Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 10/107/01. The protocol was agreed in February 2011. The assessment report began editorial review in June 2011 and was accepted for publication in March 2012. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors' report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen's Printer and Controller of HMSO 2013. This work was produced by Westwood et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
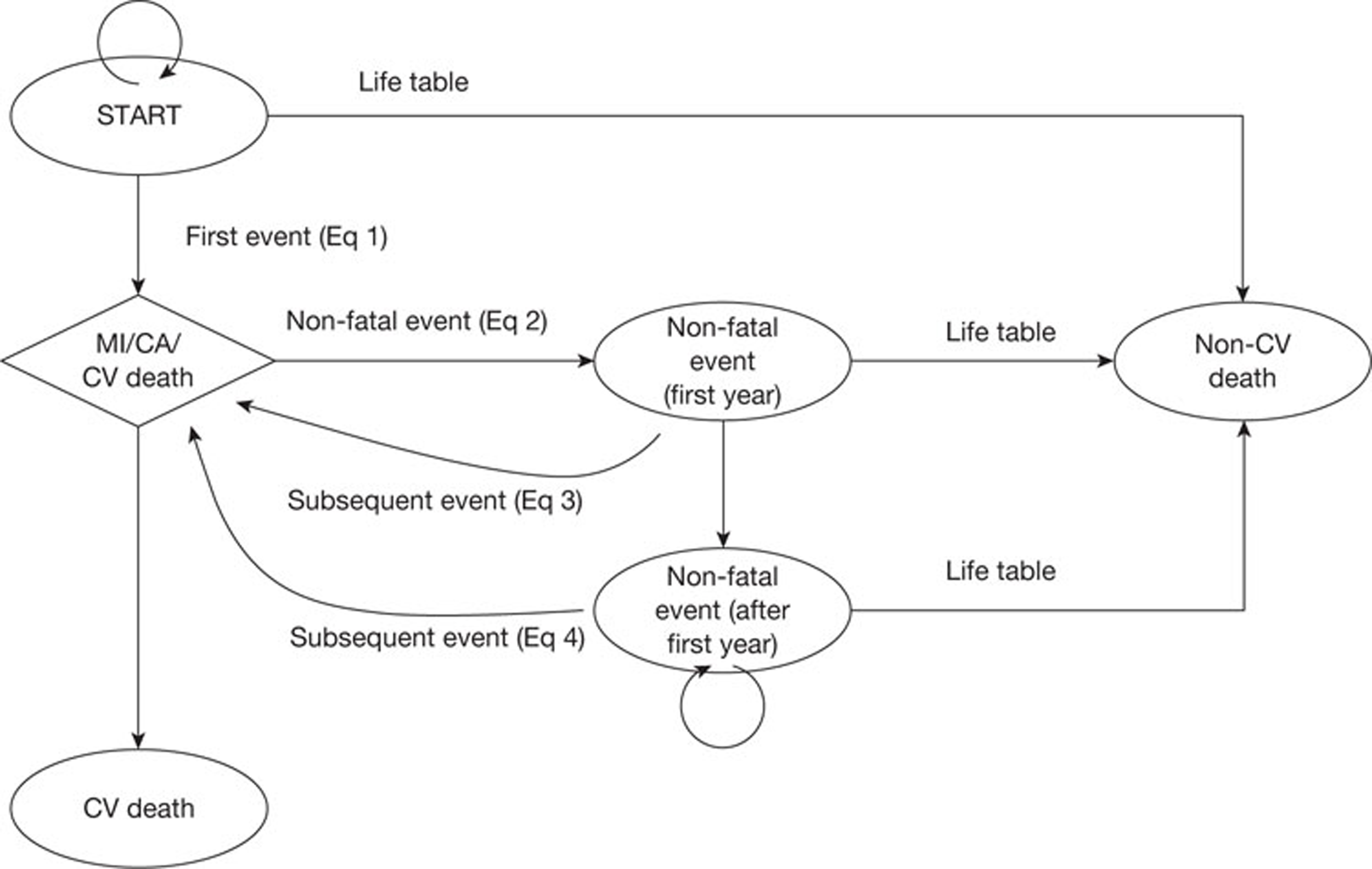
Chapter 1 Background and definition of the decision problem(s)
Conditions and aetiologies
This assessment concerns the clinical effectiveness and cost-effectiveness of cardiac computed tomography (CT), using the instruments described below (see Description of technologies under assessment) and hereafter to be referred to as ‘new-generation cardiac computed tomography (NGCCT)’. The assessment was conducted in two distinct populations. These populations were patients with known or suspected coronary artery disease (CAD), who are difficult to image using current 64-slice CT technology, and patients with complex congenital heart disease requiring additional information for treatment planning.
Coronary artery disease
Coronary artery disease is a major cause of cardiovascular (CV) disability and death in the UK. In 2007 coronary heart disease caused around 91,000 deaths in the UK (approximately 19% of deaths in men and 13% of deaths in women). 1 It is caused by narrowing of the coronary arteries, most commonly by atherosclerotic deposits of fibrous and fatty tissue, leading to a reduction in the flow of blood to the heart, angina and, ultimately, myocardial infarction (MI).
The National Institute for Health and Clinical Excellence (NICE) clinical guideline CG95 (Chest pain of recent onset) defines significant CAD as ≥ 70% diameter narrowing (stenosis) of at least one major epicardial artery segment or ≥ 50% diameter stenosis in the left main coronary artery. 2 Some factors intensify ischaemia and allow less-severe lesions (e.g. ≥ 50% diameter stenosis of one major epicardial artery segment) to produce angina, for example reduced oxygen delivery, increased oxygen demand, large mass of ischaemic myocardium or longer lesion length. Similarly, some factors reduce ischaemia and may render lesions (≥ 70% diameter stenosis of one major epicardial artery segment) asymptomatic, for example a well-developed collateral supply or small mass of ischaemic myocardium.
Invasive coronary angiography (ICA) or computed tomography coronary angiography (CTCA) are used to assess the state of the arteries and to identify significant stenosis as recommended by NICE clinical guideline CG95. 2 The guideline recommends use of a 64-slice (or above) CT scanner in patients with an estimated probability of CAD of 10–29%, who have undergone calcium scoring and who have a calcium score of between 0 and 400. The diagnostic performance of 64-slice CT has been well established; recent systematic reviews have estimated the sensitivity and specificity of 64-slice CT, for the detection of ≥ 50% coronary artery stenosis, to be 92–99% and 89–92%, respectively. 3–5 For most patients, it is therefore unlikely that the use of NGCCT would offer significant benefit over the use of a 64-slice CT scanner. However, NGCCT scanners may be beneficial in specific groups of patients who are currently difficult to image, for example those who cannot hold their breath, have an irregular or fast heartbeat or are obese, or in whom artefacts produced by high levels of coronary calcium or existing stents may reduce image quality. These patients are not currently candidates for CT imaging in routine practice, although some may be imaged in specialist centres.
In addition to enabling the assessment of otherwise difficult-to-image patients, NGCCT may reduce the radiation exposure associated with scanning. However, the benefits of reduced radiation exposure are likely to be limited in this population as patients with known or suspected CAD tend to be older adults.
Congenital heart disease
Congenital heart disease is a general term that describes birth defects that affect the heart. There are many different types of congenital heart defect. The most common simple lesions are ventricular or atrial septal defects, pulmonary or aortic stenosis and patent ductus arteriosus; more complex lesions include tetralogy of Fallot, transposition of the great arteries and even more complex single-ventricle morphologies. The incidence rate for congenital heart disease in the UK is estimated to be 1 in every 150 babies born and approximately 85% of children born with congenital heart disease respond well to treatment and will survive into adulthood. 6 Adequate visualisation of the defect is important to surgical/treatment planning, and diagnostic work-up currently comprises multiple imaging modalities, including echocardiography, invasive angiography, cardiac magnetic resonance imaging (MRI) and cardiac CT. It is likely that NGCCT would provide additional information in only a small proportion of patients with congenital heart disease, those whose conditions are particularly complex. Expert input from paediatric cardiologists has indicated that these will primarily involve lesions with a major extracardiac component that is not well imaged by echocardiography, for example pulmonary atresia with major aortopulmonary collateral arteries (MAPCA), variants of anomalous pulmonary venous drainage [total anomalous pulmonary venous drainage (TAPVD), scimitar syndrome, etc.], aortic arch abnormalities (double aortic arch, vascular ring, etc.) and lesions with both a vascular and an airway component (pulmonary artery sling, tracheal stenosis, right aortic arch with aberrant subclavian artery, etc.). Additionally, as with CAD, patients who have previously treated lesions, in whom stents or pacemakers make imaging with MRI or 64-slice CT difficult, may benefit from NGCCT.
Although there is some evidence that NGCCT may provide accurate initial diagnoses for a range of congenital heart conditions,7,8 diagnostic accuracy is not considered a relevant outcome for this assessment, as existing imaging strategies can provide accurate initial diagnoses, without the need for radiation exposure.
One further potential advantage of NGCCT over current CT scanners is the fast image acquisition time, which may allow babies and infants to be scanned without the need for a general anaesthetic. Reduced radiation dose also has the potential to decrease rates of radiation-induced cancer and infertility in later life. However, as CT scanning is most likely to be used in a single instance for treatment planning, rather than for ongoing monitoring, this impact may be reduced.
Description of technologies under assessment
This assessment has focused upon specialised cardiac applications, where NGCCT is claimed to offer potential advantages over current imaging modalities, for example decreased failure rates and improved accuracy in difficult-to-image patients. However, it should be noted that NGCCT can also be used for all routine imaging procedures in which earlier generations of CT technology are currently applied.
A detailed comparison of the technical characteristics of three of the four CT scanners included in this assessment [Brilliance iCT (Phillips Healthcare), Somatom Definition Flash (Siemens Healthcare) and Aquilion ONE (Toshiba Medical Systems)] is provided as part of a market review of advanced CT scanners for coronary angiography, by the NHS Purchasing and Supply Agency Centre for Evidence-based Purchasing (CEP). 9 There follows a brief summary of the key technical features and manufacturers' claims for each of these scanners, as well as Discovery CT750 HD, GE Healthcare (not included in the CEP report), as they may relate to the applications considered in this assessment. Summaries are presented in alphabetical order, by manufacturer name and are based largely upon product information supplied by the manufacturers.
Discovery CT750 HD, GE Healthcare
The Discovery CT750 HD is a 2 × 64-slice dual source CT scanner. There is a 40-mm-wide detector array with 64 rows of 0.625-mm elements. The Discovery CT750 HD has a gantry aperture of 70 cm, a gantry tilt of ± 30° and a gantry rotation speed of 0.35 seconds. The table has a maximum load of 227 kg and a horizontal speed of 137.5 mm/second. The maximum scan field is 50 cm.
The Discovery CT750 HD can provide a spatial resolution of 0.23 mm. It has a Gemstone™ detector that uses a fast scintillator made of a complex rare earth-based oxide with a chemical structure of garnet crystal. It has a single X-ray source, which switches between two energy levels, allowing two data sets – high energy and low energy – to be acquired simultaneously. This imaging technique is claimed to detect very small concentrations of contrast agent and be able to deliver non-contrast-like images by subtracting the detected agent from the images. It can also give a cardiac temporal resolution of 0.44 milliseconds.
The SnapShot Pulse™, a prospectively gated axial scanning technique, allows a complete picture of the heart to be captured in three or four ‘snapshots’ taken at precise patient table positions and timed to correspond to a specific phase of the cardiac cycle.
An adaptive statistical iterative reconstruction algorithm is used to enhance low contrast detection at a reduced level of radiation and to give a reduction in image noise. Other features claimed to reduce radiation dose are:
-
dynamic z-axis tracking to provide automatic and continuous correction of the X-ray beam position to block unused radiation at the beginning and end of a helical scan
-
filters to reduce noise providing dose reduction while maintaining image quality and spatial resolution
-
three-dimensional dose modulation to facilitate dose protocol medication to individual patients.
Brilliance iCT, Philips Healthcare
The Philips Brilliance iCT is a new-generation, 256-slice multidetector CT scanner. It has 128 × 0.625 mm detector rows providing a total z-axis coverage of 80 mm per rotation. Each detector row is double sampled to increase spatial resolution. In cardiac step and shoot mode the Brilliance iCT can capture an image of the heart in two heart beats. It has a gantry rotation time of 0.27 seconds, a gantry aperture of 70 cm, a maximum table load of 204 kg (with an option to increase to maximum load to 295 kg) and a 50-cm scan field.
The Brilliance iCT has several features designed to manage radiation dose. It uses filters to reduce dose through absorption of unwanted X-rays and to provide a uniform dose delivery across the scan field. It uses automatic current selection to enable individualised dose optimisation. It has a collimator which is claimed to lower patient exposure during helical scanning by removing radiation at the beginning and end, which would not contribute to image formation.
Additional technical features and claims:
-
It is claimed that the X-ray tube gives improved image quality and spatial resolution, particularly in patients with high BMIs.
-
A 120-kW generator is claimed to maximise the image quality of short scans.
-
NanoPanel detectors, claimed to reduce electronic noise, enabling fast, low-dose scans with high spatial resolution (up to 24 line pairs per centimetre).
-
iDose iterative reconstruction technique, claimed to facilitate low-dose imaging and provide faster data reconstruction.
-
It is claimed that when using low-dose step-and-shoot imaging, patients with heart rates of up to 75 beats per minute (b.p.m.) can be imaged successfully.
Somatom Definition Flash, Siemens Healthcare
The Somatom Definition Flash is a second-generation, dual-source 128-slice CT scanner designed to provide high-resolution images at a fast scanning speed with low-dose radiation. The scanner has two X-ray tubes and two detector arrays mounted at 95° to each other. There are 64 × 0.6 mm detector rows, giving a total z-axis coverage of 38.4 mm per rotation. Each detector row is double sampled to give 128 data channels.
The gantry opening measures 78 cm and the table has a maximum load of 220 kg as standard, with an option to increase maximum load to 300 kg. The maximum scan field is 50 cm, with an option to increase the scan field to 78 cm. The gantry has a rotation time of 0.28 seconds, which, combined with the fast table feed, results in a maximum scan speed of 458 mm/s. It is claimed that fast acquisition times may benefit uncooperative patients, such as young children and patients for whom a breath hold is difficult.
The use of two source–detector assemblies is designed to facilitate dual-energy scanning by operating the two tubes at different peak kilovoltages. The dual-energy data are acquired at the same time, which enables a temporal resolution of 75 milliseconds and allows scanning in a high-pitch helical ‘flash’ mode.
Somatom Definition Flash also has a number of features aimed to reduce the radiation load associated with imaging: ‘Flash’ mode scanning (recommended by the manufacturer for heart rates of up to 65 b.p.m.), in which it is claimed that data projections of the entire heart can be captured in approximately 250 milliseconds with a radiation dose of < 1 millisievert (mSv); selective photon shield, which filters the high-kilovoltage X-rays; and iterative reconstruction in image space (IRIS) to reconstruct an image from raw data.
To scan patients with heart rates of > 65 b.p.m. without the use of beta-blockers, the manufacturer recommends different scan modes, which are said to result in higher acquisition times and radiation doses.
Aquilion ONE, Toshiba Medical Systems
The Toshiba Aquilion ONE is a 640-slice CT scanner with 320 × 0.5 mm detector rows giving z-axis coverage of 160 mm. It is claimed that this specification allows an image of the heart can be captured within a single heart beat and reduces radiation and contrast dose. In helical scanning mode the z-axis coverage is 80 mm from 160 × 0.5 mm detector rows.
Additional technical features and claims:
-
Adaptive iterative dose reduction, claimed to produce diagnostic images with low noise levels and minimal operator input.
-
Automated parameter selection, claimed to provide consistent image quality for all patients, regardless of size.
-
PhaseXact, which automatically selects the cardiac phase that displays the least amount of motion and is claimed to improve temporal accuracy and reduce review time.
-
ConeXact volume reconstruction, which removes artefacts that are related to the wide cone angle.
-
Automatic arrhythmia rejection software, which terminates radiation exposure if abnormal heart beat is detected and acquires the next normal beat for image reconstruction.
-
Adaptive multisegment reconstruction: claimed to improve temporal resolution in patients with high or variable heart rates.
-
It is also claimed that the Aquilion ONE can perform cardiac functional analysis and anatomical analysis in one scan, reducing the need to perform multiple examinations using different modalities.
Comparators
Patients with coronary artery disease who are difficult to image using 64-slice computed tomography
In patients in whom 64-slice CT is not a viable option, NGCCT may be used to rule out significant stenosis, or to confirm significant stenosis requiring coronary artery bypass graft (CABG) and thus avoid ICA; where a percutaneous coronary intervention (PCI), i.e. balloon angioplasty with or without stent implantation, is indicated, ICA is frequently performed at the same time as the intervention. The only relevant comparator for patients with CAD is ICA.
Invasive coronary angiography is an invasive imaging technique that uses a contrast dye and X-rays to provide anatomical information about the degree of stenosis in the coronary arteries. A catheter is generally inserted into an artery in the groin and is moved up the aorta and into the coronary arteries. Once in place, the dye is injected through the catheter, and a rapid series of X-ray images are taken to show how the dye moves through the branches of the coronary arteries. Any narrowing of the arteries will show up on the X-ray images. In babies and children a general anaesthetic would be required to perform the procedure.
Despite some limitations [see Chapter 5, Strengths and limitations (clinical effectiveness)], ICA is considered the reference standard for providing anatomical information and defining the site and severity of coronary artery lesions. There are serious complications associated with the technique. However, a 1990 survey by the Society for Cardiovascular Angiography and Interventions (SCAI) included approximately 60,000 patients and indicated that the total risk, for all major complications from ICA (mortality, MI, cerebrovascular accident, arrhythmia, vascular complications, allergic reaction to contrast media, haemodynamic complications, perforation of heart chamber), is < 2%. 10,11
Invasive coronary angiography was the reference standard in our assessment of diagnostic accuracy.
Patients with congenital heart disease
In these patients, cardiac CT is likely to be used for treatment/surgical planning following diagnosis and as an add-on to imaging with echocardiography, invasive angiography and MRI. Thus, 64-slice CT is the only relevant comparator.
Multislice CT scanners (64-slice CT) combine the use of X-rays with computed analysis of a series of two-dimensional X-ray images to create three-dimensional images. The technology has been rapidly advancing, with four-slice CT scanners first appearing in 1998, 16-slice scanners in 2001 and 64-slice scanners at the end of 2004. Multislice CTCA is a minimally invasive investigation that uses a contrast dye injected through a cannula in the forearm and provides anatomical information about the degree of stenosis in the coronary arteries. Cardiac CT has particular challenges owing to the continuous motion of the heart.
Studies that compared the treatment plan and/or patient outcome, in the same group of patients, with and without CT (high definition or 64-slice), or studies that randomised patients to receive treatment based on assessment with or without CT, were considered relevant to this assessment. Diagnostic accuracy data were not considered relevant, as existing imaging strategies can provide accurate initial diagnosis.
Care pathways
Coronary artery disease
Diagnosis
NICE clinical guideline CG95 details the care pathway recommended to make a diagnosis of stable angina in people with chest pain. 2 The guideline suggests that a diagnosis of significant CAD can be made using anatomical imaging and a diagnosis of reversible myocardial ischaemia can be made using functional imaging. Both significant CAD and reversible myocardial ischaemia are treated as a diagnosis of stable angina.
The imaging strategy recommended is dependent upon the estimated pre-test probability of significant CAD. The guideline states that:
-
People with chest pain who have an estimated probability of CAD of 10–29% should be offered calcium scoring followed by CTCA if the calcium score is between 1 and 400; people with high calcium scores (> 400) are considered difficult to image using current CT technologies (64-slice CT) and are included in this assessment as one of the specified categories of ‘difficult-to-image’ CAD patients. For patients with calcium scores > 400, CG95 recommends ICA if this is considered clinically appropriate.
-
People with chest pain who have an estimated probability of CAD of 30–60% should be offered non-invasive functional imaging for myocardial ischaemia.
-
People with chest pain who have an estimated probability of CAD of 61–90% should be offered ICA if clinically appropriate and coronary revascularisation is being considered.
Where non-invasive functional imaging is to be offered the following strategies are recommended by CG95:
-
myocardial perfusion scintigraphy with single-photon emission computed tomography or
-
stress echocardiography or
-
first-pass contrast-enhanced magnetic resonance perfusion or
-
MRI for stress-induced wall motion abnormalities.
As the guideline on chest pain of recent onset is relatively new and technology advances have been occurring rapidly, it has been noted that the guideline on chest pain of recent onset has not been implemented in all cardiac centres across the UK.
Clinical management
Patients diagnosed as having significant CAD should be initially managed as having stable angina. NICE guideline CG126 provides recommendations on the management of stable angina. 12
Key recommendations from the guideline state:
-
Optimal drug treatment consists of one or two antianginal drugs as necessary plus drugs for secondary prevention of cardiovascular disease.
-
A short-acting nitrate should be offered for preventing and treating episodes of angina.
-
Aspirin 75 mg daily should be considered for the secondary prevention of CV disease, taking into account the risk of bleeding and co-morbidities.
-
Treatment with one or two antianginal drugs should be offered for the initial management of stable angina.
-
First-line treatment options for stable angina are beta-blockers and/or calcium channel blockers.
-
For people who cannot tolerate beta-blockers or calcium channel blockers, or these drugs are contraindicated, monotherapy with a long-acting nitrate – ivabradine, nicorandil or ranolazine – can be considered.
-
For people on beta-blocker or calcium-channel blocker monotherapy, whose symptoms are not controlled and the other option is contraindicated or not tolerated, one of the following can be considered as an additional drug: a long-acting nitrate, ivabradine, nicorandil or ranolazine.
-
A third drug can be considered when symptoms are not controlled with two antianginal drugs and the person is waiting for revascularisation or it is not considered appropriate or acceptable.
Management by revascularization
The NICE clinical guideline on stable angina recommends considering revascularisation for people whose symptoms are not controlled by drug treatment. Results of any functional and/or anatomical tests performed at diagnosis should be reviewed when revascularisation is being considered. ICA to guide the revascularisation strategy should be offered if not recently completed during diagnosis. Additional non-invasive or invasive functional testing may be required.
Two revascularisation strategies are available. The first strategy, CABG, involves major cardiac surgery. The second strategy, PCI, involves non-surgical widening from within the artery using a balloon catheter and may be performed with or without stent implantation. NICE technology appraisal (TA) 71 (Guidance on the use of coronary artery stents)13 and NICE TA152 (Drug-eluting stents for the treatment of CAD)14 provide recommendations on the use of stents for revascularisation in CAD.
The NICE clinical guideline on stable angina states that, where revascularisation is considered appropriate, PCI should be offered where CABG is not considered appropriate and CABG should be offered where PCI is not considered appropriate. When either procedure would be appropriate, relative risks and benefits should be explained to the patient, and where no preference is expressed it should be explained that PCI may be the more cost-effective option. Further, when either procedure would be appropriate, the potential survival advantage of CABG for people with complex multivessel disease, who are aged > 65 years and/or have diabetes, should be considered.
NICE TA7113 recommends that stents should be routinely used in patients in whom PCI is indicated. Further, NICE TA15214 states that drug-eluting stents are recommended for use in PCI for the treatment of CAD only if:
-
the target artery to be treated is of > 3 mm in calibre or the lesion is longer than 15 mm, and
-
the price difference between drug-eluting stents and bare-metal stents is no more than £300.
Congenital heart disease
Diagnosis
We are not aware of any nationally accepted guidelines on the diagnosis and management of newborns, infants and children with congenital heart disease. Other sources of information, such as NHS Choices and Patient UK, provide limited information. 15,16 They suggest that if congenital heart disease is suspected then a full clinical history of the pregnancy and the mother's health should be taken prior to investigations. This should be followed by echocardiography, which is a non-invasive procedure without ionising radiation that can provide information on the anatomy and function of the heart. Other tests such as electrocardiography (ECG), chest radiography and pulse oximetry may also be used, as clinically appropriate. Invasive angiography, CT imaging or MRI may be used, in some instances, to provide further anatomical information and to prepare for correction or palliation of the defect.
The main disadvantage of using MRI in this population is the procedure length and the need to gate the scan to both the ECG and phase of respiration; this requires babies and young children to be under general anaesthetic; however, there is no associated radiation exposure. CT imaging has the advantage of rapid acquisition time, potentially removing the need for general anaesthetic. In addition, CT images allow easier examination of the lungs and airways than is the case for MRI. The main disadvantage of CT imaging is that it is associated with radiation exposure. Further, small children may have heart rates that are too high to benefit from the low radiation modes of scanning in NGCCT.
Cardiac catheterisation and invasive angiography, which would require a general anaesthetic, is avoided whenever possible but may be required for certain lesions particularly when intravascular and intracardiac pressures and oxygen saturations are required or in preparation for catheter intervention.
As the majority of babies born with congenital heart disease now survive into adulthood, long-term monitoring and care is essential. In addition, some congenital defects may be diagnosed for the first time in adult life. The European Society of Cardiology (ESC) has recently updated its Guidelines on the Management of Adult Congenital Heart Disease. 17 Recommendations are similar to those suggested for children (above): a clinical examination followed by an ECG and pulse oximetry. Chest radiography may be performed when indicated, but is not routinely recommended. Further investigation of anatomy and physiology has shifted away from invasive studies to non-invasive protocols involving cardiovascular magnetic resonance (CMR) and CT. Cardiac catheterisation and invasive angiography is reserved for the resolution of specific anatomical and physiological questions, or for intervention. 17
Treatment and monitoring
Once congenital heart disease is diagnosed, watchful waiting, medical management, catheter intervention, invasive surgery or heart transplantation may be used to treat the condition, depending on the type of heart anomaly identified. There are several NICE Interventional Procedure Guidelines relating to the treatment of various heart defects; these are listed in Appendix 6.
For adults with congenital heart disease, medical management generally focuses on prevention or control of cardiac problems, for example heart failure, arrhythmias, hypertension, thromboembolic events and endocarditis. Sudden cardiac death is a particular concern. Further intervention may be required in people who have undergone procedures in childhood but have residual or new complications. In addition, new interventions may be required in people with conditions not previously diagnosed, or not considered severe enough to require surgery in childhood. Care of adults with congenital heart disease also needs to take into account a number of issues not directly related to treatment of the cardiac condition, including recommendations for exercise and sports, and issues around pregnancy, contraception and genetic counselling. 17
Owing to the range of conditions covered by the term ‘congenital heart defects’, a variety of different treatment and follow-up strategies may be appropriate for different conditions. For example, people with an atrial septal defect successfully treated with surgery can usually be discharged from indefinite follow-up. Patients with more complicated defects or sequelae following interventional treatment will require lifelong regular follow-up, with frequencies usually ranging from yearly to once every 5 years. 17
Chapter 2 Definition of decision problem
Overall aim of the assessment
To assess the clinical effectiveness and cost-effectiveness of cardiac CT, using Discovery CT750 HD (GE Healthcare), Brilliance iCT (Philips Healthcare), Somatom Definition Flash (Siemens Healthcare), or Aquilion ONE (Toshiba Medical Systems) in specified groups of cardiac patients.
Objectives
To determine the clinical effectiveness and cost-effectiveness of NGCCT for the diagnosis of clinically significant CAD in patients with suspected CAD (defined as those who have chest pain or have other symptoms suggestive of CAD) or known CAD (defined as those who have previously been diagnosed with CAD and whose symptoms are no longer controlled by drug treatment and/or are being considered for revascularisation), who are difficult to image accurately using 64-slice CT technology.
To determine the clinical effectiveness and cost-effectiveness of NGCCT for treatment planning in babies, infants, children and adults who are diagnosed with complex congenital heart defects.
Chapter 3 Assessment of clinical effectiveness
A systematic review was conducted to summarise the evidence on the clinical effectiveness of NGCCT, for the diagnosis of clinically significant coronary artery stenosis in difficult-to-image patient groups with known or suspected CAD, and for treatment planning in patients with complex congenital heart disease. Systematic review methods followed the principles outlined in the Centre for Reviews and Dissemination (CRD) guidance for undertaking reviews in health care and the NICE Diagnostic Assessment Programme interim methods statement. 18,19
Inclusion and exclusion criteria
Participants
Study populations eligible for inclusion were:
-
Adults (≥ 18 years) with known (previously diagnosed who have symptoms that are no longer controlled by drug treatment and/or who are being considered for revascularisation) or suspected (chest pain or other suggestive symptoms) CAD, who are difficult to image (not currently candidates for CT imaging). Difficult-to-image patient groups defined a priori were:
-
obesity [body mass index (BMI) of ≥ 30 kg/m2]
-
high levels of coronary calcium (calcium score > 400)
-
arrhythmias [including, but not limited to, atrial fibrillation (AF)]
-
high heart rate (HHR) (> 65 b.p.m.)
-
intolerance of beta-blockers
-
previous stent implantation
-
previous bypass graft(s).
-
[Difficult-to-image patients were not limited to these patient groups, but no other groups were identified during the review process. Following consultation with clinical experts, the definition of HHR (> 70 b.p.m.) specified in the protocol was broadened to avoid potential loss of relevant data, as identified studies frequently defined HHR as > 65 b.p.m.]
-
Infants, children and adults diagnosed with complex congenital heart disease, including but not limited to:
-
pulmonary atresia with MAPCA
-
variants of anomalous pulmonary venous drainage (TAPVD, scimitar syndrome, etc.)
-
aortic arch abnormalities (double aortic arch, vascular ring, etc.)
-
lesions with both a vascular and airway component (pulmonary artery sling, tracheal stenosis, right aortic arch with aberrant subclavian artery, etc.)
-
previously treated lesions where stents or pacemakers make MRI an unsuitable imaging strategy.
-
Setting
Relevant settings were secondary or tertiary care.
Interventions
Included interventions, described as ‘NGCCT’ throughout, were the following CT scanners:
-
Discovery CT750 (GE Healthcare)
-
Brilliance iCT (Philips Healthcare)
-
Somatom Definition Flash (Siemens Healthcare)
-
Aquilion ONE (Toshiba Medical systems).
No additional equivalent technologies were identified during the review process.
Comparators
The only relevant comparator for the assessment of difficult-to-image patients with CAD was ICA.
Relevant comparators, for the assessment of complex congenital heart disease, were 64-slice CT and conventional imaging (without CT).
Reference standard
Studies reporting the diagnostic accuracy of NGCCT for the detection of significant CAD were required to use ICA as the reference standard. Diagnostic accuracy was not considered a relevant outcome for studies of congenital heart disease.
Outcomes
Studies reporting the following outcomes were considered relevant for both clinical applications (CAD and congenital heart disease):
-
impact of testing on treatment plan (e.g. surgical or medical management), where information on the appropriateness of the final treatment plan was also reported
-
impact of testing on clinical outcome (e.g. angina, MI, CV mortality).
Studies reporting the following outcomes were considered relevant only for difficult-to-image patients with CAD:
-
test accuracy
-
indeterminacy (the number of patients in whom imaging failed to provide diagnostic information).
For included studies reporting any of the above outcome measures, the following outcomes were also recorded, if reported:
-
acceptability of tests to patients
-
adverse events associated with testing
-
radiation dose associated with imaging.
Study design
The following study designs were eligible for inclusion:
-
randomised or non-randomised controlled trials, in which participants were assigned to the intervention or comparator tests, for treatment planning, and outcomes were compared at follow-up
-
randomised or non-randomised controlled trials in which participants were assigned to conventional imaging only, or conventional imaging plus high definition or 64-slice CT (congenital heart disease only).
No randomised or non-randomised controlled trials were identified. Therefore, the following observational study types were considered eligible for inclusion:
-
cross-sectional test accuracy studies, where the intervention was compared with the reference standard (CAD only)
-
observational studies reporting change to treatment plan or clinical outcome subsequent to high-definition CT (CAD and congenital heart disease) or 64-slice CT (congenital heart disease only).
Cross-sectional test accuracy studies were required to report the absolute numbers of true-positive (TP), false-negative (FN), false-positive (FP) and true-negative (TN) test results, or sufficient information to allow their calculation.
The following study/publication types were excluded:
-
pre-clinical, animal and phantom studies
-
reviews, editorials, and opinion pieces
-
case reports
-
studies reporting only technical aspects of the test, or image quality
-
studies with < 10 participants.
Search strategy
Search strategies were based on target condition and intervention, as recommended in the CRD guidance for undertaking reviews in health care and the Cochrane handbook for diagnostic test accuracy reviews. 18,20,21
The following databases were searched for relevant studies from 1 January 2000 to 9 March 2011:
-
MEDLINE (2000 to February week 2 2011) (OvidSP)
-
MEDLINE In-Process and Other Non-Indexed Citations and Daily Update (2000 to 16 February 2011) (OvidSP)
-
EMBASE (2000 to week 6 2011) (OvidSP)
-
Cochrane Database of Systematic Reviews (CDSR) (The Cochrane Library Issue 1 : 2011) (Wiley)
-
Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library Issue 1 : 2011) (Wiley)
-
Database of Abstracts of Reviews of Effects (DARE) (2000 to 9 March 2011) (CRD website)
-
NHS Economic Evaluation Database (NHS EED) (2000 to 9 March 2011) (CRD website)
-
Health Technology Assessment database (HTA) (2000 to 9 March 2011) (CRD website)
-
Science Citation Index (SCI) (2000 to 5 March 2011) (Web of Science).
Supplementary searches were undertaken on the following resources to identify grey literature, completed and ongoing trials:
-
National Institutes of Health Clinicaltrials.gov (2000 to 9 March 2011) (Internet): www.clinicaltrials.gov/
-
Current Controlled Trials (2000 to 9 March 2011) (Internet): www.controlled-trials.com/
-
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (2000 to 9 March 2011) (Internet): www.who.int/ictrp/en/
Searches were undertaken to identify studies of NGCCT in the diagnosis of CAD and assessment of congenital heart disease. Search strategies were developed specifically for each database and the keywords associated with CAD and congenital heart defects were adapted according to the configuration of each database. Searches took into account generic and other product names for the intervention. No restrictions on language or publication status were applied. Limits were applied to remove animal studies. Full search strategies are reported in Appendix 1.
Electronic searches were undertaken for the following conference abstracts:
-
American College of Cardiology (ACC) (2006–10) (Internet): www.cardiosource.org/Meetings/Previous-Meetings-OLD.aspx
-
Society of Cardiovascular Computed Tomography (SCCT) (2006–10) (Internet): www.scct.org/annualmeeting/2010/index.cfm
-
European Society of Cardiology (ESC) (2006–10) (Internet): www.escardio.org/congresses/past_congresses/Pages/past-ESC-congresses.aspx
-
American Heart Association (AHA) (2007–10) (Internet):
Identified references were downloaded in EndNote X4 software (Thomson Reuters, CA, USA) for further assessment and handling.
References in retrieved articles were checked for additional studies.
Inclusion screening and data extraction
Two reviewers (MW and HR) independently screened the titles and abstracts of all reports identified by searches and any discrepancies were discussed and resolved by consensus. Full copies of all studies deemed potentially relevant, after discussion, were obtained and the same two reviewers independently assessed these for inclusion; any disagreements were resolved by consensus. Details of studies excluded at the full-paper-screening stage are presented in Appendix 5.
Studies listed in submissions from the manufacturers of NGCCT were first checked against the project reference database, in EndNote X4; any studies not already identified by our searches were screened for inclusion following the process described above. Studies referenced by manufacturers and excluded at the full-paper-screening stage are noted in Appendix 5. Appendix 5 also includes a list of studies, referenced by manufacturers, which were excluded at title and abstract screening.
Where there was uncertainty regarding possible overlap between study populations, authors were contacted for clarification.
Data were extracted on study details (study design, participant recruitment, setting, funding, stated objective, and categories of participants relevant to this assessment for whom data were reported); study participants (total number of participants, number of participants in each relevant group, study inclusion criteria, study exclusion criteria, and participant characteristics relevant to CV risk for the relevant participant groups or the whole study population); assessed technology and reference standard (technical details of the test, any use of beta-blockers prior to scanning, details of who interpreted tests and how, threshold used to define a positive test); and study results. All studies included in the review were diagnostic accuracy studies and the results extracted were unit of analysis (patient, artery or arterial segment); numbers of TP, FN, FP and TN test results; numbers of patients, arteries or segments classified as non-diagnostic by NGCCT; and radiation exposure associated with imaging. All data were extracted by one reviewer, using a piloted, standard data extraction form and checked by a second; any disagreements were resolved by consensus. Full data extraction tables are provided in Appendix 4.
Quality assessment
All studies included in the systematic review were test accuracy studies. The QUADAS tool,22 is recommended for assessing the methodological quality of test accuracy studies. 18,20 However, a revised version of QUADAS (QUADAS-2) has recently been published. 23 QUADAS-2 more closely resembles the approach and structure of the Cochrane risk of bias tool. It is structured into four key domains covering participant selection, index test, reference standard, and the flow of patients through the study (including timing of tests). Each domain is rated for risk of bias (low, high or unclear) and the tool provides signalling questions, in each domain, to aid reviewers in reaching a judgement. The participant selection, index test and reference standard domains are also, separately, rated for concerns regarding the applicability of the study to the review question (low, high or unclear). Thus, QUADAS-2 separates bias from external validity (applicability) and does not include any items which assess only reporting quality. Guidance for the use of QUADAS-2 will emphasise the need to tailor the tool to specific projects and the need to avoid the use of summary quality scores. Further information on QUADAS-2 is available at the QUADAS website: www.bris.ac.uk/quadas/quadas-2.
Review-specific guidance was produced for the use of QUADAS-2 in this assessment and is reported in Appendix 2. The version of QUADAS-2 used in this assessment included only the risk of bias components, as it was considered that the inclusion criteria matched the review question and that questions of applicability were, therefore, not relevant.
The results of the quality assessment are summarised and presented in tables and graphs in the results of the systematic review (see Chapter 3, Results) and are presented in full, by study, in Appendix 3. No diagnostic accuracy data set included in this assessment was of sufficient size to allow statistical exploration of between-study heterogeneity based on aspects of risk of bias. The findings of the quality assessment were also used to inform recommendations for future research.
Methods of analysis/synthesis
All studies included in the systematic review were test accuracy studies in difficult-to-image patients with CAD. Results were summarised by patient group (e.g. obese, HHR, high coronary calcium score, etc.) and further stratified by unit of analysis (patient, artery or arterial segment). For all included studies, the absolute numbers of TP, FN, FP and TN test results, as well as sensitivity and specificity values, with 95% confidence intervals (CIs), were presented in results tables, for each patient group reported. Data on the numbers of non-diagnostic tests and radiation exposure were also included in the results tables and described in text summaries.
Where groups of similar studies (same patient group and unit of analysis) included four or more data sets, summary receiver operating characteristic (SROC) curves and summary estimates of sensitivity and specificity, with 95% CIs, were calculated using the bivariate modelling approach;24,25 four data sets is the minimum requirement to fit models of this type. Analyses were conducted in Stata 10 (StataCorp LP, College Station, TX, USA), using the ‘metandi’ function. 26 In two cases, a bivariate model could not be fitted because the number of studies was small (four), 2 × 2 data contained one or more zero values, and between-study heterogeneity was low. In these cases, pooled estimates of sensitivity and specificity, with 95% CIs, were calculated using a random-effects model; these analyses were conducted using Meta-DiSc 1.4 (Hospital Ramon y Cajal and Universidad, Madrid, Spain)27 and forest plots were constructed, showing the sensitivity and specificity estimates from each study together with pooled estimates. No distinction was made between patients with known or suspected CAD as per-patient data sets were generally small, with low to moderate between-study heterogeneity. In addition, ‘known’ and ‘suspected’ CAD were often poorly defined by the included studies.
Between-study heterogeneity was assessed using the chi-squared test and inconsistency was quantified using the I2-statistic. 28 There were no data sets of sufficient size (minimum 10) to allow statistical exploration of sources of heterogeneity by including additional co-variables in the SROC model.
Where meta-analysis was considered unsuitable for the data identified (e.g. because of the heterogeneity and/or small numbers of studies), studies were summarised using a narrative synthesis. Text and tables were stratified by patient group.
No data were identified on the effects of NGCCT on treatment planning and/or clinical outcome, adverse events associated with testing, or acceptability of tests to patients.
Results
The literature searches of bibliographic databases identified 3986 references. After initial screening of titles and abstracts, 119 were considered to be potentially relevant and ordered for full-paper screening. A further 11 papers were ordered based on screening of submissions from industry and two studies cited in trials registry entries were also obtained. Of the total of 132 publications considered potentially relevant, five29–33 could not be obtained within the timescale of this assessment; these were held in British Library stacks, which are currently closed for asbestos removal or they were not held by the British Library. Figure 1 shows the flow of studies through the review process, and Appendix 5 provides details, with reasons for exclusions, of all publications excluded at the full-paper-screening stage.
FIGURE 1.
Flow of studies through the review process.
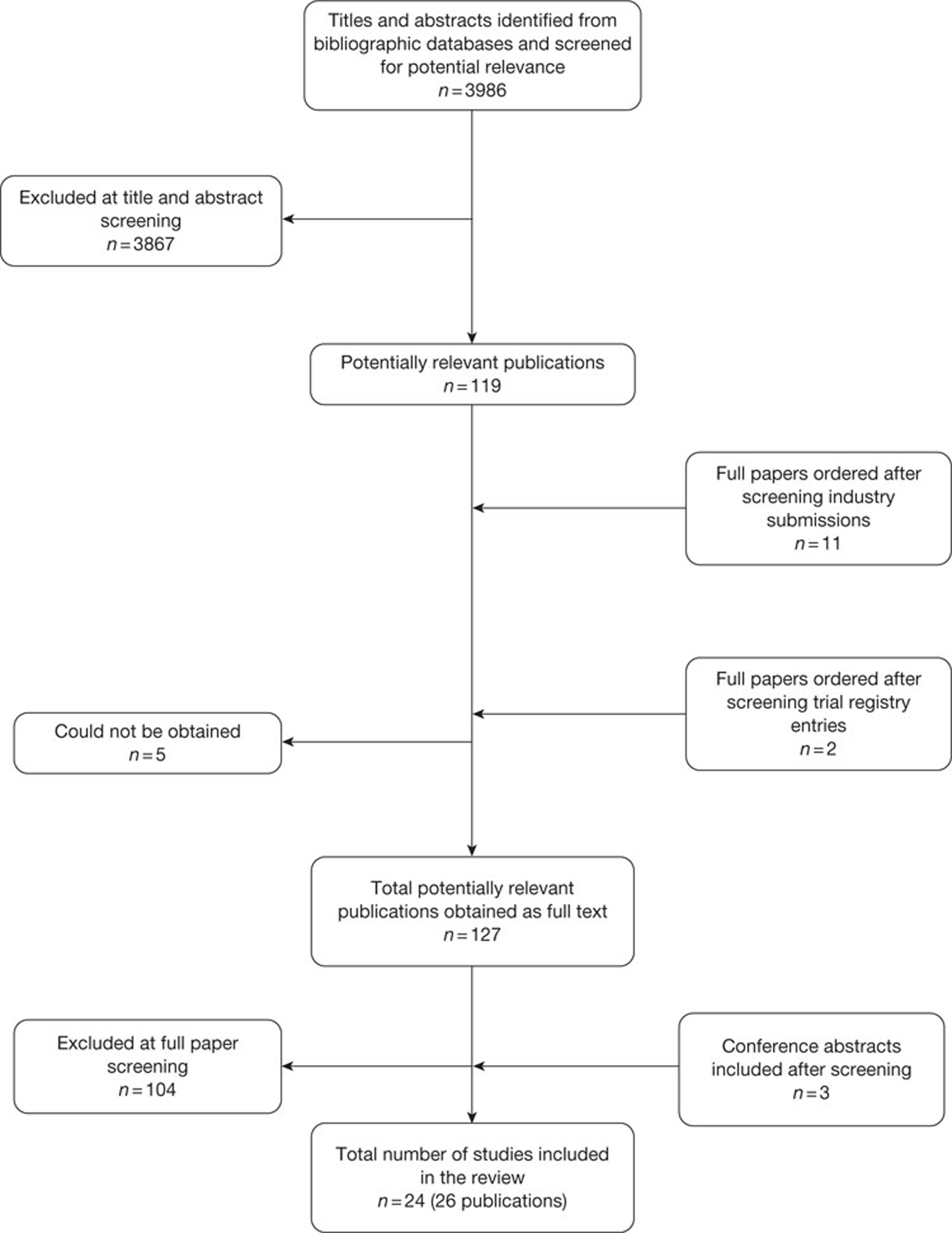
Based on the searches and inclusion screening described above, 23 publications of 21 studies were included in the review. Hand-searching of conference proceedings resulted in the inclusion of a further three studies, which were published in abstract form only (see Figure 1). 34–36 A total of 24 studies in 26 publications were, therefore, included in the review (see Table 1).
| Study ID | Study design | Objective | Obesity | HCS | Arrhythmias | HR > 65 b.p.m. | Stent(s) | Bypass | Beta-blocker intolerance |
|---|---|---|---|---|---|---|---|---|---|
| Alkadhi 201041 | Prospective diagnostic cohort | To prospectively investigate the diagnostic accuracy of dual-source CTCA in relation to BMI, vessel wall calcifications, and average HR as compared with the reference standard ICA | ✓ | ||||||
| Consecutive recruitment | |||||||||
| (Dates not reported) | |||||||||
| Single centre | |||||||||
| Switzerland | |||||||||
| Supported by the National Centre of Competence in Research, Computer Aided and Image Guided Medical Interventions of the Swiss National Science Foundation | |||||||||
| Brodoefel 200842 | Prospective diagnostic cohort | To prospectively evaluate the effect of BMI on DSCT image quality and to assess diagnostic accuracy for coronary artery stenosis, using ICA as the reference standard | ✓ | ||||||
| Recruitment not described | |||||||||
| (September 2006 to July 2007) | |||||||||
| Single centre | |||||||||
| Germany | |||||||||
| Funding not reported | |||||||||
| Brodoefel 200846 | Prospective diagnostic cohort | To prospectively evaluate the effect of heart rate, heart rate variability, and calcification on DSCT image quality and to prospectively assess diagnostic accuracy for coronary artery stenosis, using ICA as the reference standard | ✓ | ✓ | |||||
| Recruitment not described | |||||||||
| (September 2006 to March 2007) | |||||||||
| Single centre | |||||||||
| Germany | |||||||||
| Funding not reported | |||||||||
| De Graaf 201040 | Prospective? diagnostic cohort | To evaluate the diagnostic accuracy of 320-row CTA in the evaluation of significant in-stent re-stenosis. A second purpose of the study was to assess CTA stent image quality and diagnostic accuracy vs stent characteristics and heart rate during CTA image acquisition | ✓ | ||||||
| Recruitment not described | |||||||||
| (Dates not reported) | |||||||||
| Multicentre | |||||||||
| Netherlands | |||||||||
| Supported by the Dutch Technology Foundation, Applied Science Division of NWO, and the Technology Program of the Ministry of Economic Affairs; the Netherlands Heart Foundation; Boston Scientific; Biotronik; Medtronic; BMS Medical Imaging; St Jude Medical; GE Healthcare; Edwards Lifesciences | |||||||||
| LaBounty 201038 | Prospective diagnostic cohort, abstract only | To evaluate the diagnostic accuracy of high-definition (HD)-CTCA in an intent-to-diagnose analysis | ✓ | ||||||
| Consecutive recruitment | |||||||||
| (Dates not reported) | |||||||||
| Multicentre | |||||||||
| USA and Canada | |||||||||
| Funding not reported | |||||||||
| Leber 200743 | Prospective? diagnostic cohort | To assess the clinical performance of a dual X-ray source MSCT with high temporal resolution to assess coronary status in patients with an intermediate pre-test likelihood for significant CAD without using negative chronotropic pre-treatment | ✓a | ✓a | |||||
| Consecutive recruitment | |||||||||
| (July 2006 to January 2007) | |||||||||
| Single centre | |||||||||
| Germany | |||||||||
| NR | |||||||||
| Lin 201044 | Retrospective diagnostic cohort | To evaluate the ability of DSCT CA to diagnose CAD in a heterogeneous population referred to an imaging centre, including patients with irregular heart rates and significant calcification of the coronary arteries | ✓ | ||||||
| Selected patients from a consecutive series | |||||||||
| (October 2006 to June 2007) | |||||||||
| Multicentre | |||||||||
| Taiwan | |||||||||
| Funding not reported | |||||||||
| Marwan 201047 | Prospective? diagnostic cohort | To determine the diagnostic accuracy of DSCT to identify significant coronary stenosis in patients with AF referred for ICA | ✓ | ||||||
| Consecutive recruitment | |||||||||
| (Dates not reported) | |||||||||
| Single centre | |||||||||
| Germany | |||||||||
| One author received support from Siemens and Bayer Schering Pharma. The study was supported by Bundesministerium für Bildung und Forschung, Bonn, Germany | |||||||||
| Meng 200948 | Prospective? diagnostic cohort | To evaluate the diagnostic accuracy of DSCT coronary angiography, with particular focus on the effect of heart rate and calcifications | ✓ | ✓ | |||||
| Consecutive recruitment | |||||||||
| (November 2006 to November 2007) | |||||||||
| Multicentre | |||||||||
| China (PRC) | |||||||||
| Funding not reported | |||||||||
| Oncel 200749 | Prospective diagnostic cohort | To evaluate the sensitivity and specificity of dual-source CT for significant coronary stenosis (> 50% narrowing) in patients with A F, using conventional coronary angiography as the reference standard | ✓ | ||||||
| Consecutive recruitment | |||||||||
| (September 2006 to January 2007) | |||||||||
| Single-centre | |||||||||
| Turkey | |||||||||
| Funding not reported | |||||||||
| Oncel 200850 | Prospective diagnostic cohort | To assess the diagnostic performance of dual-source CT in the evaluation of coronary stent patency to determine whether or not improved temporal resolution aid in visualisation of coronary stents | ✓ | ||||||
| Consecutive recruitment | |||||||||
| (September 2006 to August 2007) | |||||||||
| Single centre | |||||||||
| Turkey | |||||||||
| Funding not reported | |||||||||
| Pflederer 200951 | Prospective? diagnostic cohort | To evaluate the accuracy of DSCT for the assessment of coronary in-stent re-stenosis | ✓ | ||||||
| Consecutive recruitment | |||||||||
| (Dates not reported) | |||||||||
| Multicentre | |||||||||
| Germany and USA | |||||||||
| Work supported by the Bundesministerium für Bildung und Forschung, Berlin Germany. One author supported by research grants from Siemens Healthcare, Erlangen, and Bayer Schering Pharma, Berlin, Germany | |||||||||
| Pflederer 201034 | Diagnostic cohort, abstract only | To assess the accuracy of DSCT to detect coronary artery stenosis in patients with previous coronary revascularisation who were scheduled for ICA | ✓ | ✓ | |||||
| Recruitment not described | |||||||||
| (Dates not reported) | |||||||||
| Single centre | |||||||||
| Germany | |||||||||
| Funding not reported | |||||||||
| Pugliese 200852 and 200753 | Prospective diagnostic cohort | To evaluate the diagnostic performance of DSCT-CA for the detection of in-stent re-stenosis in patients with angina symptoms after stent implantation | ✓b | ✓ | |||||
| Recruitment not described (April 2006 to January 2007) | |||||||||
| Single centre | |||||||||
| Netherlands | |||||||||
| Funding not reported | |||||||||
| Rist 200954 | Prospective? diagnostic cohort | To assess the image quality and diagnostic accuracy of coronary angiograms using DSCT in patients with AF | ✓ | ||||||
| Recruitment not described | |||||||||
| (Dates not reported) | |||||||||
| Single centre | |||||||||
| Germany | |||||||||
| Funding not reported | |||||||||
| Rixe 200935 | Prospective? Diagnostic cohort, abstract only | To investigate the feasibility of DSCT with a temporal resolution of 83 milliseconds for the detection of CAD in patients with AF compared with conventional quantitative coronary angiography | ✓ | ||||||
| Consecutive recruitment | |||||||||
| (Dates not reported) | |||||||||
| Single centre | |||||||||
| Germany | |||||||||
| Funding not reported | |||||||||
| Ropers 200739 | Prospective? diagnostic cohort | To assess the influence of heart rate on diagnostic accuracy of DSCT coronary angiography without beta-blocker pre-medication | ✓ | ||||||
| Consecutive recruitment | |||||||||
| (Dates not reported) | |||||||||
| Single centre | |||||||||
| Germany | |||||||||
| Funding not reported | |||||||||
| Ropers 200837 | Diagnostic cohort, abstract only | To assess the ability of DSCT to evaluate CABG patients for the presence of significant stenoses in bypass grafts and native coronary arteries | ✓ | ||||||
| Recruitment not described | |||||||||
| (Dates not reported) | |||||||||
| Single centre | |||||||||
| Germany | |||||||||
| Funding not reported | |||||||||
| Scheffel 200655 | Prospective diagnostic cohort | To assess the diagnostic accuracy of DSCT for evaluation of CAD in a population with extensive coronary artery calcifications without heart rate control | ✓ | ✓ | |||||
| Recruitment not described | |||||||||
| (Dates not reported) | |||||||||
| Single centre | |||||||||
| Switzerland | |||||||||
| Supported by the National Centre of Competence in Research, Computer Aided and Image Guided Medical Interventions of the Swiss National Science Foundation | |||||||||
| Tsiflikas 201056 and Drosch57 | Prospective? diagnostic cohort | To evaluate the diagnostic accuracy of DSCT to detect significant coronary stenoses (> 50% luminal narrowing) in patients without stable sinus rhythm in a clinical setting | ✓ | ||||||
| Recruitment not described | |||||||||
| (July 2006 to January 2008) | |||||||||
| Multicentre | |||||||||
| Netherlands | |||||||||
| Funding not reported | |||||||||
| Van Mieghem 200736 | Diagnostic cohort, abstract only | To compare ‘traditional work-up’, using exercise stress testing, myocardial perfusion imaging, stress echo or direct referral for ICA, with a CT-based strategy for the assessment of patients with recurrent chest pain after PCI | ✓ | ||||||
| Recruitment not described | |||||||||
| (Dates not reported) | |||||||||
| Single centre | |||||||||
| Netherlands | |||||||||
| Funding not reported | |||||||||
| Weustink 200958 | Prospective? diagnostic cohort | To evaluate the contribution of non-invasive dual-source CTA in the comprehensive assessment of symptomatic patients after CABG | ✓c | ✓ | |||||
| Consecutive recruitment | |||||||||
| (Dates not reported) | |||||||||
| Single centre | |||||||||
| Netherlands | |||||||||
| Funding not reported | |||||||||
| Weustink 200945 | Prospective? diagnostic cohort | To determine the effect of HRF and HRV on radiation exposure and image quality in a large cohort of patients undergoing DS CTCA with adaptive ECG pulsing, and to evaluate the impact of HRF and HRV on the diagnostic performance of DS CTCA to help detect or rule out significant stenoses in a subgroup of patients who underwent additionally conventional coronary angiography | ✓ | ||||||
| Consecutive recruitment | |||||||||
| (April 2006 to October 2008) | |||||||||
| Single centre | |||||||||
| Netherlands | |||||||||
| Funding not reported, statement of ‘no financial relationships’ | |||||||||
| Zhang 201059 | Prospective diagnostic cohort | To prospectively evaluate the accuracy of DS CTCA in diagnosing coronary artery stenosis according to CAG, and the effect of average heart rate, heart rate variability, and calcium score on the accuracy of CTCA | ✓ | ✓ | |||||
| Consecutive recruitment | |||||||||
| (December 2006 to September 2008) | |||||||||
| Multicentre | |||||||||
| China and USA | |||||||||
| Funding not reported |
All included studies were test accuracy studies conducted in patients with known or suspected CAD. No study reported data on changes to patient management or outcomes, test-related adverse events or patient preferences. No studies were identified, of patients with congenital heart disease, which met the inclusion criteria of the review.
Nineteen of the 24 included studies reported using Somatom Definition (a similar previous model of Somatom Definition Flash), and one study used Somatom Definition Flash. 34 Three studies did not specify the instrument used;36–38 the authors of one of these37 had used Somatom Definition in an earlier study, which was also included in this review,39 and another study was later confirmed by the manufacturer to have used Discovery CT750 HD. 38 The remaining study used Aquilion ONE. 40 This study assessed patients who had previous stent implantation for in-stent restenosis. 40
All included studies were published in 2006 or later.
Eleven38,39,41–46,48,55,59 of the 21 included studies reported data on difficult-to-image patients as subgroup analyses. Six of these studies39,41–45 reported sufficient information to allow calculation of the proportion of the total participants who had one or more difficult-to-image criteria; the mean percentage was 41.5% (range 28–51%). Table 1 shows the details of included studies and the specific difficult-to-image patient groups for which each publication reported data. Further details of the characteristics of study participants and the technical details of the conduct of the index test (NGCCT) and reference standard and their interpretation are reported in the data extraction tables presented in Appendix 4.
Accuracy of new-generation cardiac computed tomography for the detection of coronary artery disease in obese patients
One study42 assessed the performance of NGCCT for the detection of significant stenosis (defined as ≥ 50% vessel narrowing) in obese patients with suspected CAD or suspected progression of known CAD; obese patients were defined as those with a BMI of ≥ 30 kg/m2. This study reported high sensitivity and specificity values; however, data were only reported per arterial segment; 543 data points (segments) were derived from 44 patients; data of this type are potentially problematic in that they assume independence of data sets derived from the same patient, which is unlikely to be true in practice, and may thus result in underestimation of variance. Some patients with additional characteristics which may contribute to difficulty in imaging [13 patients who had previous bypass graft(s) were excluded from this study, but it was not clear how many, if any, of these patients were also obese]. Therefore, the potential for biased accuracy assessments due to inappropriate exclusions could not be judged. Eleven (2%) of the arterial segments assessed in this study were classified as non-diagnostic and, although these segments appear to have been included in the analysis, it was unclear how they were classified. Table 2 summarises the QUADAS-2 assessment and the results of this study are summarised in Table 3.
| Study ID | Patient selection | Index test | Reference standard | Flow and timing |
|---|---|---|---|---|
| Risk of bias | Risk of bias | Risk of bias | Risk of bias | |
| Brodoefel 200842 | ? | ↑ | ↓ | ↓ |
| Study ID | Obesity definition | Patient or segment data (n) | Index test | Reference standard | TP | FN | FP | TN | Sensitivity (%) | Specificity (%) | ND (n) | Radiation (mean ± SD) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brodoefel 200842 | ≥ 30 kg/m2 | Segment (543) | Somatom Definition (+ve test ≥ 1 stenosis ≥ 50%)b | ICA (+ve test ≥ 1 stenosis ≥ 50%) | 113 | 12 | 33 | 385 | 90.4 (95% CI 83.8 to 94.9)a | 92.1 (95% CI 89.1 to 94.5)a | Segment 11 (2.0%) | NR |
Accuracy of new-generation cardiac computed tomography for detection of coronary artery disease in patients with high calcium score
For the purpose of this assessment, levels of coronary calcium likely to result in a patient being difficult to image were classified as a high calcium score (HCS) > 400. Four studies46,48,55,59 reported 10 data sets describing the accuracy of NGCCT for the detection of CAD in patients with HCS. Three46,48,55 of the four studies reported only per-segment or per-artery accuracy data; data of this type are potentially problematic in that they assume independence of data sets derived from the same patient, which is unlikely to be true in practice, and may thus result in underestimation of variance. All studies excluded some patients with additional characteristics which may contribute to difficulty in imaging [e.g. previous bypass surgery (four studies46,48,55,59), previous stent implantation (three studies48,55,59)]. However, no study reported the numbers of excluded patients who also had HCS. Therefore, the potential for biased accuracy assessments due to inappropriate exclusions could not be judged. One study48 excluded non-diagnostic segments from its analysis; however, even if all of these segments were in the HCS patient group considered in this section, they would represent a maximum of 7% of the segments analysed; the effect of their exclusion on the reported accuracy estimates is, therefore, likely to be minimal. Table 4 summarises the QUADAS-2 assessments for these studies and Table 5 summarises individual study results.
| Study ID | Risk of bias | |||
|---|---|---|---|---|
| Patient selection | Index test | Reference standard | Flow and timing | |
| Brodoefel 200848 | ? | ↑ | ↓ | ↓ |
| Meng 200948 | ? | ↑ | ↓ | ? |
| Scheffel 200655 | ? | ↓ | ↓ | ↓ |
| Zhang 201059 | ? | ↓ | ↓ | ? |
| Study ID | HCS threshold | Patient or segment data (n) | Index test | Reference standard | TP | FN | FP | TN | Sensitivity (%) | Specificity (%) | ND (n) | Radiation (mean ± SD) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brodoefel 200846 | Calcium score > 400 | Segment (576) | Somatom Definition (+ve test ≥ 1 stenosis ≥ 50%)a | ICA (+ve test ≥ 1 stenosis ≥ 50%) | 187 | 14 | 59 | 316 | 93.0 (95% CI 88.6 to 96.1)b | 84.3 (95% CI 80.2 to 87.8)b | 92 (16.0%) | NR |
| Meng 200948 | Calcium score > 400 | Artery (135) | Somatom Definition (+ve test ≥ 1 stenosis > 50%)c | ICA (+ve test ≥ 1 stenosis > 50%) | 43 | 1 | 19 | 72 | 97.7 (95% CI 88.0 to 99.9)b | 79.1 (95% CI 69.3 to 86.9)b | NR | For total population, CT dose index 30–42 mGy |
| Segment (342) | 69 | 2 | 56 | 215 | 97.2 (95% CI 90.2 to 99.7)b | 79.3 (95% CI 74.0 to 84.0)b | Total population 25/1558 (Nr for the HCS group) | |||||
| Scheffel 200655 | ≥ 400 | Segment (206) | Somatom Definition (+ve test > 50%)a | ICA (+ve test ≥ 1 stenosis > 50%) | 49 | 2 | 8 | 147 | 96.1 (95% CI 86.5 to 99.5) | 94.8 (95% CI 90.1 to 97.8) | Noned | NR |
| Zhang 201059 | > 400 | Patients (12) | Somatom Definition (+ve test ≥ 1 stenosis ≥ 50%) | ICA (+ve test ≥ 1 stenosis ≥ 50%) | 12 | 0 | 0 | 0 | 100 | – | NR | Total (all patients in study) 61.38 ± 11.64 mGy, 16.51 ± 3.75 mSv |
| Artery (36) | 29 | 0 | 0 | 7 | 100 (95% CI 88.1 to 100)b | 100 (95% CI 59.0 to 100)b | NR | |||||
| Segment (180) | 50 | 8 | 4 | 118 | 86.2 (95% CI 74.6 to 93.9)b | 96.7 (95% CI 91.8 to 99.1)b | Total (all patients) 134/1661 (8.1%) | |||||
| Patients (12) | (+ve test ≥ 1 stenosis > 75%) | (+ve test ≥ 1 stenosis > 75%) | 10 | 1 | 0 | 1 | 90.9 (95% CI 58.7 to 99.8)b | 100 (95% CI 25.0 to 100)b | NR | |||
| Artery (36) | 17 | 3 | 1 | 15 | 85.0 (95% CI 62.1 to 96.8)b | 93.8 (95% CI 69.8 to 99.8)b | NR | |||||
| Segment (180) | 28 | 10 | 6 | 136 | 73.7 (95% CI 56.9 to 86.6)b | 95.8 (95% CI 91.0 to 98.4)b | Total (all patients) 193/1661 (11.6%) |
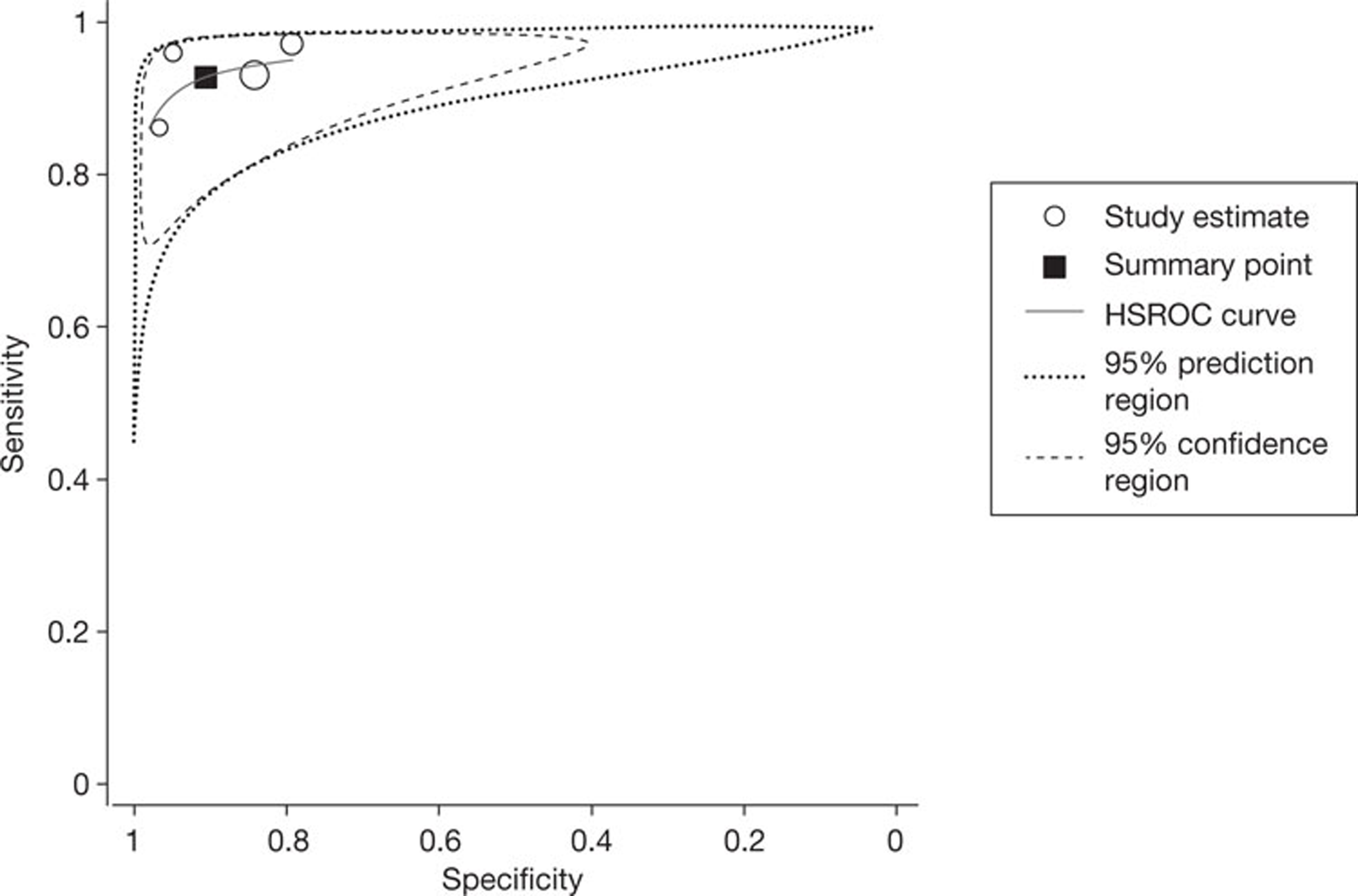
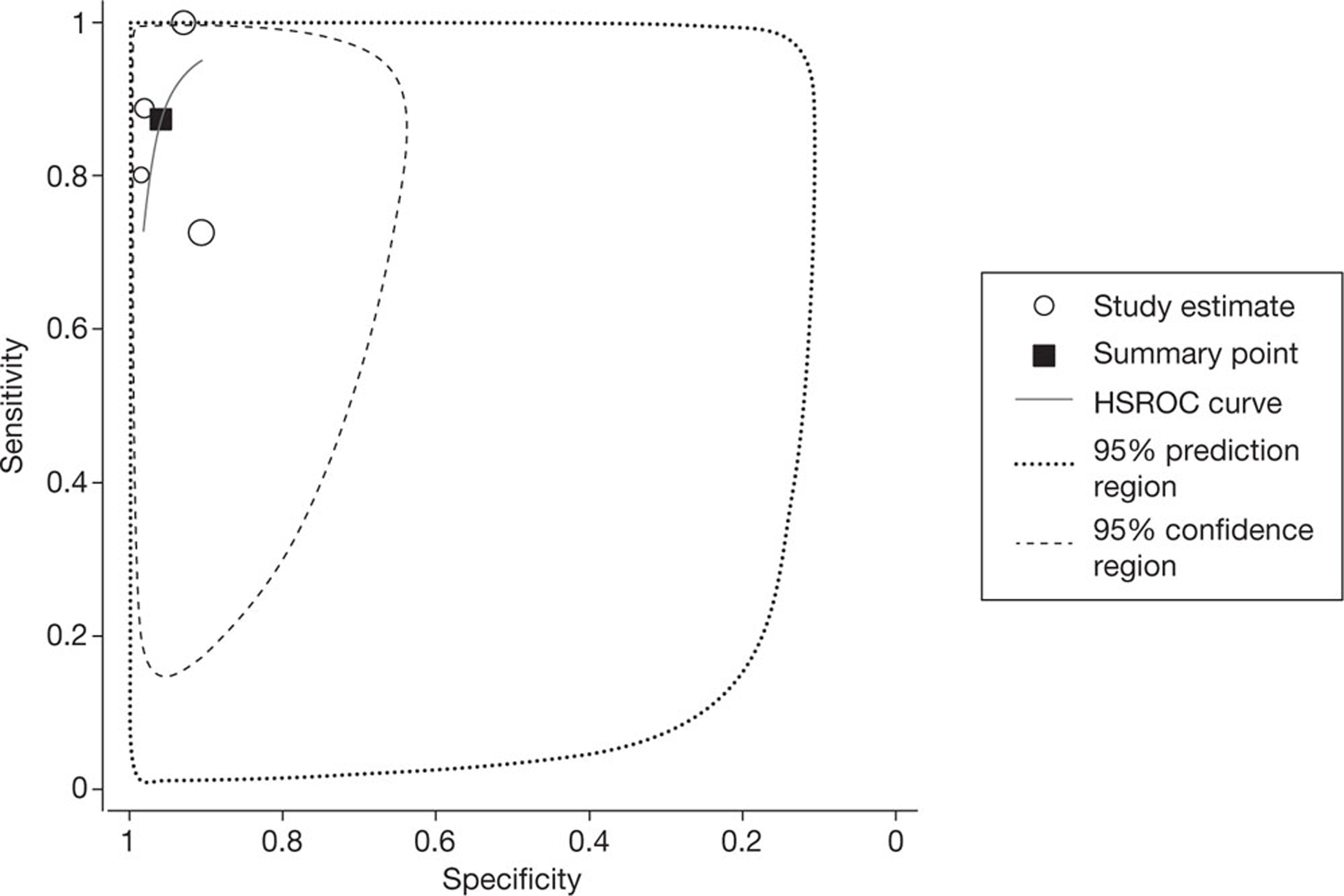
All four studies reported per-segment data, using a threshold of ≥ 50% or > 50% vessel narrowing to define significant stenosis. The pooled estimates of sensitivity and specificity, derived from these data using a bivariate model, were 92.7% (95% CI 88.3% to 95.6%) and 90.6% (95% CI 80.6% to 95.8%), respectively. The I2-statistic indicated moderate between-study heterogeneity in the estimates of sensitivity (I2 = 54.2%) and high between-study heterogeneity in the estimates of specificity (I2 = 92.2%). Figure 2 shows the associated SROC curve for per-segment data in patients with HCS; the open circles, representing individual study results, are scaled to indicate relative sample size. In contradiction with the I2-values, this plot indicates a lack of between-study heterogeneity, with individual study results ‘clustered’ in the upper left quadrant; this contradiction is indicative of the limited utility of statistic tests for heterogeneity in very small sample sizes.
FIGURE 2.
Summary receiver operating characteristic curve for per-segment data in studies of patients with HCS. HSROC, hierarchical summary receiver operating characteristic.
Two studies48,59 also reported accuracy data on a per-artery basis; these results are summarised in Table 5.
Only one study reported per-patient estimates of accuracy and these were of limited value as all 12 included patients were classified as TP using ≥ 50% vessel narrowing as the threshold to define significant stenosis. 59 This same study59 also reported data for all three units of analysis (patient, artery and segment) using a threshold of > 75% vessel narrowing to define significant stenosis; sensitivity and specificity estimates were broadly similar to those obtained using the ≥ 50% vessel narrowing threshold and are reported in Table 5. However, using the higher threshold, estimates of per-patient accuracy could be calculated, sensitivity 90.9% (95% CI 58.7% to 99.8%) and specificity 100% (95% CI 25.0% to 100%); the wide CIs reflect the very small number of patients included in the analysis.
Accuracy of new-generation cardiac computed tomography for detection of coronary artery disease in patients with arrhythmias
Five studies35,47,49,54,56 reported 10 data sets describing the accuracy of NGCCT for the detection of CAD in patients with arrhythmias. Three35,49,54 of the five studies reported using no additional (extra to the patient's normal medication) beta-blockers prior to scanning, and beta-blocker use was unclear in a fourth study. 56 The fifth study47 used beta-blockers prior to scanning in 40% of patients, and excluded 14% of otherwise eligible patients because they were unresponsive to beta-blockers and had rapid AF (> 100 b.p.m.) at the time of scanning; this study was judged to be at high risk of bias with respect to participant selection. In one study,54 only 31% of eligible patients received the reference standard and were included in the analysis; this study was judged to be at high risk of bias, with respect to the flow of patients through the study, in this case due to partial verification bias. Table 6 summarises the QUADAS-2 assessments for these studies and Table 7 summarises individual study results. All but one of these studies were conducted in patients with AF; the fifth study included patients who were ‘without stable sinus rhythm during scanning’.
| Study ID | Risk of bias | |||
|---|---|---|---|---|
| Patient selection | Index test | Reference standard | Flow and timing | |
| Marwan 201047 | ↑ | ? | ? | ↓ |
| Oncel 200749 | ↓ | ↓ | ↓ | ↓ |
| Rist 200954 | ? | ↓ | ↓ | ↑ |
| Rixe 200935 | ↓ | ? | ? | ? |
| Tsiflikas 201056 and Drosch 200857 | ? | ↑ | ↓ | ? |
| Study ID | Arrhythmia Definition | Patient or segment data (n) | Index test | Reference standard | TP | FN | FP | TN | Sensitivity (%) | Specificity (%) | ND (n) | Radiation (mean ± SD) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Marwan 201047 | All patients in AF at scan (39 permanent, 21 persistent) | Patient (60) | Somatom Definition (+ve test ≥ 1 stenosis ≥ 50)a | ICA (+ve test ≥ 1 stenosis > 50%) | 14 | 0 | 7 | 39 | 100 (95% CI 76.8 to 100)b | 84.8 (95% CI 71.1 to 93.7)b | 3 patients (5%) | Mean DLP 1186 ± 375 mGy-cm (range 630–2038 mGy-cm). Using a conversion factor of 0.014 for chest CT in adults, mean effective dose 16 ± 5 mSv |
| Artery (240) | 21 | 1 | 14 | 204 | 95.5 (95% CI 77.2 to 100)b | 93.6 (95% CI 89.5 to 96.4)b | 3 vessels (1.3%) | |||||
| Oncel 200749 | Patients with A F. All patients had irregular heart rates during scanning | Patient (15) | Somatom Definition (+ve test ≥ 1 stenosis > 50%)c | ICA (+ve test ≥ 1 stenosis > 50%) | 7 | 0 | 2 | 6 | 100 (95% CI 59.0 to 100)b | 75.0 (95% CI 34.9 to 96.8)b | NR | 13.8 ± 1.37 mSv |
| Artery (60) | 12 | 3 | 2 | 43 | 80.0 (95% CI 51.9 to 95.7)b | 95.6 (95% CI 84.9 to 99.5)b | NR | |||||
| Segment (212) | 12 | 3 | 3 | 194 | 80.0 (95% CI 51.9 to 95.7)b | 98.5 (95% CI 95.6 to 99.7)b | 13 (5.8%) | |||||
| Rist 200954 | All patients had chronic AF and irregular HR during scan | Patient (21) | Somatom Definition (+ve test ≥ 1 stenosis ≥ 50%)c | ICA (+ve test ≥ 1 stenosis ≥ 50%) | 9 | 1 | 2 | 9 | 90.0 (95% CI 55.5 to 99.7)b | 81.8 (95% CI 48.2 to 97.7b | Total population 4/68 (5.9%) | For all 68 participants, mean DLP 942.9 ± 442 mGy-cm, mean effective dose 13.28 mSv |
| Segment (283) | 16 | 2 | 5 | 260 | 88.9 (95% CI 65.3 to 98.6)b | 98.1 (95% CI 95.7 to 99.4)b | Total population 81/979 (8.3%)d | |||||
| Rixe 200935 | AF (no further details) | Patient (30) | Somatom Definition (+ve test ≥ 1 stenosis > 50%)a | ICA (+ve test ≥ 1 stenosis ≥ 50%) | 13 | 0 | 4 | 13 | 100 (95% CI 75.3 to 100)b | 76.5 (95% CI 50.1 to 93.2)b | NR | 13.5 ± 4.2 mSv |
| Segment (459) | 24 | 0 | 30 | 405 | 100 (95% CI 85.8 to 100)b | 93.1 (95% CI 90.3 to 95.3)b | 32 (7.0%) | |||||
| Tsiflikas 201056 and Drosch 200857 | Patients without stable sinus rhythm during CT scan | Segment (572)e | Somatom Definition (+ve test ≥ 1 stenosis ≥ 50%)f | ICA (+ve test ≥ 1 stenosis ≥ 50%) | 69 | 26 | 41 | 400 | 72.6 (95% CI 62.5 to 81.3)b | 90.7 (95% CI 87.6 to 93.2)b | 28 (5%) | NR |
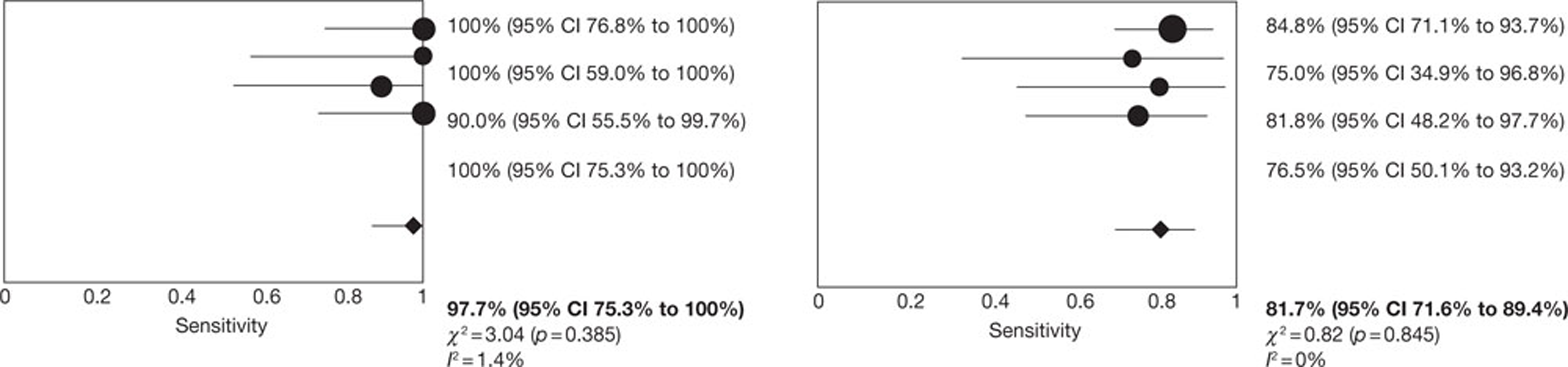
All four studies35,47,49,54,56 of patients with AF reported per-patient data. The pooled estimates of sensitivity and specificity (derived from these data using a DerSimonian–Laird random-effects model, in which 0.5 was added to all cells to allow for zero values) were 97.7% (95% CI 88.0% to 99.9%) and 81.7% (95% CI 71.6% to 89.4%), respectively. Between-study heterogeneity was low: the I2-values were 1.4% for sensitivity and zero for specificity. No SROC curve was fitted as study results were too similar. Figure 3 illustrates the per-patient sensitivity and specificity values for each study, with pooled estimates. The filled circles, representing individual studies, are scaled to indicate relative sample sizes and the wide CIs reflect the generally small sample sizes involved. One study reported the proportion of patients with AF who had non-diagnostic images (5%). 47
FIGURE 3.
Forest plot of per-patient sensitivity and specificity of NGCCT for the detection of CAD in patients with AF.
One study also reported per-artery data and these results are described in Table 7. 47
Four studies35,49,54,56 reported per-segment data. These data were more heterogeneous than was the case for the per-patient data: the I2-values were 79.6% for sensitivity and 89.5% for specificity. The pooled estimates of sensitivity and specificity, derived from these data using a bivariate model, were 87.4% (95% CI 68.3% to 95.7%) and 96.0% (95% CI 91.2% to 98.2%), respectively. Figure 4 shows the associated SROC curve for per-segment data in patients with arrhythmias, with the open circles, representing individual study results, being scaled to indicate relative sample size.
FIGURE 4.
Summary receiver operating characteristic (SROC) curve for per-segment data in studies of patients with arrhythmias. HSROC, hierarchical summary receiver operating characteristic.
Accuracy of new-generation cardiac computed tomography for detection of coronary artery disease in patients with high heart rate
Eight studies39,41,44–46,48,55,59 reported 24 data sets describing the accuracy of NGCCT for the detection of CAD in patients with HHRs. The five studies39,41,44,45,55 that reported the heart rates observed in patients classified as HHR reported mean heart rates of between 76 ± 9 and 88.8 ± 8.4 b.p.m. Three studies46,48,55 reported only per-segment or per-artery accuracy data. Data of this type are potentially problematic in that they assume independence of data sets derived from the same patient; this is unlikely to be true in practice, and may thus result in underestimation of variance. With the exception of one study,60 all studies in this group excluded patients with previous revascularisations (previous stent implantation and/or previous bypass graft); one study44 was a retrospective analysis of selected patients who had undergone both CT and ICA and was judged to be at high risk of bias. Two studies39,45 also excluded patients with AF. The first of these39 excluded > 10% of otherwise eligible participants and was, therefore, judged to be at high risk of bias with respect to participant selection. In the second of these studies45 only 48% of patients received the reference standard and were included in the analysis; this study was therefore also judged to be at high risk of bias with respect to the flow of patients through the study, owing to partial verification bias. Table 8 summarises the QUADAS-2 assessments for these studies and Table 9 summarises individual study results. Studies in this group defined HHR as ≥ 66, ≥ 65 or ≥ 70 b.p.m.; for the purposes of meta-analysis, these studies were treated as a single group assessing the accuracy of NGCCT in patients with a HR of ≥ 65 b.p.m. The baseline use of beta-blockers by study participants varied (see Appendix 4, Inclusion/exclusion criteria and participant characteristics of included studies), but all studies in this section reported that no additional beta-blockers were given prior to CT scanning.
| Study ID | Risk of bias | |||
|---|---|---|---|---|
| Patient selection | Index test | Reference standard | Flow and timing | |
| Alkadhi 200841 | ↓ | ↓ | ↓ | ↓ |
| Brodoefel 200848 | ? | ↑ | ↓ | ↓ |
| Lin 201044 | ↑ | ? | ↓ | ↓ |
| Meng 200948 | ? | ↑ | ↓ | ? |
| Ropers 200739 | ? | ↓ | ? | ↓ |
| Scheffel 200655 | ? | ↑ | ↓ | ↓ |
| Weustink 200945 | ↑ | ↓ | ↓ | ↑ |
| Zhang 201059 | ? | ↓ | ↓ | ? |
| Study ID | HR | Patient or segment data (n) | Index test | Reference standard | TP | FN | FP | TN | Sensitivity (%) | Specificity (%) | ND (n) | Radiation (mean ± SD) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alkadhi 201041 | > 66 b.p.m. | Patient (75) | Somatom Definition (+ve test ≥ 1 stenosis > 50%) | ICA (+ve test ≥ 1 stenosis > 50%) | 27 | 1 | 6 | 41 | 96.4 (95% CI 81.7 to 99.9) | 87.2 (95% CI 74.5 to 95.2) | NR | 7–9 mSv? |
| Segment (1018) | Somatom Definition (+ve test ≥ 1 stenosis > 50%)a | 118 | 5 | 32 | 863 | 95.9 (95% CI 90.8 to 98.7) | 96.4 (95% CI 95.0 to 97.5) | Segment 22 (2.2%) | ||||
| Brodoefel 200846 | > 70 b.p.m. | Segment (370) | Somatom Definition (+ve test ≥ 1 stenosis ≥ 50%)b | ICA (+ve test ≥ 1 stenosis > 50%) | 73 | 6 | 26 | 265 | 92.4 (95% CI 84.2 to 97.2)c | 91.1(95% CI 87.2 to 94.1)c | 7 (1.9%) | NR |
| Lin 201044 | ≥ 70 b.p.m. | Patient (18) | Somatom Definition (+ve test ≥ 1 stenosis > 50%) | ICA (+ve test ≥ 1 stenosis > 50%) | 11 | 0 | 4 | 3 | 100 (95% CI 71.5 to 100)c | 42.9 (95% CI 9.9 to 81.6)c | NR | NR |
| Artery (54) | 19 | 1 | 9 | 25 | 95 (95% CI 75.1 to 99.9)c | 73.5 (95% CI 55.6 to 87.1)c | NR | |||||
| Segment (223) | 31 | 4 | 18 | 170 | 88.6 (95% CI 73.3 to 96.8)c | 90.4 (95% CI 85.3 to 94.2)c | NR | |||||
| Meng 200948 | ≥ 70 b.p.m. | Artery (225) | Somatom Definition (+ve test ≥ 1 stenosis > 50%)d | ICA (+ve test ≥ 1 stenosis > 50%) | 68 | 3 | 14 | 140 | 95.8 (95% CI 88.1 to 99.1)c | 90.9 (95% CI 85.2 to 94.9)c | NR | For total population, CT dose index 30–42 mGy |
| Segment (756) | 103 | 6 | 62 | 585 | 94.5 (95% CI 88.4 to 98.0)c | 90.4 (95% CI 87.9 to 92.6)c | Total population 25/1558 (NR for the HHR group) | |||||
| Ropers 200739 | ≥ 65 b.p.m. | Patient (44) | Somatom Definition (+ve test ≥ 1 stenosis > 50%)a | ICA (+ve test > 1 stenosis > 50%) | 19 | 1 | 3 | 21 | 95 (95% CI 75.1 to 99.9)c | 87.5 (95% CI 67.6 to 97.3)c | 3 (6.8%) | Mean effective dose 15.9 ± 3.11 mSv |
| Artery (176) | 33 | 2 | 5 | 136 | 94.3 (95% CI 80.8 to 99.3)c | 96.5 (95% CI 91.9 to 98.8)c | 9 (5.1%) | |||||
| Segment (616) | 62 | 4 | 27 | 523 | 93.9 (95% CI 85.2 to 98.3)c | 95.1 (95% CI 92.9 to 96.7)c | 50 (8.1%) | |||||
| Scheffel 200655 | ≥ 70 b.p.m. | Segment (175) | Somatom Definition (+ve test ≥ 1 stenosis > 50%)b | ICA (+ve test ≥ 1 stenosis > 50%) | 19 | 1 | 3 | 152 | 95.0 (95% CI 75.1 to 99.9)c | 98.1 (95% CI 94.4 to 99.6)c | 4/175 (2.2%) | NR |
| Weustink 200945 | 66–79 b.p.m. | Patients (333, 170 underwent ICA and were included in the analysis) | Somatom Definition (+ve test ≥ 1 stenosis > 50%)b | ICA (+ve test ≥ 1 stenosis > 50%) | 116 | 1 | 7 | 46 | 99.1 (95% CI 95.3 to 100)c | 86.8 (95% CI 74.7 to 94.5)c | NR | Optimal ECG pulsing: Pitch: 0.25 ± 0.03 CTDIvol (mGy): 56.1 ± 14 CTDIw (mGy): 16.6 ± 3.5 |
| ≥ 80 b.p.m. | Patients (171, 85 underwent ICA and were included in the analysis) | 47 | 0 | 5 | 33 | 100 (95% CI 92.5 to 100)c | 86.8 (95% CI 71.9 to 95.6)c | NR | Optimal ECG pulsing: Pitch: 0.3 ± 0.04 CTDIvol (mGy): 42.7 ± 16.9 CTDIw (mGy): 14.9 ± 1 | |||
| ≥ 66 b.p.m. | Patients (504, 255 underwent ICA and were included in the analysis) | 163 | 1 | 12 | 79 | 99.4 (95% CI 6.6 to 100)c | 86.8 (95% CI 8.1 to 93.0)c | NR | NR | |||
| 66–79 b.p.m. | Segment (2613) | 240 | 21 | 71 | 2281 | 92.0 (95% CI 8.0 to 95.0)c | 97.0 (95% CI 6.2 to 97.6)c | NR | NA | |||
| ≥ 80 b.p.m. | Segment (1327) | 102 | 4 | 49 | 1172 | 96.2 (95% CI 0.6 to 99.0)c | 96.0 (95% CI 4.7 to 97.0)c | NR | NA | |||
| ≥ 66 .p.m. | Segment (3940) | 342 | 25 | 120 | 3453 | 93.2 (95% CI 0.1 to 95.5)c | 96.6 (95% CI 6.0 to 97.2)c | NR | NA | |||
| Zhang 201059 | > 70 b.p.m. | Patients (70) | Somatom Definition (+ve test ≥ 1 stenosis ≥ 50%) | ICA (+ve test ≥ 1 stenosis > 50%) | 43 | 3 | 2 | 22 | 93.5 (95% CI 2.1 to 98.6)c | 91.7 (95% CI 3.0 to 99.0)c | Total (all patients) 134/1661 (8.1%) | Total (all patients in study) 61.38 ± 11.64 mGy, 16.51 ± 3.75 mSv |
| Artery (209) | 72 | 9 | 5 | 123 | 88.9 (95% CI 80.0 to 94.8)c | 96.1 (95% CI 1.1 to 98.7)c | ||||||
| Segment (1035) | 110 | 25 | 10 | 890 | 81.5 (95% CI 73.9 to 87.6)c | 98.9 (95% CI 98.0 to 99.5)c | ||||||
| Patients (70) | (+ve test ≥ 1 stenosis > 75%) | (+ve test ≥ 1 stenosis > 75%) | 32 | 4 | 1 | 33 | 88.9 (95% CI 73.9 to 96.9)c | 97.1 (95% CI 84.7 to 99.9)c | ||||
| Artery (209) | 41 | 8 | 4 | 156 | 83.7 (95% CI 70.3 to 92.7)c | 97.5 (95% CI 93.7 to 99.3)c | ||||||
| Segment (1035) | 59 | 16 | 8 | 952 | 78.7 (95% CI 67.7 to 87.3)c | 99.2 (95% CI 98.4 to 99.6)c |
Five studies39,41,44,45,59 reported per-patient data, using a threshold of ≥ 50% or > 50% vessel narrowing to define significant stenosis. The pooled estimates of sensitivity and specificity, derived from these data using a bivariate model, were 97.7% (95% CI 93.2% to 99.3%) and 86.3% (95% CI 80.2% to 90.7%), respectively; there was moderate between-study heterogeneity in both the estimates of sensitivity (I2 = 39.0%) and the estimates of specificity (I2 = 49.8%). Figure 5 shows the SROC curve for per-patient data in patients with HHR. One study45 reported per-patient accuracy data for multiple definitions of HHR; these results are summarised in Table 9. One study39 reported the proportion of patients with HHR who had non-diagnostic images (6.8%).
FIGURE 5.
Summary receiver operating characteristic curve for per-patient data in studies of patients with HHR. HSROC, hierarchical summary receiver operating characteristic.
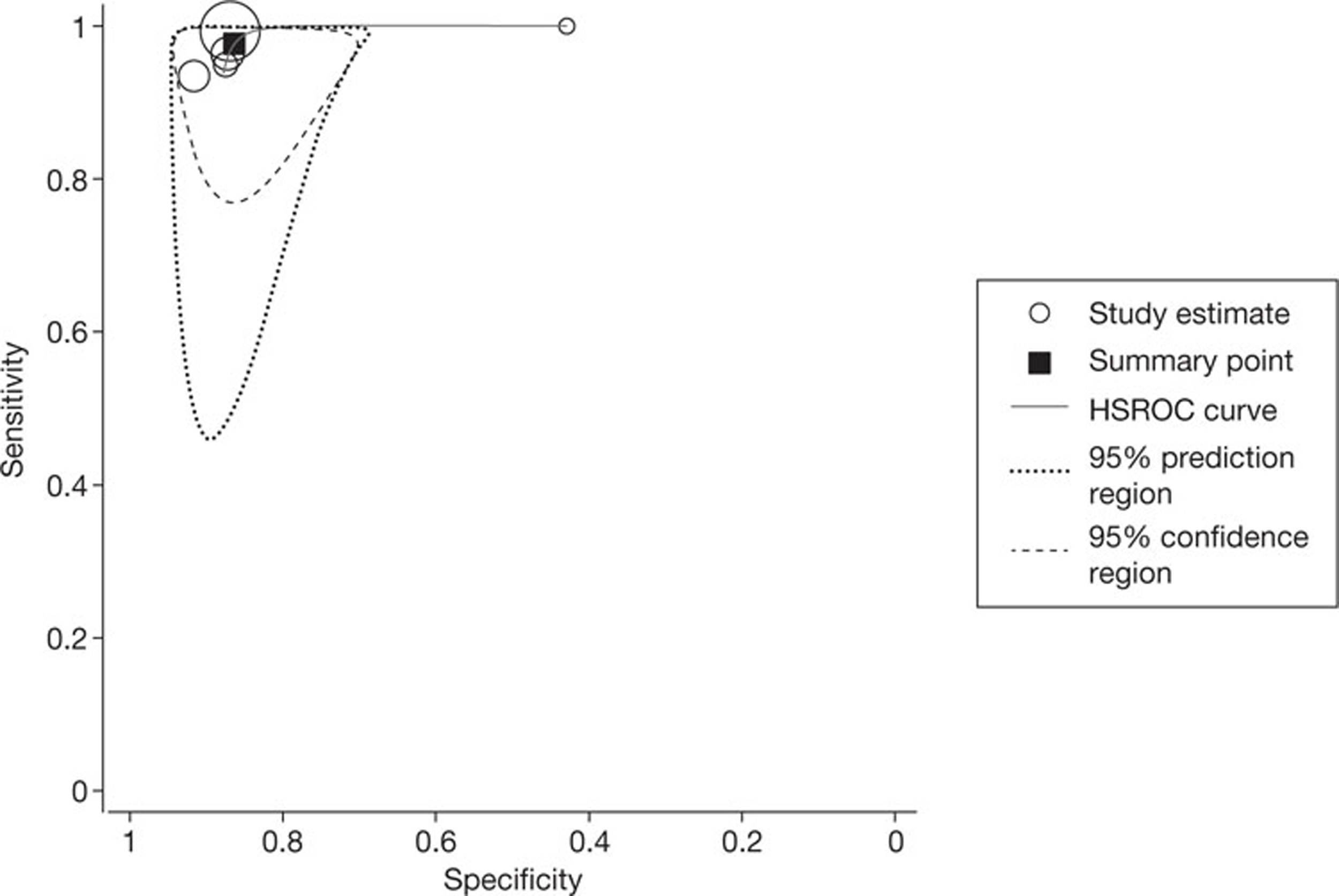
Four studies39,44,48,59 reported per-artery data, using a threshold of ≥ 50% or > 50% vessel narrowing to define significant stenosis. The pooled estimates of sensitivity and specificity, derived from these data using a bivariate model, were 93.7% (95% CI 87.8% to 96.9%) and 92.4% (95% CI 83.3% to 96.8%), respectively; between-study heterogeneity was low (zero) for the estimates of sensitivity, but high for estimates of specificity (I2 = 83.7%). Figure 6 shows the SROC curve for per-artery data in patients with HHR.
FIGURE 6.
Summary receiver operating characteristic curve for per-artery data in studies of patients with HHR. HSROC, hierarchical summary receiver operating characteristic.
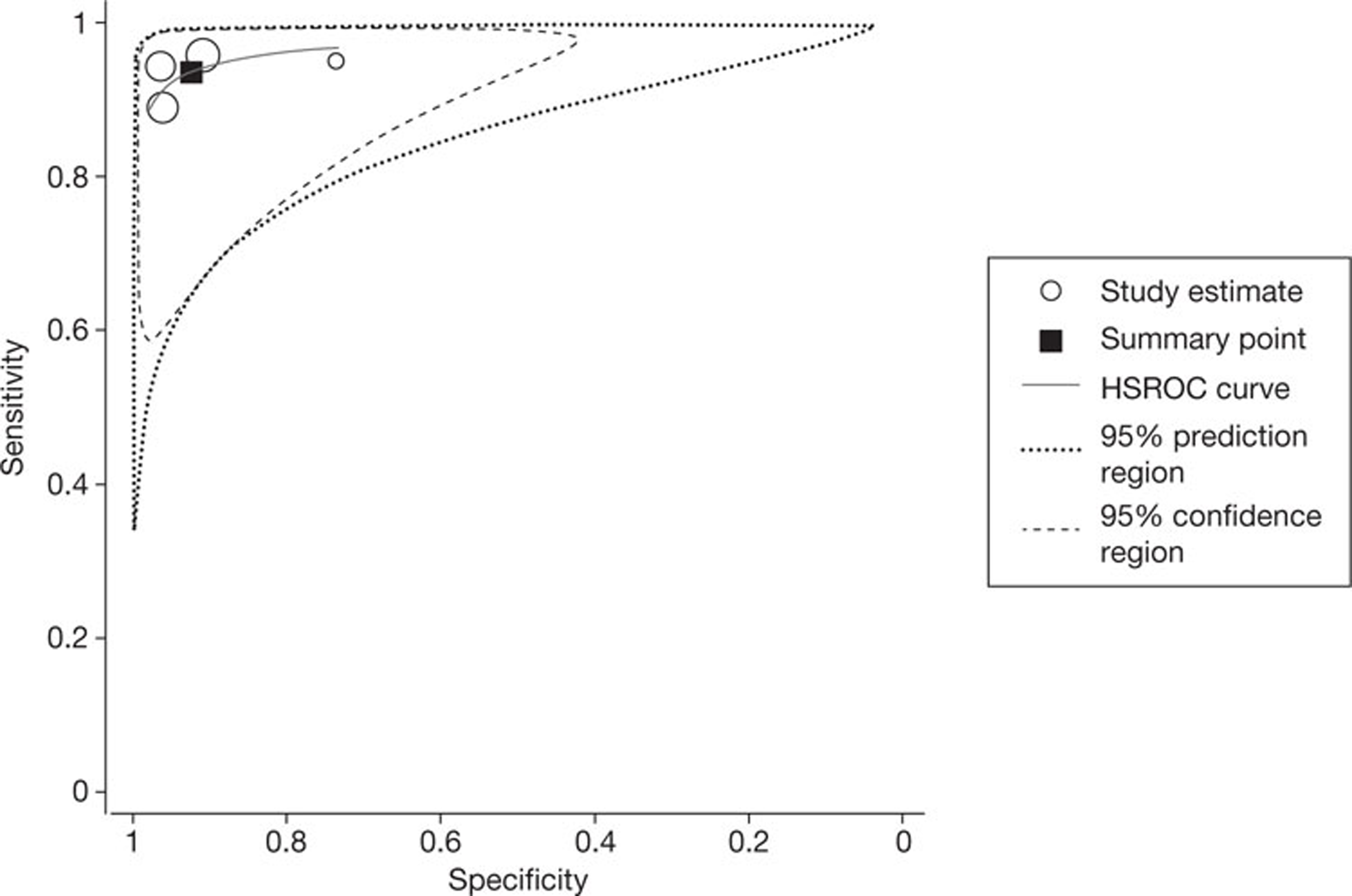
All eight studies reported accuracy data by arterial segment, using a threshold of ≥ 50% or > 50% vessel narrowing to define significant stenosis. The pooled estimates of sensitivity and specificity, derived from these data using a bivariate model, were 92.7% (95% CI 89.3% to 95.1%) and 95.7% (95% CI 92.8% to 97.4%), respectively; there was high between-study heterogeneity in both the estimates of sensitivity (I2 = 67.1%) and the estimates of specificity (I2 = 92.8%). Figure 7 shows the SROC curve for per-segment data in patients with HHR. One study45 reported per-segment accuracy data for multiple definitions of HHR; these results are summarised in Table 9.
FIGURE 7.
Summary receiver operating characteristic curve for per-segment data in studies of patients with HHR. HSROC, hierarchical summary receiver operating characteristic.
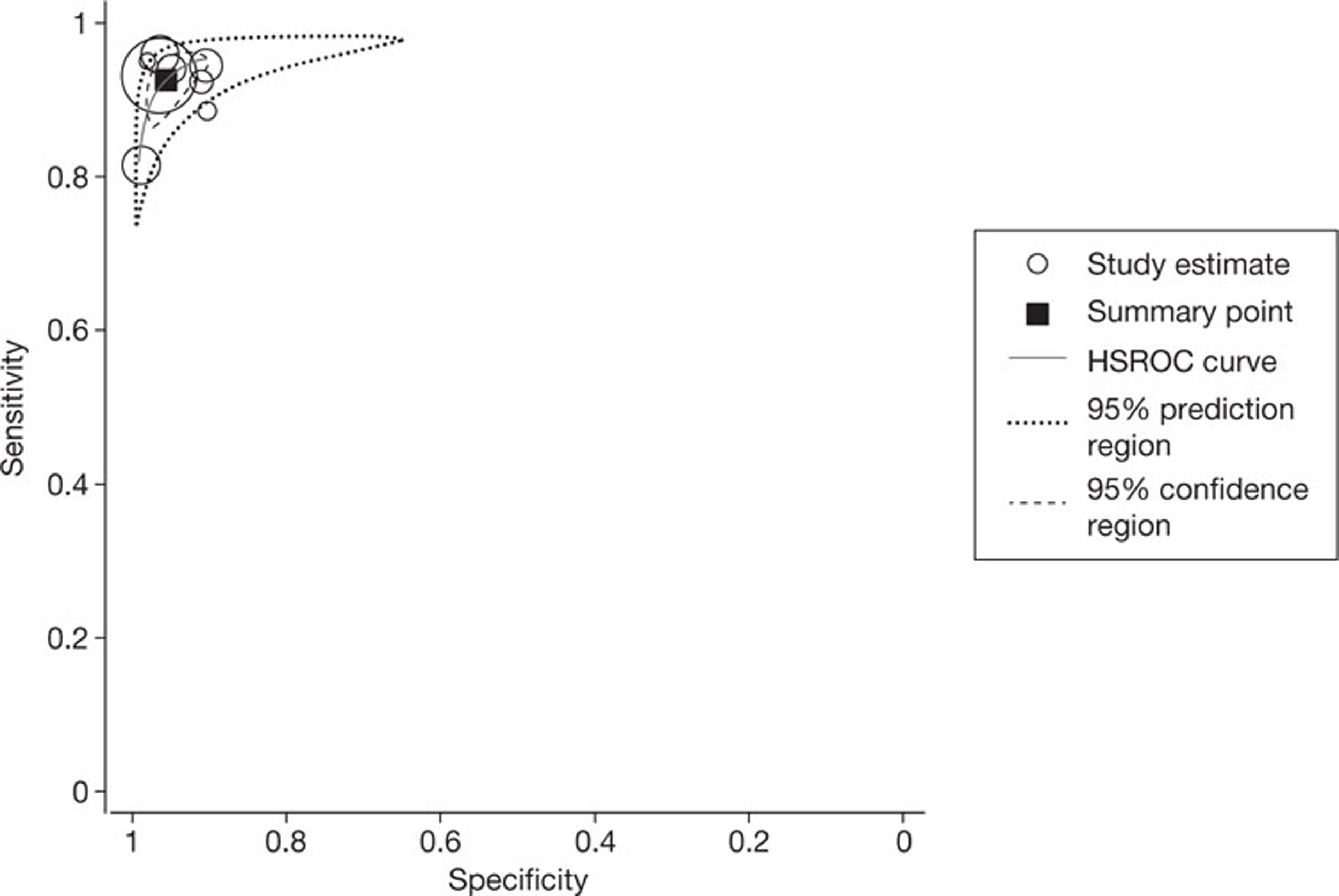
One study59 reported additional data for all three units of analysis (patient, artery and segment) using a threshold of > 75% vessel narrowing to define significant stenosis; sensitivity and specificity estimates were broadly similar to those obtained using the ≥ 50% vessel narrowing threshold and are reported in Table 9.
Accuracy of new-generation cardiac computed tomography for detection of coronary artery disease in beta-blocker intolerance
No studies of the accuracy of NGCCT for the detection of CAD in patients who were intolerant to beta-blockers were identified.
Accuracy of new-generation cardiac computed tomography for detection of coronary artery disease in stented patients
Seven studies34,36,38,40,50–52 reported 10 data sets describing the accuracy of NGCCT for the detection of CAD in patients with previous stent(s) implantation. Three studies34,38,52 reported only per-stent or stented-lesion accuracy data; data of this type are potentially problematic in that they assume independence of data sets derived from the same patient, which is unlikely to be true in practice, and may thus result in underestimation of variance. Four studies excluded some patients with additional characteristics that may contribute to difficulty in imaging. These included HHR and intolerance to beta-blockers,40 previous bypass graft36 and irregular heart rhythm/AF. 51,52 The last of these studies51 also excluded patients with stents in bypass grafts, resulting in the exclusion of > 10% of otherwise eligible participants and a classification of high risk of bias with respect to participant selection. This same study51 excluded non-diagnostic stents from its analyses; however, as the distribution of these stents between patients was not reported, their potential effect on per-patient accuracy estimates could not be assessed. Table 10 summarises the QUADAS-2 assessments for these studies and Table 11 summarises individual study results. Six34,38,40,50–52 of the seven studies considered only in-stent restenosis and the seventh36 considered both in-stent restenosis and stenosis of native vessels.
| Study ID | Risk of bias | |||
|---|---|---|---|---|
| Patient selection | Index test | Reference standard | Flow and timing | |
| De Graaf 201040 | ? | ↓ | ↓ | ↓ |
| LaBounty 201038 | ? | ↑ | ↓ | ? |
| Oncel 200850 | ? | ↓ | ↓ | ↓ |
| Pflederer 200951 | ↑ | ? | ↓ | ? |
| Pflederer 201034 | ? | ↑ | ? | ? |
| Pugliese 200852 and 200753 | ? | ↑ | ↓ | ? |
| Van Mieghem 200736 | ? | ? | ? | ? |
| Study ID | Patient or segment data (n) | Index test | Reference standard | TP | FN | FP | TN | Sensitivity (%) | Specificity (%) | ND (n) | Radiation (mean ± SD) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| De Graaf 201040 | Patient (53)a | Aquilion ONE (+ve test ≥ 1 stenosis ≥ 50%)b | ICA (+ve test ≥ 1 stenosis ≥ 50%) | 11 | 0 | 8 | 34 | 100 (95% CI 71.5 to 100)c | 81.0 (95% CI 65.9 to 91.4)c | Patients 5 (9%) | Unclear, reported for different imaging protocols. Mean dose ranged from 3.2 ± 1.1 to 16.7 ± 6.3 mSv |
| Stent (89, overlapping stents treated as a single stent) | 11 | 1 | 13 | 64 | 91.7 (95% CI 61.5 to 99.8)c | 83.1 (95% CI 72.9 to 90.7)c | Stents 7 (7.9%) | ||||
| LaBounty 201038 | Stent (54) | Unspecified 128-slice, dual source (+ve test stenosis ≥ 50%) | ICA (+ve test stenosis ≥ 50%) | 1 | 0 | 5 | 48 | 100 (95% CI 2.5 to 100)c | 90.6 (95% CI 79.3 to 96.9)c | NR | For total population, median = 3.9 mSv (IQR 1.9 to 9.1), NR for stented patients |
| (+ve test stenosis ≥ 70%) | (+ve test stenosis ≥ 70%) | 1 | 0 | 2 | 51 | 100 (95% CI 2.5 to 100)c | 96.2 (95% CI 87.0 to 99.5)c | ||||
| Oncel 200850 | Patient (35)d | Somatom Definition (+ve test ≥ 1 stenosis ≥ 50%) | ICA (+ve test ≥ 1 stenosis ≥ 50%) | 16 | 0 | 2 | 17 | 100 (95% CI 79.4 to 100)c | 89.5 (95% CI 66.9 to 97.8)c | None | CT: 12.3 ± 1.52 mSv |
| Stent (48) | 17 | 0 | 2 | 29 | 100 (95% CI 80.5 to 100)c | 93.5 (95% CI 78.6 to 99.2)c | ICA: 5.3 ± 2.76 mSv | ||||
| Pflederer 200951 | Patient (112)a,d | Somatom Definition (+ve test ≥ 1 stenosis ≥ 50)b | ICA (+ve test ≥ 1 stenosis ≥ 50%) | 17 | 2 | 17 | 76 | 89.5 (95% CI 66.9 to 98.7)c | 81.7 (95% CI 72.4 to 89.0)c | NR | 14.8 ± 4.8 mSv |
| Stent (135) | 16 | 3 | 6 | 110 | 84.2 (95% CI 60.4 to 96.6)c | 94.8 (95% CI 89.1 to 98.1)c | 15 (11%) | ||||
| Pflederer 201034 | Stent (78) | Somatom Definition (+ve test ≥ 1 stenosis > 50%) | ICA (+ve test stenosis > 50%) | 15 | 1 | 6 | 56 | 93.8 (95% CI 69.8 to 99.8)c | 90.3 (95% CI 80.1 to 96.4)c | NR | NR |
| Pugliese 200852 and 200753 | Stented lesions (178)e | Somatom Definition (+ve test ≥ 1 stenosis > 50%)b | ICA (+ve test ≥ 1 stenosis > 50%) | 37 | 2 | 11 | 128 | 94.9 (95% CI 82.7 to 99.4)c | 86.5 (95% CI 79.9 to 91.5)c | 9 (5.1%) | NR |
| Van Mieghem 200736 | Patient (33)f | DSCT (unspecified) (+ve test > 50% stenosis) | ICA (+ve test > 50% stenosis) | 28 | 1 | 2 | 2 | 96.6 (95% CI 82.2 to 99.9)c | 50.0 (95% CI 6.8 to 93.2)c | NR | NR |
Four studies36,40,50,51 reported per-patient data, using a threshold of ≥ 50% or > 50% vessel narrowing to define significant stenosis. The pooled estimates of sensitivity and specificity, derived from these data using a DerSimonian and Laird random-effects model, where 0.5 was added to all cells to allow for zero values, were 96.0% (95% CI 88.8% to 99.2%) and 81.6% (95% CI 74.7% to 87.3%), respectively. Between-study heterogeneity was low: the I2-values were 19% for sensitivity and zero for specificity. No SROC curve was fitted as study results were too similar. Figure 8 illustrates the per-patient sensitivity and specificity values for each study, with pooled estimates. One study40 reported the proportion of patients with previous stent implantation who had non-diagnostic images (9%).
FIGURE 8.
Forest plot of per-patient sensitivity and specificity of NGCCT for the detection of CAD in patients with previous stent(s).
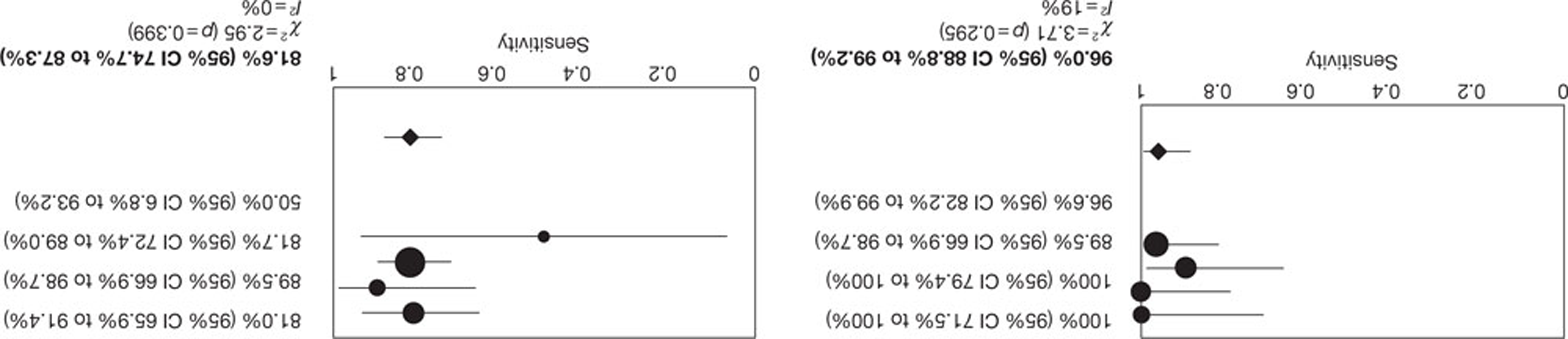
Six studies34,38,40,50–52 reported accuracy data by stent or stented lesion. The pooled estimates of sensitivity and specificity, derived from these data using a bivariate model, were 93.6% (95% CI 86.1% to 97.2%) and 91.0% (95% CI 87.3% to 93.7%), respectively; between-study heterogeneity was low (zero) for the estimates of sensitivity, and moderate for estimates of specificity (I2 = 35.1%). Figure 9 shows the SROC curve for per-stent/stented-lesion data in patients with previous stent(s). One study38 reported additional data, using a threshold of ≥ 70% narrowing to define significant in-stent restenosis; sensitivity and specificity estimates were broadly similar to those obtained using the ≥ 50% narrowing threshold and are reported in Table 11.
FIGURE 9.
Summary receiver operating characteristic curve for per stent/stented-lesion data in studies of patients with previous stent(s). HSROC, hierarchical summary receiver operating characteristic.
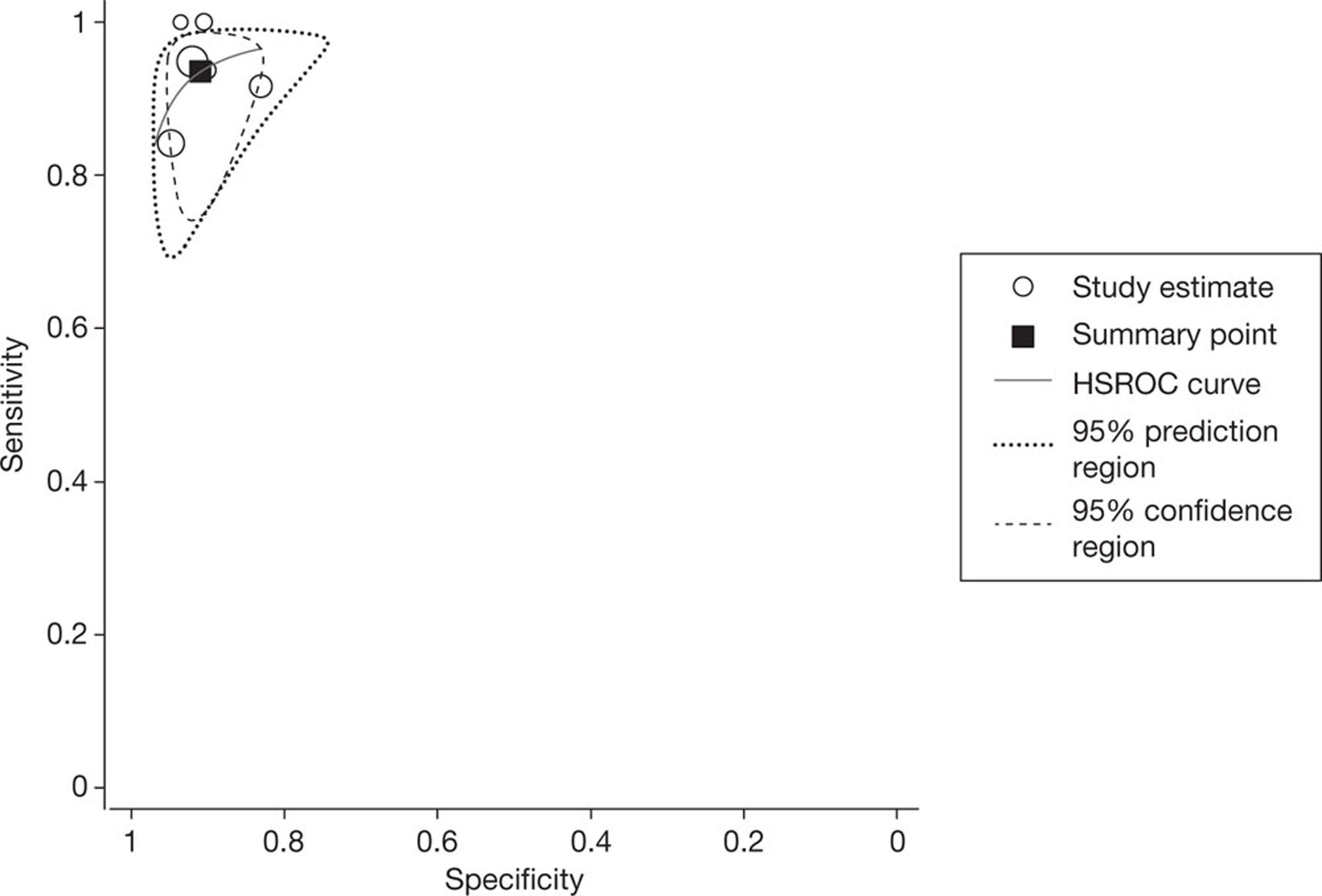
Accuracy of new-generation cardiac computed tomography for detection of coronary artery disease in patients with coronary artery bypass graft
Three studies34,37,58 reported six data sets describing the accuracy of NGCCT for the detection of CAD in patients with previous bypass graft(s). Two34,37 of the three studies included in this section were published only as conference abstracts. In these cases, the minimal methodological information reported made it difficult to assess the risk of bias; this is reflected in the high proportion of unclear (?) judgements. The study that was reported as a full paper58 reported only accuracy results per segment. Table 12 summarises the QUADAS-2 assessments for these studies. A variety of different units of analysis were used, including bypass grafts, segments of bypass grafts, segments of native vessels and/or distal run-off, and patients; results are summarised in Table 13. Only one study37 assessed the per-patient accuracy of NGCCT for the detection of any significant stenosis (≥ 50% narrowing) in a bypass graft, distal run-off, or native vessel. The per-patient sensitivity estimated from this study was 96.4% (95% CI 87.5% to 99.6%) and the per-patient specificity was 87.0% (95% CI 66.4% to 97.2%).
| Study ID | Patient selection | Index test | Reference standard | Flow and timing |
|---|---|---|---|---|
| Risk of bias | Risk of bias | Risk of bias | Risk of bias | |
| Pflederer 201034 | ? | ↑ | ? | ? |
| Ropers 200837 | ? | ? | ? | ? |
| Weustink 200958 | ↓ | ↑ | ↓ | ↓ |
| Study ID | Patient, vessel or segment data (n) | Index test | Reference standard | TP | FN | FP | TN | Sensitivity (%) | Specificity (%) | ND (n) | Radiation (mean ± SD) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pflederer 201034 | Bypass graft (42) | Somatom Definition (+ve test ≥ 1 stenosis > 50%) | ICA (+ve test stenosis > 50%) | 15 | 0 | 1 | 26 | 100 (95% CI 78.2 to 100)a | 96 (95% CI 81.0 to 99.9)a | NR | NR |
| Ropers 200837 | Bypass graft (195) | Unspecified DSCT (+ve test stenosis ≥ 50%) | ICA (+ve test stenosis ≥ 50%) | 90 | 0 | 5 | 100 | 100 (95% CI 96.0 to 100)a | 95.2 (95% CI 89.2 to 98.4)a | None | NR |
| Native coronary artery and distal run-off, segment (854) | 111 | 12 | 103 | 541 | 90.2 (95% CI 83.6 to 94.9)a | 84.0 (95% CI 80.9 to 86.8)a | 87 (10.2%) | ||||
| Patient (78) | Unspecified DSCT (+ve test ≥ 1 stenosis ≥ 50%)b | 53 | 2 | 3 | 20 | 96.4 (95% CI 87.5 to 99.6)a | 87.0 (95% CI 66.4 to 97.2)a | None | |||
| Weustink 200958 | Bypass graft, segment (152) | Somatom Definition (+ve stenosis ≥ 50%)c | ICA (+ve stenosis ≥ 50%) | 29 | 0 | 0 | 123 | 100 (95% CI88.1 to100)a | 100 (95% CI 97.0 to 100)a | NR | DLP (mGy-cm) 1.726 ± 596 |
| Native coronary artery (grafted), segment (289) | 170 | 0 | 5 | 112 | 100 (95% CI 97.9 to100)a | 95.7 (95% CI 90.3 to 98.6)a | NR | Effective dose (mSv) 22.1 ± 2.8 | |||
| Native coronary artery (non-grafted), segment (118) | 33 | 1 | 7 | 77 | 97.1 (95% CI 84.7 to 99.9)a | 91.7 (95% CI 83.6 to 96.6)a | NR | ||||
| Distal run-off, segment (142) | 19 | 1 | 0 | 122 | 95.0 (95% CI 75.1 to 99.9)a | 100 (95% CI 97.0 to 100)a | NR |
Accuracy of new-generation cardiac computed tomography for detection of coronary artery disease (multiple criteria)
Three studies reported the accuracy of NGCCT in patients with different combinations of difficult-to-image criteria. 42,52,58 Two studies52,58 only reported per segment or per lesion accuracy data. The only study58 to report per-patient data excluded non-diagnostic segments and, as it was unclear how these were distributed between patients, it was not possible to assess how their exclusion may have affected per-patient results. Table 14 summarises the QUADAS-2 assessments for these studies and Table 15 summarises individual study results. Units of analysis differed between studies and only one study43 reported per-patient data. The per-patient sensitivity estimated from this study was 91.7% (95% CI 61.5% to 99.8%) and the per-patient specificity was 88.2% (95% CI 72.5% to 96.7%), for patients with HR of > 65 b.p.m. and/or AF.
| Study ID | Risk of bias | |||
|---|---|---|---|---|
| Patient selection | Index test | Reference standard | Flow and timing | |
| Leber 200743 | ↓ | ? | ? | ? |
| Pugliese 200852and 200753 | ? | ↑ | ↓ | ? |
| Weustink 200958 | ↓ | ↑ | ↓ | ↓ |
| Study ID | Participants | Patient or segment data (n) | Index test | Reference standard | TP | FN | FP | TN | Sensitivity (%) | Specificity (%) | ND (n) | Radiation (mean ± SD) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leber 200743 | HR > 65 b.p.m. and/or AF | Patient (46) | Somatom Definition (+ve test stenosis > 50%)a | ICA (+ve test stenosis > 50%) | 11 | 1 | 4 | 30 | 91.7 (95% CI 61.5 to 99.8)b | 88.2 (95% CI 72.5 to 96.7)b | One patient | For total population, mean dose 9.6 mSv (range 7.1–12.3 mSv). No separate data reported for HHR/AF participants |
| Segment (637) | 21 | 3 | 5 | 608 | 87.5 (95% CI 67.6 to 97.3)b | 99.2 (95% CI 98.1 to 99.7)b | NR | |||||
| Pugliese 200852 and 200753 | Previous stent implantation, and HHR (≥ 70 b.p.m.) | Lesions (54) | Somatom Definition | CA | 9 | 1 | 4 | 40 | 90.0 (95% CI 55.5 to 99.7)b | 90.9 (95% CI 78.3 to 97.5)b | NR | NR |
| Weustink 200958 | Previous bypass graft and HHR (> 65 b.p.m.) | Native coronary arteries (grafted), segment (289)c | Somatom Definition (+ve stenosis ≥ 50%)d | ICA (+ve stenosis ≥ 50%) | 90 | 0 | 1 | 63 | 100 (95% CI 96.0 to 100)b | 98.4 (95% CI 91.6 to 100)b | NR | DLP (mGy-cm): 1.726 ± 596 |
| Effective dose (mSv): 22.1 ± 2.8 |
Summary
All 24 studies (26 publications, see Table 1) included in the systematic review were diagnostic test accuracy studies that reported data on the performance of NGCCT in difficult-to-image patients with known or suspected CAD. Figure 10 provides a summary of the risk of bias assessments for these studies. The majority of studies were judged to be at low risk of bias with respect to the reference standard domain of QUADAS-2; this reflects the specification, in the inclusion criteria of the review, of a single acceptable reference standard (ICA). Unclear ratings for this domain mainly reflected poor reporting of the interpretation of the reference standard and uncertainty whether or not those interpreting ICA were blinded to the index test results. The judgement of risk of bias with respect to patient selection was problematic and this is reflected in the high proportion of unclear ratings. The unclear rating frequently related to uncertainty regarding the potential impact of inappropriate exclusions. Difficult-to-image patient groups were frequently reported as subgroups within larger studies, with those who had one or more additional criteria, which may contribute further to difficulty in imaging, being excluded from the study (e.g. a study reporting data for general CAD patients and a subgroup of patients with HHR may have excluded patients with previous revascularisations). In addition, the numbers/proportion of patients excluded in this way were frequently not reported. Inclusion of multiple measurements per patient (per-arterial segment, per-artery or per-stent data) was a common problem in the index test domain. Where studies excluded non-diagnostic arterial segments from their analyses, the potential impact of these exclusions was frequently unclear because their distribution between patients was not reported. For example, if a positive test for per-patient data is defined as one or more positive segments, exclusion of a non-diagnostic segment which is actually stenosed may result in misclassification of the whole patient as TN (i.e. a reduced estimate of the number of FN patients).
FIGURE 10.
Summary of QUADAS-2 assessments.
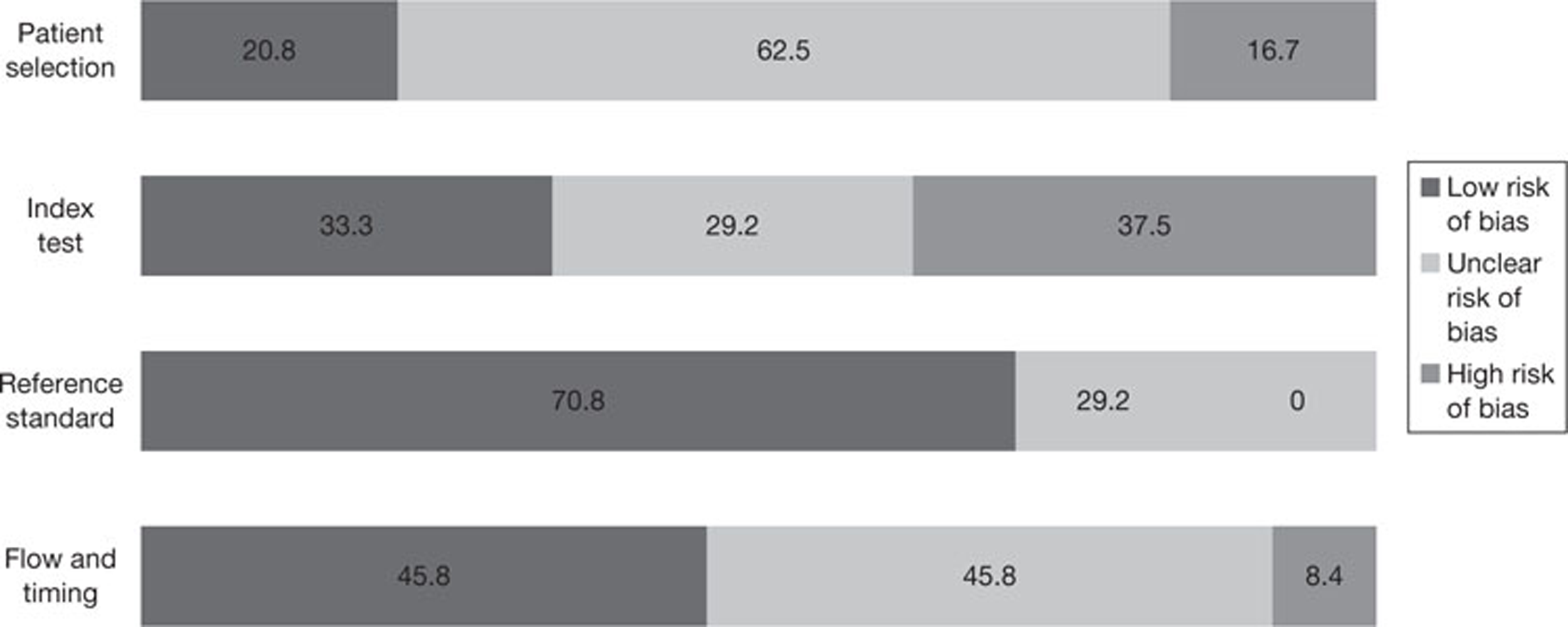
Where per-patient estimates of test accuracy were possible, these were generally high. Pooled estimates of sensitivity and specificity are summarised in Table 16. In particular, all per-patient estimates of sensitivity were > 95%, indicating that NGCCT could reliably rule out significant stenosis and thus potentially avoid invasive investigations such as ICA. Furthermore, although there were no data specifically for beta-blocker intolerant patients, it should be noted that no study reporting per-patient data for patients with HHR used additional beta-blockers prior to scanning. It may therefore be inferred that NGCCT could reasonably be used to image patients who are intolerant to beta-blockers who could not otherwise be reliably imaged by 64-slice CT.
| Patient group | Unit of analysis | No. of studies | n | Sensitivity (%) | I2 (%) | Specificity (%) | I2 (%) |
|---|---|---|---|---|---|---|---|
| Obesity (BMI ≥ 30 kg/m2) | Segment | 1 | 543 | 90.4 (95% CI 83.8 to 94.9) | NA | 92.1 (95% CI 89.1 to 94.5) | NA |
| HCS (> 400) | Segment | 4 | 1304 | 92.7 (95% CI 88.3 to 95.6) | 54.2 | 90.6 (95% CI 80.6 to 95.8) | 92.2 |
| Arrhythmias | Patient | 4 | 126 | 97.7 (95% CI 88.0 to 99.9) | 1.4 | 81.7 (95% CI 71.6 to 89.4) | 0 |
| Segment | 4 | 1526 | 87.4 (95% CI 68.3 to 95.7) | 79.6 | 96.0 (95% CI 91.2 to 98.2) | 89.5 | |
| HHR (≥ 65 b.p.m.) | Patient | 5 | 462 | 97.7 (95% CI 93.2 to 99.3) | 39.0 | 86.3 (95% CI 80.2 to 90.7) | 49.8 |
| Artery | 4 | 664 | 93.7 (95% CI 87.8 to 96.9) | 0 | 92.4 (95% CI 83.3 to 96.8) | 83.7 | |
| Segment | 8 | 8133 | 92.7 (95% CI 89.3 to 95.1) | 67.1 | 95.7 (95% CI 92.8 to 97.4) | 92.8 | |
| Previous stent implantation | Patient | 4 | 233 | 96.0 (95% CI 88.8 to 99.2) | 19.0 | 81.6 (95% CI 74.7 to 87.3) | 0 |
| Stent/stented lesion | 6 | 582 | 93.6 (95% CI 86.1 to 97.2) | 0 | 91.0 (95% CI 87.3 to 93.7) | 35.1 |
With the exception of one small study, data on the accuracy of NGCCT in patients with high coronary calcium scores, previous bypass grafts, or obesity were limited to per arterial segment or per-artery data. Sensitivity estimates remained high (> 90% in all but one study).
Data on the number of difficult-to-image patients in whom NGCCT was non-diagnostic were sparse; where numbers of non-diagnostic images were reported, these were often for the whole study population, rather than the difficult-to-image subgroup. Three studies did report subgroup-specific non-diagnostic image rates in different populations; these were 5% for patients with arrhythmias,47 6.8% for patients with HHR44 and 9% for patients with previous stent implantation. 40
Chapter 4 Assessment of cost-effectiveness
Search strategy
Searches were undertaken to identify cost-effectiveness studies of NGCCT. As with the clinical effectiveness searching, search strategies were developed specifically for each database and searches took into account generic and other product names for the intervention. No restrictions on language or publication status were applied. Limits were applied to remove animal studies. Full search strategies are reported in Appendix 1.
The following databases were searched for relevant studies from 1 January 2000 to 21 March 2011: MEDLINE (2000 to March week 2 2011) (OvidSP)
-
MEDLINE In-Process and Other Non-Indexed Citations and Daily Update (2000 to 17 March 2011) (OvidSP)
-
EMBASE (2000 to week 11 2011) (OvidSP)
-
NHS EED (2000 to 9 March 2011) (CRD website)
-
Health Economic Evaluation Database (HEED) (2000 to 9 March 2011) (Wiley) http://onlinelibrary.wiley.com/book/10.1002/9780470510933
-
Paediatric Economic Database Evaluation (PEDE) (2000 to 5 March 2011) (internet) http://pede.ccb.sickkids.ca/pede/search.jsp
Supplementary searches on catheter angiography were undertaken on the following resources to identify guidelines and guidance:
-
National Guideline Clearinghouse (NGC) (2005 to 16 March 2011) (Internet) www.guideline.gov/
-
International Guideline Library (G-I-N) (2005 to 16 March 2011) www.g-i-n.net
-
NICE guidance (up to 16 March 2011) (internet) http://guidance.nice.org.uk/
-
Turning Rsearch Into Practice (TRIP) database (2005 to 16 March 2011) (internet) www.tripdatabase.com/
-
HTA (2005 to 16 March 2011) (CRD website).
Identified references were downloaded in EndNote X4 software for further assessment and handling. References in retrieved articles were checked for additional studies.
Cost-effectiveness of new-generation cardiac computed tomography in coronary artery disease
Model structure and methodology
In order to assess the cost-effectiveness of NGCCT for difficult-to-image patient groups with CAD a model was developed. This model provides a framework for the synthesis of data from the review of clinical effectiveness of NGCCT (see Chapter 3, Results), which only consisted of accuracy data, and other relevant parameters, such as costs and effects of complications due to procedures, the long-term costs and effects of patients with CAD, and the risk of cancer from radiation exposure, in order to evaluate the potential long-term cost-effectiveness of NGCCT.
The cost-effectiveness of NGCCT for difficult-to-image patient groups is estimated for two CAD populations: the suspected CAD population and the known CAD population. Patients suspected of CAD are patients who have chest pain or other symptoms suggestive of CAD. Patients with known CAD are patients who have previously been diagnosed with CAD and whose symptoms are no longer controlled by drug treatment and/or being considered for revascularisation. The use of NGCCT has different purposes in the two CAD populations: for the suspected CAD population the purpose is to diagnose patients with CAD and for the known CAD population the purpose is to aid decision-making regarding a revascularisation.
The overall decision problem for which we aimed to develop a model can be subdivided into separate components. As for most of these components models were already available, we decided to combine five models to estimate the cost-effectiveness of the NGCCT:
-
a decision tree that models the diagnostic pathway (see below, Diagnostic model)
-
an alive–dead Markov model for ‘healthy’ patients without CAD (see below, Healthy population Markov model)
-
a simple stroke model to estimate the impact of test and treatment-related stroke (see below, Stroke model)
-
a model for the prognosis of patients with CAD (see below, EUROPA)
-
a model constructed by the Centre for Health Economics, University of York to model the impact of imaging due to radiation on cancer morbidity and mortality, hereafter referred to as the York Radiation Model (YRM)61 (see below, York Radiation Model).
The comparator used for the evaluation of suspected or known CAD in difficult-to-image patients was ICA (see Chapter 3). Three strategies were evaluated in this assessment. The first strategy (ICA only) is a strategy through which patients with suspected or known CAD only undergo an ICA. Although ICA is the reference standard test and is assumed to be 100% sensitive and specific, it is associated with a risk of serious complications, including death, non-fatal MI and stroke. NGCCT does not have a sensitivity and specificity of 100% and thus is less accurate than the ICA. The second strategy (NGCCT–ICA) evaluates the combination of cardiac CT using the new-generation technologies and ICA. Cardiac CT is first performed in all patients and patients with a positive CT scan then undergo an ICA. 3 This additional test will reveal any patients with a false-positive CT test result but it also provides other information that a CT currently does not. 3 The third strategy (NGCCT only) uses only NGCCT to diagnose patients.
The five models used in the analyses are described, in detail, below. The stochastic analyses are based on cohort simulations. To investigate decision uncertainty, second-order uncertainty microsimulations were run. All costs and effects were discounted by 3.5%. The model incorporated a lifetime horizon to estimate outcomes in terms of quality-adjusted life-years (QALYs) and costs from the perspective of the NHS. Only health effects of patients were included.
Diagnostic model
The diagnostic pathway was modelled using a modified version of the CE-MARC model, developed by Walker (University of York, 2011, personal communication) which is based on the CE-MARC study. 62 The CE-MARC study62 compared CV MRI with other diagnostic tests. Modification of the original CE-MARC model was necessary because the test strategies considered in this assessment did not correspond with the test strategies used in the original model. Furthermore, they did not include the treatment medication-only option required for our suspected CAD population. Our model identifies patients as TP, TN, FP and FN depending on the diagnostic performance of the test or test strategy and the prior likelihood of the test outcome. Furthermore, it estimates the mortality and morbidity of the tests and the interventions.
Decision trees for this process are shown in Figures 11–13 for patients with suspected CAD and in Figures 16–18 for patients with known CAD. Two versions of the diagnostic model were created because the known (two-treatment model) and suspected CAD (three-treatment model) populations are treated differently after a positive test outcome. The disease progression of the survivors of the tests and revascularisation procedures was modelled with the disease progression model (see EUROPA, below). We assumed that the tests were performed immediately after each other without any time delay.
FIGURE 11.
Coronary artery disease suspected population: ICA only.
FIGURE 12.
Coronary artery disease suspected population: NGCCT-ICA.
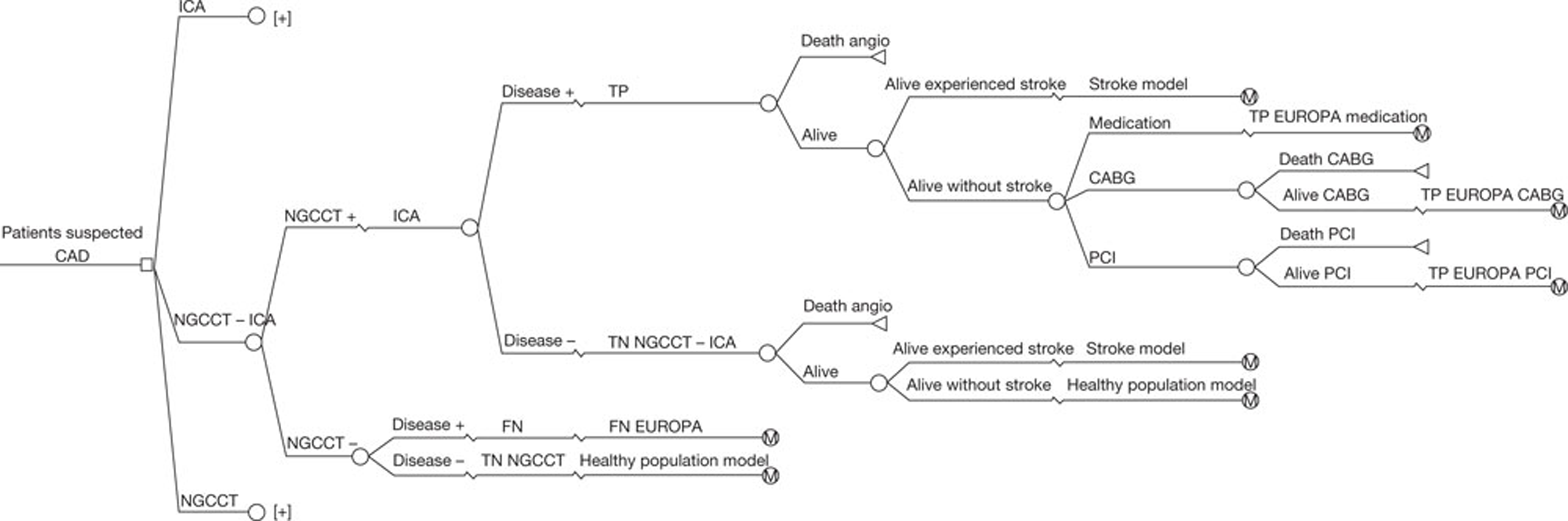
FIGURE 13.
Coronary artery disease suspected population: NGCCT.
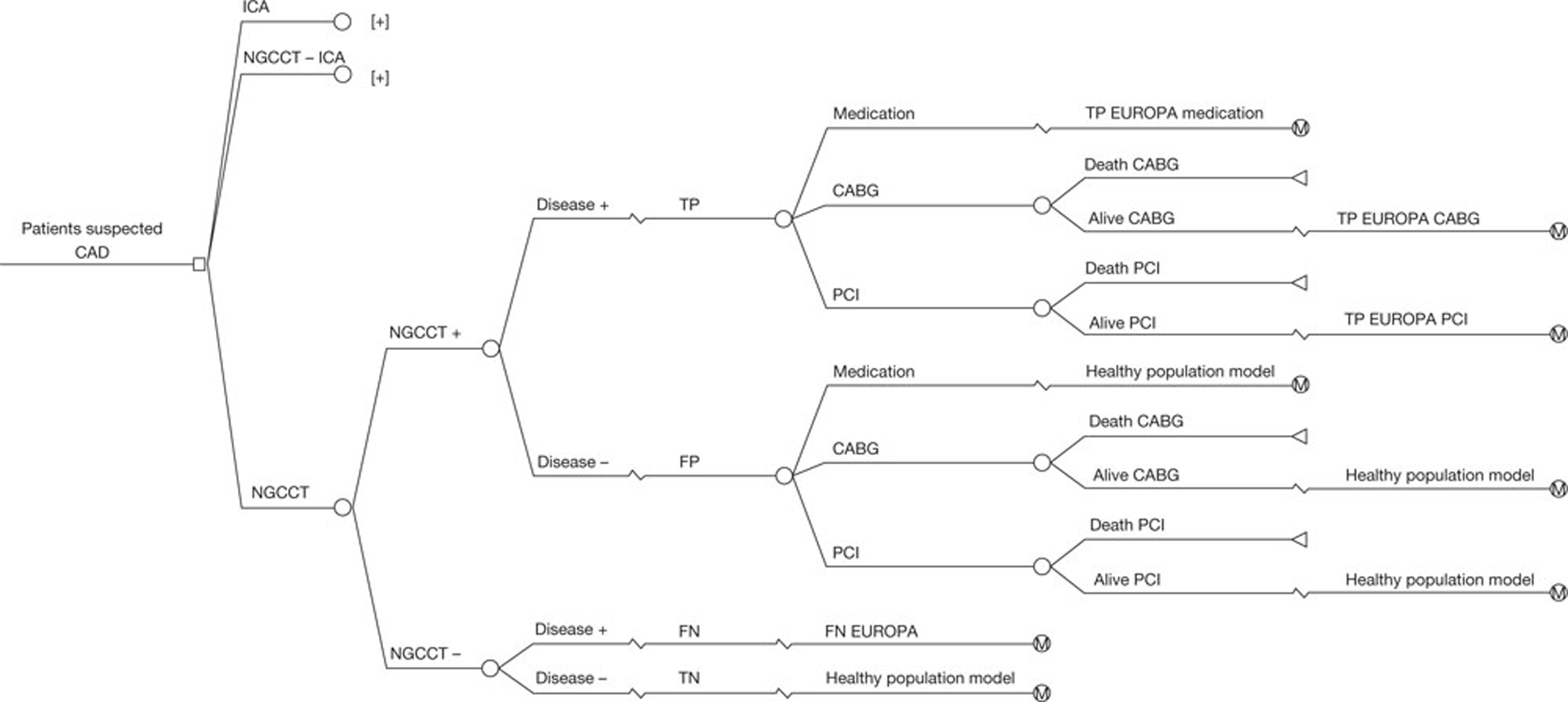
Diagnostic model for patients with suspected coronary artery disease
The purpose of testing patients with suspected CAD (based on clinical symptoms) is to diagnose those patients and give, when necessary, appropriate treatment.
The prior likelihood of having CAD in patients with suspected CAD is assumed to be 10–29%, based on the clinical guideline Chest pain of recent onset. 63 This prior likelihood is based on some patient characteristics (age, gender, diabetes, smoking and hyperlipidaemia, and either non-anginal chest pain, atypical angina or typical angina). According to the guideline, in these patients, first a CT calcium scoring is performed and the patients referred for 64-slice CT (i.e. our population) have a score of 1–400. Patients with a higher prior likelihood than 10–29% should be referred for ICA. Some difficult-to-image subgroups could have a higher prior likelihood but how much higher is unknown. Therefore, we performed a scenario analysis where the prior likelihood was set at 30% for all subgroups. Table 17 summarises the prior likelihood of CAD in the known and suspected CAD populations.
The sensitivity and specificity of ICA was assumed to be 100%, as in Mowatt et al. 3 The systematic review performed for this assessment provided the estimates of the sensitivity and specificity for the NGCCT. As described in Chapter 3, Summary, estimates of sensitivity and specificity differed for the different difficult-to-image patient groups. The sensitivity and specificity of the NGCCT in the beta-blocker-intolerant patient group were assumed to be the same as the sensitivity and specificity in patients with a HHR. As beta-blockers are used to lower the heart rate of the patients, it is not the intolerance itself that makes the patient difficult to scan but rather the fact that such a patient may have a heart rate that is too high during the scan; studies reporting per-patient sensitivity and specificity in patients with a HHR did not use beta-blockers prior to scanning. Table 18 shows the sensitivity and specificity estimates for the NGCCT in the different difficult-to-image patient groups.
| Test and population | Sensitivity (95% CI) | Specificity (95% CI) | Source |
|---|---|---|---|
| ICA: reference standard | 1 | 1 | |
| NGCCT: obesity | 0.904 (0.838 to 0.949) | 0.921 (0.891 to 0.945) | Review |
| NGCCT: high coronary calcium score | 0.927 (0.883 to 0.956) | 0.906 (0.806 to 0.958) | Review |
| NGCCT: arrhythmias | 0.977 (0.881 to 0.999) | 0.817 (0.716 to 0.894) | Review |
| NGCCT: HHR | 0.977 (0.932 to 0.993) | 0.863 (0.802 to 0.907) | Review |
| NGCCT: beta-blocker intolerance | 0.977 (0.932 to 0.993) | 0.863 (0.802 to 0.907) | Assumption |
| NGCCT: previous stented | 0.960 (0.822 to 0.999) | 0.816 (0.747 to 0.873) | Review |
| NGCCT: previous CABG | 0.964 (0.875 to 0.996) | 0.87 (0.664 to 0.972) | Review |
The result of the test and the presence of the disease determine whether a patient is classified as TP, TN, FP or FN (illustrated in Figure 14). The three strategies (ICA only, NGCCT only and NGCCT–ICA) all have other properties and therefore test outcomes differ by strategy. The four outcomes were calculated using the following formulae: TP, prior likelihood × sensitivity; TN, (1 – prior likelihood) × specificity; FP, (1 – prior likelihood) × (1 – specificity); FN, prior likelihood × (1 – sensitivity). Possible test outcomes are described by strategy.
FIGURE 14.
A 2 × 2 table for patients with suspected CAD.
| Test outcome | Disease positive | Disease negative |
|---|---|---|
| Test positive | TP | FP |
| Test negative | FN | TN |
Patients with suspected CAD who have a positive test result are thought to have CAD according to the test and need to be treated with medication only or a revascularisation. A negative test result implies that the patient with suspected CAD does not have the disease and does not need to be treated.
-
ICA-only strategy Patients diagnosed with the reference standard ICA can be defined as only TP or TN because ICA is assumed to be 100% accurate and therefore misdiagnosis is not possible.
-
NGCCT-only strategy The sensitivity and specificity of the NGCCT are not 100%, and the results of these tests can therefore define patients as TP, TN, FP or FN. For the patients who are diagnosed incorrectly the test result will have consequences. A proportion of the FNs will later be identified as TPs because patients may have persistent symptoms. However, in our model, these patients could have experienced an event [e.g. MI or cardiac arrest (CA)] before the correct diagnosis is established. The FPs may receive unnecessary treatment with its attendant consequences.
-
NGCCT–ICA strategy In this strategy, an ICA is performed to confirm a positive NGCCT scan. Therefore, all patients with a FP result for the NGCCT will subsequently be correctly classified by the ICA as TNs. As a result, these patients will not receive any unnecessary treatment. In the model, all of these patients are subsequently considered as TNs for the NGCCT–ICA strategy since the ICA correctly reclassified them. However, an ICA is not performed in patients with a negative NGCCT result. As the sensitivity of the NGCCT is not 100%, it is possible for FN results to arise from this NGCCT–ICA strategy. As with the FNs from the NGCCT-only strategy, a proportion of these FNs will be identified at a later stage.
Diagnostic model for population with known coronary artery disease
The purpose of testing patients with known CAD (defined as those who have previously been diagnosed with CAD and whose symptoms are no longer controlled by drug treatment and/or are being considered for revascularisation) is to inform revascularisation decisions.
The prior likelihood of performing a revascularisation in patients with known CAD is assumed to be 39.5%, based on the CE-MARC study (see Table 17). 64 The CE-MARC study62 calculated the cost-effectiveness of using CV MRI to determine whether or not a revascularisation is necessary. The purpose of diagnostic testing assessed in the CE-MARC study62 captures the aim of this economic evaluation for the known CAD population and therefore the prior likelihood of the CE-MARC population can be used in the diagnostic model.
The accuracy of the NGCCT for the known CAD population is assumed to be the same as for the suspected CAD population. This assumption was made because for some difficult-to-image patient groups there were no data or just one article for a known CAD population. Details of the reported inclusion criteria, for all studies included in the systematic review, are provided in Appendix 4.
A positive test result for the patient population who have previously been diagnosed with CAD and whose symptoms are no longer controlled by drug treatment and/or who are being considered for revascularisation indicates that the patient will benefit from a revascularisation and should undergo a CABG or a PCI. A negative test result for the same population implies that the patient will not benefit from a revascularisation and drug treatment only should be continued.
The same test outcomes apply to the known CAD population as previously described before for the suspected CAD population (Figure 15). Thus the ICA-only strategy will define only TP and TN because ICA is assumed to be 100% accurate. The NGCCT-only strategy gives four possible outcomes: TP, FP, TN and FN. The combined strategy (NGCCT–ICA) defines three outcomes: TP, TN and FN.
FIGURE 15.
A 2 × 2 table for patients with known CAD.
| Test Outcome | Revascularisation needed | Revascularisation not needed |
|---|---|---|
| Test positive | TP | FP |
| Test negative | FN | TN |
FIGURE 16.
Known CAD: ICA only.
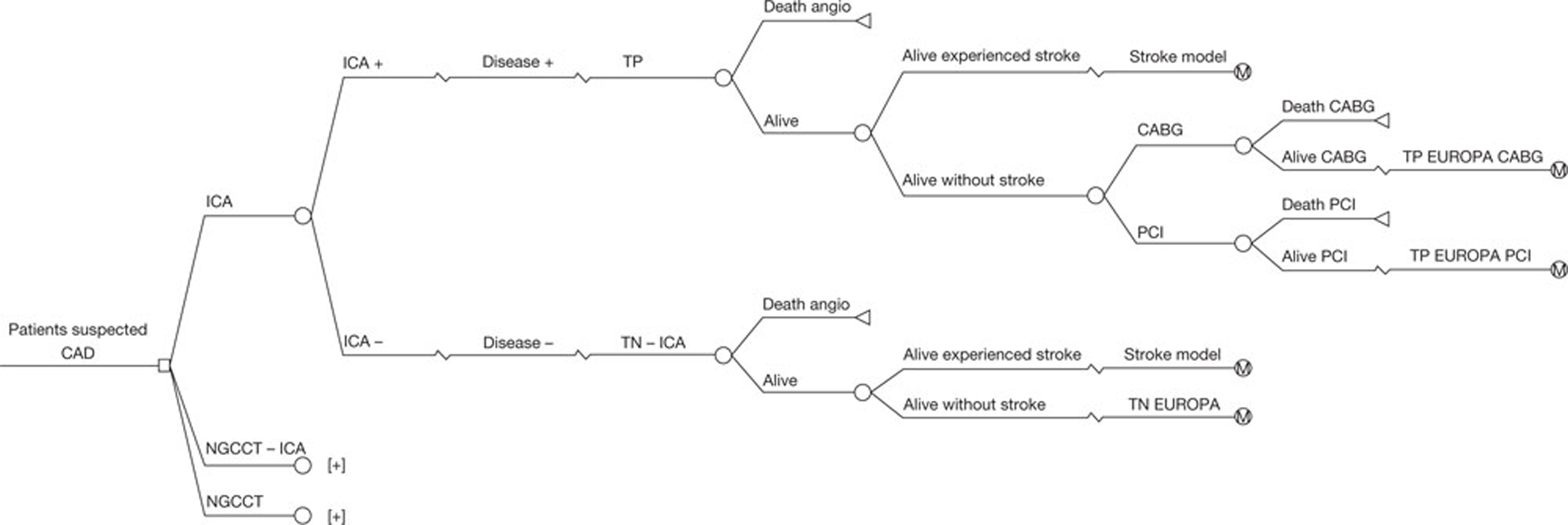
FIGURE 17.
Known CAD: NGCCT-ICA.
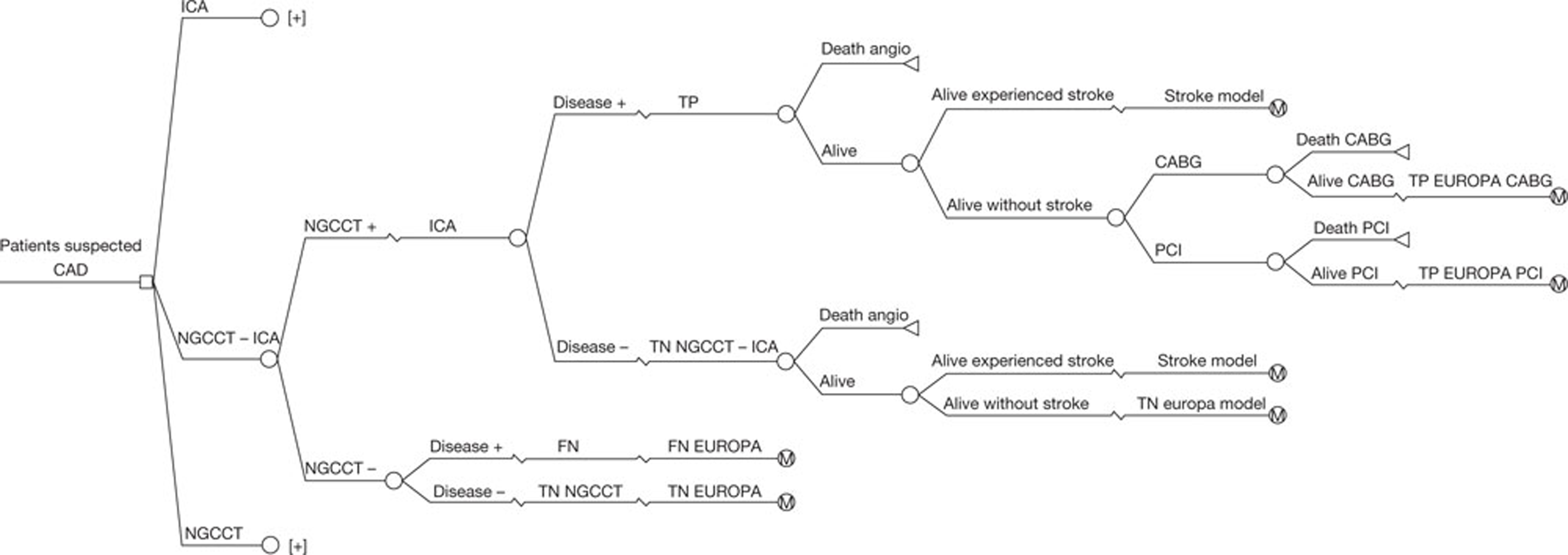
FIGURE 18.
Known CAD: NGCCT only.
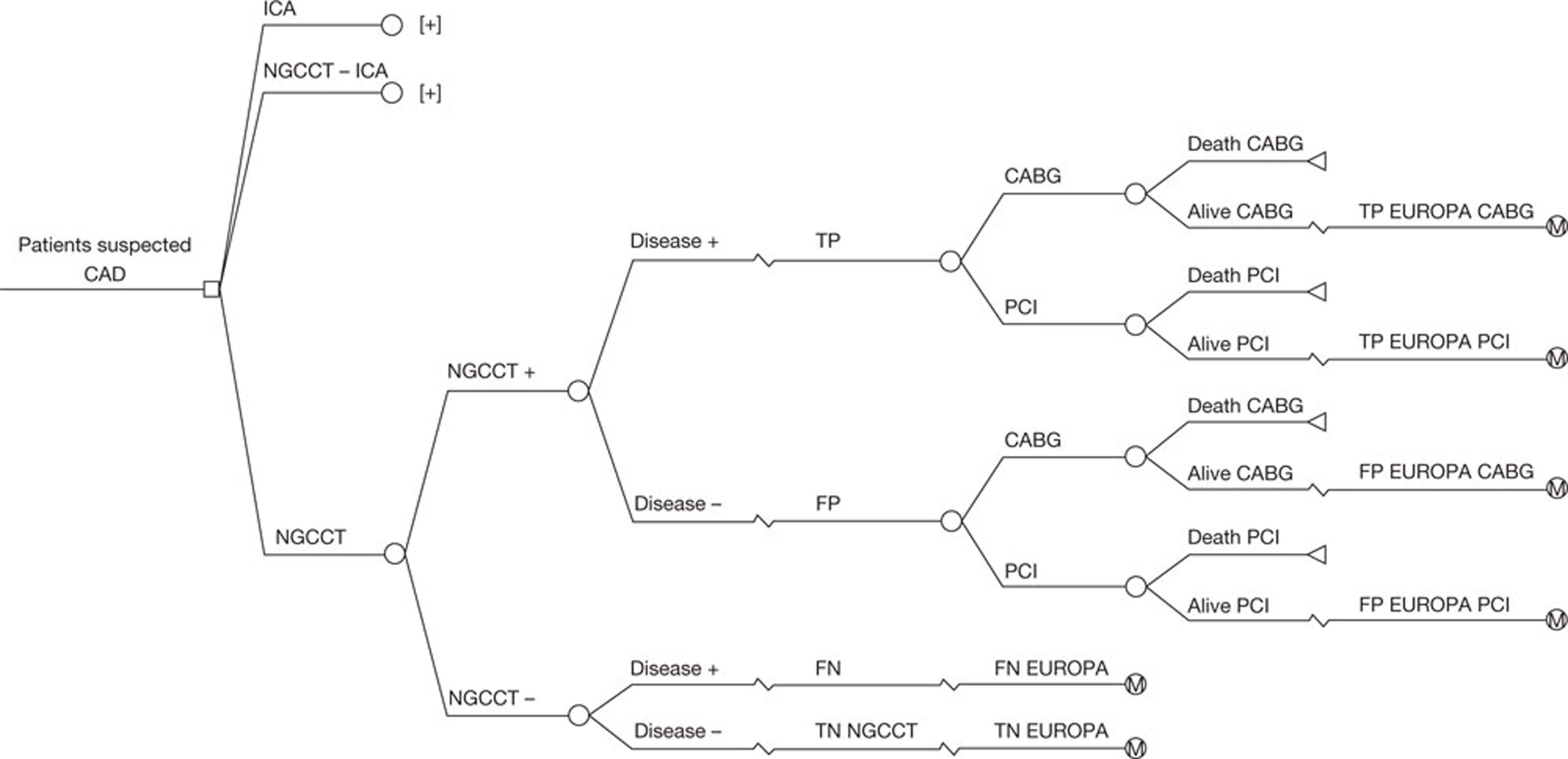
Healthy population Markov model
Patients without the disease (TN and FP from the suspected CAD population; see Table 19) were modelled with a simple alive–dead Markov model (Figure 19) based on UK life tables. 65 Based on UK life tables, patients could either die of all causes (including CV, because a negative test result does not mean that patients will never develop CAD) or stay in the ‘alive’ state. Only QALYs but no costs were calculated with this model.
| Strategy | Without angiographic and revascularisation mortality | With angiographic and revascularisation mortality | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TP | FP | TN | FN | TP | FP | TN | FN | Mortality ICA | Morbidity ICA | Mortality revascularisation | Morbidity revascularisation | |
| Obese | ||||||||||||
| ICA only | 0.2000 | – | 0.8000 | – | 0.1996 | – | 0.7994 | – | 0.0007 | 0.0006 | 0.0003 | 0.000 |
| NGCCT-ICA | 0.1808 | – | 0.8000 | 0.0192 | 0.1804 | – | 0.8000 | 0.0192 | 0.0002 | 0.0002 | 0.0002 | 0.000 |
| NGCCT only | 0.1808 | 0.0632 | 0.7368 | 0.0192 | 0.1806 | 0.0631 | 0.7368 | 0.0192 | – | – | 0.0003 | 0.001 |
| Arrhythmias | ||||||||||||
| ICA only | 0.2000 | – | 0.8000 | – | 0.1996 | – | 0.7994 | – | 0.0007 | 0.0006 | 0.0003 | 0.000 |
| NGCCT-ICA | 0.1954 | – | 0.8000 | 0.0046 | 0.1950 | – | 0.7999 | 0.0046 | 0.0002 | 0.0002 | 0.0003 | 0.000 |
| NGCCT only | 0.1954 | 0.1464 | 0.6536 | 0.0046 | 0.1951 | 0.1462 | 0.6536 | 0.0046 | – | – | 0.0005 | 0.001 |
| High coronary calcium score | ||||||||||||
| ICA only | 0.2000 | – | 0.8000 | – | 0.1996 | – | 0.7994 | – | 0.0007 | 0.0006 | 0.0003 | 0.000 |
| NGCCT-ICA | 0.1854 | – | 0.8000 | 0.0146 | 0.1851 | – | 0.7999 | 0.0145 | 0.0002 | 0.0002 | 0.0003 | 0.000 |
| NGCCT only | 0.1854 | 0.0752 | 0.7248 | 0.0146 | 0.1852 | 0.0747 | 0.7252 | 0.0145 | – | – | 0.0004 | 0.001 |
| HHR | ||||||||||||
| ICA only | 0.2000 | – | 0.8000 | – | 0.1996 | – | 0.7994 | – | 0.0007 | 0.0006 | 0.0003 | 0.000 |
| NGCCT-ICA | 0.1954 | – | 0.8000 | 0.0046 | 0.1950 | – | 0.7999 | 0.0046 | 0.0002 | 0.0002 | 0.0003 | 0.000 |
| NGCCT only | 0.1954 | 0.1096 | 0.6904 | 0.0046 | 0.1951 | 0.1095 | 0.6904 | 0.0046 | – | – | 0.0004 | 0.001 |
| Intolerance beta-blocker | ||||||||||||
| ICA only | 0.2000 | – | 0.8000 | – | 0.1996 | – | 0.7994 | – | 0.0007 | 0.0006 | 0.0003 | 0.000 |
| NGCCT-ICA | 0.1954 | – | 0.8000 | 0.0046 | 0.1950 | – | 0.7999 | 0.0046 | 0.0002 | 0.0002 | 0.0003 | 0.000 |
| NGCCT only | 0.1954 | 0.1096 | 0.6904 | 0.0046 | 0.1951 | 0.1095 | 0.6904 | 0.0046 | – | – | 0.0004 | 0.001 |
FIGURE 19.
Simple alive-dead Markov model.
Of the patients without the disease, only those with a FP test result may undergo unnecessary medical tests and procedures before the absence of CAD is established. The analyses performed in this study included the costs and health outcomes resulting from these tests and procedures in the diagnostic model. However, beyond this, there was no reason to expect any long-term difference in prognosis between patients with a TN test result and those with a FP test result. Long-term costs were therefore not included in the analyses.
Stroke model
As stated previously, ICA and revascularisations are associated with complications and one of these is stroke. The costs and health expectancy of patients who experienced a stroke due to the initial ICA or revascularisation were modelled using a simple alive–dead stroke model. Life expectancy is based on updated UK life tables, combined with a multiplier for age-specific mortality among stroke patients. 66 Costs and QALYs for stroke patients were calibrated to correspond with the results of an economic evaluation by Sandercock et al. ,66 which estimated the cost-effectiveness of thrombolytic treatment for acute ischaemic stroke compared with standard care for the NHS perspective. In particular, we assumed that stroke patients would receive thrombolytic treatment. 67
EUROPA
The EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease (EUROPA) trial assessed the ability of the ACE inhibitor perindopril to reduce CV death, MI, and CA in a broad population of patients with stable coronary heart disease and without heart failure or substantial hypertension. 68 Based on the patients in this trial, Briggs et al. 69 built a Markov model.
Patients with the disease who have not experienced a stroke due to the initial ICA or initial revascularisation, irrespective of the test outcome enter the EUROPA model. The Markov based EUROPA model predicts changes to life expectancy and QALYs for patients with CAD. These changes are calculated based on risk equations which predict the probability of events [CA, (non-)fatal MI] that patients could suffer and the mortality associated with those events. The time cycle used in the EUROPA model is 3 months.
EUROPA model structure
The EUROPA Markov model (Figure 20) consisted of five health states that were defined as absence of primary event in the EUROPA trial: ‘trial entry’, ‘CV death’, ‘non-fatal primary event in current year’, ‘history of non-fatal event (NFE)’ and ‘non-CV death’. 70 The 3-monthly transition probabilities between the different states were based on risk equations and on UK life tables on non-CV death. The risk equations consisted of several covariates based on baseline characteristics and previous conditions, such as age, gender, previous MI, diabetes mellitus, etc. The prognosis of the patients was partly dependent on the initial test outcome and treatment decision.
All patients with CAD (with the exception of those who experience non-fatal complications from ICA, PCI or CABG) enter the EUROPA model in the ‘Start’ state. A patient can either stay in this state, die from a non-CV cause (and move to the ‘Non-CV death’ state), or experience a CV event and move to the ‘CV death’ state if the event is fatal or to the state ‘non-fatal event (first year)’ if the event is not fatal. The ‘non-CV death’ and the ‘CV death’ states are both mutually absorbing states. Patients can end up in the ‘non-fatal event (first year)’ state in two different ways: by experiencing a non-fatal MI from the initial ICA or revascularisation or by experiencing a non-fatal event at a later time (modelled in the EUROPA model by the risk equations). When a patient is in the ‘non-fatal event (first year)’ state he or she can remain in this state for maximum of 1 year without experiencing a subsequent event. After that, a patient can move to the ‘non-fatal event (after first year)’ state if he or she has stayed in the ‘non-fatal event (first year)’ state for a year without experiencing a new event. Patients in the ‘non-fatal event (first year)’ can also move to the ‘Non-CV death’ state if the patient dies from a non-CV cause; the ‘CV death state’ if the patient experiences a subsequent event which is fatal (‘CV death’ state) or stay in the ‘non-fatal event (first year)’ state if the subsequent event is not fatal. A patient in the ‘non-fatal event (after first year)’ state can stay there, move to the ‘non-fatal event (first year)’ state if the patient experiences a non-fatal subsequent event, move to the ‘CV death’ state if the patient experiences a fatal subsequent event, or move to the ‘non-CV death’ state if the patient dies from a non-CV cause. The risks of events and the mortality associated with events are predicted by the risk equations. Non-CV mortality was based on UK life tables.
EUROPA model entry for population with suspected coronary artery disease
The proportions of patients classified as TP and FN entering the EUROPA model were based on the calculations using prevalence of the disease, sensitivity and specificity of the tests as defined in the diagnostic model. These proportions can vary between the three strategies. Table 19 shows intermediate results of the diagnostic model in two ways. The first part shows how the four test outcomes are represented for each strategy, each difficult-to-image patient group. The second part shows the impact of immediate procedure-related mortality and morbidity on the distribution of the test outcomes. As expected the mortality rates differ considerably between the three strategies. Patients suspected of CAD diagnosed with the ICA alone have the highest overall mortality and morbidity rate. The TN proportion is the lowest in the difficult-to-image arrhythmias group due to the low specificity. The disease progression of the TP and the FN (patients with the disease) was modelled with the EUROPA model. These two outcomes were divided into three treatment possibilities: medication, PCI or CABG. The other two test outcomes (FP and TN) were modelled through a simple alive–dead Markov model (healthy population model) based on life tables, as described above (see Healthy population Markov model).
EUROPA model entry for population with known coronary artery disease
Table 20 presents the intermediate outcomes of the three strategies for the known CAD population. The first part shows how the test outcomes are distributed in each strategy for each difficult-to-image patient group. The second part incorporates also the mortality and morbidity associated with the ICA and revascularisations. The NGCCT–ICA strategy results in the lowest mortality and morbidity rates. The prognosis of patients in all four outcomes (TP, TN, FP and FN) was modelled using the EUROPA model because all patients have CAD.
| Strategy | Without angiographic and revascularisation mortality | With angiographic and revascularisation mortality | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TP | FP | TN | FN | TP | FP | TN | FN | Mortality ICA | Morbidity ICA | Mortality revascularisation | Morbidity revascularisation | |
| Obese | ||||||||||||
| ICA only | 0.3947 | – | 0.6053 | – | 0.3915 | – | 0.6048 | – | 0.0007 | 0.0006 | 0.0030 | 0.0051 |
| NGCCT-ICA | 0.3568 | – | 0.6053 | 0.0379 | 0.3541 | – | 0.6052 | 0.0379 | 0.0001 | 0.0003 | 0.0027 | 0.0046 |
| NGCCT only | 0.3568 | 0.0478 | 0.5574 | 0.0379 | 0.3542 | 0.0475 | 0.5574 | 0.0379 | – | – | 0.0030 | 0.0052 |
| Arrhythmias | ||||||||||||
| ICA only | 0.3947 | – | 0.6053 | – | 0.3915 | – | 0.6048 | – | 0.0007 | 0.0006 | 0.0030 | 0.0051 |
| NGCCT-ICA | 0.3857 | – | 0.6053 | 0.0091 | 0.3827 | – | 0.6052 | 0.0091 | 0.0002 | 0.0003 | 0.0029 | 0.0050 |
| NGCCT only | 0.3857 | 0.1108 | 0.4945 | 0.0091 | 0.3828 | 0.1099 | 0.4945 | 0.0091 | – | – | 0.0037 | 0.0064 |
| High coronary calcium score | ||||||||||||
| ICA only | 0.3947 | – | 0.6053 | – | 0.3915 | – | 0.6048 | – | 0.0007 | 0.0006 | 0.0030 | 0.0051 |
| NGCCT-ICA | 0.3659 | – | 0.6053 | 0.0288 | 0.3633 | – | 0.6052 | 0.0286 | 0.0001 | 0.0003 | 0.0027 | 0.0047 |
| NGCCT only | 0.3659 | 0.0569 | 0.5484 | 0.0288 | 0.3634 | 0.0562 | 0.5486 | 0.0286 | – | – | 0.0032 | 0.0054 |
| HHR | ||||||||||||
| ICA only | 0.3947 | – | 0.6053 | – | 0.3915 | – | 0.6048 | – | 0.0007 | 0.0006 | 0.0030 | 0.0051 |
| NGCCT-ICA | 0.3857 | – | 0.6053 | 0.0091 | 0.3827 | – | 0.6052 | 0.0091 | 0.0001 | 0.0003 | 0.0029 | 0.0050 |
| NGCCT only | 0.3857 | 0.0829 | 0.5223 | 0.0091 | 0.3828 | 0.0823 | 0.5223 | 0.0091 | – | – | 0.0035 | 0.0060 |
| Intolerance beta-blocker | ||||||||||||
| ICA only | 0.3947 | – | 0.6053 | – | 0.3915 | – | 0.6048 | – | 0.0007 | 0.0006 | 0.0030 | 0.0051 |
| NGCCT-ICA | 0.3857 | – | 0.6053 | 0.0091 | 0.3827 | – | 0.6052 | 0.0091 | 0.0001 | 0.0003 | 0.0029 | 0.0050 |
| NGCCT only | 0.3857 | 0.0829 | 0.5223 | 0.0091 | 0.3828 | 0.0823 | 0.5223 | 0.0091 | – | – | 0.0035 | 0.0060 |
| Previous stent | ||||||||||||
| ICA only | 0.3947 | – | 0.6053 | – | 0.3915 | – | 0.6048 | – | 0.0007 | 0.0006 | 0.0030 | 0.0051 |
| NGCCT-ICA | 0.3789 | – | 0.6053 | 0.0158 | 0.3760 | – | 0.6052 | 0.0158 | 0.0002 | 0.0003 | 0.0028 | 0.0049 |
| NGCCT only | 0.3789 | 0.1114 | 0.4939 | 0.0158 | 0.3761 | 0.1105 | 0.4939 | 0.0158 | – | – | 0.0037 | 0.0063 |
| Previous CABG | ||||||||||||
| ICA only | 0.3947 | – | 0.6053 | – | 0.3915 | – | 0.6048 | – | 0.0007 | 0.0006 | 0.0030 | 0.0051 |
| NGCCT-ICA | 0.3805 | – | 0.6053 | 0.0142 | 0.3776 | – | 0.6052 | 0.0142 | 0.0001 | 0.0003 | 0.0028 | 0.0049 |
| NGCCT only | 0.3805 | 0.0787 | 0.5266 | 0.0142 | 0.3777 | 0.0781 | 0.5266 | 0.0142 | – | – | 0.0034 | 0.0059 |
Every cycle a certain proportion of the FN patients in both populations will be identified as TP based on the Canadian Cardiovascular Society (CCS) angina classification. Identified TPs will be treated and they will have the same prognosis as the TPs who were identified directly by the diagnostic test. The FNs that are still not identified have a higher chance of experiencing an event.
EUROPA model risk equation adjustments
Risk equations to predict the events for patients with CAD were based on the EUROPA trial. 68 Using the EUROPA model for the evaluation of the NGCCT in the two CAD populations (suspected and known), and for the different difficult-to-image patient groups, required some adjustment of the EUROPA model. These adjustments were necessary as the baseline characteristics of the EUROPA population were not completely comparable with the subgroups in the known and suspected CAD populations.
As shown in Figure 20, four equations were used to calculate transition probabilities between the states. The first equation based on time-to-event survival analysis estimated the probability of any event that will occur in one cycle of 3 months as a function of the following covariates: age, years older than 65, perindopril usage, smoking, previous MI, existing vascular disease [stroke, transient ischaemic attack (TIA) or peripheral vascular disease], family history of CAD, symptomatic angina or history of heart failure, systolic blood pressure, total cholesterol, obese (BMI of > 30 kg/m2), gender, nitrates usage, calcium channel blockers usage, lipid-lowering treatment, units creatinine clearance below 80 ml/minute and previous revascularisation (PCI or CABG) (Table 21). The second equation of the EUROPA model estimates the odds that the event is fatal, based on age, previous MI and total cholesterol. The third equation estimates the risk of a subsequent event in the first year after a first NFE and is based on the presence of symptomatic angina or history of heart failure. The fourth equation, which predicts the risk of a subsequent event after 1 year, is the same as the first equation except that the covariate previous MI is updated by setting the covariate previous MI at ‘1’.
| Covariates | Mean values EUROPA population: mean (SD) or % (95% CI) | Equation 1: Risk of first primary event | Equation 2: Odds that first event is fatal | Equation 3: Risk of subsequent event in first year after initial NFE | |||
|---|---|---|---|---|---|---|---|
| Coefficient | HR | Coefficient | OR | Coefficient | HR | ||
| Perindopril usage | 100% | −0.2148 | 0.8067 | ||||
| Age (years) | 60 (9) | 0.0396 | 1.0403 | ||||
| Age > 65 years | 0 | 0.0592 | 1.0610 | 0.6139 | 1.8476 | ||
| Gender | 85.4% (84.8% to 86.0%) | 0.4349 | 1.5448 | ||||
| Smoking | 15.2% (14.6% to 15.8%) | 0.3959 | 1.4858 | ||||
| Previous MI | 64.8% (64.0% to 65.6%) | 0.3675 | 1.4441 | 0.4673 | 1.5956 | ||
| Previous revascularisation | 54.9% (54.0% to 55.8%) | −0.1332 | 0.8753 | ||||
| Existing vascular disease | 9.8% (9.3% to 10.3%) | 0.5233 | 1.6876 | ||||
| Diabetes mellitus | 12.3% (11.7% to 12.9%) | 0.4005 | 1.4926 | ||||
| Family history | 27.2% (26.4% to 28.0%) | 0.1873 | 1.2060 | ||||
| Symptomatic angina | 24.5% (24.2% to 25.8%) | 0.2801 | 1.3232 | ||||
| Systolic blood pressure | 137 (15) | 0.0045 | 1.0045 | ||||
| Creatinine clearance < 80 ml/minute | 6.9 (10.3) | 0.0114 | 1.0115 | ||||
| Obesity | 21.1% (20.3% to 21.7%) | 0.3455 | 1.4127 | ||||
| Total cholesterol | 5.4 (1.0) | 0.1248 | 1.1329 | 0.1870 | 1.2056 | ||
| Use of nitrates at baseline | 44.4% (43.1% to 44.9%) | 0.3537 | 1.4243 | ||||
| Use calcium channel blockers at baseline | 32.4% (31.6% to 33.2%) | 0.1815 | 1.1990 | ||||
| Use lipid-lowering treatment at baseline | 55.9% (55.0% to 56.8%) | −0.1566 | 0.8551 | ||||
| Constant (log scale) | 1 | −12.2737 | −4.3725 | −6.459 | |||
| Ancillary parameter | 0.7 | ||||||
The risk equations consist of covariates based on the EUROPA trial and therefore baseline characteristics had to be established for the 12 subgroups (seven difficult-to-image patient groups in the known CAD population and five in the suspected CAD population). Means were used in the risk equation, as we used a cohort model. The accuracy of the NGCCT was based on the systematic review reported in Chapter 3, and this review was also used as a source to estimate the baseline characteristics of the different subgroups for use in the risk equations; details of the baseline characteristics of study populations included in the review are reported in Appendix 4. Only subgroup-specific publications were used, thus studies which determined the accuracy of the NGCCT in two or more difficult-to-image patient groups were not used. The baseline characteristics of the EUROPA population were used when information for a specific subgroup and baseline characteristic was not found; this approach assumes that there were no differences between the EUROPA population and the specific subgroup (see Table 21).
Population with suspected coronary artery disease
Baseline characteristics, such as age, gender, family history, diabetes mellitus, obesity, smoking and symptomatic angina, were collected from the articles included in the review that focused on the suspected CAD population. The richness of the information collected from the articles differed between the difficult-to-image patient groups. In all difficult-to-image patient groups except for the ‘intolerant to beta-blockers’ group, a minimum of gender and age data were found. When population specific information regarding risk-related characteristics was not found in the literature, the assumption was made that the difficult-to-image subgroup did not differ from the EUROPA population and therefore the value of the EUROPA population (see Table 21) was taken. ‘Perindopril usage’ was assumed to be 0.23 for the whole suspected CAD population. 71 We will assume that the effect of perindopril does apply for any ACE inhibitor. The covariates ‘age’, ‘age > 65 years’, ‘men (y/n)’, ‘smoking (y/n)’, ‘diabetes mellitus (y/n)’, ‘positive family history (y/n)’, ‘obese (y/n)’, ‘symptomatic angina (y/n)’ differed per difficult-to-image subgroup. No subgroup-specific information was collected for the covariates ‘systolic blood pressure’, ‘creatinine clearance’, ‘total cholesterol’ and ‘the usage of lipid-lowering treatment at baseline’. The five other covariates depended on the strategy, treatment and test outcomes. Tables 22 and 23 illustrate how proportions were assigned to the covariates. The proportion that has had an MI was based on the non-fatal complications of ICA and revascularisation. FNs in strategies 2 and 3 have not experienced an MI, revascularisation or vascular disease because they do not undergo an ICA or a revascularisation. The covariate previous revascularisation was set at 1 for the TPs treated with a revascularisation. Nitrates usage was assumed to be ‘0’ for all test outcomes. Usage of calcium channel blockers was assumed to be ‘1’ for TPs who received medical treatment. This is because, although they might actually be prescribed a beta-blocker instead,71 there was only a covariate in the risk equation for calcium channel blocker and not beta-blocker. This assumption can be justified because the efficacy of calcium channel blockers does not differ from that of beta-blockers. 71
| Explanatory baseline characteristics | Strategy 1: ICA only | Strategy 2: HDCT–ICA | Strategy 3: HDCT only | |||||
|---|---|---|---|---|---|---|---|---|
| TP revascularisation | TP medication | TP revascularisation | TP medication | FN | TP revascularisation | TP medication | FN | |
| Age, gender, positive family history, smoking, diabetes mellitus, obesity and symptomatic angina | Subgroup specific, if not available EUROPA population | Subgroup specific, if not available EUROPA population | Subgroup specific, if not available EUROPA population | Subgroup specific, if not available EUROPA population | Subgroup specific, if not available EUROPA population | Subgroup specific, if not available EUROPA population | Subgroup specific, if not available EUROPA population | Subgroup specific, if not available EUROPA population |
| Systolic blood pressure, creatinine clearance, total cholesterol, lipid-lowering treatment usage at baseline | Proportion EUROPA population | Proportion EUROPA population | Proportion EUROPA population | Proportion EUROPA population | Proportion EUROPA population | Proportion EUROPA population | Proportion EUROPA population | Proportion EUROPA population |
| ACE inhibitor usagea | 0.23 | 0.23 | 0.23 | 0.23 | 0.23 | 0.23 | 0.23 | 0.23 |
| Previous MI | MI due to ICA and revascularisation | MI due to ICA | MI due to ICA and revascularisation | MI due to ICA | 0 | MI due to revascularisation | 0 | 0 |
| Previous revascularisation | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| Existing vascular diseaseb | Stroke due to ICA and revascularisation | Stroke due to ICA | Stroke due to ICA and revascularisation | Stroke due to ICA | 0 | 0 | 0 | 0 |
| Use of nitrates at baseline | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Use calcium channel blockers at baseline | Proportion EUROPA population | 1 | Proportion EUROPA population | 1 | Proportion EUROPA population | Proportion EUROPA population | 1 | Proportion EUROPA population |
| Explanatory baseline characteristics | Obese | Arrhythmias | Beta-blockers | High coronary calcium | HHR |
|---|---|---|---|---|---|
| Age (years) | 63 | 66.11 | N/A | 63.93 | 61.91 |
| Gender | 0.659 | 0.69 | N/A | 0.75 | 0.68 |
| Positive family history | N/A | 0.17 | N/A | N/A | 0.16 |
| Smoking | 0.28 | 0.08 | N/A | N/A | 0.37 |
| Diabetes mellitus | 0.341 | 0.27 | N/A | N/A | 0.19 |
| Obesity | 1 | 0.42 | N/A | 0.37 | 0.18 |
| Symptomatic angina | N/A | N/A | N/A | N/A | 0.85 |
Population with known coronary artery disease
The procedure described above to establish the baseline characteristics for the suspected CAD population was also used for the known CAD population. No information about gender and age was available for the beta-blocker intolerance and high coronary calcium score groups. For the other groups these data were collected from the accuracy studies included in the systemic review. The covariates ‘age’, ‘age > 65 years’, ‘men (y/n)’, ‘smoking (y/n)’, ‘diabetes mellitus (y/n)’, ‘positive family history (y/n)’ and ‘obese (y/n)’ differed per difficult-to-image patient group. No subgroup-specific information was available for the covariates ‘symptomatic angina’, ‘systolic blood pressure’, ‘creatinine clearance’, ‘total cholesterol’ and ‘the usage of lipid-lowering treatment at baseline’. Perindopril intake proportion was set at 0.23, based on published data. 70 The proportion of patients experiencing an MI or the proportion where vascular disease is present was based on the EUROPA population. The proportions were not raised with ICA or revascularisation-induced MI. Nitrates usage and calcium channel blockers at baseline were not reported in the studies included in the systematic review and therefore these proportions were based on the EUROPA population (see Table 21). The proportion for previous revascularisation was set at ‘1’ for the TPs in all strategies, for the FPs in strategy 3, and for the subgroups' previous PCI and previous CABG this was set at ‘1’ for all test outcomes. The remaining proportions were set as for the EUROPA population (Tables 25 and 26).
Difficult-to-image patient group-specific data
In addition to CAD population-specific adjustments of the EUROPA risk equations, adjustments were necessary for each specific difficult-to-image patient group. It is likely that some of the reasons why patients are difficult to scan may also lead to a higher probability of a CV event.
In the obese patient group, the increased risk of events was already captured in the risk equation, as it contains a covariate for obesity. For the obese group, the covariate obesity was set at ‘1’ for all test outcomes, strategies and CAD populations.
For simplicity, we treated the difficult-to-image subgroup with a previous CABG the same as the difficult-to-image subgroup with a previous PCI. 72 The covariate ‘previous revascularisation’ is present in the first and fourth risk equations of the EUROPA model; thus, the risk of having a primary or subsequent event for these specific patient groups was captured.
For the difficult-to-image groups arrhythmias and high coronary calcium level, a relative risk (Table 24), compared with the EUROPA population, was used to adjust the risk of events. For the HCS patient group, data from an unpublished study73 were used to estimate the relative risk without correcting for other factors of experiencing primary events in patients with a coronary calcium score > 400 compared with patients without a coronary calcium score of > 400. The proportion with a coronary calcium score of > 400 in the EUROPA population was not reported and therefore the study of Shemesh et al. 74 was used to estimate a proportion assuming that the populations are comparable. We assumed that this relative risk also applies for the risk of having a subsequent event.
| Subgroup | RR female | RR male | Source | Proportion condition stable angina (%) | Source | Adjusted RR female | Adjusted RR male |
|---|---|---|---|---|---|---|---|
| Arrhythmias | 3.06 | 2.04 | Hippisley-Cox et al.75 | 19 | Banasiak (2007)76 | 2.2 | 1.7 |
| HCS | 4.58 | 4.58 | Joosen et al.73 | 49 | Shemesh (1998)74 | 1.66 | 1.66 |
| Explanatory baseline characteristics | Obese | Arrhythmias | Beta-blockers | High coronary calcium | HHR | Revascularisation |
|---|---|---|---|---|---|---|
| Age (years) | 63 | 68 | N/A | N/A | 56.2 | 65.12 |
| Gender | 0.659 | 0.71 | N/A | N/A | 0.52 | 0.69 |
| Positive family history | N/A | 0.7 | N/A | N/A | N/A | 0.39 |
| Smoking | 0.28 | N/A | N/A | N/A | N/A | 0.2858 |
| Diabetes mellitus | 0.341 | 0.2 | N/A | N/A | N/A | 0.3 |
| Obesity | 1 | 0.59 | N/A | N/A | N/A | 0.264 |
| Symptomatic angina | N/A | N/A | N/A | N/A | N/A | N/A |
| Explanatory baseline characteristics | Strategy 1: ICA only | Strategy 2: HDCT–ICA | Strategy 3: HDCT only | ||||||
|---|---|---|---|---|---|---|---|---|---|
| TP revascularisation | TN | TP revascularisation | TN | FN | TP revascularisation | TN | FN | FP revascularisation | |
| Age, gender, positive family history, smoking, diabetes mellitus, obesity and symptomatic angina | Subgroup specific, if not available EUROPA population | Subgroup specific, if not available EUROPA population | Subgroup specific, if not available EUROPA population | Subgroup specific, if not available EUROPA population | Subgroup specific, if not available EUROPA population | Subgroup specific, if not available EUROPA population | Subgroup specific, if not available EUROPA population | Subgroup specific, if not available EUROPA population | Subgroup specific, if not available EUROPA population |
| Systolic blood pressure, creatinine clearance, total cholesterol, lipid-lowering treatment usage at baseline | Proportion EUROPA population | Proportion EUROPA population | Proportion EUROPA population | Proportion EUROPA population | Proportion EUROPA population | Proportion EUROPA population | Proportion EUROPA population | Proportion EUROPA population | Proportion EUROPA population |
| ACE inhibitor usagea | 0.23 | 0.23 | 0.23 | 0.23 | 0.23 | 0.23 | 0.23 | 0.23 | 0.23 |
| Previous MI | Proportion EUROPA population | Proportion EUROPA population | Proportion EUROPA population | Proportion EUROPA population | Proportion EUROPA population | Proportion EUROPA population | Proportion EUROPA population | Proportion EUROPA population | Proportion EUROPA population |
| Previous revascularisation | 1 | Proportion EUROPA population (if subgroup is previous PCI/CABG than 1) | 1 | Proportion EUROPA population (if subgroup is previous PCI/CABG than 1) | Proportion EUROPA population (if subgroup is previous PCI/CABG than 1) | 1 | Proportion EUROPA population (if subgroup is previous PCI/CABG than 1) | Proportion EUROPA population (if subgroup is previous PCI/CABG than 1) | 1 |
| Existing vascular diseaseb | Proportion EUROPA population | Proportion EUROPA population | Proportion EUROPA population | Proportion EUROPA population | Proportion EUROPA population | Proportion EUROPA population | Proportion EUROPA population | Proportion EUROPA population | Proportion EUROPA population |
| Use of nitrates at baseline | Proportion EUROPA population | Proportion EUROPA population | Proportion EUROPA population | Proportion EUROPA population | Proportion EUROPA population | Proportion EUROPA population | Proportion EUROPA population | Proportion EUROPA population | Proportion EUROPA population |
| Use calcium channel blockers at baseline | Proportion EUROPA population | Proportion EUROPA population | Proportion EUROPA population | Proportion EUROPA population | Proportion EUROPA population | Proportion EUROPA population | Proportion EUROPA population | Proportion EUROPA population | Proportion EUROPA population |
A relative risk, compared with the EUROPA population, was also used to estimate the risk of experiencing events for the patient group with arrhythmias. The term ‘arrhythmias’ encompasses several different conditions, with AF being the most common. A relative risk was calculated, controlling for other factors for patients with arrhythmias, based on the relative risk found in the QRISK study, which investigated the relative risk of experiencing events for patients with AF against patients without AF. 75 The proportion of the patients with AF was not reported by the EUROPA study and therefore we assumed that the proportion AF in patients with CAD is 19% based on Banasiak et al. 76
No adjustments to the risk equations were necessary for the intolerant to beta-blockers patient group because it was assumed that intolerance of beta-blockers does not lead to an increased risk of experiencing events; patients undergoing a cardiac CT receive beta-blockers to lower their heart rate in order to produce images of adequate quality, not in order to prevent events. Patients with CAD will often be treated with beta-blockers but these can be replaced with calcium channel blockers and/or ACE inhibitors and therefore intolerance to beta-blockers will probably not affect prognosis.
For the patient group with HHR the risk equations were not adjusted because it was assumed that HHRs affect only the quality of CT imaging. The patient groups with HHR and intolerance to beta-blockers were modelled with the original risk equations based on the EUROPA population.
York Radiation Model
The impact of imaging-associated radiation on cancer rates and outcomes was not estimated with the EUROPA model but was with the YRM. 61 The EUROPA model takes into account only mortality and not the QALYs and costs of treatment of radiation-induced cancer. The YRM is a radiation impact model recently developed by the Technology Assessment Group of the University of York to assess the health impact of a reduction in radiation when using a new X-ray imaging system for diagnostic purposes. 61
Biological effects of radiation
The dose of ionised radiation absorbed by a body is measured in grays (Gy). However, the health-relevant (and harmful) energy absorbed depends on the tissue and type of radiation and is expressed in sieverts (Sv). Because of the small doses of imaging radiation, more often millisieverts (mSv) are used (1000 mSv = 1 Sv). Also, 1 Sv = 1 Gy × a weighting factor (e.g. for a breast scan the weighting factor is 0.05).
Exposure to ionised radiation has mainly three biological adverse effects. 77 First, radiation has a harmful effect on developing embryos when the expecting mother is exposed to radiation. This is not relevant in our application. Second, radiation exposure might affect reproductive health, i.e. radiation exposure may lead to adverse congenital health outcomes of later offspring. There is, however, no convincing evidence for this effect in humans, only in animal experiments. The third, most harmful, effect is an increased lifetime risk of cancer incidence. For low doses, sparse clinical evidence exists. A prominent source is a cohort study of Japanese atomic bomb survivors who were exposed to radiation. These data provide strong evidence of an increased cancer mortality risk at equivalent doses of > 100 mSv, good evidence of an increased risk for doses between 50 and 100 mSv, and reasonable evidence for an increased risk for doses between 10 and 50 mSv. 78
The standard epidemiological risk models use a linear relationship between radiation exposure and lifetime probability of solid cancer without assuming a threshold, i.e. even a minimal exposure is assumed to increase the lifetime risk of cancer incidence. The younger the age at exposure, the higher is the lifetime probability of cancer incidence for a given amount of radiation, partly because children have on average more life-years remaining to develop cancer. The cumulative lifetime risk of an individual for repeated exposure to radiation is calculated by summing the probabilities for lifetime cancer incidence over each exposure.
In a recent report, the Centre for Radiation, Chemical and Environmental Hazards (CRCE), formerly the National Radiological Protection Board (NRPB), of the Health Protection Agency (HPA), has calculated lifetime risks for cancer incidence by age and sex for different levels of radiation. 79 Those calculations are based on a 2007 publication of the International Commission on Radiological Protection (ICRP). 80
Structure of York Radiation Model
The calculations for health consequences of radiation exposure are based on an adjusted version of the YRM (Figure 21). The YRM consists mainly of four elements: a radiation module, a cancer module, a utility module and a main module combining all intermediate calculations.
FIGURE 21.
Stylised overview of YRM.
In the radiation module, the YRM estimates the lifetime probability of an individual, given the timing and the amount of radiation exposure. To translate the cumulative radiation dose into the probability of lifetime cancer incidence the HPA model is used (see Table 47). 80
The cancer module is based on prior research. 61 In the absence of cancer models for all types of cancer, four common cancers are modelled: lung and colorectal cancer for both sexes, breast cancer only for females, and prostate cancer only for males. For each cancer, the module contains the further expected QALYs and disease costs for patients with cancer at the average age at diagnosis (see Table 46). For each sex, these values are then combined and weighted according the relative incidence of radiation-induced cancer. For males, the weights are approximately 46% colorectal, 42% lung and 12% prostate, whereas for females the weights are 16% colorectal, 50% lung and 34% breast.
The utility module is based on data for the general UK population (see Table 49). 61 For patients who do not get cancer, the remaining lifetime QALYs from the age at first radiation exposure are calculated. For patients who do get cancer, the utility module calculates the QALYs until the age at diagnosis of cancer, i.e. the timespan without cancer.
The main module combines the outcome of the three prior modules. So for a given age at first exposure, the share of patients who get radiation-induced cancer during their lifetime is calculated. For those patients, their QALYs until age at cancer diagnosis equal the general UK population and after that the remaining QALYs and the (additional) disease costs owing to cancer are taken from the cancer module. For the rest of the patients, just the remaining QALYs based on the general UK population are calculated. These values are combined and weighted by the sex ratio of the patient population. Both QALYs and disease costs are discounted to the age at first exposure to radiation. The intervention, i.e. the reduction in radiation exposure through the comparator technology, is modelled via the reduction in the probability of lifetime radiation-induced cancer. The YRM allows to conduct a probabilistic sensitivity analysis (PSA) accounting for the uncertainties in age at cancer incidence, cancer costs and QALYs lost due to cancer.
Radiation dose and patient populations
Computer tomography is a relatively high-dose X-ray imaging technique. The effective dose, i.e. absorbed radiation dose by a patient measured in sieverts, depends on a number of factors such as age of patient, the region of the body scanned, tissue type involved, precise type of CT, and scanning protocol for the particular diagnosis in question. Furthermore, CTs are an evolving technology in which the radiation doses vary with CT generation and by manufacturer. Moreover, scanning protocols themselves change over time. In particular, multislice CTs allow for increasingly rapid scans and lower radiation doses. Although 64-slice scanners have increasingly become the standard, earlier-generation CTs are still in use.
The broad range of CT types and CT applications compels studies which aim to quantify the radiation burden attributable to CTs in the general population to measure the radiation dose by scan for a particular body region/diagnosis type, for example head or full chest, roughly differentiating only by CT type (mostly single slice vs multislice). To account for the particular diagnostic needs of the disease assessed, we conducted expert surveys to obtain the relevant dosages by scanning strategy. The results are shown in Table 52 (for patients with CAD) and Table 66 (for patients with congenital heart disease).
The results of our expert surveys are in line with the literature that focuses on general chest CTs (Table 27). A study by the NRPB for the UK, conducted in 2003, shows slightly higher results than our expert survey, as its results were mostly based on single-slice and four-slice technology,81 which usually have higher radiation doses than 64-slice technology. More recent studies, such as the UNSCEAR 2008 report, assessing the trends in worldwide radiation exposure,82 and a review article focusing on children's exposure and based on German data,83 support the overall lower radiation dose for CT64 indicated by our expert survey. Note that in Table 27 values are also presented for younger age groups, as those values are required for the analysis presented below (see Cost-effectiveness of new-generation cardiac computed tomography in congenital heart disease).
| Source | Age | |||
|---|---|---|---|---|
| 1 year | 5 years | 10 years | Adult | |
| UNSCEAR report82 (lowest and highest reported values) | 1.8–6.3 | 2.1–3.6 | 3.0–3.9 | 3.5–12.9 |
| NRPB report81 [mean (25th/75th percentile)] | 6.3 [2.9–7.9] | 3.6 [2.1–4.1] | 3.9 [2.3–4.8] | 5.8 [3.9–6.9] |
| Linet (2009)83 | 2.2 | 2.5 | 3.0 | 5.9 |
The YRM was used for the two patient populations under assessment, the patients with CAD (this section) and the congenital heart disease patients (see Cost-effectiveness of new-generation cardiac computed tomography in congenital heart disease). The adjusted version of the YRM does not model benefits of the different CT strategies, but only the harmful consequences of radiation exposure. Hence, it can be used for both patient populations without further modifications; only the key parameter age at exposure, radiation dose (dependent on type and number of scans) and sex are used. In the case of the patients with CAD, the YRM output was used for further analysis. For an overview of the radiation doses in the patient populations for the different strategies under assessment see Tables 52 and 69.
Overview of the models used
Table 28 provides an overview of which models were used for each difficult-to-image patient group within each CAD population (suspected or known). The diagnostic model was used for each subgroup and modelled separately for 100% of the patients. To estimate the extra costs and QALY loss due to radiation, the YRM was used for each subgroup for the entire population. The healthy population model was used only for the suspected CAD population to model the patients who do not have CAD (TN and FP). The known and suspected CAD populations with CAD were modelled separately using two versions of the EUROPA model. The suspected CAD population had three treatment options (PCI, CABG and medication), whereas the known CAD population could undergo only a CABG or a PCI. The difficult-to-image patient groups ‘previous CABG’ and ‘previous stent implantation’ were treated as one subgroup in the EUROPA model because Deckers et al. 72 and Briggs et al. 69 use only one coefficient in the risk equation, namely previous revascularisation. 69,72 Cost and QALYs for patients who have experienced a stroke due to the initial ICA or initial revascularisation are based on a previously conducted study by Sandercock et al. 66 Subgroup-specific costs and QALYs obtained in the stroke model were calculated by using the subgroups ‘specific age’ and ‘proportion men’.
| Subgroups | Diagnostic model | YRM | Healthy population model | EUROPA model | Stroke | |
|---|---|---|---|---|---|---|
| Two-treatment model | Three-treatment model | |||||
| Suspected CAD population | ||||||
| Obese | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Arrhythmias | ✗ | ✗ | ✗ | ✗ | ✗ | |
| High coronary calcium level | ✗ | ✗ | ✗ | ✗ | ✗ | |
| HHR | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Beta-blocker intolerant | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Known CAD population | ||||||
| Obese | ✗ | ✗ | ✗ | ✗ | ||
| Arrhythmias | ✗ | ✗ | ✗ | ✗ | ||
| High coronary calcium level | ✗ | ✗ | ✗ | ✗ | ||
| HHR | ✗ | ✗ | ✗ | ✗ | ||
| Beta-blocker intolerant | ✗ | ✗ | ✗ | ✗ | ||
| Previous stent implantation | ✗ | ✗ | ✗ | ✗ | ||
| Previous CABG | ✗ | ✗ | ✗ | ✗ | ||
Model parameters
This section describes the parameters used in the diagnostic model, the EUROPA model, the healthy population model, the YRM and the stroke model. Distributions of the parameters are presented in Table 61 and described below (see Results, Sensitivity analyses). The last section describes how the difficult-to-image patient groups were combined to get overall incremental cost-effectiveness ratio (ICER) estimates for each CAD population (suspected and known).
Diagnostic model
The diagnostic model estimates the initial costs of diagnosis and initial treatment. Mortality and morbidity associated with the treatments and the diagnostic tests were also modelled and have an impact on the effectiveness of the three strategies. The events occur at one moment in time; the diagnostic model is time independent.
Costs
The costs included in the diagnostic model were the costs for the diagnostic tests and the costs of the two revascularisation procedures (Table 31). Medication-induced costs were modelled as part of the background costs in the disease progression model. The average cost prices for the revascularisation procedures and the ICA were calculated based on the NHS reference prices 2010–11. 84 An average cost price is calculated by multiplying the number of admissions with the costs for each different specific procedure. An ICA was estimated as costing on average £1003. A CABG would cost £8280 per procedure, and £9242 in combination with a ICA. A PCI in combination with an ICA would cost £4196, and a PCI without an ICA would cost £3633 per procedure.
Given that the cost of ICA (invasive CA) was estimated using the NICE reference cost, for comparability a reference cost would have been useful for each of the different types of scan – both standard 64-slice and the NGCT. However, the only data available were for any CT, i.e. not specifically for CTCA (Table 29).
| Currency code | Currency description | Activity | National average unit cost (£) | Lower quartile unit cost (£) | Upper quartile unit cost (£) | No. of data submissions |
|---|---|---|---|---|---|---|
| RA08Z | CT scan, one area, no contrast | 535,388 | 101 | 69 | 108 | 159 |
| RA09Z | CT scan, one area with post contrast only | 200,500 | 116 | 88 | 126 | 144 |
| RA10Z | CT scan, one area, pre- and post contrast | 48,604 | 112 | 73 | 128 | 102 |
Therefore, a bottom-up costing was performed, which attempted to use the categories that the reference cost would be composed of, which are shown below (Table 30).
| Category | 64-slice | NGCCT | Source |
|---|---|---|---|
| Capital, £ | 500,000 | 1,000,000 | The ImPACT Group, 200985 |
| Maintenance per year, £ | 73,624 | 137,941 | Expert opinion |
| Scanner life, years | 10 | 10 | National Audit Office, 201186 |
| Capital per year plus maintenance per year, £ | 123,624 | 237,941 | Calculated |
| No. of scans per year | 3120 | 3120 | Calculateda |
| Scanner cost (capital plus maintenance) per scan, £ | 59.43 | 114.39 | Calculated |
| Radiographer time, hours | 0.5 | 0.5 | Expert opinion |
| Radiologist time, hours | 0.5 | 0.5 | Expert opinion |
| Radiographer cost per hour (includes overheads), £ | 40 | 40 | PSSRU, 201087 |
| Radiologist cost per hour (includes overheads), £ | 146 | 146 | PSSRU, 201087 |
| Radiographer cost per scan, £ | 20 | 20 | Calculated |
| Radiologist cost per scan, £ | 73 | 73 | Calculated |
| Total staff cost per scan, £ | 93 | 93 | Calculated |
| Total cost (scanner plus staff) per scan, £ | 132.62 | 169.26 | Calculated |
| Diagnostic test | HDCT model | |
|---|---|---|
| Cost per diagnostic test (£) | Source | |
| Coronary angiography | 1003 | NHS reference costs |
| NGCCT | 169 | Calculated (see Table 30) |
| CABG | 8280 | NHS reference costs |
| PCI | 3633 | NHS reference costs |
| CABG + ICA | 9242 | NHS reference costs |
| PCI + ICA | 4196 | NHS reference costs |
The final costs of 64-slice and NGCCT are calculated to be £132.62 and £169.26, respectively. The estimated costs of 64-slice CT are higher than the reference costs. However, this is plausible given that much of the capital cost of existing scanners is probably not included in the reference costs. This is because many scanners are actually purchased using non-NHS money, i.e. by private donations (Valerie Fone, Trust Imaging Services Manager, Royal Brompton and Harefield NHS Foundation Trust, personal communication; see Appendix 7). Also, the staff costs for CTCA are higher given the considerable use of consultant as opposed to more junior or no radiologist time. Scenario analyses will be performed for 4160 scans per year (cost price NGCCT: £150) and 2080 scans per year (cost price NGCCT: £207).
Prior likelihood
The prior likelihood for the suspected and known CAD populations is presented above (see Model structure and methodology, Diagnostic model).
Initial treatment decision
Diagnostic tests, using the NGCCT, are performed to determine if treatment is necessary for a difficult-to-image patient. The cost-effectiveness of the NGCCT was estimated for two CAD populations which are treated differently. For the assumptions concerning the treatment options for the suspected CAD population expert opinion was used.
Suspected coronary artery disease population
Patients with suspected CAD and a positive cardiac CT or ICA test result can be treated with drug therapy alone, a CABG or a PCI. The proportions undergoing either revascularisation (18.1%) or medication (81.9%) after a positive test result were based on expert opinion (Hofstra, 2011, personal communication; which was based on an unpublished study conducted in the Netherlands73). The proportion of PCI compared with CABG in patients requiring revascularisation was based on UK procedure figures, which showed a 70%:30% proportion for PCI compared with CABG. 88
Patients treated with medication only are treated with beta-blockers or calcium channel blockers. 12 When the symptoms are not controlled with one of the two drugs, then a combination can be given or a nitrate can be prescribed. A revascularisation is then considered if symptoms of patients are still uncontrolled by drug treatment alone. The proportions undergoing revascularisation or medication treatment is comparable with a previously published article based on the Euro Heart Survey, which reported a revascularisation rate of 13%. 70 Furthermore, expert opinion indicated that the results of this study were also appropriate for the difficult-to-image patient groups considered in this assessment.
Population with known coronary artery disease
Given a positive CT or ICA test for patients with known CAD, two treatment options are considered: either PCI or CABG. The proportions undergoing PCI or CABG in patients with known CAD were also assumed to be 70%:30%, based on the same expert opinion used for the suspected CAD population.
Procedure-related mortality and morbidity
Invasive coronary angiography and revascularisation are accompanied by a risk of serious complications, including stroke, non-fatal MI and death (Table 32). The mortality rates are important for the impact on QALYs of the three strategies. The strategy in which all patients will undergo an ICA has the highest test-related mortality rate and this mortality rate influences the cost-effectiveness ratio by lowering the expected QALYs.
| Complications | Batyraliev et al.90 | Chandrasekar et al.91 | West et al.89 | Smith et al.92 |
|---|---|---|---|---|
| ICA | ||||
| Total complication rate | 0.0205 | 0.0460 | 0.0074 | |
| Mortality rate | 0.0008 | 0.0043 | 0.0007 | 0.0007 |
| Cerebrovascular accident rate | 0.0006 | 0.0024 | 0.0006 | 0.0014 |
| MI rate | 0.0008 | 0.0010 | 0.00003 | |
| Other complications | 0.0182 | 0.0383 | 0.0060 | |
| PCI | ||||
| Mortality rate | 0.0029 | Rajani (2011)96 | ||
| Cerebrovascular accident rate | 0.0005 | Rajani (2011)96 | ||
| MI rate | 0.0005 | Rajani (2011)96 | ||
| CABG | ||||
| Mortality rate | 0.018 | Bridgewater (2007)93 | ||
| Cerebrovascular accident rate | 0.016 | Tarakji (2011)94 | ||
| MI rate | 0.024 | Serruys (2001)95 and Tarakji (2011)94 | ||
The complication rate used in this model is based on published data. 89 A literature search for UK guidelines for performing coronary angiography was conducted to identify a study that provided primary data on complications caused by diagnostic ICA. Seventeen UK guidelines were found and these were checked for studies presenting primary data; 17 potentially relevant studies were found. A further four primary studies89–92 were identified after checking the references of the initial 17 studies and performing a citation search. Two studies90,91 did not present a complication rate based on the UK population but were conducted in Turkey and Canada, respectively. One study92 reported a complication rate for a UK population but was based on a single centre. A multicentre study89 on diagnostic angiography in the UK (and the most recently performed study) was considered to be the most appropriate study to inform the model. This study reported a complication rate of 7.4 (95% CI 7.0 to 7.7) and a mortality rate of 0.7 (95% CI 0.6 to 0.9) per 1000 patients, based on 219,227 procedures undertaken between 1991 and 1999. The mortality rate and the cerebrovascular accident rate presented in this study were comparable with data from another of the identified studies. 90 The overall complication rate and the MI rate presented were considerably lower than those presented in the other studies. We assumed that the complication rate of coronary angiography presented by the selected study is applicable regardless of the underlying risk of CV events particularly in difficult-to-image patient groups.
Both revascularisation procedures, CABG and PCI, are associated with complications including stroke, non-fatal MI and death. These complications are included in the diagnostic model. The mortality rate (0.018) of a CABG is based on Bridgewater et al. 93 CABG-related stroke was taken from the study. 93 As there were no studies that reported CABG-related MI, we used the study by Serruys et al. 95 to give an estimate of CABG-related MI. A survival curve (patients without MI and stroke) presented in the Serruys study95 was used: at 30 days, the survival was 96%; thus, 4% experienced a stroke or a MI. As we found a stroke rate of 1.6%94 related to the procedure we used 2.4% as an estimate for CABG-related MI assuming that within 30 days after the procedure it is still related to the procedure. This could lead to an overestimation of the MI rate, because the 4% reported by Serruys et al. 95 is not related to the procedure per se.
The complication rates induced by PCI were based on the study of Rajani et al. ;96 mortality due to a PCI is 0.0029, to a MI 0.0005 and stroke due to PCI 0.0005.
Finally, it has also been suggested that the intravenous contrast used in ICA, PCI and the NGCCT may carry a small risk of contrast-induced renal failure, dialysis and mortality. 97 However, a paper reviewing this risk in CT scans showed a negligible risk. In a total of six studies in patients receiving contrast fluid for a CT, no patients needed dialysis or died out of 1175 patients. 98 Thus, we have added no complications for the NGCCT. Contrast-related mortality may be assumed to be part of overall mortality due to ICA and PCI discussed earlier. Thus, the only remaining issue is a potential underestimation of the complications of these invasive procedures. As the complication rate can be greatly influenced by taking prophylactic measures in patients who are more at risk, this additional risk is here considered to be negligible. 99
Healthy population model
The healthy population model applies only for the suspected CAD population because all patients with known CAD have a different prognosis than patients without CAD; this was modelled using the EUROPA model. The TN and the FP patients in the suspected CAD population do not have CAD and therefore modelling their ‘future’ with the EUROPA model is not appropriate. Life tables were used to predict mortality for those groups of patients assuming that these patients do not differ from the average UK population. Costs are not assigned to this Markov model.
Survival
Three-monthly, age-dependent transition probabilities were used to model mortality for TN and FP patients in the suspected CAD population. The transition probabilities were based on UK life tables for all-cause mortality (Table 33). 65 All-cause mortality life tables were used, as these patients can still develop and die from CAD in the future.
| Age (years) | All causes | |
|---|---|---|
| Male | Female | |
| 0–4 | 0.000344 | 0.00027018 |
| 5–9 | 2.43E-05 | 2.1251E-05 |
| 10–14 | 3.54E-05 | 2.6616E-05 |
| 15–19 | 0.000104 | 5.4024E-05 |
| 20–24 | 0.000159 | 6.7097E-05 |
| 25–29 | 0.00018 | 8.4161E-05 |
| 30–34 | 0.000229 | 0.00011491 |
| 35–39 | 0.00031 | 0.00016842 |
| 40–44 | 0.000445 | 0.00028385 |
| 45–49 | 0.000706 | 0.00046288 |
| 50–54 | 0.001107 | 0.00073712 |
| 55–59 | 0.001708 | 0.00112255 |
| 60–64 | 0.00288 | 0.00175231 |
| 65–69 | 0.00457 | 0.00292024 |
| 70–74 | 0.007701 | 0.00485634 |
| 75–79 | 0.013048 | 0.00881416 |
| 80–84 | 0.022073 | 0.01569499 |
| 85–89 | 0.034578 | 0.02697076 |
| 90+ | 0.059551 | 0.05399661 |
Utility for patients without coronary artery disease
Patients from the suspected CAD population with a TN or FP test outcome are patients without CAD and it is therefore assumed that the health-related quality of life (HRQoL) for these patients would be equal to the population norms by gender and age (Table 34). 100 Of course, when patients presented they must have had similar symptoms to those who actually have CAD. However, we have assumed that these symptoms resolve over time, either through spontaneous improvement or through appropriate treatment. Additionally, it should be realised that the general population utility already is based on the presence of some illness, which implies that the difference between the utility of suspected CAD population who do not have CAD and the general population may be expected to be small. QALYs are discounted with 3.5%. 97
| Age (years) | Males | Females | ||
|---|---|---|---|---|
| Mean | SE | Mean | SE | |
| 55–64 | 0.78 | 0.02 | 0.81 | 0.02 |
| 65–74 | 0.78 | 0.02 | 0.78 | 0.02 |
| 75+ | 0.75 | 0.03 | 0.71 | 0.02 |
EUROPA model
The EUROPA model models the progression of stable CAD by predicting CV events and mortality. Health-care costs were evaluated by Briggs et al. 69 from resource items collected as part of the EUROPA study68 and these are grouped, for our analysis, into three categories: background costs, NFE costs and fatal event costs. More details can be found in the technical appendix of Briggs et al. 69 During the EUROPA trial a cost data set was constructed by recording, for each patient, the costs for each year in the trial. Covariates were then defined that related to the states of the model. A linear regression model (controlling for clustering by individual) was then used to estimate the cost associated with each of the model states, together with the potential effects of other covariates. 69Table 35 shows the results of the cost regression.
| Covariate | Coefficient (£) |
|---|---|
| Proportion of the year remaining following death/censoring | −1224 |
| NFE | 11,805 |
| Non-fatal event history | 986 |
| CV fatal event | 3641 |
| Non-CV fatal event | 12,421 |
| Age | 13 |
| Existing vascular diseases | 392 |
| Diabetes mellitus | 253 |
| Symptomatic disease | 283 |
| Creatinine clearance below 80 ml/minute | 8 |
| Using nitrates at baseline | 273 |
| On calcium channel blockers at baseline | 189 |
| On lipid-lowering treatment at baseline | 121 |
| UK resource use | −107 |
| Constant | −21 |
The original cost prices of the EUROPA trial 2003–4 were updated with a price correction based on the Personal Social Services Research Unit (PSSRU) Unit Costs of Health and Social Care 2010 (PSSRU 2010). Inflation correction is 1.2077402 and costs are discounted at an annual rate of 3.5%. 101
Background costs
Background costs are costs which are applied to the trial entry state and the NFE states. The background costs are based on age, the existence of vascular diseases or diabetes mellitus, medication usage, creatinine clearance and symptomatic disease. For each combination of difficult-to-image patient group, strategy, treatment decision, test outcome and known or suspected CAD population background costs (Tables 37 and 38) were estimated with the linear regression presented in Table 35. The costs of medication for patients who are treated with medication only were included in this background cost. An example is presented below for a patient from the known CAD population who is obese and defined TP in the ICA-only strategy.
The average age of an obese patient with known CAD and a TP test outcome is 63 years; 34% have diabetes mellitus and 25% are symptomatic. Creatinine clearance below 80 ml/minute is on average 6.9 ml/minute, nitrates usage at baseline 44%, the presence of existing vascular disease is 10.1%, calcium channel blocker usage at baseline 32% and lipid-lowering therapy at baseline 55.9%. So, in total, £298.05 is assigned per cycle of 3 months as a background cost (Table 36).
| Covariate | Coefficient | Mean | Annual | Quarterly |
|---|---|---|---|---|
| Age | 13 | 63 | 819 | 204.8 |
| Existing vascular disease | 392 | 0.10 | 40.3 | 10.1 |
| Diabetes mellitus | 253 | 0.34 | 86.3 | 21.6 |
| Symptomatic angina | 283 | 0.25 | 69.3 | 17.3 |
| Creatinine clearance of < 80 ml/minute | 8 | 6.9 | 55.2 | 13.8 |
| Nitrates usage | 273 | 0.44 | 121.2 | 30.3 |
| Calcium channel blocker usage | 189 | 0.32 | 61.2 | 15.3 |
| Lipid-lowering drugs usage | 121 | 0.56 | 67.6 | 16.9 |
| UK | −107 | 1 | −107 | −26.8 |
| Constant | −21 | 1 | −21 | −5.3 |
| Total background costs (£) | 1192.2 | 298.05 |
| Strategy | Test outcome | Treatment | Obese | HHC | HHR | Intolerance beta-blocker | Arrhythmias |
|---|---|---|---|---|---|---|---|
| ICA only | TP | Revascularisation | 298.0 | 287 | 328.2 | 288.3 | 303.6 |
| TP | Medication | 329.6 | 319 | 359.8 | 319.8 | 335.1 | |
| NGCCT-ICA | TP | Revascularisation | 298.0 | 287 | 328.2 | 274.6 | 303.6 |
| TP | Medication | 329.6 | 319 | 359.8 | 319.8 | 335.1 | |
| FN | 0 | 0 | 0 | 0 | 0 | ||
| NGCCT only | TP | Revascularisation | 298.0 | 287.3 | 328.2 | 288.3 | 303.6 |
| TP | Medication | 329.5 | 318.8 | 359.7 | 319.8 | 335.1 | |
| FN | 0 | 0 | 0 | 0 | 0 |
| Strategy | Test outcome | Obese | HHC | HHR | Intolerance beta-blocker | Arrhythmias | Revascularisation |
|---|---|---|---|---|---|---|---|
| ICA only | TP | 298.0 | 274.6 | 262.2 | 274.6 | 305.4 | 302.6 |
| TN | 297.6 | 274.1 | 261.8 | 274.1 | 304.9 | 302.1 | |
| NGCCT-ICA | TP | 298.0 | 274.6 | 262.2 | 274.6 | 305.4 | 302.6 |
| TN | 297.5 | 274.0 | 261.7 | 274.0 | 304.8 | 302.1 | |
| FN | 297.5 | 274.0 | 261.7 | 274.0 | 304.8 | 302.1 | |
| NGCCT only | TP | 298.0 | 274.5 | 262.2 | 274.5 | 305.3 | 302.6 |
| TN | 297.5 | 274.0 | 261.7 | 274.0 | 304.8 | 302.1 | |
| FN | 297.5 | 274.0 | 261.7 | 274.0 | 304.8 | 302.1 | |
| FP | 298.0 | 274.5 | 262.2 | 274.5 | 305.3 | 302.6 |
Non-fatal event costs
For the year in which a NFE occurs, £11,805 was added to the background cost. For subsequent years, the additional cost was estimated as £986. In the year that a fatal CV event occurs, the additional cost was estimated as £3641. When a fatal non-CV event occurred, an additional cost of £12,421 was added.
Utilities for patients with coronary artery disease
Health-related quality-of-life estimates were assigned to the states in the Markov model based on age, gender, baseline CCS classification and whether or not the patient had undergone treatment. Patients modelled through the disease progression model are assumed to have a CCS class (Campeau102) of 2. The HRQoL estimates were based on three sources: population norm for the EQ-5D,100 EQ-5D scores per CCS class103 and treatment effect on quality of life (QoL) based on the Randomized Intervention Treatment of Angina (RITA-2) trial. 64
Combining the population norm values with the EQ-5D scores per CCS class (0–4) (Tables 39 and 40) generates relative HRQoL by CCS class and gender. Longworth's scores103 were based on a median age of 61 years and these were divided by population norms for the age group 55–64 years. To obtain HRQoL by CCS class and age, the HRQoL by CCS class was multiplied by the age-specific HRQoL scores from Kind et al. ,100 assuming that the relative HRQoL by CCS class compared with the general population would hold across all ages. This multiplication was taken for the patients with CAD at baseline (without treatment).
| Age (years) | CCS class | ||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| 55–64 | 0.81 | 0.75 | 0.60 | 0.41 | 0.36 |
| 65–74 | 0.81 | 0.75 | 0.60 | 0.41 | 0.36 |
| 75+ | 0.78 | 0.72 | 0.58 | 0.39 | 0.35 |
| Age (years) | CCS class | ||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| 55–64 | 0.8 | 0.75 | 0.60 | 0.41 | 0.36 |
| 65–74 | 0.8 | 0.72 | 0.58 | 0.39 | 0.35 |
| 75+ | 0.7 | 0.66 | 0.53 | 0.36 | 0.32 |
The RITA-2 trial provided data on the initial CCS class and the CCS class following revascularisation to estimate the HRQoL for a patient who is treated. The long-term effects of PCI and medical treatment in patients with CAD are compared in the RITA-2 trial. The baseline EQ5D score was combined with the RITA 2 trial to generate HRQoL scores by baseline CCS (i.e. CCS before treatment), age and gender following revascularisation (Tables 41 and 42). Improvement in HRQoL (a better CCS class) was estimated by combining the changes in CCS after treatment with association seen between baseline CCS and baseline HRQoL. A new HRQoL was calculated from the shifts to the other CCS classes. For example, 20% of the patients will have a better CCS class after treatment, 10% will have a worse CCS class after treatment and 70% will stay in the same CCS class. The product of the proportion and the HRQoL in each specific CCS class after treatment provided an updated HRQoL for a patient by baseline CCS class. The assumption was made that the effect of revascularisation on HRQoL continues. The same HRQoL values were used for patients treated with medication only.
| Age (years) | Before-treatment CCS class | ||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| 55–64 | 0.79 | 0.74 | 0.75 | 0.69 | 0.72 |
| 65–74 | 0.79 | 0.74 | 0.75 | 0.69 | 0.72 |
| 75+ | 0.76 | 0.72 | 0.72 | 0.66 | 0.69 |
| Age (years) | Before-treatment CCS class | ||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| 55–64 | 0.79 | 0.74 | 0.75 | 0.69 | 0.72 |
| 65–74 | 0.76 | 0.72 | 0.72 | 0.66 | 0.69 |
| 75+ | 0.69 | 0.65 | 0.65 | 0.60 | 0.63 |
A 3-monthly disutility of 0.010225104 was assigned to the non-fatal event states because an event has occurred. We assumed that the disutility owing to a MI is the same as for a cardiac arrest. Of the NFEs only 2.5% will be a cardiac arrest; thus, the impact of changes in the disutility of a cardiac arrest will be minimal.
Population with known coronary artery disease
For the suspected CAD population, the baseline HRQoL applies for the patients with CAD, but not treated with a revascularisation or medication (FNs). In the EUROPA model, after a while a FN patient with CAD could be identified and would be treated; for this identified patient the HRQoL following treatment applies. The TPs from the suspected CAD population have CAD and will be treated with a revascularisation or medication and therefore the HRQoL following treatment applies (Table 43).
| Population | Test outcome | HRQoL |
|---|---|---|
| Suspected | TP | HRQoL following treatment |
| FN | Baseline HRQoL – without treatment | |
| Known | TP | HRQoL following treatment |
| FN | Baseline HRQoL – without treatment | |
| FP | HRQoL following treatment | |
| TN | HRQoL following treatment |
Population with known coronary artery disease
Patients from the known CAD population all have CAD irrespective of their test outcome. Therefore, they are already identified and the TPs who are treated will have the HRQoL following treatment. The TNs do not need a revascularisation; therefore they have a HRQoL of being treated because we assume that these patients are in such a good state that a revascularisation is not necessary and therefore they have the highest HRQoL, namely that of treated patients. The FPs are treated with a revascularisation although this was not necessary. Therefore, we assumed that patients being FP and who are treated have the highest HRQoL, namely that of patients who are treated. The FNs need a revascularisation so the HRQoL of patients who are not treated applies for these patients (see Table 43).
Transition probabilities
Tables 44 and 45 present the 3-monthly transition probabilities for the suspected and known CAD populations for each subgroup. These transition probabilities were based on the risk equations which are explained in Model structure and methodology, EUROPA.
| Transition probabilities | Obese | Arrhythmias | HCC | HHR | Beta-blocker |
|---|---|---|---|---|---|
| Probability first trial event, TP revascularisation, strategy 1, 3-monthly | 0.0078 | 0.0113 | 0.0095 | 0.0067 | 0.0056 |
| Probability first trial event, TP revascularisation, strategy 2, 3-monthly | 0.0078 | 0.0113 | 0.0095 | 0.0067 | 0.0056 |
| Probability first trial event, TP revascularisation, strategy 3, 3-monthly | 0.0078 | 0.0113 | 0.0095 | 0.0067 | 0.0056 |
| Probability first trial event, TP medication, strategy 1, 3-monthly | 0.0100 | 0.0145 | 0.0121 | 0.0087 | 0.0073 |
| Probability first trial event, TP medication, strategy 2, 3-monthly | 0.0100 | 0.0145 | 0.0121 | 0.0087 | 0.0073 |
| Probability first trial event, TP medication, strategy 3, 3-monthly | 0.0100 | 0.0145 | 0.0121 | 0.0087 | 0.0073 |
| Probability first trial event, FN strategy 2, 3-monthly | 0.0089 | 0.0129 | 0.0107 | 0.0077 | 0.0064 |
| Probability first trial event, FN strategy 3, 3-monthly | 0.0089 | 0.0129 | 0.0107 | 0.0077 | 0.0064 |
| Probability event is fatal, TP strategy 1, medication | 0.2951 | 0.3212 | 0.3028 | 0.2861 | 0.2710 |
| Probability subsequent event is fatal, TP strategy 1, medication | 0.4004 | 0.4303 | 0.4093 | 0.3901 | 0.3723 |
| Probability event is fatal, TP strategy 2, medication | 0.2951 | 0.3212 | 0.3028 | 0.2861 | 0.2710 |
| Probability subsequent event is fatal, TP strategy 2, medication | 0.4004 | 0.4303 | 0.4093 | 0.3901 | 0.3723 |
| Probability event is fatal, TP strategy 3, medication | 0.2951 | 0.3212 | 0.3028 | 0.2861 | 0.2710 |
| Probability subsequent event is fatal, TP strategy 3, medication | 0.4004 | 0.4303 | 0.4093 | 0.3901 | 0.3723 |
| Probability event is fatal, TP strategy 1, revascularisation | 0.2958 | 0.3220 | 0.3035 | 0.2869 | 0.2750 |
| Probability subsequent event is fatal, TP strategy 1, revascularisation | 0.4004 | 0.4303 | 0.4093 | 0.3901 | 0.3723 |
| Probability event is fatal, TP strategy 2, revascularisation | 0.2958 | 0.3220 | 0.3035 | 0.2869 | 0.2750 |
| Probability subsequent event is fatal, TP strategy 2, revascularisation | 0.4004 | 0.4303 | 0.4093 | 0.3901 | 0.3723 |
| Probability event is fatal, TP strategy 3, revascularisation | 0.2958 | 0.3220 | 0.3035 | 0.2869 | 0.2750 |
| Probability subsequent event is fatal, TP strategy 3, revascularisation | 0.4004 | 0.4303 | 0.4093 | 0.3901 | 0.3723 |
| Probability event is fatal, FN strategy 2 | 0.2951 | 0.3212 | 0.3028 | 0.2861 | 0.2710 |
| Probability event is fatal, FN strategy 3 | 0.2951 | 0.3212 | 0.3028 | 0.2861 | 0.2710 |
| Probability of subsequent event within first year post event, 3 monthly | 0.0272 | 0.0272 | 0.0272 | 0.0272 | 0.0272 |
| Probability of subsequent event within first year post event, annually | 0.1046 | 0.1046 | 0.1046 | 0.1046 | 0.1046 |
| Probability subsequent event after first year, TP strategy 1, medication, 3-monthly | 0.0144 | 0.0210 | 0.0175 | 0.0125 | 0.0105 |
| Probability subsequent event after first year, TP strategy 1, revascularisation, 3-monthly | 0.0112 | 0.0163 | 0.0136 | 0.0097 | 0.0081 |
| Probability subsequent event after first year, TP strategy 2, medication, 3-monthly | 0.0144 | 0.0210 | 0.0175 | 0.0125 | 0.0105 |
| Probability subsequent event after first year, TP strategy 2, revascularisation, 3-monthly | 0.0112 | 0.0163 | 0.0136 | 0.0097 | 0.0081 |
| Probability subsequent event after first year, TP strategy 3, medication, 3-monthly | 0.0144 | 0.0210 | 0.0175 | 0.0125 | 0.0105 |
| Probability subsequent event after first year, TP strategy 3, revascularisation, 3-monthly | 0.0112 | 0.0163 | 0.0136 | 0.0097 | 0.0081 |
| Probability subsequent event after first year, FN strategy 2, 3-monthly | 0.0128 | 0.0185 | 0.0155 | 0.0110 | 0.0092 |
| Probability subsequent event after first year, FN strategy 3, 3-monthly | 0.0128 | 0.0185 | 0.0155 | 0.0110 | 0.0092 |
| Quarterly probability of a FN patient being identified as TP | 0.1930 | 0.1930 | 0.1930 | 0.1930 | 0.1930 |
| Transition probabilities | Obese | Arrhythmias | HCC | HHR | Beta-blocker | Revascularisation |
|---|---|---|---|---|---|---|
| Probability first trial event, TP strategy 1 known, 3-monthly | 0.01212 | 0.0231 | 0.0145 | 0.0076 | 0.0088 | 0.0097 |
| Probability first trial event, TN strategy 1 known, 3-monthly | 0.01286 | 0.0245 | 0.0154 | 0.0080 | 0.0093 | 0.0097 |
| Probability first trial event, TP strategy 2 known, 3-monthly | 0.01212 | 0.0231 | 0.0145 | 0.0076 | 0.0088 | 0.0097 |
| Probability first trial event, TN strategy 2 known, 3-monthly | 0.01286 | 0.0245 | 0.0154 | 0.0080 | 0.0093 | 0.0097 |
| Probability first trial event, FN strategy 2 known, 3-monthly | 0.01286 | 0.0245 | 0.0154 | 0.0080 | 0.0093 | 0.0097 |
| Probability first trial event, FP strategy 2 known, 3-monthly | 0.01286 | 0.0245 | 0.0154 | 0.0080 | 0.0093 | 0.0097 |
| Probability first trial event, TP strategy 3 known, 3-monthly | 0.01212 | 0.0231 | 0.0145 | 0.0076 | 0.0088 | 0.0097 |
| Probability first trial event, TN strategy 3 known, 3-monthly | 0.01286 | 0.0245 | 0.0154 | 0.0080 | 0.0093 | 0.0097 |
| Probability first trial event, FN strategy 3 known, 3-monthly | 0.01286 | 0.0245 | 0.0154 | 0.0080 | 0.0093 | 0.0097 |
| Probability first trial event, FP strategy 3 known, 3-monthly | 0.01212 | 0.0231 | 0.0145 | 0.0076 | 0.0088 | 0.0097 |
| Probability event is fatal, TP strategy 1 | 0.36165 | 0.4084 | 0.3347 | 0.3021 | 0.3347 | 0.3820 |
| Probability subsequent event is fatal, TP strategy 1 known | 0.40043 | 0.4487 | 0.3723 | 0.3379 | 0.3723 | 0.4216 |
| Probability event is fatal, TN strategy 1 | 0.36165 | 0.4084 | 0.3347 | 0.3021 | 0.3347 | 0.3820 |
| Probability subsequent event is fatal, TN strategy 1 known | 0.40043 | 0.4487 | 0.3723 | 0.3379 | 0.3723 | 0.4216 |
| Probability event is fatal, TP strategy 2 | 0.36165 | 0.4084 | 0.3347 | 0.3021 | 0.3347 | 0.3820 |
| Probability subsequent event is fatal, TP strategy 2 known | 0.40043 | 0.4487 | 0.3723 | 0.3379 | 0.3723 | 0.4216 |
| Probability event is fatal, TN strategy 2 | 0.36165 | 0.4084 | 0.3347 | 0.3021 | 0.3347 | 0.3820 |
| Probability subsequent event is fatal, TN strategy 2 known | 0.40043 | 0.4487 | 0.3723 | 0.3379 | 0.3723 | 0.4216 |
| Probability event is fatal, FN strategy 2 known | 0.29506 | 0.3212 | 0.3028 | 0.2861 | 0.2710 | 0.0335 |
| Probability event is fatal, TP strategy 3 | 0.36165 | 0.4084 | 0.3347 | 0.3021 | 0.3347 | 0.3820 |
| Probability subsequent event is fatal, TP strategy 3 known | 0.40043 | 0.4487 | 0.3723 | 0.3379 | 0.3723 | 0.4216 |
| Probability event is fatal, TN strategy 3 | 0.36165 | 0.4084 | 0.3347 | 0.3021 | 0.3347 | 0.3820 |
| Probability subsequent event is fatal, TN strategy 3 known | 0.40043 | 0.4487 | 0.3723 | 0.3379 | 0.3723 | 0.4216 |
| Probability event is fatal, FP strategy 3 | 0.36165 | 0.4084 | 0.3347 | 0.3021 | 0.3347 | 0.3820 |
| Probability subsequent event is fatal, FP strategy 3 known | 0.40043 | 0.4487 | 0.0524 | 0.3379 | 0.3723 | 0.4216 |
| Probability event is fatal, FN strategy 3 known | 0.36165 | 0.4084 | 0.3347 | 0.3021 | 0.3347 | 0.3820 |
| Probability of subsequent event within first year post event, 3-monthly | 0.0272 | 0.0272 | 0.0272 | 0.0272 | 0.0272 | 0.0272 |
| Probability of subsequent event within first year post event, annually | 0.1046 | 0.1046 | 0.1046 | 0.1046 | 0.1046 | 0.1046 |
| Probability subsequent event after first year, TP strategy 1, 3-monthly | 0.01378 | 0.0262 | 0.0165 | 0.0086 | 0.0100 | 0.0110 |
| Probability subsequent event after first year, TN strategy 1, 3-monthly | 0.01463 | 0.0278 | 0.0176 | 0.0091 | 0.0106 | 0.0110 |
| Probability subsequent event after first year, TP strategy 2, 3-monthly | 0.01378 | 0.0262 | 0.0165 | 0.0086 | 0.0100 | 0.0110 |
| Probability subsequent event after first year, TN strategy 2, 3-monthly | 0.01463 | 0.0278 | 0.0176 | 0.0091 | 0.0106 | 0.0110 |
| Probability subsequent event after first year, FN strategy 2, 3-monthly | 0.01463 | 0.0278 | 0.0176 | 0.0091 | 0.0106 | 0.0110 |
| Probability subsequent event after first year, FP strategy 2, 3-monthly | 0.01463 | 0.0278 | 0.0176 | 0.0091 | 0.0106 | 0.0110 |
| Probability subsequent event after first year, TP strategy 3, 3-monthly | 0.01378 | 0.0262 | 0.0165 | 0.0086 | 0.0100 | 0.0110 |
| Probability subsequent event after first year, TN strategy 3, 3-monthly | 0.01463 | 0.0278 | 0.0176 | 0.0091 | 0.0106 | 0.0110 |
| Probability subsequent event after first year, FN strategy 3, 3-monthly | 0.01463 | 0.0278 | 0.0176 | 0.0091 | 0.0106 | 0.0110 |
| Probability subsequent event after first year, FP strategy 3, 3-monthly | 0.01378 | 0.0262 | 0.0165 | 0.0086 | 0.0100 | 0.0110 |
| Quarterly probability of a FN patient being identified as TP | 0.1930 | 0.1930 | 0.1930 | 0.1930 | 0.1930 | 0.1930 |
Stroke model
The costs and effects of the patients who experience a stroke due to the initial ICA or revascularisation are modelled with a relatively simple alive–dead model based on estimates by Sandercock et al. 66 for thrombolytic therapy of stroke.
Survival
Mortality rates were based on UK life tables65 and a relative risk of 2.5 to reflect the increased risk of mortality following a stroke. 105 Survival for each subgroup modelled in this study was therefore not simply dependent on stroke but also on the average age in that subgroup.
Costs
Sandercock et al. 66 estimated a cost of approximately £6260 in the first year after a stroke. As Sandercock et al. 66 presented both 12-month and lifetime costs, we estimated the average annual costs of treating stroke patients after the first year to be approximately £3400. These costs were then inflated to reflect costs for 2009–10 and then discounted at a rate of 3.5%.
Quality-adjusted life-year
Calibration of the model to fit with the results by Sandercock et al. 66 resulted in an average health utility of 0.37. This value was combined with survival and the resulting QALYs were discounted using at a rate of 3.5%.
York Radiation Model
The following tables show the key parameters for the base-case scenario for the YRM when modelling the effect of radiation on CAD patients. Table 46 shows the mean parameter values (costs and QALY loss due to cancer) for the cancer module of the YRM. If the age at first exposure to radiation is < 40 years, the average age of incidence for breast cancer is assumed to be 40 years; for higher ages the average is assumed to be 60 years. In the CAD patient population all patients are aged > 40 years. This can be seen clearly in Table 51, with demographic characteristics of the patient population. The lifetime risk of cancer incidence by age and sex for a one-time exposure to 10 mSv, based on the HPA model, is shown in Table 47. Table 49 shows the age-specific utilities used to calculate the QALYs for non-cancer patients. Table 50 shows the life expectancy for the general population, i.e. patients who do not get cancer, based on the 2007 England and Wales life table. Note that in various tables values are presented for younger age groups, as those values are required for the analysis presented below (see Cost-effectiveness of new-generation cardiac computed tomography in congenital heart disease).
| Cancer | Age at diagnosis (years) | Costs of cancer (£) | QALYs lost due to cancer |
|---|---|---|---|
| Breast | 40 (0) | 14,990 (940) | 5.6988 (0.4533) |
| Breast | 60 (0) | 13,927 (848.11) | 3.4219 (0.311) |
| Lung | 72.2684 (0.0395) | 22,712 (440,60) | 6.8011 (0.056) |
| Colorectal | 73.72 (0.139) | 14,075 (356.00) | 3.4493 (0.1386) |
| Prostate | 74 (NA) | 12,389 (NA) | 4.6226 (NA) |
| Age at exposure (years) | Risk of all cancers (for exposure to 10 mSv) | |
|---|---|---|
| Males | Females | |
| 0–9 | 0.000999 | 0.00127 |
| 10–19 | 0.0008 | 0.000994 |
| 20–29 | 0.000623 | 0.000795 |
| 30–39 | 0.000512 | 0.000646 |
| 40–49 | 0.000422 | 0.000562 |
| 50–59 | 0.000327 | 0.000441 |
| 60–69 | 0.000223 | 0.00032 |
| 70–79 | 0.000132 | 0.000194 |
| 80–89 | 0.000055 | 0.000075 |
| 90–99 | 0.000004 | 0.000002 |
Table 52 presents the radiation doses for each of the analysed scanning strategies for patients with CAD. Te value for NGCCT is based on an expert survey (response: n = 2) for this particular patient group, whereas the average radiation doses for ICA and PCI are taken from literature. 61
For all of the scanning strategies, the uncertainty in the costs (Table 48) and remaining QALYs of the cancer module in the YRM are modelled via a PSA. Te values for the input are shown in Table 46.
| Strategy | Cost per scan (£) |
|---|---|
| CT64 | 132.62 |
| NGCCT | 169.26 |
| Age (years) | Mean utility | SD |
|---|---|---|
| < 25 | 0.94 | 0.12 |
| 25–34 | 0.93 | 0.15 |
| 35–44 | 0.91 | 0.16 |
| 45–54 | 0.85 | 0.25 |
| 55–64 | 0.80 | 0.26 |
| 65–74 | 0.78 | 0.26 |
| 75+ | 0.73 | 0.27 |
| Age (years) | Males | Females | Combined (50% male) |
|---|---|---|---|
| 0 | 77.98 | 82.09 | 80.04 |
| 10 | 68.50 | 72.53 | 70.52 |
| 20 | 58.67 | 62.63 | 60.65 |
| 30 | 49.04 | 52.80 | 50.92 |
| 40 | 39.55 | 43.07 | 41.31 |
| 50 | 30.32 | 33.61 | 31.97 |
| 60 | 21.71 | 24.63 | 23.17 |
| 70 | 14.09 | 16.35 | 15.22 |
| 80 | 7.98 | 9.36 | 8.67 |
| 90 | 4.15 | 4.59 | 4.37 |
| 100 | 2.13 | 2.22 | 2.18 |
| Subgroup | Known | Suspected | ||
|---|---|---|---|---|
| Mean age (years) | % Male | Mean age (years) | % Male | |
| Obese | 63 | 0.659 | 63 | 0.659 |
| Arrhythmias | 68 | 0.71 | 66.11 | 0.69 |
| Intolerance beta-blockers | 60 | 0.854 | 60 | 0.854 |
| Previous stents | 65 | 0.66 | ✗ | ✗ |
| Previous CABG | 66 | 0.788 | ✗ | ✗ |
| HHR | 61.91 | 0.52 | 56.2 | 0.68 |
| High coronary calcium score | 63.93 | 0.854 | 60 | 0.7503 |
| Scanning strategy | Radiation dose (mSv) |
|---|---|
| ICA | 7 |
| NGCCT | 4.5 |
| ICA–NGCCT | 11.5 |
| ICA–PCI | 22 |
| NGCCT–PCI | 19.5 |
| ICA–NGCCT–PCI | 26.5 |
Proportions of patients in difficult-to-image subgroups
Difficult-to-image patient group-specific costs and QALYs were calculated. Te aim was to calculate an overall ICER for the three strategies and for the two populations (suspected and known CAD). Expert opinion was used to gather information on the relative proportions of patients in the different difficult-to-image groups in a known or suspected CAD population. Primary data collection from patient records was considered, but due to time constraints a questionnaire distributed to experts in the field was used to derive a reasonable estimate of the relative proportions. Multiplying the relative proportions with the subgroup-specific costs and effects produced an overall ICER for the suspected CAD population and an overall ICER for the known CAD population.
The questionnaire was distributed to six experts, four of whom completed and returned it. Means are calculated from the proportions that the experts filled in. See Appendix 7 for details on the experts. Table 53 shows the relative proportions for each population. According to the experts it is impossible to have a revascularisation before the test is performed in a population with suspected CAD.
| Subgroups | Mean proportion (%) | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Average | |
| Suspected CAD population | |||||
| Obese | 26 | 15 | 14 | 10 | 16.25 |
| High-level coronary calcium | 12 | 10 | 48 | 40 | 27.50 |
| Arrhythmias | 12 | 10 | 10 | 15 | 11.75 |
| HHR | 38 | 50 | 9 | 20 | 29.25 |
| Intolerance beta-blocker | 12 | 15 | 19 | 15 | 15.25 |
| 100 | 100 | 100 | 100 | 100 | |
| Known CAD population | |||||
| Obese | 20 | 5 | 5 | 10.00 | |
| High-level coronary calcium | 12 | 20 | 45 | 25.67 | |
| Arrhythmias | 12 | 5 | 5 | 7.33 | |
| HHR | 32 | 40 | 10 | 27.33 | |
| Intolerance beta-blocker | 8 | 10 | 10 | 9.33 | |
| Previous PCI | 8 | 15 | 10 | 11.00 | |
| Previous CABG | 8 | 5 | 15 | 9.33 | |
| 100 | 100 | 100 | 100 | 100 | |
Assumptions
Using five models that were each designed for another purpose lead to some unavoidable assumptions. Assumptions made are summarised in Table 54.
| Assumptions | Reference |
|---|---|
| General assumptions | |
| A mean BMI is transformed to obesity percentage assuming a normal distribution | |
| The suspected CAD group cannot have had a previous revascularisation | Questionnaire |
| Proportion PCI–CABG is 70–30% | |
| Diagnostic model general | |
| An ICA is performed only after a positive HDCT test outcome in the strategy HDCT–ICA | |
| ICA is the gold standard with a 100% sensitivity and 100% specificity | |
| When a PCI is performed after an ICA, the mortality of PCI only is used. Assumption is that a PCI is performed at the same time as ICA | |
| All diagnostic tests are performed immediately after each other without any time delay | |
| The most relevant complications of an ICA and PCI/CABG are mortality, non-fatal MI or cerebrovascular accident | |
| The sensitivity and specificity of the HDCT in patients intolerant of beta-blockers is assumed to be the same as for the subgroup with a HHR | |
| Accuracy estimates are the same for the suspected and known population | |
| Complication rates of revascularisation and ICA are assumed to be the same in all difficult-to-image subgroups | |
| Patients treated with a revascularisation are treated with a CABG or a PCI. The proportion is 30% and 70%, respectively | |
| Diagnostic model suspected population | |
| Patients suspected with CAD with the disease and with a positive test outcome have three treatment options: CABG/PCI or medication. A revascularisation is performed in 15% of the patients and 85% of the patients receive medication | Hofstra (personal communication) |
| Prior likelihood of patients suspected of CAD is 10–29% | NICE CG9563 |
| Diagnostic model known population | |
| Patients with known CAD with a positive test outcome have two treatment options: CABG/PCI | |
| Prior likelihood that a known patient would benefit from a revascularisation is 0.395 | CE-MARC64 |
| EUROPA model | |
| The difficult-to-image indications CABG and PCI are treated as one indication in the EUROPA model. The covariate ‘previous revascularisation’ captures the impact of an previous revascularisation on the risk of experiencing an event | |
| The covariates of the risk equation of the EUROPA study are appropriate for the known and suspected CAD population | |
| Primary events predicted with the EUROPA model are cardiac arrest, non-fatal MI and death | |
| The input values for the risk equations were based on the systematic review if available, otherwise they were based on the EUROPA population | |
| Relative risks are used to update the risk equations of the EUROPA model for the subgroups: high coronary calcium, HHR and arrhythmias | |
| Patients intolerant for beta-blockers do not have an increased risk of experiencing events. Beta-blockers are provided to make interpretable images and not to prevent events. Patients intolerant for beta-blockers can also receive calcium channel blockers to reduce events as an alternative | |
| The risk of experiencing a non-fatal MI, cardiac arrest or mortality is for the subgroup obesity captured in the risk equation by the covariate obese | |
| A relative risk based on Hofstra et al. is used to update the risk equation for the difficult-to-image subgroup high coronary calcium level | |
| Proportion HCC in the EUROPA trial is assumed to be the same as in the study … | |
| A relative risk based on the QRISK study is used to update the risk equation for the difficult-to-image subgroup ‘arrhythmias’ | |
| AF is taken as an proxy for the difficult-to-image subgroup arrhythmias because AF is the most common type of arrhythmia | British Heart Foundation1 |
| Proportion AF in EUROPA population is assumed to be the same as in study … | |
| It is assumed that the conditions of the subgroups HHR and beta-blockers intolerant do not have an impact on the transition probabilities | |
| Age- and CCS-specific HRQoL values based on Longworth et al. 2005,102 Kind et al. 1999100 and the RITA-2 trial give good estimates for (un)treated patients with CAD | |
| Disutility for experiencing a cardiac arrest is assumed to be the same as for a non-fatal MI | |
| Patients with a positive test outcome who will be treated with medication will be treated with a calcium channel blocker. Calcium channel blocker usage is a covariate in the risk equation. Normally patients with CAD will receive a calcium channel blocker or a beta-blocker. The clinical effectiveness of these two drugs is comparable and therefore we assume that the HR is the same. Even when a combination of both drugs is given the HR will probably the same because we assume that a second drug will only be given when the first was not (fully) effective | |
| EUROPA suspected CAD | |
| The input values for the risk equations for the suspected group are based on the accuracy studies performed on the suspected population. If suspected specific input values are not available then studies which combine suspected and known CAD are used. If combined studies are not available the input values will be based on the EUROPA population | |
| Proportion MI in the risk equation is based on the non-fatal complications due to the initial revascularisation or ICA | |
| Patients are not treated with nitrates at baseline | |
| ACE inhibitor usage at baseline 23% | |
| EUROPA known CAD | |
| The input values for the risk equations for the known group are based on the accuracy studies performed based on known CAD population. If known specific input values are not available then the input values will be based on the EUROPA population | |
| All patients with known CAD are modelled with the EUROPA model irrespective of the test outcome | |
| ACE inhibitor usage at baseline 23% | Daly (2005)70 |
| Proportion MI in risk equation is based on the EUROPA population; the proportion is not raised with the ICA and initial revascularisation-induced MI | |
| A HRQoL value following treatment is assigned to patients with the test outcomes FPs and TNs | |
| Alive–dead model | |
| TN and FP modelled with the life death model with all-cause mortality probabilities | |
| Stroke model | |
| Patients are treated with thrombolytic agents | |
Results
Initially the costs of using the NGCCT instead of an ICA are lower but what is the influence of the lower sensitivity and specificity on the effectiveness side and the costs side? The cost-effectiveness of the three strategies is described below. First intermediate results are given for the three strategies for each subgroup.
Intermediate outcomes
In addition to the cost-effectiveness of the NGCCT, intermediate outcomes in terms of mortality, morbidity and the percentages of correct diagnostic classification (TP, FP, TN and FN) are also important. Tables 55 and 56 show, for both CAD populations and for each difficult-to-image group, these three intermediate outcomes.
| Strategy | Proportion correct classification | Misclassification | Mortality tests | Morbidity tests | Mortality revascularisation | Morbidity revascularisation | |
|---|---|---|---|---|---|---|---|
| FPs | FNs | ||||||
| Obese | |||||||
| ICA only | 100.0 | – | – | 0.0007 | 0.0006 | 0.0003 | 0.0005 |
| NGCCT-ICA | 98.1 | – | 1.9 | 0.0002 | 0.0002 | 0.0002 | 0.0004 |
| NGCCT only | 91.8 | 6.3 | 1.9 | – | – | 0.0003 | 0.0006 |
| Arrhythmias | |||||||
| ICA only | 100.0 | – | – | 0.0007 | 0.0006 | 0.0003 | 0.0005 |
| NGCCT-ICA | 99.5 | 0.5 | 0.0002 | 0.0002 | 0.0003 | 0.0005 | |
| NGCCT only | 84.9 | 14.6 | 0.5 | – | – | 0.0005 | 0.0008 |
| High coronary calcium score | |||||||
| ICA only | 100.0 | – | – | 0.0007 | 0.0006 | 0.0003 | 0.0005 |
| NGCCT-ICA | 98.5 | – | 1.5 | 0.0002 | 0.0002 | 0.0003 | 0.0004 |
| NGCCT only | 91.0 | 7.5 | 1.5 | – | – | 0.0004 | 0.0006 |
| HHR | |||||||
| ICA only | 100.0 | – | – | 0.0007 | 0.0006 | 0.0003 | 0.0005 |
| NGCCT-ICA | 99.5 | – | 0.5 | 0.0002 | 0.0002 | 0.0003 | 0.0005 |
| NGCCT only | 88.6 | 11.0 | 0.5 | – | – | 0.0004 | 0.0007 |
| Intolerance beta-blocker | |||||||
| ICA only | 100.0 | – | – | 0.0007 | 0.0006 | 0.0003 | 0.0005 |
| NGCCT-ICA | 99.5 | – | 0.5 | 0.0002 | 0.0002 | 0.0003 | 0.0005 |
| NGCCT only | 88.6 | 11.0 | 0.5 | – | – | 0.0004 | 0.0007 |
| Strategy | Proportion correct classification (%) | Misclassification (%) | Mortality tests | Morbidity tests | Mortality revascularisation | Morbidity revascularisation | |
|---|---|---|---|---|---|---|---|
| FPs | FNs | ||||||
| Obese | |||||||
| ICA only | 100.0 | – | – | 0.0007 | 0.0006 | 0.0030 | 0.0051 |
| NGCCT-ICA | 96.2 | – | 3.8 | 0.0001 | 0.0003 | 0.0027 | 0.0046 |
| NGCCT only | 91.4 | 4.8 | 3.8 | – | – | 0.0030 | 0.0052 |
| Arrhythmias | |||||||
| ICA only | 100.0 | – | – | 0.0007 | 0.0006 | 0.0030 | 0.0051 |
| NGCCT-ICA | 99.1 | – | 0.9 | 0.0002 | 0.0003 | 0.0029 | 0.0050 |
| NGCCT only | 88.0 | 11.1 | 0.9 | – | – | 0.0037 | 0.0064 |
| High coronary calcium score | |||||||
| ICA only | 100.0 | – | – | 0.0007 | 0.0006 | 0.0030 | 0.0051 |
| NGCCT-ICA | 97.1 | – | 2.9 | 0.0001 | 0.0003 | 0.0027 | 0.0047 |
| NGCCT only | 91.4 | 5.7 | 2.9 | – | – | 0.0032 | 0.0054 |
| HHR | |||||||
| ICA only | 100.0 | – | – | 0.0007 | 0.0006 | 0.0030 | 0.0051 |
| NGCCT-ICA | 99.1 | – | 0.9 | 0.0001 | 0.0003 | 0.0029 | 0.0050 |
| NGCCT only | 90.8 | 8.3 | 0.9 | – | – | 0.0035 | 0.0060 |
| Intolerance to beta-blocker | |||||||
| ICA only | 100.0 | – | – | 0.0007 | 0.0006 | 0.0030 | 0.0051 |
| NGCCT-ICA | 99.1 | – | 0.9 | 0.0001 | 0.0003 | 0.0029 | 0.0050 |
| NGCCT only | 90.8 | 8.3 | 0.9 | – | – | 0.0035 | 0.0060 |
| Previous stent | |||||||
| ICA only | 100.0 | – | – | 0.0007 | 0.0006 | 0.0030 | 0.0051 |
| NGCCT-ICA | 98.4 | – | 1.6 | 0.0002 | 0.0003 | 0.0028 | 0.0049 |
| NGCCT only | 87.3 | 11.1 | 1.6 | – | – | 0.0037 | 0.0063 |
| Previous CABG | |||||||
| ICA only | 100.0 | – | – | 0.0007 | 0.0006 | 0.0030 | 0.0051 |
| NGCCT-ICA | 98.6 | – | 1.4 | 0.0001 | 0.0003 | 0.0028 | 0.0049 |
| NGCCT only | 90.7 | 7.9 | 1.4 | – | – | 0.0034 | 0.0059 |
Population with suspected coronary artery disease
As expected, the ICA had 100% correct diagnostic classification due to the assumption of 100% sensitivity and 100% specificity. Unfortunately, this comes with higher mortality and morbidity rates due to the complications of the test itself. The strategy where each patient will undergo an ICA had the highest test-induced mortality and morbidity rate, and the strategy that uses only the NGCCT to diagnose patients has test-induced mortality and morbidity rates of zero. Conversely, revascularisation-induced mortality and morbidity rates were highest in the NGCCT-only strategy due to the FPs who undergo unnecessary revascularisations with the associated complications. The NGCCT–ICA strategy had the lowest revascularisation-induced mortality and morbidity rates because only TPs are treated and the FNs who are not correctly diagnosed will not receive a revascularisation where they should have. The NGCCT-only strategy has the lowest overall mortality rate in the suspected population. The NGCCT-only strategy, as expected, had the lowest correct classification proportion.
Population with known coronary artery disease
The same results apply for the known CAD population; the ICA classifies 100% of patients correctly, the ICA strategy has the highest test mortality and morbidity rates and the NGCCT-only strategy has the highest revascularisation mortality and morbidity. However, in the known population the overall mortality and morbidity is lowest in the NGCCT–ICA strategy. ICA only has the highest overall mortality and morbidity rate.
Costs per model
Table 57 shows the costs assigned to the patients in the diagnostic model, the EUROPA model, the YRM and costs from the stroke model per subgroup. The presented costs are after including the probabilities; adding the cost per model gives the total costs.
| Strategy | Diagnostic model | EUROPA model | YRMa | Stroke model | Total | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Suspected | Known | Suspected | Known | Suspected | Known | Suspected | Known | Suspected | Known | |
| Obese | ||||||||||
| ICA only | 1174 | 2867 | 5747 | 26,676 | 2.6 | 3.9 | 44 | 147 | 6968 | 29,694 |
| NGCCT-ICA | 568 | 2252 | 5709 | 26,806 | 2.3 | 3.8 | 18 | 116 | 6297 | 29,177 |
| NGCCT only | 405 | 2360 | 5686 | 26,776 | 1.7 | 3.0 | 13 | 116 | 6106 | 29,254 |
| Arrhythmias | ||||||||||
| ICA only | 1175 | 2869 | 5569 | 24,436 | 2.8 | 4.4 | 39 | 119 | 6785 | 27,428 |
| NGCCT-ICA | 675 | 2450 | 5530 | 24,529 | 2.7 | 4.7 | 19 | 101 | 6227 | 27,084 |
| NGCCT only | 536 | 3115 | 5524 | 24,493 | 1.9 | 3.8 | 16 | 114 | 6077 | 27,726 |
| HHR | ||||||||||
| ICA only | 1172 | 2866 | 6111 | 27,405 | 2.8 | 4.0 | 56 | 159 | 7342 | 30,434 |
| NGCCT-ICA | 640 | 2455 | 6089 | 27,484 | 2.7 | 4.3 | 26 | 136 | 6758 | 30,080 |
| NGCCT only | 484 | 2864 | 6089 | 27,463 | 1.9 | 3.4 | 20 | 146 | 6595 | 30,477 |
| High coronary calcium score | ||||||||||
| ICA only | 1172 | 2867 | 5577 | 28,126 | 2.2 | 3.5 | 49 | 148 | 6801 | 31,145 |
| NGCCT-ICA | 591 | 2321 | 5528 | 28,216 | 2.0 | 3.6 | 21 | 120 | 6142 | 30,661 |
| NGCCT only | 430 | 2525 | 5515 | 28,188 | 1.5 | 2.8 | 15 | 123 | 5962 | 30,839 |
| Intolerance to beta-blockers | ||||||||||
| ICA only | 1173 | 2869 | 5791 | 26,303 | 2.0 | 3.1 | 49 | 164 | 7016 | 29,339 |
| NGCCT-ICA | 643 | 2457 | 5763 | 26,371 | 1.9 | 3.3 | 23 | 141 | 6430 | 28,972 |
| NGCCT only | 485 | 2862 | 5775 | 26,339 | 1.4 | 2.6 | 18 | 150 | 6279 | 29,354 |
| Previous stents | ||||||||||
| ICA only | – | 2868 | – | 25,443 | – | 4.1 | – | 136 | – | 28,450 |
| NGCCT-ICA | – | 2378 | – | 25,562 | – | 4.3 | – | 112 | – | 28,056 |
| NGCCT only | – | 3020 | – | 25,522 | – | 3.5 | – | 127 | – | 28,672 |
| Previous CABG | ||||||||||
| ICA only | – | 2867 | – | 25,465 | – | 4.0 | – | 130 | – | 28,466 |
| NGCCT-ICA | – | 2405 | – | 25,570 | – | 4.1 | – | 109 | – | 28,088 |
| NGCCT only | – | 2892 | – | 25,540 | – | 3.3 | – | 118 | – | 28,554 |
Population with suspected coronary artery disease
Most of the costs in the EUROPA model do not differ significantly between the three strategies. The difference in costs between the strategies is mainly due to the difference in the costs in the diagnostic model. The ICA-only strategy has the highest costs in the diagnostic model because the test itself is much more expensive than NGCCT. The impact of treating FPs unnecessary with a revascularisation in the NGCCT-only strategy is marginal because the proportion that receives a revascularisation is just 18%. The incremental cost induced due to radiation is lowest in the NGCCT-only strategy because the radiation dose is lowest in the NGCCT-only strategy. Also, not surprisingly, the costs in the stroke model are the highest for the ICA-only strategy due to the largest proportion having non-fatal complications of the initial ICA and revascularisations.
Population with known coronary artery disease
In the known population, the costs in the diagnostic model are still the highest for the ICA-only strategy. However, the NGCCT–ICA strategy instead of the NGCCT-only strategy has the lowest cost in the diagnostic model. This is different from the suspected CAD population because the treatment decision differs between the two models. The known FPs of the NGCCT-only strategy are always treated with a revascularisation with accompanying extra costs. In the suspected CAD population, only 18% of FPs receive a revascularisation and, as medication costs are modelled in the EUROPA model, it will lead to fewer costs for the FPs.
The same applies for the stroke model because the non-fatal complication rate of the NGGCT-only strategy in the known group is higher than that of the NGCCT–ICA strategy and in the suspected population the NGGCT-ICA has a higher non-fatal complication rate. The proportion of the suspected CAD population that receives a revascularisation after a positive test is 18%, and corresponding proportion in the known population is 100%, therefore the proportion that experience a stroke due to the revascularisation is higher in the known population.
Quality-adjusted life-years per model
Table 58 shows an overall QALY estimate and a separate QALY estimate per model for every strategy, subgroup and population. The presented QALYs are after including the probabilities; adding up the QALYs of the different models leads to the total QALYs per strategy. The YRM provides disutilities, as it induces QALY loss due to radiation.
| Strategy | EUROPA model | Healthy population model | YRMa | Stroke model | Total | ||||
|---|---|---|---|---|---|---|---|---|---|
| Suspected | Known | Suspected | Suspected | Known | Suspected | Known | Suspected | Known | |
| Obese | |||||||||
| ICA only | 1.89 | 8.85 | 8.62 | −0.0007 | −0.0011 | 0.0025 | 0.0082 | 10.519 | 8.857 |
| NGCCT-ICA | 1.87 | 8.87 | 8.63 | −0.0007 | −0.0011 | 0.0010 | 0.0065 | 10.508 | 8.872 |
| NGCCT only | 1.87 | 8.86 | 8.63 | −0.0005 | −0.0009 | 0.0007 | 0.0065 | 10.508 | 8.869 |
| Arrhythmias | |||||||||
| ICA only | 1.67 | 6.54 | 7.78 | −0.0008 | −0.0013 | 0.0022 | 0.0068 | 9.448 | 6.545 |
| NGCCT-ICA | 1.63 | 6.58 | 7.79 | −0.0008 | −0.0014 | 0.0011 | 0.0058 | 9.419 | 6.588 |
| NGCCT only | 1.63 | 6.59 | 7.79 | −0.0006 | −0.0011 | 0.0009 | 0.0065 | 9.420 | 6.595 |
| HHR | |||||||||
| ICA only | 1.98 | 11.21 | 8.99 | −0.0008 | −0.0012 | 0.0030 | 0.0088 | 10.969 | 11.223 |
| NGCCT-ICA | 1.97 | 11.24 | 9.00 | −0.0008 | −0.0012 | 0.0014 | 0.0075 | 10.968 | 11.242 |
| NGCCT only | 1.97 | 11.23 | 9.00 | −0.0006 | −0.0010 | 0.0011 | 0.0080 | 10.967 | 11.233 |
| High coronary calcium score | |||||||||
| ICA only | 1.79 | 9.26 | 8.42 | −0.0010 | −0.0010 | 0.0027 | 0.0083 | 10.210 | 9.271 |
| NGCCT-ICA | 1.78 | 9.30 | 8.43 | −0.0010 | −0.0010 | 0.0011 | 0.0067 | 10.202 | 9.306 |
| NGCCT only | 1.78 | 9.30 | 8.43 | −0.0008 | −0.0008 | 0.0008 | 0.0069 | 10.201 | 9.301 |
| Intolerance to beta-blockers | |||||||||
| ICA only | 2.11 | 10.01 | 9.43 | −0.0006 | −0.0009 | 0.0027 | 0.0090 | 11.541 | 10.016 |
| NGCCT-ICA | 2.10 | 10.04 | 9.44 | −0.0006 | −0.0009 | 0.0012 | 0.0077 | 11.540 | 10.042 |
| NGCCT only | 2.10 | 10.03 | 9.44 | −0.0004 | −0.0007 | 0.0010 | 0.0083 | 11.542 | 10.039 |
| Previous stents | |||||||||
| ICA only | – | 8.72 | – | – | −0.0012 | – | 0.0077 | – | 8.724 |
| NGCCT-ICA | – | 8.73 | – | – | −0.0012 | – | 0.0063 | – | 8.737 |
| NGCCT only | – | 8.74 | – | – | −0.0010 | – | 0.0072 | – | 8.744 |
| Previous CABG | |||||||||
| ICA only | – | 8.71 | – | – | −0.0011 | – | 0.0074 | – | 8.719 |
| NGCCT-ICA | – | 8.72 | – | – | −0.0012 | – | 0.0062 | – | 8.725 |
| NGCCT only | – | 8.72 | – | – | −0.0010 | – | 0.0067 | – | 8.725 |
Population with suspected coronary artery disease
In the EUROPA model the ICA-only strategy obtains, in every difficult-to-image patient group, the highest number of QALYs. This is because of the lower HRQoL FNs experienced in the NGCCT-only strategy and in the NGCCT–ICA strategy owing to lower sensitivity and specificity of the NGCCT. FNs do not occur in the ICA-only strategy; they will all be classified as TP with a higher HRQoL. The QALYs in the healthy population model are the lowest in the ICA-only population because the proportion of TNs is the lowest for this strategy. The NGCCT–ICA and NGCCT-only strategies have larger proportion in the TNs because less ICA-related mortality occurs. Table 19 shows the four test outcomes; the proportion that is modelled with the healthy population model is the sum of the proportions classified as TN and FP. The QALYs from the stroke model are highest in the ICA-only strategy because in this strategy the largest proportion of patients is modelled with this model due to the highest morbidity induced by the initial treatment and initial ICA.
Population with known coronary artery disease
In the known population there is little difference between the three strategies, as all test outcomes are modelled with the EUROPA model. In the known population, every patient has CAD and therefore the healthy population model is not used for this population. In all cases, the ICA only has the lowest QALYs in the EUROPA model. This could be because ICA only has the largest overall mortality rate and therefore fewer people are modelled with the EUROPA model. The morbidity rate was the highest for the ICA-only strategy and therefore it accumulates the highest number of QALYs in the stroke model. The NGCCT–ICA strategy has the lowest morbidity rate and therefore it obtains fewer QALYs than the other strategies in the stroke model. More QALYs obtained in the stroke model can lead to less QALY gain in the EUROPA model; as the HRQoL in the stroke model is lower than in the EUROPA model, the higher complication rate of ICA is not favourable for the ICA-only strategy. The disutilities associated with the YRM are the largest (Table 58 shows no difference between the first two strategies but this is due to rounding) for the ICA-only strategy owing to the higher radiation dose of the ICA compared with the NGCCT.
Cost-effectiveness
The aim of this assessment was to estimate the cost-effectiveness of the NGCCT in difficult-to-image patients for a suspected and for a known CAD population. ICERs are presented below for the suspected CAD population (see Table 59) and for the known CAD population (see Table 60). The cost-effectiveness is based on probabilistic modelling as the models are non-linear. After running the subgroup-specific probabilistic sensitivity analyses we combined them into one population by using each subgroup-specific costs and effects (mean and SE), the correlations between the costs and effects, and the relative frequencies of the subgroups. The uncertainty regarding these relative frequencies was included in the probabilistic analyses. The relative proportions were based on expert opinion, as described above (see Proportions of patients in difficult-to-image subgroups and Table 53).
| Strategy | Costs (£) | QALYs | iCosts | iQALYs | ICER | ||
|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | ||||
| Obese | |||||||
| NGCCT–ICA | 6297 | 1237 | 10.508 | 0.167 | |||
| NGCCT only | 6106 | 1202 | 10.508 | 0.167 | −191 | 0.000 | Dominates NGCCT–ICA |
| ICA only | 6968 | 1217 | 10.519 | 0.163 | 862 | 0.011 | 81,318 |
| Arrhythmias | |||||||
| NGCCT–ICA | 6227 | 1190 | 9.419 | 0.171 | |||
| NGCCT only | 6077 | 1161 | 9.420 | 0.171 | −150 | 0.000 | Dominates NGCCT–ICA |
| ICA only | 6785 | 1205 | 9.448 | 0.166 | 708 | 0.029 | 24,645 |
| HHR | |||||||
| NGCCT only | 6595 | 1256 | 10.967 | 0.156 | |||
| NGCCT–ICA | 6758 | 1289 | 10.968 | 0.157 | 162 | 0.001 | 312,047 |
| ICA only | 7342 | 1263 | 10.969 | 0.155 | 584 | 0.001 | 440,057 |
| HCC | |||||||
| NGCCT only | 5962 | 1168 | 10.201 | 0.169 | |||
| NGCCT–ICA | 6142 | 1248 | 10.202 | 0.169 | 180 | 0.001 | 205,536 |
| ICA only | 6801 | 1189 | 10.210 | 0.167 | 659 | 0.008 | 80,446 |
| Intolerance to beta-blockers | |||||||
| NGCCT–ICA | 6430 | 1320 | 11.540 | 0.151 | |||
| ICA only | 7016 | 1242 | 11.541 | 0.148 | 586 | 0.001 | 972,803 |
| NGCCT only | 6279 | 1240 | 11.542 | 0.151 | −736 | 0.001 | Dominant |
| Suspected overall | |||||||
| NGCCT only | 5808 | 573 | 10.588 | 0.109 | |||
| NGCCT–ICA | 5950 | 589 | 10.590 | 0.109 | 142 | 0.002 | 71,000 |
| ICA only | 6534 | 572 | 10.597 | 0.107 | 584 | 0.007 | 83,429 |
| Strategy | Costs (£) | QALYs | iCosts | iQALYs | ICER | ||
|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | ||||
| Obese | |||||||
| ICA only | 29,694 | 928 | 8.857 | 0.464 | |||
| NGCCT only | 29,254 | 924 | 8.869 | 0.477 | −439 | 0.012 | Dominates ICA only |
| NGCCT-ICA | 29,177 | 920 | 8.872 | 0.460 | −77 | 0.003 | Dominant |
| Arrhythmias | |||||||
| ICA only | 27,428 | 908 | 6.545 | 0.504 | |||
| NGCCT-ICA | 27,084 | 916 | 6.588 | 0.503 | −344 | 0.043 | Dominates ICA only |
| NGCCT only | 27,726 | 971 | 6.595 | 0.499 | 642 | 0.007 | 90,683 |
| HHR | |||||||
| ICA only | 30,434 | 1169 | 11.223 | 0.381 | |||
| NGCCT only | 30,477 | 1190 | 11.233 | 0.377 | 43 | 0.011 | 4021 |
| NGCCT-ICA | 30,080 | 1184 | 11.242 | 0.378 | −397 | 0.009 | Dominant |
| HCS | |||||||
| ICA only | 31,145 | 1079 | 9.271 | 0.538 | |||
| NGCCT only | 30,839 | 1103 | 9.301 | 0.533 | −306 | 0.030 | Dominates ICA only |
| NGCCT-ICA | 30,661 | 1075 | 9.306 | 0.539 | −178 | 0.005 | Dominant |
| Intolerance to beta-blocker | |||||||
| ICA only | 29,339 | 986 | 10.016 | 0.392 | |||
| NGCCT only | 29,354 | 1004 | 10.039 | 0.392 | 14 | 0.024 | 610 |
| NGCCT-ICA | 28,972 | 988 | 10.042 | 0.394 | −381 | 0.003 | Dominant |
| Previous stents | |||||||
| ICA only | 28,450 | 842 | 8.724 | 0.364 | |||
| NGCCT-ICA | 28,056 | 855 | 8.737 | 0.358 | −394 | 0.013 | Dominates ICA only |
| NGCCT only | 28,672 | 888 | 8.744 | 0.354 | 617 | 0.007 | 93,526 |
| Previous CABG | |||||||
| ICA only | 28,466 | 844 | 8.719 | 0.363 | |||
| NGCCT-ICA | 28,088 | 859 | 8.725 | 0.360 | −378 | 0.006 | Dominates ICA only |
| NGCCT only | 28,554 | 1028 | 8.725 | 0.359 | 466 | 0.000 | 2,943,850 |
| Known overall | |||||||
| ICA only | 28,234 | 502 | 9.516 | 0.288 | |||
| NGCCT-ICA | 27,785 | 531 | 9.537 | 0.283 | −449 | 0.022 | Dominates ICA only |
| NGCCT only | 28,228 | 498 | 9.538 | 0.286 | 443 | 0.001 | 726,230 |
Population with suspected coronary artery disease
Table 59 presents very small differences in QALYs; however, the ICA-only strategy is in general more effective than the other two strategies. In most subgroups, the NGCCT–ICA strategy achieves fewer QALYs than the other strategies. The ICA-only strategy is the most expensive strategy; the NGCCT-only strategy is cost saving compared with the other strategies. The negative incremental costs of the NGCCT-only strategy are due to the lower costs in the diagnostic model. The lower costs in the diagnostic model are the result of the large difference between the cost prices of the NGCCT and the ICA. After combining the results of the subgroups, we see that the NGCCT-only strategy might be considered the most attractive. The ICER of NGCCT–ICA compared with NGCCT only is so high (£71,000) that, given conventional willingness-to-pay threshold of £20,000–30,000, it is unlikely that commissioners of health care would consider this a cost-effective use of NHS resources.
Population with known coronary artery disease
In the known CAD population the cost-effectiveness differed by subgroup (Table 60). The NGCCT–ICA and the NGCCT-only strategies are, in all subgroups, more effective than the ICA-only strategy. In the subgroups obese, HCS, HHR, and beta-blocker intolerance, the NGCCT–ICA strategy dominated the other strategies, being more effective and of lower cost than the other two strategies. In all subgroups, the NGCCT–ICA strategy was less expensive than the other strategies. When results of the subgroups are combined, the most attractive strategy would be to perform a NGCCT with ICA; this scenario yields the highest cost saving, and dominates ICA only. The ICER of NGCCT only compared with NGCCT–ICA is so high (£726,230) that it is unlikely to be considered cost-effective, given conventional willingness-to-pay threshold of £20,000 to £30,000.
Sensitivity analyses
Probabilistic sensitivity analyses were performed to explore the robustness of the outcomes. The NGCCT accuracy parameters, the prior likelihood of CAD for both populations, treatment decisions, complication and mortality rates, cost of events, cost of radiation, disutilities due to radiation, the QoL and transition rates in the disease progression model are varied in the sensitivity analysis. The test accuracy parameters of the ICA were not varied in the sensitivity analysis. Cost-effectiveness acceptability curves are presented in this section per population after combining the difficult-to-image subgroups into one population group. Table 61 presents the distributions of the parameters. Subgroup-specific parameters such as sensitivity, specificity, etc., are presented for only the obese subgroup of the suspected CAD population.
| Parameter | Distribution | Mean | SE | Alpha | Beta |
|---|---|---|---|---|---|
| Logit of sensitivity (obese 0,904) | Normal | 2.24 | 0.33 | ||
| Logit of specificity (obese 0,921) | Normal | 2.46 | 0.19 | ||
| Prior likelihood of suspected CAD | Beta | 0.2 | 20 | 80 | |
| Prior likelihood of known CAD | Beta | 0.395 | 296 | 454 | |
| Proportion of patients receiving revascularisation (CAD-suspected population) | Beta | 0.181 | 50 | 227 | |
| ICA mortality | Beta | 0.0007 | 155 | 211,490 | |
| PCI mortality | Beta | 0.0029 | 11 | 3849 | |
| CABG mortality | Beta | 0.018 | 47 | 2552 | |
| ICA non-fatal complications | Beta | 0.00064 | 136 | 211,509 | |
| PCI non-fatal complications | Beta | 0.001 | 4 | 3856 | |
| CABG non-fatal complications | Beta | 0.04 | 24 | 581 | |
| Proportion MI of non-fatal complications ICA | Beta | 0.052 | 7 | 129 | |
| Proportion MI of non-fatal complications PCI | Beta | 0.5 | 50 | 50 | |
| Proportion MI of non-fatal complications CABG | Beta | 0.6 | 60 | 40 | |
| Transition probabilities (TP ICA-only suspected, obese) | |||||
| Risk equation 1: risk of first primary event | Logistic regression: Cholesky decomposition | 0.0078 | |||
| Risk equation 2: odds that first event is fatal | Logit: Cholesky decomposition | 0.2950 | |||
| Risk equation 3: risk of subsequent event in first year after initial NFE | Weibull regression: Cholesky decomposition | 0.0272 | |||
| Risk equation 4: subsequent event after 1 year | Logit: Cholesky decomposition | 0.0112 | |||
| Background costs | Regression: Cholesky decomposition | ||||
| YRM incremental costs (obese 26.5 mSv vs 0 mSv) | Normal | 9.194 | 0.1305 | ||
| YRM incremental effects (obese 26.5 mSv vs 0 mSv) | Normal | −0.0026 | 0.000029 | ||
| Annual disutility due to MI or cardiac arrest | Normal | 0.0409 | 0.0002 | ||
| Cost of events | |||||
| NFE | Ordinary least squares regression | 11,805 | |||
| NFE history | Ordinary least squares regression | 986 | |||
| CV fatal event | Ordinary least squares regression | 3641 | |||
| Non-CV fatal event | Ordinary least squares regression | 12,421 | |||
| QALYs disease progression model | |||||
| Population norms (male, 65–74 years) | Beta | 388 | 109 | ||
| After treatment QoL | Dirichlet | ||||
The acceptability curves in Figures 22 and 23 are in line with the base-case results presented in Tables 59 and 60. In the suspected population, in the range of thresholds of < £30,000, the NGCCT-only strategy has the highest probability of being cost-effective. Once thresholds are > £70,000, the three different strategies are equivalent. For the known CAD patients, the NGCCT–ICA strategy has the highest probability of being cost-effective, over the whole range of thresholds, while the ICA-only strategy has always the smallest probability of being cost-effective.
FIGURE 22.
Suspected CAD population: CEAC.
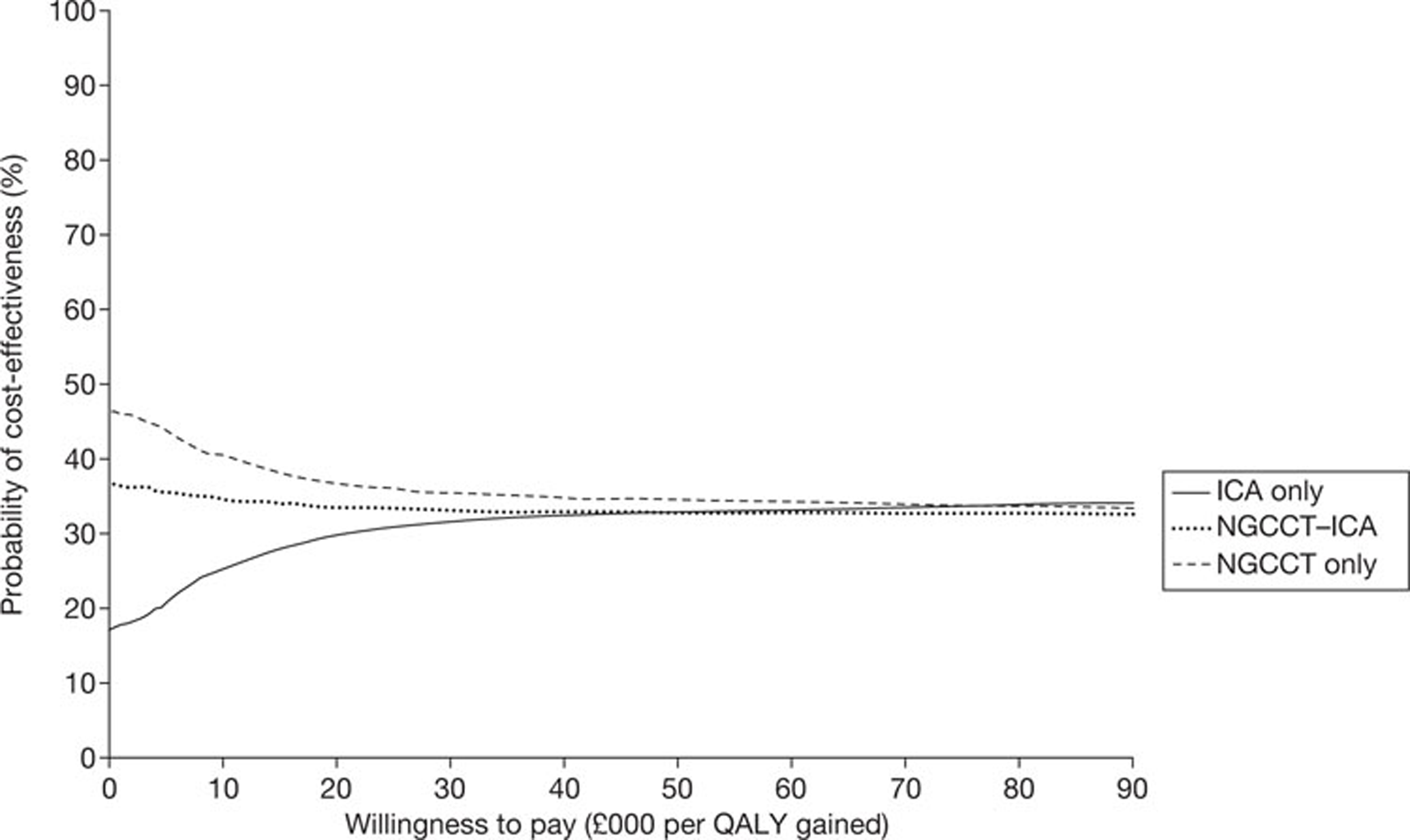
FIGURE 23.
Known CAD population: CEAC.
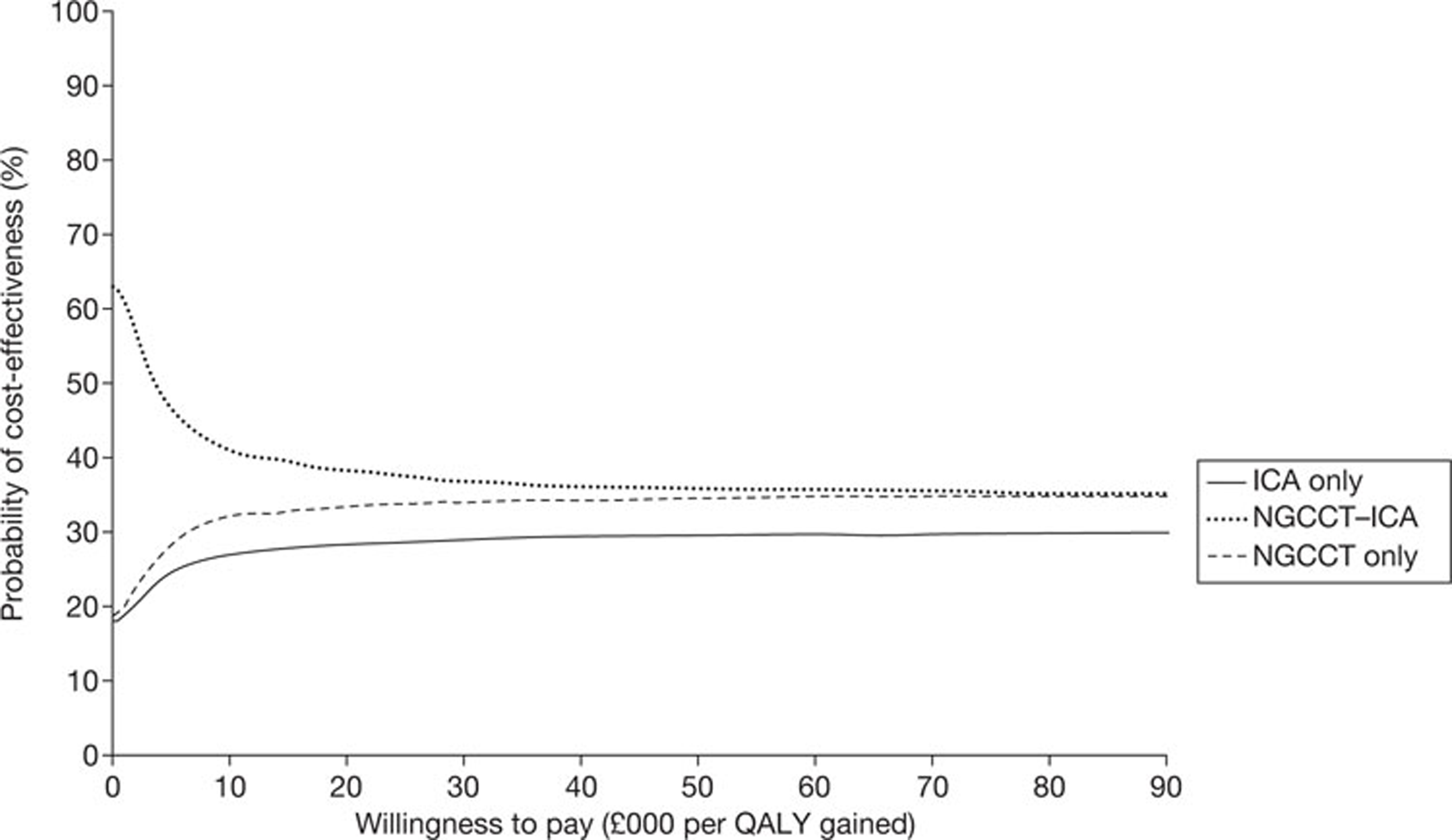
Scenario analyses
Scenario analyses based on a probabilistic analysis were performed to estimate the influence of the cost price of the NGCCT, the prior likelihood of the CAD suspected population, and the influence of the complication rates on the cost-effectiveness. In the first two scenarios, the cost price of the NGCCT is fixed at £150 and at £207, respectively. All other parameters are varied as in the PSA. Tables 62 and 63 show the results for the lower cost price of the NGCCT in both CAD populations for each subgroup. Tables 64 and 65 present the results of the higher cost price.
| Strategy | Costs (£) | QALYs | iCosts | iQALYs | ICER | ||
|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | ||||
| Obese | |||||||
| NGCCT-ICA | 6295 | 1191 | 10.507 | 0.165 | |||
| NGCCT only | 6102 | 1157 | 10.510 | 0.166 | −193 | 0.003 | Dominates NGCCT–ICA |
| ICA only | 6988 | 1169 | 10.516 | 0.160 | 886 | 0.006 | 145,092 |
| Arrhythmias | |||||||
| NGCCT only | 6023 | 1160 | 9.421 | 0.172 | |||
| NGCCT-ICA | 6172 | 1189 | 9.423 | 0.172 | 148 | 0.001 | 144,492 |
| ICA only | 6741 | 1205 | 9.449 | 0.168 | 569 | 0.027 | 21,258 |
| HHR | |||||||
| NGCCT-ICA | 6771 | 1286 | 10.961 | 0.157 | |||
| NGCCT only | 6604 | 1255 | 10.964 | 0.156 | −167 | 0.003 | Dominates NGCCT–ICA |
| ICA only | 7372 | 1257 | 10.964 | 0.152 | 768 | 0.000 | 5,182,062 |
| HCS | |||||||
| NGCCT-ICA | 6167 | 1220 | 10.199 | 0.170 | |||
| NGCCT only | 5978 | 1156 | 10.199 | 0.170 | −189 | 0.000 | Dominates NGCCT–ICA |
| ICA only | 6837 | 1172 | 10.206 | 0.169 | 859 | 0.007 | 123,267 |
| Intolerance beta-blockers | |||||||
| ICA only | 6997 | 1203 | 11.544 | 0.150 | |||
| NGCCT-ICA | 6374 | 1282 | 11.545 | 0.153 | −624 | 0.001 | Dominates ICA only |
| NGCCT only | 6243 | 1191 | 11.545 | 0.151 | −131 | 0.001 | Dominant |
| Suspected overall | |||||||
| NGCCT-ICA | 5980 | 580 | 10.59 | 0.11 | |||
| NGCCT only | 5819 | 559 | 10.59 | 0.11 | −161 | 0.002 | Dominates NGCCT–ICA |
| ICA only | 6572 | 567 | 10.60 | 0.11 | 753 | 0.006 | 125,500 |
| Strategy | Costs (£) | QALYs | iCosts | iQALYs | ICER | ||
|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | ||||
| Obese | |||||||
| ICA only | 29,705 | 930 | 8.853 | 0.463 | |||
| NGCCT-ICA | 29,163 | 918 | 8.871 | 0.463 | −542 | 0.019 | Dominates ICA only |
| NGCCT only | 29,241 | 920 | 8.877 | 0.459 | 78 | 0.006 | 13,597 |
| Arrhythmias | |||||||
| ICA only | 27,453 | 888 | 6.560 | 0.505 | |||
| NGCCT only | 27,085 | 899 | 6.591 | 0.507 | −368 | 0.031 | Dominates ICA only |
| NGCCT-ICA | 27,729 | 947 | 6.603 | 0.488 | 644 | 0.012 | 52,655 |
| HHR | |||||||
| ICA only | 30,458 | 1194 | 11.229 | 0.383 | |||
| NGCCT only | 30,451 | 1181 | 11.251 | 0.372 | −6 | 0.022 | Dominates ICA only |
| NGCCT-ICA | 30,056 | 1175 | 11.262 | 0.379 | −395 | 0.010 | Dominant |
| HCS | |||||||
| ICA only | 31,133 | 1073 | 9.276 | 0.531 | |||
| NGCCT-ICA | 30,629 | 1074 | 9.308 | 0.539 | −504 | 0.032 | Dominates ICA only |
| NGCCT only | 30,809 | 1081 | 9.314 | 0.530 | 179 | 0.006 | 29,531 |
| Intolerance to beta-blockers | |||||||
| ICA only | 29,333 | 981 | 10.025 | 0.390 | |||
| NGCCT only | 29,347 | 998 | 10.033 | 0.394 | 14 | 0.008 | 1640 |
| NGCCT-ICA | 28,972 | 982 | 10.034 | 0.394 | −375 | 0.001 | Dominant |
| Previous stent | |||||||
| ICA only | 28,454 | 843 | 8.725 | 0.364 | |||
| NGCCT only | 28,664 | 875 | 8.727 | 0.361 | 210 | 0.001 | 147,862 |
| NGCCT-ICA | 28,043 | 845 | 8.729 | 0.357 | −620 | 0.002 | Dominant |
| Previous CABG | |||||||
| ICA only | 28,452 | 839 | 8.722 | 0.365 | |||
| NGCCT-ICA | 28,051 | 847 | 8.733 | 0.361 | −401 | 0.010 | Dominates ICA only |
| NGCCT only | 28,518 | 1030 | 8.735 | 0.374 | 468 | 0.003 | 166,672 |
| Known overall | |||||||
| ICA only | 28,121 | 501 | 9.52 | 0.29 | |||
| NGCCT only | 28,302 | 500 | 9.54 | 0.29 | 181 | 0.021 | 8748 |
| NGCCT-ICA | 27,818 | 499 | 9.55 | 0.29 | −484 | 0.004 | Dominant |
| Strategy | Costs (£) | QALYs | iCosts | iQALYs | ICER | ||
|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | ||||
| Obese | |||||||
| NGCCT only | 6132 | 1195 | 10.509 | 0.171 | |||
| NGCCT-ICA | 6319 | 1228 | 10.511 | 0.167 | 187 | 0.002 | 88,132 |
| ICA only | 6960 | 1209 | 10.516 | 0.165 | 641 | 0.005 | 129,189 |
| Arrhythmias | |||||||
| NGCCT only | 6071 | 1178 | 9.418 | 0.175 | |||
| NGCCT-ICA | 6221 | 1207 | 9.419 | 0.173 | 149 | 0.001 | 171,745 |
| ICA only | 6737 | 1216 | 9.445 | 0.168 | 517 | 0.026 | 19,545 |
| HHR | |||||||
| NGCCT-ICA | 6828 | 1320 | 10.966 | 0.158 | |||
| ICA only | 7372 | 1293 | 10.967 | 0.155 | 544 | 0.001 | 481,876 |
| NGCCT only | 6660 | 1286 | 10.968 | 0.157 | −711 | 0.001 | Dominant |
| HCS | |||||||
| NGCCT-ICA | 6189 | 1230 | 10.201 | 0.172 | |||
| NGCCT only | 6004 | 1154 | 10.203 | 0.170 | −185 | 0.002 | Dominates NGCCT–ICA |
| ICA only | 6804 | 1170 | 10.210 | 0.169 | 800 | 0.008 | 102,208 |
| Intolerance beta-blockers | |||||||
| NGCCT-ICA | 6455 | 1298 | 11.541 | 0.150 | |||
| ICA only | 7009 | 1217 | 11.542 | 0.149 | 554 | 0.000 | 6,278,463 |
| NGCCT only | 6312 | 1218 | 11.542 | 0.152 | −697 | 0.000 | Dominant |
| Suspected overall | |||||||
| NGCCT-ICA | 5979 | 591 | 10.586 | 0.109 | |||
| NGCCT only | 5813 | 557 | 10.590 | 0.110 | −166 | 0.004 | Dominates NGCCT–ICA |
| ICA only | 6519 | 578 | 10.593 | 0.109 | 706 | 0.003 | 235,333 |
| Strategy | Costs (£) | QALYs | iCosts | iQALYs | ICER | ||
|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | ||||
| Obese | |||||||
| ICA only | 29,710 | 935 | 8.847 | 0.471 | |||
| NGCCT-ICA | 29,238 | 928 | 8.851 | 0.469 | −471 | 0.004 | Dominates ICA only |
| NGCCT only | 29,309 | 928 | 8.870 | 0.463 | 70 | 0.019 | 3727 |
| Arrhythmias | |||||||
| ICA only | 27,437 | 898 | 6.567 | 0.498 | |||
| NGCCT only | 27,762 | 941 | 6.592 | 0.502 | 325 | 0.025 | 12,894 |
| NGCCT-ICA | 27,127 | 904 | 6.602 | 0.495 | −635 | 0.010 | Dominant |
| HHR | |||||||
| ICA only | 30,418 | 1161 | 11.226 | 0.379 | |||
| NGCCT-ICA | 30,094 | 1157 | 11.248 | 0.377 | −324 | 0.022 | Dominates ICA only |
| NGCCT only | 30,465 | 1174 | 11.249 | 0.378 | 371 | 0.001 | 295,660 |
| HCS | |||||||
| ICA only | 31,132 | 1062 | 9.262 | 0.549 | |||
| NGCCT only | 30,865 | 1084 | 9.302 | 0.545 | −267 | 0.040 | Dominates ICA only |
| NGCCT-ICA | 30,685 | 1058 | 9.302 | 0.543 | −181 | 0.000 | Dominant |
| Intolerance to beta-blockers | |||||||
| ICA only | 29,346 | 998 | 10.013 | 0.401 | |||
| NGCCT-ICA | 29,023 | 1005 | 10.033 | 0.398 | −324 | 0.020 | Dominates ICA only |
| NGCCT only | 29,385 | 1014 | 10.046 | 0.387 | 362 | 0.014 | 26,423 |
| Previous stent | |||||||
| ICA only | 28,461 | 843 | 8.727 | 0.359 | |||
| NGCCT only | 28,729 | 884 | 8.729 | 0.360 | 268 | 0.003 | 100,271 |
| NGCCT-ICA | 28,103 | 854 | 8.739 | 0.354 | −626 | 0.009 | Dominant |
| Previous CABG | |||||||
| ICA only | 28,473 | 845 | 8.722 | 0.364 | |||
| NGCCT only | 28,598 | 1025 | 8.734 | 0.357 | 125 | 0.012 | 10,450 |
| NGCCT-ICA | 28,117 | 851 | 8.744 | 0.367 | −481 | 0.010 | Dominant |
| Known overall | |||||||
| ICA only | 28,268 | 510 | 9.52 | 0.29 | |||
| NGCCT-ICA | 27,920 | 494 | 9.54 | 0.28 | −348 | 0.020 | Dominates ICA only |
| NGCCT only | 28,296 | 511 | 9.54 | 0.29 | 376 | 0.004 | 103,297 |
The prior likelihood of the suspected population was increased to 0.3. Table 66 presents the results of this scenario analysis.
| Strategy | Costs (£) | QALYs | iCosts | iQALYs | ICER | ||
|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | ||||
| Obese | |||||||
| NGCCT-ICA | 9314.3 | 308.61 | 10.366 | 0.172 | |||
| NGCCT only | 9028.2 | 301.17 | 10.37 | 0.1723 | −286 | 0.004 | Dominates NGCCT–ICA |
| ICA only | 9927.5 | 327.33 | 10.388 | 0.1669 | 899 | 0.018 | 50,007 |
| Arrhythmias | |||||||
| NGCCT-ICA | 9124.8 | 301.97 | 9.2579 | 0.1771 | |||
| NGCCT only | 8895.4 | 307.44 | 9.2593 | 0.1773 | −229 | 0.001 | Dominates NGCCT–ICA |
| ICA only | 9612.3 | 529.68 | 9.3023 | 0.1726 | 717 | 0.043 | 16,655 |
| HHR | |||||||
| NGCCT-ICA | 10,036 | 326.32 | 10.828 | 0.1568 | |||
| NGCCT only | 9786.7 | 330.37 | 10.83 | 0.1572 | −249 | 0.002 | Dominates NGCCT–ICA |
| ICA only | 10,538 | 332.76 | 10.84 | 0.1544 | 752 | 0.009 | 80,684 |
| HCS | |||||||
| NGCCT only | 8839.8 | 303.35 | 10.036 | 0.1776 | |||
| NGCCT-ICA | 9111.6 | 546.38 | 10.039 | 0.1771 | 272 | 0.003 | 82,843 |
| ICA only | 9706 | 317.08 | 10.056 | 0.1711 | 594 | 0.017 | 34,761 |
| Intolerance to beta-blockers | |||||||
| NGCCT-ICA | 9453.2 | 639.39 | 11.413 | 0.1482 | |||
| NGCCT only | 9238 | 332.08 | 11.418 | 0.1503 | −215 | 0.005 | Dominates NGCCT–ICA |
| ICA only | 9984.8 | 344.16 | 11.419 | 0.1457 | 747 | 0.000 | 5,935,679 |
| Suspected overall | |||||||
| NGCCT only | 9061 | 172 | 10.44 | 0.11 | |||
| NGCCT-ICA | 9355 | 232 | 10.44 | 0.11 | 294 | 0.001 | 294,000 |
| ICA only | 9790 | 182 | 10.46 | 0.11 | 435 | 0.015 | 29,000 |
Worst-case and best-case scenario analyses were performed to show the influence of the revascularisation and test complications on the cost-effectiveness. The influence of the rates on the cost-effectiveness in the suspected CAD population is shown below; see Tables 67 and 68.
| Strategy | Costs (£) | QALYs | iCosts | iQALYs | ICER | ||
|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | ||||
| Obese | |||||||
| NGCCT--ICA | 6288 | 1207 | 10.503 | 0.167 | |||
| NGCCT only | 6097 | 1174 | 10.505 | 0.166 | −192 | 0.002 | Dominates NGCCT–ICA |
| ICA only | 6965 | 1191 | 10.512 | 0.164 | 868 | 0.006 | 138,953 |
| Arrhythmias | |||||||
| NGCCT only | 6051 | 1144 | 9.420 | 0.174 | |||
| NGCCT-ICA | 6199 | 1170 | 9.423 | 0.174 | 147 | 0.003 | 52,093 |
| ICA only | 6746 | 1184 | 9.448 | 0.168 | 547 | 0.025 | 22,017 |
| HHR | |||||||
| ICA only | 7373 | 1256 | 10.962 | 0.154 | |||
| NGCCT-ICA | 6785 | 1285 | 10.963 | 0.156 | −587 | 0.001 | Dominates ICA only |
| NGCCT only | 6619 | 1249 | 10.963 | 0.156 | −166 | 0.000 | Dominant |
| High coronary calcium score | |||||||
| NGCCT-ICA | 6167 | 1221 | 10.196 | 0.171 | |||
| NGCCT only | 5983 | 1146 | 10.197 | 0.172 | −184 | 0.001 | Dominates NGCCT–ICA |
| ICA only | 6823 | 1161 | 10.203 | 0.167 | 841 | 0.006 | 141,072 |
| Intolerance to beta-blockers | |||||||
| ICA only | 7001 | 1200 | 11.539 | 0.150 | |||
| NGCCT-ICA | 6401 | 1279 | 11.540 | 0.152 | −601 | 0.001 | Dominates ICA only |
| NGCCT only | 6266 | 1202 | 11.541 | 0.153 | −135 | 0.001 | Dominant |
| Suspected overall | |||||||
| NGCCT only | 5795 | 553 | 10.585 | 0.109 | |||
| NGCCT-ICA | 5962 | 576 | 10.587 | 0.111 | 167 | 0.002 | 83,500 |
| ICA only | 6547 | 565 | 10.591 | 0.108 | 585 | 0.004 | 146,250 |
| Strategy | Costs (£) | QALYs | iCosts | iQALYs | ICER | ||
|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | ||||
| Obese | |||||||
| NGCCT-ICA | 6285 | 1225 | 10.514 | 0.163 | |||
| NGCCT only | 6093 | 1191 | 10.515 | 0.163 | −192 | 0.001 | Dominates NGCCT-ICA |
| ICA only | 6957 | 1208 | 10.522 | 0.160 | 864 | 0.007 | 122,501 |
| Arrhythmias | |||||||
| NGCCT only | 6050 | 1148 | 9.426 | 0.174 | |||
| NGCCT-ICA | 6200 | 1176 | 9.426 | 0.175 | 150 | 0.001 | 290,135 |
| ICA only | 6745 | 1183 | 9.455 | 0.168 | 545 | 0.029 | 18,689 |
| HHR | |||||||
| NGCCT-ICA | 6811 | 1297 | 10.967 | 0.158 | |||
| NGCCT only | 6645 | 1267 | 10.968 | 0.158 | −166 | 0.001 | Dominates NGCCT-ICA |
| ICA only | 7389 | 1269 | 10.970 | 0.155 | 744 | 0.002 | 366,638 |
| High coronary calcium score | |||||||
| NGCCT only | 5991 | 1152 | 10.199 | 0.171 | |||
| NGCCT-ICA | 6175 | 1220 | 10.200 | 0.171 | 184 | 0.000 | 512,161 |
| ICA only | 6824 | 1164 | 10.210 | 0.166 | 649 | 0.011 | 60,086 |
| Intolerance to beta-blockers | |||||||
| NGCCT-ICA | 6406 | 1284 | 11.545 | 0.151 | |||
| NGCCT only | 6272 | 1204 | 11.545 | 0.149 | −134 | 0.000 | Dominates NGCCT-ICA |
| ICA only | 7002 | 1207 | 11.546 | 0.148 | 730 | 0.001 | 583,943 |
| Suspected overall | |||||||
| NGCCT-ICA | 5992 | 586 | 10.590 | 0.110 | |||
| NGCCT only | 5800 | 557 | 10.591 | 0.108 | −192 | 0.001 | Dominates NGCCT-ICA |
| ICA only | 6579 | 571 | 10.600 | 0.106 | 779 | 0.009 | 86,556 |
Scenario analysis: new-generation cardiac computed tomography £150, coronary artery disease
A lower cost price means that the NGCCT–ICA and the NGCCT-only strategies become less expensive. The overall results do not change.
Scenario analysis: new-generation cardiac computed tomography £207, coronary artery disease
This scenario shows the impact of a higher NGCCT cost price on the cost-effectiveness. There is little change in the incremental costs, even when the cost of the NGCCT increases. In the suspected population the ICA-only strategy is still the most expensive strategy and NGCCT only the least expensive strategy. The higher price of the NGCCT led to a change in cost rank in the known CAD population. In the base case ICA only was the most expensive strategy but when the price is increased the NGCCT-only strategy is the most expensive strategy. Based on the ICER, for the suspected population NGCCT only remains the most favourable strategy, whereas for the known population the most favourable strategy remains NGCCT–ICA.
Scenario: prior likelihood, suspected population 0.3
‘ICA only’ is still the most expensive strategy and it gains the most QALYs. However, a higher prior likelihood leads to an increase in costs and a decrease in QALYs for all strategies. A higher prior likelihood means that more patients will have CAD and therefore more patients must be treated, which leads to higher costs. Furthermore, fewer patients will be modelled with the healthy population model resulting in a decrease in QALYs and more costs in the EUROPA model. With regards to the ICER, the NGCCT-only strategy remains the most favourable.
Scenario analysis complication rates
In the best-case scenario (Table 67) for the NGCCT, the complication rates are set at the upper limit of the 95% CI. ICA only is still the most effective strategy. However, the incremental QALYs gained by the ICA-only strategy have become smaller in comparison with the base-case analysis. As the ICA induces more complications than the NGCCT, this scenario analysis can be seen as the best-case scenario for the NGCCT strategies.
In the worst-case scenario (Table 68) for the NGCCT, the complication rates are set at the lower limit of the 95% CI, the ICA-only strategy is the most effective strategy. The incremental QALYs gained by ICA only increased compared with the base-case analysis. When assessing the balance between costs and effects, in both scenarios NGCCT only remains the most favourable strategy.
Scenario analysis covariates used in risk equation for obese subgroup
A study by Oreopoulos et al. 106 examines the association between obesity and HRQoL in patients with CAD. It gives a good representation of an obese population with CAD (BMI of 25–30 kg/m2, n = 2310; BMI of 30–35 kg/m2, n = 1331; BMI of 35–40 kg/m2, n = 446; BMI of > 40 kg/m2, n = 178). The baseline characteristics that were found in the Oreopoulos et al. study106 are similar to the baseline characteristics used in our model. The baseline characteristics in the model are based on the systematic review and on the EUROPA trial. Not all covariates for the risk equations are presented in the Oreopoulos et al. study106 but gender, diabetes, existing vascular disease and previous MI are presented. We have performed scenario analyses within the obese group to study the effect of changing these covariates. The baseline values used in the obese known subgroup are existing vascular disease (stroke, TIA and peripheral vascular disease) 9.8%, female 34.1%, previous MI 64.7% and diabetes milletus proportion 34.1%. These values were changed to the following: existing vascular disease 7.5% and 13.5%; female 30%; previous MI 50% and diabetes milletus proportion 60%. These analyses (results not shown) show that these changes have no impact on our conclusions.
Cost-effectiveness of new-generation cardiac computed tomography in congenital heart disease
Model structure
The main model structure of the YRM for patients with congenital heart disease is identical to the structure discussed in detail above (see York Radiation Model). For the patients with congenital heart disease a number of scenario analyses were conducted, for example varying the age of cancer incidence. These are variations only in key parameters, not in the model structure. Further details are provided below. Regarding the potentially repetitive nature of the imaging in patients with congenital heart disease, experts emphasised that, owing to radiation exposure considerations, these patients are mostly imaged with echocardiography and MRI. We therefore assumed that the NGCCT would be used in a single instance for treatment planning, rather than for ongoing monitoring.
Model parameters
Base case
In the base case for patients with congenital heart disease, the key parameters of the YRM (i.e. utility, costs per scan, probability of cancer incidence given radiation, and cancers models) remain the same as for patients with CAD. The only difference is in the radiation doses for patients with congenital heart disease. These were based on an expert opinion, accounting for the particular diagnostic circumstances of patients with congenital heart disease (Table 69). We used these results to define five different age groups: 1-year-olds (infants), 5- to 10-year-olds (young children) and 25- to 35-year-olds (adults).
| Age group | CT64 | NGCCT |
|---|---|---|
| Very small children | 1.6 (1–4) | 0.8 (0.5–2) |
| Medium-sized children | 3 (1–8) | 1.5 (0.5–4) |
| Adults | 6 (4–25) | 3 (1–12) |
Patients with congenital heart disease can suffer from a range of cyanotic or non-cyanotic heart diseases. The timing of diagnosis and treatment and, hence, the use of a CT, depends on the particular lesion in question, but in most cases occurs in the first years of life. Depending on the lesion, further investigations and treatment might be necessary later in life. For aortic arch abnormalities (double aortic arch, vascular ring), for example a CT is undertaken at the time of diagnosis, usually in the first year of life. Similarly, for pulmonary atresia with MAPCAs either echocardiography, followed by cardiac catheterisation with invasive angiography or cross-sectional imaging (MRI or CT), is carried out in the first year of life and then again as required but often at the age of 2 or 3 years; for total anomalous pulmonary venous drainage/scimitar, echocardiography followed by cross-sectional imaging (MRI or CT) is undertaken at time of diagnosis and often again immediately before surgery (age 2–3 years). For lesions with both a vascular and airway component a CT may be carried out at diagnosis, which is usually soon after birth. In some cases, where a lesion has been previously treated using stents or pacemakers, MRI is unsuitable and patients require the use of CT when clinically indicated.
No clear evidence exists on to what extent NGCCT reduces the radiation dose at each scan. The general, NGCCT favourable assumption, based on information from one expert (see Appendix 7) was to assume a reduction of 50% compared with standard 64-slice CT.
Scenario analysis
In the scenario analyses a number of key parameters for patients with congenital heart disease were varied. These were (a) using the minimum radiation dose, (b) the maximum radiation dose, (c) an earlier age at cancer diagnosis, and (d) using the Biological Effects of Ionizing Radiation (BEIR) model for the effects of radiation on cancer incidence. Lastly, we ran (e) a scenario combining the least favourable assumption for the comparator, i.e. an NGCCT-friendly scenario that uses maximum radiation dose for a 64-slice CT scan, early onset of cancer, and the BEIR cancer radiation model.
The values for the (a) minimum and (b) maximum scenarios were based on the data shown in Table 64. The values for (c), the earlier age at cancer incidence scenario, were taken from the cancer model in the YRM. 61 The earlier age with the corresponding disease costs and remaining QALYs is shown in Table 70. Note that for the age group of patients with congenital heart disease (age at exposure < 40 years), the YRM takes the incidence age of 40 years for breast cancer by default. The values for the BEIR model (Table 71) were published by the National Research Council for a 1999 US population. 107 The BEIR study developed a more conservative risk model to estimate the relationship between exposure to ionising radiation and harmful health effects, primarily based on the cancer incidence data from the Life Span Study for the period 1958–98 and based on Dosimetry System 2002 (DSO2) dosimetry data. 61
| Cancer | Age at diagnosis (years) | Costs of cancer (£) | QALYs lost due to cancer |
|---|---|---|---|
| Lung | 55 | 22,331 | 1.2145 |
| Colorectal | 55 | 14,321 | 3.8124 |
| Prostate | 55 | 12,389 | 2.6152 |
| Age at exposure (years) | Risk of all cancers (for exposure to 10 mSv) | |
|---|---|---|
| Male | Female | |
| 1 | 0.002414 | 0.004497 |
| 5 | 0.001816 | 0.003377 |
| 10 | 0.001445 | 0.002611 |
| 25 | 0.000832 | 0.001356 |
| 35 | 0.000667 | 0.000976 |
| 60 | 0.000489 | 0.000586 |
For all of the scenarios, the uncertainty in the costs and remaining QALYs of the cancer module are modelled via a PSA. The values for this are shown in Table 46. For prostate cancer no data for the uncertainty exists. In addition, we varied for all scenarios (including the base case) the price of a 64-slice CT scan; the alternative value is shown in Table 72.
| Strategy | Costs per scan (£) |
|---|---|
| 64-slice CT | 105.55 |
Base-case results
Table 73 shows the intermediate result of the probability of lifetime cancer incidence for a given patient, group for the average radiation dose and the ranges as given by expert survey (HPA radiation/cancer model, assuming 50% male patients). The probability depends on overall radiation dose and age at exposure. Table 74 shows the absolute QALYs for each age group by scanner type. NGCCT leads to higher overall QALYs because of the lower probability of cancer. The number of patients needed to be scanned in each age group to gain 1 QALY (in absolute terms) is shown in Table 75.
| Age (years) | 64-slice CT | NGCCT | Difference |
|---|---|---|---|
| 1 | 0.00018 | 0.00000908 | 0.0000907 |
| 5 | 0.00034 | 0.00017 | 0.0001702 |
| 10 | 0.000269 | 0.000135 | 0.0001345 |
| 25 | 0.000425 | 0.000213 | 0.0002127 |
| 35 | 0.000347 | 0.000174 | 0.0001737 |
| Age (years) | 64-slice CT | NGCCT | Difference |
|---|---|---|---|
| 1 | 24.696847 (0.000007) | 24.696918 (0.000003) | −0.000071 |
| 5 | 24.377658 (0.000014) | 24.377807 (0.000007) | −0.000149 |
| 10 | 23.911911 (0.000012) | 23.912049 (0.000006) | −0.000138 |
| 25 | 21.930976 (0.000032) | 21.931331 (0.000016) | −0.000355 |
| 35 | 20.042644 (0.000035) | 20.043041 (0.000016) | −0.000397 |
| Age (years) | Difference in QALYs between NGCCT and CT64 | No. of patients to be scanned |
|---|---|---|
| 1 | −0.000071 | 14,085 |
| 5 | −0.00015 | 6711 |
| 10 | −0.00014 | 7246 |
| 25 | −0.00036 | 2817 |
| 35 | −0.0004 | 2519 |
The costs caused by radiation-attributable cancer are shown in Table 76. Table 77 shows the maximum admissible cost that makes an NGCCT cost-effective, only accounting for the costs of radiation-induced cancer, for two different threshold values, i.e. a willingness to pay per gained QALY of £20,000 or £30,000, respectively. Table 78 shows the ICERS for the base-case scenario using two different costs for a 64-slice CT scan (£132.66 and £105.55, respectively); the price for the NGCCT is identical in both cases.
| Age (years) | CT64 | NGCCT | Difference |
|---|---|---|---|
| 1 | 0.42 (0.002873076) | 0.21 (0.001513261) | 0.21 |
| 5 | 0.89 (0.006429484) | 0.45 (0.003215453) | 0.44 |
| 10 | 0.83 (0.005951270) | 0.41 (0.003132579) | 0.42 |
| 25 | 2.15 (0.016340907) | 1.07 (0.008268757) | 1.08 |
| 35 | 2.41 (0.020106730) | 1.20 (0.010022409) | 1.21 |
| Age (years) | Threshold value (£) | |
|---|---|---|
| 20,000 | 30,000 | |
| 1 | 1.62 | 2.32 |
| 5 | 3.43 | 4.92 |
| 10 | 3.18 | 4.56 |
| 25 | 8.16 | 11.70 |
| 35 | 9.13 | 13.10 |
| Age (years) | ICER, price per CT64 scan | |
|---|---|---|
| £133 | £106 | |
| 1 | 521,377 | 908,786 |
| 5 | 244,196 | 426,830 |
| 10 | 266,617 | 465,842 |
| 25 | 100,351 | 176,730 |
| 35 | 90,088 | 158,905 |
Sensitivity analysis and scenario analysis results
In this section the results for the sensitivity analysis and different scenario analysis are presented. In the sensitivity analysis the inputs for the age at cancer incidence, expected disease costs and the expected remaining QALYs are varied (for details see Table 46). The key parameters for the scenario analysis are outlined above.
Table 79 shows the intermediate results of the probability of lifetime cancer incidence given radiation dose and age at exposure for the five patient groups using the BEIR model, and assuming 50% male patients.
| Age (years) | CT64 | NGCCT | Difference |
|---|---|---|---|
| 1 | 0.0005528 | 0.0002764 | 0.000276 |
| 5 | 0.000779 | 0.0003895 | 0.00039 |
| 10 | 0.0006084 | 0.0003042 | 0.000304 |
| 25 | 0.0006561 | 0.0003281 | 0.000328 |
| 35 | 0.0004928 | 0.0002464 | 0.000246 |
Sensitivity analysis
In Figure 24 the cost-effectiveness plane for the five different age groups of the base-case scenario is shown. The sensitivity analysis accounts for the uncertainty of the mean age of incidence, disease cost of cancer, and remaining QALYs in the YRM cancer module. In Table 80, selected summary statistics of the outcome distribution of the PSA are shown.
FIGURE 24.
Cost-effectiveness plane for PSA of base-case scenario for five different age groups (note: origin not included).
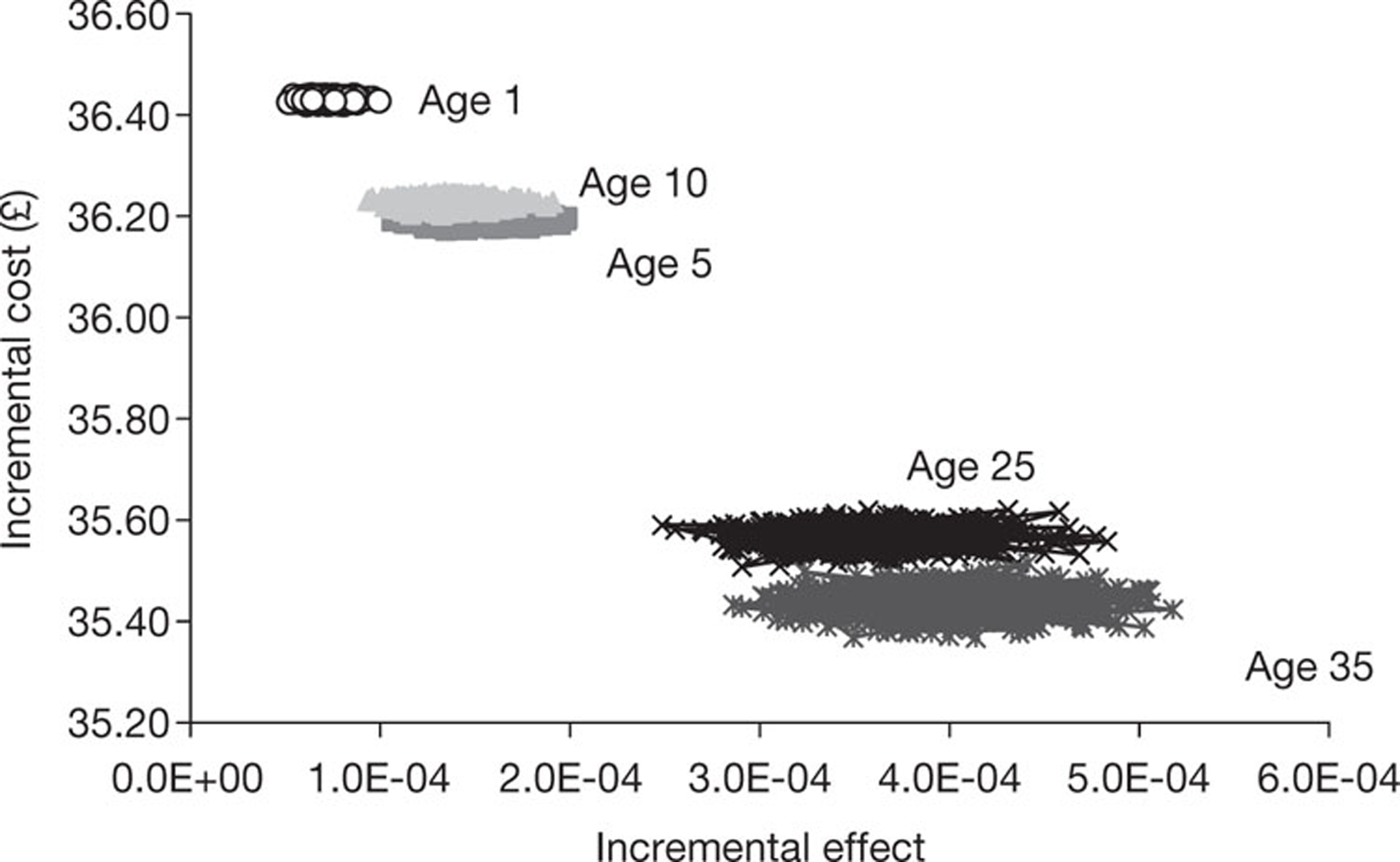
| Age (years) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 10 | 25 | 35 | |||||||||||
| Inc. effects | Inc. costs (£) | ICER (£) | Inc. effects | Inc. costs (£) | ICER (£) | Inc. effects | Inc. costs (£) | ICER | Inc. effects (£) | Inc. costs | ICER (£) | Inc. effects | Inc. costs (£) | ICER (£) | |
| Mean | 0.000071 | 36.37 | 521,377 | 0.000150 | 36.16 | 244,196 | 0.000137 | 36.19 | 266,617 | 0.000357 | 35.53 | 100,351 | 0.000396 | 35.40 | 90,088 |
| SD | 0.000008 | 1.31 | 56,292 | 0.000016 | 1.14 | 26,507 | 0.000014 | 1.14 | 28,460 | 0.000036 | 1.12 | 10,101 | 0.000039 | 1.12 | 8940 |
| Median | 0.000070 | 36.43 | 519,204 | 0.000148 | 36.19 | 243,816 | 0.000137 | 36.23 | 264,819 | 0.000357 | 35.57 | 99,765 | 0.000395 | 35.44 | 89,765 |
| 2.5th percentile | 0.000057 | 36.42 | 425,904 | 0.000121 | 36.18 | 198,225 | 0.000111 | 36.21 | 220,195 | 0.000290 | 35.53 | 83,231 | 0.000324 | 35.39 | 74,202 |
| 97.5th percentile | 0.000085 | 36.44 | 640,876 | 0.000182 | 36.21 | 298,016 | 0.000164 | 36.24 | 324,597 | 0.000427 | 35.60 | 122,568 | 0.000477 | 35.48 | 108,808 |
| Minimum | 0.000007 | 0.00 | 54,377 | 0.000016 | 0.01 | 25,622 | 0.000014 | 0.01 | 27,425 | 0.000034 | 0.02 | 9698 | 0.000037 | 0.02 | 8570 |
| Maximum | 0.000099 | 36.44 | 708,734 | 0.000198 | 36.22 | 342,328 | 0.000190 | 36.25 | 388,872 | 0.000483 | 35.62 | 143,456 | 0.000517 | 35.51 | 124,102 |
Scenario analysis
In this section the results of the five different scenario analyses are shown. These were (a) using the minimum radiation dose, (b) the maximum radiation dose, (c) an earlier age at cancer diagnosis and (d) using the BEIR model for the effects of radiation on cancer incidence. Lastly, we ran (e) a scenario combining the least favourable assumption for the comparator, i.e. an NGCCT-friendly scenario that uses maximum radiation dose for a 64-slice CT scan, early onset of cancer, and the BEIR cancer-radiation model.
Tables 81 and 82 show the disease in the costs of radiation-induced cancer and the expected absolute QALYs for each age group in the five different scenario analyses. The corresponding differences are reported in Tables 83 and 84.
| Age (years) | (a) Minimum | (b) Maximum | (c) Early cancer | (d) BEIR model | (e) NGCCT friendly | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CT64 | NGCCT | CT64 | NGCCT | CT64 | NGCCT | CT64 | NGCCT | CT64 | NGCCT | |
| 1 | 0.37 (0.00339) | 0.18 (0.00163) | 1.46 (0.01365) | 0.73 (0.00701) | 0.59 (0.00533) | 0.29 (0.00278) | 1.35 (0.008707) | 0.67 (0.004586) | 4.57 (0.039829) | 2.28 (0.020124) |
| 5 | 0.42 (0.00405) | 0.21 (0.00200) | 3.35 (0.03124) | 1.67 (0.01585) | 1.25 (0.01217) | 0.63 (0.00566) | 2.16 (0.014509) | 1.08 (0.006838) | 7.85 (0.067654) | 3.92 (0.033607) |
| 10 | 0.39 (0.00379) | 0.20 (0.00188) | 3.13 (0.03076) | 1.56 (0.01484) | 1.17 (0.01135) | 0.59 (0.00576) | 1.97 (0.013421) | 0.99 (0.006649) | 7.24 (0.064880) | 3.62 (0.033030) |
| 25 | 2.06 (0.01986) | 1.03 (0.01018) | 12.87 (0.12919) | 6.44 (0.06335) | 3.09 (0.03095) | 1.54 (0.01509) | 3.43 (0.024985) | 1.72 (0.012502) | 20.16 (0.190711) | 10.08 (0.094329) |
| 35 | 2.35 (0.02469) | 1.18 (0.01215) | 14.70 (0.15442) | 7.35 (0.07540) | 3.53 (0.03579) | 1.76 (0.01790) | 3.49 (0.028303) | 1.74 (0.013855) | 21.04 (0.212029) | 10.52 (0.106989) |
| Age (years) | (a) Minimum | (b) Maximum | (c) Early cancer | (d) BEIR model | (e) NGCCT friendly | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CT64 | NGCCT | CT64 | NGCCT | CT64 | NGCCT | CT64 | NGCCT | CT64 | NGCCT | |
| 1 | 24.6337635 (0.0000041) | 24.6338588 (0.0000022) | 24.6331895 (0.0000172) | 24.6335726 (0.0000088) | 24.6336489 (0.0000069) | 24.6338016 (0.0000034) | 24.696526 (0.000024) | 24.696758 (0.000012) | 24.631637 (0.000061) | 24.632797 (0.000029) |
| 5 | 24.3045185 (0.0000047) | 24.3046284 (0.0000024) | 24.3029834 (0.0000374) | 24.3038604 (0.0000188) | 24.3040802 (0.0000139) | 24.3044091 (0.0000072) | 24.377222 (0.000038) | 24.377589 (0.000019) | 24.300726 (0.000100) | 24.302736 (0.000049) |
| 10 | 23.8239917 (0.0000042) | 23.8240945 (0.0000021) | 23.8225465 (0.0000334) | 23.8233723 (0.0000168) | 23.8235788 (0.0000131) | 23.8238886 (0.0000063) | 23.911517 (0.000033) | 23.911853 (0.000016) | 23.820483 (0.000089) | 23.822340 (0.000043) |
| 25 | 21.7794578 (0.0000225) | 21.7800067 (0.0000107) | 21.7737154 (0.0001281) | 21.7771307 (0.0000656) | 21.7789115 (0.0000314) | 21.7797323 (0.0000157) | 21.930540 (0.000056) | 21.931112 (0.000027) | 21.770003 (0.000222) | 21.775281 (0.000115) |
| 35 | 19.8228249 (0.0000219) | 19.8234595 (0.0000113) | 19.8161715 (0.0001418) | 19.8201349 (0.0000692) | 19.8221934 (0.0000330) | 19.8231420 (0.0000165) | 20.042283 (0.000050) | 20.042860 (0.000025) | 19.812870 (0.000212) | 19.818485 (0.000101) |
| Age (years) | Costs (£) | ||||
|---|---|---|---|---|---|
| (a) Minimum | (b) Maximum | (c) Early cancer | (d) BEIR model | (e) NGCCT friendly | |
| 1 | −0.19 | −0.73 | −0.30 | −0.68 | −2.29 |
| 5 | −0.21 | −1.68 | −0.62 | −1.08 | −3.93 |
| 10 | −0.19 | −1.57 | −0.58 | −0.98 | −3.62 |
| 25 | −1.03 | −6.43 | −1.55 | −1.71 | −10.08 |
| 35 | −1.17 | −7.35 | −1.77 | −1.75 | −10.52 |
| Age (years) | (a) Minimum | (b) Maximum | (c) Early cancer | (d) BEIR model | (e) NGCCT friendly |
|---|---|---|---|---|---|
| 1 | 0.000095 | 0.000383 | 0.000153 | 0.000232 | 0.001160 |
| 5 | 0.000110 | 0.000877 | 0.000329 | 0.000367 | 0.002010 |
| 10 | 0.000103 | 0.000826 | 0.000310 | 0.000336 | 0.001857 |
| 25 | 0.000549 | 0.003415 | 0.000821 | 0.000572 | 0.005278 |
| 35 | 0.000635 | 0.003963 | 0.000949 | 0.000577 | 0.005615 |
Tables 85 and 86 show the maximum admissible cost that makes an NGCCT cost-effective for two different threshold values, i.e. a willingness to pay per gained QALY £20,000 or £30,000, respectively. Tables 87 and 88 report the ICERs for the scenario analyses in each age group, for a 64-slice CT price of £132.62 and £105.55, respectively.
| Age (years) | Cost £) | ||||
|---|---|---|---|---|---|
| (a) Minimum | (b) Maximum | (c) Early cancer | (d) BEIR model | (e) NGCCT friendly | |
| 1 | 1.01 | 4.02 | 3.35 | 5.32 | 25.47 |
| 5 | 3.43 | 9.11 | 7.21 | 8.43 | 44.13 |
| 10 | 3.18 | 8.44 | 6.78 | 7.69 | 40.76 |
| 25 | 8.16 | 34.35 | 17.96 | 13.16 | 115.66 |
| 35 | 9.13 | 37.90 | 20.74 | 13.28 | 122.82 |
| Age (years) | (a) Minimum | (b) Maximum | (c) Early cancer | (d) BEIR model | (e) Benign |
|---|---|---|---|---|---|
| CT64 | CT64 | CT64 | CT64 | CT64 | |
| 1 | 1.45 | 5.77 | 4.87 | 7.64 | 37.07 |
| 5 | 1.64 | 13.07 | 10.49 | 12.11 | 64.24 |
| 10 | 1.53 | 12.11 | 9.88 | 11.04 | 59.33 |
| 25 | 7.87 | 49.28 | 26.17 | 18.89 | 168.44 |
| 35 | 8.71 | 54.34 | 30.22 | 19.05 | 178.97 |
| Age (years) | (a) Minimum | (b) Maximum | (c) Early cancer | (d) BEIR model | (e) NGCCT friendly |
|---|---|---|---|---|---|
| 1 | 785,466 | 194,919 | 224,93 | 154,879 | 27,907 |
| 5 | 692,360 | 84,492 | 103,409 | 96,738 | 15,279 |
| 10 | 745,225 | 91,383 | 109,900 | 106,332 | 16,705 |
| 25 | 142,272 | 20,197 | 40,323 | 61,025 | 4653 |
| 35 | 128,361 | 18,018 | 34,658 | 60,489 | 4297 |
| Age (years) | (a) Minimum | (b) Maximum | (c) Early cancer | (d) BEIR model | (e) NGCCT friendly |
|---|---|---|---|---|---|
| 1 | 1,447,128 | 361,003 | 415,310 | 271,448 | 52,980 |
| 5 | 1,275,892 | 157,915 | 191,792 | 170,375 | 29,738 |
| 10 | 1,373,115 | 170,589 | 203,716 | 187,061 | 32,361 |
| 25 | 264,186 | 39,659 | 75,742 | 108,327 | 10,160 |
| 35 | 238,635 | 35,695 | 65,303 | 107,413 | 9474 |
Only in the NGCCT-friendly scenario do the ICERs decrease significantly, ranging from £28,000 per QALY gained for the youngest patients to £4300 per QALY gained for the adult patients. Looking at Tables 83 and 84, it is clear that of all key parameters, setting the radiation dose to the maximum of the range given by the expert has the highest impact on the cancer-related costs to be saved and QALYs to be gained. However, this upper value of the range of 25 mSv should be regarded with caution. It is very likely that the expert has implied a range of values ever used in his/her patient population, and it is very unlikely that it was implied that the average dosage could range from 4 to 25 mSv. The fact that for all other scenarios the ICER remains > £30,000 indicates that, even with the uncertainty about the various assumptions in mind, it can reasonably be concluded that the use of NGCCT instead of 64-slice CT in order to reduce radiation exposure is not cost-effective in this patient group.
Summary
In this chapter, we assessed the cost-effectiveness of NGCCT in two different populations (Table 89). The first is the comparison of NGCCT compared with ICA in difficult-to-image CAD patients and the second is the comparison of NGCCT compared with 64-slice CT in patients with congenital heart disease.
| Strategy | Costs (£) | QALYs | iCosts | iQALYs | ICER |
|---|---|---|---|---|---|
| Suspected CAD | |||||
| NGCCT only | 5808 | 10.588 | |||
| NGCCT–ICA | 5950 | 10.590 | 142 | 0.002 | 71,000 |
| ICA only | 6534 | 10.597 | 584 | 0.007 | 83,429 |
| Known | |||||
| ICA only | 28,234 | 9.516 | |||
| NGCCT–ICA | 27,785 | 9.537 | −449 | 0.022 | Dominates ICA only |
| NGCCT only | 28,228 | 9.538 | 443 | 0.001 | 726,230 |
The CAD population was divided into two subpopulations: the suspected CAD population and the known CAD population. Patients suspected of CAD are patients who have chest pain or other symptoms suggestive of CAD. Patients with known CAD are patients who have previously been diagnosed with CAD and whose symptoms are no longer controlled by drug treatment and/or being considered for revascularisation. The use of NGCCT has different purposes in the two CAD populations: for the suspected CAD population the purpose is to diagnose patients with CAD and for the known CAD population the purpose is to aid decision-making regarding a revascularisation.
For the CAD population, five different models were combined to estimate the cost-effectiveness of the NGCCT:
-
a decision tree that models the diagnostic pathway
-
an alive–dead Markov model for ‘healthy’ patients without CAD65
-
a stroke model to estimate the impact of test and treatment-related stroke
-
a model for the prognosis of patients with CAD (the EUROPA model)69
-
a model to assess the impact of imaging due to radiation on cancer morbidity and mortality. 61
The last of these five models, the YRM, was also used to assess the cost-effectiveness of the use of NGCCT to lower radiation exposure in patients with congenital heart disease.
The health economic analysis of the use of NGCCT in difficult-to-image patients with CAD showed that the use of NGCCT instead of invasive CA may be considered cost-effective. In patients with suspected CAD, the NGCCT-only strategy might be considered the most attractive. The ICER of NGCCT–ICA compared with NGCCT only is so high (£71,000) that it is unlikely to be considered cost-effective, given a conventional willingness-to-pay threshold of £20,000 to £30,000. In patients with known CAD, the most attractive strategy would be to perform a NGCCT with ICA; this scenario yields the highest cost saving and dominates ICA only. The ICER of NGCCT only compared with NGCCT–ICA is so high (£726,230) that it is unlikely to be considered cost-effective.
When taking uncertainty into account, these findings are confirmed. In the suspected population, in the range of thresholds of < £70,000, the NGCCT-only strategy has the highest probability of being cost-effective. For thresholds above £70,000, the three different strategies are more or less equivalent. For the patients with known CAD, the NGCCT–ICA strategy has the highest probability of being cost-effective, over the whole range of thresholds, whereas the ICA-only strategy has always the smallest probability of being cost-effective.
The key drivers behind these results are the percentage of patients being misclassified (as a results of test accuracy data and prevalence of disease) and the complication rate for ICA and revascularisation (see Table 55). In the ICA-only strategy, all patients are at risk for ICA-induced morbidity and mortality, whereas the TPs are also at risk for the revascularisation-induced morbidity and mortality. In the NGCCT-only strategy, misclassification leads to FPs who undergo unnecessary revascularisations with the associated complications, whereas ICA complications cannot occur. Overall, in the population of suspected CAD, the NGCCT-only strategy has the lowest overall mortality rate – less than half of that of ICA only. To some extent, the same results apply for the known CAD population; here the overall mortality and morbidity is lowest in the NGCCT–ICA strategy. ICA only has the highest overall mortality and morbidity rate, regardless of the population.
As noted previously, it is important to realise that the percentage of patients being misclassified is a function of both diagnostic accuracy and the prior likelihood. If the prior likelihood increases, the percentage of FNs also increases while the percentage of FPs decreases. This explains to some extent why the results for the suspected CAD population are slightly different than for the known CAD population, even though for both populations the same accuracy was assumed.
Currently, there is uncertainty about the estimate of the cost price of a NGCCT scan, as we had to make various assumptions. Therefore, we performed a scenario analysis changing this cost price to £207 per scan, and this did not alter our conclusions.
The disaggregated results in Tables 57 and 58 show that the inclusion of the reduced radiation effects has only very minimal impact on the outcomes.
The cost-effectiveness analysis of the use of NGCCT in congenital heart disease showed that, when only considering the radiation exposure, the use of NGCCT instead of 64-slice CT is not cost-effective in this group. The ICER ranged from £521,000 per QALY gained for the youngest patients to £90,000 per QALY gained for the adult patients. The reduction in radiation by replacing a single 64-slice CT scan by a NGCCT scan is small and leads to only a minor decrease in radiation-related cancer incidence, therefore it cannot justify the additional costs of the NGCCT scan.
Various scenarios were explored to assess the impact of the main assumptions. Only in the most unlikely scenario, i.e. an average radiation dose of 25 mSV for a 64-slice CT, do the ICERs decrease significantly. The fact that for all other scenarios the ICER remains > £30,000 indicates that, even with the uncertainty about the various assumptions in mind, it can reasonably be concluded that the use of NGCCT instead of 64-slice CT in order to reduce radiation exposure is not cost-effective in this patient group.
Chapter 5 Discussion
Statement of principal findings
Clinical effectiveness
All 24 studies (26 publications) included in the systematic review were diagnostic test accuracy studies that reported data on the performance of NGCCT in difficult-to-image patients with known or suspected CAD.
Where per-patient estimates of test accuracy were possible, these were generally high. The pooled estimates of sensitivity were 97.7% (95% CI 88.0% to 99.9%), 97.7% (95% CI 93.2% to 99.3%) and 96.0% (95% CI 88.8% to 99.2%), for patients with arrhythmias, patients with HHRs and patients with previous stent implantation(s), respectively. The corresponding pooled estimates of specificity were 81.7% (95% CI 71.6% to 89.4%), 86.3% (95% CI 80.2% to 90.7%) and 81.6% (95% CI 74.7% to 87.3%), respectively. The high per-patient estimates of sensitivity (> 95%) indicate that NGCCT could be used to reliably rule out significant stenosis and thus potentially avoid invasive investigations such as ICA in these patient groups. Furthermore, although there were no data specifically for beta-blocker-intolerant patients, it should be noted that no study reporting per-patient data for patients with HHRs used additional beta-blockers before imaging. Therefore, it may be inferred that NGCCT could reasonably be used to image patients who are intolerant to beta-blockers who could not otherwise be reliably imaged by 64-slice CT. With the exception of one small study, data on the accuracy of NGCCT in patients with high coronary calcium scores, previous bypass grafts, or obesity were limited to per-arterial segment or per-artery data. Sensitivity estimates remained high (> 90% in all but one study).
The majority of studies were judged to be at low risk of bias with respect to the reference standard domain of QUADAS-2; this reflects the specification, in the inclusion criteria of the review, of a single acceptable reference standard (ICA). Unclear ratings for this domain mainly reflected poor reporting of the interpretation of the reference standard and uncertainty as to whether or not those interpreting ICA were blinded to the index test results. The judgement of risk of bias with respect to patient selection was problematic and this is reflected in the high proportion of unclear ratings. The unclear rating frequently related to uncertainty surrounding the potential impact of inappropriate exclusions. Difficult-to-image patient groups were frequently reported as subgroups within larger studies, with those who had one or more additional criteria that may contribute further to difficulty in imaging being excluded from the study (e.g. a study reporting data for patients with HHR may have excluded patients with previous revascularisations). In addition, the numbers/proportion of patients excluded in this way were frequently not reported. Inclusion of multiple measurements per patient (per-arterial segment, per-artery or per-stent data) was a common problem in the index test domain. Where studies excluded non-diagnostic arterial segments from their analyses, the potential impact of these exclusions was frequently unclear because their distribution between patients was not reported.
No study reported data on changes to patient management or outcomes, test-related adverse events or patient preferences. No studies were identified of patients with congenital heart disease which met the inclusion criteria of the review.
Cost-effectiveness
The health economic analysis of the use of NGCCT in difficult-to-image patients with CAD showed that the use of NGCCT instead of invasive CA may be considered cost-effective. In patients with suspected CAD, the NGCCT-only strategy might be considered the most attractive. The ICER of NGCCT–ICA compared with NGCCT only is so high (£71,000) that it is unlikely to be considered cost-effective given a willingness-to-pay threshold of £20,000–30,000 per additional QALY. In patients with known CAD, the most attractive strategy would be to perform a NGCCT with ICA; this scenario yields the highest cost saving and dominates ICA only. The ICER of NGCCT only compared with NGCCT–ICA is so high (£726,230) that it is unlikely to be considered cost-effective. When taking uncertainty into account, these findings were confirmed. In the suspected population, in the range of thresholds of < £70,000, the NGCCT-only strategy has the highest probability of being cost-effective. For thresholds above £70,000, the three different strategies are more or less equivalent. For the patients with known CAD, the NGCCT–ICA strategy has the highest probability of being cost-effective over the whole range of thresholds, whereas the ICA-only strategy always has the smallest probability of being cost-effective.
The key drivers behind these results are the percentage of patients being misclassified (a function of both diagnostic accuracy and the prior likelihood) and the complication rate for ICA and revascularisation. Overall, in the population of suspected CAD, the NGCCT-only strategy has the lowest overall procedure-induced mortality rate, less than half that of ICA only. To some extent, the same results apply for the known CAD population; here the overall procedure-induced mortality and morbidity is lowest in the NGCCT–ICA strategy. ICA only has the highest overall procedure-induced mortality and morbidity rate. There is currently uncertainty about the estimate of the cost price of a NGCCT scan. Therefore, we performed a scenario analysis changing this cost price to £207 per scan, and this did not alter our conclusions.
The inclusion of the reduced radiation effects achievable using NGCCT compared with ICA has only very minimal impact on the outcomes.
The cost-effectiveness analysis of the use of NGCCT in congenital heart disease showed that, when only considering the radiation exposure, the use of NGCCT instead of 64-slice CT is not cost-effective in this group. The ICER ranged from £521,000 per QALY gained for the youngest patients to £90,000 per QALY gained for the adult patients. The reduction in radiation by replacing a single 64-slice CT scan by a NGCCT scan is small and leads to only a minor decrease in radiation-related cancer incidence, therefore it cannot justify the additional costs of the NGCCT scan.
Various scenarios were explored to assess the impact of the main assumptions. Only in the most unlikely scenario, i.e. an average radiation dose of 25 mSV for a 64-slice CT, do the ICERs decrease significantly. The fact that for all other scenarios the ICER remains > £30,000 indicates that, even with the uncertainty about the various assumptions in mind, it can reasonably be concluded that the use of NGCCT instead of 64-slice CT in order to reduce radiation exposure is not cost-effective in this patient group.
Strengths and limitations of assessment
Clinical effectiveness
Extensive literature searches were conducted in an attempt to maximise retrieval of relevant studies. These included electronic searches of a variety of bibliographic databases, as well as screening of clinical trials registers and conference abstracts to identify unpublished studies. Because of the known difficulties in identifying test accuracy studies using study design-related search terms,21 search strategies were developed to maximise sensitivity at the expense of reduced specificity. Thus, large numbers of citations were identified and screened, many of which did not meet the inclusion criteria of the review.
The possibility of publication bias remains a potential problem for all systematic reviews. Considerations may differ for systematic reviews of test accuracy studies. It is relatively simple to define a positive result for studies of treatment, for example a significant difference between the treatment and control groups, which favours treatment. This is not the case for test accuracy studies, which measure agreement between index test and reference standard. It would seem likely that studies finding greater agreement (high estimates of sensitivity and specificity) will be published more often. In addition, test accuracy data are often collected as part of routine clinical practice, or by retrospective review of records; test accuracy studies are not subject to the formal registration procedures applied to randomised controlled trials and are therefore more easily discarded when results appear unfavourable. The extent to which publication bias occurs in studies of test accuracy remains unclear; however, simulation studies have indicated that the effect of publication bias on meta-analytic estimates of test accuracy is minimal. 108 Formal assessment of publication bias in systematic reviews of test accuracy studies remains problematic and reliability is limited. 108 We did not undertake a statistical assessment of publication bias in this review. However, our search strategy included a variety of routes to identify unpublished studies and resulted in the inclusion of a number of conference abstracts, in which little documentation of study methodology and findings could be found.
Clear inclusion criteria were specified in the protocol for this review. Eligibility of studies for inclusion is therefore transparent. In addition, we have provided specific reasons for excluding any of the studies considered potentially relevant at initial citation screening (see Appendix 5). The review process followed recommended methods to minimise the potential for error and/or bias;18 studies were independently screened for inclusion by two reviewers and data extraction and quality assessment were undertaken by one reviewer and checked by a second. Any disagreements were resolved by consensus.
All studies included in the review were test accuracy studies. Methodological quality was therefore assessed using QUADAS-2. The QUADAS tool is recommended for assessing the methodological quality of test accuracy studies,18,20 and has been widely adopted by researchers and key organisations such as The Cochrane Collaboration, NICE in the UK, and the Institut für Qualität and Wirtschaftlichkeit im Gesundheitswesen (IQWiG) in Germany. It has been mentioned in more than 200 abstracts on the DARE database and has been cited more than 500 times. However, user experience and feedback have suggested potential improvements. A revised version of QUADAS (QUADAS-2) has recently been published. QUADAS-2 more closely resembles the approach and structure of the Cochrane risk of bias tool. It is structured into four key domains covering participant selection, index test, reference standard, and the flow of patients through the study (including timing of tests). 23 Each domain is rated for risk of bias (low, high or unclear) and the tool provides signalling questions, in each domain, to help reviewers in reaching a judgement. The participant selection, index test and reference standard domain are also separately rated for concerns regarding the applicability of the study to the review question (low, high or unclear). However, our assessment included only the risk of bias components of QUADAS-2, as it was considered that the inclusion criteria for this review were very specific to the review question and that questions of applicability were, therefore, not relevant. The review-specific guidance used in our QUADAS-2 assessment is reported in Appendix 2. We reported the results of our risk of bias assessment in full (see Appendix 3) and in summary in the results (see Chapter 3, Results). However, the usefulness of this assessment was limited by poor reporting of primary study methods.
There were a number of areas where problems caused by unclear reporting might be considered specific to this review. Because our assessment of test accuracy in patients with known or suspected CAD concerned only specific groups of patients who are known to be difficult to image using current (64-slice) CT technologies, the data included in our review were frequently derived from subgroup analysis reported as part of larger studies conducted in a general population of patients with CAD. One consequence of this was that patients with one or more additional criteria that might contribute further to difficulty in imaging were often excluded from these studies, for example a study of patients with suspected CAD that reported subgroup data for patients with HHRs might have excluded patients with previous revascularisations. In this scenario, judgement of the risk of bias is further complicated because, although the study may have reported the total number of patients excluded because of previous revascularisation, it is unlikely to have reported how many of these patients were in the HHR subgroup. It is therefore unclear what proportion of the relevant patient group (those with HHRs) have been inappropriately excluded. A further consideration in this review was the way in which data were reported, as many studies reported per-artery, per-stented lesion or per-segment data. These types of within-patient ‘clustered’ data are a common feature of test accuracy studies and are likely to result in a correlation between results within each patient, which should be accounted for in any statistical analyses. 109 Uncorrected estimates of sensitivity and specificity derived from such data are likely to be accurate, but imprecision will be underestimated. 109 The handling of non-diagnostic segments was also a particular issue for studies included in this review. The classification of non-diagnostic segments as positive for significant stenosis was adopted by many studies. If a patient is considered test positive when one or more segments with significant stenosis are identified, using this strategy will minimise the number of FN patients at the expense of increasing FPs. Thus, if NGCCT is being used to rule out patients from further invasive investigation, this strategy might reasonably be considered the most appropriate representation of how the test would be used in practice. However, it may result in overestimations of the sensitivity of NGCCT. By contrast, some studies in this review excluded non-diagnostic segments from their analyses. This approach is likely to produce inflated per-segment estimates of sensitivity and specificity and, if numbers of non-diagnostic segments or patients are not reported, ignores an important aspect of the practical utility of the test. For per-patient data, when a positive test is defined as one or more positive segments, exclusion of a non-diagnostic segment that is actually stenosed may result in misclassification of a positive patient as TN (if this is the only stenosed segment) or may have no effect (if multiple segments are stenosed).
Hierarchical or bivariate models are considered the optimal methods for estimating SROC curves. 18 Wherever possible, we have used the bivariate model24 to generate pooled estimates of sensitivity and specificity for each difficult-to-image patient group considered. This model analyses sensitivity and specificity jointly, retaining the paired nature of the original data, and has been shown to produce equivalent results to the hierarchical SROC model in the absence of other study-level covariates. 25 There were no data sets of sufficient size (minimum 10) to allow statistical exploration of sources of heterogeneity by including additional covariables in the SROC model. In cases where a bivariate model could not be fitted because the number of studies was small (four), 2 × 2 data contained one or more zero values, and between-study heterogeneity was low, pooled estimates of sensitivity and specificity, with 95% CIs, were calculated using a random-effects model. In view of the known problems with meta-analysis of likelihood ratios with a bivariate model,110 we have not included summary likelihood ratios and have instead adopted sensitivity and specificity as the primary outcomes for our review. 110
Assessments of the diagnostic accuracy of NGCCT are underpinned by the assumption that the reference standard (ICA), against which NGCCT is being evaluated, is 100% sensitive and 100% specific. ICA has some limitations in that it can only provide information about abnormalities that narrow the vessel lumen; it is limited in its ability to accurately define the aetiology of the obstruction or to detect the presence of early atherosclerotic disease. 11 When stenosis is present on ICA, pathological analyses almost always confirm findings, i.e. the assumption of 100% specificity is generally valid. However, the converse is not true; pathological studies have suggested that angiography underestimates the extent and severity of stenosis,111–115 and the assumption of 100% sensitivity is therefore weaker. Several factors contribute to this problem: ICA provides two-dimensional visualisation, whereas coronary lesions are often geometrically complex; an adaptive phenomenon known as coronary remodelling (an outward displacement of the external vessel wall to compensate for narrowing), which occurs in the early stages of disease and may conceal atheroma on ICA; and frequent absence of a normal reference segment (in the presence of diffuse reference segment disease, per cent stenosis will underestimate the true extent of vessel narrowing). 11 If the assumption of 100% sensitivity for ICA does not hold and FNs do occur, one possible consequence for accuracy studies that use ICA as the reference standard would be underestimation of the true specificity of the index test. This would occur if the index test is better able to detect early stage or other disease missed by ICA and the numbers of FP index test results are thus overestimated. However, despite its limitations, ACC/AHA guidelines state that coronary angiography remains the accepted reference standard for assessment of anatomical coronary disease. 11
The clinical applicability of accuracy data included in this review may have some limitations. NICE guidance on the assessment and diagnosis of recent onset chest pain or discomfort of suspected cardiac origin defines significant CAD on ICA as ≥ 70% diameter stenosis of at least one major epicardial artery segment or ≥ 50% diameter stenosis in the left main coronary artery. 63 By contrast, almost all of the studies included in this review considered the accuracy of NGCCT for the detection of significant CAD, which was defined as ≥ 50% diameter, regardless of the arteries assessed. However, the two studies that presented additional data for a threshold of > 75% diameter reduction59 or ≥ 70% diameter reduction38 both gave similar estimates of sensitivity and specificity for these thresholds and the 50% threshold.
The majority of included studies reported no information on funding; three40,47,51 reported funding from NGCCT manufacturers.
Cost-effectiveness
In this study, we brought together various existing models, which have already been validated through peer review, to inform the assessment of the cost-effectiveness of NGCCT in difficult-to-image patients with CAD. The advantage of combining five different models into one overall model is that the combined model is broad enough to describe as well as possible the whole range from diagnostics to clinical pathway to complications and radiation. A disadvantage is that some of the models were developed for other study populations. The existing models needed to be adjusted for the known and suspected CAD difficult-to-image subgroups which introduces additional uncertainty.
We included procedure-induced morbidity, as well as mortality, as this is an important aspect of ICA. Throughout the model, we have used evidence to inform parameters that was UK relevant and as up to date and high quality as possible. Where evidence was not available through published studies or databases, for example for population characteristics, we used the most likely and plausible ranges based on expert opinion.
We found that the main drivers of our cost-effectiveness results were accuracy, prior likelihood and the complication rate for ICA, PCI and CABG. The uncertainty around the accuracy estimates was not very large, given the reasonably large number of studies conducted. However, as noted above (see Model structure and methodology), some limitations apply to these estimates. The estimates of the prior likelihood that we used were not derived from any studies. For the suspected CAD group the estimate was based on the clinical guideline for chest pain of recent onset63 and for the known CAD group on the value assumed in the CE-MARC study. 64 For the suspected CAD group, the likelihood estimate is actually more an assumption than an estimate. According to the NICE clinical guideline (CG95)63 on the assessment and diagnosis of stable chest pain of recent onset, CT scans mainly play a part in the diagnostic path of patients with a prior likelihood of CAD of 10–29% and a non-zero calcium score. This likelihood is based on presence of certain clinical symptoms (suggestive of angina), and the risk factors age, gender, diabetes, smoking and hyperlipidaemia. For the likelihood estimate in the known CAD population, it is not entirely certain that the CE-MARC study and our study consider the exact same patient population. It is therefore possible that the actual prior likelihood in our known CAD population differs from that currently assumed in our model. Cost-effectiveness modelling for this assessment was based on patients with a prior likelihood of CAD of 10–29%, in accordance with the scope that was based on current NICE guidance. This guidance currently recommends ICA as the first-line investigation in patients where the estimated likelihood of CAD is 61–90% and functional imaging as the first-line investigation in patients where the estimated likelihood of CAD is 30–60%. Although the studies included in the systematic review component of this assessment rarely reported CAD risk factors separately for difficult-to-image patients (see Appendix 4), there was some indication that a significant proportion of these patients may be in the higher (30–90%) likelihood of CAD categories. With this consideration in mind and given the apparent accuracy of NGCCT in these populations, further modelling for higher prior likelihoods of CAD could be considered to inform future updates to NICE guidance.
Information on the final main driver, the complication rates, was derived from various sources. As the rate of MI resulting from a CABG was not available from data included in the literature review conducted for this assessment, we combined two studies identified for the purpose. 94,95 The overall complication rate (MI and stroke) taken from Serruys et al. 95 is based on a RCT. The authors presented only overall complication rates at 1 year of follow-up, and it seems likely that all of the reported events cannot fully be attributed to the procedure itself. Therefore, we used a 30-day complication rate based on the published survival curve, assuming that complications occurring in the first 30 days are induced by the procedure. An overestimation of the overall complication rate could have occurred. To estimate the MI rate, we subtracted the stroke rate reported by Tarakji et al. 94 from the overall complication rate presented in Serruys et al. 95 This method could have led to an inaccurate estimation of the MI rate for CABG. In contrast, the ICA-related mortality and morbidity were derived from an observational study in the UK, in which complications of diagnostic ICA were reported over a period of 10 years in 41 cardiac centres. 89 Thus, the reliability of the complication rates for ICA used in this model may be expected to be higher than for revascularisation. The British Cardiovascular Intervention Society (BCIS) was contacted to investigate the possibility of obtaining audit data on ICA-related complications; however, these data were not available at the time of our assessment. The PCI-induced morbidity reported in the BCIS database was approximately equal to that used in our model. The BCIS reported that PCI-related mortality was towards the lower limit of the CI applied in our sensitivity analyses. Given that our conclusions were unaltered if we took all uncertainty into account, using the BCIS data would not have changed these conclusions.
It was reassuring to see that the results were very similar across different subgroups of difficult-to-image patients. Had there been clear differences between the groups, questions would need to be answered in relation to implementation, i.e. do we recommend NGCCT for all difficult-to-image patients or only to a smaller subset. Furthermore, because the subgroup-specific outcomes were so similar, the impact of the relative weight of each subgroup, which was based on expert opinion, became small.
For the assessment of the cost-effectiveness of NGCCT in congenital heart disease, an important limitation is the fact that the current analysis considers only the effects of the lower radiation dose. However, we expect that inclusion of other factors, such as improved treatment planning, would have a limited impact on the current outcomes. An important reason for this is that it is likely that treatment (planning) be improved in only a fraction of patients, and in only a fraction of these would that lead on to improved health outcomes or reduction of costs.
Uncertainties
Clinical effectiveness
A major assumption underpinning this assessment is that the accuracy of NGCCT in the general population of patients with known or suspected CAD is equivalent to or better than that of 64-slice CT. The accuracy of 64-slice CT in the general population has been well established; recent systematic reviews have estimated the sensitivity and specificity of 64-slice CT, for the detection of ≥ 50% coronary artery stenosis, to be 92–99% and 89–92%, respectively. 3–5 It is therefore possible, although unlikely, that the use of NGCCT scanners would offer significant benefit over the use of a 64-slice CT scanner for most patients. There remains, however, the possibility that the radiation dose reduction protocols associated with NGCCT may negatively affect test accuracy. It was not part of the objectives of this review to systematically assess the accuracy of NGCCT in the general CAD population. However, a non-systematic sample of 10 studies which were excluded from the review at the full-paper-screening stage and which reported accuracy data in their abstracts indicated sensitivity and specificity estimates of 87–100% and 73–98%, respectively. 116–125
None of the categories of difficult-to-image patients considered in this review was evaluated in large numbers of studies; the maximum was eight studies for patients with HHRs. Data were particularly sparse for obese patients and patients with previous bypass graft(s). There were no data specifically for beta-blocker-intolerant patients. However, it should be noted that no study reporting per-patient data for patients with HHRs used additional beta-blockers before scanning. It may therefore be inferred from the performance of NGCCT in patients with HHRs that these technologies could reasonably be used to image patients who are intolerant to beta-blockers who could not otherwise be reliably imaged by 64-slice CT.
As noted above (see Strengths and limitations of assessment, Clinical effectiveness), the effect on test accuracy of multiple difficult-to-image criteria within patients remains uncertain. Only two studies included in this review52,58 reported data for patients with two distinct difficult-to-image criteria (HHR and previous revascularisation). Both of these studies reported sensitivity and specificity values > 90% and both excluded patients with arrhythmias.
In addition to test accuracy, an important consideration for the practical utility of NGCCT in difficult-to-image patient groups is the proportion of these patients in whom NGCCT imaging is non-diagnostic. Few of the studies in this assessment reported these data; where numbers of non-diagnostic images were reported, these were often for the whole study population, rather than the difficult-to-image subgroup. Three studies did report subgroup-specific non-diagnostic image rates in different populations; these were 5% for patients with arrhythmias,47 6.8% for patients with HHR39 and 9% for patients with previous stent implantation. 40 Although these studies indicate that the proportions of otherwise difficult-to-image patients who would remain ‘non-diagnostic’, even with the use of NGCCT, are likely to be low, further studies are needed to confirm this.
It should be further noted that although this review provides reasonable evidence on the accuracy of NGCCT in difficult-to-image patients groups, no studies were identified which reported the effects of scanning with NGCCT on patient management or outcomes in these patients. The ultimate aim of any research on clinical tests should be to determine impact upon patient management and outcome. These data are essential to fully inform both clinical decision-making and policy decision-making.
We were unable to identify any studies reporting data on the effects of NGCCT scanning on management and outcomes for patients with congenital heart disease. The potential impact of the introduction of NGCCT in this patient group therefore remains an unknown quantity. In practice, if NGCCT were to be introduced on the basis of evidence of its effectiveness and cost-effectiveness in difficult-to-image patients with known or suspected CAD, it is likely that these scanners would also be used opportunistically in patients with complex congenital heart disease.
This assessment treats the specified NGCCT scanners [Discovery CT750 HD (GE Healthcare), Brilliance iCT (Philips Healthcare), Somatom Definition Flash (Siemens Healthcare) and Aquilion ONE (Toshiba Medical Systems)] as equivalent technologies. However, it should be noted that 20 of the 24 studies included in the systematic review reported using Somatom Definition; three studies did not specify the instrument used,36–38 although the authors of one of these37 had used Somatom Definition in an earlier study, which was also included in this review. 39 One study reported using Aquilion ONE for the assessment of in-stent restenosis40 and found per-patient estimates of sensitivity and specificity of 100% (95% CI 71.5% to 100%) and 81.0% (95% CI 65.9% to 91.4%), consistent with the reported estimates for Somatom.
Cost-effectiveness
As noted above (see Uncertainties, Clinical effectiveness), we have assumed the accuracy of the various NGCCTs to be the same. In the health economic analysis, the same assumption has been made regarding radiation dosages and cost prices. Potential differences in any of these factors might lead to different conclusions for the various NGCCTs.
An important part of the CAD model, i.e. the EUROPA model, is based on risk equations that enabled the calculation of patient-specific transition probabilities. However, we applied the model to a cohort of ‘average’ patients, all with the average age, for a certain percentage male, for a certain percentage currently using calcium channel blockers, etc. This was done because the combination of five separate models used to model the current decision problem made patient-level simulation impossible. As a result, we removed one source of variation: the results that we found may well be different for certain subgroups of patients, such as younger or older patients.
An important factor in the final results in the CAD population is the percentage of patients misclassified. In the ICA strategy this percentage is ‘0’, whereas the NGCCT strategies both lead to patients incorrectly classified as negative. In the model it has been assumed that these patients will in time be correctly identified as positive. A key benefit of correct identification is the increased HRQoL of a TN compared with a FN during this period, as well as the marginally reduced risk of experiencing a CV event. Therefore, an accurate estimate of the time until correct identification is important, but will be difficult to obtain. Probably the best source of information at this time would be expert elicitation, but this has its own difficulties, as the cardiologists would need to be able to distinguish between those who were originally misidentified (i.e. true FN) and those who were originally correctly identified as not having CAD (TN) but who developed CAD in the interim.
Chapter 6 Conclusions
Implications for service provision
The results of our systematic review suggest that NGCCT may provide sufficiently accurate anatomical information for the diagnosis and assessment of CAD in some or all difficult-to-image patient groups. These technologies may be particularly useful in ruling out patients from further invasive investigations. However, data were sparse, particularly for obese patients, patients with high coronary calcium and those with previous bypass grafts.
The limited available data indicate that the proportions of otherwise difficult-to-image patients in whom imaging would remain ‘non-diagnostic’, even with the use of NGCCT, are likely to be low. However, further studies are needed to confirm this.
In a recent report it was stated that, in the next 3 years, half of the CT scanners and MRIs in the UK will need to be replaced. 86 Assuming that our cost price estimate for NGCCT is realistic, the results of the economic evaluation of new-generation cardiac CT suggest that it is likely to be considered cost-effective for difficult-to-image patients with CAD, at current levels of willingness to pay in the NHS. Although ICA can diagnose these patients with certainty, this comes at the cost of procedure-induced mortality and morbidity. Overall, taking uncertainty into account, we may conclude that strategies including NGCCT are cost saving while yielding approximately the same number of QALYs. Whether NGCCT should be used with or without ICA depends on the CAD population. However, it is important to remember that our results are valid only within the group of difficult-to-image patients with CAD; they are not be extrapolated to the whole population of patients with known or suspected CAD, as for these patients non-invasive 64-slice CT remains a good option.
Suggested research priorities
All studies included in our systematic review were test accuracy studies conducted in difficult-to-image patient groups with known or suspected CAD. The test accuracy study design compares the results of a new test (index test) with those of the reference standard (which are assumed always to be correct); it is therefore inherently not capable of comparing tests in terms of their ultimate impact on patient outcome. The studies included in this review compare NGCCT with the reference standard (ICA) purely in terms of its ability to detect a predefined level of stenosis (usually 50%). They do not provide any indication of the contribution of NGCCT to therapeutic decision-making or subsequent impact on patient outcomes. The ideal study to address these questions would be a large, multicentre RCT, in which patients are randomised to receive therapeutic planning and/or treatment based on different imaging strategies (e.g. NGCCT, ICA, or NGCCT and ICA); evaluation in more than one centre is preferred, in order to minimise performance bias. Recognising that the establishment of large-scale RCTs is particularly problematic in rapidly evolving fields such as vascular imaging, one possible compromise strategy might be to establish a multicentre tracker study. Such a study should enable the collection of data comparing numbers of misdiagnoses, clinical outcomes and HRQoL resulting from alternative imaging strategies. Such a study would also be the ideal set-up to provide a more robust assessment of the cost-effectiveness of the various diagnostic strategies.
In addition, test accuracy data were relatively sparse and further, high-quality accuracy studies, particularly for obese patients, patients with high coronary calcium and those with previous bypass grafts are needed to confirm the findings of our systematic review. Studies should include and fully report details of patients with more than one difficult-to-image criterion, so that the important issues of the potential cumulative impact on accuracy of multiple criteria can be fully assessed. Studies should also report the numbers of patients in whom NGCCT is non-diagnostic. QUADAS-2 assessment highlighted limitations in the reporting of many studies included in our review; future evaluations of NGCCT should follow the STARD guidelines for reporting test accuracy studies. 126,127
This assessment was unable to identify any studies that assessed changes to patient management/outcome (subsequent to NGCCT) in patients with complex congenital heart disease. If NGCCT is introduced on the basis of evidence in CAD patients and is opportunistically used in congenital heart disease patients, ‘before-and-after’ population studies might offer some insight into the impact of introducing NGCCT upon treatment decisions and/or outcomes for patients with complex conditions. When well designed, such studies might also inform the cost-effectiveness of NGCCT in this population.
In the clinical guideline Chest pain of recent onset,63 one of the recommendations was to establish a national registry for people who are undergoing initial assessment for stable angina. 63 It was mentioned that accurate assessment of the likelihood of coronary disease is needed to inform the cost-effective choice of investigative technologies. The data on which the estimated likelihood of CAD are currently based date from 1979 in a US population and may not be applicable to contemporary UK populations. We saw in our study that the prior likelihood of CAD is one of the main drivers of the cost-effectiveness results, and thus, such a registry could increase robustness of the health economic findings.
Acknowledgements
The authors acknowledge the clinical advice and expert opinion provided by Owen Miller, Consultant in Paediatric and Fetal Cardiology, Evelina Children's Hospital, Guy's and St Thomas' NHS Foundation Trust; Ruth Clarke, Trainee Consultant Radiographer, Mid Yorkshire NHS Trust; Francesca Pugliese, Senior Lecturer & Consultant Radiologist, Barts and the London NHS Trust; Ramesh De Silva, Consultant Interventional Cardiologist, Bedford Hospital NHS Trust; Carl Roobottom, Professor of Radiology and Consultant Radiologist, Plymouth Hospitals NHS Trust; Simon Padley, Consultant Radiologist, Royal Brompton and Harefield NHS Foundation trust; and Leo Hofstra, Professor of Cardiology, University Hospital Maastricht, Netherlands.
The authors would also like to acknowledge statistical advice provided by Roger Harbord, Medical Statistician, School of Social and Community Medicine, University of Bristol, and example NHS costs information for cardiac CT provided by Valerie Fone, Trust Imaging Services Manager, Royal Brompton and Harefield NHS Foundation Trust.
Thanks also to Claire McKenna, Simon Walker and Mark Sculpher, Centre for Health Economics, University of York, UK, for making the CE-MARC, EUROPA and York Radiation Models available.
Contribution of authors
Marie Westwood and Heike Raatz planned and performed the systematic review and interpretation of evidence.
Maiwenn Al, Laura Burgers, Ken Redekop and Stefan Lhachimi planned and performed the cost-effectiveness analyses and interpreted results.
Nigel Armstrong contributed to planning and interpretation of cost-effectiveness analysis and acquisition of input data for modelling.
Kate Misso devised and performed the literature searches and provided information support to the project.
Jos Kleijnen and Hans Severens provided senior advice and support to the systematic review and cost-effectiveness analyses, respectively.
All parties were involved in drafting and/or commenting on the report.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- UK coronary heart disease statistics 2009–10. London: BHF; 2010.
- Cooper A, Calvert N, Skinner J, Sawyer L, Sparrow K, Timmis A, et al. Chest pain of recent onset: assessment and diagnosis of recent onset chest pain or discomfort of suspected cardiac origin. London: National Clinical Guideline Centre for Acute and Chronic Conditions; 2010.
- Mowatt G, Cummins E, Waugh N, Walker S, Cook J, Jia X, et al. Systematic review of the clinical effectiveness and cost-effectiveness of 64-slice or higher computed tomography angiography as an alternative to invasive coronary angiography in the investigation of coronary artery disease. Health Technol Assess 2008;12.
- Schuetz GM, Zacharopoulou NM, Schlattmann P, Dewey M. Meta-analysis: noninvasive coronary angiography using computed tomography versus magnetic resonance imaging. Ann Intern Med 2010;152:167-77.
- Khan R, Rawal S, Eisenberg MJ. Transitioning from 16-slice to 64-slice multidetector computed tomography for the assessment of coronary artery disease: are we really making progress?. Can J Cardiol 2009;25:533-42.
- British Heart Foundation . Incidence of Congenital Heart Disease 2005. www.heartstats.org/datapage.asp?id=3395 (accessed 12 January 2011).
- Cheng Z, Wang X, Duan Y, Wu L, Wu D, Chao B, et al. Low-dose prospective ECG-triggering dual-source CT angiography in infants and children with complex congenital heart disease: first experience. Eur Radiol 2010;20:2503-11.
- Hope SA, Crossett M, Nasis A, Seneviratne S. Early experience with Aquilion One 320 slice computed tomography scanner in paediatric and congenital heart disease n.d.
- Market review: advanced CT scanners for coronary angiography. CEP10043. London: Centre for Evidence-based Purchasing; 2010.
- Noto TJ, Johnson LW, Krone R, Weaver WF, Clark DA, Kramer JR, et al. Cardiac catheterization 1990: a report of the Registry of the Society for Cardiac Angiography and Interventions (SCA&I). Cathet Cardiovasc Diagn 1991;24:75-83.
- Scanlon PJ, Faxon DP, Audet AM, Carabello B, Dehmer GJ, Eagle KA, et al. ACC/AHA guidelines for coronary angiography. A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on Coronary Angiography). Developed in collaboration with the Society for Cardiac Angiography and Interventions. J Am Coll Cardiol 1999;33:1756-824.
- Management of stable angina. London: NICE; 2011.
- Ischaemic heart disease: coronary artery stents. London: NICE; 2003.
- Drug-eluting stents for the treatment of coronary artery disease. London: NICE; 2008.
- Congenital heart disease. 2009.
- Tidy C. Congenital heart disease in children. Leeds: Patient UK; 2011.
- Baumgartner H, Bonhoeffer P, De Groot NM, de Haan F, Deanfield JE, Galie N, et al. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J 2010;31:2915-57.
- Systematic reviews: CRD's guidance for undertaking reviews in health care. York: University of York; 2009.
- Diagnostics Assessment Programme: interim methods statement (version 2). London: NICE; 2010.
- Cochrane Diagnostic Test Accuracy Working Group . Cochrane Collaboration. Handbook for DTA Reviews 2011. http://srdta.cochrane.org/handbook-dta-reviews (accessed 12 January 2011).
- Whiting P, Westwood M, Beynon R, Burke M, Sterne JA, Glanville J. Inclusion of methodological filters in searches for diagnostic test accuracy studies misses relevant studies. J Clin Epidemiol 2011;64:602-7.
- Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3.
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36.
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005;58:982-90.
- Harbord RM, Deeks JJ, Egger M, Whiting P, Sterne JA. Unification of models for meta-analysis of diagnostic accuracy studies. Biostatistics 2006;1:1-21.
- Harbord RM, Whiting P. Metandi: meta-analysis of diagnostic accuracy using hierarchical logistic regression. Stata J 2009;9:211-29.
- Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol 2006;6.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58.
- Paul N, Nguyen E, Doyle D, Crean A, Bret P. Prospective ECG gated low dose CT angiography with 320-MDCT. Presented at 2nd World Congress of Thoracic Imaging and Diagnosis in Chest Disease, Valencia, Spain, 30 May to 2 June 2009. J Thorac Imaging 2009;24.
- Sun ML. Diagnostic accuracy of dual-source CT coronary angiography with prospective ECG triggering on different heart rate patients. Cardiology 2010;117:114-15.
- Leschka S, Scheffel H, Husmann L, Wildermuth S, Marincek B, Kaufmann PA, et al. Influence of body-mass-index, calcium-score and heart heart rate on the accuracy of DSCT-coronary angiography. Dtsch Med Wochenschr 2007;132.
- Stinn B, Leschka S, Schmid F, Thurnheer M, Schultes B, Wildermuth S. CT-coronary angiography: Improvement of evaluation quality of coronary artery for morbid obesity with the new Dual-Source CT in comparison to 64-lines CT. Dtsch Med Wochenschr 2007;132.
- Kepka C, Opolski MP, Kruk M, Pregowski J, Michalowska I, Demkow M, et al. Dual-source computed tomography angiography in patients after bypass grafting: comparison with invasive coronary angiography. Postep Kardiol Inter 2010;6:12-20.
- Pflederer T, Seltmann M, Marwan M, Ropers D, Daniel WG, Achenbach S. Detection of coronary artery stenoses in patients with previous coronary revascularization by 128-slice dual source computed tomography. (Abstract 953). Presented at European Society of Cardiology, ESC Congress, Stockholm, Sweden, 28 August to 1 September 2010. Eur Heart J 2010;31.
- Rixe J, Rolf A, Erkapic D, Koch A, Moellmann H, Nef H, et al. Usefulness of dual-source computed tomography for the detection of coronary artery disease in patients with arrhythmia: comparison to invasive angiography. (Abstract P1364). Eur Heart J Suppl 2009;30.
- Van Mieghem CA, Weustink A, Meijboom B, Pugliese F, Serruys PW, de Feyter PJ. Abstract 2328: clinical value of ct coronary angiography in symptomatic patients after percutaneous coronary intervention. Circulation 2007;116.
- Ropers D, Ropers U, Pflederer T, Bachmann S, Daniel WG, Achenbach S. Accuracy of dual source CT coronary angiography in patients after coronary bypass surgery: comparison to invasive angiography in 78 patients. J Am Coll Cardiol 2008;51.
- LaBounty T, Leipsic J, Mancini GBJ, Heilbron B, Patel S, Kazerooni E, et al. A prospective multicenter study evaluating the diagnostic accuracy of high-definition coronary computed tomographic angiography: an intent-to-diagnose analysis. Presented at American College of Cardiology's 59th Annual Scientific Session and i2 Summit: Innovation in Intervention, 2010 14–16 Mar; Atlanta, United States. J Am Coll Cardiol 2010;55.
- Ropers U, Ropers D, Pflederer T, Anders K, Kuettner A, Stilianakis NI, et al. Influence of heart rate on the diagnostic accuracy of dual-source computed tomography coronary angiography. J Am Coll Cardiol 2007;50:2393-8.
- de Graaf FR, Schuijf JD, van Velzen JE, Boogers MJ, Kroft LJ, de Roos A, et al. Diagnostic accuracy of 320-row multidetector computed tomography coronary angiography to noninvasively assess in-stent restenosis. Invest Radiol 2010;45:331-40.
- Alkadhi H, Scheffel H, Desbiolles L, Gaemperli O, Stolzmann P, Plass A, et al. Dual-source computed tomography coronary angiography: influence of obesity, calcium load, and heart rate on diagnostic accuracy. Eur Heart J 2008;29:766-76.
- Brodoefel H, Tsiflikas I, Burgstahler C, Reimann A, Thomas C, Schroeder S, et al. Cardiac dual-source computed tomography: effect of body mass index on image quality and diagnostic accuracy. Invest Radiol 2008;43:712-18.
- Leber AW, Johnson T, Becker A, von Ziegler F, Tittus J, Nikolaou K, et al. Diagnostic accuracy of dual-source multi-slice CT-coronary angiography in patients with an intermediate pretest likelihood for coronary artery disease. Eur Heart J 2007;28:2354-60.
- Lin CJ, Hsu JC, Lai Y, Wang KL, Lee JY, Li AH, et al. Diagnostic accuracy of dual-source CT coronary angiography in a population unselected for degree of coronary artery calcification and without heart rate modification. Clin Radiol 2010;65:109-17.
- Weustink AC, Neefjes LA, Kyrzopoulos S, Van Straten M, Eu RN, Meijboom WB, et al. Impact of heart rate frequency and variability on radiation exposure, image quality, and diagnostic performance in dual-source spiral CT coronary angiography. Radiology 2009;253:672-80.
- Brodoefel H, Burgstahler C, Tsiflikas I, Reimann A, Schroeder S, Claussen CD, et al. Dual-source CT: effect of heart rate, heart rate variability, and calcification on image quality and diagnostic accuracy. Radiology 2008;247:346-55.
- Marwan M, Pflederer T, Schepis T, Lang A, Muschiol G, Ropers D, et al. Accuracy of dual-source computed tomography to identify significant coronary artery disease in patients with atrial fibrillation: comparison with coronary angiography. Eur Heart J 2010;31:2230-7.
- Meng L, Cui L, Cheng Y, Wu X, Tang Y, Wang Y, et al. Effect of heart rate and coronary calcification on the diagnostic accuracy of the dual-source CT coronary angiography in patients with suspected coronary artery disease. Korean J Radiol 2009;10:347-54.
- Oncel D, Oncel G, Tastan A. Effectiveness of dual-source CT coronary angiography for the evaluation of coronary artery disease in patients with atrial fibrillation: initial experience. Radiology 2007;245:703-11.
- Oncel D, Oncel G, Tastan A, Tamci B. Evaluation of coronary stent patency and in-stent restenosis with dual-source CT coronary angiography without heart rate control. AJR Am J Roentgenol 2008;191:56-63.
- Pflederer T, Marwan M, Renz A, Bachmann S, Ropers D, Kuettner A, et al. Noninvasive assessment of coronary in-stent restenosis by dual-source computed tomography. Am J Cardiol 2009;103:812-17.
- Pugliese F, Weustink AC, Van Mieghem C, Alberghina F, Otsuka M, Meijboom WB, et al. Dual source coronary computed tomography angiography for detecting in-stent restenosis. Heart 2008;94:848-54.
- Pugliese F, Alberghina F, Weustink AC, Otsuka M, Mollet NR, de Feyter PJ, et al. Dual-source computed tomography angiography for the assessment of in-stent restenosis in coronary arteries. AJR Am J Roentgenol 2007;188.
- Rist C, Johnson TR, Mueller-Starck J, Arnoldi E, Saam T, Becker A, et al. Noninvasive coronary angiography using dual-source computed tomography in patients with atrial fibrillation. Invest Radiol 2009;44:159-67.
- Scheffel H, Alkadhi H, Plass A, Vachenauer R, Desbiolles L, Gaemperli O, et al. Accuracy of dual-source CT coronary angiography: first experience in a high pre-test probability population without heart rate control. Eur Radiol 2006;16:2739-47.
- Tsiflikas I, Drosch T, Brodoefel H, Thomas C, Reimann A, Till A, et al. Diagnostic accuracy and image quality of cardiac dual-source computed tomography in patients with arrhythmia. Int J Cardiol 2010;143:79-85.
- Drosch T, Reimann A, Brodoefel H, Tsiflikas I, Thomas C, Heuschmid M, et al. Diagnostic accuracy and image quality of cardiac dual-source computed tomography in patients with arrhythmia. Circulation 2008;118.
- Weustink AC, Nieman K, Pugliese F, Mollet NR, Meijboom BW, van Mieghem C, et al. Diagnostic accuracy of computed tomography angiography in patients after bypass grafting. Comparison with invasive coronary angiography. JACC Cardiovasc Imaging 2009;2:816-24.
- Zhang LJ, Wu SY, Wang J, Lu Y, Zhang ZL, Jiang SS, et al. Diagnostic accuracy of dual-source CT coronary angiography: the effect of average heart rate, heart rate variability, and calcium score in a clinical perspective. Acta Radiol 2010;51:727-40.
- de Jonge GJ, van Ooijen PMA, Piers LH, Dikkers R, Tio RA, Willems TP, et al. Visualization of anomalous coronary arteries on dual-source computed tomography. Eur Radiol 2008;18:2425-32.
- McKenna C, Wade R, Faria R, Yang H, Stirk L, Gummerson N, et al. EOS 2D 3D X-Ray Imaging System: A Diagnostics Assessment Report 2011. http://guidance.nice.org.uk/DT/1/AssessmentReport/pdf/English (accessed 1 June 2011).
- Greenwood JP, Maredia N, Radjenovic A, Brown JM, Nixon J, Farrin AJ, et al. Clinical evaluation of magnetic resonance imaging in coronary heart disease: the CE-MARC study. Trials 2009;10.
- Chest pain of recent onset: assessment and diagnosis of recent onset chest pain or discomfort of suspected cardiac origin. London: NICE; 2010.
- Walker S. CE-MARC cost-effectiveness analysis model. York: Centre for Health Economics, University of York; 2011.
- Review of the Registrar General on deaths by cause, sex, and age, in England and Wales. London: HMSO; 2006.
- Sandercock P, Berge E, Dennis M, Forbes J, Hand P, Kwan J, et al. Cost-effectiveness of thrombolysis with recombinant tissue plasminogen activator for acute ischemic stroke assessed by a model based on UKNHS costs. Stroke 2004;35:1490-7.
- Stroke: diagnosis and initial management of acute stroke and transient ischaemic attack (TIA). London: NICE; 2008.
- Fox KM, Bertrand M, Ferrari R, Remme WJ, Simoons ML, Remme WJ, et al. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study). Lancet 2003;362:782-8.
- Briggs A, Mihaylova B, Sculpher M, Hall A, Wolstenholme J, Simoons M, et al. Cost effectiveness of perindopril in reducing cardiovascular events in patients with stable coronary artery disease using data from the EUROPA study. Heart 2007;93:1081-6.
- Daly CA, Clemens F, Sendon JLL, Tavazzi L, Boersma E, Danchin N, et al. The initial management of stable angina in Europe, from the Euro Heart Survey: a description of pharmacological management and revascularization strategies initiated within the first month of presentation to a cardiologist in the Euro Heart Survey of Stable Angina. Eur Heart J 2005;26:1011-22.
- Management of stable angina. London: NICE; 2010.
- Deckers JW, Goedhart DM, Boersma E, Briggs A, Bertrand M, Ferrari R, et al. Treatment benefit by perindopril in patients with stable coronary artery disease at different levels of risk. Eur Heart J 2006;27:796-801.
- Joosen I, Versteylen M, Laufer E, Winkens M, Narula J, Hofstra L. The use of Framingham risk score, coronary calcium score and coronary computed tomographic angiography in patients with stable chest pain. Maastricht: Maastricht University Medical Center; 2011.
- Shemesh J, Stroh CI, Tenenbaum A, Hod H, Boyko V, Fisman EZ, et al. Comparison of coronary calcium in stable angina pectoris and in first acute myocardial infarction utilizing double helical computerized tomography. Am J Cardiol 1998;81:271-5.
- Hippisley-Cox J, Coupland C, Vinogradova Y, Robson J, Minhas R, Sheikh A, et al. Predicting cardiovascular risk in England and Wales: prospective derivation and validation of QRISK2. BMJ 2008;336:1475-82.
- Banasiak W, Pociupany R, Wilkins A, Ponikowski P. Characteristics of patients with coronary artery disease managed on an outpatient basis in the population of Poland. Results of the multicentre RECENT trial. Kardiol Pol 2007;65:132-42.
- Hall EJ, Brenner DJ. Cancer risks from diagnostic radiology. Br J Radiol 2008;81:362-78.
- Brenner D, Elliston C, Hall E, Berdon W. Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol 2001;176:289-96.
- Wall B, Hillier M, Haylock R, Hart D, Jansen J, Shrimpton P. Radiation Risks from Medical X-Ray Examinations As a Function of the Age and Sex of the Patient 2011.
- International Commission on Radiological Protection (ICRP) . The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann ICRP 2007;37:1-332.
- Shrimpton P, Hillier M, Lewis M, Dunn M. Doses from computed tomography (CT) examinations in the UK: 2003 review. Didcot: National Radiological Protection Board; 2005.
- United Nations Scientific Committee on the Effects of Atomic Radiation . Sources and Effects of Ionizing Radiation 2010. www.unscear.org/unscear/en/publications/2008_1.html (accessed 16 May 2011).
- Linet MS, Kim KP, Rajaraman P. Children's exposure to diagnostic medical radiation and cancer risk: epidemiologic and dosimetric considerations. Pediatr Radiol 2009;39:4-26.
- NHS Reference costs 2010/11. London: Department of Health; 2010.
- Buyer's guide: multi-slice CT scanners. CEP08007. London: Centre for Evidence-Based Purchasing; 2009.
- Managing high value capital equipment in the NHS in England. London: The Stationery Office; 2011.
- Unit Costs of Health and Social Care. Canterbury: University of Kent; 2010.
- Hospital Episode Statistics: healthcare resource groups – HRG version 3 [Excel spreadsheet]. London: Health and Social Care Information Centre; 2010.
- West R, Ellis G, Brooks N, Joint Audit Comm British Cardiac S. Complications of diagnostic cardiac catheterisation: results from a confidential inquiry into cardiac catheter complications. Heart 2006;92:810-14.
- Batyraliev T, Ayalp MR, Sercelik A, Karben Z, Dinler G, Besnili F, et al. Complications of cardiac catheterization: a single-center study. Angiology 2005;56:75-80.
- Chandrasekar B, Doucet S, Bilodeau L, Crepeau J, deGuise P, Gregoire J, et al. Complications of cardiac catheterization in the current era: a single-center experience. Catheter Cardiovasc Interv 2001;52:289-95.
- Smith LDR, Spyer G, Dean JW. Audit of cardiac catheterisation in a district general hospital: implications for training. Heart 1999;81:461-4.
- Bridgewater B, Grayson AD, Brooks N, Grotte G, Fabri BM, Au J, et al. Has the publication of cardiac surgery outcome data been associated with changes in practice in northwest England: an analysis of 25,730 patients undergoing CABG surgery under 30 surgeons over eight years. Heart 2007;93:744-8.
- Tarakji KG, Sabik JF, 3rd, Bhudia SK, Batizy LH, Blackstone EH. Temporal onset, risk factors, and outcomes associated with stroke after coronary artery bypass grafting. JAMA 2011;305:381-90.
- Serruys PW, Unger F, Sousa JE, Jatene A, Bonnier HJRM, Schonberger JPAM, et al. Comparison of coronary-artery bypass surgery and stenting for the treatment of multivessel disease. N Engl J Med 2001;344:1117-24.
- Rajani R, Lindblom M, Dixon G, Khawaja M, Hildick-Smith D, Holmberg S, et al. Evolving trends in percutaneous coronary intervention. Br J Cardiol 2011;18:73-6.
- Namasivayam S, Kalra MK, Torres WE, Small WC. Adverse reactions to intravenous iodinated contrast media: a primer for radiologists. Emerg Radiol 2006;12:210-15.
- Katzberg RW, Newhouse JH. Intravenous contrast medium-induced nephrotoxicity: is the medical risk really as great as we have come to believe?. Radiology 2010;256:21-8.
- Stacul F, van der Molen AJ, Reimer P, Webb JA, Thomsen HS, Morcos SK, et al. Contrast Media Safety Committee of European Society of Urogenital Radiology (ESUR). Contrast induced nephropathy: updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol 2011;21:2527-41.
- Kind P, Hardman G, Macran S. UK population norms for EQ-5D. York: Centre for Health Economics; 1999.
- Guide to the methods of technology appraisal. London: NICE; 2008.
- Campeau L. Letter: Grading of angina pectoris. Circulation 1976;54:522-3.
- Longworth L, Buxton MJ, Sculpher M, Smith DH. Estimating utility data from clinical indicators for patients with stable angina. Eur J Health Econ 2005;6:347-53.
- Sullivan PW, Ghushchyan V. Preference-based EQ-5D index scores for chronic conditions in the United States. Med Decis Making 2006;26:410-20.
- Hankey GJ, Jamrozik K, Broadhurst RJ, Forbes S, Burvill PW, Anderson CS, et al. Five-year survival after first-ever stroke and related prognostic factors in the Perth Community Stroke Study. Stroke 2000;31:2080-6.
- Oreopoulos A, Padwal R, McAlister FA, Ezekowitz J, Sharma AM, Kalantar-Zadeh K, et al. Association between obesity and health-related quality of life in patients with coronary artery disease. Int J Obes 2010;34:1434-41.
- Health risks from exposure to low levels of ionizing radiation: BEIR VII Phase 2. Washington, DC: National Academies Press; 2006.
- Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 2005;58:882-93.
- Zhou X, McClish D, Obuchowski NA. Statistical methods in diagnostic medicine. New York, NY: Wiley Interscience; 2002.
- Zwinderman AH, Bossuyt PM. We should not pool diagnostic likelihood ratios in systematic reviews. Stat Med 2008;27:687-97.
- Blankenhorn DH, Curry PJ. The accuracy of arteriography and ultrasound imaging for atherosclerosis measurement. A review. Arch Pathol Lab Med 1982;106:483-9.
- Arnett EN, Isner JM, Redwood DR, Kent KM, Baker WP, Ackerstein H, et al. Coronary artery narrowing in coronary heart disease: comparison of cineangiographic and necropsy findings. Ann Intern Med 1979;91:350-6.
- Roberts WC, Jones AA. Quantitation of coronary arterial narrowing at necropsy in sudden coronary death: analysis of 31 patients and comparison with 25 control subjects. Am J Cardiol 1979;44.
- Grondin CM, Dyrda I, Pasternac A, Campeau L, Bourassa MG, Lesperance J. Discrepancies between cineangiographic and postmortem findings in patients with coronary artery disease and recent myocardial revascularization. Circulation 1974;49:703-8.
- Vlodaver Z, Frech R, Van Tassel RA, Edwards JE. Correlation of the antemortem coronary arteriogram and the postmortem specimen. Circulation 1973;47:162-9.
- Arnoldi E, Ramos-Duran L, Abro JA, Zwerner PL, Nikolaou K, Reiser MF, et al. Coronary CT angiography using prospective ECG triggering. Radiologe 2010;50:500-6.
- Burgstahler C, Reimann A, Drosch T, Heuschmid M, Brodoefel H, Tsiflikas I, et al. Cardiac dual-source computed tomography in patients with severe coronary calcifications and a high prevalence of coronary artery disease. J Cardiovasc Comput Tomogr 2007;1:143-51.
- Chen BX, Ma FY, Wen ZY, Luo W, Zhao XZ, Kang F, et al. Diagnostic value of 128-slice CT coronary angiography in comparison with invasive coronary angiography. Zhonghua Xin Xue Guan Bing Za Zhi 2008;36:223-8.
- Chen HW, Fang XM, Hu XY, Bao J, Hu CH, Chen Y, et al. Efficacy of dual-source CT coronary angiography in evaluating coronary stenosis: initial experience. Clin Imaging 2010;34:165-71.
- Chen S-Y, Su Y-S, Xie P-Y, Xu S-l, Fang Y-Q, Huang A-R. Clinical value of dual-source CT in evaluating coronary artery disease. Nan Fang Yi Ke Da Xue Xue Bao 2010;30:2125-7.
- Fang XM, Chen HW, Hu XY, Bao J, Chen Y, Yang ZY, et al. Dual-source CT coronary angiography without heart rate or rhythm control in comparison with conventional coronary angiography. Int J Cardiovasc Imaging 2010;26:323-31.
- Herzog BA, Husmann L, Burkhard N, Gaemperli O, Valenta I, Tatsugami F, et al. Accuracy of low-dose computed tomography coronary angiography using prospective electrocardiogram-triggering: first clinical experience. Eur Heart J 2008;29:3037-42.
- Heuschmid M, Burgstahler C, Reimann A, Brodoefel H, Mysal I, Haeberle E, et al. Usefulness of noninvasive cardiac imaging using dual-source computed tomography in an unselected population with high prevalence of coronary artery disease. Am J Cardiol 2007;100:587-92.
- Johnson TR, Nikolaou K, Busch S, Leber AW, Becker A, Wintersperger BJ, et al. Diagnostic accuracy of dual-source computed tomography in the diagnosis of coronary artery disease. Invest Radiol 2007;42:684-91.
- Nance JW, Jr, Bastarrika G, Kang DK, Ruzsics B, Vogt S, Schmidt B, et al. High-temporal resolution dual-energy computed tomography of the heart using a novel hybrid image reconstruction algorithm: initial experience. J Comput Assist Tomogr 2011;35:119-25.
- Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. BMJ 2003;326:41-4.
- Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Ann Intern Med 2003;138.
- Morise AP, Haddad WJ, Beckner D. Development and validation of a clinical score to estimate the probability of coronary artery disease in men and women presenting with suspected coronary disease. Am J Med 1997;102:350-6.
Appendix 1 Literature search strategies
Appendix 2 Study-specific guide to completion of QUADAS-2
Study-specific guide to completion of QUADAS-2 (PDF download)
Appendix 3 Quality assessment: QUADAS-2 results
Appendix 4 Data extraction tables
Appendix 5 List of excluded studies with rationale
Appendix 6 The National Institute for Health and Clinical Excellence guidance relevant to treatment of congenital heart disease in childhood
The National Institute for Health and Clinical Excellence guidance relevant to treatment of congenital heart disease in childhood (PDF download)
Appendix 7 Details of input provided by experts
Appendix 8 Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist
Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist (PDF download)
Appendix 9 Protocol
Glossary
- Acute chest pain
- Chest pain/discomfort which has occurred recently and may still be present, is of suspected cardiac origin and may be due to acute myocardial infarction or unstable angina.
- Calcium scoring
- A technique by which the extent of calcification in the coronary arteries is measured and scored. This does not necessarily reflect the degree of stenosis.
- Congenital heart defect
- A defect in the structure of the heart and great vessels that is present at birth.
- Coronary angiography
- An invasive diagnostic test that provides anatomical information about the degree of stenosis (narrowing) in a coronary artery. It involves manipulation of cardiac catheters from an artery in the arm or top of the leg. A contrast medium is injected into the coronary arteries, and the flow of contrast in the artery is monitored by taking a rapid series of radiographs. It is considered the reference standard for providing anatomical information and defining the site and severity of coronary artery lesions.
- Coronary artery
- An artery that supplies the myocardium.
- Coronary artery disease
- A condition in which atheromatous plaque builds up inside the coronary artery, leading to narrowing of the arteries, which may be sufficient to restrict blood flow and cause myocardial ischaemia.
- Cost-effectiveness analysis
- An economic analysis that converts effects into health terms and describes the costs for additional health gain.
- Decision modelling
- A theoretical construct that allows the comparison of the relationship between costs and outcomes of alternative health-care interventions.
- False-negative
- Incorrect negative test result – number of diseased persons with a negative test result.
- False-positive
- Incorrect positive test result – number of non-diseased persons with a positive test result.
- Gantry
- Found in computed tomography machines, a gantry rotates around a patient for cross-sectional views.
- Incremental cost-effectiveness ratio
- The difference in the mean costs of two interventions in the population of interest divided by the difference in the mean outcomes in the population of interest.
- Index test
- The test of which performance is being evaluated.
- Major aortopulmonary collateral arteries
- Arteries that develop to supply blood to the lungs when native pulmonary circulation is underdeveloped. Instead of coming from the pulmonary trunk, blood supply usually develops from the aorta and other systemic arteries.
- Markov model
- An analytic method particularly suited to modelling repeated events, or the progression of a chronic disease over time.
- Material separation
- The contrast resolution of the image between the iodine agent and the soft tissues. Improved material separation enables a lower dose of contrast agent to be used.
- Meta-analysis
- Statistical techniques used to combine the results of two or more studies and obtain a combined estimate of effect.
- Meta-regression
- Statistical technique used to explore the relationship between study characteristics and study results.
- Multislice computed tomography coronary angiography
- A non-invasive investigation that provides coronary calcium scoring and anatomical information about the degree of stenosis (narrowing) in the coronary arteries. The scanner has a special X-ray tube and rotation speed, and as the technology has advanced the number of slices in each rotation has increased. A dual-source scanner has two pairs of X-ray sources and multislice detectors mounted at 90° to each other.
- Myocardial perfusion scintigraphy with single-photon emission computed tomography
- Myocardial perfusion scintigraphy involves injecting small amounts of radioactive tracer to evaluate perfusion of the myocardium via the coronary arteries at stress and at rest. The distribution of the radioactive tracer is imaged using a gamma camera. In single-photon emission computed tomography (SPECT) the camera rotates round the patient and the raw data processed to obtain tomographic images of the myocardium. Cardiovascular stress may be induced by either pharmacological agents or exercise.
- Opportunity costs
- The cost of forgone outcomes that could have been achieved through alternative investments.
- Patent ductus arteriosus
- The ductus arteriosus is a duct or passage in the heart that is meant to close shortly after birth. In cases of patent ductus arteriosus, the duct fails to completely close, which means that some oxygen-rich blood leaks through the duct, into the pulmonary valve and into the lungs.
- Publication bias
- Bias arising from the preferential publication of studies with statistically significant results.
- Pulmonary artery sling
- A rare condition in which the left pulmonary artery anomalously originates from a normally positioned right pulmonary artery.
- Quality of life
- An individual's emotional, social and physical well-being, and his or her ability to perform the ordinary tasks of living.
- Quality-adjusted life-year
- A measure of health gain, used in economic evaluations, in which survival duration is weighted or adjusted by the patient's quality of life during the survival period.
- Receiver operating characteristic curve
- A graph that illustrates the trade-offs between sensitivity and specificity, which result from varying the diagnostic threshold.
- Reference standard
- The best currently available diagnostic test(s), against which the index test is compared.
- Scimitar syndrome
- A rare congenital heart defect characterised by anomalous venous return (partial or total) from the right lung. The name scimitar syndrome refers to the curvilinear pattern, seen on a chest radiograph, of the pulmonary veins that drain into the inferior vena cava.
- Sensitivity
- Proportion of people with the target disorder who have a positive test result.
- Septal defects (atrial or ventricular)
- A group of common congenital anomalies consisting of a hole in the septum (the wall) between the chambers of the heart. The hole may be between the left and right atria or the left and right ventricles. The result is that the blood cannot circulate as it should and the heart has to compensate by working harder.
- Specificity
- Proportion of people without the target disorder who have a negative test result.
- Stable angina
- There are no case definitions of stable angina that have been agreed internationally. The working definition of angina is a symptom of myocardial ischaemia that is recognised clinically by its character, its location and its relation to provocative stimuli. Angina is usually caused by obstructive coronary artery disease that is sufficiently severe to restrict oxygen delivery to the cardiac myocytes. Generally speaking, angiographic luminal obstruction estimated at ≥ 70% is regarded as ‘severe’ and likely to be a cause of angina, but this will depend on other factors.
- Stenosis
- A narrowing of the arteries leading to a reduction in blood flow. May be due to the build-up of atherosclerotic deposits of fibrous and fatty tissue or may be a congenital defect.
- Stress echocardiography
- An ultrasound examination of the heart. Exercise or pharmacological stress may be used to look for reversible systolic regional wall motion abnormalities consistent with the development of myocardial ischaemia.
- Stress magnetic resonance imaging
- Magnetic resonance imaging (MRI) is a diagnostic procedure that uses radio waves in a strong magnetic field. The pattern of electromagnetic energy released is detected and analysed by a computer to generate detailed images of the heart. Stress MRI is a specific application in which a contrast agent is used to detect myocardial blood flow at stress and at rest. Pharmacological stress is used to induce cardiovascular stress.
- Tetralogy of Fallot
- A complex congenital heart defect condition comprising a ventricular septal defect, pulmonary obstruction, a displaced aorta and an enlarged right ventricle.
- Total anomalous pulmonary venous drainage
- A rare cyanotic congenital heart defect in which all four pulmonary veins are incorrectly positioned and make anomalous connections to the systemic venous circulation. All pulmonary veins, draining blood from the lungs, should normally be connected to the left atrium; in total anomalous pulmonary venous drainage they drain into the right atrium, usually via systemic venous circulation.
- Transposition of great arteries
- A congenital heart defect in which the aorta and pulmonary artery are transposed so that the aorta arises from the right ventricle and the pulmonary artery arises from the left ventricle. This leads to oxygen-low blood being pumped around the body.
- True-negative
- Correct negative test result – number of non-diseases persons with a negative test result.
- True-positive
- Correct positive test result – number of diseased persons with a positive test result.
- Vascular ring
- A congenital defect in which there is abnormal formation of the aorta and/or its surrounding blood vessels. The trachea and oesophagus are completely encircled by a ring formed by these vessels, which can lead to breathing and digestive problems.
- Unstable angina
- New onset chest pain/discomfort, or abrupt deterioration in previously stable angina, with chest pain/discomfort occurring frequently and with little or no exertion, and often with prolonged episodes. This often presents in the same way as myocardial infarction but without biomarker evidence of myocardial necrosis.
- z-axis
- The direction that the scanning table travels in (i.e. head to toe).
List of abbreviations
- ACC
- American College of Cardiology
- AF
- atrial fibrillation
- AHA
- American Heart Association
- BCIS
- British Cardiovascular Intervention Society
- BMI
- body mass index
- b.p.m.
- beats per minute
- CABG
- coronary artery bypass graft
- CAD
- coronary artery disease
- CCS
- Canadian Cardiovascular Society
- CEP
- Centre for Evidence-based Purchasing
- CI
- confidence interval
- CMR
- cardiovascular magnetic resonance
- CRD
- Centre for Reviews and Dissemination
- CT
- computed tomography
- CTA
- computed tomography angiography
- CTCA
- computed tomography coronary angiography
- CV
- cardiovascular
- DLP
- dose-length product
- DSCT
- dual-source computed tomography
- ECG
- electrocardiogram
- EQ-5D
- European Quality of Life-5 Dimensions
- ESC
- European Society of Cardiology
- FN
- false-negative
- FP
- false-positive
- HCS
- high calcium score
- HDCT
- high-definition computed tomography
- HHR
- high heart rate
- HPA
- Health Protection Agency
- HR
- hazard ratio
- HRF
- heart rate frequency
- HRQoL
- health-related quality of life
- HRV
- heart rate variability
- ICA
- invasive coronary angiography
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- MAPCA
- major aortopulmonary collateral arteries
- MI
- myocardial infarction
- MRI
- magnetic resonance imaging
- MSCT
- multislice computed tomography
- N/A
- not available
- NA
- not applicable
- NFE
- non-fatal event
- NGCCT
- new-generation cardiac computed tomography
- NGCCT-ICA
- ICA after positive NGCCT
- NR
- not reported
- OR
- odds ratio
- PCI
- percutaneous coronary intervention
- PSA
- probabilistic sensitivity analysis
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- SCCT
- Society of Cardiovascular Computed Tomography
- SD
- standard deviation
- SE
- standard error
- SPECT
- single photon emission computed tomography
- SROC
- summary receiver operating characteristic
- TAPVD
- total anomalous pulmonary venous drainage
- TIA
- transient ischaemic attack
- TN
- true-negative
- TP
- true-positive
- YRM
- York Radiation Model
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has only been used once, or it is a non-standard abbreviation used only in figures/tables/appendices in which case the abbreviation is defined in the figure or table legend.