Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 01/18/01. The contractual start date was in September 2002. The draft report began editorial review in February 2011 and was accepted for publication in August 2012. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors' report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen's Printer and Controller of HMSO 2013. This work was produced by Duffy et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background to and evolution of FH01
The historical situation
In the late 1980s, the UK National Health Service Breast Screening Programme (NHSBSP) was instituted. 1 In the first instance it offered mammography every 3 years to women aged 50–64 years. The lower age limit was based on trial results suggesting a greater impact in women aged > 50 years. The age range has since expanded to 50–70 years, and is in the process of further expansion to 47–73 years.
In the 1990s, there was considerable controversy about the benefit of breast screening with mammography in women aged 40–49 years. 2,3 There was evidence that the intervention in this age group could confer a reduction in mortality from breast cancer,4 but with the following qualifications. First, the benefit was more difficult to achieve because of radiologically denser breast tissue and more rapid progression of tumours in younger women. Secondly, owing to the much lower incidence of breast cancer in women aged 40–49 years than in women aged > 50 years, the absolute benefit was likely to be markedly lower than that achieved by screening older women.
At the same time, discovery of high-risk gene mutations and the generally increased level of awareness of breast cancer was leading to a growing body of women concerned about their risk of breast cancer because of diagnosis of the disease among their relatives. 5–7
Risk triage based on family history was already becoming common practice in the late 1990s and early 2000s. 8 There is a large population with a family history which does not increase risk substantially above that of the general female population. For this population, no particular intervention is indicated. There is also a very small population with a high-risk mutation identified in the family or with such a strong family history as to have a serious probability of a mutation. For these women, magnetic resonance imaging (MRI) surveillance was under investigation during the late 1990s and early 2000s. 9,10 Potential surgical interventions were also options, including prophylactic mastectomy and prophylactic oophorectomy. 11,12
There is a third population of intermediate risk, whose family history is sufficiently strong as to confer a level of individual risk around three times the risk of the general population, but not sufficiently strong as to give rise to suspicion of a high-risk gene mutation. For this population, towards the end of the last century, it was not clear what the appropriate management strategy should be, but one option was to offer mammographic surveillance at an earlier age and a greater frequency (usually annually, but in some centres biennially) than that provided by the NHSBSP. 13,14 This service was already being provided in a non-standardised and sporadic fashion, and in the early 2000s it remained unevaluated.
A survey of British Association of Surgeons in Oncology (BASO) breast units indicated that 96% of units offered regular mammography to women aged < 50 years with a family history, but 12% of these had no written inclusion criteria. Practice varied considerably around the country. There was, however, evidence that screening in this group could achieve at least the same detection capability as in the NHSBSP, with detection rates at screening almost double the interval cancer rates. 13
Contemporaneously, the science and technology of individual risk prediction was progressing rapidly. 15–17 There was now a serious possibility of a standardised surveillance service for family history subjects below the NHSBSP age, with rigorous inclusion criteria based on familial risk.
Conception of FH01
Against this background, there was considerable interest in the early years of the twenty-first century in the evaluation of mammographic surveillance of women aged < 50 years with a family history of breast cancer. The ultimate aim of screening asymptomatic women for breast cancer is to prevent deaths from the disease by the mechanism of detection at an early stage when treatment is more likely to be curative. 18 The ideal evaluation design would be a randomised trial in which one group is randomised to the offer of surveillance and the other to usual care, with death from breast cancer as end point. 4,18
There were a number of circumstances mitigating against this strategy. First, with survival from breast cancer having improved markedly throughout the 1990s, a trial based on mortality would need very large numbers, a very long follow-up or both. Power calculations indicated that such a randomised trial would require approximately 30,000 subjects followed up for 15 years. In the meantime, many centres would continue to provide the mammographic surveillance service, unevaluated, as it was perceived as prudent clinical practice. It was clear that a more rapid and economically viable evaluation was necessary.
Secondly, a feasibility study including a survey of breast units revealed a distinct lack of equipoise on the part of the clinicians providing breast services. The consensus was that randomly allocating a substantial proportion of the population to no surveillance would be clinically imprudent. There was also a prevailing opinion that with the clear mortality reduction in women aged > 50 years in the randomised trials,19 and the evidence for a slightly weaker but still worthwhile benefit in women aged 40–49 years,2,4 it was no great leap of faith to expect a similar benefit in younger women with a family history.
As a result of the lack of equipoise it was evident that we could not design an evaluation in which part of the population received no surveillance at all. Suggested randomised designs included a comparison of annual with 3-yearly mammography and a trial of mammography compared with clinical breast examination. These were considered, but were not adopted, on the basis that the question of interest at the time was ‘Does mammographic surveillance save lives in women aged < 50 years at enhanced familial risk, in comparison with no surveillance?’.
This left the study team with a difficult design problem. However, some time previously Professor Howard Cuckle had proposed a single-arm evaluation based on quality measures of screening, a positive result being defined as achieving realisations of these measures similar to those in the intervention arms of the randomised trials. This was taken as a basis for the design of the study, hereafter referred to as FH01, but there was a perceived need for an evaluation which gave an estimate of the intervention's effect on the clinical outcome, breast cancer mortality.
This in turn necessitated resolving two further issues: first, how to estimate the future breast cancer mortality in the cohort since, as mentioned above, it would take many years to accrue large numbers of breast cancer deaths; second, how to estimate the future breast cancer mortality if the surveillance had not taken place. For the first of these, it had already been observed and validated that the pathological characteristics size, node status and grade of breast cancers were excellent predictors of death from the disease. 20,21 Thus, it would be possible to predict the numbers of breast cancer deaths from the tumour data. This has two advantages: first, the end point is observable at the time of diagnosis, long in advance of the time of death; and, second, with the standard error dependent on all cancers, not only fatal ones, the statistical power is greater.
As for the second question, an external comparison group was indicated. After considerable thought, it was decided that the main comparison group would be the control group in the UK Breast Screening Age Trial (Age Trial),22 as this population was of similar age to the putative FH01 cohort, and was not undergoing surveillance. It was appreciated that the comparison would have to be adjusted for the differing risk profiles of the two populations: the Age Trial recruits would be from the general female population, whereas the FH01 cohort would be at enhanced familial risk. The development of individual risk prediction models meant that this was a practicable option. 15–17
This gave rise to one further problem of design and research ethics. In order to make the adjustment for the different risk profiles, we needed risk-factor data on the Age Trial population. Because of the prevailing ethics and governance environment at the time the Age Trial was initiated, the controls had never been contacted and did not necessarily know that they were in a screening trial. Contacting them at the start of FH01 to elicit risk factor information on breast cancer was not considered practical and might not survive ethical scrutiny. However, as part of an epidemiological study under way, the required risk factor information was available from a subset of the Age Trial intervention group. Since randomisation would be expected to render the intervention and control groups comparable with respect to risk factors, this was considered sufficient for purposes of adjusting comparisons for underlying risk.
A final consideration was that the aim was to evaluate rather than change current practice. We did stipulate that annual mammographic surveillance was the target for our evaluation, so that some centres offering 2-yearly surveillance could not participate without changing, but aside from that, the design was non-prescriptive, with the following basic features:
-
a single cohort of women at moderately increased risk due to family history of breast cancer
-
annual mammography for 5 years
-
ideally aged 40–44 years at recruitment, so that they would still be aged < 50 years after 5 years
-
end points of the size, node status and grade of the tumours diagnosed, plus the expected future breast cancer mortality based on these tumour features; and
-
comparison of these end points with the Age Trial control group, adjusted for underlying risk.
Timelines
Detailed design of the study is reported in Chapter 2. The study began on 1 January 2003 and was planned to end in 2009. Owing to slower recruitment than expected, the study was extended to 2010, when the predicted mortality results were published,23 and the cohort will now be followed up for actual mortality.
Chapter 2 Design, planned analysis and study size
Basic design and end points
FH01 was designed as a single-arm cohort study (see Appendix 1 for protocol and Appendix 2 for protocol of the accompanying blood study). The intervention to be evaluated was annual mammography (or at any rate, with the interval not slipping beyond 18 months) for 5 years. We targeted women aged 40–44 years at recruitment so that after 5 years of mammography they would still be in the age range 40–49 years. This was to avoid arguments about ‘age creep’: the theory propounded about randomised trials of screening that the apparent benefits of screening in women aged 40–49 years at randomisation were actually due to screening activity taking place after the recruits had passed their 50th birthdays. 20 It should be noted that there is little empirical evidence for the phenomenon,21,24 but it was considered prudent to head off the issue by design if possible.
It was specified that, to be eligible, women had to satisfy at least one of the following family history criteria:
-
one first-degree female relative with breast cancer diagnosed at ≤ 40 years of age
-
one first-degree female relative with bilateral breast cancer diagnosed at < 50 years of age
-
two first-degree or one first- and one second-degree female relative, both with breast cancer diagnosed at ≤ 60 years of age (same side of family)
-
one first- or second-degree female relative with breast and ovarian cancer, with the first cancer diagnosed at ≤ 60 years of age
-
three first- or second-degree female relatives with breast or ovarian cancer at any age (same side of family)
-
one first-degree male relative with breast cancer at any age
-
paternal history of a minimum of two second-degree relatives (father's first-degree relatives) with breast cancer at ≤ 50 years of age, or one with breast cancer at ≤ 50 years of age and an ovarian cancer (any age), or paternal uncle/grandfather with breast cancer at < 50 years of age.
A first-degree female relative is defined as mother, sister or daughter. A second-degree female relative is defined as granddaughter, grandmother, aunt or niece. Exclusion criteria were:
-
inability to give written informed consent
-
pregnancy
-
age < 40 years
-
proven breast cancer or ductal carcinoma in situ (DCIS)
-
previous bilateral prophylactic mastectomy
-
presence of a breast cancer type 1 (BRCA1) or breast cancer type 2 (BRCA2) mutation in the family in women who have been tested negative for the mutation.
On the basis of the inclusion criteria, the study group was anticipated to have at least a 3% probability of breast cancer between ages 40 and 49 years, inclusive. Although BRCA1- and BRCA2-positive cases were not explicitly excluded, the moderate-risk criteria implied that relatively few FH01 recruits would be BRCA positive.
The information sheet for potential recruits is given as Appendix 3. The information for primary care professionals is given in Appendix 4.
For reasons noted in Chapter 1, the primary end points were the size, node status and histological grade of tumours diagnosed and the projected mortality from these. The primary comparison group was the control group of the Age Trial. We planned to adjust the comparison for differences between the FH01 cohort and the Age Trial population in underlying risk of breast cancer. The adjustment was made by calculating the expected 10-year absolute incidence of breast cancer17 for each population (FH01 and Age Trial controls), and dividing the projected rate of breast cancer for each group by its expected incidence. We also planned to compare the FH01 results with those from other, historical family history cohorts undergoing little or no surveillance. 25 Further details are given in Planned statistical analyses.
Collaborating units were expected to offer annual (or at least 18-monthly) two-view mammography and to:
-
operate a breast cancer unit in line with the recommendations of the British Breast Group and the BASO guidelines for surgeons in the treatment of symptomatic breast disease26
-
have experience in mammography in symptomatic women aged < 50 years
-
either participate in the NHSBSP or offer mammographic services at a level consistent with the quality standards set out by the NHSBSP
-
have a clearly defined referral line for high-risk women to a regional clinical genetics service
-
have at least one member of the multidisciplinary team trained in pedigree construction and interpretation, and risk analysis.
Study size
We had originally designed FH01 to have the power for the same comparison of tumour attributes and the consequent expected mortality in two risk-stratified subgroups. As a result, the planned sample size at the initiation of the study was 10,000. A monitoring visit by the funding body noted that recruitment had been poor in the early years, and recommended changing the target to a more modest study size designed to have adequate power for the cohort as a whole, with no regard for subgroup analyses. For this target, assuming use of the controls in the Age Trial as a comparison group, an important planned comparison was the incidence of node-positive tumours in the FH01 cohort with that expected from the comparison group, taking into account the different underlying incidences in the two groups. From the Swedish Two-County Study controls, we would expect an unscreened tumour series in the age group 40–49 years to be node-positive in 42% of cases. 27 In the Age Trial control group, with 7 years of cancer incidence in 106,000 women,22 we conservatively expected around 742 cancers, and therefore 311 (42%) node-positive tumours.
Results from the Two-County Study suggest a screening sensitivity of 83% and a mean sojourn time (average duration of the preclinical screen-detectable period) of 2.44 years in women aged 40–49 years. 24 This suggests that with a 1-year interval there would be 77% screen-detected cancers, of which 11% would be node positive. We assume that the interval cancers would have the same 42% node-positive cancer rate as an unscreened group, giving an overall 18% node-positive cancer rate. Thus, the comparison anticipated is between a group with 42% node-positive cancers and one with 18% node-positive cancers. This would correspond, on the basis of the relative fatality of node-positive and node-negative cancers, to long-term survival of 64% compared with 74%, with a relative risk (RR) in the FH01 cohort of 0.72. A 5-year incidence rate at around 4 per 1000 per year (due to high familial risk) would mean a total incidence of node-positive disease of 3.6 per 1000 (0.18 × 0.004 × 5). For 90% power to detect a difference in incidence of node-positive cancers of 3.6 per 1000 and 8.4 per 1000 (0.0036 × 0.42/0.18) as significant, and allowing a 5% increase in standard errors as a result of adjustment for different underlying risk in the two populations, we would require 6000 women and 120 cancers. Thus, we aimed to recruit 6000 women and expected to be in a position to analyse the data and report after an average of 5 years' observation.
Planned statistical analyses
The data proforma is given in Appendix 5. As noted above, the primary analyses planned were the comparison of the prognostic variables tumour size, lymph node status and histological grade, and the consequent predicted breast cancer mortality, between the FH01 cohort and the Age Trial controls. Secondary comparisons with other historical data sets were anticipated. Categorical variables were compared between the FH01 tumours and comparison groups using the chi-squared test. Continuous variables were compared using the t-test. We calculated the Nottingham Prognostic Index (NPI) score for invasive cases as a + b + c, where
-
a = 0.2 × size in cm
-
b = 1 if node-negative, 2 if 1–3 positive nodes, 3 if ≥ 4 positive nodes; and
-
c = histological grade (1, 2 or 3).
From this, we estimated the 10-year survival as shown by Blamey et al.,28 who regressed 10-year survival on NPI, splitting their data set in two for cross-validation purposes. They obtained two quadratic equations for the prediction of survival from NPI, which gave very similar predictions. Here we use the average of their two equations, giving an estimate S of average 10-year per cent survival as a function of NPI, denoted N in equation (1).
For the FH01 cohort and the Age Trial control group, we then calculated the absolute expected rate of tumours proving fatal over 10 years, and divided this in each case by the underlying risk in the two populations, calculated from family history and other risk-factor data using the absolute risk model of Tyrer et al. 17 The Tyrer et al. 17 model has been independently validated and shown to predict risk with accuracy. 29 The absolute risk was calculated directly on all of the FH01 recruits. For the Age Trial comparison group, for the ethics and governance reasons outlined in Chapter 1, a more indirect process had to be used. There were no risk-factor data on the Age Trial control group, and there were ethical problems with contacting members of this group to ascertain risk factors. However, in another, unrelated study, a subset of the Age Trial intervention group had undergone risk factor ascertainment. We therefore used this study population to estimate the underlying risk in the Age Trial control group, on the basis that, owing to the randomisation, the risk profiles of members of the study group would be the same as those of the control group.
Thus, we were able to calculate the RR of absolute mortality, corrected for the different risk profiles of the two populations. This was done by dividing the expected death rates by Tyrer et al.'s17 independent estimates of the underlying incidence. The corrected RR was:
where d1, P1 and r1 are the expected deaths, person-years of observation and underlying 10-year average risk in the FH01 cohort, and d2, P2 and r2 the corresponding quantities in the Age Trial control group. The numerator and denominator of the RR are simply the ratios of expected death rates from the NPI to the expected incidences based on Tyrer et al.'s17 model, to adjust for the fact that the FH01 cohort has higher underlying risk than the Age Trial population. Again, note that r1 was calculated directly from the FH01 subjects, whereas r2 was calculated from a subgroup of 3001 members of the study group of the Age Trial. Note that the division by the expected incidence figures, r1 and r2, calculated from an independently derived algorithm, is a safeguard against lead time, length bias and overdiagnosis. Actual incidence in the FH01 cohort would have been potentially susceptible to these biasing factors, but independently estimated incidence is not.
The variance of the logarithm of RR is:
where f is the predicted case fatality rate from the NPI, that is, the complement of the predicted survival, and n is the number of invasive cancer cases in FH01.
The standard error of the logarithm of RR was then calculated as s, the square root of the variance and the 95% confidence interval (CI) on ln(RR) calculated as:
The end points of the interval were then transformed exponentially to give the 95% CI on RR.
In addition to the Age Trial controls, we also compared the FH01 results with those from a Dutch series of 238 breast cancers (all invasive) in women largely not subject to surveillance, with a family history of breast cancer and not BRCA1 or BRCA2 positive. 25 However, for the Dutch series, we had only published tabular results. We did not have risk-factor data on which to calculate the absolute risks and correct the comparison for these.
From the published tabular data on tumour size, node status and grade in the Dutch study, we calculated average NPI and, to be conservative, the maximum possible standard error on this consistent with the tabular data. The predicted average 10-year survival and its standard error in the Dutch study were calculated from the average NPI, using the second-order Taylor approximation. 30
It had originally been planned to compare our results with those of a French series of tumours in women with a family history who had not been subject to mammographic surveillance. However, preliminary results suggested that geographical and temporal confounding factors were rendering the comparison unreliable, in that our results would have seemed too good to be true. For example, 62% of the tumours in the French series were of size > 20 mm. We therefore abandoned this planned analysis.
One further analysis of clinical outcome was performed. From the rates of screen-detected and interval cancers by node status, we estimated the parameters of progression by node status in a Markov process. From these we estimated the cancers by node status which would have been expected to occur if the surveillance had not taken place. These were then combined with 20-year fatality rates of cancer by node status in the Swedish Two-County Trial of breast screening27 to estimate long-term mortality from breast cancer with and without mammographic surveillance. These results were used in the health economic analysis.
Other analyses planned included summaries of screening activity and negative outcomes of the surveillance, including false-positives, benign surgery cases and radiation dose. The study was accompanied by a psychosocial evaluation, which was funded separately and has already been published in detail. 31–34 FH01 also incorporated radiology and pathology reviews, and an economic evaluation. These are also briefly reported on in the following chapters.
Recruitment
Recruitment took place between 16 January 2003 and 28 February 2007, with a total of 6710 women in the study. Figure 1 shows recruitment over time. A number of centres remained ineligible for the study because of an ongoing policy of 2-yearly surveillance. Recruitment began slowly, but accelerated steadily. In the first year, around 600 women were recruited, in the second year, around 1200, and in each of the third and fourth years > 2000. A number of measures were taken to improve recruitment during the course of the study, including expansion of the eligible centres to include Scotland and Northern Ireland, personal visits and other contacts to potentially high-recruitment centres and the institution of regional co-ordinators to take local responsibility for recruitment and data capture. Although these measures did bear fruit in terms of improved accrual, it is not clear which particular measures were the most effective. It is likely that if we had taken these measures from the study's inception, recruitment would have been considerably faster. However, the pattern of slow recruitment in the early months, gradually accelerating, is common in large population studies.
FIGURE 1.
FH01 cumulative recruitment over time.
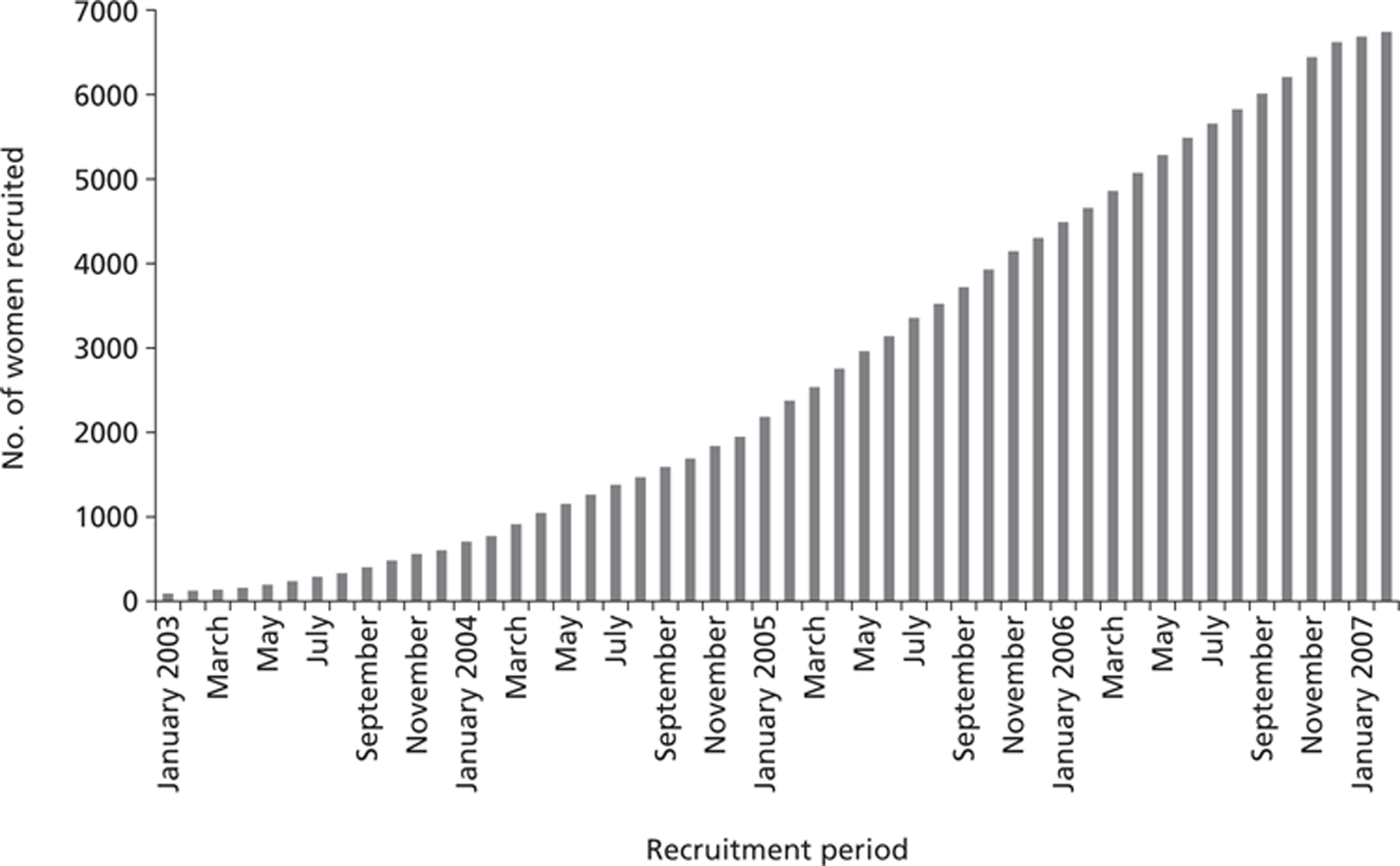
Table 1 shows recruitment by individual centre. Interestingly, Scotland, Wales and Northern Ireland contributed around 30% of recruits. Within England, major recruiting centres were the Withington Community Hospital Manchester, Nottingham City Hospital and the Jarvis Breast Screening and Diagnostic Centre, Guildford. In Wales, women were recruited in a single genetics service covering the entire country and within a single research network, although surveillance took place at three sites.
| Hospital name | Total no. of patients recruited |
|---|---|
| Aberdeen Royal Infirmary | 116 |
| Addenbrooke's Hospital | 113 |
| Airedale General Hospital | 31 |
| Ardmillan Breast Screening Centre | 308 |
| Ayr Hospital | 3 |
| Barnsley District General Hospital | 40 |
| Basildon Hospital | 83 |
| Brighton General Hospital | 47 |
| Burnley General Hospital | 3 |
| Charing Cross Hospital, London | 2 |
| City Hospital, Birmingham | 62 |
| Countess of Chester Hospital | 68 |
| Coventry and Warwickshire Hospital | 71 |
| Craigavon Area Hospital | 33 |
| Crosshouse Hospital, Kilmarnock | 9 |
| Cumberland Infirmary | 3 |
| Darlington Memorial Hospital | 12 |
| Derby City General Hospital | 145 |
| Derriford Hospital | 26 |
| Elizabeth Garrett Anderson and Obstetrics Hospital, London | 43 |
| Frenchay Hospital | 149 |
| Glasgow Royal Infirmary | 16 |
| Guy's Hospital, London | 36 |
| Hairmyres Hospital/RAH | 6 |
| Hinchingbrooke Hospital | 69 |
| Hope Hospital | 37 |
| Ipswich Hospital | 41 |
| James Paget Hospital | 7 |
| Jarvis Screening Centre, Guildford | 231 |
| Kettering General Hospital | 59 |
| Leighton Hospital, Crewe | 50 |
| Macclesfield District General | 80 |
| Mayday Hospital, London | 9 |
| Medway Hospital | 29 |
| Milton Keynes General Hospital | 7 |
| New Cross Hospital, Wolverhampton | 43 |
| Newcastle General Hospital | 103 |
| Ninewells Hospital, Dundee | 49 |
| North Hampshire Hospital | 9 |
| Northampton General Hospital | 45 |
| Northwick Park Hospital, London | 34 |
| Nottingham City Hospital | 270 |
| Parapet Breast Screening Centre, Windsor | 145 |
| Princess Royal Hospital, Telford | 73 |
| Queen Alexandras Hospital, Portsmouth | 190 |
| Queen Elizabeth Hospital, Gateshead | 6 |
| Queen Mary Hospital, Roehampton | 2 |
| Queen Mary's Hospital, Sidcup | 31 |
| Queen's Hospital Burton | 16 |
| Royal Cornwall Hospital (Treliske) | 107 |
| Royal Devon and Exeter Hospital | 94 |
| Royal Free Hospital, London | 25 |
| Royal Liverpool Hospital | 76 |
| Royal Marsden Hospital, London | 229 |
| Royal United Hospital, Bath | 181 |
| Sandwell Hospital, Birmingham | 9 |
| Scarborough General Hospital | 41 |
| Southend Hospital | 110 |
| Southport and Formby District General Hospital | 52 |
| St Bartholomew's Hospital, London | 44 |
| St George's Hospital, London | 109 |
| St James Hospital, Leeds | 100 |
| St Mary's Hospital, London | 23 |
| Stobhill Hospital, Glasgow | 30 |
| University Hospital Aintree | 31 |
| University Hospital of North Tees | 4 |
| Victoria Infirmary, Glasgow | 80 |
| Wales | 1116 |
| West Suffolk Hospital | 12 |
| Western Infirmary, Glasgow | 263 |
| Weston-super-Mare Hospital | 20 |
| Whiston Hospital | 26 |
| Wishaw General Hospital | 73 |
| Withington Hospital | 755 |
| Worthing Hospital | 40 |
| Total | 6710 |
It should be noted that the slow recruitment in the first 2 years was not because of unwillingness of eligible women to participate in the study. In five centres polled, four reported that participation rates in excess of 90%, and one of 65%. The phenomenon derived from a combination of the delay in centres joining the study, largely because of ethics and governance formalities, and the fact that many centres had relatively few eligible women.
The comparison populations
The Age Trial randomised 53,890 (study group) women aged 40–42 in the general population years to invitation to annual mammography for 7 years, and 106,971 (control group) women of the same age to usual care. 22 Recruitment took place between 1991 and 1999. As noted above, our aim was to compare the pathological characteristics and the corresponding predicted 10-year mortality between the cancers diagnosed in FH01 and those diagnosed in the control group of the Age Trial, during 622,127 person-years of follow-up. Thus, our comparison group would be of similar ages to the FH01 population and would not have been offered mammographic surveillance. The group would, however, have general population risk, whereas the FH01 population would be at enhanced risk of breast cancer owing to family history. To adjust for this, we used risk-factor data on 3001 subjects within the Age Trial study group, assuming equal underlying risk between the study and the control group due to the randomisation. We estimated the 10-year probabilities of breast cancer in the Age Trial and FH01 recruits, and corrected our estimate of relative mortality for these.
The Dutch comparison series comprised 238 breast cancers in women with a family history of the disease but without a known BRCA mutation, during the period 1980–2004. Ages at diagnosis ranged from 25 to 77 years. The cases were from a population largely not undergoing surveillance. Thus, the Dutch series was comparable with FH01 with respect to family history, and was mostly not subject to mammography. However, the age range was much wider in the Dutch series.
Chapter 3 Baseline characteristics of the recruited population and the Age Trial comparison population
Demographic and risk-factor data
When considering the baseline status of the populations under study, it is as well to be reminded of the basic analysis plan. The projected analysis was as follows:
-
Obtain the pathological tumour size, lymph node status and histological grade of tumours diagnosed in FH01.
-
Obtain the same data from the Age Trial control group.
-
From each, calculate the NPI score and the consequent estimated numbers of deaths within 10 years. 28
-
In each group, divide the expected numbers of breast cancer deaths by the corresponding person-years.
-
Calculate the average absolute predicted 10-year breast cancer incidence in each group:17 for FH01 directly using the risk-factor data on the FH01 recruits, for the Age Trial controls using the data on 3001 Age Trial study group members.
-
Divide the rates calculated in point 4 (above) by their corresponding breast cancer incidence figures. The ratio of the resulting figures is an estimate of the relative breast cancer mortality in the two populations, corrected for their different underlying breast cancer risks.
The necessity of points 5 and 6 lends considerable importance to the risk factor status in both the FH01 and the comparison populations.
Table 2 shows the recruited FH01 population by baseline epidemiological characteristics. Of the 6710 recruits, 91% were aged between 40 and 44 years at recruitment. Owing to the eligibility criteria, there are considerably higher proportions with relatives affected with breast cancer than in the general population in this age group.
| Factor | Category | No. (%) |
|---|---|---|
| Age (years) | < 40 | 185 (3) |
| 40 | 1578 (24) | |
| 41 | 1180 (17) | |
| 42 | 1144 (17) | |
| 43 | 1160 (17) | |
| 44 | 1070 (16) | |
| 45+ | 393 (6) | |
| Total | 6710 (100) | |
| Parity | 0 | 1071 (17) |
| 1 | 1068 (17) | |
| 2 | 2623 (41) | |
| 3+ | 1586 (25) | |
| Not known | 362 | |
| Age at first pregnancy (years) | < 20 | 564 (11) |
| 20-24 | 1413 (28) | |
| 25-29 | 1767 (34) | |
| 30-34 | 981 (19) | |
| 35+ | 396 (8) | |
| Not known/nulliparous | 1589 | |
| Age at menarche (years) | < 13 | 2592 (44) |
| 13+ | 3362 (56) | |
| Not known | 756 | |
| Menopausal status | Premenopausa | 4650 (90) |
| Post/perimenopausa | 492 (10) | |
| Not known | 1568 | |
| Age at menopause (years) (if applicable) | < 40 | 202 (63) |
| 40+ | 119 (37) | |
| Not known | 171 | |
| HRT use | Never | 4508 (93) |
| Yes, now | 228 (5) | |
| Yes, previously | 119 (2) | |
| Not known | 1855 | |
| Mother have breast cancer | No | 2178 (34) |
| Yes | 4300 (66) | |
| Sister have breast cancer | No | 4486 (69) |
| Yes | 1992 (31) | |
| Relative with breast cancer before age 40 years? | No | 3660 (57) |
| Yes | 2781 (43) | |
| Affected relative data missing | 232a | |
| Previous mammography? | No | 1761 (28) |
| Yes | 4605 (72) | |
| Not known | 344 | |
| Previous breast biopsy? | No | 4320 (88) |
| Yes | 616 (12) | |
| Not known | 1774 |
As expected, there were very few BRCA1- or BRCA2-positive recruits. For 374 subjects, there had been a test for a BRCA1 mutation in the family, of whom 82 (1% of recruits) had a positive test. For 3546 recruits, there had been no testing in the family. For the remaining 2790 recruits, BRCA1-testing status in the family was unknown. For the vast majority of these subjects the mutation would not have been tested for in the family. Of the 82 recruits with a positive test in the family, 14 recruits had themselves tested positive. Similar figures apply for BRCA2. For 3539 recruits, there had been no testing in the family. For 284 recruits, there had been a test in the family, of which 65 cases (1%) had a positive test. Of those recruits with a positive BRCA2 test in the family, 21 had themselves tested positive. Eight subjects were excluded on the basis of a positive mutation in the family, but a negative personal test.
Table 3 shows selected comparisons with the 3001 Age Trial recruits used for determining the baseline risk in the Age Trial control group. It should be noted that although the cancers in the Age Trial occurred in the same broad age group as in FH01, the Age Trial subjects in the subsample of 3001 were interviewed for risk factors some years after recruitment to the age trial, so that 30% of them were aged ≥ 50 years at the time of interview. As a result, reported menopausal status, age at menopause and hormone replacement therapy (HRT) use, were all substantially different for the Age Trial recruits (data not shown). Although the Age Trial recruits had slightly but significantly higher parity than the FH01 subjects, the most striking differences, as expected, were for family history. Almost 10 times as many FH01 subjects had an affected mother and 15 times as many an affected sister. These differences emphasise the need to adjust the mortality comparison for the different underlying risks in the two populations.
| Factor | Category | FH01, no. (%) | Age Trial, no. (%) | Signifcance |
|---|---|---|---|---|
| Age at menarche (years) | < 13 | 2592 (44) | 1239 (43) | p = 0.5 |
| 13+ | 3362 (56) | 1652 (57) | ||
| Parity | 0 | 1071 (17) | 411 (14) | p < 0.001 |
| 1 | 1068 (17) | 422 (14) | ||
| 2 | 2623 (41) | 1475 (49) | ||
| 3+ | 1586 (25) | 693 (23) | ||
| Mother have breast cancer | No | 2178 (34) | 2786 (93) | p < 0.001 |
| Yes | 4300 (66) | 215 (7) | ||
| Sister have breast cancer | No | 4486 (69) | 2617 (98) | p < 0.001 |
| Yes | 1992 (31) | 67 (2) |
Apart from family history, we had limited risk-factor data within FH01 and, in particular, did not have weight and height data. However, since 90% of the subjects were premenopausal, this did not seriously affect the risk prediction.
Projected breast cancer risk in FH01 and in the Age Trial
As estimated from the Tyrer–Cuzick programme,17 the average 10-year risk of the FH01 recruits was 6.3% (95% CI 6.2% to 6.4%). This is estimated to be 2.61 times the population risk for this age group. Of the 6710 recruits, 6251 (93%) were estimated to have a 10-year risk of at least 3%, as targeted by the eligibility criteria. It should be borne in mind that this 10-year risk is not for the 10 years aged 40–49 years inclusive, but for the 10 years from age at recruitment, hence the rather high cumulative risk.
The average 10-year risk of the 3001 Age Trial subjects estimated using the Tyrer–Cuzick programme17 was 2.4% (95% CI 2.3% to 2.5%); this is estimated to be 0.96 times the population risk. As the Age Trial subjects were from the general population, this was to be anticipated.
Chapter 4 Surveillance activity and outcomes
Mammography episodes
Table 4 shows the number of prevalence and incidence surveillance episodes. For our purposes, a prevalence episode is defined as the individual's first ever screen (not necessarily the first screen within FH01), and an incidence episode any subsequent screen. There were 2068 prevalence episodes and 28,488 incidence episodes, giving a total of 30,556 screens in the study. Recall rates were 8% at prevalence episodes and 6% at incidence, with corresponding cancer detection rates of 5 per 1000 and 4 per 1000, respectively. False-positive rates were 7% and 5% at prevalence and incidence episodes, respectively.
| Episode type | No. of episodes | Recall for assessment (%) | Cancers detected (no. per thousand) |
|---|---|---|---|
| Prevalence | 2068 | 165 (8) | 10 (5) |
| Incidence | 28,488 | 1639 (6) | 112 (4) |
| Total | 30,556 | 1804 (6) | 122 (4) |
Of the 30,556 mammographic surveillance episodes, 11,503 (38%) were accompanied by clinical breast examination and 2598 (9%) by ultrasound. Taking only those episodes where the clinical examination or ultrasound were done before knowledge of the mammogram result, to exclude those cases where the additional examination might have been prompted by the mammogram result, there was no indication of an increased detection rate from the clinical examination, but there was a result suggestive of increased detection due to ultrasound. For the 8002 episodes in which clinical examination took place before knowledge of the mammogram result, there were 33 cancers detected, almost exactly equal to the overall average of 4 per 1000. For the 400 episodes in which ultrasound examination took place before knowledge of the mammogram result, seven cancers were detected, 18 per 1000: a highly significant difference from the remaining episodes (p = 0.001). Being based on only seven cancers, however, this result requires confirmation in a larger data set.
One centre had to suspend surveillance for 2 years because of funding difficulties, but rejoined the study when these were resolved. All cancers from all centres are included in the analyses in later chapters on the intention-to-treat principle. Average time since last mammogram for screen-detected cases was 13 months [standard deviation (SD) 5 months]. Average time for interval cancers was also 13 months (SD 8 months). For cancers diagnosed after failure to attend most recent screen, the average time since last mammogram was 19 months (SD 5 months). Ninety-two per cent of incidence screen-detected cancers were detected at a screen within 18 months of the previous screen.
Numbers of screens attended are shown in Table 5. There was an average of 4.6 screens per person. Sixty per cent of subjects had five screens or more. It is likely that the 97 women (1%) for whom we have no record of mammography are a result of missing data, as it is likely that they would have had at least one mammography episode around the time of recruitment. Of the 23,943 second or subsequent screens within FH01, we had data on dates of screen and previous FH01 screen on 23,913 (99.9%) of episodes. The average interval for these was 13 months (SD 5 months). Five per cent (1263 subjects) had a longer interval than 18 months, the maximum specified in the protocol.
| No. of screens attended | No. of subjects (%) |
|---|---|
| 0 | 97 (1) |
| 1 | 356 (5) |
| 2 | 421 (6) |
| 3 | 582 (9) |
| 4 | 1292 (19) |
| 5 | 2153 (32) |
| 6 | 1146 (17) |
| 7 | 507 (8) |
| 8 | 150 (2) |
| 9 | 6 (< 1) |
Percutaneous and surgical biopsies
Of those recalled for assessment, 21% (387 out of 1804) had percutaneous biopsy (for the most part core biopsies). Of the 122 screen-detected cancers, 113 (93%) had a percutaneous biopsy and therefore a preoperative cancer diagnosis. There were 93 women who had surgery or open biopsy for what transpired to be normal or benign disease: six at prevalence screen and 87 at incidence screens. This gave a ratio of 4 : 3 of screen-detected cancers to benign surgery cases, and a ratio of just under 2 : 1 for all cancers to benign surgery cases.
Radiation exposure
We had radiation dose data from two centres on 190 women screened at least once between January 2004 and December 2010, with a total of 666 screening episodes. Table 6 shows radiation doses experienced by these women. At the first episode, 38 (14%) women had single-view mammography. All had two-view mammography in subsequent episodes. Average doses ranged from 1.7 to 2.0 mGy for mediolateral oblique views, and from 1.5 to 1.8 mGy for craniocaudal views. The doses declined at successive episodes. Mean breast thickness was 54 mm (range 18–95 mm) for mediolateral oblique views and 50 mm (range 16–88 mm) for craniocaudal. At the first episode, 12% of women had a mean glandular dose (MGD) > 2.5 mGy, declining to 5% at the fourth episode. Doses from mediolateral oblique views were more likely to exceed the standard than from craniocaudal.
| Screening episode | Mediolateral oblique views | Craniocaudal views | ||
|---|---|---|---|---|
| No. | MGD (mGy), mean (range) | No. | MGD (mGy), mean (range) | |
| 1 | 277 | 1.9 (0.6-6.5) | 239 | 1.8 (0.6–5.0) |
| 2 | 229 | 2.0 (0.8-4.8) | 229 | 1.8 (0.9–4.4) |
| 3 | 110 | 2.0 (0.8-4.8) | 110 | 1.8 (0.9–5.5) |
| 4 | 37 | 1.7 (1.1-3.2) | 37 | 1.5 (1.0–2.8) |
| 5 | 10 | 1.8 (1.3-2.6) | 10 | 1.6 (1.3–2.1) |
| 6 | 3 | 1.6 (1.3-1.8) | 3 | 1.5 (1.3–2.0) |
| Overall | 666 | 1.9 (0.8-4.4) | 628 | 1.7 (0.6–5.0) |
Comparison with the National Health Service Breast Screening Programme standards
The standard for recall rate at prevalence screen in the NHSBSP is a rate of < 10%. The achieved rate in the national programme is 8.7%35 and in FH01 it was 8%. For incidence screens, the NHSBSP standard is a maximum of 7%, with 3.4% achieved in the NHSBSP and 6% achieved in FH01. In terms of cancer detection rates, a direct comparison is not possible, as the underlying incidence of FH01 differs from that of the general population in the target age range of the NHSBSP (although invasive and in situ cancer detection rates in FH01 do exceed the minimum NHSBSP standards for both prevalence and incidence screens). The benign biopsy rate was 2.7 per 1000, exceeding the NHSBSP standard of 2.0 per 1000. On the other hand, the preoperative diagnosis rate of screen-detected cancers was 93%, well above the national minimum standard of 80%.
In our radiation dose substudy on 277 participants and 666 mammographic episodes, the vast majority of radiation doses were within the national standard of 2.5 mGy as MGD per flm. 36 Possibly because of the higher breast density in this age group, a small number did exceed the national standard. Exposures were slightly lower than observed in the Age Trial. 37
Chapter 5 Cancers diagnosed, end points and efficacy
Cancers diagnosed
In total, there were 165 cancers diagnosed in 37,025 person-years of observation, a rate of 4.45 per 1000 person-years. Table 7 shows the cancers diagnosed in FH01 by age and mode of detection, notified to the data centre before 16 December 2010. The dates of diagnosis ranged from 19 June 2003 to 8 December 2010. The average age at diagnosis was 45 years (SD 2.2 years).
| Detection mode | Age group, no. (%) | ||
|---|---|---|---|
| < 45 years | 45+ years | Total | |
| Prevalence screen | 9 (90) | 1 (10) | 10 (100) |
| Incidence screen | 53 (47) | 59 (53) | 112 (100) |
| Interval cancer | 16 (41) | 23 (59) | 39 (100) |
| Non-attendera | 1 (25) | 3 (75) | 4 (100) |
| Total | 79 (48) | 86 (52) | 165 (100) |
Although > 90% of subjects were aged < 45 years at recruitment, the majority of tumours were diagnosed after age 45 years. Overall, 122 out of 165 (74%) of the cancers were diagnosed at screening, 122 out of 161 in those actually attending, giving a programme sensitivity of 76%. In those women aged < 45 years, the programme sensitivity was 78% and in those women aged ≥ 45 years it was 70%.
Table 8 shows the cancers by invasive status and detection mode. There were 120 (73%) invasive cancers, 44 (26%) in situ and one with invasive status unknown. The one woman with unknown invasive status was detected at an incidence screen. Almost exactly 33% of screen-detected cancers and 9% of symptomatic tumours were DCIS. Correspondingly, 81 of 120 (68%) invasive tumours and 40 out of 44 (91%) DCIS cases were screen detected.
| Detection mode | Invasive status (%) | ||
|---|---|---|---|
| Invasive | DCIS | Total | |
| Prevalence screen | 7 (70) | 3 (30) | 10 (100) |
| Incidence screen | 74 (67) | 37 (33) | 111 (100) |
| Interval cancer | 36 (92) | 3 (8) | 39 (100) |
| Non-attender | 3 (75) | 1 (25) | 4 (100) |
| Total | 120 (73) | 44 (27) | 164 (100) |
Table 9 shows the pathological characteristics of the 120 invasive cancers, cross-classified by detection mode (screening or symptomatic). Relatively high proportions were small (42% were < 15 mm in maximum diameter) and node-negative (68%). The screen-detected cancers were smaller and more likely to be node-negative than the symptomatic tumours. They were also slightly more likely to be oestrogen receptor- and progesterone receptor-positive, suggesting an element of length bias. However, the distributions of histological grade and type were very similar for screen-detected and symptomatic tumours.
| Factor | Category | Screen detected (%) | Symptomatic (%) | Total (%) |
|---|---|---|---|---|
| Tumour size (mm) | < 15 | 37 (48) | 12 (31) | 49 (42) |
| 15-20 | 18 (23) | 14 (37) | 32 (28) | |
| 21-30 | 17 (22) | 9 (24) | 26 (22) | |
| 31-50 | 5 (6) | 1 (3) | 6 (5) | |
| 51+ | 1 (1) | 2 (5) | 3 (3) | |
| NK | 3 | 1 | 4 | |
| Lymph node status | Negative | 55 (73) | 22 (58) | 77 (68) |
| 1–3 positive nodes | 14 (19) | 12 (32) | 26 (23) | |
| 4+ positive nodes | 6 (8) | 4 (10) | 10 (9) | |
| NK | 6 | 1 | 7 | |
| Histological grade | 1 | 15 (19) | 8 (22) | 23 (20) |
| 2 | 29 (36) | 13 (35) | 42 (36) | |
| 3 | 36 (45) | 16 (43) | 52 (44) | |
| NK | 1 | 2 | 3 | |
| Histological type | Ducta | 71 (90) | 31 (88) | 102 (90) |
| Lobular | 4 (5) | 2 (6) | 6 (5) | |
| Other | 4 (5) | 2 (6) | 6 (5) | |
| NK | 2 | 4 | 6 | |
| Oestrogen receptor status | Negative | 13 (18) | 10 (29) | 23 (21) |
| Positive | 61 (82) | 24 (71) | 85 (79) | |
| NK | 7 | 5 | 12 | |
| Progesterone receptor status | Negative | 12 (21) | 10 (34) | 22 (25) |
| Positive | 46 (79) | 19 (66) | 65 (75) | |
| NK | 23 | 10 | 33 |
Comparison with the Age Trial and Dutch series and estimated efficacy of the surveillance
Table 10 gives the pathological attributes of the tumours diagnosed in FH01, in the Age Trial control group and in the non-BRCA family history cases from the Dutch study. 22,25 Invasive cancers in FH01 were significantly smaller (p = 0.004), less likely to be node-positive (p = 0.003) and of more favourable histological grade (p = 0.002) than the Age Trial controls. They were also significantly less likely to be node-positive than the Dutch cancers (p = 0.005), but did not differ significantly from the Dutch tumours in terms of size (p = 0.2). The grade distribution of the FH01 cancers was more favourable than that of the Dutch tumours, with borderline significance (p = 0.05).
| Factor | Category | No. (%) in FH01 | No. (%) in Age Trial controls22 | No. (%) in non-BRCA Dutch cases25 |
|---|---|---|---|---|
| Tumour size | <20 mm | 81 (70) | 397 (55) | 145 (63) |
| > 20 mm | 35 (30) | 321 (45) | 87 (37) | |
| NK | 4 | 37 | 6 | |
| Node status | Negative | 77 (68) | 306 (53) | 121 (52) |
| Positive | 36 (32) | 276 (47) | 111 (48) | |
| NK | 7 | 173 | 6 | |
| Grade | 1 | 23 (20) | 53 (8) | 20 (8) |
| 2 | 42 (36) | 285 (43) | 56 (31) | |
| 3 | 52 (44) | 324 (49) | 101 (61) | |
| NK | 3 | 93 | 61 | |
| Average NPI score ( | 95% CI) | 3.98 (3.76 to 4.20) | 4.53 (4.44 to 4.62) | 4.62 (4.43 to 4.81) |
Average NPI28 score in the FH01 tumours was 3.98 (95% CI 3.76 to 4.20). This differed significantly (p < 0.001) from the average NPI score of 4.53 (95% CI 4.44 to 4.62) in the Age Trial controls, and from the average NPI score of 4.62 (95% CI 4.43 to 4.81) in the Dutch series (p < 0.001).
The predicted average 10-year survival rates from the NPI score were 84% (95% CI 81% to 87%), 73% (95% CI 71% to 75%) and 71% (95% CI 68% to 74%) for the FH01 tumours, the Age Trial control group tumours and the Dutch series, respectively.
For the absolute mortality comparison, the fatality rates (complement of the survival rates) were applied to the 120 invasive tumours in FH01 and the 755 in the Age Trial control group,22 to give 19 and 204 expected deaths, respectively. With person-years for FH01 and the Age Trial controls of 37,025 and 622,127, respectively, and underlying 10-year average breast cancer risks from Tyrer et al. 's method17 of 6.3% [standard error (SE) 0.02%] and 2.4% (SE 0.03%), we have:
The 95% CI on RR is 0.37 to 0.98. Thus, there was a significant (p = 0.04) reduction in predicted mortality as a result of the surveillance in FH01. The expected number of breast cancer deaths in the absence of the surveillance is 19/0.60 = 32. Thus, we estimated that 13 breast cancer deaths were prevented as a result of 30,556 surveillance episodes and 4.3 deaths avoided per 10,000 screening episodes. Note that this figure pertains only to deaths avoided within 10 years of diagnosis, and so is conservative.
The comparison with the Dutch series would give a rather larger reduction in mortality, 45%.
Internal estimation of the mortality effect using Markov modeling
The broad strategy in internal estimation of the effect of the surveillance on mortality was to:
-
Estimate the parameters of a Markov model of disease progression by lymph node status.
-
From this, estimate the numbers of node-positive and node-negative cancers which would have been diagnosed during the surveillance period in the absence of the mammographic surveillance (comparison group).
-
Combine these with 20-year survival data by node status to estimate the year by year expected numbers of deaths in the cohort if the surveillance had not taken place.
-
Combine the observed cancers in the cohort by node status with the same survival data, to obtain the expected numbers of deaths in each year in the cancers actually observed.
-
Accumulate the differences between points 3 and 4 (above) to estimate the reduction in mortality and life-years saved as a result of the surveillance over 20 years of follow-up from diagnosis.
A number of issues arise in this activity. Firstly, we obtained conservative estimates of life-years saved by only including in the comparison group those cancers which would have arisen in the period of surveillance in our cohort in the absence of screening. Thus, we will be working with a larger number of cancers in the real FH01 cohort than in the notional comparison group because of lead time.
Our Markov model was estimated from the data on prevalence screen, incidence screen and interval cancers, as in Day and Walter. 38 We performed the estimation twice, first using only the invasive tumours with known node status, and second including DCIS and cases with nodes unknown as node-negative.
Table 11 shows the data used to estimate the parameters of the Markov model. Table 12 shows the resulting progression parameters estimated by the two strategies. The latter can then be applied to estimate the probability of node-positive and node-negative disease in the absence of surveillance. Because the time of ‘birth’ of tumours into the presymptomatic phase is unknown and could predate entry to the study by several years, we follow Day and Walter38 in approximating the inception of tumours as a uniform annual rate, but use the traditional Markov assumption of exponential rates for all other transitions. If parameters are named as in Table 12, the probability of breast cancer during the average 5.52 years of observation is:
| Detection mode | No. screened | Cancers by node status | |||
|---|---|---|---|---|---|
| Node-negative | Node-positive | DCIS | Node status unknown | ||
| Prevalence screen | 2068 | 4 | 2 | 3 | 1 |
| Incidence screen | 28,488 | 51 | 18 | 37 | 6 |
| Interval | – | 20 | 15 | 3 | 1 |
| Non-attender | – | 2 | 1 | 1 | 0 |
| Transition | Parameter symbol | Estimate (95% CI) | |
|---|---|---|---|
| Strategy 1 | Strategy 2 | ||
| No disease to asymptomatic N– | λ1 | 0.0039 (0.0037 to 0.0042) | 0.0042 (0.0034 to 0.0051) |
| Asymptomatic N− to symptomatic N− | λ2 | 0.6047 (0.4314 to 0.8477) | 0.3535 (0.1026 to 1.2180) |
| Asymptomatic N− to asymptomatic N+ | λ3 | 1.0660 (0.8182 to 1.3889) | 0.4015 (0.1013 to 1.5905) |
| Asymptomatic N+ to symptomatic N+ | λ4 | 1.7598 (1.0694 to 2.8959) | 2.0634 (0.1084 to 39.2740) |
This would equal 0.0215 in the first estimation strategy and 0.0232 in the second, giving total numbers of cancers as 144 and 156, respectively. The numbers are smaller than the 165 observed in our cohort as a result of the detection of additional cancers in FH01 because of lead time, as noted above. To be conservative, we base our estimates on these numbers of cancers, potentially inflating the estimated numbers of breast cancer deaths in our cohort.
The expected number of node-negative cancers is:
This simplifies to:
This is equal to 0.0069 under the first estimation strategy and 0.0083 under the second, yielding estimated numbers of node-negative cancers of 46 and 56, respectively. The corresponding expected numbers of node-positive cancers are calculated by subtraction as 144 − 46 = 98 and 156 − 56 = 100.
Table 13 shows the survival of 642 node-positive and 1557 node-negative tumours in the Swedish Two-County Trial,27 by year since diagnosis, up to 20 years' follow-up. If we apply these to the numbers of node-positive and node-negative tumours in our FH01 cohort, we have 46 deaths from breast cancer expected. For the comparison group with no surveillance, we expect 67 deaths under estimation strategy 1 and 70 deaths under strategy 2. These correspond to a reduction in breast cancer mortality of between 31% and 34% and an absolute benefit of between 6.9 and 7.8 deaths prevented over 20 years of follow-up per 10,000 screening episodes. The corresponding year-on-year differences give 387 life-years saved by the surveillance under strategy 1 and 427 under strategy 2. Lagging the comparison group figures by a year's lead time would give 320 and 357 life-years saved. The combined effect of this lag, the estimated additional cancers in the FH01 cohort and the stratification by node status together give a complete and likely conservative adjustment for lead time. In terms of quality adjustment, these additional life-years saved would be spent with a diagnosis of breast cancer.
| Time since diagnosis (years) | Survival (%) | |
|---|---|---|
| Node negative | Node positive | |
| 0 | 100.0 | 100.0 |
| 1 | 99.8 | 89.7 |
| 2 | 99.0 | 79.6 |
| 3 | 97.5 | 73.7 |
| 4 | 96.1 | 69.5 |
| 5 | 94.8 | 63.7 |
| 6 | 93.5 | 60.0 |
| 7 | 92.7 | 56.6 |
| 8 | 91.5 | 54.0 |
| 9 | 90.1 | 52.1 |
| 10 | 89.0 | 50.3 |
| 11 | 88.1 | 49.0 |
| 12 | 87.2 | 47.7 |
| 13 | 86.5 | 46.1 |
| 14 | 85.8 | 45.8 |
| 15 | 84.7 | 44.2 |
| 16 | 83.3 | 43.4 |
| 17 | 82.4 | 41.9 |
| 18 | 81.8 | 41.2 |
| 19 | 81.3 | 40.3 |
| 20 | 81.3 | 40.3 |
Economic evaluation
Having derived the life-years saved in Internal estimation of the mortality effect using Markov modelling, we now:
-
tabulate the screening and diagnostic activity undergone in the study as a result of the surveillance
-
tabulate the treatment activity
-
tabulate the treatment activity by node status and apply this to the expected cancers by node status in the absence of surveillance, in point 2 above
-
cost the activities; and
-
calculate difference in costs corresponding to the life-years saved, after quality adjustment.
Table 14 shows the screening and diagnostic activity in the FH01 cohort. There were 30,554 screening episodes, generating 1803 assessment clinic visits, with the consequent investigations shown in Table 14. Note that we do not include primary care costs in either the FH01 cohort or the notional comparison groups. The procedures associated with screen detection were directly observed in the cohort. Since we did not have information on negative symptomatic consultations, we used data from 16,603 symptomatic breast clinic visits reported by Britton et al. 39 to estimate these. In the 16,603 symptomatic clinic visits, 1235 (7.4%) breast cancers were diagnosed, suggesting 13.44 visits for each cancer diagnosed. This would imply 578 symptomatic clinic visits in the FH01 cohort, with the numbers of procedures incurred estimated from the proportions observed by Britton et al. 39
| Detection mode | Procedure/investigation | Quantity |
|---|---|---|
| Screening | Screening mammography episodes | 30,554 |
| Ultrasound at clinic | 1390 | |
| Mammography at clinic | 1803 | |
| Clinical examination at clinic | 1372 | |
| Percutaneous biopsies | 411 | |
| Open biopsies | 162 | |
| Symptomatic | Ultrasound at clinic | 377 |
| Mammography at clinic | 383 | |
| Clinical examination at clinic | 577 | |
| Percutaneous biopsies | 98 | |
| Open biopsies | 5 |
We also used the Britton et al. 39 data to estimate the diagnostic activity taking place in the notional comparison groups. With estimation strategy 1, we would expect 1935 clinic visits (13.44 × 144) and with strategy 2, 2097 (13.44 × 156). Corresponding expected numbers of procedures also calculated from the proportions in Britton et al. 39 are shown in Table 15. Since the diagnostic activity took place in the twenty-first century, the majority of percutaneous biopsies are likely to be core biopsies.
| Estimation strategy | Procedure/investigation | Quantity |
|---|---|---|
| 1 (144 cancers) | Ultrasound at clinic | 1264 |
| Mammography at clinic | 1283 | |
| Clinical examination at clinic | 1933 | |
| Percutaneous biopsies | 329 | |
| Open biopsies | 15 | |
| 2 (156 cancers) | Ultrasound at clinic | 1369 |
| Mammography at clinic | 1390 | |
| Clinical examination at clinic | 2095 | |
| Percutaneous biopsies | 356 | |
| Open biopsies | 17 |
Table 16 shows the treatments used in the 165 cancers in FH01. Four women had no surgery recorded, possibly because diagnostic open biopsy was judged to have removed the tumour. Table 17 shows the percentage of women by surgical and adjuvant treatment for node-negative and node-positive tumours separately. These were used to estimate the numbers receiving the various treatments in the comparison group, for the two estimation strategies (Table 18).
| Treatment | Quantity |
|---|---|
| Mastectomy | 65 |
| Local excision (sometimes referred to as lumpectomy) | 96 |
| No surgery recorded | 4 |
| Radiotherapy | 68 |
| Hormone therapy (almost invariably tamoxifen) | 64 |
| Chemotherapy | 57 |
| Treatment | Per cent treated | |
|---|---|---|
| Node-negative cases (includes DCIS) | Node-positive cases | |
| Mastectomy | 34 | 41 |
| Local excision | 63 | 59 |
| No surgery | 3 | 0 |
| Radiotherapy | 36 | 59 |
| Hormone therapy | 34 | 54 |
| Chemotherapy | 25 | 68 |
| Treatment | Strategy 1 (144 cancers) | Strategy 2 (156 cancers) |
|---|---|---|
| Mastectomy | 56 | 60 |
| Local excision | 87 | 94 |
| No surgery | 1 | 2 |
| Radiotherapy | 74 | 79 |
| Hormone therapy | 69 | 73 |
| Chemotherapy | 78 | 82 |
The ranges of estimated benefits in terms of life-years saved were 320–387 for estimation strategy 1 and 357–427 for estimation strategy 2. To be conservative, we used the lower points of these ranges, 320 and 357. The total cost-incurring items are shown in Table 19, as observed in FH01 and for the two estimation strategies for the comparison group not subject to surveillance. Note that the estimated life-years saved are all spent with breast cancer.
| Item | FH01 cohort | Comparison group estimation 1 | Comparison group estimation 2 |
|---|---|---|---|
| Mammograms | 30554 | 1283 | 1390 |
| Ultrasound exams | 1767 | 1264 | 1369 |
| Clinical exams | 1949 | 1933 | 2095 |
| Core biopsy | 509 | 329 | 356 |
| Open biopsy | 167 | 15 | 17 |
| Mastectomy | 65 | 56 | 60 |
| Lumpectomy | 96 | 87 | 94 |
| Radiotherapy | 68 | 74 | 79 |
| Tamoxifen | 64 | 69 | 73 |
| Chemotherapy | 57 | 78 | 82 |
The costs were derived from the Department of Health's national schedule of costs for trusts and primary care trusts augmented with costs estimated from research studies where necessary. 40–45 The life-years saved were quality adjusted by a factor of 0.8 since all would be spent with a diagnosis of and with the consequences of treatment for breast cancer. Results are shown in Table 20. We also calculated 95% CIs on the incremental cost-effectiveness ratios (ICERs) by Monte Carlo simulation, using the dispersion data for the cost variables and assuming a normal distribution assumption. The estimated ICERs for the two estimation strategies were, respectively, £5450 (95% CI £4154 to £7878) and £4435 (95% CI £3426 to £6234) per quality-adjusted life-year (QALY) saved. 46 If we discount the future benefits achieved by 2% per annum, this would increase the ICERs by approximately 20%, giving ICERs of £6540 and £5322, respectively.
| Item | Unit cost/QA coefficient | Sources | Costs for groups (£) | ||
|---|---|---|---|---|---|
| FH01 cohort | Comparison group estimation 1 | Comparison group estimation 2 | |||
| Mammogram | 47 | 43–45 | 1,436,038 | 60,301 | 65,330 |
| Ultrasound examination | 76 | 41, 43 | 134,292 | 96,064 | 104,044 |
| Clinical examination | 161 | 41 | 313,789 | 311,213 | 337,295 |
| Core biopsy | 149 | 42, 43, 45 | 75,841 | 49,021 | 53,044 |
| Open biopsy | 216 | 41 | 36,072 | 3240 | 3672 |
| Mastectomy | 7,342 | 41 | 477,230 | 411,152 | 440,520 |
| Lumpectomy | 2,023 | 41 | 194,208 | 176,001 | 190,162 |
| Radiotherapy | 2,479 | 45 | 168,572 | 183,446 | 195,841 |
| Tamoxifen | 155 | 45 | 9920 | 10,695 | 11,315 |
| Chemotherapy | 7,127 | 40 | 406,239 | 555,906 | 584,414 |
| Total cost | 3,252,201 | 1,857,039 | 1,985,637 | ||
| Net cost over comparators | 1,395,162 | 1,266,564 | |||
| Minimum life-year gains over comparators | 320 | 357 | |||
| QALYs | 0.80 | 46 | 256 | 286 | |
| ICER, cost per QALY | 5450 | 4435 | |||
Withdrawals
Table 21 shows withdrawals or women censored from the study with reasons for withdrawal. There was a total of 165 women censored because of confirmed breast cancer and 534 (8%) withdrawals. The most common reason for withdrawal was removal from the local programme because of non-attendance (47% of withdrawals). Only 35 women withdrew because of change of genetic status (including eight with BRCA mutations in the family but a negative personal test) and 24 because of risk-reducing surgery.
| Reason for withdrawal | Number of withdrawals or censored |
|---|---|
| Change of genetic status | 35 |
| Removed from local programme because of non-attendance | 252 |
| Diagnosed with breast cancer | 159 |
| Breast cancer – unconfirmed | 1 |
| Died of breast cancer | 6 |
| Died of other cause | 27 |
| Moved out of area | 90 |
| Refused further surveillance | 35 |
| Risk-reducing surgery | 24 |
| Other | 70 |
| Total | 699 |
There were six deaths from breast cancer. One woman did not receive surgery and so had no pathology data. Of the remaining five women, four were node positive at diagnosis, two were of grade 3, two were of grade 2 and one was of grade 1. Average size was 21 mm.
Discussion and implications
The results here indicate a significant 31–45% reduction in predicted breast cancer mortality due to the annual (or approximately annual) mammographic surveillance in women at moderately increased risk due to family history. This translates to 4.3 breast cancer deaths prevented within 10 years of diagnosis per 10,000 screening episodes and to 6.9–7.8 deaths prevented over 20 years for the same number of screening episodes. Our economic analysis suggests that the intervention is cost-effective in UK terms and is unlikely to exceed £7878 per QALY.
The results differ slightly from those published in 2010. 23 This is because of increased follow-up and further tumours diagnosed, pursuit and checking of baseline data and more accurate estimation of the person-years at risk.
The major limitations of this study are the absence of a control group (a consequence of insufficient equipoise) and the use of predicted rather than actual mortality. Mortality was predicted using the NPI, which is a combination of the pathological size, node status and grade of the tumours, and also from the Markov model for node status. We did originally approach breast centres offering family services, proposing a randomised trial, but met with negative responses. The clinicians in the breast units felt that there was insufficient equipoise. The consensus was that it was ethically dubious to randomise intermediate-risk subjects to no surveillance. Alternative designs were considered, such as randomised trials of mammography against clinical breast examination and different mammographic frequencies, but it was felt that these did not answer the question of interest: is mammographic surveillance saving lives in comparison with no surveillance? These designs, with a mortality end point in subjects all of whom were receiving some surveillance, would also have entailed recruiting very large numbers and follow-up for 10–20 years to observe the breast cancer deaths. However, it was also felt that evaluation results were needed in the next few years, and not several decades hence; it was therefore necessary to identify a design and analysis that allowed surveillance to continue in all subjects and that delivered a timely end point. It has to be acknowledged that these considerations did dictate a less definitive and less straightforwardly interpretable study than a randomised trial.
We corrected our comparison with the Age Trial control group for the independently calculated 10-year risk of breast cancer rather than the observed incidence, to avoid length bias and overdiagnosis bias. The estimated incidences are higher than those observed, owing to the former being based on a 10-year period rather than the observed periods of 5–6 years on average. This means that the estimated risks necessarily pertain to a higher average age and, therefore, a higher average incidence. The proportional difference is greater for the Age Trial controls (2.4 per 1000 per year vs 1.3 per 1000 per year) than for the FH01 cohort (6.3 per 1000 per year vs 4.5 per 1000 per year), so the reduction in mortality is underestimated rather than overestimated. Also, given the fact that the observed incidence is if anything lower than expected, there is no evidence of serious overdiagnosis.
Although the age ranges of the comparison groups differ from that in FH01, the main comparison group, the Age Trial control group, is similar to that of FH01, with ages at diagnosis of 40–49 years compared with 40–50 years in FH01. The faster growth rates of tumours in younger women47 might mean that our comparison with the Dutch series is indeed conservative.
Also, although the epoch of diagnosis is earlier in both comparison groups, there was no indication of a change in prognostic attributes with time in the Age Trial controls. No significant trends in node-positive rates (p = 0.89), tumour size (p = 0.54) or histological grade (p = 0.56) were observed. Prior to 1996, 48% of cases were node positive. Thereafter, the proportion was 47%. The corresponding proportions of invasive tumours of size ≥ 20 mm were 56% and 53%, and of grade 3 tumours 48% and 49%. With improvements in treatment over time, a comparison of actual mortality would be confounded with treatment effects. However, use of the projected mortality from the tumour attributes is unaffected by treatment. It might be expected that the actual breast cancer mortality in the FH01 cohort will be lower than projected mortality, owing to improved treatment. It should also be noted that the predicted mortality for both comparison groups was close to but slightly lower than the observed. For the Age Trial controls the actual survival was approximately 71% and for the Dutch series 70%, suggesting that our results may in fact be conservative. 25,48
We specifically targeted the age range 40–44 years at recruitment so that the subjects would contribute 5 years of observation before reaching age 50 years, thus avoiding arguments about ‘age creep’. 20 This also rendered our group comparable with the Age Trial controls who were recruited at ages 40–42 years and followed up for 10 years (in our case, the recruits were mainly aged 40–44 years and followed up for an average of 5.52 years). The age range of the Dutch cases varies widely and the comparison with the latter must be interpreted much more cautiously.
The basic principles of our estimates of the effect on breast cancer mortality are (1) that the effects of screening on tumour size, node status and grade are predictive of its effect on future mortality, and (2) that node status alone or in combination with other factors as in the NPI gives accurate prediction of survival from breast cancer. These have both been subject to empirical validation. Blamey et al. 28 estimated the effect of NPI on 10-year survival in two mutually exclusive tumour sets and found that the two graphs of dependence were virtually overlapping. Table 22 shows the RR of node-positive disease together with the RR of mortality (study vs control group) in the results from the randomised trials of breast screening. 48–54 Clearly, the effect of screening on node-positive disease is closely reflected in its effect on breast cancer mortality.
| Trial48–54 | RR mortality | RR node-positive |
|---|---|---|
| HIP Greater New York | 0.77 | 0.85 |
| Malmöa | 0.78 | 0.83 |
| Two-County | 0.68 | 0.73 |
| Edinburgh | 0.71 | 0.81 |
| Stockholm | 0.90 | 0.82 |
| NBSS1 | 1.06 | 1.20 |
| NBSS2 | 1.02 | 1.09 |
| Gothenburg | 0.76 | 0.80 |
| Age Trial | 0.83 | 0.89 |
It could be argued that the comparisons are confounded by other tumour attributes than size, node status and grade. However, biological variables such as hormone receptor status are strongly correlated with size, node status and grade. 55 Also, Dawson et al. 56 investigated the effects of 11 biological variables on survival in breast cancer and found that they added little to NPI in explaining the survival advantage of screen-detected cancers.
It is also of interest to compare the attributes of the FH01 cancers with those diagnosed in the NHSBSP. In those attending for surveillance in FH01, 24% of cancers were interval cancers and the remainder were screen detected. In the West Midlands Screening Histories project in 2002, around 41% of cancers in attenders to the national programme were interval cancers. 57Table 23 shows the proportions of node-positive, size > 20 mm, and grade 3 invasive cancers in FH01 and in 14,672 invasive tumours in the West Midlands project,58 for interval cancers and screen-detected cancers separately.
| Attribute | Per cent with attribute in screen-detected cancers | Per cent with attribute in interval cancers | ||
|---|---|---|---|---|
| FH01 | NHSBSP West Midlands | FH01 | NHSBSP West Midlands | |
| Node positive | 27 | 22 | 43 | 39 |
| Tumour size > 20 mm | 29 | 21 | 34 | 44 |
| Grade 3 | 45 | 17 | 47 | 37 |
Results for node status were similar for the two series, each showing a substantially lower rate of node-positive disease in the screen-detected tumours. There was a smaller difference in the FH01 series for tumours of size > 20 mm. Reflecting the younger age at diagnosis, the FH01 tumours, including the screen-detected tumours, were much more likely to be grade 3. This may explain the smaller effect on tumour size in FH01.
A relatively high proportion of cancers diagnosed in FH01 were DCIS, 26% overall and 33% of screen-detected cancers, compared with approximately 20% in the NHSBSP. Table 24 shows the size and grade of DCIS cases detected at surveillance compared with 700 cases detected in the NHSBSP in the West Midlands. 59 A higher proportion of large and high-grade tumours was observed in FH01, again possibly because of the younger age at presentation. The high grade of the FH01 tumours suggests that the high rate of DCIS is unlikely to be because of overdiagnosis.
| Attribute | Category | Per cent (screen-detected cancers only) | |
|---|---|---|---|
| FH01 | NHSBSP West Midlands | ||
| Size | ≤ 20 mm | 57 | 88 |
| > 20 mm | 43 | 12 | |
| Grade | Low | 8 | 12 |
| Intermediate | 25 | 30 | |
| High | 67 | 58 | |
In conclusion, our most conservative estimate of the cost per QALY saved was £6450, which is less expensive than combined mammography and MRI in BRCA1 mutation carriers. 60 The conclusion of this work is that annual mammography surveillance for women at moderate familial risk is both clinically effective and cost-effective.
Chapter 6 Radiology and pathology reviews
Introduction
The intention to carry out radiology and pathology reviews was noted in the original FH01 protocol, although their design and conduct was not specified. In this chapter, we summarise the protocols of the reviews, report progress on the reviews and give some preliminary results. The reviews are substantial pieces of work, involving the collation from multiple centres of radiological images and biological material. Consequently, they are still ongoing, but there are already some interesting observations, notably for the radiology review.
Radiology review
Radiology review is standard practice in major screening studies. 61,62 This radiology review has two components: a rereading of mammograms of cancers and selected non-cancer cases for radiological features and their correlation with pathological and biological features; and a case–control study of mammographic density. The first component of the review includes:
-
Determination of the observed radiological features of the cancers on mammography, to identify diagnostic features with verified poor outlook either on histology, biological features or outcome (in the long term, survival and disease-free survival).
-
Radiological audit to identify those tumours which could have been detected at a screen previous to the diagnosis, (i.e. potential false-negatives), with a review of the reasons for failure of mammographic diagnosis. This is particularly relevant to the 26% interval cancers.
-
Comparison with other age groups or risk profiles, including:
-
the NHSBSP for women aged 50–70 years – essentially postmenopausal women
-
women in the UK study comparing MRI with mammography. These women have a high probability of carrying BRCA1 or BRCA2 mutations because of intensity of family history. These are women aged < 50 years, similar to FH01, but with a considerably higher level of risk63
-
the ongoing FH02 study – women aged 35–39 years at elevated risk due to family history.
-
The review includes the films of the cancers including their previous mammograms, as in the radiology reviews of the previous breast screening studies such as the Age Trial and the Breast Screening Frequency Trial. 61,62 The review differs from that of previous studies in three important respects. First, it includes mammograms from subjects who never developed cancer during the study, two per cancer matched for age, centre and date of screening. Second, the X-rays are digitised (where not already digital) so that reviewers can view the mammograms without either readers or films having to travel. Third, the review includes the density study mentioned above.
Digitisation of the analogue mammograms was by Array Corporation's 2905 X-ray film digitiser (Array Corporation USA, Hampton, NH, USA), which gives a pixel size of 3600 by 4800 and DICOM resolution of 1 mm = 20 pixels, equivalent to 12 bit. The DICOM images were converted to bitmap images as this format is the most suitable for uploading to the web. This conversion reduces the resolution to 8 bit. Digital mammograms were anonymised, assigned a unique study number, then converted to bitmap (8 bit) format and uploaded to the web.
The formatted and anonymised images were uploaded into Image-box, version 1 (University of Southampton, Southampton, UK), where there is further compression of the images to 550 by 900 pixels. We used the image database developed for the Prospective study of Outcomes in Sporadic and Hereditary breast cancer (POSH) radiological review. 64 This is web based and was developed by Kevin Wheeler of Southampton University, who is employed by Professor James Batchelor, in the Department of Computer Science.
Features of the POSH database include:
-
It is web based and password controlled.
-
It incorporates anonymised scanned mammographic and ultrasound images.
-
Recording sheets appear online to match images and screening events under examination.
-
It enables a greater number of radiologists to participate and can therefore be opened up to volunteers from the centres.
-
It allows for two readers per study.
-
A third reader arbitrates on any differences (we have a limited list of final decision arbitrators). For each field the final observation is adopted if both initial readers agree or two out of three after arbitration agree. If all three differ, we take as the final observation the decision of the third reader.
-
The database uses Breast Imaging-Reporting and Data System (BI-RADS) terms so that it is suitable for publication in American journals.
-
Studies are reviewed starting with the latest screening episode and progressing backwards through the series so that any information on the site of the cancers can be used to look for subtle earlier signs (in the same way as interval cancer audit is generally done).
A major outcome of the first component of the radiology review is radiological audit of cancers arising in FH01 – could these have been picked up at previous screens, and what are the key radiological signs of this? The inclusion of the non-cancers will give us information about specificity of earlier signs of malignancy, yielding an estimate of the likely effect on false-positives of a change in practice or training. Other outcomes include a quality assessment of both radiology and radiography in FH01. It should be noted, however, that with the advent of digital mammography, accuracy will improve in any case for this young population with relatively dense breast tissue.
The second component of the radiology review is a case–control study of breast density, again using at least two age-matched controls per cancer case. The major aim is to determine whether or not breast density is a risk factor in this population at enhanced familial risk, as it is in the general population. 65 Secondary outcomes will be the determination of whether per cent density or absolute dense area is the better predictor of breast cancer risk in this population, and how far in advance of diagnosis does density predict risk. Density was measured by the Cumulus interactive threshold computer program version 4 (University of Toronto, Toronto, Canada),66 operated by a single radiologist (Ruth Warren) with extensive experience in reading mammograms for density, both visually and using Cumulus. The program yields measures of dense area and total breast area. From these, the per cent dense area and the non-dense area can be calculated.
Density was read on digitised mammograms as described above. We have digitised mammograms for 103 cancer cases and 231 disease-free controls. The multiple readings for earlier mammographic signs of malignancy, quality assessment and radiology/pathology/biology correlation is ongoing. Density readings were available for 101 cases and 228 controls. Some results of the density study are already available. Table 25 shows the dense area, total breast area and per cent density for cases and controls. The cases have slightly higher values than the controls for all three measures, but especially so for absolute dense area. As expected, per cent density was negatively correlated with age, although this was of borderline significance (correlation coefficient −0.10; p = 0.06).
Formal analysis was by conditional logistic regression, taking into account the individual matching of cases and controls.
| Group | Mean (SD) dense area in cm2 | Mean (SD) total breast area in cm2 | Mean (SD) per cent density |
|---|---|---|---|
| Controls | 47 (32) | 152 (66) | 34 (19) |
| Cases | 59 (41) | 162 (74) | 37 (19) |
There was a significant increase in risk of cancer with absolute breast density after adjustment for menopausal status (p = 0.03), with an 8% increase per 10 cm2 of dense tissue [odds ratio (OR) = 1.08, 95% CI 1.01 to 1.19]. The difference was more marked in premenopausal women (p = 0.008), defined as having had a menstrual period within the last 6 months. There was a 12% increase in risk per 10 cm2 of dense tissue (OR = 1.12, 95% CI 1.02 to 1.22). This remained significant after adjusting for HRT, age at menarche, parity and age at first birth (OR = 1.15, 95% CI 1.02 to 1.29; p = 0.01). A non-significant decrease in risk was observed in postmenopausal women; however, only 25 (8%) women were postmenopausal.
Per cent breast density did not have a significant effect on risk, regardless of menopausal status, unless adjusted for total breast area. In terms of both significance (p = 0.008 vs p = 0.03) and the magnitude of the standardised regression coefficient (0.41 vs 0.34), absolute dense area was a stronger predictor of risk than total area-adjusted per cent density.
Further analysis will focus on time between the mammographic examination from which density was estimated and diagnosis of cancer, on association of density with other breast cancer risk factors, and on the combined effects of density and other factors on breast cancer risk. In the meantime, the conclusions from the density component of the radiology review are that absolute density is a stronger predictor of breast cancer risk than per cent density in this population, and that absolute density increases risk in addition to the effect of other breast cancer risk factors. There is suggestive evidence that the effect is stronger in premenopausal women.
Pathology review
This will be a standardised review of conventional histopathological features (grade, type, size, etc.), which will be compared with the original pathology laboratory determination. In addition, this gives an opportunity to record other morphological features that are increasingly recognised as important characteristics of specific tumour subtypes, such as central scar formation, lymphocytic response, pushing or infiltrative tumour margin and degree of stromal response. 67 In addition, features of ‘background’ non-involved breast tissue will be documented, which will be of relevance to the linked radiology review (see Radiology review above). It is suggested, therefore, that all haematoxylin- and eosin-stained slides for each case are requested for review. The pathology review form is shown in Figure 2.
FIGURE 2.
Pathology review form.
This is similar to the pathology reviews carried out in the various UK breast screening trials. However, in addition to the standard pathological variables, we also propose to stain the tumour samples for the recently discovered molecular subtypes of tumour, to determine the underlying aggressiveness of cancers occurring in the FH01 risk group. An ‘intrinsic gene set’ identified by Perou et al. 68 and validated by Sortie et al. 69 has led to the recognition of five ‘molecular’ subgroups: luminal (Lum) A, Lum B + C, human epidermal growth factor receptor-2 (HER-2) positive, basal and ‘normal-like’. These molecular subgroups of breast cancers have been shown to differ in their clinical behaviour, with HER-2-positive and basal groups exhibiting the poorest prognosis. BRCA1-associated breast cancers frequently exhibit a basal phenotype,70 but basal tumours are also more common in non-BRCA-associated cancers arising in young women,71 and may be associated with loss of BRCA function through other mechanisms such as gene methylation. 72 There is, however, growing evidence that the basal subtype of breast cancer may not be a single entity,73,74 and different subsets may differ in their clinical behaviour and in potential therapeutic targets. Recent research identifying molecules that are highly effective at targeting BRCA-null tumours (and therefore potentially all or a subgroup of basal tumours) underlines the importance of accurately establishing the molecular phenotype of breast cancers. 75 This study will employ a wide panel of markers that should better identify biologically important tumour subsets. In addition, whole sections will be analysed for BRCA1-methylation status (methodology already optimised).
As a comparison group, we will interrogate the POSH database64 to identify a cohort of age-matched non-family history cases on which the same analysis will be performed as part of the POSH study.
The tumour samples are still being collated, which is proving a demanding job in terms of administration and governance, with material transfer agreements being required for > 70 sites. For one centre with seven cancers, the tumour material has been transferred to Barts Health and the rereading of invasive status and grade is 100% in agreement with that in the original pathology laboratory, and correlation of tumour size with that of the original laboratory is 0.9997.
Chapter 7 Follow-up data
Follow-up questionnaire
We planned to send a follow-up questionnaire to all subjects who had completed 5 years in the study. The purpose of this was to update risk factor information, in particular family history, and to check on the reliability of the data by estimating agreement/disagreement rates between the first and second enquiry. For example, although parity and number of affected relatives might both increase over the period of the study, they cannot decrease. The approval of clinical staff managing the subject's surveillance was sought before sending the questionnaire. The items of information in the questionnaire were as follows:
-
ever in employment (yes/no)
-
educational level attained
-
weight and height
-
parity
-
breastfeeding
-
menopausal status/age at menopause
-
HRT use
-
tamoxifen use
-
update to family history (relatives diagnosed with breast cancer since recruitment).
Results
So far, 5462 follow-up questionnaires have been sent out and 2760 (51%) returned. Data have been entered and successfully linked with FH01 baseline data for 2705 (98%) of these. Table 26 shows the distributions of risk factors reported in the follow-up questionnaire. Understandably, the proportion of postmenopausal subjects is higher in the follow-up survey than at baseline, and the parity distributions are similar (see Chapter 3, Table 2). Substantial numbers reported a relative diagnosed with cancer since their initial family history was taken at recruitment, 13% reporting a diagnosis in their mothers and 9% in their sisters.
| Factor | Category | No. of subjects (%) |
|---|---|---|
| Body mass index (kg/m2) | < 20 | 103 (4) |
| 20-24.9 | 951 (41) | |
| 25-29.9 | 779 (33) | |
| 30+ | 511 (22) | |
| Not known | 361 | |
| Parity | 0 | 366 (14) |
| 1 | 444 (17) | |
| 2 | 1191 (46) | |
| 3+ | 569 (23) | |
| Not known | 135 | |
| Ever breastfed? | No | 1705 (75) |
| Yes | 578 (25) | |
| Not known | 422 | |
| Menopausal status | Premenopausa | 1932 (72) |
| Postmenopausa | 737 (28) | |
| Not known | 36 | |
| HRT use? | No | 2430 (90) |
| Ye s | 256 (10) | |
| Not known | 19 | |
| Tamoxifen use? | No | 2581 (99) |
| Yes | 27 (1) | |
| Not known | 97 | |
| Mother diagnosed with breast cancer since recruitment? | No | 2347 (87) |
| Yes | 358 (13) | |
| Sister diagnosed with breast cancer since recruitment? | No | 2455 (91) |
| Yes | 250 (9) |
Table 27 shows the individual baseline responses tabulated against the follow-up responses for the binary variables menopausal status and HRT use. Table 28 gives the baseline responses for parity cross-tabulated with the corresponding follow-up responses. For menopausal status, the agreement rate was 80%. It is plausible that the 402 subjects whose status was premenopausal at baseline and postmenopausal at follow-up did indeed change their status during the study. The eight subjects (< 1%) who reported being postmenopausal at baseline but premenopausal at follow-up suggest a small element of response error at one or both occasions. Results for HRT use suggest a similarly small error rate.
| Factor | Baseline response | Follow-up response (cell %) | |
|---|---|---|---|
| No | Yes | ||
| Postmenopausal? | No | 1491 (74) | 402 (20) |
| Yes | 8 (< 1) | 123 (6) | |
| HRT use (ever)? | No | 1653 (89) | 80 (4) |
| Yes | 23 (1) | 104 (6) | |
| Parity reported at baseline | Parity reported at follow-up (cell %) | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3+ | |
| 0 | 325 (13) | 21 (1) | 17 (1) | 9 (< 1) |
| 1 | 6 (< 1) | 368 (15) | 51 (2) | 9 (< 1) |
| 2 | 9 (< 1) | 29 (1) | 1012 (41) | 34 (1) |
| 3+ | 3 (< 1) | 10 (< 1) | 58 (2) | 489 (20) |
For parity, there was 89% agreement and the small numbers with lower parity reported at follow-up than at baseline (115 subjects, 5%) suggest a small degree of response error for this factor.
The family history factors are rather more difficult to interpret. For maternal breast cancer, 13% report a diagnosis since their original family history was taken at recruitment. For sisters, the figure is 9%. Table 29 shows the original baseline response tabulated against the report of new diagnoses in the follow-up questionnaire. The vast majority of those reporting a new diagnosis in mother or sister already had such a report at baseline, on average 5–6 years before. Although some of the reports may pertain to recurrences or new primaries in the same relative, or to cancer in different sisters, it is likely that the reports refer to the original cancer in the affected relative. The subjects were posted the questionnaire and did not have their baseline responses to hand when completing it. There has been at least one study where a postal questionnaire was considered adequate for taking a family history of colorectal cancer. 76 However, another study investigating personal history of all cancers found rather poor sensitivity of a postal questionnaire. 77 The results here suggest that if a family history is to be updated without a face-to-face interview, then it would be reasonable simply to request the entire family history again, rather than ask the individual to qualify the reported history with respect to previous responses.
| Factor | Baseline response | Report of new diagnosis at follow-up (cell %) | |
|---|---|---|---|
| No | Yes | ||
| Mother have breast cancer? | No | 727 (28) | 16 (< 1) |
| Yes | 1548 (59) | 334 (13) | |
| Sister have breast cancer? | No | 1811 (69) | 40 (1) |
| Yes | 574 (22) | 200 (8) | |
Chapter 8 Related studies
Psychological impact of mammography screening for women under 50 years with a family history of breast cancer
The study of the Psychological Impact of maMMography Screening for women under 50 with a family history of breast cancer (PIMMS) was carried out independently of FH01, but was considered a companion study, with several members of management committees in common. PIMMS was completed before FH01, and has been published extensively. 31–34,78 The major findings of PIMMS were:
-
Gaps in knowledge on the part of screened women were noted, with respect to their own level of lifetime risk and the sensitivity of the screening test.
-
Women who received an immediate all-clear result after mammography experienced a decrease in cancer worry and negative psychological consequences immediately after their screening result, whereas those women recalled for further tests did not. However, recalled women experienced a significant increase in cancer worry which was present at 1-month follow-up. This was not significant for women who only received a non-invasive procedure at recall.
-
By 6 months' follow-up, cancer-specific distress had reduced significantly in both groups. Recalled women did not have higher levels of worry than non-recalled women at 6 months.
-
Worry was significantly stronger at the woman's first screen in the programme.
-
Changes in levels of distress pre and postscreening between women with an immediate all-clear and those who received an all-clear after further tests were significantly different, but in absolute terms the difference was not large.
-
By far, the strongest predictor of worry at an individual level following a screening test was the level of worry experienced before the test. Prescreening worry explained 55–60% of the variability in postscreening worry.
-
Recalled women reported significantly greater positive psychological consequences of screening immediately after their final result and were also more positive about the benefits of screening compared with women who received an immediate all-clear result.
-
Women diagnosed with breast cancer in the programme reported a sense of reassurance from screening prior to diagnosis.
From this it was concluded that women who are recalled for further tests do not appear to be harmed by screening and the study findings suggest that they view any distress caused by recall as an acceptable part of screening.
FH01 blood study
For many years, there has been interest in identifying a blood test indicative of either presence of or very high risk of breast cancer. Although the breast screening programme in the UK serves the population well,79 there is scope for improvement in sensitivity of screening for those subjects with radiologically dense breast tissue. High breast density both increases risk of breast cancer and impairs the sensitivity of mammography. 80 A population such as FH01 is potentially fertile ground for discovery or validation of such markers, as it is relatively young and so has a substantial proportion of subjects with dense breasts, but it has higher incidence than the general population of the same age.
Two blood-testing strategies which show considerable promise are a gene expression test carried out on extracted ribonucleic acid (RNA) from peripheral blood,81 and a serum test for a panel of oncoantibodies. 82
In addition, there is considerable interest in epigenetic markers. 83 Accordingly, we have set up a prospective study taking blood samples from FH01 recruits, and follow-up for subsequent occurrence of breast cancer.
The objectives can be summarised as:
-
Prospective estimation of sensitivity of the gene expression and antibody tests in subjects with a significant family history of breast cancer.
-
Prospective estimation of specificity of the tests in subjects with a significant family history of breast cancer.
-
If the test proves sensitive to breast cancer in this setting, estimation of how far in advance of diagnosis a positive test is observed.
-
Do the answers to questions 1–3 (above) differ between those subjects with dense breasts and those with fatty-replaced breasts?
-
What is the typical profile of this moderate familial risk group with respect to the epigenetic markers?
-
Do any of the epigenetic markers act as early markers of the development of breast cancer in this population?
From the highest recruiting breast cancer clinics for FH01 we propose to recruit 4000 subjects so far free of cancer. These subjects will be asked to provide a blood sample every 12 months for 2 years (at baseline, 12 months and 24 months). In addition to mammographic surveillance within FH01, the subjects are flagged with the Medical Research Information Service, so that all breast cancers, whether detected at surveillance or outwith the study, will be ascertained. A dedicated member of staff at each centre will be trained and responsible for taking all samples.
Blood samples will be collected into three tubes. RNA for the gene expression test will be extracted from two of the tubes in an external laboratory. The extracted RNA will be analysed only for those who are subsequently diagnosed with breast cancer and for 10 control subjects for each breast cancer case. Results will then be analysed for questions 1–4 in the objectives summary above.
Serum from the third tube of each blood sample will be separated and stored for epigenetic testing, and for the onco-antibody assays. Storage will be at < −70 °C. So far we have recruited 132 subjects in four centres and taken 480 blood samples. The pace of progress of the study depends on funding applications currently under way. Further details are given in the blood study protocol (see Appendix 2).
FH02
FH02 is a study of mammographic surveillance in women aged 35–39 years at increased familial risk of breast cancer. The National Institute for Health and Clinical Excellence recommended that such surveillance in this age group only take place in a research context. The aim is to recruit 2800 women satisfying similar family history criteria to FH01. The study end points are detection and interval cancer rates. FH02 is funded by the Breast Cancer Campaign. The funding decision was strongly influenced by the positive results from FH01. To date, 1911 women have been recruited in 32 centres and 17 cancers have been diagnosed.
Chapter 9 Implications of the results
Implications for care policy
The first question to consider is whether or not the study results are sufficiently robust to give indications for policy. FH01 was a volunteer-based, single-arm cohort study. The issue of the volunteer population gives rise to the issue of selection bias. However, in the five centres polled, four reported participation rates in excess of 90%. The early delay in recruitment was due to centre difficulties rather than refusal of women approached to join the study. In addition, the volunteer population reflects practice in the management of women at increased familial risk. There is no unsolicited invitation to surveillance, as there is in the NHSBSP. Women managed by the genetics and family history services have sought advice of their own volition. In terms of the study design, the analytical strategy has dealt with this by adjusting external comparisons for underlying risk using independent estimates, by supplementing this with internal estimation of the future effect and by use of well-validated predictors of breast cancer mortality. It is therefore likely that the results with respect to the effect of the surveillance on future breast cancer mortality are reliable and can inform policy.
The primary implication of the results of FH01 is that the provision of annual mammographic surveillance services from age 40 years to women at moderately increased familial risk of breast cancer is both clinically effective and cost-effective. The policy can be expected to confer at least a 25% reduction in ultimate mortality from the disease in the population offered the service. We estimate that the policy will cost between £4435 and £5450 per QALY saved.
A number of centres practised clinical breast examination and/or ultrasound in addition to mammography. Our results did not indicate any substantially marginal additional benefit of clinical examination, but did suggest that use of ultrasound boosted the detection rates. This is consistent with the age of this population and the associated high prevalence of dense breast tissue.
It should also be noted that at the stage of recruitment, we encountered a wide variation across the country in policies for managing this risk group. It may be that a standardisation of policy, with nationwide co-ordination, would not only enhance the surveillance service for this population, but also bring economies of scale.
The human costs in terms of false-positives and radiation exposure are within the standards of the NHSBSP, as are detection rates. Although the proportion of cancers with preoperative diagnosis is well within the national standard, there is still a rather high rate of benign open biopsies. This needs future monitoring and improvement. Also, although the recall rates for assessment conform to national standards, they are somewhat high. The figures are 8% at prevalence screen and 6% at incidence, corresponding to positive predictive values of 6% and 7%, respectively. To minimise anxiety for this population, the issue might be addressed by immediate interpretation of mammograms with additional imaging, including ultrasound, at the same visit, to eliminate a proportion of the false-positives. This would imply the surveillance taking place at static units, which is generally the case in this risk group. Also, digital mammography was not universally used in FH01, but it is reasonable to assume that it will be in the near future. This may improve the accuracy of the examination. In addition, the advent of digital mammography is likely to improve sensitivity and reduce interval cancer rates.
A reasonable model for management of women aged 40–49 years (or 40–47 years bearing in mind the age expansion of the national programme) would be to have a risk assessment performed by a genetics service or at a specialist breast centre. If there is no or only mild family history, the subject may be reassured and recommended to have no additional surveillance but to be breast aware until she qualifies for the NHSBSP. For those women at high risk, that is, those women with a strong probability of a BRCA1 or BRCA2 mutation, there are management strategies including MRI surveillance and possible surgical interventions. Those women at moderate risk might be referred to the screening programme for an earlier starting age for screening, and an annual interval until age 50 years.
The results of the density component indicate that at least in premenopausal women, breast density adds to information on risk within this moderate-risk population, independently of other risk factors. Although measurement of density is currently labour intensive, with the advent of digital mammography, the scope for development and exploitation of fully automatic computerised density measurement will increase. 84
This begs the question: how to define the moderate-risk group? Resources are limited and the call on these resources will depend on the minimum-risk criterion to qualify for surveillance. From the risk estimates in our data on the FH01 moderate-risk cohort and in the general population sample from the Age Trial, we have estimated the proportion of the population aged 40–49 years who would qualify for the surveillance, for various eligibility criteria based on risk relative to the general population. These are shown in Table 30, along with minimum approximate absolute risks at age 40–49 years and total number of subjects eligible in the UK. The last is based on an approximate estimated female population of 4.5 million women aged 40–49 years in the UK. 85Table 30 also shows the percentage of FH01 subjects who would qualify by each cut-off.
| RR cut-off | Approximate cumulative 10-year risk, age 40–49 years | Per cent of population qualifying | Number of UK women aged 40–49 years qualifying | Per cent of FH01 subjects qualifying |
|---|---|---|---|---|
| 2 | 3% | 10 | 450,000 | 70 |
| 2.5 | 3.75% | 7 | 315,000 | 45 |
| 3 | 4.5% | 4 | 180,000 | 25 |
| 4 | 6% | 1 | 45,000 | 9 |
A cut-off of three to four times the population risk would seem reasonable. Given that only a proportion of the eligible population is likely to come forward, a reasonable cut-off, such as a risk of three times the population risk, would involve an increase of < 10% in the mammography activity in the NHSBSP. 36 A cut-off of four times the population risk would involve only a marginal increase in activity, but this would exclude a large number of women with a substantially higher risk than the population. Surveys in the USA find 7–12% prevalence of moderate familial risk,86,87 but their definition of moderate is likely to include what would be considered mild risk in a UK context.
The results of the follow-up study suggest that in using a self-administered postal questionnaire to update family history, it would be prudent to request the full family history again. In many centres, this is the policy even when family history is reascertained and risk assessment updated by direct interview with a health professional.
Implications for research
First, it should be noted that much of the mammographic activity in FH01 used traditional film mammography and this is steadily changing to digital. There is a need for research to assess the impact that this will have on mammographic accuracy in this population. Indeed, there is a need for research on the optimal way of delivering surveillance, minimising both false-negatives and false-positives, and dealing with the latter in a way which minimises anxiety and other human costs.
Second, there will always be pressure to streamline any risk-based surveillance system. In view of the unsatisfactory family history information in the follow-up study, there is need for research to further improve self-administered family history questionnaires. There will also be room for improvement in delineating individual risk. When standardised risk assessment and referral to the programme are under way, there will be an opportunity to validate and, if necessary, amend the individual risk prediction algorithm.
Third, the results of the breast density part of the radiology review suggest that density adds to risk prediction in this group already at enhanced familial risk, and are consistent with a stronger effect on risk of absolute dense area on the mammogram than of per cent dense area. This issue remains unresolved. 88,89 The increased risk with density appeared to be confined to premenopausal women but we had too few postmenopausal women to conclude this with any certainty. These results indicate that density probably has a role in risk management in the medium term, but in the meantime the following issues should be targets of future research:
-
The effect of density in larger postmenopausal populations at increased familial risk of breast cancer, taking account of other risk factors.
-
Investigation of whether or not automatic methods of breast density can reliably replace traditional methods.
-
Confirmation of the better risk prediction using absolute dense area rather than per cent density.
-
Detailed quantification of the marginal addition to risk information conferred by density.
Overall conclusion
Annual mammographic surveillance in women aged 40–49 years at moderate familial risk of breast cancer results in a significant reduction in future deaths from breast cancer, at reasonable financial and human cost.
Acknowledgements
This is dedicated to the memory of our colleague Joan Austoker.
The research would not have been possible without the women who agreed to be recruited to FH01. The following colleagues contributed to this research.
FH01 Steering Committee Caroline Boggis, John Burn, Jack Cuzick, Bob Haward, Anthony Howell, Robert Mansel, Hazel Marshall Cork, John Robertson, Julietta Patnick, Paul Pharoah, Anne Robinson and Stephen Sutton.
Radiology review Masako Kataoka and Penelope Moyle.
Pathology review Louise Jones.
Psychosocial studies Joan Austoker, Alison Clements and Eila Watson.
Radiation exposure Ken Young.
Co-ordination Phil Duffy, Rhian Gabe, Lorraine Roberts, Ailsa Taylor and Iqbal Warsi.
Informatics and data-analysis Adam Brentnall.
Biological studies Fiona Dungey, Attila Lorincz and Judith Offman.
Collaborators Marion Adams, Jenny Affen, Mary Aldous, Amir Al-Dabbagh, Alison Allen, Alison Ames, Riccardo Audisio, Sue Ashworth, Alison Barnes, Paula Botham, Roger Brookstein, David Brown, Sheila Bullard, Robert Carpenter, Wendy Chorley, Donna Christensen, Cathy Coleman, Christine Coe, David St John Collier, Julie Cooke, Timothy G Cooke (deceased), Ruth Crichton, Samantha Crockett, Diana Dalgliesh, Mary Davies, Caroline Deacon, Fiona Douglas, Sarah Drummond, Steve Ebbs, Jane Edwardson, Jacqueline Elliott, Sian Evans, Cathy Farnon, J Ferguson, Gillian Fowler, Lynda Fumagali, Nick Gallegos, Claire Gaskell, Jonathan Gay, W David George, Fiona Gilbert, Kate Gower Thomas, Rosemary Greenhalgh, Dimitri Hadjiminas, Douglas Hansell, Claudia Harding-Mackean, Sue Hartup, Janet Hayman, Philippa Hill, Christopher Hinton, Shirley Hodgson, Sue Holcombe, Martin Hogg, Catherine Hubbard, Louise Izatt, Chris Jacobs, Sabah Jmor, Irene Jobson, Reshma Kanani, Margaret Kent, Katherine Knight, Alison Lannigan, Celia Lewis, Stephanie MacArthur, Chris Marano, Lee Martin, Duncan Matheson, Andrew Maurice, Jane McClement, Jan McCarrick, Mary Milne, Caroline Mortimer, Leigh Moss, A Nejim, Dierdre Pallister, Viviene Parr, Joan Paterson, Karen Pearson, Simerjit Rai, Jenny Ramm, Oduru Ravisekar, Fiona Read, Paul Ridley, Nicola Roche, Linda Rockall, Colin Rogers, Mark Rogers, Neil Rothnie, Gary Rubin, Zahida Saad, Lynne Sears, Mike Shere, Heather Shires, Isabel dos Santos Silva, C Simpson, D Smith, Sheila Stallard, Kerstin Stepp-Schuh, A Stebbing, James Steel, R Stewart, Mandy Stone, Olga Strukowska, Elizabeth Tee, Amanda Taylor, William Teh, Alastair Thompson, WD Thompson, WO Thompson, Rosemary Toye, Philip Turton, Luna Vishwanath, Alison Waghorn, Joanne Walsh, Michael Williams, Jenny Williamson, Jenny Wise, Martin Wise, Phillipa Whitford and Charles Zammit.
Contribution of authors
Stephen W Duffy was joint principal investigator (PI), supervised the statistical analysis and drafted the report.
James Mackay was joint PI, took responsibility of clinical aspects of the study and contributed to editing the report.
Sue Thomas co-ordinated the study, participated in data-analysis and contributed to editing the report.
Elaine Anderson took a leading role in study conduct in Scotland, participated in study management nationally and contributed to editing the report.
Tony HH Chen carried out the Markov modelling and contributed to editing the report.
Ian Ellis led on pathological aspects and contributed to editing the report.
Gareth Evans led on clinical genetics aspects, participated in study management nationally and contributed to editing the report.
Hilary Fielder was a co-applicant, took a leading role in study conduct in Wales and guardianship of the study data, participated in study management nationally and contributed to editing the report.
Rosemary Fox took a leading role in study conduct in Wales and guardianship of the study data, participated in study management nationally and contributed to editing the report.
Gerald Gui took a leading role in study conduct in London, participated in study management nationally and contributed to editing the report.
Douglas Macmillan was one of the team initiating the study, participated in study management nationally and contributed to editing the report.
Sue Moss participated in study management nationally, took a leading role in comparison with the Age Trial data and contributed to editing the report.
Cerilan Rogers was a co-applicant, was one of the team initiating the study, took the lead in the first 2 years on study conduct in Wales and guardianship of the study data, participated in management of the study nationally and contributed to editing the report.
Mark Sibbering contributed to management of the study nationally, led on surgical aspects and contributed to editing the report.
Matthew Wallis took a leading role in radiological aspects and contributed to editing the report.
Ruth Warren led the radiology review, participated in study management nationally and contributed to editing the report.
Eila Watson took a leading role in psychosocial aspects of the study and contributed to editing the report.
David Whynes carried out the health economic evaluation and contributed to editing the report.
Prue Allgood took a leading role in study co-ordination in London, in the blood and radiation dose substudies and contributed to editing the report.
Jack Caunt was responsible for informatics aspects, data management and processing, and contributed to editing the report.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
Publications
Mackay J, Rogers C, Fielder H, Blamey R, Macmillan D, Boggis C, et al. Development of a protocol for evaluation of mammographic surveillance services in women under 50 with a family history of breast cancer. J Epidemiol Biostat 2001;6:365–9.
FH01 management committee, steering committee and collaborators. The challenge of evaluating annual mammography screening for young women with a family history of breast cancer. J Med Screening 2006;13:177–82.
FH01 Collaborative Groups. Mammographic surveillance in women younger than 50 years who have a family history of breast cancer: tumour characteristics and projected effect on mortality in the prospective, single-arm, FH01 study. Lancet Oncol 2010;11:1127–34.
References
- Breast and cervical screening: the first 20 years. Sheffield: NHSCSP; 2008.
- Hendrick RE, Smith RA, Rutledge JH III, Smart CR. Benefit of screening mammography in women aged 40–49: a new meta-analysis of randomized controlled trials. J Natl Cancer Inst Monographs 1997;22:87-92.
- Fletcher SW. Breast cancer screening among women in their forties: an overview of the issues. J Natl Cancer Inst Monographs 1997;22:5-9.
- Organising committee and collaborators . Breast-cancer screening with mammography in women aged 40–49 years. Int J Cancer 1996;68:693-9. http://dx.doi.org/10.1002/(SICI)1097-0215(19961211)68:6<693::AID-IJC1>3.0.CO;2-Z.
- Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 1994;266:66-71. http://dx.doi.org/10.1126/science.7545954.
- Wooster R, Bignell G, Lancaster J, Swift S, Seal S, Mangion J, et al. Identification of the breast cancer susceptibility gene BRCA2. Nature 1995;378:789-92. http://dx.doi.org/10.1038/378789a0.
- Evans DG, Lalloo F. Risk assessment and management of high risk familial breast cancer. J Med Genet 2002;39:865-71. http://dx.doi.org/10.1136/jmg.39.12.865.
- Eccles DM, Evans DGR, Mackay J. UK Cancer Family Study Group . Guidelines for a genetic risk based approach to advising women with family history of breast cancer. J Med Genet 2000;37:203-9. http://dx.doi.org/10.1136/jmg.37.3.203.
- Leach MO, Boggis CR, Dixon AK, Easton DF, Eeles RA, Evans DG, et al. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS). Lancet 2005;365:1769-78. http://dx.doi.org/10.1016/S0140-6736(05)66481-1.
- Kriege M, Brekelmans CT, Boetes C, Besnard PE, Zonderland HM, Obdeijn IM, et al. Efficacy of MRI and mammography for breast cancer screening in women with a familial or genetic predisposition. N Engl J Med 2004;351:427-37. http://dx.doi.org/10.1056/NEJMoa031759.
- Salhab M, Bismohun S, Mokbel K. Risk-reducing strategies for women carrying BRCA1/2 mutations with a focus on prophylactic surgery. BMC Women'S Health 2010;10. http://dx.doi.org/10.1186/1472-6874-10-28.
- Eisen A, Lubinski J, Klijn J, Moller P, Lynch HT, Offit K, et al. Breast cancer risk following bilateral oophorectomy in BRCA1 and BRCA2 mutation carriers: an international case–control study. J Clin Oncol 2005;23:7491-6. http://dx.doi.org/10.1200/JCO.2004.00.7138.
- Macmillan RD. Screening women with a family history of breast cancer- results from the British Familial Breast Cancer Group. Eur J Surg Oncol 2000;26:149-52. http://dx.doi.org/10.1053/ejso.1999.0759.
- Mackay J, Rogers C, Fielder H, Blamey R, Macmillan D, Boggis C, et al. Development of a protocol for evaluation of mammographic surveillance services in women under 50 with a family history of breast cancer. J Epidemiol Biostat 2001;6:365-9. http://dx.doi.org/10.1080/135952201753337086.
- Claus EB, Risch N, Thompson WD. Genetic analysis of breast cancer in the cancer and steroid hormone study. AM J Hum Genet 1991;48:232-42.
- Decarli A, Calza S, Masala G. Gail model for prediction of absolute risk of invasive breast cancer: independent evaluation in the Florence-European Investigation into Cancer and Nutrition cohort. J Natl Cancer Inst 2006;98:1686-93. http://dx.doi.org/10.1093/jnci/djj463.
- Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med 2004;23:1111-30. http://dx.doi.org/10.1002/sim.1668.
- Smith RA, Duffy SW, Gabe R, Tabar L, Yen AM, Chen TH. The randomized trials of breast cancer screening: what have we learned?. Radiol Clin N Amer 2004;42:793-806. http://dx.doi.org/10.1016/j.rcl.2004.06.014.
- Wald NJ, Chamberlain J, Hackshaw A. European Society of Mastology Consensus Conference on breast cancer screening: report of the evaluation committee. Br J Radiol 1994;67:925-33. http://dx.doi.org/10.1259/0007-1285-67-802-925.
- FH01 management committee, steering committee and collaborators . The challenge of evaluating annual mammography screening for young women with a family history of breast cancer. J Med Screening 2006;13:177-82.
- Tabár L, Duffy SW, Chen HH. Re: Quantitative interpretation of age-specific mortality reductions from the Swedish Breast Cancer-Screening Trials. J Natl Cancer Inst 1996;88:52-5. http://dx.doi.org/10.1093/jnci/88.1.52-a.
- Moss S, Waller M, Anderson TJ, Cuckle H. Randomised controlled trial of mammographic screening in women from age 40: predicted mortality based on surrogate outcome measures. Br J Cancer 2005;92:955-60. http://dx.doi.org/10.1038/sj.bjc.6602395.
- FH01 Collaborative Groups . Mammographic surveillance in women younger than 50 years who have a family history of breast cancer: tumour characteristics and projected effect on mortality in the prospective, single-arm, FH01 study. Lancet Oncol 2010;11:1127-34. http://dx.doi.org/10.1016/S1470-2045(10)70263-1.
- Bjurstam N, Björneld L, Warwick J, Sala E, Duffy SW, Nyström L, et al. The Gothenburg Breast Screening Trial. Cancer 2003;97:2387-96. http://dx.doi.org/10.1002/cncr.11361.
- Brekelmans CTM, Tilanus-Linthorst MMA, Seynaeve C, vd Ouweland A, Menke-Pluymers MB, Bartels CC, et al. Tumour characteristics, survival and prognostic factors of hereditary breast cancer from BRCA2-, BCRA1- and non-BRCA12 families as compared to sporadic breast cancer cases. Eur J Cancer 2007;43:867-76. http://dx.doi.org/10.1016/j.ejca.2006.12.009.
- Blamey RW. The British Association of Surgical Oncology guidelines for surgeons in the management of symptomatic breast disease in the UK. Eur J Surg Oncol 1998;24:464-76. http://dx.doi.org/10.1016/S0748-7983(98)93104-3.
- Tabar L, Fagerberg G, Duffy SW, Day NE, Gad A, Grontoft O. Update of the Swedish Two-County program of mammographic screening for breast cancer. Radiol Clin N Amer 1992;30:187-210.
- Blamey RW, Ellis IO, Pinder SE, Lee AH, Macmillan RD, Morgan DA, et al. Survival of invasive breast cancer according to the Nottingham Prognostic Index in cases diagnosed in 1990–99. Eur J Cancer 2007;43:1548-55. http://dx.doi.org/10.1016/j.ejca.2007.01.016.
- Amir E, Evans DG, Shenton A, Lalloo F, Moran A, Boggis C, et al. Evaluation of breast cancer risk assessment packages in the family history evaluation and screening programme. J Med Genet 2003;40:807-14. http://dx.doi.org/10.1136/jmg.40.11.807.
- Casella G, Berger RL. Statistical inference. Belmont, CA: Duxbury Press; 2002.
- Tyndel S, Clements A, Bankhead C, Henderson BJ, Brain K, Watson E, et al. Mammographic screening for young women with a family history of breast cancer: knowledge and views of those at risk. Br J Cancer 2008;99:1007-12. http://dx.doi.org/10.1038/sj.bjc.6604672.
- Brain K, Henderson BJ, Tyndel S, Bankhead C, Watson E, Clements A, et al. Predictors of breast cancer-related distress following mammography screening in younger women on a family history breast screening programme. Psychooncol 2008;17:1180-8. http://dx.doi.org/10.1002/pon.1355.
- Tyndel S, Austoker J, Henderson BJ, Brain K, Bankhead C, Clements A, et al. What is the psychological impact of mammographic screening on younger women with a family history of breast cancer? Findings from a prospective cohort study by the PIMMS Management Group. J Clin Oncol 2007;25:3823-30. http://dx.doi.org/10.1200/JCO.2007.11.0437.
- Henderson BJ, Tyndel S, Brain K, Clements A, Bankhead C, Austoker J, et al. Factors associated with breast cancer-specific distress in younger women participating in a family history mammography screening programme. Psychooncol 2008;17:74-82. http://dx.doi.org/10.1002/pon.1201.
- NHS Breast Screening Programme annual review 2010. Sheffield: NHSBSP; 2010.
- Quality assurance guidelines for mammography including radiographic quality control. Sheffield: NHS Cancer Screening Programmes; 2006.
- Young KC. Radiation doses in the UK trial of breast screening in women aged 40–48 years. Br J Radiol 2002;75:362-70.
- Day NE, Walter SD. Simplified models of screening for chronic disease: estimation procedures from mass screening programmes. Biometrics 1984;40:1-14. http://dx.doi.org/10.2307/2530739.
- Britton P, Warwick J, Wallis MG, O’Keeffe S, Taylor K, Sinnatamby R, et al. Measuring the accuracy of diagnostic imaging in symptomatic breast patients: team and individual performance. Br J Radiol 2012;85:415-22. http://dx.doi.org/10.1259/bjr/32906819.
- Campbell HE, Epstein D, Bloomfield D, Griffin S, Manca A, Yarnold J, et al. The cost-effectiveness of adjuvant chemotherapy for early breast cancer: a comparison of no chemotherapy and first, second, and third generation regimens for patients with differing prognoses. Eur J Cancer 2011;47:2517-30. http://dx.doi.org/10.1016/j.ejca.2011.06.019.
- National schedule of reference costs 2010–11 for NHS trusts and PCTs combined, dh_131148. London: DoH; 2011.
- Guerriero C, Gillan MGC, Cairns J, Wallis MG, Gilbert FJ. Is computer-aided detection cost-effective in screening mammography? A model based on the CADET 2 study. BMC Health Services Res 2011;11. http://dx.doi.org/10.1186/1472-6963-11-11.
- Madan J, Rawdin A, Stevenson M, Tappenden P. A rapid-response economic evaluation of the UK NHS cancer reform strategy breast cancer screening program extension via a plausible bounds approach. Value Health 2010;13:215-21. http://dx.doi.org/10.1111/j.1524-4733.2009.00667.x.
- Familial breast cancer costing report, CG041. London: NICE; 2006.
- Robertson C, Arcot Ragupathy SK, Boachie C, Dixon JM, Fraser C, Hernández R, et al. The clinical effectiveness and cost-effectiveness of different surveillance mammography regimens after the treatment for primary breast cancer: systematic reviews, registry database analyses and economic evaluation. Health Technol Assess 2011;15.
- Turnbull LW, Brown SR, Olivier C, Harvey I, Brown J, Drew P, et al. Multicentre randomised controlled trial examining the cost-effectiveness of contrast-enhanced high field magnetic resonance imaging in women with primary breast cancer scheduled for wide local excision (COMICE). Health Technol Assess 2010;14.
- Maurice A, Evans DGR, Shenton A, Ashcroft L, Baildam A, Barr L, et al. Screening younger women with a family history of breast cancer – does early detection improve outcome?. Eur J Cancer 2006;42:1385-90. http://dx.doi.org/10.1016/j.ejca.2006.01.055.
- Moss S, Cuckle H, Evans A, Johns L, Waller M, Bobrow L. Effect of mammography screening from age 40 years on breast cancer mortality at 10 years' follow-up: a randomised controlled trial. Lancet 2006;368:2053-60. http://dx.doi.org/10.1016/S0140-6736(06)69834-6.
- Nyström L, Andersson I, Bjurstam N, Frisell J, Nordenskjold B, Rutqvist LE. Long term effects of mammography screening: updated overview of the Swedish randomised trials. Lancet 2002;359:909-19. http://dx.doi.org/10.1016/S0140-6736(02)08020-0.
- Miller AB, To T, Baines CJ, Wall C. The Canadian National Breast Screening Study-1: breast cancer mortality after 11 to 16 years of follow-up. A randomized screening trial of mammography in women age 40 to 49 years. Ann Intern Med 2002;137:305-12.
- Miller AB, To T, Baines CJ, Wall C. Canadian National Breast Screening Study-2: 13-year results of a randomized trial in women aged 50–59 years. J Natl Cancer Inst 2000;92:1490-9. http://dx.doi.org/10.1093/jnci/92.18.1490.
- Tabar L, Vitak B, Chen HH, Duffy SW, Smith RA. The Swedish Two-County Trial twenty years later: updated mortality results and new insights from long term follow-up. Radiol Clin N Amer 2000;38:625-51.
- Alexander FE, Anderson TJ, Brown HK, Forrest AP, Hepburn W, Kirkpatrick AE, et al. 14 years of follow-up from the Edinburgh randomised trial of breast-cancer screening. Lancet 1999;353:1903-8. http://dx.doi.org/10.1016/S0140-6736(98)07413-3.
- Shapiro S, Venet W, Strax P, Venet L. Periodic screening for breast cancer: the health insurance project and its sequelae 1963–86. Baltimore, MD: Johns Hopkins UP; 1988.
- Pinder SE, Wencyk PM, Naylor HE, Bell JA, Elston CW, Robertson JF, et al. The assessment of multiple variables on breast carcinoma fine needle aspiration (FNA) cytology specimens: method, preliminary results and prognostic associations. Cytopathol 1995;6:316-24. http://dx.doi.org/10.1111/j.1365-2303.1995.tb00577.x.
- Dawson SJ, Duffy SW, Blows FM, Driver KE, Provenzano E, LeQuesne J, et al. Molecular characteristics of screen detected vs symptomatic breast cancers and their impact on survival. Br J Cancer 2009;101:1338-44. http://dx.doi.org/10.1038/sj.bjc.6605317.
- Lawrence G, O’Sullivan E, Kearins O, Tappenden N, Martin K, Wallis M, et al. Screening histories of invasive breast cancers diagnosed 1989–2006 in the West Midlands, UK: variation with time and impact on survival. J Med Screen 2009;16:186-92. http://dx.doi.org/10.1258/jms.2009.009040.
- Nagtegaal ID, Allgood PC, Duffy SW, Kearins O, Sullivan EO, Tappenden N, et al. Prognosis and pathology of screen-detected carcinomas: how different are they?. Cancer 2011;117:1360-8. http://dx.doi.org/10.1002/cncr.25613.
- Wallis MG, Clements K, Kearins O, Ball G, Mccartney J, Lawrence GM. The effect of DCIS grade on rate, type and time to recurrence after 15 years of follow-up of screen-detected DCIS. Br J Cancer 2012;106:1611-17. http://dx.doi.org/10.1038/bjc.2012.151.
- Norman RP, Evans DG, Easton DF, Young KC. The cost-utility of magnetic resonance imaging for breast cancer in BRCA1 mutation carriers aged 30–49. Eur J Health Econ 2007;8:137-44. http://dx.doi.org/10.1007/s10198-007-0042-9.
- Evans AJ, Kutt E, Record C, Waller M, Moss S. Radiological findings of screen-detected cancers in a multi-centre randomized controlled trial of mammographic screening in women from age 40 to 48 years. Clin Radiol 2006;61:784-8. http://dx.doi.org/10.1016/j.crad.2006.04.013.
- Warren RM, Young JR, McLean L, Lyons K, Wilson AR, Evans A, et al. Radiology review of the UKCCCR Breast Screening Frequency Trial: potential improvements in sensitivity and lead time of radiological signs. Clin Radiol 2003;58:128-32. http://dx.doi.org/10.1053/crad.2002.1132.
- Gilbert FJ, Warren R, Kwan-Lim G, Thompson DJ, Eeles RA, Evans DG, et al. Cancers in BRCA1 and BRCA2 carriers and in women at high risk for breast cancer: MR imaging and mammographic features. Radiology 2009;252:358-68. http://dx.doi.org/10.1148/radiol.2522081032.
- Eccles D, Gerty S, Simmonds P, Hammond V, Ennis S, Altman DG. Prospective study of outcomes in sporadic and hereditary breast cancer (POSH): study protocol. Cancer 2007;7.
- McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2006;15:1159-69. http://dx.doi.org/10.1158/1055-9965.EPI-06-0034.
- Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med 2007;356:227-36. http://dx.doi.org/10.1056/NEJMoa062790.
- Fulford LG, Easton DF, Reis-Filho JS, Sofronis A, Gillett CE, Lakhani SR, et al. Specific morphological features predictive for the basal phenotype in grade III invasive ductal carcinoma of the breast. Histopathology 2006;49:22-34. http://dx.doi.org/10.1111/j.1365-2559.2006.02453.x.
- Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature 2000;406:747-52. http://dx.doi.org/10.1038/35021093.
- Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumour subclasses with clinical implications. Proc Natl Acad Sci USA 2001;98:10,869-74. http://dx.doi.org/10.1073/pnas.191367098.
- Foulkes WD, Stefansson IM, Chappuis PO, Bégin LR, Goffin JR, Wong N, et al. Germline BRCA1 mutations and the basal epithelial phenotype. J Natl Cancer Inst 2003;99:1482-5. http://dx.doi.org/10.1093/jnci/djg050.
- Millikan RC, Newman B, Tse CK, Moorman PG, Conway K, Dressler LG, et al. Epidemiology of basal like breast cancer. Breast Cancer Res Treat 2008;109:123-39. http://dx.doi.org/10.1007/s10549-007-9632-6.
- Turner NC, Reis-Filho JS, Russell AM, Springall RJ, Ryder K, Steele D, et al. BRCA1 dysfunction is sporadic basal-like breast cancer. Oncogene 2007;26:2126-32. http://dx.doi.org/10.1038/sj.onc.1210014.
- Sotiriou C, Neo SY, McShane LM, Korn EL, Long PM, Jazaeri A, et al. Breast cancer classification and prognosis based on gene expression profiles from a population based study. Proc Natl Acad Sci USA 2003;100:10,393-8. http://dx.doi.org/10.1073/pnas.1732912100.
- Teschendorff AE, Journée M, Absil PA, Sepulchre R, Caldas C. Elucidating the altered transcriptional programs in breast cancer using independent component analysis. PLoS Comp Biol 2007;3. http://dx.doi.org/10.1371/journal.pcbi.0030161.
- McCabe N, Turner NC, Lord CJ, Kluzek K, Bialkowska A, Swift S, et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res 2006;66:8109-15. http://dx.doi.org/10.1158/0008-5472.CAN-06-0140.
- House W, Sharp D, Sheridan E. Identifying and screening patients at high risk of colorectal cancer in general practice. J Med Screen 1999;6:205-8.
- Schrijvers CT, Stronks K, van de Mheen DH, Coebergh JW, Mackenbach JP. Validation of cancer prevalence data from a postal survey by comparison with cancer registry records. Am J Epidemiol 1994;139:408-14.
- Clements A, Henderson B, Tyndel S, Evans G, Brain K, Austoker J, et al. Diagnosed with breast cancer while on a family history screening programme: an exploratory qualitative study. Eur J Cancer Care 2008;17:245-52. http://dx.doi.org/10.1111/j.1365-2354.2007.00837.x.
- Duffy SW, Tabar L, Olsen AH, Vitak B, Allgood PC, Chen TH, et al. Absolute numbers of lives saved and overdiagnosis in breast cancer screening, from a randomized trial and from the Breast Screening Programme in England. J Med Screening 2010;17:25-30. http://dx.doi.org/10.1258/jms.2009.009094.
- Stone J, Ding J, Warren RM, Duffy SW. Predicting breast cancer risk using mammographic density measurements from both mammogram sides and views. Breast Cancer Res Treat 2010;124:551-4. http://dx.doi.org/10.1007/s10549-010-0976-y.
- Aarøe J, Lindahl T, Dumeaux V, Saebø S, Tobin D, Hagen N, et al. Gene expression profiling of peripheral blood cells for early detection of breast cancer. Breast Cancer Res 2010;12. http://dx.doi.org/10.1186/bcr2472.
- Chapman C, Murray A, Chakrabarti J, Thorpe A, Woolston C, Sahin U, et al. Autoantibodies in breast cancer: their use as an aid to early diagnosis. Ann Oncol 2007;18:868-73. http://dx.doi.org/10.1093/annonc/mdm007.
- Rønneberg JA, Fleischer T, Solvang HK, Nordgard SH, Edvardsen H, Potapenko I, et al. Methylation profiling with a panel of cancer related genes: association with estrogen receptor, TP53 mutation status and expression subtypes in sporadic breast cancer. Mol Oncol 2011;5:61-76. http://dx.doi.org/10.1016/j.molonc.2010.11.004.
- Understanding R2 Quantra 1.3. Bedford, MA: Hologic Inc.; 2009.
- UK National Statistics n.d. www.statistics.gov.uk/ (accessed 26 November 2012).
- Scheuner MT, McNeel TS, Freedman AN. Population prevalence of familial cancer and common hereditary cancer syndromes. The 2005 California Health Interview Survey. Genet Med 2010;12:726-35. http://dx.doi.org/10.1097/GIM.0b013e3181f30e9e.
- O’Neill SM, Rubinstein WS, Wang C, Yoon PW, Acheson LS, Rothrock N, et al. Familial risk for common diseases in primary care: the Family Healthware Impact Trial. Am J Prev Med 2009;36:506-14. http://dx.doi.org/10.1016/j.amepre.2009.03.002.
- Stone J, Ding J, Warren RM, Duffy SW, Hopper JL. Using mammographic density to predict breast cancer risk: dense area or percentage dense area. Breast Cancer Res 2010;12. http://dx.doi.org/10.1186/bcr2778.
- Wong CS, Lim GH, Gao F, Jakes RW, Offman J, Chia KS, et al. Mammographic density and its interaction with other breast cancer risk factors in an Asian population. Br J Cancer 2011;104:871-4. http://dx.doi.org/10.1038/sj.bjc.6606085.
Appendix 1 Protocol of FH01
Appendix 2 FH01 blood study protocol
Appendix 3 Information sheet used in England and Wales for potential FH01 recruits
Information sheet used in England and Wales for potential FH01 recruits (PDF download)
Appendix 4 Information sheet for primary care staff
Appendix 5 Data proformas
List of abbreviations
- Age Trial
- UK Breast Screening Age Trial
- BASO
- British Association of Surgeons in Oncology
- BRCA1
- breast cancer type 1 gene
- BRCA2
- breast cancer type 2 gene
- CI
- confidence interval
- DCIS
- ductal carcinoma in situ
- HRT
- hormone replacement therapy
- ICER
- incremental cost-effectiveness ratio
- MGD
- mean glandular dose
- MRI
- magnetic resonance imaging
- NHSBSP
- National Health Service Breast Screening Programme
- NPI
- Nottingham Prognostic Index
- OR
- odds ratio
- PIMMS
- Psychological Impact of maMMography Screening for women under 50 with a family history of breast cancer
- POSH
- Prospective study of Outcomes in Sporadic and Hereditary breast cancer
- QALY
- quality-adjusted life-year
- RR
- relative risk
- SD
- standard deviation
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.