Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 10/110/01. The contractual start date was in January 2011. The draft report began editorial review in April 2011 and was accepted for publication in August 2012. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors' report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
The following authors have no conflicts of interest: RO, MP, SB, AH, DM and PB. SR has received training support for TAVI from Edwards Life Sciences. SK has received training support from both Edwards Life Sciences and Medtronic and is a TAVI proctor (Medtronic CoreValve).
Permissions
Copyright statement
© Queen's Printer and Controller of HMSO 2013. This work was produced by Orlando et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Aortic stenosis
Aetiology
Aortic stenosis (AS) is a condition in which the aortic valve becomes progressively narrowed, leading to gradual obstruction of left ventricular outflow. The most common form, occurring in about 80% of cases, is degenerative. 1 This primarily presents as calcific AS2 and shares a similar developmental process and risk factors to atherosclerosis. 2–4 Risk factors for the development of AS include male sex, hypertension, elevated low-density lipoproteins, diabetes, cholesterol, smoking and a family history of heart disease. 1,5 Patients with chronic AS may remain asymptomatic for many years but, in most patients, symptoms of disease eventually develop. AS is usually considered in terms of its haemodynamic and symptomatic disease severity.
Haemodynamic diagnosis and severity measurement can be conducted using echocardiography, Doppler studies and cardiac catheterisation. 2,5 Severity can be assessed in terms of the aortic valve area (AVA), peak aortic jet velocity (Vmax) and transaortic valve gradient, severe AS being defined as AVA < 1 cm2, Vmax > 4 m/second and mean transaortic valve gradient > 40 mmHg. 6
Clinical symptoms of AS include chest pain or breathlessness on exertion, angina, dizziness and syncope, and patients may suffer sudden death. 2,5 The New York Heart Association (NYHA) heart failure classification is used to classify the severity of symptoms, ranging from class I, in which the patient has no limitation in daily physical activity, to class IV, in which the patient is breathless at rest. Of patients with valvular heart disease, ∼30% are in each of the classes I, II and III and ∼10% are in class IV. 1 Most patients with severe AS display symptoms of disease. However, this is not always the case and some patients with haemodynamically severe AS are asymptomatic. 7
Epidemiology
Aortic stenosis is the most common valvular heart disease in Western countries. 2,3,5 The majority of people treated for AS are > 60 years of age1 and the prevalence of AS has been found to be 2% in those ≥ 65 years, 3% in those ≥ 75 years8 and 4% in those ≥ 85 years. 9 The prevalence of valve disease is strongly linked to population ageing10 and the prevalence of AS is therefore likely to increase in developed countries such as the UK because of the increasing proportion of elderly people in those populations.
Natural history
The progression of AS is very variable, with some patients living a long time with stable, asymptomatic disease. However, once symptoms develop, risk of death is high. 2,11 Symptoms may develop in patients of any age but most commonly present in patients in their 60s. 11
In patients with severe symptomatic AS, in the absence of treatment, survival rates have been shown to display a sharp decrease, independent of age at symptom onset. 11 Mortality risk is about 2% per month4 and typical survival is < 2 to 3 years. 5,12 Two-year mortality is approximately 50%13 and 3-year mortality approximately 75%. 4 In a trial which enrolled patients unsuitable for surgical intervention, the 1-year mortality under standard therapy was approximately 50%. 14 This higher mortality is not unexpected given the patient group involved.
In asymptomatic patients, sudden death is rare2 but there is a small risk that symptoms will develop very rapidly and the patient will die suddenly or even that death will occur suddenly without the onset of clinical symptoms. The risk of sudden death in asymptomatic patients has been estimated to be around 1% per year. 2,7
Treatment of severe aortic stenosis
Surgical aortic valve replacement
Patients
Surgical aortic valve replacement (SAVR) is considered the treatment of choice for patients with severe symptomatic AS and is recommended in all patients who are candidates for surgery. 2 Some researchers argue that asymptomatic patients should also be considered for surgical intervention. 4,7 However, the disease-related risk of sudden death (∼1%7) may be low compared with the risks associated with surgery4 and, as it is often difficult to accurately standardise haemodynamic measures of severity,4 it may be difficult to identify particularly severe asymptomatic patients in whom surgery would be indicated. In the UK, SAVR has increased from 1900 cases per annum in 1999 to 4250 cases in 2008. 15 The mean age of patients undergoing SAVR has risen from 68 years in 1994 to 73.5 years in 2008. 15 Since 1999, the percentage of patients > 80 years of age undergoing SAVR has more than doubled from 6% to 13%. 15
Procedure
Surgical aortic valve replacement is a major surgical procedure. It involves division of the sternum to achieve adequate exposure of the heart and aorta for the procedure. During SAVR, cardiopulmonary bypass is used to maintain the circulation and blood oxygenation. The approach to the diseased valve is via the ascending aorta and the valve is replaced with a prosthetic valve. Following closure of the aorta, coronary artery perfusion is re-established. Cardiopulmonary bypass support is then gradually withdrawn and the patient's heart resumes control of the circulation.
Mechanical or biological prosthesis can be used for SAVR. Historically, approximately half of patients undergoing SAVR for AS received mechanical and half received biological valves. 1 However, there has been an increasing trend towards the use of biological valves. In 2004, 60% of SAVRs used biological valves; in 2008 this had risen to 73%. 15 This reflects not only an increase in mean age of this group of patients, but also that data have shown better longevity for the modern generation of biological valves. 15 Mechanical valves may be more durable, but they require lifelong anticoagulation therapy with monitoring and are usually more appropriate for younger patients, whereas biological valves may be more appropriate in older patients with shorter anticipated lifespans. Some guidelines have suggested a threshold of 65 years of age for choosing a biological rather than mechanical valve, but in the UK and mainland Europe the trend has been towards a threshold of 70 or 75 years of age. 1
Outcomes
Early studies (pre 1966) of patients undergoing SAVR give rates of procedural mortality of around 4–20%. 11 However, the risks associated with surgical intervention have decreased and, more recently, operative mortality of isolated SAVR is typically around 2–5%. 1,5,13,15 Operative mortality may be around 5–15% in older patients. 2,16
With surgical intervention, overall patient survival is prolonged. In a meta-analysis of SAVR studies, postprocedural mortality risk was estimated as 4.3% per year. 17 Postsurgical projections have been shown to track healthy life expectancy11 and, where SAVR is successful, rates of long-term survival may be similar to those of age-matched populations without AS. 2,4,5
The success of SAVR in elderly patients may be anticipated to be lower than in younger patients as age-associated comorbidities have a large impact on procedural and long-term risks. 18 However, studies restricted to elderly patients (≥ 80 years) undergoing SAVR have also shown positive outcomes. Rates of 5-year survival have been estimated to be around 50–70% and these may compare well with rates of life expectancy of the same-aged general population. 15,18
In many studies, around 50% of patients undergoing SAVR are also undergoing additional procedures [usually coronary artery bypass grafting (CABG)]. 19–21 In a large cohort of patients undergoing SAVR (n = 4131), the presence of additional procedures was shown to be an independent predictor of adverse outcome [odds ratio (OR) 1.81, 95% confidence interval (CI) 1.4 to 2.3]. 21 Mortality is approximately doubled in patients receiving SAVR combined with CABG compared with those receiving SAVR alone. 15,19 As operative and long-term risk is likely to be substantially higher for patients undergoing concomitant procedures, data from retrospective surgical series, that are not restricted to isolated SAVR, may tend to underestimate the effectiveness of SAVR.
Medical management
Patients
Patients with severe symptomatic AS deemed ineligible for surgery are treated with medical therapy. 2 In some cases, patients may be potentially eligible for invasive intervention but choose to be treated medically.
Treatments
Since lipids are involved in fibrosis, calcification and subsequent stenosis, lipid-lowering agents, such as hydroxymethylglutaryl-coenzyme A reductase inhibitors or statins, may be used to potentially slow the progression of AS. 5 Other medications, such as angiotensin-converting enzyme (ACE) inhibitors and bisphosphonates, have also been identified as potentially useful for the treatment of AS. 5
Outcomes
In patients with severe AS, medical management is considered to bring little benefit. 4 Studies have shown variable results and medical therapy has not been demonstrated to alter the natural history of AS. 2,5 In a study of severe symptomatic AS patients who, as a result of ineligibility for transcatheter aortic valve implantation (TAVI) or SAVR or patient preference, underwent medical treatment, 1- and 1.5-year overall survival rates were 63% and 58% respectively. 22 These patients were old [mean age 81.7 years, standard deviation (SD) 8.7 years] and at particularly high risk [mean logistic European System for Cardiac Operative Risk Evaluation (EuroSCORE) 35.1%, SD 22.3%] and there was a high prevalence of comorbidities. Although overall survival was low, as the prognosis of this patient group was poor, it may be that medical treatment brought some benefit. However, in the absence of controlled data, it is difficult to judge whether or not medical management contributes additional survival for patients with severe AS.
Balloon aortic valvuloplasty
Patients
Balloon aortic valvuloplasty has been used as an alternative approach in patients who are not fit for surgery, but this is usually tailored to the patient. Balloon aortic valvuloplasty can be associated with poor outcomes, but in specific patients who are haemodynamically unstable it may serve as a bridge to surgery or TAVI, or as a palliative measure where surgery is contraindicated. 2,3,23
Procedure
Balloon aortic valvuloplasty is the use of a balloon catheter to attempt to increase the size of the valve opening and improve blood flow. The catheter is passed into the narrowed aortic valve and then inflated to dilate the valve. 24
Outcomes
Historical cohort data show that balloon valvuloplasty results in complications in around 10% of cases2 and is associated with high rates of mortality and morbidity. 3,23 Restenosis is common, with recurrence of symptoms within a few months,3,23 and balloon valvuloplasty is thought to have little impact on the natural history of AS. 2,18
Transcatheter aortic valve implantation
Patients
Transcatheter aortic valve implantation is a relatively new technique for the treatment of AS and, at the time of this report, it was considered for use in patients who are deemed ineligible or too high risk for surgery. Where possible, it is often used in preference to medical treatment or balloon valvuloplasty. Since the work reported here has been completed, new National Institute for Health and Care Excellence (NICE) guidance25 has been issued allowing TAVI in the following circumstances:
For patients with aortic stenosis who are considered to be unsuitable for surgical aortic valve replacement. . .TAVI may be used with normal arrangements for clinical governance, consent and audit.
For patients with aortic stenosis for whom SAVR is considered suitable but to pose a high risk. . .TAVI should only be used with special arrangements for clinical governance, consent and data collection or research.
For patients with aortic stenosis for whom SAVR is considered suitable and not to pose a high risk. . .TAVI should only be used in the context of research.
Procedure
Valve implantation is achieved by keyhole catheter technique (without bypass and open-heart surgery) in which the new valve, loaded within a catheter, is delivered with radiographic guidance into the native valve. In the process, the old, diseased valve is displaced and the new TAVI valve is implanted within it. The first percutaneous aortic valve implantation was conducted in 200226 and, since then, more sophisticated delivery systems have been developed. 27 Based on data from 2007, the TAVI procedure takes around 2 to 2.5 hours. 28 Clinical advice is that procedure time may have reduced slightly since then in some cases, as a result of equipment development and increased familiarity with the procedure. TAVI may be undertaken in a number of ways.
Access routes
A number of approaches to percutaneous heart valve replacements have been developed. The femoral artery [retrograde transfemoral (TF)], subclavian artery, and transapical (TA; antegrade transapical) and direct aortic approaches are the most commonly used. 13 More recently, there has been increased uptake of the direct aortic route in TAVI cases with a concomitant reduction in the TA approach.
For the TF approach, a catheter, introduced through the groin, is passed up through the femoral and iliac arteries to the aorta and aortic valve. 13 There may be difficulty with this approach, especially where there is a high degree of atherosclerosis, because the catheter that must be passed through the iliac artery and aorta is large. However, good clinical outcomes have resulted in the TF approach being favoured by some cardiologists. 27 TA aortic valve replacement has been developed for patients who are unsuited to the femoral approach (unsuitable aortic or iliac artery anatomy), although it may be used more widely. 13 TA is conducted by a left thoracotomy incision without cardiopulmonary bypass but requires a general anaesthetic. 13 Alternative vascular access routes more commonly used for TAVI include the left subclavian and direct aortic routes. The latter is used in patients in whom there is no suitable TF or subclavian access. TAVI is normally performed under general anaesthesia although, for the TF approach, sedation and local anaesthesia are used in suitable patients. 10
The basis for selection of a route for TAVI is complex and involves consideration of many factors relating to access to the vessel site, the extent of disease in the peripheral vasculature and other factors such as respiratory status. 28 There also appears to be a certain degree of subjective influence. For example, in the European Placement of AoRtic TraNscathetER Valves (PARTNER) trial, rates of TF versus TA varied widely between centres, with TF being used in 16.7–66.7% of patients in different centres. 28
Although it is likely that patient characteristics will determine which approach is more appropriate, currently there is no evidence to suggest whether or not one approach is better. From a systematic review of the literature,13 rates of implantation success for femoral and TA approaches (93% and 94% respectively) and 30-day survival (90% and 88% respectively) appear similar. It is recommended that decisions are based on the expertise of those conducting procedures and the condition of the patient. 10
Transcatheter aortic valve implantation valves
Currently, there are two valves in clinical use for TAVI: the Edwards SAPIEN Transcatheter Heart Valve (Edwards Lifesciences, Irvine, CA, USA) and the Medtronic CoreValve ReValving System (Medtronic, Minneapolis, MN, USA). 13 These valves are implanted in different ways. The balloon-expandable (SAPIEN) valve is delivered retrogradely through a steerable guiding catheter through the aorta to the aortic annulus. The CoreValve is a self-expanding device loaded within a special delivery catheter. The valve is delivered using standard transcatheter technique and advanced into the aortic annulus and deployed by controlled self-expansion. 27 Both valve types may be implanted using the TF approach. The CoreValve device can also be inserted via the left subclavian and direct aortic routes. The Edwards SAPIEN valve can be inserted via the TA route and in some cases via the direct aortic route. 27 There is no consensus as to the comparative effectiveness of these types of valves. In a systematic review of TAVI studies, rates of implantation success and 30-day survival appeared similar for both and no definitive conclusions could be drawn. 13 As the number of procedures increases, further investigation will be possible. The only real difference so far apparent between the valves is a higher proportion of patients requiring pacemaker implantation after receiving CoreValve. 29
Replacement aortic valves used for transcatheter implantation vary in size and an appropriate size is selected to match the size of the patient. Prosthesis–patient mismatch (PPM) describes the condition when the prosthetic valve that has been fitted is too small in relation to the size of the aortic annulus. 30 The degree of mismatch may be severe or moderate depending on the size of the indexed effective orifice area (IEOA); an IEOA of ≤ 0.85 cm2/m2 represents a moderate mismatch and an IEOA of < 0.65 cm2/m2 represents a severe mismatch. 15,30 A study of patients undergoing TAVI successfully found that 16% of patients had severe and 23% had moderate PPM following the procedure. 30 PPM may be encountered following TAVI or SAVR. Based on haemodynamic data, it is generally believed that PPM is less of a problem in TAVI than in SAVR. From their systematic review of SAVR PPM studies, Urso et al. 15 concluded that severe PPM could be a predictor of short- and mid-term mortality among patients undergoing SAVR and that a moderate PPM was only likely to be a predictor of early- and mid-term mortality in patients with poor ejection fraction. As severe mismatch appears to affect a reasonable proportion of TAVI patients, and there is reasonable evidence that it affects risk of mortality, the selection of the most appropriate sized valve for TAVI is important. This is also important to minimise the incidence of paravalvular leakage and aortic root rupture.
As this is a relatively new procedure, the long-term durability of TAVI valves is also currently unknown. 10 However, with the potential use of TAVI in a wider range of patients and the increasing length of postprocedural life expectancy, valve durability may become an important factor for consideration.
Outcomes
In centres experienced in conducting TAVIs, procedural success may be around 90% or more and closely linked to experience, with greater learning resulting in better patient selection and outcomes. 10 Some studies have shown high rates of success and, in a systematic review of TAVI, more recent studies showed procedural mortality rates of 0–10%. 13 Thirty-day mortality may range from 5% to 18%. 10,13,29,31,32
Studies of long-term mortality show 2-year survival rates of 70–80%. 10,29 In most TAVI studies, the majority of patients are > 80 years of age and rates of comorbidities are high. 10,29 Thus, long-term mortality rates cannot be directly compared with those from SAVR series. 31 It is also unclear to what extent rates of observed long-term survival depart from the natural rate of survival in patients of this age and health status.
Other important outcomes include paravalvular regurgitation, pacemaker requirement, effects on quality of life (QoL) and adverse events. Moderate to severe paravalvular regurgitation is higher in TAVI than in SAVR patients at 30 days, 1 year and 2 years. 33
Rates of pacemaker requirement appear to be somewhat higher in TAVI than in SAVR patients. 33 Furthermore, results from the UK TAVI registry showed pacemaker requirement of 24% for CoreValve and 7% for Edwards SAPIEN. 29
Transcatheter aortic valve implantation is thought to give important improvements in QoL. Studies have shown significant improvements in QoL from baseline following TAVI34 and significant improvements compared with control patients receiving medical therapy (additional data provided by US PARTNER study investigators on request14). Evidence suggests that intervention with TAVI improves QoL. However, the degree to which improvements are related to an intervention being conducted [some medical patients also showed sustained improvement in QoL (additional data14)] and the degree to which improvements would bring patients' QoL in line with that of people who had not suffered from AS are uncertain.
Although TAVI has shown good outcomes in terms of overall survival and QoL, some associated adverse outcomes have been highlighted:10,29
-
major vascular complications with the TF approach compared with other routes
-
long-term consequences of paravalvular leaks, even if mild to moderate regurgitation is considered not to have significant consequences in the short term
-
atrioventricular block, the incidence, timing and predictors of which need to be identified more precisely.
Current treatment guidelines
In 2008, NICE published its initial guidance for the use of TAVI in patients with AS; this was updated in 2012. As well as defining the relevant patient groups (see Transcatheter aortic valve implantation, Patients), the following guidance was given:25
Clinicians wishing to undertake TAVI for patients with aortic stenosis for whom SAVR is considered suitable but to pose a high risk. . .should take the following actions.
Inform the clinical governance leads in their Trusts.
Ensure that patients understand the risk of stroke and death, and the uncertainty about the procedure's efficacy in the long term. Provide them with clear written information. In addition, the use of NICE's information for patients (‘Understanding NICE guidance’) is recommended.
Patient selection should be carried out by a multidisciplinary team including interventional cardiologists, cardiac surgeons, a cardiac anaesthetist and an expert in cardiac imaging. The multidisciplinary team should determine the risk level for each patient.
TAVI is a technically challenging procedure that should be performed only by clinicians and teams with special training and experience in complex endovascular cardiac interventions. Units undertaking this procedure should have both cardiac and vascular surgical support for emergency treatment of complications.
NICE encourages further research into TAVI for aortic stenosis. In particular, NICE encourages clinicians to enter all suitable patients into the UK TAVI trial. Information from research trials that will be useful for future guidance includes patient selection criteria and comparisons between TAVI and SAVR in patients who would be suitable for either procedure. Outcomes should include incidence of stroke and other adverse events, symptom relief, quality of life, occurrence of aortic regurgitation, and valve durability in the short and long term.
Other treatment-related guidance has been issued by the European Society of Cardiology (ESC), European Association of Cardio-Thoracic Surgery and the European Association of Percutaneous Cardiovascular Interventions. Guidelines have been set out for the use of SAVR, balloon valvuloplasty and medical treatments2 and TAVI10 in patients with severe AS. The following is recommended:
For surgical aortic valve replacement:
-
Early valve replacement in all symptomatic patients with severe AS who are otherwise candidates for surgery.
-
Early elective surgery, at the asymptomatic stage can only be recommended in selected patients, at low operative risk.
For balloon valvuloplasty:
-
Balloon valvuloplasty can be considered as a bridge to surgery in haemodynamically unstable patients in whom surgery is associated with high risk or in patients with symptomatic severe AS who require urgent major non-cardiac surgery.
-
Occasionally, balloon valvuloplasty could be considered as a palliative measure in individual cases when surgery is contraindicated because of severe comorbidities.
For medical management:
-
Patients who are unsuitable candidates for surgery may be treated with digitalis, diuretics, ACE inhibitors or angiotensin receptor blockers if they are experiencing heart failure.
For transcatheter aortic valve implantation:
-
Patient selection should involve a multidisciplinary consultation between cardiologists, surgeons, imaging specialists, anaesthesiologists and possibly other specialists if necessary.
-
TAVI is indicated in patients with pure or predominant AS.
-
TAVI should be performed only in patients with severe AS.
-
At present, TAVI should be proposed only for patients with severe symptoms that can definitely be attributed to valve disease.
-
TAVI should currently be restricted to patients at high risk or in whom surgery is contraindicated.
-
It is premature to consider using TAVI in patients who are good surgical candidates.
-
TAVI should not be performed in patients whose life expectancy is < 1 year, and who should be managed conservatively.
Current policy and demand
In 2005–6 there were 10,396 hospital diagnoses of AS in England. 35 If it is assumed that this incidence is typical and that most of these cases will, at some point, go on to present as severe AS, it can be estimated that around 10,000 people are assessed for severe AS each year.
A position statement, published in November 2010 by the British Cardiovascular Intervention Society (BCIS) and the Society for Cardiothoracic Surgery (SCTS),36 states that ‘TAVI should currently be reserved for patients who have been considered by a multidisciplinary team (including 2 surgeons and 2 interventional cardiologists) who consider the risk/benefit ratio of open heart surgery and TAVI to favour TAVI’.
A large number of patients with severe AS may be at low procedural risk and be referred directly for SAVR. However, for patients considered to be high risk for SAVR, the projected use of TAVI is unclear. For this latter patient population, the balance of treatment with SAVR and TAVI may depend on many factors (see Choice of treatments), some of which will change with time. The number of TAVI procedures in the UK has increased, from 67 people in 2007 and 272 in 2008, to 533 in 2009 (P Ludman, UK TAVI Registry presentation on behalf of UK TAVI registry group, 2010, personal communication). TAVI is currently used relatively infrequently and it appears likely that, although the exact uptake is unclear, the number of TAVI procedures in the UK is likely to rise over time.
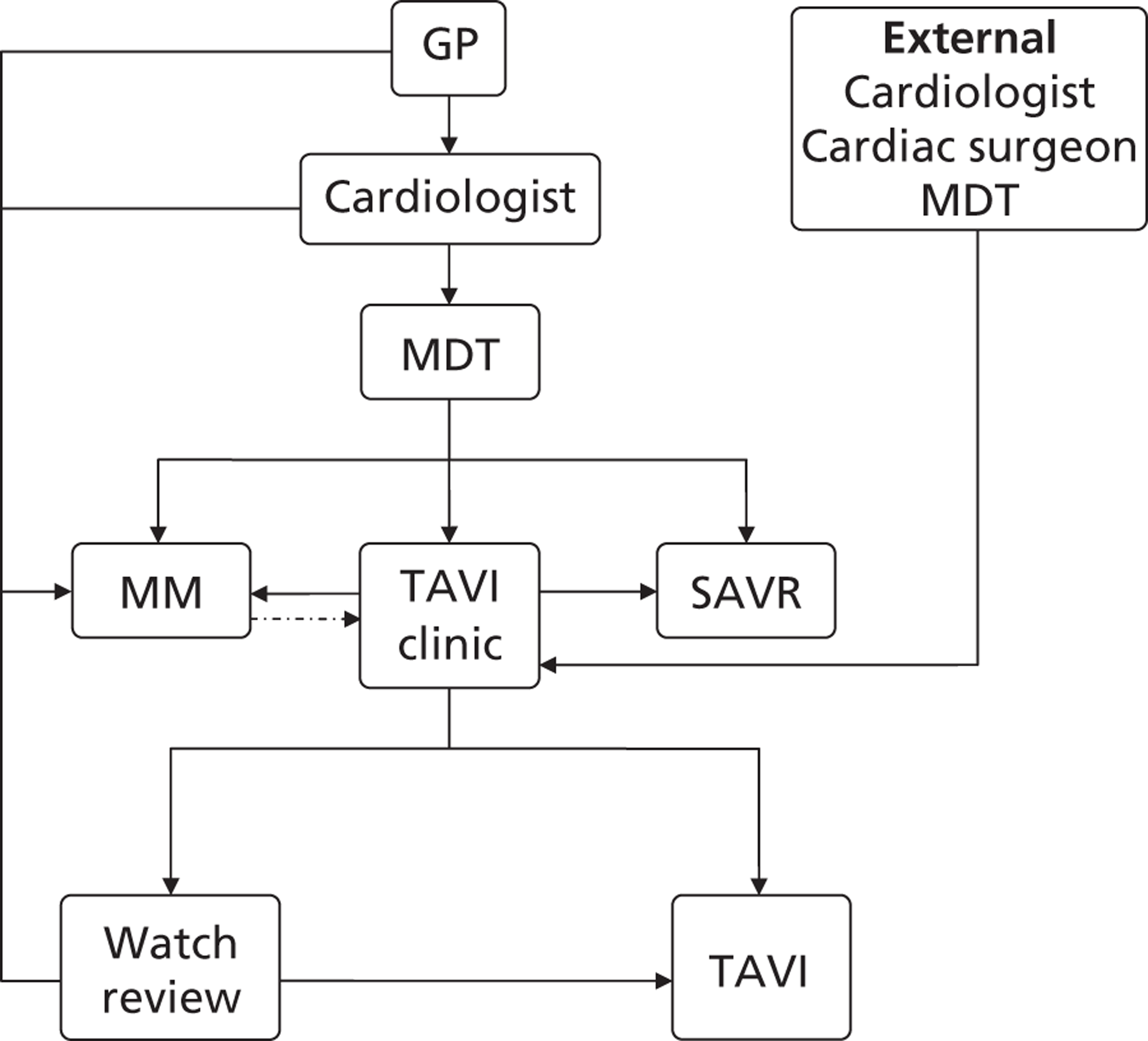
Patient pathway
The patient pathway is likely to vary between centres and may depend on staff resources and the length of time that centres have been undertaking SAVRs or TAVIs. Referral may often take the following course. Patients with pre-diagnosed AS may be under the watch of their general practitioner (GP). On the development of symptomatic disease that is judged to be severe, or on the initial presentation of a patient with severe symptomatic AS, patients may be referred to a cardiologist for further/initial investigation and assessment. If the cardiologist believes further intervention to be appropriate, patients are referred to a multidisciplinary team (MDT) to discuss treatment options. The MDT usually consists of cardiology and surgical specialists and an anaesthetist, with expertise and experience in the treatment of AS and TAVI/SAVR techniques. Together, this team discusses each patient individually and comes to a consensus decision about the best course of treatment.
Where patients are deemed eligible for conventional SAVR, they are referred for surgical assessment. Where patients are considered too high risk for SAVR, they may be considered for medical management or, if they are judged as potential candidates for TAVI, referred to a TAVI clinic. Here, there is further assessment to determine the suitability of TAVI and, where appropriate, the best mode of entry. In some cases, where further discussion has deemed that a patient is fit to undergo surgery, these patients may be referred back for SAVR. Where doctor assessment or patient preference deems that TAVI is not appropriate or desirable, patients may be referred for medical management. Patients may be placed under review by the TAVI clinic and it is possible that balloon aortic valvuloplasty may be used at this point as a proof of concept that TAVI may be undertaken successfully.
There may be considerable fluidity in the referral process between medical and surgical teams, with patients originally assigned to one intervention (medical management, TAVI or surgery) subsequently being referred for another. Patient preference will also play a role in the referral process and may ultimately be the factor that determines which treatment is undertaken; for example, a patient offered TAVI might decline it and accept medical management instead. Figure 1 outlines an example of a possible referral pathway for patients with severe AS.
FIGURE 1.
Example patient referral pathway for patients with severe AS.
Choice of treatments
A variety of factors are taken into account in the decision-making, and the choice between SAVR, TAVI and medical treatment depends on the relative risks associated with procedures/treatments, the potential gains in terms of extended lifespan and QoL, and the preferences of the patient and medical team. Factors governing these decisions are likely to include risk scores, comorbidities, patient age, patient preference, and the expertise and preference of medical staff.
Risk scores
In order to guide decisions around surgical intervention, several risk-scoring systems have been developed to predict operative mortality. The two systems for estimating risk for SAVR in most common use37 are the EuroSCORE38,39 and the Society of Thoracic Surgeons Predicted Risk Of Mortality (STS-PROM)40 (http://209.220.160.181/STSWebRiskCalc261/de.aspx). There are two forms of EuroSCORE: the simple (or additive) version and the logistic EuroSCORE (LES). Details of the components of these scoring systems are given in Appendix 1. In Europe the EuroSCORE is more commonly used, whereas in the USA the STS-PROM score is more commonly used.
Risk-scoring systems are commonly used but investigators have highlighted their limitations for accurately determining individual patient risk and the EuroSCORE has been particularly criticised. 10,37,41 For the development of the EuroSCORE system, patients undergoing SAVR represented only a proportion (17%) of the patient population and other patients underwent different cardiac operations. 38 However, for the development of the STS-PROM score, patients undergoing SAVR for AS were modelled separately. 40 There are particular concerns around the validity of risk-scoring systems in high-risk AS patients as these patients are particularly heterogeneous in terms of their comorbidities. 10 Studies suggest that the EuroSCORE may predict operative mortality to be more than three times that of observed mortality. 37,41 All risk-scoring systems have been shown to have failings, but some authors suggest that the STS-PROM system provides more accurate estimates of operative risk than the EuroSCORE system. 37,42–44
Despite their limitations, risk scores may be useful guides as they incorporate many of the factors that are important predictors of SAVR procedural risk and therefore help to simplify the estimation of overall risk. For severe AS patients with low-risk scores and without other comorbidities, SAVR is likely to be the appropriate course of intervention. In these patients, risk scoring may be sufficient to give a reasonable risk assessment and, given the recommendations to use SAVR where possible, decisions may be reasonably straightforward. Equally, in patients who have extremely high-risk scores and substantial comorbidities, any interventional procedure (SAVR or TAVI) may be considered inappropriate and medical management may be the treatment of choice.
However, in many patients with elevated risk scores and/or some comorbidities, the choice between surgical intervention, TAVI and medical management is less straightforward. A cut-off of LES > 20% and STS-PROM score > 10% are often used to define patients in whom surgery may be contraindicated. 45 Scores of this type have been used as enrolment criteria in randomised controlled trials. 14 However, many surgeons recognise that this is unrealistic in everyday practice and are willing to operate on patients of higher estimated surgical risk. As such, there is unlikely to be a suitable cut-off in risk score that can be used to determine whether surgery, TAVI or medical therapy is more appropriate. In part, this is due to the nature of scoring systems, but also the important impact of additional morbidities and age on procedural risk, as well as estimated life gain and consideration of the preferences of the patient and medical team.
Where estimated procedural risk is thought to be too high for SAVR, TAVI may be appropriate and provide a less invasive intervention. However, estimations of surgical risk from scoring systems cannot be extrapolated to procedural risks associated with TAVI. 41 In a study of estimated procedural risk and outcome, there was no difference in LES between patients who died during TAVI (LES 23.2 ± 15) and those who survived (LES 25 ± 15). 41 Piazza et al. 46 also showed that the EuroSCORE had no predictive power [area under the receiver operating characteristic (ROC) curve 0.49 (area under curve of 0.5 represents no predictive power, i.e. represents a 50% chance of rightly predicting TAVI procedural mortality and equal to random chance)] and that the STS-PROM system performed poorly (area under ROC curve 0.69) in determining which patients would suffer a fatal event due to the TAVI procedure. Currently, there is no risk-scoring system for predicting procedural mortality associated with TAVI. 37 There may be important risk factors for TAVI that are not associated with SAVR and risk factors that are important for SAVR that do not influence TAVI. As TAVI may increasingly be used in patients potentially eligible for surgery, the development of a TAVI scoring system may be important to weigh up the relative risks of each procedure.
Comorbidities
Risk-scoring systems are useful for making decisions about the best course of treatment, but the influence of some comorbidities may not be fully taken into account. These factors, despite being incorporated into the risk calculation, may also be considered independently by those assessing surgical risk. For example, increased operative mortality may be associated with age, advanced stage of heart disease, whether attested by heart failure, NYHA class IV, decreased left ventricular ejection fraction, atrial fibrillation, chronic pulmonary disease, renal insufficiency, associated atherosclerosis of coronary or peripheral arteries, and previous bypass or valve surgery. 2,18 These factors may have a greater influence than that predicted by risk-scoring systems and be considered independently in the decision-making process.
There may also be important factors that are not included in surgical risk scores due to their rarer occurrence but which may impact procedural risk, for example porcelain aorta, liver disease, prior radiation therapy, neurocognitive dysfunction, chest wall deformity, highly compromised respiratory function and frailty. 18,37,45 When considering TAVI, adequate vascular access is one of the most important determinants of procedural success and, where there is tortuosity with marked calcification, it can be difficult to move the large sheaths down the aorta. 45 The presence of left ventricular dysfunction may exclude patients from undergoing TAVI and, where there is very severe left ventricular dysfunction and low left ventricular contractility reserve, patients may be less able to respond to the postprocedural drop in blood pressure and cardiac output. 45 The decision around the use of TAVI and the route of administration (TF or otherwise) is also taken after consideration of a number of patient anatomical characteristics:
-
size of ascending aorta, horizontal aorta
-
sigmoid septum
-
calcification of aortic valve aortic root
-
size of iliac and femoral arteries
-
calcification of aorta
-
tortuosity of iliac and femoral arteries.
Associated procedures
In many patients with severe AS, other surgical procedures, such as CABG or mitral valve replacement, are indicated. CABG is conducted in up to 50% of patients undergoing SAVR. 19–21,47 It is unlikely that TAVI would be performed alongside any procedure involving open chest surgery, whereas some other procedures such as those involving percutaneous access could be performed before or during TAVI.
Patient age
Patient age has implications for the most appropriate mode of treatment since the benefits in terms of life-years gained for older patients are likely to be less than for younger patients. However, age in itself may not be a contraindication for invasive procedures. Severe AS increasingly presents in patients over 80 years but, although many of these patients are not referred for surgery, SAVR can potentially extend overall survival and improve QoL. 2 Good outcomes have been observed in older patients undergoing SAVR20 and it is generally considered that age alone should not be used as a criterion for surgical eligibility. 2,4,48 TAVI has primarily been used in patients considered ineligible for surgery due to age and comorbidities. Studies of these patients (often mean age > 80 years) have shown low rates of procedural and 30-day mortality and reasonable long-term survival. 10,13
SAVR may be the appropriate treatment option for older patients with severe AS with no comorbidities. However, if life expectancy is lower, either SAVR or TAVI may be inappropriate. It has been recommended that TAVI is not conducted in patients whose life expectancy is < 1 year. 10 Similarly, for SAVR, where life expectancy is short, the appropriateness of invasive intervention is considered in terms of the balance between procedural risks and the potential gain in survival and QoL.
Patient preference
Up to one-third of elderly, symptomatic AS patients are not referred for surgery because of high surgical risk or patient refusal. 3,13,23 As decisions are likely to result from combined clinician/patient discussion, it may be difficult to accurately estimate the numbers of procedures avoided solely due to patient preference. In studies of patients with severe AS, it has been estimated that 14–23% did not undergo SAVR solely because of patient refusal49–52 and investigators have highlighted the importance of clinician–patient communication in conveying to older patients the potential survival benefits associated with SAVR. 49 The availability of TAVI may in future provide a procedure agreeable to those patients declining the opportunity to undergo SAVR, although this is currently contrary to UK guidelines (see Current treatment guidelines).
Referral for surgery
The most appropriate course of treatment is often unclear, and there is likely to be some degree of subjectivity in the decision-making process. For example, in the US PARTNER trial of patients assessed to be inoperable and randomised to medical therapy, 10% went on to undergo SAVR in the following year. 14 In another study, of patients who had not initially been proposed for surgery due to comorbidities, 75% subsequently went on to have SAVR in the following months. 52 Decisions may be influenced by the opinions and preferences of the assessing physician or surgeon. A limiting factor for SAVR may be the referral of patients for surgical assessment. In a study of patients with severe AS, 69% were not referred for surgical assessment and did not undergo SAVR. 53 All of the other patients, who were assessed by cardiothoracic surgeons, went on to have SAVR,53 suggesting that surgical referral was the limiting factor in determining patient treatment.
With the development of the TAVI procedure, it is now recommended that MDTs assess each patient to determine their suitability for SAVR, TAVI or medical management. 25 With the increasing use of MDTs for patient assessment, surgical referral may no longer be a limiting factor for SAVR and the results of this have been observed. When comparing rates between the years before and after the introduction of TAVI, US investigators noted a 64–74% increase in rates of surgical referral and a 44% increase in SAVRs, whereas the number of un-operated AS patients fell from 51% to 41%. 51
The impact of TAVI on the number of SAVRs in a UK centre has also been studied. 54 At the University Hospital of South Manchester (UHSM), in the 2 years after the introduction of the TAVI service, there was a 37% increase in the numbers of SAVR procedures, as shown in Figure 2.
FIGURE 2.
Surgical aortic valve replacement activities at the UHSM from January 2005 to December 2009. Redrawn from Grant et al. 54
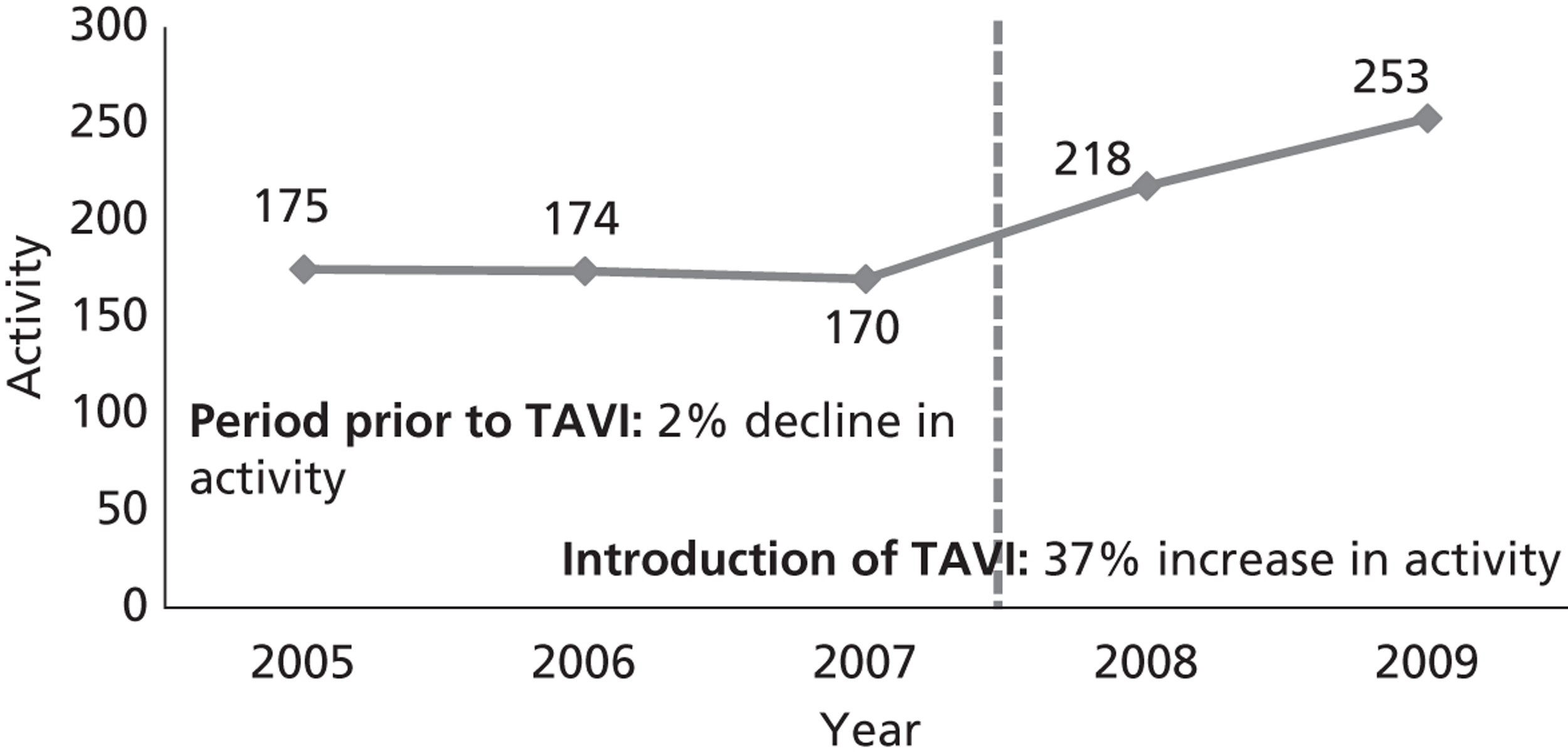
Study investigators concluded that this increase was largely due to the increased awareness of treatment options and referral for SAVR. The authors also highlight the importance of the MDT in this process. Patients are considered first for SAVR, before being referred for TAVI. However, in practice, some patients are initially considered ineligible for SAVR and referred for TAVI, but after reassessment by the MDT may subsequently receive SAVR. 54 This has been demonstrated in other series of patients referred for TAVI, of whom 15%55 or 20%56 went on to undergo SAVR.
The increased use of MDTs in the setting of AS treatment decision-making may have had a beneficial impact in providing balanced, multidisciplinary assessments. It has been suggested that surgeons be the gatekeepers of TAVI, assessing the risk of standard SAVR and referring for TAVI if they believe the risks of conventional surgery to be too high. 57 However, assessment by MDTs with both surgical and medical expertise may more effectively bring to light all possible issues for selecting the best mode of treatment.
Post-transcatheter aortic valve implantation/surgical treatment and surveillance
Immediately after TAVI has been conducted, aortography and, when available, transoesophageal echocardiography or transthoracic echocardiography are performed to assess whether or not there is aortic regurgitation, to assess the patency of the coronary arteries, and to rule out complications such as haemopericardium and aortic dissection. 10 Haemodynamic measurements are also assessed using pressure recordings and/or echocardiography. 10
It is recommended that, following TAVI, patients should stay in intensive care for at least 24 hours and be closely monitored for several days, especially with regard to haemodynamics, vascular access, rhythm disturbances and renal function. 10 UK data show that the mean duration of hospital stay following SAVR is 10–11 days. 58 In the case of elderly patients (> 80 years), the average length of postprocedural hospital stay is around 13 days. 16
After discharge, patients may be referred for a programme of rehabilitation. This consists of exercise sessions aimed at reducing symptoms associated with AS and improving physical performance. 59,60 However, rehabilitation may not be routinely offered to patients who have undergone aortic valve replacement (Birmingham cardiac rehabilitation services, 2011, personal communication) and, where programmes are recommended, uptake may be low. 16
Chapter 2 Rationale and objectives
In light of the increasing use of TAVI in many UK institutions, it is important to establish the cost-effectiveness of this procedure and to assess in which types of patients it may be cost-effective. The aim of this report was to present any existing literature on the cost-effectiveness of TAVI and to conduct a cost-effectiveness analysis to model the costs and benefits of scenarios where the availability of TAVI varies.
This report aims to address the following questions:
-
What does the literature say regarding the cost-effectiveness of TAVI?
-
Is making TAVI available to patients who are contraindicated or high risk for SAVR a cost-effective policy (compared with TAVI being unavailable)?
-
Is TAVI cost-effective under the current policy, i.e. limited to patients unsuitable for SAVR?
-
Is TAVI cost-effective if the threshold for indication is lowered (to include patients at lower risk for SAVR)?
-
Is TAVI availability a patient prioritisation policy?
-
What is the future potential for this technology?
In order to address these objectives, a systematic review of cost-effectiveness studies was conducted and a cost-effectiveness model was built to model the possible scenarios for TAVI availability as follows:
-
TAVI unavailable
-
TAVI available for patients contraindicated or high risk for SAVR
-
TAVI available for patients both suitable and unsuitable for SAVR.
Chapter 3 Cost-effectiveness review
Searches
Initial scoping searches were carried out in October 2010 to assess the volume and type of literature relating to TAVI for AS. A comprehensive search of the literature for studies of the costs, cost-effectiveness and QoL associated with the use of TAVI was conducted without language restrictions (see Appendix 2). The search was an update to the Bazian report, published in May 2008,35 and searches were therefore undertaken from 2007 to November 2010.
-
Bibliographic databases: MEDLINE (Ovid) 1950 to November week 3 2010, EMBASE (Ovid) 1980 to 2010 week 46, The Cochrane Library (Wiley) Health Technology Assessment (HTA), Database of Abstracts of Reviews of Effects (DARE) and NHS Economic Evaluation Database (NHS EED) databases 2010 Issue 4 and the York Centre for Reviews and Dissemination (CRD) HTA, DARE and NHS EED databases (October 2010).
-
A range of guideline resources including National Library of Guidelines, National Guidelines Clearinghouse and Guidelines International Network.
-
UK Clinical Research Network (UKCRN), Current Controlled Trials metaRegister and International Standard Randomised Controlled Trial Number (ISRCTN), World Health Organization (WHO) International Trials Register and ClinicalTrials.gov for ongoing trials.
-
Conference proceedings and annual meetings abstracts (BCIS, ESC, American College of Cardiology, American Heart Association), British Library's Electronic Table of Contents (Zetoc) conference proceedings and the Index to Theses for unpublished material.
-
Internet/grey literature searches.
-
Manufacturers' websites.
-
Consultation with experts.
In addition to this search, any studies relating to cost-effectiveness obtained through the more general search for model parameters (see Chapter 4, Identifying studies for model parameters) were identified and included in the review of cost-effectiveness studies.
Study selection, data extraction and quality assessment
The inclusion and exclusion criteria applied to the economic searches are shown in Table 1.
| Study design | Cost–consequence analysis, cost–benefit analysis, cost–utility analysis, cost studies, QoL studies |
| Population | Patients with severe AS who are considered high risk for undergoing surgery |
| Intervention | TAVI |
| Comparator | Medical management or SAVR |
| Outcome | Survival-years, QoL estimates, cost estimates, cost-effectiveness |
Title or abstract screening was undertaken by an experienced health economist. Inclusion and exclusion criteria were applied to abstracts, and where studies appeared potentially relevant full-paper copies were obtained. Hard copies of the selected studies were then further inspected to determine inclusion or exclusion from the review. Data extraction of relevant cost-effectiveness studies was conducted to obtain details of:
-
study characteristics: study question, form of economic analysis, population, interventions, comparators, perspective, time horizon and modelling used
-
clinical effectiveness and cost parameters, such as effectiveness data, health state valuations (utilities), resource-use data, unit cost data, price year, discounting, key assumptions and productivity costs
-
results and sensitivity analysis.
Eligible economic evaluation studies were assessed regarding their applicability and methodological quality using the quality assessment checklist by Drummond et al. 61
Results
No published evidence was found regarding the cost-effectiveness of TAVI compared with SAVR or medical management. A number of unpublished developmental economic models were identified. These were conference presentations62–64 and could not therefore be formally assessed. A report by Murphy et al. 62 was used for an advice statement by the Scottish Health Technologies Group. 65 However, the published abstract62 does not give any numerical estimates of cost-effectiveness. Watt et al. 63 quote a base-case incremental cost-effectiveness ratio (ICER) of £23,357.94 per quality-adjusted life-year (QALY) for TAVI compared with medical management based on a 10-year time horizon, with a probability of 94.6% that TAVI is cost-effective at a threshold of £30,000 per QALY. Diage et al. 64 used a 3-year time horizon and gave base-case ICERs of $7269 per QALY (£4700 per QALY) for TAVI against medical management, and $66,375 per QALY (£43,000 per QALY) for SAVR compared with TAVI.
A report by Bazian35 presents a partial economic evaluation (summary shown in Table 2), but only costs and outcomes of the use of TAVI are described and no comparison with alternative treatments is made.
| Author, year | Bazian Ltd, 2008 |
| Title | Percutaneous aortic valve replacement for severe aortic stenosis |
| Patient characteristic | Severe AS patients |
| Intervention | TAVI |
| Comparator | None |
| Form of analysis | Cost–outcome description |
| Model used | Local clinical and cost impact of PAVR in the region |
| Time horizon of model | 1 year |
| Cost year and currency | 2008, GBP |
| Base-case results | Cost of the procedure is given as approximately £18,000 |
A cost-effectiveness analysis based on the PARTNER trial Cohort B14 was published in journal form in 2012, beyond the date for the review above. For completeness, it should be noted that this US-based analysis gave base-case ICERs for TAVI against standard therapy of $50,200 (in 2012 it was approximately equal to £31,000) per life-year gained or $61,889 (£39,000) per QALY. 66
Chapter 4 Cost-effectiveness model
Model description
The purpose of the model is to assess the cost-effectiveness of TAVI compared with standard therapy in patients who require aortic valve replacement but are high risk or not fit for conventional surgery (SAVR). The model is built from a policy perspective. Accordingly, the decision options are TAVI available compared with TAVI not available. The model uses a 25-year time horizon – effectively covering all patients' remaining lifetime – to allow for differences in survival between the different options in the model.
It is not the purpose of this model to inform the decisions that must be made as part of the referral process; possible referral pathways have been described in Chapter 1, Current policy and demand. From the modelling point of view, it is assumed that some policy is in place from which it can – at least in principle – be determined what proportion of any patient group will be expected to follow any given pathway. Alternative referral policies are considered in the sensitivity analysis.
The model is structured in two parts: the first part relating to the selection of treatment and the second part relating to the results of treatment.
Selection of treatment
This part of the model is a schematic representation of the range of patient groups being compared. It takes the form of a decision tree, as illustrated in Figure 3. The first division in the tree represents the policy choice of whether or not TAVI is available. The whole patient group is taken to follow each of these choices in turn, but then subdivided at later nodes. It is convenient for modelling purposes to divide first by the underlying condition of the patient, even though this may not become known to any clinician until later (or, indeed, at all). Thus, patients are divided into those who are suitable for surgery and those who are unsuitable (too high risk and/or other reason such as suffering from porcelain aorta). The proportions following each of these branches must be the same for the two policy options (TAVI available or not available). Within these groups (suitable or unsuitable for surgery), patients are then divided according to the treatment actually selected. On clinical advice, and in the interests of simplicity, the possibility of balloon aortic valvuloplasty in the model has not been included.
FIGURE 3.
Selection of treatments for AS patients. The model structure takes the form of a decision tree. The first division of the tree represents the policy choice of whether or not TAVI is available. The next division is the true condition of the patient in terms of surgical risk. Patients then divide according to the treatment actually given. There are eight different pathways, labelled A to H. For example, pathway A represents the case where TAVI is available; the patient is suitable for, and receives, SAVR. Pathway B represents patients who are suitable for surgery, but receive TAVI. MM, medical management.
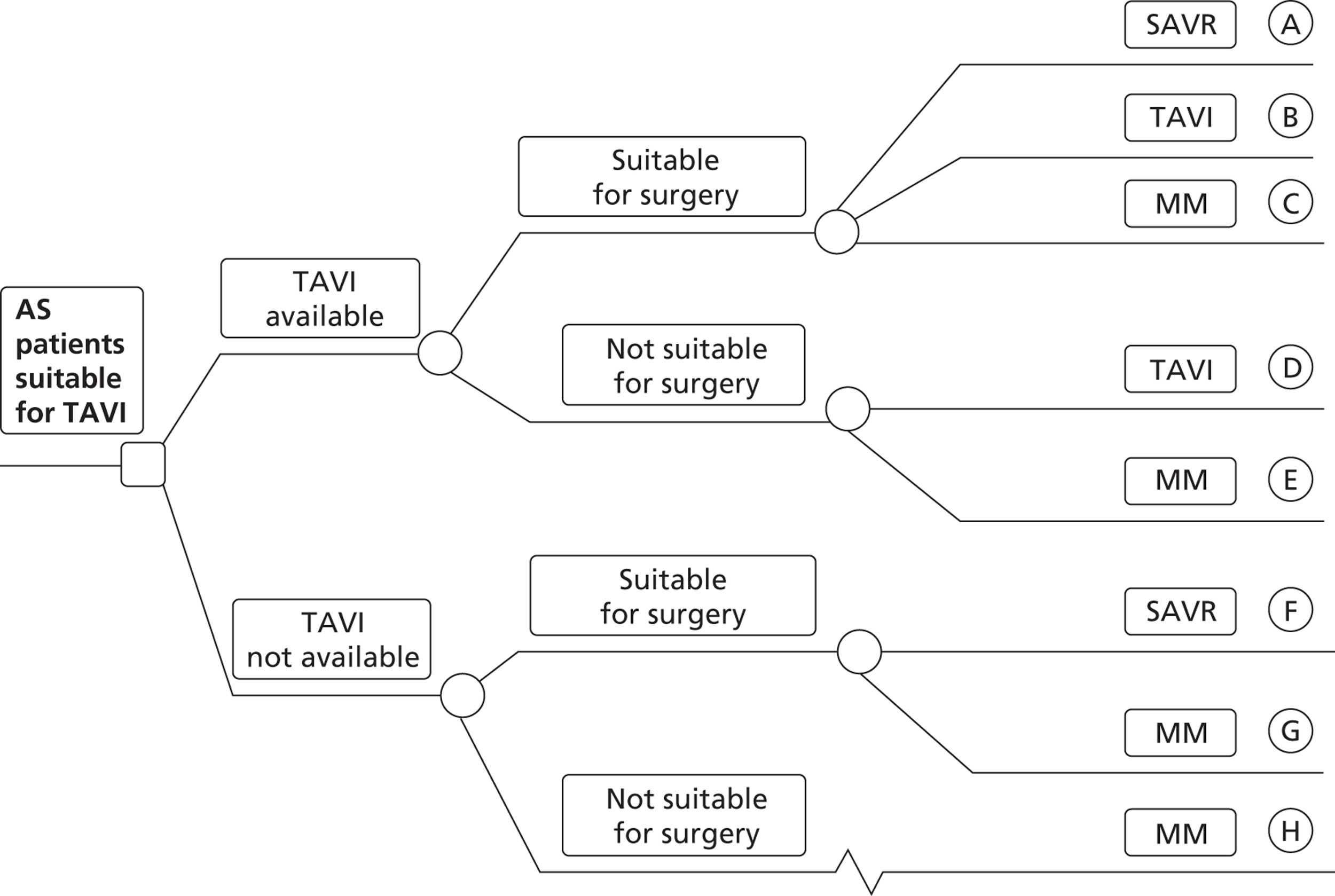
So, for example, in the absence of TAVI, patients are either suitable for SAVR or not. Those who are suitable for SAVR may receive it (group F) or may not for a reason other than clinical risk (group G), an example being patients who choose not to undergo such invasive surgery. This group (G) would still receive medical management in the absence of TAVI, as would those who are unsuitable for SAVR (group H).
If TAVI is available, patients suitable for SAVR could receive any of SAVR (group A), TAVI (group B) or – for similar reasons to those given above – medical management (group C). Those not suitable for SAVR could receive either TAVI (group D) or medical management (group E).
This grouping of potential patients allows for all possible policy options relating to the use of TAVI, including the possibility that TAVI could be offered as an alternative to SAVR in certain patients.
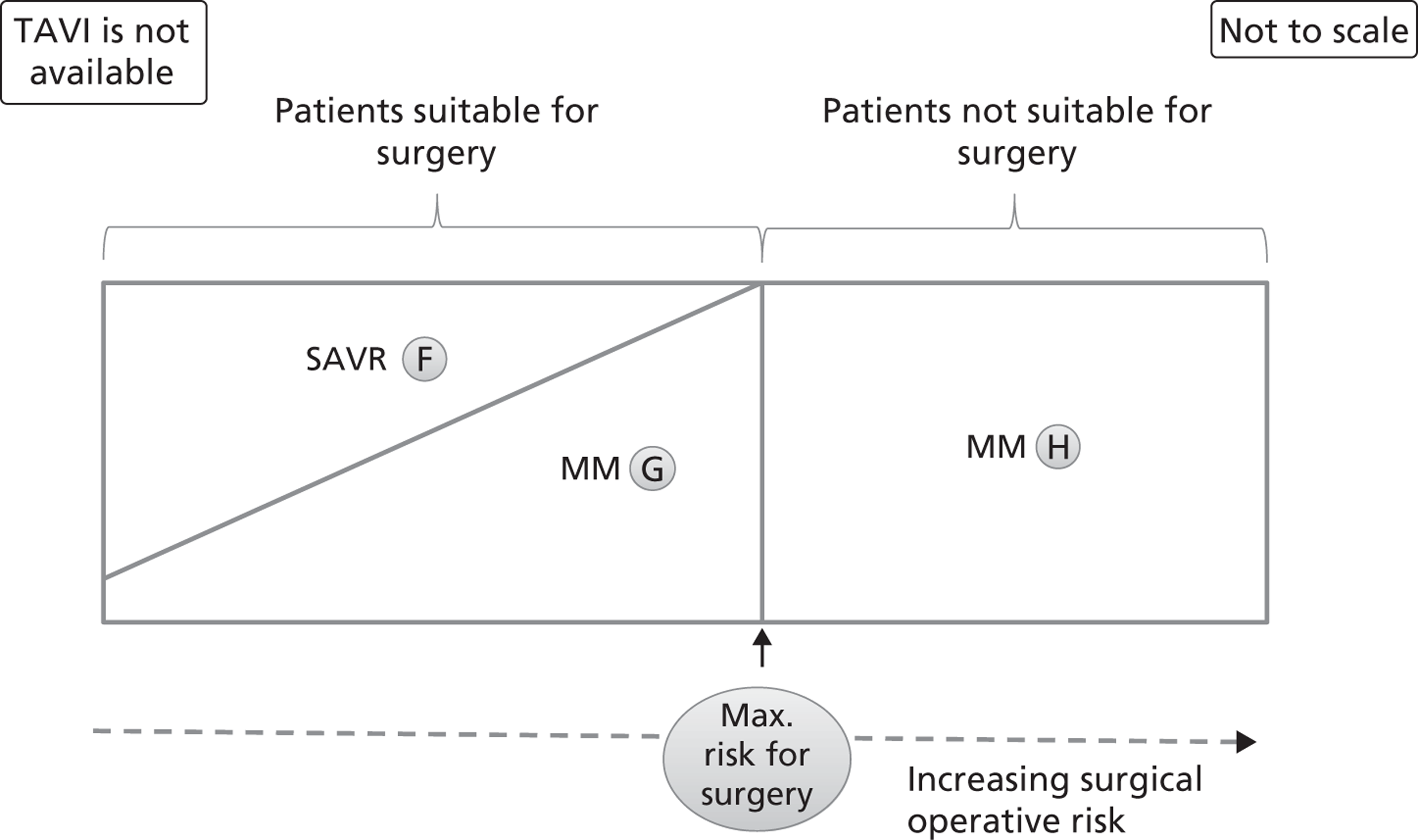
These populations can be envisaged diagrammatically in terms of SAVR risk. For example, Figure 4 shows the distribution of patients in the case where TAVI is not available (groups F to H). The area of each region approximates to the proportion of patients following each possible pathway. The horizontal scale represents schematically the proportion of patients in order of increasing surgical risk. It is stressed that this surgical risk is not intended to be simply the value of some risk score, but is intended to take account of clinical features and other personal characteristics. It is assumed that the probability of surgery being chosen decreases with increased operative risk and that surgery is never offered if the risk is above a threshold. The diagram is drawn to reflect the possibility that a patient at minimum surgical risk may still end up with medical management.
FIGURE 4.
Distribution of patients when TAVI is not available. This graph represents the possible clinical treatment options for AS patients in the absence of TAVI. The patients are distributed from left to right according to their increasing surgical operative risk, based on a risk score adjusted for individual patient characteristics. Patients suitable and unsuitable for surgery are differentiated by a cut-off value that is the maximum acceptable risk for surgery. Each area (labelled F to H) represents the approximate proportion of patients who follow the appropriate arm of the decision tree in Figure 3. MM, medical management.
The availability of TAVI can be incorporated into this diagrammatic framework. Figure 5 shows the distribution of patients when TAVI is available. Some patients suitable for SAVR may receive TAVI; this could include both patients who would receive SAVR in the absence of TAVI and some patients who choose not to undergo surgery. Some patients unsuitable for SAVR would receive TAVI rather than medical management. In the base-case analysis, this last group represents 90% of the patients receiving TAVI, a further 9% being those who would have received SAVR.
FIGURE 5.
Distribution of patients when TAVI is available subject to a minimum SAVR risk. This graph represents the possible clinical treatment options for AS patients when TAVI is available only for patients who are above a minimum surgical risk. The patients are distributed from left to right according to their increasing surgical operative risk, based on a risk score adjusted for individual patient characteristics. Patients suitable and unsuitable for surgery are differentiated by a cut-off value that is the maximum acceptable risk for surgery. Each area (labelled A to E) represents the approximate proportion of patients who follow the appropriate arm of the decision tree in Figure 3. MM, medical management.
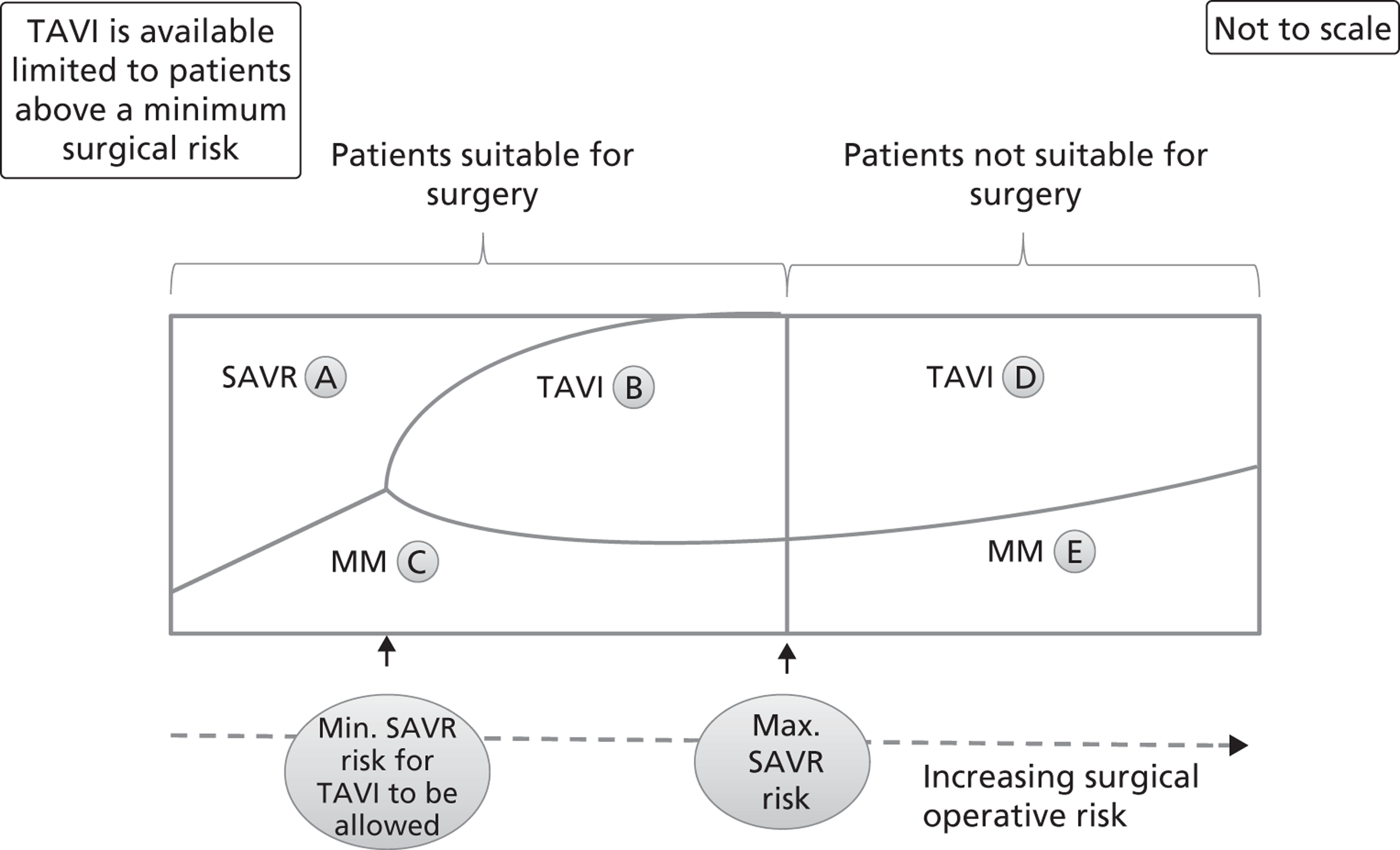
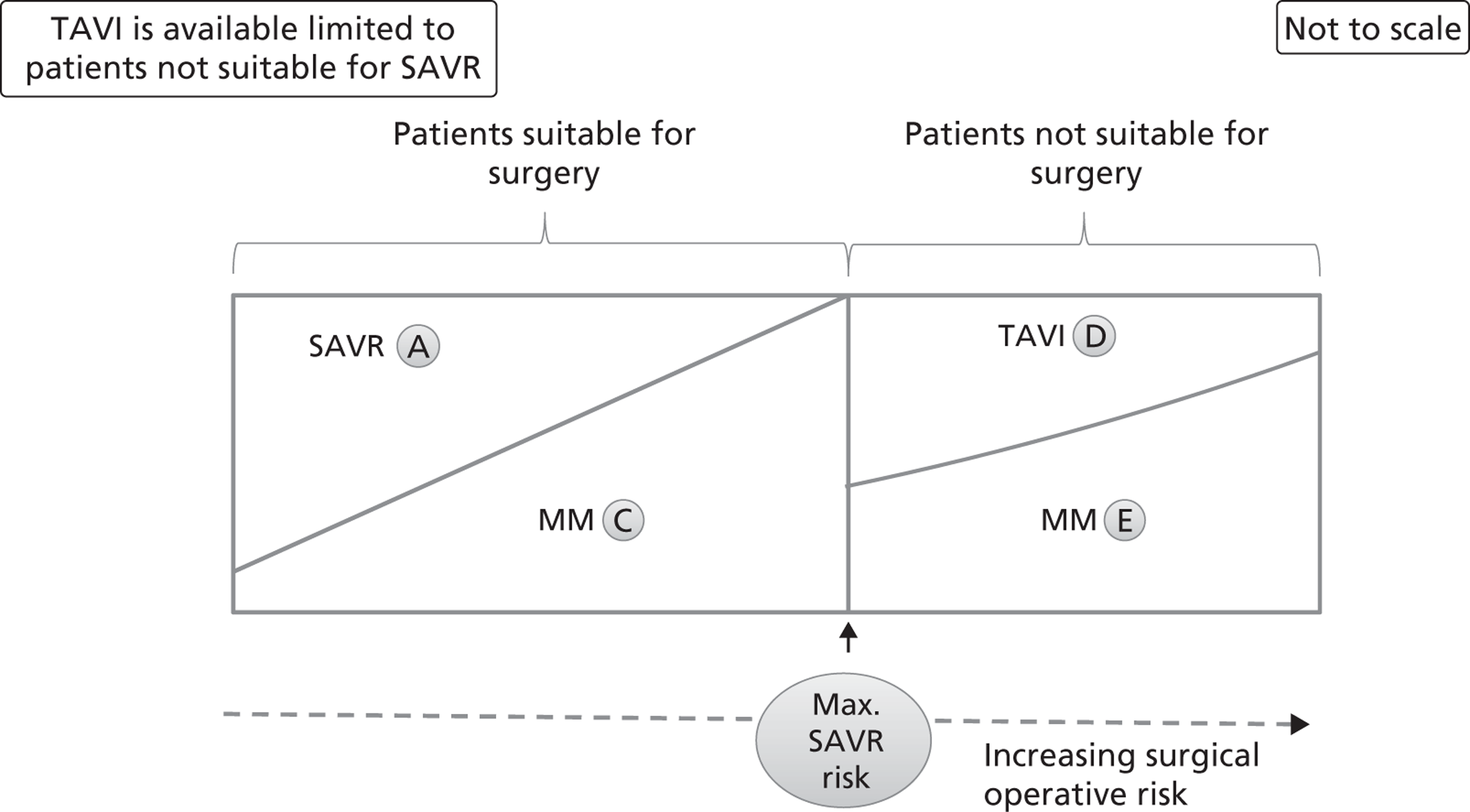
Figure 5 is drawn on the assumption that there is a threshold SAVR risk below which TAVI would not be allowed. This may be considered as a middle case where TAVI is available. Two extreme cases are possible. Removing the threshold SAVR risk and allowing TAVI to be used without restriction is illustrated in Figure 6. The opposite extreme, where TAVI is only available for patients in whom SAVR is contraindicated, is shown in Figure 7.
FIGURE 6.
Distribution of patients when TAVI is available with no restriction. This graph represents the possible clinical treatment options for AS patients when TAVI is available for all of them. The patients are distributed from left to right according to their increasing surgical operative risk, based on a risk score adjusted for individual patient characteristics. Patients suitable and unsuitable for surgery are differentiated by a cut-off value that is the maximum acceptable risk for surgery. Each area (labelled A to E) represents the approximate proportion of patients who follow the appropriate arm of the decision tree in Figure 3. MM, medical management.
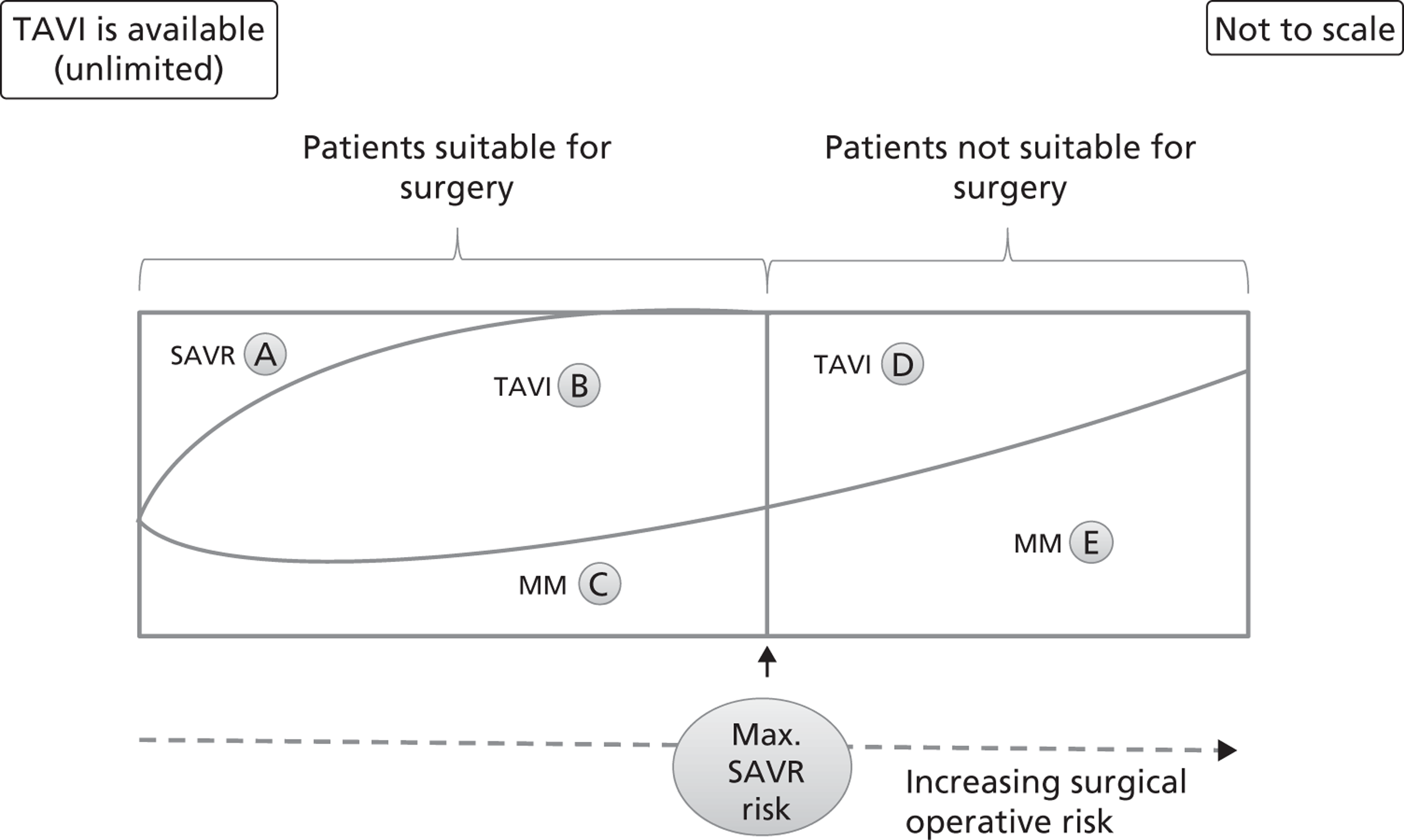
FIGURE 7.
Distribution of patients when TAVI is available only to patients in whom SAVR is contraindicated. This graph represents the possible clinical treatment options for AS patients when TAVI is available only to those for whom SAVR is contraindicated, and is shown as a theoretical extreme, which is unlikely to represent true practice. The patients are distributed from left to right according to their increasing surgical operative risk, based on a risk score adjusted for individual patient characteristics. Patients suitable and unsuitable for surgery are differentiated by a cut-off value that is the maximum acceptable risk for surgery. Each area (labelled A to E) represents the approximate proportion of patients who follow the appropriate arm of the decision tree in Figure 3. Note that compared with the distributions in Figure 5 and Figure 6, there is no group B in this diagram. MM, medical management.
Results of treatment
For each patient group, the results of treatment were calculated by estimating overall survival and survival free of hospitalisation. QoL scores were attached to the two possible health states hospital-free survival and other survival, the latter state including all surviving patients who had undergone at least one episode of hospitalisation after initial treatment. Monthly costs were attached to each health state, as well as a cost for first hospitalisation following initial treatment. For simplicity, further hospitalisation was not modelled explicitly, with the costs and QoL scores for the other survival state intended to represent an average of all patients in that state. As there is no explicit provision in the model for the effect of long-term adverse events on QoL, this is assumed to be included in the data on which the QoL calculations are based. Average monthly costs and QoL were accumulated to estimate the total expected costs and QALYs per patient, applying an annual discount rate of 3.5% to both costs and outcomes.
The modelling framework allows for separate estimates to be made for each of the patient groups described above and is shown in Figures 3–7. In practice, a single estimate was used for the two TAVI groups (B and D), and a (different) single estimate was used for the four medical management groups (C, E, G and H). However, it was possible to estimate the mortality separately for low and moderate-risk groups for SAVR, and this was used where appropriate to give separate estimates for the two SAVR groups (A and F).
Identifying studies for model parameters
A search was conducted to update the Bazian report, which was published in May 2008. 35 A systematic literature search for systematic reviews and primary studies of TAVI for AS without language restrictions was undertaken in November 2010 (see Appendix 2). Search strategies were based on the 2008 Bazian report searches35 and a date limit of 2007 to November 2010 was used to cover the period since publication of that report. The following sources were searched:
-
Bibliographic databases: MEDLINE (Ovid) 1950 to October week 2 2010 and 1950 to November week 3 2010, MEDLINE In-Process & Other Non-Indexed Citations (Ovid) at 24 November 2010, EMBASE (Ovid) 1980 to 2010 week 47, The Cochrane Library (Wiley) Cochrane Database of Systematic Reviews (CDSR) 2010 Issue 11, The Cochrane Library (Wiley) HTA, DARE and NHS EED databases 2010 Issue 4 and the York CRD HTA, DARE and NHS EED databases (October 2010) for background reviews and other studies as well as QoL studies, economic evaluations and cost-effectiveness models.
-
A range of guideline resources including National Library of Guidelines, National Guidelines Clearinghouse and Guidelines International Network.
-
UKCRN, Current Controlled Trials metaRegister and ISRCTN, WHO International Trials Register and ClinicalTrials.gov for ongoing trials.
-
Conference proceedings and annual meetings abstracts (BCIS, ESC, American College of Cardiology, American Heart Association), Zetoc conference proceedings and the Index to Theses for unpublished material.
-
Internet/grey literature searches.
-
Manufacturers' websites.
-
Consultation with experts.
Any papers related to QoL identified through the cost-effectiveness and QoL searches (see Chapter 3, Searches) were also used to inform parameters for the cost-effectiveness model. Additional targeted searches (bibliographic and internet based) were performed on an ad hoc basis to seek information to populate specific parameters identified (expected and observed mortality rates for surgical aortic valve replacement, information regarding risk stratification and rehabilitation costs for heart surgery patients).
Transcatheter aortic valve implantation model data and inputs
This section of the report describes how the data available were used to produce model inputs.
Fitting survival curves
Data were available for various populations in terms of both overall survival and hospitalisation-free survival. In all cases, the follow-up time was considerably shorter than is required for the modelling. To extrapolate these curves forward in time, it is necessary to fit a parametric curve to the data. Visual inspection of survival curves shows a decreasing hazard with time. However, this cannot be sustained in the longer term, as the general population mortality risk increases with age. It is necessary to represent the long-term mortality that can be specifically attributed to AS using a function that can be approximated by a parametric form suitable for extrapolation. Without making any assumptions, the overall survival S(t) at time t can be written as:
where k is the 30-day survival; f(t) is a function to be determined, and is related to the long-term mortality specific to AS; and g(t) is the general population survival.
For TAVI and medical management, survival curves are available for both overall survival and hospitalisation-free survival for up to 2 years from the PARTNER trial (Leon et al. , figure 1). 14 Reading from the Kaplan–Meier curves, the values in Table 3 were obtained. The entries in the first three columns were taken directly from the source; the remaining columns show the result of the process used to infer the function f(t).
It is convenient to fit a Weibull distribution for f(t) taking the form:
where a and b are parameters to be determined. Here a is a dimensionless shape parameter: if a = 1 then the distribution reduces to the exponential distribution with a constant hazard. If a < 1 then the hazard decreases with time, while if a > 1 the hazard increases with time. For a fixed value of a, the mean survival time represented by f(t) is proportional to the scale parameter b.
| Time (months) | Actual values | Relative to 1-month survival | Relative to 1-month and general population survival | |||
|---|---|---|---|---|---|---|
| Overall survival | Hospitalisation-free survival | Overall survival | Hospitalisation-free survival | Overall survival | Hospitalisation-free survival | |
| 1 | 0.950 | 0.894 | N/A | N/A | N/A | N/A |
| 6 | 0.766 | 0.656 | 0.806 | 0.734 | 0.821 | 0.748 |
| 12 | 0.694 | 0.578 | 0.730 | 0.647 | 0.759 | 0.672 |
| 18 | 0.625 | 0.516 | 0.658 | 0.577 | 0.698 | 0.612 |
| 24 | 0.569 | 0.444 | 0.599 | 0.496 | 0.649 | 0.538 |
Fitting a Weibull distribution to a given set of observed values can be done by transforming the equation into the form:
and estimating values of a and b to minimise the sum of the squares of the errors in the above equation. In line with the principle of parsimony in modelling, a common value of the shape parameter a is to be preferred whenever an assumption of proportional hazards can be considered at all reasonable. A reasonable fit can be obtained to the last two columns in Table 3 by using a = 0.550 in each case, with b = 116 for overall survival, and b = 60.9 for hospitalisation-free survival. The comparison between the observed and fitted values can be seen in Table 4.
| Time (months) | Overall survival | Hospitalisation-free survival | ||
|---|---|---|---|---|
| Observed | Fitted | Observed | Fitted | |
| 6 | 0.821 | 0.822 | 0.748 | 0.756 |
| 12 | 0.759 | 0.751 | 0.672 | 0.664 |
| 18 | 0.698 | 0.699 | 0.612 | 0.599 |
| 24 | 0.649 | 0.657 | 0.538 | 0.549 |
Similar calculations were performed for the medical management group, again using the values from the PARTNER trial. 14 For surgical aortic valve replacement, other data sources had to be used. 42,67 Patients undergoing SAVR were divided into two groups: low risk (LES < 10)68 and moderate risk (LES between 10 and 20). 42,67 Separate curves were fitted for these groups. Two data sources were available for moderate risk and these were combined.
Full details of the relevant calculations are in Appendix 3. The parameters for the various curves fitted are shown in Table 5. Although the 30-day survival is slightly higher for the moderate-risk group than for the low-risk group, the long-term survival is much higher in the low-risk group. This difference is of little importance in the modelling, as almost all of the results are based only on the moderate-risk group.
As an illustration of how these numbers are then used in the model, consider the population at 12 months, for which time the relevant general population survival is 0.963. For TAVI, we have
From these figures, a proportion 0.686 – 0.571 = 0.115 of the original population is alive but has had at least one repeat hospitalisation. Note that the modelled values differ slightly from the observed figures in Table 3 for the same reason as the fitted values in Table 4 differ from the observed values in that table.
For medical management, there is no additional mortality assumed in the first month and the calculation at 12 months is as follows:
From these figures, a proportion 0.524 − 0.297 = 0.227 of the original population is alive, but has had at least one repeat hospitalisation.
| Parameter | SAVR (low risk) | SAVR (moderate risk) | TAVI | Medical management |
|---|---|---|---|---|
| Survival at 30 days | 0.925 | 0.927 | 0.950 | Not required (see notes) |
| Hospitalisation-free survival at 30 days | 0.847 | 0.850 | 0.894 | Not required (see notes) |
| a for both overall and hospitalisation-free survival | 1.15 | 1.15 | 0.550 | 0.821 |
| b for overall survival (months) | 613 | 160 | 116 | 22.0 |
| b for hospitalisation-free survival (months) | 450 | 117 | 60.9 | 9.83 |
Quality-of-life scores
The model requires average QoL scores for survivors with or without repeat hospitalisation. The available information from the PARTNER trial14 does not include direct estimates of these QoL scores. These must therefore be estimated. This was done using the two-stage process described below.
First, the PARTNER trial14 reports results in the form of proportions in each of the four NYHA classes at 6 months and 1 year. QoL scores (utilities) were obtained by reading from figure 3 in the paper of Maliwa et al. 69 From these figures, the mean QoL scores for the TAVI group at each time could be obtained as shown in Table 6. These are weighted averages of the QoL scores, weighted by the proportion of TAVI patients in each class at the relevant time. These mean QoL scores are also weighted averages of the QoL scores for survivors with or without repeat hospitalisation, weighted by the number of TAVI patients in each category at the relevant time. The PARTNER trial14 has also reported these numbers, which are also shown in Table 6.
The second stage of the calculation involves solving a pair of linear simultaneous equations, to identify the QoL scores for survivors with or without repeat hospitalisation, using the overall average QoL scores at each time, and the proportions of patients in each category. The values obtained were 0.717 for survival without repeat hospitalisation and 0.579 for survival with repeat hospitalisation. It should be noted that these point estimates are subject to considerable uncertainty given the nature of the method used.
To estimate the QoL under medical management, the standard therapy arm of the PARTNER trial14 was used. When this was done using a similar process to that described above, the average QoL scores at 6 months and 1 year were estimated to be 0.545 and 0.585 respectively. However, the PARTNER trial14 reports a higher proportion of repeat hospitalisation in the standard therapy arm among survivors at 1 year than at 6 months. If there were a lower QoL in survivors with repeat hospitalisation than in survivors without repeat hospitalisation, the average QoL should be lower at 1 year than at 6 months. As this is not the case, it is not possible to obtain sensible estimates of QoL scores for the two states separately for the medical management arm of the model using the same method as for TAVI. Instead, a single figure of 0.565 was found by averaging the 6-month and 1-year results.
Although the methods used to obtain the QoL scores are far from ideal, the results obtained seem reasonable. For patients undergoing SAVR, the same values were used as for TAVI in the absence of any alternative data source. However, in the first month of the model, account is taken of the time in hospital and post-surgery recovery. For SAVR, QALYs accumulated were reduced by 50%, whereas for TAVI they were reduced by 25% to reflect the lesser invasiveness of the procedure.
| Class | QoL score | Proportions of TAVI patients in each class | |
|---|---|---|---|
| 6 months | 1 year | ||
| I | 0.844 | 0.292 | 0.343 |
| II | 0.699 | 0.486 | 0.361 |
| III | 0.553 | 0.130 | 0.217 |
| IV | 0.408 | 0.092 | 0.080 |
| Average QoL score | 0.696 | 0.694 | |
| Survivors without repeat hospitalisation | 0.717a | 117 | 102 |
| Survivors with repeat hospitalisation | 0.579a | 21 | 20 |
Costs
The costing perspective was NHS, given that the vast majority of the patients are over retirement age; the price year was 2010. 70 Costs in the model are detailed below. They are made up of the short-term costs associated with the procedures, and then longer-term costs resulting from follow-up appointments, medication and repeat hospitalisation. All costs and QALYs were discounted at 3.5% from the starting point.
Short-term costs
Procedure costs shown in Table 7 include the procedure itself, hospital stay and costs of dealing with short-term adverse events. Costs for the procedure are based on the South Central report,71 to which costs for adverse events are added. Using a range of sources for the frequency of adverse events, attempts have been made to account for those adverse events that involve extra cost, as detailed in Appendix 4. Some adverse events (such as prolonged ventilation) essentially require additional stay in hospital. It was assumed that the time taken is already included in the mean length of stay, so that it would be double counting to include an additional cost for these events.
| Cost (£) | SAVR | TAVI |
|---|---|---|
| Procedure including hospital staya | 18,111.25 | 24,000.00 |
| Adverse eventsb | 1075.30 | 1078.98 |
| Total | 19,193.55 | 25,078.98 |
Longer-term costs
Follow-up appointments for SAVR and TAVI are taken to occur at 1 month after treatment, then at 6 months, 12 months and every 12 months thereafter. For SAVR, this was taken simply as a cardiologist appointment (costed at £218 from reference costs). For TAVI, the cost of electrocardiography and echocardiography (£99 in total) was added.
Rehospitalisation was costed as treatment for a cardiac valve disorder (£2046 from NHS reference costs70), plus the cost of an extended stay in a cardiology ward (£396.16 per day based on the Bazian report32 inflated to 2010 prices). The average additional stay was assumed to be 6 days for SAVR or TAVI and 14 days for medical management, based on clinical advice. No definitive costs for rehabilitation were found. No rehabilitation costs were included in the base case. However, the possibility of rehabilitation costs was considered as part of a general sensitivity analysis concerning the unit costs of each procedure (see Chapter 4, Resource use in the first month).
Base-case analysis
For the base-case analysis, it was assumed that any patients receiving SAVR or medical management when TAVI was available would receive the same treatment if TAVI was not available. Hence, any results for such patients would cancel out of the incremental analysis, and such patients were therefore excluded from the model. Thus, the only patients modelled were those receiving TAVI when it was available. The comparator treatment for these patients was either SAVR or medical management (the treatment that would have been given in the absence of TAVI). Based on clinical advice, it was assumed that 10% of TAVI patients were suitable for SAVR, and that 90% of these would have received SAVR in the absence of TAVI; the remaining 10% would have received medical management in this case. As noted earlier in Chapter 4 (see Costs), SAVR patients were divided into low and moderate risk. The base-case assumption was that patients who were suitable for SAVR and considered for TAVI would be drawn from the moderate-risk SAVR group. In a sensitivity analysis, half of these patients were low risk and half were moderate risk.
For convenience, the total number of patients was set at 1000. Then the numbers of patients in each of the patient groups defined in Model description were as shown in Table 8. (The total number of patients in the model is arbitrary; changing this would not alter the per patient results as long as the proportion of patients in each group was maintained.)
| Distribution of modelled patients under the assumption that TAVI is available | ||
|---|---|---|
| Group | n | Comments |
| A | 0 | This group consists of patients who would receive SAVR regardless of the availability of TAVI so were excluded from the model |
| B | 100 | These are patients who are deemed suitable for SAVR but would receive TAVI if it were available |
| C | 0 | This group consists of patients who are deemed suitable for SAVR but would still receive medical management even if TAVI were available so were excluded from the model |
| D | 900 | These are patients who are deemed unsuitable for SAVR but would receive TAVI if it were available |
| E | 0 | This group consists of patients who are deemed unsuitable for SAVR but would still receive medical management even if TAVI were available so were excluded from the model |
| Distribution of modelled patients under the assumption that TAVI is unavailable | ||
| Group | n | Comments |
| F | 90 | These are patients who would receive SAVR if TAVI were not available |
| G | 10 | These are patients who are deemed suitable for SAVR but would receive medical management if TAVI were not available |
| H | 900 | These are patients who are deemed unsuitable for SAVR and would therefore necessarily receive medical management if TAVI were not available |
When the model was run over a 25-year time horizon with base-case parameters and the distribution of patients shown in Table 8, the results were obtained as shown in Table 9 (the overall results for all patients modelled), Table 10 (results only for patients suitable for SAVR) and Table 11 (results only for patients not suitable for SAVR).
| Weighted average across all groups for given policy option | Overall cost per patient (£) | Overall QALYs per patient |
|---|---|---|
| TAVI available | 27,833 | 2.8530 |
| TAVI not available | 5305 | 1.2291 |
| Difference | 22,528 | 1.6239 |
| ICER (£ per QALY) | 13,900 |
| Weighted average across all groups for given policy option | Overall cost per patient (£) | Overall QALYs per patient |
|---|---|---|
| TAVI available | 27,833 | 2.8530 |
| TAVI not available | 19,871 | 3.4617 |
| Difference | 7963 | −0.6087 |
| In this case the TAVI not available option dominates the TAVI available option | ||
| Weighted average across all groups for given policy option | Overall cost per patient (£) | Overall QALYs per patient |
|---|---|---|
| TAVI available | 27,833 | 2.8530 |
| TAVI not available | 3687 | 0.9810 |
| Difference | 24,147 | 1.8720 |
| ICER (£ per QALY) | 12,900 |
Note that for the patients suitable for SAVR, the base-case results (see Table 10) suggest that TAVI would be overall more costly and less effective than the comparator. This effect is known as simple dominance and, in such a case, it is not appropriate to quote the numerical value of the ICER. 72 These results assume a time horizon of 25 years. Varying the time horizon from 2 to 25 years produces the results shown in Figure 8. The ICERs for the whole patient group and for the patients unsuitable for SAVR decrease with time. The ICER for patients suitable for SAVR is not shown because the dominance relationship applies across all time horizons considered. Note that the overall results are very similar to the results for the patients unsuitable for SAVR because these patients form the vast majority of the patients in the model.
FIGURE 8.
Base-case results across a range of time horizons. a, ICER not shown for patients suitable for SAVR as TAVI is dominated by comparator for all time horizons.
Deterministic sensitivity analysis
This section reports the results of a number of different forms of one-way sensitivity analysis. These are divided into two groups. In the first group, different assumptions about the selection of patients for treatment are made. Detailed results are reported, followed by a summary table for this group, from which all the results can be readily compared with the base case. After that, the effect of varying the parameters related to the results from the modelled treatments is explored. In each section, all base-case assumptions are assumed to apply unless explicitly varied.
Varying the comparator with transcatheter aortic valve implantation for patients suitable for surgical aortic valve replacement
In the base-case analysis, it was assumed that 10% of TAVI cases would be patients suitable for SAVR, and that 90% of these would actually receive SAVR if TAVI were not available. Suppose that, instead, only 50% of these patients would receive SAVR. Compared with the base case, this scenario reflects what would happen if patients who receive TAVI but would have been suitable for SAVR are drawn in higher numbers from those who would not actually have received SAVR, for example due to personal choice not to undergo SAVR. The overall results and results for the suitable for SAVR group are then as shown in Tables 12 and 13. Results for the unsuitable for SAVR group are not shown, as they are unchanged from the base-case results.
| Weighted average across all groups for given policy option | Overall cost per patient (£) | Overall QALYs per patient |
|---|---|---|
| TAVI available | 27,833 | 2.8530 |
| TAVI not available | 4586 | 1.1188 |
| Difference | 23,248 | 1.7342 |
| ICER (£ per QALY) | 13,400 | |
| Weighted average across all groups for given policy option | Overall cost per patient (£) | Overall QALYs per patient |
|---|---|---|
| TAVI available | 27,833 | 2.8530 |
| TAVI not available | 12,678 | 2.3592 |
| Difference | 15,156 | 0.4938 |
| ICER (£ per QALY) | 30,700 | |
Figure 9 shows the results of varying the time horizon in this case. At all time horizons, TAVI is now more effective, but still more costly, than the comparator, and so a positive ICER is shown. As in the base-case results, the overall results are similar to the results for patients unsuitable for SAVR.
FIGURE 9.
Varying the time horizon when only 50% of TAVI patients suitable for SAVR would actually have received SAVR.
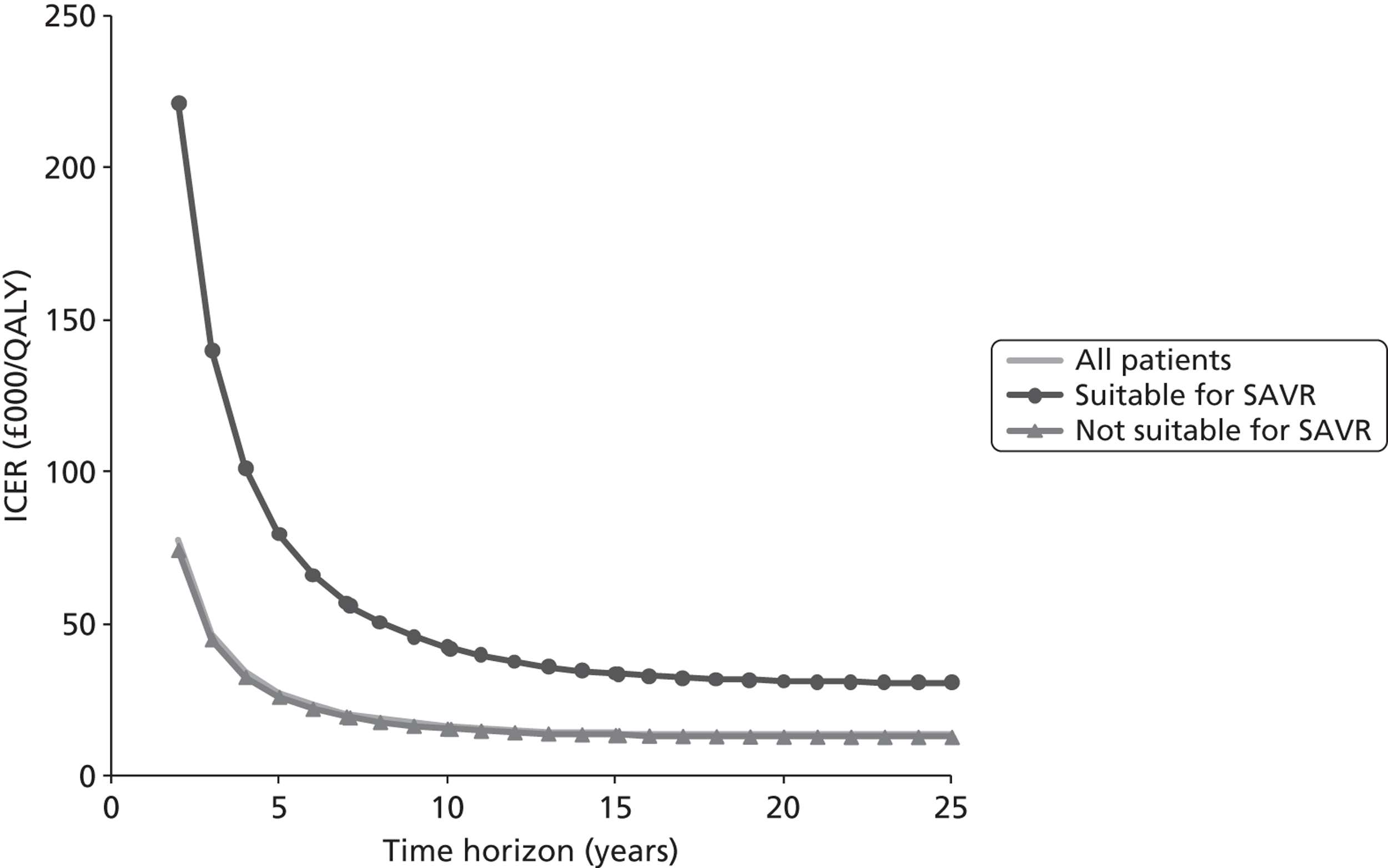
An extension of this sensitivity analysis is to consider the patients suitable for SAVR and vary the proportion of them who would actually receive SAVR across the whole range from 0% to 100%. The effect of this is shown in Figure 10. This is based on a 25-year time horizon. The results for the unsuitable for SAVR group do not vary, whereas the results for the overall group remain close to those results throughout. The results for the suitable for SAVR group vary considerably according to the proportion who would actually have received SAVR. The ICER goes over £20,000 per QALY when this proportion reaches 36%, and over £30,000 per QALY at 50%. The ICER becomes very large as the proportion exceeds 67% and the TAVI option is dominated by the comparator when the proportion is 68% or higher.
FIGURE 10.
Varying the percentage of suitable patients receiving SAVR in the absence of TAVI.
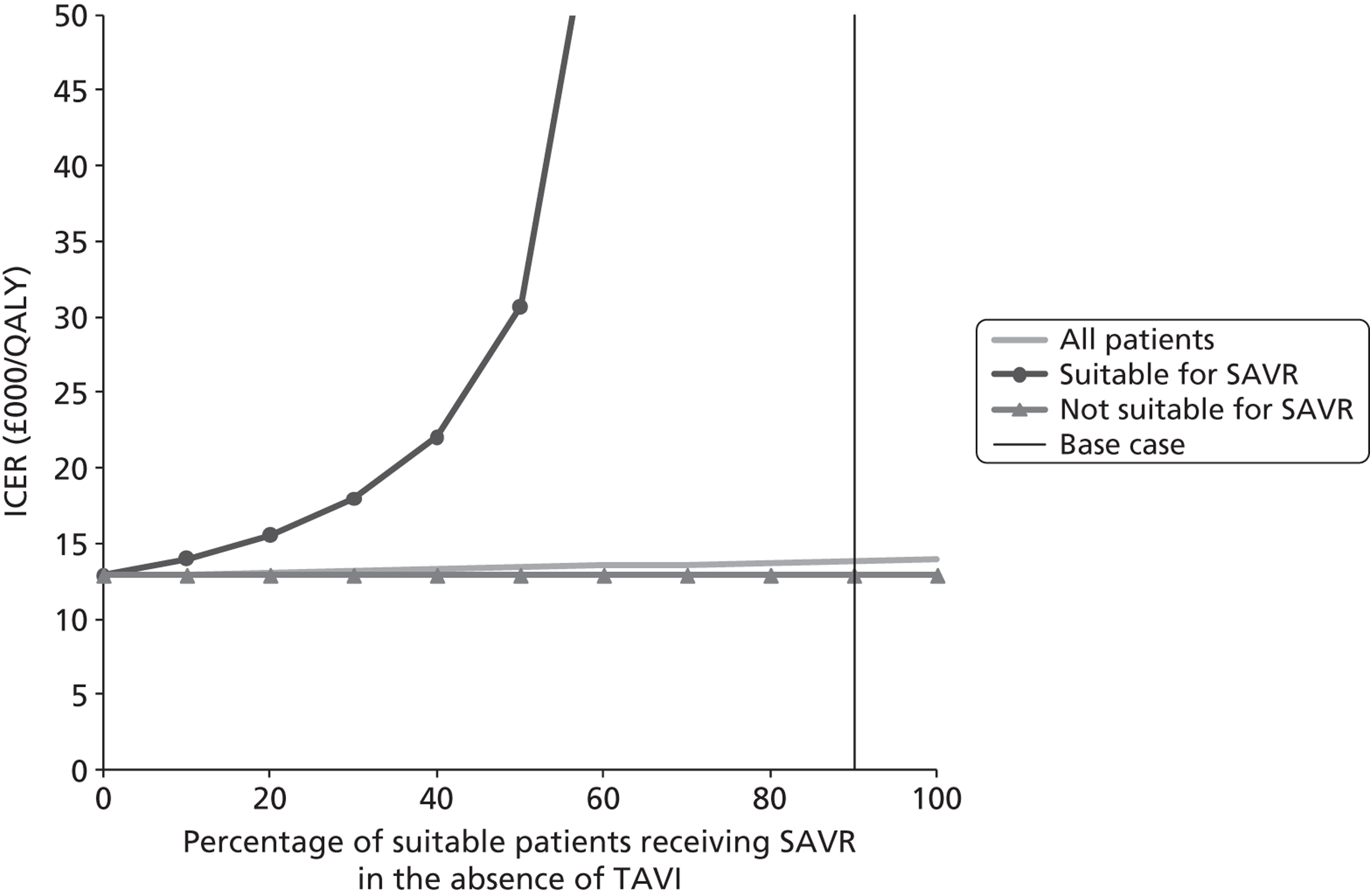
Varying the eligibility criteria for transcatheter aortic valve implantation
This analysis relates to Figures 6 and 7 in Model description, where the eligibility for TAVI is either extended to all AS patients or restricted to patients who are deemed completely unsuitable for surgery. In fact there is no need for any further analysis in the latter case, as it is covered by the results in Table 11, with an ICER of £12,900 per QALY. If TAVI is extended to a wider range of patients with moderate risk, then the results for the two patient groups separately are still as in Table 10 and Table 11, but the overall results change. Table 14 shows the results in the case where equal numbers of TAVI patients are drawn from the two patient groups suitable and unsuitable for SAVR, assuming that 90% of TAVI patients would receive SAVR in the absence of TAVI. The overall ICER increases from its base-case value of £13,700 per QALY to £25,400 per QALY. The overall ICER crosses £20,000 per QALY when the proportion suitable for SAVR is around 40%, and £30,000 per QALY at around 55%. Finally, if as many as 76% of TAVI cases came from the group of patients suitable for SAVR, the net effect of introducing TAVI would be a reduction in overall QALYs at an increased overall cost.
| Weighted average across all groups for given policy option | Overall cost per patient (£) | Overall QALYs per patient |
|---|---|---|
| TAVI available | 27,833 | 2.8530 |
| TAVI not available | 11,779 | 2.2213 |
| Difference | 16,055 | 0.6316 |
| ICER (£ per QALY) | 25,400 | |
The above analysis assumes that all patients receiving TAVI instead of SAVR would be taken from the moderate-risk group. If low-risk patients were also included, the results for TAVI would be worse. For example, if TAVI patients were drawn in equal numbers from those suitable and unsuitable for SAVR, and those suitable for surgery were drawn in equal numbers from low- and moderate-risk patients, then the overall ICER would increase to nearly £48,000 per QALY (Table 15).
| Weighted average across all groups for given policy option | Overall cost per patient (£) | Overall QALYs per patient |
|---|---|---|
| TAVI available | 27,833 | 2.8530 |
| TAVI not available | 11,766 | 2.5160 |
| Difference | 16,067 | 0.3370 |
| ICER (£ per QALY) | 47,700 | |
Considering the effect of increased referrals
In the background section of this report, it was noted that a possible effect of TAVI availability is that additional patients might be referred for investigation and end up receiving SAVR when they might have had medical management in the absence of TAVI. As a speculative analysis, suppose that the total number of patients receiving SAVR is unchanged as a result of the introduction of TAVI. In the base-case analysis, 90 patients receive TAVI when it is available but would receive SAVR when TAVI is not available (see Table 8). Therefore, to maintain the total numbers receiving SAVR would involve including in the modelled population an additional 90 patients receiving SAVR in the TAVI available strategy and medical management in the TAVI not available strategy. Assuming that all of these patients are drawn from the group with moderate SAVR risk, the results both overall and for patients suitable for SAVR are shown in Table 16 and Table 17 respectively.
| Weighted average across all groups for given policy option | Overall cost per patient (£) | Overall QALYs per patient |
|---|---|---|
| TAVI available | 27,324 | 2.9260 |
| TAVI not available | 5171 | 1.2086 |
| Difference | 22,153 | 1.7174 |
| ICER (£ per QALY) | 12,900 | |
| Weighted average across all groups for given policy option | Overall cost per patient (£) | Overall QALYs per patient |
|---|---|---|
| TAVI available | 24,913 | 3.2719 |
| TAVI not available | 12,205 | 2.2866 |
| Difference | 12,709 | 0.9853 |
| ICER (£ per QALY) | 12,900 | |
As can be seen, the results change dramatically and suggest that TAVI availability would be cost-effective in all groups. In fact, in this case, it can be seen that the ICER for TAVI availability is the same for patients suitable for SAVR as it is for patients not suitable for SAVR. This is not a coincidence: what is now happening is that TAVI is effectively displacing medical management only in both groups. Different people receive SAVR in the two arms of the model, but they are assumed to be taken from a homogeneous population, and so the change in identity of the SAVR patients has no effect on the model results.
The above analysis assumes that all SAVR patients are drawn from the moderate-risk group. If the additional SAVR patients are drawn in equal numbers from low- and moderate-risk groups, whereas the patients moving from SAVR to TAVI are still taken only from the moderate-risk group, the ICER for the patients suitable for SAVR drops even further to around £9800 per QALY.
It may well be the case that the number of patients receiving SAVR in place of medical management is considerably lower than the number receiving TAVI in place of SAVR. The ICER for TAVI availability in the group suitable for SAVR goes over £20,000 per QALY when approximately 60% of the patients switching from SAVR to TAVI are replaced by patients switching from medical management to SAVR, assuming again that all SAVR patients are moderate risk.
Summary of sensitivity analysis results for patient selection
Table 18 summarises the results presented in detail in the previous three sections. The results given here show that the overall ICER and the ICER for patients suitable for SAVR are highly sensitive to the rules for selection of patients for TAVI.
| Scenario | Overall ICER | ICER for patients suitable for SAVR | ICER for patients not suitable for SAVR |
|---|---|---|---|
| Base case. The assumption here is that 90% of the TAVI patients were unsuitable for SAVR and that 9% would receive SAVR in the absence of TAVI, whereas 1% would be deemed suitable for SAVR but not receive it. All SAVR patients are assumed to be moderate risk | 13,700 | D | 12,900 |
| The assumption here is that 90% of the TAVI patients were unsuitable for SAVR and that 5% would receive SAVR in the absence of TAVI, whereas 5% would be deemed suitable for SAVR but not receive it. All SAVR patients are assumed to be moderate risk | 13,400 | 30,700 | 12,900 |
| The assumption here is that 50% of the TAVI patients were unsuitable for SAVR and that 45% would receive SAVR in the absence of TAVI, whereas 5% would be deemed suitable for SAVR but not receive it. All SAVR patients are assumed to be moderate risk | 25,400 | D | 12,900 |
| The assumption here is that 50% of the TAVI patients were unsuitable for SAVR and that 45% would receive SAVR in the absence of TAVI, whereas 5% would be deemed suitable for SAVR but not receive it. In this analysis, half of the patients deemed suitable for SAVR were assumed to come from the low-risk group | 47,700 | D | 12,900 |
| The assumption here is that 90% of the TAVI patients were unsuitable for SAVR and that 9% would receive SAVR in the absence of TAVI, whereas 1% would be deemed suitable for SAVR but not receive it. However, it is also assumed that a number of moderate-risk patients receive SAVR when TAVI is available but medical management when TAVI is not available, so that the total number of patients receiving SAVR is not changed. All SAVR patients are assumed to be moderate risk | 12,900 | 12,900 | 12,900 |
Allowing for the possibility of repeat transcatheter aortic valve implantation
As noted in the background section, the long-term durability of TAVI valves is currently unknown. The possibility that replacement TAVI may be necessary was considered by adding the cost of an additional TAVI to all surviving patients at a given time from the start of the model. On clinical advice, the times chosen were 7, 10 and 15 years. It is acknowledged that this is a very simplistic approach, but it at least gives some indication of the scale of the potential problem. The results are shown in Figures 11, 12 and 13. In each case, the part of the graph for a time horizon up to the point of valve replacement is the same as for the base-case result shown in Figure 8. There is then a sudden increase in the ICER as the cost of replacement TAVI is included in the analysis. The overall results are not greatly affected by this change to the model. Indeed, in the case of replacement after 15 years the effect of the change is hardly noticeable on the graph. This reflects an overall modelled survival rate of 7% at that time, together with the effect of discounting. In all cases, the TAVI available strategy is dominated by the TAVI not available strategy for patients suitable for SAVR, regardless of the time horizon of the model.
FIGURE 11.
Including cost of replacement TAVI for all patients after 7 years. a, ICER not shown for patients suitable for SAVR as TAVI is dominated by comparator for all time horizons.
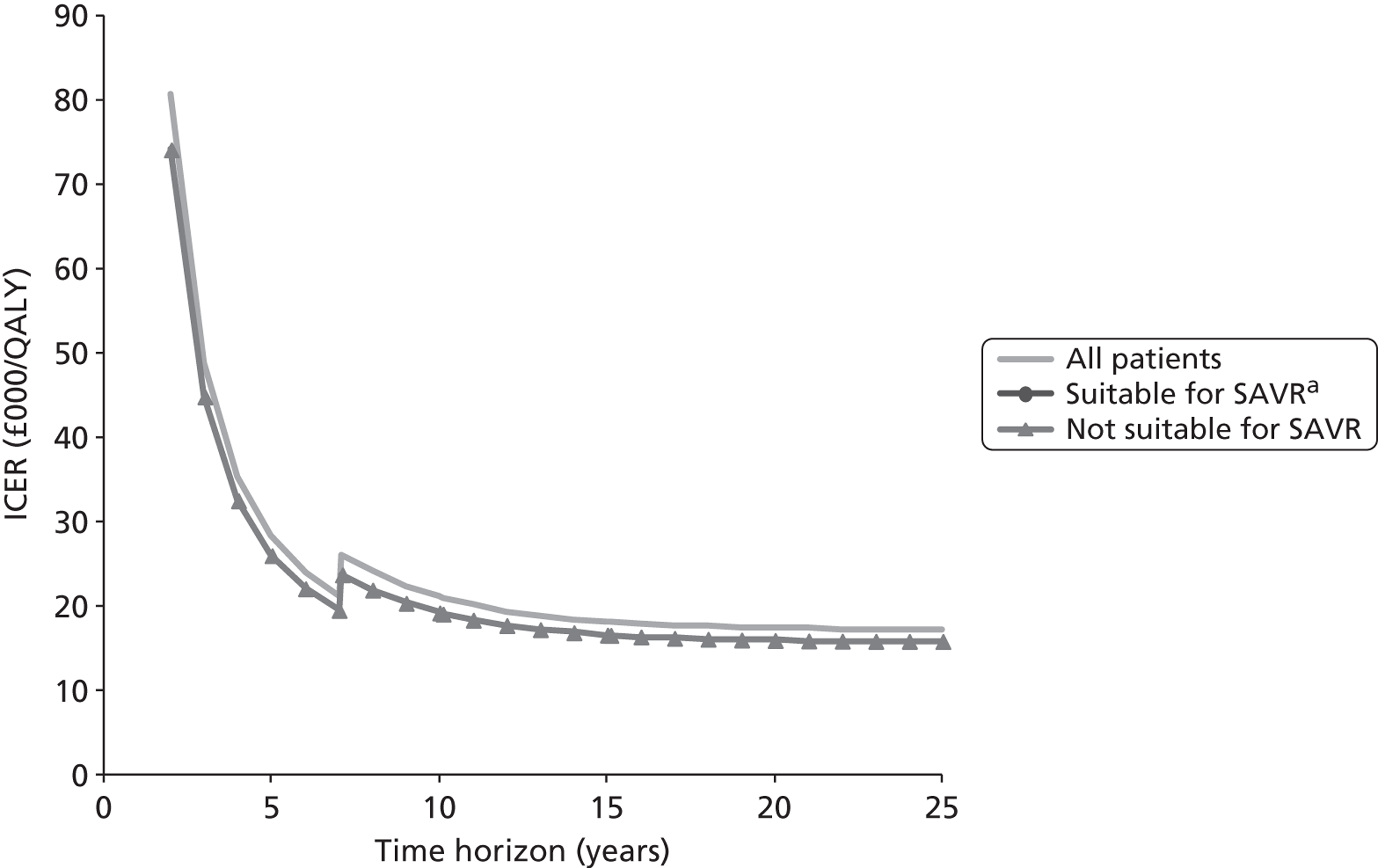
FIGURE 12.
Including cost of replacement TAVI for all patients after 10 years. a, ICER not shown for patients suitable for SAVR as TAVI is dominated by comparator for all time horizons.
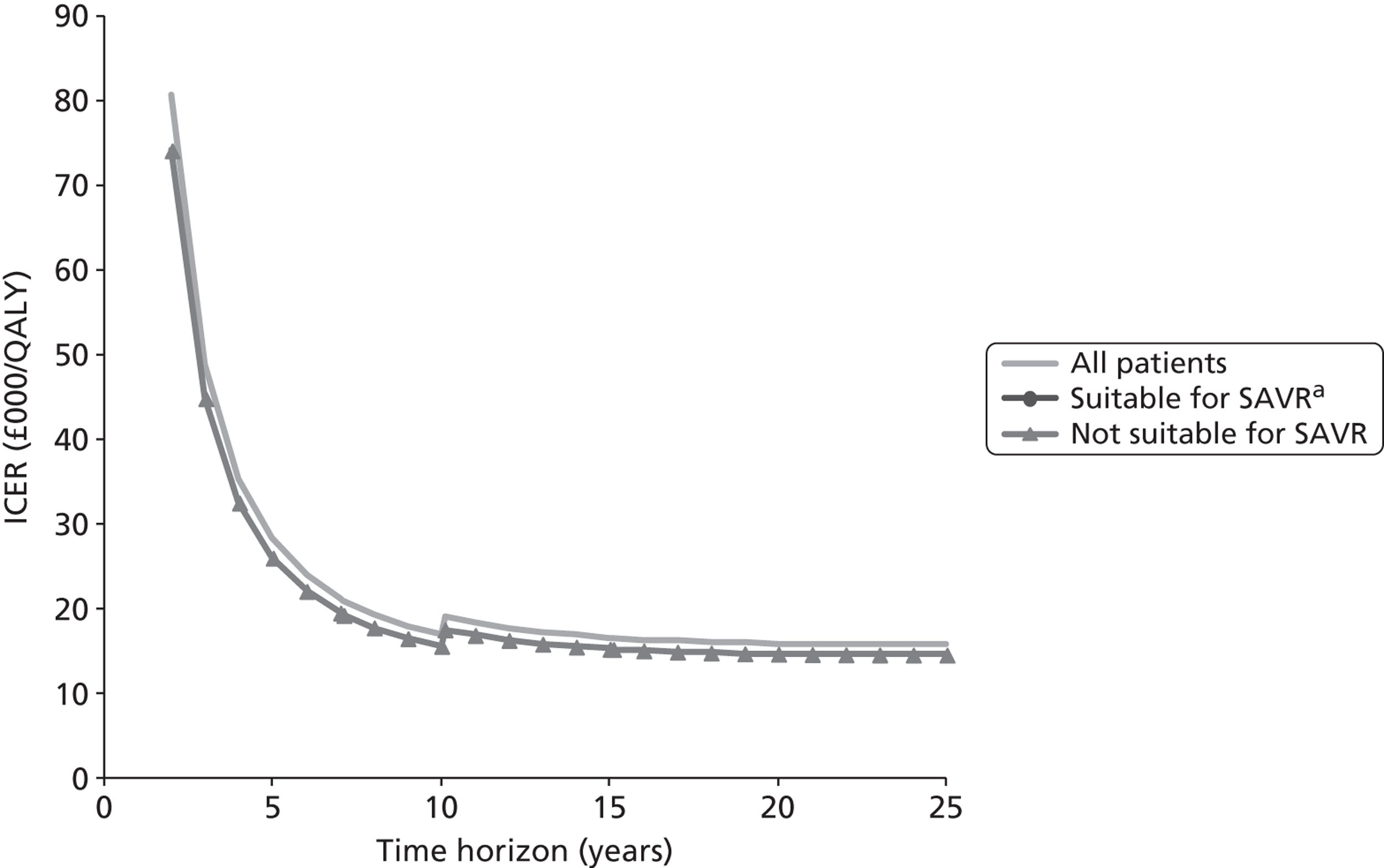
FIGURE 13.
Including cost of replacement TAVI for all patients after 15 years. a, ICER not shown for patients suitable for SAVR as TAVI is dominated by comparator for all time horizons.
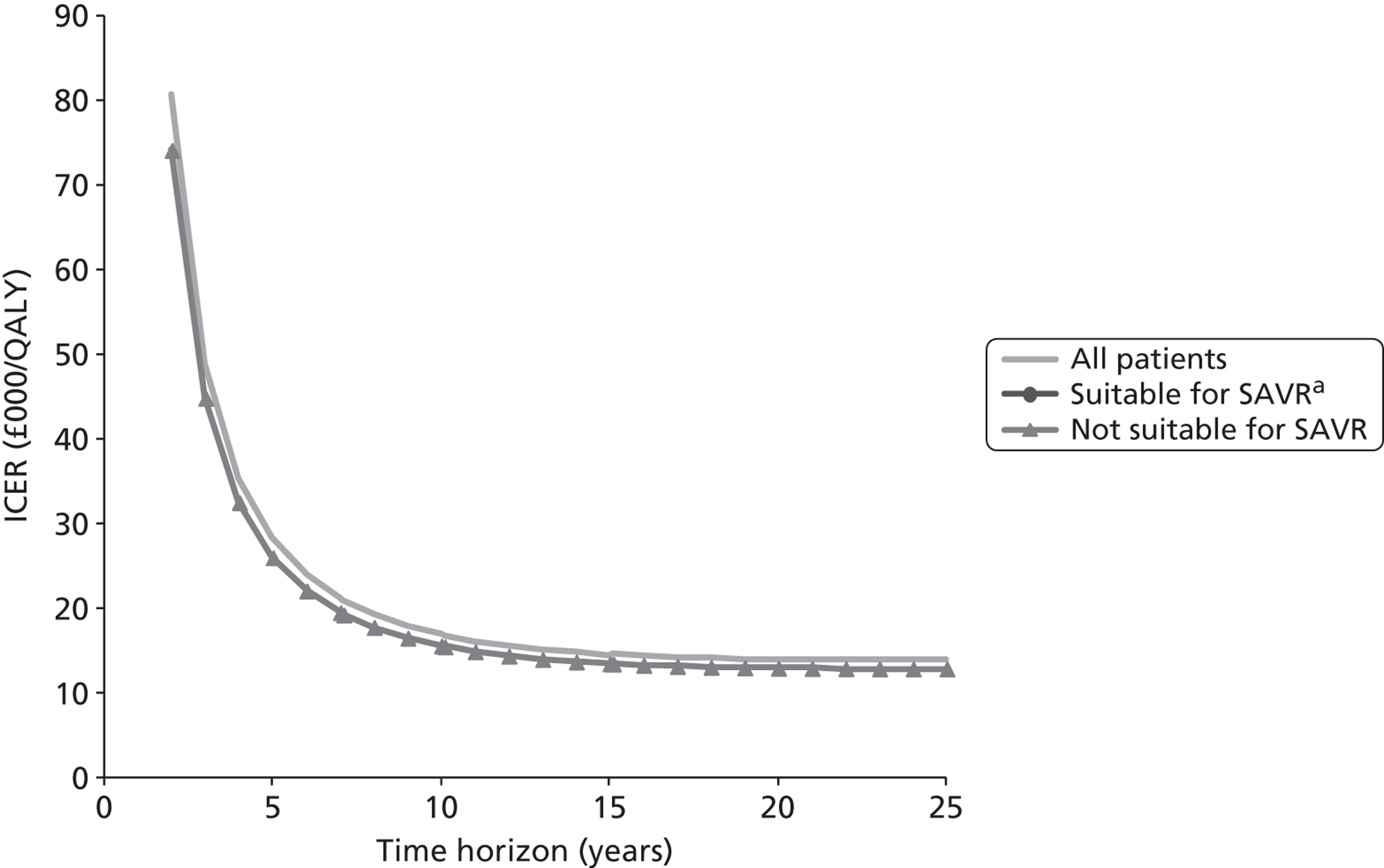
Resource use in first month
There is considerable uncertainty about a number of the inputs from which the first month's costs for SAVR and TAVI were estimated. For example, in the base-case analysis, an estimated mean length of stay in hospital following SAVR of 13.36 days was used, made up of 3.1 days in an intensive care unit (ICU) and 10.26 days on a cardiology ward. Data from the Adult Cardiac Surgery Database58 give a mean stay of 10.7 days. If we use this figure for the total length of stay and keep the assumption of 3.1 days in an ICU, this reduces the mean days on a cardiology ward by 2.66 days, reducing the cost of SAVR by 2.66 × £396.16 = £1053.79. This clearly has no effect on the results for the patients not suitable for SAVR. For the patients suitable for SAVR, the average cost per patient for the TAVI not available strategy is reduced. Since this strategy was already less costly and more effective than the TAVI available strategy in our base-case analysis, the same must be true with the change in assumption. There is a small effect on the overall ICER, but the rounded value remains at £13,900 per QALY.
In the same way, any change in the assumption about frequency of short-term adverse events or inclusion of rehabilitation costs would have the same effect as an adjustment in the unit cost of the relevant treatment. Table 19 and Table 20 show the effects of changes in the unit cost assigned to SAVR and TAVI respectively.
| Change to cost of SAVR | Overall ICER | ICER for patients suitable for SAVR | ICER for patients not suitable for SAVR |
|---|---|---|---|
| Reduce by £3000 | 14,000 | D | 12,900 |
| Reduce by £2000 | 14,000 | D | 12,900 |
| Reduce by £1000 | 13,900 | D | 12,900 |
| Base case | 13,900 | D | 12,900 |
| Increase by £1000 | 13,800 | D | 12,900 |
| Increase by £2000 | 13,800 | D | 12,900 |
| Increase by £3000 | 13,700 | D | 12,900 |
| Change to cost of TAVI | Overall ICER | ICER for patients suitable for SAVR | ICER for patients not suitable for SAVR |
|---|---|---|---|
| Reduce by £3000 | 12,000 | D | 11,300 |
| Reduce by £2000 | 12,600 | D | 11,800 |
| Reduce by £1000 | 13,300 | D | 12,400 |
| Base case | 13,900 | D | 12,900 |
| Increase by £1000 | 14,500 | D | 13,400 |
| Increase by £2000 | 15,100 | D | 14,000 |
| Increase by £3000 | 15,700 | D | 14,500 |
In all cases explored, the TAVI not available strategy is less costly and more effective than TAVI available for patients suitable for SAVR. The results in Table 19 necessarily show no change in the ICER for patients not suitable for SAVR and only small changes in the overall results. The variation shown in Table 20 is somewhat greater, since the change in TAVI cost affects a larger number of patients in the model. However, the results still do not change greatly for fairly substantial changes in the unit cost assigned to TAVI.
Cost of repeat hospitalisation
In the base-case analysis, the cost of repeat hospitalisation was assumed to be approximately £4400 for SAVR and TAVI patients, but £7600 for medical management patients. If the lower figure is also used for medical management patients, the ICER for patients unsuitable for SAVR increases from £12,900 per QALY to £13,600 per QALY, and overall from £13,900 per QALY to £14,700 per QALY. Similarly, if the higher figure is used for all patients, these ICERs change to £13,300 and £14,300 respectively. In either case, the TAVI available option is still dominated for patients suitable for SAVR.
Short-term mortality
In this analysis, the short-term mortality was adjusted for each of SAVR and TAVI separately. For SAVR, the 1-month mortality was given as 7.3%, with a 95% CI from 4.3% to 11.0%. These results were obtained from combining the results of studies by Leontyev67 (10.7%) and Wendt42 (4.6%). For TAVI, the 1-month mortality was given as 5.0%, with a 95% CI from 2.3% to 8.7%. 14 Applying each of these figures individually gives the results in Table 21. They show very little variation from the base case.
| Scenario | Overall ICER | ICER for patients suitable for SAVR | ICER for patients not suitable for SAVR |
|---|---|---|---|
| Base case (7.3% for SAVR, 5.0% for TAVI) | 13,900 | D | 12,900 |
| Low mortality (4.3%) for SAVR | 14,000 | D | 12,900 |
| High mortality (11.0%) for SAVR | 13,700 | D | 12,900 |
| Low mortality (2.3%) for TAVI | 13,300 | D | 12,400 |
| High mortality (8.7%) for TAVI | 14,800 | D | 13,600 |
Long-term mortality
The methods used to estimate the long-term survival curves do not naturally produce CIs. For deterministic analysis, an arbitrary decision has been taken to apply multipliers to shape and scale parameters of the Weibull distributions separately. Multiplying the scale parameter by a constant effectively multiplies the mean of the underlying distribution by that same constant; the effect of multiplying the scale parameter is not easily described. Shape parameters are naturally on a more limited range than scale parameters. Accordingly, multipliers of 0.8 and 1.2 have been used for shape parameters, while multipliers of 0.6 and 1.4 have been used for scale parameters. These correspond roughly to the 95% limits used in the probabilistic sensitivity analysis shown in Probabilistic sensitivity analysis. The results are in Table 22.
| Scenario | Overall ICER | ICER for patients suitable for SAVR | ICER for patients not suitable for SAVR |
|---|---|---|---|
| Base case | 13,900 | D | 12,900 |
| Multiply shape parameter for SAVR by 0.8 | 13,700 | D | 12,900 |
| Multiply shape parameter for SAVR by 1.2 | 14,000 | D | 12,900 |
| Multiply scale parameter for SAVR by 0.6 | 13,300 | 75,300 | 12,900 |
| Multiply scale parameter for SAVR by 1.4 | 14,200 | D | 12,900 |
| Multiply shape parameter for TAVI by 0.8 | 14,700 | D | 13,600 |
| Multiply shape parameter for TAVI by 1.2 | 13,200 | D | 12,400 |
| Multiply scale parameter for TAVI by 0.6 | 20,100 | D | 17,700 |
| Multiply scale parameter for TAVI by 1.4 | 11,600 | D | 11,000 |
| Multiply shape parameter for MM by 0.8 | 14,800 | D | 13,800 |
| Multiply shape parameter for MM by 1.2 | 13,300 | D | 12,300 |
| Multiply scale parameter for MM by 0.6 | 11,700 | D | 11,000 |
| Multiply scale parameter for MM by 1.4 | 16,500 | D | 15,200 |
These results are not highly sensitive to the changes in the shape parameter, but are more sensitive to the changes made in the scale parameter. In particular, if the overall survival (relative to population survival) following SAVR is reduced by 40%, it is no longer the case that the TAVI not available strategy is more effective than the TAVI available strategy for patients suitable for SAVR. In this single scenario, TAVI available is both more costly and more effective than the comparator, but still well above the upper NICE threshold of £30,000 per QALY.
Quality-of-life scores
The deterministic sensitivity analysis for QoL scores is based on the idea of using 95% limits of an appropriate distribution to represent the uncertainty. There is no theoretically correct distribution for these parameters and it is necessary to make a pragmatic decision. It is reasonable to suppose that all QoL scores used in the model represent states better than death, and so the QoL scores must be between 0 and 1. Further, it is reasonable to suppose that there is a very low probability that the scores will be at either extreme of possibility.
A convenient distribution for QoL scores is a beta distribution. This has two parameters a and b: the mean of the distribution is equal to a/(a + b) and (for a fixed mean), the variance reduces as a and b increase. Ensuring that there is a very low probability of taking extreme values requires that both a and b parameters are at least equal to two. The approach taken here is to allow the maximum possible variance subject to this condition. There are three QoL parameters in the model:
u1 is the QoL for hospitalisation-free survival following SAVR or TAVI;
u2 is the QoL for post-hospitalisation survival following SAVR or TAVI; and
u3 is the QoL for survival following medical management.
Consider first the QoL u1 following surgery (SAVR or TAVI), but without repeat hospitalisation, for which the point estimate is 0.717. Since 0.717 > 0.5, we will have a > b, so it is appropriate to take b = 2 and then calculate a from the equation
giving the answer a = 5.06. This distribution has 95% limits 0.363 to 0.957.
For post-hospitalisation QoL after SAVR or TAVI u2 it is important to keep the value lower than the QoL score u1 for hospitalisation-free survival. It is therefore not appropriate to sample u2 directly from a beta distribution. Instead, it makes sense to use a beta distribution for the ratio between the two QoL scores. The point estimate for this is given by
so the ratio can be taken to follow a beta(8.37,2) distribution, which has mean 0.807 as required. The 95% limits of this beta distribution are 0.532 and 0.973. These correspond to QoL scores u2 of 0.532 × 0.717 = 0.381 and 0.973 × 0.717 = 0.697 respectively when u1 takes its base-case value of 0.717.
Similarly, it is reasonable to suppose that the QoL for medical management u3 should be lower than u1. However, there is no compelling reason why u3 should be either higher or lower than u2. Accordingly, it is sensible to apply a beta distribution to the ratio of u3 to u1, in the same way as for u2. This time the point estimate for the ratio is 0.788, which is the mean of a beta(7.44,2) distribution. The 95% limits for this distribution are 0.494 and 0.970, giving 95% limits for u3 of 0.354 and 0.695.
For the univariate analysis shown in Table 23, each of the QoL scores was taken to its lower and upper 95% limits. When u1 was varied, the other two scores were adjusted in proportion, but when u2 and u3 were varied, each of the other scores was kept at its base-case value.
The results show that the overall ICER and the ICER for patients not suitable for SAVR varied somewhat but remained below £20,000 per QALY in all cases, except the one in which the QoL scores were reduced to very low – and perhaps unrealistically low – values. In all cases, the option TAVI available was dominated for patients suitable for SAVR.
| Scenarioa | Overall ICER | ICER for patients suitable for SAVR | ICER for patients not suitable for SAVR |
|---|---|---|---|
| Base case (0.717, 0.579, 0.565) | 13,900 | D | 12,900 |
| Low value for hospitalisation-free survival following SAVR or TAVI (0.363, 0.293, 0.286) | 27,400 | D | 25,500 |
| High value for hospitalisation-free survival following SAVR or TAVI (0.957, 0.773, 0.754) | 10,400 | D | 9,700 |
| Low value for post-hospitalisation survival following SAVR or TAVI (0.717, 0.381, 0.565) | 15,800 | D | 14,600 |
| High value for post-hospitalisation survival following SAVR or TAVI (0.717, 0.697, 0.565) | 12,900 | D | 12,000 |
| Low value for survival following medical management (0.717, 0.579, 0.354) | 11,500 | D | 10,800 |
| High value for survival following medical management (0.717, 0.579, 0.695) | 15,900 | D | 14,700 |
Summary of sensitivity analysis for parameters related to results of treatment
In all but two of the cases considered, the overall ICER for TAVI and the ICER for TAVI applied to patients not suitable for SAVR remained below £20,000 per QALY, while the TAVI available option was more costly and less effective than the comparator for patients suitable for SAVR. The only case in which the ICERs for TAVI (overall or for patients not suitable for SAVR) rose above £20,000 per QALY was one in which the QoL scores were reduced to extremely, and almost certainly unrealistically, low values. The only case in which TAVI available was not less effective than the comparator was when the long-term survival following SAVR was reduced substantially.
While the univariate sensitivity analysis shown in this section gives an idea of the relative importance of each parameter in the model, it will usually underestimate the overall uncertainty. The next section of this report describes an analysis in which multiple parameters are varied simultaneously.
Probabilistic sensitivity analysis
Probabilistic sensitivity analysis is a method for assessing the overall effects of parameter uncertainty in a model. Parameters are sampled from a joint distribution and the resulting distribution of model outputs can be plotted on an incremental cost-effectiveness plane. For the TAVI model, parameter distributions were used as shown in Table 24. The general principle is that the distributions used here are the ones whose approximate 95% limits were used for the deterministic sensitivity analysis in the sections from Resource use in first month to Quality-of-life scores.
| Parameter | Distributiona | Comments |
|---|---|---|
| Adjustment to total cost of SAVR and TAVI | Normal(0,£1500) | Adjustment added to base-case value; independent adjustments for each treatment |
| 30-day survival SAVR (moderate risk) | Beta(216,17) | Theoretically correct distribution based on data67,42 |
| 30-day survival TAVI | Beta(170,9) | Theoretically correct distribution based on data14 |
| Shape parameters for Weibull distributions | Log-normal(1,0.1) | Base-case values for each treatment independently multiplied by exp(y), where y was sampled from a normal distribution with mean 0 and SD 0.1 |
| Scale parameters for Weibull distributions | Log-normal(1,0.2) | Base-case values for each treatment independently multiplied by exp(y), where y was sampled from a normal distribution with mean 0 and SD 0.2 |
| QoL scores | Beta distributions | Combined in the way described in the text |
For QoL scores, the beta distributions described in Quality-of-life scores were used. Values u1, v2 and v3 were sampled independently from beta distributions with parameters (5.06,2), (8.37,2) and (7.44,2) respectively. Then
u1 is the QoL for hospitalisation-free survival following SAVR or TAVI;
u2 = v2u1 is the QoL for post-hospitalisation survival following SAVR or TAVI; and
u3 = v3u1 is the QoL for survival following medical management.
The full justification for this approach may be found in Quality-of-life scores.
Figures 14, 15 and 16 show the incremental cost-effectiveness planes when 1000 samples were drawn from the distributions described and run through the model. The results are summarised in the cost-effectiveness acceptability curves in Figure 17.
FIGURE 14.
Incremental cost-effectiveness plane for overall results. The assumption here is that 90% of the TAVI patients were unsuitable for SAVR and that 9% would receive SAVR in the absence of TAVI, whereas 1% would be deemed suitable for SAVR but not receive it. All SAVR patients are assumed to be moderate risk.
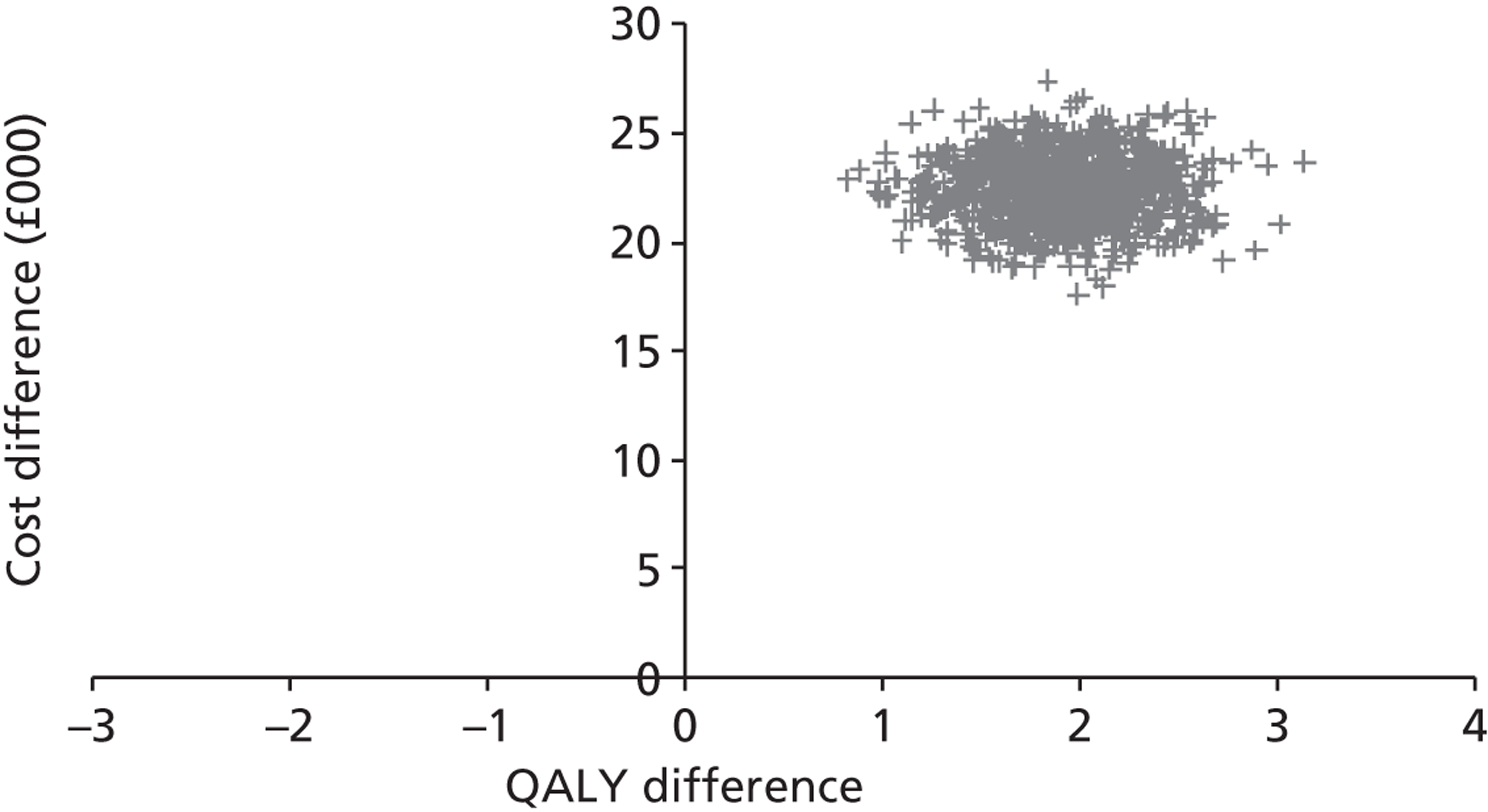
FIGURE 15.
Incremental cost-effectiveness plane for patients suitable for SAVR, of whom 90% would receive SAVR in the absence of TAVI, the other 10% receiving medical management. All SAVR patients are assumed to be moderate risk.
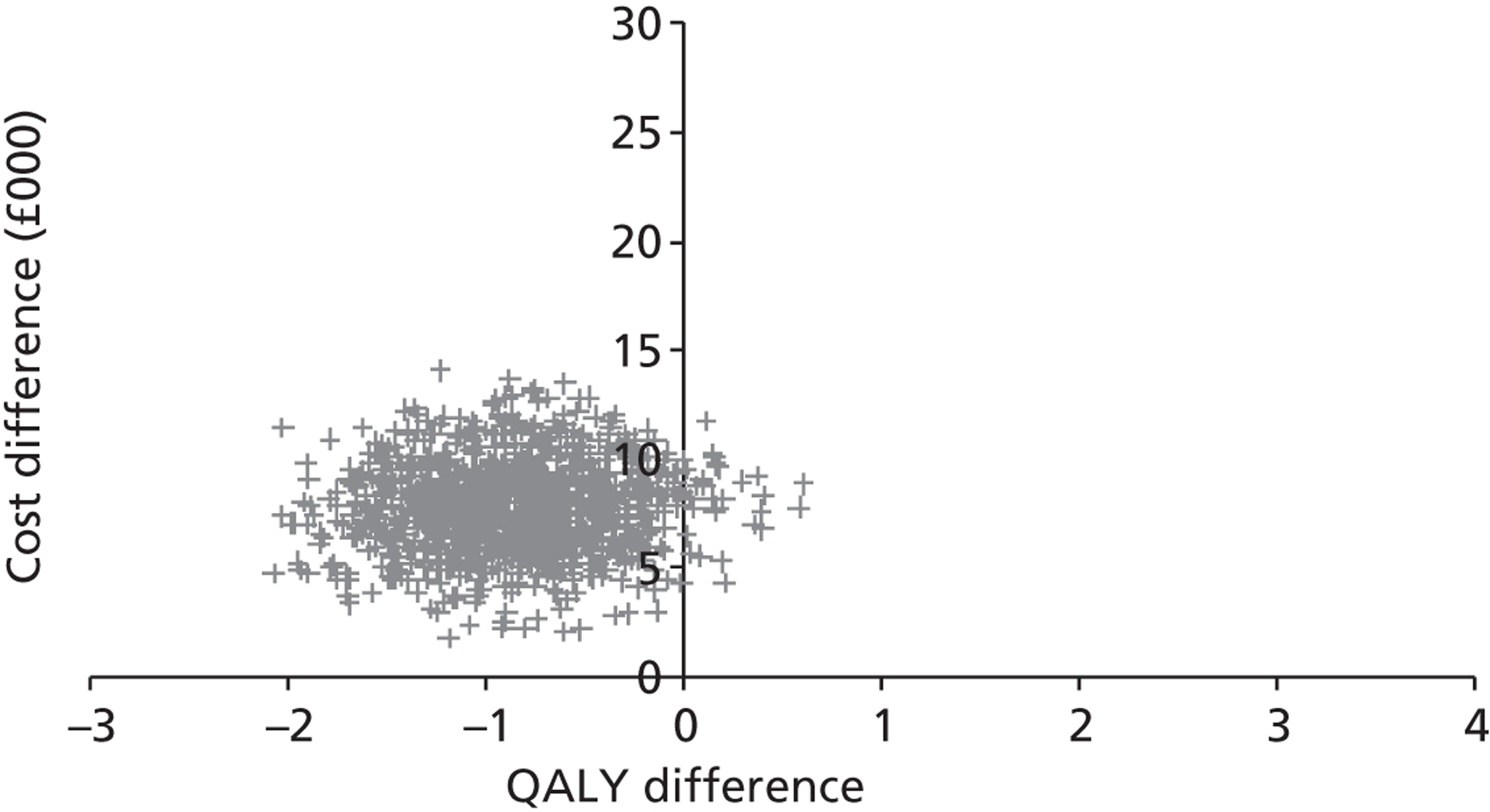
FIGURE 16.
Incremental cost-effectiveness plane for patients deemed unsuitable for SAVR, all of whom would receive medical management in the absence of TAVI.
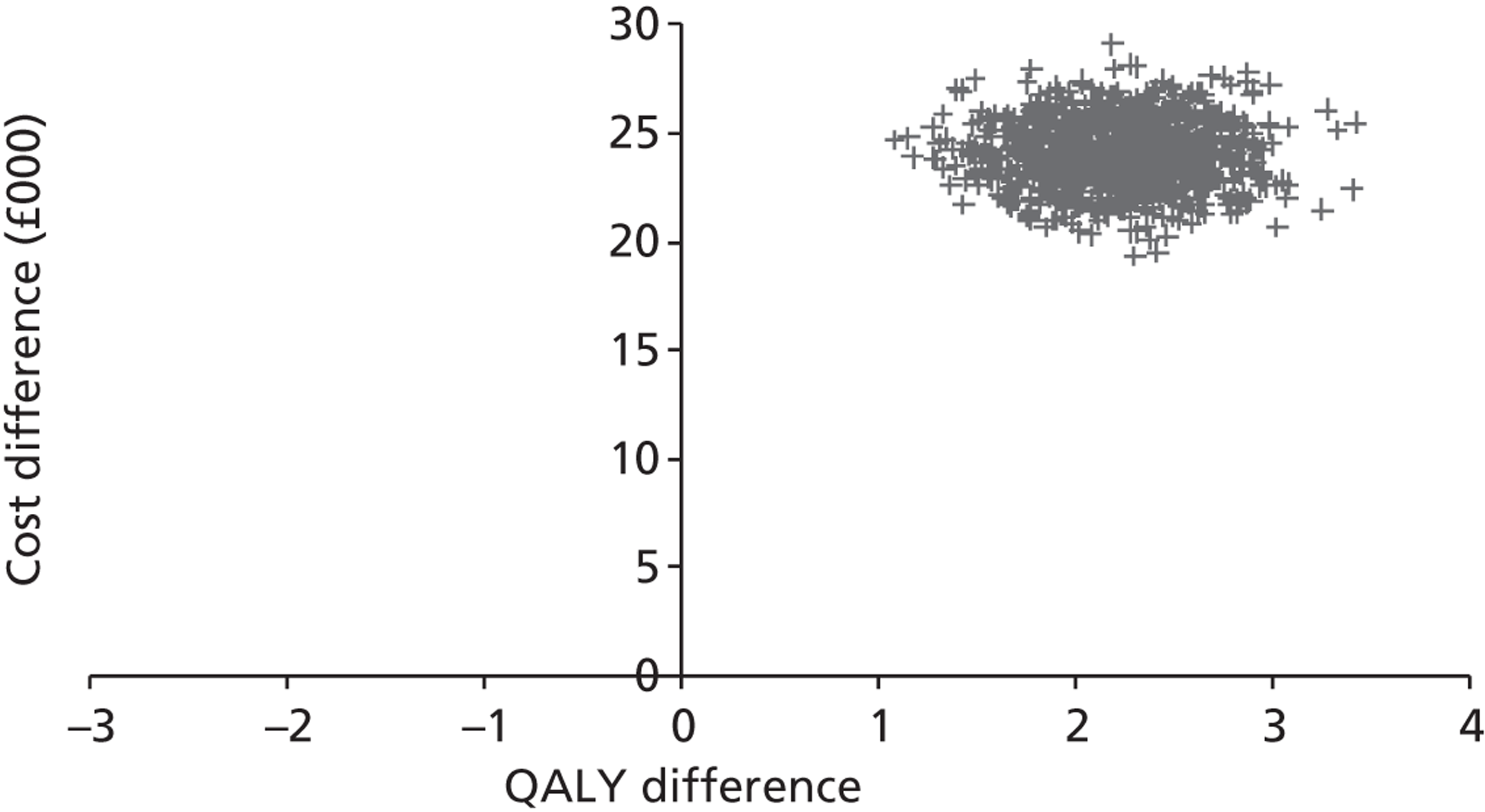
FIGURE 17.
Cost-effectiveness acceptability curves. The assumption here is that 90% of the TAVI patients were unsuitable for SAVR and that 9% would receive SAVR in the absence of TAVI, whereas 1% would be deemed suitable for SAVR but not receive it. All SAVR patients are assumed to be moderate risk.
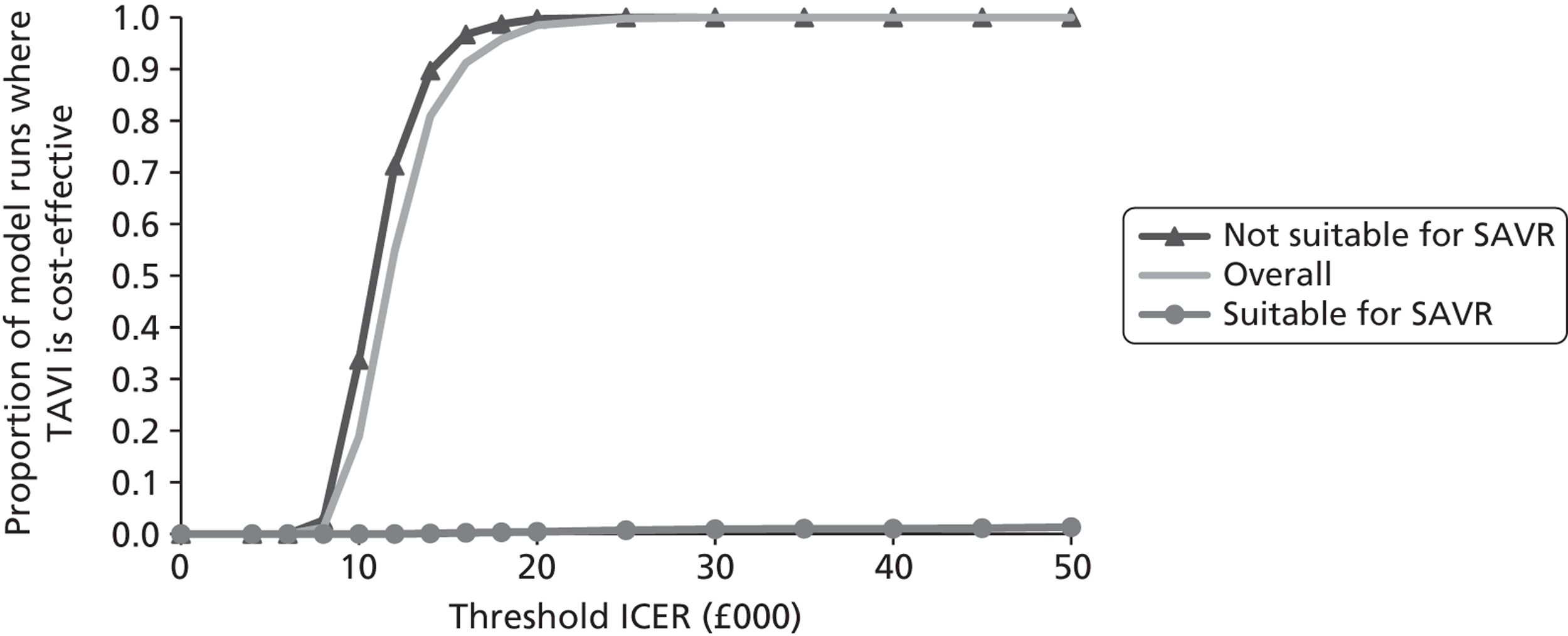
Figure 17 shows the proportion of model runs favouring TAVI at a range of possible threshold ICERs up to £50,000 per QALY. In the case where the model structure is known to be adequate and all the uncertainty is properly represented in the parameter distributions, this proportion may be interpreted in a Bayesian framework as the probability that TAVI is cost-effective at that threshold. Such an interpretation would be unjustified in this case. However, it may be said that the modelling in this report is supportive of the adoption of TAVI at any threshold ICER of £20,000 per QALY or higher for patients deemed unsuitable for SAVR. This modelling does not support the use of TAVI in patients suitable for SAVR: in 97% of model runs, TAVI is less effective than the comparator, and the cost-effectiveness acceptability curve accordingly does not rise above 0.03 however high the threshold ICER is set.
The above analysis was conducted on the basis that 90% of TAVI patients would be unsuitable for TAVI. Reducing this figure to 50% leaves the results for each patient group separately unchanged, but the overall results are now shown in the scatterplot in Figure 18 and the changed cost-effectiveness acceptability curve in Figure 19. These results are considerably less favourable to TAVI, and may be interpreted as showing the need for caution in extending the use of TAVI to patients deemed suitable for SAVR.
FIGURE 18.
Incremental cost-effectiveness plane for overall results under changed assumption. The assumption here is that 50% of the TAVI patients were unsuitable for SAVR and that 45% would receive SAVR in the absence of TAVI, whereas 5% would be deemed suitable for SAVR but not receive it. All SAVR patients are assumed to be moderate risk.
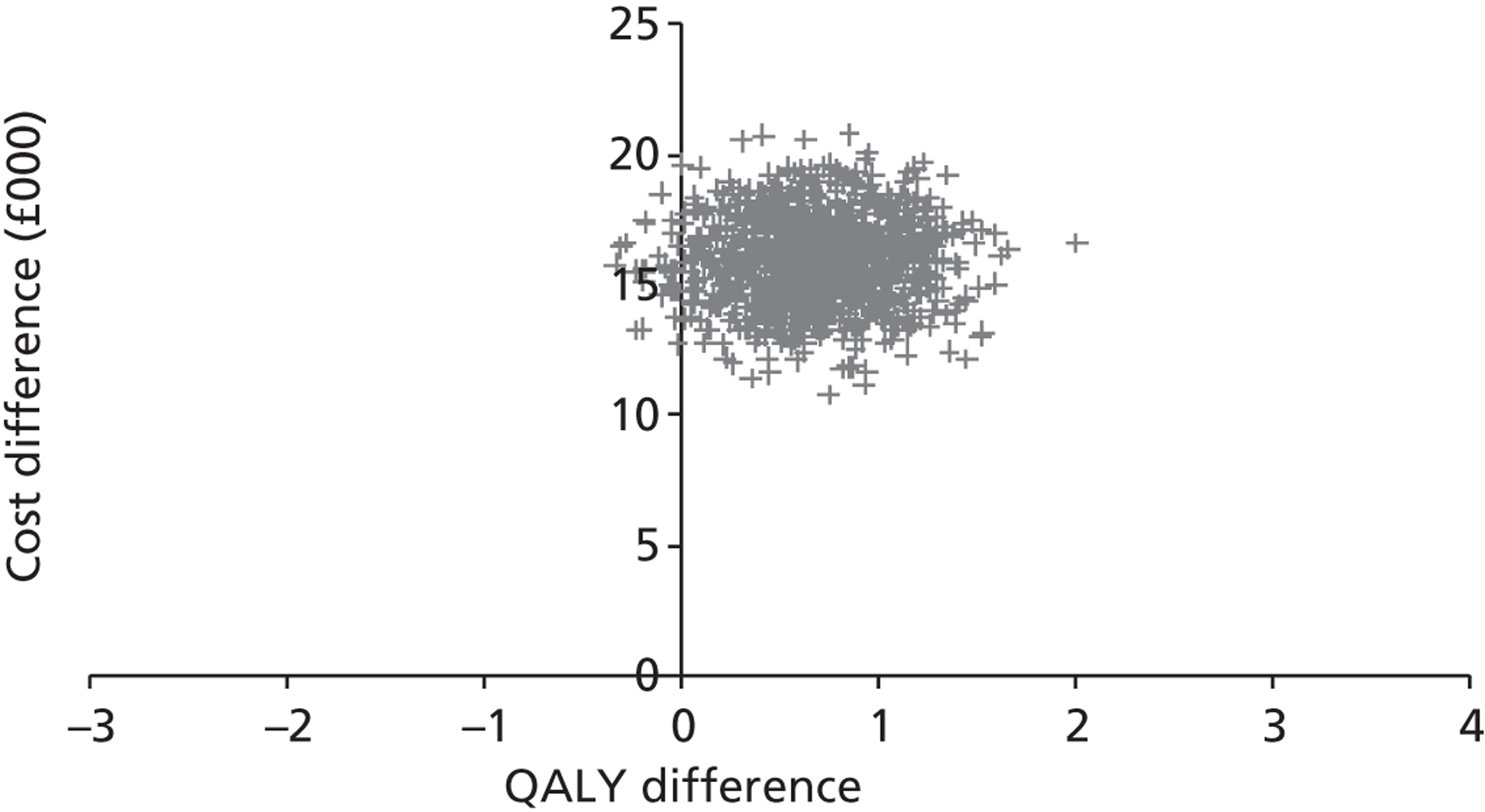
FIGURE 19.
Cost-effectiveness acceptability curves under changed assumption. The assumption here is that 50% of the TAVI patients were unsuitable for SAVR and that 45% would receive SAVR in the absence of TAVI, whereas 5% would be deemed suitable for SAVR but not receive it. All SAVR patients are assumed to be moderate risk.
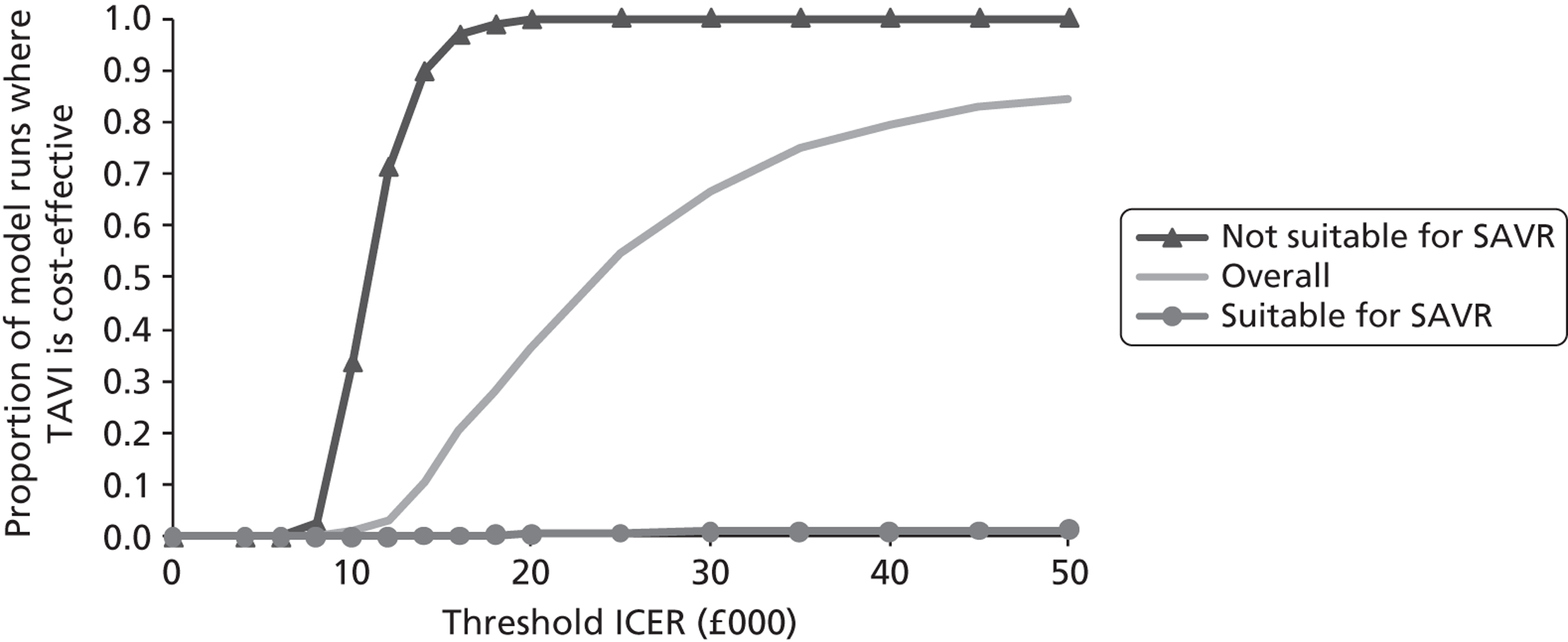
Summary of model results
The model has been run under a wide range of assumptions, comparing two policy options: one when TAVI is available and the other when TAVI is not available. Results have been estimated for the overall effect, and separately for patients who were, or were not, deemed suitable for SAVR.
Dealing first with the patients not suitable for SAVR, the comparison in this case is simply between TAVI and medical management. The base-case results show TAVI as more costly but more effective than the comparator, with an ICER of £12,900 per QALY. This result was robust to a number of changes in the model. In the deterministic sensitivity analysis, the only case in which the ICER exceeded £20,000 per QALY was when the QoL scores were taken to an extremely low value. The ICER was below £20,000 per QALY for over 99% of model runs in the probabilistic sensitivity analysis.
On the other hand, for patients suitable for SAVR, the comparator with TAVI is a mixture of SAVR and medical management. In this case, TAVI is both more costly and less effective than the comparator in the base-case analysis, which assumes that the vast majority of patients in this group would receive SAVR in the absence of TAVI. The base-case result is robust to a number of changes in the assumptions about the effects of treatment, but highly sensitive to assumptions about the proportion of patients receiving SAVR in the comparator arm of the model.
Overall results in the base-case analysis are close to the results for patients not suitable for SAVR, as would be expected given that these patients are assumed to form the majority of the modelled population. When the use of TAVI is extended to include a larger number of patients suitable for SAVR, the overall results become less favourable for TAVI.
Chapter 5 Discussion and conclusions
At the time this modelling work was carried out, no other economic model for TAVI had been published, although the results from some previous modelling had been presented. The modelling methods and results reported here are offered as an equal first model in this clinical area and are intended to contribute to a bank of knowledge about how TAVI can and should be modelled. The choice of patient health states for the model was based on the results reported in the PARTNER trial:14 surviving patients were classified according to whether or not they had undergone further hospitalisation. No attempt was made to subdivide the group of patients who had been rehospitalised at least once. With greater access to patient-level data, a more detailed representation of patient status after first rehospitalisation could be modelled.
The UK TAVI registry is likely to collect appropriate data for populating the model for the population groups in which TAVI is the chosen treatment. However, care will need to be taken in interpreting the results if such data are used for TAVI alone without making a proper estimate, preferably based on a randomised study, of how those patients would have fared under an alternative treatment.
Although a detailed assessment of short-term adverse events has been attempted, it should be noted that the impact of changes in the assumptions here has been shown to be small compared with other features of the model.
The results given here for TAVI compared with medical management in patients unsuitable for surgery are reasonably robust and suggest that TAVI is likely to be cost-effective in those patients. On the other hand, for patients in whom the alternative is surgery, the results suggest that TAVI could be both more costly and less effective than SAVR. However, these results are not based on randomised data and could easily be upset by the results of trials reporting after this work was carried out. The work in this report was undertaken before the publication of relevant randomised trial results.
The overall results suggest that the total effect of introducing TAVI falls within conventional standards of cost-effectiveness. It should be stressed that this depends on the assumption that a very substantial majority of TAVI patients will be those who are unsuitable for surgery. From a decision-theoretic point of view, the overall results should not be used to guide any decision, but the decision to allow TAVI for different patient groups should be taken separately for each group based on the results for that patient group alone. However, in practice a decision to allow TAVI for patients deemed unsuitable for surgery is likely to lead to TAVI being offered to some patients who would have received surgery in the absence of TAVI; it is helpful to quantify the importance of this possibility.
Comparison with other published results
The only cost-effectiveness results known to have been published at the time of this work were in the form of conference abstracts. 62–64 Work by Murphy et al. 65 formed part of the evidence for the Scottish Health Technologies Group advice statement, which did not recommend the use of TAVI. However, the published abstract62 does not give any numerical estimates of cost-effectiveness. Work by Watt et al. 63 compares TAVI against medical management only, using a 10-year time horizon. Their work states a base-case ICER of approximately £23,000 per QALY with a probability of approximately 95% that TAVI is cost-effective at a threshold ICER of £30,000 per QALY. The results in the current report are somewhat more favourable to TAVI. The results given by Diage et al. 64 are based on a 3-year time horizon. It is not clear what patient group was modelled, and the results cannot be considered as comparable with those reported here.
Strengths
The main strength of the current work is that it is based on a clear model structure based directly on patient survival curves (both for overall survival and, except for SAVR, for hospitalisation-free survival). The assumptions behind the modelling are generally very transparent, and the effects of alternative assumptions can be readily found, as has been shown in the sensitivity analysis. The comparison between TAVI and medical management for patients unsuitable for surgery is based on the only randomised data available at the time of producing this report. Results for the part of the PARTNER trial comparing TAVI against SAVR were not in the public domain at the time of the production of this report.
The model allows for consideration of different referral strategies. In particular, it allows for consideration of the possibility that TAVI availability might lead to some patients receiving SAVR when they might otherwise have had medical management. The results suggest that the overall cost-effectiveness of TAVI is highly sensitive to this possibility.
Limitations
There are considerable limitations involved in this analysis. The most obvious of these relates to the limited availability of data at the time the work in this report was undertaken. For example, as mentioned above, only part of the PARTNER trial results were in the public domain. Sources of non-randomised data such as the UK TAVI registry were not readily accessible during the time frame of this report. One consequence of the limited data was to restrict the model structure, in which all living patients are classified into one of only two health states, depending on whether or not they had undergone repeat hospitalisation. It would be preferable to include a wider range of health states in the model, subject to the availability of appropriate data. A further consequence is that the modelling depends on the PARTNER Cohort B data, and the extent to which this can be applied to the UK TAVI/medical management population is debatable.
A further important limitation of the model is that it is based on long-term extrapolation of survival curves, which were often based on real data for only 2 years. In particular, it is not known how long TAVI will last for a patient, and only highly simplistic methods have been used to test the importance of this feature of the model.
The model uses data for SAVR based on entirely different studies from those used for the other treatments in the model. These were the best available at the time of carrying out the analysis, but it would clearly be an advantage if randomised data were used. Such randomised data became available after the work in this report was undertaken.
The methods used to estimate QoL scores for the various health states in the model are seriously unstable; although the numbers actually used may be reasonable, there is considerable scope for improvement in the reliability of these estimates.
Although the patient selection process for TAVI includes an appreciable amount of clinicians' time, this has not been costed into the analysis.
Implications for health care
The results in this report suggest that TAVI is a cost-effective treatment when the comparator is medical management. However, the evidence and modelling in this report do not suggest that TAVI should be widely used as an alternative to SAVR.
Research recommendations
The various initial models, including those produced for manufacturers and the Scottish Health Technologies Group, need to be reconciled with each other and with the model introduced in this report. The sensitivity analysis from each of these models should give an indication as to the importance of the various features of an agreed model.
The data being generated while this report was under construction should be included where appropriate in any revised analysis based on an agreed model structure.
Future data collection, including any future trials, should be designed with economic evaluation in mind. In particular, once a set of relevant health states for future modelling is agreed, every effort should be made to obtain realistic and reasonable QoL scores for those health states.
Acknowledgements
We would like to thank Dr P Ludman for clinical advice.
Contributions of authors
R Orlando and P Barton co-ordinated the design of the economic model.
R Orlando, M Pennant and A Hassan carried out the data extraction and checking.
S Rooney and S Khogali provided clinical advice throughout the project.
S Bayliss designed search strategies and undertook the searches.
R Orlando and P Barton reviewed the economic literature.
P Barton coded the model and led the analysis of the results.
D Moore was the senior reviewer on this report and provided project management advice.
All authors contributed to the drafting of the report.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Iung B, Baron G, Butchart EG, Delahaye F, Gohlke-Barwolf C, Levang OW, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003;24:1231-43. http://dx.doi.org/10.1016/S0195-668X(03)00201-X.
- Vahanian A, Baumgartner H, Bax J, Butchart E, Dion R, Filippatos G, et al. Guidelines on the management of valvular heart disease: The Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J 2007;28:230-68. http://dx.doi.org/10.1093/eurheartj/ehm095.
- Sagie A, Dvir D. Recent advances in medical treatment and percutaneous, transapical and surgical interventions in aortic-valve stenosis. F1000 Med Rep 2009;1:1-5. http://dx.doi.org/10.3410/M1-26.
- Carabello BA, Paulus WJ. Aortic stenosis. Lancet 2009;373:956-66. http://dx.doi.org/10.1016/S0140-6736(09)60211-7.
- Nathaniel S, Saligram S, Innasimuthu AL. Aortic stenosis: An update. World J Cardiol 2010;2:135-9. http://dx.doi.org/10.4330/wjc.v2.i6.135.
- Bonow RO, Carabello BA, Chatterjee K, de Leon ACJ, Faxon DP, Freed MD, et al. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease. J Am Coll Cardiol 2008;52:e1-142. http://dx.doi.org/10.1016/j.jacc.2008.05.007.
- Iung B, Vahanian A. Epidemiology of valvular heart disease in the adult. Nature Rev Cardiol 2011;8:162-72. http://dx.doi.org/10.1038/nrcardio.2010.202.
- Lindroos M, Kupari M, Heikkila J, Tilvis R. Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J Am Coll Cardiol 1993;21:1220-5. http://dx.doi.org/10.1016/0735-1097(93)90249-Z.
- Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol 1997;29:630-4. http://dx.doi.org/10.1016/S0735-1097(96)00563-3.
- Vahanian A, Alfieri O, Al-Attar N, Antunes M, Bax J, Cormier B, et al. Transcatheter valve implantation for patients with aortic stenosis: a position statement from the European Association of Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg 2008;34:1-8. http://dx.doi.org/10.1016/j.ejcts.2008.04.039.
- Ross J, Braunwald E. Aortic stenosis. Circulation 1968;38:61-7. http://dx.doi.org/10.1161/01.CIR.38.1S5.V-61.
- Bonow RO, Carabello B, de Leon ACJ, Edmunds LH, Fedderly BJ, Freed MD, et al. Guidelines for the management of patients with valvular heart disease: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients with Valvular Heart Disease). Circulation 1998;98:1949-84. http://dx.doi.org/10.1161/01.CIR.98.18.1949.
- Coeytaux RR, Williams JW, Gray RN, Wang A. Percutaneous heart valve replacement for aortic stenosis: state of the evidence. Ann Intern Med 2010;153:314-24. http://dx.doi.org/10.7326/0003-4819-153-5-201009070-00267.
- Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597-607. http://dx.doi.org/10.1056/NEJMoa1008232.
- Urso S, Sadaba R, Aldamiz-Echevarria G. Is patient-prosthesis mismatch an independent risk factor for early and mid-term overall mortality in adult patients undergoing aortic valve replacement?. Interact Cardiovasc Thorac Surg 2009;9:510-18. http://dx.doi.org/10.1510/icvts.2009.207597.
- Vicchio M, Della Corte A, De Santo LS, De Feo M, Caianiello G, Scardone M, et al. Tissue versus mechanical prostheses: quality of life in octogenarians. Ann Thoracic Surgery 2008;85:1290-5. http://dx.doi.org/10.1016/j.athoracsur.2007.12.039.
- Tjang YS, van Hees Y, Korfer R, Grobbee DE, van der Heijden GJ. Predictors of mortality after aortic valve replacement. Eur J Cardiothorac Surg 2007;32:469-74. http://dx.doi.org/10.1016/j.ejcts.2007.06.012.
- Iung B. Management of the elderly patient with aortic stenosis. Heart 2008;94:519-24. http://dx.doi.org/10.1136/hrt.2007.122804.
- Levin IL, Olivecrona GK, Thulin LI, Olsson SB. Aortic valve replacement in patients older than 85 years: outcomes and the effect on their quality of life. Coron Artery Dis 1998;9:373-80. http://dx.doi.org/10.1097/00019501-199809060-00009.
- Olsson M, Janfjall H, Orth-Gomer K, Unden A, Rosenqvist M. Quality of life in octogenarians after valve replacement due to aortic stenosis. A prospective comparison with younger patients. Eur Heart J 1996;17:583-9. http://dx.doi.org/10.1093/oxfordjournals.eurheartj.a014912.
- Walther T, Rastan A, Falk V, Lehmann S, Garbade J, Funkat AK, et al. Patient prosthesis mismatch affects short- and long-term outcomes after aortic valve replacement. Eur J Cardiothorac Surg 2006;30:15-9. http://dx.doi.org/10.1016/j.ejcts.2006.04.007.
- Ben-Dor I, Pichard AD, Satler LF, Okubagzi P, Torguson R, Xue Z, et al. Clinical profile, treatment assignment and clinical outcome of patients with severe aortic stenosis not eligible to participate in a clinical trial of percutaneous aortic valve replacement. Am J Cardiol 2010;105:857-61. http://dx.doi.org/10.1016/j.amjcard.2009.11.045.
- Hudorovic N. Aortic valve surgery: what is the future?. Int J Surg 2008;6:169-74. http://dx.doi.org/10.1016/j.ijsu.2007.04.009.
- National Institute for Health and Care Excellence . Balloon Valvuloplasty for Aortic Valve Stenosis in Adults and Children n.d. http://publications.nice.org.uk/balloon-valvuloplasty-for-aortic-valve-stenosis-in-adults-and-children-ipg78 (accessed 23 July 2012).
- National Institute for Health and Care Excellence . IPG421: Transcatheter Aortic Valve Implantation for Aortic Stenosis n.d. http://publications.nice.org.uk/transcatheter-aortic-valve-implantation-for-aortic-stenosis-ipg421/guidance (accessed 23 July 2012).
- Cribier A, Eltchaninoff H, Bash A, Borenstein N, Tron C, Bauer F, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 2002;106:3006-8. http://dx.doi.org/10.1161/01.CIR.0000047200.36165.B8.
- Webb JG, Lichtenstein S. Transcatheter percutaneous and transapical aortic valve replacement. Semin Thorac Cardiovasc Surg 2007;19:304-10. http://dx.doi.org/10.1053/j.semtcvs.2007.11.001.
- Lefevre T, Kappetein AP, Wolner E, Nataf P, Thomas M, Schachinger V, et al. One year follow-up of the multi-centre European PARTNER transcatheter heart valve study. Eur Heart J 2011;32:148-57. http://dx.doi.org/10.1093/eurheartj/ehq427.
- Moat N, Ludman P, de Belder M, Bridgewater B, Cunningham A, Young C, et al. Long-term outcomes after transcatheter aortic valve implantation in high-risk patients with severe aortic stenosis: the UK TAVI (United Kingdom transcatheter aortic valve implantation) Registry. J Am Coll Cardiol 2011;58:2130-8.
- Tzikas A, Piazza N, Geleijnse ML, Van Mieghem NM, Nuis RJ, Schultz C, et al. Prosthesis-patient mismatch after transcatheter aortic valve implantation with the medtronic CoreValve system in patients with aortic stenosis. Am J Cardiol 2010;106:255-60. http://dx.doi.org/10.1016/j.amjcard.2010.02.036.
- Ruel M, Labinaz M. Transcatheter aortic-valve replacement: a cardiac surgeon and cardiologist team perspective. Curr Opin Cardiol 2010;25:107-13. http://dx.doi.org/10.1097/HCO.0b013e328335fff4.
- Thomas M, Schymik G, Walther T, Himbert D, Lefevre T, Treede H, et al. Thirty-day results of the SAPIEN aortic Bioprosthesis European Outcome (SOURCE) Registry: A European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation 2010;122:62-9. http://dx.doi.org/10.1161/CIRCULATIONAHA.109.907402.
- Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187-98. http://dx.doi.org/10.1056/NEJMoa1103510.
- Krane M, Deutsch MA, Bleiziffer S, Schneider L, Ruge H, Mazzitelli D, et al. Quality of life among patients undergoing transcatheter aortic valve implantation. Am Heart J 2010;160:451-7. http://dx.doi.org/10.1016/j.ahj.2010.05.038.
- Percutaneous aortic valve replacement for severe aortic stenosis. Part A: Technology assessment and impact model for East Midlands Specialist Commissioning Group. London: Bazian Ltd; 2008.
- British Cardiovascular Intervention Society and the Society of Cardiothoracic Surgeons . Transcatheter Aortic Valve Implantation (TAVI): A Position Statement of the British Cardiovascular Intervention Society (BCIS) and the Society of Cardiothoracic Surgeons (SCTS) 2010. www.bcis.org.uk/resources/documents/BCIS%20SCTS%20position%20statement.pdf (accessed 22 March 2013).
- Mack MJ. Risk scores for predicting outcomes in valvular heart disease: how useful?. Curr Cardiol Rep 2011;13:107-12. http://dx.doi.org/10.1007/s11886-010-0167-9.
- Roques F, Nashef SA, Michel P, Gauducheau E, de Vincentiis C, Baudet E, et al. Risk factors and outcome in European cardiac surgery: analysis of the EuroSCORE multinational database of 19030 patients. Eur J Cardiothorac Surg 1999;15:816-22. http://dx.doi.org/10.1016/S1010-7940(99)00106-2.
- Nashef SA, Roques F, Michel P, Gauducheau E, Lemeshow S, Salamon R. European system for cardiac operative risk evaluation (EuroSCORE). Eur J Cardiothorac Surg 1999;16:9-13. http://dx.doi.org/10.1016/S1010-7940(99)00134-7.
- O’Brien SM, Shahian DM, Filardo G, Ferraris VA, Haan CK, Rich JB, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 2 – isolated valve surgery. Ann Thorac Surg 2009;88:23-42. http://dx.doi.org/10.1016/j.athoracsur.2009.05.056.
- Ranucci M, Guarracino F, Castelvecchio S, Baldassarri R, Covello RD, Landoni G, et al. Surgical and transcatheter aortic valve procedures. The limits of risk scores. Interact Cardiovasc Thorac Surg 2010;11:138-41. http://dx.doi.org/10.1510/icvts.2010.236141.
- Wendt D, Osswald BR, Kayser K, Thielmann M, Tossios P, Massoudy P, et al. Society of Thoracic Surgeons score is superior to the EuroSCORE determining mortality in high risk patients undergoing isolated aortic valve replacement. Ann Thorac Surg 2009;88:468-74. http://dx.doi.org/10.1016/j.athoracsur.2009.04.059.
- Frilling B, von Renteln-Kruse W, Riess FC. Evaluation of operative risk in elderly patients undergoing aortic valve replacement: the predictive value of operative risk scores. Cardiology 2010;116:213-18. http://dx.doi.org/10.1159/000319703.
- Dewey TM, Brown D, Ryan WH, Herbert MA, Prince SL, Mack MJ. Reliability of risk algorithms in predicting early and late operative outcomes in high-risk patients undergoing aortic valve replacement. J Thorac Cardiovasc Surg 2008;135:180-7. http://dx.doi.org/10.1016/j.jtcvs.2007.09.011.
- Ben-Dor I, Waksman R, Satler LF, Pichard AD. Patient selection–risk assessment and anatomical selection criteria for patients undergoing transfemoral aortic valve implantation. Cardiovasc Revasc Med 2010;11:124-36. http://dx.doi.org/10.1016/j.carrev.2009.03.005.
- Piazza N, Wenaweser P, van Gameren M, Pilgrim T, Tsikas A, Otten A, et al. Relationship between the logistic EuroSCORE and the Society of Thoracic Surgeons Predicted Risk of Mortality score in patients implanted with the CoreValve ReValving system – a Bern-Rotterdam Study. Am Heart J 2010;159:323-9. http://dx.doi.org/10.1016/j.ahj.2009.11.026.
- Chiappini B, Camurri N, Loforte A, Di Marco L, Di Bartolomeo R, Marinelli G. Outcome after aortic valve replacement in octogenarians. Ann Thorac Surg 2004;78:85-9. http://dx.doi.org/10.1016/j.athoracsur.2003.12.060.
- Nugteren LB, Sandau KE. Critical review of health-related quality of life studies of patients with aortic stenosis. J Cardiovasc Nurs 2010;25:25-39.
- Kojodjojo P, Gohil N, Barker D, Youssefi P, Salukhe TV, Choong A, et al. Outcomes of elderly patients aged 80 and over with symptomatic, severe aortic stenosis: impact of patient’s choice of refusing aortic valve replacement on survival. QJM 2008;101:567-73. http://dx.doi.org/10.1093/qjmed/hcn052.
- Bakaeen FG, Chu D, Ratcliffe M, Gopaldas RR, Blaustein AS, Venkat R, et al. Severe aortic stenosis in a veteran population: treatment considerations and survival. Ann Thorac Surg 2010;89:453-8. http://dx.doi.org/10.1016/j.athoracsur.2009.10.033.
- Malaisrie SC, Tuday E, Lapin B, Wang E, Lee R, McGee EC, et al. Transcatheter aortic valve implantation decreases the rate of unoperated aortic stenosis. Eur J Cardiothorac Surg 2011;40:43-8. http://dx.doi.org/10.1016/j.ejcts.2010.11.031.
- Pierard S, Seldrum S, de Meester C, Pasquet A, Gerber B, Vancraeynest D, et al. Incidence, determinants, and prognostic impact of operative refusal or denial in octogenarians with severe aortic stenosis. Ann Thorac Surg 2011;91:1107-12. http://dx.doi.org/10.1016/j.athoracsur.2010.12.052.
- Freed BH, Sugeng L, Furlong K, Mor-Avi V, Raman J, Jeevanandam V, et al. Reasons for nonadherence to guidelines for aortic valve replacement in patients with severe aortic stenosis and potential solutions. Am J Cardiol 2010;105:1339-42. http://dx.doi.org/10.1016/j.amjcard.2009.12.056.
- Grant SW, Devbhandari MP, Grayson AD, Dimarakis I, Kadir I, Saravanan DM, et al. What is the impact of providing a transcatheter aortic valve implantation service on conventional aortic valve surgical activity: patient risk factors and outcomes in the first 2 years. Heart 2010;96:1633-7. http://dx.doi.org/10.1136/hrt.2010.203661.
- Dewey TM, Brown DL, Das TS, Ryan WH, Fowler JE, Hoffman SD, et al. High-risk patients referred for transcatheter aortic valve implantation: management and outcomes. Ann Thorac Surg 2008;86:1450-6. http://dx.doi.org/10.1016/j.athoracsur.2008.07.043.
- Kapadia SR, Goel SS, Svensson L, Roselli E, Savage RM, Wallace L, et al. Characterization and outcome of patients with severe symptomatic aortic stenosis referred for percutaneous aortic valve replacement. J Thorac Cardiovasc Surg 2009;137:1430-5. http://dx.doi.org/10.1016/j.jtcvs.2008.12.030.
- Thomas M, Wendler O. Transcatheter aortic valve implantation (TAVI): how to interpret the data and what data is required?. Eurointervention 2009;5:25-7. http://dx.doi.org/10.4244/EIJV5I1A4.
- Bridgewater B, Keogh B, Kinsman R, Walton P. Sixth National Adult Cardiac Surgical Database Report 2008.
- Stewart KJ, Badenhop D, Brubaker PH, Keteyian SJ, King M. Cardiac rehabilitation following percutaneous revascularization, heart transplant, heart valve surgery, and for chronic heart failure. Chest 2003;123:2104-11. http://dx.doi.org/10.1378/chest.123.6.2104.
- Sire S. Physical training and occupational rehabilitation after aortic valve replacement. Eur Heart J 1987;8:1215-20.
- Drummond M, Sculpher M, Torrance G, O’Brien B, Stoddard G. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2005.
- Murphy A, Briggs A, Fenwick E. n.d.
- Watt M, Mealing S, Sculpher M. n.d.
- Diage T, Martin P, Payyanad R, Wolfrath D, Chih M, Almany SL. Cost–benefit analysis of transfemoral aortic valve implantation (TAVI) for treatment of aortic stenosis (TCT-490). J Am Coll Cardiol 2010;56.
- Scottish Health Technologies Group . Transcatheter Aortic Valve Implantation: Advice Statement 2011. www.healthcareimprovementscotland.org/our_work/technologies_and_medicines/shtg_advice_statements/advice_statement_005-11.aspx (accessed 22 March 2013).
- Reynolds MR, Magnuson EA, Wang K, Lei Y, Vilain K, Walczak J, et al. Cost-effectiveness of transcatheter aortic valve replacement compared with standard care among inoperable patients with severe aortic stenosis. Circulation 2012;125:1102-9. http://dx.doi.org/10.1161/CIRCULATIONAHA.111.054072.
- Leontyev S, Walther T, Borger MA, Lehmann S, Funkat AK, Rastan A, et al. Aortic valve replacement in octogenarians: utility of risk stratification with EuroSCORE. Ann Thorac Surg 2009;87:1440-5. http://dx.doi.org/10.1016/j.athoracsur.2009.01.057.
- Bollati M, Tizzani E, Moretti C, Sciuto F, Omede P, Zoccai GB, et al. The future of new aortic valve replacement approaches. Future Cardiol 2010;6:351-60. http://dx.doi.org/10.2217/fca.10.14.
- Maliwa MA, van der Heijden GJ, Bots ML, van Hout BA, Casselman FP, van Swieten H, et al. Quality of life and NYHA class 30 years after mechanical aortic valve replacement. Cardiovasc Surg 2003;11:381-7. http://dx.doi.org/10.1016/S0967-2109(03)00030-9.
- NHS reference costs 2009–2010. Department of Health; 2011.
- Meeson B. Model service specification for Transcatheter Aortic Valve Implantation. NHS South Central Cardiovascular Network. Portsmouth: South Central Cardiovascular Network; 2010.
- Gray AM, Clarke PM, Wolstenholme J, Wordsworth S. Applied methods of cost-effectiveness analysis in health care. Oxford Handbooks in Health Economics Evaluation. Oxford: Oxford University Press; 2010.
- Svensson LG, Dewey T, Kapadia S, Roselli EE, Stewart A, Williams M, et al. United States feasibility study of transcatheter insertion of a stented aortic valve by the left ventricular apex. Ann Thorac Surg 2008;86:46-54. http://dx.doi.org/10.1016/j.athoracsur.2008.04.049.
- Walther T, Schuler G, Borger MA, Kempfert J, Seeburger J, Ruckert Y, et al. Transapical aortic valve implantation in 100 consecutive patients: comparison to propensity-matched conventional aortic valve replacement. Eur Heart J 2010;31:1398-403. http://dx.doi.org/10.1093/eurheartj/ehq060.
- Thielmann M, Wendt D, Eggebrecht H, Kahlert P, Massoudy P, Kamler M, et al. Transcatheter aortic valve implantation in patients with very high risk for conventional aortic valve replacement. Ann Thorac Surg 2009;88:1468-74. http://dx.doi.org/10.1016/j.athoracsur.2009.07.033.
- Maillet JM, Somme D, Hennel E, Lessana A, Saint-Jean O, Brodaty D. Frailty after aortic valve replacement (AVR) in octogenarians. Arch Gerontol Geriatr 2009;48:391-6. http://dx.doi.org/10.1016/j.archger.2008.03.010.
- Berry C, Asgar A, Lamarche Y, Marcheix B, Couture P, Basmadjian A, et al. Novel therapeutic aspects of percutaneous aortic valve replacement with the 21F CoreValve Revalving System. Catheter Cardiovasc Interv 2007;70:610-16. http://dx.doi.org/10.1002/ccd.21282.
- Kalavrouziotis D, Li D, Buth KJ, Légaré JF. The European System for Cardiac Operative Risk Evaluation (EuroSCORE) is not appropriate for withholding surgery in high-risk patients with aortic stenosis: a retrospective cohort study. J Cardiothorac Surg 2009;4:1-8. http://dx.doi.org/10.1186/1749-8090-4-32.
- Marcheix B, Lamarche Y, Berry C, Asgar A, Laborde J-C, Basmadjian A, et al. Surgical aspects of endovascular retrograde implantation of the aortic CoreValve bioprosthesis in high-risk older patients with severe symptomatic aortic stenosis. J Thorac Cardiovasc Surg 2007;134:1150-6. http://dx.doi.org/10.1016/j.jtcvs.2007.07.031.
- Kolh P, Kerzmann A, Honore C, Comte L, Limet R. Aortic valve surgery in octogenarians: predictive factors for operative and long-term results. Eur J Cardiothorac Surg 2007;31:600-6. http://dx.doi.org/10.1016/j.ejcts.2007.01.003.
- Osten MD, Feindel C, Greutmann M, Chamberlain K, Meineri M, Rubin B, et al. Transcatheter aortic valve implantation for high risk patients with severe aortic stenosis using the Edwards Sapien balloon-expandable bioprosthesis: a single centre study with immediate and medium-term outcomes. Catheter Cardiovasc Interv 2010;75:475-85. http://dx.doi.org/10.1002/ccd.22291.
- Eltchaninoff H. Evaluation before transcatheter aortic valve implantation. Arch Cardiovasc Dis 2010;103:205-6. http://dx.doi.org/10.1016/j.acvd.2010.03.005.
- Webb JG, Pasupati S, Humphries K, Thompson C, Altwegg L, Moss R, et al. Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation 2007;116:755-63. http://dx.doi.org/10.1161/CIRCULATIONAHA.107.698258.
- Walther T, Simon P, Dewey T, Wimmer-Greinecker G, Falk V, Kasimir MT, et al. Transapical minimally invasive aortic valve implantation: multicenter experience. Circulation 2007;116:I240-5. http://dx.doi.org/10.1161/CIRCULATIONAHA.106.677237.
- Covello RD, Maj G, Landoni G, Maisano F, Michev I, Guarracino F, et al. Anesthetic management of percutaneous aortic valve implantation: focus on challenges encountered and proposed solutions. J Cardiothorac Vasc Anesth 2009;23:280-5. http://dx.doi.org/10.1053/j.jvca.2008.12.017.
- Dworakowski R, MacCarthy PA, Monaghan M, Redwood S, El-Gamel A, Young C, et al. Transcatheter aortic valve implantation for severe aortic stenosis – a new paradigm for multidisciplinary intervention: a prospective cohort study. Am Heart J 2010;160:237-43.
- John D, Buellesfeld L, Yuecel S, Mueller R, Latsios G, Beucher H, et al. Correlation of Device landing zone calcification and acute procedural success in patients undergoing transcatheter aortic valve implantations with the self-expanding CoreValve prosthesis. JACC: Cardiovasc Interv 2010;3:233-43. http://dx.doi.org/10.1016/j.jcin.2009.11.015.
- Grube E, Buellesfeld L, Mueller R, Sauren B, Zickmann B, Nair D, et al. Progress and current status of percutaneous aortic valve replacement: results of three device generations of the CoreValve Revalving system. Circulation: Cardiovasc Interv 2008;1:167-75. http://dx.doi.org/10.1161/CIRCINTERVENTIONS.108.819839.
- Grube E, Schuler G, Buellesfeld L, Gerckens U, Linke A, Wenaweser P, et al. Percutaneous aortic valve replacement for severe aortic stenosis in high-risk patients using the second- and current third-generation self-expanding CoreValve prosthesis: device success and 30-day clinical outcome. J Am Coll Cardiol 2007;50:69-76.
- Buellesfeld L, Wenaweser P, Gerckens U, Mueller R, Sauren B, Latsios G, et al. Transcatheter aortic valve implantation: predictors of procedural success – the Siegburg-Bern experience. Eur Heart J 2010;31:984-91. http://dx.doi.org/10.1093/eurheartj/ehp570.
- Bojara W, Mumme A, Gerckens U, Lindstaedt M, Gotzmann M, Germing A, et al. Implantation of the CoreValve self-expanding valve prosthesis via a subclavian artery approach: a case report. Clin Res Cardiol 2009;98:201-4. http://dx.doi.org/10.1007/s00392-009-0750-5.
- Bleiziffer S, Ruge H, Mazzitelli D, Schreiber C, Hutter A, Laborde JC, et al. Results of percutaneous and transapical transcatheter aortic valve implantation performed by a surgical team. Eur J Cardiothorac Surg 2009;35:615-20. http://dx.doi.org/10.1016/j.ejcts.2008.12.041.
- Schofer J, Schluter M, Treede H, Franzen OW, Tubler T, Pascotto A, et al. Retrograde transarterial implantation of a nonmetallic aortic valve prosthesis in high-surgical-risk patients with severe aortic stenosis: a first-in-man feasibility and safety study. Circulation: Cardiovasc Interv 2008;1:126-33. http://dx.doi.org/10.1161/CIRCINTERVENTIONS.108.800607.
- Walther T, Falk V, Borger MA, Kempfert J, Ender J, Linke A, et al. Transapical aortic valve implantation in patients requiring redo surgery. Eur J Cardiothorac Surg 2009;36:231-4. http://dx.doi.org/10.1016/j.ejcts.2009.02.016.
- Zierer A, Wimmer-Greinecker G, Martens S, Moritz A, Doss M. The transapical approach for aortic valve implantation. J Thorac Cardiovasc Surg 2008;136:948-53. http://dx.doi.org/10.1016/j.jtcvs.2008.06.028.
- Pasic M, Unbehaun A, Dreysse S, Drews T, Buz S, Kukucka M, et al. Transapical aortic valve implantation in 175 consecutive patients: excellent outcome in very high-risk patients. J Am Coll Cardiol 2010;56:813-20.
- Baan J, Yong ZY, Koch KT, Henriques JPS, Bouma BJ, de Hert SG, et al. Percutaneous implantation of the CoreValve aortic valve prosthesis in patients at high risk or rejected for surgical valve replacement: Clinical evaluation and feasibility of the procedure in the first 30 patients in the AMC-UvA. Netherlands Heart J 2010;18:18-24.
- Descoutures F, Himbert D, Lepage L, Iung B, Detaint D, Tchetche D, et al. Contemporary surgical or percutaneous management of severe aortic stenosis in the elderly. Eur Heart J 2008;29:1410-17. http://dx.doi.org/10.1093/eurheartj/ehn081.
- Rodes-Cabau J, Houde C, Perron J, Benson LN, Pibarot P. Delayed improvement in valve hemodynamic performance after percutaneous pulmonary valve implantation. Ann Thorac Surg 2008;85:1787-8. http://dx.doi.org/10.1016/j.athoracsur.2007.11.007.
- Behan M, Haworth P, Hutchinson N, Trivedi U, Laborde JC, Hildick-Smith D. Percutaneous aortic valve implants under sedation: our initial experience. Catheter Cardiovasc Interv 2008;72:1012-15. http://dx.doi.org/10.1002/ccd.21777.
- Pederzolli N, Agostini F, Fiorani V, Tappainer E, Nocchi A, Manfredi J, et al. Postendocarditis mitral valve aneurysm. J Cardiovasc Med 2009;10:259-60. http://dx.doi.org/10.2459/JCM.0b013e32831fb22a.
- Ussia GP, Mulè M, Barbanti M, Cammalleri V, Scarabelli M, Immè S, et al. Quality of life assessment after percutaneous aortic valve implantation. Eur Heart J 2009;30:1790-6. http://dx.doi.org/10.1093/eurheartj/ehp171.
- NHS reference costs 2006–07. Department of Health; 2008.
Appendix 1 Risk-scoring systems
The EuroSCORE
The EuroSCORE has been developed to estimate procedural mortality risks associated with cardiac surgery in adult patients. 38,40 The system was first developed using data from 19,030 patients undergoing cardiac surgery under cardiopulmonary bypass. 38 A large number of preoperative (n = 68) and operative (n = 29) risk factors were considered and those variables showing significant associations in a model of operative risk were included in the risk-scoring system. 38 The additive EuroSCORE was proposed and this system assigns weights to the various surgical risk factors relating to patient characteristics, type and severity of the cardiac disease and type and extent of the surgical procedure. The LES has since been developed to provide greater precision in high-risk patients. 37 Although this system was first developed to estimate operative mortality in general cardiac surgery,38 it is also applied specifically for the assessment of patients undergoing SAVR and is widely used in Europe.
The Society of Thoracic Surgeons Predicted Risk Of Mortality scoring system
The STS-PROM scoring system was developed using patients (n = 67,292) undergoing SAVR from 2002–6 in the USA. 40 The model was first developed on 60% of these patients and the other 40% of patients were used as a validation sample. 40 Final model coefficients were then estimated using the whole population sample. The STS score calculator can be accessed online (http://209.220.160.181/STSWebRiskCalc261/de.aspx) and gives estimates of risks of operative mortality, morbidity and development of complications, the length of postoperative hospital stay and the risk of needing to reoperate.
Appendix 2 Transcatheter aortic valve implantation search strategies
Searches for studies of cost-effectiveness, costs and quality of life
The Cochrane Library (Wiley) 2010 Issue 4 (NHS Economic Evaluation Database)
Search strategy
Database: MEDLINE (Ovid) 1950 to week 3, November 2010
Search strategy
-
((transcatheter or percutaneous or transaortic or transfemoral or transarterial or transapical or transluminal) adj3 (implant$ or valv$ or insert$ or replac$ or approach$ or surgery)).mp.
-
(TAVI or TAVR).mp.
-
(PAVI or PAVR).mp.
-
Edwards Sapien.mp.
-
Cribier Edwards.mp.
-
Core Valve.mp.
-
corevalve.mp.
-
Heart Valve Prosthesis/
-
or/1-8
-
exp Aortic Valve Stenosis/
-
aortic valve stenosis.mp.
-
valvular aortic stenosis.mp.
-
exp Heart Valve Diseases/
-
aortic valv$ disease$.mp.
-
heart valve disease$.mp.
-
or/10-15
-
9 and 16
-
limit 17 to yr="2007 - 2010"
-
economics/
-
exp "costs and cost analysis"/
-
cost of illness/
-
exp health care costs/
-
economic value of life/
-
exp economics medical/
-
exp economics hospital/
-
economics pharmaceutical/
-
exp "fees and charges"/
-
(econom$ or cost or costs or costly or costing or price or pricing or pharmacoeconomic$).tw.
-
(expenditure$ not energy).tw.
-
(value adj1 money).tw.
-
budget$.tw.
-
or/19-31
-
18 and 32
Database: EMBASE (Ovid) 1980 to week 46, 2010
Search strategy
-
((transcatheter or percutaneous or transaortic or transfemoral or transarterial or transapical or transluminal) adj3 (implant$ or valv$ or insert$ or replac$ or approach$ or surgery)).mp.
-
(TAVI or TAVR).mp.
-
(PAVI or PAVR).mp.
-
Edwards Sapien.mp.
-
Cribier Edwards.mp.
-
Core Valve.mp.
-
corevalve.mp.
-
exp heart valve prosthesis/
-
or/1-8
-
exp aorta valve stenosis/
-
aortic valve stenosis.mp.
-
valvular aortic stenosis.mp.
-
aorta valve disease/
-
heart valve disease$.mp.
-
or/10-14
-
9 and 15
-
limit 16 to yr="2007 - 2010"
-
cost benefit analysis/
-
cost effectiveness analysis/
-
cost minimization analysis/
-
cost utility analysis/
-
economic evaluation/
-
(cost or costs or costed or costly or costing).tw.
-
(economic$ or pharmacoeconomic$ or price$ or pricing).tw.
-
(technology adj assessment$).tw.
-
or/18-25
-
17 and 26
Database: MEDLINE (Ovid) 1950 to week 3, November 2010
Search strategy
-
((transcatheter or percutaneous or transaortic or transfemoral or transarterial or transapical or transluminal) adj3 (implant$ or valv$ or insert$ or replac$ or approach$ or surgery)).mp.
-
(TAVI or TAVR).mp.
-
(PAVI or PAVR).mp.
-
Edwards Sapien.mp.
-
Cribier Edwards.mp.
-
Core Valve.mp.
-
corevalve.mp.
-
Heart Valve Prosthesis/
-
or/1-8
-
exp Aortic Valve Stenosis/
-
aortic valve stenosis.mp.
-
valvular aortic stenosis.mp.
-
exp Heart Valve Diseases/
-
aortic valv$ disease$.mp.
-
heart valve disease$.mp.
-
or/10-15
-
9 and 16
-
limit 17 to yr="2007 - 2010"
-
decision support techniques/
-
markov.mp.
-
exp models economic/
-
decision analysis.mp.
-
cost benefit analysis/
-
or/19-23
-
18 and 24
Database: MEDLINE (Ovid) 1950 to week 3, November 2010
Search strategy
-
((transcatheter or percutaneous or transaortic or transfemoral or transarterial or transapical or transluminal) adj3 (implant$ or valv$ or insert$ or replac$ or approach$ or surgery)).mp.
-
(TAVI or TAVR).mp.
-
(PAVI or PAVR).mp.
-
Edwards Sapien.mp.
-
Cribier Edwards.mp.
-
Core Valve.mp.
-
corevalve.mp.
-
Heart Valve Prosthesis/
-
or/1-8
-
exp Aortic Valve Stenosis/
-
aortic valve stenosis.mp.
-
valvular aortic stenosis.mp.
-
exp Heart Valve Diseases/
-
aortic valv$ disease$.mp.
-
heart valve disease$.mp.
-
or/10-15
-
9 and 16
-
limit 17 to yr="2007 - 2010"
-
quality of life/
-
life style/
-
health status/
-
health status indicators/
-
or/19-22
-
18 and 23
-
16 and 22
-
limit 25 to yr="2007 - 2010"
-
24 or 26
Database: EMBASE (Ovid) 1980 to week 46, 2010
Search strategy
-
((transcatheter or percutaneous or transaortic or transfemoral or transarterial or transapical or transluminal) adj3 (implant$ or valv$ or insert$ or replac$ or approach$ or surgery)).mp.
-
(TAVI or TAVR).mp.
-
(PAVI or PAVR).mp.
-
Edwards Sapien.mp.
-
Cribier Edwards.mp.
-
Core Valve.mp.
-
corevalve.mp.
-
exp heart valve prosthesis/
-
or/1-8
-
exp aorta valve stenosis/
-
aortic valve stenosis.mp.
-
valvular aortic stenosis.mp.
-
aorta valve disease/
-
heart valve disease$.mp.
-
or/10-14
-
9 and 15
-
limit 16 to yr="2007 - 2010"
-
quality adjusted life.ti,ab.
-
(qaly$ or qald$ or qale$).mp. or qtime$.ti,ab.
-
disability adjusted life.ti,ab.
-
daly$.tw.
-
health status indicators/
-
(sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirtysix or short form thirty six).tw.
-
(sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).tw.
-
(sf12 or sf 12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).tw.
-
(sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen).tw.
-
(sf20 or sf 20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).tw.
-
(euroqol or euro qol or eq5d or eq 5d).tw.
-
(hql or hqol or h qol or hrqol or hr qol).tw.
-
(hye or hyes).tw.
-
health$ year$ equivalent$.tw.
-
health utili$.tw.
-
rosser.tw.
-
quality of wellbeing.tw.
-
quality of well being.tw.
-
qwb.tw.
-
willingness to pay.tw.
-
standard gamble$.tw.
-
time trade off.tw.
-
time tradeoff.tw.
-
tto.tw.
-
cost utility study.tw.
-
cost utility studies.tw.
-
VAS.tw.
-
"Quality of Life"/
-
value of life.mp.
-
quality adjusted life year$.mp.
-
or/18-47
-
17 and 48
-
15 and 48
-
limit 50 to yr="2007 - 2010"
-
49 or 51
General searches for primary studies and systematic reviews
Database: MEDLINE (Ovid) 1950 to week 2, October 2010
Search strategy
-
((transcatheter or percutaneous or transarterial or transapical) adj3 (implant$ or valvuloplasty or insert$ or replac$ or approach$ or surgery)).mp.
-
TAVI.mp.
-
((transcatheter or transaortic) adj2 valve$).mp.
-
or/1-3
-
exp Aortic Valve Stenosis/
-
aortic valve stenosis.mp.
-
valvular aortic stenosis.mp.
-
exp Heart Valve Diseases/
-
aortic valve disease.mp.
-
or/5-9
-
4 and 10
-
limit 11 to "reviews (specificity)"
-
limit 11 to "reviews (optimized)"
Database: MEDLINE (Ovid) In-Process & Other Non-Indexed Citations 24 November 2010
Search strategy
-
((transcatheter or percutaneous or transaortic or transfemoral or transarterial or transapical or transluminal) adj3 (implant$ or valv$ or insert$ or replac$ or approach$ or surgery)).mp.
-
(TAVI or TAVR).mp.
-
(PAVI or PAVR).mp.
-
Edwards Sapien.mp.
-
Cribier Edwards.mp.
-
Core Valve.mp.
-
corevalve.mp.
-
or/1-7
-
aortic valve stenosis.mp.
-
valvular aortic stenosis.mp.
-
aortic valv$ disease$.mp.
-
heart valve disease$.mp.
-
or/9-12
-
8 and 13
-
limit 14 to yr="2007 - 2010"
Database: MEDLINE (Ovid) 1950 to week 3, November 2010
Search strategy
-
((transcatheter or percutaneous or transaortic or transfemoral or transarterial or transapical or transluminal) adj3 (implant$ or valv$ or insert$ or replac$ or approach$ or surgery)).mp.
-
(TAVI or TAVR).mp.
-
(PAVI or PAVR).mp.
-
Edwards Sapien.mp.
-
Cribier Edwards.mp.
-
Core Valve.mp.
-
corevalve.mp.
-
Heart Valve Prosthesis/
-
or/1-8
-
exp Aortic Valve Stenosis/
-
aortic valve stenosis.mp.
-
valvular aortic stenosis.mp.
-
exp Heart Valve Diseases/
-
aortic valv$ disease$.mp.
-
heart valve disease$.mp.
-
or/10-15
-
9 and 16
-
limit 17 to yr="2007 - 2010"
-
limit 18 to "therapy (sensitivity)"
-
(cohort$ or case or trial$ or open-label or retrospective or prospective or long term or follow up or outcome$).mp.
-
18 and 20
-
19 or 21
Database: EMBASE (Ovid) 1980 to week 47, 2010
Search strategy
-
((transcatheter or percutaneous or transaortic or transfemoral or transarterial or transapical or transluminal) adj3 (implant$ or valv$ or insert$ or replac$ or approach$ or surgery)).mp.
-
(TAVI or TAVR).mp.
-
(PAVI or PAVR).mp.
-
Edwards Sapien.mp.
-
Cribier Edwards.mp.
-
Core Valve.mp.
-
corevalve.mp.
-
exp heart valve prosthesis/
-
or/1-8
-
exp aorta valve stenosis/
-
aortic valve stenosis.mp.
-
valvular aortic stenosis.mp.
-
aorta valve disease/
-
heart valve disease$.mp.
-
or/10-14
-
9 and 15
-
limit 16 to yr="2007 - 2010"
-
limit 17 to "treatment (2 or more terms high sensitivity)"
The Cochrane Library (Wiley) 2010 Issue 4 (Cochrane Central Register of Controlled Trials, Database of Abstracts of Reviews of Effects, Health Technology Assessment) Issue11 (Cochrane Database of Systematic Reviews)
Search strategy
Additional targeted searches
Database: MEDLINE (Ovid) 1948 to week 1, January 2011
Search strategy
-
risk stratification.mp.
-
(heart adj2 valve$).mp.
-
1 and 2
Database: MEDLINE (Ovid) 1948 to week 4, January 2011
Search strategy
-
PARTNER.mp.
-
quality of life.mp. or exp "Quality of Life"/
-
(surgery or surgical).mp.
-
aortic valve$.mp. or exp Aortic Valve/
-
1 and 2 and 3 and 4
-
1 and 2 and 3
-
2 and 3 and 4
-
limit 7 to "therapy (sensitivity)"
Database: MEDLINE (Ovid) 1950 to week 4, October 2010
Search strategy
-
new york heart classification.mp.
-
aortic stenosis.mp. or exp Aortic Valve Stenosis/
-
(new york adj2 heart).mp.
-
2 and 3
Appendix 3 Fitting survival curves for surgical aortic valve replacement and medical management
The methods used for fitting survival curves have been described in the main part of the report, based on the notion that the overall survival S(t) at time t can be written as
where k is the 30-day survival; f(t) is a function to be determined, and which is related to the long-term mortality specific to AS; and g(t) is the general population survival.
For medical management, the same procedure was followed as for TAVI using data from the PARTNER trial,14 except that no special account was taken of survival in the first month. Table 25 shows the calculations for the survival f(t) relative to general population survival.
| Time (months) | Actual values | Relative to general population survival | ||
|---|---|---|---|---|
| Overall survival | Hospitalisation-free survival | Overall survival | Hospitalisation-free survival | |
| 6 | 0.698 | 0.516 | 0.711 | 0.525 |
| 12 | 0.494 | 0.297 | 0.513 | 0.308 |
| 18 | 0.401 | 0.188 | 0.426 | 0.199 |
| 24 | 0.346 | 0.103 | 0.375 | 0.112 |
As with TAVI, a Weibull distribution was fitted to the observed values by transforming the equation into the form
and estimating values of a and b to minimise the sum of the squares of the errors in the above equation. In line with the principle of parsimony in modelling, a common value of the shape parameter a is to be preferred whenever an assumption of proportional hazards can be considered at all reasonable. A reasonable fit can be obtained to the last two columns in Table 25 by using a = 0.821 in each case, with b = 22.0 for overall survival, and b = 9.83 for hospitalisation-free survival. The comparison between the observed and fitted values can be seen in Table 26.
| Time (months) | Overall survival | Hospitalisation-free survival | ||
|---|---|---|---|---|
| Observed | Fitted | Observed | Fitted | |
| 6 | 0.711 | 0.709 | 0.525 | 0.513 |
| 12 | 0.513 | 0.544 | 0.308 | 0.308 |
| 18 | 0.426 | 0.428 | 0.199 | 0.194 |
| 24 | 0.375 | 0.341 | 0.112 | 0.125 |
For SAVR, data were only available for overall survival. The only sources identified which gave survival over a range of follow up times were Leontyev,67 who gave survival curves for low- and moderate-risk groups, and Wendt,42 who gave survival curves for the moderate-risk group only. Here, low risk was defined as a LES < 10, whereas moderate risk was defined as a LES between 10 and 20. Table 27 shows the transformation of the survival data for each of these groups separately. For moderate-risk groups, the data were combined by minimising the total sum of squared errors across the two sources. 42,67
| Results from Leontyev67 | ||||||
|---|---|---|---|---|---|---|
| Time (months) | Actual values | Relative to 1-month survival | Relative to 1-month and general population survival | |||
| Low risk | Moderate risk | Low risk | Moderate risk | Low risk | Moderate risk | |
| 1 | 0.925 | 0.893 | N/A | N/A | N/A | N/A |
| 12 | N/A | 0.78 | N/A | 0.873 | N/A | 0.907 |
| 36 | 0.80 | 0.70 | 0.865 | 0.784 | 0.982 | 0.890 |
| 60 | 0.70 | 0.53 | 0.757 | 0.594 | 0.960 | 0.753 |
| 96 | 0.38 | 0.33 | 0.411 | 0.370 | 0.651 | 0.586 |
| Results from Wendt42 | ||||||
| Time (months) | Actual values | Relative to 1-month survival | Relative to 1-month and general population survival | |||
| Low risk | Moderate risk | Low risk | Moderate risk | Low risk | Moderate risk | |
| 1 | N/A | 0.954 | N/A | N/A | N/A | N/A |
| 12 | N/A | 0.861 | N/A | 0.903 | N/A | 0.937 |
| 24 | N/A | 0.808 | N/A | 0.847 | N/A | 0.918 |
| 60 | N/A | 0.548 | N/A | 0.574 | N/A | 0.729 |
Then, the values in the last two columns of Table 27 were taken as estimates of two separate Weibull functions with a common shape parameter. The best fit was obtained with a shape parameter a = 1.15 and scale parameters b = 613 for low risk and b = 160 for moderate risk. For the modelled 30-day survival, the observed figure (0.925) was used for low risk, and the weighted average of the two observed figures (0.927) was used for moderate risk. The small anomaly here is of little importance as the low-risk values were used only in a small part of the sensitivity analysis.
There remains the question of how to handle hospitalisation-free survival for SAVR. In the absence of anything better, this was projected from overall survival to match the projection from TAVI. For the 30-day survival, the OR between overall and hospitalisation-free survival was calculated as 2.24; this was then applied to the overall SAVR survival to give estimates of hospitalisation-free 30-day survival as 0.847 (low risk) and 0.850 (moderate risk), carrying over the small anomaly between these figures. For long-term survival, a similar calculation was undertaken using hazard ratio, applied to the function f(t) which represents long-term survival relative to the general population survival. The hazard ratio between the relevant curves for TAVI was calculated as 1.43; this gave parameters for the hospitalisation-free survival f(t) under SAVR as b = 450 for low risk and b = 117 for moderate risk.
Appendix 4 Details of costing
Table 28 reports the incidence of adverse events within 30 days of the procedure for both TAVI and SAVR. The studies shown are those identified from the searches which report the relevant adverse event. Only one of the studies in this table is a randomised controlled trial (Leon et al., 201014). The other studies are observational or case studies. For each study reporting a particular adverse event, the total number of patients is shown, together with the number and proportion of those patients in whom the adverse event occurred. Overall figures are used as an estimate of the incidence of each event. In some cases, no studies were found for SAVR; the relevant sections show results only for TAVI.
| Author | Patients, N | AE occurred, n | Incidence of AE |
|---|---|---|---|
| Cerebral damage stroke due to a thromboembolic event | |||
| TAVI | |||
| Svensson et al. 200873 | 40 | 2 | 0.05 |
| Thielmann et al. 200975 | 39 | 1 | 0.03 |
| Berry et al. 200777 | 13 | 1 | 0.08 |
| Marcheix et al. 200779 | 10 | 2 | 0.20 |
| Leon et al. 2010 (PARTNER trial)14 | 179 | 12 | 0.07 |
| Osten et al. 201081 | 46 | 3 | 0.07 |
| Eltchaninoff et al. 201082 | 244 | 9 | 0.04 |
| Webb et al. 200783 | 50 | 2 | 0.04 |
| Walther et al. 200784 | 59 | 2 | 0.03 |
| Covello et al. 200985 | 69 | 1 | 0.01 |
| Dworakowski et al. 201086 | 151 | 9 | 0.06 |
| John et al. 201087 | 100 | 1 | 0.01 |
| Grube et al. 200888 | 136 | 4 | 0.03 |
| Grube et al. 200789 | 86 | 9 | 0.10 |
| Buellesfeld et al. 201090 | 168 | 6 | 0.04 |
| Krane et al. 201034 | 99 | 4 | 0.04 |
| Gotzman et al. 200991 | 44 | 1 | 0.02 |
| Bleiziffer et al. 200992 | 137 | 7 | 0.05 |
| Dewey et al. 200855 | 21 | 3 | 0.14 |
| Ludman 201037 | 872 | 34 | 0.04 |
| Schofer et al. 200893 | 15 | 1 | 0.07 |
| Total | 2578 | 114 | 0.04 |
| SAVR | |||
| Walther et al. 201074 | 100 | 2 | 0.02 |
| Maillet et al. 200976 | 84 | 5 | 0.06 |
| Kalavrouziotis et al. 200978 | 1184 | 27 | 0.02 |
| Kalavrouziotis et al. 200978 | 237 | 12 | 0.05 |
| Kolh et al. 200780 | 220 | 4 | 0.02 |
| Total | 1825 | 50 | 0.03 |
| MI (including coronary occlusion) | |||
| TAVI | |||
| Svensson et al. 200873 | 40 | 7 | 0.18 |
| Leon et al. 201014 | 179 | 1 | 0.01 |
| Webb et al. 200783 | 50 | 1 | 0.02 |
| Dworakowski et al. 201086 | 151 | 1 | 0.01 |
| Walther et al. 200994 | 25 | 1 | 0.04 |
| John et al. 201087 | 100 | 1 | 0.01 |
| Grube et al. 200888 | 136 | 2 | 0.01 |
| Grube et al. 200789 | 86 | 1 | 0.01 |
| Buellesfeld et al. 201090 | 168 | 3 | 0.02 |
| Krane et al. 201034 | 99 | 3 | 0.03 |
| Eltchaninoff et al. 201082 | 244 | 3 | 0.01 |
| Zierer et al. 200895 | 26 | 2 | 0.08 |
| Pasic et al. 201096 | 175 | 1 | 0.01 |
| Ludman 201037 | 872 | 11 | 0.01 |
| Schofer et al. 200893 | 15 | 1 | 0.07 |
| Total | 2366 | 39 | 0.02 |
| SAVR | |||
| Maillet et al. 200976 | 84 | 3 | 0.04 |
| Total | 84 | 3 | 0.04 |
| Arrhythmia (atrial fibrillation, bradyarrythmia, heart conduction disorders) | |||
| TAVI | |||
| Leon et al. 201014 | 179 | 1 | 0.01 |
| Dewey et al. 200844 | 21 | 4 | 0.19 |
| Marcheix et al. 200779 | 10 | 2 | 0.20 |
| Thielmann et al. 200975 | 39 | 3 | 0.08 |
| Zierer et al. 200895 | 26 | 5 | 0.19 |
| Covello et al. 200985 | 18 | 1 | 0.06 |
| Walther et al. 200784 | 59 | 18 | 0.31 |
| Baan et al. 201097 | 30 | 1 | 0.03 |
| Total | 382 | 35 | 0.09 |
| SAVR | |||
| Dewey et al. 200844 | 16 | 6 | 0.38 |
| Maillet et al. 200976 | 84 | 38 | 0.45 |
| Kalavrouziotis et al. 200978 | 1184 | 336 | 0.28 |
| Kalavrouziotis et al. 200978 | 237 | 73 | 0.31 |
| Kolh et al. 200780 | 220 | 53 | 0.24 |
| Total | 1741 | 506 | 0.29 |
| Cardiac tamponade | |||
| TAVI | |||
| Baan et al. 201097 | 30 | 2 | 0.07 |
| Gotzman et al. 200991 | 51 | 1 | 0.02 |
| Krane et al. 201034 | 99 | 1 | 0.01 |
| Grube et al. 200789 | 86 | 9 | 0.10 |
| Grube et al. 200888 | 136 | 2 | 0.01 |
| Webb et al. 200783 | 50 | 1 | 0.02 |
| Eltchaninoff et al. 201082 | 244 | 3 | 0.01 |
| Rodes-Cabua et al. 200899 | 22 | 1 | 0.05 |
| Descoutures et al. 200898 | 12 | 1 | 0.08 |
| Thielmann et al. 200975 | 39 | 1 | 0.03 |
| Total | 769 | 22 | 0.03 |
| SAVR | |||
| Descoutures et al. 200898 | 12 | 2 | 0.17 |
| Bridgewater et al. 200858 | 14,980 | 823 | 0.05 |
| Total | 14,992 | 825 | 0.06 |
| Bleeding | |||
| TAVI | |||
| Baan et al. 201097 | 30 | 1 | 0.03 |
| Rodes-Cabua et al. 200899 | 22 | 1 | 0.05 |
| Descoutures et al. 200898 | 12 | 2 | 0.17 |
| Marcheix et al. 200779 | 10 | 2 | 0.20 |
| Berry et al. 200777 | 11 | 2 | 0.18 |
| Pasic et al. 201096 | 175 | 3 | 0.02 |
| Osten et al. 201081 | 46 | 7 | 0.15 |
| Leon et al. 201014 | 179 | 30 | 0.17 |
| Total | 485 | 48 | 0.10 |
| SAVR | |||
| Dewey et al. 200844 | 16 | 1 | 0.06 |
| Maillet et al. 200976 | 84 | 6 | 0.07 |
| Total | 100 | 7 | 0.07 |
| Pacemaker | |||
| TAVI | |||
| Gotzman et al. 200991 | 51 | 26 | 0.51 |
| Behan et al. 2008100 | 12 | 3 | 0.25 |
| Eltchaninoff et al. 201082 | 244 | 29 | 0.12 |
| Osten et al. 201081 | 46 | 4 | 0.09 |
| Ussia et al. 2009102 | 39 | 5 | 0.13 |
| Marcheix et al. 200779 | 10 | 3 | 0.30 |
| Thielmann et al. 200975 | 39 | 4 | 0.10 |
| Leon et al. 201014 | 179 | 6 | 0.03 |
| Walther et al. 201074 | 100 | 9 | 0.09 |
| Total | 720 | 89 | 0.12 |
| SAVR | |||
| Kalavrouziotis et al. 200978 | 1184 | 64 | 0.05 |
| Kalavrouziotis et al. 200978 | 237 | 24 | 0.10 |
| Total | 1421 | 88 | 0.06 |
| Cardiogenic shock | |||
| TAVI | |||
| Baan et al. 201097 | 30 | 1 | 0.03 |
| Valve embolization | |||
| TAVI | |||
| Osten et al. 201081 | 46 | 1 | 0.02 |
| Leon et al. 201014 | 179 | 1 | 0.01 |
| Thielmann et al. 200975 | 39 | 1 | 0.03 |
| Total | 264 | 3 | 0.01 |
| Respiratory failure | |||
| TAVI | |||
| Baan et al. 201097 | 30 | 3 | 0.10 |
| Behan et al. 2008100 | 12 | 1 | 0.08 |
| Marcheix et al. 200779 | 10 | 1 | 0.10 |
| Walther et al. 200995 | 25 | 2 | 0.08 |
| Total | 77 | 7 | 0.09 |
| Renal failure | |||
| TAVI | |||
| Krane et al. 201034 | 99 | 15 | 0.15 |
| Dworakowski et al. 201086 | 151 | 50 | 0.33 |
| Covello et al. 200985 | 18 | 1 | 0.06 |
| Osten et al. 201081 | 46 | 1 | 0.02 |
| Dewey et al. 200844 | 21 | 1 | 0.05 |
| Marcheix et al. 200779 | 10 | 1 | 0.10 |
| Leon et al. 201014 | 179 | 2 | 0.01 |
| Total | 524 | 71 | 0.14 |
| SAVR | |||
| Dewey et al. 200844 | 16 | 3 | 0.19 |
| Maillet et al. 200976 | 84 | 10 | 0.12 |
| Total | 100 | 13 | 0.13 |
| Vascular complications | |||
| TAVI | |||
| Gotzman et al. 200991 | 51 | 6 | 0.12 |
| Krane et al. 201034 | 99 | 12 | 0.12 |
| Thielmann et al. 200975 | 39 | 5 | 0.13 |
| Dworakowski et al. 201086 | 151 | 13 | 0.09 |
| Marcheix et al. 200779 | 10 | 3 | 0.30 |
| Behan et al. 2008100 | 12 | 1 | 0.08 |
| Leon et al. 201014 | 179 | 55 | 0.31 |
| Descoutures et al. 200898 | 12 | 2 | 0.17 |
| Pasic et al. 201096 | 175 | 1 | 0.01 |
| Total | 728 | 98 | 0.13 |
Table 29 reports the costing of short-term adverse events. For each event, the relevant NHS reference cost descriptions are given. 70 The incidence for each event is taken from Table 28; rounded figures are shown in the table, but unrounded figures were used to calculate the cost of each event. Where no data for SAVR were available, assumptions were made based on clinical advice from SR. These are shown in footnotes to the table. The total cost figures are incorporated in the main report in Table 7.
| Event | NHS reference cost 2009–1070 | Unit cost (£) | Probability SAVR | Probability TAVI | SAVR cost (£) | TAVI cost (£) |
|---|---|---|---|---|---|---|
| Stroke | Stroke PS28A | 549.00 | 0.03 | 0.04 | 15.04 | 24.28 |
| Stroke PS28B | ||||||
| Rehab for Stroke VC04Z | ||||||
| MI | MI EB10Z | 1685.00 | 0.04 | 0.02 | 60.18 | 27.77 |
| Rehab MI VC38Z | ||||||
| Arrhythmia | Arrhythmia or Conduction Disorders without CC EB07I | 794.00 | 0.29 | 0.09 | 230.77 | 72.75 |
| Cardiac tamponade | Other Non-Complex Cardiac Surgery EA40Z | 6216.00 | 0.06 | 0.03 | 342.06 | 177.83 |
| Bleeding | Haemorrhage/laceration/bleeding PS21A/B/C | 248.00 | 0.07 | 0.10 | 17.36 | 24.54 |
| Pacemaker | Pace 1 – Single chamber or Implantable Diagnostic Device EA03Z | 2886.00 | 0.06 | 0.12 | 178.72 | 356.74 |
| Heart failure or shock | Heart failure or shock EB03H-EB03I | 1929.00 | 0.03a | 0.03 | 57.87 | 64.30 |
| Valve embolisation | Cardiac Valve Disorders EB06Z | 2046.00 | 0a | 0.01 | 0.00 | 23.25 |
| Respiratory failure | Respiratory Failure without Intubation without CC DZ27F | 1149.00 | 0.09a | 0.09 | 104.45 | 104.45 |
| Renal dialysis | Haemodialysis/Filtration 19 years and over LC02A | 188.00 | 0.13 | 0.14 | 24.44 | 25.47 |
| Vascular complication | Major Cranial, Visceral or Blood Vessel Injury without CC HA82C | 1366.00 | 0.03a | 0.13 | 44.40 | 177.58 |
| Total AE cost | 1075.29 | 1078.98 | ||||
Table 30 shows the total procedure cost including hospital stay, but excluding adverse events for which an additional cost was incurred. The ICU cost has been calculated from the NHS reference cost list 2006–7103 inflated to 2009–10. 70 Cardio ward daily cost has been taken from the 2008 Bazian report35 and inflated to 2009–10. The number of days for SAVR patients spent in ICU has been taken from the NHS South Central Cardiovascular Network 2010;71 this number has been subtracted from the average in-hospital stay of SAVR patients of 13.36 days (based on the literature search16,67,76,80,98). The number of days for TAVI patients spent in ICU and in cardio ward has been taken from NHS South Central Cardiovascular Network 2010;71 however, this cost is included in the total package cost for TAVI of £24,000, reported from the same source. The cost of SAVR procedure, £10,097, is the cost for single cardiac valve procedure (EA17Z) from the NHS reference list cost 2009–10. 70 The total cost figures are incorporated in the main report in Table 7.
| Hospitalisation cost | Unit cost (£) | Days (SAVR), n | Days (TAVI), n | Total cost hospitalisation (SAVR) (£) | Total cost hospitalisation (TAVI) (£) |
|---|---|---|---|---|---|
| ICU | 1276.35 | 3.1 | 1 | 3956.68 | 1276.35 |
| Cardio ward | 396.16 | 10.26 | 5 | 4064.58 | 1980.79 |
| Total cost of hospital stay | 8021.25 | 3257.14 | |||
| Cost of procedure | 10,097.00 | ||||
| Total cost per package including hospital stay | 18,118.25 | 24,000.00 |
Table 31 reports the rehospitalisation cost per patient, assuming that the patient is hospitalised for a cardiac valve disorder and the length of stay depends on the functionality of the valve (see below the justification of in-hospital stay). Details are given below the table.
| Rehospitalisation | Total rehospitalisation cost per patient (£) | Days, n | Cardio ward (cost in £ per day) | Cardiac valve disorder (cost in £ per day) |
|---|---|---|---|---|
| SAVR and TAVI 1 weeka (6 days) | 4422.95 | 6 | 396.16 | 2046.00 |
| MM 2 weeksb (14 days) | 7592.21 | 14 | 396.16 | 2046.00 |
Appendix 5 Protocol
Short Report protocol
Cost effectiveness of Transcatheter Aortic Valve Implantation (TAVI) for Aortic Stenosis in patients who cannot undergo surgery
Produced by: West Midlands Health Technology Assessment Collaboration
Unit of Health Economics and Unit of Public Health, Epidemiology & Biostatistics
University of Birmingham
Edgbaston
Birmingham B15 2TT
Authors: Rosanna Orlando1
Mary Pennant2
Stephen Rooney3
Sue Bayliss2
Ahamedali Hassan2
David Moore2
Pelham Barton1
1Unit of Health Economics, University of Birmingham
2Unit of Public Health, Epidemiology & Biostatistics, University of Birmingham
3Department of Cardiac Surgery, Queen Elizabeth Hospital, Birmingham
Correspondence to: Pelham Barton, Unit of Health Economics, University of Birmingham, Edgbaston, Birmingham B15 2TT, UK.
Date Completed: 30 November 2010.
This report was commissioned by the NIHR HTA Programme as project number 10/110/01.
The views expressed in this report are those of the authors and not necessarily those of the NIHR HTA Programme. Any errors are the responsibility of the authors.
Conflicts of interest: The following authors have no conflicts of interest: RO, MP, SB, DM, PB. SR has received training support for TAVI from Edwards.
Assessment Question
The objective of this short report is to determine the cost-effectiveness of transcatheter aortic valve implantation (TAVI) compared to Standard Therapy in patients who require aortic valve replacement but are high risk or not fit for conventional surgery.
Background
Aortic stenosis is the commonest indication for aortic valve replacement. The majority of cases of aortic stenosis are secondary to either calcific degeneration or congenital bicuspid aortic valve.
Aortic stenosis
Aortic stenosis is a degenerative condition in which the aortic valve becomes progressively narrowed leading to gradual obstruction of left ventricular outflow at the level of the aortic valve. The prevalence of this pathology increases with age, the majority of people treated being above 60 years of age. 1 Chronic aortic stenosis causes pressure overloading of the left ventricle which becomes hypertrophied to maintain the stroke volume and cardiac output. Under these circumstances the patient may remain asymptomatic for many years. However with longstanding hypertrophy the ventricle will eventually become less compliant and symptoms of breathlessness, angina or collapse will develop. In the late stages of aortic stenosis the left ventricle will dilate. For patients who present in congestive heart failure mean survival is less than a year.
Different outcome measures are used to assess the severity of the condition and these are based on clinical assessment. They include the following:
-
The New York Heart Association (NYHA) heart failure classification is used to classify the severity of breathlessness: from class I, in which the patient has no limitation in daily physical activity, to class IV, in which the patient is breathless at rest. NYHA is a functional classification system linking the patient's symptoms and quality of life to normal life qualities (www.americanheart.org).
-
Haemodynamic assessment may involve measurement by echocardiography and/or Doppler. Aortic valve area (cm2) is assessed relative to body surface area (in m2). Aortic valve area < 0.6 cm2/m2 indicates severe aortic stenosis.
-
Transaortic gradient (mmHg) measures the blood volume flow rate through the aortic valve. Peak transaortic valve gradient > 64 mmHg and mean transaortic valve gradient > 40 mmHg indicates severe aortic stenosis. 2
Treatments for aortic stenosis (in the absence of TAVI)
Surgical aortic valve replacement is the reference treatment for aortic stenosis with around 60,000 operations conducted in Europe annually. 1 Surgical aortic valve replacement involves replacing the diseased valve with a prosthetic mechanical or biological valve through a median sternotomy and using cardiopulmonary bypass. This surgical procedure consists of an incision along the sternum, after which the sternum itself is divided to provide access to the heart and lungs for surgery. The cardiopulmonary bypass is a circulatory support technique that temporarily takes over the function of the heart and lungs during surgery, maintaining the circulation of blood and oxygen delivery to the body's tissues. 2
Surgical risk of existing treatments
Surgical aortic valve replacement carries a very high risk for some patients, particularly those who are elderly and/or who suffer from concomitant illnesses. Risk factors include age over 80, previous cardiac surgery, chronic obstructive airways disease, peripheral vascular disease, previous stroke with residual deficit, poor left ventricular function, renal failure, diabetes and hypertension. 3
Surgical risk assessment tools
-
The European System for Cardiac Operative Risk Evaluation (EuroSCORE) calculates the predictive operative mortality of patients that undergo cardiac surgery. For high-risk patients, the more accurate logistic EuroSCORE is used. This model is most commonly used in Europe; this type of scoring system also considers particular combinations of the risk factors (www.euroscore.org). EuroSCORE is limited in risk stratifying patients for aortic valve replacement as it is essentially designed to risk stratify patients undergoing CABG. However it is the most widely used system in the UK. It is also straightforward to use – by completing an online scoresheet with various patient factors the logistic EuroSCORE will be provided for you in the form of a predicted mortality percentage.
-
The STS score is a risk model developed by the Society of Thoracic Surgeons based on clinical and demographic data in an adult population and used to predict operative mortality and morbidity after cardiac surgery. This scoring system has been developed in USA. The high surgical risk is defined by an STS risk score of 10% or higher (on a scale of 0% to 100%, with higher scores indicating greater surgical risk). The model is based on clinical and epidemiological data of a given population who have received cardiac surgery (www.sts.org). This is quite a sophisticated model that gives not only mortality risk but also risk of major morbidity such as prolonged ventilation, stroke and renal failure
-
The Ambler Risk score has been developed in UK and is able to predict the risk of in-hospital mortality for patients undergoing heart valve surgery. This scoring system seems to be the more accurate on target population considered in this report. In real practice surgeons evaluate the surgery mortality risk according to more than one risk-scoring system and different associated clinical conditions. (http://www.ucl.ac.uk/statistics/research/riskmodel/index.html)
The British Cardiovascular Intervention Society (BCIS) and the Society for Cardiothoracic Surgery (SCTS) state that the patient's operative risk should be assessed by a multidisciplinary team (MDT). The multidisciplinary team should comprise two cardiac surgeons, two interventional cardiologists, an imaging specialist, cardiothoracic anaesthetists and experienced nurses who assess the cost/benefit ratio of open heart surgery and TAVI. The usual “High risk” patient eligible for TAVI will have a logistic EuroSCORE of ≥ 20 or an STS score of ≥ 10. 2
Interventions which do not involve open surgery
Percutaneous balloon valvuloplasty has a limited role in the treatment of severe aortic stenosis in adult patients. It may be considered to provide palliative treatment for patients with considerable co-morbidity as relief of symptoms is likely to be temporary. 2
Transcatheter aortic valve implantation
Transcatheter Aortic Valve Implantation (TAVI) is a procedure used as an alternative to open heart surgery for people with severe aortic stenosis. In highly developed nations, the request for valve surgery is increasing among older people, who may present with more co-morbidities and a higher incidence of concomitant coronary artery disease. TAVI may allow aortic valve replacement to be undertaken in some of these patients who would previously have been considered too high risk for aortic valve replacement. This is highly specialised technology and is relatively new: the first human case was in 2002. There is a UK TAVI registry; the number of TAVI per year included in this registry was 67 in 2007, 272 in 2008 and 533 in 2009. 4 TAVI is currently restricted to high-risk patients with severe aortic stenosis and absolute contraindications for surgery. 5
TAVI aims to implant a bioprosthetic aortic valve at the site of the native aortic valve through a percutaneous route. The choice of the vascular route has been developed in order to reduce surgical trauma and the use of cardiopulmonary bypass associated with valve replacement. During the procedure, a biological valve is crimped into a delivery catheter. The delivery catheter is inserted either in the femoral artery through a small incision at the top of the leg (known as transfemoral, percutaneous, endovascular and transluminal approach) or between the ribs through the apex of the heart (known as transapical or transventricular approach). The valve is guided to the heart using radiological visual guidance. Usually the route of choice is the transfemoral as this is deemed to be the least invasive for the patient. The transapical route is used if the transfemoral route is limited by atherosceloris or small calibre. 2
A balloon catheter is advanced via the arterial system or the left ventricle over a guide wire and positioned within the opening of the aortic valve. The existing aortic valve is dilated in order to make room for the prosthetic valve. The new valve, mounted on a metal stent, is manipulated into position and is either self expanding or deployed using balloon inflation. Deployment leads to obliteration of the existing aortic valve.
TAVI may be carried out under general anaesthesia or spinal aneasthesia. The procedure requires a combination of echocardiography and fluoroscopic imaging to ensure accurate deployment of the valve. Prophylactic antibiotics and anticoagulation medication are administered before and during the procedure. 2
Surgeons and cardiologists involved in TAVI undergo dedicated training in both patient assessment as well as the clinical procedure. In addition the valve companies provide a high level of technical support and proctors with considerable experience until the new centre has enough experience to run an independent programme.
Cost implications
TAVI is an expensive procedure, requiring a multidisciplinary team and substantial equipment to be available during the procedure. The approximate cost of the procedure has been estimated at £18,000. 1 However, successful implantation may reduce the need for later hospitalisation, so there is likely to be some cost saving to offset against the cost of the procedure.
Economic Evaluation
The objective of this short report is to determine the cost-effectiveness of transcatheter aortic valve implantation (TAVI) compared to Standard Therapy in patients who require aortic valve replacement but are high risk or not fit for conventional surgery. The cost-effectiveness analysis will adopt the perspective of the NHS. An incremental cost-effectiveness analysis will be conducted, with survival-years as the main measure of the efficacy of the technology. The result will be presented as cost per additional year gained and if possible as cost per quality adjusted life year (QALY) gained. 6
Cost effectiveness review
Initial scoping searches have been carried out to assess the volume and type of literature relating to TAVI for aortic stenosis.
National TAVI and aortic valve replacement registry is the most updated source of data. A trial published on the New England Journal of Medicine in October 20107 and other cohort studies summarised in Annals of Internal Medicine8 also in 2010 represent relevant updated evidence about short term effectiveness. Ongoing trials have been found into the TAVI clinical trials registers searches.
A model published by Bazian in May 20081 contains TAVI cost data, the model is not a cost-effectiveness analysis but it contains both procedure-related costs and costs that may be incurred during one year of follow up for both TAVI and medical therapy.
Bazian reported that they had found no published studies or models that had assessed the cost-effectiveness of TAVI. We will carry out an appropriate search to determine whether this is still the case. If any relevant studies or models are found, we will appraise them using standard criteria9. Although we do not expect to find any/many studies, if any robust evaluations are identified, we will use them to inform our model.
Economic model
An economic model will be designed to represent the pathway of patients with severe symptomatic aortic stenosis who are high risk or unfit for surgery compared to standard therapy.
The standard therapy comparator will differ depending on the type of patient. For patients at high risk, but not contraindicated for surgery, the most appropriate comparator may be surgery whereas, for patients contraindicated for surgery, other therapies will be more appropriate comparators. It is recognised that there is likely to be considerable areas of grey where the most appropriate treatment route for a particular patients is debatable. However, in order to make modelling for this project feasible, two separate patient groups will be considered. As was done in the PARTNER trial7, the two patient groups will be assumed to be distinct:
Group 1 – Patients who are at high risk but are not contraindicated for surgery. The comparator for this group will be surgery.
Group 2 – Patients who are contraindicated for surgery. The comparator for this group will be other forms of standard therapy (not surgery).
Before and during the development of the model, a steering group, with expertise in TAVI and surgical techniques and other possible comparators, will be consulted to give guidance to the technical team on clinical pathways, treatment strategies and other factors that may influence the structure and content of the model. This group will also give clinical guidance to inform the suitability of model parameters. This group is currently being formed under the direction of our clinical expert (SR).
As far as possible, the model will be populated with data derived from the literature. Data required for the model based economic evaluation may include:
Safety data:
-
In hospital mortality and procedure related complications. Those data will refer to intra-, post- and peri-procedure (in the latter, beginning with the patient's emergence from anaesthesia and continuing through the time required for the acute effects of the aesthetic and surgical procedures to abate). This procedure safety period will be extended from the time of hospitalisation for surgery to the time of discharge.
-
Impact of procedural learning curve over procedural success and outcome for patients.
-
Impact of choice of the specific valve and delivery methods on clinical outcome.
-
Effect of age on peri-operative complication and post-operative quality of life.
-
Vascular complication rate due to malfunctioning of the aortic valve and/or developed consequent to the TAVI procedure.
-
Morbidity related to TAVI and medical therapy.
-
Bleeding and renal insufficiency with the procedure and without it.
-
Stroke, transient ischaemic attack, and myocardial infarction.
-
Complications with associated treatments.
Effectiveness data:
-
Survival Rate short term and long term.
-
Overall impact of medical treatment and TAVI on health related quality of life, expressed as Quality Adjusted Life Years (QALYs) if possible.
-
Procedural success rate (successful implantation of aortic valve).
-
Haemodynamic improvements.
-
Cardiac symptomatic improvement (measured as NYHA).
Costs:
-
Procedural hospitalisation costs: the equipment, other resource use and costs associated with TAVI procedure (the procedure is generally performed in a hospital, in a cardiac operating theatre or a hybrid operating room: sterilised and equipped with specific instrumentation needed for the procedure).
-
Time and resources associated with multidisciplinary staff involved in the procedure's performance (cardiac surgeon, interventional cardiologist, anaesthetist, operating room assistant, echographist).
-
Diagnosis, admission and maintenance costs of patients going under TAVI procedure and of those who stay on standard therapy (these costs will include hospital stay, ongoing medical management and readmission costs per patient).
-
Repeat hospitalisation due to aortic stenosis or complications of the valve procedure (valve-related hospitalisation rate).
-
Outpatient resource utilisation.
Literature searches to obtain data
In order to obtain data to populate the model, our starting point will be the 2008 Bazian report1, the trial as reported in the NEJM,7 and the review of observational studies in the Annals of Internal Medicine. 8
We will update the searches in the Bazian report to ensure that up to date information is included. We consider these searches to be sufficiently robust. Wherever possible, model parameters will be chosen from studies where the data is most relevant to the decision problem.
Additional searches
Additional targeted searches may be performed on an ad hoc basis to seek information to populate parameters identified by the modellers that have not been obtained by other means.
Such information may include unit costs or prices, required to be attached to each resource item so that the overall cost per patient can be calculated. Some hospital resource utilisation and costs data are reported in the Bazian report1 but the two main sources will be the “Unit cost of health and social care10” published by (PSSRU) Personal Social Services Research Unit 2009 and the NHS Reference Cost. 11
Other sources of data
It is anticipated that data from controlled trials on long term outcomes and quality of life may not be found in the literature searches. Additionally, there does not appear to be data available from controlled trials of the effectiveness of TAVI in patients who are high risk, but not contraindicated, for surgery (patient group 2 in the model).
In order to address these points of concern, we have made contact with the study author of the PARTNER trial. Findings for one arm of this randomised controlled trial are currently published. 7 The published data is for patients who are contraindicated for surgery (patient group 1 in the model). The study author has provided us with data on patient quality of life at 1 year.
The other arm of the PARTNER trial involves the randomisation of patients who are high risk, but not contraindicated, for surgery (patient group 2 in the model). We have requested data from this arm of the trial. These results are due to be presented in spring 2011 and, if it is available before March 2011, the authors have provisionally agreed to provide us with that data.
A further source of data may be the national cardiac surgery register. We will take advice from the steering group whether this is a useful source of data for the model and, if so, how best to access the relevant information.
Model structure and approach
The likely model structure will be based on a decision tree representing the short-term effects of TAVI. Longer term effects will be incorporated in Markov processes with a monthly time cycle. TreeAge software will be used – this software is appropriate for the model structure proposed.
Markov models are able to represent clinical situations where patients change health states or experience recurrent events over a long period of time. 12 Health states to be included in this model are likely to be based on repeat hospitalisation, mainly due to left ventricular failure.
In the base-case analysis, the time horizon for the model will approximate a lifetime model. Alternative analysis will be run with a shorter time horizon of 2 years to reflect the information available from trial data. 7
An incremental approach will be adopted with a focus on additional costs and gain in benefits. Discounting adjustments will be made to reflect the differential timing of costs and outcomes in terms of extension to the length of life associated with the procedure.
Sensitivity analysis and presentation of results
Both deterministic and probabilistic sensitivity analysis will be conducted. Deterministic analysis will include consideration of alternative scenarios and may also include unvaried sensitivity analysis in which key parameters are individually varied within their plausible range. This will help us to find the parameters that drive uncertainty.
Probabilistic sensitivity analysis considers overall parameter uncertainty by constructing distributions for values of model parameters, either singly or jointly, as required to allow for correlation between uncertainties in parameters. 13 Results will be presented in graphical formats including cost-effectiveness scatter plots and cost-effectiveness acceptability curves. 14
Project timetable
The proposed duration for this project is 5 months.
| Five months Project Task | Nov 2010 | Dec 2010 | Jan 2011 | Feb 2011 | Mar 2011 |
| Preview protocol development | |||||
| Searching and collecting studies | |||||
| Study assessment, data extraction | |||||
| Evidence synthesis | |||||
| Economic modelling | |||||
| Progress report to NCCHTA | |||||
| Writing draft report | |||||
| Internal peer review | |||||
| Final report and paper writing |
References
- Percutaneous Aortic Valve Replacement for Severe Aortic Stenosis. Bazian Ltd Report 2008.
- Transcatheter aortic valve implantation for aortic stenosis: guidance. National Institute for Health and Clinical Excellence. London: NICE; 2008.
- Ambler G, Omar RZ, Royston P, Kinsman R, Keogh BE, Taylor KM. Generic, simple risk stratification model for heart valve surgery. Circulation 2005;112:224-31.
- Fludman P. UK TAVI Registry Presentation on Behalf of UK Registry Group EuroPCR British Cardiovascular Intervention Society Department of Health n.d. URL: http://www.sbhci.org.br/pdf/euro_pcr2010/26.05/artigo4_26_05.pdf.
- A position statement of the British Cardiovascular Intervention Society (BCIS) and the British Society for Cardiothoracic Surgery (SCTS). London: BCIS, SCTS; 2008.
- Drummond MF, O’Brien B, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2005.
- Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al. Transcatheter aortic valve implantation for aortic stenosis in patients who cannot undergo surgery. NEJM 2010;363:1597-60.
- Coeytaux RR, Williams JW, Grey RN, Wang A. Percutaneous heart valve replacement for aortic stenosis: state of the evidence. Annals of Internal Medicine 2010;153:314-2.
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. BMJ 1996;313:275-83.
- NHS Reference Cost. Department of Health; 2010.
- Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Dec Making 1993;13:322-8.
- Felli JC, Hazen GB. Sensitivity Analysis and the Expected Value of Perfect information. Medical Decision Making 1998;18:95-109.
- Claxton K, Neumann PJ, Araki S, Weinstein MC. Bayesian Value-of-Information Analysis: An application to a policy model of Alzheimer’s disease. International Journal of Technology Assessment in Health Care 2001;17:38-55.
List of abbreviations
- ACE
- angiotensin-converting enzyme
- AS
- aortic stenosis
- AVA
- aortic valve area
- BCIS
- British Cardiovascular Intervention Society
- CABG
- coronary artery bypass grafting
- CI
- confidence interval
- CRD
- Centre for Reviews and Dissemination
- DARE
- Database of Abstracts of Reviews of Effects
- ESC
- European Society of Cardiology
- EuroSCORE
- European System for Cardiac Operative Risk Evaluation
- GP
- general practitioner
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- ICU
- intensive care unit
- IEOA
- indexed effective orifice area
- ISRCTN
- International Standard Randomised Controlled Trial Number
- LES
- logisitic EuroSCORE
- MDT
- multidisciplinary team
- MESH
- medical subject heading
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- NYHA
- New York Heart Association
- OR
- odds ratio
- PARTNER
- European Placement of AoRtic TraNscathetER valves trial
- PPM
- prosthesis–patient mismatch
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- ROC
- receiver operating characteristic
- SAVR
- surgical aortic valve replacement
- SD
- standard deviation
- STS-PROM
- Society of Thoracic Surgeons Predicted Risk Of Mortality
- TAVI
- transcatheter aortic valve implantation
- TA
- transapical
- TF
- transfemoral
- UKCRN
- UK Clinical Research Network
- UHSM
- University Hospital of South Manchester
- WHO
- World Health Organization
- Zetoc
- British Library's Electronic Table of Contents
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.