considerations familiar from other sections of this report, such as the likelihood of research being conducted.
Issues specific to ‘only in research’ recommendations
To tease out the ethical issues raised by OIR it will be useful to construct an illustrative case. Suppose that NICE appraises a new treatment for which there is strong evidence of effectiveness – that is, the innovation is known to be clinically superior to existing treatments – but there is considerable uncertainty over its cost-effectiveness. Whether or not the new treatment would prove to be cost-effective depends not only on how expensive it is but also on how much health benefit it would produce – that is, quantification of the health benefit – compared with standard treatment. NICE might consider an OIR judgement in these circumstances to establish more exactly the size of benefit that, in turn, is deemed necessary to establish cost-effectiveness. For example, NICE could approve the new treatment only in the context of a RCT comprising two trial arms, the innovative treatment arm and the standard treatment arm. Crucially, on this decision, patients outside the trial, and participants randomly allocated to the standard arm of the RCT, would be denied what is almost certainly the better treatment for their condition. This scenario creates a number of important ethical issues.
Equipoise
A criterion established in research ethics for the legitimacy of carrying out a RCT is that there is substantial uncertainty as to which of the treatments being compared – that is, an innovative treatment and a standard treatment – is the more effective. This is sometimes known as the principle of equipoise. The principle is meant to capture the intuition that no one – patient or participant – should knowingly be offered less than the best treatment for their condition. OIR decisions may be made when there is such substantial uncertainty. In such cases, the principle is respected and ethical review of the relevant research poses only issues already considered as standard by researchers and Research Ethics Committees (RECs). However, in the situation envisaged here, NICE is considering recommending OIR when the intervention in question is clearly superior to alternatives, but the degree of its superiority remains uncertain, and so its cost-effectiveness is uncertain. Evidently, in this scenario researchers are not in equipoise about the relative effectiveness of the two interventions. The substantial uncertainty relates to whether the more effective, but more expensive, treatment produces sufficient extra benefit compared with alternatives for it to be recommended by NICE, but it does not relate to whether it is more effective. Is it permissible to flout the principle of equipoise concerning effectiveness and give an OIR decision in such circumstances?
An intuitive response is that patients are harmed by an OIR decision that denies them the best-known treatment for their condition in the interests of research. Theoretical support for this intuition is provided by the well-known principle of maleficence: above all, do no harm. But harm-based objections to OIR are inconclusive for two reasons. First, the concept of harm is contested. The three main accounts define someone being harmed by contrast with, respectively, (1) their state before the harm was perpetrated, (2) the state they could have been in and (3) a minimum or baseline standard of well-being. When clinicians and patients are not sufficiently uncertain about the effectiveness of the treatments being compared, patients denied a better treatment by OIR are harmed according to the definition of harm based on (2) because they are put in a worse state than was possible. But the patients are not harmed according to the understanding of harm based on (1) and (3) because they will receive the standard NHS treatment (i.e. the same treatment as patients who do not take part in the research, or the same treatment as all NHS patients would have received had NICE rejected the treatment rather than approved it as OIR). So, harm-based objections to OIR are as inconclusive as the current debate on the definition of harm. Second, harm-based objections to OIR are question-begging, in the following way. Suppose that there was a consensus on the nature of harm and, further, on the fact that OIR without equipoise harms some patients. To conclude that this makes OIR impermissible is to assume that the harm in question outweighs the benefits of research. But this is precisely what is in dispute, namely, the relative values of the benefits of early approval and of further research. Given NICE values, principles and practices, it is perfectly feasible to conclude that the harm perpetrated by OIR is justified by the benefits to future patients of a better evidence base for allocation decisions.
There are more fruitful lines of thought about OIR without equipoise. First, the principle of equipoise itself is under considerable strain from pressures that have nothing to do with OIR and AWR. For example, it has been argued that it is permissible to trial less than the best-known treatment for human immunodeficiency virus in developing countries unable to afford the most effective interventions. 85 It is generally accepted that, as a result of such pressures, the principle of equipoise needs to be refashioned. NICE can trade on this by asking, ‘About what must researchers be uncertain?’. The traditional requirement is that researchers must be in equipoise about the best-known treatment; but, NICE might argue, the really salient uncertainty is not about effectiveness per se, but about the extent of effectiveness, and hence about the cost-effectiveness. Because, in the scenario envisaged here, the OIR recommendation is made precisely because of uncertainty about the extent of effectiveness, the refashioned principle of equipoise is respected. This argument is part of a larger research ethics question about what is required in terms of equipoise, so will not be addressed here. In the present context there is a more important practical consideration to emphasise. A RCT required by OIR would have to be reviewed by a REC. RECs are used to requiring traditional equipoise (i.e. substantial uncertainty about which is the better treatment). So, if RECs are to approve research of the kind considered here, in which researchers are not in equipoise as traditionally understood, they will have to be informed of, and agree with, the rationale for conducting these distinctive studies.
Another way of looking at this issue is as follows. If NICE does not advise OIR then it must advise either to approve or to not approve. If it advises not to approve, because although the treatment is clinically the more effective it is judged not to be sufficiently effective to be cost-effective, then no one receives this treatment on the NHS. Compared with that situation, an OIR decision would benefit some patients and harm none. If NICE was to approve the treatment then all patients for whom the treatment is relevant would benefit and, by comparison, an OIR decision would harm some patients [on definition (2) above]. But other patients might be unfairly harmed by the decision to approve because if, in fact, the treatment is not sufficiently effective to be cost-effective the opportunity costs of providing the treatment outweigh the benefits. And because the relevant research is not being carried out, it will remain unknown that this is the case. In any event it would be problematic for a REC to refuse to sanction the type of research we are considering once NICE had made an OIR decision because that condemns both present and future populations to receive only the inferior treatment, whether or not the more effective treatment is sufficiently effective.
Coercion
Another research ethics principle relevant to the sort of OIR decision under discussion is that competent patients have the right to consent to participate in, and withdraw from, a research project. This is akin to the competent patient’s right to consent to treatment, both rights being underpinned by the principle of respect for individual autonomy. Conversely, it is impermissible to coerce competent patients to participate in research. In OIR the patient can have the more effective intervention on the NHS only if he or she agrees to be a research participant. Does this coerce patients to participate in the trial?
Any research study involving care clinically superior to that available on the NHS provides an incentive to enrol in that study. Whether or not such an incentive constitutes coercion depends on whether or not the patient is being presented with a threat as opposed to an offer to participate. Importantly, in the sort of OIR decisions under discussion, patients who do not receive the new treatment – that is, patients not enrolled on the trial, and participants not allocated to the new treatment arm – will receive standard NHS care. Arguably, then, the trial provides an offer, namely, the chance of receiving better than standard treatment, as opposed to any threat, and so does not constitute coercion. To clarify, suppose, by contrast, that patients will be refused access to normal NHS care unless they agree to participate in the research; this would present a threat as opposed to an offer to participate, and thereby constitute coercion. In sum, providing that standard research ethics requirements are met – principally, that prospective participants are properly informed about, and give valid consent to participate in, the trial, and that if they do not participate their access to standard NHS care will not be affected – the research required by OIR is not coercive and respects the principle of individual autonomy because patients retain the right to choose whether or not to accept the chance of better than standard treatment offered by the trial.
Equity
A further ethical worry is that OIR decisions result in inequity because participants in one arm of the trial receive better treatment than both those in the other arm and those not participating in the research. It is important to distinguish two versions of this inequity charge. The first is that it is always wrong to allocate health resources in ways that will lead to an unequal distribution of health benefits. This version of the inequity worry is bound to founder in this context because NICE’s values and principles, which are taken as granted here, entail that limited health resources will and should be allocated to maximise benefit to the whole population, even at the expense of subgroups within it. So, the fact that OIR results in an unequal distribution of health benefits can be justified by NICE’s principles if the research will provide sufficiently valuable evidence.
A second version of this inequity worry is more involved. Under OIR, some patients will receive the intervention (paid for by the NHS) and others will not, simply because the former happen to be in a position to participate in the research. Many of the factors that determine access to treatment through participation in research – such as geographical location, socioeconomic status and patient characteristics – should not be considered relevant to whether patients have access to the treatments being studied. This is a significant consideration but one that can be overridden. Specifically, in deciding whether or not an OIR recommendation is ethically acceptable, a judgement would need to be made whether or not the benefits of such a recommendation outweigh the lack of equity (although, in fulfilling an OIR recommendation, it would always be important to minimise such inequities).
Issues specific to ‘approval with research’ recommendations
‘Approval with research’ raises a problem about consent, which is related to the discussion of coercion above. AWR also raises a further distinctive issue centring on the likelihood of the research taking place.
Consent
Two established principles of medical ethics are that competent patients have a right to confidentiality (including a right to decide whether or not to disclose their personal medical information) and a right to informed consent to participate in research (including a right to decline to participate in, or to decide to withdraw from, a study with impunity). It might be argued that some AWR decisions transgress these rights because the required research may involve collecting data on long-term outcomes and adverse events on patient registries (or some similar system of epidemiological data collection) without the explicit consent of patients. This argument is unsound. For one thing, in many cases a patient’s decision to have a treatment that was approved with research may imply consent to the relevant data collection. Furthermore, although it comprises personal medical information, the data collected will be anonymous, ameliorating concerns about breaching confidentiality. Finally, this type of data collection that may be required by an AWR decision is equivalent to current large-scale epidemiological research studies that are considered ethically permissible. So, concerning consent, and on grounds of consistency, AWR should be permitted as long as the research is conducted to the ethical standards normally required of data collection of this sort.
Incentivising research
A further ethical issue raised specifically by AWR recommendations involves the mechanisms used to give ‘teeth’ to the research requirement: how will NICE ensure that the relevant research is carried out? One option has a subtle ethical dimension. NICE might threaten that, if the research is not satisfactorily completed (e.g. by the relevant manufacturing company), the intervention would cease to be made available on the NHS. Although this would provide an incentive for the manufacturer to carry out the research, it raises the following problem. At time T(1), the AWR decision is made, that is, the intervention is funded by the NHS. Suppose that, at time T(2) – the time when NICE reconsiders the decision to fund – the research has not been carried out (or has failed to provide any further relevant information). At time T(2) NICE will be making a decision on exactly the same information and evidence as at T(1). In this case it would seem that NICE should make exactly the same decision, namely, to provide the intervention on the NHS. But if NICE decides to reject the intervention (on the grounds, for example, that the manufacturer had failed to carry out the relevant research) then patients could claim unfairness. The unfairness is that they were provided with the treatment on the NHS between T(1) and T(2) but not after T(2) even though the evidence is exactly the same in both situations.
This is a serious problem for AWR decisions but it is not essentially an ethical matter. Rather, it is another point at which the ethical discussion segues into practical considerations familiar from other sections of this report. Specifically, various problems will result from the research condition put on an AWR decision not being met, and not just the ethical quandary outlined here. This situation should be avoided by doing everything reasonable to ensure that the relevant research will be conducted and reported. In fact, the risk that the ethical issue described here will arise if the research condition is not met can be used to put further pressure on whoever is responsible for conducting the research.
Summary of ethical analysis
Although they create numerous ethical challenges, OIR and AWR judgements are ethically permissible given the values and principles that underpin standard NICE practices. Nonetheless, this ethical analysis has identified some issues requiring further thought, which are highlighted in this summary.
Futurity
The fact that OIR and AWR trade off the interests of present and future populations is justified given current NICE values, principles and practices. Nonetheless, such decisions rely on research in the future being conducted, reported and useful. Future events are less predictable the more distant they are from the present and so the benefits of early approval are more secure than the value of future research. This might justify approving clinically beneficial treatments even though further research to determine their degree of effectiveness would be valuable.
Research Ethics Committees
‘Only in research’ decisions when clinicians and researchers would not be in equipoise about the effectiveness (as opposed to the cost-effectiveness) of an intervention are consistent with current NICE values, principles and practices, and further justified by the pressure brought on the principle of equipoise from elsewhere. Nonetheless, RECs are used to requiring traditional equipoise, that is, uncertainty about effectiveness. So, RECs may disallow trials required by an OIR decision unless they are informed of, and agree with, the rationale for contravening the traditional principle of equipoise.
Informing participants
Participants in trials required by an OIR decision are not coerced. Nonetheless, patients are strongly incentivised to participate by the prospect of being allocated to the new treatment arm of a trial. Two things must be made very clear to prospective participants: that participation does not guarantee that the new treatment will be received and that standard NHS care will be neither denied nor compromised if a patient declines to participate in, or decides to withdraw from, a study.
Unfair access to a trial
The inequity involved in an OIR decision is justifiable given current NICE values, principles and practices. Criteria for access to the treatment trial have to be established. Nonetheless, thought should be given to how to minimise unfairness. For example, if geographical location is a criterion of access, over-representation of certain regions at the expense of others should be avoided.
Registries
Evidence development required by AWR may involve a health data registry. These evidence development mechanisms should comply with standard epidemiological research ethics practices. For example, explicit consent to provide data should be collected whenever this is feasible, data collection and storage should comply with current data protection legislation and data should be anonymous.
Incentivising research
There is an important practical question as to whether or not an AWR research requirement will be met. One option is to incentivise researchers by threatening to withdraw approval if the research fails to report. This has a subtle ethical dimension: to carry out the threat would be unfair on future patients because the evidence base has not changed since the original AWR judgement. This is a form of inequity that strengthens the requirement to ensure that research conditions on decisions to approve treatments are met.
Chapter 4 A review of ‘only in research’/‘approval with research’ recommendations in NICE technology appraisal guidance
Introduction
The National Institute for Health and Clinical Excellence issues technology appraisal guidance on the use of new and existing health technologies in the NHS. The recommendations are formulated by independent Appraisal Committees following a review of evidence and submissions from interested parties on the technology of interest. Since its inception, NICE has occasionally issued guidance that includes recommendations for further research as an alternative to binary ‘accept’ or ‘reject’ decisions. Previous studies that have considered the use of NICE research recommendations have tended to focus on the OIR-type recommendations. 3 It remains unclear whether the remit of NICE includes provision for issuing guidance in which approval is conditional on research being conducted (AWR). For example, NICE has no specific budget for research funding to accompany its recommendations to the NHS. In addition, the organisations that are responsible for implementing NICE guidance also do not have dedicated funding for NICE-recommended research. Nevertheless, AWR-type recommendations appear to have been issued by NICE in the past.
The National Institute for Health and Clinical Excellence provides its committees with general guidance on the health technology assessment methodologies and social value judgements it considers to be most appropriate for the formulation of NICE guidance. 1,83 These documents refer to the possibility of issuing recommendations in the context of research. The document describing the social value judgements that NICE expects its Advisory Committees to consider phrases the possibility of issuing research recommendations quite broadly in a way that could capture both OIR- and AWR-type recommendations: ‘NICE’s advisory bodies may recommend the use of the intervention within a research programme if this will provide more information about its effectiveness, safety or cost’. 83 However, the Guide to the Methods of Technology Appraisal refers more specifically to OIR-type recommendations and states that ‘the Appraisal Committee may recommend that particular interventions are used within the NHS only in the context of research’. 1 Also, NICE has recently categorised its guidance into four groups (recommended, optimised, only in research and not recommended) and does not include an AWR category.
In its guidance to Advisory Committees, NICE specifies four issues that should be considered when recommending further research within the guidance. These are (1) whether or not the intervention is reasonably likely to benefit patients and the public, (2) how easily the research can be set up or whether it is already planned or in progress, (3) how likely the research is to provide further evidence and (4) whether or not the research is good value for money. 1 However, it is not clear to what extent these criteria have been considered in the formulation of NICE guidance. In addition, there is no guidance on when NICE advisory bodies should consider recommending research rather than ‘accept’ or ‘reject’ decisions.
The main aim of this review was to identify where OIR/AWR recommendations were made or considered in the development of NICE guidance. Secondary aims were to identify the considerations that led to the recommendations for further research and to assess the implementation of the OIR/AWR recommendations based on reviews of published guidance.
Methods
Inclusion and exclusion criteria
All NICE technology appraisal draft and final guidance up to January 2010 was considered for inclusion in the review. NICE guidance documents are published in a standardised format, with the guidance to the NHS presented in section 1. The rest of the document provides an overview of the evidence (sections 2 and 3), an explanation of how the evidence was interpreted by the committee (section 4), additional information to assist the implementation of the guidance (section 5) and a list of recommended related research (section 6). The research recommendations in section 6 do not form part of the formal guidance to the NHS. The committee’s final recommendations [Final Appraisal Determinations (FADs)] are made publicly available and can be appealed by specific stakeholders before becoming final guidance to the NHS. In 2002 the NICE process was amended to also publish draft guidance [Appraisal Consultation Documents (ACDs)] for public consultation. This review included both final published guidance and draft guidance. In some cases multiple ACDs or FADs were issued by NICE when guidance changed following consultation; in these cases all ACDs and FADs, along with the final guidance, were reviewed.
The criterion for inclusion was guidance from the Technology Appraisal Programme that recommends research, or refers to ongoing or planned research, in the guidance section of the documents. The research recommendations could be framed either as OIR or AWR based on the following definitions for this review:
-
OIR – a recommendation which states in the guidance section that the technology should not be used routinely unless it is in the context of further research
-
AWR – a recommendation which states in the guidance section that the technology should be used routinely and which recommends further research is conducted.
Only documents that have been made publicly available are included [specifically, ACDs for Technology Appraisals (TAs) TA1–43, except TA32, were not made publicly available]. Documents that have been publicly released but later removed from the NICE website are included in the review (e.g. guidance that has been replaced by a subsequent review) and have been obtained directly from NICE where appropriate. Draft recommendations that request further clarification or analysis from the sponsor of the technology (sometimes referred to as ‘minded no’ recommendations in the STA process) are excluded as they usually require the reanalysis of existing data rather than additional data collection. The documents containing OIR/AWR recommendations were cross-checked with a list of appraisals including OIR recommendations complied by NICE to check for potential omissions (Sarah Garner, Associate Director of Research and Development, 4 March 2010, personal communication).
Data extraction and analysis
Data from each draft and final guidance document that included OIR and/or AWR recommendations were extracted using a template developed for the project (see Appendix 3). The template was developed following the review of the literature described in Chapter 2, and iteratively alongside the development of the framework described in Chapter 3. Data extracted included information on the appraisal process, information on the technology, reported and accepted incremental cost-effectiveness ratios (ICERs) and the reasons for including an OIR or AWR recommendation in the guidance.
Data were extracted by one reviewer (JY) and a sample cross-checked by another reviewer (LL). The data were analysed to identify common characteristics of appraisals that included OIR and/or AWR recommendations. When OIR/AWR recommendations changed between draft and final guidance, explanations for the change were reviewed and assessed. In addition, the extracted data were analysed to assess the extent to which the assessments proposed in the framework in Chapter 3 were evident in informing the OIR/AWR recommendation in the draft or final guidance. Specifically, the following assessments were considered:
-
significant irrecoverable costs
-
cost-effectiveness of the technologies
-
need for further research and the type of evidence requested
-
possibility of conducting research with and without approval
-
impact of price on the considerations
-
resolution of uncertainties over time
-
relative costs and benefits of research
-
other considerations.
Significant irrecoverable costs
The guidance was reviewed for consideration of significant irrecoverable costs and explanations of their impact on the recommendations. It became clear early on that there would be little or no mention of these costs in the guidance, therefore an additional step was taken to assess whether or not the technologies were likely to incur irrecoverable costs. A possible indication of whether or not a technology has irrecoverable costs is if the directive to make NHS funding available for the treatment has been extended. Usually the directive is for funding to be available within 3 months of guidance being issued; however, this period can be extended if there are issues relating to implementation (e.g. a need to amend the NHS infrastructure or a need for significant training of staff to deliver the technology). The OIR/AWR guidance was cross-checked against the appraisals that have received extensions to the 3-month directive, and associated documents from the Department of Health website were reviewed for information on the reason for the extension.
Cost-effectiveness of the technologies
The NICE Guide to the Methods of Technology Appraisal1 states that all appraisals should include an assessment of cost-effectiveness as a standard part of the NICE appraisal process. ICERs reported as considered most plausible by the committee and ICERs reported by the Evidence Review Group (ERG) or Assessment Group (AG) were extracted and classified according to the NICE threshold range for cost-effectiveness: < £20,000; £20,000–30,000 and > £30,000 per additional QALY gained. 1 In addition, consideration was given to whether or not reference was made to if the committee considered the technology to be a cost-effective use of NHS resources.
Need for further research and the type of evidence requested
The reasons for OIR/AWR recommendations and the type of evidence requested were reviewed from the ‘Committee’s Considerations’ section in the guidance documents. Potential reasons for inclusion of OIR/AWR recommendations were identified at the outset after reviewing the preliminary findings of the systematic review and the draft framework for assessment. The following categories for which a need for further evidence was considered necessary were included:
-
clinical effectiveness:
-
– relative clinical effectiveness
-
– data on the natural history/progression of disease
-
– long-term data
-
– relative clinical effectiveness for the OIR/AWR population
-
– adverse effects
-
– data to substantiate mechanism of treatment action
-
-
cost-effectiveness:
-
– to resolve uncertainty in cost-effectiveness estimates
-
– cost-effectiveness data with an appropriate comparator
-
– quality-of-life data
-
– cost data
-
-
other uncertainties:
-
– budget impact
-
– investment and irreversible costs
-
– potential impact on ongoing research.
-
Possibility of conducting research with and without approval
The data were reviewed for reference to the possibility of conducting research and the potential impact of the recommendations on ongoing or future research. In addition, the type of research design requested (experimental or observational) was recorded. Although an imperfect proxy, this can give an indication of the possibility of conducting research because, as discussed in Chapter 3, it may be more difficult to obtain data on relative effectiveness if a technology has already been recommended for routine use as patients or their clinicians may be unwilling to participate in randomised trials that include the previous (‘old’) standard care.
Impact of price on the considerations
Standard NICE technology appraisal methodology is to consider the list price of technologies [e.g. as reported in the British National Formulary (BNF) for drugs] but not to take account of possible future price changes or local discounts. 1 However, national discounts such as those offered through patient access schemes may be considered. 86 The aim of these schemes is to ‘improve the cost effectiveness of a medicine and therefore allow NICE to recommend treatments it would otherwise have found too costly’. Manufacturers may formally offer a reduction in the price of the technology to the NHS or may offer other schemes that reduce the overall cost of the technology to the NHS (e.g. by providing some courses of treatment at no cost). Although a formal process for patient access schemes was introduced in 2009, ‘access’ or ‘risk-sharing’ schemes have previously been adopted, for example the Department of Health risk-sharing scheme for beta-interferon. 87 However, unlike the multiple sclerosis scheme, data collection is not necessarily required for NICE patient access schemes, which can take many different forms (e.g. a simple reduction in price). Information on patient access schemes for OIR/AWR guidance was collected as part of the review.
Resolution of uncertainties over time
References to possible changes over time that could resolve the key uncertainties identified by the committee were sought from the guidance documents as well as the impact of these considerations on the guidance.
Relative costs and benefits of research
Reference in the guidance documents to consideration of the relative costs and benefits of research were sought. This could include formal value of information-type analyses or qualitative assessments.
Review of implementation of the ‘only in research’/‘approval with research’ recommendations
Each piece of NICE guidance is considered for review at a specified length of time after publication (usually 3 years). To examine the impact of OIR/AWR recommendations on evidence collection and future guidance we considered guidance that included OIR/AWR recommendations and that had been updated by NICE. The documents were examined for changes in the evidence base available between original appraisal and review that were considered by the committee, and for changes to the decisions reflected in the guidance.
Results
Use of ‘only in research’ and ‘approval with research’ recommendations in NICE technology appraisal guidance
Of the 184 appraisals conducted up to January 2010, 40 included OIR/AWR recommendations in the draft and/or final guidance. In total, 29 FADs and 31 ACDs were issued relating to these 40 appraisals and which included OIR/AWR recommendations. Multiple ACDs were released for some appraisals; the 31 ACDs with OIR/AWR recommendations arise from 25 appraisals. All of the 29 FADs relate to the final guidance to the NHS (i.e. there were no changes to the recommendations between publication of the FAD and it becoming guidance). Table 2 shows the frequency of OIR and AWR recommendations in the guidance documents. A list of all appraisals including OIR and AWR recommendations is provided in Appendix 3.
The research recommendations in the guidance most commonly took the form of OIR guidance. The terminology used in the OIR guidance differed between appraisals. Some guidance was specific about the type of research recommended whereas other guidance was much more general. For example, TA588 on the introduction of liquid-based cytology for cervical cancer screening is very specific in the research it recommends; it includes a whole host of recommendations and refers to ‘a programme of pilot implementation projects’ that should evaluate a range of impacts including ‘the effect on test results’, ‘the extent to which productivity improvements in cytology laboratories are realised in routine practice’ and ‘the impact in the primary care setting’. However, the guidance for a subgroup of patients in TA16389 simply states that ‘infliximab should only be used for the treatment of acute exacerbations of active ulcerative colitis in clinical trials’. It could be inferred that the more specific recommendations are recommending the research in a more positive way, whereas some of the more general recommendations could be seen to be akin to a diluted reject decision sometimes referred to as a ‘polite no’. Some guidance also refers to research ongoing at the time that the guidance was published. For example, TA890 on hearing aids states that ‘There is insufficient robust scientific evidence to support the nationwide introduction of digital hearing aids at present. Evidence regarding the benefits of digital devices as compared with the current NHS range and to more sophisticated analogue devices, is expected to be available after the completion of research projects currently being undertaken in the UK.’
Despite not being a formal category of guidance used by NICE, a handful of appraisals took the form of AWR guidance, that is, they recommended the technology but also recommended further research in the guidance section. One example of an AWR recommendation in final guidance comes from TA11391 on the use of inhaled insulin. The guidance section recommends the use of inhaled insulin for a specific subgroup of patients and also includes the statement that ‘Data on the use of inhaled insulin according to this guidance should be collected as part of a coordinated prospective observational study.’ Another example of AWR guidance included very specific recommendations for further research. TA3692 on treatments for rheumatoid arthritis recommended within the guidance that ‘All clinicians prescribing etanercept or infliximab should (with the patient’s consent) register the patient with the Biologics Registry established by the British Society for Rheumatology (BSR) and forward information on dosage, outcome and toxicity on a 6-monthly basis.’
Changes to the inclusion or exclusion of OIR/AWR recommendations between draft and final guidance were more common than suggested by the summary numbers in Table 2. Eleven appraisals included OIR/AWR recommendations in the draft guidance but not in the final guidance. Three appraisals included OIR/AWR recommendations in the final guidance but not in the draft guidance (ACDs were unavailable for a further 12 appraisals). The reported issues that led to the changes are explored further later in this chapter.
| Recommendation | Draft guidance | Final guidance |
|---|---|---|
| OIR | 26 | 25 |
| AWR | 5 | 4 |
| Total | 31 | 29 |
Most pieces of NICE guidance included several recommendations. These related to multiple technologies, multiple indications or different settings for the use of the technology. Over half of the OIR/AWR recommendations specified the need for further research in specified subgroups of patients (52% of OIR/AWR recommendations in final guidance documents). In approximately a quarter of cases, the OIR/AWR recommendations targeted a subset of the technologies included in the appraisal.
Table 3 shows the frequency of OIR/AWR recommendations by year since the beginning of the Technology Appraisal Programme, and the total number of final OIR/AWR recommendations as a proportion of the total number of pieces of final guidance that year. Sixteen per cent of all final guidance included an OIR/AWR recommendation. It appears that OIR/AWR guidance has been issued less frequently over the last few years. No final guidance included OIR/AWR recommendations in 2007, which is also the year that the STA process was introduced. Differences in the frequency of OIR/AWR recommendations were observed between the two NICE appraisal processes. Of appraisals issued through the multiple technology appraisal (MTA) process, OIR or AWR recommendations were included in draft guidance of 23 appraisals and final guidance of 28 appraisals. These 28 appraisals account for 19% of all final guidance issued within the MTA process. In the STA process only two ACDs and one piece of final guidance contained OIR/AWR recommendations. This accounts for just 2% of all final guidance issued through the STA process up to the time that the review was conducted.
| Publication year | Draft guidance | Final guidance | Total no. of pieces of final guidance | % of final guidance including an OIR/AWR recommendation | ||||
|---|---|---|---|---|---|---|---|---|
| OIR | AWR | Total | OIR | AWR | Total | |||
| 2000 | N/A | N/A | N/A | 6 | 0 | 6 | 17 | 35 |
| 2001 | N/A | N/A | N/A | 2 | 0 | 2 | 14 | 14 |
| 2002 | 4 | 2 | 6 | 3 | 3 | 6 | 23 | 26 |
| 2003 | 3 | 0 | 3 | 4 | 0 | 4 | 19 | 21 |
| 2004 | 2 | 0 | 2 | 1 | 0 | 1 | 13 | 8 |
| 2005 | 6 | 1 | 7 | 3 | 0 | 3 | 7 | 43 |
| 2006 | 5 | 1 | 6 | 3 | 1 | 4 | 19 | 21 |
| 2007 | 2 | 1 | 3 | 0 | 0 | 0 | 21 | 0 |
| 2008 | 4 | 0 | 4 | 2 | 0 | 2 | 32 | 6 |
| 2009 | 0 | 0 | 0 | 1 | 0 | 1 | 19 | 5 |
| Total | 26 | 5 | 31 | 25 | 4 | 29 | 184 | 16 |
The data were examined for differences in the use of OIR/AWR recommendations according to general disease area and the type of technology under appraisal. In absolute terms OIR/AWR recommendations were more common for cancer treatments (n = 10), accounting for over a third of all of the OIR/AWR recommendations in final guidance, followed by musculoskeletal conditions (n = 7), which accounted for almost a quarter of cases identified (Table 4). However, NICE has appraised a large number of treatments for cancer: 28% of all published appraisals over the review period. Only 7% of all NICE technology appraisal guidance has related to musculoskeletal conditions and so it appears that a disproportionate amount of this guidance has included OIR/AWR recommendations compared with appraisals for other conditions.
| Disease area | Draft guidance, n | Final guidance, n |
|---|---|---|
| Musculoskeletal | 6 | 7 |
| Cancer | 8 | 10 |
| Mental health and behavioural conditions | 3 | 3 |
| Eye | 1 | 1 |
| Ear and nose | 1 | 1 |
| Infectious diseases | 1 | 1 |
| Mouth and dental | 0 | 1 |
| Urogenital | 0 | 1 |
| Endocrine, nutritional and metabolic | 2 | 1 |
| Digestive system | 1 | 1 |
| Cardiovascular/central nervous system | 2 | 2 |
| Total | 25 | 29 |
Just over half of the final guidance with OIR/AWR recommendations related to the appraisal of drugs (n = 16; 55%). However, taking into account the total number of pieces of drug guidance published, the use of OIR/AWR appears to be on average less common for drug appraisals: 11% of all drug appraisals within the period contained OIR/AWR compared with 47% of all guidance on therapeutic or surgical procedures and 27% of all guidance on devices.
Consideration of irrecoverable costs
Investment and irrecoverable costs were not explicitly quantified in any of the guidance documents and there were no documented considerations relating to these costs in the OIR/AWR guidance. This suggests either that the technologies reviewed by NICE do not have significant irrecoverable costs or that such costs do not explicitly influence decision-making. Three of the technology appraisals that included OIR/AWR guidance (TA60,93 TA68,94 TA9795) had their funding direction extended and, although the rationale for this is not explicit on the Department of Health website, the characteristics of the technologies suggest that it could be due, at least in part, to a need for significant additional training for staff. In addition, one appraisal explicitly noted some concerns around training although this was not directly cited as a reason for issuing the OIR guidance. TA5196 (an earlier version of TA9795) on computerised cognitive behavioural therapy (CCBT) stated that ‘Further information is required about the extent of training needed and circumstances under which different staff could provide support for users of CCBT.’ Thus, it would appear that some of the technologies considered in the Technology Appraisal Programme are associated with significant irrecoverable costs even if these have not previously been explicitly incorporated into the decision-making process.
Cost-effectiveness of technologies
As expected given the NICE process, all OIR/AWR appraisals included a consideration of the cost-effectiveness of the technologies. Most of the guidance documents reported several different estimates of incremental cost-effectiveness based on analyses submitted by different stakeholders relating to different uses of the technology or based on different sets of assumptions or evidence. However, a formal assessment of cost-effectiveness was not always conducted or reported in the ACD or FAD for the use of the technology specified in the OIR/AWR recommendation. Table 5 shows the ICERs (incremental cost per QALY gained) for the overall population and for the specific OIR/AWR indication where this differs. The table distinguishes between the base-case estimates of the ICER submitted by the AG/ERG in their original analysis and the estimate of the ICER considered most plausible by the Appraisal Committee following appraisal of all of the evidence submitted to NICE and noted in the guidance documents.
| Incremental cost per QALY | OIR/AWR indication | Total population | ||
|---|---|---|---|---|
| Committee’s preferred estimate | AG/ERG’s estimate | Committee’s preferred estimate | AG/ERG’s estimate | |
| Not reported | 30 (68) | 22 (50) | 23 (52) | 10 (23) |
| Dominates | 0 | 1 (2) | 0 | 1 (2) |
| ICER < £20,000 | 0 | 2 (5) | 1 (2) | 4 (9) |
| ICER £20,000–30,000 | 4 (9) | 1 (2) | 5 (11) | 3 (7) |
| ICER > £30,000 | 9 (20) | 15 (34) | 12 (27) | 22 (50) |
| Dominated | 0 | 2 (5) | 0 | 1 (2) |
| Other | 1 (2) | 1 (2) | 3 (7) | 3 (7) |
| Totala | 44 (100) | 44 (100) | 44 (100) | 44 (100) |
Most documents did not cite the ICER considered by the Appraisal Committee to be most realistic. The ERG/AG estimates were more frequently reported and it is likely that they were available in supporting documents and were not directly referred to in the ACD or FAD. ICERs for technologies with OIR/AWR recommendations were frequently > £30,000 (the upper bound of the NICE threshold range). The ‘other’ category includes ICERs that were reported using non-QALY-based outcome metrics and ICERs presented as a range that could not be classified into the categories. For example, TA588 on the use of liquid-based cytology reported ICERs of £1100 and £2500 per life-year gained depending on the length of the screening interval. When ICERs were not directly reported, there was usually an indication of whether the technology was considered to be cost-effective. For example, TA6597 states that ‘The clinical and cost effectiveness of rituximab in patients with localised disease has not been established’ and TA4498 states that ‘Appraisal Committee believed that metal on metal hip resurfacing arthroplasty was likely to be of similar cost effectiveness to conventional total hip replacements in people who were expected to outlive the device’.
Table 6 shows the numbers of technologies stated to be cost-effective when used in the context of the OIR/AWR recommendation according to the final guidance document. In most cases an OIR recommendation was issued and the technology was on average not cost-effective. There were two examples of an OIR recommendation being used when the technology was probably cost-effective based on the accepted analyses. Both of these appraisals (TA588 on liquid-based cytology and TA5196 on CCBT) requested that pilot implementation programmes be undertaken before routine introduction of the technologies in the NHS. The technology was considered likely to be cost-effective in three of the four cases in which an AWR recommendation was issued. In the remaining appraisal including an AWR recommendation [TA3692 – etanercept (Enbrel®, Wyeth) and infliximab (Remicade®, Schering-Plough) for rheumatoid arthritis], the ICERs were £27,000–35,000, close to, or above, the upper end of the cost-effectiveness threshold range.
| Cost-effectiveness | OIR, n | AWR, n | Total, n |
|---|---|---|---|
| Considered cost-effective | 2 | 3 | 5 |
| Not considered cost-effective | 23 | 1 | 24 |
| Total | 25 | 4 | 29 |
Need for further evidence and type of evidence requested
The rationales for issuing the OIR/AWR recommendations as stated in the guidance documents are summarised in Table 7 (note that multiple reasons were cited in some cases). Three of the four appraisals that did not provide a rationale were issued before the committee’s considerations were routinely described in the documents. The OIR recommendation in the other appraisal (TA7599 on hepatitis C) related to three specific subgroups of patients; two subgroups were not referred to in the committee’s considerations at all and no evidence was noted for the third subgroup.
| Reason for requesting further research | Draft guidance, n | Final guidance, n |
|---|---|---|
| None stated | 1 | 4 |
| Clinical effectiveness | ||
| Need for more evidence on relative effectiveness in the whole population | 19 | 16 |
| Need for data on relative effectiveness in the target OIR population | 15 | 9 |
| Need for longer-term data | 13 | 7 |
| Need for information on adverse effects | 6 | 4 |
| Need for data on natural history/progression of disease | 2 | 0 |
| Need further evidence to support mechanism of treatment action | 4 | 3 |
| Cost-effectiveness | ||
| Uncertainty in cost-effectiveness estimates | 13 | 6 |
| Need for cost-effectiveness data with an appropriate comparator | 2 | 2 |
| Need for more data on quality-of-life impact | 6 | 3 |
| Need for more data on costs | 1 | 1 |
| Other uncertainties | ||
| Budget impact | 0 | 0 |
| Investment and reversal costs | 0 | 0 |
| Potential impact on ongoing research | 0 | 0 |
A need for further evidence on the relative effectiveness of the intervention in the overall population or the OIR/AWR subgroup was the most commonly cited reason for issuing the OIR/AWR recommendation. A need for longer-term data was also frequently cited. Uncertainty in the cost-effectiveness estimates was also a common consideration; however, in all cases this was coupled with a need for further clinical evidence. Concern about the budget impact of introducing the technology, investment and reversal costs and the potential impact on ongoing research did not lead to the OIR/AWR recommendation in any of the appraisals.
In some appraisals the stated rationale for the OIR/AWR recommendation changed between draft and final guidance. For example, the use of infliximab for the treatment of acute exacerbations of ulcerative colitis (TA16389) was recommended OIR in both the ACD and the FAD. However, the final guidance cited only poor relative effectiveness evidence as a rationale for the OIR recommendation whereas the draft guidance also cited a need for other types of evidence such as information on adverse effects and cost-effectiveness data.
Possibility of conducting research with and without approval
No appraisals cited a concern for the impact of recommendations on ongoing trials as a direct rationale for the OIR/AWR guidance. In addition, the possibility of conducting research was not explicitly noted in most appraisals. Decision-makers may be more likely to use OIR recommendations than AWR when data on relative effectiveness are required. A lack of sufficient evidence on relative effectiveness was cited in 19 pieces of final guidance identified in the review and most of these included OIR recommendations (i.e. including those that stated a need for more evidence on relative effectiveness for the whole population or for evidence on relative effectiveness for the target OIR population in Table 7). The exception to this was TA11391 (inhaled insulin), which included an AWR recommendation for a highly selective subgroup of patients, and which noted the committee consideration that data on relative effect would be most appropriately collected through a disease register.
Experimental research designs, such as RCTs, were more frequently referred to, and were slightly more common in draft guidance (Table 8). Two appraisals cited a need for further evidence on relative effectiveness in the final guidance but recommended observational studies because of anticipated difficulties in conducting RCTs in the specific OIR patient population (TA37100) or indication (TA167101). There were changes in the recommended type of research between draft and final guidance, which were mainly due to changes in the target OIR/AWR population (e.g. TA6894) or changes to be less specific about the research design (e.g. TA89102).
| Type of research | Draft guidance, n (%) | Final guidance, n (%) |
|---|---|---|
| Experimental | 21 (68) | 14 (48) |
| Observational | 5 (16) | 7 (24) |
| Unclear (or both) | 5 (16) | 8 (28) |
| Total | 31 (100) | 29 (100) |
Overall, reference to the likelihood of research being conducted was rare in the guidance documents. However, there was one notable exception in which the likelihood of further research being conducted led to a change in the guidance: the ACD for TA129103 stated that bortezomib monotherapy was not recommended except for use in well-designed clinical studies; however, this recommendation was removed in the FAD, which noted that no further studies of bortezomib were planned and highlighted a difficulty of conducting further research in the group of patients to which the guidance related (this guidance was later further amended following the offer of a patient access scheme).
The impact of price on ‘only in research’/‘approval with research’ recommendations
Price changes or discounts featured in two of the appraisals including OIR/AWR recommendations. A patient access scheme was incorporated into TA129103 and the guidance changed from reject to approval. This appraisal had previously included an OIR recommendation in draft guidance but was changed to reject prior to the offer of the access scheme as described in the previous section. In the second appraisal, an OIR recommendation was revised to approval after the committee revised their estimates of cost-effectiveness based on discounted prices of the technology along with further information on quality-of-life improvements (TA166104 on cochlear implants).
Other considerations
Considerations around whether uncertainties in the evidence base would resolve over time were not explicitly mentioned as reasons for issuing OIR or AWR recommendations. In addition, the relative costs and benefits of conducting research were not reported as considerations of the committee when formulating its research recommendations.
Review of implementation of the ‘only in research’/‘approval with research’ recommendations
Among the appraisals with OIR/AWR recommendations in the final guidance, 10 were later reviewed by NICE, including two that were incorporated into NICE clinical guidelines. Table 9 provides details of the appraisals and whether or not additional evidence was provided and the change to the OIR/AWR recommendation (new evidence for other recommendations included within the guidance is not noted in the table).
| Original | Review | Additional evidence provided for the OIR/AWR indication | Summary of change to OIR/AWR guidance |
|---|---|---|---|
| TA588 | TA69107 | New evidence available. Pilot implementation programmes were requested in the OIR. A Scottish implementation study and other evidence became available | OIR removed: technology recommended |
| TA6105 | TA30108 | No additional evidence presented | OIR amended: TA30108 includes an OIR recommendation for a more restricted indication |
| TA16109 | TA89102 | Updated RCT data and new non-RCT evidence | OIR unchanged (some amendments to types of evidence required) |
| TA17110 | TA105111 | New evidence (RCTs) available | OIR removed: technology recommended |
| TA30108 | CG81112 | New evidence (RCT and registry data) available | OIR removed: CG81112 did not include the OIR indication in the scope of the guideline |
| TA33106 | TA93113 | No new RCTs but updated adverse effect data | OIR unchanged |
| TA3692 | TA130114 (only in ACD) | New RCT and registry data available | AWR removed: technology recommended. A new OIR for another use of the drugs in ACD, but this was removed in the FAD (no guidance provided for this use of the drugs) |
| TA37100 | TA137115 | No new evidence presented | OIR removed: technology recommended |
| TA5196 | TA9795 | New evidence (RCT and non-RCT) available | OIR amended: original OIR was for CCBT as a class; amended OIR was for specific CCBT packages |
| TA72116 | CG79117 | New evidence (RCTs) available | OIR unchanged |
In the majority of reviewed appraisals (n = 7), new evidence informing the OIR/AWR recommendation was available for the review. In four of these reviews, the OIR or AWR restriction was removed and the technology was recommended routinely. In two cases the additional evidence was considered insufficient to warrant a change in the OIR recommendation. In the remaining appraisal the OIR was revised so that some technologies within the class were recommended routinely whereas OIR recommendations were issued for others (TA5196 on CCBT). In all cases the changes in the guidance were owing to the new data relating to the evidence gap identified in the OIR/AWR recommendation.
In three cases no new evidence was provided on the OIR/AWR indication. For the review of TA6,105 no new RCT data were available for the OIR recommendation, which was made more restrictive in the review guidance. New evidence on clinical effectiveness was not available for the review of TA33,106 but further information on adverse effects was provided. This was considered inadequate and no change was made to the OIR recommendation. The OIR recommendation was removed from the review of TA37100 despite a lack of new evidence presented. The documents state that the reasons for this were a reduction in demand for the drug in this setting (it had since become licensed and NICE approved for treatment of an earlier stage of disease) and concerns about the feasibility of future data collection.
Conclusions
The National Institute for Health and Clinical Excellence has issued OIR/AWR recommendations in 16% of its published technology appraisal guidance. These recommendations have most frequently taken the form of OIR recommendations. OIR recommendations have mainly been issued for technologies considered not to be cost-effective by the Appraisal Committee; however, there has been occasional use of OIR for technologies for which the best available evidence suggests that they may be cost-effective: liquid-based cytology for cervical cancer screening and CCBT. In both of these cases, the implementation of routine use of the technologies in the NHS could have required fairly substantial infrastructure or training requirements and possibly significant irreversible costs. In addition, in both cases the recommended research referred to ‘pilot implementation projects’. Changes in the evidence base of reviewed appraisals show some limited success in the conduct of research recommended in OIR/AWR guidance; however, it is unclear if the research can be directly attributable to the NICE recommendations.
The most common reason cited for the OIR/AWR recommendations was the need for further evidence on relative effectiveness. Although uncertainty over the cost-effectiveness of the OIR/AWR technologies was cited in several appraisals, this was always accompanied by concern about the uncertainty around the clinical effectiveness. Some of the assessments recommended in the proposed framework do not appear to be currently considered when formulating guidance. Consequently it is not possible to categorise the recommendations into the 35 possible decision pathways described in Chapter 3. The lack of explicit consideration given to irrecoverable costs when formulating OIR/AWR guidance is notable. It seems unlikely that such costs do not occur in the provision of the technologies considered by NICE, particularly given that the introduction of some of the technologies has required changes to the existing provision of NHS infrastructure that have been expected to take longer than the standard 3 months.
The National Institute of Health and Clinical Excellence has recently issued a categorisation of all of its technology appraisal guidance. There are some differences between the NICE categorisation and the results of this review because of differences in the definitions employed. The most notable differences relate to the classification of AWR guidance. NICE does not use the terminology of AWR in its classification system. However, this review has identified a small number of appraisals that apparently fall into this category of approving a technology for use, and also recommending research within the guidance to the NHS. In all of these cases, observational studies and/or data collection through disease registers were recommended. The NICE categorisation refers only to final guidance. Of the four pieces of final guidance identified in this review, NICE categorised one as recommended, two as optimised and one as OIR. The lack of a formal category of AWR guidance from NICE most likely reflects its remit, which is to make recommendations on the best of technologies within the NHS rather than to make recommendations on research to research funders. Despite this, there is clearly ambiguity in the terminology used in the guidance and differences in the interpretation of recommendations for research made within the guidance section of the documents.
One striking finding from this review is the decline in use of OIR/AWR recommendations over the past 5 years. The decline in the use of OIR/AWR recommendations coincides with the introduction of the STA process in 2006. Only one appraisal conducted through this process – which is now the most commonly used route for new technologies – included an OIR recommendation in the final guidance and none included AWR recommendations. At first glance this may appear to be counterintuitive. Technologies appraised through this process are usually new and therefore have a more limited evidence base than technologies appraised through the MTA process. However, it could also be that the STA process has started to shift the burden of proof of effectiveness and cost-effectiveness onto manufacturers and sponsors of technologies and recommendations to the NHS regarding the research of these technologies are seen as less relevant. It could also be linked to an increased opportunity to negotiate on the costs of technologies through patient access schemes or to tighter time and resource constraints in the production of STA guidance. The rarity of OIR/AWR recommendations in the STA process does not fully account for the reduction over time and there has been a decline in OIR/AWR recommendations within the MTA process. There is no clear reason for this from the documentation included in this review. However, insights gained from discussion with NICE stakeholders at workshops held as part of this research have suggested that it may be in line with other developments since the establishment of NICE – including refinements to its processes and the phrasing of the guidance. In addition, a possible explanation may include a realisation of the difficulties of developing and implementing OIR/AWR recommendations – perhaps because of difficulties in getting research funders to implement the recommendations.
One limitation of this analysis has been the reliance on the documented considerations of the Appraisal Committee in formulating its recommendations and whether these, in some cases fairly brief, summaries fully reflect all of the considerations that led to the recommendations and specifically the recommendations for research. In addition, identifying the ICERs related to the OIR/AWR indication and considered by the committee to be the most plausible was difficult as it was not always clearly stated and/or available from the documentation, although the clarity of reporting of ICERs has improved in the more recent documentation. Finally, focusing on the reviews as an indication of the success of the research recommendations could bias towards a positive finding as a lack of new evidence could have led to the postponement of planned reviews. However, information from the NICE website suggests that research to potentially inform a review is being conducted in most of the appraisals including OIR/AWR recommendations. Sixteen OIR/AWR appraisals have been considered for review: six have been postponed pending the reporting of ongoing research and a further six are ongoing or scheduled. Only two reviews have been cancelled because of a lack of new evidence and a further two have been cancelled because of the technology becoming obsolete.
Recent developments at NICE have increased the potential for further research alongside approval, although the uptake remains limited. One opportunity has arisen through the supplementary guidance to NICE committees about technologies used to treat patients at the end of life. 118 This guidance describes criteria for when the committees should consider departing from the usual criteria for cost-effectiveness. In addition, the guidance states that when recommending a treatment under the end-of-life criteria, NICE ‘will normally recommend to the Department of Health that it should give consideration to a data collection exercise for treatment recommended for use on the basis of the criteria set out in Section 2. The purpose of this will be to assess the extent to which the anticipated survival gains are evident when the treatments involved are used in routine practice. The outcome of this exercise will be evaluated when the guidance for that treatment is reviewed.’ However, in practice the uptake of this recommendation appears to be limited and in an early review of the policy it was noted that implementation of such schemes had proven problematic and was likely to be particularly difficult for non-cancer treatments. 119 Another opportunity has arisen through the introduction of patient access schemes in 2009; however, although these allow for additional data collection, it is not a requirement. 86
The National Institute of Health and Clinical Excellence has recently issued a methods manual for the identification and prioritisation of research recommendations. 120 Although the guidance is aimed at the research recommendations provided outside of the main guidance to the NHS in the NICE documentation, the process also briefly refers to research recommendations in the guidance. A process is described whereby the Advisory Committees identify the ‘key’ evidence gaps, which are then translated into brief research questions by NICE staff in collaboration or consultation with other stakeholders. Prioritisation is conducted by NICE staff based on the ‘existing state of the evidence’ and reviewed at an annual meeting with the NIHR Evaluation, Trials and Studies Coordinating Centre. No further recommendations on when research recommendations should be included in guidance are described.
This review has revealed that NICE has used OIR/AWR since its inception, but these recommendations appear to be on the decline. Consideration of cost-effectiveness is routine within the appraisal process, and consideration of whether further research is needed and the type of evidence required appears to be frequently considered for OIR/AWR appraisals. However, some of the assessments outlined in the proposed framework do not appear to be currently considered, most notably whether or not significant irrecoverable costs are likely and whether or not the benefits of the proposed research outweigh the costs.
Chapter 5 Informing the assessments
The key principles and assessments that are needed when considering OIR or AWR guidance have been outlined in Chapter 2. How these assessments might be made and whether the existing methods of appraisal are sufficient, or whether additional information, evidence and analysis might be useful, were not addressed. In this chapter we outline additional information and evidence that might be useful and a range of methods of analysis that could be used to inform each of the assessments and decisions within the algorithm. We take existing methods of NICE appraisal as an accepted starting point and focus instead on exploring what additional information and analysis might feasibly be included in appraisal and how it might be interpreted to inform the judgements required using a series of case studies. We also consider whether this type of additional information and analysis might be routinely required within appraisal or only when OIR or AWR appears to be particularly relevant, for example more sophisticated additional analysis might be required only if it is established that further research might in principle be worthwhile.
Full details of each of the case studies are reported in Appendices 7–10. A separate technical appendix (see Appendix 6) is also provided that describes in detail how to carry out the calculations that can inform each of the assessments described in this chapter.
A checklist of assessment
The possible sequences of assessment and decision that lead to particular categories and types of guidance were represented as an algorithm in Figures 1 and 2 and Figures 26–28 in Appendix 2. The sequence of judgements required can be summarised as a simple checklist that could be considered by the Technology Appraisal Review (TAR) team/ERG and Appraisal Committee as well as manufacturers during appraisal. There are two checklists: one for technologies expected to be cost-effective (Table 10A) and one for those not expected to be cost-effective (Table 10B) based on the balance of existing evidence and current effective prices. The only difference between the checklists is at point 4, where, for technologies expected to be cost-effective, the judgement is whether the research is possible with approval whereas a judgement of whether research is possible without approval is required if the technology is not expected to be cost-effective.
| Point | Assessment | Judgement | |
|---|---|---|---|
| Yes | No | ||
| 1 | Is it cost-effective? | Yes | |
| 2 | Are there significant irrecoverable costs? | ||
| 3 | Does more research seem worthwhile? | ||
| 4 | Is the research possible with approval? | ||
| 5 | Will other sources of uncertainty resolve over time? | ||
| 6 | Are the benefits of research greater than the costs? | ||
| 7 | Are the benefits of approval greater than the costs? | ||
| Point | Assessment | Judgement | |
|---|---|---|---|
| Yes | No | ||
| 1 | Is it cost-effective? | No | |
| 2 | Are there significant irrecoverable costs? | ||
| 3 | Does more research seem worthwhile? | ||
| 4 | Is the research possible without approval? | ||
| 5 | Will other sources of uncertainty resolve over time? | ||
| 6 | Are the benefits of research greater than the costs? | ||
| 7 | Are the benefits of approval greater than the costs? | ||
Each of the seven points on the checklist relate to the sequence of decision nodes that fully describe the algorithm in Appendix 2. Therefore, each sequence of ‘yes’ or ‘no’ judgements defines a single pathway leading to a particular type and category of guidance (the type and category of guidance implied by each combination is described in Table 32, Appendix 4). However, all seven assessments do not necessarily need to be undertaken because sometimes earlier decisions will lead directly to guidance. For example, a ‘no’ at point 3 always leads directly to either approve or reject and hence further assessment is unnecessary. Similarly, a ‘no’ at point 6 also leads directly to approve or reject if there are no significant irrecoverable costs associated with the technology (see Table 32, Appendix 4).
Introduction to case studies
The objective of developing a series of case studies was to (1) demonstrate how the key principles and assessments might inform the development of guidance through application of the checklist and (2) establish whether existing methods of appraisal are sufficient, or whether (and when) additional information and analysis might be useful.
Selection of case studies
Case studies were selected to ensure that the full range of possible analysis was feasible within the time and resource constraints of this research project, while exploring situations in which OIR or AWR might be particularly relevant and challenging. Therefore, de novo analysis or substantial reanalysis of original assessments is not possible. Nor would it be necessary or informative as one of the objectives is to explore what additional information and analysis might be required. For this reason candidate case studies that met the following feasibility criteria were considered: (1) the economic analysis was regarded as a suitable basis for developing guidance, (2) an analysis of uncertainty in expected cost-effectiveness [probabilistic sensitivity analysis (PSA) as specified in the NICE reference case] was conducted and (3) ready access was available to the electronic versions of the models that informed guidance.
There are three groups of potential case studies in which the key principles and assessment described above might have influenced guidance: (1) when OIR or AWR was included in the FAD, (2) when OIR or AWR was considered during appraisal (e.g. included in the ACD or section 6 of the technology appraisal guidance) and (3) when OIR or AWR was not obviously considered at any stage. As well as examples of AWR for technologies expected to be cost-effective and OIR for those not, there are also a number of particularly interesting ways in which guidance might be influenced by these additional considerations. For technologies expected to be cost-effective these include (1) OIR rather than approve when research is not possible with approval and (2) OIR or even reject rather than AWR or approve even if research is possible with approval because there are significant irrecoverable costs.
To fully explore the implications of these principles and assessments it is useful to select case studies that reflect the range of possible and interesting characteristics. For example, technologies (1) that are and are not expected to be cost-effective, (2) with and without irrecoverable costs, (3) for which other sources of uncertainty are and are not present, (4) for which the research needed is and is not possible with approval, (5) that are non-pharmaceutical interventions and (6) that are appraised under the MTA and STA process. Four case studies will not be enough to demonstrate the full range of possible combinations of interesting characteristics or illustrate all of the potential impacts of interest. Therefore, in selecting case studies there was a need to balance feasibility and coverage of those characteristics of greatest interest.
Background to the case studies
The following four case studies were selected. A range of additional information was sought and further analysis conducted to inform the sequence of assessment and judgements required when completing the OIR/AWR checklist in Tables 10A and 10B.
Enhanced external counterpulsation for chronic stable angina
The NIHR HTA programme identified enhanced external counterpulsation (EECP) as an important topic and commissioned a short report to examine the clinical effectiveness and cost-effectiveness of EECP as an adjunct to standard therapy in patients with chronic stable angina. Although the topic was not ultimately considered by NICE it was commissioned in the same way and with the same resources as other assessment reports that inform NICE guidance. The assessment followed the NICE reference case and is consistent with the type of analysis that would have been required in a MTA appraisal. Like other MTA TARs it was published in full as a HTA monograph. 75,121
Enhanced external counterpulsation is a non-invasive procedure (adjunct to standard therapy) used to provide symptomatic relief from stable angina. The analysis compares EECP with standard therapy alone. RCT evidence suggests an improvement in health-related quality of life (HRQoL) with EECP at 12 months. 121 To characterise the uncertainty associated with possible longer durations of treatment effect, formal elicitation of expert clinical judgement was undertaken. This provided an estimate of the probability, with uncertainty, of continuing to respond to treatment with EECP in subsequent years.
The possible pathways through the algorithm that the EECP case study illustrates are reported in Figure 30 in Appendix 4. In this case study the new technology is expected to be cost-effective but with potentially significant irrecoverable costs. These irrecoverable costs include both (1) long-lived costs associated with the purchase of equipment and (2) large initial per-patient treatment costs, combined with a chronic condition in which a decision not to treat a particular patient with EECP can be changed at a later date (decisions are not irreversible) when research reports or other events occur. Consequently, these irrecoverable costs might influence the category of guidance, for example OIR rather than approve. This case study also provides an opportunity to explore the impact of research design (length of follow-up) on guidance and to examine the potential role of elicitation rather than extreme scenarios in characterising uncertainty.
Clopidogrel for the management of patients with non-ST segment elevation acute coronary syndromes
The use of clopidogrel (CLOP) (for up to 12 months) in combination with low-dose aspirin was recommended by NICE following a MTA appraisal for patients with non-ST segment elevation acute coronary syndrome (NSTE-ACS) presenting with a moderate to high risk of ischaemic events (TA80122 in 2004 and updated in 2010 in CG94123). In TA80 the Appraisal Committee considered 12-month or lifetime treatment with CLOP but recommended research to inform optimal treatment duration. The original assessment report had included an analysis of shorter treatment durations (< 12 months) and the NIHR HTA programme subsequently commissioned additional reanalysis based on this original work to inform this research recommendation in 2009. This case study is based on the reanalysis of TA80 undertaken in 2009, which included standard therapy compared with four alternative treatment durations of CLOP of 1, 3, 6 and 12 months. Importantly, although the case study is based on the later reanalysis of TA80, the analysis considered here has been undertaken from the standpoint of the original TA80 appraisal, asking what assessments might have been made at that time when standard therapy was low-dose aspirin.
The research recommendation was made in section 6 of TA80; therefore, this case study is not an example of AWR at FAD but an example of AWR considered during appraisal. The possible pathways through the algorithm that the CLOP case study illustrates are reported in Figure 29 in Appendix 4, with the new technology expected to be cost-effective and have no significant irrecoverable costs. The CLOP case study also illustrates a number of other important characteristics, including (1) the impact that other sources of uncertainty (price change following patent expiry) can have on the value of further research, (2) the interpretation of analyses in which there are multiple alternatives and (3) the use of scenarios to represent alternative but credible assumptions.
Omalizumab for the treatment of severe persistent allergic asthma in children aged 6–11 years
The use of omalizumab (OMAL) for the treatment of severe persistent allergic asthma in children aged 6–11 years was not recommended by NICE following a STA appraisal (TA201124 in 2010). The analysis compared OMAL as an add-on to standard care compared with standard care alone. The primary analysis was based on a prespecified severe asthma population within an international, multicentre, placebo-controlled RCT. However, a high-risk subgroup within this population (recent hospitalisation for an asthma exacerbation) was also identified post hoc.
Omalizumab was not found to be cost-effective in either the severe or severe/high-risk population. However, a RCT was recommended comparing OMAL with oral corticosteroids (OCS) in children to establish reduction in OCS use. This was made in section 6 of TA201; therefore, OMAL is not an example of OIR at FAD but an example of OIR considered during appraisal. The possible pathways through the algorithm that the OMAL case study illustrates are reported in Figure 31 in Appendix 4, with the new technology expected not to be cost-effective and to have no significant irrecoverable costs. OMAL also illustrates assessment in small patient populations (rare disease) and how subgroup analysis can be considered.
Etanercept, infliximab and adalimumab for patients with active and progressive psoriatic arthritis
Following a MTA appraisal (TA199125 in 2010) the use of biologic treatment with etanercept, infliximab and adalimumab (Humira®, Abbott) was recommended by NICE for patients with active and progressive psoriatic arthritis (PsA) and who have an inadequate response to standard treatment, including two conventional disease-modifying antirheumatic drugs (DMARDs). However, the guidance also recommended that treatment should start with the least expensive drug, taking account of dose, route of administration and price. This guidance updated an earlier MTA appraisal in 2006 (TA104126) that had recommended etanercept and restricted guidance on the use of infliximab to only those patients shown to be either intolerant or contraindicated to etanercept. a The analysis in this case study is from the standpoint of TA199, using the updated model that included new evidence and adalimumab as an additional comparator. At this point NICE guidance recommended etanercept and so the first question posed in the checklist can be interpreted as whether or not the other technologies available (infliximab, adalimumab or palliative care) are expected to be cost-effective compared with etanercept.
In section 6 of TA199 the importance of data from patient registries on long-term outcomes and adverse events was highlighted; therefore, the PsA case study is not an example of AWR at FAD but an example of AWR considered during appraisal. The possible pathways through the algorithm that the PsA case study illustrates are reported in Figure 32 in Appendix 4. In this case study the alternatives to etanercept are not expected to be cost-effective. However, etanercept as well as infliximab and adalimumab have potentially significant irrecoverable costs because of the high initial per-patient treatment costs, combined with a chronic condition in which treatment decisions are not irreversible. The PsA case study, like the EECP case study, also provides an opportunity to examine the potential role of elicitation in the appraisal process.
Is it cost-effective and what are the risks?
The judgements made at points 1 and 2 of the checklist are critical because, although neither leads directly to a particular category of guidance, they determine the subsequent path that might be taken, sometimes avoiding further and potentially complex assessments. For example, the absence of significant irrecoverable costs means that only four out of the 12 possible pathways require all seven assessments to be made (see Table 32, Appendix 4).
Point 1: Is it expected to be cost-effective?
The sequence of assessments starts with cost-effectiveness and the expected impact on population NHEs, that is, at the following point in the algorithm:
This requires an assessment of expected cost-effectiveness based on the balance of the evidence and analysis currently available. Methods to estimate expected cost-effectiveness are well established within the NICE appraisal process and are extensively described in the Guide to Methods of Technology Appraisal. 1,b Commonly, expected cost-effectiveness is summarised and presented using ICERs. Equivalently, but more usefully in this context, cost-effectiveness can be expressed in terms of expected NHEs, which can be expressed per-patient treated or for a population of patients. This is especially important when later assessments require a comparison of benefits for current and future patient populations and when assessing the significance of irrecoverable costs (see Point 2: Are there significant irrecoverable cost?). All of the information required to express expected cost-effectiveness in these ways is available from the type of analysis already undertaken during appraisal.
Cost-effectiveness at the patient level
Estimates of the expected NHS costs and QALYs for each patient treated over an appropriate time horizon – the ‘patient time horizon’ – can be summarised as an ICER, which must be compared with a cost-effectiveness threshold to judge cost-effectiveness too. c Equivalently, this can be expressed as the per-patient NHE of each intervention, that is, the difference between any health gained and health forgone elsewhere. d
Technologies expected to be cost-effective
The results for EECP are summarised in Table 11. There are only two alternatives (EECP and standard care) and so only one ICER. EECP is just expected to be cost-effective at a threshold of £20,000 per QALY. e Consequently, the NHEs of EECP are greater than those of standard care but the difference per patient treated (the incremental NHE) is small.
| Treatment | Cost (£) | QALYs | ICER (£/QALY) | Cost-effectiveness threshold £20,000 per QALY | Cost-effectiveness threshold £30,000 per QALY | ||
|---|---|---|---|---|---|---|---|
| NHE, QALYs (£) | Incremental NHE, QALYs (£) | NHE, QALYs (£) | Incremental NHE, QALYs (£) | ||||
| EECP | 4744 | 7.6045 | 19,391 | 7.3673 (147,346) | 0.0074 (149) | 7.4464 (223,391) | 0.0865 (2595) |
| Standard care | – | 7.3598 | – | 7.3598 (147,197) | – | 7.3598 (220,795) | – |
It is also important to consider how NHEs accumulate over time or the investment profile per patient treated with EECP. Figure 3 illustrates the cumulative incremental NHE over the patient time horizon. The initial per-patient costs of EECP are high and are far in excess of the immediate health benefits in the initial period of treatment. These negative NHEs are gradually offset by positive NHEs in later periods. In this case, it is only after 14 years that the initial losses are compensated by later gains, that is, EECP is not expected to break-even until 14 years from initial treatment. It is only beyond 30 years that the modest incremental NHEs reported in Table 11 are eventually achieved. f
FIGURE 3.
Cumulative incremental NHEs of EECP over the patient time horizon.
Multiple alternatives
Similar analysis can be conducted when there are more than two alternatives. For example, in the CLOP case study four treatment durations as well as current NHS treatment (aspirin alone) were considered at the time of TA80. 122 The results in Table 12 indicate that 12-month treatment with CLOP is expected to be cost-effective, although the difference in NHE between 12 and 6 months’ treatment is small.
| Treatment | Cost (£) | QALYs | ICER (£/QALY) | Cost-effectiveness threshold £20,000 per QALY | Cost-effectiveness threshold £30,000 per QALY |
|---|---|---|---|---|---|
| NHE, QALYs (£) | NHE, QALYs (£) | ||||
| CLOP12 | 20,127 | 8.122 | 18,663 | 7.115 (142,307) | 7.451 (223,525) |
| CLOP6 | 19,860 | 8.107 | 10,477 | 7.114 (142,288) | 7.445 (223,362) |
| CLOP3 | 19,712 | 8.093 | 9396 | 7.108 (142,154) | 7.436 (223,087) |
| CLOP1 | 19,598 | 8.081 | 4961 | 7.101 (142,025) | 7.428 (222,837) |
| NHS | 19,502 | 8.062 | – | 7.087 (141,734) | 7.412 (222,353) |
The investment profile of CLOP, per patient treated, is illustrated in Figure 4. The per-patient costs of CLOP are in excess of the health benefits during the period of treatment. These negative NHEs are eventually offset by positive NHEs in later periods. In this case, it is only after 5 years that 12 months of treatment with CLOP breaks-even against current NHS care and it is not until 21 years that it is better than a shorter treatment duration of 6 months. Notice that shorter treatment durations with CLOP offer a much less ‘risky profile’, for example the break-even point for 1 month of treatment is 2 years against current NHS care.
FIGURE 4.
Cumulative incremental NHEs of CLOP over the patient time horizon.
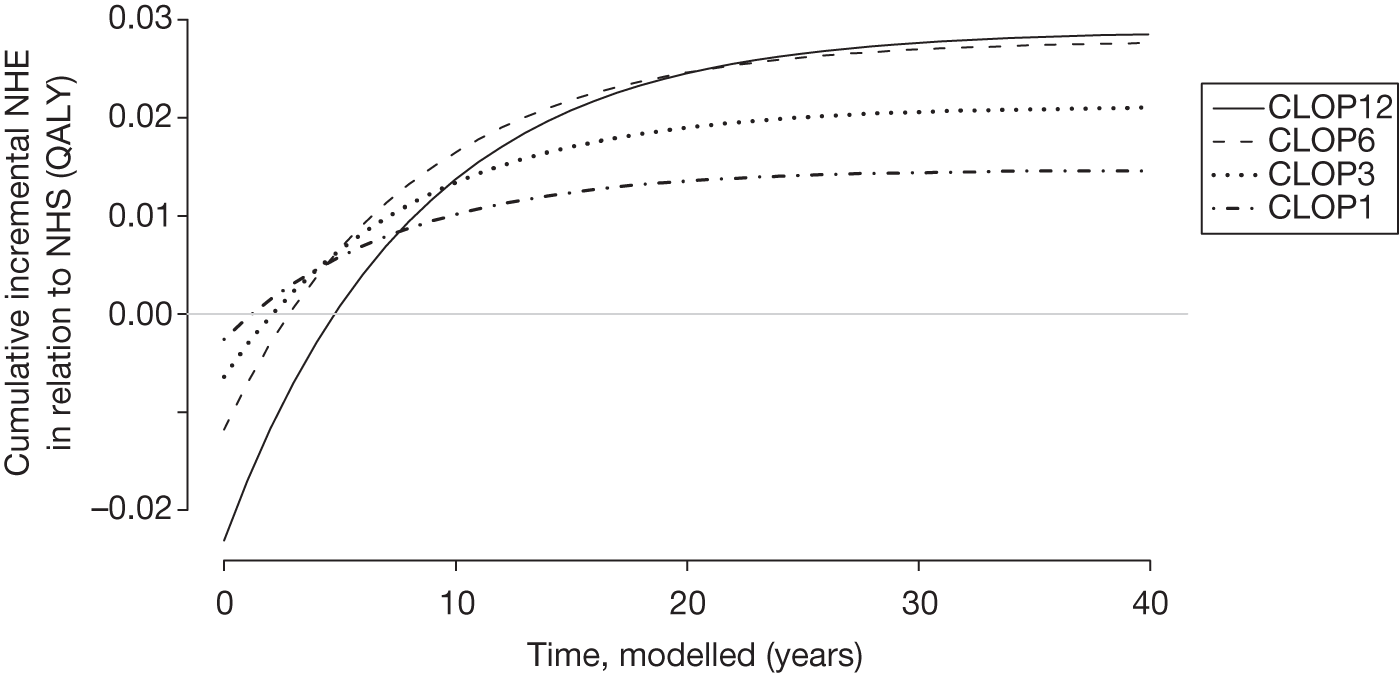
Technologies not expected to be cost-effective
The ICER for OMAL in Table 13 is greater than the threshold and so it is not expected to be cost-effective compared with standard care alone. Consequently, the incremental NHE of OMAL is negative.
| Treatment | Cost (£) | QALYs | ICER (£/QALY) | Cost-effectiveness threshold £20,000 per QALY | Cost-effectiveness threshold £30,000 per QALY | ||
|---|---|---|---|---|---|---|---|
| NHE, QALYs (£) | Incremental NHE, QALYs (£) | NHE, QALYs (£) | Incremental NHE, QALYs (£) | ||||
| OMAL + standard care | 94,992 | 16.64 | 93,844 | 11.8861 (237,721) | –2.1908 (–43,815) | 13.4693 (404,078) | –1.2627 (–37,882) |
| Standard care | 39,310 | 16.04 | – | 14.0768 (281,536) | – | 14.7320 (441,960) | – |
The per-patient investment profile for OMAL is illustrated in Figure 5 and shows that it is always expected to offer negative NHEs compared with standard care over the entire patient time horizon, that is, the high costs of treatment are never compensated by future health gains. In this example, the initial treatment costs with OMAL continue for 10 years (10 years is assumed to represent the duration that a patient would continue to receive treatment with OMAL) with health effects predominately while on treatment. Therefore, OMAL is not so much a ‘risky purchase’ but one that is simply not cost-effective at its current price.
FIGURE 5.
Cumulative incremental NHEs of OMAL over the patient time horizon.
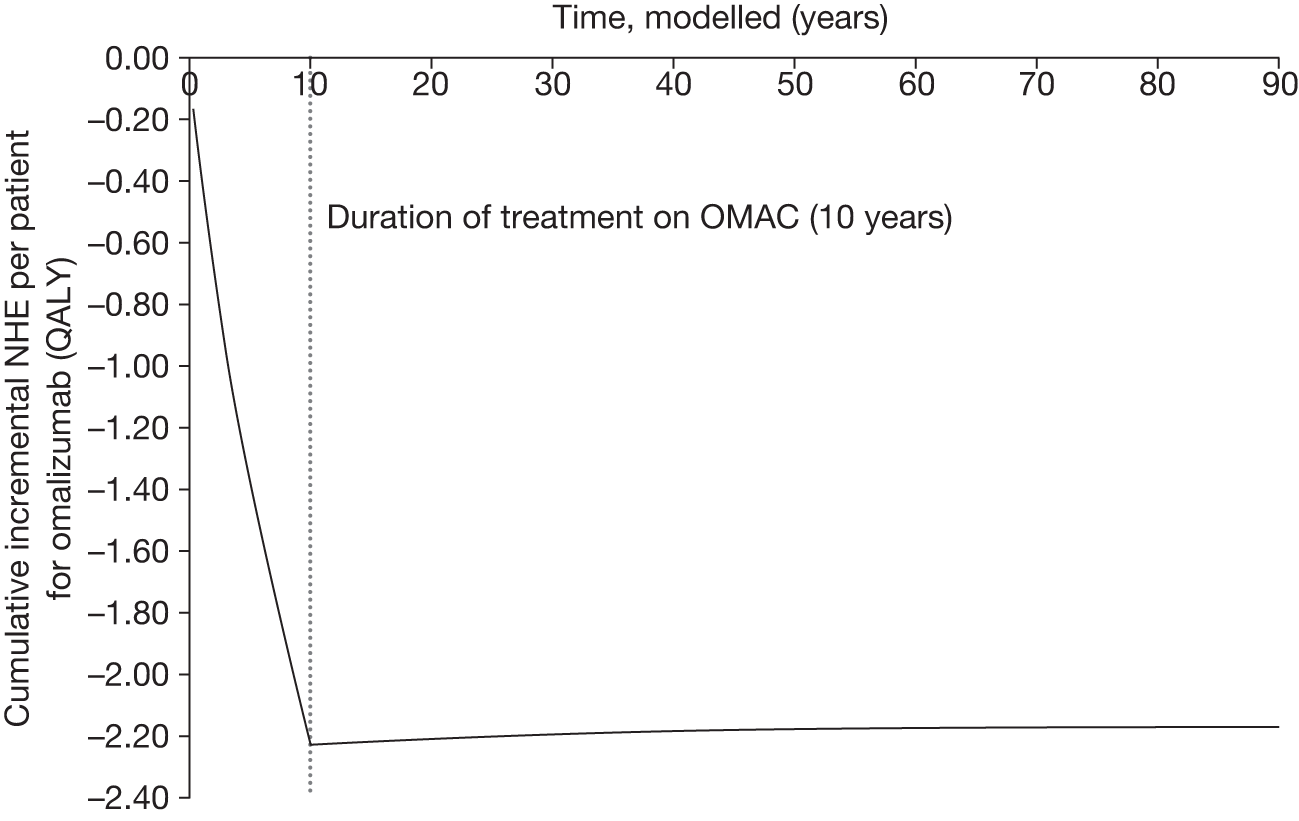
Multiple alternatives
Psoriatic arthritis offers an example in which the alternatives to the treatment already recommended by NICE (etanercept at the time of TA199125) are not expected to be cost-effective, that is, the results in Table 14 indicate that etanercept is expected to be cost-effective. Notice that although adalimumab is less effective than etanercept it is also cheaper; however, the resource savings it offers do not compensate for the reduction in health benefits.
| Treatment | Cost (£) | QALYs | ICER (£/QALY) | Cost-effectiveness threshold £20,000 per QALY | Cost-effectiveness threshold £30,000 per QALY |
|---|---|---|---|---|---|
| NHE, QALYs (£) | NHE, QALYs (£) | ||||
| 1: Infliximab | 90,343 | 7.269 | 60,965 | 2.752 (5504) | 4.258 (8516) |
| 2: Etanercept | 78,150 | 7.069 | 17,733 | 3.161 (6322) | 4.464 (8928) |
| 3: Adalimumab | 72,972 | 6.777 | 14,622 | 3.129 (6258) | 4.345 (8690) |
| 4: Palliative care | 51,800 | 5.329 | – | 2.739 (5478) | 3.602 (7204) |
Consequently, the investment profiles of the alternatives to etanercept, illustrated in Figure 6, differ in appearance. However, all of the biologic treatments for PsA have high initial costs, which are only gradually compensated for by later health benefits. Adalimumab, etanercept and infliximab all ultimately offer positive NHEs compared with palliative care but only break-even at 17, 17.5 and 34.5 years, respectively. Adalimumab offers a slightly less risky profile than etanercept and so it is only at 21.25 years that etanercept is expected to offer the highest NHE.
FIGURE 6.
Cumulative incremental NHEs in PsA over the patient time horizon.
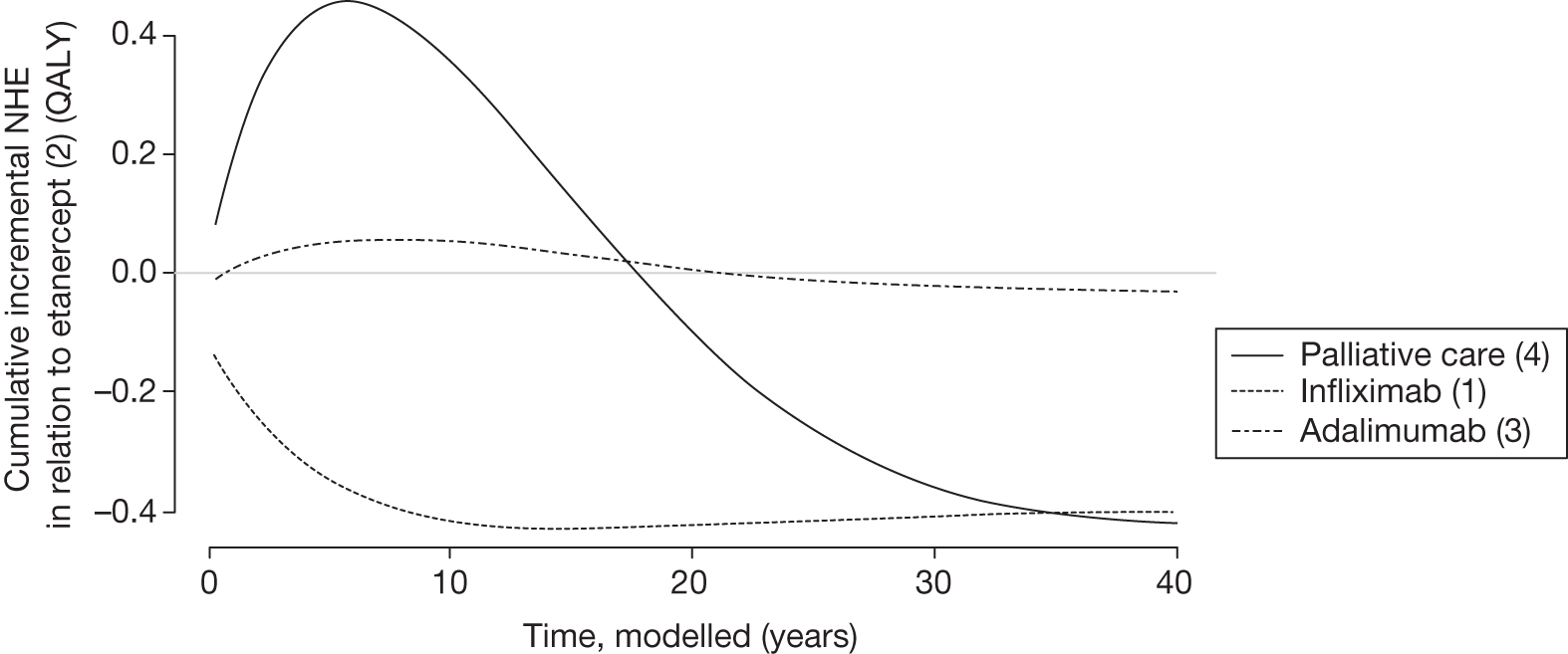
Cost-effectiveness at the population level
Per-patient NHEs can also be expressed for the population of current and future patients. This requires information about prevalence and future incidence of the target population (already required in appraisal). It also requires a judgement about the time horizon over which the technology will be used. This ‘technology time horizon’ ought to reflect the period over which the technology is likely to be part of clinical practice and generate the expected NHEs. g An estimate of the scale of the total population NHEs and how they cumulate over time is important for subsequent assessments, including (1) when the NHE for current patient populations must be compared with the benefits to future patients and (2) when the treatment decision can be changed so that the irrecoverable opportunity costs of initially negative NHEs become significant, that is, might influence the category of guidance.
For example, there is a large prevalent population (n = 109,800) eligible for EECP relative to future incident populations (n = 9500 per annum) in this chronic condition. The total population NHEs, assuming that the technology will be used to treat prevalent and incident patients over 10 years, are reported in Table 15. The expected cost-effectiveness is unchanged (the ICER is the same as in Table 11) but the incremental NHE, although small per patient, is more significant at a population level because of the size of the target population.
| Treatment | Costs (£M) | QALYs | ICER (£/QALY) | Cost-effectiveness threshold £20,000 per QALY | Cost-effectiveness threshold £30,000 per QALY | ||
|---|---|---|---|---|---|---|---|
| NHE, QALYs (£M) | Incremental NHE, QALYs (£M) | NHE, QALYs (£M) | Incremental NHE, QALYs (£M) | ||||
| EECP | 896 | 1,435,787 | 19,391 | 1,391,001 (27,820) | 1405 (28) | 1,405,930 (42,177) | 16,334 (490) |
| Standard care | – | 1,389,596 | – | 1,389,596 (27,792) | 1,389,596 (41,688) | ||
The investment profile for EECP when used to treat patients over 10 years is illustrated in Figure 7. At a population level it is not until 17 years (rather than 14 years at a patient level) that initial losses are compensated for by later gains and EECP breaks even. In other words, EECP appears a more risky investment when evaluated at a population rather than an individual level. This is because, although each patient treated with EECP is expected to offer the same profile of NHEs as shown in Figure 3, the negative NHEs associated with patients incident and treated in year 10 will not be offset by later gains until year 24. The population-level investment profile would exhibit greater risk (break-even later) if the prevalent population was smaller relative to the incident population and/or the technology time horizon was longer. For example, the break-even point extends to 23 years when the technology time horizon is increased to 20 years.
FIGURE 7.
Cumulative incremental NHEs of EECP for the population.
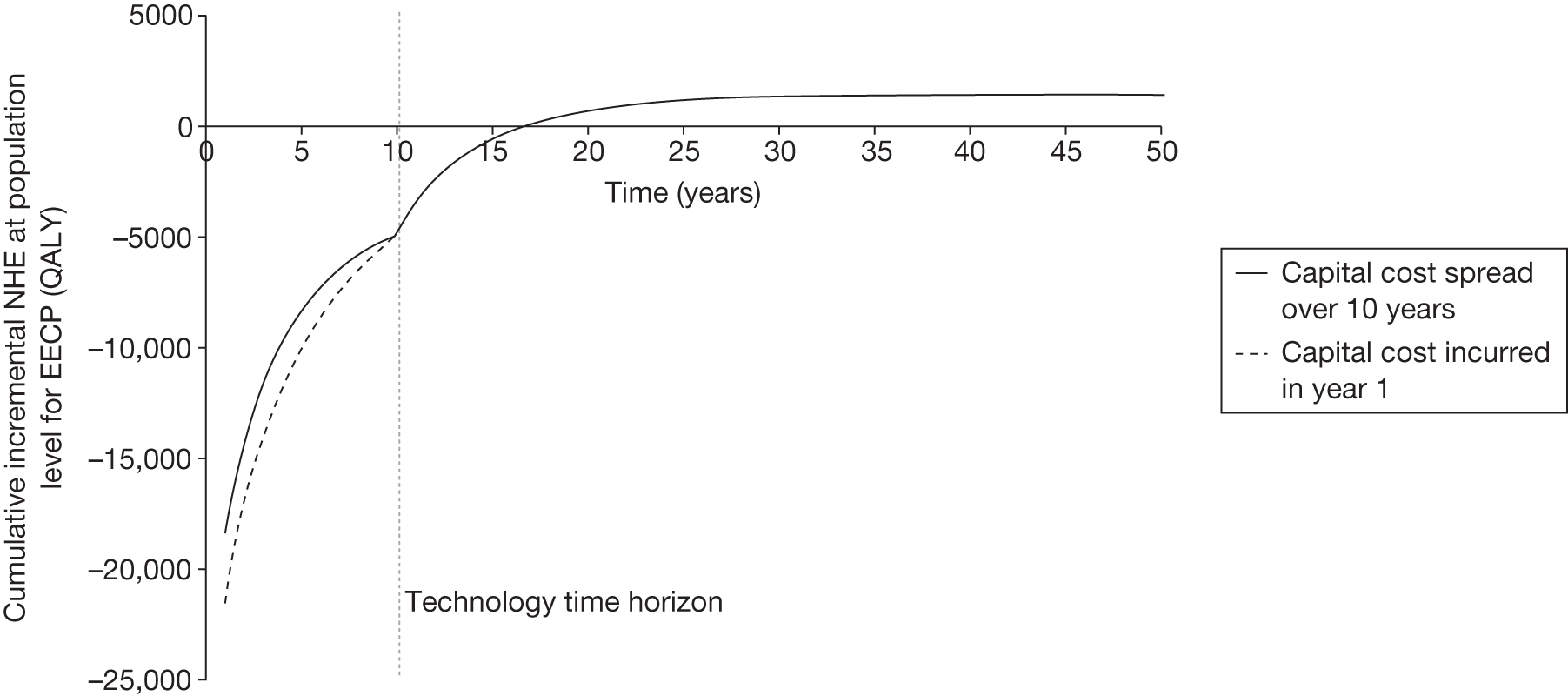
The effect on the other case studies of assessment at the population level is similar to the effect on the EECP case study. It simply increases the magnitude of differences in per-patient NHE (to a greater extent for longer technology time horizons) but leaves expected cost-effectiveness unchanged. However, the investment profiles at a population level also differ, exhibiting greater ‘risk’ indicated by later break-even points for the same reasons as for EECP. For example, the break-even points for CLOP when evaluated at a population level are reported in Table 16. At a technology time horizon of 10 years it is only at 11 years, rather than 5 years for a single patient, that 12 months of CLOP treatment breaks even against current NHS care and not until 27 years (rather than 21 years) that 12 months of CLOP treatment is better than a shorter treatment duration of 6 months. Even the shorter durations of treatment offer a ‘risky profile’, for example the break-even point for 1 month of treatment against current NHS care is 4 years (rather than 2 years).
| Technology time horizon | Treatment | Incremental NHE, QALYs (£M) | Break-even point (years) | ||
|---|---|---|---|---|---|
| 12 months vs 6 months | 12 months vs NHS | 1 month vs NHS | |||
| 5 years | CLOP12 | 269 (5.4) | 24 | 8 | 4 |
| CLOP6 | 1881 (37.6) | ||||
| CLOP3 | 1804 (36.1) | ||||
| CLOP1 | 4073 (81.5) | ||||
| NHS | – | ||||
| 10 years | CLOP12 | 495 (9.9) | 27 | 11 | 4 |
| CLOP6 | 3465 (69.3) | ||||
| CLOP3 | 3324 (66.5) | ||||
| CLOP1 | 7502 (150) | ||||
| NHS | – | ||||
| 15 years | CLOP12 | 686 (13.7) | 30 | 12 | 4 |
| CLOP6 | 4799 (96) | ||||
| CLOP3 | 4603 (92.1) | ||||
| CLOP1 | 10,389 (207.8) | ||||
| NHS | – | ||||
| 20 years | CLOP12 | 846 (16.9) | 33 | 12 | 4 |
| CLOP6 | 5921 (118.4) | ||||
| CLOP3 | 5680 (113.6) | ||||
| CLOP1 | 12,820 (256.4) | ||||
| NHS | – | ||||
Point 2: Are there significant irrecoverable costs?
The second point on the checklist requires (1) an assessment of whether there are irrecoverable costs and (2) a judgement of their potential significance, that is, at the following point in the algorithm:
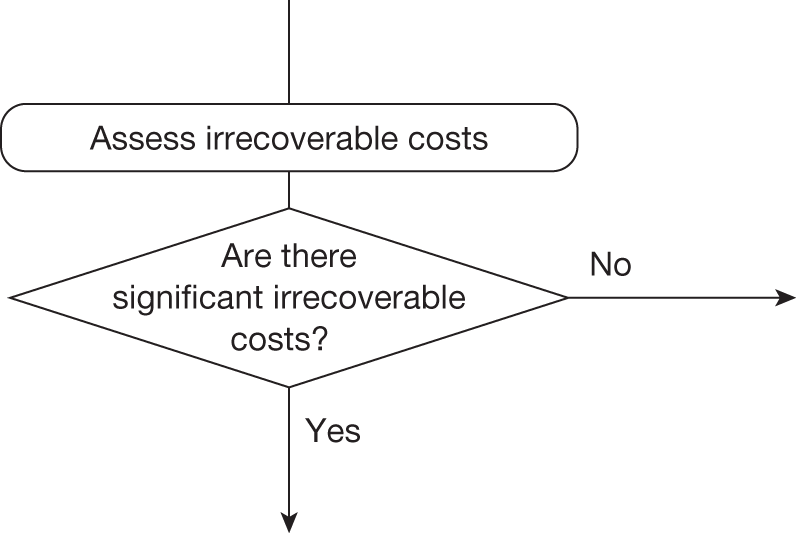
Irrecoverable costs are those that once committed cannot be recovered if guidance is changed at a later date (see Appendix 6, Are there significant irrecoverable costs?). Irrecoverable costs are most commonly thought of as upfront or capital costs of new facilities or equipment with long life expectancy (they might also include any practitioner training and the costs of implementation efforts). In NICE appraisal these types of cost are first annuitisedh and then allocated pro rata to the number of patients likely to be treated during the lifetime of the equipment. That is, capital costs are treated as if they are paid per patient treated over the lifetime of the equipment. If guidance remains unchanged throughout this period (i.e. research does not report or other sources of uncertainty do not resolve) then this common assumption has no influence. However, should guidance change (initial approval is withdrawn) before the end of the lifetime of the equipment then, although future patents will no longer use the technology, the cost of the equipment allocated to them cannot be recovered. The possibility that initial guidance might change and its impact on expected costs needs to be considered before costs are made irrecoverable through approval or AWR. The impact of irrecoverable costs will tend to be greater if they represent a greater proportion of the total costs, if guidance is more likely to change and if guidance is more likely to change in the near rather than distant future.
Enhanced external counterpulsation is the only case study in which these types of cost are present to any great extent because treatment requires capital investment in the EECP machines themselves. The expected per-patient and population costs reported in Tables 11 and 15 allocated this capital cost in the usual way (i.e. annuitised over the 10-year lifetime of the machines and allocated to the number of patients treated each year). The irrecoverable costs are reported separately in Table 17 and represent 19% of the total. However, this will have no influence on expected cost-effectiveness as long as guidance does not change during the lifetime of the equipment. i
| Treatment | Capital cost (£) | Non-capital cost (£) | QALYs | ICER (£/QALY) | Cost-effectiveness threshold £20,000 per QALY | |
|---|---|---|---|---|---|---|
| NHE, QALYs (£M) | Incremental NHE, QALYs (£M) | |||||
| EECP | 170,304,591 | 725,408,798 | 1,435,787 | 19,391 | 1,391,001 (27,820) | 1405 (28) |
| Standard care | – | – | 1,389,596 | – | 1,389,596 (27,792) | – |
Investment profile of net health effects
Even in the absence of capital costs of equipment and facilities, NHEs accumulate over time at both a patient and a population level. With the possible exception of OMALj the analysis in the previous section indicates a common pattern of initially negative NHEs that are only gradually offset by positive NHEs in later periods. Therefore, approval or AWR commonly commits opportunity costs of negative NHE that are irrecoverable. For OMAL, the profile of NHEs at a patient level did not exhibit significant irrecoverable opportunity costs. Assessment at a population level and for longer technology time horizons simply increases the magnitude of the expected negative NHE; therefore, there are no irrecoverable costs in this case study.
Are irrecoverable costs likely to be significant?
Whether or not irrecoverable costs are significant, that is, might influence guidance, depends critically on whether guidance is likely to change and whether that is more likely in the near or in the distant future. That will depend on whether research is likely to be undertaken and when it is likely to report, as well as on other events that might occur, for example a change in price following patent expiry. These are assessed later, at points 5 and 6 in the checklist. However, the potential significance of any irrecoverable costs can be assessed at this point. For example, capital costs can be judged based on the proportion of total population costs that are irrecoverable for this reason as well as their scale relative to the additional population NHE offered (e.g. see Table 17).
Judging the potential significance of the investment profiles of NHEs is more nuanced. It depends whether treatment decisions for individual patients are irreversible, which in part depends on the nature of the disease. For example, in an acute condition the decision to treat a particular presenting patient with a technology cannot be reconsidered at a later date – it is irreversible. Of course, it is possible that the later benefits are not realised but it is also possible that they will realise more (the profiles of NHEs in Figures 3–6 are the average over these possibilities). Similarly, the possibility that guidance might change in the future (e.g. research suggests that the longer-term benefits will not offset initial losses) will not influence the irreversible decision to treat a presenting patient with a technology that is expected to be cost-effective before the research reports.
Implication for the case studies
Clopidogrel is a treatment for acute coronary syndromes and, although decisions about treatment and its duration are not irreversible in the very short run, over the time scales more likely for research being conducted (and reporting) or other events occurring that would change guidance they can be regarded as such. Therefore, although the investment profile of CLOP (at a patient and more so at a population level) exhibits irrecoverable costs these should not be judged significant in the sense that they have little potential to influence guidance. There are also no significant irrecoverable costs associated with OMAL but for different reasons; although treatment decisions are reversible in this chronic condition, any irrecoverable costs appear very limited (see Figure 5).
Both EECP and the biologics in PsA are for chronic conditions in which the decision to treat a particular patient can be changed at some later date (decisions are not irreversible). Therefore, the type of investment profile of NHEs at a patient and population level is significant because, instead of committing irrecoverable costs by deciding to use technologies expected to be cost-effective now, the decision and commitment of costs can be made later, after research reports, other events occur and/or guidance changes. Of course, proper account must be taken of the impact of withholding initiation of treatment on expected health benefits and costs (see Point 7: Are the benefits of approval greater than the costs?), for example some patients who might have been treated may not survive to benefit from the results of the research or disease may have irreversibly progressed so that the expected health benefits are lower. 75
Enhanced external counterpulsation is the only case in which both types of irrecoverable costs are potentially significant. Figure 7 illustrates the impact of accounting for the actual timing of expenditure on EECP machines rather than treating it as if it was paid when each patient was treated, that is, expenditure is treated like a consumable cost by spreading the capital cost over 10 years. k If approval of EECP might be withdrawn before 10 years, the potential losses in NHEs will be greater than initially indicated in Figure 7 because the equipment costs allocated to treating future patients cannot be recovered. The earlier such a change might occur the greater the additional loss. The impact of these possibilities should be considered at point 7 of the checklist before guidance to approve or AWR commits both types of irrecoverable costs.
Pricing and irrecoverable costs
The importance of irrecoverable treatment costs when they may be potentially significant should also consider the scale of initially negative NHEs and the duration of such losses, that is, how long until the technology breaks even for an individual patient and for the population of patients who are likely to be treated if it is approved. Health technologies with patent protection are more likely to be priced close to the point at which the expected incremental NHEs are close to zero, that is, the ICER is close to or equal to the threshold. A value-based pricing scheme would formalise these existing incentives. A technology that is only just expected to be cost-effective will not break-even until close to the end of the patient time horizon and for much longer for the population of patents likely to benefit from its use (up to the technology time horizon plus the patient time horizon – less if patent expiry and cheaper generic enter before the technology time horizon). Therefore, those technologies already priced close to the threshold, and all new technologies considered in a value-based pricing scheme, will tend to increase the scale of irrecoverable costs committed by approval, making OIR or reject more likely even when a technology is just expected to be cost-effective at point 1 of the checklist. l
Is further research required?
The judgements made at points 3 and 4 of the checklist are critical because if more research is not judged to be worthwhile no further assessments are required (unless there are significant irrecoverable costs; see Table 32 in Appendix 4). If research is worthwhile, the type of evidence needed and whether or not the research required to generate it can be conducted, while the technology is approved will determine whether or not AWR or OIR is a possibility.
Point 3: Does more research seem worthwhile?
The third point on the checklist requires an assessment of the potential benefits of conducting further research, that is, at the following point in the algorithm:
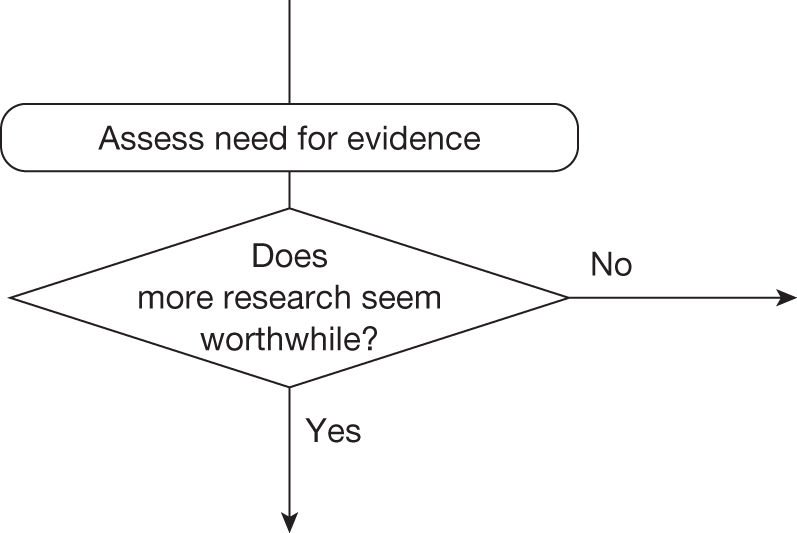
This requires judgements about (1) how uncertain a decision to approve or reject a technology might be based on the estimates of expected cost-effectiveness and (2) whether or not the scale of the likely consequences of this uncertainty might justify further research. Some assessment of the potential consequences of uncertainty is important because it indicates the scale of the population NHEs that could be gained if the uncertainty surrounding this decision could be resolved immediately, that is, it represents an expected upper bound on the benefits of more research. m If the potential benefits of further research are unlikely to justify the costs, then a judgement that more research does not seem worthwhile will lead directly to guidance in the following circumstances (extracted from Table 32 in Appendix 4):
| Assessment | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Guidance | |
|---|---|---|---|---|---|---|---|---|---|
| Pathway number { | 6 | Yes | No | No | – | – | – | – | Approve4 |
| 12 | No | No | No | – | – | – | – | Reject4 | |
| 35 | No | Yes | No | – | – | – | – | Reject11 |
Assessing the consequences of uncertainty
Some assessment is required of (1) how uncertain a decision based on expected cost-effectiveness might be and (2) what the consequences, in terms of population NHEs, are likely to be if an incorrect decision is made.
Enhanced external counterpulsation is expected to be cost-effective compared with standard care (Table 18 and see Table 11), but the estimates of cost and QALYs are uncertain and so there is a chance that a decision to approve EECP based on existing evidence will be incorrect, that is, standard care might offer greater NHEs. Some assessment of the likely consequences of approving EECP when standard care might be better could be based on the difference in expected NHEs, that is, the expected incremental population NHEs reported in Tables 15 and 18). This is illustrated in Figure 8 in which a judgement about the probability that a decision based on expected cost-effectiveness is correct translates into expected consequences based on the expected incremental population NHE. For example, if the decision was judged to be 100% certain then there are no consequences and so there would be nothing to be gained by more research; however, as the probability that the decision is correct becomes less certain, the expected consequences (and hence potential value of more research) increase.
| Treatment | ICER (£/QALY) | Cost-effectiveness threshold £20,000 per QALY | Cost-effectiveness threshold £30,000 per QALY | ||||
|---|---|---|---|---|---|---|---|
| Incremental NHE, QALYs (£M) | Probability cost-effective | Expected consequences, QALYs (£M) | Incremental NHE, QALYs (£M) | Probability cost-effective | Expected consequences, QALYs (£M) | ||
| EECP | 19,391 | 1405 (28.1) | 0.428 | 9287 (185.7) | 1,405,930 (490) | 0.700 | 2774 (83.2) |
| Standard care | – | 0.572 | – | 0.300 | |||
FIGURE 8.
Probability that EECP is cost-effective and the consequences of uncertainty.
This judgement of how uncertain a decision might be can be informed by the PSA already used to estimate costs and QALYs and required as part of the NICE reference case. 127,128 The probability that EECP is cost-effective is 0.428 (see Table 18),n which would translate into approximately 800 QALYs (see Figure 8) over the technology time horizon,o based on the expected or average difference between NHEs. However, the difference in NHEs when EECP is not the correct decision is not necessarily the average. In fact, it is very unlikely to be the average and such estimates may substantially under- or overestimate the expected consequences of uncertainty. p
The same probabilistic analysis can be used to record the difference between the NHEs of EECP and the NHEs of standard care and the frequency of such errors (i.e. the proportion of simulations in which standard care offers greater NHEs). The distribution of consequences associated with these errors is illustrated in Figure 9. Commonly there are no consequences, because EECP is the correct decision (i.e. there is a 42.8% chance of zero consequences). However, when EECP offers lower NHEs than standard care (a 57.2% chance) there are consequences in terms of NHEs forgone. Figure 9 illustrates that the consequences of errors may be relatively small, for example 9% of the simulations have consequences of < 5000 QALYs. However, consequences may also be very large, for example there is a small chance (5.7%) that they are > 30,000 QALYs. The average over this distribution provides the correct estimate of the expected consequences of uncertainty, which in this case is 9287 QALYs. q
FIGURE 9.
Distribution of the consequences of uncertainty for EECP.
These expected consequences can be interpreted as an estimate of the population NHEs over the technology time horizon that could be gained if the uncertainty surrounding this decision could be resolved immediately, that is, it indicates an expected upper bound on the benefits of more research. 127,129,r The consequences can also be expressed as the equivalent NHS resources required to generate the same population NHEs (£185.7M in Table 18). They will increase with the size of the patient population and the technology time horizon. In the case of EECP the consequences fall with the cost-effectiveness threshold because a decision to approve EECP will be less uncertain (see Table 18). A judgement at this point that more research might be worthwhile seems reasonable because the upper bound on its potential benefits exceeds the likely costs.
Multiple alternatives
Similar analysis can be conducted when there are more than two alternatives but greater difficulties are encountered unless the results of the PSA are used to assess both uncertainty and its consequences. For example, in the CLOP case study, 12 months’ treatment with CLOP is expected to be cost-effective but this is also uncertain. A judgement is required about the chance that 12 months of treatment is incorrect and if so which of the other four alternatives are likely to offer a higher NHE and how much higher. In other words, for decisions involving multiple alternatives, a judgement is required on the level of uncertainty surrounding the decision, how this uncertainty is distributed across the various alternatives and what the consequences are likely to be. The results of the PSA can inform this judgement. The probabilities that each of the five alternatives is cost-effective are reported in Table 19. This indicates that 12 months’ treatment is uncertain (probability that it is incorrect is 0.476). However, much of this probability of error is allocated to 6 months’ treatment with CLOP (0.18) for which the difference in NHEs is likely to be relatively modest.
| Treatment | ICER (£/QALY) | Cost-effectiveness threshold £20,000 per QALY | Cost-effectiveness threshold £30,000 per QALY | ||||
|---|---|---|---|---|---|---|---|
| Incremental NHE,a QALYs (£M) | Probability cost-effective | Expected consequences, QALYs (£M) | Incremental NHE,a QALYs (£M) | Probability cost-effective | Expected consequences, QALYs (£M) | ||
| CLOP12 | 18,663 | 495 (9.9) | 0.524 | 5194 (103.9) | 2798 (56.0) | 0.677 | 3657 (109.7) |
| CLOP6 | 10,477 | 3465 (69.3) | 0.180 | 4736 (94.7) | 0.092 | ||
| CLOP3 | 9396 | 3324 (66.5) | 0.018 | 4305 (86.1) | 0.009 | ||
| CLOP1 | 4961 | 7502 (150.0) | 0.075 | 8327 (166.5) | 0.052 | ||
| NHS | – | – | 0.202 | – | 0.170 | ||
The distribution of consequences is illustrated in Figure 10. Most commonly (52.4%) there are no consequences because 12 months’ treatment with CLOP is the correct decision. When it is not, there is a greater chance of relatively small consequences of error (30% are < 10,000 QALYs), which occur predominantly when 6 months’ treatment duration offers the highest NHE (18% chance). There is a small chance of much larger consequences (< 5% chance that they are > 30,000 QALYs). These occur only when standard NHS treatment offers the highest NHE. Overall, there is an almost 20% chance that NHS treatment offers a higher NHE, that is, there remains important uncertainty about the cost-effectiveness of CLOP itself, not just the duration of treatment. The expected consequence of uncertainty (5194 QALYs) is simply the average over this distribution. Again, this can be interpreted as an estimate of the population NHE that could be gained over the time horizon of this technology if the uncertainty about treatment and its duration could be immediately resolved. Therefore, like EECP, a judgement at this point that more research might be worthwhile seems reasonable because the potential benefits exceed the likely costs.
FIGURE 10.
Distribution of the consequences of uncertainty for CLOP.
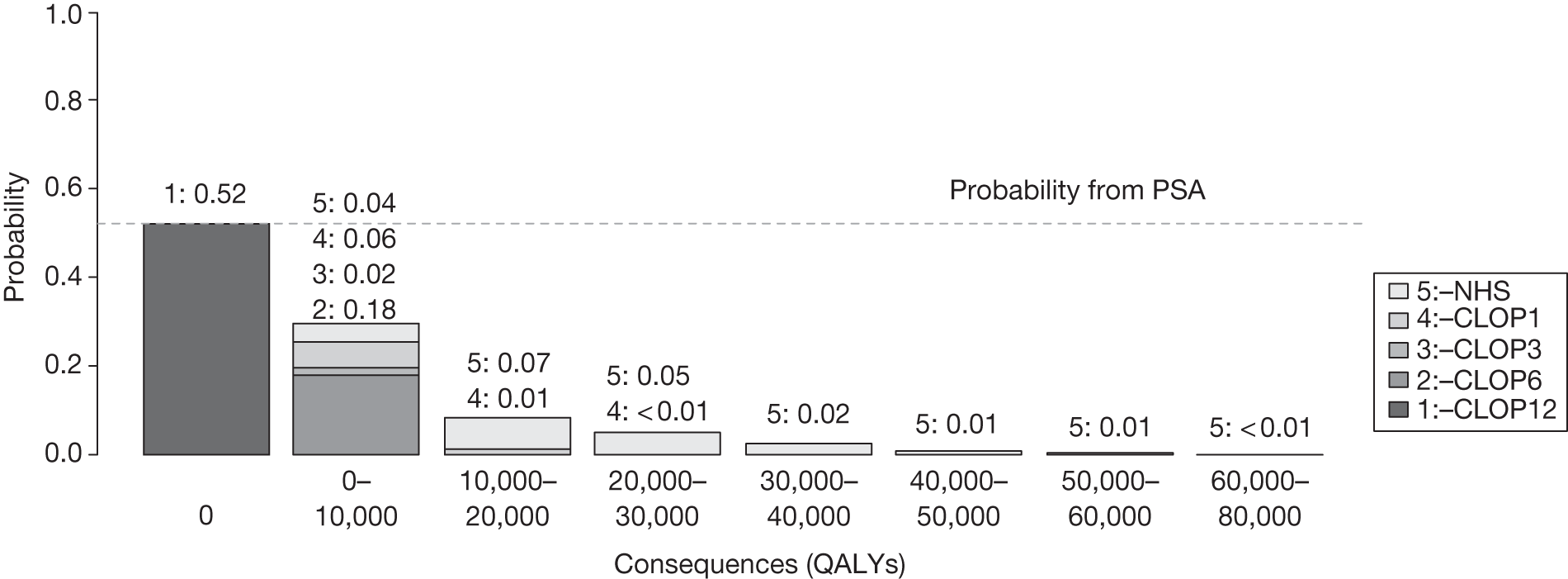
Psoriatic arthritis provides a similar picture to that of CLOP, in which approval of the alternative (etanercept), which is expected to be cost-effective, is uncertain (probability that approval is incorrect is 0.557), but in this case most of this probability of error is associated with palliative care (probability of 0.4 that it is cost-effective). Again, there is a greater chance of relatively small consequences (19% are < 28,000 QALYs), most of which occur when adalimumab has the highest NHE, and a smaller chance of very large consequences (4.7% chance that they are > 138,000 QALYs), which occur when palliative care offers the greatest NHE. The expected consequences of uncertainty and the upper bound on the population NHE that might be gained by immediately resolving uncertainty (35,342 QALYs or £707M over the technology time horizon) supports a judgement that more research may be worthwhile.
Analysis of subgroups
Omalizumab was not expected to be cost-effective based on existing evidence. The ICER in Table 13 was substantially greater than the threshold and a decision to reject this technology does not appear uncertain. This judgement is supported by the results of PSA (the probability that OMAL is cost-effective is zero in Table 20). Therefore, a decision to reject (‘Reject4’ in the algorithm) is not uncertain; there are no consequences of uncertainty and nothing to be gained by more research. However, it is possible to consider a high-risk subgroup within this population. Subgroups, once credibly defined, need to be considered in the same way, starting at point 1 on the checklist, that is, entering at the top of the algorithm (see Appendix 6, Is further research required?, Subgroups). Although the ICER for this high-risk subgroup is somewhat lower, it is still significantly higher than the threshold. The results of PSA suggest that even at a threshold of £30,000 the probability that OMAL is cost-effective is very small and the upper bound on the gains from more research are very limited (10.61 QALYs). Therefore, even after an analysis of subgroups OMAL is not expected to be cost-effective and more research does not seem worthwhile. OMAL can be rejected at this point and no further assessment is required.
| Treatment | ICER (£/QALY) | Cost-effectiveness threshold £20,000 per QALY | Cost-effectiveness threshold £30,000 per QALY | ||||
|---|---|---|---|---|---|---|---|
| Incremental NHE, QALYs (£M) | Probability cost-effective | Expected consequences, QALYs (£) | Incremental NHE, QALYs (£M) | Probability cost-effective | Expected consequences, QALYs (£) | ||
| Severe population | |||||||
| OMAL + standard care | 93,844 | –5789 (–116) | 0.0 | 0 | –3337 (–100) | 0.0 | 0.0 |
| Standard care | – | 1.0 | – | 1.0 | |||
| High-risk subgroup | |||||||
| OMAL + standard care | 69,463 | –3851 (–77) | 0.0 | 0 | –2048 (–61) | 0.013 | 10.61 (0.32) |
| Standard care | – | 1.0 | – | 0.987 | |||
Alternative scenarios
There are often alternative views about the quality and relevance of evidence as well as other assumptions that might be made when estimating expected costs and QALYs. These are commonly presented as separate scenarios, with estimates of costs and QALY presented for each. Much of the deliberation by the Appraisal Committee often surrounds the scientific value judgements required to judge the credibility of the alternative assumptions represented by the scenarios. The type of probabilistic analysis reported represents the uncertainty within each scenario and will be sufficient to indicate the potential benefits of research when only one scenario is regarded as credible. However, when more than one scenario might be credible and carry some ‘weight’, there will be uncertainty between as well as within scenarios. The ‘weighting’ of scenarios can be made explicit by assigning probabilities to represent how credible each is believed to be. The weighted average of costs and QALYs across scenarios can easily be calculated (see Appendix 6, Is further research required?, Alternative scenarios). It is also tempting to take a simple weighted average of the expected consequences of uncertainty across these scenarios as well. However, a simple weighted average may under- or overestimate the combined consequences of uncertainty within and between scenarios (see Appendix 11, Why averaging scenarios may be misleading). 130 The correct estimate requires the probabilities (weights) to be applied directly to the simulated output from PSA rather than to the mean values. Although this does not require additional simulation and is quick and easy to implement, it does require either that the probabilities are made explicit in advance or that estimates be presented for a range of probabilities that might represent the judgement of the Appraisal Committee following deliberation.
For example, the CLOP analysis presented above assumes a constant relative treatment effect for different durations of treatment (scenario A). An alternative assumption (scenario B) was that the relative treatment effect also differed by duration based on the data reported in the Scottish Intercollegiate Guidelines Network (SIGN) guidelines. This alternative assumption made longer durations less cost-effective and reduced the expected consequences of uncertainty from 5195 to 3969 QALYs. Although scenario A was regarded as more credible by the Appraisal Committee, scenario B might nevertheless carry some weight or have some probability associated with it. In this case the simple weighted average of expected consequences (linear combination of mean estimates) is very similar to the correct estimate based on weighting the output of PSA in Figure 11. This also shows how these estimates can be presented for a range of probabilities.
FIGURE 11.
Expected consequences of uncertainty with alternative scenarios: CLOP case study.
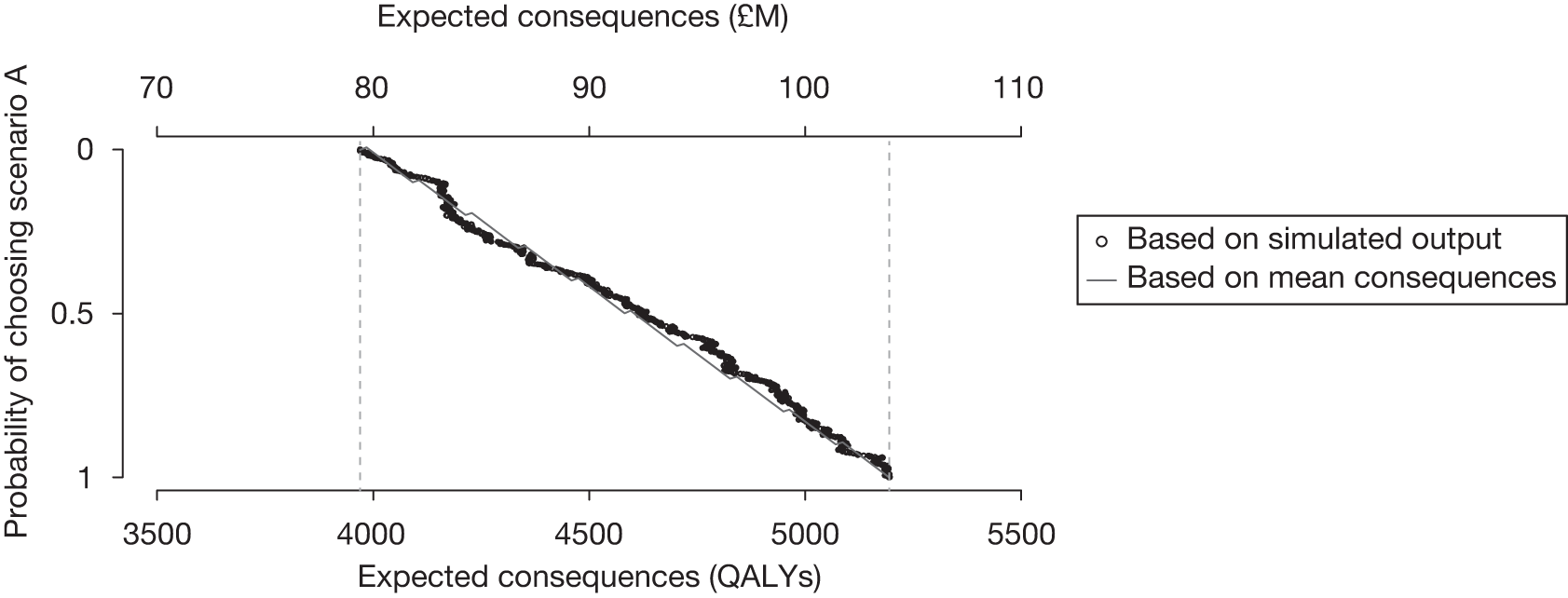
An alternative assumption of a common class effect across the three biologics was considered in the PsA case study (scenario B) but was judged less credible than the analysis that allowed differential effects (scenario A). The alternative scenario made etanercept less likely to be cost-effective and increased the expected consequences of uncertainty from 34,930 to 38,521 QALYs (Figure 12). In this case a simple weighted average of expected consequences based on the probability assigned to each scenario is, in general, lower than the correct estimate of expected consequences based on the output from PSA.
FIGURE 12.
Expected consequences of uncertainty with alternative scenarios: PsA case study.
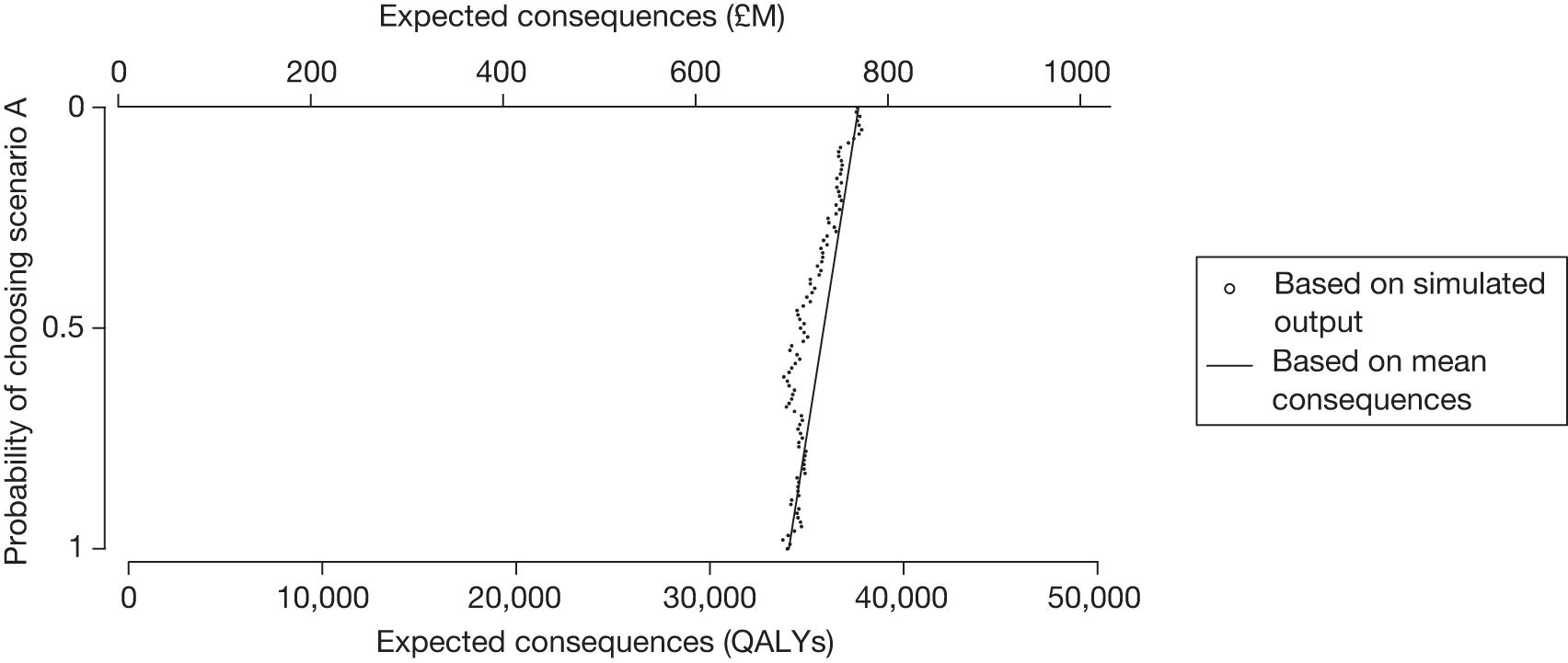
Elicitation
The single RCT of EECP showed evidence of improvements in quality of life at 12 months;121 however, the degree to which these are sustained in the long run is uncertain. Rather than make alternative assumptions and present extreme scenarios, formal elicitation of the judgement of clinical experts about the likelihood of QALY gains in subsequent years was undertaken. s The uncertainty in these elicited values is included in the estimates of the expected consequences of uncertainty reported in Table 18, which might otherwise have been represented by alternative scenarios, for example no QALY benefits beyond 12 months could be assumed for scenario A, benefits sustained for a patient’s lifetime for scenario B and benefits sustained for 4 years for scenario C. The results of elicitation implied probabilities of 0.243, 0.353 and 0.404 associated with each of these scenarios, respectively. A simple weighted average of the expected consequences within each scenario using these probabilities (1442 QALYs) significantly underestimates both the estimate of expected consequences based on all of the information from elicitation (9287 QAYs) and the estimate based on weighting scenarios using the simulated output rather than the mean estimates (13,081 QALYs). This illustrates that (1) a simple weighted average of expected consequences may be misleading and (2) elicitation may provide a richer characterisation of uncertainty as well the probabilities associated with alternative assumptions (see Appendix 11, Why averaging scenarios may be misleading for further discussion).
Point 4: Is research possible with approval?
The fourth point on the checklist requires an assessment of what type of evidence is needed and a judgement of whether the research required to generate it can be conducted while the technology is approved, that is, at the following point in the algorithm:
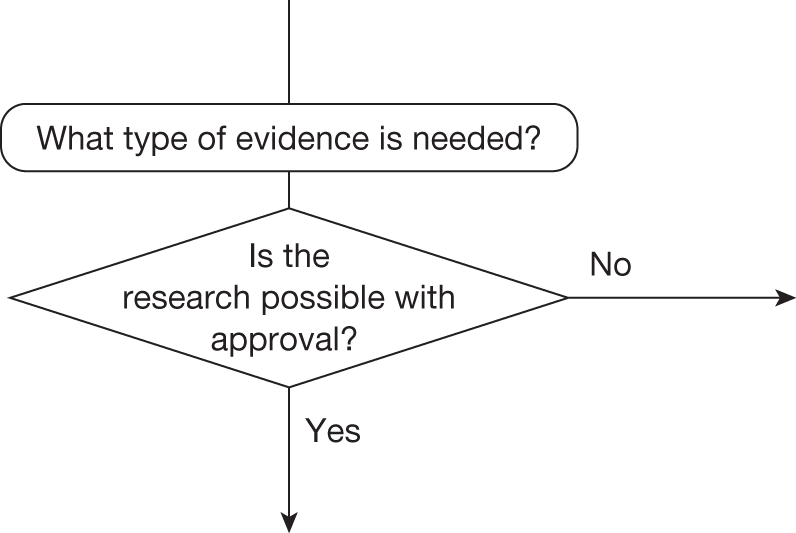
Although the decision at this point does not lead directly to guidance, it does determine whether AWR or OIR is a possibility. This judgement will depend, in part, on whether the type of evidence that is needed will require an experimental research design. For example, more precise estimates of relative treatment effect are likely to require a RCT if the dangers of selection bias are to be avoided; however, further RCTs for this particular indication and patient group are unlikely to be possible once a technology is approved for widespread NHS use.
This requires judgements about (1) how important particular types of parameters (inputs to the economic model) are to estimates of cost and QALYs, (2) what values these parameters would have to take to change a decision based on expected cost-effectiveness, (3) how likely is it that parameters might take such values and (4) what would be the consequences if they did, that is, what might be gained in terms of population NHE if the uncertainty in the values of these parameters could be immediately resolved (see Appendix 6, Is the research possible if the intervention is approved?).
Assessing the importance of parameters
The type of economic model used to estimate expected cost-effectiveness in NICE appraisal specifies the relationship between the inputs (the parameters) and the outputs (costs and QALYs). A simple summary of the direction and strength of these relationships can be provided by calculating elasticities for each, that is, the proportionate change in the NHE of each alternative, and differences in NHE, owing to a 1% change in the value of the parameter, for example those parameters with high elasticities (especially with respect to differences in NHE) might be regarded as more ‘important’. These elasticities are presented for the CLOP case study in Table 21. They give some indication of (1) the relative importance of parameters for certain comparisons (e.g. RR_death seems particularly important for all comparisons), (2) those parameters that are of no or very limited importance (e.g. parameters 1–6 in the comparison of 12 and 6 months’ treatment) and (3) the direction of the relationship (e.g. the elasticity for C_Well is negative indicating that if the costs of NHS care in the well state are greater, then 12 months’ treatment will be less cost-effective than 6 months’ treatment or current NHS care).
| Parameter | Elasticity over the NHEs (QALYs) of | Elasticity over the incremental NHEs (QALYs) of | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CLOP12 | CLOP6 | CLOP3 | CLOP1 | NHS | CLOP12 vs NHS | CLOP12 vs CLOP6 | CLOP12 vs alla | |||
| Natural history | 1 | P_die_0.1 | –0.208 | –0.207 | –0.207 | –0.207 | –0.222 | 0.014 | – | 0.003 |
| 2 | P_NFMI_0.1 | –0.012 | –0.012 | –0.011 | –0.011 | –0.015 | 0.004 | – | – | |
| 3 | P_die_1.3 | –0.137 | –0.137 | –0.137 | –0.147 | –0.145 | 0.008 | – | 0.004 | |
| 4 | P_NFMI_1.3 | –0.002 | –0.002 | –0.002 | –0.002 | –0.002 | 0.001 | – | – | |
| 5 | P_die_3.6 | –0.146 | –0.146 | –0.157 | –0.157 | –0.154 | 0.008 | – | 0.007 | |
| 6 | P_NFMI_3.6 | –0.005 | –0.005 | –0.007 | –0.007 | –0.007 | 0.002 | – | 0.001 | |
| 7 | P_die_6.12 | –0.148 | –0.159 | –0.158 | –0.157 | –0.155 | 0.007 | 0.011 | 0.010 | |
| 8 | P_NFMI_6.12 | –0.005 | –0.007 | –0.007 | –0.007 | –0.007 | 0.002 | 0.002 | 0.002 | |
| 9 | TP_AC | –0.121 | –0.120 | –0.120 | –0.120 | –0.118 | –0.003 | –0.001 | –0.002 | |
| 10 | TP_AD | –3.637 | –3.622 | –3.604 | –3.594 | –3.541 | –0.096 | –0.016 | –0.047 | |
| 11 | TP_CD | –0.233 | –0.235 | –0.239 | –0.240 | –0.253 | 0.020 | 0.002 | 0.009 | |
| 12 | TP_BD | –0.586 | –0.593 | –0.602 | –0.605 | –0.641 | 0.055 | 0.007 | 0.024 | |
| Utilities | 13 | U_Well | 0.746 | 0.745 | 0.743 | 0.742 | 0.737 | 0.009 | 0.001 | 0.004 |
| 14 | U_Well1 | 6.090 | 6.064 | 6.034 | 6.017 | 5.929 | 0.160 | 0.026 | 0.079 | |
| 15 | U_NFMI | 0.133 | 0.134 | 0.136 | 0.136 | 0.144 | –0.011 | –0.001 | –0.005 | |
| 16 | U_PostMI | 1.138 | 1.150 | 1.165 | 1.171 | 1.236 | –0.099 | –0.012 | –0.043 | |
| Relative effect | 17 | RR_death | –0.639 | –0.491 | –0.344 | –0.207 | – | –0.641 | –0.150 | –0.380 |
| 18 | RR_NFMI | –0.024 | –0.018 | –0.013 | –0.011 | – | –0.025 | –0.006 | –0.014 | |
| Costs | 19 | C_Well | –0.740 | –0.737 | –0.733 | –0.731 | –0.720 | –0.019 | –0.003 | –0.009 |
| 20 | C_MI_LT | –0.051 | –0.052 | –0.053 | –0.053 | –0.056 | 0.004 | 0.001 | 0.002 | |
| 21 | C_PostMI | –0.142 | –0.143 | –0.145 | –0.146 | –0.154 | 0.012 | 0.002 | 0.005 | |
| 22 | TC_Well_Dead | –0.027 | –0.027 | –0.027 | –0.027 | –0.027 | – | – | – | |
| 23 | C_t1 | –0.045 | – | – | – | – | –0.045 | –0.045 | –0.045 | |
| 24 | C_t2 | – | –0.033 | – | – | – | – | 0.033 | 0.008 | |
| 25 | C_t3 | – | – | –0.026 | – | – | – | – | 0.007 | |
| 26 | C_t4 | – | – | – | –0.022 | – | – | – | 0.005 | |
| 27 | C_t5 | – | – | – | – | –0.016 | 0.016 | – | 0.004 | |
Although these measures of importance are more instructive than a series of arbitrary one-way sensitivity analyses, they do not directly help the assessment of what values parameters must take to change decisions and how likely such values might be. A simple summary of the values that particular parameters must take to make each of the alternatives cost-effective can also be provided. These ‘threshold values’ for parameters are presented for the CLOP case study in Table 22. This provides additional information to the elasticities in Table 21, for example there are only six parameters that could possibly take values that would lead to current NHS care (without CLOP) generating higher NHEs than 12 months of treatment with CLOP. However, although instructive, such ‘threshold values’ do not indicate how likely it is that the threshold will be crossed or the combined effect of groups of related parameters.
| Parameter | Mean value | CLOP12 | CLOP6 | CLOP3 | CLOP1 | NHS | ||
|---|---|---|---|---|---|---|---|---|
| Natural history | 1 | P_die_0.1 | 0.032 | 0 to 0.10 | 0.11 to 0.54 | 0.54 to 0.63 | 0.63 to 1 | – |
| 2 | P_NFMI_0.1 | 0.040 | 0 to 0.14 | 0.14 to 0.71 | 0.71 to 0.82 | 0.82 to 1 | – | |
| 3 | P_die_1.3 | 0.022 | 0 to 0.10 | 0.10 to 0.55 | 0.55 to 1 | – | – | |
| 4 | P_NFMI_1.3 | 0.004 | 0 to 0.10 | 0.10 to 0.7 | 0.7 to 1 | – | – | |
| 5 | P_die_3.6 | 0.023 | 0.01 to 0.10 | 0.10 to 1 | 0 to 0.01 | – | – | |
| 6 | P_NFMI_3.6 | 0.011 | 0 to 0.11 | 0.11 to 1 | – | – | – | |
| 7 | P_die_6.12 | 0.024 | 0.02 to 1 | 0 to 0.02 | – | – | – | |
| 8 | P_NFMI_6.12 | 0.009 | 0.005 to 1 | 0 to 0.005 | – | – | – | |
| 9 | TP_AC | 0.018 | 0 to 0.06 | 0.06 to 1 | – | – | – | |
| 10 | TP_AD | 0.072 | 0 to 0.08 | 0.08 to 0.10 | – | – | 0.10 to 1 | |
| 11 | TP_CD | 0.188 | 0.12 to 1 | 0 to 0.12 | – | – | – | |
| 12 | TP_BD | 0.07 | 0.06 to 1 | 0.04 to 0.06 | – | – | 0 to 0.04 | |
| Utilities | 13 | U_Well | 0.798 | 0.29 to 1 | 0 to 0.29 | – | – | – |
| 14 | U_Well1 | 0.930 | 0.90 to 1 | 0.74 to 0.90 | – | – | 0 to 0.74 | |
| 15 | U_NFMI | 0.801 | 0 to 1 | – | – | – | – | |
| 16 | U_PostMI | 0.931 | 0 to 1 | – | – | – | – | |
| Relative effect | 17 | RR_death | 0.931 | 0 to 0.93 | 0.94 to 0.97 | 0.97 to 0.98 | 0.98 to 0.99 | 1.00 to max.a |
| 18 | RR_NFMI | 0.710 | 0 to 0.82 | 0.83 to 1.55 | 1.56 to 1.83 | – | 1.84 to max.a | |
| Costs | 19 | C_Well | 2061.5 | 0 to 2690 | 2690 to 5611 | – | – | 5611 to max.a |
| 20 | C_MI_LT | 6050 | 0 to max.a | – | – | – | – | |
| 21 | C_PostMI | 2309.7 | 870 to max.a | 0 to 870 | – | – | – | |
| 22 | TC_Well_Dead | 871.5 | 0 to 20,474 | 20,474 to max.a | – | – | – | |
| 23 | C_t1 | 895.1 | 0 to 910 | 910 to max.a | – | – | – | |
| 24 | C_t2 | 651.6 | 630 to max.a | 0 to 630 | – | – | – | |
| 25 | C_t3 | 524.2 | 370 to max.a | – | 0 to 370 | – | – | |
| 26 | C_t4 | 434.8 | 150 to max.a | – | – | 0 to 150 | – | |
| 27 | C_t5 | 329.8 | 0 to max. | – | – | – | – | |
Assessment of uncertainty
The judgement about how likely it is that parameters might take values that will change the technology expected to be cost-effective can be informed by the results of PSA. This is because the distributions assigned to parameters in PSA describe how uncertain the parameter estimates are, such that they ought to reflect the amount and quality of existing evidence. The probabilities that parameters might take values that would lead to each of the alternatives being cost-effective are reported for the CLOP case study in Table 23. This essentially decomposes the overall probabilities reported in Table 19 into the contribution that each parameter makes. t Interestingly, it indicates that it is uncertainty in the estimate of relative effect (RR_death) that contributes most to the probability of error associated with 12 months of treatment. It is the only parameter that (alone) might take values that could make any of the other alternatives cost-effective. It is also worth noting that there is a very small chance that cost in the ‘well state’ (C_Well) might be sufficiently high that standard NHS care would be cost-effective (i.e. a 3% chance that the cost of this state exceeds the threshold value of £5611 required for standard NHS care to have the highest NHE). In other words, if the NHS costs associated with the ‘well state’ are higher than the mean value then any cost savings associated with moving more patients more quickly to the well state [and thus avoiding the costs of additional events such as non-fatal myocardial infarction (NFMI)] will tend to be lower.
| Parameter | CLOP12 | CLOP6 | CLOP3 | CLOP1 | NHS | ||
|---|---|---|---|---|---|---|---|
| Natural history | 1 | P_die_0.1 | 1 | – | – | – | – |
| 2 | P_NFMI_0.1 | 1 | – | – | – | – | |
| 3 | P_die_1.3 | 1 | – | – | – | – | |
| 4 | P_NFMI_1.3 | 1 | – | – | – | – | |
| 5 | P_die_3.6 | 1 | – | – | – | – | |
| 6 | P_NFMI_3.6 | 1 | – | – | – | – | |
| 7 | P_die_6.12 | 0.65 | 0.35 | – | – | – | |
| 8 | P_NFMI_6.12 | 0.91 | 0.09 | – | – | – | |
| 9 | TP_AC | 1 | – | – | – | – | |
| 10 | TP_AD | 0.83 | 0.17 | – | – | – | |
| 11 | TP_CD | 1 | – | – | – | – | |
| 12 | TP_BD | 0.85 | 0.15 | – | – | – | |
| Utilities | 13 | U_Well | 1 | – | – | – | – |
| 14 | U_Well1 | 0.94 | 0.06 | – | – | – | |
| 15 | U_NFMI | 1 | – | – | – | – | |
| 16 | U_PostMI | 1 | – | – | – | – | |
| Relative effect | 17 | RR_death | 0.55 | 0.18 | 0.01 | 0.10 | 0.16 |
| 18 | RR_NFMI | 0.97 | 0.03 | – | – | – | |
| Costs | 19 | C_Well | 0.78 | 0.19 | – | – | 0.03 |
| 20 | C_MI_LT | 1 | – | – | – | – | |
| 21 | C_PostMI | 0.89 | 0.11 | – | – | – | |
| 22 | TC_Well_Dead | 1 | – | – | – | – | |
| 23 | C_t1 | 0.95 | 0.05 | – | – | – | |
| 24 | C_t2 | 0.99 | 0.01 | – | – | – | |
| 25 | C_t3 | 1 | – | – | – | – | |
| 26 | C_t4 | 1 | – | – | – | – | |
| 27 | C_t5 | 1 | – | – | – | – | |
What type of evidence is needed?
Although an understanding of uncertainty and the importance of parameters separately is helpful, an assessment of the likely consequences of this uncertainty, and therefore what might be potentially gained in terms of population NHEs if uncertainty could be immediately resolved, is required. This assessment can directly inform the judgement of what evidence is needed and whether or not the type of research required to generate it will be possible with approval. As in the previous section on whether or not more research seems worthwhile, the results of PSA can inform this judgement as estimates of the expected consequences of uncertainty associated with each parameter combine both uncertainty in its potential values and its importance in terms of changing decisions and the resulting differences in NHEs (see Appendix 6, What type of evidence is needed?). The expected consequences of uncertainty associated with each parameter in the CLOP case study are reported in Table 24. This decomposes the overall expected consequences reported in Table 19 into the contribution that each parameter makes. It also identifies which of the alternatives to 12-month treatment might offer a higher NHE. u Table 24 confirms that it is uncertainty in the estimate of relative effect (RR_death) that contributes most and for which there is potentially the most to be gained by resolving this uncertainty through additional research (4433 QALYs or £88.7M). Since more precise estimates of relative effects are likely to require a RCT, a judgement that the type of research needed will not be possible if a 12-month treatment duration is approved may be reasonable. However, the potential benefits of resolving the uncertainty associated with other groups of parameters, for example costs (547 QALYs or £10.9M) and the natural history (369 QALYs or £7.4M), might mean that other types of cheaper, non-experimental research could be worthwhile as well or might be conducted before commissioning potential expensive experimental research that may take some time to complete and report. 131,v
| Parameter | Expected consequences (QALYs) | |||||||
|---|---|---|---|---|---|---|---|---|
| Decomposed by treatment choice | Overall | |||||||
| CLOP12 | CLOP6 | CLOP3 | CLOP1 | NHS | ||||
| Natural history a | 1 | P_die_0.1 | 0 | – | – | – | – | 0 |
| 2 | P_NFMI_0.1 | 0 | – | – | – | – | 0 | |
| 3 | P_die_1.3 | 0 | – | – | – | – | 0 | |
| 4 | P_NFMI_1.3 | 0 | – | – | – | – | 0 | |
| 5 | P_die_3.6 | 0 | – | – | – | – | 0 | |
| 6 | P_NFMI_3.6 | 0 | – | – | – | – | 0 | |
| 7 | P_die_6.12 | 0 | 250 | – | – | – | 250 | |
| 8 | P_NFMI_6.12 | 0 | 9 | – | – | – | 9 | |
| 9 | TP_AC | 0 | – | – | – | – | 0 | |
| 10 | TP_AD | 0 | 47 | – | – | – | 47 | |
| 11 | TP_CD | 0 | – | – | – | – | 0 | |
| 12 | TP_BD | 0 | 35 | – | – | – | 35 | |
| Utilities a | 13 | U_Well | 0 | – | – | – | – | 0 |
| 14 | U_Well1 | 0 | 10 | – | – | – | 10 | |
| 15 | U_NFMI | 0 | – | – | – | – | 0 | |
| 16 | U_PostMI | 0 | – | – | – | – | 0 | |
| Relative effect a | 17 | RR_death | 0 | 284 | 16 | 518 | 3614 | 4433 |
| 18 | RR_NFMI | 0 | 3 | – | – | – | 3 | |
| Costs a | 19 | C_Well | 0 | 153 | – | – | 321 | 474 |
| 20 | C_MI_LT | 0 | – | – | – | – | 0 | |
| 21 | C_PostMI | 0 | 8 | – | – | – | 8 | |
| 22 | TC_Well_Dead | 0 | – | – | – | – | 0 | |
| 23 | C_t1 | 0 | 8 | – | – | – | 8 | |
| 24 | C_t2 | 0 | 0 | – | – | – | 0 | |
| 25 | C_t3 | 0 | – | – | – | – | 0 | |
| 26 | C_t4 | 0 | – | – | – | – | 0 | |
| 27 | C_t5 | 0 | – | – | – | – | 0 | |
The full results tables for the other case studies are provided in Appendices 7, 9 and 10. In summary, the EECP case study provides a similar pattern of results with the most significant consequences of uncertainty associated with parameters related to relative treatment effect, suggesting that the research needed might not be possible following approval of EECP. Interestingly, although the probability of sustaining the QALY benefits of EECP in the long run is very uncertain, the greater potential value is in more precise estimates of QALY gains in the first 12 months (8511 QALYs or £170M, respectively).
With regard to the PsA case study, on the other hand, the greater potential value is associated with uncertainty over the natural history of Health Assessment Questionnaire (HAQ) progression (8697 QALYs or £17.4M) rather than relative treatment effect (1201 QALYs or £2.4M). Although this might suggest that AWR, which recommended research on HAQ progression, is possible and worthwhile, the combined potential benefit of resolving uncertainty associated with natural history [both in HAQ and Psoriatic Arthritis Response Criteria (PsARC)] and treatment effect together is much greater than the ‘sum of its parts’. w This suggests that both types of research could be conducted while etanercept continues to be approved but infliximab and adalimumab are not, that is, a possible OIR rather than reject for infliximab and adalimumab but AWR for etanercept.
Implications of between-scenario uncertainty
In Point 3: Does more research seem worthwhile?, Alternative scenarios the contribution that alternative scenarios might make to the overall expected consequences of uncertainty and therefore the potential gains from further evidence was considered and discussed. In situations in which more than one scenario might be regarded as credible there will be uncertainty between as well as within each of the scenarios. It was demonstrated that an assessment of the combined consequences of both sources of uncertainty requires ‘weights’ (probabilities) to be assigned to represent their credibility, which can then be applied directly to the simulated output from PSA (see Appendix 11, Why averaging scenarios may be misleading). However, the same analysis can also be used to identify the expected consequences of uncertainty associated with the alternative scenarios themselves, that is, what might be gained if evidence could immediately distinguish which scenario was ‘true’. This can help to inform the assessment of what type of evidence might be needed and whether the research required to generate it is likely to be possible once a technology is approved for widespread NHS use.
For example, in the CLOP analysis, scenarios A and B (treatment effect was constant or differed by treatment duration, respectively) were associated with expected consequences of uncertainty of 5195 and 3969 QALYs, respectively. If both scenarios were regarded as equally likely, the overall expected consequences of uncertainty (combining consequences within and between scenarios) would be 4667 QALYs. However, the expected consequences of uncertainty associated with the two alternative scenarios themselves and what might be potentially gained if the uncertainty between them could be immediately resolved is relatively modest at 85 QALYs, that is, most of what might be gained from further evidence is associated with the parameters in Table 24 rather than the alternative scenarios. This suggests that more evidence about overall relative effect on mortality is more important than resolving uncertainty about whether or not such an effect differs by treatment duration.
In the EECP case study, formal elicitation of the judgement of clinical experts about whether or not observed QALY gains at 12 months are likely to be sustained in subsequent years was undertaken. Because the uncertainty in these elicited values was incorporated into the analysis in the same way as for other parameters, the use of alternative scenarios was not necessary. However, in Point 3: Does more research seem worthwhile?, scenarios were used to illustrate the type of analysis that, without elicitation, might otherwise have been required. The scenarios included A, no QALY benefits beyond 12 months; B, benefits sustained for a patient’s lifetime; and C, benefits sustained for 4 years. The results of elicitation implied probabilities of 0.243, 0.353 and 0.404 associated with each of these scenarios, respectively. Based on these ‘weights’ for each scenario the overall expected consequences of uncertainty (combining the consequences within and between scenarios) would be 14,146 QALYs. In this case the expected consequences of uncertainty between the scenarios (13,202 QALYs) are much greater than what might be potentially gained from resolving the uncertainty within each scenario (1765 QALYs). Therefore, unlike in the CLOP case study, most of what might be gained from further evidence about EECP (in the absence of formal elicitation) would be evidence that could help distinguish between the scenarios rather than the parameters associated with each.
Do the benefits of research exceed the costs?
The judgements made at points 5 and 6 of the checklist are critical because if the benefits of research are not judged to exceed the costs then no further assessment are required (unless there are significant irrecoverable costs; see Table 32 in Appendix 4). If they are judged to exceed the costs and research can be conducted with approval then AWR would be appropriate. However, other sources of uncertainty need to be assessed first because they will influence the potential benefits of research and, even when research is not conducted, they will also influence the appropriate category of guidance when there are significant irrecoverable costs.
Point 5: Will other sources of uncertainty resolve over time?
The fifth point on the checklist requires an assessment of whether or not changes are likely to occur in the future that will influence the cost-effectiveness of the alternative technologies and the potential benefits of research, that is, at the following point in the algorithm:
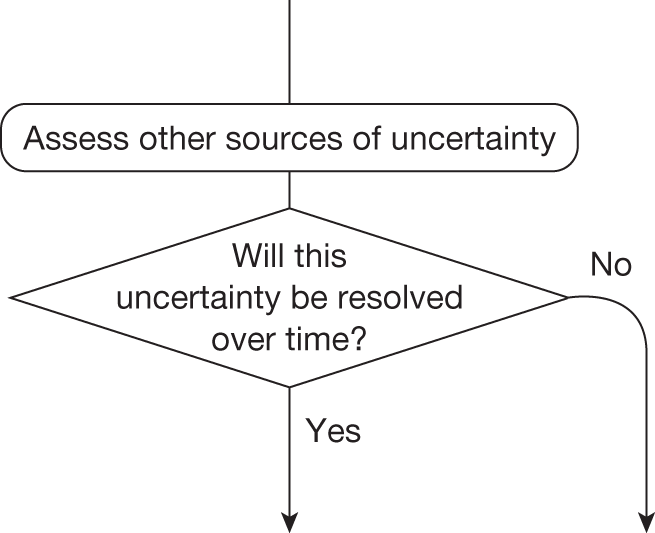
The judgement made at this point will influence the potential benefits of research and therefore subsequent decisions that lead directly to a particular category of guidance (see point 6 below). Even when research was not considered worthwhile (at point 3) the presence of other sources of uncertainty will determine whether or not significant irrecoverable costs are likely to influence the category of guidance. In some circumstances it can lead directly to guidance, that is, if there are no other sources of uncertainty even significant irrecoverable costs will have no influence and a technology that is expected to be cost-effective can be approved:
| Assessment | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Guidance | |
|---|---|---|---|---|---|---|---|---|---|
| Pathway number { | 29 | Yes | Yes | No | – | No | – | – | Approve12 |
This assessment requires information about (1) changes in prices of the technology and its comparators, (2) the emergence of new technologies that might make existing ones obsolete or change their cost-effectiveness and (3) other relevant research reporting (see Appendix 6, Impact of other sources of uncertainty). A number of potential sources of information and evidence were examined to inform this assessment for each case study (see Appendix 4 for the full details of the sources and searches conducted). However, many potentially useful sources were proprietary or public access was restricted, making it surprisingly difficult to inform these assessments with publically available information. When information and estimates were available they were often not directly relevant to a UK context.
Changes in the prices of the technology and its comparators
Changes in prices influence not only expected cost-effectiveness but also uncertainty and the potential benefits of research to future patients, for example if the price of a technology expected to be cost-effective is likely to fall significantly just before research reports the potential benefits will not be realised because approval of the technology will be less uncertain and there may be much less or little to gain from the results of the research. This assessment requires information about when major changes in prices are likely and some evidence about the likely extent of the changes. A major event in the life cycle of a pharmaceutical technology is the date at which the patent expires and cheaper generic versions of the brand become available. Although the date of patent expiry is, of course, known, it is surprisingly difficult to obtain the relevant date for particular products in the UK from publically available sources. Evidence of the extent to which the prices of generic versions are below the original brand price is also difficult to obtain and likely to differ by health-care system, type of technology, indication and time since patent expiry. Therefore, the estimate reported by the OFT that, on average, generic prices tend to be 25% of the original price was used in the subsequent analysis. 12
At the time of TA80122 the patent for CLOP was expected to expire 7 years later and subsequent analysis assumes that at that time equivalent generic prices will be 25% of the original price of CLOP at the time of TA80. x Although it was possible in the PsA case study to find patent expiry dates for etanercept (Enbrel®, Wyeth), infliximab (Remicade®, Schering-Plough) and adalimumab (Humira®, Abbott) in the USA (2012, 2014 and 2017, respectively), they were not available for the UK on the national patent database (Intellectual Property Office). It is even more difficult to locate patent information relevant to devices such as EECP because a device may only have a CE mark, which, unlike a patent, does not offer protection and can be renewed every 10 years. Any patent is likely to relate to some aspect of the device rather than the device itself. Although prices may change over time they can also be relatively stable but with incremental innovation of the original device. Again, this is likely to differ by health-care system, technology and indication. For these reasons future changes in prices are only quantitatively explored in the subsequent analysis of the CLOP case study in Point 6: Are the benefits of research greater than the costs?. There is a need to consider how access to the type of information required during NICE appraisal can be provided and how estimates of likely changes in prices relevant to the UK can be made readily available if these assessments are to be routinely made.
Entry of new technologies
The entry of a new technology will tend to change the relative cost-effectiveness of the alternatives, influencing how uncertain a decision to approve the original technology will be for future patients and the potential gains from research. For example, the entry of a new technology may make the existing technology that is expected to be cost-effective obsolete (no longer the most cost-effective alternative) or may make it more cost-effective and decisions less uncertain when used in combination with the new technology. A number of potential sources of information were examined to identify new technologies relevant to the indications that were likely to become available. These included a variety of sources related to NICE topic selection, information about licence applications and clinical research in phases I, II and III as well as evidence of the probability that earlier phase research leads to entry (probability of successful licence) and the likely time of entry (time to launch from initiating phases I, II and III research). Again, this information and evidence is fragmented, and in some cases restricted, for example NIHR Horizon Scanning Centre. Nevertheless, the information that was available indicated that one new technology relevant to CLOP and one relevant to PsA might have been expected to enter. Information about these technologies was limited and so scenarios are used to explore the implication for CLOP and PsA in Point 6: Are the benefits of research greater than the costs?.
Other research reporting of the technology and its comparators
Research that is already under way, commissioned or likely to be undertaken, whether in the UK or elsewhere, is relevant for two reasons: first, if it is research based in the UK then guidance might impact on recruitment and the successful completion of this research (see Point 7: Are the benefits of approval greater than the costs?); second, when this research reports that there is a chance that it will change the estimates of cost-effectiveness and resolve some of the current uncertainties. In other words, there is little to be gained by recommending OIR or AWR if the uncertainty is likely to be resolved in the near future when other research reports. A number of potential sources of information were examined to identify clinical research under way at the time of the relevant appraisal, including national and international trial registries and other databases that report NHS-funded research and not just clinical trials (e.g. National Research Register and UK Clinical Research Network). Despite an assiduous search no records relevant to the case studies were identified. This may suggest that no other research was ongoing or expected for these comparators in these indications, or it may indicate that currently available sources are fragmented, incomplete and/or difficult to access.
Point 6: Are the benefits of research greater than the costs?
The sixth point on the checklist requires a reassessment of the potential benefits of conducting further research that were initially considered at point 3, and a judgement of whether the benefits of research are likely to exceed the costs, that is, at the following point in the algorithm:
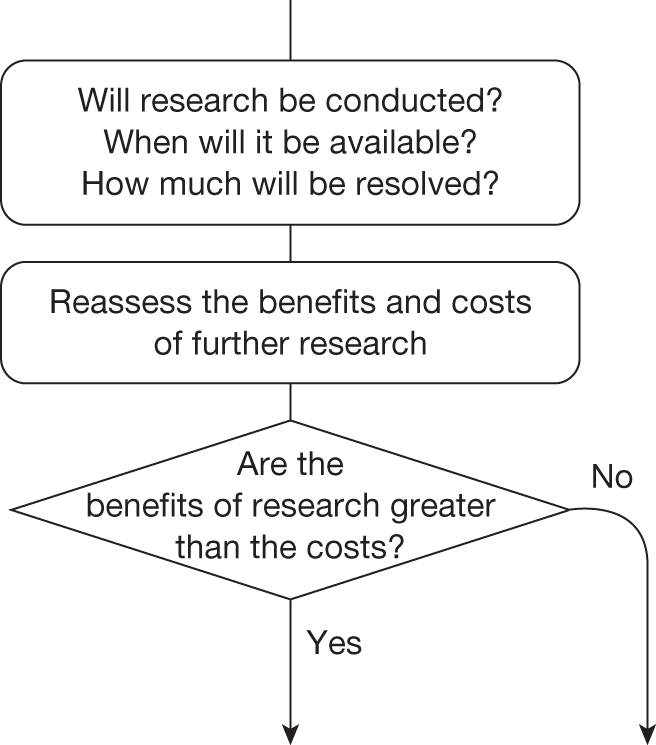
A judgement about whether or not the potential benefits of research identified earlier (see Is further research required?) will be realised requires an assessment of (1) whether or not the type of research that is required is likely to be conducted, (2) if conducted, when the results are likely to be available, (3) how much uncertainty is likely to be resolved and (4) the likely impact of the other sources of uncertainty identified in Point 5: Will other sources of uncertainty resolve over time? on the longer-term benefits of research (see Will the research be conducted?, How long until the research reports?, How much of the uncertainty will the research resolve? and How do other sources of uncertainty impact on the need for evidence?, Appendix 6, respectively).
The decision at this point may not necessarily lead directly to guidance, for example when the benefits of research exceed the costs but research is not possible with approval or there are significant irrecoverable costs. Which category of guidance will ultimately be appropriate will depend on whether or not the benefits of approval are judged to exceed the costs, that is, point 7 of the checklist (see next section). However, in many other circumstances the decision at this point will lead directly to a particular category of guidance. These circumstances or pathways through the algorithm are detailed below (extracted from Table 32 in Appendix 4):
| Assessment | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Guidance | |
|---|---|---|---|---|---|---|---|---|---|
| Pathway number { | 1 | Yes | No | Yes | Yes | Yes/no | Yes | – | AWR1 |
| 2 | Yes | No | Yes | Yes | Yes/no | No | – | Approve1 | |
| 5 | Yes | No | Yes | No | Yes/no | No | – | Approve3 | |
| 7 | No | No | Yes | Yes | Yes/no | Yes | – | OIR2 | |
| 8 | No | No | Yes | Yes | Yes/no | No | – | Reject1 | |
| 11 | No | No | Yes | No | Yes/no | No | – | Reject3 | |
| 19 | Yes | Yes | Yes | Yes | No | No | – | Approve6 | |
| 26 | Yes | Yes | Yes | No | No | No | – | Approve10 | |
| 30 | No | Yes | Yes | Yes | Yes/no | Yes | – | OIR7 | |
| 31 | No | Yes | Yes | Yes | Yes/no | No | – | Reject8 | |
| 34 | No | Yes | Yes | No | Yes/no | No | – | Reject10 |
The expected consequences of uncertainty reported in Point 3: Does more research seem worthwhile? represented the NHE that could be gained over the lifetime of the technology if the uncertainty surrounding the decision based on expected cost-effectiveness could be immediately and completely resolved. This represents an upper bound on the potential benefits of research for a number of reasons: (1) research, although recommended, might not be commissioned and/or recruit and report, (2) any research will take some time to complete before results are available and (3) not all of the uncertainty is likely to be resolved. In addition, future events (identified in Point 5: Will other sources of uncertainty resolve over time?) might change the NHE expected to be gained by future patient populations. Finally, the expected benefits of research once properly reassessed must be compared with the likely costs.
Will the research be conducted?
Even if research is recommended in OIR or AWR it might not be undertaken by manufacturers or commissioned by research funders. Even if undertaken or commissioned there is no guarantee that research will be able to recruit or it may not complete for other reasons (see Appendix 6, Will the research be conducted?). The expected consequences of uncertainty for CLOP and EECP reported in Point 3: Does more research seem worthwhile? are illustrated in Figures 13 and 14, respectively, for a range of probabilities that research will be successfully undertaken. This indicates that the potential gains depend on a judgement of whether the research recommended as part of OIR or AWR will be successfully completed. This also illustrates that the cost of research (in this case considered to be either £1.5M or £10M) can be compared directly with the potential benefits by either (1) expressing the potential gains in population NHE as the equivalent NHS resources (i.e. the resources that would be required to generate the same NHE) or (2) expressing the cost of research in terms of the QALYs that could be gained elsewhere in the NHS by using the same resources to provide access to health care.
FIGURE 13.
Expected potential benefits of research: CLOP case study.
FIGURE 14.
Expected potential benefits of research: EECP case study.
When will it be available?
Research, even if commissioned and successfully completed, will take time to commission, complete and report. Therefore, any assessment of the potential benefits should account for the fact that patient populations will not benefit from the results of research until they are available and change clinical practice. y Whether or not those patients who are prevalent while research is under way will be able to benefit from the results will depend on whether treatment decisions for presenting patients are irreversible or not (see Point 2: Are there significant irrecoverable costs?). If treatment decisions are irreversible, for example in the CLOP case study, it is only those patients incident after the research reports who will realise any of the potential benefits. In contrast, treatment decisions in EECP are not irreversible (it is a chronic condition) and so, although patients prevalent while research is undertaken will not benefit immediately, those who survive can benefit from the results once the research is completed. How long research might take to report will depend in part on the design (follow-up, sample size and end points), recruitment rate and size of the eligible patient population, as well as on how efficient the organisation and data collection might be. The potential value of research in CLOP and EECP over a range of possible time horizons is reported in Figures 15 and 16, respectively. In both cases the potential value of further research declines with increase in time to research reporting. This relationship gives some indication of the value of improving the timeliness of research through, for example, investment in research infrastructure or adopting a research design that, although offering less potential benefits, can be conducted more quickly. It also indicates the value of efforts to ensure that any revised NICE guidance following OIR or AWR is more quickly and more completely implemented.
FIGURE 15.
Potential value of research by time to report: CLOP case study.
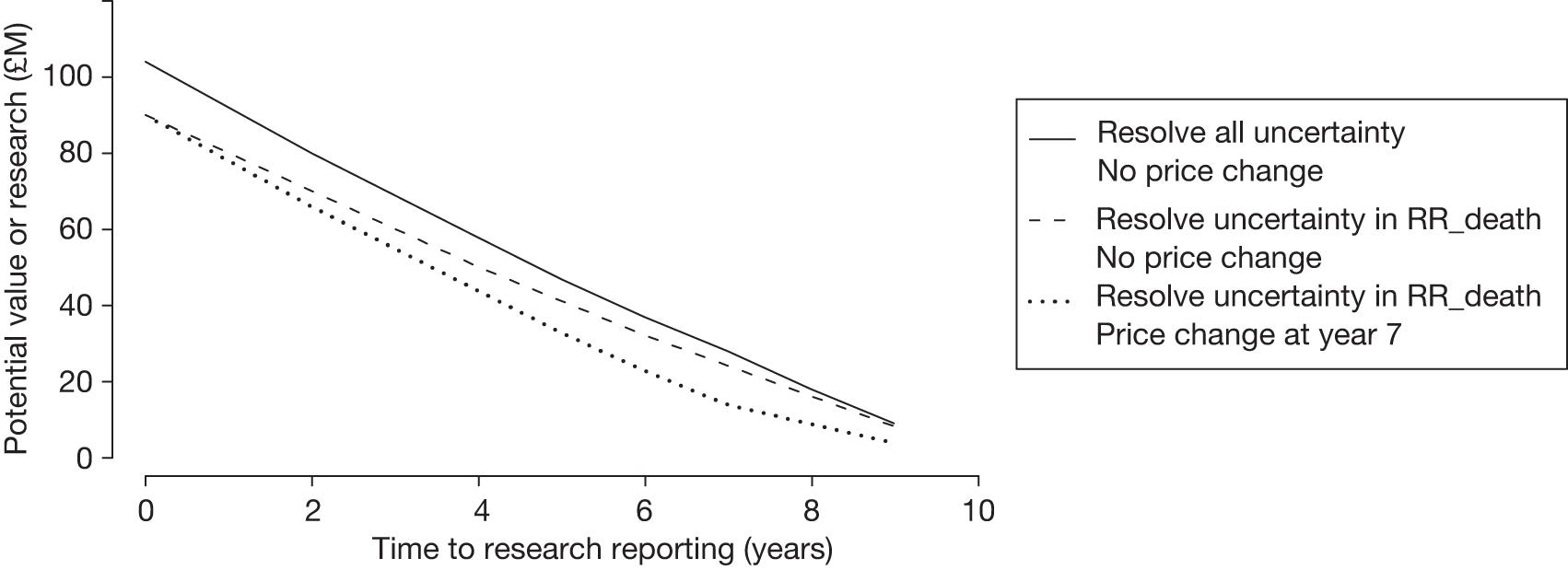
FIGURE 16.
Potential benefits of research by time to report: EECP case study.
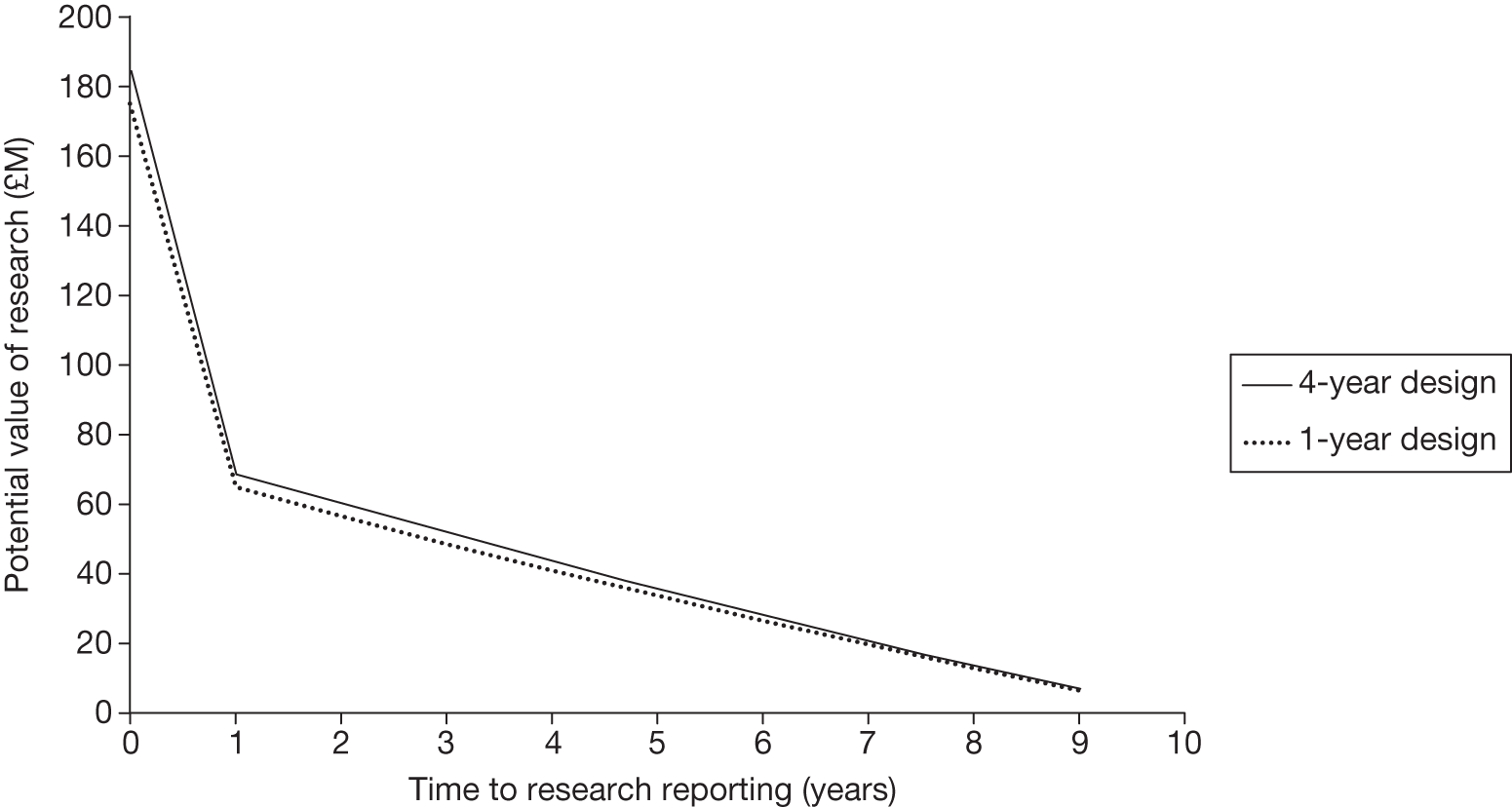
How much will be resolved?
Most research will not inform all of the parameters that determine expected cost and QALYs but usually a subset of them. Therefore, the potential benefits of research that might be conducted will not be the total expected cost of uncertainty surrounding expected cost-effectiveness but some part of it (see Appendix 6, What type of evidence is needed?). In Assesstment of uncertainty the potential benefits of different types of evidence were assessed. In the CLOP case study it was additional evidence about relative treatment effects that was most valuable and therefore experimental research may be required to provide a more precise estimate of RR_death. The potential value of research that resolved only uncertainty about this relative treatment effect over a range of times to report is also represented in Figure 15 (denoted by the legend ‘resolve uncertainty in RR_death’). Although the potential value of research is lower at every time point, unless research is likely to take more than 8 years the potential value is still likely to exceed the costs. In the EECP case study there was most benefit to be gained by resolving the uncertainty in the improvement in quality of life at 12 months, which in common with the CLOP case study is likely to require an experimental research design. Figure 16 represents the potential benefits of alternative trial designs with either 1 or 4 years of follow-up (1-, 2-, 3- and 4-year follow-up designs were evaluated; see Appendices 7–10). Although longer follow-up offers greater potential benefits they are relatively small compared with the loss of potential value if longer follow-up delays the time until research findings are available, that is, a 4-year design will require a minimum of 4 years to complete. Again, as long as research reports before 8 years the potential benefits are likely to exceed the costs.
What is the impact of other sources of uncertainty?
In Point 5: Will other sources of uncertainty resolve over time? the information that was publically available identified that the patent for CLOP was due to expire 7 years after the appraisal. Based on the OFT estimate that generic prices tend to be 25% of the original brand price, this other source of uncertainty can be integrated quantitatively when estimating the potential value of research over the lifetime of the technology. In this case a significant fall in price in year 7 will substantially reduce the uncertainty surrounding 12 months of treatment with CLOP. Therefore, after year 7 there is less to be gained from resolving uncertainty and so the potential and value of research findings for patients incident after year 7 are reduced. The effect of a price change on research that could potentially resolve uncertainty in cost, natural history and utilities as well as relative effect is also illustrated in Figure 15. The potential value of the research is lower whenever the research reports because it includes the value to future as well as current patient populations. Nevertheless, even if research did not report until 7 years the potential value is likely to exceed the costs. The expected price reduction reduces the potential value of research at each time point, for example, from 174,519 QALYs when research is immediately available (see Figure 17) to 165,701 with a price change at year 7. z
FIGURE 17.
Potential value of research by time to report and other sources of uncertainty: CLOP case study.
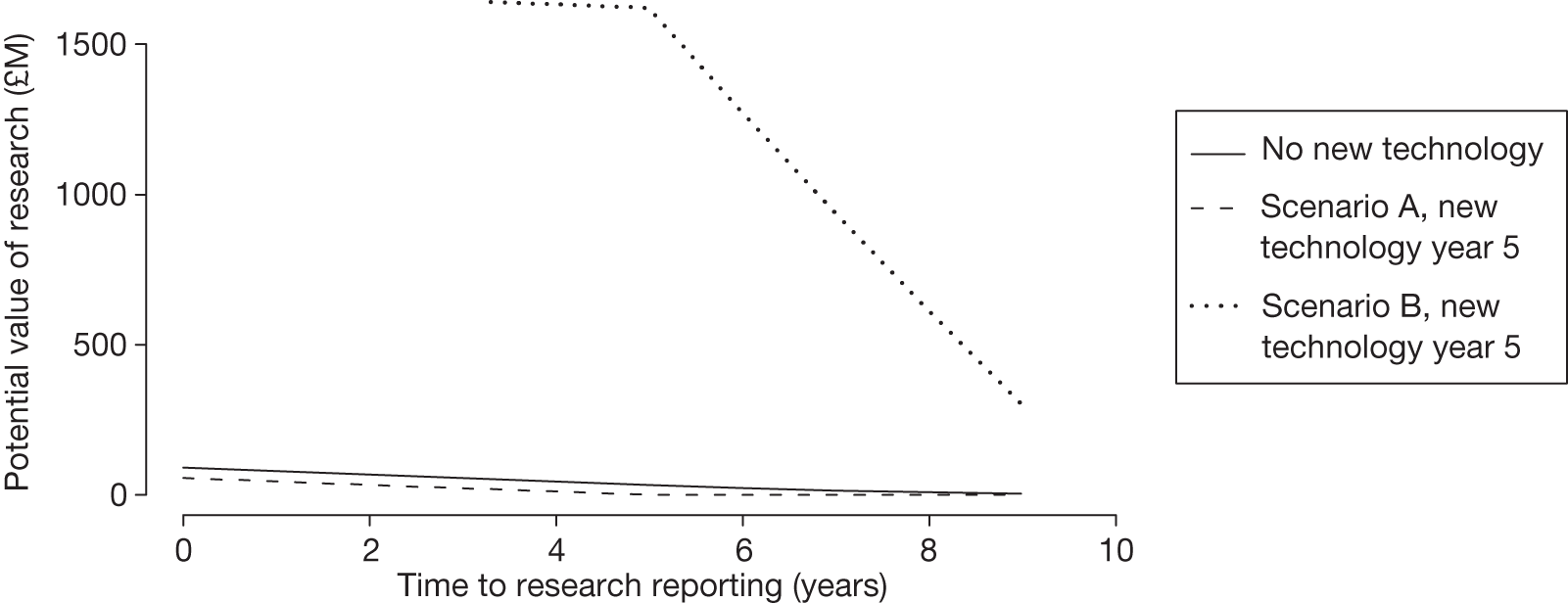
There was some evidence of possible entry of a new technology (comparator) in the indication described in the CLOP case study. However, there was limited information on its characteristics. Therefore, two alternative but somewhat extreme scenarios are illustrated in Figure 17. In scenario A the new technology enters at year 5 and makes CLOP entirely obsolete, that is, not cost-effective and not uncertain (equivalent to a shorter technology time horizon of 5 years). At this point there is no value in the evidence generated by research about CLOP. aa In these circumstances research is likely to be worthwhile only if it reports quickly. In scenario B the new technology has a similar NHE to 12 months of treatment with CLOPab and the uncertainty surrounding its expected cost-effectiveness is also similar. Now research about CLOP has more potential value in the future because it will also help resolve some of the uncertainty in the choice between CLOP and the new technology for patients who become incident after that time. Although there was no evidence of new technologies emerging in EECP, the same scenarios are explored as the development and launch of new devices are more difficult to identify in advance. The impact on the potential value of research is illustrated in Figure 18, demonstrating similar qualitative effects as for CLOP. In scenario A (EECP becomes obsolete) the potential benefits of further research about EECP are likely to exceed the costs only if the research reports quickly. Nevertheless, even in this extreme scenario the benefits of research with only 1 year of follow-up are likely to exceed the costs as long as it reports before 4 years.
FIGURE 18.
Potential value of research by time to report and other sources of uncertainty: EECP case study. These potential values are based on a 1-year follow-up design.
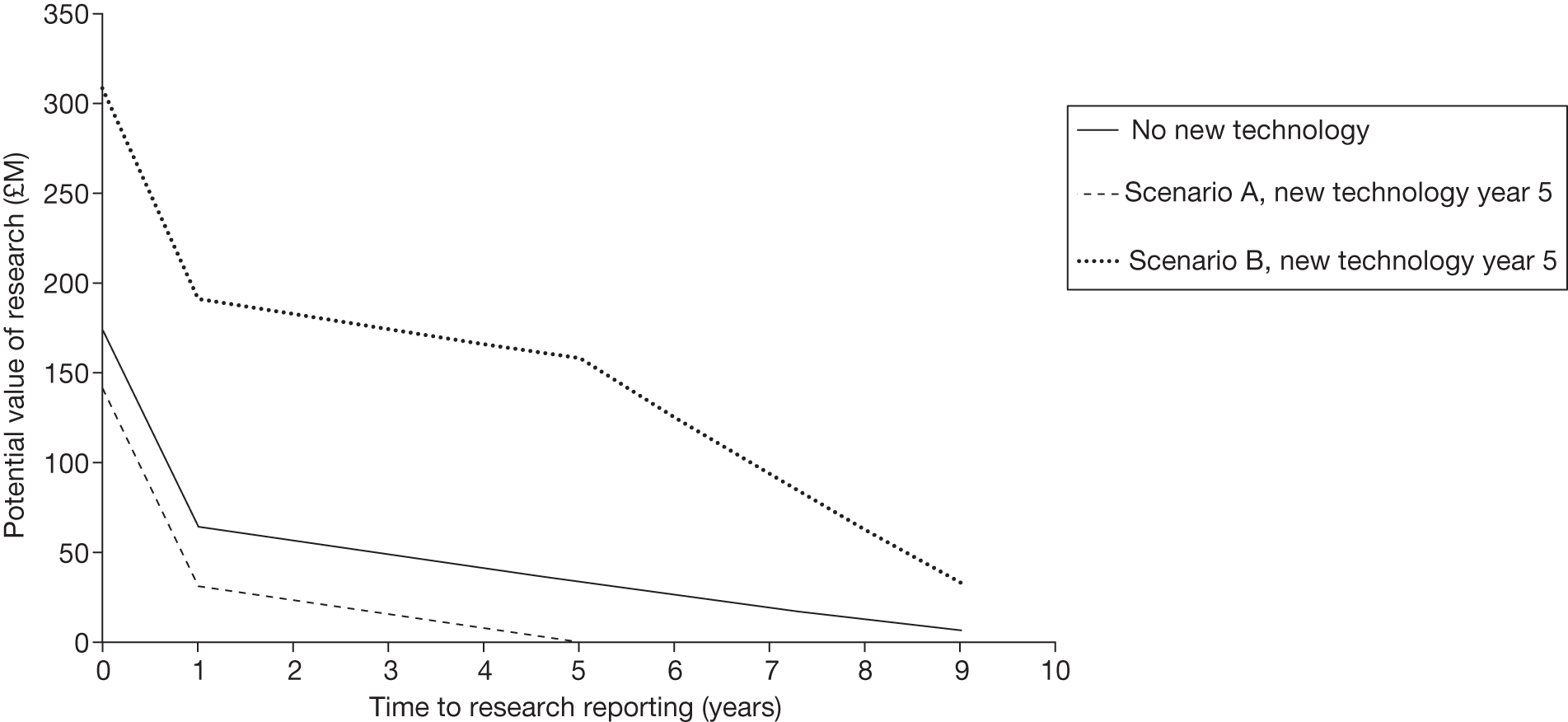
The potential value of research presented in these figures, even after accounting for the type of evidence, follow-up and time until research reports, should still be regarded as an upper bound to the value that is likely to be realised by actual research for two reasons: (1) even well-designed research with large sample sizes will not fully resolve the uncertainty in the value that a parameter might take, especially in specific target populations and in particular (future) contexts and (2) insofar as implementation of NICE guidance is not ‘perfect’ and all clinical practice might not immediately respond to the results of research, the full benefits will be realised only over time or with additional implementation efforts. For these reasons a judgement of whether or not benefits of research are likely to exceed the costs might be made conservatively, requiring evidence that even in pessimistic scenarios the research would still be worthwhile.
Point 7: Are the benefits of approval greater than the costs?
The seventh and final point on the checklist requires an assessment and comparison of the benefits and costs of early approval. The costs of approval are not financial ones but opportunity costs and will include the potential value of any research that may be forgone as a consequence, for example if the research needed cannot be conducted once the technology is approved for use. They will also include any costs that are irrecoverably committed by approval. As well as the capital costs of equipment and facilities (or training and learning), they will also include the irrecoverable opportunity costs of initially negative NHEs (if treatment decisions are not irreversible – see the discussion in Chapter 3, Technologies with significant irrecoverable costs and Chapter 5, Point 1: Is it expected to be cost-effective? and Point 2: Are there significant irrecoverable costs?). A judgement of whether or not the benefits of approval and early access for current patients are likely to exceed the opportunity costs for future patients is required, that is, at the following point in the algorithm:
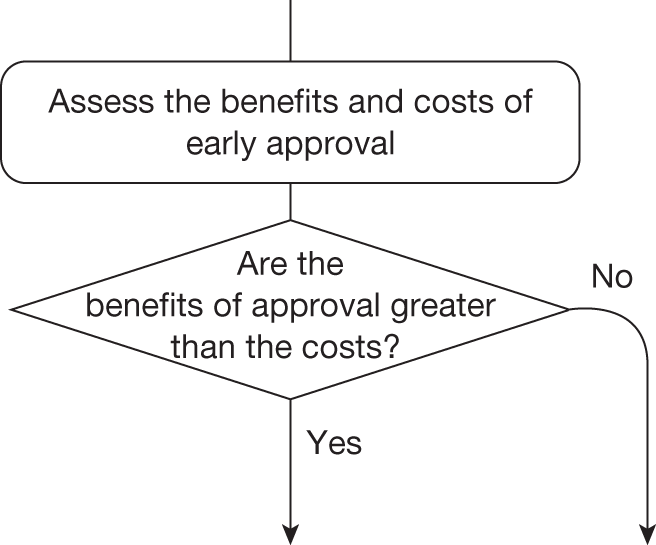
The decision at this point always leads directly to guidance, with all remaining possible pathways allocated to a particular type and category of guidance. These remaining (20) pathways through the algorithm are detailed below (extracted from Table 32 in Appendix 4):
| Assessment | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Guidance | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Pathway number { | 3 | Yes | No | Yes | No | Yes/no | Yes | Yes | Approve2 | |
| 4 | Yes | No | Yes | No | Yes/no | Yes | No | OIR1 | ||
| 9 | No | No | Yes | No | Yes/no | Yes | Yes | AWR2 | ||
| 10 | No | No | Yes | No | Yes/no | Yes | No | Reject2 | ||
| 13 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | AWR3 | } Significant irrecoverable costs | |
| 14 | Yes | Yes | Yes | Yes | Yes | Yes | No | OIR3 | ||
| 15 | Yes | Yes | Yes | Yes | Yes | No | Yes | Approve5 | ||
| 16 | Yes | Yes | Yes | Yes | Yes | No | No | Reject5 | ||
| 17 | Yes | Yes | Yes | Yes | No | Yes | Yes | AWR4 | ||
| 18 | Yes | Yes | Yes | Yes | No | Yes | No | OIR4 | ||
| 20 | Yes | Yes | Yes | No | Yes | Yes | Yes | Approve7 | ||
| 21 | Yes | Yes | Yes | No | Yes | Yes | No | OIR5 | ||
| 22 | Yes | Yes | Yes | No | Yes | No | Yes | Approve8 | ||
| 23 | Yes | Yes | Yes | No | Yes | No | No | Reject6 | ||
| 24 | Yes | Yes | Yes | No | No | Yes | Yes | Approve9 | ||
| 25 | Yes | Yes | Yes | No | No | Yes | No | OIR6 | ||
| 27 | Yes | Yes | No | – | Yes | – | Yes | Approve11 | ||
| 28 | Yes | Yes | No | – | Yes | – | No | Reject7 | ||
| 32 | No | Yes | Yes | No | Yes/No | Yes | Yes | AWR5 | ||
| 33 | No | Yes | Yes | No | Yes/No | Yes | No | Reject9 |
Technologies without significant irrecoverable costs
Only 4 of the 20 possible pathways illustrated above are associated with technologies without significant irrecoverable costs. In these four pathways either (1) research was not considered possible with approval for those expected to be cost-effective (i.e. ‘Approve2’ or ‘OIR1’) or (2) research was not possible without approval for those not expected to be cost-effective (i.e. ‘AWR2’ or ‘Reject2’). The CLOP case study provides an example of the former. It is research that would provide more precise estimates of the relative effect of CLOP and of shorter treatment durations that is potentially valuable (see Point 4: Is research possible without approval?). As a consequence, the type of experimental design that is likely to be needed is unlikely to be possible if 12 months of treatment with CLOP is already approved for widespread NHS use. Although treatment with CLOP does commit initially negative NHEs that are irrecoverable, these should not be regarded as significant as the treatment decision for a presenting patient is irreversible in relevant time frames (see Point 2: Are there significant irrecoverable costs?). Therefore, AWR may not be possible and so the benefits of early access to 12 months of treatment with CLOP (approval) must be compared with the potential value of OIR.
‘Only in research’ is more likely to offer greater expected NHEs than approve if the research can be conducted quickly and report sooner, as fewer patients forgo their access to CLOP and more can have their treatment choice informed by the research findings. This is illustrated in Figure 19, which reports the difference between approve and OIR in population NHEs over a range of times for when the research recommended in OIR might report. This takes account of both the expected changes in price at year 7 and research costs of £10M. It shows that OIR will be appropriate only if the research reports within 3 years of appraisal (T* = 3) because beyond this time the NHEs forgone by withholding access to CLOP will exceed the potential gains to future patients.
FIGURE 19.
Population NHEs of approve and OIR for time to research reporting: CLOP case study. T* is the time to research reporting at which the NHEs of OIR and approve are equal. That is, for a time to report of less than T* OIR offers higher NHEs than approve and for a time to report of more than T* approve offers higher NHEs than OIR.
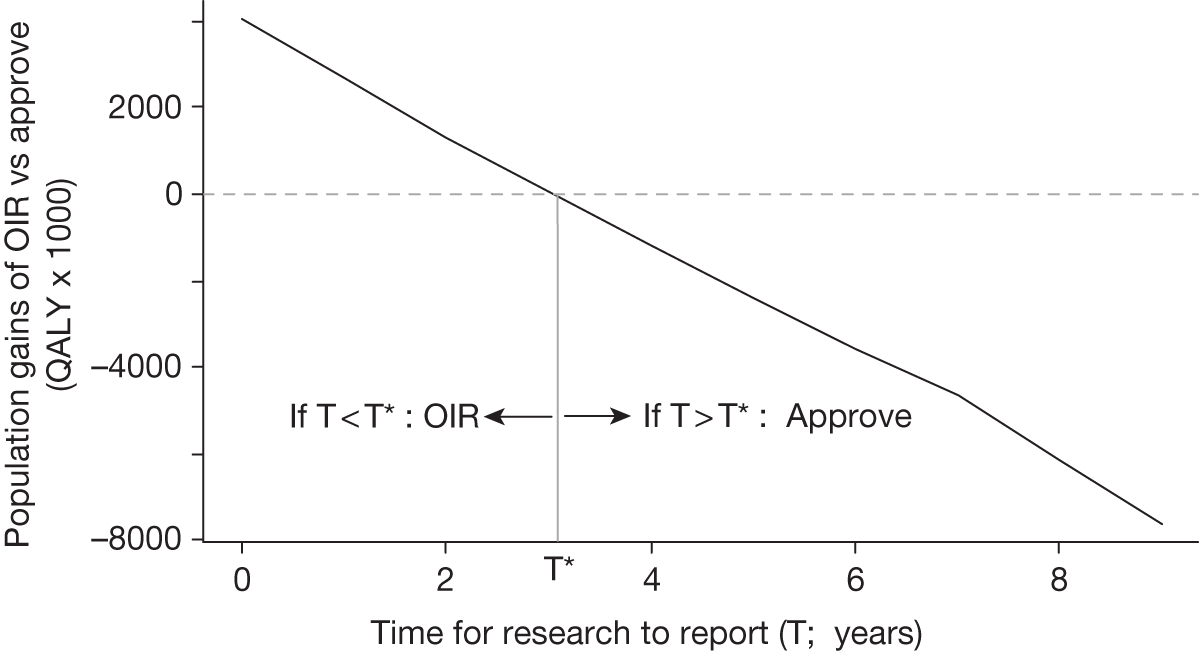
The trade-off between NHEs for current patients and NHEs for future patients that lies behind Figure 19 is illustrated in Figure 20 using undiscounted values for ease of exposition. It illustrates the (per-period) population NHEs of approval and OIR if the research recommended as part of OIR reports at year 3. At this point the initial losses of NHEs caused by restricting access to CLOP (area A) start to be offset by the potential gains from the research findings (area B). The price change at year 7 increases the NHEs of approval (i.e. CLOP is more cost-effective) but on balance reduces the NHEs of OIR. In other words, because CLOP is more cost-effective and offers greater NHEs the evidence generated by the research is less valuable because the choice of treatment and duration is less uncertain (see Point 5: Will other sources of uncertainty resolve over time? and Point 6: Are the benefits of research greater than the costs?). With research reporting at 3 years the initial losses of OIR (area A) are just offset by the later gains (area B) so T* = 3. If research reported earlier than 3 years (area A < area B) OIR would be appropriate but if it reported later than 3 years (area A > area B) approve would be more appropriate.
FIGURE 20.
Population NHEs of approve and OIR at T*: CLOP case study.
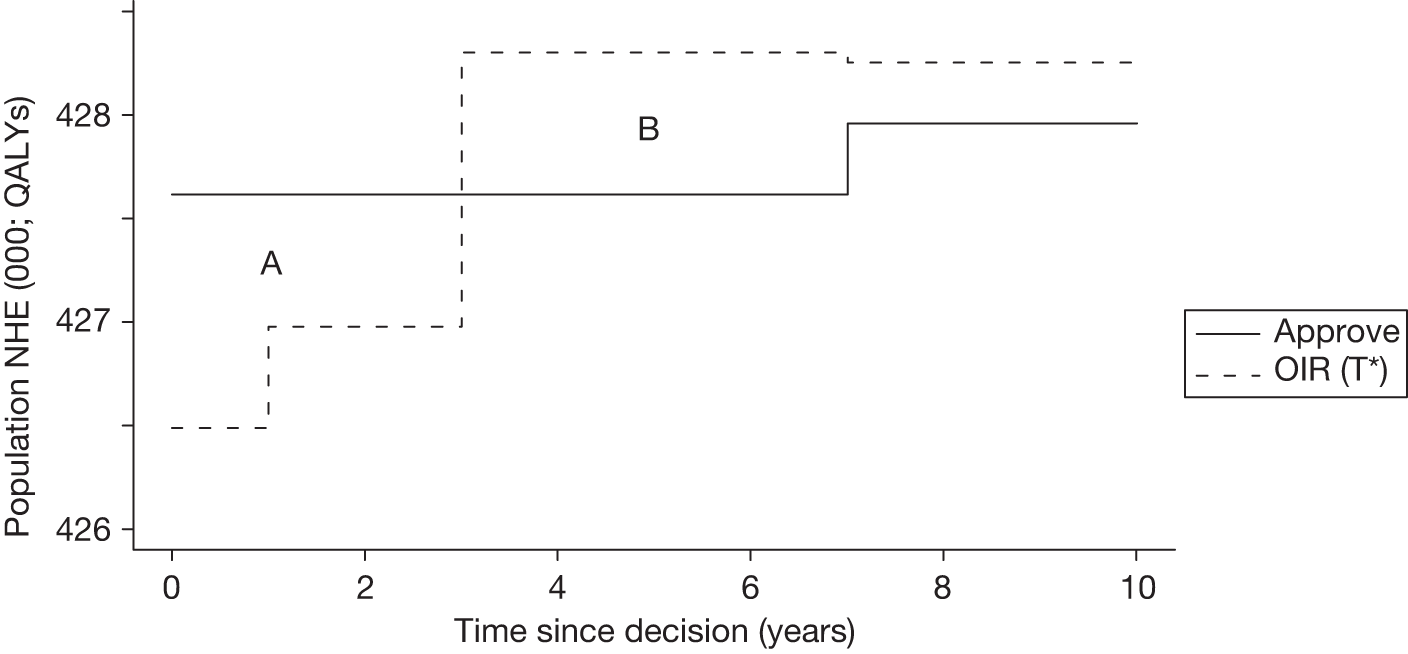
However, there is no guarantee that the research recommended as part of OIR guidance will be conducted by manufacturers or commissioned by research funders. Even if it is, it is not certain that it will be successfully completed (see discussion in Point 6: Are the benefits of research greater than the costs?). Therefore, the probability that research will report at a particular time also needs to be considered. The implications of considering whether the recommended research will be conducted and when it might report are illustrated in Figure 21, which presents a boundary for when OIR might be appropriate or when approval should be granted. For example, if research is certain to report but will take 4 years, or when it will take only 1 year but has only a 50% chance of reporting, OIR would not be appropriate and 12 months of treatment with CLOP should be approved, that is, points that fall to the north-east of the boundary. Points to the south-west of the boundary indicate that OIR might be appropriate (see Appendix 6, When might it be commissioned and report).
FIGURE 21.
An OIR or approve boundary: CLOP case study.
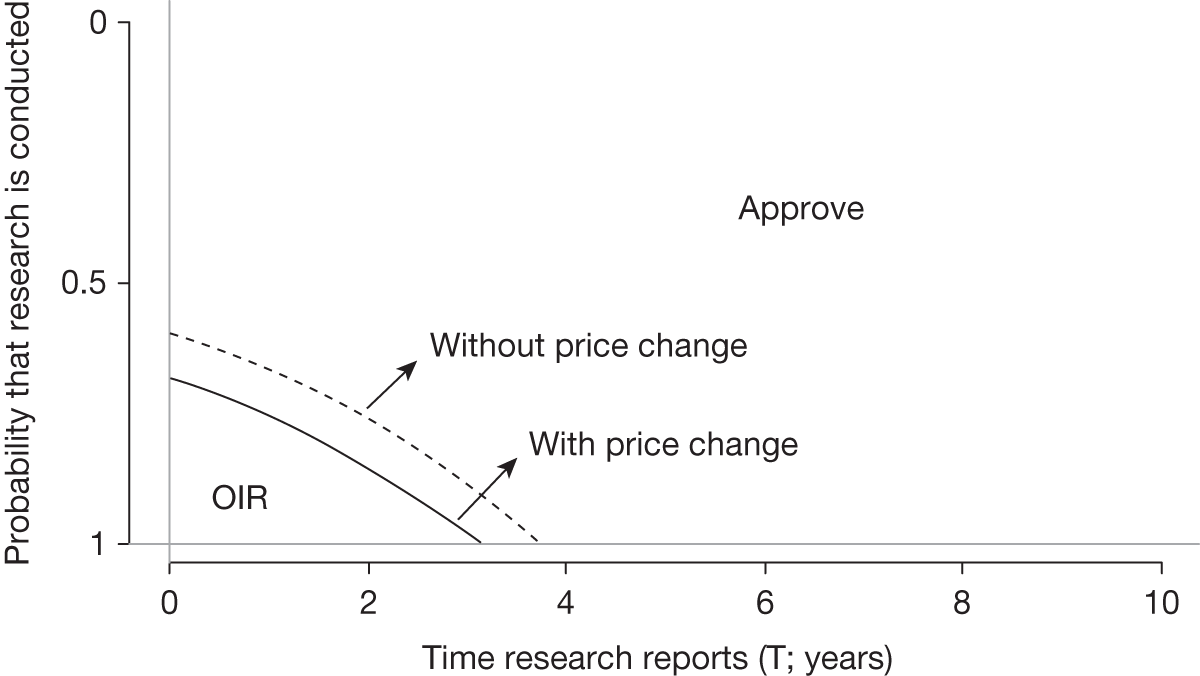
However, the estimates of the potential value of additional evidence on which these boundaries are based are still likely to overestimate the value that will be realised by research (see discussion in Point 6: Are the benefits of research greater than the costs?); they represent a necessary condition for OIR. Therefore, OIR guidance should require a conservative judgement that the point is almost certain to be below the boundary rather than on balance close to it. For the same reason, points anywhere above the boundary represent a sufficient condition for approval. The boundary when the change in price is included is to the south-west, reflecting the lower potential value of research, and OIR guidance, once CLOP becomes more cost-effective. In this case it seems unlikely that the type of research required could report quickly enough and with sufficient confidence that OIR would be appropriate. Therefore, these assessments would support a judgement that the benefits of approval are likely to exceed the opportunity costs, and ‘Approve2’ (pathway 3 for CLOP in Table 32, Appendix 4) would seem more appropriate.
The assessments that have been undertaken for CLOP can be brought together to consider (1) what would be the value of being able to conduct research while CLOP is approved (value of AWR) and (2) what would be the value of making evidence that is needed by the NHS available at launch. These questions can be informed by the results already presented elsewhere but these results are also reported together in Table 25.
| NHE for time to research reporting | Approve | OIR | AWR* | Reject | Value of AWR | Uncertainty resolved at launch | Value of evidence at launch |
|---|---|---|---|---|---|---|---|
| NHEs in QALYs | |||||||
| T < T* (T = 2) | 3,680,187 | 3,681,480 | 3,682,995 | 3,671,660 | 1515 | 3,684,181 | 2701 |
| T > T* (T = 7) | 3,680,187 | 3,675,487 | 3,680,362 | 3,671,660 | 175 | 3,684,181 | 3994 |
| NHEs in £M | |||||||
| T < T* (T = 2) | 73,604 | 73,630 | 73,660 | 73,433 | 30 | 73,684 | 54 |
| T > T* (T = 7) | 73,604 | 73,510 | 73,607 | 73,433 | 4 | 73,684 | 80 |
The difference in NHEs between AWR (if it was possible) and the next best feasible policy, which is OIR when T < T* and approve when T > T*, is £30M and £4M, respectively. This difference represents the value to the NHS of being able to conduct research while CLOP is approved for use, for example to inform whether investment in better data collection, registries or information systems is worthwhile that might make this possible. ac The difference in population NHEs if all uncertainty had been resolved before appraisal (at launch) and the next best available policy, OIR for T < T* and approve for T > T*, is £54M and £80M, respectively. This difference represents the value to the NHS of having access to the evidence needed at launch. This can also directly inform policies that might make better and more relevant evidence available.
It is also possible to consider the commercial as well as NHS value in each of the cells of this table. The value of early evidence at launch can then be considered from the perspective of the manufacturer (the expected revenue streams), taking account of prices (see discussion of price in Chapter 3, Changes in effective prices and Incentives for evaluative research) and expected volumes over the remaining patent life and technology time horizon. Together with estimates of the costs of conducting research by manufacturers or through public funding, this assessment might inform when manufacturers might be expected to conduct the research needed (e.g. high commercial value that exceeds the cost to manufacturers) or when the NHS might be expected to undertake it (e.g. low commercial value but high potential value to the NHS that exceeds the costs to the NHS). When the research is worthwhile from both a commercial and a NHS perspective the question of who should conduct, or pay for, the research might be informed by which sector has a comparative advantage, that is, which can gain the most net social value. Of course, the value to the NHS and to manufacturers will depend, to a large extent, on what type of flexible pricing arrangements and value-based pricing schemes might be in place. The question will also turn on how any agreements can be made and incentive consistent contracts written and enforced.
Technologies with significant irrecoverable costs
Most of the possible pathways illustrated above are associated with technologies with significant irrecoverable costs (16 out of the remaining 20). This is because, even when research is possible with approval (or even when not needed), the impact of committing irrecoverable costs through AWR (or approval) must be considered and so OIR (or reject) remains a possibility (see Appendix 6, What is the impact of irrecoverable costs?). The EECP case study provides an example of this, in which research that would provide more precise estimates of the effect of treatment on quality of life accounts for all of the potential value (see Point 4: Is research possible with approval?). EECP does commit both capital costs associated with long-lived equipment and initially negative per-patient NHEs. Unlike in the CLOP case study these irrecoverable opportunity costs at a patient level are significant because treatment choice for a presenting patient is not irreversible over relevant time frames (see Point 2: Are there significant irrecoverable costs?). As a consequence, even if research is possible with approval it is not clear that AWR would be appropriate, because OIR avoids the commitment of irrecoverable costs until research finding are available and a more informed decision can be made. ad
Research is possible with approval
Even when research is possible with approval OIR offers greater expected NHEs than AWR as long as research reports before 9 years (Figure 22). This is because the consequences (losses of population NHE) of committing both aspects of irrecoverable costs through AWR are greater than the NHEs forgone by restricting access to EECP through OIR. The costs of research have not been included because they are incurred with both AWR and OIR guidance. ae
FIGURE 22.
Population NHEs of AWR and OIR for time to research reporting: EECP case study.
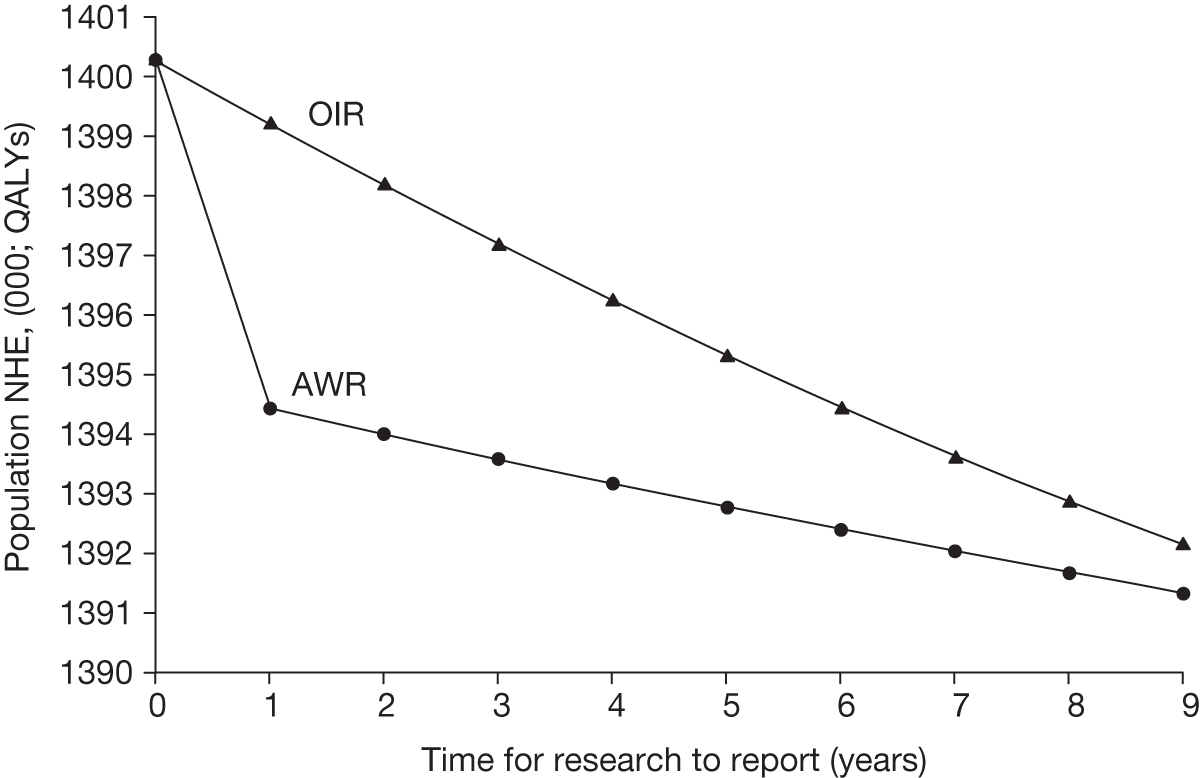
As previously for the CLOP case study, there is no guarantee that the research recommended as part of OIR or AWR guidance will be conducted and research report. A boundary for when OIR rather than AWR might be appropriate is illustrated in Figure 23 for four research designs with differing times of follow-up. A 2-year follow-up will generate evidence with the lowest potential value (so the boundary is to the south-west) but it is likely to report sooner. Therefore, OIR might be appropriate even if the probability that the research will be conducted and report is relatively low. In this case it seems likely that the type of research required could report quickly enough and with sufficient confidence that OIR would be appropriate even though the research could be conducted while EECP is approved. Therefore, these assessments would support a judgement that the benefits of approval (through AWR) are unlikely to exceed the opportunity costs (the NHEs of OIR) and so ‘OIR4’ (pathway 18 in Table 32, Appendix 4) rather than ‘AWR4’ (pathway 17) would seem more appropriate.
FIGURE 23.
An OIR or AWR boundary: EECP case study.
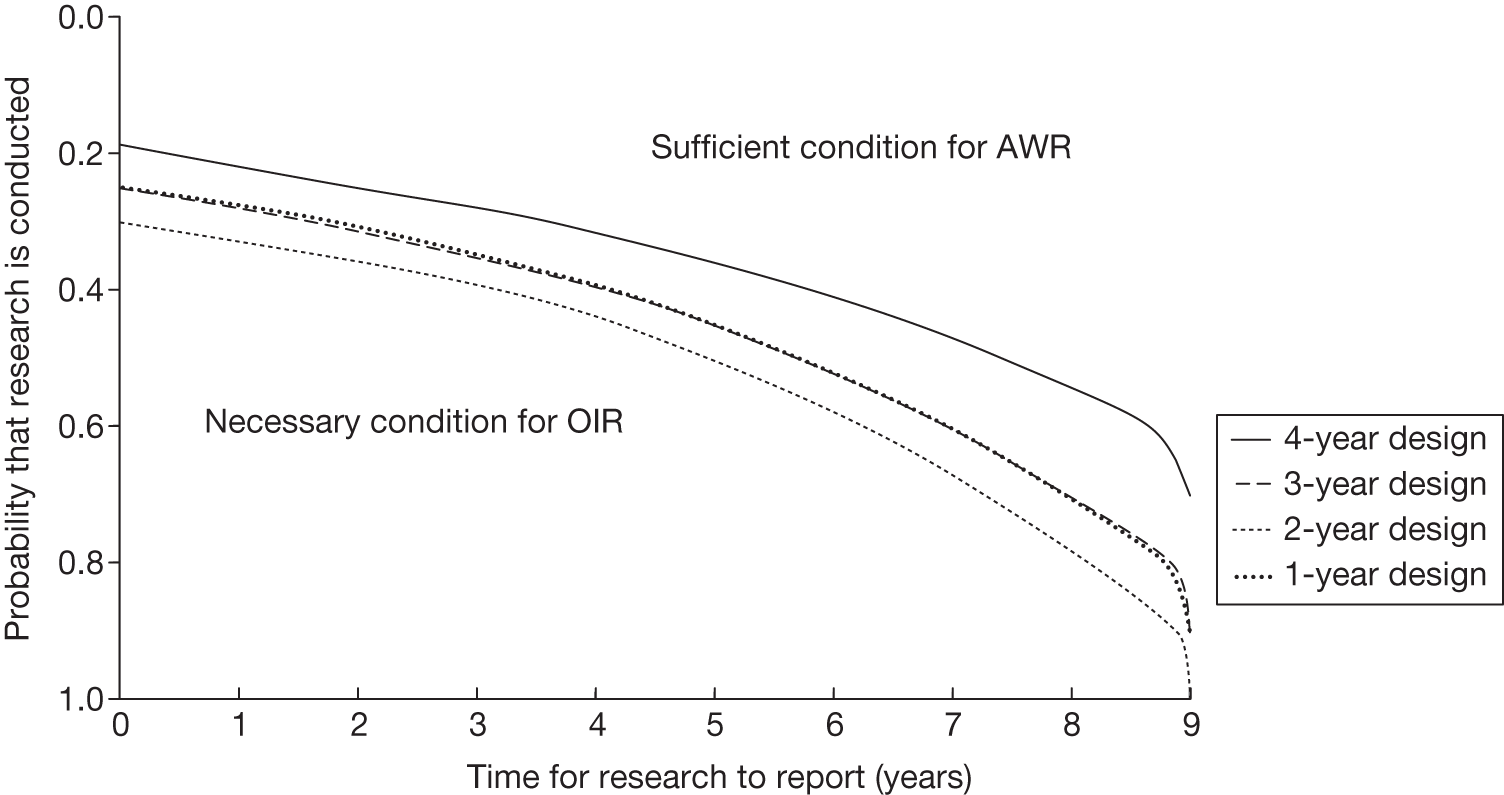
Research is not possible with approval
For the general reasons discussed in Chapter 3 and those specific to EECP discussed in Point 4: Is research possible with approval?, the type of experimental research required to robustly estimate the effect of EECP on quality of life is unlikely to be possible once it is approved and in widespread use. Now approval (now through approve rather than AWR) not only commits the type of irrecoverable costs discussed above but also means that the potential value of evidence to future patients must be forgone. This is reflected in Figure 24 in which the difference between OIR and approve is always greater than the difference between OIR and AWR in Figure 22. It suggests that as long as the cost of the research exceeds the difference between OIR and approve, when it is expected to report, OIR rather than approve would be appropriate. This is also reflected in the boundaries for OIR and approve reported in Figure 25. These boundaries are always to the north-east of the OIR/AWR boundaries reported in Figure 23, again reflecting the fact that approval not only commits irrecoverable costs but also forgoes the potential value of evidence that might have been generated through an OIR recommendation. These assessments would support a judgement that the benefits of approval are unlikely to exceed the opportunity costs (the NHEs of OIR) and so ‘OIR6’ (pathway 25 in Table 32, Appendix 4) rather than ‘Approve9’ (pathway 24) would be more appropriate.
FIGURE 24.
Population NHEs of approve and OIR for time to research reporting: EECP case study.
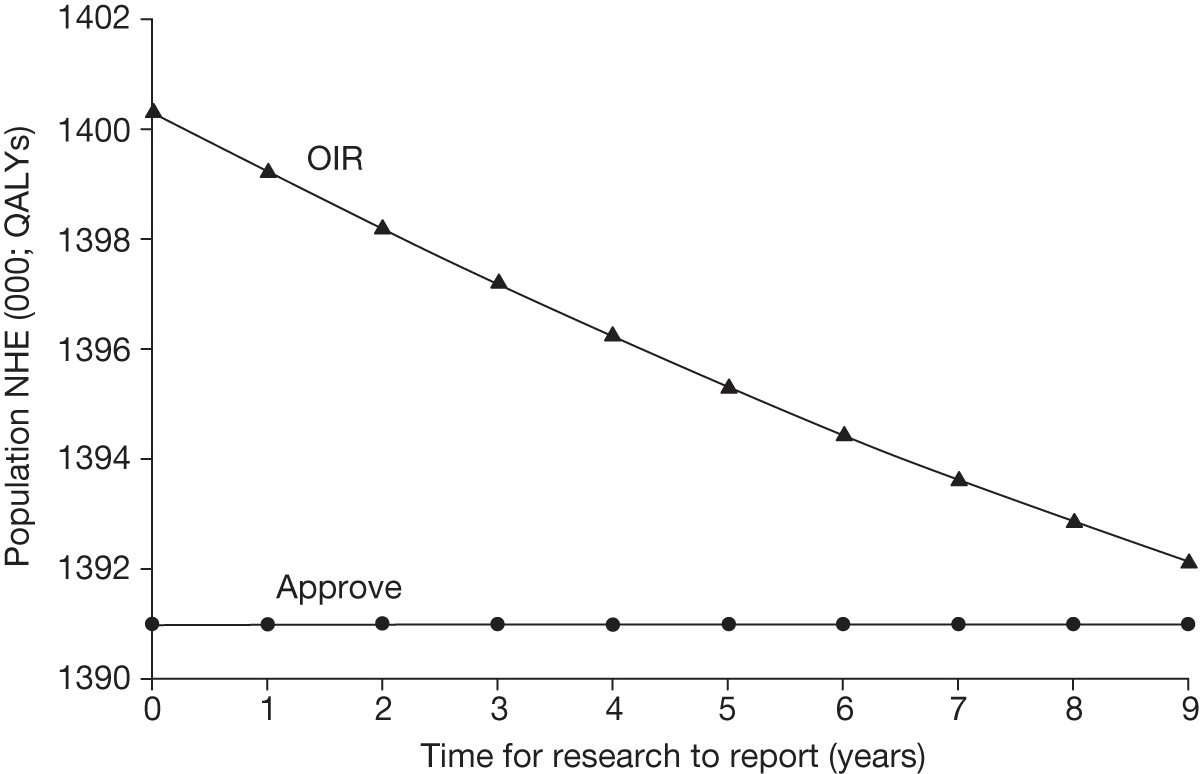
FIGURE 25.
An OIR or approve boundary: EECP case study.
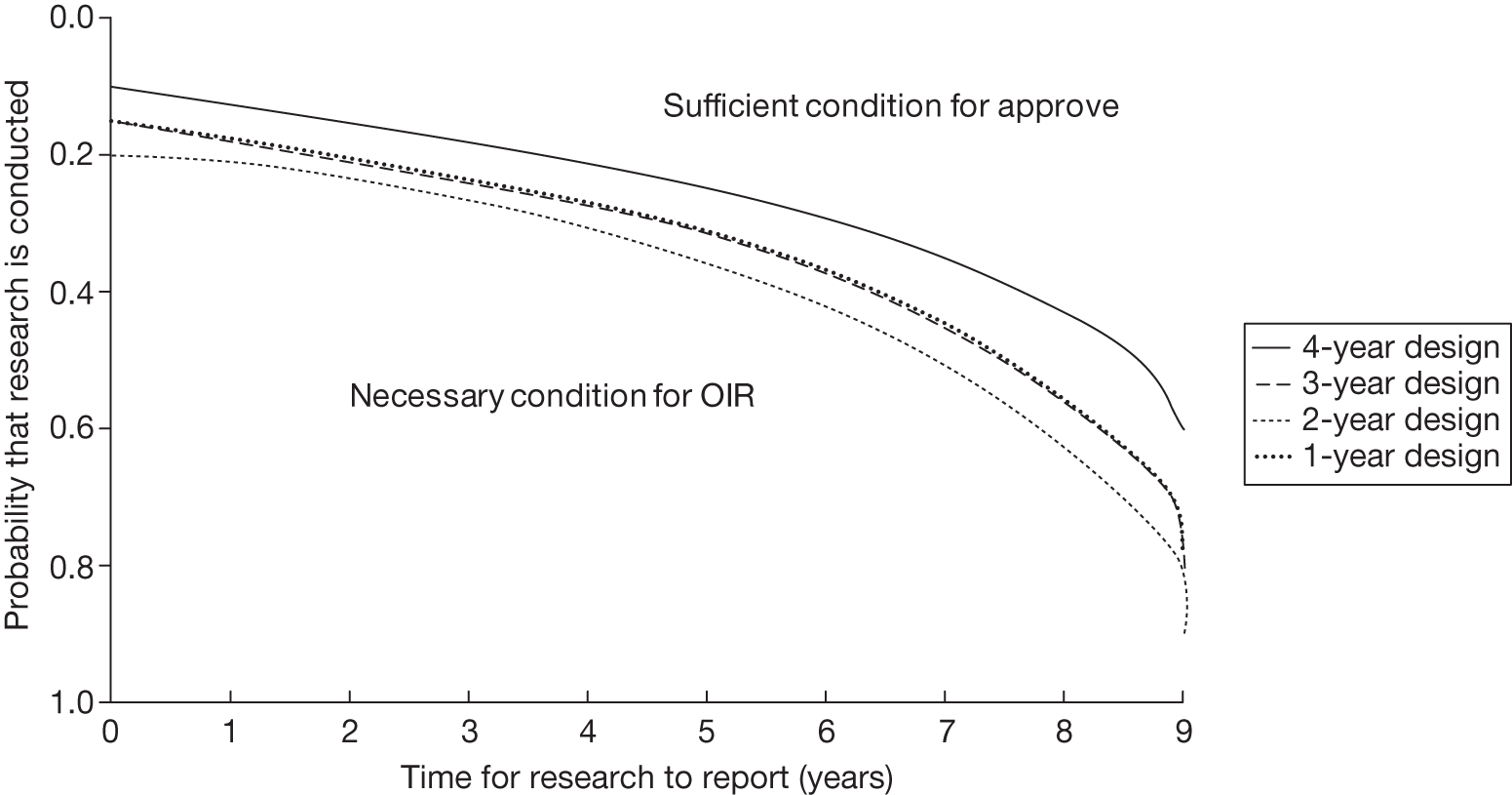
As with the CLOP case study, the assessments that have been undertaken for EECP are bought together in Table 26 and can help inform the same policy questions: (1) what would be the value of being able to conduct research while EECP is approved? and (2) what would be the value of making the evidence that is needed by the NHS available at launch? In this case, because of the irrecoverable costs associated with EECP, there is no value to the NHS of being able to conduct research while EECP is approved for use. In fact, these figures are negative, indicating that even if AWR was possible it would not be appropriate. However, as in the CLOP case study, there is value to the NHS of having the evidence needed before appraisal. This value, expressed in the equivalent NHS resources, depends on how long it would otherwise have taken for an OIR recommendation to deliver the same evidence, for example £62M if 3 years and £134M if 7 years. As with the CLOP case study, these assessments can also inform policies that might make better and more relevant evidence available and answer the questions of how to provide the evidence needed at the right time and who might contribute most to providing this evidence.
| NHE for time to research reporting | Approve | OIR | AWR* | Reject | Value of AWR | Uncertainty resolved at launch | Value of evidence at launch |
|---|---|---|---|---|---|---|---|
| NHEs in QALYs | |||||||
| T = 3 | 1,391,001 | 1,397,192 | 1,393,578 | 1,389,596 | –3614 | 1,400,288 | 3096 |
| T = 7 | 1,391,001 | 1,393,608 | 1,392,030 | 1,389,596 | –1578 | 1,400,288 | 6680 |
| NHEs in £M | |||||||
| T = 3 | 27,820 | 27,944 | 27,872 | 27,792 | –72 | 28,006 | 62 |
| T = 7 | 27,820 | 27,872 | 27,841 | 27,792 | –32 | 28,006 | 134 |
Chapter 6 Implications for policy, process and methods
This final chapter of the main report draws together some of the implications that a more explicit assessment of OIR and AWR might have for policy (e.g. NICE guidance and drug pricing), the process of appraisal (e.g. greater involvement of research commissioners) and methods of appraisal (e.g. should additional information, evidence and analysis be required). Within each of the broad topics discussed below, we try to distinguish between policy issues directly relevant to the NICE remit (at a ‘higher level’ than the process and methods of technology appraisal); those that might be most relevant to other bodies (e.g. Department of Health, Department for Business, Innovation & Skills) and stakeholders; and specific issues for the process and the methods of technology appraisal. It constitutes a brief discussion of possibilities that NICE and others might choose to take forward. How a consideration of uncertainty and the need for evidence might influence value-based pricing and the impact of patient access schemes on OIR and AWR guidance is also considered. This discussion draws on the feedback provided at both workshops, which included a wide range of relevant stakeholders, including members of NICE and its Advisory Committees (including lay members and other NICE programmes), patient representatives, manufacturers, and research and NHS commissioners, as well as relevant academics. The feedback from both workshops has been summarised and is available online (see www.york.ac.uk/che/research/teehta/workshops/only-in-research-workshop//).
Key principles and assessments needed
The key principles and assessments needed fall into four broad areas:
-
expected cost-effectiveness and population NHEs (including benefits, harms and NHS costs)
-
the need for evidence and whether the type of research required can be conducted once a technology is approved for widespread use
-
whether or not there are sources of uncertainty that cannot be resolved by research but only over time
-
whether or not there are significant (opportunity) costs that will be committed and cannot be recovered once the technology is approved.
Guidance will depend on the combined effect of all of these assessments because they influence whether the benefits of research are likely to exceed the costs and whether any benefits of early approval are greater than the costs of withholding approval until additional research is conducted or other sources of uncertainty are resolved. There was general consensus that the key principles represented by a sequence of assessments and judgements (see Chapter 3 and the algorithm in Appendix 2) were the relevant ones, complete and in an appropriate order. In response to feedback from the first workshop this sequence of assessment and judgement was summarised as a simple 7-point checklist (see Chapter 5, A checklist of assessment) that could be considered by the TAR groups and Appraisal Committees as well as manufacturers during appraisal.
Other NICE programmes
The principles outlined in Chapter 3 and the checklist do not presuppose how the assessments required might be informed and judgements made. Distinguishing principles from methods of analysis in this way meant that there was a general consensus that the principles and the checklist might be useful in other NICE programmes, whilst recognising that how the assessment might be made is likely to differ. It was recognised that some amendments might be required when cost-effectiveness is not a consideration (e.g. in interventional procedures) or when only technologies that are expected to be cost saving to the NHS are considered (e.g. the Medical Technologies Evaluation Programme). Similarly, the complexity of multiple outcomes in public health and the greater scope and complexity of decision problems in clinical guidelines offer greater challenges for quantitative assessment but do not change the key principles and considerations. The relative paucity of evidence and the speed of innovation in devices are likely to influence how the assessment might be made. On the other hand, it was considered by some workshop participants that the more iterative process of appraisal in some other NICE programmes (e.g. clinical guidelines) might make it easier to integrate the assessments required in the checklist. Further research, using case studies from these other NICE programmes, would be required to identify how best the assessments might be informed given the programme-specific challenges, the existing methods of appraisal and the resource and time constraints of their current processes.
The checklist was subsequently applied to a series of four case studies (three pharmaceuticals from the Technology Appraisal Programme and one device from the HTA programme; see Chapter 5, Introduction to the case studies). These applications allow an examination of how each of the assessments might be made based on the type of evidence and analysis currently provided in NICE technology appraisal and how they might be better informed with a range of additional information and/or analyses. The possible implications for process and methods of appraisal are discussed below.
The scope of ‘only in research’ and ‘approval with research’ recommendations
Categories and type of guidance
Each sequence of assessment and decision leads to different categories and ‘types’ of guidance for technologies with differing characteristics, indications and target populations, that is, each sequence of ‘yes’ or ‘no’ judgements in the checklist defines a single pathway leading to a particular type and category of guidance (see Table 32, Appendix 4). The different types of apparently similar guidance illustrate how the same category of guidance (e.g. approve, AWR, OIR or reject) might be arrived at in different ways, helping to identify the particular combinations of considerations that might underpin guidance, contributing to the transparency of the appraisal process. There was a general view that the application of the checklist would improve transparency in communicating the considerations that underpin guidance but that this alone would not be sufficient, especially in situations in which OIR guidance was made for a technology that was, on balance, expected to be cost-effective. Evidence of how, not just what, assessments and judgements were made would be required (see What additional information and analysis might be required?).
These principles suggest that the categories of guidance available to NICE have wider application than is reflected in previous guidance (see the review of NICE guidance in Chapter 4). For example, there are five different types of OIR guidance that may be appropriate when a technology is expected to be cost-effective (see Table 1). Indeed, OIR may be appropriate even when research is possible with approval if there are significant irrecoverable costs. AWR can be considered only when research is possible with approval but reject remains a possibility even for a cost-effective technology if there are irrecoverable costs. Therefore, the full range of categories of guidance (OIR and reject as well as AWR and approve) ought to be considered for technologies that, on the balance of existing evidence and current prices, are expected to be cost-effective. It is only approval that can be ruled out if a technology is not expected to be cost-effective, that is, cost-effectiveness is necessary but not sufficient for approval but lack of cost-effectiveness is neither necessary nor sufficient for rejection.
Scope of ‘only in research’ recommendations
Importantly, which category of guidance will be appropriate depends only partly on an assessment of expected cost-effectiveness and hence this assessment should be regarded only as an initial step in formulating guidance. Guidance will depend on a number of other key assessments, which include (1) the need for evidence, (2) whether or not the type of research required is possible with approval, (3) the expected longer-term benefits and costs of the type of research likely to be conducted, (4) the impact of other sources of uncertainty that will resolve over time and (5) the significance of any irrecoverable costs.
In general, it was accepted that as well as AWR for technologies expected to be cost-effective and OIR for those not, there are other important circumstances when OIR should be considered. In particular, for technologies expected to be cost-effective, OIR rather than approve may be appropriate when research is not possible with approval and OIR or even reject rather than AWR or approve may be appropriate even if research is possible with approval when there are significant irrecoverable costs. The ethical implications of such decisions were set out in Chapter 3 (see Social value judgements and ethical principles) and are discussed below.
Implications of patient access schemes and value-based pricing
‘Only in research’, ‘approval with research’ and effective price
Any change in the effective price of the technology, either through patient access schemes (which offer some form of discount that reduces NHS costs) or through direct price changes (possibly negotiated though a future value-based pricing scheme), will affect the key assessments leading to different categories of guidance. For example, provisional OIR guidance for a technology that is expected to be cost-effective might be revised to approve with a sufficient price reduction because the benefits of early approval will be greater and uncertainty about its cost-effectiveness and the value of additional evidence will tend to be lower. Similarly, AWR might be revised to approve if the consequences of uncertainty and what might be gained from additional evidence is sufficiently reduced. Equally, provisional guidance to reject a technology that is not expected to be cost-effective might be revised to OIR if the reduction in price (although not sufficient to make it cost-effective) made a reject decision more uncertain and hence research worthwhile.
Therefore, consideration of the effect of price changes on OIR and AWR is needed when assessing the potential impact of patient access schemes and more direct price negotiation through value-based pricing. However, there are limits to the effects of price reductions as even at a zero price the technology might not be cost-effective and/or further research may still be required if there is a lack of confidence that the technology is effective (harms may not be compensated for by benefits) and/or it imposes other non-acquisition costs on the NHS.
Implications for value-based pricing
The price at which the technology would just be expected to be cost-effective is commonly regarded as the value-based price for the technology. It is the maximum price that the NHS can afford to pay without imposing negative health effects. This describes the threshold price below which approve rather than reject would be appropriate when (1) OIR or AWR guidance is not available to the decision-maker, (2) there is no uncertainty over cost-effectiveness or (3) the research, if needed, can be conducted with approval and there are no irrecoverable costs. In all other circumstances there are a number of other value-based prices, each representing the threshold price below which guidance would change. Importantly, once uncertainty and the need for evidence as well as the impact of irrecoverable costs are recognised, the threshold price that would lead to approval will always be lower than or equal to a single value-based price based on expected cost-effectiveness alone, that is, disregarding uncertainty over costs and effects.
For example, when research could be conducted without approval but not with it, there will be a price threshold above which reject rather than OIR would be appropriate and a lower price threshold below which approve rather than OIR would be appropriate. This approve/OIR threshold price will always be lower than a value-based price based on expected cost-effectiveness. If a technology also imposes significant irrecoverable costs then this threshold price will be lower still. There may also be up to three threshold prices as all four categories of guidance might become relevant at different prices.
Value-based pricing and irrecoverable costs
Health technologies with patent protection are more likely to be priced close to the point at which the ICER is close to or equal to the threshold, that is, expected incremental NHEs are close to zero. A value-based pricing scheme would formalise these existing incentives so technologies will tend to impose greater irrecoverable opportunity costs (initially negative NHEs). A technology that is only just expected to be cost-effective will not break-even until close to the end of the patient time horizon and not for much longer for the population of patients likely to benefit from its use. Therefore, those technologies already priced close to the threshold, and all new technologies considered in a value-based pricing scheme, will tend to increase the scale of irrecoverable costs committed by approval. If these irrecoverable costs are significant (because treatment decisions are not irreversible) then the price threshold for unrestricted access (i.e. approve or AWR) will be lower than the price at which the technology is just expected to be cost-effective, even if research can be conducted with approval (see Chapter 3, Issues specific to ‘only in research’ recommendations). Further research is required to establish how these considerations could be integrated within a practical value-based pricing scheme. In addition, irrecoverable opportunity costs are commonly associated with many health technologies that offer future benefits following treatment. The significance of these types of irrecoverable costs is not widely recognised and further research to demonstrate their potential impact more generally is needed.
Retaining ‘only in research’ as part of value-based pricing
Even in circumstances in which price negotiation becomes possible alongside NICE appraisal, it will be important to retain OIR and AWR as available categories of guidance for two reasons. First, there is no guarantee that manufacturers will always agree to the lower price below which approval rather than OIR or AWR would be appropriate. For example, manufacturers will be unwilling to agree prices that are below marginal production costs. They will also be unwilling to lower UK prices if they can be referenced by other countries and have an impact on global revenues. Second, and possibly more importantly, there may be many circumstances when there is no effective price reduction that would make approval appropriate. For example, reject or OIR guidance may still be appropriate even if the effective price of a technology is zero if there is substantial uncertainty about its effectiveness (and/or potential for harms) or if the technology imposes other non-acquisition costs on the NHS.
Incentives for evaluative research
Flexible pricing agreements
Consideration of OIR and AWR recommendations provides a link between uncertainty, evidence and price which might appropriately align incentives for manufacturers conducting the type of evaluative research that would be most valuable for the NHS. The current PPRS provides for flexible pricing agreements in which price is revised once the research reports and the results are known, increasing prices if the evidence suggests that benefits were originally underestimated or reducing prices if benefits were overestimated. This means that manufacturers retain an incentive to conduct further evaluative research if they believe that there are additional benefits that could not be evidenced at launch. Publically funded evaluative research, however, will still be required when these incentives are insufficient and especially in those cases in which the original evidence is likely to have overestimated the benefits or underestimated the potential for harm. However, it should be noted that linking effective prices to the results of publically funded research means that the NHS will benefit (realise the value of evidence) only if the results lead to a lower price or more restrictive guidance because the technology is found not to be cost-effective (thus avoiding the losses associated with negative NHEs). Manufacturers will, however, be able to appropriate the value of evidence through higher prices when it suggests that NHEs were originally underestimated. Even under current arrangements this value can be appropriated when the technology is reappraised by NICE. For example, patient access schemes, which offer an effective discount to the NHS, can be withdrawn or approval might be made less restrictive when a technology is reappraised following further research.
Incentives and early advice
It is important that policy provides (or at least does not undermine) appropriate incentives for manufacturers to conduct the type of research needed to support NICE guidance at launch. The use of OIR and AWR guidance, and its link to price, provides a clear signal and an incentive for manufacturers to ensure that the type of evidence that cannot be acquired following approval is available and sufficient at launch (e.g. relative effectiveness and subtle but important differences in side effect profiles). Therefore, a predictable OIR and AWR policy signals what type of evidence is likely to be most important at an early stage. It offers manufacturers a choice, to (1) accept OIR guidance at a higher price but restricted volume, (2) reduce the effective price to achieve approval, or AWR when that is possible, or (3) conduct the evaluative research at an earlier stage so that additional evidence is not required at launch.
Other things being equal, those new technologies that are supported by more, better-quality and relevant evidence will be more likely to be approved and at higher prices than those that are not. Therefore, greater consideration of OIR and AWR guidance will tend to reward those manufacturers who have invested in good-quality and relevant evidence with earlier approval of their technology. It was also suggested that the checklist of assessments could be used within the NICE Scientific Advice Programme, providing advice to manufacturers at an early stage before technology appraisal.
Who should conduct ‘approval with research’ and ‘only in research’ research?
Consideration of how the NHS and manufacturers are likely to share the value of evidence might inform whether manufacturers should be expected to conduct the research specified in AWR or OIR guidance. Alternatively, manufacturers might be expected to make some contribution to the costs of publically funded research that may ultimately benefit their product. As well as an assessment of how the value of additional evidence is likely to be shared between the NHS and the manufacturers, it was widely recognised that two other issues need to be considered. First, the resource constraints on publically funded research may mean that other research priorities (often without commercial interest) may be more valuable to the NHS (see How should the assessments be undertaken?). Second, the success of AWR recommendations when manufacturers are asked to conduct the research will depend on whether or not NICE and/or the Department of Health are able to establish incentive-consistent contractual arrangements as part of an AWR recommendation, that is, arrangements that can be monitored and enforced with credible penalties to ensure that agreed research is conducted and in the way intended. It was widely recognised that, at present, NICE does not have a credible mechanism to ensure that the type of research recommended in AWR guidance would actually be undertaken by manufacturers. Removing approval of a technology simply because recommended research has not been conducted was not considered an ethical or a credible threat. Further research could consider what type of feasible contractual arrangements would offer credible threats that would make AWR more likely to be successful. This work could include more detailed examination of linking an AWR agreement to price penalties within a value-based pricing scheme.
Although OIR provides a greater incentive to undertake research, it was recognised that there may be circumstances when manufacturers would nonetheless choose not to undertake it, that is, accepting an effective reject. If the research is also not a sufficient priority to secure public funding, then approval rather than OIR (an effective reject in these circumstances) would be appropriate only at the lower approve/OIR price threshold. Many workshop participants took the view that it would be better if all AWR and OIR research was publically funded rather than undertaken by manufacturers to ensure wide availability of research findings and provide confidence that commercial interests do not influence its design, conduct and reporting. It was suggested that the costs of publically funded research might be recovered directly from manufacturers or indirectly through other price discounts. Such arrangements might be mutually beneficial in some circumstances (e.g. if the costs of publically funded research are lower), while allowing wider access to the data generated and more transparency and accountability in the conduct of the research.
Further research could examine the relative costs of conducting this type of evaluative research and explore feasible mechanisms for recovering the costs of publically funded research from manufacturers. It should also include an examination of how the value of research is likely to be shared between manufacturers and the NHS in different circumstances and under different price renegotiation rules. The pay-offs for the different policies reported in Tables 25 and 26 are from an NHS perspective only. It would be interesting to estimate the commercial pay-offs associated with each, which could start to inform when manufacturers or public bodies might be expected to conduct or pay for the research recommended as part of AWR or OIR guidance.
Value of ‘approval with research’ and evidence at launch
The assessments that need to be made (especially in Chapter 5, Point 6: Are the benefits of research greater than the costs? and Point 7: Are the benefits of approval greater than the costs?) can be used to consider (1) what would be the value of being able to conduct research while a technology is approved (value of AWR), (2) what would be the value of making evidence that is needed by the NHS available at launch and (3) what is the value of being able to acquire evidence more quickly.
The difference in population NHEs between AWR and the next best feasible policy represents the value to the NHS of being able to conduct research while a technology is approved for use. This can inform investment decisions in better data collection, registries or information systems that might make AWR possible. Importantly, this value will differ by technology and will depend on the scale and significance of irrecoverable costs (if they are sufficiently high there will be no value in AWR – see Chapter 5, Technologies with significant irrecoverable costs). The difference in population NHEs between resolving all uncertainty before appraisal and the next best available policy represents the value to the NHS of having access to the evidence needed at launch. This might inform a range of policies, such as early advice, public investment in transitional and evaluative research earlier in the development process and other incentives for research and development. Understanding the relationship between the time taken for research to report and the value of the evidence to future populations can help to inform (1) investments that might make research findings more quickly available, (2) the trade-off implicit in the choice of alternative research designs (i.e. greater precision or timeliness) and (3) research prioritisation (identifying those areas in which if research is to be undertaken there must be confidence that it can report quickly).
The value of early evidence at launch and AWR can also be considered from the perspective of the manufacturer (the expected revenue streams), taking account of prices and expected volumes over the remaining patent life and technology time horizon. Together with estimates of the costs of conducting research by manufacturers or through public funding, this assessment might inform when manufacturers might be expected to conduct the research needed (e.g. high commercial value that exceeds the costs to manufacturers) or when the NHS might be expected to undertake it (e.g. low commercial value but high potential value to the NHS that exceeds the costs to the NHS). In some circumstances, the value to manufacturers and to the NHS will exceed their respective costs. The question of who should conduct, or pay for, the research might then be informed by which sector has the comparative advantage, that is, which sector can offer greater gains in net social value. The value to the NHS and to manufacturers will depend, to a large extent, on the type of pricing arrangements that might be in place (see Implications of patient access schemes and value-based pricing) and how any agreements that might be made can be enforced with incentive-consistent contracts. As well as understanding the pay-offs from an NHS perspective, these policy questions could be better informed with further research to estimate the likely commercial pay-offs and possible contractual arrangements that might align incentives and make AWR possible.
Social value judgements and ethical principles
Although OIR and AWR recommendations pose important ethical issues, the types of judgements required were considered ethically permissible and consistent with the social values and principles that underpin existing NICE appraisal. In general, no significant concerns about ethical issues or the types of social value judgements required when applying the checklist were raised during the workshops. Nonetheless, the analysis of the ethical issues did identify some that might require further consideration and investigation. In particular, it was recognised that an OIR recommendation may be made when clinicians and researchers would not be in equipoise about the effectiveness (as opposed to the cost-effectiveness) of an intervention. Therefore, RECs may disallow trials required by OIR guidance unless they are informed of, and agree with, the rationale for contravening the traditional principle of equipoise. This ought to be reflected in an assessment of the likelihood of research being commissioned and reporting. However, more research on how a useful notion of equipoise might be informed by the types of social decisions that bodies like NICE have to make may be valuable. The social values that underpin NICE appraisal evolve. For example, the government’s plans for a value-based pricing scheme include an assessment of severity, unmet need and wider social benefits as well as QALY gains. Irrespective of how these different aspects of outcome might be included, some assessment of uncertainty and the need for evidence will still be required. Therefore, the principles of what assessments are required appear robust to such changes. Insofar as social values can be expressed quantitatively as ‘weights’, the assessments required can still be informed in the ways described in Chapter 5 but based on a boarder measure of net social (rather than health) effects.
Some potential for inequity was recognised when OIR guidance might mean that patients are less likely to be included in the research. Criteria for access to the treatment trial need to be established, mindful of how unfairness might be minimised. Whether or not an AWR recommendation will be undertaken by manufacturers (see discussion in Incentives for evaluative research) also has an ethical dimension. One option is to incentivise research by threatening to withdraw approval if the research fails to report. However, to carry out the threat would be unfair on future patients because the evidence base would not have changed. This potential for inequity means that NICE has no credible threat to ensure that the research recommended as part of AWR guidance will be undertaken in the way intended. It strengthens the case for establishing enforceable, incentive-consistent contractual arrangements or acknowledging that AWR may not be possible and so OIR is the only effective policy option.
How should the assessments be undertaken?
Order and sequence of assessments
The order of the assessments in the checklist relates to the sequence of decision nodes that fully describe the algorithm in Appendix 2. This order of considerations means that all seven assessments do not necessarily need to be made when an earlier judgement (e.g. at points 3, 5 and 6) can lead directly to guidance. In addition, the early assessment of the scale and potential significance of irrecoverable costs (at point 2) can avoid further and more complex assessment later.
There was a general consensus that the order was appropriate and that all of the assessments do not need to be routinely made for every appraisal, especially at points 6 and 7, which would need to be made only when the Appraisal Committee was considering OIR or AWR as a possibility. However, the assessment of whether, in principle, further research might be worthwhile and what type of evidence might be required would need to be undertaken routinely (additional information or analysis that might inform these assessments is discussed in What additional information and analysis might be required?). For example, at point 3 a judgement that more research does not seem worthwhile will lead directly to guidance (see Point 3: Does more research seem worthwhile?). However, if research may be worthwhile, some indication of the type of evidence needed would also be useful for those making an assessment of the prospects of research (see next section) and whether or not the type of research required to generate the evidence would be possible with approval. Therefore, routine assessment up to point 4 of the checklist (see Point 4: Is research possible with approval?) would seem appropriate before others with expertise and responsibility for research design and commissioning consider the prospects of the type of research needed. In fact, some assessment of other sources of uncertainty, such as future price changes, entry of new technologies and research reporting, which influence the future value of research, probably ought to be undertaken routinely as well to inform these deliberations. A number of participants suggested that this type of information, required to make an assessment at point 5 of the checklist, ought to be collected at a much earlier stage in the appraisal process – ideally at scoping.
One model for an efficient order of assessment would be to consider points 1–5 routinely (possibly with information required for point 5 collected at scoping). The Appraisal Committee would then be in a position to either rule out OIR or AWR and issue guidance in the usual way or indicate in the ACD that OIR or AWR was provisionally recommended subject to advice from a Research Advisory Committee and subsequent analysis to support an assessment of points 6 and 7 of the checklist at FAD. This model would avoid unnecessary analysis and assessment and incorporate the judgements of the research community without necessarily adding to the time that an appraisal might take. However, it does pose some questions about who will conduct the additional work between ACD and FAD and whether this is consistent with current contractual arrangements with the TAR groups or whether this work might be undertaken by the Decision Support Unit. This is a matter that NICE and NIHR are best placed to resolve.
Assessing the prospects of research
When considering OIR or AWR guidance there must be some assessment of (1) the type of research needed to address the key uncertainties, (2) whether or not this will be regarded as ethical and can be undertaken while the technology is approved for use, (3) whether or not it is likely to be a priority for public funding and be commissioned and (4) when it is likely to report.
Although the NICE appraisal process may be well suited to identifying the need for evidence when assessing cost-effectiveness, these other critical assessments (the type of research and its priority) are not necessarily ones for which NICE and its Advisory Committees, as currently constituted, have particular expertise, not least because they reflect the decisions of those responsible for research design, prioritisation and commissioning. Without sufficient co-ordination between these communities there is a danger that OIR or AWR guidance could be issued when the type of research required would either not be regarded as ethical or feasible or not of sufficient priority compared with other competing research needs to be commissioned. Because publically funded research is also budget constrained, it is perfectly possible that research that might be valuable from a wider NHS perspective might nevertheless not be a priority if other more valuable research might be displaced. This might be a particular concern if there is a possibility that the research could be undertaken by the manufacturer rather than displacing other research without a commercial interest. Therefore, a decision of whether OIR or AWR research should be undertaken by the manufacturer or through publically funded research is one that NICE cannot properly take alone (see Incentives for evaluative research).
Some judgement about how the research community might respond to OIR or AWR recommendations when NICE is formulating guidance is clearly possible, but more informed judgements and better decisions are likely to be possible through greater involvement of the research community. For example, a Research Advisory Committee could be constituted that could consider provisional OIR or AWR guidance (at ACD), making recommendations about the type of research needed and its ethics, feasibility and likely priority during the consultation period before final appraisal and guidance. It might also make recommendations about whether research should be publically funded or undertaken by the manufacturer with appropriate contractual arrangements (which may require the involvement of the Department of Health at some stage). Such recommendations might be informed by the type of analysis described in Chapter 5, Point 6: Are the benefits of research greater than the costs? and Point 7: Are the benefits of approval greater than the costs? and discussed in Incentives for evaluative research above. There are of course many different ways in which greater co-ordination might be achieved. However, as some of the assessments that must be made in formulating OIR or AWR guidance are, in fact, research decisions that fall outside the NICE remit, it would seem sensible to draw on the expertise of those involved in, and responsible for, these types of research decisions to help make these assessments, for example translating the need for particular types of evidence into a need for particular types of research, its cost, relative priority, likelihood of success and when it is likely to report. There was a general consensus among workshop participants that greater involvement of research commissioners when NICE is considering AWR or OIR recommendations would be useful. However, the finer detail of how the process of appraisal might need to be amended to accommodate this is for NICE to consider and may require an update to the methods and process of technology appraisal.
Reappraisal
A number of participants pointed out that the triggers for reappraisal need to be linked to OIR and AWR guidance in two respects: (1) to ensure that guidance is reconsidered once research findings are available and (2) to reconsider guidance if the type of research anticipated as part of AWR or OIR guidance is not undertaken and there is little prospect that it will be, for example when there is no agreement with a manufacturer or publically funded research was not prioritised or commissioned. In addition, other changes (prices, entry of new technologies and new evidence) that might mean that research is no longer required ought to be included as criteria for reappraisal, to be considered by NICE when prioritising reappraisals. This suggests that the criteria for reappraisal and the process by which they are judged by NICE might need to be amended. Some consideration also needs to be given to the unintended consequences of reconsidering OIR before sufficient time has elapsed to encourage manufacturers to undertake research or publically funded research to be prioritised and commissioned.
What additional information and analysis might be required?
Population net health effects
In current NICE appraisal cost-effectiveness is most commonly summarised in terms of ICERs. However, such summary measures give little indication of the scale of the expected NHEs for current and future patient populations, which is a key assessment when considering OIR and AWR. For this reason cost-effectiveness was presented in terms of NHEs per patient treated and for the population of patients over time, which provides information in a way that is directly relevant to the assessments that need to be made (especially at points 2 and 7 for the checklist – see Chapter 5, Point 1: Is it expected to be cost-effective?). All of the information required to express expected cost-effectiveness in these ways is already available during appraisal and they are entirely equivalent to the more familiar ICER.
Irrecoverable costs and time horizons
Amending how irrecoverable capital costs are incorporated into the estimates of expected costs poses few technical difficulties. Some of the judgements required are already made in current methods of appraisal, for example the patient time horizon is specified in existing economic models and the expected lifetime of equipment is assumed when annuitising capital costs. The technology time horizon is not currently explicitly considered and some judgement by the Appraisal Committee supported by sensitivity analysis as well as expert clinical views is likely to be required. Considering the significance of the irrecoverable opportunity costs of initially negative NHEs of a technology (the investment profile) is more nuanced (see Chapter 3, Issues specific to OIR recommendations) and their precise effect will depend on assessments made at points 5 and 6, which can be supported by additional analysis using the type of economic models developed during appraisal. However, some early indication of their potential importance can be based on their scale relative to expected NHEs, the point at which initial losses are expected to be compensated by later gains, whether or not treatment decisions are reversible and what opportunities to improve health might be forgone by a delay to initiating treatment.
Assessing the consequences of uncertainty
The judgement at point 3 of the checklist requires some assessment of (1) how uncertain a decision based on expected cost-effectiveness might be and (2) what the consequences, in terms of population NHEs, are likely to be if an incorrect decision is made. The methods of analysis presented in Chapter 5, Point 3: Does more research seem worthwhile? attempt to decompose this assessment into a series of steps, each presenting what is already available within current methods of appraisal but in ways that can more directly inform the assessment required. Unfortunately, a simple proxy measure of the expected consequences based on expected costs and effects is not useful because it provides neither an upper or a lower bound, that is, it may seriously under- or overestimate what might be gained through further research. However, the information required to estimate the expected consequences of uncertainty and decompose them (see Figure 10) is already available in existing probabilistic analysis but is generally unused. Such estimates do require some judgement (implicit in all research decisions) about the period over which research findings will continue to be useful. Again, such judgements can be supported by sensitivity analysis as well as expert views, for example from a Research Advisory Committee. The implications of scenarios and other sources of uncertainty are discussed below.
What type of evidence is required?
This assessment at point 4 of the checklist requires judgements about (1) how important particular types of parameters (inputs to the economic model) are to estimates of cost and QALYs, (2) what values these parameters would have to take to change a decision based on expected cost-effectiveness, (3) how likely is it that parameters might take such values and (4) what would be the consequences if they did, that is, what might be gained in terms of population NHEs if the uncertainty in the values of these parameters could be immediately resolved. The methods of analysis presented in Chapter 5, Point 4: Is research possible without approval? decompose this assessment into this series of steps, presenting in turn what is already available within current methods of appraisal but in ways that more directly inform the assessment required. It is only when assessing the consequences of uncertainty associated with particular parameters that additional analysis (using the results of existing probabilistic analysis) is required to provide quantitative estimates. There are circumstances when this additional computation is a significant burden although, increasingly, suitable simplifications and approximations are available.
Uncertainty between scenarios
It was recognised by participants that uncertainty in the parameters included in probabilistic analysis generally do not represent all sources of uncertainty. Commonly there is also uncertainty between alternative assumptions or judgements that might be made, often represented by alternative scenarios. How the consequences of uncertainty between as well as within scenarios can be presented was explored in Chapter 5, Point 3: Does more research seem worthwhile? and Point 4: Is research possible without approval?. Properly accounting for this source of uncertainty requires ‘weights’ (probabilities) representing the credibility of each scenario to be applied directly to the simulated output from probabilistic analysis. Although this does not require additional simulation and is quick and easy to implement, it does require that either the probabilities are made explicit in advance or estimates are presented for a range of probabilities that might represent the judgement of the Appraisal Committee following deliberation. Rather than make alternative assumptions and present extreme scenarios, formal elicitation of the judgement of clinical experts about the unknown parameters for which assumptions are required is possible. Such elicitation may provide a richer characterisation of uncertainty than weighting alternative scenarios but would require relevant experts to be identified in advance and the Appraisal Committee to accept their judgements. Alternatively, this type of analysis might be possible in real time during Appraisal Committee meetings using recent initiatives such as the Transparent Interactive Decision Interrogator, so the quantitative implications of the judgements of the Appraisal Committee could be represented. 132
Additional information
The current appraisal process generally already provides the information and much of the analysis required to complete all of the analysis reported in Chapter 5 and as a consequence requires little additional resource. However, the information required to assess whether or not other sources of uncertainty will resolve over time (point 5 on the checklist) is not commonly sought as part of NICE appraisal. This information includes (1) likely changes in prices of the technology and its comparators (e.g. patent expiry and likely generic prices), (2) the emergence of new technologies that might make existing ones obsolete or change their cost-effectiveness and (3) other relevant research reporting. A number of potential sources of information and evidence were examined to inform this assessment for each case study (see Chapter 5, Point 5: Will other sources of uncertainty resolve over time? and Appendix 4 for full details of the sources and searches conducted). However, many potentially useful sources were proprietary or public access was restricted, making it surprisingly difficult to inform these assessments from the information that is currently publically available. When information and estimates were available they were often not complete or directly relevant to a UK context. NICE may need to consider how ERGs, TAR groups and manufacturers can be provided with access to this type of information or whether the Institute should extract this type of information itself at an early stage of appraisal, for example at scoping or topic selection, as suggested by a number of participants.
Who should conduct the analysis?
Whether or not the ERG/TAR group or the manufacturers should be primarily responsible for conducting any additional analysis to inform these assessments was a question raised by a number of participants. Some were concerned that this analysis should be undertaken independently and others that ERGs might not have sufficient resources and time to properly interrogate submissions that include such analysis. Because the STA process bases appraisal primarily on manufacturers’ submissions, any additional analysis would need to be included in the submissions and be reviewed by the ERG. Although the time and resource implications of the additional analysis are limited, even if all of the quantitative analysis in Chapter 5 was undertaken (most is already required but is sometimes presented in different forms), more explicit consideration of OIR and AWR and the link to price would make the critique of how uncertainty and its consequences have been characterised more important. An assessment of whether or not the point estimate of cost-effectiveness is reasonable is inevitably a more limited task than also assessing whether or not the uncertainty surrounding that assessment is credible. Any additional burden on TAR groups, ERGs and manufacturers might be eased with clear guidance on the details of how analysis should be conducted and presented and what common assumptions are deemed reasonable (e.g. time horizons) and the provision of additional information by NICE. Others also suggested that greater opportunities for iteration between ERGs and manufacturers might be helpful as well as considering only points 6 and 7 on the checklist when NICE has recommended AWR or OIR at ACD and after advice from a Research Advisory Committee (see Implications of patient access schemes and value-based pricing).
Implementing changes informing assessments
Although it was recognised that most of the analysis presented in Chapter 5 was appropriate and by and large simply used existing analysis to more directly inform the assessment required (consequently with limited resource implications), a number of participants felt that this might not be self-evident to Appraisal Committee members, ERGs/TAR groups and manufacturers. They suggested that some ‘training’ activities might be considered to ensure that appropriate skills were in place and that any additional analysis or change in the way that cost-effectiveness is presented is properly conducted and interpreted appropriately.
Acknowledgements
We would like to thank the members of our Advisory Group, Kalipso Chalkidou, Iain Chalmers, Sarah Garner, Chris Henshall, John Hutton, Catherine Law, Jonathan Michaels, Bhash Naidoo, Jon Nicholl, Hans Severens, Ken Stein, Sean Tunis and Tom Walley, who have provided invaluable feedback and comment throughout this research project. We would especially like to thank Steve Holland and Tony Hope, as ethics advisors to the project, and Iain Chalmers, as a member of the Advisory Group, for their overall contribution but in particular for their contribution to the section of the report that discusses social value judgements and ethical principles. We would like to acknowledge that they have substantially revised the original version of this discussion presented in the briefing documents to the first workshop. It is their revised version that is presented in Chapter 3 of this report. We would also like to thank all those who participated in the two workshops, whose engagement and feedback were invaluable. We would especially like to thank Peter Littlejohns for suggesting this topic to the MRC/NIHR Methodology Research Programme and his support and encouragement throughout, including chairing both workshops. Of course, the views expressed in this report, as well as any errors or omissions, are the sole responsibility of the authors.
Contribution of authors
KC, SP and LL secured the funding for this research. All authors contributed to the concepts and analysis and participated in drafting this report. KC drafted Chapters 1, 3, 5 and 6. SP and LB drafted Chapter 2. LL and JY drafted Chapter 4. MS, CM and LB were responsible for the case study analyses. SG and ES drafted Appendix 6. Appendices 7–11 were drafted by LB, SG, CM, MS and ES.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, MRC or the Department of Health.
Notes
Chapter 3
Chapter 5
Appendix 5
Appendix 6
Appendix 7
Appendix 8
Appendix 9
Appendix 10
Appendix 11
References
- NICE . Guide to the Methods of Technology Appraisal 2008.
- Tunis S, Pearson S. Coverage options for promising technologies: Medicare’s ‘coverage with evidence development’. Health Aff 2006;25:1218-30.
- Chalkidou K, Hoy A, Littlejohns P. Making a decision to wait for more evidence: when the National Institute for Health and Clinical Excellence recommends a technology only in the context of research. J R Soc Med 2007;100:453-60.
- Hutton J, Trueman P, Henshall C. Coverage with evidence development: an examination of conceptual and policy issues. Int J Technol Assess Health Care 2007;23:425-35.
- Tunis S, Chalkidou K, . Coverage with evidence development: a very good beginning, but much to be done. Int J Technol Assess Health Care 2007;23:432-5.
- Eckermann S, Willan AR. The option value of delay in health technology assessment. Med Decis Making 2008;28:300-5.
- Palmer S, Smith P. Incorporating option values into the economic evaluation of health care technologies. J Health Econ 2000;19:755-66.
- Stafinski T, McCabe CJ, Menon D. Funding the unfundable: mechanisms for managing uncertainty in decisions on the introduction of new and innovative technologies into healthcare systems. Pharmacoeconomics 2010;28:113-42.
- Cooksey D. A review of UK health research funding. London: HM Treasury; 2006.
- House of Commons Health Committee . The Influence of the Pharmaceutical Industry: Fourth Report of Session 2004–2005 2005.
- NICE Citizens Council . ‘Only in research’ 2007.
- Office of Fair Trading (OFT) . The Pharmaceutical Price Regulation Scheme. An OFT Market Study 2007.
- Brezis M, Lehmann LS. Medicare requirement for research participation. JAMA 2006;296.
- Gafni A, Birch S. Building bridges between academic research and policy formulation: when costing less means costing more. Pharmacoeconomics 2007;25:523-8.
- Goeree R, O’Reilly D, Tarride JE, Bowen JM, Blackhouse G, Campbell K, et al. Being led down the wrong garden PATH: the importance of knowledge and facts for the crossroads. Pharmacoeconomics 2007;25:528-32.
- Groeneveld PW. Medicare requirement for research participation. JAMA 2006;296:2923-4.
- Bowen JM, Patterson LL, O’Reilly D, Hopkins RB, Blackhouse G, Burke N, et al. Conditionally funded field evaluations and practical trial design within a health technology assessment framework. J Am Coll Radiol 2009;6:324-31.
- Briggs A, Ritchie K, Fenwick E, Chalkidou K, Littlejohns P. Access with evidence development in the UK: past experience, current initiatives and future potential. Pharmacoeconomics 2010;28:163-70.
- Carbonneil C, Quentin F, Lee-Robin SH. European network for Health Technology Assessment (EUnetHTA) . A common policy framework for evidence generation on promising health technologies. Int J Technol Assess Health Care 2009;25:56-67.
- Goeree R, Levin L. Building bridges between academic research and policy formulation: the PRUFE framework – an integral part of Ontario’s evidence-based HTPA process. Pharmacoeconomics 2006;24:1143-56.
- McCabe CJ, Stafinski T, Edlin R, Menon D. Access with evidence development schemes: a framework for description and evaluation. Pharmacoeconomics 2010;28:143-52.
- Miller W. Value-based coverage policy in the United States and the United Kingdom: different paths to a common goal. Washington, DC: National Health Policy Forum; 2006.
- Towse A, Garrison LP. Can’t get no satisfaction? Will pay for performance help?: toward an economic framework for understanding performance-based risk-sharing agreements for innovative medical products. Pharmacoeconomics 2010;28:93-102.
- Trueman P, Grainger DL, Downs KE. Coverage with evidence development: applications and issues. Int J Technol Assess Health Care 2010;26:79-85.
- Carlson JJ, Sullivan SD, Garrison LP, Neumann PJ, Veenstra DL. Linking payment to health outcomes: a taxonomy and examination of performance-based reimbursement schemes between healthcare payers and manufacturers. Health Policy 2010;96:179-90.
- Chalmers I. Addressing uncertainties about the effects of treatments offered to NHS patients: whose responsibility?. J R Soc Med 2007;100:440-1.
- Ramsey SD, Sullivan SD. Evidence, economics, and emphysema: Medicare’s long journey with lung volume reduction surgery. Health Aff 2005;24:55-66.
- Wallner P, Konski A. A changing paradigm in the study and adoption of emerging health care technologies: coverage with evidence development. J Am Coll Radiol 2008;5:1125-9.
- Chalkidou K, Lord J, Fischer A, Littlejohns P. Evidence-based decision making: when should we wait for more information?. Health Aff 2008;27:1642-53.
- Tunis S, Whicher D. The National Oncologic PET Registry: lessons learned for coverage with evidence development. J Am Coll Radiol 2009;6:360-5.
- Medicare’s coverage with evidence development: a policy-making tool in evolution . J Oncol Pract 2007;3:296-301.
- Kamerow D. Paying for promising but unproven technologies: is the policy on ‘coverage with evidence development’ a helpful way forward?. BMJ 2007;335.
- Mohr PE, Tunis SR. Access with evidence development: the US experience. Pharmacoeconomics 2010;28:153-62.
- Carino T, Williams RD II, Colbert AM, Bridger P. Medicare’s coverage of colorectal cancer drugs: a case study in evidence development and policy. Health Aff 2006;25:1231-9.
- Brice J. Imaging research efforts place focus on clinical effectiveness. Research to Practice 2008. www.diagnosticimaging.com/display/article/113619/1180672.
- Breckenridge A, Walley T. Risk sharing and payment by results. Clin Pharmacol Ther 2008;83:666-7.
- Carino T, Sheingold S, Tunis S. Using clinical trials as a condition of coverage: lessons from the National Emphysema Treatment Trial. Clinical Trials 2004;1:108-21.
- Dhalla IA, Garner S, Chalkidou K, Littlejohns P. Perspectives on the National Institute for Health and Clinical Excellence’s recommendations to use health technologies only in research. Int J Technol Assess Health Care 2009;25:272-80.
- Garber A. Is having more preapproval data the best way to assure drug safety?. Health Aff 2008;5:371-3.
- Krich TB. CMS new coverage with evidence policy. J BioLaw Bus 2007;10:43-4.
- Lindsay MJ, Siegel BA, Tunis SR, Hillner BE, Shields AF, Carey BP, et al. The National Oncologic PET Registry: expanded Medicare coverage for PET under coverage with evidence development. AJR Am J Roentgenol 2007;188:1109-13.
- Macdonald F. Coverage With Evidence Development: A Personal, Industry View 2008. www.docstoc.com/docs/105704904/Coverage-With-Evidence-Development.
- Menon D, McCabe CJ, Stafinski T, Edlin R. signatories to the Consensus Statement. Principles of design of access with evidence development approaches: a consensus statement from the Banff Summit. Pharmacoeconomics 2010;28:109-11.
- Neumann PJ, Kamae MS, Palmer JA. Medicare’s national coverage decisions for technologies, 1999–2007. Health Aff 2008;27:1620-31.
- Niezen M, de Bont A, Stolk E, Eyek A, Niessen L, Stoevalaar H. Conditional reimbursement with the Dutch drug policy. Health Policy 2007;84:39-50.
- Pearson SD, Miller FG, Emanuel EJ. Medicare’s requirement for research participation as a condition of coverage: is it ethical?. JAMA 2006;296:988-91.
- Reed S, Shea A, Schulman K. Economic implications of potential changes to regulatory and reimbursement policies for medical devices. J Gen Intern Med 2008;23:50-6.
- Straube BM. How changes in the Medicare coverage process have facilitated the spread of new technologies. Health Aff 2005.
- Thornton S. Drug price reform in the UK: debunking the myths. Health Econ 2007;16:981-92.
- Whalley GA, Gamble GD, Walsh HJ, Wright SP, Agewall S, Sharpe N, et al. Effect of tissue harmonic imaging and contrast upon between observer and test–retest reproducibility of left ventricular ejection fraction measurement in patients with heart failure. Eur J Heart Fail 2004;6:85-93.
- NICE and AHRQ . Managing Uncertainty in Healthcare 2008. www.nice.org.uk/aboutnice/howwework/researchanddevelopment/managinguncertaintyinhealthcare.jsp.
- Department of Health . Faster Access to Modern Treatment: How NICE Appraisal Will Work 1999.
- NHS Quality Improvement Scotland . Coverage With Evidence Development in NHS Scotland 2008.
- Office for Life Sciences, HM Government . Life Sciences Blueprint 2009.
- Centers for Medicare & Medicaid Services . Medicare and Medicaid programs; conditions for coverage for organ procurement organizations (OPOs). Final rule. Fed Regist 2006;71:30981-1054.
- Mechanic R, Zinner D. Optimizing information under coverage with evidence development. Waltham, MA: The Health Industry Forum; 2007.
- Slutsky J. CED for Promising Technologies: USA 2008.
- Sansom L. Medicines Use: What’s Next for the PBS n.d.
- Chalkidou K. ‘Only in research’: a polite ‘no’ or a valuable policy option?. Birmingham: NICE; 2006.
- Reeves B. Verteporfin Photodynamic Therapy (PDT) Cohort Study for the UK ‘coverage With Evidence development’ 2008.
- Garrison L, Sullivan S, Carlson J, Bresnahan B, Carlson R, Neumann P, et al. Performance-based risk-sharing reimbursement agreements for new medical products. Washington, DC: University of Washington; 2008.
- Culyer A, McCabe C, Briggs A, Claxton K, Buxton M, Akehurst R, et al. Searching for a threshold, not setting one: the role of the National Institute for Health and Clinical Excellence. J Health Serv Res Policy 2007;12:56-8.
- Phelps CE, Mushlin AI. On the (near) equivalence of cost-effectiveness and cost–benefit analyses. Int J Technol Assess Health Care 1991;7:12-21.
- Stinnett AA, Mullahy J. Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decis Making 1998;18:S68-80.
- Claxton K, Briggs A, Buxton M, Culyer A, McCabe C, Walker S, et al. Value based pricing for NHS drugs: an opportunity not to be missed?. BMJ 2008;336:251-4.
- McCabe C, Claxton K, Culyer AJ. The NICE cost-effectiveness threshold: what it is and what that means. Pharmacoeconomics 2008;26:733-44.
- Claxton K. The irrelevance of inference: a decision-making approach to the stochastic evaluation of health care technologies. J Health Econ 1999;18:342-64.
- Claxton K, Cohen J, Neumann P. When is evidence sufficient?. Health Aff 2005;24:93-101.
- Claxton K, Sculpher M, Drummond M. A rational framework for decision making by the National Institute for Clinical Excellence. Lancet 2002;360:711-15.
- Griffin SC, Claxton KP, Palmer SJ, Sculpher MJ. Dangerous omissions: the consequences of ignoring decision uncertainty. Health Econ 2011;20:212-24.
- Philips Z, Claxton K, Palmer S. The half-life of truth: what are appropriate time horizons for research decisions?. Med Decis Making 2008;28:287-99.
- Eckermann S, Willan A. Expected value of information and decision making in HTA. Health Econ 2006;16:195-209.
- Fenwick E, Claxton K, Sculpher M. The value of implementation and the value of information: combined and uneven development. Med Decis Making 2008;28:21-32.
- Hoomans T, Fenwick EA, Palmer S, Claxton K. Value of information and value of implementation: application of an analytic framework to inform resource allocation decisions in metastatic hormone-refractory prostate cancer. Value Health 2009;12:315-24.
- McKenna C, Claxton K. Addressing adoption and research design decisions simultaneously: the role of value of sample information analysis. Med Decis Making 2011;31:853-65.
- Basu A. Economics of individualization in comparative effectiveness research and a basis for a patient-centered health care. J Health Econ 2011;30:549-59.
- Claxton K, Sculpher M, Carroll S. Value-based pricing for pharmaceuticals: its role, specification and prospects in a newly devolved NHS. York: Centre for Health Economics, University of York; 2011.
- Department of Health . A New Value-Based Approach to the Pricing of Branded Medicines: A Consultation 2010.
- Department of Health . A New Value-Based Approach to the Pricing of Branded Medicines: Government Response to Consultation 2010.
- Claxton K. OFT, VBP: QED?. Health Econ 2007;16:545-58.
- Department of Health . The Pharmaceutical Price Regulation Scheme 2008.
- Chalkidou K, Walley T, Culyer A, Littlejohns P, Hoy A. Evidence-informed evidence-making. J Health Serv Res Policy 2008;13:167-73.
- NICE . Social Value Judgements Guideline. Principles for the Development of NICE Guidance 2008.
- Kumar A, Soares H, Wells R, Clarke M, Hozo I, Bleyer A, et al. Are experimental treatments for cancer in children superior to established treatments? Observational study of randomised controlled trials by the Children’s Oncology Group. BMJ 2005;331.
- Levine RJ. The ‘best proven therapeutic method’ standard in clinical trials in technologically developing countries. IRB 1998;20:5-9.
- NICE . Process for Advising on the Feasibility of Implementing a Patient Access Scheme 2009.
- Department of Health . Health Service Circular 2002 004 – Cost Effective Provision of Disease Modifying Therapies for People With Multiple Sclerosis 2002.
- NICE . The Use of Liquid-Based Cytology for Cervical Screening 2003.
- NICE . Infliximab for Acute Exacerbations of Ulcerative Colitis 2008.
- NICE . Hearing Disability – Hearing Aids (withdrawn) 2000.
- NICE . Inhaled Insulin for the Treatment of Type 1 and Type 2 Diabetes 2006.
- NICE . Guidance on the Use of Etanercept and Infliximab for the Treatment of Rheumatoid Arthritis 2002.
- NICE . Guidance on the Use of Patient-Education Models for Diabetes 2003.
- NICE . Guidance on the Use of Photodynamic Therapy for Age-Related Muscular Degeneration 2003.
- NICE . Computerised Cognitive Behaviour Therapy for Depression and Anxiety: Guidance 2006.
- NICE . Computerised Cognitive Behaviour Therapy (CCBT) for the Treatment of Depression and Anxiety 2002.
- NICE . Rituximab for Aggressive Non-Hodgkin’s Lymphoma 2003.
- NICE . Guidance on the Use of Metal on Metal Hip Resurfacing Arthroplasty 2002.
- NICE . Interferon Alfa (pegylated and Non-Pegylated) and Ribavirin for the Treatment of Chronic Hepatitis C 2004.
- NICE . Lymphoma (follicular Non-Hodgkin’s) – Rituximab 2002.
- NICE . Endovascular stent–grafts for the Treatment of Abdominal Aortic Aneurysms 2009.
- NICE . The Use of Autologous Chondrocyte Implantation for the Treatment of Cartilage Defects in Knee Joints 2005.
- NICE . Bortezomib Monotherapy for Relapsed Multiple Myeloma 2007.
- NICE . Cochlear Implants for Severe to Profound Deafness in Children and Adults 2009.
- NICE . Taxanes for the Treatment of Breast Cancer 2000.
- NICE . Guidance on the Use of Irinotecan, Oxaliplatin and Raltitrexed for the Treatment of Advanced Colorectal Cancer 2002.
- NICE . Cervical Cancer – Cervical Screening (review) 2003.
- NICE . Taxanes for the Treatment of Breast Cancer 2001.
- NICE . Knee Joints (defective) – Autologous Cartilage Transplantation: Guidance 2000.
- NICE . Guidance on the Use of Laparoscopic Surgery for Colorectal Cancer 2000.
- NICE . Laparoscopic Surgery for Colorectal Cancer 2006.
- NICE . Advanced Breast Cancer: NICE Guideline 2009.
- NICE . Colorectal Cancer (advanced) – Irinotecan, Oxaliplatin and Raltitrexed 2005.
- NICE . Rheumatoid Arthritis – Adalimumab, Etanercept and Infliximab 2007.
- NICE . Rituximab for the Treatment of Relapsed or Refractory Stage III or IV Follicular Non-Hodgkin’s Lymphoma 2008.
- NICE . Rheumatoid Arthritis – Anakinra 2003.
- NICE . Rheumatoid Arthritis: The Management of Rheumatoid Arthritis in Adults 2009.
- NICE . Supplementary Guidance to the Appraisal Committees: Appraising Life-Extending, End of Life Treatment 2009. www.nice.org.uk (accessed 18 February 2011).
- NICE . Report to the NICE Board: Update Report on the Application of the ‘end-of-life’ Supplementary Advice in Health Technology Appraisals 2009. www.nice.org.uk (accessed 18 February 2011).
- NICE . Research Recommendations Process and Methods Guide 2011.
- McKenna C, McDaid C, Suekarran S, Hawkins N, Claxton K, Light K, et al. Enhanced external counterpulsation for the treatment of stable angina and heart failure: a systematic review and economic analysis. Health Technol Assess 2009;13.
- NICE . Clopidogrel in the Treatment of Non-ST-Segment-Elevation Acute Coronary Syndrome 2004.
- NICE . Unstable Angina and NSTEMI: The Early Management of Unstable Angina and Non-ST-Segment-Elevation Myocardial Infarction 2010.
- NICE . Omalizumab for the Treatment of Severe Persistent Allergic Asthma in Children Aged 6 to 11 Years. NICE Technology Appraisal Guidance 201 2010. www.nice.org.uk/nicemedia/live/13256/51345/51345.pdf (accessed 22 July 2011).
- NICE . Etanercept, Infliximab and Adalimumab for the Treatment of Psoriatic Arthritis 2010.
- NICE . Etanercept and Infliximab for the Treatment of Psoriatic Arthritis 2006.
- Claxton K. Exploring uncertainty in cost-effectiveness results. Pharmacoeconomics 2008;926:781-98.
- Claxton K, Sculpher M, McCabe C, Briggs AH, Akehurst R, Buxton M, et al. Probabilistic sensitivity analysis for NICE technology assessment: not an optional extra. Health Econ 2005;14:339-47.
- Claxton K, Sculpher M. Using value of information analysis to prioritise health research: some lessons from recent UK experience. Pharmacoeconomics 2006;24:1055-68.
- Price MJ, Welton NJ, Briggs AH, Ades AE. Model averaging in the presence of structural uncertainty about treatment effects: influence on treatment decision and expected value of information. Value Health 2011;14:205-18.
- Griffin S, Welton N, Claxton K. Exploring the research decision space: the expected value of information for sequential research designs. Med Decis Making 2010;30:155-62.
- Bujkiewicz S, Jones HE, Lai MC, Cooper NJ, Hawkins N, Squires H, et al. Development of a transparent interactive decision interrogator to facilitate the decision-making process in health care. Value Health 2011;14:768-76.
- Kary W, Bergthold L, Talbott M, Aubry W. Coverage for evidence development: a conceptual frame. Baltimore, MD: Center for Medical Technology Policy; 2009.
- NICE . Guidance on the Selection of Prostheses for Primary Total Hip Replacement 2000.
- NICE . Guidance on the Use of Temozolomide for the Treatment of Recurrent Malignant Glioma (brain Cancer) 2001.
- NICE . Etanercept for the Treatment of Juvenile Idiopathic Arthritis 2002.
- NICE . Imatinib for Chronic Myeloid Leukaemia 2002.
- NICE . Guidance on the Use of Imatinib for Chronic Myeloid Leukaemia 2003.
- NICE . Imatinib for the Treatment of Unresectable and Or Metastatic Gastro-Intestinal Stromal Tumours 2004.
- NICE . HealOzone for the Treatment of Tooth Decay (occlusal Pit and Fissure Caries and Root Caries) 2005.
- NICE . Immunosuppressive Therapy for Renal Transplantation in Children and Adolescents 2006.
- NICE . Donepezil, Galantamine, Rivastigmine (review) and Memantine for the Treatment of Alzheimer’s Disease 2007.
- NICE . Carmustine Implants and Temozolomide for the Treatment of Newly Diagnosed High Grade Glioma 2007.
- NICE . Pemetrexed Disodium for the Treatment of Mesothelioma 2008.
- NICE . Erythropoetin (alpha and Beta) and Darbepoetin for the Treatment of Cancer-Treatment Induced Anaemia 2008.
- NICE . Adalimumab, Etanercept and Infliximab for Ankylosing Spondylitis 2008.
- NICE . Spinal Cord Stimulation for Chronic Pain of Neuropathic or Ischaemic Origin 2008.
- Rogowski W, Burch J, Palmer S, Craigs C, Golder S, Woolacott N. The effect of different treatment durations of clopidogrel in patients with non-ST-segment elevation acute coronary syndromes: a systematic review and value of information analysis. Health Technol Assess 2009;13.
- Shuchman M. Delaying generic competition – corporate payoffs and the future of Plavix. N Engl J Med 2006;355:1297-300.
- DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ 2003;22:151-85.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien B, Stoddart GL. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2005.
- Briggs AH, Sculpher MJ, Claxton K. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2006.
- Felli JC, Hazen GB. Sensitivity analysis and the expected value of perfect information. Med Decis Making 1998;18:95-109.
- Claxton K, Posnett J. An economic approach to clinical trial design and research priority-setting. Health Econ 1996;5:513-24.
- Bojke L, Claxton K, Sculpher M, Palmer S. Characterizing structural uncertainty in decision-analytic models: a review and application of methods. Value Health 2009;12:739-49.
- Hoeting JA, Madigan D, Raftery AE, Volinsky CT. Bayesian model averaging: a tutorial. Stat Sci 1999;14:382-417.
- Ades AE, Lu G, Claxton K. Expected value of sample information calculations in medical decision modeling. Med Decis Making 2004;24:207-27.
- Willan A, Eckermann S. Optimal clinical trial design using value of information methods with imperfect implementation. Health Econ 2010;19:549-61.
- Allender S, Peto V, Scarborough P, Boxer A, Rayner M. Morbidity. London: British Heart Foundation; 2007.
- Arora R, Chou T, Jain D, Fleishman B, Crawford L, McKiernan T, et al. The multicenter study of enhanced external counterpulsation (MUST-EECP): effect of EECP on exercise-induced myocardial ischemia and anginal episodes. J Am Coll Cardiol 1999;33:1833-40.
- Arora R, Chou T, Jain D, Fleishman B, Crawford L, McKiernan T, et al. Effects of enhanced external counterpulsation on health-related quality of life continue 12 months after treatment: a substudy of the Multicenter Study of Enhanced External Counterpulsation. J Investig Med 2002;50:25-32.
- Lawson WE, Hui JCK, Soroff HS, Zheng ZS, Kayden DS, Sasvary D, et al. Efficacy of enhanced external counterpulsation in the treatment of angina pectoris. Am J Cardiol 1992;70:859-62.
- Lawson WE, Hui JCK, Zheng ZS, Oster Z, Katz JP, Diggs P, et al. Three-year sustained benefit from enhanced external counterpulsation in chronic angina pectoris. Am J Cardiol 1995;75:840-1.
- Scottish Intercollegiate Guidelines Network (SIGN) . Management of Stable Angina: A National Clinical Guideline 2007.
- Sinvhal RM, Gowda RM, Khan IA. Enhanced external counterpulsation for refractory angina pectoris. Heart 2003;89:830-3.
- Bonetti PO, Holmes DR, Lerman A, Barsness GW. Enhanced external counterpulsation for ischemic heart disease: what’s behind the curtain?. J Am Coll Cardiol 2003;41:1918-25.
- O’Hagan A, Buck CE, Daneshkhah A, Eiser JR, Garthwaite PH, Jenkinson DJ, et al. Uncertain judgements. Eliciting experts’ probabilities. Chichester: Wiley; 2006.
- van Noortwijk JM, Dekker A, Cooke RM, Mazzuchi TA. Expert judgment in maintenance optimization. IEEE Trans Reliabil 1992;41:427-32.
- Cooke RM. Experts in uncertainty: opinion and subjective probability in science. New York, NY: Oxford University Press; 1991.
- Briggs A, Mihaylova B, Sculpher M, Hall A, Wolstenholme J, Simoons M, et al. Cost effectiveness of perindopril in reducing cardiovascular events in patients with stable coronary artery disease using data from the EUROPA study. Heart 2007;93:1081-6.
- Office for National Statistics . Interim Life Tables 2007. www.gad.gov.uk/Demography%20Data/Life%20Tables/Interim_life_tables.html (accessed 23 June 2011).
- Michaels A, Barsness G, Soran O, Kelsey S, Kennard E, Hui J, et al. Frequency and efficacy of repeat enhanced external counterpulsation for stable angina pectoris (from the International EECP Patient Registry). Am J Cardiol 2005;95:394-7.
- Vasogenics price list (effective from April 2007) n.d.
- National Institute for Health and Clinical Excellence Centre for Health Technology Evaluation . Evaluation Pathway Programme for Medical Technologies 2010. www.nice.org.uk/media/5C8/62/ProductsConsideredByMTAC220211.pdf (accessed 10 December 2010).
- Main C, Palmer S, Griffin S, Jones L, Orton V, Sculpher M, et al. Clopidogrel used in combination with aspirin compared with aspirin alone in the treatment of non-ST-segment-elevation acute coronary syndromes: a systematic review and economic evaluation. Health Technol Assess 2004;8.
- Peters RJ, Mehta SR, Fox KAA, Zhao F, Lewis BS, Kopecky SL, et al. Effects of aspirin dose when used alone or in combination with clopidogrel in patients with acute coronary syndromes: observations from the Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) study. Circulation 2003;108:1682-7.
- Morais J. Insights from CURE: using clopidogrel on top of standard therapy. Cerebrovasc Dis 2002;13:17-21.
- Yusuf S, Mehta SR, Zhao F, Gersh BJ, Commerford PJ, Blumenthal M, et al. Early and late effects of clopidogrel in patients with acute coronary syndromes. Circulation 2003;107:966-72.
- Budaj A, Yusuf S, Mehta SR, Fox KAA, Tognoni G, Zhao F, et al. Benefit of clopidogrel in patients with acute coronary syndromes without ST-segment elevation in various risk groups. Circulation 2002;106:1622-6.
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KAA, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001;345:494-502.
- Keltai M, Tonelli M, Mann JF, Sitkei E, Lewis BS, Hawken S, et al. Renal function and outcomes in acute coronary syndrome: impact of clopidogrel. Eur J Cardiovasc Prev Rehabil 2007;14:312-18.
- Scottish Intercollegiate Guidelines Network (SIGN) . Acute Coronary Syndromes: A National Clinical Guideline 2007.
- Karnon J, Bakhai A, Brennan A, Pandor A, Flather M, Warren E, et al. A cost–utility analysis of clopidogrel in patients with non-ST-segment-elevation acute coronary syndromes in the UK. Int J Cardiol 2006;109:307-16.
- British Medical Association and Royal Pharmaceutical Society of Great Britain . British National Formulary 2007.
- Collinson, J, Flather MD, Fox KAA, Findlay I, Rodrigues E, Dooley P, et al. Eur Heart J 2000;21:1450-7.
- Lanier B, Bridges T, Kulus M, Taylor AF, Berhane I, Vidaurre CF. Omalizumab for the treatment of exacerbations in children with inadequately controlled allergic (IgE-mediated) asthma. J Allergy Clin Immunol 2009;124:1210-16.
- Asthma UK. For Journalists: Key Facts & Statistics 2010. www.asthma.org.uk/news_media/media_resources/for_journalists_key.html (accessed 3 June 2011).
- Novartis Pharmaceuticals UK Ltd . Xolair (omalizumab) for Severe Persistent Allergic Asthma in Children Aged 6–≪ 12 Years. Manufacturer Submission of Evidence 2010.
- Scottish Intercollegiate Guidelines Network (SIGN) . British Guideline on the Management of Asthma; A National Clinical Guideline 2009.
- Walker S, Burch J, McKenna C, Wright K, Griffin S, Woolacott N. Omalizumab for the treatment of severe persistent allergic asthma in children aged 6 to 11 years. London: NICE; 2010.
- Dewilde S, Turk F, Tambour M, Sandstrom T. The economic value of anti-IgE in severe persistent, IgE-mediated (allergic) asthma patients: adaptation of INNOVATE to Sweden. Curr Med Res Opin 2006;22:1765-76.
- Humbert M, Beasley R, Ayres J, Slavin R, Hebert J, Bousquet J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy 2005;60:309-16.
- Watson L, Turk F, James P, Holgate ST. Factors associated with mortality after an asthma admission: a national United Kingdom database analysis. Respir Med 2007;101:1659-64.
- Lloyd A, Price D, Brown R. The impact of asthma exacerbations on health-related quality of life in moderate to severe asthma patients in the UK. Prim Care Respir J 2007;16:22-7.
- Kyle S, Chandler D, Griffiths CE, Helliwell P, Lewis J, McInnes I, et al. Guideline for anti-TNF-alpha therapy in psoriatic arthritis. Rheumatology 2005;44:390-7.
- Symmons DP, Barrett EM, Bankhead CR, Scott DG, Silman AJ. The incidence of rheumatoid arthritis in the United Kingdom: results from the Norfolk Arthritis Register. Rheumatology 1994;33:735-9.
- Mease PJ, Goffe BS, Metz J, VanderStoep A, Finck B, Burge DJ. Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trial. Lancet 2000;356:385-90.
- Mease PJ, Kivitz AJ, Burch FX, Siegel EL, Cohen SB, Ory P, et al. Etanercept treatment of psoriatic arthritis: safety, efficacy, and effect on disease progression. Arthritis Rheum 2004;50:2264-72.
- Mease PJ, Kivitz AJ, Burch FX, Siegel EL, Cohen SB, Ory P, et al. Continued inhibition of radiographic progression in patients with psoriatic arthritis following 2 years of treatment with etanercept. J Rheumatol 2006;33:712-21.
- Fleischmann R, Baumgartner SW, Weisman MH, Liu T, White B, Peloso P. Long term safety of etanercept in elderly subjects with rheumatic diseases. Ann Rheum Dis 2006;65:379-84.
- Lebwohl M, Gottlieb A, Goffe BS, Jahreis A. Etanercept in psoriatic arthritis: sustained improvement in skin and joint disease and inhibition of radiographic progression at 2 years. J Am Acad Dermatol 2005;52.
- Wanke LA, Mease PJ, Gottlieb AB. Sustained improvement in functional status and vitality of psoriatic arthritis patients treated with etanercept. J Invest Dermatol 2004;122.
- Mease PJ, Woolley JM, Singh A, Tsuji W, Dunn M, Chiou CF. Patient-reported outcomes in a randomized comparison of etanercept and placebo for treatment of psoriatic arthritis. Ann Rheum Dis 2006;65.
- Centocor . A Multicenter Placebo-Controlled, Double-Blind, Randomised Study of Anti-TNF Chimeric Monoclonal Antibody (cA2, Infliximab) in Patients With Active Psoriatic Arthritis (IMPACT): Protocol No. P02114 2003.
- Schering-Plough Ltd . Remicade in the Treatment of Psoriatic Arthritis in the United Kingdom: A Submission to the National Institute for Clinical Excellence 2004.
- Antoni CE, Kavanaugh A, Kirkham B, Tutuncu Z, Burmester GR, Schneider U, et al. Sustained benefits of infliximab therapy for dermatologic and articular manifestations of psoriatic arthritis: results from the infliximab multinational psoriatic arthritis controlled trial (IMPACT). Arthritis Rheum 2005;52:1227-36.
- Antoni CE, Kavanaugh A, van der Heijde D, Beutler A, Keenan G, Zhou B, et al. Two-year efficacy and safety of infliximab treatment in patients with active psoriatic arthritis: findings of the Infliximab Multinational Psoriatic Arthritis Controlled Trial (IMPACT). J Rheumatol 2008;35:869-76.
- Kavanaugh A, Antoni CE, Gladman D, Wassenberg S, Zhou B, Beutler A, et al. The Infliximab Multinational Psoriatic Arthritis Controlled Trial (IMPACT): results of radiographic analyses after 1 year. Ann Rheum Dis 2006;65:1038-43.
- Kavanaugh A, Kruegar GG, Birbara C, Halter D, de Vlam K, Zhou B, et al. Treatment with infliximab is associated with ‘major clinical response’ in psoriatic arthritis patients treated with infliximab: analysis of two double-blind placebo controlled trials. Arthritis Rheum 2005;52.
- Kavanaugh A, Kruegar GG, Birbara C, Halter D, Geusens P, de Vlam K, et al. Treatment with infliximab is associated with ‘major clinical response’ in psoriatic arthritis patients treated with infliximab: analysis of two double-blind placebo controlled trials. J Invest Dermatol 2007;127.
- Antoni CE, Kavanaugh A, Kirkham B, Tutuncu ZN, Burmester G, Schneider U, et al. 2-year data: infliximab maintains clinical response in psoriatic arthritis patients – data from the Infliximab Multinational Psoriatic Arthritis Controlled Trial (IMPACT). Arthritis Rheum 2005;52.
- Antoni CE, Kavanaugh A, Gladman D, Wassenberg S, Zhou B, Beutler A, et al. The infliximab multinational psoriatic arthritis controlled trial (IMPACT): results of radiographic analyses after 1 year. Ann Rheum Dis 2005;64.
- Antoni CE, Kavanaugh A, Kirkham B, Burmester G, Manger B, Tutuncu Z, et al. The infliximab multinational psoriatic arthritis controlled trial (IMPACT): substantial efficacy on synovitis and psoriatic lesions with or without concomitant DMARD therapy. Ann Rheum Dis 2003;62.
- Antoni CE, Kavanaugh A, Kirkham B, Burmester G, Manger B, Schneider U, et al. The infliximab multinational psoriatic arthritis controlled trial (IMPACT1): the concomitant use of DMARDs does not influence the efficacy and safety of infliximab over a one year period. Ann Rheum Dis 2004;63:411-12.
- Antoni C, Krueger G, de Vlam K, Birbara C, Beutler A, Guzzo C, et al. Infliximab improves signs and symptoms of psoriatic arthritis: results of the IMPACT 2 trial. Ann Rheum Dis 2005;64:1150-7.
- van der Heijde D, Kavanaugh A, Gladman DD, Antoni C, Krueger GG, Guzzo C, et al. Infliximab inhibits progression of radiographic damage in patients with active psoriatic arthritis through one year of treatment: results from the induction and maintenance psoriatic arthritis clinical trial 2. Arthritis Rheum 2007;56:2698-707.
- Kavanaugh A, Krueger GG, Beutler A, Guzzo C, Zhou B, Dooley LT, et al. Infliximab maintains a high degree of clinical response in patients with active psoriatic arthritis through 1 year of treatment: results from the IMPACT 2 trial. Ann Rheum Dis 2007;66:498-505.
- Kavanaugh A, Antoni C, Mease P, Gladman D, Yan S, Bala M, et al. Effect of infliximab therapy on employment, time lost from work, and productivity in patients with psoriatic arthritis. J Rheumatol 2006;33:2254-9.
- Kavanaugh A, Antoni C, Krueger GG, Yan S, Bala M, Dooley LT, et al. Infliximab improves health related quality of life and physical function in patients with psoriatic arthritis. Ann Rheum Dis 2006;65:471-7.
- Papp K, Menter A, Antoni C, Kavanaugh A, Gottlieb AB, Paulin Y, et al. Rapid and persistent improvement of psoriasis in patients with active psoriatic arthritis treated with infliximab: 24-week results of the impact 2 trial. J Eur Acad Dermatol Venereol 2004;18.
- Kavanauagh A, Krueger GG, De Vlam K, Birbara C, Beutler A, Guzzo C, et al. Infliximab significantly improves joint and skin involvement in psoriatic arthritis to a substantial extent and irrespective of baseline joint involvement or MTX use: analysis of clinical response from the IMPACT2 trial. Arthritis Rheum 2004;50.
- Mease PJ, Gladman DD, Ritchlin CT, Ruderman EM, Steinfeld SD, Choy EH, et al. Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum 2005;52:3279-89.
- Mease PJ, Ory P, Sharp JT, Ritchlin CT, Van den Bosch F, Wellborne F, et al. Adalimumab for long-term treatment of psoriatic arthritis: 2-year data from the Adalimumab Effectiveness in Psoriatic Arthritis Trial (ADEPT). Ann Rheum Dis 2009;68:702-9.
- Gladman DD, Mease PJ, Ritchlin CT, Choy EH, Sharp JT, Ory PA, et al. Adalimumab for long-term treatment of psoriatic arthritis: forty-eight week data from the adalimumab effectiveness in psoriatic arthritis trial. Arthritis Rheum 2007;56:476-88.
- Gladman DD, Mease PJ, Cifaldi MA, Perdok RJ, Sasso E, Medich J. Adalimumab improves joint-related and skin-related functional impairment in patients with psoriatic arthritis: patient-reported outcomes of the Adalimumab Effectiveness in Psoriatic Arthritis Trial. Ann Rheum Dis 2007;66:163-8.
- Choy E, Gladman D, Sasso E. Efficacy of adalimumab by disease duration in psoriatic arthritis: subanalysis of ADEPT. J Am Acad Dermatol 2007;56.
- Gladman D, Mease P, Ritchlin C, Okun M. PASI-100 is associated with better dermatology-specific patient-reported outcomes compared with PASI-75e99 in adalimumab-treated patients with psoriatic arthritis: subanalysis of ADEPT. J Am Acad Dermatol 2007;56.
- Gladman D, Mease P, Kavanaugh A, Weinberg M. Adalimumab is efficacious in treating skin disease in psoriatic arthritis: subanalysis of moderate versus severe psoriasis in the ADEPT trial. J Am Acad Dermatol 2006;54:AB10-11.
- Mease P, Gladman D, Ritchlin C, Weinberg M. Clinical efficacy, safety and inhibition of joint destruction of adalimumab in the treatment of moderate to severe psoriatic arthritis: 48-week results of the ADEPT trial. J Am Acad Dermatol 2006;54.
- Mease P, Sharp J, Ory P, Gladman D, Ritchlin C, Choy E, et al. Adalimumab treatment effects on radiographic progression of joint disease in patients with psoriatic arthritis: results from ADEPT. J Eur Acad Dermatol Venereol 2005;19.
- Genovese MC, Mease PJ, Thomson GT, Kivitz AJ, Perdok RJ, Weinberg MA, et al. Safety and efficacy of adalimumab in treatment of patients with psoriatic arthritis who had failed disease modifying antirheumatic drug therapy. J Rheumatol 2007;34:1040-50.
- Sutton A, Ades AE, Cooper N, Abrams K. Use of indirect and mixed treatment comparisons for technology assessment. Pharmacoeconomics 2008;26:753-67.
- Rodgers M, Epstein D, Bojke L, Yang H, Craig D, Fonseca T, et al. Etanercept, infliximab and adalimumab for the treatment of psoriatic arthritis: a systematic review and economic evaluation. Health Technol Assess 2011;15.
- Kobelt G, Jönsson L, Lindgren P, Young A, Eberhardt K. Modelling the progression of rheumatoid arthritis: a two-country model to estimate costs to estimate costs and consequences of rheumatoid arthritis. Arthritis Rheum 2002;46:2310-19.
- Department of Health . Reference Costs 2007–2008 2009. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyandGuidance/DH_09895.
- Symmons DP, Silman AJ. The Norfolk Arthritis Register (NOAR). Clin Exp Rheumtol 2003;21:S94-9.
- Yang H, Epstein D, Bojke L, Craig D, Light K, Bruce I, et al. Evidence Review group’s Report: Golimumab for the Treatment of Psoriatic Arthritis. 2010.
- Brennan A, Kharroubi S, O’Hagan A, Chilcott J. Calculating partial expected value of perfect information via Monte Carlo sampling algorithms. Med Decis Making 2007;27:448-70.
Appendix 1 Literature review, searches and results
Initial set of key papers
Policy papers
-
1. Cooksey D. A review of UK health research funding. London: The Stationery Office; 2006.
-
2. Centers for Medicare & Medicaid Services. National coverage determinations with data collection as a condition of coverage: coverage with evidence development. 2006. URL: www.cms.gov/Medicare/Coverage/Coverage-with-Evidence-Development/index.html.
-
3. House of Commons Health Committee, National Institute for Health and Clinical Excellence. Report no. HC 27–1. London: The Stationary Office; 2008. Chapter 6.
-
4. Mechanic R, Zinner D. Optimizing information under coverage with evidence development. Health Industry Forum Policy Brief. 24 October 2007. URL: http://healthforum.brandeis.edu/meetings/materials/2007-24-Oct./Policy-Brief.pdf.
-
5. NICE Citizens Council. Only in research. London: NICE; 2007.
-
6. NHS Quality Improvement Scotland. Coverage with evidence development in NHS Scotland. Discussion paper. 2008. URL: www.Healthcareimprovementscotland.org/previous_resources/hta_report/evidence_development.aspx.
-
7. Office of Fair Trading. The pharmaceutical price regulation scheme. An OFT market study. London: OFT; 2007. Chapters 4 and 5.
-
8. Office for Life Sciences, HM Government. Life sciences blueprint. A statement from the Office for Life Sciences. July 2009. URL: www.medilink.co.uk/Libraries/connect/Life-sciences-2009-blueprint.sflb.ashx.
Discussion and application papers
-
9. Medicare’s coverage with evidence development: a policy-making tool in evolution. J Oncol Pract 2007;3:296–301.
-
10. Briggs A, Ritchie K, Fenwick E, Chalkidou K, Littlejohns P. Access with evidence development in the UK: past experience, current initiatives and future potential. Pharmacoeconomics 2010;28:163–70.
-
11. Carino T, Sheingold S, Tunis S. Using clinical trials as a condition of coverage: lessons from the National Emphysema Treatment Trial. Clin Trials 2004;1:108–21.
-
12. Chalmers R. Addressing uncertainties about the effects of treatments offered to NHS patients: whose responsibility? J R Soc Med 2007;100:440–1
-
13. Chalkidou K. ‘Only in research’: a polite ‘no’ or a valuable policy option? Birmingham: NICE; 2006.
-
14. Chalkidou K, Hoy A, Littlejohns P. Making a decision to wait for more evidence: when the National Institute for Health and Clinical Excellence recommends a technology only in the context of research. J R Soc Med 2007;100:453–60.
-
15. Chalkidou K, Lord J, Fischer A, Littlejohns P. Evidence-based decision making: when should we wait for more information? Health Aff 2008;27:1642–53.
-
16. Dhalla IF, Garner S. Perspectives on the National Institute for Health and Clinical Excellence’s recommendations to use technologies only in research. Int J Technol Assess Health Care 2009;25:272–80.
-
17. Garrison L, Sullivan S, Carlson J, Bresnahan B, Carlson R, Neumann P, et al. Performance-based risk-sharing reimbursement agreements for new medical products. Washington, DC: University of Washington; 2008.
-
18. Kary W, Bergthold L, Talbott M, Aubry W. Coverage for evidence development: a conceptual frame. Baltimore, MD: Center for Medical Technology Policy; 2009.
-
19. Macdonald F. Coverage with evidence development: a personal, industry view. Presentation slides. 19 September 2008. URL: www.docstoc.com/docs/105704904/Coverage-With-Evidence-Development.
-
20. McCabe CJ, Stafinski T, Edlin R, Menon D. Access with evidence development schemes: a framework for description and evaluation. Pharmacoeconomics 2010;28:143–52.
-
21. Menon D, McCabe CJ, Stafinski T, Edlin R, signatories to the Consensus Statement. Principles of design of access with evidence development approaches: a consensus statement from the Banff Summit. Pharmacoeconomics 2010;28:109–11.
-
22. Miller FG, Pearson SD. Coverage with evidence development: ethical issues and policy implications. Med Care 2008;46:746–51.
-
23. Mohr P, Tunis SR. Access with evidence development: the US experience. Pharmacoeconomics 2010;28:153–62.
-
24. Hutton J, Trueman P, Henshall C. Coverage with evidence development: an examination of conceptual and policy issues. Int J Technol Assess Health Care 2007;23:425–35.
-
25. Pearson SD, Miller FG, Emanuel EJ. Medicare’s requirement for research participation as a condition of coverage: is it ethical? JAMA 2006;296:988–91.
-
26. Stafinski T, McCabe C, Menon D. Funding the unfundable: mechanisms for managing uncertainty in decisions on the introduction of new and innovative technologies into healthcare systems. Pharmacoeconomics 2010;28:113–42.
-
27. Towse A, Garrison LP. Can’t get no satisfaction? Will pay for performance help?: toward an economic framework for understanding performance-based risk-sharing agreements for innovative medical products. Pharmacoeconomics 2010;28:93–102.
-
28. Trueman P. Coverage with evidence development: applications and issues. Int J Technol Assess Health Care 2010;26:79–85.
-
29. Tunis SR, Pearson SD. Coverage options for promising technologies: Medicare’s ‘coverage with evidence development’. Health Aff 2006;25:1218–30.
-
30. Tunis SR, Chalkidou K. Coverage with evidence development: a very good beginning, but much to be done. Commentary to Hutton et al. Int J Technol Assess Health Care 2007;23:432–5.
-
31. Tunis S, Whicher D. The National Oncologic PET Registry: lessons learned for coverage with evidence development. J Am Coll Radiol 2009;6:360–5.
Search strategies
Traditional literature searches
Scoping search
Ovid MEDLINE(R) –1996 to week 2 February 2010
Searched via Ovid, 18 February 2010.
Medical subject heading (MeSH) terms:
-
1. Health Policy/ (24,246)
-
2. Decision Making/ (32,178)
-
3. exp policy making/ (10,169)
-
4. Insurance Coverage/ (5950)
-
5. Insurance, Health/ (8791)
-
6. Insurance, Health, Reimbursement/ (3860)
-
7. Insurance Benefits/ (1246)
-
8. Evidence-Based Medicine/ (35,935)
-
9. uncertainty/ (2644)
-
10. Medicare/ (14,219)
-
11. Reimbursement Mechanisms/ (4658)
-
12. Technology Assessment, Biomedical/ (4437)
-
13. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 (132,518)
Key terms:
-
14. ((access$ or cover$ or approv$ or reimburs$ or fund$ or licens$ or licenc$ or list$) adj (with or in) adj (evidence or condition$)).ti,ab. (30)
-
15. “only in research”.ti,ab. (26)
-
16. “only with research”.ti,ab. (2)
-
17. “in the context of research”.ti,ab. (125)
-
18. “condition$ of coverage$”.ti,ab. (57)
-
19. “condition$ of reimburse$”.ti,ab. (13)
-
20. 14 or 15 or 16 or 17 or 18 or 19 (251)
Words near to words terms:
-
21. ((interim or condition$ or restrict$ or evidence) adj2 (fund$ or reimburse$ or cover$ or approv$ or list$ or access$ or licens$ or licenc$)).ti,ab. (3602)
-
22. 13 and 20 (82)
-
23. limit 22 to yr = “1999 - 2010” (77)
-
24. limit 20 to yr = “1999 - 2010” (223)
-
25. 13 and 21 (443)
-
26. limit 25 to yr = “1999 - 2010” (404)
-
27. limit 21 to yr = “1999 - 2010” (3082)
Results were then deduplicated so that only additional records not found by the preceding search were added to the library.
| Search | Results | Results after deduplication |
|---|---|---|
| Key terms AND MeSH | 77 | 77 |
| Key terms only | 223 | 146 |
| near to terms AND MeSH | 404 | 341 |
| near to terms only | 3082 | 2648 |
Results were reviewed and the final strategy adopted was to use only the ‘Key terms’ searches, with several additions. The ‘near to terms’ retrieved few relevant records and additional key terms were added to ensure that these were captured. The relatively low number of results meant that combining ‘Key terms’ with MeSH terms to reduce the results set was not necessary.
Final search strategies
Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) –1950 to present
Searched via Ovid, 24 February 2010.
-
((access$ or cover$ or approv$ or reimburs$ or fund$ or licens$ or licenc$ or list$) adj (with or in) adj (evidence or condition$)).ti,ab. (45)
-
“only in research”.ti,ab. (43)
-
“only with research”.ti,ab. (2)
-
“in the context of research”.ti,ab. (158)
-
“condition$ of coverage$”.ti,ab. (82)
-
“condition$ of reimburse$”.ti,ab. (19)
-
(condition$ adj fund$).ti,ab. (21)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 (365)
-
limit 8 to yr = “1999 - 2010” (258)
EMBASE –1980 to week 7 2010
Searched via Ovid, 24 February 2010.
-
((access$ or cover$ or approv$ or reimburs$ or fund$ or licens$ or licenc$ or list$) adj (with or in) adj (evidence or condition$)).ti,ab. (36)
-
“only in research”.ti,ab. (38)
-
“only with research”.ti,ab. (1)
-
“in the context of research”.ti,ab. (108)
-
“condition$ of coverage$”.ti,ab. (39)
-
“condition$ of reimburse$”.ti,ab. (13)
-
(condition$ adj fund$).ti,ab. (20)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 (252)
-
limit 8 to yr = “1999 - 2010” (182)
The Cochrane Library [Cochrane Database of Systematic Reviews (CDSR), Database of Abstracts of Reviews of Effects (DARE), HTA database, NHS Economic Evaluation Database (NHS EED)]
Searched via www3.interscience.wiley.com/tools/citex, 24 February 2010.
-
#1 ((access* or cover* or approv* or reimburs* or fund* or licens* or licenc* or list*) NEXT (with or in) NEXT (evidence or condition$)) (1)
-
#2 “only in research” (1)
-
#3 “only with research” (0)
-
#4 “in the context of research” (4)
-
#5 “condition* of coverage*” (0)
-
#6 “condition* of reimburse*” (0)
-
#7 condition* NEXT fund* (22)
-
#8 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7), from 1999 to 2010 (22)
EconLit –1969 to February 2010
Searched via Ovid, 24 February 2010.
-
((access$ or cover$ or approv$ or reimburs$ or fund$ or licens$ or licenc$ or list$) adj (with or in) adj (evidence or condition$)).ti,ab. (0)
-
“only in research”.ti,ab. (0)
-
“only with research”.ti,ab. (0)
-
“in the context of research”.ti,ab. (15)
-
“condition$ of coverage$”.ti,ab. (12)
-
“condition$ of reimburse$”.ti,ab. (4)
-
(condition$ adj fund$).ti,ab. (7)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 (38)
-
limit 8 to yr = “1999 - 2010” (37)
Cumulative Index to Nursing and Allied Health Literature
Searched via EBSCO, 24 February 2010.
Results limited to 1999 onwards.
-
TX “access* with evidence” or TX “coverage* with evidence” or TX “approv* with evidence” or TX “reimburs* with evidence” or TX “fund* with evidence” or TX “licenc* with evidence” or TX “licens* with evidence” and TX “list* with evidence”
-
TX “access* in evidence” or TX “coverage* in evidence” or TX “approv* in evidence” or TX “reimburs* in evidence” or TX “fund* in evidence” or TX “licenc* in evidence” or TX “licens* in evidence” and TX “list* in evidence”
-
TX “access* with condition*” or TX “coverage* with condition*” or TX “approv* with condition*” or TX “reimburs* with condition*” or TX “fund* with condition*” or TX “licenc* with condition*” or TX “licens* with condition*” and TX “list* with condition*”
-
TX “access* in condition*” or TX “coverage* in condition*” or TX “approv* in condition*” or TX “reimburs* in condition*” or TX “fund* in condition*” or TX “licenc* in condition*” or TX “licens* in condition*” and TX “list* in condition*”
-
TX “only in research”
-
TX “only with research”
-
TX “in the context of research”
-
TX “condition* of coverage*”
-
TX “condition* of reimburse*”
-
TX “condition* fund*”
-
1 or 2 or 3 or 3 or 5 or 6 or 7 or 8 or 9 or 10 (378)
Grey literature searches
New York Academy of Medicine Grey Literature Report
Searched via www.nyam.org/library/pages/grey_literature_report, 8 March 2010.
““ti,phr: access with evidence or ti,phr: coverage with evidence or ti,phr: approval with evidence or ti,phr: approved with evidence or ti,phr: reimbursement with evidence or ti,phr: reimbursed with evidence or ti,phr: reimburse with evidence or ti,phr: funding with evidence or ti,phr: funded with evidence or ti,phr: licenced with evidence or ti,phr: licensed with evidence or ti,phr: licensing with evidence or ti,phr: licencing with evidence or ti,phr: licence with evidence or ti,phr: license with evidence or ti,phr: list with evidence or ti,phr: lists with evidence or ti,phr: listed with evidence or ti,phr: access in evidence or ti,phr: coverage in evidence or ti,phr: approval in evidence or ti,phr: reimbursement in evidence or ti,phr: reimbursed in evidence or ti,phr: reimburse in evidence or ti,phr: funding in evidence or ti,phr: funded in evidence or ti,phr: licenced in evidence or ti,phr: licensed in evidence or ti,phr: licensing in evidence or ti,phr: licencing in evidence or ti,phr: licence in evidence or ti,phr: license in evidence or ti,phr: list in evidence or ti,phr: lists in evidence or ti,phr: listed in evidence mc-ccode:GREYLIT” ”
“ti,phr: access with conditions or ti,phr: coverage with conditions or ti,phr: approval with conditions or ti,phr: approved with conditions or ti,phr: reimbursement with conditions or ti,phr: reimbursed with conditions or ti,phr: reimburse with conditions or ti,phr: funding with conditions or ti,phr: funded with conditions or ti,phr: licenced with conditions or ti,phr: licensed with conditions or ti,phr: licensing with conditions or ti,phr: licencing with conditions or ti,phr: licence with conditions or ti,phr: license with conditions or ti,phr: list with conditions or ti,phr: lists with conditions or ti,phr: listed with conditions mc-ccode:GREYLIT”
““ti,phr: access in conditions or ti,phr: coverage in conditions or ti,phr: approval in conditions or ti,phr: approved in conditions or ti,phr: reimbursement in conditions or ti,phr: reimbursed in conditions or ti,phr: reimburse in conditions or ti,phr: funding in conditions or ti,phr: funded in conditions or ti,phr: licenced in conditions or ti,phr: licensed in conditions or ti,phr: licensing in conditions or ti,phr: licencing in conditions or ti,phr: licence in conditions or ti,phr: license in conditions or ti,phr: list in conditions or ti,phr: lists in conditions or ti,phr: listed in conditions mc-ccode:GREYLIT” ”
“ti,phr: only in research or ti,phr: only with research or ti,phr: OIR mc-ccode:GREYLIT”
“ti,phr: in the context of research or ti,phr: condition of coverage or ti,phr: conditions of coverage or ti,phr: condition of reimbursement or ti,phr: conditions of reimbursement or ti,phr: conditional funding or ti,phr: conditionally funded or ti,phr: conditional funds mc-ccode:GREYLIT”
IDEAS – University of Connecticut Department of Economics
Searched via http://ideas.repec.org/search.html, 9 March 2010.
Phrase searching restricted to papers, chapters, books and software components, for following phrases:
-
access with evidence
-
coverage with evidence
-
approval with evidence
-
approved with evidence
-
reimbursement with evidence
-
reimbursed with evidence
-
reimburse with evidence
-
funding with evidence
-
funded with evidence
-
licenced with evidence
-
licensed with evidence
-
licensing with evidence
-
licencing with evidence
-
licence with evidence
-
license with evidence
-
list with evidence
-
lists with evidence
-
listed with evidence
-
access in evidence
-
coverage in evidence
-
approval in evidence
-
approved in evidence
-
reimbursement in evidence
-
reimbursed in evidence
-
reimburse in evidence
-
funding in evidence
-
funded in evidence
-
licenced in evidence
-
licensed in evidence
-
licensing in evidence
-
licencing in evidence
-
licence in evidence
-
license in evidence
-
list in evidence
-
lists in evidence
-
listed in evidence
-
access with conditions
-
coverage with conditions
-
approval with conditions
-
approved with conditions
-
reimbursement with conditions
-
reimbursed with conditions
-
reimburse with conditions
-
funding with conditions
-
funded with conditions
-
licenced with conditions
-
licensed with conditions
-
licensing with conditions
-
licencing with conditions
-
licence with conditions
-
license with conditions
-
list with conditions
-
lists with conditions
-
listed with conditions
-
access in conditions
-
coverage in conditions
-
approval in conditions
-
approved in conditions
-
reimbursement in conditions
-
reimbursed in conditions
-
reimburse in conditions
-
funding in conditions
-
funded in conditions
-
licenced in conditions
-
licensed in conditions
-
licensing in conditions
-
licencing in conditions
-
licence in conditions
-
license in conditions
-
list in conditions
-
lists in conditions
-
listed in conditions
-
in the context of research
-
condition of coverage
-
conditions of coverage
-
condition of reimbursement
-
conditions of reimbursement
-
conditional funding
-
conditionally funded
-
conditional funds
Note: the phrases ‘only in research’ and ‘only with research’ were not searchable as ‘only’, ‘in’ and ‘with’ are all stopwords and stopwords are applied even when phrase searching is selected. Search therefore searches for ‘research’ only and retrieves over 24,000 records.
In total, 64 results were saved to MRC OIR Project/Literature Search/IDEAS results.docx. These were screened and none was found to be relevant.
Results
| Database | Results | Results after deduplication |
|---|---|---|
| MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations | 258 | 253 |
| EMBASE | 182 | 50 |
| CDSR | 2 | 2 |
| DARE | 7 | 7 |
| HTA | 2 | 2 |
| NHS EED | 9 | 9 |
| EconLit | 37 | 35 |
| CINAHL | 378 | 317 |
| New York Academy of Medicine | 0 | 0 |
| IDEAS | 0 | 0 |
| Total | 875 | 675 |
Websites searched for policy documents
| Organisation | Web link | Search terms |
|---|---|---|
| UK sources | ||
| Department of Health | www.dh.gov.uk/en/Publicationsandstatistics/index.htm |
Coverage with evidence Only in research Free scan of website |
| NICE | Via www.evidence.nhs.uk |
Coverage with evidence Only in research Free scan of website |
| HM Treasury | http://archive.treasury.gov.uk/ |
Free scan of subject headings – linked to news releases Free scan of current news releases and reports |
| House of Commons Health Committee | www.parliament.uk/parliamentary_committees/health_committee/health_committee_reports_and_publications.cfm | Free scan of the reports and publication web pages |
| NICE Citizens Council | www.nice.org.uk/aboutnice/howwework/citizenscouncil/reports.jsp | All of the available reports (13) were scanned |
| Office of Fair Trading | www.oft.gov.uk/advice_and_resources/publications/ | Free scan of reports section of publications |
| Office for Life Sciences | www.bis.gov.uk/publications |
Only in research Coverage with evidence Free scan by subject |
| NHS Scotland and NHS Quality Improvement Scotland |
Only in research Coverage with evidence Free scan of publications |
|
| Health Industry Forum | http://healthforum.brandeis.edu/publications/index.html | Free scan of publications list |
| Department for Business, Innovation & Skills | www.berr.gov.uk/publications/reports/index.html |
Only in research Coverage with evidence Managed entry Also free scan by subject |
| Non-UK sources | ||
| Centers for Medicare & Medicaid | www.cms.hhs.gov/home/rsds.asp | Free scan of the Research, Statistics, Data & Systems web pages |
| Center for Medical Technology Policy | www.cmtpnet.org/recent-articles | Free scan of publications list |
| Organisation for Economic Co-operation and Development (OECD) | www.oecd.org/publications/0,3353,en_2649_201185_1_1_1_1_1,00.html |
Only in research Coverage with evidence Free scan by ‘Health’ and ‘Economics’ subjects |
| European network for Health Technology Assessment (EUnetHTA) | www.eunethta.eu | Free scan of website and publications section |
Summary of search results
Table 27 summarises the total number of references identified, the number identified for full screening and the final number from each source included in the review. From the total number of references retrieved by the searches, potentially relevant references were initially identified by screening titles and abstracts. Full copies of all of these references were then obtained and subjected to full screening. References were included in the review if they explicitly discussed any of the following issues relating to OIR/AWR: terminology, taxonomy, general themes and/or principles including evidence collection, investment and reversal costs, changing prices and ethical considerations. In addition, case study applications were included if any of the above issues were discussed in detail as part of the application or interpretation of the results.
| Source | Total no. of references retrieved | No. of relevant references (full screening) | No. of references included in the review |
|---|---|---|---|
| Original list of key references | 31 | ||
| Traditional literature search | 675 | 56 | 27 (18 of which already in original key paper list) |
| Grey literature search | 64 | 0 | 0 |
| Citation search | 122 | 16 | 7 (2 of which already identified from original key paper list) |
| Policy websites | 280 | 14 | 11 (9 of which already identified from original key paper list and systematic searches) |
| Advisory Group references | 7 | 5 | 5 (4 of which are already in original key paper list) |
| Other sources |
HTAi interest group = 5 PATH interest group = 4 Stafinski et al. review8 = 68 |
HTAi interest group = 5 PATH interest group = 4 Stafinski et al. review = 4 |
HTAi interest group = 3 PATH interest group = 4 Stafinski et al. review8 = 2 |
Of the 675 references identified using the traditional systematic search, full papers were obtained for only 56 despite a relatively inclusive approach being adopted (any mention of OIR/AWR or related terms in the titles or abstracts). Of these, 27 references were eventually included as part of the review (18 of which had been previously identified as part of the initial set of key papers used for the citation searches). Although this is fewer than the number of references identified in the traditional literature in the Stafinski et al. review8 (n = 68), it simply reflects the fact that the literature discussing general principles and themes relevant to OIR/AWR is considerably smaller than the literature reporting specific applications and case studies.
In total, 64 potentially relevant references were identified from the grey literature searches. However, after full screening, none of these references was included in the final review. The absence of grey literature discussing general principles and themes contrasts with the findings from the Stafinski review8 in which the majority of case studies were located from grey literature sources. Although this difference could be due to the more limited range of grey literature databases searched here, it seems more likely that the reporting format of case study applications (typically in the form of presentation slides) means that these would be identified only through grey literature searches. In addition, it should be noted that several references from the grey literature were already identified from searches of other sources through the HTAi interest group, PATH website and additional Advisory Group suggestions.
The citation searches identified 122 potentially relevant references. After final screening, five additional references were deemed relevant for inclusion in the review. From the remainder of the searches undertaken, a further two references were identified from the policy websites51,56 (Table 28), one from the Advisory Group suggestions, four from the PATH interest group and three from the HTAi interest group. In addition, by cross-referencing with the results from the Stafinski et al. paper,8 four additional references were identified, two of which were journal articles not previously identified in the traditional systematic search. Table 29 gives the full list of included references and areas covered.
| Organisation | Potentially relevant references | Included references |
|---|---|---|
| UK based | ||
| NICE |
‘Coverage with evidence’ = 196 under NICE and economics subsections ‘Only in research’ = 45 under NICE subsection |
1 |
| NICE Citizens Council | 1 | 1 (already included in list of key papers) |
| Department of Health | 0 | 0 |
| HM Treasury | 0 | 0 |
| House of Commons Health Committee | 3 | 1 (already included in list of key papers) |
| Health Industry Forum | 5 | 2 (1 of which already included in list of key papers) |
| Office of Fair Trading | 0 | 0 |
| Office for Life Sciences | 1 | 1 (already included in list of key papers) |
| NHS Scotland and NHS Quality Improvement Scotland | 0 | 0 |
| Department for Business, Innovation & Skills |
Search terms: ‘only in research’ and ‘evidence’ = 19 ‘Coverage evidence’ = 0 Managed entry = 0 Free scan = 1 |
0 |
| Non-UK based | ||
| Centers for Medicare & Medicaid | 1 | 1 (already included in list of key papers) |
| Center for Medical Technology Policy | 7 | 7 (all of which already identified from key papers and traditional searches) |
| Organisation for Economic Co-operation and Development (OECD) | 1 | 0 |
| European network for Health Technology Assessment (EUnetHTA) | 0 | 0 |
| Reference | Terminology and taxonomy | General themes | Specific issues | Examples(s) of application | |||
|---|---|---|---|---|---|---|---|
| Evidence collection | Investment and reversal costs | Changing price of technology | Ethical issues/social value judgements | ||||
| Journal articles | |||||||
| 1. Anon 200731 | ✓ | ✓ | ✓ | ✓ | |||
| 2. Bowen 200917 | ✓ | ✓ | ✓ | ✓ | |||
| 3. Breckenridge 200836 | ✓ | ✓ | |||||
| 4. Brezis 200613 | ✓ | ✓ | |||||
| 5. Brice 200835 | ✓ | ✓ | |||||
| 6. Briggs 201018 | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| 7. Carbonneil 200919 | ✓ | ✓ | |||||
| 8. Carino 200437 | ✓ | ✓ | |||||
| 9. Carino 200634 | ✓ | ✓ | ✓ | ||||
| 10. Carlson 201025 | ✓ | ✓ | ✓ | ||||
| 11. Chalkidou 20073 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| 12. Chalkidou 200829 | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| 13. Chalmers 200726 | ✓ | ✓ | ✓ | ||||
| 14. Dhalla 200938 | ✓ | ✓ | ✓ | ✓ | |||
| 15. Gafni 200714 | ✓ | ✓ | ✓ | ||||
| 16. Garber 200839 | ✓ | ✓ | |||||
| 17. Goeree 200620 | ✓ | ||||||
| 18. Goeree 200715 | ✓ | ✓ | |||||
| 19. Groeneveld 200616 | ✓ | ||||||
| 20. Hutton 20074 | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| 21. Kamerow 200732 | ✓ | ✓ | |||||
| 22. Krich 200740 | ✓ | ✓ | ✓ | ||||
| 23. Lindsay 200741 | ✓ | ✓ | ✓ | ✓ | |||
| 24. Macdonald 200842 | ✓ | ✓ | ✓ | ✓ | |||
| 25. McCabe 201021 | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| 26. Menon 201043 | ✓ | ✓ | |||||
| 27. Miller 200622 | ✓ | ✓ | ✓ | ✓ | |||
| 28. Mohr 201033 | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| 29. Neumann 200844 | ✓ | ✓ | ✓ | ||||
| 30. Niezen 200745 | ✓ | ||||||
| 31. Pearson 200646 | ✓ | ✓ | ✓ | ||||
| 32. Ramsey 200527 | ✓ | ✓ | |||||
| 33. Reed 200847 | ✓ | ✓ | ✓ | ✓ | |||
| 34. Stafinski 20108 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| 35. Straube 200548 | ✓ | ✓ | ✓ | ||||
| 36. Thornton 200749 | ✓ | ✓ | ✓ | ✓ | |||
| 37. Towse 201023 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| 38. Trueman 201024 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| 39. Tunis 20062 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| 40. Tunis 20075 | ✓ | ✓ | ✓ | ||||
| 41. Tunis 200930 | ✓ | ✓ | |||||
| 42. Wallner 200828 | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| 43. Whalley 200450 | ✓ | ||||||
| Policy documents | |||||||
| 44. Centers for Medicare & Medicaid Services 200655 | ✓ | ✓ | ✓ | ||||
| 45. Cooksey 20069 | ✓ | ✓ | ✓ | ||||
| 46. Department of Health 199952 | ✓ | ✓ | |||||
| 47. House of Commons Health Committee 200510 | ✓ | ✓ | ✓ | ✓ | |||
| 48. Mechanic 200756 | ✓ | ✓ | ✓ | ||||
| 49. Miller 200622 | ✓ | ✓ | ✓ | ||||
| 50. NHS Quality Improvement Scotland 200853 | ✓ | ✓ | ✓ | ✓ | |||
| 51. NICE and AHRQ 200851 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| 52. NICE Citizens Council 200711 | ✓ | ✓ | ✓ | ✓ | |||
| 53. Office for Life Sciences 200954 | ✓ | ✓ | |||||
| 54. Office of Fair Trading 200712 | ✓ | ✓ | ✓ | ||||
| Other sources | |||||||
| 55. Chalkidou 200659 | ✓ | ✓ | ✓ | ||||
| 56. CMTP 2009133 | ✓ | ✓ | ✓ | ||||
| 57. Reeves 200860 | ✓ | ✓ | ✓ | ||||
| 58. Sansom 200758 | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| 59. Slutsky 200857 | ✓ | ✓ | ✓ | ✓ | |||
Appendix 2 An algorithm for ‘only in research’ and ‘approval with research’ decisions
FIGURE 26.
Part I:Technologies without significant irrecoverable costs.
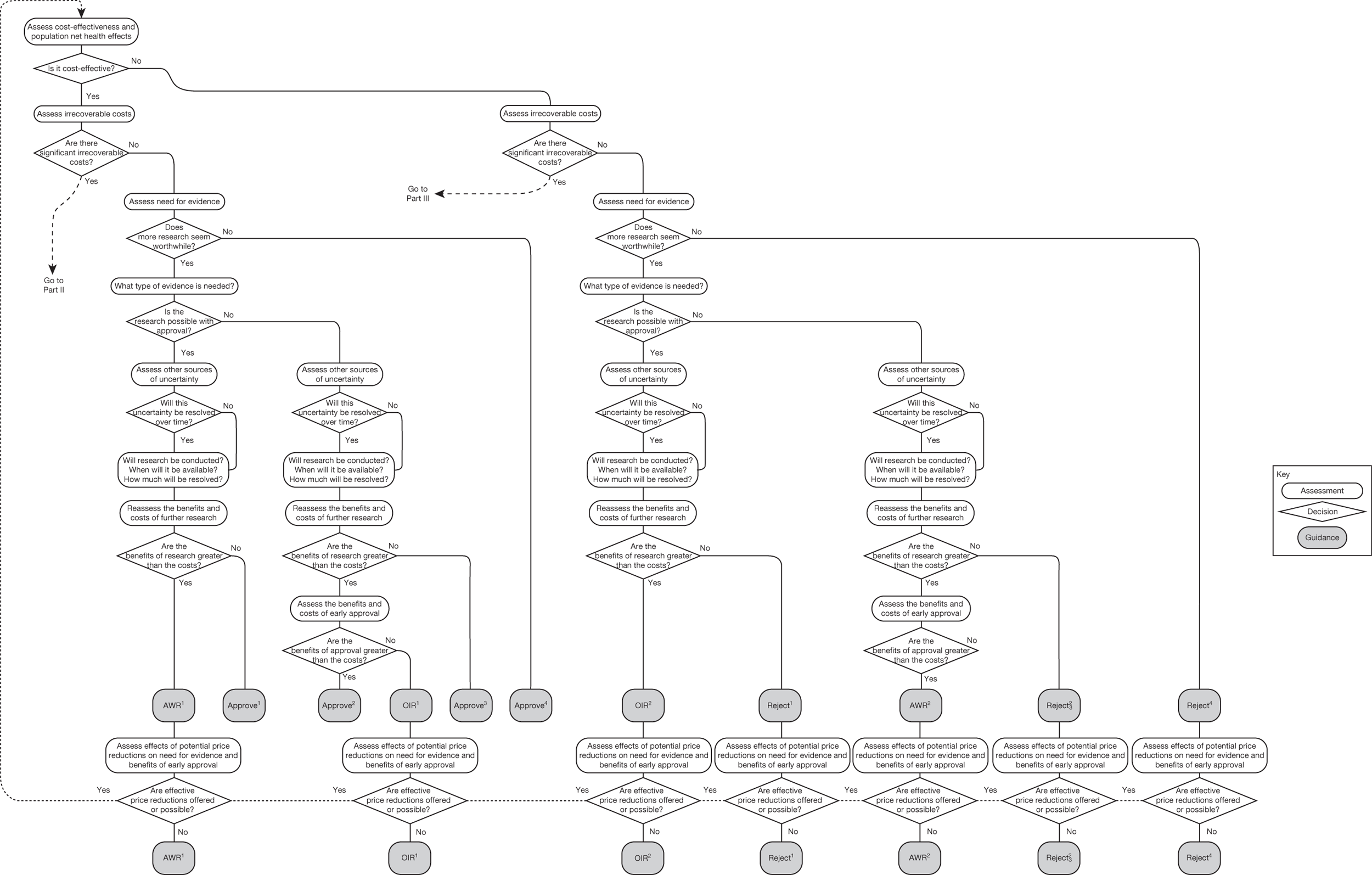
FIGURE 27.
Part II: Technologies with significant irrecoverable costs, expected to be cost-effective and research needed.
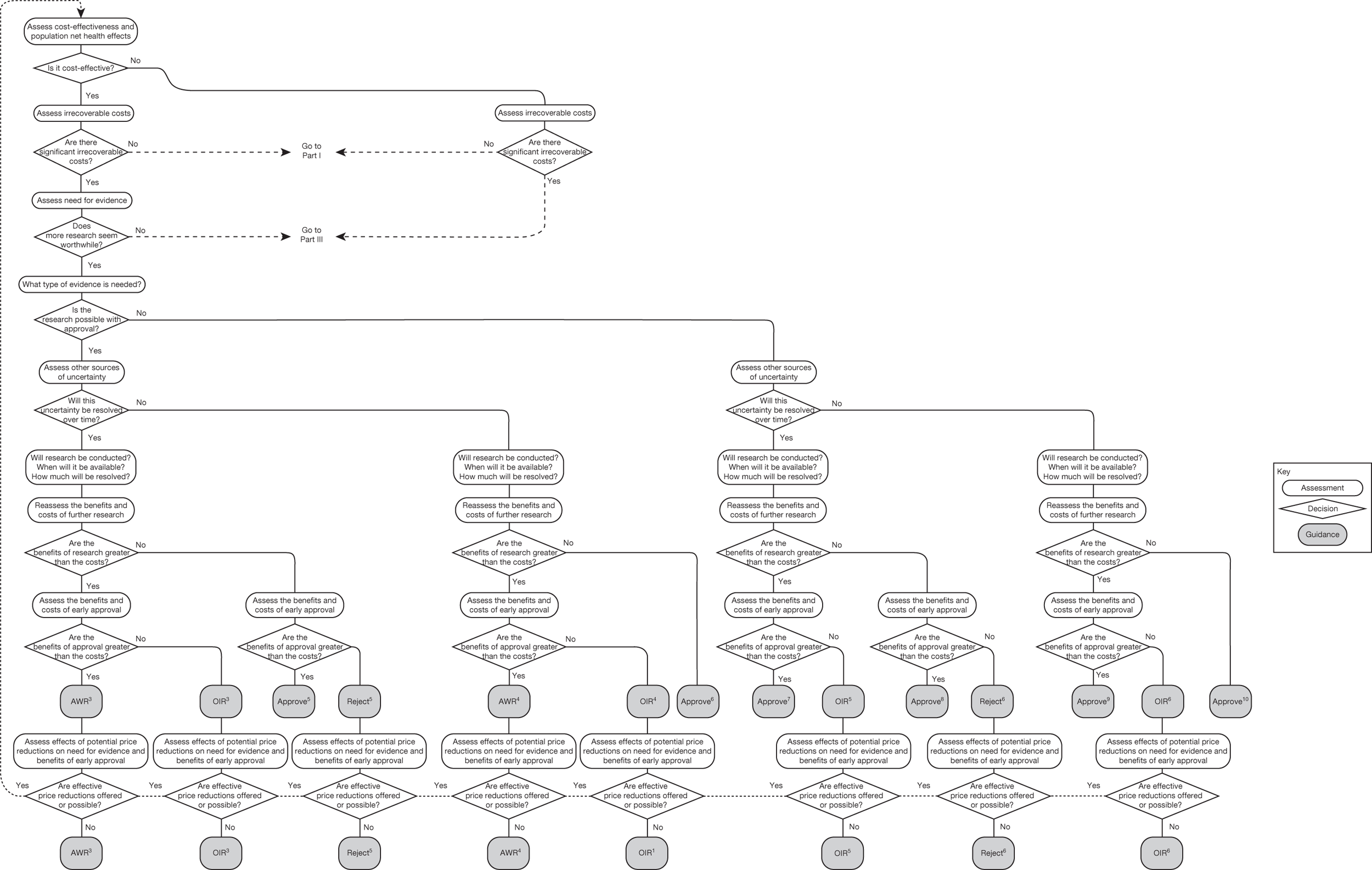
FIGURE 28.
Part III:Technologies with significant irrecoverable costs, not expected to be cost-effective or research not needed.
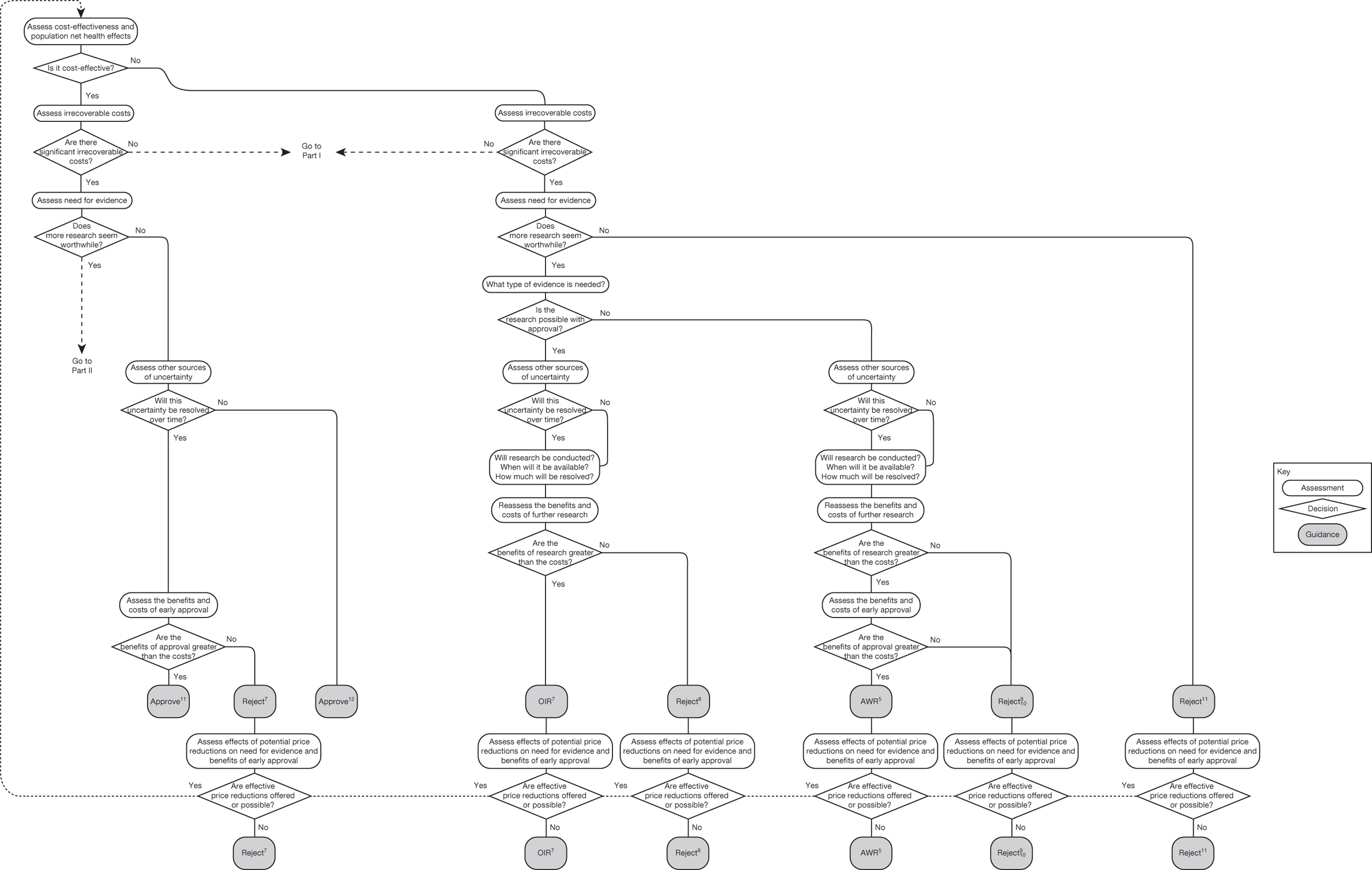
Appendix 3 Data extraction
| No. | Technology appraisal subject | Date | ACD/FAD |
|---|---|---|---|
| TA2134 | Prostheses for primary total hip replacement | April 2000 | FAD onlya |
| TA588 | Cervical cancer – liquid based cytology | May 2000 | FAD onlya |
| TA6105 | Taxanes for breast cancer | June 2000 | FAD onlya |
| TA890 | Hearing aid technology | July 2000 | FAD onlya |
| TA16109 | Knee joints (defective) – autologous cartilage transplantation | December 2000 | FAD onlya |
| TA17110 | Colorectal cancer – laparoscopic surgery | December 2000 | FAD onlya |
| TA23135 | Recurrent malignant glioma (brain cancer) – temozolomide | April 2001 | FAD onlya |
| TA30108 | Taxanes for breast cancer | September 2001 | FAD onlya |
| TA33106 | Colorectal cancer (advanced) – irinotecan, oxaliplatin and raltitrexed (Tomudex®, Hospira) | March 2002 | FAD onlya |
| TA35136 | Arthritis (juvenile idiopathic) – etanercept | March 2002 | FAD onlya |
| TA3692 | Rheumatoid arthritis – etanercept and infliximab | March 2002 | FAD onlya |
| TA37100 | Lymphoma (follicular non-Hodgkin’s) – rituximab (MabThera®, Roche) | March 2002 | FAD onlya |
| TA4498 | Hip disease – metal on metal hip resurfacing | June 2002 | ACD and FAD |
| TA50137 | Leukaemia (chronic myeloid) – imatinib (Gilvec®, Novartis) | May 2002 | ACD only |
| TA5196 | Depression and anxiety – computerised cognitive behaviour therapy | October 2002 | ACD and FAD |
| TA6093 | Diabetes – patient education models | November 2002 | ACD only |
| TA6597 | Aggressive non-Hodgkin’s lymphoma – rituximab | September 2003 | ACD and FAD |
| TA6894 | Macular degeneration (age-related) – photodynamic therapy | September 2003 | ACD and FAD |
| TA70138 | Leukaemia (chronic myeloid) – imatinib | October 2003 | FAD only |
| TA72116 | Rheumatoid arthritis – anakinra (Kieret®, Swedish Orphan) | November 2003 | ACD and FAD |
| TA7599 | Hepatitis C – pegylated interferons, ribavirin and alfa interferon | January 2004 | ACD and FAD |
| TA86139 | Gastro-intestinal stromal tumours (GIST) – imatinib | May 2004 | ACD only |
| TA89102 | Cartilage injury – autologous chondrocyte implantation (ACI) | May 2005 | ACD and FAD |
| TA92140 | Tooth decay – HealOzone | July 2005 | FAD only |
| TA93113 | Colorectal cancer (advanced) – irinotecan, oxaliplatin and raltitrexed | August 2005 | ACD and FAD |
| TA9795 | Depression and anxiety – computerised cognitive behavioural therapy | February 2006 | ACD and FAD |
| TA99141 | Immunosuppressive therapy for renal transplantation in children | April 2006 | FAD only |
| TA104126 | Psoriatic arthritis – etanercept and infliximab | June 2005 | ACD only |
| TA111142 | Alzheimer’s disease – donepezil (Aricept®, Eisai), galantamine (Reminyl® XL, Shire), rivastigmine (Exelon®, Novartis) and memantine (Ebixa®, Lundbeck) | November 2006 (update 2009) | ACD and FAD |
| TA11391 | Diabetes (type 1 and 2) – inhaled insulin | December 2006 | ACD and FAD |
| TA121143 | Glioma – carmustine implants and temozolomide | December 2005 | ACD only |
| TA129103 | Multiple myeloma – bortezomib (Velcade®, Janssen) | July 2006 | ACD only |
| TA130114 | Rheumatoid arthritis – adalimumab, etanercept and infliximab | February 2006 | ACD only |
| TA135144 | Mesothelioma – pemetrexed disodium | March 2006 | ACD only |
| TA142145 | Anaemia – erythropoietin (alpha and beta) and darbepoetin | July 2005 | ACD only |
| TA143146 | Ankylosing spondylitis – adalimumab, etanercept and infliximab | July 2007 | ACD only |
| TA159147 | Pain (chronic neuropathic or ischaemic) – spinal cord stimulation | October 2008 | ACD and FAD |
| TA16389 | Ulcerative colitis (acute exacerbations) – infliximab | December 2008 | ACD and FAD |
| TA166104 | Hearing impairment – cochlear implants | December 2007 | ACD only |
| TA167101 | Abdominal aortic aneurysm – endovascular stent-grafts | February 2009 | ACD and FAD |
| 1. Appraisal information | |
|---|---|
| (a) | Appraisal number |
| (b) | Appraisal title |
| (c) | Publication date |
| (d) | Is the appraisal a review of previous guidance? |
| (e) | Has the guidance been subsequently reviewed? |
| (f) | Appraisal process (STA or MTA) |
| (g) | Disease area |
| (h) | Monotherapy or combination therapy |
| 2. Recommendations | |
| (i) | Type of recommendation (OIR or AWR) |
| (j) | OIR recommendation |
| (k) | Type of research recommended (experimental or observational) |
| (l) | Applied to a subgroup? |
| (m) | Patient access scheme offered? |
| (n) | Patient access scheme accepted? |
| (o) | Years until proposed review date |
| 3. Background information on the condition | |
| (p) | Incidence and prevalence of the condition |
| (q) | Total population affected |
| (r) | Type of technology (drug, device, diagnostic, procedure, etc.) |
| (s) | Alternative active treatment available? |
| 4. Cost-effectiveness data | |
| (t) | Manufacturer/sponsor estimates of the: |
| ICER for the whole population | |
| ICER for the OIR/AWR subgroup (where applicable) | |
| Probability cost-effective at £20,000 and £30,000 | |
| (u) | AG/ERG estimates of the: |
| ICER for the whole population | |
| ICER for the OIR/AWR subgroup (where applicable) | |
| Probability cost-effective at £20,000 and £30,000 | |
| (v) | Committee’s best estimate of the cost-effectiveness (mean ICER and/or range) |
| 5. Committee rational for OIR/AWR recommendation | |
| (w) | Evidence base |
| No evidence | |
| Poor or insufficient evidence | |
| Evidence suggests less effective than alternative | |
| No or limited data on target population | |
| No change in evidence base since previous appraisal | |
| No or limited evidence on safety or adverse effects | |
| No or limited evidence to support mechanism of action | |
| (x) | Cost-effectiveness |
| Cost-effectiveness not assessed against appropriate comparator | |
| Considered unlikely to be cost-effective | |
| Uncertainty around cost-effectiveness too high | |
| (y) | Investment/irreversibility |
| Overall costs too high | |
| Irreversible/sunk costs too high | |
| Concern about potential impact on ongoing or future research | |
Appendix 4 Types and categories of guidance for case studies
| Assessment | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Guidance | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Yes | No | Yes | Yes | Yes/no | Yes | – | AWR1 | } Part I of the algorithm |
| 2 | Yes | No | Yes | Yes | Yes/no | No | – | Approve1 | |
| 3 | Yes | No | Yes | No | Yes/no | Yes | Yes | Approve2 | |
| 4 | Yes | No | Yes | No | Yes/no | Yes | No | OIR1 | |
| 5 | Yes | No | Yes | No | Yes/no | No | – | Approve3 | |
| 6 | Yes | No | No | – | – | – | – | Approve4 | |
| 7 | No | No | Yes | Yes | Yes/no | Yes | – | OIR2 | |
| 8 | No | No | Yes | Yes | Yes/no | No | – | Reject1 | |
| 9 | No | No | Yes | No | Yes/no | Yes | Yes | AWR2 | |
| 10 | No | No | Yes | No | Yes/no | Yes | No | Reject2 | |
| 11 | No | No | Yes | No | Yes/no | No | – | Reject3 | |
| 12 | No | No | No | – | – | – | – | Reject4 | |
| 13 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | AWR3 | } Part II of the algorithm |
| 14 | Yes | Yes | Yes | Yes | Yes | Yes | No | OIR3 | |
| 15 | Yes | Yes | Yes | Yes | Yes | No | Yes | Approve5 | |
| 16 | Yes | Yes | Yes | Yes | Yes | No | No | Reject5 | |
| 17 | Yes | Yes | Yes | Yes | No | Yes | Yes | AWR4 | |
| 18 | Yes | Yes | Yes | Yes | No | Yes | No | OIR4 | |
| 19 | Yes | Yes | Yes | Yes | No | No | – | Approve6 | |
| 20 | Yes | Yes | Yes | No | Yes | Yes | Yes | Approve7 | |
| 21 | Yes | Yes | Yes | No | Yes | Yes | No | OIR5 | |
| 22 | Yes | Yes | Yes | No | Yes | No | Yes | Approve8 | |
| 23 | Yes | Yes | Yes | No | Yes | No | No | Reject6 | |
| 24 | Yes | Yes | Yes | No | No | Yes | Yes | Approve9 | |
| 25 | Yes | Yes | Yes | No | No | Yes | No | OIR6 | |
| 26 | Yes | Yes | Yes | No | No | No | – | Approve10 | |
| 27 | Yes | Yes | No | – | Yes | – | Yes | Approve11 | } Part III of the algorithm |
| 28 | Yes | Yes | No | – | Yes | – | No | Reject7 | |
| 29 | Yes | Yes | No | – | No | – | – | Approve12 | |
| 30 | No | Yes | Yes | Yes | Yes/no | Yes | – | OIR7 | |
| 31 | No | Yes | Yes | Yes | Yes/no | No | – | Reject8 | |
| 32 | No | Yes | Yes | No | Yes/no | Yes | Yes | AWR5 | |
| 33 | No | Yes | Yes | No | Yes/no | Yes | No | Reject9 | |
| 34 | No | Yes | Yes | No | Yes/no | No | – | Reject10 | |
| 35 | No | Yes | No | – | – | – | – | Reject11 |
FIGURE 29.
Possible pathways for CLOP.
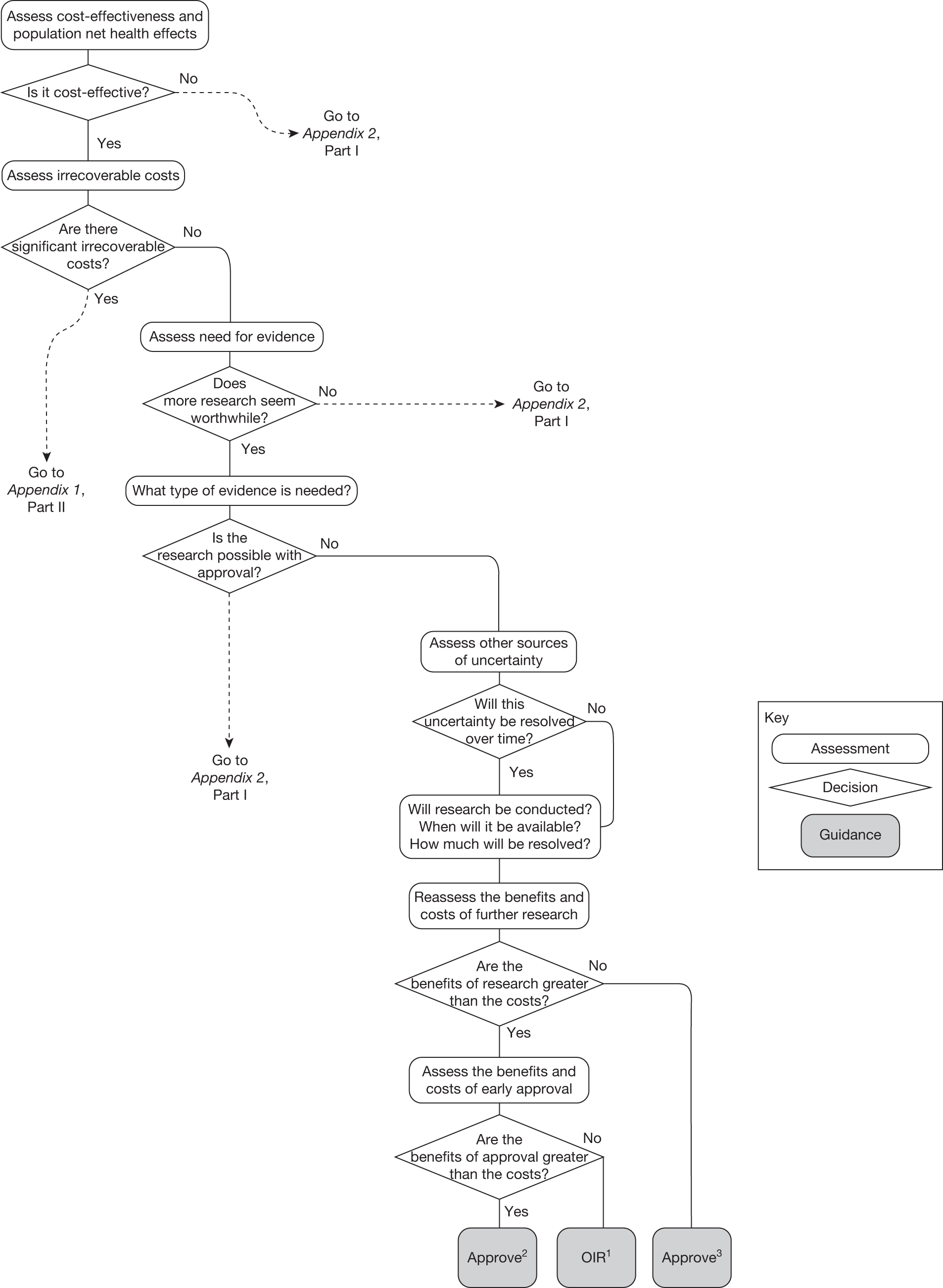
FIGURE 30.
Possible pathways for EECP.
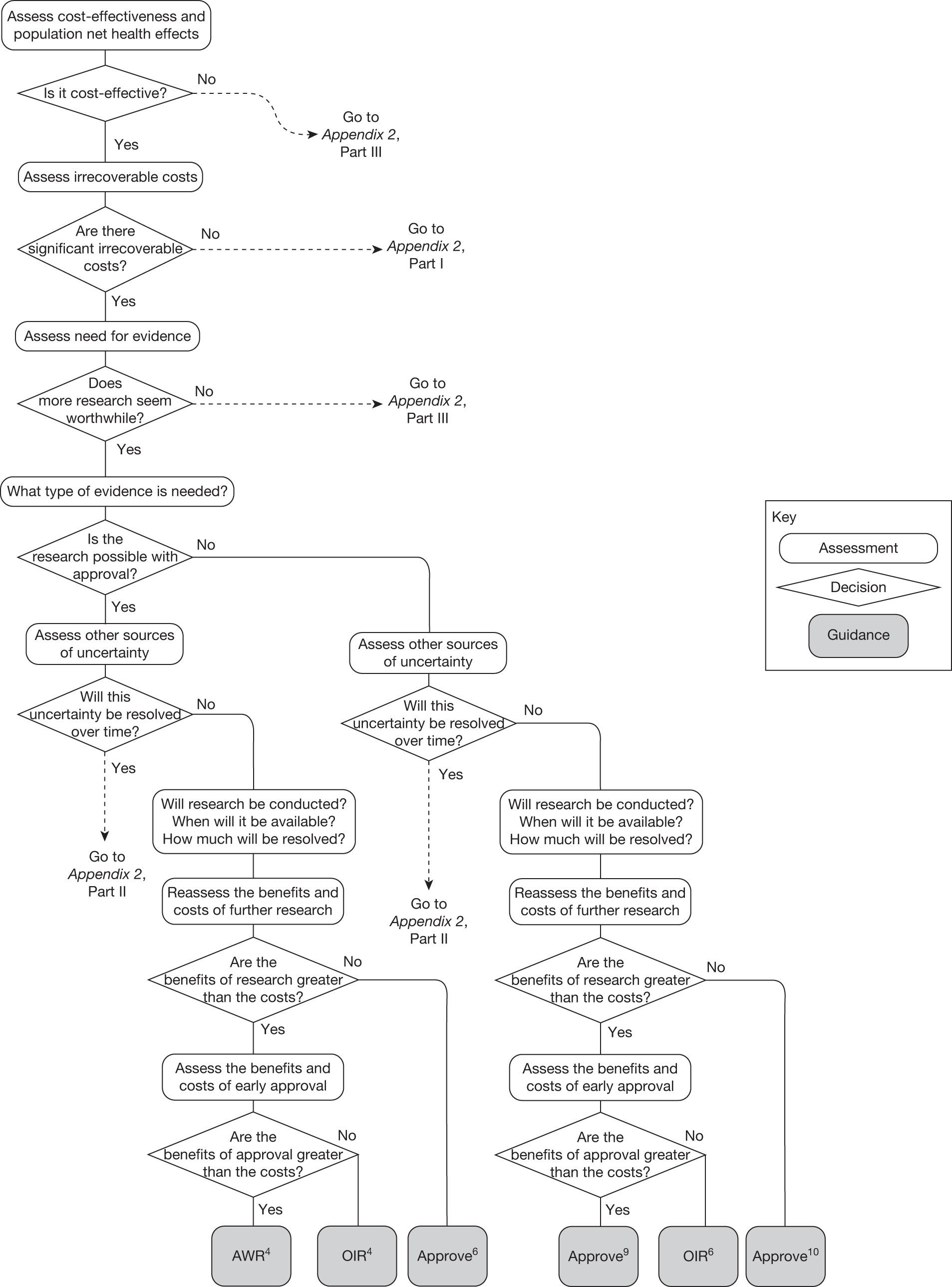
FIGURE 31.
Possible pathways for OMAL.
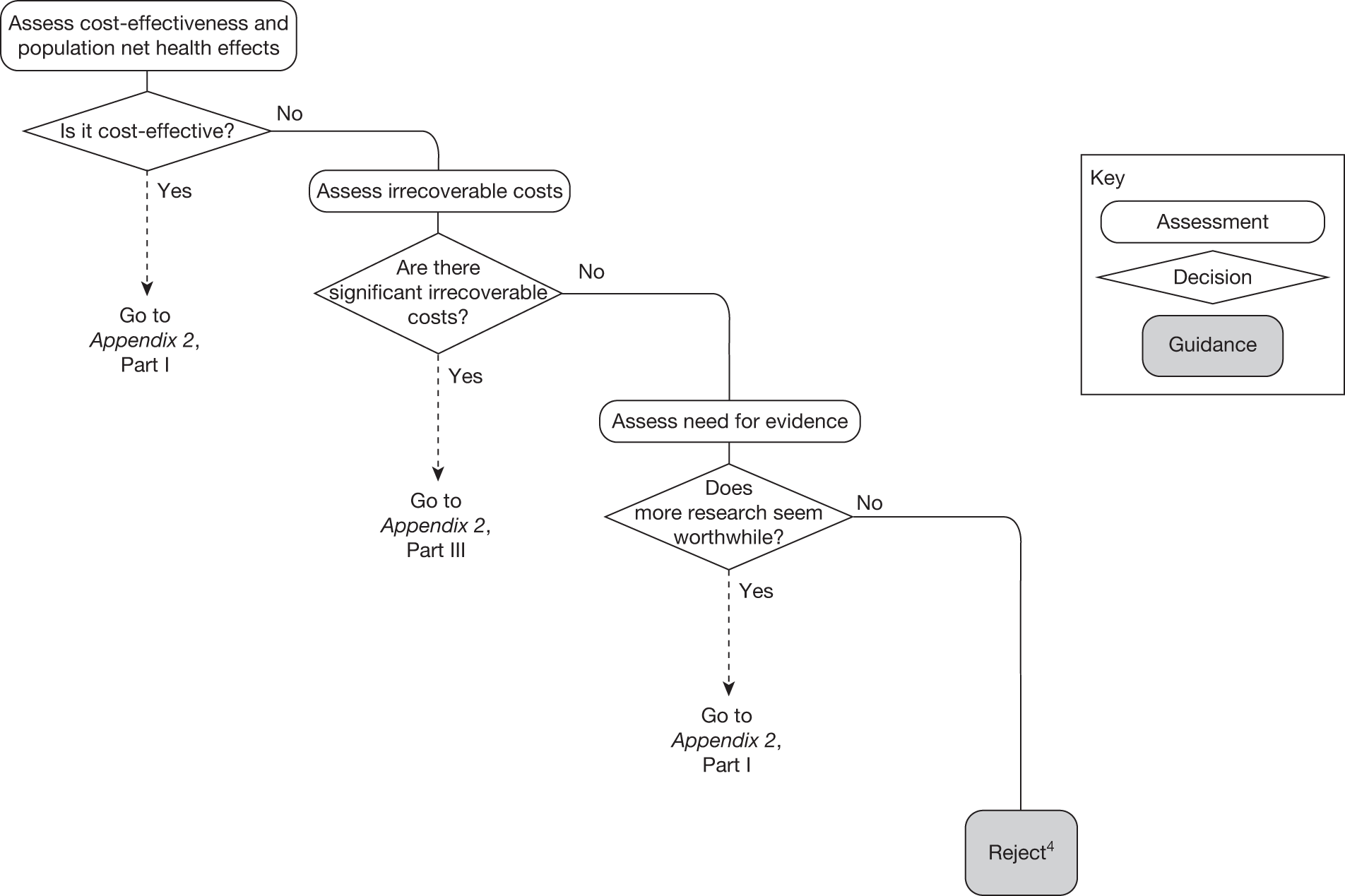
FIGURE 32.
Possible pathways for PsA.
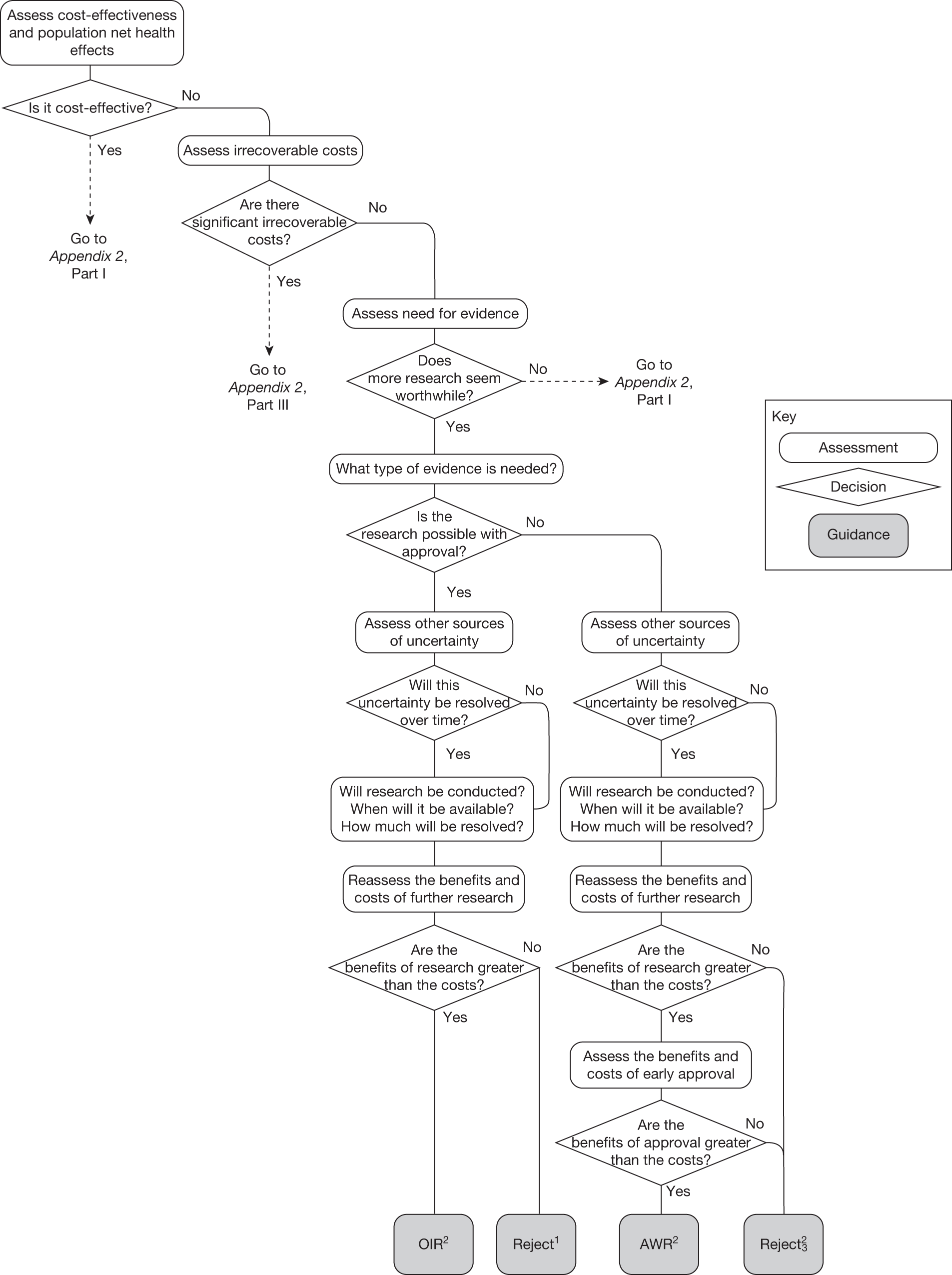
Other sources of uncertainty: searches for sources of additional information
The searches for sources of information to complete the assessments in Chapter 5 are detailed in the following sections. Specifically they describe sources for each type of information required, including access details.
Changes in the prices of the technology and its comparators
The anticipated time for drugs coming off patent is available from a number of web-based sources. These include:
-
MPA Business Services Limited (www.mpasearch.co.uk/mpadatasearch). This requires registration and a fee for generating information, which is approximately £25–500 for a single molecule.
-
Horizon scanning (www.ukmicentral.nhs.uk/pressupp/pe.htm) includes a patent database that details the dates of drug patent issue. This service is available only to NHS staff or through specific project permission.
-
The national patent database (www.ipo.gov.uk/patent/p-find/p-find-number.htm) provides the date of patent registration. The appropriate patent can be found using the INID code (on the patent page) or using http://gb.espacenet.com/search97cgi/s97_cgi.exe?Action%20=%20FormGen%26Template%20=%20gb/en/quick.hts to search by drug name or summary of product characteristics. In the UK a patent typically lasts for 20 years from the application date shown on the front page of the patent; thus, 20 years from this date will give the full-term expiry date. One potential complication is that for pharmaceuticals this can be extended up to a further 5 years by gaining a Supplementary Protection Certificate (SPC).
-
In the USA Google Patent finder is available (www.google.com/googlepatents/about.html).
For non-drugs (medical interventions, etc.) there is less information. Many medical devices will have a trademark/CE mark (see www.ipo.gov.uk/tm/t-find/t-find-text/ to search by trademark number or free text). Trademarks can last indefinitely as long as they are renewed every 10 years (www.ipo.gov.uk/tm.htm). Any patent is likely to relate to some aspect of the device rather than the device itself. Although prices may change over time they can also be relatively stable but with incremental innovation of the original device. Again, this is likely to differ by health-care system, technology and indication.
Regarding the extent of change there is again very little published information on the likely impact of generics. The OFT estimated that, on average, generic prices tend to be 25% of the original brand price before patent expiry. 12 A recent HTA model148 also used the assumption that the generic price is equal to 25% of the original product price. A US study149 previously saw the cost of CLOP at 80% of the branded cost (this was deemed inappropriate for the UK). These general figures are, however, unlikely to be entirely accurate as reductions in prices are likely to be location specific and differ by type of technology. This cannot be quantified but needs to be recognised when interpreting the likely cost of generics.
Entry of new technologies
To identify any relevant new technologies that may be emerging there are a number of sources available:
-
The topic selection by NICE gives some indication as to what drugs will soon be given licences in the UK. The topic selection panel minutes are available at www.nice.org.uk/getinvolved/topicselection/considerationpanels/minutes/minutes.jsp.
-
Also, proposed technology appraisals are given at www.nice.org.uk/ourguidance/niceguidancebytype/technologyappraisals/proposedappraisals/nowave.jsp. Technology appraisal topics not being progressed by NICE are available at www.nice.org.uk/getinvolved/suggestatopic/outcomes/Topics.jsp.
-
New drugs suggested by https://www.ukpharmascan.org.uk/login. These are available only to pharmaceutical companies and certain NHS staff.
-
Medical technologies in the Medical Technology Evaluation Programme (www.nice.org.uk/aboutnice/whatwedo/aboutmedicaltechnologies/notifyaproducttoep.jsp). Contains a list of technologies reviewed by the Medical Technologies Advisory Committee (MTAC) up to a certain date.
-
Information about licence applications: new applications or change of use in progress are available through NHS horizon scanning (www.ukmi.nhs.uk/applications/NDO/dbSearch.asp). Requires registration to access ‘new drugs online’. Can be searched by drug name or by BNF chapter. Search by developmental stage (licensed, Phase 1/2/3, licensed but not launched, application filled, etc.).
-
Non-UK regulators such as the FDA (www.fda.gov/Drugs/informationondrugs/ucm079436.htm) have databases containing upcoming competitors.
Many of the databases contain information on Phase II trials and so it may be necessary to try to predict the number of these that will then proceed to Phase III and approval. The OFT life cycle of drug development shows a 3-year period between initiation of Phase II and Medicines and Healthcare products Regulatory Agency (MHRA)/European Medicines Agency (EMEA) approval. 12 The same document also gives a period of 4.6 years from the initiation of Phase II to the end of phase III (taken from DiMasi et al. 150 but it is unclear which figures this is using). In the paper by DiMasi et al. 150 a period of 9.9 years from initiation of Phase II to the beginning of approval stage in the US market is quoted. The paper also presents the probability of approval by clinical phase (Table 33). Using these figures the OFT states that on average 21.5% of drugs entering clinical trials (Phases I, II and III) will go through to market approval.
| Clinical phase | Probability of entering phase |
|---|---|
| Phase I | 100 |
| Phase II | 71 |
| Phase III | 31.4 |
Other research reporting the technology and its comparators
There are a number of sources available to search for clinical research ongoing at the time of appraisal. These include:
-
clinical trial registers (www.controlled-trials.com/, www.clinicaltrials.gov/)
-
the National Research Register (NRR) Archive (www.nihr.ac.uk/Pages/NRRArchive.aspx)
-
ClinicalTrials.gov (www.clinicaltrials.gov)
-
the World Health Organization (WHO) ICTRP (www.who.int/ictrp/en/).
These databases report on the sample size, end points, trial type, comparators, start date and anticipated reporting date.
In addition, there are a number of non-clinical registries and outcomes databases. These include:
-
The NRR Archive (www.nihr.ac.uk/Pages/NRRArchive.aspx) – this covers all UK NHS-funded research not just trials. The NRR stopped being updated in 2007.
-
The current equivalent is the UK Clinical Research Network (UKCRN) Study Portfolio (http://public.ukcrn.org.uk/search/). The type of research that is eligible to be included in the Portfolio database varies depending whether it is funded in England, Wales, Scotland or Northern Ireland, but details of eligibility criteria are available at www.crncc.nihr.ac.uk/about_us/processes/portfolio/p_eligibility.
Appendix 5 Funding brief
MRC-NIHR METHODOLOGY RESEARCH PROGRAMME: NEEDS-LED RESEARCH
FUNDING BRIEF
Informing a decision framework for when NICE should recommend the use of health technologiesa only in the context of an appropriately designed programme of evidence development (‘only in research’)
1. BACKGROUND
Introduction
In its initial instructions to NICE, the Department of Health made provision for the Technology Appraisal Committee to ‘recommend that further research is carried out to see whether the potential promise of the intervention can be realised, indicate in broad terms the questions this research should address and advise clinicians that, in the meantime, they should only use the new intervention as part of a well-designed programme of research intended to answer these questions’. b
There is controversy about the terminology used to refer to these decisions. To date the term ‘only in research’ (OIR) has been used. Also parallels have been drawn with the concept of ‘Coverage with Evidence Development’, which is used in the US (Hutton et al. , [3]).
Of the 133 technology appraisal guidance documents issued by NICE between 1999 and the end of 2007, 21 (26%) contained one or more ‘OIR’ recommendations. In practice, such ‘OIR’ decisions have been made when there are important uncertainties about the effectiveness and cost-effectiveness of an intervention [1]. It is however acknowledged that ‘OIR’ decisions have potential costs, benefits and risks; both the individual decisions themselves and the principle of an ‘OIR’ decision option have been subject to international debate [2].
Importance of topic
Recently there have been a number of calls for NICE to use the ‘OIR’ decision option more often [2;4;5] [1]. There has also been criticism that ‘OIR’ is used as a money saving option or to avoid a clear ‘no’ decision [2].
While NICE’s R&D Committee has advised on the issues that need to be addressed in making an OIR recommendation, there are at present no decision criteria as to when ‘OIR’ might be an appropriate option; each recommendation has been made on a case-by case basis. This research is very timely because of recent moves to assess new technologies closer to the point of licensing. At this point, the evidence base is likely to be immature and as a result greater uncertainty about the comparative effectiveness and overall value of the technology [1;3].
Research needs
The primary research need is to inform the key principles that could be taken into consideration by NICE Appraisal Committees to enable them to determine whether an ‘OIR’ decision is appropriate. The methodological and process implications of providing the necessary technology-specific data on each of these principles should also be considered.
The main research output that is required is a list of key principles and recommendations about how these principles could be operationalised within a structured decision framework that NICE could employ when considering an ‘OIR’ decision. The research should also explore the possibility of using these principles as the basis of a ‘checklist’ to provide transparency in ‘OIR’ decision-making. This could include one or more pilot studies based on appraisals in progress (or proposed) to test possible decision-making approaches for ‘OIR’. It could also involve an evaluation of how Value of Information methods can be integrated into the decision-making framework.
A second research objective is to investigate how ‘OIR’ recommendations are currently implemented in practice and examine barriers and potential solutions including any changes to the research infrastructure. This will necessitate examination of previous ‘OIR’ recommendations.
An optional objective would be to explore the terminology used to describe ‘OIR’ recommendations. If opted for, then the acceptability of different options should be tested through consultation.
Other relevant initiatives and/or activities
Some preliminary work into the ‘OIR’ option has already been undertaken. NICE consulted its Citizen’s Council; a group of 30 people who bring the views of the public to NICE decision-making. The council made a series of recommendations about the circumstances in which it felt it is justified for NICE to recommend that an intervention is used only in the context of research. NICE has also obtained the views of other stakeholders through a workshop, held in collaboration with the American Agency for Healthcare Research and Quality (AHRQ)c and a series of interviews with industry, academia, government, the NHS, independent research funding agencies, and consultants (publication in progress). Internationally the concepts have been discussed by participants at the Health Technology Assessment International (HTAI)_Policy Forum in February 2007 [3] and the US Centres for Medicare and Medicaid Services [8]). A HTAI special interest policy group has been established.
There have been a number of academic publications exploring the ‘OIR’ option including that by Chalkidou et al. [1] and Hutton et al. [3]. There has also been research into potential methodologies including ‘Value of Information’ methods [10–13] and Options Theory [15]. Whilst not mandatory, the option to use VoI methods has been included in the NICE guide to methods of technology appraisal [14]. It is recognised that a quantitative value of information approaches cannot be used in isolation to determine whether an ‘OIR’ decision would be appropriate.
2. RESEARCH REQUIRED
Important issues to consider
Research is required to fulfil the needs identified in section 1.3 above.
Previous work has identified a number of potential themes that could be explored further. Note this is not an exhaustive list and further exploration of more recent research undertaken by NICE and others is required.
-
The potential research:
-
– Will the research reduce uncertainties about the effects of a technology (including side effects) to a level at which a clear-cut decision could be made?
-
– Will the research be timely, feasible and relevant (to the required patient groups)?
-
– Would the research represent value for money?
-
– What is the role of formal VoI methodology?
-
-
The consequences of making the wrong decision:
-
– Are the sunk costs associated with a ‘yes’ decision particularly high such that it would be uneconomical to reverse the decision?
-
– In terms of current research: would a ‘yes’ decision result in existing research being terminated early, possibly due to recruitment difficulties?
-
– In terms of future research: what is the value of the research foregone as a result of a ‘yes’ or ‘no’ decision?
-
-
Equity and ethical concerns:
-
– Would an ‘OIR’ decision effectively limit the technology to those who can access a research centre?
-
– Should ‘the benefit of the doubt’ be given to technologies for life threatening conditions where no other treatment exists (the rule of rescue)?
-
– Would a decision violate the social value judgements guidance applied by NICE [9]?
-
In addition, it will be necessary to consider the opportunity cost of delaying a coverage decision for further research in terms of the potential health gains foregone by delaying access to a cost-effective intervention.
Methodological approaches to be used
The MRP panel recognises the need to allow applicants to identify the most appropriate methodological approach for this study and the approach to be taken should be clearly justified. It is anticipated that a mixture of quantitative and qualitative primary and secondary research will be required. The research will need to address both the theoretical and practical considerations of ‘OIR’ decisions.
Applicants must ensure that the proposed research is congruent with the 2008 Methods for Technology Appraisal [14] and Social Value Judgements [9]. It is possible that more than one proposal will be funded.
Applicants may wish to consider undertaking a review of principles and methods currently used in, or advocated for, ‘OIR’ decisions, both in the UK and internationally.
Applicants should also include a retrospective ‘case-study’ analysis of ‘OIR’ decisions made by NICE to identify what principles have previously been taken into account when an ‘OIR’ decision has been made. It may also be necessary to explore instances when an ‘OIR’ decision was not determined to be appropriate.
Establishing and maintaining a dialogue with NICE and its Appraisal committees and other stakeholders, including patient groups and industry, will be essential. Where necessary, NICE will assist the research team to gain access to the necessary information and expertise.
This analysis may include consideration of how the ‘OIR’ decision-making process can help to define the nature of the research required (e.g. study design; key variables to be assessed). The pros and cons of possible approaches should be identified, in order to provide recommendations for the principles to be included in a structured decision-making process and how such a process can be operationalised by NICE Appraisal Committees. There is scope for this decision-making process to be supported by a ‘checklist’.
3. MANAGEMENT OF STUDY
Applicants should specify and justify: (1) the resources requested for the research and (2) an appropriate timescale within which the research can be completed.
4. REFERENCES
- Chalkidou K, Hoy A, Littlejohns P. Making a decision to wait for more evidence: When the National Institute for Health and Clinical Excellence recommends a technology only in the context of research. J R Soc Med 2007;100:453-60.
- NICE Citizen’s Council . Only in Research 2007.
- Hutton J, Trueman P, Henshall C. Coverage with evidence development: An examination of conceptual and policy issues. Int J Technol Assess Health Care 2007;23:425-35.
- Cooksey D. A review of UK health research funding. London: HM Treasury; 2006.
- House of Commons Health Committee . National Institute for Health and Clinical Excellence 2008:27-1.
- Tunis SR, Chalkidou K, . Coverage with evidence development: A very good beginning, but much to be done. Int J Technol Assess Health Care 2007;23:432-5.
- CMO . On the State of the Public Health: Annual Report of the Chief Medical Officer 2005 2006.
- Centers for Medicare and Medicaid Services . National Coverage Determinations With Data Collection As a Condition of Coverage: Coverage With Evidence Development 2006.
- NICE . Principles for the Development of NICE Guidance 2005.
- Claxton K, Ginnelly L, Sculpher M, Philips Z, Palmer S. A pilot study on the use of decision theory and value of information analysis as part of the NHS Health Technology Assessment programme. Health Technol Assess 2004;8.
- Eckermann S, Willan AR. Expected value of information and decision making in HTA. Health Econ 2007;16:195-209.
- Tappenden P, Chilcott JB, Eggington S, Oakley J, McCabe C. Methods for expected value of information analysis in complex health economic models: developments on the health economics of interferon-β and glatiramer acetate for multiple sclerosis. Health Technol Assess 2004;8.
- Girling A, Freeman G, Gordon JP, Poole-Wilson P, Scott DA, Lilford RJ. Modeling payback from research into the efficacy of left-ventricular assist devices as destination therapy. Int J Technol Assess Health Care 2007;32:269-77.
- NICE . Guide to the Methods of Technology Appraisal 2004.
- Palmer S, Smith PC. Incorporating option values into the economic evaluation of health care technologies. J Health Econ 2000;19:755-66.
Appendix 6 Technical appendix
Overview
The purpose of this appendix is to describe more formally the core principles of the framework developed to include OIR or AWR recommendations alongside approve and reject policies in deciding on the use of health technologies. This appendix will describe how to carry out the calculations that can inform each of the assessments described in Chapter 5 in order to quantify the net health impact of alternative policy decisions. Established methods for cost-effectiveness analysis and value of information analysis are drawn together using a consistent set of notation throughout to describe a general algebraic framework. A simple numerical example is used to demonstrate the application of this general algebraic framework, illustrating how the pay-offs from the alternative policy options of approve, AWR, OIR and reject can be estimated. Special consideration is given to the impact of price at submission on the estimated pay-off and how this can alter the rank order of the alternative policy options.
Notation
This appendix makes use of formal notation throughout to illustrate how the pay-off from the alternative policy decisions can be calculated. Table 34 shows the terms used, their definitions and the sections in this appendix where they are described in detail.
| Symbol | Definition | Section reference |
|---|---|---|
| h( ) | Health benefits | Is the intervention cost-effective? |
| j = 1,2,. . .,J | Each j represents an alternative out of a set of J mutually exclusive comparator interventions | Is the intervention cost-effective? |
| k | Cost-effectiveness threshold | Is the intervention cost-effective? |
| c( ) | Costs of interventions that fall on the budget for health care | Is the intervention cost-effective? |
| θ | Set of uncertain parameters used to estimate costs and health effects and that are amenable to further research | Is the intervention cost-effective?, Cost-effectiveness at the patient level |
| NHE( ) | Net health effects | Is the intervention cost-effective?, Cost-effectiveness at the patient level |
| Pop | The total effective number of patients who would receive an intervention | Is the intervention cost-effective?, Cost-effectiveness at the population level |
| P | The prevalent population eligible for treatment at the time of the reimbursement decision | Is the intervention cost-effective?, Cost-effectiveness at the population level |
| I t | The incident population per unit time | Is the intervention cost-effective?, Cost-effectiveness at the population level |
| d | Discount rate | Is the intervention cost-effective?, Cost-effectiveness at the population level |
| T | Time horizon | Is the intervention cost-effective?, Cost-effectiveness at the population level |
| C( ) | Investment cost | |
| EVPI θ | The maximum improvement in NHEs that could be achieved for an individual patient by further research on θ | Is further research required?, Consequences of uncertainty at the patient level |
| Π ( ) | Policy pay-off in terms of NHEs | Is further research required?, Consequences of uncertainty at the population level |
| s | Set of uncertain scenario assumptions used to estimate NHEs | Is further research required?, Alternative scenarios |
| R | The amount of health displaced as a result of the cost of further research | Does more research seem worthwhile? |
| τ | Time at which the results of research will be available | How long until the research reports? |
| α | Probability of further research | Will the research be conducted? |
| n | Number of patient recruited to a research study | How much of the uncertainty will the research resolve? |
| θi | Parameter i out of the set of uncertain parameters θ | What type of evidence is needed? |
| τ_ | Time at which the results of research on parameter i will be available | What type of evidence is needed? |
| R i | The amount of health displaced as a result of the cost of further research to inform parameter i | What type of research is possible if the technology is approved? |
| γ | Uncertain parameter not able to be informed by further research but that will resolve over time | Impact of other sources of uncertainty |
Is the intervention cost-effective and what are the risks?
Is the intervention cost-effective?
Within the health-care sector the outcome of interest for economic evaluation has generally been regarded as some measure of health, h. To facilitate comparisons between alternative health interventions, j = 1,2,. . .,J, it is necessary to have a single index measure of health that captures both length of life and quality of life. The opportunity cost of investing resources in providing a particular health intervention can be characterised in terms of the health that could have been generated with the next best alternative use of those same resources. By utilising the additional cost that would displace one unit of health gain elsewhere in the health-care system, k, it is possible to describe costs that fall on the health-care sector budget, c, in terms of health benefits,
forgone. The cost-effectiveness of a particular health-care intervention can be assessed by estimating its associated costs and health outcomes and determining whether or not, given the cost-effectiveness threshold k, reimbursing the intervention would lead to an expected increase in population health benefits. The assessment is the same regardless of whether the intervention is new or is already reimbursed as part of current activities, that is, the decision to discontinue funding an existing activity can be made on the same basis as the decision to reimburse a new intervention.
Figure 33 shows an intervention that generates two additional units of health compared with the next best alternative. For every extra £20,000 that is spent on this intervention there will be a loss of one unit of health from the displaced activities.
FIGURE 33.
Value, cost-effectiveness and net benefit. P*, price that results in no change in population health.
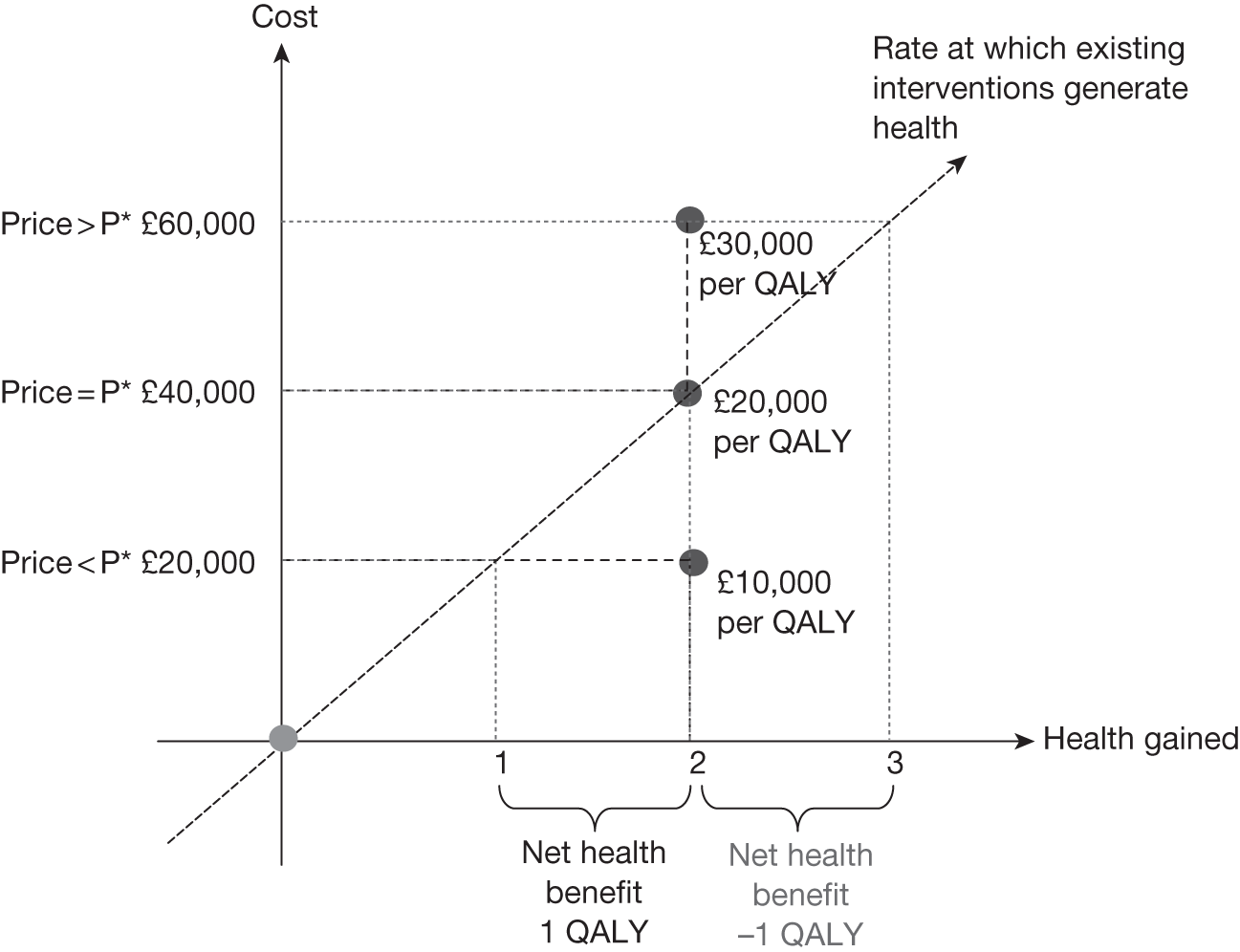
If the price of the intervention is £40,000, then approving it for use within the health-care system would generate two additional units of health but displace two units elsewhere and so the net effect would be no change in population health. If the price of the intervention is £60,000, then the decision to approve it for reimbursement would displace more health than would be gained, resulting in a net loss in population health. However, if the price is £20,000 then approving the intervention would displace less health than it generated, resulting in a net gain in health, and in these circumstances the intervention could be regarded as providing value for money. a
Cost-effectiveness at the patient level
The estimation of expected costs and health outcomes will be informed by the available evidence, θ, and for each intervention j can be expressed in terms of NHEs:
Health and cost outcomes that occur in the future should be adjusted to net present values using an appropriate discount rate. The relevant time horizon over which to assess the health and cost impacts must be long enough to capture any differences between the alternative interventions. 150 The decision-maker in charge of the health-care budget can maximise population health by choosing the intervention that maximises expected NHEs:
Thus, an intervention, j, can be determined to be cost-effective if it would maximise expected NHEsb out of the range of mutually exclusive alternatives, J, relevant to the decision problem. 67 NHEs make cost-effectiveness comparisons and calculations numerically simple as they embody the objective to maximise health gains from available resources simply by maximising NHEs. 63,64 Of course, the caveat to this is that one must first be satisfied that costs, health outcomes and opportunity costs were calculated appropriately and that they take account of all factors relevant to the viewpoint of the decision-maker, such as equity considerations about the value of health gains to different beneficiaries. c
If we take as a starting point NHEs that are deemed to be accurate and reliable we can utilise a simple numerical example to demonstrate the processes and intuition behind conducting formal, evidence-based evaluations to support the range of assessments a decision-maker may wish to make to inform reimbursement decisions and recommendations for further research. The numerical example is introduced in Table 35, which presents the health outcomes and costs for two interventions (j = 1,2). The health outcomes, h, and costs, c, are assessed according to their timing for the individual receiving each intervention by considering two consecutive time periods. NHEs are calculated given k = £20,000. The numbers in the table represent net present values. The evidence on which the estimates of cost and health outcomes are based is represented by an uncertain parameter θ, which can take one of four possible true underlying values, each of which would lead to a different estimation of the NHEs associated with each intervention. The final rows of each part of Table 35 give the expected values given the uncertainty in how θ will resolve.
| Intervention 1 | |||||||
|---|---|---|---|---|---|---|---|
| θ | Period 1 | Period 2 | Total | ||||
| h(1,θ) | c(1,θ) | NHE(1,θ) | h(1,θ) | c(1,θ) | NHE(1,θ) | NHE(1,θ) | |
| 1 | 0.35 | 2875 | 0.21 | 0.35 | £2875 | 0.21 | 0.41 |
| 2 | 0.20 | 3250 | 0.04 | 0.20 | £3250 | 0.04 | 0.08 |
| 3 | 0.45 | 3000 | 0.30 | 0.45 | £3000 | 0.30 | 0.60 |
| 4 | 0.65 | 2750 | 0.51 | 0.65 | £2750 | 0.51 | 1.03 |
| E θ | 0.41 | 2969 | 0.26 | 0.41 | £2969 | 0.26 | 0.53 |
| Intervention 2 | |||||||
| θ | Period 1 | Period 2 | Total | ||||
| h(2,θ) | c(2,θ) | NHE(2,θ) | h(2,θ) | c(2,θ) | NHE(2,θ) | NHE(2,θ) | |
| 1 | 0.50 | 10,250 | 0.01 | 0.50 | £250 | 0.49 | 0.48 |
| 2 | 0.65 | 11,250 | 0.09 | 0.65 | £1250 | 0.59 | 0.68 |
| 3 | 0.55 | 12,250 | –0.06 | 0.55 | £2250 | 0.44 | 0.38 |
| 4 | 0.70 | 11,500 | 0.13 | 0.70 | £1500 | 0.63 | 0.75 |
| E θ | 0.60 | 11,313 | 0.03 | 0.60 | £1313 | 0.53 | 0.57 |
In this example there are differences between the interventions in both time periods and so the relevant time horizon for assessing the cost-effectiveness of either intervention comprises both periods one and two. Figure 34 illustrates how the accrual of expected NHEs changes over time with each intervention from the perspective of an individual patient.
FIGURE 34.
Accrual of expected NHEs over time.
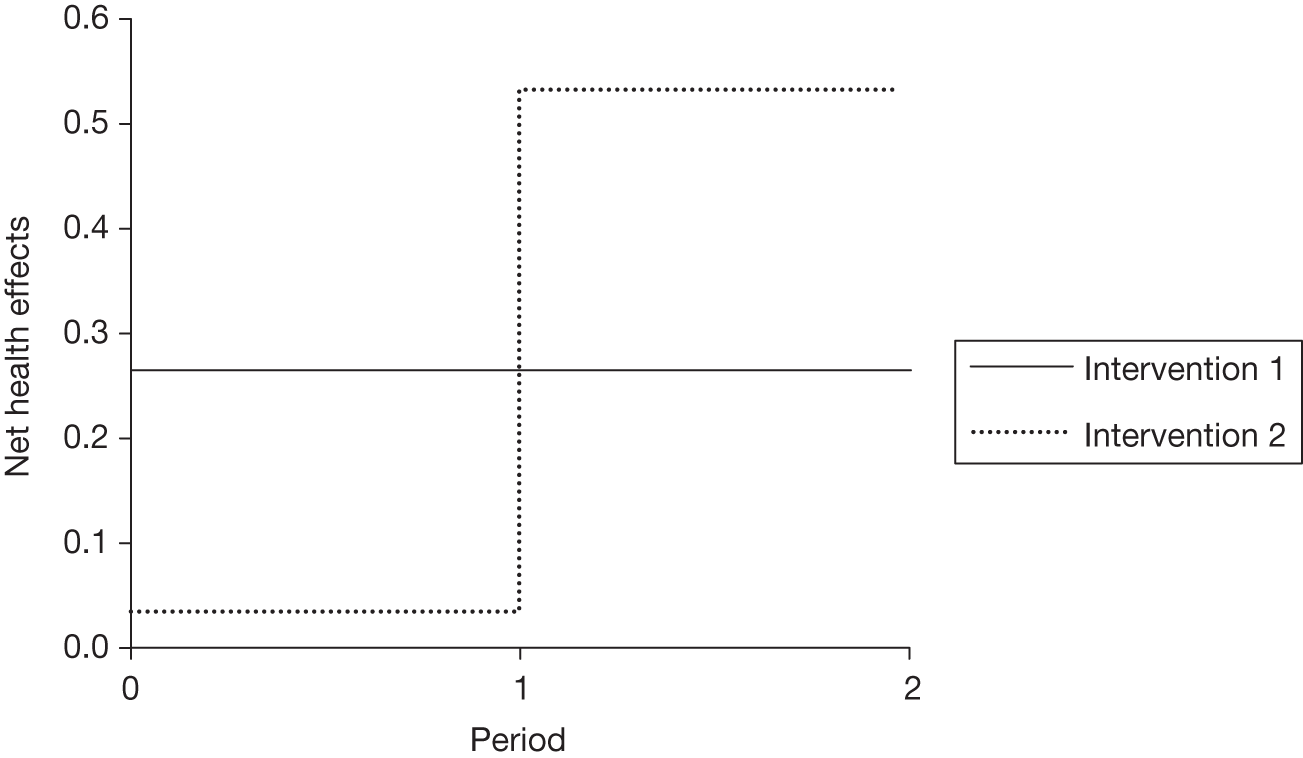
In Table 35 it can be seen that with intervention 2 the costs are loaded upfront. A comparison of the expected NHEs in period 1 would suggest that intervention 1 is cost-effective. However, the NHEs in period 2 indicate that intervention 2 is cost-effective. The gains offered by intervention 2 in the second period are sufficient to ensure that, overall, intervention 2 is cost-effective. In Figure 34 the area under the curve for intervention 2 is greater than the area under the curve for intervention 1. If treatment cannot or will not be withdrawn once initiated, then the timing of the costs is irrelevant to the reimbursement decision, and this can be based on the summation of the NHEs across the time periods, as shown in Table 36. In the following section (Are there significant irrecoverable costs?) we discuss the additional considerations that must be made when the NHEs that accrue vary over time and treatment may be withdrawn.
| θ | Intervention 1 | Intervention 2 | ||||
|---|---|---|---|---|---|---|
| h(1,θ) | c(1,θ) (£) | NHE(1,θ) | h(2,θ) | c(2,θ) | NHE(2,θ) | |
| 1 | 0.70 | 5750 | 0.41 | 1.00 | £10,500 | 0.48 |
| 2 | 0.40 | 6500 | 0.08 | 1.30 | £12,500 | 0.68 |
| 3 | 0.90 | 6000 | 0.60 | 1.10 | £14,500 | 0.38 |
| 4 | 1.30 | 5500 | 1.03 | 1.40 | £13,000 | 0.75 |
| E θ | 0.83 | 5938 | 0.53 | 1.20 | £12,625 | 0.57 |
To apply Equation 2 to determine which intervention is cost-effective we look at the final row of Table 36, which gives the expected NHEs of each intervention given the uncertainty in how θ will resolve. A comparison of these expected NHEs indicates that intervention 2 is cost-effective. Approving intervention 2 rather than intervention 1 would be expected to offer an additional 0.04 units of net health benefit per patient treated.
Cost-effectiveness at the population level
To judge the scale of the improvement in health gains that might result from a decision to provide either intervention it is necessary to consider the number of individuals who would receive treatment. The total population health effects associated with each intervention are found by multiplying the per-patient NHE by the total number of patients who would receive each intervention during the time period for which they would be utilised. This population, Pop, is a function of the prevalence and incidence of the disease per unit of time, P and It, summed over the relevant time horizon, TPop, taking account of the decision-maker’s rate of time preference for health gains, d:
The time horizon, TPop, over which the interventions would be utilised may be uncertain, requiring that a range of possible values be considered. 53 The time horizon for determining the relevant population size and the scale of the NHEs is different from the time horizon for assessing the cost-effectiveness of an intervention for an individual as was described in the previous section.
The size of population does not alter the assessment of which treatment is cost-effective; however, it may be necessary to know the size of the population to estimate some elements of costs or health outcomes. The resources required to provide an intervention may include those purchased and utilised on a per-patient basis and those that are shared across individuals. The number of patients who would utilise a joint resource determines how the costs can be apportioned at a per-patient level. For resource items that are shared between patients over successive time periods the total cost, C, can be converted to an equivalent cost per time period by dividing through by the annuity factor,
which is a function of the lifetime of the resource item, TC, and the decision-maker’s rate of time preference, d. This cost per time period,
can then be divided by the number of patients expected to utilise the resource in each time period to give the per-patient cost, c. 151 The per-patient costs of these joint resource items are thereby incorporated in the evaluation of cost-effectiveness at the individual level.
Table 37 uses the individual NHEs from Table 36 and multiplies them by the population of patients who could receive treatment over three consecutive time periods. That is, it multiplies Equation 2 by Equation 3 evaluated over three consecutive time periods. Because the example deals with an acute disease the size of the population is determined by the incidence of the disease (It = 100). For simplicity we assume a discount rate of zero (d = 0). The time period over which the interventions could be utilised is 10 years (TPop = 10). This gives a total patient population, Pop, of 1000.
| θ | Population 1 | Population 2 | Population 3 | |||
|---|---|---|---|---|---|---|
| NHE(1,θ) | NHE(2,θ) | NHE(1,θ) | NHE(2,θ) | NHE(1,θ) | NHE(2,θ) | |
| 1 | 2475 | 2850 | 825 | 950 | 825 | 950 |
| 2 | 450 | 4050 | 150 | 1350 | 150 | 1350 |
| 3 | 3600 | 2250 | 1200 | 750 | 1200 | 750 |
| 4 | 6150 | 4500 | 2050 | 1500 | 2050 | 1500 |
| E θ | 3169 | 3413 | 1056 | 1138 | 1056 | 1138 |
The time horizon over which the interventions could be utilised (TPop = 10) is split into three periods by considering two points in time at which reimbursement decisions can be revised (t = 6,8). For each population there remain the same two alternative interventions. In this example, applying Equation 3, population 1 consists of 600 patients and populations 2 and 3 each consist of 200 patients:
If nothing is expected to occur that would change the assessment of cost-effectiveness between each population the same decision will be made for each. The total overall population is Pop = Pop1 + Pop2 + Pop3. Approving intervention 2 would be expected to offer 3413 + 1138 + 1138 = 5689 units of net health benefit in the whole patient population, which represents an additional 408 units of population NHE compared with that offered by intervention 1 (3169 + 1056 + 1056 = 5281).
Analysis of subgroups
The relevant patient population is one that is homogeneous with respect to characteristics that affect the treatment decision. If the overall population is heterogeneous with respect to factors on which the treatment decision can be based and that affect the estimated NHEs of interventions it must first be split into homogeneous subgroups. 152 The cost-effectiveness of treatments must be evaluated separately in each distinct subgroup for which the treatment can be used, by applying the assessments described in Chapter 5. This allows different decisions to be made for each subgroup. When there are differences in the most cost-effective intervention between subgroups, basing decisions on the average across the whole population would lead to lower expected population net health benefits.
How does price affect cost-effectiveness?
Reducing price will make an intervention appear more cost-effective by increasing its expected NHEs. A straight discount will translate directly into increased NHEs. When more complex schemes are used to reduce the effective price of an intervention, the costs associated with setting up and utilising the scheme and the level of compliance must also be taken into account in the assessment of cost-effectiveness.
Are there significant irrecoverable costs?
When resources are committed in an earlier time period to that in which the ensuing benefits are realised these represent an investment. Similarly, investment may occur in health terms when patients incur initial health losses with the expectation of subsequent health gains. A typical example of this would be undergoing surgery to reduce the risk of death from coronary artery disease. Investment costs are irrecoverable if, once committed, they cannot be recovered should the decision to utilise an intervention be revised. This can be important if the decision is altered at a time earlier to that over which the expected future health gains were calculated in the assessment of cost-effectiveness described in Chapter 5. For example, if investment costs are irrecoverable and the decision is altered before the end of the anticipated life span of that investment the equivalent annual cost will have been calculated incorrectly and the number of patients who utilised the resource may be lower, meaning that the per-patient costs were underestimated when assessing cost-effectiveness. The irrecoverable investment costs will have been weighted against benefits that were not realised. However, in the case of surgery, once the patient has received the treatment a change of guidance does not alter his or her investment profile (the change of guidance does not modify the expected future change in health benefits attributed to having had surgery) and so does not have the same impact. The potential significance of irrecoverable costs can be judged:
-
according to whether or not estimates of cost-effectiveness would alter if the decision were to be revised sooner than anticipated
-
by assessing the probability that the decision might be altered earlier than expected
-
by comparing their size in health terms as a proportion of the total NHEs.
An assessment of irrecoverable costs requires some consideration of how health effects and costs accrue over time. This was shown in Figure 34 at the patient level and an equivalent diagram can be constructed at the population level. It is then possible to determine a break-even point, T*, the earliest time at which the treatment selected based on accumulated population NHEs up to T* would match the treatment selected based on accumulated population NHEs up to TPop. Certain interventions may exhibit zero irrecoverable costs whereby health benefits are attained simultaneously with costs. If irrecoverable costs are a large proportion of overall NHEs this may imply a large initial negative NHE to be offset by future gains. If T* is low then it will be less likely that guidance will change before the cumulative incremental NHEs of adopting the intervention become positive. This would indicate that irrecoverable costs were not significant as they would have little influence on the alternative policy pay-offs. However, if T* is large the impact of irrecoverable costs must be judged alongside an assessment of the likelihood that guidance will change, and this is addressed further later in this appendix (see Impact of other sources of uncertainty and What is the impact of irrecoverable costs?).
Suppose that the cost of intervention 2 includes an upfront irrecoverable investment of £20M. In health terms this upfront cost would be expected to displace health activities that would have generated 1000 net health benefits:C(2)=£20,000,000k=1000. d
In Table 38 the NHE of this irrecoverable cost is separated out from the remaining costs and health effects. e
| θ | Irrecoverable costs | Population 1 | Population 2 | Population 3 | ||||
|---|---|---|---|---|---|---|---|---|
| NHE(1,θ) | NHE(2,θ) | NHE(1,θ) | NHE(2,θ) | NHE(1,θ) | NHE(2,θ) | NHE(1,θ) | NHE(2,θ) | |
| 1 | 0 | –1000 | 2475 | 3450 | 825 | 1550 | 825 | 1550 |
| 2 | 0 | –1000 | 450 | 4650 | 150 | 1950 | 150 | 1950 |
| 3 | 0 | –1000 | 3600 | 2850 | 1200 | 1350 | 1200 | 1350 |
| 4 | 0 | –1000 | 6150 | 5100 | 2050 | 2100 | 2050 | 2100 |
| E θ | 0 | –1000 | 3169 | 4013 | 1056 | 1338 | 1056 | 1338 |
Irrecoverable costs as a proportion of total NHEs for intervention 2 are 1000/5689 = 18%. If Intervention 2 is selected and the decision is unchanged over the time horizon TPop = 10 = TC, the impact of irrecoverable costs will have been adequately represented. The total NHEs from intervention 2 are 4013 + 1338 + 1338 – 1000 = 5689, which matches that calculated in Cost-effectiveness at the population level and is greater than that offered by intervention 1 (3169 + 1056 + 1056 = 5281). However, if the decision were changed earlier, at t = 6 (after population 1), the accumulated population NHEs for intervention 2 would be 4013 – 1000 = 3013, which is less than the NHEs that could have been realised with intervention 1 (3169). f If the guidance is altered at a time t < TC, the investment costs will have been underestimated and the decision to approve intervention 2 may have been in error. If the decision is changed at t = 8 (after population 2), the accumulated population NHEs for intervention 2 would be equal to 4013 + 1338 – 1000 = 4351, which is greater than that offered by intervention 1 over the same time horizon (3169 + 1056 = 4225) and so intervention 2 has ‘broken even’. In this example T* = 7.1.
Is further research required?
The resource implications and health outcomes used to inform reimbursement decisions about the use of treatments within the health-care sector cannot be known with absolute certainty. Uncertainties stem from the reliance on sample information and the choice of methods to combine information from different sources and to translate what that body of information implies for the particular decision problem under consideration. Cost-effectiveness analysis can be used to estimate which of a set of mutually exclusive interventions might offer the greatest health gain and to describe the impact of uncertainty on those estimates. 153 Table 35 shows a representation of the output from a cost-effectiveness analysis comparing two alternative interventions, j = 1,2. The range of plausible true values supported by the evidence used to calculate the costs, c, and health outcomes, h, for a typical patient who receives either intervention are described by θ, which can be labelled as a parameter or input that can take one of four possible values. As described in the previous section (see Is the intervention cost-effective and what are the risks?), cost-effectiveness can be determined by assessing expected NHEs. Tables 35–37 also characterise the uncertainty associated with this decision by showing the range of values that θ could take and the NHEs that would result from those values.
A decision based on expected NHEs could be regarded as being in error if the true value of θ suggests that the alternative intervention to the one reimbursed would have offered greater health gains. The probability that a decision to recommend a particular intervention is in error is equal to the probability of observing a value of θ for which a different intervention would maximise NHEs. Knowing more about θ enables a decision-maker to reduce the cost of uncertainty by reducing the possibility of error. By avoiding reimbursing an intervention that turns out to be cost-ineffective, the decision-maker would expect better health gains overall. 154
Consequences of uncertainty at the patient level
The best a decision-maker could do would be to select the intervention that maximised health gains for a particular realisation of θ, [inline]. Given the range of possible values for θ and the likelihood of observing those values it is possible to calculate the expected NHEs associated with this error-free choice:
The difference between the NHEs that could have been achieved without error (see Equation 4 ) and the best choice based on current information (see Equation 2 ) describes the health consequences of this uncertainty at the individual patient level. This represents the maximum improvement in NHEs that could result from further research on θ and is often described as the EVPI:
Table 39 repeats the NHEs per patient treated from Table 36 and in the final column presents the maximum NHE for each realization of θ. The final row shows the expected values assuming that each realisation of θ is equally likely.
| θ | NHE(1,θ) | NHE(2,θ) | Max. NHE(j,θ) |
|---|---|---|---|
| 1 | 0.41 | 0.48 | 0.48 |
| 2 | 0.08 | 0.68 | 0.68 |
| 3 | 0.60 | 0.38 | 0.38 |
| 4 | 1.03 | 0.75 | 1.03 |
| E θ | 0.53 | 0.57 | 0.69 |
As before, the expected value of intervention 2 is higher than that of intervention 1: EθNHE(1,θ) < EθNHE(2,θ). This suggests that if θ is unknown it is better to use intervention 2. However, if θ were known to be 3 or 4 then it would be better to use intervention 1 because NHE(2,3) < NHE(1,3), NHE(2,4) < NHE(1,4), whereas if θ were known to be 1 or 2 then it would be better to use intervention 2 because NHE(1,1) < NHE(2,1), NHE(1,2) < NHE(2,2). In this case the error probability is 0.5 (50%). The expected value of being able to make a decision knowing the exact value of θ is higher than the expected value of approving either intervention 1 or 2, suggesting that there is value in resolving θ:g EVPIθ = 0.69 – 0.57 = 0.12.
Consequences of uncertainty at the population level
To judge the scale of the improvement in health benefits that might result from a decision to conduct further research it is necessary to consider the number of individuals who could benefit. 53 In other words, it is necessary to estimate the number of times a decision is made that utilises the information generated. This will be a function of the prevalence and incidence of the disease in question as shown in Equation 3, but it may also be a function of the extent of other diseases for which the information may be relevant. Any process for evaluating the value for money of interventions or research will involve assumptions and value judgements about the quality and relevancy of available evidence and which analytical methods are appropriate for determining what that evidence implies for the decision problem at hand. Also, there can be ‘unknown unknowns’, unexpected future events that we know can occur from past experience but that cannot be predicted or parameterised within the analysis. The relevant time horizon over which the information will be utilised, TEVPI, can differ from the time horizon over which any particular intervention will be utilised, TPop. 53
For simplicity, in the example we assume that the time horizon for information, TEVPI, is coincident with the time horizon for the technology, TPop, and from this point denote both with T. We also assume that the relevant evidence is utilised only to make decisions between the two alternative interventions so far considered and therefore is utilised in the same number of decisions as the number of patients who receive treatment with either intervention. Table 40 shows the individual-level NHEs from Table 39 multiplied by the number of individuals who could benefit from either intervention or any additional information.
| θ | Population 1 | Population 2 | Population 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NHE(1,θ) | NHE(2,θ) | Max. NHE | NHE(1,θ) | NHE(2,θ) | Max. NHE | NHE(1,θ) | NHE(2,θ) | Max. NHE | |
| 1 | 2475 | 2850 | 2850 | 825 | 950 | 950 | 825 | 950 | 950 |
| 2 | 450 | 4050 | 4050 | 150 | 1350 | 1350 | 150 | 1350 | 1350 |
| 3 | 3600 | 2250 | 3600 | 1200 | 750 | 1200 | 1200 | 750 | 1200 |
| 4 | 6150 | 4500 | 6150 | 2050 | 1500 | 2050 | 2050 | 1500 | 2050 |
| E θ | 3169 | 3413 | 4163 | 1056 | 1138 | 1388 | 1056 | 1138 | 1388 |
Using Table 40 consider three simple policy options: to outright approve the new intervention (approve), to commission research (research) or to outright reject the new intervention (reject). The pay-offs from each policy in terms of the NHEs that accrue to each of the three patient populations [Π(Pop1), Π(Pop2), Π(Pop3)] and the total pay-off across all three populations (Π) are shown in Table 41.
| Policies | ∏(Pop1) | ∏(Pop2) | ∏(Pop3) | ∏ |
|---|---|---|---|---|
| 1. Approve | 3413 | 1138 | 1138 | 5688 |
| 2. Reject | 3169 | 1056 | 1056 | 5281 |
| 3. Research | 4163 | 1388 | 1388 | 6938 |
The total NHE of approving and implementing j = 2 in all populations, in this example 3413 + 1138 + 1138 = 5688, is more generally given by:
The total NHE of rejecting and continuing to implement j = 1 in all populations, in this example 3169 + 1056 + 1056 = 5281, is more generally given by:
As the NHE of j = 2 is higher than that of j = 1, the decision to approve is preferred to the decision to reject. The total NHE of being able to choose the appropriate treatment for each value of θ, in this example 4163 + 1388 + 1388 = 6938, is given by:
The most beneficial policy option is therefore research, which allows the decision-maker to implement treatment based on knowing θ, resulting in total expected NHEs of 6938. The difference between the pay-off from research and the next best pay-off from approve describes the maximum value of further research and is equivalent to multiplying Equation 5 by the size of the population to benefit (see Equation 3 ): EVPIθ. Pop = ΠResearch – ΠApprove = – 6938 – 5688 = 1250.
Subgroups
Just as cost-effectiveness can be assessed separately in different subgroups, so too can the value of further research. However, it may be necessary to consider whether information collected in one subgroup can be used to inform decisions in other subgroups. In these circumstances the number of patients who will benefit from the information can differ from the number of patients expected to benefit from each separate treatment decision. This should be reflected in calculating the value of further research.
Alternative scenarios
The example so far has considered uncertainty in the true value of θ, but uncertainty may also exist in how θ can be used to calculate cost and health outcomes. If there exists an alternative plausible characterisation of h and c as a function of θ this can produce modelling or structural uncertainty. 130,155,156 The alternative modelling or structural assumptions can be used to describe a series of scenarios within which expected NHEs will be estimated differently. From the decision-maker’s perspective, the NHEs will now be a function of the choice of treatment, parameter uncertainty and the choice of scenario, NHE(j,θ,s). The assessment of cost-effectiveness in Equation 2 is now made by taking the expectation of NHEs across the parameter uncertainty and the range and likelihood of the alternative scenarios, maxjEθEsNHE(j,θ,s).
The best a decision-maker could do would be to select the intervention that maximised health gains for a particular realisation of θ and s. The expected NHEs associated with this error-free choice given in Equation 4 become EθEsmaxjNHE(j,θ,s).
The added value of further research on parameter and scenario uncertainty can then be evaluated based on the results that integrate both parameter and scenario uncertainty. h
Table 42 presents the NHEs for each intervention based on competing plausible forms of the functions h(j,θ,2) and c(j,θ,2) to those that were presented in Table 40 [which can be characterised as having presented h(j,θ,1) and c(j,θ,1)].
| θ | Population 1 | Population 2 | Population 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NHE(1,θ) | NHE(2,θ) | Max. NHE | NHE(1,θ) | NHE(2,θ) | Max. NHE | NHE(1,θ) | NHE(2,θ) | Max. NHE | |
| 1 | 2475 | 1950 | 2475 | 825 | 650 | 825 | 825 | 650 | 825 |
| 2 | 450 | 3150 | 3150 | 150 | 1050 | 1050 | 150 | 1050 | 1050 |
| 3 | 3600 | 1350 | 3600 | 1200 | 450 | 1200 | 1200 | 450 | 1200 |
| 4 | 6150 | 3600 | 6150 | 2050 | 1200 | 2050 | 2050 | 1200 | 2050 |
| E θ | 3169 | 2513 | 3844 | 1056 | 838 | 1281 | 1056 | 838 | 1281 |
In this alternative scenario the NHEs of intervention 2 are estimated to be smaller than those associated with intervention 1 and so it no longer appears cost-effective. Within this scenario, the value of resolving θ is EVPIθ|s = 2·Pop = 6406 – 5281 = 1125, which differs to the value within scenario 1 (see Table 40) of EVPIθ|s = 1·Pop = 1250.
If either scenario is equally plausible, the expected NHEs of each intervention overall can be found by averaging the NHEs from each scenario. For example, the expected NHEs of approving intervention 2, accounting for two equally likely scenarios, s, are calculated based on a 50% chance of achieving the NHEs from Table 40 and a 50% chance of achieving the NHEs shown in Table 42: ΠApprove = EθEsNHE(2,θ,s) × Pop = 0.5(3413 + 1138 + 1138) + 0.5(2513 + 838 + 838) = 4938
As the expected population NHEs of intervention 1 are the same in both scenarios, ΠReject = EθEsNHE(1,θ,s) × Pop = 5281; this would suggest that intervention 2 was not cost-effective (ΠApprove < ΠReject).
Further research could be expected to resolve both parameter and structural uncertainty, meaning that the decision-maker could select the treatment that would provide the maximum NHEs given particular values of θ and s. When θ = 1 the maximum NHE is associated with intervention 2 in scenario 1 (see Table 40) and intervention 1 in scenario 2 (see Table 42). When θ = 2 the maximum NHE is associated with intervention 2 in both scenarios. When θ = 3 the maximum NHE is associated with intervention 1 in both scenarios. Averaging across these maximum values gives ΠResearch=EθEsmaxjNHE(j,θ,s)=6672. The value of research that would resolve both parameter and decision uncertainty is found by comparing EθEsmaxjNHE(j,θ,s) to the next best achievable pay-off of 5281. Thus, the value of research that would resolve both parameter and scenario uncertainty is 6672 – 5281 = 1391.
For the remainder of this appendix we assume that there is no alternative plausible set of modelling assumptions.
Does more research seem worthwhile?
Although resolving decision uncertainty results in the most beneficial outcome, whether or not further research should be undertaken will depend on the costs of carrying out the research and who will be responsible for these costs (e.g. the manufacturer or public funding bodies). When the costs of research fall on the budget constraint for providing health care this will displace other activities that could have generated health. This research cost must be subtracted from the policy pay-off from conducting further research. If the maximum value of research does not exceed the expected costs this is a sufficient condition to decide against investing in further research. 67
The value of research is established by comparing policy 3, research, with the next best alternative, which is policy 1, approve the new technology intervention 2. At the patient level this is equivalent to the EVPIθ detailed in Equation 5. Scaling up by the size of the population that would benefit from any additional information generated gives a maximum value for research of 1250 (EVPIθ × Pop). Using the same threshold, k = £20,000, the costs of research can be expressed in terms of health displaced, R. If the research costs are expected to exceed 1250 units of health benefit (or in monetary terms £25M), then policy 1 would be more beneficial than policy 3. Similarly, this would provide a sufficient condition for the decision to avoid the analysis time required for estimating more accurately the true value of research, which is discussed further in the following section (see How long until the research reports?). More generally, the pay-off from conducting additional research can be calculated as:
How does price affect the need for evidence?
If the decision to immediately approve an intervention does not seem to be the most valuable policy option, the manufacturer can alter the balance between the alternative decisions by adjusting the price. Reducing price will make an intervention appear more cost-effective by increasing its expected NHEs. A price change will also affect the value of conducting further research. 70 The subtraction of a constant does not reduce the variance in NHEs for a given intervention, but it will shift the distribution of the NHEs expected from the new intervention relative to the next best alternative and hence alter the distribution of incremental NHEs. This can change the probability of error and/or the value of the health benefits forgone if the wrong intervention were reimbursed, and can make research appear either more or less valuable. When price change reduces (increases) the proportion of incremental NHEs that are less than zero, the decision uncertainty is reduced (increased) following a price reduction. For an intervention that is expected to be cost-effective a price reduction will generally reduce the value of research because the probability of error and the health forgone as a consequence of error will both fall. This is because the health forgone given that the decision to reimburse was wrong is calculated by comparing the NHEs of the intervention that was expected to be cost-effective with the NHEs of an alternative, and this difference will have been reduced by the price reduction. For an intervention that is not expected to be cost-effective a price reduction will initially increase the probability of error but may reduce the health forgone as a consequence of error, and the overall impact on the value of further research is more difficult to predict. This is because the alternative interventions compared to calculate the amount of health forgone may not always have included the intervention that was subject to a change in price. As price is bounded at zero there is a limit to how much price adjustments can be used to increase NHEs and alter estimates of the value of research.
For example, suppose a research design is proposed costing £20M. If this money were diverted from existing health-care activities it would be expected to displace 1000 units of health benefit (R = £20M/k = 1000). On the basis of Table 41 research would seem to be the most valuable policy option, offering an expected pay-off in NHEs of ΠResearch = 6938 – 1000 = 5938. However, if the price of intervention 2 dropped from £1000 to £800 the pay-offs from the alternative policy options would alter. The impact of the price change is shown in Table 43.
| θ | Intervention 1 | Intervention 2 | Max. NHE | ||||
|---|---|---|---|---|---|---|---|
| h(1,θ) | c(1,θ) | NHE(1,θ) | h(2,θ) | c(2,θ) | NHE(2,θ) | ||
| 1 | 0.70 | £5750 | 0.41 | 1.00 | £8500 | 0.58 | 0.58 |
| 2 | 0.40 | £6500 | 0.08 | 1.30 | £10,500 | 0.78 | 0.78 |
| 3 | 0.90 | £6000 | 0.60 | 1.10 | £12,500 | 0.48 | 0.60 |
| 4 | 1.30 | £5500 | 1.03 | 1.40 | £11,000 | 0.85 | 1.03 |
| E θ | 0.83 | £5938 | 0.53 | 1.20 | £10,625 | 0.67 | 0.85 |
The expected NHEs for an individual receiving intervention 2 have increased from 0.57 to 0.67. There is still value in knowing θ because if θ were known to be equal to 3 or 4 then intervention 1 offers greater NHEs. After multiplying by the size of the population to benefit, the pay-offs from the policy options incorporating the new expected NHEs are shown in Table 44.
| Policies | ∏(Pop1) | ∏(Pop2) | ∏(Pop3) | ∏ |
|---|---|---|---|---|
| 1. Approve | 4013 | 1338 | 1338 | 6688 |
| 2. Reject | 3169 | 1056 | 1056 | 5281 |
| 3. Research | 4463 | 1488 | 1488 | 7438 |
After the price change the maximum value of research for the population has fallen to EVPIθ × Pop = 7438 – 6688 = 750, and this is no longer sufficient to outweigh the health benefits displaced by imposing research costs on the budget constraint. In this particular example, a price reduction has created a sufficient condition for immediate approval without further research. The threshold price for intervention 2 at which the optimal policy decision changes from research to approve is £900.
Figure 35 displays the pay-offs in terms of NHEs from the policy options in Table 41 as a function of the price of intervention 2, assuming that R = 1000.
FIGURE 35.
Impact of price on optimal policy decision (immediate research).
The pay-off from reject is unaffected by the price whereas the pay-off from immediate approval is a linear function of the price of intervention 2. When the price of intervention 2 exceeds £1081 it is no longer cost-effective (ΠReject > ΠApprove). The pay-off from research is a non-linear function of price in this example. The closer the price is to £1081, the more similar the NHEs from both treatments, hence the greater the decision uncertainty and the probability of error but the smaller the opportunity cost of selecting the wrong alternative. The policy with the highest pay-off switches as the price of intervention 2 increases. The figure shows how the optimal policy switches from approve to research at a price of £900. Once the price exceeds £1400 the optimal policy switches from research to reject.
Notice from Tables 36 and 43 that intervention 2 is associated with better health gains than intervention 1 for every value of θ. In other words, intervention 2 is known to be more effective than intervention 1. The uncertainty around whether intervention 2 is more cost-effective stems from whether the magnitude of the health gains is sufficient to justify the additional expense of intervention 2. A price reduction directly addresses this uncertainty by reducing the price of intervention 2, but does not have any impact on the health outcomes expected for patients treated with each intervention. However, for some comparisons the uncertainty around whether or not the new intervention is cost-effective will stem from if it improves or worsens health in the patients that receive treatment. A price reduction will still have the impact of making such an intervention appear more cost-effective because it subtracts a constant from the cost of an intervention, and this constant could be equivalently expressed in health terms (by utilising the threshold, k).
How long until the research reports?
The results of further research will in general not be available immediately, meaning that there exists a population of patients for whom the treatment decision must be made based on current evidence. Thus, there is a need to make a reimbursement decision for this initial population (approve or reject) in combination with a decision about whether or not to collect further research. This means that research alone is not a policy option, and the relevant alternatives are approve (without further research), approve with further research (AWR), OIR (i.e. reject and conduct further research) and reject.
The following general notation is for calculating the policy pay-offs when research will report at time t = τ. The pay-off from approve remains as described in Equation 6. If research was undertaken after approval (AWR) then the initial population receives the NHEs associated with intervention 2, but subsequent populations receive the maximum NHE because the uncertainty is resolved:
If research is undertaken after reject (OIR) then the initial population receives the NHE associated with intervention 1 whereas subsequent populations receive the maximum NHE:
If intervention 2 is rejected and no further research is undertaken then each population will receive the NHE associated with intervention 1 as described in Equation 7.
The numbers in Table 45 are calculated from Table 40 and show the policy pay-offs when research would report at τ = 6 (results of research available for populations 2 and 3). i For example, if research was undertaken after approval then population 1 receives the NHEs associated with intervention 2, but populations 2 and 3 receive the maximum NHE because the uncertainty is resolved. If research is undertaken after rejecting intervention 2 then population 1 receives the NHE associated with intervention 1 and populations 2 and 3 receive the maximum NHE. If intervention 2 is rejected and no further research is undertaken then each population will receive the NHE associated with intervention 1.
| Policies | ∏(Pop1) | ∏(Pop2) | ∏(Pop3) | ∏ |
|---|---|---|---|---|
| 1. Approve (no research) | 3413 | 1138 | 1138 | 5688 |
| 2. AWR (approve and research) | 3413 | 1388 | 1388 | 6188 |
| 3. OIR (reject and research) | 3169 | 1388 | 1388 | 5944 |
| 4. Reject (no research) | 3169 | 1056 | 1056 | 5281 |
In this example research is better than not doing research as different values of θ imply different reimbursement decisions. As was noted earlier (see Does more research seem worthwhile?, How does price affect the need for evidence?) there is a 50% chance that once the results of research are known the intervention regarded as cost-effective will change. As τ < T this implies a 50% chance that guidance will change before the end of the lifetime of the technology following a policy decision of AWR or OIR. If we assume that research costs do not fall on the budget constraint for health care then AWR will always dominate approve and OIR will always dominate reject. The value in collecting further evidence is demonstrated by the fact that the total expected pay-off in terms of health effects from policy 2 exceeds that of policy 1 (ΠAWR > ΠApprove), and similarly the expected health benefits from policy 3 exceed those offered by policy 4 (ΠOIR > ΠReject). Given the simple example presented so far, AWR will always dominate OIR when the new alternative is regarded as cost-effective (and vice versa when the new alternative is regarded as cost-ineffective).
However, given a cost of research of R = 1000 that does fall on the budget constraint for health care, the additional net health benefits from research are insufficient to outweigh the opportunity cost of gathering that information. When research costs are subtracted from the policies that incorporate research in Table 45 the pay-off from policy 1 exceeds that from policy 2 ΠApprove = 5688 compared with ΠAWR – R = 6188 – 1000 = 5188) and the pay-off from policy 4 exceeds that from policy 3. It is still better to approve than reject because intervention 2 is cost-effective in this example.
How does price affect the choice between approve, ‘approval with research’, ‘only in research’ and reject?
Recall from Does more research seem worthwhile?, How does price affect the need for evidence? that for a cost-effective intervention a price reduction will generally reduce the value of research and hence improve the pay-off from approve relative to AWR. Also, a price reduction makes an intervention appear more cost-effective, improving the pay-off from approve relative to reject (see Is the intervention cost-effective?, How does price affect cost-effectiveness?). The NHEs that accrue to patients after research reports are assumed to be the same in AWR and OIR (although this will be relaxed later; see Will the research be conducted?), and so the threshold price at which the pay-offs from AWR and OIR are equivalent is the same as the threshold price for cost-effectiveness, that is, where the pay-offs from approve and reject are equivalent. However, depending on the value of further research and how price change affects this value, for a technology that is not expected to be cost-effective the initial decision may be reject or OIR and as the price falls the optimal decision could switch between reject and OIR before changing to AWR or straight to approve when the price is low enough for the technology to be regarded as cost-effective.
The pay-offs in Table 45 are based on a price of £1000 for intervention 2. Figure 36 shows how the optimal policy changes as the price of intervention 2 changes, assuming research costs of R = 1000.
FIGURE 36.
Impact of price on optimal policy decision (research available Population 2).
The policies that entail research (AWR and OIR) do not offer the highest pay-off for any price of intervention 2 and hence the optimal policy switches from approve to reject at a price of £1081. Above this price approve (AWR) ceases to dominate reject (OIR) because intervention 2 is no longer cost-effective. When the difference between AWR and approve, or between OIR and reject, reaches its nadir it is equal to minus the cost of research (e.g. ΠAWR –ΠApprove = –R), which indicates that there is no cost of uncertainty. When the difference is not equal to minus the cost of research, this indicates that there were benefits of research but these were insufficient to outweigh those costs.
How does time to research affect the choice between approve, ‘approval with research’, ‘only in research’ and reject?
The numbers in Table 46 are also calculated from Table 40. In this case further research takes longer to report (τ = 8) and so only population 3 benefits from resolving θ.
| Policies | ∏(Pop1) | ∏(Pop2) | ∏(Pop3) | ∏ |
|---|---|---|---|---|
| 1. Approve (no research) | 3413 | 1138 | 1138 | 5688 |
| 2. AWR (approve and research) | 3413 | 1138 | 1388 | 5938 |
| 3. OIR (reject and research) | 3169 | 1056 | 1388 | 5613 |
| 4. Reject (no research) | 3169 | 1056 | 1056 | 5281 |
Table 46 demonstrates that the benefits of research decrease if it takes longer to report. As the population that benefits from research decreases the decision-maker is more likely to select policy 1 over policy 2 or policy 4 over policy 3.
Recall that in the simple example presented so far, AWR will always dominate OIR when the new alternative is regarded as cost-effective and OIR will always dominate AWR when the new alternative is regarded as cost-ineffective. This will not always be the case. In the next section we consider the interaction between the reimbursement decision and the ability to conduct the required type of research.
Is the research possible if the intervention is approved?
The prospects of acquiring information through research may be affected by the decision to approve for a number of reasons: (1) the adoption of a technology removes incentives on the manufacturer to conduct further research, (2) the early diffusion of a technology means that future clinical trials are less likely to be supported or regarded as ethical by the clinical community, even when public funds are made available for such research and (3) patients are unlikely to enrol in clinical trials once they have unrestricted access to the new technology. By rejecting a new technology in favour of current practice, the prospects for future research are unaffected: the manufacturer of the new technology retains an incentive to conduct research to obtain approval and no change in clinical practice occurs to damage existing trials or remove the ethical basis for future research. 70
If the new intervention is expected to be cost-effective but research is not possible if the new intervention is approved then policy 2 in Table 45 is no longer available. Even though the new intervention is cost-effective it may be preferable to reject and then carry out research (OIR) rather approving with no research (approve). This is the case in Table 45 where the pay-off from policy 3 exceeds the pay-off from policy 1. In general, the balance between OIR and approve will depend on whether or not the increase in population health benefits that arises from resolving uncertainty exceeds the increase in population health benefits from approving the new technology in the population of patients who receive treatment before research reporting. The size of this trade-off is determined by the extent of cost-effectiveness, the amount of time it takes for research to report or the size of the populations affected and the value of resolving uncertainty. If research takes longer to report, as in Table 46, then policy 3 no longer dominates policy 1 and a sufficient condition for immediate approval is met. In the case in which the new technology is not cost-effective the optimal decision is unchanged by the fact that evidence cannot be gained after approval, as it depends only on whether there is an additional benefit of carrying out further research (see Is further research required?).
Some types of uncertainty, such as long-term safety, are easier to resolve if the new technology is approved. If it is the case that research cannot be undertaken without approval then policy 3 in Table 45 is not an option. If the new intervention is cost-effective the decision does not change and the choice between policies 1 and 2 depends on the value of resolving the uncertainty. If, however, the new intervention is not cost-effective it might be the case that policy 2, AWR, is preferred to policy 4, reject with no research. This would occur if the value of resolving the uncertainty is greater than the health benefits lost from displaced activities as a result of approving an intervention that is not expected to be cost-effective. In the later section What type of evidence is needed? we consider the different types of uncertainty that may require different research designs and how these may be affected differently by the decision to approve a technology for widespread use.
Will the research be conducted?
As already discussed, the potential value of research depends on the likelihood that it will be conducted and the timing of when it will report. For reasons just described, in some cases further research is not possible if the new technology is approved. In other cases the research might be possible but may still not be conducted if the decision-maker must rely on other agents to commission or complete the required research. For example, the manufacturer may not have an incentive to conduct socially valuable research if it would not increase profits, and this is more likely for a technology that has already been approved. Even once research is under way studies may fail or remain unreported because of reasons outside the decision-maker’s control.
To incorporate the uncertainty of whether research will be conducted and/or reported we introduce α, the probability that the results of further research will become available. In Table 45 it is assumed that α = 1, but if it is < 1 then the reduced probability of research means that the expected NHEs from policies 2 and 4 will be lower. In reality policy 2 is weighted by the probability of no research being undertaken.
The expected NHEs for policies that entail further evidence collection (AWR and OIR) incorporate the expected benefits received by patients after research reports. The pay-offs from these policies were calculated in How long until research reports? in which the benefits of research expected to report at time τ were estimated within Equations 10 and 11 by
Incorporating the probability that research will be conducted, this expression now becomes, in the case of AWR:
and in the case of OIR:
Given that the number of potentially worthwhile research proposals exceeds available research funding, the probability of any particular proposal being funded will depend on its value relative to the relevant alternative research proposals. Because of the interaction between the adoption decision and the prospects for further research, as discussed in Is the research possible if the intervention is approved?, the probability of research following a decision to approve, αApprove, may differ from the probability of research following a decision to reject, αReject. If αApprove > αReject then AWR would be expected to yield higher NHEs for patients after research has reported than OIR. The section Is the research possible if the intervention is approved? considers the extreme situation in which αApprove = 0 and αReject = 1 and AWR is not an option, but if 0 < αApprove < αReject the pay-off from AWR could be either higher or lower than that of OIR depending on the trade-off between greater upfront benefits for patients receiving a more cost-effective intervention and lower expected benefits from further research for later populations.
When might it be commissioned and report?
As previously discussed (see How long until research reports?), the length of time until research reports, τ, affects the size of the population that benefits from immediate approval or rejection and the size of the population that benefits from further research. Previously we considered τ to be at t = 6 (research available for population 2) in Table 45 or at t = 8 (research available for population 3) in Table 46. As discussed in the previous section, future research may be regarded as a chance event, with a probability of occurrence, α, that can be < 1. Based on an assessment of cost-effectiveness and an assessment of the value of further research it is possible to describe the range of possible values of τ and α for which the NHEs of immediate approval will exceed those of initial rejection of a given intervention.
Figure 37 shows an example in which the possibility of research if the new technology is approved is zero (αApprove = 0). When this is the case policy option 2 in Tables 45 and 46 is no longer available. An initial decision to reject the new intervention (e.g. reject or OIR) is assumed to not affect the prospect for research, but research is not certain to proceed for the reasons discussed earlier. For combinations of τ and αReject located to the north-east of the boundary in Figure 37, the benefits of immediate approval exceed the health benefits forgone from the reduced possibility of research, and a necessary condition for immediate approval is met. This is because the pay-off from approve exceeds the policy pay-off from OIR based on the maximum value of further research that would resolve all of the uncertainty that was characterised in the evaluation of NHEs. So in Table 46, when research is available for population 3 and is certain to occur, the policy to approve exceeds the value of OIR. The point at which τ = 2 and αReject = 1 is to the north-east of the boundary and a sufficient condition for immediate approval is met. If research takes a shorter time to report, as in Table 45, then as more patients stand to benefit from the additional information OIR increases in value relative to approve. The point at which τ = 1 and αReject = 1 is located inside the boundary and a sufficient condition for immediate approval is not met.
FIGURE 37.
A sufficient boundary for immediate approval.
The value of α can be inferred from system factors and previous experience. The value of α will be higher if the decision-making body determining approval also has the remit to commission further research and higher still if it has access to funding. In the scenario in which only the manufacturer has funding for research, incentives must be properly aligned to assume that α will be high. Even when research is under way, α may still not be equal to 1 if there is a chance that the research will not be reported. These factors will also influence the time until research reports. Research requiring large populations or undertaken in diseases with a low incidence and prevalence may be hindered by recruitment. Certain research questions will require longer follow-up and different types of research may be more complicated and time-consuming to execute (see What type of evidence is needed?).
Figure 37 allows decision-makers to incorporate their own values for τ and α. Alternatively, a quantitative analysis could describe the values of τ and α supported by available evidence by assigning to them probability distributions, which could be correlated if appropriate. 70 These could be utilised in the calculation of policy pay-offs described in Will the research be conducted? to estimate the pay-off from AWR or OIR conditional on τ and α. The decision-maker can then compare the expected policy pay-offs. However, this type of analysis provides a sufficient but not necessary condition for approval, that is, if approve offers the highest pay-off then it will be optimal to approve the intervention, but in some cases in which approve does not offer the highest pay-off it would still have been optimal to approve. Equivalently, it only provides a necessary condition for OIR because the research, if and when it does report, will not resolve all uncertainty. In other words, when OIR or AWR offers the highest pay-off it is not always the case that it is the optimal policy. Therefore, a sufficient condition for AWR or OIR would require consideration of how much uncertainty is likely to be resolved by the research.
How much of the uncertainty will the research resolve?
So far we have discussed the assessments that can be made about the value of further evidence. The assessments describe the value of further evidence that would eliminate all uncertainty. This is the maximum value that additional information could be expected to provide. A single study, no matter the size, is unlikely to take all of the measurements of interest to inform a cost-effectiveness analysis that draws together information on costs, quality of life, prognosis and efficacy, and this issue is addressed in the following section, which discusses the assessments required to describe the value of further evidence that would eliminate all uncertainty for only some of the elements of the decision problem. In practice, of course, new evidence would be expected to lessen rather than eliminate uncertainty. 157 When taking measurements from a sample of the relevant population, a larger sample would produce a more certain result, but unless we measure the entire population some measurement uncertainty will remain. However, it is important to note that this value uncertainty does not necessarily result in decision uncertainty. 153 Regarding Table 36, if further research reduced the uncertainty in θ so that it was known to take a value of either 1 or 2, then measurement error would remain but the decision uncertainty would be eliminated. Intervention 2 would have higher NHEs than intervention 1 no matter how θ resolved and the probability of error would be 0. Finally, there exist sources of uncertainty that cannot be resolved through further research. Although some of these may be resolved over time (see Impact of other sources of uncertainty), others will remain.
The process of assessing the value of sample information is simply that of asking what changes we could expect in our decisions if we obtained further information from additional research. The first step is to predict the possible outcome of this hypothetical new research. The second step is to incorporate the new evidence with the existing evidence to get an updated estimate of expected costs and health outcomes in order to record the difference in the NHEs we would expect from a revised decision compared with our original decision. Finally, as we could imagine many possible outcomes for the new study, we must repeat steps one and two until the full range of possible outcomes is adequately described. We can then compare the expected value of our revised decision with additional sample information with the value of the decision based on current evidence. 157 Although this process may be conceptually straightforward, in current practice with current computing capabilities it can entail very long analysis times, making it sometimes impractical for informing decision-making in a timely manner. This is especially true when considering the task of evaluating the value of the full range of possible study designs that could potentially inform the decision problem.
The expected gain in health from the additional information provided by future studies must be balanced against the cost of those studies. The notion of who would bear the costs of research was discussed in Does more research seem worthwhile?, but regardless of this the opportunity costs to patients included in research must be accounted for. Additional experimental research may assign recruited patients to receive interventions that they would otherwise not have had access to. The benefits that accrue to these research participants may differ from the benefits that accrue to those in the wider population, and so should also be assessed separately. If a RCT comparing two interventions recruits a total of n patients with a 1 : 1 allocation procedure then 0. 5n patients will have different expected NHEs from the remaining Pop – 0. 5n, and this should be reflected in any calculations. It may also be necessary to consider whether additional investment is required to ensure that the results of research will be translated into clinical practice. 73,158
Recall from the section Is further research required? that it is possible to establish a necessary condition for immediate approval on the basis of establishing the value of research that would eliminate all uncertainty. Thus, in Figure 37, if the decision-maker suspects that the likelihood and timing of further research are such that the pay-off from approve would be above the boundary, the intervention should be approved for immediate use. However, for points inside the boundary a sufficient condition for OIR is not met. Points inside a boundary that was drawn based on the expected value of sample information would provide a sufficient condition for OIR, but this boundary may not be estimable within reasonable analysis time for reasons already described. Nonetheless, an assessment of the additional value that research would generate could be used to draw a boundary that provides a necessary and sufficient condition for immediate approval, as shown by the dashed line in Figure 38. Such information could be a useful guide to decision-makers in possession of the expected value of a particular proposed study and would allow them to incorporate different views on the timing and likelihood of that research study.
FIGURE 38.
Necessary and sufficient boundaries for immediate approval.
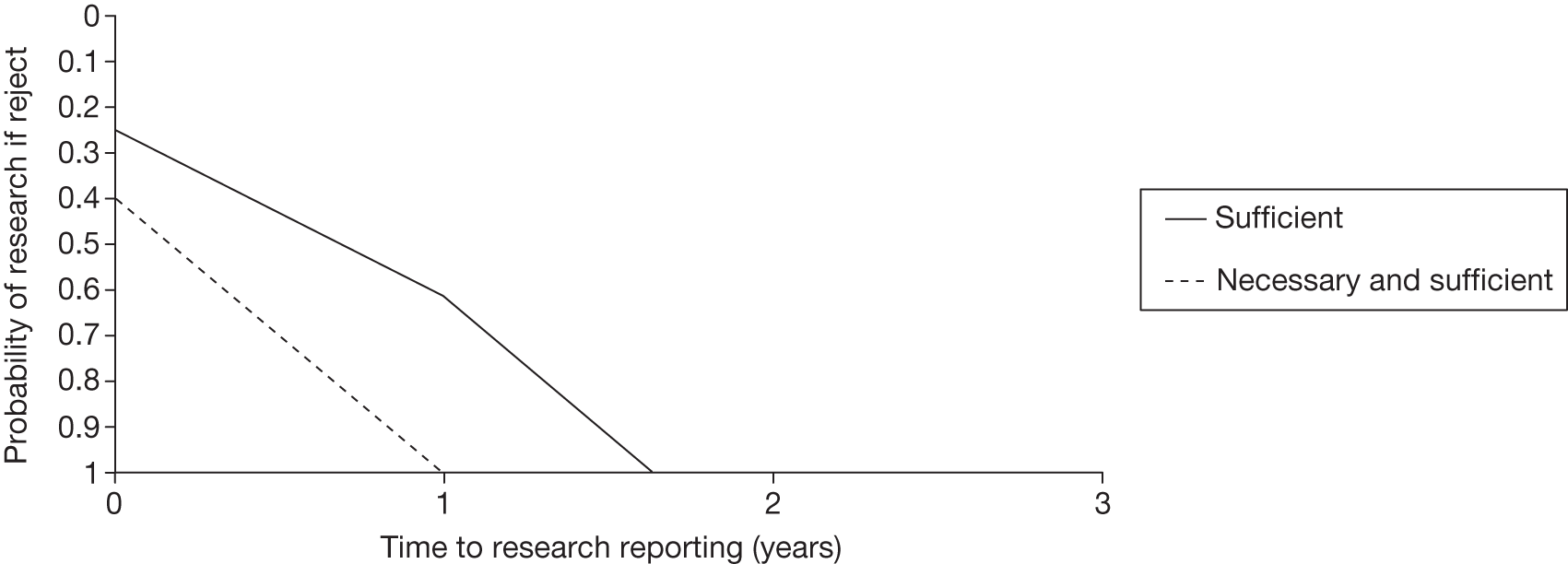
Alternatively, given a pair of values for τ and α, it is possible to calculate what value of sample information would generate a boundary line that would pass through that point. This describes the minimum additional value that further research must generate to make OIR the optimal policy decision. In other words, when the sufficient condition for approval is not met we could ask how few additional health benefits would have to be generated from further sample information to mean that a given point would be to the north-east of a boundary based on sample information.
In Figure 38, if the decision-maker believes that research would be available for population 2 and thinks that there is an 100% chance of research if they reject the new technology, they would not be convinced that the benefits of immediate approval could exceed the value of evidence forgone should approval reduce the probability of research to zero (see Table 45 and Figure 37; τ = 1 and αReject = 1 lies to the south-west of the boundary). However, this boundary is drawn considering the maximum benefit of further evidence, with which a policy of OIR generates 5944 – 5688 = 256 additional units of NHE relative to approve. As can be seen from Table 45, the policy of OIR generates an opportunity cost of 3169 – 3413 = –244 in the first period. However, after research has reported for populations 2 and 3 it generates additional expected NHEs of (1388 + 1388) – (1138 + 1138) = 500, outweighing the initial loss. New evidence must generate additional value of at least 244 units of NHE to outweigh the initial opportunity cost for OIR to dominate approve. The decision-maker must then assess whether they believe that new research would provide greater health benefits than this cut-off.
How does price affect the need for evidence?
In Does more research seem worthwhile? it was shown that reducing the price of a new technology would increase the benefits of immediate approval and reduce the benefits of further research, shifting the optimal policy decision from OIR or AWR to approve. The impact of a price reduction is to shift the sufficient boundary for immediate approval to the south-west. This is shown in Figure 39.
FIGURE 39.
Impact of price change on sufficient boundary for immediate approval.
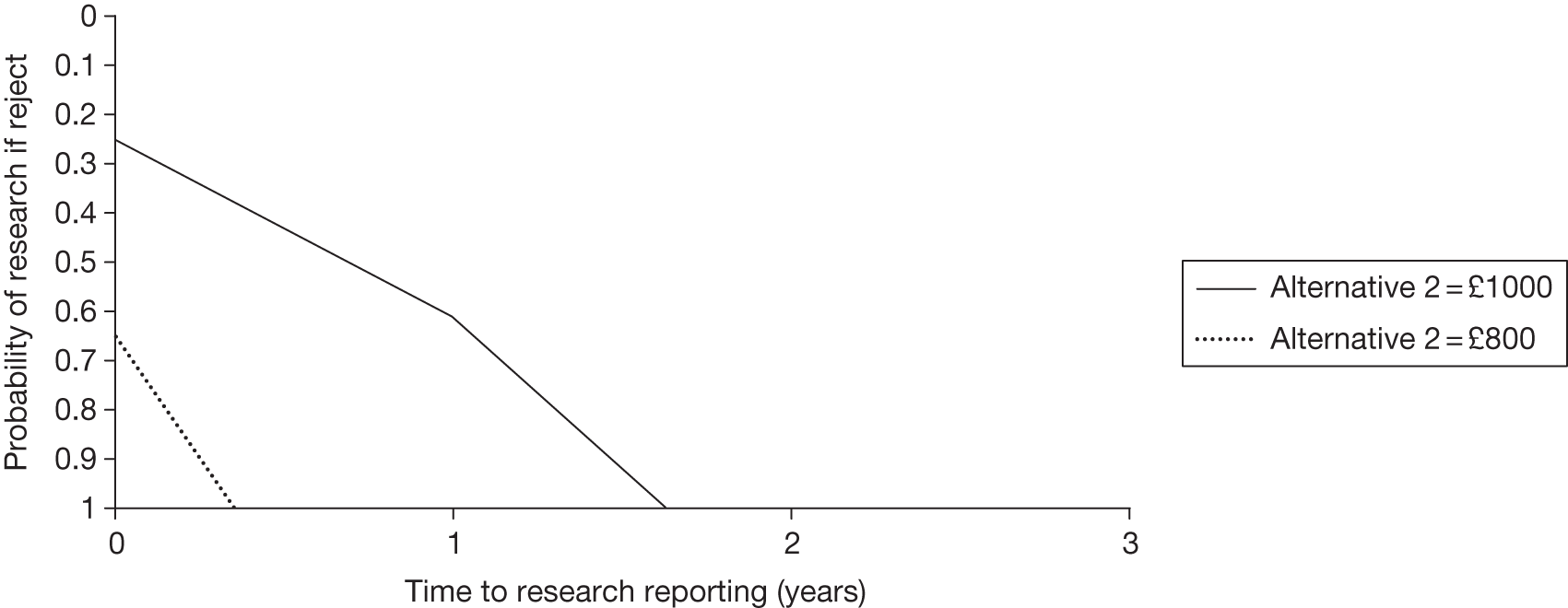
The price change from £1000 to £800 means that the point at which τ = 1 and αReject = 1 goes from being south-west of the original boundary to being north-east of the new boundary, and a sufficient condition for immediate approval is met.
How does current uncertainty affect the need for evidence?
The manufacturer of an intervention can also move this boundary for immediate approval by providing additional evidence to support the use of the technology. Reducing current uncertainty causes the added value of gathering further evidence to diminish, shifting the boundary to the south-west. Suppose that the manufacturer of intervention 2 supplied the results of an additional study that reduced the uncertainty about the increase in NHEs relative to intervention 1. Table 47 shows the NHEs associated with each intervention incorporating the additional information.
| θ | Intervention 1 | Intervention 2 | Max. NHE | ||||
|---|---|---|---|---|---|---|---|
| h(1,θ) | c(1,θ) | NHE(1,θ) | h(2,θ) | c(2,θ) (£) | NHE(2,θ) | ||
| 1 | 0.70 | £5750 | 0.41 | 1.0 | £11,000 | 0.45 | 0.45 |
| 2 | 0.40 | £6500 | 0.08 | 1.2 | £15,000 | 0.45 | 0.45 |
| 3 | 0.90 | £6000 | 0.60 | 1.3 | £14,000 | 0.60 | 0.60 |
| 4 | 1.30 | £5500 | 1.03 | 1.3 | £10,500 | 0.78 | 1.03 |
| E θ | 0.83 | £5938 | 0.53 | 1.2 | £12,625 | 0.57 | 0.63 |
The expected health outcomes and costs of intervention 2 are similar to those in Table 36 but the uncertainty is reduced. Additional information would now lead to a change in decision only if θ = 4, and so the error probability has fallen from 0.5 to 0.25. The consequences of this uncertainty have fallen to less than 0.06 units of NHE per individual. Figure 40 shows the impact of this on the boundary for immediate approval, after multiplying these individual NHEs by the relevant population to benefit.
FIGURE 40.
Impact of additional evidence on sufficient boundary for immediate approval.
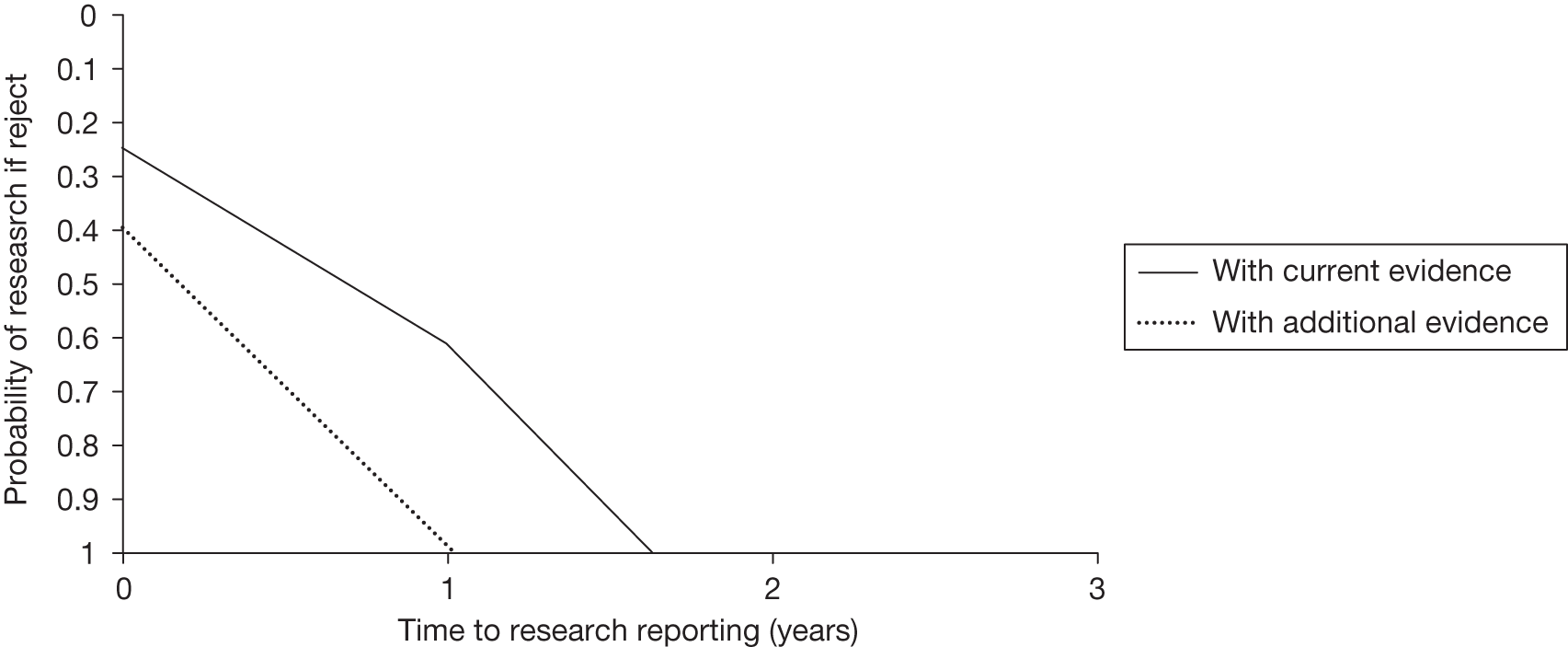
In this example the additional evidence in Table 47 shifts the boundary to the south-west, but not by enough to make immediate approval appear optimal when τ = 1 and αReject = 1. In other words, the policy of OIR would dominate the policy of approve. However, this boundary is drawn based on the maximum value of further research, and as discussed earlier it is shown that a boundary based on the actual value of further research would lie further to the south-west. Unless the decision-maker believed that further research would provide NHEs very close to this maximum value, the reduction in current uncertainty would result in a sufficient and necessary condition for immediate approval.
What type of evidence is needed?
Earlier we discussed how to determine whether further research is worthwhile (see Is further research required?). It would also be useful to know what type of additional evidence would be most valuable. In this section we expand our established framework to show the value of different types of information and the importance of the sequence of data collection. This information can be used to prioritise data collection but also becomes important in determining a decision to approve if certain types of research are not possible with approval (see Is the research possible if the intervention is approved?).
We can expand Table 39 to describe a situation in which the available evidence is made up of two uncertain parameters, θ = θ1,θ2, resulting in Table 48. The first row of Table 48, when θ = 1, is a result of θ1 = 1 and θ2 = 1. In this example, if θ2 = 1 then intervention 2 is preferred if θ1 = 1 but intervention 1 is preferred if θ1 = 2.
| θ | θ1 | θ2 | NHE(1,θ) | NHE(2,θ) | Max. NHE |
|---|---|---|---|---|---|
| 1 | 1 | 1 | 0.41 | 0.48 | 0.48 |
| 2 | 2 | 0.08 | 0.68 | 0.68 | |
| 3 | 2 | 1 | 0.60 | 0.38 | 0.60 |
| 4 | 2 | 1.03 | 0.75 | 1.03 | |
| E θ | 0.53 | 0.57 | 0.69 |
As was done previously in Table 37, in Table 49 we multiply the individual NHEs by the size of the relevant patient population (given by Equation 3 ), split into three time periods.
| θ | θ1 | θ2 | Population 1 | Population 2 | Population 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NHE(1,θ) | NHE(2,θ) | Max. NHE | NHE(1,θ) | NHE(2,θ) | Max. NHE | NHE(1,θ) | NHE(2,θ) | Max. NHE | |||
| 1 | 1 | 1 | 2475 | 2850 | 2850 | 825 | 950 | 950 | 825 | 950 | 950 |
| 2 | 2 | 450 | 4050 | 4050 | 150 | 1350 | 1350 | 150 | 1350 | 1350 | |
| 3 | 2 | 1 | 3600 | 2250 | 3600 | 1200 | 750 | 1200 | 1200 | 750 | 1200 |
| 4 | 2 | 6150 | 4500 | 6150 | 2050 | 1500 | 2050 | 2050 | 1500 | 2050 | |
| E θ | 3169 | 3413 | 4163 | 1056 | 1138 | 1388 | 1056 | 1138 | 1388 | ||
The expected values assume that each value is equally likely for both θ1 and θ2. With more than one type of uncertainty it is possible that further research would gather information on a subset of the available evidence. To determine the benefit associated with resolving only θ1 we find the expected NHEs of each intervention for each possible value of θ1. In this case the benefit associated with θ1 = 1 in population 1 for intervention 1,Eθ2|θ1=1NHE(1,θ1=1,θ2). ∑t=16It(1+d)t,is the average of 2475 and 450, which is equal to 1463 and the NHE associated with θ1 = 1 in population 1 for intervention 2, Eθ2|θ1=1NHE(2,θ1=1,θ2). ∑t=16It(1+d)t,is the average of 2850 and 4050, which is equal to 3450. The full range of effects is calculated and shown in Table 50 for the three patient populations. Knowing whether θ1 would resolve at 1 or 2 would allow the decision-maker to obtain the maximum net benefits of Eθ1maxjEθ2|θ1NHE(j,θ1,θ2). Pop=4163+1388+1388=6938.
| θ1 | Population 1 | Population 2 | Population 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NHE(1,θ1) | NHE(2,θ1) | Max. NHE | NHE(1,θ1) | NHE(2,θ1) | Max. NHE | NHE(1,θ1) | NHE(2,θ1) | Max. NHE | |
| 1 | 1463 | 3450 | 3450 | 488 | 1150 | 1150 | 488 | 1150 | 1150 |
| 2 | 4875 | 3375 | 4875 | 1625 | 1125 | 1625 | 1625 | 1125 | 1625 |
| E θ | 3169 | 3413 | 4163 | 1056 | 1138 | 1388 | 1056 | 1138 | 1388 |
The benefit of resolving uncertainty in θ2 can be calculated in the same way and is shown in Table 51. Knowing whether or not θ2 would resolve at 1 or 2 would allow the decision-maker to obtain the maximum NHEs of 3656 + 1219 + 1219 = 6094. In both tables the expected NHEs of approving the cost-effective intervention 2, based on current evidence, is unchanged at 3413 + 1138 + 1138 = 5688. However, the gain in expected NHEs from research on θ1 is greater than the expected gain from further research on θ2.
| θ2 | Population 1 | Population 2 | Population 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NHE(1,θ2) | NHE(2,θ2) | Max. NHE | NHE(1,θ2) | NHE(2,θ2) | Max. NHE | NHE(1,θ2) | NHE(2,θ2) | Max. NHE | |
| 1 | 3038 | 2550 | 3038 | 1013 | 850 | 1013 | 1013 | 850 | 1013 |
| 2 | 3300 | 4275 | 4275 | 1100 | 1425 | 1425 | 1100 | 1425 | 1425 |
| E θ | 3169 | 3413 | 3656 | 1056 | 1138 | 1219 | 1056 | 1138 | 1219 |
Previously, Tables 44–46 considered the potential pay-offs from research that would resolve all uncertainty, θ, that is, both θ1 and θ2. Assuming that research designs to resolve θ1 or θ2 individually would be available for population 2 (τ1 = τ2 = 6), and that research is certain to report (α = 1), we consider an expanded range of potential adoption and research decisions in Table 52.
| Policies | ∏(Pop1) | ∏(Pop2) | ∏(Pop3) | ∏ |
|---|---|---|---|---|
| 1. Approve | 3413 | 1138 | 1138 | 5688 |
| 2. AWR(θ1) | 3413 | 1388 | 1388 | 6188 |
| 3. AWR(θ2) | 3413 | 1219 | 1219 | 5850 |
| 4. AWR(θ) | 3413 | 1388 | 1388 | 6188 |
| 5. OIR(θ) | 3169 | 1388 | 1388 | 5944 |
| 6. OIR(θ1) | 3169 | 1388 | 1388 | 5944 |
| 7. OIR(θ2) | 3169 | 1219 | 1219 | 5606 |
| 8. Reject | 3169 | 1056 | 1056 | 5281 |
The policies and results for 1, 4, 5 and 8 are the same as those presented in Table 45. The NHEs for policies 2 and 6 are calculated by assuming that only θ1 is resolved in populations 2 and 3 (see Table 50). The NHEs for policies 3 and 7 are calculated by assuming that only θ2 is resolved in populations 2 and 3 (see Table 51). There are now a range of possible OIR and AWR decisions that differ in the type of additional evidence sought. For example, the policy to reject intervention 2 and conduct further research on θ1 can be denoted OIR(θ1). The policy to reject intervention 2 and conduct research on all (both) parameters is OIR(θ). Policies 2–4 represent AWR decisions whereas policies 5–7 represent OIR decisions.
The results reported in Table 52 suggest that there is no additional benefit in resolving θ2 over and above that realised if θ1 is resolved. The most beneficial policy is to approve and then research θ1, that is, an AWR(θ1) decision.
The formal notation for the additional reimbursement and research decisions described in Table 52 are:
-
Approve: see Equation 6
-
Approve and research θ1:[Equation 14]ΠAWR(θ1)=maxjEθNHE(j,θ). ∑t=1τ1It(1+d)t+Eθ1maxjEθ2NHE(j,θ). ∑t=τ1TIt(1+d)t
-
Approve and research θ2:[Equation 15]ΠAWR(θ2)=maxjEθNHE(j,θ). ∑t=1τ2It(1+d)t+Eθ2maxjEθ1NHE(j,θ). ∑t=τ2TIt(1+d)t
-
Approve and research θ (both θ1 and θ2): see Equation 10
-
Reject and research θ1 and θ2: see Equation 11
-
Reject and research θ1:[Equation 16]ΠOIR(θ1)=EθNHE(1,θ). ∑t=1τ1It(1+d)t+Eθ1maxjEθ2NHE(j,θ). ∑t=τ1TIt(1+d)t
-
Reject and research θ2:[Equation 17]ΠApprove=maxjEθNHE(j,θ). Pop
-
Reject: see Equation 7.
What type of research is possible if the technology is approved?
The section Is the research possible if the intervention is approved? discussed the fact that some types of research may not be possible once a new intervention is approved for widespread use. In this example if research to inform θ1 is not possible once the new intervention is approved, then policies 2 and 4 in Table 52 are not options. For example, this type of trade-off may arise if a RCT is the appropriate research design for gathering further information on θ1, whereas an observational study could inform θ2. The decision-maker must choose between immediate approval, with further research possible only on θ2, or rejection and the potential to conduct further research on either or both types of uncertainty. In these circumstances the expected pay-offs in health effects associated with policies 5 and 6 (ΠOIR(θ),ΠOIR(θ1)) are greater than those of policies 1 and 3 (ΠApprove,ΠAWR(θ2)), that is, it is more beneficial to utilise OIR and reject in order to allow research about θ1 than to approve or utilise AWR restricted to research that would inform only θ2. The expected health gains from conducting the more valuable research design exceed the opportunity cost of delaying access to the new intervention.
If the observational study design to inform θ2 is viable only following approval, then policies 5 and 7 in Table 52 are no longer options. The decision-maker may be faced with the choice between approving the new intervention in order to obtain information on θ2 and rejecting in order to obtain information on θ1. In this example, policy 6 (ΠOIR(θ1=5944)) is more valuable than policy 3 (ΠAWR(θ2=5850)). However, this balance could be altered if the research designs impose different costs on the budget for health care. For example, if research to inform θ2 costs £200,000(R2=£200,000k=10)whereas research to inform θ1 costs £4M (R1 = 200) then the pay-off from AWR with further research on θ2, ΠAWR(θ2)−R2=5850−10=5840, would offer greater expected NHEs than policy 6, ΠOIR(θ1)−R1=5944−200=5744.
How does price affect the type of research required?
Figure 41 shows the frontier of optimal policy choices as a function of the price of intervention 2 and with research costs of R1 = 200, R2 = 10 and R1,2 = 210. The frontier shows that the choice between approve, reject, AWR and OIR is sensitive to the price of intervention 2.
FIGURE 41.
Optimal policy frontier.
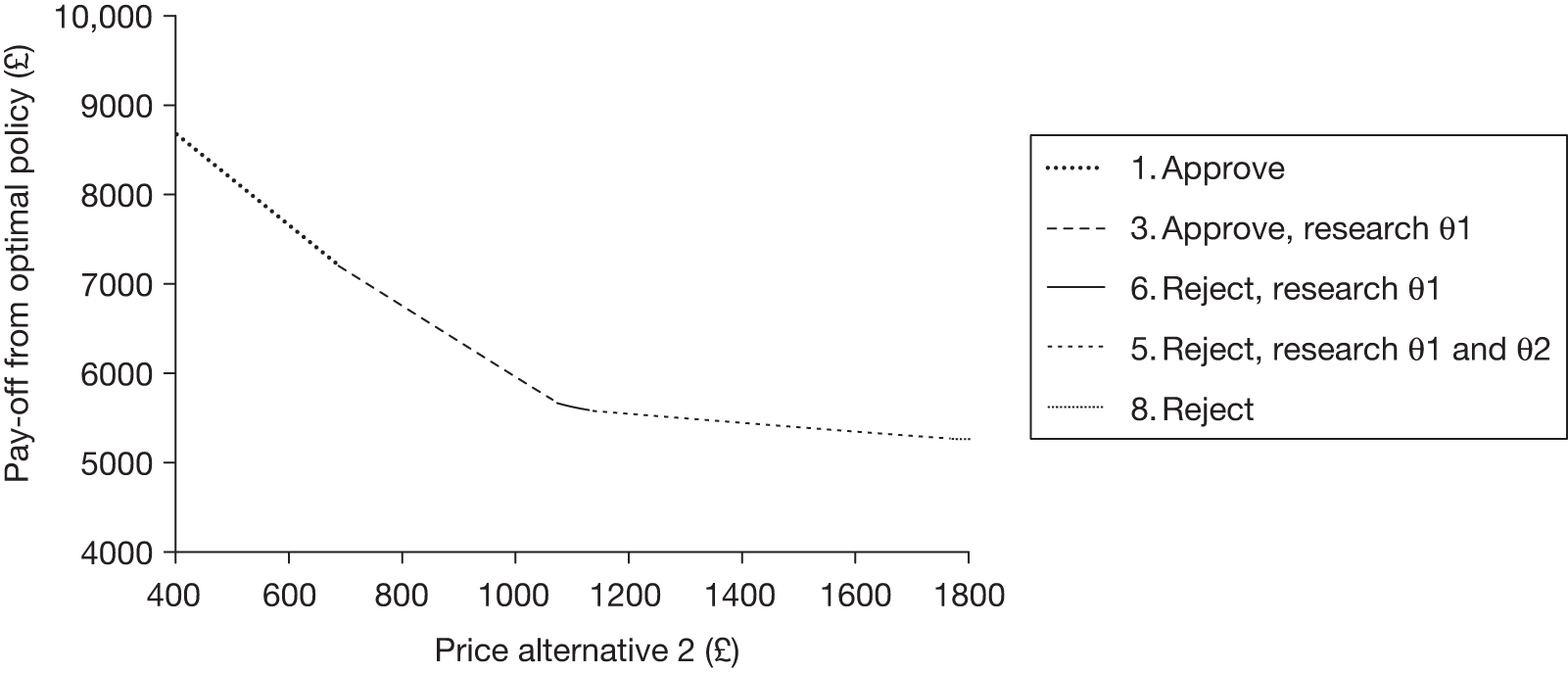
When the price of intervention 2 is less than £700, the benefits from additional research would not exceed the costs and approve is the optimal policy. As the price of intervention 2 rises to between £700 and £1081 the consequences of decision uncertainty increase to the point that the benefits of further research on θ1 outweigh the costs and AWR(θ1) is the optimal policy. For prices between £1081 and £1145 intervention 2 is no longer expected to be cost-effective, but further research to inform θ1 still appears worthwhile so that OIR(θ1) is the optimal policy. The type of research required remains the same for prices between £700 and £1145, but the initial decision to approve or reject prior to research reporting is altered. For prices between £1145 and £1780 intervention 2 is still not expected to be cost-effective, but the benefits of further research are altered such that the type of research required under OIR is now research into both θ1 and θ2. When the price of intervention 2 exceeds £1780 the consequences of uncertainty in the decision to reject intervention 2 are reduced to the point at which further research no longer appears worthwhile.
Does the sequence of research have implications for ‘approval with research’ and ‘only in research’?
The policy set evaluated in Table 52 describes the choice between no research or a single research study of various designs. However, the possible research decisions also include the different sequences in which different types of research could be conducted. The choice of sequence will depend on the benefits and costs of different types of research and the impact of resolving some uncertainties on the importance of others that remain unresolved. For example, quick and relatively cheap research about a key parameter (which influences the importance of others) might, in some circumstances, show that a very expensive trial which would take a considerable time to report might no longer be required. The benefits of a sequential research design are that the latter pieces of research will be conducted only if it still appears valuable conditional on the results of the earlier pieces, thus allowing the decision-maker to avoid some research costs, R. 131 Note that the time until research reports and the likelihood of it reporting must be calculated for each potential research design. The choice of sequence will also be modified by whether particular types of research are possible with and without approval.
With two types of uncertainty there are two possible sequences for research that can be evaluated alongside one-off research decisions. Research initiated immediately would report at τ1 for θ1, or τ2 if instead it was designed to inform θ2. It is also possible to consider the probability of research separately for each possible research design (see Is further research required?). However, in this example we assume that research is guaranteed to report (α1 = α2 = α1,2 = 1). If further research into θ2 is initiated after research on θ1 had reported at time τ1, that research would report at time τ1 + τ2. So decisions earlier than τ1 must be made before the results of any research are known. Decisions made between τ1 and τ1 + τ2 can utilise the additional information generated for θ1. Decisions made after τ1 + τ2 can utilise the additional information generated for both θ1 and θ2.
If research on parameter i will cost Ri then after having conducted research into θ1 the decision to proceed with research on θ2 is informed by:
That is, the benefits of research into θ2 will be available at time τ1 + τ2, and these must be compared with the benefits of continuing with a decision based on current information available at time τ1, that is, to maximise expected NHEs with perfect knowledge of θ1.
After having conducted research into θ2 the decision to proceed with research on θ1 is informed by:
Let us suppose that the costs of research are R1 = 200 and R2 = 10. Table 52 indicates that there is no added value to further research on θ2 following research into θ1 (the pay-offs from policies 2 and 4 are the same, as are the pay-offs from policies 5 and 6). Therefore, the probability of proceeding to incur research costs for θ2 in a sequential design to investigate first θ1 then θ2 is zero:
The pay-off from a sequential design to inform θ1 then θ2 is simply the same as the pay-off from a one-off research design for θ1.
However, a comparison of the pay-offs from policies 3 and 4 or policies 5 and 7 suggests that there is additional value from resolving θ1 after θ2 is known. To calculate whether research would proceed on θ1 we need to return to Table 49 to calculate the value of further research and compare this with the costs of research, R1 = 200. We must establish whether further research to resolve θ1 for population 3 would proceed once research had identified the true value of θ2 for population 2. First, we consider the case in which research identifies that θ2 = 1. Notice from Table 50 that if the value of θ2 is known to be 1, the NHEs of intervention 1 in population 3 are the average of 825 and 1200,Eθ1NHE(1,θ1,θ2=1)∑t=8TIt(1+d)t=1013,the NHEs of intervention 2 in population 3 are the average of 950 and 750,Eθ1NHE(2,θ1,θ2=1)∑t=8TIt(1+d)t=850,and the maximum NHEs in population 3 are the average of 950 and 1200,maxjEθ1NHE(j,θ1,θ2=1)∑t=8TIt(1+d)t=1075.
The additional NHEs from research are not sufficient to outweigh the opportunity cost of that research,[maxjEθ1NHE(j,θ1,θ2=1)−Eθ1NHE(1,θ1,θ2=1)]∑t=8TIt(1+d)t<R1,and the optimal policy is to reject. Now we make the same calculations for the case in which research identifies that θ2 = 2. The NHEs of intervention 1 in population 3 are the average of 150 and 2050,Eθ1NHE(1,θ1,θ2=2)∑t=8TIt(1+d)t=1100,the NHEs of intervention 2 in population 3 are the average of 1350 and 1500,Eθ1NHE(2,θ1,θ2=2)∑t=8TIt(1+d)t=1425,and the maximum NHEs in population 3 are the average of 1350 and 2050,maxjEθ1NHE(j,θ1,θ2=2)∑t=8TIt(1+d)t=1700.
The additional NHEs from research now outweigh the opportunity cost of that research,
and the optimal policy is to continue to research θ1. In summary, if θ2 = 1 research on θ1 will not proceed, whereas if θ2 = 2 research on θ1 will proceed. Each value of θ2 is equally likely and so the probability of continuing to research on θ1 is 0.5 (50%). This means that the expected research costs for the sequential research design (R2 + 0.5R1) are less than the expected research costs for a decision to conduct both research studies (R2 + R1). Table 53 shows the full set of expected research costs alongside the policy pay-offs from the full range of policies that incorporate AWR and OIR with sequential research designs.
| Policies | ∏(Pop1) | ∏(Pop2) | ∏(Pop3) | R | ∏ – R |
|---|---|---|---|---|---|
| 1. Approve | 3413 | 1138 | 1138 | 0 | 5688 |
| 2. AWR(θ1) | 3413 | 1388 | 1388 | 200 | 5988 |
| 3. AWR(θ2) | 3413 | 1219 | 1219 | 10 | 5840 |
| 4. AWR(θ1 then θ2) | 3413 | 1388 | 1388 | 200 | 5988 |
| 5. AWR(θ2 then θ1) | 3413 | 1219 | 1378 | 110 | 5899 |
| 6. AWR(θ) | 3413 | 1388 | 1388 | 210 | 5978 |
| 7. OIR(θ) | 3169 | 1388 | 1388 | 210 | 5734 |
| 8. OIR(θ1 then θ2) | 3169 | 1388 | 1388 | 200 | 5744 |
| 9. OIR(θ2 then θ1) | 3169 | 1219 | 1378 | 110 | 5655 |
| 10. OIR(θ1) | 3169 | 1388 | 1388 | 200 | 5744 |
| 11. OIR(θ2) | 3169 | 1219 | 1219 | 10 | 5596 |
| 12. Reject | 3169 | 1056 | 1056 | 0 | 5281 |
The general notation for calculating the pay-offs from the additional policies described in Table 53 is:
-
Approve: see Equation 6
-
Approve and research θ1: see Equation 14
-
Approve and research θ2: see Equation 15
-
Approve and research θ1 then θ2: utilise Equations 14 and 18:[Equation 20]ΠAWR(θ1 then θ2)=maxjEθNHE(j,θ). ∑t=1τ1It(1+d)t+Eθ1maxjEθ2NHE(j,θ). ∑t=τ1τ2It(1+d)t−R1+Eθ1max{maxjEθ2NHE(j,θ). ∑t=τ2TIt(1+d)t,Eθ2|θ1maxjNHE(j,θ). ∑t=τ2TIt(1+d)t−R2}
-
Approve and research θ2 then θ1: utilise Equations 15 and 19:[Equation 21]ΠAWR(θ1 then θ2)=maxjEθNHE(j,θ). ∑t=1τ1It(1+d)t+Eθ2maxjEθ1NHE(j,θ). ∑t=τ1τ2It(1+d)t−R2+Eθ2max{maxjEθ1NHE(j,θ). ∑t=τ2TIt(1+d)t,Eθ1|θ2maxjNHE(j,θ). ∑t=τ2TIt(1+d)t−R1}
-
Approve and research θ (both θ1 and θ2): see Equation 10
-
Reject and research θ1: see Equation 16
-
Reject and research θ2: see Equation 17
-
Reject and research θ1 then θ2: utilise Equations 16 and 18:[Equation 22]ΠOIR(θ1 then θ2)=EθNHE(1,θ). ∑t=1τ1It(1+d)t+Eθ1maxjEθ2NHE(j,θ). ∑t=τ1τ2It(1+d)t−R1+Eθ1max{maxjEθ2NHE(j,θ). ∑t=τ2TIt(1+d)t,Eθ2|θ1maxjNHE(j,θ). ∑t=τ2TIt(1+d)t−R2}
-
Reject and research θ2 then θ1: utilise Equations 17 and 19:[Equation 23]ΠOIR(θ2 then θ1)=EθNHE(1,θ). ∑t=1τ1It(1+d)t+Eθ2maxjEθ1NHE(j,θ). ∑t=τ1τ2It(1+d)t−R2+Eθ2max{maxjEθ1NHE(j,θ). ∑t=τ2TIt(1+d)t,Eθ1|θ2maxjNHE(j,θ). ∑t=τ2TIt(1+d)t−R1}
-
Reject and research θ (both θ1 and θ2): see Equation 11
-
Reject: see Equation 7.
The consideration of research costs must be incorporated in order to determine the pay-offs from the sequential research designs. If manufacturers bear the research costs such that they no longer fall on the budget for health care, further knowledge on θ1 would be regarded as valuable whether θ2 = 1 or 2. However, when research costs do fall on the budget for health care then if θ2 = 1 research on θ1 would not proceed and the decision-maker would not be able to obtain the maximum possible NHEs for population 3, EθmaxjNHE(j,θ), only the maximum conditional on θ2, Eθ2maxjEθ1NHE(j,θ). In this example the smaller expected research costs from a sequential design to research first θ2 then θ1 (AWR policy 5 or OIR policy 10) are not sufficient to outweigh the benefit of earlier research into θ1 (AWR policies 2, 4 and 6 or OIR policies 7, 8 and 10).
Recall from earlier that some types of research might not be possible once a technology is approved for widespread use (see Is the research possible if the intervention is approved?). If it is not possible to conduct research into θ1 following approval then policies 2, 4, 5 and 6 in Table 53 are no longer available. In this example, the sequential OIR designs that would provide additional information on θ1 do not alter the conclusion that AWR(θ2) offers the greatest expected pay-off.
Impact of other sources of uncertainty
The section Is further research required? explained why there may be value in resolving uncertainty through further research, and the section What type of evidence is needed? discussed the range of assessments that would have to be made about the appropriate research design. However, some uncertain elements that determine the expected costs and health outcomes of technologies will not be resolvable through further research. If it is possible that further information may be revealed over time on these other sources of uncertainty then they too can have an impact on the appropriate policy choice and so this possibility must be assessed. One example of a source of uncertainty that is not resolvable through research is the price of a health-care technology following patent expiry. The decision-maker may have good knowledge of the time at which the patent is due to expire but be uncertain of the impact that this and the potential for generic entry will have on the price. In this section we consider the impact of such an event on the appropriate policy choice.
For example, the patent for intervention 2 is known to expire at time t = 6 and at this point one of two possible outcomes will occur. Either there will be immediate generic entry to the market (a state of the world represented by γ = 1) or the manufacturer might successfully protect its exclusive market position (a state of the world represented by γ = 2). In this simple example both states of the world are deemed to be equally likely. As before, if the decision-maker must select an intervention before further information about generic entry is known, they should choose the technology with the greatest expected NHEs: maxjEγNHE(j,γ).
After the patent has expired and the decision-maker knows whether or not a lower-priced version of the drug will be available the intervention can be chosen conditional on the outcome of the event maxjNHE(j,γ), and the expected value of this decision is EγmaxjNHE(j,γ). Table 54 shows the expected NHEs for each intervention depending on the resolution of the uncertain parameter, γ. Note that when cost-effectiveness is assessed as described earlier (see Is the intervention cost-effective and what are the risks?) it is assessed on the basis of either the monopoly price or the generic price. It would be inappropriate to incorporate the expected price (i.e. the average of these two prices) in the assessment of cost-effectiveness, as basing decisions on this expected price would in general offer fewer NHEs than basing decisions on the known current price within each decision period. The example so far has assumed that the price of intervention 2 remains unchanged at the monopoly price and so the numbers equate to those for γ = 2.
| θ | Population 1 | Population 2 | Population 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NHE(1,θ) | NHE(2,θ) | Max. NHE | NHE(1,θ) | NHE(2,θ) | Max. NHE | NHE(1,θ) | NHE(2,θ) | Max. NHE | |
| 1 | 3169 | 3413 | 3413 | 1056 | 1738 | 1738 | 1056 | 1738 | 1738 |
| 2 | 3169 | 3413 | 3413 | 1056 | 1138 | 1138 | 1056 | 1138 | 1138 |
| E γ | 3169 | 3413 | 3413 | 1056 | 1438 | 1438 | 1056 | 1438 | 1438 |
Table 55 presents the pay-offs from possible policy options. As the uncertainty about whether or not generic entry will occur cannot be resolved through research, the decision to initiate research is no longer relevant. However, as the uncertainty is expected to resolve over time the reimbursement decision could be re-evaluated and reversed.
| Policies | ∏(Pop1) | ∏(Pop2) | ∏(Pop3) | ∏ |
|---|---|---|---|---|
| 1. Approve | 3413 | 1438 | 1438 | 6288 |
| 2. Reversal (approve then revise) | 3413 | 1438 | 1438 | 6288 |
| 3. Delay (reject then revise) | 3169 | 1438 | 1438 | 6044 |
| 4. Reject | 3169 | 1056 | 1056 | 5281 |
The general notation for the pay-offs included in Table 55 are as follows:
-
Approve with no change of guidance:[Equation 24]ΠApprove=maxjEγNHE(j,γ). Pop
-
Reversal (approve then revise guidance after patent expiry):[Equation 25]ΠReversal=EγNHE(2,γ). ∑t=1τIt(1+d)t+EγmaxjNHE(j,γ). ∑t=τTIt(1+d)t
-
Delay (reject then revise guidance after patent expiry):[Equation 26]ΠDelay=EγNHE(1,γ). ∑t=1τIt(1+d)t+EγmaxjNHE(j,γ). ∑t=τTIt(1+d)t
-
Reject with no change of guidance:[Equation 27]ΠReject=EγNHE(1,γ). Pop
When a new intervention is initially regarded as cost-effective, the potential for a price reduction will not change that assessment: intervention 2 goes from being cost-effective to being even more cost-effective. The NHEs of approve in Table 55 exceed the value in Table 40 whereas the NHEs of reject are unaffected. It is for this reason that the option to initially approve the new intervention and then reverse the decision after the price change is not valuable. Likewise, the option to delay approval until after the price change is known generates fewer expected net health benefits than approve. However, the option to revise the decision may be relevant if the resolution of the other source of uncertainty would make intervention 2 appear less, rather than more, cost-effective.
How does price affect the impact of other sources of uncertainty?
If the monopoly price is higher (£1500) as shown in Table 56 and intervention 2 is not cost-effective then a policy to reject the new intervention appears more valuable than a policy to approve. However, the potential change in price means that once the uncertainty is resolved approval may appear cost-effective, and so policy 3 to initially reject and then approve if the price falls after patent expiry offers the greatest pay-off: ΠDelay = 3169 + 1397 + 1397 = 5963.
| Policies | ∏(Pop1) | ∏(Pop2) | ∏(Pop3) | ∏ |
|---|---|---|---|---|
| 1. Approve | 1913 | 1188 | 1188 | 4288 |
| 2. Reversal (approve then revise) | 1913 | 1397 | 1397 | 4706 |
| 3. Delay (reject then revise) | 3169 | 1397 | 1397 | 5963 |
| 4. Reject | 3169 | 1056 | 1056 | 5281 |
Some might regard this as a policy to delay the decision whereas it is in fact reject followed by a reassessment of the value of the new intervention should the price drop. This policy to delay approval (policy 3) offers 5963 – 5281 = 682 additional expected NHEs compared with the next best policy of reject. In this example there is a 50% chance that guidance will change at t = 6, that is, before the end of the expected lifetime of the intervention, TPop = 10.
Figure 42 shows how the optimal policy changes with the monopoly price of intervention 2 (the plotted values correspond to those in Table 55 with a 50% probability of a generic price of £400 after one period).
FIGURE 42.
Impact of monopoly price intervention 2 on optimal policy decision.
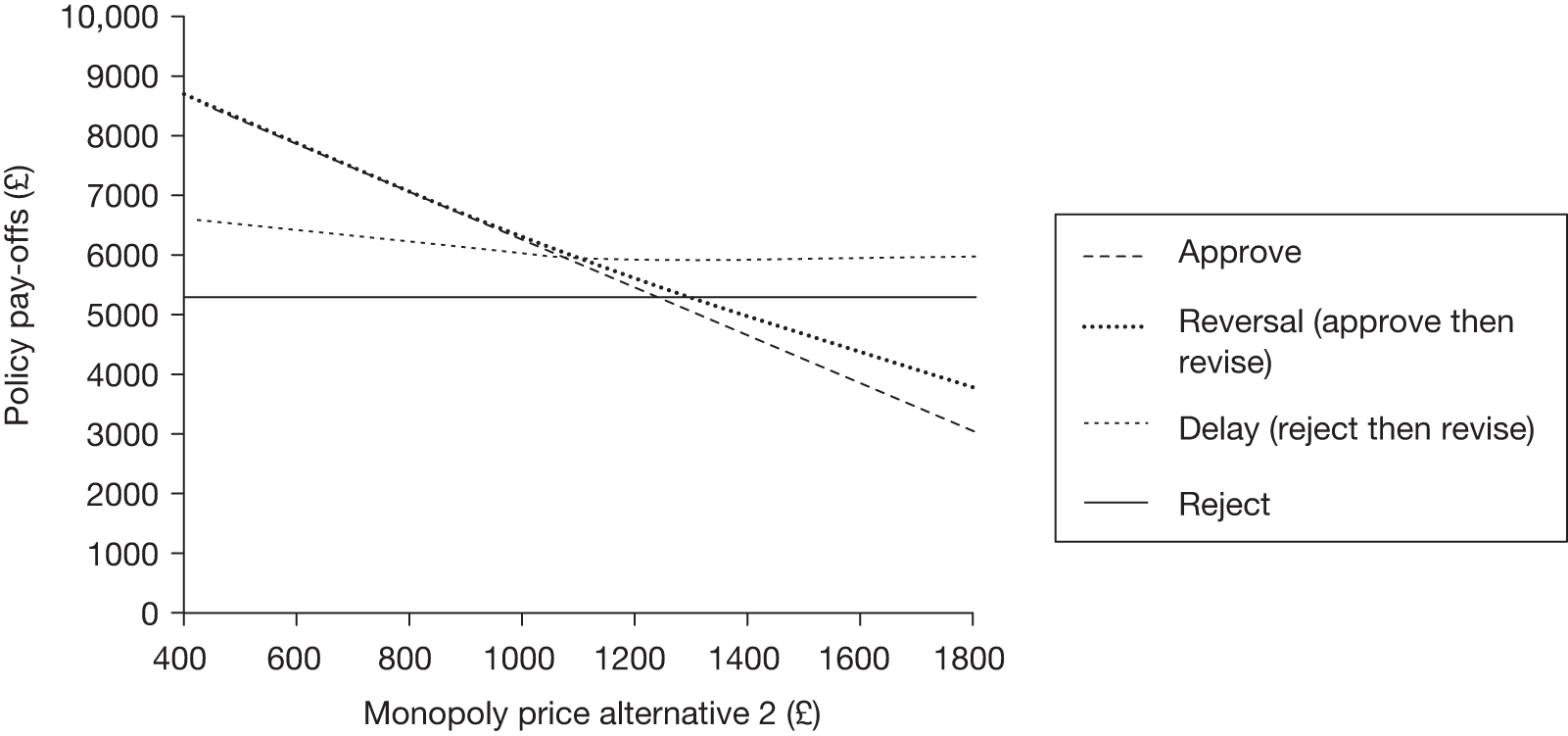
For prices below £1081 the optimal policy is to approve, and the opportunity to revise the decision (Reversal) does not offer additional value because intervention 2 is cost-effective regardless of whether or not there is generic entry. Under these circumstances the resolution of the other source of uncertainty would not be expected to lead to a change in guidance. Once the price exceeds £1081 intervention 2 is not cost-effective unless the price falls with generic entry, and so the policy of reject then revise (delay) offers the greatest pay-off. In this price range the resolution of the other source of uncertainty could potentially lead to a change of guidance. Thus, the impact of a price change can be to alter the probability that guidance will be changed in the future in response to the resolution of other sources of uncertainty over time.
How does the impact of other sources of uncertainty vary with their magnitude?
So far the probability of generic entry has been assumed to be 50%. If the probability of generic entry is lower, for example 10% as in Table 57, the additional expected health effects from delaying the decision will be reduced.
| Policies | ∏(Pop1) | ∏(Pop2) | ∏(Pop3) | ∏ |
|---|---|---|---|---|
| 1. Approve | 1913 | 748 | 748 | 3408 |
| 2. Reversal (approve then revise) | 1913 | 1124 | 1124 | 4161 |
| 3. Delay (reject then revise) | 3169 | 1124 | 1124 | 5418 |
| 4. Reject | 3169 | 1056 | 1056 | 5281 |
However, notice that the pay-offs from all policies in which the intervention might be approved have fallen because there is less chance of a large improvement in expected NHEs from intervention 2 when generic entry is less likely. The impact of altering the level of uncertainty about the possibility of generic entry is greatest for approve (no revision), for which the decision cannot be altered in the light of additional information.
How do other sources of uncertainty impact on the need for evidence?
When regarded in isolation, a source of uncertainty that will resolve over time illustrates that decision-makers may have an opportunity to maximise health benefits by revising their reimbursement decision in light of additional information. However, other sources of uncertainty can also impact on decisions about whether further research is valuable. Thus, when such a source of uncertainty is identified, it is necessary to reassess the value of further research in the presence of these uncertain events that may be resolved over time. That is, the assessments described in Is further research required? and What type of evidence is needed? must be combined with those described in Impact of other sources of uncertainty. To illustrate how this can be achieved, Table 58 combines Tables 49 and 54 to show all possible value combinations for θ conditional on different values for γ.
| θ | θ | θ1 | θ2 | Population 1 | Population 2 | Population 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NHE(1,θ,θ) | NHE(2,θ,θ) | Max. NHE | NHE(1,θ,θ) | NHE(2,θ,θ) | Max. NHE | NHE(1,θ,θ) | NHE(2,θ,θ) | Max. NHE | ||||
| 1 | 1 | 1 | 1 | 2475 | 2850 | 2850 | 825 | 950 | 950 | 825 | 950 | 950 |
| 2 | 2 | 450 | 4050 | 4050 | 150 | 1350 | 1350 | 150 | 1350 | 1350 | ||
| 3 | 2 | 1 | 3600 | 2250 | 3600 | 1200 | 750 | 1200 | 1200 | 750 | 1200 | |
| 4 | 2 | 6150 | 4500 | 6150 | 2050 | 1500 | 2050 | 2050 | 1500 | 2050 | ||
| 2 | 1 | 1 | 1 | 2475 | 2850 | 2850 | 825 | 1550 | 1550 | 825 | 1550 | 1550 |
| 2 | 2 | 450 | 4050 | 4050 | 150 | 1950 | 1950 | 150 | 1950 | 1950 | ||
| 3 | 2 | 1 | 3600 | 2250 | 3600 | 1200 | 1350 | 1350 | 1200 | 1350 | 1350 | |
| 4 | 2 | 6150 | 4500 | 6150 | 2050 | 2100 | 2100 | 2050 | 2100 | 2100 | ||
| E θγ | 3169 | 3413 | 4163 | 1056 | 1438 | 1563 | 1056 | 1438 | 1563 | |||
Table 59 repeats the policy options from Table 53 in light of the other source of uncertainty regarding the potential price reduction. However, in this example we assume that the costs of research do not fall on the budget for health care and so R1 = R2 = R1,2 = 0.
| Policies | ∏(Pop1) | ∏(Pop2) | ∏(Pop3) | ∏ |
|---|---|---|---|---|
| 1. Approve | 3413 | 1438 | 1438 | 6288 |
| 2. AWR(θ1) | 3413 | 1538 | 1538 | 6488 |
| 3. AWR(θ2) | 3413 | 1438 | 1438 | 6288 |
| 4. AWR(θ1 then θ2) | 3413 | 1538 | 1563 | 6513 |
| 5. AWR(θ2 then θ1) | 3413 | 1438 | 1563 | 6413 |
| 6. AWR(θ) | 3413 | 1563 | 1563 | 6538 |
| 7. OIR(θ) | 3169 | 1563 | 1563 | 6294 |
| 8. OIR(θ1 then θ2) | 3169 | 1538 | 1563 | 6269 |
| 9. OIR(θ2 then θ1) | 3169 | 1438 | 1563 | 6169 |
| 10. OIR(θ1) | 3169 | 1538 | 1538 | 6244 |
| 11. OIR(θ2) | 3169 | 1438 | 1438 | 6044 |
| 12. Reject | 3169 | 1056 | 1056 | 5281 |
When a new intervention is regarded as cost-effective, the potential for a price reduction can increase the benefits of adoption relative to the benefits of further research. Once other sources of uncertainty are resolved, the probability of a wrong decision (see Is further research required?) is altered and hence the value of research will change. For example, in Table 49 additional information about θ1,θ2 would lead to a different reimbursement decision for two out of the four possible value combinations, giving an error probability of 50%. However, when also considering the possibility of a price reduction, there are eight possible value combinations shown in Table 58, and once that other source of uncertainty is resolved (for populations 2 and 3) additional information about θ1,θ2 would lead to a different reimbursement decision for only two of these, leading to an error probability of 25%. However, a reduction in the probability of a wrong decision does not necessarily imply that the value of further evidence will fall, as the resolution of other sources of uncertainty can also impact on the consequences of a wrong decision.
Whereas in Table 53 policy 10 [OIR(θ1)] was regarded as more valuable than approve without evidence collection (policy 1), the prospect of a price reduction means that the ranking of these policies is reversed in Table 59. The additional information about θ1 generated by policy 10 would be available only after the price reduction was known. At this point additional information about θ1 is less valuable relative to approval and the gain in expected NHEs is insufficient to outweigh the opportunity cost of denying earlier access to intervention 2 in order to conduct the research. Hence, the resolution of this other source of uncertainty makes an OIR decision to collect further information on θ1 no longer valuable.
Other sources of uncertainty can also have the effect of increasing the value of certain types of research. For example, in Table 59 there was no additional value to researching θ2 once research on θ1 is completed. However, given a reduction in price (γ = 1) further information about θ2 would lead to different adoption decisions for population 3 in Table 59, and hence the value of policies 4 and 8 is increased such that [inline] and [inline].
So although the prospect of a future price reduction could make early adoption appear more valuable, it could also increase the value of further research. When further research is not possible after the intervention is approved (see Is the research possible if the intervention is approved? and What type of research is possible if the intervention is approved?) it is possible that on balance this other source of uncertainty could make OIR policies more attractive relative to approve even though the intervention is expected to be more cost-effective. So, for example, if research is not possible on θ1 following approval, then policies 2, 4, 5 and 6 in Table 59 are no longer available. Now it is preferable to use OIR(θ1,θ2), that is, to reject in order to conduct further research on both θ1 and θ2 rather than to utilise AWR to approve and conduct research only on θ2.
What is the impact of irrecoverable costs?
Irrecoverable costs were introduced in the section Are there significant irrecoverable costs? in which it was noted that the expenditure of investment costs occurs in an earlier time period to that in which the ensuing benefits are realised. Investment costs may be incurred when the decision is made to commence treatment for each individual patient. Alternatively they may be at the level of the health-care system when the decision is made to approve an intervention for widespread use. Reversal costs are incurred when the decision is made to discontinue using a particular health-care technology. Similar to investment costs they can be at the individual patient level or at the level of the health-care system. For example, the decision to cease a pharmacological treatment for a chronic condition may require additional health-care contacts to monitor withdrawal at an individual level. At the health-care system level the decision to disinvest in a technology, that is, to no longer reimburse it, may need to be disseminated in order to alter clinical practice. Investment or reversal costs may be in terms of health effects rather than expenditure on capital equipment. 6,7
When investment costs, C, such as expenditure on capital equipment, are incorporated by annuitisation (see Are there significant irrecoverable costs?), they are weighed against the health outcomes in the same way as any other costs, that is, the total investment cost is allocated over time and the patient population as if it was actually incurred (in small parts) per patient in each period. The total expected costs of approving the intervention will be correctly estimated as long as this decision is not revised earlier than expected because of other events that may change the expected costs and health outcomes of this or comparator technologies. Therefore, it should be apparent that investment and reversal costs will have a particular impact (other than on expected costs) only if research is likely to report or other sources of uncertainty resolve in the near or distant future.
Investment costs are incurred at the point of approving a technology for use within the health service, and their introduction reduces the benefits of approve relative to reject. If investment costs are high enough, this may lead us to reject an intervention that was regarded as cost-effective before considering those costs. If there is an additional irrecoverable cost of £20M associated with intervention 2, this would be expected to displace activities that could have produced 1000 health benefits
Thus, the NHEs from approving the new intervention in Table 41 need to be reduced by this amount, from 5688 to 4688 (assuming that they have not been incorporated by annuitisation in the estimation of individual NHEs). This would mean that the benefits of approve no longer exceed the benefits of reject (5281) and consideration of investment costs means that the new alternative no longer appears cost-effective. Of course, additional costs that were previously not included in an analysis will increase expected costs. However, the question is, in what circumstances will the irrecoverable nature of these costs impact on the approval and research decisions?
The impact of irrecoverable costs with other sources of uncertainty
In the section Impact of other sources of uncertainty we discussed the impact of resolving uncertain events over time on the expected costs and health effects of alternative interventions, and the possibility that this may lead to changes in the reimbursement decision over time. This means that the health outcomes from approving a technology may accrue over a shorter time period and thus will be reduced in magnitude relative to the irrecoverable costs.
Reversal costs are incurred at the point of removing an existing intervention from use within the health service (e.g. discontinuing the intervention that is current practice). These are often omitted from cost-effectiveness analysis, which simply compares the marginal benefit of one intervention over another. However, once we consider that the reimbursement decision may change over time, these should be incorporated in the same way as investment costs before the point at which the decision to approve the intervention is made. The difference between investment and reversal costs relates to the time at which they are incurred, but also to the likelihood of their occurring. Although the decision to approve a technology will necessarily lead to the imposition of investment costs (probability = 1), it is only if that decision is later changed that reversal costs are incurred (probability ≤ 1).
Table 55 showed the benefit of alternative policy decisions in the presence of uncertainty surrounding the future price of intervention 2. With this price change, the most beneficial policy was approve with NHEs of 6288. Table 60 shows the value of the same policy options from Table 55 but with an irrecoverable cost of C(2) = 1000 attached to the decision to approve intervention 2.
| Policies | ∏(Pop1) | ∏(Pop2) | ∏(Pop3) | E C (2) | ∏ |
|---|---|---|---|---|---|
| 1. Approve | 3413 | 1438 | 1438 | 1000 | 5288 |
| 2. Reversal (approve then revise) | 3413 | 1438 | 1438 | 1000 | 5288 |
| 3. Delay (reject then revise) | 3169 | 1397 | 1397 | 500 | 5463 |
| 4. Reject | 3169 | 1056 | 1056 | 0 | 5281 |
It can be seen that overall the health effects across all populations from approving the new intervention outweigh the investment costs (approve is more valuable than reject). However, the policy to reject the intervention until the outcome of the price change is known (delay) is now increased in value relative to approve. This is because if we reject initially, the health effects from approving the new intervention in populations 2 and 3 in the absence of a price change (γ = 2) are not enough to exceed the irrecoverable costs,NHE(2,γ=2)∑t=6TIt(1+d)t−C(2)<NHE(1,γ=2)∑t=6TIt(1+d)t(1138 + 1138 – 1000 < 1056 + 1056), and so if this state of the world arises we still reject. However, if generic entry does occur (γ = 1), the increased health gains from approving the new technology are enough to exceed the irrecoverable costs,NHE(2,γ=1)∑t=6TIt(1+d)t−C(2)<NHE(1,γ=1)∑t=6TIt(1+d)t(1738 + 1738 – 1000 > 1056 + 1056), and so we would choose to approve at this point. The decision to approve immediately is optimal if the price drops, and delaying approval allows the decision-maker to incur the irrecoverable costs only if this is actually what happens (p = 0.5), rather than incurring the irrecoverable costs regardless of how γ is resolved (p = 1). Therefore, the expected irrecoverable costs associated with the policy to reject then revise (delay), EC(2) = 500, are lower than the expected irrecoverable costs associated with approve, where EC(2) = 1000.
The pay-off from the optimal policy in the presence of irrecoverable costs can be found using:
where C(j) represents investment costs; these fall to zero in the second time period (t ≥ τ) if intervention j was selected in the first time period (t ≤ τ). For C(j) that are reversal costs these become positive in the second time period (t ≥ τ) only if intervention j is selected in the first time period (t ≤ τ) then rejected in the second.
The impact of irrecoverable costs when further research is conducted
In the section Is further research required? we have discussed how to assess if further evidence is valuable for reducing uncertainty and shown that further research is valuable when the results of that research could indicate a change in the reimbursement decision. This implies again (see Impact of other sources of uncertainty) that the health effects from approving an intervention may be accrued over a shorter time period, and will be reduced in value relative to the irrecoverable costs. The impact of irrecoverable costs must be incorporated at the point at which an intervention is approved, which may be after research reports. When the irrecoverable costs are incurred after research reports, the remaining intervention lifetime is shorter and so health effects from approval will accrue to a smaller population compared with immediate approval. Table 61 shows the policy options previously shown in Table 53 but now with irrecoverable costs included and when the research costs do not fall on the budget for health care.
| Policies | ∏(Pop1) | ∏(Pop2) | ∏(Pop3) | E C (2) | ∏ |
|---|---|---|---|---|---|
| 1. Approve | 3413 | 1138 | 1138 | 1000 | 4688 |
| 2. AWR(θ1) | 3413 | 1388 | 1388 | 1000 | 5188 |
| 3. AWR(θ2) | 3413 | 1219 | 1219 | 1000 | 4850 |
| 4. AWR(θ1 then θ2) | 3413 | 1388 | 1388 | 1000 | 5188 |
| 5. AWR(θ2 then θ1) | 3413 | 1219 | 1388 | 1000 | 5019 |
| 6. AWR(θ) | 3413 | 1388 | 1388 | 1000 | 5188 |
| 7. OIR(θ) | 3169 | 1356 | 1356 | 250 | 5631 |
| 8. OIR(θ1 then θ2) | 3169 | 1388 | 1388 | 500 | 5444 |
| 9. OIR(θ2 then θ1) | 3169 | 1056 | 1356 | 250 | 5331 |
| 10. OIR(θ1) | 3169 | 1388 | 1388 | 500 | 5444 |
| 11. OIR(θ2) | 3169 | 1056 | 1056 | 0 | 5281 |
| 12. Reject | 3169 | 1056 | 1056 | 0 | 5281 |
The expected irrecoverable cost, EC(2), is determined by the probability that intervention 2 will be approved. In policies 1–6 intervention 2 is approved immediately and so irrecoverable costs are incurred with a probability equal to one. In policy 12 intervention 2 is never approved and so irrecoverable costs are never incurred.
In policy 7, OIR(θ), intervention 1 is provided to population 1 and the results of research on both parameters are available in population 2. Investing in intervention 2 at this point would allow the health effects to accrue to patients in populations 2 and 3. Comparing these benefits previously reported in Table 49 with the irrecoverable costs indicates that for three of the four possible combinations of θ1 and θ2 the decision-maker would continue to reject intervention 2. It is only if θ1 = 1 and θ2 = 2 that the benefits of intervention 2 in populations 2 and 3 outweigh the irrecoverable costs and the decision-maker would approve. This outcome has a probability of 0.25 and so the expected irrecoverable costs of policy 7 are 250. However, note that the pay-offs from policy 7 differ from those from the same policy in Table 52 because the probability of approval given perfect knowledge of θ is reduced from 0.5 to 0.25 once additional irrecoverable costs are introduced.
In policy 10, OIR(θ1), intervention 1 is provided to population 1 and the results of research to inform θ1 are available in population 2. From Table 50 it would appear optimal at this point to change the decision to approve if θ1 = 1 but not if θ1 = 2; hence, the expected irrecoverable cost for OIR(θ1) is EC(2) = 500.
In policy 11, OIR(θ2), intervention 1 is provided to population 1 and the results of research to inform θ2 are available in population 2. The values reported in Table 51 illustrate that, regardless of how θ2 resolves, the decision-maker would not choose to approve intervention 2. This is because the additional NHEs from approving intervention 2 if θ2 = 2 are reduced by the irrecoverable costs (1425 + 1425 – 1000 = 1850) and are less than the NHEs from continuing to provide intervention 1 (1100 + 1100 = 2200). The expected irrecoverable costs of policy 11 are therefore equal to zero, the additional value generated by the research on θ2 is zero and the pay-off is equivalent to that of policy 12 (ΠReject = EγNHE(1,γ). Pop. The impact of the irrecoverable costs is to reduce the value of research that previously would have appeared worthwhile.
In policy 8, OIR(θ1 then θ2), the results of research to inform θ1 are available in population 2. At this point it would appear optimal to change the decision to approve intervention 2 if θ1 = 1 but not if θ1 = 2 (see Table 50). The results of research to inform θ2 are available in population 3. If θ1 is already known to be 1, the irrecoverable costs will have been sunk and the decision-maker must simply choose whether to continue to reimburse intervention 2 or opt for intervention 1. In this example, the decision-maker would choose to continue to reimburse intervention 2 if θ1 = 1 no matter how θ2 resolves (see Table 49). However, if θ1 is already known to be 2, the investment will not have yet been made. In this example, if θ1 = 2 the decision-maker would continue to reject intervention 2 no matter how θ2 resolves. The probability of incurring the irrecoverable costs is therefore given by the probability that θ1 = 1, that is, 0.5.
Summary
Each section of this technical appendix describes how the pay-off from alternative policy decisions can be quantified in the presence of uncertainty that can be resolved through research, uncertainty that will resolve over time and irrecoverable costs. The impact of various combinations of these factors has been demonstrated in a simple framework. The general notation should allow readers to combine relevant sections of the report in order to make the assessments required to inform a range of reimbursement and research decisions.
Appendix 7 Enhanced external counterpulsation for chronic stable angina
Introduction
The NIHR HTA programme identified EECP as an important topic and commissioned a short report to examine the clinical effectiveness and cost-effectiveness of EECP as an adjunct to standard therapy in patients with chronic stable angina. Although the topic was not ultimately considered by NICE it was commissioned in the same way and with the same resources as other assessment reports that inform NICE guidance. The assessment followed the NICE reference case1 and is consistent with the type of analysis that would have been required in a MTA appraisal. Like other MTA appraisal reports it was published in full as a HTA monograph. 121
The case study of EECP is used to demonstrate how the key principles and assessments in Chapter 5 could inform the development of guidance for the use of EECP. The following sections include a background to EECP and a detailed examination of the sequence of assessments and decisions that lead to a particular category and type of guidance for EECP.
Background to the case study
Angina is a condition most commonly caused by coronary artery atherosclerosis with flow-limiting plaques that impede blood flow to the myocardium. Its prevalence in the UK is estimated to be approximately 700,000 men aged between 55 and 75 years and 400,000 women, with about 52,000 new cases per year in men and 43,000 in women. 159 The use of EECP as a treatment for angina is increasing steadily worldwide following reports of sustained benefit. 160–163 EECP has been utilised mainly in patients not suitable for coronary revascularisation or in those who have chosen not to undergo revascularisation. 164,165 It is estimated that about 10% of angina patients could potentially benefit from EECP (Michael Chester, Liverpool Hope University, UK, 2008, personal communication), which implies a prevalence of about 110,000 and an annual incidence of 9500.
Enhanced external counterpulsation results in upfront costs of treatment but the potential quality-of-life benefits through improved symptoms of angina and long-term relief from symptoms may outweigh the costs compared with not giving the therapy. Although EECP was not ultimately considered by NICE, the NIHR HTA programme identified EECP as an important topic for research and commissioned it in the same way as other assessment reports.
Intervention and population
Enhanced external counterpulsation is a non-invasive procedure used to provide symptomatic relief from stable angina. 160,162,163 Long inflatable pressure cuffs are wrapped around a patient’s calves, lower thighs and upper thighs. The cuffs are inflated and deflated to increase blood flow to the coronary arteries and decrease peripheral vascular resistance and cardiac workload. 165 It typically involves 35 1-hour treatment sessions over a period of 4–7 weeks. 166 EECP as an adjunct to standard therapy is compared with standard therapy alone.
The population of interest is patients with stable angina. Because stable angina is a chronic condition, the prevalent population while any research is being conducted can benefit from the information and switch treatment if they survive and remain eligible when the results of the research reports.
Evidence on clinical effectiveness
A review of clinical effectiveness identified only one RCT [the Multicenter Study of Enhanced External Counterpulsation (MUST-EECP) trial160,161] comparing active with inactive EECP. This trial showed evidence of improved HRQoL from EECP (see Table 62); however, follow-up was limited to 12 months and so the degree to which improvement in HRQoL from EECP is sustained beyond 12 months is uncertain. To characterise the uncertainty associated with possible durations of treatment effect, formal elicitation of expert clinical judgement was undertaken. 167 Five experts with experience and knowledge of EECP in the UK independently completed an Microsoft Excel-based exercise that elicited their judgements about the likelihood of sustaining HRQoL benefits from EECP in subsequent years. a Because the uncertainty associated with any judgement is critical, a frequency chart format to represent a distribution was adopted. 168 The results from each expert were linearly pooled, with equal weight, providing the probability of continuing to respond to treatment in subsequent years. 169 The uncertainty associated with the pooled estimates was characterised by fitting beta distributions to pooled responses. See Table 62 for a summary of the mean values and distributions for the probability of sustaining HRQoL benefits in each year.
| Parameter | Mean value | Prior distribution | Source |
|---|---|---|---|
| Baseline patient characteristics | |||
| Age | 64 years | Fixed | MUST-EECP trial161 |
| HRQoL improvement from baseline to 1 year after treatment | |||
| EECP relative to standard therapy | 0.0717 | Beta(α = 3.641, β = 47.139)a | MUST-EECP trial161 |
| Probability of sustaining HRQoL benefits in subsequent yearsb | |||
| Year 2 | 0.757 | Beta(α = 8.028, β = 2.577) | Expert elicitation121 |
| Year 3 | 0.742 | Beta(α = 5.589, β = 1.943) | Expert elicitation121 |
| Year 4 | 0.719 | Beta(α = 4.413, β = 1.726) | Expert elicitation121 |
| Year 5 onwards | 0.719 | Beta(α = 4.413, β = 1.726) | Expert elicitation121 |
| Probability of repeat EECP sessions | |||
| Per year (decreased exponentially) | 0.099 | Beta(α = 194, β = 884) | Michaels et al.172 |
| Resource use and unit costs | |||
| Capital cost of EECP machine | £90,000 | Fixed | K Miles, 2008, personal communication |
| Capital cost per annum (annuitised over 10 years) | £10,822 | Fixed | |
| Equipment replacement costs | |||
| One set of cuffs per year | £139 | Fixed | Vasogenics173 |
| One set of hoses per year | £76 | Fixed | Vasogenics173 |
| Pleth per every 2 years | £53 | Fixed | Vasogenics173 |
| Consumables per patient | |||
| Ultrasound scan | £75 | Fixed | Vasogenics173 |
| Trousers | £16 | Fixed | Vasogenics173 |
| Gel | £8 | Fixed | Vasogenics173 |
| ECG electrodes | £110 | Fixed | Vasogenics173 |
| Staffing and overhead costs per patient (based on 12 patients per year) | £3214 | Fixed | W Sheedy, Castle Hill Hospital, East Yorkshire, UK, 2008, personal communication |
| Total cost of EECP per patient | £4347 | Fixed | |
Decision model
A probabilistic decision-analytic model was developed to estimate the expected cost-effectiveness of EECP and associated uncertainty. The decision model was structured to capture the HRQoL benefits and costs associated with EECP up to a period of 12 months and to project these benefits to a lifetime time horizon relevant to this patient population. Three health states were defined: (1) a ‘responders’ state represented patients who continued to sustain HRQoL benefits from EECP in each yearly cycle, (2) a ‘non-responders’ state represented patients who lost their initial HRQoL benefits from treatment and reverted back to baseline HRQoL and (3) ‘dead’ represented deaths from disease-specific cardiovascular causes170 and other causes informed by UK life tables. 171 In the first year after treatment it was assumed that patients achieved the HRQoL benefits reported in the 12-month follow-up of MUST-EECP. 161 After 12 months the proportion of patients responding to treatment was based on the results of the elicitation exercise. After year 4, experts did not expect the probability of sustained response to be different in subsequent years. Therefore, a beta distribution, equivalent to that fitted to pooled year 4 responses, was assigned to year 5 onwards.
Some patients require additional ‘top-up’ sessions, which are generally given to help sustain the long-term benefits of treatment. Based on the study by Michaels et al. ,172 18% of patients required repeat EECP within 2 years of the initial course of treatment. This was converted to an annual probability and decreased exponentially over time to reflect the diminishing attempts to continue to retreat if the patient is not responding. Resource use and costs associated with EECP were based on published unit costs. The cost for repeat procedures was based on an average of 10 additional sessions. Table 62 summarises the key parameters for the decision model, the distributions assigned and the sources of evidence used. The analysis is conducted from the perspective of the NHS and Personal Social Services (PSS) and costs and outcomes are discounted at a rate of 3.5% per annum. 1
Key features and possible pathways
Enhanced external counterpulsation results in large upfront costs of treatment but the potential quality-of-life benefits through improved symptoms and long-term relief from symptoms may outweigh the costs when compared with not giving the treatment. The technology has a range of interesting characteristics that can be used to explore the implications of the principles and assessments outlined in Chapter 5:
-
EECP is a medical device rather than a pharmaceutical.
-
There is an element of investment cost associated with the purchase of equipment.
-
EECP has large initial upfront costs of treatment (£4347 per patient), which are irrecoverable once treated.
-
The impact of investment and irrecoverable costs on guidance can be fully explored.
-
Formal elicitation methods are combined with Bayesian decision theory to determine a necessary and sufficient condition for conducting further research. This provides an opportunity to examine the potential role of elicitation rather than extreme scenarios to characterise uncertainty. It also provides an opportunity to examine the impact of the research design, in terms of length of follow-up, on guidance.
-
Angina is a chronic condition so while research is conducted the prevalent population can still benefit from the information and switch treatment based on the results of the research.
The possible pathways through the algorithm are reported in Figure 30 in Appendix 4. EECP is expected to be cost-effective but with potentially significant irrecoverable costs. These irrecoverable costs include both (1) long-lived costs associated with the purchase of equipment and (2) large initial per-patient treatment costs, combined with a chronic condition in which a decision not to treat a particular patient with EECP can be changed at a later date (decisions are not irreversible) when research reports or other events occur. Consequently, these irrecoverable costs might influence the category of guidance, for example OIR rather than approve.
The following sections examine each of the seven points on the checklist relating to the possible sequence of assessments and decisions that lead to a particular category and type of guidance for EECP.
Is it cost-effective and what are the risks?
Point 1: Is it expected to be cost-effective?
The sequence of assessments starts with cost-effectiveness and the expected impact on population NHEs, that is, at the following point in the algorithm:
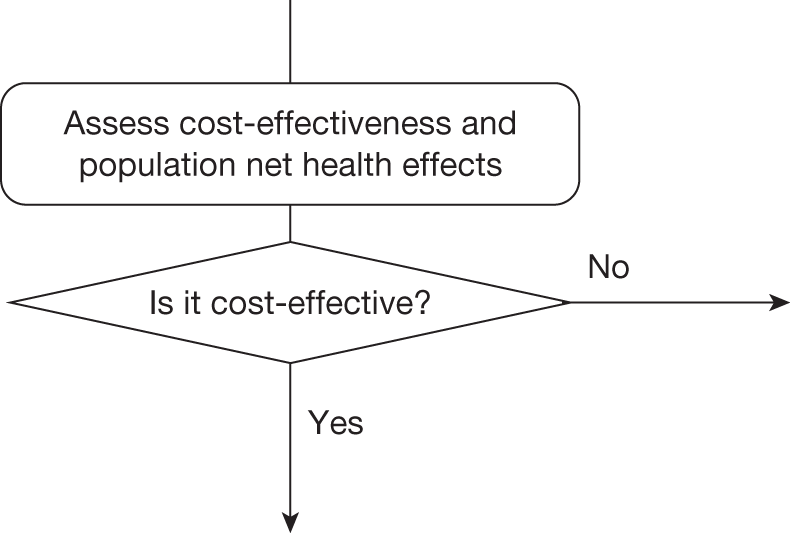
Cost-effectiveness at the patient level
Table 63 summarises the expected cost-effectiveness of EECP per patient treated. EECP as an adjunct to standard therapy is compared with standard therapy alone. Only the additional costs and effects of EECP over and above those of standard therapy are considered in the analysis. The expected incremental difference in lifetime costs and QALYs of EECP relative to standard therapy is £4744 and 0.2447 QALYs, respectively, giving an ICER of £19,391. Therefore, EECP is just expected to be cost-effective at a threshold of £20,000 per QALY. b The NHE of EECP is greater than that of standard care but the difference per patient treated (the incremental NHE) is small. This difference of 0.0074 and 0.0865 QALYs at thresholds of £20,000 and £30,000 per QALY, respectively, represents the expected benefit per patient of immediate approval of EECP based on current evidence or, alternatively, the opportunity cost per patient of withholding approval of EECP if, for example, additional research is required that could not be conducted if it is approved.
| Treatment | Cost (£) | QALYs | ICER (£/QALY) | Cost-effectiveness threshold £20,000 per QALY | Cost-effectiveness threshold £30,000 per QALY | ||
|---|---|---|---|---|---|---|---|
| NHE, QALYs (£) | Incremental NHE, QALYs (£) | NHE, QALYs (£) | Incremental NHE, QALY (£) | ||||
| EECP | 4744 | 7.6045 | 19,391 | 7.3673 (147,346) | 0.0074 (149) | 7.4464 (223,391) | 0.0865 (2595) |
| Standard care | – | 7.3598 | – | 7.3598 (147,197) | – | 7.3598 (220,795) | – |
As discussed in Chapter 5, Is it cost-effective and what are the risks?, it is also important to consider how NHEs accumulate over time or the investment profile per patient treated with EECP. Figure 43 illustrates the cumulative incremental NHE over the patient time horizon of 50 years. The initial per-patient costs of EECP are high and are far in excess of the immediate health benefits in the initial period of treatment. These negative NHEs are gradually offset by positive NHEs in later periods. In this case, it is only after 14 years that the initial losses are compensated by later gains, that is, EECP doesn’t break-even until 14 years from initial treatment. It is only beyond 30 years that the modest incremental NHEs reported in Table 63 are eventually achieved.
FIGURE 43.
Cumulative incremental NHEs of EECP over the patient time horizon.
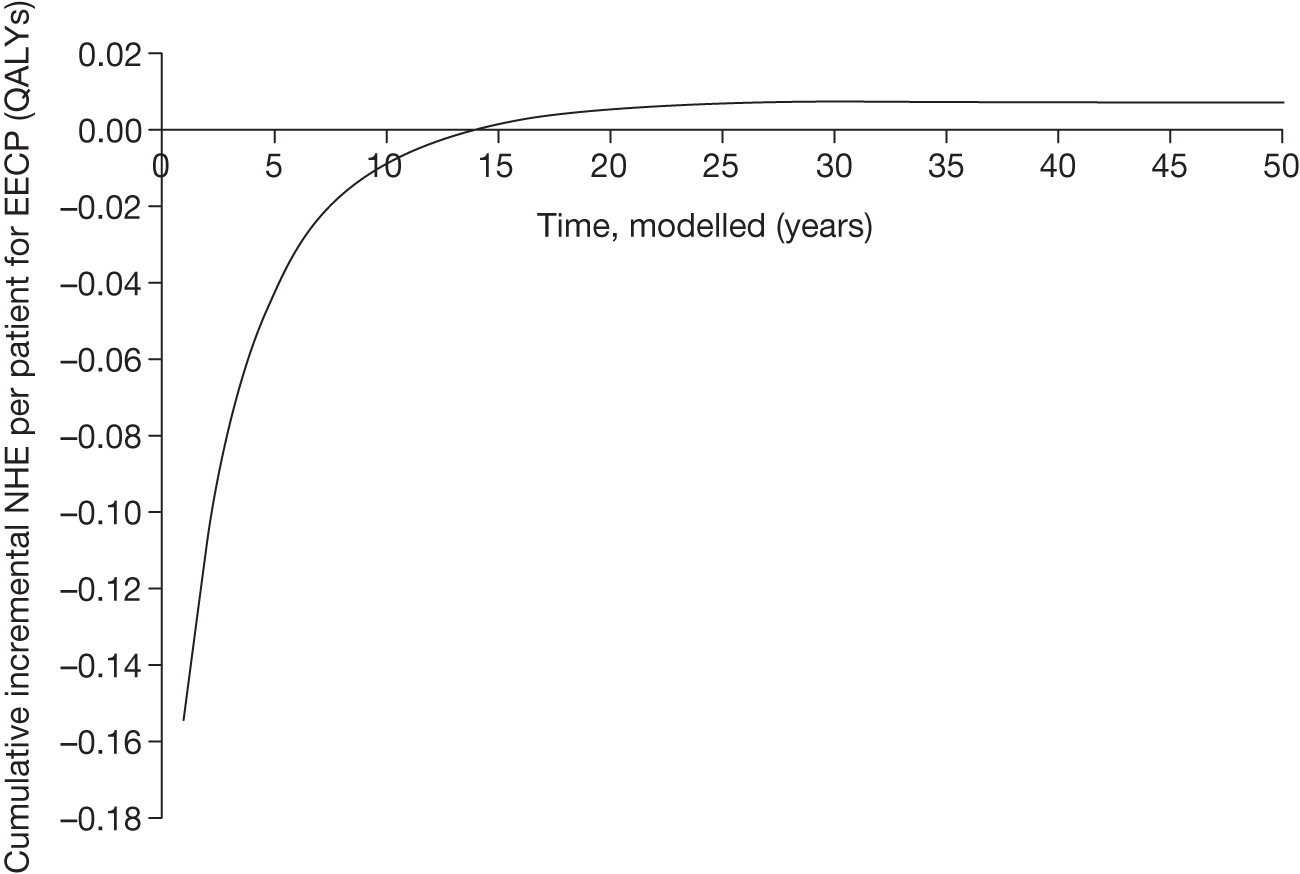
Cost-effectiveness at the population level
Per patient NHEs can also be expressed for the population of current and future patients. This requires information about prevalence and future incidence of the target population. It also requires a judgement about the time horizon over which the technology will be used. An estimate of the scale of the total population NHEs and how they cumulate over time is important for subsequent assessments, including: (i) where the NHE for current patient populations must be compared with the benefits to future patients; and (ii) where the treatment decision can be changed such that the irrecoverable opportunity costs of initially negative NHE become significant, i.e., might influence the category of guidance.
The British Heart Foundation estimates that the prevalence of angina in the UK is just under 1.1 million and the annual incidence is around 95,000. 159 Assuming that 10% of angina patients can potentially benefit from EECP (Michael Chester, personal communication, 2008) this implies a prevalent population of 109,800 and an annual incidence of 9500. The total population NHEs, assuming that the technology will be used to treat prevalent and incident patients over 10 years, are reported in Table 64. Expected cost-effectiveness is unchanged (ICER is the same as in Table 63) but the incremental NHEs, although small per patient, are more significant at a population level at 1405 and 16,334 QALYs at thresholds of £20,000 and £30,000 per QALY, respectively.
| Treatment | Costs (£M) | QALYs | ICER (£/QALY) | Cost-effectiveness threshold £20,000 per QALY | Cost-effectiveness threshold £30,000 per QALY | ||
|---|---|---|---|---|---|---|---|
| NHE, QALYs (£M) | Incremental NHE, QALYs (£M) | NHE, QALYs (£M) | Incremental NHE, QALYs (£M) | ||||
| EECP | 896 | 1,435,787 | 19,391 | 1,391,001 (27,820) | 1405 (28) | 1,405,930 (42,177) | 16,334 (490) |
| Standard care | – | 1,389,596 | – | 1,389,596 (27,792) | 1,389,596 (41,688) | ||
The investment profile for EECP when used to treat patients over 10 years is illustrated in Figure 44. At a population level it is not until 17 years (rather than 14 years at a patient level) that initial losses are compensated for by later gains and EECP breaks even. In other words, EECP appears a more risky investment when evaluated at a population rather than at an individual level. This is because, although each patient treated with EECP is expected to offer the same profile of NHEs shown in Figure 43, the negative NHEs associated with patients incident and treated in year 10 will not be offset by later gains until year 24. The population-level investment profile would exhibit greater risk (break-even later) if the prevalent population was smaller relative to the incident population and/or the technology time horizon was longer.
FIGURE 44.
Cumulative incremental NHEs of EECP for the population.
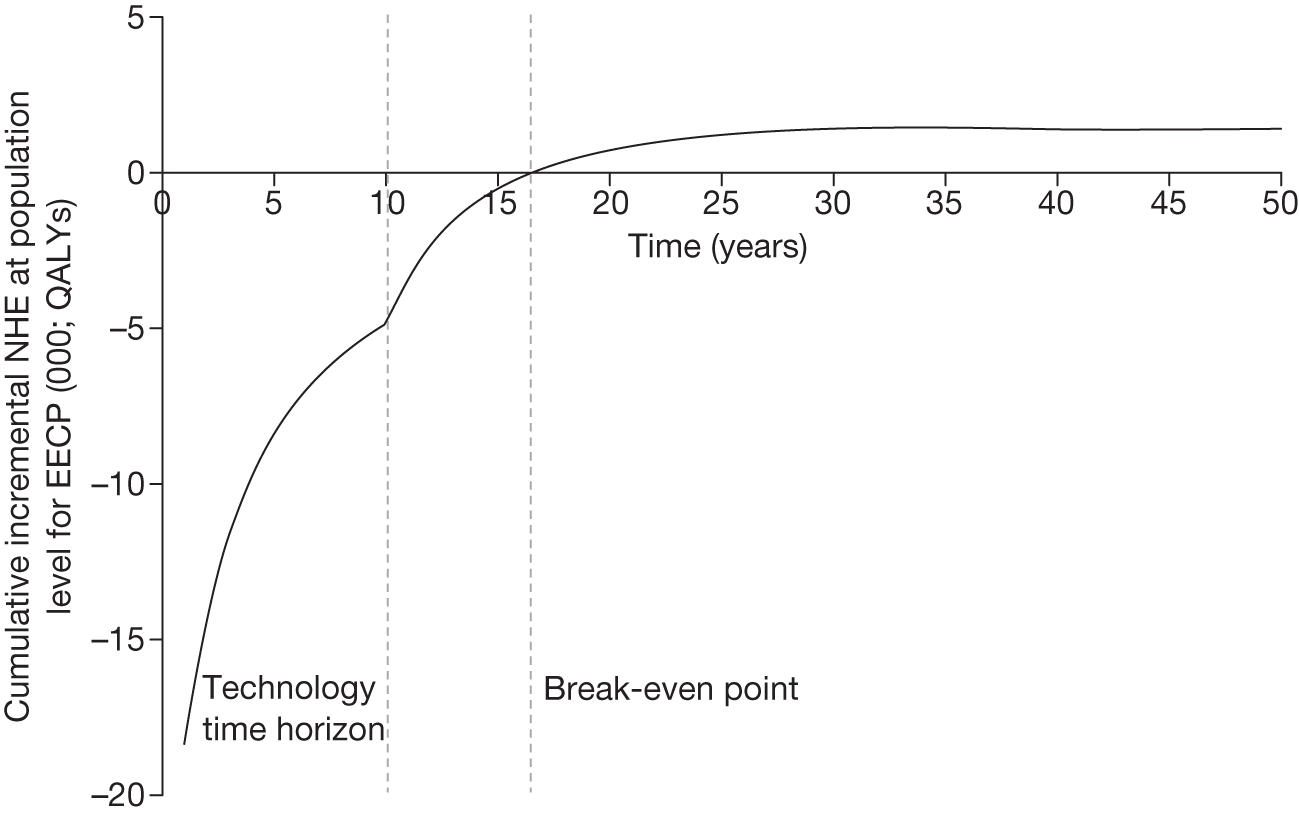
The time horizon of 10 years was chosen as a proxy for a complex and uncertain process of future changes in new technologies, prices and evidence. 53 The impact of different technology time horizons on NHE is illustrated in Table 65. The break-even point extends to 23 years when the technology time horizon is increased to 20 years. Therefore, the investment profile for EECP appears more risky the longer the time horizon of the technology.
| Technology time horizon (years) | Incremental NHE, QALYs (£M) | Break-even point (years) |
|---|---|---|
| 5 | 1137 (22.7) | 15 |
| 10 | 1405 (28.1) | 17 |
| 15 | 1632 (32.6) | 20 |
| 20 | 1822 (36.4) | 23 |
Point 2: Are there significant irrecoverable costs?
The second point on the checklist requires (1) an assessment of whether or not there are irrecoverable costs and (2) a judgement of their potential significance, that is, at the following point in the algorithm:
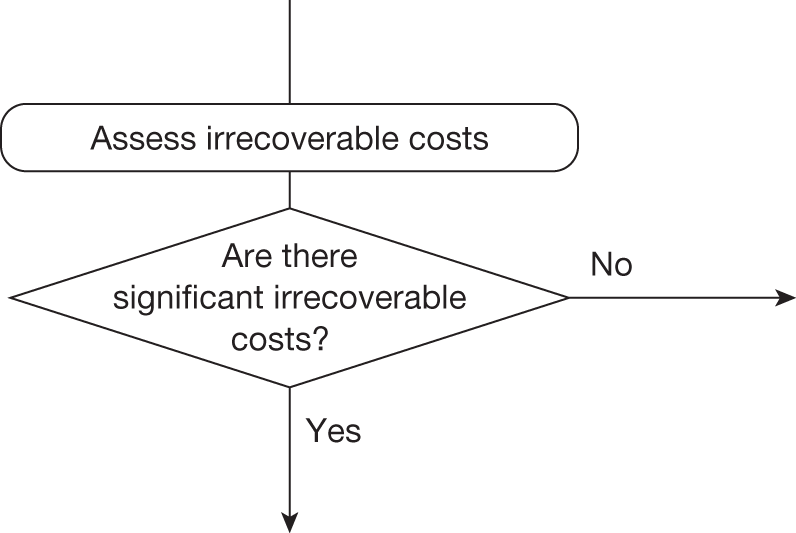
Identifying the irrecoverable costs
Irrecoverable costs are those that, once committed, cannot be recovered if guidance is changed at a later date. Irrecoverable costs are most commonly thought of as ‘upfront’ or capital costs of new facilities or equipment with long life expectancy. In the case of EECP, treatment requires capital investment in the EECP machines. The expected per-patient and population costs reported in Tables 63 and 64 allocated this capital cost by annuitising the cost over the 10-year lifetime of the equipment at a rate of 3.5% per annum and allocating it to the number of patients treated each year (see Table 61). These irrecoverable costs are reported separately in Table 66 and represent 19% of the total; however, this will have no influence on expected cost-effectiveness as long as guidance does not change during the lifetime of the equipment. Figure 45 shows the effect on population NHEs when these capital costs are incurred in full in the first year rather than allocated per patient over the lifetime of the equipment. The cumulative incremental NHE for EECP is more negative in the first 10 years but the investment profile for EECP is no more risky when the capital cost is incurred up front. The investment profile would be expected to exhibit greater risk if the lifetime of the equipment was extended beyond 10 years.
| Treatment | Capital cost (£) | Non-capital cost (£) | QALYs | ICER (£/QALY) | Cost-effectiveness threshold £20,000 per QALY | |
|---|---|---|---|---|---|---|
| NHE, QALYs (£M) | Incremental NHE, QALYs (£M) | |||||
| EECP | 170,304,591 | 725,408,798 | 1,435,787 | 19,391 | 1,391,001 (27,820) | 1405 (28) |
| Standard care | – | – | 1,389,596 | – | 1,389,596 (27,792) | – |
FIGURE 45.
Cumulative incremental NHEs with capital costs incurred in year 1.
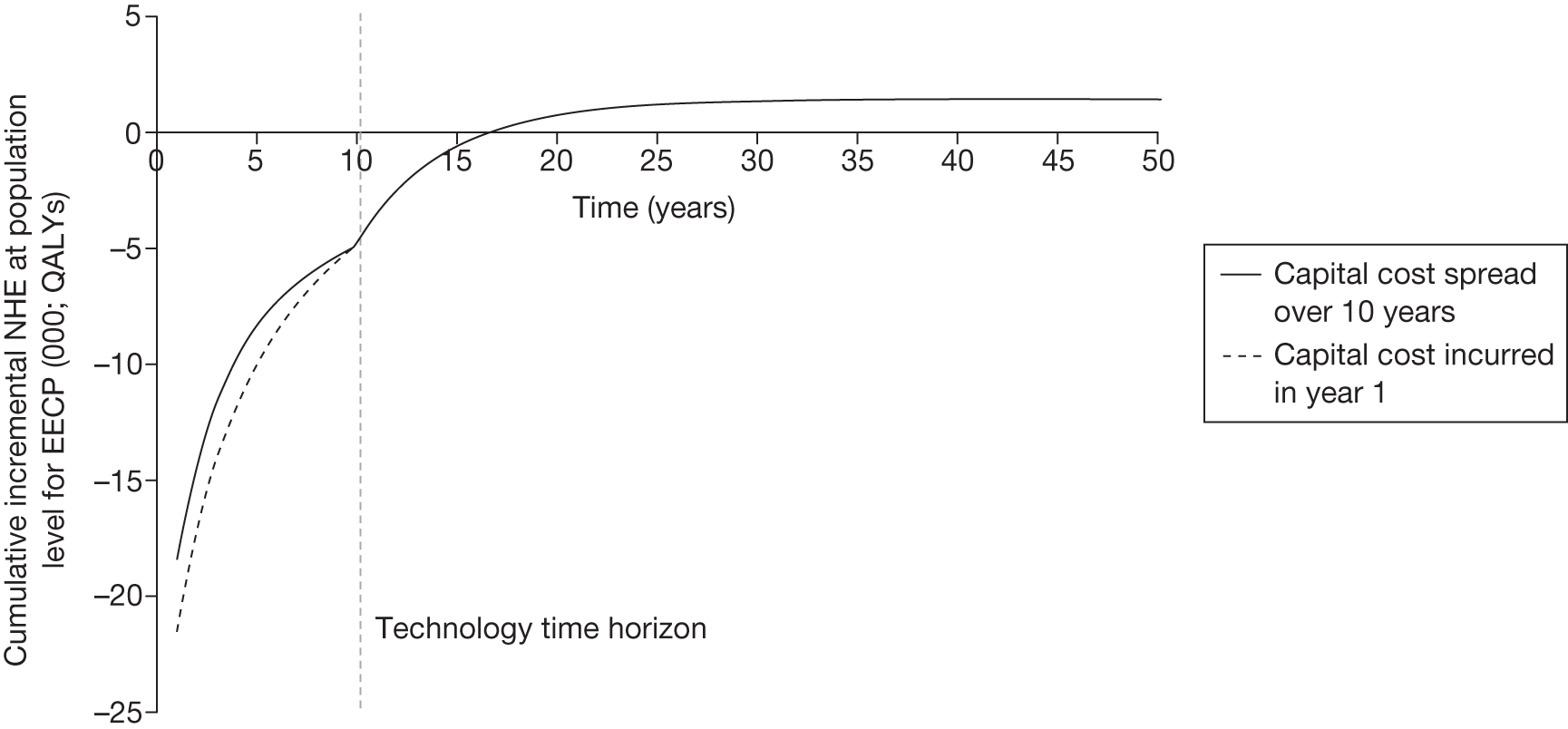
Even in the absence of capital costs of EECP, NHEs accumulate over time. Therefore, approval or AWR commits opportunity costs of negative NHEs that are irrecoverable.
Are they likely to be significant?
The significance of the irrecoverable capital costs can be judged based on the proportion of the total population cost that they represent as well as their scale relative to the additional population NHEs offered. The irrecoverable capital cost of EECP represents 19% of the total cost (see Table 66) and could be considered significant. Furthermore, even in the absence of the capital costs EECP exhibits a profile of NHEs in which approval or AWR commits opportunity costs of negative NHEs that are irrecoverable. Irrecoverable costs include situations in which initial losses (negative NHEs) of a technology are offset by later gains (positive NHEs). If early approval is revised, for example because research reveals that the technology is not as effective as expected, then initial losses will have been incurred but they will not be compensated for by later gains. For example, if research reports before the break-even point of 17 years (i.e. before losses are recouped) there is a chance that the results will indicate that EECP is not in fact cost-effective, approval will be withdrawn and patients will be switched from EECP to standard care; initial losses will have been incurred but they will not be compensated for by the later gains that were originally expected. The impact of this investment profile of cumulative incremental NHEs will be greater when a decision to approve is more likely to change and in the more immediate future, that is, when it is more uncertain and when research will be conducted and report in the near future.
Enhanced external counterpulsation is for a chronic condition in which the decision to treat a particular patient can be changed at some later date (decisions are not irreversible). Therefore, the type of investment profile of NHEs at a patient and population level is significant because, instead of committing irrecoverable costs by deciding to use the technology expected to be cost-effective now, the decision and commitment of costs can be made later, after research reports, other events occur and/or guidance changes.
Figure 45 illustrates the impact of accounting for the actual timing of expenditure on EECP machines rather than treating it as if it was paid when each patient was treated, that is, expenditure is treated like a consumable cost by spreading the capital cost over 10 years. c If approval of EECP might be withdrawn before 10 years, the potential losses in NHEs will be greater than initially indicated in Figure 45 because the equipment costs allocated to treating future patients cannot be recovered. The earlier such a change might occur the greater the additional loss. The impact of these possibilities should be considered at point 7 of the checklist before guidance to approve or AWR commits both types of irrecoverable costs.
Types and categories of guidance resulting from points 1 and 2
Points 1 and 2 of the checklist do not lead directly to a category or type of guidance. The sequence of assessments and decisions that ultimately lead to guidance starts with cost-effectiveness, expected impact on population NHEs and significance of irrecoverable costs. In the case of EECP, the technology is expected to be cost-effective and has significant irrecoverable costs. Table 67 summarises the categories and types of guidance that could ultimately result from the sequence of assessments up to point 2 (see Table 32 in Appendix 4).
| Assessment | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Guidance |
|---|---|---|---|---|---|---|---|---|
| 13 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | AWR3 |
| 14 | Yes | Yes | Yes | Yes | Yes | Yes | No | OIR3 |
| 15 | Yes | Yes | Yes | Yes | Yes | No | Yes | Approve5 |
| 16 | Yes | Yes | Yes | Yes | Yes | No | No | Reject5 |
| 17 | Yes | Yes | Yes | Yes | No | Yes | Yes | AWR4 |
| 18 | Yes | Yes | Yes | Yes | No | Yes | No | OIR4 |
| 19 | Yes | Yes | Yes | Yes | No | No | – | Approve6 |
| 20 | Yes | Yes | Yes | No | Yes | Yes | Yes | Approve7 |
| 21 | Yes | Yes | Yes | No | Yes | Yes | No | OIR5 |
| 22 | Yes | Yes | Yes | No | Yes | No | Yes | Approve8 |
| 23 | Yes | Yes | Yes | No | Yes | No | No | Reject6 |
| 24 | Yes | Yes | Yes | No | No | Yes | Yes | Approve9 |
| 25 | Yes | Yes | Yes | No | No | Yes | No | OIR6 |
| 26 | Yes | Yes | Yes | No | No | No | – | Approve10 |
Is further research required?
Point 3: Does more research seem worthwhile?
The third point on the checklist requires an assessment of the potential benefits of conducting further research, that is, at the following point in the algorithm:
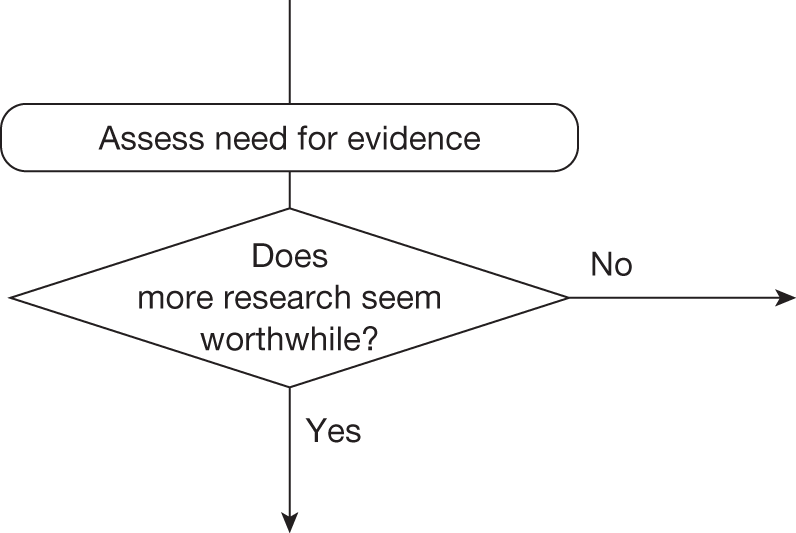
This requires judgements about (1) how uncertain a decision to approve or reject EECP might be based on the estimates of expected cost-effectiveness and (2) whether or not the scale of the likely consequences of this uncertainty might justify further research. Some assessment of the potential consequences of uncertainty is important because it indicates the population NHEs that could be gained if the uncertainty surrounding this decision could be resolved immediately, that is, it represents an expected upper bound on the benefits of more research. d
Assessing the consequences of uncertainty
Enhanced external counterpulsation is expected to be cost-effective compared with standard care (see Tables 63 and 64) but the estimates of cost and QALYs are uncertain so there is a chance that a decision to approve EECP based on existing evidence will be incorrect, that is, standard care might offer greater NHEs. Some assessment of the likely consequences of approving EECP when standard care might be better could be based on the difference in expected NHEs, that is, the expected incremental population NHEs reported in Table 63. The simplest approach would be to weight the average NHEs for EECP and standard care (reported in Table 63) by a judgement of the probability of an incorrect decision. For example, if the decision was judged to be 100% certain then there are no consequences and so there would be nothing to be gained by more research; however, as the probability that the decision is correct becomes less certain, the expected consequences (and hence potential value of more research) increase. Table 68 shows the expected consequences of uncertainty based on a weighting of average NHEs.
| Probability decision is correct | Cost-effectiveness threshold £20,000 per QALY | Cost-effectiveness threshold £30,000 per QALY |
|---|---|---|
| Expected consequences, QALYs (£M) | Expected consequences, QALYs (£M) | |
| 1.00 | 0 (0) | 0 (0) |
| 0.99 | 14 (0.3) | 163 (4.9) |
| 0.95 | 70 (1.4) | 817 (25) |
| 0.90 | 141 (2.8) | 1633 (49) |
| 0.75 | 351 (7.0) | 4083 (123) |
| 0.50 | 703 (14) | 8167 (245) |
| 0.25 | 1054 (21) | 12,250 (368) |
| 0.10 | 1265 (25) | 14,701 (441) |
| 0.05 | 1335 (27) | 15,517 (466) |
| 0.01 | 1391 (28) | 16,171 (485) |
| 0.00 | 1405 (28) | 16,334 (490) |
A judgement of how uncertain a decision might be can be informed by the PSA already used to estimate costs and QALYs. The probability that EECP is cost-effective is 0.428 at £20,000 per QALY and 0.700 at £30,000 per QALY (Table 69). e This translates into approximately 800 QALYs (Figure 46) based on the expected or average difference in NHEs at a threshold of £20,000 per QALY.
FIGURE 46.
Probability that EECP is cost-effective and the consequences of uncertainty at £20,000.
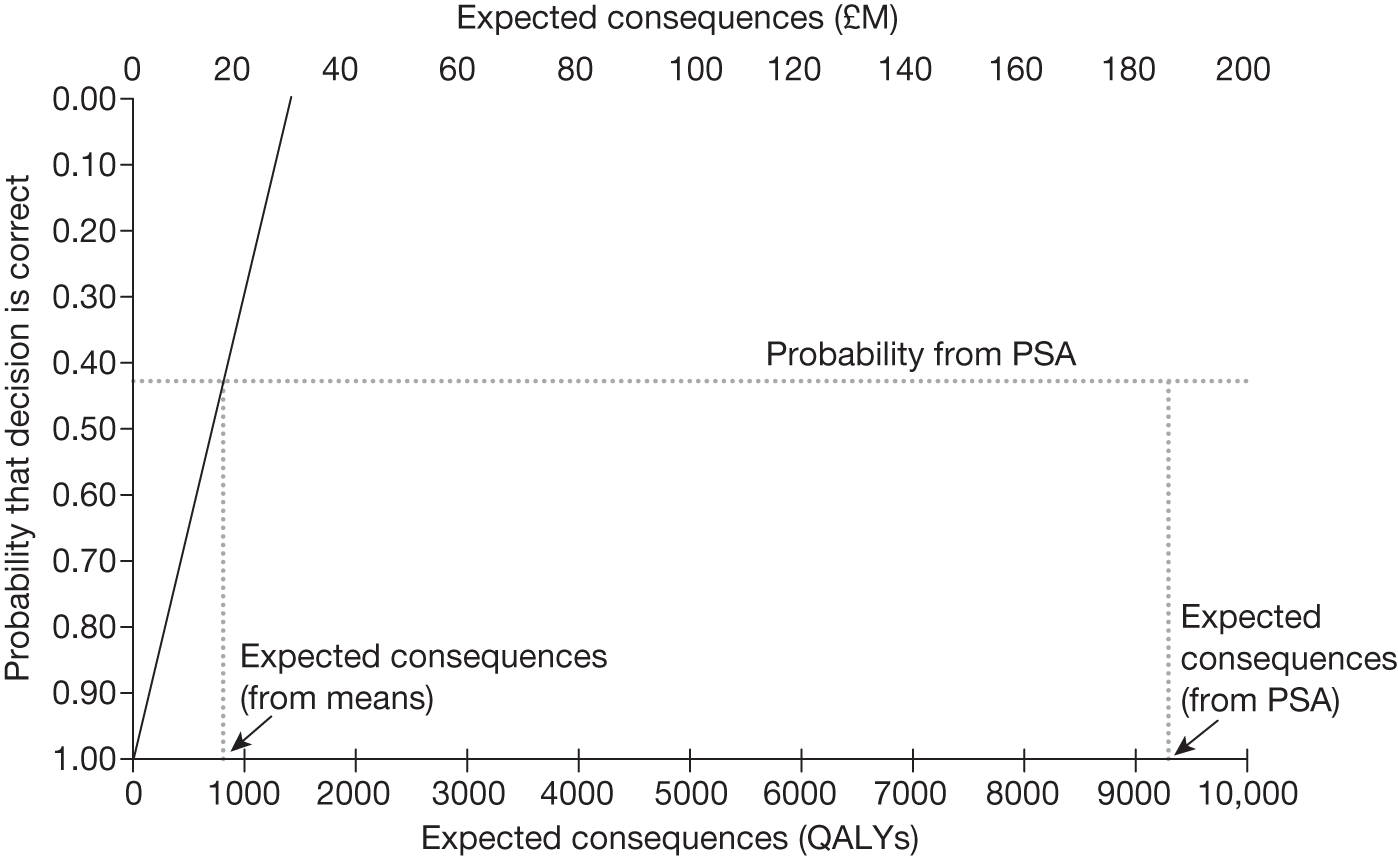
However, the difference in NHEs when EECP is not the correct decision is not necessarily the average (see Appendix 11, Why the consequences of uncertainty differ from mean incremental effects). In fact, it is very unlikely to be the average and such estimates may substantially under- or overestimate the expected consequences of uncertainty. At £20,000 per QALY, the estimate of 800 QALYs based on mean incremental population NHEs is a substantial underestimate of the expected consequences of 9287 QALYs (see Table 69 and Figure 46) based on the distribution of uncertainty from the PSA. Similarly, the estimate of 4900 QALYs at a threshold of £30,000 per QALY (Figure 47) is an overestimate of the expected consequences of 2774 QALYs (see Table 69) based on the distribution of uncertainty from the PSA. Therefore, it is only appropriate to conduct an analysis of the expected consequences of uncertainty based on the PSA rather than use a simple estimate of average NHEs, which can be misleading.
| Treatment | ICER (£/QALY) | Cost-effectiveness threshold £20,000 per QALY | Cost-effectiveness threshold £30,000 per QALY | ||||
|---|---|---|---|---|---|---|---|
| Incremental NHE, QALYs (£M) | Probability cost-effective | Expected consequences, QALYs (£M) | Incremental NHE, QALYs (£M) | Probability cost-effective | Expected consequences, QALYs (£M) | ||
| EECP | 19,391 | 1405 (28.1) | 0.428 | 9287 (185.7) | 1,405,930 (490) | 0.700 | 2774 (83.2) |
| Standard care | – | 0.572 | – | 0.300 | |||
FIGURE 47.
Probability that EECP is cost-effective and the consequences of uncertainty at £30,000.
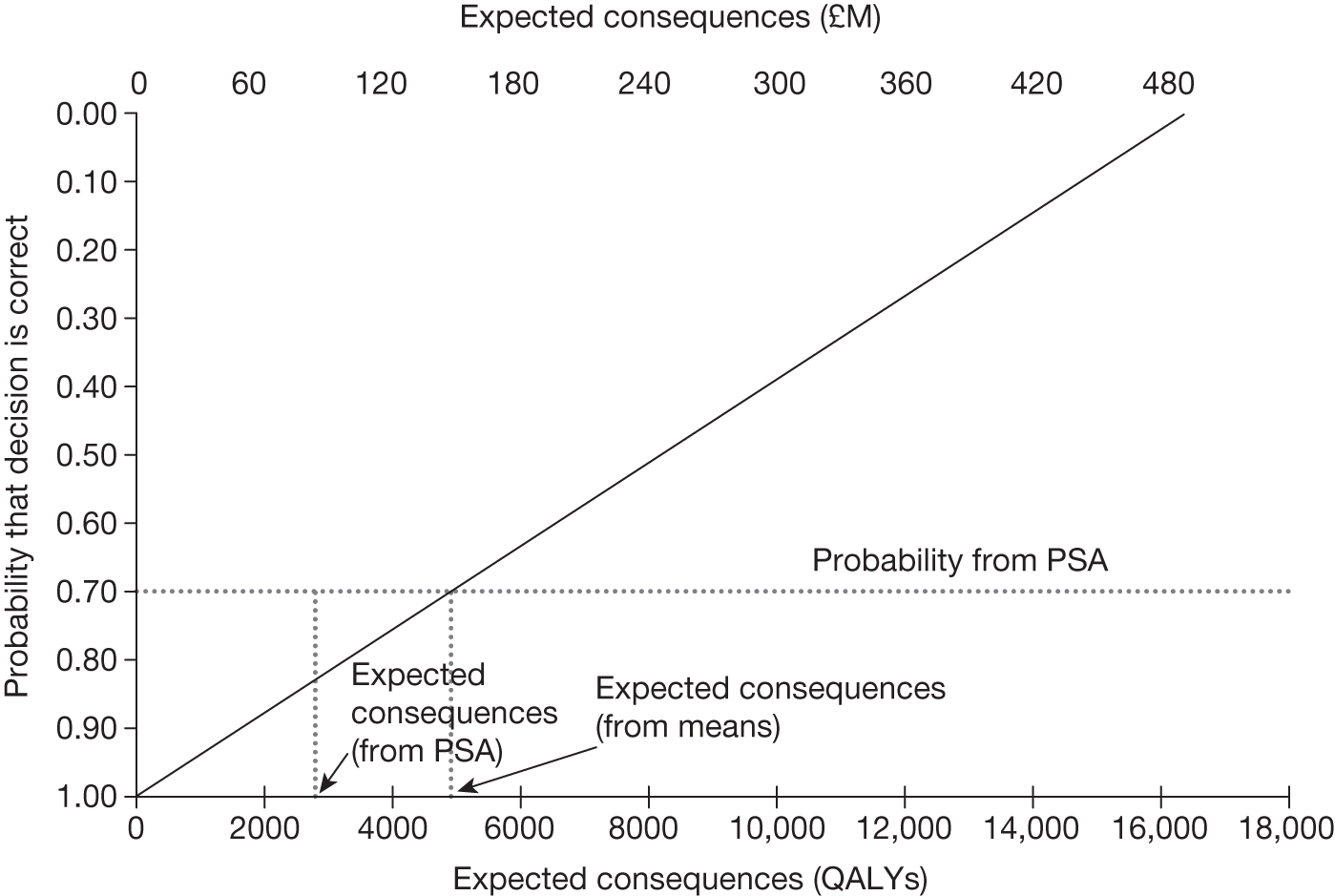
The same PSA can be used to record the frequency of errors in the decision to approve EECP. Figure 48 shows the distribution of consequences of uncertainty for EECP. Most commonly there are no consequences because EECP is the correct decision 42.8% of the time. When EECP offers lower NHEs than standard care the consequences of error may be relatively small, for example 9% are < 5000 QALYs. However, they may be very large, although this is less likely, for example there is a small chance (5.7%) that they are > 30,000 QALYs. The average over this distribution provides the expected consequences of uncertainty (9287 QALYs).
FIGURE 48.
Distribution of the consequences of uncertainty for EECP.
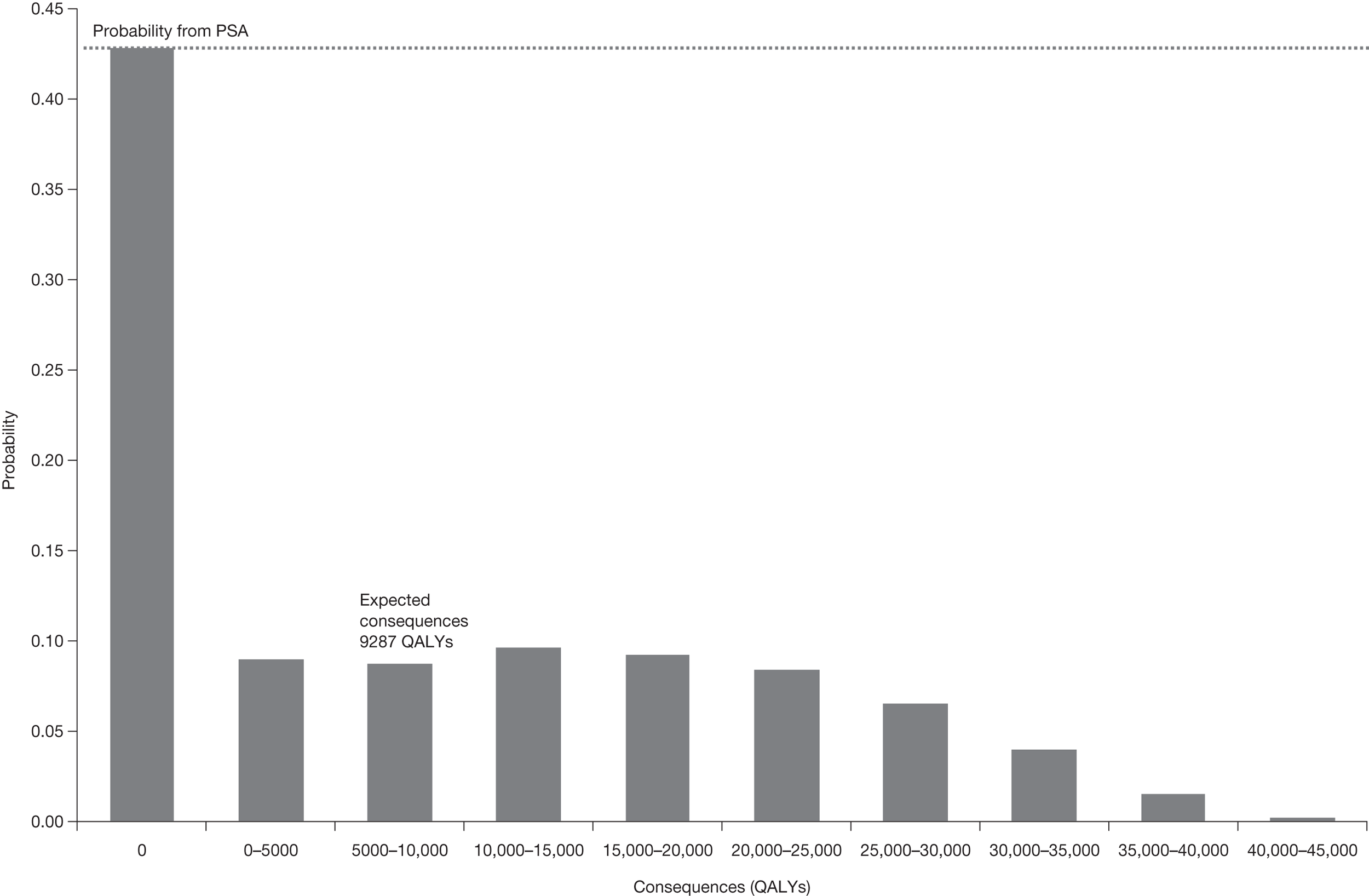
These expected consequences can be interpreted as an estimate of the population NHEs over the technology time horizon that could be gained if the uncertainty surrounding this decision could be resolved immediately, that is, it indicates an expected upper bound on the benefits of more research. The consequences can also be expressed as the equivalent NHS resources required to generate the same population NHEs (£185.7M in Table 69). In the case of EECP the consequences fall with the cost-effectiveness threshold because a decision to approve EECP will be less uncertain (see Table 69). A judgement at this point that more research might be worthwhile seems reasonable as the upper bound on its potential benefits exceeds the likely costs.
The time horizon over which evidence generated by research about a technology might be valuable may be longer (or shorter) than the period over which the technology is used. In this case, the technology time horizon is assumed to be equal to the time horizon for the benefits of research, that is, 10 years. Table 70 shows the expected consequences of uncertainty for different technology time horizons. The consequences increase with the technology time horizon and will also increase with the size of the patient population.
| Technology time horizon, years | Expected consequences, QALYs (£M) |
|---|---|
| 5 | 7511 (150) |
| 10 | 9287 (186) |
| 15 | 10,783 (216) |
| 20 | 12,042 (241) |
Analysis of subgroups
There are no relevant subgroups in the EECP case study.
Alternative scenarios
The uncertainty described above reflects uncertainty within the set of assumptions used to estimate expected costs and QALYs. However, there are often alternative views about the assumptions, which are usually presented as separate scenarios. When more than one scenario might be credible and carry some ‘weight’, there will be uncertainty between as well as within scenarios. The single RCT of EECP showed evidence of improvements in quality of life at 12 months; however, the degree to which these are sustained in the long run is uncertain. Rather than make alternative assumptions and present extreme scenarios, formal elicitation of the judgements of clinical experts about the likelihood of QALY gains in subsequent years was undertaken. The uncertainty in these elicited values is included in the estimates of the expected consequences of uncertainty reported above. However, the analysis could be presented as alternative scenarios:
-
scenario A – HRQoL benefits are sustained for only 1 year (first 12 months)
-
scenario B – HRQoL benefits are sustained for a patient’s lifetime
-
scenario C – HRQoL benefits are sustained for 4 years.
Scenarios A and B are extreme scenarios whereas scenario C lies somewhere between the two extremes. Table 71 presents the cost-effectiveness of EECP for the alternative scenarios. EECP is not expected to be cost-effective under scenario A whereas it is highly cost-effective under scenario B. For scenario C, EECP would not be regarded as cost-effective at a threshold of £20,000 per QALY but could be approved for use, based on current evidence, at a threshold of £30,000 per QALY. However, as above, some assessment of the likely consequences of approving EECP or standard care when the alternative treatment might be better should be informed by the PSA. Table 72 shows the expected consequences if the decision to approve the technology expected to be cost-effective turns out to be wrong. Under scenarios A and B the expected consequences are small (1.4 and 394 QALYs at a threshold of £20,000 per QALY, respectively) because there is less uncertainty about the decision (99.95% and 96.5% chance of no error, respectively); however, scenario C is more uncertain with expected consequences of 3228 QALYs.
| Treatment | Cost (£) | QALYs | ICER (£/QALY) | Cost-effectiveness threshold £20,000 per QALY | Cost-effectiveness threshold £30,000 per QALY | ||
|---|---|---|---|---|---|---|---|
| NHE, QALYs (£M) | Incremental NHE, QALYs (£M) | NHE, QALYs (£M) | Incremental NHE, QALYs (£M) | ||||
| Scenario A | |||||||
| EECP | 861,758,393 | 1,401,330 | 65,292 | 1,358,242 (27,165) | –29,889 (–598) | 1,372,605 (41,178) | –15,527 (–466) |
| Standard care | – | 1,388,132 | – | 1,388,132 (27,763) | – | 1,388,132 (41,644) | – |
| Scenario B | |||||||
| EECP | 966,199,093 | 1,555,685 | 5767 | 1,507,375 (30,147) | 119,243 (2385) | 1,523,478 (45,704) | 135,347 (4060) |
| Standard care | – | 1,388,132 | – | 1,388,132 (27,763) | – | 1,388,132 (41,644) | – |
| Scenario C | |||||||
| EECP | 886,474,497 | 1,423,008 | 26,531 | 1,378,685 (27,574) | –10,911 (–218) | 1,393,459 (41,804) | 3864 (116) |
| Standard care | – | 1,389,596 | – | 1,389,596 (27,792) | – | 1,389,596 (41,688) | – |
| Treatment | ICER (£/QALY) | Cost-effectiveness threshold £20,000 per QALY | Cost-effectiveness threshold £30,000 per QALY | ||||
|---|---|---|---|---|---|---|---|
| Incremental NHE, QALYs (£) | Probability cost−effective | Expected consequences, QALYs (£M) | Incremental NHE, QALYs (£) | Probability cost−effective | Expected consequences, QALYs (£M) | ||
| Scenario A | |||||||
| EECP | 65,292 | –29,889 (–598) | 0.0005 | 1.4 (0.028) | –15,527 (–466) | 0.0278 | 117 (3.5) |
| Standard care | – | – | 0.9995 | – | 0.9722 | ||
| Scenario B | |||||||
| EECP | 5767 | 119,243 (2385) | 0.9649 | 394 (7.8) | 135,347 (4060) | 0.9905 | 67 (2.0) |
| Standard care | – | – | 0.0351 | – | 0.0095 | ||
| Scenario C | |||||||
| EECP | 26,531 | –10,911 (–218) | 0.2256 | 3228 (64) | 3864 (116) | 0.5261 | 4715 (141) |
| Standard care | – | – | 0.7744 | – | 0.4739 | ||
The expected consequences of uncertainty within each scenario are sufficient to indicate the potential benefits of research when only one scenario is regarded as credible. However, when more than one scenario might be credible and carry some ‘weight’, there will be uncertainty between as well as within scenarios. The ‘weighting’ of scenarios can be made explicit by assigning probabilities to represent how credible each is believed to be. The weighted average of costs and QALYs across scenarios can easily be calculated. It is tempting to take a simple weighted average of the expected consequences of uncertainty across the scenarios. However, a simple weighted average may under- or overestimate the combined consequences of uncertainty within and between scenarios. The correct estimate requires the probabilities (weights) to be applied directly to the simulated output from PSA rather than to the mean values. Table 73 shows that the simple weighted average of expected consequences (linear combination of mean estimates) substantially underestimates the correct estimate based on weighting the output of PSA.
| Probability of scenario C | Probability of scenario A | Probability of scenario B | Expected consequences, QALYs (£M) | |
|---|---|---|---|---|
| Weighted | PSA | |||
| 1.00 | 0.00 | 0.00 | 3228 (64) | 3228 (64) |
| 0.90 | 0.05 | 0.05 | 2925 (59) | 8966 (179) |
| 0.80 | 0.10 | 0.10 | 2622 (52) | 14,369 (287) |
| 0.70 | 0.15 | 0.15 | 2319 (46) | 14,426 (289) |
| 0.60 | 0.20 | 0.20 | 2016 (40) | 14,480 (290) |
| 0.50 | 0.25 | 0.25 | 1713 (34) | 14,580 (292) |
| 0.40 | 0.30 | 0.30 | 1410 (28) | 14,700 (294) |
| 0.30 | 0.35 | 0.35 | 1107 (22) | 14,849 (297) |
| 0.20 | 0.40 | 0.40 | 804 (16) | 14,972 (299) |
| 0.10 | 0.45 | 0.45 | 501 (10) | 15,039 (301) |
| 0.00 | 0.50 | 0.50 | 198 (4) | 15,106 (302) |
The results of the elicitation can be used to provide an estimate of the ‘weighting’ of scenarios. The elicitation implied probabilities of 0.243, 0.353 and 0.404 associated with scenarios A, B and C, respectively. A simple weighted average of the expected consequences within each scenario using these probabilities (1442 QALYs) significantly underestimates both the estimate of expected consequences based on all of the information from elicitation (9287 QALYs) and the estimate based on weighting the simulated output rather than the mean estimates (13,081 QALYs). This illustrates (1) that a simple weighted average of expected consequences may be misleading and (2) that elicitation may provide a richer characterisation of uncertainty as well as the probabilities associated with alternative assumptions (see Appendix 11, Why averaging scenarios may be misleading).
Point 4: Is research possible with approval?
The fourth point on the checklist requires an assessment of what type of evidence is needed and a judgement of whether or not the research required to generate it can be conducted while the technology is approved, i.e., at the following point in the algorithm:
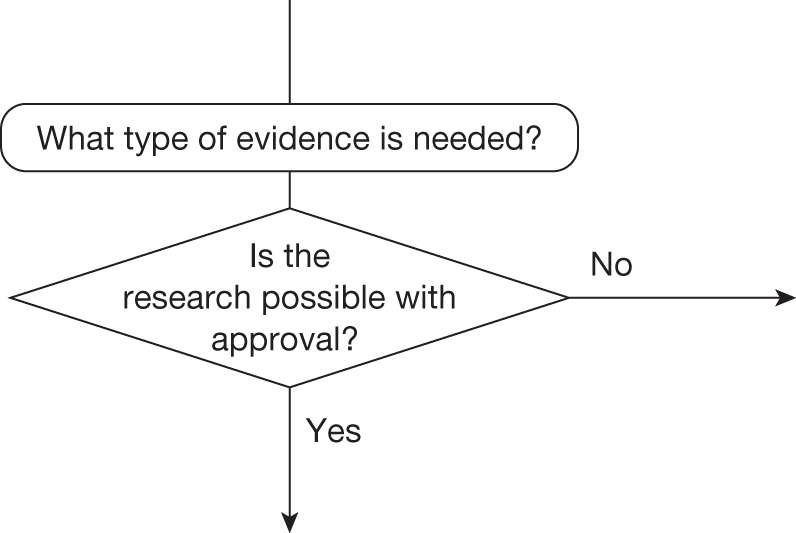
This requires judgements about (1) how important particular types of parameters are to estimates of cost and QALYs, (2) what values these parameters would have to take to change a decision based on expected cost-effectiveness, (3) how likely it is that parameters might take such values and (4) what would be the consequences if they did, that is, what might be gained in terms of population NHEs if the uncertainty in the values of these parameters could be immediately resolved.
Assessing the importance of parameters
The expected cost-effectiveness of EECP is based on the relationship between the input parameters (see Table 62) and outputs of cost and QALYs. A simple summary of the direction and strength of these relationships can be provided by the elasticity, that is, the proportionate change in NHEs due to a 1% change in the value of the parameter (Table 74). Parameters with high elasticities (especially with respect to differences in NHE) might be regarded as more ‘important’.
| Parameter | Elasticity for NHEs, QALYs | Elasticity for incremental NHEs, QALYs | |
|---|---|---|---|
| EECP | Standard care | ||
| HRQoL increment in first year from EECP | –0.6419 | –0.8899 | 0.2480 |
| Probability of sustaining HRQoL benefits in year 2 (elicited) | 0.1682 | 0.0000 | 0.1682 |
| Probability of sustaining HRQoL benefits in year 3 (elicited) | 0.1218 | 0.0000 | 0.1218 |
| Probability of sustaining HRQoL benefits in year 4 (elicited) | 0.0881 | 0.0000 | 0.0881 |
| Probability of sustaining HRQoL benefits in subsequent years (group of elicited parameters) | 0.3726 | 0.0000 | 0.3726 |
| 2-year probability of requiring repeat EECP sessions | –0.0165 | 0.0000 | –0.0165 |
| Cost of EECP | –0.2372 | 0.0000 | –0.2372 |
Although these measures of importance are more instructive than a series of arbitrary one-way sensitivity analyses, they do not directly help the assessment of what values parameters must take to change decisions and how likely such values might be. A simple summary of the values that particular parameters must take to make each of the alternatives cost-effective can also be provided (Table 75); however, although instructive, such ‘threshold values’ do not indicate how likely it is that the threshold will be crossed.
| Parameter | Mean value | EECP | Standard care |
|---|---|---|---|
| HRQoL increment in first year from EECP | 0.0717 | 0.0686 to max. | 0–0.0685 |
| Probability of sustaining HRQoL benefits in year 2 (elicited) | 0.7570 | 0.7123–1 | 0–0.7122 |
| Probability of sustaining HRQoL benefits in year 3 (elicited) | 0.7420 | 0.6782–1 | 0–0.6781 |
| Probability of sustaining HRQoL benefits in year 4 (elicited) | 0.7188 | 0.6419–1 | 0–0.6418 |
| 2-year probability of requiring repeat EECP sessions | 0.1780 | 0–0.2680 | 0.2681–1 |
| Cost of EECP | £4347 | £0–4486 | £4487 to max. |
Assessment of uncertainty
The judgement about how likely it is that parameters might take values that will change the technology expected to be cost-effective can be informed by the results of the PSA. The distributions assigned to the parameters in PSA describe how uncertain the parameter estimates are, such that they ought to reflect the amount and quality of existing evidence. The probability that each parameter might take values that would lead to each of the alternatives being cost-effective is reported in Table 76 for the uncertain parameters.
| Parameters | EECP | Standard care |
|---|---|---|
| HRQoL increment in first year from EECP | 0.454 | 0.546 |
| Probability of sustaining HRQoL benefits in year 2 (elicited) | 0.627 | 0.373 |
| Probability of sustaining HRQoL benefits in year 3 (elicited) | 0.653 | 0.347 |
| Probability of sustaining HRQoL benefits in year 4 (elicited) | 0.661 | 0.339 |
| 2-year probability of requiring repeat EECP sessions | 1.000 | 0.000 |
What type of evidence is needed?
An assessment of the likely consequences of the uncertainty described above is required. This assessment can directly inform the judgement of what evidence is needed and whether or not the type of research required to generate it will be possible with approval. The expected consequences of uncertainty associated with each parameter are reported in Table 77. This decomposes the overall expected consequences into the contribution that each parameter (or group of parameters) makes. Note that the overall expected consequences of uncertainty will not, in general, equal the sum of the expected consequences for each of the parameters (or groups of parameters) separately. This is because the overall consequences take account of the joint effect of uncertainty in all parameters simultaneously. Even if parameters are independent they will be related to differences in NHEs in different ways (see Table 74); sometimes the effect of uncertainty in one parameter may, to some extent, substitute for or complement the effect of uncertainty in the others.
| Parameters | Expected consequences, QALYs (£M) |
|---|---|
| HRQoL increment in first year from EECP | 8511 (170) |
| Probability of sustaining HRQoL benefits in year 2 (elicited) | 1644 (33) |
| Probability of sustaining HRQoL benefits in year 3 (elicited) | 1398 (28) |
| Probability of sustaining HRQoL benefits in year 4 (elicited) | 1058 (21) |
| Probability of sustaining HRQoL benefits in subsequent years (group of elicited parameters) | 2709 (54) |
| 2-year probability of requiring repeat EECP sessions | 0 (0) |
| Overall expected consequences of uncertainty | 9440 (189) |
The most significant consequences of uncertainty associated with parameters relate to the relative treatment effect of EECP in the first year in terms of its improvement of HRQoL. Interestingly, although the probability of sustaining the QALY benefits of EECP in the long run is very uncertain, the greater part of potential value is in more precise estimates of QALY gains in the first 12 months (2709 QALYs or £54M and 8511 QALYs or £170M, respectively).
Because more precise estimates of the relative treatment effect are required research needed might not be possible for the reasons discussed in Chapter 5. If it is possible, more precise estimates of treatment effect either in the short or in the longer term will require an experimental design if selection bias is to be avoided. The results in Table 77 suggest that a further RCT may well be worthwhile; however, whether such a trial should have a follow-up of 12 months or a longer follow-up duration depends on if the additional benefits of a longer follow-up to more precisely estimate duration of effect exceed the additional opportunity costs of a more costly and lengthy trial (see later sections).
Implications of between-scenario uncertainty
Earlier the contribution that alternative scenarios might make to the overall expected consequences of uncertainty and therefore the potential gains from further evidence was considered. In situations in which more than one scenario might be regarded as credible there will be uncertainty between as well as within each of the scenarios. It was demonstrated in Alternative scenarios that an assessment of the combined consequences of both sources of uncertainty requires ‘weights’ (probabilities) to be assigned to represent their credibility, which can then be applied directly to the simulated output from PSA (see Appendix 11, Why averaging scenarios may be misleading). The same analysis can also be used to identify the expected consequences of uncertainty associated with the alternative scenarios themselves, that is, what might be gained if evidence could immediately distinguish which scenario was ‘true’. This can help to inform the assessment of what type of evidence might be needed and whether or not the research required to generate it is likely to be possible once a technology is approved for widespread NHS use.
In Alternative scenarios three scenarios were considered: A – HRQoL benefits are sustained for only 12 months; B – HRQoL benefits are sustained for a patient’s lifetime; and C – HRQoL benefits are sustained for 4 years. Table 78 shows the expected consequences of uncertainty within scenarios, between scenarios and combining consequences within and between scenarios. The expected consequences of the uncertainty between scenarios are much greater than the expected consequences within scenarios, suggesting that further evidence about EECP that could help to distinguish between the scenarios is more valuable than the parameters associated with each.
| Probability of scenario C | Probability of scenario A | Probability of scenario B | Expected consequences, QALYs (£M) | ||
|---|---|---|---|---|---|
| Within scenarios | Between scenarios | Within and between scenarios | |||
| 1.00 | 0.00 | 0.00 | 3228 (65) | 0 (0) | 3228 (65) |
| 0.90 | 0.05 | 0.05 | 4281 (86) | 5962 (119) | 8887 (178) |
| 0.80 | 0.10 | 0.10 | 6380 (128) | 11,718 (234) | 14,340 (287) |
| 0.70 | 0.15 | 0.15 | 4406 (88) | 12,121 (242) | 14,440 (289) |
| 0.60 | 0.20 | 0.20 | 3273 (65) | 12,524 (250) | 14,540 (291) |
| 0.50 | 0.25 | 0.25 | 2637 (53) | 12,928 (259) | 14,641 (293) |
| 0.40 | 0.30 | 0.30 | 2279 (46) | 13,331 (267) | 14,741 (295) |
| 0.30 | 0.35 | 0.35 | 2078 (42) | 13,735 (275) | 14,841 (297) |
| 0.20 | 0.40 | 0.40 | 1972 (39) | 14,138 (283) | 14,942 (299) |
| 0.10 | 0.45 | 0.45 | 1940 (39) | 14,541 (291) | 15,042 (301) |
| 0.00 | 0.50 | 0.50 | 1947 (39) | 14,945 (299) | 15,142 (303) |
Formal elicitation of the judgement of clinical experts about whether observed QALY gains at 12 months are likely to be sustained in subsequent years was undertaken. Because the uncertainty in these elicited values was incorporated into the analysis in the same way as other parameters, the use of alternative scenarios was not necessary. The results of elicitation implied probabilities of 0.243, 0.353 and 0.404 associated with scenarios A, B and C, respectively. Based on these ‘weights’ for each scenario the overall expected consequences of uncertainty (combining the consequences within and between scenarios) would be 14,146 QALYs. The expected consequences of uncertainty between the scenarios (13,202 QALYs) are much greater than what might be potentially gained from resolving the uncertainty within each scenario (1765 QALYs). Therefore, most of what might be gained from further evidence about EECP (in the absence of formal elicitation) would be evidence that could help distinguish between the scenarios rather than the parameters associated with each.
Types and categories of guidance resulting from points 3 and 4
Points 3 and 4 of the checklist are critical because if research is not judged to be worthwhile no further assessments are required. In the case of EECP, more research appears to be worthwhile; the expected consequences of uncertainty are high. Whether or not the research required to generate the evidence needed can be conducted while the technology is approved for widespread use will determine whether AWR or OIR is a possibility. Table 79 summarises the categories and types of guidance that could ultimately result from the sequence of assessments up to point 4.
| Assessment | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Guidance |
|---|---|---|---|---|---|---|---|---|
| 13 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | AWR3 |
| 14 | Yes | Yes | Yes | Yes | Yes | Yes | No | OIR3 |
| 15 | Yes | Yes | Yes | Yes | Yes | No | Yes | Approve5 |
| 16 | Yes | Yes | Yes | Yes | Yes | No | No | Reject5 |
| 17 | Yes | Yes | Yes | Yes | No | Yes | Yes | AWR4 |
| 18 | Yes | Yes | Yes | Yes | No | Yes | No | OIR4 |
| 19 | Yes | Yes | Yes | Yes | No | No | – | Approve6 |
| 20 | Yes | Yes | Yes | No | Yes | Yes | Yes | Approve7 |
| 21 | Yes | Yes | Yes | No | Yes | Yes | No | OIR5 |
| 22 | Yes | Yes | Yes | No | Yes | No | Yes | Approve8 |
| 23 | Yes | Yes | Yes | No | Yes | No | No | Reject6 |
| 24 | Yes | Yes | Yes | No | No | Yes | Yes | Approve9 |
| 25 | Yes | Yes | Yes | No | No | Yes | No | OIR6 |
| 26 | Yes | Yes | Yes | No | No | No | – | Approve10 |
Do the benefits of research exceed the costs?
Point 5: Will other sources of uncertainty resolve over time?
The fifth point on the checklist requires an assessment of whether or not changes are likely to occur in the future that will influence the cost-effectiveness of the alternative technologies and the potential benefits of research, that is, at the following point in the algorithm:
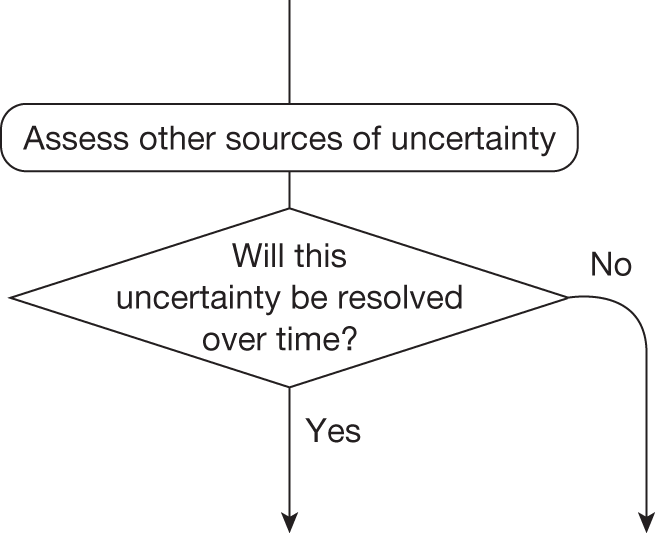
This assessment requires information about (1) changes in the prices of the technology and its comparators, (2) the emergence of new technologies that might make existing ones obsolete or change their cost-effectiveness and (3) other relevant research reporting. A number of potential sources of information and evidence were examined to inform this assessment (see Appendix 4 for full details of the sources and searches conducted).
Changes in the prices of the technology and its comparators
Changes in prices influence not only expected cost-effectiveness but also uncertainty and the potential benefits of research to future patients, for example if the price of a technology expected to be cost-effective is likely to fall significantly just before research reports the potential benefits will not be realised because approval of the technology will be less uncertain and there may be much less or little to gain from the results of the research. This assessment requires information about when major changes in prices are likely and some evidence about the likely extent of any change. It is very difficult to locate information on changes in price relevant to devices such as EECP as a device may have only a CE mark, which, unlike a patent, does not offer protection and can be renewed every 10 years. Any patent is likely to relate to some aspect of the device rather than the device itself. EECP was filed in 1995, renewed in 2005 and is up for renewal in 2015. The manufacturer (Vasogenics, Sapphire House, Albion Road, Bradford, West Yorkshire, UK) is still heavily marketing EECP so it is fair to assume that it will renew in 2015. There are no current comparators to EECP (apart from standard care). For these reasons a change in price for EECP is not anticipated and is not explored further.
Entry of new technologies
The entry of a new technology may make the existing technology that is expected to be cost-effective obsolete (no longer the most cost-effective alternative). Even when it does not, it will tend to change the relative cost-effectiveness of the alternatives, influencing how uncertain a decision to approve the original technology will be for future patients and the potential gains from research. A number of potential sources of information were examined to identify new technologies relevant to stable angina that were likely to become available. In particular, products considered by the MTAC as part of the Evaluation Pathway Programme for Medical Technologies at NICE were searched using the term ‘angina’. 174 No relevant records were found. Although there is no evidence of new technologies emerging in EECP, different scenarios are explored in Point 6: Are the benefits of research greater than the costs? as the development and launch of new devices are more difficult to identify in advance.
Other research reporting of the technology and its comparators
Research that is already under way, commissioned or likely to be undertaken, whether in the UK or elsewhere, is relevant. A number of potential sources of information were examined to identify clinical research under way at the time of the assessment for EECP including national and international trial registries (ClinicalTrials.gov, WHO ICTRP and Current Controlled Trials) as well as other databases that report NHS-funded research and not just clinical trials (e.g. NRR and UKCRN). Despite an assiduous search no records relevant to EECP were identified.
Point 6: Are the benefits of research greater than the costs?
The sixth point on the checklist requires a judgement of whether the potential benefits of conducting further research (initially considered at point 3) are likely to exceed the costs, that is, at the following point in the algorithm:
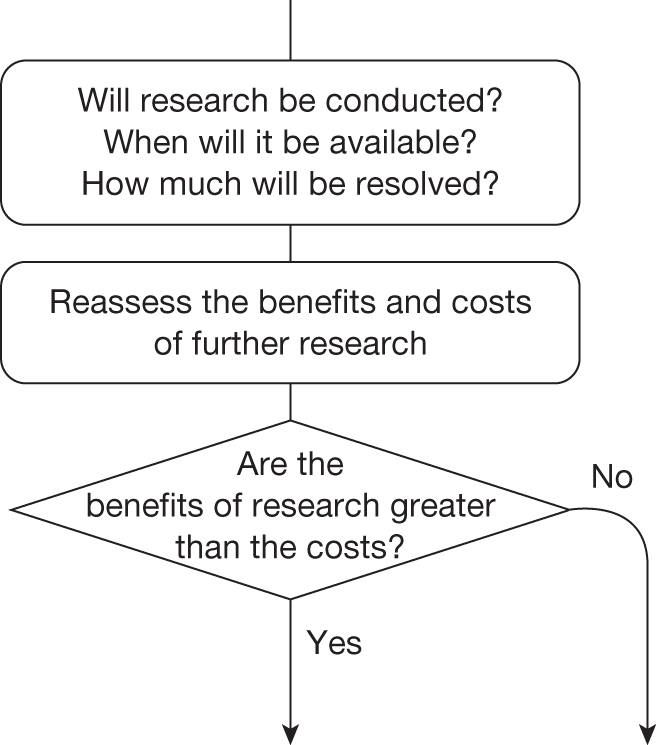
This requires an assessment of (1) whether or not the type of research that is required is likely to be conducted, (2) if conducted, when the results are likely to be available, (3) how much uncertainty is likely to be resolved and (4) the likely impact of any other sources of uncertainty on the longer-term benefits of research.
Will the research be conducted?
Even if research is recommended in OIR or AWR, it might not be undertaken by manufacturers or commissioned by research funders and there is no guarantee that research will be able to recruit or complete. The expected consequences of uncertainty for EECP reported in Point 3: Does more research seem worthwhile? are illustrated in Figure 49 for a range of probabilities that research will be successfully undertaken. The potential gains from research depend on a judgement of whether the research recommended as part of OIR or AWR will be successfully completed. The cost of research (in this case considered to be either £1.5M or £10M) can be compared directly with the potential benefits by either expressing the potential gains in population NHEs as the equivalent NHS resources (i.e. the resources that would be required to generate the same NHEs) or expressing the cost of research in terms of the QALYs that could be gained elsewhere in the NHS by using the same resources to provide access to health care.
FIGURE 49.
Expected potential benefits of research.
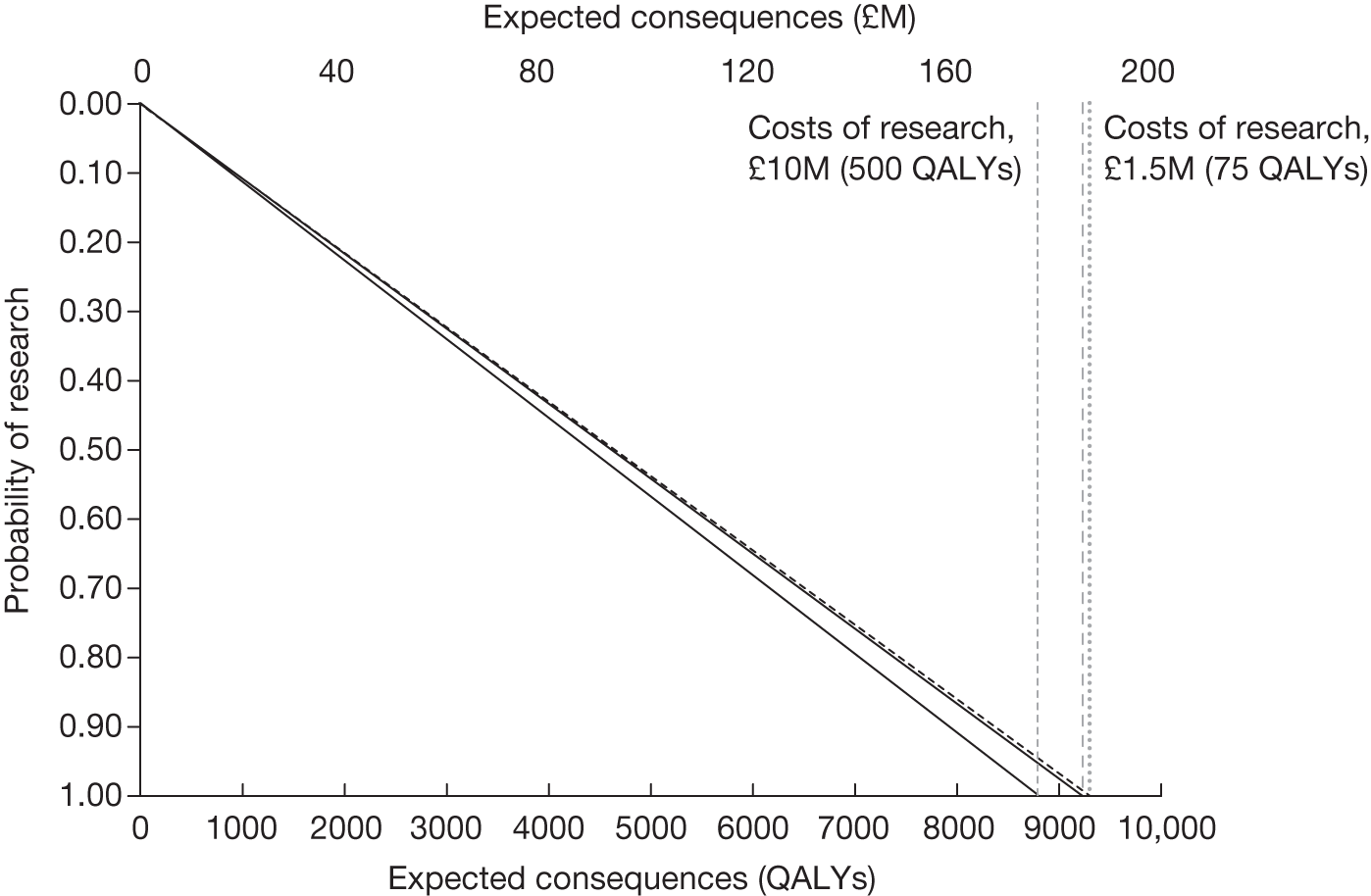
When will it be available?
Research, even if commissioned and successfully completed, will take time to complete and report. Therefore, any assessment of the potential benefits should account for the fact that patient populations will not benefit from the results of research until they are available. If treatment decisions are irreversible (e.g. an acute indication) then it is only those patients who are incident after the research reports who will realise any of the potential benefits. However, for treatment decisions that are not irreversible, which is the case for EECP as it is a chronic condition, prevalent and incident patients can benefit from the results of the research. Patients prevalent while the research is undertaken will not benefit immediately but those who survive can benefit from the results once the research is completed. Figure 50 shows the expected benefits of research for EECP for a range of probabilities that research will be successfully undertaken and for different times for research to report. Again, the cost of research (considered to be £1.5M) can be compared directly with the potential benefits by the time that it takes for research to report.
FIGURE 50.
Expected potential benefits of research by time for research to report.
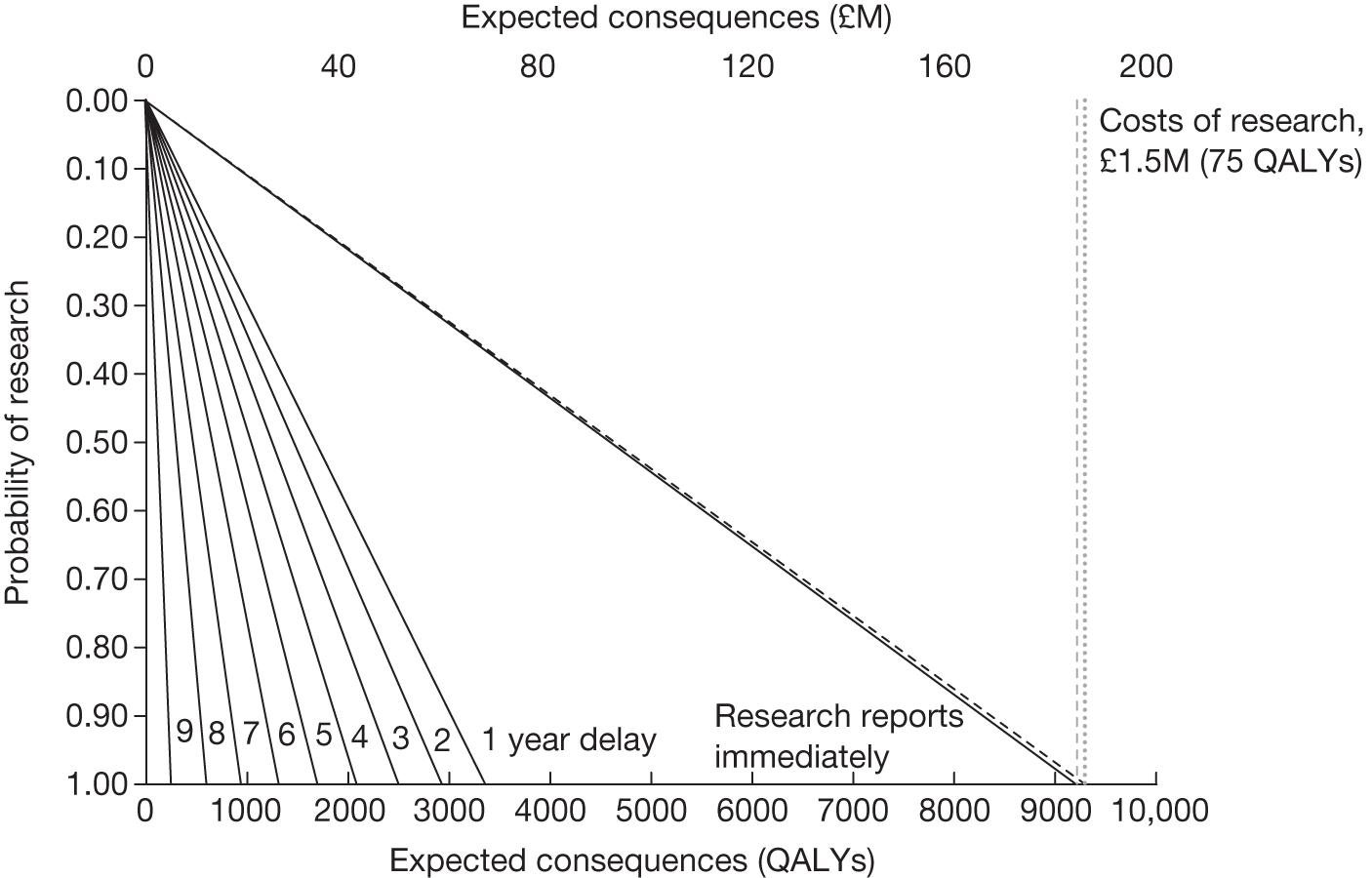
How long research might take to report will depend in part on the design (follow-up, sample size and end points), recruitment rates and size of the eligible patient population, as well as on how efficient the organisation and data collection might be. The potential value of research for EECP over a range of possible time horizons is reported in Figure 51 for different research designs (length of follow-up). The potential value of further research declines with the time to research reporting. The value of research is always higher for designs with longer follow-up as the research is able to resolve more of the uncertainty surrounding the approval of EECP; however, the differences are small in this case, but the longer the follow-up design the longer the time it takes for the research to report. This relationship gives some indication of the value of improving the timeliness of research through, for example, investment in research infrastructure or adopting a research design.
FIGURE 51.
Potential benefits of research by time to report for different research designs.
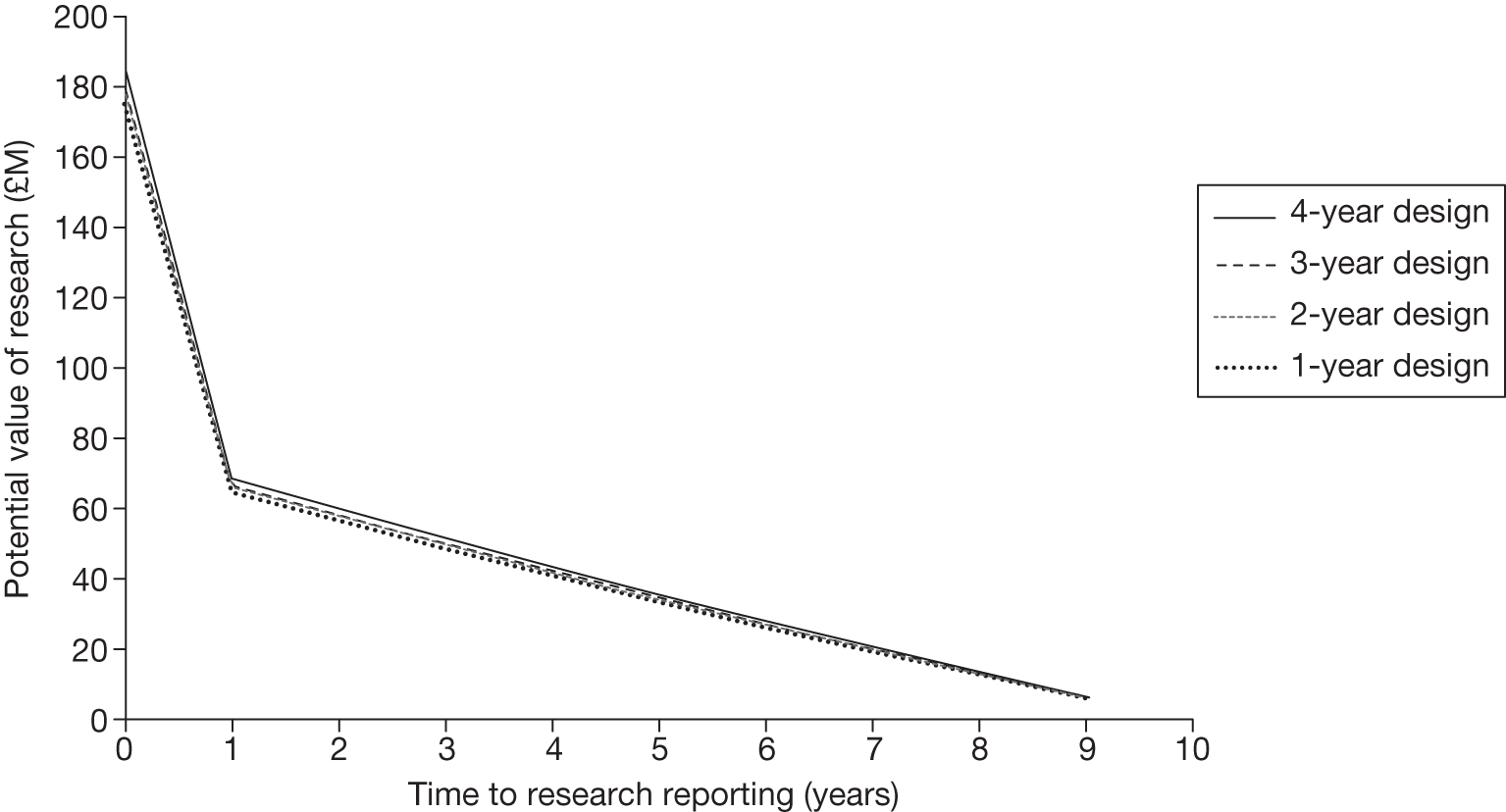
How much will be resolved?
How much of the uncertainty will be resolved depends on the type of research likely to be undertaken. In Point 4: Is research possible with approval? the potential benefits of different types of evidence were assessed. In the case of EECP, most benefit can be gained by resolving the uncertainty in the improvement in quality of life at 12 months. This would require a 1-year follow-up design, with research reporting after 1 year. Resolving more of the uncertainty would require a longer follow-up and hence longer follow-up designs. For example, a trial with a 2-year follow-up will provide information about improvements in quality of life at 12 months and about the sustained duration of these improvements from 12 months to 2 years. Similarly, a trial with an even longer follow-up will provide information about the sustained duration in subsequent years. Table 80 reports the potential benefits of alternative research designs of 1–4 years of follow-up. Although longer follow-up offers greater potential benefits, they are relatively small compared with the loss of potential value if longer follow-up delays the time until research findings are available, for example a 4-year design will require a minimum of 4 years to complete; however, as long as research reports before 8 years the potential benefits are likely to exceed the costs.
| Time until research reports, years | Potential benefits of research, QALYs (£M) | |||
|---|---|---|---|---|
| 4-year design | 3-year design | 2-year design | 1-year design | |
| Immediately | 9287 (186) | 8987 (180) | 8944 (179) | 8756 (175) |
| 1 | 3435 (69) | 3324 (67) | 3308 (66) | 3238 (65) |
| 2 | 2999 (60) | 2902 (58) | 2888 (58) | 2827 (57) |
| 3 | 2577 (52) | 2494 (50) | 2482 (50) | 2430 (49) |
| 4 | 2170 (43) | 2100 (42) | 2090 (42) | 2046 (41) |
| 5 | 1776 (36) | 1719 (34) | 1711 (34) | 1675 (34) |
| 6 | 1396 (28) | 1351 (27) | 1345 (27) | 1316 (26) |
| 7 | 1029 (21) | 996 (20) | 991 (20) | 970 (19) |
| 8 | 674 (14) | 652 (13) | 649 (13) | 636 (13) |
| 9 | 331 (7) | 321 (6) | 319 (6) | 312 (6) |
| 10 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
What is the impact of other sources of uncertainty?
In Point 5: Will other sources of uncertainty resolve over time?, no information was identified regarding other sources of uncertainty for EECP. A change in price for EECP is not anticipated and there is no evidence of new technologies emerging. However, given that the development and launch of new devices are more difficult to identify in advance, two alternative scenarios are considered in Figure 52. In scenario A the new technology enters at year 5 and makes EECP entirely obsolete, that is, not cost-effective and not uncertain – equivalent to a shorter technology horizon of 5 years. At this point there is no value in the evidence generated by research about EECP. The potential benefits of further research about EECP are likely to exceed the costs only if the research reports quickly. Nevertheless, even in this extreme scenario the benefits of research with only 1 year of follow-up are likely to exceed the costs as long as it reports before 4 years. In scenario B the new technology enters at year 5 and has similar NHEs to treatment with EECP and the uncertainty surrounding its expected cost-effectiveness is also similar. Now research about EECP has more potential value in the future because it will also help to resolve some of the uncertainty in the choice between EECP and the new technology.
FIGURE 52.
Potential value of research by time to report and other sources of uncertainty. These potential values are based on a 1-year follow-up design.
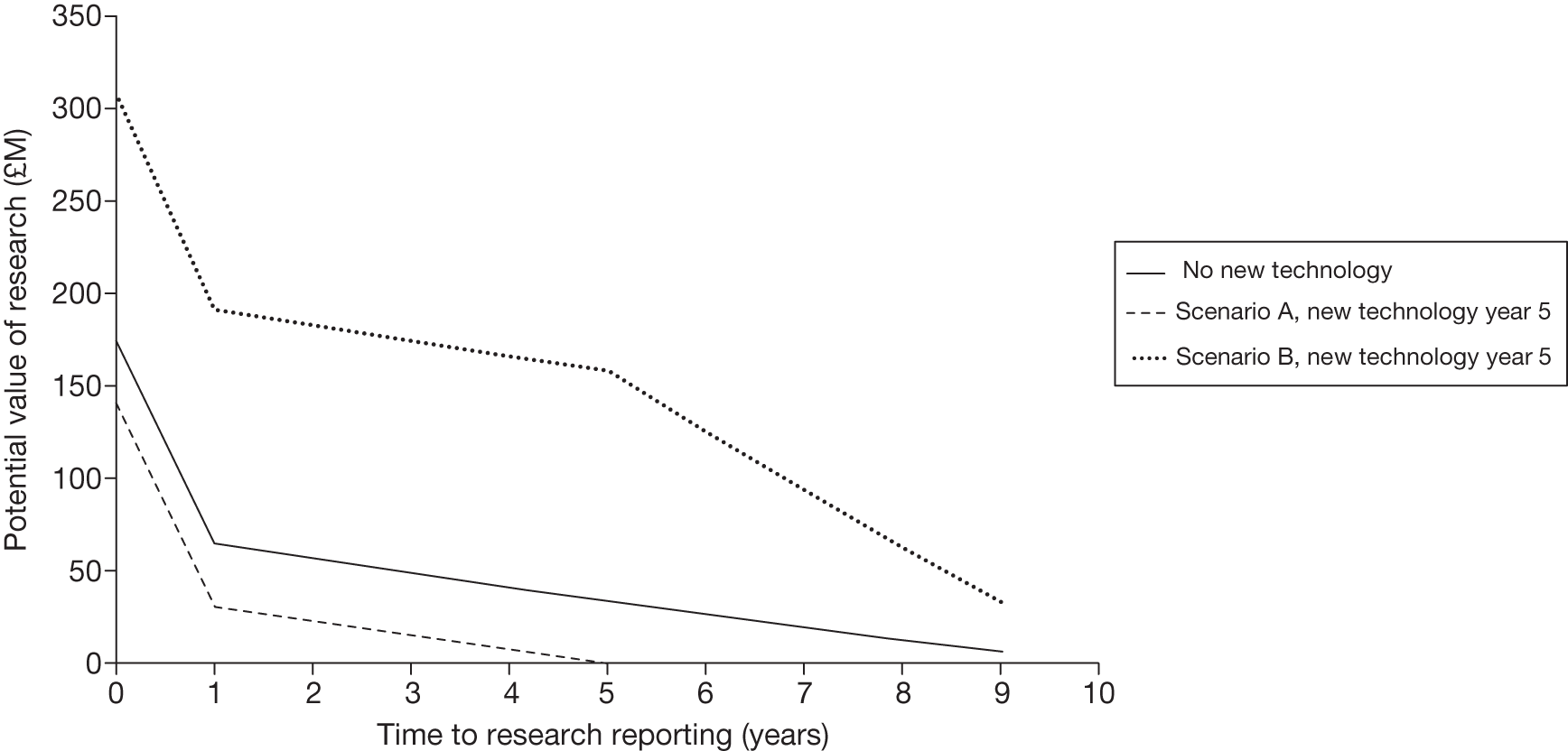
The potential value of research presented in Figure 52 should be regarded as an upper bound to the value that is likely to be realised by actual research for two reasons: (1) even well-designed research with large sample sizes will not fully resolve the uncertainty in the value that a parameter might take and (2) insofar as implementation of NICE guidance is not ‘perfect’ and all clinical practice might not immediately respond to the results of research, the full benefits will only be realised over time or with additional implementation efforts. For these reasons a judgement of whether or not benefits of research are likely to exceed the costs might be made conservatively, requiring evidence that, even in pessimistic scenarios, the research would still be worthwhile.
Types and categories of guidance resulting from points 5 and 6
The judgements made at points 5 and 6 of the checklist are critical because if the benefits of research are not judged to exceed the costs then no further assessments are required (unless there are significant irrecoverable costs, as in the case of EECP). For EECP, no other sources of uncertainty were identified at point 5 and the potential value of research is likely to exceed the costs. Therefore, the categories and types of guidance that could ultimately result from the sequence of assessments up to point 6 are AWR4, OIR4, Approve9 and OIR6 (Table 81).
| Assessment | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Guidance |
|---|---|---|---|---|---|---|---|---|
| 17 | Yes | Yes | Yes | Yes | No | Yes | Yes | AWR4 |
| 18 | Yes | Yes | Yes | Yes | No | Yes | No | OIR4 |
| 19 | Yes | Yes | Yes | Yes | No | No | – | Approve6 |
| 24 | Yes | Yes | Yes | No | No | Yes | Yes | Approve9 |
| 25 | Yes | Yes | Yes | No | No | Yes | No | OIR6 |
| 26 | Yes | Yes | Yes | No | No | No | – | Approve10 |
Are the benefits of approval greater than the costs?
Point 7: Are the benefits of approval greater than the costs?
The seventh and final point on the checklist requires an assessment and comparison of the benefits and costs of early approval. The costs of approval include the potential value of any research that may be forgone as a consequence of approving EECP and any costs that are irrecoverably committed by approval. A judgement of whether or not the benefits of approval and early access for current patients are likely to exceed the opportunity costs for future patients is required, that is, at the following point in the algorithm:
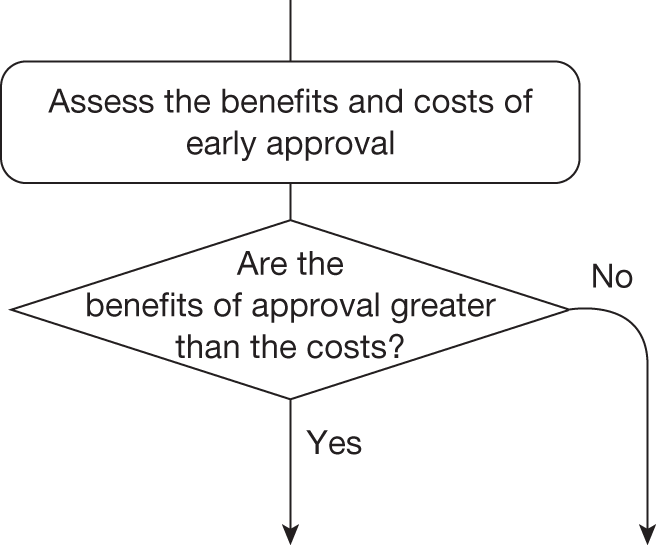
The decision at this point always leads directly to a particular category and type of guidance. For EECP, this could be AWR, OIR or approve (see Table 81).
Technologies with significant irrecoverable costs and research is possible with approval
Even when research is possible with approval, the impact of committing irrecoverable costs through AWR (or approval) must be considered, so OIR remains a possibility. EECP commits capital costs associated with long-lived equipment as well as initially negative per-patient NHEs. These irrecoverable opportunity costs at a patient level are significant because treatment choice for a presenting patient is not irreversible over relevant time frames (see Point 2: Are there significant irrecoverable cost?). As a consequence, even if research is possible with approval it is not clear that AWR would be appropriate, because OIR avoids the commitment of irrecoverable costs until research findings are available and a more informed decision can be made.
Figure 53 shows the expected NHEs of AWR and OIR over a range of possible time horizons for when the research recommended might report. OIR offers greater expected NHEs than AWR as long as research reports before 9 years. This is because the consequences (losses of population NHEs) of committing both aspects of irrecoverable costs through AWR are greater than the NHEs forgone by restricting access to EECP through OIR. The costs of research have not been included because they are incurred with both AWR and OIR guidance. f
FIGURE 53.
Population NHEs of AWR and OIR by time to research reporting.
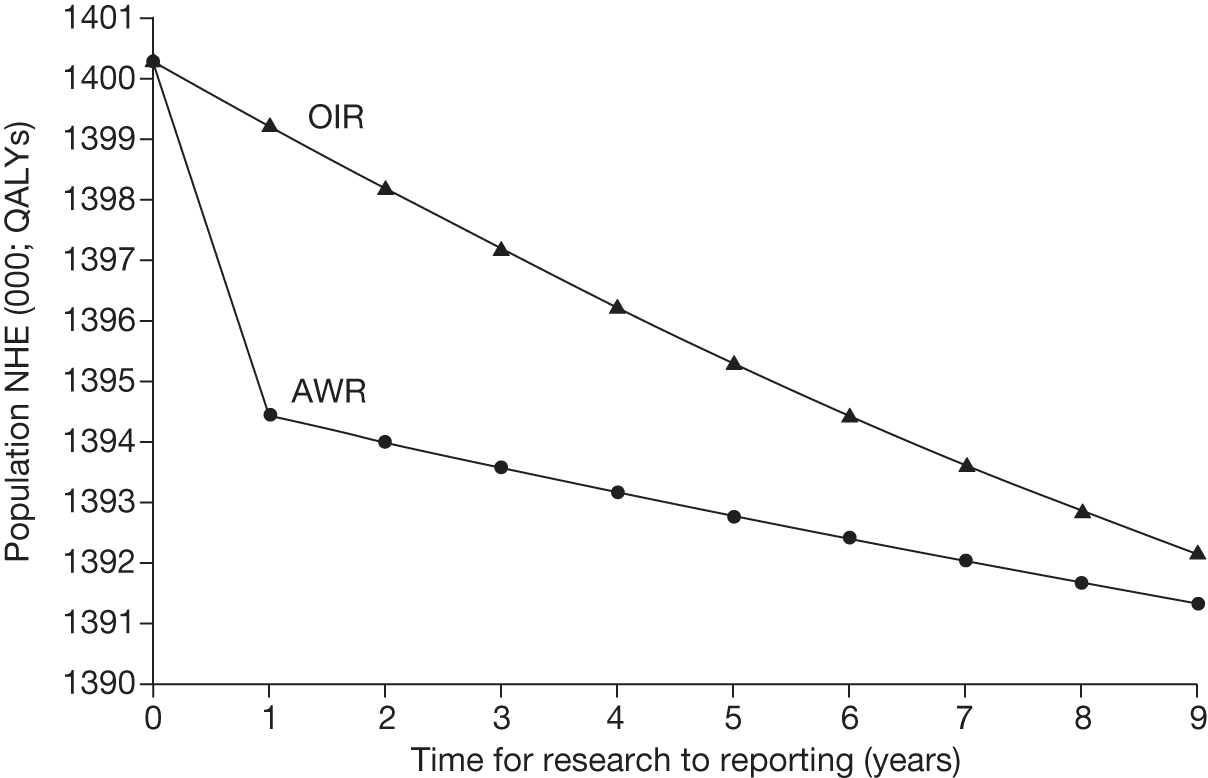
However, there is no guarantee that the research recommended as part of OIR or AWR guidance will be conducted by manufacturers or commissioned by research funders. Even if it is, it is not certain that it will be successfully completed. Therefore, the probability that research will report at a particular time also needs to be considered. The implications of considering whether the recommended research will be conducted and when it might report are illustrated in Figure 54, which presents a boundary for when OIR rather than AWR might be appropriate. The boundary is illustrated for four research designs with differing lengths of follow-up. Designs with a shorter follow-up might be preferred if the additional benefits of a longer follow-up are less than the additional opportunity costs of waiting longer until the research reports. In this case it seems likely that the type of research required could report quickly enough for all research designs and with sufficient confidence that OIR would be appropriate, even though the research could be conducted while EECP is approved. Therefore, these assessments would support a judgement that the benefits of approval (through AWR) are unlikely to exceed the opportunity costs (the NHEs of OIR) and so OIR4 (pathway 18, see Table 81) rather than AWR4 (pathway 17, see Table 81) would be more appropriate.
FIGURE 54.
An OIR or AWR boundary.
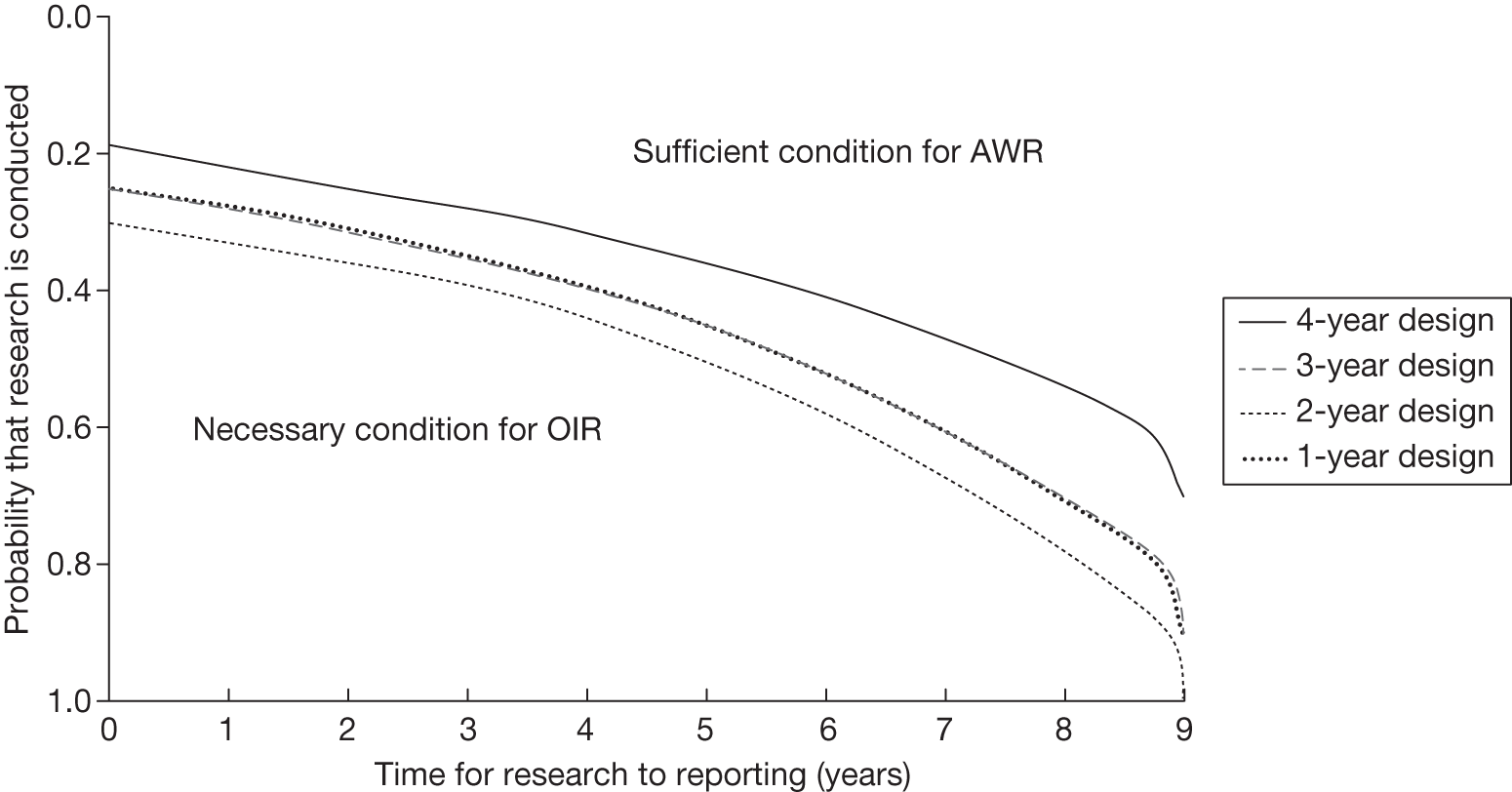
Table 82 summarises the population NHEs over the technology time horizon for different policies for a 4-year design. This can be used to help inform the policy questions: (1) what would be the value of being able to conduct research while EECP is approved? and (2) what would be the value of making the evidence that is needed by the NHS available at launch? In this case, because of the irrecoverable costs associated with EECP, there is no value to the NHS of being able to conduct research while EECP is approved for use. In fact, these figures are negative, indicating that even if AWR was possible it would not be appropriate. The difference in NHEs between OIR and AWR (the next best policy, if it were possible) represents the value to the NHS of withholding approval until the research is conducted (i.e. £72M if 3 years). The difference in population NHEs between all uncertainty resolved prior to appraisal (at launch) and the next best available policy represents the value to the NHS of having access to the evidence needed at launch. The value, expressed in the equivalent NHS resources, depends on how long it would otherwise have taken for an OIR recommendation to deliver the same evidence, for example £62M if 3 years and £134M if 7 years.
| NHE for time to research reporting | Approve | OIR | AWR | Reject | Value of AWR | Uncertainty resolved at launch | Value of evidence at launch |
|---|---|---|---|---|---|---|---|
| NHEs in QALYs | |||||||
| T = 3 | 1,391,001 | 1,397,192 | 1,393,578 | 1,389,596 | –3614 | 1,400,288 | 3096 |
| T = 7 | 1,391,001 | 1,393,608 | 1,392,030 | 1,389,596 | –1578 | 1,400,288 | 6680 |
| NHEs in £M | |||||||
| T = 3 | 27,820 | 27,944 | 27,872 | 27,792 | –72 | 28,006 | 62 |
| T = 7 | 27,820 | 27,872 | 27,841 | 27,792 | –32 | 28,006 | 134 |
Technologies with significant irrecoverable costs and research is not possible with approval
For the reasons discussed earlier, the type of experimental research required to robustly estimate the effect of EECP on quality of life is unlikely to be possible once EECP is approved and in widespread use; therefore, research may not be possible with approval. In this case, approve (rather than AWR) is an alternative option. However, approve not only commits the type of irrecoverable costs discussed above but also means that the potential value of evidence to future patients must also be forgone. This is reflected in Figure 55 in which the difference between OIR and approve is always greater than that between OIR and AWR in Figure 53. It suggests that as long as the cost of the research exceeds the difference between OIR and approve, when it is expected to report, OIR rather than approve would be appropriate. This is also reflected in the boundaries for OIR and approve reported in Figure 56. The boundaries for the different research designs are always to the north-east of the OIR/AWR boundaries reported in Figure 54, again reflecting the fact that approval not only commits irrecoverable costs but also forgoes the potential value of evidence that might have been generated through an OIR recommendation. These assessments would support a judgement that the benefits of approval are unlikely to exceed the opportunity costs (the NHEs of OIR) and so ‘OIR6’ (pathway 25, see Table 81) rather than ‘Approve9’ (pathway 24, see Table 81) would be more appropriate.
FIGURE 55.
Population NHEs of approve and OIR by time to research reporting.
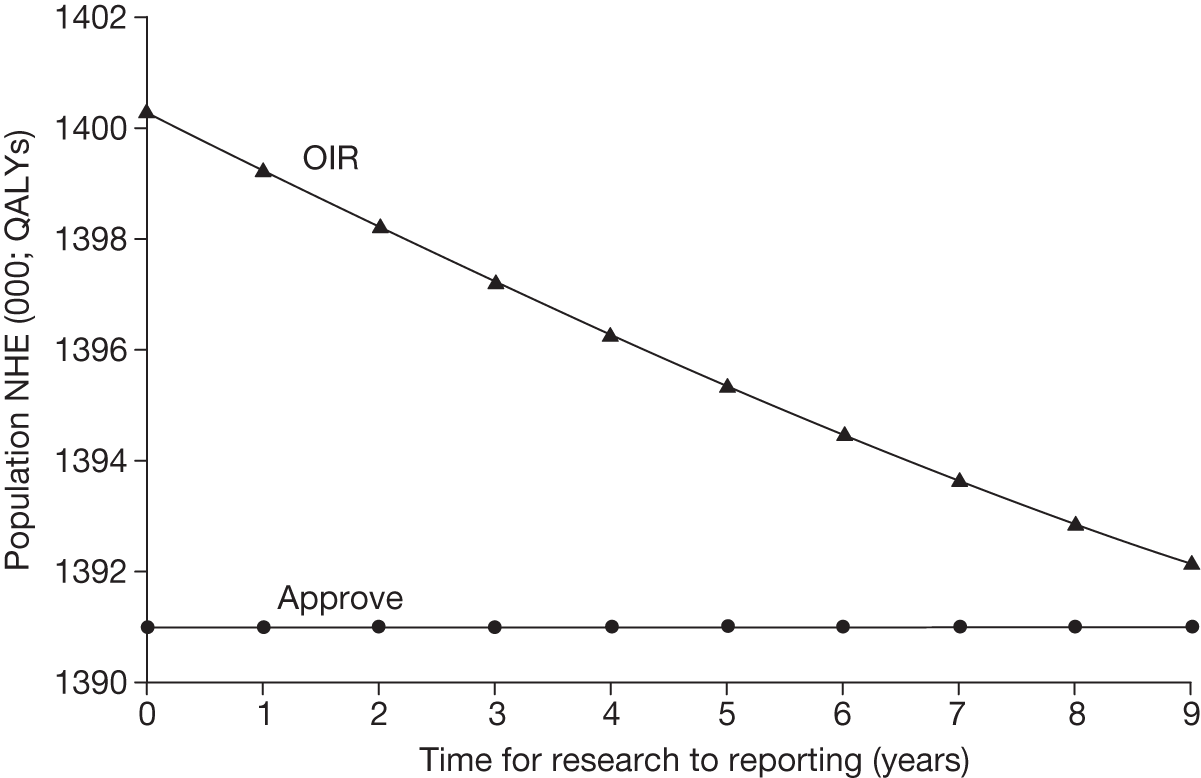
FIGURE 56.
An OIR or approve boundary.
Types and categories of guidance resulting from point 7
The decision at this point always leads directly to guidance, allocating all remaining possible pathways to a particular type and category of guidance. The resulting guidance for EECP is summarised in Table 83. Guidance will depend on whether or not EECP can be approved for widespread use while research is conducted (AWR); however, as demonstrated above, even when research is possible with approval, OIR appears to offer greater expected NHEs than AWR or approve as long as research reports before 9 years. This is largely because the consequences of committing significant irrecoverable costs through AWR or approve are greater than the NHEs forgone by restricting access to EECP through OIR.
| Assessment | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Guidance |
|---|---|---|---|---|---|---|---|---|
| 17 | Yes | Yes | Yes | Yes | No | Yes | Yes | AWR4 |
| 18 | Yes | Yes | Yes | Yes | No | Yes | No | OIR4 |
| 24 | Yes | Yes | Yes | No | No | Yes | Yes | Approve9 |
| 25 | Yes | Yes | Yes | No | No | Yes | No | OIR6 |
Appendix 8 Clopidogrel for the management of patients with non-ST segment elevation acute coronary syndromes
Introduction
The use of CLOP (for up to 12 months) in combination with low-dose aspirin was recommended by NICE following a MTA appraisal for patients with NSTE-ACS presenting with a moderate to high risk of ischaemic events (TA80122 in 2004). In TA80 the Appraisal Committee considered 12 months’ or lifetime treatment with CLOP, but recommended research to inform optimal treatment duration. The original report had included an analysis of shorter treatment durations (< 12 months) and the NIHR HTA programme subsequently commissioned additional reanalysis based on the original work to inform the research recommendation in 2009. This case study is based on the reanalysis of TA80 undertaken in 2009, which included standard therapy compared with four alternative treatment durations of CLOP of 1, 3, 6 and 12 months. This analysis informed clinical guideline CG94123 in 2010. Importantly, although this case study is based on the later reanalysis of TA80, the analysis considered here has been undertaken from the standpoint of the original TA80 appraisal and asks what assessments might have been made at that time when standard therapy was low-dose aspirin.
Background to the case study
An acute coronary syndrome is a set of symptoms of myocardial ischaemia occurring because of the presence of occlusion thrombi in the arteries because of the fissuring or rupturing of atheromatous plaques. NSTE-ACS can be classed as either unstable angina or non-ST elevation myocardial infarction (NSTEMI). NSTE-ACS are associated with a high risk of death or ischaemic complications, and antiplatelet agents such as CLOP are effective at reducing the risk of further ischaemic events.
In recommending CLOP in TA80,122 NICE used a published report of analyses undertaken by the Centre for Reviews and Dissemination (CRD) and the Centre for Health Economics (CHE). 175 This evaluation assessed the clinical effectiveness and cost-effectiveness of CLOP in combination with aspirin for people with NSTE-ACS, the results of which were later updated by Rogowski et al. 148 in 2009. The optimal duration of CLOP treatment was assessed at this later stage and an analysis of the value of further research was undertaken. The update of the MTA appraisal was used to inform NICE’s clinical guidelines (CG94123) in 2010.
The model and methods used in the evaluation by Rogowski et al. 148 were used here to illustrate the information and associated analyses that would have been required to inform a decision process linking adoption with further evidence collection. Despite using the model and methods of Rogowski et al. ,148 the analysis has been undertaken from the standpoint of the original TA80122 appraisal. Given the objectives of exploring what additional information and analysis might be required, de novo or substantial reanalysis of original assessments was not undertaken in the current work.
Interventions and population
The population of interest includes patients with NSTE-ACS presenting with a moderate to high risk of ischaemic events. Treatment should start immediately after the acute event; thus, prevalent cases are not assumed eligible for treatment, only incident cases. The incidence of NSTE-ACS in the UK was estimated to be 60,000 patients per year,148 reflecting the size of the population eligible to receive treatment annually. This figure is assumed to be non-stochastic in further analyses.
The treatment strategies considered in this case study were:
-
CLOP12: treatment with CLOP as an adjunct to standard therapy for 12 months
-
CLOP6: treatment with CLOP as an adjunct to standard therapy for 6 months
-
CLOP3: treatment with CLOP as an adjunct to standard therapy for 3 months
-
CLOP1: treatment with CLOP as an adjunct to standard therapy for 1 month
-
NHS: lifetime treatment with standard therapy alone (including aspirin).
Lifetime treatment with standard therapy is considered here as the treatment established in the NHS, as we aim to recreate the decision problem set out in 2004 (TA80122). A possible decision to use CLOP will thus represent a change in guidance. Given that the evaluation undertaken was used to inform NICE’s decision-making, this study used the NHS perspective and a discount rate of 3.5% for future costs and QALYs. 1
Evidence on clinical effectiveness
The Clopidogrel in Unstable Angina to Prevent Recurrent Events (CURE) trial established the effectiveness of CLOP in patients with NSTE-ACS. 176–181 This was a multicentre, double-blind RCT that recruited 12,562 patients who presented within 24 hours of experiencing an NSTE-ACS event. The trial compared CLOP (300 mg initially followed by 75 mg daily) in combination with aspirin (75–325 mg/day) with placebo plus aspirin (75–325 mg/day). Results show that CLOP was significantly more effective than placebo at reducing the risk of the composite outcome of cardiovascular death, NFMI or stroke. The proportion of patients experiencing the composite outcome was greater in the aspirin-only group at 30 days [relative risk (RR) 0.79; 95% confidence interval (CI) 0.67 to 0.92] and from 30 days to 12 months (RR 0.82; 95% CI 0.70 to 0.95). The results also suggested that the benefits of CLOP may be most apparent within the first 3 months of treatment. This was investigated further using an exploratory post hoc analysis of the CURE data (comprising non-randomised comparisons), presented in the SIGN guidelines (no. 93). 182 This analysis showed that, within the CURE trial, the event rate was statistically significantly lower in the CLOP group for the periods 0–1 month, 1–3 months and 0–12 months, but not for the periods 3–6 months, 6–9 months and 9–12 months.
Decision model
The model used by Rogowski et al. 148 combines a short-term decision tree and a Markov model representing the longer term. The short-term tree characterises the period up to 12 months following an acute coronary syndrome, tracking for possible myocardial infarctions (MIs) or death. The Markov model was defined to have four states (well, death, MI and post MI). The model was run for a period of 40 years, the ‘patient time horizon’, using yearly cycles. The patient time horizon is the time horizon over which costs and benefits are likely to differ for an individual patient and was here assumed to approximate the lifetime of the patients. A summary of the parameters used to define the decision model is shown in Table 84.
| Parameter | Description | Source | Distribution | ||
|---|---|---|---|---|---|
| Natural history | 1 | P_die_0.1 | Short term: probability of death, 0–1 months | PRAIS-UK data11 | Dirichlet |
| 2 | P_NFMI_0.1 | Short term: probability of a NFMI, 0–1 months | |||
| 3 | P_die_1.3 | Short term: probability of death, 1–3 months | Dirichlet | ||
| 4 | P_NFMI_1.3 | Short term: probability of a NFMI, 1–3 months | |||
| 5 | P_die_3.6 | Short term: probability of death, 3–6 months | Dirichlet | ||
| 6 | P_NFMI_3.6 | Short term: probability of a NFMI, 3–6 months | |||
| 7 | P_die_6.12 | Short term: probability of death, 6–12 months | Dirichlet | ||
| 8 | P_NFMI_6.12 | Short term: probability of a NFMI, 6–12 months | |||
| 9 | TP_AC | Long term: annual TP – well(A) to MI(C) | Main et al. 2004175 | Normal (log-hazard) | |
| 10 | TP_AD | Long term: annual TP – MI(C) to dead(D) | Normal (log-hazard) | ||
| 11 | TP_CD | Long term: annual TP – MI(C) to dead(D) | Normal (log-hazard) | ||
| 12 | TP_BD | Long term: annual TP – post MI(B) to dead(D) | Normal (log-hazard) | ||
| Relative effects | 17 | RR_death | Treatment effects (RR) – all-cause mortality | CURE trial176–181 | Normal (log-RR) |
| 18 | RR_NFMI | Treatment effects (RR) – NFMI | Normal (log-RR) | ||
| Utilities | 13 | U_Well | Utility weights per health state: IHD year 1 | Karnon et al. 2006183 | Beta |
| 14 | U_Well1 | Utility weights per health state: post IHD | Beta | ||
| 15 | U_NFMI | Utility weights per health state: MI year 1 | Beta | ||
| 16 | U_POSTMI | Utility weights per health state: post MI | Beta | ||
| Costs | 19 | C_Well | Cost per health state: IHD year 1 | a | a |
| 20 | C_MI_LT | Cost per health state: MI year 1 | a | a | |
| 21 | C_PostMI | Cost per health state: post MI | a | a | |
| 22 | TC_Well_Dead | Cost per health state: transitions to death | a | a | |
| 23 to 27 | C_clop12, . . ., C_NHS | Cost per treatment strategy | a | a | |
| 28 | Clop_cost | Cost per mg of CLOP (2100 mg, 2007) | BNF 2007184 | Constant | |
The probabilities of death and NFMI applied in the first year were derived from the Prospective Registry of Acute Ischaemic Syndrome in the UK (PRAIS-UK), an observational UK cohort registry of 1046 patients with ACS. 185 Treatment with CLOP was assumed to prevent death and NFMI and this was parameterised using relative treatment effects. These were applied throughout the duration of the CLOP treatment period (in the short-term model), within which these were constant; hence, any benefits of treatment with CLOP were assumed to stop at the time of withdrawal, and patients were modelled to rebound to the same prognosis as an equivalent patient on aspirin alone (NHS standard care).
The long-term model was used to quantify the remaining QALYs and costs of patients once they exited the short-term model. The rate at which transitions happen within the long-term Markov model were parameterised using transition probabilities. These transitions were estimated using the Nottingham Heart Attack Register (NHAR) data (n = 1279) (for further details see Rogowski et al. 148) and were defined as independent of treatment; hence, CLOP was assumed to not impact directly on the long-term outcomes of patients.
Utility parameters were derived using published evidence from Karnon et al. 183 Costs and resource use categories considered were treatment related, adverse event related, those associated with health states and those associated with possible revascularisation (tracked in the short-term model). The specificities of how these were considered are detailed in the main report. 99 In the current assessment, to allow tractability in the further analysis and clarity in the presentation of results, the number of model parameters was reduced by using the resource use and cost parameters in the original model to derive costs per health state and costs per treatment.
Inputs in the model were assumed to be uncertain and the model was run probabilistically using Monte Carlo simulation (5000 simulations). Expected cost-effectiveness was determined using these probabilistic results, as established within the NICE appraisal process. a The use of probabilistic analysis also allows uncertainty over model parameters to be translated into uncertainty in the overall results.
Key features and possible pathways
The CLOP case study focuses on evaluating alternative durations of treatment with CLOP alongside lifetime treatment with standard therapy as well as standard therapy alone (current NHS care) for the management of patients with NSTE-ACS. This is an acute condition and so only incident populations after research reports can benefit from the information.
A research recommendation was made in section 5 of the FAD in TA80122 regarding uncertainty over the duration of treatment; therefore, CLOP is not an example of AWR at FAD but an example of AWR considered during appraisal. In an update of the assessments undertaken,99 alternative assumptions about the way that treatment effects were modelled were also evaluated.
Using the CLOP case study we aim to demonstrate how the key principles and assessments could inform the development of guidance regarding the use of CLOP through application of the checklist developed in this project. We also aim to illustrate how existing methods of appraisal can be applied to this case study and also what additional information and analysis (and when) can be useful for the Appraisal Committee to undertake the proposed judgements.
The possible pathways through the algorithm that the CLOP case study illustrates are reported in Figure 29 in Appendix 4, with the new technology expected to be cost-effective and with no significant irrecoverable costs. The CLOP case study also illustrates a number of other important characteristics, including (1) the impact that other sources of uncertainty (price change following patent expiry) can have on the value of further research, (2) the interpretation of analyses when there are multiple alternatives and (3) the use of scenarios to represent alternative but credible assumptions. The following sections examine each of the seven points on the checklist relating to the possible sequence of assessments and decisions, which lead to a particular category and type of guidance for CLOP.
Is it cost-effective and what are the risks?
Point 1: Is it expected to be cost-effective?
The sequence of assessments starts with cost-effectiveness and the expected impact on population NHEs, that is, at the following point in the algorithm:
The assessment of expected cost-effectiveness is made based on the balance of the evidence and analysis currently available. Commonly, expected cost-effectiveness is summarised and presented using ICERs. Equivalently, but more usefully in this context, cost-effectiveness can be expressed in terms of expected NHEs, which can be expressed per patient treated or for a population of patients. All of the information required to express expected cost-effectiveness in these ways is already available during appraisal.
Cost-effectiveness at the patient level
Estimates of the expected NHS costs and QALYs for each patient treated over an appropriate time horizon – the ‘patient time horizon’ – can be summarised as the per patient NHEs of each intervention, that is, the difference between any health gained and health forgone elsewhere.
The results for CLOP are summarised in Table 85. b There are more than two alternatives: four treatment durations as well as current NHS treatment (aspirin alone) were considered. The results indicate that 12-month treatment with CLOP is expected to be cost-effective at a threshold of £20,000 per QALY,c although the difference in NHEs between 12 months and 6 months of treatment is small. Consequently, the NHEs of 12 months’ treatment with CLOP are greater than the NHEs of 6 months’ treatment with CLOP but the difference per patient treated (the incremental NHE) is small.
| Treatment | Cost (£) | QALYs | ICER (£/QALY) | Cost-effectiveness threshold £20,000 per QALY | Cost-effectiveness threshold £30,000 per QALY |
|---|---|---|---|---|---|
| NHE, QALYs (£) | NHE, QALYs (£) | ||||
| CLOP12 | 20,127 | 8.122 | 18,663 | 7.115 (142,307) | 7.451 (223,525) |
| CLOP6 | 19,860 | 8.107 | 10,477 | 7.114 (142,288) | 7.445 (223,362) |
| CLOP3 | 19,712 | 8.093 | 9396 | 7.108 (142,154) | 7.436 (223,087) |
| CLOP1 | 19,598 | 8.081 | 4961 | 7.101 (142,025) | 7.428 (222,837) |
| NHS | 19,502 | 8.062 | – | 7.087 (141,734) | 7.412 (222,353) |
It is also important to consider how NHEs accumulate over time or the investment profile per patient treated with CLOP. Figure 57 illustrates the cumulative incremental NHEs over the patient time horizon. The per patient costs of CLOP are in excess of the health benefits during the period of treatment. These negative NHEs are eventually offset by positive NHEs in later periods. In this case, it is only after 5 years that 12 months of treatment with CLOP breaks even against current NHS care and it is not until 21 years that it is better than a shorter treatment duration of 6 months. Notice that shorter treatment durations with CLOP offer a much less ‘risky profile’, for example the break-even point for 1 month of treatment is 2 years against current NHS care.
FIGURE 57.
Cumulative incremental NHEs of CLOP over the patient time horizon.
Cost-effectiveness at the population level
Per patient NHEs can also be expressed for the population of current and future patients. This requires information about prevalence and future incidence of the target population (already required in appraisal). It also requires a judgement about the time horizon over which the technology will be used. This ‘technology time horizon’ ought to reflect the period over which the technology is likely to be part of clinical practice and generate the expected NHEs. d An estimate of the scale of the total population NHEs and how they cumulate over time is important for subsequent assessments, including (1) when the NHEs for current patient populations must be compared with the benefits to future patients and (2) when the treatment decision can be changed so the irrecoverable costs of initially negative NHEs become significant.
Given the acute nature of the condition being assessed in the CLOP case study, it is assumed that the prevalent population is not significant and that only incident populations are eligible for treatment with CLOP. The total population NHEs, assuming that the technology will be used to treat incident patients over 10 years, are reported in Table 86. Expected cost-effectiveness is unchanged (ICERs are the same as in Table 85) but the incremental NHEs, although small per patient, are more significant at a population level.
| Treatment | Cost (£) | QALYs | ICER (£/QALY) | Cost-effectiveness threshold £20,000 per QALY | Cost-effectiveness threshold £30,000 per QALY |
|---|---|---|---|---|---|
| NHE, QALYs (£M) | NHE, QALYs (£M) | ||||
| CLOP12 | 10,394,830,647 | 4,194,554 | 18,663 | 3,674,813 (73,496) | 3,848,060 (115,442) |
| CLOP6 | 10,256,672,674 | 4,187,151 | 10,477 | 3,674,318 (73,486) | 3,845,262 (115,358) |
| CLOP3 | 10,180,425,730 | 4,179,874 | 9396 | 3,670,853 (73,417) | 3,840,526 (115,216) |
| CLOP1 | 10,121,529,942 | 4,173,605 | 4961 | 3,667,529 (73,351) | 3,836,221 (115,087) |
| NHS | 10,072,035,344 | 4,163,629 | – | 3,660,027 (73,201) | 3,827,894 (114,837) |
The investment profile for CLOP when used to treat patients over 10 years is illustrated in Figure 58. At a population level it is not until 11 years (rather than 5 years at a patient level) that initial losses are compensated for by later gains and CLOP (12 months of treatment) breaks even against current NHS care. It is not until 27 years (rather than 21 years at a patient level) that 12 months of treatment is better than a shorter treatment duration of 6 months. In other words, CLOP appears a more risky investment when evaluated at a population rather than an individual level. This is because, although each patient treated with CLOP is expected to offer the same profile of NHEs shown in Figure 57, the negative NHEs associated with patients who are incident and treated in year 10 will not be offset by later gains.
FIGURE 58.
Cumulative incremental NHEs of CLOP for the population. Incremental NHEs are presented for the different treatment strategies in relation to standard NHS care. A 10-year technology time horizon is assumed, indicated by the grey shading.
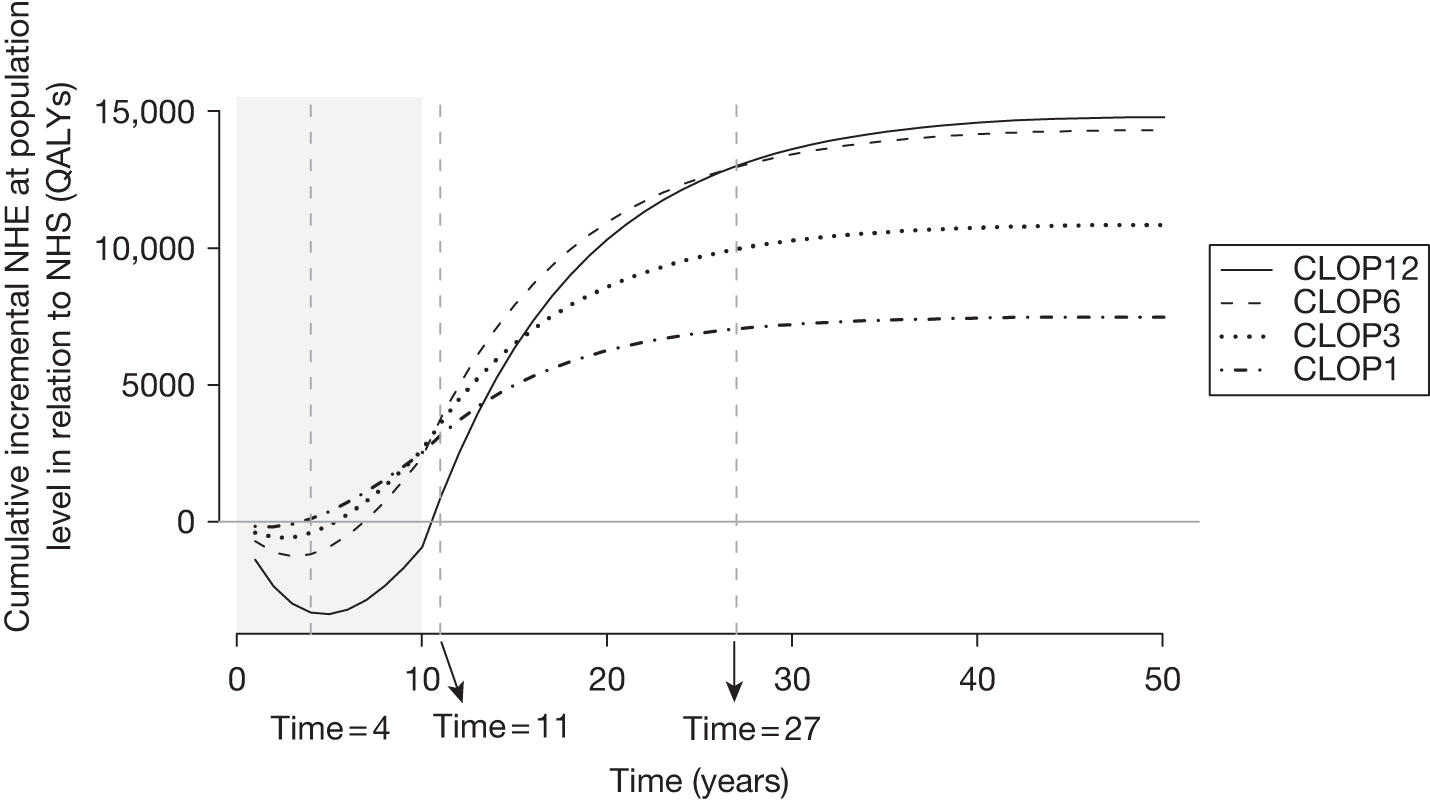
Table 87 shows that the population-level investment profile would exhibit greater risk (break-even later) if the technology time horizon was longer. For example, the break-even point for 12 months of treatment with CLOP against 6 months of treatment extends to 33 years when the technology time horizon is increased to 20 years. Shorter durations of treatment still offer a ‘risky profile’, for example the break-even point for 1 month of treatment compared with current NHS care is 4 years.
| Technology time horizon | Treatment | Incremental NHE, QALYs (£M) | Break-even point (years) | ||
|---|---|---|---|---|---|
| 12 months vs 6 months | 12 months vs NHS | 1 month vs NHS | |||
| 5 years | CLOP12 | 269 (5.4) | 24 | 8 | 4 |
| CLOP6 | 1881 (37.6) | ||||
| CLOP3 | 1804 (36.1) | ||||
| CLOP1 | 4073 (81.5) | ||||
| NHS | – | ||||
| 10 years | CLOP12 | 495 (9.9) | 27 | 11 | 4 |
| CLOP6 | 3465 (69.3) | ||||
| CLOP3 | 3324 (66.5) | ||||
| CLOP1 | 7502 (150) | ||||
| NHS | – | ||||
| 15 years | CLOP12 | 686 (13.7) | 30 | 12 | 4 |
| CLOP6 | 4799 (96) | ||||
| CLOP3 | 4603 (92.1) | ||||
| CLOP1 | 10,389 (207.8) | ||||
| NHS | – | ||||
| 20 years | CLOP12 | 846 (16.9) | 33 | 12 | 4 |
| CLOP6 | 5921 (118.4) | ||||
| CLOP3 | 5680 (113.6) | ||||
| CLOP1 | 12,820 (256.4) | ||||
| NHS | – | ||||
Point 2: Are there significant irrecoverable costs?
The second point on the checklist requires (1) an assessment of whether there are irrecoverable costs and (2) a judgement of their potential significance, that is, at the following point in the algorithm:
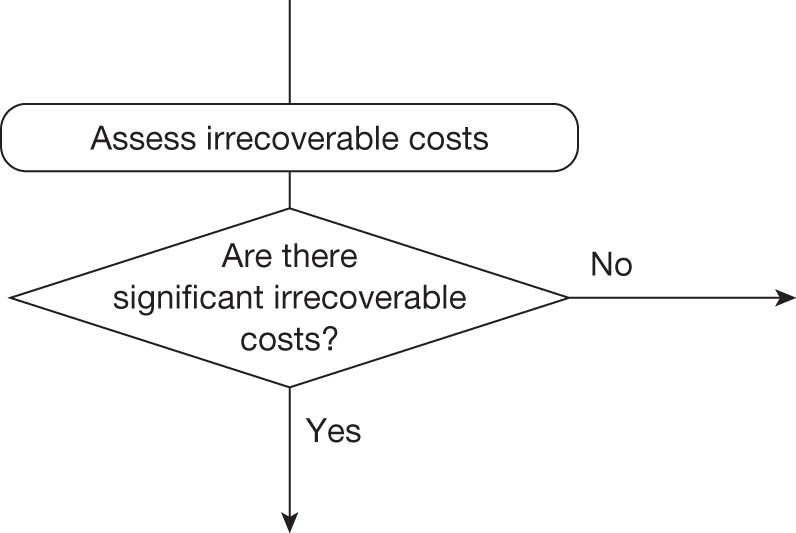
Irrecoverable costs are those that, once committed, cannot be recovered if guidance is changed at a later date. Irrecoverable costs are most commonly thought of as ‘upfront’ or capital costs of new facilities or equipment with long life expectancy (they might also include any practitioner training and the costs of implementation efforts). In the CLOP case study, no capital costs were included in the analysis.
However, even in the absence of capital costs of equipment and facilities, NHEs accumulate over time both at a patient and a population level. The analysis in Point 1: Is it expected to be cost-effective? (see Figure 58) indicates a common pattern of initially negative NHEs that are only gradually offset by positive NHEs in later periods. The investment profile of CLOP (at a patient and more so at a population level) thus exhibits irrecoverable costs. Therefore, in the case of an approval or AWR recommendation, opportunity costs of negative NHEs are committed that may be irrecoverable.
Are they likely to be significant?
Whether or not irrecoverable costs are significant (i.e. might influence guidance) depends critically on whether or not guidance is likely to change and whether that is more likely in the near or distant future. That will depend on whether or not research is likely to be undertaken and when it is likely to report, as well as on other events that might occur, for example a change in price following patent expiry. These are assessed later, at points 5 and 6 on the checklist. However, the potential significance of any irrecoverable costs can be assessed at this point.
Judging the potential significance of the investment profiles of NHEs is more nuanced. It depends whether treatment decisions for individual patients are irreversible, which in part depends on the nature of the disease. CLOP is a treatment for ACS and, although decisions about treatment and its duration are not irreversible in the short run, over the time scales more likely for research being conducted (and reporting) or other events occurring that could change guidance they can be regarded as such. Of course, it is possible that the later benefits are not realised but it is also possible that they will realise more (the profiles of NHEs in Figure 57 are the average over these possibilities). Similarly, the possibility that guidance might change in the future (e.g. research suggests that the longer-term benefits will not offset initial losses) will not influence the irreversible decision to treat a presenting patient with a technology that is expected to be cost-effective prior to the research reporting. Therefore, although the investment profile of CLOP exhibits irrecoverable costs these should not be judged significant in the sense that they have little potential to influence guidance.
Types and categories of guidance resulting from points 1 and 2
Points 1 and 2 of the checklist do not lead directly to a category or type of guidance. The sequence of assessments and decisions, which ultimately lead to guidance, starts with cost-effectiveness, expected impact on population NHEs and significance of irrecoverable costs. In the case of CLOP, the technology is expected to be cost-effective and it does not have significant irrecoverable costs. Although treatment with CLOP does commit initially negative NHEs that are irrecoverable, these should not be regarded as significant as the treatment decision for a presenting patient is irreversible in relevant time frames.
Table 88 summarises the categories and types of guidance that could ultimately result from the sequence of assessments up to point 2 (see Table 32 in Appendix 4).
| Assessment | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Guidance |
|---|---|---|---|---|---|---|---|---|
| 1 | Yes | No | Yes | Yes | Yes/no | Yes | – | AWR1 |
| 2 | Yes | No | Yes | Yes | Yes/no | No | – | Approve1 |
| 3 | Yes | No | Yes | No | Yes/no | Yes | Yes | Approve2 |
| 4 | Yes | No | Yes | No | Yes/no | Yes | No | OIR1 |
| 5 | Yes | No | Yes | No | Yes/no | No | – | Approve3 |
| 6 | Yes | No | No | – | – | – | – | Approve4 |
Is further research required?
Point 3: Does more research seem worthwhile?
The third point on the checklist requires an assessment of the potential benefits of conducting further research, that is, at the following point in the algorithm:
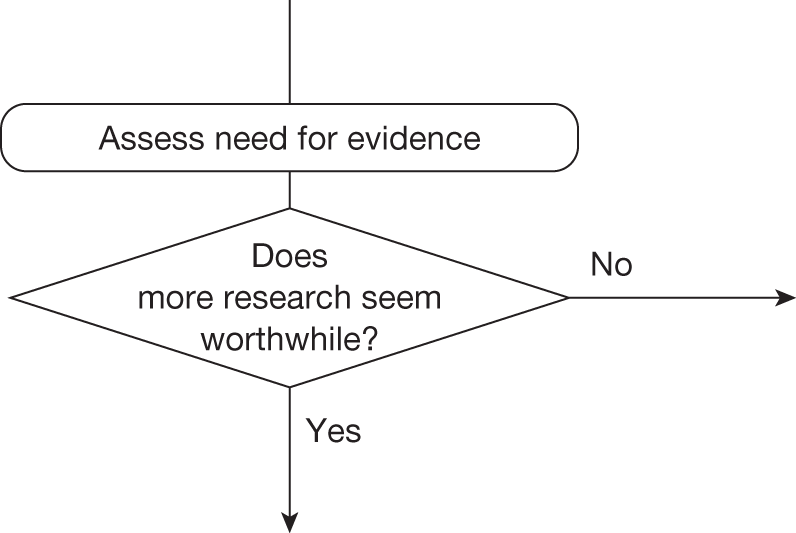
This requires judgements about (1) how uncertain a decision to approve or reject a technology might be based on the estimates of expected cost-effectiveness and (2) whether the scale of the likely consequences of this uncertainty might justify further research. Some assessment of the potential consequences of uncertainty is important because it indicates the scale of the population NHEs, over the technology time horizon, that could be gained if the uncertainty surrounding this decision could be resolved immediately, that is, it represents an expected upper bound on the benefits of more research. e
Assessing the consequences of uncertainty
In the CLOP case study 12 months of treatment is expected to be cost-effective (see Tables 85 and 86), but the estimates of costs and QALYs are uncertain so there is a chance that a decision to approve CLOP based on existing evidence will be incorrect, that is, other treatment options might offer greater NHEs. A judgement is required about the chance that 12 months of treatment is incorrect and if so which of the other four alternatives is likely to offer higher NHEs and how much higher. In other words, for decisions involving multiple alternatives, a judgement is required on the level of uncertainty surrounding the decision, how this uncertainty is distributed across the various alternatives and what the consequences are likely to be.
Some assessment of the likely consequences of approving CLOP under uncertainty could be based on the difference in expected NHEs, that is, the expected incremental population NHEs reported in Table 87. The simplest approach could be to take the difference in expected NHEs for CLOP and standard NHS care and weight it by a judgement of the probability that the decision is correct, that is, allocate the consequences of an incorrect decision to NHS standard care. f For example, if the decision was judged to be 100% certain (the probability that the decision is correct would be 1) then there are no consequences and so there would be nothing to be gained by more research; however, as the decision becomes more uncertain, the expected consequences (and hence potential value of more research) increases.
In the presence of multiple treatment alternatives, there are other options than to allocate all of the consequences of an erroneous decision to NHS standard care. We have examined four alternative ways:
-
The consequences are assigned to NHS standard care.
-
The consequences are assigned to CLOP6, the next best treatment.
-
Equal shares of the consequences are assigned to treatments other than CLOP12.
-
The consequences are assigned to treatments other than CLOP12 based on the probability of the alternative treatments being cost-effective from PSA. Note that this uses the PSA only to inform the magnitude of the consequences of a wrong decision; a judgement on the likelihood of adopting CLOP being the incorrect decision is still required.
The results of these alternative analyses are illustrated in Table 89 in which a judgement about the probability that a decision based on expected cost-effectiveness is correct translates into expected consequences based on expected incremental population NHEs.
| Probability decision is correct, p | Cost-effectiveness threshold £20,000 per QALY | Cost-effectiveness threshold £30,000 per QALY | ||||||
|---|---|---|---|---|---|---|---|---|
| Expected consequences, QALYs (£M) | Expected consequences, QALY (£M) | |||||||
| (1) | (2) | (3) | (4) | (1) | (2) | (3) | (4) | |
| 1 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 0.99 | 148 (3) | 5 (0.1) | 66 (1.3) | 37 (0.7) | 202 (4.0) | 28 (0.6) | 106 (2.1) | 56 (1.1) |
| 0.95 | 739 (14.8) | 25 (0.5) | 332 (6.6) | 185 (3.7) | 1008 20.2) | 140 (2.8) | 529 (10.6) | 281 (5.6) |
| 0.90 | 1479 (29.6) | 50 (1.0) | 663 (13.3) | 370 (7.4) | 2017 40.3) | 280 (5.6) | 1058 (21.2) | 561 (11.2) |
| 0.75 | 3696 (73.9) | 124 (2.5) | 1658 (33.2) | 925 (18.5) | 5041 (100.8) | 699 (14) | 2646 (52.9) | 1403 (28.1) |
| 0.50 | 7393 (147.9) | 248 (5.0) | 3316 (66.3) | 1850 (37) | 10,083 (201.7) | 1399 (28) | 5292 (105.8) | 2806 (56.1) |
| 0.25 | 11,089 (221.8) | 371 (7.4) | 4973 (99.5) | 2776 (55.5) | 15,124 (302.5) | 2098 (42) | 7938 (158.8) | 4209 (84.2) |
| 0.10 | 13,307 (266.1) | 446 (8.9) | 5968 (119.4) | 3331 (66.6) | 18,149 (363) | 2518 (50.4) | 9525 (190.5) | 5050 (101) |
| 0.05 | 14,046 (280.9) | 470 (9.4) | 6299 (126) | 3516 (70.3) | 19,157 (383.1) | 2658 (53.2) | 10,055 (201.1) | 5331 (106.6) |
| 0.01 | 14,638 (292.8) | 490 (9.8) | 6565 (131.3) | 3664 (73.3) | 19,964 (399.3) | 2770 (55.4) | 10,478 (209.6) | 5555 (111.1) |
| 0 | 14,786 (295.7) | 495 (9.9) | 6631 (132.6) | 3701 (74) | 20,165 (403.3) | 2798 (56) | 10,584 (211.7) | 5611 (112.2) |
This judgement, of how uncertain a decision might be, can be informed by the PSA already used to estimate costs and QALYs and required as part of the NICE reference case. The probabilities that each of the five alternatives is cost-effective are reported in Table 90. The probability that 12 months of treatment is cost-effective is 0.524, which would translate into 1723 QALYs over the technology time horizon (assumed to be 10 years) based on the expected or average difference between NHEs (Figure 59). The time horizon over which evidence generated by research about a technology might be valuable may be longer (or shorter) than the period over which the technology is used; therefore, the technology time horizon and the time horizon for the benefits of research may differ. For simplicity, in this case study we have assumed these to be equal.
| Treatment | ICER (£/QALY) | Cost-effectiveness threshold £20,000 per QALY | Cost-effectiveness threshold £30,000 per QALY | ||||
|---|---|---|---|---|---|---|---|
| Incremental NHE,a QALYs (£M) | Probability cost-effective | Expected consequences, QALYs (£M) | Incremental NHE,a QALYs (£M) | Probability cost-effective | Expected consequences, QALYs (£M) | ||
| CLOP12 | 18,663 | 495 (9.9) | 0.524 | 5194 (103.9) | 2798 (56.0) | 0.677 | 3657 (109.7) |
| CLOP6 | 10,477 | 3465 (69.3) | 0.180 | 4736 (94.7) | 0.092 | ||
| CLOP3 | 9396 | 3324 (66.5) | 0.018 | 4305 (86.1) | 0.009 | ||
| CLOP1 | 4961 | 7502 (150.0) | 0.075 | 8327 (166.5) | 0.052 | ||
| NHS | – | – | 0.202 | – | 0.170 | ||
FIGURE 59.
Probability that CLOP is cost-effective and the consequences of uncertainty. The expected consequences of uncertainty (from means) are from the results of alternative analysis (4) in Table 89.
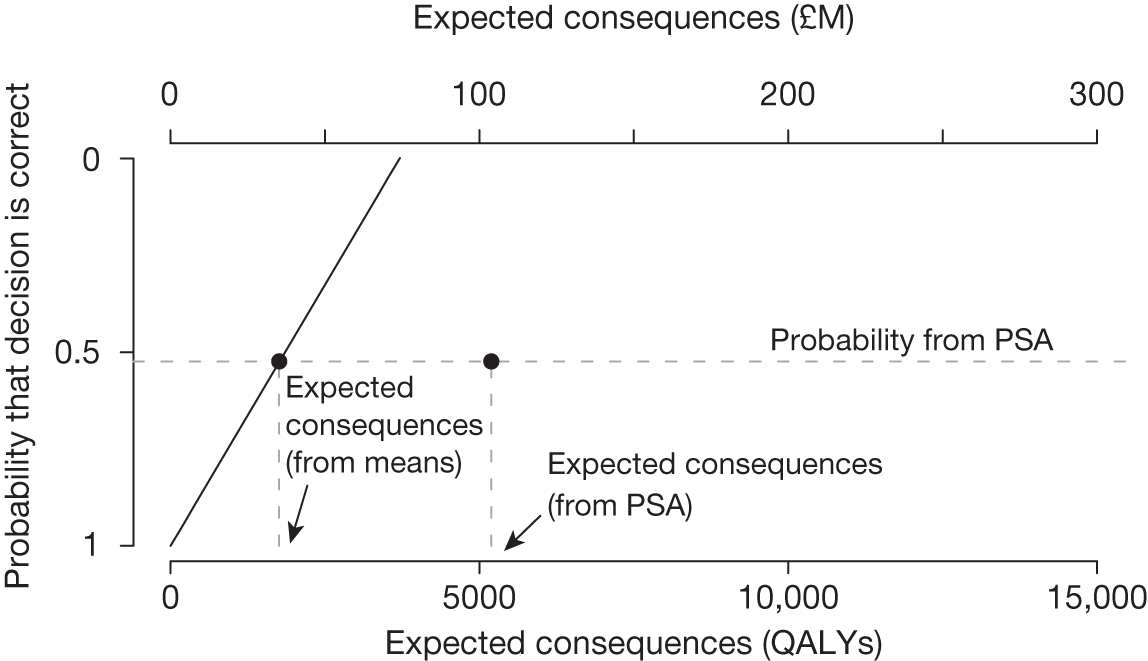
The previous analyses assumed that the difference in NHEs when CLOP is not the correct decision is the average; however, this is not necessarily true and estimates based on means may substantially under- or overestimate the expected consequences of uncertainty (see Appendix 11, Why the consequences of uncertainty differ from mean incremental effects). To accurately record the difference between the NHEs of CLOP and alternative treatments, and the frequency of such errors, the probabilistic analysis can be used. This distribution of consequences from PSA is illustrated in Figure 60. Most commonly (52.4%) there are no consequences because 12 months of treatment with CLOP is the correct decision. When it is not, there is a greater chance of relatively small consequences (30% are < 10,000 QALYs), which occur predominantly when 6 months of treatment offers the highest NHEs. However, there is a small chance of larger consequences (< 5% chance that they are > 30,000 QALYs) when standard NHS treatment offers the highest NHEs, that is, there remains important uncertainty about the cost-effectiveness of treatment itself, not just its duration.
FIGURE 60.
Distribution of the consequences of uncertainty for CLOP.
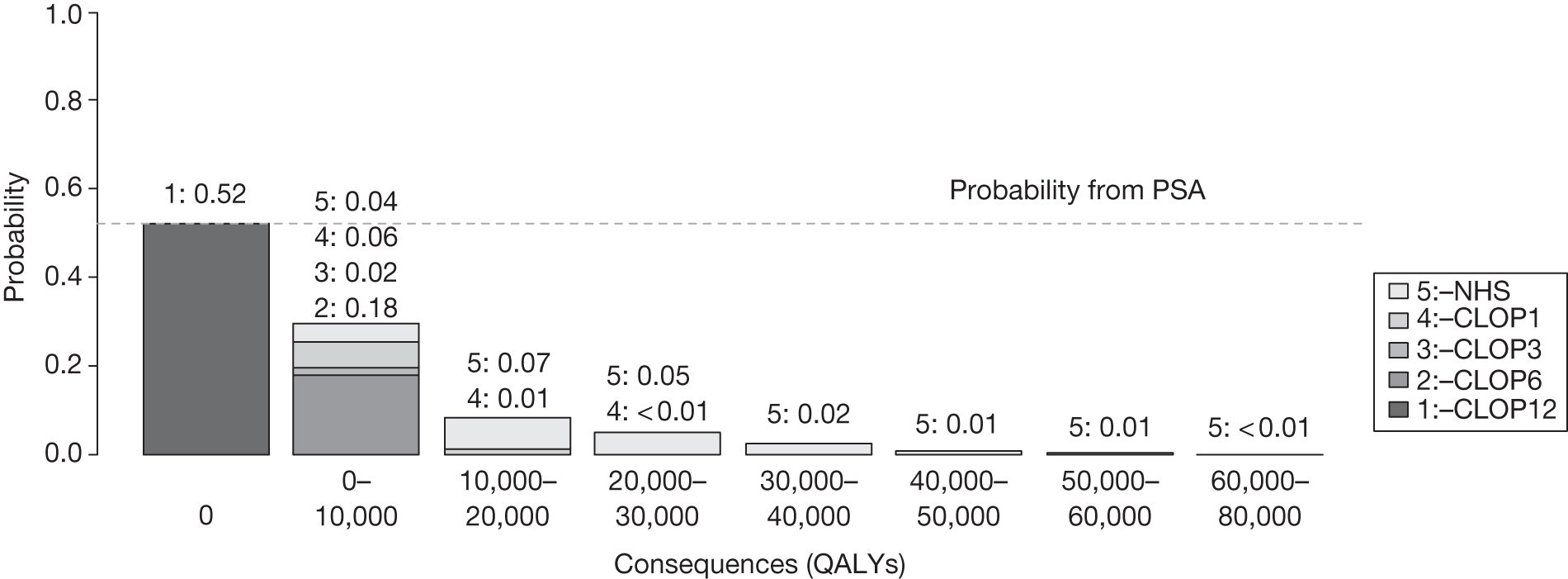
The average over this distribution provides the expected consequences of uncertainty from PSA, which in this case is 5194 QALYs (see Table 90). This is substantially greater than the estimate of 1723 QALYs based on mean incremental population NHEs, demonstrating that such simple estimates may be misleading (see Figure 59). The consequences can also be expressed as the equivalent NHS resources required to generate the same population NHEs: £103.9M (see Table 90). At a higher cost-effectiveness threshold (£30,000 per QALY) the consequences fall because a decision to approve CLOP will be less uncertain (see Table 90).
The value of the expected consequences of uncertainty can be interpreted as an estimate of the population NHEs that could be gained, over the time horizon of this technology, if the uncertainty about treatment and its duration could be immediately resolved; it indicates an expected upper bound on the benefits of more research. g A judgement at this point that more research might be worthwhile seems reasonable as the potential benefits exceed the likely costs.
The expected consequences increase with the technology time horizon and size of the patient population. Table 91 evaluates the consequences of uncertainty (using PSA) for alternative technology time horizons.
| Technology time horizon, years | Expected consequences, QALYs (£M) |
|---|---|
| 5 | 2819 (56.4) |
| 10 | 5194 (103.9) |
| 15 | 7193 (143.9) |
| 20 | 8876 (117.5) |
Analysis of subgroups
There were no relevant subgroups in the CLOP case study.
Alternative scenarios
The probabilistic analysis reported above reflects mainly the uncertainty over input parameters, given a set of assumptions made when estimating expected costs and QALYs. However, there are often alternative views not considered initially about, for example, the quality and relevance of evidence as well as other assumptions that might have been made. These are commonly presented as separate scenarios, with estimates of costs and QALYs presented for each. In the CLOP case study, base-case analyses assume a constant relative treatment effect for different durations of treatment. An alternative assumption (scenario B) was that the relative treatment effect also differed by duration based on the data reported in the SIGN guidelines. Tables 92 and 93 present a summary of the cost-effectiveness, decision uncertainty and expected consequences of uncertainty for the alternative scenarios. The alternative assumption that relative treatment effects differ by duration made longer durations less cost-effective and reduced the expected consequences of uncertainty from 5195 to 3969 QALYs.
| Treatment | Cost (£) | Health effects, QALYs | ICER (£/QALY) | Cost-effectiveness threshold £20,000 per QALY | Cost-effectiveness threshold £30,000 per QALY |
|---|---|---|---|---|---|
| NHE, QALYs (£M) | NHE, QALYs (£M) | ||||
| Scenario A (base case) | |||||
| CLOP12 | 10,394,830,647 | 4,194,554 | 18,663 | 3,674,813 (73,496) | 3,848,060 (115,442) |
| CLOP6 | 10,256,672,674 | 4,187,151 | 10,477 | 3 674,318 (73,486) | 3,845,262 (115,358) |
| CLOP3 | 10,180,425,730 | 4,179,874 | 9396 | 3,670,853 (73,417) | 3,840,526 (115,216) |
| CLOP1 | 10,121,529,942 | 4,173,605 | 4961 | 3,667,529 (73,351) | 3,836,221 (115,087) |
| NHS | 10,072,035,344 | 4,163,629 | – | 3,660,027 (73,201) | 3,827,894 (114,837) |
| Scenario B | |||||
| CLOP12 | 10,377,895,363 | 4,236,359 | 20,494 | 3,717,464 (74,349) | 3,890,429 (116,713) |
| CLOP6 | 10,236,295,027 | 4,229,449 | 11,963 | 3,717,635 (74,353) | 3,888,240 (116,647) |
| CLOP3 | 10,154,505,201 | 4,222,613 | 4087 | 3,714,887 (74,298) | 3,884,129 (116,524) |
| CLOP1 | 10,044,958,670 | 4,195,806 | 3601 | 3,693,558 (73,871) | 3,860,974 (115,829) |
| NHS | 9,941,658,953 | 4,167,119 | – | 3,670,036 (73,401) | 3,835,730 (115,072) |
| Treatment | ICER (£/QALY) | Cost-effectiveness threshold £20,000 per QALY | Cost-effectiveness threshold £30,000 per QALY | ||||
|---|---|---|---|---|---|---|---|
| Incremental NHE,a QALYs (£M) | Probability cost−effective | Expected consequences, QALYs (£M) | Incremental NHE,a QALYs (£M) | Probability cost−effective | Expected consequences, QALYs (£M) | ||
| Scenario A (base case) | |||||||
| CLOP12 | 18,663 | 495 (9.9) | 0.524 | 5194 (103.9) | 2798 (56.0) | 0.677 | 3657 (109.7) |
| CLOP6 | 10,477 | 3465 (69.3) | 0.180 | 4736 (94.7) | 0.092 | ||
| CLOP3 | 9396 | 3324 (66.5) | 0.018 | 4305 (86.1) | 0.009 | ||
| CLOP1 | 4961 | 7502 (150.0) | 0.075 | 8327 (166.5) | 0.052 | ||
| NHS | – | – | 0.202 | – | 0.170 | ||
| Scenario B | |||||||
| CLOP12 | 20,494 | –171 (–3.4) | 0.435 | 3969 (79.4) | 2189 (43.8) | 0.564 | 2871 (86.1) |
| CLOP6 | 11,963 | 2747 (54.9) | 0.327 | 4110 (82.2) | 0.268 | ||
| CLOP3 | 4087 | 21,329 (426.6) | 0.237 | 23,155 (463.1) | 0.168 | ||
| CLOP1 | 3601 | 23,522 (470.4) | 0.001 | 25,244 (504.9) | 0.000 | ||
| NHS | – | – | 0.000 | – | 0.000 | ||
The uncertainty within each scenario will be sufficient to indicate the potential benefits of research only when this scenario is the only one regarded as credible. However, when more than one scenario might be credible and carry some ‘weight’, there will be uncertainty between as well as within scenarios. Much of the deliberation by the Appraisal Committee often surrounds the scientific value judgements required to judge the credibility of the alternative assumptions represented by the scenarios. The ‘weighting’ of scenarios can be made explicit by assigning probabilities to represent how credible each is believed to be. The weighted average of costs and QALYs across scenarios can easily be calculated. It is also tempting to take a simple weighted average of the expected consequences of uncertainty across these scenarios as well. However, a simple weighted average may under- or overestimate the combined consequences of the uncertainty within and between scenarios. The correct estimate requires the probabilities (weights) to be applied in a way that correctly identifies the consequences of uncertainty (for further details refer to Appendix 11, Why averaging scenarios may be misleading). Although this does not require additional simulation and is quick and easy to implement, it does require that either the probabilities are made explicit in advance or that estimates be presented for a range of probabilities that might represent the judgement of the Appraisal Committee following deliberation.
In the CLOP case study, although scenario A was regarded as more credible by the Appraisal Committee, scenario B might nevertheless carry some weight or have some probability associated with it. Figure 61 shows that, in this case study, the simple weighted average of expected consequences (linear combination of mean estimates) approximates reasonably the correct estimate based on weighting the output of PSA. This figure also shows how these estimates can be presented for a range of probabilities.
FIGURE 61.
Expected consequences of uncertainty with alternative scenarios: CLOP case study.
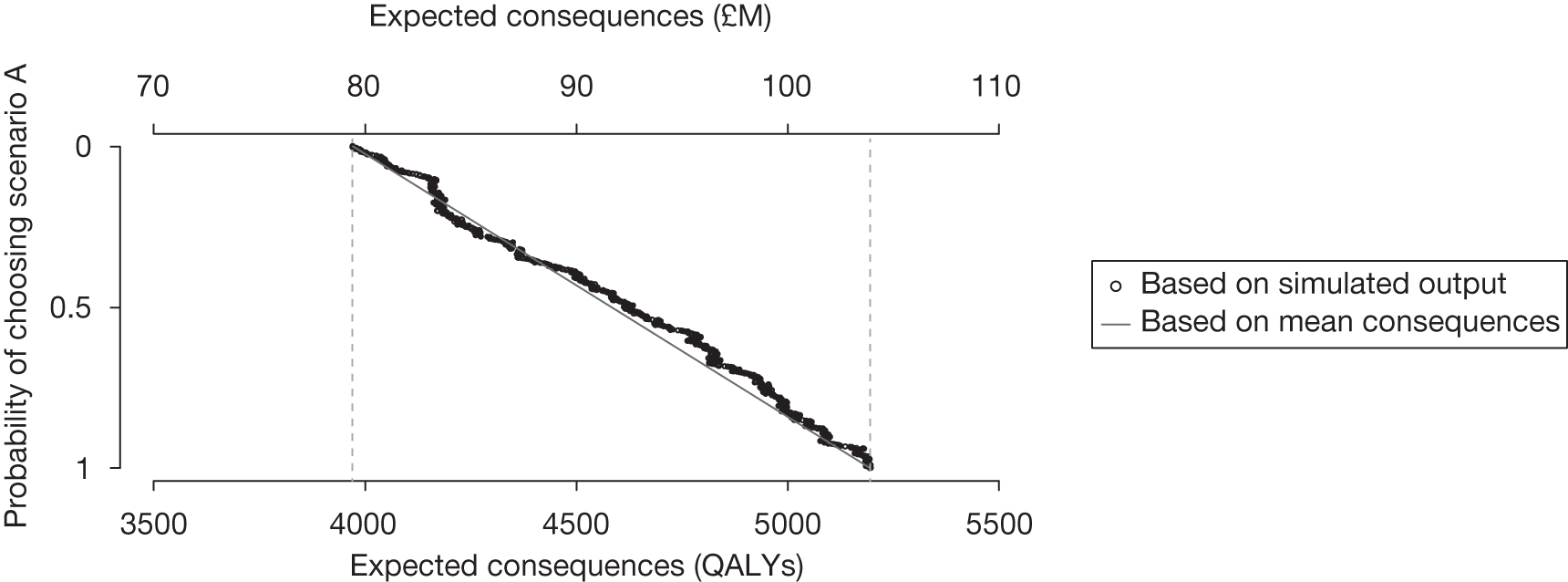
Closed-form solutions can also correctly estimate the consequences of within- and between-scenario uncertainty (Figure 62) and can be used instead of weighting the output of PSA.
FIGURE 62.
Expected consequences of uncertainty with alternative scenarios: CLOP case study.
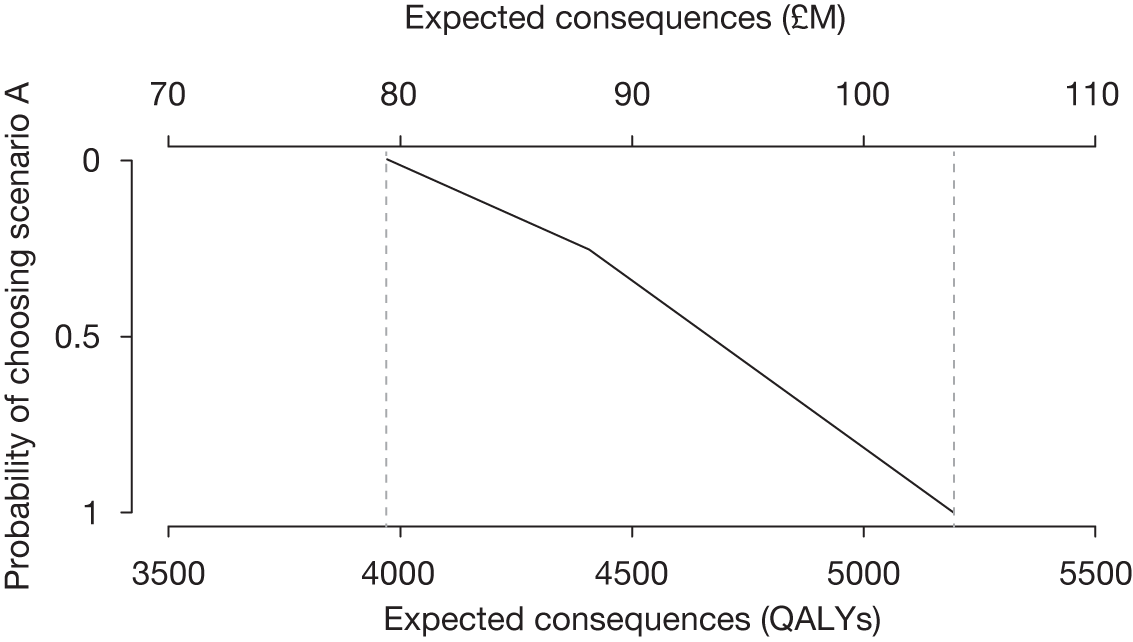
Point 4: Is research possible with approval?
The fourth point on the checklist requires an assessment of the type of evidence that is needed and a judgement of whether or not the research required to generate it can be conducted while the technology is approved. Although the decision at this point does not lead directly to guidance, it does determine whether AWR or OIR recommendations are possibilities. This judgement will depend, in part, on whether or not the type of evidence that is needed will require an experimental research design. For example, more precise estimates of relative treatment effect are likely to require a RCT if the dangers of selection bias are to be avoided. However, further RCTs for this particular indication and patient group are unlikely to be possible once a technology is approved for widespread NHS use.
This assessment requires judgements about (1) how important particular types of parameters (inputs to the economic model) are to estimates of cost and QALYs, (2) what values these parameters would have to take to change a decision based on expected cost-effectiveness, (3) how likely it is that parameters might take such values and (4) what would be the consequences if they did, that is, what might be gained in terms of population NHEs if the uncertainty in the values of these parameters could be immediately resolved.
Assessing the importance of parameters
The type of economic model used to estimate expected cost-effectiveness in NICE appraisal specifies the relationship between the inputs (the parameters) and the outputs (costs and QALYs). A simple summary of the direction and strength of these relationships can be provided by calculating elasticities for each, that is, the proportionate change in the NHEs of each alternative, and differences in NHEs, owing to a 1% change in the value of the parameter. The elasticities regarding parameters of the CLOP case study are presented in Table 94. Those parameters with high elasticities in absolute value (especially with respect to differences in NHEs) might be regarded as more ‘important’; see as an example the elasticity for RR_death.
| Parameter | Elasticity over the NHEs (QALYs) of | Elasticity over the incremental NHEs (QALYs) of | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CLOP12 | CLOP6 | CLOP3 | CLOP1 | NHS | CLOP12 vs NHS | CLOP12 vs CLOP6 | CLOP12 vs alla | |||
| Natural history | 1 | P_die_0.1 | –0.208 | –0.207 | –0.207 | –0.207 | –0.222 | 0.014 | – | 0.003 |
| 2 | P_NFMI_0.1 | –0.012 | –0.012 | –0.011 | –0.011 | –0.015 | 0.004 | – | – | |
| 3 | P_die_1.3 | –0.137 | –0.137 | –0.137 | –0.147 | –0.145 | 0.008 | – | 0.004 | |
| 4 | P_NFMI_1.3 | –0.002 | –0.002 | –0.002 | –0.002 | –0.002 | 0.001 | – | – | |
| 5 | P_die_3.6 | –0.146 | –0.146 | –0.157 | –0.157 | –0.154 | 0.008 | – | 0.007 | |
| 6 | P_NFMI_3.6 | –0.005 | –0.005 | –0.007 | –0.007 | –0.007 | 0.002 | – | 0.001 | |
| 7 | P_die_6.12 | –0.148 | –0.159 | –0.158 | –0.157 | –0.155 | 0.007 | 0.011 | 0.010 | |
| 8 | P_NFMI_6.12 | –0.005 | –0.007 | –0.007 | –0.007 | –0.007 | 0.002 | 0.002 | 0.002 | |
| 9 | TP_AC | –0.121 | –0.120 | –0.120 | –0.120 | –0.118 | –0.003 | –0.001 | –0.002 | |
| 10 | TP_AD | –3.637 | –3.622 | –3.604 | –3.594 | –3.541 | –0.096 | –0.016 | –0.047 | |
| 11 | TP_CD | –0.233 | –0.235 | –0.239 | –0.240 | –0.253 | 0.020 | 0.002 | 0.009 | |
| 12 | TP_BD | –0.586 | –0.593 | –0.602 | –0.605 | –0.641 | 0.055 | 0.007 | 0.024 | |
| Utilities | 13 | U_Well | 0.746 | 0.745 | 0.743 | 0.742 | 0.737 | 0.009 | 0.001 | 0.004 |
| 14 | U_Well1 | 6.090 | 6.064 | 6.034 | 6.017 | 5.929 | 0.160 | 0.026 | 0.079 | |
| 15 | U_NFMI | 0.133 | 0.134 | 0.136 | 0.136 | 0.144 | –0.011 | –0.001 | –0.005 | |
| 16 | U_PostMI | 1.138 | 1.150 | 1.165 | 1.171 | 1.236 | –0.099 | –0.012 | –0.043 | |
| Relative effect | 17 | RR_death | –0.639 | –0.491 | –0.344 | –0.207 | – | –0.641 | –0.150 | –0.380 |
| 18 | RR_NFMI | –0.024 | –0.018 | –0.013 | –0.011 | – | –0.025 | –0.006 | –0.014 | |
| Costs | 19 | C_Well | –0.740 | –0.737 | –0.733 | –0.731 | –0.720 | –0.019 | –0.003 | –0.009 |
| 20 | C_MI_LT | –0.051 | –0.052 | –0.053 | –0.053 | –0.056 | 0.004 | 0.001 | 0.002 | |
| 21 | C_PostMI | –0.142 | –0.143 | –0.145 | –0.146 | –0.154 | 0.012 | 0.002 | 0.005 | |
| 22 | TC_Well_Dead | –0.027 | –0.027 | –0.027 | –0.027 | –0.027 | – | – | – | |
| 23 | C_t1 | –0.045 | – | – | – | – | –0.045 | –0.045 | –0.045 | |
| 24 | C_t2 | – | –0.033 | – | – | – | – | 0.033 | 0.008 | |
| 25 | C_t3 | – | – | –0.026 | – | – | – | – | 0.007 | |
| 26 | C_t4 | – | – | – | –0.022 | – | – | – | 0.005 | |
| 27 | C_t5 | – | – | – | – | –0.016 | 0.016 | – | 0.004 | |
Although these measures of importance are more instructive than a series of arbitrary one-way sensitivity analyses, it does not directly help the assessment of what values parameters must take to change decisions and how likely such values might be. A simple summary of the values that particular parameters must take to make each of the alternatives cost-effective can also be provided. For CLOP this information is in Table 95. It shows, for example, that if P_die_0.1 is > 0.10, treatments other than 12 months of treatment with CLOP are deemed cost-effective. However, although instructive, such ‘threshold values’ do not indicate how likely it is that a threshold will be crossed or the combined effect of groups of related parameters.
| Parameter | Mean value | CLOP12 | CLOP6 | CLOP3 | CLOP1 | NHS | ||
|---|---|---|---|---|---|---|---|---|
| Natural history | 1 | P_die_0.1 | 0.032 | 0 to 0.10 | 0.11 to 0.54 | 0.54 to 0.63 | 0.63 to 1 | – |
| 2 | P_NFMI_0.1 | 0.040 | 0 to 0.14 | 0.14 to 0.71 | 0.71 to 0.82 | 0.82 to 1 | – | |
| 3 | P_die_1.3 | 0.022 | 0 to 0.10 | 0.10 to 0.55 | 0.55 to 1 | – | – | |
| 4 | P_NFMI_1.3 | 0.004 | 0 to 0.10 | 0.10 to 0.7 | 0.7 to 1 | – | – | |
| 5 | P_die_3.6 | 0.023 | 0.01 to 0.10 | 0.10 to 1 | 0 to 0.01 | – | – | |
| 6 | P_NFMI_3.6 | 0.011 | 0 to 0.11 | 0.11 to 1 | – | – | – | |
| 7 | P_die_6.12 | 0.024 | 0.02 to 1 | 0 to 0.02 | – | – | – | |
| 8 | P_NFMI_6.12 | 0.009 | 0.005 to 1 | 0 to 0.005 | – | – | – | |
| 9 | TP_AC | 0.018 | 0 to 0.06 | 0.06 to 1 | – | – | – | |
| 10 | TP_AD | 0.072 | 0 to 0.08 | 0.08 to 0.10 | – | – | 0.10 to 1 | |
| 11 | TP_CD | 0.188 | 0.12 to 1 | 0 to 0.12 | – | – | – | |
| 12 | TP_BD | 0.070 | 0.06 to 1 | 0.04 to 0.06 | – | – | 0 to 0.04 | |
| Utilities | 13 | U_Well | 0.798 | 0.29 to 1 | 0 to 0.29 | – | – | – |
| 14 | U_Well1 | 0.930 | 0.90 to 1 | 0.74 to 0.90 | – | – | 0 to 0.74 | |
| 15 | U_NFMI | 0.801 | 0 to 1 | – | – | – | – | |
| 16 | U_PostMI | 0.931 | 0 to 1 | – | – | – | – | |
| Relative effect | 17 | RR_death | 0.931 | 0 to 0.93 | 0.94 to 0.97 | 0.97 to 0.98 | 0.98 to 0.99 | 1.00 to max.a |
| 18 | RR_NFMI | 0.710 | 0 to 0.82 | 0.83 to 1.55 | 1.56 to 1.83 | – | 1.84 to max.a | |
| Costs | 19 | C_Well | 2061.5 | 0 to 2690 | 2690 to 5611 | – | – | 5611 to max.a |
| 20 | C_MI_LT | 6050 | 0 to max.a | – | – | – | – | |
| 21 | C_PostMI | 2309.7 | 870 to max.a | 0 to 870 | – | – | – | |
| 22 | TC_Well_Dead | 871.5 | 0 to 20,474 | 20,474 to max.a | – | – | – | |
| 23 | C_t1 | 895.1 | 0 to 910 | 910 to max.a | – | – | – | |
| 24 | C_t2 | 651.6 | 630 to max.a | 0 to 630 | – | – | – | |
| 25 | C_t3 | 524.2 | 370 to max.a | – | 0 to 370 | – | – | |
| 26 | C_t4 | 434.8 | 150 to max.a | – | – | 0 to 150 | – | |
| 27 | C_t5 | 329.8 | 0 to max. | – | – | – | – | |
Assessment of uncertainty
The judgement about how likely it is that parameters might take values that will change the technology expected to be cost-effective can be informed by the results of probabilistic analysis. This is because the distributions assigned to parameters in PSA describe how uncertain the parameter estimates are, such that they ought to reflect the amount and quality of exiting evidence. The probabilities that each parameter might take values that would lead to each of the alternatives being cost-effective are reported for the CLOP case study in Table 96. This essentially decomposes the overall probabilities reported in Table 91 into the contribution that each parameter makes. h Interestingly, it indicates that it is uncertainty in the estimates of relative effect (RR_death, parameter 17) that contributes most to the probability of error associated with 12 months of treatment. It is the only parameter that might take values which could make any of the other alternatives cost-effective. To better visualise this, the histogram in Figure 63, showing the distribution of values that this parameter may take, is shaded to highlight the ranges of values that lead to different adoption decisions. Despite contributing to decision uncertainty, we are still unclear on whether or not there are significant consequences from these shifts in the adoption decision.
| Parameter | CLOP12 | CLOP6 | CLOP3 | CLOP1 | NHS | ||
|---|---|---|---|---|---|---|---|
| Natural history | 1 | P_die_0.1 | 1 | – | – | – | – |
| 2 | P_NFMI_0.1 | 1 | – | – | – | – | |
| 3 | P_die_1.3 | 1 | – | – | – | – | |
| 4 | P_NFMI_1.3 | 1 | – | – | – | – | |
| 5 | P_die_3.6 | 1 | – | – | – | – | |
| 6 | P_NFMI_3.6 | 1 | – | – | – | – | |
| 7 | P_die_6.12 | 0.65 | 0.35 | – | – | – | |
| 8 | P_NFMI_6.12 | 0.91 | 0.09 | – | – | – | |
| 9 | TP_AC | 1 | – | – | – | – | |
| 10 | TP_AD | 0.83 | 0.17 | – | – | – | |
| 11 | TP_CD | 1 | – | – | – | – | |
| 12 | TP_BD | 0.85 | 0.15 | – | – | – | |
| Utilities | 13 | U_Well | 1 | – | – | – | – |
| 14 | U_Well1 | 0.94 | 0.06 | – | – | – | |
| 15 | U_NFMI | 1 | – | – | – | – | |
| 16 | U_PostMI | 1 | – | – | – | – | |
| Relative effect | 17 | RR_death | 0.55 | 0.18 | 0.01 | 0.10 | 0.16 |
| 18 | RR_NFMI | 0.97 | 0.03 | – | – | – | |
| Costs | 19 | C_Well | 0.78 | 0.19 | – | – | 0.03 |
| 20 | C_MI_LT | 1 | – | – | – | – | |
| 21 | C_PostMI | 0.89 | 0.11 | – | – | – | |
| 22 | TC_Well_Dead | 1 | – | – | – | – | |
| 23 | C_t1 | 0.95 | 0.05 | – | – | – | |
| 24 | C_t2 | 0.99 | 0.01 | – | – | – | |
| 25 | C_t3 | 1 | – | – | – | – | |
| 26 | C_t4 | 1 | – | – | – | – | |
| 27 | C_t5 | 1 | – | – | – | – | |
FIGURE 63.
Histogram of the values of parameter RR_death: CLOP case study.
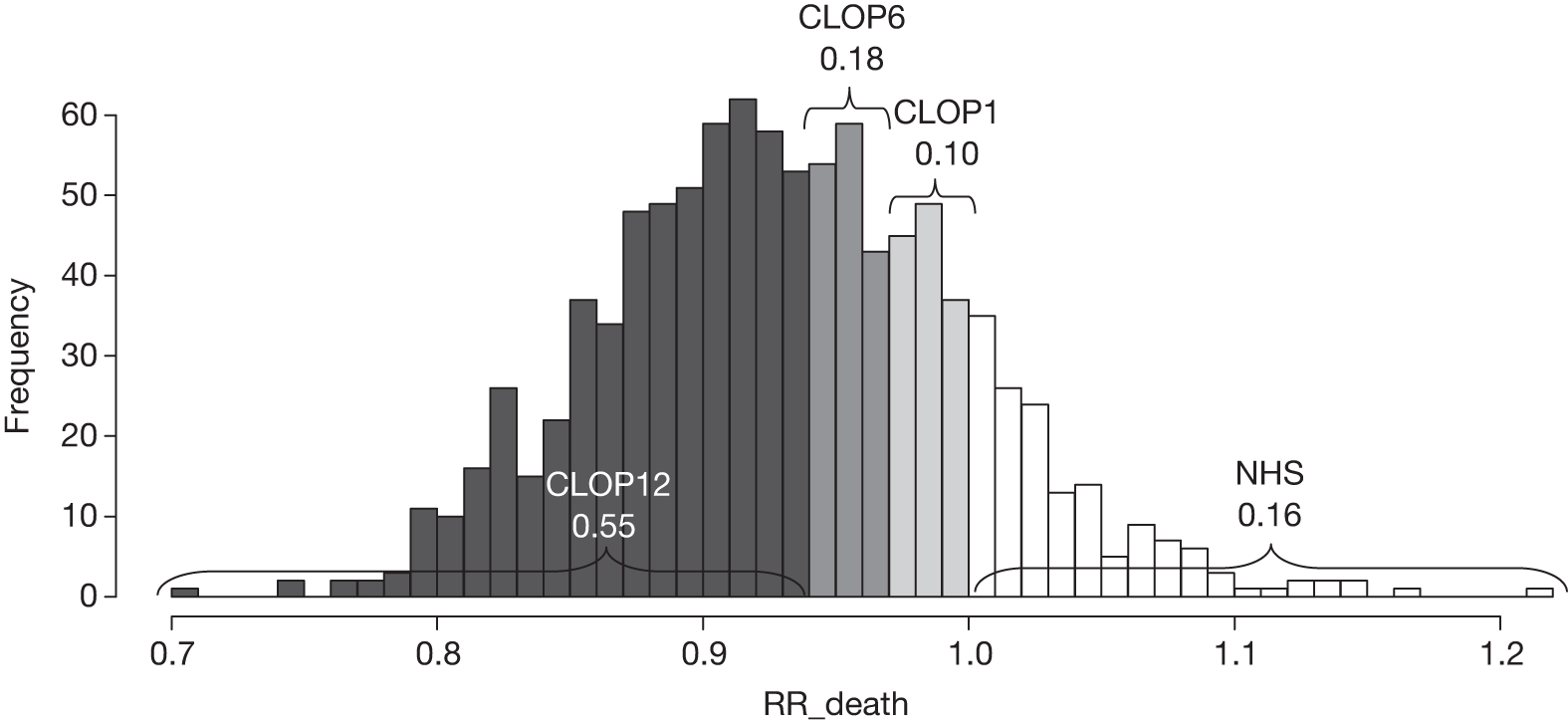
What type of evidence is needed?
Although an understanding of uncertainty and the importance of parameters separately is helpful, an assessment of the likely consequences of this uncertainty, and therefore what might potentially be gained in terms of population NHEs if uncertainty could be immediately resolved, is required. This assessment can directly inform the judgement of what evidence is needed and whether the type of research required to generate it will be possible with approval. As in the section on Point 3: Does more research seem worthwhile?, the results of PSA can inform this judgement as estimates of the expected consequences of uncertainty associated with each parameter combine both uncertainty in their potential values and their importance in terms of changing decisions and differences in NHEs. The distribution of consequences owing to uncertainty in specific parameters is illustrated for the parameter RR_death in Figure 64. There is a likelihood of 45% of values of RR_death leading to 12 months of treatment with CLOP not being the correct decision (100% – 55%; see Table 97). The average over this distribution provides the expected consequences of uncertainty for the parameter RR_death, which in this case is 4433 QALYs (or £88.7M).
FIGURE 64.
Distribution of consequences due to uncertainty in RR_death in the CLOP case study.
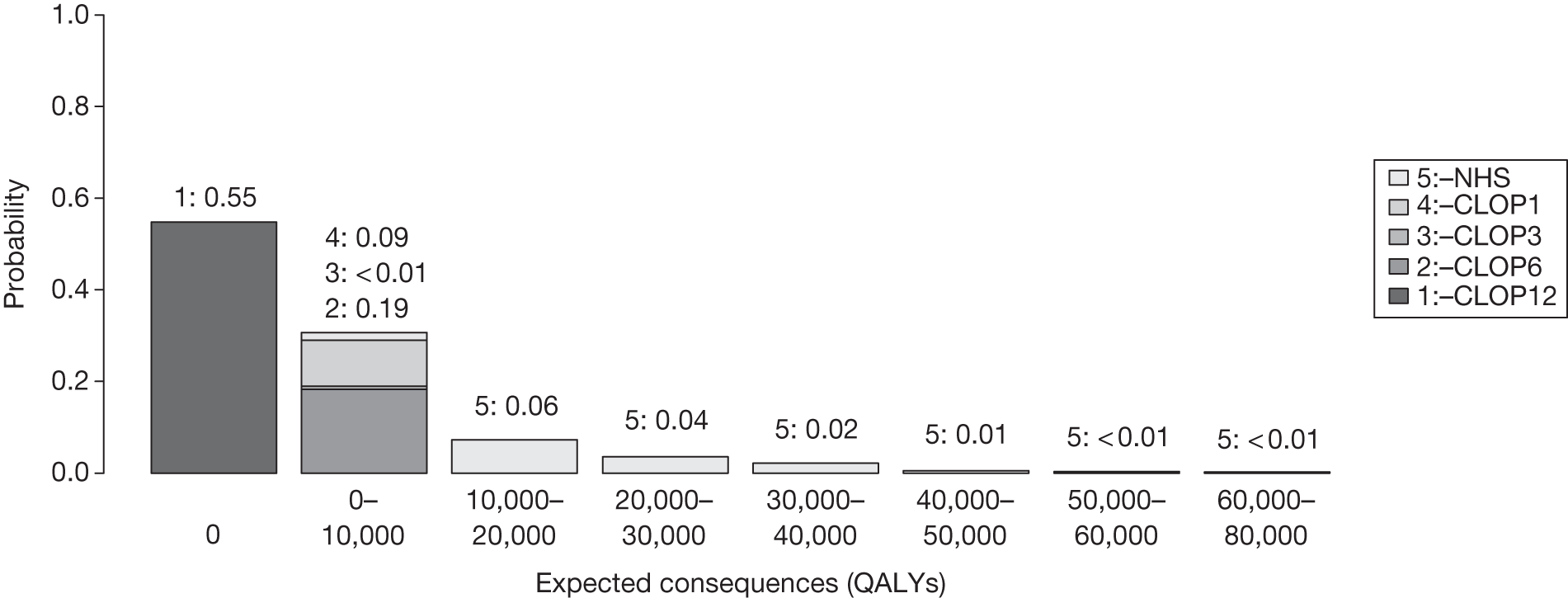
| Parameter | Expected consequences (QALYs) | |||||||
|---|---|---|---|---|---|---|---|---|
| Decomposed by treatment choice | Overall | |||||||
| CLOP12 | CLOP6 | CLOP3 | CLOP1 | NHS | ||||
| Natural history a | 1 | P_die_0.1 | 0 | – | – | – | – | 0 |
| 2 | P_NFMI_0.1 | 0 | – | – | – | – | 0 | |
| 3 | P_die_1.3 | 0 | – | – | – | – | 0 | |
| 4 | P_NFMI_1.3 | 0 | – | – | – | – | 0 | |
| 5 | P_die_3.6 | 0 | – | – | – | – | 0 | |
| 6 | P_NFMI_3.6 | 0 | – | – | – | – | 0 | |
| 7 | P_die_6.12 | 0 | 250 | – | – | – | 250 | |
| 8 | P_NFMI_6.12 | 0 | 9 | – | – | – | 9 | |
| 9 | TP_AC | 0 | – | – | – | – | 0 | |
| 10 | TP_AD | 0 | 47 | – | – | – | 47 | |
| 11 | TP_CD | 0 | – | – | – | – | 0 | |
| 12 | TP_BD | 0 | 35 | – | – | – | 35 | |
| Utilities a | 13 | U_Well | 0 | – | – | – | – | 0 |
| 14 | U_Well1 | 0 | 10 | – | – | – | 10 | |
| 15 | U_NFMI | 0 | – | – | – | – | 0 | |
| 16 | U_PostMI | 0 | – | – | – | – | 0 | |
| Relative effect a | 17 | RR_death | 0 | 284 | 16 | 518 | 3614 | 4433 |
| 18 | RR_NFMI | 0 | 3 | – | – | – | 3 | |
| Costs a | 19 | C_Well | 0 | 153 | – | – | 321 | 474 |
| 20 | C_MI_LT | 0 | – | – | – | – | 0 | |
| 21 | C_PostMI | 0 | 8 | – | – | – | 8 | |
| 22 | TC_Well_Dead | 0 | – | – | – | – | 0 | |
| 23 | C_t1 | 0 | 8 | – | – | – | 8 | |
| 24 | C_t2 | 0 | 0 | – | – | – | 0 | |
| 25 | C_t3 | 0 | – | – | – | – | 0 | |
| 26 | C_t4 | 0 | – | – | – | – | 0 | |
| 27 | C_t5 | 0 | – | – | – | – | 0 | |
The expected consequences of uncertainty associated with each parameter in the CLOP case study are reported in Table 97. This decomposes the overall expected consequences reported in Table 93 into the contribution that each parameter makes and which other alternatives might offer higher NHEs than 12 months of treatment. i It confirms that it is uncertainty in the estimates of relative effect (RR_death) that contributes most and for which there is potentially the most to be gained by resolving this uncertainty through additional research (4433 QALYs or £88.7M).
Because more precise estimates of relative effects are likely to require a RCT, a judgement that the type of research needed will not be possible if 12 months of treatment with CLOP is approved may be reasonable. However, the potential benefits of resolving the uncertainty associated with other groups of parameters, for example costs and natural history (Table 98), might mean that other types of cheaper, non-experimental research could be worthwhile as well. j
| Group of parameters | Expected consequences, QALYs (£M) |
|---|---|
| Natural history | 369 (7.4) |
| Relative effects | 4504 (90.1) |
| Utilities | 15 (0.3) |
| Costs | 547 (10.9) |
Implications of between-scenario uncertainty
In Point 3: Does more research seem worthwhile?, Alternative scenarios the contribution that alternative scenarios might make to the overall expected consequences of uncertainty and therefore the potential gains from further evidence was considered and discussed. In situations in which more than one scenario might be regarded as credible there will be uncertainty between as well as within each of the scenarios. It was demonstrated that an assessment of the combined consequences of both sources of uncertainty requires ‘weights’ (probabilities) to be assigned to represent their credibility, which can then be applied directly to the simulated output from PSA (see Appendix 11, Why averaging scenarios may be misleading). However, the same analysis can also be used to identify the expected consequences of uncertainty associated with the alternative scenarios themselves, that is, what might be gained if evidence could immediately distinguish which scenario was ‘true’. This can help to inform the assessment of what type of evidence might be needed and whether the research required to generate it is likely to be possible once a technology is approved for widespread NHS use.
In the CLOP analysis, scenarios A and B (treatment effect was constant or differed by treatment duration respectively) were associated with expected consequences of uncertainty of 5195 and 3969 QALYs respectively (Table 99 and see Point 3: Does more research seem worthwhile?, Alternative scenarios). If both scenarios were regarded as equally likely, the overall expected consequences of uncertainty (combining consequences within and between scenarios) would be 4667 QALYs. However, the expected consequences of uncertainty associated with the two alternative scenarios themselves and what might be potentially gained if the uncertainty between them could be immediately resolved is relatively modest at 85 QALYs, that is, most of what might be gained from further evidence is associated with the parameters in Table 97 rather than the alternative scenarios. This suggests that more evidence about the overall relative effect on mortality is more important than resolving uncertainty about whether such an effect differs by treatment duration.
| Consequences of uncertainty on | QALYs (£M) |
|---|---|
| Parameters, within scenario A (base case) | 5194 (103.9) |
| Parameters, within scenario B | 3969 (79.4) |
| Parameters, considering between-scenario uncertainty | 2356 (47.1) |
| Between-scenario uncertainty, considering uncertainty within scenarios | 85 (1.7) |
| Parameters and scenario uncertainty | 4667 (13.3) |
Types and categories of guidance resulting from points 3 and 4
Points 3 and 4 of the checklist are critical because if research is not judged to be worthwhile then no further assessments are required. In the case of CLOP, more research appears to be worthwhile and the expected consequences of uncertainty are high.
In CLOP, research was not considered possible with approval. It is research that would provide more precise estimates of the relative effect of CLOP and of shorter treatment durations that is potentially valuable (see Point 4: Is research possible with approval?). As a consequence, the type of experimental design that is likely to be needed is unlikely to be possible if 12 months of treatment with CLOP is already approved for widespread NHS use.
Table 100 summarises the categories and types of guidance that could ultimately result from the sequence of assessments up to point 4.
| Assessment | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Guidance |
| 3 | Yes | No | Yes | No | Yes/no | Yes | Yes | Approve2 |
| 4 | Yes | No | Yes | No | Yes/no | Yes | No | OIR1 |
| 5 | Yes | No | Yes | No | Yes/no | No | – | Approve3 |
Do the benefits of research exceed the costs?
Point 5: Will other sources of uncertainty resolve over time?
The fifth point on the checklist requires an assessment of whether or not changes are likely to occur in the future that will influence the cost-effectiveness of the alternative technologies and the potential benefits of research, that is, at the following point in the algorithm:
This assessment requires information about (1) changes in the prices of the technology and its comparators, (2) the emergence of new technologies that might make existing ones obsolete or change their cost-effectiveness and (3) other relevant research reporting. A number of potential sources of information and evidence were examined to inform this assessment for each case study (the full details of the sources and searches conducted are reported in Appendix 4).
Changes in the prices of the technology and its comparators
Changes in prices influence not only expected cost-effectiveness but also uncertainty and the potential benefits of research to future patients, for example if the price of a technology expected to be cost-effective is likely to fall significantly just before research reports the potential benefits will not be realised because approval of the technology will be less uncertain and there may be much less or little to gain from the results of the research. This assessment requires information about when major changes in prices are likely and some evidence about the likely extent of any changes. A major event in the life cycle of a pharmaceutical technology is the date at which the patient expires and cheaper generic versions of the brand become available. At the time of TA80122 the patent for CLOP was expected to expire 7 years later and subsequent analysis assumes that at that time equivalent generic prices will be 25% of the original price of CLOP at the time of TA80. k
Entry of new technologies
The entry of a new technology may make the existing technology that is expected to be cost-effective obsolete (no longer the most cost-effective alternative). Even when it does not, it will tend to change the relative cost-effectiveness of the alternatives, influencing how uncertain a decision to approve the original technology will be for future patients and the potential gains from research. The information that was available indicated that one new technology relevant to CLOP might have been expected to enter. Information about this technology was limited so scenarios are used in Point 6: Are the benefits of research greater than the costs? to explore the implications for CLOP.
Other research reporting the technology and its comparators
Research that is already under way, commissioned or likely to be undertaken, whether in the UK or elsewhere, is relevant for the reasons discussed in this section. Despite an assiduous search no records relevant to CLOP were identified.
Point 6: Are the benefits of research greater than the costs?
The sixth point on the checklist requires a judgement of whether or not the potential benefits of conducting further research (initially considered at point 3) are likely to exceed the costs, that is, at the following point in the algorithm:
This requires an assessment of (1) whether or not the type of research that is required is likely to be conducted, (2) if conducted, when the results are likely to be available, (3) how much uncertainty is likely to be resolved and (4) the likely impact of other sources of uncertainty (identified in Point 5: Will other sources of uncertainty resolve over time?) on the longer-term benefits of research.
Will the research be conducted?
Even if research is recommended in OIR or AWR, it might not be undertaken by manufacturers or commissioned by research funders. Even if undertaken or commissioned, there is no guarantee that research will be able to recruit or it may not complete for other reasons. The expected consequences of uncertainty for CLOP reported in Point 3: Does more research seem worthwhile? are illustrated in Figure 65 for a range of probabilities that research will be successfully undertaken. This indicates that the potential gains depend on a judgement of whether the research recommended as part of OIR will be successfully completed. It also illustrates that the cost of research (in this case considered to be either £1.5M or £10M) can be compared directly with the potential benefits by either expressing the potential gains in population NHEs as the equivalent NHS resources (i.e. the resources that would be required to generate the same NHEs) or expressing the cost of research in terms of the QALYs that could be gained elsewhere in the NHS by using the same resources to provide access to health care.
FIGURE 65.
Expected potential benefits of research: CLOP case study.
When will it be available?
Research, even if commissioned and successfully completed, will take time to complete and report; therefore, any assessment of the potential benefits should account for the fact that patient populations will not benefit from the results of research until they are available. Whether or not those patients who are prevalent while research is under way will be able to benefit from the results will depend on whether or not treatment decisions for presenting patients are irreversible (see Point 2: Are there significant irrecoverable costs?). In the CLOP case study, because the indication is acute, it is only those patients who are incident after the research reports who will realise any of the potential benefits. Treatment decisions are thus irreversible in the CLOP case study.
The potential value of research in CLOP over a range of possible time horizons for research to report is reported in Figure 66. How long research might take to report will depend in part on the design (follow-up, sample size and end points), recruitment rates and size of the eligible patient population, as well as on how efficient the organisation and data collection might be. The potential value of further research declines with the time to research reporting. This relationship gives some indication of the value of improving the timeliness of research through, for example, investment in research infrastructure or by adopting a research design that, although offering less potential benefits, can be conducted more quickly.
FIGURE 66.
Potential value of research by time to report: CLOP case study.
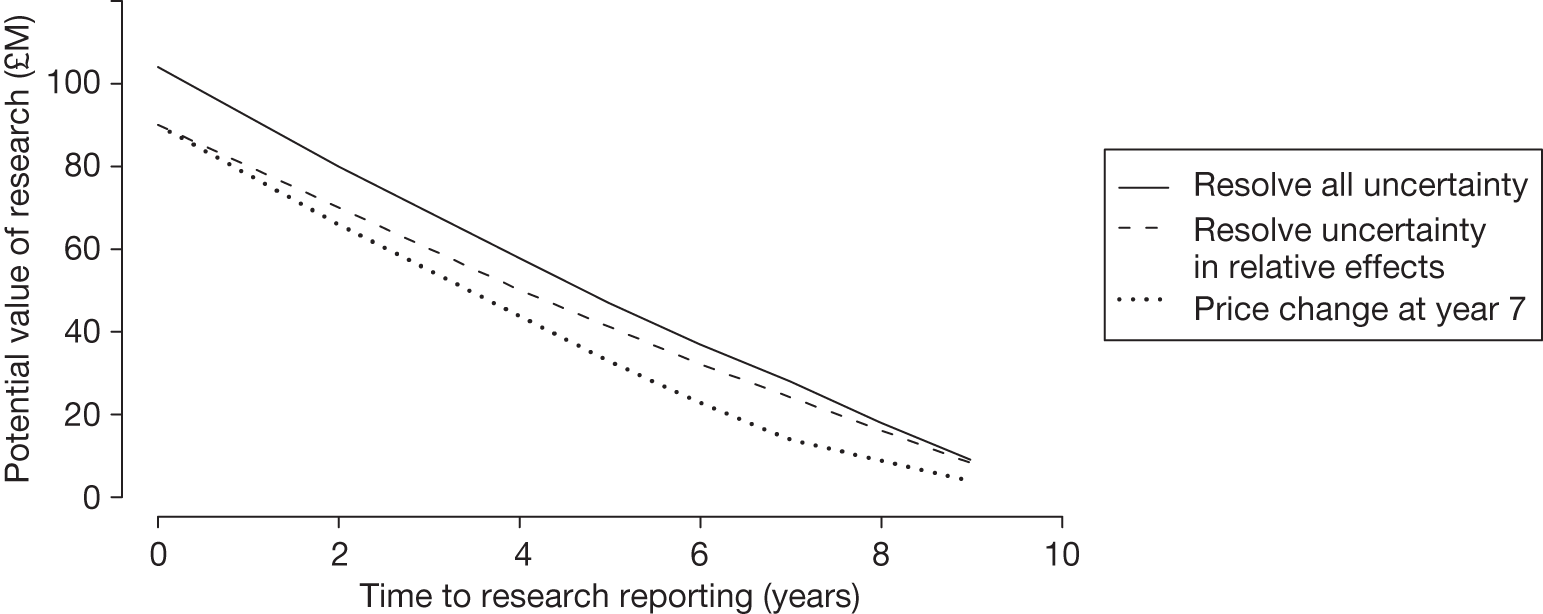
How much will be resolved?
Most research will not inform all of the parameters that determine expected costs and QALYs but usually a subset of them; therefore, the potential benefits of research that might be conducted will not be the total expected cost of uncertainty surrounding expected cost-effectiveness but some part of it. Earlier the potential benefits of different types of evidence were assessed. In the CLOP case study it was additional evidence about relative treatment effects that was most valuable and therefore experimental research may be required to provide more precise estimates of RR_death. The potential value of research that resolved uncertainty only about this relative treatment effect over a range of times to report is also represented in Figure 66. Although the potential value of research is lower at every time point, unless research is likely to take > 8 years the potential value is still likely to exceed the costs. Table 101 reports the potential benefits of research for groups of parameters by time to report.
| Time until research reports, years | Potential value of research, QALYs (£M) | ||||
|---|---|---|---|---|---|
| Natural history | Relative effects | Utilities | Costs | Scenario uncertainty | |
| Immediately | 369 (7.4) | 4504 (90.1) | 15 (0.3) | 547 (10.9) | 85 (1.7) |
| 1 | 326 (6.5) | 3981 (79.6) | 13 (0.3) | 483 (9.7) | 75 (1.5) |
| 2 | 285 (5.7) | 3475 (69.5) | 12 (0.2) | 422 (8.4) | 65 (1.3) |
| 3 | 245 (4.9) | 2987 (59.7) | 10 (0.2) | 363 (7.2) | 56 (1.1) |
| 4 | 206 (4.1) | 2515 (50.3) | 8 (0.2) | 305 (6.1) | 47 (0.9) |
| 5 | 169 (3.4) | 2059 (41.1) | 7 (0.1) | 250 (5.0) | 39 (0.8) |
| 6 | 133 (2.7) | 1618 (32.4) | 5 (0.1) | 197 (3.9) | 30 (0.6) |
| 7 | 98 (1.9) | 1193 (23.8) | 4 (0.1) | 145 (2.9) | 22 (0.5) |
| 8 | 64 (1.3) | 781 (15.6) | 3 (0.1) | 95 (1.9) | 15 (0.3) |
| 9 | 31 (0.6) | 384 (8) | 1 (0) | 47 (0.9) | 7 (0.1) |
What is the impact of other sources of uncertainty?
In Point 5: Will other sources of uncertainty resolve over time? the information that was publically available identified that the patent for CLOP was due to expire 7 years after the appraisal. Based on the OFT estimate that generic prices tend to be 25% of the original brand price,12 this other source of uncertainty that will resolve over time can be integrated quantitatively when estimating the potential value of research over the lifetime of the technology. In this case a significant fall in price in year 7 will substantially reduce the uncertainty surrounding 12 months of treatment with CLOP. Therefore, after year 7 there is less to be gained from resolving uncertainty and so the potential and value of research findings for patients who are incident after year 7 are thereby reduced. The effect of a price change on research that could potentially resolve all uncertainty (costs, natural history and utilities as well as relative effect) is also illustrated in Figure 66. The potential value of the research is lower whenever the research reports. This is because after the price reduction the value of research is lower, and calculations include the value to future as well as current patient populations. Nevertheless, even if research did not report until 7 years the potential value (676 QALY or £14M) is likely to exceed the costs. The impact of the time of introduction of the discount is analysed in Table 102. This shows that if both the price change and research reporting are immediate the value of research is 2552 QALY (or 51M).
| Time until research reports, years | Value of research for possible times of patent expiry, QALYs (£M) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No price change | 9 years | 8 years | 7 years | 6 years | 5 years | 4 years | 3 years | 2 years | 1 year | Immediate price change | |
| Immediately | 5194 (104) | 4969 (99) | 4736 (95) | 4495 (90) | 4245 (85) | 3986 (80) | 3719 (74) | 3442 (69) | 3156 (63) | 2859 (57) | 2552 (51) |
| 1 | 4591 (92) | 4366 (87) | 4132 (83) | 3891 (78) | 3641 (73) | 3383 (68) | 3116 (62) | 2839 (57) | 2552 (51) | 2256 (45) | 2256 (45) |
| 2 | 4008 (80) | 3783 (76) | 3549 (71) | 3308 (66) | 3058 (61) | 2800 (56) | 2533 (51) | 2256 (45) | 1969 (39) | 1969 (39) | 1969 (39) |
| 3 | 3444 (69) | 3219 (64) | 2986 (60) | 2745 (55) | 2495 (50) | 2237 (45) | 1969 (39) | 1692 (34) | 1692 (34) | 1692 (34) | 1692 (34) |
| 4 | 2900 (58) | 2675 (53) | 2442 (49) | 2201 (44) | 1951 (39) | 1692 (34) | 1425 (28) | 1425 (28) | 1425 (28) | 1425 (28) | 1425 (28) |
| 5 | 2374 (47) | 2149 (43) | 1916 (38) | 1675 (33) | 1425 (29) | 1167 (23) | 1167 (23) | 1167 (23) | 1167 (23) | 1167 (23) | 1167 (23) |
| 6 | 1866 (37) | 1641 (33) | 1408 (28) | 1167 (23) | 917 (18) | 917 (18) | 917 (18) | 917 (18) | 917 (18) | 917 (18) | 917 (18) |
| 7 | 1375 (28) | 1150 (23) | 917 (18) | 676 (14) | 676 (14) | 676 (14) | 676 (14) | 676 (14) | 676 (14) | 676 (14) | 676 (14) |
| 8 | 901 (18) | 676 (14) | 443 (9) | 443 (9) | 443 (9) | 443 (9) | 443 (9) | 443 (9) | 443 (9) | 443 (9) | 443 (9) |
| 9 | 443 (9) | 218 (4) | 218 (4) | 218 (4) | 218 (4) | 218 (4) | 218 (4) | 218 (4) | 218 (4) | 218 (4) | 218 (4) |
There was some evidence of the possible entry of a new technology (comparator) in the indication described in the CLOP case study; however, there was limited information on its characteristics. Therefore, two alternative but somewhat extreme scenarios are illustrated in Figure 67. In scenario A the new technology enters at year 5 and makes CLOP entirely obsolete, that is, not cost-effective and not uncertain – equivalent to a shorter technology horizon of 5 years. At this point there is no value in the evidence generated by research about CLOP. l In these circumstances the potential value of research is likely to exceed its costs only if it reports quickly. In scenario B the new technology has similar NHEs to 12 months of treatment with CLOPm and the uncertainty surrounding its expected cost-effectiveness is also similar. Now research about CLOP has more potential value in the future because it will also help resolve some of the uncertainty in the choice between CLOP and the new technology for patients who become incident after that time.
FIGURE 67.
Potential value of research and other sources of uncertainty: CLOP case study. Assumes that a prince change will occur at year 7 because of patent expiry.
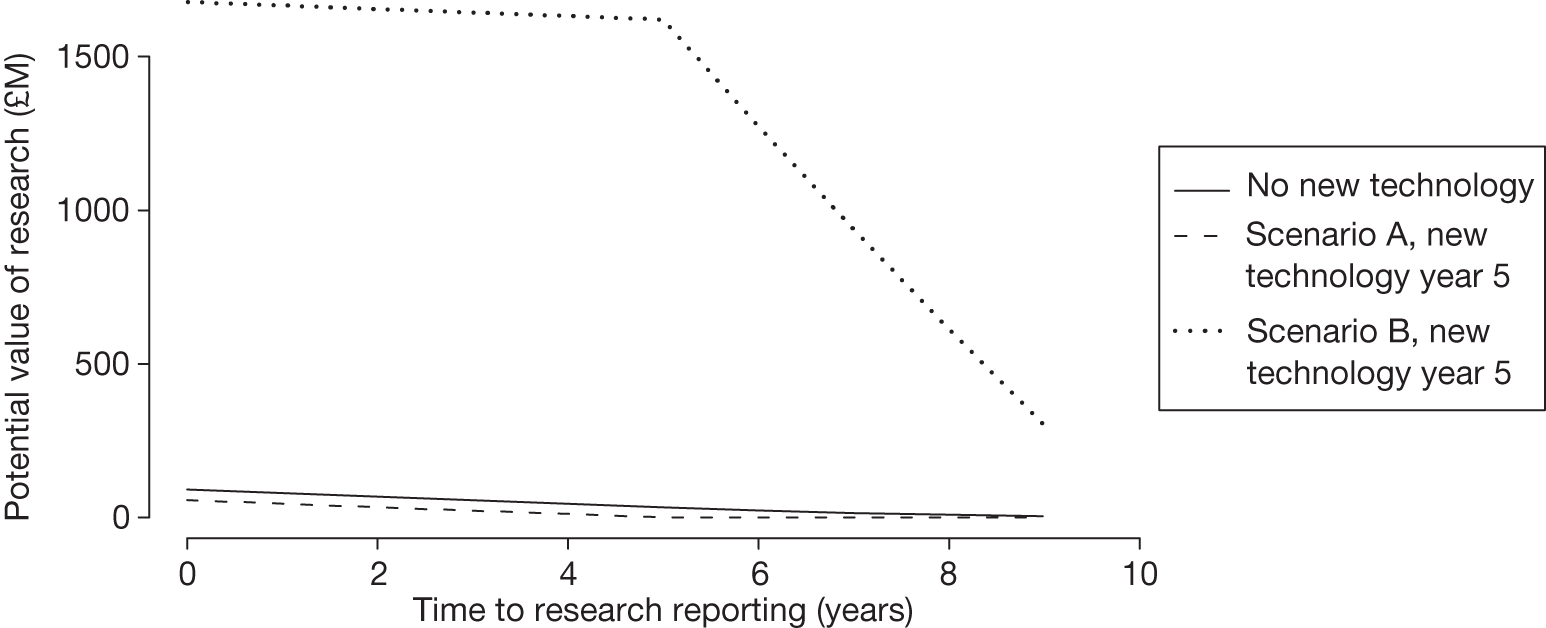
Tables 103 and 104 evaluate alternative times of introduction of the new technology in scenarios A and B, given a change in the price of CLOP at year 7 because of patent expiry.
| Time until research reports, years | Value of research for possible times of introduction of a new technology, QALYs (£M) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Not introduced | 9 years | 8 years | 7 years | 6 years | 5 years | 4 years | 3 years | 2 years | 1 year | Immediate introduction | |
| Immediately | 4495 (90) | 4277 (86) | 4052 (81) | 3819 (76) | 3328 (67) | 2820 (56) | 2294 (46) | 1750 (35) | 1186 (24) | 603 (12) | 0 (0) |
| 1 | 3891 (78) | 3674 (73) | 3448 (69) | 3215 (64) | 2725 (54) | 2216 (44) | 1691 (34) | 1146 (23) | 583 (12) | 0 (0) | 0 (0) |
| 2 | 3308 (66) | 3091 (62) | 2865 (57) | 2632 (53) | 2142 (43) | 1633 (33) | 1108 (22) | 563 (11) | 0 (0) | 0 (0) | 0 (0) |
| 3 | 2745 (55) | 2527 (51) | 2302 (46) | 2069 (41) | 1578 (32) | 1070 (21) | 544 (11) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 4 | 2201 (44) | 1983 (40) | 1758 (35) | 1525 (30) | 1034 (21) | 526 (11) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 5 | 1675 (33) | 1457 (29) | 1232 (25) | 999 (20) | 508 (10) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 6 | 1167 (23) | 949 (19) | 724 (14) | 491 (10) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 7 | 676 (14) | 458 (9) | 233 (5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 8 | 443 (9) | 225 (5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 9 | 218 (4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Time until research reports, years | Value of research for possible times of introduction of a new technology, QALYs (£M) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Not introduced | 9 years | 8 years | 7 years | 6 years | 5 years | 4 years | 3 years | 2 years | 1 year | Immediate introduction | |
| Immediately | 4495 (90) | 18,020 (360) | 32,018 (640) | 46,507 (930) | 61,253 (1225) | 76,515 (1530) | 92,311 (1846) | 108,660 (2173) | 127,024 (2541) | 146,030 (2921) | 165,701 (3314) |
| 1 | 3891 (78) | 17,416 (348) | 31,415 (628) | 45,904 (918) | 60,649 (1213) | 75,912 (1518) | 91,708 (1834) | 108,057 (2161) | 126,420 (2528) | 145,426 (2908) | 145,426 (2908) |
| 2 | 3308 (66) | 16,833 (337) | 30,832 (617) | 45,321 (906) | 60,066 (1201) | 75,328 (1507) | 91,125 (1822) | 107,474 (2149) | 125,837 (2517) | 125,837 (2517) | 125,837 (2517) |
| 3 | 2745 (55) | 16,270 (325) | 30,269 (605) | 44,757 (895) | 59,503 (1190) | 74,765 (1495) | 90,561 (1811) | 106,910 (2138) | 106,910 (2138) | 106,910 (2138) | 106,910 (2138) |
| 4 | 2201 (44) | 15,726 (314) | 29,724 (594) | 44,213 (884) | 58,959 (1179) | 74,221 (1484) | 90,017 (1800) | 90,017 (1800) | 90,017 (1800) | 90,017 (1800) | 90,017 (1800) |
| 5 | 1675 (33) | 15,200 (304) | 29,199 (584) | 43,687 (874) | 58,433 (1169) | 73,695 (1474) | 73,695 (1474) | 73,695 (1474) | 73,695 (1474) | 73,695 (1474) | 73,695 (1474) |
| 6 | 1167 (23) | 14,692 (294) | 28,690 (574) | 43,179 (864) | 57,925 (1159) | 57,925 (1159) | 57,925 (1159) | 57,925 (1159) | 57,925 (1159) | 57,925 (1159) | 57,925 (1159) |
| 7 | 676 (14) | 14,201 (284) | 28,200 (564) | 42,688 (854) | 42,688 (854) | 42,688 (854) | 42,688 (854) | 42,688 (854) | 42,688 (854) | 42,688 (854) | 42,688 (854) |
| 8 | 443 (9) | 13,968 (279) | 27,966 (5,59) | 27,966 (560) | 27,966 (560) | 27,966 (560) | 27,966 (560) | 27,966 (560) | 27,966 (560) | 27,966 (560) | 27,966 (560) |
| 9 | 218 (4) | 13,743 (275) | 13,743 (275) | 13,743 (275) | 13,743 (275) | 13,743 (275) | 13,743 (275) | 13,743 (275) | 13,743 (275) | 13,743 (275) | 13,743 (275) |
If the expected price reduction does not occur the potential value of research at each time point for both scenarios increases, for example for scenario B from 165,701 with a price change at year 7 to 174,519 QALYs without a price change when research is immediately available. n
The potential value of research presented in these figures, even after accounting for the type of evidence, follow-up and time until research reports, should still be regarded as an upper bound to the value that is likely to be realised by actual research for two reasons: (1) even well-designed research with large sample sizes will not fully resolve the uncertainty in the value that a parameter might take, especially in specific target populations and in a particular (future) context and (2) insofar as implementation of NICE guidance is not ‘perfect’ and all clinical practice might not immediately respond to the results of research, the full benefits will be realised only over time or with additional implementation efforts. For these reasons a judgement of whether benefits of research are likely to exceed the costs might be made conservatively, requiring evidence that, even in pessimistic scenarios, the research would still be worthwhile.
Types and categories of guidance resulting from points 5 and 6
The judgements made at points 5 and 6 of the checklist are critical because if the benefits of research are not judged to exceed the costs then no further assessment is required. In the CLOP case study, other sources of uncertainty will resolve over time, but the benefits of research still seem likely to be greater than the costs. Table 105 summarises the categories and types of guidance that could ultimately result from the sequence of assessments up to point 6.
| Assessment | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Guidance |
|---|---|---|---|---|---|---|---|---|
| 3 | Yes | No | Yes | No | Yes | Yes | Yes | Approve2 |
| 4 | Yes | No | Yes | No | Yes | Yes | No | OIR1 |
Are the benefits of approval greater than the costs?
Point 7: Are the benefits of approval greater than the costs?
The seventh and final point on the checklist requires an assessment and comparison of the benefits and costs of early approval. The costs of approval are not financial ones but opportunity costs and will include the potential value of any research that may be forgone as a consequence, for example if the research needed cannot be conducted once the technology is approved for use. They will also include any costs that are irrecoverably committed by approval. As well as the capital costs of equipment and facilities (or training and learning), they will also include the irrecoverable opportunity costs of initially negative NHEs (if treatment decisions are not irreversible – see the discussion in Point 1: Is it expected to be cost-effective?). A judgement of whether or not the benefits of approval and early access for current patients are likely to exceed the opportunity costs for future patients is required, that is, at the following point in the algorithm:
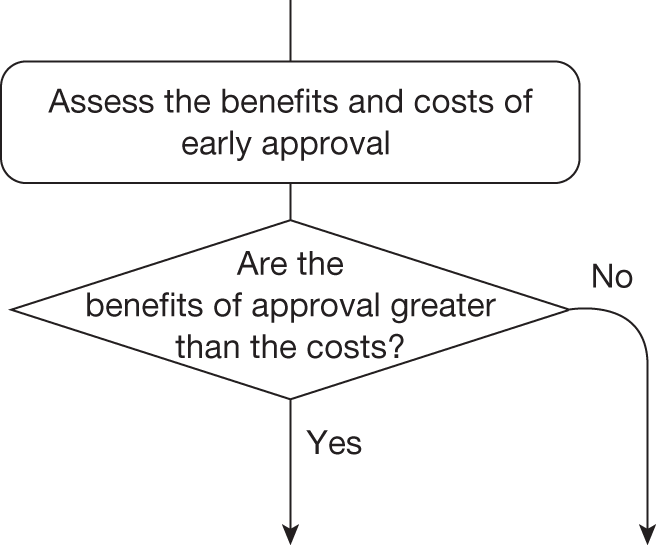
The decision at this point always leads directly to guidance, allocating all remaining possible pathways to a particular type and category of guidance.
An OIR recommendation is more likely to offer greater expected NHEs than approve if the research can be conducted quickly and report sooner, as fewer patients forgo access to CLOP and more can have treatment choice informed by the research findings. This is illustrated in Figure 68, which reports the difference between approve and OIRo in population NHEs over a range of times for when the research recommended in OIR might report. (Under OIR access to CLOP is not granted until research reports. In calculations we assumed that, before research reports, patients accrue an average NHE estimated using treatments other than CLOP. In averaging weights derived from the PSA results were used.) This takes account of both the expected changes in price at year 7 and research costs of £10M. It shows that OIR will be appropriate only if the research reports within 3 years of appraisal (T* = 3) because beyond this time the NHEs forgone by withholding access to CLOP will exceed the potential gains to future patients. p
FIGURE 68.
Population NHEs of approve and OIR by time to research reporting: CLOP case study. Assumes that a price change will occur at year 7 as a result of expiry of the patent.
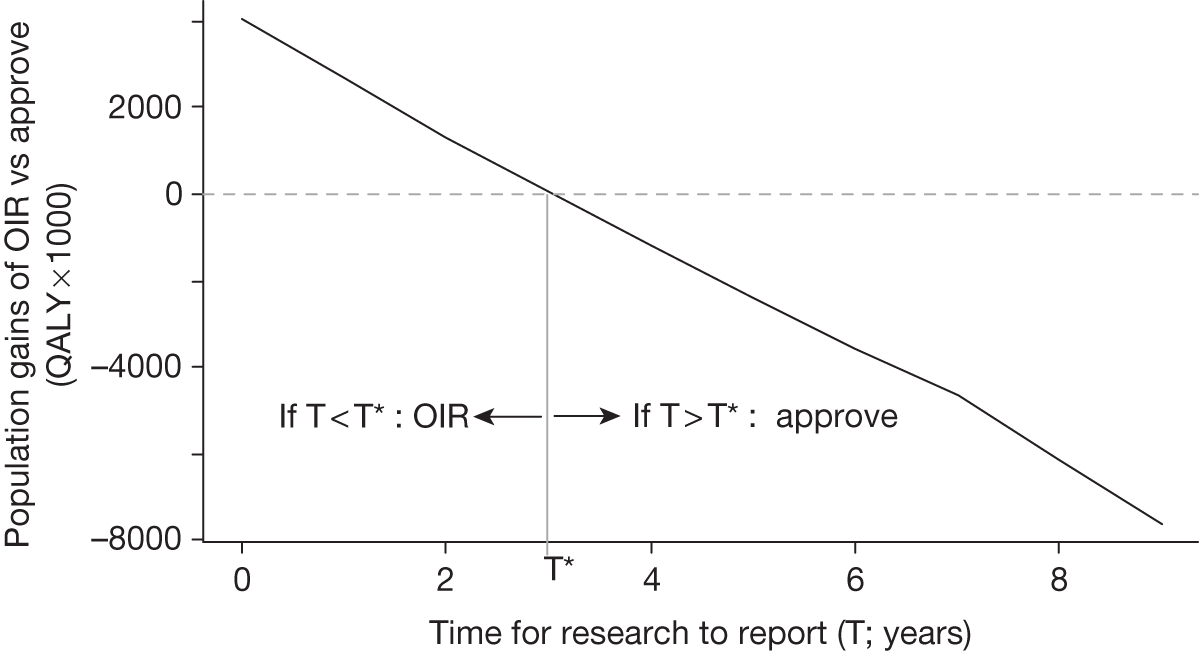
The trade-off between NHEs for current and future patients that lies behind Figure 68 is illustrated in Figure 69 using undiscounted values for ease of exposition. It illustrates the (per-period) population NHEs of approval and OIR if the research recommended as part of OIR reports at year 3. At this point, the initial losses of NHEs, caused by restricting access to CLOP (area A), start to be offset by the potential gains from the research findings (area B). The price change at year 7 increases the NHEs of approval (i.e. CLOP is more cost-effective) but on balance reduces the NHEs of OIR, that is, although CLOP is more cost-effective and offers greater NHEs the evidence generated by the research reporting is less valuable because the choice of treatment and duration is less uncertain. With research reporting at 3 years the initial losses of OIR (area A) are just offset by the later gains (area B) so T* = 3. If research reported earlier than 3 years (area A < area B) OIR would be appropriate but if research reported later than 3 years (area A > area B) approve would be more appropriate.
FIGURE 69.
Population NHEs of approve and OIR at T*: CLOP case study.
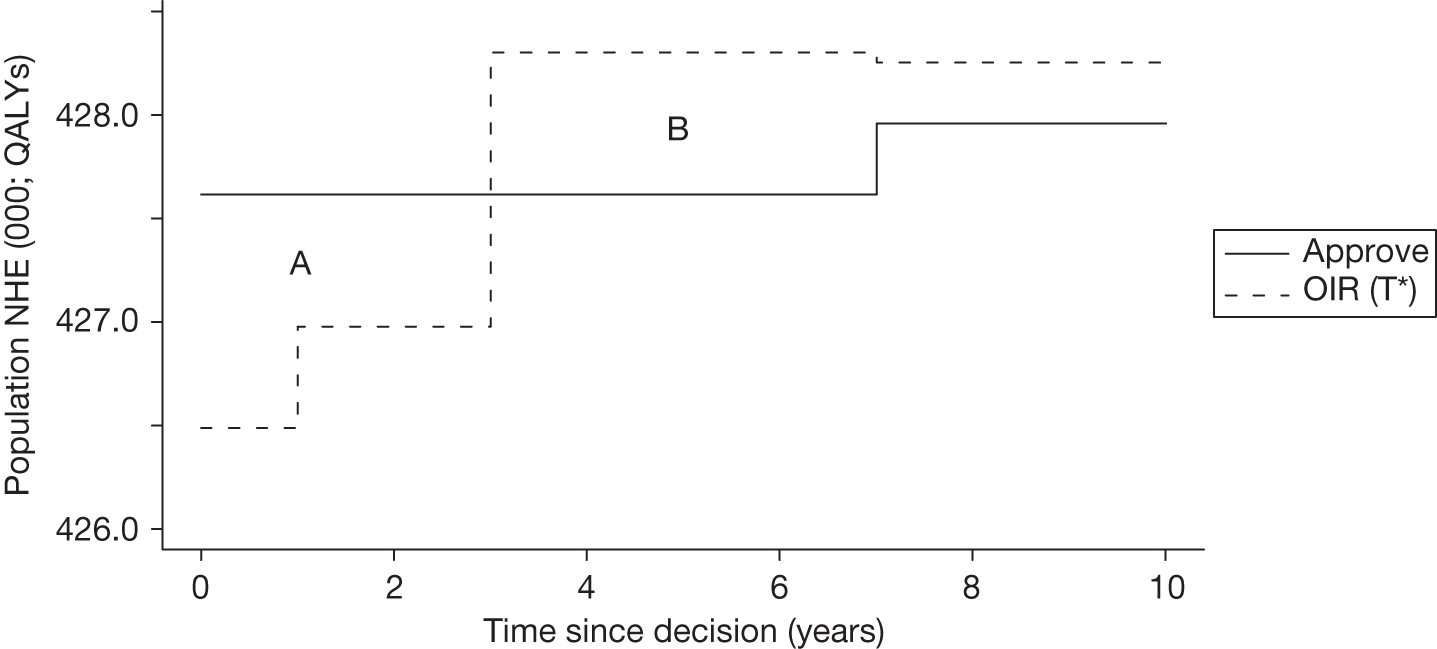
The investment profile of alternative policies on the use of CLOP to treat patients over 10 years is illustrated in Figure 70 in relation to rejecting CLOP. As expected, all regimens start by having initial losses (in OIR policies this is because of the initial investment in research); however, in policies in which research reports earlier these initial losses are quickly compensated for by gains obtained from better treatment decisions after research reports.
FIGURE 70.
Time profile of approve and OIR: CLOP case study.
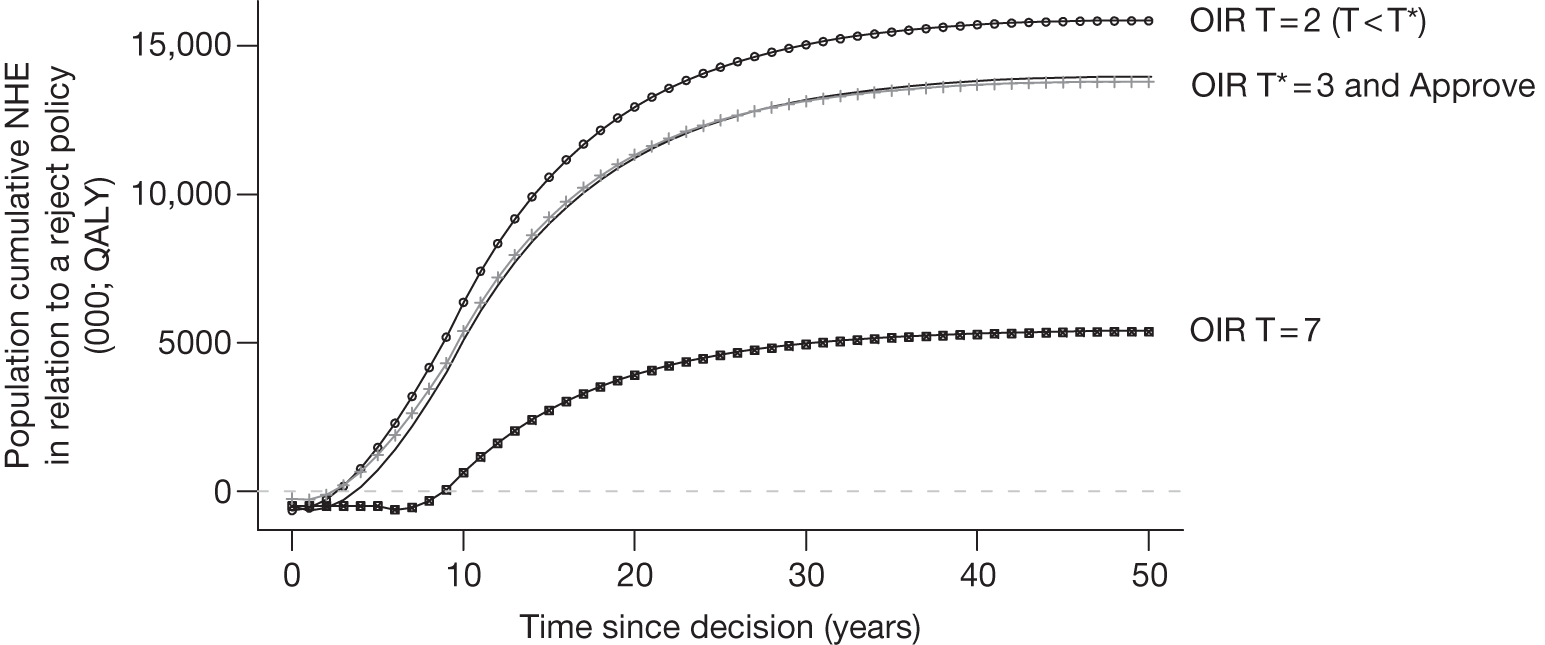
There is no guarantee that the research recommended as part of OIR guidance will be conducted by manufacturers or commissioned by research funders. Even if it is, it is not certain that it will be successfully completed (see discussion in Point 6: Are the benefits of research greater than the costs?). Therefore, the probability that research will report at a particular time also needs to be considered. The implications of considering whether the recommended research will be conducted and when it might report are illustrated in Figure 71, which presents a boundary for when OIR might be appropriate or when approval should be granted. For example, if research is certain to report but will take 4 years, or when it will only take 1 year but has only a 50% chance of reporting, then OIR would not be appropriate and 12-month treatment with CLOP should be approved, that is, these points fall to the north-east of this boundary. Points to the south-west of the boundary indicate that OIR might be appropriate.
FIGURE 71.
An OIR or approve boundary: CLOP case study.
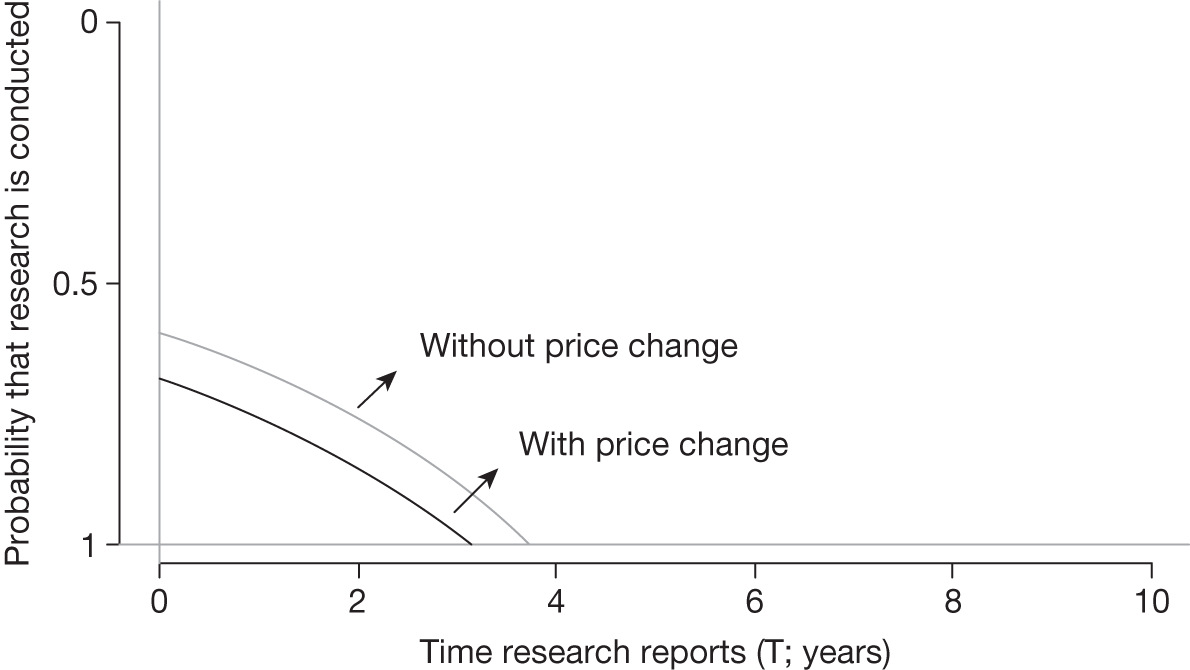
However, the estimates of the potential value of research on which these boundaries are based are still likely to overestimate the value that will be realised by research (see discussion in Point 6: Are the benefits of research greater than the costs?); they represent a necessary condition for OIR. Therefore, OIR guidance should require a conservative judgement that the point is almost certain to be below the boundary, rather than on balance close to it. For the same reason, points anywhere above the boundary represent a sufficient condition for approval. The boundary when the change in price is included is to the south-west, reflecting the lower potential value of research, and OIR guidance, once CLOP becomes more cost-effective (see Figure 71). In this case it seems unlikely that the type of research required could report quickly enough and with sufficient confidence that OIR would be appropriate. Therefore, these assessments would support a judgement that the benefits of approval are likely to exceed the opportunity costs, and ‘Approve2’ (pathway 3 for CLOP) would be more appropriate.
The assessments that have been undertaken for CLOP can be brought together to consider (1) what would be the value of being able to conduct research while CLOP is approved and (2) what would be the value of making evidence that is needed by the NHS available at launch. These questions can be informed by the results already presented elsewhere but also reported in Table 106.
| Approve | OIR | AWR* | Reject | Value of AWR | Uncertainty resolved at launch | Value of evidence at launch | |
|---|---|---|---|---|---|---|---|
| NHEs in QALYs | |||||||
| T < T* (T = 2) | 3,680,187 | 3,681,480 | 3,682,995 | 3,671,660 | 1515 | 3,684,181 | 2701 |
| T > T* (T = 7) | 3,680,187 | 3,675,487 | 3,680,362 | 3,671,660 | 175 | 3,684,181 | 3994 |
| NHEs in £M | |||||||
| T < T* (T = 2) | 73,604 | 73,630 | 73,660 | 73,433 | 30 | 73,684 | 54 |
| T > T* (T = 7) | 73,604 | 73,510 | 73,607 | 73,433 | 4 | 73,684 | 80 |
The difference in population NHEs between AWR (if it had been possible) and the next best feasible policy (i.e. OIR for T < T* = £30M and approve T > T* = £4M) represents the value to the NHS of being able to conduct research while CLOP is approved for use, for example informing whether investment in better data collection, registries or information systems might make this possible. The difference in population NHEs between the case when all uncertainty had been resolved before appraisal (at launch) and the next best available policy (i.e. OIR for T < T* = £54M and approve for T > T* = £80M) represents the value to the NHS of having access to the evidence needed at launch. This can inform policies that might make better and more relevant evidence available.
Types and categories of guidance resulting from point 7
The decision at this point always leads directly to guidance, allocating all remaining possible pathways to a particular type and category of guidance. In CLOP, research was not considered possible with approval (i.e. ‘Approve2’ or ‘OIR1’). It is research that would provide more precise estimates of the relative effects of CLOP and of shorter treatment durations that is potentially valuable (see Point 4: Is research possible with approval?). As a consequence, the type of experimental design that is likely to be needed is unlikely to be possible if 12 months of treatment with CLOP is already approved for widespread NHS use. Although treatment with CLOP does commit initially negative NHEs that are irrecoverable, these should not be regarded as significant as the treatment decision for a presenting patient is irreversible in relevant time frames (see Point 2: Are there significant irrecoverable costs?). Therefore, AWR is not considered possible and so the benefits of early access to 12 months of treatment with CLOP (approval) must be compared with the potential value of OIR. The analysis of point 7 indicates that if research reports earlier than 3 years OIR would be appropriate; otherwise, approve would be more appropriate.
Appendix 9 Omalizumab for the treatment of severe persistent allergic asthma in children aged 6–11 years
Introduction
The use of OMAL for the treatment of severe persistent allergic asthma in children aged 6–11 years was not recommended by NICE following a STA appraisal (TA201124 in 2010). The analysis compared OMAL as an add-on to standard therapy with standard therapy alone. The primary analysis was based on a prespecified severe asthma population within an international, multicentre, placebo-controlled RCT;186 however, a high-risk subgroup within this population (recent hospitalisation for an asthma exacerbation) was also identified post hoc.
Omalizumab was not found to be cost-effective in either the severe or the severe/high-risk population. However, a RCT was recommended in section 6 of TA201124 comparing OMAL with OCS in children to establish reduction in OCS use. The OMAL case study is used to demonstrate how the key principles and assessments in section 5 could inform the development of guidance for the use of OMAL. The following sections include a background to OMAL and a detailed examination of the sequence of assessments and decisions that lead to a particular category and type of guidance for OMAL. The analysis is from the standpoint of TA201; therefore, the case study can be used to see what the decision would have been if the checklist had been used.
Background to the case study
Asthma affects approximately 1.1 million children in the UK187 and within this group there is a small but very significant number of children with severe symptoms whose asthma control remains poor despite best available therapy. 188 These children receive frequent or maintenance doses of OCS together with other controller medications. Clinical guidelines specify that treatment should aim to control asthma using the lowest possible OCS dose and, if possible, stop OCS treatment completely. 189
The scope for the STA appraisal was the clinical effectiveness and cost-effectiveness of OMAL, within its licensed indication, for the treatment of severe persistent allergic asthma in children aged 6–11 years. OMAL is licensed as an add-on to existing therapy in patients aged 6 to < 12 years with severe, persistent allergic immunoglobulin E (IgE)-mediated asthma whose condition remains uncontrolled despite best standard care with high-dose inhaled corticosteroids (ICS) and long-acting beta agonists. The independent ERG190 critiqued the evidence submitted by the manufacturer on the clinical effectiveness and cost-effectiveness of OMAL.
Intervention and population
The population of interest is children aged 6–11 years with severe persistent allergic asthma. Within this population, a high-risk subgroup is also identified, defined by a recent (within the previous year) hospitalisation for an asthma exacerbation. A post hoc subgroup analysis was conducted as part of the STA submission by using a subpopulation of patients in the main IA-05 trial. 186 Both populations are considered in the case study analysis below. It is estimated that there is an annual incidence of 307 children in the UK with severe persistent allergic asthma who remain uncontrolled despite best available therapy and who would meet the criteria for therapy with OMAL. 188
Omalizumab is administered by subcutaneous injection every 4 or 2 weeks. The permitted dosage depends on body weight and baseline levels of IgE. Evidence of treatment effectiveness must be observed at 16 weeks for treatment to be continued. Treatment duration on OMAL is assumed to be 10 years. 188 OMAL is compared as an add-on to standard therapy with standard therapy alone. Lifetime treatment with standard therapy is considered. The analysis is conducted from the perspective of the NHS and PSS and costs and outcomes are discounted at a rate of 3.5% per annum. 1
Evidence on clinical effectiveness
The evidence on clinical effectiveness is primarily based on the results of an international, multicentre, placebo-controlled RCT in children aged 6–11 years with allergic asthma – study IA-05. 186 A subpopulation with more severe asthma was specified prospectively – referred to as IA-05 EUP. The primary analysis was conducted in the IA-05 EUP population. A high-risk subgroup (defined by a recent hospitalisation for an asthma exacerbation) was also specified and a post hoc analysis of efficacy used – referred to as the EUP hospitalisation subgroup. OMAL treatment was associated with a statistically significant reduction in the rate of clinically significant exacerbations but not clinically significant severe exacerbations. 188 OMAL use has been demonstrated to have only numerically small but statistically insignificant reductions in ICS use. There is limited evidence of a reduction in OCS use with OMAL.
Decision model
The manufacturer’s STA submission included a Markov decision-analytic model. The model comprised six health states: (1) day-to-day symptoms with OMAL, (2) day-to-day symptoms with standard therapy, (3) clinically significant exacerbation, (4) clinically significant severe exacerbation, (5) asthma-related death, and (6) death from all causes. The model has been described in detail elsewhere. 188,191 Patients start in the appropriate day-to-day symptoms state based on treatment received. Treatment effectiveness was based on reduction in the rate of exacerbations and quality of life as a result of day-to-day symptoms. Movement between states was derived from the IA-05 EUP population186 and three other studies. 192–194 Adverse events were not included in the model. Table 107 summarises the key parameters and sources used in the decision model.
| Parameter | Mean value | Distribution | Source |
|---|---|---|---|
| Treatment effectiveness | |||
| Exacerbation rate per person for initial 24 weeks | |||
| Standard therapy | 1.939 | Log-normal | IA-05 EUP data186 |
| OMAL | 1.363 | Log-normal | IA-05 EUP data186 |
| Exacerbation rates per year per person for OMAL | 0.519 | Log-normal | IA-05 EUP data186 |
| Percentage of exacerbations that are severe, OMAL | 27.30% | Beta | IA-05 EUP data186 |
| Exacerbation rates per year per person for standard therapy | 2.028 | Log-normal | IA-05 EUP data186 |
| Percentage of exacerbations that are severe, standard therapy | 22.90% | Beta | IA-05 EUP data186 |
| Proportion of OMAL responders | 74.20% | Beta | IA-05 EUP data186 |
| Relative risk for exacerbations, OMAL responders | 0.256 | Log-normal | IA-05 EUP data186 |
| Relative risk for exacerbations, non-responders (same as standard therapy) | 1 | Fixed | IA-05 EUP data186 |
| Exacerbation-related deaths by age (same for OMAL and standard therapy) | |||
| 0–11 years | 0.09% | Beta | Watson et al. 2007193 |
| 12–16 years | 0.32% | Beta | Watson et al. 2007193 |
| 17–44 years | 0.38% | Beta | Watson et al. 2007193 |
| 45+ years | 2.47% | Beta | Watson et al. 2007193 |
| HRQoL | |||
| Utility for standard therapy and non-responders all ages | 0.669 | Beta | Humbert et al. 2005192 |
| Utility for OMAL responders aged < 12 years | 0.669 | Beta | Humbert et al. 2005192 |
| Utility for OMAL responders aged 12+ years | 0.779 | Beta | Humbert et al. 2005192 |
| Utility for clinically significant exacerbation | 0.572 | Beta | Lloyd et al. 2007194 |
| Utility for clinically significant severe exacerbation | 0.326 | Beta | Lloyd et al. 2007194 |
| Resource use and unit costs | |||
| OMAL and standard therapy | See STA submission188 | Fixed | – |
Lifetime costs and QALYs were determined by comparing lifetime standard therapy costs with costs of 10 years of OMAL add-on therapy followed by standard therapy. Only patients who were judged by physicians to have responded to OMAL after 16 weeks of therapy continued with treatment; non-responders to OMAL reverted to standard therapy at 16 weeks. The 28-week exacerbation rates (clinically significant and clinically significant severe) from IA-05 EUP were annualised for standard therapy and OMAL responders. 186 Death because of asthma was based on the probability reported by Watson et al. 193 and varied by age.
Most inputs in the model were assumed to be uncertain and the model was run probabilistically using Monte Carlo simulation (1000 simulations). Expected cost-effectiveness was determined using these probabilistic results as established within the NICE appraisal process. a The use of probabilistic analysis also allows uncertainty over model parameters to be translated into uncertainty in the overall results.
Key features and possible pathways
Omalizumab was not found to be cost-effective in either the severe or the severe/high-risk population. However, a RCT was recommended comparing OMAL with OCS in children to establish reduction in OCS use. This was made in section 6 of TA201;124 therefore, OMAL is not an example of OIR at FAD but an example of OIR considered during appraisal. OMAL provides some interesting characteristics, which can be used to explore the implications of the principles and assessments outlined in Chapter 5:
-
small patient population (approximately 300 children per annum) – rare condition
-
consideration of subgroup analysis (inclusion of a high-risk hospitalisation subgroup)
-
NICE decision to reject
-
RCT recommended comparing OMAL with OCS in children to establish reduction in use of OCS in the FAD (but not an OIR decision).
The possible pathways through the algorithm that OMAL illustrates are reported in Figure 31 in Appendix 4. OMAL is not expected to be cost-effective and there are no significant irrecoverable costs. The following sections examine the points on the checklist relating to the possible sequence of assessments and decisions that lead to a particular category and type of guidance for OMAL.
Is it cost-effective and what are the risks?
Point 1: Is it expected to be cost-effective?
The sequence of assessments starts with cost-effectiveness and the expected impact on population NHEs, that is, at the following point in the algorithm:
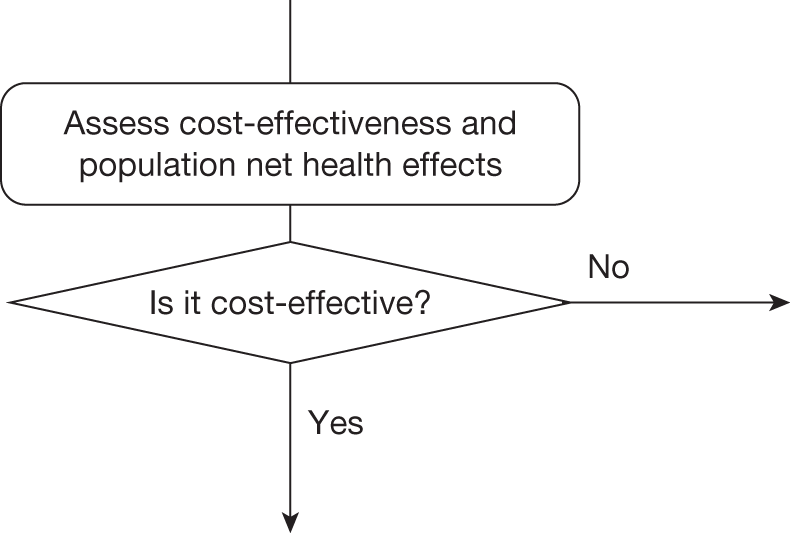
Cost-effectiveness at the patient level
Table 108 summarises the expected cost-effectiveness of OMAL per patient treated. OMAL as an adjunct to standard therapy is compared with standard therapy alone. The expected incremental differences in lifetime costs and QALYs of OMAL relative to standard therapy are £55,682 and 0.5933 QALYs, respectively, giving an ICER of £93,844. The ICER for OMAL is greater than the threshold of £20,000 per QALY;b therefore, OMAL is not expected to be cost-effective compared with standard therapy alone. Consequently, the incremental NHE of OMAL is negative.
| Treatment | Cost (£) | QALYs | ICER (£/QALY) | Cost-effectiveness threshold £20,000 per QALY | Cost-effectiveness threshold £30,000 per QALY | ||
|---|---|---|---|---|---|---|---|
| NHE, QALYs (£) | Incremental NHE, QALYs (£) | NHE, QALYs (£) | Incremental NHE, QALYs (£) | ||||
| OMAL + standard care | 94,992 | 16.64 | 93,844 | 11.8861 (237,721) | –2.1908 (–43,815) | 13.4693 (404,078) | –1.2627 (–37,882) |
| Standard care | 39,310 | 16.04 | – | 14.0768 (281,536) | – | 14.7320 (441,960) | – |
The per-patient investment profile for OMAL is illustrated in Figure 72 and shows that it is always expected to offer negative NHEs compared with standard care over the entire patient time horizon of 90 years, that is, the high costs of treatment are never compensated by future health gains. In this example, the initial treatment costs with OMAL continue for 10 years (10 years is assumed to represent the duration for which a patient would continue to receive treatment with OMAL) with health effects predominately obtained while on treatment. OMAL does not represent a ‘risky purchase’ but one that is simply not cost-effective at its current price.
FIGURE 72.
Cumulative incremental NHEs of OMAL over the patient time horizon.
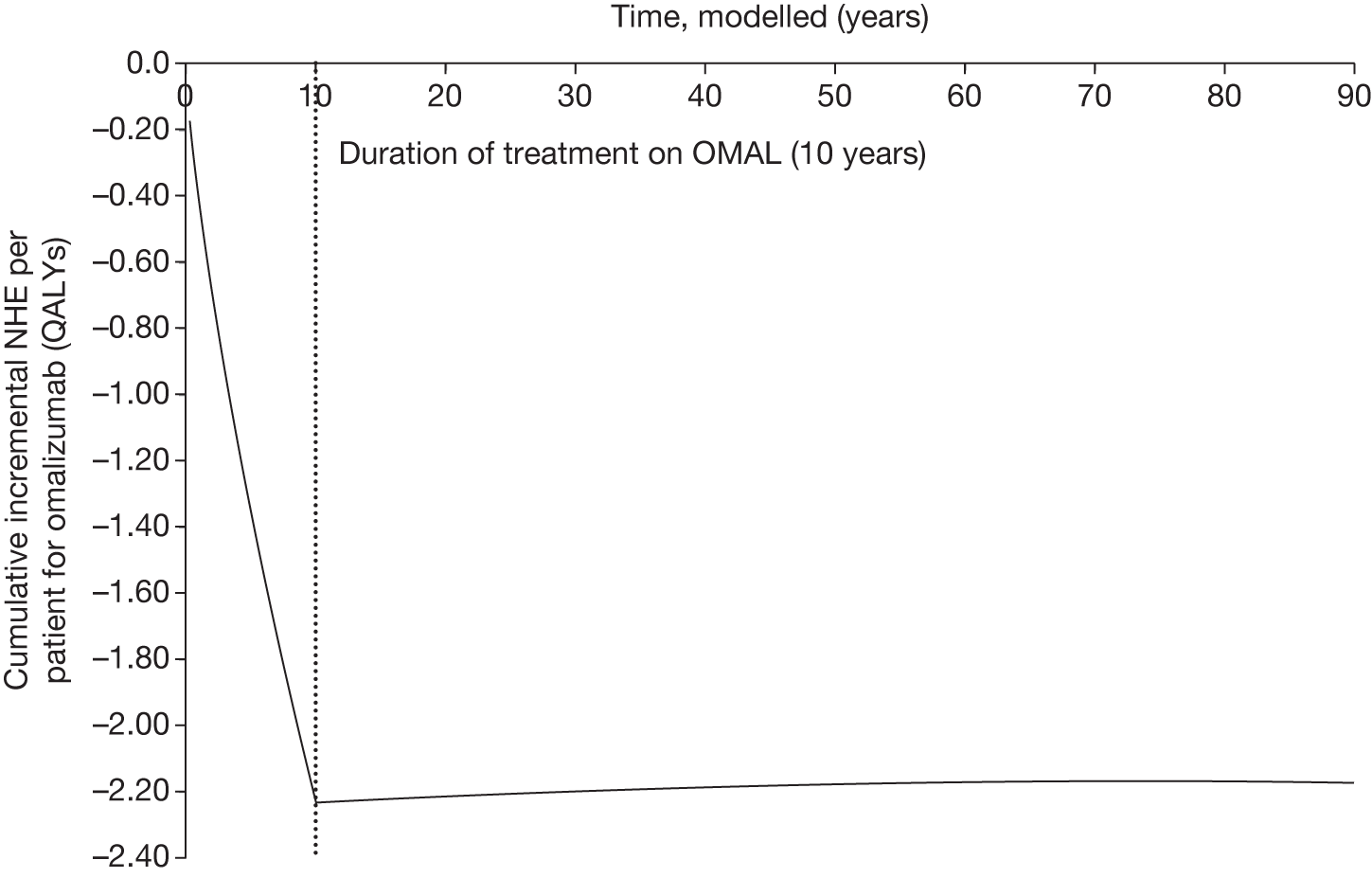
Cost-effectiveness at the population level
Per-patient NHEs can also be expressed for the population of current and future patients. This requires information about the prevalence and future incidence of the target population. It also requires a judgement about the time horizon over which the technology will be used. It is estimated that there are only 307 children in the UK per year with severe persistent allergic asthma who remain uncontrolled despite best available therapy and who would meet the criteria for therapy with OMAL. 188 As part of the budget impact assessment included with the manufacturer’s STA submission, the expected uptake rate of OMAL in the first 5 years is assumed to be < 307 patients per year (Table 109). Market uptake is assumed to be 3.9% in the first year then 11.7%, 23.1%, 30.6% and 32.6% for years 2–5, respectively. This results in a patient pool of only 12 patients in year 1 rising to 100 patients in year 5. Table 110 shows the total population NHE assuming that OMAL will be used over 10 years for (1) the estimated patient population in Table 109 for the first 5 years and an incident population of 307 patients per year thereafter and (2) an incident population of 307 patients per year over the 10-year period. The expected cost-effectiveness is unchanged (ICER is the same as in Table 108) but the incremental NHE is more negative and significant at a population level. Clearly, as more patients are treated OMAL appears even less cost-effective as the high costs of treatment are never compensated by future health gains.
| Year | Expected uptake rate (%) | Expected population starting on OMAL | Expected population on standard therapy |
|---|---|---|---|
| 2010 | 3.9 | 12 | 295 |
| 2011 | 11.7 | 36 | 271 |
| 2012 | 23.1 | 71 | 236 |
| 2013 | 30.6 | 94 | 213 |
| 2014 | 32.6 | 100 | 207 |
| Treatment | Costs (£M) | QALYs | ICER (£/QALY) | Cost-effectiveness threshold £20,000 per QALY | Cost-effectiveness threshold £30,000 per QALY | ||
|---|---|---|---|---|---|---|---|
| NHE, QALYs (£M) | Incremental NHE, QALYs (£M) | NHE, QALYs (£M) | Incremental NHE, QALYs (£M) | ||||
| Based on expected uptake rate | |||||||
| OMAL + standard care | 142 | 24,836 | 93,844 | 17,745 (355) | –3271 (–65) | 20,108 (603) | –1885 (–57) |
| Standard care | 59 | 23,950 | – | 21,015 (420) | – | 21,994 (660) | – |
| Based on incidence of 307 patients per year | |||||||
| OMAL + standard care | 251 | 43,961 | 93,844 | 31,410 (628) | –5789 (–116) | 35,593 (1,068) | –3337 (–100) |
| Standard care | 104 | 42,393 | – | 37,199 (744) | – | 38,930 (1,168) | – |
The investment profile for OMAL when used to treat patients over 10 years is illustrated in Figure 73. At a population level there are large losses that are never compensated for by later health gains. Unlike the other case studies, OMAL does not appear a more risky investment when evaluated at a population level; it is simply not cost-effective and never breaks even with standard therapy.
FIGURE 73.
Cumulative incremental NHEs of OMAL for the population.
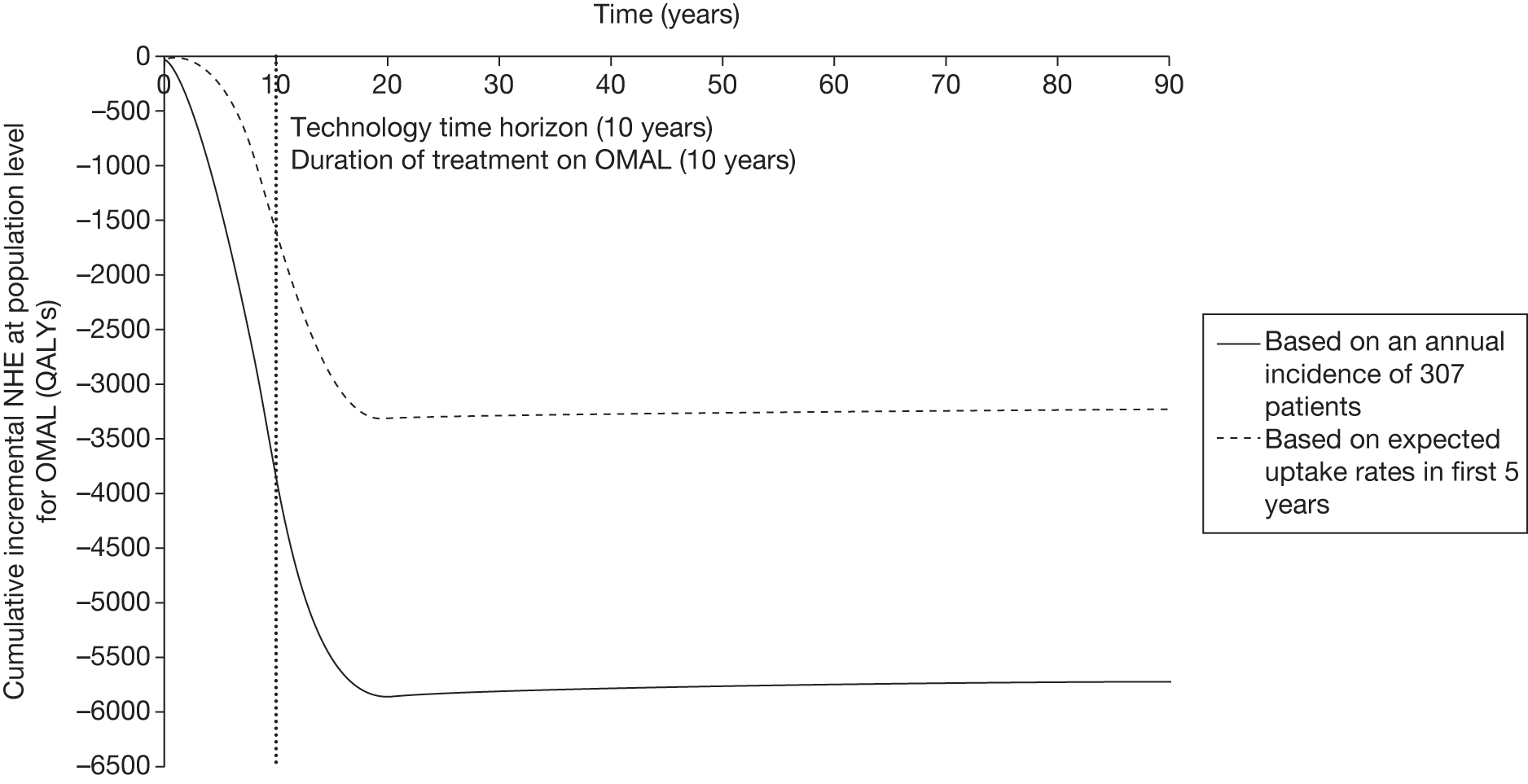
The time horizon of 10 years was chosen as a proxy for a complex and uncertain process of future changes in new technologies, prices and evidence. 71 The impact of different technology time horizons on NHEs is illustrated in Table 111. The incremental NHE becomes more negative when the technology time horizon is increased to 20 years. For a shorter duration of 5 years the losses are smaller but still amount to 3143 QALYs for a population of 307 patients per annum.
| Technology time horizon, years | Incremental NHE, QALYs (£M) | |
|---|---|---|
| Annual incidence of 307 patients | Expected uptake rate in first 5 years | |
| 5 | –3143 (–63) | –624 (–12) |
| 10 | –5789 (–116) | –3271 (–65) |
| 15 | –8017 (–160) | –5499 (–110) |
| 20 | –9893 (–198) | –7375 (–147) |
Point 2: Are there significant irrecoverable costs?
The second point on the checklist requires (1) an assessment of whether or not there are irrecoverable costs and (2) a judgement of their potential significance, that is, at the following point in the algorithm:
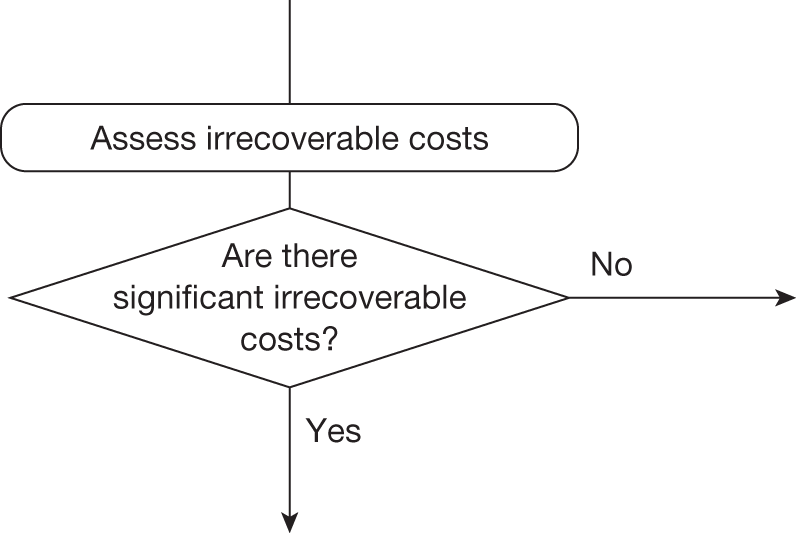
Identifying any significant irrecoverable costs
Irrecoverable costs are those that, once committed, cannot be recovered if guidance is changed at a later date. In the case of OMAL there are no capital investment costs associated with equipment or facilities. Drug costs associated with treatment occur annually per patient treated. In the absence of capital investment costs, NHEs accumulate over time at both a patient and a population level. For OMAL the profile of NHEs at a patient level (see Figure 72) exhibits negative NHEs in all time periods. Assessment at a population level and for longer technology time horizons simply increases the magnitude of the expected negative NHEs. Therefore, there are no irrecoverable costs associated with OMAL.
Types and categories of guidance resulting from points 1 and 2
Points 1 and 2 of the checklist do not lead directly to a category or type of guidance. The sequence of assessments and decisions, which ultimately lead to guidance, starts with cost-effectiveness, expected impact on population NHEs and significance of irrecoverable costs. In the case of OMAL, the technology is not expected to be cost-effective and there are no significant irrecoverable costs. Table 112 summarises the categories and types of guidance that could ultimately result from the sequence of assessments up to point 2 (see Table 32 in Appendix 4).
| Assessment | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Guidance |
|---|---|---|---|---|---|---|---|---|
| 7 | No | No | Yes | Yes | Yes/no | Yes | – | OIR2 |
| 8 | No | No | Yes | Yes | Yes/no | No | – | Reject1 |
| 9 | No | No | Yes | No | Yes/no | Yes | Yes | AWR2 |
| 10 | No | No | Yes | No | Yes/no | Yes | No | Reject2 |
| 11 | No | No | Yes | No | Yes/no | No | – | Reject3 |
| 12 | No | No | No | – | – | – | – | Reject4 |
Is further research required?
Point 3: Does more research seem worthwhile?
The third point on the checklist requires an assessment of the potential benefits of conducting further research, that is, at the following point in the algorithm:
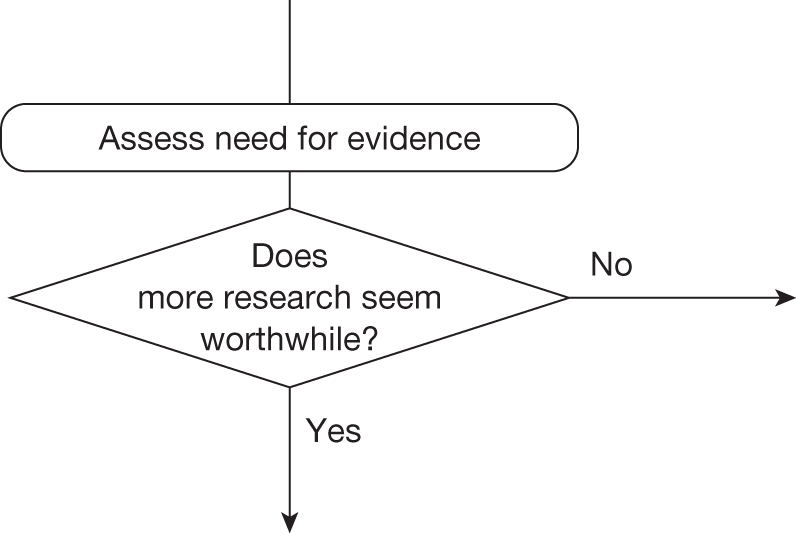
This requires judgements about (1) how uncertain a decision to approve or reject OMAL might be based on the estimates of expected cost-effectiveness and (2) whether or not the scale of the likely consequences of this uncertainty might justify further research. If the potential benefits of further research are unlikely to justify the costs, a judgement that more research does not seem worthwhile will lead directly to guidance.
Assessing the consequences of uncertainty
Omalizumab is not expected to be cost-effective compared with standard therapy but the estimates of costs and QALYs are uncertain so there is a chance that a decision to approve standard therapy rather than OMAL based on existing evidence will be incorrect, that is, OMAL might offer greater NHEs. Some assessment of the likely consequences of approving standard therapy when OMAL might be better could be based on the difference in expected NHEs, that is, the expected incremental population NHEs reported in Table 110. The simplest approach would be to weight the average NHEs for OMAL and standard therapy (reported in Table 110) by a judgement of the probability of an incorrect decision. For example, if the decision was judged to be 100% certain then there are no consequences and so there would be nothing to be gained by more research; however, as the probability that the decision is correct becomes less certain, the expected consequences (and hence potential value of more research) increase. Table 113 shows the expected consequences of uncertainty based on a weighting of average NHEs. c
| Probability decision is correct, p | Cost-effectiveness threshold £20,000 per QALY | Cost-effectiveness threshold £30,000 per QALY |
|---|---|---|
| Expected consequences, QALYs (£M) | Expected consequences, QALYs (£M) | |
| 1.00 | 0 (0) | 0 (0) |
| 0.99 | 58 (1) | 33 (1) |
| 0.95 | 289 (6) | 167 (5) |
| 0.90 | 579 (12) | 334 (10) |
| 0.75 | 1447 (29) | 834 (25) |
| 0.50 | 2895 (58) | 1668 (50) |
| 0.25 | 4342 (87) | 2503 (75) |
| 0.10 | 5210 (104) | 3003 (90) |
| 0.05 | 5500 (110) | 3170 (95) |
| 0.01 | 5731 (115) | 3303 (99) |
| 0.00 | 5789 (116) | 3337 (100) |
A judgement of how uncertain a decision might be can be informed by the PSA already used to estimate costs and QALYs. The probability that standard therapy is cost-effective rather than OMAL is 1.00 at both £20,000 and £30,000 per QALY (Table 114). An estimate of the expected consequences of uncertainty should be based on the distribution of uncertainty from the PSA rather than on a simple estimate of average NHEs. Table 114 shows that the expected consequences of uncertainty are 0.00; therefore, at the upper and lower bounds for the range that NICE has adopted for the threshold there is insufficient uncertainty to suggest that OMAL might be cost-effective.
| Treatment | ICER (£/QALY) | Cost-effectiveness threshold £20,000 per QALY | Cost-effectiveness threshold £30,000 per QALY | ||||
|---|---|---|---|---|---|---|---|
| Incremental NHE, QALYs (£M) | Probability cost-effective | Expected consequences, QALYs (£M) | Incremental NHE, QALYs (£M) | Probability cost-effective | Expected consequences, QALYs (£M) | ||
| OMAL + standard care | 93,844 | –5789 (–116) | 0.000 | 0.000 (0) | –3337 (–100) | 0.000 | 0.000 (0) |
| Standard care | – | 1.000 | – | 1.000 | |||
The expected consequences provide an upper bound on the benefits of more research. Because the frequency of error is zero, there is no uncertainty. Therefore, a decision to reject OMAL is not uncertain; there are no consequences of uncertainty and nothing to be gained by more research.
Alternative scenarios
The uncertainty described above reflects uncertainty within the set of assumptions used to estimate expected costs and QALYs. However, there are often alternative views about the assumptions, which are usually presented as separate scenarios. For OMAL, the STA submission considered scenarios that would generate the most favourable ICERs but still plausibly reflect the benefits seen by paediatric asthma specialists in clinical practice. The scenarios included (1) a longer treatment duration, increased from 10 years to 20 years, (2) utility benefits in day-to-day symptoms for all age groups (the previous base-case analysis assumed a utility benefit for ages > 12 years only) and (3) an increase in mortality rate owing to clinically significant severe exacerbations of 100%. These scenarios were incorporated additively, that is, each scenario was considered in addition to the previous one. Table 115 presents the cost-effectiveness of OMAL and the expected consequences of uncertainty for the alternative scenarios. Despite a fall in the ICER for OMAL from £93,844 to £62,049 with the addition of the three favourable assumptions, a decision to reject OMAL does not appear uncertain; the probability that OMAL is cost-effective is zero and there are no consequences of uncertainty or value to be gained by more research.
| Additive scenario | ICER (£/QALY) | Incremental NHE, QALYs (£M) | Probability cost-effective | Expected consequences, QALYs (£M) |
|---|---|---|---|---|
| Base case | 93,844 | –5789 (–116) | 0.0000 | 0.0000 (0) |
| + 20-year treatment duration | 78,324 | –9198 (–184) | 0.0000 | 0.0000 (0) |
| + utility difference in day-to-day symptoms for all ages | 67,525 | –8721 (–174) | 0.0000 | 0.0000 (0) |
| + mortality rate owing to clinically significant severe exacerbations increased by 100% | 62,049 | –8377 (–168) | 0.0000 | 0.0000 (0) |
Types and categories of guidance resulting from point 3
Point 3 of the checklist is critical because if research is not judged to be worthwhile no further assessments are required. For OMAL, the ICER is substantially greater than the threshold and the NHE is always negative. A decision to reject OMAL is not uncertain and there are no consequences of uncertainty and nothing to be gained by additional research. Table 116 summarises the category and type of guidance for OMAL.
| Assessment | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Guidance |
|---|---|---|---|---|---|---|---|---|
| 12 | No | No | No | – | – | – | – | Reject4 |
Analysis of subgroups
Subgroups, once credibly defined, need to be considered in the same way as the base-case population. This involves starting at point 1 of the checklist, that is, entering at the top of the algorithm, and working through the sequence of assessments from point 1 to point 7 in the same way as for the primary base-case analysis. This can be used to identify whether or not different guidance should be arrived at for the subgroup population. It will also identify whether or not additional research is required in the specific patient subgroup that may not otherwise have been conducted for the base-case population.
For OMAL a high-risk subgroup was identified from within the base-case population. This was defined by a recent (within the previous year) hospitalisation for an asthma exacerbation. A post hoc subgroup analysis was conducted as part of the STA submission by using a subpopulation of patients in the main IA-05 trial. 186 Guidance for this subgroup is considered using the same assessments as for the base-case population. Consideration is given below to each of the checklist points.
Point 1: Is it expected to be cost-effective?
Cost-effectiveness at the patient level
Table 117 summarises the expected cost-effectiveness of OMAL per patient treated for the subgroup population. The expected incremental differences in lifetime costs and QALYs of OMAL relative to standard therapy are £40,927 and 0.5892 QALYs, respectively, giving an ICER of £69,463. The ICER for OMAL in the subgroup population is much lower than that in the base-case population but it is still greater than the threshold of £20,000 per QALY; therefore, OMAL is not expected to be cost-effective compared with standard therapy alone.
| Treatment | Cost (£) | QALYs | ICER (£/QALY) | Cost-effectiveness threshold £20,000 per QALY | Cost-effectiveness threshold £30,000 per QALY | ||
|---|---|---|---|---|---|---|---|
| NHE, QALYs (£) | Incremental NHE, QALYs (£) | NHE, QALYs (£) | Incremental NHE, QALYs (£) | ||||
| OMAL + standard care | 82,681 | 15.11 | 69,463 | 10.9793 (219,585) | –1.4571 (–29,143) | 12.3573 (370,718) | –0.7750 (–23,251) |
| Standard care | 41,754 | 14.52 | – | 12.4364 (248,728) | – | 13.1323 (393,969) | – |
The per-patient investment profile for OMAL is illustrated in Figure 74 for the subgroup population. It shows that OMAL is always expected to offer negative NHEs compared with standard therapy over the entire patient time horizon of 90 years, that is, the high costs of treatment are never compensated for by future health gains in this subpopulation either. OMAL is not expected to be cost-effective at its current price.
FIGURE 74.
Cumulative incremental NHEs of OMAL over the patient time horizon for the subgroup.
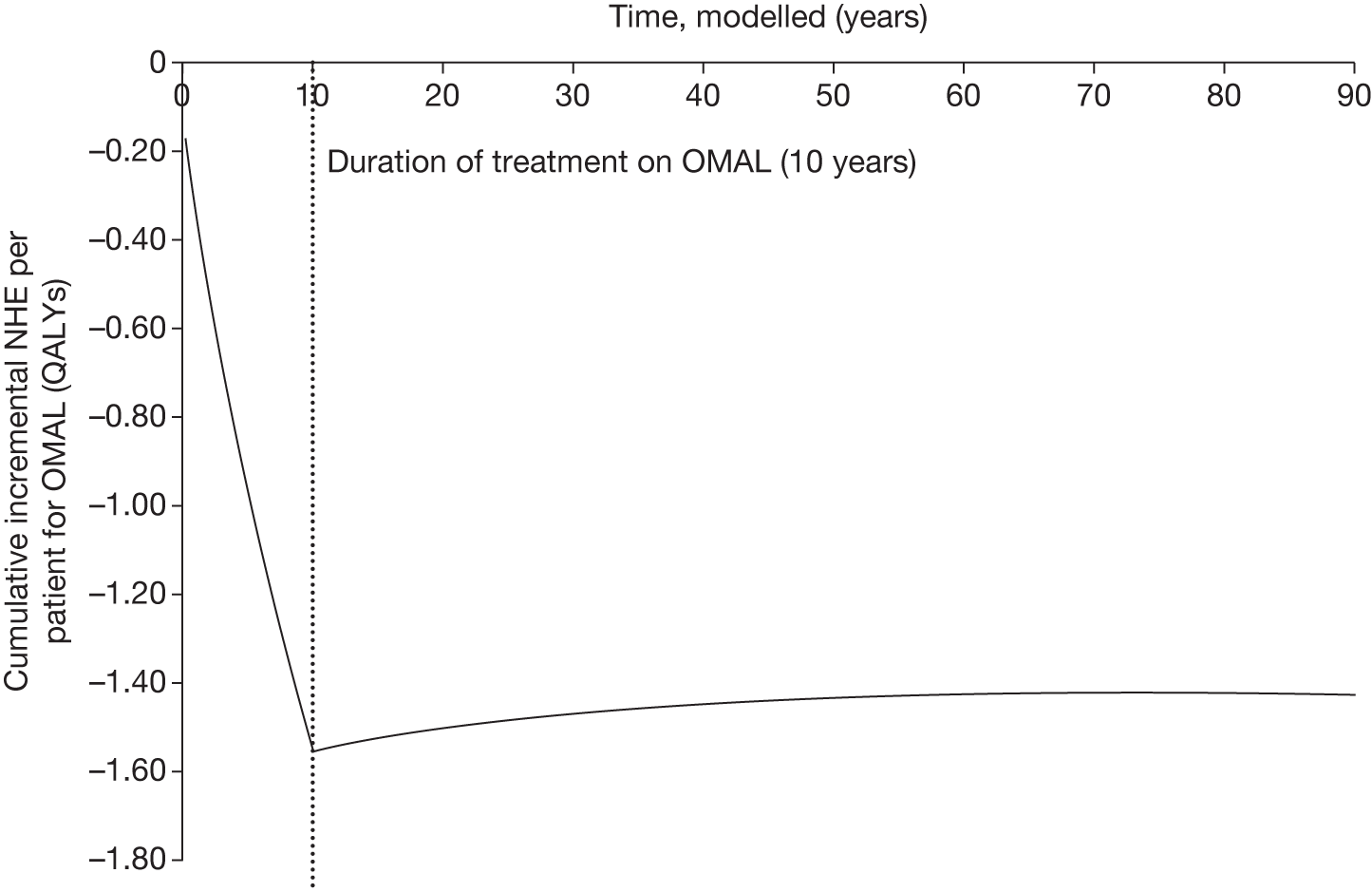
Cost-effectiveness at the population level
Table 118 shows the total population NHEs over 10 years, assuming that OMAL will be used to treat the estimated patient population in Table 109 (based on uptake rates for the first 5 years and then 307 patients per year thereafter) rather than an incident population of 307 patients per year. The lower estimate of population size is used to reflect the fact that the high-risk subgroup would be expected to include fewer patients. The incremental NHE is less negative for the subgroup population than in the base case because of fewer patients and a lower differential effect between OMAL and standard therapy. Figure 75 shows the investment profile for OMAL over 10 years. As seen above, the large losses are never compensated for by future health gains. OMAL is not cost-effective and never breaks even with standard therapy.
| Treatment | Costs (£M) | QALYs | ICER (£/QALY) | NHE, QALYs (£M) | Incremental NHE, QALYs (£M) | NHE, QALYs (£M) | Incremental NHE, QALYs (£M) |
|---|---|---|---|---|---|---|---|
| OMAL + standard care | 123 | 22,563 | 69,463 | 16,391 (328) | –2175 (–44) | 18,448 (553) | –1157 (–35) |
| Standard care | 62 | 21,683 | – | 18,566 (371) | – | 19,605 (588) | – |
FIGURE 75.
Cumulative incremental NHEs of OMAL for the subgroup population.
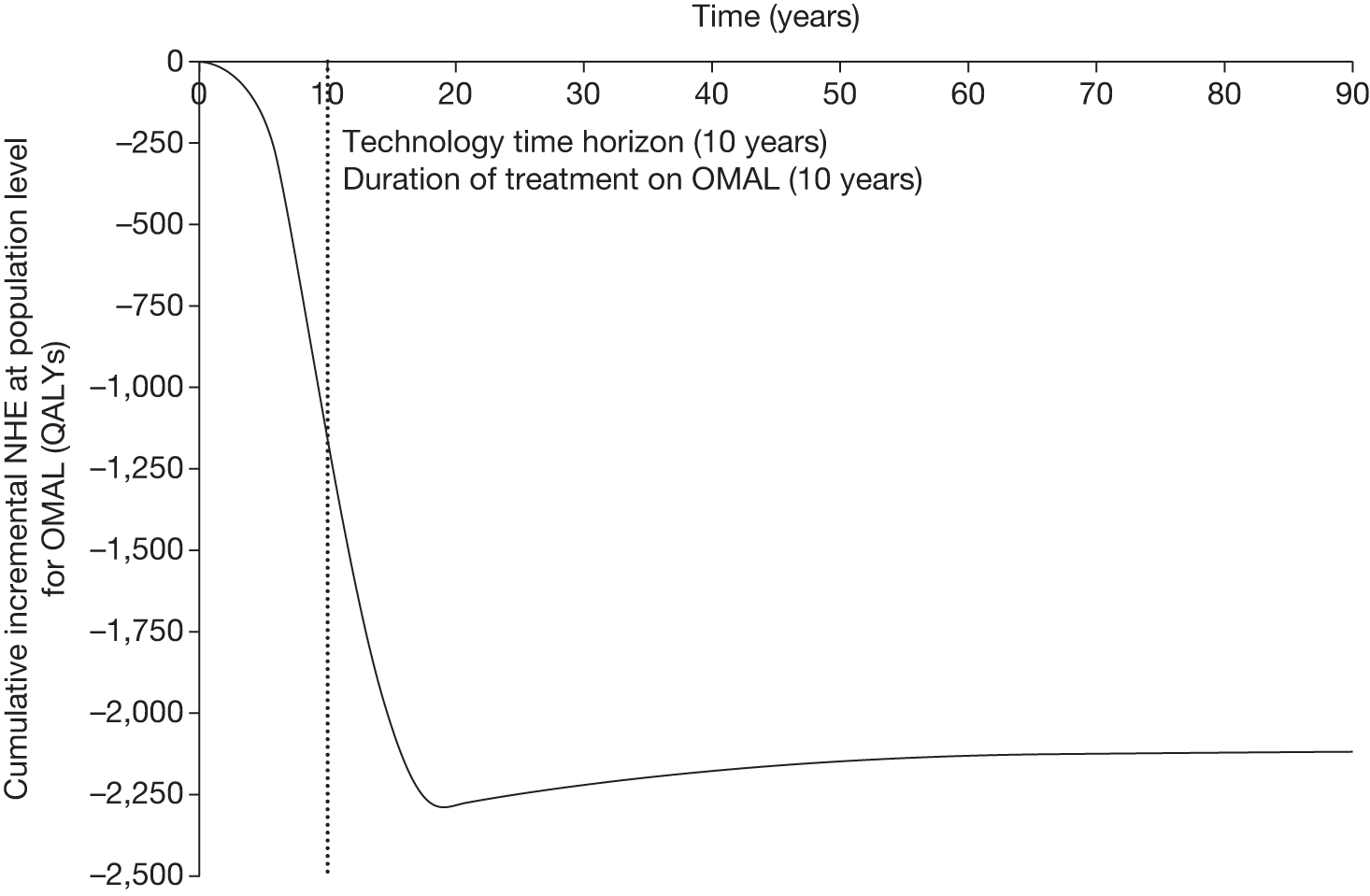
Point 2: Are there significant irrecoverable costs?
Omalizumub does not exhibit significant irrecoverable opportunity costs (see Point 2: Are there significant irrecoverable costs?). There are no additional irrecoverable costs associated with the subgroup population.
Point 3: Does more research seem worthwhile?
Omalizumub is not expected to be cost-effective based on existing evidence in the high-risk subgroup. Although the ICER is lower than in the base-case population, it is still significantly higher than the threshold. An assessment of the likely consequences of approving standard therapy when OMAL might be better is shown in Table 119. The results of PSA suggest that even at a threshold of £30,000 per QALY the probability that OMAL is cost-effective is very small (0.013) and the upper bound on the gains from more research is very limited (5.99 QALYs). Therefore, even after an analysis of subgroups OMAL is not expected to be cost-effective and more research does not seem worthwhile.
| Treatment | ICER (£/QALY) | Cost-effectiveness threshold £20,000 per QALY | Cost-effectiveness threshold £30,000 per QALY | ||||
|---|---|---|---|---|---|---|---|
| Incremental NHE, QALYs (£M) | Probability cost-effective | Expected consequences, QALYs (£M) | Incremental NHE, QALYs (£M) | Probability cost-effective | Expected consequences, QALYs (£M) | ||
| OMAL + standard care | 69,463 | –2175 (–44) | 0.000 | 0.000 (0) | –1157 (–35) | 0.013 | 5.99 (0.12) |
| Standard care | – | 1.000 | – | 0.987 | |||
Table 120 shows the expected consequences of uncertainty in the subgroup with the alternative scenarios (see Point 3: Does more research seem worthwhile?, Alternative scenarios). Even under the most favourable assumptions, a decision to reject OMAL does not appear uncertain. There are only very small consequences of uncertainty or value to be gained by more research (0.624 QALYs) under the most favourable assumption for OMAL.
| Additive scenario | ICER (£/QALY) | Incremental NHE, QALYs (£M) | Probability cost-effective | Expected consequences, QALYs (£M) |
|---|---|---|---|---|
| Base-case subgroup | 69,463 | –2175 (–44) | 0.000 | 0.000 (0) |
| + 20-year treatment duration | 59,096 | –3399 (–68) | 0.000 | 0.000 (0) |
| + utility difference in day-to-day symptoms for all ages | 53,269 | –3171 (–63) | 0.000 | 0.000 (0) |
| + mortality rates owing to clinically significant severe exacerbations increased by 100% | 44,921 | –2861 (–57) | 0.002 | 0.624 (0.01) |
Types and categories of guidance resulting from subgroup analysis
The subgroup analysis was considered in the same way as for the base-case population; it started at point 1 of the checklist and worked through the same sequence of assessments as in the primary analysis. For the high-risk subgroup, OMAL is not expected to be cost-effective and there is no uncertainty. OMAL can be rejected at this point and no further assessment is required. Table 121 summarises the category and type of guidance for OMAL in the high-risk subgroup.
| Assessment | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Guidance |
|---|---|---|---|---|---|---|---|---|
| 12 | No | No | No | – | – | – | – | Reject4 |
Appendix 10 Etanercept, infliximab and adalimumab for the treatment of psoriatic arthritis
Introduction
Following a MTA appraisal (TA199125 in 2010), the use of biologic treatment with etanercept, infliximab and adalimumab was recommended by NICE for patients with active and progressive PsA. However, the guidance also recommended that treatment should start with the least expensive biologic, taking account of dose, route of administration and price. This guidance updated an earlier MTA appraisal in 2006 (TA104126), which had recommended etanercept and restricted guidance on the use of infliximab to only those patients shown to be either intolerant or contraindicated to etanercept. a
In considering the results of the cost-effectiveness analysis, the NICE Appraisal Committee expressed concern about any differential effectiveness between treatments (specifically adalimumab and etanercept, which are biologically similar). They felt that a ‘class effect’ may be a more reasonable assumption. A scenario making this assumption (applying the same initial response and HAQ gain to adalimumab and etanercept) was therefore presented at the second appraisal meeting. In addition, in section 6 of TA199 the importance of data on long-term outcomes and adverse events from patient registries was highlighted. Therefore, PsA is not an example of AWR at FAD but an example of AWR considered during appraisal.
The analysis in this case study is from the standpoint of TA199 using the updated model that included new evidence and adalimumab as an additional comparator. At this point NICE guidance recommended etanercept and so the first question posed in the checklist can be interpreted as whether or not the other technologies available (infliximab, adalimumab or palliative care) are expected to be cost-effective compared with etanercept.
Background to the case study
Interventions and population
The three biologics (etanercept, infliximab and adalimumab) are compared with palliative care. No sequencing of treatments is considered; instead, if patients withdraw from any of the active treatments they will instead receive palliative care for the remainder of their lifetime. If patients do not withdraw from treatment (because of adverse events or lack of efficacy) they will remain on active treatment for the remainder of their lifetime, that is, PsA is a chronic disease.
The population of interest includes those patients with active and progressive PsA who have an inadequate response to standard treatment, including two conventional DMARDs (BSR criteria). 195 There is little information about the incidence of PsA in the UK; therefore, in the absence of a better alternative, based on clinical expert opinion the annual incidence of PsA was estimated at one-third of that for rheumatoid arthritis,196 that is, 12,000 per year. As patients are unlikely to switch treatments once well controlled, a prevalent population was not considered relevant.
Evidence on clinical effectiveness
The review of clinical effectiveness identified six RCTs comparing one of the three biologics with placebo. 197–231 To synthesise all of these trials a Bayesian mixed-treatment comparison232 was used to generate the following parameters for the decision model (for each biologic and for palliative care): probability of PsARC response at 12 weeks, probability of achieving Psoriasis Area Severity Index (PASI) 50, 75 and 90 responses at 12 weeks, and the associated HAQ for PSARC responder/non-responder. 233
The trials, however, are limited in follow-up and therefore the longer-term effectiveness of the biologics and the impact on HAQ scores of withdrawing from biologics are not available. Expert elicitation was used in the model to generate estimates of the progression of HAQ for patients continuing on biologics and the progression of HAQ following withdrawal from treatment (rebound). 233 Table 122 shows the probabilistic input parameters used in the model and their distributions.
| Description | Variable name | Mean | SE | Distribution | Source |
|---|---|---|---|---|---|
| Change in utility for 1 unit change in HAQ | U_HAQ | –0.298 | 0.006 | Normal | Rodgers et al. 2001233 |
| Change in utility for 1 unit change in PASI | U_PASI | –0.004 | 0.0003 | Normal | Rodgers et al. 2001233 |
| Change in cost for 1 unit change in HAQ | C_HAQ | 187 | 21 | Normal | Kobelt et al. 2002234 |
| 3-month cost for mild-to-moderate psoriasis if uncontrolled by biologics | C_psoriasis | 198 | 9 | Normal | Department of Health 2009235 |
| Change in HAQ while not on treatment per 3-month period | LT_NH_HAQ | 0.018 | 0.007 | Gamma236 | Norfolk Arthritis Register (NOAR) database236 |
| Log-withdrawal rate from biologics per year | Withdraw | –1.823 | 0.2044 | Normal | Registry data233 |
| Probability of PsARC response on placebo | PSARC_NH | 0.249 | 0.0384 | Beta | Evidence synthesis |
| Change in HAQ given a PsARC response on placebo | HAQ_NH | –0.218 | 0.0465 | Normal | |
| Probability of PASI 50 response on placebo | PASI_50_NH | 0.130 | 0.021 | Beta | |
| Probability of PASI 75 response on placebo | PASI_75_NH | 0.044 | 0.009 | Beta | |
| Probability of PASI 90 response on placebo | PASI_90_NH | 0.016 | 0.004 | Beta | |
| Probability of PsARC response on biologic_etanercept | PSARC_treat_E | 0.713 | 0.071 | Beta | |
| Probability of PsARC response on biologic_infliximab | PSARC_treat_I | 0.795 | 0.058 | Beta | |
| Probability of PsARC response on biologic_adalimumab | PSARC_treat_A | 0.587 | 0.072 | Beta | |
| Change in HAQ in first 3 months given no PsARC response of biologic_etanercept | HAQ_noPSARC_treat_E | –0.185 | 0.102 | Beta | |
| Change in HAQ in first 3 months given no PsARC response of biologic_infliximab | HAQ_noPSARC_treat_I | –0.190 | 0.073 | Beta | |
| Change in HAQ in first 3 months given no PsARC response of biologic_adalimumab | HAQ_noPSARC_treat_A | –0.064 | 0.064 | Beta | |
| Change in HAQ in first 3 months given PsARC response of biologic_etanercept | HAQ_treat_E | –0.623 | 0.095 | Beta | |
| Change in HAQ in first 3 months given PsARC response of biologic_infliximab | HAQ_treat_I | –0.652 | 0.072 | Beta | |
| Change in HAQ in first 3 months given PsARC response of biologic_adalimumab | HAQ_treat_A | –0.423 | 0.061 | Beta | |
| Probability of PASI 50 response on biologic_etanercept | PASI_50_treat_E | 0.4026 | 0.0916 | Beta | |
| Probability of PASI 50 response on biologic_infliximab | PASI_50_treat_I | 0.9128 | 0.0374 | Beta | |
| Probability of PASI 50 response on biologic_adalimumab | PASI_50_treat_A | 0.7383 | 0.0853 | Beta | |
| Probability of PASI 75 response on biologic_etanercept | PASI_75_treat_E | 0.1768 | 0.0586 | Beta | |
| Probability of PASI 75 response on biologic_infliximab | PASI_75_treat_I | 0.7687 | 0.0795 | Beta | |
| Probability of PASI 75 response on biologic_adalimumab | PASI_75_treat_A | 0.4772 | 0.1085 | Beta | |
| Probability of PASI 90 response on biologic_etanercept | PASI_90_treat_E | 0.0737 | 0.0292 | Beta | |
| Probability of PASI 90 response on biologic_infliximab | PASI_90_treat_I | 0.5571 | 0.1088 | Beta | |
| Probability of PASI 90 response on biologic_adalimumab | PASI_90_treat_A | 0.2571 | 0.0863 | Beta |
Decision model
A probabilistic cohort model was developed to estimate costs and QALYs and explore any uncertainties.
These processes are inherently non-linear in terms of the relationship between model inputs and outputs (costs and QALYs) (see Appendix 11, Model linearity and correlation between parameters). The model was run for a period of 40 cycles (time horizon of the model, 3-monthly cycles) and probabilistic analysis was used (Monte Carlo simulation was run for 5000 iterations). The discount rate was 3.5%.
The initial response of the drug is defined in the model using PsARC for joints and PASI 75 for psoriasis; these parameters were estimated using a Bayesian evidence synthesis. Given a response (or no response) there is an associated impact on functional status, defined using the HAQ, for the arthritis aspect of the disease. The expected change in PASI is modelled as a constant. The model was implemented in R.
Patients can withdraw from biologics at any point after the initial response phase (first 12 weeks). If they withdraw during the initial response phase this is regarded as a ‘no response’. Patients will receive palliative care after withdrawal at any stage. On withdrawing from treatment, it is assumed that mean PASI returns to its initial score at baseline (rebound equal to initial gain). There is considerable uncertainty about change in HAQ associated with withdrawal (rebound). Previous modelling work assumed that rebound of HAQ follows either of two alternative scenarios, with no data to inform which scenario is the more likely: rebound equal to initial gain and rebound equal to natural history. In TA199125 this rebound assumption is informed by an expert opinion elicitation exercise conducted with five experts.
Key features and possible pathways
Biologics are not associated with any large upfront costs of treatment as treatment costs are incurred on a monthly basis according to whether or not the patient is still receiving treatment. The gains in quality-of-life benefits are also apparent throughout, with an initial gain in quality of life (through HAQ and PASI) associated with those who are treatment responders, as assessed at 3 months. The technology does, however, have a number of interesting characteristics, which can be used to explore the implications of the principles and assessments outlined in Chapter 5:
-
Etanercept, infliximab and adalimumab all have potentially significant irrecoverable costs because of the high per-patient treatment costs combined with a chronic condition in which treatment decisions are not irreversible. PsA is a chronic condition in which patients are unlikely to switch treatment once maintained. Switching will happen only if patients withdraw from treatment because of adverse events or lack of efficacy.
-
There is uncertainty about the effectiveness of biologics. A separate scenario was assessed that assumed a class effect for the biologics etanercept and adalimumab. This provides an opportunity to examine the role of exploring separate extreme scenarios to characterise uncertainty.
The possible pathways through the algorithm are reported in Figure 32 in Appendix 4. The alternatives to etanercept (adalimumab, infliximab and palliative care) are not expected to be cost-effective. There are, however, a number of uncertainties. These uncertainties might influence the category of guidance, for example OIR or AWR rather than reject.
The following sections examine each of the seven points on the checklist relating to the possible sequence of assessments and decisions that lead to a particular category and type of guidance for PSA.
Is it cost-effective and what are the risks?
The judgements made at points 1 and 2 of the checklist are critical because, although neither leads directly to a particular category of guidance, they determine the subsequent path that might be taken, sometimes avoiding further and potentially complex assessments. For example, the absence of significant irrecoverable costs means that only four out of the 12 possible pathways require all seven assessments to be made (see Appendix 4).
Point 1: Is it expected to be cost-effective?
The sequence of assessments starts with cost-effectiveness and the expected impact on population NHEs, that is, at the following point in the algorithm:
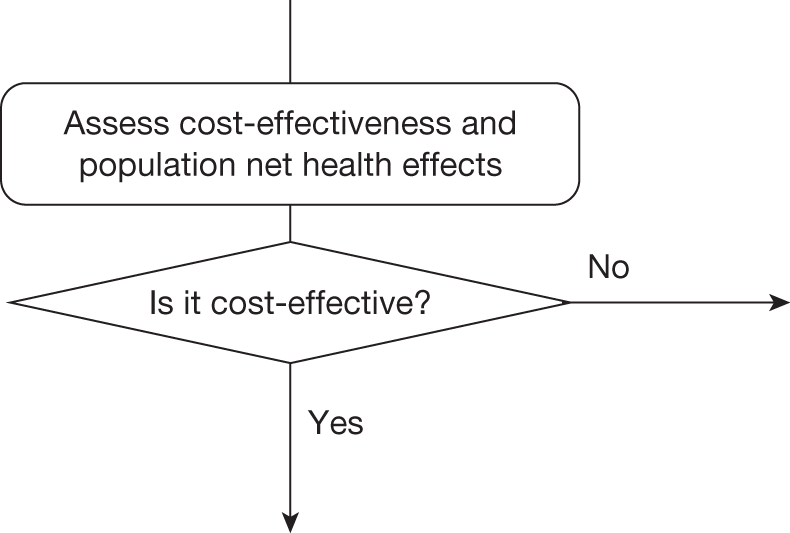
This requires an assessment of expected cost-effectiveness based on the balance of the evidence and analysis currently available. Methods to estimate expected cost-effectiveness are well established within the NICE appraisal process and are extensively described in the Guide to Methods of Technology Appraisal. 1,b Commonly, expected cost-effectiveness is summarised and presented using ICERs. Equivalently, but more usefully in this context, cost-effectiveness can be expressed in terms of expected NHEs, which can be expressed per patient treated or for a population of patients. This is especially important when later assessments require a comparison of benefits to current or future patient populations and when assessing the significance of irrecoverable costs (see Cost-effectiveness at the population level). All of the information required to express expected cost-effectiveness in these ways is already available during appraisal.
Cost-effectiveness at the patient level
Estimates of the expected NHS costs and QALYs for each patient treated over an appropriate time horizon – the ‘patient time horizon’c – can be summarised as an ICER, which must be compared with a cost-effectiveness threshold to judge cost-effectiveness. Equivalently, this can be expressed as the per-patient NHE of each intervention, that is, the difference between any health gained and health forgone elsewhere.
For the PsA case study, the treatment already recommended by NICE (etanercept at the time of TA199125) is expected to be cost-effective (Table 123). d Other alternatives to etanercept (adalimumab, infliximab and palliative care) are not expected to be cost-effective. Although adalimumab is less effective than etanercept, it is also cheaper; however, the resource savings it offers do not compensate for the reduction in health benefits (for an ICER of around £20,000 per QALY). Infliximab is more effective but it is also much more expensive. These increased costs are, however, not justified by the QALY gains.
| Treatment | Cost (£) | QALYs | ICER (£/QALY) | Cost-effectiveness threshold £20,000 per QALY | Cost-effectiveness threshold £30,000 per QALY |
|---|---|---|---|---|---|
| NHE, QALYs (£) | NHE, QALYs (£) | ||||
| 1: Infliximab | 90,343 | 7.269 | 60,965 | 2.752 (5504) | 4.258 (8516) |
| 2: Etanercept | 78,150 | 7.069 | 17,733 | 3.161 (6322) | 4.464 (8928) |
| 3: Adalimumab | 72,972 | 6.777 | 14,622 | 3.129 (6258) | 4.345 (8690) |
| 4: Palliative care | 51,800 | 5.329 | – | 2.739 (5478) | 3.602 (7204) |
The investment profiles of the alternatives to etanercept, illustrated in Figure 76 relative to etanercept, differ in appearance. All of the biologic treatments for PsA (etanercept, infliximab and adalimumab) have high initial costs, which are only gradually compensated for by later health benefits. Palliative care offers a positive NHE initially compared with etanercept. As the time horizon of the model increases the NHE of palliative care compared with etanercept becomes negative; this happens at 19 years. Adalimumab also offers positive NHEs in the short run compared with etanercept (lower costs) so it is only at 21.25 years that etanercept is expected to offer the highest NHE (all others have negative NHEs relative to this). Infliximab is always a risky strategy and does not break-even within a reasonable modelled time horizon (40 years).
FIGURE 76.
Cumulative incremental NHEs in PsA over the patient time horizon.
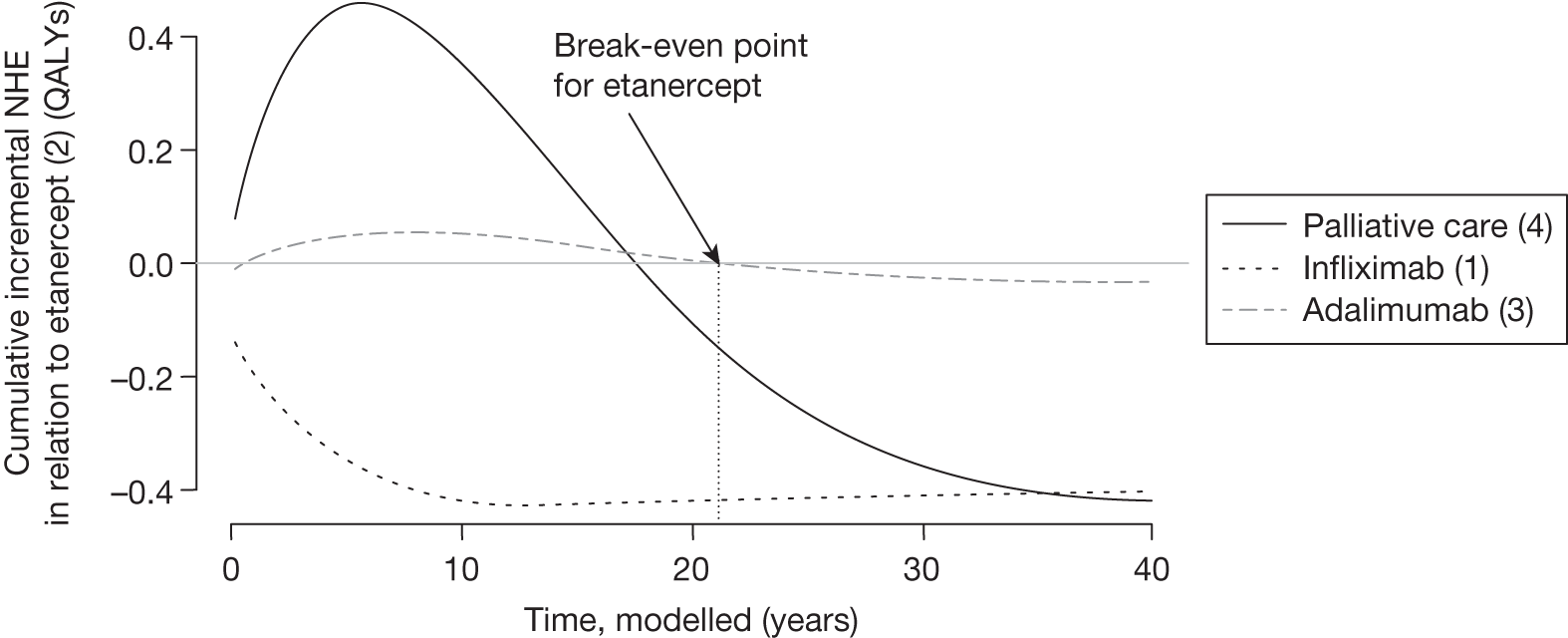
Etanercept, infliximab and adalimumab all therefore have potentially significant irrecoverable costs because of the high per-patient treatment costs combined with a chronic condition in which treatment decisions are not irreversible.
Cost-effectiveness at the population level
Per-patient NHEs can also be expressed for the population of current and future patients. This requires information about prevalence and future incidence of the target population (already required in appraisal). It also requires a judgement about the time horizon over which the technology will be used. This ‘technology time horizon’ ought to reflect the period over which the technology is likely to be part of clinical practice and generate the expected NHEs. e An estimate of the scale of the total population NHEs and how they cumulate over time is important for subsequent assessments, including (1) when the NHEs for current patient populations must be compared with the benefits to future patients and (2) when the treatment decision can be changed so the irrecoverable costs of initially negative NHEs become significant.
In the PsA case study there is no prevalent population eligible for adalimumab, infliximab or palliative care if it is approved. This is because the prevalent population will not switch treatments whilst maintained on their current therapy. They will switch only if they relapse (withdraw from treatment because of adverse events or lack of efficacy). The total population NHEs, assuming that the technology will be used to treat only incident patients (12,000 per year) over 10 years, are reported in Table 124. The expected cost-effectiveness is unchanged (the ICER is the same as that in Table 121) but the incremental NHEs, although small per patient, are more significant at a population level.
| Treatment | Cost (£M) | QALYs | ICER (£/QALY) | Cost-effectiveness threshold £20,000 per QALY | Cost-effectiveness threshold £30,000 per QALY | ||
|---|---|---|---|---|---|---|---|
| NHE, QALYs (£M) | Incremental NHE, QALYs (£M) | NHE, QALYs (£M) | Incremental NHE, QALYs (£M) | ||||
| 1: Infliximab | 9332 | 750,874 | 60,965 | 284,288 (5686) | –42,227 (–845) | 439,816 (13,194) | –21,237 (–637) |
| 2: Etanercept | 8072 | 730,128 | 17,733 | 326,515 (6530) | – | 461,053 (13,832) | – |
| 3: Adalimumab | 7537 | 700,037 | 14,622 | 323,162 (6463) | –3353 (–67) | 448,787 (13,464) | –12,266 (–368) |
| 4: Palliative care | 5351 | 550,422 | – | 282,894 (5658) | –43,621 (–872) | 372,070 (11,162) | –88,983 (–2669) |
The investment profile for PsA when patients are treated over 10 years is illustrated in Figure 77. The NHEs for infliximab, adalimumab and palliative care are calculated relative to etanercept (standard NHS care). At a population level it is not until 26 years (rather than 21.25 years at a patient level) that initial losses are compensated for by later gains and etanercept breaks even (all other NHEs are negative relative to etanercept213). In other words, etanercept appears a more risky investment when evaluated at a population rather than at an individual level. This is because, although each patient treated with etanercept is expected to offer the same profile of NHEs shown in Figure 76, the negative NHEs associated with patients who are incident and treated in year 10 will not be offset by later gains until year 26. The incremental NHEs and break-even points when evaluated at a population level are reported in Table 125 over different time horizons. These are reported for adalimumab compared with palliative care, palliative care compared with etanercept, palliative care compared with infliximab and adalimumab compared with etanercept. The table shows that as the technology time horizon increases treatment with biologics becomes a more risky prospect and the NHE of palliative care remains positive for longer. At a technology time horizon of 10 years it is only at 18.75 years that current NHS care (etanercept) breaks even against palliative care and not until 22 years that it is better than adalimumab.
FIGURE 77.
Cumulative incremental NHEs in PsA for the population (in relation to standard care, etanercept).
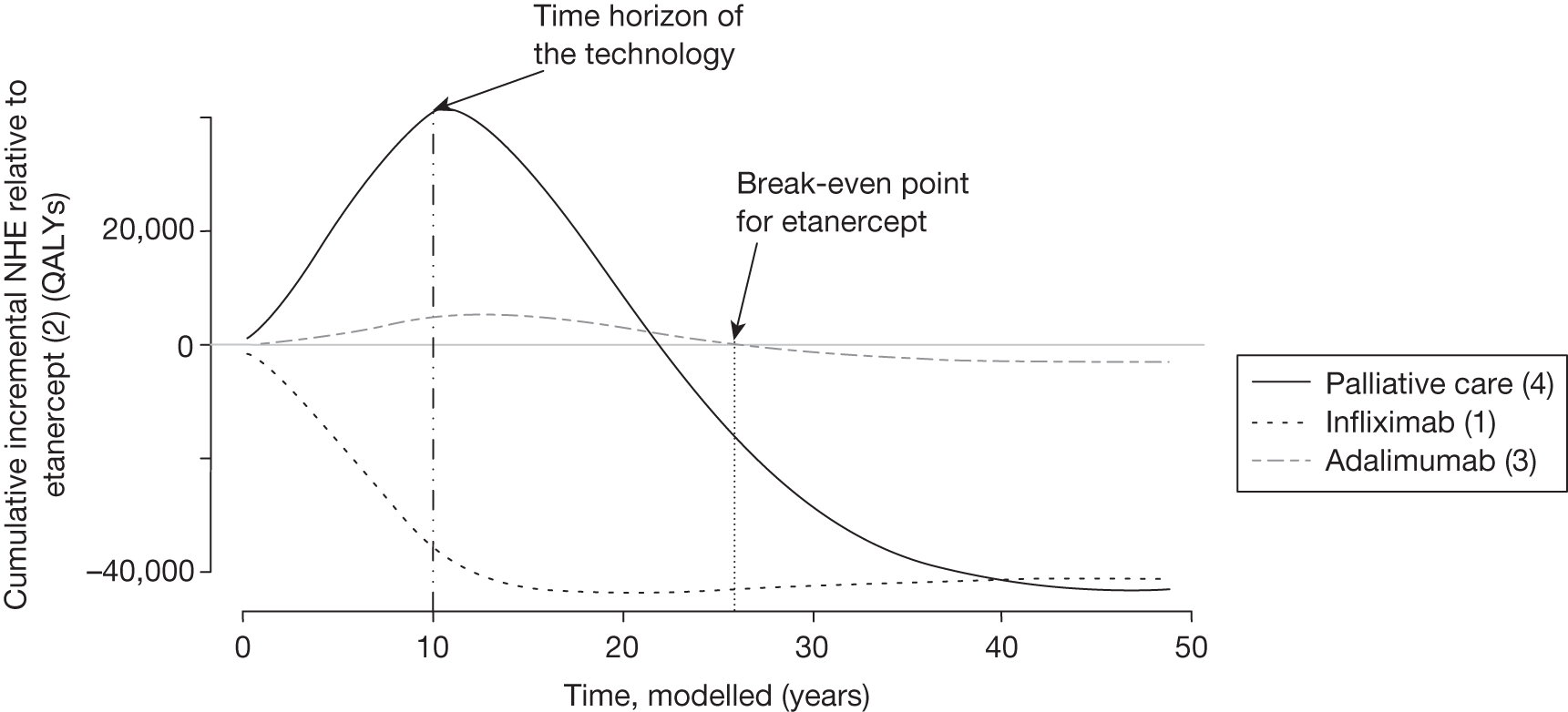
| Technology time horizon | Treatment | Incremental NHE (compared with etanercept), QALYs | Break-even point (years) | ||
|---|---|---|---|---|---|
| vs 4: Palliative care | vs 2: Etanercept | vs 1: Infliximab | |||
| 5 years | 1: Infliximab | –22,925 (–459) | |||
| 2: Etanercept | – | ||||
| 3: Adalimumab | –1820 (–25) | 17 | 22 | ||
| 4: Palliative care | –23,681 (–710) | 18.25 | 36.25 | ||
| 10 years | 1: Infliximab | –42,227 (–1267) | |||
| 2: Etanercept | – | ||||
| 3: Adalimumab | –3353 (–101) | 18.5 | 22 | ||
| 4: Palliative care | –43,624 (–1309) | 18.75 | 36.25 | ||
| 15 years | 1: Infliximab | –58,480 (–1754) | |||
| 2: Etanercept | – | ||||
| 3: Adalimumab | –4643 (–139) | 19 | 22.5 | ||
| 4: Palliative care | –60,410 (–1812) | 19.5 | 36.5 | ||
| 20 years | 1: Infliximab | –72,163 (–2165) | |||
| 2: Etanercept | – | ||||
| 3: Adalimumab | –5730 (–172) | 19.5 | 23.75 | ||
| 4: Palliative care | –77,545 (–2326) | 20 | 37 | ||
Point 2: Are there significant irrecoverable costs?
The second point on the checklist requires (1) an assessment of whether or not there are irrecoverable costs and (2) a judgement of their potential significance, that is, at the following point in the algorithm:
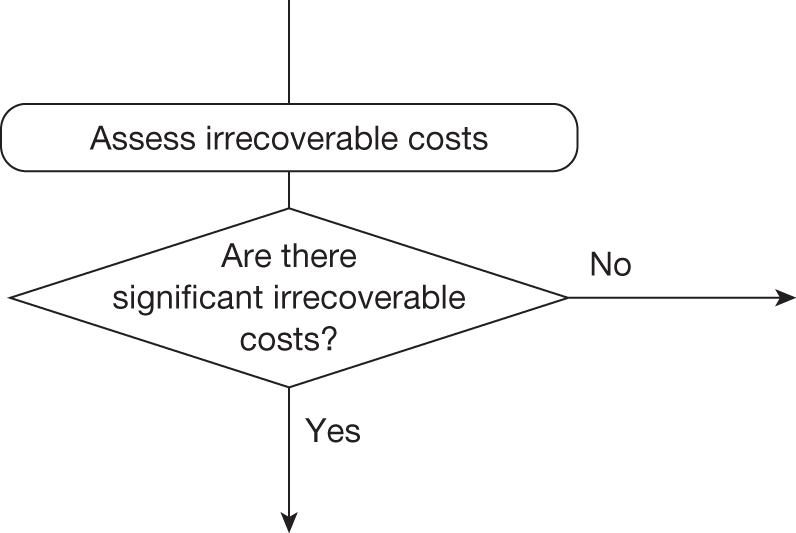
Identifying the irrecoverable costs
Irrecoverable costs are those that once committed cannot be recovered if guidance is changed at a later date (see Chapter 5 and Appendix 11, Accounting for the investment profile of displaced interventions). The impact of irrecoverable costs will tend to be greater if they represent a greater proportion of the total costs and if guidance is more likely to change and to change in the near future.
Even in the absence of capital costs of equipment and facilities, NHEs accumulate over time at both a patient and a population level. The analysis in Point 1: Is it expected to be cost-effective? indicates a common pattern of initially negative NHEs that are only gradually offset by positive NHE in later periods. Therefore, AWR may commit opportunity costs of negative NHEs that are irrecoverable.
Are they likely to be significant?
Whether or not irrecoverable costs are significant (i.e. might influence guidance) depends critically on whether guidance is likely to change and whether that is more likely in the near or in the distant future. That will depend on whether research is likely to be undertaken and when it is likely to report, as well as on other events that might occur, for example a change in price following patent expiry. These are assessed later, in points 5 and 6; however, the potential significance of any irrecoverable costs can be assessed at this point.
The potential significance of the investment profiles of NHEs depends on if treatment decisions for individual patients are irreversible, which in part depends on the nature of the disease. For example, in an acute condition the decision to treat a particular presenting patient with a technology cannot be reconsidered at a later date – it is irreversible with associated reversal costs. Of course, it is possible that the later benefits are not realised but it is also possible that they will realise more (the profiles of NHEs in Figures 76 and 77 are the average over these possibilities). Similarly, the possibility that guidance might change in the future (e.g. research suggests that the longer-term benefits will not offset initial losses) will not influence the irreversible decision to treat a presenting patient with a technology that is expected to be cost-effective prior to the research reporting.
Psoriatic arthritis is a chronic condition in which the decision to treat a particular patient cannot be changed at some later date (decisions are assumed irreversible). Therefore, the NHS will commit to irrecoverable costs by deciding not to use technologies not expected to be cost-effective now [if they have less irrecoverable costs than standard care (etanercept)]. This is the case for adalimumab and palliative care for which the NHEs are initially higher than for etanercept (Figure 77 shows less irrecoverable costs for adalimumab and palliative care initially). For infliximab, however, the NHEs are lower than for etanercept; therefore, deciding not to use infliximab will reduce the loss in NHEs (as the initial loss in NHE is lower for etanercept). In deciding if these costs are likely to be significant, however, it is important to note that, in the example of PsA, decisions cannot be reversed (patients remain on treatment if they are responding). Therefore, patients will still experience the positive NHEs that occur later in the modelled time horizon (resulting from the recommended treatment, etanercept). In conclusion, therefore, there are no significant investment or reversal costs for this case study.
Types and categories of guidance resulting from points 1 and 2
Points 1 and 2 of the checklist do not lead directly to a category or type of guidance. The sequence of assessments and decisions, which ultimately lead to guidance, starts with cost-effectiveness, expected impact on population NHEs and significance of irrecoverable costs. In the case of PsA, alternatives to etanercept are not expected to be cost-effective and there are no significant irrecoverable costs. Table 126 summarises the categories and types of guidance that could ultimately result from the sequence of assessments up to point 2.
| Assessment | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Guidance |
|---|---|---|---|---|---|---|---|---|
| 7 | No | No | Yes | Yes | Yes/no | Yes | – | OIR2 |
| 8 | No | No | Yes | Yes | Yes/no | No | – | Reject1 |
| 9 | No | No | Yes | No | Yes/no | Yes | Yes | AWR2 |
| 10 | No | No | Yes | No | Yes/no | Yes | No | Reject2 |
| 11 | No | No | Yes | No | Yes/no | No | – | Reject3 |
| 12 | No | No | No | – | – | – | – | Reject4 |
Is further research required?
Point 3: Does more research seem worthwhile?
The third point on the checklist requires an assessment of the potential benefits of conducting further research, that is, at the following point in the algorithm:
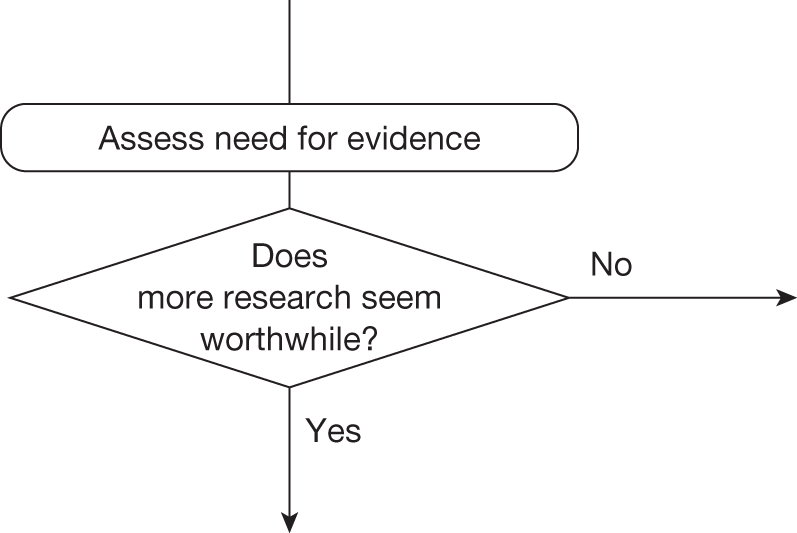
This requires judgements about (1) how uncertain a decision to approve or reject a technology might be based on the estimates of expected cost-effectiveness and (2) whether or not the scale of the likely consequences of this uncertainty might justify further research.
The judgements made at points 3 and 4 of the checklist are critical because if more research is not judged to be worthwhile then no further assessments are required (unless there are significant irrecoverable costs; see Appendix 4). If research is worthwhile then what type of evidence is needed and whether the research required to generate it can be conducted while the technology is approved will determine whether AWR or OIR is a possibility.
Assessing the consequences of uncertainty
Infliximab, palliative care and adalimumab are not expected to be cost-effective compared with standard care (see Tables 123 and 124) but the estimates of costs and QALYs are uncertain so there is a chance that a decision to reject these treatments based on existing evidence will be incorrect, that is, infliximab, palliative care or adalimumab might offer greater NHEs. Some assessment of the likely consequences of rejecting infliximab, palliative care and adalimumab might be based on the difference in expected NHEs, that is, the expected incremental population NHEs reported in Table 124. However, as there are more than two alternatives, a judgement is required about the chance that using standard care (etanercept) is incorrect and if so which of the other three alternatives are likely to offer higher NHEs and how much higher. In other words, for decisions involving multiple alternatives, a judgement is required on the level of uncertainty surrounding the decision, how this uncertainty is distributed across the various alternatives and what the consequences are likely to be.
The simplest approach could be to take the difference in expected NHEs between adalimumab, palliative care or infliximab and standard NHS care and weight it by a judgement of the probability that the decision is correct, that is, allocate the consequences of an incorrect decision to NHS standard care. For example, if the decision was judged to be 100% certain (the probability that the decision is correct would be 1) then there are no consequences and so there would be nothing to be gained by more research; however, as the decision becomes more uncertain, the expected consequences (and hence the potential value of more research) increase.
In the presence of multiple treatment alternatives there are other options than to allocate all of the consequences of an erroneous decision to NHS standard care. We have examined four alternative ways:
-
The consequences are assigned to NHS standard care (etanercept).
-
The consequences are assigned to adalimumab, the next best treatment.
-
Equal shares of the consequences are assigned to treatments other than NHS standard care (etanercept).
-
The consequences are assigned to treatments other than NHS standard care (etanercept) based on the probability of the alternative treatments being cost-effective from PSA. Note that this uses the PSA only to inform the magnitude of the consequences of a wrong decision; a judgement on the likelihood of adopting etanercept being the incorrect decision is still required.
The results of these alternative analyses are illustrated in Table 127 in which a judgement about the probability that a decision based on expected cost-effectiveness is correct translates into expected consequences based on expected incremental population NHEs.
| Error probability, p | Cost-effectiveness threshold £20,000 per QALY | Cost-effectiveness threshold £20,000 per QALY | ||||||
|---|---|---|---|---|---|---|---|---|
| NHEs for the population, QALYs | NHEs for the population, £ | |||||||
| 1: Infliximab | 2: Etanercept | 3: Adalimumab | 4: Palliative care | 1: Infliximab | 2: Etanercept | 3: Adalimumab | 4: Palliative care | |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0.01 | 0 | 34 | 297 | 133 | 0 | 670,500 | 5,946,720 | 2,669,666 |
| 0.05 | 0 | 168 | 1487 | 667 | 0 | 3,352,540 | 29,733,600 | 13,348,331 |
| 0.1 | 0 | 335 | 2973 | 1335 | 0 | 6,705,080 | 59,467,220 | 26,696,661 |
| 0.25 | 0 | 838 | 7433 | 3337 | 0 | 16,762,700 | 148,668,040 | 66,741,653 |
| 0.5 | 0 | 1676 | 14,867 | 6674 | 0 | 33,525,420 | 297,336,080 | 133,483,307 |
| 0.75 | 0 | 2514 | 22,300 | 10,011 | 0 | 50,288,120 | 446,004,120 | 200,224,960 |
| 0.99 | 0 | 3319 | 29,436 | 13,215 | 0 | 66,380,320 | 588,725,440 | 264,296,947 |
| 1 | 0 | 3353 | 29,734 | 13,348 | 0 | 67,050,820 | 594,672,160 | 266,966,613 |
The expected consequences of a wrong decision are high in scenarios (2), (3) and (4). There are no consequences of a wrong decision if all of the error gets assigned to treatment 2 (NHS standard care) because treatment 2 is the most cost-effective treatment (NHEs calculated relative to this).
The judgement regarding how uncertain a decision might be can be informed by the PSA already used to estimate costs and QALYs. The probabilities that each of the three alternatives is cost-effective are reported in Table 128. This shows that although infliximab, palliative care and adalimumab are not considered to be cost-effective there is a non-zero probability that this judgement is incorrect (they are indeed cost-effective). At a threshold of £20,000, the probabilities that the three treatments are cost-effective are 0.012, 0.146 and 0.399 for infliximab, adalimumab and palliative care, respectively (Figure 78). At a threshold of £30,000, the probabilities that the three treatments are cost-effective are 0.113, 0.103 and 0.280 for infliximab, adalimumab and palliative care, respectively.
| Treatment | ICER (£/QALY) | Cost-effectiveness threshold £20,000 per QALY | Cost-effectiveness threshold £30,000 per QALY | ||||
|---|---|---|---|---|---|---|---|
| Incremental net health effect,a QALYs (£M) | Probability cost-effective | Expected consequences, QALYs (£M) | Incremental net health effect,a QALYs (£M) | Probability cost-effective | Expected consequences, QALYs (£M) | ||
| 1: Infliximab | 60,965 | –42,240 (–844) | 0.012 | 35,341 (707) | –21,279 (–425) | 0.113 | 18,079 (542) |
| 2: Etanercept | 17,733 | 3306 (66) | 0.443 | 12,292 (245) | 0.502 | ||
| 3: Adalimumab | 14,622 | 40,284 (805) | 0.146 | 76,746 (1534) | 0.103 | ||
| 4: Palliative care | – | – | 0.399 | – | 0.280 | ||
FIGURE 78.
Probability that etanercept is cost-effective and the consequences of uncertainty.
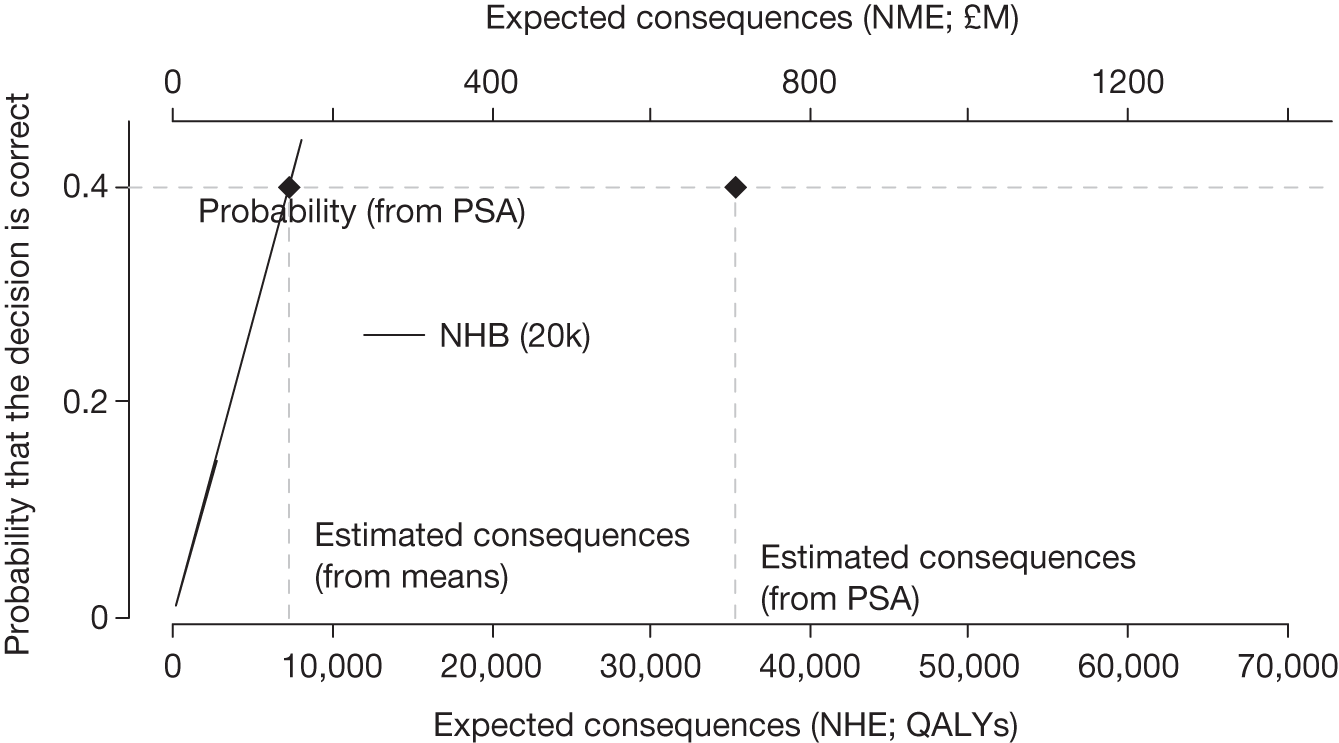
The previous analysis assumed that the differences in NHEs when etanercept is not cost-effective are the averages; however, this is not necessarily true and estimates based on means may substantially under- or overestimate the expected consequences of uncertainty (see Appendix 11, Why the consequences of uncertainty differ from mean incremental effects). To accurately record the differences between the NHEs of the alternative treatments and the probability that these errors will occur, the results from the PSA can be used. The distribution of consequences is illustrated in Figure 79. Most commonly (44.3%) there are no consequences because etanercept is the correct decision. When it is not, there is a greater chance of relatively small consequences (19% are < 27,250 QALYs), which occur predominantly when adalimumab offers the highest NHEs. There is a small chance of larger consequences (< 0.1% chance that they are > 190,750 QALYs), when palliative care offers the highest NHEs, that is, there remains important uncertainty about the cost-effectiveness of treatment. The expected consequence of uncertainty (35,341 QALYs or £706M) is simply the average over this distribution. Again, this can be interpreted as an estimate of the population NHEs that could be gained over the time horizon of this technology if the uncertainties could be immediately resolved. A judgement at this point that more research might be worthwhile seems reasonable, as the potential benefits exceed the likely costs.
FIGURE 79.
Distribution of the consequences of uncertainty in PsA.
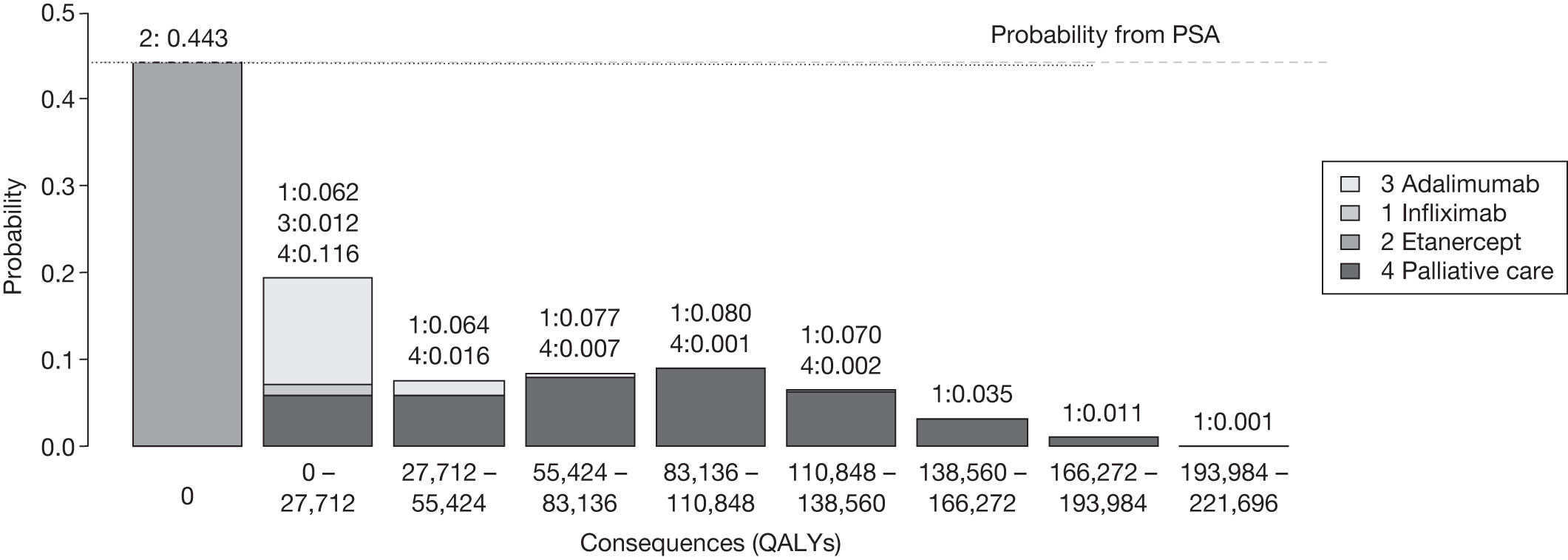
The time horizon over which evidence generated by research about a technology might be valuable may be longer (or shorter) than the period over which the technology is used. In this case the technology time horizon is assumed to be equal to the time horizon for the benefits of research, that is, 10 years. Table 129 shows the expected consequences for different technology time horizons. The consequences increase with the technology time horizon and will also increase with the size of the patient population.
| Technology time horizon, years | Expected consequences, QALYs (£M) |
|---|---|
| 5 | 19,186 (3837) |
| 10 | 35,341 (7068) |
| 15 | 48,943 (9788) |
| 20 | 60,396 (12,079) |
Alternative scenarios
There are often alternative views about the quality and relevance of evidence as well as other assumptions that might be made when estimating expected costs and QALYs. These are commonly presented as separate scenarios, with estimates of costs and QALYs presented for each. Much of the deliberation by the NICE Appraisal Committee often surrounds the scientific value judgements required to judge the credibility of the alternative assumptions represented by the scenarios. The type of probabilistic analysis reported represents the uncertainty within each scenario and will be sufficient to indicate the potential benefits of research when only one scenario is regarded as credible. However, when more than one scenario might be credible and carry some ‘weight’, there will be uncertainty between as well as within scenarios. The ‘weighting’ of scenarios can be made explicit by assigning probabilities to represent how credible each is believed to be. The weighted average of costs and QALYs across scenarios can easily be calculated. It is also tempting to take a simple weighted average of the expected consequences of uncertainty across these scenarios as well; however, a simple weighted average may under- or overestimate the combined consequences of uncertainty within and between scenarios. The correct estimate requires the probabilities (weights) to be applied directly to the simulated output from PSA rather than to the mean values. Although this does not require additional simulation and is quick and easy to implement, it does require either that the probabilities are made explicit in advance or that estimates are presented for a range of probabilities that might represent the judgement of the Appraisal Committee following deliberation.
An alternative assumption of a common class effect across the three biologics was considered in the PsA case study (scenario B) but this was judged less credible than the analysis that allowed differential effects (scenario A). The alternative scenario made etanercept less likely to be cost-effective (Table 130) and increased the expected consequences of uncertainty from 34,930 to 38,521 QALYs (Figure 80). In this case a simple weighted average of expected consequences based on the probability assigned to each scenario is, in general, lower than the correct estimate of expected consequences based on the output from PSA.
| Treatment | ICER (£/QALY) | Cost-effectiveness threshold £20,000 per QALY | Cost-effectiveness threshold £30,000 per QALY | ||||
|---|---|---|---|---|---|---|---|
| Incremental NHE, QALYs (£M) | Probability cost-effective | Expected consequences, QALYs (£M) | Incremental NHE, QALYs (£M) | Probability cost-effective | Expected consequences, QALYs (£M) | ||
| Scenario A | |||||||
| 1: Infliximab | 60,965 | –42,240 (–844) | 0.012 | 35,341 (707) | –21,279 (–425) | 0.113 | 18,079 (542) |
| 2: Etanercept | 17,733 | 3306 (66) | 0.443 | 12,292 (245) | 0.502 | ||
| 3: Adalimumab | 14,622 | 40,284 (805) | 0.146 | 76,746 (1534) | 0.103 | ||
| 4: Palliative care | – | – | 0.399 | – | 0.280 | ||
| Scenario B | |||||||
| 1: Infliximab | 59,208 | –42,349 (–846) | 0.003 | 38,521 (770) | –21,071 (–421) | 0.065 | 22,008 (660) |
| 2: Etanercept | 32,630 | –309 (–6.1) | 0.306 | 0 | 0.332 | ||
| 3: Adalimumab | 15,036 | 4451 (8.9) | 0.293 | 89,451 (1789) | 0.329 | ||
| 4: Palliative care | – | – | 0.398 | – | 0.274 | ||
FIGURE 80.
Expected consequences of uncertainty with alternative scenarios: PsA case study.
Point 4: Is research possible with approval?
The fourth point on the checklist requires an assessment of what type of evidence is needed and a judgement of whether or not the research required to generate it can be conducted while the technology is approved, that is, at the following point in the algorithm:
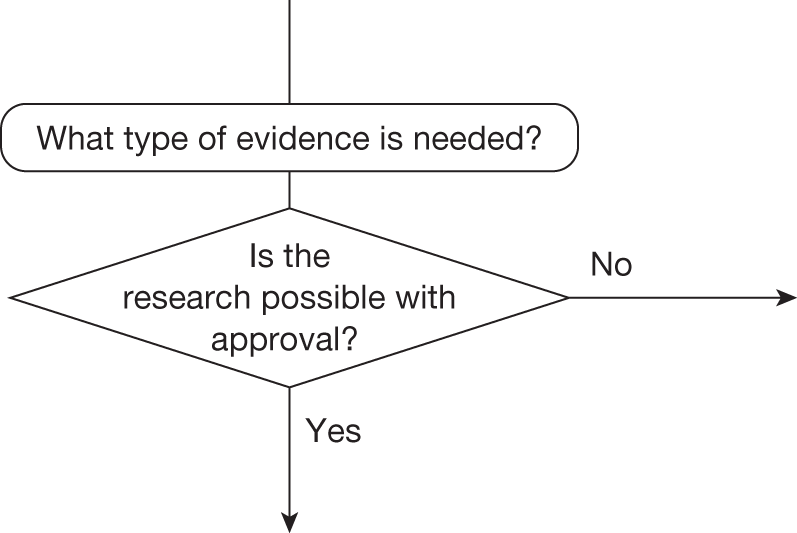
This requires judgements about (1) how important particular types of parameters are to estimates of costs and QALYs, (2) what values these parameters would have to take to change a decision based on expected cost-effectiveness, (3) how likely is it that parameters might take such values and (4) what would be the consequences if they did, that is, what might be gained in terms of population NHEs if the uncertainty in the values of these parameters could be immediately resolved.
Assessing the importance of parameters
The expected cost-effectiveness of infliximab, adalimumab and palliative care is based on the relationship between the input parameters (see Table 122) and outputs of costs and QALYs. A simple summary of the direction and strength of these relationships can be provided by the elasticity, that is, the proportionate change in NHEs owing to a 1% change in the value of a parameter (Table 131). Parameters with high elasticities (especially with respect to differences in NHEs) might be regarded as more ‘important’.
| Parameter | Elasticity over the NHEs (QALYs) of | Elasticity over the incremental NHEs (QALYs) of | ||||||
|---|---|---|---|---|---|---|---|---|
| 1: Infliximab | 2: Etanercept | 3: Adalimumab | 4: Palliative care | 2: Etanercept vs 4: Palliative care | 2: Etanercept vs all | |||
| Natural history | 1 | LT_NH_HAQ | –0.15445 | –0.08421 | –0.2139 | –0.04515 | –0.0167 | –0.24492 |
| 2 | PsARC_NH | – | – | – | –0.004 | – | – | |
| 3 | HAQ_NH | – | – | – | –0.004 | – | – | |
| 4 | PASI_50_NH | – | – | – | –0.003 | – | – | |
| 5 | PASI_75_NH | – | – | – | –0.004 | – | – | |
| 6 | PASI_90_NH | – | – | – | –0.004 | – | – | |
| 7 | U_HAQ | –0.22571 | –0.16120 | –0.10134 | –0.13878 | –0.01850 | –0.22297 | |
| 8 | U_PASI | –0.15774 | –0.09167 | –0.02711 | –0.05061 | –0.01766 | –0.25942 | |
| Treatment effect | 9 | Withdrawal | –0.19082 | –0.12534 | –0.06514 | –0.09596 | –0.01781 | –0.23562 |
| 10 | Prob_PSARC | –0.15254 | –0.07236 | –0.01781 | – | –0.01478 | –0.27423 | |
| 11 | HAQ_noPSARC | –0.15338 | –0.08665 | –0.02275 | – | –0.01750 | –0.38879 | |
| 12 | HAQ_resp | –0.15469 | –0.08569 | –0.02213 | – | –0.01698 | –0.37380 | |
| 13 | PASI_50 | –0.15314 | –0.08650 | –0.02213 | – | –0.01752 | –0.39059 | |
| 14 | PASI_75 | –0.15236 | –0.08627 | –0.02165 | – | –0.01766 | –0.39315 | |
| 15 | PASI_90 | –0.15330 | –0.08665 | –0.02235 | – | –0.01752 | –0.39095 | |
| Cost | 16 | C_psoriasis | –0.16242 | –0.09599 | –0.03224 | –0.05698 | –0.01757 | –0.25331 |
| 17 | C_HAQ | –0.15838 | –0.09263 | –0.02796 | –0.05167 | –0.01774 | –0.26011 | |
Although these measures of importance are more instructive than a series of arbitrary one-way sensitivity analyses, they do not directly help the assessment of what values parameters must take to change decisions and how likely such values might be. A simple summary of the values that particular parameters must take to make each of the alternatives cost-effective can also be provided (Table 132). It shows, for example, that if HAQ-_resp is valued between –0.371 and –0.661 for adalimumab (treatment 3) or at –0.838 for infliximab (treatment 1), treatments other than etanercept (treatment 2) are deemed cost-effective. However, although instructive, such ‘threshold values’ do not indicate how likely it is that the threshold will be crossed.
| Parameter | Mean value | 1: Infliximab | 2: Etanercept | 3: Adalimumab | 4: Palliative care | ||
|---|---|---|---|---|---|---|---|
| Natural history a | 1 | LT_NH_HAQ | 0.018 | – | 0.014 to 0.044 | – | 0.004 to 0.014 |
| 2 | PsARC_NH | 0.249 | – | 0.001 to 0.920 | – | – | |
| 3 | HAQ_NH | –0.218 | – | –0.961 to 0 | – | – | |
| 4 | PASI_50_NH | 0.130 | – | 0 to 0.934 | – | – | |
| 5 | PASI_75_NH | 0.044 | – | 0 to 0.688 | – | – | |
| 6 | PASI_90_NH | 0.016 | – | 0 to 0.530 | – | – | |
| 7 | U_HAQ | –0.298 | – | –0.279 to 0.315 | – | – | |
| 8 | U_PASI | –0.004 | – | –0.003 to 0.005 | – | – | |
| Treatment effect | 9 | Withdrawal | –1.823 | – | –1.189 to 2.711 | – | – |
| 10 | Prob_PSARCb | E: 0.713 | – | 0.464 to 0.883 | – | – | |
| A: 0.587 | |||||||
| I: 0.795 | |||||||
| 11 | HAQ_noPSARC | E: –0.190 | – | –0.122 to 0.478 | – | – | |
| A: –0.130 | |||||||
| I: –0.194 | |||||||
| 12 | HAQ_resp | E: –0.630 | –0.838 | –0.493 to 0.930 | –0.371 to 0.661 | – | |
| A: –0.103 | |||||||
| I: –0.650 | |||||||
| 13 | PASI_50 | E: 0.403 | – | 0.120 to 0.738 | – | – | |
| A: 0.738 | |||||||
| I: 0.913 | |||||||
| 14 | PASI_75 | E: 0.177 | – | 0.044 to 0.434 | – | – | |
| A: 0.477 | |||||||
| I: 0.769 | |||||||
| 15 | PASI_90 | E: 0.074 | – | 0.015 to 0.206 | – | – | |
| A: 0.257 | |||||||
| I: 0.557 | |||||||
| Cost | 16 | C_psoriasis | 198 | – | 129.11 to 250.30 | – | – |
| 17 | C_HAQ | 187 | – | 166.38 to 222.44 | – | – | |
The judgement about how likely it is that parameters might take values that will change the technology expected to be cost-effective can be informed by the results of the PSA. The distributions assigned to the parameters in PSA describe how uncertain the parameter estimates are, such that they ought to reflect the amount and quality of existing evidence. The probability that each parameter might take values that would lead to each of the alternatives being cost-effective is reported in Table 133 for the uncertain parameters. This essentially decomposes the overall probabilities reported in Table 130 into the contribution that each parameter makes. It indicates that it is uncertainty in the estimates of natural history of HAQ progression that contributes most to the probability of error associated with etanercept. At certain values of this parameter treatment 4 (palliative care) would be considered cost-effective. In addition, uncertainty regarding the treatment effect (measured using HAQ) also contributes to the probability of error. At certain values treatment 3 (adalimumab) would be considered cost-effective.
| Parameter | 1: Infliximab | 2: Etanercept | 3: Adalimumab | 4: Palliative care | ||
|---|---|---|---|---|---|---|
| Natural history | 1 | LT_NH_HAQ | 0.646 | – | – | 0.354 |
| 2 | PsARC_NH | – | 1 | – | – | |
| 3 | HAQ_NH | – | 1 | – | – | |
| 4 | PASI_50_NH | – | 1 | – | – | |
| 5 | PASI_75_NH | – | 1 | – | – | |
| 6 | PASI_90_NH | – | 1 | – | – | |
| 7 | U_HAQ | – | 1 | – | – | |
| 8 | U_PASI | – | 1 | – | – | |
| Treatment effect | 9 | Withdrawal | – | 1 | – | – |
| 10 | Prob_PSARC | – | 1 | – | – | |
| 11 | HAQ_noPSARC | – | 1 | – | – | |
| 12 | HAQ_resp | 0 | 0.812 | 0.187 | – | |
| 13 | PASI_50 | – | 1 | – | – | |
| 14 | PASI_75 | – | 1 | – | – | |
| 15 | PASI_90 | – | 1 | – | – | |
| Cost | 16 | C_psoriasis | – | 1 | – | – |
| 17 | C_HAQ | – | 1 | – | – | |
What type of evidence is needed?
An assessment of the likely consequences of the uncertainty described above is required. This assessment can directly inform the judgement of what evidence is needed and whether or not the type of research required to generate it will be possible with approval. The expected consequences of uncertainty associated with each parameter are reported in Table 134. This decomposes the overall expected consequences into the contribution that each parameter (or group of parameters) makes. Note that the overall expected consequences of uncertainty will not, in general, equal the sum of the expected consequences for each of the parameters (or groups of parameters) separately. This is because the overall consequences take account of the joint effect of uncertainty in all parameters simultaneously. Even if parameters are independent they will be related to differences in NHEs in different ways (see Table 134); sometimes the effect of uncertainty in one parameter may, to some extent, substitute for or complement the effect of uncertainty in the others. Table 134 confirms that it is uncertainty regarding the natural history of progression and the treatment effect (HAQ gain for biologics) that contributes towards the value of further research. There is potentially the most to be gained by resolving this uncertainty through additional research (8694 and 1201 QALYs or £17M and £2.4M, respectively).
| Parameter | Expected consequences at the population level (based on PSA), decomposed by treatment choice, QALYs | ||||||
|---|---|---|---|---|---|---|---|
| 1: Infliximab | 2: Etanercept | 3: Adalimumab | 4: Palliative care | Overall | |||
| Natural history | 1 | LT_NH_HAQ | – | – | – | 8694 | 8694 |
| 2 | PsARC_NH | – | – | – | – | – | |
| 3 | HAQ_NH | – | – | – | – | – | |
| 4 | PASI_50_NH | – | – | – | – | – | |
| 5 | PASI_75_NH | – | – | – | – | – | |
| 6 | PASI_90_NH | – | – | – | – | – | |
| 7 | U_HAQ | – | – | – | – | – | |
| 8 | U_PASI | – | – | – | – | – | |
| Treatment effect | 9 | Withdrawal | – | – | – | – | – |
| 10 | Prob_PSARC | – | – | – | – | – | |
| 11 | HAQ_noPSARC | – | – | – | – | – | |
| 12 | HAQ_resp | – | – | 1201 | – | 1201 | |
| 13 | PASI_50 | – | – | – | – | – | |
| 14 | PASI_75 | – | – | – | – | – | |
| 15 | PASI_90 | – | – | – | – | – | |
| Cost | 16 | C_psoriasis | – | – | – | – | – |
| 17 | C_HAQ | – | – | – | – | – | |
The potential benefits of resolving the uncertainty associated with groups of parameters are presented in Table 135. The most significant consequences of uncertainty associated with parameters relate to the natural history of PsA (8697 QALYs or £17.4M). Value is also associated with uncertainty regarding the treatment effect (1201 QALYs or £2.4M).
| Group of parameters | Overall expected consequences, QALYs | Overall expected consequences, costs (£M) |
|---|---|---|
| Natural history | 8694 | 17.4 |
| Utility | 0 | 0 |
| Treatment effect | 1201 | 2.4 |
| Costs | 0 | 0 |
The results in Table 135 suggest that a further observational (registry) study may well be worthwhile. In addition, there may be reason to believe that a study looking at the effectiveness of adalimumab, infliximab and etanercept in terms of the HAQ gain for responders might be worthwhile. It is likely that this research would have to be undertaken as part of a randomised trial design (RCT). Non-randomised designs, although still imposing significant costs, are likely to be cheaper than a RCT. It is therefore reasonable to assume that the research associated with the greatest value (natural history of progression) could be undertaken if infliximab, palliative care or adalimumab were approved for use in the NHS.
Types and categories of guidance resulting from points 3 and 4
Points 3 and 4 of the checklist are critical because if research is not judged to be worthwhile then no further assessments are required. In the case of PsA, more research appears to be worthwhile; the expected consequences of uncertainty are high. Whether the research required to generate the evidence needed can be conducted while the technology is approved for widespread use will determine whether AWR or OIR is a possibility. Table 136 summarises the categories and types of guidance that could ultimately result from the sequence of assessments up to point 4.
| Assessment | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Guidance |
|---|---|---|---|---|---|---|---|---|
| 7 | No | No | Yes | Yes | Yes/no | Yes | – | OIR2 |
| 8 | No | No | Yes | Yes | Yes/no | No | – | Reject1 |
Do the benefits of research exceed the costs?
The judgements made at points 5 and 6 of the checklist are critical because if the benefits of research are not judged to exceed the costs then no further assessments are required (unless there are significant irrecoverable costs; see Appendix 4). If they are and research can be conducted with approval, then AWR would be appropriate; however, other sources of uncertainty need to be assessed first as they will influence the potential benefits of research and, even when research is not conducted, they will also influence the appropriate category of guidance when there are significant irrecoverable costs.
Point 5: Will other sources of uncertainty resolve over time?
The fifth point on the checklist requires an assessment of whether or not changes are likely to occur in the future that will influence the cost-effectiveness of the alternative technologies and the potential benefits of research, that is, at the following point in the algorithm:
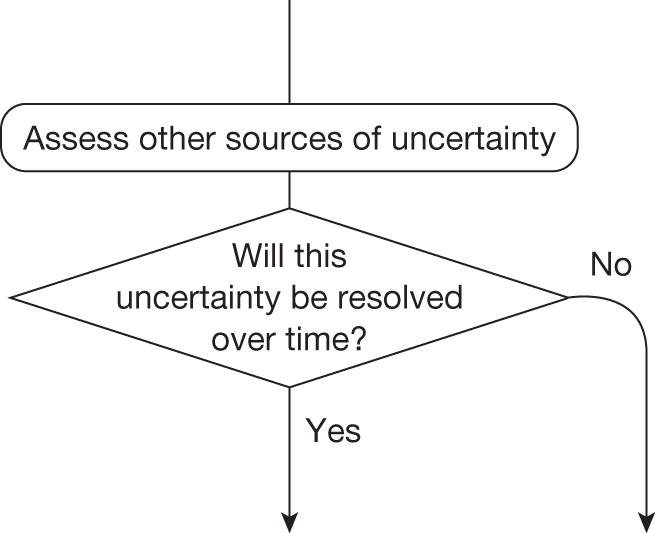
This assessment requires information about (1) changes in the prices of the technology and its comparators, (2) the emergence of new technologies that might make existing ones obsolete or change their cost-effectiveness and (3) other relevant research reporting. A number of potential sources of information and evidence were examined to inform this assessment for each case study (see Appendix 4). However, many potentially useful sources were either proprietary or public access was restricted, making it surprisingly difficult to inform these assessments with publically available information. When information and estimates were available they were often not directly relevant to a UK context.
Changes in the prices of the technology and its comparators
Changes in prices influence not only expected cost-effectiveness but also uncertainty and the potential benefits of research to future patients, for example if the price of a technology expected to be cost-effective is likely to fall significantly just before research reports the potential benefits will not be realised because approval of the technology will be less uncertain and there may be much less or little to gain from the results of the research. This assessment requires information about when major changes in prices are likely and some evidence about the likely extent of any changes. A major event in the life cycle of a pharmaceutical technology is the date at which the patent expires and cheaper generic versions of the brand become available. Although the date of patent expiry is, of course, known, it is surprisingly difficult to obtain the relevant date for particular products in the UK from publically available sources. Evidence of the extent to which the prices of generic versions are below the original brand price is also difficult to obtain and likely to differ by health-care system, type of technology, indication and time since patent expiry.
Although it was possible in the PsA case study to find patent expiry dates for etanercept (Enbrel), infliximab (Remicade) and adalimumab (Humira) in the USA (2012, 2014 and 2017, respectively) they were not available for the UK on the national patent database (Intellectual Property Office). There is a need to consider how access to the type of information required during NICE appraisal can be provided and how estimates of likely changes in prices relevant to the UK can be made readily available, if these assessments are to be routinely made.
Entry of new technologies
The entry of a new technology may make the existing technology that is expected to be cost-effective obsolete (no longer the most cost-effective alternative). Even when it does not, it will tend to change the relative cost-effectiveness of the alternatives, influencing how uncertain a decision to approve the original technology will be for future patients and the potential gains from research. A number of potential sources of information were examined to identify new technologies relevant to the indications that were likely to become available. These included a variety of sources related to NICE topic selection, information about licence applications and clinical research in Phases I, II and III as well as evidence of the probability that earlier phase research leads to entry (probability of successful licence) and the likely time of entry (time to launch from initiating Phase I, II and III research). Again, this information and evidence is fragmented and in some cases restricted, for example NHS Horizon Scanning Centre9.
It is known from the STA update (golimumab)237 that a new comparator was available within 1 year of TA199. 125 There is a single trial for this comparing golimumab with placebo. Results showed that golimumab was not as effective as other strategies. To update the searches from this period onwards, new licence applications since the golimumab STA were searched using www.ukmi.nhs.uk/applications/NDO/dbSearch.asp. The terms ‘etanercept’, ‘infliximab’ and ‘adalimumab’ were used. A total of 742 records were found of which two were relevant (apremilast and certolizumab); however, these were not ongoing at the time of the NICE appraisal.
As no new evidence would have been available at the time of TA199125 (golimumab trial anticipated but results not known), information about the technology is assumed to be limited. As such, scenarios are used in Point 6: Are the benefits of research greater then the costs? to explore the implications of this.
Other research reporting of the technology and its comparators
Research that is already under way, commissioned or likely to be undertaken, whether in the UK or elsewhere, is relevant for two reasons. First, if it is research based in the UK then guidance might impact on recruitment and the successful completion of this research. Second, when this research reports there is a chance that it will change the estimates of cost-effectiveness and resolve some of the current uncertainties. In other words, there is little to be gained by recommending OIR or AWR if the uncertainty is likely to be resolved in the near future when other research reports. A number of potential sources of information were examined to identify clinical research under way at the time of the relevant appraisal, including national and international trial registries (e.g. ClinicalTrials.gov, WHO ICTRP, Current Controlled Trials) and other databases that report NHS-funded research and not just clinical trials (e.g. NRR and UKCRN). Searches were also conducted for the recent STA. 237
Despite an assiduous search no relevant records were identified. This may suggest that no other research was ongoing or expected for these comparators in these indications or it may indicate that currently available sources are fragmented, incomplete and/or difficult to access.
Point 6: Are the benefits of research greater than the costs?
The sixth point on the checklist requires a judgement on whether or not the potential benefits of conducting further research (initially considered at point 3) are likely to exceed the costs, that is, at the following point in the algorithm:
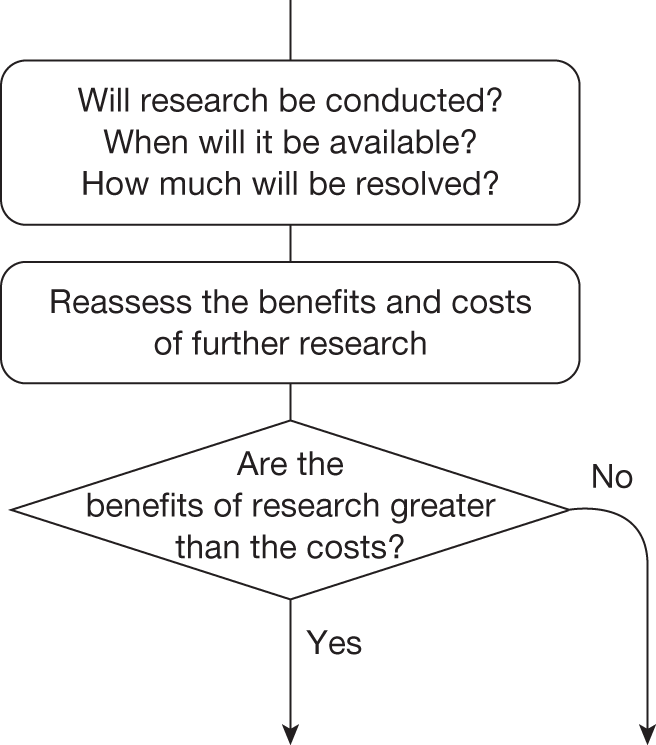
This requires an assessment of (1) whether or not the type of research that is required is likely to be conducted, (2) if conducted, when the results are likely to be available, (3) how much uncertainty is likely to be resolved and (4) the likely impact of any other sources of uncertainty on the longer-term benefits of research.
Will the research be conducted?
Even if research is recommended, it might not be undertaken by manufacturers or commissioned by research funders, and there is no guarantee that research will be able to recruit or complete. The expected consequences of uncertainty for PsA reported in Point 3: Does more research seem worthwhile? are illustrated in Figure 81 for a range of probabilities that research will be successfully undertaken. The potential gains from research depend on a judgement of whether the research recommended as part of OIR or AWR will be successfully completed. The cost of research (in this case considered to be either £1.5M or £10M) can be compared directly with the potential benefits by either expressing the potential gains in population NHEs as the equivalent NHS resources (i.e. the resources that would be required to generate the same NHEs) or expressing the cost of research in terms of the QALYs that could be gained elsewhere in the NHS by using the same resources to provide access to health care. Figure 81 shows the expected benefits of research minus its costs. Even assuming a cost of £10M for research the benefits of research outweigh this at all probabilities > 0 of research being conducted.
FIGURE 81.
Expected potential benefits of research.
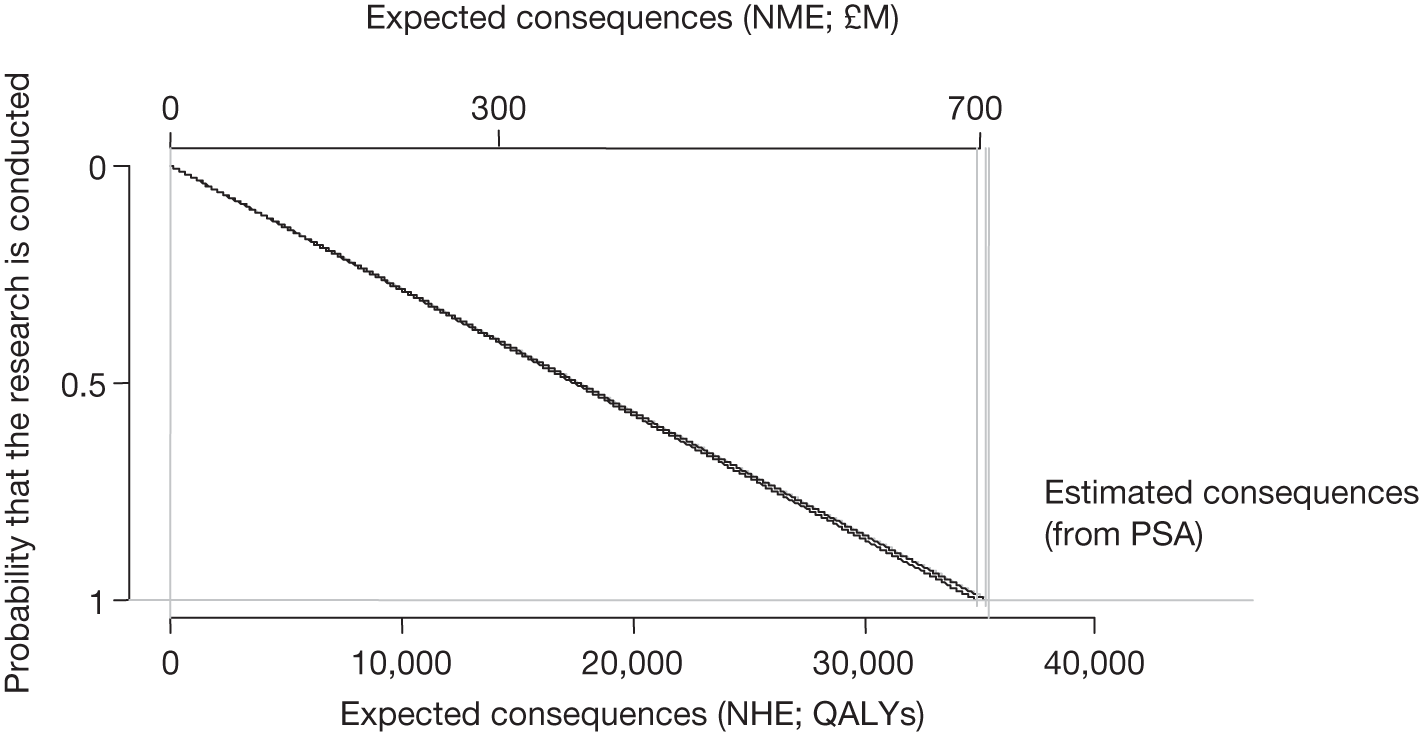
When will it be available?
Research, even if commissioned and successfully completed, will take time to complete and report; therefore, any assessment of the potential benefits should account for the fact that patient populations will not benefit from the results of research until they are available. If treatment decisions are irreversible (e.g. an acute indication or a irreversible chronic condition such as PsA) then it is only those patients who are incident after the research reports who will realise any of the potential benefits. For treatment decisions that are not irreversible, prevalent and incident patients can benefit from the results of the research. Patients prevalent while the research is undertaken will not benefit immediately but those who survive can benefit from the results once the research is completed.
How long research might take to report will depend in part on the design (follow-up, sample size and end points), recruitment rates and size of the eligible patient population, as well as on how efficient the organisation and data collection might be.
The time needed for research to report may be unknown. Table 137 shows that the time to report affects the maximum that one should be willing to invest in research. If research will take 5 years to report the maximum one should be willing to invest decreases by around £323M. Figure 82 shows the boundary for the maximum that one should be willing to invest in research.
| Time from adoption to research reporting, years | Maximum cost of research, £M |
|---|---|
| 0 (instant reporting) | 707 |
| 1 | 647 |
| 2 | 584 |
| 3 | 520 |
| 4 | 453 |
| 5 | 384 |
| 6 | 312 |
| 7 | 238 |
| 8 | 161 |
| 9 | 82 |
FIGURE 82.
Boundary for the maximum costs that one should be willing to invest in research by time to research reporting.
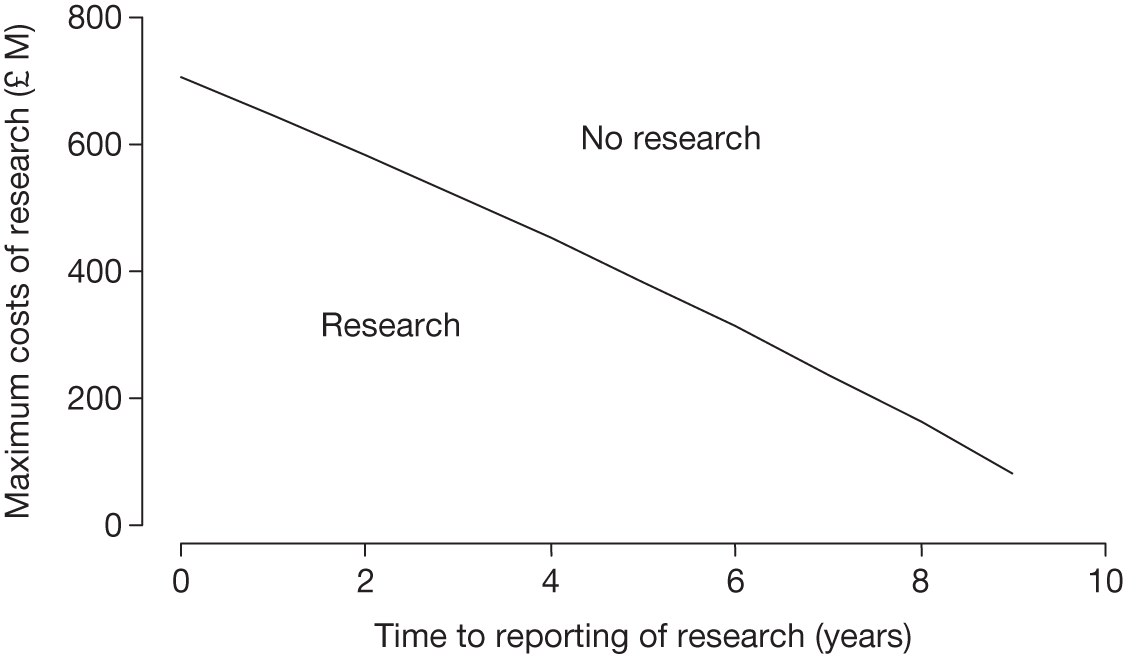
How much will be resolved?
How much of the uncertainty will be resolved depends on the type of research likely to be undertaken. In Point 4: Is research possible with approval? the potential benefits of different types of evidence were assessed. In the case of PsA, the most benefit can be gained by resolving the uncertainty regarding the natural history of PsA progression (measured using the HAQ). Table 138 reports the potential benefits of research by delay in research reporting. As long as research reports before 4 years, the potential benefits are likely to exceed the costs. This table shows that one should be willing to invest the most in research relating to the natural history of disease progression (measured using the HAQ). This is associated with the highest value across all potential delays to research reporting.
| Time from adoption to research reporting | Maximum cost of research informing specific parameter sets, QALYs (£M) | |||
|---|---|---|---|---|
| Natural history | Treatment effect | Utilities | Costs | |
| Immediate | 8694 (17.40) | 1201 (2.40) | 0 | 0 |
| 1 | 7683 (15.38) | 1061 (2.12) | 0 | 0 |
| 2 | 6708 (13.42) | 926 (1.85) | 0 | 0 |
| 3 | 5765 (11.53) | 796 (1.59) | 0 | 0 |
| 4 | 4854 (9.71) | 670 (1.34) | 0 | 0 |
| 5 | 3974 (7.95) | 549 (1.09) | 0 | 0 |
| 6 | 3123 (6.25) | 431 (0.86) | 0 | 0 |
| 7 | 2302 (4.61) | 372 (0.63) | 0 | 0 |
| 8 | 1508 (3.02) | 208 (0.42) | 0 | 0 |
| 9 | 741 (1.48) | 102 (0.20) | 0 | 0 |
What is the impact of other sources of uncertainty?
In Point 5: Will other sources of uncertainty resolve over time?, no information was identified relating to a change in price or other research reporting. There is evidence of new technologies emerging for PsA although it was unclear at the time of TA199125 what the impact of this would be. Different scenarios are therefore explored below. In scenario A the new technology enters at year 5 and makes etanercept entirely obsolete, that is, not cost-effective and not uncertain. At this point there is no value in the evidence generated by research about PsA; therefore, in these circumstances the potential value of research is likely to exceed its costs only if it reports quickly. In scenario B the new technology enters at year 5 and has similar NHEs to etanercept and the uncertainty surrounding its expected cost-effectiveness is also similar. Now research about PsA has more potential value in the future as it will also help resolve some of the uncertainty in the choice between etanercept and the new technology for patients who become incident after that time.
Tables 139 and 140 evaluate alternative times of introduction of the new technology in scenarios A and B. Under scenario B there is still some value in conducting further research even when research will not report until some point in the future. This is greater if the new technology is available sooner rather than later. It must be noted that the potential values of research presented in these tables, even after accounting for the type of evidence, follow-up and time until research reports, should still be regarded as upper bounds to the values that are likely to be realised by actual research for two reasons: (1) even well-designed research with large sample sizes will not fully resolve the uncertainty in the value that a parameter might take, especially in specific target populations and in a particular (future) context, and (2) insofar as implementation of NICE guidance is not ‘perfect’ and all clinical practice might not immediately respond to the results of research, the full benefits will be realised only over time or with additional implementation efforts. For these reasons a judgement of whether or not the benefits of research are likely to exceed the costs might be made conservatively, requiring evidence that, even in pessimistic scenarios, the research would still be worthwhile.
| Time from adoption to research reporting, years | Maximum cost of research for possible times of introduction of a new technology, £M | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Not introduced | 9 years | 8 years | 7 years | 6 years | 5 years | 4 years | 3 years | 2 years | 1 year | Immediate introduction | |
| 0 (instant reporting) | 706.83 | 646.58 | 584.22 | 519.68 | 452.87 | 383.73 | 312.17 | 238.11 | 161.50 | 82.10 | 0 |
| 1 | 624.71 | 564.46 | 502.10 | 437.56 | 370.76 | 301.62 | 230.06 | 155.99 | 79.33 | 0 | 0 |
| 2 | 545.37 | 485.12 | 422.76 | 358.22 | 291.42 | 222.28 | 150.72 | 76.65 | 0 | 0 | 0 |
| 3 | 468.72 | 408.47 | 346.11 | 281.56 | 214.76 | 145.62 | 74.06 | 0 | 0 | 0 | 0 |
| 4 | 394.65 | 334.40 | 272.04 | 207.50 | 140.70 | 71.56 | 0 | 0 | 0 | 0 | 0 |
| 5 | 323.09 | 262.84 | 200.48 | 135.94 | 69.14 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | 253.95 | 193.70 | 131.94 | 66.80 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | 187.15 | 126.90 | 64.54 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 8 | 122.61 | 62.36 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 9 | 60.25 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Time from adoption to research reporting, years | Maximum cost of research for possible times of introduction of a new technology, £M | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Not introduced | 9 years | 8 years | 7 years | 6 years | 5 years | 4 years | 3 years | 2 years | 1 year | Immediate introduction | |
| 0 (instant reporting) | 706.8 | 1140.0 | 1588.7 | 2052.9 | 2533.4 | 3030.7 | 3545.4 | 4078.1 | 4629.4 | 5200.1 | 5790.7 |
| 1 | 624.7 | 1058.0 | 1506.6 | 1970.8 | 2451.3 | 2948.5 | 3463.2 | 3996.0 | 4547.3 | 5118.0 | 5118.0 |
| 2 | 545.3 | 978.7 | 1427.2 | 1891.4 | 2371.9 | 2869.2 | 3383.9 | 3916.0 | 4468.0 | 4468.0 | 4468.0 |
| 3 | 468.7 | 902.0 | 1350.6 | 1814.8 | 2295.3 | 2792.5 | 3307.2 | 3840.0 | 3840.0 | 3840.0 | 3840.0 |
| 4 | 394.6 | 828.0 | 1276.5 | 1740.7 | 2221.2 | 2718.5 | 3233.2 | 3233.2 | 3233.2 | 3233.2 | 3233.2 |
| 5 | 323 | 756.4 | 1204.9 | 1669.2 | 2149.6 | 2646.9 | 2646.9 | 2646.9 | 2646.9 | 2646.9 | 2646.9 |
| 6 | 253.9 | 687.3 | 1135.8 | 1600.0 | 2080.5 | 2080.5 | 2080.5 | 2080.5 | 2080.5 | 2080.5 | 2080.5 |
| 7 | 187.1 | 620.5 | 1069.0 | 1533.2 | 1533.2 | 1533.2 | 1533.2 | 1533.2 | 1533.2 | 1533.2 | 1533.2 |
| 8 | 122.6 | 555.9 | 1004.4 | 1004.4 | 1004.4 | 1004.4 | 1004.4 | 1004.4 | 1004.4 | 1004.4 | 1004.4 |
| 9 | 60.2 | 493.6 | 493.6 | 493.6 | 493.6 | 493.6 | 493.6 | 493.6 | 493.6 | 493.6 | 493.6 |
Types and categories of guidance resulting from points 5 and 6
The assessment that the benefits of research are likely to exceed the costs leads to a final decision at point 6 in the checklist, therefore negating the need to make the assessments at point 7 (Table 141). The decision is to recommend OIR. The model parameter associated with the greatest value is the progression of disease, as measured using the HAQ. There is also some value associated with establishing the effectiveness of biologics, measured using HAQ response. The first of these uncertainties could be addressed by commissioning an observational study, perhaps a registry. The second issue is likely to require a RCT.
| Assessment | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Guidance |
|---|---|---|---|---|---|---|---|---|
| 7 | No | No | Yes | Yes | Yes | Yes | – | OIR2 |
Appendix 11 Technical notes to the case studies
Model linearity and correlation between parameters
Evaluating cost-effectiveness and the need for further research (and its value) using a decision model may pose analytical challenges if, for example, the model is non-linear or there is correlation between input parameters. In a linear model that does not feature correlation between parameters it is possible to calculate cost-effectiveness using a deterministic analysis, and the value of further research using one loop of simulation, otherwise more burdensome simulation procedures may need to be implemented. Independence between parameters (or the absence of correlation) is important if parameters need to be evaluated separately for their impact on decision uncertainty and the value of further research. a The existence of correlation depends greatly on how the input parameters were estimated; however, correlation may not have a significant impact on the results (i.e. in the assessment of linearity and in estimating the value of further research for specific parameters). An investigation of model linearity is required if it is proposed to use a deterministic analysis to inform the assessments described in Chapter 5. An investigation of correlation is required to know whether or not a deterministic analysis may be appropriate in some circumstances, and also to know what information can be presented to inform the assessments at points 3, 4 and 6 of the checklist described in Chapter 5.
Exploring model linearity
Why is model linearity important?
A decision model is a function used to calculate NHEs based on a set of P input parameters θ = {θ1,θ2,. . .,θp}. The model can be represented as a function g(.) such that NHE = g(θ). The input parameters are uncertain and thus in estimating the expected NHEs the following expression needs to be evaluated: E[NHE] = E[g(θ)]. It is current practice that this expectation is evaluated using a Monte Carlo simulation procedure (PSA), which entails (1) simulating from the distribution of the parameters, (2) evaluating the NHEs for each set of simulated parameters and (3) calculating the expectation by averaging the NHEs obtained from the simulations. Although the single Monte Carlo simulation procedure required to estimate cost-effectiveness is usually not too burdensome, the need to evaluate nested expectations in estimating the value of further research (for specific parameters) implies using nested simulations and therefore a much higher computation time. b If the analysis becomes unfeasible, analysts may either choose not to proceed or choose to apply simplifying assumptions (e.g. reduce the number of Monte Carlo simulations used).
The calculation of expected NHEs can however be simplified if g(.) is linear or multilinear in its parameters. In this case, E[g(θ)] is equal to g(E[θ]) and hence expected NHEs can be computed in a single calculation with the parameters set to their expected values (deterministic analysis), in this case eliminating the need for simulation procedures. However, the presence of non-linearity implies that the desired model output cannot be expressed as a function of the parameters set to their expected values, and proceeding with such a deterministic analysis will return biased results.
When is a model linear or multilinear?
Linearity happens when g(.) represents a sum (or subtraction) of parameters (e.g. g(θ1,θ2) = θ1 + θ2, in which case E[θ1 + θ2] = E[θ1] + E[θ2]). For this relation to sustain, it is not necessary that the summands are mutually independent. When the NHE function contains products of (independent) parameters, g(.) is said to be multilinear. If, for example, the NHE can be calculated using the product of θ1 and θ2, that is, g(θ1,θ2) = θ1 × θ2, then E[θ1 × θ2] is equal to E[θ1] × E[θ2]. This is valid only if θ1 and θ2 are independent. However, if the covariance (correlation) between these parameters is close to zero, this relation might be assumed to sustain.
How can model linearity be evaluated?
Evaluating model linearity is fairly straightforward if g(.), the decision model, can be defined using algebraic descriptions. In this case, even if the model is apparently non-linear there may be a way to express the conditional NHEs as a linear (or multilinear) function of transformations of parameters by rearranging the model equations. When the model function is complex it may be more difficult to express it algebraically, which impedes a direct evaluation of linearity.
In complex models, evaluating non-linearity can be carried out by comparing deterministic and probabilistic results. A first step may be to evaluate its impact over the overall cost-effectiveness results (NHEs). For this, the expected NHEs using probabilistic, that is, E[f(θ)], and deterministic, that is, f(E[θ]), estimates can be obtained and compared. Sufficient Monte Carlo simulations must be run to obtain a stable probabilistic estimate of E[f(θ)]. If this differs significantly from the deterministic estimate, it can be said that the model is non-linear. It may happen that the bias differs but is the same for all strategies. In this case, the existence of non-linearity may not be significant in the sense that the conclusions drawn from the cost-effectiveness analysis will not change. Observing that the deterministic and probabilistic estimates do not differ in term of expected NHEs does not ensure that the model is linear, as the estimates of the value of resolving all uncertainty in a specific parameter may still be affected. It is thus important to also evaluate linearity using the expected NHEs when conditioned on specific values of a parameter of interest (spanning through its plausible range). c Estimates of the consequences of uncertainty calculated using or not using the assumption of linearity can also be compared.
If the assumption of linearity is rejected then model outputs should be estimated on the basis of probabilistic analysis. The evaluations of linearity proposed here require that some probabilistic analysis be conducted; however, it could potentially reduce computation time if, conditional on the model appearing to be linear, additional analysis could be based on deterministic results. Note again that observing a difference between deterministic and probabilistic results indicates non-linearity but not observing a difference does not guarantee linearity.
Exploring correlation
The existence of correlation between parameters depends on how these input parameters were estimated. Examples of when correlated quantities are used to inform a model may be when more than one coefficient derived using a regression analysis is used to predict prognosis, or potentially when multiple results from an evidence synthesis procedure are used.
Why is correlation important?
Correlation is important because under certain conditions it may determine non-linearity (see Exploring model linearity). Also, it may determine how the value of resolving uncertainty on specific parameters is evaluated. In undertaking these calculations it is necessary to evaluate the expected NHEs when the parameters of interest take particular values while the remaining parameters are still uncertain (conditional NHE estimates, as described in Exploring model linearity; see notes a and b). Thus, in the presence of correlation, the distribution of the remaining parameters depends on the value assumed for the parameter(s) of interest, and this needs to be accounted for. On some occasions we may know these conditional distributions, in which case we may be able to explicitly use these in the estimation. d For example, coefficients from a linear regression analysis are commonly assumed multivariate normally distributed, where regression estimates inform both the means and the variance/covariance matrix. However, in other cases, information on the conditional distribution of sets of parameters may not be easy to obtain, for example when we have no access to more detailed results of analysis, or when correlation is implicitly introduced in the analyses, for example through the use of a random effect across parameters when relative treatment effects for multiple treatments were obtained jointly in a mixed-treatment comparison.
Note that if the parameters θC are probabilistically independent of the parameters θI, correlation does not play a part in computations, even if the subset of parameters in θC are not independent between themselves or the subset of parameters in θI are not independent between themselves. Parameters that are possibly correlated can thus be grouped (e.g. natural history parameters can be evaluated separately from treatment effect parameters) to avoid dealing explicitly with correlation. e In this case, however, conclusions over individual parameters cannot be drawn. In some situations it may be important to evaluate the parameters individually, especially if there is the need to further evaluate specific research designs (e.g. comparators for RCTs). In this context, correlation between parameters cannot be ignored as the results of investigations will be biased. If we suspect that there may be correlation between the parameter(s) of interest and its complementary set, and if this cannot be explicitly accounted for, it may be worth evaluating the presence of correlation and its impact on the results,f instead of proceeding to assume independence.
How can correlation and its impact be evaluated?
An evaluation of the existence and impact of correlation can be conducted by exploring:
-
Between-parameter correlation: The issue is that when non-monotonic transformations of parameters are used to define the model evaluating correlation between the untransformed parameters may not be informative.
-
Impact of correlation in cost-effectiveness: Consider two possibly correlated parameters, θ1 and θ2. We will examine the case in which the NHE of treatment A is a function of θ1 and the NHE of treatment B is a function of θ2. In the absence of correlation, alternative values of θ1 should not impact on cost-effectiveness estimates of treatment B – only through correlation the latter may change. g
-
Impact of correlation in decision uncertainty: The previous assessments aim to evaluate the impact of correlation on the values assumed by the expected NHEs. However, even small changes in the expected NHEs due to correlation can meaningfully impact on the adopt decision and/or on the maximum NHEs attained. It may be tempting to assess the impact of correlation by calculating the value of resolving uncertainty for potentially correlated parameters individually (i.e. assuming independence), summing these and comparing this sum with the estimate obtained by assessing the value of resolving uncertainty for the group of potentially correlated parameters; however, even in the presence of independence these values may not be equal.
As with the explorations proposed for linearity, the absence of evidence of correlation does not mean that there is no significant correlation; thus, care is needed in using the assumption of independence in further analysis.
Accounting for the investment profile of displaced interventions
When an intervention is approved that is more costly than its comparator the additional cost of the new intervention must be funded at the expense of other interventions in the NHS. Thus, the approval of a new intervention displaces the comparator, a less effective or more costly intervention used to treat the same disease, and other interventions in the NHS used to treat other diseases. The amount of this additional displacement depends on the difference in budget impact between the newly approved intervention and its comparator. The opportunity cost of new interventions has been well described in the literature and is the key economic argument for needing a threshold in a budget-constrained system. If the new intervention is more cost-effective than the intervention displaced then the health system efficiency has improved.
As funders make decisions about providing a new intervention they are interested not only in the total costs and health effects but also in how these costs and health effects are accrued over time. The accrual of costs and health effects can be shown using the investment profile previously described. Additional research or other sources of uncertainty resolving over time can cause the reimbursement decision to be reversed before the end of the lifetime of the intervention (the time horizon that was used in assessing cost-effectiveness). For some interventions a change of guidance would not prevent the investment profile from being completed; however, in those circumstances in which the accumulation of NHEs would cease at the point of a change in guidance, the investment profile can inform the decision-maker about the potential risk in terms of the NHEs accumulated to that point.
The incremental investment profiles used in this report take into account the timing of the costs and health effects of both the new intervention and its comparator(s); however, the incremental investment profile does not take into account the investment profiles of the additionally displaced non-comparator interventions because at the time of approval it is unknown which current interventions will be displaced. Because the investment profiles of the displaced interventions are not known it is important to assess the implicit assumptions being used and their implications.
In the investment profiles presented in the main report and the case studies (see Appendices 7–10) we consider the overall NHEs of the displaced interventions using the cost-effectiveness threshold (k). What we have not taken into account is how the overall NHEs of the displaced interventions are accrued over time.
For many interventions upfront costs are offset over time by future health benefits. Table 142 shows the population costs (C), health effects (H) and NHEs for two mutually exclusive interventions. The lifetime of use for the interventions is assumed to be 10 years, after which they are assumed to be obsolete.
| Year | New intervention | Comparator | Incremental NHE | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C (£) | H | NHE | Cumulative NHE | C (£) | H | NHE | Cumulative NHE | Per period | Cumulative | |
| 1 | 10,000 | 0.30 | –0.20 | –0.20 | 6000 | 0.30 | 0.00 | 0.00 | –0.20 | –0.20 |
| 2 | 10,000 | 0.30 | –0.20 | –0.40 | 6000 | 0.30 | 0.00 | 0.00 | –0.20 | –0.40 |
| 3 | 1000 | 0.32 | 0.27 | –0.13 | 1000 | 0.25 | 0.20 | 0.20 | 0.07 | –0.33 |
| 4 | 1000 | 0.34 | 0.29 | 0.16 | 1000 | 0.25 | 0.20 | 0.40 | 0.09 | –0.24 |
| 5 | 1000 | 0.36 | 0.31 | 0.47 | 1000 | 0.25 | 0.20 | 0.60 | 0.11 | –0.13 |
| 6 | 1000 | 0.38 | 0.33 | 0.80 | 1000 | 0.25 | 0.20 | 0.80 | 0.13 | 0.00 |
| 7 | 1000 | 0.40 | 0.35 | 1.15 | 1000 | 0.25 | 0.20 | 1.00 | 0.15 | 0.15 |
| 8 | 1000 | 0.40 | 0.35 | 1.50 | 1000 | 0.25 | 0.20 | 1.20 | 0.15 | 0.30 |
| 9 | 1000 | 0.40 | 0.35 | 1.85 | 1000 | 0.25 | 0.20 | 1.40 | 0.15 | 0.45 |
| 10 | 1000 | 0.40 | 0.35 | 2.20 | 1000 | 0.25 | 0.20 | 1.60 | 0.15 | 0.60 |
| Total | 28,000 | 3.60 | 2.20 | 20,000 | 2.60 | 1.60 | 0.60 | |||
Figure 83 shows the cumulative population NHEs for the two interventions from Table 142. The cumulative NHE of a new intervention must be higher than that of its comparators for it to be considered cost-effective. This means that the incremental cumulative NHE of the new intervention relative to the comparator will be positive at the end of the time horizon of interest.
FIGURE 83.
Cumulative NHEs of new and comparator interventions.
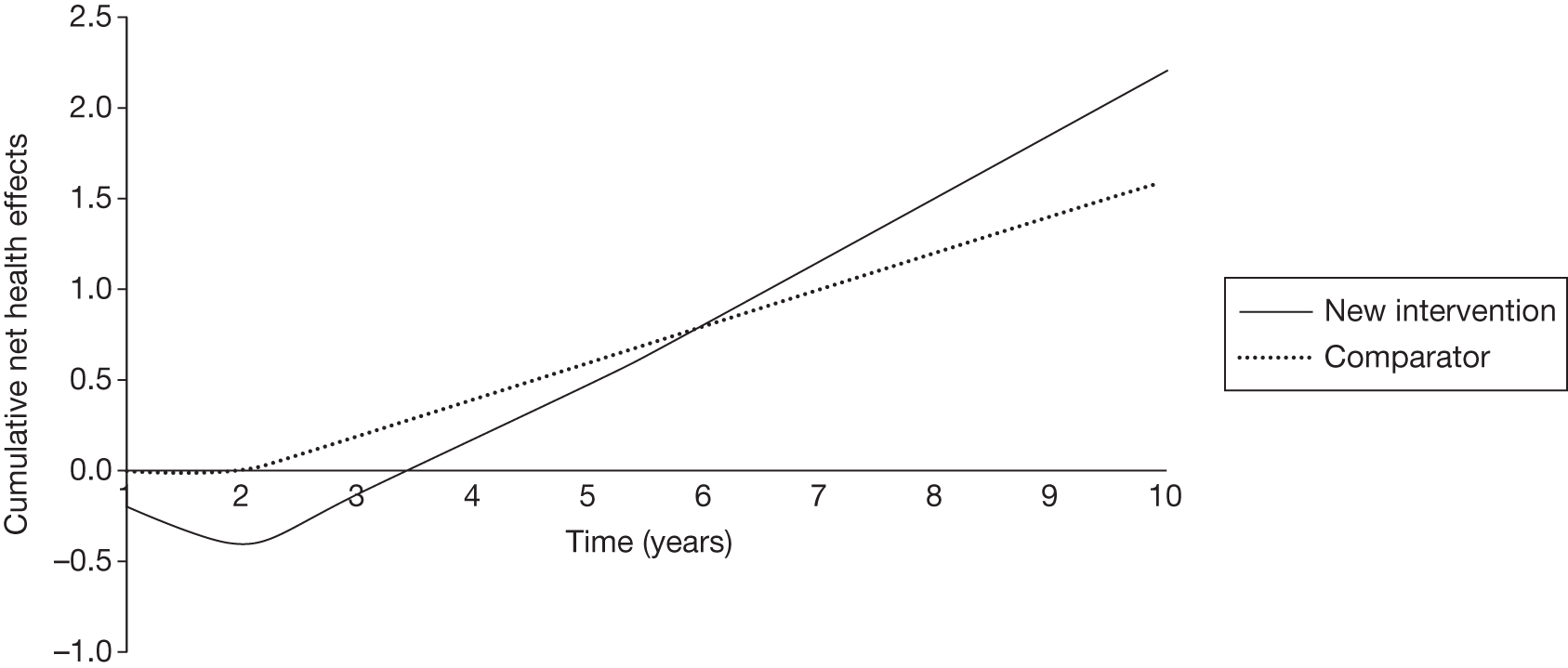
Figure 83 shows that the NHEs of the new intervention do not become positive until year 4. The cumulative NHEs from the new intervention do not exceed those offered by the comparator until year 6. As can be seen from Table 142, discontinuing the existing comparator would release £20,000 to fund the new intervention, which leaves a shortfall of £8000. Additional interventions must be displaced to fund this £8000. h Table 143 shows three alternative scenarios for how the additional displaced interventions accrue incremental NHEs over time (relative to the relevant next best alternative comparator intervention).
| Year | C (£) | H | NHE | Cumulative NHE |
|---|---|---|---|---|
| Scenario 1: more risky | ||||
| 1 | 800 | –0.06 | –0.10 | –0.10 |
| 2 | 800 | –0.06 | –0.10 | –0.20 |
| 3 | 800 | –0.06 | –0.10 | –0.30 |
| 4 | 800 | –0.01 | –0.05 | –0.35 |
| 5 | 800 | 0.04 | 0.00 | –0.35 |
| 6 | 800 | 0.04 | 0.00 | –0.35 |
| 7 | 800 | 0.09 | 0.05 | –0.30 |
| 8 | 800 | 0.14 | 0.10 | –0.20 |
| 9 | 800 | 0.14 | 0.10 | –0.10 |
| 10 | 800 | 0.14 | 0.10 | 0.00 |
| Total | 8000 | 0.40 | 0.00 | |
| Scenario 2: no risk | ||||
| 1 | 800 | 0.04 | 0.00 | 0.00 |
| 2 | 800 | 0.04 | 0.00 | 0.00 |
| 3 | 800 | 0.04 | 0.00 | 0.00 |
| 4 | 800 | 0.04 | 0.00 | 0.00 |
| 5 | 800 | 0.04 | 0.00 | 0.00 |
| 6 | 800 | 0.04 | 0.00 | 0.00 |
| 7 | 800 | 0.04 | 0.00 | 0.00 |
| 8 | 800 | 0.04 | 0.00 | 0.00 |
| 9 | 800 | 0.04 | 0.00 | 0.00 |
| 10 | 800 | 0.04 | 0.00 | 0.00 |
| Total | 8000 | 0.40 | 0.00 | |
| Scenario 3: less risky | ||||
| 1 | 800 | 0.14 | 0.10 | 0.10 |
| 2 | 800 | 0.14 | 0.10 | 0.20 |
| 3 | 800 | 0.14 | 0.10 | 0.30 |
| 4 | 800 | 0.09 | 0.05 | 0.35 |
| 5 | 800 | 0.04 | 0.00 | 0.35 |
| 6 | 800 | 0.04 | 0.00 | 0.35 |
| 7 | 800 | –0.01 | –0.05 | 0.30 |
| 8 | 800 | –0.06 | –0.10 | 0.20 |
| 9 | 800 | –0.06 | –0.10 | 0.10 |
| 10 | 800 | –0.06 | –0.10 | 0.00 |
| Total | 8000 | 0.40 | 0.00 | |
In scenario 1 the additional displaced interventions could be described as ‘more risky’ as they do not break-even until year 10. In scenario 2 the accumulation of NHEs is constant over time and so the interventions could be described as ‘no risk’. In scenario 3 the additional displaced interventions initially have positive incremental NHEs and so could be described as ‘less risky’. Figure 84 shows the cumulative incremental NHE curves of average displaced interventions for each of these scenarios. Note that the cumulative incremental NHEs of the additional displaced interventions are always equal to zero at year 10, which is consistent with the assumption underlying the use of a cost-effectiveness threshold.
FIGURE 84.
Cumulative incremental NHEs of additional displaced interventions.
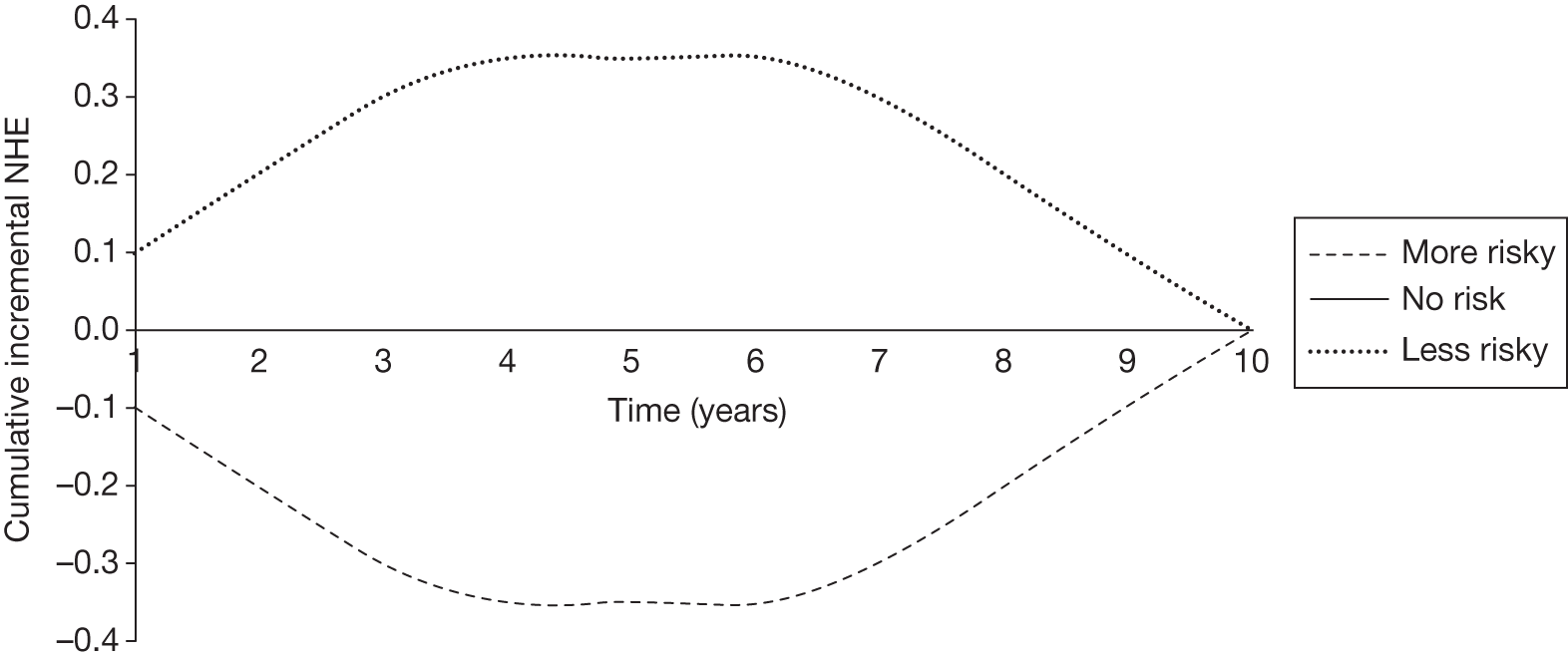
Figure 85 shows the incremental NHE curve for the new intervention relative to its comparator (solid black line). This incremental curve is equivalent to those used in the main report and case studies (see Appendices 7–10), which take into account the timing of the incremental NHEs relative to the comparator and the opportunity costs of the additional displaced interventions, but not the timing of the NHEs of the displaced interventions. This would suggest that the break-even point at which the cumulative NHEs became positive was at year 6. By considering different risk profiles of the displaced interventions we can see the difference in effect on the accumulation of NHEs, taking into account the total displaced interventions. If the investment profile of the displaced interventions is constant then the inclusion of the displaced interventions has no effect on the investment profile of the new intervention. If the investment profile of the displaced interventions is initially positive then the break-even point of the new intervention investment profile has been underestimated by not considering the displaced interventions. If the investment profile of the additional displaced interventions is initially negative (‘more risky’), the break-even point will have been overestimated.
FIGURE 85.
Cumulative incremental NHEs of the new intervention.
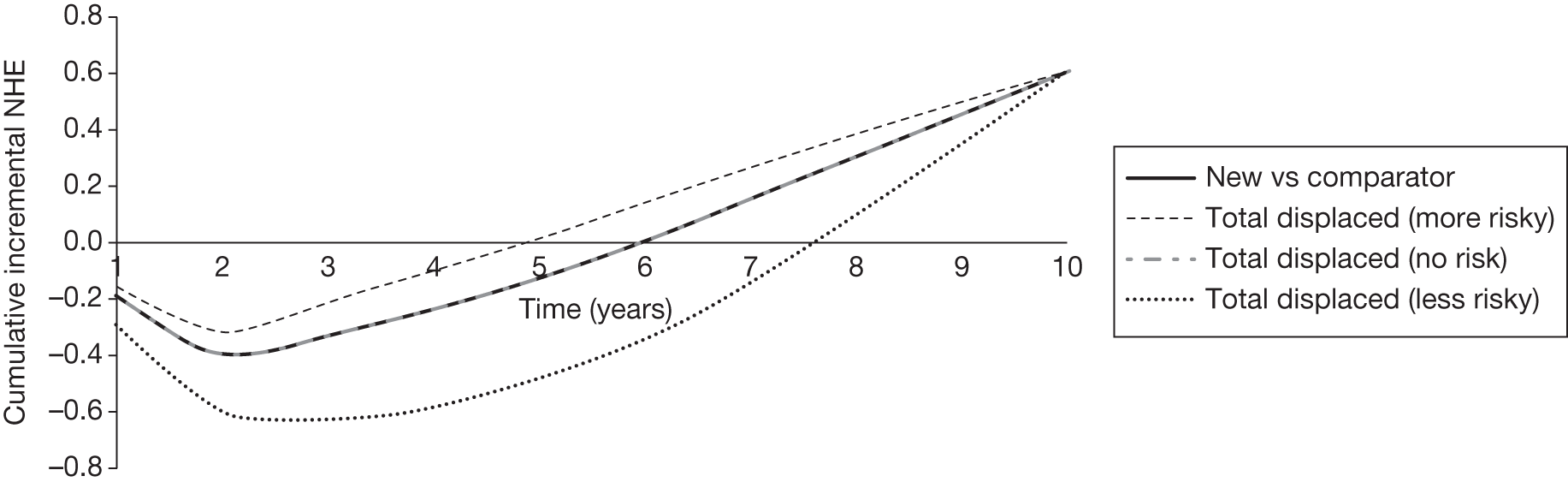
From the examples demonstrated the investment profile and break-even point of the new intervention is affected by its budget impact compared with the cost of the displaced interventions. The larger this difference the more interventions will be displaced and the more influence displaced interventions will have on the new intervention’s risk profile. The investment profile of the new intervention is also affected by the shape of the investment profile of the displaced intervention. If the displaced intervention has a non-constant investment profile then the current evaluation will change by whether it is more or less risky than the displaced intervention. If the displaced intervention has a riskier profile than the new intervention then the investment profile of the new intervention will be underestimated by not taking into account the displaced intervention. If the displaced intervention has a less risky profile then the investment profile of the new intervention will be overestimated by not taking into account the displaced intervention. And if there is no risk to the displaced intervention then the investment profile of the new intervention is not affected.
It is tempting to think that displaced interventions are likely to have reached the positive NHEs of their investment profile and that the risk of new interventions is being underestimated; however, all previous NHEs of displaced interventions must be considered sunk and the investment profile should be considered starting at the point of the decision moving forward, thus for the incident population of those who would receive the displaced intervention.
Why the consequences of uncertainty differ from mean incremental effects
A decision based on expected NHEs may be incorrect if the true value of the underlying evidence suggests that an alternative intervention to the one reimbursed would have offered greater health gains. The probability that a decision to recommend a particular intervention is incorrect is equal to the probability of observing a state of the world in which a different intervention would maximise NHEs. The cost of making such an error, measured as health forgone, is determined by the difference in NHEs between the intervention that was selected and the best that could have been achieved. Thus, the expected cost of uncertainty, regarding the choice of the optimum treatment, is a function of the probability of making such an error and the amount of health forgone if the wrong treatment were selected. By avoiding reimbursement of an intervention that turns out to be cost-ineffective, the decision-maker would expect better health gains overall. As such, the expected cost of uncertainty describes the magnitude of the health gains that could be achieved by eliminating the decision uncertainty. An estimate of the cost of uncertainty is required to inform the assessments made from point 3 of the checklist described in Chapter 5.
Estimating the cost of uncertainty
Assessing the cost of uncertainty requires estimates of the probability that the decision will be incorrect and the size of the associated health losses. Consider the simple example in Table 144 comparing two alternative interventions, j = 1,2. The available evidence (parameters) used to calculate the NHEs for a typical patient who receives either intervention is described by θ. This parameter can take one of four possible values.
| θ | NHE(1,θ) | NHE(2,θ) | Max. NHE |
|---|---|---|---|
| 1 | 0.4 | 0.5 | 0.5 |
| 2 | 0.1 | 0.7 | 0.7 |
| 3 | 0.6 | 0.4 | 0.6 |
| 4 | 1.0 | 0.8 | 1.0 |
| E θ | 0.5 | 0.6 | 0.7 |
Intervention 2 has the highest expected NHEs, maxjEθNHE(j,θ)=0.6, and would therefore be regarded as cost-effective based on current evidence. The incremental net health benefit from reimbursing intervention 2 instead of intervention 1 would be EθNHE(2,θ) – EθNHE(1,θ) = 0.6 – 0.5 = 0.1.
The probability of error and the consequences of that error in terms of health forgone can be estimated directly from the results of PSA. If, subsequent to intervention 2 being reimbursed, it was learned that the true value of θ was actually 3 or 4 (not 1 or 2), the decision based on current evidence would have been incorrect as intervention 1 would have offered greater NHEs and should have been reimbursed instead of intervention 2. The probability of making an error is therefore 0.5 (50%), assuming that all values of θ are equally likely. The consequences of this error in terms of health forgone are described by EθNHE(1,3) – EθNHE(2,3) = 0.6 – 0.4 = 0.2 for θ = 3 and EθNHE(1,4) – EθNHE(2,4) = 1.0 – 0.8 = 0.2 for θ = 4. This gives an expected health loss of 0.2. This expected health loss from making the wrong decision must be multiplied by the probability of error (50%) to express it in terms of the expected cost of uncertainty: 0.5 × 0.2 = 0.1.
In the absence of a probabilistic analysis, an assessment of expected NHEs can be made based on a deterministic analysis if the underlying model is linear or is multilinear with independent parameters. However, a deterministic analysis provides no information on the probability and consequences of error, and so an assessment of the cost of uncertainty would have to be made on the basis of additional, perhaps informal, considerations. Note that in this example the expected health loss if intervention 2 was selected in error is 0.2, which is greater than the incremental NHE of intervention 2 compared with intervention 1 across all values of θ (0.1). In general, the difference between the expected NHEs of competing interventions cannot provide a good approximation of the consequences of uncertainty.
Why averaging scenarios may be misleading
One source of uncertainty in estimating the true value of the expected costs and health outcomes associated with alternative interventions has been described variously as modelling, structural or scenario uncertainty. Scenario uncertainty arises when alternative plausible modelling assumptions can be made. Potential sources of scenario uncertainty include alternative assumptions about which sources of evidence are relevant to the decision problem, the choice of mathematical model to estimate model parameters and the type and structure of the decision model that is used to generate estimates of costs and health outcomes. Unless the scenario uncertainty can be characterised by a parameter within the decision model, its impact on decision uncertainty will not be reflected in routine PSA based on Monte Carlo simulation and further analytical processes are required. This section describes in more detail how to estimate appropriately the value of further research in the presence of both parameter and scenario uncertainty. This is relevant when providing information for the assessments required at points 3 and 4 of the checklist described in Chapter 5.
How to estimate cost-effectiveness in the presence of scenario uncertainty
The estimation of expected costs, c, and health outcomes, h, will be informed by the available evidence, θ, and the set of assumptions, s, that characterise a particular scenario. The results of a cost-effectiveness analysis for each alternative intervention, j, can be expressed in terms of NHEs,
where k is the cost-effectiveness threshold.
The assessment of cost-effectiveness is made by taking the expectation of NHEs across the parameter uncertainty and the range and likelihood of the alternative scenarios, EθEsNHE(j,θ,s).
Parameter uncertainty (uncertainty as to the true value of θ) can be characterised by assigning probability distributions to the model parameters. This allows the results of the model to be evaluated by PSA; a Monte Carlo simulation procedure can be used to repeatedly sample from those distributions a set of model inputs, and for each set calculate the corresponding model outputs. The expectation of the model outputs is found by averaging across the results of the Monte Carlo simulation. If scenario uncertainty has not been characterised by a probability distribution and sampled simultaneously alongside the parameter values, that Monte Carlo simulation procedure will estimate the expectation of NHEs across parameter uncertainty within a single scenario, for example EθNHE(j,θ,s = 1). The Monte Carlo simulation procedure must then be repeated for each possible scenario. The expected NHEs across parameter uncertainty from each scenario can then be combined utilising the likelihood of each possible scenario to describe the expected NHEs across both parameter and scenario uncertainty.
How to estimate the value of research in the presence of scenario uncertainty
The best that a decision-maker could do would be to select the intervention that maximised health gains for a particular realisation of θ and s. The expected NHE associated with this error-free choice is EθEsmaxjNHE(j,θ,s). The maximum value of further research that would eliminate all uncertainty, including scenario uncertainty, is EVPIθ,s=EθEsmaxjNHE(j,θ,s)−maxjEθEsNHE(j,θ,s).
Given that we believe a certain scenario to be true, for example scenario 1, the maximum value of further research to eliminate parameter (θ) uncertainty is
Combining the expected value of further research within each possible scenario using the likelihood of each scenario would not describe the value of research that would eliminate both scenario and parameter uncertainty. When alternative scenarios would suggest that different interventions would be expected to be cost-effective the NHEs of choosing the best intervention when integrating both parameter and modelling uncertainty cannot be found by averaging the NHEs of choosing the best intervention within each scenario. This is because
Averaging across the scenarios in this way ignores scenario uncertainty as it assumes that the decision-maker can select alternative treatments based on knowing how the scenario uncertainty is resolved. It is only when scenario uncertainty is not associated with decision uncertainty, that is, when the same intervention would be identified as cost-effective in all scenarios, that this would produce an unbiased estimate of the value of further research.
An appropriate method by which to evaluate the value of further research that would eliminate both parameter and scenario uncertainty would be to stack the Monte Carlo simulations, ensuring that the proportion of Monte Carlo simulations selected from the PSA within each scenario corresponds to the likelihood of that scenario being correct. To produce representative results this may require a larger number of simulations than would be selected based on consideration of parameter uncertainty alone, particularly if the likelihood of any particular scenario is low.
Measures quantifying the maximum value of eliminating uncertainty in either parameters or scenarios can also be of importance. The maximum value of further research to eliminate parameter (θ) uncertainty when scenario uncertainty is present is EVPIθ=EθmaxjEsNHE(j,θ,s)−maxjEθEsNHE(j,θ,s). The maximum value of further research to eliminate scenario uncertainty in the presence of parameter uncertainty is EVPIs=EsmaxjEθNHE(j,θ,s)−maxjEθEsNHE(j,θ,s).
Example
Table 145 presents the NHEs for each intervention based on competing plausible forms of the function NHE(j,θ,s); s = 1,2. It is assumed that each value of θ is equally likely and each scenario is equally plausible. Therefore, the expectation across parameter or scenario uncertainty can be found by averaging across the relevant set of results.
| θ | Overall population | ||
|---|---|---|---|
| NHE(1,θ) | NHE(2,θ) | Max. NHE | |
| Scenario 1 (base case) | |||
| 1 | 4125 | 3250 | 4125 |
| 2 | 750 | 5250 | 5250 |
| 3 | 6000 | 2250 | 6000 |
| 4 | 10,250 | 6000 | 10,250 |
| E θ | 5281 | 4188 | 6406 |
| Scenario 2 | |||
| 1 | 4125 | 4750 | 4750 |
| 2 | 750 | 6750 | 6750 |
| 3 | 6000 | 3750 | 6000 |
| 4 | 10,250 | 7500 | 10,250 |
| E θ | 5281 | 5688 | 6938 |
| Average across scenarios | |||
| 1 | 4125 | 4000 | 4125 |
| 2 | 750 | 6000 | 6000 |
| 3 | 6000 | 3000 | 6000 |
| 4 | 10,250 | 6750 | 10,250 |
| E θ | 5281 | 4938 | 6594 |
Based on scenario 1 it would appear that intervention 1 is cost-effective as it offers the greatest expected NHEs: maxjEθNHE(j,θ,s=1)=5281; however, in the alternative scenario 2 the NHEs of intervention 2 are estimated to be larger than those associated with intervention 1 and so intervention 1 no longer appears cost-effective: maxjEθNHE(j,θ,s=2)=5688. In the presence of both scenario and parameter uncertainty it would appear that intervention 1 is cost-effective: maxjEθEsNHE(j,θ,s)=5281. Averaging across the expected NHEs from within each scenario result would be equivalent to assuming that the decision-maker could select a different intervention based on how the scenario uncertainty resolved, giving expected NHEs of EsmaxjEθNHE(j,θ,s)=5485.
Table 146 presents the value of further research for a range of possible research questions.
| NHE | Value of further research | Calculation |
|---|---|---|
| EVPI θ|s = 1 | 1125 | 6406 – max.(5281, 4188) |
| EVPI θ|s = 2 | 1250 | 6938 – max.(5281, 5688) |
| EVPI θ | 1313 | 6594 – max.(5281, 4938) |
| EVPI s | 203 | Mean[max.(5281, 4188), max.(5281, 5688)] – max.(5281, 4938) |
| EVPI θ,s | 1391 | Mean(6406, 6938) – max(5281, 4938) |
The value of resolving parameter uncertainty in scenario 1 is 1125. In scenario 2 this value is 1250. Note that in this example the value of research to resolve parameter uncertainty is estimated to be greater when it takes account of scenario uncertainty (1313), even though the research would not resolve the scenario uncertainty. The value of resolving only scenario uncertainty is 203. The value of resolving both parameter and scenario uncertainty in this example is 1391.
List of abbreviations
- ACD
- Appraisal Consultation Document
- AG
- Assessment Group
- AHRQ
- Agency for Healthcare Research and Quality
- AWR
- approval with research
- BNF
- British National Formulary
- BSR
- British Society for Rheumatology
- CAD
- coverage with appropriate determination
- CCBT
- computerised cognitive behavioural therapy
- CED
- coverage with evidence development
- CFFE
- conditionally funded field evaluation
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CLOP
- clopidogrel
- CMS
- Centers for Medicare & Medicaid Services
- CSP
- coverage with study participation
- CURE
- Clopidogrel in Unstable Angina to Prevent Recurrent Events
- DMARD
- disease-modifying antirheumatic drug
- EECP
- enhanced external counterpulsation
- ERG
- Evidence Review Group
- EVPI
- expected value of perfect information
- FAD
- Final Appraisal Determination
- HAQ
- Health Assessment Questionnaire
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- HTAi
- Health Technology Assessment international
- ICER
- incremental cost-effectiveness ratio
- ICS
- inhaled corticosteroids
- ICTRP
- International Clinical Trials Registry Platform
- IgE
- immunoglobulin E
- MeSH
- medical subject heading
- MI
- myocardial infarction
- MRC
- Medical Research Council
- MTA
- multiple technology appraisal
- MTAC
- Medical Technologies Advisory Committee
- MUST-EECP
- Multicenter Study of Enhanced External Counterpulsation
- NCD
- national coverage determination
- NETT
- National Emphysema Treatment Trial
- NFMI
- non-fatal myocardial infarction
- NHEs
- net health effects
- NICE
- National Institute for Health and Clinical Excellence
- NIHR
- National Institute for Health Research
- NRR
- National Research Register
- NSTE-ACS
- non-ST segment elevation acute coronary syndrome
- OCS
- oral corticosteroids
- OFT
- Office of Fair Trading
- OIR
- only in research
- OMAL
- omalizumab
- OWR
- only with research
- PASI
- Psoriasis Area Severity Index
- PATH
- Programs for Assessment of Technology in Health
- PPRS
- Pharmaceutical Price Regulation Scheme
- PSA
- probabilistic sensitivity analysis
- PsA
- psoriatic arthritis
- PsARC
- Psoriatic Arthritis Response Criteria
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- SIGN
- Scottish Intercollegiate Guidelines Network
- STA
- single technology appraisal
- TA
- Technology Appraisal
- TAR
- Technology Appraisal Review
- UKCRN
- UK Clinical Research Network
- WHO
- World Health Organization
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, Department of Pharmacology and Therapeutics, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, Department of Pharmacology and Therapeutics, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Professor of Bio-Statistics, Department of Public Health and Epidemiology, University of Birmingham
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, Department of Pharmacology and Therapeutics, University of Liverpool
-
Professor Zarko Alfirevic, Head of Department for Women’s and Children’s Health, Institute of Translational Medicine, University of Liverpool
-
Professor Judith Bliss, Director of ICR-Clinical Trials and Statistics Unit, The Institute of Cancer Research
-
Professor David Fitzmaurice, Professor of Primary Care Research, Department of Primary Care Clinical Sciences, University of Birmingham
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, Department of Specialist Radiology, University College Hospital, London
-
Professor Angela Harden, Professor of Community and Family Health, Institute for Health and Human Development, University of East London
-
Dr Joanne Lord, Reader, Health Economics Research Group, Brunel University
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor Dion Morton, Professor of Surgery, Academic Department of Surgery, University of Birmingham
-
Professor Gail Mountain, Professor of Health Services Research, Rehabilitation and Assistive Technologies Group, University of Sheffield
-
Professor Irwin Nazareth, Professor of Primary Care and Head of Department, Department of Primary Care and Population Sciences, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norrie, Director, Centre for Healthcare Randomised Trials, Health Services Research Unit, University of Aberdeen
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Peter Tyrer, Professor of Community Psychiatry, Centre for Mental Health, Imperial College London
-
Professor Martin Underwood, Professor of Primary Care Research, Warwick Medical School, University of Warwick
-
Professor Caroline Watkins, Professor of Stroke and Older People’s Care, Chair of UK Forum for Stroke Training, Stroke Practice Research Unit, University of Central Lancashire
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Tom Foulks, Medical Research Council
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Diana Baralle, Consultant and Senior Lecturer in Clinical Genetics, University of Southampton
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Public contributor
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Nicola Lennard, Senior Medical Officer, MHRA
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Public contributor
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Public contributor
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Matthew Thompson, Senior Clinical Scientist and GP, Department of Primary Health Care, University of Oxford
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Joanna Jenkinson, Board Secretary, Neurosciences and Mental Health Board (NMHB), Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Public contributor
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire County Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Mrs Jean Thurston, Public contributor
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Public contributor
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Public contributor
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Public contributor
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Public contributor
-
Mr Sankaran Chandra Sekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Public contributor
-
Mr Seumas Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Dr Matthew Wilson, Consultant Anaesthetist, Sheffield Teaching Hospitals NHS Foundation Trust
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan, Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Public contributor
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer, Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Public contributor
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Public contributor
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Public contributor
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health