Notes
Article history paragraph text
The research reported in this issue of the journal was funded by the HTA programme as project number 10/91/01. The contractual start date was in June 2011. The draft report began editorial review in January 2012 and was accepted for publication in October 2012. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors' report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen's Printer and Controller of HMSO 2013. This work was produced by Sutcliffe et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Scientific summary
Background
The spine is a common site for bone metastasis for a number of cancers. Spinal metastases may grow to cause weakness and fracture of a vertebra or compression of the spinal nerve cord. Spinal cord compression (SCC) carries a risk of paralysis of body structures below the level of compression, compromising limb movement and bladder, bowel and sexual functioning. Early targeted treatment might prevent, reduce or delay serious unwanted outcomes. Diagnostic methods include plain radiography, myelography, magnetic resonance imaging (MRI), computerised tomography (CT), radionuclide bone scanning (scintigraphy with technetium-99m-labelled diphosphonates), single-photon emission CT and positron emission tomography (PET).
These might serve several purposes: (1) to inform the choice about potential pre-emptive intervention(s) so as to avoid or delay complication and more radical surgical intervention; (2) to bring forward radical interventions before patient health deteriorates too far; and (3) to categorise patients into those more or less suitable for earlier or later radical intervention. However, there is uncertainty about the effectiveness of these diagnostic techniques.
Main question
To undertake a systematic review to examine the natural history of metastatic spinal lesions and to identify patients at high risk of vertebral fracture and SCC.
Methods
Searches were undertaken from inception to June 2011 in 13 electronic bibliographic databases (e.g. MEDLINE, EMBASE, Cochrane Database of Systematic Reviews, etc.). Evidence was also retrieved through contact with experts, scrutiny of references of included studies, and other relevant resources. The search strategy covered the concepts of metastasis, the spine and adults. No study type or publication type restrictions were applied, as all types of study involving all languages were screened for potential inclusion. The titles and abstracts of retrieved studies were examined for inclusion by two reviewers independently. Disagreement was resolved by retrieval of the full publication and consensus agreement. Included studies involved adult patients with vertebral metastases, at risk of developing (or who had developed) metastatic spinal cord compression, vertebral collapse or progression of vertebral collapse. Natural history was taken to mean the progression of spinal metastases from inception to resolution independent of the influence of intervention. Diagnostic/prognostic methods included clinical features and/or imaging technologies. Full data were extracted independently by one reviewer. All included studies were reviewed by a second researcher with disagreements resolved by discussion. A quality assessment instrument was used to assess bias in six domains: study population, attrition, prognostic factor measurement, outcome measurement, confounding measurement, and account and analysis. Data were tabulated and discussed in a narrative review.
Results
Searches
In all, 2425 potentially relevant articles were identified; 31 met the inclusion criteria. Seventeen studies reported retrospective data, 10 were prospective studies, three were other study designs and one was a systematic review. There were no randomised controlled trials (RCTs). The approximate overall number of participants was 7888 and 5782 were included in analyses. Sample sizes analysed ranged from 41 to 859. Cancers reported on were: lung (n = 3), prostate (n = 6), breast (n = 7), mixed cancers (n = 13) and unclear (n = 1).
Quality assessment
Included studies were generally of poor methodological quality and suffered from missing data, lack of transparency and clarity of reporting, particularly regarding participant selection. No studies tested the performance of identified risk factors in a cohort independent of the one in which the factors had been identified. Almost all made use of medical records and/or stored scan images rather than using data collection techniques specifically designed for research purposes.
Summary of findings of included studies
We did not identify any epidemiological study with a primary aim of investigating the natural history of spinal metastases. Most studies looked at factors associated with survival. Identification of prognostic factors for intermediate outcomes (SCC or vertebral collapse) was often an incidental objective. Ninety-three prognostic factors were reported as statistically significant in predicting risk of vertebral fracture or SCC in the 30 included primary studies.
Consideration of quantitative results from the studies does not easily allow generation of a coherent numerical summary: studies were heterogeneous, especially with regard to population, results were not consistent between studies and study results almost universally lacked corroboration from other independent studies. Below we summarise the major findings; these should be viewed with caution while bearing in mind the caveats regarding quality of studies and the general lack of replication of results.
Summary of prostate cancer studies
None of the included prostate cancer studies provided a description of the natural history of spinal metastases.
Only 409 patients were included in the six prostate cancer studies identified, and the underlying populations, diagnostic interventions methodology and transparency of reporting of these studies varied. This made interpretation of findings difficult. Selection bias was a potential problem in almost all studies, particularly because they all used routine medical records for data collection. In the prostate cancer studies, high tumour grade, high metastatic load and long time on hormone therapy were associated with increased risk of SCC. Studies reported that the more spinal metastases that were present, and the longer a patient was at risk, the greater the chance of clinically occult SCC. It was suggested that the time a patient is on hormone therapy may be a proxy for risk of occult compression.
In one investigation of castration-resistant metastatic prostate cancer, risk of SCC before death was 24% and was 2.37 times greater with high-grade cancer than with low-grade cancer (Gleason score ≥ 7 compared with < 7) (p = 0.003). A further investigation reported that patients with six or more bone lesions were at greater risk of SCC than those with fewer than six lesions [odds ratio (OR) 2.9, 95% confidence interval (CI) 1.012 to 8.35; p = 0.047]. Among these patients, median time from initial MRI for suspected SCC to development of neurological deficit was 896 days (95% CI 13 to 986 days).
However, prostate cancer studies were heterogeneous, results were not consistent between studies and study results almost universally lacked corroboration from further independent studies.
Results from the prostate cancer studies also imply that:
-
Patients with a high-risk bone scan may benefit from MRI screening of the spine aimed at early detection and treatment of occult subarachnoid space compression/SCC.
-
‘Total involvement of vertebra’, according to scintigraphy, appears to be highly discriminatory for subsequent SCC.
Summary of breast cancer studies
None of the studies described the natural history of spinal metastases derived from breast cancer.
The seven included studies were disparate in terms of population, imaging procedures and study aims, and some provided limited information on these factors. In an early study, a positive test result from myelography for suspected epidural SCC was associated with a positive bone scan (p < 0.001), bone pain (p < 0.001), and paraesthesia (p = 0.009). Among breast cancer patients who underwent CT for suspected SCC, multiple logistic regression identified four independent variables predictive of a positive test: bone metastases ≥ 2 years (OR 3.0, 95% CI 1.2 to 7.6; p = 0.02); metastatic disease at initial diagnosis (OR 3.4, 95% CI 1.0 to 11.4; p = 0.05); objective weakness (OR 3.8, 95% CI 1.5 to 9.5; p = 0.005); and vertebral compression fracture on spine radiograph (OR 2.6, 95% CI 1.0 to 6.5; p = 0.05). A Japanese study of breast cancer patients following primary surgery using Cox's regression analysis reported that the risk of developing bone metastases was associated with tumour/node/metastasis (TNM) tumour stage [hazard ratio (HR) 1.615, 95% CI 1.322 to 1.973; p < 0.0001]; N (nodal) stage classification (HR 2.128, 95% CI 1.381 to 3.279; p = 0.0006); presence of metastases to axillary lymph nodes (p = 0.0006); and the presence of metastases in important organs (HR 7.502, 95% CI 5.100 to 11.036; p < 0.0001). Of patients who developed skeletal metastases, 82% exhibited spinal metastases and 14% of these developed paralysis. The median time between detection of skeletal metastases and development of SCC was 4.4 (range 2–72) months.
A consideration of quantitative results from the breast cancer studies does not easily allow generation of a coherent numerical summary; as with prostate cancer, studies were heterogeneous, especially with regard to populations, results were not consistent between studies and, almost universally, study results lacked independent corroboration.
The following results should therefore be viewed with caution:
-
A positive bone scan, back pain, paraesthesia and bladder/bowel dysfunction at the time of myelography were more common in patients with a positive myelogram than in those with a negative myelogram.
-
Objective weakness in patients with suspected SCC was predictive for SCC but estimates of sensitivity and specificity for this were low.
-
Stratification of patients suspected of SCC according to the number of independent risk factors (see above: e.g. stage, grade, duration of risk and bone metastasis) identified a high-risk group with an 85% probability of CT-positive SCC.
-
TNM classification stages were identified as risk factors in one study.
-
Longer survival was a risk factor for vertebral fracture and for SCC.
-
Two biomechanical studies examined in vitro power of vertebral load-bearing capacity estimates for predicting vertebral fracture and were reported to have superior specificity to an alternative method; however, this is, of course, not practicable in the clinical setting.
Results from time-to-event analyses are difficult to generalise because of the different populations studied and the uncertainty regarding representativeness.
Summary of lung cancer studies
The three included studies used retrospective methods and routinely collected case note data. Two studies investigated patients with non-small cell lung cancer (NSCLC) and recruited a substantial number of participants (642 with advanced disease and 273 with bone metastases).
Among patients with advanced NSCLC who received chemotherapy, the occurrence of skeletal-related events (SREs; i.e. fracture, SCC, requirement for bone surgery or radiotherapy, or hypocalcaemia causing death or requiring emergency treatment) was reported to be associated with the load of bone metastases (OR 3.08, 95% CI 1.60 to 5.94 for single bone metastasis; OR 4.27, 95% CI 2.66 to 6.86 for multiple bone metastases). Among patients with more than one bone metastasis, the median time from start of chemotherapy to occurrence of first SRE was 19.7 months (95% CI 14.5 to 24.9 months). In another study of patients with advanced small cell lung cancer with skeletal metastases, multivariate analysis identified ‘ever smoked’ as significantly associated with risk of a SRE (OR 2.8, 95% CI 1.32 to 6.00).
For lung cancer, findings included:
-
The greater the number of bone metastases, the greater is the risk of a SRE.
-
There was an increased likelihood of SREs with smoking, lack of history of treatment with epidermal growth factor receptor tyrosine kinase inhibitors, poor Eastern Cooperative Oncology Group (ECOG) status and non-adenocarcinoma.
Again prognostic factors identified were not validated in other independent populations.
Summary of studies involving a variety of cancers
Thirteen studies investigated mixed primary tumour types. Patients with breast, prostate and lung cancers provided the majority of participants; however, it is important to note that the relative contribution of different tumour types varied considerably from study to study. A very broad range of factors was investigated. Among patients who received surgery for SCC a retrospective analysis identified that vertebral body compression fractures were associated with presurgery chemotherapy (OR 2.283, 95% CI 1.064 to 4.898; p = 0.03), primary breast cancer (OR 4.179, 95% CI 1.457 to 11.983; p = 0.008), thoracic involvement (OR 3.505, 95% CI 1.343 to 9.143; p = 0.01) and anterior cord compression (OR 3.213, 95% CI 1.416 to 7.293; p = 0.005). In another study, thecal sac compression was associated with abnormal neurological examination (OR 3.0, 95% CI 1.6 to 10.4; p = 0.004), stage IV cancer at initial diagnosis (OR 2.8, 95% CI 1.40 to 7.7; p = 0.006), known vertebral metastases (OR 2.8, 95% CI 1.4 to 8.2; p = 0.008) and middle or upper back pain (OR 2.7, 95% CI 1.4 to 9.1; p = 0.010).
Findings common to several of these mixed cancer studies included:
-
Primary tumour type was a risk factor for vertebral collapse and SCC recurrence in three studies.
-
Patient health status was a factor in SCC recurrence.
-
Degree of tumour occupancy of the vertebral body was predictive for fracture.
-
Two studies identified combinations of risk factors to predict individual SCC risk with high probability – five factors delivered a probability of 87% and combination of three or four factors gave a probability of 81%.
-
An empirical algorithm for prediction of fracture in vertebrae harbouring predominantly lytic metastases was found potentially useful, as were other proposed models.
Missing data, lack of transparency and clarity of reporting, particularly regarding participant selection, mean that in general the validity of findings was uncertain. No studies tested the performance of identified predictors or risk factors in an independent cohort.
Discussion
We undertook a systematic review to examine the natural history of metastatic spinal lesions and to identify patients at high risk of vertebral fracture and SCC. We identified 31 studies in three different cancer areas of which 13 studies had populations with several different cancers represented.
Overall summary of results
We did not identify any epidemiological study with a primary aim of investigating the natural history of spinal metastases.
The evidence presented in this report suggests that the greater the extent of invasion of any one vertebra by metastases, the more likely spinal fracture is to occur. In addition, the more spinal metastases present and the longer a patient is at risk, the greater the chance of SCC. There is an increased risk of developing SCC if a cancer has already spread to the bones. Clinicians are unlikely to have been unaware of these factors and much of the research reported here appears to add little to current knowledge. Several included studies, with populations with a mix of cancer types, identified cancer type itself as a significant factor in predicting SCC, but it remains difficult to determine the difference in risk as a result of the type of cancer (e.g. breast, lung or prostate cancer) and these studies are liable to suffer from residual bias.
Three studies attempted to combine risk factors into algorithms predictive for occurrence of an event. These appeared to have modest discriminatory power but were not tested in independent samples.
Included studies were of poor methodological quality and made use of medical records and/or stored scan images rather than using data collection techniques specifically designed for research purposes.
Imaging methods used for detection of and screening for SCC and/or vertebral fracture have changed over the duration of the studies described. Formal comparison of different imaging procedures was rarely undertaken and we found no RCTs. It is clear that investigations now favour MRI and CT over myelography only and/or plain radiography. Bone scanning (e.g. scintigraphy) were widely employed but PET was not used in any of the included studies. The development and routine availability of machines with faster throughput and better performance (e.g. resolution) may change practice.
The considerable variability in the prognostic factor categories, the quality of studies, the lack of studies for some categories and changes in practice over the time period to which the studies relate have all made it difficult to provide clear conclusions as to which factors might currently offer the most potential to identify patients at high risk of vertebral fracture and SCC.
Strengths and limitations
We identified a large volume of literature and all papers were read and sifted by two reviewers. We used a rigorous search strategy in a large number of databases. A large number of papers were sifted at full paper stage. Nevertheless, our κ-statistic at 0.74 was acceptable. Owing to the poor reporting of the natural history we are unable to draw any conclusions on this aspect of the review. As far as prognostic factors are concerned, heterogeneity precluded the use of meta-analysis.
Implications for research
There is a need for:
-
Prospective randomised designs of the clinical effectiveness and cost-effectiveness of identification and subsequent treatment of patients at high risk of vertebral collapse and SCC. These trials should be undertaken for diagnostic methods such as bone scintigraphy and particularly for serial MRI, to identify patient groups who are most likely to benefit from early detection and treatment, and the value of, and optimal frequency of MRI screening for populations.
-
Service Delivery and Organisation research on MRI and scanning (in tandem with research studies on use of MRI to monitor progression) in order to understand best methods for maximising use of MRI scanners (e.g. to investigate variation in need, and optimal location, throughput and staffing, etc.).
-
Investigation of prognostic algorithms designed to calculate the probability of a specified event using high-quality prospective studies, involving defined populations, randomly selected and clearly identified samples, and with blinding of investigators.
-
Higher-quality prospective studies to investigate and confirm previous findings on risk factors for progression or spinal collapse, as opposed to survival. These could usefully feed into work on prognostic algorithms.
-
Methodological research to improve prognosis research.
Implications for clinical practice
The major factors that should be taken into account when considering a patient for further investigation and potential treatment when at risk of SCC, progression or spinal collapse have not altered from those identified in 2008 NICE guideline 75.
Conclusions
This report has identified a large number of studies reporting limited evidence on risk factors for progression or spinal collapse for patients with spinal metastases. Evidence is generally of poor quality. Rigorous research is now needed on best diagnostic methods for patients with spinal metastases to identify those patients at high risk of vertebral fracture and SCC.
Funding
Funding for this study was provided by the Health Technology Assessment programme of the National Institute for Health Research.
Chapter 1 Introduction
When a cancer spreads to a new and different site in the body it very often locates in the bony skeleton. The commonest place for these new cancers in bone is in one or more vertebrae, in which case they are called spinal metastases. Sometimes these spinal metastases do not cause symptoms; however, they can be a source of severe pain or weakness in the vertebrae, which may fracture. Spinal metastases may grow so that the spinal nerve cord that runs through the length of the vertebral column is compressed. In this report we concentrate mainly on bony metastases in the spine. Although rarer, metastases may also grow in the extradural space, causing metastatic spinal cord compression (SCC). 1
When vertebrae fracture, the spine may become bent or twisted, making everyday movements more difficult, and there is a danger that vertebral fracture and collapse may also cause compression of the spinal cord. Compression of the spinal cord carries with it the risk of paralysis of body structures below the level of compression. If it were possible to predict which vertebrae were more likely to fracture, then early targeted treatment might prevent, reduce or delay such events and the serious unwanted outcomes that can result.
This report aimed to examine the natural history of metastatic spinal lesions and to identify patients at high risk of progression or spinal collapse. The use of these technologies might serve several purposes: (1) to inform the choice of potential pre-emptive intervention(s) so as to avoid or delay more radical surgical intervention; (2) to bring forward radical interventions before patients' health deteriorates to the extent that they are no longer suitable candidates for intervention; and (3) to categorise patients into those more or less suitable for earlier or later radical intervention.
The first chapter examines the different types of cancer, pathological and clinical manifestation of spinal metastases, investigations, treatment, prognosis and current service cost.
Background
Cancer is the second most common cause of death in the UK and it constituted 29% of all deaths registered in England and Wales in 2010. 2 Cancer of the lung, colorectum, breast and prostate are responsible for the majority of incident cancer and cancer deaths in the UK (Tables 1 and 2). 3,4 In 2009, lung cancer and colorectal cancer were the leading causes of cancer death in both sexes (24% of all deaths in males and 21% of all deaths in females for lung cancer; 10.5% in males and 10% in females for colorectal cancer) (see Table 1). The second most common causes of cancer death by sex were breast cancer in women and prostate cancer in men, constituting approximately 7.5% and 6.6% of all cancer deaths in the UK, respectively. 5 In 2008, breast cancer (15%) was the most commonly diagnosed cancer in the UK followed by cancer of the lung (13.2%), the colorectum (12.9%) and then the prostate (12%). 4
| Cancer type | Male, n (%) | Female, n (%) | Total, n (%) |
|---|---|---|---|
| Lung cancer | 19,724 (24.08) | 15,265 (20.61) | 34,989 (22.41) |
| Colorectal cancera | 8600 (10.48) | 7308 (9.86) | 15,908 (10.19) |
| Breast cancer | 77 (0.09) | 11,651 (15.73) | 11,728 (7.51) |
| Prostate cancer | 10,382 (12.65) | – | 10,382 (6.65) |
| Other cancers | 43,251 (52.70) | 39,832 (53.80) | 83,083 (53.24) |
| All cancer deaths | 82,034 | 74,056 | 156,090 |
| Cancer type | Male | Female | Overall | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of new cases | European AS rate per 100,000 | Rank in UK | Number of new cases | European AS rate per 100,000 | Rank in UK | Number of new cases | European AS rate per 100,000 | Rank in UK | |
| Breast cancer | 341 | 0.9 | – | 47,693 | 123.9 | 1 | 48,034 | 65.2 | 1 |
| Lung cancer | 22,846 | 59.4 | 2 | 17,960 | 38.8 | 2 | 40,806 | 47.8 | 2 |
| Colorectal cancera | 22,097 | 58.5 | 3 | 17,894 | 37.8 | 3 | 39,991 | 47.2 | 3 |
| Prostate cancer | 37,051 | 97.9 | 1 | – | – | – | 37,051 | – | 4 |
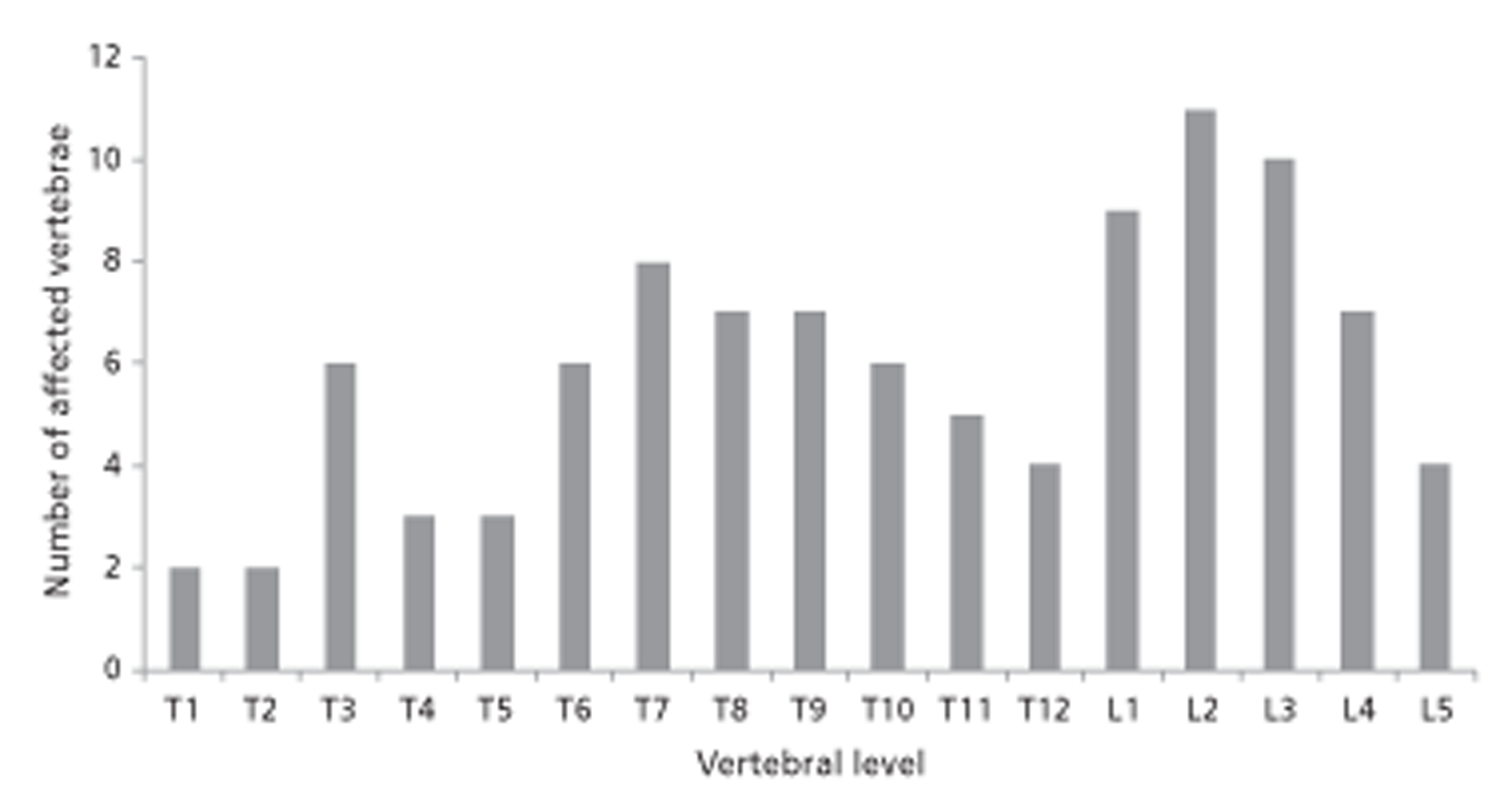
In most cases, death occurs as a result of metastases and complications rather than the primary tumour. 6 The most common site of metastases is the liver, followed by lung and bone. 7,8 Approximately 70% of all bone metastases are in the spine. 8 It is reported that 60–70% of patients with systemic cancer develop spinal metastasis, although only 10% are symptomatic. 9 The thoracic vertebrae (60–80%) are the most frequently involved sites, followed by lumbar (15–30%) and cervical vertebrae (< 10%) (Figure 1). 7,10 It is estimated that almost half of patients with spinal metastasis will have metastases at multiple levels of the spine. 11
FIGURE 1.
Spinal cord. Reproduced with permission from CancerHelp UK, the patient information website of Cancer Research UK. URL: http://cancerhelp.cancerresearchuk.org.
Anatomically, spinal metastases can be classed as intradural (intramedullary or extramedullary) or extradural. 12 Approximately 95% of extradural lesions are either pure epidural lesions (rare) or those arising initially from the vertebra but migrating to the thecal sac. 7
Cancer cells spread to the spine through various mechanisms – via the arterial system, Batson's venous plexus or cerebrospinal fluid (CSF) and directly from paraspinous disease. 10 In most cases, the posterior ventral body is the initial site of involvement. In > 90% of patients, spinal metastases are extradural, most often arising in the vertebral column and then extending into the epidural space. Spinal metastases very rarely involve the intradural and intramedullary regions of the spine. 10
The average time from original diagnosis of cancer to development of spinal metastases has been estimated to be 32 months and the average time from detection of spinal metastases to spinal compression approximately 27 months. 13 It is reported that median overall survival of patients with spinal metastases is 7 months (ranging between 3 and 16 months), although in those with epidural metastases median overall survival is between 3 and 6 months. Overall survival depends mainly on type of primary tumour. 8 Two-year survival rate is lowest for lung cancer (≈ 9%) but higher for breast and prostate cancer (≈ 44%). 8 It has been estimated that only between 10% and 20% of patients with spinal metastases are alive 2 years after diagnosis. 8
Although spinal metastases can occur in any age group, they are most commonly seen in individuals aged between 40 and 70 years. 10 It has been suggested that the incidence of spinal metastasis is comparatively higher in males than in females probably because of higher incidence of prostate cancer relative to breast cancer. 10
Spinal metastases can lead to significant morbidity and reduction in quality of life owing to SCC, which can result in para- or quadriplegia, severe bone pain and pathological fractures. 7,10 Between 5% and 20% of patients with spinal metastases develop metastatic SCC during the course of their disease. 8,14 An early study estimated average survival for patients with SCC to be between 3 and 7 months, with a 36% probability of survival to 12 months. 13 Therefore, early diagnosis of spinal metastases is important. 15 It can help clinicians to manage disease and delay complications. 15 However, there are disputes regarding the specificity and sensitivity of the different diagnostic techniques currently available. 8 Some authors have also developed different models that can be used to predict collapse of the metastatic vertebrae.
The current review aims to explore the natural history of metastatic spinal lesions and to evaluate evidence on technologies for identifying patients at high risk of vertebral fracture and SCC.
Types of cancer
Breast cancer
Breast cancer is the most common cancer among women in the UK. Approximately 48,000 women were diagnosed with breast cancer in 2008 (see Table 2). 4 The European age-standardised (AS) incidence rate for breast cancer was reported as 124 per 100,000 in the UK in 2008. 3 Using the adjusted for multiple primaries (AMP) method, Cancer Research UK reported the lifetime risk of breast cancer to be one in eight for women and one in 1014 for men. 16
Although there are a number of risk factors, increasing age is one of the most important. 16 Approximately 81% of breast cancer cases were diagnosed in those aged > 50 years and almost half occurred between 50 and 69 years of age. 16
Bone is the most common site for metastases in breast cancer. 17 It is suggested that cancer cells metastasise directly to the bone via blood from some anatomical sites. Some studies have found that venous blood from the breast drains to the vena cava and also into a vertebral venous plexus. 18 The latter drains blood to the skeletal system, and this partly explains the likelihood of spread to bones. 18 There are other factors that influence the pattern of metastases, such as the molecular and cellular biological characteristics of breast cancer cells and of the tissues of the metastatic sites. 19
The extent of the disease is measured using the tumour/node/metastasis (TNM) classification. In this classification, ‘T’ refers to the size of the tumour, ‘N’ refers to spread of the tumour to lymph nodes and ‘M’ refers to distant metastases. 20 The treatment and prognosis of patients with breast cancer depend on the extent of the disease. 20 There are three types of receptors expressed on breast cancer cells, namely oestrogen receptor, progesterone receptor and human epidermal growth factor receptor 2. 20 Treatment of patients also depends on these receptors. 20
Approximately 70% of patients with metastatic breast cancer will have bone metastases. 19 A retrospective study of all patients with histologically confirmed diagnosis of carcinoma of the breast attending the Clinical Oncology Unit at Guy's Hospital in London reported that approximately 69% of the patients had radiological evidence of skeletal metastases before death. 21 Another large population-based cohort study in Denmark carried out over 9 years (between 1999 and 2007) reported a lower incidence rate of bone metastases. Researchers estimated the overall and annual incidence of bone metastases and skeletal-related events (SREs) in newly diagnosed breast cancer patients. 22 The authors found that 0.5% of patients had bone metastases at the time of primary diagnosis. The 1-year, 3-year and 5-year cumulative incidences of bone metastases among these patients were found to be 1.9% [95% confidence interval (CI) 1.7% to 2.0%], 3.4% (95% CI 3.2% to 3.6%) and 4.7% (95% CI 4.4% to 4.9%), respectively. 22 One study conducted in Canada reported a similar incidence rate of bone metastases. This study included a cohort of women (n = 1608) with invasive breast cancer treated in a hospital between 1987 and 1997 to evaluate the patterns of metastatic spread in different types of breast cancer. In this study, the risk of developing bone metastases within 10 years after diagnosis was 7–9% for all types of breast cancer. 23
Median survival from diagnosis of bone metastases from breast cancer is measurable in years; in contrast median survival from lung cancer is measured in months. 18 The prognosis for breast cancer is mainly dependent on co-existing non-osseous metastatic disease. In a retrospective study of 859 patients with bone metastases from breast cancer seen at one hospital between 1975 and 1991, a median survival of 34 months was reported in those with only bone metastases compared with a median survival of 5.5 months in those with bone and liver metastases. 24 Subsequent occurrence of extraosseous metastases in those with breast cancer and metastases confined to bone significantly affects survival. Median survival in those who developed extraosseous metastasis was 1.6 years compared with the median survival of 2.1 years in those with no extraosseous metastasis (p < 0.001). 25
Ten-year survival in patients with early-stage breast cancer who are diagnosed early is approximately 85%, because of advances in combination therapy. 19 Those who survive are thought to go through repeated periods of remission and progression. 19 The progression stage is responsible for significant morbidity, which may manifest itself clinically as pain, pathological fractures, SCC and hypercalcaemia. The occurrence of these events is seen to be highly influenced by whether or not patients are on treatment. Walkington and Coleman19 found that the skeletal events occurred more frequently if the patients were not receiving bone-targeted therapies.
Lung cancer
Lung cancer is the second most commonly diagnosed cancer in the UK after breast cancer (see Table 2). 4 In 2008, approximately 40,806 new cases of lung cancer were diagnosed. 4 The European AS incidence rate for lung cancer in the UK was found to be 47.8 per 100,000 in 2008. 3 Using the AMP method, Cancer Research UK calculated the risk of lung cancer to be 1 in 14 for men and 1 in 19 for women. 26 The incidence is reported to be high in Scotland and northern England and lower in Wales, the Midlands and southern England. 26 Cigarette smoking is the most important risk factor for lung cancer. 27 The National Institute for Health and Care Excellence (NICE)27 reported that since the 1970s there has been a 25% reduction in the number of men who smoke, whereas the number of women smoking has increased considerably, leading to an increased number of deaths among women. 27 Therefore, if the cause of death is considered according to sex, then in 2009 lung cancer was the number one cause of cancer deaths in women, followed by breast and colorectal cancer (see Table 1). 5
Lung cancer is rare in those aged < 40 years and the risk increases after this age, with 87% of cases in people aged > 60 years. 26 Incidence rates are highest in those aged 80–84 years. 26
Histologically, lung cancer can be categorised into two types: approximately 20% are small cell lung cancer (SCLC) and the remaining 80% are non-small cell lung cancer (NSCLC). 27 There are three main types of NSCLC, namely squamous cell carcinoma, adenocarcinoma and large cell carcinoma, constituting approximately 35%, 27% and 10% of all NSCLC, respectively. 27 In those who smoke cigarettes, all four types of lung cancer are common, whereas, in those who do not smoke, adenocarcinoma is common. 26 Adenocarcinoma is now the most common type of lung cancer seen in North America, and it has been suggested that this could be due to the increasing reduction in cigarette smoking and a change in pathological classification. 28 In Europe, squamous cell carcinoma is the most common type of lung cancer. 26
Approximately 22% of all cancer deaths in the UK are caused by lung cancer. 5 In England and Wales, the 1-year survival rates of cancer in men and women are 27% and 30%, respectively, while the 5-year survival rates are 7% and 9%, respectively. 26 Survival rate is low compared with other cancers, mainly because lung cancer is often diagnosed at an advanced stage. 26 It has been estimated that the 5-year survival rate for those diagnosed with stage 1A NSCLC would be 54–80% whereas for those in stage 1B it would be 38–65%. 26
Bone is a common site of metastasis in lung cancer. It is reported that approximately 15–30% of patients with lung cancer will have bone metastases. 29 Approximately 30–40% of patients with advanced lung cancer will develop bone metastases during the course of their disease, resulting in a significant negative impact on both morbidity and survival. 30
Prostate cancer
Prostate cancer is the most incident cancer in men3 and the second most common cause of deaths in men (see Tables 1 and 2). 5 In 2008, approximately 37,051 men were diagnosed with prostate cancer in the UK. 4 The European AS incidence rate was 97.9 per 100,000 in 2008. 3 Approximately 13% of cancer deaths in 2009 were due to prostate cancer. 5 Using the current probability method, Cancer Research UK found that in 2008 the lifetime risk in the UK of being diagnosed with prostate cancer was 1 in 9. 31
Incidence rates of prostate cancer have increased over time, and it has been suggested that this is the result of better detection techniques and testing methods. 31,32 One study stated that, if men lived long enough, all of them would be likely to die with histological evidence of the disease present. 33 However, in fact only about 3% of men die of prostate cancer. 32
Prostate cancer risk increases with increasing age. Incidence rates are almost five times higher in men aged 75–79 years than in those aged 55–59 years (751 per 100,000 vs. 155 per 100,000 of population). 31
Prostate cancer survival depends on stage of disease. Five-year survival rate in men with localised disease is > 90%, whereas in those with metastatic disease it is approximately 30%. 34
Factors such as TNM classification stage, Gleason score and prostate-specific antigen (PSA) levels are used as predictive factors in prostate cancer. 32 The TNM classification is the most important of them. 32 It is used to stage the disease: T stage is used to indicate the extent of primary tumour, N stage is used to indicate if the disease has spread to local lymph nodes and M stage is used to describe the absence or presence of distant metastasis. 32 Based on this classification, if the cancer is found to have spread to lymph nodes and distant sites, then the prognosis is poor. 32
Gleason score is an international grading system used to grade biopsy specimens histologically on the basis of architectural differentiation of tumour cells which, in turn, can predict lymph node metastases. 32 A score of ≥ 7 indicates that the tumour has metastasised to lymph nodes and the prognosis is poor. 32
Prostate-specific antigen is a protein released by both normal and malignant prostate cells. 32,35 As serum PSA levels can rise in a number of conditions other than malignancy, such as infection and benign enlargement of the prostate,32,35 it has been suggested that PSA testing is not a good marker for this condition. 32,35
The prostate is a small gland located below the bladder and in front of the rectum that helps in production of fluid for semen. 36 It is divided into several zones but cancer mainly originates from the peripheral area. It is estimated that approximately 95% of prostate cancers are adenocarcinoma. 33
Prostate cancer is caused by genetic mutation. 37 Owing to mutation, control of normal proliferation and differentiation of prostate cells is lost, and this in turn leads to abnormal accumulation of a large number of abnormal cells. 37 These cells accumulate and become a localised tumour. In the majority of cases, it takes many years for a cancer to become large enough to be detected clinically and even longer to spread either locally or to distant sites. Progression of the prostate tumour is dependent on androgen levels, especially levels of testosterone and dihydrotestosterone. Therefore, to delay progression, antiandrogen treatment (ADT) is given. This leads to chemical castration, which can hinder tumour growth. Over time genetic mutation may ensue and the tumour may become even more or less susceptible to androgen levels. 38 The tumour may continue to grow even when blood testosterone levels are low or negligible. Tumours that respond to ADT are known as castration-sensitive prostate cancer, and those that no longer respond are known as castration-resistant prostate cancer. The latter is also known as hormone-resistant prostate cancer.
Other solid tumours
The other most important cancer in the UK is colorectal cancer. In 2008, approximately 39,991 new cases were registered (22,097 in males and 17,894 in females). 4 Colorectal cancer is the second most common cause of cancer deaths in the UK,5 and 10% of all cancer deaths in 2009 were due to colorectal cancer (see Table 1). 5 It is reported that approximately 25% of patients with colorectal cancer have metastatic disease at the time of initial presentation. 39 The staging of colorectal cancer is undertaken using the Dukes' classification and more recently using the TNM classification. 40 Survival depends on stage of disease. Five-year survival is > 90% in those diagnosed with Dukes' stage A disease compared with 7% in those diagnosed at a later stage. 40 Usually cancers of the colorectum metastasise to liver and peritoneum. 41 In 6–10% of cases, the cancer may metastasise to bone. 41,42 Cancers of thyroid, kidney and bladder have also all been found to metastasise to bone (Table 3). 18
| Primary tumour | Incidence of bone metastases | |
|---|---|---|
| Roodman 200429 | Coleman 200618 (post-mortem examination) | |
| Breast | ≈ 70% with advanced disease | 73% |
| Prostate | ≈ 70% with advanced disease | 68% |
| Others | 15–30% in cancers of lung, colon, stomach, bladder, uterus, rectum, thyroid and kidney | Thyroid: 42%; kidney: 35%; lung: 36%; gastrointestinal: 5% |
Pathophysiology of bone metastasis
Bone is one of the commonest sites for metastasis in cancer. 8,10 Post-mortem examination of patients dying with a diagnosis of breast or prostate cancer revealed that about 70% had evidence of metastatic bone disease. 43 High percentages have also been observed for thyroid, kidney and lung carcinomas. 18
Mechanism of metastasis
There are three mechanisms by which a cancer can disseminate in the body: (1) direct seeding of body cavities or surfaces, (2) lymphatic spread and (3) haematogenous spread. 44 Direct dissemination of tumour cells is rare. It can, however, occur during surgery. 45 A direct seeding of body cavities and surfaces may occur when a tumour penetrates into a natural cavity. Most commonly involved is the peritoneal cavity, although other cavities such as the pleural, pericardial, subarachnoid and joint space can also be affected. Ovarian carcinoma is the best example of this type of metastasis, in which cancer cells spread to the peritoneal surface as a result of serosal invasion or perforation by cancer. 46
The initial dissemination of cancer occurs via the lymphatic system following the natural route of lymphatic drainage to local lymph nodes, which can act to prevent onward spread for a while. 44 For example, breast cancer disseminates into the axillary, infraclavicular and supraclavicular nodes. 47 Lung cancer of the major respiratory passages usually spreads to the perihilar tracheobronchial and mediastinal nodes. 48 In some cases, local lymph nodes may be spared because of venous–lymphatic anastomoses or because of obliteration of the lymphatic pathway by inflammation or radiation; however, this can lead to lymphoedema. 49
The most important method of spread to bone is via the circulatory system, particularly the venous system. 11 The retrograde venous route is probably the most important cause of metastasis to vertebrae. 11 There is a communication between veins of the breast and the plexus of Batson in the thoracic region and therefore cancers of the breast and lungs often metastasise to thoracic vertebrae. 11 Lungs drain their blood through pulmonary veins to the left side of the heart, which can therefore disseminate lung cancer cells to all parts of the body. 11 The prostate drains through the pelvic plexus into the lumbar region so cancers of the prostate metastasise to lumbosacral vertebrae. 11 Cancer of the bowel metastasises first to liver and lungs via the portal and caval system, respectively. 11
Some cancers such as renal cell carcinomas and hepatocellular carcinomas invade veins directly. 50,51 In renal cell carcinomas, cancer invades the renal vein, after which it grows within the vein up to the inferior vena cava. 51 In hepatocellular carcinomas, cancer often penetrates portal and hepatic radicles and then grows to penetrate the main venous channels. 50
Organ-specific metastasis
There are certain cancers that show an organ-specific pattern of spread. For example, cancers of breast and prostate usually metastasise to bone. 52 In order to explain this propensity of certain cancers to metastasise to specific organs, a ‘seed and soil’ hypothesis, first explained by Paget in 1889, is used. 53
Paget suggested that distribution of secondary growth does not happen by chance, but a relationship between tumour cells (referred to as ‘seed’) and host cells (referred to as ‘soil’) is the main reason why certain types of cancer metastasise to specific organs. 52,53 Blood flow in red marrow is very high and so it provides considerable opportunity for tumour cells to metastasise (seeding). Factors such as growth factors, hormones and cytokines provide a suitable environment for tumour growth/metastasis to take root in bone. 54,55 Another surgeon, James Ewing, challenged Paget's hypothesis and suggested that this type of metastasis occurs as the result of a certain circulatory pattern between cancer and specific organs. 52 Currently it is acknowledged that both hypotheses are important in understanding the pathogenesis of organ-specific metastasis. 52
Molecular mechanism
Metastatic bone diseases are often classified as osteolytic or osteoblastic; however, lesions can be made up of both components, i.e. osteoclasts and osteoblasts. 29,55,56 Osteoclasts originate from precursor cells of the monocyte–macrophage lineage whereas osteoblasts arise from mesenchymal stem cells. 29 The following description is based on the review by Roodman. 29
In an individual with no cancer, bone remodels itself via a synchronised process of osteoblast and osteoclast activity on trabecular surfaces and within the Haversian system. 29 First, resorption of bone byosteoclast occurs and then new bone is formed at the same location by osteoblasts. 29 However, when tumour cells metastasise to bones, this normal remodelling sequence is disrupted and, depending on the type of cancer, either osteoblastic or osteoclastic activity becomes predominant. In breast cancer, osteolytic lesions are predominant although at least a quarter of lesions are thought to be osteoblastic. 29 In prostate cancer, most lesions are osteoblastic in nature. It should, however, be noted that a lesion can contain both osteoblasts and osteoclasts. 55 The difference between the two types of lesions is evident only during radiological examinations: osteoclastic lesions appear lytic, osteoblastic lesions appear sclerotic and, when both components are present, lesion appears mixed. 57
In normal bone, several systemic hormones and locally produced cytokines are responsible for the formation and activity of osteoclasts and osteoblasts. 29 For these cells to develop properly, a suitable microenvironment is necessary, which is provided by macrophage colony-stimulating factor and receptor activator of nuclear factor-κB ligand (RANKL). 29 RANKL is a type of tumour necrosis factor present on the surface of osteoblasts and stromal cells. 29 Factors such as parathyroid hormone (PTH), 1,25-dihydroxyvitamin D3, prostaglandins and interleukins stimulate the formation of osteoclasts by increasing the expression of RANKL. 29 RANKL binds the RANK receptor on osteoclast precursors and forms osteoblasts via the nuclear factor-κB and Jun N-terminal kinase pathways. 29 Another type of tumour necrosis factor receptor, osteoprotegerin (known as decoy receptor), is also present in the bone marrow. It inhibits the differentiation and resorption of osteoclasts. The ratio of RANKL and osteoprotegerin regulates the formation and activity of osteoblasts. 29 The differentiation of osteoblasts is less well understood than that of osteoclasts. 29 Runx-2 (core-binding factor α1), a transcription factor, is important for differentiation of osteoblasts. It stimulates genes related to osteoblastic differentiation. Factors such as PTH, prostaglandins, cytokines, platelet-derived growth factor, corticosteroids and interleukins regulate the formation of osteoblasts. 29
Once cancer cells reach bone marrow, production of osteoclasts is increased. This increment is initiated by a factor called PTH-related peptide (PTHRP) produced by tumour cells. 29 When released, PTHRP binds to a receptor that is the same as that for PTH, called PTHR1, which activates RANKL on marrow stromal cells. The receptor then increases production of osteoclasts, which cause bone resorption. 29 This cycle supports tumour growth in the bone. 55 PTHRP is secreted by breast cancer cells, prostate cancer cells and other solid tumours. 29 During bone resorption, growth factors (transforming growth factor-β) and calcium stored in the bone matrix are released. 29,55 The transforming growth factor-β released during bone resorption further stimulates production of the PTHRP by the cancer cells. 29 This type of osteoclastic metastasis is predominantly seen in breast cancer. Other factors that also induce osteoclastic activity in breast cancer patients are interleukin 6, prostaglandin E2, macrophage colony-stimulating factor, interleukin 1 and tumour necrosis factor-α. 29
The mechanism and factors involved in osteoblastic metastasis are not well known. 29 In prostate cancer, a large number of fibroblastic growth factors have been found. Another growth factor, endothelin-1, is found at increased levels in patients with prostate cancer and is also found in breast cancer patients. Both fibroblastic growth factors and endothelin-1 have been found to stimulate bone formation in vivo29,58 and are also shown to cause osteoblastic activity in prostate cancer. In prostate cancer, other factors are also found to contribute to bone metastasis. 29 PC3 (prostate cancer) cells produce a factor similar to urokinase-type plasminogen activator, which increases bone metastasis. 29 PSA is a factor that blocks tumour-induced bone resorption and also activates growth factors such as insulin-like growth factors I and II or transforming growth factor-β released during bone metastasis. 29
In summary, several signalling pathways operate in controlling bone formation and breakdown and these are influenced by the activity of metastatic cells.
Clinical manifestation of spinal metastases
Spinal metastases can lead to a considerable number of complications. 57 They may cause bone pain, fractures, motor or sensory dysfunction and also symptoms associated with systemic disease. 59 On examination, a patient may show signs of systemic disease such as weight loss and anaemia. 57,59 Patients may also show signs of nerve root impingement or SCC. 59 In some, a palpable mass may also be found, especially in the case of large sacral metastases. 59
Pain is the most common manifestation in patients with spinal metastasis. 10,57,59 It is estimated that approximately 80–95% of patients will complain of pain. 10,57,59 However, pain will be the initial symptom of spinal metastasis in only about 10% of patients. 59 Patients with spinal metastases can have one of the three types of pain, i.e. local pain, mechanical pain or radicular pain. 10,59 It is believed that local pain occurs as a result of periosteal stretching or increasing length of the spine or enlargement of epidural venous plexuses. 10,59 This pain is often termed ‘night’ or ‘nocturnal’ pain as the patient feels better during activity. 10,59 It is aggravated on percussion or palpation and is often described as ‘gnawing’ or ‘aching’ pain. 10,59 It is often relieved by taking anti-inflammatory medication or corticosteroids. 59 Mechanical pain results from instability of the spine, which happens when metastases affect the vertebral body of the spine. The strain to support muscles and tendons increases under these conditions. 59 Therefore, mechanical pain is aggravated during movement and activity. 10,59 This pain, in contrast to nocturnal pain, is relieved only by lying down, often on one side. Stabilisation of the spine using braces or fixators can improve a patient's quality of life remarkably. 59 Radicular pain occurs when a tumour compresses or invades nerve roots and can also result from pathological fractures. 10,59 Pain is usually sharp, shooting or stabbing in nature59 and often radiates towards limb, chest or upper abdomen. 18 An intense or burning type of pain is felt when a nerve root is impeded by intradural extramedullary metastases. 59
Motor dysfunction is the second most commonly found clinical manifestation in patients with spinal metastasis. 10,59 It is estimated that approximately 35–75% of patients will present with this dysfunction. 10 Again this happens as the result of direct compression of nerves and nerve roots by tumour or fragments of bones resulting from pathological fracture. 59 This causes myelopathy, radiculopathy or sometimes a combination of both, which clinically manifests itself as a weakness of muscles. 59 Patients may also complain of heaviness at their extremities and when clinically examined, motor dysfunctions will be found. 10,59
Some patients may also present with sensory dysfunction; however, motor dysfunction and pain in the corresponding dermatomes are always present. 10,59 Sensory dysfunctions include anaesthesia, hyperaesthesia and paraesthesia.
Metastatic spinal cord compression
Metastatic spinal cord compression (MSCC ) is the most serious complication that can occur in patients with spinal metastasis. 60 It is defined as ‘compression of the dural sac and its contents (spinal cord and/or cauda equina) by an extradural tumour mass’ (Figure 2). 60
FIGURE 2.
A tumour causing SCC. Reproduced with permission from CancerHelp UK, the patient information website of Cancer Research UK. URL: http://cancerhelp.cancerresearchuk.org.
It is estimated that approximately 10 people per 100,000 per year will develop this complication. 60 It is a critical condition that requires emergency care to prevent loss of neurological function and to reverse established deficits. 61 Surgical indications can include bony compression and spinal instability. 62
The patient can have a range of symptoms. Approximately 60–85% of patients will have weakness of muscles. 59 In addition, patients may have autonomic disturbances that include abnormalities of bowel, bladder and sexual function. 59,60 Initially, patients will often present with numbness and anaesthesia of the parts distal to the metastases. 18 Symptoms such as urinary retention, incontinence and impotence occur late in the disease. 18 The most common autonomic abnormality found in patients with MSCC is bladder dysfunction, often clinically presenting as urinary retention. 59 The degree of bladder dysfunction is directly associated with the degree of motor dysfunction. If a patient with motor dysfunction is not treated, they may progress to complete paralysis. 59
Investigations
Diagnosis
Patients with suspected spinal metastasis should be evaluated with a detailed medical history, clinical examination and laboratory tests. 8,59,63
Spinal metastases may be asymptomatic and detected during routine examination, but suspicious clinical examination or suggestive symptoms are more likely to lead to investigation and detection. Patients can have a plethora of symptoms, which include pain, weight loss, weakness, and neurological and organ dysfunction. 59 Details of different types of pain have been described in previous sections. The laboratory examination includes blood cell counts, urine examination, liver function, creatinine level and PSA. 59,63
Imaging and detection
In those patients undergoing surgery or other interventions, assessment of bowel and bladder function, motor weakness and sensory deficits is important as they determine outcomes such as healing and risk of infection.63 Imaging technologies such as ultrasonography and computerised tomography (CT) of the abdomen and chest may be helpful in localising primary neoplasms.
Biopsy of the tumour and examination of the CSF are more useful when the source of the primary tumour is unknown. 8,59 The CT-guided needle biopsy is safe and a reliable method. 59 However, where lesions are small, it may not be possible to collect an appropriate sample. 8 In these patients an open biopsy is better. 8
A broad range of imaging techniques is available to the clinician, for example plain radiography, myelography, magnetic resonance imaging (MRI), CT, radionuclide bone scan, single-photon emission CT (SPECT) and positron emission tomography (PET). 63 MRI of the entire spinal axis is likely to be the gold standard for evaluation of vertebral metastasis. 64,65 MRI of the entire spinal axis provides images of masses, distortion of CSF spaces and various metastases and therefore is better than CT. 10 However, CT with sagittal, coronal and three-dimensional reconstruction allows detailed evaluation of the bony anatomy of the spine, allowing preoperative and intraoperative surgical planning and postoperative consideration. 66,67 In addition, CT also provides images of vertebral arteries, and the characteristics, extent and overall instability of a fracture. It has been suggested that CT usually complements the findings of MRI. 10 Myelography may be used in patients who are unable to undergo MRI because of metallic implants or foreign bodies. 8
There is active discussion in the literature regarding which method or combination of methods (e.g. integrated CT/PET) is most useful and appropriate; nevertheless, no method achieves 100% sensitivity or specificity in identification of patients at high risk of vertebral collapse and SCC. It has been reported that if lesions are examined using three methods, i.e. plain radiography, CT and MRI, then sensitivity and specificity ranges between 85% and 100%. 8
Plain radiography can be useful in identifying vertebral body collapse, pedicle erosion, osteoblastic and osteolytic lesions, and pathological fracture–dislocation. 68,69 However, it is not a reliable diagnostic tool for a number of reasons: (1) vertebral body collapse is frequently seen in non-neoplastic conditions, (2) 30–40% of bone must be eroded before lesions are visible on plain radiography, and (3) in most cases, lesions are seen only after half of the vertebral body is affected. Despite these problems it is estimated that approximately 90% of patients with symptomatic disease show abnormal changes on plain radiography. 70,71
Other imaging techniques such as bone scan, SPECT and PET with 18F-fluorodeoxyglucose are used to diagnose and evaluate vertebral metastases. PET with 18F-fluorodeoxyglucose has been found by some investigators to be as accurate as MRI. 72
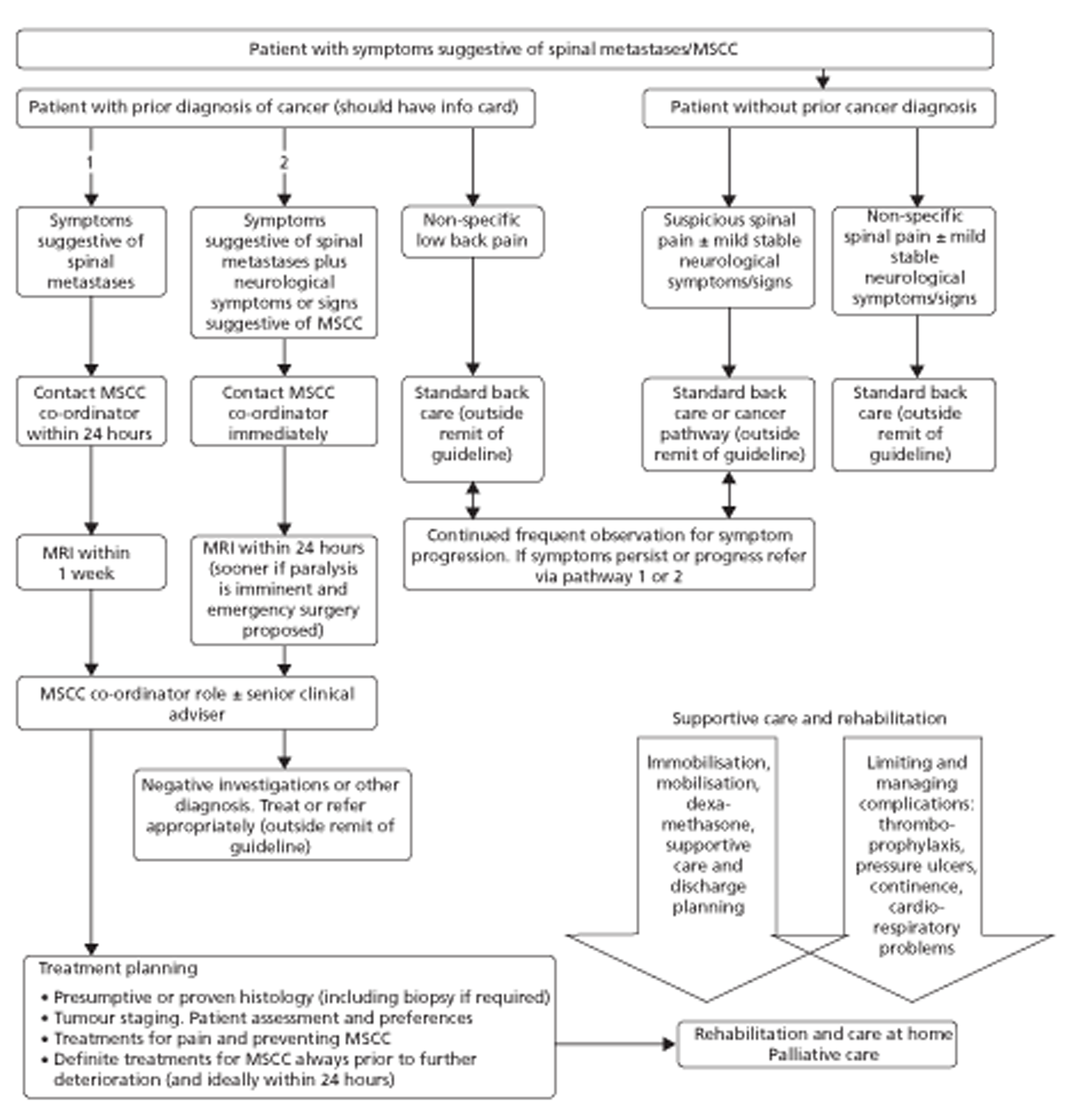
There appear to be no guidelines that recommend specific imaging modalities; however, NICE clinical guideline 75, for diagnosis and management of adults at risk of or with MSCC, states that MRI should be undertaken very soon after diagnosis or suspected diagnosis. 15 The guideline reports that in patients in whom MIR is contraindicated, CT with three-plane reconstruction should be performed. Finally, the guideline states that plain radiography should not be used to confirm or exclude the diagnosis of spinal metastases or MSCC. 15 In cases of spinal pain suggestive of spinal metastases, NICE states that MRI should be carried out as early as possible to deploy definitive treatment within 1 week of developing these symptoms. However, in cases of spinal pain suggestive of MSCC or neurological function deterioration, MRI should be undertaken within 24 hours as this is a medical emergency15 (see Figure 3).
FIGURE 3.
Patient treatment pathways for diagnosis and management of adults at risk of or with MSCC. Adapted from Metastatic spinal cord compression: Diagnosis and management of adults at risk of or with metastatic spinal cord compression. 2008. Available from: http://guidance.nice.org.uk/CG75/Guidance/pdf/English (accessed April 2011). 15
Treatment
The treatment of metastatic spinal tumours typically involves multiple interventions such as surgery, medical therapy and radiation. 63 Interdisciplinary collaboration is essential to allow each patient's treatment to be tailored to the overall prognosis,8 and therefore treatment of these patients involves a variety of specialties, namely medicine, surgery, oncology, neurology and rehabilitation medicine. 59 Owing to the heterogeneity of tumour pathology, patients' condition and the anatomical extent of disease, it remains difficult to provide a consensus about treatment. As therapy is not curative, treatment, in most cases, is focused on improving a patient's quality of life and restoring neurological function or preventing further deterioration, reducing pain and stabilising the spine mechanically. 59,63 Radiation therapy and different forms of surgery are the primary methods for treating SCC. High-dose steroids are administered with radiation treatment and tapered gradually with completion of treatment. 61 Surgical interventions include decompression and fixation of the spinal joints. 62,73
National Institute for Health and Care Excellence clinical guideline for management of spinal cord compression
In November 2008, NICE issued a clinical guideline for the diagnosis and management of adults at risk of or with MSCC. 15 The guidelines contained treatment algorithms for patients with symptoms suggestive of spinal metastases. The guideline proposed the patient treatment pathways shown in Figure 3.
Treatment of patients with spinal metastases and MSCC can be broadly divided into three pathways. 15
Treatment of patients with spinal metastases and prevention of metastatic spinal cord compression
Patients with painful spinal metastases should be offered conventional analgesics, i.e. non-steroidal anti-inflammatory drugs. Those patients with intractable pain should be considered for specialist pain care that includes invasive procedures and neurosurgical interventions. Patients with spinal metastases from breast and prostate cancer should be offered bisphosphonates to alleviate pain and reduce the risk of pathological fracture/collapse of the spine. Those patients with non-mechanical spinal pain should be given single-fraction palliative radiotherapy. This should also be considered in those who are completely paralysed. In asymptomatic patients, radiotherapy should not be administered.
Two vertebral augmentation techniques, vertebroplasty and kyphoplasty, should be considered in those with mechanical spinal pain resistant to conventional analgesics and no evidence of MSCC or spinal instability.
Surgery should be preferred when there is evidence of progressive disease mainly to prevent MSCC. It should also be considered in those with spinal metastases and mechanical pain resistant to conventional analgesics and in those with evidence of spinal instability.
Treatment of threatened spinal cord in patients with metastatic spinal cord compression
In patients with severe mechanical pain suggestive of spinal instability or those with neurological symptoms or signs suggestive of MSCC, the spine should be stabilised. Patients should be monitored regularly, especially during sitting from supine to 60 degrees. If patients continue to deteriorate, then they should revert back to the lying position or to the position in which there is minimal pain/neurological symptoms. In those patients not suitable for definitive treatment, the aim of the treatment should be helping the patient to achieve a comfortable position and mobilisation. This is usually achieved by using orthoses.
Corticosteroids should be given to all patients with MSCC unless contraindicated. Dexamethasone at 16 mg as a loading dose should be given followed by a short course of 16 mg dexamethasone daily until definitive treatment is employed. After definitive treatment, the dose of dexamethasone should be reduced gradually over 5–7 days and then stopped. In those patients in whom symptoms have deteriorated, the dose of dexamethasone can be increased temporarily.
Definitive treatment of metastatic spinal cord compression
The definitive treatment should be given as early as possible, ideally within 24 hours of the diagnosis of MSCC. Before this, diagnosis of primary location of the tumour should be made. In addition, an attempt should be made to study the extent of the disease. A scoring system such as the Tokuhashi scoring system and the American Society of Anesthesiologists grading for overall patient condition should be used to assess whether surgery is appropriate.
Surgery
Surgery should be considered only if it would increase the patient's survival by > 3 months. The aim of this treatment is to decompress the spinal cord and stabilise the spine. Posterior decompression alone should be used only in cases of isolated epidural tumour or neural arch metastases without bony instability. In those in whom metastasis involves the vertebral body and who are therefore at increased risk of spinal instability, posterior decompression by internal fixation, with or without bone grafting, should be carried out. Reconstruction of the vertebral body should be carried out in patients with MSCC and vertebral body involvement who are expected to survive < 1 year, whereas in those expected to survive > 1 year, reconstruction of the vertebral body with anterior bone graft should be undertaken. In rare circumstances such as solitary renal or thyroid metastasis following complete staging, en bloc excisional surgery should be carried out.
Radiotherapy
Patients unsuitable for surgery should receive radiotherapy within 24 hours, 7 days a week. Fractionated radiotherapy is the definitive treatment of choice for patients with epidural tumour without neurological dysfunction, mechanical pain or spinal instability. It is also an appropriate first-line treatment for patients with good prognosis. Radiotherapy should not be given to patients with MSCC who are waiting for surgery but fractionated radiotherapy should be offered to all patients postoperatively once their wound has healed.
Supportive care and rehabilitation
Supportive care includes thromboprophylaxis, management of pressure ulcers, bladder and bowel continence, circulatory and respiratory functions and access to specialist rehabilitation care at home. 15
Radiation
The aim of radiation is to alleviate pain and to prevent recurrence and tumour growth. 74 It is indicated when the spine is stable, if the tumour is radiosensitive and the patient's neurological condition is stable, or if the patient is in poor medical condition or has a life expectancy < 3–6 months and has had complete paraplegia for > 24 hours. 74
According to a recent review,74 conventional external beam radiation is the most commonly used radiotherapy in patients with spinal metastasis. Often radiotherapy is used in combination with surgical treatment as this is useful in preventing local recurrence. 74 It should, however, be noted that radiation adversely affects surgical outcomes by delaying wound healing and/or delaying fusion of the joints. Thus, radiation is now usually not given before surgery. It is given either as a single fraction or as multiple fractions. Usually it is administered in 10 fractions, which is equivalent to 3000 cGy. 74
Patients aged < 65 years with radio-resistant tumours and with signs of MSCC are treated with surgery and adjuvant radiotherapy. The latter is used to prevent local recurrence of the tumour. 74 Currently, in those with low-grade compression, a single-fraction treatment is given. 74
Recently, new approaches, such as intensity-modulated radiotherapy (IMRT),75 or stereotactic body radiotherapy, have been suggested for the treatment of vertebral metastases. 76
Systemic therapies
Corticosteroids
Intravenous or oral corticosteroids have been found to provide improvement or resolution of neurological symptoms and pain in patients with epidural spinal metastases. 63 It should be noted that there is no standard dosage regimen for corticosteroids. They are often used before surgery. 63
In patients with MSCC undergoing surgical decompression, corticosteroids are often used in combination with radiotherapy. 74
Bisphosphonates and denosumab
Bisphosphonates are known to impair osteoclastic activity and so they reduce tumour-related resorption of bone. 10,57 Currently, bisphosphonates are used to alleviate metastatic bone pain and to reduce SREs such as pathological fractures, hypercalcaemia and MSCC. Bisphosphonates are also used to reduce the frequency of surgery and radiation therapy. 10,57 Bisphosphonates such as pamidronate (Aredia®; Novartis Pharmaceutitcals Corporation), clodronate (Bonefos®, Clasteon®, Loron®; Bayer), ibandronate (Bondronat®; F. Hoffmann-La Roche Ltd), alendronate (Fosamax®; Merck Sharp & Dohme Corporation) and zoledronate (Aclasta®; Novartis) have all been found to be effective in the treatment of hypercalcaemia. 57 Although radiotherapy is the main treatment for reducing bone pain, bisphosphonates can be used as an alternative therapy, which in turn will considerably reduce the frequency of radiotherapy. 10,57 The effect of bisphosphonates on pain is not dependent on the nature or type of the tumour (i.e. sclerotic or lytic). 57 The efficacy of these drugs has been seen in breast cancer, multiple myeloma and other osteolytic metastases. 57 Although bisphosphonates have been found to be effective in preventing skeletal-related complications, they are not so effective in reducing pain in patients with prostate cancer. 77
Recently, monoclonal antibody therapy with denosumab, a specific inhibitor of RANKL, has been found to be effective in delaying and preventing SREs. 77
Chemotherapy
The benefits of chemotherapy are limited in spinal metastases, as patients are usually at a late stage of disease. 57 Chemotherapy can be given on its own or in combination with surgery and hormonal therapy. 10
Radioisotopes
Radioisotopes are administered systematically and act as local radiation therapy to the spine. 10 Radioisotopes include strontium-89 and rhenium-186. Although radioisotopes are found to reduce pain in patients with spinal metastases, these can cause irreversible bone marrow suppression and, for this reason, they are recommended for use in those with good marrow function and in whom no other treatment is available. 10
Surgery
The main aims of surgery are to remove the tumour, to achieve spinal stability and to reconstruct the vertebral column. 7 Surgery may also help with diagnosing the origin of the tumour and in relieving neurological symptoms. 7 In those with solitary renal cell carcinoma metastases, surgery can increase disease-free survival. 80 Current indications for surgery are (1) radioresistant tumour such as renal or colon carcinoma, (2) evidence of neurological function deterioration or tumour progression despite radiotherapy, (3) radiological images showing fragments of bone in the spinal canal, (4) spine instability due to fracture and causing pain and neurological deficit, (5) neurological deficit for > 24 hours, or significant MSCC, and (6) life expectancy of at least 3 months. 7,74
Different scoring systems have been developed to select patients who will benefit from surgery such as those developed by Tokuhashi et al. 81 Prognostic predictions for these patients after surgery can also be made using these scoring systems. 81 Details of this have been given below (see Prognosis).
The surgical approach to remove a tumour or to decompress neurons in spinal metastases depends on various elements such as the spinal segment involved and the location and histological characteristics of the tumour. 59 In most cases, metastases occur in the vertebral body of the spine and therefore an anterior approach has been used by many surgeons to remove the tumour, and to decompress and then stabilise the spine. 74 An anterior approach is appropriate if the cervical spine is involved. Other approaches such as anterolateral cervical with sternotomy or thoracotomy are preferred when the upper thoracic spine is affected. 82 During these techniques great vessels in the thorax can obstruct access to the spine and newer approaches have been developed such as transpedicular posterior or posterolateral approaches.
Vertebral augmentation
Two techniques, percutaneous vertebroplasty and kyphoplasty, initially developed for treatment of painful vertebral haemangiomas, are now used effectively in treating painful pathological fractures caused by metastatic spinal disease. 59 Vertebroplasty involves an injection of polymethylmethacrylate (PMMA) into the compression fracture whereas in kyphoplasty an inflatable balloon is placed in the vertebral body and PMMA is injected. 83,84 Although these interventions can lead to significant pain reduction and greater mobility,83,84 they are contraindicated in SCC because of pathological fractures as they do not relieve cord compression. 59 Complications of these techniques include leakage of PMMA, misplacement of PMMA and haematogenous embolisation of PMMA to the lungs. 59
Prognosis
Several types of prognostic studies have been undertaken to explore the prognosis of spinal metastases. These studies will be the focus of this current short report. Prognostic studies serve several purposes, for example to inform choice of potential pre-emptive intervention(s) so as to avoid or delay more radical surgical intervention; to bring forward radical interventions before patients' health deteriorates to the extent that they are no longer suitable candidates for interventions; and to categorise patients into those more or less suitable for earlier or later radical intervention.
Prognostic studies comprise four types:
-
attempts to determine the risk factors that allow prediction of overall survival (e.g. scoring schemes such as those of Tokuhashi et al. 85 and Tomita et al. 86)
-
the identification of patients most suitable for surgical intervention; some of these studies are specific for metastases derived from particular primary tumours (e.g. lung, breast)
-
attempts to identify risk factors important in determining the survival of patients after surgical intervention for SCC and/or vertebral compression fracture(s)87 (e.g. vertebrectomy and reconstruction, vertebroplasty, kyphoplasty, radiofrequency ablation)
-
assessment of risk factors using clinical or imaging technologies for progression of metastatic spinal metastases to SCC and/or to vertebral compression fracture(s). 88,89
Early studies by Yamashita et al. 90 documented longer survival in patients with spinal or pelvic metastatic cancer lesions compared with those with appendicular lesions or both. Tokuhashi et al. 81 developed a scoring system involving six parameters to determine survival after surgery for metastatic spinal tumours: (1) general condition; (2) number of vertebral metastases; (3) number of metastases to internal organs; (4) number of metastases to extraspinal bone; (5) primary site; and (6) severity of spinal cord injury. Scores of 9 out of a possible 12 indicated a good prognosis for patients whereas scores < 5 indicated a worse prognosis. 81 Tomita et al. 86 developed a similar scoring system based on (1) primary tumour site, (2) presence of visceral metastases and (3) number of bone metastases. In contrast to Tokuhashi et al. ,81 in this system, a lower score indicates a better prognosis. 86
van der Linden et al. 91 analysed response to radiotherapy in a cohort of patients with painful spinal metastases and without neurological impairment. Patient characteristics such as Karnofsky performance score, primary tumour site, number of visceral metastases, etc., were studied for their prognostic value in predicting survival. The points were awarded as follows: (1) 2, 1 and 0 points were given for Karnofsky performance score of 80–100, 50–70 and 10–40, respectively; (2) 3, 2, 1 and 0 points were given for breast cancer, prostate cancer, lung cancer and other types of cancer, respectively; and (3) in the presence of visceral metastases 1 point was given, and 0 points if they were absent. Three prognostic groups were formed: Group A with scores between 0 and 3, Group B with scores between 4 and 5, and Group C with a total score of 6. The median overall survival in Groups A, B and C was found to be 3 months, 9 months and 18.7 months, respectively. Patients in Group C had breast cancer with good performance and no metastases to organs. 91
Sioutos et al. 92 studied a cohort of patients with spinal metastases from solid tumours and epidural compression of the spinal cord who underwent surgical decompression of the spinal cord and radiotherapy. Patient characteristics such as anatomical site of primary carcinoma, preoperative neurological deficit, extent of disease, number of vertebral metastases, site of cord compression and age were explored if they predicted survival. In the study, it was found that patients with renal cell carcinoma survived longer than those with breast, prostate, lung or colon cancer. Patients with single vertebral body metastasis survived comparatively longer than those with multiple vertebral body metastases. The presence of leg strength between 0/5 and 3/5, lung or colon cancer, and multiple vertebral metastases all had a negative impact on survival; however, factors such as extent of disease, age and location of tumour had no apparent impact on overall survival of patients. 92
Ambulatory status, age < 60 years and single vertebral segment involvement have also been found to be independent predictors of good outcome. 92–95 Furthermore, Weigel et al. 95 reported a significant association between a postoperative Karnofsky scale and duration of survival.
Bauer and Wedin96 studied survival of patients with spinal metastases after surgery. The survival of the patient was found to be associated with metastatic load, location of tumour and presence of pathological fracture. On multivariate regression analysis, some factors such as pathological fracture, metastasis to brain or viscera, and lung cancer were found to be negative prognostic factors while single skeletal metastases and breast or kidney cancer were positive variables. 96
All of these scoring systems relate to survival, which is not one of the outcome measures included in this current review. According to a recent review, they have recently been assessed as having limited predictive value. 8
Current service cost
Economic impact of skeletal complications
There is a large burden on health-care resources from bone metastases and their complications. The cost also increases because of the multidisciplinary approach required to manage such patients. For example, Botteman et al. 97 used NHS perspective costs to compare relative cost and cost-effectiveness of commonly used bisphosphonates versus no therapy for the management of SREs in breast cancer patients with bone metastasis and receiving chemotherapy or hormone therapy. The authors took different types of costs into consideration such as hospital cost (including cost of vertebral fracture, non-vertebral fracture, hypercalcaemia, radiotherapy, orthopaedic surgery), community care cost, monthly cost of bone pain (including cost of medical consultant, palliative care nurse, district nurse, social work assistant) and cost of drugs. 97 The paper reports a mean cost of £18,662 over the mean survival of 2 years with no bisphosphonate therapy, and states that the use of bisphosphonates can be cost-saving and cost-effective in reducing SREs without influencing survival. The mean cost of using zoledronic acid over the mean survival of 2 years was £16,396. 97
Another study showed that zoledronic acid may be cost-effective in lung cancer patients with bone metastases, with the mean drug cost (£1473) being slightly lower than costs associated with additional SREs (£1562) incurred in an untreated population. 98
Recently denosumab, a monoclonal antibody, has been found to prevent and delay SREs. 77 In the UK, it is currently indicated for the prevention of osteoporotic fractures in postmenopausal women99 and for the treatment of bone loss associated with hormone ablation in men with prostate cancer. 100 The recommended dose is 60 mg every 6 months via subcutaneous injection. It is available as Prolia® manufactured by Amgen (Thousand Oaks, CA, USA) and costs £183 for a 1-ml (60 mg/ml) prefilled syringe. 100 On 18 November 2010, the US Food and Drug Administration approved denosumab (trade name Xgeva®, Amgen) for the prevention of SREs in patients with bone metastases from solid tumours. 101
For this, the recommended dose of denosumab is 120 mg every 4 weeks subcutaneously,101 giving an annual cost of approximately £4770 in the UK.
A retrospective observational study reported high costs of treating SREs in lung cancer. 102 Of 534 patients identified with lung cancer and bone metastases, 295 (55%) experienced one or more SREs over a mean follow-up of 5.6 months, whereas 25% of patients had two or more SREs. Costs of treatment of SREs were estimated to be approximately $9500. Total medical care costs were almost $28,000 in patients with SREs and were significantly higher than in patients without SREs (p < 0.001). Radiation therapy accounted for 55% of the treatment cost (compared with 25% for bone surgery), and 54% of costs were due to inpatient hospitalisation. 102
These examples most importantly show that the management of a patient with malignant skeletal metastases is associated with appreciable consumption of resources.
Summary
Metastases to the spine occur commonly in commonly occurring cancers, such as breast, prostate and colorectal cancers.
Spinal metastases can lead to significant morbidity and reduction in quality of life due to SCC, which can result in paraplegia or quadriplegia, severe bone pain and pathological fractures. An early study estimated average survival for patients with SCC to be between 3 and 7 months, with a 36% probability of survival to 12 months. Spinal metastases are costly. Early diagnosis is important, helping clinicians to manage disease and delay complications. There is uncertainty about the specificity and sensitivity of the different diagnostic techniques currently available. Prognostic models have been developed to predict overall survival.
Chapter 2 Methodology
A protocol was produced and approved by the Health Technology Assessment (HTA) Programme before the start of this review. It is available on the HTA Programme website (www.hta.ac.uk/project/2553.asp).
Search strategies
The search aimed to identify all references relating to the natural history of metastatic spinal lesions and the identification of patients at high risk of vertebral fracture and SCC through the use of various technologies. The search strategy involved searching electronic bibliographic databases; contact with experts in the field; and scrutiny of references of included studies. An iterative procedure was used to develop the search strategy, with input from clinical advisors, an experienced information specialist and previous HTA and systematic reviews (e.g. Cooper et al. ,103 National Collaborating Centre for Cancer15 and Sutcliffe et al. 104). Copies of the search strategies used in the main electronic databases are provided in Appendix 1.
The searches were undertaken in June 2011. Searches were performed in MEDLINE; MEDLINE In-Process & Other Non-Indexed Citations; EMBASE; Cochrane Database of Systematic Reviews; CENTRAL; Database of Abstract of Reviews of Effects (DARE); NHS Economic Evaluation Database (EED); HTA databases [NHS Centre for Reviews and Dissemination (CRD)]; Science Citation Index and Conference Proceedings (Web of Science); UK Clinical Research Network (UKCRN) Portfolio Database; Current Controlled Trials; and ClinicalTrials.gov.
The search strategy covered the concepts of metastases, spine and adults (see Appendix 1) and was intentionally kept broad to cover natural history, diagnostic and prognostic factors.
In addition, the reference lists of relevant articles were checked and various health services research-related resources were consulted via the internet. These included HTA organisations, guideline-producing bodies, generic research and trials registers. Citation searches of included studies were undertaken using the Web of Science citation search facility. The reference lists of included studies, and relevant review articles were also checked.
Search restrictions
No study type or publication type restrictions were applied, as all types of study involving all languages were screened for potential inclusion.
Inclusion of relevant studies
Titles and abstracts of retrieved studies were examined for inclusion by two reviewers independently. Disagreement was resolved by retrieval of the full publication and consensus agreement. The following inclusion and exclusion criteria were used.
Study design
Randomised controlled trials (RCTs), systematic reviews, prospective or retrospective case series, cohort or case–control studies (case studies were excluded).
Population
Adult patients with vertebral metastases at risk of developing (or who have developed) MSCC, vertebral collapse or progression of vertebral collapse.
Intervention/technologies
Diagnostic/prognostic methods, including clinical features and/or imaging technologies [MRI, CT, PET, technetium-99m (99Tcm) scintigraphy, radiography], and natural history.
Comparator
None or another diagnostic/prognostic method.
Outcomes
Spinal cord compression, vertebral compression, vertebral collapse or progression of vertebral collapse.
Exclusion criteria
-
Animal models and post-mortem studies.
-
Preclinical and biological studies.
-
Editorials, opinions.
-
Reports published as meeting abstracts only, where insufficient methodological details are reported to allow critical appraisal of study quality.
-
Studies not in English, French and German.
-
Studies where a majority of patients (> 50%) are suffering from multiple myeloma.
-
Studies predicting overall survival as the only outcome measure.
Data extraction strategy
The full data were extracted independently by one reviewer using a data extraction form informed by the NHS CRD105 and previous HTAs involving prognosis (e.g. Sutcliffe et al. ;104 see Appendix 3). All included studies were reviewed by a second researcher, and any disagreements were resolved by discussion. Further discrepancies were resolved by discussion, with involvement of a third reviewer when necessary. Summary tables were developed that list all clinical assessments, imaging and other technologies that may inform prognosis of metastatic spinal lesions reported in the literature, with details of their prognostic value, where adequate information was available. In view of the early publication date of some included studies, and in the context of a short report, it was not considered feasible to contact authors for data or for clarification. Data have been extracted from relevant copyright figures and used to redraw graphs; as this procedure is not exact these graphs are used for illustrative purposes only.
Quality assessment strategy
Quality assessment of included studies was informed using the guidelines suggested by Hayden et al. 106 as appropriate for prognosis studies (see Appendix 2) and modified as necessary according to Sutcliffe et al. 104 (further details are provided below and in Appendix 3).
Hayden et al. 106 appraised how authors of reviews of prognostic studies had assessed study quality and provided recommendations as to the domains that should be included, and also the questions that might contribute to the assessment of each domain. Domains proposed by Hayden et al. 106 to assess potential biases in prognostic studies were (1) study population; (2) study attrition; (3) prognostic factor measurement; (4) outcome measurement; (5) confounding measurement and account; and (6) analysis.
Within each of these categories, Hayden et al. 106 proposed a series of additional questions to help assess the extent of possible biases. In line with the previous HTA work undertaken by Sutcliffe et al. ,104 we have adapted these questions for the current disease area, the types of studies available, and also to clarify the meaning of each question in the context of the short report. The resulting quality assessment tool is provided in Appendix 3. Systematic reviews were quality assessed using an adapted checklist proposed by the NHS CRD105 (see Appendix 3).
In total there were 16 questions; they included an overall question on the conclusion for each domain. Each question was scored as yes (Y), no (N), partly clear (P), unsure (UN) or not applicable (NA). The quality of each study was assessed by at least two of the three members of the research team (PS, MC, DS). Regular discussion meetings were arranged to resolve uncertainty between reviewers who completed the quality assessment. The third team member attended the meetings when agreement could not be reached.
The following section provides a brief summary of issues used to appraise the quality under each of the domains proposed by Hayden et al. 106
Study population
We assessed whether a study reported sufficient information on the principal factors known to affect patient prognosis so that it would be clear to which population the results were applicable. 104
Treatment
The reporting of the principal treatment and diagnostic or prognostic tool and also the proportion of patients who had had treatment were evaluated.
Recruitment dates
The time period during which patients were recruited was established.
Baseline characteristics
Known prognostic factors were included, for example whether there were differences between studies in terms of the stages of the cancers.
Study attrition
Following preliminary scoping work, it became apparent that many included studies would be retrospective; therefore, the assessment of attrition was likely to be relevant to this short report. The total number of patients from the study population and reasons for patient exclusion were noted.
Prognostic factor measurement
This domain was assessed in terms of whether a well-defined and reproducible method of extraction and measurement was reported. In particular, whether the authors provided a description of the measurement of the factors prognostic of MSCC, vertebral collapse or progression of vertebral collapse.
Outcome measurement
This domain was assessed in terms of whether the outcomes were clearly defined by the authors.
Confounding measurements
This domain was assessed in terms of whether the authors had provided any measurements of potential confounding factors. In particular, reviewers assessed whether bisphosphonates and tamoxifen had been clearly reported in the study population as both influence the rate of bone fracture at sites of metastases, so that if these treatments are not considered in identifying predictors they may confound treatments.
Analysis
This domain was assessed in terms of whether an adequate description of the analysis and sufficient data were provided.
Methods of analysis/synthesis
Data were tabulated and discussed in a narrative review. Summary tables for each included paper were provided. Each tumour type was looked at separately.
Chapter 3 Results
The following section provides a summary of the search results, a quality assessment and a detailed description of the included studies for each cancer group.
Result of searches
Natural history studies
No epidemiological studies were identified that had a primary aim of evaluating the natural history of spinal metastases. In the Discussion section of this report we include an evaluation of what can be inferred about the natural history of spinal metastases from the prognostic studies we identified and which we evaluate in the following section.
Number of studies identified
The flow chart outlining the process of identifying relevant literature can be found in Figure 4. Following the removal of duplicates, the searches identified 2425 potentially relevant articles. A total of 2089 articles did not meet our inclusion criteria and were removed at title and abstract sift, leaving a total of 336 articles to be further investigated. Of these, 305 were removed at full-paper sift, resulting in 31 articles that met the inclusion criteria. Appendix 4 lists included papers at full sift.
FIGURE 4.
Flow diagram.
Kappa statistic
A κ-statistic was calculated for the sifting of the 336 articles examined at full text by the two reviewers (PS and MC). Tables 4 and 5 provide a summary of the κ-statistic calculations. The resulting κ was 0.7033.
| Category | Observer MC | Total | ||
|---|---|---|---|---|
| + | − | |||
| Observer PS | ||||
| + | A = 28 | B = 9 | A + B = 37 | |
| − | C = 11 | D = 288 | C + D = 299 | |
| Total | A + C = 39 | B + D = 297 | A + B + C + D = 336 | |
| Agreement | Expected agreement | Kappa | SE | z | Prob > z |
|---|---|---|---|---|---|
| 94.05% | 79.94% | 0.7033 | 0.0545 | 12.90 | 0.0000 |
Number of studies excluded
A list of the 305 articles that were excluded at full paper sift with reasons for exclusion is provided in Appendix 5. Table 6 provides a summary of the main reasons for excluding papers at full-paper sift. The most common reason for exclusion was related to outcome measures.
| Reasons for exclusion | n |
|---|---|
| Outcome measures did not meet inclusion criteria | 266 |
| Review | 22 |
| Abstract | 6 |
| Case reports | 6 |
| Editorial | 3 |
| Animal study | 1 |
| Letter | 1 |
Prognostic factors identified
A broad range of factors (93 in total) were reported as significant in prediction of MSCC; vertebral collapse or progression of vertebral collapse were reported across the 31 included studies (Table 7). Many prognostic factors were mentioned by only a small number of studies, or in some cases by a single study. It was not possible to examine the potential issues of publication bias or selective outcome reporting.
| Age |
| Age < 60 years |
| Alkaline phosphatase |
| Altered sensation |
| Amount of vertebral body occupied by tumour |
| Anterior cord compressiona |
| Back paina |
| Bladder and bowel dysfunction |
| Blastic-type tumour |
| Bone metastases diagnosed > 1 year earlier |
| Bone metastases previously diagnosed |
| Bone metastasis |
| Bone only |
| Bone scan extent of disease score |
| Cervical level |
| Complaint of inability to walk |
| Complete resection |
| Costovertebral joint destruction |
| Cross-sectional area within the vertebral body |
| CT appearance |
| Duration of hormonal therapy before study entry |
| ECOG status 2/3, vertebral body fracture on most recent plain radiograph |
| Elective surgery |
| Ever smoked |
| Extraspinal metastases |
| Favourable tumour histology |
| Focal radiographic abnormalities |
| Gleason score |
| Good general health status |
| Haemoglobin concentration |
| High PSA level at the time of initial MRI |
| Histology of non-adenocarcinoma |
| History of local pain |
| History of radicular pain |
| History of radiotherapy before chemotherapy |
| History of weakness |
| Increased deep tendon reflexes |
| Increasing number of spinal levels |
| Known bone metastases spinal level |
| LBC/BM |
| Lesion location |
| Lesions located between t10 and sacrum |
| Log transformed PSA |
| Low number of affected vertebral bodies |
| Lytic lesions |
| Lytic-type tumour |
| Male sexa |
| Metastatic disease at initial diagnosis |
| Motor deficit |
| MRI multiple bone metastases |
| Neurological examination (abnormal neurological examination) |
| Neurological abnormalities |
| No history of EGFR TKI therapy |
| Number of spinal metastases |
| Objective weakness |
| Older age |
| Pain (tumour, mechanical, radicular) |
| Paraparesis |
| Paraesthesia |
| Progesterone receptor status |
| Positive vertebral plain films |
| Posterior vertebral heights |
| Preoperative chemotherapya |
| Preoperative radiation |
| Presence of back pain |
| Primary breast cancera |
| Prostatic acid phosphatase |
| Performance status of 2–3 |
| PSA |
| Radicular pain |
| Radicular weakness |
| Sensory deficits |
| Sensory level or dermatomal loss on examination |
| Short PSA doubling time < 3 months |
| Soloway grade 4 |
| Stage IV cancer at initial diagnosis |
| Symmetrical fractures with fragments |
| Thoracic spine involvementa |
| Time interval from the diagnosis of the primary tumour |
| Total involvement of vertebra |
| Tumour size and pedicle destruction in the thoracolumbar |
| and lumbar spine (T10–L5) |
| Tumour size in the thoracic region |
| Tumour involvement of > 50% |
| Tumour size |
| Undifferentiated tumours |
| Upper lumbar |
| Urinary and bowel symptoms |
| Vertebrae with > 80% body infiltration |
| Vertebral axial displacement |
| Vertebral bulge |
| Vertebral compression fracture on spine radiograph |
| Visceral metastases |
| Weakness or difficulty in walking |
Anterior cord compression, back pain, male sex, preoperative chemotherapy, primary breast cancer and thoracic spine involvement were reported in two studies. Furthermore, the most commonly reported factor was related to tumour characteristics and was found to be significant for 11 factors in eight studies;88,89,109,110,113,117,126,129 however, the definition of tumour characteristics varied between the different studies [e.g. amount of vertebral body occupied by tumour,88 overall tumour size and pedicle destruction in the thoracolumbar and lumbar spine (T10–L5),89 tumour size in the thoracic region,89 blastic-type tumour,109 lytic-type tumour,109 tumour pain,109 favourable tumour histology,117 time interval from diagnosis of the primary tumour,113 total involvement of vertebra,129 tumour involvement of > 50%,110 undifferentiated tumours126].
Description of included studies
The following section summarises the main characteristics of the 30 included studies24,88,89,107–133 which are listed in Tables 8 and 9. (The systematic review is be discussed separately.)
| Author, year | Sample selected (n) | Sample analysed (n) | Study design | Mean age (years) | Median age (years) | Range age (years) | Male (n) | Female (n) |
|---|---|---|---|---|---|---|---|---|
| Bayley 2001107 | 68 | 68 | Prospective study | NR | 71 | 50–84 | 68 | 0 |
| Bernat 1983108 | 133 | Unclear | Retrospective data comparison study | NR | 61 | 7–85 | 77 | 56 |
| Chaichana 2009109 | 216 | 162 | Retrospective review | 58 | NR | NR | 95 | 67 |
| Fisher 2010110 | NA | NA | Modified Delphi technique | NA | NA | NA | NA | NA |
| Goldman 1989111 | 616 | 610 | Retrospective analysis of records | Unclear | Unclear | Unclear | Unclear | Unclear |
| Harrison 1985112 | 78 | 78 | Retrospective case series | 51 | 51 | 22–75 | 0 | 78 |
| Helweg-Larsen 2000113 | 153 | 153 | Prospective study | NR | Females = 64 (36–88) years; males = 71 (26–92) years | 26–92 | 78 | 75 |
| Helweg-Larsen 1995114 | 107 | 107 | Prospective study | NR | 66 | 34–91 | 53 | 54 |
| Huddart 1997115 | 69 | 69 | Retrospective analysis of patient records | NR | NR | NR | NR | NR |
| Husband 2001116 | 280 | 201 | Prospective study | NR | 67 | 23–89 | 158 | 122 |
| Klekamp 1998117 | 101 | 106 | Prospective study | 62 | NR | NR | NR | NR |
| Kuban 1986118 | 41 | 41 | Case series | NR | 68 | 50–90 | 611 | 0 |
| Levack 2002119 | 319 | 319 | Prospective observational study | NR | 65 | NR | 203 | 116 |
| Lu 1998120 | Unclear | 93 | Retrospective analysis/study | NR | 52.9 | 29.8–77.3 | 0 | 93 |
| Lu 2005121 | Unclear | 134 | Prospective study | NR | 61.5 | 30.9–84.8 | NR | NR |
| McCloskey 1993122 | 100 controls and 163 women | 100 controls and 163 women | Prospective study criteria | 59 | NR | 30–75 | 0 | 263 |
| Oka 2006123 | 695 | 695 | Retrospective cohort study | 53.1 | NR | 24–88 | 4 | 691 |
| Plunkett 200024 | 1437 | 859 | Retrospective analysis/study | Unclear | Unclear | Unclear | Unclear | Unclear |
| Rose 200988 | 62 | 62 | Prospective study | 62 | Unclear | Unclear | 38 | 24 |
| Roth 2004124 | 560 | 72 | Retrospective study design | NR | NR | NR | 34 | 38 |
| Sekine 2009125 | 642 | 642 | Retrospective study | NR | Patients without SREs = 61 years; Patients with SREs = 59.5 years | Patients without SREs = 24–86 years; Patients with SREs = 26–77 years | 402 | 240 |
| Shah 2003126 | 213 | Unclear | Retrospective cohort study | 58 | NR | 20–90 | 26 | 27 |
| Snyder 2005127 | Unclear | 106 | Prospective study | NR | NR | NR | 0 | 106 |
| Snyder 2009128 | 94 | 94 | Prospective observational | 55 | NR | NR | 0 | 94 |
| Soerdjbalie-Maikoe 2004129 | 84 | 84 | Retrospective observational | NR | NR | NR | 84 | 0 |
| Sun 2011130 | 1166 | 273 | Retrospective observational | NR | NR | Unclear | 60.1% | 39.9% |
| Talcott 1999131 | 258 | 258 | Retrospective cohort | NR | 56.5 | 18–83 | 39% | 61% |
| Taneichi 199789 | 53 | 53 | Retrospective study | 59.7 | NR | 43–80 | NR | NR |
| Venkitaraman 2007132 | 150 | 150 | Retrospective study | NR | 69 | 50–88 | 150 | 0 |
| Venkitaraman 2010133 | 130 | 130 | Retrospective study | NR | 70 | 50–88 | 130 | 0 |
| Author, year | Cancers | Interventions | Medications | Spinal level | |||
|---|---|---|---|---|---|---|---|
| Cervical | Thoracic | Lumbar | Other | ||||
| Bayley 2001107 | Prostate | Bone scans, radiographs, sagittal T1-weighted spin-echo sequence | Hormone therapy, analgesics, acetaminophen, non-steroidal anti-inflammatory medications, narcotic analgesics | 3 | 20 | 8 | Clinically occult SAS compression/SCC was identified in 22 patients. SAS compression alone in 12 patients and frank compression of the spinal cord or cauda equina in 10 patients. Nine of 22 patients had SAS compression/SCC at two discontinuous vertebral levels |
| Bernat 1983108 | Lung, breast, prostate, lymphoma, colon/rectal, melanoma, kidney and ureter, bladder, other, unknown | CSF examination, radiography, vertebral radiographs, bone scans, and myelograms | NR | 9% of 47 | 50% of 47 (23 or 24) | Sacral 31% of 47 | 15 cauda equine |
| Chaichana 2009109 | Lung, breast, prostate, renal, haematopoietic, thyroid, gastrointestinal, melanoma and non-renal genitourinary system | MRI, CT, intraoperative recordings | NR | 35 | 114 | 49 | Cervicothoracic 22; thoracolumbar 24 |
| Fisher 2010110 | Unclear | Unclear | NR | Unclear | Unclear | Unclear | Unclear |
| Goldman 1989111 | Small cell lung cancer | Laminectomy and decompression of spinal cord, chest radiography, liver function tests, liver ultrasound scan, isotope bone scan and isotope or CT | Dexamethasone, chemotherapy with doxorubicin (Adriamycin®) and methotrexate | 17 | 61 | Unclear | Thoracic or thoracic and lumbar spine in 61 cases and lumbosacral spine alone in 43 cases |
| Harrison 1985112 | Breast | Patient records, prior bone scans, skeletal radiographs, myelography, panmyelography | NR | M+ 17/42 M− 11/36 | M+ 33/42 M− 15/36 | M+ 33/42 M− 12/36 | M− bone 19/36 |
| Helweg-Larsen 2000113 | Breast carcinoma, prostatic carcinoma, NSCLC, small cell lung cancer, solid tumours | Myelographic evidence, MRI scanning | NR | 7 (4%) cases | 102 (67%) cases | 0 | Lumbosacral in 44 (29%) cases |
| Helweg-Larsen 1995114 | Breast, adenocarcinoma of the prostate, tumour of the lung and other solid tumours | Myelography alone or myelography combined with postmyelography, CT | NR | Unclear | Unclear | Unclear | Unclear |
| Huddart 1997115 | Prostate | Myelography with or without MRI/CT; plain radiography | High-dose steroids, hormone therapy if not hormone resistant, radiotherapy | 5 | 57 | 20 | |
| Husband 2001116 | Breast, prostate, bronchus, haematological, urinary tract, gastrointestinal tract, unknown primary, other | Plain radiographs of the whole spine | NR | 15 | 160 | 71 | |
| Klekamp 1998117 | Breast, prostate, thyroid, kidney, unknown primary tumour, lung, colon, melanoma, urogenital tract, pleura mesothelioma, teratoma, gallbladder | Plain radiographs, CT, myelography, MRI | ‘Adjuvant’ therapy administered postoperatively to 60% (radiation ± hormone therapy/chemotherapy | 12 | 62 | 24 | |
| Kuban 1986118 | Biopsy-proved adenocarcinoma of the prostate | Radioisotopic bone scans, plain films and myelograms | NR | 12 | 21 | 14 | Cervical and thoracic 1 (2.4%); cervicothoracic junction 1 (2.4%); thoracic and lumbar 2 (4.9%) |
| Levack 2002119 | Lung, prostate and breast, gastrointestinal, haematological origin (myeloma, lymphoma, chronic lymphatic leukaemia). In 23 cases (7%) the site of primary tumour was never identified | MRI, plain films, isotope bone scintigraphy | Strong opioids | 7% | 68% | 21% | Sacral 4% |
| Lu 1998120 | Breast | Spinal CT, MRI, myelography and spine radiography | NR | 6% | 67% | 55% | Sacral 3% |
| Lu 2005121 | Breast, lung, prostate, non- Hodgkin's lymphoma, multiple myeloma, others | MRI of the spine, sagittal T1- and/or T2- weighted images of the spine with selected axial images | NR | 6 | 64 | 30 | Sacral 6% |
| McCloskey 1993122 | Breast | Radiographs | NR | Unclear | Unclear | Unclear | Unclear |
| Oka 2006123 | Breast | Bone scintigraphy, chest radiography, chest CT, liver ultrasonography, abdominal CT, cranial CT or MRI (or any combination thereof) | NR | Unclear | Unclear | Unclear | Unclear |
| Plunkett 200024 | Breast | Bone scans, radiographs, histology | Endocrine therapy | Unclear | Unclear | Unclear | Unclear |
| Rose 200988 | Renal cell, melanoma, prostate, sarcoma, colorectal, cholangiocarcinoma, thyroid, NSCLC, breast, other | Spinal MRI or CT, myelography | Bisphosphonate therapy, narcotics | 6 | 47 | 18 | 46 sites were lytic (65%), 13 were sclerotic (18%) and 12 were mixed (17%) |
| Roth 2004124 | Breast, lung, colon, prostate, lymphoma multiple myeloma, renal, other, unknown | CT | NR | 0 | 48 | 44 | 0 |
| Sekine 2009125 | Advanced NSCLC | Unclear | Zoledronic acid (bisphosphonates) | Unclear | Unclear | Unclear | Unclear |
| Shah 2003126 | Breast, lung, prostate, renal, undifferentiated, others | MRI | NR | 6 | 16 | 16 | Whole spine 79; thoracolumbar 39; cervicothoracic 8 |
| Snyder 2005127 | Metastatic breast cancer to the spine | Transaxial CT | NR | Unclear | Unclear | Unclear | Unclear |
| Snyder 2009128 | Breast | Axial CT | NR | Unclear | Unclear | Unclear | Unclear |
| Soerdjbalie-Maikoe 2004129 | Prostate | Bone scintigraphy, scintigraphy images | Hormone therapy, estramustine (Estracyt®; Pharmacia) | 2 | 14 | 6 | |
| Sun 2011130 | NSCLC | Unclear | Bisphosphonates: pamidronate, zoledronic acid | Unclear | Unclear | Unclear | Unclear |
| Talcott 1999131 | Breast, NSCLC, prostate, sarcoma, other | CT, myelography, MRI | Palliative radiotherapy, prior hormonal and chemotherapies | Unclear | Unclear | Unclear | Unclear |
| Taneichi 199789 | Breast, lung, prostate, renal, hepatocellular, gastric, colon, malignant meningioma, malignant fibrous histocytoma, rhabdomyosarcoma, leiomyosarcoma, malignant lymphoma, ureter cancer, adrenal cancer, unknown | CT of the spine | NR | Unclear | Unclear | Unclear | Unclear |
| Venkitaraman 2007132 | Prostate | MRI | NR | Unclear | 20 | 21 | Unclear |
| Venkitaraman 2010133 | Prostate | MRI | NR | Unclear | Unclear | Unclear | Unclear |
A large proportion of the included studies evaluated retrospective data (n = 17); however, other study designs were also reported: (1) prospective study (n = 11); (2) case series (n = 1); and (3) a review and modified Delphi technique (n = 1). The reviewers reported difficulties in calculating the number of participants who were selected and analysed. The approximate overall number of participants selected in the included studies was 7888 (four studies did not provide this information110,120,121,127) and 5782 were analysed (three studies did not provide this information108,110,126). The analysed sample sizes ranged from 41 to 859. Table 8 shows the range of male and female participants involved in these studies. The ranges of ages across studies were 7–92 years.
The types of cancers reported included lung alone (n = 3); prostate alone (n = 6); breast alone (n = 7); mixed cancers (n = 13); and unclear (n = 1). There was often limited reporting of the mean and median ages. Use of a broad range of technologies was reported. These included CT, bone scanning, bone scintigraphy, CSF examination, chest CT, liver ultrasonography, chest radiography, intraoperative recording, isotope bone scanning, isotope bone scintigraphy, isotope tomography, liver function tests, MRI, myelography, panmyelography, patient records, X-rays, sagittal T1- and/or T2-weighted images of the spine with selected axial images, and scintigraphy images. Some technologies were unclear. Eleven studies24,88,107,111,115,117,119,125,129–131 reported on medication use; for example, three studies88,125,130 reported the use of bisphosphonates. There was a lack of clarity about the spinal level of involvement in 12 included studies. 24,89,110,114,122,123,125,127,130,131,133
Quality assessment
Each study was evaluated according to six subheadings (study population, study attrition, prognostic factor measurement, outcome measurement, confounding measurement and account, and analysis). An overall quality score was not provided for each paper. Rather the quality assessment tool enabled the two reviewers to identify factors for consideration when interpreting the findings from each study. Appendix 8, Table 37 provides a summary of the 16 questions considered under the six subheadings.
Study population
The majority of the 30 included studies24,88,89,107–133 (the review by Loblaw et al. 62 is discussed separately) either adequately reported (n = 1724,109,112–116,120,121,123–126,130–133) or partly reported (n = 1188,89,107,108,111,117–119,122,128,129) the inclusion and exclusion criteria (including treatment, start/finish date, recruitment). However, two studies110,127 did not provide sufficient information on inclusion and exclusion criteria. 110,127 The baseline study sample (i.e. individuals entering the study) was adequately described for key characteristics (n = 1788,89,107,108,115,116,119–121,123,125,126,128,130–133) or partly described (n = 824,111–114,117,122,124) among the included papers. A further five studies109,110,118,127,129 provided limited information on the characteristics of the sample (e.g. sampling frame). Overall, in 11 studies115,116,120,121,123,125,126,130–133 the population of interest was sufficiently represented on key characteristics to limit potential bias, with partial bias in a further 17 studies. 24,88,89,109,111–114,117–119,122,124,128,129 Two studies110,127 provided such limited information on overall study population that the potential for bias could not be assessed.
Study attrition
The majority of studies (n = 2724,88,89,107,108,111–133) reported exclusions due to missing data at baseline, although two studies did not (n = 2109,110), and in one case the reviewers were unsure. 127 Compared with missing data reported at baseline, fewer studies reported (n = 2224,88,89,107,111–116,119–126,129–133) or partly reported (n = 1122) exclusions at follow-up. Some studies did not provide any details about exclusions from trials because of missing data at follow-up (n = 3108–110) and this was not considered appropriate in two studies117,118 or the reviewers were unsure. 127,128 None of the studies reported a clear statement as to the possible effect on the results from missing data. Overall, study quality related to the loss to follow-up was considered adequate in the majority of studies (n = 2388,89,107,111–121,123–126,129–133), partly adequate in four studies (n = 424,108,122,128), not adequate (n = 2109,110), or reviewers were unsure (n = 1127).The quality of reporting the study attrition was adequate and many studies provided details about exclusions for missing data at baseline and follow-up.
Prognostic factor measurement
A clear definition of prognostic factors was provided (e.g. extraction method, measurement described) in the majority of studies (n = 2288,89,107,108,112,116,118,133). For eight studies the definition of prognostic factors was only partly reported. 24,109–111,113–115,117 There was excellent reporting of the specified instrument and personnel for measurement of predictive factors in 27 studies. 88,89,107–109,111–117,119–133 In three studies, this was only partly reported. 24,110,118 Continuous variables or appropriate cut-off points were reported in two studies (n = 288,129) and partly reported in 14. 89,109,110,115,117,120–123,126,130–133 Three studies did not provide sufficient information about the continuous variables or appropriate cut-off points. 24,118,119 In six studies107,108,124,125,127,128 there was a lack of clarity about continuous variables or appropriate cut-off points, and this was not applicable in five studies. 111–114,116 There was a lack of reporting of blinding across the majority of studies (n = 2988,89,107–123,125–133) or reporting of blinding was unclear (n = 1124). Overall, in five studies88,120,125,129,131 measurement of prognostic factors of interest was sufficient to limit bias. In 25 studies24,89,107–119,121–124,126–128,130–133 the prognostic factor(s) of interest were only ‘partly’ measured to limited potential bias. Therefore, the majority of included studies provided incomplete reporting of prognostic factor measurement.
Outcome measurement
A large number of studies provided an adequate (n = 2088,89,107,108,112,115,116,118,120–123,125–133) or partly adequate (n = 1024,109–111,113–115,117,119,124) definition of the outcomes measured (SCC, vertebral compression, vertebral collapse or progression of vertebral collapse).
Confounding measurement and account
Four studies88,107,109,123 adequately met and six studies115,117,120,125,130,131 partly met the criteria for whether or not confounding factors (e.g. bisphosphonate use) had appropriately been accounted for. A further 18 studies24,89,108,110–114,116,118,121,122,124,126–128,132,133 provided insufficient information or the information was unclear (n = 2119,129). In general, there was poor reporting of the possible confounding measures and how they were accounted for.
Analysis
There was sufficient presentation of data to assess the adequacy of analysis in 19 studies24,88,107–109,112,115–117,119–123,125,128,131–133 and to partly assess the adequacy of analysis in 11 studies. 89,110,111,113,114,118,124,126,127,129,130 For a large proportion of the included studies the selected statistical analysis was considered adequate (n = 2224,88,89,107–109,112,113,115,117,120–128,131–133) or partly adequate (n = 5110,114,116,129,130) for the design of the study. Statistical analysis was not considered adequate in three studies. 111,118,119 Overall, the quality of the statistical analysis was considered appropriate in the majority of included studies.
Table 10 provides a summary of the summed quality assessment for the overall questions (see Appendix 6 – shaded boxes). Five studies were considered to be of high quality88,120,123,125,131 as they scored ‘yes’ on five of the six overall quality assessment questions. Five studies were considered to be of poor quality24,110,111,114,119 as they scored ‘yes’ on one or none of the six overall quality assessment questions. Twenty studies89,107–109,112,113,115–118,121,122,124,126,127–130,132,133 were considered to be of intermediate quality because they scored ‘yes’ on between two and four of the six overall quality assessment questions.
| Author, year | Yes | Partly | No | Unsure | NA |
|---|---|---|---|---|---|
| Lu 1998120 | 5 | 1 | 0 | 0 | 0 |
| Oka 2006123 | 5 | 1 | 0 | 0 | 0 |
| Rose 200988 | 5 | 1 | 0 | 0 | 0 |
| Sekine 2009125 | 5 | 1 | 0 | 0 | 0 |
| Talcott 1999131 | 5 | 1 | 0 | 0 | 0 |
| Bayley 2001107 | 4 | 2 | 0 | 0 | 0 |
| Lu 2005121 | 4 | 1 | 1 | 0 | 0 |
| Shah 2003126 | 4 | 1 | 1 | 0 | 0 |
| Venkitaraman 2007132 | 4 | 1 | 1 | 0 | 0 |
| Venkitaraman 2010133 | 4 | 1 | 1 | 0 | 0 |
| Harrison 1985112 | 3 | 2 | 1 | 0 | 0 |
| Huddart 1997115 | 3 | 3 | 0 | 0 | 0 |
| Husband 2001116 | 3 | 2 | 1 | 0 | 0 |
| Soerdjbalie-Maikoe 2004129 | 3 | 2 | 0 | 1 | 0 |
| Sun 2011130 | 3 | 3 | 0 | 0 | 0 |
| Taneichi 199789 | 3 | 2 | 1 | 0 | 0 |
| Bernat 1983108 | 2 | 3 | 1 | 0 | 0 |
| Chaichana 2009109 | 2 | 3 | 1 | 0 | 0 |
| Helweg-Larsen 2000113 | 2 | 3 | 1 | 0 | 0 |
| Klekamp 1998117 | 2 | 4 | 0 | 0 | 0 |
| Kuban 1986118 | 2 | 2 | 2 | 0 | 0 |
| McCloskey 1993122 | 2 | 3 | 1 | 0 | 0 |
| Roth 2004124 | 2 | 3 | 1 | 0 | 0 |
| Snyder 2005127 | 2 | 1 | 2 | 1 | 0 |
| Snyder 2009128 | 2 | 3 | 1 | 0 | 0 |
| Goldman 1989111 | 1 | 3 | 2 | 0 | 0 |
| Helweg-Larsen 1995114 | 1 | 4 | 1 | 0 | 0 |
| Levack 2002119 | 1 | 3 | 1 | 1 | 0 |
| Plunkett 200024 | 1 | 4 | 1 | 0 | 0 |
| Fisher 2010110 | 0 | 3 | 3 | 0 | 0 |
Summary of overall quality assessment
This section has shown that the included studies varied in terms of quality on ratings of study population, study attrition, prognostic factor measurement, outcome measurement, confounding measurement and account, and analysis. Study populations were adequately reported although none of the included studies provided a statement of the possible effect on the results of missing data. Loss to follow-up and study attrition information reported were considered adequate in the majority of studies but for a large number of studies ratings for the measurement of prognostic factors of interest were considered only ‘partly’ adequate to limit potential bias. None of the included studies provided information about blinding of investigators (e.g. clinical outcomes assessors), representing a considerable weakness in methodology. A large number of studies provided a clear definition of the outcome, although there was a lack of consistency in the type and definitions of outcomes reported. There was poor reporting of possible confounding factors (e.g. bisphosphonate use) and, when they were reported, how confounders were accounted for. The quality of the statistical analysis was considered appropriate in the majority of included studies.
Summary of systematic review evidence
The only review included in the current short report was undertaken by Loblaw et al. 62 The Critical Appraisal Skills Programme (CASP) systematic review checklist134 was used to critically appraise the quality of the review (see Appendix 6 for full details). In summary, the review aimed to address a large number of questions, which resulted in a broad range of study designs being included. It was difficult to determine whether all the included studies met the authors' inclusion criteria. No quality assessment was undertaken of the included papers and data from the studies were not clearly displayed to allow a clear comparison. MRI was the preferred imaging technique and conclusions were proposed that treatment for patients with MSCC should consider pretreatment ambulatory status, comorbidities, technical surgical factors, the presence of bony compression and spinal instability, potential surgical complications, potential radiotherapy reactions and patient preferences. Given the limited discussion of the populations in each included study and the lack of quality assessment, it is difficult to draw strong conclusions as to the application of these findings. Although the review discussed issues related to adverse events, there was a lack of consideration of the costs of treatment diagnosis and management of malignant extradural SCC, and the consequential outcomes of false-positive and false-negative predictions or diagnoses.
Data synthesis
The heterogeneous nature of the studies precluded the use of meta-analysis. One of the main sources of heterogeneity was in the measures of outcome, as is commonly found. 135
General considerations
The primary aim of many of the included studies was to identify prognostic factors for survival; the analysis of influential factors for intermediate outcomes, such as SCC or vertebral collapse, was often an incidental objective.
A large number of included studies enrolled patients who had different primary tumours; more than five types of cancer were included in some studies. Several of these mixed cancer studies (e.g. breast, prostate, colorectal, etc.) indicate that the type of primary tumour might itself be a prognostic factor for SCC and/or vertebral collapse. As the factors influencing development of spinal metastases, and of the unwanted outcomes that develop from them, can be expected to differ between various primary cancers, the interpretation of results from these ‘mixed tumour’ studies is problematic. The relative importance of identified prognostic factors may reflect only that the characteristics of the most frequent cancers in the sample analysed are potentially subject to both lead time and length time bias because of differential rates of diagnosis, progression and growth of different cancers.
Studies in which the whole sample population was diagnosed with prostate cancer
Bayley et al. (2001)107
Relevant aim
The aim of this study was to identify risk factors for occult subarachnoid space (SAS) compression or SCC in patients judged to be at risk according to clinical, radiography or bone scan parameters. Occult SAS compression or SCC was established using MRI.
Design and method
This study investigated outpatients with metastatic prostate cancer at a single Canadian hospital (n = 68). All had previous evidence of spinal metastases, but had no neurological indication or signs of SAS compression or SCC; 64 out of 68 had received continuous hormone therapy and 61 had hormone-refractory disease (indicated by rising PSA levels). The authors described the sample as cross-sectional; however, as patients were approached at the physicians' discretion, the sample was probably one of convenience. All patients were examined by bone scintigraphy within 1 week of study entry. Follow-up ranged from 1 to 47 months (median 8 months). Thirty patients underwent plain radiographic examination: 22 for back pain and eight at the physicians' discretion. The timing of radiography was not reported.
Results
MRI was used to identify patients with occult SAS compression/SCC; the timing of the MRI was not stated. The criteria used to establish occult compression are shown in Box 1.
Impingement of the SAS by metastatic tumour involving vertebrae
Distortion or collapse of vertebrae with impingement of the SAS by bone fragments
Frank compression of spinal cord or cauda equina by either of the above
Occult compression was identified in 22 out of 68 patients; all cases were due to direct extension of tumour from the vertebral body. Ten patients had frank compression of the cauda equina or the spinal cord, and 12 of the SAS alone. Nine of 22 had compression at two separate vertebral levels. The disposition of compressions was cervical in three patients, thoracic in 20 patients and lumbar in eight patients. Plain radiographs were not informative for detection of occult compression.
Clinically evident SCC developed during follow-up in 4 of the 46 patients in whom no occult compression was apparent at MRI (the authors quote actuarial risk at 1 and 2 years using Kaplan–Meier analysis; however, with only four events it is unlikely that this analysis is meaningful). The 22 patients with occult compression were treated with appropriate radiotherapy; the post-MRI occurrence of neurological compression in these 22 patients was not reported.
Logistic regression was used to identify risk factors for occult SAS compression/SCC. Of the candidate factors examined in univariate regression, haemoglobin, duration of continuous hormone therapy before study entry and bone scan extent of disease (EOD) score (extent of disease according to number of bone metastases according to the Soloway et al. method136) were significantly associated with occult compression (p = 0.04, p = 0.03 and p = 0.015, respectively), whereas no association was found for Gleason score, alkaline phosphatase, PSA, prostatic acid phosphatase, presence of back pain or use of narcotic analgesics. In multivariate regression, only EOD and duration of hormone therapy were significantly associated with occult compression (p = 0.02 and p = 0.04, respectively).
Author conclusion
Heavy load of spinal metastases, as indicated by scintigraphy, and duration of continuous treatment with hormone therapy are predictive factors for presence of occult SAS compression or SCC. Such patients, although lacking neurological abnormality and signs of compression, would probably benefit from early MRI for occult compression, which if positive for compression should be followed by radiotherapy treatment before the development of symptomatic compression.
Reviewer conclusions
Patients with a high-risk bone scan may benefit from MRI of the spine aimed at early detection and treatment of occult SAS compression/SCC. The reported results are as would be intuitively expected, so the more spinal metastases that are present and the longer a patient is at risk, the greater the chance of clinically occult SCC. The time a patient is on hormone therapy is a proxy for how long he or she is at risk of occult compression. The quantitative estimates of risk probably do not add much value to this conclusion other than suggesting that spinal load is more influential than time at risk; it can be hypothesised that time at risk interacting with the individual patient's propensity for metastases to reach the spine will govern the spinal load. What this study does not address is the probability that occult SCC becomes patently symptomatic SCC, and how long after occult SCC is detected this occurs.
Huddart et al. (1997)115
Relevant aim
The aim of this study was to identify risk factors for recurrence of SCC at an old or new site in prostate cancer patients with previous diagnosis of SCC.
Design and method
The main focus of this retrospective study was to identify prognostic factors for survival and for good response to therapy, after diagnosis of SCC in prostate cancer patients with SCC treated at the Royal Marsden Hospital between 1984 and 1992. Sixty-nine patients with SCC were identified from a review of medical records of (1) participants in a previous study of hormone-resistant prostate cancer; (2) those who had undergone spinal MRI; and (3) those who had undergone spinal irradiation. The total number of records reviewed was not stated. SCC was established by myelography or MRI in 66 patients and from plain radiographs in three patients. No information was provided about patients with negative assessments for suspected SCC. Thirteen of 69 patients had SCC at presentation; the median time from diagnosis of prostate cancer to detection of SCC in the remaining 56 patients was 586 days. Most patients (n = 52) had received hormone therapy. Evidence of vertebral collapse at the site of cord compression was present in 24 patients. MRI identified more patients with multiple sites of SCC than did myelography. Fifty-seven patients were given radiotherapy after SCC diagnosis and 13 received surgery.
Results
Neurological relapse (from various causes including 13 second occurrences of SCC) was observed in 20 out of 69 patients. A second SCC at the same site occurred in eight patients and at a new site in five patients.
None of the following potentially predictive factors were associated with the occurrence of neurological relapse: presenting characteristics; haemoglobin; the number of lesions evident by bone scan; hormonal status or method of diagnosis; radiation dose for first SCC. The paper provides a Kaplan–Meier analysis of the cumulative probability of neurological relapse. The methodology used for this was unclear and no ‘at risk’ table was provided; one interpretation is that all 69 patients were included and many were censored at time of death if no relapse had occurred.
Author conclusions
No significant factor was identified for risk of future relapse. An early improvement in motor power is a strong predictor of subsequent functional improvement. MRI detects additional sites of asymptomatic SCC which makes it the investigation of choice.
Reviewer conclusions
No significant predictive factor was identified for risk of future relapse (i.e. second SCC) but the sample was so small that there was little power in the analysis. The actuarial analysis of time to relapse was difficult to interpret because of a lack of methodological detail and ambiguity about the equivalence of SCC and neurological relapse.
Kuban et al. (1986)118
Relevant aim
The aim was to determine and analyse, with reference to primary tumour stage and differentiation at diagnosis, the interval between primary diagnosis and SCC, the interval between radiographic evidence of bony metastasis and cord impingement, and the survival period after spinal cord compromise.
Design and method
Forty-one patients with biopsy-proven adenocarcinoma of the prostate who presented with MSCC, or who subsequently developed MSCC, were identified from a total of 611 prostate cancer patients seen at a single centre over a period from 1975 to 1983. Mean and median age was 68 (range 50–90) years. Primary tumours were classified according to a modified Gleason system and patients were classified according to the Fowler–Whitmore staging system. SCC was established by myelography (n = 36) and by clinical findings (n = 5), and by bone scan- or plain radiography-detected lesions at the level of compression. Of the 41 patients with SCC, 3, 11 and 27 were classified as stages A, B and C at the time of diagnosis of the primary tumour. The spinal locations of the SCCs were reported as follows: cervical, two patients; thoracic, 21 patients; lumbar, 14 patients; cervical and thoracic, one patient; cervicothoracic junction, one patient; thoracic and lumbar, two patients.
Results
The median time between primary diagnosis and SCC was 24 months, with 5 out of 41 patients presenting with SCC; there was no clear relationship with tumour grade at diagnosis. The median time from detection of bone metastases to SCC was 15.5 months, with 6 out of 41 patients having bone metastases first observed at the diagnosis of SCC. A second SCC developed in six patients during follow-up, at 6, 6, 8, 19, 21 or 23 months after the first SCC.
Author conclusions
Overall, tumour stage and differentiation were poor predictors of prognosis once a diagnosis of cord compression was established. MSCC secondary to adenocarcinoma of the prostate most frequently occurs in a thoracic location in patients with poorly differentiated disease at diagnosis. The mechanism of cord involvement appears to begin with osseous vertebral metastasis, progressing to extradural compromise with a median interval that is independent of tumour grade. The prognosis following spinal cord involvement remains dismal in the majority of cases.
Reviewer conclusions
This paper did not look at predictive factors of SCC other than tumour grade at diagnosis of primary tumour and this had no detectably significant influence on median time to SCC detection. Kaplan–Meier analysis was not used.
Soerdjbalie-Maikoe et al. (2004)129
Relevant aim
The study aimed to determine if high-resolution bone scintigraphy at the time of diagnosis of hormone-refractory metastatic prostate cancer has added prognostic value compared with prevailing PSA concentrations and tumour staging (Gleason grading) for SCC-free survival. The authors were also interested in prognosis for overall survival.
Design and method
This was a retrospective study of 84 patients with histologically confirmed diagnosis of hormone-resistant metastatic prostate cancer (treatments had included orchidectomy, luteinising hormone-releasing hormone agonist or antiandrogens). Hormone treatment was stopped for 23 patients. Patients were followed up till death. Tumours were assessed according to Gleason grading. Various criteria were used to establish hormone refractoriness. Palliative care treatments included radiotherapy, radionuclide therapy (89Sr), nitrogen-containing bisphosphonate olpadronate and analgesia.
Whole-body high-resolution bone scintigraphy was undertaken using 99Tcm-labelled methylene-diphosphonate. Bone scans were scored according to the Soloway scoring system; in addition, metastasis of vertebrae was classified according to degree of involvement as total or partial. Scintigrams were interpreted by two independent observers. Observations were related to subsequent development of SCC established clinically according to impaired motor or sensory function confirmed by MRI or CT at the appropriate spinal level. ‘Skeletal event-free survival’ was defined as survival without SCC.
Kaplan–Meier analysis of overall survival and of SCC-free survival was undertaken with Cox regression to investigate the relationship between the relative risk (RR) of SCC according to variables including PSA (log-transformed), serum alkaline phosphatase (log-transformed), Soloway grade, age, degree of vertebral involvement and Gleason score.
Results
Mean survival after hormone refractoriness was 8.6 [standard deviation (SD) 10.6] months. Twenty of 84 patients developed SCC 3 days to 10 months after refractoriness was established. Six patients experienced SCC at lumbar level, and 14 at thoracic level; four of the latter also had SCC at another level, two at lumbar level and two at cervical level.
Mean Gleason score was 7.5. When Gleason score was dichotomised to ≥ 7 or < 7, the former was found to be significantly associated with shorter SCC-free survival and overall survival (median 6.1 vs. 12.3 months; p < 0.05). Median overall survival of patients with Gleason scores ≥ 7 and < 7 was 6.8 months and 12.7 months, respectively (p < 0.03). RR (Gleason ≥ 7 vs. < 7) was 1.89 (95% CI 1.02 to 3.53) for mortality and 1.76 (95% CI 0.95 to 3.28) for SCC-free survival. RRs remained significant after adjusting for confounders: RR = 2.33 (p = 0.013) for mortality and RR = 2.37 (p = 0.003) for SCC.
Serum PSA and alkaline phosphatase activity were elevated in all patients (mean values of 511 ± 1035 μg/l and 402 ± 503 U/l, respectively). Log-transformed PSA concentrations were significantly predictive of SCC-free survival (RR = 1.21, 95% CI 1.07 to 1.36; p = 0.003) and survival (RR = 1.17, 95% CI 1.04 to 1.32; p = 0.01) but log-transformed serum alkaline phosphatase activity and age were not.
The unadjusted RR for SCC was significantly associated with Soloway grade (p = 0.031), i.e. patients with heavier metastatic skeletal load were more likely to sustain SCC (the reported results are summarised in Table 11). However, after adjustment for confounders (PSA and alkaline phosphatase concentrations and age), statistical significance was greatly reduced to p = 0.35 (similarly, for survival, p = 0.008 became p = 0.09 after adjustment).
The unadjusted RR for SCC-free survival among Soloway grade 4 patients was significantly greater than that for grade 1 patients (this also applied for overall survival).
| Soloway gradea | Patients, n | RR survival (95% CI) | p-value | RR SCC-free survival (95% CI) | p-value | ||
|---|---|---|---|---|---|---|---|
| 0 | |||||||
| 1 | 11 | 1 | 1 | ||||
| 2 | 23 | 2.29 | (1.03 to 5.07) | 1.96 | (0.89 to 4.29) | ||
| 3 | 24 | 1.85 | (0.84 to 4.07) | 1.72 | (0.79 to 3.75) | ||
| 4 | 26 | 3.66 | (1.67 to 8.06) | 3.03 | (1.40 to 6.56) | ||
| 0.008 | 0.031 | ||||||
For the ‘new method’ of assessing total or partial vertebral involvement at progression, the sensitivity and specificity were 0.90 and 0.94, respectively (based on 2 × 2 table values shown in Table 12 derived from table 2 of published paper). The positive predictive value for a totally involved vertebra was therefore 82% and the positive likelihood ratio (LR) was 14.4. The SCC pre-test probability of ≈ 0.24 is raised to a post-test probability of ≈ 0.82 by a positive total involvement test.
| Soloway gradea | SCC | No SCC | Total |
|---|---|---|---|
| Total involvement | 18 | 4 | 22 |
| Partial involvement | 2 | 60 | 62 |
| Total | 20 | 64 | 84 |
Author conclusions
The data demonstrate that bone scintigraphy performed at the time of development of refractoriness to hormone therapy is of high predictive value for inherent risk of subsequent SCC.
Reviewer conclusions
There is no indication of how the 84 patients were selected. It is possible that different patients received various treatments likely to influence the probability of SCC (e.g. bisphosphonates), but it is not clear if these were accounted for in Cox regression analyses. Although the ‘total involvement of vertebra’ according to scintigraphy appeared to be highly sensitive and specific for subsequent SCC, the study lacks sufficient rigour to be confident of this result; in particular, participant selection was unclear, progression criteria were not defined precisely and no details were given of the method of discriminating total from partial vertebral involvement except that two independent assessors were involved. The frequency of investigator disagreements was not reported, and the level of concordance and how investigator disagreement was handled were not mentioned. It is not altogether clear whether the assessment was conducted before or after SCC was determined to have occurred, and whether scintigraphy assessors and MRI/CT assessors were reciprocally blind to each other's results.
Venkitaraman et al. (2007)132
Relevant aim
The aim of the study was to identify clinical factors that predict a high risk for SCC in metastatic prostate cancer patients with MRI-suspected overt or occult SCC who have no functional neurological deficit.
Design and method
This was a retrospective study based on a single institution's medical records of 570 consecutive patients with prostate cancer who underwent MRI of the spine between January 2001 and May 2005. Patients with skeletal metastases were included if their MRI indicated SCC in the absence of neurological deficit. Patients were excluded if they had experienced previous SCC. In all, 150 patients were included. Their median age was 69 (range 50–88) years, 112 out of 150 were hormone refractory, median time since diagnosis was 41.3 (range 3.13–213) months and from start of hormone treatment was 26.8 (range 0.1–157.5) months. Gleason scores ranged from 6 to 10 (G6, n = 23; G7, n = 36; G8, n = 26; G9, n = 29; G10, n = 9). Back pain was present in 72% of patients.
Whole-spine MRI was conducted and patients were classified as (1) ‘overt SCC’, defined as involvement or compression of either the spinal cord or the cauda equina by an epidural or an intramedullary mass lesion; (2) ‘occult SCC’, defined as metastatic disease causing impingement, indentation or loss of definition of the thecal sac; or (3) no SCC. Categories (1) and (2) were considered together as radiological SCC (rSCC).
Binary univariate and multivariate logistic regression were used to identify independent clinical risk factors for rSCC.
Results
Of the 150 patients, 41 (27.33%) had rSCC, 24 (16%) had overt rSCC and 17 (11.3%) had occult rSCC. Seven patients had rSCC at multiple non-contiguous sites; 20 had compression in the thoracic spinal level and 21 in the lumbosacral region.
On univariate analysis, significant determinants of rSCC were found to be extensive bone metastases (six or more bone lesions; p = 0.005) and back pain (p = 0.002), whereas age (p = 0.97), time from diagnosis (p = 0.52), metastasis at diagnosis (p = 0.535), Gleason score (p = 0.34), hormone refractory status (p = 0.158), time from starting hormonal treatment (p = 0.96) and PSA at the time of MRI (p = 0.855) did not predict rSCC. Univariate regression results are summarised in Table 13.
| Factor | n | OR (95% CI) | p-value |
|---|---|---|---|
| Back pain | |||
| No | 42 | 1 | 0.002 |
| Yes | 108 | 7.05 (2.04 to 24.36) | |
| Extensive bone metastasis | |||
| No, < 6 lesions | 45 | 1 | 0.005 |
| Yes, ≥ 6 lesions | 104 | 4.24 (1.54 to 11.67) | |
| Age (years) | |||
| < 70 | 76 | 1 | 0.97 |
| ≥ 70 | 74 | 0.97 (0.47 to 1.99) | |
| Haemoglobin below normal (< 13 g/dl) | |||
| No | 57 | 1 | 0.61 |
| Yes | 86 | 1.21 (0.57 to 2.56) | |
| Calcium (mmol/l) | |||
| < 2.27 | 66 | 1 | 0.32 |
| ≥ 2.27 | 74 | 0.69 (0.33 to 1.44) | |
| Initial PSA (ng/ml) | |||
| < 52 | 70 | 1 | 0.68 |
| ≥ 52 | 73 | 1.17 (0.56 to 2.44) | |
| PSA at MRI (ng/ml) | |||
| < 71 | 75 | 1 | 0.85 |
| ≥ 71 | 75 | 0.94 (0.46 to 1.92) | |
| Hormone refractory | |||
| No | 38 | 1 | 0.16 |
| Yes | 112 | 1.93 (0.77 to 4.81) | |
| T stage | |||
| < T3 | 19 | 1 | 0.19 |
| ≥ T3 | 103 | 2.40 (0.65 to 8.84) | |
| N stage | |||
| N0 | 58 | 1 | 0.19 |
| N1 | 40 | 0.51 (0.19 to 1.38) | |
| Composite Gleason score | |||
| < 8 | 61 | 1 | 0.34 |
| ≥ 8 | 64 | 0.68 (0.31 to 1.49) | |
| Primary Gleason grade | |||
| < 4 | 41 | 1 | 0.36 |
| ≥ 4 | 84 | 0.68 (0.31 to 1.54) | |
| Metastasis at diagnosis | |||
| No | 67 | 1 | 0.53 |
| Yes | 83 | 0.80 (0.39 to 1.64) | |
| Serum alkaline phosphatase | |||
| No | 42 | 1 | 0.93 |
| Yes | 104 | 0.97 (0.44 to 2.14) | |
| Time from diagnosis | |||
| < 3.4 years | 76 | 1 | 0.52 |
| ≥ 3.4 years | 74 | 1.27 (0.62 to 2.61) | |
| Time from start of hormone therapy | |||
| < 2 years | 70 | 1 | 0.96 |
| ≥ 2 years | 80 | 1.02 (0.50 to 2.09) | |
On multivariate analysis, back pain [odds ratio (OR) 5.1, 95% CI 1.44 to 18.25; p = 0.012] and extensive bone metastasis (OR 2.9, 95% CI 1.01 to 8.35; p = 0.047) were significant independent predictors of rSCC. One variable, PSA at the time of MRI (median PSA 402 vs. 98 ng/ml), was significantly different in the patients who had overt SCC and those who had occult SCC [hazard ratio (HR) 1.005, 95% CI 1.001 to 1.009; p = 0.014].
Author conclusions
A significant proportion (27.3%) of patients with metastatic prostate cancer may harbour overt or occult SCC in the absence of functional neurological deficit. MRI of the spine for the early diagnosis of SCC may be considered useful in patients with extensive skeletal metastasis and back pain.
Reviewer conclusions
Magnetic resonance imaging of the spine in patients with extensive skeletal metastasis and back pain but lacking neurological deficit may lead to early detection of SCC. Since 72% of the selected population had back pain, and rSCC may occur in patients without back pain (although relevant data were not provided), it appears that MRI of all patients with extensive skeletal metastases might represent a factor for consideration.
Venkitaraman et al. (2010)133
Relevant aim
The authors' stated aims were to determine the optimal frequency of MRI of the spine required to detect clinically occult rSCC and to determine the incidence of neurological deficit in patients with metastatic prostate cancer. The rSCC was defined as involvement or compression of either the spinal cord or the cauda equina by an epidural or an intramedullary mass/lesion, or metastatic disease causing impingement, indentation or loss of definition of the thecal sac.
Design and method
This was a retrospective study based on a single institution's medical records of 570 consecutive patients with prostate cancer who underwent MRI of the spine between January 2001 and May 2005. Initial MRI was requested by the physician when patients were considered at high risk of SCC. These appear to be the same patients as those in Venkitaraman et al. 2007. 132 From these 570, 130 patients (median age 70 years, range 50–88 years) with castration-resistant disease and skeletal metastases in whom MRI was indicative of suspected clinically occult SCC (i.e. an absence of neurological deficit) were included. Patients were excluded if they had experienced previous SCC. Median times since diagnosis and the start of hormone treatment were 1355 (range 219–6412) days and 917 (range 100–3332) days, respectively. The selection criteria differed from those in the previous 2007 study in that some patients in the latter were not hormone refractory. Median follow-up after initial MRI was 11 (range 1–50) months. Patients with rSCC received radiotherapy to the site of the lesion and some received bisphosphonate treatment as indicated by the treating physician.
Kaplan–Meier analysis was used to investigate neurological deficit-free survival (NDFS) (i.e. the time to development of neurological deficit); those patients who did not develop neurological deficit were censored at time of death or at end of follow-up. Cox's multivariate regression was used to identify influential risk factors for neurological deficit. The following variables were tested: rSCC during first MRI; PSA level at the time of initial MRI; PSA doubling time; radiotherapy; and back pain.
Results
Median overall survival was 416 (95% CI 23 to 987) days.
The median time to development of neurological deficit was 896 (95% CI 13 to 986) days (Figure 5). Thirty-seven (28.5%) of the 130 patients had rSCC at initial MRI. When patients were dichotomised into those with (n = 37) or without (n = 93) rSCC at the first MRI, the latter exhibited slower development of deficit (Figure 6) but this did not reach statistical significance (p = 0.103 by log-rank test); median times were 657 (95% CI 23 to 1103) days and 896 (95% CI 13 to 986) days, respectively.
FIGURE 5.
Time to development of neurological deficit after initial MRI (patients' initial MRI requested because of physicians' judgement of a high risk of SCC). Patients were censored either at the time of death or at the time of last follow-up for surviving patients who had not developed neurological deficit. Data read from published graph (Venkitaraman 2010133).
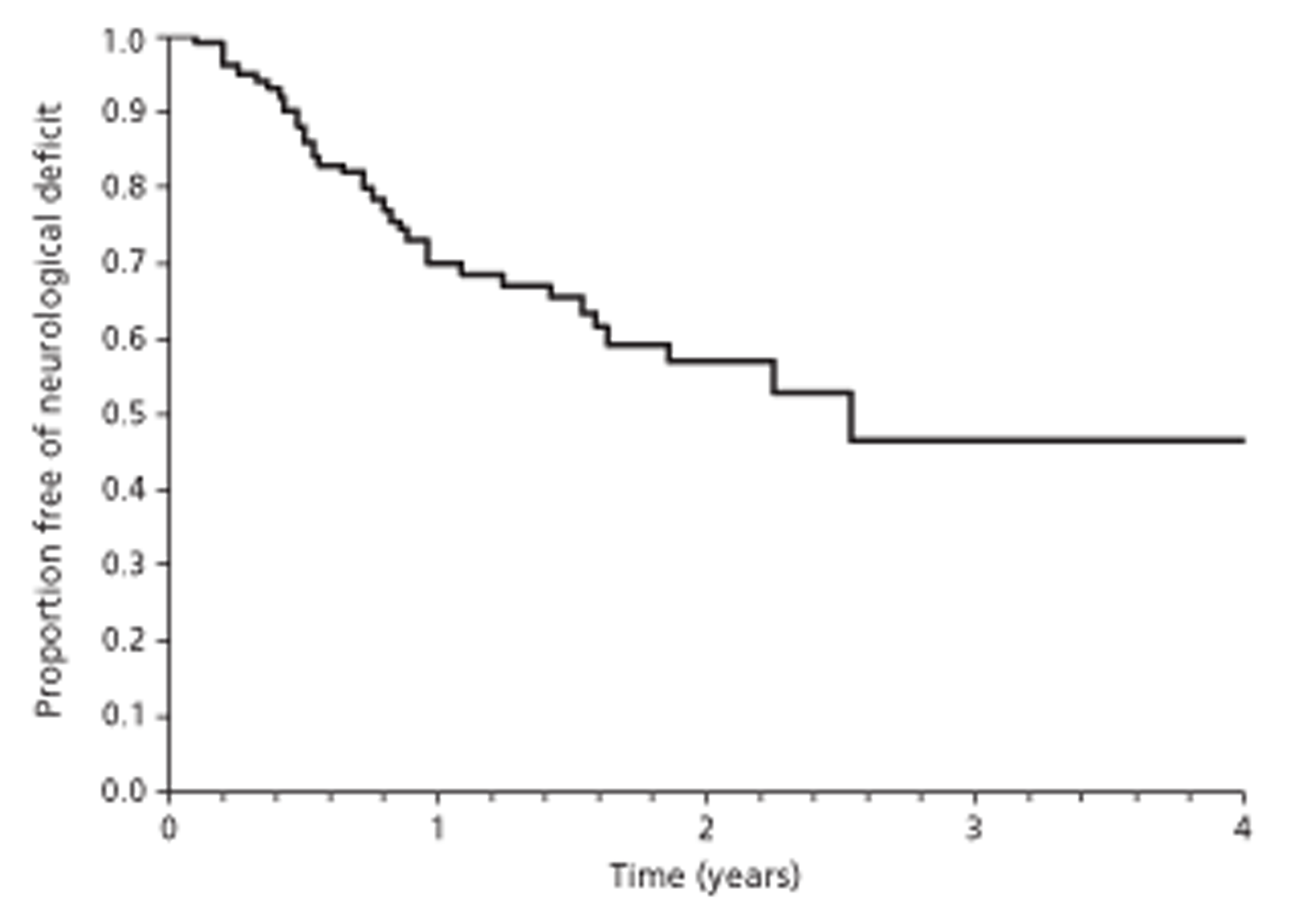
FIGURE 6.
Time to development of neurological deficit according to rSCC at initial MRI. Patients were censored either at the time of death or at the time of last follow-up for surviving patients who had not developed neurological deficit. Data read from published graph (Venkitaraman 2010133).
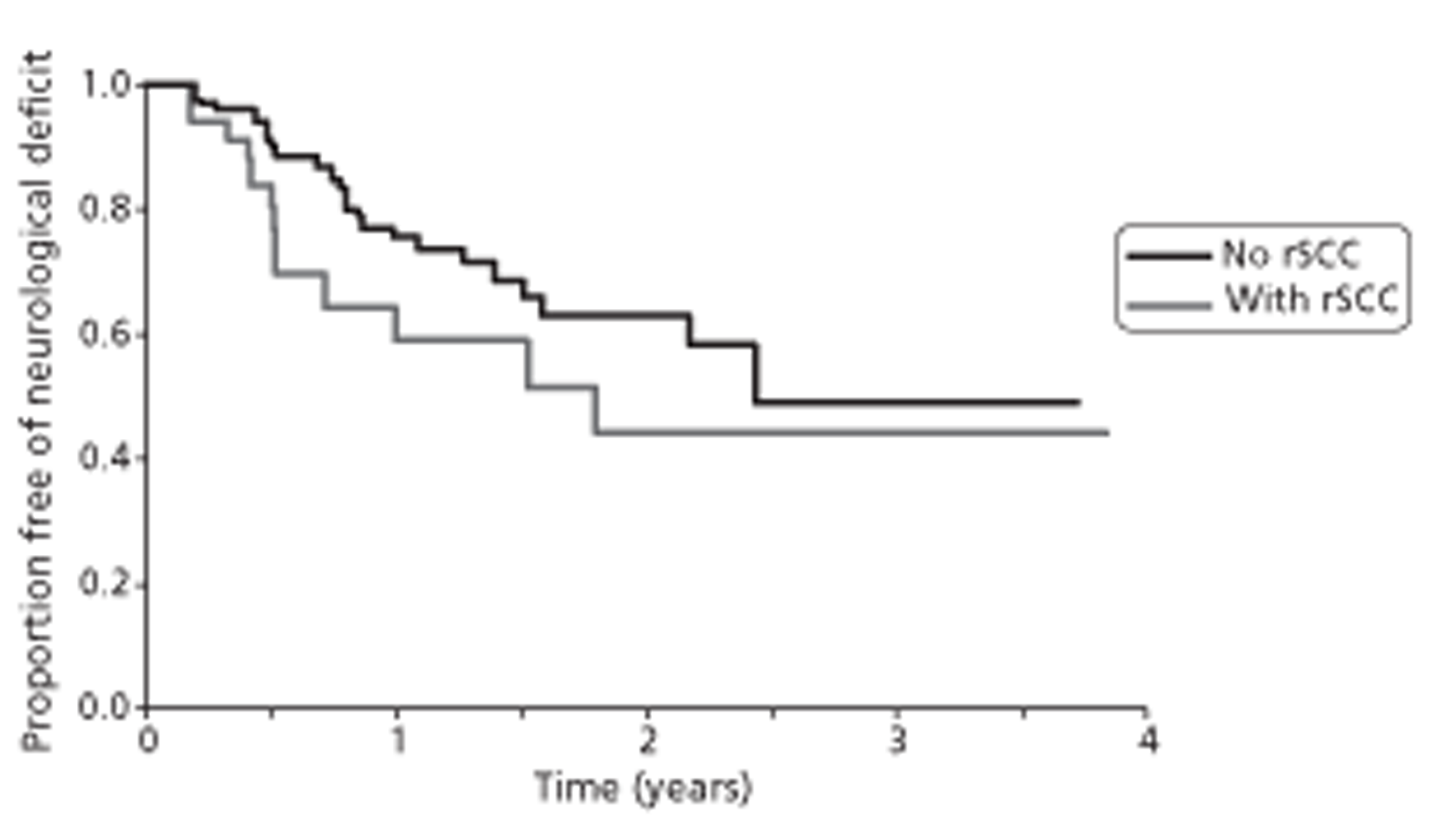
Of the 37 patients who had rSCC during initial MRI, 10 developed a repeat rSCC on MRI follow-up after a median time of 161 (95% CI 63 to 259) days. In 6 out of 10 of these patients, recurrence was at the same site as initial rSCC and radiotherapy. Six of the 37 patients (16.2%) developed irreversible paraparesis on follow-up.
Of the 93 patients without rSCC at initial MRI, 20 (21.5%) developed SCC during follow-up. The median time to development of an rSCC for patients with no rSCC on initial MRI was 283 (95% CI 229 to 337) days, and 8 out of 93 (8.6%) developed paraparesis during follow-up.
High PSA level at the time of initial MRI (HR 2.04, 95% CI 1.05 to 3.96; p = 0.035) and short PSA doubling time (< 3 months) (HR 0.40, 95% CI 0.19 to 0.79; p = 0.009) were found to significantly predict adverse NDFS on univariate analysis, but rSCC on initial MRI (p = 0.11), radiotherapy (p = 0.1) and back pain (p = 0.059) did not attain statistical significance. On multivariate analysis, only a rapid PSA doubling time (< 3 months) independently predicted future neurological deficit (p = 0.042).
The authors tabulated the actuarial NDFS at several time points for patients with and without back pain and for patients with and without rSCC at initial MRI. These data are summarised graphically in Figure 7.
FIGURE 7.
Actuarial deficit-free survival according to (a) back pain and (b) rSCC at initial MRI.
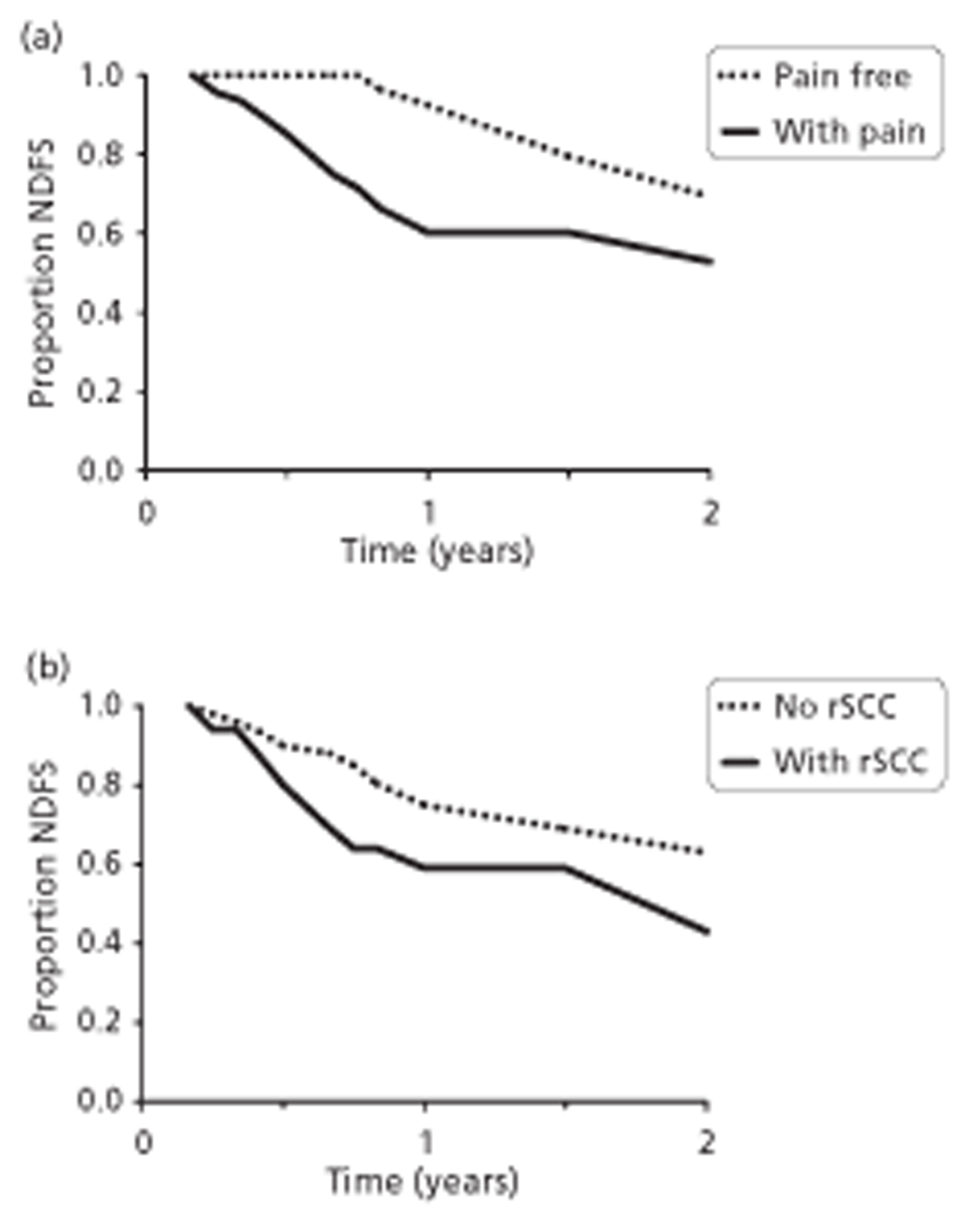
Author conclusions
Imaging by MRI of the spine can be used to detect asymptomatic rSCC in patients with castration-resistant prostate cancer. Serial estimations are required to maintain a low incidence of clinical SCC. If serial MRI is to be used to detect rSCC in 90% of patients before the development of neurological signs, the optimum frequency for scanning depends on the subset of patients studied.
Reviewer conclusions
The study findings are consistent with the fairly obvious conclusion that, among castration-resistant prostate cancer patients lacking neurological deficit, those in whom MRI is suggestive of occult SCC (i.e. with rSCC) will develop neurological deficit sooner than those patients whose MRI scan is negative for occult SCC. Only 37 (28%) of patients had occult SCC so the study lacked power. Rapid escalation of serum PSA was found to be associated with increased risk of neurological deficit.
Summary of prostate cancer studies
None of the included prostate cancer studies provided a description of the natural history of spinal metastases.
The six included studies varied in methodology and transparency, and this resulted in difficulties in interpreting the findings reported. In particular, it was often difficult to ascertain how study samples were selected. In three studies (those by Bayley et al. 107 and Venkitaraman et al. 132,133) patient participation depended on physicians' decisions (e.g. regarding requirement for MRI), but the criteria for decision-making were not clear. In the study by Huddart et al. ,115 an investigation conducted at the same centre as the Venkitaraman studies132,133 but a decade earlier, participants had been diagnosed with SCC; however, it was not clear if this was a subsample of such patients at the centre or a complete set. The report of Soerdjbalie-Maikoe et al. 129 gave no information regarding sampling frame. In the study by Kuban et al. 118 both sampling frame and selection procedure were fully described.
Patient populations differed with regard to degree of progression of their prostate cancer so that looking for coherence of results across studies should be undertaken with caution. In the study by Bayley et al. ,107 patients had metastatic prostate cancer with neurological deficit. In two studies (Kuban et al. 118 and Huddart et al. 115) metastatic patients with SCC were examined. Venkitaraman et al. 132 investigated patients with SCC but no neurological deficit, whereas in two studies (Venkitaraman et al. 133 and Soerdjbalie- Maikoe et al. 129) patients had progressed to become castration resistant. A further complication arises because previous and current treatments and the timing of their implementation, likely to affect the natural progression of the spinal metastases and to influence the identity of potential prognostic factors, varied between studies.
All studies used medical records to ascertain measures of and presence of risk factors. These records were not collected for the purposes of the studies according to a structured framework that was applied equitably to each participant. Furthermore, the completeness of information content within the records was indeterminate. The six studies together included only 409 patients.
The results from these studies imply that patients with a high-risk bone scan may benefit from MRI of the spine aimed at early detection and treatment of occult SAS compression/SCC. The more spinal metastases present, and the longer a patient is at risk, the greater the chance of clinically occult SCC. The time a patient is on hormone therapy may be a proxy for how long he or she is at risk of occult compression. ‘Total involvement of vertebra’, according to scintigraphy, appeared to be highly discriminatory for subsequent SCC. 129 Other studies reported no significant predictive factors for risk of future relapse (i.e. second SCC). Time-to-event analyses were difficult to generalise because of the different populations studied and uncertainty regarding their representativeness. The validity of the risk factors identified in these studies did not appear to have been tested in an independent population selected according to similar criteria.
Studies in which the whole sample population was diagnosed with breast cancer
Harrison et al. (1985)112
Relevant aim
The aim of this study was to determine the correlation of specific symptoms and signs with epidural spinal cord compression (ESCC) in patients suspected of having ESCC, and to compare the roles of skeletal radiographs, radionuclide scans and CSF analyses in the diagnosis of SCC.
Design and method
This was a retrospective study based on case records from a single centre. Consecutive patients with a positive diagnosis of breast cancer from records dated between January 1977 and July 1982 were selected if they had received myelography for suspected SCC secondary to metastatic breast cancer. The number with suspected SCC who did not receive myelography was not reported.
Results
In 42 of the 78 included patients (age range 22–75 years), myelography was positive for ESCC (with complete block in 21); myelography was negative in 36 patients. In all 42 myelogram-positive patients, bone scans were also positive for spinal metastases, and most had three or more of these at different sites. Bone scans were positive for spinal metastases in 11 of the 36 patients with negative myelograms. Seven of the patients with a negative myelogram were found not to have metastatic disease.
There was no statistical difference between myelogram-positive and myelogram-negative groups in the distribution of spinal metastases as detected by bone scan or in the proportion with visceral metastases (Table 14). The distribution of spinal metastases in patients not undergoing myelography is unknown. The distribution of various signs and symptoms of SCC between patients with positive and negative myelograms indicated that back pain, paraesthesias and bladder/bowel dysfunction were more common in the myelogram-positive group (p≤ 0.05 by chi-squared test; Table 15). The number of metastatic sites (0, 1, 2, ≥ 3) and the type of dominant site (bone, visceral, soft tissue, none) were also significantly associated with a positive myelogram. Plain radiographs were analysed in only 13 of the 36 patients with negative myelograms and therefore comparison between groups based on radiographic findings is unlikely to be meaningful.
| Variable | Positive myelogram (n = 42 patients) | Negative myelogram (n = 36 patients) | p-value from statistical testa for difference |
|---|---|---|---|
| Positive bone scan | 42/42 | 19/36 | < 0.001 |
| Positive bone scan for spinal metastases | 42/42 | 16/36 | < 0.001 |
| Cervical involvement | 17/42 | 11/36 | 0.149 |
| Thoracic involvement | 33/42 | 15/36 | 0.484 |
| Lumbar involvement | 33/42 | 12/36 | 0.418 |
| Visceral metastases | |||
| Liver | 6/42 | 5/36 | 0.96 |
| Lung | 13/42 | 10/36 | 0.76 |
| Brain | 3/42 | 6/36 | 0.29 |
| Signs and symptoms of ESCC | |||
| Back pain | 39/42 | 20/36 | < 0.001 |
| Radicular pain | 24/42 | 13/36 | 0.06 |
| Paraesthesias | 24/42 | 10/36 | 0.009 |
| Extremity weakness | 28/42 | 22/36 | 0.61 |
| Bladder/bowel dysfunction | 18/42 | 8/36 | 0.05 |
| Variable | TSC | No TSC | Total |
|---|---|---|---|
| Positive for objective weakness | 20 | 30 | 50 |
| Negative for objective weakness | 13 | 60 | 73 |
| Total | 33 | 90 | 123 |
Author conclusion
Specific signs and symptoms may predict SCC. Back pain, radicular pain, paraesthesias and even bladder and bowel dysfunction are seen in patients without cord lesions. Myelography remains the most precise tool for diagnosing spinal cord lesions.
Reviewer conclusions
In a comparison of patients in whom myelography for suspected ESCC was positive or negative, chi-squared tests indicated that a positive bone scan, back pain, paraesthesia and bladder/bowel dysfunction at the time of myelography were more common in patients with a positive myelogram than those with a negative myelogram. The discriminatory power of these signs or symptoms to distinguish patients with or without ESCC was poor because they were common in both groups.
Lu et al. (1998)120 (see also Talcott et al., 1999)131
Relevant aim
The aim of this study was to examine potential clinical neurological and oncological risk factors for CT-established SCC in breast cancer patients with suspected SCC.
Design and method
This was a retrospective study of 123 episodes of suspected SCC encountered at a single centre over a 2.5-year period from 1985 to 1988. In all, 405 episodes of suspected SCC were recorded; the number of breast cancer patients among these was not reported. Sixty-three episodes were excluded for various reasons including radiotherapy to the suspected site of SCC within 1 year of the index CT scan. Of the remaining 342 episodes, 146 occurred in breast cancer patients; 23 episodes were excluded because of incomplete medical records. The 123 included episodes involved 93 patients with a median age of 52.9 (range 29.8–77.3) years, of whom 98% had previously established metastatic disease and 89% had skeletal involvement. The spinal level of the suspected episodes was reported as lumbar 38%, thoracic 35%, cervical 18%, sacral 1% and various combinations (8%).
All patients received CT scans; problematic cases were examined with MRI when this became available. Bone scans were performed in 99% of episodes and plain spinal radiography was undertaken in 72%.
Univariate analysis (Fisher's exact test) was used to test for association of potential clinical, neurological and oncological factors predictive of SCC. Stepwise multivariable logistic regression was used to identify independent risk factors for SCC and Kaplan–Meier analysis examined survival of SCC-positive and -negative patients after the index CT scan.
Results
Of 123 episodes (93 patients), the index CT scan revealed spinal cord (or cauda equina) displacement in 14 (11%), thecal sac compression (TSC) without displacement in 19 (16%), epidural cancer without displacement or compression in 21 (17%) and epidural cancer in 69 (56%). Depending on the definition of clinically important metastatic ESCC, the authors estimate that the CT scan was positive in between 11% and 44% of episodes (any epidural disease). In their analysis of predictors, the authors took clinically important metastatic ESCC to be TSC or thecal compression (equivalent to 33 of 123 episodes, 27%).
In univariate analysis, predictors for a positive CT index scan for clinically important metastatic ESCC were: known bone or vertebral metastases for ≥ 1 year; metastatic breast cancer at initial diagnosis; previous spine radiotherapy; objective weakness; increased deep tendon reflexes; and abnormal plantar reflex. These were weak predictors; the best was objective weakness with specificity of only 67% and positive predictive value of 40%. Given these data it is possible to populate a 2 × 2 table for this predictor, as shown in Table 15.
Some variables tested occurred so infrequently in the population that they could not reach statistical significance as predictors.
In multiple logistic regression analysis, four independent predictors of TSC were identified as follows: known bone metastases ≥ 2 years (OR 3.0, 95% CI 1.2 to 7.6; p = 0.02); metastatic disease at initial diagnosis (OR 3.4, 95% CI 1.0 to 11.4; p = 0.05); objective weakness (OR 3.8, 95% CI 1.5 to 9.5; p = 0.005); and vertebral compression fracture on spine radiograph (OR 2.6, 95% CI 1.0 to 6.5; p = 0.05). These four predictors stratified episodes into subgroups with widely varying risk of positive TSC scans, ranging from 12% (no risk factors) to 85% (≥ 3 risk factors) as shown in Table 16. Of the 123 episodes, 11% were associated with three or more risk factors relative to a prevalence of 27% for TSC.
| Number of significant predictors | Number of episodes | Number of episodes with TSC | Number of episodes with no TSC | LR with TSC/without TSC | Post-test probability of TSCa |
|---|---|---|---|---|---|
| 0 | 33 (0.27) | 4 (0.12) | 29 (0.32) | 0.376 | 0.121 |
| 1 | 52 (0.42) | 9 (0.27) | 43 (0.48) | 0.571 | 0.173 |
| 2 | 25 (0.20) | 9 (0.27) | 16 (0.18) | 1.534 | 0.360 |
| 3 or 4 | 13 (0.11) | 11 (0.33) | 2 (0.02) | 15.00 | 0.850 |
| Total | 123 (1.0) | 33 (1.0) | 90 (1.0) |
Author conclusions
The results suggest that evaluation of breast cancer patients with suspected SCC might include clinical information about disease course, in addition to neurological examination and previous imaging studies. If confirmed, these predictors may help clinicians to assess risk in this patient population.
Reviewer conclusions
A combination of three or four of the four identified risk factors predicted TSC with a probability of 85% in this population of breast cancer patients. In the current context the presence of any two of these would probably lead to physicians requesting MRI examination, and so their predictive utility would appear to be limited as these patients are obvious cases for further imaging examination. The study by Talcott et al. 131 examined CT scans for suspected SCC over the same period (1985–8) at the same institute; Talcott et al. included several primary cancers, of which breast cancer represented 42% of 258 patients (n = 108); it appears that data for most or all of the patients in Lu et al. 120 were also included in the Talcott study. 131
McCloskey et al. (1993)122
Relevant aim
The aim was to develop a radiological method to assess vertebral deformity in women and to employ the method to estimate the incidence and prevalence of vertebral deformity in a population of women with breast cancer.
Design and method
This study used radiographs of the spinal vertebrae of 100 randomly selected normal women (aged 45–50 years) to measure the anterior, posterior and central heights of vertebrae T4 to L5; these measures were termed A, P and C. The predicted posterior height (PP) of a given vertebra was estimated using the mean p-values for adjacent vertebrae (for the population of 100 women). The predicted height for each vertebra (for 100 women) could then be compared with its measured height (for 100 women) to give a ratio and its SD. For the posterior height, this was termed the posterior to predicted posterior height (P/PP) ratio. All P/PP ratios were very close to unity (listed as 1.000) and SDs were mostly in the range 0.04–0.06 and varied somewhat between each specific vertebra. This indicated that estimating the predicted height of a vertebra from the heights of normal adjacent vertebrae was a reasonably accurate procedure. The ratios A/P and C/P and their SDs were also calculated. The authors tested the within- and between-observer reproducibility of the procedure and judged that it was satisfactory.
The ratios P/PP, A/P and C/P were plotted in a normal probability plot and each was shown to have a normal distribution. Given these ratios and their normal distributions, the authors defined criteria for the presence of deformity in a given vertebra in terms of how many SDs the ratios for a scrutinised vertebra departed from the mean observed among the 100 normal women (taking the SD specific for that vertebra as determined for the 100 normal women). The number of standard variations required (e.g. 2.5, 3, 3.5, 4, 4.5) could be varied in determining the presence or absence of deformity and so various cut-off thresholds for deformity could be investigated. Criteria for four types of deformity (central collapse, anterior wedge, posterior wedge and crush deformity) were defined in terms of the combination of ratios required to be satisfied according to appropriate SDs for different cut-offs.
An estimate of the specificity of a particular cut-off threshold was made by examining the vertebrae of the 100 normal women and assuming a prevalence of zero and that a vertebra below the mean according to the defined threshold represented a false-positive (Figure 8 and cell B in Table 17). This allowed the population of cells B and D in the 2 × 2 table and thence the calculation of specificity.
FIGURE 8.
Illustration of the proportion of false-positives among normal vertebrae at a defined threshold.
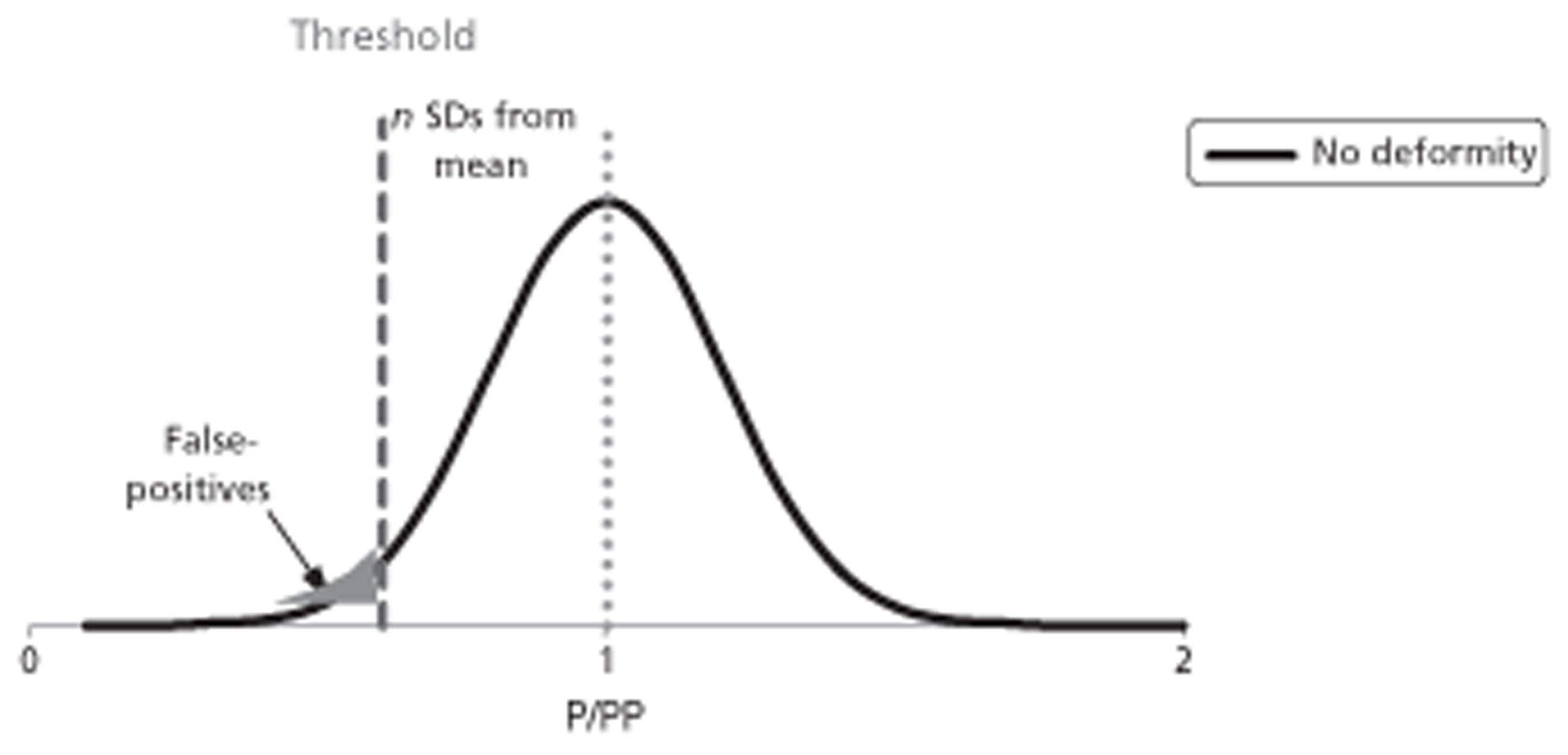
| Deformity present | Deformity absent | ||
|---|---|---|---|
| Test positive | A | B | |
| Test negative | C | D | |
| Total | 0 | 100 | 100 |
Vertebrae of 163 women with breast cancer (aged 30–75 years) and with skeletal metastases were examined and classified according to the presence of vertebral deformity using A/P, C/P, P/PP ratio criteria and five SD cut-offs ranging from 2.5 to 4.5. After 6 months the vertebrae were re-examined in 121 of these women and newly developed deformities were estimated. These evaluations allowed estimates of point prevalence and 6-month incidence rates of vertebral deformity for this population. For the breast cancer population false-positives (cell B in Table 17) were defined as those with ratios above the mean (for normal women) by a defined number of SDs (depending on the threshold cut-off).
Results
For normal vertebrae the P/PP ratio was normally distributed with a mean of 1.00; SDs of the P/PP ratio varied from vertebra to vertebra, suggesting the need to use site-level criteria in determining the presence of deformity. The SDs for the P/PP ratio were similar whether the PP was made using measures from one or from four adjacent vertebrae (un-deformed). Using a cut-off of 3 standard deviations, the prevalence of vertebral deformity in women with breast cancer and skeletal metastases was 46%.
Author conclusions
The technique developed for assessment of vertebral deformities is robust and rapid, and has minimal effects on sensitivity while maximising specificity. The method was able to detect minor vertebral deformities, which subsequently progress, and there is a close relationship between existence of deformities and subsequent rate of deformity in breast cancer.
Reviewer conclusions
Radiography coupled with vertebral measurements and the use of the criteria developed by the authors allowed specific detection of vertebral deformity in women with breast cancer and skeletal metastases. Such detection before the development of frank neurological involvement could be useful. Radiography of the spine is not now used in the comprehensive way reported in this study and whether the procedures developed could be applied using CT or MRI images is uncertain; however, the loss of ‘length’ dimension in a diseased vertebra, relative to the value of that dimension expected from measurement of healthy adjacent vertebrae, is currently used to determine if fracture is present.
Oka et al. (2006)123
Relevant aim
This study attempted to identify prognostic factors for bone metastases.
Design and method
The main focus of this study was to provide basic data on the incidence of bone and spinal metastases and SCC of Japanese breast cancer patients treated with endocrine therapy or chemotherapy following primary surgery in a single institution and to calculate the survival rate after breast surgery, bone or spinal metastasis, and paralysis due to cord compression, using the Kaplan–Meier analysis.
This was a retrospective study of 695 patients with breast cancer (four men, 691 women) who underwent radical surgery at a single centre between January 1990 and December 1996; mean age at surgery was 53.1 (range 24–88) years. Forty-two patients had other concurrent cancer. One hundred and three patients had visceral metastases at baseline and 592 had no visceral metastases.
Various imaging methods were employed including bone scintigraphy, chest radiography, chest CT, liver ultrasonography, abdominal CT, cranial CT and MRI (or any combination of these). A Cox proportional hazards model was used to identify prognostic factors for the development of skeletal metastases.
Results
Bone metastases were observed in 148 patients at the end of the observation period (all received chemotherapy; 44 patients received endocrine therapy before the metastases). The survival of these patients after surgery was much worse than that of the 547 patients who did not develop skeletal metastases (5-year survival 45.8% vs. 89.9%). The interval between surgical treatment and the development of bone metastases ranged from 0 to 130 months (median 19 months). Kaplan–Meier analysis indicated that after surgical treatment of breast cancers, bone metastases developed in 18.1% of the patients over 5 years and in 24.7% of the patients over 10 years (Figure 9).
FIGURE 9.
Time to development of skeletal metastases after surgery for breast cancer. Data read from published graph (Oka 2006123); Weibull distribution fitted to the first 10.5 years of data. Number at risk at t0 assumed to be 605.
The risk factors for development of bone metastases identified using Cox's regression were tumour stages evaluated by TNM classification (HR 1.615, 95% CI 1.322 to 1.973; p < 0.0001); nodal (N) stage classification (HR 2.128, 95% CI 1.381 to 3.279; p = 0.0006); the presence or absence of metastases to axillary lymph nodes (p = 0.0006); and the presence or absence of metastases to important organs (HR 7.502, 95% CI 5.100 to 11.036; p < 0.0001). By the end of the observation period spinal metastases were observed in 121 of the 148 patients who developed skeletal metastases, and 17 out of 121 of these developed paralysis due to SCC. The time between detection of skeletal metastases and development of SCC ranged from 2 to 72 months with a median of 4.4 months.
The study investigated factors prognostic for survival after surgery and survival after development of skeletal metastases. Postsurgery prognostic factors included tumour stages evaluated by TNM classification (HR 1.346, 95% CI 1.099 to 1.648; p = 0.004); N stage classification (HR 1.524, 95% CI 1.030 to 2.257; p = 0.03); the presence or absence of metastases to axillary lymph nodes (p = 0.03); presence or absence of metastases to important organs (HR 3.356, 95% CI 2.226 to 5.060; p < 0.0001); presence or absence of oestrogen receptors (HR 1.686, 95% CI 1.102 to 2.580; p = 0.02); presence or absence of progesterone receptors (HR 1.954, 95% CI 1.274 to 2.997; p = 0.002); and the presence or absence of bone metastases (HR 3.704, 95% CI 2.415 to 5.682; p < 0.0001).
Prognostic factors for survival after development of bone metastases included the presence or absence of metastases to important organs (HR 2.379, 95% CI 1.484 to 3.815; p = 0.0003) and the presence or absence of progesterone receptors (HR 2.689, 95% CI 1.553 to 4.657; p = 0.0004). Statistically, there were no factors significantly associated with the prognosis of breast cancer patients with paralysis due to cord compression (only 17 patients available for analysis).
Author conclusions
To detect predictive factors of long survival after paralysis and establish indications for surgery, a comparative study among large groups of patients with paralysis and with different backgrounds is needed.
Reviewer conclusions
Risk factors for the development of skeletal metastases were tumour stage (TNM classification), N stage classification, metastases to axillary lymph nodes and visceral metastases. The prognostic factors for survival after development of bone metastases were visceral metastases and presence of progesterone receptors.
Plunkett et al. (2000)24
Relevant aim
The aim was to identify factors that predict complications from skeletal disease in patients with bone metastases from advanced breast cancer.
This was a retrospective study of breast cancer patients with bone metastases identified between 1975 and 1991 at one centre. Of 1437 patients so identified, 111 were followed up elsewhere and 460 were diagnosed elsewhere and their records contained too little information for inclusion in the study. The notes for 7 patients (0.5%) were not found. The remaining 859 patients were included. Patients were divided into four groups according to sites of disease at the time of diagnosis of bone metastases as follows: (1) bone metastases only (n = 243, 28%); (2) bone and soft tissue disease only (n = 268, 31%); (3) bone and pleuropulmonary disease, with or without soft tissue disease (n = 237, 28%); (4) bone and liver metastases, with or without soft tissue or pleuropulmonary disease (n = 111, 13%). Patients were monitored with scintigraphy and radiography; myelography was not mentioned.
Results
The time from diagnosis of skeletal metastases to vertebral fracture was shortest in the bone-only group (p < 0.0017). Figure 10 illustrates the shape of the reported Kaplan–Meier plots for each group. In addition, patients with bone-only disease developed SCC more rapidly than patients in other groups (p = 0.01). Thirty-six patients with bone-only disease at diagnosis of bone metastases (15%) developed cord compression compared with 2–6% of patients in the other groups. Bone scan evidence of metastases in the spine did not predict for subsequent development of cord compression.
FIGURE 10.
Time to development of skeletal fracture according to patient's subgroup.
Survival from diagnosis of bone metastases was significantly greater for patients with bone disease only at diagnosis of skeletal metastases (p < 0.001), and was shortest for patients with concomitant liver metastases (median survival 5.5 months).
There were no differences between the groups in the time to pathological long bone fractures. However, since patients with bone disease only at diagnosis of skeletal disease lived longest, most fractures occurred in this group. Of a total of 243 such patients, 42 (17%) developed a pathological long bone fracture (i.e. 1 in 5.8 patients), compared with 5 of 111 shorter-lived patients with bone and liver disease (5%) (i.e. 1 in 22.2 patients).
Author conclusions
The results suggest that patients with disease confined to the skeleton at the diagnosis of bone metastases are most likely to develop skeletal-related complications from advanced breast cancer. Such patients may benefit most from treatment with bisphosphonates.
Reviewer conclusions
The study does not give detailed information regarding participants such as age or time since diagnosis. In breast cancer patients diagnosed with bone metastases, longer survival is a risk factor for vertebral fracture and for SCC, and longer survival is associated with lack of disease at sites additional to the skeleton, especially liver disease and to a lesser extent pleuropulmonary disease.
Snyder et al. (2005)127
Relevant aim
The aim was to investigate if prognostic factors identified ex vivo using structural rigidity analysis of transaxial CT image data predict in vivo vertebral fracture in cancer patients with spinal metastases more effectively than contemporary clinical and radiographic guidelines published by Taneichi et al. 89
Design and method
This study describes the development of the authors' biomechanical model for prediction of fracture. The model estimates the load-bearing capacity of vertebrae using transaxial CT scans. It was tested in 106 women with breast cancer metastatic to the spine who were followed up for 4 months after CT scan to monitor vertebral fracture (4 months was selected as a sufficiently short period for the influence of any tumour progression that might occur to minimally affect risk).
The fracture risk index (FRI) was calculated for each vertebra between T8 and L5 using two different load scenarios for each patient: (1) lifting a 10-kg mass and (2) rising from a chair. A FRI > 1 predicts that fracture would occur during the applied load condition. The accuracy of FRI was compared with the radiographic criteria according to the Taneichi et al. guidelines,89 which predict fracture on the basis of size and location estimates of the spinal tumour. Investigators blinded to the predictions used MRI and radiography to establish the incidence of actual fractures using standard criteria.
Results
Over the 4-month period, 10 out of 106 patients suffered one or more new vertebral fractures. Both the CT-based structural rigidity analysis and the Taneichi criteria predicted that these 10 patients were at increased fracture risk (sensitivity = 100% for either method, threshold set so there are no false-negatives).
However, the CT rigidity analysis was better at predicting which patients would not fracture an affected vertebra (specificity = 49% when FRI > 1 for lifting a 10-kg mass) compared with the Taneichi CT criteria (specificity = 20%). With sensitivity at 100%, negative predictive ratios are not calculable (infinitely large). The calculated positive LRs were modest for both methods (1.96 and 1.25, respectively), as also were the positive predictive ratios (0.17 and 0.11).
When the load-carrying capacity of the vertebra was normalised by the patient's body mass index (BMI; kg/m2) and the threshold for predicting vertebral fracture set to achieve 100% sensitivity, the specificity for predicting no vertebral fracture was improved to 69% (i.e. a false-positive rate of 31%). This is illustrated in purely diagrammatic form in Figure 11. The negative LR becomes 0.25 and positive LR 3.2.
FIGURE 11.
Diagrammatic representation of threshold set to 100% sensitivity and 69% specificity. The dashed vertical line represents the load capacity threshold for discriminating predicted fracture and non-fracture.
By logistic regression the estimated RR for fracture based on FRI > 1 was 4.2 (95% CI 1.4 to 12.8; p < 0.001). When controlling for BMI (kg/m2), the adjusted RR for fracture based on FRI > 1 was 7.9 (95% CI 1.8 to 34.5; p < 0.001).
Author conclusions
A CT-based structural rigidity analysis was as sensitive but significantly more specific than the best radiographic guidelines for estimating metastatic cancer vertebral fracture risk.
Reviewer conclusions
The paper has inadequate information in terms of patient population. The number of events (10 patients with fractures) was small. The study compares sensitivity and specificity of CT-based structural rigidity analysis against available guidelines (Taneichi et al. 89); neither method performed well in this population.
Snyder et al. (2009)128
Relevant aim
These authors aimed to compare CT-based structural rigidity analysis with current standards for prediction of spinal fracture in women with breast cancer with spinal metastases. The current standard is implied to be plain radiography used with an empirically derived logistic regression analysis based on size and location of vertebral metastases observed by axial CT scanning (Taneichi's algorithm).
Design and method
The records of 1024 breast cancer patients at a single institute were reviewed to identify study participants. Ninety-four patients were included; patients were excluded if records indicated neural compromise (due to metastases in brain or spinal cord), withdrawal, relocation, death, previous fracture at metastatic or adjacent site, surgical treatment for impending fracture or fractured bones due to significant trauma. Fifty-one per cent of included patients were postmenopausal, many with co-existent osteopenia. It was unclear when CT and radiographic examinations were conducted relative to time of selection into the study.
Axial CT scans were used to estimate rigidity, a product of bone tissue modulus and geometry. It had been previously established (in an ex vivo study) that the force needed to fracture vertebrae is proportional to the weakest cross-section through the affected bone; thus, the scans were used to identify the cross-sectional structural rigidity with weakest resistance to axial (EA), or bending (EI) loads (that is the minimal axial load and bending load rigidities for each vertebra). From this, the load-bearing capacity (LBC) of the vertebra in combined axial compression and forward bending was also estimated using ‘beam theory’. The LBC was standardised on BMI (kg/m2) (LBC/BMI). The rate of fractures over the next 4 months was recorded by independent investigators.
Results
The value for each of the four parameters (EI, EA, LBC, LBC/BMI) in each of the 247 vertebrae was estimated. There were 11 fractures over 4 months (236 vertebrae did not fracture). Fractures were distributed as shown in Table 18.
| Group | Vertebral level | Number involved | Number fractured | Percentage fractured |
|---|---|---|---|---|
| 1 | T8 | 33 | 0 | 0 |
| 2 | T9–L1 | 93 | 10 | 11 |
| 3 | L2–L5 | 82 | 1 | 1 |
| 4 | L5 | 39 | 0 | 0 |
| Total | T8–L5 | 247 | 11 | 4 |
The value for each of the four parameters in each of the 247 observed vertebrae was calculated. From these values, for each parameter the maximum value for the 11 fractured vertebrae was selected as the diagnostic threshold for that parameter. For example, for LBC/BMI, the maximum value among fractured vertebrae was 46.5. As all other fractured vertebrae had values < 46.5, using this as the threshold meant that all fractures would be detected; the resulting sensitivity was 100%. Of the 236 unfractured vertebrae, 74 also had a LBC/BMI < 46.5, giving a specificity of 68.6% [(236 − 74)/236, which was reported as 70%, possibly based on patients rather than vertebrae]. Should a successful treatment be available that prevented fracture, these results imply a number needed to treat of ≈ 7.7.
Using the same procedure for LBC, EI and EA, the specificities were 44%, 53% and 55% (all sensitivities at 100%), respectively. Using Taneichi's algorithm and sensitivity set at 100%, specificity was only 20% (i.e. very many false-positives).
The authors provided a receiver operating characteristic (ROC) curve for LBC/BMI showing how sensitivity and specificity were affected by changing (reducing) the value of the cut-off (Figure 12). Hence, as cut-off fell below 46.5, < 100% of the fractures were detected, but there were fewer false-positives and so specificity improved. The area under the ROC curves was estimated using a binomial semi-parametric model. The results were Taneichi, 0.6; LBC, 0.82; EI, 0.80; EA, 0.68; LBC/BMI, 0.84. Corresponding p-values for the comparison with chance (tossing a coin; area under the curve = 0.5) were 0.25, 0.001, 0.001, 0.002 and < 0.001, respectively.
FIGURE 12.
Receiver operating characteristic curves for LBC/BMI and radiological prediction. Data read from published graph (Synder 2009128).
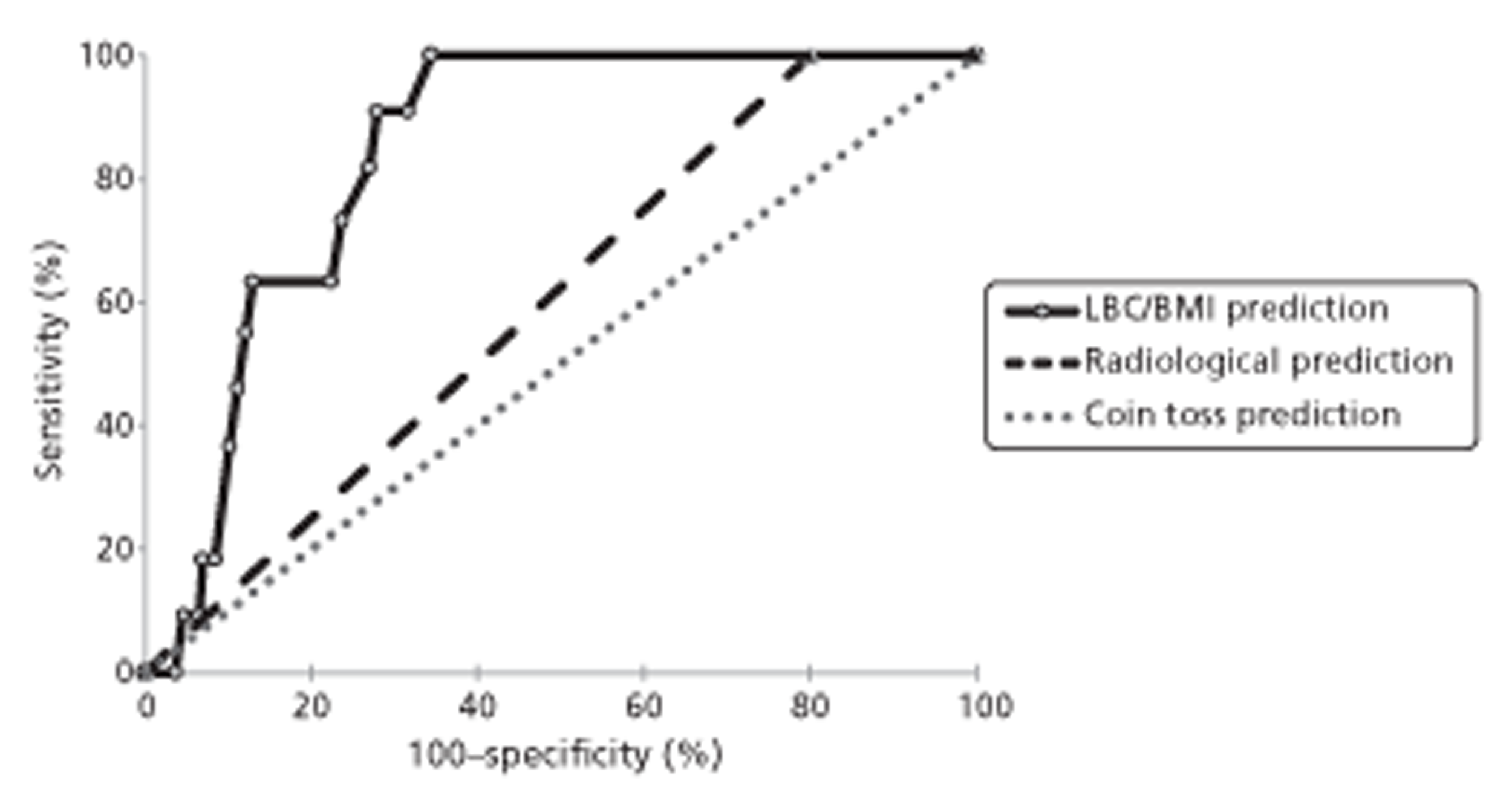
Author conclusions
The CT-based structural rigidity analysis is as sensitive as, and significantly more specific than, current radiographic criteria for predicting vertebral fracture in breast cancer.
Reviewer conclusions
Patient selection was not fully described; it is possible that sensitivities and specificities could vary depending on stage of vertebral invasion by metastases, so selection of participants is important. From T8 to L5 for 94 women provides at least 658 potential vertebrae examined; 247 were used for parameter calculations, but it was not reported how these were selected (i.e. whether these were all those identified with metastases or a proportion of them). It is unclear if the patients in this study overlapped with those in the Snyder 2005127 study considered above. There were only 11 events (fractures); to retain 100% sensitivity with more events would probably require moving the threshold to a higher value, thereby probably increasing the rate of false-positives and reducing specificity.
Summary of breast cancer studies
None of the studies described the natural history of spinal metastases derived from breast cancer.
The seven included studies were disparate in terms of population, imaging procedures and study aims. In the study by Harrison et al. ,112 participants with suspected SCC underwent myelography and an attempt was made to identify risk factors associated with positive and negative myelography tests. Lu et al. 120 examined 93 patients with suspected SCC and identified clinical and oncological features associated with a positive CT scan for SCC. Oka et al. 123 searched for risk factors associated with development of bone metastases in 695 breast cancer patients, and another study (Plunkett et al. 24) looked for factors associated with skeletal events in breast cancer patients with bone metastases. McCloskey et al. 122 investigated how dimensional measures (e.g. vertebral height) made in vertebrae with metastases and in adjacent intact vertebrae could be used in the diagnosis of vertebral fracture/collapse, while the two biomechanical studies (Snyder et al. 127,128) examined the power of vertebral LBC estimates for predicting vertebral fracture, comparing the specificity of the method with that of Taneichi et al. 89
The results from Harrison et al. 112 imply that a positive bone scan, back pain, paraesthesia and bladder/bowel dysfunction at the time of myelography were more common in patients with a positive myelogram than in those with a negative myelogram. Another study, by Lu et al. ,120 found that objective weakness in patients with suspected SCC was predictive for SCC; however, the calculated estimates of sensitivity and specificity were very modest. Stratification of patients suspected of having SCC according to the number of independent risk factors identified a high-risk group with an 85% probability of CT-positive SCC. Oka et al. 123 identified T stage (TNM classification), N stage classification, metastases to axillary lymph nodes and visceral metastases as risk factors for the development of skeletal metastases. In breast cancer patients diagnosed with bone metastases, one study, by Plunkett et al. ,24 observed that longer survival was found to be a risk factor for vertebral fracture and for SCC.
According to Snyder et al. ,127,128 the ‘vertebral load bearing capacity algorithm’ developed by the authors had superior specificity to the method used by Taneichi et al. 89 for predicting vertebral collapse.
The included studies generally provided limited information about the patient population and selection criteria. Results from time-to-event analyses are difficult to generalise because of the different populations studied and uncertainty regarding their representativeness.
Studies in which the whole sample population was diagnosed with lung cancer (non-small cell lung cancer or small cell lung cancer)
Sekine et al. (2009)125
Relevant aim
The stated aim was to identify the risk factors for SREs in patients with advanced NSCLC.
Design and method This was a retrospective study of 642 NSCLC patients. According to the report, for inclusion in the study, patients required a histological or cytological diagnosis of NSCLC, stage IV disease or postoperative recurrence with distant metastases, and ‘no prior chemotherapy’ or ‘chemotherapy prescribed by the National Cancer Center Hospital between 2000 and 2006’. These criteria may therefore have excluded patients who received certain sorts of chemotherapy not prescribed by the National Cancer Center Hospital between 2000 and 2006. However, all 642 patients were described as having received first-line chemotherapy as follows: platinum based, n = 429; gefitinib (Iressa®, AstraZeneca), n = 117; third-generation monotherapy, n = 47; non-platinum doublets, n = 9. Patients were excluded if they had postoperative local recurrence without distant metastases. Forty-three patients received zoledronic acid (Aclasta®; Novartis) either before (n = 26) or after (n = 17) the development of SREs.
At initial diagnosis 399 had no bone metastases, 63 had a single bone metastasis and 180 had multiple bone metastases. Disease progression was observed in 580 out of 642 patients; the initial site of progression was bone in 78 and other than bone in 502.
SREs were defined as (1) pathological fractures, (2) SCC, (3) requirement for radiation therapy, (4) requirement for surgery to the bone, (5) requirement for radiological intervention to the bone, and (6) hypercalcaemia of malignancy that was either fatal or required emergency treatment. Association of baseline characteristics with development of SREs was examined in univariate analysis and multivariate logistic regression. Cox's proportional hazards model was used to identify risk factors for time to event. Kaplan–Meier analysis was used to investigate the time to first SRE after commencement of chemotherapy.
Results
A total of 118 (18.4%) patients developed SREs during or after initial chemotherapy (107 required radiotherapy to bone, five developed hypercalcaemia of malignancy, three developed compression fracture of vertebrae, two required surgical treatment of the bone and one underwent radiofrequency ablation therapy to bone).
In univariate analysis the number of bone metastases (none, single or multiple) at initial diagnosis (p < 0.001) and history of radiotherapy to bone before chemotherapy (p = 0.001) were associated with the development of SREs, whereas sex, age, performance status and cancer histology were not (Table 19). However, multivariate analysis using a logistic regression model showed that the number of bone metastases was strongly associated with the occurrence of SREs (OR 3.08, 95% CI 1.60 to 5.94, for single bone metastasis; OR 4.27, 95% CI 2.66 to 6.86, for multiple bone metastases), whereas radiotherapy to the bone before the chemotherapy was not (OR 1.43, 95% CI 0.69 to 2.97).
| Characteristics | Patients without SREs | Patients with SREs | p-value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Number of patients | 524 | 81.6 | 118 | 18.4 | |
| Sex | |||||
| Male | 325 | 80.8 | 77 | 19.2 | 0.53 |
| Female | 199 | 82.9 | 41 | 17.1 | |
| Age, median (range) | 61 | 24–86 | 59.5 | 26–77 | 0.083 |
| Performance status | |||||
| 0 | 163 | 82.7 | 34 | 17.3 | 0.68 |
| 1 | 335 | 81.5 | 76 | 18.5 | |
| 2–3 | 26 | 76.5 | 8 | 23.5 | |
| Histology | |||||
| Adenocarcinoma | 419 | 80.6 | 101 | 19.4 | 0.16 |
| Non-adenocarcinoma | 105 | 86.1 | 17 | 13.9 | |
| Bone metastases | |||||
| None | 358 | 89.7 | 41 | 10.3 | < 0.001 |
| Single | 46 | 73.0 | 17 | 27.0 | |
| Multiple | 120 | 66.7 | 60 | 33.3 | |
| Radiotherapy to the bone before chemotherapy? | |||||
| No | 499 | 82.9 | 103 | 17.1 | 0.001 |
| Yes | 25 | 62.5 | 15 | 37.5 | |
Of patients who had no bone metastasis at diagnosis, only 10.3% developed SREs, whereas 27% of patients with a single bone metastasis and 33% of patients with multiple bone metastases developed SREs during their clinical course (p < 0.001); the median follow-up for SREs was 10.4 (range 0.1–77) months.
In univariate analysis the time to development of SREs was associated with sex, performance status, number of bone metastases at diagnosis and history of radiotherapy to bone before chemotherapy. However, on multivariate analysis a history of radiotherapy to bone was not associated with the development of SREs (Table 20).
| Analysis | HRs (95% CI) | |
|---|---|---|
| Time to the first SRE | Univariate | Multivariate |
| Sex | ||
| Female | 1 | 1 |
| Male | 1.47 (1.01 to 2.15) | 1.44 (0.98 to 2.11) |
| Performance status | ||
| 0 | 1 | 1 |
| 1 | 1.43 (0.96 to 2.15) | 1.15 (0.76 to 1.74) |
| 2–3 | 3.73 (1.71 to 8.14) | 2.21 (0.97 to 5.03) |
| Bone metastases | ||
| None | 1 | 1 |
| Single | 3.26 (1.85 to 5.75) | 3.00 (1.68–5.35) |
| Multiple | 4.98 (3.33 to 7.44) | 4.43 (2.91 to 6.76) |
| Radiotherapy to the bone before chemotherapy? | ||
| No | 1 | 1 |
| Yes | 3.39 (1.97 to 5.86) | 1.39 (0.77 to 2.49) |
Kaplan–Meier analysis indicated that the time to first SRE was shorter for those with multiple bone metastases at diagnosis than those with a single metastasis or none; the relationship published is illustrated in Figure 13.
FIGURE 13.
Time from start of chemotherapy to first skeletal event according to bone metastases at diagnosis. Data read from published graph (Sekine 2009125). Patients with no event by end of follow-up were censored at that time.
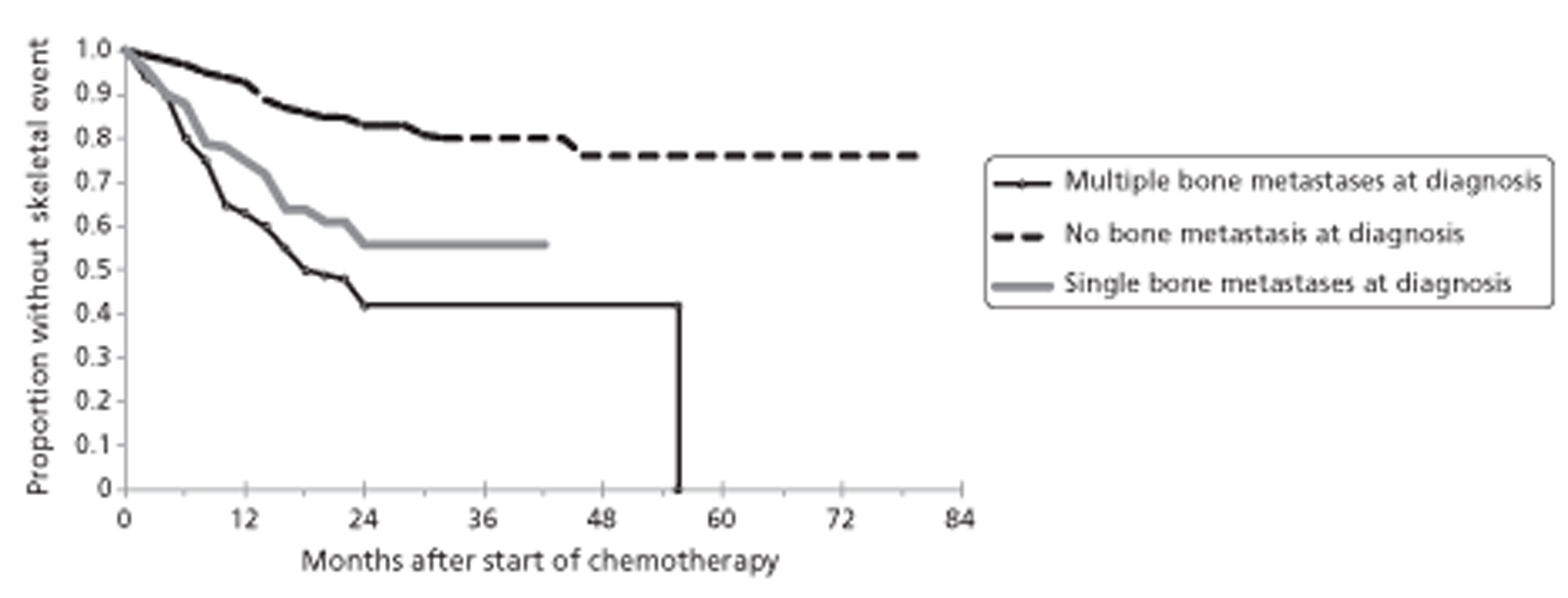
For the analysis of SRE-free survival, a SRE or death was taken as an event and patients without an event by end of follow-up were censored. In multivariate analysis, SRE-free survival was strongly associated with performance status (compared with zero performance status: HR 1.47, 95% CI 1.15 to 1.89, for performance status 1; and OR 3.72, 95% CI 2.31 to 5.98, for performance status 2 or 3). The median SRE-free survival was 23.5 months (95% CI 18.6 to 28.5 months) in patients with performance status of 0, 13.1 months (95% CI 10.4 to 15.8 months) in patients with performance status of 1 and 5.2 months (95% CI 1.0 to 9.4 months) in patients with performance status of 2 or 3 (p < 0.001). To a lesser extent male sex and multiple bone metastases at diagnosis were also indicators of poor SRE-free survival.
Author conclusions
The presence of multiple bone metastases was significantly associated with the development of SRE in patients with advanced NSCLC treated by systemic chemotherapy. The factor ‘multiple bone metastases’ was identified as a risk factor for the development of SREs as assessed by all three parameters, and was, therefore, considered as a definite risk factor for the development of SREs. Male sex and poor performance status may be additional risk factors for the development of SREs in these patients. Male sex and poor performance status were significant risk factors influencing the SRE-free survival, marginally significant in relation to the time to the first SRE, and not significant in relation to the presence of SRE.
Reviewer conclusions
This was a large study with homogeneous mixed cases and more statistical power than many others. The definition of SRE included a number of clinical presentations so it is difficult to distinguish the number of occurrences related to spines. The study does not report number of spinal metastases. A small proportion of participants used bisphosphonates, a drug that prevents loss of bone mass/delayed SREs. It is probably not a surprising finding that the greater the number of bone metastases, the greater the risk of a SRE.
Sun et al. (2011)130
Relevant aim
The study aimed to identify clinical factors that can predict SREs in patients with advanced NSCLC.
Design and method
Patients were identified from medical records of consecutive diagnoses of advanced NSCLC at a single centre between January 2006 and March 2009 (n = 1166). From these, 273 patients with bone metastases secondary to NSCLC were identified from imaging (e.g. scintigraphy and PET) and biopsy records. Clinical data were obtained from the date of primary diagnosis to 31 October 2009; median follow-up was 11 (range 0.7–46.0) months. Of the 273 included patients, 242 were diagnosed with bone metastases at the time of NSCLC diagnosis. Bone metastases were found at multiple locations in most patients. A total of 528 locations were identified (221 to the spine).
The authors investigated the following potential risk factors for their association with SREs: sex; ever a smoker; adenocarcinoma/non-adenocarcinoma; no history of therapy with an epidermal growth factor receptor tyrosine kinase inhibitor (EGFR TKI) such as gefitinib; Eastern Cooperative Oncology Group (ECOG) status; BMI (kg/m2); and age. These were evaluated in univariate analysis and multivariate logistic regression for an association with the risk of experiencing a first SRE (i.e. at least one event), for the time to first SRE using Kaplan–Meier methods, and for the risk of recurrent SREs (> 21 days after the preceding event) using survival-adjusted multiple event analysis. 137
Results
Of the 273 patients analysed, 171 experienced at least one SRE, and 46 had multiple SREs. A total of 229 SREs developed of which 65 occurred before any systemic treatment was received. The most frequent site of SREs was the spine (55.2%). The pattern of SREs was complex: radiotherapy in 169 cases (73.9%), cord compression with vertebral fracture in 14 cases (6.1%), cord compression without definitive vertebral fracture in 14 cases (6.1%), pathological fracture in 30 cases (13.1%), and one case each of prophylactic surgery for impending fracture and hypercalcaemia (0.8%).
In multivariate analysis, only ‘ever smoked’ was associated with significantly higher SRE risk (OR 2.8, 95% CI 1.32 to 6.00). The same result was obtained if patients who received bisphosphonate therapy were omitted from the analysis. For median time to first SRE in multivariate analysis (Table 21) the following variables were associated with shorter median time to first SRE: no history of therapy with a EGFR TKI such as gefitinib; ever smoked; and histology of non-adenocarcinoma.
| Characteristic | Number of patients (%) | Time to event (months) | Univariate, p-value | Multivariate HR (95% CI) | p-value | ||
|---|---|---|---|---|---|---|---|
| Total | 273 | (100) | 8.9 | ||||
| Sex | |||||||
| Male | 164 | (60.1) | 6.3 | 0.15 | NA | – | |
| Female | 109 | (39.9) | 11.6 | ||||
| Age | |||||||
| 50 years | 196 | (71.8) | 8.2 | 0.64 | NA | – | |
| < 50 years | 77 | (28.2) | 10.1 | ||||
| BMI (kg/m2) | |||||||
| ≥ 25 | 67 | (24.5) | 10.4 | 0.18 | NA | ||
| 20 to < 25 | 166 | (60.8) | 10.1 | ||||
| < 20 | 40 | (14.7) | 3.9 | ||||
| Smoking | |||||||
| Ever | 138 | (50.5) | 5.2 | 0.004 | 1.75 | (1.05 to 2.92) | 0.03 |
| Never | 135 | (49.5) | 11.6 | ||||
| Performance status | |||||||
| ECOG 0, 1 | 209 | (76.6) | 10.0 | 0.23 | NA | ||
| ECOG 2, 3 | 64 | (23.4) | 5.3 | ||||
| Histology | |||||||
| Non-adenocarcinoma | 72 | (26.4) | 3.1 | < 0.001 | 1.59 | (1.14 to 2.22) | 0.007 |
| Adenocarcinoma | 201 | (73.6) | 11.4 | ||||
| History of EGFR TKI therapy | |||||||
| No | 81 | (29.7) | 3.3 | < 0.001 | 2.12 | (1.49 to 3.00) | < 0.001 |
| Yes | 192 | (70.3) | 11.8 | ||||
The same three factors (no history of therapy with a EGFR TKI, ever smoked and histology of non-adenocarcinoma) and also ECOG status 2/3 were significantly associated with increased risk of multiple events (separated by at least 21 days) (Table 22).
| Characteristics | HR | 95% CI | p-value |
|---|---|---|---|
| Ever a smoker (vs. never a smoker) | 1.601 | 1.034 to 2.479 | 0.035 |
| Non-adenocarcinoma (vs. adenocarcinoma) | 1.498 | 1.116 to 2.011 | 0.007 |
| Performance status ECOG 2, 3 (vs. ECOG 0, 1) | 1.458 | 1.074 to 1.980 | 0.016 |
| No history of treatment with EGFR TKI (vs. history of treatment with EGFR TKI) | 1.937 | 1.428 to 2.627 | < 0.001 |
| Female (vs. male) | 1.382 | 0.879 to 2.170 | 0.161 |
Significantly more SREs per cycle of treatment occurred during cytotoxic therapy than during EGFR TKI therapy; however, the authors draw attention to the potential pitfall for interpretation in that systemic therapies did not necessarily precede SRE in all cases.
Author conclusions
This study suggests that metastatic NSCLC patients with characteristics such as ever having smoked, no history of EGFR TKI therapy, poor ECOG status and non-adenocarcinoma are more likely to suffer SREs.
Reviewer conclusions
SREs appear to have been classified as pathological fracture, SCC with or without vertebral fracture, need for radiation or surgery to bone and hypercalcaemia of malignancy. The risk factors identified may well apply equally to SCC and/or vertebral fracture alone; however, this would need to be investigated using the appropriate narrower definition of an event. This is one of the few studies that considered the risk of repeated events.
Goldman et al. (1989)111
Relevant aim
The aim was to undertake an analysis of medical records to define factors predictive of SCC.
Design and method
This was a retrospective analysis of medical records of patients who participated in an RCT that investigated the effectiveness of chemotherapy regimens for SCLC between 1982 and 1986. In this trial, participants (n = 616) were randomised to four or eight courses of vincristine (Oncovin®, Genus), cyclophosphamide (trade names Endoxan, Cytoxan, Neosar, Procytox, Revimmune) and etoposide [etoposide phosphate or VP-16 (current brand name Etopophos)]. On relapse, patients were further randomised to standard care or to further therapy with doxorubicin INN (trade name Adriamycin; also known as hydroxydaunorubicin) and methotrexate. The cases of SCC (n = 24, 3.8% of 616 patients; age range 42–64 years; 20 male) consisted of those trial participants who according to medical records had a diagnosis of SCC at the start of the trial or who developed SCC during follow-up. SCC was assessed on clinical grounds of signs and symptoms. Of the 24 with SCC, myelography was performed in only 11.
Results
Of 616 patients, 500 had undergone bone scanning at presentation (presumed to be study entry). Among these, scanning was judged to be positive for spinal metastases in 131 patients; 9 of these 131 patients (6.9%) had a diagnosis of SCC at some time. Hence, 15 patients (the 24 patients with SCC minus the 9) had SCC at some time but either did not have a scan or had a negative scan. If all 15 had negative scans for spinal metastases then the percentage of any-time SCC patients who had negative scans at presentation is 4.1%. This is somewhat lower than the 6.9% SCC associated with positive scans for spinal metastases. If the 15 SCC patients not detected with a positive spinal bone scan were proportionately distributed among all non-positive scan patients (616 − 131 = 485) then percentage of any-time SCC patients providing negative scans for spinal metastases reduces to 3.1%, half that for positive scans indicating a likely association of spinal metastases with present or future SCC. Two of the patients who did not have bone scans had plain radiographs showing vertebral collapse.
Of 24 patients (3.9% of 616) who presented with back pain and a positive spinal bone scan, nine (38%) had SCC at some time. Of 32 patients presenting with cerebral metastases, 12.5% had SCC at some time. Of 87 patients who relapsed with cerebral metastases, 8% had SCC at some time.
Author conclusion
Patients with the combination of cerebral metastases and a positive bone scan had a 25% chance of developing SCC. It may be possible to select patients who should receive radiotherapy to the spine to try to prevent the development of this complication.
Reviewer conclusions
This is an early study with only 24 cases of SCC. Not all SCC cases were confirmed by myelography and the study may have predated wide use of CT or MRI. Multiple logistic regression was not performed and chemotherapy (some patients received very heavy loads of cytotoxic agents), a potentially influential confounder for risk of SCC, was not considered; subsequent studies have indicated that such treatments might affect frequency of SCC. The authors' conclusion regarding the combination of positive bone scan and cerebral metastases as a discriminatory risk factor should be viewed with caution. First, as cerebral metastases and SCC could occur at any time during follow-up, there is no assurance that cerebral metastases preceded SCC. Second, as shown in the 2 × 2 table below (Table 23), viewed as a diagnostic test for SCC, the combination (positive bone scan for spinal metastases plus cerebral metastases) has a sensitivity of only 25% based on the reported results. The positive predictive value is 0.25. The positive LR, that is the ratio of those with SCC to those without SCC returning a positive test, is 0.25/0.03 = 8.33. With a prevalence of 4.1% (24/592) this provides a probability of 0.25 (25%) of having SCC should the test result be positive (pre-test odds = 24/592; post-test odds = (24/592) × 8.33 = 0.338; post-test probability = 1.338/0.338 = 0.252). Patients with a positive test would therefore require further imaging before treatment decisions could be safely undertaken.
| SCC | No SCC | Total | |
|---|---|---|---|
| Positive for combination | 6 | 18 | 24 |
| Negative for combination | 18 | 574 | 592 |
| Total | 24 | 592 | 616 |
Summary of lung cancer studies
Two of the three included studies (Sekine et al. 125 and Sun et al. 130) investigated patients with NSCLC and recruited a substantial number of participants (642 with advanced disease and 273 with bone metastases, respectively). Sekine et al. 125 found that the greater the number of bone metastases the greater the risk of a SRE. Sun et al. 130 found that smoking, no history of treatment with EGFR TK inhibitors, poor ECOG status and non-adenocarcinoma were associated with more likely occurrence of SREs. Sun et al. 130 also considered the risk of repeated events.
In an early study by Goldman et al. ,111 616 SCLC patients with and without SCC were investigated. A combination of cerebral metastases and a positive bone scan were reported to provide a post-test 25% probability for developing SCC, an improvement on the pre-test probability of 0.039; however, this result should be viewed with caution because it was unclear if cerebral metastases actually preceded SCC.
These were retrospective studies that depended on retrieval of information from medical records not designed for, and possibly not suitable for, the study questions addressed. Caution is needed in generalising the conclusions across and beyond the included studies. The prognostic factors identified have not been validated in other independent populations.
Studies in which the population was diagnosed with a variety of cancers
Bernat et al. (1983)108
Relevant aim
The study aimed to identify risk factors for SCC so as to distinguish between those who would benefit from myelography and those who would not.
Design and method
This retrospective study examined medical records of patients discharged from two hospitals during a period from 1975 to 1980 and identified patients who (1) had clinically suspected epidural compression from ‘metastatic cancer’ and (2) had undergone myelography to confirm or exclude the clinical diagnosis. Myelograms were considered positive for compression if ≥ 80% of the SAS was obliterated. Patient age ranged from 7 to 85 years.
A total of 133 patients were included. They had diagnoses of carcinoma, sarcoma and lymphoma as follows: lung (n = 40), breast (n = 27), prostate (n = 15), lymphoma (n = 12), colon/rectal (n = 6), melanoma (n = 6), kidney and ureter (n = 5), bladder (n = 3), other (n = 15) and unknown (n = 6) (two patients had primaries at two different sites).
Results
The paper reported that 62 and 71 patients, respectively, had positive and negative myelograms. The ratio of positive to negative images varied with primary cancer; for example, 20 of 27 and 3 of 15 myelograms were positive in breast cancer and prostate cancer patients, respectively. Compressions were commonest in the thoracic region, followed by the lumbosacral and cervical regions. Of compressions to the spinal cord, 30 of 47 were complete and 17 were incomplete; there were 15 patients with primarily cauda equina compression.
Stepwise logistic regression was used to examine the association of 13 variables with a positive myelogram. The precise identity of these variables was unclear. The authors selected the following as the most influential variables (p-values were not reported): positive vertebral plain radiographs; sensory level or dermatomal loss on examination; history of local pain; older age; history of weakness; history of radicular pain; male sex; paraparesis or radicular weakness on examination. These predictors were used to calculate the predicted probability of a positive myelogram (compression) for each patient who had compression (i.e. observed compression) and for each who had no compression (observed negative myelogram). Figure 1 of the paper presented the frequency of patients with predicted probabilities of a positive myelogram ranging in 10% steps of probability from 0–0.09 to 0.9–1. Unfortunately, the number of patients with observed positive myelograms shown in the figure fell short of those reported to have positive myelograms (i.e. 62 positive myelograms reported, 53 patients in the figure); this discrepancy was not explained but it is possible the missing patients represent those with incomplete data for logistic regression.
Author conclusion
Attempts to identify symptoms and signs that might increase diagnostic ability were not successful. Logistic regression analysis was used to separate two groups; however, overlap in scores of those with and without compression resulted in difficulty in selecting a useful cut-off point.
Reviewer conclusions
The study sample selected is difficult to define; it will reflect different physicians' decisions about a clinical diagnosis of compression, likely to vary from study centre to study centre and perhaps through time (e.g. 30 years ago vs. now). There was a considerable mix of different cancers in the study sample; any predictive variable(s) uncovered are likely to reflect the mixed cases and would be difficult to generalise to particular conditions or to spinal metastases in general. As different primary tumours manifest at different times, the influence of age as a predictor could relate to mixed cases rather than risk of compression. It is unclear if the type of primary tumour was explored as a predictive variable: p-values were not reported for the logistic regression and there appeared to be errors in the application of the regression results to the study population.
Chaichana et al. (2009)109
Relevant aim
To understand factors associated with pathological vertebral body compression fractures in patients with metastatic epidural SCC [SCC caused by an epidural mass (EM)].
Design and method
This retrospective study examined medical records of patients who had received surgery for SCC at a single tertiary care centre between 1996 and 2007. Inclusion required MRI evidence of spinal cord displacement by an EM. Patients with more than one discrete lesion, brain metastases, cauda equina or spinal root compression were excluded.
The report implied that, of 216 patients who may have been included, data for 162 were analysed (implying a possibility of ≈ 25% missing data). Patients with vertebral fracture who did not receive surgery were not identified or quantified.
The primary cancer diagnoses among the 162 included patients were various, reported as follows: lung (n = 26, 16%), breast (n = 26, 16%), prostate (n = 20, 12%), renal (n = 21, 13%) and haematopoietic (n = 28, 17%). Other sources included thyroid, gastrointestinal, melanoma and non-renal genitourinary system. The distribution of spinal metastases was described as: cervical, n = 35; thoracic, n = 114; lumbar, n = 49; cervicothoracic, n = 22; and thoracolumbar, n = 24.
Results
Univariate logistic regression identified the following variables that were associated with presurgery vertebral fracture: sensory deficit (p = 0.02), presurgery chemotherapy (p = 0.03), primary breast cancer (p = 0.02), thoracic involvement (p < 0.001), number of spinal levels involved (p = 0.1), number of spinal metastases (p = 0.07) and anterior location (p = 0.005). Variables found not to be associated with vertebral fracture according to univariate regression (p > 0.1) included age, pain symptoms, motor deficit, lytic-type tumour, blastic-type tumour and extraspinal metastases. After multivariate logistic regression, presurgery chemotherapy (OR 2.283, 95% CI 1.064 to 4.898; p = 0.03), primary breast cancer (OR 4.179, 95% CI 1.457 to 11.983; p = 0.008), thoracic involvement (OR 3.505, 95% CI 1.343 to 9.143; p = 0.01) and anterior cord compression (OR 3.213, 95% CI 1.416 to 7.293; p = 0.005) were found to be independently associated with vertebral body compression fractures.
Author conclusion
The factors strongly associated with preoperative compression fractures include lack of sensory deficits, primary breast cancer, anterior spine metastases, thoracic spine involvement, preoperative chemotherapy and possibly preoperative radiation therapy.
Reviewer conclusions
The study sample selected may be unrepresentative of patients with SCC who are at risk of or who have vertebral body compression fractures, as those patients who did not receive surgery were not included; this may or may not be a sufficient proportion to bias results. A further concern is that it appears that 25% of relevant data were missing; however, the report lacks clarity on this. There was a considerable mix of different cancers in the study sample; any predictive variable(s) uncovered are likely to reflect the mixed cases. It would be difficult to generalise to particular conditions or to patients with SCC in general.
Helweg-Larsen et al. (2000)113
Relevant aim
The parts of this study relevant to this report were (1) an investigation of whether tumour type influenced the time from cancer diagnosis to diagnosis of spinal cord or nerve root compression and the clinical severity of the compression; and (2) the identification of prognostic factors for subsequent recurrence of compression at a second site. The authors' main aim focused on the analysis of the prognostic significance of various clinical and radiological variables for ambulatory function and survival following treatment for spinal cord or nerve root compression.
Design and method
This was a prospective study of 153 consecutive patients recruited over a 3.5-year period if they had a diagnosis of SCC or nerve root compression due to intraspinal metastases from a known solid malignant tumour. All 153 patients had SCC or nerve root compression confirmed by myelography (some received CT imaging). Patients were followed up from diagnosis of compression to death or for a minimum of 11 months. The primary cancer diagnoses were breast carcinoma in 56 patients (37%), prostatic carcinoma in 43 (28%), NSCLC in 18 (12%), SCLC in nine (6%), and other solid tumours in 27 (17%) patients. The distribution of compressions was cervical, seven (4%) cases; thoracic, 102 (67%) cases; and lumbosacral, 44 (29%) cases.
Results
The main results concerned predictors of overall survival and of ambulatory status after treatment for compression and these are outside the remit of this short report. The type of primary tumour was found to be a predictor of the time from diagnosis of cancer to the time of the first compression (p < 0.0005 in multivariate analysis), and also of the severity of the compression in terms of patients' ambulatory status at the time of diagnosis of compression. Of the tumour types examined, the shortest time to compression was found for lung cancer and the longest for breast cancer. Kaplan–Meier analyses of this outcome were not shown.
New compression at a different site was observed in 14 of 153 patients. In an analysis that lacked power because of small sample size, it was found that primary tumour type was not a predictor of recurrence. The median time to new recurrence after the first compression was 4.5 (range 1–25.4) months.
Author conclusions
There was a significant association (p = 0.016) between time interval from diagnosis of primary tumour until development of SCC and type of primary tumour. Pretreatment ambulatory function of SCC patients is a main determinant for post-treatment gait function. Survival time is short, especially in non-ambulatory patients, and can be improved only by restoration of gait function in non-ambulatory patients by immediate treatment.
Reviewer conclusions
Primary tumour type influences the time to SCC and patient walking status at time of confirmation of SCC. An inference that follows for studies with populations of mixed cancer type is that the length of time from primary diagnosis to study entry will influence the results of any analysis of prognostic factors predicting compression or vertebral fracture. There will therefore be considerable difficulties in interpretation of results from studies with a case-mix of patients with different cancer types and various delays between primary diagnosis and study entry.
Helweg-Larsen et al. (1995)114
Relevant aim
A stated aim of this study was to compare the risk of a recurrence of spinal cord or root compression among patients with different numbers of spinal metastases detected at the time of the diagnosis of the first compression.
Design and method
This was a prospective study of patients recruited over a 3.5-year period. The study included 107 consecutive patients with myelographically verified metastatic SCC or spinal root compression from a histologically verified solid tumour. The report states that all patients received radiotherapy after myelographic diagnosis, but also that ‘only those epidural lesions causing neurological signs or symptoms were irradiated’. The primary cancer diagnoses were reported as breast carcinoma in 42 patients, prostatic adenocarcinoma in 28 patients, lung cancer in 21 patients and other solid tumours in 16 patients. Multiple epidural lesions were observed in 37 (35%) patients; in one patient there were four separate lesions, in eight patients there were three lesions, and in 28 there were two separate lesions.
Results
Recurrence of compression was observed in 8 of 107 patients. There was no difference in risk of a second compression between patients with a single metastasis at the time of the confirmatory myelogram for first compression (five recurrence events among 70 patients at risk) and those with multiple metastases at the confirmatory myelogram (three recurrence events among 37 patients at risk). The overall survival was superior for those who experienced recurrence (n = 8, median 9.2 months) than for those with no recurrence (n = 99, median 3.5 months). Unsurprisingly, this indicates that a predictor for recurrence is prolonged survival and that identifying patients with recurrence tends to select those with longer survival.
Author conclusions
Only symptomatic epidural metastases should be irradiated, and all patients treated should be followed regularly and observed for a second SCC.
Reviewer conclusions
The number of recurrence events (n = 8) was too small to allow for meaningful investigation of prognostic factors predicting recurrence. Unsurprisingly, patients who survive longer are more at risk of recurrence.
Husband et al. (2001)116
Relevant aim
These authors aimed to assess indicators that might identify those patients with physician-suspected MSCC in whom MRI examination can be forgone.
Design and method
This was a prospective study of 280 consecutive patients, recruited over 2 years at a single centre, who underwent MRI for suspected SCC. Of 362 potentially eligible patients, 51 were excluded because they had MRI at other centres, and 31 were excluded because they did not undergo MRI for various reasons (e.g. unavailable scanner). The primary cancer diagnoses among the 280 included patients were various and reported as follows: breast 65, prostate 57, bronchus 72, haematological 23, urinary tract 21, gastrointestinal tract 13, unknown primary 12 and other 17 patients.
Patients received MRI, plain radiography and neurological assessment. The relative timing of the different imaging modalities was not reported.
Results
The presence of focal abnormality on radiographs together with neurological signs consistent with compression at that level was taken as positive diagnosis by radiography. On this basis, 104 out of 280 patients were judged positive; however, 13 had received previous radiotherapy at that site and were classified as MRI-mandatory because of previous therapy. The remaining 91 patients were classified as MRI non-mandatory. The remaining 176 out of 280 patients were negative by radiography plus neurological signs and were classified as MRI-mandatory.
Of the 280 MRI scans undertaken, 201 were positive for MSCC (186 extradural, five intradural but extramedullary and 10 intramedullary), and 79 were negative (of these, 19 showed no MRI abnormality and 60 showed various abnormalities). MSCC was observed at one level in 161 patients, at two levels in 36 patients and in three regions in four patients. The sites at which MSCCs were observed were cervical in 15 patients, thoracic in 160 patients and lumbar/sacral in 71 patients.
The diagnostic/prognostic performance of plain radiographs plus neurological examination for the diagnosis of MSCC was compared with MRI (the latter taken as gold standard), and specificity, sensitivity and positive and negative predictive values were calculated and reported. The results were presented in table 9 of the paper. Of the 91 patients classified as ‘non-mandatory MRI’ based on positive radiography plus neurological signs, 89 were positive for MSCC according to MRI, leaving two as MRI negative (i.e. false-positives). Of the 189 patients classified as ‘mandatory MRI’ based on negative radiography plus neurological signs (or by virtue of previous treatment), 112 were positive for MSCC by MRI. This should leave 77 true-negatives (189 − 112) by radiography; however, the table presents this number as 87, giving a total number of patients of 290 (10 more than included in the study). The reason for this discrepancy is unclear. The text states that for this analysis ‘thecal sac compression without SCC’ was viewed as a negative MRI, but it appears that these patients may have been double counted. The reported sensitivity, specificity and positive and negative predictive values were 44%, 98%, 98% and 44%, respectively. Values calculated on a total of 280 patients become 97% for specificity and 41% for negative predictive value.
Author conclusions
Although focal radiographic abnormalities with consistent neurological findings, when present, accurately predicted the presence and level of MSCC, whole-spine MRI is indicated in most patients with suspected MSCC because the additional information may alter the management plan. The primary tumour is not helpful in predicting which patients will have more than one site of compression, although this is uncommon in tumours of haematological origin.
Reviewer conclusions
The predictive/diagnostic performance of radiography plus neurological signs was poor (sensitivity only 44%), with more false-negatives than true-positives, but with a positive predictive value of 97%. However, predictive values are highly dependent on the prevalence of the condition in the population examined; here the prevalence was 69%, which tends to strongly favour a high positive predictive value, as illustrated in Figure 14.
FIGURE 14.
Strong dependence of positive predictive value (PPV) on prevalence. The curve shows the relationship between prevalence and positive predictive value when sensitivity and specificity are 44% and 97%, respectively, as in the study by Husband et al. 116 The straight line is when sensitivity and specificity are both set at 50%.
Klekamp and Samii (1998)117
Relevant aim
The aim was to identify factors that might predict local recurrent disease (i.e. of spinal metastases).
Design and method
The main focus of this paper was to identify variables associated with prolonged survival or a favourable postoperative neurological status in patients who have received surgery for spinal metastases.
This was a prospective study of 101 patients (with a total of 106 spinal metastases) who received surgery for spinal metastases at a single hospital between 1977 and 1996. Patients were recruited from a total of 740 patients who had received spinal tumour treatment. The number of patients with spinal metastases not in receipt of surgery was unclear. The primary cancer diagnoses resulting in 106 spinal metastases were reported as breast 17, prostate 15, thyroid 9, kidney 12, unknown primary tumour 25, lung 17, colon 5, melanoma 2, urogenital tract 1, pleural mesothelioma 1, teratoma 1 and gallbladder 1. The time interval between cancer diagnosis and diagnosis of spinal metastases ranged from 2 days to 5 years (mean 4 months; SD 6 months). Spinal metastases were distributed as follows: cervical, 12 patients; thoracic, 62 patients; lumbar, 24 patients; and sacral, three patients. Various imaging methods were employed to establish the diagnosis of spinal metastasis. Health status was monitored according to the authors' published scoring system based on that of Karnofsky; scores ranged from 0 to 5.
Results
The 106 spinal metastases were subdivided according to primary tumour into ‘long survival prognosis’ (n = 53; breast, prostate, thyroid, kidney) and ‘short survival prognosis’ (n = 53; lung, colon, melanoma, urinogenital, mesothelioma, teratoma, gallbladder). The rationale for this subdivision was not elaborated. Kaplan–Meier analysis was used to investigate time to local recurrence and multiple logistic regression was used to identify influential risk variables for recurrence.
The absolute number of postsurgery local recurrences was not reported. According to Kaplan–Meier analysis, recurrence of spinal metastases leading to neurological deterioration (implying the presence of SCC) was observed in 57.9% of spinal metastases within 6 months of surgery, 69.3% within 1 year and 96% within 4 years (no risk table was provided; it is assumed that patients who died before recurrence were censored at time of death). Figure 3 of the study depicts the time to recurrence for ‘short survival prognosis’ and for ‘long survival prognosis’ patients; time to recurrence was much shorter for the latter. The reported relationship is represented in Figure 15. It is unclear if the prognostic subgroups were specified a priori. Similarly, time to recurrence was shorter for patients with better health status (< 3) than for those with scores ≥ 3. The relationship is represented in Figure 16.
FIGURE 15.
Time to local recurrence reported by Klekamp and Samii. 117 Data read from graph and fitted with Weibull distributions.
FIGURE 16.
Time to local recurrence reported by Klekamp and Samii. 117 Data read from graph and fitted with Weibull distributions.
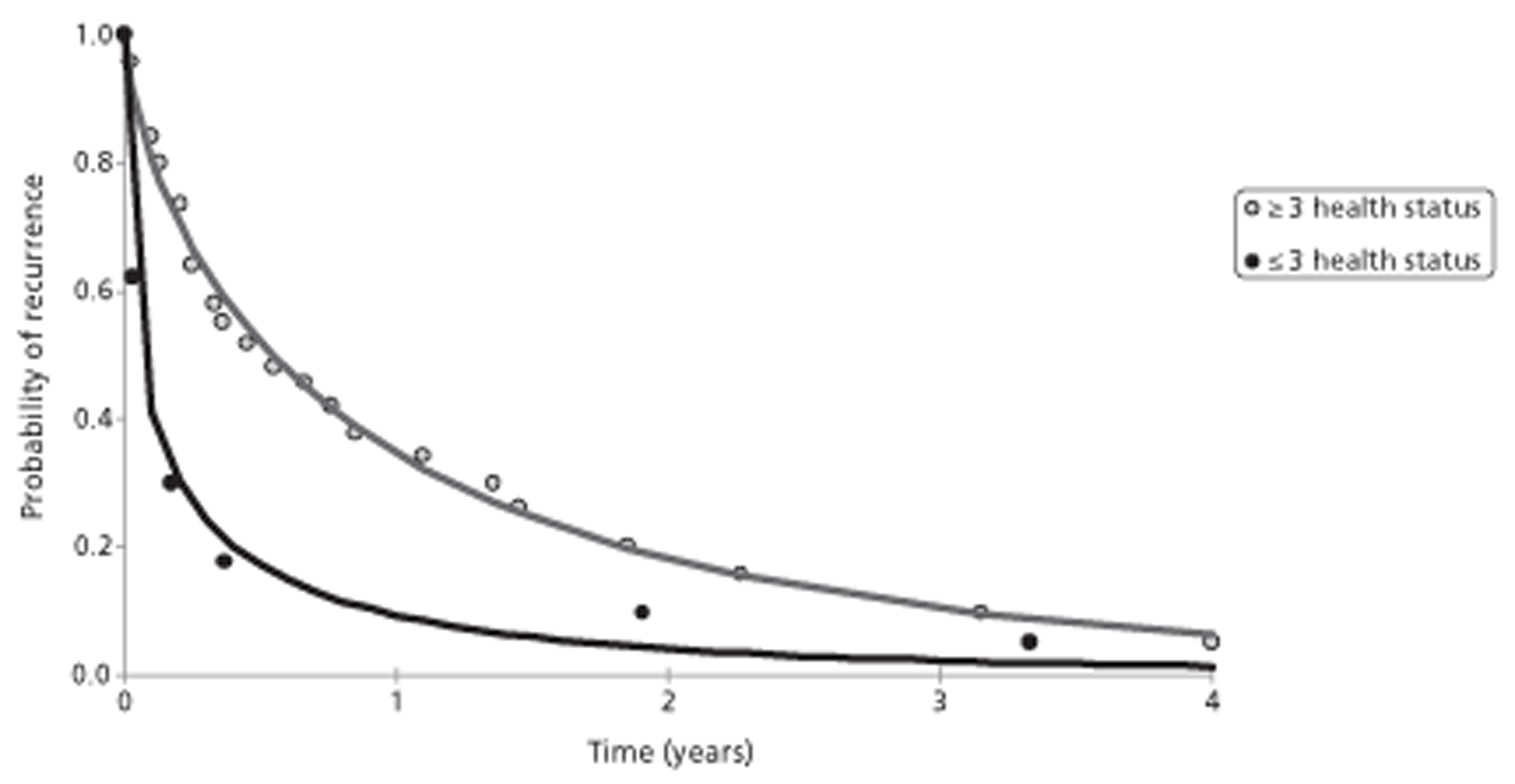
Multiple logistic regression identified that long postoperative recurrence-free survival was associated with the following variables: favourable tumour histology (i.e. tumours in the long survival prognosis group category), tumours at cervical spine level, low number of affected vertebral bodies, good general health status, complete resection at surgery, and elective surgery (as distinct from emergency surgery; 70% of patients received emergency surgery). Adjuvant postoperative therapy, length of history and age did not show a significant influence on local metastatic recurrence rate.
Author conclusions
The authors' conclusions were largely based on a literature survey and concerned survival, treatment modalities and recommendations of treatment pathways as follows:
-
Patients in good health condition and living independently should undergo surgery for spinal metastasis if neurological symptoms are present. Postoperatively, adjuvant therapy should be initiated.
-
Patients with neurological symptoms but in poor condition, requiring hospitalisation for their cancerous disease independent of spinal metastasis, should not be operated on but should be offered radiotherapy and/or chemotherapy primarily.
-
Patients with spinal instability due to metastatic disease require stabilisation to achieve a satisfactory neurological outcome. However, a surgical procedure has to be tailored according to life expectancy and health status of the patient.
-
Patients without neurological symptoms or instability should undergo radiotherapy primarily.
-
Patients who deteriorate after or despite primary radiotherapy may be candidates for surgery, but more complications and higher mortality rates should be expected.
Reviewer conclusions
The patient population was very heterogeneous and the study spanned two decades, during which imaging and treatment modalities will have changed. Patients not judged suitable for surgery were excluded and therefore the study population represents physicians' judgements and may be particular to time and place. Unsurprisingly, type of primary tumour and patient's health status were identified as factors that influence the reappearance of spinal metastases after surgery. As recurrences were associated with neurological deficit it is probable that these factors will also be associated with SCC or vertebral collapse.
Levack et al. (2002)119
Relevant aim
The authors aimed to quantify the incidence of clinical signs and symptoms of malignant SCC, or of cauda equina compression, among patients with confirmed diagnosis of compression and to assess the utility of various imaging procedures for the diagnosis of malignant compression. The authors focused on the nature and onset of patient symptoms relative to the time of diagnosis, and on the reasons for delays in diagnosis.
Design and method
This was a prospective observational study of 319 patients with SCC or cauda equina compressions (with a total of 324 compressions) recruited at three centres between January 1998 and April 1999. Compression was mostly confirmed by MRI. The primary cancer diagnoses were reported as lung, prostate and breast, together accounting for 59% of all cases. Tumours were from the gastrointestinal tract in 10% of cases (32) while 10% were of haematological origin (myeloma, lymphoma, chronic lymphatic leukaemia) and in 23 cases (7%) the site of the primary tumour was never identified. Median age was 65 years and 203 out of 319 participants were male. The spinal level of compressions was reported to be cervical in 7% of cases, thoracic in 68%, lumbar in 21% and sacral in 4%. In 55 of 324 compressions, compression of more than one site was detected. In 72 out of 319 patients, the malignant compression was the presenting symptom; the remaining 247 patients were known to have cancer at the time of compression diagnosis. Diagnoses of malignant compression were mainly made on weekdays (average of 18.3% per day for all diagnoses) whereas weekend days only accounted for 4.3% per day, implying a few days' delay in diagnosis for a proportion of patients, presumably reflecting the lack of access to MRI outside the working week.
The proportions of patients with various clinical signs and symptoms of malignant compression, and in some instances the timing of their onset, were reported; however, no formal regression analysis of predictive factors or Kaplan–Meier analyses were undertaken. Various imaging methods were employed, including plain radiography, bone scintigraphy, CT and MRI. The accuracy of different modalities for diagnosis of malignant compression was compared.
Results
The reported frequencies of signs and symptoms are summarised in Table 24.
| Self-reported sign or symptoma | Proportion experiencing it | Timing before diagnosis |
|---|---|---|
| Pain (spinal nerve root or localised) | 94% | Median duration 90 days (IQR 37–205 days) |
| Root pain | 79% | |
| Root pain alone | 35% (n = 86) | |
| Back pain alone | 44% (n = 110) | |
| Progressive pain and latterly severe | 84% (n = 197) | |
| Pain rated as ‘worst imagined pain’ | 29% | |
| Unable to walk at diagnosis | 18% | |
| Falls before diagnosis | Common | |
| Weakness before diagnosis | 85% (n = 210) | Median duration 20 days (IQR 7–132 days) |
| Altered sensation before diagnosis | 68% (n = 168) | Median duration 12 days (IQR 4–41 days) |
| Problem passing urine | 56% (n = 139) | |
| Urinary incontinence | 15% | |
| Frequency | 6% | |
| Urgency | 3% | |
| Hesitancy | 14% | |
| Bowel problems | 74% (n = 183) | |
| Constipation (possibly opioid-related) | 64% (n = 164) | |
| First relevant symptom | 100% | Median duration 66 days (IQR 37–205 days) |
| Clinical assessmentb | ||
| Weakness | 84% (n = 272) | |
| Sensory abnormality | 58% (n = 187) | |
| Abnormality at noted level | 52% (n = 169) | |
Pain was experienced by 94% of patients. Various categories of pain were common, and pain was found not to be the predictive factor of malignant cord compression; there was considerable discordance between the spinal level of pain and the structural level of compression. Eighteen per cent of patients were unable to walk by the time a diagnosis was made. There was no association between ability to walk and the patient's self-reported pain level (p = 0.99). Most clinical indicators were so common in this sample that they had little potential power as predictors.
The clinical level of sensory abnormality corresponded poorly with the level of cord compression identified on MRI scans. Considering the whole study population of 324 compressions, a sensory level was of value in identifying the level of compression in only 16%.
Factors contributing to delays in diagnosis of compression included slow general practitioner referral for patients not already known to have cancer, delay in referral after first appearance of signs or symptoms [median 66 days; interquartile range (IQR) 37–205 days], and delay between referral and definitive diagnosis (median 15 days; IQR 3–66 days). The rate of diagnosis of malignant cord compression increased through the week and was maximal on a Friday and low on weekend days.
Plain radiographs were obtained for 57% of compressions before diagnosis. Vertebral collapse (defined as ≥ 50% loss of vertebral height) was seen in 60 out of 187 (32%) plain films, yielding a sensitivity of only 32%. In 39 of these, the level of compression was confirmed on MRI. Hence, correctly predicted compression level was found in only 21% of radiographs. The most common request was for lumbar spine radiography, whereas the commonest site of compression was the thoracic spine.
Bone scintigraphy for back pain was performed in 139 patients. In 49, spinal hotspots suggestive of extensive bone destruction were identified, and in 26 of these the site corresponded to the level of compression as identified by MRI, yielding a true-positive rate of only 19% (26/139).
Author conclusions
Patients who developed spinal metastases were at risk of irreversible spinal cord damage. Weakness and sensory abnormalities were reported late and identified even later, despite patients having reported pain for a considerable time. Plain films and bone scans accurately predicted the level of compression in only 21% and 19% of cases, respectively. The only accurate investigation to establish the presence and site of a compressive lesion was MRI. Certain categories of patients are at risk of malignant cord compression, in particular, patients who are already known to have cancer when they first develop pain or who are > 50 years of age, and those with breast or prostate cancer with known bone metastases.
Reviewer conclusions
The paper looked at clinical symptoms, clinical signs and different technologies. The clinical symptoms and signs examined were found to be common in the selected population and therefore lacked discriminatory predictive power. MRI was judged to be the best available technology for detecting malignant compression and, relative to MRI, both plain radiography and bone scintigraphy were judged to perform badly.
Loblaw et al. (2005)62
Relevant aim
One aim was to identify and to assess the utility predictive models for MSCC, and similarly to compare imaging modalities for investigation of suspected MSCC (for previous more detailed discussion of concerns in relation to quality considerations for this systematic review, see Summary of systematic review evidence).
Design and method
The study was a systematic review that focused on the following seven questions concerning malignant SCC: (1) What are the clinical symptoms of MSCC? (2) What is the optimal approach for investigating suspected MSCC? (3) Is there a role for systemic corticosteroids in the management of MSCC, and if there is, what is the optimal dose? (4) What are the indications for surgery in the management of MSCC? (5) What are the indications for radiotherapy in the management of MSCC? (6) Is there an optimal dose prescription for radiotherapy? and (7) What are the treatment options for recurrent MSCC in an area previously irradiated?
Results
Fifty published studies were included and were reviewed by narrative description. Those publications that considered predictors of SCC or of vertebral collapse are discussed elsewhere in this short report.
Six studies were reviewed that addressed the relative utility of myelography and MRI for the investigation of suspected MSCC. These indicated that whole-spine MRI should be used for patients.
Author conclusions
Predictive risk models may help to define patients at higher risk of developing cord compression, but optimal screening strategy for a population and intervention have not been elucidated. Back pain was not predictive of MSCC. Treatment for patients with MSCC should consider presence of bony compression and spinal instability comorbidities, pretreatment ambulatory status, technical surgical factors, potential radiotherapy reactions, patient preferences and potential surgical complications.
Reviewer conclusions
Different factors such as inability to walk, increased deep tendon reflexes, compression fractures on radiographs of the spine, bone metastases present, bone metastases diagnosed > 1 year earlier and age < 60 years were found to be of some predictive value for MSCC. Back pain was found not to be predictive of MSCC.
Lu et al. (2005)121
Relevant aim
The aim was to identify independent clinical predictors of MRI-established SCC in cancer patients through the analysis of potential risk factors.
Design and method
This prospective study was a review of cancer patients with suspected SCC who were evaluated by MRI at two centres over the period from July 1998 to March 1999. Inclusion required consent by the physician ordering MRI. The patients included in the study had pathologically confirmed cancer diagnosis, no metastatic epidural cancer over the previous 12 months, age ≥ 18 years, and gave consent to a brief interview within 7 days of the scan. The patient interviews were conducted by one physician and focused on numerous factors experienced before MRI. Interviews were also conducted with the physicians ordering the scans; most patients were not blind to the results of the MRI. Of 167 eligible episodes of suspected SCC, a total of 136 episodes of suspected SCC among 134 patients were investigated by interview. The primary cancer diagnoses were reported as breast (n = 33; 24%), lung (n = 33; 24%), prostate (n = 21; 15%), non-Hodgkin's lymphoma (n = 8; 6%), multiple myeloma (n = 6; 4%) and others (n = 35, 26%). Median age was 61.5 (range 30.9–84.8) years.
Univariate analysis using Fisher's exact test was used to estimate the association of variables with a positive MRI test for SCC. Multivariate stepwise logistic regression was used to identify significant independent predictors of SCC.
Results
Clinically important metastatic epidural SCC was defined as any TSC with or without spinal cord displacement. MRI demonstrated 50 episodes of TSC reported at the following spinal levels: cervical 6%; thoracic 64%; lumbar 30%; sacral 6%. Vertebral metastases without TSC were seen in 46 episodes and no vertebral metastases in 40 episodes. In univariate analysis, back pain was not associated with TSC (92% of episodes were associated with back pain). Four independent variables predictive of TSC were identified by multivariate regression as follows: abnormal neurological examination (OR 3.0, 95% CI 1.6 to 10.4; p = 0.004); stage IV cancer at initial diagnosis (OR 2.8, 95% CI 1.4 to 7.7; p = 0.006); known vertebral metastases (OR 2.8, 95% CI 1.4 to 8.2; p = 0.008); and middle or upper back pain (OR 2.7, 95% CI 1.4 to 9.1; p = 0.010). These four predictors stratified patients experiencing episodes into subgroups with varying risks of TSC, ranging from 8% (no risk factors) to 81% (three or four risk factors), as summarised in Table 25. Only 19% of the episodes were associated with three or four risk factors relative to a prevalence of TSC of 36.7%.
| Number of significant predictors | Number of episodes | Number of episodes with TSC | Number of episodes with no TSC | LR with TSC/without TSC | Post-test probability of TSCa |
|---|---|---|---|---|---|
| 0 | 26 (0.19) | 2 (0.04) | 24 (0.28) | 0.143 | 0.077 |
| 1 | 38 (0.28) | 7 (0.14) | 31 (0.36) | 0.388 | 0.184 |
| 2 | 46 (0.34) | 20 (0.40) | 26 (0.30) | 1.534 | 0.435 |
| 3 or 4 | 26 (0.19) | 21 (0.42) | 5 (0.05) | 15.00 | 0.808 |
| Total | 136 (1.0) | 50 (1.0) | 86 (1.0) |
Among the episodes not associated with abnormal neurological examination (n = 100), middle or upper back pain and stage IV cancer at initial diagnosis were found to be independent predictive variables. In this population these variables stratified patients experiencing episodes into subgroups with varying risks of TSC, ranging from 11% (no risk factors) to 69% (both risk factors), as summarised in Table 26. Only 13% of episodes in this population exhibited both risk factors.
| Number of significant predictors | Number of episodes | Number of episodes with TSC | Number of episodes with no TSC | LR with TSC/without TSC | Post-test probability of TSCa |
|---|---|---|---|---|---|
| 0 | 36 (0.36) | 4 (0.14) | 32 (0.46) | 0.292 | 0.111 |
| 1 | 51 (0.51) | 17 (0.57) | 34 (0.49) | 1.163 | 0.333 |
| 2 | 13 (0.13) | 9 (0.30) | 4 (0.06) | 5.25 | 0.692 |
| Total | 100 (1.0) | 30 (1.0) | 70 (1.0) |
Author conclusions
The results confirmed earlier retrospective studies indicating that evaluation of cancer patients with suspected SCC should be based on clinical information that includes cancer-related history, symptom data and presence of pertinent neurological signs. Predictors may help clinicians to assess risk in this patient population.
Reviewer conclusions
The selected population included several different types of cancers and so the four identified risk factors need to be tested in both similar and different mixed case populations to determine the generalisability of the findings. The primary tumour type and treatment with bisphosphonates or other interventions (except radiotherapy to the level of suspected SCC) which might influence the identity of predictive variables do not appear to have been included in the regression analyses.
Rose et al. (2009)88
Relevant aim
The aim of this study was to evaluate prospectively the probability of vertebral fracture in patients who have received single-dose image-guided intensity-modulated radiotherapy (IG-IMRT) for spinal metastases, and also to identify risk factors for such fracture.
Design and method
Image-guided-intensity-modulated radiotherapy is a recently developed therapeutic option for patients with spinal tumours. The authors noticed that a number of patients sustained vertebral fractures after IG-IMRT so they undertook a prospective study to monitor the incidence and risk factors for post-therapy fracture. The study included 71 lesions occurring in 62 patients given IG-IMRT for spinal tumours. The primary cancer types for the 71 lesions were reported as follows: renal cell, n = 14; melanoma, n = 9; prostate, n = 9; sarcoma, n = 7; colorectal, n = 6; cholangiocarcinoma, n = 5; thyroid, n = 5; non-small cell lung, n = 5; breast, n = 4; and other, n = 7. The spinal distributions of the treated lesions were reported as follows: cervical, n = 6; thoracic, n = 47; and lumbosacral, n = 18. The treated sites were classified on CT appearance as lytic (n = 46; 65%), sclerotic (n = 13; 18%) or mixed (n = 12; 17%). The sites were also classified according to the percentage of the vertebral body occupied by the lesion, as follows: 0–20% of the vertebral body occupied, n = 26 lesions (37%); 21–40% occupied, n = 18 (25%); 41–60% occupied, n = 10 (14%); 61–80% occupied, n = 7 (10%); and > 80% occupied, n = 10 lesions (14%).
After IG-IMRT patients were followed up using MRI at 2 months and then at 3-month to 4-month intervals. Multiple logistic regression and Cox's proportional hazard models were used to identify factors associated with fracture. Kaplan–Meier analysis was used to determine time to fracture. Fractures were classified as new or progressive; a progressive fracture represented a worsening, after IG-IMRT, of a lesion in which vertebral deformity or end-plate infraction existed at the time of IG-IMRT therapy. The following potential risk factors for fracture were examined: location of the lesion; size of the lesion (tumour occupancy in vertebral body); type of lesion (lytic, sclerotic or mixed); appearance of the lesion in CT; obesity; local kyphosis; bisphosphonate use; IG-IMRT radiation dose; presence of baseline fracture; and histology of fracture.
Results
Fracture progression was found in 27 vertebral bodies (39%). Multivariate logistic regression analysis showed that CT appearance (lytic or sclerotic/mixed), lesion location and amount of vertebral body occupied by tumour independently predicted fracture progression. Lesions located between T10 and the sacrum were 4.6 times more likely to fracture than were lesions above T10 (95% CI 1.1 to 19.7 times more likely). Lytic lesions were 6.8 times more likely to fracture than were sclerotic and mixed lesions (95% CI 1.4 to 33.3 times more likely). As the amount of vertebral body occupied by tumour increased, the odds of fracture increased.
Obesity, local kyphosis, bisphosphonate use, baseline presence of vertebral deformity and IG-IMRT radiation dose were not associated with increased risk of fracture. The presence of baseline fracture was not associated with new fracture development or progression. There was no clear correlation between histology and risk of fracture.
Median time to fracture taken from the Kaplan–Meier analysis was reported to be 25 months. Figure 17 illustrates the relationship and shows data read from the graph. A Weibull fit generated a median time to fracture of 25.02 months.
FIGURE 17.
Time to progressive fracture. Data read from graph (Rose 200980). Solid line represents a Weibull distribution fit to extracted data.
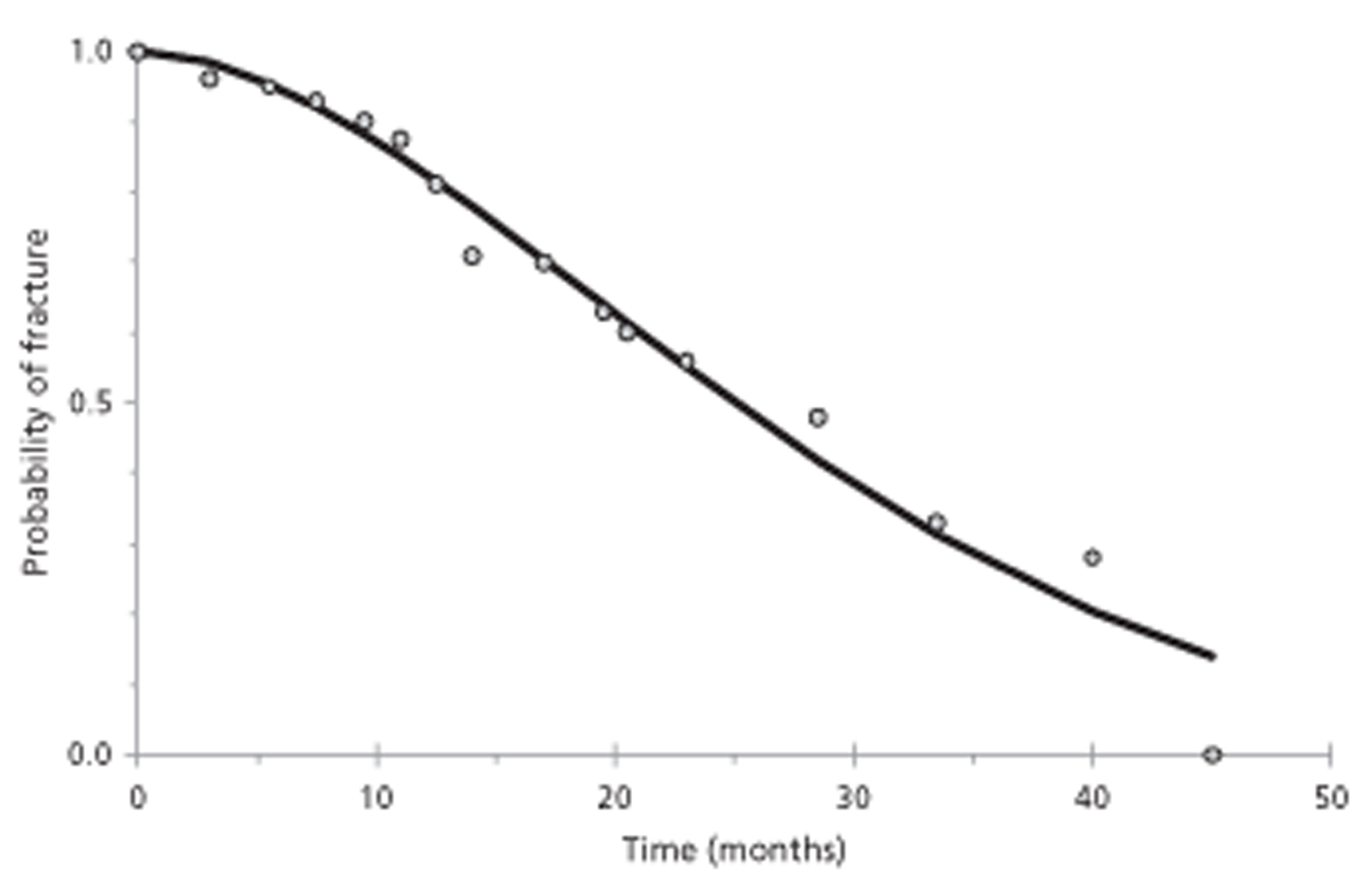
The median time to fracture for lytic lesions was reported to be 19 months, whereas the median time in sclerotic or mixed lesions was 32 months (p < 0.05). Figure 18 illustrates the time-to-event relationship and shows data read from the published graph. Weibull fits generated median times to fracture of 18.9 months for lytic lesions and 36.9 months for sclerotic/mixed lesions.
FIGURE 18.
Time to fracture for lytic and sclerotic or mixed lesions. Data read from published graph (Rose 200980). Numbers at risk assumed to be 46 and 25, respectively, and numbers of events assumed to be 23 and four, respectively. Solid lines represent fits using Weibull distributions; dashed line uses a Gompertz distribution and dotted line a log-log distribution.
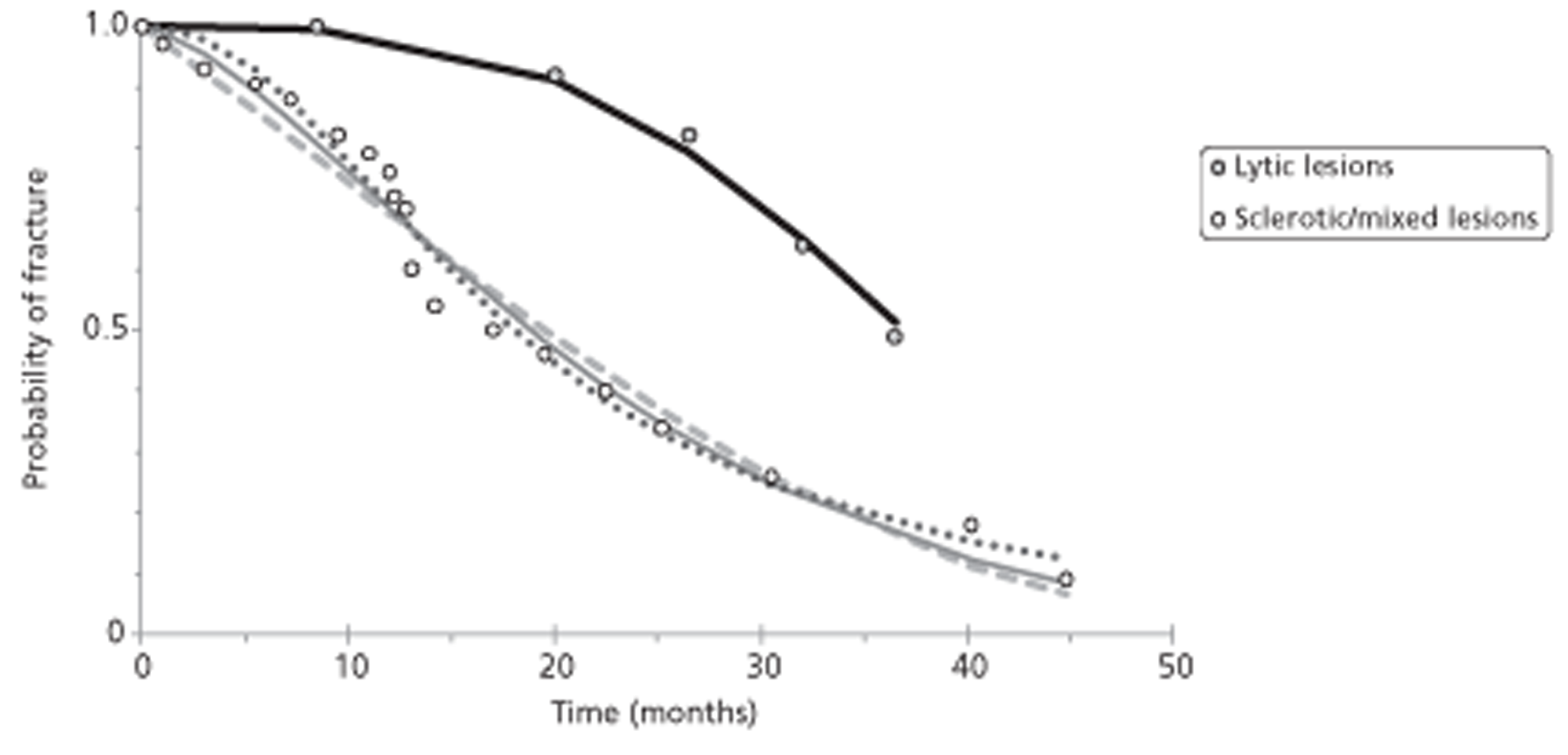
By stratifying lesions according to location, median time to fracture changed significantly. The median time to fracture with lesions between T10 and the sacrum was 20 months, and for lesions located higher in the spine it was 35 months (p < 0.05). Stratification according to the amount of the vertebral body occupied by the lesion also resulted in significantly different fracture probability functions (p < 0.02).
In the multivariate proportional hazards regression model, only lytic appearance (HR 3.8, 95% CI 1.3 to 11.4) and lesions that occupied 41–60% of the vertebral body (HR 3.9, 95% CI 1.1 to 14.2) were associated with a statistically significant increase in the HR.
The Karnofsky performance status at final follow-up was 80%. The median change in Karnofsky performance status among patients with fracture progression was 10%, and among patients without fracture progression was 0% (p < 0.03).
Author conclusions
The study identifies a high risk of vertebral fracture after single-fraction IG-IMRT to spinal metastases. Lytic disease involving > 40% of the vertebral body and location at or below T10 confers a high risk of fracture, the presence of which yields significantly poorer clinical outcomes.
Reviewer conclusions
The study explores fracture risk after single-fraction IG-IMRT treatment. Risk of progressive fracture after IG-IMRT was appreciable; poor prognosis for fracture appeared to be associated with lytic lesions, those occupying > 40% of the vertebral body and greater load on the deformed vertebra (as indicated by greater risk for lesions below T10). This was a small study with potentially very important findings but because of quality considerations its validity is difficult to evaluate.
Roth et al. (2004)124
Relevant aim
The study aimed to assess the predictive utility of biomechanically derived models to accurately predict the risk of vertebral burst fracture in the metastatic spine, and to generate simple methods to obtain the required data needed to make such risk assessment of burst fracture.
Design and method
This was a retrospective study of all cancer patients seen at a single centre between September 1998 and November 2001 who, on the basis of available CT imaging, were considered to have osteolytic metastases of the thoracic or lumbar spine. Vertebrae were classified as not fractured or as bearing burst or wedge fractures.
Of 560 potentially eligible patients with spinal metastases, 117 had suitable CT imaging and, of these, 72 (34 male and 38 female) harboured osteolytic spinal metastases (48 thoracic and 44 lumbar) and were included. Of the 92 metastatic vertebrae, 21 (23%) harboured fractures (17 burst fractures and four compression fractures), and 71 (77%) were not fractured. The primary cancer diagnoses were reported as follows: breast, n = 23; lung, n = 7; colon, n = 3; prostate, n = 5; lymphoma, n = 6; multiple myeloma, n = 5; renal, n = 4; other, n = 10; and unknown, n = 9.
The following estimates were made for each vertebra: vertebral body volume; minimal cross-sectional area; tumour volume (as a percentage of the vertebral body volume); apparent bone mineral density; tumour volume in the pedicle (dichotomised as intact or involved) and intervertebral disc quality (dichotomised as healthy or degenerated); pressure load based on patient weight, activity level and apparent cross-sectional area of the vertebra; and estimated proportion of body weight above the vertebral level. Loading rate, dichotomised as high or normal, was also recorded. For fractured vertebrae the minimal sectional area was estimated from that of adjacent intact vertebrae. The data estimates were used in biomechanical models, developed in a previous study, so as to determine the risk of burst fracture for each vertebra. The predicted outcome could then be compared with the known presence or absence of a burst fracture.
Results
The most accurate predictor of burst fracture was a model of vertebral bulge using only the spinal load-bearing capacity (constant pressure load). At an appropriate threshold (5.04, with a margin of 0.37) this had sensitivity and specificity of 1 for distinguishing burst-fractured vertebrae from unfractured or wedge-fractured vertebrae, and in logistic regression a Hosmer–Lemeshow test value of 1. Burst fracture prediction using vertebral axial displacement and tumour size were also good predictors. None of the models performed well at distinguishing unfractured from fractured (burst or wedge) vertebrae.
Author conclusions
Fracture prediction was optimised using the vertebral bulge model considering only load-bearing capacity with a specificity, sensitivity and CI of 1 to yield a clear threshold for burst fracture risk. Fracture prediction in the other two models, vertebral axial displacement considering only load-bearing capacity and tumour size, was also strong, with receiver-operator curve values of 0.992 and 0.988, respectively. The predictive power of these models can provide useful clinical information for prophylactic decision-making.
Reviewer conclusions
As indicated by the authors, the operator inputs required to undertake the modelling described are considerable and the methods used required relatively sophisticated digital scanning equipment, which may not be widely available. The development of automated systems may be required for the necessary data collection to become routine. Although prediction of burst fractures was impressive, the number of samples included was small and the validity of the results needs testing in a larger sample and in different populations.
Shah et al. (2003)126
Relevant aim
The aim of this study was to identify risk factors for metastatic vertebral fracture and epidural impingement.
Design and method
This was a retrospective study of metastatic cancer patients with spinal metastases. Patients were excluded if the primary tumour was a myeloma, lymphoma or other tumour of haematopoietic origin, if MRI was carried out within 30 days of a surgical intervention or if MRI demonstrated a metallic implant.
A random sampling method was reported and was used to select two samples from a population of MRI-evaluated patients with spinal metastases seen at one university hospital between October 1992 and June 1998. The first sample was used to estimate the incidence of vertebral fracture and risk factors for fracture; the second sample was selected from patients who presented with vertebral fracture and was used to investigate progression from normal shape, patterns of fracture and progression to epidural impingement.
The first sample comprised 53 patients (mean age 58 years, SD 26 years; 26/53 male) with images by MRI of 756 vertebrae. The primary tumours were reported as follows: breast, n = 14 patients (26.4%); lung, n = 13 (24.5%); prostate, n = 9 (17%); renal, n = 7 (13.2%); undifferentiated, n = 3 (5.7%); and others, n = 7 (13.2%). Metastatic lesions were observed in 253 out of 756 vertebrae (33.4%). In 114 of these, an isolated zone of lysis or of osteoblastic new bone could be identified; these lesions were classified as circumscribed. Of circumscribed lesions, 104 (91.2%) were confined to the vertebral body (excluding arch and pedicles). The metastatic lesions were observed most commonly among lumbar and posterior thoracic vertebrae.
The second sample comprised 67 patients presenting with vertebral fractures (113 fractured vertebrae). Twenty-two fractures were found to have no metastatic infiltration and were not analysed further, leaving a final sample of 91 fractured vertebrae.
Results
The risk of vertebral fracture among infiltrated vertebrae was greatest for upper lumbar (L1–L3) vertebrae relative to other vertebrae (RR 1.95, 95% CI 1.12 to 3.38; p = 0.017), and for undifferentiated tumours relative to other tumours (RR 7.36, 95% CI 2.69 to 20.12; p = 0.001). Prostate metastases were associated with the smallest risk of fractures (RR 0.21, 95% CI 0.082 to 0.535; p = 0.001).
MRI follow-up identified 23 normally shaped vertebrae that progressed to fracture. According to a Cox's proportional hazards model, greater fracture risk was noted in vertebrae with > 80% vertebral body infiltration relative to less infiltration (HR 4.60, 95% CI 1.66 to 12.71).
The number of spinal levels affected by metastasis was weakly correlated with the number of fractured vertebrae in an individual patient (r = 0.325). There was no significant correlation between metastatic involvement of one or both pedicles with fractures (p = 0.43).
Fractures were classified by the authors as (1) symmetrical compression wedge fracture (with ‘delta fragments’); (2) symmetrical compression fracture with no ‘delta fragments’; (3) lateral compression fracture; or (4) anterior compression fracture. Those classified as wedge fractures had a greater tendency to progress to migration into the epidural space.
Author conclusions
Fracture risk was greatest for upper lumbar and undifferentiated tumours. Fracture risk was substantially increased in vertebrae with > 80% body infiltration, and symmetrical fractures with fragments were associated with the greatest risk of epidural impingement.
Reviewer conclusions
The reported results are perhaps unsurprising in that those vertebrae tending to bear greater load and sustaining greater metastatic infiltration are more likely to fracture. Similarly, fractures generating bony fragments might be expected to cause more serious epidural penetration. The finding that risk of fracture appears to vary with primary tumour diagnosis is of interest for this report in that it implies that results with mixed cancer type populations might be viewed as largely reflecting the proportional contribution of the different cancer types.
Talcott et al. (1999)131
Relevant aim
The aim was to examine potential clinical neurological and oncological risk factors for CT-established SCC in metastatic cancer patients with suspected SCC.
Design and method
This was a retrospective study of medical and CT scan records accumulated between 1 February 1985 and 30 September 1988 at a single centre. Patients were included if a CT scan had been conducted for clinically suspected SCC (where SCC = SCC or cauda equina syndrome); this was termed the index scan. Patients were excluded if CT was not carried out for suspected SCC or if they had a previous diagnosis of SCC.
Of 405 index scans identified from records, 342 (in 258 patients) were included for analysis. The reasons for exclusion of 63 scans were reported. Mean age at study entry was 56.5 years. Primary tumour diagnosis was reported as breast in 42% of patients; NSCLC in 14%; prostate in 9%; sarcoma in 5%; and other in 30%.
The time period, study centre, number of index scans identified and the number excluded (n = 63) correspond to the Lu et al. 1998 study120 (described earlier); however, Lu et al. investigated only breast cancer patients (number reported as 93). Talcott et al. report on 258 patients, of whom 42% had diagnosis of breast cancer (n = 108); it is likely that most of the breast cancer patients in this study are identical to those reported by Lu et al. 120
The spinal level of the suspected episodes was reported as L3 or L4 in 43% of 342 index scans and T13 in 30% of 342 index scans. Uncertain scans (< 5%) were followed up by myelography or MRI. Most patients received imaging before the index CT, mostly to document metastases to bone, especially spine. Plain film radiographs immediately preceded 250 of the 342 index scans; vertebral lesions (lytic 29%, blastic 16%, mixed 20%) were seen in 68% of the plain films and compression fractures were seen in 30%.
Results
Twenty-two variables were examined in univariate or multivariate logistic regression for association with SCC. Several definitions of SCC were employed: TSC; spinal cord or cauda equina displacement (SCD); TSC + SCD; EM; SCD + TCD + EM.
A positive diagnosis at index scan depended on the formal definition of SCC used. For TSC, 29 out of 342 scans were positive; for SCD, 43 out of 342; for EM only, 52 out of 342; for TSC + SCD, 72 out of 342; for TSC + SCD + EM, 124 out of 342; and for TSC + SCD at index or within the 90-day follow-up, 80 out of 342. If local radiation at the site of suspected SCC within 90 days of a negative index CT, as an indication of SCC, then 169 out of 342 (49%) index scan episodes were positive. The reported associations of variables with SCC in univariate logistic regression is summarised in Table 27.
| Variable | Patients with SCC if variable is | OR (95% CI) | p-value | ||
|---|---|---|---|---|---|
| Present | Absent | ||||
| Bone metastases previously diagnosed | 27 | 8 | 4.1 | (1.7 to 9.8) | 0.002 |
| Vertebral body fracture on most recent plain radiograph | 42 | 18 | 3.3 | (1.9 to 5.7) | < 0.0005 |
| Increased deep tendon reflexes | 43 | 20 | 3 | (1.6 to 5.6) | 0.001 |
| Complaint of inability to walk | 39 | 21 | 2.4 | (1.3 to 4.7) | 0.008 |
| Bone metastases diagnosed 1 year prior | 34 | 18 | 2.4 | (1.4 to 4.0) | 0.001 |
| Bone metastases diagnosed 90 days prior | 30 | 15 | 2.3 | (1.4 to 4.0) | 0.002 |
| Bone metastases diagnosed 2 years prior | 37 | 20 | 2.3 | (1.3 to 4.2) | 0.006 |
| Bone metastases diagnosed 6 months prior | 31 | 17 | 2.2 | (1.3 to 3.7) | 0.003 |
| Prior radiotherapy elsewhere in the spine | 37 | 20 | 2.3 | (1.3 to 4.1) | 0.005 |
| Spine metastases diagnosed 6 months prior | 32 | 17 | 2.3 | (1.4 to 3.8) | 0.001 |
| Spine metastases diagnosed 90 days prior | 31 | 16 | 2.3 | (1.4 to 3.8) | 0.002 |
| Spine metastases previously diagnosed | 27 | 14 | 2.2 | (1.2 to 4.2) | 0.01 |
| Spine metastases diagnosed 1 year prior | 34 | 19 | 2.2 | (1.3 to 3.7) | 0.003 |
| Spine metastases diagnosed 2 years prior | 36 | 21 | 2.1 | (1.1 to 3.9) | 0.022 |
| Prior radiotherapy at the suspected spinal site | 38 | 22 | 2.2 | (1.1 to 4.7) | 0.04 |
| Complaint of bowel or bladder dysfunction | 37 | 21 | 2 | (1.1 to 4.2) | 0.02 |
| Any vertebral body lesion on most recent plain radiograph | 30 | 17 | 2.1 | (1.2 to 3.6) | 0.004 |
| Vertebral body lytic lesion on most recent plain radiograph | 32 | 19 | 2.1 | (1.2 to 3.4) | 0.006 |
| Abnormal plantar reflex | 35 | 21 | 2 | (1.1 to 3.7) | 0.02 |
| Weakness on physical examination | 31 | 18 | 2 | (1.2 to 3.3) | 0.008 |
| Complaint of sensory loss | 33 | 21 | 1.9 | (1.1 to 3.3) | 0.02 |
| Sensory deficit on physical examination | 31 | 21 | 1.8 | (1.0 to 3.0) | 0.04 |
In multivariate logistic regression six variables were significantly associated with TSC at index or during 90 days of follow-up. These are summarised in Table 28.
| Variable | OR (95% CI) | p-value | |
|---|---|---|---|
| Vertebral body fracture on most recent plain radiograph | 2.7 | (1.6 to 5.1) | < 0.0005 |
| Bone metastases previously diagnosed | 2.6 | (1.0 to 6.7) | 0.05 |
| Complaint of inability to walk | 2.3 | (1.1 to 4.7) | 0.02 |
| Increased deep tendon reflexes | 2.3 | (1.2 to 4.6) | 0.02 |
| Bone metastases diagnosed 1 year prior | 1.8 | (1.0 to 3.2) | 0.04 |
| Age < 60 years | 1.8 | (1.0 to 3.2) | 0.05 |
The authors counted the number of these six risk factors present in each patient and related this to the occurrence of TSC at index scan or within 90 days. The results are summarised in Table 29 together with the post-test probability of having TSC. According to these results, the 23% pre-test probability of TSC (within 90 days of the index scan) is raised to 87% for a patient who exhibits five risk factors.
| Total risk factors | Patients in group | Number with TSC (%) | Number without TSC (%) | LR (with TSC/without TSC) | Post-test probability of TSC |
|---|---|---|---|---|---|
| 0 | 24 | 1 (4) | 23 (96) | 0.1424 | 0.042 |
| 1 | 63 | 6 (10) | 57 (90) | 0.3447 | 0.095 |
| 2 | 121 | 25 (21) | 96 (79) | 0.8529 | 0.207 |
| 3 | 92 | 21 (23) | 71 (77) | 0.9687 | 0.228 |
| 4 | 27 | 14 (52) | 13 (48) | 3.5269 | 0.519 |
| 5 | 15 | 13 (87) | 2 (13) | 21.2875 | 0.867 |
| Total | 342 | 80 (23) | 262 (77) |
Author conclusions
The clinical history of patients' cancer contributes independently to risk assessment. The prevalence of SCC depends on definition used and whether short-term clinical follow-up is included.
Reviewer conclusions
The authors' main conclusion is justified. The 23% prevalence of TSC among the CT index scans may not be surprising because patients were selected for suspected SCC. Several of the risk factors identified were also probably unsurprising as they included vertebral fracture on most recent radiograph (250 of the 342 index scans were immediately preceded by plain radiograph and fracture is known to be highly associated with SCC); previously diagnosed bone metastases (these may have progressed for a long time before the index scan, and although breast cancer and other patients may have osteoporosis, which might independently lead to bone fractures, it is unlikely that SCC will occur without bone metastasis in this selected population); complaint of inability to walk (a well-known symptom of SCC); increased deep tendon reflex; and age < 60 years. Spinal imaging has advanced since this study was conducted (1985–8) so that some of the findings might be interpreted differently in the current context.
Taneichi et al. (1997)89
Relevant aim
In an attempt to accurately diagnose impending collapse of vertebrae with metastatic invasion, the authors determined the risk factors for vertebral collapse, estimated the predicted probability of collapse under various states of metastatic vertebral involvement and established criteria for impending collapse.
Design and method
Fifty-three patients, with (n = 40) or without vertebral collapse, harbouring 100 thoracic or lumbar metastatic tumours were studied. The patients' average age was 59.7 years (SD 8.8, range 43–80 years). The sampling frame was not reported but it is implied that patients visited a single centre. The vertebrae were selected if they satisfied the following conditions: they contained purely or predominantly osteolytic metastatic lesions; there were no end-plate fractures in adjacent vertebrae; tomography (sagittal and coronal plane) and CT had been performed within 1 week of an initial plain radiographic examination, and images were judged to be qualified for detailed analysis; and radiographic examinations were performed before biopsy, radiation therapy or surgical treatment (e.g. laminectomy).
A variety of primary cancers were reported to be associated with the 100 vertebrae, as follows: renal cell carcinoma, n = 17; breast, n = 15; prostate, n = 15; hepatocellular, n = 13; lung, n = 8; oesophageal, n = 4; thyroid, n = 3; gastric, n = 3; colon, n = 2; melanoma, n = 2; fibrous sarcoma, n = 1; rhabdosarcoma, n = 1; leiomyosarcoma, n = 1; lymphoma, n = 1; ureter, n = 1; adrenal, n = 1; and unknown, n = 12. The distribution of affected vertebrae was as shown in Figure 19 and was reported as equally split between T4 to T10 (n = 50) and T11 to L4 (n = 50).
The potential risk factors for vertebral collapse examined reflected size and disposition of the metastatic lesion in the affected vertebra. Four factors were estimated. Factor [1] was the percentage tumour occupancy (% TO) of the vertebral body. To estimate %TO the CT images were examined using computer software so as to gain measures of A, the most extensive cross-sectional area of the tumour, and B, the cross-sectional area of the adjacent unaffected vertebra measured in the same plain as A. The %TO then = 100 × A/B; if A in the affected vertebra could not be reasonably measured then the intact part of the vertebral body C was measured, and a value for A was obtained indirectly as B–C. Factors [2], [3] and [4] were dichotomised according to CT image estimates of the presence of destruction of, respectively, the pedicle, the posterior elements (not including the pedicle); and the costovertebral joint (T vertebrae only).
Vertebral collapse was defined as (1) a vertebra with fractures of the end-plate adjacent to the osteolytic lesion, and (2) a vertebra with reduction of vertebral body height because of pathological fractures of the anterior and/or lateral cortex of the vertebral body. Vertebral body height was considered to be reduced when the height of the affected vertebral body was < 90% of the estimated original height. This was calculated from an average of the corresponding measurements at adjacent unaffected levels above and below the metastatic vertebra.
The authors developed a multivariate logistic regression model to determine the associations between the occurrence of vertebral collapse and the four risk factors. The predicted probability of vertebral body collapse in various states of metastatic vertebral involvement was estimated using the developed model. A set of criteria for ‘impending vertebral body collapse’ was suggested.
Results
There were no differences between T and L groups in the frequency of risk factors in collapsed and intact vertebrae, nor in the %TO of collapsed vertebrae (Table 30).
| Groups T1 to T10 | Groups T11 to L5 | ||||
|---|---|---|---|---|---|
| Present | Absent | Present | Absent | ||
| Vertebral body collapse | 24 | 26 | 26 | 24 | |
| [1] | %TO (SD) | 40.8 (24.8) | 40.3 (24.1) | ||
| [2] | Pedicle destruction | 15 | 35 | 14 | 36 |
| [3] | Posterior element destruction | 22 | 28 | 12 | 38 |
| [4] | Costovertebral joint destruction | 28 | 22 | - | - |
The results of multivariate logistic regression for the T and L group vertebrae are summarised in Tables 31 and 32.
| Risk factor | OR | 95% CI | p-value |
|---|---|---|---|
| [1] %TO | |||
| 10% increment | 2.44 | 1.07 to 5.55 | 0.032 |
| 20% increment | 5.93 | 1.14 to 30.77 | |
| 30% increment | 14.44 | 1.22 to 170.65 | |
| [2] Pedicle destruction | 1.73 | 0.10 to 28.75 | 0.703 |
| [3] Posterior element destruction | 1.17 | 0.13 to 10.63 | 0.886 |
| [4] Costovertebral joint destruction | 10.17 | 1.43 to 72.45 | 0.021 |
| Risk factor | OR | 95% CI | p-value |
|---|---|---|---|
| [1] %TO | |||
| 10% increment | 4.35 | 1.73 to 10.93 | 0.002 |
| 20% increment | 18.92 | 3.00 to 119.39 | |
| 30% increment | 82.27 | 5.19 to 1305.53 | |
| 40% increment | 357.81 | 8.98 to 14,254.10 | |
| [2] Pedicle destruction | 297.08 | 4.11 to 21,474.90 | 0.009 |
| [3] Posterior element destruction | 0.03 | 0.00 to 1.00 | 0.027 |
For group T vertebrae, the strongest association was between costovertebral joint destruction and vertebral collapse (OR 10.17; p = 0.021). The tumour size (%TO) was also associated with the risk of vertebral collapse (p = 0.032) with an OR of 2.44 for every 10% increment in %TO. Destruction of the pedicle and other posterior elements was not associated with collapse (OR 1.73; p = 0.703, and OR 1.17; p = 0.886, respectively).
For group L, the two most important risk factors for vertebral body collapse were %TO (OR of every 10% increment in %TO 4.35; p = 0.002) and pedicle destruction (OR 297.08; p = 0.009). Destruction of the posterior elements was inversely correlated with the risk of collapse (OR 0.03; p = 0.027).
Based on these results the authors developed the following equations to describe the probability of vertebral collapse in group T and group L:
where [1], [2], [3] and [4] refer to risk factors listed in Table 32 and take values [1], 0–100 and [2], [3] and [4], 0 or 1.
where [1], [2] and [3] refer to risk factors.
Examples of the reported predicted probabilities of fracture for the T group, calculated according to the equation above, are shown in Table 33.
| T group vertebral type | Value | % involvement | |||||
|---|---|---|---|---|---|---|---|
| %TO | [1] value | 30 | 60 | 30 | 60 | 30 | 60 |
| Costovertebral | [4] value | 0 | 0 | 1 | 1 | 1 | 1 |
| Pedicle | [2] value | 0 | 0 | 0 | 0 | 1 | 1 |
| Posterior | [3] value | 0 | 0 | 0 | 0 | 0 | 1 |
| Reported odds | |||||||
| Odds T collapse | −1.927 | 0.743 | 0.392 | 3.062 | 0.938 | 3.769 | |
| Exp (odds T collapse) | 0.1456 | 2.1022 | 1.4799 | 21.370 | 2.5548 | 43.336 | |
| 1 + exp (odds T collapse) | 1.1456 | 3.1022 | 2.4799 | 22.370 | 3.5548 | 44.336 | |
| Probability of fracture | 0.127083 | 0.677652 | 0.596764 | 0.955298 | 0.718695 | 0.977445 | |
| Results by factor | |||||||
| Factor | [1] | [2] | [3] | [4] | Constant | ||
| Coefficient | 0.089 | 0.546 | 0.161 | 2.319 | 4.597 | ||
Similar reported predicted probabilities of fracture for the L group are shown in Table 34.
| L group vertebral type | Value | % involvement | ||||||
|---|---|---|---|---|---|---|---|---|
| %TO | [1] value | 20 | 30 | 40 | 40 | 60 | 5 | 20 |
| Pedicle | [2] value | 0 | 0 | 0 | 1 | 1 | 1 | 1 |
| Posterior | [3] value | 0 | 0 | 0 | 0 | 1 | 1 | 1 |
| Reported odds | ||||||||
| Odds L collapse | −2.552 | −1.082 | 0.388 | 6.082 | 5.413 | −2.672 | −0.467 | |
| Exp (odds L collapse) | 0.077926 | 0.338917 | 1.47403 | 437.9041 | 224.3035 | 0.069114 | 0.62688 | |
| 1 + exp (odds L collapse) | 1.077926 | 1.338917 | 2.47403 | 438.9041 | 225.3035 | 1.069114 | 1.62688 | |
| Probability of fracture | 0.072 | 0.253 | 0.596 | 0.998 | 0.995 | 0.064 | 0.385 | |
| Results by factor | ||||||||
| Factor | [1] | [2] | [3] | Constant | ||||
| Coefficient | 0.147 | 5.694 | 3.609 | 5.492 | ||||
On the basis of these predictors, the criteria of impending collapse in group T were defined by the authors as (1) 50–60% (%TO) involvement of the vertebral body with no destruction of the other structures; and (2) 25–30% (%TO) involvement of the vertebral body with costovertebral joint destruction. In group L, the criteria were defined as (1) 35–40% (%TO) involvement of the vertebral body with no destruction of the other structures; and (2) 20–25% (%TO) involvement of the vertebral body with destruction of the posterior elements including the pedicle.
Author conclusions
With respect to the timing and occurrence of vertebral collapse, there is a distinct discrepancy between the thoracic and thoracolumbar or lumbar spine. When a prophylactic treatment is required, the optimum timing and method of treatment should be selected according to the level and extent of the metastatic vertebral involvement.
Reviewer conclusions
This study is more complete than many in that it develops empirical equations for prediction of fracture. The study selected only intraspinal tumour-related factors as risk factors for collapse; extraspinal factors such as age and sex were not considered. Any effect exerted from different primary types was not explored. Intraspinal factors such as costovertebral joint destruction and tumour size in the thoracic region were found to be significant risk factors. Factors such as tumour size and pedicle destruction were found to be significant risk factors in the thoracolumbar and lumbar spine. The equations developed need testing prospectively in different populations with spinal metastases.
Summary of studies involving a variety of cancers
There were 13 studies in which the sample was constructed from participants with a variety of different primary tumour types. The variation in samples in this regard is summarised in Figure 20. The study by Levack et al. 119 provided insufficient detail to determine the frequency of each major tumour type. In most studies, patients with breast, prostate and lung cancers constituted the majority of participants; however, this was not invariably so and the relative contribution from these three common cancer types was variable from study to study. Attempts to identify symptoms and signs that might increase diagnostic ability were not always successful in the included studies (e.g. Levack et al. 119). Because of the very broad range of factors investigated (see Table 7), this summary focuses on clinically significant findings common to several studies.
FIGURE 20.
The frequency of different cancers in the included case-mix studies. GI, gastrointestinal.
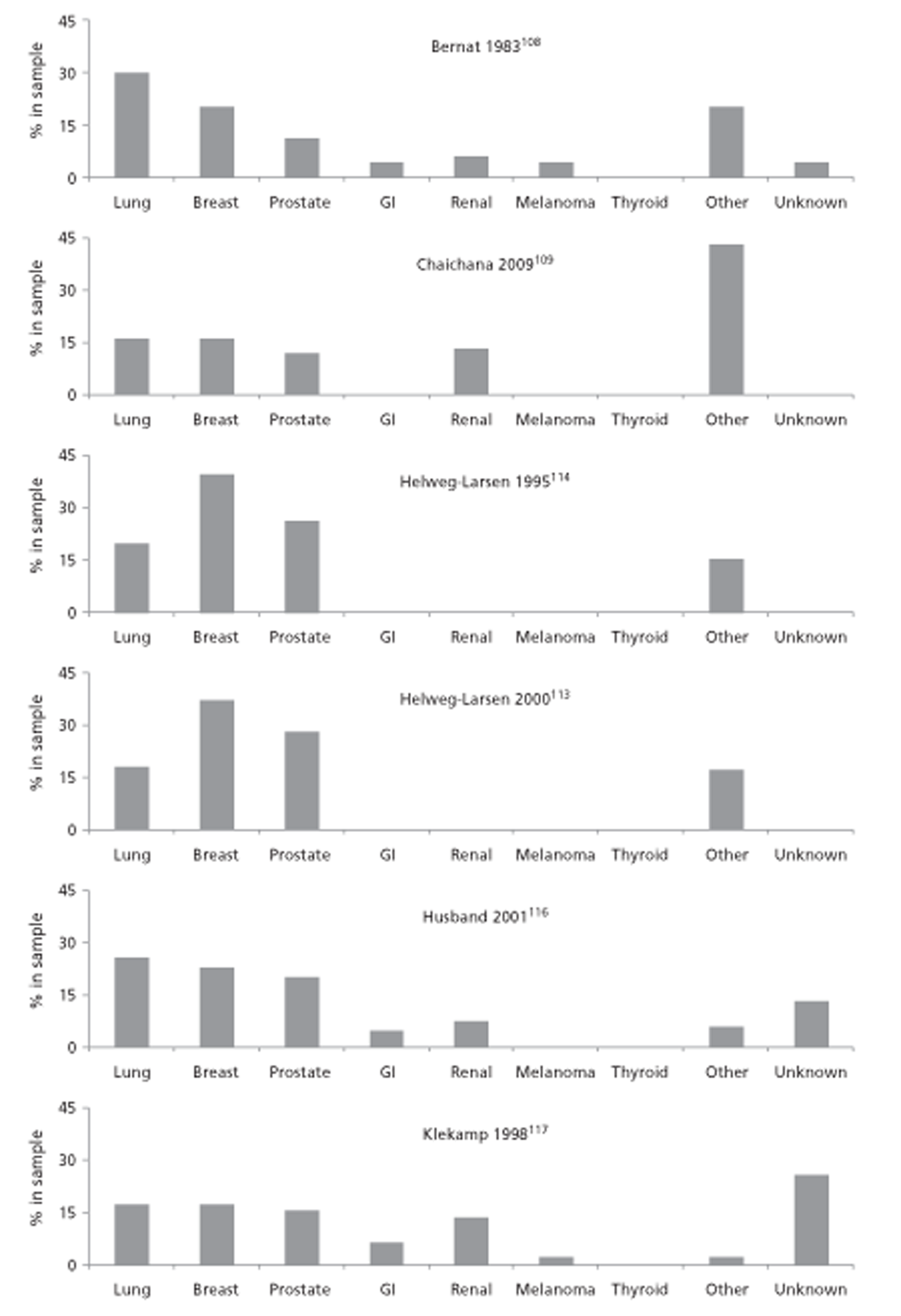
Two studies (Chaichana et al. 109 and Shah et al. 126) found that primary tumour type was a risk factor for vertebral collapse. Similarly, three studies (Helweg-Larsen et al. ,114 Helweg-Larsen et al. 113 and Klekamp et al. 117) found that primary tumour type was a risk factor for SCC recurrence, and Klekamp et al. 117 found that patient health status was also influential. Three studies (Roth et al. ,124 Rose et al. 88 and Taneichi et al. 89) all found that degree of tumour occupancy of the vertebral body was predictive for fracture.
Two studies (Lu et al. 121 and Talcott et al. 131) were able to identify risk factors which in combination in a single individual predicted SCC with high probability (five factors present delivered a probability of 87%131 and combination of three or four factors gave a probability of 81%121). Taneichi et al. 89 constructed an empirical algorithm for prediction of fracture in vertebrae harbouring predominantly lytic metastases. CT images were used to estimate tumour occupancy of the vertebral body and the presence or absence of various manifestations of vertebral damage. Two predictive models were developed, one for T4 to T10 and one for T11 to L4. The proposed models gave potential utility but were not widely used in the included studies. According to a citation index the paper has been cited 53 times since publication.
Missing data and a lack of transparency and clarity in reporting, particularly regarding participant selection, mean that in general the validity of findings was uncertain. No studies tested the performance of identified risk factors in a cohort independent of that in which the factors had been identified.
An inference that follows from the finding that risk of fracture appears to vary with primary tumour diagnosis is that the mix of cancer types in these case-mix studies, and the length of time from primary diagnosis to study entry, will influence the results of any analysis of prognostic factors. This leads to considerable difficulty interpreting the results from these studies. Potential confounding effects of primary tumour type, age and treatment with bisphosphonates or other interventions should have been considered when attempting to identify predictive variables.
Expert opinion paper
Fisher et al. (2010)110
Relevant aim
A stated objective of this study was ‘To use an evidence-based medicine process using the best available literature and expert opinion consensus to develop a comprehensive classification system to diagnose neoplastic spinal instability’. The authors comment that spinal stability may be defined as the ability of the spine to maintain its degree of motion while simultaneously preventing pain, neurological deficit and abnormal angulation.
Design and method
The authors (34 members of a Spine Oncology Study Group) used evidence provided by two systematic reviews undertaken by members of the group, and attempted to integrate this with expert opinion through a modified Delphi technique to generate through consensus of best evidence and expert opinion a classification system to define neoplastic spinal instability. The outcome was a spinal instability scoring system (Spine Instability Neoplastic Score). The authors believe that the Spine Instability Neoplastic Score, together with a patient's overall prognosis and physical condition, should be taken into account when considering interventions (e.g. surgery) that might be appropriate.
One systematic review was referenced as ‘in press’: (1) Fehlings MD, Furlan J, Bilsky M, et al. Defining oncologic instability of the cervical spinecan the available evidence guide clinical practice? Spine (in press). This study was not retrieved in our searches. A study by Weber et al. 52 was excluded from this short report because the outcome measures did not meet the inclusion criteria (see Appendix 5). Most of the studies included in the Weber systematic review were conducted with animal models, cadaver vertebrae or computer modelling and were not concerned with testing prognostic variables in humans with spinal metastases.
Results
The authors developed a Spine Instability Neoplastic Score for a series of variables that may be present in a particular patient. The variables were classified under six categories and are summarised in Table 35.
| Component | Score | Commentary |
|---|---|---|
| Spine location | ||
| Junctional (occiput–C2, C7–T2, T11–L1, L5–S1) | 3 | Spine location is scored based on global variations in the spinal architecture. Junctional regions include occipitocervical (C0–C2), cervicothoracic (C7–T2), thoracolumbar (T11–L1) and lumbosacral (L5–S1) regions. Mobile segments include those not in the junctional regions and those that do not articulate with the rib cage. Semi-rigid segments are non-junctional segments in the thoracic region that articulate with the rib cage. Rigid segments are parts of the non-junctional sacral spine (S2–S4) |
| Mobile spine (C3–C6, L2–L4) | 2 | |
| Semi-rigid (T3–T10) | 1 | |
| Rigid (S2–S5) | 0 | |
| Pain relief with recumbence and/or pain with movement/loading of the spine | ||
| Yes | 3 | Mechanical or postural pain is scored in this section. Relief with recumbency supports a structural or mechanical component |
| No (occasional pain but not mechanical) | 1 | |
| Pain-free lesion | 0 | |
| Bone lesion quality | ||
| Lytic | 2 | This category is meant to describe spinal alignment between motion segments that are affected by tumour. Scoring of de novo deformity such as kyphosis and/or scoliosis requires knowledge of prior imaging or may be assessed with upright compared with supine radiographs |
| Mixed lytic/blastic | 1 | |
| Blastic | 0 | |
| Radiographic spinal alignment score | ||
| Subluxation/translation present | 4 | This category is meant to describe spinal alignment between motion segments that are affected by tumour. Scoring of de novo deformity such as kyphosis and/or scoliosis requires knowledge of prior imaging or may be assessed with upright compared with supine radiographs |
| De novo deformity (kyphosis/scoliosis) | 2 | |
| Normal alignment | 0 | |
| Vertebral body collapse score | ||
| < 50% collapse | 3 | Presence and extent of vertebral body height collapse are used to assign a contribution of the score to the anterior and middle columns |
| > 50% collapse | 2 | |
| No collapse with > 50% body involved | 1 | |
| None of the above | 0 | |
| Posterolateral involvement of spinal elements (facet, pedicle, or costovertebral joint fracture or replacement with tumour) | ||
| Bilateral | 3 | The ‘posterolateral elements of the spine’ component of the score allows contribution from the posterior elements including pedicles, facets and costovertebral joints. Bilateral involvement is scored as greater than double the contribution of unilateral involvement because of the destabilising nature of its effects |
| Unilateral | 1 | |
| None of the above | 0 | |
Author conclusions
The Spine Instability Neoplastic Score was found to be a comprehensive classification system with content validity that could potentially guide clinicians in identifying when patients with neoplastic disease of the spine may benefit from surgical consultation. The Spine Instability Neoplastic Score might aid surgeons in assessing the key components of spinal instability due to neoplasia and may become a prognostic tool for surgical decision-making when put in context with other key elements such as neurological symptoms, extent of disease, prognosis, patient health factors, oncological subtype and radiosensitivity of the tumour.
Reviewer conclusions
It is difficult to discern how the findings of the systematic reviews fed into the study findings. In essence, this study is an expert opinion piece, and as such its quality is difficult to gauge using conventional assessment procedures. As with other studies included in this short report, the potentially prognostic variables identified require testing quantitatively in appropriate relevant populations; the clear difference between this study and others is that the prognostic variables deemed important have been identified on the basis of evidence synthesis by expert opinion.
Overall summary of results
No studies were identified that primarily aimed at describing the natural history of spinal metastases. It was not possible to examine the full text of all 2425 retrieved studies; consequently, natural history descriptions might exist but the titles (and abstracts) of such publications failed to reveal this fact readily. Because progression of spinal metastases will be influenced by the many different interventions that can affect host bone and/or resident metastases, and because spinal metastases vary according to tumour type and the general condition of the patient, we think a description of a natural history of spinal metastases is problematic. The relatively poor imaging methods available in early studies, in which interventions were minimal, means that it is unlikely that these would provide representative cohorts of patients and findings generalisable to current practice.
Imaging methods used for detection of SCC and/or vertebral fracture changed over the duration of the studies described. Formal comparison of different imaging procedures was rarely undertaken. We found no RCTs. It is clear that investigators favoured MRI and CT over myelography and/or plain radiography. Bone scans were widely employed but PET was not used in any of the included studies. The development and routine availability of machines with faster throughput and better performance (e.g. resolution) may change practice.
The included studies provided some evidence regarding factors that influence the risk of vertebral fracture and/or SCC. In general, these risk factors were unsurprising and would be familiar to clinicians charged with the care of patients with spinal metastases. They included the following: the number of spinal metastases (or skeletal metastases); the time of exposure to spinal metastases (i.e. survival); type of primary tumour and whether the spinal metastasis is lytic or blastic; the degree of occupancy of the vertebral body by the metastasis and its distribution. Some studies (e.g. Taneichi et al. ,89 Snyder et al. 128 and Roth et al. 124) attempted to combine risk factors into a decision rule that developed a probability for occurrence of an event. These appeared to have modest discriminatory power and were not tested by the authors in a population independent of that in which they were developed. Generally, the included studies made use of medical records and/or stored scan images to identify and quantify potential risk factors, and this information had not been collected specifically for the reported investigation.
Chapter 4 Discussion
The present report aimed to examine the natural history of metastatic spinal lesions and to identify patients at high risk of vertebral fracture and SCC. We did not find any epidemiological evidence with a primary aim of evaluating the natural history of spinal metastases. This review therefore focused on studies of spinal metastatic disease and candidate prognostic factors to predict undesirable outcomes for individuals or their vertebrae.
Summary of background
The overall effects of MSCC can be devastating. Spinal metastases can lead to significant morbidity and reduction in quality of life due to SCC or collapse. Compression of the spinal cord carries with it the risk of paralysis of body structures below the level of compression. If it were possible to predict which vertebrae were more likely to fracture, then early targeted treatment might prevent, reduce or delay such events and the serious unwanted outcomes that might result. There are many diagnostic methods available, including plain radiography, myelography, MRI, CT, radionuclide bone scan, SPECT and PET. However, uncertainty surrounds the effectiveness of these diagnostic techniques.
Summary of methods
Evidence was retrieved through searches during June 2011 in 13 electronic bibliographic databases, contact with experts in the field, scrutiny of references of included studies, and checking various health-service research-related resources. The search strategy covered the concepts of metastatic spinal lesions, adults, natural history, outcomes, technologies and prognosis. No study type or publication type restrictions were applied, as all types of study involving all languages were screened for potential inclusion. The titles and abstracts of retrieved studies were examined for inclusion by two reviewers independently. Disagreement was resolved by retrieval of the full publication and consensus agreement. Included studies involved adult patients with vertebral metastases at risk of developing (or who had developed) MSCC, vertebral collapse or progression of vertebral collapse and involved diagnostic/prognostic methods, including clinical features and/or imaging technologies. The full data were extracted independently by one reviewer. All extracted data were reviewed by a second researcher, and any disagreements were resolved by discussion. A quality assessment instrument was used to assess bias in six domains: study population, attrition, prognostic factor measurement, outcome measurement, confounding measurement, and account and analysis. Data were tabulated and discussed in a narrative review. Summary tables for each included paper were provided. Each tumour type was looked at separately.
Summary of principal findings
Searches
Comprehensive searches identified 2425 potentially relevant articles. Of these, 30 primary studies and one systematic review met the inclusion criteria. Seventeen studies reported retrospective data, 10 were prospective studies and three were other study designs. There were no RCTs. The approximate overall number of participants selected was 7888 and sample sizes analysed ranged from 41 to 859. Types of cancers reported were lung alone (n = 3), prostate alone (n = 6), breast alone (n = 7), mixed cancers (n = 13) and unclear (n = 1). We did not identify any epidemiological studies with a primary aim of investigating the natural history of spinal metastases.
Quality assessment
Five studies were considered to be of relatively high quality88,120,123,125,131 as they scored ‘yes’ on five of the six overall quality assessment questions developed for this review. Two additional studies are recognised by the reviewers to be of particular relevance and of reasonable quality, those by Venkitaraman et al. 133 and Taneichi et al. 89
Limitations in the evidence base
We identified some key limitations with the evidence in this review including:
-
limited information about the patient population and selection criteria
-
poor reporting of methods for estimating values for prognostic factors
-
failure to justify missing data or perform sensitivity analyses around the effects of missing data
-
limited reporting of multivariate models
-
time-to-event analyses failed to indicate numbers at risk at different time intervals
-
failure to consider potentially influential confounders for risk of SCC (e.g. primary tumour type) and treatments (e.g. with bisphosphonates).
There was a lack of coherence of potential predictive factors investigated between research groups. Across studies there was a lack of consistent methodology with regard to sampling procedure, populations (disease stage), treatments received for primary cancer or metastases, or definitions of outcome measures.
Further sources of uncertainty result from the small number of participants in the majority of studies and the small number of studies between which populations were comparable.
Natural history
We were unable to draw strong conclusions on natural history because of the limited information available.
The natural history of progression of skeletal metastasis suggests that all patients with occult SCC on MRI, if untreated, may progress to develop neurological deficit. 133 The risk of SCC and of the recurrence of cord compression increases with longer survival.
If the natural history of a condition is taken to be a description of how it progresses in the absence of influential interventions then the available information does not allow a precise or detailed description of the natural history of spinal metastases. Factors contributing to this situation include the following:
-
Many interventions, encompassing a wide variety of actions, have been developed and employed for treatment of primary tumours and specifically for skeletal metastases. These, to a greater or lesser extent, alter the natural progression of spinal metastases; they may inhibit or block the progressive growth of the metastasis, or they may alter bone metabolism (e.g. tamoxifen, bisphosphonates, denosumab, cytotoxic drugs, radiation) so that unwanted sequelae are more or less likely.
-
Early studies on the natural progression of spinal metastases in which the influence of interventions may be minimal are unlikely to provide samples that are currently useful. This is partly because imaging has changed and also because clinical practice at that time would tend to identify more advanced cases.
-
Spinal metastases can arise from a wide variety of primary cancer types and so do not represent a single entity. The natural history of metastases is likely to reflect characteristics of the primary tumour from which they arise. Some of the mixed cancer studies included in this report show that the type of primary tumour may be important for the development of SCC and/or vertebral collapse from spinal metastases.
The primary aim of many of the included studies was to identify prognostic factors for survival, the analysis of influential factors for intermediate outcomes; SCC or vertebral collapse was often an incidental objective.
Prognostic factors for vertebral collapse or spinal cord compression
In the 30 primary studies a total of 93 prognostic factors were reported as statistically significant in predicting the risk of progression and/or spinal collapse. The considerable variability in the prognostic factor categories, the quality of studies, the lack of studies for some categories, and changes in practice over the time period to which the studies relate have all made it difficult to provide clear conclusions as to which factors might currently offer the most potential to identify patients at high risk of vertebral fracture and SCC.
The evidence presented in this report suggests that the greater the extent of invasion of any one vertebra by metastases, the more likely spinal fracture is to occur, and the more spinal metastases present and the longer a patient is at risk, the greater the chance of SCC. In addition, there is an increased risk of developing SCC if a cancer has already spread to the bones. Clinicians are likely to have been aware of these factors and much of the research reported here appears to add little to current knowledge. Several included studies with mixed case populations identified cancer type as a significant factor in predicting SCC, but it remains difficult to determine a precise increment in risk as a result of the type of cancer (e.g. breast, lung or prostate cancer) and these studies are liable to suffer from both length and lead-time bias.
A broad range of factors was associated with preoperative compression fractures and MSCC. These included sensory deficits, primary breast cancer, anterior spine metastases, inability to walk, increased deep tendon reflexes, longer time interval from diagnosis of primary tumour until development of SCC, longer-surviving patients, type of primary tumour, thoracic spine involvement, preoperative chemotherapy, tumour size and pedicle destruction, focal radiographic abnormalities with consistent neurological findings, patient's health status and, possibly, preoperative radiation therapy.
Some specific prognostic factors were only identified by a few studies (or in some cases by a single study). The most commonly reported factor was related to tumour characteristics and was found to be significant for 11 factors in eight studies;88,89,109,110,113,117,126,129 however, the definition of tumour characteristics varied between the different studies [e.g. amount of vertebral body occupied by tumour,88 overall tumour size and pedicle destruction in the thoracolumbar and lumbar spine (TH10-l5),89 tumour size in the thoracic region,89 blastic-type tumour,109 lytic-type tumour,109 tumour pain,109 favourable tumour histology,117 time interval from diagnosis of the primary tumour,113 total involvement of vertebra,129 tumour involvement of > 50%,110 undifferentiated tumours126].
In addition to the 93 prognostic factors reported as statistically significant in one or more of the 30 included studies, a further large number of potential prognostic factors were identified in the included studies.
As far as diagnostic interventions were concerned, MRI was reported to be the best available technology for detecting malignant compression and, relative to MRI, plain radiography and bone scintigraphy were judged to perform badly.
Prognostic factors by cancer type
Summary of prostate cancer studies
None of the included prostate cancer studies provided a description of the natural history of spinal metastases.
The six included studies varied in methodology and transparency, and this resulted in difficulties in interpreting the findings reported. In particular, it was often difficult to ascertain how study samples were selected. In three studies (by Bayley et al. 107 and Venkitaraman et al. 132,133) patient participation depended on physicians' decisions (e.g. regarding requirement for MRI), but the criteria for decision-making were not clear. In Huddart et al. ,115 an investigation conducted at the same centre as the Venkitaraman studies,132,133 but a decade earlier, participants had been diagnosed with SCC; however, it was not clear if this was a subsample of such patients at the centre or a complete set. The report of Soerdjbalie-Maikoe et al. 129 gave no information regarding sampling frame. In Kuban et al. 118 both sampling frame and selection procedure were fully described.
Patient populations differed with regard to degree of progression of their prostate cancer so that looking for coherence of results across studies should be undertaken with caution. In the study by Bayley et al. 107 patients had metastatic prostate cancer with neurological deficit. In two studies (by Kuban et al. 118 and Huddart et al. 115) metastatic patients with SCC were examined. Venkitaraman et al. 132 investigated patients with SCC but no neurological deficit, whereas in two studies (by Venkitaraman et al. 133 and Soerdjbalie- Maikoe et al. 129) patients had progressed to become castration resistant. A further complication arises because previous and current treatments and the timing of their implementation, both of which are likely to affect the natural progression of the spinal metastases and to influence the identity of potential prognostic factors, varied between studies.
All studies used medical records to ascertain measures of and presence of risk factors. These records are not collected for the purposes of the studies according to a structured framework that was applied equitably to each participant. Furthermore, the completeness of information content within the records was indeterminate. The six studies together included only 409 patients.
In one investigation of castration-resistant metastatic prostate cancer, risk of SCC before death was 24% and was 2.37 times greater with high-grade cancer than with low-grade cancer (Gleason score ≥ 7 compared with < 7) (p = 0.003). A further investigation reported that patients with six or more bone lesions were at greater risk of SCC than those with fewer than six lesions (OR 2.9, 95% CI 1.012 to 8.35; p = 0.047). For these patients median time from initial MRI for suspected SCC to development of neurological deficit was 896 days (95% CI 13 to 986 days).
The results from these studies imply the following:
-
Patients with a high-risk bone scan may benefit from MRI investigations of the spine aimed at early detection and treatment of occult SAS compression/SCC.
-
The more spinal metastases present, and the longer a patient is at risk, the greater the chance of clinically occult SCC.
-
The time a patient is on hormone therapy may be a proxy for how long they are at risk of occult compression.
-
‘Total involvement of vertebra’, according to scintigraphy, appeared to be highly discriminatory for subsequent SCC (Soerdjbalie-Maikoe et al. 129).
-
Time-to-event analyses were difficult to generalise because of the different populations studied and uncertainty regarding their representativeness.
-
The validity of the risk factors identified in these studies did not appear to have been tested in an independent population selected according to similar criteria.
-
No significant predictive factors were identified for risk of future relapse (i.e. second SCC).
Summary of breast cancer studies
None of the studies described the natural history of spinal metastases derived from breast cancer.
The seven included studies were disparate in terms of population, imaging procedures and study aims. Harrison et al. 's participants112 with suspected SCC underwent myelographic imaging and an attempt was made to identify risk factors associated with positive and negative myelograms. Lu et al. 120 examined 93 patients with suspected SCC and identified clinical and oncological features associated with a positive CT scan for SCC. Oka et al. 123 searched for risk factors associated with development of bone metastases in 695 breast cancer patients and another study (by Plunkett et al. 24) looked for factors associated with skeletal events in breast cancer patients with bone metastases. McCloskey et al. 122 investigated how dimensional measures (e.g. vertebral height) made in vertebrae with metastases and in adjacent intact vertebrae could be used in the diagnosis of vertebral fracture/collapse while the two biomechanical studies (by Snyder et al. 127,128) examined the power of vertebral load-bearing capacity estimates for predicting vertebral fracture, comparing the specificity of the method with that of Taneichi et al. 89
In the early study by Harrison et al. 112 a positive myelogram for suspected epidural SCC was associated with a positive bone scan (p < 0.001), bone pain (p < 0.001) and paraesthesias (p = 0.009). Among breast cancer patients who underwent a CT for suspected SCC, multiple logistic regression identified four independent variables predictive of a positive test: bone metastases ≥ 2 years (OR 3.0, 95% CI 1.2 to 7.6; p = 0.02); metastatic disease at initial diagnosis (OR 3.4, 95% CI 1.0 to 11.4; p = 0.05); objective weakness (OR 3.8, 95% CI 1.5 to 9.5; p = 0.005); and vertebral compression fracture on spine radiograph (OR 2.6, 95% CI 1.0 to 6.5; p = 0.05). A Japanese Cox's regression study of breast cancer patients following primary surgery indicated that the risk of developing bone metastases was associated with TNM T stage (HR 1.615, 95% CI 1.322 to 1.973; p < 0.0001); N stage classification (HR 2.128, 95% CI 1.381 to 3.279; p = 0.0006); presence of metastases to axillary lymph nodes (p = 0.0006); and the presence of metastases in important organs (HR 7.502, 95% CI 5.100 to 11.036; p < 0.0001). Of patients who developed skeletal metastases, 82% exhibited spinal metastases, and 14% of these developed paralysis. The median time between detection of skeletal metastases and development of SCC was 4.4 (range 2–72) months.
A consideration of quantitative results from the breast cancer studies does not easily allow generation of a coherent numerical summary; as with prostate cancer, studies were heterogeneous especially with regard to populations, results were not consistent between studies, and almost universally, study results lacked independent corroboration.
The results summarised below should therefore be viewed with caution:
-
A positive bone scan, back pain, paraesthesia and bladder/bowel dysfunction at the time of myelography were more common in patients with a positive myelogram than in those with a negative myelogram (Harrison et al. 112).
-
Objective weakness in patients with suspected SCC was predictive for SCC, although calculated estimates of sensitivity and specificity were very modest (Lu et al. 120).
-
Stratification of patients suspected of SCC according to the number of independent risk factors identified a high-risk group with an 85% probability of CT-positive SCC (Lu et al. 120).
-
TNM classification stages were identified as risk factors – N stage classification, metastases to axillary lymph nodes and visceral metastases for the development of skeletal metastases (Oka et al. 123).
-
Longer survival was found to be a risk factor for vertebral fracture and for spinal cord compression (Plunkett et al. 24).
-
The ‘vertebral load bearing capacity algorithm’ developed by Snyder et al. 127,128 was reported as having superior specificity to the method used by Taneichi et al. 89 for predicting vertebral collapse.
The included studies generally provided limited information about the patient population and selection criteria. Results from time-to-event analyses are difficult to generalise because of the different populations studied and uncertainty regarding their representativeness.
Summary of lung cancer studies
Two of the three included studies (by Sekine et al. 125 and Sun et al. 130) investigated patients with NSCLC and recruited a substantial number of participants (642 with advanced disease and 273 with bone metastases). Goldman et al. 111 studied SCLC.
Among patients with advanced NSCLC who received chemotherapy, the occurrence of SREs (i.e. fracture, SCC, requirement for bone surgery or radiotherapy, or hypocalcaemia causing death or requiring emergency treatment) was reported to be associated with the load of bone metastases (OR 3.08, 95% CI 1.60 to 5.94 for single bone metastasis; OR 4.27, 95% CI 2.66 to 6.86 for multiple bone metastases). Among patients with more than one bone metastasis, the median time from start of chemotherapy to occurrence of first SRE was 19.7 months (95% CI 14.5 to 24.9 months). Another study of advanced SCLC patients with skeletal metastases multivariate analysis identified ‘ever smoked’ as significantly associated with risk of a SRE (OR 2.8, 95% CI 1.32 to 6.00).
Findings included:
-
The greater the number of bone metastases, the greater the risk of a SRE (Sekine et al. 125).
-
Smoking, no history of treatment with EGFR TKIs, poor ECOG status and non-adenocarcinoma were associated with more likely occurrence of SREs (Sun et al. 130).
-
For patients with and without SCC, a combination of cerebral metastases and a positive bone scan were reported to provide a post-test 25% probability for developing SCC, an improvement on the pre-test probability of 0.039. However, this result should be viewed with caution because it was unclear if cerebral metastases actually preceded SCC (Goldman et al. 111).
These were retrospective studies that depended on retrieval of information from medical records not designed for and possibly not suitable for the study questions addressed. Caution is needed in generalising the conclusions across and beyond the included studies. The prognostic factors identified have not been validated in other independent populations.
Summary of studies involving a variety of cancers
Thirteen studies88,89,108,109,113,114,116,117,119,121,124,126,131 investigated mixed primary tumour types. Patients with breast, prostate and lung cancers provided the majority of participants; however, it is important to note that the relative contribution of different tumour types varied considerably from study to study. A very broad range of factors was investigated. The variation in samples in this regard is summarised in Figure 20. The study by Levack et al. 119 provided insufficient detail to determine the frequency of each major tumour type in the sample. In most studies patients with breast, prostate and lung cancers provided the majority of participants; however, this was not invariably so, and the relative contribution from these three common cancer types was variable from study to study. Attempts to identify symptoms and signs that might increase diagnostic ability were not always successful in the included studies (e.g. the study by Levack et al. 119). Because of the very broad range of factors investigated (see Table 7) this summary focuses on significant findings common to several studies.
Among patients who received surgery for SCC, a retrospective analysis identified that vertebral body compression fractures were associated with presurgery chemotherapy (OR 2.283, 95% CI 1.064 to 4.898; p = 0.03), primary breast cancer (OR 4.179, 95% CI 1.457 to 11.983; p = 0.008), thoracic involvement (OR 3.505, 95% CI 1.343 to 9.143; p = 0.01) and anterior cord compression (OR 3.213, 95% CI 1.416 to 7.293; p = 0.005). In another study, TSC was associated with abnormal neurological examination (OR 3.0, 95% CI 1.6 to 10.4; p = 0.004), stage IV cancer at initial diagnosis (OR 2.8, 95% CI 1.40 to 7.7; p = 0.006), known vertebral metastases (OR 2.8, 95% CI 1.4 to 8.2; p = 0.008) and middle or upper back pain (OR 2.7, 95% CI 1.4 to 9.1; p = 0.010).
Findings common to several of these mixed cancer studies included:
-
Primary tumour type was a risk factor for vertebral collapse in two studies (by Chaichana et al. 109 and Shah et al. 126).
-
Primary tumour type was also a risk factor for SCC recurrence in three studies (by Helweg-Larsen et al. 113,114 and Klekamp and Samii117).
-
Patient health status was also a factor in SCC recurrence (by Klekamp and Samii117).
-
Degree of tumour occupancy of the vertebral body was predictive for fracture in the studies by Roth et al. ,124 Rose et al. 88 and Taneichi et al. 89.
-
Two studies (by Lu et al. 121 and Talcott et al. 131) were able to identify risk factors which in combination in a single individual predicted SCC with high probability – five factors present delivered a probability of 87%131 and a combination of three or four factors gave a probability of 81%. 12
-
Taneichi et al. 89 constructed an empirical algorithm for prediction of fracture in vertebrae harbouring predominantly lytic metastases, which was found to be potentially useful, as were other proposed models.
Missing data and a lack of transparency and clarity in reporting, particularly regarding participant selection, mean that in general the validity of findings was uncertain. No studies tested the performance of identified risk factors in a cohort independent of that in which the factors had been identified.
An inference that follows from the finding that risk of fracture appears to vary with primary tumour diagnosis is that the mix of cancer types in these studies, and the length of time from primary diagnosis to study entry, will influence the results of any analysis of prognostic factors. This leads to considerable difficulty interpreting the results from these studies. Potential confounding effects of primary tumour type, age and treatment with bisphosphonates or other interventions should have been considered when attempting to identify predictive variables.
Overall evaluation of the results
We did not identify any epidemiological study with a primary aim of investigating the natural history of spinal metastases. Because the progression of spinal metastases, from inception to complications, will be influenced by the use of the many different interventions that can affect host bone and/or resident metastases, and because spinal metastases vary according to tumour type and the general condition of the patient, a description of a natural history of spinal metastases is problematic. Relatively poor imaging methods were available in the early studies and interventions were minimal. This means that these studies are likely to provide unrepresentative cohorts with spinal metastases detected at late stages of development.
Imaging methods used for detection of SCC and/or vertebral fracture have changed over the duration of the studies described. Formal comparison of different imaging procedures was rarely undertaken and we found no RCTs. It is clear that investigations now favour MRI and CT over myelography only and/or plain radiography. Bone scans were widely employed but PET was not used in any of the included studies. The development and routine availability of machines with faster throughput and better performance (e.g. resolution) may change practice.
The included studies provided some evidence regarding factors that influence the risk of vertebral fracture and/or SCC. In general, these risk factors were unsurprising and would be familiar to clinicians charged with the care of patients with spinal metastases. They include the following: number of spinal metastases (or skeletal metastases); the time of exposure to spinal metastases (i.e. survival); type of primary tumour and whether the spinal metastasis is lytic or blastic; and the degree of occupancy of the vertebral body by the metastasis and its distribution. Three studies attempted to combine risk factors into a decision rule that developed a probability for occurrence of an event. These appeared to have modest discriminatory power but were not tested by the authors in a population independent of that in which they were developed. Generally, included studies were of poor methodological quality and made use of medical records and/or stored scan images rather than using data collection techniques specifically designed for research purposes.
Strengths and limitations of this review
Many bibliographic databases were searched and a large volume of literature was sifted by two reviewers. We used a rigorous search strategy in a large number of databases to identify research papers. Reviewers had difficulties in determining whether or not a paper met the inclusion criteria at abstract level and therefore a large number of papers needed to be sifted at full-paper stage. Nevertheless, our κ-statistic at 0.74 was acceptable. We have summarised a large volume of research. We used a detailed quality assessment process using a dedicated prognostic factors quality assessment framework developed by one of our team and an in-depth analysis (where possible) by cancer type.
Unfortunately, the relatively poor quality and methodology of the papers retrieved, coupled with the variability of underlying patient populations investigated, makes it difficult to draw overall and generalisable conclusions about development of vertebral fracture and SCC. It was not possible to examine the full text of all 2425 retrieved studies; consequently, natural history descriptions might exist but the titles (and abstracts) of such publications failed to reveal this fact readily. At full paper it was difficult to identify relevant information related to the prediction of spinal collapse. The sifting process was time-consuming as it required detailed scrutiny and evaluation of a large number of papers.
It is a weakness that owing to lack of reports of the natural history we are unable to draw any conclusions on this aspect of the review. As far as prognostic factors are concerned, the heterogeneity between studies prevented the use of meta-analysis and again, because of this it is difficult to summarise findings.
Research needs
Clear conclusions cannot currently be drawn from the evidence to identify patients at high risk of vertebral fracture and SCC, either clinically or using imaging investigations. Prospective clinical studies are needed to define those patients who are more likely to present with fractures and to establish functional outcomes and cost-effectiveness of identification and treatment of these patients. MRI is often used for diagnosing SCC and it would be useful to know which patients are at high risk of SCC and are most likely to benefit from early detection and treatment.
In the absence of good predictors, repeated imaging using MRI to monitor progression may offer the best route to identifying patients who can benefit from intervention. Venkitaraman et al. 133 proposed further research to investigate the statistically significant positive effect of early detection of SCC using serial screening MRI. They suggested that the effect of early treatment on neurological function and on survival in metastatic castration-resistant prostate cancer patients needed to be explored with a prospective randomised study involving a quality of life and health economic analysis. The NICE guidelines on SCC also state that MRI screening for SCC in asymptomatic high-risk patients is a promising area for further clinical research. 15 The evidence presented in this short report also supports this conclusion. We would suggest that research on the optimal frequency for MRI screening would also be beneficial.
Personal communication (Professor Charles Hutchinson, University of Warwick, 2011, personal communication) from our clinical expert has suggested that ≈ 10% of patients with metastases of the spine are currently re-imaged using MRI. (This information is taken from a database of 24,991 MRI patients' images obtained over the last 5 years in one large hospital in the UK; 1175 patients had metastatic disease of the spine and 125 had repeat scans.) This proportion of patients suggests that the practice of re-imaging is current and it is likely to be increasing. However, given that MRI is an expensive and sometimes limited resource,107 it may be useful to undertake Service Delivery and Organisation research on MRI scans and scanning (in tandem with research studies on use of MRI to monitor progression) to understand the best methods for maximising use of MRI scanners (e.g. to investigate variation in need, and optimal location, throughput and staffing, etc.).
Several included studies involved prognostic algorithms (see Lu et al. ,120 Talcott et al. 131 and Taneichi et al. 89) designed to calculate the probability of a specified event. These findings could be explored further in high-quality prospective studies, involving defined populations, randomly selected and clearly identified samples, and with blinding of investigators.
A very broad range of factors was associated with preoperative compression fractures and MSCC, including lack of sensory deficits; primary breast cancer; anterior spine metastases; inability to walk; increased deep tendon reflexes; time interval from diagnosis of primary tumour until development of SCC; longer surviving patients; type of primary tumour; thoracic spine involvement; preoperative chemotherapy; tumour size and pedicle destruction; focal radiographic abnormalities with consistent neurological findings; patient's health status; and possibly preoperative radiation therapy. Many of the included studies focused on specific regions of the spine (e.g. cervical and thoracolumbar). These results are not necessarily generalisable to all regions of the spine and should be treated with caution. Higher-quality prospective studies would be valuable on these risk factors of progression or spinal collapse, as opposed to survival, and these could usefully feed into work on prognostic algorithms.
Methodologically, suggestions for improving primary135,138 and secondary139,140 prognosis research are increasingly being reported. Furthermore, the statistical interpretations of prognostic findings, in terms of survival, are being considered. 141 There have been a number of publications reporting the development, validation and impact of prognostic models. 142–147 Recently, Henriksson et al. 148 developed a new risk equation from a Swedish cohort and carried out external validation in a smaller UK data set of patients waiting for coronary artery surgery. A lifetime time horizon was used and risk of cardiovascular events was extrapolated from the Swedish data using a Markov model. Although the authors recognise that the risk score requires further validation and refinement, this provides a useful example of how researchers might consider assessing cost-effectiveness of prognostic factors with decision models to enable prioritisation of patients waiting for treatment. 148 Importantly, the authors recognised several limitations that might be considered in further research; for example, the RR estimates for specific factors might be inflated because of publication bias or because of inadequate adjustment for the routinely recorded factors known to relate to prognostic factors and outcomes.
The Cochrane Prognosis Methods Group has also been highly influential in the conduct of systematic reviews of prognosis (see http://prognosismethods.cochrane.org/, accessed 1 December 2011). 149 This group has recognised that there are many issues particularly pertinent for systematic reviews of prognosis, for example (1) lack of clarity with indexing of studies for bibliographic searches; (2) low quality of primary studies; (3) poor reporting; and (4) difficulties in pooling results across research designs, analyses and presentations of results. All these factors have implications for future primary and secondary research of spinal metastases and risks of spinal collapse and fracture.
Ordered summary of research needs
There is a need for:
-
prospective randomised designs to establish clinical and quality of life outcomes and cost-effectiveness of identification and treatment of patients at high risk of vertebral collapse and SCC, using bone scintigraphy and serial MRI to identify patient groups who are most likely to benefit from early detection and treatment, and the value and optimal frequency of MRI screening for populations
-
Service Delivery and Organisation research on MRI scans and scanning (in tandem with research studies on use of MRI to monitor progression) to understand best methods for maximising use of MRI scanners (e.g. to investigate variation in need, and optimal location, throughput and staffing, etc.)
-
investigation of prognostic algorithms designed to calculate the probability of a specified event using high-quality prospective studies, involving defined populations, randomly selected and clearly identified samples, and with blinding of investigators
-
higher-quality prospective studies to investigate and confirm previous findings on risk factors for progression or spinal collapse, as opposed to survival – these could usefully feed into work on prognostic algorithms
-
methodological research to improve prognosis research.
Implications for clinical practice
This review has provided data on a large number of prognostic factors. Some may warrant further consideration although the weak discriminatory power of most is not encouraging for clinicians wishing to use the research in practice to guide selection for surgery or other interventions. A patient's likelihood of development of severe neurological complications is the most important consideration and there is potential for rapid and/or sustained improvement in quality of life after timely intervention. In the absence of good predictors, repeated imaging (e.g. MRI) to monitor progression may offer the best route to identifying patients who can benefit in this way. Spinal instability is a key component in treatment decision-making for spinal oncology patients, although it is poorly defined in the literature and there is a lack of current guidelines to support definition of the degree of spinal instability in the setting of spinal metastases. 110 However, in making surgical treatment decisions, stability is only one factor in the process. General health, tumour histology, overall prognosis, duration of disease symptomatology, neurology and patient choice clearly warrant consideration.
The major factors which should be taken into account when considering a patient for further investigation and potential treatment when at risk of SCC, progression or spinal collapse have not altered from those identified in 2008 NICE guideline 75. 15
Our clinical experts have directed us to the cost information provided in the NICE guideline 75. 15 For further details on cost, see Appendix 9.
Chapter 5 Conclusions
This report has identified a large number of potentially relevant factors reported across 31 studies but the evidence base is generally poor. There was a lack of consistency in methodology and rigour in studies reported. There was limited evidence from studies with a primary aim of investigating risk factors for progression or spinal collapse, with more focus within studies directed at predictive factors for overall survival. There was a lack of sample frame definition and selection, variations in the stage of disease, mixed cancers, reliance on retrospective data with no RCTs and a repeated failure to test risk factors in another population. Although we have identified many limitations in the current evidence base, these findings should be considered carefully when developing further research in this area.
Natural history
Our extensive sifting of retrieved studies failed to identify appropriate studies. A definition of the natural history of a medical condition is ‘The timeline of a morbid condition from onset–inception to resolution; the course of a particular disease if it is not treated or manipulated in any way’ (http://medical-dictionary.thefreedictionary.com/natural+history+of+disease, accessed December 2011). A description of the progression of spinal metastases from inception to the development of undesirable sequelae, un-influenced by interventions, is problematic because these patients receive many types of intervention that affect both tumour characteristics and the structure and biochemistry of host bone. Changes over time mean that very early studies in which intervention may have been minimal would no longer be useful because of inadequacies in the frequency and resolution of imaging modalities.
Prognostic studies
The quality of studies was generally limited. No RCTs were identified and study designs were such that the results were susceptible to biases (especially selection bias) and analyses were susceptible to confounding from unrecorded or unanalysed variables.
The body of evidence provided by the studies was not strong; very many potentially prognostic variables were investigated, but testing of these beyond the population in which they were developed was minimal. In those investigations that developed prognostic algorithms, or risk probabilities according to stratification of risk factors, it appeared that the discriminatory performance of the models was modest.
Imaging modalities
It is clear from many studies that the current clinical consensus favours MRI and CT imaging modalities for the investigation of SCC and vertebral fracture. Myelography appears reserved for ‘difficult’ cases and plain radiography for preliminary investigation. Formal comparison of modalities was not convincingly undertaken among the included studies. Bone scintigraphy was widely used in the studies included in this review and may be the method of choice for readily establishing the load of spinal and other bone metastases, but there is no evidence currently to support this. In practice the choice of imaging modality may well be influenced by availability of appropriate instruments at the time required. The development and routine availability of machines or practices with increased or faster throughput and better performance (e.g. resolution) may change practice.
Clinical importance of spinal metastases
Early diagnosis and treatment of SCC is essential for the preservation of neurological function. 107 However, diagnosis of SCC is frequently not established until significant neurological deficit is present, by which time functional recovery may be difficult. At this stage treatment may need to be undertaken as an emergency, often with reduced efficiency and at increased cost. Therefore, early diagnosis of SCC before the development of symptoms may allow for treatment to preserve neurological function in some patients who might otherwise be left with significant problems. This may in turn result in more efficient management of diagnostic and therapeutic staff and facilities, and reduce long-term costs of caring for disabled patients. However, none of the identified studies discussed costs or cost-effectiveness.
The included studies, and many other publications discussed in the introduction of this report, testify to the serious consequences that may arise from spinal metastases and the impact of these on the quality of life of patients. We consider that further research is needed in this area. The desirability of good predictors of unwanted sequelae from spinal metastases is clear; however, this review suggests that good-quality evidence on either natural history or on technologies for identifying patients at high risk of vertebral fracture and SCC does not currently exist.
Acknowledgements
We would like to thank Professor Charles Greenough, Trauma and Orthopaedics, South Tees NHS Trust; Professor Charles E Hutchinson, Professor of Radiology, University Hospitals Coventry and Warwickshire; and Professor Martin Underwood, Head of Division of Health Sciences, Professor of Primary Care Research, University of Warwick.
Contributions of authors
Paul Sutcliffe (Senior Research Fellow) and Martin Connock (Senior Research Fellow) co-ordinated the review.
Rachel Court (Information Specialist) developed the search strategy and undertook searches.
Paul Sutcliffe and Martin Connock screened the search results; they screened retrieved papers against inclusion criteria, appraised the quality of papers and abstracted data from papers.
Paul Sutcliffe, Martin Connock and Ngianga-Bakwin Kandala (Principal Research Fellow) analysed the data.
Deepson Shyangdan (Research Fellow), Paul Sutcliffe and Martin Connock wrote the report.
Aileen Clarke (Professor of Public Health & Health Services Research) provided advice on design and analysis, wrote the summary and provided comments on the report.
About Warwick Evidence
Warwick Evidence is a Health Technology Assessment Group, located in Warwick Medical School, working in close collaboration with the NHS to support the further development of knowledge-based health services. Warwick Evidence brings together experts in clinical effectiveness and cost-effectiveness reviewing, health economics and modelling.
Disclaimer text
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Gilbert RW, Kim JH, Posner JB. Epidural spinal cord compression from metastatic tumor: diagnosis and treatment. Ann Neurol 1978;3:40-51. http://dx.doi.org/10.1002/ana.410030107.
- Office for National Statistics . Statistical Bulletin: Births and Deaths in England and Wales, 2010 n.d. www.ons.gov.uk/ons/rel/vsob1/death-reg-sum-tables/2009--final-/births-and-deaths-summary-tables-stats-bulletin---2009.pdf (accessed 11 December 2011).
- Cancer Research UK . Latest UK Cancer Incidence (2008) and Mortality (2008) Summary 2011. http://info.cancerresearchuk.org/prod_consump/groups/cr_common/@nre/@sta/documents/generalcontent/cr_072108.pdf (accessed 9 December 2011).
- Cancer Research UK . CancerStats Incidence 2008 – UK 2011. http://info.cancerresearchuk.org/prod_consump/groups/cr_common/@nre/@sta/documents/generalcontent/cr_072111.pdf (accessed 9 December 2011).
- Cancer Research UK . Deaths from Common Cancers – UK Mortality Statistics 2011. http://info.cancerresearchuk.org/cancerstats/mortality/cancerdeaths/ (accessed 9 December 2011).
- Singh S, Singh G, Singh G, Rabbani SA. Bone metastasis: experimental and clinical therapeutics. New York, NY: Humana Press Inc.; 2005.
- Bartels RHMA, van der Linden YM, van der Graaf WTA. Spinal extradural metastasis: review of current treatment options. CA Cancer J Clinicians 2008;58:245-59. http://dx.doi.org/10.3322/CA.2007.0016.
- Delank KS, Wendtner C, Eich HT, Eysel P. The treatment of spinal metastases. Dtsch Arzt Int 2011;108:71-U27.
- Tse V. Spinal Metastases and Metastatic Disease to the Spine and Related Structures 2009. http://emedicine.medscape.com/article/1157987-overview (accessed April 2011).
- Harel R, Angelov L. Spine metastases: current treatments and future directions. Eur J Cancer 2010;46:2696-707. http://dx.doi.org/10.1016/j.ejca.2010.04.025.
- Maccauro G, Spinelli MS, Mauro S, Perisano C, Graci C, Rosa MA. Physiopathology of spine metastasis. Int J Surg Oncol 2011:1-8. http://dx.doi.org/10.1155/2011/107969.
- Perrin RG, Laxton AW. Metastatic spine disease: epidemiology, pathophysiology, and evaluation of patients. Neurosurg Clin N Am 2004;15. http://dx.doi.org/10.1016/j.nec.2004.04.018.
- Boogerd W, Vandersande JJ. Diagnosis and treatment of spinal-cord compression in malignant disease. Cancer Treat Rev 1993;19:129-50. http://dx.doi.org/10.1016/0305-7372(93)90031-L.
- Rades D, Stalpers LJ, Veninga T, Schulte R, Hoskin PJ, Obralic N, et al. Evaluation of five radiation schedules and prognostic factors for metastatic spinal cord compression. J Clin Oncol 2005;23:3366-75. http://dx.doi.org/10.1200/JCO.2005.04.754.
- National Institute for Health and Care Excellence (NICE), National Collaborating Centre for Cancer . National Institute for Health and Care Excellence Clinical Guideline 75. Metastatic Spinal Cord Compression: Diagnosis and Management of Adults at Risk of or With Metastatic Spinal Cord Compression 2008. http://guidance.nice.org.uk/C975/Guidance/pdf/English (accessed April 2011).
- Cancer Research UK . Breast Cancer – UK Incidence Statistics 2011. http://info.cancerresearchuk.org/cancerstats/types/breast/incidence/ (accessed 8 December 2011).
- Bendre M, Gaddy D, Nicholas RW, Suva LJ. Breast cancer metastasis to bone: it is not all about PTHrP. Clin Orthop Relat Res 2003;415:S39-S45. http://dx.doi.org/10.1097/01.blo.0000093844.72468.f4.
- Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 2006;12:6243s-9s. http://dx.doi.org/10.1158/1078-0432.CCR-06-0931.
- Walkington L, Coleman RE. Advances in management of bone disease in breast cancer. Bone 2011;48:80-7. http://dx.doi.org/10.1016/j.bone.2010.05.037.
- Cancer Research UK . Types of Treatment for Breast Cancer n.d. www.cancerresearchuk.org/cancer-help/type/breast-cancer/treatment/which-treatment-for-breast-cancer (accessed 8 December 2011).
- Coleman RE, Rubens RD. The clinical course of bone metastases from breast cancer. Br J Cancer 1987;55:61-6. http://dx.doi.org/10.1038/bjc.1987.13.
- Jensen AO, Jacobsen JB, Norgaard M, Yong M, Fryzek JP, Sorensen HT. Incidence of bone metastases and skeletal-related events in breast cancer patients: a population-based cohort study in Denmark. BMC Cancer 2011;11. http://dx.doi.org/10.1186/1471-2407-11-29.
- Dent R, Hanna WM, Trudeau M, Rawlinson E, Sun P, Narod SA. Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res Treat 2009;115:423-8. http://dx.doi.org/10.1007/s10549-008-0086-2.
- Plunkett TA, Smith P, Rubens RD. Risk of complications from bone metastases in breast cancer: implications for management. Eur J Cancer 2000;36:476-82. http://dx.doi.org/10.1016/S0959-8049(99)00331-7.
- Coleman RE, Smith P, Rubens RD. Clinical course and prognostic factors following bone recurrence from breast cancer. Br J Cancer 1998;77:336-40. http://dx.doi.org/10.1038/bjc.1998.52.
- Cancer Research UK . Lung Cancer – UK Incidence Statistics 2011. http://info.cancerresearchuk.org/cancerstats/types/lung/incidence/ (accessed 9 December 2011).
- National Institute for Health and Care Excellence clinical guideline 121 – lung cancer. The diagnosis and treatment of lung cancer. London: NICE; 2011.
- Charloux A, Quoix E, Wolkove N, Small D, Pauli G, Kreisman H. The increasing incidence of lung adenocarcinoma: reality or artefact? A review of the epidemiology of lung adenocarcinoma. Int J Epidemiol 1997;26:14-23. http://dx.doi.org/10.1093/ije/26.1.14.
- Roodman GD. Mechanisms of bone metastasis. N Engl J Med 2004;350:1655-64. http://dx.doi.org/10.1056/NEJMra030831.
- Al Husaini H, Wheatley-Price P, Clemons M, Shepherd FA. Prevention and management of bone metastases in lung cancer: a review. J Thorac Oncol 2009;4:251-9. http://dx.doi.org/10.1097/JTO.0b013e31819518fc.
- Cancer Research UK . Prostate Cancer – UK Incidence Statistics 2011. http://info.cancerresearchuk.org/cancerstats/types/prostate/incidence/ (accessed 9 December 2011).
- National Institute for Health and Clincial Excellence Clinical guideline 58 – prostate cancer: diagnosis and treatment. London: NICE; 2008.
- Selley S, Donovan J, Faulkner A, Coast J, Gillatt D. Diagnosis, management and screening of early localised prostate cancer. Health Technol Assess 1997;1.
- Cancer Research UK . Prostate Cancer – Survival Statistics 2010. http://info.cancerresearchuk.org/cancerstats/types/prostate/survival/ (accessed 9 December 2011).
- Thompson IM, Ankerst DP, Chi C, Goodman PJ, Tangen CM, Lucia MS, et al. Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst 2006;98:529-34. http://dx.doi.org/10.1093/jnci/djj131.
- Cancer Research UK . Treating Prostate Cancer n.d. www.cancerresearchuk.org/cancer-help/type/prostate-cancer/treatment/ (accessed 8 December 2011).
- Mimeault M, Mehta PP, Hauke R, Batra SK. Functions of normal and malignant prostatic stem/progenitor cells in tissue regeneration and cancer progression and novel targeting therapies. Endocr Rev 2008;29:234-52. http://dx.doi.org/10.1210/er.2007-0040.
- Balk SP, Knudsen KE. AR, the cell cycle, and prostate cancer. Nucl Recept Signal 2008;6.
- Clinical guideline 131 – colorectal cancer. The diagnosis and management of colorectal cancer. London: NICE; 2011.
- Cancer Research UK . Bowel Cancer – Survival Statistics 2009. http://info.cancerresearchuk.org/cancerstats/types/bowel/survival/ (accessed 9 December 2011).
- Sundermeyer ML, Meropol NJ, Rogatko A, Wang H, Cohen SJ. Changing patterns of bone and brain metastases in patients with colorectal cancer. Clin Colorectal Cancer 2005;5:108-13. http://dx.doi.org/10.3816/CCC.2005.n.022.
- Kanthan R, Loewy J, Kanthan SC. Skeletal metastases in colorectal carcinomas: a Saskatchewan profile. Dis Colon Rectum 1999;42:1592-7. http://dx.doi.org/10.1007/BF02236213.
- Galasko CSB, Weiss L, Gilbert HA. Bone metastasis. Boston, MA: G.K. Hall; 1981.
- Stacker SA, Baldwin ME, Achen MG. The role of tumor lymphangiogenesis in metastatic spread. FASEB J 2002;16:922-34. http://dx.doi.org/10.1096/fj.01-0945rev.
- Schoppmeyer K, Fruhauf N, Oldhafer K, Seeber S, Kasimir-Bauer S. Tumor cell dissemination in colon cancer does not predict extrahepatic recurrence in patients undergoing surgery for hepatic metastases. Oncol Rep 2006;15:449-54.
- Carmignani CP, Sugarbaker TA, Bromley CM, Sugarbaker PH. Intraperitoneal cancer dissemination: mechanisms of the patterns of spread. Cancer Metastasis Rev 2003;22:465-72. http://dx.doi.org/10.1023/A:1023791229361.
- Eubank WB, Mankoff DA, Vesselle HJ, Eary JF, Schubert EK, Dunnwald LK, et al. Detection of locoregional and distant recurrences in breast cancer patients by using FDG PET. Radiographics 2002;22:5-17.
- Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest 1997;111:1718-23. http://dx.doi.org/10.1378/chest.111.6.1718.
- Robless P, Lim J, Geroulakos G. Lymphoedema. Surgery 2010;28:268-72.
- Chung JW, Park JH, Han JK, Choi BI, Han MC. Hepatocellular carcinoma and portal vein invasion: results of treatment with transcatheter oily chemoembolization. AJR Am J Roentgenol 1995;165:315-21.
- Skinner DG, Pritchett TR, Lieskovsky G, Boyd SD, Stiles QR. Vena caval involvement by renal cell carcinoma. Surgical resection provides meaningful long-term survival. Ann Surg 1989;210:387-92. http://dx.doi.org/10.1097/00000658-198909000-00014.
- Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2002;2:563-72. http://dx.doi.org/10.1038/nrc865.
- Paget S. The distribution of secondary growths in cancer of the breast. Cancer Metastasis Rev 1889;8:98-101.
- Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860-7. http://dx.doi.org/10.1038/nature01322.
- Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nat Rev Cancer 2011;11:411-25. http://dx.doi.org/10.1038/nrc3055.
- Smith HS. Painful osseous metastases. Pain Physician 2011;14:E373-E403.
- Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev 2001;27:165-76. http://dx.doi.org/10.1053/ctrv.2000.0210.
- Dunstan CR, Boyce R, Boyce BF, Garrett IR, Izbicka E, Burgess WH, et al. Systemic administration of acidic fibroblast growth factor (FGF-1) prevents bone loss and increases new bone formation in ovariectomized rats. J Bone Miner Res 1999;14:953-9. http://dx.doi.org/10.1359/jbmr.1999.14.6.953.
- Sciubba DM, Petteys RJ, Dekutoski MB, Fisher CG, Fehlings MG, Ondra SL, et al. Diagnosis and management of metastatic spine disease. A review. J Neurosurg Spine 2010;13:94-108. http://dx.doi.org/10.3171/2010.3.SPINE09202.
- Kilbride L, Cox M, Kennedy CM, Lee SH, Grant R. Metastatic spinal cord compression: a review of practice and care. J Clin Nursing 2010;19:1767-83. http://dx.doi.org/10.1111/j.1365-2702.2010.03236.x.
- Sejpal SV, Bhate A, Small W. Palliative radiation therapy in the management of brain metastases, spinal cord compression, and bone metastases. Semin Intervent Radiol 2007;24:363-74. http://dx.doi.org/10.1055/s-2007-992324.
- Loblaw DA, Perry J, Chambers A, Laperriere NJ. Systematic review of the diagnosis and management of malignant extradural spinal cord compression: the cancer care Ontario practice guidelines initiative's neuro-oncology disease site group. J Clin Oncol 2005;23:2028-37. http://dx.doi.org/10.1200/JCO.2005.00.067.
- Ecker RD, Endo T, Wetjen NM, Krauss WE. Diagnosis and treatment of vertebral column metastases. Mayo Clin Proc 2005;80:1177-86. http://dx.doi.org/10.4065/80.9.1177.
- Cook AM, Lau TN, Tomlinson MJ, Vaidya M, Wakeley CJ, Goddard P. Magnetic resonance imaging of the whole spine in suspected malignant spinal cord compression: impact on management. Clin Oncol (R Coll Radiol) 1998;10:39-43. http://dx.doi.org/10.1016/S0936-6555(98)80111-8.
- Loughrey GJ, Collins CD, Todd SM, Brown NM, Johnson RJ. Magnetic resonance imaging in the management of suspected spinal canal disease in patients with known malignancy. Clin Radiol 2000;55:849-55. http://dx.doi.org/10.1053/crad.2000.0547.
- Ohmori K, Kawaguchi Y, Kanamori M, Ishihara H, Takagi H, Kimura T. Image-guided anterior thoracolumbar corpectomy: a report of three cases. Spine (Phila Pa 1976) 2001;26:1197-201.
- Tacke J, Klein HM, Bertalanffy H, Mayfrank L, Thron A, Gilsbach JM, et al. Clinical significance of three-dimensional helical CT in neurosurgery. Minim Invasive Neurosurg 1997;40:30-5. http://dx.doi.org/10.1055/s-2008-1053411.
- Algra PR, Heimans JJ, Valk J, Nauta JJ, Lachniet M, Van KB. Do metastases in vertebrae begin in the body or the pedicles? Imaging study in 45 patients. AJR Am J Roentgenol 1992;158:1275-9.
- Asdourian PL, Weidenbaum M, Dewald RL, Hammerberg KW, Ramsey RG. The pattern of vertebral involvement in metastatic vertebral breast cancer. Clin Orthop Relat Res 1990;250:164-70.
- Stark RJ, Henson RA, Evans SJ. Spinal metastases. A retrospective survey from a general hospital. Brain 1982;105:189-213. http://dx.doi.org/10.1093/brain/105.1.189.
- Rodichok LD, Harper GR, Ruckdeschel JC, Price A, Roberson G, Barron KD, et al. Early diagnosis of spinal epidural metastases. Am J Med 1981;70:1181-8. http://dx.doi.org/10.1016/0002-9343(81)90825-1.
- Schirrmeister H, Glatting G, Hetzel J, Nussle K, Arslandemir C, Buck AK, et al. Prospective evaluation of the clinical value of planar bone scans, SPECT, and (18)F-labeled NaF PET in newly diagnosed lung cancer. J Nucl Med 2001;42:1800-4.
- Prasad D, Schiff D. Malignant spinal-cord compression. Lancet Oncol 2005;6:15-24.
- Eleraky M, Papanastassiou I, Vrionis FD. Management of metastatic spine disease. Curr Opin Support Palliat Care 2010;4:182-8. http://dx.doi.org/10.1097/SPC.0b013e32833d2fdd.
- Inoue T, Oh RJ, Shiomi H. New approach for treatment of vertebral metastases using intensity-modulated radiotherapy. Strahlenther Onkol 2011;187:108-13. http://dx.doi.org/10.1007/s00066-010-2187-1.
- Chang EL, Shiu AS, Mendel E, Mathews LA, Mahajan A, Allen PK, et al. Phase I/II study of stereotactic body radiotherapy for spinal metastasis and its pattern of failure. J Neurosurg Spine 2007;7:151-60. http://dx.doi.org/10.3171/SPI-07/08/151.
- Body JJ. New developments for treatment and prevention of bone metastases. Curr Opin Oncol 2011;23:338-42. http://dx.doi.org/10.1097/CCO.0b013e328347918b.
- Danila DC, Morris MJ, de Bono JS, Ryan CJ, Denmeade SR, Smith MR, et al. Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J Clin Oncol 2010;28:1496-501. http://dx.doi.org/10.1200/JCO.2009.25.9259.
- Reid AH, Attard G, Danila DC, Oommen NB, Olmos D, Fong PC, et al. Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. J Clin Oncol 2010;28:1489-95. http://dx.doi.org/10.1200/JCO.2009.24.6819.
- Jackson RJ, Gokaslan ZL, Loh SCA. Metastatic renal cell carcinoma of the spine: surgical treatment and results. J Neurosurg 2001;94:18-24.
- Tokuhashi Y, Matsuzaki H, Toriyama S, Kawano H, Ohsaka S. Scoring system for the preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila PA 1976) 1990;15:1110-13.
- Cohen ZR, Fourney DR, Gokaslan ZL, Walsh GL, Rhines LD. Anterior stabilization of the upper thoracic spine via an ‘interaortocaval subinnominate window’: case report and description of operative technique. J Spinal Disord Tech 2004;17:543-8. http://dx.doi.org/10.1097/01.bsd.0000117541.10843.c9.
- Fourney DR, Schomer DF, Nader R, Chlan-Fourney J, Suki D, Ahrar K, et al. Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients. J Neurosurg 2003;98:21-30.
- Wenger M. Vertebroplasty for metastasis. Med Oncol 2003;20:203-9. http://dx.doi.org/10.1385/MO:20:3:203.
- Tokuhashi Y, Ajiro Y, Umezawa N. Outcome of treatment for spinal metastases using scoring system for preoperative evaluation of prognosis. Spine 2009;34:69-73. http://dx.doi.org/10.1097/BRS.0b013e3181913f19.
- Tomita K, Kawahara N, Kobayashi T, Yoshida A, Murakami H, Akamaru T. Surgical strategy for spinal metastases. Spine 2001;26:298-306. http://dx.doi.org/10.1097/00007632-200102010-00016.
- Chaichana KL, Pendleton C, Sciubba DM, Wolinsky JP, Gokaslan ZL. Outcome following decompressive surgery for different histological types of metastatic tumors causing epidural spinal cord compression. Clinical article. J Neurosurg Spine 2009;11:56-63. http://dx.doi.org/10.3171/2009.1.SPINE08657.
- Rose PS, Laufer I, Boland PJ, Hanover A, Bilsky MH, Yamada J, et al. Risk of fracture after single fraction image-guided intensity-modulated radiation therapy to spinal metastases. J Clin Oncol 2009;27:5075-9. http://dx.doi.org/10.1200/JCO.2008.19.3508.
- Taneichi H, Kaneda K, Takeda N, Abumi K, Satoh S. Risk factors and probability of vertebral body collapse in metastases of the thoracic and lumbar spine. Spine 1997;22:239-45. http://dx.doi.org/10.1097/00007632-199702010-00002.
- Yamashita K, Denno K, Ueda T, Komatsubara Y, Kotake T, Usami M, et al. Prognostic significance of bone metastases in patients with metastatic prostate cancer. Cancer 1993;71:1297-302. http://dx.doi.org/10.1002/1097-0142(19930215)71:4<1297::AID-CNCR2820710421>3.0.CO;2-S.
- van der Linden YM, Dijkstra SP, Vonk EJ, Marijnen CA, Leer JW. Dutch Bone Metastasis Study Group . Prediction of survival in patients with metastases in the spinal column: results based on a randomized trial of radiotherapy. Cancer 2005;103:320-8. http://dx.doi.org/10.1002/cncr.20756.
- Sioutos PJ, Arbit E, Meshulam CF, Galicich JH. Spinal metastases from solid tumors. Analysis of factors affecting survival. Cancer 1995;76:1453-9. http://dx.doi.org/10.1002/1097-0142(19951015)76:8⟨1453::AID-CNCR2820760824⟩3.0.CO;2-T.
- Enkaoua EA, Doursounian L, Chatellier G, Mabesoone F, Aimard T, Saillant G. Vertebral metastases: a critical appreciation of the preoperative prognostic tokuhashi score in a series of 71 cases. Spine (Phila PA 1976) 1997;22:2293-8.
- Sundaresan N, Galicich JH, Bains MS, Martini N, Beattie EJ. Vertebral body resection in the treatment of cancer involving the spine. Cancer 1984;53:1393-6. http://dx.doi.org/10.1002/1097-0142(19840315)53:6⟨1393::AID-CNCR2820530629⟩3.0.CO;2-0.
- Weigel B, Maghsudi M, Neumann C, Kretschmer R, Muller FJ, Nerlich M. Surgical management of symptomatic spinal metastases. Postoperative outcome and quality of life. Spine 1999;24:2240-6. http://dx.doi.org/10.1097/00007632-199911010-00012.
- Bauer HC, Wedin R. Survival after surgery for spinal and extremity metastases. Prognostication in 241 patients. Acta Orthop Scand 1995;66:143-6. http://dx.doi.org/10.3109/17453679508995508.
- Botteman M, Barghout V, Stephens J, Hay J, Brandman J, Aapro M. Cost effectiveness of bisphosphonates in the management of breast cancer patients with bone metastases. Ann Oncol 2006;17:1072-82. http://dx.doi.org/10.1093/annonc/mdl093.
- Marfatia AA, Botteman MF, Foley I, Brandman J, Langer CJ. Economic value of zoledronic acid compared with placebo in the treatment of skeletal metastases in patients with solid tumors: the case of the United Kingdom (UK). ASCO Annual Meeting Proceedings (Post-Meeting Edition). J Clin Oncol 2007;25.
- NICE technology appraisal guidance 204. Denosumab for the prevention of osteoporotic fractures in postmenopausal women. London: NICE; 2010.
- British national formulary. London: BMA and RPS; 2011.
- U.S. Food and Drug Administration . Denosumab (Prolia) 2012. www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm272420.htm (accessed 23 December 2011).
- Delea TE, McKiernan J, Brandman J, Edelsberg J, Sung J, Raut M, et al. Impact of skeletal complications on total medical care costs among patients with bone metastases of lung cancer. J Thorac Oncol 2006;1:571-6. http://dx.doi.org/10.1097/01243894-200607000-00012.
- Cooper K, Meng Y, Harnan S, Ward S, Fitzgerald P. Positron emission tomography (PET) and magnetic resonance imaging (MRI) for the assessment of axillary lymph node metastases in early breast cancer: systematic review and economic evaluation. Health Technol Assess 2011;15.
- Sutcliffe P, Hummel S, Simpson E, Young T, Rees A, Wilkinson A, et al. Use of classical and novel biomarkers as prognostic risk factors for localised prostate cancer: a systematic review. Health Technol Assess 2009;13.
- Khan KS, Ter Riet G, Galnville J, Snowdon AJ, Kleijnen J. CRD's guidance for carrying out or commissioning reviews. York: NHS Centre for Reviews and Dissemination (CRD), University of York; 2001.
- Hayden JA, Cote P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med 2006;144:427-37.
- Bayley A, Milosevic M, Blend R, Logue J, Gospodarowicz M, Boxen I, et al. A prospective study of factors predicting clinically occult spinal cord compression in patients with metastatic prostate carcinoma. Cancer 2001;92:303-10. http://dx.doi.org/10.1002/1097-0142(20010715)92:2⟨303::AID-CNCR1323⟩3.0.CO;2-F.
- Bernat JL, Greenberg ER, Barrett J. Suspected epidural compression of the spinal cord and cauda equina by metastatic carcinoma. Clinical diagnosis and survival. Cancer 1983;51:1953-7. http://dx.doi.org/10.1002/1097-0142(19830515)51:10⟨1953::AID-CNCR2820511035⟩3.0.CO;2-D.
- Chaichana KL, Pendleton C, Wolinsky JP, Gokaslan ZL, Sciubba DM. Vertebral compression fractures in patients presenting with metastatic epidural spinal cord compression. Neurosurgery 2009;65:267-74. http://dx.doi.org/10.1227/01.NEU.0000349919.31636.05.
- Fisher CG, DiPaola CP, Ryken TC, Bilsky MH, Shaffrey CI, Berven SH, et al. A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine 2010;35:E1221-9. http://dx.doi.org/10.1097/BRS.0b013e3181e16ae2.
- Goldman JM, Ash CM, Souhami RL, Geddes DM, Harper PG, Spiro SG, et al. Spinal cord compression in small cell lung cancer: a retrospective study of 610 patients. Br J Cancer 1989;59:591-3. http://dx.doi.org/10.1038/bjc.1989.119.
- Harrison KM, Muss HB, Ball MR, McWhorter M, Case D. Spinal cord compression in breast cancer. Cancer 1985;55:2839-44. http://dx.doi.org/10.1002/1097-0142(19850615)55:12⟨2839::AID-CNCR2820551222⟩3.0.CO;2-B.
- Helweg-Larsen S, Sorensen PS, Kreiner S. Prognostic factors in metastatic spinal cord compression: a prospective study using multivariate analysis of variables influencing survival and gait function in 153 patients. Int J Radiat Oncol Biol Phys 2000;46:1163-9. http://dx.doi.org/10.1016/S0360-3016(99)00333-8.
- Helweg-Larsen S, Hansen SW, Sorensen PS. Second occurrence of symptomatic metastatic spinal cord compression and findings of multiple spinal epidural metastases. Int J Radiat Oncol Biol Phys 1995;33:595-8. http://dx.doi.org/10.1016/0360-3016(95)00199-9.
- Huddart RA, Rajan B, Law M, Meyer L, Dearnaley DP. Spinal cord compression in prostate cancer: treatment outcome and prognostic factors. Radiother Oncol 1997;44:229-36. http://dx.doi.org/10.1016/S0167-8140(97)00112-6.
- Husband DJ, Grant KA, Romaniuk CS. MRI in the diagnosis and treatment of suspected malignant spinal cord compression. Br J Radiol 2001;74:15-23.
- Klekamp J, Samii H. Surgical results for spinal metastases. Acta Neurochir 1998;140:957-67. http://dx.doi.org/10.1007/s007010050199.
- Kuban DA, el-Mahdi AM, Sigfred SV, Schellhammer PF, Babb TJ. Characteristics of spinal cord compression in adenocarcinoma of prostate. Urology 1986;28:364-9. http://dx.doi.org/10.1016/0090-4295(86)90062-2.
- Levack P, Graham J, Collie D, Grant R, Kidd J, Kunkler I, et al. Don't wait for a sensory level–listen to the symptoms: a prospective audit of the delays in diagnosis of malignant cord compression. Clin Oncol (R Coll Radiol) 2002;14:472-80. http://dx.doi.org/10.1053/clon.2002.0098.
- Lu C, Stomper PC, Drislane FW, Wen PY, Block CC, Humphrey CC, et al. Suspected spinal cord compression in breast cancer patients: a multidisciplinary risk assessment. Breast Cancer Res Treat 1998;51:121-31. http://dx.doi.org/10.1023/A:1006002823626.
- Lu C, Gonzalez RG, Jolesz FA, Wen PY, Talcott JA. Suspected spinal cord compression in cancer patients: a multidisciplinary risk assessment. J Support Oncol 2005;3:305-12.
- McCloskey EV, Spector TD, Eyres KS, Fern ED, Orourke N, Vasikaran SM, et al. The assessment of vertebral deformity – a method for use in population studies and clinical-trials. Osteoporos Int 1993;3:138-47. http://dx.doi.org/10.1007/BF01623275.
- Oka H, Kondoh T, Seichi A, Hozumi T, Nakamura K. Incidence and prognostic factors of Japanese breast cancer patients with bone metastasis. J Orthop Sci 2006;11:13-9. http://dx.doi.org/10.1007/s00776-005-0966-9.
- Roth SE, Mousavi P, Finkelstein J, Chow E, Kreder H, Whyne CM. Metastatic burst fracture risk prediction using biomechanically based equations. Clin Orthop Relat Res 2004:83-90. http://dx.doi.org/10.1097/00003086-200402000-00015.
- Sekine I, Nokihara H, Yamamoto N, Kunitoh H, Ohe Y, Tamura T. Risk factors for skeletal-related events in patients with non-small cell lung cancer treated by chemotherapy. Lung Cancer 2009;65:219-22. http://dx.doi.org/10.1016/j.lungcan.2008.10.026.
- Shah AN, Pietrobon R, Richardson WJ, Myers BS. Patterns of tumor spread and risk of fracture and epidural impingement in metastatic vertebrae. J Spinal Disord Tech 2003;16:83-9. http://dx.doi.org/10.1097/00024720-200302000-00013.
- Snyder BD, Hipp JA, Nazarian A. Non-invasive prediction of fracture risk due to benign and metastatic skeletal defects. Warrendale, PA: Materials Research Society; 2005.
- Snyder BD, Cordio MA, Nazarian A, Kwak SD, Chang DJ, Entezari V, et al. Noninvasive prediction of fracture risk in patients with metastatic cancer to the spine. Clin Cancer Res 2009;15:7676-83. http://dx.doi.org/10.1158/1078-0432.CCR-09-0420.
- Soerdjbalie-Maikoe V, Pelger RCM, Nijeholt GABL, Arndt JW, Zwinderman AH, Bril H, et al. Bone scintigraphy predicts the risk of spinal cord compression in hormone-refractory prostate cancer. Eur J Nucl Med Mol Imaging 2004;31:958-63. http://dx.doi.org/10.1007/s00259-004-1479-z.
- Sun J-M, Ahn JS, Lee S, Kim JA, Lee J, Park YH, et al. Predictors of skeletal-related events in non-small cell lung cancer patients with bone metastases. Lung Cancer 2011;71:89-93. http://dx.doi.org/10.1016/j.lungcan.2010.04.003.
- Talcott JA, Stomper PC, Drislane FW, Wen PY, Block CC, Humphrey CC, et al. Assessing suspected spinal cord compression: a multidisciplinary outcomes analysis of 342 episodes. Support Care Cancer 1999;7:31-8. http://dx.doi.org/10.1007/s005200050220.
- Venkitaraman R, Sohaib SA, Barbachano Y, Parker CC, Khoo V, Huddart RA, et al. Detection of occult spinal cord compression with magnetic resonance imaging of the spine. Clin Oncol (R Coll Radiol) 2007;19:528-31. http://dx.doi.org/10.1016/j.clon.2007.04.001.
- Venkitaraman R, Sohaib SA, Barbachano Y, Parker CC, Huddart RA, Horwich A, et al. Frequency of screening magnetic resonance imaging to detect occult spinal cord compromise and to prevent neurological deficit in metastatic castration-resistant prostate cancer. Clin Oncol (R Coll Radiol) 2010;22:147-52. http://dx.doi.org/10.1016/j.clon.2009.11.007.
- Serefhanoglu S, Goker H, Aksu S, Buyukasik Y, Sayinalp N, Haznedaroglu IC, et al. Spinal myeloid sarcoma in two non-leukemic patients. Intern Med 2010;49:2493-7. http://dx.doi.org/10.2169/internalmedicine.49.3878.
- Hemingway H, Riley RD, Altman DG. Ten steps towards improving prognosis research. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b4184.
- Soloway MS, Hardeman SW, Hickey D, Raymond J, Todd B, Soloway S, et al. Stratification of patients with metastatic prostate cancer based on extent of disease on initial bone scan. Cancer 1988;61:195-202. http://dx.doi.org/10.1002/1097-0142(19880101)61:1⟨195::AID-CNCR2820610133⟩3.0.CO;2-Y.
- Wei LJ, Lin DY, Weissfeld L. Regression analysis of multivariate incomplete failure time data by modeling marginal distributions. J Am Statistical Assoc 1989;84:1065-73. http://dx.doi.org/10.1080/01621459.1989.10478873.
- Moons KG, Royston P, Vergouwe Y, Grobbee DE, Altman DG. Prognosis and prognostic research: what, why, and how?. BMJ 2009;338. http://dx.doi.org/10.1136/bmj.b375.
- Wilczynski NL, Haynes RB. Optimal search strategies for detecting clinically sound prognostic studies in EMBASE: an analytic survey. J Am Med Inform Assoc 2005;12:481-5. http://dx.doi.org/10.1197/jamia.M1752.
- Wilczynski NL, Haynes RB. Developing optimal search strategies for detecting clinically sound prognostic studies in MEDLINE: an analytic survey. BMC Med 2004;2. http://dx.doi.org/10.1186/1741-7015-2-23.
- Royston P, Parmar MK, Altman DG. Visualizing length of survival in time-to-event studies: a complement to Kaplan–Meier plots. J Natl Cancer Inst 2008;100:92-7. http://dx.doi.org/10.1093/jnci/djm265.
- Royston P, Moons KG, Altman DG, Vergouwe Y. Prognosis and prognostic research: developing a prognostic model. BMJ 2009;338. http://dx.doi.org/10.1136/bmj.b604.
- Altman DG, Vergouwe Y, Royston P, Moons KG. Prognosis and prognostic research: validating a prognostic model. BMJ 2009;338. http://dx.doi.org/10.1136/bmj.b605.
- Moons KG, Altman DG, Vergouwe Y, Royston P. Prognosis and prognostic research: application and impact of prognostic models in clinical practice. BMJ 2009;338. http://dx.doi.org/10.1136/bmj.b606.
- Mallett S, Royston P, Waters R, Dutton S, Altman DG. Reporting performance of prognostic models in cancer: a review. BMC Med 2010;8. http://dx.doi.org/10.1186/1741-7015-8-21.
- Mallett S, Royston P, Dutton S, Waters R, Altman DG. Reporting methods in studies developing prognostic models in cancer: a review. BMC Med 2010;8. http://dx.doi.org/10.1186/1741-7015-8-20.
- Altman DG. Prognostic models: a methodological framework and review of models for breast cancer. Cancer Invest 2009;27:235-43. http://dx.doi.org/10.1080/07357900802572110.
- Henriksson M, Palmer S, Chen R, Damant J, Fitzpatrick NK, Abrams K, et al. Assessing the cost effectiveness of using prognostic biomarkers with decision models: case study in prioritising patients waiting for coronary artery surgery. BMJ 2010;340. http://dx.doi.org/10.1136/bmj.b5606.
- Riley RD, Ridley G, Williams K, Altman DG, Hayden J, de Vet HC. Prognosis research: toward evidence-based results and a Cochrane methods group. J Clin Epidemiol 2007;60:863-5. http://dx.doi.org/10.1016/j.jclinepi.2007.02.004.
- Kyzas PA, Loizou KT, Ioannidis JP. Selective reporting biases in cancer prognostic factor studies. J Natl Cancer Inst 2005;97:1043-55. http://dx.doi.org/10.1093/jnci/dji184.
- Altman DG. Systematic reviews of evaluations of prognostic variables. BMJ 2001;323:224-8. http://dx.doi.org/10.1136/bmj.323.7306.224.
- Altman DG, Royston P. What do we mean by validating a prognostic model?. Stat Med 2000;19:453-73. http://dx.doi.org/10.1002/(SICI)1097-0258(20000229)19:4⟨453::AID-SIM350⟩3.0.CO;2-5.
- Braitman LE, Davidoff F. Predicting clinical states in individual patients. Ann Intern Med 1996;12:406-12.
- Counsell C, Dennis M. Systematic review of prognostic models in patients with acute stroke. Cerebrovasc Dis 2001;12:159-70. http://dx.doi.org/10.1159/000047699.
- Jacob M, Lewsey JD, Sharpin C, Gimson A, Rela M, van der Meulen JH. Systematic review and validation of prognostic models in liver transplantation. Liver Transpl 2005;11:814-25. http://dx.doi.org/10.1002/lt.20456.
- Laupacis A, Wells G, Richardson WS, Tugwell P. Users' guides to the medical literature. V. How to use an article about prognosis. Evidence-Based Medicine Working Group. JAMA 1994;272:234-7. http://dx.doi.org/10.1001/jama.1994.03520030076032.
Appendix 1 Record of searches undertaken
Appendix 2 Assessment of risk of bias in prognostic studies (Hayden et al.)
Assessment of risk of bias in prognostic studies (Hayden et al. ) (PDF download)
Appendix 3 Quality assessment
Appendix 4 Included papers at full sift (n = 31)
Appendix 5 Reasons for exclusion at full sift (n = 305)
Reasons for exclusion at full sift ( n = 305) (PDF download)
Appendix 6 Quality assessment forms: extracted data for each study
Quality assessment forms: extracted data for each study (PDF download)
Appendix 7 Data extraction tables
Appendix 8 Quality assessment results
Appendix 9 Cost information relative to the treatment of spinal metastases
Cost information relative to the treatment of spinal metastases (PDF download)
Appendix 10 Short report protocol
Glossary
- Aetiology
- Study of the factors involved in the development of a disease.
- Biochemical
- Involving chemical processes in living organisms.
- Biopsy
- Sampling of tissue from a specific area of the body (e.g. the prostate) to check for abnormalities such as cancer.
- Brachytherapy
- Form of radiation therapy involving radioactive seeds that emit radiation while implanted to help destroy the cancer.
- Cancer
- Growth of abnormal cells in the body in an uncontrolled manner.
- Epidemiology
- Study of the causes, distribution and control of disease in populations.
- Grade
- Degree of severity of a cancer.
- Heterogeneous (heterogeneity)
- A diverse mixture of different kinds or subgroups.
- Hormone therapy
- Use of hormones, hormone analogues and specific surgical techniques to treat a disease.
- Natural history
- The timeline of a morbid condition from onset–inception to resolution; the course of a particular disease if it is not treated or manipulated in any way.
- Prognosis
- Potential clinical outlook or chance of recovery based on the status and likely course of the disease.
- Progression
- Continuing growth of a cancer.
- Radiation therapy
- Use of X-rays and other types of radiation to destroy malignant tissue and cells.
- Recurrence
- Reappearance of disease.
- Risk
- Probability or chance that a specific event will or will not happen.
- Stage
- Term used to define the size and physical extent of a cancer.
- Staging
- Process of determining extent of disease in a patient from all available information, e.g. Whitmore–Jewett staging classification and more detailed TNM (tumour/node/metastasis) classification.
List of abbreviations
- ADT
- antiandrogen treatment
- AMP
- adjusted for multiple primaries
- AS
- age standardised
- AUC
- area under the curve
- BMI
- body mass index
- CI
- confidence interval
- CSF
- cerebrospinal fluid
- CT
- computerised tomography
- CTRA
- CT-based structural rigidity analysis
- DARE
- Database of Abstracts of Reviews of Effects
- EA
- axial load
- ECOG
- Eastern Cooperative Oncology Group
- EGFR TKI
- epidermal growth factor receptor tyrosine kinase inhibitor
- EI
- bending load
- EM
- epidural mass
- EOD
- extent of disease
- ESCC
- epidural spinal cord compression
- FRI
- fracture risk index
- HR
- hazard ratio
- HTA
- Health Technology Assessment
- IG-IMRT
- image-guided intensity-modulated radiotherapy
- IMRT
- intensity-modulated radiotherapy
- IQR
- interquartile range
- LBC
- load-bearing capacity
- LR
- likelihood ratio
- MESCC
- metastatic epidural spinal cord compression
- MRI
- magnetic resonance imaging
- MSCC
- metastatic spinal cord compression
- N
- nodal
- NCRI
- National Cancer Research Institute
- NDFS
- neurological deficit-free survival
- NHS CRD
- NHS Centre for Reviews and Dissemination
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- NSCLC
- non-small cell lung cancer
- OR
- odds ratio
- P/PP
- posterior to predicted posterior height ratio
- PP
- predicted posterior height
- PET
- positron emission tomography
- PMMA
- polymethylmethacrylate
- PSA
- prostate-specific antigen
- PTH
- parathyroid hormone
- PTHRP
- parathyroid hormone-related peptide
- RANKL
- receptor activator of nuclear factor-κB ligand
- RCT
- randomised controlled trial
- ROC
- receiver operating characteristic
- RR
- relative risk
- rSCC
- radiological spinal cord compression
- SAS
- subarachnoid space
- SCC
- spinal cord compression
- SCD
- spinal cord or cauda equina displacement
- SCLC
- small cell lung cancer
- SD
- standard deviation
- SPECT
- single-photon emission computerised tomography
- SRE
- skeletal-related event
- 99Tcm
- technetium-99m
- T10-L5
- thoracolumbar and lumbar spine
- TNM
- tumour/node/metastasis
- %TO
- percentage tumour occupancy
- TSC
- thecal sac compression
- UKCRN
- UK Clinical Research Network