Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 05/503/10. The contractual start date was in December 2007. The draft report began editorial review in April 2012 and was accepted for publication in October 2012. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors' report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen's Printer and Controller of HMSO 2013. This work was produced by Powell et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background
Acute asthma continues to be one of the main reasons for acute hospital admission in children, and accounts for much morbidity, anxiety, stress, and time off school and work for the families of children with asthma. 1 The Department of Health has targeted respiratory disease as an area for improved management. 2 The British Thoracic Society and Scottish Intercollegiate Guideline Network (BTS/SIGN)3 have developed an evidence-based guideline for the management of asthma. It offers comprehensive guidance on the acute and chronic management of asthma in children and adults, but the document highlights the paucity of good information to guide the management of a number of clinical situations. Nowhere is this more striking than in the management of acute asthma, for which the recommended treatment for children (< 16 years old) differs markedly from that for adults (≥ 16 years) in those who are unresponsive to initial standard treatment – a reflection of the evidence base in the different age groups.
The guideline recommends that the initial management in children is inhaled β2-agonists and ipratropium and systemic corticosteroids. This is similar to the initial management in adults. Oxygen saturation of < 92% while breathing room air at presentation is noted to be an indicator of more severe asthma, as is oxygen saturation of < 92% at 20 minutes after inhaled β2-agonists. For children of > 5 years of age who do not respond to initial treatment, it is recommended that clinicians consider intravenous bronchodilator therapy – initially, salbutamol followed by a continuous infusion, then intravenous aminophylline followed by infusion. There is little evidence for an intravenous bronchodilator of choice. Furthermore, although the guideline recognises that intravenous magnesium sulphate (MgSO4) is a safe treatment for patients with acute asthma, with no side effects up to doses of 100 mg/kg, it concedes that its place in management is not yet established. MgSO4 is not recommended for children aged ≤ 5 years. The BTS guidelines3 recommend intravenous MgSO4 in the initial management of severe acute asthma in adults but, as there is a lack of evidence in children, it is not currently recommended as first-line intravenous treatment in paediatric care. 3 There are no current paediatric recommendations concerning nebulised MgSO4.
There is clear evidence that MgSO4 has bronchodilator effects in acute asthma. 4 The BTS guidelines state that experience suggests that intravenous and the nebulised routes are both safe ways of administering MgSO4 in adults. Further trial results are awaited in adults. 5 A single dose of intravenous MgSO4 of a dose of 1.2–2 g in an infusion over 20 minutes is safe and effective improving lung function in adults with acute severe asthma. Safety and efficacy at higher dosages in adults have not been assessed. There is some concern about higher doses causing muscle weakness and respiratory failure. Nebulised MgSO4 in doses of 135–1152 mg in combination with β2-agonists shows a trend towards reduction in the number of hospital admissions and is mentioned as a possible treatment in adults. 6,7 In marked contrast with the paediatric recommendations, intravenous aminophylline and intravenous β2-agonists have limited use in adults, with recommendations that these interventions are reserved for ventilated patients and those in extremis. 3 The final recommendation from BTS/SIGN3 is that more studies are needed regarding the route, frequency and dose in adults for MgSO4. The recommendations from the Cochrane review of 20056 and the 2007 systematic review by Mohammed and Goodacre4 are that more studies are needed in both adults and children to identify exactly how MgSO4 (intravenous or inhaled) should be used.
Rationale
Mechanisms
The use of MgSO4 for acute asthma was first described in 1936, and since then there has been increasing evidence for its use in adults and children with asthma. 3 There are a number of proposed mechanisms for its actions. In vitro studies demonstrate an inhibitory effect of MgSO4 on contraction of bronchial smooth muscle, and the release of acetylcholine in cholinergic nerve terminals and of histamine from mast cells. 6 There is evidence that MgSO4 may act as an anti-inflammatory agent by inhibiting the neutrophil respiratory burst in adults with asthma. 8 The main effect of MgSO4 is that it blocks the calcium ion influx to the smooth muscles of the respiratory system9 and bronchodilatation occurs.
Clinical evidence for magnesium sulphate as a bronchodilator
Intravenous magnesium sulphate
The Acute Asthma and Magnesium Study Group has demonstrated the efficacy of intravenous MgSO4 in severe acute asthma in adults. 10 In a multicentre randomised placebo-controlled trial of 248 adults with acute asthma and a forced expiratory volume in 1 second (FEV1) of < 30% predicted, intravenous administration of 2 mg of MgSO4 as an adjunct to the standard therapy resulted in significant benefit in FEV1 of nearly 5%. The effect appeared greatest in those with the most severe asthma, with a difference of 10% in FEV1 between MgSO4- and placebo-treated groups, thus the recommendations set out in the BTS guidelines. 3 A Cochrane review of intravenous treatment with MgSO411 supports this evidence and recommendation. Intravenous administration of MgSO4 requires careful monitoring because peripheral vasodilatation and systolic hypotension can occur in association with flushing, nausea and venous phlebitis at the site of infusion. Consequently, interest has grown in the use of nebulised MgSO4 in acute asthma.
Nebulised magnesium sulphate
Nebulised MgSO4 does not appear to act as a bronchodilator in subjects with stable chronic asthma. 12,13 However, in acute exacerbations in subjects between the age of 12 and 60 years with moderate to severe acute asthma, the response to nebulised MgSO4 appears to be of similar magnitude as the response to salbutamol, as defined by changes in peak expiratory flow rate (PEFR). 14
Initial therapeutic trials of nebulised MgSO4 administered as an adjunct to nebulised salbutamol gave conflicting results in adults. In a small study of 35 adults, Nannini et al. demonstrated a significantly greater improvement in PEFR at 20 minutes after administration in patients receiving nebulised MgSO4 in addition to nebulised salbutamol than with nebulised isotonic saline and salbutamol. 15 A report in adults with severe acute asthma with an FEV1 of < 30% of that predicted, 30 minutes after initial administration of salbutamol via a nebuliser, demonstrated a significant benefit in FEV1 for those receiving MgSO4 compared with isotonic saline. 16 In contrast, Bessmertny et al. could show no evidence of benefit in 74 adults with moderately severe asthma. 17
The most recent Cochrane review of nebulised MgSO4 examined only six randomised controlled trials (RCTs) involving a total of 296 patients. 6 Four studies15–18 compared nebulised MgSO4 plus a β2-agonist with a β2-agonist plus placebo, and two studies14,19 compared MgSO4 with a β2-agonist alone. Three15–17 of the six studies14–19 involved adults exclusively: those by Bessmertny et al. (18–65 years),17 Hughes (16–65 years)16 and Nannini et al. (> 18 years). 15 Of the remaining three studies,14,18,19 one included a mix of adult and paediatric patients aged 12–60 years14 and there were two paediatric studies18,19 that included patients aged 5–17 years.
The two paediatric studies18,19 that used nebulised MgSO4 both have methodological deficits. However, the results of the studies show that nebulised MgSO4 appears to have a similar bronchodilator effect in acute asthma in childhood, although the magnitude and duration may not be as great as salbutamol when directly compared. 19 There appears to be an additive effect when inhaled MgSO4 is combined with salbutamol. 18
Meral19 examined two groups of 20 children with mean ages of 10.6 years and 11 years (range 8–13 years) with a severe exacerbation of asthma. In a RCT, patients received either 2 ml of MgSO4 (280 mmol/l, tonicity 258 mOsm, pH 6.7) nebulised over 15–20 minutes or inhaled salbutamol (note: no salbutamol was given in the MgSO4 group). Clinical score and PEFR were measured at 5, 15, 30, 60, 180, 240 and 360 minutes after treatment. Lung function at 5, 60 and 360 minutes was significantly greater in the salbutamol group. 19 This study19 had an unclear randomisation and blinding procedure, had a questionable outcome measure (owing to the lack of reproducibility and reliability of PEFR) and unclear inclusion and exclusion criteria. 20
Mahajan et al. 18 included 62 patients, aged 5–17 years, with severe acute asthma in a double-blind, randomised, placebo-controlled trial. Using FEV1 at 10 and 20 minutes after treatment and admission rates as outcomes along with a clinical score, they administered 2.5 ml of isotonic MgSO4 (6.3% solution) with salbutamol (2.5-mg nebule) or salbutamol with normal saline. One dose of the study medication was used and they demonstrated a significant improvement in FEV1 at 10 and 20 minutes after treatment with MgSO4 and salbutamol combined. 18
The overall conclusions from this review were that the use of nebulised inhaled MgSO4 in addition to β2-agonists in the treatment of an acute asthma exacerbation appears to have benefits with respect to improved pulmonary function [standard mean difference (SMD) 0.23 [95% confidence interval (CI) – 0.03 to 0.50]; four studies]. 6 The benefit was significantly greater in more severe asthma exacerbations [SMD 0.55 (95% CI 0.12 to 0.98)] but overall there were insufficient data, particularly in children, to make firm recommendations. Most importantly, there were no adverse events (AEs) reported and so the other important conclusion was that nebulised MgSO4 treatment was safe. 5 Thus, conclusions regarding treatment with nebulised MgSO4 were difficult to draw.
Mohammed and Goodacre4 completed a systematic review in 2007 and identified three more studies involving nebulised MgSO4. There were no new exclusively paediatric publications. There was one new adult study by Kokturk et al. 21 in 2005 (18–60 years) and two studies22,23 including mixed populations of adults and adolescents: Aggarwal et al. 22 (13–60 years) and Drobina et al. 23 (12–60 years). These three studies21–23 contributed a further 236 patients bringing the overall total to 532.
Kokturk et al. 21 examined 26 patients (18- to 60-year-olds) in a randomised, single-blinded trial. They examined PEFR up to 240 minutes post randomisation and admissions as their main outcomes of interest. They examined moderate to severe exacerbations and compared MgSO4 (2.5 ml of 6.3%) and salbutamol (2.5 ml) with saline as placebo and salbutamol. This small study21 suggested there is no benefit to be gained from adding MgSO4 to salbutamol in terms of PEFR or number of hospital admissions.
Aggarwal et al. 22 went on to study 100 patients (aged 13–60 years). The mean age of the patients studied was 46 years in both the intervention and the control group, which would suggest that the study was unlikely to have contained many adolescents. The authors examined PEFR up to 120 minutes post randomisation and admissions as the main outcomes and looked at severe to life-threatening acute asthma. They compared nebulised salbutamol (1 ml) with nebulised MgSO4 (1 ml of 500 mg), three doses in 1 hour, with saline and distilled water as placebo. The patients were randomised using a random number table and the study was double-blind. The investigators found no difference in outcomes between the two groups and concluded that there is no therapeutic benefit to be gained from adding MgSO4 to the standard treatment regimen. 22 Drobina et al. 23 (findings published in abstract only) examined 110 patients (12–60 years) with mild to severe asthma, again using PEFR and admissions as the primary outcomes. The intervention group received 150 mg of MgSO4 (0.3 ml of 50% MgSO4) added to each nebulised dose of medication. The control group received nebulised treatments of salbutamol 0.5% (5 mg/ml) combined with 0.5 mg of ipratropium bromide 0.02% inhalation solution. This study showed no evidence of an effect of adding MgSO4 on the above outcomes. 23
These further three studies21–23 with 236 patients thus found no evidence of an effect. Based on these findings, along with those of the other six studies, the reviewers concluded, that, in adolescents and adults, there is only weak evidence that the use of nebulised MgSO4 has an effect on respiratory function [SMD 0.17 (95% CI – 0.02 to 0.36); p = 0.09] or hospital admission [relative risk (RR) 0.68 (95% CI 0.46 to 1.02); p = 0.06]. These effects were clearly weaker that the results from the 2005 Cochrane review. 6 The reviewers felt able to draw an overall conclusion of the paediatric evidence based on the two paediatric studies. They concluded that there was no evidence of a significant effect of the addition of MgSO4 to standard treatment on respiratory function [SMD – 0.26 (95% CI – 1.49 to 0.98); p = 0.69] or hospital admission [RR 2.0 (95% CI 0.19 to 20.93); p = 0.56]. This conclusion did not differ significantly from the results of the Cochrane review in 2005. 6 Assessment of the risk of outcome reporting bias in the latest systematic review4 led to a sensitivity analysis adjusting for the suspected bias; the results24 suggested that the conclusions of the review were robust to this problem.
Risks and benefits
Risks
All six studies14–19 reported in the Cochrane review reported no serious adverse events (SAEs) in either arm. 6 The risk of SAEs was low in the studies comparing (1) MgSO4 with β2-agonists [risk difference (RD) 0.00 (95% CI – 0.11 to 0.11)] and (2) MgSO4 with a β2-agonist to a β2-agonist alone (RD 0.00; 95% CI – 0.03 to 0.03). The risk of SAEs was low and appeared to be even lower in patients treated with MgSO4 – either alone [RD – 0.17 (95% CI – 0.41 to 0.06)] or in combination with β2-agonists (RD – 0.09; 95% CI – 0.24 to 0.06). In the three extra papers in the Mohammed review,4 Aggarwal et al. 22 and Kokturk et al. 21 reported no significant AEs and Drobina et al. 23 made no comment (see Appendix 1, Table 38).
A systematic review (not published) of the adverse effects of inhaled MgSO4 in children was undertaken by the University of Liverpool for this study and identified two studies,25,26 not included in the Cochrane review,6 containing at most 18 further children. There were no reported AEs (see Table 1). These extra studies were not RCTs of MgSO4 during an acute asthma attack but they did report the effects of administering nebulised MgSO4, thus AEs could be examined. 25,26
In the MAGNET pilot study (Ashtekar et al. ;27 EudraCT no. 2004-003825-29), a total of 25 eligible patients were identified for inclusion into the study over a 3-month period. Of these, 17 gave informed consent to be randomised to receive nebulised magnesium or placebo in addition to salbutamol and ipratropium. All individuals received the treatment to which they were randomised. Seven patients who were randomised to active treatment and 10 patients to placebo. MAGNET27 found that there were no differences between the two groups when comparing the median Asthma Severity Score (ASS)28–30 after three nebulised treatments and the area under the curve (AUC) analysis of the ASS for the six time points. 27 There were insufficient numbers to make a significant comment about the efficacy of nebulised MgSO4 from the pilot study, the main aim of which was to test recruitment, administration and outcome assessment feasibility.
Two children (both of whom received MgSO4) had mild AEs. One child had transient facial flushing and, although asymptomatic, a blood pressure reading appeared low. The blood pressure was immediately remeasured and was then normal. Another child had transient tingling of the fingers. 27
Benefits
As described in detail above, five studies14–16,18–19 showed a benefit to using nebulised MgSO4 in some preparation, whereas four studies17,21–23 showed no benefit. There was heterogeneity between trials regarding study design, dose given, intervention comparison, primary outcomes and exclusion criteria (see Appendix 1, Tables 37–39), There was a non-statistically significant improvement in pulmonary function between patients whose treatments included nebulised MgSO4 in addition to β2-agonists [SMD 0.23 (95% CI – 0.03 to 0.50), four studies] and hospitalisations were similar between the groups [RR 0.69 (95% CI 0.42 to 1.12), three studies]. Subgroup analyses demonstrated statistically significant differences in lung function improvements with nebulised MgSO4 in addition to a β2-agonist in patients with severe exacerbations of their asthma [SMD 0.55 (95% CI 0.12 to 0.98)].
However, only one study16 reported the effect of three doses of MgSO4 nebulised with salbutamol in patients with severe asthma. In the study reported by Hughes,16 three nebulised treatments of MgSO4 mixed with salbutamol were given at 30-minute intervals to adults with severe asthma, and resulted in a twofold greater increase in FEV1 than the same dose of salbutamol administered with isotonic saline nebuliser solution; this enhanced bronchodilator response was associated with a significant reduction in hospital admission rates [RR 0.61 (95% CI 0.37 to 0.99), p = 0.04]. Only one study23 had used nebulised ipratropium bromide as well as salbutamol as standard treatment,23 which is certainly the current recommendation from the BTS for children and for adults. 3
The University of Liverpool systematic review also investigated the efficacy of nebulised MgSO4 in children. The findings are summarised in Table 1.
| Study | AEs in MgSO4 group | Efficacy |
|---|---|---|
| Rolla 198725 | Measured: not stated | No difference in lung function |
| Reported: no mention of AE in results/discussion | Improvement in airway responsiveness | |
| Rolla 198826 | Measured: not stated | Inhaled doses of > 0.1 mmol led to improvement in bronchial hyper-responsiveness |
| Reported: ‘no patient experienced side effects’ | ||
| Meral 199619 | Measured: ‘subjects were evaluated for possible adverse effects’ | PEFR: MgSO4 group better after 5 minutes, then back to pre-Mg measurement by 6 hours. Control group had sustained improvement at 6 hours. At 6 hours control group PEFR was better than magnesium group. Respiratory distress score: no difference between groups |
| Reported: in discussion – ‘No adverse reaction in either group as the heart rate and blood pressure did not change’ | ||
| Mangat 199814 | Measured: blood pressure, arrhythmia; hyporeflexia, respiratory depression | Patients treated with nebulised MgSO4 improved in terms of bronchodilatation and Fischl score.31 However, this effect was not significantly different to that of the group given nebulised salbutamol |
| Reported: (not stated whether these occurred in adults or children) – one transient hypotension (spontaneously resolved); no hyporeflexia | Note: the study report does not report the paediatric results separately from the adult results | |
| Mahajan 200418 | Measured: tremors, headaches, nausea, vomiting, hyporeflexia | FEV1 absolute: improvement at 10 minutes significantly better than in control group (p < 0.03); at 20 minutes no difference between groups |
| Reported: ‘none of the patients in either group showed any side effects’ | FEV1% predicted: no difference between groups |
At the beginning of recruitment to MAGNETIC, this was the current published evidence. We have currently completed a further update of the Cochrane review6 using the Cochrane review methodology, and this has now been published. 32 At the time of this report there were a total of 16 published studies of randomised controlled study design in acute asthma, with a total of 838 patients (439 subjects who had completed an intervention with MgSO4 and 399 who were control subjects in studies). The seven studies27,33–38 published since Mohammed 2007, or earlier studies not included in Mohammed's systematic review, are three studies involving adults exclusively;33–35 one study including adults and paediatric patients;36 two studies that enrolled children27,37 and, one study38 in which the age of participants was not stated. The data from these studies will be discussed in Chapter 5 of this report. The features of these 16 studies14–19,21–23,27,33–38 are presented in Appendix 1 in three tables but they are clearly heterogeneous in study design, population examined, intervention administered and outcomes measured.
Thus there is a need for a large study examining the addition of nebulised MgSO4 in children with acute severe asthma compared with standard treatment in a placebo-controlled double-blind randomised manner. MAGNETIC is a randomised placebo-controlled multicentre trial of the use of nebulised MgSO4 in severe acute asthma in childhood in patients who show a poor response to maximal conventional aerosol treatment.
Objective
Primary objective
Does nebulised MgSO4, used as an adjunct to nebulised salbutamol and ipratropium bromide for 1 hour in children with severe asthma, result in a clinical improvement compared with nebulised salbutamol, ipratropium bromide and placebo?
Secondary objectives
Does nebulised MgSO4, used as an adjunct to nebulised salbutamol and ipratropium bromide for 1 hour in children with severe asthma, compared with nebulised salbutamol, ipratropium bromide and placebo, have an effect on:
-
(a) clinical outcomes in terms of additional treatment/management while in hospital, and length of stay (LOS) in hospital
-
(b) patient outcomes in terms of quality of life (QoL), time off school and health-care resource usage over the following month
-
(c) parent outcomes in terms of time off work over the following month
-
(d) costs and cost-effectiveness for the NHS and Personal Social Services and, more broadly, for society?
Chapter 2 Methods
Objective
The objective of the MAGNETIC trial was to assess whether the addition of magnesium to standard treatment for acute severe asthma in children resulted in a clinical improvement compared with standard treatment alone.
Design
This was designed as a prospective, controlled, double-blind, multicentre RCT comparing the effects of nebulised MgSO4 with placebo for children presenting to secondary care with an acute severe asthma exacerbation.
Participants
Using the Medicines for Children Research Network (MCRN), 30 centres were identified. The network now covers most regions in England. Adding the Northern Ireland Research Network, the Scottish MCRN and the one site in Wales (Cardiff), we established (via an initial feasibility study) that each centre would be likely to able to recruit sufficient patients with severe acute asthma for the numbers required for the study. These centres all received patients with acute asthma into their unit's unscheduled care service and this may be in the form of a visit to emergency department (ED) or a children's assessment unit (CAU) or both. The patient inclusion and exclusion criteria for the MAGNETIC trial were as follows.
Inclusion criteria
Potential participants for the study could be between the ages of 2 years and 16 years. They could have had a previous history and diagnosis of asthma and be on treatment but could also be patients who have presented for the first time with acute asthma as per BTS/SIGN definitions. 3 Subjects could be recruited in either an ED or a CAU in secondary care. The main clinical definition for inclusion was severe acute asthma as defined by the BTS/SIGN guidelines. 3
For children of ≥ 6 years, severe acute asthma is based on at least one of the following criteria being met:
-
(a) oxygen saturations of < 92% while breathing room air
-
(b) too breathless to talk
-
(c) heart rate of > 120 beats per minute (b.p.m.)
-
(d) respiratory rate of > 30 breaths per minute
-
(e) use of accessory neck muscles.
For children aged 2–5 years, severe acute asthma is based on at least one of the following criteria being met:
-
(a) oxygen saturations of < 92% while breathing room air
-
(b) too breathless to talk
-
(c) heart rate of > 130 b.p.m.
-
(d) respiratory rate of > 50 breaths per minute
-
(e) use of accessory neck muscles.
Exclusion criteria
-
(a) Co-existing respiratory disease, such as cystic fibrosis or chronic lung disease of prematurity.
-
(b) Severe renal disease.
-
(c) Severe liver disease.
-
(d) Known pregnancy.
-
(e) Known previous reaction to magnesium.
-
(f) Inability to give informed consent.
-
(g) Previous randomisation into the MAGNETIC trial.
-
(h) Life-threatening symptoms.
-
(i) Current or previous (in the 3 months preceding screening) involvement with a trial of a medicinal product.
Interventions
Patients were randomised to receive nebulised salbutamol 2.5 mg (aged 2–5 years) or 5 mg (aged ≥ 6 years) and ipratropium bromide 0.25 mg mixed with either 2.5 ml of isotonic MgSO4 (250 mmol/l, tonicity 289 mOsm; 151 mg per dose) or 2.5 ml of isotonic saline on three occasions at approximately 20-minute intervals. There is currently no specific agreed dose of MgSO4 for use in children. 4 The MgSO4 dose for this study was chosen based on the doses described in the published paper by Hughes in 2003,16 as they were shown to be effective and safe in acute asthma in an adult population. 16 The magnesium solution needs to be isotonic as hypertonic and hypotonic solutions may cause bronchoconstriction. 16 The doses used in the published paediatric studies were both isotonic [Meral,19 2 ml of isotonic MgSO4 (280 mmol/l, tonicity 258 mOsm, 116 mg/dose); Mahajan et al. ,18 2.5 ml of isotonic (6.3%) MgSO4, 145 mg/dose)]. The frequency of the dosing was based on the three doses of bronchodilators (salbutamol and ipratropium) in the first hour of treatment as recommended by BTS,3 with the MgSO4 or placebo added. Use of various doses is described in the clinical effectiveness literature (see Appendix 1 and discussion in Chapter 5).
Study procedures
Patients were identified on presentation to EDs or CAUs and assessed against the study inclusion criteria. If they fulfilled the severity criteria as defined by the BTS definition,3 the Yung ASS was recorded. 30 Patients were then given a nebuliser containing salbutamol and/or ipratropium bromide (variations allowed; as per site routine clinical practice) and parents/guardians were then approached and asked for their informed consent.
Following this initial nebuliser the patient was re-assessed against the inclusion criteria and the ASS recorded again. Patients were eligible for randomisation provided at least one of the inclusion criteria of the severe asthma BTS definition3 were met and informed consent had been obtained from the parent and if appropriate assent from the child.
Patients were randomised to receive either 2.5 ml of isotonic MgSO4 (250 mmol/l, tonicity 289 mOsm; 151 mg per dose) or 2.5 ml of isotonic saline via nebuliser on three occasions at approximately 20-minute intervals. Each nebuliser also contained salbutamol 2.5 mg (children aged 2–5 years) or 5 mg (children aged ≥ 6 years) and ipratropium bromide 0.25 mg in both the active and placebo groups. It was planned that as soon as they were randomised then the treatment would start.
The ASS was measured at approximately 20 (T20, after first treatment nebuliser), 40 (T40, after second treatment nebuliser), 60 (T60, after third treatment nebuliser), 120, 180 and 240 minutes post randomisation. AEs, concomitant medication, oxygen saturation, respiratory rate and blood pressure were also recorded at these assessment points.
Following the conclusion of 4-hour follow-up, AEs were monitored and data collection continued until discharge from hospital to assess secondary outcome measures. Parents and patients (if aged ≥ 5years) were contacted by the research team and asked to complete postal questionnaires 1 month after their hospital visit in order to collect health-related QoL and health economics data. The schedule of study procedures is shown in Table 2, see below.
| Procedures | Screening | Randomisationa | Minutes post randomisation: | During admission | Before discharge | 1-month follow-up | Premature discontinuation | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 20 | 40 | 60 | 120 | 180 | 240 | ||||||||
| Signed consent form | ✗ | ||||||||||||
| Assessment of eligibility criteria | ✗ | ✗ | |||||||||||
| Yung ASS | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||||
| Assignment to study treatment | ✗ | ||||||||||||
| Review of medical history | ✗ | ✗ | |||||||||||
| Review of concomitant medications | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| Study interventionb | ✗ | ✗ | ✗ | ||||||||||
| Blood pressure, SaO2, respiration rate | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||||
| PedsQL™ Asthma Module | ✗ | ||||||||||||
| EQ-5D | ✗ | ||||||||||||
| Health economics questionnaires | ✗ | ||||||||||||
| Physical examination | Complete | ✗ | ✗ | ||||||||||
| Symptom-directed | (✗) | (✗) | (✗) | (✗) | (✗) | (✗) | (✗) | (✗) | (✗) | ||||
| Assessment of AEs | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||||
Procedures for assessment
Efficacy
Asthma severity was assessed using a validated score, the Yung ASS,28–30 which comprises three clinical signs: wheezing, accessory muscle use and heart rate. Each component has a minimal score of zero and a maximum of 3. The total score is a sum of each component, giving a minimum score of zero and a maximum of 9. The score has been validated as a measure of asthma severity in children including the younger age group, has been demonstrated to be reproducible and reliable,29 with good interobserver agreement, and correlates well with oxygen saturation and FEV1. 30 This score is clinically easy to use and involves some of the standard assessments, used routinely by medical and nursing staff while managing children with acute asthma. The ASS assessment was carried out by a clinician or by a nurse who was appropriately trained to make the necessary observations in the opinion of the principal investigator for that site.
Safety
Patient status was monitored for 4 hours post randomisation. Oxygen saturation, respiratory rate and blood pressure were recorded twice during screening, approximately 20, 40 and 60 minutes post randomisation, and follow-up checks at 120, 180 and 240 minutes post randomisation. The research team were prompted to check for AEs at each assessment point, by reviewing physiological parameters such as blood pressure and asking about known side effects, for example facial flushing. AEs were followed up until discharge from hospital.
Health economics and quality of life
The case report form (CRF) used by the clinical team at each site recorded each child's NHS resource use from randomisation to discharge from hospital. The 1-month follow-up postal questionnaire collected QoL [Paediatric Quality of Life Inventory (PedsQL™) Asthma Module and European Quality of Life-5 Dimensions (EQ-5D) questionnaires] and health economics (NHS and non-NHS) data from discharge to 1 month post randomisation (see Appendices 2 and 9).
Outcomes
There are many and varied primary outcomes to choose from in acute asthma studies. 39 There are no agreed core outcomes for use in acute asthma studies in ether adult or paediatric studies, and so there is huge variation in the primary and secondary outcomes reported. 4,6 In the nebulised MgSO4 literature, numerous and varied outcomes (see Appendix 1, Table 38) are reported. Measurements of lung function in children recorded during an acute attack or in those in whom lung function has never previously been measured are too unreliable to use accurately. 40 Thus, an ASS appears to be a clinically relevant score to use in children to avoid the need for measuring lung function. The main problem is there are over 20 asthma severity scores39,41,42 all with different qualities. We chose the most validated and easiest to use – the Yung ASS. 30 The choice of the ASS is discussed further in Chapter 5. As there was evidence that the response to inhaled MgSO4 is within the first hour of treatment4,7,19 we decided to measure the primary outcome as the ASS at 60 minutes post treatment (T60) and then hourly up to 240 (T240) minutes post treatment to establish if there is a sustained effect. We also measured respiratory rate, heart rate, oxygen saturation in air and blood pressure as objective measurements. There are a number of secondary outcomes that we collected based on the most common secondary outcomes measured in acute asthma studies. 39 ‘Stepping down’ of treatment at 1 hour describes the decision to change from nebulisers to spacers, a proxy for the treating clinician making a judgement that the child is getting better having presented with severe exacerbation. In a study of 36 EDs in Australia including 720 patients with acute asthma, 50% of those with acute asthma who presented as a severe exacerbation improved sufficiently to be classified as to be moderate at 1 hour after treatment was started, thus potentially able to change from nebulisers to spacers. 43
Primary outcome
The primary end point was the ASS after 60 minutes of treatment. It was defined as ASS at T60.
Secondary outcomes
Clinical (during hospitalisation):
-
‘stepping down’ of treatment at 1 hour (the ‘stepping down’ of treatment at 1 hour is defined by the change to metered dose inhaler (MDI)/spacer combination only or no further treatment until discharge)
-
number and frequency of additional salbutamol administrations
-
LOS in hospital
-
requirement for intravenous bronchodilator treatment
-
intubation and/or admission to a paediatric intensive care unit (PICU).
Patient and parental outcomes at follow-up (1 month):
-
paediatric QoL (PedsQL™ Asthma Module parental report for all children and self-completion if aged > 5 years, EQ-5D)
-
time off school/nursery for the child
-
health-care resource usage [e.g. general practitioner (GP) visits, additional prescribing]
-
time off work (related to child's illness).
Sample size calculation
In order to detect a difference between the two treatment groups at T60 of 0.5 points on the ASS at a 5% significance level with 80% power, 500 children were required to participate in the trial. This assumes a standard deviation (SD) = 1.95 based on a similar population in Australia. 30 The SD was estimated from the Cardiff pilot study (EudraCT no. 2004–003825–29) to be 1.7. We took the larger SD estimate in order to be conservative. The ASS can range from zero to 9. A difference of 0.5 was deemed by the research and Trial Management Group (TMG) members to be the minimum worthwhile clinically important difference to be detected. This sample size will also be sufficient to identify an increase in the number of children being ‘stepped down’ in terms of medication after 1 hour of treatment from 50–63% with 80% power at a 5% significance level. Sample size calculations were undertaken using nQuery Advisor software (Statistical Solutions, Saugus, MA, USA), version 4. 44
Randomisation and blinding
Randomisation lists were generated in Stata Statistical Software (StataCorp LP, College Station, TX, USA), release 9, using block randomisation with random variable blocks length 2 and 4 and a 1 : 1 ratio of treatment allocation. Randomisation was stratified by centre. Treatment packs were identical in appearance and numbered in sequential order in the format XXXYYY (X = site code, Y = sequential number beginning with 001). Each pack contained three vials of 2.5 ml of MgSO4 or placebo, manufactured and quality controlled and QP released by St Mary's Pharmaceutical Unit, Cardiff, UK [MA (IMP) 35929] (IMP, Investigational Medicinal Product). Centres used their own stock of salbutamol and ipratropium bromide.
Data management
The data were recorded on standardised CRFs designed collaboratively by the TMG. These were returned to the MCRN CTU and the data entered on to a validated electronic study database [InferMed MACRO version 3 (InferMed, London)] by trained staff. Confirmation of patient recruitment was by receipt of a fully signed consent form. Each CRF was checked for adherence to the trial protocol and for missing and/or erroneous values. Discrepancies were queried with study sites to obtain the correct data or obtain reasons, where possible, for missing data/errors. Data entry accuracy checks were performed on 100% of primary outcome data, ‘LOS’, ‘admission to PICU/intubation’ and ‘need for IV treatment’. Checks were performed by a member of staff independent from the trial. Levels of missing data were monitored throughout and strategies developed to minimise occurrence; however; as much information as possible were collected about the reasons for missing data.
Statistical methods
Internal pilot
To ensure the appropriateness of the SD used in the sample size calculation it was planned to undertake an internal pilot after the first 30 children had been randomised and completed follow-up. This blinded internal pilot was not deemed to have any significant impact on the final analysis and no between-group comparisons were made. If the SD had been found to be smaller than that used in the sample size calculation, suggesting that fewer patients were required than initially proposed, then no action would be taken and the size of the study would remain as planned. If the SD was found to be larger than assumed, suggesting the need for more patients, then, on the advice of the Independent Data and Safety Monitoring Committee (IDSMC), the Trial Steering Committee (TSC) would have aimed to increase recruitment and consider implications for funding and existing resources.
Interim analysis
To estimate the effect of nebulised MgSO4 for the primary efficacy outcome, a single interim analysis adopting the Haybittle–Peto45 approach was planned after approximately 250 children had been randomised, with 99.9% CIs calculated for the effect estimate. This method was chosen to ensure that interim efficacy results would have to be extreme before early termination would be recommended in order to be convincing to the clinical community. The method also minimises controversy regarding interpretation of the results from estimation and hypothesis testing at the final analysis, and no inflation factor needs to be applied to the sample size using this approach.
Study statistical analysis plan
All analyses were conducted according to the statistical analysis plan (SAP) (see Appendix 3), which provides a detailed and comprehensive description of the main, pre-planned analyses for the study. Analyses were performed with standard statistical software (SAS version 9.3, SAS Institute Inc., Cary, NC, USA) apart from joint modelling (undertaken as a sensitivity analysis for examining the effect of missing primary outcome data) that was undertaken using the R language, version 2.15.2 (The R Foundation for Statistical Computing, Vienna, Austria) (http://cran.r-project.org/). The software for joint modelling (JoineR library; 2.13.0 version, the R Foundation for Statistical Computing, Vienna, Austina) has been validated through simulations in variety of settings representing different correlation patterns between longitudinal and survival processes. The main features of the SAP are summarised below.
The CONSORT (Consolidated Standards of Reporting Trials) flow diagram is used to summarise representativeness of the study sample and patient throughput in the trial. It was planned to collect screening data, and hence efforts were regularly made to encourage the return of screening logs.
Baseline characteristics are presented by treatment group and overall, with continuous variables summarised in terms of means (SD) or medians [interquartile range (IQR)] depending on the degree of skewness, and categorical variables presented in terms of numbers (%) per category. The intention-to-treat (ITT) principle is used with a two sided p-value of 0.05 (5% level) for statistical significance and 95% CIs for the relative treatment effect reported throughout.
The primary outcome is presented with means and SDs at T60 for each treatment group. Analysis of covariance (ANCOVA) is used to present results adjusted for baseline ASS value. The reasons for missing primary outcome data are provided with the results of the sensitivity analyses which are used to investigate the robustness of the primary outcome results to missing data (see Appendix 5). The chief investigator classified the information on the reason for missing ASS data and was blind to the treatment group allocation. Key baseline characteristics for those with observed ASS at T60 are compared between treatment groups, and differences in key baseline characteristics between patients with observed and missing ASS at T60 are also investigated (see Appendix 5) to assess whether missingness affects the randomisation balance and plausibility of the missing completely at random (MCAR) assumption. Sensitivity analysis was also performed to examine a centre effect (see Appendix 6).
All continuous secondary outcomes that were non-normally distributed are summarised in terms of medians and IQR for each treatment group, and compared using the Mann–Whitney U-test. When a secondary outcome is categorical, the two treatment groups are compared using a chi-squared test.
The chief investigator classified information on the reason for PICU admission/intubation in terms of whether it was likely to be related to trial treatment, queries regarding whether children had stepped down at 1 hour, and AEs and SAEs, blind to treatment group allocation. A statistical test comparing the percentage of children suffering an AE in each arm has not been performed for two reasons: (1) this analysis would assume the AEs are of equal importance; and (2) no hypotheses on AEs were set out upfront before the blind had been broken.
Protocol amendments
Protocol amendments are summarised in Appendix 4. In summary, the main amendments following those made to obtain Multicentre Research Ethics Committee (MREC) and Medicines and Healthcare products Regulatory Agency (MHRA) approval were to include additional principal investigators and participating centres. No major changes to the study procedures were made during the trial.
Health economics analysis plan
The economic evaluation aimed to assess the cost-effectiveness of nebulised MgSO4 in the management of severe acute asthma in children based on the data collected within the MAGNETIC trial.
Treating children with asthma is likely to have at least two economic research aspects, which both relate to clinical effectiveness. The first is the short-term side effects and relief from primary symptoms and direct consequences of the condition on costs and health-related QoL. The second is the medium- and long-term effects in terms of reduced disability and any medium- and long-term adverse reactions from treatment. This study focused on the short- and medium-term costs and consequences of nebulised MgSO4 in the management of severe acute asthma in children. The study protocol had allowed for extrapolation of costs and consequences over a longer time horizon if the results had demonstrated a difference in medium-term outcomes. This longer-term modelling would have been based on the natural history of the disease and additional evidence from the literature in the event that the trial yielded significant benefits for MgSO4.
The primary analysis (base case) took the perspective of the NHS and Personal Social Services46 and, consequently, the costs incurred by children's families or education services were excluded from the base-case analysis. A sensitivity analysis took a wider societal perspective that included broader economic costs, including costs incurred by children's families at the time of treatment and during the 4 weeks thereafter.
Two main analyses of incremental cost-effectiveness were conducted. The first analysis comprised a cost-effectiveness analysis (CEA) calculating the incremental cost per unit change in ASS after 60 minutes of treatment, whereas the second comprised a cost–utility analysis (CUA) calculating the incremental cost per quality-adjusted life-year (QALY) gained through treatment.
Collection of resource-use data
Data were collected about all significant health service and broader societal resource inputs over the 1-month time horizon of the study (i.e. over the period between randomisation and 1 month post randomisation). These data were obtained through two principal means. First, the study CRFs captured all resource use related to the child's primary hospital attendance(s) including diagnosis and treatment as well as transfers between wards and hospitals. Specifically, individualised resource use was estimated for the resources associated with the primary ED/CAU attendance, admissions to inpatient wards [classified as PICU, high-dependency unit (HDU), general paediatric ward (GM)], duration of intubation during the hospital admission(s), duration of mechanical ventilation during the hospital admission(s), surgical procedures performed during the hospital admission(s), tests or investigations performed during the hospital admission(s), additional bronchodilator medication, concomitant medications, and resources associated with AEs. Duration of resource use for significant resource items during the ED/CAU attendance and hospital admission(s) was recorded. Second, economic questionnaires were posted to the main parent of each child approximately 1 month post randomisation. The questionnaires recorded the children's resource use during the period between completion of ED/CUA or hospital discharge and 1 month post randomisation (see Appendix 9). The data collected in the postal questionnaires recorded direct non-medical costs borne by parents and carers as a result of attending hospital with the child during their ED/CAU and/or hospital admission(s). These direct non-medical costs covered travel costs, child care costs, expenses incurred while in hospital, and other direct non-medical expenses. The parent-completed questionnaires also recorded the children's use of prescribed inhalers, other prescribed medicines, privately purchased over-the-counter medications, and non-hospital community health and social services, as well as their hospital outpatient attendances and hospital re-admissions (by type of ward). Finally, the parent-completed questionnaires recorded direct non-medical costs borne by parents and carers, as well as their self-reported lost earnings, as a result of the child's asthma during the period between completion of ED/CAU or hospital discharge and 1 month post randomisation. The 1-month economic questionnaire had been piloted among members of the lay panels of the MCRN to ascertain its acceptability, comprehension and reliability, and reminder letters were sent to parents to increase the response and completion rates. All resource-use data were entered directly from the postal questionnaires into the MACRO trial database, with in-built safeguards against inconsistent entries, and then verified by dual coding.
Valuation of resource-use cost data
Unit costs for resources used by children who participated in the study were obtained from a variety of primary and secondary sources, with the majority obtained from secondary sources. All unit costs used followed recent guidelines on costing health and social care services as part of an economic evaluation. 46,47 Where necessary, secondary information was obtained from ad hoc studies reported in the literature. Unit costs of hospital and community health-care costs were largely derived from national sources and took account of the cost of the health professionals' qualifications. 48 Some costs were valued using the NHS Reference Costs (2009–10), a catalogue of costs compiled by the Department of Health in England. 49 Drug costs were obtained from the British National Formulary (BNF). 50 Costs for individual preparations were used as well as costs for chemical entities, i.e. drugs were grouped by chemical entity and unit costs for these chemical entities were calculated (Prescription Cost Analysis 2010). 49 The values attached to direct non-medical costs borne by parents and carers and their lost earnings were those provided by the parents completing the 1-month economic questionnaire. Lost earnings were not valued if annual or compassionate leave was taken as a result of the child's health state. All costs were expressed in pounds sterling and valued at 2009–10 prices. None of the costs were inflated or deflated for use in the economic evaluation. For the base-case analyses, unit costs were combined with resource volumes to obtain a net cost per child covering all categories of hospital and community health and social services. In one of several sensitivity analyses, these costs were supplemented with the range of costs incurred by family members and carers in the course of treatment and follow-up (societal perspective adopted). Further details on the methods used to value resource use are provided in Appendix 2.
Calculation of utilities and quality-adjusted life-years
Parents of children aged ≥ 5 years were asked to describe their children's QoL at 1 month after participation in the MAGNETIC trial using the proxy version of the EuroQol EQ-5D instrument. 51 The EQ-5D is the generic, multiattribute, preference-based measure preferred by National Institute for Health and Care Excellence (NICE) for broader cost-effectiveness comparative purposes. 46 The EQ-5D consists of two principal measurement components. The first is a descriptive system, which defines health-related QoL in terms of five dimensions: ‘mobility’, ‘self care’, ‘usual activities’, ‘pain/discomfort’ and ‘anxiety/depression’. Responses in each dimension are divided into three ordinal levels coded: (1) no problems; (2) some or moderate problems; and (3) severe or extreme problems. A total of 243 health states are generated by the EQ-5D descriptive system. For the purposes of this study, the York A1 tariff was applied to each set of responses to the descriptive system to generate an EQ-5D utility score at 1 month for each child. 52 The York A1 tariff set was derived from a survey of the adult UK population (n = 3337), which used the time trade-off valuation method to estimate utility scores for a subset of 45 EQ-5D health states, with the remainder of the EQ-5D health states subsequently valued through the estimation of a multivariate model. 52 Resulting utility scores range from scores – 0.59 to 1.0, with ‘0’ representing death and ‘1’ representing full health. Utilities values of < 0 indicate health states worse than death. The second measurement component of the EQ-5D, the vertical visual analogue scale ranging from 100 (best imaginable health state) to 0 (worst imaginable health state), was not included in MAGNETIC.
There is limited evidence of the psychometric properties of the EQ-5D in young children. 53 Consequently, analyses were conducted to ‘map’ or ‘cross-walk’ responses to the PedsQL™ Asthma Module on to EQ-5D utility scores. These mapping models were developed on the basis of data collected for 5- to 16-year-old children for whom both EQ-5D and PedsQL™ responses were available; the resulting mapping algorithms were used to estimate EQ-5D utility scores for 2- to 4-year-old children in MAGNETIC for whom the validated toddler module of the PedsQL™ Asthma Scales had been completed. A number of models were used to develop these mapping algorithms in keeping with current methodological guidance for mapping between non-preference-based and preference-based measures of health status. 54,55
Model 1: ordinary least squares using PedsQL™ total score
It was assumed that there was a linear relationship between the PedsQL™ total score and the EQ-5D utility score with a high score on the PedsQL™ correlated with a high score on the EQ-5D measure and vice versa. An ordinary least squares (OLS) model was used to examine the existence of such a relationship between the PedsQL™ total score and the EQ-5D utility score. The dependent variable, the EQ-5D utility score, was measured on its natural scale (i.e. – 0.594 to 1). The PedsQL™ total score was measured on a (0–100) scale. Covariates for age and gender were also included in the model.
Model 2: ordinary least squares using the PedsQL™ subscales
A simple model that includes the PedsQL™ total score may not be able to explain the variation between PedsQL™ and EQ-5D responses, as the relationship between the two may be more complex. The PedsQL™ total score can be broken down into four subscales: asthma symptoms, treatment problems, worry and communication; using information from these subscales may result in a model that provides a better fit. The simple OLS model can therefore be improved by using the four subscales of the PedsQL™ as independent variables in place of the PedsQL™ total score. As in model 1, the dependent variable (EQ-5D utility score) was measured on its natural scale and the PedsQL™ subscale scores were each measured on a (0–100) scale. Covariates for age and gender were also included in the model. We explored whether multicollinearity was present in our mapping model 2, which included PedsQL™ subscale scores and age and gender as explanatory variables. The mean variance inflation factor in this model was estimated at 1.72, well below the threshold value of 10 that is normally indicative of multicollinearity. Moreover, there is a now a wealth of evidence in the published literature confirming the four-factor conceptually derived measurement model for the PedsQL™ scales (www.pedsql.org).
Model 3: ordinary least squares using the PedsQL™ subscales with squared terms and interactions
A multiple OLS regression model was used to examine the relationship between the EQ-5D utility score and the four PedsQL™ subscale scores, squared subscale scores and interaction terms derived using the product of subscale scores. The dependent variable (EQ-5D utility score) was measured on its natural scale and the PedsQL™ subscale scores were measured on a (0–100) scale. The model was defined as:
where i = 1, 2, . . . , n represents individual respondents, j = 1, 2, . . . , and m represents the four different subscales. The dependent variable, y, represents the EQ-5D utility score, x represents the vector of PedsQL™ subscales, r represents the vector of squared terms, z represents the vector of interaction terms and εij represents the error term. This is an additive model that imposes no restrictions on the relationship between dimensions. The squared terms are designed to pick up non-linearities in the relationship between dimension scores and the EQ-5D utility score. The interaction terms are considered important as the dimensions are not additive. Covariates for age and gender were also included in this model.
The best-fitting model of the three was identified on the basis of the highest explanatory power in terms of the lowest Akaike information criterion (AIC) statistic. This model was used to make the EQ-5D predictions for the 2- to 4-year-old children in MAGNETIC. The accuracy of the predictions were tested by carrying out a within sample validation and the root-mean-squared error (RMSE) (a recommended measure of predictive ability) was calculated for each model. 54
Baseline utility data were not collected because trial participants were enrolled in ED/CUA with minimal data collection and concomitant concerns surrounding family intrusions at such a sensitive time. To estimate QALYs, it was necessary to impute baseline utility data based on secondary evidence. A physician panel made up of two respiratory nurses and a consultant mapped the ASS scores on to EQ-5D health states from which baseline utility scores were estimated. In the base-case analysis, ASS scores of 1–3 were mapped on to an EQ-5D health state of 11111; ASS scores of 4–6 were mapped on to an EQ-5D health state of 22222; and ASS scores of 7–9 were mapped on to an EQ-5D health state of 33333. These mappings were varied as part of the sensitivity analyses (see Chapter 4 for details).
The number of QALYs accrued over the 1-month follow-up period was calculated using linear interpolation between the baseline and follow-up utility score. It is likely that children return to the EQ-5D health state reported at 1 month earlier than that time; however, it is acknowledged that this depends in part on the number of asthma attacks that have occurred since treatment. Consequently, the base-case analysis assumed that the EQ-5D health state had been achieved immediately following hospital discharge, while a sensitivity analysis applied linear interpolation of the utility scores over the follow-up period. In order to account for potential baseline imbalances between the trial groups, adjustments were made to the QALY estimates by simply subtracting each child's baseline utility value from their on-treatment utilities before calculating QALYs. This method effectively indexes the utilities relative to baseline.
Missing data
Multiple imputation was used to impute missing data and avoid biases associated with complete case analysis. 56 Missing data was a particular issue for costs and utility scores collected at the 1-month follow-up. The MICE (Multiple Imputation by Chained Equations) algorithm within R statistical software version 2.13 (R Development Core Team) was used to impute missing data for the following variables: total health and social care costs based on data combined from the CRFs and from parental questionnaires; total societal costs based on data combined from the CRFs and from parental questionnaires; QALY estimates based on linear interpolation, assuming that the health gain was achieved immediately following hospital discharge; and QALY estimates based on linear interpolation assuming that the health gain was achieved linearly over the follow-up period. Age, sex and treatment allocation were included as explanatory variables in the imputation models. Costs up to completion of ED/CUA attendance or hospital discharge were included as an additional explanatory variable in the models that imputed values for total health and social care costs and total societal costs over the 1-month time horizon. The ‘match’ option within ‘ice’ was used for utilities and costs as this algorithm is less dependent on assumptions of normality than default options. Five imputed data sets were generated.
Cost-effectiveness analytic models
As described above, the primary clinical outcome measure for the study was ASS at T60. Assessment severity score data were collected both before (as part of screening) and during the trial (prior to randomisation and at T20, T40, T60 and when necessary thereafter). The assessment severity score at T60 was the primary clinical outcome pre-specified in the protocol and this was also used in the CEA. In the CEA, the incremental cost-effectiveness ratio (ICER) was calculated as the difference in average costs (ΔC) divided by the difference in average effects (ΔE) and expressed as the incremental cost per unit change in ASS at T60. A separate CUA was performed, the results of which were expressed in terms of incremental cost per QALY gained. The time horizon for the measurement and valuation of costs and health outcomes within the CEA covered the period between randomisation and discharge from the ED/CUA or the hospital where the child was admitted to an inpatient ward immediately following ED/CUA attendance. The time horizon for the measurement and valuation of costs and health outcomes within the CUA covered the period between randomisation and 1 month post randomisation. No discounting of costs or benefits was applied as the time horizon was < 12 months.
Independent-sample t-tests were used to test for differences in resource use, costs, utility scores and QALYs between treatment groups. All statistical tests were two-tailed. Multiple regression was used to estimate the differences in total cost between the magnesium and placebo groups and to adjust for potential confounders, including the covariates incorporated into the main clinical analyses. For the generalised linear model (GLM) on costs, a gamma distribution and identity link function was selected in preference to alternative distributional forms and link functions on the basis of its low AIC statistic.
The five imputed data sets generated through multiple imputation were bootstrapped separately in Microsoft Excel 2003 (Microsoft Corporation, Redmond, WA, USA) and the results were subsequently combined56 to calculate standard errors (SEs) around mean costs and effects that incorporate uncertainty around imputed values as well as sampling variation. SEs were used to calculate 95% CIs around total and incremental costs, incremental effects and QALYs based on Student's t-distribution. Cost-effectiveness acceptability curves (CEACs) showing the probability that magnesium is cost-effective relative to placebo at a range of ceiling ratios were generated, based on the proportion of bootstrap replicates (across all five imputed data sets) with positive incremental net benefits. 57,58 For the purposes of the CEA, incremental net benefit was defined as the unit reduction in ASS multiplied by the cost-effectiveness threshold for this clinical outcome minus the incremental cost, where the ceiling ratio (or cost-effectiveness threshold) represents the maximum society is willing or able to pay for each unit reduction in ASS. For the purposes of the CUA, incremental net benefit was defined as the incremental QALY gain multiplied by the ceiling ratio minus the incremental cost, where the ceiling ratio (or threshold) represents the maximum that society is willing or able to pay for each additional QALY. Unless otherwise stated, all statements about cost-effectiveness are based on a £20,000 per QALY gained threshold. The probability that magnesium is less costly or more effective than no treatment was based on the proportion of bootstrap replicates that had negative incremental costs or positive incremental health benefits (unit reduction in ASS for the purposes of the CEA: QALYs for the purposes of the CUA), respectively.
Several sensitivity analyses were undertaken to assess the impact of areas of uncertainty surrounding components of the economic evaluation. These included the following for purposes of the CEA: (1) performing a complete case (rather than multiple imputation) analysis, which limited the CEA to the children for whom complete information on both costs and ASS were available; (2) varying the per diem costs for inpatient stays in paediatric wards (PICU, HDU, GM); (3) assuming that part of a day spent by a child in an inpatient ward equated to a proportional period for costing purposes and that, consequently, the vacated inpatient bed would be filled immediately; (4) assuming that part of a day spent by a child in an inpatient ward equated to a full 24-hour period for costing purposes and that, consequently, the inpatient bed would not be filled until the end of that 24-hour period; and (5) varying the average cost of an ED/CUA attendance. The sensitivity analyses included the following for purposes of the CUA: (1) performing a complete case (rather than multiple imputation) analysis, which limited the CUA to the children for whom complete information on both costs and QALYs was available; (2) assuming linear interpolation of health utilities over entire follow-up period; (3) assuming baseline ASS scores mapped on to EQ-5D health states with lower utility scores than in the baseline analysis (ASS scores of 1–3 mapped on to an EQ-5D health state of 11222; ASS scores of 4–6 mapped on to an EQ-5D health state of 22333; and ASS scores of 7–9 were mapped on to an EQ-5D health state of 33333); (4) assuming baseline ASS mapped on to EQ-5D health states with higher utility scores than in the baseline analysis (ASS of 1–3 mapped on to an EQ-5D health state of 11111; ASS of 4–6 mapped on to an EQ-5D health state of 22111; and ASS of 7–9 were mapped on to an EQ-5D health state of 33222); and (5) adopting a societal perspective rather than a NHS and Personal Social Services perspective.
Chapter 3 Results
Participant flow and recruitment
Five hundred and eight children were randomised from 30 centres throughout the UK (one centre in Wales, two in Scotland, two in Northern Ireland and 25 in England).
The first child was recruited on 14 December 2008 and the last child was randomised on 21 March 2011. Table 3 shows all of the 30 recruiting centres, the date the site was initiated, the target recruitment, the number of participants randomised, the date of the first randomisation and the date of the last randomisation. All 30 centres randomised at least one participant.
Five further centres were at different stages of opening for recruitment at the end of the study (Royal Alexander Children's Hospital, Brighton; Fairfield Hospital, Bury; Leighton Hospital, Crewe; Whiston Hospital, Prescot, Liverpool; Morriston Hospital, Swansea; Royal Hospital for Sick Children, Belfast) but did not randomise any children.
Screening data
Sites were requested to prospectively record each potentially eligible child on a screening log and return this to the Clinical Trials Unit (CTU) on a monthly basis. The log recorded the time and date of presentation, whether or not the child was screened/eligible, and whether or not he/she was then randomised. Reasons for screen failures/non-randomisation were also requested.
Unfortunately, few centres complied, with the majority citing that collection of this information prospectively was too onerous for staff. In instances in which the logs were received, they were often sent sporadically and were poorly completed, not recording children who were missed for trial eligibility assessment.
Efforts were regularly made to encourage return [supported on one occasion by the MRCN local research networks (LRNs)], as screening information was the primary way to objectively assess barriers to recruitment in underperforming sites. Another option given was to record the information retrospectively by review of departmental records; however, again the majority of centres stated they did not have the resources to do this on a regular basis.
Recruitment rates
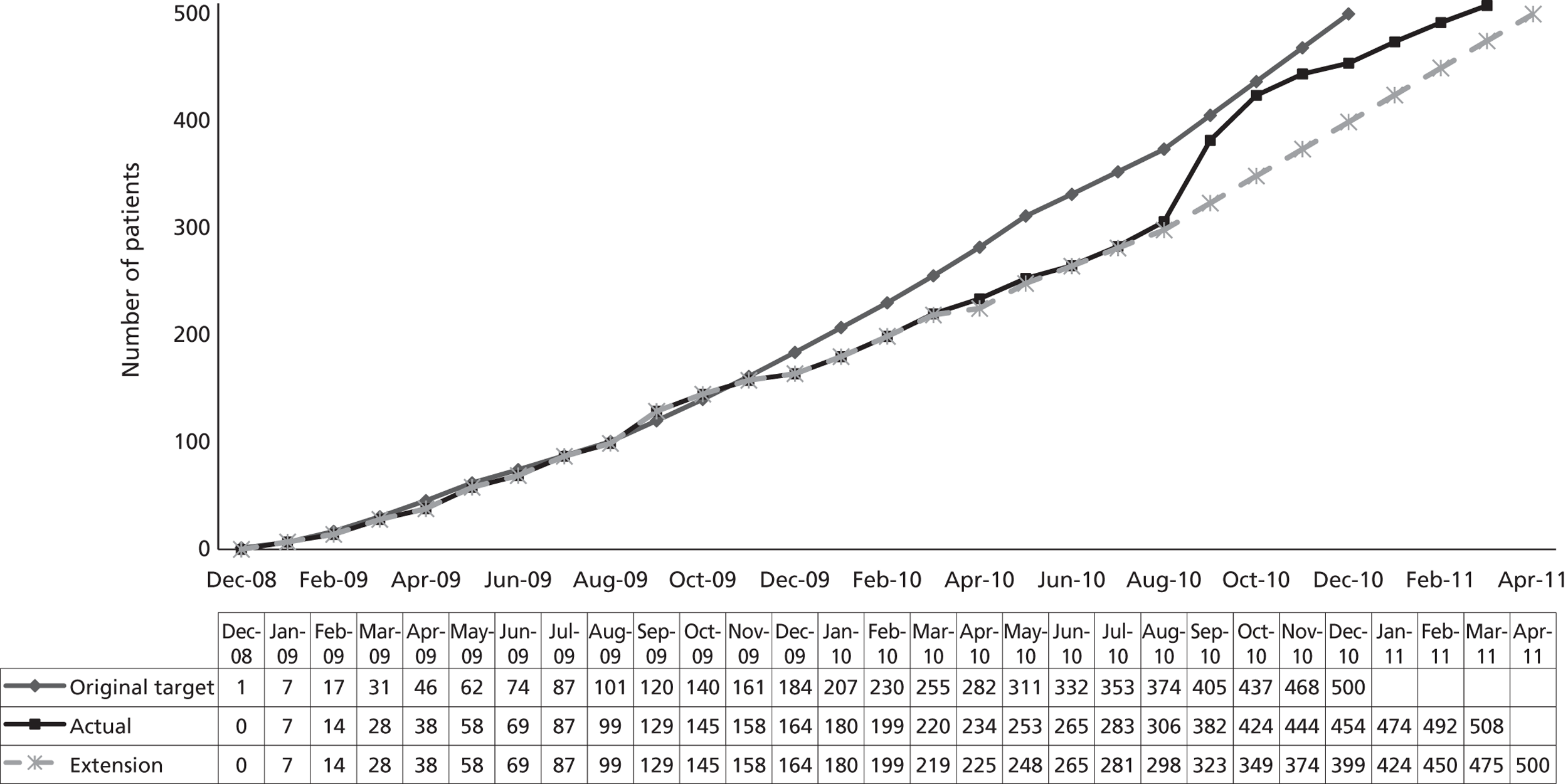
The study target sample size of 500 was expected to have been achieved within a 24-month recruitment period. The actual recruitment was somewhat slower than anticipated (Figure 1), being achieved within 28 months. Reasons for the slower than expected recruitment include the time taken to obtain approvals and undertake training at centres (specifically, good clinical practice training, required to consent children to the trial), rotation of middle-grade medical staff responsible for obtaining consent at many centres (again a training issue), and the seasonal fluctuations in asthma presentations.
The recruitment period of the trial was extended for 5 months in August 2010, and recruitment rates improved following intervention of the MCRN LRNs who conducted a feasibility survey to identify additional recruiting centres. Throughout the trial, at different stages of the study, the LRNs ran MAGNETIC promotions to keep up the profile of the study. For example, Nottingham invested extra resources to boost recruitment in March 2010 with the theme of MAGNETIC March.
FIGURE 1.
Expected vs. actual recruitment rates.
| Centre | Date site initiated | Target recruitment | No. randomised | Date of first randomisation | Date of final randomisation |
|---|---|---|---|---|---|
| St Thomas' Hospital | 4 December 2008 | 30 | 26 | 2 January 2009 | 17 March 2011 |
| Royal Devon and Exeter Hospital | 4 December 2008 | 30 | 33 | 5 January 2009 | 20 March 2011 |
| Derbyshire Children's Hospital | 17 December 2008 | 20 | 21 | 20 February 2009 | 17 January 2011 |
| Tameside General Hospital | 17 December 2008 | 10 | 3 | 14 January 2009 | 27 October 2009 |
| Leicester Royal Infirmary | 9 January 2009 | 20 | 20 | 23 July 2009 | 8 July 2010 |
| Royal Albert Edward Infirmary, Wigan | 9 January 2009 | 18 | 20 | 2 March 2009 | 25 February 2011 |
| Queens Hospital, Burton | 9 January 2009 | 20 | 21 | 13 February 2009 | 14 November 2010 |
| University Hospital of Wales | 9 January 2009 | 25 | 31 | 5 February 2009 | 18 January 2011 |
| Royal London Hospital | 9 January 2009 | 12 | 11 | 2 April 2009 | 21 November 2010 |
| Countess of Chester Hospital | 21 January 2009 | 16 | 26 | 30 July 2009 | 15 March 2011 |
| Macclesfield District General Hospital | 21 January 2009 | 25 | 28 | 17 February 2009 | 5 March 2011 |
| Royal Hospital for Sick Children, Glasgow | 29 January 2009 | 30 | 22 | 14 April 2009 | 21 December 2010 |
| Sheffield Children's Hospital | 29 January 2009 | 20 | 14 | 28 May 2009 | 19 November 2010 |
| Preston Royal Infirmary | 29 January 2009 | 14 | 12 | 4 August 2009 | 6 February 2011 |
| Bristol Royal Children's Hospital | 16 April 2009 | 30 | 37 | 27 April 2009 | 15 March 2011 |
| Queen's Medical Centre Nottingham | 6 May 2009 | 20 | 20 | 29 June 2009 | 22 November 2010 |
| Victoria Hospital Blackpool | 6 May 2009 | 17 | 7 | 26 June 2009 | 1 February 2011 |
| Ormskirk and District Hospital | 12 May 2009 | 20 | 30 | 5 June 2009 | 9 June 2011 |
| Wythenshawe Hospital | 16 September 2009 | 10 | 3 | 15 December 2009 | 6 December 2010 |
| Birmingham Children's Hospital | 2 October 2009 | 15 | 14 | 28 November 2009 | 23 February 2011 |
| University Hospital of North Staffordshire | 3 November 2009 | 18 | 19 | 28 January 2010 | 9 February 2011 |
| Craigavon Area Hospital | 14 November 2009 | 13 | 9 | 29 January 2010 | 24 January 2011 |
| Birmingham Heartlands Hospital | 18 January 2010 | 15 | 4 | 28 March 2010 | 11 May 2010 |
| Royal Aberdeen Children's Hospital | 1 April 2010 | 16 | 11 | 8 June 2010 | 27 January 2011 |
| University Hospital North Tees | 30 April 2010 | 18 | 17 | 22 May 2010 | 6 March 2011 |
| University Hospital Lewisham | 30 April 2010 | 15 | 14 | 30 May 2010 | 7 March 2011 |
| Altnagelvin Area Hospital | 9 June 2010 | 10 | 14 | 15 August 2010 | 2 February 2011 |
| Southampton General Hospital | 2 July 2010 | 10 | 6 | 29 July 2010 | 14 October 2010 |
| Royal Manchester Children's Hospital | 23 August 2010 | 10 | 10 | 27 August 2010 | 28 January 2011 |
| Royal Cornwall Hospital | 7 December 2010 | 8 | 5 | 9 February 2011 | 16 March 2011 |
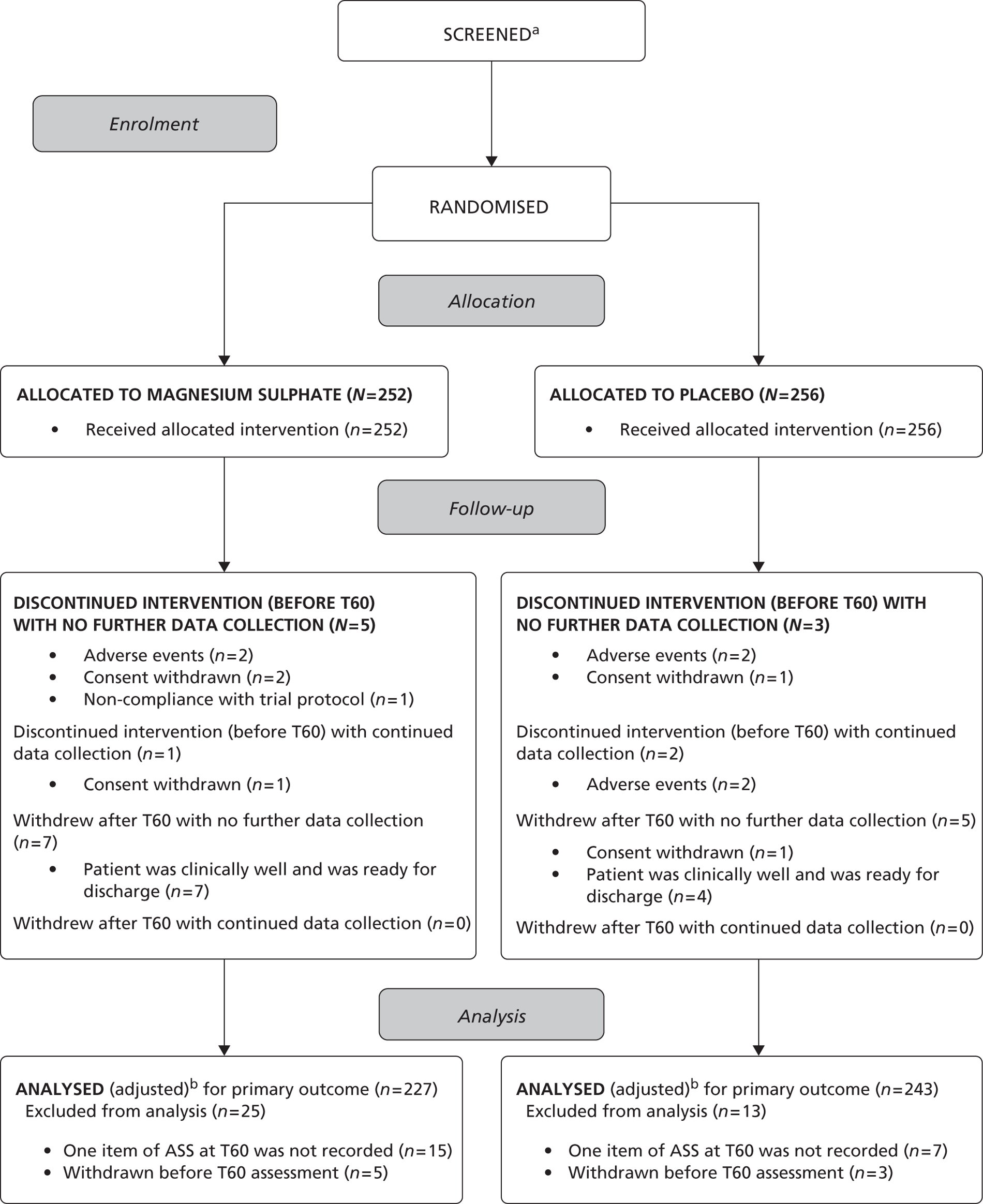
The flow of children
The flow of children through the trial is represented in the CONSORT flow diagram in Figure 2. Five hundred and eight children were randomised: 339 patients from EDs and 169 from paediatric assessment units (PAUs), 252 to the magnesium group and 256 to the placebo group.
In total, 13 children withdrew in the magnesium group; six children discontinued the intervention (withdrew before T60 assessment) and five out of six children did not provide data for the primary outcome analysis; seven children withdrew after T60 assessment and only one child continued to provide further data following withdrawal. In total, 10 children withdrew from the placebo group; five children discontinued the intervention (withdrew before T60 assessment) and three out of five did not provide data for the primary outcome analysis; five children withdrew after T60 assessment and none continued to provide further data following withdrawal. In total, 25 children on magnesium and 13 children on placebo did not have data to contribute to the analysis of the primary outcome. Consequently, 227 children were analysed for the primary outcome in the magnesium group, and 243 children were analysed for the primary outcome in the placebo group.
FIGURE 2.
Consort flow diagram. (a) Few centres complied, with the majority citing that collection of this information prospectively was too onerous for staff. In instances where the logs were received, they were often sent sporadically and were poorly completed and not recording children who were missed. (b) Analysed unadjusted for baseline ASS.
Baseline comparability of randomised groups
Table 4 shows that the baseline characteristics of the 508 randomised participants were similar, with no differences deemed clinically significant.
Participants ranged in age between 1 and 15 years, with the median age similar in both the treatment groups as well as their median age at asthma onset. There were no gender differences between the groups. There were also no differences in current treatment taken for their asthma, treatment given before presentation for the acute attack or previous admissions for acute asthma.
The mean ASS at baseline was almost identical in the two treatment groups. There were no physiological differences in presentation heart rate, respiratory rate or blood pressure or oxygen therapy required at admission.
Most (69%) children were randomised between 0900 and 1700 hours. This is clearly when most of the research staff were around to recruit patients. There were three categories of duration of most recent asthma attack, with the most frequent duration being between 6 and 24 hours.
| Baseline characteristic | Magnesium (n = 252) | Placebo (n = 256) | Total (n = 508) |
|---|---|---|---|
| Age (years): median (IQR), range | 4.0 (3.0–7.0), 2–15 | 4.0 (3.0–7.0), 1–15 | 4.0 (3.0–7.0), 1–15 |
| Male, n (%) | 143 (57) | 150 (59) | 293 (58) |
| Age (years) at asthma onset: | (n = 165) | (n = 168) | (n = 333) |
| Median (IQR), range | 2.0 (1.0–3.0), 0–11 | 2.0 (1.0–3.0), 0–10 | 2.0 (1.0–3.0), 0–11 |
| Undiagnosed, n (%) | 79 (31) | 76 (30) | 155 (31) |
| Missing, n (%) | 8 (3) | 12 (5) | 20 (4) |
| Time of day that randomisation occurred: n (%) | |||
| 0900–1700 | 181 (72) | 168 (66) | 349 (69) |
| 1700–2200 | 49 (19) | 59 (23) | 108 (21) |
| 2200–0900 | 22 (9) | 29 (11) | 51 (10) |
| ASS at baseline | (n = 248) | (n = 254) | (n = 502) |
| Mean (SD), range | 5.7 (1.3), 2–9 | 5.8 (1.4), 2–9 | 5.7 (1.4), 2–9 |
| Previous admissions for asthma: n (%) | (n = 250) | (n = 255) | (n = 505) |
| 0 | 101 (40) | 99 (39) | 200 (40) |
| 1–4 | 101 (40) | 95 (37) | 196 (39) |
| > 4 | 48 (20) | 61 (24) | 109 (21) |
| Duration of the most recent asthma attack: n (%) | (n = 251) | (n = 254) | (n = 505) |
| For the last few days | 54 (22) | 54 (21) | 108 (21) |
| For the last 24 hours | 162 (64) | 162 (64) | 324 (64) |
| For the last 6 hours or less | 35 (14) | 38 (15) | 73 (15) |
| Current asthma medication: n (%) (can be > 1) | |||
| Undiagnosed | 79 | 76 | 155 |
| Diagnosed | 173 | 180 | 353 |
| None | 7 (2) | 1 (0) | 8 (1) |
| Short-acting β2-agonists | 196 (51) | 207 (53) | 403 (52) |
| Inhaled corticosteroids | 106 (28) | 109 (28) | 215 (28) |
| Long-acting β2-agonists | 11 (3) | 19 (5) | 30 (4) |
| Long-acting β2-agonist/steroid combination | 15 (4) | 14 (4) | 29 (4) |
| Leukotriene receptor antagonists | 28 (7) | 28 (7) | 56 (7) |
| Oral steroids | 6 (2) | 2 (0) | 8 (1) |
| Othera | 8 (2) | 7 (2) | 15 (2) |
| Nothing ticked (V1 CRF)b | 5 (1) | 6 (1) | 11 (1) |
| Allergy history: n (%) (can be more than one) | |||
| None/nothing ticked | 118 (40) | 123 (39) | 241 (39) |
| Hay fever | 38 (13) | 61 (19) | 99 (16) |
| Eczema | 97 (33) | 91 (29) | 188 (31) |
| Food allergy | 41 (14) | 42 (13) | 83 (14) |
| Treatment received pre-admission: | |||
| Steroids only | 21 (8) | 25 (10) | 46 (9) |
| Nebulisers only | 68 (27) | 72 (28) | 140 (27) |
| Both steroids and nebulisers | 47 (19) | 55 (21) | 102 (20) |
| Yes, but neither steroids nor nebulisers | 20 (8) | 17 (7) | 37 (7) |
| Not known | 3 (1) | 10 (4) | 13 (3) |
| None | 79 (31) | 73 (29) | 152 (30) |
| Nothing ticked (V1 CRF) | 10 (4) | 3 (1) | 13 (3) |
| Other treatment missing (V1 CRF) | 4 (2) | 1 (0) | 5 (1) |
| Nebuliser received before randomisation: n (%) | (n = 250) | (n = 254) | (n = 504) |
| Salbutamol | 106 (42) | 101 (40) | 207 (41) |
| Salbutamol + ipratropium | 144 (58) | 150 (59) | 294 (58) |
| Not given | 0 (0) | 3 (1) | 3 (1) |
| SaO2 (%), mean (SD), range | (n = 250) | (n = 253) | (n = 503) |
| 93.8 (3.5), 84–100 | 93.4 (3.4), 81–100 | 93.6 (3.4), 81–100 | |
| Blood pressure (mmHg), mean (SD), range | (n = 210) | (n = 211) | (n = 421) |
| Systolic | 109.5 (14.1), 62–163 | 112.7 (12.5), 70–172 | 111.1 (13.4), 62–172 |
| Diastolic | 65.5 (11.6), 30–105 | 66.3 (12.7), 34–123 | 65.9 (12.2), 30–123 |
| Respiratory rate (breaths per minute), mean (SD), range | (n = 247) | (n = 250) | (n = 497) |
| 43.2 (10.5), 20–72 | 42.5 (10.9), 20–70 | 42.9 (10.7), 20–72 | |
| Oxygen therapy, n (%) | (n = 241) | (n = 247) | (n = 488) |
| Yes | 94 (37) | 98 (38) | 192 (38) |
| No | 147 (63) | 149 (62) | 296 (62) |
Timing of treatment administration
Each child was randomised to receive nebulised salbutamol 2.5 mg (aged 2–5 years) or 5 mg (aged ≥ 6 years) and ipratropium bromide 0.25 mg mixed with either 2.5 ml of isotonic MgSO4 (250 mmol/l, tonicity 289 mOsm; 151 mg per dose) or 2.5 ml of isotonic saline on three occasions at 20-minute intervals. No dose modification of the study treatment was permitted and dosing was continued in the event of deterioration of the child's condition unless cessation of therapy was deemed necessary by the clinician or if consent for the trial was withdrawn.
Table 5 shows treatment details for all randomised children. There was no clinically significant deviation in mean prescribed times between the treatment groups on any of the three occasions.
There were 246 and 250 children who received all three treatments in the magnesium and placebo groups respectively. It was expected that all three trial treatments should have been received within approximately 1 hour; however, in some cases, treatments were administered slightly late. Based on the fact that the prescription time of each treatment was reported, and not the time of the end of the third treatment, it was expected that the time between first and third treatments should be 40 minutes but an allowance of an additional 15 minutes would be tolerable. Therefore, if the above timing was > 55 minutes, this was defined as a deviation outside the acceptable window (see Table 6). There were 53 children who received their third treatment at > 55 minutes after randomisation. Note that this is a change to the proposed deviation outlined in the SAP, and was made prior to unblinding and any comparative analysis (see Appendix 3 for more details).
| Treatment details | Prescribed time (minutes) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Magnesium | Placebo | Total | |||||||
| Firsta | Secondb | Thirdc | Firsta | Secondb | Thirdc | Firsta | Secondb | Thirdc | |
| No. treated | 252 | 248 | 246 | 255 | 252 | 250 | 507 | 500 | 496 |
| Timing of treatment | |||||||||
| Mean (SD) | 5.3 (8.4) | 23.6 (5.9) | 23.7 (6.8) | 6.4 (8.1) | 23.1 (5.1) | 23.0 (5.5) | 5.8 (8.3) | 23.4 (5.5) | 23.3 (6.2) |
| Range | 0–65 | 10–65 | 10–65 | 0–40 | 5–40 | 14–60 | 0–65 | 5–65 | 10–65 |
Unblinding of randomised treatments
The treatment allocation for two children was unblinded during the course of the trial (one in the magnesium group and one in the placebo group; see Table 14). One child (magnesium group) was unblinded to enable treatment of a SAE; however, the event was considered to be unlikely to be related to trial medication. One child (placebo group) was unblinded after resolution of a SAE as parents wished to be notified of their child's treatment allocation.
Protocol deviations
There were 14 children who did not receive nebulised treatment during screening pre-randomisation. Two children aged 15 and 23 months were recruited. One child was recruited twice. Further protocol deviations were classified in Table 6 and summarised for each treatment group. There is no imbalance across treatment groups.
| Protocol specification | No. of deviations (%) | |
|---|---|---|
| Magnesium | Placebo | |
| Inclusion criteria – two children aged 15 and 23 months were recruited | 0a | 2 (1) |
| Exclusion criteria – one child was recruited twice | 0a | 2 (1) |
| Treatment regime | ||
| Allocation (did not receive full trial treatment as per protocol) | 7 (3) | 12 (5) |
| Timingb (deviations outside acceptable timing window) | 24 (10) | 29 (12) |
| Primary outcome data (deviation in the method of assessment) | 0 | 0 |
| Secondary outcome data (deviation in the method of assessment) | ||
| Clinical outcomes | 0 | 0 |
| Child and parental outcomes at 1-month follow-up | 0 | 0 |
Internal pilot and interim analysis
To ensure the appropriateness of the SD used in the sample size calculation, we undertook an internal pilot after the first 36 children had been randomised and completed follow-up. The SD estimated from a sample of 26 patients with complete ASS data at T60 (ranging from 2 to 7) was 1.4. As there were 10 patients with missing ASS at T60, and these could plausibly include both extremes of the possible ASS range (0–9), this may be an underestimate of the true value, so we undertook a sensitivity analysis. Nine of the ten patients with missing ASS at T60 had T40 data and the mean value of ASS of these records was 5.56. Among those 26 patients who did have ASS at T60, 25 had T40 data and the mean value of ASS of these records was 5.32. So, on average, T40 ASS was slightly higher among those who had a missing ASS at T60 measurement. The IDSMC did not consider that the missing observations would have a substantial impact on the SD, which was lower than the value assumed for the original power calculation. The IDSMC recommended no change to the sample size based on these results.
Furthermore, a blinded interim analysis was undertaken after 262 children had been randomised. ANCOVA adjusted for baseline ASS and independent samples t-test were performed, and the mean differences and 99.9% CIs were reported in the closed section of the IDSMC report; blinded results as presented to the IDSMC are shown in Table 7.
| Treatment I (n = 123) | Treatment J (n = 115) | Mean difference (99.9% CI) | Adjusted mean difference (99.9% CI) |
|---|---|---|---|
| 4.97 | 4.66 | – 0.307a (– 0.922 to 0.308) | – 0.356 (– 0.923 to 0.211) |
The IDSMC noted that the difference in ASS at T60 was less than the minimum critical difference value of 0.5 on which the sample size was based. There were no substantial risk–benefit concerns, and continued recruitment and conduct of the trial was recommended.
Analysis of primary outcome
The results for the final analysis of the primary outcome are presented in Table 8. The mean difference in ASS at T60 between the two treatment groups, magnesium minus placebo, adjusting for baseline ASS, was – 0.25 points (95% CI – 0.48 to – 0.02 points), i.e. magnesium appears to lower the score. However, although the difference between the treatment groups was statistically significant, the 95% CI lies above the minimum clinically important difference of 0.5 points defined prior to the trial. Diagnostic plots for the analysis of the primary outcome data are presented in Appendix 7. There is no evidence of violation of model assumptions.
| Outcome | Magnesium (nm = 228): T60 mean (SD), range | Placebo (np = 244): T60 mean (SD), range | Estimate (95% CI), p-value | |
|---|---|---|---|---|
| Difference in mean: nm = 228, np = 244 | Adjusted difference in mean: nm = 227, np = 243 | |||
| ASS | 4.72 (1.37), 2–9 | 4.95 (1.40), 2–9 | – 0.24 (– 0.49 to 0.02), p = 0.066 | – 0.25 (– 0.48 to – 0.02), p = 0.034 |
Key baseline characteristics for those with observed ASS at T60 are presented in Appendix 5, Table 40, and show no differences between the treatment groups, which implies that the patients with missing outcomes do not affect the randomisation balance. There is no evidence of a difference in key baseline characteristics between patients with observed and missing ASS at T60 (see Appendix 5, Table 41), indicating plausibility of the MCAR assumption.
The reasons for missing primary outcome data are provided in Appendix 5 (see Reasons for exclusion of children from primary outcome analysis) with the results of the sensitivity analyses (see Sensitivity analyses of missing primary outcome). The problem of non-ignorable missing ASS data is addressed through joint modelling of the longitudinal data and the time to drop out from the study [Appendix 5, see Sensitivity analysis (3)]. Sensitivity analyses did not suggest substantially different conclusions to those above.
A sensitivity analysis to test the robustness of ignoring the centre effect in the primary analysis is presented in Appendix 6. Both random-effects analysis of variance and fixed-effect models indicated a significant main effect of centre but there is no evidence that the treatment effect varies by centre.
Analysis of secondary outcomes
Area under the curve for Asthma Severity Score over three time intervals
The results for the AUC analysis for ASS at 20, 40 and 60 minutes are presented in Table 9. Figure 3 shows the mean longitudinal profiles for each group. All three values of ASS were available for 462 (91%) children. The mean difference in AUC between the two treatment groups was 8.1 points (95% CI – 20.8 to 4.6 points) lower in the magnesium group. However, the difference between the treatment groups was not statistically significant.
| Outcome | Magnesium: AUC mean (SD), range | Placebo: AUC mean (SD), range | Difference in mean: estimate (95% CI), p-value |
|---|---|---|---|
| AUC: nm = 223, np = 239 | 316.1 (68.4), 160–520 | 324.2 (70.7), 110–540 | – 8.1 (– 20.8 to 4.6), p = 0.210 |
FIGURE 3.
Mean longitudinal profiles.
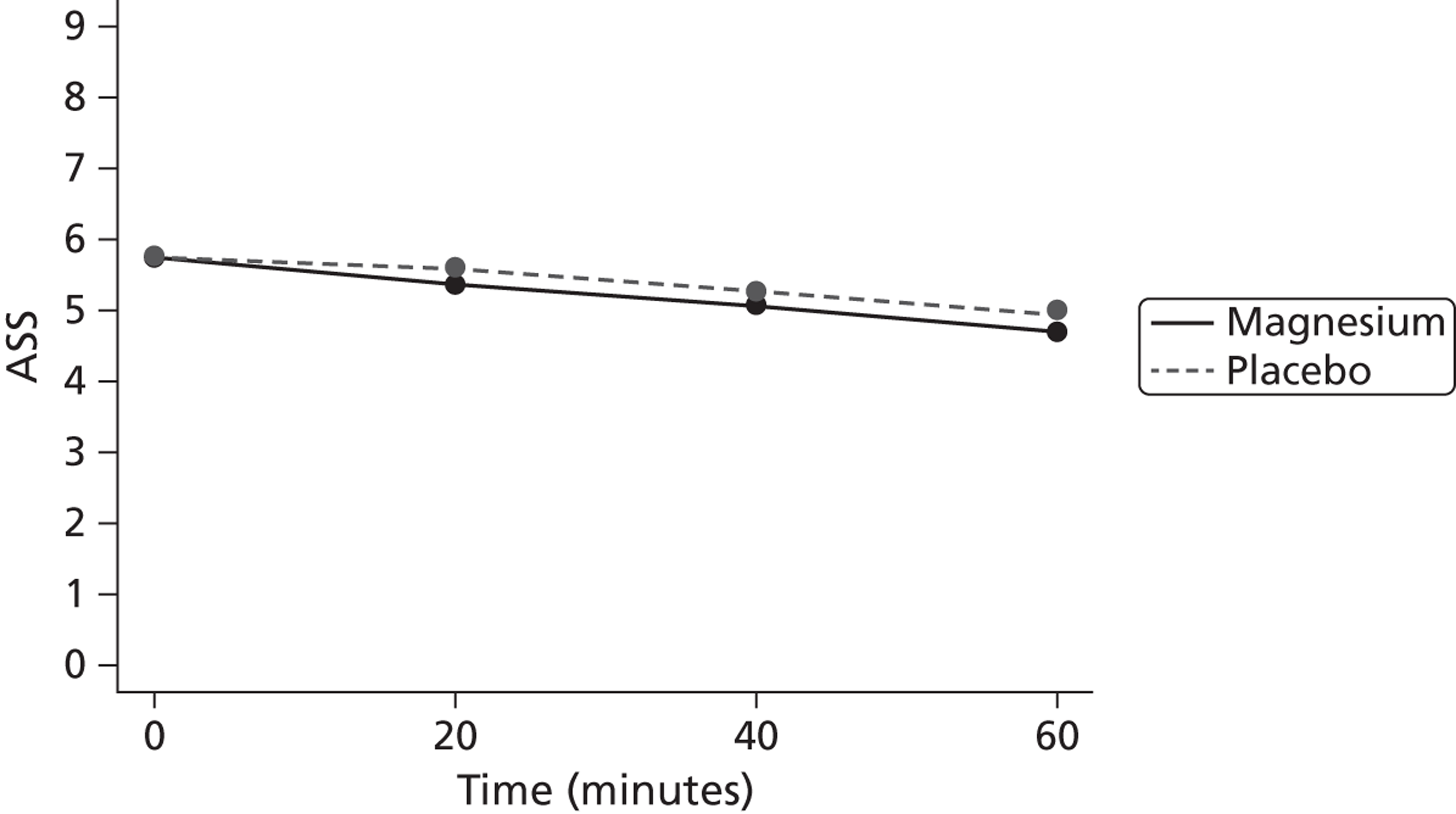
Analysis of secondary efficacy clinical outcomes
There were five secondary efficacy clinical outcomes: ‘stepping down’ of treatment at 1 hour, number of additional salbutamol administrations, LOS in hospital, requirement for intravenous bronchodilator treatment and intubation and/or admission to a PICU. Results are shown in Table 10.
The ‘stepping down’ of treatment at 1 hour was defined by the no treatment or MDI spacer only until discharge. The proportion of child stepping down at 1 hour was slightly higher in magnesium group; however, the results did not show a statistically significant difference between the two treatment groups. We abandoned a detailed analysis of stepping down of treatment as a primary outcome, as it became apparent that the definition was not clear and varied from centre to centre.
The total number of additional salbutamol administrations was slightly lower in the magnesium group; however, the results did not show a statistically significant difference between the two treatment groups.
The LOS in hospital was defined by the time from randomisation to trial treatment to discharge from hospital. The median LOS for children in magnesium group is 26 hours, whereas that for placebo was 27 hours. The results did not show a statistically significant difference between the two treatment groups.
The proportion of children requiring of intravenous bronchodilator treatment was slightly lower in the magnesium group; however, the results did not show a statistically significant difference between the two treatment groups.
The proportion of children requiring intubation and/or admission to a PICU was slightly higher in the magnesium group; however, the results did not show a statistically significant difference between the two treatment groups. There was only one child who required intubation in the study and this child was in the placebo group.
Although children in the magnesium group showed favourable secondary outcomes compared with the placebo group, none of the differences reached statistical significance. As presented in Appendix 6, as there is no evidence that the treatment effect varies by centre, no sensitivity analyses for the centre-specific outcomes (‘stepping down’ of treatment at 1 hour, progression to intravenous treatment, intubation and/or admittance to PICU) were undertaken to account for centre characteristics. Histograms for continuous secondary outcome data are presented in Appendix 7.
| Secondary outcome | Magnesium | Placebo | Estimate (95% CI), p-value |
|---|---|---|---|
| Proportion (%) stepping down treatment at 1 hour: nm = 248, np = 253 | 82/248 (33) | 76/253 (30) | 0.03 (– 0.05 to 0.11), p = 0.527 |
| No. of additional salbutamol administrations [median (IQR)]: nm = 247, np = 253 | 8 (4 to 14) | 9 (4 to 17) | – 1.0 (– 2.00 to 0.00), p = 0.236 |
| LOS (hours) in hospital [median (IQR)]: nm = 251, np = 254 | 26.3 (17.4 to 44.8) | 27.1 (19.2 to 47.6) | – 1.8 (– 4.80 to 0.70), p = 0.166 |
| Proportion (%) requiring intravenous bronchodilator treatment: nm = 249, np = 255 | 24/249 (10) | 30/255 (12) | – 0.02 (– 0.07 to 0.03), p = 0.527 |
| Proportion (%) requiring intubation and/or admission to a PICU:a nm = 251, np = 254 | 22/251 (9) | 15/254 (6) | 0.03 (– 0.02 to 0.07), p = 0.283 |
Assessing the evidence for treatment–covariate interactions
There is evidence that the more severe the exacerbation of asthma, the more likely a better response to magnesium. 4,6,31 Our hypothesis would be that the effect of the addition of magnesium would be greater in those with more severe disease. We thus took the saturation level of oxygen in haemoglobin, as measured in arterial blood (SaO2) level at presentation to be the best marker of severity to examine as a treatment covariate,3 there is evidence that as magnesium acts as a smooth muscle bronchodilator and that the early response is affected by nebulised magnesium to a greater extent than the later more inflammatory response;59 a further hypothesis would be that those with a shorter duration of attack may have a better response to treatment.
Other factors, such as age or gender, may affect the response but a number of possible interactions could be argued. Prognostic factors affecting response could thus be examined in further analysis and could not be justified at this stage.
Treatment–covariate interactions were thus investigated for two clinically important baseline covariates: duration of the most recent asthma attack and SaO2 level. This is a change to the proposed analysis outlined in SAP (see Appendix 3 for more details). The models were adjusted for treatment group, baseline ASS and the baseline covariate of interest. The results are presented in Table 11, and predicted treatment–covariate interactions are shown graphically in Figures 4 and 5. Both treatment–covariate interactions are statistically significant. The model including the duration of the most recent asthma attack indicates a trend towards the effect of magnesium being greater, and clinically significant, if given within the first 6 hours of the onset of the attack. As both ASS and SaO2 are measures of severity, we have also investigated a second model for SaO2 level, excluding baseline ASS. Both models indicate that magnesium appears beneficial for lower SaO2 level (more severe) but no difference for higher SaO2 level (less severe).
| Variable | Estimate (95% CI), p-value | |
|---|---|---|
| Models with main effects only | Models with treatment–covariate interaction effects | |
| Duration of the most recent asthma attack | ||
| Intercept | 2.62 (2.07 to 3.17), p < 0.0001 | 2.52 (1.94 to 3.10), p < 0.0001 |
| Magnesium | – 0.28 (– 0.51 to – 0.04), p = 0.020 | 0.01 (– 0.48 to 0.51), p = 0.955 |
| ASS at baseline | 0.40 (0.32 to 0.49), p < 0.0001 | 0.40 (0.31 to 0.48), p < 0.0001 |
| For the last 6 hours or less vs. for the last few days | – 0.34 (– 0.74 to 0.06), p = 0.099 | 0.03 (– 0.51 to 0.57), p = 0.920 |
| For the last 24 hours vs. for the last few days | 0.10 (– 0.19 to 0.39), p = 0.490 | 0.24 (– 0.16 to 0.64), p = 0.250 |
| Marginal effect of attack duration p = 0.040 | ||
| For the last 6 hours or less vs. for the last few days* magnesium | – 0.79 (– 1.58 to -0.00), p = 0.049 | |
| For the last 24 hours vs. for the last few days* magnesium | – 0.28 (– 0.85 to 0.30), p = 0.346 | |
| Marginal effect of attack duration* magnesium, p = 0.143 | ||
| SaO2 (model 1) | ||
| Intercept | 5.28 (2.01 to 8.56), p = 0.002 | 8.70 (4.16 to 13.24), p < 0.001 |
| Magnesium | – 0.23 (– 046 to 0.01), p = 0.055 | – 7.11 (– 13.49 to – 0.74), p = 0.029 |
| ASS at baseline | 0.38 (0.29 to 0.46), p < 0.0001 | 0.37 (0.28 to 0.46), p < 0.0001 |
| SaO2 | – 0.03 (– 0.06 to 0.01), p = 0.124 | – 0.06 (– 0.11 to – 0.02), p = 0.010 |
| SaO2* magnesium | 0.07 (0.01 to 0.14), p = 0.034 | |
| SaO2 (model 2: without ASS at baseline) | ||
| Intercept | 8.24 (4.82 to 11.66), p < 0.0001 | 12.19 (7.39 to 16.98), p < 0.0001 |
| Magnesium | – 0.21 (– 0.46 to 0.04), p = 0.095 | – 8.17 (– 14. 99 to – 1.36), p = 0.019 |
| SaO2 | – 0.04 (– 0.07 to 0.00), p = 0.058 | – 0.08 (– 0.13 to – 0.03), p = 0.003 |
| SaO2* magnesium | 0.08 (0.01 to 0.16), p = 0.022 | |
FIGURE 4.
Predicted scores for treatment: duration of the most recent asthma attack interaction effect.
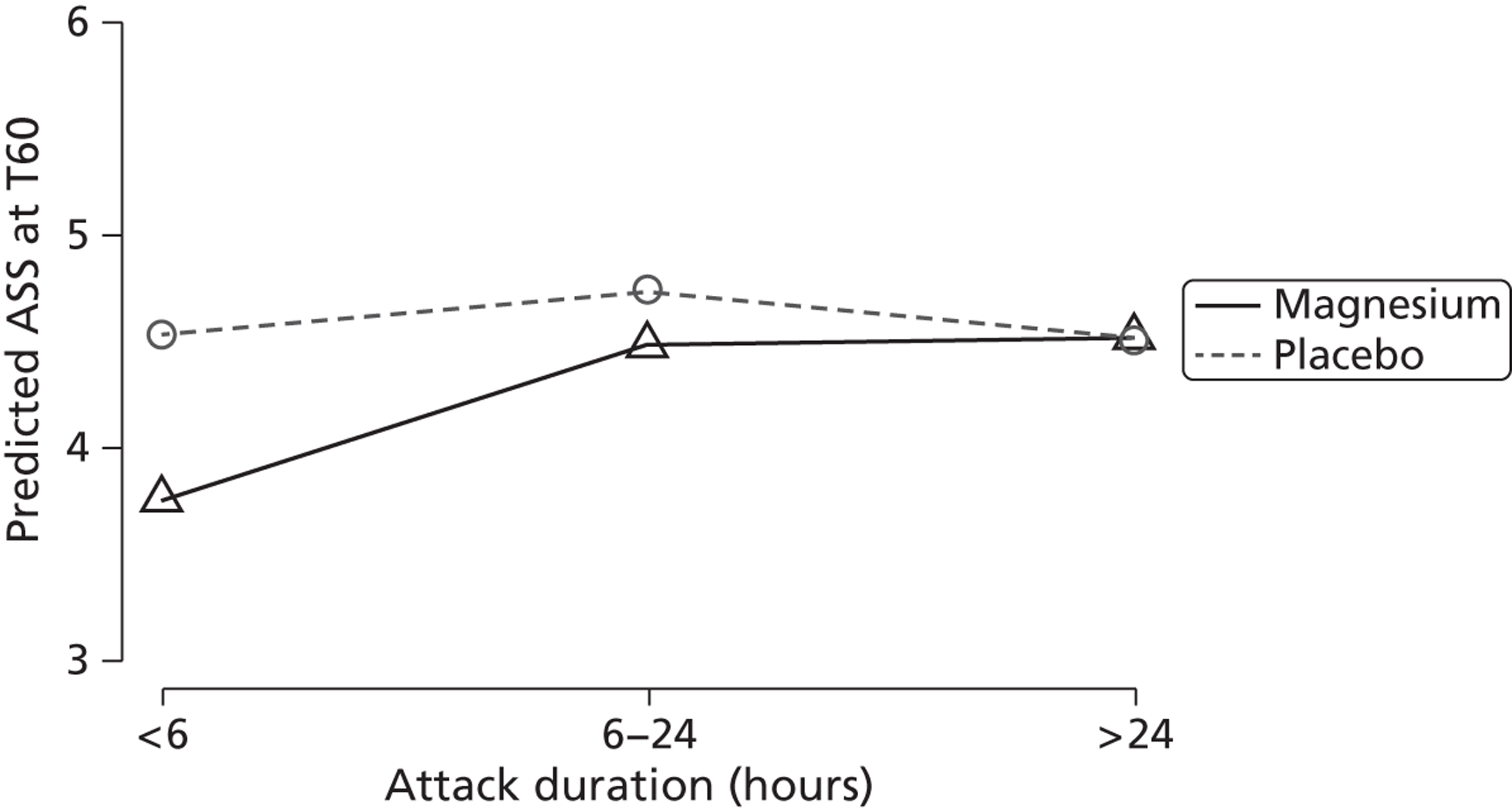
FIGURE 5.
Predicted scores for treatment: SaO2 interaction effect.
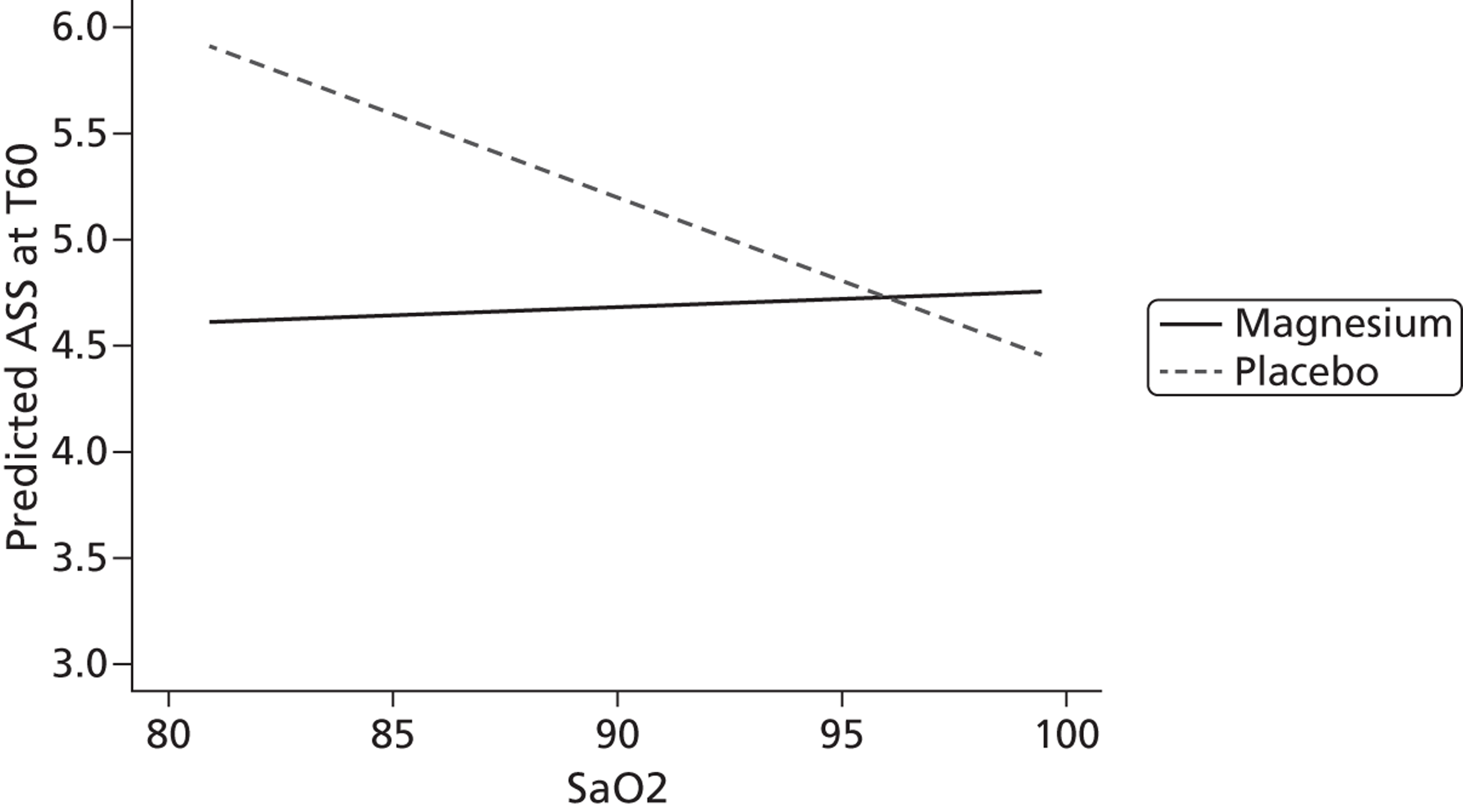
Safety outcomes
Adverse effects were assessed during follow-up checks at 2, 3 and 4 hours after the final study treatment.
For the analysis of safety outcomes, all children who have received at least one dose of the study drug and were available for follow-up were included. One patient did not receive the study drug.
Adverse events
The number (and percentage) of children experiencing each AE is presented for each treatment arm in Table 12. Serious AEs were not included in this section but will be discussed in more detail in the next section. Table 12 presents AEs categorised by severity. For each child, only the maximum severity experienced of each type of AE is displayed. There were 21 types of AEs (abdominal pain, asymptomatic hypotension, back pain, blood per rectum, chest pain, diarrhoea, dizziness, drowsiness, facial flushing, feet cramp, fever, headache, hypokalaemia, itchy face, jitteriness, nausea, sleepiness, teeth whitening, urticarial rash, vacant episode, vomiting).
A statistical test comparing the percentage of children suffering an AE in each arm has not been performed for two reasons: (1) this analysis would assume the AEs are of equal importance and (2) no hypotheses on AEs were set out upfront before the blind has been broken.
The results in Tables 12 and 13 do not appear to suggest there are any important increases in any event in either of the treatment groups.
| Event | Magnesium | Placebo | Total | |||
|---|---|---|---|---|---|---|
| Children [n = 47/252 (19%)]: n (%) | Events (n = 47) | Children [n = 52/255 (20%)]: n (%) | Events (n = 59) | Children [n = 99/507 (19%)]: n (%) | Events (n = 106) | |
| Abdominal pain | 2 (0.8) | 2 | 2 (0.8) | 2 | 4 (0.8) | 4 |
| Asymptomatic hypotension | 1 (0.4) | 1 | 2 (0.8) | 2 | 3 (0.6) | 3 |
| Back pain | 0 (0.0) | 0 | 1 (0.4) | 1 | 1 (0.2) | 1 |
| Blood per rectum | 0 (0.0) | 0 | 1 (0.4) | 1 | 1 (0.2) | 1 |
| Chest pain | 1 (0.4) | 1 | 2 (0.8) | 3 | 3 (1.2) | 4 |
| Diarrhoea | 0 (0.0) | 0 | 1 (0.4) | 1 | 1 (0.2) | 1 |
| Dizziness | 1 (0.4) | 1 | 0 (0.0) | 0 | 1 (0.2) | 1 |
| Drowsiness | 1 (0.4) | 1 | 0 (0.0) | 0 | 1 (0.2) | 1 |
| Facial flushing | 2 (0.8) | 2 | 3 (1.2) | 3 | 5 (1.0) | 5 |
| Feet cramp | 0 (0.0) | 0 | 1 (0.4) | 1 | 1 (0.2) | 1 |
| Fever | 8 (3.2) | 8 | 5 (2.0) | 5 | 13 (2.6) | 13 |
| Headache | 5 (2.0) | 5 | 1 (0.4) | 1 | 6 (1.2) | 6 |
| Hypokalaemia | 0 (0.0) | 0 | 1 (0.4) | 1 | 1 (0.2) | 1 |
| Itchy face | 0 (0.0) | 0 | 1 (0.4) | 1 | 1 (0.2) | 1 |
| Jitteriness | 1 (0.4) | 1 | 0 (0.0) | 0 | 1 (0.2) | 1 |
| Nausea | 4 (1.6) | 4 | 2 (0.8) | 2 | 6 (1.2) | 6 |
| Sleepa | 0 (0.0) | 0 | 1 (0.4) | 1 | 1 (0.2) | 1 |
| Teeth whitening | 0 (0.0) | 0 | 1 (0.4) | 1 | 1 (0.2) | 1 |
| Urticarial rash | 0 (0.0) | 0 | 1 (0.4) | 2 | 1 (0.2) | 2 |
| Vacant episode | 0 (0.0) | 0 | 2 (0.8) | 2 | 2 (0.4) | 2 |
| Vomiting | 21 (8.3) | 21 | 24 (9.4) | 29 | 45 (8.9) | 50 |
| Event | Severitya | No. of events | No. of childrenb | ||||
|---|---|---|---|---|---|---|---|
| Magnesium | Placebo | Total | Magnesium [n = 47/252 (19%)]: n (%) | Placebo [n = 52/255 (20%)]: n (%) | Total [n = 99/507 (19%)]: n (%) | ||
| Abdominal pain | Mild | 2 | 2 | 4 | 2 (0.8) | 2 (0.8) | 4 (0.8) |
| Moderate | 0 | 0 | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Asymptomatic hypotension | Mild | 1 | 1 | 2 | 1 (0.4) | 1 (0.4) | 2 (0.4) |
| Moderate | 0 | 1 | 1 | 0 (0.0) | 1 (0.4) | 1 (0.2) | |
| Back pain | Mild | 0 | 1 | 1 | 0 (0.0) | 1 (0.4) | 1 (0.2) |
| Moderate | 0 | 0 | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Blood per rectum | Mild | 0 | 1 | 1 | 0 (0.0) | 1 (0.4) | 1 (0.2) |
| Moderate | 0 | 0 | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Chest pain | Mild | 1 | 2 | 3 | 1 (0.4) | 1 (0.4) | 2 (0.4) |
| Moderate | 0 | 1 | 1 | 0 (0.0) | 1 (0.4) | 1 (0.2) | |
| Diarrhoea | Mild | 0 | 1 | 1 | 0 (0.0) | 1 (0.4) | 1 (0.2) |
| Moderate | 0 | 0 | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Dizziness | Mild | 1 | 0 | 1 | 1 (0.4) | 0 (0.0) | 1 (0.2) |
| Moderate | 0 | 0 | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Drowsiness | Mild | 1 | 0 | 1 | 1 (0.4) | 0 (0.0) | 1 (0.2) |
| Moderate | 0 | 0 | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Facial flushing | Mild | 2 | 1 | 3 | 2 (0.8) | 1 (0.4) | 3 (0.6) |
| Moderate | 0 | 2 | 2 | 0 (0.0) | 2 (0.8) | 2 (0.4) | |
| Feet cramp | Mild | 0 | 1 | 1 | 0 (0.0) | 1 (0.4) | 1 (0.2) |
| Moderate | 0 | 0 | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Fever | Mild | 7 | 5 | 12 | 7 (2.8) | 5 (2.0) | 12 (2.4) |
| Moderate | 1 | 0 | 1 | 1 (0.4) | 0 (0.0) | 1 (0.2) | |
| Headache | Mild | 5 | 1 | 6 | 5 (2.0) | 1 (0.4) | 6 (1.2) |
| Moderate | 0 | 0 | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Hypokalaemia | Mild | 0 | 1 | 1 | 0 (0.0) | 1 (0.4) | 1 (0.2) |
| Moderate | 0 | 0 | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Itchy face | Mild | 0 | 1 | 1 | 0 (0.0) | 1 (0.4) | 1 (0.2) |
| Moderate | 0 | 0 | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Jitteriness | Mild | 1 | 0 | 1 | 1 (0.4) | 0 (0.0) | 1 (0.2) |
| Moderate | 0 | 0 | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Nausea | Mild | 4 | 2 | 6 | 4 (1.6) | 2 (0.8) | 6 (1.2) |
| Moderate | 0 | 0 | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Sleep | Mild | 0 | 1 | 1 | 0 (0.0) | 1 (0.4) | 1 (0.2) |
| Moderate | 0 | 0 | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Teeth whitening | Mild | 0 | 1 | 1 | 0 (0.0) | 1 (0.4) | 1 (0.2) |
| Moderate | 0 | 0 | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Urticarial rash | Mild | 0 | 2 | 2 | 0 (0.0) | 1 (0.4) | 1 (0.2) |
| Moderate | 0 | 0 | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Vacant episode | Mild | 0 | 1 | 1 | 0 (0.0) | 1 (0.4) | 1 (0.2) |
| Moderate | 0 | 1 | 1 | 0 (0.0) | 1 (0.4) | 1 (0.2) | |
| Vomiting | Mild | 20 | 24 | 44 | 20 (7.9) | 21 (8.2) | 41 (8.1) |
| Moderate | 1 | 5 | 6 | 1 (0.4) | 3 (1.2) | 4 (0.8) | |
Serious adverse events and suspected unexpected serious adverse reactions
There were 15 SAEs (three on magnesium, 12 on placebo) but no suspected unexpected serious adverse reactions (SUSARs) during the course of the trial. The same child reported increased bronchospasm on two occasions during follow-up. One child who was admitted to PICU was subsequently admitted to hospital twice owing to worsening symptoms. Seven SAEs were deemed to be unrelated, seven unlikely to be related and one possibly related. Full details are shown in Table 14.
| No. | Treatment allocation | Description | Seriousness | Severity | Relationship | Expectedness | Cause | Outcome | Child Status | Unblinded |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Placebo | Low SaO2 level (< 86%)/silent chest/cyanosis | Medically significant/important; required hospitalisation; Immediately life-threatening; prolonged existing hospitalisation | Severe | Possibly | Unexpected | Disease under study | Resolved | Withdrawn from treatment | Yes |
| 2 | Placebo | Child had glycosuria and high blood sugars of > 20 mmol/l | Medically significant/important | Mild | Unrelated | Unexpected | Prior or concomitant treatment | Resolved | Completed trial | No |
| 3 | Placebo | Chest infection | Prolonged existing hospitalisation | Moderate | Unrelated | Unexpected | Other illness | Resolved | Completed trial | No |
| 4 | Magnesium | Child deterioration, developing silent chest, vomiting | Required hospitalisation | Severe | Unlikely | Unexpected | Disease under study | Resolved | Withdrawn from treatment | Yes |
| 5 | Placebo | Increased bronchospasm | Medically significant/important; prolonged existing hospitalisation | Mild | Unlikely | Unexpected | Disease under study | Resolved | Completed trial | No |
| 6 | Placebo | Increased bronchospasm | Medically significant/important; prolonged existing hospitalisation | Moderate | Unlikely | Unexpected | Disease under study | Resolved | Completed trial | No |
| 7 | Placebo | Admission to PICU as HDU child – increased wheeze, respiratory rate and air entry | Prolonged existing hospitalisation | Moderate | Unlikely | Expected | Disease under study | Resolved | Completed trial | No |
| 8 | Magnesium | Viral pneumonia | Required hospitalisation; prolonged existing hospitalisation | Mild | Unrelated | Unexpected | Other illness | Ongoing at final follow-up | Continuing in trial | No |
| 9 | Magnesium | Admission to PICU because of clinical deterioration and nebuliser poor compliance | Prolonged existing hospitalisation | Moderate | Unlikely | Expected | Disease under study | Resolved | Completed trial | No |
| 10 | Placebo | Bronchiectasis | Medically significant/important | Mild | Unrelated | Unexpected | Other illness | Ongoing at final follow-up | Completed trial | No |
| 11 | Placebo | Admission to PICU as symptoms not improving | Prolonged existing hospitalisation | Mild | Unrelated | Unexpected | Disease under study | Resolved | Completed trial | No |
| 12 | Placebo | Re-admitted to hospital | Required hospitalisation | Mild | Unlikely | Unexpected | Disease under study | Resolved with sequelae | Continuing in trial | No |
| 13 | Placebo | Re-admitted to hospital | Required hospitalisation | Mild | Unrelated | Expected | Disease under study | Resolved with sequelae | Completed trial | No |
| 14 | Placebo | Worsening of asthma, required aminophylline | Prolonged existing hospitalisation | Moderate | Unrelated | Expected | Disease under study | Resolved | Continuing in trial | No |
| 15 | Placebo | Deterioration in asthma, requiring intravenous drugs | Medically significant/important | Moderate | Unlikely | Expected | Disease under study | Resolved | Continuing in trial | No |
Withdrawals
There were a total of 20 withdrawals from the study with no further data collection; eight in the placebo group and 12 in the magnesium group. There were three further withdrawals with continued data collection: two in the placebo group and one in the magnesium group. The reasons for withdrawal are shown in Tables 15 and 16 by time point. The number in parentheses is the number of occurrences for each reason.
| Treatment allocation | Reason for withdrawal from study | T0 | T20 | T40 | T60 | T120 | T180 | T240 |
|---|---|---|---|---|---|---|---|---|
| Magnesium | Child was clinically well and was ready for discharge | ✗ (2) | ✗ (5) | |||||
| Placebo | Child was clinically well and was ready for discharge | ✗ (2) | ✗ (2) | |||||
| Placebo | SAE [low SaO2 level (< 86%)/silent chest/cyanosis] | ✗ | ||||||
| Magnesium | AE (hypotension) | ✗ | ||||||
| Placebo | AE (sleep) | ✗ | ||||||
| Placebo | Mother withdrew consent (child's father not present, mother was tired, tearful and unsure) | ✗ | ||||||
| Placebo | Self-discharged (parent felt they could provide required treatment at home) | ✗ | ||||||
| Magnesium | Child did not like the taste of nebuliser | ✗ | ||||||
| Magnesium | Child not tolerating nebulisers, becoming distressed | ✗ (2) | ||||||
| Magnesium | Non-compliance with protocol | ✗ |
| Treatment allocation | Reason for withdrawal from study | T0 | T20 | T40 | T60 | T120 | T180 | T240 |
|---|---|---|---|---|---|---|---|---|
| Magnesium | SAE (developed silent chest) | ✗ | ||||||
| Placebo | AE (vacant episode) | ✗ | ||||||
| Placebo | AE (vomiting) | ✗ |
Chapter 4 Results of economic evaluation
Analysis of resource use and costs
Table 17 provides a summary of the resource-use values for each arm of the trial; results are presented separately for the magnesium and placebo groups. There were no statistically significant differences between the trial arms in any category of resource use with the exception of number of children who had contact with community health-care services and number of children who had a full blood count analysis.
Adverse event costs represented the least costly resource category in both trial arms (£0.35 and £0.73 for the magnesium and placebo groups, respectively; Table 18), whereas initial hospital admissions represented the most costly resource category (£765.20 and £748.93 for the magnesium and placebo groups, respectively; Table 19). Statistical analysis revealed that, at the 5% level, there were no significant differences between the two trial groups in any cost category with the exception of the cost of the experimental intervention. Table 19 shows the costs of non-NHS resource use for both the magnesium and placebo groups.
Mean total health service costs including magnesium during the period between randomisation and discharge from the ED or CAU, or the hospital where the child was admitted to an inpatient ward immediately following attendance, was £908 in the magnesium group, compared with £863 in the placebo group, generating a mean cost difference of £45 that was not statistically significant (p = 0.63) (see Table 20). When multiple imputation was used to impute all missing data over this time horizon, mean total health service costs were £897 in the magnesium group, compared with £882 in the placebo group, generating a mean cost difference of £15 that was not statistically significant (p = 0.87) (see Table 22). When the time horizon of the economic evaluation extended to 1 month post randomisation, mean total health and social services costs were £1056 in the magnesium group, compared with £1126 in the placebo group, generating a mean cost difference of £70 (complete case analysis) (see Table 32). When multiple imputation was used to impute all missing data over the 1-month time horizon, mean total health and social service costs were £1009 in the magnesium group, compared with £1014 in the placebo group, generating a mean cost difference of £5 (see Table 34).
| Resource | Magnesium: n (%) | Placebo: n (%) | p-valuea | ||||
|---|---|---|---|---|---|---|---|
| NHS and social care resources from randomisation to discharge [resource use based on complete case data (n = 252 for magnesium and n = 256 for placebo)] | |||||||
| Initial hospital inpatient admissions | 232 (92) | 245 (96) | 0.097 | ||||
| Chest radiography | 72 (29) | 83 (33) | 0.386 | ||||
| Lung function | 2 (1) | 4 (2) | 0.686 | ||||
| Electrolytes | 33 (13) | 48 (19) | 0.090 | ||||
| Blood culture | 13 (5) | 21 (8) | 0.214 | ||||
| Full blood count | 30 (12) | 49 (19) | 0.028 | ||||
| NHS and social care resources from discharge to 4 weeks [resource use based on complete case data (n = 118 for magnesium and n = 112 for placebo)] | |||||||
| Hospital re-admissions (asthma) | 8 (7) | 8 (7) | 1.000 | ||||
| Outpatient visits | 20 (17) | 28 (25) | 0.146 | ||||
| Community health service contacts | 42 (36) | 56 (50) | 0.033 | ||||
| Medications prescribed | 51 (43) | 51 (46) | 0.791 | ||||
| Inhalers prescribed | 111 (94) | 107 (96) | 0.769 | ||||
| Magnesium | Placebo | Difference | |||||
| Mean | SEa | Mean | SE | Mean | SE | p-valueb | |
| Days off school (days off school based on complete case data (n = 89 for magnesium and n = 80 for placebo) | |||||||
| Full days off school | 2.28 | 0.303 | 2.35 | 0.389 | – 0.69 | 0.488 | 0.889 |
| Half days off school | 0.73 | 0.237 | 0.68 | 0.186 | 0.055 | 0.301 | 0.855 |
| Total days off school | 2.65 | 0.314 | 2.69 | 3.380 | – 0.414 | – 0.492 | 0.933 |
| Resource | Magnesium | Placebo | Difference | ||||
|---|---|---|---|---|---|---|---|
| Mean | SEa | Mean | SE | Mean | SE | p-valuea | |
| NHS and social care costs: from randomisation to discharge [costs (£) based on complete case data (n = 252 for magnesium and n = 256 for placebo)] | |||||||
| (Initial) hospital admissions | 765.20 | 68.40 | 748.93 | 57.60 | 16.26 | 89.42 | 0.856 |
| ED/CUA attendances only | 128.30 | 0.58 | 129.53 | 0.43 | – 1.23 | 0.72 | 0.880 |
| Intervention costs | 1.79 | 0.15 | 1.42 | 0.15 | 0.36 | 0.22 | 0.000 |
| AEs costs | 0.35 | 0.15 | 0.73 | 0.25 | – 0.38 | 0.29 | 0.191 |
| Total cost of care up to discharge | 896.53 | 68.61 | 881.50 | 57.70 | 15.02 | 89.65 | 0.867 |
| NHS and social care costs: from discharge up to 4 weeks post randomisation [costs (£) based on complete case data (n = 118 for magnesium and n = 112 for placebo)] | |||||||
| Hospital re-admissions costs | 71.73 | 28.45 | 52.57 | 20.80 | 19.16 | 35.24 | 0.587 |
| Outpatient attendances costs | 23.22 | 4.98 | 39.98 | 7.56 | – 16.76 | 9.06 | 0.066 |
| Community health service costs | 14.95 | 2.35 | 19.23 | 2.25 | – 4.28 | 3.25 | 0.189 |
| Medications prescribed | 6.32 | 1.45 | 6.48 | 1.18 | – 0.16 | 1.87 | 0.932 |
| Inhalers prescribed | 22.03 | 1.90 | 22.56 | 1.90 | – 0.53 | 2.68 | 0.843 |
| Total cost of care up to discharge and including 1-month data | 1064.96 | 100.15 | 1118.65 | 110.14 | – 53.68 | 148.87 | 0.719 |
| Total non-NHS costs | 91.57 | 13.12 | 83.52 | 16.36 | 8.04 | 20.97 | 0.702 |
| Total societal costs | 1156.53 | 103.90 | 1202.17 | 115.92 | – 45.63 | 155.67 | 0.770 |
| Resource | Magnesium | Placebo | Difference | ||||
|---|---|---|---|---|---|---|---|
| Mean | SEa | Mean | SE | Mean | SE | p-valuea | |
| Non-NHS costs up to 4 weeks post randomisation [costs (£) based on complete case data (n = 118 for magnesium and n = 112 for placebo)] | |||||||
| Initial hospital visit: travel costs (parents) | 16.89 | 4.07 | 12.07 | 1.30 | 4.83 | 4.27 | 0.261 |
| Initial hospital visit: travel costs (others) | 8.80 | 1.30 | 12.19 | 2.18 | – 3.39 | 2.53 | 0.182 |
| Initial hospital visit: expenses (e.g. lost pay, child care, snacks) | 48.43 | 8.42 | 47.29 | 12.81 | 1.14 | 15.33 | 0.941 |
| Additional costs after discharge from hospital (e.g. travel, lost pay, child care) | 16.30 | 5.43 | 9.35 | 3.30 | 6.94 | 6.36 | 0.276 |
| Additional cost of over-the-counter medicines after discharge from hospital | 1.14 | 0.32 | 2.61 | 0.87 | – 1.47 | 0.932 | 0.116 |
Results of the cost-effectiveness analysis
Complete case analysis
The CEA evaluated the cost-effectiveness of magnesium in terms of natural units, calculating the incremental cost per unit decrement in ASS after 60 minutes of treatment. The time horizon for the CEA covered the period between randomisation and discharge from the ED or CAU, or the hospital where the child was admitted to an inpatient ward immediately following attendance. The incremental cost-effectiveness of magnesium is shown in Table 20 for the 472 children (228 receiving magnesium and 244 receiving placebo) for whom we had complete cost and outcomes data. Within the base-case analysis, the average cost was £908 in the magnesium group, compared with £863 in the placebo group, generating a mean cost difference of £45. The costs presented in Table 20 differ from those presented in Table 22, as the latter represents a multiple imputation analysis including all 508 trial participants. There was no statistically significant difference in costs between the two trial groups, with 36.6% of bootstrap replicates finding magnesium to be less costly than placebo.
In the base-case analysis, the incremental cost-effectiveness of magnesium was estimated at £189 per unit decrement in ASS. However, there was substantial stochastic uncertainty around this finding. The variability around the base-case estimates of cost-effectiveness is shown in Figure 6. Although the majority (54.3%) of the bootstrapped replications of the ICER fall in the north-east quadrant of the cost-effectiveness plane, some bootstrapped replications fall in the other three quadrants of the cost-effectiveness plane. As a result, a meaningful ordering of the bootstrapped replications required to make the CI surrounding the ICER interpretable is very difficult. Under these circumstances, CEACs provide an appropriate approach to representing the uncertainty surrounding the ICER. The CEAC curve for the primary clinical outcome measure is displayed in Figure 7. The CEAC shown in Figure 7 indicates that the higher the value decision-makers place on an additional unit decrement in ASS after 60 minutes of treatment, the higher the probability that magnesium will be cost-effective. At the notional cost-effectiveness threshold (or ceiling ratio) of £1000 per unit decrement in ASS, the probability that use of magnesium is cost-effective is 75.1%. Although no previous research has shown how much society or the NHS may or should be willing to pay to reduce the ASS, the economic burden of impairment in children with severe asthma is likely to be significant. 60 If decision-makers are willing to pay £5000 per unit decrement in ASS, the probability that use of magnesium is cost-effective increases to 85.5%.
Mean net benefits were estimated for alternative cost-effectiveness thresholds per unit decrement in ASS (Table 21). Assuming that the cost-effectiveness threshold equals £1000 per unit decrement in ASS generates a mean net benefit to the health service attributable to magnesium of £170 (i.e. on average, there is a net gain to the health service in monetary terms). This is analogous to stating that if the actual health benefit of magnesium, in terms of the reduction in ASS, is multiplied by an assumed willingness to pay of £1000 per unit decrement in ASS, and the net cost is subtracted, then the benefit to the NHS of adopting magnesium is, on average, positive in monetary terms. Note, however, that the 95% CI surrounding the mean net benefit (– 362 to 678) includes negative values, i.e. there is a possibility of a net monetary loss associated with adopting magnesium (see Table 20). If the cost-effectiveness threshold is increased as high as £5000 per unit decrement in ASS, the mean net benefit increases to £1066 (95% CI – £945 to £3058).
Sensitivity analyses were conducted to determine the impact of changing particular parameter values or assumptions on the size of the ICER (see Tables 20 and 21; see Figure 7). Assuming that higher level inpatient care was valued per diem, using the NHS reference cost for paediatric high-dependency care reduced the mean cost difference between the trial arms to £18 and increased the probability that magnesium is cost-effective to 81.5% at a £1000 cost-effectiveness threshold (mean ICER £78; north-east quadrant of cost-effectiveness plane). In contrast, assuming that higher-level inpatient care was valued per diem, using the NHS reference cost for paediatric intensive care increased the mean cost difference between the trial arms to £77, and reduced the probability that magnesium is cost-effective to 68.3% at a £1000 cost-effectiveness threshold (mean ICER £327; north-east quadrant). Assuming that part of a day spent by a child in an inpatient ward equated to a proportional period for costing purposes and that, consequently, the vacated inpatient bed would be filled immediately reduced the mean cost difference between the trial arms to £30, and increased the probability that magnesium is cost-effective to 81.0% at a £1000 cost-effectiveness threshold (mean ICER £126; north-east quadrant). Assuming that part of a day spent by a child in an inpatient ward equated to a full 24-hour period for costing purposes and that, consequently, the inpatient bed would not be filled until the end of that 24-hour period, and varying the average cost of an ED attendance and general medical ward admission, each had less impact on the cost-effectiveness results. CEACs generated following each sensitivity analysis are shown in Figure 7. Estimates of net monetary benefits for notional cost-effectiveness thresholds per unit decrement in ASS are shown in Table 21 for each sensitivity analysis. For example, assuming that the cost-effectiveness threshold equals £1000 per unit decrement in ASS and that higher-level inpatient care was valued per diem, using the NHS reference cost for paediatric high-dependency care generates a mean net benefit to the health service attributable to magnesium of £225 (i.e. on average, there is a net gain to the health service in monetary terms).
| Analysisa | Mean costs (95% CI) | Mean effects (95% CI) | ICER (£) | Probability that magnesium is | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Magnesium (£) | Placebo (£) | Difference (£) | Magnesium (£) | Placebo (£) | Difference (£) | More effective (%)b | Less costly (%)b | Cost-effective (%)b,c | Cost-effective (%)b,d | ||
| Base case | 908 (764 and 1052) | 863 (752 and 975) | 45 (– 138 and 227) | 4.72 (4.54 and 4.90) | 4.95 (4.78 and 5.13) | 0.24e (– 0.02 and 0.49) | 189 | 88.00 | 36.60 | 75.10 | 85.50 |
| Higher level inpatient care valued using NHS cost for paediatric high-dependency care (£886) | 813 (708 and 918) | 794 (718 and 871) | 18 (– 111 and 148) | 4.72 (4.54 and 4.90) | 4.95 (4.78 and 5.13) | 0.24 (– 0.02 and 0.49) | 78 | 89.50 | 41.90 | 81.50 | 87.80 |
| Higher level inpatient care valued using NHS cost for paediatric intensive care (£2225) | 1027 (826 and 1227) | 950 (790 and 1109) | 77 (– 179 and 333) | 4.72 (4.54 and 4.90) | 4.95 (4.78 and 5.13) | 0.24 (– 0.02 and 0.49) | 327 | 89.20 | 32.60 | 68.30 | 85.90 |
| Exact LOS used | 783 (649 and 917) | 753 (651 and 855) | 30 (– 139 and 198) | 4.72 (4.54 and 4.90) | 4.95 (4.78 and 5.13) | 0.24 (– 0.02 and 0.49) | 126 | 89.50 | 42.60 | 81.00 | 87.50 |
| LOS rounded up to full days | 1019 (867 and 1172) | 964 (844 and 1084) | 56 (– 139 and 250) | 4.72 (4.54 and 4.90) | 4.95 (4.78 and 5.13) | 0.24 (– 0.02 and 0.49) | 233 | 88.90 | 35.00 | 74.70 | 86.70 |
| NHS reference costs used to value A&E department visit | 876 (732 and 1020) | 831 (719 and 942) | 45 (– 136 and 227) | 4.72 (4.54 and 4.90) | 4.95 (4.78 and 5.13) | 0.24 (– 0.02 and 0.49) | 193 | 87.70 | 36.70 | 76.50 | 85.40 |
| NHS reference costs used to value stay on GM ward | 943 (797 and 1090) | 900 (787 and 1013) | 43 (– 142 and 228) | 4.72 (4.54 and 4.90) | 4.95 (4.78 and 5.13) | 0.24 (– 0.02 and 0.49) | 184 | 90.30 | 38.50 | 78.10 | 88.70 |
| Analysisa | Mean monetary net benefit (95% CI) | ||||||
|---|---|---|---|---|---|---|---|
| Value of threshold (£) | Base case (£) | Higher-level inpatient care valued using NHS cost for paediatric high-dependency care (£886) | Higher-level inpatient care valued using NHS cost for paediatric intensive care (£2225) | Exact LOS used (£) | LOS rounded up to full days (£) | NHS reference costs used to value A&E department visit (£) | NHS reference costs used to value stay on GM ward (£) |
| £0 | – 54 (– 347 and 214) | – 21 (– 247 to 184) | – 87 (– 493 and 303) | – 26 (– 280 and 233) | – 59 (– 353 and 229) | – 49 (– 346 and 217) | – 49 (– 332 and 217) |
| £500 | 58 (– 321 and 412) | 102 (– 223 and 391) | 31 (– 398 and 514) | 93 (– 254 and 427) | 55 (– 300 and 416) | 65 (– 307 and 421) | 76 (– 285 and 416) |
| £1000 | 170 (– 362 and 678) | 225 (– 260 and 686) | 149 (– 391 and 779) | 211 (– 298 and 688) | 169 (– 312 and 690) | 179 (– 358 and 687) | 201 (– 295 and 693) |
| £1500 | 282 (– 410 and 977) | 349 (– 320 and 988) | 267 (– 406 and 1073) | 329 (– 360 and 992) | 282 (– 363 and 1022) | 293 (– 403 and 957) | 326 (– 326 and 988) |
| £2000 | 394 (– 499 and 1263) | 472 (– 383 and 1284) | 385 (– 464 and 1344) | 447 (– 415 and 1316) | 396 (– 428 and 1332) | 407 (– 470 and 1228) | 450 (– 371 and 1302) |
| £2500 | 506 (– 570 and 1554) | 595 (– 451 and 1596) | 503 (– 493 and 1635) | 565 (– 470 and 1617) | 510 (– 524 and 1629) | 521 (– 525 and 1512) | 575 (– 418 and 1637) |
| £3000 | 618 (– 648 and 1845) | 718 (– 516 and 1922) | 621 (– 571 and 1928) | 684 (– 560 and 1922) | 624 (595 and 1962) | 635 (– 588 and 1813) | 700 (– 468 and 1956) |
| £3500 | 730 (– 708 and 2139) | 842 (– 584 and 2232) | 739 (– 641 and 2250) | 802 (– 650 and 2228) | 737 (– 675 and 2302) | 749 (– 655 and 2100) | 825 (– 524 and 2273) |
| £4000 | 842 (– 807 and 2438) | 965 (– 629 and 2546) | 857 (– 709 and 2541) | 920 (– 731 and 2558) | 851 (– 754 and 2634) | 863 (– 721 and 2402) | 950 (– 579 and 2588) |
| £4500 | 954 (– 874 and 2734) | 1088 (– 680 and 2867) | 975 (– 784 and 2889) | 1038 (– 811 and 2888) | 965 (– 830 and 2949) | 977 (– 796 and 2726) | 1075 (– 634 and 2904) |
| £5000 | 1066 (– 945 and 3058) | 1212 (– 767 and 3174) | 1092 (– 864 and 3184) | 1156 (– 98 and 3198) | 1079 (– 908 and 3273) | 1091 (– 868 and 2999) | 1199 (– 689 and 3228) |
FIGURE 6.
Cost-effectiveness plane for CEA base-case analysis: complete case analyses.
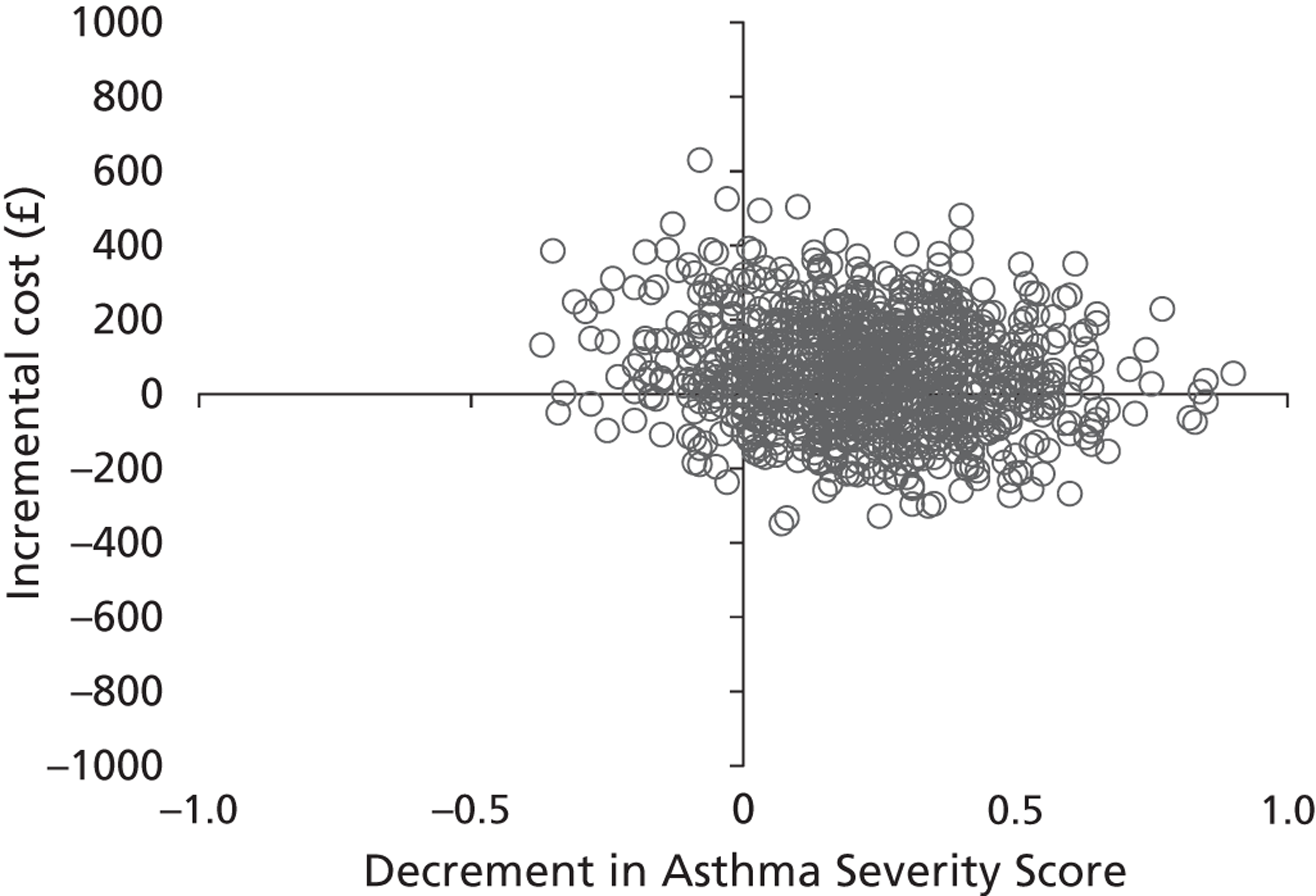
FIGURE 7.
Cost-effectiveness acceptability curves for CEA base-case analyses and sensitivity analyses: complete case analyses. a, Each CEAC shows the probability that magnesium is cost-effective with changes in the amount that society is willing to pay for a unit reduction in the asthma ASS. A&E, a lower NHS reference cost applied to A&E department attendances; GM, a higher per diem cost applied to general paediatric ward care; HDU, a lower per diem cost applied to higher-level care; LOS exact, part of a day spent by a child in an inpatient ward equated to a proportional period for costing purposes; LOS full, part of a day spent by a child in an inpatient ward equated to a full 24-hour period for costing purposes; PICU, a higher per diem cost applied to higher-level care.
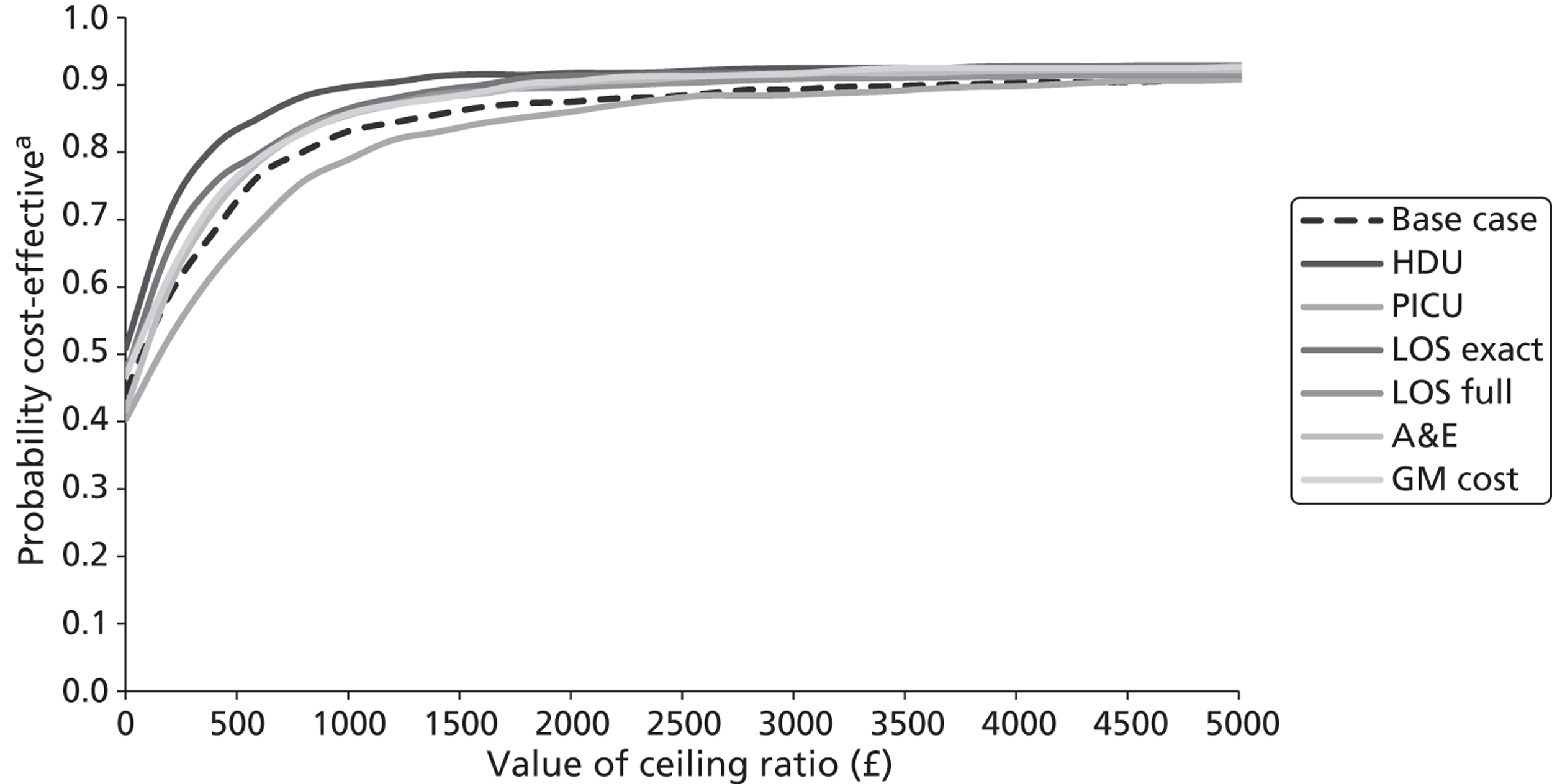
Analyses following multiple imputation
The CEA, expressed in terms of incremental cost per unit decrement in ASS after 60 minutes of treatment, was repeated for all 508 trial participants (252 receiving magnesium and 256 receiving placebo) following multiple imputation of missing cost and outcomes data. As with the complete case analysis, the time horizon for this analysis covered the period between randomisation and discharge from the ED, or the hospital where the child was admitted to an inpatient ward immediately following attendance. The incremental cost-effectiveness of magnesium is shown in Table 22. Within the base-case analysis, the average cost was £897 in the magnesium group compared with £882 in the placebo group, generating a mean cost difference of £15. There was no statistically significant difference in costs between the two trial groups, with 44.9% of bootstrap replicates finding magnesium to be less costly than placebo.
In the base-case analysis, the incremental cost-effectiveness of magnesium was estimated at £52 per unit decrement in ASS (north-east quadrant of cost-effectiveness plane). However, as in the complete case analysis, substantial stochastic uncertainty surrounded this finding. This is displayed in the cost-effectiveness plane in Figure 8. The CEAC shown in Figure 9 indicates that at the notional cost-effectiveness threshold of £1000 per unit decrement in ASS, the probability that use of magnesium is cost-effective is 83.1%. If decision-makers are willing to pay £5000 per unit decrement in ASS, the probability that use of magnesium is cost-effective increases to 90.8%. Mean net benefits were also estimated for alternative cost-effectiveness thresholds per unit decrement in ASS following the multiple imputation procedures (Table 23). Assuming that the cost-effectiveness threshold equals £1000 per unit decrement in ASS generates a mean net benefit to the health service attributable to magnesium of £266 (95% CI – £275 to £805). If the cost-effectiveness threshold is increased as high as £5000 per unit decrement in ASS, the mean net benefit increases to £1420 (95% CI – £523 to £3440).
Finally, sensitivity analyses were conducted to determine the impact of changing particular parameter values or assumptions on the ICER (see Tables 22 and 23; see Figure 9). Assuming that higher level inpatient care was valued per diem, using the NHS reference cost for paediatric high-dependency care reduced the mean cost difference between the trial arms to £1, and increased the probability that magnesium is cost-effective to 89.7% at a £1000 cost-effectiveness threshold (mean ICER – £2; south-east quadrant of cost-effectiveness plane). In contrast, assuming that higher-level inpatient care was valued per diem, using the NHS reference cost for paediatric intensive care increased the mean cost difference between the trial arms to £35 and reduced the probability that magnesium is cost-effective to 78.9% at a £1000 cost-effectiveness threshold (mean ICER £119; north-east quadrant of cost-effectiveness plane). Assuming that part of a day spent by a child in an inpatient ward equated to a proportional period for costing purposes and that, consequently, the vacated inpatient bed would be filled immediately reduced the mean cost difference between the trial arms to £4, and increased the probability that magnesium is cost-effective to 86.5% at a £1000 cost-effectiveness threshold (mean ICER £14; north-east quadrant of cost-effectiveness plane). Assuming that part of a day spent by a child in an inpatient ward equated to a full 24-hour period for costing purposes and that, consequently, the inpatient bed would not be filled until the end of that 24-hour period, and varying the average cost of an ED attendance and general medical ward admission, had less impact on the cost-effectiveness results. CEACs generated following each sensitivity analysis are shown in Figure 9. Estimates of net monetary benefits for notional cost-effectiveness thresholds per unit decrement in ASS are shown in Table 23 for each sensitivity analysis.
| Analysisa | Mean costs (95% CI) | Mean effects (95% CI) | ICER (£) | Probability active treatment is | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Magnesium (£) | Placebo (£) | Difference (£) | Magnesium (£) | Placebo (£) | Difference (£) | More effective (%)b | Less costly (%)b | Cost-effective (%)b,c | Cost-effective (%)b,d | ||
| Base case | 897 (762 to 1031) | 882 (768 to 995) | 15 (– 161 to 191) | 4.66 (4.49 to 4.83) | 4.95 (4.78 to 5.12) | 0.29e (0.05 to 0.53) | 52 | 92.30 | 44.90 | 83.10 | 90.80 |
| Higher level inpatient care valued using NHS cost for paediatric high-dependency care (£886) | 802 (704 to 900) | 802 (726 to 879) | – 1 (– 125 to 123) | 4.66 (4.49 to 4.83) | 4.95 (4.78 to 5.12) | 0.29 (0.05 to 0.53) | – 2 | 93.00 | 51.90 | 89.70 | 92.50 |
| Higher level inpatient care valued using NHS cost for paediatric intensive care (£2225) | 1015 (827 to 1202) | 980 (816 to 1144) | 35 (– 214 to 283) | 4.66 (4.49 to 4.83) | 4.95 (4.78 to 5.12) | 0.29 (0.05 to 0.53) | 119 | 92.40 | 40.90 | 78.90 | 90.80 |
| Exact LOS used | 773 (649 to 898) | 769 (666 to 873) | 4 (– 158 to 166) | 4.66 (4.49 to 4.83) | 4.95 (4.78 to 5.12) | 0.29 (0.05 to 0.53) | 14 | 93.30 | 47.70 | 86.50 | 92.90 |
| LOS rounded up to full days | 1005 (861 to 1148) | 985 (862 to 1108) | 20 (– 169 to 208) | 4.66 (4.49 to 4.83) | 4.95 (4.78 to 5.12) | 0.29 (0.05 to 0.53) | 68 | 92.90 | 48.30 | 85.50 | 91.40 |
| NHS reference costs used to value A&E department visit | 865 (730 to 999) | 849 (736 to 962) | 16 (– 159 to 192) | 4.66 (4.49 to 4.83) | 4.95 (4.78 to 5.12) | 0.29 (0.05 to 0.53) | 55 | 93.80 | 42.00 | 85.50 | 92.20 |
| NHS reference costs used to value stay on GM ward | 931 (794 to 1068) | 918 (804 to 1032) | 14 (– 165 to 192) | 4.66 (4.49 to 4.83) | 4.95 (4.78 to 5.12) | 0.29 (0.05 to 0.53) | 47 | 92.90 | 47.50 | 85.70 | 92.70 |
| Analysisa | Mean monetary net benefit (95% CI) | ||||||
|---|---|---|---|---|---|---|---|
| Value of threshold (£) | Base case (£) | Higher-level inpatient care valued using NHS cost for paediatric high-dependency care (£886) | Higher level inpatient care valued using NHS cost for paediatric intensive care (£2225) | Exact LOS used (£) | LOS rounded up to full days (£) | NHS reference costs used to value A&E department visit (£) | NHS reference costs used to value stay on GM ward (£) |
| 0 | – 23 (– 310 and 249) | – 1 (– 200 to 191) | – 42 (– 441 to 366) | – 3 (– 284 to 260) | – 12 (– 318 to 285) | – 26 (– 312 to 251) | – 13 (– 307 to 261) |
| 500 | 121 (– 266 to 493) | 142 (– 162 to 413) | 97 (– 379 to 562) | 142 (– 187 to 491) | 134 (– 230 to 510) | 120 (– 250 to 473) | 133 (– 237 to 492) |
| 1000 | 266 (– 275 to 805) | 284 (– 189 to 693) | 235 (– 364 to 842) | 288 (– 184 to 794) | 279 (– 224 to 799) | 266 (– 266 to 763) | 279 (– 237 to 799) |
| 1500 | 410 (– 304 to 1135) | 426 (– 211 to 993) | 374 (– 350 to 1128) | 434 (190 to 1112) | 425 (– 229 to 1014) | 412 (– 280 to 1066) | 426 (– 274 to 1135) |
| 2000 | 554 (– 338 to 1473) | 569 (– 255 to 1315) | 512 (– 387 to 1452) | 579 (– 223 to 1435) | 570 (– 259 to 1413) | 557 (– 303 to 1393) | 572 (– 311 to 1459) |
| 2500 | 699 (– 362 to 1797) | 711 (– 285 to 1635) | 651 (– 444 to 1778) | 725 (– 254 to 1775) | 715 (– 299 to 1722) | 703 (– 348 to 1719) | 718 (– 343 to 1793) |
| 3000 | 843 (– 400 to 2125) | 853 (– 311 to 1957) | 790 (– 485 to 2075) | 871 (– 287 to 2120) | 861 (– 325 to 2030) | 849 (– 397 to 2039) | 864 (– 385 to 2125) |
| 3500 | 987 (– 422 to 2463) | 996 (– 354 to 2278) | 928 (– 554 to 2393) | 1016 (– 315 to 2452) | 1006 (– 376 to 2357) | 995 (– 442 to 2382) | 1011 (– 422 to 2457) |
| 4000 | 1132 (– 465 to 2774) | 1138 (– 386 to 2597) | 1067 (– 619 to 2731) | 1162 (– 325 to 2764) | 1152 (– 422 to 2678) | 1141 (– 478 to 2725) | 1157 (– 460 to 2789) |
| 4500 | 1276 (– 494 to 3095) | 1280 (– 413 to 2903) | 1206 (– 678 to 3070) | 1308 (– 373 to 3087) | 1297 (– 468 to 2999) | 1287 (– 529 to 3057) | 1303 (– 496 to 3142) |
| 5000 | 1420 (– 523 to 3440) | 1423 (– 440 to 3229) | 1344 (– 736 to 3384) | 1453 (– 395 to 3416) | 1442 (– 514 to 3315) | 1433 (– 583 to 3390) | 1449 (– 530 to 3501) |
FIGURE 8.
Cost-effectiveness plane for CEA base-case analysis: analyses following multiple imputation.
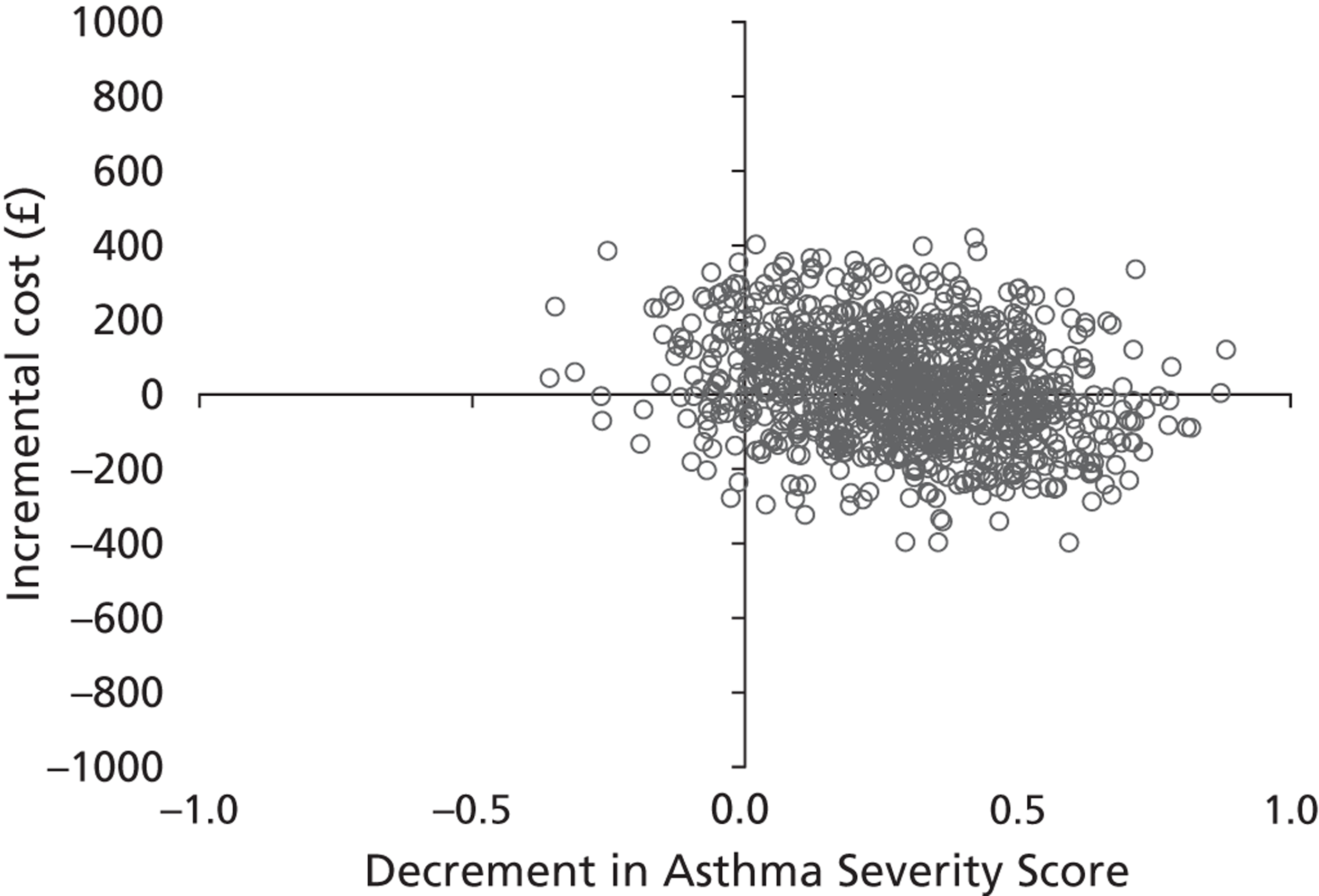
FIGURE 9.
Cost-effectiveness acceptability curves for CEA base-case analyses and sensitivity analyses: analyses following multiple imputation. a, Each CEAC shows the probability that magnesium is cost-effective with changes in the amount that society is willing to pay for a unit reduction in the asthma ASS. A&E, a lower NHS reference cost applied to A&E department attendances; GM, a higher per diem cost applied to general paediatric ward care; HDU, a lower per diem cost applied to higher level care; LOS exact, part of a day spent by a child in an inpatient ward equated to a proportional period for costing purposes; LOS full, part of a day spent by a child in an inpatient ward equated to a full 24-hour period for costing purposes; PICU, a higher per diem cost applied to higher level care.
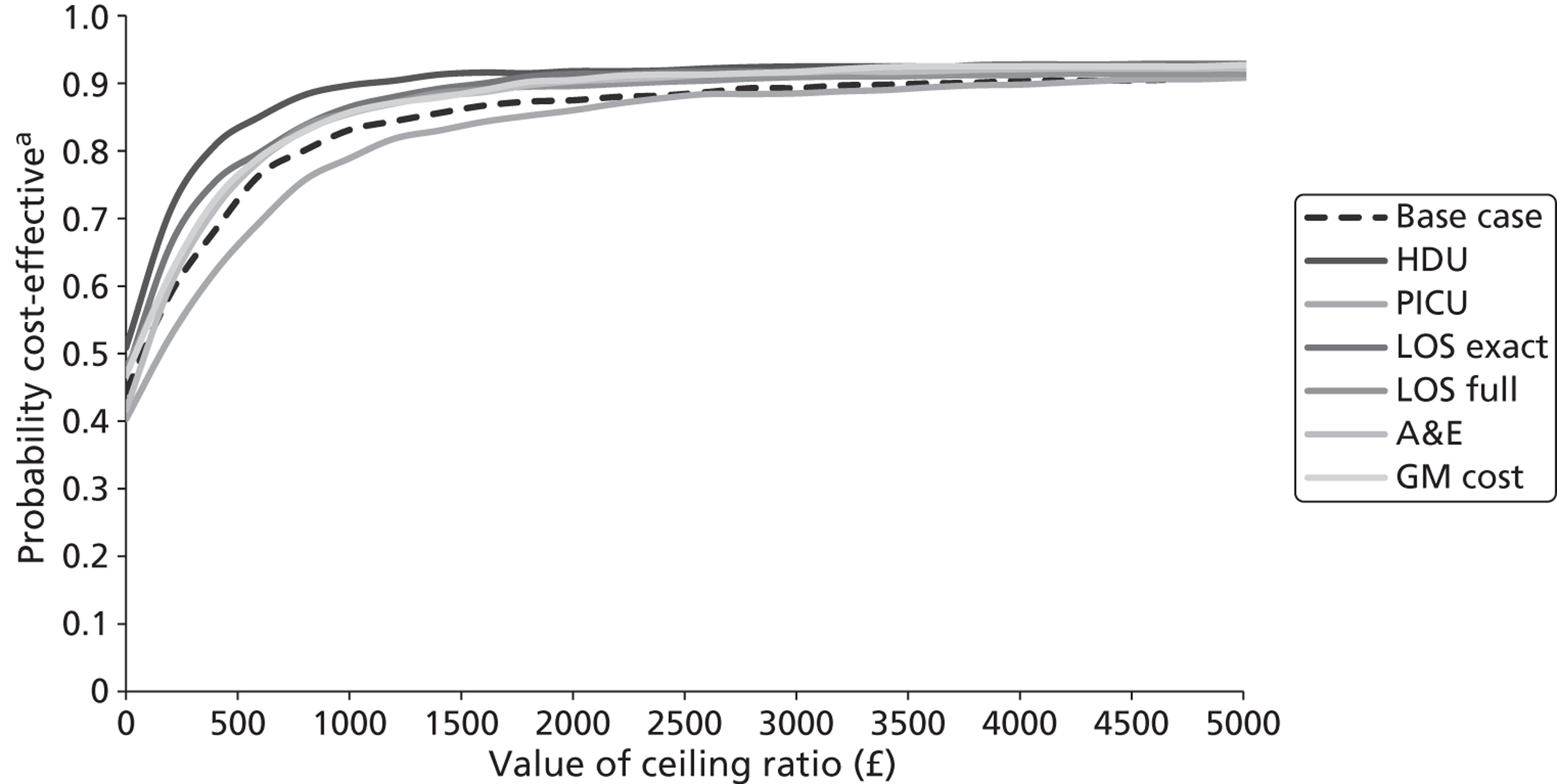
Analysis of health-related quality of life and utility measures
Parents were asked to describe the QoL of their children at 1 month using the PedsQL™ Asthma Scales. In addition, children aged ≥ 5 years were asked to describe their own health-related QoL at 1 month with the help of a parent or guardian using the PedsQL™ Asthma Scales. The PedsQL™ was designed to provide a modular approach to measuring QoL in healthy children and adolescents, as well as those with acute and chronic health conditions, across the broadest empirically feasible age groups. Of particular relevance is that, unlike other widely used non-preference-based measures of health-related QoL designed for childhood, such as the KIDSCREEN and Child Health Questionnaire, the PedsQL™ has been validated for use in children of < 5 years. 61 The PedsQL™ Asthma Scales comprise parallel child self-report [ages 5–7 years (young child), 8–12 years (child) and 13–18 years (adolescent)] and parent proxy-report [ages 2–4 years (toddler), 5–7 years (young child), 8–12 years (child) and 13–18 years (adolescent)] formats. The items for each of the age-specific modules and self-report or proxy-report formats are essentially identical, differing only in terms of developmentally appropriate language, or first or third person tense. The PedsQL™ Asthma Scales contain 28 items covering asthma symptoms (11 items), treatment problems (11 items), worry (three items) and communication (three items). A five-point response scale is utilised across each item (0 = never a problem; 1 = almost never a problem; 2 = sometimes a problem; 3 = often a problem; 4 = almost always a problem) (for self-reports by young children a three-point response scale is utilised). Items are reverse scored and linearly transformed to a 0–100 scale (0 = 100, 1 = 75, 2 = 50, 3 = 25, 4 = 0) with higher scores indicating improved QoL. For subscale and total scores, the mean is computed as the sum across all items divided by the number of items answered, thereby accounting for missing data.
Of the 508 1-month postal questionnaires sent to parents, 230 (45%) questionnaires were returned (118 from the magnesium group and 112 from the placebo group). In both groups, the majority (> 70%) of the questionnaires were returned to the research team within 60 days. The 1-month postal questionnaire was carefully designed to ensure that parents were fully aware of the time period under consideration for each question in the questionnaire.
A total of 228 parents completed the PedsQL™ Asthma Scales as part of the 1-month postal questionnaire; 116 in the magnesium arm of the trial and 112 in the placebo arm of the trial. There were no significant differences in baseline clinical and sociodemographic characteristics between the trial groups for whom parent-reported PedsQL™ Asthma Scales data were provided. The mean score on the asthma symptoms, treatment problems, worry and communication subscales was 63.90, 83.57, 73.19 and 77.33, respectively, in the magnesium arm, and 59.55, 80.35, 75.04 and 75.00, respectively, in the placebo arm (Table 24). The mean (SE) total parent-reported PedsQL™ asthma score was 73.92 (1.56) in the magnesium arm and 70.24 (1.63) in the placebo arm (p = 0.104). The distributions of parent-reported PedsQL™ asthma subscale and total scores across quartiles of the relevant scales are shown in Table 25. A total of 52 (45%) children in the magnesium arm had a total parent-reported PedsQL™ asthma score of ≥ 76 compared with 38 (34%) in the placebo arm.
| Subscale | No. of items | Magnesium (n = 116) | Placebo (n = 112) | p-valueb | ||
|---|---|---|---|---|---|---|
| n | Mean (SE) | n | Mean (SE) | |||
| Asthma symptoms | 11 | 114 | 63.90 (1.98) | 109 | 59.55 (1.96) | 0.1202 |
| Treatment problems | 11 | 116 | 83.57 (1.55) | 109 | 80.35 (1.64) | 0.1566 |
| Worry | 3 | 115 | 73.19 (2.83) | 109 | 75.04 (2.72) | 0.5763 |
| Communication | 3 | 111 | 77.33 (2.59) | 106 | 75.00 (2.72) | 0.5322 |
| Total scale score | 28 | 109 | 73.92 (1.56) | 103 | 70.24 (1.63) | 0.1042 |
| PedsQL™ subscale/total scale scores | Score (n, %) | |||||||
|---|---|---|---|---|---|---|---|---|
| Magnesium (n = 116) | Placebo (n = 112) | |||||||
| 0 to < 26 | 26 to < 51 | 51 to < 76 | 76 to 100 | 0 to < 26 | 26 to < 51 | 51 to < 76 | 76 to 100 | |
| Asthma symptoms | 5 (4) | 27 (23) | 42 (36) | 40 (34) | 6 (5) | 32 (29) | 43 (38) | 28 (25) |
| Treatment problems | 0 (0) | 6 (5) | 24 (21) | 86 (74) | 2 (2) | 5 (4) | 33 (29) | 69 (62) |
| Worry | 13 (11) | 19 (16) | 19 (16) | 64 (55) | 9 (8) | 16 (14) | 25 (22) | 59 (53) |
| Communication | 8 (7) | 18 (16) | 22 (19) | 63 (54) | 10 (9) | 18 (16) | 23 (21) | 55 (49) |
| Total scale score | 0 (0) | 10 (9) | 47 (41) | 52 (45) | 2 (2) | 9 (8) | 54 (48) | 38 (34) |
A total of 93 children aged ≥ 5 years separately completed the PedsQL™ Asthma Scales as part of the 1-month postal questionnaire; 47 in the magnesium arm of the trial and 46 in the placebo arm of the trial. There were no significant differences in baseline clinical and sociodemographic characteristics between the trial groups for whom child-reported PedsQL™ Asthma Scales data were provided. The mean score on the asthma symptoms, treatment problems, worry and communication subscales was 53.69, 74.67, 67.57 and 67.02, respectively, in the magnesium arm, and 53.44, 75.62, 68.60 and 57.75, respectively, in the placebo arm (Table 26). The mean (SE) total child-reported PedsQL™ asthma score was 65.48 (2.68) in the magnesium arm and 64.02 (2.67) in the placebo arm (p = 0.701). The distributions of child-reported PedsQL™ asthma subscale and total scores across quartiles of the relevant scales are shown in Table 27. A total of 14 (30%) children in the magnesium arm had a total child-reported PedsQL™ asthma score of ≥ 76 compared with 11 (24%) in the placebo arm.
| Subscale | No. of items | Magnesium (n = 47) | Placebo (n = 46) | p-valueb | ||
|---|---|---|---|---|---|---|
| n | Mean (SE) | n | Mean (SE) | |||
| Asthma symptoms | 11 | 47 | 56.39 (3.52) | 45 | 53.44 (3.04) | 0.5273 |
| Treatment problems | 11 | 47 | 74.67 (2.59) | 45 | 75.62 (2.65) | 0.7990 |
| Worry | 3 | 46 | 67.57 (4.02) | 43 | 68.60 (3.61) | 0.8493 |
| Communication | 3 | 47 | 67.02 (4.05) | 43 | 57.75 (5.03) | 0.1546 |
| Total scale score | 28 | 46 | 65.48 (2.68) | 43 | 64.02 (2.67) | 0.7013 |
| PedsQL™ subscale/total scale scores | Score (n/%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Magnesium (n = 47) | Placebo (n = 46) | |||||||
| 0 to < 26 | 26 to < 51 | 51 to < 76 | 76 to 100 | 0 to < 26 | 26 to < 51 | 51 to < 76 | 76 to 100 | |
| Asthma symptoms | 4 (9) | 18 (38) | 11 (23) | 14 (30) | 3 (7) | 17 (37) | 20 (43) | 5 (11) |
| Treatment problems | 0 (0) | 7 (15) | 15 (32) | 25 (53) | 0 (0) | 3 (7) | 19 (41) | 23 (50) |
| Worry | 4 (9) | 12 (26) | 14 (30) | 16 (34) | 2 (4) | 11 (24) | 13 (28) | 17 (37) |
| Communication | 4 (9) | 16 (34) | 9 (19) | 18 (38) | 10 (22) | 13 (28) | 5 (11) | 15 (33) |
| Total scale score | 0 (0) | 13 (28) | 19 (40) | 14 (30) | 1 (2) | 5 (11) | 26 (57) | 11 (24) |
Ordinary least squares regressions were conducted using the total child-reported PedsQL™ asthma score (model 1) and total parent-reported PedsQL™ asthma score (model 2) as the dependent variables (Table 28). Potential confounders replicated the covariates incorporated into the main clinical analyses. Robust Ses were estimated to account for potential heteroscedasticity in the distribution of residuals. Following controls for clinical and sociodemographic covariates, magnesium was associated with a 1.33 increase in the total child-reported PedsQL™ asthma score (p = 0.734) and a 4.84 increase in the total parent-reported PedsQL™ asthma score (p = 0.043). In model 2, no other clinical or sociodemographic covariate was a significant predictor of the total PedsQL™ asthma score. We do not consider there to be a clinically plausible reason why there may be a relationship between PedsQL™ and late night admission.
| Variable (unit) | Self-reported PedsQL™ (child completeda) | Proxy PedsQL™ (parent completedb) | ||||
|---|---|---|---|---|---|---|
| Fully adjusted β (robust SE) | p > |t| | (95% CI) | Fully adjusted β (robust SE) | p > |t| | (95% CI) | |
| Trial arm (referent = placebo) | ||||||
| Magnesium | 1.336 (3.911) | 0.734 | – 6.455 to 9.126 | 4.836 (2.372) | 0.043 | 0.156 to 9.515 |
| Age (years) | 0.598 (0.569) | 0.296 | – 0.534 to 1.731 | – 0.648 (0.414) | 0.119 | – 1.464 to 0.169 |
| Gender (referent = female) | ||||||
| Male | 9.200 (4.219) | 0.032 | 0.796 to 17.603 | 3.584 (2.408) | 0.138 | – 1.167 to 8.335 |
| Duration of most recent asthma attack (referent = last ≤ 6 hours) | ||||||
| For the last few days | – 10.738 (4.616) | 0.023 | – 19.933 to – 1.543 | – 0.539 (3.294) | 0.870 | – 7.038 to 5.959 |
| For the last 24 hours | – 11.611 (5.927) | 0.054 | – 23.417 to 0.196 | – 1.615 (3.956) | 0.684 | – 9.419 to 6.190 |
| SaO2 (value) | 0.472 (0.601) | 0.435 | – 0.726 to 1.670 | 0.290 (0.342) | 0.398 | – 0.385 to 0.964 |
| Assessment at baseline (severity score) | 2.188 (1.561) | 0.165 | – 0.922 to 5.299 | 1.619 (1.027) | 0.117 | – 0.407 to 3.645 |
| Respiratory rate | 0.206 (0.194) | 0.291 | – 0.180 to 0.591 | 0.006 (0.160) | 0.971 | – 0.311 to 0.322 |
| Oxygen therapy required (referent = no) | ||||||
| Yes | – 0.401 (3.910) | 0.919 | – 8.190 to 7.389 | 0.952 (2.498) | 0.704 | – 3.976 to 5.880 |
| Time of day randomisation occurred (referent = 0000–1700) | ||||||
| 1701–2200 | 4.112 (4.078) | 0.317 | – 4.013 to 12.236 | 2.659 (2.400) | 0.270 | – 2.078 to 7.395 |
| 2201–0859 | 14.612 (4.815) | 0.003 | 5.021 to 24.203 | 4.674 (3.646) | 0.201 | – 2.59 to 11.868 |
Parents of children aged ≥ 5 years were asked to describe the QoL of their children at 1 month using the proxy version of the EuroQol EQ-5D instrument. The EQ-5D is the generic, multiattribute, preference-based measure preferred by NICE for broader cost-effectiveness comparative purposes. 46 The parents were asked to complete only the EQ-5D descriptive system, which defines QoL in terms of five dimensions: ‘mobility’, ‘self-care’, ‘usual activities’, ‘pain/discomfort’ and ‘anxiety/depression’, and not the separate EQ-5D visual analogue scale. Responses in each dimension of the descriptive system are divided into three ordinal levels coded (1) no problems; (2) some or moderate problems; and (3) severe or extreme problems. For the purposes of this study, the York A1 tariff was applied to each set of responses to the descriptive system to generate an EQ-5D utility score at 1 month for each child. 52
A total of 89 parents of children aged ≥ 5 years completed the proxy version of the EuroQol EQ-5D as part of the 1-month postal questionnaire: 46 in the magnesium arm of the trial and 43 in the placebo arm of the trial. There were no significant differences in baseline clinical and sociodemographic characteristics between the trial groups for whom parent-reported EQ-5D data were provided. The mean (SE) EQ-5D utility score was 0.86 (0.04) in the magnesium arm and 0.88 (0.04) in the placebo arm (p = 0.710). Table 29 shows the distribution of functional levels across the five EQ-5D dimensions for the two trial groups. Table 30 shows suboptimal levels of function within EQ-5D dimensions by trial group. There were no significant differences in suboptimal level of function across EQ-5D dimensions between the trial groups. Finally, two alternative methods of multivariate analysis were used to model the association between EQ-5D utility scores (dependent variables) and trial intervention: OLS and Tobit (Table 31). OLS regression is the most widely used estimator in the literature. It relies on the Gauss–Markov assumptions about the data and variables used in the model, which need to be met in order to produce unbiased estimators. Tobit regression was used to account for the non-trivial proportion of the study population with maximum EQ-5D utility scores. Potential confounders replicated the covariates incorporated into the main clinical analyses. In both the OLS and Tobit regressions, magnesium was associated with non-significant reductions in the mean EQ-5D utility score at 1 month: 0.023 and 0.100, respectively. There were no significant associations between any of the clinical and sociodemographic covariates incorporated into both models and the EQ-5D utility score.
| EQ-5D dimension | Magnesium (n = 46): n (%) | Placebo (n = 43): n (%) |
|---|---|---|
| Mobility | ||
| Level 1 | 38 (82.6) | 38 (88.4) |
| Level 2 | 7 (15.2) | 5 (11.6) |
| Level 3 | 0 (0.0) | 0 (0.0) |
| Missing | 1 (2.2) | 0 (0.0) |
| Self-care | ||
| Level 1 | 38 (82.6) | 39 (90.7) |
| Level 2 | 4 (8.7) | 2 (4.7) |
| Level 3 | 2 (4.3) | 1 (2.3) |
| Missing | 2 (13.0) | 1 (2.3) |
| Usual activities | ||
| Level 1 | 32 (69.6) | 37 (86.0) |
| Level 2 | 12 (26.1) | 5 (11.6) |
| Level 3 | 0 (0.0) | 1 (2.3) |
| Missing | 2 (4.3) | 0 (0.0) |
| Pain/discomfort | ||
| Level 1 | 31 (67.4) | 33 (76.7) |
| Level 2 | 12 (26.1) | 9 (20.9) |
| Level 3 | 1 (2.2) | 1 (2.3) |
| Missing | 2 (4.3) | 0 (0.0) |
| Anxiety/depression | ||
| Level 1 | 33 (71.7) | 34 (79.1) |
| Level 2 | 10 (21.7) | 7 (16.3) |
| Level 3 | 0 (0.0) | 2 (4.7) |
| Missing | 3 (6.5) | 0 (0.0) |
| Dimension | Magnesium (n = 46): n (%) | Placebo (n = 43): n (%) | p-valuec |
|---|---|---|---|
| Mobility | 7 (15.2) | 5 (11.6) | 0.758 |
| Self-care | 6 (13.0) | 3 (7.0) | 0.485 |
| Usual activities | 12 (26.1) | 6 (14.0) | 0.186 |
| Pain/discomfort | 13 (28.3) | 10 (23.3) | 0.628 |
| Anxiety/depression | 10 (21.7) | 9 (20.9) | 1.000 |
| Variable (unit) | OLS | Tobit | ||||
|---|---|---|---|---|---|---|
| Fully adjusted β (robust SE) | p > |t| | 95% CI | Fully adjusted β (robust SE) | p > |t| | 95% CI | |
| Trial arm (referent = placebo) | ||||||
| Magnesium | – 0.023 (0.062) | 0.705 | – 0.146 to 0.099 | – 0.100 (0.126) | 0.430 | – 0.351 to 0.151 |
| Age (years) | 0.012 (0.011) | 0.277 | – 0.010 to 0.033 | 0.011 (0.021) | 0.603 | – 0.031 to 0.053 |
| Gender (referent = female) | ||||||
| Male | 0.074 (0.067) | 0.272 | – 0.059 to 0.207 | 0.107 (0.136) | 0.433 | – 0.164 to 0.378 |
| Duration of most recent asthma attack (referent = last ≤ 6 hours) | ||||||
| For the last few days | – 0.054 (0.048) | 0.265 | – 0.150 to 0.042 | – 0.182 (0.197) | 0.359 | – 0.574 to 0.211 |
| For the last 24 hours | – 0.132 (0.076) | 0.088 | – 0.284 to 0.020 | – 0.408 (0.211) | 0.057 | – 0.828 to 0.012 |
| SaO2 (value) | – 0.007 (0.006) | 0.236 | – 0.020 to 0.005 | – 0.015 (0.017) | 0.374 | – 0.048 to 0.018 |
| Assessment at baseline (severity score) | 0.036 (0.025) | 0.165 | – 0.015 to 0.086 | 0.077 (0.051) | 0.135 | – 0.025 to 0.178 |
| Respiratory rate | 0.002 (0.002) | 0.436 | – 0.003 to 0.006 | 0.006 (0.007) | 0.383 | – 0.008 to 0.020 |
| Oxygen therapy required (referent = no) | ||||||
| Yes | – 0.030 (0.064) | 0.636 | – 0.158 to 0.097 | – 0.096 (0.132) | 0.468 | – 0.358 to 0.166 |
| Time of day that randomisation occurred (referent = 0900–1700) | ||||||
| 1701–2200 | – 0.068 (0.056) | 0.227 | – 0.043 to 0.180 | 0.263 (0.147) | 0.078 | – 0.031 to 0.556 |
| 2201–0859 | 0.060 (0.077) | 0.437 | – 0.094 to 0.214 | 0.210 (0.245) | 0.394 | – 0.278 to 0.697 |
A number of mapping models were developed on the basis of data collected for 5- to 16-year-old children for whom both EQ-5D and PedsQL™ responses were available. The best fitting model, in terms of the lowest RMSE and lowest AIC statistic, was model 3 (described in Chapter 2), namely an OLS model that incorporated the four PedsQL™ subscale scores, squared PedsQL™ subscale scores and interaction terms derived using the product of two PedsQL™ subscale scores, as well as age and gender, as independent variables. Mapping algorithms developed from this model were used to estimate EQ-5D utility scores for 2- to 4-year-old children in MAGNETIC for whom the validated toddler module of the PedsQL™ Asthma Scales had been completed; the RMSE for this preferred model – model 3 – was 0.026 compared with 0.039 for model 1 and 0.038 for model 2.
Following this estimation procedure for health utilities at 1 month, QALY estimates were available for a total of 218 children: 111 in the magnesium arm of the trial and 107 in the placebo arm of the trial. By contrast, the multiple imputation procedure filled all missing values for both costs and health utilities.
Results of the cost–utility analysis
Complete case analysis
The CUA evaluated the cost–utility of magnesium in terms of QALYs, a preference-based measure of health outcome recommended by decision-makers such as NICE for cost-effectiveness comparative purposes. The time horizon for the CUA covered the period between randomisation and 1 month post randomisation. The incremental cost–utility of magnesium is initially shown in Table 32 for the 218 children (111 receiving magnesium and 107 receiving placebo) for whom we had complete cost and QALY data over the 1-month time horizon. Within the base-case analysis, the average cost was £1056 in the magnesium group compared with £1126 in the placebo group, generating a mean cost saving of £70. The costs presented in Table 32 differ from those presented in Table 34, as the latter represents a multiple imputation analysis including all 508 trial participants.
In the base-case analysis, the incremental cost–utility of magnesium was estimated at £175,598 per QALY gained (south-west quadrant of cost-effectiveness plane). The magnitude of this ICER is being driven by the small baseline-adjusted QALY difference between the trial groups (– 0.0004; denominator of ICER). Moreover, there was substantial stochastic uncertainty around this finding. The variability around the base-case estimates of cost–utility is shown in Figure 10. Although the majority of the bootstrapped replications of the ICER fall in the south-west quadrant of the cost-effectiveness plane (representing lower costs but poorer outcomes), some bootstrapped replications fall in the other three quadrants of the cost-effectiveness plane. The CEAC for the QALY outcome measure is displayed in Figure 11. The CEAC shown in Figure 11 indicates that the probability that use of magnesium is cost-effective varies between 60% and 70%, depending on the value of the cost-effectiveness threshold. If decision-makers are willing to pay £20,000 per additional QALY (NICE 2008),46 the probability that use of magnesium is cost-effective is 67.6%.
Mean net benefits were estimated for alternative cost-effectiveness thresholds per QALY gain (Table 33). Assuming that the cost-effectiveness threshold equals £20,000 per QALY gain generates a mean net benefit to the health service attributable to magnesium of £63 (i.e. on average, there is a net gain to the health service in monetary terms). This is analogous to stating that if the actual health benefit of magnesium, in terms of QALY gain, is multiplied by an assumed willingness to pay of £20,000 per QALY gained, and the net cost is subtracted, then the benefit to the NHS of adopting magnesium is, on average, positive in monetary terms. Note, however, that, as with the CEA results, the 95% CI surrounding the mean net benefit (– 219 to 334) includes negative values, i.e. there is a possibility of a net monetary loss associated with adopting magnesium (see Table 33). If the cost-effectiveness threshold is increased as high as £100,000 per QALY gain, there is little effect on mean net benefit.
Sensitivity analyses were conducted to determine the impact of changing particular parameter values or assumptions on the ICER (see Tables 32 and 33; see Figure 11). Assuming linear interpolation of health utilities over the entire follow-up period, rather than assuming that the health gain was achieved immediately following hospital discharge, had the largest effect on the ICER. This assumption increased the mean baseline-adjusted QALY difference between the trial groups to – 0.005, and reduced the probability that magnesium is cost-effective to 40.6% at a £20,000 cost-effectiveness threshold (mean ICER £13,607; south-west quadrant of cost-effectiveness plane). In contrast, assuming baseline ASS mapped on to EQ-5D health states with higher utility scores than in the baseline analysis increased the probability that magnesium is cost-effective to 68.2% at a £20,000 cost-effectiveness threshold (mean ICER £240,906; south-west quadrant of cost-effectiveness plane). Assuming that baseline ASS mapped on to EQ-5D health states with lower utility scores than in the baseline analysis, and adopting a societal perspective for the economic evaluation, only slightly reduced the probability that magnesium is cost-effective. CEACs generated following each sensitivity analysis are shown in Figure 11. Estimates of net monetary benefits for notional cost-effectiveness thresholds per QALY gain are shown in Table 33 for each sensitivity analysis.
| Analysisa | Mean costs (95% CI) | Mean QALYs gained relative to baseline utility (95% CI) | Incremental cost/QALY | Probability that magnesium is: | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Magnesium (£) | Placebo (£) | Difference (£) | Magnesium | Placebo | Difference | More effective (%) | Less costly (%) | Cost-effective at a £20,000 cost-effectiveness threshold (%) | ||
| Base casea | 1056 (855 to 1256) | 1126 (904 to 1347) | – 70 (– 369 to 228) | 0.00133 (0.00098 to 0.00169) | 0.00173 (0.00131 to 0.00216) | – 0.00040 (– 0.00095 to 0.00015) | 175,598 | 8.5 | 69.1 | 67.6 |
| Linearb (U) | 1056 (855 to 1256) | 1126 (904 to 1347) | – 70 (– 369 to 228) | 0.02530 (0.02060 to 0.02999) | 0.03047 (0.02539 to 0.03555) | – 0.00517 (– 0.01209 to 0.00174) | 13,607 | 7.0 | 67.3 | 40.6 |
| Lower (U) | 1056 (855 to 1256) | 1126 (904 to 1347) | – 70 (– 369 to 228) | 0.00236 (0.00198 to 0.00275) | 0.00268 (0.00225 to 0.00312) | – 0.00032 (– 0.00090 to 0.00026) | 219,930 | 14.4 | 66.4 | 64.4 |
| Higher (U) | 1056 (855 to 1256) | 1126 (904 to 1347) | – 70 (– 369 to 228) | 0.00073 (0.00048 to 0.00099) | 0.00102 (0.00072 to 0.00133) | – 0.00029 (– 0.00069 to 0.00011) | 240,906 | 7.6 | 69.6 | 68.2 |
| Societal perspective | 1145 (937 to 1352) | 1211 (977 to 1443) | – 66 (– 378 to 246) | 0.00133 (0.00098 to 0.00169) | 0.00173 (0.00131 to 0.00216) | – 0.00040 (– 0.00095 to 0.00015) | 164,303 | 8.1 | 64.1 | 63.2 |
| Analysis:a value of threshold (£) | Base case: % cost-effective | Base case analysis: mean net benefit (95% CI) | Linear (U):b % cost-effective | Linear (U): mean net benefit (95% CI) | Lower (U): % cost-effective | Lower (U): mean net benefit (95% CI) | Higher (U): % cost-effective | Higher (U): mean net benefit (95% CI) | Societal: % cost-effective | Societal: mean net benefit (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 69.10 | 71 (– 215 to 351) | 67.30 | 69 (– 249 to 362) | 66.4 | 66 (– 229 to 377) | 69.6 | 79 (– 218 to 369) | 64.1 | 61 (– 247 to 360) |
| 10,000 | 68.20 | 67 (– 217 to 342) | 53.80 | 17 (– 292 to 324) | 65.5 | 63 (– 230 to 372) | 69.0 | 76 (– 222 to 367) | 63.8 | 57 (– 248 to 353) |
| 20,000 | 67.60 | 63 (– 219 to 334) | 40.60 | – 36 (– 354 to 293) | 64.4 | 60 (– 228 to 365) | 68.2 | 73 (– 226 to 364) | 63.20 | 53 (– 249 to 347) |
| 30,000 | 66.20 | 59 (– 219 to 327) | 30.90 | – 89 (– 441 to 266) | 63.8 | 57 (– 231 to 360) | 67.5 | 70 (– 229 to 361) | 62.90 | 49 (– 250 to 340) |
| 40,000 | 65.50 | 55 (– 220 to 321) | 23.20 | – 141 (– 548 to 250) | 63.3 | 53 (– 228 to 351) | 66.7 | 67 (– 231 to 356) | 61.9 | 45 (– 251 to 333) |
| 50,000 | 64.80 | 52 (– 221 to 315) | 19.30 | – 194 (– 661 to 248) | 62.6 | 50 (– 225 to 343) | 66 | 64 (– 234 to 351) | 61.10 | 41 (– 252 to 327) |
| 60,000 | 64.10 | 48 (– 223 to 309) | 16.20 | – 246 (– 771 to 234) | 62.0 | 47 (– 222 to 337) | 64.9 | 61 (– 235 to 345) | 60.0 | 37 (– 257 to 323) |
| 70,000 | 63.60 | 44 (– 224 to 303) | 14.70 | – 299 (– 872 to 233) | 61.30 | 44 (– 222 to 331) | 64.1 | 58 (– 234 to 342) | 59.3 | 33 (– 257 to 320) |
| 80,000 | 62.20 | 40 (– 227 to 299) | 12.90 | – 352 (– 978 to 259) | 60.10 | 41 (– 222 to 325) | 63 | 55 (– 233 to 338) | 58.0 | 29 (– 254 to 317) |
| 90,000 | 60.90 | 36 (– 232 to 296) | 11.40 | – 404 (– 1081 to 260) | 59.80 | 37 (– 222 to 317) | 62.10 | 53 (– 231 to 333) | 56.80 | 25 (– 255 to 310) |
| 100,000 | 60.00 | 32 (– 236 to 291) | 10.80 | – 457 (– 1200 to 269) | 59.10 | 34 (– 223 to 309) | 61.4 | 50 (– 232 to 329) | 55.40 | 21 (– 258 to 303) |
FIGURE 10.
Cost-effectiveness plane for CUA base-case analysis: complete case analyses.
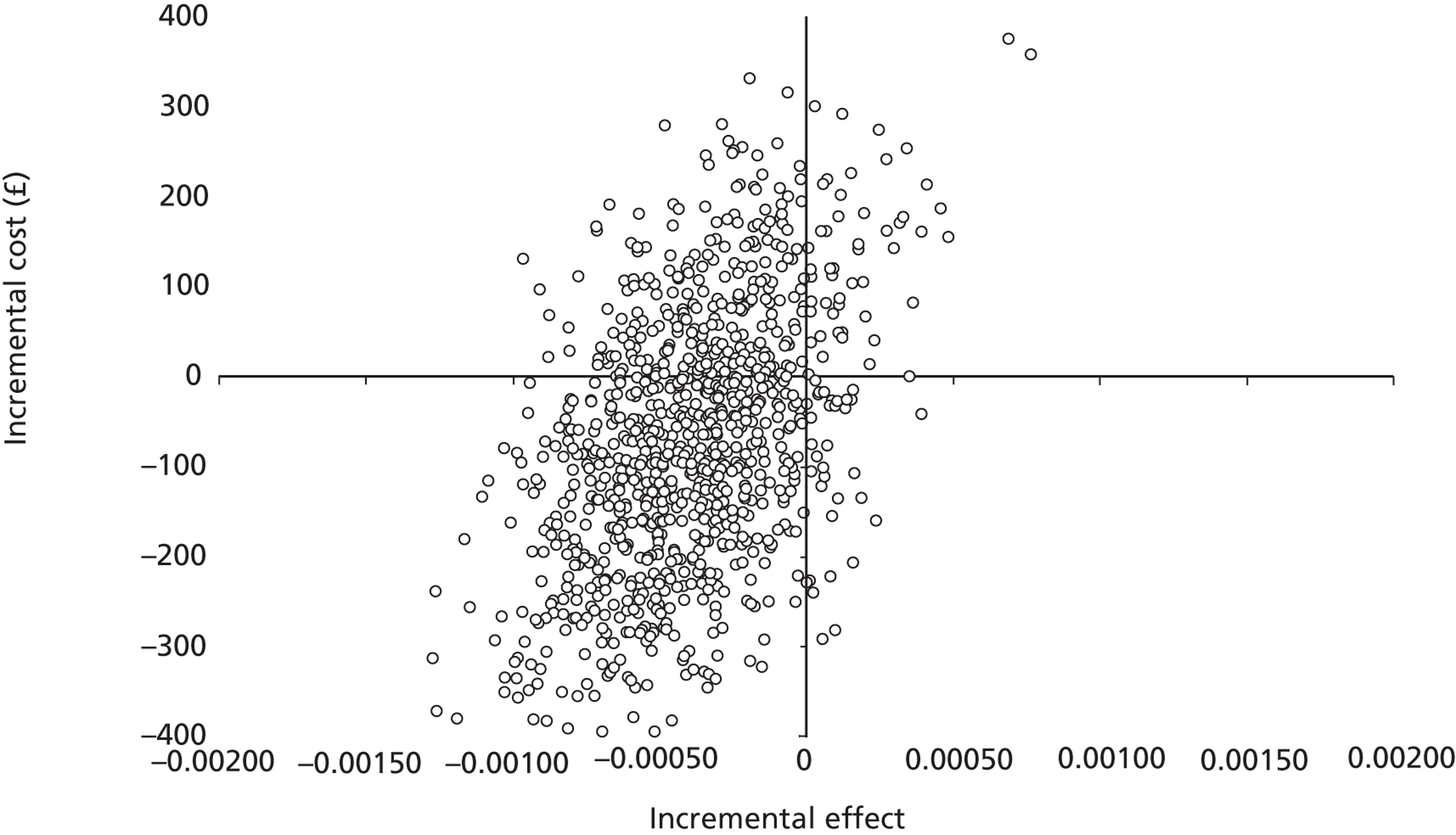
FIGURE 11.
Cost-effectiveness acceptability curves for CUA base-case analyses and sensitivity analyses: complete case analyses. a, Each CEAC shows the probability that magnesium is cost-effective with changes in the amount that society is willing to pay for a QALY. A&E, a lower NHS reference cost applied to A&E department attendances; GM, a higher per diem cost applied to general paediatric ward care; HDU, a lower per diem cost applied to higher level care; LOS exact, part of a day spent by a child in an inpatient ward equated to a proportional period for costing purposes; LOS full, part of a day spent by a child in an inpatient ward equated to a full 24-hour period for costing purposes; PICU, a higher per diem cost applied to higher level care.
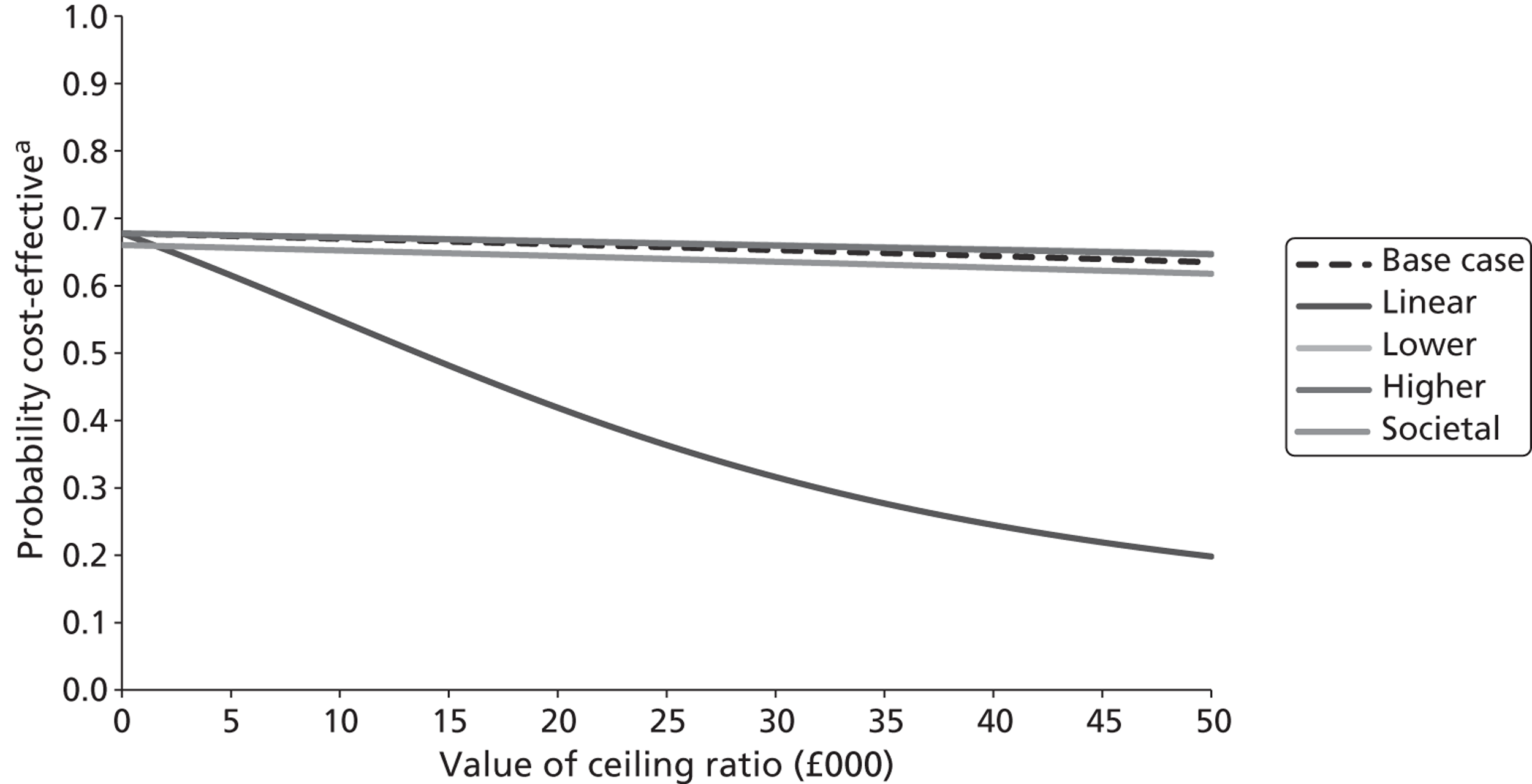
Analyses following multiple imputation
The CUA, expressed in terms of incremental cost per QALY gained, was repeated for all 508 trial participants (252 receiving magnesium and 256 receiving placebo) following multiple imputation of missing cost and outcomes data. As with the complete case analysis, the time horizon for this analysis covered the period between randomisation and 1 month post randomisation. The incremental cost–utility of magnesium is shown in Table 34. Within the base-case analysis, the average cost was £1009 in the magnesium group compared with £1014 in the placebo group, generating a mean cost saving of £5. There was no statistically significant difference in costs between the two trial groups, with 49.0% of bootstrap replicates finding magnesium to be less costly than placebo.
In the base-case analysis, the incremental cost–utility of magnesium was estimated at £11,886 per QALY gained (south-west quadrant of cost-effectiveness plane). However, as in the complete case analysis, substantial stochastic uncertainty surrounded this finding. This is displayed in the cost-effectiveness plane in Figure 12. The CEAC shown in Figure 13 indicates that, at the notional cost-effectiveness threshold of £20,000 per QALY gained, the probability that use of magnesium is cost-effective is 50.9%. If the cost-effectiveness threshold is increased to £30,000 per QALY gained, there is little effect on the probability of cost-effectiveness. Mean net benefits were also estimated for alternative cost-effectiveness thresholds per QALY gained following the multiple imputation procedures (Table 35). Assuming that the cost-effectiveness threshold equals £20,000 per QALY gained generates a mean net loss to the health service attributable to magnesium of £2 (95% CI – £171 to £168). If the cost-effectiveness threshold is increased to £30,000 per QALY gained, the mean net loss to the health service attributable to magnesium increases to £6 (95% CI – £173 to £162).
Finally, sensitivity analyses were conducted to determine the impact of changing particular parameter values or assumptions on the ICER (see Tables 34 and 35, and Figure 13). As in the complete case analysis, assuming linear interpolation of health utilities over the entire follow-up period, rather than assuming that the health gain, was achieved immediately following hospital discharge, had the largest effect on the ICER. This assumption increased the mean QALY difference between the trial groups to – 0.006, and reduced the probability that magnesium is cost-effective to 14.6% at a £20,000 cost-effectiveness threshold (mean ICER £816; south-west quadrant of cost-effectiveness plane). Assuming that baseline ASS mapped on to EQ-5D health states with either lower or higher utility scores than in the baseline analysis, and adopting a societal perspective for the economic evaluation, each had less impact on the cost–utility results. CEACs generated following each sensitivity analysis are shown in Figure 13. Estimates of net monetary benefits for notional cost-effectiveness thresholds per QALY gain are shown in Table 35 for each sensitivity analysis.
| Analysisa | Mean costs (95% CI) | Mean QALYs gained relative to baseline utility (95% CI) | Incremental cost/QALY | Probability that magnesium is: | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Magnesium (£) | Placebo (£) | Difference (£) | Magnesium | Placebo | Difference | More effective (%) | Less costly (%) | Cost-effective at a £20,000 cost-effectiveness threshold (%) | ||
| Base case | 1009 (877 to 1140) | 1014 (895 to 1131) | – 5 (– 181 to 172) | 0.00138 (0.00116 to 0.00159) | 0.00176 (0.00153 to 0.00200) | – 0.00038 (– 0.00070 to – 0.00007) | 11,886 | 1.0 | 51.0 | 50.9 |
| Linear (U) | 1009 (877 to 1140) | 1014 (895 to 1131) | – 5 (– 181 to 172) | 0.02458 (0.02161 to 0.02755) | 0.03018 (0.02709 to 0.03326) | – 0.00560 (– 0.00988 to – 0.00132) | 816 | 0.8 | 50.4 | 14.6 |
| Lower (U) | 1009 (877 to 1140) | 1014 (895 to 1131) | – 5 (– 181 to 172) | 0.00257 (0.00235 to 0.00278) | 0.00275 (0.00253 to 0.00298) | – 0.00019 (– 0.00050 to 0.00013) | 24,562 | 14.2 | 53.0 | 50.6 |
| Higher (U) | 1009 (877 to 1140) | 1014 (895 to 1131) | – 5 (– 181 to 172) | 0.00063 (0.00048 to 0.00077) | 0.00088 (0.00071 to 0.00105) | – 0.00025 (– 0.00047 to – 0.00003) | 18,088 | 1.6 | 51.4 | 49.6 |
| Societal perspective | 1111 (975 to 1246) | 1112 (987 to 1236) | – 1 (– 185 to 183) | 0.00138 (0.00116 to 0.00159) | 0.00176 (0.00153 to 0.00200) | – 0.00038 (– 0.00070 to – 0.00007) | 2390 | 0.7 | 50.5 | 48.4 |
| Analysis: a value of threshold (£) | Base case: % cost-effective | Base case analysis: mean net benefit (95% CI) | Linear (U):b % cost-effective | Linear (U): mean net benefit (95% CI) | Lower (U): % cost-effective | Lower (U): mean net benefit (95% CI) | Higher (U): % cost-effective | Higher (U): mean net benefit (95% CI) | Societal: % cost-effective | Societal: mean net benefit (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 53.7 | 6 (– 165 to 177) | 54.5 | 11 (– 163 to 197) | 52.5 | 2 (– 173 to 164) | 51.2 | 4 (– 169 to 172) | 51.3 | 1 (– 185 to 187) |
| 10,000 | 52.5 | 2 (– 168 to 173) | 31.0 | – 45 (– 223 to 138) | 51.3 | 0 (– 174 to 161) | 50.3 | 1 (– 171 to 169) | 49.9 | – 3 (– 186 to 182) |
| 20,000 | 50.9 | – 2 (– 171 to 168) | 14.6 | – 101 (– 291 to 89) | 50.6 | – 1 (– 175 to 159) | 49.6 | – 1 (– 173 to 166) | 48.4 | – 6 (– 188 to 178) |
| 30,000 | 49.4 | – 6 (– 173 to 162) | 8.3 | – 158 (– 367 to 56) | 49.8 | – 3 (– 175 to 156) | 48.7 | – 4 (– 175 to 162) | 46.7 | – 10 (– 192 to 172) |
| 40,000 | 48.2 | – 9 (– 175 to 157) | 4.6 | – 214 (– 453 to 26) | 48.6 | – 5 (– 175 to 154) | 47.8 | – 6 (– 178 to 158) | 44.5 | – 14 (– 199 to 166) |
| 50,000 | 46.8 | – 13 (– 177 to 153) | 2.6 | – 271 (– 534 to 4) | 47.8 | – 7 (– 175 to 151) | 46.8 | – 9 (– 182 to 155) | 43.1 | – 18 (– 204 to 160) |
| 60,000 | 45.1 | – 17 (– 179 to 151) | 1.7 | – 327 (– 621 to – 27) | 47.4 | – 9 (– 175 to 148) | 45.4 | – 11 (– 184 to 153) | 40.8 | – 22 (– 205 to 154) |
| 70,000 | 43.3 | – 21 (– 182 to 148) | 1.2 | – 384 (– 716 to – 51) | 46.3 | – 11 (– 175 to 145) | 44.2 | – 14 (– 185 to 151) | 39.1 | – 25 (– 207 to 149) |
| 80,000 | 41.1 | – 24 (– 185 to 144) | 1.0 | – 440 (– 798 to – 68) | 45.2 | – 12 (– 176 to 144) | 43.1 | – 16 (– 188 to 148) | 37.4 | – 29 (– 211 to 146) |
| 90,000 | 38.7 | – 28 (– 188 to 140) | 0.8 | – 496 (– 903 to – 90) | 44.7 | – 14 (– 177 to 143) | 41.5 | – 19 (– 190 to 146) | 36.0 | – 33 (– 213 to 141) |
| 100,000 | 36.7 | – 32 (– 192 to 136) | 0.7 | – 553 (– 1006 to – 110) | 43.5 | – 16 (– 178 to 142) | 41.0 | – 21 (– 194 to 143) | 35.0 | – 37 (– 218 to 138) |
FIGURE 12.
Cost-effectiveness plane for CUA base-case analysis: analyses following multiple imputation.
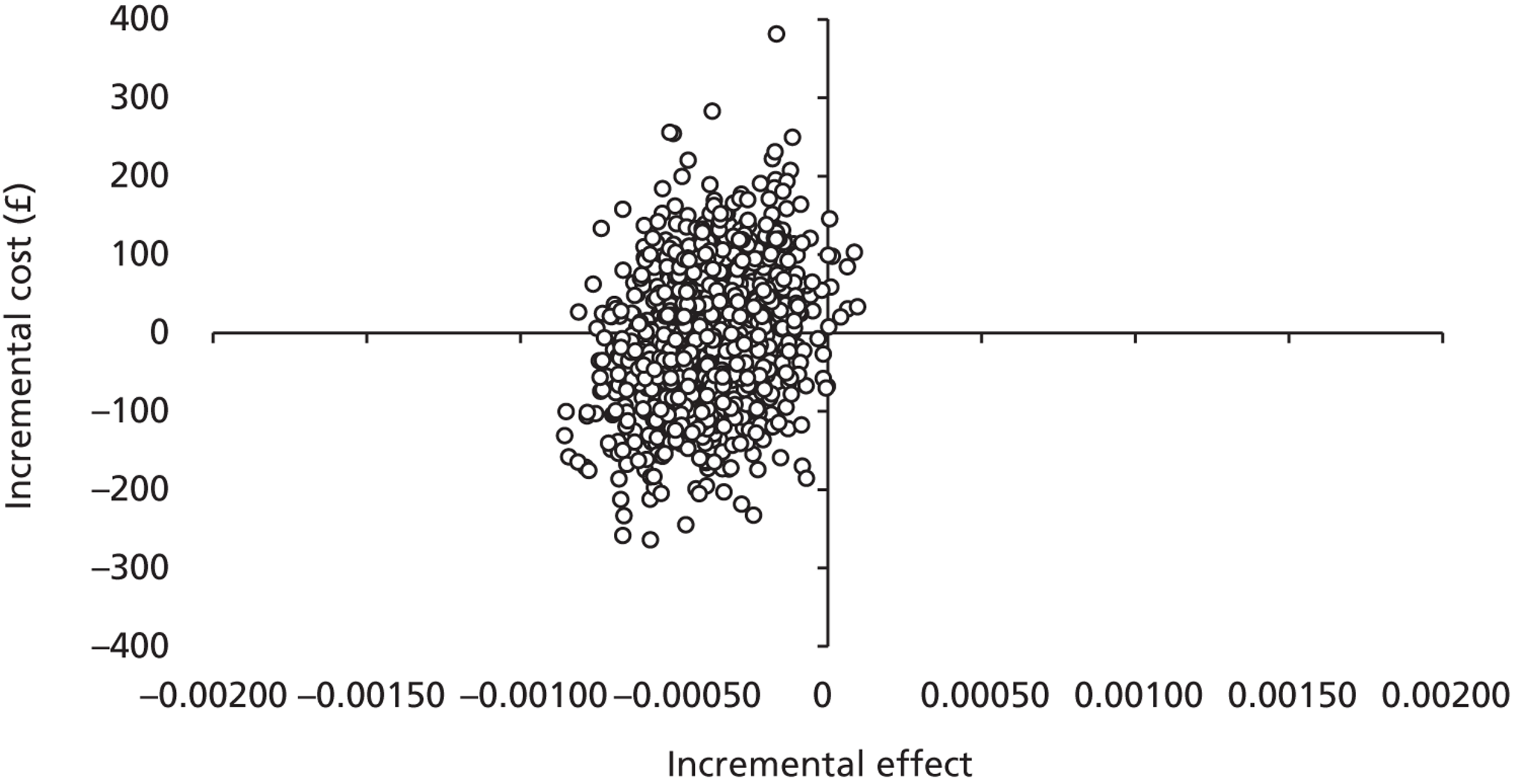
FIGURE 13.
Cost-effectiveness acceptability curves for CUA base-cases analyses and sensitivity analyses: analyses following multiple imputation. a, Each CEAC shows the probability that magnesium is cost-effective with changes in the amount that society is willing to pay for a QALY. A&E, a lower NHS reference cost applied to A&E department attendances; GM, a higher per diem cost applied to general paediatric ward care; HDU, a lower per diem cost applied to higher level care; LOS exact, part of a day spent by a child in an inpatient ward equated to a proportional period for costing purposes; LOS full, part of a day spent by a child in an inpatient ward equated to a full 24-hour period for costing purposes; PICU, a higher per diem cost applied to higher level care.
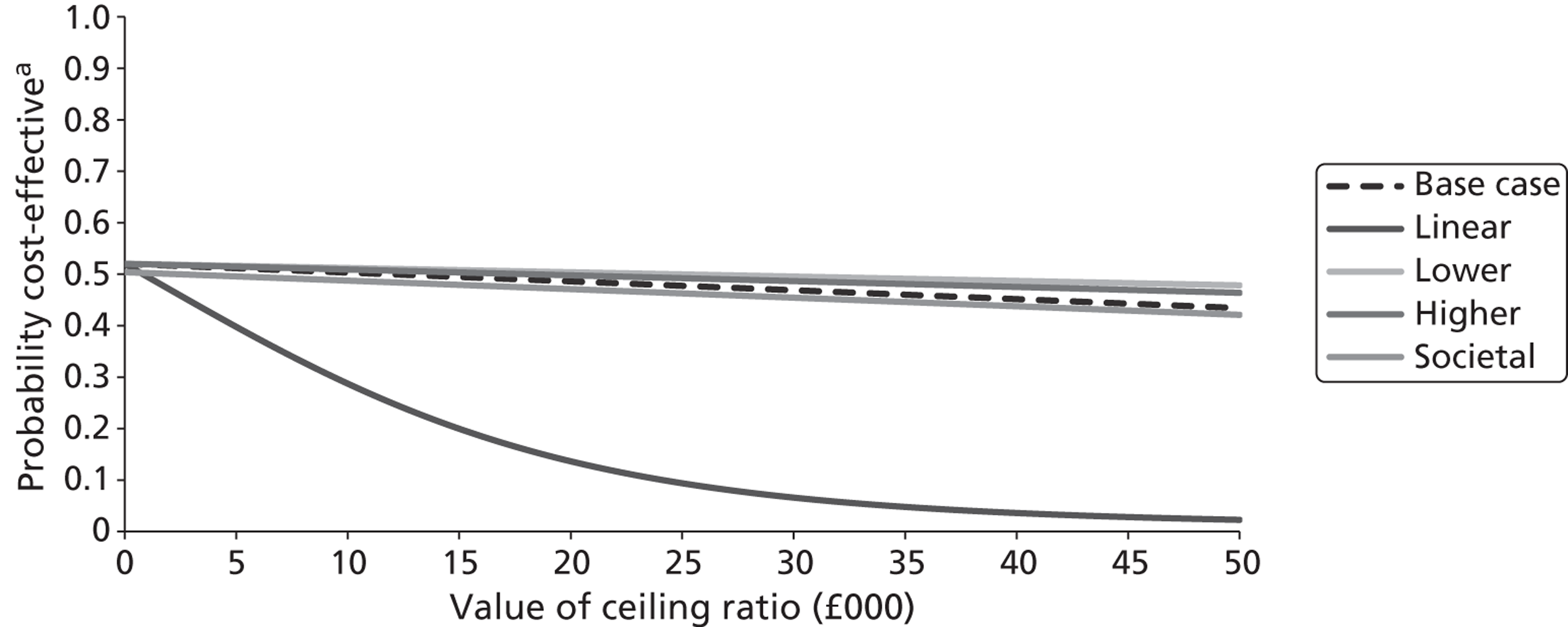
Generalised linear model on costs
For the GLMs performed on costs, a gamma distribution and identity link function was selected in preference to alternative distributional forms and link functions on the basis of its low AIC statistic. Table 36 summarises the results of three GLM models that regressed costs on intervention mode as well the prespecified sociodemographic and clinical covariates. Robust SEs were estimated to account for potential heteroscedasticity in the distribution of residuals. In model 1, NHS costs to discharge from the ED or CAU, or the hospital where the child was admitted to an inpatient ward immediately following attendance, acted as the dependent variable. In model 2, NHS and Personal Social Services costs to 1 month acted as the dependent variable, whereas in model 3 societal costs to 1 month acted as the dependent variable. In all three models, the use of magnesium did not have a significant effect on economic costs. All three models revealed that male gender is associated with increased economic costs, whereas increased SaO2 values at baseline are associated with reduced economic costs.
| Variable (unit) | NHS costs up to discharge | NHS and PSS costs to 1 month | Societal costs to 1 month | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Fully adjusted β (robust SE) | p > |t| | 95% CI | Fully adjusted β (robust SE) | p > |t| | 95% CI | Fully adjusted β (robust SE) | p > |t| | 95% CI | |
| Trial arm (referent = placebo) | |||||||||
| Magnesium | – 0.40 (64.33) | 0.995 | – 126.48 to 125.69 | – 13.60 (64.83) | 0.834 | – 140.66 to 113.47 | – 25.17 (65.90) | 0.702 | – 154.34 to 103.99 |
| Age (years) | 54.35 (13.83) | 0.000 | 27.25 to 81.45 | 62.11 (14.24) | 0.000 | 34.20 to 90.02 | 72.47 (14.64) | 0.000 | 43.77 to 101.17 |
| Gender (referent = female) | |||||||||
| Male | – 26.55 (59.99) | 0.658 | – 144.13 to 91.02 | – 97.69 (66.65) | 0.143 | – 228.33 to 32.95 | – 158.03 (70.49) | 0.025 | – 296.18 to – 19.88 |
| Duration of most recent asthma attack (referent = last ≤ 6 hours) | |||||||||
| For the last few days | 2.48 (89.84) | 0.978 | – 173.59 to 178.56 | 8.68 (94.81) | 0.927 | – 177.14 to 194.50 | 2.87 (93.50) | 0.976 | – 180.39 to 186.13 |
| For the last 24 hours | 42.47 (104.63) | 0.685 | – 162.62 to 247.55 | 19.18 (111.55) | 0.863 | – 199.46 to 237.82 | 38.40 (111.08) | 0.730 | – 179.31 to 256.11 |
| SaO2 (value) | – 36.84 (9.99) | 0.000 | – 56.42 to – 17.26 | – 40.66 (10.13) | 0.000 | – 60.51 to – 20.81 | – 45.37 (10.63) | 0.000 | – 66.21 to – 24.54 |
| Assessment at baseline (severity score) | 50.23 (22.25) | 0.024 | 6.63 to 93.83 | 51.84 (24.53) | 0.035 | 3.77 to 99.91 | 52.43 (24.40) | 0.032 | 4.60 to 100.26 |
| Respiratory rate | 235.90 (80.91) | 0.004 | – 1.48 to 12.47 | 6.41 (3.74) | 0.087 | – 0.93 to 13.74 | 6.76 (3.90) | 0.083 | – 0.88 to 14.40 |
| Oxygen therapy required (referent = no) | |||||||||
| Yes | – 0.030 (0.064) | 0.636 | 77.32 to 394.47 | 235.47 (81.69) | 0.004 | 75.35 to 395.58 | 267.18 (90.62) | 0.003 | 89.57 to 444.79 |
| Time of day randomisation occurred (referent = 0900–1700) | |||||||||
| 1701–2200 | – 72.40 (53.98) | 0.180 | – 178.20 to 33.40 | – 104.17 (57.83) | 0.072 | – 217.51 to 9.17 | – 87.17 (60.20) | 0.148 | 205.15 to 30.82 |
| 2201–0859 | 375.15 (276.52) | 0.175 | – 166.82 to 917.11 | 246.30 (236.33) | 0.297 | – 216.90 to 709.51 | 308.35 (246.70) | 0.211 | – 175.17 to 791.88 |
Chapter 5 Discussion
Main findings
MAGNETIC is the largest, randomised, double-blind, placebo-controlled study examining standard inhaled bronchodilator therapy in acute severe asthma to date in children aged between 2 and 16 years. The study compares the addition of three doses of nebulised isotonic MgSO4 or placebo (isotonic saline) to standard treatment in children aged between 2 and 16 years. The study has shown a statistically significant difference in ASS at 60 minutes post treatment in favour of the magnesium treatment, after three doses of nebulised isotonic magnesium, given as an adjuvant to the standard therapy of nebulised salbutamol and ipratropium bromide administered three times in the first hour of treatment at presentation to secondary care as per the BTS/SIGN guidelines. 3 This effect on ASS continues to be statistically significant up to 240 minutes post initial treatment.
Overall, the size of the effect at T60 adjusted for ASS at presentation (see Table 8), although statistically significant is only 0.25 (95% CI 0.02 to 0.48) of a difference in the ASS scale. This is unlikely to be a clinically meaningful difference. This statistically significant difference continues over the 240 minutes (see Appendix 5, Table 46) but again, of minimum clinical significance at 0.20 (95 CI% 0.01 to 0.40).
However, this effect is more marked in children who have had a more severe exacerbation (as defined by oxygen saturation in air on presentation) and in those children who have had a shorter duration of symptoms of their exacerbation (as defined by parental report) of < 6 hours. Thus there is a more marked effect on improvement of ASS that is more likely to be clinically significant.
The magnesium regimen in this study, three doses in the first hour, did not show any statistically significant difference in need for intravenous bronchodilator therapy, admission to intensive care, length of stay in hospital, admission rate or number of doses of salbutamol given after the initial treatment of the first hour compared with standard treatment. The main side effects reported during the study associated were flushing, vomiting, headache and asymptomatic self-correcting and transient hypotension. There was no important difference between the groups. There were no severe unexpected AEs associated with the use of MgSO4.
We would conclude that in children with acute severe asthma, nebulised isotonic MgSO4 might be added without harm to the initial regimen combined with ipratropium bromide and salbutamol, especially in those children with a more severe episode and a short history of deterioration of symptoms.
Strengths of the study
Study design
The study was a pragmatic study, using the standard BTS/SIGN guidelines for treating acute asthma,3 recruiting patients from 30 centres across the UK. Although there are data to show that guidelines are not always followed completely,62 we felt that a randomised placebo-controlled study designed around a current treatment regimen and current practice was more likely to be completed successfully. We defined acute severe asthma using the BTS definitions for severe asthma: a usable, nationally accepted, published definition. On presentation each patient was treated for their acute symptoms with nebulised bronchodilator (salbutamol with and without ipratropium), while informed consent was obtained with randomisation and the first study treatment given within 30 minutes. This was a similar study design to the study of Hughes et al. 16 and was noted to be a safe approach to recruiting. Patient status was monitored for safety for 4 hours post randomisation. Oxygen saturation, respiratory rate and blood pressure were recorded twice during screening, approximately 20, 40 and 60 minutes post randomisation, and follow-up checks at 120, 180 and 240 minutes. The research team was prompted to check for AEs at each assessment point. AEs were followed up until discharge from hospital.
The randomisation process occurred where the study drug was manufactured before distribution to each study centre. There was random sequence generation in variable-sized blocks and adequate allocation concealment and so low risk of selection bias. The study was blinded to patients, researchers, clinicians, parents and study personnel, and so there was a low risk of performance bias. Outcome assessment was also blinded to all so there was a low risk of detection bias. These data were followed up as much as possible but there were incomplete outcome data, especially the 1-month health economic data, for which the return rate was only 50%, so there is the potential for attrition bias. The data remained blinded to all those analysing the data and only when the SAP was completed successfully, were the data unblinded.
There were no differences in baseline characteristics of our two groups following the randomisation process. This reinforces the internal validity of the study results. Using the LRNs of the MCRN allowed the study to recruit patients from a combination of smaller general hospitals, larger general hospitals as well as tertiary paediatric centres. We recruited patients from both EDs and CAUs – this makes our data more generalisable to the typical clinical situations in which acute asthma presents in the UK.
The involvement of the LRNs was crucial to the success of the trial, offering organisation and support to recruitment. The use of a central CTU, with a dedicated trials manager, data manager and statistical support improved the quality of data. Finally, regular meetings of the TMG, TSC and IDSMC ensured regular research governance and guidance for successful completion of the study over the 2 years of recruitment.
Outcomes
The power of the study was calculated on the basis of the ASS reported by Bishop and Yung29,30 as the primary outcome of interest. It comprises three clinical signs: wheezing, accessory muscle use and heart rate with the total score a sum of each component, giving a minimum score of 0 and a maximum score of 9. Although there are over 20 ASSs,39,41,42 the Bishop and Yung score is well validated and easy to use, and allows comparability with other studies. 63 The score has been validated as a measure of asthma severity in children, demonstrated to be reproducible and reliable29 with good interobserver agreement, and correlates well with severity as defined by oxygen saturations at presentation and FEV1 at presentation. 30 This score is clinically easy to use and involves standard assessments, used routinely by medical and nursing staff while managing acute asthma.
In order to detect a difference between the two groups at 60 minutes post treatment of 0.5 points on the ASS at the 5% significance level with 80% power, 500 children were required. A difference of 0.5 in ASS was deemed to be the minimum worthwhile clinically important difference to be detected by the research team. There are no studies demonstrating what is a clinically relevant change in an ASS to the patient. As a group of experienced clinicians and researchers we felt a change of 0.5 would be an important difference. There is no evidence base to underpin this pragmatic decision and one of the future studies generated from this work would be to examine what is a clinically relevant change to the patient. Thus the main issue is the clinical relevance of a statistically significant difference in an ASS – this question remains a challenge to those working in acute asthma research.
Severity of asthma exacerbation in the children recruited
We used the BTS definition of ‘severe’ acute asthma and our initial concern was that we were recruiting children into the study who may only have been included because of their tachycardia, especially the younger children. This is an aspect of this definition identified previously as a concern needing further exploration. 64 We did not have comprehensive screening data of the population presenting at the recruitment centres and so external validity of our population could be a concern.
However, data from a national audit of UK asthma admissions of 9428 children, by Davies et al. ,64 between 1998 and 2005, and a recent update of this national audit from November 2011 (J Paton, Royal Hospital for Sick Children, Glasgow, March 2012, personal communication), suggest that we have identified a more severe group of patients. Although the presenting arterial oxygen saturation in air was 94% (IQR 91–96%) in the national audit64 and in this population was slightly lower at 93.6% (range 81–100%), the use of intravenous bronchodilators as a marker of severity, was 4–5% in the national audit64 and in the same level in November 2011 and in this population was 11% (see Table 10). So we feel that we have identified a group of children with acute severe asthma, which does represent the more severe end of the asthma exacerbation population presenting to unscheduled care facilities in the UK and thus our study has external validity.
We now have a data set of over 500 acute episodes of asthma, which will allow us to explore the BTS definitions of severity further, as has been suggested by Davies et al. 64 The magnesium effect is most marked in those children with a more severe exacerbation, as defined here by oxygen saturation in air at presentation. With further analysis of our data using a receiver operating characteristic (ROC) curve we may be able to define where the cut-off point in oxygen saturation at presentation may be to gain maximal effect from the addition of magnesium.
Treatment–covariate interactions
In the initial SAP (see Appendix 3), the plan was to formally test a treatment–covariate interaction for the effect of age by including the interaction term in a regression model. Exploratory analysis was planned to examine the impact on any treatment effect of other factors, such as gender or presenting clinical signs. However, blinded to the results, the treatment–covariate interaction hypotheses were discussed further by the statistical and clinical leads (PW, RD, CP, ID) and several changes to the SAP were made as we felt that this approach would be more robust (see Appendix 3, Changes to statistical analysis plan). Treatment–covariate interactions were investigated for two clinically important baseline covariates, SaO2 level at presentation and duration of symptoms of the asthma attack. Other factors, such as age or gender, may affect the response but a number of possible patterns of interaction could be argued. Prognostic factors affecting response will be examined in further analysis outside the scope of this report.
Oxygen saturation at presentation
There is evidence that the more severe the exacerbation of asthma, the more likely a child will have a better response to magnesium. 4,6,31 Our hypothesis would be that the effect of the addition of magnesium to the standard regimen would be greater in those with more severe disease. We thus took SaO2 level at presentation to be the best marker of severity to examine as a potential treatment effect modifier. 3 Further exploration of this relationship will be undertaken outside this report, where we investigate heart rate and respiratory rate in relation to age and response to magnesium.
Duration of attack
The second hypothesis was that the shorter the duration of symptoms then the more marked response to magnesium. This hypothesis is based on the concepts of phenotypes of acute asthma and an understanding of the proposed mechanism for the effect of magnesium on the acutely constricted airway.
-
(a) There has been a suggestion in the adult literature that there are at least two phenotypes of acute asthma – so-called rapid-onset acute asthma (ROAA) and slow-onset acute asthma (SOAA). 65 Definitions used by this prospective study of 403 adults with severe acute asthma (defined as PEFR < 50% predicted at presentation) are that ROAA is < 6 hours' duration of symptoms and SOAA is > 6 hours' symptoms. Their hypothesis is that prolonged symptoms may give an indication of more airway inflammation and the shorter duration may suggest prominent airway smooth muscle contraction,65 the latter responding more rapidly to treatment. 66 The incidence of ROAA in this severe group of acute asthma was 11.3%. 67 Barr et al. 67 demonstrated in 800 adult patients with acute severe asthma (defined as PEFR of < 50% predicted) 14% (95% CI 11% to 16%) had ROAA. 67 Martin et al. 68 demonstrated a prevalence of 17% of ROAA in a study of 30 children with near fatal asthma attacks. Our study recruited 15% of children with an exacerbation with < 6 hours' symptom duration. Three categories (< 6 hours, < 24 hours and > 24 hours) were established by the research team to define duration of attack. These data were collected and recorded based on parental report, which may be subject to recall bias and previous experience of acute asthma attacks; however, we considered that these data and definitions were sufficiently robust to be able to explore the duration of attack effect.
-
(b) Nebulised magnesium acts as a smooth muscle bronchodilator as described previously. In a guinea pig model of acute asthma (triggered by histamine challenge) the main effect of nebulised magnesium is in the early phase of bronchoconstriction, where a greater bronchodilator response is evident compared with the later more inflammatory phase in which the effect is less marked. 59
Thus we felt that the hypothesis that the effect of magnesium may be more marked in those with a shorter duration of attack and shorter duration of symptoms was justified. The concept of phenotypes of acute asthma in children needs to be explored further and will be investigated using these data outside this report.
Longitudinal assessment
We also assessed the effects of the addition of magnesium to changes in the ASS over 240 minutes. So, rather than a cross-sectional measurement at T60 we were able to see the effect, longitudinally up to 4 hours after treatment. This is a novel approach to assessing ASS and has not been presented in the acute asthma literature before. 39 Longitudinal ASS data were summarised by the AUC. The AUC is a summary measure that integrates repeated assessments over the duration of the treatment.
Figure 3 illustrates the mean longitudinal profiles over the first hour. There was no significant difference in AUC over the first hour during the treatment regimen (p = 0.21; see Table 9). However when we examined the effect over 240 minutes, even accounting for missing values and dropouts we can demonstrate that the statistically significant effect seen at the cross sectional T60 measurement (see Table 8) is sustained up to 240 minutes (see Appendix 5, Figures 15 and 16, and Table 46). Again, the clinical significance of this difference is unlikely to be important [treatment effect on ASS 0.20 (95% CI 0.01 to 0.40)], but it does emphasise that there is a pharmacological effect and that this is sustained over 240 minutes in the overall group. This effect would need to be explored further to examine the magnitude and length of the effect in those with a more severe attack and shorter duration of symptoms. The data from MAGNETIC will allow further exploration of the AUC as a potential core outcome for future acute asthma studies.
Secondary outcomes
We examined secondary outcomes frequently measured in acute asthma studies32,39 (see Appendix 1): need for intravenous bronchodilator therapy, need for PICU admission and intubation, stepping down of treatment after 1 hour, length of stay and additional bronchodilators given. We found no evidence of a difference between those who received magnesium and those who received standard therapy. No paediatric studies of nebulised magnesium have found any evidence of differences in these outcomes but none, including the current study, are powered individually to do so.
The only ‘new’ outcome reported in this study is the ‘stepping down’ of treatment from nebuliser to spacer. We were unable to detect a difference between the two groups: 33% in the magnesium group and 30% in the placebo group (p = 0.53). In the study by Kelly et al. ,43 among 720 patients (adults and children) presenting to 36 EDs in Australia, asthma severity improved from severe to moderate after an hour of treatment in 50%, resulting in a change from nebuliser to spacers – thus ‘step-down’. Stepping down is thus considered to be a proxy for the clinician considering the child to be clinically better and is based, rather than on a score, on a clinician's subjective impression. However, the fact that only one-third stepped down at 1 hour in this study would suggest that we have a group of children with more severe acute asthma attacks than the mixed population of all levels of severity in those presenting to EDs in the Kelly et al. study;43 the mixed age groups and wider spectrum of severity may explain the difference. This concept of stepping down of treatment needs to be further developed for further studies in acute asthma.
An outcome that we did not analyse is the concept of mean duration in supplemental oxygen. Khashabi 200837 (presented in abstract form only) examined 40 children with acute asthma (mean age 3.55 years) but found no difference in an ASS 1 hour after two doses of either nebulised magnesium and salbutamol or salbutamol and placebo, but they did find a difference in mean duration of supplemental oxygen therapy (not defined in the abstract): 15.2 hours (95% CI 9.3 to 21.5 hours) compared with 19.0 hours (95% CI 12.4 to 25.8 hours). 37 This outcome should be defined and explored further in future studies of acute asthma.
Centre effect
A sensitivity analysis was performed to investigate the robustness of ignoring a centre effect in the primary analysis. Two models were fitted when the centre was treated as either a fixed effect or as a random effect. Both models were adjusted for baseline ASS. Reassuringly, there was no evidence that the treatment effect varies by centre (see Appendix 6, Table 47).
Timing of treatment administration
There could be concern that there was significant variation in the timing of the administration of the study medication in the two groups. Reassuringly, there was no clinically significant deviation in the mean prescribed times between the treatment groups on any of the three occasions (see Table 5) and the mean time to administration in both groups was 5.8 (SD 8.3) minutes after randomisation, 23.4 (SD 5.5) minutes after the first dose and 23.3 (SD 6.2) minutes after the second dose, which was as per the protocol. We had previously stated that 15 minutes leeway was clinically acceptable, and Table 6 has shown that only 53/508 instances were considered to be protocol deviations, with 10% in the magnesium group and 12% in the placebo group.
Potential limitations
Dose of magnesium given
We used the same dose of isotonic MgSO4 for all ages on each of the three administrations in the first hour (2.5 ml of 250 mmol/l, tonicity 289 mOsm, 151 mg per dose). This was the dose used by Hughes et al. 16 in their adult study of 52 patients where they demonstrated a significant effect in lung function improvement at 90 minutes post treatment. 16
The ideal dose for children has not yet been clarified and whether the dose needs to be changed with age/weight or whether one standard dose is sufficient, modulated by the child's tidal volume, is yet to be ascertained. There is clearly a dose–response effect in the guinea pig model of magnesium effect and bronchoconstriction59 with guinea pigs with stable tidal volumes but the examination of this issue has not had any exploration in this acute asthma literature.
In the nebulised magnesium studies including children, so far one dose has been used for all ages but these have differed in frequency, formulation and combination with other bronchodilators (see Appendix 1, Table 39). This illustrates how difficult it is to make any comparison and firm conclusion when comparing the literature. 32 This is also a similar research consideration in the adult data.
-
Aggarwal et al. 22 (ages 13–60 years, n = 110) 1 ml MgSO4 (500 mg) three doses 20 minutes apart with β2-agonist; total magnesium used: 1500 mg (3 × 500 mg).
-
Ashtekar et al. 27 and this study, MAGNETIC (ages 2–16 years, n = 508) 2.5 ml of 250 mmol/l, tonicity 289 mOsm, 151 mg per dose; total magnesium used: 453 mg (3 × 151 mg).
-
Drobina et al. 23 (ages 12–60 years, n = 110) 125 mg MgSO4 0.25 ml of 50% solution (three doses 20 minutes apart with β2-agonist; total magnesium used: 375 mg (3 × 125 mg).
-
Khashabi et al. 37 (ages mean age 3.55 years) two doses of isotonic MgSO4 not stated.
-
Mangat 199814 (ages 12–60 years, n = 33) 3 ml (95 mg) MgSO4 (four doses, 20 minutes apart) compared with β2-agonist; total magnesium used: 380 mg (4 × 95 mg).
-
Mahajan et al. 18 (ages 5–17 years, n = 62) 2.5 ml isotonic MgSO4 solution (6.3%) with single dose of β2-agonist (dose).
-
Meral 199619 (ages < 16 years, n = 40) 2 ml of MgSO4 280 mmol/l).
No studies have examined the use of frequent doses of nebulised magnesium outside the first hour of treatment. Dose used and frequency given need further research in the clinical setting of acute asthma in children.
Different nebulisers and outputs
This was a pragmatic study and thus did not define a standard nebuliser for each centre but they were all oxygen driven from wall oxygen supplies. We felt that in order to produce a generalisable result we should use what is currently being used in the EDs and CAUs in the UK. Each centre used the same type of nebuliser for all patients within that centre, but different types of nebuliser were used in different centres. There are some American data to suggest that there is variable output from different nebulisers. 69 The PARI LC Star® (PARI Respiratory Equipment, Midlothian, VA, USA) had an appropriate particle size distribution but a very slow aerosol output rate. The Omron MicroAir® [Clement Clarke International (CCI), Harlow, UK] had an even slower output rate and a larger particle size distribution, which would be inappropriate for smaller children. In vitro lung deposition with the Aeroneb Go with Idehaler (Aerogen, Galway, Ireland) was 16.0 ± 0.4 mg/minute in older children and approximately one-fifth of that in toddlers. This presumably relates to lung deposition and not necessarily therapeutic effect; some effect may be due to absorption across mucous membranes and independent of lung deposition. Their conclusion was that the Aeroneb Go with Idehaler was the ideal one for a nebulised magnesium study currently under way in the USA.
Unblinding of randomised treatments during the study and protocol deviations and missing values
Unblinding of randomised treatments during the study
The treatment allocation was unblinded during the course of the trial for only two children, one in each group (see Table 14), and the children were withdrawn from the study owing to SAEs that both resolved. Both events were considered to be unlikely to be related to the study medication and will not have affected the outcome of the study.
Protocol deviations
Table 6 illustrates the protocol deviations that occurred and these were related to the timing of administration (53), age of patient (2), recruitment more than once (1), and pretreatment with spacers rather than with nebulisers (14). These were thus few and not likely to represent any danger to the children. It was reassuring that there was no imbalance across treatment groups.
Missing values
Although we achieved the expected recruitment rates, there were concerns about the missing values in the data collated in the CRFs early on in the course of recruitment. The concern was that these missing values could influence the conclusions of the study.
Primary outcome data
There were 36 (7%) children recruited into the study who had insufficient data to complete an ASS at T60 (see Appendix 5, Table 41). The reasons for the missing components of the ASS in these 36 cases are illustrated in Appendix 5, Table 42. The main issues were missing components of the ASS in 22 (4.3%) of cases. This illustrates how well the training of the ASS by the PI and research nurses in the study was completed. The lack of difference in the key baseline characteristics between observed patients and those missing at T60 indicates the plausibility of the MCAR assumption.
Three sensitivity analyses were performed (see Appendix 5, Tables 43–45) to explore this assumption:
-
reason for missingness (see Appendix 5, Table 43); adjusted difference in mean [– 0.32 (95% CI – 0.56 to – 0.08), p < 0.01]
-
multiple imputations (see Appendix 5, Table 44); adjusted difference in mean [– 0.28 (95% CI – 0.51 to – 0.05)]
-
joint modelling of the longitudinal first 60 minutes' data (see Appendix 5, Table 45).
Thus the sensitivity analysis did not suggest a substantially different conclusion from the assumption that the missing values were missing at random and they did not influence the final conclusion of the analysis.
Longitudinal data
The relationship between the ASS and dropout from the study over the entire length of the study was examined by joint modelling. In Appendix 5, Figures 15 and 16, and Table 46 illustrate that the dropout in the magnesium group was due to those subjects getting better (see Figure 16) and not getting worse. This does not affect the final conclusion that the effect of magnesium on a ASS of 0.2 (95% CI 0.01 to 0.40) over the 240 minutes is sustained statistically. Thus the effect of any missing value in either treatment arm does not significantly affect the conclusions from the study.
Safety
There were no major safety issues of clinical concern reported and this study suggests that the doses and frequency given in this regimen can be considered safe. We did not measure the serum levels of magnesium, but there are adult data to suggest70 that it is safe not to do so. However, if further studies were to be undertaken using higher or more frequent doses in children, concerns over safety might mandate the measurement of serum magnesium levels and pharmacokinetic studies with dose–response measurements may be necessary.
The AEs reported in the study 99/507 (19.5%) were mainly mild and of similar magnitude in both groups; magnesium 19% and placebo 20%. Vomiting was the most commonly reported feature in both groups; magnesium 8.3% and placebo 9.4%. Headache was reported more commonly in the magnesium group (2% compared with the 0.4% in the placebo group). Further analysis of these AE may be useful; if the vomiting and headaches were related to the use of intravenous bronchodilators (e.g. aminophylline), especially the vomiting, then the incidence related to the magnesium may even be reduced further.
There were 15 SAEs (three on magnesium and 12 on placebo), only one of which was considered to be possibly related to the study drug, but this was a child in the placebo group (see Table 14). There were no SUSARs. One can thus conclude that, although the study was not powered to identify every difference in AE and SAE or rates of SUSAR, the administration of nebulised magnesium at these doses and frequency is safe. This is supported by the data from all published 16 studies using nebulised MgSO4 (see Appendix 1, Table 3932).
Comparison with other studies
MAGNETIC is the first clinical trial of such size to address standard treatment as per BTS guidelines with the addition of nebulised magnesium in the UK. The conclusion from the systematic review by Mohammed and Goodacre4 was that ‘insufficient data exist to draw reliable conclusions regarding the role of nebulised MgSO4 in children’.
Only two paediatric studies18,19 were included in this review and the conclusion was based on the lack of significant effect on respiratory function (SMD 20.26; 95% CI – 1.49 to 0.98; p = 0.69) or hospital admission (RR 2.0; 95% CI 0.19 to 20.93; p = 0.56) in children. But these two studies18,19 were of insufficient power and methodological rigour to draw any other conclusion. Our data are of adequate power and reliability, and are sufficiently generalisable, to suggest that there is a significant clinical effect on acute asthma using nebulised MgSO4, especially in severe exacerbations of short duration. There are sufficient data in this study to suggest that the addition of nebulised magnesium to the standard regimen for acute severe asthma in children is justified.
Almost universally in the published studies showing a beneficial effect of the addition of magnesium to standard treatment, it is the more severe patients – both adults and children – who gain the most benefit. 4,6,32 The conclusion from the MAGNETIC study is therefore supported by the literature and firms up the recommendation that can be given about the use of nebulised magnesium in severe acute asthma in children.
We have shown a more marked effect of nebulised magnesium on children with a shorter duration of symptoms. There is little published evidence on different phenotypes of acute asthma, and the MAGNETIC data set will enable us to explore this topic outside the scope of this report. As described above (see Duration of attack), we generated the hypothesis that response may be more marked in those children with a shorter duration of symptoms, based on data suggesting different phenotypes of acute asthma and an understanding of how magnesium may work. The main criticism about the definition used here could be that the duration of symptoms is defined by parental report and these could be subject to bias from a number of areas: experience of symptoms previously and length of diagnosis of asthma, responsiveness of parents to getting medical help and recognising symptoms, some children may have had only their first attack of wheezing and so parental understanding may be variable, and what constitutes the onset of symptoms may be different in different families; all may have an effect on the reporting of onset of symptoms.
Data from asthma mortality studies also suggest at least two mechanisms for death in acute asthma. These two mechanisms may highlight the two different phases of an acute asthma response – an immediate asthma response followed by a later response – which are well-described phases in airway compromise seen in exercise-induced and methacholine and histamine challenge test-induced airway constriction. 71 Slow-onset cases fatality have shown to be more eosinophilic inflammatory-mediated response and the more sudden onset a more neutrophil-mediated response with acute bronchospasm. 72 More recent data73 suggest that there are different inflammatory profiles during acute asthma in children and adults. Although a small study, it suggested that adult acute asthma was more likely to be neutrophil driven, whereas in children it was more likely to be eosinophilic. Indeed, this group has suggested that there are a number of phenotypes: eosinophilic, neutrophilic, mixed granulocytic and paucigranulocytic asthma. 74 The frequency in acute childhood asthma has not yet been determined but there is sufficient evidence to suggest there may well be different mechanisms during an acute episode to warrant exploration of our data.
Finally, as described in Chapter 1, magnesium appears to work at a number of levels in acute asthma. It may affect the inflammatory process in asthma, especially attenuating neutrophil burst associated with an asthma response and thus acting as an anti-inflammatory agent. 8 Indeed, in a guinea pig model of asthma developed by part of this current research group, a reduction in neutrophil numbers has been demonstrated. 58 Again, this would support the concept of examining those children with a shorter duration of symptoms, perhaps neutrophil mediated, responding differently to those with a longer duration of symptoms.
Thus, we have demonstrated a greater effect in those children who have had a shorter duration of exacerbation, supporting the animal model's implication that nebulised magnesium has more of an effect on the early asthma bronchoconstriction response. When one examines the conflicting literature in the adult nebulised magnesium studies, this becomes evident. Aggarwal et al. ,22 in a RCT of reasonable power, found no effect in 100 adults with acute asthma. However, in both study groups asthma history preceding their recruitment to the study averaged 72 hours; thus, the later inflammatory response may have predominated in those subjects, explaining the lack of clinical response. 22 A recent study by Gallegos-Solórzano et al. 36 found a significant difference in response adding nebulised magnesium in a RCT involving 60 patients, and their duration of attack was shorter – between 15 and 23 hours – again demonstrating a shorter duration of exacerbation associated with improved lung function, post-treatment oxygen saturation and a reduced admission rate.
In a low-powered RCT by Kokturk et al. 21 involving 26 patients, no difference in PEFR or clinical scores was seen when nebulised magnesium was added to a standardised regimen. The duration of attack was not reported and thus the relationship between duration of attack and response cannot be commented on. There were also no data on the duration of attack in the Hughes study. 16
Mahajan et al. 18 studied 62 children in whom lung function had shown a minimal short-term response to nebulised magnesium. The average duration of attack of 42 hours was in both groups, shorter than in the subjects in the study by Aggarwal et al. ,22 but still longer than in the children in our study, who showed a more marked clinical response.
The topic of phenotypes of acute asthma and this apparently more marked response to magnesium needs further exploration outside the scope of this report.
Health economics data
The economic evaluation undertaken alongside the MAGNETIC trial compared the addition of MgSO4 to standard treatment with standard treatment only in children with acute severe asthma who presented at a hospital ED or CAU. It represents, to our knowledge, the first economic evaluation of MgSO4 in children with asthma. The economic evaluation was conducted according to nationally-agreed design and reporting standards. 46,47 The economic evaluation has three key strengths. First, it is based on prospective collection of cost and QoL data from the MAGNETIC trial, which recruited over 500 children from the UK; this means that the source of the data is likely to be reliable and appropriate to inform health-care decision-making in the NHS. Second, some of the approaches used to measure children's QoL outcomes in the CUA are novel and perhaps will pave the way for future empirical research into the measurement of QoL of children with asthma. Third, there has been a substantial collection of non-NHS data from patients in the trial. Describing the results of the economic evaluation from the perspective of the NHS and Personal Social Services and from the wider societal perspective means that decision-makers can make a more informed choice when deciding whether or not to invest scarce health-care resources in treatments for children with acute severe asthma.
The cost and outcomes data collected in the MAGNETIC trial were analysed within two alternative frameworks: (1) a CEA that used the child's ASS score as the health outcome of interest and (2) a CUA that used the child's QALY profile as the health outcome of interest. A series of sensitivity analyses were carried out for each analysis to account for uncertainty surrounding key components of the economic evaluation; in addition, the implications of missing data were explored via multiple imputation analyses and the results were incorporated into both the CEA and the CUA.
In the CEA, the economic evaluation was restricted to the time period from randomisation to hospital discharge and the perspective was that of the NHS and Personal Social Services. As resource-use data were collected via the trial CRFs, complete health economics data were available for analyses and we are therefore confident that we have been able to identify, measure and value resource use reliably for both groups of children. There were no statistically significant differences demonstrated between the magnesium and the placebo groups for any of the cost categories except for the cost of the study intervention. However, there was a statistically significant difference in ASS at T60 between the groups (the primary outcome of the MAGNETIC trial) in favour of the MAGNETIC group. Consequently, the results of the CEA demonstrate that adding magnesium to standard treatment yields a relatively high probability (75%) that magnesium is cost-effective at a threshold of £1000. Increasing the cost-effectiveness threshold illustrates that adding magnesium to standard treatment becomes increasingly cost-effective; at a threshold of £5000, the probability that magnesium is cost-effective increases to 85.5%. Clearly, how much society or the NHS may or should be willing to pay to reduce a child's ASS is unknown and this is the challenge faced by health-care decision-makers. Future preference elicitation studies in this area should aid their decision-making. The results of the sensitivity analysis confirm that the probability of magnesium being cost-effective compared with no magnesium in the base-case analysis is robust; probabilities of cost-effectiveness range from 68.3% (applying a higher PICU cost to higher-level inpatient care) to 81.5% (applying a lower HDU cost to higher-level inpatient care). The results of the multiple imputation analyses support the findings of the base-case CEA and show that the likelihood that magnesium is cost-effective ranges between 78.9% and 89.7% at threshold of £1000.
In the CUA, the economic evaluation was covered a longer time horizon than the CEA; costs and benefits were analysed from randomisation to 1 month after the child's initial visit to ED/CUA. The base-case CUA was undertaken from the perspective of the NHS and Personal Social Services. None of the NHS costs was found to be statistically significantly different between the two groups. Initially, the CUA was restricted to the trial population for whom questionnaires were returned and so the CUA was based on data from fewer children than the CEA (230 vs. 508, respectively); the full population was included in the CUA using multiple imputation methods. In the base-case analysis for the CUA, the ICER is high at £175,598 per QALY gained. The size of the ICER is largely driven by the very small mean difference in QALY scores between the two trial groups; there is a 0.0004 difference in QALYs in favour of the placebo group. However, the results of the base-line CUA demonstrate that adding magnesium to standard treatment is likely to yield probabilities of 60–70% of cost-effectiveness across thresholds ranging from £0 to £100,000. At a cost-effectiveness threshold of £20,000 per QALY gained, the results of the sensitivity analysis show that the conclusion of the base-line CUA is relatively robust and that the only parameter change that leads to a relatively low (40%) probability of cost-effectiveness is related to the assumption that the EQ-5D health state has not been immediately achieved following hospital discharge; clinical opinion is that the EQ-5D health state is likely to be achieved following discharge. The results of the sensitivity analysis which uses societal (NHS, Personal Social Services, families and carer) rather than NHS costs only support the conclusion of the base-line CUA that adding magnesium to standard treatment is likely to be cost-effective at the £20,000 per QALY threshold. The results following multiple imputation analyses are less favourable showing lower probabilities of cost-effectiveness as thresholds increase.
As always, a number of caveats should be noted when interpreting the results of any economic evaluation.
First, in both the CEA and the CUA there is considerable stochastic uncertainty around the size of the base-case ICERs; this means that it is important to focus on the interpretation of the results of the CEA and the CUA, as illustrated by the CEACs. When results show that the size of the ICER is uncertain, it is more meaningful to state how likely the intervention is to be cost-effective compared with the control, rather than affirming that the intervention is or is not cost-effective. The CEAC offers a means of communicating the inherent uncertainty around the size of the ICER and simultaneously offers health-care decision-makers a foundation to support any decision made.
Second, another limitation of the economic evaluation is that the QoL and cost data describing health status and resource use from hospital discharge to 1 month post randomisation are available only from the returned and completed parental questionnaires. This means that the data cannot be verified and reliability is determined by the parent or carer's recollection of events during the 1-month period after discharge from hospital; however, asking parents to recall events related to their children that took place in the previous 4 weeks is considered to be reasonable. As the aim of treatment with magnesium is to quickly reduce the ASS, there is further confidence in the reliability of the post-discharge data, as there were no statistically significant differences in the majority of QoL and economic outcomes that were explored.
The third limitation relates to the nature and quantity of the QoL data collected from children in the MAGNETIC trial and there are three distinct but related issues to consider. Owing to the design of the MAGNETIC trial, the only clinical outcome that it was possible to measure at screening and randomisation as well as post treatment was the ASS; the EQ-5D was measured uniquely 1 month after treatment. In order to generate before treatment QALY scores for children, the baseline ASS for each patient was translated into a baseline EQ-5D score by the health economics research team taking advice from asthma experts (doctor and nurses) who routinely treat children with asthma. Clearly, it would have been preferable to have baseline EQ-5D scores for all children but as this was not possible owing to ethical considerations, converting the ASS score in this way was considered to be a valid approach. Next, post-treatment EQ-5D scores were not available for all patients and it was necessary for the research team to map data from completed PedsQL™ Asthma Modules to the EQ-5D scoring system in order to generate QALYs that could be incorporated into the economic evaluation (for those patients with PedsQL™ data but without EQ-5D data). It was also necessary to map data from completed PedsQL™ Asthma Modules to the EQ-5D scoring system for those children of < 5 years whose parents/carers completed the EQ-5D questionnaire while unaware that they were not required to do so.
Finally, the choice of EQ-5D scores used in the sensitivity analysis requires further discussion. The research team considered that it was appropriate to vary the before treatment QALY values used in the base-case analyses in order to check that the translation from ASS to QALY was reasonable and that the CUA results held firm when QALY values were increased or decreased slightly. The range of variation for the baseline EQ-5D scores was dictated by experts (and not directly informed by the experience of children in the trial or elsewhere) but it is anticipated that it reflects the experience of children with slightly higher or lower ASS and therefore offers an analytical check on the appropriateness of the original before treatment utility values used. There is a final general concern there are some aspects of health status relevant to young children that are not captured by either the EQ-5D or the PedsQL™ Asthma Module. However, until both generic and specific QoL instruments are designed to successfully reflect experiences across all stages of childhood, health economists have to rely on the available, but often constrained, measures for the purposes of economic evaluation.
In conclusion, the results of our base-case analyses suggest that from an NHS and Personal Social Services perspective, the addition of magnesium to standard treatment is likely to be cost-effective compared with standard treatment only. The results of both sets of analyses (CEA and CUA) show that the probability of magnesium being cost-effective is over 60% at cost-effectiveness thresholds of £1000 per unit decrement in ASS and £20,000 per QALY gained, respectively, and is highest in the CEA. It is anticipated those data collected on the costs and QoL of children with acute severe asthma as part of the MAGNETIC trial will be used to inform future economic evaluations and other empirical research studies in this area.
Conclusions
This study has had extremely and rigorous management of all aspects of research governance, the recruitment process, data collection, data analysis and examination of the results before unblinding. Despite the possible limitations of the study discussed above, the defence of the limitations and the strength of the study would suggest that the study has good external and internal validity.
Interpretation
There are sufficient data in this study to support the use of nebulised isotonic MgSO4 at the dose of 151 mg given three times in the first hour of treatment as an adjuvant to standard treatment, when a child presents with an acute episode of severe asthma. The response is likely to be more marked in those children with more severe attacks and with a shorter duration of exacerbation. Although the study was not powered to demonstrate this, the data certainly support the hypotheses that nebulised magnesium has a greater clinical effect in children who have more severe exacerbation with shorter duration of symptoms.
Implications for health care
The results of the base-case economic analyses suggest that, from an NHS and Personal Social Services perspective, the addition of magnesium to standard treatment may be cost-effective compared with standard treatment only, though there remains substantial uncertainty around this finding. The results of both sets of analyses (CEA and CUA) show that the probability of magnesium being cost-effective is over 60% at cost-effectiveness thresholds of £1000 per unit decrement in ASS and £20,000 per QALY gained respectively; it is noted that for some parameter variations this probability is much lower, reflecting the impact of variation on the small differences in QALY and costs seen in this trial.
Recommendations for research
Further studies on dose–response at different ages and frequency of administration during an attack are required. The effect on secondary outcomes such as need for intravenous bronchodilators and PICU admissions and length of stay with different nebulised magnesium treatment regimen (dose and frequency) needs further exploration. The concept of different phenotypes and severity where the use of nebulised magnesium can be tailored to the features of the exacerbation needs further exploration.
Currently, three further analyses are planned using these data:
-
exploration of the relationship between ASS and the BTS definition of acute severe asthma
-
assessment of the value of the AUC analysis of ASS
-
examination of the concept of acute phenotypes of asthma in children and the response to treatment.
It may be that these data are sufficient to recommend that nebulised magnesium is added to standard treatment, particularly in those who have a severe attack and those with a short history. Further studies of dose–response pharmacokinetics and frequency of doses, nebuliser use, compatibility studies and animal models to clarify the mechanisms of magnesium use are also to be considered.
Setting trial in context of existing research
The results of this large study are timely. One large study in adults, the 3MG study, is coming to a conclusion4 and another paediatric study in the USA is currently under way. 69 There are limited trial data in children, with only four published studies14,18,19,27 (including the pilot study MAGNET27). This is the largest study of nebulised MgSO4 in children to date. These data will add further evidence, which may help to improve and strengthen the recommendations of national and international guidelines on the management of acute asthma in childhood.
Chapter 6 Other information
Registration
Identifying numbers:
-
HTA 05/503/10
-
ISRCTN81456894
-
EudraCT no. 2007–006227–12
-
MREC 07/H1010/101.
Protocol
The MAGNETIC trial protocol is available from www.hta.ac.uk/project/1615.asp (accessed October 2011).
Funding
This trial was funded by the NIHR Health Technology Assessment programme.
Acknowledgements
The MAGNETIC TMG are very grateful to all of the principal investigators, research practitioners, site pharmacists and participating families for their contribution, commitment and enthusiasm, and Professor Chris Butler for initial assistance with the protocol development and grant application. The MAGNETIC TMG is also grateful to the following: Hannah Short, MAGNETIC data manager; Kamran Khan for help with the economic analysis; the MCRN LRNs in England; ScotMCN in Scotland; CRC Cymru in Wales; the TSC (chaired by Professor Ian Russell) and the Data and Safety Monitoring and Committee (chaired by Professor David Jones) for their support and work throughout the study.
Contribution of authors
Dr Colin Powell, chief investigator.
Dr Ruwanthi Kolamunnage-Dona (trial statistician) was a member of the TMG, performed the statistical analyses and reviewed a draft of the report.
Dr Angela Boland was a member of the TMG, performed the health economic analyses and reviewed a draft of the report.
Professor Stavros Petrou (Professor of Health Economics, Warwick University) led the health economics team, contributed to the design of the study and reviewed a draft of the report.
Mr John Lowe was the trial co-ordinator, a member of the TMG and he prepared the report for publication.
Dr Iolo Doull (Consultant Respiratory Paediatrician) was a member of the TMG, contributed to the design and conduct of the study, and reviewed a draft of the report.
Professor Kerenza Hood (Director, South East Wales Trials Unit) was a member of the TMG, contributed to the design and conduct of the study, and reviewed a draft of the report.
Professor Paula Williamson (Director, MCRN CTU) was a member of the TMG, led the statistical team, contributed to the design and conduct of the study, and reviewed a draft of the report.
MAGNETIC Study Group
Dr Colin PowellPrincipal investigatorUniversity Hospital of Wales, CardiffAnn RussellResearch nurseAnwen HowellsResearch nurseDr David LevyPrincipal investigatorTameside General HospitalSonia KeaneResearch nurseClaire FishResearch nurseDr Faisal Al-ZidgaliPrincipal investigatorWythenshawe HospitalSue LangworthWard managerAnne CookResearch nurseZoe ThomasResearch nurseDr Kate GoldbergPrincipal investigatorBlackpool Victoria HospitalJacqueline WoodsResearch nurseJacqueline BradleyResearch nurseDr Anil ShenoyPrincipal investigatorRoyal Albert Edward Infirmary WiganHelena PradyResearch nurseJane HowellResearch nurseJamie DolanResearch nurseDr Dhia MahmoodPrincipal investigatorRoyal Preston HospitalJacqueline WoodsResearch nurseDr Huw ThomasPrincipal investigatorBristol Royal Children's HospitalVictoria PayneResearch nurseTracey BinghamResearch nursePhoebe MoulsdaleResearch nurseDr Adrian HarrisPrincipal investigatorRoyal Devon and Exeter HospitalSu WilkinsResearch nurseMichelle CurtisResearch nurseLayla PriceResearch nurseSue WardResearch nurseDr Ravi JayaramPrincipal investigatorCountess of Chester HospitalCaroline BurchettResearch nurseDr Ang HoPrincipal investigatorMacclesfield District General HospitalJoanne ShippeyResearch nurseDr Sharryn GardnerPrincipal investigatorOrmskirk and District General HospitalDr Matouk ZbaedaPrincipal investigatorZena HaslamResearch nurseMoira MorrisonResearch nurseDr Patrick UkwadePrincipal investigatorBirmingham Heartlands HospitalMark SmithAdvanced nurse practitionerIndy BirakResearch nurseMark SmithResearch nurseDr Kathleen BerryPrincipal investigatorBirmingham Children's HospitalMark SmithResearch nurseIndy BirakResearch nurseDr Tina NewtonPrincipal investigatorUniversity Hospital of North StaffordshireHilary ShepleyResearch nurseKatharine BeamondResearch nurseDr Mansoor AhmedPrincipal investigatorQueens Hospital, BurtonJane MaidenResearch nurseStephanie RawlinsResearch nurseDr Richard BrookerPrincipal investigatorAberdeen Children's HospitalLindsay CameronResearch nurseDr James PatonPrincipal investigatorRoyal Hospital for Sick Children, GlasgowVincent ChouduryLead A&E consultantChristine KerrPersonal assistant to principal investigatorEmma ScobieResearch nurseDr Hitesh PandyaPrincipal investigatorLeicester Royal InfirmaryMelanie McFeetersConsultant nurseSamantha HuntResearch nurseDr Will CarrollPrincipal investigatorDerbyshire Children's HospitalCoral SmithResearch nurseVanessa UnsworthResearch nurseSamantha JonesResearch nurseNicola WatsonResearch nurseDr Chris FitzsimmonsPrincipal investigatorSheffield Children's HospitalDr Mark EverardPrincipal investigatorKaty TreherneResearch nurseAlyson BarberResearch nurseDr Clare DieppePrincipal investigatorQueens Medical Centre NottinghamProfessor Harish VyasPrincipal investigatorHelen SmithResearch nurseYvonne HootonResearch nurseDr John CriddlePrincipal investigatorSt Thomas' HospitalLorraine HodsdonResearch nurseVictoria TimmsResearch nurseDr Ami ParikhPrincipal investigatorRoyal London HospitalFrances LingResearch nurseGemma BarryResearch nursePauleen WaitheResearch nurseDr Jane BayreutherPrincipal investigatorUniversity Hospital LewishamEniola NsirimResearch nurseDr Michael SmithPrincipal investigatorCraigavon Area HospitalSara GilpinResearch nurseDr Damien ArmstrongPrincipal investigatorAltnagelvin Area HospitalJulie BrownResearch nurseDr Katherine PotierPrincipal investigatorManchester Children's HospitalAnne CookResearch nurseHelena PradyResearch nurseDr Hazel EvansPrincipal investigatorSouthampton General HospitalCilla LongResearch nurseMichelle CaseyResearch nurseDr Anil TuladharPrincipal investigatorUniversity Hospital North TeesDr Venkata PaturiSub investigatorCatherine Tarn NozedarResearch nurse
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Sennhauser F. The burden of asthma in children. Paediatr Resp Rev 2005;6:2-7. http://dx.doi.org/10.1016/j.prrv.2004.11.001.
- Department of Health (DoH) n.d. www.dh.gov.uk (accessed 12 April 2013).
- British Guideline on the Management of Asthma. A national clinical guideline. London: BTS; 2011.
- Mohammed S, Goodacre S. Intravenous and nebulised magnesium sulphate for acute asthma: systematic review and meta-analysis. EMJ 2007;24:823-30. http://dx.doi.org/10.1136/emj.2007.052050.
- Goodacre S. The 3Mg Study: a randomised trial of intravenous or nebulised magnesium sulphate versus placebo for acute severe asthma n.d. www.sheffield.ac.uk/scharr/sections/hsr/emergency/3mg (accessed March 2012).
- Blitz M. Inhaled magnesium sulphate in the treatment of acute asthma (a review). Cochrane Database Syst Rev 2005;3.
- Blitz M, Blitz S, Hughes R, Diner B, Beasley R, Knopp J, et al. Aerosolised magnesium sulphate for acute asthma: a systematic review. Chest 2005;128:337-44.
- Cairns CB, Kraft M. Magnesium attenuates the neutrophil respiratory burst in adult asthmatic patients. Acad Emerg Med 1996;3:1093-7. http://dx.doi.org/10.1111/j.1553-2712.1996.tb03366.x.
- Gourgoulianis KI, Chatziparasidis G, Chatziefthimiou A, Molyvdas PA. Magnesium as a relaxing factor of airway smooth muscles. J Aerosol Med 2001;14:301-7. http://dx.doi.org/10.1089/089426801316970259.
- Silverman R. Intravenous magnesium sulphate in the treatment of acute severe asthma. A multi-centre randomised controlled trial. Chest 2002;122:489-97.
- Rowe BH, Bretzlaff JA, Bourdon C, Bota GW, Camargo CA. Magnesium sulfate for treating exacerbations of acute asthma in the emergency department. Cochrane Database Syst Rev 2000;2. http://dx.doi.org/10.1002/14651858.CD001490.
- Hill J, Britton J. Dose-response relationship and time-course of the effect of inhaled magnesium sulphate on airflow in normal and asthmatic subjects. Br J Clin Pharmacol 1995;40:539-44. http://dx.doi.org/10.1111/j.1365-2125.1995.tb05798.x.
- Zanddsteeg AMG, Hirman P, Pamsa HR, Yska JP, ten Brinke A. Effect of MgSO4 on FEV1 in stable severe asthma patients with chronic airflow limitation. Magnesium Res 2009;22:256-61.
- Mangat H. Nebulised magnesium sulphate versus nebulised salbutamol in acute bronchial asthma: a clinical trial. Eur Resp J 1998;38:341-4.
- Nannini L, Pendino J, Corna R, Mannarino S, Quispe R. Magnesium sulphate as a vehicle for nebulised salbutamol in acute asthma. Am J Med 2000;108:193-7.
- Hughes R. The use of isotonic nebulised magnesium as an adjuvant to salbutamol. Lancet 2003;361:2114-17. http://dx.doi.org/10.1016/S0140-6736(03)13721-X.
- Bessmertny O, DiGregorio R, Cohen H, Becker E, Looney D, Golden J, et al. A randomized clinical trial of nebulised magnesium sulphate in addition to albuterol in the treatment of acute mild-to-moderate asthma exacerbations in adults. Ann Emerg Med 2002;39:585-91.
- Mahajan P, Haritos D, Rosenberg N, Thomas R. Comparison of nebulised magnesium sulphate plus albuterol plus saline in children with exacerbations of mild to moderate asthma. J Emerg Med 2004;27:21-5.
- Meral A. Inhalation therapy with MgSO4. Turk J Pediatr 1996;38:169-75.
- Costello J, Howes M. Nebulised magnesium in asthma. Emerg Med J 2004;21:586-7. http://dx.doi.org/10.1136/emj.2004.017939.
- Kokturk N, Turktas H, Kara P, Mullaoglu S, Yilmaz F, Karamercan A. A randomized clinical trial of magnesium sulphate as a vehicle for nebulized salbutamol in the treatment of moderate to severe asthma attacks. Pulm Pharmacol Ther 2005;18:416-21. http://dx.doi.org/10.1016/j.pupt.2005.03.003.
- Aggarwal P, Sharad S, Handa R, Dwiwedi SN, Irshad M. Comparison of nebulised magnesium sulphate and salbutamol combined with salbutamol alone in the treatment of acute bronchial asthma: a randomised study. Emerg Med J 2006;23:358-62. http://dx.doi.org/10.1136/emj.2005.026203.
- Drobina BJ, Kostic MA, Roos JA. Nebulized magnesium has no benefit in the treatment of acute asthma in the emergency department. Acad Emerg Med 2006;13. http://dx.doi.org/10.1197/j.aem.2006.03.047.
- Dwan K, Gamble C, Kolamunnage-Dona R, Mohammed S, Powell C, Williamson P. Assessing the potential for reporting bias in a review: a tutorial. Trials 2010;11:52-6. http://dx.doi.org/10.1186/1745-6215-11-52.
- Rolla G, Bucca C, Arossa W, Bugiani M. Magnesium attenuates methacholine-induced bronchoconstriction in asthmatics. Magnesium 1987;6:201-4.
- Rolla G, Bucca C, Caria E. Dose-related effect of inhaled magnesium-sulfate on histamine bronchial challenge in asthmatics. Drugs Exp Clin Res 1988;14:609-12.
- Ashtekar CS, Powell C, Hood K, Doull I. Magnesium nebuliser trial (MAGNET): a randomised double-blind placebo controlled pilot study in severe acute asthma. Arch Dis Child 2008;93:A100-6.
- Conway S. Admission to hospital with asthma. Arch Dis Child 1985;60:636-9. http://dx.doi.org/10.1136/adc.60.7.636.
- Bishop J, Carlin J, Nolan T. Evaluation of the properties and reliability of the clinical severity scale for acute asthma in children. J Clin Epidemiol 1992;45:71-6. http://dx.doi.org/10.1016/0895-4356(92)90190-X.
- Yung M, South M, Byrt T. Evaluation of an asthma severity score. JPCH 1996;32:261-4. http://dx.doi.org/10.1111/j.1440-1754.1996.tb01567.x.
- Fischl, MA, Pitchenik A, Gardner LB. An index predicting relapse and need for hospitalization in patients with acute bronchial asthma. N Engl J Med 1981;305:783-9. http://dx.doi.org/10.1056/NEJM198110013051402.
- Powell C, Dwan K, Milan SJ, Beasley R, Hughes R, Knopp-Sihota JA, et al. Inhaled magnesium sulfate in the treatment of acute asthma. Cochrane Database Syst Rev 2012;12. http://dx.doi.org/10.1002/14651858.CD003898.pub5.
- Abreu-Gonzalez J, Rodríguez-Díaz CY. Magnesium and bronchodilator effect of beta-adrenergic. Am J Resp Crit Care Med 2002;165.
- Gaur SN, Singh A, Kumar R. Evaluating role of inhaled magnesium sulphate as an adjunct to salbutamol and ipratropium in severe acute asthma. Chest 2008;134.
- Gallegos-Solórzano MC, Pérez-Padilla R, Hernández-Zenteno RJ. Usefulness of inhaled magnesium sulfate in the co-adjuvant management of severe asthma crisis in an emergency department. Pulm Pharmacol Ther 2010;23:432-7.
- Neki NS. A comparative clinical efficacy and safety profile of inhaled magnesium sulphate and salbutamol nebulisations in severe asthma. Indian J Allergy Asthma Immunol 2006;20.
- Khashabi J, Asadolahi S, Karamiyar M, Salari Lak S. European Respiratory Society Annual Congress. Berlin; 2008.
- Dadhich P, Tailor M, Gupta ML, Gupta R. Magnesium sulphate nebulisation in acute severe asthma. Indian J Allergy Asthma Immunol 2005;19.
- Rafai Z. Systematic Review of Outcomes in Acute Asthma in Childhood 2012.
- Gorelick MH, Stevens MW, Schultz T, Scribano PV. Difficulty in obtaining peak expiratory flow measurements in children with acute asthma. Pediatr Emerg Care 2004;20:22-6. http://dx.doi.org/10.1097/01.pec.0000106239.72265.16.
- Van der Mindt DA, Nagelkerke AF, Bouter LM, Dankert-Roelse JE, Veerman JP. Clinical scores for acute asthma in preschool children: a review of the literature. Epidemiology 1994;47:635-46.
- Birken CS, Parkin PC, Macarthur C. Asthma severity scores for preschoolers displayed weaknesses in reliability, validity and responsiveness. J Clin Epidemiol 2004;57:1177-81. http://dx.doi.org/10.1016/j.jclinepi.2004.02.016.
- Kelly AM, Kerr D, Powell C. Is severity assessment after one hour of treatment better for predicting the need for admission in acute asthma?. Respir Med 2004;98:777-81. http://dx.doi.org/10.1016/j.rmed.2004.01.008.
- Elashoff JD. nQuery Advisor Version 4.0 User's Guide. Los Angeles, CA: Statistical Solutions; 2000.
- Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. Part I: Introduction and design. Br J Cancer 1976;34:585-612. http://dx.doi.org/10.1038/bjc.1976.220.
- National Institute for Health and Care Excellence (NICE) . Guide to the methods of technology appraisal. Sections 5.4.1 to 5.4.4 2008. www.nice.org.uk/media/B52/A7/TAMethodsGuideUpdatedJune2008.pdf (accessed 12 April 2013).
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2005.
- Curtis L. Unit costs of health and social care. Canterbury: PSSRU, University of Kent; 2009.
- NHS reference costs 2009-2010. London: DoH; 2011.
- British Medical Association and Royal Pharmaceutical Society of Great Britain . British National Formulary 2010. www.bnf.org/bnf/ (accessed 12 April 2013).
- Kind P, Hardman G, Macran S. The University of York, Centre for Health Economics discussion paper 172. 1999. Centre for Health Economics, University of York; 1999.
- Dolan P, Gudex C, Kind P, Williams A. A social tariff for EuroQol: Results from a UK general population survey. York: Centre for Health Economics, University of York; 1995.
- Petrou S. Methodological issues raised by preference-based approaches to measuring the health status of children. Health Econ 2003;12:697-702. http://dx.doi.org/10.1002/hec.775.
- Brazier JE, Yang Y, Tsuchiya A, Rowen DL. A review of studies mapping (or cross walking) non-preference based measures of health to generic preference-based measures. Eur J Health Econ 2010;11:215-25. http://dx.doi.org/10.1007/s10198-009-0168-z.
- Longworth L, Rowen D. NICE DSU technical support document 10. Sheffield: Decision Support Unit, ScHARR, University of Sheffield; 2011.
- Briggs A, Clark T, Wolstenholme J, Clarke P. Missing … presumed at random: cost-analysis of incomplete data. Health Econ 2003;12:377-92. http://dx.doi.org/10.1002/hec.766.
- Briggs AH, Gray AM. Handling uncertainty when performing economic evaluation of healthcare interventions. Health Technol Assess 1999;3.
- Stinnett AA, Mullahy J. Net health benefits: A new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decis Making 1998;18:S65-80. http://dx.doi.org/10.1177/0272989X9801800209.
- Turner D, Broadley K, Ford, Kidd E, Powell C. Nebulised magnesium sulphate bronchodilator and bronchoprotective?. London: British Pharmacological Society Proceedings; 2011.
- Szefler SJ, Zeiger RS, Haselkorn T, Mink DR, Kamath TV, Fish JE, et al. Economic burden of impairment in children with severe or difficult-to-treat asthma. Ann Allergy Asthma Immunol 2011;107:110-19. http://dx.doi.org/10.1016/j.anai.2011.04.008.
- Eiser C, Morse R. Quality-of-life measures in chronic diseases of childhood. Health Technol Assess 2001;5.
- Babl FE, Sherrif N, Borland M, Acworth J, Neutze J, Krieser D, et al. Paediatric acute asthma management in Australia and New Zealand: practice patterns in the context of clinical practice guidelines. Arch Disease Child 2008;93:307-12. http://dx.doi.org/10.1136/adc.2007.125062.
- Yung M, South M. Randomised controlled trial of aminophylline for severe acute asthma. Arch Dis Child 1998;79:405-10. http://dx.doi.org/10.1136/adc.79.5.405.
- Davies G, Paton J, Beaton S , Young D, Lenney W. Children admitted with acute wheeze/asthma during. November 1998–2005: a national UK audit. Arch Dis Child 2008;93:952-8. http://dx.doi.org/10.1136/adc.2007.133868.
- Rodrigo G, Rodrigo C. Rapid onset asthma attack: a prospective cohort study about characteristics and response to emergency department treatment. Chest 2000;118:1547-52. http://dx.doi.org/10.1378/chest.118.6.1547.
- Woodruff P, Edmond S, Singh A, Carmargo C. Sudden onset severe acute asthma: clinical features and response to therapy. Acad Emerg Med 1998;5:695-701. http://dx.doi.org/10.1111/j.1553-2712.1998.tb02488.x.
- Barr R, Woodruff P, Clark S, Camargo C. Sudden onset asthma exacerbations: clinical features, response to therapy and 2-week follow up. Eur Resp J 2000;15:266-73. http://dx.doi.org/10.1034/j.1399-3003.2000.15b08.x.
- Martin A, Campbell D, Gluyas P, Coates J, Ruffin R, Roder D, et al. Characteristics of near fatal asthma in childhood. Pediatr Pulmonol 1995;20:1-8. http://dx.doi.org/10.1002/ppul.1950200102.
- Coates AL, Leung K, Vecellio L, Schuh S. Testing of nebulizers for delivering magnesium sulfate to pediatric asthma patients in the emergency department. Respir Care 2011;56:314-18. http://dx.doi.org/10.4187/respcare.00826.
- DiGregorio RV, Bessmertny O, Pringle G, Cohen H. Effect of nebulized magnesium sulfate on serum magnesium levels in asthmatic patients. ASHP Midyear Clinical Meeting 1999 n.d.;34.
- Coates JE. Lung function: assessment and application to medicine. Oxford: Blackwell Scientific Publication; 1993.
- Sur S, Cotty T, Kephart G, Hyma B, Colby T, Reed C, et al. Sudden onset asthma. A distinct entity with few eosinophils and more neutrophils in the airway mucosa. Am Rev Resp Dis 1993;148:713-19.
- Wang F, He X, Baines K, Gunawardhana L, Simpson J, Li F, et al. Different inflammatory phenotypes in adults and children with acute asthma. Eur Resp J 2011;38:567-74. http://dx.doi.org/10.1183/09031936.00170110.
- Simpson J, McElduff P, Gobson P. Assessment and reproducibility of non-eosinophilic asthma using induced sputum. Respiration 2010;79:147-51. http://dx.doi.org/10.1159/000245899.
- Davis WJ, Pang LM, Chernack WJ, Mellins RB. Terbutaline in the treatment of acute asthma in childhood. Chest 1977;72:614-17. http://dx.doi.org/10.1378/chest.72.5.614.
- Friede T, Keisser M. Sample size recalculation for binary data in internal pilot study designs: a review. Biometrical J 2006;48:537-55.
- European Medicines Agency (EMA) (The European Agency for the Evaluation of Medical Products) . Points to Consider on Adjustments for Baseline Covariates 2003. www.emea.europa.eu/pdfs/human/ewp/286399en.pdf (accessed 12 April 2013).
- Pham B, Cranney A, Boers M, Verhoeven AC, Wells G, Tugwell P. Validity of area-under-the-curve analysis to summarize effect in rheumatoid arthritis clinical trials. J Rheumatol 1999;27:712-16.
- Matthews JNS, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ 1990;300:230-5. http://dx.doi.org/10.1136/bmj.300.6719.230.
- Efron B, Tibshirani R. An introduction to the bootstrap. New York, NY: Chapman and Hall; 1993.
- Wood AM, White IR, Thompson SG. Are missing outcome data adequately handled? A review of published randomized controlled trials in major medical journals. Clin Trials 2004;1:368-76. http://dx.doi.org/10.1191/1740774504cn032oa.
- van Buuren S, Oudshoorn K. Technical report PG/VGZ/99.054. Leiden: TNO Prevention and Health; 1999.
- Unnebrink K, Windeler J. Sensitivity analysis by worst and best case assessment: is it really sensitive?. Drug Inf J 1999;33:835-9. http://dx.doi.org/10.1177/009286159903300324.
- Wood AM, White IR, Hillsdon M, Carpenter J. Comparison of imputation and modelling methods in the analysis of a physical activity trial with missing outcomes. Int J Epidemiol 2005;34:89-9. http://dx.doi.org/10.1093/ije/dyh297.
- Henderson R, Diggle P, Dobson A. Joint modelling of longitudinal measurements and event time data. Biostatistics 2000;1:465-80. http://dx.doi.org/10.1093/biostatistics/1.4.465.
- Clarke M, Hopewell S, Chalmers I. Reports of clinical trials should begin and end with up-to-date systematic reviews of other relevant evidence: a status report. J R Soc Med 2007;100:187-90. http://dx.doi.org/10.1258/jrsm.100.4.187.
Appendix 1 Summary of the features of the 16 published randomised controlled trials on nebulised magnesium up to 2012
| Study | Severity | Based on | Adult/mixed/paediatric (years) |
|---|---|---|---|
| Abreu-Gonzalez 200233 | Moderate | FEV1 and PEFR at baseline | Adults (?) |
| Aggarwal 200622 | Severe and life-threatening | BTS definition clinical features and PEFR | Mixed (13–60) |
| Ashtekar 200827 | Severe | BTS definition clinical features | Paediatric (2–16) |
| Bessmertny 200217 | Moderate to severe | PEFR between 40% and 80% | Adults (18–65) |
| Dadhich 200538 | Severe | PEFR < 50% | Adults (?) |
| Drobina 200623 | Unclear | Used PEFR and clinical signs | Adults (?) |
| Gallegos-Solórzano 201035 | Moderate to severe | FEV1 < 60% | Adults > 18 |
| Gaur 200834 | Severe | FEV1 < 30% | Adults (18–60) |
| Hughes 200316 | Severe | FEV1 < 50% | Adults (16–65) |
| Khashabi 200837 | Unclear | Clinically defined as respiratory distress | Paediatric (mean age 3.55 years) |
| Kokturk 200521 | Moderate to severe | Clinical scores and PEFR | Adults (18–60) |
| Mahajan 200418 | Moderate to severe | FEV1 between 45% and 75% | Paediatric (5–17) |
| Mangat 199814 | Moderate to severe | PEFR < 300 l/minute | Mixed (12–60) |
| Meral 199619 | Moderate to severe | PEFR < 75% | Paediatric (? age) |
| Nannini 200015 | Severe | PEFR < 50% | Adult (> 18) |
| Neki 200636 | Severe | FEV1 < 40% or PEFR < 300 l/minute | Adult (15–60) |
| Study | Presentation to which department? | Origin | Primary outcome(s) | Side effects (patients in study) | Pharmaceutical exclusions | Other interventions |
|---|---|---|---|---|---|---|
| Abreu-Gonzalez 200233 | Not clear | Tenerife, Spain | FEV1 and PEFR | None documented (24) | None documented | None documented |
| Aggarwal 200622 | ED | New Delhi, India | PEFR | Palpitations (salbutamol/Mg 13, and salbutamol/placebo 11) and tremors (7 and 7). Nothing else noted (100) | None documented | Clinicians free to administer steroids, more salbutamol if needed – intravenous hydrocortisone |
| Ashtekar 200827 | CAU after GP referral | Cardiff, Wales | ASS (Yung 1996) | One given magnesium had tingling in fingers and another one TSLH with facial flushing (17) | None documented | 2 mg/kg prednisolone |
| Bessmertny 200217 | ED | Brooklyn, USA | FEV1 (% predicted) | No SAEs noted (74) | No theophylline or anticholinergic drugs 2 hours prior to presentation | 2 mg/kg hydrocortisone 6 hourly |
| Dadhich 200538 | ED | Ajmer, India | PEFR | ‘Side effects were self limiting’ (71) | Not stated | Not stated |
| Drobina 200623 | ED | USA | PEFR and admissions | No comment (110) | Not stated | 50 mg oral prednisolone |
| Gallegos-Solórzano 201035 | ED | Mexico City, Mexico | Percentage change FEV1, O2 post treatment and admission rates | dry and bitter mouth in magnesium group (1) and dizziness (one in each) (60) | Use of steroids prior to presentation | 1 mg/kg/day for 10 days' prednisolone |
| Gaur 200834 | ED | Delhi, India | FEV1 | None documented (60) | None stated | Intravenous hydrocortisone |
| Hughes 200316 | ED | Wellington, New Zealand | FEV1 | None reported (52) | None | 100 mg hydrocortisone |
| Khashabi 200837 | Unclear | Urmia, Iran | Mean duration of O2 therapy and respiratory distress score (? which one) | There were no side effects (40) | Not stated | Not stated |
| Kokturk 200521 | ED | Gazi University, Ankara, Turkey | PEFR difference | TSLH in magnesium group (2) and palpitation (1) in salbutamol only group. No other side effect reported (26) | None mentioned | 1 mg/kg prednisolone to all but additional theophylline, anticholinergic drugs and salbutamol given at clinician's discretion |
| Mahajan 200418 | ED | Detroit, USA | Percentage change in FEV1 | No side effects occurred (62) | Having received steroids, ipratropium or theophylline in the last 3 days | 2 mg/kg prednisolone |
| Mangat 199814 | ED | St John's College, Agra, India | PEFR, Fischl index score and admissions | TSLH (1), palpitation (1), tremors (2), all in control group and only one TSLH n in magnesium group (33) | Oral parenteral bronchodilators (6 hours) steroids (last 12 hours) | 100 mg intravenous hydrocortisone |
| Meral 199619 | Not clear | Izmir, Turkey | Percentage change in PEFR | No side effects noted (blood pressure and heart rate monitored) (40) | No medication taken in the previous 12 hours (β2-agonists and theophylline) | No other medication given |
| ASS (Davis–Leffert–Dabbous score)75 | ||||||
| Nannini Jr 200015 | ED | Four hospitals in Argentina | PEFR and admissions | None observed (35) | Oral or parenteral steroids in the last 7 days | None stated |
| Neki 200636 | Not clear | Amritsar, Punjab, India | PEFR, RR and Fischl index | Not commented on (40) | Oral, inhaled or parenteral bronchodilators in past 6 hours and steroids in last 12 hours | All given 100 mg intravenous hydrocortisone |
| Study | Magnesium dose | Mixed with | Control comparison | Mixed with |
|---|---|---|---|---|
| Abreu-Gonzalez 2002,33 24 patients | 2 ml MgSO4 (isotonic), 13 patients | 400 μg salbutamol (? once) | 2 ml of a physiological serum of an inhaled form, 11 patients | 400 μg salbutamol |
| Aggarwal 2006,22 100 patients | 1 ml of 500 mg/ml MgSO4, AS – 29, ALT – 21 | 1 ml salbutamol (? dose) | 7.5 ml normal saline, AS – 30, ALT – 20 | 1 ml salbutamol (? dose) |
| 8 ml distilled water | 1.5 ml distilled water | |||
| (295 mOsm/kg) × 3 in 1 hour (ultrasonic nebuliser) | (287 mOsm/kg) × 3 in 1 hour | |||
| Ashtekar 2008,27 17 patients | 2.5 ml isotonic MgSO4 sulphate (151 mg/dose), seven patients | 500 μg ipratropium bromide | 2.5 ml of isotonic saline, 10 patients | 500 μg ipratropium bromide |
| 2.5 mg salbutamol or 5 mg salbutamol (2–5 and > 5 years) | 2.5 mg salbutamol or 5 mg salbutamol (2–5 and > 5 years) | |||
| Three times in 1 hour | Three times in 1 hour | |||
| Bessmertny 2002,17 74 patients | MgSO4 (384 mg), 34 patients (three withdrawn) | Followed by (i.e. not mixed) salbutamol 2.5 mg/ml | Normal saline (no volume documented), 34 patients (three withdrawn) | Followed by (i.e. not mixed) salbutamol 2.5 mg/ml |
| Three times in 1 hour | Three times in 1 hour | |||
| Dadhich 2005,38 71 patients | Gp A n = 24 salbutamol | No doses in any group | ||
| Gp B n = 26 salbutamol and magnesium | ||||
| Gp C n = 21 magnesium alone | ||||
| Drobina 2006,23 110 patients | 150 mg MgSO4 (0.3 ml of 50% MgSO4 heptahydrate), 60 patients (from Mohammed and Goodacre4) | Salbutamol sulphate (0.5%) 5 mg/ml and 0.5 mg ipratropium bromide (0.02% inhalation solution) | No placebo so volume will be less, i.e. blinding may be an issue, 50 patients (from Goodacre) | Salbutamol sulphate (0.5%) 5 mg/ml) and 0.5 mg ipratropium bromide (0.02% inhalation solution) |
| Unclear how frequent | ||||
| Gallegos-Solórzano 2010,35 112 patients (60 completed) | 3 ml (333 mg) of 10% isotonic MgSO4 (1g/10 ml), 60 randomised, 30 completed | 2.5 mg salbutamol and 500 μg ipratropium | 3 ml of isotonic saline, 52 randomised, 30 completed | 2.5 mg salbutamol and 500 μg ipratropium |
| Three doses in 1 hour | Three doses in 1 hour | |||
| Gaur 2008,34 60 patients | 3 ml (3.2 g%), 30 patients isotonic MgSO4, 30 patients | Salbutamol and ipratropium (no doses cited) | Saline as placebo, 30 patients | Salbutamol and ipratropium (no doses cited) |
| No comment about frequency | No comment about frequency | |||
| Hughes 2003,16 52 patients | 2.5 ml isotonic MgSO4 (250 mmol/l 151 mg), 28 patients | 2.5 mg salbutamol, patients unable to distinguish solutions | 2.5 ml normal saline, 24 patients | 2.5 mg salbutamol |
| Three times every 30 minutes | Three times every 30 minutes | |||
| Khashabi 2008,37 40 patients | Isotonic MgSO4, (? dose), unclear how many | Salbutamol (? dose) | 2.5 ml normal saline, unclear how many | Salbutamol (? dose) |
| Possible two doses | Possibly two doses | |||
| Kokturk 2005,21 26 patients | Isotonic MgSO4 (2.5 ml), 14 patients | Salbutamol (? dose) | 2.5 ml normal saline, 12 patients | Salbutamol (? dose) |
| Three doses in 1 hour then hourly for the rest of the 4 hours) | Three doses in 1 hour then hourly for the rest of the 4 hours | |||
| Mahajan 2004,18 62 patients | 2.5 ml isotonic (6.3%) MgSO4 solution, 31 patients | Salbutamol 2.5mg, one dose only | 2.5 ml normal saline, 31 patients | Salbutamol 2.5 mg, one dose only |
| Mangat 1998,14 33 patients | 3.2% solution MgSO4 = 95 mg, 16 patients | Four doses every 20 minutes | 3 ml (2.5 mg) salbutamol, 17 patients | Four doses every 20 minutes |
| Meral 1996,19 40 patients | 2 ml MgSO4 (280 mmol/l 258 mOsm, pH 6.7), 20 patients | ? one dose given over 10–15 minutes | Salbutamol 2.5 mg in 2.5 ml, 20 patients | ? one dose given over 10–15 minutes |
| Nannini Jr 2000,15 35 patients | 3 ml isotonic MgSO4 (286 mOsm, 7.5%, 225 mg), 19 patients | 0.5 ml 2.5 mg salbutamol, ? one dose given only | 3 ml normal saline, 16 patients | 0.5 ml 2.5 mg salbutamol, ? one dose given only |
| Neki 2006,36 40 patients | 20 patients, 3.2 g% MgSO4, 20 patients | Four doses, 20 minutes apart | 3 ml of ?25 mg salbutamol, 20 patients | Four doses 20 minutes apart |
| Total: 896 randomised but 33 interventions and 25 control subjects withdrawn after randomisation, so total completed studies = 838 | 452 + the Drobina study23 presumed 20 = 472, minus the 33 withdrawals = 439 completed intervention studies | 404 + the Drobina study23 presumed 20 = 424 minus the 25 control subjects withdrawn = 399 completed the control studies |
Appendix 2 Summary of methods of resource-use valuation
| From randomisation to discharge | |
|---|---|
| Intervention | Only the unit costs of magnesium, salbutamol and ipratropium were estimated. No consumable costs were included in total cost estimates. Cost source: BNF 6050 |
| Not all patients received the full dose of the intervention/placebo. Full data were available from the CRF to ensure that all doses were costed appropriately. Dosages were estimated in accordance with age of the child | |
| A&E department visit | All children incurred the cost of an A&E department visit. The cost estimate used in the analysis depended on whether or not the child was admitted to hospital as a result of attendance |
Cost source: PSSRU 201048
|
|
| Hospital stay | Hospital stays were divided into two categories: per diem general medical ward and per diem HDU/PICU |
| The per diem general medical ward cost (£368) was taken from the NHS reference costs 2009–1049 (DZF15F-Asthma without complications without intubation). This closely matched a general ward per diem estimate of £348, provided by the Finance/Accounts Department of Alder Hey Hospital, Liverpool | |
| As the difference between HDU and PICU costs was large, a weighted average of the two costs was estimated | |
| Cost source: NHS reference costs 2009–1049 (Critical Care Paediatric Bed-days) | |
| HDU cost: XB07Z (£868) | |
| PICU cost: XB05Z (£2225) | |
| Weighted average: (£1471.96) | |
| In the base case, total general medical ward stay and total HDU/PICU stay were estimated in terms of hours and minutes. If a child had spent more than 12 hours in a ward, a full per diem cost was applied. If a child had spent < 12 hours in a ward, a half day cost was applied. Full days incurred the full per diem cost | |
| The duration, and therefore cost, of inpatient stay is a key driver in the economic evaluation and required careful consideration in the sensitivity analysis where various approaches were used to test the robustness of the economic evaluation results to changes in the cost of hospital inpatient admission | |
| In the sensitivity analysis, a cost of £392 was used (NHS reference costs 2009–10,49 DZ15E-Asthma with complications without intubation) to estimate the cost of a per diem general medical ward stay; the weighted average cost was replaced by the HDU cost (low estimate) and the PICU cost (high estimate); hours and minutes of inpatient stays on either/both wards were costed exactly, i.e. taking account of fractions of time and, finally, all inpatient stays of < 12 hours were not costed in the analysis | |
| AEs | The cost of concomitant medications used to treat AEs were estimated using Prescription Costs Analysis data (2010).49 The costs of additional days in hospital as a result of an AE were included in the hospital stay costs up until discharge |
| From discharge to 4 weeks post randomisation | |
| Medication costs | All medication costs were estimated using the net ingredient cost per prescription stated in the Prescription Cost Analysis (2010) data.49 For all medications, the total for chemical entity value was used |
| Inhaler costs | All inhaler-related costs were estimated using the net ingredient cost per prescription stated in the Prescription Cost Analysis (2010) data.49 For all items, the total for chemical entity value was used |
| Overnight hospital stay | All overnight stay costs were estimated using per diem general medical ward cost (£368) from the NHS reference costs 2009–1049 (DZF15F-Asthma without complications without intubation). This closely matched a general ward per diem estimate of £348 provided by the Finance/Accounts Department of Alder Hey Hospital, Liverpool |
| Outpatient attendance | All costs were taken from PSSRU Unit Costs of Health Care 201048 |
Outpatient attendance costs were divided into three separate cost categories:
|
|
| Non-hospital costs | A variety of sources were used to estimate non-hospital costs |
The following costs were taken from the Unit Costs of Health Care (PSSRU 2010):48
|
|
| *Cost of telephone call was estimated using the GP surgery to telephone call cost ratio (0.61) using practice nurse surgery visit cost | |
| ** Cost of telephone call was estimated using the GP home visit to telephone call cost ratio (0.18) using health visitor home visit cost | |
The following costs were taken from NHS Reference Costs 2009–2010:49
|
|
| In the sensitivity analysis, the NHS reference cost (2009–10)49 out of hours walk-in appointment cost of £45 was used (VB09Z, category 1 investigation with one to two significant treatments) | |
| Non-NHS costs | |
| Travel | As recorded by the respondent. Travel costs included car parking fees, petrol/fuel costs, public transport fares, taxi fares and ‘other’ costs |
| Travel costs were only estimated in relation to the time period from the child's initial hospital visit up until discharge | |
| Estimates were presented for parent/carer of the child, partner of parent/carer of the child and relatives/friend of the child | |
| Expenses | As recorded by the respondent. Expenses costs were only estimated in relation to the time period from initial hospital visit to discharge |
| Expenses were those costs resulting from lost earnings, child care costs, hospital expenses (e.g. snacks/gifts) and ‘other’ costs | |
| Estimates were presented for parent/carer of the child, partner of parent/carer of the child and relatives/friends of the child | |
| Extras | As recorded by the respondent. Extras were only estimated in relation to the time period from discharge to 4 weeks post randomisation |
| Extras were those costs resulting from visits to the family doctor or hospital, and included travel costs, lost earnings due to taking time off work, child care costs and ‘other’ expenses. Expenses also included a specific ‘other’ cost category, for example, help with housework, telephone bills, special equipment for child or ‘other’ expenses | |
| Estimates were presented for parent/carer of the child, partner of parent/carer of the child and relatives/friends of the child | |
| Over-the-counter medicines | As recorded by the respondent. In a few cases only the names of the medicines were stated. If this medicine had already been mentioned by other respondents then an average cost was used. If the medicine had not already been mentioned by other respondents, then costs were taken from Boots (www.boots.com) or Chemist Direct (www.chemistdirect.co.uk). All internet costs were accessed in 2012 |
Appendix 3 Statistical analysis plan
Introduction
The SAP provides a detailed and comprehensive description of the main, preplanned analyses for the study ‘MAGnesium NEbuliser Trial In Children (MAGNETIC) – A randomised, placebo-controlled study of nebulised magnesium in acute severe asthma in children’. This study is carried out in accordance with the World Medical Association Declaration of Helsinki (1964) and the Tokyo (1975), Venice (1983), Hong Kong (1989) and South Africa (1996) amendments and will be conducted in compliance with the protocol, MCRN CTU Standard Operating Procedures and EU Directive 2001/20/EC, transposed into UK law as the UK Statutory Instrument 2004 No 1031: Medicines for Human Use (Clinical Trials) Regulations 2004.
These planned analyses will be performed by the trial statistician. The analysis results will be described in a statistical analysis report, to be used as the basis of the primary research publications according to the study publication plan.
All analyses are performed with standard statistical software (R or SAS). More specialised software in R will be used in the joint analysis of longitudinal and time to event data. The final analysis data sets, programs and outputs are archived following good clinical practice guidelines (ICHE9). The testing and validation of the statistical analysis programs will be performed following the relevant standard operation procedure.
Design
Study design
This is a multicentre, randomised, placebo-controlled study involving 20–25 sites throughout the UK that plans to recruit 500 children, 250 into each of the study arms. All patients recruited into the study will have standard treatment as per BTS guidelines plus either nebulised MgSO4 (2.5 ml of isotonic nebulised MgSO4) or placebo (2.5 ml of isotonic nebulised saline). Each site randomises patients to one of two treatment arms in a 1 : 1 ratio.
Study objectives
The main objective is to compare the ASS at 1 hour of children with acute severe asthma given nebulised MgSO4 when used as an adjunct to nebulised salbutamol and ipratropium bromide to those given nebulised salbutamol, ipratropium bromide and placebo. The proportion of patients who required a ‘stepping up’ of medication at 1 hour, progression to intravenous treatment, intubation and/or admittance to HDU/PICU will be compared between the two groups.
Secondary objectives are:
Does nebulised MgSO4 used as an adjunct to nebulised salbutamol and ipratropium bromide for 1 hour in children with acute severe asthma, when compared with nebulised salbutamol, ipratropium bromide and placebo, have an effect on:
-
(a) clinical outcomes in terms of additional treatment/management while in hospital
-
(b) length of stay in hospital
-
(c) patient outcomes in terms of quality of life, time off school and health-care resource usage over the following month
-
(d) parent outcomes in terms of time off work over the following month
-
(e) overall cost to the NHS and society.
Primary and secondary outcomes
Primary outcome
Asthma Severity Score after 60 minutes of treatment.
Secondary outcomes
Clinical (during hospitalisation):
-
‘stepping down’ of treatment at 1 hour, i.e. changed to having hourly treatment after the initial three 20-minute nebulisers
-
number and frequency of additional salbutamol administrations
-
length of stay in hospital
-
requirement for intravenous bronchodilator treatment
-
intubation and/or admission to a PICU.
Patient outcomes at follow-up (1 month):
-
paediatric quality of life (PedsQL™) asthma module parental report for all children and self-completion if aged > 5 years, EQ-5D
-
time off school/nursery
-
health-care resource usage (e.g. GP visits, additional prescribing).
Parent outcomes at follow-up (1 month):
-
time off work (related to child's illness).
Inclusion/exclusion criteria
Inclusion criteria
Severe acute asthma as defined by the BTS/SIGN guidelines. 3
For children ≥ 6 years, severe asthma is based on at least one of the following criteria being met:
-
(a) oxygen saturations of < 92% while breathing room air
-
(b) too breathless to talk
-
(c) heart rate greater than 120 b.p.m.
-
(d) respiratory rate of > 30 breaths per minute
-
(e) use of accessory neck muscles.
For children aged 2–5 years, severe asthma is based on at least one of the following criteria being met:
-
(a) oxygen saturations of < 92% while breathing room air
-
(b) too breathless to talk
-
(c) heart rate greater than 130 b.p.m.
-
(d) respiratory rate > 50 breaths per minute
-
(e) use of accessory neck muscles.
Exclusion criteria
-
(a) coexisting respiratory disease such as cystic fibrosis or chronic lung disease of prematurity
-
(b) severe renal disease
-
(c) severe liver disease
-
(d) known to be pregnant
-
(e) known to have had a reaction to magnesium previously
-
(f) parents who are unable to give informed consent
-
(g) previously randomised into MAGNETIC trial
-
(h) patients who present with life-threatening symptoms
-
(i) previously or currently involved with a trial of a medicinal product in the 3 months preceding screening.
Sample size
In order to detect a difference between the two groups at 60 minutes post treatment of 0.5 points on the ASS at a 5% significance level with 80% power, 500 children are required. This assumes an SD of 1.95, based on a similar population in Australia. 30 The SD was estimated from the Cardiff pilot study27 (EudraCT number: 2004–003825–29) to be 1.7. The target of 500 children will stand. ASS can range from 0 to 9. A difference of 0.5 is deemed to be the minimum worthwhile clinically important difference to be detected. It is a relatively small difference given the low cost and perceived good safety profile of the intervention.
Recruitment
The date the first patient recruited was 3 January 2009. Expected date of end of recruitment and expected date of end of follow-up will be 31 October 2010 and 31 December 2010, respectively. There are 30 sites recruiting patients into the trial and the proposed recruitment targets are given in Table 1.
| Recruiting centre | Minimum target accrual per centre |
|---|---|
| Royal Devon and Exeter Hospital | 20 |
| Leicester Royal Infirmary | 20 |
| Royal Albert Edward Infirmary, Wigan | 20 |
| St Thomas' Hospital | 20 |
| Whiston Hospital | 10 |
| Blackpool Victoria Hospital | 20 |
| Countess of Chester Hospital | 10 |
| Birmingham Heartlands Hospital | 20 |
| Bristol Royal Hospital for Children | 20 |
| Birmingham Children's Hospital | 20 |
| Royal London Hospital | 20 |
| Royal Preston Hospital | 20 |
| Derbyshire Children's Hospital | 20 |
| Wythenshawe Hospital | 20 |
| Queens Hospital, Burton on Trent | 20 |
| Ormskirk District General Hospital | 10 |
| Queens Medical Centre, Nottingham | 20 |
| Leighton Hospital | 10 |
| Sheffield Children's Hospital | 20 |
| Macclesfield District General Hospital | 10 |
| Singleton Hospital, Swansea | 10 |
| Royal Aberdeen Children's Hospital | 20 |
| Royal Hospital for Sick Children, Glasgow | 20 |
| Fairfield General Hospital | 20 |
| Tameside General Hospital | 10 |
| Craigavon Area Hospital | 10 |
| North Staffordshire | 20 |
| University Hospital of Wales | 20 |
| Altnagelvin Area Hospital | 10 |
| Antrim Area Hospital | 10 |
Description of study population
Representativeness of study sample and patient throughput
Details of patients assessed for eligibility, those who meet the study inclusion criteria, those who are eligible and randomised, those who are eligible but not randomised (with reasons as far as possible), those who withdraw from the study after randomisation (with reasons as far as possible) and those who are lost to follow-up (with reasons as far as possible) will be summarised in a CONSORT flow diagram. Eligible patients who are randomised will be described with respect to demographic details and history (gender, age at randomisation, age at asthma onset, current asthma medication, allergy history, previous admission for asthma, duration of the most recent asthma attack, treatment/nebulisers received pre-admission and ASS, SaO2, blood pressure, respiratory rate, oxygen therapy at baseline). The number of ineligible patients randomised will be reported.
Baseline comparability of randomised groups
Patients in each treatment group (magnesium and placebo) will be described separately with respect to gender, age at randomisation, age at asthma onset, current asthma medication, allergy history, previous admission for asthma, duration of the most recent asthma attack, treatment/nebulisers/steroids received pre-admission and ASS, SaO2, blood pressure, respiratory rate, oxygen therapy at baseline. Tests of statistical significance will not be undertaken for baseline characteristics; rather the clinical importance of any imbalance will be noted.
Follow-up assessments and losses to follow-up
The number (and percentage) of patients with scheduled follow-up assessments at 20, 40, 60, 120, 180 and 240 minutes post randomisation will be reported by treatment group. The number lost to follow-up within each treatment group will be reported and reasons where known will be documented in the CONSORT flow diagram. Any deaths and their causes will be reported. Any unblinded events will be reported. The rate of patient and parent outcome questionnaires return at one month will be reported by treatment group.
Description of compliance with therapy
In this study, treatment should be directly observed. Deviations from intended treatment (e.g. withdrawals from randomised treatment) will be summarised for each treatment group. The distribution of timing of treatment administration will be summarised by treatment groups.
Trial monitoring
Internal pilot
The SD that was used for the original sample size calculation will be checked after approximately 30 patients have been randomised.
The only outcome data that will be analysed within the interim analyses will be the primary outcome of the study which is defined in the protocol as the ASS after 60 minutes of treatment.
This blinded internal pilot will not have any significant impact on the final analysis. 76
Interim analysis plan
In order to estimate the effect of nebulised MgSO4 for the primary efficacy outcome at each interim and final analysis, the Haybittle–Peto approach will be employed for one interim analysis, planned after approximately 250 children have been randomised, with 99.9% CIs calculated for the effect estimate. This method has been chosen to ensure that interim efficacy results would have to be extreme before early termination is recommended in order to be convincing to the clinical community. The method also minimises controversy regarding interpretation of the results from estimation and hypothesis testing at the final analysis. No inflation factor needs to be applied to the sample size using this approach.
If the trial is stopped early then the analysis will contain all the patients that have been randomised up until that point. The procedures that are described in the statistical quality assurance standard operating procedure will all be implemented before and after the interim analyses.
Unblinding of randomisation treatments
The number of patients who were unblinded will be reported for each treatment group and the reasons as to why they were unblinded will be recorded. Unblinding envelopes for the remaining patients will be checked to ensure they were not opened or tampered with.
Patients groups for analysis
Intention-to-treat analysis of efficacy outcomes
To provide a pragmatic comparison of the policies of the different drug treatments, the principle of invention to treat, as far as is practically possible, will be the main strategy of analysis adopted for the primary end point. These analyses will be conducted on all patients who have primary outcome data, assigned to the two treatment groups – magnesium or placebo as randomised – regardless of the study treatment or non-study treatment received. A sensitivity analysis will be applied for any missing primary outcome data (see Data analysis, Analysis of missing primary outcome data, below).
Analysis of safety outcomes
For the analysis of safety outcomes, all patients who have received at least one dose of the study drug and were available for follow-up will be included. Patients will be included in the treatment group they actually received.
Data analysis
Analysis of primary efficacy outcome
The primary endpoint is the ASS at T60.
The primary analysis will follow the ITT approach. The hypothesis of no difference between the two treatment arms will be tested using ANCOVA. A p-value of 0.05 (5% level) will be used to declare statistical significance and 95% CIs of the estimated effects will be reported. The primary analysis using ANCOVA will not adjust for any missing data. However, reasons for missing outcome data will be reported and a sensitivity analysis will be undertaken (see Data analysis, Analysis of missing primary outcome data, below).
The assumptions that are made when using ANCOVA (i.e. normality of ASS at treatment levels, homogeneity of variance, homogeneity of regression slopes, linear regression) will be assessed. Histogram of ASS will be plotted for checking normality and a suitable transformation (e.g. square root, log) will be considered to correct non-normally distributed data. Levene's test will be used to test the assumption of homogeneity of variance. Assumptions of linear regression (magnitude of the scatter of the points is the same throughout the length of regression line) and homogeneity of regression slopes (direction and strength of this relationship must be similar in each treatment group) will be detected by examining simple scatterplots between ASS and covariates. If unequal variances, non-linearity and/or non-parallel slopes are present, a suitable transformation of ASS will be used to improve the linearity and to promote equality of the variances.
Randomisation is stratified by centre; however, owing to the large number of small centres, centre will not be included in the model as a covariate, and this is due to the fact that including a large number of small centres may lead to unreliable estimates of the treatment effect and p-values that may be too large or too small. 77 To test the robustness of ignoring the centre effect in the primary analysis, sensitivity analyses will be performed. A GLM type II analysis will be carried out with treatment, centre and treatment-by-centre interaction and baseline measurement included as covariates. Centre will be treated as both fixed and random in separate analyses to assess if there is any effect of this assumption. If the sensitivity analysis suggests the results are not robust to how centre is handled in analysis, centre characteristics (e.g. university hospital, DHS, specialist centre) will be explored further.
All longitudinal ASS data collected will be used in a secondary analysis, with a resulting increase in power. Longitudinal ASS data will be summarised by the AUC. The AUC is a summary measure that integrates repeated assessments of a patient's end point over the duration of the treatment. AUC measures preserved discriminant validity in treatment comparisons and reported more precise treatment effect estimates. 78,79 As the study drug is aimed to lower the ASS over three time intervals, AUC is the most appropriate measure for the treatment comparison.
Analysis of secondary efficacy clinical outcomes
The five clinical secondary outcomes of interest are:
-
‘stepping down’ of treatment at 1 hour
-
number and frequency of additional salbutamol administrations
-
length of stay in hospital
-
requirement for intravenous bronchodilator treatment
-
intubation and/or admission to a PICU.
The proportion of patients who required a ‘stepping up’ of medication at 1 hour, progression to intravenous treatment, intubation and/or admittance to HDU/PICU will be compared between the two arms using a chi-squared test. As these are centre-specific outcomes, a sensitivity analysis will be undertaken to account for centre characteristics.
The mean (SD) or median (IQR) of number (frequency) of additional salbutamol administrations will be computed depending on whether it is skewed or not, and compared across treatment groups using a t-test or Mann–Whitney U-test.
Summaries of length of stay in hospital will be presented as means (SDs) or medians (IQRs) depending on whether it is normally distributed or not, and compared across treatment groups.
A formal test of a treatment–covariate interaction will be conducted for the effect of age (2–5 years and ≥ 6 years) by including the interaction term in a regression model. Exploratory analysis will be conducted as to the impact on any treatment effect of other factors such as gender or presenting clinical signs.
A p-value of 0.05 (5% level) will be used to declare statistical significance and 95% CIs of the estimated effects will be reported.
Analysis of secondary outcomes of quality-of-life and health economic measures at 1 month
There are four patient/parent secondary outcomes at 1-month follow-up of interest:
-
paediatric quality of life (PedsQL™) asthma module parental report for all children and self-completion if aged > 5 years, EQ-5D)
-
time off school/nursery
-
health-care resource usage (e.g. GP visits, additional prescribing)
-
time off work (related to child's illness).
Independent-sample t-tests will be used to test for differences in resource use, costs, utility scores (generated by the EQ-5D multiattribute utility measure), and QALYs between treatment groups. All statistical tests will be two-tailed and considered statistically significant at p-value of < 0.05.
Handling missing health economic data
The ICE command within Stata (version 10.0) will be used to impute missing data for economic outcomes. Following the methods of Briggs et al. 56 for handling missing data, five imputed data sets will be generated through multiple imputation using non-parametric bootstrapping80 in Microsoft Excel 2003 (Microsoft Corporation, Redmond, WA, USA) and the results will be combined using equations described by Briggs et al. 56 to calculate SEs around mean costs and effects that incorporate uncertainty around imputed values as well as sampling variation. SEs will be used to calculate 95% CIs around total and incremental costs and QALYs based on Student's t-distribution.
Cost-effectiveness acceptability curves (CEACs)57 showing the probability that nebulised MgSO4 is cost-effective relative to placebo at a range of ceiling ratios will be generated based on the proportion of bootstrap replicates (across all five imputed data sets) with positive incremental net benefits. 58 Incremental net benefit can be defined as the incremental QALY gain multiplied by the ceiling ratio minus the incremental cost58 where the ceiling ratio (or threshold) represents the maximum society is willing or able to pay for each additional QALY. All statements about cost-effectiveness will be based on a £20,000 per QALY gained threshold. The probability of nebulised MgSO4 being less costly or more effective will be based on the proportion of bootstrap replicates that have negative incremental costs or positive incremental benefits, respectively. No discounting will be applied to costs and health effects as the time horizon for the economic evaluation will be < 1 year.
A series of multiway and probabilistic sensitivity analyses will be performed to explore the implications of uncertainty surrounding variables with a degree of uncertainty.
Analysis of missing primary outcome data
Three nebulised study treatments will be given at T0, T20 and T40. The primary analysis will be of the ASS at T60. To investigate how sensitive the results of the primary analysis are to missing data a number of strategies will be used. These sensitivity analyses will involve joint modelling as well as imputing values for missing ASS at T60.
These sensitivity analyses will be carried out as secondary analyses of the study data. The results of these analyses will be compared with the relative effect of missing data on the conclusions of the primary analysis.
Description of missing data
The proportion of patients with missing outcome data will be reported by treatment arm together with reasons for missingness.
Further descriptions of the missing outcome data will be reported in terms of:
-
Differences in key baseline characteristics between treatment arms in those with observed ASS T60.
This description will be used to assess whether the patients with missing outcomes affect the randomisation balance. 81
-
Differences in key baseline characteristics between patients with observed and missing ASS T60.
This description will be used to assess the plausibility of the MCAR assumption. 81
Imputation
If missingness is due to an administrative reason (e.g. staff involved were called to an emergency), missing ASS at T60 will not be imputed. Such values are missing for reasons unrelated to any inference we wish to draw about the intervention and hence MCAR. Otherwise, missing values will be imputed depending on the reason for the data being missing.
-
Impute with worst-case value: If the reason for missingness is related to the patient's poor condition (e.g. death, study withdrawal owing to severity by clinician), the missing ASS at T60 will be replaced by the worst possible score for the ASS. ASS is measured on a scale between 0 and 9 (where severity increases with score); hence a missing value would be replaced with a ‘9’.
-
Impute with best-case value: If missingness is due to study withdrawal by parent/self discharge (e.g. parent felt child was well enough to go home), the missing value is replaced by the lowest score that the patient experiences at T0, T20 and T40.
-
Model-based imputation: If the reason for missingness is not available, missing values will be (multiply) imputed by MICE82 algorithm conditional on all available values at T0, T20 and T40. MICE iterates through values at each time point, modelling each conditional on the others. The imputations themselves are predicted values from a regression model, with the appropriate random error included. MICE is available as an stand-alone package (WinMICE), and also in R (mice library) and SAS. As ASS is a numerical score, imputations can be generated using predictive mean matching (PMM) method.
Both (1) and (2) are ad hoc approaches, so rarely lead to unbiased estimates of the treatment effects. 81,83,84 Approach (3) is based on the MAR (missing at random) assumption. 81
Joint modelling
The problem of non-ignorable missing ASS data will be addressed through a more advanced analysis of joint modelling of the longitudinal data and the time to dropout from the study. 85 In this analysis, patients who did not dropout from the study will be censored at the time of discharge from hospital. Dropout owing to reasons related to treatment will be treated as potentially informative, and dropout due to other reasons as a censored follow-up time.
Mean profile plots will be drawn which provide a visual representation of the variation patients may experience in terms of their ASS over time. By reversing the time axis, variation in ASS of an individual prior to informative dropout from the study will be examined.
Description of safety outcomes
Safety analysis
All AEs and SAEs reported by the clinical investigator will be presented, identified by treatment group. AEs will be grouped according to a pre-specified AE coding system and tabulated. The number (and percentage) of patients experiencing each AE/SAE will be presented for each treatment arm categorised by severity. For each patient, only the maximum severity experienced of each type of AE will be displayed. The number (and percentage) of occurrences of each AE/SAE will also be presented for each treatment arm. No formal statistical testing will be undertaken.
| No. | AE (expected/unexpected) | Severity | Arm | Total no. of patients | |
|---|---|---|---|---|---|
| Treatment A: n (%) | Treatment B: n (%) | ||||
| 1 | Facial flushing (E) | Mild | |||
| Moderate | |||||
| Severe | |||||
| 2 | Tachycardia (U) | Mild | |||
| Moderate | |||||
| Severe | |||||
| No. | Treatment | Description | Severity | Relationship to study drug | Expectedness | Cause | Outcome | Patient status | Unblinded | |
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | |||||||||
| 1 | ||||||||||
| 2 | ||||||||||
Reporting protocol deviations
Protocol deviations will be classified according to the following table and summarised for each treatment group. They will be compared across treatment groups and any imbalance will be investigated.
| Protocol specification | Potential deviation(s) | Impact | Justification (in terms of whether bias is likely in the assessment of response) |
|---|---|---|---|
| Inclusion criteria | |||
For children aged ≥ 6 years, severe asthma is based on at least one of the following criteria being met:
|
None of the specified severe asthma criteria | Major | The severity of asthma is likely to influence response |
For children aged 2–5 years of age, severe asthma is based on at least one of the following criteria being met:
|
None of the specified severe asthma criteria | Major | The severity of asthma is likely to influence response |
| Exclusion criteria | |||
| Patient suffering from life-threatening symptoms | Patient suffering from life-threatening symptoms | Major | Patient may not be able to metabolise drug effectively thus affecting response |
| Patient has co-existing severe renal or liver disease | Patient has co-existing severe renal or liver disease | Major | May affect efficacy of study drug and potentially increase incidence of AEs |
| Patient known to have had a previous reaction to magnesium | Patient known to have had a previous reaction to magnesium | Major | True effect of magnesium on fetus is not known |
| Patient known to be pregnant | Patient known to be pregnant | Major | Co-existing disease may adversely affect efficacy of study drug |
| Patient have co-existing respiratory disease (except asthma) | Patient have co-existing respiratory disease (except asthma) | Major | Cannot be sure of effect of potential drug interactions on efficacy and/or safety of study drug |
| Patient been involved in a trial of a medicinal product within last 30 months | Patient been involved in a trial of a medicinal product within last 3 months | Major | May affect the way of patient response in patient-reported outcomes, which may introduce bias and affect generalisability of results |
| Patient previously been randomised into the MAGNETIC trial | Patient previously been randomised into the MAGNETIC trial | Minor | Arbitrary cut-off level, no physiological reason |
| Patient aged ≥ 16 years | Patient aged ≥ 16 years | Minor | Patient may not be able to metabolise drug effectively thus affecting response |
| Treatment regime | |||
| Allocation | Patient did not receive full trial treatment as per protocol | Major | May affect ASS and outcome data |
| Timing | Deviations outside acceptable timing window (T = 60 + 15 minutes) without explanation | Minor | May shorten or lengthen treatment period |
| TMG to review cases blind to allocation to determine whether minor/major deviation | |||
| Primary outcome data | Deviation in the method of assessment | Major | Introduce bias in the assessment of response |
| Assessment of ASS at T60 | |||
| Secondary outcome data | |||
| ‘Stepping down’ of treatment at 1 hour | Deviation in the method of assessment | Major | Introduce bias in the assessment of response |
| No. and frequency of additional salbutamol administration | |||
| Requirement for intravenous bronchodilator treatment | |||
| Intubation and/or admission to a PICU | |||
| Length of stay in hospital | |||
| Patient and parental outcomes at 1-month follow-up | If the questionnaire is returned too long after 1 month and we are not confident that the data relate to 1 month | Major | Introduce bias in the assessment of response |
Setting results in context of previous research
We will integrate the results of this trial within the context of an up-to-date systematic review of relevant evidence from other trials. 86 We will refer the results of this trial to the latest existing systematic review of nebulised magnesium in children with asthma. 4 This review concluded that further trials of nebulised MgSO4 in children were needed. More recent trials not included in this review will be identified and reviewed.
A1 Changes to Statistical Analysis Plan
Section 7.2: One change
(1) Treatment–covariate interactions
Treatment–covariate interactions were investigated for two clinically important baseline covariates, duration of the most recent asthma attack and SaO2, owing to reasons explained above (see Chapter 3, Assessing the evidence for treatment–covariate interactions, in the report). It was originally planned to conduct a formal test of a treatment–covariate interaction for the effect of age. Although age may affect the response, a number of possible interactions could be argued.
Section 9: One change
(1) Timing of treatment regimes
Protocol deviation was originally defined as deviations outside acceptable timing window (T = 60 + 15 minutes) without explanation. However, because the prescription time of each treatment was reported rather than the time of the end of the third treatment, it was only possible to determine the difference in prescription times between the first and third treatment which should be ≤ 55 (40 + 15) minutes. Therefore, if this timing was > 55 minutes, this was defined as a deviation outside the acceptable window.
Appendix 4 Details of protocol amendments
Final protocol, version 6.1, 18 January 2010
Amendments from version 6.0 (23 July 2009) to version 6.1 (18 January 2010)
Amendments from version 5.0 (19 September 2008) to version 6.0 (23 July 2009)
Amendments from version 4.0 (18 April 2008) to version 5.0 (19 September 2008)
Amendments from version 3.0 (3 March 2008) to version 4.0 (18 April 2008)
Amendments from version 2.0 (18 January 2008) to version 3.0 (03 March 2008)
Amendments from version 1.0 (23 November 2007) to version 2.0 (18 January 2008)
Appendix 5 Description of missing primary outcome data and sensitivity analyses
| Baseline characteristic | Magnesium (n = 228) | Placebo (n = 244) |
|---|---|---|
| Age (years): median (IQR), range | 4.0 (3.0–7.0), 2–15 | 4.0 (3.0–7.0), 1–15 |
| Male, n (%) | 128 (56) | 144 (59) |
| Time of day that randomisation occurred, n (%) | ||
| 0900–1700 | 164 (72) | 161 (66) |
| 1700–2200 | 44 (19) | 57 (23) |
| 2200–0900 | 20 (9) | 26 (11) |
| ASS at baseline | (n = 227) | (n = 243) |
| Mean (SD), range | 5.8 (1.3), 3–9 | 5.8 (1.4), 2–9 |
| Duration of the most recent asthma attack, n (%) | (n = 227) | (n = 242) |
| For the last few days | 48 (21) | 54 (22) |
| For the last 24 hours | 149 (66) | 150 (62) |
| For the last 6 hours or less | 30 (13) | 38 (16) |
| SaO2 (%) | (n = 227) | (n = 241) |
| Mean (SD), range | 93.7 (3.5), 84–100 | 93.4 (3.4), 81–100 |
| Respiratory rate (breaths per minute) | (n = 225) | (n = 238) |
| Mean (SD), range | 43.5 (10.5), 20–72 | 42.4 (10.8), 20–70 |
| Oxygen therapy, n (%) | (n = 222) | (n = 235) |
| Yes | 88 (40) | 94 (40) |
| No | 134 (60) | 141 (60) |
| Baseline characteristic | Observed ASS at T60 (n = 472) | Missing ASS at T60 (n = 36) |
|---|---|---|
| Age (years): median (IQR), range | 4.0 (3.0–7.0), 1–15 | 5.5 (3.0–8.0), 2–13 |
| Male, n (%) | 272 (59) | 21 (57) |
| Time of day that randomisation occurred, n (%) | ||
| 0900–1700 | 325 (69) | 24 (67) |
| 1700–2200 | 101 (21) | 7 (19) |
| 2200–0900 | 46 (10) | 5 (14) |
| ASS at baseline | (n = 470) | (n = 32) |
| Mean (SD), range | 5.8 (1.3), 2–9 | 5.0 (1.3), 2–7 |
| Duration of the most recent asthma attack (N = 469): n (%) | ||
| For the last few days | 102 (22) | 6 (17) |
| For the last 24 hours | 299 (64) | 25 (69) |
| For the last 6 hours or less | 68 (14) | 5 (14) |
| SaO2 (%) | (n = 468) | (n = 35) |
| Mean (SD), range | 93.5 (3.4), 81–100 | 94.4 (3.5), 84–100 |
| Respiratory rate (breaths per minute) | (n = 463) | (n = 34) |
| Mean (SD), range | 43.0 (10.6), 20–72 | 41.6 (11.5), 25–70 |
| Oxygen therapy, n (%) | (n = 457) | (n = 31) |
| Yes | 182 (40) | 10 (32) |
| No | 275 (60) | 21 (68) |
Reasons for exclusion of children from primary outcome analysis
There were 25 children in the magnesium group who did not contribute data for the adjusted analysis of the primary outcome of ASS at T60. There were 13 children in the placebo group who did not contribute data for the adjusted analysis of the primary outcome. Four children (three from the magnesium group and one from the placebo group) could not contribute ASS data at either baseline or T60.
| Reason for missing data | Magnesium | Placebo | ||
|---|---|---|---|---|
| T0 | T60 | T0 | T60 | |
| No. of children | No. of children | No. of children | No. of children | |
| Heart rate was not recorded | 1 | 7 | 0 | 2 |
| Muscle use was not recorded | 1 | 6 | 0 | 4 |
| Wheeze was not recorded | 0 | 2 | 0 | 1 |
| Withdrawn from study | 1 | 4 | 0 | 3a |
| Non-compliance with trial protocol | 1 | 0 | 0 | 0 |
| Reason not known | 0 | 3 | 0 | 2 |
| Data not available | 0 | 2 | 2 | 0 |
| Total | 4b | 24 | 2c | 12 |
Sensitivity analyses of missing primary outcome
Sensitivity analyses were carried out to investigate the robustness of the conclusions concerning the analysis of the primary outcome to assumptions about the missing data. In the analysis in Table 8, it is assumed that the data are missing at random. Sensitivity of results to those cases with missing data for the primary outcome was assessed by three methods.
Sensitivity analysis (1)
First, if the reason for missingness of ASS at T60 was related to good status, the missing value was replaced by ‘0’ (for three children) in the sensitivity analysis; if the reason was related to poor status, it was replaced by ‘9’ (for one child); if the reason was unlikely to be related to status or unknown, it stays as missing (for 32 children). The results of this sensitivity analysis are presented in Table 43.
| Outcome | T60 mean (SD), range | Estimate (95% CI), p-value | ||
|---|---|---|---|---|
| Magnesium: nm = 231 | Placebo: np = 245 | Difference in mean: nm = 231, np = 245 | Adjusted difference in mean: nm = 230, np = 244 | |
| ASS | 4.66 (1.46), 0–9 | 4.97 (1.42), 2–9 | – 0.31 (– 0.57 to – 0.05), p = 0.0183 | – 0.32 (– 0.56 to – 0.08), p = 0.0091 |
The statistical significance of the adjusted analysis remained unchanged; however, the minimum clinically importance difference of 0.5 points is now contained within the 95% CI.
Sensitivity analysis (2)
Secondly, a model-based imputation of MICE (see statistical analysis plan in Appendix 3, Data analysis, Imputation) was used to impute missing ASS values at T60 conditional on all available values at T0, T20 and T40. The R-language library ‘mice’ is used in this analysis. Five imputations were performed in sequence and during each imputation the missing values are imputed, and at the end of the imputations (all five in this case), the values are averaged together to take into account the variance of the missing values. The averaged final data set is used to compute the mean difference in ASS at T60 between the two treatment groups, magnesium minus placebo, adjusting for baseline ASS. The results are presented in Table 44.
| Outcome | T60 mean (SD), range: | Estimate (95% CI), p-value | ||
|---|---|---|---|---|
| Magnesium (nm = 252) | Placebo (np = 256) | Difference in mean (nm = 252, np = 256) | Adjusted difference in mean (nm = 252, np = 256) | |
| ASS | 4.66 (1.37), 2–9 | 4.95 (1.40), 2–9 | – 0.29 (– 0.53 to – 0.04), p = 0.0214 | – 0.28 (– 0.51 to – 0.05), p = 0.0164 |
The statistical significance of the adjusted analysis remained unchanged. The minimum clinically importance difference of 0.5 points is just contained within the 95% CI.
Sensitivity analysis (3)
Third, the problem of non-ignorable missing ASS data was addressed through joint modelling of the longitudinal data and the time to dropout from the study. In this analysis, children who withdrew from the study were considered as ‘dropouts’ and the time (at T0, T20, T40 or T60) they withdrew is taken as the time of event (dropout). Those who did not drop out from the study before T60 were censored at T60. In the joint analysis, dropout was modelled as potentially informative given ASS data. Therefore, the joint model combines the information from the dropout pattern (time-to-event analysis) and ASS over time (longitudinal data analysis).
Figure 3 (see Chapter 3, Area under the curve for asthma severity score over three time intervals) shows the mean longitudinal profiles over T0 to T60. As shown in Figure 3, the mean profiles are almost identical for both magnesium and placebo groups. However, this pattern could be an artefact of selective dropout, and it would be a biased comparison between the groups unless it is adjusted with joint modelling.
Asthma severity score data at T0 were not available for six children and their records were excluded from this analysis. Note that these six observations were not dropouts but rather the first observation over the longitudinal process was missing. There were 40 dropouts (19 at T40, 12 at T20 and 9 at T0) and 462 were censored at T60. The mean profiles prior to dropout are presented in Figure 14, which tends to show that dropout in the magnesium group occurred because patients get better (most children were clinically well and ready to discharge, as shown in Table 15), whereas dropout in placebo occurred is because patients get worse. The results from the joint model are presented in Table 45.
FIGURE 14.
Mean profiles prior to dropout. (a) Magnesium. (b) Placebo.
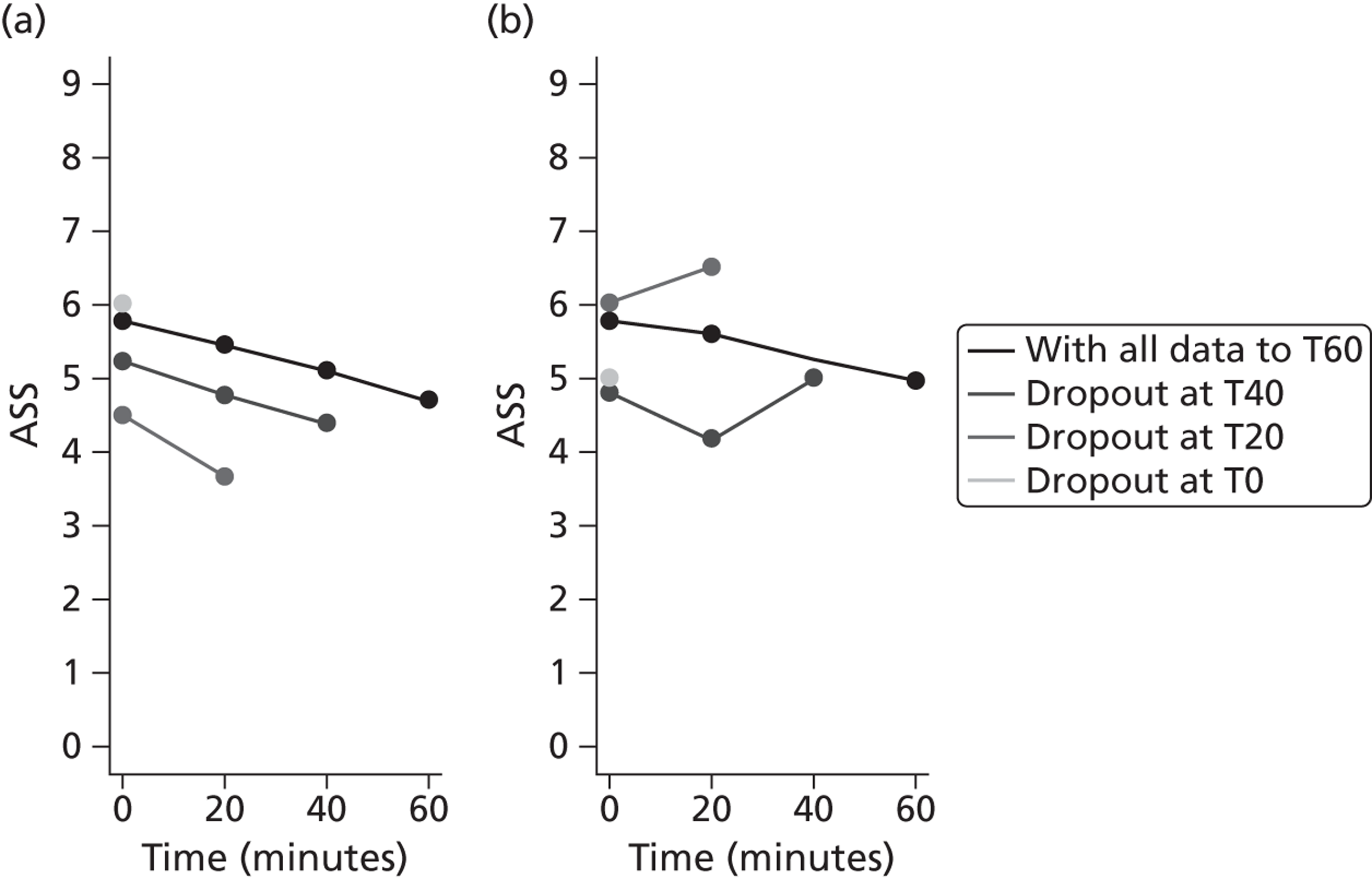
| Variable | Estimate (95% CI) |
|---|---|
| Longitudinal ASS | |
| Intercept | 5.84 (5.69 to 5.99) |
| Time | – 0.02 (– 0.02 to – 0.01) |
| Magnesium | – 0.16 (– 0.34 to 0.05) |
| Dropout | |
| Magnesium | 0.55 (– 0.10 to 1.30), HR = 1.73 (95% CI 0.90 to 3.66) |
| γ | – 0.38 (– 0.75 to – 0.05) |
The joint analysis of longitudinal ASS and dropout show a statistically significant association between ASS and dropout (95% CI for the parameter γ does not include zero).
The relationship between ASS and dropout over entire follow-up is also examined through joint modelling. In this case, the dropout pattern is as follows: 31 at T120, 30 at T180, 27 at T60, 19 at T40, 12 at T20 and 9 at T0, and 374 were censored at T240. The longitudinal mean profiles over T0 to T240 are shown in Figure 15 and the longitudinal mean profiles prior to dropout are shown in Figure 16. Pattern in Figure 15 remains the same as that in Figure 3 over entire follow-up, however comparison of between groups in this setting may be biased as explained above. Figure 16 shows similar pattern to Figure 14 that dropout in the magnesium group is due to children get better and ready to discharge. The results from the joint model are presented in Table 46. The analysis still shows a statistically significant association between ASS and dropout (95% CI for the parameter γ does not include zero).
FIGURE 15.
Mean profiles over entire follow-up.
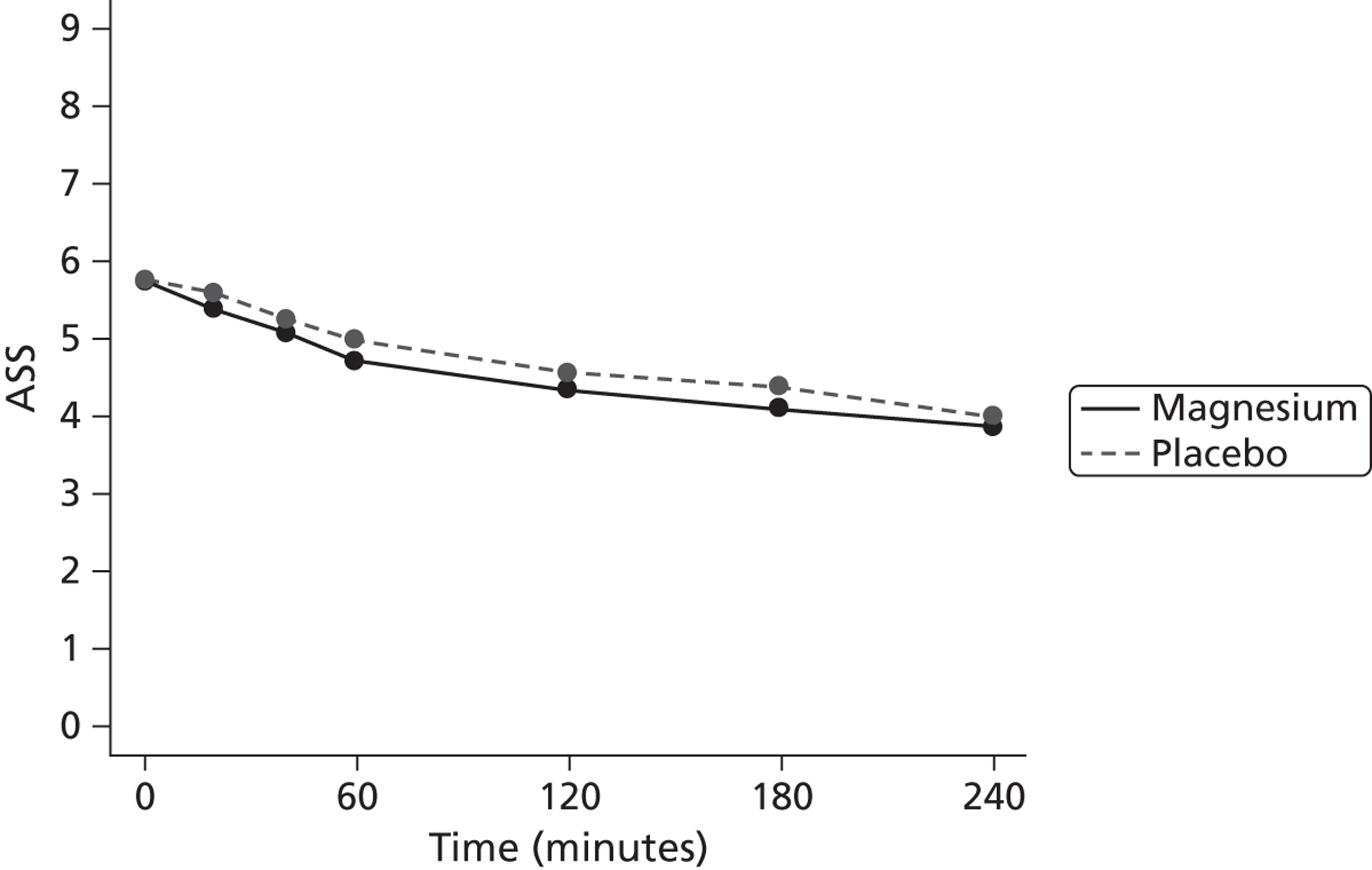
FIGURE 16.
Mean profiles prior to dropout over entire follow-up. (a) Magnesium. (b) Placebo.
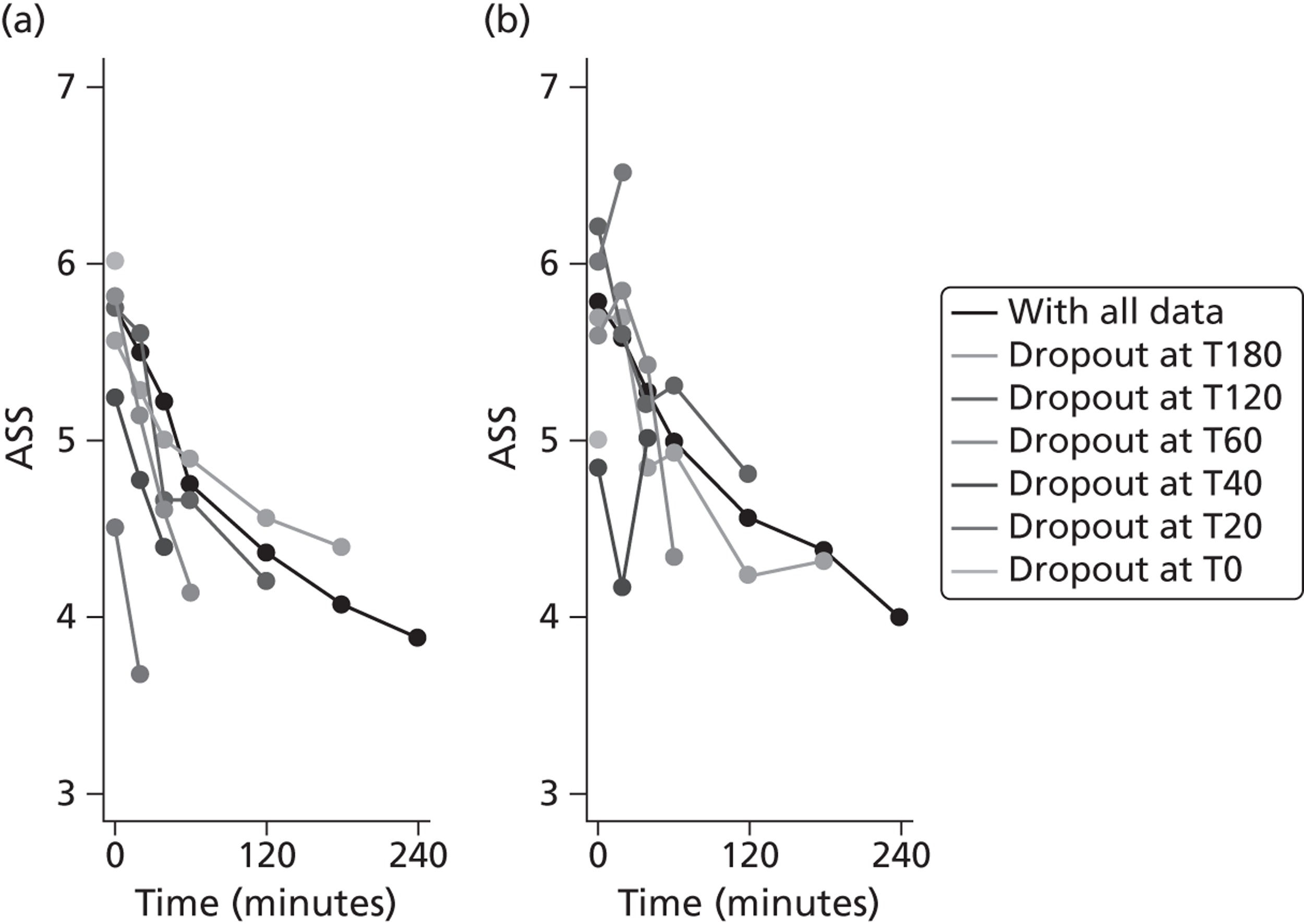
| Variable | Estimate (95% CI) |
|---|---|
| Longitudinal ASS | |
| Intercept | 5.62 (5.47 to 5.75) |
| Time | – 0.01 (– 0.008 to – 0.007) |
| Magnesium | – 0.20 (– 0.40 to – 0.01) |
| Dropout | |
| Magnesium | 0.53 (0.18 to 0.92), HR = 1.70 (95% CI 1.20 to 2.51) |
| γ | – 0.18 (– 0.39 to – 0.002) |
Appendix 6 Sensitivity analyses for centre effect
A sensitivity analysis was performed to investigate the robustness of ignoring any centre effect in the primary analysis. Two models were fitted: in the first model centre was treated as a fixed effect, and in a second model it was treated as a random effect. The second model determines the appropriate F-tests based on centre and treatment–centre interaction being treated as random effects. Type II SS computes the estimates for the main effects. Type III SS computes the estimates for fixed or random centre–treatment interaction effect if entered last into the model. Both models were also adjusted for baseline ASS. The results are presented in Table 47.
Both random-effects analysis of variance and the fixed-effects model indicated significant main effect of centre, but there is no evidence that the treatment effect varies by centre.
| Variable | Model 1: fixed effects | Model 2: random effects | |
|---|---|---|---|
| F-value, Type II SS, p-value | F-value, Type III SS, p-value | F-value, Type III SS, p-value | |
| Treatment | 5.53, 8.47, p = 0.0191 | 1.83, 2.81, p = 0.1766 | 2.38, 2.81, p = 0.1265 |
| Centre | 2.56, 113.87, p < 0.0001 | 2.31, 102.81, p = 0.0002 | 3.61, 102.81, p = 0.0005 |
| ASS at T0 | 66.72, 102.18, p < 0.0001 | 66.72, 102.18, p < 0.0001 | 66.72, 102.18, p < 0.0001 |
| Treatment–centre interaction | 0.64, 28.51, p = 0.9262 | 0.64, 28.51, p = 0.9262 | |
Appendix 7 Diagnostic plots for primary outcome data analysis and histograms of continuous secondary outcomes
FIGURE 17.
Histogram of ASS at T60 to check normality assumption.
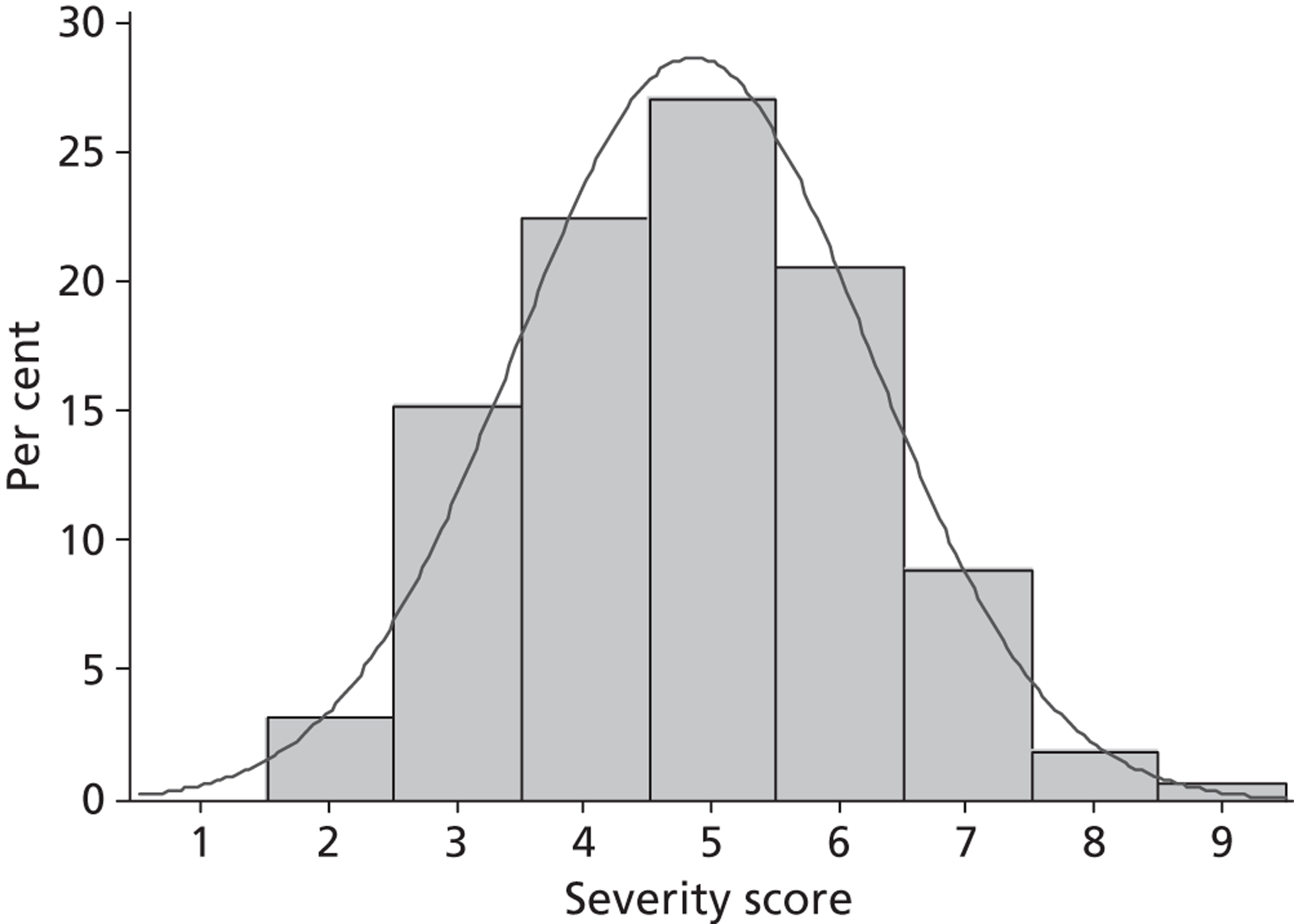
FIGURE 18.
Plot of residuals vs. fitted values to check for heteroscedasticity.
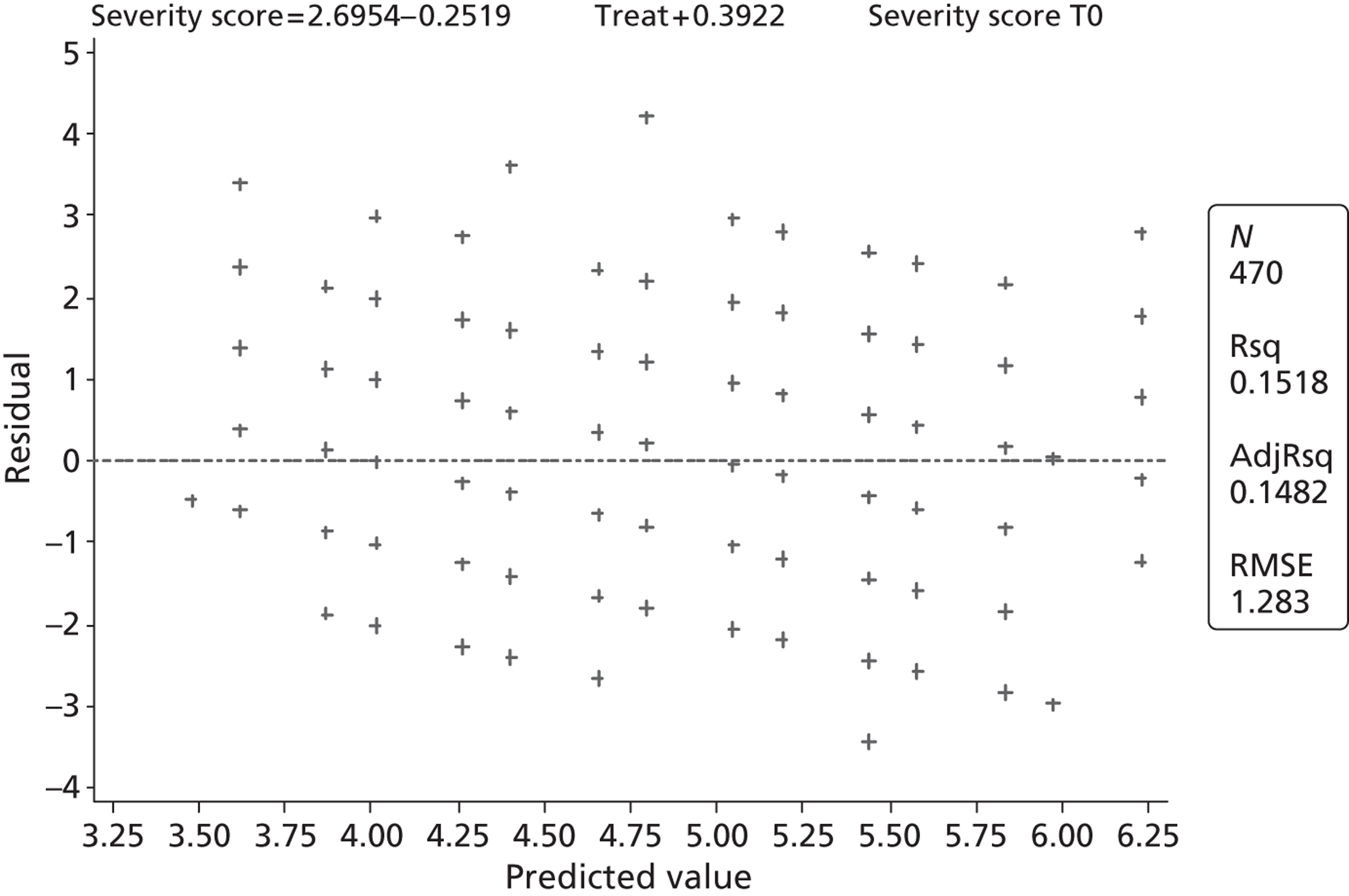
FIGURE 19.
Index plot to check for correlation between observations.
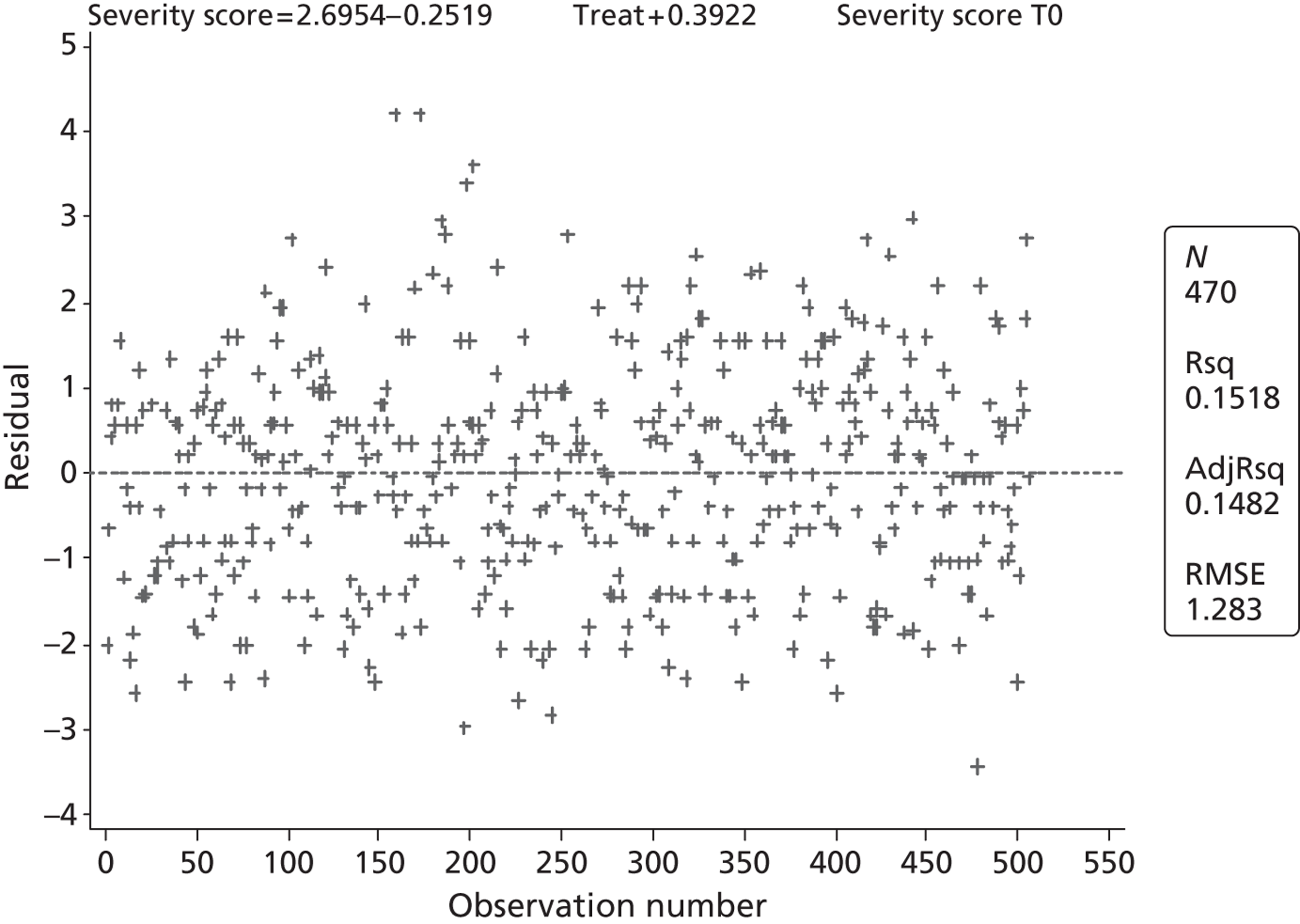
FIGURE 20.
Q–Q plot to check normality of residuals.
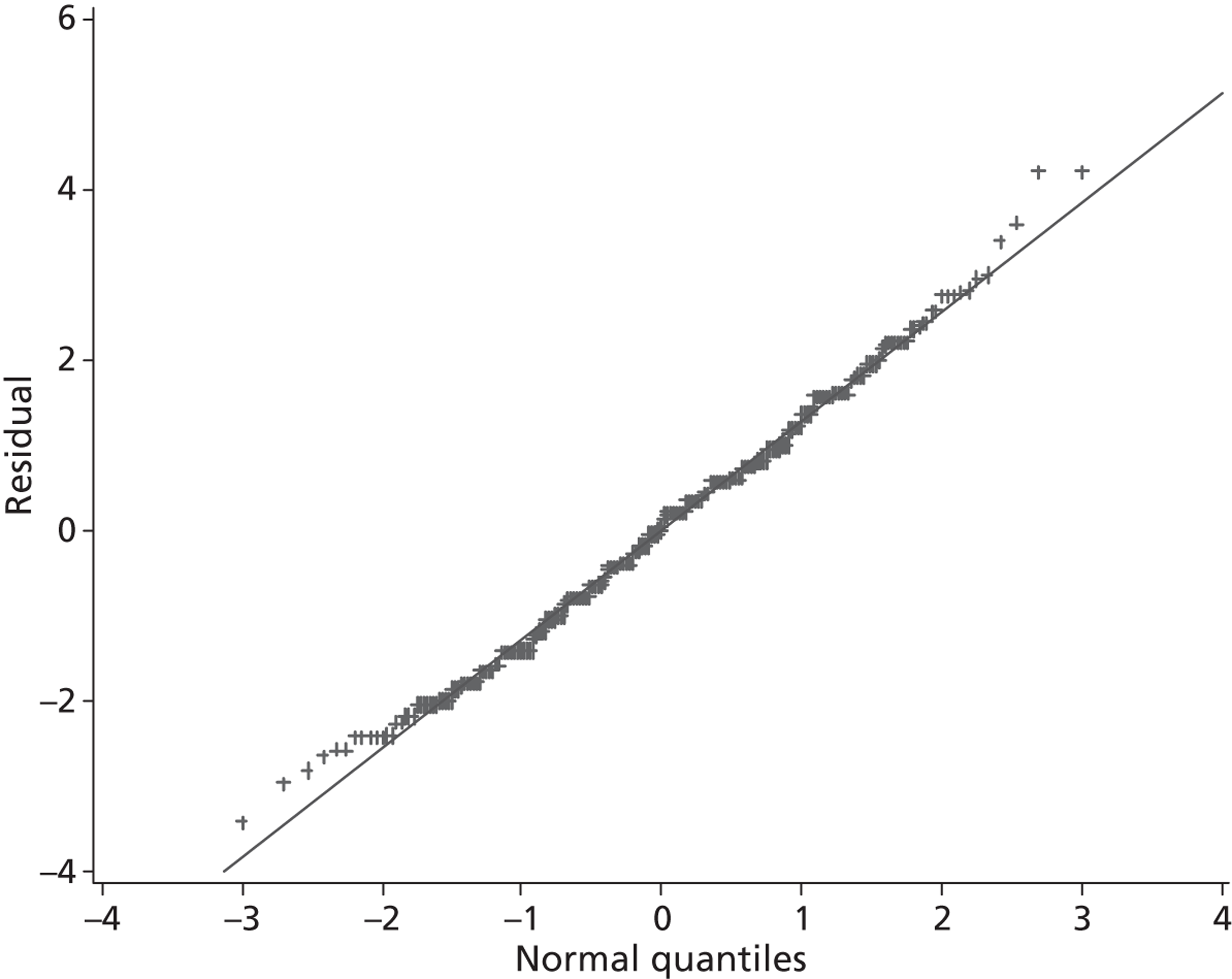
Histograms of continuous secondary outcomes
FIGURE 21.
Number of salbutamol administrations.
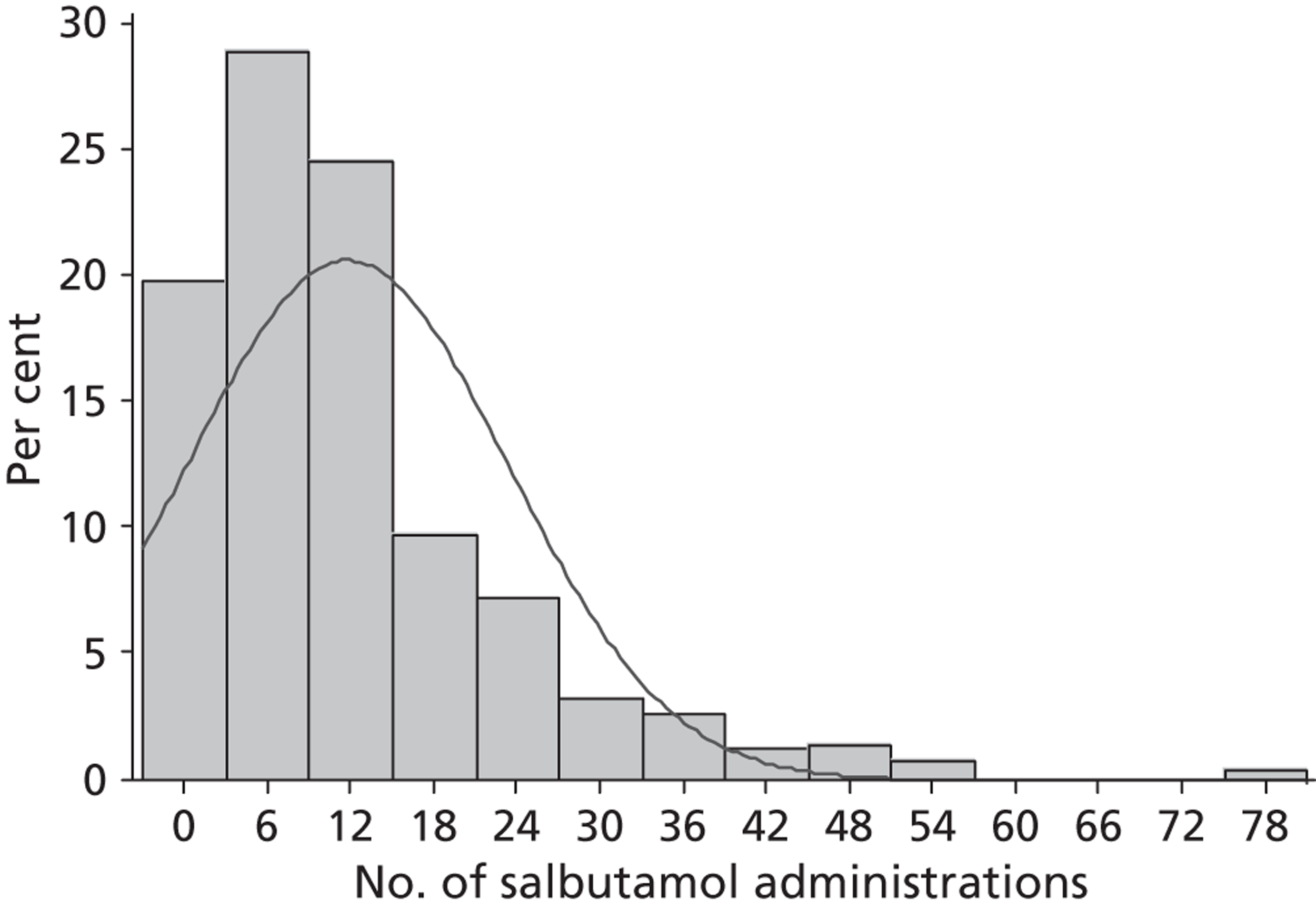
FIGURE 22.
Length of stay in hours.
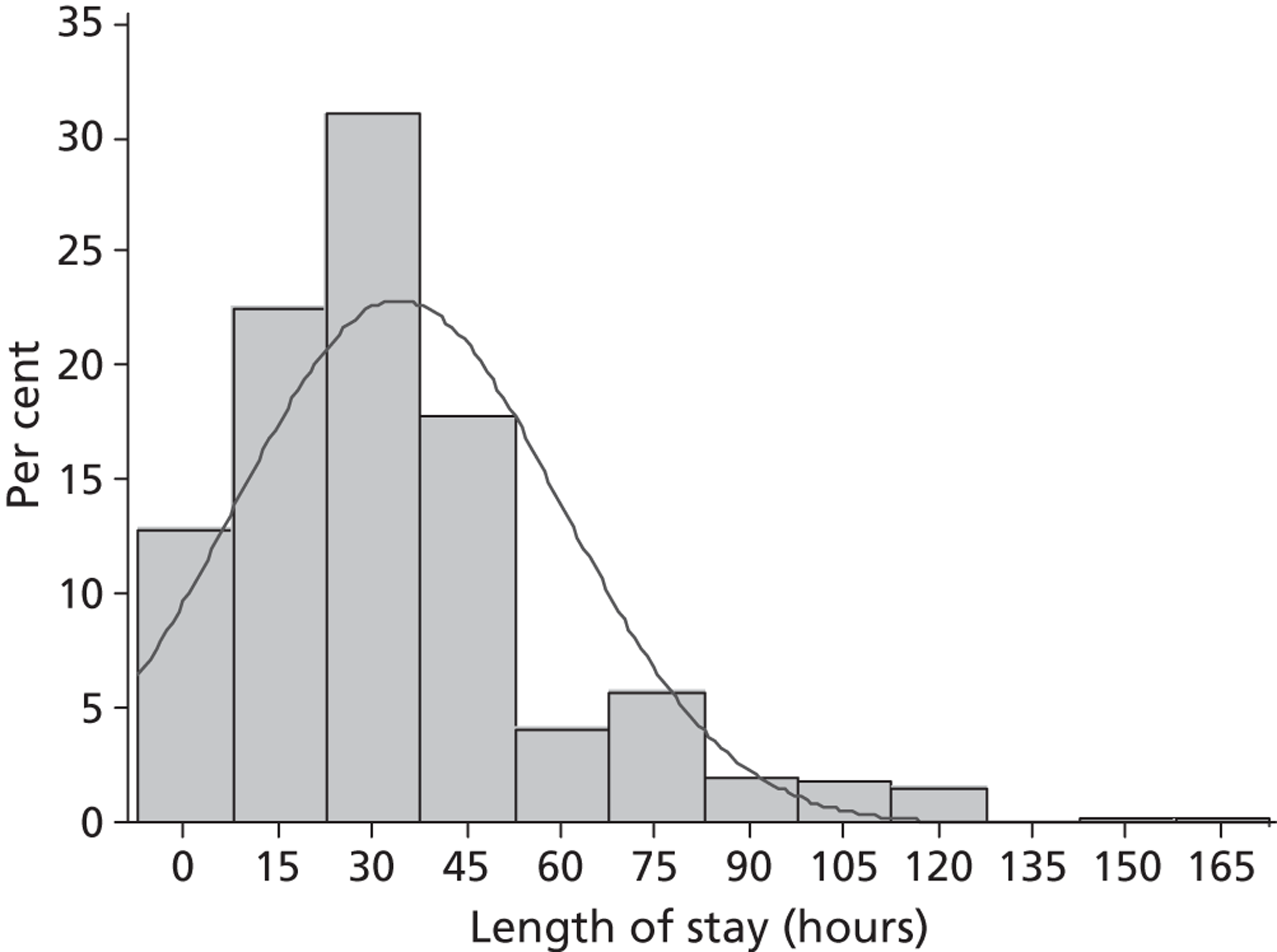
Appendix 8 Patient information sheets
Appendix 9 Health economics questionnaire
Appendix 10 Protocol
List of abbreviations
- A&E
- accident and emergency
- AE
- adverse event
- AIC
- Akaike information criterion
- ANCOVA
- analysis of covariance
- ASS
- Asthma Severity Score
- AUC
- area under the curve
- BNF
- British National Formulary
- b.p.m.
- beats per minute
- BTS
- British Thoracic Society
- CAU
- children's assessment unit
- CEA
- cost-effectiveness analysis
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- CTU
- Clinical Trials Unit (refers to MCRN CTU)
- CUA
- cost–utility analysis
- ED
- emergency department
- EQ-5D
- European Quality of Life-5 Dimensions
- FEV1
- forced expiratory volume in 1 second
- GLM
- generalised linear model
- GM
- general paediatric ward
- GP
- general practitioner
- HDU
- high-dependency unit
- ICER
- incremental cost-effectiveness ratio
- IDSMC
- Independent Data and Safety Monitoring Committee
- IMP
- Investigational Medicinal Product
- IQR
- interquartile range
- ITT
- intention to treat
- LOS
- length of stay
- LRN
- local research network
- MCAR
- missing completely at random
- MDI
- metered dose inhaler
- MCRN
- Medicines for Children Research Network
- MCRN CTU
- Medicines for Children Clinical Trials Unit
- MICE
- Multiple Imputation by Chained Equations
- MgSO4
- magnesium sulphate
- MREC
- Multicentre Research Ethics Committee
- RMSE
- root-mean-squared error
- NICE
- National Institute for Health and Care Excellence
- OLS
- ordinary least squares
- PAU
- paediatric assessment unit
- PedsQL™
- Paediatric Quality of Life Inventory
- PEFR
- peak expiratory flow rate
- PICU
- paediatric intensive care unit
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- RD
- risk difference
- ROAA
- rapid-onset acute asthma
- ROC
- receiver operating characteristic
- RR
- relative risk
- SAE
- serious adverse event
- SaO2
- the saturation level of oxygen in haemoglobin, as measured in arterial blood
- SAP
- statistical analysis plan
- SD
- standard deviation
- SE
- standard error
- SIGN
- Scottish Intercollegiate Guideline Network
- SMD
- standard mean difference
- SOAA
- slow-onset acute asthma
- SUSAR
- suspected unexpected serious adverse reaction
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee