Notes
Article history paragraph text
The research reported in this issue of the journal was funded by the HTA programme as project number 07/31/02. The contractual start date was in November 20008. The draft report began editorial review in October 2011 and was accepted for publication in May 2012. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors' report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen's Printer and Controller of HMSO 2013. This work was produced by Molassiotis et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Literature review
Existing research
Significant developments in antiemetic therapy over the past two decades have improved the control of chemotherapy-related vomiting. By contrast, chemotherapy-related nausea, both acute and delayed, is still a significant problem in clinical practice, with 42–52% of patients experiencing nausea on any one day in routine practice. 1 Surprisingly, despite improvements in the management of vomiting, postchemotherapy nausea seems to have increased. 2 Furthermore, clinicians often underestimate the experience of nausea, especially with regards to delayed nausea. 3,4
Chemotherapy-induced nausea and vomiting can have a profound effect on the cancer treatment experience5 and is associated with negative effects on daily life and overall quality of life, including in relation to food intake, weight loss, social interactions, dehydration, difficulty with sleeping and anxiety. 5,6 In a qualitative study of patients' experiences, unmanaged nausea was constant in some patients and made them exhausted for long periods after chemotherapy, making recovery between cycles longer. 5 The impact of nausea is greater than that of vomiting,7 and nausea has proven to be more difficult to control. The direct and indirect costs of the experience of nausea and vomiting, especially of delayed symptoms, are considerable. 8 Antiemetic trials have traditionally focused primarily on vomiting and emetic episodes, on which the effectiveness of many antiemetic drugs is judged. Little attention has been directed to the concept of chemotherapy-induced nausea despite the fact that it is increasingly recognised that nausea and vomiting are related but separate entities. 9,10 The need for these two symptoms to be treated as two separate entities is strongly advocated. 10
The reasons behind this incomplete management of chemotherapy-induced nausea and vomiting are multifaceted. They include health professionals' limited understanding of the complex concept of chemotherapy-induced nausea and vomiting and its different phases; limited assessment in clinical practice of chemotherapy-induced nausea and vomiting and its risk factors; using more emetogenic chemotherapy protocols than in the past; not understanding clearly all the pathways involved in the development of chemotherapy-induced nausea and vomiting; and more focus given to the vomiting experience than nausea in clinical trials. 11
As antiemetic medications do not fully control nausea during chemotherapy, non-pharmacological interventions in addition to antiemetics have been tested over the years, especially in the 1980s, including relaxation techniques, coping preparation, imagery and distraction techniques, with positive results in most studies. 12 Acupuncture and its non-invasive form of acupressure have been tested several times after the classic early work of Dundee et al. 13,14 In a literature search of MEDLINE, PubMed and Cumulative Index to Nursing and Allied Health Literature (CINAHL) using the key words ‘acupressure’, ‘nausea’, ‘vomiting’, ‘emesis’, ‘chemotherapy’, ‘cancer’ and combinations, between 1990 and May 2005, we have identified 10 studies specific to oncology, reported elsewhere,15 with 7 out of 10 studies showing positive results and a further two approaching statistical significance. These studies have used a variety of acupressure methods, such as the ReliefBand® [a small battery-operated transcutaneous electrical nerve stimulation (TENS) device designed to stimulate the P6 acupoint];16–18 an acupressure wristband (a small elastic band with a round plastic button applying constant mild pressure on the P6 acupoint);19–21 and direct pressure on the P6 acupoint22 or P6 and ST36 acupoints together. 23 Most studies had small sample sizes of 18–50 patients. The largest study to date (n = 739) testing acupressure and acustimulation showed improvements in nausea and vomiting in men and a similar trend in women to reduce acute symptoms only, although the latter did not reach statistical significance. 24 No improvement in nausea/vomiting was shown in a small study by Roscoe et al. 25 in women with breast cancer using acustimulation (ReliefBand) wristbands. These two studies are suggestive of a possible gender effect. However, most past studies are hampered by small sample sizes, the wide variety of (non-standardised) antiemetics used, differences in the risk factors for nausea and vomiting in these samples, the range of emetogenicity of chemotherapy regimens used and sampling issues. A recent Cochrane systematic review26 of the literature highlights that acupressure reduces acute nausea but not delayed nausea, and has no benefit for vomiting. However, the review was primarily focused on acupuncture rather than acupressure, all different methods of acupressure were examined together and the results regarding specifically vomiting are questionable (as many of the studies included in the review had samples with little, if any, vomiting across experimental and control groups).
Our own work
Over the past 8 years the lead applicant has developed a programme of research in the management of chemotherapy-induced nausea and vomiting that feeds into the current application. This has involved the assessment of the effectiveness of non-pharmacological interventions for the management of chemotherapy-induced nausea and vomiting including progressive muscle relaxation training and imagery techniques;27 pilot testing of acupressure;15 identification of risk factors for chemotherapy-induced nausea and vomiting development such as age, gender and anxiety;28,29 the management of anticipatory nausea and vomiting;30,31 the development of international clinical guidelines for managing chemotherapy-induced nausea and vomiting32,33 and radiation-induced nausea and vomiting;34,35 exploration and further clarification of the concept of chemotherapy-induced nausea as a separate entity from vomiting;36 assessment of chemotherapy-induced nausea and vomiting levels in current clinical practice in the UK;37,38 and development of a chemotherapy-induced nausea and vomiting-relevant clinical scale for the assessment of acute and delayed symptoms. 39 This last is the only chemotherapy-specific scale available to date. In our qualitative study of the experience of chemotherapy-related nausea in 17 patients with cancer in the UK and USA,36 nausea was described as a distressing and complex symptom. Preliminary evidence indicates that nausea is part of a cluster of symptoms. Self-management techniques, such as dietary strategies and distraction techniques, were rooted in participants' understanding of nausea and their beliefs about what caused nausea. Although self-management was common in almost all patients, acupressure was not one of the approaches used. In an observational prospective evaluation using patient self-reports, 102 patients with cancer receiving their first chemotherapy treatment participated. 37 Participants were followed up for four cycles of chemotherapy, providing a total of 272 assessments of nausea and vomiting. The results indicated that acute vomiting was experienced by 15.7% of the patients in cycle 1 and delayed vomiting by 14.7%, whereas acute nausea was present in 37.3% of the patients and delayed nausea in 47.1%, which increased over the four cycles. Moderately emetogenic chemotherapy had the highest incidence of chemotherapy-induced nausea and vomiting, and acute symptoms were more controlled than delayed symptoms. The data suggested that, although vomiting is relatively well controlled, nausea is a significant problem in practice; it also highlighted the high cost of inappropriate use of antiemetics, which was £17,524 for every 100 patients treated over four cycles. 37
Chapter 2 Research objectives
Primary objective
-
1. To assess the clinical effectiveness of self-acupressure using wristbands in addition to standard care compared with standard care with sham acupressure wristbands and standard care alone in the management of chemotherapy-induced (acute and delayed) nausea.
Secondary objectives
-
2. To assess the cost-effectiveness and extent of use of usual care in patients using acupressure wristbands in addition to standard care compared with that in patients receiving standard care with sham acupressure wristbands and standard care alone for the management of chemotherapy-induced nausea.
-
3. To assess the quality of life in patients using acupressure wristbands in addition to standard care compared with that in patients receiving standard care with sham acupressure wristbands and standard care alone in the management of chemotherapy-induced nausea and vomiting.
-
4. To assess the clinical effectiveness of self-acupressure using wristbands in addition to standard care compared with that in patients receiving standard care with sham acupressure wristbands and standard care alone in the management of chemotherapy-induced (acute and delayed) vomiting.
-
5. To ascertain for which emetogenic level of chemotherapy regimen (i.e. high, moderate or low) self-acupressure using wristbands in addition to standard care is more or less effective in terms of nausea compared with patients receiving standard care with sham acupressure wristbands and standard care alone.
-
6. To ascertain whether or not any improvement in chemotherapy-induced nausea and vomiting from using acupressure wristbands is different between men and women.
-
7. To ascertain whether or not there is an age effect from the use of acupressure wristbands in relation to chemotherapy-induced nausea and vomiting.
Chapter 3 Research methods
Design of the study
The study was a randomised controlled trial with three arms (Assessment of Nausea in Chemotherapy Research or ANCHoR). The three arms consisted of usual care plus (1) self-administered acupressure wristbands, (2) sham acupressure wristbands and (3) no additional treatment. The duration of the patients' involvement was for four cycles of chemotherapy, as after four cycles patients not responding to the given chemotherapy may discontinue it, may be offered a different chemotherapy regimen or a different treatment plan, or may be offered supportive care only.
Subjects were allocated to the trial groups through computer-generated randomisation carried out remotely by the trials unit of the Christie NHS Foundation Trust, Manchester. The randomisation method used consisted of minimisation with a random element (stochastic minimisation), balancing for gender,29,40 age (16–24, > 24–50, > 50 years)29,41 and three levels of emetogenic chemotherapy [low, moderate and high according to international American Society of Clinical Oncology (ASCO) and Multinational Association of Supportive Care in Cancer (MASCC) classifications]. 32,42
Biases were minimised through (1) exclusion criteria that leave out some of the factors and sources of nausea and vomiting in cancer patients other than chemotherapy (i.e. intestinal obstruction); (2) the use of covariates for variables that are closely linked with nausea and cannot be excluded as they are present in a large proportion of the population (i.e. anxiety),29,43 to be incorporated during the data analysis as a covariate in analysis of covariance (ANCOVA) models; and (3) the use of stratification for other key risk factors for nausea development during chemotherapy (i.e. age, gender) at the randomisation stage. Stratification, prior to randomisation, is important to ensure that known prognostic factors are equally distributed before measuring the treatment-related variables.
Pilot study using this design
We had carried out a two-arm pilot study of 36 breast cancer patients comparing acupressure wristbands (plus antiemetics) with standard antiemetics only. 15 The present trial has been based on methods tested in this pilot study. Although it is acknowledged that this study was limited, key findings suggested that acupressure improved the nausea experience as well as nausea and vomiting occurrence and distress across the first 5 days of chemotherapy. Improvements were higher in relation to nausea than in relation to vomiting. Mean overall percentage of improvement (pre to post assessment) in the experimental subjects was 44.5%. The study showed that an acupressure trial is feasible, with high levels of compliance (only one patient stopped using the wristband, because of arm swelling), although one-third of the patients did not return completed assessments. The lack of follow-up techniques (i.e. reminder letters), which was due to time constraints, is partly responsible for this and is acknowledged as a limitation of the pilot study. However, missing data in the returned assessments were almost non-existent, and patient logs for acupressure usage were fully completed.
Sham acupressure and acupressure have also been used in another pilot trial that we have carried out recently for the management of cancer-related fatigue;44 patients in the sham group who were informed that they were receiving one of two combinations of (acu)points were blinded until the end of the trial and this group had little improvement compared with the real acupressure group, suggesting that this technique was a credible placebo and thus capable of minimising the likely effect of placebo on the study's findings.
Experimental and control interventions
The study was a Phase III pragmatic randomised trial.
The target population was a heterogeneous group of cancer patients meeting the inclusion criteria and about to receive chemotherapy of high, moderate and low emetogenic potential. Heterogeneity is important to address issues of response to different types of emetogenic chemotherapy, as well as by gender and age, as past literature highlights that these are important in assessing the effectiveness of treatments for chemotherapy-related nausea and vomiting. Minimally emetogenic chemotherapy was not included, as clinical guidelines recommend no antiemetic treatment and the nausea/vomiting level is < 10%.
In the acupressure group, in addition to standard antiemetics, patients were provided with a pair of widely available acupressure wristbands. These bands are elastic wristbands with a 1-cm protruding round plastic button (stud). They are available in two sizes, standard and large. Patients wore the wristband with the stud pressing the P6 acupoint, which is located on the anterior surface of the forearm, approximately a three-finger width up from the crease of the wrist between the tendons of the palmaris longus and flexor carpi radialis. An instruction sheet with a picture of acupoint P6 and how to locate the point was also provided to patients. Patients were provided with a pair of acupressure wristbands and instructed to wear them on both arms and take them off only when showering/bathing. Patients were instructed to wear the wristbands from the morning before chemotherapy administration and for the subsequent 6 days (total 7 days). No other complementary therapy use was recommended during the course of acupressure (although any such use was documented).
In the sham acupressure group, in addition to standard antiemetics, patients were provided with a pair of identical-appearing wristbands, with the only difference being that the sham wristband had the button on the exterior of the wristband and patients were instructed to wear the wristband with the button away from what is the P6 point. There has been an ongoing scientific debate on what constitutes an appropriate sham treatment, and it has been acknowledged that there is no sham method in acupuncture and acupressure studies that can be widely accepted as the optimal method. It is increasingly believed that sham acupuncture/acupressure designs cannot detect the whole placebo effect and may generate false-negative results,45–48 depending on the method used. We had debated the appropriateness of other sham methods but either they were not blinded enough for the purposes of the trial (i.e. they were slightly dissimilar to real acupressure wristbands) or they could be perceived as treatments themselves (i.e. acupressure at other points in the forearm or elsewhere where we had no information as to an effect on the experience of nausea). Patients in the clinics could also talk to each other and realise that they have different interventions or check the P6 point on the internet. Hence, we resolved to use an acupressure technique that appeared to be exactly the same as the active treatment with the only exception being the place of the stud on the wristband (interior or exterior to the band) used. This was also agreed by practitioners who had been consulted about their views on the most appropriate sham method. Furthermore, although it was acknowledged that many patients may have heard of the use of such wristbands, the results of our pilot study suggested that their knowledge of acupressure wristbands would be limited. 15 In addition, the results of our qualitative study on self-management of chemotherapy-induced nausea and vomiting suggested that acupressure was not commonly used by patients. 36 An assessment of blinding at the end of the trial was not conducted as patients had not been informed of the use of both sham and real acupressure bands during the trial, but had instead been informed that two different types of wristbands were being evaluated, with the approval of the ethics committee. Clinicians did not know the patients' group allocation.
The control group received standard antiemetics alone. Standard antiemetics for all three groups were based on ASCO and MASCC international antiemetic guidelines with the exception of neurokinin 1 (NK1) receptor antagonists [e.g. aprepitant (Emend®, Merck)] recommended in highly emetic chemotherapy, which were not available in the NHS. Hence, for highly emetic chemotherapy, patients received a 5-hydroxytryptamine type 3 (5-HT3) receptor antagonist [i.e. ondansetron 8 mg (Zofran®, GlaxoSmithKline)] and dexamethasone 8 mg intravenously before chemotherapy and the same orally for 3 days post chemotherapy; for moderately emetogenic chemotherapy a 5-HT3 receptor antagonist (ondansetron 8 mg) and dexamethasone 8 mg intravenously before chemotherapy and a 5-HT3 receptor antagonist or dexamethasone for 2 days post chemotherapy; and for low emetogenic chemotherapy dexamethasone 8 mg before chemotherapy and no other treatment post chemotherapy]. 32,42 All patients received rescue antiemetics if nausea and/or vomiting was persistent and they failed to respond to the antiemetic treatment (i.e. severe nausea or more than five vomiting episodes), based on the experience of each clinician (as agreed guidelines for rescue antiemetics had not been developed to date).
Inclusion/exclusion criteria
Inclusion criteria
-
Patients scheduled to receive their first chemotherapy cycle.
-
Patients scheduled to receive chemotherapy with high, moderate and low emetogenic potential (as per ASCO and MASCC classifications).
-
Patients scheduled to receive a chemotherapy regimen given as a single or a multiple administration repeated in 2-, 3- or 4-week cycles.
-
Patients who were acupressure wristband naive (in terms of never having tried for themselves such a wristband, although they may have seen or heard about such wristbands).
-
Patients of either gender and > 16 years.
-
Patients with any cancer diagnosis receiving chemotherapy without concurrent use of radiotherapy.
-
Patients receiving chemotherapy as outpatients or inpatients.
-
Patients who were willing to participate in the study and be randomised into one of the three study groups.
Exclusion criteria
-
Patients scheduled to receive radiotherapy concurrently with chemotherapy.
-
Patients unable to self-care (i.e. unable to use wristbands appropriately; mental incapacity preventing continuous and optimal use of wristbands) as judged by the investigators.
-
Patients with liver disease (as nausea is a common presenting symptom).
-
Patients with metabolic risk factors for nausea (i.e. electrolyte imbalances causing nausea/vomiting).
-
Patients with mechanical risk factors for nausea (i.e. intestinal obstruction).
-
Patients experiencing nausea and/or vomiting resulting from the use of opioids.
-
Patients with lymphoedematous arms.
-
Patients with chronic alcohol use (chronic alcohol use is associated with minimal levels of nausea and/or vomiting).
Proposed sample size
In our pilot study15 the mean score for nausea experience averaged over 5 days was 2.79 [weighted average standard deviation (SD) 3.15] in the control group and 1.45 (weighted average SD 2.76) in the intervention group. At least 135 participants per arm would be required to detect this pair-wise difference between arms using a t-test with a conservative Bonferroni-adjusted significance level of 0.05/3 = 0.017 at a power of 90%. The pilot study suggested an attrition rate of 33% and so, initially, at least 202 participants would be required per arm. As the SDs are much larger than the means in the pilot data, they are suggestive of highly skewed distributions; hence, the equivalent non-parametric test (the Mann–Whitney test) will be used. As the asymptotic relative efficiency of the Mann–Whitney test is at worst 0.864, the sample size for a Mann–Whitney test is equal, in the worst case, to the sample size for the t-test divided by 0.864. This would increase the required sample size to 156 per arm before attrition and 233 after attrition, equalling 699 across the three arms.
Recalculation of sample size requirements during the trial
Because of a slower recruitment rate than envisaged initially, it was felt necessary to reconsider the sample size requirements. The Clinical Trials Unit analysed the first 141 cases that provided complete data over all four cycles. The SD for this cohort of patients was 2.75, slightly lower than the SD of 3 that we included in the initial power calculations. We had also calculated the power of the study to 90%, whereas the standard power in most studies is 80%. We adjusted the power down to 80%, which is the standard power accepted. With these adjustments the sample required was 480 participants. This change was agreed with both the Data Monitoring and Ethics Committee (DMEC) and the Health Technology Assessment (HTA) programme.
There were 361 cases with data on the primary outcome (117, 118 and 126 in the standard care only, sham acupressure and real acupressure arms respectively). Pair-wise trial arm comparisons were planned and as the data were skewed a non-parametric test (Mann–Whitney) was used, and this has an asymptotic relative efficiency of at worst 0.864 compared with the t-test. Thus, our effective sample sizes are around 117 × 0.864, which is about 100. With such a sample size there is approximately 80% power to detect a standardised difference in means of 0.46 in a two-tailed test at the 0.017 level of significance.
Recruitment took place in the largest single-site cancer centre in the UK and cancer units or centres of district general hospitals and university hospitals, including the Christie NHS Foundation Trust and its peripheral clinics where chemotherapy is administered (Royal Oldham Hospital, Tameside General Hospital, Leighton Hospital, Stepping Hill Hospital at Stockport, Macclesfield District General Hospital, North Manchester General Hospital), the Royal Liverpool Hospital, Clatterbridge Centre for Oncology, St Helens Hospital, Southport General Infirmary and three cancer units associated with the University of Plymouth (South Devon Healthcare NHS Foundation Trust, Plymouth Hospitals NHS Trust, Royal Cornwall Hospitals NHS Trust). Available statistics from the Christie NHS Foundation Trust alone had shown that around 9000 patients receive chemotherapy every year, with approximately two-thirds of these patients receiving chemotherapy in 3-week cycles. Recruitment rates were based on a similar antiemetic study that we had conducted over four cycles of chemotherapy,37 which had taken 6 months to recruit 102 patients, with 65% retained over the four cycles of chemotherapy. Based on similar recruitment levels at each of the 14 sites listed above, we had estimated that recruitment would be completed in 16 months, with a further 3 months required to complete the follow-up of the final patients. Three dedicated researchers and 30 cancer network research nurses recruited patients and collected the data. Data collection was audited regularly and discussed with the Trial Steering Committee and the DMEC.
Statistical analysis
Descriptive statistics have been estimated for all baseline sociodemographic and clinical variables by arm and for outcome variables (scores on nausea and vomiting subscales) by arm. The association between baseline sociodemographic or clinical variables and outcome variables has been assessed using between-group tests or correlations depending on skewness. Primary outcome variables have been compared between the arms using t-tests, one-way analysis of variance (ANOVA), Mann–Whitney tests and Kruskal–Wallis tests, bearing in mind any skewness in the data. Ordinal regression models were employed to permit covariate-adjusted analyses of a grouped version of the primary outcome. An extension of the proportional odds regression model was used for longitudinal analyses over cycles and this was fitted with a generalised estimating equation (GEE) approach. An intention-to-treat analysis model has been followed. As the primary outcome variable was assessed over several days repeatedly, an aggregate score of all assessments in each cycle was calculated before any modelling analysis.
The effect of missing values was assessed by comparing the numbers and percentages of participants with missing values in the three arms of the study; differences in baseline variables between participants with observed and missing outcomes in each arm; and, for participants with observed outcomes, differences in baseline variables between the three arms. There were no clear associations between known predictors of nausea and cases missing the nausea primary outcome. This fact along with the highly non-normal distribution of the primary response (for which imputation methods are not so well developed) informed our decision not to apply multiple imputation analyses.
Randomisation method
The trial arm allocation method was minimisation with a random element over the margins of three factors: gender, age (16–24, > 24–50, > 50 years) and emetogenic risk (low, moderate, high). The first 20 cases were allocated completely at random and thereafter the allocation probability vector was (0.6, 0.3, 0.1) to the arms that would result in the least to the most imbalance respectively. Researchers telephoned the randomisation office staff with the patient details and the staff used an in-house program to obtain the allocated trial arm.
Outcome measures
Primary outcome
Rhodes Index of Nausea, Vomiting and Retching
The Rhodes Index of Nausea, Vomiting and Retching49 is an eight-item validated scale measuring nausea and vomiting experience, incidence and severity. In this study the nausea experience subscale has been used (as shown in Appendix 1) for power calculations of the sample size, using the mean score across all assessment days in each cycle as the end point. From the nausea experience score, incidence and severity can also be isolated. Scores can range from 0 to 12, with higher scores indicating higher levels of the symptom experience. This scale, taking 1–2 minutes to complete, was scored daily from the day before chemotherapy (to capture any anticipatory nausea) up to 7 days post chemotherapy, that is, eight assessments per cycle.
Secondary outcomes
Multinational Association of Supportive Care in Cancer Antiemesis Tool
The MASCC Antiemesis Tool (MAT)39 is an eight-item scale that assesses in a simple way both acute and delayed nausea and vomiting incidence and extent and was designed specifically for chemotherapy-related nausea and vomiting. This short clinical scale has shown satisfactory internal reliability (alpha = 0.77), contrasted-groups and concurrent validity, and high recall of events up to 3 weeks post chemotherapy. The MAT is designed to be used once per cycle, with retrospective patient recall of events, minimising the patient burden. Factor analysis has clearly identified three factors, namely vomiting, acute nausea and delayed nausea. 39 The scale (see Appendix 2) was completed at day 10 of each cycle (i.e. four assessments).
Functional Assessment of Cancer Therapy – General quality-of-life scale
The Functional Assessment of Cancer Therapy – General (FACT-G)50 is a well-validated quality-of-life scale focusing on functional assessment. This functional scale has provided not only quality-of-life indications, but also changes in other symptoms/side effects that may have resulted from any improved management of nausea (e.g. appetite). High internal consistency and construct validity have been reported in past studies using the FACT scales in various cancer populations. Completion time is about 5 minutes. This scale (see Appendix 3) was completed at baseline and then at day 10 of each cycle (i.e. five assessments).
Hospital Anxiety and Depression Scale
The Hospital Anxiety and Depression Scale51 is a 14-item scale assessing anxiety with seven items and depression with a further seven items. Each item is answered on a 4-point scale (0–3). Scores on each subscale thus range between 0 (no symptoms) and 21 (numerous and severe symptoms). There are separate scores for anxiety and depression. In this study the anxiety subscale was obtained at baseline (see Appendix 4), with the score used as a covariate in the final statistical analysis of the data, as anxiety is a key risk factor for the development of nausea/vomiting. 29,43 This scale has been used extensively with cancer patients as a screening tool and has been reported to have excellent psychometric properties. Completion time is approximately 2–5 minutes.
Patient expectations of nausea/vomiting
As this is a key risk factor identified in the literature,29,43 a two-item scale was developed assessing patient expectations for nausea and vomiting, measured on a 10-point ordinal scale. We have used the same measurement approach elsewhere in the past52 although no validation of these two items has formally taken place. This was incorporated in the final analysis of outcomes. Patients were also asked how much they believed that the acupressure wristbands had helped them alleviate nausea and how much faith they had in complementary therapies, also using 10-point scales (see Appendix 5).
Sociodemographic and disease/treatment variables
Sociodemographic and treatment characteristics were obtained from the patients' records and the patients themselves (see Appendix 6). These included gender, age, educational level, marital status, experience with nausea in the past (such as during pregnancy, motion sickness or nausea when eating certain foods), use of/experience with other complementary therapies in the past, cancer diagnosis, stage of disease and chemotherapy protocol used and dosage. Such a questionnaire had already been developed by the team and used in the past in other nausea/vomiting studies. 15,29 Medication use (standard and rescue antiemetics) during study participation was obtained from pharmacy records. Furthermore, although not formally required, researchers asked patients about any side effects (or patients could volunteer side effect information) and these were recorded in a descriptive manner.
Assessment scales were provided to patients for self-completion at home; completed forms were returned to researchers using a prepaid self-addressed envelope. Patients were asked to complete their daily assessments of nausea at the same time in the evening to have a consistent time frame for measuring change. Patients were reminded to return their completed scales when attending for chemotherapy and were also contacted at an early stage during the trial when the researcher would remind them of the instructions for completing and returning the scales. Table 1 shows the timing of the completion of the study assessment forms.
| Assessment scale | Baseline assessment | Chemotherapy days −1, 0, 1, 2, 3, 4, 5, 6×four cycles | Chemotherapy day 10×four cycles | End of study participation |
|---|---|---|---|---|
| Rhodes Index of Nausea, Vomiting and Retching | ✗ | |||
| MAT | ✗ | |||
| FACT-G | ✗ | ✗ | ||
| Hospital Anxiety and Depression Scale | ✗ | ✗ | ||
| Patient expectations questionnaire | ✗ | |||
| Sociodemographic variables | ✗ | |||
| Disease/treatment variables | ✗ | ✗ | ||
| Health economics assessment | ✗ | ✗ | ✗ | ✗ |
Wristband compliance and audit
Patients who had been randomised to the acupressure and sham acupressure groups were also asked to provide information about the length of time that they had worn their wristbands on the day of chemotherapy and the 6 subsequent days. A wristband compliance questionnaire (see Appendix 7) was given to the patients per cycle to complete and return in the prepaid envelope together with the other completed scales.
In addition to compliance with wearing wristbands, it was important to determine whether or not patients were wearing the wristbands correctly according to the instructions they had been given. A wristband audit was conducted at two Manchester sites (Christie NHS Foundation Trust and Macclesfield District Hospital) and two Liverpool sites (Clatterbridge Centre for Oncology and the Royal Liverpool Hospital). Research nurses who were involved with patient recruitment to the trial assessed how correctly patients were wearing their wristbands when they attended for chemotherapy treatment. In total, observations were carried out for 35 pairs of wristbands. An audit proforma is shown in Appendix 8.
The trial did not come under Medicines and Healthcare products Regulatory Agency (MHRA) regulations as it was not a clinical trial of an investigational medicinal product as defined by EU Directive 2001/20/EC; a procedure for the reporting of serious adverse events was therefore not required. However, patients were regularly asked about their experiences with regard to wearing wristbands (e.g. when attending for chemotherapy and during routine telephone communication during the trial) and about any problems associated with the wristbands (e.g. if a larger size was required, if the wristband caused any kind of discomfort, if the wristband became damaged). These were logged by the researchers, with appropriate advice offered to patients.
Measurement of costs
Costs were identified, measured and valued using a microcosting approach (in which each component of resource use was identified, estimated and a unit cost derived from market prices and national estimates53). The cost analysis was performed from the perspective of the health service provider and from a societal perspective. Included in the health-care provider costs were those accrued by the acute trusts and the primary care trusts. Costs to the patients and their families, including social care, were considered as the additional costs for society. Indirect costs in terms of workdays lost were also included.
Data were collected prospectively and retrospectively using multiple sources including patient records and patient self-reported questionnaires (see Appendices 9 and 10). The questionnaires reported health service utilisation subsequent to and as a result of chemotherapy-induced nausea/vomiting (e.g. GP visits), patient out-of-pocket expenses such as over-the-counter medicines or transport and the use of services in the social sector such as home help and support from family and friends. Valuation of resource items including hospital resources (e.g. bed-days and staff time) and community resources (e.g. GP visits, home help) was carried out using national estimates;53 market prices were assigned to medication; non-market items, specifically patient time and informal help provided by family and friends, were valued using market wage rates; and out-of-pocket expenses (e.g. bus fares) were also calculated.
In more detail, direct medical costs were defined as the costs of prophylactic or rescue antiemetic medications, drug administration devices, staff time associated with preparing and administering medication and tending to patients with chemotherapy-induced nausea and vomiting, hospitalisations due to chemotherapy-induced nausea and vomiting, hospital outpatient or GP visits due to chemotherapy-induced nausea and vomiting and over-the-counter medications or other complementary therapies. Direct non-medical costs were those for transportation and need for assistance, such as additional childcare. Indirect costs were based exclusively on the number of workdays lost due to chemotherapy-induced nausea and vomiting. Costs that were not included in this evaluation were costs for chemotherapy agents, preplanned visits or hospitalisations for the purpose of chemotherapy administration, and diagnostic and laboratory tests, and other patient management costs not directly related to chemotherapy-induced nausea and vomiting.
Analysis of economic data
The total cost of each arm of the trial was calculated by combining the resource use and unit cost data. No discounting was necessary given the time period of data collection (< 1 year). Sensitivity analysis was carried out to account for uncertainty when estimates of cost data were used. Differences in costs between the three arms were tested for using independent sample t-tests. Cost data in each of the arms were analysed alongside the quality-of-life measures with the data combined and analysed using cost-effectiveness ratios (i.e. the difference in costs between alternatives relative to the difference in effectiveness between the same alternatives). Cost per quality-adjusted life-year (QALY) data are presented.
Nested qualitative study
There was an exploratory nested qualitative study within the trial that explored patients' reasons for consenting to take part in the trial and their experiences of participating in a randomised controlled trial for acupressure wristbands.
A number of qualitative studies have explored patients' experiences of receiving treatment with acupuncture, and their findings have suggested that the treatment is associated with eliciting benefits beyond the alleviation of the patients' presenting condition. 54–56 These expanded effects of care include improvements in physical/mental health and emotional well-being and changes in personal identity and lifestyle, and can result in patients ‘feeling normal again’ and ‘regaining their lives’. Despite the burgeoning qualitative research exploring the experiences of patients receiving acupuncture, to date no study has been conducted to explore the experiences of users of acupressure or acupressure wristbands. To address this gap in the evidence base, a nested qualitative study was conducted with patients taking part in the main trial.
Objectives
-
To outline patients' experiences of using acupressure wristbands.
-
To outline the reasons why patients consent to take part in a clinical trial of acupressure wristbands.
-
To outline patients' experiences of taking part in a randomised controlled trial of acupressure wristbands.
Sample
A purposive sample of patients who had taken part in the clinical trial of the effectiveness and cost-effectiveness of acupressure wristbands for chemotherapy-induced nausea and vomiting participated in one-to-one semistructured interviews. Patients were recruited from each of the three geographical sites, represented all three study arms, had either high or low scores for the item about their expectation of effect from the wristbands and either had or did not have experience using complementary therapies in general.
Methodology
Interviews were conducted by three members of the research team and were directed by a topic guide. Topic guides were updated throughout the study to incorporate emerging themes. Interviews were audiotaped and transcribed verbatim. Interviews lasted for between 30 and 70 minutes. Transcripts were analysed thematically using framework analysis, a manual, matrix method, which facilitates thematic and cross-case interpretation. 57,58 Analysis proceeded in five stages:
-
familiarisation – transcripts were read and reread by members of the research team until they became familiar with and immersed in the data
-
identification of the thematic framework – key issues, concepts and themes arising from the data were identified and grouped thematically to construct a conceptual framework
-
indexing – two of the research team independently applied the thematic framework to the same transcript to explore any differences in application; the thematic framework was then applied systematically to all of the data
-
charting – thematic matrices were constructed for all identified categories/subcategories to further summarise and synthesise the indexed data
-
detection, categorisation and classification – the original research questions were reconsidered and the charts examined in order to define concepts, map the range and nature of phenomena, find any associations and provide explanations.
Research governance
The sponsor of the study was the University of Manchester. The MHRA has confirmed that this trial does not fall under the Medicines for Human Use (Clinical Trials) Regulations 2004, as described earlier.
Trial Steering Committee
A Trial Steering Committee was formed, which was chaired by a patient representative. Other members included a medically trained expert in chemotherapy nausea who was independent of the study, an acupressure practitioner, a representative of the trials unit (statistician), the lead applicant and two of the co-applicants, one of whom was the study's user/co-applicant. This committee was responsible for trial safety and assurance of scientific validity and was convened four times: once after completion of the preparatory part of the trial, twice more during the recruitment phase and once after data cleaning and before data analysis.
Data Monitoring and Ethics Committee
An independent DMEC was set up by the Christie NHS Foundation Trust Clinical Trials Unit in accordance with standard procedures to review accruing trial data on a regular basis and also to ensure that a sufficient number of patients were enrolled, reporting back to and guiding the Trial Steering Committee and also reporting to the HTA programme through the Trial Steering Committee. The members of this committee were not linked to the study in any way.
Trial management
The Christie NHS Foundation Trust Clinical Trials Unit was responsible for data management. It is based within the research and development division of the Christie NHS Foundation Trust. The unit manages international, national and local studies and its portfolio during the conduct of our trial included three international studies, 14 randomised studies and 12 other studies. These included a number of studies with funding from Cancer Research UK through the Clinical Trials Advisory and Awards Committee (CTAAC) route. The unit has a strategic alliance with the Medical Research Council Clinical Trials Unit in London, and the development and operation of the Christie unit is supported by the MRC unit.
The Clinical Trials Unit has robust governance and management systems that have been subjected to a MHRA Good Clinical Practice inspection, with the inspectors describing the systems for the management of clinical trials as ‘robust’. This study was conducted in accordance with the unit's standard operating procedures; these cover all aspects of the management of clinical trials and the unit can assure funders that the studies supported are managed within a quality framework that has been reviewed by the MHRA. The unit has been responsible for the monitoring of the trial to ensure that it is conducted in accordance with the protocol, research governance framework and applicable regulations. A full list of standard operating procedures is available on request and on the unit's website. The unit had the capacity and capability to support a trial of this nature (indeed it has in its portfolio another national study on complementary therapies using acupuncture) and was able to identify a project lead to oversee the work of the data manager. The data manager was responsible for ensuring that the data generated by the study were reviewed appropriately by the DMEC, and the trials supported by the unit are typically reviewed through these mechanisms. Randomisation and statistical analysis have been supported by the unit's statisticians.
Service user involvement
Service users were involved at three levels. The first has been at the development phase of this proposal, with the contribution of the chairperson of the National Cancer Research Institute (NCRI) Consumer Liaison Group, who was a named co-applicant in the study, and reviews by expert patients. The second level was monitoring the trial project and guiding it within its scientific framework through chairing and participating in the Trial Steering Committee and the DMEC. Finally, users have advised us in planning appropriate patient-focused dissemination of the trial results at the end of the study. For reviews, contacts, active involvement and access, the research partners' strategy and mechanisms through the NCRI Cancer Experiences Collaborative have been utilised.
Chapter 4 Main findings
Patient recruitment
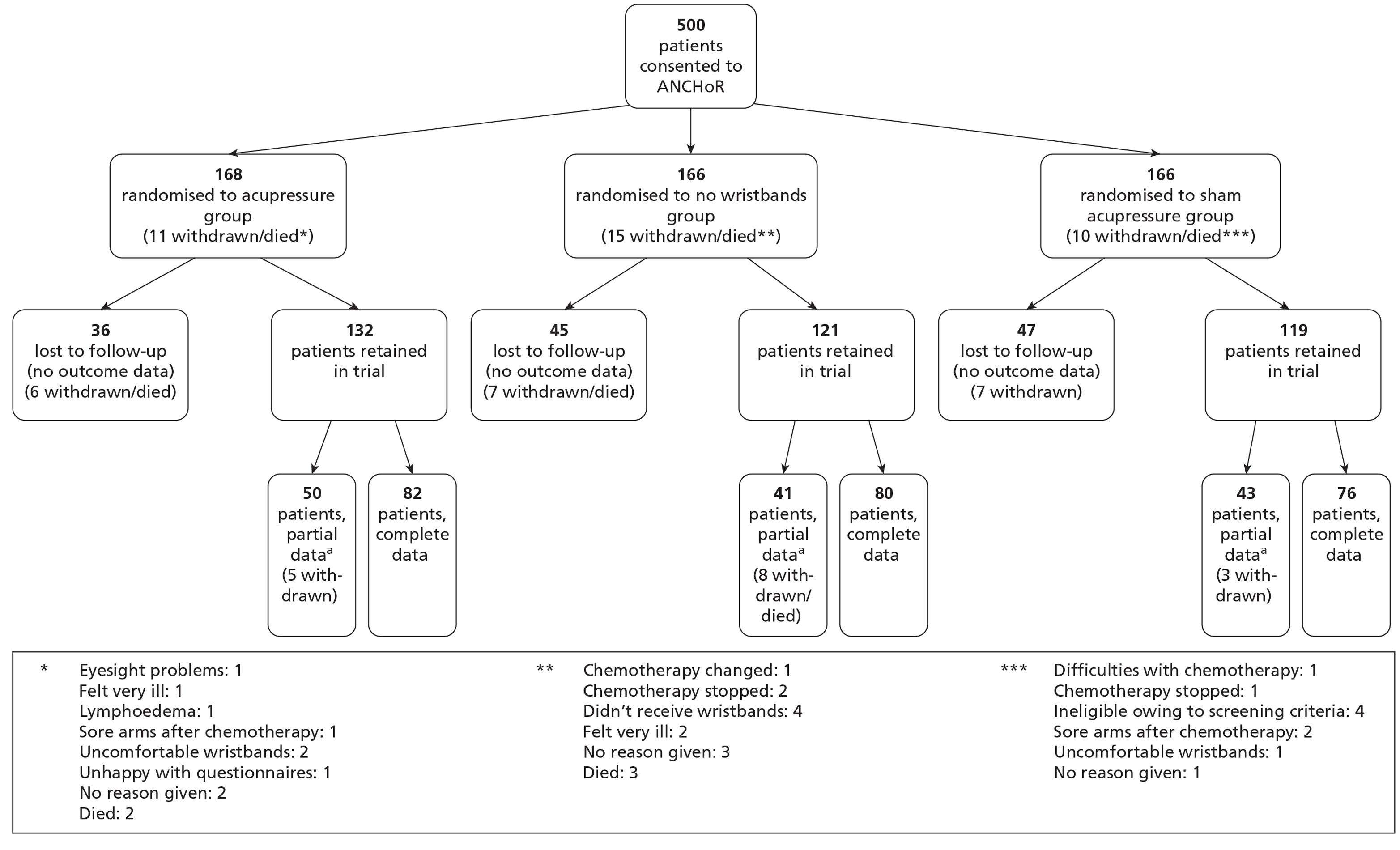
Three main study centres were involved in patient recruitment, namely Manchester, Liverpool and Plymouth. Recruitment began with a month-long pilot phase in Manchester at the Christie NHS Foundation Trust to test recruitment processes. The first case was randomised on 23 March 2009. A phased recruitment launch followed in the two remaining centres with patient recruitment starting in Liverpool in May 2009 and in Plymouth in June 2009. The last case was randomised on 15 October 2010. In total, 14 hospital sites were involved in recruitment (Table 2) and 500 cases were randomised (166 standard care, 166 sham acupressure and 168 acupressure).
| Main centre and total number of patients recruited | Breakdown of recruitment per study site |
|---|---|
| Manchester (244 patients randomised over 76 weeks) | 119 from Christie NHS Foundation Trust (including initial pilot phase) |
| 50 from Royal Oldham Hospital | |
| 1 from Tameside General Hospital | |
| 5 from Leighton Hospital | |
| 39 from Stepping Hill Hospital | |
| 26 from Macclesfield District General Hospital | |
| 4 from North Manchester General Hospital | |
| Liverpool (161 patients randomised over 71 weeks) | 33 from Clatterbridge Centre for Oncology |
| 79 from Royal Liverpool Hospital | |
| 41 from St Helens Hospital | |
| 8 from Southport General Infirmary | |
| Plymouth (95 patients randomised over 64 weeks) | 2 from Torbay District General Hospital |
| 88 from Derriford Hospital | |
| 5 from Royal Cornwall Hospital |
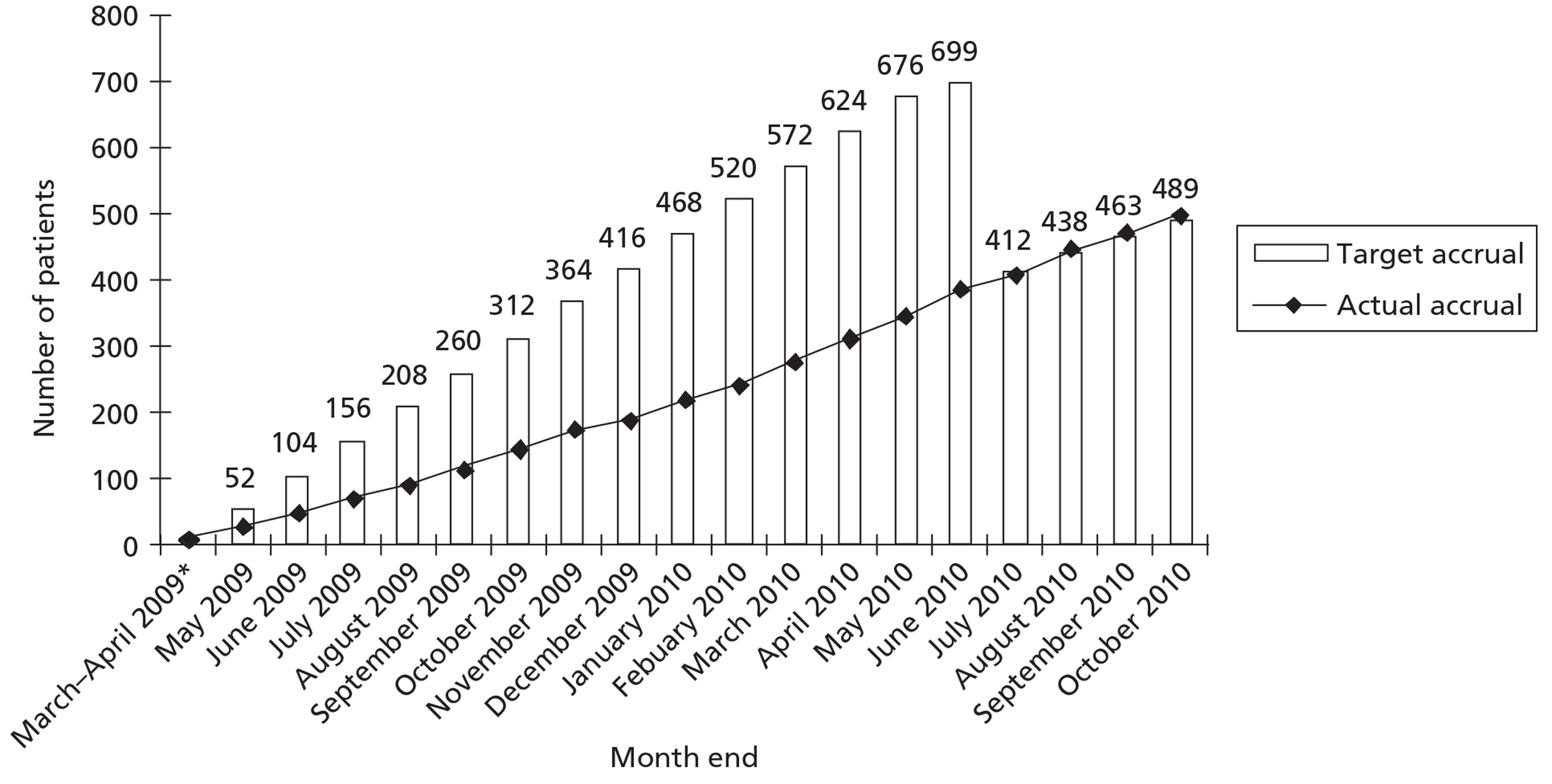
Monthly recruitment for the trial is given in Figure 1.
FIGURE 1.
Trial recruitment by month. Monthly target accrual was recalculated after sample size adjustment in May 2010, with the agreement of the HTA team. Power was initially calculated to 90% but as recruitment was slower than expected the sample size was recalculated to 80% power.
A CONSORT flow diagram (Figure 2) shows the numbers of participants recruited and randomly assigned to the three trial arms and who received the intended interventions and were analysed for the primary outcome.
FIGURE 2.
Patient recruitment into the ANCHoR trial: Manchester, Liverpool and Plymouth sites, March 2009 to October 2010. a, Partial data indicate data collected from less than the complete dataset (baseline plus four cycles of chemotherapy), that is, when at least one assessment was missing. Data represented in this figure include the pilot phase, March–April 2009.
Descriptive statistics by trial arm
The majority of the participants were female, married and aged > 50 years. The key diagnoses of the sample included breast and colorectal cancer and the majority had received moderately emetogenic chemotherapy (including anthracycline-based chemotherapy). Other sociodemographic and clinical data are provided in Table 3.
| None (n) | Sham bands (n) | Acupressure bands (n) | |
|---|---|---|---|
| Gender | |||
| Male | 38 | 37 | 39 |
| Female | 128 | 129 | 129 |
| Age group (years) | |||
| ≤ 50 | 51 | 55 | 54 |
| 51 + | 115 | 111 | 114 |
| Marital status | |||
| Single | 18 | 15 | 24 |
| Married | 85 | 83 | 88 |
| Divorced or separated | 20 | 27 | 21 |
| Widowed | 13 | 16 | 11 |
| Missing | 30 | 25 | 24 |
| Educational attainment | |||
| Primary school | 0 | 3 | 2 |
| Secondary school | 68 | 74 | 69 |
| College/diploma | 37 | 26 | 43 |
| University/degree | 20 | 14 | 17 |
| Postgraduate | 6 | 12 | 8 |
| Missing | 35 | 37 | 29 |
| Ethnic origin | |||
| Caucasian | 111 | 110 | 121 |
| Black | 0 | 1 | 2 |
| Asian/Chinese | 2 | 2 | 2 |
| Mixed | 1 | 3 | 0 |
| Missing | 52 | 50 | 43 |
| Religious affiliation | |||
| Christian | 114 | 106 | 122 |
| Muslim | 1 | 2 | 3 |
| Hindu | 0 | 1 | 0 |
| None | 17 | 18 | 12 |
| Prefers not to say | 1 | 4 | 2 |
| Other | 5 | 8 | 7 |
| Missing | 28 | 27 | 22 |
| Occupational group | |||
| Professional | 40 | 44 | 36 |
| Managerial and technical | 17 | 16 | 23 |
| Skilled non-manual | 14 | 11 | 16 |
| Skilled manual | 14 | 12 | 11 |
| Unskilled | 14 | 16 | 15 |
| Not applicable | 28 | 33 | 43 |
| Missing | 39 | 34 | 24 |
| Occupational status59 | |||
| Employed full-time | 50 | 36 | 33 |
| Employed part-time | 22 | 17 | 26 |
| Retired | 46 | 50 | 51 |
| Unemployed | 2 | 4 | 3 |
| Casual worker | 0 | 0 | 1 |
| Not working due to ill health | 10 | 20 | 17 |
| Housewife | 5 | 11 | 10 |
| Other | 2 | 3 | 4 |
| Missing | 29 | 25 | 23 |
| Smoking history | |||
| Never | 63 | 62 | 66 |
| Previously | 56 | 51 | 62 |
| Current | 20 | 25 | 18 |
| Missing | 27 | 28 | 22 |
| Cancer diagnosis | |||
| Breast | 89 | 90 | 82 |
| Bowel (colon and rectum) | 25 | 27 | 19 |
| Gynaecological | 20 | 15 | 21 |
| Lung | 7 | 10 | 21 |
| Lymphoma | 6 | 2 | 7 |
| Oesophagus | 4 | 5 | 2 |
| Stomach | 2 | 3 | 1 |
| Pancreas | 1 | 1 | 4 |
| Melanoma | 2 | 2 | 1 |
| Bladder | 1 | 1 | 2 |
| Gall bladder | 0 | 1 | 1 |
| Head and neck | 1 | 1 | 0 |
| Endocrine/thymus | 0 | 1 | 1 |
| Prostate | 1 | 0 | 1 |
| Child: Ewing's sarcoma | 1 | 1 | 0 |
| Child: non-Hodgkin's lymphoma | 0 | 1 | 0 |
| Missing | 6 | 5 | 5 |
| Emetogenic risk | |||
| Low | 13 | 11 | 12 |
| Moderate | 107 | 111 | 111 |
| High | 46 | 44 | 45 |
Assessment of missing data for the primary outcome
A total of 500 cases were randomised, but there are data for only 361 of these cases for the primary outcome, i.e. the primary outcome is missing in around 28% of cases. Table 4 illustrates, for the remaining 497 cases, the proportion of cases in which the primary outcome is missing by various factors thought to influence nausea propensity (three cases were allocated to ‘No acupressure’ but there are no records in the trial database for these three cases, i.e. no completed screening forms and no returned data form). There are no marked associations between any of these factors and the probability of the primary outcome being missing.
| Factor | Level | Missing primary outcome, n/N (%) | p-valuea |
|---|---|---|---|
| Trial arm | None | 46/163 (28)b | 0.69 |
| Sham bands | 48/166 (29) | ||
| Acupressure bands | 42/168 (25) | ||
| Age (years) | ≤ 50 | 42/160 (26) | 0.78 |
| 51 + | 94/337 (28) | ||
| Gender | Male | 35/114 (31) | 0.43 |
| Female | 101/383 (26) | ||
| Emetogenic risk | Low | 12/36 (33) | 0.69 |
| Moderate | 87/327 (27) | ||
| High | 37/134 (28) | ||
| 2-weekly CT | No | 122/451 (27) | 0.75 |
| Yes | 14/46 (30) | ||
| Baseline anxiety (83 missing) | Normal (0–7) | 50/250 (20) | 0.16 |
| Borderline (8–10) | 22/76 (29) | ||
| Case (11–21) | 24/88 (27) | ||
| Nausea expectation (84 missing) | 0–3 | 24/100 (24) | 0.80 |
| 4–6 | 52/224 (23) | ||
| 7–10 | 18/89 (20) |
Inevitably there is a further dropout of cases with each cycle, as illustrated in Table 5.
| Dropout status | None | Sham bands | Acupressure bands | Total |
|---|---|---|---|---|
| Immediate (no outcome data) | 45 | 47 | 36 | 128 |
| Complete (all outcome data) | 80 | 76 | 82 | 238 |
| Cycle 1 then dropped out | 12 | 14 | 14 | 40 |
| Cycles 1 and 2 then dropped out | 11 | 17 | 12 | 40 |
| Cycles 1–3 then dropped out | 14 | 11 | 12 | 37 |
| Intermittent data | 4 | 1 | 12 | 17 |
| Total | 166 | 166 | 168 | 500 |
Table 6 shows the mean nausea experience of the patients using the Rhodes Index of Nausea, Vomiting and Retching scale. Scores range from 0 to 12, with higher scores indicating higher levels of nausea. Both the sham and acupressure arms experienced less nausea than the standard care arm, although the difference did not reach statistical significance. It is important to note that these mean values represent very low levels of nausea. Only at cycle 4 did the results become significant (p < 0.05), with the acupressure group reporting no nausea experience.
| Cycle | Nonea | Sham bandsa | Acupressure bandsa |
|---|---|---|---|
| 1 | 1.43 (0, 0, 3.71, 8.57) (n = 117) | 0.57 (0, 0, 2.64, 9.17) (n = 118) | 1.0 (0, 0, 2.97, 7.50) (n = 126) |
| 2 | 1.71 (0, 0, 3.43, 10.14) (n = 109) | 0.71 (0, 0, 2.14, 10.29) (n = 105) | 0.93 (0, 0, 3.43, 9.57) (n = 114) |
| 3 | 1.14 (0, 0, 3.86, 11.86) (n = 96) | 0.71 (0, 0, 2.29, 9.71) (n = 88) | 0.43 (0, 0, 3.00, 10.14) (n = 103) |
| 4 | 1.14 (0, 0, 4.00, 9.14) (n = 81) | 0.43 (0, 0, 2.43, 8.57) (n = 77) | 0.00 (0, 0, 1.82, 9.86) (n = 90) |
Tables 7 and 8 show the frequencies of the nausea and vomiting experience, respectively, categorised over five ranges of experience.
| Score | ||||||
|---|---|---|---|---|---|---|
| 0 | (0–2] | (2,4] | (4,6] | > 6 | NA | |
| Cycle 1 | ||||||
| None | 34 | 35 | 21 | 14 | 13 | 49 |
| Sham bands | 36 | 47 | 19 | 8 | 8 | 48 |
| Acupressure bands | 41 | 38 | 28 | 11 | 8 | 42 |
| Cycle 2 | ||||||
| None | 32 | 29 | 27 | 9 | 12 | 57 |
| Sham bands | 36 | 41 | 13 | 10 | 5 | 61 |
| Acupressure bands | 43 | 29 | 19 | 10 | 13 | 54 |
| Cycle 3 | ||||||
| None | 33 | 22 | 19 | 14 | 8 | 70 |
| Sham bands | 33 | 31 | 10 | 9 | 5 | 78 |
| Acupressure bands | 43 | 29 | 11 | 8 | 12 | 65 |
| Cycle 4 | ||||||
| None | 27 | 23 | 13 | 10 | 8 | 85 |
| Sham bands | 36 | 16 | 15 | 7 | 3 | 89 |
| Acupressure bands | 53 | 17 | 8 | 6 | 6 | 78 |
| Score | ||||||
|---|---|---|---|---|---|---|
| 0 | (0–1] | (1,2] | (2,3] | > 3 | NA | |
| Cycle 1 | ||||||
| None | 76 | 24 | 11 | 4 | 3 | 48 |
| Sham bands | 80 | 27 | 7 | 3 | 1 | 48 |
| Acupressure bands | 85 | 24 | 9 | 7 | 1 | 42 |
| Cycle 2 | ||||||
| None | 74 | 20 | 9 | 2 | 3 | 58 |
| Sham bands | 74 | 21 | 6 | 4 | 0 | 61 |
| Acupressure bands | 90 | 16 | 2 | 4 | 2 | 54 |
| Cycle 3 | ||||||
| None | 75 | 13 | 3 | 0 | 5 | 70 |
| Sham bands | 68 | 15 | 4 | 0 | 1 | 78 |
| Acupressure bands | 81 | 15 | 3 | 1 | 3 | 65 |
| Cycle 4 | ||||||
| None | 60 | 11 | 3 | 5 | 2 | 85 |
| Sham bands | 61 | 12 | 1 | 2 | 1 | 89 |
| Acupressure bands | 76 | 9 | 3 | 1 | 1 | 78 |
Tables 9 and 10 present descriptive data of acute and delayed nausea, respectively, based on the MAT scale, showing similar results to those obtained with the Rhodes Index of Nausea, Vomiting and Retching scale. Tables 11 and 12 report the data for acute and delayed vomiting, respectively, based on the MAT scale.
| Score | ||||||
|---|---|---|---|---|---|---|
| 0 | 1–3 | 4–6 | 7–9 | 10 | NA | |
| Cycle 1 | ||||||
| None | 63 | 20 | 14 | 11 | 9 | 49 |
| Sham bands | 65 | 33 | 9 | 8 | 5 | 46 |
| Acupressure bands | 72 | 29 | 14 | 8 | 7 | 38 |
| Cycle 2 | ||||||
| None | 56 | 28 | 14 | 6 | 6 | 56 |
| Sham bands | 71 | 21 | 4 | 8 | 2 | 60 |
| Acupressure bands | 68 | 25 | 7 | 8 | 8 | 52 |
| Cycle 3 | ||||||
| None | 52 | 24 | 10 | 7 | 3 | 70 |
| Sham bands | 53 | 21 | 9 | 4 | 2 | 77 |
| Acupressure bands | 65 | 15 | 10 | 8 | 4 | 66 |
| Cycle 4 | ||||||
| None | 44 | 17 | 10 | 7 | 3 | 85 |
| Sham bands | 49 | 16 | 8 | 5 | 1 | 87 |
| Acupressure bands | 61 | 12 | 8 | 4 | 3 | 80 |
| Score | ||||||
|---|---|---|---|---|---|---|
| 0 | 1–3 | 4–6 | 7–9 | 10 | NA | |
| Cycle 1 | ||||||
| None | 49 | 24 | 21 | 13 | 11 | 48 |
| Sham bands | 61 | 25 | 14 | 7 | 9 | 50 |
| Acupressure bands | 58 | 30 | 25 | 8 | 7 | 40 |
| Cycle 2 | ||||||
| None | 43 | 23 | 22 | 11 | 6 | 61 |
| Sham bands | 54 | 24 | 17 | 7 | 2 | 62 |
| Acupressure bands | 57 | 28 | 17 | 9 | 5 | 52 |
| Cycle 3 | ||||||
| None | 43 | 22 | 15 | 8 | 5 | 73 |
| Sham bands | 42 | 26 | 14 | 4 | 3 | 77 |
| Acupressure bands | 56 | 19 | 13 | 10 | 4 | 66 |
| Cycle 4 | ||||||
| None | 35 | 20 | 10 | 8 | 6 | 87 |
| Sham bands | 44 | 16 | 13 | 4 | 1 | 88 |
| Acupressure bands | 57 | 14 | 6 | 7 | 2 | 82 |
| Score | ||||||
|---|---|---|---|---|---|---|
| 0 | 1–3 | 4–6 | 7–9 | 10 | NA | |
| Cycle 1 | ||||||
| None | 99 | 10 | 4 | 2 | 2 | 49 |
| Sham bands | 106 | 12 | 1 | 1 | 1 | 45 |
| Acupressure bands | 110 | 11 | 5 | 1 | 3 | 38 |
| Cycle 2 | ||||||
| None | 97 | 8 | 1 | 4 | 0 | 56 |
| Sham bands | 94 | 7 | 4 | 1 | 0 | 60 |
| Acupressure bands | 101 | 9 | 2 | 0 | 3 | 53 |
| Cycle 3 | ||||||
| None | 91 | 3 | 1 | 1 | 1 | 69 |
| Sham bands | 81 | 5 | 1 | 1 | 0 | 60 |
| Acupressure bands | 91 | 8 | 3 | 0 | 0 | 66 |
| Cycle 4 | ||||||
| None | 75 | 4 | 1 | 1 | 0 | 85 |
| Sham bands | 72 | 6 | 0 | 1 | 0 | 87 |
| Acupressure bands | 86 | 3 | 0 | 1 | 0 | 78 |
| Score | ||||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | NA | |
| Cycle 1 | ||||||
| None | 99 | 9 | 6 | 2 | 3 | 47 |
| Sham bands | 103 | 7 | 3 | 2 | 2 | 49 |
| Acupressure bands | 108 | 8 | 7 | 2 | 3 | 40 |
| Cycle 2 | ||||||
| None | 94 | 7 | 3 | 0 | 2 | 60 |
| Sham bands | 98 | 3 | 2 | 1 | 1 | 61 |
| Acupressure bands | 104 | 7 | 3 | 0 | 2 | 52 |
| Cycle 3 | ||||||
| None | 86 | 2 | 2 | 1 | 2 | 73 |
| Sham bands | 80 | 5 | 2 | 1 | 1 | 77 |
| Acupressure bands | 90 | 6 | 2 | 2 | 1 | 67 |
| Cycle 4 | ||||||
| None | 71 | 2 | 2 | 2 | 3 | 86 |
| Sham bands | 71 | 3 | 2 | 2 | 0 | 88 |
| Acupressure bands | 80 | 4 | 1 | 1 | 0 | 82 |
Primary outcome analysis
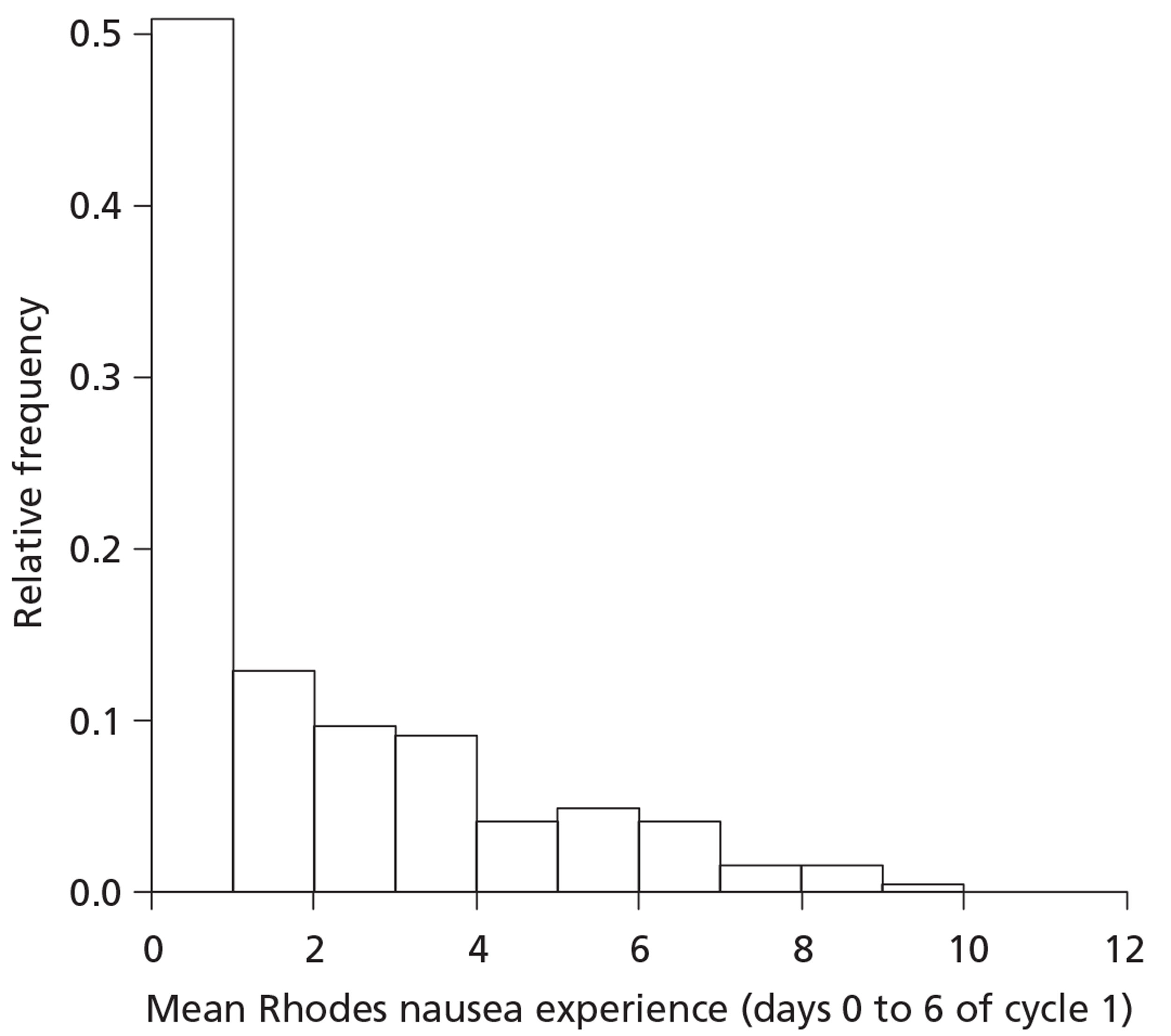
The primary outcome is the mean nausea experience (days 0–6) for cycle 1 measured using the Rhodes Index of Nausea, Vomiting and Retching scale. As can be seen from Figure 3 the data are highly skewed. The possible range of scores is from 0 to 12, but, in fact, 111/361 (31%) are exactly zero and around half of all values are < 1. No transformation would be successful in normalising such a distribution.
FIGURE 3.
Primary outcome distribution (n = 361). The intervals are [0,1], (1,2], (2,3], … [11,12]. The first and last intervals are closed at both ends and intervals in between are open at their lower bound and closed at their upper bound.
The distribution by trial arm is shown in Figure 4. Because of the highly skewed distribution, the non-parametric Kruskal–Wallis test has been used for the primary comparison of the trial arms. This overall test is non-significant (p = 0.14).
FIGURE 4.
Box and whisker plot of the primary outcome by trial arm.
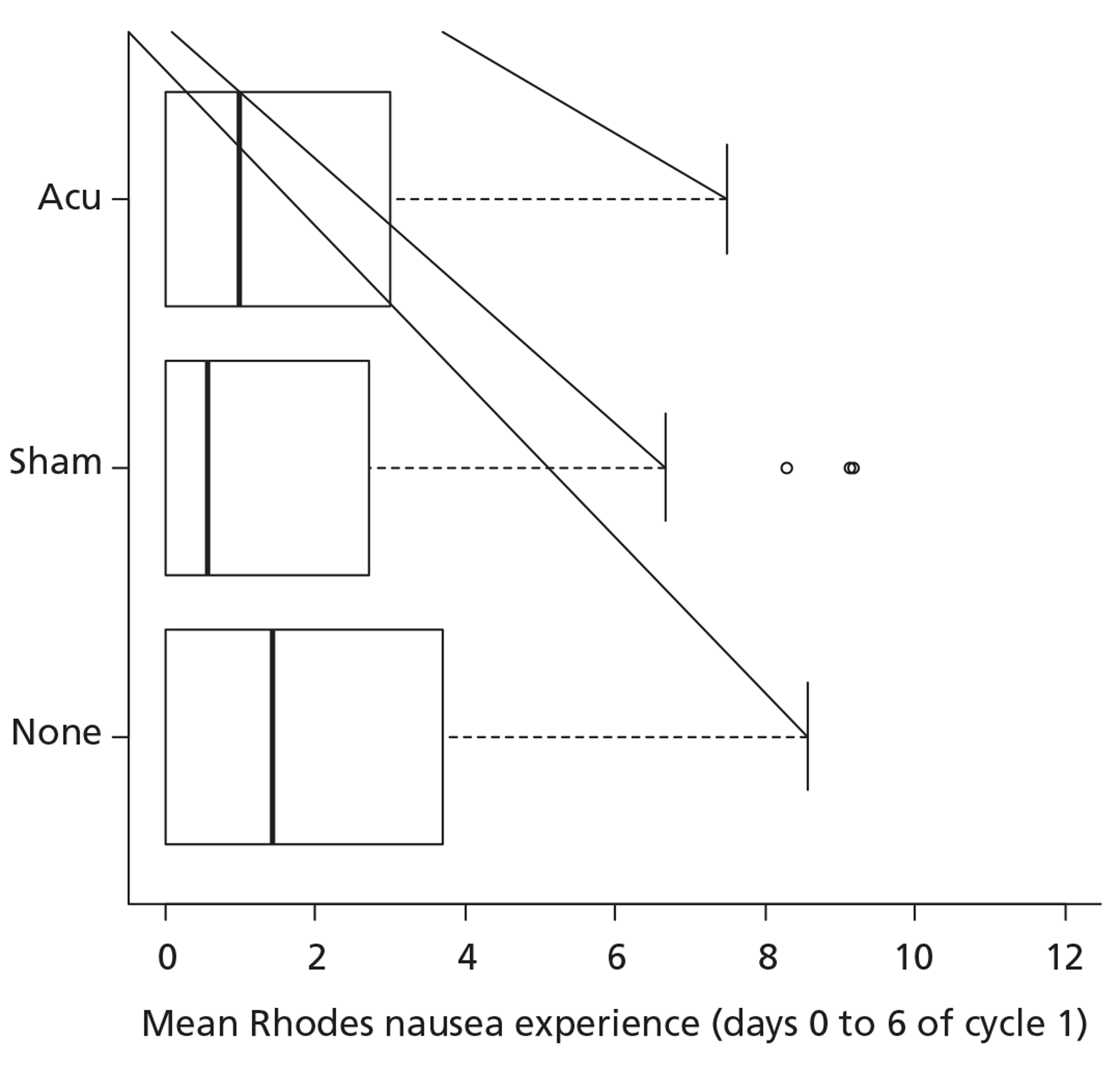
Provision for pair-wise comparisons (Mann–Whitney U-tests) with a Bonferroni adjustment was made in the trial design: none compared with acupressure (p = 0.23), none compared with sham acupressure (p = 0.05), sham acupressure compared with acupressure (p = 0.40). It should be noted that the reference value for statistical significance is 0.017 and so none of these pair-wise comparisons is statistically significant.
Regression analyses for the nausea primary outcome data
As noted previously, the data are highly skewed and no transformation will be successful in normalising the distribution. The approach adopted here is to group the values into five ordered categories and to utilise regression methods for ordinal data. The categories were chosen after inspection of Figure 3. The first category, ‘Zero’, was chosen as it represented no nausea at all and a large fraction of cases fell into this category (31%). The choice of the other categories was somewhat arbitrary. Five categories is fairly typical for ordinal regression models in the literature and it is desirable that no category has a very small frequency. Category 5 has a broad range but includes only 8% of cases; subdividing it further would be counterproductive. Having chosen the lowest and highest categories the remaining three were simply selected to have equal width. Table 13 shows the categories and the frequencies by trial arm (n = 361).
| Category | Nausea score | None | Sham bands | Acupressure bands |
|---|---|---|---|---|
| 1 | 0 | 34 | 36 | 41 |
| 2 | (0, 2) | 35 | 47 | 38 |
| 3 | (2, 4) | 21 | 19 | 28 |
| 4 | (4, 6) | 14 | 8 | 11 |
| 5 | (6, 12) | 13 | 8 | 8 |
The main tool used was the proportional odds regression model:60
where k = 1, 2, 3, 4 denotes category; i = 1, 2, …, n denotes case; Yi is the response category for the ith case; xi is a vector of covariate values for the ith case (including the trial arm); αk is an intercept term for the kth category; β is a vector of parameters to be estimated; and Logit(p) = log[p/(1 – p)].
Unadjusted fit
The snippet of output in Table 14 is for an unadjusted fit using all of the available data (n = 361).
| Coefficients | Definition | Value | Standard error | t-value |
|---|---|---|---|---|
| ArmSham | (Sham = 1, otherwise = 0) | 0.3354 | 0.2356 | 1.424 |
| ArmAcupressure | (Acu = 1, otherwise = 0) | 0.2418 | 0.2334 | 1.036 |
| Intercepts | Value | Standard error | t-value | |
| 1|2 | −1.0138 | 0.1836 | −5.5203 | |
| 2|3 | 0.3777 | 0.1775 | 2.1281 | |
| 3|4 | 1.3821 | 0.1943 | 7.1138 | |
| 4|5 | 2.2506 | 0.2343 | 9.6047 | |
These data show that:
-
the likelihood ratio test of the proportional odds assumption is non-significant (p = 0.51) meaning that there is no good evidence against this assumption
-
the likelihood ratio test for the trial arm effects is non-significant (p = 0.34)
-
the estimated odds ratio (OR) of a lower (i.e. better) score for acupressure than for the control is e0.2418 = 1.27 [95% confidence interval (CI) 0.80 to 2.03]
-
the estimated OR of a lower (i.e. better) score for sham acupressure than for the control is e0.3354 = 1.40 (95% CI 0.87 to 2.24).
Hence, patients in the acupressure arm are 27% more likely to have less nausea/vomiting than patients in the control arm and patients in the sham arm are 40% more likely to have less nausea/vomiting than patients in the control arm.
Adjusted fit (age, gender and emetogenic risk)
The snippet of output presented in Table 15 is for an adjusted fit using all of the available data (n = 361).
| Coefficients | Value | Standard error | t-value |
|---|---|---|---|
| ArmSham | 0.3520 | 0.2400 | 1.4670 |
| ArmAcu | 0.1689 | 0.2368 | 0.7131 |
| Age51Plus | 0.7445 | 0.2141 | 3.4766 |
| GenderMale | 0.7554 | 0.2692 | 2.8060 |
| EmetogenicRiskLow | 1.3783 | 0.4918 | 2.8025 |
| EmetogenicRiskModerate | 0.3369 | 0.2164 | 1.5572 |
| Intercepts | Value | Standard error | t-value |
| 1|2 | −2.0399 | 0.2793 | −7.3029 |
| 2|3 | −0.4924 | 0.2579 | −1.9094 |
| 3|4 | 0.5786 | 0.2627 | 2.2021 |
| 4|5 | 1.4753 | 0.2909 | 5.0720 |
Table 16 shows the results of the regression analysis assessing variables that affect the primary outcome of nausea. It indicates that, irrespective of arm allocation, subjects who were > 50 years old and male had a better nausea outcome. Also, the emetogenicity of the drug was a significant factor in the nausea outcome.
| Term | df | p-value |
|---|---|---|
| Arm | 2 | 0.34 |
| Age ≥ 51 years | 1 | 0.0005 |
| Male | 1 | 0.005 |
| Emetogenic risk group | 2 | 0.013 |
These data show that:
-
the likelihood ratio test of the proportional odds assumption is non-significant (p = 0.66) meaning that there is no good evidence against this assumption
-
the likelihood ratio test for the trial arm effects after adjustment for age group, gender and emetogenic risk group is non-significant (p = 0.34)
-
the estimated OR of a lower (i.e. better) score for acupressure than for the control (for identical age group, gender and emetogenic risk group) is e0.1689 = 1.18 (95% CI 0.74 to 1.90)
-
the estimated OR of a lower (i.e. better) score for sham acupressure than for the control (for identical age group, gender and emetogenic risk group) is e0.3520 = 1.42 (95% CI 0.88 to 2.30).
Hence, considering the three risk factors presented in Table 16 and the adjusted data presented earlier, patients in the acupressure arm are 18% more likely to have less nausea/vomiting than patients in the control arm and patients in the sham arm are 42% more likely to have less nausea/vomiting than patients in the control arm.
It is of interest to consider the impact of adding a trial arm by term interaction effect to the fitted model for each of age group, gender and emetogenic risk group in turn. A regression analysis presents the findings of this analysis (Table 17).
| Interaction term | df | p-value |
|---|---|---|
| Arm × (Age ≥ 51) | 2 | 0.70 |
| Arm × Male | 2 | 0.002 |
| Arm × (Emetogenic risk group) | 4 | 0.88 |
This indicates that there is evidence to suggest that treatment effects may vary with gender. This merits further investigation (see Simple illustration of primary outcome by trial arm and gender).
Adjusted fit (age, gender, emetogenic risk, cycle frequency, anxiety and nausea expectation)
There were further values missing for the baseline Hospital Anxiety and Depression Scale anxiety score and the pretreatment nausea expectation score. Hence, the snippet of output presented in Table 18 is for an adjusted fit using all of the available data (n = 315).
| Coefficients | Value | Standard error | t-value |
|---|---|---|---|
| ArmSham | 0.26417 | 0.25973 | 1.0171 |
| ArmAcu | 0.12936 | 0.25220 | 0.5129 |
| Age51Plus | 0.51111 | 0.22929 | 2.2291 |
| GenderMale | 0.67565 | 0.32595 | 2.0729 |
| EmetogenicRiskLow | 1.23203 | 0.56228 | 2.1911 |
| EmetogenicRiskModerate | 0.29651 | 0.23076 | 1.2849 |
| TwoWeekly | −0.06794 | 0.41474 | −0.1638 |
| Anxiety | −0.03628 | 0.02613 | −1.3887 |
| NauseaExpectation | −0.15455 | 0.05046 | −3.0627 |
| Intercepts | Value | Standard error | t-value |
| 1|2 | −0.8360 | 0.3876 | −2.1567 |
| 2|3 | 0.7305 | 0.3872 | 1.8868 |
| 3|4 | 1.8205 | 0.4011 | 4.5389 |
| 4|5 | 2.8123 | 0.4293 | 6.5504 |
A regression analysis, presented in Table 19, shows that age > 50 years, male gender and lower expectation of nausea are significantly linked with lower levels of nausea. The role of the emetogenicity of the chemotherapy in this analysis was borderline non-significant.
| Interaction term | df | p-value |
|---|---|---|
| Arm | 2 | 0.60 |
| Age ≥ 51 years | 1 | 0.025 |
| Male | 1 | 0.038 |
| Emetogenic risk group | 2 | 0.067 |
| 2-weekly CT | 1 | 0.87 |
| Anxiety | 1 | 0.16 |
| Nausea expectation | 1 | 0.002 |
These data show that:
-
the likelihood ratio test of the proportional odds assumption is non-significant (p = 0.31), meaning that there is no good evidence against this assumption
-
the likelihood ratio test for the trial arm effects after adjustment for age group, gender, emetogenic risk group, cycle frequency, anxiety and nausea expectation is non-significant (p = 0.60)
-
the estimated OR of a lower (i.e. better) score for acupressure than for the control (for identical values of the other covariates in the model) is e0.1294 = 1.14 (95% CI 0.69 to 1.88)
-
the estimated OR of a lower (i.e. better) score for sham acupressure than for the control (for identical values of the other covariates in the model) is e0.2642 = 1.30 (95% CI 0.77 to 2.19).
Hence, considering the risk factors presented in Table 19 and the adjusted data presented earlier in this section, patients in the acupressure arm are 14% more likely to have less nausea/vomiting than patients in the control arm, and patients in the sham arm are 30% more likely to have less nausea/vomiting than patients in the control arm.
It is of interest to consider the impact of adding a trial arm by term interaction effect to the fitted model for each of age group, gender, emetogenic risk group, cycle frequency, anxiety and nausea expectation in turn. The results of this analysis are presented in Table 20 and show that only gender (with anxiety being a borderline non-significant variable) is significantly linked with the primary outcome, once again indicating that there is some evidence to suggest that treatment effects may vary with gender. This merits further investigation (see Simple illustration of primary outcome by trial arm and gender).
| Interaction term | df | p-value |
|---|---|---|
| Arm × (Age ≥ 51) | 2 | 0.78 |
| Arm × Male | 2 | 0.023 |
| Arm × Emetogenic risk group) | 4 | 0.93 |
| Arm × (2-weekly) | 2 | 0.14 |
| Arm × Anxiety | 2 | 0.08 |
| Arm × (Nausea expectation) | 2 | 0.16 |
Simple illustration of primary outcome by trial arm and gender
The regression analyses above suggest that there may be a trial arm by gender interaction. To illustrate this, we may return to the box and whisker plots by trial arm further broken down by gender as shown in Figures 5 and 6. Given that we have seen a statistically significant interaction in the ordinal regression analyses, there is some justification to repeat the Kruskal–Wallis tests separately within the two gender strata. This yields significant results for men (p = 0.04; n = 79) and for women (p = 0.01; n = 282). Descriptively, the difference appears to be with the sham arm having a beneficial effect for women but a detrimental effect for men. This is a post hoc analysis and should be interpreted with due caution especially as there are a limited number of men.
FIGURE 5.
Box and whisker plot of the primary outcome by trial arm for men.
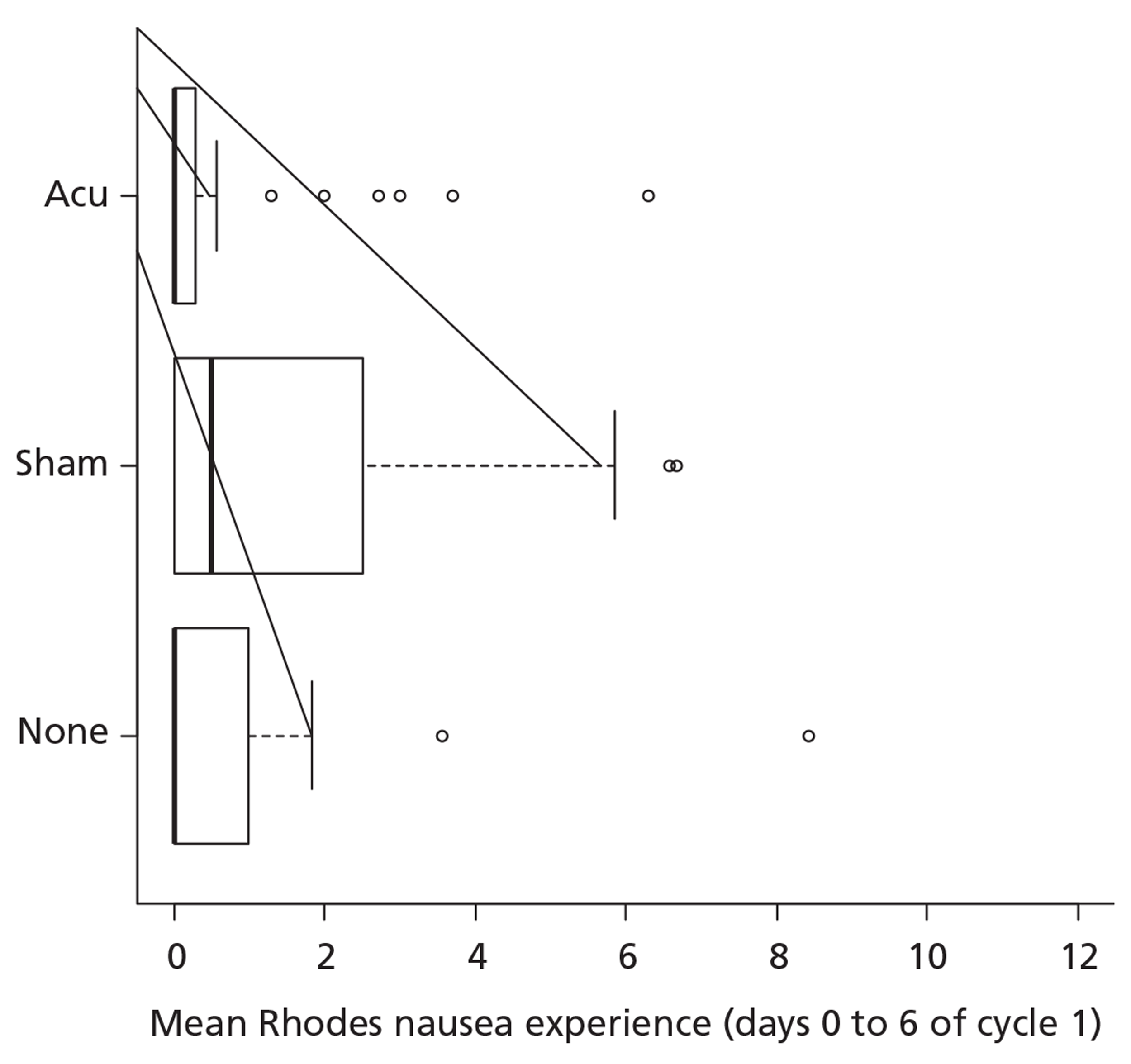
FIGURE 6.
Box and whisker plot of the primary outcome by trial arm for women.
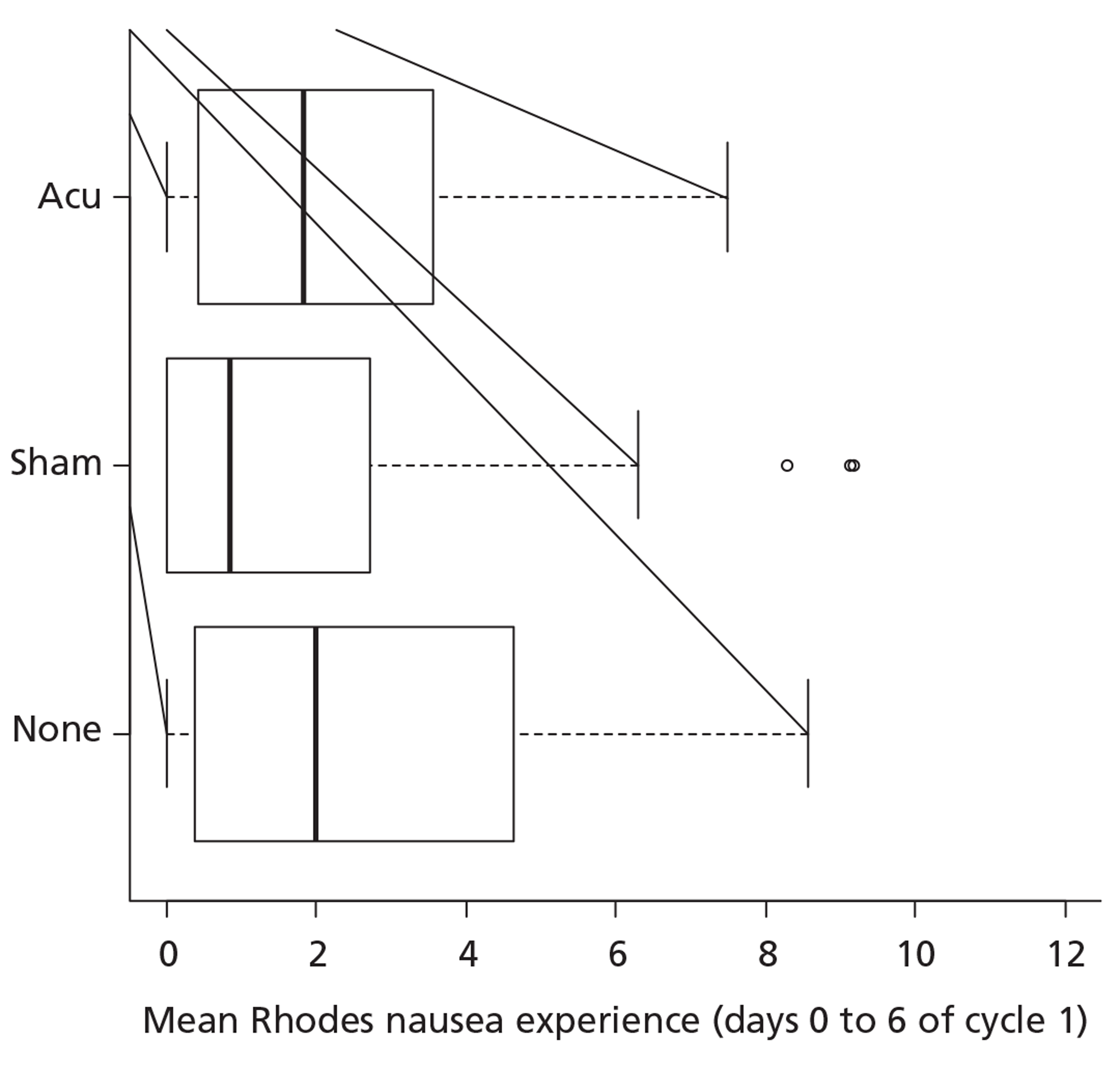
Longitudinal regression analyses of mean Rhodes Index of Nausea, Vomiting and Retching nausea experience scores
The mean Rhodes Index of Nausea, Vomiting and Retching nausea experience scores (days 0–6) have also been calculated for cycles 2, 3 and 4. Once again, the scores for each cycle have been grouped in the same manner as previously to a 5-point ordinal scale. These repeated ordinal data have been analysed using an extension of the proportional odds regression model described previously, this time fitted using a GEE approach. 61 A working autoregressive correlation structure has been assumed, but the regression parameters are robust to this assumption. All reported Wald tests have used the robust covariance estimates.
Unadjusted fit
First, a trial arm by cycle model was fitted and the interaction term was tested for significance (Wald test chi-squared on 6 df, p = 0.25). There being no formal evidence for different treatment effects with cycle, the simpler trial arm plus cycle model was fitted as presented in Table 21.
| Coefficients | Estimate | Naive standard error | Naive z | Robust standard error | Robust z |
|---|---|---|---|---|---|
| Intercept 1|2 | −0.9794 | 0.1399 | −6.9980 | 0.1613 | −6.069 |
| Intercept 2|3 | 0.2440 | 0.1357 | 1.7972 | 0.1567 | 1.557 |
| Intercept 3|4 | 1.1257 | 0.1439 | 7.8185 | 0.1628 | 6.910 |
| Intercept 4|5 | 1.9900 | 0.1675 | 11.8760 | 0.1876 | 10.607 |
| Sham | 0.3823 | 0.1603 | 2.3851 | 0.1977 | 1.933 |
| Acu | 0.4255 | 0.1563 | 2.7225 | 0.2023 | 2.103 |
| Cycle2 | 0.0406 | 0.1124 | 0.3609 | 0.0899 | 0.451 |
| Cycle3 | 0.1680 | 0.1341 | 1.2530 | 0.0973 | 1.725 |
| Cycle4 | 0.4708 | 0.1472 | 3.1984 | 0.1199 | 3.925 |
-
The likelihood ratio test of the proportional odds assumption is non-significant (p = 0.27), meaning that there is no good evidence against this assumption.
-
The estimated correlation parameter (95% CI) is 0.345 (0.001 to 0.997), that is, mild autocorrelation with a very wide CI.
-
The Wald test for the trial arm effects on 2 df is of borderline significance (p = 0.07).
-
The estimated OR of a lower (i.e. better) score for acupressure than for the control is e0.4255 = 1.53 (95% CI 1.12 to 2.09).
-
The estimated OR of a lower (i.e. better) score for sham acupressure than for the control is e0.3823 = 1.47 (95% CI 1.06 to 2.02).
-
The GEE approach is valid with an ignorable missing response mechanism (missing completely at random). All results should be interpreted with this in mind and sensitivity analyses with respect to missing data mechanisms will be performed.
Trial arm effects by gender from adjusted regression analyses
The regression analyses described previously for the cycle 1 nausea primary outcome revealed a trial arm by gender interaction. A parallel longitudinal analysis also displayed the same interaction (data not shown). A useful summary of the trial arm effects is given in Tables 22 (cycle 1) and 23 (cycle-averaged data). The OR terms are the odds of a lower (i.e. better) nausea score for each of categories 1–4 in the 5-point ordinal scale described previously.
| Effect | OR estimatea | 95% CI |
|---|---|---|
| Sham acupressure compared with none (men) | 0.35 | 0.12 to 1.03 |
| Sham acupressure compared with none (women) | 2.02 | 1.19 to 3.42 |
| Acupressure compared with none (men) | 1.27 | 0.40 to 4.08 |
| Acupressure compared with none (women) | 1.17 | 0.70 to 1.95 |
| Effect | OR estimatea | 95% CI |
|---|---|---|
| Sham acupressure compared with none (men) | 0.66 | 0.29 to 1.48 |
| Sham acupressure compared with none (women) | 1.71 | 1.10 to 2.65 |
| Acupressure compared with none (men) | 1.47 | 0.59 to 3.68 |
| Acupressure compared with none (women) | 1.44 | 0.93 to 2.23 |
From these OR estimates and CIs we see that there was a statistically significant benefit for women in the sham arm (but not for men for whom the effect was in fact reversed). Both men and women appear to have enjoyed a benefit of similar magnitude in the acupressure arm but this is not formally statistically significant. The same caveats as mentioned previously apply, that is, this is a post hoc analysis, there are a relatively small number of men and assumptions have been made about missing data mechanisms.
Longitudinal analyses of secondary outcomes
Multinational Association of Supportive Care in Cancer Antiemesis Tool: acute and delayed nausea
Acute and delayed nausea were both scored from 0 to 10 and were highly skewed with large proportions on the 0 extreme. For regression analysis new ordered factors with five levels were created as in Table 24.
| Group | Score |
|---|---|
| None | 0 |
| Mild | 1–3 |
| Moderate | 4–6 |
| High | 7–9 |
| Severe | 10 |
The regression analyses followed a similar approach to that employed for nausea experience measured using the Rhodes Index of Nausea, Vomiting and Retching scale as described earlier. For both outcomes there was no evidence of an arm by cycle interaction but both outcomes exhibited evidence of an arm by gender interaction. Tables 25 and 26 show the arm effect estimates for acute and delayed nausea respectively.
| Effect | OR estimatea | 95% CI |
|---|---|---|
| Sham acupressure compared with none (men) | 0.32 | 0.12 to 0.82 |
| Sham acupressure compared with none (women) | 1.62 | 1.04 to 2.53 |
| Acupressure compared with none (men) | 0.63 | 0.21 to 1.96 |
| Acupressure compared with none (women) | 1.27 | 0.82 to 1.96 |
| Effect | OR estimatea | 95% CI |
|---|---|---|
| Sham acupressure compared with none (men) | 0.54 | 0.21 to 1.40 |
| Sham acupressure compared with none (women) | 1.74 | 1.12 to 2.68 |
| Acupressure compared with none (men) | 0.99 | 0.37 to 2.69 |
| Acupressure compared with none (women) | 1.49 | 0.97 to 2.28 |
Multinational Association of Supportive Care in Cancer Antiemesis Tool: acute and delayed vomiting
The mean (days 0–6) MAT vomiting experience data were highly skewed. The data were grouped 0, (0,1), (1,2), (2,3) and > 3. When analysed with a longitudinal proportional odds model there was no evidence of any trial arm effects (p = 0.47, Wald test).
The MAT scale acute vomiting data were recorded as the number of times vomiting occurred in the 24 hours since chemotherapy. Descriptively there was no difference between the trial arms. The MAT scale delayed vomiting data were recorded as the number of days on which vomiting occurred (0–4). When analysed with a longitudinal proportional odds model there was no evidence of any trial arm effects (p = 0.69, Wald test).
Longitudinal analyses for nausea and vomiting using post hoc dichotomies
In previous analyses these outcomes were mapped to a 5-point ordinal scale and proportional odds models were fitted. In each case a test of the proportional odds assumption was made and found to be non-significant. Essentially that implies that the odds of a lower score for a variable (notably trial arm) does not depend on the particular cut point. Most cases have low scores for nausea and vomiting and it is felt that a simple dichotomy may give an adequate simpler description (Tables 27–32 provide data for the Rhodes Index of Nausea, Vomiting and Retching and the MAT scale). Longitudinal binomial models were fitted (cycle + arm using the GEE approach) with a general working correlation structure and a Wald test employed using the robust covariance estimates to assess the trial arm effects. The reason for the dichotomies stated above is that the models for ordinal data typically consider the cumulative logits (CL), that is, the log-odds of a lower score. With the five categories used for the nausea outcome we consider four such CLs evaluated at the category upper limits:
| Cycle 1, n/N (%) | Cycle 2, n/N (%) | Cycle 3, n/N (%) | Cycle 4, n/N (%) | |
|---|---|---|---|---|
| None | 69/117 (59) | 61/109 (56) | 55/96 (57) | 50/81 (62) |
| Sham bands | 83/118 (70) | 77/105 (73) | 64/88 (73) | 52/74 (70) |
| Acupressure bands | 79/126 (63) | 72/114 (63) | 72/103 (70) | 70/90 (78) |
| Cycle 1, n/N (%) | Cycle 2, n/N (%) | Cycle 3, n/N (%) | Cycle 4, n/N (%) | |
|---|---|---|---|---|
| None | 100/118 (85) | 94/108 (87) | 88/96 (92) | 71/81 (88) |
| Sham bands | 107/119 (90) | 95/105 (90) | 83/88 (94) | 73/77 (95) |
| Acupressure bands | 109/126 (87) | 106/114 (93) | 96/103 (93) | 85/90 (94) |
| Cycle 1, n/N (%) | Cycle 2, n/N (%) | Cycle 3, n/N (%) | Cycle 4, n/N (%) | |
|---|---|---|---|---|
| None | 83/117 (71) | 84/110 (76) | 76/96 (79) | 61/81 (75) |
| Sham bands | 98/120 (82) | 92/106 (87) | 74/89 (83) | 65/79 (82) |
| Acupressure bands | 101/130 (78) | 93/116 (80) | 80/102 (78) | 73/88 (83) |
| Cycle 1, n/N (%) | Cycle 2, n/N (%) | Cycle 3, n/N (%) | Cycle 4, n/N (%) | |
|---|---|---|---|---|
| None | 73/118 (62) | 66/105 (63) | 65/93 (70) | 55/79 (70) |
| Sham bands | 86/116 (74) | 78/105 (74) | 68/89 (76) | 60/78 (77) |
| Acupressure bands | 88/128 (69) | 85/116 (73) | 75/102 (74) | 71/86 (83) |
| Cycle 1, n/N (%) | Cycle 2, n/N (%) | Cycle 3, n/N (%) | Cycle 4, n/N (%) | |
|---|---|---|---|---|
| None | 99/117 (85) | 97/110 (88) | 91/97 (94) | 75/81 (93) |
| Sham bands | 106/121 (88) | 94/106 (89) | 81/88 (92) | 72/79 (91) |
| Acupressure bands | 110/130 (85) | 101/115 (88) | 91/102 (89) | 86/90 (96) |
| Cycle 1, n/N (%) | Cycle 2, n/N (%) | Cycle 3, n/N (%) | Cycle 4, n/N (%) | |
|---|---|---|---|---|
| None | 99/119 (83) | 94/106 (89) | 86/93 (92) | 71/80 (89) |
| Sham bands | 103/117 (88) | 98/105 (93) | 80/89 (90) | 71/78 (91) |
| Acupressure bands | 108/128 (84) | 104/116 (90) | 90/101 (89) | 80/86 (93) |
In a regression context we aim to see how covariates affect these CLs. In an unconstrained model there would be a term for each covariate for each of the four response categories. Nausea experience and vomiting experience are each scored from 0 to 12, whereas MAT is scored from 0 to 10. All of these distributions are markedly skewed, with those for vomiting being even more skewed than those for nausea. Five categories were selected for each but the definitions of these were permitted to vary from variable to variable. As the proportional odds assumption was not violated for each variable, the results should not be too sensitive to the particular dichotomy used.
The Wald test for the arm effects gave p = 0.08 with the ORs of the lower score being estimated at 1.62 for sham acupressure and 1.46 for acupressure compared with no bands.
The Wald test for the arm effects gave p = 0.19 with the ORs of the lower score being estimated at 1.74 for sham acupressure and 1.46 for acupressure compared with no bands.
The Wald test for the arm effects gave p = 0.18 with the ORs of the lower score being estimated at 1.60 for sham acupressure and 1.29 for acupressure compared with no bands.
The Wald test for the arm effects gave p = 0.13 with the ORs of the lower score being estimated at 1.51 for sham acupressure and 1.43 for acupressure compared with no bands.
The Wald test for the arm effects gave p = 0.98, with the ORs of the lower score being estimated at 1.05 for sham acupressure and 1.00 for acupressure compared with no bands.
The Wald test for the arm effects gave p = 0.69, with the ORs of the lower score being estimated at 1.28 for sham acupressure and 1.05 for acupressure compared with no bands.
Results for the Hospital Anxiety and Depression Scale and the Functional Assessment of Cancer Therapy – General scale
These two scales were assessed at baseline and at cycles 1–4. Longitudinal linear models were fitted (GEE approach using unstructured covariance matrices) to the cycles 1–4 data using the relevant baseline variable as a covariate along with factors representing cycle and arm. There was no evidence of any trial arm effects on mean values, as can be seen from Table 33.
| Variable | Scale | Cycle arm p-value (model 1)a | Arm p-value (model 2)b | Sham bandsc | Acupressure bandsc |
|---|---|---|---|---|---|
| Anxiety | 0–21 | 0.34 | 0.48 | 0.11 (0.38) | −0.35 (0.37) |
| Depression | 0–21 | 0.15 | 0.40 | 0.02 (0.37) | 0.45 (0.36) |
| PWB | 0–28 | 0.07 | 0.71 | 0.48 (0.67) | 0.02 (0.66) |
| SFWB | 0–28 | 0.37 | 0.82 | −0.13 (0.46) | −0.24 (0.39) |
| EWB | 0–24 | 0.80 | 0.77 | 0.05 (0.39) | 0.26 (0.38) |
| FWB | 0–28 | 0.39 | 0.86 | 0.11 (0.68) | 0.35 (0.65) |
| FACT-G | 0–108 | 0.71 | 0.81 | 0.92 (1.67) | −0.06 (1.62) |
Scale reliability
A descriptive measure of scale reliability (Cronbach's alpha) has been calculated at baseline. FACT scales may be calculated with four or more completed items; however, for Cronbach's alpha analysis all items must be completed. As can be seen in Table 34, all scales used in the study had very good reliability values.
| Scale | Number of items | Time point | n complete | Cronbach's alpha |
|---|---|---|---|---|
| Rhodes Index of Nausea, Vomiting and Retching nausea experience | 3 | Cycle 1 day 1 (day following CT) | 356 | 0.915 |
| Rhodes Index of Nausea, Vomiting and Retching vomiting experience | 3 | Cycle 1 day 1 (day following CT) | 358 | 0.883 |
| Hospital Anxiety and Depression Scale: anxiety | 7 | Baseline | 414 | 0.884 |
| Hospital Anxiety and Depression Scale: depression | 7 | Baseline | 420 | 0.824 |
| FACT-G: PWB | 7 | Baseline | 381 | 0.742 |
| FACT-G: SFWB | 7 | Baseline | 215 | 0.777 |
| FACT-G: SFWB | 6a | Baseline | 377 | 0.844 |
| FACT-G: EWB | 6 | Baseline | 397 | 0.790 |
| FACT-G: FWB | 7 | Baseline | 354 | 0.863 |
| FACT-G from four subscales | 4 | Baseline | 363 | 0.743 |
Wristband audit
In total, 35 ‘wrist pairs’ were observed during the months August–November 2010 in the outpatient departments at four trial sites (Royal Oldham Hospital, Macclesfield District General Hospital, Liverpool Royal Hospital and Clatterbridge Centre for Oncology).
The vast majority of observations indicated that both wristbands (i.e. on both left and right wrists) were being worn correctly. In two instances a patient was observed to be wearing only one of a pair of wristbands on arrival at the outpatient department, with one patient having a swollen left arm and the other patient having removed one wristband in advance of chemotherapy administration with the intention of wearing the wristband after the chemotherapy had been given. Eight patients were not wearing their wristbands, with one patient having swollen hands and the remaining seven patients stating that they intended to wear their wristbands after chemotherapy had been administered. Three patients who were using spare wristbands offered to them by the research nurse correctly demonstrated their use.
Only four of the 68 wristbands observed (i.e. for left and right wrists) were positioned incorrectly. Three of these were worn incorrectly on the right wrist and one was worn incorrectly on the left wrist. Figure 7 shows the distribution of wristband observations.
FIGURE 7.
Wristband observations during the audit (n = 70).
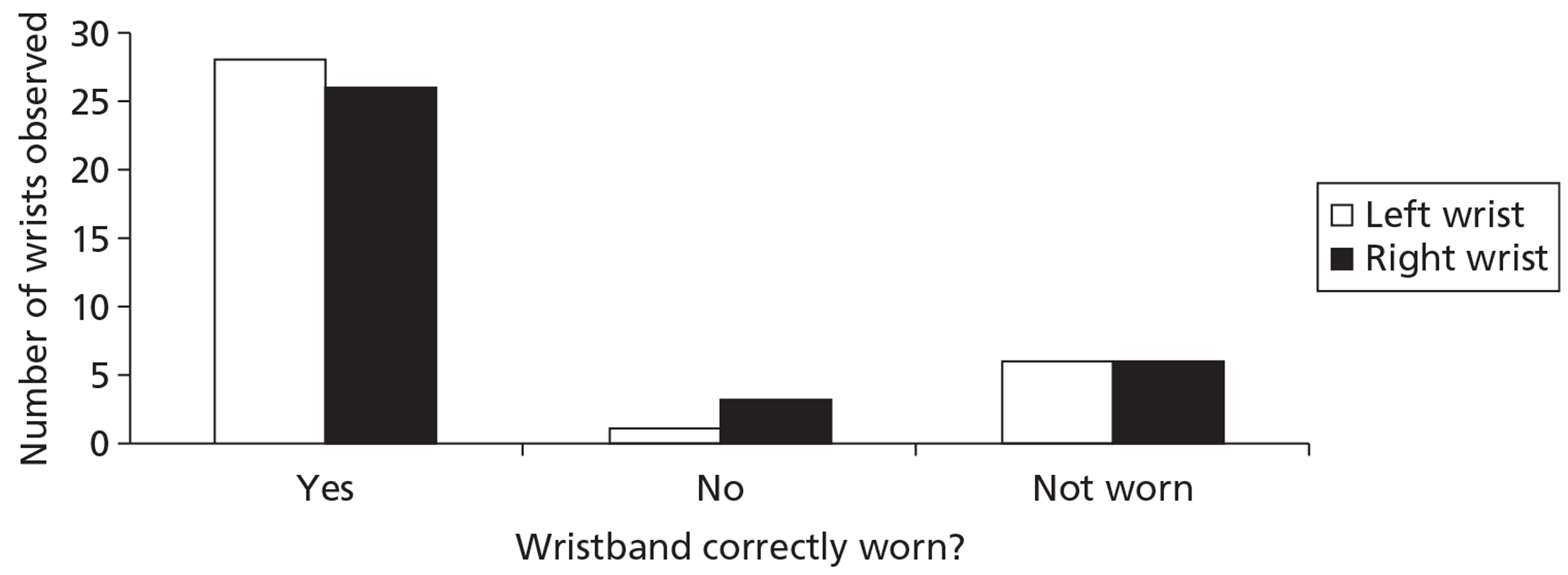
The condition of the wristbands was also noted by the research nurses for a total of 25 pairs. Six wristbands were in poor condition: two pairs of wristbands (one pair being worn for the fourth cycle of chemotherapy and one pair being worn for the second cycle of chemotherapy) were in generally poor condition and one pair of wristbands being worn for the third cycle of chemotherapy had loose acupressure buttons on both bands. All of these bands were replaced. In total, 22 pairs of wristbands were recorded as being in good condition.
Side effects from wearing the wristbands
A small number of patients in the trial reported problems with wearing their wristbands:
-
One patient stated that the wristbands felt ‘too tight’ – this patient was offered larger wristbands and was advised not to continue wearing the bands if the large bands also felt tight.
-
Six patients reported that their hands and/or arms had become swollen after receiving chemotherapy – two of these patients wanted to try wearing larger bands but all patients were advised not to wear the bands while the swelling persisted.
-
One patient reported a painful arm after receiving chemotherapy, which made wearing the wristband uncomfortable – this patient was advised not to wear the wristband on the painful arm until the pain had subsided.
-
One patient reported some swelling of their arms after wearing the wristbands – this patient was advised not to wear the wristbands but was also advised to attempt to wear the wristbands at a later stage once the swelling had subsided but to discontinue using the wristbands if swelling occurred on the second attempt to wear the bands.
-
Two patients stated that they had experienced some ‘irritation’ from wearing the wristbands – both patients were advised to discontinue wearing them.
-
Two patients stated that they had experienced some ‘sensitivity’ to the wristbands – both patients were advised to discontinue wearing them.
-
One patient reported experiencing ‘intolerance’ to wearing the wristbands – the patient was advised to discontinue wearing them.
-
One patient reported that the wristbands felt ‘uncomfortable’ – this patient was offered a pair of large-sized wristbands but these still felt ‘uncomfortable’ and so the patient was advised to stop wearing them.
In all of the cases above, patients were reminded to complete their wristband compliance questionnaires accordingly.
Chapter 5 Health economics data
Context of the data
Aim and perspective
The aim was to assess the cost-effectiveness of self-acupressure using wristbands in addition to standard care compared with standard care with sham acupressure wristbands and standard care alone in the management of chemotherapy-induced (acute and delayed) nausea. The study adopted a NHS perspective in relation to cost evaluation to inform health policy relating to the use of acupressure bands in chemotherapy patients. A societal perspective for costs was adopted for sensitivity analysis. Reporting made reference to economic evaluation good practice. 62
Time frame
Costs and benefits were calculated for the study period (four cycles of chemotherapy) only, which was 21 days. As the trial has a time frame of less than a year neither costs nor benefits were discounted.
Resource use and costs
Resource-use data collected in the trial and unit costs are presented in Appendix 11. These include number of visits to the GP, practice nurse, pharmacist, health visitor and specialist nurse, number of consultations during hospital stays and medication use.
Data were collected prospectively and retrospectively using multiple sources including patient records and patient self-reported questionnaires. The questionnaires report health service utilisation subsequent to and as a result of chemotherapy-induced nausea and vomiting. Direct medical costs incurred by the NHS and social services are defined as the costs of prophylactic or rescue antiemetic medications, drug administration devices, staff time associated with preparing and administering medication and tending to patients with chemotherapy-induced nausea and vomiting, hospitalisations due to chemotherapy-induced nausea and vomiting and hospital outpatient or GP visits due to chemotherapy-induced nausea and vomiting. The analysis did not include costs for chemotherapy agents, preplanned visits or hospitalisations for the purpose of chemotherapy administration and diagnostic and laboratory tests, and other patient management costs not directly related to chemotherapy-induced nausea and vomiting. The resource-use questionnaire completed by patients asked them to record only health service resource usage that was as a result of chemotherapy-related nausea or vomiting.
Appendix 12 lists each of the drugs that were prescribed within the study. The Commercial Medicines Unit (CMU) electronic Market Information Tool (eMIT)63 was used to cost the drugs; however, when drugs were not listed on eMIT, costs were taken from the British National Formulary (BNF). 64 Expert opinion was elicited on the standard practice for antiemetics during chemotherapy and the standard dose for each of the drugs. As Zofran is the brand name of ondansetron we used this (higher) cost only when it was stated that Zofran was prescribed. All other ondansetron prescriptions were costed at the eMIT price for ondansetron.
The total cost of each arm of the trial is calculated by combining the resource use and unit cost data along with the cost of drugs and the price of the acupressure band. There were a number of assumptions that were made when analysing the cost data. The cost of the sham acupressure band was assumed to be the same as the cost of the acupressure band. If the patient did not fill in the resource-use form (left it blank), we assumed that the data were missing. Hospitalisations that led to one face-to-face contact were assumed to represent a short stay and those with more than one face-to-face contact were assumed to represent a long stay.
Assumptions relating to the reported medication use are indicated in the following section.
Assumptions made on antiemetic use
-
All patients received a standard care:
-
moderate/high emetogenic risk: domperidone 20 mg four times a day for 7 days; ondansetron 8 mg intravenously on day of chemotherapy, 8 mg orally twice a day for 3 days; dexamethasone 8 mg intravenously on day of chemotherapy, 8 mg orally for 1 day, 4 mg for 1 day, 2 mg for 1 day.
-
low emetogenic risk: domperidone 20 mg four times a day for 7 days.
-
-
Prolonged course = double the number of days on the antiemetic.
-
If form stated ‘as required’ = 7 days on stated drug.
-
Assumed that all medication was tablet form unless stated.
-
When medication details were not provided (only ‘antiemetics given’ stated), assumed standard care.
-
When doses were not recorded expert opinion was used on average normal dose of each drug.
-
If not stated assume that next cycle is the same as the previous cycle.
Societal costs
Costs from the societal perspective were calculated by combining productivity loss and loss of earnings due to work absence and out-of-pocket expenses incurred as a result of chemotherapy-induced nausea and vomiting. For productivity loss we employed a human capital approach using the gross median weekly pay rate (£499) for full-time employees from the 2010 Annual Survey of Hours and Earnings65 and divided this by five. This was then reduced to 80% to represent the fact that absence from work will not lead to a proportionate loss of productivity,66 giving a daily productivity loss cost of £79.84. As there were a lot of missing data it was assumed that these reflected zero costs incurred.
Outcome measurement and valuation
Participant health-related quality of life was assessed using the European Quality of Life-5 Dimensions (EQ-5D),67 which was included along with the patient resource-use questionnaires. Differences between the randomised groups at follow-up with respect to EQ-5D scores were investigated using non-parametric Mann–Whitney U-tests as the data were not normally distributed. Change scores over time were evaluated using independent t-tests.
Participant responses to the EQ-5D questionnaire were converted to health-state utility values using the UK tariff values68 and an area under the curve approach. These values were then multiplied by duration (21 days) in each health state and divided by 365 to estimate QALYs. QALYs represent a quality-weighted survival value in which 1 QALY is the equivalent of 1 year of full health. Average QALYs between adjacent time points were calculated to generate smoothed estimates between time points. QALYs were calculated as:
where T1, T2m, T3m, T4m and T5m are time points of assessment in the trial, that is, baseline and end of cycles 1, 2, 3 and 4 respectively.
Missing data
Respondents who fail to complete individual items of the EQ-5D are not allocated a utility index score.
The last observation carried forward (LOCF) method was employed to deal with missing time point data. In this trial there were four cycles of chemotherapy after the baseline observation. If a patient dropped out of the study after the third week, this value was then ‘carried forward’ and assumed to be the score for the fourth cycle missing data point. Similarly, if a patient dropped out after baseline this value was carried forward to the first cycle of chemotherapy. Scores were carried forward to one consecutive time point only. Patients in whom more than one consecutive score was missing were omitted from the analyses. Scores were not carried backwards – that is, those without baseline scores did not have their first follow-up score carried backwards. The advantage of the LOCF approach is that it minimises the number of subjects who are eliminated from the analysis and it allows the analysis to examine the trends over time, rather than focusing simply on the end point. Assuming that scores are expected to improve over time, LOCF may be a conservative way of dealing with missing data. A full case analysis was conducted in the sensitivity analyses in which participants were excluded if they had missing scores from any time point.
We used the same method for missing resource-use data, carrying forward the last observation only once if there were missing data for the next time point. Patients were omitted from the analyses if they had more than one missing time point.
Economic evaluation
Descriptive statistics of costs and EQ-5D scores were calculated by subgroup. Parametric tests (Student's t-tests and ANOVA tests) and non-parametric tests (Wilcoxon signed-rank tests, Mann–Whitney tests and Kruskal–Wallis ANOVA tests) were conducted to evaluate differences in individual characteristics and health-related quality-of-life scores between groups. Mann–Whitney U-tests were used to evaluate the significance of differences in costs between groups as this test is less likely to be influenced by outliers. For the economic evaluation only costs of patients who had a QALY value were included.
The outcome of the cost-effectiveness analysis was an incremental cost per QALY. 61,69 We present incremental cost-effectiveness ratios (ICERs)61,69 representing the ratios of the incremental cost and incremental benefits (QALYs) between acupressure and sham acupressure, between acupressure and standard care and between sham acupressure and standard care. The ICER represents the additional cost per one unit of outcome gained, in this case per QALY gained for each intervention compared with its next best alternative. As a guideline rule, the National Institute for Health and Clinical Excellence (NICE) accepts as cost-effective those interventions with an ICER of < £20,000 per QALY. NICE states that, in general, if a treatment costs > £30,000 per QALY it would not be considered cost-effective. We also present cost-effectiveness plane scatter plots illustrating the uncertainty surrounding the cost-effectiveness estimates.
The cost-effectiveness planes were derived using bootstrapping with replacement. This stochastic uncertainty analysis involved running 10,000 bootstrapped estimates of the incremental costs and QALYs. The bootstrap approach is a non-parametric method that treats the original sample as though it were the population and draws multiple random samples from the original sample. Cost-effectiveness acceptability curves were also generated to illustrate the probability that each treatment would be cost-effective given a range of acceptable threshold values. 70
Sensitivity analyses were carried out to account for uncertainty in the cost and EQ-5D values. We performed the sensitivity analysis by adding and subtracting 20% of the costs and assessing the subsequent impact on the ICERs. The value of 20% is essentially arbitrary but it was considered likely to represent any uncertainty that might exist in parameter values. We performed a sensitivity analysis on the QALYs by conducting a full-case analysis on EQ-5D scores. In contrast to the LOCF, no missing data are imputed and patients with any missing data are omitted from the analyses. Sensitivity analyses were also carried out based on using an average group cost for those outliers with high costs and by adopting a societal perspective for costs, including productivity losses and lost earnings.
Net benefit values71 were generated to enable more traditional analysis of therapy efficacy. Net benefit is a composite representation of cost-effectiveness and willingness to pay that is derived by rearranging the ICER calculation and incorporating the willingness-to-pay threshold value (in this case the £20,000 per incremental QALY threshold of NICE). Net monetary benefit (NMB) is derived for each patient as:
The decision rule becomes to adopt an intervention if the NMB > 0. Because values are transformed from ratio to linear we can subsequently employ standard regression models on the data. Net benefit regression allows us to control for covariates and baseline between-group differences. 72 We applied the variables used for the primary end point regression analyses to NMB data with treatment arm included as an independent variable. Univariate ANOVA and linear regression models were generated.
Results
Data from 450 patients (157 acupressure, 146 standard care, 147 sham acupressure) were included in the base-case analysis.
Resource use and costs
There was very little reported resource use within the trial; thus, caution should be exercised in interpreting the results as a small number of high-resource-use consumers or a few instances of high-cost resource use may be unduly influencing the overall results. Table 35 shows the number of times each of the health service resources was used by trial arm. The acupressure group used the fewest resources and the standard care group used the most.
| Services | Acupressure | Standard care | Sham acupressure |
|---|---|---|---|
| GP surgery visit | 5 | 6 | 5 |
| GP home visit | 3 | 4 | 3 |
| District nurse | 4 | 11 | 17 |
| Contact with oncology hotline for advice | 6 | 11 | 9 |
| Contact with hospital oncology nurse clinician for advice | 9 | 9 | 6 |
| Contact with hospital oncology clinic for advice | 5 | 8 | 7 |
| Hospital inpatient stay | 10 | 14 | 9 |
| Hospital A&E department | 3 | 1 | 2 |
| Hospital general outpatient clinic | 1 | 0 | 2 |
| Other services | 3 | 1 | 1 |
| Total instances of resource use | 49 | 65 | 61 |
In terms of the total NHS resource-use cost, the acupressure group used the least number of resources and also had the lowest cost. Mean total drug costs (including antiemetics and other drugs prescribed for chemotherapy-induced nausea and vomiting and antiemetic use outside of the routine pathway in each arm of the trial) for the groups were £37.07 (SD £101.72) for the acupressure group, £51.66 (SD £150.02) for the standard care group and £24.03 (SD £18.70) for the sham acupressure group. Mean total costs (drug costs plus all other NHS costs plus band costs) for the acupressure group were lower than those for the standard care group or the sham acupressure group (Table 36). ‘All other NHS costs’ include the resource use from Table 35. The NHS costs for the sham group appear higher than those for the other groups and this seems to be driven by a higher number of district nurse visits and a few patients in the sham arm of the trial having longer inpatient stays. There were no significant differences in costs between any of the treatment arms according to the t-tests; this was true for the whole sample as well as for the subsample who also had QALY data. Mean values were distorted by a small number of high-cost outlying patients leading to high levels of uncertainty around the mean cost estimates. Median total costs were £30.92, £22.72 and £30.92 for the acupressure, standard care and sham acupressure groups respectively. Mann–Whitney tests indicated that the standard care group had significantly lower total costs than the acupressure and sham acupressure groups (both p < 0.001); there was no significant difference in costs between the acupressure and sham acupressure groups (p = 0.585). No adjustments for multiple testing were considered necessary. Figure 8 shows the cost distribution for the standard care group and illustrates the presence of a small number of high-cost outliers. A similar pattern was observed for the other two treatment arms.
| Acupressure (£), mean (SD) | Standard care (£), mean (SD) | Sham acupressure (£), mean (SD) | |
|---|---|---|---|
| n | 72 | 62 | 60 |
| Drug cost – cycle 1 | 5.47 (1.40) | 5.60 (0.65) | 5.17 (1.55) |
| Drug cost – cycle 2 | 11.71 (36.35) | 15.36 (50.43) | 5.15 (1.55) |
| Drug cost – cycle 3 | 9.92 (33.34) | 15.39 (49.77) | 6.86 (8.86) |
| Drug cost – cycle 4 | 9.96 (33.34) | 15.31 (49.80) | 6.86 (8.86) |
| Total drug cost | 37.07 (101.72) | 51.66 (150.02) | 24.03 (18.70) |
| All other NHS costs – baseline | 2.92 (17.56) | 0.05 (0.0) | 17.29 (124.90) |
| All other NHS costs – cycle 1 | 8.52 (34.91) | 21.73 (76.90) | 9.35 (38.74) |
| All other NHS costs – cycle 2 | 9.78 (44.44) | 9.18 (44.48) | 13.27 (86.59) |
| All other NHS costs – cycle 3 | 4.17 (25.80) | 16.06 (73.89) | 52.13 (303.28) |
| All other NHS costs – cycle 4 | 0.0 (0.0) | 12.50 (80.47) | 37.66 (283.54) |
| All other NHS costs total | 25.39 (81.07) | 59.52 (171.78) | 129.69 (604.28) |
| Band cost | 8.20 (0.00) | 0.00 (0.00) | 8.20 (0.00) |
| Total cost | 70.66 (129.75) | 111.18 (262.68) | 161.92 (604.57) |
FIGURE 8.
Total cost distribution for the standard care group.
Table 37 includes costs from the societal perspective. Lost earnings and productivity losses to employers through sickness absence appeared higher in the standard care group than in either of the band groups. Overall societal costs are similar for the band groups and significantly higher (over double) for the standard care group. However, as with the health-care costs, this result is partly driven by a small number of high-cost outlying individuals, making robust conclusions difficult.
| Acupressure (£), mean (SD) | Standard care (£), mean (SD) | Sham acupressure (£), mean (SD) | |
|---|---|---|---|
| n | 72 | 62 | 60 |
| Total days out of work | 1.18 (6.77) | 2.82 (9.89) | 0.75 (3.45) |
| Total lost earnings | 54.17 (391.44) | 241.42 (1068.43) | 31.50 (244.00) |
| Total expenditure | 0.18 (1.15) | 0.10 (0.53) | 0.00 (0.00) |
| Social cost – baseline | 0.00 (0.00) | 19.82 (104.46) | 0.00 (0.00) |
| Social cost – cycle 1 | 48.95 (255.68) | 226.73 (922.55) | 19.89 (101.05) |
| Social cost – cycle 2 | 52.70 (329.40) | 97.17 (347.41) | 25.21 (112.07) |
| Social cost – cycle 3 | 46.95 (374.77) | 69.77 (295.67) | 27.80 (159.23) |
| Social cost – cycle 4 | 0.00 (0.00) | 53.37 (286.99) | 18.48 (143.18) |
| Total social cost | 148.60 (925.90) | 466.87 (1490.27) | 91.38 (472.80) |
| Total societal costs (social cost + health-care costs) | 219.26 (983.56) | 578.00 (1557.79) | 253.30 (760.40) |
Utility data
Tables 38 and 39 show the numbers of valid and missing EQ-5D questionnaires for each cycle between the three arms of the study when no scores are imputed and when values are imputed respectively. Mean EQ-5D scores are included for each cycle. Both the acupressure and sham acupressure groups showed a slight increase in EQ-5D from baseline to cycle 4 of chemotherapy, whereas the standard care group showed a slight decrease. In general, the results indicate small fluctuations in utility values throughout the trial period and four chemotherapy cycles, making it difficult to draw conclusions about the impact of chemotherapy-induced nausea and vomiting and its treatments on generic health-related quality of life. All of the observed changes were below the minimally important difference for the EQ-5D (which is estimated to range from 0.1 to 0.1273). Table 40 provides the mean EQ-5D change scores between baseline and the follow-up points for the LOCF analysis; these were the values employed in the QALY calculations. Independent sample t-tests indicated that the changes in EQ-5D score over time were not statistically significant.
| Time point | Acupressure | Standard care | Sham acupressure | |
|---|---|---|---|---|
| EQ-5D baseline | Mean (SD) EQ-5D score | 0.764 (0.226) | 0.775 (0.210) | 0.774 (0.246) |
| n valid (missing) | 133 (24) | 126 (20) | 121 (26) | |
| EQ-5D cycle 1 | Mean (SD) EQ-5D score | 0.778 (0.215) | 0.784 (0.244) | 0.788 (0.254) |
| n valid (missing) | 110 (47) | 101 (45) | 99 (48) | |
| EQ-5D cycle 2 | Mean (SD) EQ-5D score | 0.788 (0.190) | 0.764 (0.253) | 0.799 (0.209) |
| n valid (missing) | 93 (64) | 89 (57) | 76 (71) | |
| EQ-5D cycle 3 | Mean (SD) EQ-5D score | 0.732 (0.334) | 0.748 (0.237) | 0.806 (0.249) |
| n valid (missing) | 79 (78) | 72 (74) | 70 (77) | |
| EQ-5D cycle 4 | Mean (SD) EQ-5D score | 0.806 (0.176) | 0.752 (0.255) | 0.820 (0.266) |
| n valid (missing) | 53 (104) | 40 (106) | 44 (103) |
| Time point | Acupressure | Standard care | Sham acupressure | |
|---|---|---|---|---|
| EQ-5D baseline | Mean (SD) EQ-5D score | 0.764 (0.226) | 0.775 (0.210) | 0.774 (0.246) |
| n valid (missing) | 133 (24) | 126 (20) | 121 (26) | |
| EQ-5D cycle 1 | Mean (SD) EQ-5D score | 0.748 (0.244) | 0.765 (0.244) | 0.785 (0.265) |
| n valid (missing) | 148 (9) | 141 (5) | 138 (9) | |
| EQ-5D cycle 2 | Mean (SD) EQ-5D score | 0.776 (0.217) | 0.763 (0.252) | 0.779 (0.255) |
| n valid (missing) | 111 (46) | 103 (43) | 104 (43) | |
| EQ-5D cycle 3 | Mean (SD) EQ-5D score | 0.735 (0.314) | 0.746 (0.249) | 0.797 (0.258) |
| n valid (missing) | 96 (61) | 92 (54) | 77 (70) | |
| EQ-5D cycle 4 | Mean(SD) | 0.763 (0.291) | 0.749 (0.245) | 0.806 (0.246) |
| n valid (missing) | 81 (76) | 73 (73) | 71 (76) |
| Time point | Acupressurea | Standard carea | Sham acupressurea | |
|---|---|---|---|---|
| Baseline to cycle 1 | Mean | −0.0086 | −0.0113 | 0.0209 |
| n | 133 | 126 | 121 | |
| Baseline to cycle 2 | Mean | −0.0186 | −0.0503 | 0.0170 |
| n | 96 | 88 | 86 | |
| Baseline to cycle 3 | Mean | −0.0639 | −0.0637 | −0.0144 |
| n | 85 | 79 | 66 | |
| Baseline to cycle 4 | Mean | −0.0401 | −0.0649 | 0.0050 |
| n | 74 | 63 | 60 |
Statistical tests indicated that there were no significant differences between EQ-5D scores at baseline for the three treatment arms. This being the case, baseline adjustments were not required. At baseline 10.0% (7/70) of the missing EQ-5D values were excluded as a result of one missing response on the questionnaire; 3.6% (5/140) of the missing values in cycle 1 were due to missing one response, 1.6% (3/192) in cycle 2 were missing one response, 0.9% (2/229) in cycle 3 were missing one response and 0.3% (1/313) in cycle 4 were missing one response.
Economic evaluation results regarding quality-adjusted life-year gains
Table 41 shows the total costs and QALY gains for each of the three treatment arms. Differences in QALY gains between groups were minimal and suggest only negligible health benefits of acupressure over standard care. The sham acupressure group had the highest QALY gains over the trial period with the standard care group having the lowest. The mean total cost was highest for the sham acupressure group and lowest for the acupressure group. The high SDs for the cost estimates indicate the presence of a few outlying individuals who incurred significant health service costs.
| Acupressure, mean (SD) | Standard care, mean (SD) | Sham acupressure, mean (SD) | |
|---|---|---|---|
| n | 72 | 62 | 60 |
| Total QALY gain | 0.270 (0.062) | 0.265 (0.072) | 0.283 (0.058) |
| Total cost (£) | 70.66 (129.75) | 111.13 (262.68) | 161.92 (604.57) |
Table 42 provides the cost-effectiveness results, showing the incremental costs and benefits as well as the ICERs for each arm of the trial. Interpretation should be tempered by considering the high level of uncertainty surrounding the cost estimates and the negligible between-group differences observed in QALY gains. The standard care group was dominated by acupressure as it had higher costs and lower QALY gains. The results suggest that acupressure might be cost saving compared with standard care alone. The acupressure group had lower costs than the sham acupressure group; however, the sham group had higher, albeit negligible, QALY gains.
| Analysis | Incremental cost (£) | Incremental QALY gain | ICER (£) |
|---|---|---|---|
| Acupressure vs standard care | −40.47 | 0.005 | −7494.44 (acupressure dominates) |
| Acupressure vs sham acupressure | −91.26 | −0.012 | 7359.68 |
| Sham acupressure vs standard care | 50.79 | 0.018 | 2853.37 |
Table 43 shows the sensitivity analyses that were carried out as well as the results from the non-parametric bootstrapping. The analysis adding 20% to the costs supports the base-case analysis as acupressure still dominates the standard care group. Subgroup analyses by emetogenic risk group were not possible as a very small proportion of patients was rated as high or low emetogenic risk and a large majority was rated as moderate.
| Analysis | Incremental cost (£) | Incremental QALY gain | ICER (£) |
|---|---|---|---|
| +20% of costs | |||
| Acupressure vs standard care | −48.56 | 0.005 | −8993.33 (acupressure dominates) |
| Acupressure vs sham acupressure | −109.51 | −0.012 | 8831.61 |
| Sham acupressure vs standard care | 60.95 | 0.018 | 3424.04 |
| −20% of costs | |||
| Acupressure vs standard care | −32.38 | 0.005 | −5995.56 (acupressure dominates) |
| Acupressure vs sham acupressure | −73.01 | −0.012 | 5887.74 |
| Sham acupressure vs standard care | 40.63 | 0.018 | 2282.70 |
| EQ-5D full-case scenario | |||
| Acupressure vs standard care | −111.51 | 0.009 | −12,319.31 (acupressure dominates) |
| Acupressure vs sham acupressure | −153.92 | −0.018 | 8538.87 |
| Sham acupressure vs standard care | 42.41 | 0.027 | 1566.25 |
| Excluding cost outliers | |||
| Acupressure vs standard care | −7.58 | 0.005 | −1403.70 |
| Acupressure vs sham acupressure | −9.68 | −0.012 | 780.65 |
| Sham acupressure vs standard care | 2.10 | 0.018 | 117.98 |
| Societal cost perspective | |||
| Acupressure vs standard care | −358.74 | 0.005 | −66,433.33 (acupressure dominates) |
| Acupressure vs sham acupressure | −34.04 | −0.012 | 2745.16 |
| Sham acupressure vs standard care | −324.70 | 0.018 | −18,241.57 (sham acupressure dominates) |
| Bootstrapping | |||
| Acupressure vs standard care | −40.64 | 0.005 | −9025.53 (acupressure dominates) |
| Acupressure vs sham acupressure | −90.26 | −0.013 | 7163.27 |
Including only patients who had completed EQ-5D scores at every time point did not significantly change the ICER. The mean cost and QALY gain estimates from the bootstrapping yield similar ICER results to those of the deterministic base-case scenario. Acupressure still dominates standard care only and is cheaper but marginally less effective than sham acupressure. Excluding cost outliers or including societal costs does not alter the outcome of the key comparisons.
Figures 9 and 10 are cost-effectiveness planes for acupressure compared with standard care and acupressure compared with sham acupressure respectively. For acupressure compared with standard care (see Figure 9), the 1000 sample estimates are spread mainly in the south-east and south-west quadrants, suggesting that acupressure is likely to be cost saving. A high proportion of iteration results are in the south-east quadrant suggesting that acupressure is also likely to lead to a higher quality of life; however, QALY gains are minimal. For acupressure compared with sham acupressure (see Figure 10), most of the sample iterations lie in the south-west quadrant, suggesting that acupressure is less effective (leads to reduced quality of life) than sham acupressure but leads to cost savings.
FIGURE 9.
Cost-effectiveness plane for acupressure compared with standard care.
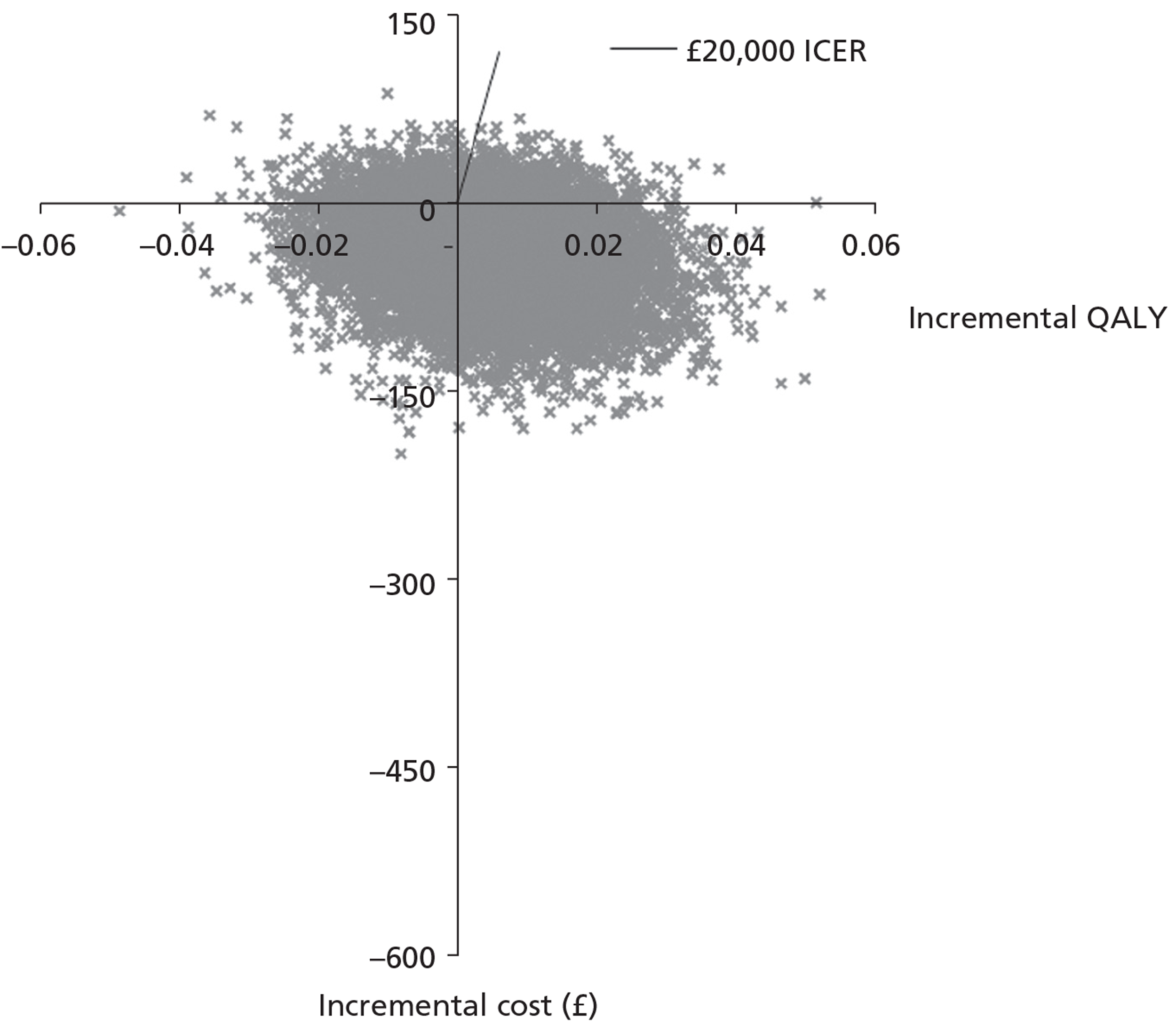
FIGURE 10.
Cost-effectiveness plane for acupressure compared with sham acupressure.
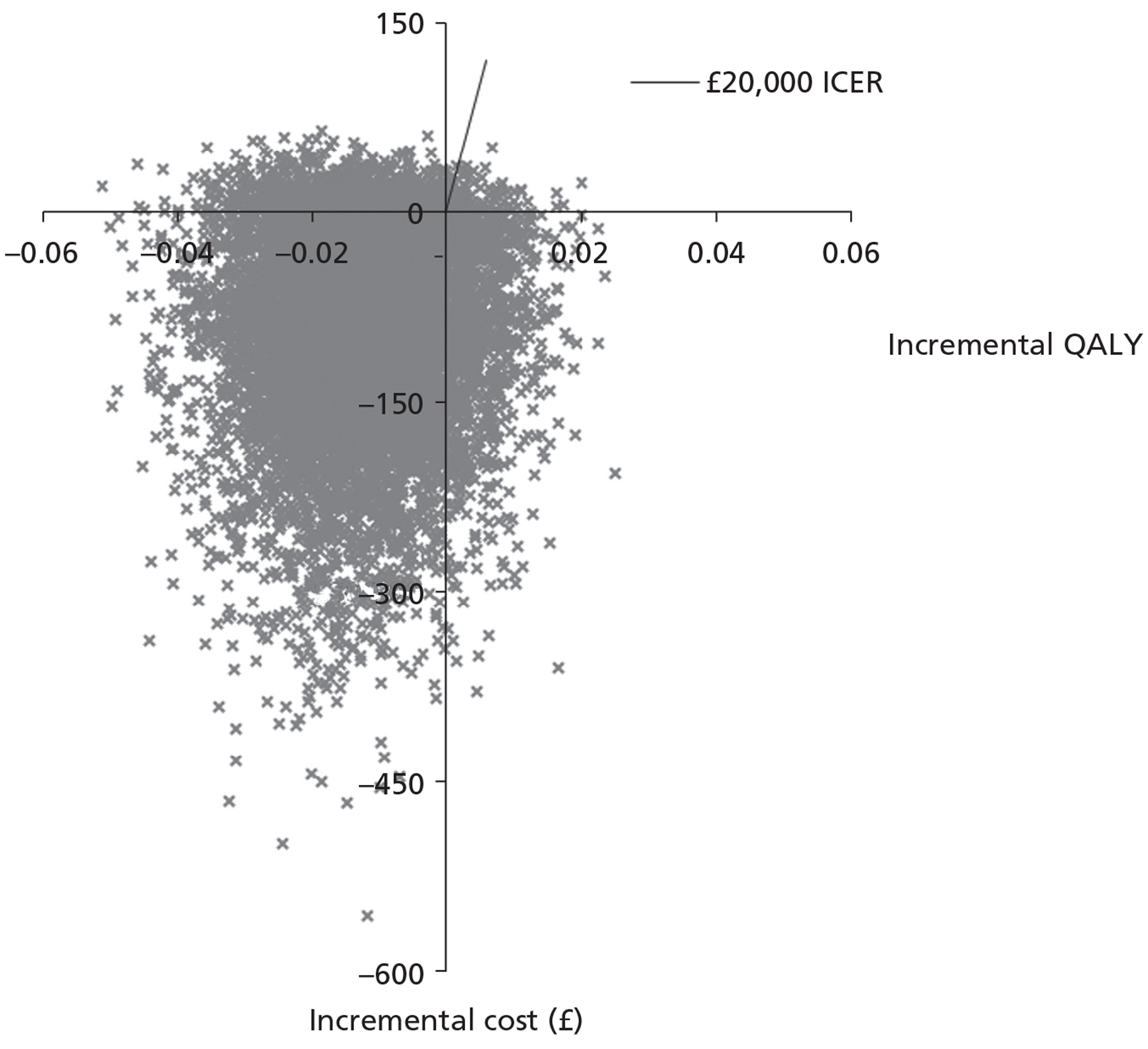
Figures 11 and 12 are the cost-effectiveness acceptability curves showing the probability that each treatment is a cost-effective choice given a range of cost-effectiveness willingness-to-pay thresholds. Figure 11 compares standard care with acupressure. Using the NICE threshold of £20,000 the probability that acupressure is cost-effective is 0.70 compared with standard care. Figure 12 compares all three treatments. At the NICE threshold of £20,000, sham acupressure appears more likely to be the cost-effective option (p = 0.71) than acupressure (p = 0.20). Only at low willingness-to-pay thresholds (< £7000) does acupressure become the most likely option to be cost-effective. However, considering the negligible QALY gains observed in the study, the low levels of resource use, the low median health-care costs and the leverage of a few high-cost outliers, any results must be treated with caution. It is difficult to make robust claims about the comparative cost-effectiveness of any of the therapy arms.
FIGURE 11.
Cost-effectiveness acceptability curves for acupressure compared with standard care.
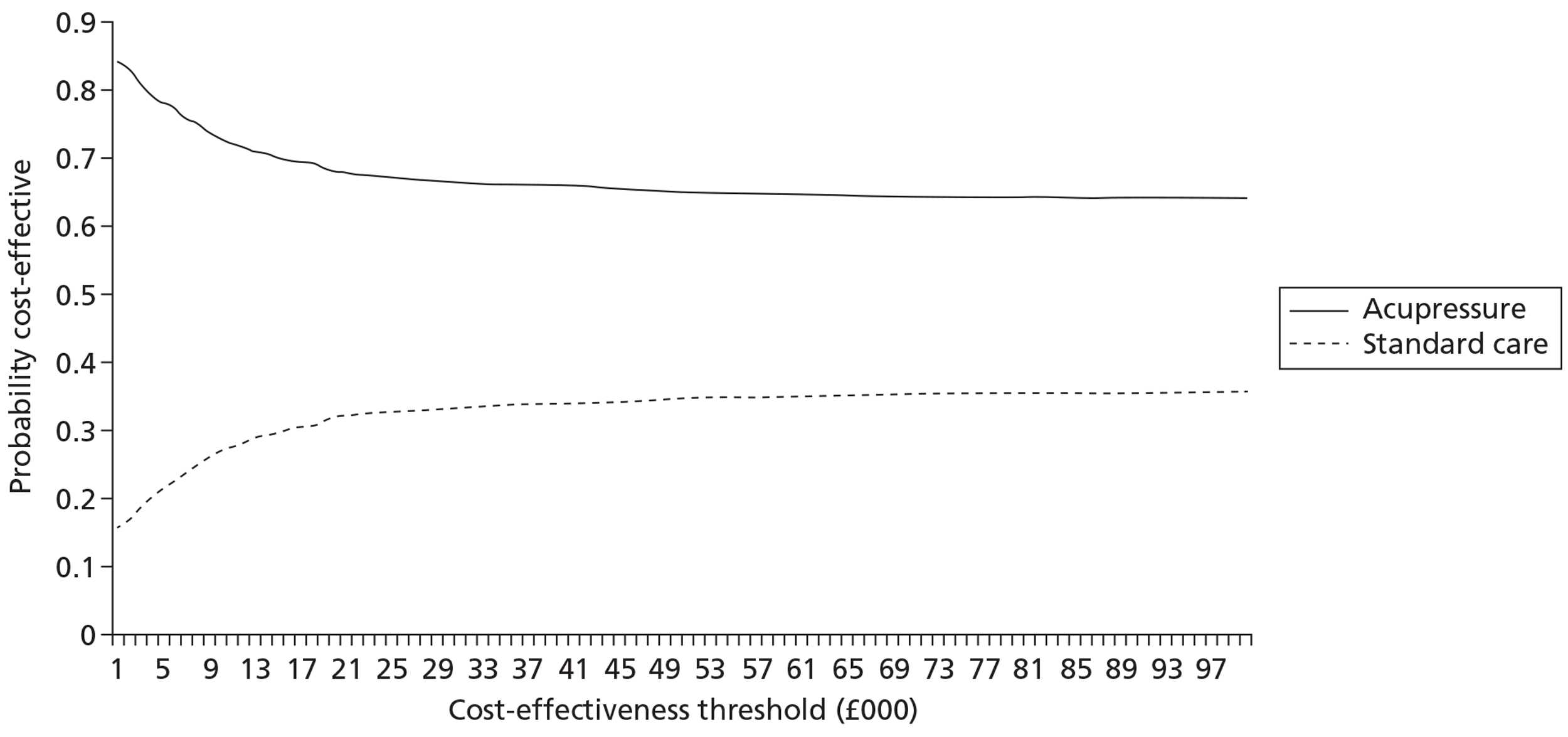
FIGURE 12.
Cost-effectiveness acceptability curves for all three treatments.
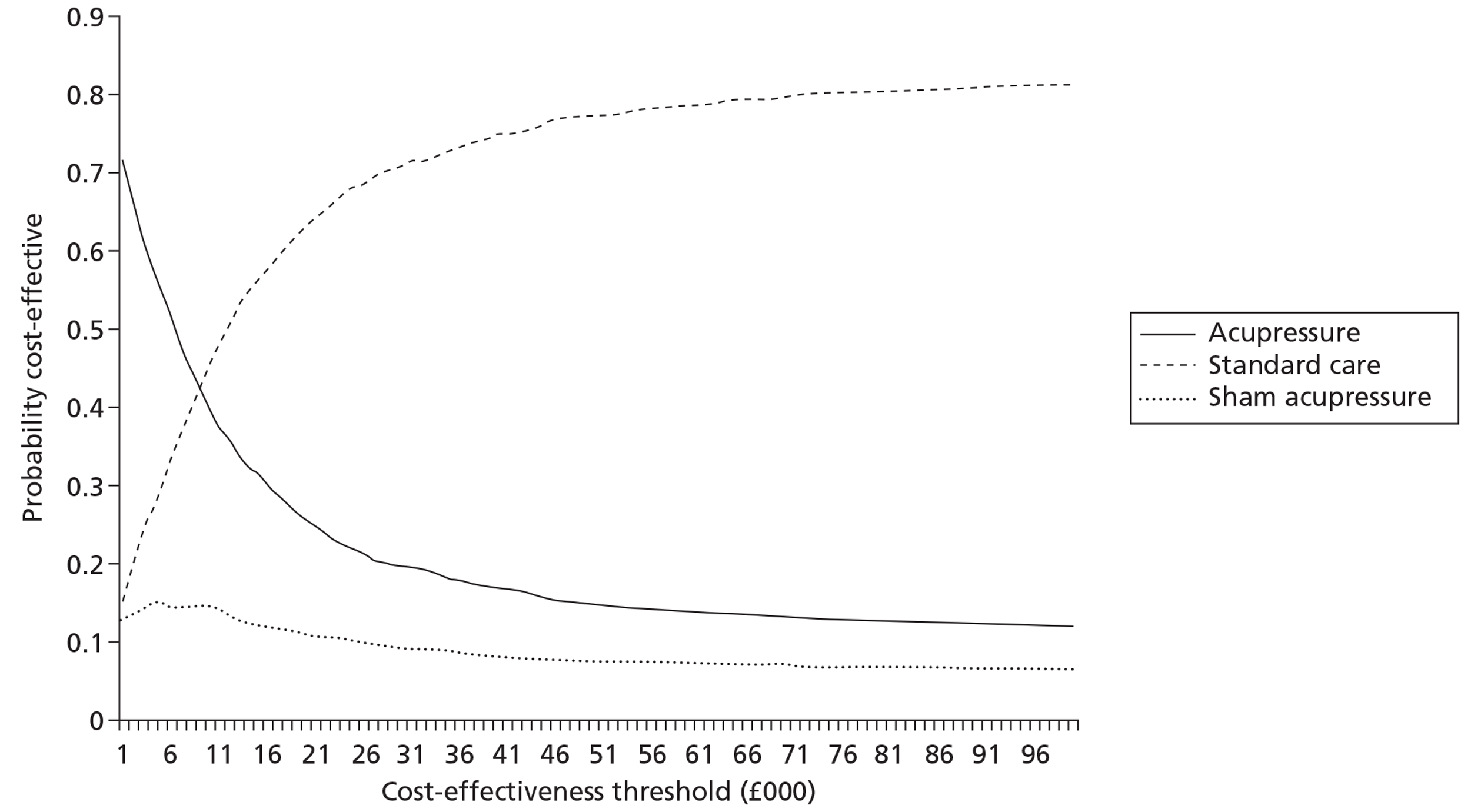
Net monetary benefit
Net monetary benefit was calculated for each patient and was found to be generally positive. NMB means (SD) were 5333 (1288), 5184 (1521) and 5489 (1261) for acupressure, standard care and sham acupressure respectively. Univariate ANOVA and linear regression models using age group, gender, emetogenic risk group, treatment allocation and baseline EQ-5D scores as covariates and independent variables were run to predict NMB.
Treatment allocation was not found to be a significant predictor of NMB. Only baseline EQ-5D score was found to be a significant contributor to the models. This is unsurprising as NMB is based partially on QALY values derived from the EQ-5D.
Chapter 6 Qualitative data
Objectives
-
To outline patients' experiences of using acupressure wristbands.
-
To outline the reasons why patients consent to take part in a clinical trial of acupressure wristbands.
-
To outline patients' experiences of taking part in a randomised controlled trial of acupressure wristbands.
Findings
Sample
A total of 26 patients participated. Nine patients were recruited from Greater Manchester, nine from Merseyside and eight from Plymouth; in total, 10 patients had received true acupressure, nine had received sham acupressure and seven had not received any acupressure. The age range of the patients was 35–79 years (mean 55 years) and seven were male and 19 were female. Nine of the sample were classified as receiving chemotherapy of high emetogenic potential, 12 as receiving chemotherapy of medium emetogenic potential and five as receiving chemotherapy of low emetogenic potential. How much nausea participants expected to experience during their chemotherapy treatment ranged from 0 to 10 (mean 5.8), with 0 being ‘not at all’ and 10 being ‘very frequently’; participants' belief that the acupressure wristbands would help manage their sickness ranged from 2 to 10 (mean 6.9), with 0 being ‘not at all’ and 10 being ‘will help me a lot’.
Themes
Deciding to participate
The majority of patients indicated that, after the initial approach by members of the research team, the decision to participate was immediate. In the time before consenting to take part (24+ hours), most did, however, discuss their intention to participate with significant others, typically partners and close family. Interestingly, patients attending one hospital appear to have been informed that if they participated in clinical trials it generated additional funds for the hospital, which appears to have influenced some patients to participate.
Well part of it he [consultant] did say that obviously it would be useful but also at the same time he did explain that obviously the more people that took part then you know it helped their side of things as well, like it got funded or something.
P318
I didn't hesitate.
P382
Patients identified a range of factors influencing their decision to participate in the trial of acupressure wristbands. Undoubtedly the main motivational factor influencing patients was a desire to ‘give something back’. Almost all participants expressed an awareness of the fact that, without the willingness of patients to engage in research studies, it would be impossible for cancer care treatments and services to advance. In particular, it was the altruistic act of attempting to improve the care of future cancer patients that motivated patients to assist in evaluating whether or not acupressure wristbands are effective for chemotherapy-related nausea and vomiting.
My reasons were as I'd had various treatments obviously associated with the diagnosis I'd had earlier that year it was partly that each time I had treatment I did reflect on people that had gone before me who from their experience and their participation in possible research that that perhaps made things easier for me in my experience. So it was a small way of trying to help for the future. That was kind of in my head, that I just wanted to try and be part of this and hopefully that would help people like me in the future.
P358
Well I thought if it helps somebody else in the future that is the only way they are going to find out the answers.
P411
Patients were aware that chemotherapy treatment is associated with a number of adverse side effects, including nausea and vomiting. A desire to limit their own experience of nausea and vomiting during their chemotherapy treatment was also a strong motivational factor for almost all patients. Of key importance was the fact that those patients participating in the trial may receive wristbands in addition to the antiemetic medication they would have received as part of their conventional care.
I just thought if I was to be going to be sick then it might be helpful.
P318
Because I could see all the list of things that you have … the after effects, you get tummy upsets and stuff like that. Then I thought, well, if that's going to help me I don't want to keep being sick, if it does help me I'll have a go.
P351
A minority of patients indicated that the lack of any perceived risk of adverse effects from taking part in the trial was also a motivational factor when deciding to participate. Some of these patients elaborated, comparing participating in the trial of acupressure wristbands with participating in a pharmaceutical trial – specifically that the decision to participate would likely have been more considered if the trial had been for a new drug as opposed to a ‘natural’ remedy because of a perceived greater likelihood of adverse effects.
There would be no side effects, there would be nothing, there was, it was no detriment really …. I know you have got to help with these things, I think possibly yes [if pharmaceutical trial] I would have done but I would have had to know a lot more about it and a lot more in depth about it.
P280
Perceptions and experiences of complementary therapies
As part of the interview process participants were asked about their views and experiences of complementary and alternative medicine. Most patients had only limited personal experience of complementary and alternative medicine treatments before taking part in the trial; however, a number indicated that they had received additional complementary and alternative medicine treatments during their cancer treatment, such as reflexology, massage and reiki.
Some patients were aware of the current lack of an evidence base underpinning many complementary and alternative medicine treatments. Despite this patients frequently described themselves as ‘open-minded’ with regards to complementary and alternative medicine treatments and their effectiveness, often citing the successful treatment of family/friends or the long history of some of the treatments, with comments such as ‘it's been around for years, so there must be something in it’ being common. Complementary and alternative medicine treatments were also perceived as being ‘natural’, ‘safe’ treatments associated with fewer side effects than conventional medicine. However, some patients, often those who had used complementary and alternative medicine treatments previously, held preconceived beliefs that many complementary and alternative medicine interventions were beneficial to patients whereas others held an opinion that complementary and alternative medicine treatments were of little or no benefit to patients. For some, particularly those with a belief in the effectiveness and value of complementary and alternative medicine treatments, these preconceptions were identified as a further motivational factor when deciding to participate. Interestingly, some patients indicated that their involvement in the trial, or use of complementary and alternative medicine during chemotherapy, had altered their perception of complementary and alternative medicine; typically, their personal experiences of using complementary and alternative medicine made them more accepting of its potential value to patients.
I think a lot of people don't realise that it's beneficial and it helps. So yeah I think if more people realised and realised what help it does, you think oh complementary therapy it's neither here nor there, but actually it does help you relax and you can forget about your troubles and what you've gone through and it's just, it's so relaxing and so nice. You just feel as though you could, you are just a million miles away.
P280
I can't say whether it totally worked for me but for some people it must really assist them. Not it's the way we have got to go, if it can save taking drugs, save ailments and acupuncture has been going on for quite a few years now [laughs] there must be something in it, for it to still keep going you know and so I have never experienced the needles or anything like that, but I understand and appreciate it must work because it is still going after what hundreds of years?
P334
Most patients saw the role of complementary and alternative medicine as being in combination with conventional medicine. Indeed, some patients indicated that they would be accepting of complementary and alternative medicine only if it was provided within the NHS, or if they were advised to use it by a treating conventional health-care practitioner. Irrespective of previously held views or experiences of complementary and alternative medicine interventions, participants were near unanimous in the importance they placed on research into complementary and alternative medicine, highlighting the importance of establishing whether or not treatments are effective for patients to use. There was also a view among some that there should be a greater integration of complementary and alternative medicine therapies and conventional therapies provided within the NHS but that this increasing integration should be underpinned by an emerging evidence base.
Yes I think any alternative therapy should be looked into, or to run not necessarily on its own but to run alongside you know, medicine.
P219
I think it's very important [research into complementary and alternative medicine]. Yes. Because I see complementary medicine as being non-invasive, as being an aid to conventional medicines, or an add-on to conventional medicines. Yes.
P302
Experience of taking part in the trial
Only a couple of participants had any previous experience of taking part in research before agreeing to take part in the trial of acupressure wristbands. Interviewed patients typically indicated that they felt that they had a good understanding of the study before consenting to participate, with many recounting the written and verbal information they had received before consenting.
Yes I knew what it entailed and it was explained to me quite well.
P411
Part of this was being given information on the randomisation process to receive wristbands A, wristbands B or treatment as normal. Patients were typically pragmatic in their perception of the process, exemplified by P280 who commented: ‘you are either going to be lucky if you get the wristbands and it might help, you are unlucky you don't get anything and you just carry on as you would do if you wasn't on the trial’.
However, despite this pragmatic approach, many allocated to treatment as normal still indicated that they felt ‘disappointment’ at not receiving wristbands.
Many participants expressed feeling daunted on initially receiving the paperwork for the trial. As one participant stated, ‘I thought oh crumbs [laughter]. I thought oh God every day.’ However, these feelings typically subsided once patients familiarised themselves with the forms and realised that forms were extensively duplicated. Participants generally felt that the included questionnaires were easy to follow, with comments such as ‘pretty straightforward’ being common. Although the questionnaires were easy to complete, a few patients felt that they were a little onerous, making comments such as ‘they were perhaps, perhaps a little bit too lengthy maybe’. Patients were asked to complete forms for the first 7 days following chemotherapy and on day 10. Some indicated that they failed to complete all of the paperwork on the designated days, in particular that lapses in memory, or poor health, led to forms sometimes being completed retrospectively. In some instances this could involve forms being completed up to 1 week after the date on which they were supposed to be completed. It was the difficulty of completing the paperwork when experiencing the adverse effects of chemotherapy that most patients felt resulted in some patients dropping out of the trial.
Some of the days when I was really, really poorly with the chemo I couldn't even get out of bed to even fill it in I'll be honest, but I knew each day how bad I was, how sick I was. So, you know, was able to fill it in accurately because I knew that at the beginning I was so poorly and so sick and by the end of it you are kind of coming round, so I knew from as soon as I come round and I was out of bed I filled it in.
P280
A number of patients felt that they had experienced positive outcomes as a result of taking part in the trial. Linked to patients' motivations for taking part, most frequent was a sense of well-being as a result of feeling that they had completed an altruistic act and helped others. In addition, some patients felt that the process of completing the trial paperwork had been of benefit to them, specifically that it provided them with some control at a time when they felt a lack of control over their cancer experience or that they gained benefit from being able to reflect on how their symptoms had improved during previous cycles of chemotherapy. In contrast, some patients indicated that they had experienced some negative effects from completing the paperwork, chiefly that reading and rating their level of nausea and vomiting at a time when they were feeling nauseous had at times worsened their experience.
I think taking part in the trial is quite, it makes you feel better actually because it is a useful tool and it's going to be of use for other people in the future …. Yes because it makes you feel better doesn't it if you feel you are contributing something.
P250
I just completed the forms as requested and it was no hassle at all. And actually it helped because it was something positive to do, on certain days and ticking the boxes and all that sort of thing, I felt because I think part of having cancer is you lose control, and I am quite, the sort of person that likes to be in control and this is enabling me a little bit of control back, so that part I quite enjoyed actually.
P317
I know this sounds silly, funny almost, but some of the questions actually don't help you when you're not feeling well. You actually go oh, I feel sick reading out how many cups sort of, you know, sick.
P358
Experience of using the wristbands
Those patients who were allocated to receive either true or sham wristbands during the trial were asked about their experiences of using them. Almost all patients interviewed appeared to have worn the wristbands as instructed, keeping them on for at least 7 days following chemotherapy and removing them only when washing or bathing. Indeed, some patients continued using the wristbands after the 7-day period, and many also continued using the wristbands after the four chemotherapy cycles included in the trial. Patients were asked to demonstrate how they wore the bands, with most of those interviewed apparently wearing the wristbands in the correct position. The exceptions were two patients allocated to receive sham wristbands, who were found to have worn them inappropriately. One patient wore the wristbands inside out (meaning that slight pressure would have been applied to the point) and the other patient indicated that he had manually applied pressure to acupoint P6.
Many of the patients interviewed indicated that they had experienced only mild nausea or vomiting during the trial. A number of patients were pragmatic about the extent to which the wristbands were responsible for this, highlighting the fact that there could be a number of other reasons, including their antiemetic medication, basic constitution or just luck. However, many patients, including some sham-treated patients, believed the wristbands to have had a positive impact on their nausea and vomiting. There was a perception and belief that the wristbands were, at least in part, responsible for the lack of nausea and vomiting they had experienced. In some cases this resulted from the patients' experiences during the trial, such as noticing that nausea was greater when the wristbands were not worn. The wristbands were not seen as being any more or less effective at different times of the day or at different points in the patients' chemotherapy treatment. There were also no additional benefits reported from wearing the wristbands beyond the alleviation of nausea and vomiting.
Until the trial is complete you can't really say whether it helped you or not can you …. I wore them, yes I wore them each time I had treatment yes. And erm … yes I feel as though I benefited from them, and I think I possibly would have been more sick than what I was. I felt the bands did help in that respect. Yes.
P219
I must say I think, having learnt that I didn't used to wear them for the first 2 weeks at first, but then I would be feeling a bit sick and I would think goodness me, why am I feeling so sick and then I would put them on and it would improve and one day I was feeling sick with them on, but they weren't in the right place (laughs) I was looking at them and they weren't in the right place, so there is a reason you know, this is why I have got such belief in them now.
P334
Patients reported that they had not experienced any restrictions from wearing the wristbands in terms of everyday activities, other than showering/bathing/washing dishes. As P297 commented, ‘I don't have many household chores, but no not at all, not at all. I just, take them off when I have a bath or a shower, other than that no they are not intrusive at all‘. For most patients the wristbands were seen as comfortable to wear, although a few patients reported that they had experienced minor irritations, such as the wristbands feeling tight or painful, or the wrists becoming itchy. All reported adverse side effects were generally deemed minor and acceptable.
I find sometimes my, it gets, my skin gets quite red, and they rub and I am sure it's because they are so badly made inside, I think that is the contributing factor.
P318
I did find the bobble that went into your arm painful a few times, and I sort of had to wriggle it around just sort of release the pressure on my arm just for a few minutes, rub my arm and then put it back in again.
P334
A number of patients highlighted the fact that they had been questioned by others, both known to them and not, regarding their wearing of the trial wristbands. Many were unconcerned by the views of others. As P210 commented, ‘if they don't like it, that's their problem … didn't influence me whatsoever’. However, a few patients highlighted that they had inhibitions about wearing them in the company of others or had received negative responses from others to their wearing of the wristbands. When asked about this, some compared the minor impact of wearing wristbands with such things as having to wear a headscarf or have surgical pointers on their body.
It's a, it's a silly point but they are there all the time, so you do sort of start pulling your sleeves down because you don't want people to see them, you know it looks rather strange walking round with two wristbands on … Well everybody knew about them, because I, you know you chat to people and people ask you, and so, I would just tell people but if you went for a meal or if you were out somewhere socially where people didn't know you, you would feel slightly embarrassed. These two bands, that look as though you should be in the gym.
P302
Irrespective of perceptions of effectiveness, almost all patients indicated that they would recommend the wristbands to other chemotherapy patients, or to patients experiencing nausea and vomiting from other causes. This related to the fact that, irrespective of whether or not the wristbands were of benefit for nausea and vomiting, they were not associated with any negative or adverse effects.
There isn't a reason not to wear them. Personally yes. So, yes I would recommend them yes.
P194
Chapter 7 Discussion
Key quantitative findings
Despite the higher proportion of patients showing no nausea in the acupressure group, the results of the trial show that there were no statistically significant differences between the three trial arms in relation to nausea experience. Patients in both wristbands arms had a higher OR for improving their nausea experience than those in the standard care arm, with the sham arm having a higher OR than the acupressure arm. There was a significant gender effect with women in both wristband groups showing significant improvements compared with men. Health-care utilisation was lower in the wristband arms than in the standard care only arm.
Other trials in the past have also shown no significant effects from the use of acupressure in relation to nausea and vomiting management during chemotherapy administration. A review by Lee and Frazier74 examined the results of seven trials of acupressure, with four having positive results and three negative, highlighting that the overall effect of acupressure is strongly suggestive but not conclusive. No significant differences were reported in another trial of 160 women with regards to acute nausea and vomiting, although significant differences were reported with regards to delayed nausea and vomiting. 75 In the largest trial of its kind (n = 739), Roscoe et al. 24 showed that patients experienced less nausea in the first day of chemotherapy in the acupressure arms but there were no significant differences in relation to delayed symptoms. Also, the authors identified a strong gender effect, with men in an acustimulation arm improving but not women, which is in contrast to the results of the current trial. Roscoe et al. 17 also showed, in a small sample of 27 patients (25 women and two men), no statistically significant differences in average severity of nausea between acustimulation of the P6 point, sham acustimulation and standard care; however, the data showed a difference close to statistical significance in the severity of delayed nausea between active acustimulation and no acustimulation (p = 0.06). In addition, patients took fewer antinausea pills during the active acustimulation cycle of this experiment than during the no acustimulation phase (p < 0.05). Negative results have been shown in relation to acupressure and nausea/vomiting symptoms in a large trial of 340 women during labour and delivery. 76 In a trial of acupuncture compared with sham acupuncture during radiotherapy,77 the authors showed less nausea and vomiting in both the real and the sham acupuncture arms than in the standard care arm and justified this as due to non-specific effects of general care or high patient expectancy, which may partly explain our results too.
Key issues in most of the past studies showing positive results include the lack of standardised antiemetic use in the trial participants and inclusion of only or mostly female subjects. If our trial had included only the female subsample, the results would have also been positive. Also, it seems that the vast majority of positive studies in the literature include small sample sizes (< 100 participants), whereas the negative studies (or partly negative) have much larger sample sizes; this suggests that effects observed in methodologically weaker studies cannot always be sustained when larger and more robust trials are carried out. Furthermore, other studies in the past have shown that expectancy,24,78 age and anxiety28,29 together with the antiemetic potential of the chemotherapy are important predictors of, and can affect, the outcome of acupressure, but in our trial, although unidimensionally these were also important, in a multivariate model only gender showed a significant effect.
Our findings suggest a placebo or non-specific effect of the intervention arms. Placebo effects are viewed as a form of interpersonal healing, distinct from spontaneous natural healing or technological healing that depends on physiologically active pharmacological products or procedures. 79 Alkaissi et al. 80 have suggested that acupressure does indeed have a placebo effect in relation to nausea after 24 hours, although correct stimulation of the P6 point is needed to observe decreased rescue antiemetic use and decreased vomiting. Research also suggests that there are different placebo responses, each of which may be influenced by different psychological and neurobiological mechanisms depending on the context in which the placebo is given. 81 The literature also shows that placebos have actual biological effects on the brain and body and are more than response biases. The review by Price et al. 81 concludes that placebo effects reflect mind–brain–body relationships and as such we should not ‘resort to eliminative materialism or forms of dualism that completely divide the mind from the body’ (p. 586).
Trials of acupressure pose a specific problem with regards to the blinding and the choice of placebo, particularly when outcome measures are subjective. We have chosen to use the same wristbands in both the real and the sham groups so that they look identical, with the real acupressure group instructed to have the button pressing the P6 point and the sham group instructed to have the button pointing away from the P6 point on the other side of the arm. We observed during the interviews that some patients in the sham acupressure group (two out of nine) used the wristbands as in the acupressure group because they had looked on the internet or saw others wearing them properly. This may have contaminated our results. It was not possible to create a wristband that would look identical to the real one but which would not have a button or which would exert no pressure. These bands were elastic bands and, as reported by Sinha et al. 76 (through observations from their colleagues in the Department of Industrial and Manufacturing Engineering, Penn State University, PA, USA), elastic bands result in some pressure. This suggests that the pressure of the band in the area proximal to the P6 point, irrespective of the presence of a button pressing the P6 point, may have produced some positive results.
Our sample had generally low levels of nausea and/or vomiting. This may be due to the fact that we have standardised antiemetic use in our study and an inclusion criterion was receiving antiemetics as per MASCC antiemetic guidelines. This low level of experienced symptoms may be a reason for not showing significant differences in the current trial, as we have shown in another observational study of nearly 1000 patients that use of antiemetics during chemotherapy according to MASCC guidelines is associated with significantly improved nausea/vomiting symptoms. 52
A limitation of the trial may be the missing data for the primary outcome. However, the proportion of cases missing the primary outcome (28%) is of a similar order to that anticipated at the design stage (33%). Originally 90% power had been planned for a standardised difference in means of around 0.48. For pragmatic reasons the study power was reduced to 80% so that it could complete in a reasonable time frame. The attained power that the final sample size with complete data for the primary outcome (n = 361) delivered was 80% for a standardised difference in means of 0.46. Also, another limitation that needs to be carefully considered in future trials is the choice of sham wristbands, which in our case may not have been the most optimal design.
Health-care utilisation data
Although there was no statistically significant effect of acupressure over sham acupressure and no acupressure, the health economics part of the trial was run concurrently with the effectiveness part of the trial. Through health economic analyses in negative trials it is possible that intervention benefits that are not apparent in traditional efficacy end points may register in QALYs, as this is a different composite outcome. Current thinking in respect of economic analyses in cases in which there is no clear differential effect/effectiveness of the intervention being assessed is that a full cost-effectiveness analysis is undertaken and uncertainty accounted for. Even given that the EQ-5D changes were below those levels that are minimally important and were not significant, and the QALY gains were minimal (for acupressure vs standard care), one cannot evaluate the cost-effectiveness without considering costs. 82,83
Mean costs resulting from NHS resource use were consistently higher for the standard care only group than for the acupressure group; this finding was relatively robust to sensitivity analyses. However, because very little resource use was recorded in the study and the results may have been unduly influenced by outliers with high costs, this finding is relatively uncertain and must be treated with caution. The results from the EQ-5D score analysis revealed no significant differences in utility changes over time between treatment arms and QALY gains throughout the study were negligible.
The acupressure bands group appears to have reduced health-care resource use while realising negligible improvements in quality of life compared with standard care alone. A rapid review of the literature found no studies including costs of chemotherapy-induced nausea and vomiting to the NHS, precluding comparison with previous research. Neither did the review yield any previous studies reporting EQ-5D scores for this patient group. In the present study the EQ-5D analysis showed no differences in utility between groups. There also appeared to be little overall impact of chemotherapy as no utility score changes between any cycle exceeded (or came close to exceeding) the minimal important difference identified for the measure. EQ-5D scores for older patients at the final chemotherapy cycle were similar to the population norm,84 but younger patients appeared to experience a greater utility decrement relative to norms. The mean utility for both the 25–50 and ≥ 50 age groups was 0.77 after cycle 4 compared with population norms of 0.90 for the 25–54 age group and 0.79 for the 55–74 age group. It is possible that the EQ-5D is not a sensitive enough measure to capture quality-of-life benefits that may be experienced as a result of reduced chemotherapy-induced nausea and vomiting, although a cancer-specific quality-of-life measure (FACT-G) also failed to detect any between-group differences. There is also a non-significant baseline difference in utility scores between groups, which may explain differential QALY gains over the trial period.
There were several limitations of this economic evaluation. We made assumptions regarding drug doses and antiemetics drug type because of missing data and also made assumptions regarding length of hospital stay. Assumptions were also necessary regarding the standard care of each of the patients, relying on expert opinion and assuming that care was the same across centres. It is possible that more expensive (branded) antiemetic treatments may have been used but not captured as not all prescribing information was available. Different sites may have different protocols relating to antiemetic prescription as a standard therapy. However, the ICERs and the resulting conclusions regarding the cost-effectiveness of acupressure were robust to sensitivity analyses and stochastic bootstrapping.
When calculating the EQ-5D missing data we employed the LOCF approach; this method assumes that the participant's EQ-5D response would have been stable between each cycle, rather than declining or improving. It also assumes that missing values are ‘missing completely at random’ (i.e. that the probability of dropout is not related to variables such as disease severity, symptoms or drug side effects). As such, the LOCF method may lead to bias in the results. However, assumptions about both costs and utilities were tested in the sensitivity analyses. Finally, as stated above, it is possible that the EQ-5D is an inappropriate measure to capture health benefits incurred as a result of reduced nausea and that a cancer-specific measure may be more suitable. 85 However, cost–utility analyses and the EQ-5D are compliant with the NICE reference case. Future research should consider cancer-specific utility measurement and comparisons with generic utility values. As the occurrence and impact of chemotherapy-induced nausea and vomiting varies over time in relation to chemotherapy receipt, quality-of-life impact depends on the time of measure completion during the cycle. Thus, research exploring patients' health status on a daily basis throughout the course of a chemotherapy cycle may be warranted. Given the influence of high-cost outliers in the analyses, greater exploration of what is driving these costs and indeed whether or not they relate to chemotherapy-induced nausea and vomiting may be worthwhile; this may involve qualitative follow-up of high-resource-use individuals.
Qualitative data
The nested qualitative study aimed to explore patients' reasons for taking part in a clinical trial of acupressure wristbands and their experiences of using acupressure wristbands and taking part in the trial of acupressure wristbands. The key findings from these data suggest that patients in the wristband arms perceived the bands to be effective and felt more in control of their nausea and vomiting experience.
The motivations that patients identified for participating in the trial are congruent with the findings of previous research. Paterson et al. 86 conducted a nested qualitative study exploring the experiences of patients with migraines receiving acupuncture within a randomised controlled trial. Similar to the present study, they found that patients felt that acupuncture was ‘worth a try’, with patients being eager for symptom relief and having a desire to help with research for altruistic motives, although the theme ‘giving something back’ appears to be much more prevalent within the present study. This may be a consequence of the greater morbidity associated with cancer, although further research would need to be conducted to confirm whether or not this is the case.
Patients were largely satisfied with the organisation and running of the trial. The process of recruitment was generally perceived as straightforward and to have been adequately described. Interestingly, the completion of trial paperwork was seen as being of benefit by some patients, a finding again consistent with previous research. 86 Where the current study findings appear to differ from previous research is in the explicit feeling of ‘well-being’ that many patients experienced as a direct result of knowing that their participation in the trial might lead to the improvement of care for future cancer patients.
Previous qualitative research exploring the experiences of patients receiving treatment with acupuncture suggests that patients experience a range of benefits beyond the alleviation of their presenting condition, including improvements in overall well-being, sleep pattern and energy levels. 54–56 A number of patients who received wristbands in the present study indicated that they had experienced only minor levels of nausea and vomiting during their chemotherapy. Some patients – those receiving both true and sham wristbands – attributed the wristbands as having had a positive impact on the level of nausea and vomiting experienced. However, none of the patients who participated in interviews associated the acupressure wristbands with eliciting benefits beyond the relief of chemotherapy-related nausea and vomiting. This may suggest that a greater stimulation of acupuncture points is required to elicit these effects or that the contextual factors within the acupuncture consultation, such as the interaction between the practitioner and the patient or consultation setting, may work in conjunction with the stimulation of acupuncture points to elicit these expanded effects of care.
The wristbands were not associated with any significant adverse effects and patients found them easy to wear and to be associated only with limited social inhibitions. Importantly, patients appear to have worn the wristbands in the correct location and for the period of time instructed. However, the data revealed that two of the nine sham acupressure group patients had worn their wristbands inappropriately (based on their trial arm allocation), either wearing them inside out or applying pressure manually to the acupoint. Also of importance is the apparent delay in some patients completing the trial paperwork, relying on memory to fill in the forms. Both of these may represent confounding variables, which if generalisable to other patients in the trial could have serious implications for the results of the trial.
Conclusions and research recommendations
Despite several acupressure antiemetic trials suggesting a beneficial effect, the trial heterogeneity and inconsistent findings prevented any definitive conclusions being drawn. Our study, using a strong methodological design and standardisation of antiemetics, showed no significant differences between acupressure and sham acupressure wristbands for the management of nausea and vomiting during chemotherapy. Nevertheless, the somewhat improved, albeit not statistically significant, levels of nausea in both wristband arms needs some attention, although, as minimally important differences in relation to chemotherapy-related nausea and vomiting are currently not established, some caution is necessary here. The use of wristbands led to lower health-care utilisation (although this did not reach statistical significance). Bands are well accepted and are low cost and safe additions to antiemetic drugs, but the ethical aspects of suggesting the use of potentially non-effective interventions that lead to lower health-care costs and health-care utilisation need some careful consideration. There is a sufficiently encouraging signal and a suggestion of potential health resource-use benefits to justify exploration of acupressure in further trials using both no intervention and sham acupressure controls. Questions that need to be answered in the future include whether or not other forms of acupressure such as regular finger acupressure or Korean hand acupressure could be more effective than wristband acupressure. A meta-analysis of existing data on acupressure wristbands may be an appropriate way to provide a more concrete answer to the question of whether or not acupressure wristbands are effective in managing nausea and/or vomiting during chemotherapy.
Acknowledgements
Trial manager
Wanda Russell, Christie NHS Foundation Trust
Liverpool lead researcher
John Hughes, Royal Liverpool Hospital
Plymouth lead researcher
Matthew Breckons, Derriford Hospital
Research nurses (National Cancer Resarch Institute recruiters to the Assessment of Nausea in Chemotherapy Research trial)
Tina Pritchard, Christie NHS Foundation Trust
Joanne Allen, Christie NHS Foundation Trust
Wendy Cook, Royal Oldham Hospital
Ruth Halford, Royal Oldham Hospital
Stephanie Ridgway, Tameside General Hospital
Elly Anscombe, Stepping Hill Hospital
Sam Corcoran, Stepping Hill Hospital
Sarah McKenna, Stepping Hill Hospital
Lisa Hardstaff, Macclesfield District General Hospital
Barbara Townley, Macclesfield District General Hospital
Claire Gaskell, Macclesfield District General Hospital
Sarah Entwistle, Macclesfield District General Hospital
Sam Jole, North Manchester General Hospital
Deborah Fleetwood, Royal Liverpool Hospital
Alison Hassall, Clatterbridge Centre for Oncology
Lynne Dickinson, Clatterbridge Centre for Oncology
Holly Donaghy, St Helens Hospital
Frances Lawton, St Helens Hospital
Julie Griffiths, Southport General Infirmary
Ann Wearing, Southport General Infirmary
Ingrid Koehler, Torbay District General Hospital
Julie Pascoe, Derriford Hospital
Nicola Donlin, Derriford Hospital
Caroline Snelgrove, Derriford Hospital
Laura Evenden, Derriford Hospital
Elizabeth Whitby , Derriford Hospital
Helen Smith, Derriford Hospital
Rachel Keast, Royal Cornwall Hospital (Treliske)
Sophie Mepham, Royal Cornwall Hospital (Treliske)
Darren Beech, Royal Cornwall Hospital (Treliske)
Research and Development and administrative staff
Charlotte Lever, Christie NHS Foundation Trust
Joanne Johnson, Royal Oldham Hospital
Philippa Hill, Macclesfield District General Hospital
Joy Bailey, Macclesfield District General Hospital
Marilyn McCurrie, Macclesfield District General Hospital
Edith Curran, North Manchester General Hospital
Tracie Cocks, Stepping Hill Hospital
Cancer Research network managers and support staff
Sue Dyde, Christie NHS Foundation Trust
Zoe Coombe, Christie NHS Foundation Trust
Richard Jones, Christie NHS Foundation Trust
Trial Steering Committee for the trial
Professor Kinta Beaver (independent chairperson, University of Central Lancashire)
Olive Craven (independent member)
Dai Roberts (independent member)
Anne Urmston (patient representative)
Data Management and Ethics Committee for the trial
Simon Rogers
David Ryder
John Belcher (patient representative)
April Matthews (patient representative)
Baio Tang
Health economics support (lead Dr Claire Hulme)
Chantelle Brown, University of Leeds
David Meads, University of Leeds
Contribution of authors
Professor Alex Molassiotis (Professor of Cancer and Supportive Care, University of Manchester) conceived and designed the initial plan of the study, had overall responsibility for the study, analysed the data together with David Ryder (Senior Statistician, Christie NHS Foundation Trust, who led on the statistical analysis) and drafted the final report. Dr Wanda Russell (Clinical Trials Manager, University of Manchester) was responsible for the day-to-day operationalisation and management of the study, liaison with the study sites, recruitment at the Manchester sites, quality assurance, producing reports and preparing for meetings and liaising with the National Cancer Research Network (NCRN) database. Dr John Hughes (Research Associate, University of Liverpool) and Dr Matt Breckons (Research Associate, University of Plymouth) were responsible for recruitment and trial management at the Liverpool and Plymouth sites respectively. Dr John Hughes also carried out the qualitative interviews (together with Dr Russell and Dr Breckons) and led the analysis of the qualitative element of the trial. The co-applicants Professor Mari Lloyd-Williams (Professor of Palliative Medicine, University of Liverpool), Professor Janet Richardson (Professor of Health Services Research, University of Plymouth), Dr Sarah Brearley (Lecturer, Lancaster University) and Dr Adam Garrow (Research Fellow, University of Salford) were involved in all stages of the work – design, analysis and commenting on and drafting sections of the final report. Professor Lloyd-Williams and Professor Richardson were also leads for the trial in Liverpool and Plymouth respectively. Dr Malcolm Campbell (Senior Lecture in Statistics, University of Manchester) was responsible for the initial statistical design of the trial and oversaw the statistical elements throughout the trial. Dr Claire Hulme (Director of the Academic Unit of Health Economics, University of Leeds) was the lead for the cost-effectiveness part of the trial, was involved with the design and conduct of this part of the trial and led on the analysis of the health economics data.
The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Department of Health.
Publication
Molassiotis A, Russell W, Hughes J, Breckons M, Lloyd-Williams M, Richardson J, et al. The effectiveness of acupressure for the control and management of chemotherapy-related acute and delayed nausea: a randomized controlled trial [published online ahead of print April 19 2013]. J Pain Symptom Manage 2013; in press. http://dx.doi.org/10.1016/j.jpainsymman.2013.03.007
Disclaimer
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Glaus A, Knipping C, Morant R, Böhme C, Lebert B, Glawogger B, et al. Chemotherapy-induced nausea and vomiting in routine practice: a European perspective. Support Care Cancer 2004;12:708-15.
- Roscoe JA, Morrow GR, Hickok JT, Stern RM. Nausea and vomiting remain a significant clinical problem: trends over time in controlling chemotherapy-induced nausea and vomiting in 1413 patients treated in the community. J Pain Symptom Manage 2000;20:113-21.
- Grunberg SM. Chemotherapy-induced nausea and vomiting: prevention, detection, and treatment – how are we doing?. J Support Oncol 2004;2:1-10.
- Liau CT, Chu NM, Liu HE, Deuson R, Chen JS. Incidence of chemotherapy-induced nausea and vomiting in Taiwan: physicians' and nurses' estimation vs patients' reported outcomes. Support Care Cancer 2005;13:277-86.
- Bergkvist K, Wengstrom Y. Symptom experiences during chemotherapy treatment – with focus on nausea and vomiting. Eur J Oncol Nurs 2006;10:21-9.
- Foubert J, Vaessen G. Nausea: the neglected symptom?. Eur J Oncol Nurs 2005;9:21-32.
- Griffin AM, Butow PN, Coates AS, Childs AM, Ellis PM, Dunn SM, et al. On the receiving end. V: patient perceptions of the side effects of cancer chemotherapy in 1993. Ann Oncol 1996;7:189-95.
- Ihbe-Heffinger A, Ehlken B, Bernard R, Berger K, Peschel C, Eichler HG, et al. The impact of delayed chemotherapy-induced nausea and vomiting on patients, health resource utilization and costs in German cancer centers. Ann Oncol 2004;15:526-36.
- ASHP therapeutic guidelines on the pharmacologic management of nausea and vomiting in adult and pediatric patients receiving chemotherapy or radiation therapy or undergoing surgery. Am J Health Syst Pharm 1999;56:729-64.
- Miller M, Kearney N. Chemotherapy-related nausea and vomiting – past reflections, present practice and future management. Eur J Cancer Care 2004;13:71-8.
- Molassiotis A. Managing nausea and vomiting after cancer treatments: patients still suffer unnecessarily. Eur J Oncol Nurs 2005;9:4-5.
- Burish TG, Tope DM. Psychological techniques for controlling the adverse side effects of cancer chemotherapy: findings from a decade of research. J Pain Symptom Manage 1992;7:287-301.
- Dundee JW, Ghaly RG, Fitzpatrick KT, Abram WP, Lynch GA. Acupuncture prophylaxis of cancer chemotherapy-induced sickness. J R Soc Med 1989;82:268-71.
- Dundee JW, Ghaly RG, Fitzpatrick KT, Lynch GA, Abram WP. Acupuncture to prevent cisplatin-associated vomiting. Lancet 1987;1.
- Molassiotis A, Helin AM, Dabbour R, Hummerston S. The effects of P6 acupressure in the prophylaxis of chemotherapy-related nausea and vomiting in breast cancer patients. Complement Ther Med 2007;15:3-12.
- Pearl ML, Fischer M, McCauley DL, Valea FA, Chalas E. Transcutaneous electrical nerve stimulation as an adjunct for controlling chemotherapy-induced nausea and vomiting in gynecologic oncology patients. Cancer Nurs 1999;22:307-11.
- Roscoe JA, Morrow GR, Bushunow P, Tian L, Matteson S. Acustimulation wristbands for the relief of chemotherapy-induced nausea. Altern Ther Health Med 2002;8:56-63.
- Treish I, Shord S, Valgus J. Randomised double-blind study of the Reliefband as an adjunct to standard antiemetics in patients receiving moderately-high to highly emetogenic chemotherapy. Support Care Cancer 2003;11:516-21.
- Dundee JW, Yang J. Prolongation of the antiemetic action of P6 acupuncture by acupressure in patients having cancer chemotherapy. J R Soc Med 1990;83:360-2.
- Dundee JW, Yang J, McMillan C. Non-invasive stimulation of the P6 (Neiguan) antiemetic acupuncture point in cancer chemotherapy. J R Soc Med 1991;84:210-12.
- Wright LD. The use of motion sickness bands to control nausea and vomiting in a group of hospice patients. Am J Hosp Palliat Care 2005;22:49-53.
- Shin YH, Kim TI, Shin MS, Juon HS. Effect of acupressure on nausea and vomiting during chemotherapy cycle for Korean postoperative stomach cancer patients. Cancer Nurs 2004;27:267-74.
- Dibble SL, Chapman J, Mack KA, Shih AS. Acupressure for nausea: results of a pilot study. Oncol Nurs Forum 2000;27:41-7.
- Roscoe JA, Morrow GR, Hickok JT, Bushunow P, Pierce HI, Flynn PJ, et al. The efficacy of acupressure and acustimulation wrist bands for the relief of chemotherapy-induced nausea and vomiting. A University of Rochester Cancer Center Community Clinical Oncology Program multicenter study. J Pain Symptom Manage 2003;26:731-42.
- Roscoe JA, Matteson SE, Morrow GR, Hickok JT, Bushunow P, Griggs J, et al. Acustimulation wrist bands are not effective for the control of chemotherapy-induced nausea in women with breast cancer. J Pain Symptom Manage 2005;29:376-84.
- Ezzo JM, Richardson MA, Vickers A, Allen C, Dibble SL, Issell BF, et al. Acupuncture-point stimulation for chemotherapy-induced nausea or vomiting. Cochrane Database Syst Rev 2006;2.
- Molassiotis A, Yung HP, Yam BMC, Chan FYS, Mok TSK. The effectiveness of progressive muscle relaxation training in managing chemotherapy-induced nausea and vomiting in Chinese breast cancer patients: a randomized controlled trial. Support Care Cancer 2002;10:237-46.
- Olver I, Molassiotis A, Aapro M, Herrstedt J, Grunberg S, Morrow G. Antiemetic research: future directions. Support Care Cancer 2011;19:S49-55.
- Molassiotis A, Yam BM, Yung H, Chan FY, Mok TS. Pretreatment factors predicting the development of postchemotherapy nausea and vomiting in Chinese breast cancer patients. Support Care Cancer 2002;10:139-45.
- Aapro MS, Molassiotis A, Olver I. Anticipatory nausea and vomiting. Support Care Cancer 2005;13:117-21.
- Roscoe JA, Morrow GR, Aapro MS, Molassiotis A, Olver I. Anticipatory nausea and vomiting. Support Care Cancer 2011;19:1533-8.
- Roila F, Hesketh PJ, Herrstedt J. Antiemetic Subcommitte of the Multinational Association of Supportive Care in Cancer. Prevention of chemotherapy- and radiotherapy-induced emesis: results of the 2004 Perugia International Antiemetic Consensus Conference. Ann Oncol 2006;17:20-8.
- Roila F, Herrstedt J, Aapro M, Gralla RJ, Einhorn LH, Ballatori E, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol 2010;21:v232-43.
- Maranzano E, Feyer PCh, Molassiotis A, Rossi R, Clark-Snow RA, Olver I, et al. Evidence-based recommendations for the use of antiemetics in radiotherapy. Radiother Oncol 2005;76:227-33.
- Feyer PC, Maranzano E, Molassiotis A, Roila F, Clark-Snow RA, Jordan K, et al. Radiotherapy-induced nausea and vomiting (RINV): MASCC/ESMO guideline for antiemetics in radiotherapy: update 2009. Support Care Cancer 2011;19:S5-14.
- Molassiotis A, Stricker CT, Eaby B, Velders L, Coventry PA. Understanding the concept of chemotherapy-related nausea: the patient experience. Eur J Cancer Care 2008;17:444-53.
- Molassiotis A, Saunders MP, Valle J, Wilson G, Lorigan P, Wardley A, et al. A prospective observational study of chemotherapy-related nausea and vomiting in routine practice in a UK cancer centre. Support Care Cancer 2008;16:201-8.
- Molassiotis A, Brearley SG, Stamataki Z. Use of antiemetics in the management of chemotherapy-related nausea and vomiting in current UK practice. Support Care Cancer 2011;19:949-56.
- Molassiotis A, Coventry PA, Stricker CT, Clements C, Eaby B, Velders L, et al. Validation and psychometric properties of a short clinical scale to measure chemotherapy-induced nausea and vomiting: the MASCC Antiemesis Tool (MAT). J Pain Symptom Manage 2007;34:148-59.
- du Bois A, Meerpohl HG, Vach W, Kommoss FG, Fenzl E, Pfleiderer A. Course, patterns, and risk-factors for chemotherapy-induced emesis in cisplatin-pretreated patients: a study with ondansetron. Eur J Cancer 1992;28:450-7.
- Morrow GR, Lindke J, Black PM. Predicting development of anticipatory nausea in cancer patients: prospective examination of eight clinical characteristics. J Pain Symptom Manage 1991;6:215-23.
- Kris MG, Hesketh PJ, Somerfield MR, Feyer P, Clark-Snow R, . American Society of Clinical Oncology . American Society of Clinical Oncology guideline for antiemetics in oncology: update 2006. J Clin Oncol 2006;24:2932-47.
- Andrykowski MA, Gregg ME. The role of psychological variables in post-chemotherapy nausea: anxiety and expectation. Psychosom Med 1992;54:48-5.
- Molassiotis A, Sylt P, Diggins H. The management of cancer-related fatigue after chemotherapy with acupuncture and acupressure: a randomized controlled trial. Complement Ther Med 2007;15:228-37.
- Paterson C, Dieppe P. Characteristic and incidental (placebo) effects in complex interventions such as acupuncture. BMJ 2005;330:1202-5.
- Kaptchuk TJ. Uptake of alternative medicine. Lancet 1996;347.
- Mason S, Tovey P, Long AF. Evaluating complementary medicine: methodological challenges of randomised controlled trials. BMJ 2002;325:832-4.
- White AR, Filshie J, Cummings TM. Clinical trials of acupuncture: consensus recommendations for optimal treatment, sham controls and blinding. Complement Ther Med 2001;9:237-45.
- Rhodes VA, McDaniel RW. The Index of Nausea, Vomiting, and Retching: a new format of the Index of Nausea and Vomiting. Oncol Nurs Forum 1999;26:889-94.
- Fairclough DL, Cella DF. Functional Assessment of Cancer Therapy (FACT-G): non-response to individual questions. Qual Life Res 1996;5:321-9.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70.
- Aapro M, Molassiotis A, Dicato M, Pelaez I, Rodriguez Lescure A, Pastorelli D, et al. PEER investigators . The effect of guideline-consistent antiemetic therapy on chemotherapy-induced nausea and vomiting (CINV): the Pan European Emesis Registry (PEER). Ann Oncol 2012;23:1986-92.
- Curtis L, Netten A. Unit costs of health and social care 2010. Canterbury: PSSRU, University of Kent; 2010.
- Hughes JG, Goldbart J, Fairhurst E, Knowles K. Exploring acupuncturists' perceptions of treating patients with rheumatoid arthritis. Complement Ther Med 2007;15:101-8.
- Hughes JG. ‘When I first started going I was going in on my knees, but I came out and I was skipping': exploring rheumatoid arthritis patients' perceptions of receiving treatment with acupuncture. Complement Ther Med 2009;17:269-73.
- Paterson C. Measuring changes in self-concept: a qualitative evaluation of outcome questionnaires in people having acupuncture for their chronic health problems. BMC Complement Altern Med 2006;6.
- Ritchie J, Lewis J. Qualitative research practice. London: Sage; 2003.
- Patton MQ. Qualitative research and evaluation methods. Thousand Oaks, CA: Sage; 2002.
- Office for National Statistics . The National Statistics Socio-Economic Classification: User Manual n.d. www.ons.gov.uk/ons/dcp14858_179140.xml (accessed October 2012).
- Venables WN, Ripley BD. Modern applied statistics with S. New York, NY: Springer; 2002.
- Parsons NR, Edmondson RN, Gilmour SG. A generalized estimating equation method for fitting autocorrelated ordinal score data with an application in horticultural research. Appl Stat 2006;55:507-24.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2005.
- Department of Health Commercial Medicines Unit . Electronic Market Information Tool (eMIT) n.d. http://cmu.dh.gov.uk/electronic-market-information-tool-emit/ (accessed October 2012).
- British national formulary. No. 56, September 2008. London: BMA and RPS; 2008.
- Office for National Statistics . 2010 Annual Survey of Hours and Earnings 2010. www.ons.gov.uk (accessed October 2012).
- Koopmanschap MA, Rutten FF. A practical guide for calculating indirect costs of disease. Pharmacoeconomics 1996;10:460-6.
- EQ-5D . What Is EQ-5D n.d. www.euroqol.org/ (accessed 22 February 2012).
- Dolan P. Modelling valuations for health states: the effect of duration. Health Policy 1996;38:189-203.
- Richardson G, Manca A. Calculation of quality adjusted life years in the published literature: a review of methodology and transparency. Health Econ 2004;13:1203-10.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87.
- Stinnett AA, Mullahy J. Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decis Making 1998;18:S68-80.
- Hoch JS, Briggs AH, Willan AR. Something old, something new, something borrowed, something blue: a framework for the marriage of health econometrics and cost-effectiveness analysis. Health Econ 2002;11:415-30.
- Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes 2007;5.
- Lee EJ, Frazier SK. The efficacy of acupressure for symptom management: a systematic review. J Pain Symptom Manage 2011;42:589-603.
- Dibble SL, Luce J, Cooper BA, Israel J, Cohen M, Nussey B, et al. Acupressure for chemotherapy-induced nausea and vomiting: a randomized clinical trial. Oncol Nurs Forum 2007;34:813-20.
- Sinha A, Paech MJ, Thew ME, Rhodes M, Luscombe K, Nathan E. A randomised, double-blinded, placebo-controlled study of acupressure wristbands for the prevention of nausea and vomiting during labour and delivery. Int J Obstet Anesth 2011;20:110-17.
- Enblom A, Lekander M, Hammar M, Johnsson A, Onelov E, Ingvar M, et al. Getting the grip of nonspecific treatment effects: emesis in patients randomised to acupuncture or sham compared to patients receiving standard care. PloS One 2011;6.
- Roscoe JA, O’Neill M, Jean-Pierre P, Heckler CE, Kaptchuk TJ, Bushunow P, et al. An exploratory study on the effects of an expectancy manipulation on chemotherapy-related nausea. J Pain Symptom Manage 2010;40:379-90.
- Miller FG, Colloca L, Kaptchuk TJ. The placebo effect: illness and interpersonal healing. Perspect Biol Med 2009;52:518-39.
- Alkaissi A, Stalnert M, Kalman S. Effect and placebo effect of acupressure (P6) on nausea and vomiting after outpatient gynaecological surgery. Acta Anaesthesiol Scand 1999;43:270-4.
- Price DD, Finiss DG, Benedetti F. A comprehensive review of the placebo effect: recent advances and current thought. Annu Rev Psychol 2008;59:565-90.
- Briggs AH, O’Brien BJ. The death of cost-minimisation analysis?. Health Econ 2001;10:179-84.
- Dakin H, Wordsworth S. Cost-minimisation analysis versus cost-effectiveness analysis, revisited [published online ahead of print 22 November 2011]. Health Econ 2011. doi:10.1002/hec.1812.
- Kind P, Hardman G, Macran S. UK population norms for EQ-5D. York: University of York, Centre for Health Economics; 1999.
- Gaugris S, Craig BM, King MT, Rowen D, Brazier J, Young T, et al. Deriving a preference-based measure for cancer using the EORTC QLQ-C30. Value Health 2011;14:721-31.
- Paterson C, Zheng Z, Xue C, Wang Y. ‘Playing their parts': the experiences of participants in a randomized sham-controlled acupuncture trial. J Altern Complement Med 2008;14:199-208.
- NHS reference costs 2009–2010. London: DoH; 2011.
Appendix 1 Study scales: Rhodes Index of Nausea, Vomiting and Retching
Study scales: Rhodes Index of Nausea, Vomiting and Retching (PDF download)
Appendix 2 Study scales: Multinational Association of Supportive Care in Cancer Antiemesis
Study scales: Multinational Association of Supportive Care in Cancer Antiemesis (PDF download)
Appendix 3 Study scales: Functional Assessment of Cancer Treatment – General scale
Study scales: Functional Assessment of Cancer Treatment – General scale (PDF download)
Appendix 4 Study scales: Hospital Anxiety and Depression Scale
Study scales: Hospital Anxiety and Depression Scale (PDF download)
Appendix 5 Study scales: expectations and complementary therapy questionnaire
Study scales: expectations and complementary therapy questionnaire (PDF download)
Appendix 6 Study scales: sociodemographic characteristics questionnaire
Study scales: sociodemographic characteristics questionnaire (PDF download)
Appendix 7 Study scales: acupressure wristband compliance questionnaire
Study scales: acupressure wristband compliance questionnaire (PDF download)
Appendix 8 Wristband audit observation log
Appendix 9 Health economics baseline questionnaire
Appendix 10 Health economics questionnaire
Appendix 11 Unit costs for the health economics analysis
Appendix 12 Drug costs used for the health economic analysis
Drug costs used for the health economic analysis (PDF download)
Appendix 13 Protocol
List of abbreviations
- ANCHoR
- Assessment of Nausea in Chemotherapy Research
- ANOVA
- analysis of variance
- ASCO
- American Society of Clinical Oncology
- BNF
- British National Formulary
- CI
- confidence interval
- CL
- cumulative logit
- DMEC
- Data Monitoring and Ethics Committee
- eMIT
- electronic Market Information Tool
- EQ-5D
- European Quality of Life-5 Dimensions
- FACT-G
- Functional Assessment of Cancer Therapy – General
- GEE
- generalised estimating equation
- 5-HT3
- 5-hydroxytryptamine type 3
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- LOCF
- last observation carried forward
- MASCC
- Multinational Association of Supportive Care in Cancer
- MAT
- Multinational Association of Supportive Care in Cancer Antiemesis Tool
- MHRA
- Medicines and Healthcare products Regulatory Agency
- NCRI
- National Cancer Research Institute
- NICE
- National Institute for Health and Clinical Excellence
- NMB
- net monetary benefit
- OR
- odds ratio
- QALY
- quality-adjusted life-year
- SD
- standard deviation
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.