Notes
Article history
This issue of Health Technology Assessment contains a project originally commissioned by the MRC but managed by the Efficacy and Mechanism Evaluation Programme. The EME programme was created as part of the National Institute for Health Research (NIHR) and the Medical Research Council (MRC) coordinated strategy for clinical trials. The EME programme is funded by the MRC and NIHR, with contributions from the CSO in Scotland and NISCHR in Wales and the HSC R&D, Public Health Agency in Northern Ireland. It is managed by the NIHR Evaluation, Trials and Studies Coordinating Centre (NETSCC) based at the University of Southampton.
The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors' report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from the material published in this report.
Permissions
Copyright statement
© Queen's Printer and Controller of HMSO 2013. This work was produced by Forster et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Stroke remains a major health problem in the 21st century, with incidence rates of 1.65 per 1000 population for first-ever strokes. 1 After the recommended hospital admission, most patients are discharged home with some residual disability. 2 There is considerable reliance on informal caregivers, usually family members, to provide assistance with activities of daily living (ADL), including bathing, dressing, and toileting after hospital discharge. 3 For some, this informal care avoids or delays admission to institutional care, and the economic value of the informal care provided is considerable. 4 Indeed, it is suggested that the economic costs of informal care total £2.4B annually, almost equivalent to the hospital and social care costs that total £2.8B annually. 5
This burden of care also has an important effect on caregivers' physical and psychosocial well-being,6 with up to 48% of caregivers reporting health problems, two-thirds a decline in social life7 and high self-reported levels of strain. With the current emphasis on shorter hospital stays, caregivers will play an increasingly important role in the care and continued rehabilitation of patients after stroke. The successful adjustment of patients and their caregivers to the aftermath of stroke is clearly interlinked. Caregivers have an important role in enhancing patients' rehabilitation, and coping strategies that lead to negative experiences are associated with increasing dependence. 8 The caregivers of patients with poor physical and emotional states often have poor emotional outcomes themselves. 9 Effective interventions directed at caregivers of stroke patients are essential, as they may not only improve their own health but may also improve the recovery and adjustment of the stroke patient. 10 However, despite the physical, psychological and social consequences of caregiving, its economic cost to society and its importance in patient recovery, caregivers' central role is often given low priority in the management of stroke. 11
Previous research
A range of systematic reviews of qualitative7,12 and quantitative13 stroke literature have confirmed the diversity, complexity and frequency of problems faced by patients and caregivers during recovery from, and adjustment to, a disabling stroke. A Cochrane review has summarised the effectiveness of non-pharmacological interventions for caregivers of stroke survivors in reducing caregiver burden or enhancing caregiver well-being. 14 In eight randomised controlled trials (RCTs) involving 1007 participants, interventions evaluated were categorised as support and information provision;15–18 caregiver training programme;19 and psychoeducational. 20–24 Three of these studies were inpatient interventions;15,17,19 one was started as inpatient and crossed over into the community,21 two were conducted post discharge in the non-acute phase,18,22 and the location of delivery of two was unclear,16,20 although participants were recruited as inpatients. The comparator in six of the studies was usual care,15–19,21 one study used a crossover wait list design20 and one study used written information on stress management. 22
Only one study – the caregiver training programme19 – was seen to have a significant effect on reducing caregiver burden. The combined results of the support and information provision,15–18 and the psychoeducational20–22 interventions revealed no impact on caregiver burden.
A number of secondary outcomes were examined by the studies. Two studies (one support and information provision16 and one psychoeducational20) assessed global measures of stress or distress, and revealed no significant benefit of the intervention over usual care. One study (caregiver training programme19) assessed anxiety, and the analysis conducted by the reviewers revealed no significant effect of the intervention on caregiver anxiety. Of the five studies measuring depression,16,18–21 only the caregiver training programme19 was seen to have a significant beneficial effect. Three studies15,16,19 (the caregiver training programme19 and two support and information provision interventions15,16) assessed health-related quality of life. The caregiver training programme19 demonstrated a significant improvement in the intervention group, one support and information provision study showed no significant benefit,15 and the second support and information provision study16 showed significant improvements in 5 out of 8 SF-36 health domains. The review authors conclude that, with limited available studies the caregiver training programme19 was the most promising intervention. However, the evidence for this was from one single-centre RCT. 19
The conclusions of the Cochrane review support the findings of earlier reviews of stroke caregiver interventions:10,23,24 that previous studies have methodological limitations; further robust evaluation is required; and the effects of caregiver interventions on the patient also need to be assessed.
In the Cochrane review, the caregiver training programme,19 which included education and some practical ‘hands-on’ skills training, was identified as having the most potential to benefit both caregivers and patients. This is supported by evidence from our Cochrane review of information provision (n = 17 trials), which concluded that stroke education programmes improve patient and carer knowledge of stroke, aspects of patient satisfaction, and reduce patient depression scores. 25,26 There was also some evidence that education interventions that actively involve patients and carers and include planned follow-up for clarification and reinforcement are likely to be more successful than simply providing information. 25,26 Furthermore, caregivers have identified the information and skills training required to implement physical care as their most important pre-discharge needs. 27 Although caregiver support is a key component of stroke unit care, caregivers report that there are missed opportunities for structured skills training prior to the stroke patients discharge,28 and that support, as currently provided, is not compatible with their expressed needs, and their ability to care is not assessed. 10,27
Caregiver training programme
Literature searches conducted by the authors prior to publication of the Cochrane review on the effectiveness of caregiver interventions identified no effective early training programmes for caregivers of patients after stroke, other than the caregiver training programme evaluated by Kalra et al. 19 in an individually randomised single-centre study. This intervention – the London Stroke Carers Training Course (LSCTC) – was a systematic and structured training programme for caregivers, which included assessment in competencies in skills essential for the day-to-day management of disabled stroke survivors. The LSCTC was based on a survey among stroke caregivers, asking them to identify major problems experienced after hospital discharge. Although many reported satisfaction with involvement in discharge planning, most found themselves unprepared for the task of providing ‘hands-on’ care at home. The components of the LSCTC were therefore devised to address the knowledge and skills required to effectively care for stroke patients on discharge from hospital.
The single-centre study participants were 300 patients and caregivers admitted to a stroke rehabilitation unit in south London, UK. 19 Patients and caregivers were block randomised to receive either usual care or the LSCTC prior to discharge home. The primary outcome was the cost to health and social services during the first year of stroke. Total health and social care costs over 1 year were significantly lower in the intervention group, with a mean difference of £4043. 29 This cost difference was largely due to a shorter length of stay for patients in the intervention group than in the control group. There was no difference in quality-adjusted life-years (QALYs) in caregivers. 29 Significant secondary outcomes for caregivers receiving the intervention included a reduction in caregiver burden (as measured by the Caregiver Burden Scale; CBS30), improved quality of life [EuroQol 5-dimension health-state measure: European Quality of Life-5 Dimensions (EQ-5D) visual analogue scale31] and mood [Hospital Anxiety and Depression Scale (HADS)32]. There was no significant difference in social activity levels [Frenchay Activities Index (FAI)33]. For the patients whose caregivers had received the training, there was a significant improvement in quality of life (EQ-5D visual analogue scale) and mood (HADS). No significant differences were seen in patients' physical recovery (Barthel Index34 and modified Rankin Scale35), mortality or institutionalisation. 19
Justification for the current study
There were important limitations to the generalisability of the findings of the study of Kalra et al. 19 The LSCTC was tested in a single centre, delivered by the LSCTC development team, who might be expected to have heightened motivation and expertise, and the patient population was predominantly recruited from a middle-class suburban area, and might be more responsive to a training and education programme. In addition, having demonstrated benefit for caregivers on a range of domains, it was important to evaluate the effectiveness of the LSCTC programme on improving patient outcomes. The aim of the TRACS (Training Caregivers after Stroke) trial was to assess the effectiveness of the LSCTC on patient outcomes (once embedded in usual practice) in stroke units across the UK, thereby testing wider generalisability in settings in which the population, health and social care provision differ.
Chapter 2 The intervention: the London Stroke Carers Training Course
Development of the modified London Stroke Carers Training Course
The LSCTC consists of 14 core caregiver competencies that required training and testing. These competencies were important knowledge/skills that informal caregivers would need to be able to care effectively for the stroke patient on discharge home, for example demonstrating understanding of what a stroke is; knowledge of the patient-specific problems associated with stroke (which may be related to speech, mobility, memory, diet and swallowing, vision and reading, washing and dressing, transfer and walking); knowledge of how to manage and provide support for personal ADLs, including continence management if required. Modification of the LSCTC was required for the multicentre TRACS trial to allow the intervention to be implemented in different NHS settings, and by members of the stroke rehabilitation units' (SRUs') multidisciplinary teams (MDTs) with a range of skills and expertise. The LSCTC was modified and a training programme for staff was developed by the original LSCTC MDT (AM, MW and JS), based on what had worked in the single-centre study and what could be transferred to other settings. The modified LSCTC maintained the original structure of 14 core competencies; six of the training components were listed as mandatory, requiring the MDT to train all caregivers on these items, and the remaining eight components were to be completed as appropriate dependent on each individual patient's ability and their caregiver's needs. A full description of the 14 training components is provided in Table 1. To facilitate replicable delivery, a training manual was created, which described in detail the objective of each training component and recommendations on how to deliver the information/training, resources available (i.e. relevant stroke association information leaflets), and suggested ways to assess the caregivers competency (verbal/observations, etc.). A summary of the underpinning principles was also provided; for example, training should be individualised to the caregivers at the required level and should be based on the needs of the patient. Further modifications were made following pilot use of the modified LSCTC by the SRUs randomised to the intervention.
London Stroke Carers Training Course Caregiver training record
To support the training and standardisation of delivery of the LSCTC, a structured training record was created, which listed the 14 training components and provided a section to indicate whether or not that particular component was mandatory/appropriate for the individual carer. If mandatory/appropriate, the component could be ticked off once the training had been given, and the caregiver's competency could be signed off once assessed. A further section allowed documentation of the total time taken to deliver the training. On the final page, space was provided to document progress of training and so the record acted as a work in progress throughout the patient's stay and a way of communicating training progress and ongoing needs throughout the team. For each caregiver the MDT staff were asked to complete a training record. A copy of the training record can be seen in Appendix 1.
| The caregiver has demonstrated a knowledge and understanding of: | |
|---|---|
| 1 | His/her relative having had a stroke (mandatory) |
| 2 | What a stroke is (mandatory) |
| 3 | His/her relative's specific stroke-related problems. Possible incomplete recovery and residual unresolved problems: |
|
|
| (as appropriate) | |
| 4 | The importance of a healthy lifestyle and secondary preventions: |
|
|
| (mandatory) | |
| 5 | Dietary needs and feeding techniques: |
|
|
| (as appropriate) | |
| 6 | How to communicate with dysphasic relative (as appropriate) |
| 7 | How to manage relative's personal washing, dressing, toiletry needs (as appropriate) |
| 8 | The importance of limb positioning and the management of pressure areas and skin integrity (as appropriate) |
| 9 | Continence management (as appropriate) |
| 10 | Bowel management, fluid and dietary intake for the prevention of constipation (as appropriate) |
| 11 | Appropriate techniques and ability in: |
|
|
| (as appropriate) | |
| 12 | The importance of compliance with medication (including supervision of self-medication or routine medication) (mandatory) |
| 13 | Post-discharge arrangements and where and whom to seek help from after discharge (mandatory) |
| 14 | Adapting the knowledge and skills taught to the home environment following discharge (follow-up visit or telephone call) (mandatory) |
Training of the intervention
Two training sessions were provided for the SRUs randomised to deliver the LSCTC. The training days were delivered by the original LSCTC development team and were held over 2 days, 1 month apart. The same sessions were repeated twice; once in Leeds for the Yorkshire and North West centres and once in London for the London and the South East and South West Peninsula centres. The aim of the first training day was to ensure that the MDTs delivering the LSCTC were clear about what they needed to deliver to the caregivers, and to consider how best to implement the training within their local unit. The day covered, through presentations and group workshops, the background to the LSCTC, the training components and suggested delivery and use of the training records (see Appendix 2). This training day was filmed and provided the basis for a training CD to be used to cascade the training to all staff on the SRUs. Staff were provided with the training manual (see Appendix 3).
After the first session, the attendees were required to cascade the LSCTC training to the rest of the MDT on their SRUs. The LSCTC was then piloted on a small number of caregivers on each SRU, including completion of the training record. A second training day was held approximately 1 month later. This session allowed open discussion on possible refinement of delivery of the LSCTC by the MDT and modification of the training manual and records (see Appendix 2). After this meeting the LSCTC was gradually implemented as a part of standard practice on the SRUs. All centres then received a visit from the TRACS trial manager who used the completed training records as a basis for discussions on structure and process. Further local training sessions were arranged if necessary to provide feedback and support, and discuss any problems with LSCTC provision. The programme was then delivered for a duration of 24 months while the TRACS trial recruitment took place.
Training attendees
The TRACS team suggested that at least two key MDT members from each of the 18 intervention centres attended the initial training days. In practice, 1–13 members from each centre attended, with an average of three attendees per centre. The attendees came from a range of disciplines, primarily senior physiotherapists, senior occupational therapists and senior nurses, but also included Band 5 nurses, consultant physicians and senior speech and language therapists. The attendees were identified as ‘TRACS champions,’ who had responsibility to cascade the intervention within their site.
All centres were offered a local refresher course by the TRACS trial manager midway through the trial in September–October 2008. In total, 13 out of 18 centres received this refresher training; four centres said that they were completing the LSCTC successfully and did not require further training [three such centres did have good return rates of the training records, one centre (294) had not yet returned the records so compliance could not be assessed]. One centre remained non-compliant and refused further training. As the TRACS trial recruitment period was extended for an additional 10 months, a further central training day was provided in London in August 2009; staff from 11 centres attended this day.
London Stroke Carers Training Course delivery
The LSCTC was designed to be delivered to caregivers while the patient was an inpatient with one ‘follow through’ session provided in person or by telephone after hospital discharge. The training was individualised to the caregivers' required level of understanding. The timing of the sessions was not dictated, and could begin at anytime from admission, and throughout the patients stay, depending on when the team felt it appropriate for that particular caregiver, along with other factors, such as varying lengths of stay and local procedures. It was recognised that different components could be covered in a number of different ways, by different professionals depending on individual circumstances.
A key component of the LSCTC was the requirement to check each caregiver's competency on each of the training components delivered and for an appropriate member of the MDT to ‘sign off’ the competency as achieved. Competency was defined as ‘The caregiver has taken on board the knowledge/skills required to be able to deliver the support that that patient needs’. Training would continue until the caregiver was deemed competent (or until it was agreed by the MDT that the caregiver was unable to become competent). This allowed the level of training to be both individualised to the caregiver and standardised across SRUs.
Monitoring of delivery
The completed LSCTC training record for all trial participants was returned to the trial manager and was included as a standard monitoring report to the Trial Management Group (TMG) and Trial Steering Committee (TSC). This enabled monitoring of compliance with the intervention delivery in the SRUs, and the time taken and competencies achieved by each caregiver. In instances where there were concerns about SRU compliance, the trial manager directly engaged with the sites to explore difficulties. The chief investigator wrote to the local principal investigators (PIs) of two units to express concerns.
Chapter 3 Methods
Trial design
Training Caregivers After Stroke was a pragmatic, multicentre, cluster RCT designed to evaluate whether or not a structured, competency-based training programme for caregivers (LSCTC) improved physical and psychological outcomes for patients and their caregivers after disabling stroke and to determine if such a training programme was cost-effective.
Training Caregivers After Stroke was designed as a pragmatic trial. Thus the eligibility criteria were broad and inclusive, the intervention was highly flexible in application and was delivered in a full range of SRUs by the local MDT staff. Monitoring of participant and practitioner compliance/adherence was unobtrusive with no special strategies to improve compliance. Outcomes were objective and meaningful to patients. The primary analysis was intention to treat (ITT) as a test of whether or not the treatment worked in the context of all inherent real-life noise. 36
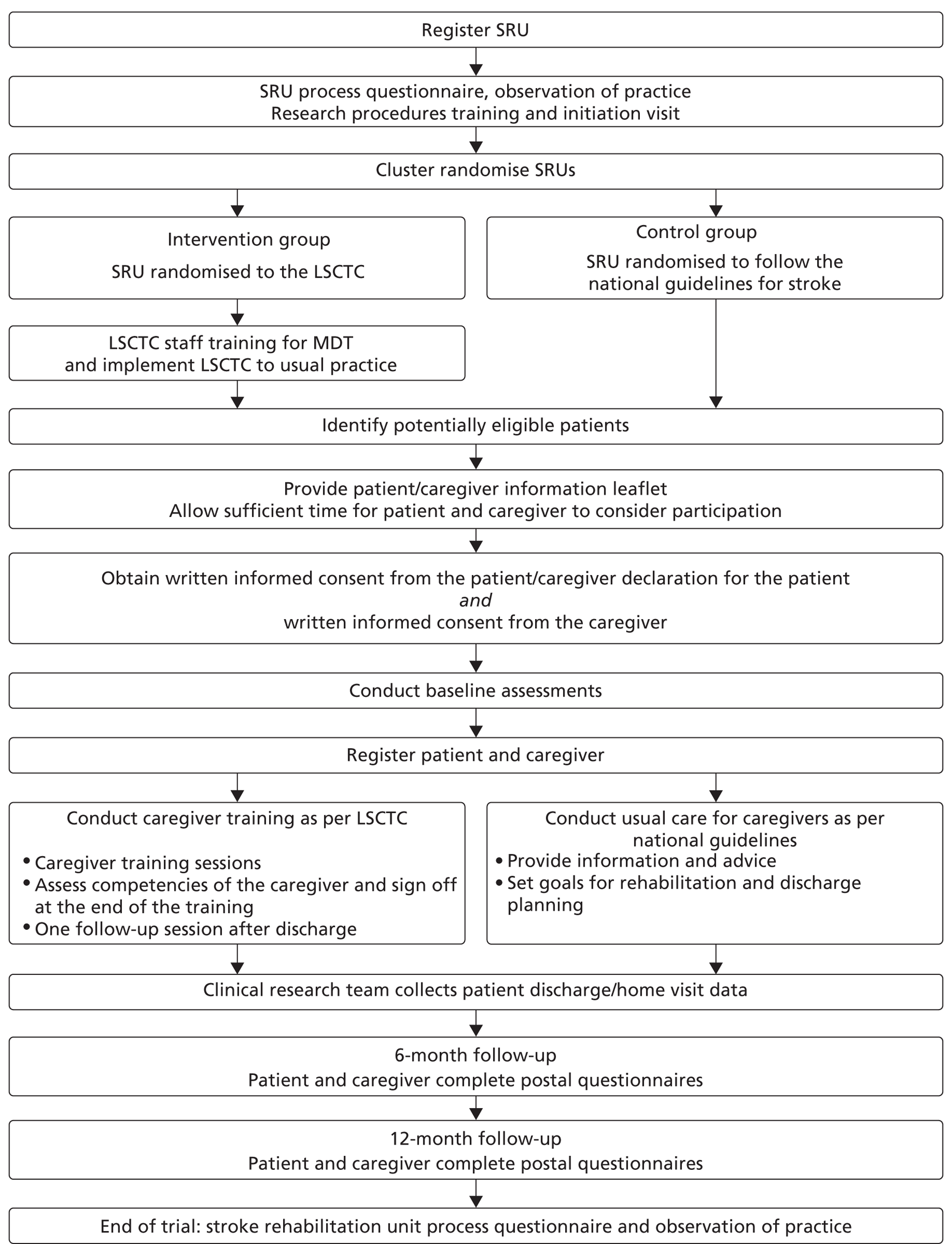
Figure 1 summarises the study methods, and the study protocol can be viewed in Appendix 4.
Justification of a cluster randomised design
The cluster randomised trial design was purposely selected to reduce between-group treatment contamination. Within the pragmatic trial, the LSCTC intervention was incorporated into usual practice and delivered by the whole MDT. If randomisation had been at the level of individual patients, the MDT would have had to operate two approaches (usual care and the LSCTC) with an associated high risk of between-group contamination as it would not have been possible to blind members of the MDT, thus it seemed likely that the new care process would have been extended to patients in the usual care group. Randomisation was therefore at the level of the (service) stroke unit. In order to minimise selection bias, there was a clear separation between the provision of the intervention by clinical staff and the recruitment and consent of patients and caregivers by research practitioners.
Primary objectives
The primary patient objective of the trial was to determine whether or not the provision of the LSCTC improved functional independence. The primary caregiver objective was to determine whether or not the provision of the LSCTC reduced burden for caregivers.
Secondary objectives
The secondary objectives were to determine whether or not the provision of the LSCTC (1) improved physical and psychological patient outcomes in the long term, (2) improved physical and psychological caregivers outcomes and (3) was cost-effective based on (a) patient outcomes, from both health/social care and societal perspectives and (b) caregiver outcomes, from a health-care perspective.
FIGURE 1.
Study flow chart.
Stroke unit (cluster) eligibility
Stroke rehabilitation units were eligible to participate in the TRACS trial if they met four out of five key criteria used to define a stroke unit, as suggested by the Royal College of Physicians of London (RCP) for the National Sentinel Stroke Audit (NSSA) 2006. 37 The five key criteria are (1) consultant physician with responsibility for stroke, (2) formal links with patient and caregiver organisations, (3) MDT meetings at least weekly to plan patient care, (4) provision of information to patients about stroke and (5) continuing education programmes for staff. Additional eligibility criteria were that a substantial number of patients on the unit had a diagnosis of stroke, the unit was able to deliver the LSCTC and the majority of patients were discharged to a permanent place of residence.
Randomisation and stratification
Cluster randomisation of the 36 eligible SRUs was performed centrally at the Clinical Trials Research Unit (CTRU). SRUs were randomised on a 1:1 basis to either the intervention or the control group. The randomisation was stratified by geographical region (Yorkshire, the North West, the South West Peninsula, and London and the South East) and quality of care (defined as being on and above, or below, the median on the key 12-indicator score of the 2006 NSSA37). Block randomisation was used to ensure these important covariates were balanced between the arms of the trial.
Intervention units
In SRUs randomised to the intervention group, usual care was augmented by provision of a modified LSCTC programme (as described in Chapter 2), incorporated into ward practice and delivered to all patients on the SRU if a caregiver was available. Recruitment was opened 4–6 months after the initial training meeting, providing sufficient time for the implementation of the LSCTC into standard ward practice.
Control units
Stroke Rehabilitation Units randomised to the control arm were asked to continue with their usual care, based on National Clinical Guidelines. 38,39 As a minimum, this care involved:
-
weekly MDT meetings
-
information provision to patients and carers
-
ad hoc training of skills to caregivers (e.g. percutaneous endoscopic gastrostomy feeds, transfers, etc.).
Stroke rehabilitation units in the control arm opened to recruitment at the same time as the intervention centres.
Details of usual involvement of patients and caregivers on the SRUs was collected via interviews with senior staff prior to randomisation, and during and at the end of participant recruitment (details below).
Process information
Process data were collected before, during and after recruitment at each participating SRU to monitor any changes in eligibility in SRUs, and in the process of care that prepared patients and caregivers for discharge in SRUs. Data were collected on the NSSA scores completed during the trial (2006 and 2008); ward type (combined acute and rehabilitation or rehabilitation); number of stroke beds; MDT staff ratios; use of community and early supported discharge stroke teams; and usual MDT working. Senior MDT staff (where possible, therapy and nursing staff) were asked open-ended questions to describe usual ward practice and discharge preparation, and how patients and caregivers were involved with this. Responses were recorded on the first visit, and on following visits the initial responses were cross-checked with the new responses and any changes/additions/losses to service updated. Any such changes were monitored by the trial manager through visits to the centres and discussions with the researchers, PIs and MDTs, and were reported back to the TMG for discussion and decision-making.
Participant eligibility
Patients were eligible for TRACS if they had a confirmed primary diagnosis of new stroke, were medically stable, were likely to return home with residual disability, and had a caregiver available, willing and able to provide support after discharge. The caregiver was defined as the main person, other than health, social, or voluntary care provider, helping with ADL and/or advocating on behalf of the patient. Written informed patient consent/caregiver declaration and caregiver consent were obtained prior to any trial-specific procedures. Patient and caregiver dyads were excluded if the patient was in need of palliative care, if discharge was planned within 1 week of admission to the SRU, or if the patient or caregiver was previously registered to the trial.
Blinding
Participant recruitment and baseline assessments were undertaken by researchers independent of the clinical MDT. The clinical MDTs in both the intervention and control arms conducted the LSCTC/usual care with all eligible patients' caregivers whether or not they consented to study procedures. The MDTs were not informed of which patients/caregivers consented to study procedures. Participants were blinded to the SRUs allocation.
Participant recruitment
Screening
All patients admitted to the SRUs were screened for eligibility for the TRACS trial. The researchers completed a screening log that included reasons why patients were not eligible, reasons for which patients/caregivers did not consent and length of stay and anonymous patient demographic information (age, ethnicity, usual living circumstances, and relationship to caregiver). The screening data permitted monitoring of rates of identification, recruitment and refusals at all sites, as well as a comparison of the patient populations being admitted and recruited into the two arms of the study.
Recruitment
To avoid selection bias, participants were recruited by researchers from the Stroke Research Network (SRN) who were independent of the clinical MDT. Where researchers worked part-time as clinical staff, they were not permitted to recruit into TRACS, and other SRN researchers came into that SRU at least weekly to screen and recruit participants into the study.
Written informed consent was obtained from both patients and caregivers. When patients were unable to provide written consent owing to stroke-related disability and/or a lack of mental capacity, a caregiver declaration was obtained. For patients who were unable to consent for themselves, this study complies with the Mental Capacity Act 2005. 40 In such cases, the caregiver acted as consultee.
Patients and caregivers in both arms of the study consented to data collection and questionnaire completion.
Registration
To be registered into the study, the patient and caregiver dyad must have provided written informed consent and completed the baseline questionnaires. The researcher was also required to collect all necessary baseline information after consent but prior to registration. Registration was performed centrally using an automated 24-hour telephone registration system at the CTRU, University of Leeds, Leeds, UK.
Withdrawal
Patients and caregivers were free to withdraw at any time from the study without giving reasons and without prejudicing the patient's treatment. Where patients or caregivers requested to withdraw from the study procedures, there was clarification of whether this was withdrawal from postal follow-up or from medical records searches or both.
Primary outcomes
The primary patient outcome was functional independence measured at 6 months using the Nottingham Extended Activities of Daily Living (NEADL) scale. 41,42
The primary caregiver outcome was caregiver burden measured at 6 months using the CBS. 30
Secondary outcomes
Secondary patient outcomes included self-reported measures of mood (HADS32); health state (EQ-5D);31,43,44 ADLs (Barthel Index);34,45 functional ability and health-related quality of life [Stroke Impact Scale (SIS)];46–51 death; hospital readmission and institutionalisation, all measured at both 6 and 12 months after recruitment, and functional independence (NEADL) at 12 months.
Secondary caregiver outcomes included self-reported measures of social restriction (FAI);33,52 mood (HADS); health state (EQ-5D); death; hospitalisation and institutionalisation at 6 and 12 months, and caregiver burden (CBS) at 12 months.
The cost-effectiveness of the LSCTC for both patients and caregivers was also assessed. Resource use was measured using the self-completed Client Service Receipt Inventory (CSRI). 29,53 Hospital records were checked for patient hospital readmissions and caregiver hospital admissions at 6 and 12 months post registration.
Assessment instruments
Nottingham Extended Activities of Daily Living Scale
Functional independence was measured using the NEADL scale. 41,42 It was designed as a postal questionnaire and assesses aspects of physical and social independence performance across 22 items [score range is from 0 (low independence) to 66 (high independence)] grouped in four categories (mobility, kitchen, domestic and leisure activities). It has been widely used as an outcome measure in rehabilitation trials. 54,55 It has proven validity, reliability56 and has demonstrated responsiveness to change and able to discriminate between services. 57
Hospital Anxiety and Depression Scale
Both patients and caregivers mood was assessed using the 14-component HADS. 32 It was initially developed as an instrument to identify anxiety disorders and depression in medical outpatients,32 but has since proven to exhibit wider generalisability. 58 HADS score is reported from 0 (normal level of anxiety/depression) to 21 (abnormal level of anxiety/depression).
EuroQol 5-dimension health-state measure
The non-disease-specific EQ-5D instrument31,43,44 was used to evaluate health-related quality of life of both patients and caregivers via a six-component questionnaire. It was developed to yield a fundamental index of health, which can be used to calculate QALY gains and, thus, facilitates the health economic evaluation. EQ-5D is scored from –0.59 (worst possible health state) to 1 (full health).
Barthel Index
Patient ADL and mobility were assessed using the Barthel Index. 34,45 This instrument was used to evaluate patients' disability and level of dependence on their caregiver via assessment of their ability in bathing, transferring from bed to chair, dressing, feeding, mobility, climbing stairs, toilet use, grooming, and bladder and bowl continence. Barthel Index is scored 0 (dependent) to 20 (independent).
Stroke Impact Scale
Functional ability and health-related quality of life of the patients was measured using the SIS. 46,47,50,51 This scale consists of eight components measuring strength, memory and thinking, emotion, communication, activities and independent ADL, mobility, hand function, and social participation. It was developed for use as a self-reporting questionnaire, which has proven to be reliable, valid and sensitive to change. 49,51 SIS has also been validated for use as a postal questionnaire. 48 Each domain is scored from 0 (worst) to 100 (best).
Client Service Receipt Inventory
Data on patient sociodemographics and use of health and other formal care services and informal care were collected using a CSRI validated for use with stroke patients. 29,53 A reduced form of this instrument was used with caregivers.
Caregiver Burden Scale
Caregiver burden was measured using a proven and reliable CBS. 30 This 22-item scale assesses various aspects of caregiver burden including general strain, isolation, disappointment, emotional involvement and environment. CBS is scored from 22 to 88, with a higher score representing a more subjective burden.
Frenchay Activities Index
The social restriction on caregivers was assessed using the FAI. 33,52 Although initially validated to assess the activities of acute stoke patients, this assessment instrument is applicable to caregivers of patients with disabling stroke. 19 FAI scores are from 0 (inactive) to 45 (highly active).
Caregiver time logs and training records
Multidisciplinary team staff in the control centres were requested to complete caregiver time logs (see Appendix 1) for two periods of 3 months during recruitment. These logs recorded the time that all MDT staff spent with patients' caregivers. MDT staff in the intervention centres were requested to complete training records (see Appendix 1) throughout the trial as a part of the LSCTC intervention. The training records logged the time that all MDT staff spent delivering the LSCTC with patient's caregivers. The control and intervention logs of MDT time were used to assess the costs associated with delivering the LSCTC. The training records were also used to monitor compliance with the LSCTC in each intervention centre. Caregiver time logs and training records were completed on all eligible patients' caregivers; the logs for caregivers who did not consent to trial procedures were returned to the trial team without any identification on them.
Further data on the costs of implementing the LSCTC were collected by logging the time taken for all MDT staff to attend the training days and for all MDT staff to cascade/receive cascaded training on the SRUs.
Baseline and follow-up data
Baseline patient information collected included demographic details (age, sex, ethnicity, living circumstances, relationship of patient to the caregiver, education and employment), pre-stroke Barthel Index, modified Rankin Scale, classification of stroke, language ability, Six-item Cognitive Impairment Test (6CIT)59 and the Edinburgh stroke case-mix factors. 60 Caregiver information collected included demographic details (age, sex, ethnicity, education and employment) and modified Rankin Scale. The baseline patient questionnaire included pre-stroke NEADL scale, HADS, EQ-5D, Barthel Index, SIS46–48,50,51 and CSRI. The caregiver questionnaire included FAI, HADS, EQ-5D and the CSRI (details below).
The 6- and 12-month questionnaires for the patients collected the same measurements as the baseline questionnaire. At 12 months an additional question was included to ask if the patient had been aware of receiving different treatment because of this research. The 6- and 12-month questionnaires for the caregivers collected the same measurements as the baseline questionnaire, and also included the CBS and questions relating to caregiver preparation to care for their relative/friend at the time of their discharge from hospital (6 months only); if the caregiver was still caring for the patient; caregiver's stroke knowledge;61,62 and if the caregiver had been aware of receiving/being denied an enhanced training package (at 12 months only).
Researchers at each site collected patient and caregiver hospital readmissions and deaths using the local health records.
Procedures for data collection
Baseline questionnaires were completed by the patient and caregiver after consent, but prior to registration. Where patients were unable to complete the baseline questionnaire owing to stroke-related disabilities, a friend/relative could complete the questionnaire using the patient's verbal responses. Where the patient could not understand the questions and/or communicate responses, a friend/relative could complete the questionnaire on their behalf. Details of proxy completion were collected and comparisons between the two arms of the study were undertaken. MDT staff and the researchers were not permitted to help with questionnaire completion. Data were collected by the researcher after consent but prior to registration from both the patient and the caregiver. Discharge details and the occurrence of any expected adverse events (AEs) or serious AEs (SAEs) were collected at the point of discharge.
Patients and caregivers were followed-up by postal questionnaires at 6 and 12 months post registration administered by CTRU. CTRU staff were blinded to the allocation of the SRUs.
Postal reminders were used if the questionnaires were not returned within 2 weeks, followed by a telephone reminder 2 weeks later if the questionnaire had still not been received. If the questionnaire was still outstanding, then, where possible, a telephone interview was conducted to obtain the primary outcomes. General practitioner (GP) checks confirmed that the patient and caregiver were alive prior to contact. If the patient had died, then no further follow-up was undertaken. If the caregiver had died, then patient follow-up was still undertaken.
The cut-off point for questionnaires to be considered for primary end point at 6 months was set at 10 months since registration: follow-up for patients and caregivers started at 6 months post registration.
Intervention compliance
The training records were used to evaluate each intervention centres compliance with the intervention. A definition of compliance was agreed by the LSCTC development team independent of the research team. Following consideration of what was felt to be a minimal acceptable level of training input for each caregiver and recognising that heterogeneity of the patient and caregiver dyads. Compliance was defined as follows:
It was indicated on the training record that training on all six mandatory components was delivered and competency achieved by the caregiver, and/or the training record was signed off by a member of the MDT, indicating that all necessary training had been delivered and competency achieved.
Sample size
The original target recruitment was 900 patient and caregiver dyads, 25 dyads from 36 SRUs. The sample size calculations assumed that a clinically relevant difference was six points [as defined in the Trial of Occupational Therapy And Leisure (TOTAL) study54,63] in the patient primary outcome measure (NEADL). A range of three to nine points was taken to be a clinically relevant difference in previous studies. We have defined six points as a difference of clinical relevance to the patient and caregiver (patient requiring less help in at least two activities) and also substantive enough to influence commissioners to change service delivery. Thirty-six stroke rehabilitation units, each recruiting 25 patients, would result in 450 patients in each group and provide close to 90% power at 5% significance level to detect a clinically relevant difference of six points on the NEADL scale [scored 0–66, standard deviation (SD) 18]. The sample size incorporates an inflation factor of 1.9 owing to clustering [cluster size of 19 after loss to follow-up; intracluster correlation coefficient (ICC) no greater than 0.0564] and 25% loss to follow-up. The assumption that the ICC would be no larger than 0.05 was based on methodological research65 showing that ICCs for patient outcomes in the community are generally < 0.05. A sample size of 900 patients provided more than 85% power at the 5% significance level to detect an effect size of one-third in any of the other outcomes. Such an effect size is usually considered moderate. So, for instance, this ensured more than 85% power to detect a difference of 4.3 points on the CBS at 6 months, assuming the same variability as in the single-centre study19 (i.e. SD of 12.9 at 6 months).
Revised sample size
The power of the trial was, however, adversely affected by a slightly higher than expected loss to follow-up and unequal cluster sizes. By estimating maximum and minimum cluster sizes66 the predicted imbalance decreased the power by 1–3%. To preserve final power of close to 90%, the trial target was increased to between 950 and 1000 patient and caregiver dyads, with a maximum of 35 dyads from each of the 36 SRUs to compensate for low recruitment at some centres.
Analysis methods
All data analyses were conducted to a prespecified analysis plan. All data analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA). All hypothesis testing was performed at the 5% two-sided significance level. Analysis of health economic data was performed using SPSS (SPSS Inc., Chicago, IL, USA).
Populations
The ITT population was defined as all patients registered for active follow-up regardless of non-compliance with the intervention. All patients (and the corresponding caregivers) within a stroke unit were analysed according to the intervention that stroke unit was randomised to. All analyses and data summaries were carried out using the ITT population. Patients whose written informed consent had not been received were not included in this population. The analysis population included all patients returning 6-month questionnaires.
Baseline characteristics
The baseline characteristics of the ITT population were tabulated using frequencies and summary statistics for each treatment group. Two-sample t-tests to compare percentages and means, weighted by the number of patients in treatment and control centres, were used to detect potential bias in recruitment.
Primary analysis
For all analyses, means and 95% confidence intervals (CIs) together with values of unadjusted and adjusted ICCs are reported. Summaries from raw and predicted data from the final model are provided.
Patients
The primary analysis was based on a complete case analysis, with no substitution for missing outcome data. The ITT analysis included all patients with a valid 6-month NEADL score. The 6-month NEADL score was compared between the intervention and control groups using two-level multilevel modelling, with patients and SRUs being the level one and level two units, respectively. The model was adjusted for:
-
The following patient-level covariates (level 1) Patient baseline NEADL score, sex, caregiver's education (age caregiver left education: ≤ 16 years, > 16 years) and caregiver baseline HADS score, the Edinburgh stroke case-mix adjuster (which includes age; whether or not patient lived alone before the stroke; whether or not the patient was independent in everyday activities before stroke; whether or not the patient can talk or he/she orientated in time, place and person; whether or not the patient can lift both arms; whether or not the patient can walk without help from another person), and
-
The following stroke unit-level covariates (level 2) The key 12-indicator score, geographical region and number of beds in each centre.
A number of sensitivity analyses were used to examine the robustness of the conclusions of the primary analysis. First, the analysis was undertaken including patients who died and assumed a NEADL score of 0 (worst possible outcome). Second, an analysis without proxy responses was performed to assess the impact of proxy responses. Third, the time of completion of 6-month questionnaires was compared between both arms using a t-test and, if significant, a sensitivity analysis would be undertaken to determine if the results were influenced by patients responding late in the follow-up period.
Caregivers
The primary analysis of caregiver outcomes was based on a complete case analysis with no substitution for missing data. The ITT analysis included all caregivers with a valid 6-month CBS score. The 6-month CBS was compared between the intervention and control groups using two-level multilevel modelling, with patients and SRUs being the level 1 and level 2 units, respectively. The model was adjusted for:
-
The following caregiver-level covariates (level 1): caregiver baseline HADS anxiety and depression scores, age, sex, caregiver's education (age caregiver left education: ≤ 16 years, > 16 years).
-
The following stroke unit-level covariates (level 2): the key 12 indicator score, geographical region, and number of beds in each centre. [In the analysis, the age that the caregiver left education is used as a binary covariate ‘≤ 16 years’ and ‘> 16 years’. Unknown and missing categories were investigated and based on caregiver baseline data compared with the general characteristics; these were imputed accordingly (in all instances they fell into the category ‘Age caregiver left education “≤ 16 years ”’).]
The sensitivity analysis relating to time of questionnaire completion was repeated for caregivers.
Secondary analyses
All analyses of secondary end points were conducted on the ITT population. Means, 95% CIs and values of unadjusted and adjusted ICCs are reported. Summaries from raw and predicted data from the final model are provided.
Two-level random intercept models were used, with patients being level one and stroke units being level two. Fixed parts were patient-level covariates (level one), stroke unit-level covariates (level two) and treatment. Data were assumed missing at random.
Patient end points at 6 months
The 6-month HADS scores, EQ-5D score, Barthel Index and SIS were summarised by treatment group. For SIS, the score was summarised for each domain separately and included the four physical domains as one (strength, hand function, mobility and ADL/instrumental ADL).
The 6-month HADS scores, EQ-5D score, Barthel Index and SIS were compared between the intervention and control groups using two-level multilevel modelling, with patients and stroke rehabilitation units being the level 1 and level 2 units, respectively. The model was adjusted for the same variables as in the primary patient end point apart from the baseline NEADL score. The baseline HADS scores, EQ-5D score, Barthel Index and SIS were used when comparing the 6-month HADS scores, EQ-5D score, Barthel index and SIS, respectively, between the two groups.
Patient end points at 12 months
The 12-month NEADL score, HADS scores, EQ-5D score, Barthel Index and SIS were summarised by treatment group.
The 12-month NEADL score, HADS scores, EQ-5D score, Barthel Index and SIS were compared between the intervention and control groups using the same process as for end points at 6 months.
Caregiver end points at 6 months
The 6-month FAI, HADS and EQ-5D scores were summarised by treatment group.
The 6-month FAI, HADS and EQ-5D scores were compared between the intervention and control groups using two-level multilevel modelling, with patients and stroke rehabilitation units being the level 1 and level 2 units, respectively. The model for HADS scores was adjusted for the same variables as in the primary caregiver end point. The rest was adjusted for the same variables as in the primary caregiver end point apart from the baseline HADS scores. The baseline FAI and EQ-5D score was when comparing the 6-month FAI and EQ-5D score, respectively, between the two groups.
Caregiver end points at 12 months
The 12-month CBS, FAI, HADS and EQ-5D scores were summarised by treatment group.
The 12-month CBS, FAI, HADS and EQ-5D scores were compared between the intervention and control groups using the same process as for caregivers at 6 months.
Process data
Data in intervention and control sites collected before trial commenced and during trial were summarised by the trial manager to ascertain whether or not care has changed over the course of the trial. During the trial, the number of training records received from the SRUs was summarised. The data from the training records were summarised for all intervention patients in terms of the number of mandatory and non-mandatory components delivered and the number of these where the caregiver achieved competence.
The proportion of training records compliant with the intervention by the SRU was summarised. The number of caregivers achieving competence for each component was also summarised.
The relationship between patient and caregiver outcome and compliance with intervention was explored. Number of mandatory components achieved as per training records completion was summarised.
Safety
Falls – the number of patient falls between registration and discharge were summarised by arm and centre. The number of falls that resulted in a SAE was presented by arm as well as the mean number of falls (of those patients who fell).
The percentage of patients and caregivers who died from any cause between registration and 12-month follow-up were summarised by arm and centre and the cause of death was presented. No statistical comparison between the intervention and control groups was undertaken. The number of patients' and caregivers' admissions or readmissions (self-reported and researcher completed) to a hospital, nursing home or residential care home, was summarised at the 6- and 12-month follow-ups by arm. No statistical comparison between the intervention and control groups was undertaken. Related and unexpected serious adverse events (RUSAEs) were listed and detailed, up to, and including, the 12-month follow-up.
Economic evaluation
The purpose of the economic evaluation was to examine the cost-effectiveness and cost–utility of the LSCTC. There were two parallel economic evaluations: one for patients and another for caregivers. The primary economic evaluation was cost-effectiveness analyses based on the patient and caregiver primary outcome measures (NEADL and CBS, respectively). The secondary economic evaluation was cost–utility analyses based on QALYs.
Perspective
The patient and caregiver evaluations were each undertaken from (a) a health and social care cost perspective and (b) a societal cost perspective. Health and social care costs included nursing/residential care; hospital inpatient, outpatient, day hospital and accident and emergency services; and primary care/community-based health/social care services. Societal costs included all of these categories plus informal care costs.
Time horizon
In keeping with the outcomes analyses, the cost-effectiveness and cost–utility analyses were primarily focused on findings at 6 months. We further examined costs and outcomes at 12 months and over one year to enable a more direct comparison of findings with the single-centre study on which this one was based. 19,29 One-year costs were estimated as the sum of costs from the 6- and 12-month assessments and 1-year QALYs were the sum of QALYs at 6 and 12 months. The time horizon was limited to 1 year because we focused on within-trial costs only.
Resource-use data
Data on the use of health and social care and informal care were collected at the individual-level using a CSRI29 that was specifically adapted for use with stroke patients. A reduced version of this instrument was used with caregivers, containing questions about their use of core health and social care services and informal care that they provided. For both patients and caregivers, the CSRI was administered as a face-to-face interview at the baseline assessment (with reference to the previous 3 months) and then as a self-complete postal questionnaire alongside other measures at 6 and 12 months (with reference to the time since previous assessment).
Costs
Individual-level resource-use quantities were combined with unit costs to calculate a cost per participant. Unit cost estimates, their sources and any assumptions made for their estimation are in Appendix 5 and are summarised in Table 2. National unit costs were used where possible to represent the geographical spread of the sites and to facilitate the generalisability of results.
| Category | Unit | Unit cost (£, 2009–10) |
|---|---|---|
| Residential care home stay | Night | 74 |
| Nursing home stay | Night | 73 |
| Inpatient services: stroke, acute | Bed-day | 294 |
| Inpatient services: stroke, acute | Stay | 2808 |
| Inpatient services: stroke, rehabilitation | Bed-day | 361 |
| Inpatient services: other | Bed-day | Range 110–1229 |
| Inpatient services: other | Stay | Range 110–3877 |
| Day hospital/day-case services | Activity | Range 368–1149 |
| Outpatient services and procedures | Visit | Range 5–785 |
| Primary care/community-based services | Contact | Range 6–129 |
| Primary care/community-based services | Hour | Range 23–158 |
| Primary care/community-based services | Item | Range 3–6 |
| Value of caregiver time: average wage | Hour | 15 |
| Value of caregiver time: home care worker | Hour | 28 |
Costs of the initial stroke admission were estimated by multiplying length of stay by the unit cost for a non-elective long-stay unit cost from the 2009–10 NHS reference costs67 (£294). If either the admission or discharge date was missing, then an average admission cost for the same service was applied (£2808).
Costs of other hospital admissions were estimated similarly by mapping participant-reported specialty or reason for the admission to Healthcare Resource Groups (HRGs) and then applying weighted average non-elective, long-stay bed-day or admission costs for each of those HRGs. Where participants did not state a specialty, the reason for the admission was used to infer specialty. Alternative assumptions were necessary for a few specialties that did not readily fit into a HRG (see Appendix 5).
Where multiple specialties were reported for one admission, an average weighted unit cost across all relevant specialties was used. For admissions with no information on specialty or reason, an average cost of all HRGs was applied. Outpatient costs were estimated using the same approach.
The patient CSRI included a question asking respondents to report use of any other services not covered by the previous questions. Many responses to this question were for services already itemised in the instrument. We report these total ‘other’ costs separately rather than amalgamate them into the specific resource categories.
Informal care represents an important input to the health of people with stroke. We estimated the monetary value of such inputs using the opportunity cost approach,68 which involves valuing caregivers' time according to the opportunities they have forgone owing to time spent care giving. Often the opportunity forgone is paid employment and so the monetary value attached to this could be wages forgone. For care provided by the main caregiver enrolled in the trial, we distinguished opportunity costs as either lost employment or lost leisure on the basis of their employment status at each assessment. Where it was assumed that the caregiver could otherwise have been working (those working part-time and those unemployed and seeking work), we applied the national average wage. Where it was assumed that the caregiver was unlikely to instead be working (those working full-time, at home not seeking work, retired, redundant/early retired, unable to work and students), we applied an estimate of the cost of leisure time. For anyone whose employment status was ‘other’ or missing (and for other caregivers), we applied the average of the two unit costs. We assumed that if the main caregiver lived with the patient, all reported live-in informal care inputs were by that caregiver and that all live-out inputs were provided by others. Conversely, if the main caregiver did not live with the patient, we assumed that all live-out informal care inputs were provided by that person and that all live-in inputs were provided by others.
The cost of the LSCTC was incorporated into the evaluation. We included only the costs of its development and staff training, not the cost of delivery to caregivers, as the latter is inherently included in the unit cost applied to the stroke admission given that it was part of routine practice in the intervention arm stroke units. Developing and delivering the staff training to ensure that ward staff were competent in delivering the LSCTC to caregivers was a multistage process consisting of the following key resource components:
The project team:
-
developing the staff training package
-
preparing and delivering four core training days
-
preparing and delivering one refresher training day
-
delivering local refresher training sessions at all intervention sites.
Ward staff:
-
attending the core training days
-
attending the refresher training day
-
receiving local refresher training
-
delivering cascading training session to other ward staff
-
attending cascaded training session.
There were three elements of data collection to measure the resources associated with each of these components. First, resources associated with all main training events were recorded by the staff associated with delivering those events. Second, ward staff attending these events and then cascading that training to other staff on their ward recorded the time, profession and salary band for all of the staff involved with delivering and receiving that cascaded training. Third, ward staff providing training inputs to caregivers recorded those time inputs on a training record. Resulting cost estimates are summarised in Table 3, with further details of each component described in Appendix 6.
| Staff training component | Costs (£, 2009–10 prices) |
|---|---|
| 1. Core training and refresher training: development | 7680 |
| 2. Core training: preparation | 3554 |
| 3. Refresher training: preparation | 753 |
| 4. Core training: delivery | 23,317 |
| 5. Refresher training: delivery | 5603 |
| 6. Local refresher visits: delivery | 16,593 |
| 7. Ward staff time | 45,077 |
| Total including development costs | 102,577 |
| Total excluding development costs | 94,897 |
| Cost per minute of input to caregivers, including development costs | 0.60 |
| Cost per minute of input to caregivers, excluding development costs | 0.56 |
We transformed the total cost of the development and staff training into an average cost per minute of input to caregivers to enable the cost of LSCTC to vary at the individual level according to inputs provided, rather than be a fixed cost across all participants. We calculated this as follows. First, we multiplied the average amount of time spent with each caregiver in the trial intervention arm (136 minutes) by the total number of eligible patients identified during the screening/recruitment process (n = 1256) to estimate the total caregiver input time that the LSCTC development and staff training potentially ‘purchased’ (170,816 minutes/2846 hours). We then divided the total training cost (£102,577 including development costs) by this total input time to estimate the training cost per minute of caregiver input provided (£0.60). This cost per minute was applied to each intervention arm participant according to the amount of time input provided by ward staff to the caregiver.
Total costs were computed for each patient and caregiver at each assessment point (baseline, 6 months, 12 months and 1 year), from both perspectives. It was not necessary to discount costs or outcomes because the evaluation covered only 1 year. All unit costs were standardised at 2009–10 levels, where relevant.
Outcome measures for the economic evaluation
Patients:
-
NEADL score at 6 months (primary patient outcome measure) and 12 months
-
QALYs between baseline and 6 months, between 6 months and 12 months and over 1 year.
Caregivers:
-
CBS at 6 months (primary caregiver outcome measure) and 12 months
-
QALYs between baseline and 6 months, between 6 months and 12 months and after 1 year.
The concept of utility refers to the value of a particular level of health status (or improvement on level of health status) and can be measured by the preferences of individuals or society for any set of health outcomes. The most common value-based measure of health outcomes used in cost–utility analysis is the QALY. 69 The quality adjustment is based on a set of utilities, one for each possible predefined health state. QALYs are calculated by multiplying the preference value for a particular health state by the time spent in it. For example, if a health state associated with receiving a particular intervention is valued at 0.6, 2 years in that health state equates to 1.2 QALYs. Results of cost–utility analyses are usually expressed in terms of additional cost per additional QALY gained by undertaking one intervention instead of another. In this study, health states were measured using the EQ-5D31 at baseline, 6 months and 12 months. Utility weights from a UK general population survey70 were attached to health states at each time point, with appropriate adjustments for the period of time involved. Utility weights and QALY gains were not estimated for those who died (or were lost to follow-up), as such cases were not included in the primary analysis and would anyway have missing resource-use/cost data. QALYs were then estimated using linear interpolation to calculate the area under the QALY curve as follows:
Cost-effectiveness and cost–utility analyses
Cost-effectiveness/cost–utility analysis is concerned with linking costs with outcomes and comparisons between two or more alternatives.
The primary economic evaluation at 6 months, the cost-effectiveness analyses, involved examining the following four cost–outcome combinations between the two randomisation groups.
Patients:
-
the additional cost per additional point improvement on the NEADL scale from the health and social care perspective
-
the additional cost per additional point improvement on the NEADL scale from the societal perspective.
Caregivers:
-
the additional cost per additional point improvement on the CBS from the health and social care perspective
-
the additional cost per additional point improvement on the CBS from the societal perspective.
The secondary economic evaluation at 6 months, the cost–utility analyses, involved the following four further cost–outcome combinations:
Patients:
-
the additional cost per additional QALY from the health and social care perspective
-
the additional cost per additional QALY from the societal perspective.
Caregivers:
-
the additional cost per additional QALY from the health and social care perspective
-
the additional cost per additional QALY from the societal perspective.
Further considering cost-effectiveness and cost–utility over 1 year involved examining these cost–outcome combinations once more. There were thus a total of 16 cost–outcome combinations to consider.
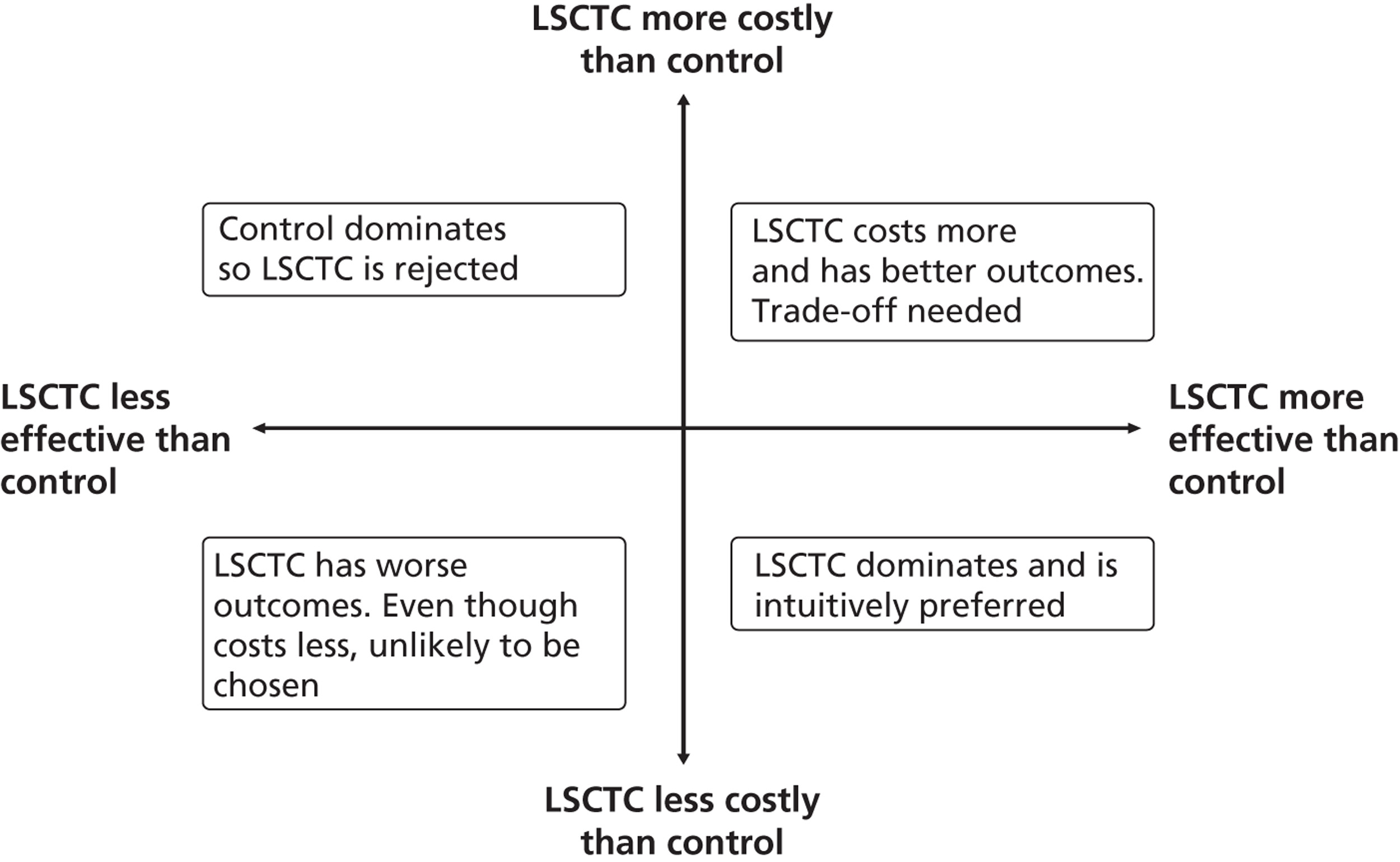
Cost-outcome comparisons between the intervention and control groups can produce one of four outcomes:
-
If costs are lower and outcomes are higher for one group, then it is considered to ‘dominate’ the other and is clearly more cost-effective.
-
If outcomes are similar between groups, then the one with lower costs can be regarded as more cost-effective (or if costs are similar between groups then the one with better outcomes is regarded as more cost-effective).
-
If both costs and outcomes are lower for one group, then there are value judgements involved in trading off outcomes for cost savings.
-
If both costs and outcomes are higher for one group, then it falls on relevant decision-makers to decide whether or not the additional benefits are worth paying for. In this scenario, results of a cost-effectiveness/cost–utility analysis are generally expressed as incremental cost-effectiveness ratios (ICERs), which represent the additional cost associated with one additional unit of the outcome for one person; these are calculated by dividing the mean difference in cost by the mean difference in outcome.
Each of these scenarios can be represented on a cost-effectiveness plane (Figure 2), in which the vertical axis represents the additional costs of one intervention against another, and the horizontal axis represents the additional outcomes. The location of a co-ordinate representing incremental cost and incremental outcome indicates which of the four scenarios the cost-effectiveness finding falls into.
FIGURE 2.
Cost-effectiveness plane.
We constructed cost-effectiveness planes using non-parametric bootstrapped regressions (5000 replications, with replacement) of study group upon 6-month health and social care costs, patient and caregiver QALYs, total NEADL score and total CBS score, in turn, with cluster adjustment for centre and baseline values of the dependent variable (except in the case of CBS), age and sex included as covariates. The resulting coefficients of group differences were saved and plotted using scatter graphs (Stata version 10.1, StataCorp LP, College Station, TX, USA) in relevant cost–outcome combinations.
We calculated ICERs for any cost–outcome combinations where the intervention group had higher costs and better outcomes (the top-right quadrant of the cost-effectiveness plane). Although ICERs have been one of the most common ways of presenting the results of cost-effectiveness analyses, they carry two important limitations. First, they are point estimates that do not provide information about the uncertainty surrounding the estimates, a problem that is compounded with the complexity of examining CIs around ICERs. Second, their use to decision-makers is limited as they provide no information about what an ‘acceptable’ level of cost-effectiveness would be, or whether or not the likelihood of cost-effectiveness would differ according to how much decision-makers would be willing to invest. Both of these limitations can be addressed by cost-effectiveness acceptability curves (CEACs) based on the net benefit approach. 71 We use CEACs alongside cost-effectiveness planes to examine any uncertainty surrounding the cost-effectiveness and cost–utility results.
Net benefits provide a single summary monetary measure of costs and outcomes for each individual (removing the need to examine ratios) and account for the value (λ) that a decision-maker would be willing to pay for a greater net benefit. They are calculated as follows:
For each cost–outcome combination, a series of net benefits were calculated for a range of relevant λ values. For the QALY-based analyses, these values ranged from £0 to £50,000 (£10,000 increments), to incorporate the £20,000–30,000 per QALY gain threshold range currently specified for National Institute for Health and Care Excellence (NICE) decision-making in England and Wales. 72 For the NEADL- and CBS-based analyses, the ‘acceptable threshold’ is unclear so we examined a range that incorporated NEADL- and CBS-related ICER values at 6 months, £0–2000 (£500 increments). Net benefits were then compared at the level of randomisation group using non-parametric bootstrapped regressions (5000 replications, with replacement) of study group upon net benefit, with a cluster adjustment for centre and baseline values of costs from the relevant perspective, age and sex, baseline utility for QALY-based CEACs and baseline NEADL score for NEADL-based CEACs included as covariates. For each value of λ, the proportion of iterations indicating a higher net benefit for the LSCTC group were calculated and plotted. These plots formed the CEACs, which represent the probability of the LSCTC group being cost-effective compared with the control for a range of values that a decision-maker would be willing to pay for an additional unit of the NEADL score, CBS score and QALYs.
Statistical analyses
All cost and QALY data are reported as mean values with SDs. To accommodate a cluster randomisation design, differences in costs between groups were tested by multilevel modelling using the xtreg procedure in Stata 10.1, from which we report 95% CIs and p-values for the differences in means. Baseline values of the dependent variable, age and sex (plus baseline NEADL score for patients) were included as covariates. Individuals were analysed according to the group to which they were randomised. As described above, net benefit comparisons for the purpose of CEAC constructions were undertaken using non-parametric bootstrapped regressions (5000 replications, with replacement), controlling for site clusters.
Missing data
The base-case economic evaluation was based on available cases (i.e. it did not impute missing data due to loss of follow-up) under the assumption that loss of follow-up was missing at random. However, we report CSRI and EQ-5D completion rates and describe baseline characteristics of those with and without these data at the primary end point – 6 months.
Resource-use data from the CSRI formed the basis of the total cost calculations for each participant. Self-complete applications of such complex instruments inevitably include missing items on returned questionnaires and to allow the computation of total costs that reflect variations in resource use rather than variations in data completeness, we imputed missing cost items on returned CSRIs as follows. If there was no report of use of a particular resource, we assumed that it was not used and thus imputed a zero cost. If a participant reported using a resource but not the quantity used, we imputed the cost of that resource use based on the mean cost for participants with data for that item at the same assessment point and in the same randomisation group (this was done separately for patients and caregivers). All such imputations were made to the cost data, rather than the resource-use data. Therefore, resource-use data are based on data availability for each item with no imputation for missing values.
Missing data transformations (or lack of) for the NEADL scale and CBS were as for the main outcomes analyses. Missing EQ-5D data were not imputed.
Sensitivity analyses
We altered some key assumptions made in the economic evaluation to explore their consequences for the results at the primary end point – 6 months.
Our first sensitivity analysis concerned the LSCTC development and staff training costs. Training record return rates were low so there were a significant number of intervention arm participants with missing data on ward staff time inputs to caregivers. As we estimated individual-level LSCTC development and staff training costs on the basis of time spent with caregivers, we were unable to allocate any LSCTC costs to many participants. For analysis purposes, such individuals were allocated a zero LSCTC cost. This may obviously have led us to underestimate the contribution of LSCTC costs to total care costs. We therefore examined the effect on patient and caregiver total health and social care costs at the primary end point, 6 months, of imputing LSCTC costs for intervention group participants with missing training records. Imputation values were based on the mean LSCTC cost for those with training record data (£81.90).
In the base-case analyses, we estimated informal care costs using the opportunity cost approach. However, there are controversies in valuing informal care,73 and it is important to explore alternative approaches given the notable size of informal care costs in this group. Therefore, our second sensitivity analysis examined the effect of adopting a replacement cost approach to informal care, i.e. the cost of replacing informal care inputs with paid professionals. We applied the cost of a local authority home care worker and examined the effect on patient and caregiver total societal costs at the primary end point – 6 months.
Our final two sensitivity analyses examined the effect of loss of follow-up by imputing missing patient and caregiver health and social care costs and QALYs at the primary end point, 6 months. We used the multiple imputation procedure in Stata 10.1. Cost imputations were based on key variables expected to predict follow-up costs: randomisation group, sex, baseline age and baseline total health and social care costs. Predictor variables for QALY imputations were the same except that they included baseline utility score rather than cost.
For each of these four sensitivity analyses, we report the same statistics around means and mean differences (and the same covariates for comparisons of means) as for the base-case analyses, and we also examine the impact of the alternative scenarios on CEACs.
Definition of end of trial
The end of the trial was defined as the date that the last 12-month postal questionnaire was completed.
Adverse events and safety monitoring
Events such as patient falls and caregiver musculoskeletal injury represent an inherent consequence of an active rehabilitation process and, therefore, cannot be entirely avoided. Similarly, in this patient population, acute illness resulting in hospitalisation, new medical problems and deterioration of existing medical problems were expected. In recognition of this, events fulfilling the definition of an AE or an expected SAE were not reported in this study, with the following exceptions: patient falls with or without fracture that occurred at any time between the date of consent and date of discharge; patient and/or caregiver death (SAE); patient and/or caregiver hospital admissions and readmissions for any reason (SAE); patient and/or caregiver institutionalisation (AE); and patient and/or caregiver treatment on an emergency outpatient basis (AE). Summaries of the above expected AEs and SAEs by treatment arm were reported quarterly to the Data Monitoring and Ethics Committee (DMEC).
Data monitoring
Data were monitored for quality and completeness by the CTRU, using established verification, validation and checking processes. Missing data (except patient and caregiver completed data items collected via the baseline and postal questionnaires) were chased until received, confirmed as not available, or the trial was at analysis.
Data Monitoring and Ethics Committee
An independent DMEC was established to review the safety and ethics of the trial. Detailed unblinded reports were prepared for the DMEC during set-up and annually during the recruitment and intervention periods. SAEs were summarised by treatment group in a quarterly report sent to the DMEC, to enable monitoring of safety rates between control and intervention sites.
Trial Steering Committee
A TSC was established to provide overall supervision of the trial, in particular trial progress, adherence to protocol, patient safety and consideration of new information. The committee met once during the set-up period and 6-monthly thereafter for the duration of the trial.
Trial Management Group
The TMG consisted of the report authors, who met monthly during study set-up and recruitment. The TMG monitored rates of recruitment and refusals at sites and between study arms, LSCTC compliance in the intervention centres, data quality and compliance, protocol adherence, and potential changes to the process of care at centres.
Ethical approval
The study protocol was approved by Leeds Research Ethics Committee (REC), reference 07/Q1205/12. Local REC approval and research and development (R&D) approval was obtained at each NHS trust prior to cluster randomisation.
Amendments to the study protocol following commencement of recruitment
Sample size
Originally the recruitment target was 900 patients, 25 dyads from each of the 36 participating SRUs. However, as described in the sample size section above, the power of the trial was adversely affected by a higher than expected loss to follow-up (expected to be closer to 30% than the predicted 25%) and unequal cluster sizes. By estimating maximum and minimum cluster sizes74 the predicted imbalance decreased the power by 1–3%. To preserve final power of close to 90%, the trial target was increased to between 950 and 1000 patient and caregiver dyads. The maximum number of dyads that could be recruited from each SRU was raised to 35 to compensate for some low-recruiting centres. In one SRU, the upper limit was increased to 38 to compensate for a very low questionnaire response rate at this centre.
Inclusion criteria
Deletion of the words ‘competent to undergo training’ from the definition of a caregiver, as caregivers in the control arm will not undergo structured training.
Informed consent
The statement ‘patients and caregivers will be given at least 24 hours to consider participation’ has been replaced with ‘patients and caregivers will be given sufficient time to consider participation’.
In accordance with The Mental Capacity Act 2005,40 the term ‘caregiver assent’ has been replaced throughout the protocol with the preferred recommended term ‘caregiver declaration’.
Data collection
Addition of residential/nursing home option to the living circumstances of patients collected on the screening log.
An additional question has been inserted into the 12-month follow-up questionnaire asking if the patient was aware of receiving/being denied better treatment because of this research.
The question asking if the caregiver was aware of receiving/being denied an enhanced training package will be collected only at 12-month (not 6-month) follow-up.
Typo: Deletion of the modified Rankin Score from the data being collected on the screening log.
Typo: The ITT statement has been amended to clarify that all patients and caregivers who are registered into the study will be considered as part of the ITT population and efforts will be made to follow them up when appropriate.
References for the stroke knowledge question have been added. 36,37
Literature review search strategy
Literature searches were completed using the Cumulative Index to Nursing and Allied Health Literature (CINAHL), EBSCO, The Cochrane Library (Wiley Online Library), EMBASE (OVID), MEDLINE (OVID), and PsycINFO (OVID), from January 1999 to November 2011. The International Standard Randomised Controlled Trial Number (ISRCTN) registry was also searched manually. Appendix 7 provides the full search strategy used.
Chapter 4 Results
Recruitment and randomisation of stroke rehabilitation units
Cluster-level recruitment
Forty-nine SRUs expressed an interest in TRACS, and 41 SRUs completed feasibility questionnaires and were assessed for eligibility. Of these 41 SRUs, two did not meet the centre eligibility criteria and three satisfied the centre eligibility criteria, but did not complete ethics/R&D approval processes in time to be entered for randomisation.
The 36 SRUs were randomised equally between control and intervention, stratified by geographical region and NSSA score. After randomisation, the characteristics of the SRUs were found to be balanced between random allocations (Table 4). Once randomised, all 36 SRUs participated throughout the course of the trial period (see Figures 5 and 6).
Participant flow and recruitment
Screening for trial participation
Patient and caregiver dyads were identified and recruited between February 2008 and February 2010. Overall, 14,370 patients went through the screening process, 7067 in intervention and 7303 in control sites, and 12,510 patients were assessed for eligibility, 6205 in intervention and 6305 in control sites. Of these, 2675 (21.4%) were deemed eligible and 1024 consented (8.2% of assessed, 38.3% of eligible), 490 in intervention and 479 in control sites (see Figure 3 and Table 5). Reasons for non-registration of patients are summarised in Figure 3. The number of assessed patients varied between SRUs from 149 to 831 patients (Table 6).
In total, 930 (7.4% of assessed, 34.8% of eligible) patients were registered into the trial (see Table 5). The number of patient and caregiver dyads recruited per SRU ranged from 13 to 38 per centre. The proportion of recruited dyads out of assessed ranged from 3.6% to 18.6% per SRU.
Characteristics of screened participants
Table 7 summarises the characteristics of screened patients. The groups were well balanced with respect to baseline characteristics.
The mean age of screened patients was 74.4 (SD 13.39) years and the average length of hospital stay was 27.9 (SD 33.07) days. In total, there were 5967 (47.7%) males; the majority of patients were white (11,628, 92.9%); 4922 (39.3%) patients lived alone; 6227 (49.8%) patients co-habited and 691 (5.5%) were nursing or residential care home residents.
The caregiver resided with 5015 (40.1%) patients. In most cases, the caregiver was the patient's family member: partner in 5047 (40.3%) and offspring in 3587 (28.7%) cases.
| Centre | Intervention (n = 18) | Control (n = 18) |
|---|---|---|
| Geographical region, n (%) | ||
| North West | 6 (33.3) | 6 (33.3) |
| London and the South East | 4 (22.2) | 4 (22.2) |
| South West Peninsula | 3 (16.7) | 3 (16.7) |
| Yorkshire | 5 (27.8) | 5 (27.8) |
| NSSA 2006 score | ||
| Mean (SD) | 67.8 (13.09) | 68.5 (13.55) |
| Median | 68.0 | 68.5 |
| No. of beds | ||
| Mean (SD) | 18.6 (7.40) | 18.5 (7.68) |
FIGURE 3.
Flow of patients from admission to registration: by treatment arm. Percentage is calculated out of ‘assessed patients’, apart from ‘assessed’, for which percentage is calculated out of ‘admitted’.
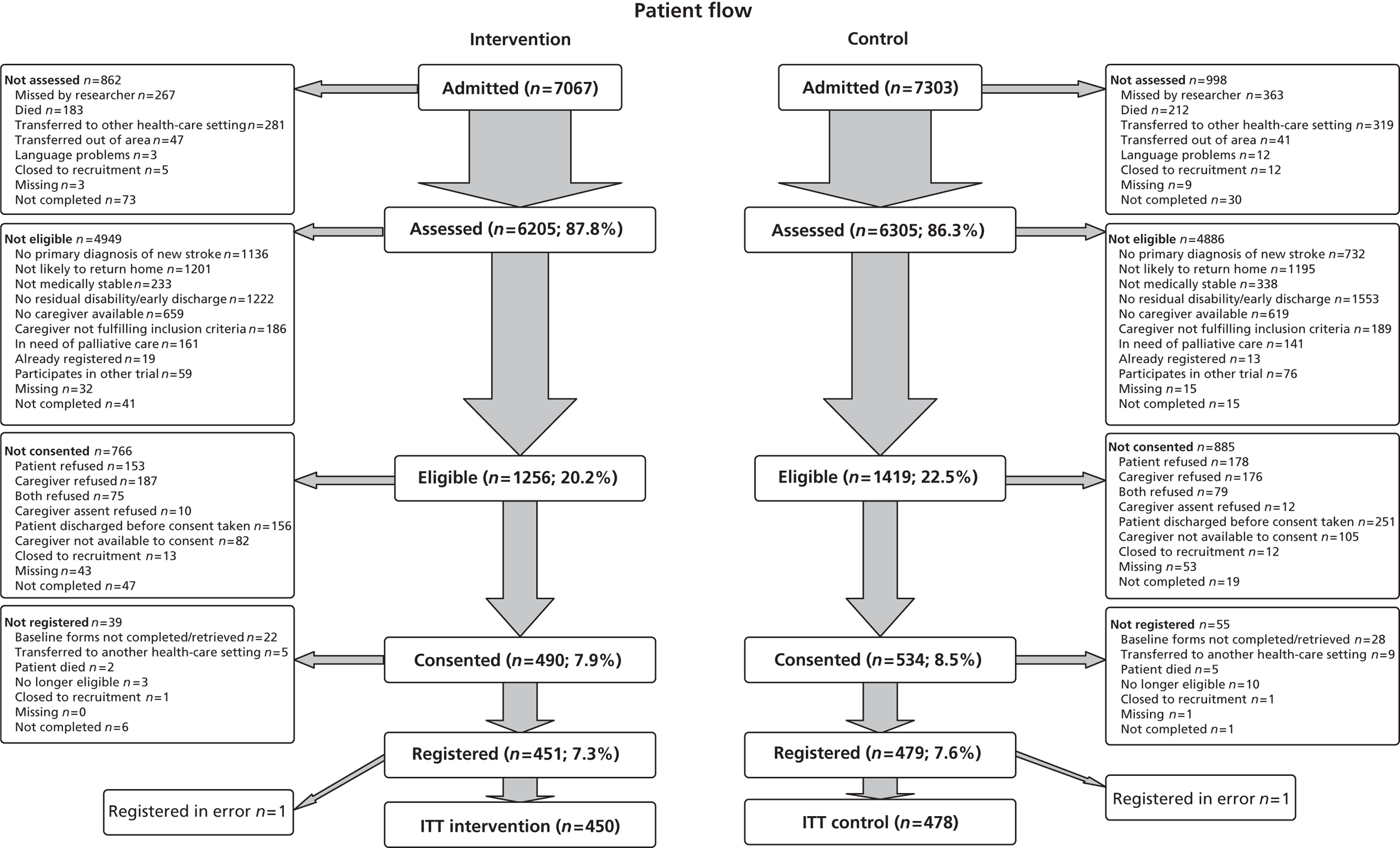
| Admitted, n | Assessed/screened, n (%)a | Eligible, n (%) | Consented, n (%) | %b | Registered, n (%) |
|---|---|---|---|---|---|
| Intervention | |||||
| 7067 | 6205 (87.8) | 1256 (20.2) | 490 (7.9) | 39.0 | 451 (7.3) |
| Control | |||||
| 7303 | 6305 (86.3) | 1419 (22.5) | 534 (8.5) | 37.6 | 479 (7.6) |
| Total | |||||
| 14,370 | 12,510 (87.1) | 2675 (21.4) | 1024 (8.2) | 38.3 | 930 (7.4) |
| Centre | Admitted, n | Assessed/screened, n (%)a | Eligible, n (%) | Consented, n (%) | Consented, %b | Registered, n (%) |
|---|---|---|---|---|---|---|
| Intervention | ||||||
| 1 | 183 | 149 (81.4) | 45 (30.2) | 23 (15.4) | 51.1 | 20 (13.4) |
| 2 | 178 | 173 (97.2) | 52 (30.1) | 21 (12.1) | 40.4 | 21 (12.1) |
| 3 | 766 | 637 (83.2) | 71 (11.1) | 24 (3.8) | 33.8 | 24 (3.8) |
| 4 | 865 | 831 (96.1) | 75 (9.0) | 33 (4.0) | 44.0 | 30 (3.6) |
| 5 | 611 | 579 (94.8) | 60 (10.4) | 35 (6.0) | 58.3 | 33 (5.7) |
| 6 | 219 | 211 (96.3) | 85 (40.3) | 38 (18.0) | 44.7 | 32 (15.2) |
| 7 | 563 | 445 (79.0) | 84 (18.9) | 33 (7.4) | 39.3 | 27 (6.1) |
| 8 | 230 | 222 (96.5) | 53 (23.9) | 25 (11.3) | 47.2 | 23 (10.4) |
| 9 | 376 | 360 (95.7) | 115 (31.9) | 30 (8.3) | 26.1 | 29 (8.1) |
| 10 | 542 | 362 (66.8) | 67 (18.5) | 25 (6.9) | 37.3 | 25 (6.9) |
| 11 | 318 | 194 (61.0) | 30 (15.5) | 16 (8.2) | 53.3 | 16 (8.2) |
| 12 | 256 | 227 (88.7) | 106 (46.7) | 38 (16.7) | 35.8 | 35 (15.4) |
| 13 | 167 | 162 (97.0) | 70 (43.2) | 16 (9.9) | 22.9 | 14 (8.6) |
| 14 | 373 | 362 (97.1) | 107 (29.6) | 38 (10.5) | 35.5 | 35 (9.7) |
| 15 | 673 | 645 (95.8) | 69 (10.7) | 24 (3.7) | 34.8 | 19 (2.9) |
| 16 | 277 | 227 (81.9) | 55 (24.2) | 17 (7.5) | 30.9 | 17 (7.5) |
| 17 | 202 | 171 (84.7) | 44 (25.7) | 27 (15.8) | 61.4 | 25 (14.6) |
| 18 | 268 | 248 (92.5) | 68 (27.4) | 27 (10.9) | 39.7 | 26 (10.5) |
| Total | 7067 | 6205 (87.8) | 1256 (20.2) | 490 (7.9) | 39.0 | 451 (7.3) |
| Control | ||||||
| 19 | 314 | 310 (98.7) | 74 (23.9) | 39 (12.6) | 52.7 | 34 (11.0) |
| 20 | 929 | 720 (77.5) | 135 (18.8) | 32 (4.4) | 23.7 | 27 (3.8) |
| 21 | 244 | 235 (96.3) | 84 (35.7) | 36 (15.3) | 42.9 | 31 (13.2) |
| 22 | 260 | 225 (86.5) | 44 (19.6) | 20 (8.9) | 45.5 | 18 (8.0) |
| 23 | 514 | 403 (78.4) | 78 (19.4) | 26 (6.5) | 33.3 | 25 (6.2) |
| 24 | 373 | 284 (76.1) | 54 (19.0) | 13 (4.6) | 24.1 | 12 (4.2) |
| 25 | 688 | 566 (82.3) | 88 (15.5) | 44 (7.8) | 50.0 | 38 (6.7) |
| 26 | 189 | 182 (96.3) | 65 (35.7) | 25 (13.7) | 38.5 | 23 (12.6) |
| 27 | 285 | 270 (94.7) | 92 (34.1) | 35 (13.0) | 38.0 | 35 (13.0) |
| 28 | 354 | 265 (74.9) | 85 (32.1) | 27 (10.2) | 31.8 | 23 (8.7) |
| 29 | 775 | 747 (96.4) | 103 (13.8) | 37 (5.0) | 35.9 | 30 (4.0) |
| 30 | 311 | 285 (91.6) | 56 (19.6) | 17 (6.0) | 30.4 | 13 (4.6) |
| 31 | 188 | 158 (84.0) | 46 (29.1) | 28 (17.7) | 60.9 | 26 (16.5) |
| 32 | 679 | 529 (77.9) | 107 (20.2) | 29 (5.5) | 27.1 | 25 (4.7) |
| 33 | 513 | 473 (92.2) | 58 (12.3) | 21 (4.4) | 36.2 | 17 (3.6) |
| 34 | 188 | 188 (100) | 67 (35.6) | 37 (19.7) | 55.2 | 35 (18.6) |
| 35 | 261 | 249 (95.4) | 84 (33.7) | 32 (12.9) | 38.1 | 32 (12.9) |
| 36 | 238 | 216 (90.8) | 99 (45.8) | 36 (16.7) | 36.4 | 35 (16.2) |
| Total | 7303 | 6305 (86.3) | 1419 (22.5) | 534 (8.5) | 37.6 | 479 (7.6) |
| Overall total | 14,370 | 12,510 (87.1) | 2675 (21.4) | 1024 (8.2) | 38.3 | 930 (7.4) |
| Baseline characteristics | Intervention (N = 6205) | Control (N = 6305) | Total (N = 12,510) |
|---|---|---|---|
| Age (years): mean (SD) | 74.1 (13.72), n = 6154 | 74.7 (13.04), n = 6224 | 74.4 (13.39), n = 12,378 |
| Length of hospital stay (days): mean (SD) | 27.3 (34.47), n = 5860 | 28.5 (31.65), n = 6076 | 27.9 (33.07), n = 11,936 |
| Sex (n, %), male | 2931 (47.2) | 3036 (48.2) | 5967 (47.7) |
| Ethnicity (n, %), white | 5742 (92.5) | 5886 (93.4) | 11,628 (92.9) |
| Living circumstances (n, %): | |||
| Lives alone | 2518 (40.6) | 2404 (38.1) | 4922 (39.3) |
| Co-habits | 3013 (48.6) | 3214 (51.0) | 6227 (49.8) |
| Nursing/residential care home | 368 (5.9) | 323 (5.1) | 691 (5.5) |
| Missing | 306 (4.9) | 364 (5.8) | 670 (5.4) |
| Co-resident caregiver (n, %) | 2356 (38.0) | 2659 (42.2) | 5015 (40.1) |
| The caregiver is the patients . . . (n, %): | |||
| Partner | 2528 (40.7) | 2519 (40.0) | 5047 (40.3) |
| Daughter/son | 1914 (30.8) | 1673 (26.5) | 3587 (28.7) |
| Other relative | 421 (6.8) | 375 (5.9) | 796 (6.3) |
| Other non-relative | 144 (2.3) | 157 (2.5) | 301 (2.4) |
| No carer | 887 (14.3) | 1078 (17.1) | 1965 (15.7) |
| Missing | 311 (5.0) | 503 (8.0) | 814 (6.5) |
Sample size
In total, 930 patients and their caregivers were registered between February 2008 and February 2010 (Figure 4). The number of patient and caregiver dyads recruited per SRU ranged from 13 to 38 per centre. Although, this is above the original target sample size and below the revised sample size of 950 patients and caregivers, it still provides us with over 80% power to detect a clinically relevant difference of six points on the NEADL scale.
Analysis populations
Confirmation of written informed consent could not be obtained centrally for two patients (one in each of the two randomised groups); therefore, these patients were not included in the ITT population.
The ITT population contains a total of 928 patients (intervention n = 450, control n = 478).
Trial conduct
A CONSORT (Consolidated Standards of Reporting Trials) flow diagram of trial progress and loss to follow-up for SRUs (clusters) and individual participants (patients and caregivers) is presented in Figures 5 (patients) and 6 (caregivers).
FIGURE 4.
Accrual into TRACS trial: monthly, overall, cumulative and target accrual.
FIGURE 5.
Clusters and patients.
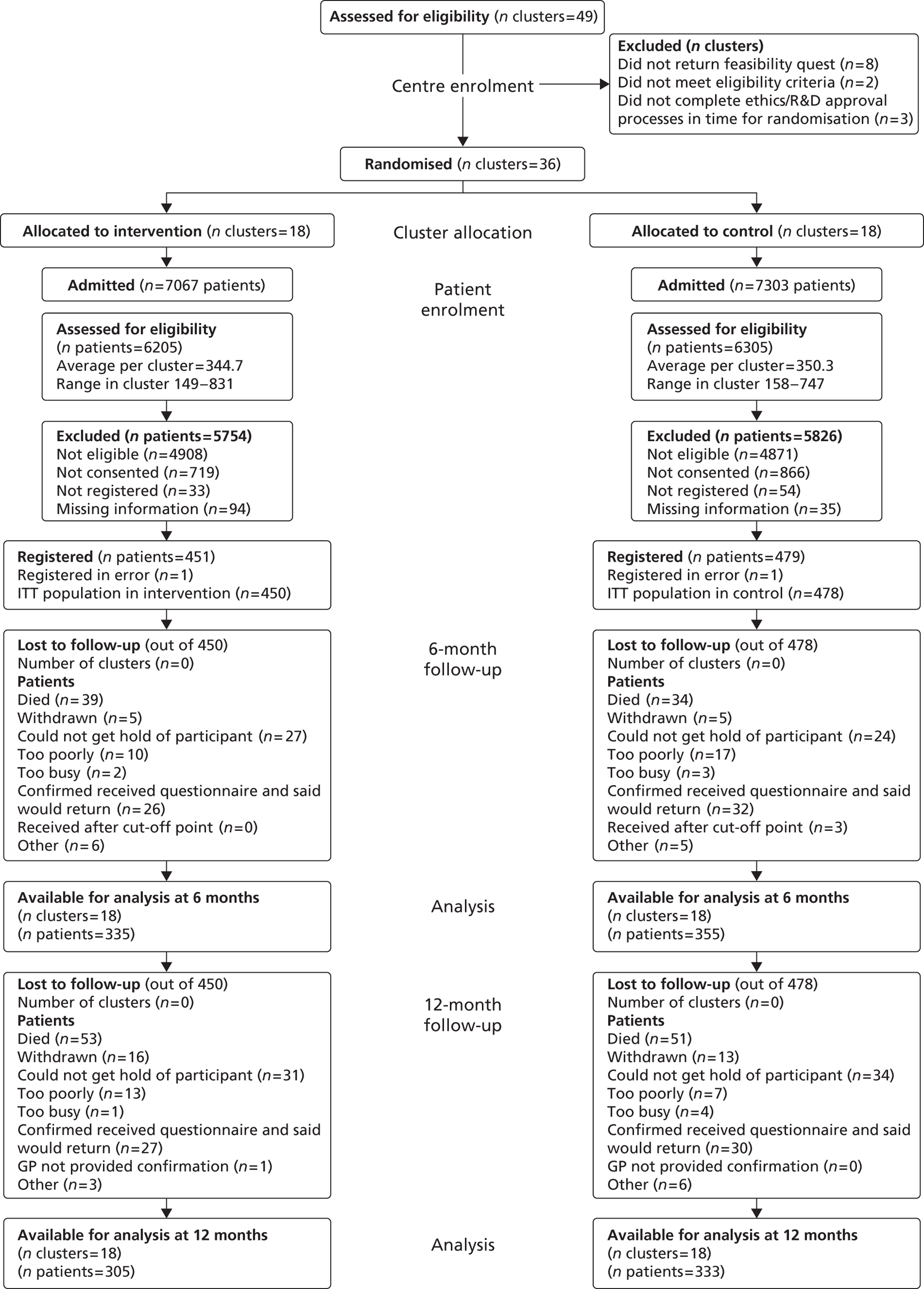
FIGURE 6.
Clusters and caregivers.
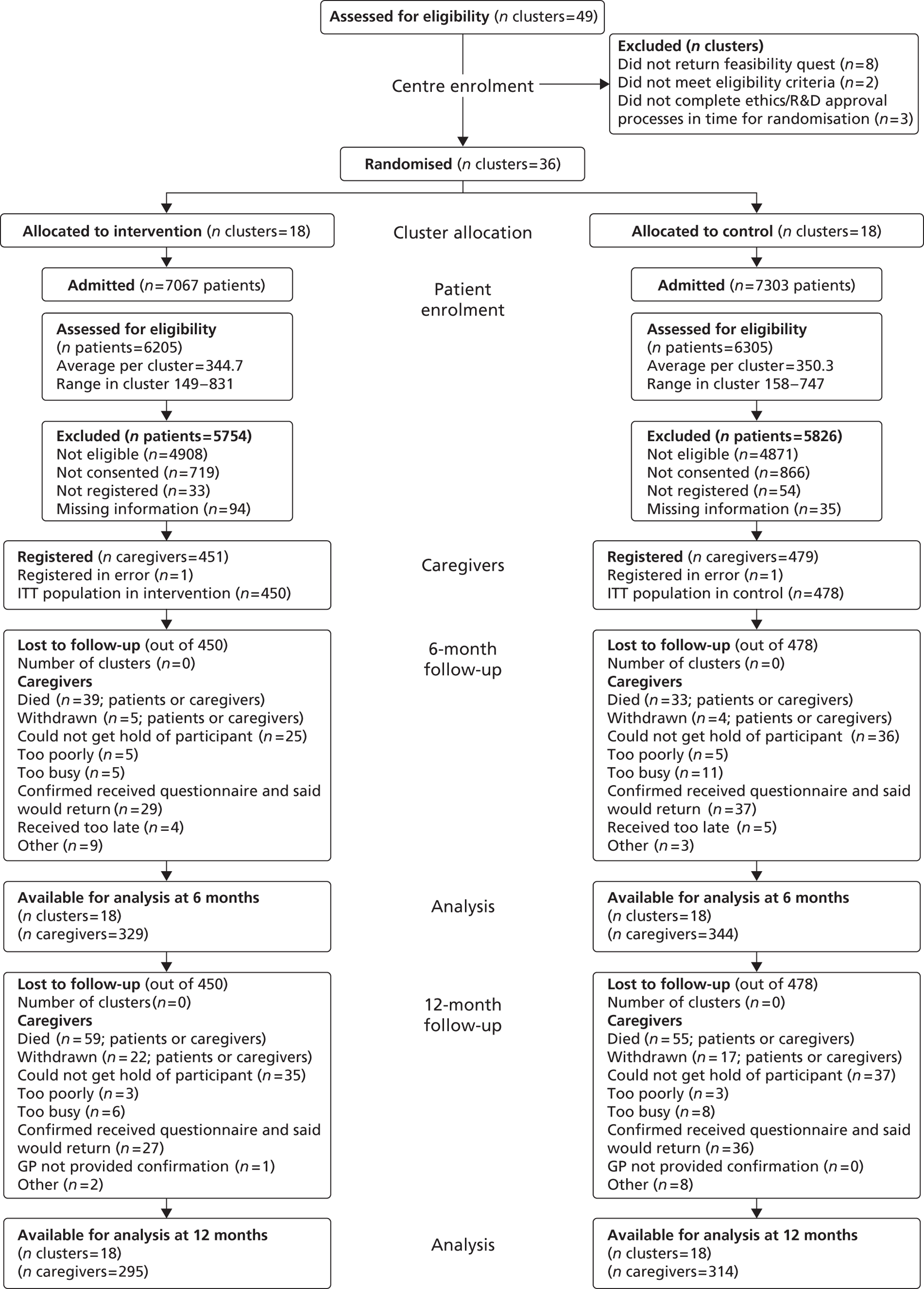
Baseline data
Patient summaries
Baseline characteristics and clinical details of the ITT population are presented in Tables 8–11. The two groups were well balanced with respect to these characteristics.
The mean age at registration was 71.0 (SD 12.76) years and 71.3 (SD 12.18) years in the intervention and control groups, respectively. There were more males than females: 257 (57.1%) in the intervention group and 262 (54.8%) in the control group, respectively. The majority of the participants were of white ethnic background: 429 (95.3%) in the intervention group and 444 (92.9%) in the control group. Most patients had a formal education: 427 (94.9%) in the intervention group and 457 (95.6%) in the control group.
At the time of registration, most patients were retired: 311 (69.1%) and 337 (70.5%) in the intervention and control groups, respectively, followed by patients in full employment, 80 (17.8%) in the intervention group and 77 (16.1%) in the control group.
The majority of patients' preferred language was English, 439 (97.6%) in the intervention group and 467 (97.7%) in the control group.
In most cases, the caregiver was the patient's close relative: partner [314 (69.8%) in intervention and 315 (65.9%) in control] or offspring [118 (26.2%) in intervention and 135 (28.2%) in control]. A small proportion of patients lived alone before the stroke: 66 (14.7%) in intervention and 85 (17.8%) in control groups.
The majority of the patients had received no more education since leaving school [270 (60%) intervention; 291 (61.1%) control] and the majority of the patients left education by the age of 16 years [328 (72.9%) intervention; 381 (79.7%) control].
The clinical details of the patient population include the clinical and pathological classification of current stroke and patient abilities following stroke. The majority of patients had ischaemic stroke [380 (84.4%) intervention; 401 (83.9%) control]. Some patients had experienced a previous stroke: 71 (15.8%) in intervention and 96 (20.1%) in control. The number of patients with dysphasia was similar across both groups: 118 (26.2%) intervention and 112 (23.4%) in control.
Researcher-completed patient baseline scores for the Barthel Index, modified Rankin Scale and 6CIT are displayed in Table 12. Scores were similar across both groups. Over one-third of patients able to complete the cognitive assessment (6CIT) demonstrated a cognitive impairment [135 (36.5%) intervention; 145 (37.1%) control].
Patient-completed baseline questionnaires
Patient-completed scores for Barthel Index, NEADL scale, HADS, EQ-5D and SIS are displayed in Tables 13–14. Scores were also similar across both groups. The HADS questionnaire was also summarised categorically: 171 intervention patients (38.0%) and 210 control patients (43.9%) had raised anxiety levels, whereas 188 (41.8%) intervention patients and 209 (43.7%) control patients had raised levels of depression.
| Demographics | Intervention (N = 450) | Control (N = 478) |
|---|---|---|
| Age (years), mean (SD) | 71.0 (12.76), n = 450 | 71.3 (12.18), n = 478 |
| Sex, n (%): male | 257 (57.1) | 262 (54.8) |
| Ethnicity, n (%): white | 429 (95.3) | 444 (92.9) |
| Formal education, n (%): yes | 427 (94.9) | 457 (95.6) |
| Employment, n (%) | ||
| Retired | 311 (69.1) | 337 (70.5) |
| Working full time (≥ 30 hours per week) | 80 (17.8) | 77 (16.1) |
| Working part time (< 30 hours per week) | 18 (4.0) | 21 (4.4) |
| Unable to work (for medical and other reasons) | 17 (3.8) | 16 (3.3) |
| Othera | 24 (5.3) | 27 (5.6) |
| Demographics | Intervention (N = 450) | Control (N = 478) |
|---|---|---|
| Patient's preferred language, n (%) | ||
| English | 439 (97.6) | 467 (97.7) |
| If other, speaks and understands English? | ||
| Yes | 6 (1.3) | 3 (0.6) |
| No | 5 (1.1) | 8 (1.7) |
| Patient–caregiver relationship, n (%) | ||
| Partner | 314 (69.8) | 315 (65.9) |
| Daughter/son | 118 (26.2) | 135 (28.2) |
| Other relative | 17 (3.8) | 23 (4.8) |
| Other non-relative | 1 (0.2) | 5 (1.0) |
| Did the patient live alone before the stroke? n (%) | ||
| Yes | 66 (14.7) | 85 (17.8) |
| Demographics | Intervention (N = 450) | Control (N = 478) |
|---|---|---|
| More education since leaving school, n (%) | ||
| Yes | 156 (34.7) | 163 (34.1) |
| No | 270 (60.0) | 292 (61.1) |
| No formal education | 23 (5.1) | 21 (4.4) |
| Missing | 1 (0.2) | 2 (0.4) |
| Age patient left education, n (%) | ||
| ≤ 16 years | 328 (72.9) | 381 (79.7) |
| > 16 years | 97 (21.6) | 76 (15.9) |
| No formal education | 23 (5.1) | 21 (4.4) |
| Unknown | 1 (0.2) | 0 (0.0) |
| Missing | 1 (0.2) | 0 (0.0) |
| Stroke details/ability | Intervention (N = 450) | Control (N = 478) |
|---|---|---|
| Pathological classification of current stroke, n (%) | ||
| Cerebral infarction | 380 (84.4) | 401 (83.9) |
| Primary intracerebral haemorrhage | 56 (12.4) | 72 (15.1) |
| Subarachnoid haemorrhagea | 6 (1.3) | 5 (1.0) |
| Other | 7 (1.6) | 0 (0.0) |
| Missing | 1 (0.2) | 0 (0.0) |
| Clinical classification of stroke symptoms, n (%) | ||
| Left hemiparesis | 217 (48.2) | 253 (52.9) |
| Right hemiparesis | 203 (45.1) | 187 (39.1) |
| Brain stem | 16 (3.6) | 20 (4.2) |
| Other | 14 (3.1) | 18 (3.8) |
| Has the patient had a previous stroke? n (%) | ||
| Yes | 71 (15.8) | 96 (20.1) |
| No | 376 (83.6) | 381 (79.7) |
| Missing | 3 (0.7) | 1 (0.2) |
| Orientated in time, place and person, n (%) | ||
| Yes | 380 (84.4) | 397 (83.1) |
| No | 70 (15.6) | 81 (16.9) |
| Able to lift both arms off the bed, n (%) | ||
| Yes | 303 (67.3) | 327 (68.4) |
| No | 146 (32.4) | 151 (31.6) |
| Missing | 1 (0.2) | 0 (0.0) |
| Able to walk without help from others, n (%) | ||
| Yes | 148 (32.9) | 159 (33.3) |
| No | 301 (66.9) | 319 (66.7) |
| Missing | 1 (0.2) | 0 (0.0) |
| Patient's language ability, n (%) | ||
| Normal | 290 (64.4) | 307 (64.2) |
| Dysphasia | 118 (26.2) | 112 (23.4) |
| Dysarthria | 42 (9.3) | 58 (12.1) |
| Missing | 0 (0.0%) | 1 (0.2) |
| Does the patient intend to live alone after discharge? n (%) | ||
| No | 399 (88.7) | 401 (83.9) |
| Are the patient and caregiver intending to live together after discharge? n (%) | ||
| Yes | 376 (83.6) | 386 (80.8) |
| Index/scale | Intervention (N = 450) | Control (N = 478) |
|---|---|---|
| Barthel Index pre stroke (0–20)a, n (%) | ||
| Mean (SD) | 19.2 (2.22), n = 450 | 18.8 (2.77), n = 478 |
| 6CIT (0–28)b, n (%) | ||
| Number (%) of 6CIT scores recorded (only patients who were able to complete those) | 370 (82.2) | 391 (81.8) |
| Normal | 235 (63.5) | 246 (62.9) |
| Impaired | 135 (36.5) | 145 (37.1) |
| Modified Rankin Scale (0–5)c, n (%) | ||
| Missing | 3 (0.7) | 0 (0.0) |
| 0 | 1 (0.2) | 3 (0.6) |
| 1 | 3 (0.7) | 4 (0.8) |
| 2 | 44 (9.8) | 43 (9.0) |
| 3 | 137 (30.4) | 148 (31.0) |
| 4 | 244 (54.2) | 258 (54.0) |
| 5 | 18 (4.0) | 22 (4.6) |
| Index/scale | Intervention (N = 450) | Control (N = 478) |
|---|---|---|
| NEADL score (0–66)a | ||
| Mean (SD) | 52.0 (15.77), n = 443 | 52.3 (15.80), n = 474 |
| Barthel Index post stroke (0–20)b | ||
| Mean (SD) | 12.2 (5.38), n = 442 | 12.6 (5.45), n = 473 |
| HADS (0–21)c, n (%) | ||
| HADS Anxiety score | ||
| Normal | 256 (56.9) | 254 (53.1) |
| Borderline abnormal | 83 (18.4) | 98 (20.5) |
| Abnormal | 88 (19.6) | 112 (23.4) |
| Missing | 23 (5.1) | 14 (2.9) |
| Mean (SD) | 6.7 (4.47), n = 427 | 7.3 (4.65), n = 464 |
| HADS Depression score | ||
| Normal | 241 (53.6) | 254 (53.1) |
| Borderline abnormal | 77 (17.1) | 86 (18.0) |
| Abnormal | 111 (24.7) | 123 (25.7) |
| Missing | 21 (4.7) | 15 (3.1) |
| Mean (SD) | 7.3 (4.68), n = 429 | 7.6 (4.81), n = 463 |
| EQ-5D indexd (–0.59, 1) | ||
| Mean (SD) | 0.360 (0.375), n = 426 | 0.380 (0.357), n = 459 |
| SIS | Intervention (N = 450) | Control (N = 478) |
|---|---|---|
| SIS (0–100)a | ||
| Strength score | ||
| Mean (SD) | 42.4 (27.00), n = 414 | 45.7 (29.14), n = 440 |
| ADL score | ||
| Mean (SD) | 43.6 (27.23), n = 431 | 46.3 (26.72), n = 466 |
| Mobility score | ||
| Mean (SD) | 41.1 (29.29), n = 424 | 43.2 (29.06), n = 459 |
| Hand function score | ||
| Mean (SD) | 26.9 (33.00), n = 426 | 28.6 (34.02), n = 440 |
| Physical domains score | ||
| Mean (SD) | 39.7 (25.19), n = 434 | 42.3 (25.47), n = 466 |
| Memory score | ||
| Mean (SD) | 66.7 (29.85), n = 431 | 67.9 (29.74), n = 467 |
| Mood score | ||
| Mean (SD) | 69.0 (18.48), n = 425 | 69.7 (17.80), n = 462 |
| Communication score | ||
| Mean (SD) | 76.4 (28.02), n = 433 | 76.9 (28.85), n = 471 |
Caregiver summaries
The caregiver baseline demographics of the ITT population are displayed in Tables 15–17. The mean caregiver age was 61.1 (SD 14.64) years and 60.8 (SD 13.91) years in the intervention and control groups, respectively. In contrast with patients, more females than males were acting as caregivers in both groups: 310 (68.9%) in the intervention group and 325 (68.0%) in the control group. The majority of caregivers were of white ethnic background: 430 (95.6%) in the intervention group and 446 (93.3%) in the control group.
A large proportion of caregivers were retired [195 (43.3%) in the intervention group and 221 (46.2%) in the control group], followed by caregivers in full- and part-time employment [187 (41.5%) in the intervention group and 188 (39.3%) in the control group].
The majority of caregivers had formal education [435 (96.7%) in intervention and 464 (97.1%) in control], with most caregivers leaving education by the age of 16 years [317 (70.4%) in the intervention group and 339 (70.9%) in the control group].
Nearly all caregivers' preferred language was English [447 (99.3%) in intervention and 475 (99.4%) in control]. The majority of caregivers lived with the patient during the last 12 months [356 (79.1%) in the intervention group and 364 (76.2%) in the control group].
Researcher completed caregiver modified Rankin Scale scores are summarised in Table 18. The majority of caregivers had a score of 0 or 1 [427 (94.9%) in the intervention group and 453 (94.7%) in the control group].
The baseline scores in FAI, HADS and EQ-5D completed by caregivers show similarities between the treatment groups (Table 19). The HADS questionnaire was summarised categorically. A sizeable proportion of caregivers showed raised levels of anxiety at baseline [236 (52.5%) in the intervention group and 238 (49.8%) in the control group], whereas 122 (27.1%) intervention caregivers and 122 (25.5%) control caregivers had raised levels of depression.
| Demographics | Intervention (N = 450) | Control (N = 478) |
|---|---|---|
| Age (years), mean (SD) | 61.1 (14.64), n = 450 | 60.8 (13.91), n = 478 |
| Sex, n (%): male | 140 (31.1) | 153 (32.0) |
| Ethnicity, n (%): white | 430 (95.6) | 446 (93.3) |
| Formal education, n (%): yes | 435 (96.7) | 464 (97.1) |
| Employment, n (%) | ||
| Retired | 195 (43.3) | 221 (46.2) |
| Working full-time (≥ 30 hours per week) | 127 (28.2) | 123 (25.7) |
| Working part time (< 30 hours per week) | 60 (13.3) | 65 (13.6) |
| Othera | 68 (15.1) | 69 (14.4) |
| Demographics | Intervention (N = 450) | Control (N = 478) |
|---|---|---|
| Caregiver's preferred language,a n (%) | ||
| English | 447 (99.3) | 475 (99.4) |
| In the last 12 months, has the caregiver resided with the patient? n (%) | ||
| Yes | 356 (79.1) | 364 (76.2) |
| Demographics | Intervention (N = 450) | Control (N = 478) |
|---|---|---|
| More education since leaving school, n (%) | ||
| Yes | 199 (44.2) | 211 (44.1) |
| No | 233 (51.8) | 245 (51.3) |
| No formal education | 15 (3.3) | 13 (2.7) |
| Missing | 3 (0.7) | 9 (1.9) |
| Age caregiver left education,a n (%) | ||
| ≤ 16 years | 317 (70.4) | 339 (70.9) |
| > 16 years | 133 (29.6) | 139 (29.1) |
| Modified Rankin Scale (0–5a) | Intervention (N = 450), n (%) | Control (N = 478), n (%) |
|---|---|---|
| 0 | 387 (86.0) | 385 (80.5) |
| 1 | 40 (8.9) | 68 (14.2) |
| 2 | 16 (3.6) | 19 (4.0) |
| 3 | 6 (1.3) | 4 (0.8) |
| 4 | 1 (0.2) | 2 (0.4) |
| Index/scale | Intervention (N = 450) | Control (N = 478) |
|---|---|---|
| FAI (range 0–45)a | ||
| Mean (SD) | 32.9 (7.99), n = 441 | 33.5 (7.40), n = 470 |
| HADS (0–21b), n (%) | ||
| HADS Anxiety score, n (%) | ||
| Normal | 206 (45.8) | 232 (48.5) |
| Borderline abnormal | 94 (20.9) | 90 (18.8) |
| Abnormal | 142 (31.6) | 148 (31.0) |
| Missing | 8 (1.8) | 8 (1.7) |
| Mean (SD) | 8.3 (4.87), n = 442 | 8.0 (4.74), n = 470 |
| HADS Depression score, n (%) | ||
| Normal | 320 (71.1) | 349 (73.0) |
| Borderline abnormal | 69 (15.3) | 81 (16.9) |
| Abnormal | 53 (11.8) | 41 (8.6) |
| Missing | 8 (1.8) | 7 (1.5) |
| Mean (SD) | 5.2 (4.25), n = 442 | 5.0 (3.89), n = 471 |
| EQ-5D index (–0.59, 1)c | ||
| Mean (SD) | 0.797 (0.232), n = 438 | 0.791 (0.245), n = 471 |
Cluster-level balance
Two-sample t-tests weighted by inverse number of patients in each centre were used to compare baseline characteristics of patients and caregivers between intervention and control groups. These tests showed that there are no statistically significant differences between patients (Tables 20 and 21) or caregivers (Tables 22 and 23).
| Patient baseline details | Total | Intervention, mean (SD) | Control, mean (SD) | p-value (two-sided) |
|---|---|---|---|---|
| Barthel Index pre stroke score | 36 | 19.3 (0.12) | 18.7 (0.21) | 0.0536 |
| 6CIT score | 36 | 7.9 (0.49) | 8.3 (0.61) | 0.6082 |
| Age (years) | 36 | 70.2 (0.86) | 71.1 (0.87) | 0.5326 |
| Barthel Index post-stroke score | 36 | 12.1 (0.26) | 12.8 (0.30) | 0.1467 |
| NEADL pre-stroke score | 36 | 52.8 (0.75) | 51.2 (1.03) | 0.2848 |
| SIS Physical score | 36 | 38.3 (1.47) | 42.1 (1.29) | 0.0994 |
| SIS Memory score | 36 | 66.4 (1.88) | 68.1 (2.01) | 0.5731 |
| SIS Mood score | 36 | 68.3 (1.07) | 70.6 (0.88) | 0.1437 |
| SIS Communication score | 36 | 77.0 (1.39) | 77.4 (1.45) | 0.8697 |
| SIS Recovery score | 36 | 44.3 (1.41) | 48.3 (1.23) | 0.0739 |
| EQ-5D score | 36 | 0.4 (0.02) | 0.4 (0.02) | 0.4499 |
| HADS Anxiety score | 36 | 7.0 (0.30) | 7.1 (0.20) | 0.9262 |
| HADS Depression score | 36 | 7.5 (0.28) | 7.4 (0.24) | 0.8535 |
| Patient baseline details | Total | Intervention, % (SD) | Control, % (SD) | p-value (two-sided) |
|---|---|---|---|---|
| Sex: male | 36 | 55.4 (2.03) | 54.7 (1.80) | 0.8162 |
| Ethnicity: white | 36 | 95.4 (1.79) | 94.8 (2.17) | 0.8674 |
| Left education aged ≤ 16 years | 36 | 70.5 (3.43) | 76.1 (3.30) | 0.305 |
| Independent before this stroke: yes | 36 | 92.0 (1.28) | 89.1 (1.74) | 0.24 |
| Lived alone before this stroke: yes | 36 | 14.5 (1.99) | 16.3 (2.29) | 0.622 |
| Intends to live alone after discharge: no | 36 | 89.9 (1.63) | 84.7 (2.23) | 0.1033 |
| Pathological classification of stroke: cerebral infarction | 36 | 84.8 (1.57) | 85.2 (1.52) | 0.8897 |
| Clinical classification of stroke: left hemiparesis | 36 | 48.4 (2.50) | 54.0 (2.28) | 0.1579 |
| Previous stroke: no | 36 | 84.2 (2.17) | 78.7 (1.95) | 0.1093 |
| Patient can talk and orientated | 36 | 85.4 (2.38) | 85.5 (2.42) | 0.9663 |
| Can lift both arms off the bed | 36 | 67.7 (3.77) | 67.1 (3.17) | 0.9096 |
| Patient can walk without help of others | 36 | 32.0 (3.31) | 34.5 (2.84) | 0.6283 |
| Normal language ability | 36 | 63.7 (3.63) | 66.0 (2.95) | 0.6728 |
| Caregiver baseline details | Total | Intervention, mean (SD) | Control, mean (SD) | p-value (two-sided) |
|---|---|---|---|---|
| Age (years) | 36 | 60.9 (0.95) | 61.3 (1.48) | 0.8541 |
| EQ-5D score | 36 | 0.8 (0.02) | 0.8 (0.01) | 0.838 |
| HADS Anxiety score | 36 | 8.3 (0.27) | 8.0 (0.22) | 0.4811 |
| HADS Depression score | 36 | 5.4 (0.27) | 5.0 (0.14) | 0.2081 |
| FAI score | 36 | 33.1 (0.39) | 33.3 (0.37) | 0.7845 |
| Caregiver baseline details | Total | Intervention, % (SD) | Control, % (SD) | p-value (two-sided) |
|---|---|---|---|---|
| Sex: male | 36 | 32.8 (2.22) | 32.2 (2.01) | 0.8656 |
| Ethnicity: white | 36 | 95.5 (1.82) | 95.0 (2.11) | 0.8713 |
| In full-time employment | 36 | 30.4 (1.96) | 26.4 (2.94) | 0.3358 |
| Modified Rankin scale = 0 | 36 | 87.9 (2.24) | 82.3 (2.21) | 0.1303 |
Trial outcomes
Questionnaire follow-up
Efforts were made to follow all patients and caregivers when appropriate. For primary end point, the cut-off period was set at 10 months. With this cut-off time-point, only three patients' questionnaires and 10 caregivers' questionnaires were excluded.
Patients
At 6 months for patient primary end point, the overall response rate for received questionnaires was 74.4% in the intervention group and 74.3% in the control group, 74.4% overall. A detailed summary of the reasons as to why questionnaires were not received is shown in Table 24. The main reasons for not returning the questionnaires were patients' deaths [73 (7.9%)], patients confirming that they would return questionnaire but questionnaire was never received [58 (6.3%)] and inability to get hold of participant [51 (5.5%)].
The response rate at 12 months was 67.8% in the intervention group and 69.6% in the control group, 68.8% in total (Table 25). The main reasons for not returning the questionnaires at this time-point were patients' deaths [104 (11.2%)], inability to get hold of participant [65 (7.0%)] and patients confirming that they would return questionnaire but questionnaire was never received [54 (6.1%)].
| Patient follow-up | Intervention (N = 450), n (%) | Control (N = 478), n (%) | Total (N = 928), n (%) |
|---|---|---|---|
| Questionnaire | |||
| Received | 329 (73.1) | 348 (72.8) | 677 (73.0) |
| Received over the telephone | 6 (1.3) | 7 (1.5) | 13 (1.4) |
| Patient died | 39 (8.7) | 34 (7.1) | 73 (7.9) |
| Patient withdrew | 5 (1.1) | 5 (1.0) | 10 (1.1) |
| Received too late | 0 (0.0) | 3 (0.6) | 3 (0.3) |
| Questionnaire not returned | 71 (15.8) | 81 (16.9) | 152 (16.4) |
| Reasons questionnaires not received | |||
| Too poorly | 10 (2.2) | 17 (3.6) | 27 (2.9) |
| Could not get hold of participant | 27 (6.0) | 24 (5.0) | 51 (5.5) |
| Confirmed received questionnaire and said that would return | 26 (5.8) | 32 (6.7) | 58 (6.3) |
| Too busy | 2 (0.4) | 3 (0.6) | 5 (0.5) |
| Other | 6 (1.3) | 5 (1.0) | 11 (1.2) |
| Patient follow-up | Intervention (N = 450), n (%) | Control (N = 478), n (%) | Total (N = 928), n (%) |
|---|---|---|---|
| Questionnaire | |||
| Received | 300 (66.7) | 329 (68.8) | 629 (67.8) |
| Received over the telephone | 5 (1.1) | 4 (0.8) | 9 (1.0) |
| Patient died | 53 (11.8) | 51 (10.7) | 104 (11.2) |
| Patient withdrew | 16 (3.6) | 13 (2.7) | 29 (3.1) |
| GP not provided confirmation (patient) | 1 (0.2) | 0 (0.0) | 1 (0.1) |
| Questionnaire not returned | 75 (16.7) | 81 (16.9) | 156 (16.8) |
| Reasons questionnaires not received | |||
| Too poorly | 13 (2.9) | 7 (1.5) | 20 (2.2) |
| Could not get hold of participant | 31 (6.9) | 34 (7.1) | 65 (7.0) |
| Confirmed received questionnaire and said that would return | 27 (6.0) | 30 (6.3) | 57 (6.1) |
| Too busy | 1 (0.2) | 4 (0.8) | 5 (0.5) |
| Other | 3 (0.7) | 6 (1.3) | 9 (1.0) |
Caregivers
Table 26 displays the response rate for caregivers' questionnaires at 6 months. Overall, 72.5% were received, 73.1% in the intervention and 72.0% in the control group. The main reasons for questionnaires not received were patients' deaths [70 (7.5%)], caregiver said that they would return questionnaire but questionnaire was never received [66 (7.1%)] and inability to get hold of caregiver [61 (6.6%)].
The response rates for caregivers' questionnaires at 12 months are summarised in Table 27. The number of received questionnaires at 12 months was lower than at 6 months. Overall, 65.6% were received: 65.6% in the intervention group and 65.7% in the control group. The main reasons for questionnaires not received were patients' deaths [104 (11.2%)], not being able to get hold of participant [72 (7.8%)] and caregiver said that they would return questionnaire but questionnaire was never received [63 (6.8%)].
| Caregiver follow-up | Intervention (N = 450), n (%) | Control (N = 478 ), n (%) | Total (N = 928), n (%) |
|---|---|---|---|
| Questionnaire | |||
| Received | 327 (72.7) | 336 (70.3) | 663 (71.4) |
| Received over the telephone | 2 (0.4) | 8 (1.7) | 10 (1.1) |
| Patient died | 37 (8.2) | 33 (6.9) | 70 (7.5) |
| Caregiver died | 2 (0.4) | 0 (0.0) | 2 (0.2) |
| Both withdrew | 5 (1.1) | 4 (0.8) | 9 (1.0) |
| Received too late | 4 (0.9) | 5 (1.0) | 9 (1.0) |
| Questionnaire not returned | 73 (16.2) | 92 (19.2) | 165 (17.8) |
| Reasons questionnaires not received | |||
| Too poorly | 5 (1.1) | 5 (1.0) | 10 (1.1) |
| Could not get hold of participant | 25 (5.6) | 36 (7.5) | 61 (6.6) |
| Confirmed received questionnaire and said that would return | 29 (6.4) | 37 (7.7) | 66 (7.1) |
| Too busy | 5 (1.1) | 11 (2.3) | 16 (1.7) |
| Other | 9 (2.0) | 3 (0.6) | 12 (1.3) |
| Caregiver follow-up | Intervention (N = 450), n (%) | Control (N = 478), n (%) | Total (N = 928), n (%) |
|---|---|---|---|
| Questionnaire | |||
| Received | 292 (64.9) | 313 (65.5) | 605 (65.2) |
| Received over the telephone | 3 (0.7) | 1 (0.2) | 4 (0.4) |
| Patient died | 53 (11.8) | 51 (10.7) | 104 (11.2) |
| Caregiver died | 6 (1.3) | 4 (0.8) | 10 (1.1) |
| Patient withdrew | 1 (0.2) | 1 (0.2) | 2 (0.2) |
| Caregiver withdrew | 8 (1.8) | 5 (1.0) | 13 (1.4) |
| Both withdrew | 13 (2.9) | 11 (2.3) | 24 (2.6) |
| GP not provided confirmation (patient) | 1 (0.2) | 0 (0.0) | 1 (0.1) |
| Questionnaire not returned | 73 (16.2) | 92 (19.2) | 165 (17.8) |
| Reasons questionnaires not received | |||
| Too poorly | 3 (0.7) | 3 (0.6) | 6 (0.6) |
| Could not get hold of participant | 35 (7.8) | 37 (7.7) | 72 (7.8) |
| Confirmed received questionnaire and said that would return | 27 (6.0) | 36 (7.5) | 63 (6.8) |
| Too busy | 6 (1.3) | 8 (1.7) | 14 (1.5) |
| Other | 2 (0.4) | 8 (1.7) | 10 (1.1) |
Proxy responses
Proxy responses, where the entire questionnaire was completed on patients' behalf without consulting them, were anticipated. At baseline, 63 (6.8%) of questionnaires were via a proxy response; at 6 months and 12 months, 37 (5.5%) and 41 (6.5%), respectively, were via a proxy response (Table 28).
| Questionnaire | Intervention, n (%) | Control, n (%) | Total, n (%) |
|---|---|---|---|
| Baseline | 33 (7.3), n = 450 | 30 (6.3), n = 478 | 63 (6.8), n = 928 |
| 6 months | 15 (4.5), n = 330 | 22 (6.3), n = 348 | 37 (5.5), n = 678 |
| 12 months | 22 (7.3), n = 301 | 19 (5.8), n = 330 | 41 (6.5), n = 631 |
Primary outcomes
Patients
Table 29 summarises the unadjusted NEADL scores for patients. Overall, NEADL scores were similar between the two treatment groups; the mean score decreased at 6 months post stroke when compared with pre-stroke level and minimally increased at 12 months.
Mean adjusted NEADL scores for 6 months were calculated and shown in Table 30, adjusted for patient- and SRU-level covariates (see Chapter 3). Results were similar to the unadjusted results, the adjusted NEADL mean score for the intervention was 27.4 [standard error (SE) 1.00] and for the control 27.6 (SE 0.99), with a mean difference of –0.2 points [95% confidence interval (CI) –3.0 to 2.5 points; p = 0.866] and an adjusted ICC of 0.027, indicating that there is no evidence of a statistically significant difference between the treatment groups in NEADL scores at 6 months.
In the trial protocol, a clinically relevant difference was defined as 6 (SD 18) points on the NEADL scale. Given these results, differences between the groups at 6 months were minimal and did not reach either clinical or statistical significance.
Caregivers
Table 31 summarises the unadjusted CBS scores and subscales for caregivers. Overall, the total and subscales CBS scores were similar between the groups at 6 and 12 months.
The mean adjusted CBS scores at 6 months were calculated, Table 32, adjusted for patient-level and SRU-level covariates (see Chapter 3). Results were similar to the unadjusted results, the adjusted CBS mean score for intervention was 45.5 (SE 0.83) and for control 45.0 (SE 0.83), with a mean difference of 0.5 points (95% CI –1.7 to 2.7 points; p = 0.660) and adjusted ICC of 0.013, indicating that there is no evidence of statistically significant difference between the groups in caregiver burden at 6 months.
| Patients' NEADL scores: unadjusted scores | ||||||
|---|---|---|---|---|---|---|
| Questionnaire | Baselinea | 6 months | 12 months | |||
| Intervention, mean (SD), n | Control, mean (SD), n | Intervention, mean (SD), n | Control, mean (SD), n | Intervention, mean (SD), n | Control, mean (SD), n | |
| NEADL | 52.0 (15.77), 443 | 52.3 (15.80), 474 | 26.9 (17.68), 330 | 26.6 (17.60), 348 | 28.7 (18.49), 301 | 28.3 (17.86), 330 |
| Patients' primary outcome at 6 months – adjusted score | |||||||
|---|---|---|---|---|---|---|---|
| Questionnaire | Intervention, mean (SE), n | Control, mean (SE), n | Difference (SE) | 95% CI of the difference | p-value | Unadjusted ICC | Adjusted ICC |
| NEADL | 27.4 (1.00), 330 | 27.6 (0.99), 348 | –0.2 (1.34) | (–3.0 to 2.5) | 0.866 | 0.016 | 0.027 |
| CBS: caregivers' questionnaire scores: unadjusted means | ||||
|---|---|---|---|---|
| Questionnaire | 6 months | 12 months | ||
| Intervention, mean (SD), n | Control, mean (SD), n | Intervention, mean (SD), n | Control, mean (SD), n | |
| Total CBSa score (0–88) | 46.3 (14.97), 325 | 45.8 (14.30), 340 | 45.9 (15.01), 291 | 45.2 (14.22), 314 |
| Subscales | ||||
| General strain | 2.3 (0.81), 325 | 2.3 (0.787), 340 | 2.3 (0.82), 291 | 2.3 (0.79), 314 |
| Isolation | 2.2 (0.82), 324 | 2.2 (0.86), 340 | 2.2 (0.79), 291 | 2.2 (0.83), 314 |
| Disappointment | 2.2 (0.83), 325 | 2.2 (0.78), 343 | 2.2 (0.81), 291 | 2.1 (0.76), 314 |
| Emotional involvement | 1.6 (0.67), 325 | 1.6 (0.67), 340 | 1.6 (0.71), 291 | 1.7 (0.70), 314 |
| Environment | 1.8 (0.71), 324 | 1.7 (0.67), 340 | 1.7 (0.72), 291 | 1.6 (0.65), 314 |
| CBS: caregivers' primary end point at 6 months: adjusted score | |||||||
|---|---|---|---|---|---|---|---|
| Questionnaire | Intervention, mean (SE), n | Control, mean (SE), n | Difference (SE) | 95% CI of the difference | p-value | Unadjusted ICC | Adjusted ICC |
| Total CBS score | 45.5 (0.83), 325 | 45.0 (0.83), 340 | 0.5 (1.08) | (–1.7 to 2.7) | 0.660 | 0.019 | 0.013 |
Sensitivity analyses
Sensitivity analyses for patient primary end point
A sensitivity analysis including patients who had died was undertaken and assumed that these patients had a NEADL score of 0. This sensitivity analysis showed results similar to the primary analysis: the adjusted scores were similar between the groups; the adjusted NEADL mean score for the intervention group was 24.2 and for the control group was 25.1, with –0.9 (95% CI –3.5 to 1.8; p = 0.507) point difference and adjusted ICC of 0.019, again indicating no evidence of a difference between the groups (see Table 80).
A sensitivity analysis without proxy responses was performed to assess the impact of proxy responses on the analysis of the primary end point. This sensitivity analysis also showed results similar to the primary analysis: the adjusted mean NEADL score for the intervention group was 28.2 points and for the control group was 28.6 points, with –0.4 (95% CI –3.4 to 2.5; p = 0.766) point difference and adjusted ICC of 0.038 (see Appendix 8, Table 80).
Sensitivity analyses for both patient and caregiver primary end point
The time of completion of questionnaires for primary end points was compared between arms. No statistically significant differences were found for patients (difference –1.2 days, 95% CI –3.4 to 1.0 days; p = 0.2862) or caregivers (difference 0 days, 95% CI –2.1 to 2.2 days; p = 0.9836) (see Table 81).
Secondary outcomes
Patients
Unadjusted Barthel Index, EQ-5D, HADS and SIS mean scores are shown in Table 33. Mean scores, differences in means, 95% CIs, p-values, unadjusted and adjusted ICCs for the questionnaires adjusted for patient- and SRU-level covariates are displayed in Tables 34 and 35. Overall, at both 6 and 12 months there was no evidence of statistically significant differences in patients' physical and psychological outcomes between intervention and control groups.
Caregivers
Unadjusted HADS, EQ-5D and FAI mean scores are shown in Table 36. Mean scores for the questionnaires, differences in means, 95% CIs, p-values, unadjusted and adjusted ICCs adjusted for caregiver- and SRU-level covariates at 6 months are displayed in Tables 37 and 38. Overall, at both 6 and 12 months, there was no evidence of statistically significant differences in caregivers' physical and psychological outcomes between intervention and control groups.
| Patients' questionnaire scores: unadjusted means | ||||||
|---|---|---|---|---|---|---|
| Questionnaire | Baseline | 6 months | 12 months | |||
| Intervention, mean (SD), n | Control, mean (SD), n | Intervention, mean (SD), n | Control, mean (SD), n | Intervention, mean (SD), n | Control, mean (SD), n | |
| Barthel Index pre stroke | 19.2 (2.22), 450 | 18.8 (2.77), 478 | N/A | N/A | N/A | N/A |
| Barthel Index post stroke | 12.2 (5.38), 442 | 12.6 (5.45), 473 | 14.1 (5.14), 323 | 13.9 (5.13), 346 | 14.4 (5.07), 297 | 14.4 (5.02), 325 |
| EQ-5D | 0.360 (0.375), 426 | 0.380 (0.357), 459 | 0.439 (0.344), 319 | 0.443 (0.348), 334 | 0.473 (0.345), 287 | 0.465 (0.344), 311 |
| HADS Anxiety | 6.7 (4.471), 427 | 7.3 (4.66), 464 | 6.7 (4.61), 323 | 6.8 (4.27), 340 | 6.3 (4.36), 294 | 6.7 (4.32), 318 |
| HADS Depression | 7.3 (4.68), 429 | 7.6 (4.81), 463 | 7.4 (4.37), 323 | 7.5 (4.32), 341 | 7.1 (4.36), 294 | 7.5 (4.27), 320 |
| SIS Strengtha | 42.4 (27.00), 414 | 45.7 (29.14), 440 | 48.1 (25.36), 294 | 49.0 (26.56), 318 | 48.7 (25.17), 275 | 50.2 (27.94), 303 |
| SIS Activitya | 43.6 (27.23), 431 | 46.3 (26.72), 466 | 54.2 (27.13), 324 | 53.6 (27.12), 345 | 56.5 (27.47), 296 | 54.1 (27.98), 324 |
| SIS Mobilitya | 41.1 (29.29), 424 | 43.2 (29.06), 459 | 57.6 (28.32), 323 | 57.0 (27.50), 341 | 58.5 (27.96), 295 | 57.6 (27.60), 319 |
| SIS Handa | 26.9 (33.00), 426 | 28.6 (34.02), 440 | 38.3 (35.93), 316 | 38.4 (36.09), 332 | 40.7 (36.35), 293 | 39.0 (36.32), 310 |
| SIS Physical | 39.7 (25.19), 434 | 42.3 (25.47), 466 | 51.7 (25.81), 323 | 51.6 (26.28), 342 | 53.3 (25.63), 295 | 52.0 (26.47), 320 |
| SIS Memory | 66.7 (29.85), 431 | 67.9 (29.74), 467 | 68.9 (27.58), 317 | 68.9 (28.11), 343 | 70.9 (27.79), 293 | 68.7 (27.04), 320 |
| SIS Mood | 69.0 (18.48), 425 | 69.7 (17.80), 462 | 69.3 (18.71), 316 | 67.9 (18.42), 338 | 68.5 (17.90), 285 | 67.4 (18.68), 318 |
| SIS Communication | 76.4 (28.02), 433 | 76.9 (28.85), 471 | 79.2 (24.72), 321 | 79.7 (25.09), 340 | 78.3 (25.19), 296 | 78.0 (25.90), 322 |
| SIS Recover | 45.5 (23.62), 382 | 49.2 (23.42), 403 | 52.6 (23.77), 255 | 53.1 (23.78), 293 | 54.3 (23.94), 245 | 54.6 (24.11), 274 |
| SIS Social Participationb | N/A | N/A | 49.3 (29.13), 307 | 50.0 (30.68), 329 | 52.9 (30.30), 286 | 52.3 (31.21), 309 |
| Questionnaire | Intervention, mean (SE), n | Control, mean (SE), n | Difference (SE) | 95% CI of the difference | p-value | Unadjusted ICC | Adjusted ICC |
|---|---|---|---|---|---|---|---|
| Barthel Index | 14.2 (0.24), 323 | 14.1 (0.23), 346 | 0.1 (0.31) | –0.6 to 0.7 | 0.825 | 0.001 | 0.000 |
| EQ-5D | 0.441 (0.0170), 319 | 0.443 (0.0169), 334 | –0.002 (0.0225) | –0.048 to 0.045 | 0.946 | 0.009 | 0.000 |
| HADS Anxiety | 6.7 (0.22), 323 | 6.6 (0.21), 340 | 0.1 (0.29) | –0.5 to 0.7 | 0.629 | 0.000 | 0.000 |
| HADS Depression | 7.3 (0.22), 323 | 7.2 (0.21), 341 | 0.1 (0.29) | –0.5 to 0.7 | 0.759 | 0.000 | 0.000 |
| SIS Physical | 52.7 (1.10), 323 | 52.0 (1.08), 342 | 0.7 (1.46) | –2.3 to 3.7 | 0.641 | 0.004 | 0.001 |
| SIS Memory | 70.1 (1.26), 317 | 70.4 (1.23), 343 | –0.3 (1.66) | –3.7 to 3.1 | 0.836 | 0.000 | 0.000 |
| SIS Mood | 70.1 (0.99), 316 | 68.6 (0.96), 338 | 1.5 (1.30) | –1.1 to 4.2 | 0.244 | 0.000 | 0.000 |
| SIS Communication | 80.1 (1.07), 321 | 80.9 (1.05), 340 | –0.8 (1.41) | –3.6 to 2.1 | 0.582 | 0.008 | 0.000 |
| SIS Recover | 54.0 (1.72), 255 | 53.9 (1.67), 293 | 0.1 (2.30) | –4.6 to 4.8 | 0.974 | 0.014 | 0.038 |
| SIS Social Participation | 49.5 (1.98), 307 | 50.6 (1.97), 329 | –1.1 (2.67) | –6.6 to 4.4 | 0.683 | 0.025 | 0.026 |
| Questionnaire | Intervention, mean (SE), n | Control, mean (SE), n | Difference (SE) | 95% CI of the difference | p-value | Unadjusted ICC | Adjusted ICC |
|---|---|---|---|---|---|---|---|
| NEADL | 29.6 (0.98), 301 | 29.1 (0.95), 330 | 0.5 (1.30) | –2.2 to 3.2 | 0.696 | 0.002 | 0.015 |
| Barthel Index | 14.6 (0.25), 297 | 14.4 (0.24), 325 | 0.2 (0.33) | –0.5 to 0.8 | 0.595 | 0.002 | 0.000 |
| EQ-5D | 0.487 (0.0187), 287 | 0.458 (0.0184), 311 | 0.028 (0.0248) | –0.022 to 0.079 | 0.252 | 0.007 | 0.006 |
| HADS Anxiety | 6.4 (0.23), 294 | 6.6 (0.22), 318 | –0.2 (0.30) | –0.9 to 0.3 | 0.355 | 0.000 | 0.000 |
| HADS Depression | 6.9 (0.25), 294 | 7.3 (0.25), 320 | –0.4 (0.33) | –1.1 to 0.3 | 0.191 | 0.001 | 0.014 |
| SIS Physical | 54.5 (1.18), 295 | 52.0 (1.14), 320 | 2.4 (1.56) | –0.8 to 5.6 | 0.121 | 0.001 | 0.000 |
| SIS Memory | 71.8 (1.35), 293 | 69.3 (1.31), 320 | 2.5 (1.78) | –1.1 to 6.1 | 0.162 | 0.000 | 0.000 |
| SIS Mood | 68.6 (1.02), 285 | 67.2 (0.98), 318 | 1.4 (1.34) | –1.3 to 4.2 | 0.287 | 0.000 | 0.000 |
| SIS Communication | 79.7 (1.14), 296 | 78.5 (1.10), 322 | 1.3 (1.50) | –1.8 to 4.4 | 0.390 | 0.000 | 0.000 |
| SIS Recover | 56.1 (1.43), 245 | 55.4 (1.41), 274 | 0.8 (1.88) | –3.1 to 4.6 | 0.683 | 0.008 | 0.000 |
| SIS Social Participation | 53.4 (1.78), 286 | 52.5 (1.75) 309 | 0.9 (2.37) | –3.9 to 5.8 | 0.700 | 0.000 | 0.000 |
| Caregivers' questionnaire scores: unadjusted means | ||||||
|---|---|---|---|---|---|---|
| Questionnaire | Baseline | 6 months | 12 months | |||
| Intervention, mean (SD), n | Control, mean (SD), n | Intervention, mean (SD), n | Control, mean (SD), n | Intervention, mean (SD), n | Control, mean (SD), n | |
| HADS Anxiety | 8.3 (4.87), 442 | 8.0 (4.74), 470 | 7.1 (4.59), 318 | 7.5 (4.53), 334 | 7.2 (4.53), 286 | 7.4 (4.66), 311 |
| HADS Depression | 5.2 (4.25), 442 | 5.0 (3.89), 471 | 5.3 (4.12), 318 | 5.6 (4.33), 334 | 5.4 (4.27), 286 | 5.4 (4.19), 312 |
| EQ-5D | 0.797 (0.232), 438 | 0.791 (0.245), 471 | 0.785 (0.244), 317 | 0.781 (0.225), 333 | 0.809 (0.200), 284 | 0.769 (0.240), 312 |
| FAI | 32.9 (7.99), 441 | 33.5 (7.40), 470 | 31.5 (7.01), 321 | 32.3 (6.87), 333 | 31.8 (6.85), 286 | 32.4 (7.14), 313 |
| Questionnaire | Intervention, mean (SE), n | Control, mean (SE), n | Difference (SE) | 95% CI of the difference | p-value | Unadjusted ICC | Adjusted ICC |
|---|---|---|---|---|---|---|---|
| HADS Anxiety | 7.0 (0.23), 318 | 7.5 (0.23), 334 | –0.5 (0.30) | –1.2 to 0.1 | 0.084 | 0.013 | 0.016 |
| HADS Depression | 5.2 (0.22), 318 | 5.5 (0.22), 334 | –0.3 (0.28) | –0.9 to 0.3 | 0.308 | 0.006 | 0.013 |
| EQ-5D | 0.777 (0.0114), 317 | 0.790 (0.0114), 333 | –0.014 (0.0147) | –0.044 to 0.016 | 0.358 | 0.000 | 0.000 |
| FAI | 31.4 (0.40), 321 | 32.2 (0.40), 333 | –0.8 (0.51) | –1.82 to 0.26 | 0.136 | 0.000 | 0.000 |
| Questionnaire | Intervention, mean (SE), n | Control, mean (SE), n | Difference (SE) | 95% CI of the difference | p-value | Unadjusted ICC | Adjusted ICC |
|---|---|---|---|---|---|---|---|
| Total CBS score | 44.8 (0.97), 291 | 43.8 (0.96), 314 | 1.0 (1.27) | –1.6 to 3.6 | 0.435 | 0.037 | 0.032 |
| HADS Anxiety | 6.9 (0.26), 286 | 7.0 (0.26), 311 | –0.1 (0.34) | –0.9 to 0.5 | 0.636 | 0.018 | 0.024 |
| HADS Depression | 5.2 (0.22), 286 | 5.2 (0.22), 312 | –0.0 (0.28) | –0.6 to 0.5 | 0.889 | 0.010 | 0.000 |
| EQ-5D | 0.806 (0.0122), 284 | 0.787 (0.0119), 312 | 0.019 (0.0154) | –0.013 to 0.050 | 0.240 | 0.000 | 0.000 |
| FAI | 31.9 (0.41), 286 | 32.6 (0.41), 313 | –0.7 (0.52) | –1.7 to 0.4 | 0.217 | 0.022 | 0.000 |
Compliance
Intervention compliance
This section summarises the training records documented by the MDT in intervention centres and provides data related to the delivery of intervention. The number of training records completed and available for analysis is displayed in Table 39. There were 124 (27.6%) training records not completed by the sites.
In total, 196 (43.6%) records were defined as intervention compliant. Table 40 provides a summary of intervention compliance by SRUs; the percentage of compliant records varied from 0.0% to 92.9%. The number of mandatory components achieved by caregivers is summarised in Table 41. Appendix 8 (see Tables 75 and 76) summarises the number of non-mandatory components achieved by caregivers.
| Training records completed | Registered (N = 450), n (%) |
|---|---|
| Yes | 326 (72.4) |
| Confirmed not completed by site | 96 (21.3) |
| Confirmed lost by site | 27 (6.0) |
| Missing | 1 (0.2) |
| SRU | Registered patients | Intervention compliant, n (%) |
|---|---|---|
| Intervention | 450 | 196 (43.6) |
| 1 | 20 | 0 (0.0) |
| 2 | 21 | 13 (61.9) |
| 3 | 24 | 16 (66.7) |
| 4 | 30 | 11 (36.7) |
| 5 | 32 | 0 (0.0) |
| 6 | 32 | 2 (6.3) |
| 7 | 27 | 8 (29.6) |
| 8 | 23 | 20 (87.0) |
| 9 | 29 | 22 (75.9) |
| 10 | 25 | 3 (12.0) |
| 11 | 16 | 14 (87.5) |
| 12 | 35 | 3 (8.6) |
| 13 | 14 | 13 (92.9) |
| 14 | 35 | 25 (71.4) |
| 15 | 19 | 7 (36.8) |
| 16 | 17 | 13 (76.5) |
| 17 | 25 | 6 (24.0) |
| 18 | 26 | 20 (76.9) |
| No. of components achieved | Registered (N = 450), n (%) | Cumulative, n (%) |
|---|---|---|
| 6 | 96 (21.3) | 96 (21.3) |
| 5 | 56 (12.4) | 152 (33.8) |
| 4 | 31 (6.9) | 183 (40.7) |
| 3 | 34 (7.6) | 217 (48.2) |
| 2 | 29 (6.4) | 246 (54.7) |
| 1 | 28 (6.2) | 274 (60.9) |
| 0a | 52 (11.6) | 326 (72.4) |
| Missing | 124 (27.6) | 450 (100.0) |
Intervention compliance and participant outcomes
The relationship between centre-level compliance with the intervention and mean patient NEADL score at 6 months is displayed in Figure 7 and mean caregiver CBS score at 6 months is in Figure 8. The tables show no evidence of higher levels of patient independence or lower level of caregiver burden in the SRUs with better levels of intervention compliance.
FIGURE 7.
Nottingham Extended Activities of Daily Living scores at 6 months and site compliance with intervention. Plot of means with SD bars.
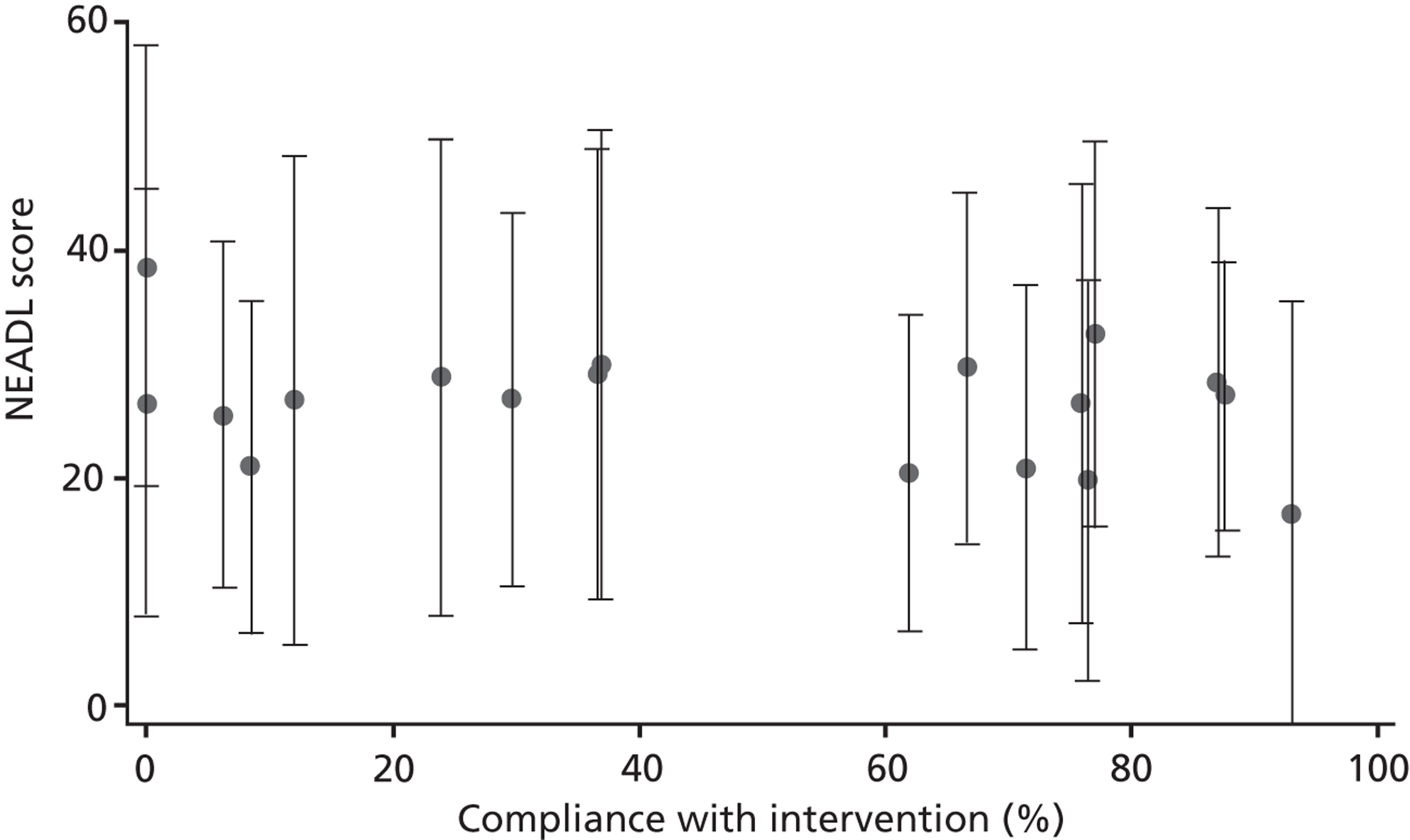
FIGURE 8.
Caregiver Burden Scale scores at 6 months and site compliance with intervention. Plot of means with SD bars.
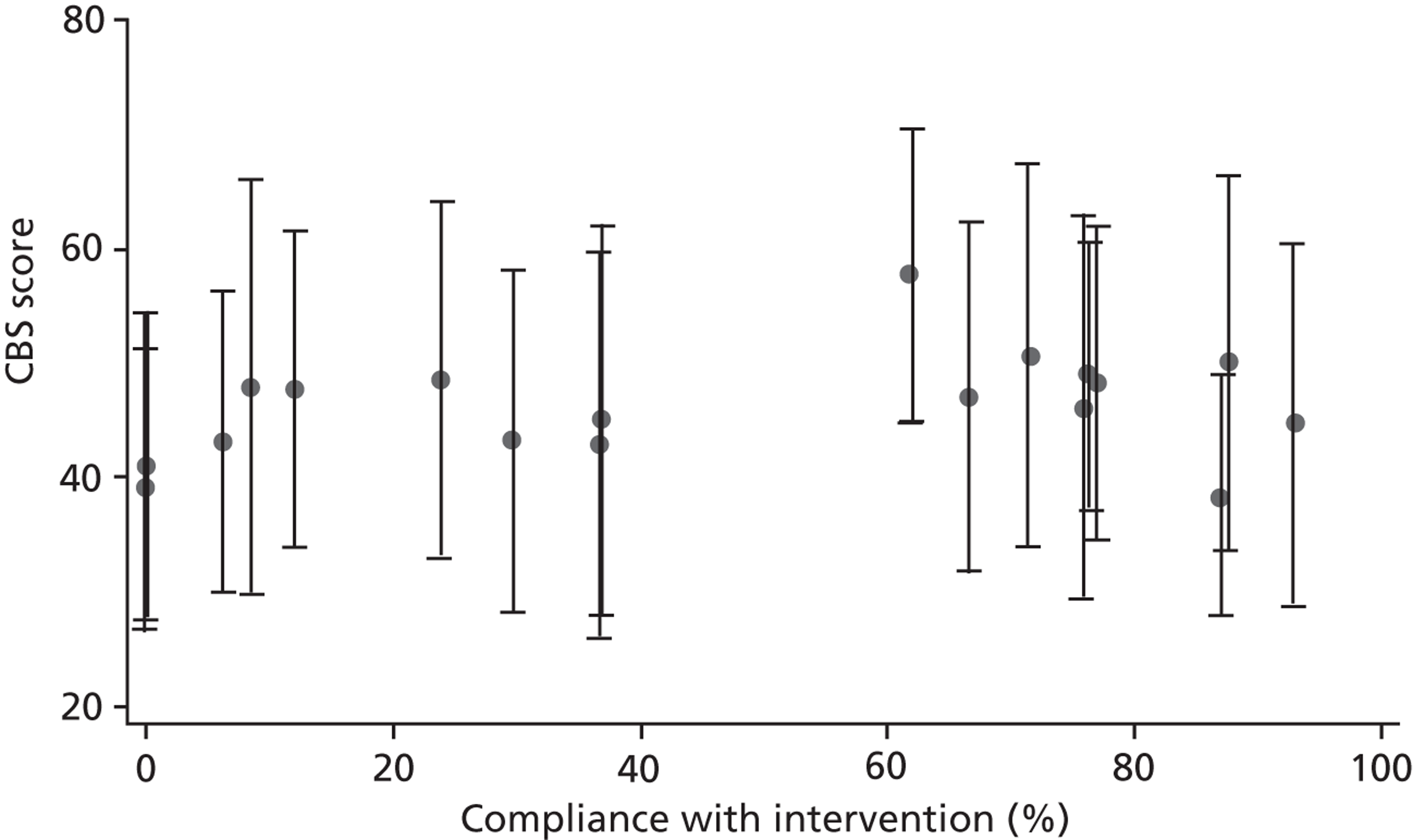
Control group compliance
A summary of the numbers of time logs completed by MDTs in control SRUs is shown in Table 42 and by site in Appendix 8 (see Table 77).
| Time logs completed | Registered (N = 478), n (%) |
|---|---|
| Yes | 211 (44.1) |
| No | 5 (1.0) |
| Not required | 174 (36.4) |
| Confirmed not completed by site | 79 (16.5) |
| Confirmed lost by site | 9 (1.9) |
Time spent with caregivers
The overall time spent with caregivers was similar in the two groups [median of 118 minutes (range 10 to 900 minutes) in the intervention group (training records) and 133 minutes (range 1 to 1130 minutes) in control group (time logs)] (Table 43; Figures 9 and 10).
| Time spent with caregiver (minutes) | Intervention | Control |
|---|---|---|
| n | 214 | 180 |
| Mean (SD) | 136.5 (118.12) | 200.3 (189.12) |
| Median (range) | 117.5 (10–900) | 132.5 (1–1130) |
| Missing | 236 | 298 |
FIGURE 9.
Overall time spent with caregiver: intervention.
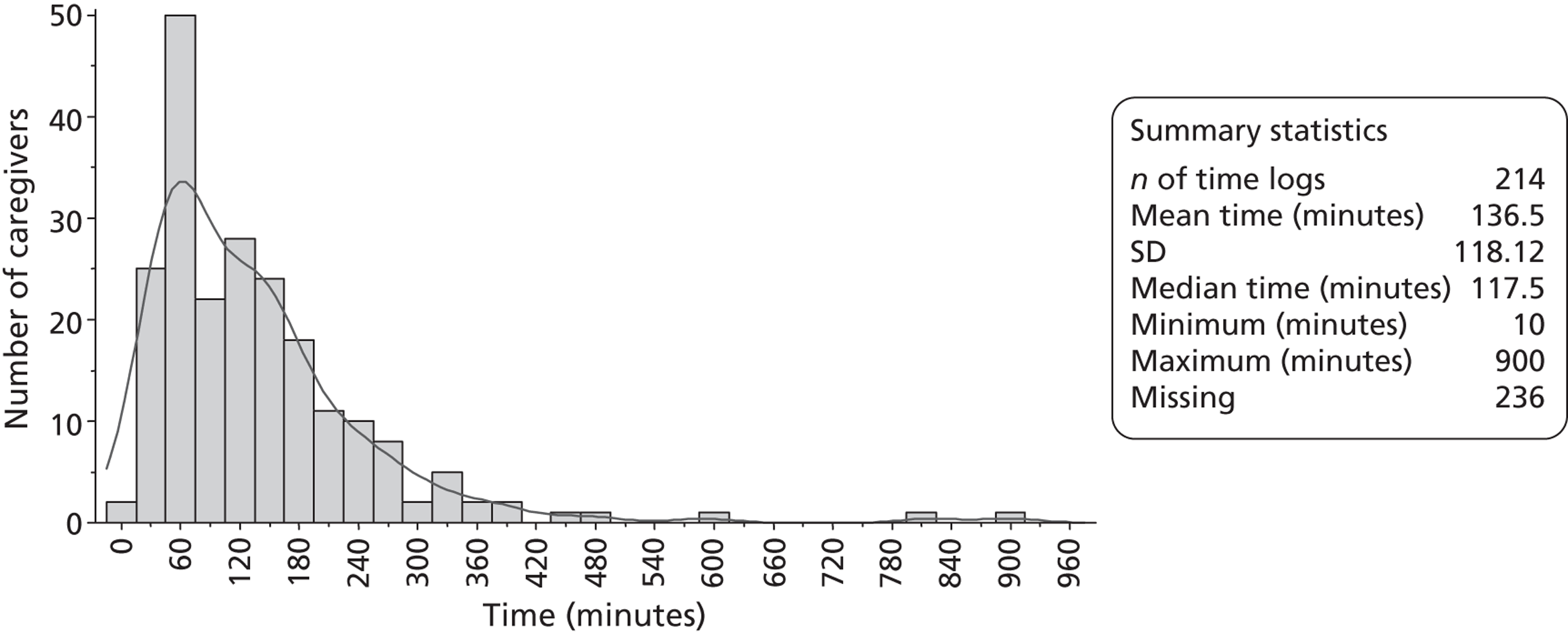
FIGURE 10.
Overall time spent with carergiver: control.
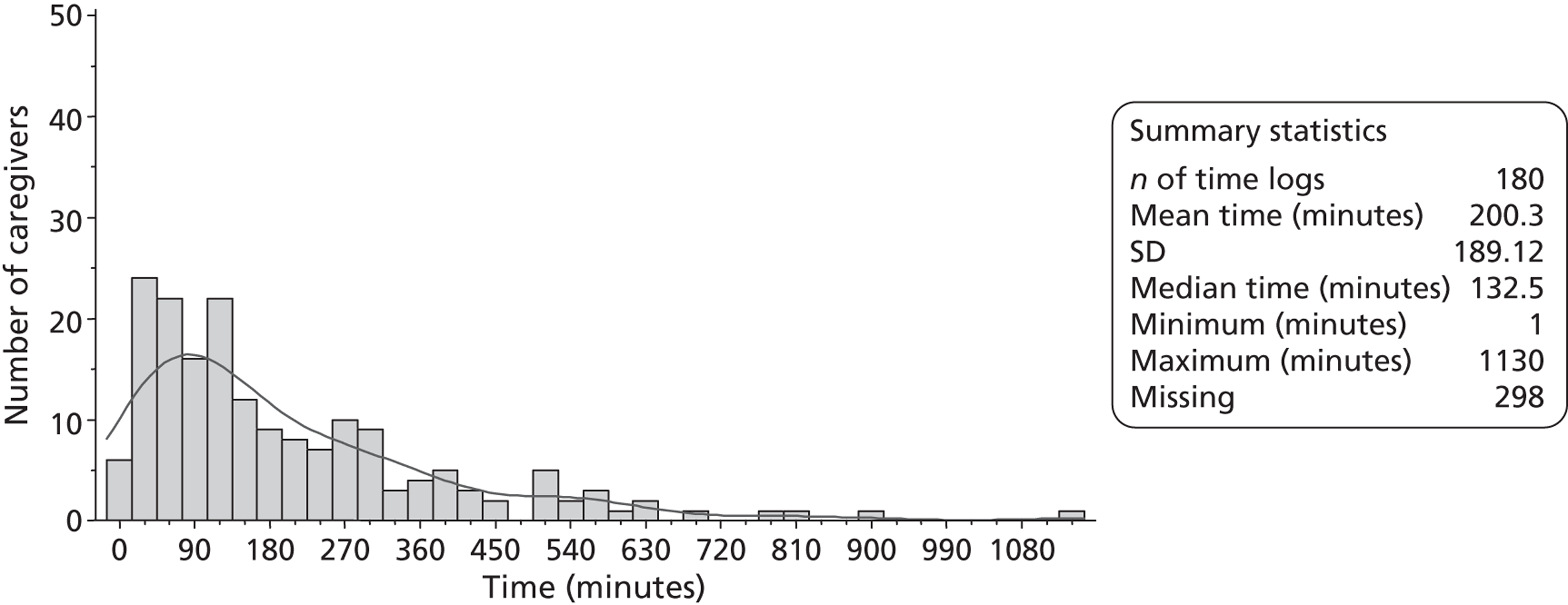
Patient safety: expected adverse events/serious adverse events
Falls
Summaries of the number of patients with falls, number of reported patients' falls and number of SAEs by arm are shown in Table 44 (and by SRUs in Appendix 8, Table 78). In each group there were 35 patients who fell one or more times. The mean number of falls per patient who fell was 1.4 (SE 0.88) in the intervention group and 1.2 (SE 0.76) in the control group. Two falls in the intervention group and three in the control group resulted in SAEs.
No RUSAEs were reported.
| Summaries of patients' falls between registration and discharge | Intervention (N = 45) | Control (N = 478) |
|---|---|---|
| Missing form, n (%) | 1 (0.2) | 0 (0.0) |
| No. of discharge forms received, n (%) | 449 (99.8) | 478 (100.0) |
| No. of patients who fell one or more times, n (%) | 35 (7.8) | 35 (7.3) |
| No. of reported falls | 50 | 42 |
| Mean no. (SE) of falls | 1.4 (0.88) | 1.2 (0.76) |
| Median | 1.0 | 1.0 |
| Range | (1–5) | (1–5) |
| No. of reported SAEs | 2 | 3 |
| Mean no. (SE) of falls that resulted in SAEs (of those who fell) | 0.1 (0.24) | 0.1 (0.28) |
| Median | 0.0 | 0.0 |
| Range | (0–1) | (0–1) |
Deaths
The numbers of patients' and caregivers' deaths by treatment arm are shown in Table 45. There were 12 patients that died before discharge; six in each group. There were 41 (9.1%) patient deaths in the intervention group and 35 (7.3%) in the control group by 6 months and a further 12 (2.7%) in intervention and 20 (4.2%) in control by 12 months.
Overall, six (1.3%) caregivers deaths in intervention and four (0.8%) in control were reported. If the patient died, the caregiver was no longer followed up.
| Deaths | Intervention (N = 450), n (%) | Control (N = 478), n (%) |
|---|---|---|
| No. of patient deaths | ||
| 6 months | 41 (9.1) | 35 (7.3) |
| 12 months | 12 (2.7) | 20 (4.2) |
| No. of caregiver deaths | ||
| 6 months | 2 (0.4) | 1 (0.2) |
| 12 months | 4 (0.9) | 3 (0.6) |
| Overall | ||
| No. of patient deaths | 53 (11.8) | 55 (11.5) |
| No. of caregiver deaths | 6 (1.3) | 4 (0.8) |
Process data
A summary of the process data collected by the trial manager on the visits before (2007) and after (2009/2010) recruitment at both control and intervention centres is provided in Appendix 8, Table 85. Data collected during recruitment (2008) did not vary from that collected after recruitment had completed. The organisational structure and the process of care on the majority of stroke units did not change significantly throughout the trial. Significant changes to the standard process of care of involving patients and their carers during the inpatient stay was seen in just one centre. Four other centres experienced significant changes to their organisational structure, which did not impact upon the process of care of involving patients and their caregivers. A brief description of these centres is provided in Appendix 8, Table 86.
Chapter 5 Economic evaluation
Client Service Receipt Inventory and European Quality of Life-5 Dimensions completion rates
Tables 46 and 47 summarise CSRI and EQ-5D completion rates, respectively, at each assessment point. Both measures had similar completion rates at each assessment point and rates were balanced between the intervention and control arms. Table 48 characterises subsamples with both cost and outcome data at 6 months, a necessary requirement for inclusion in the CEAC-based analyses. Although differences were not explored statistically, the baseline characteristics of patients and caregivers with the necessary data at 6 months appeared similar to those of the full sample. Therefore, results based on those followed up are likely to generally be representative of the full sample.
| Patients | Caregivers | ||||
|---|---|---|---|---|---|
| Baseline n (%) | 6 months n (%) | 12 months n (%) | Baseline n (%) | 6 months n (%) | 12 months n (%) |
| Intervention (n = 450) | |||||
| 442 (98) | 327 (73) | 298 (66) | 442 (98) | 327 (73) | 286 (64) |
| Control (n = 478) | |||||
| 474 (99) | 348 (73) | 327 (68) | 474 (99) | 340 (71) | 313 (66) |
| Total (n = 928) | |||||
| 916 (99) | 675 (73) | 625 (67) | 916 (99) | 667 (72) | 599 (65) |
| Patients | Caregivers | ||||
|---|---|---|---|---|---|
| Baseline n (%) | 6 months n (%) | 12 months n (%) | Baseline n (%) | 6 months n (%) | 12 months n (%) |
| Intervention (n = 450) | |||||
| 426 (95) | 319 (71) | 287 (64) | 438 (97) | 323 (72) | 284 (63) |
| Control (n = 478) | |||||
| 459 (96) | 337 (71) | 311 (65) | 471 (99) | 338 (71) | 313 (65) |
| Total (n = 928) | |||||
| 885 (95) | 656 (71) | 598 (64) | 909 (98) | 661 (71) | 597 (64) |
| Baseline characteristics | Full sample (n = 928) | Subsample with both costs and NEADL data at 6 months (n = 663) | Subsample with both costs and QALY data at 6 months: (n = 630) | |||
|---|---|---|---|---|---|---|
| Valid n | Valid n | Valid n | ||||
| Patients | ||||||
| Age (years), mean | 928 | 71 | 663 | 71 | 630 | 71 |
| Male, n (%) | 928 | 519 (56) | 663 | 384 (58) | 630 | 365 (58) |
| Baseline NEADL total score | 917 | 52.15 | 659 | 53.53 | – | – |
| Baseline utility score | 885 | 0.37 | – | – | 630 | 0.40 |
| Full sample (n = 928) | Subsample with both costs and CBS data at 6 months (n = 652) | Subsample with both costs and QALY data at 6 months (n = 649) | ||||
| Valid n | Valid n | Valid n | ||||
| Caregivers | ||||||
| Age (years), mean | 928 | 61 | 652 | 62 | 649 | 62 |
| Female, n (%) | 928 | 635 (68) | 652 | 448 (69) | 649 | 444 (68) |
| Baseline utility score | 909 | 0.79 | – | – | 649 | 0.80 |
Resource use
Resource-use differences were not compared statistically, firstly because the economic evaluation was focused on costs and cost-effectiveness and, secondly, to avoid problems associated with multiple testing. Therefore, resource-use patterns are described without statistical comparisons.
Tables 49–54 show resource use at each assessment point. These tables are limited to inpatient services plus other items used by at least 10% of responders in either trial arm at that time point. Full resource-use data are provided in Appendix 9. The length of the initial stroke admission was similar in both groups (see Table 50). Other resource use also appeared broadly comparable between the two groups at baseline, 6 months and 12 months. As could be expected, patients' use of inpatient services (other than the stroke admission), outpatient services, hospital physiotherapy and hospital occupational therapy increased during the post-stroke period compared with baseline. It is also interesting to note that caregiver's use of inpatient and outpatient services increased notably during the post-stroke period. With regards to community-based services, patients most commonly used dentist, chiropodist and optician services at all three time points. Services most used by caregivers were outpatient services, GP, practice nurse and repeat prescriptions. In comparison with formal care inputs, patient informal care rates were very high. Care to patients from non-resident informal caregivers increased at each time point.
| Resource | Unit | Intervention (N = 450) | Control (N = 478) | ||||
|---|---|---|---|---|---|---|---|
| N users/valid n | Meanb | SD | N users/valid n | Meanb | SD | ||
| Inpatient services | Bed-days | 42/441 | 14 | 21 | 67/472 | 11 | 14 |
| Outpatient services | Activities/visits | 111/437 | 2 | 2 | 126/466 | 2 | 3 |
| Community-based services | |||||||
| GP | |||||||
| Surgery visit | Visits | 225/408 | 2 | 2 | 251/428 | 2 | 2 |
| Practice nurse | |||||||
| Surgery visit | Visits | 111/383 | 2 | 1 | 124/392 | 2 | 2 |
| Repeat prescription | Occurrences | 235/393 | 3 | 1 | 294/417 | 2 | 2 |
| Chiropodist | Contacts | 58/411 | 2 | 1 | 63/436 | 2 | 2 |
| Dentist | Contacts | 61/410 | 1 | 1 | 65/433 | 2 | 1 |
| Optician | Contacts | 62/408 | 1 | < 1 | 79/431 | 1 | 1 |
| Informal care from co-residents | |||||||
| Personal care | Hours | 55/427 | 170 | 361 | 67/458 | 203 | 419 |
| Providing transport | Hours | 83/430 | 101 | 322 | 94/453 | 83 | 151 |
| Preparing meals | Hours | 110/429 | 156 | 258 | 127/454 | 125 | 111 |
| Housework/laundry | Hours | 110/426 | 111 | 260 | 113/458 | 98 | 112 |
| DIY | Hours | 53/420 | 127 | 446 | 68/446 | 40 | 47 |
| Gardening | Hours | 87/423 | 73 | 289 | 90/447 | 45 | 57 |
| Shopping | Hours | 117/429 | 77 | 247 | 130/453 | 55 | 51 |
| Outings | Hours | 82/428 | 106 | 327 | 92/447 | 60 | 48 |
| Socialising | Hours | 104/427 | 270 | 387 | 128/451 | 407 | 576 |
| Help managing finances | Hours | 99/430 | 64 | 275 | 104/455 | 37 | 34 |
| Informal care from non-residents | |||||||
| Providing transport | Hours | 43/421 | 32 | 44 | 63/443 | 38 | 52 |
| Housework/laundry | Hours | 38/423 | 48 | 65 | 52/443 | 43 | 56 |
| Gardening | Hours | 26/421 | 21 | 21 | 50/437 | 19 | 21 |
| Shopping | Hours | 46/423 | 32 | 32 | 57/441 | 28 | 27 |
| Outings | Hours | 45/423 | 36 | 50 | 57/441 | 41 | 39 |
| Socialising | Hours | 53/419 | 113 | 196 | 69/442 | 140 | 343 |
| Resource | Unit | Intervention (N = 450) | Control (N = 478) | ||||
|---|---|---|---|---|---|---|---|
| N users/valid n | Meanb | SD | N users/valid n | Meanb | SD | ||
| Initial stroke admission | Bed-days | 448/448 | 45 | 33 | 478/478 | 42 | 29 |
| Other inpatient services | Bed-days | 56/319 | 12 | 22 | 65/338 | 8 | 10 |
| A&E | Occurrences | 52/311 | 2 | 1 | 63/338 | 1 | 1 |
| Outpatient services | Activities/visits | 178/308 | 3 | 4 | 158/328 | 3 | 5 |
| Physiotherapist, hospitalc | Visits | 97/258 | 9 | 16 | 114/289 | 9 | 78 |
| Occupational therapist, hospitalc | Visits | 30/235 | 4 | 3 | 50/267 | 8 | 12 |
| Community-based services | |||||||
| GP | |||||||
| Surgery visit | Visits | 177/270 | 3 | 2. | 202/301 | 3 | 2 |
| Home visit | Visits | 124/252 | 2 | 2 | 135/267 | 2 | 2 |
| Telephone call | Calls | 85/230 | 2 | 1 | 95/247 | 2 | 2 |
| Practice nurse | |||||||
| Surgery visit | Visits | 116/241 | 3 | 3 | 116/250 | 3 | 3 |
| Physiotherapist | |||||||
| Home visit | Visits | 159/264 | 9 | 8 | 158/285 | 11 | 11 |
| Occupational therapist | |||||||
| Home visit | Visits | 164/270 | 8 | 9 | 144/284 | 6 | 8 |
| Speech and language therapist | |||||||
| Home visit | Visits | 65/262 | 7 | 8 | 65/271 | 7 | 6 |
| Social worker | |||||||
| Home visit | Visits | 65/274 | 2 | 2 | 70/297 | 2 | 2 |
| Telephone call | Calls | 31/274 | 4 | 2 | 49/297 | 3 | 1 |
| Repeat prescription | Occurrences | 208/264 | 5 | 3 | 253/288 | 5 | 3 |
| Community/district nurse | Contacts | 112/261 | 5 | 4 | 108/277 | 6 | 7 |
| Chiropodist | Contacts | 60/242 | 2 | 1 | 84/274 | 2 | 1 |
| Dentist | Contacts | 58/243 | 2 | 1 | 79/261 | 2 | 1 |
| Optician | Contacts | 66/247 | 1 | 1 | 92/264 | 2 | 1 |
| Home help personal care | Visits | 71/275 | 87 | 134 | 70/297 | 81 | 117 |
| Informal care from co-residents | |||||||
| Personal care | Hours | 206/302 | 226 | 332 | 211/317 | 324 | 449 |
| Providing transport | Hours | 190/286 | 130 | 223 | 193/304 | 138 | 159 |
| Preparing meals | Hours | 224/295 | 286 | 292 | 237/316 | 305 | 213 |
| Housework/laundry | Hours | 228/299 | 220 | 293 | 239/313 | 238 | 198 |
| DIY | Hours | 119/272 | 108 | 321 | 139/300 | 67 | 90 |
| Gardening | Hours | 159/284 | 91 | 228 | 166/304 | 73 | 69 |
| Shopping | Hours | 225/300 | 108 | 202 | 231/315 | 133 | 116 |
| Outings | Hours | 182/288 | 127 | 223 | 187/310 | 132 | 122 |
| Socialising | Hours | 213/289 | 980 | 1293 | 213/307 | 775 | 936 |
| Help managing finances | Hours | 205/296 | 90 | 212 | 203/312 | 125 | 267 |
| Informal care from non-residents | |||||||
| Personal care | Hours | 52/281 | 83 | 168 | 59/308 | 133 | 143 |
| Providing transport | Hours | 102/282 | 50 | 113 | 118/311 | 45 | 55 |
| Preparing meals | Hours | 53/278 | 28 | 28 | 57/305 | 95 | 131 |
| Housework/laundry | Hours | 54/279 | 55 | 62 | 67/305 | 101 | 103 |
| DIY | Hours | 56/274 | 27 | 30 | 48/303 | 36 | 59 |
| Gardening | Hours | 61/276 | 26 | 22 | 56/304 | 25 | 31 |
| Shopping | Hours | 75/280 | 45 | 64 | 81/306 | 52 | 44 |
| Outings | Hours | 96/285 | 47 | 69 | 86/305 | 67 | 82 |
| Socialising | Hours | 113/281 | 105 | 138 | 110/300 | 122 | 141 |
| Help managing finances | Hours | 43/278 | 29 | 20 | 53/304 | 62 | 93 |
| Resource | Unit | Intervention (N = 450) | Control (N = 478) | ||||
|---|---|---|---|---|---|---|---|
| N users/valid n | Meanb | SD | N users/valid n | Meanb | SD | ||
| Inpatient services | Bed-days | 43/288 | 9 | 12 | 58/312 | 9 | 12 |
| A&E | Occurrences | 48/284 | 2 | 1 | 52/311 | 2 | 1 |
| Outpatient services | Activities/visits | 126/281 | 3 | 2 | 130/310 | 3 | 3 |
| Physiotherapist, hospitalc | Visits | 61/244 | 9 | 8 | 58/284 | 9 | 7 |
| Community-based services | |||||||
| GP | |||||||
| Surgery visit | Visits | 167/246 | 3 | 2 | 197/276 | 3 | 2 |
| Home visit | Visits | 86/227 | 2 | 2 | 86/237 | 2 | 2 |
| Telephone call | Calls | 56/202 | 2 | 2 | 69/232 | 3 | 4 |
| Practice nurse | |||||||
| Surgery visit | Visits | 110/223 | 2 | 3 | 135/248 | 3 | 3 |
| Physiotherapist | |||||||
| Home visit | Visits | 54/232 | 8 | 11 | 44/265 | 6 | 8 |
| Repeat prescription | Occurrences | 191/234 | 5 | 4 | 226/265 | 5 | 3 |
| Community/district nurse | Contacts | 67/233 | 6 | 8 | 82/275 | 6 | 9 |
| Chiropodist | Contacts | 78/241 | 2 | 1 | 80/273 | 2 | 2 |
| Dentist | Contacts | 84/231 | 2 | 1 | 73/259 | 2 | 1 |
| Optician | Contacts | 69/223 | 2 | 1 | 75/261 | 1 | 1 |
| Informal care from co-residents | |||||||
| Personal care | Hours | 153/263 | 256 | 376 | 170/285 | 319 | 352 |
| Providing transport | Hours | 139/253 | 110 | 104 | 158/281 | 150 | 146 |
| Preparing meals | Hours | 175/263 | 281 | 157 | 206/287 | 318 | 216 |
| Housework/laundry | Hours | 170/258 | 207 | 159 | 200/280 | 185 | 154 |
| DIY | Hours | 97/247 | 46 | 45 | 116/268 | 56 | 61 |
| Gardening | Hours | 123/250 | 74 | 87 | 134/273 | 73 | 87 |
| Shopping | Hours | 174/257 | 101 | 85 | 183/279 | 115 | 93 |
| Outings | Hours | 143/251 | 113 | 102 | 152/277 | 138 | 135 |
| Socialising | Hours | 163/251 | 646 | 694 | 165/282 | 866 | 1000 |
| Help managing finances | Hours | 161/259 | 145 | 491 | 163/283 | 76 | 80 |
| Informal care from non-residents | |||||||
| Providing transport | Hours | 73/254 | 50 | 47 | 86/284 | 40 | 52 |
| Preparing meals | Hours | 39/255 | 70 | 59 | 50/286 | 91 | 137 |
| Housework/laundry | Hours | 40/255 | 82 | 65 | 60/287 | 88 | 107 |
| Gardening | Hours | 50/251 | 27 | 24 | 52/281 | 46 | 74 |
| Shopping | Hours | 44/256 | 59 | 62 | 70/287 | 43 | 43 |
| Outings | Hours | 63/256 | 39 | 48 | 79/287 | 42 | 60 |
| Socialising | Hours | 77/252 | 124 | 149 | 90/286 | 114 | 168 |
| Resource | Unit | Intervention (N = 450) | Control (N = 478) | ||||
|---|---|---|---|---|---|---|---|
| N users/valid n | Meanb | SD | N users/valid n | Meanb | SD | ||
| Inpatient services | Bed-days | 7/440 | < 1 | 1 | 16/470 | < 1 | 4 |
| Outpatient services | Activities/visits | 88/440 | 2 | 2 | 92/470 | 2 | 2 |
| Community-based services | |||||||
| GP | |||||||
| Surgery visit | Visits | 199/425 | 2 | 1 | 228/460 | 2 | 1 |
| Practice nurse | |||||||
| Surgery visit | Visits | 120/404 | 2 | 1 | 101/430 | 2 | 3 |
| Repeat prescription | Occurrences | 214/415 | 2 | 1 | 229/443 | 2 | 1 |
| Informal care provided to patient | |||||||
| Personal care | Hours | 72/420 | 117 | 173 | 105/451 | 83 | 107 |
| Providing transport | Hours | 141/419 | 58 | 98 | 158/448 | 58 | 72 |
| Preparing meals | Hours | 228/426 | 104 | 96 | 240/455 | 124 | 209 |
| Housework/laundry | Hours | 229/421 | 77 | 114 | 254/452 | 87 | 113 |
| DIY | Hours | 88/412 | 35 | 79 | 101/442 | 30 | 44 |
| Gardening | Hours | 159/423 | 32 | 64 | 158/451 | 34 | 35 |
| Shopping | Hours | 233/422 | 44 | 59 | 264/452 | 45 | 50 |
| Outings | Hours | 140/414 | 60 | 87 | 163/443 | 65 | 83 |
| Socialising | Hours | 239/425 | 281 | 409 | 269/448 | 274 | 400 |
| Help managing finances | Hours | 175/425 | 27 | 57 | 193/450 | 35 | 46 |
| Resource | Unit | Intervention (N = 450) | Control (N = 478) | ||||
|---|---|---|---|---|---|---|---|
| N users/valid n | Meanb | SD | N users/valid n | Meanb | SD | ||
| Inpatient services | Bed-days | 20/320 | < 1 | 2 | 15/335 | < 1 | 1 |
| Outpatient services | Activities/visits | 83/315 | 3 | 3 | 93/326 | 3 | 2 |
| Community-based services | |||||||
| GP | |||||||
| Surgery visit | Visits | 176/290 | 3 | 2 | 197/318 | 3 | 2 |
| Practice nurse | |||||||
| Surgery visit | Visits | 116/267 | 2 | 2 | 111/287 | 2 | 2 |
| Repeat prescription | Occurrences | 152/273 | 4 | 2 | 178/299 | 5 | 3 |
| Informal care provided to patient | |||||||
| Personal care | Hours | 238/306 | 222 | 332 | 243/323 | 370 | 601 |
| Providing transport | Hours | 201/295 | 140 | 220 | 201/309 | 144 | 172 |
| Preparing meals | Hours | 282/310 | 253 | 239 | 295/330 | 286 | 227 |
| Housework/laundry | Hours | 278/310 | 182 | 202 | 295/325 | 218 | 223 |
| DIY | Hours | 130/285 | 99 | 311 | 151/290 | 56 | 62 |
| Gardening | Hours | 181/294 | 82 | 210 | 196/307 | 64 | 71 |
| Shopping | Hours | 281/307 | 109 | 182 | 297/321 | 111 | 107 |
| Outings | Hours | 205/293 | 114 | 204 | 214/310 | 123 | 132 |
| Socialising | Hours | 271/303 | 741 | 984 | 262/317 | 679 | 1023 |
| Help managing finances | Hours | 258/309 | 70 | 172 | 268/317 | 72 | 96 |
| Resource | Unit | Intervention (N = 450) | Control (N = 478) | ||||
|---|---|---|---|---|---|---|---|
| N users/valid n | Meanb | SD | N users/valid n | Meanb | SD | ||
| Inpatient services | Bed-day | 18/282 | < 1 | 2 | 15/306 | < 1 | 3 |
| Outpatient services | Activities/visits | 71/275 | 3 | 3 | 82/303 | 2 | 2 |
| Community-based services | |||||||
| GP | |||||||
| Surgery visit | Visits | 151/263 | 2 | 2 | 191/290 | 2 | 2 |
| Practice nurse | |||||||
| Surgery visit | Visits | 105/239 | 2 | 1 | 124/263 | 2 | 2 |
| Repeat prescription | Occurrences | 139/238 | 4 | 2 | 164/264 | 4 | 2 |
| Informal care provided to patient | |||||||
| Personal care | Hours | 187/256 | 244 | 346 | 207/288 | 279 | 398 |
| Providing transport | Hours | 173/251 | 108 | 107 | 188/279 | 150 | 149 |
| Preparing meals | Hours | 243/265 | 265 | 197 | 265/296 | 298 | 206 |
| Housework/laundry | Hours | 229/260 | 195 | 181 | 257/289 | 192 | 179 |
| DIY | Hours | 129/241 | 47 | 70 | 136/268 | 47 | 48 |
| Gardening | Hours | 155/252 | 70 | 87 | 182/280 | 77 | 136 |
| Shopping | Hours | 233/257 | 107 | 96 | 259/286 | 150 | 252 |
| Outings | Hours | 181/244 | 104 | 103 | 196/280 | 147 | 149 |
| Socialising | Hours | 229/256 | 644. | 767 | 234/282 | 667 | 883 |
| Help managing finances | Hours | 222/264 | 61 | 88 | 225/290 | 84 | 107 |
Costs and quality-adjusted life-years
The mean cost of the LSCTC training and development was £39 (Table 55). This is the mean across the whole intervention group, including those allocated zero costs for either receiving no LSCTC inputs or with missing data regarding such inputs. The mean cost among only those receiving inputs was £82.
The mean cost of the initial stroke admission was similar between groups (mean difference £1243, 95% CI –£1533 to £4019; see Table 55). Total health and social care and societal costs were broadly similar between both randomisation groups at all assessment points (Tables 56–58), except that caregivers in the intervention arm had higher health and social care costs at 6 months (+£207, 95% CI £5 to £408; see Table 58). This difference was no longer present at 12 months and was not apparent when costs from the two assessment points were combined as 1-year costs. There were also no differences in QALYs for patients or caregivers at any of the assessment points (Table 59). Although the direction of the between-group difference is opposite in the patient (positive) and caregiver (negative) evaluations the mean differences are so small that they are essentially zero. It should also be noted that comparisons of costs and outcomes do not account for the correlation between these parameters.
| Intervention (n = 450) | Control (n = 478) | Intervention – controla | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Resource | Valid n | Mean | SD | Valid n | Mean | SD | Mean difference | 95% CI | p-value |
| LSCTC development and staff training | 450 | 39 | 64 | 478 | 0 | – | 41 | 26 to 57 | 0.000 |
| Stroke admission | 450 | 13,127 | 9670 | 478 | 12,471 | 8666 | 1243 | –1533 to 4019 | 0.380 |
| Institutionalisation | |||||||||
| Baseline | 442 | 11 | 209 | 474 | 18 | 231 | –7 | –36 to 21 | 0.608 |
| 6 months | 327 | 274 | 1516 | 348 | 159 | 619 | 137 | –32 to 306 | 0.113 |
| 12 months | 298 | 162 | 631 | 327 | 194 | 785 | –17 | –129 to 95 | 0.766 |
| Secondary care | |||||||||
| Baseline | 442 | 625 | 2832 | 474 | 824 | 2892 | –199 | –670 to 173 | 0.294 |
| 6 months | 327 | 1150 | 3547 | 348 | 1121 | 2541 | 56 | –410 to 522 | 0.813 |
| 12 months | 298 | 822 | 2027 | 327 | 986 | 2468 | –124 | –522 to 273 | 0.540 |
| Community-based services | |||||||||
| Baseline | 442 | 208 | 500 | 474 | 206 | 450 | 2 | –59 to 64 | 0.946 |
| 6 months | 327 | 1380 | 1777 | 348 | 1317 | 1652 | 57 | –260 to 373 | 0.726 |
| 12 months | 298 | 1042 | 1690 | 327 | 1267 | 2418 | –212 | –689 to 265 | 0.383 |
| Other health and social care services | |||||||||
| Baseline | 442 | 10 | 107 | 474 | 6 | 53 | 4 | –7 to 15 | 0.451 |
| 6 months | 327 | 9 | 45 | 348 | 26 | 260 | –18 | –47 to 11 | 0.221 |
| 12 months | 298 | 9 | 66 | 327 | 19 | 140 | –12 | –34 to 9 | 0.269 |
| Informal care | |||||||||
| Baseline | 442 | 2390 | 10,102 | 474 | 2086 | 3698 | 304 | –668 to 1277 | 0.539 |
| 6 months | 327 | 11,033 | 11,783 | 348 | 10,841 | 8721 | 300 | –1216 to 1817 | 0.698 |
| 12 months | 298 | 8404 | 9126 | 327 | 9323 | 8582 | –872 | –2449 to 704 | 0.278 |
| Resource | Intervention (n = 450) | Control (n = 478) | Intervention – controla | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Valid n | Mean | SD | Valid n | Mean | SD | Mean difference | 95% CI | p-value | |
| LSCTC development and staff training | 450 | 39 | 64 | 478 | 0 | – | 41 | 26 to 57 | 0.000 |
| Institutionalisation | |||||||||
| Baseline | Not assessed at baseline | Not assessed at baseline | |||||||
| 6 months | 327 | 0 | – | 340 | 0 | – | – | – | – |
| 12 months | 286 | 0 | – | 313 | 1 | 6 | –1 | –2 to 0.4 | 0.248 |
| Secondary care | |||||||||
| Baseline | 442 | 109 | 373 | 474 | 373 | 4130 | –276 | –661 to 109 | 0.161 |
| 6 months | 327 | 364 | 1719 | 340 | 209 | 616 | 166 | –30 to 362 | 0.096 |
| 12 months | 286 | 241 | 935 | 313 | 284 | 1331 | –31 | –217 to 155 | 0.742 |
| Community-based services | |||||||||
| Baseline | 442 | 61 | 91 | 474 | 62 | 94 | –1 | –13 to 11 | 0.835 |
| 6 months | 327 | 118 | 157 | 340 | 123 | 151 | –5 | –37 to 28 | 0.781 |
| 12 months | 286 | 100 | 132 | 313 | 116 | 141 | –16 | –39 to 8 | 0.196 |
| Informal care | |||||||||
| Baseline | 442 | 2570 | 4166 | 474 | 2606 | 3042 | –49 | –518 to 419 | 0.836 |
| 6 months | 327 | 10,626 | 8950 | 340 | 10,949 | 8836 | –145 | –1471 to 1180 | 0.830 |
| 12 months | 286 | 9370 | 7094 | 313 | 10,055 | 7125 | –643 | –1758 to 471 | 0.258 |
| Costs | Intervention (n = 450) | Control (n = 478) | Intervention – controla | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Valid n | Mean | SD | Valid n | Mean | SD | Mean difference | 95% CI | p-value | |
| Total health and social care | |||||||||
| Baseline | 442 | 855 | 2974 | 474 | 1054 | 2975 | –200 | –585 to 186 | 0.310 |
| 6 monthsb | 327 | 15,861 | 11,565 | 348 | 15,541 | 10,234 | 1184 | –1505 to 3872 | 0.388 |
| 12 months | 298 | 2037 | 2804 | 327 | 2465 | 4041 | –347 | –1119 to 425 | 0.378 |
| 1 yearb | 272 | 17,406 | 12,741 | 298 | 17,752 | 12,235 | 563 | –2986 to 4112 | 0.756 |
| Total societal | |||||||||
| Baseline | 442 | 3243 | 10,642 | 474 | 3140 | 4952 | 105 | –959 to 1168 | 0.847 |
| 6 monthsb | 327 | 26,894 | 16,832 | 348 | 26,381 | 14,274 | 1127 | –1681 to 3935 | 0.432 |
| 12 months | 298 | 10,440 | 9889 | 327 | 11,788 | 9738 | –1234 | –2953 to 485 | 0.159 |
| 1 yearb | 272 | 37,453 | 23,667 | 298 | 37,884 | 19,993 | 167 | –4163 to 4497 | 0.940 |
| Costs | Intervention (n = 450) | Control (n = 478) | Intervention – controla | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Valid n | Mean | SD | Valid n | Mean | SD | Mean difference | 95% CI | p-value | |
| Total health and social care | |||||||||
| Baseline | 442 | 170 | 396 | 474 | 435 | 4131 | –277 | –663 to 108 | 0.159 |
| 6 monthsb | 327 | 525 | 1756 | 340 | 331 | 672 | 207 | 5 to 408 | 0.045 |
| 12 months | 286 | 341 | 993 | 313 | 401 | 1375 | –47 | –241 to 147 | 0.636 |
| 1 yearb | 268 | 785 | 1604 | 285 | 708 | 1785 | 96 | –186 to 379 | 0.505 |
| Total societal | |||||||||
| Baseline | 442 | 2741 | 4204 | 474 | 3041 | 5057 | –327 | –926 to 273 | 0.286 |
| 6 monthsb | 327 | 11,151 | 9084 | 340 | 11,280 | 8902 | 99 | –1248 to 1446 | 0.885 |
| 12 months | 286 | 9711 | 7119 | 313 | 10,455 | 7247 | –644 | –1777 to 489 | 0.265 |
| 1 yearb | 268 | 21,147 | 14,434 | 285 | 22,024 | 13,774 | –574 | –3112 to 1964 | 0.658 |
| QALYs | Intervention (n = 450) | Control (n = 478) | Intervention – controlb | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Valid n | Mean | SD | Valid n | Mean | SD | Mean difference | 95% CI | p-value | |
| Patients, utility scores | |||||||||
| Baseline | 426 | 0.36 | 0.37 | 459 | 0.40 | 0.36 | –0.02 | –0.08 to 0.04 | 0.538 |
| 6 months | 319 | 0.44 | 0.34 | 337 | 0.44 | 0.35 | 0.00 | –0.04 to 0.05 | 0.835 |
| 12 months | 287 | 0.47 | 0.35 | 311 | 0.46 | 0.34 | 0.03 | –0.02 to 0.08 | 0.290 |
| Patients to QALYs | |||||||||
| 6 months | 309 | 0.21 | 0.16 | 324 | 0.21 | 0.15 | 0.00 | –0.01 to 0.01 | 0.835 |
| 12 months | 258 | 0.24 | 0.16 | 276 | 0.23 | 0.16 | 0.01 | –0.01 to 0.03 | 0.443 |
| 1 year | 249 | 0.46 | 0.30 | 266 | 0.45 | 0.30 | 0.01 | –0.02 to 0.05 | 0.520 |
| Caregivers to utility scores | |||||||||
| Baseline | 438 | 0.80 | 0.23 | 471 | 0.79 | 0.25 | 0.01 | –0.03 to 0.04 | 0.660 |
| 6 months | 323 | 0.78 | 0.24 | 338 | 0.78 | 0.23 | –0.02 | –0.04 to 0.01 | 0.239 |
| 12 months | 284 | 0.81 | 0.20 | 313 | 0.77 | 0.24 | 0.02 | –0.01 to 0.05 | 0.236 |
| Caregivers to QALYs | |||||||||
| 6 months | 317 | 0.40 | 0.10 | 333 | 0.39 | 0.11 | –0.00 | –0.01 to 0.0 | 0.239 |
| 12 months | 262 | 0.40 | 0.10 | 283 | 0.39 | 0.11 | –0.00 | –0.01 to 0.01 | 0.950 |
| 1 year | 257 | 0.80 | 0.19 | 279 | 0.78 | 0.20 | –0.00 | –0.02 to 0.02 | 0.674 |
Sensitivity analyses
All sensitivity analyses confirmed conclusions from the base-case conclusions except for sensitivity analysis 2, adopting the replacement cost approach for informal care, which led to the between group difference in mean societal costs for caregivers changing from £99 to –£831. However, although the direction of difference changed, the difference between groups remained statistically non-significant. Substituting the base-case value with this new value for the consideration of related ICERs (Table 60) would result in the intervention group dominating the control group based on CBS scores (the only case of the intervention group's dominance at the primary end point of 6 months) and an unlikely trade-off of lower costs for fewer QALYs.
Cost-effectiveness and cost–utility
Of the 16 cost–outcome combinations examined for the cost-effectiveness and cost–utility analyses, none was based on statistically significant between-group differences for both cost and outcome elements. The intervention group ‘dominated’ in seven combinations, the control group ‘dominated’ in five, the intervention group had both higher costs and better outcomes in nine, and the remaining three involved an unlikely trade-off of lower costs for worse outcomes. Where relevant, indicative ICERs are presented for information (Table 61), but these and the term ‘dominates’ should be interpreted with caution for most combinations given the small magnitude and lack of statistical significance in differences in costs or outcomes except for caregivers having higher health and social care costs at 6 months. ICERs ranged from £96 for an additional point improvement on the CBS based on 1-year health and social care costs for caregivers to £1.18M for an additional patient QALY based on their health and social care costs at 6 months.
Cost-effectiveness planes show that although differences in patient health and social care costs, NEADL scores (Figure 11) and QALYs (Figure 12) between the two groups do vary around the point estimates, they are strongly centred around zero, i.e. no difference in either costs or outcomes. In contrast, the caregiver cost-effectiveness planes suggest that health and social care costs are higher in the intervention group and, while CBS differences are clustered around zero (i.e. no difference; Figure 13), QALYs differences are clustered to the left of zero (i.e. lower in the intervention group; Figure 14).
Figures 15–18 show probabilities that the intervention group is cost-effective compared with the control group. In the patient evaluation, probabilities of cost-effectiveness were similar from both cost perspectives. Maximum probabilities of cost-effectiveness for the threshold ranges examined for each outcome measure were 51% at £2000 for an additional point improvement on the NEADL scale (see Figure 15) and 34% for an additional QALY (this was the same across all QALY thresholds examined) (see Figure 16). In the caregiver evaluation, probabilities of cost-effectiveness were higher based on the societal perspective than for the health and social care perspective, given that the latter costs were higher in the intervention group compared with the control group. Probabilities of cost-effectiveness were reasonable for CBS point improvements (see Figure 17), up to a maximum of 62% and 68% from the health and social care and societal perspectives, respectively, at the maximum threshold examined (£2000), although it is unknown what the willingness to pay for a CBS point improvement would be in practice. However, as for the patient evaluation, probabilities of cost-effectiveness based on QALYs were low (see Figure 18), not exceeding 2% from the health and social care perspective for the range examined. Thus, the intervention is unlikely to be considered cost-effective from either patient or caregiver perspectives at current policy thresholds of £20,000–30,000 per QALY gained.
| Analysis | Intervention (n = 450) | Control (n = 478) | Intervention – controla | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Valid n | Mean | (SD) | Valid n | Mean | (SD) | Mean difference | 95% CI | p-value | |
| Sensitivity analysis 1: effect on total health and social care costs at 6 months of imputing missing LSCTC costs | |||||||||
| Patient base case | 327 | 15,861 | 11,565 | 348 | 15,541 | 10,234 | 1184 | –1505 to 3872 | 0.388 |
| Patient sensitivity analysis | 327 | 15,904 | 11,560 | 348 | 15,541 | 10,234 | 1126 | –1464 to 3916 | 0.372 |
| Caregiver base case | 327 | 525 | 1756 | 340 | 331 | 672 | 207 | 5 to 408 | 0.045 |
| Caregiver sensitivity analysis | 327 | 568 | 1758 | 340 | 331 | 672 | 249 | 47 to 451 | 0.015 |
| Sensitivity analysis 2: effect on total societal costs at 6 months of adopting the replacement cost approach for informal care | |||||||||
| Patient base case | 327 | 26,894 | 16,832 | 348 | 26,381 | 14,274 | 1127 | –1681 to 3935 | 0.432 |
| Patient sensitivity analysis | 327 | 60,386 | 45,696 | 348 | 59,066 | 37,289 | 1860 | –4377 to 8097 | 0.559 |
| Caregiver base case | 327 | 11,151 | 9084 | 340 | 11,280 | 8902 | 99 | –1248 to 1446 | 0.885 |
| Caregiver sensitivity analysis | 327 | 43,117 | 34,614 | 340 | 44,759 | 31,573 | –831 | –6886 to 5224 | 0.788 |
| Sensitivity analysis 3: effect on total health and social care costs at 6 months of imputing missing cost data at 6 months | |||||||||
| Patient base case | 327 | 15,861 | 11,565 | 348 | 15,541 | 10,234 | 1184 | –1505 to 3872 | 0.388 |
| Patient sensitivity analysis | 450 | 15,875 | 9857 | 478 | 15,550 | 8736 | 995 | –1275 to 3267 | 0.390 |
| Caregiver base case | 327 | 525 | 1756 | 340 | 331 | 672 | 207 | 5 to 408 | 0.045 |
| Caregiver sensitivity analysis | 450 | 536 | 1497 | 478 | 348 | 702 | 207 | 62 to 353 | 0.005 |
| Sensitivity analysis 4: effect on total QALYs at 6 months of imputing missing QALYs at 6 months | |||||||||
| Patient base case | 309 | 0.21 | 0.16 | 324 | 0.21 | 0.15 | 0.00 | –0.01 to 0.01 | 0.835 |
| Patient sensitivity analysis | 450 | 0.20 | 0.15 | 478 | 0.20 | 0.15 | 0.00 | –0.01 to 0.01 | 0.744 |
| Caregiver base case | 317 | 0.40 | 0.10 | 333 | 0.39 | 0.11 | –0.00 | –0.01 to 0.00 | 0.239 |
| Caregiver sensitivity analysis | 450 | 0.39 | 0.10 | 478 | 0.39 | 0.11 | –0.00 | –0.01 to .000 | 0.098 |
| Cost perspective | Additional cost per additional point on the NEADL scale | Additional cost per additional point on the CBS | Additional cost per additional QALY |
|---|---|---|---|
| Patients, 6 months | |||
| Health and social care | Control dominates (£1184/–0.2) | N/A | £1,184,000 (£1184/0.001) |
| Societal | Control dominates (£1127/–0.2) | N/A | £1,127,000 (£1127/0.001) |
| Patients, 1 year | |||
| Health and social care | 1126 (£563/0.5) | N/A | £51,182 (£563/0.011) |
| Societal | 334 (£167/0.5) | N/A | £15,182 (£167/0.011) |
| Caregivers, 6 months | |||
| Health and social care | N/A | 414 (£207/0.5) | Control dominates (£207/–0.004) |
| Societal | N/A | 198 (£99/0.5) | Control dominates (£99/–0.004) |
| Caregivers, 1 year | |||
| Health and social care | N/A | £96 (£96/1) | Control dominates (£96/–0.004) |
| Societal | N/A | Intervention dominates (–£574/1) | Not applicable (–£574/–0.004) |
FIGURE 11.
Patients: cost-effectiveness plane of incremental total health and social care costs and point changes on the NEADL scale at 6 months.
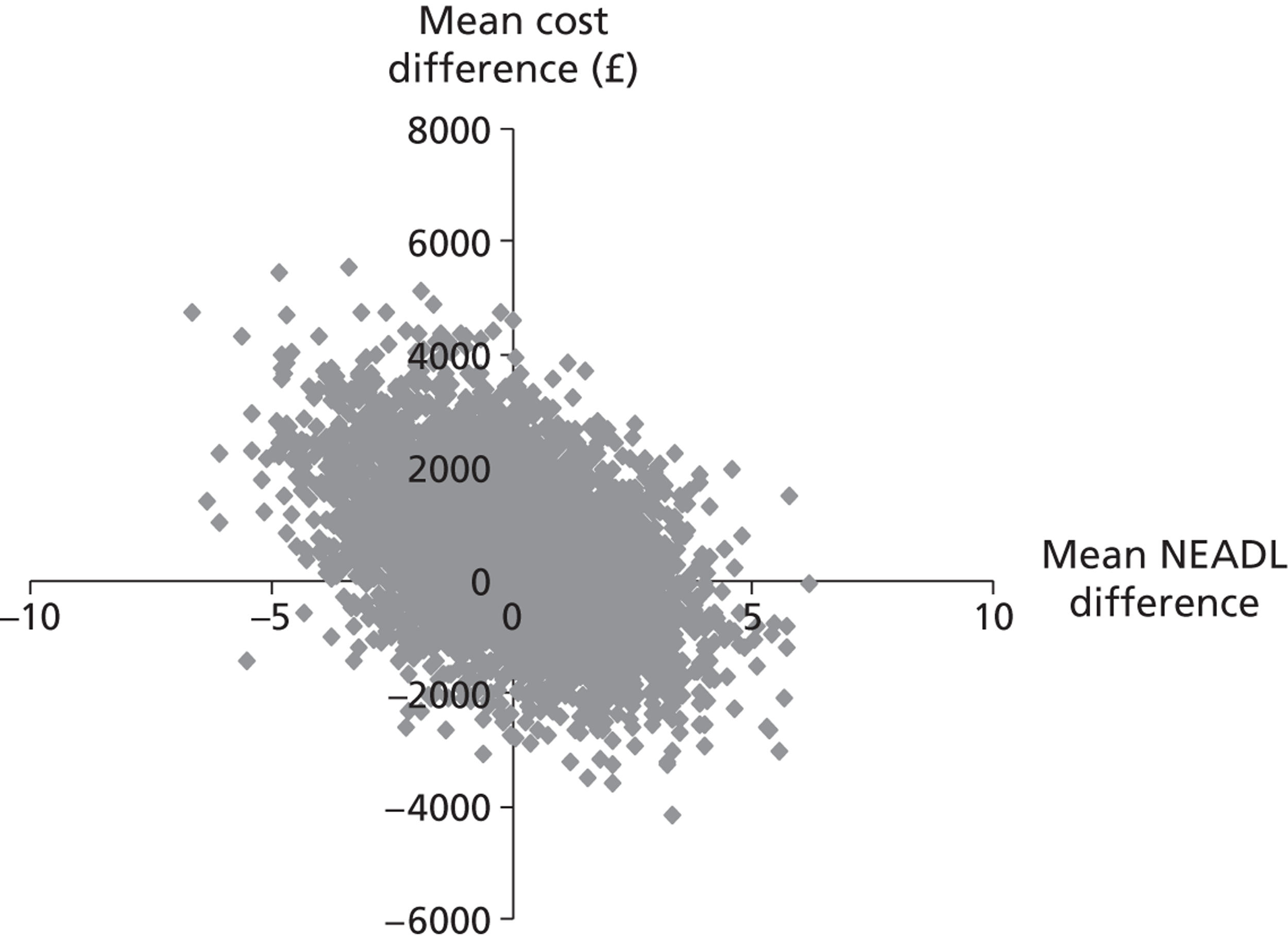
FIGURE 12.
Patients: cost-effectiveness plane of incremental total health and social care costs and QALYs at 6 months.
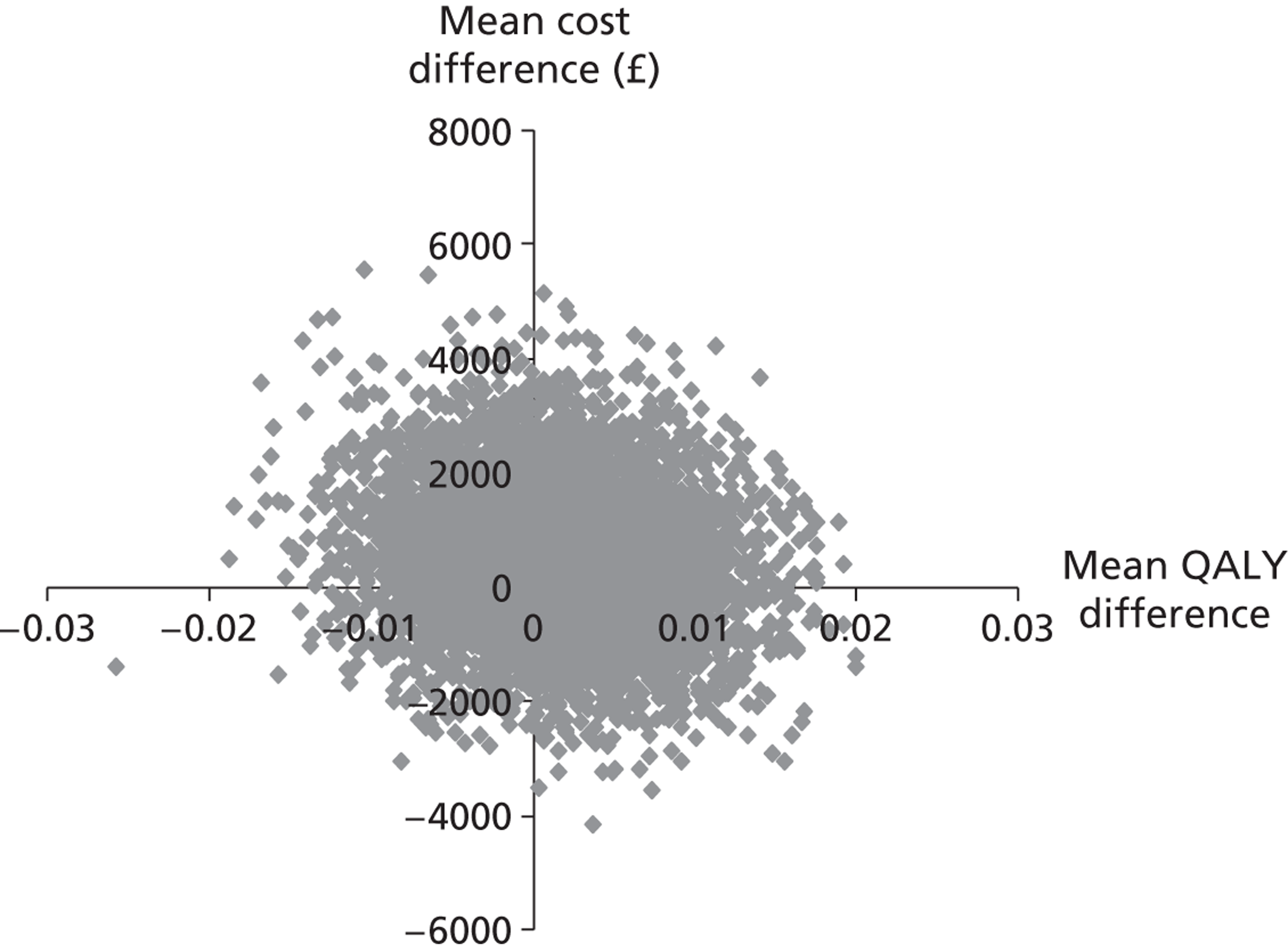
FIGURE 13.
Caregivers: cost-effectiveness plane of incremental total health and social care costs and point changes on the CBS at 6 months.
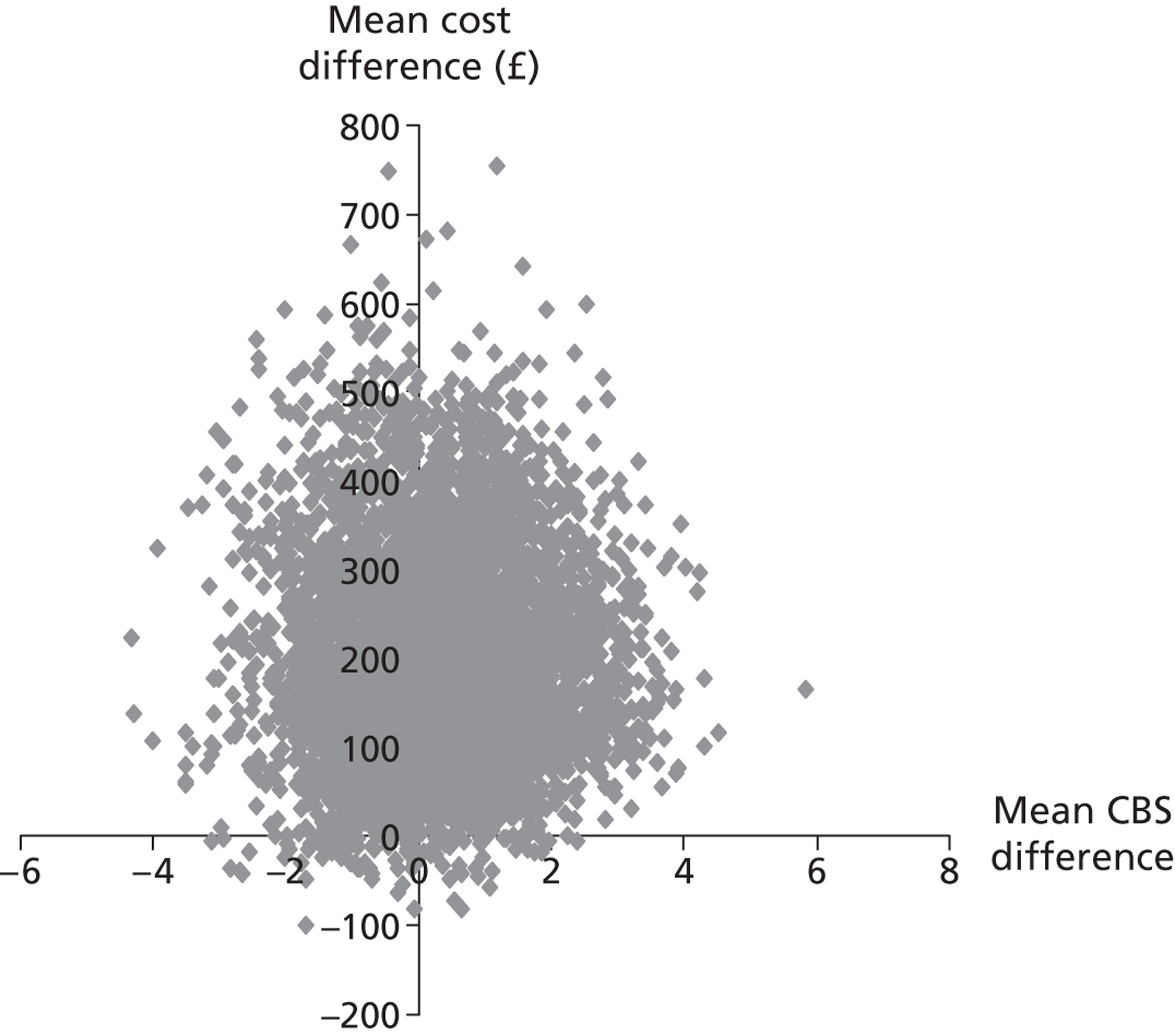
FIGURE 14.
Caregivers: cost-effectiveness plane of incremental total health and social care costs and QALYs at 6 months.
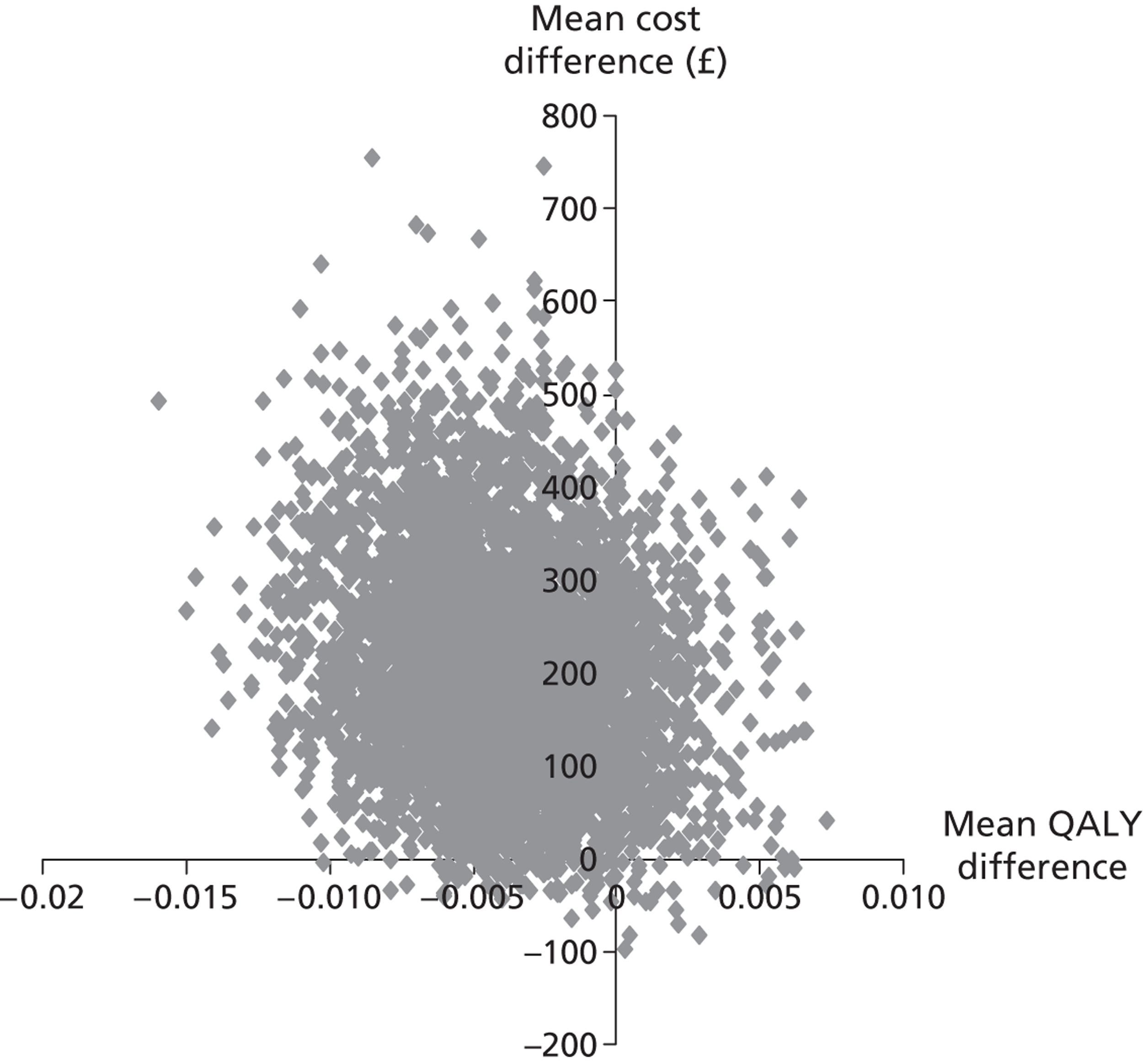
FIGURE 15.
Patients: probability that the intervention is cost-effective compared with the control at 6 months, from each cost perspective, for a range of willingness-to-pay values for an additional point improvement on the NEADL scale.
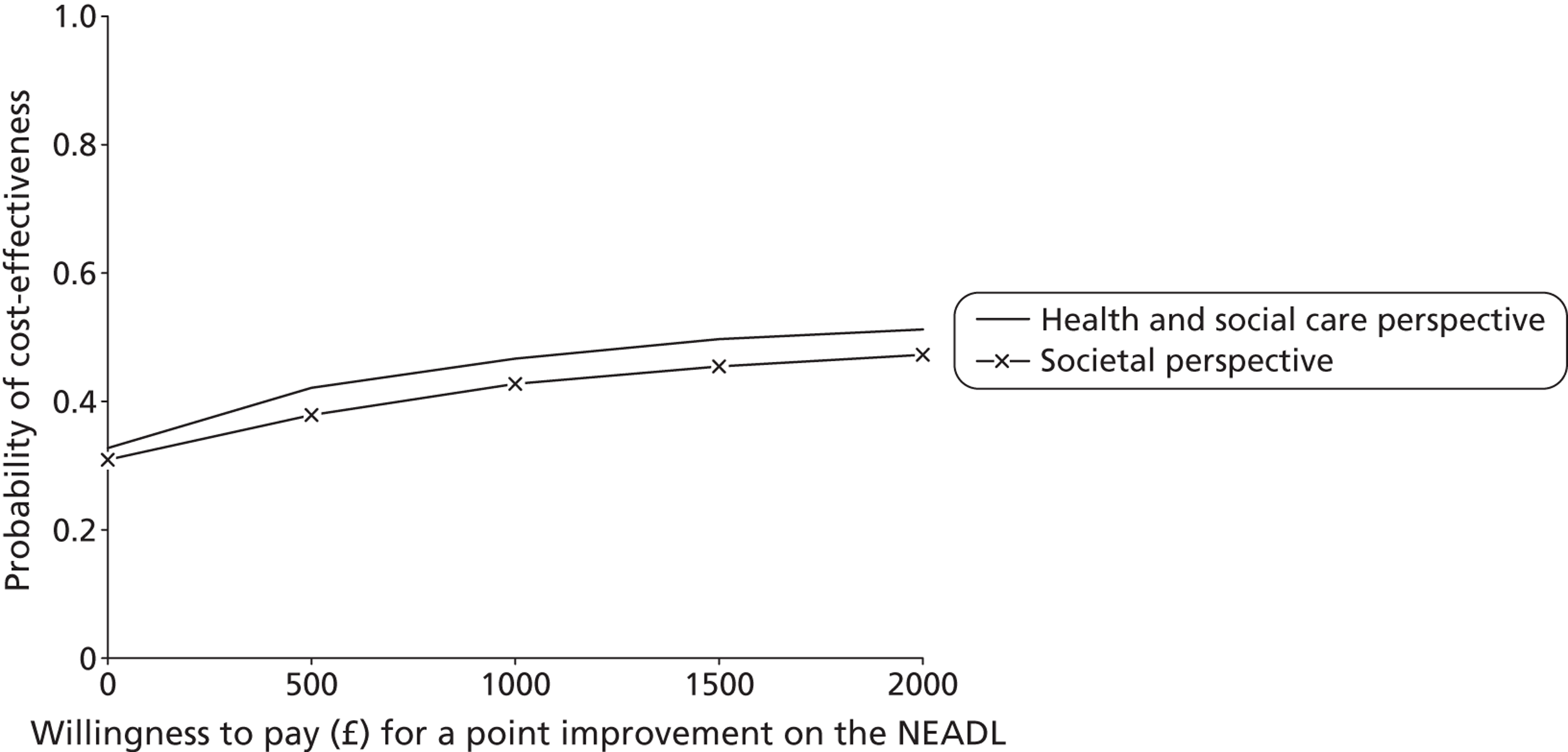
FIGURE 16.
Patients: probability that the intervention is cost-effective compared with the control at 6 months, from each cost perspective, for a range of willingness-to-pay values for an additional QALY.
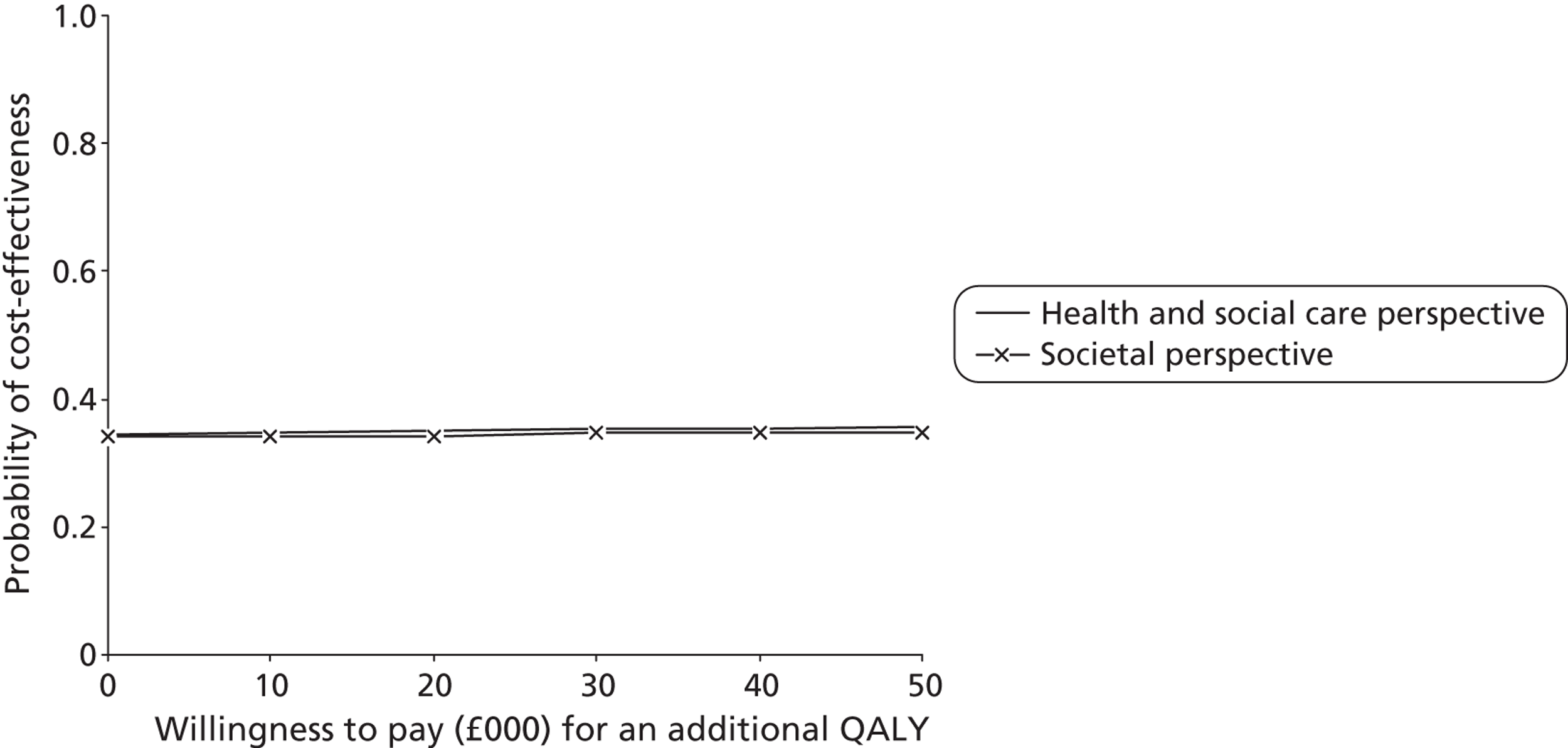
FIGURE 17.
Caregivers: probability that the intervention is cost-effective compared with the control at 6 months, from each cost perspective, for a range of willingness-to-pay values for an additional point improvement on the CBS.
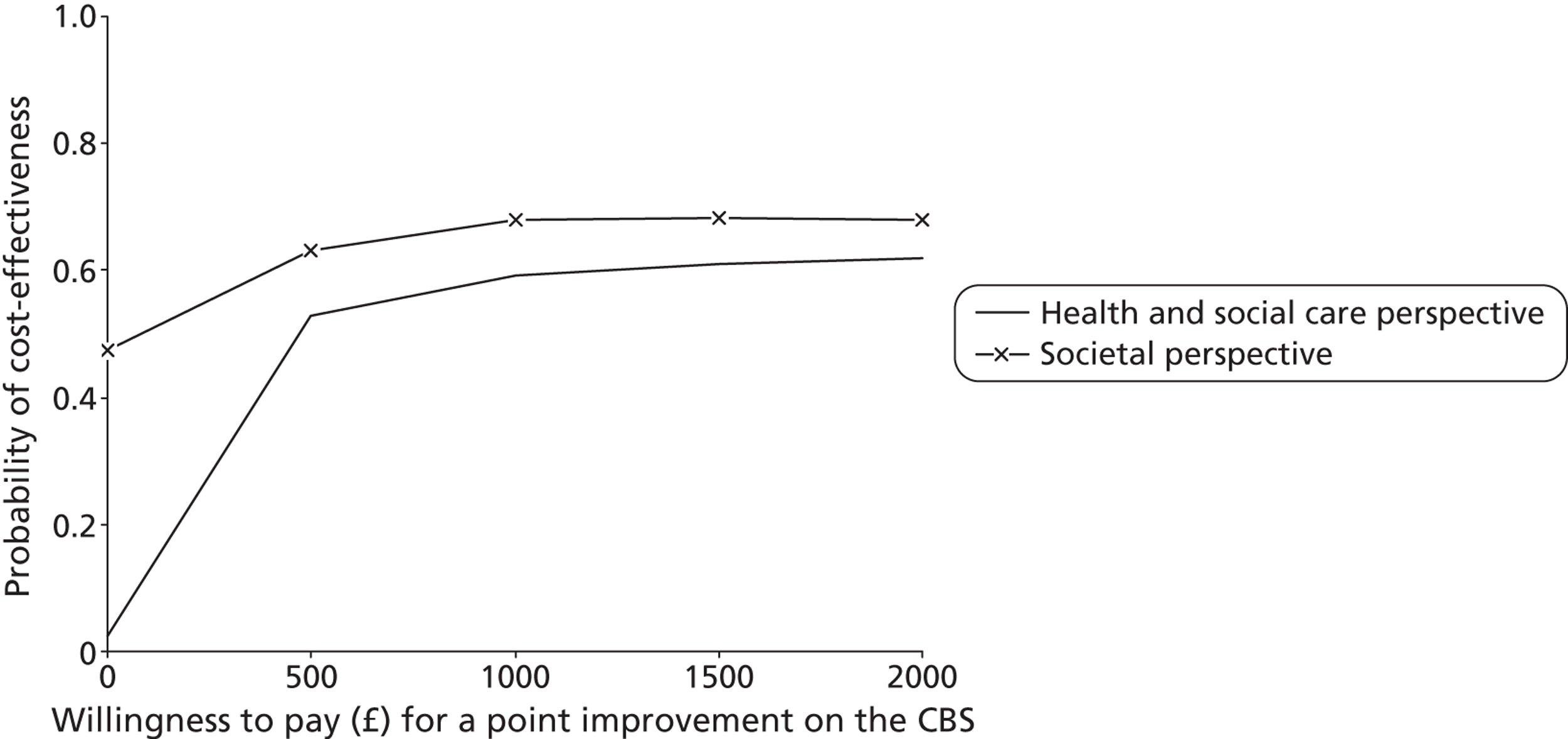
FIGURE 18.
Caregivers: probability that the intervention is cost-effective compared with the control at 6 months, from each cost perspective, for a range of willingness-to-pay values for an additional QALY.
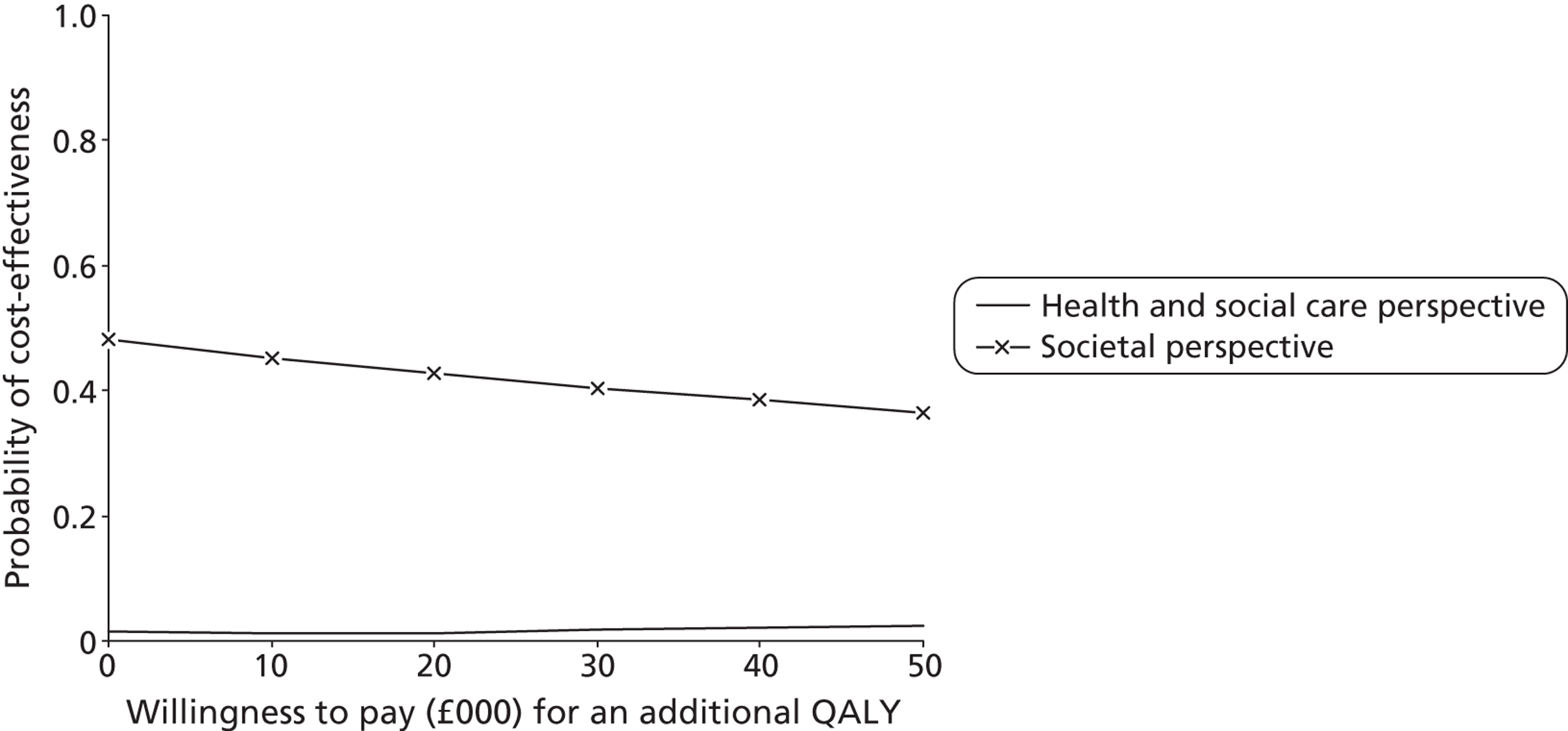
Chapter 6 Discussion
Key findings
The TRACS trial was a pragmatic, multicentre cluster RCT of a complex intervention. The trial was designed to evaluate the effectiveness of the LSCTC (LSCTC compared with usual care when implemented in SRUs across the UK in different health-care settings, with different patient populations). The trial evaluated whether the LSCTC improved physical and psychological outcomes for patients and their caregivers after disabling stroke, and sought to determine if such a training programme was cost-effective. Eighteen SRUs were randomised to implement the intervention as a part of their standard practice, and 18 SRUs were randomised to continue usual practice. A total of 928 patient and caregiver dyads were recruited into the trial: 450 in the intervention arm and 478 in the control arm.
Primary outcomes
Stroke patients attending intervention SRUs did not demonstrate a clinically significant improvement in functional independence compared with stroke patients attending the control SRUs at 6 months post registration. The burden for caregivers of stroke patients attending intervention SRUs was not significantly different to that of caregivers of stroke patients attending the control SRUs at 6 months. There was no difference in patient recovery or caregiver burden between the intervention and control groups.
Secondary outcomes
There were no statistically significant differences in stroke patients' mood, health state, functional ability and health-related quality of life or in the number of deaths, hospital readmissions or institutionalisations between the intervention and control arms at both 6 and 12 months post registration. Similarly, there were no statistically significant differences in caregivers' social restriction, mood, health state, or any difference in deaths, hospitalisation and institutionalisation at 6 or 12 months for those caregivers of patients attending intervention SRUs compared with those attending control SRUs. There was no difference in the outcome of patients' and caregivers' physical and psychological well-being between the intervention and control groups.
Economic evaluation
The economic evaluation suggests that from a patient perspective, health and social care costs, societal costs and outcomes are similar for the intervention and control groups at 6 months, 12 months and over 1 year. CEACs based on the net benefit approach, which accounted for uncertainty around point estimates of differences and potential willingness-to-pay thresholds of up to £2000 per point improvement on the NEADL scale and up to £50,000 for an additional QALY, suggest that the intervention group is less likely to be cost-effective than the control group.
From a caregiver perspective, societal costs and outcomes are similar between the two groups at 6 months, 12 months and over 1 year. Health and social care costs were on average £207 higher (95% CI £5 to £408) in the intervention group at 6 months, but this difference was no longer apparent at 12 months or over 1 year. Caregiver CEACs suggested that the intervention group is less likely to be cost-effective than the control group based on QALYs for thresholds up to £50,000 per QALY gain, but, based on the CBS, it has between 53% and 63% probability of cost-effectiveness for thresholds of £500 to £2000 per point improvement on this scale from the health and social care perspective and 63–68% from the societal perspective. The value of a one point change in this measure and the willingness to pay for it from a policy-making viewpoint is unclear.
Informal care costs were considerable in size and notably increased total care costs. However, conclusions were broadly similar from both health and social care and societal perspectives.
The ability of the EQ-5D to detect changes in quality of life in these patient and caregiver groups is unclear and needs further exploration.
Safety
Training of caregivers was not detrimental to patients. No unexpected SAEs occurred in the trial, and the number of falls between registration and discharge from the SRU were minimal in both control and intervention arms.
Comparison of Training Caregivers After Stroke with the single-centre study
The LSCTC was evaluated in a single-centre RCT of 300 patients by Kalra et al. 19 This study reported a significant cost reduction for those patients treated using the LSCTC, as well as a significant reduction in caregiver burden, and improved quality of life and mood for both patients and their caregivers. The TRACS trial assessed the LSCTC using a multicentre, pragmatic design, wherein the LSCTC was implemented as a part of standard practice and delivered by all members of the MDT, and its effectiveness was assessed across different health-care settings and a larger heterogeneous population. The large multicentre pragmatic RCT did not replicate the findings of the single-centre study. The LSCTC does not provide any benefit to patients' long-term recovery or psychological well-being, nor does it reduce caregivers' burden or enhance psychological well-being compared with usual care. The LSCTC was found to be no more cost-effective than standard care.
It is important to note that the recruitment of patients in the single-centre study took place almost 10 years ago. Since that time stroke guidance has highlighted the importance of involving caregivers throughout the stroke patients stay on the SRU. 39,75 Comparison of the caregiver burden scores in the single-centre study at 3 months (intervention median = 43; control median = 51) and the TRACS trial at 6 months (intervention mean = 46.3; control mean = 45.7) suggest that caregivers may receive more support, resulting in less burden. However, although some of the differences identified in the single-centre study may have been lost as general stroke care has improved, we believe that it is unlikely that standard care has improved to such a degree that caregivers needs have been successfully met. Indeed, the caregiver burden scores in both the intervention and control arms indicate a level of burden that still needs addressing, with a score of 45 relating to a high level of caregiver burden.
Strengths and limitations of the study
The TRACS trial is the largest stroke rehabilitation trial completed to date (worldwide), with 930 patient and caregiver dyads registered to the trial. It successfully recruited to target, demonstrating that large, multicentre cluster RCTs of stroke rehabilitation are feasible.
The TRACs trial was one of the first large multicentre stroke rehabilitation trials to use the new SRN. The establishment of the local SRNs helped to determine the geographical regions selected for participation in the TRACS trial. The availability of SRN researchers in hospitals across entire regions allowed TRACS centres to be recruited from an excellent spread of locations and health service types – from rural community hospitals in the South West Peninsula to large urban acute hospitals in central London. The SRN researchers assisted with the successful minimisation of selection bias in the TRACS trial, allowing recruitment by research staff that was entirely independent of the clinical MDTs. The invaluable support of the SRN no doubt ensured the successful recruitment of clusters and participants to target in the TRACS trial. In addition, the design of the TRACS trial has been a pioneer in bridging the chasm between clinical staff and research, by involving members of the MDTs in the research process. Many of the PIs in TRACS were senior therapists/nurses, and all participating MDTs have received an insight into the research process as a consequence of taking part in this pragmatic trial.
Study design
The TRACS trial followed closely the Medical Research Council (MRC) guidance on the evaluation of a complex intervention: a cluster RCT design was chosen as the most appropriate design for the evaluation of the LSCTC; outcome measures were carefully considered; an economic evaluation was conducted; and process data were collected. In addition, a parallel, complementary process evaluation study [funded by the National Institute for Health Research (NIHR) Research for patient benefit funding stream] was conducted to examine the nature and influence of SRU contexts on team- and patient-focused practices, on implementation processes of the LSCTC, and on the beliefs, understanding and actions of health professionals, stroke survivors and caregivers. The findings of this study have proved invaluable in interpreting the TRACS trial results and are discussed further below.
Internal validity
Clusters
Thirty-six clusters were randomised equally between control and intervention study arms, stratified by geographical region and NSSA score. Following randomisation, the characteristics of the SRUs were found to be well balanced. No clusters were lost to follow-up during the trial. However, some units struggled to achieve the target recruitment of 25 patient and caregiver dyads. Low recruitment in some SRUs was compensated for by increasing the recruitment target in high-recruiting SRUs. The loss of power in the trial caused by the unequal cluster sizes was compensated for by increasing the target recruitment beyond 900.
The process data collected in the TRACS trial indicates that, aside from the implementation of the LSCTC, there were few changes to the process of care affecting the involvement of patients and caregivers on SRUs throughout the course of the trial. More specialised stroke community teams and early supported discharge teams have emerged since the beginning of the trial but were still only present in one-third of the participating SRUs, and were balanced between study arms. Changes in the process of care take a long time to implement, and have not influenced the overall standards of care in the control and intervention SRUs taking part in the TRACS trial.
Recruitment
Recruitment of patients and caregivers commenced after cluster randomisation of the SRUs. The large sample size required in this trial, and the restricted length of hospital stay for all stroke patients, meant that it was not possible to identify and recruit the entire trial cohort prior to cluster randomisation. Therefore, the trial was carefully designed to avoid selection bias. Researchers independent of the clinical teams were used to screen and recruit participants. Attempts were made to ensure that the MDTs were unaware of which patients' and caregivers' had consented to provide trial data in study procedures, and MDT staff in the intervention arm were required to complete the LSCTC for all eligible participants regardless of trial participation. All participants were blinded to the SRUs treatment allocation. These design features proved successful as both the numbers of participants recruited and their baseline characteristics were well balanced between the study arms, demonstrating a lack of selection bias in the recruitment of participants. The follow-up rate of 75% at 6 months required for the power calculation was nearly achieved in the trial, with an actual follow-up rate of 74.4% for patients and 72.5% for caregivers.
Generalisability
Four disparate geographical regions ensured a good representation of different health-care settings (acute/community hospitals in rural and urban settings) and different SRU set-ups (combined acute and rehabilitation, and rehabilitation). The eligibility criteria in TRACS were kept to a minimum in keeping with the pragmatic trial design, to ensure that a representative stroke patient population was recruited. Patients with language and cognitive impairment were included, and there was no ‘cut-off’ level of age or disability. The results of the TRACS trial should be generalisable to all stroke patients returning home requiring support from informal caregivers, and to these informal caregivers, in SRUs across the UK.
Implementation of a complex intervention
As a pragmatic trial, the intervention was implemented as it would have been with any service initiative within the NHS. The challenges of implementation were considered carefully in designing the delivery of the training, and included choosing a method that (1) would be acceptable and feasible to MDT staff and NHS management; (2) could easily be replicated in SRUs across the UK at the end of the trial; and (3) would allow successful implementation of the LSCTC programme. Training was provided at two national training days for each intervention site, during which practical issues of implementation were discussed. This was supported by a training manual, CD and training records. The SRUs were asked to implement and embed the intervention on the wards over a 4- to 6-month period prior to the start of patient recruitment. The intent was that the training was cascaded down by staff who attended the training day to other staff on the wards. It may be that this commonly used ‘cascade’ method was not as effective as we would have wished and that other methods for implementing such service improvements should be considered.
The lack of in-depth piloting of the modified LSCTC and its implementation may be viewed as a weakness of the present study. Although piloting was conducted on a sample of caregivers in each of the intervention centres prior to the second training day, this may not have allowed sufficient insight into the complexities involved in successfully implementing a complex intervention as a part of standard care.
Intervention compliance
The intervention was accepted well by the staff attending the training days, with recognition of the importance of each of the core competencies, demonstrating ‘face validity’ for the intervention. A component of the intervention was the completion of the TRACS training record (see Appendix 1). Completed training records were returned to the trial manager and included as a standard monitoring report to the TMG and TSC. This enabled us to monitor the intervention delivery and the compliance for each patient in terms of competencies obtained. We felt that this level of monitoring was in keeping with the pragmatic trial design. Concerns about compliance were explored by the trial manager. In two instances for which compliance was very poor, letters were sent from the chief investigator to the local PIs and these were followed up with visits from the trial manager. Higher-level monitoring, for example, by check visits to the intervention units, would be potentially threatening to participating staff, probably observe unrepresentative practice, provide only a few observations and be research resource intensive.
The completion and return of this record varied across the SRUs, with an overall compliance rate of 43.6% (range 0.0–92.9%). These data indicate that some units did not implement the LSCTC as robustly as envisaged; however, half of the participating centres had a compliance rating of over 60% (one-third > 75%). The measure of compliance, provided by the TRACS development team, was defined as completion of all six mandatory components of the training or sign-off of the training record by clinical staff. This may be a strict assessment of compliance. Compliance rates are compatible with other trials evaluating complex interventions.
To our knowledge, no other study has evaluated a novel, complex intervention delivered by the whole MDT on a stroke unit. Moreover, few studies have assessed the compliance of the interventions that they are evaluating. Four previous studies15,17,19,76 have investigated inpatient interventions delivered by a specialist individual or team on a SRU. However, only one of these studies measured and reported compliance with the intervention. In this study, completed by Larson et al. ,15 compliance (defined as patient attendance at a minimum of five out of the six education sessions offered) was 50%. It is disappointing that some centres do not appear to have implemented the intervention consistently, but it is emphasised that the compliance analysis show no evidence of higher levels of patient independence or lower level of caregiver burden in the SRUs with better levels of intervention compliance.
Complementary Process Evaluation Study (separate papers in preparation)
A complementary, independent process evaluation study was conducted alongside the TRACS trial. This study investigated the implementation of the LSCTC, and the views of MDT staff, patients and caregivers on the LSCTC in a sample of six intervention SRUs. Observations and interviews were also completed in four control SRUs so that comparisons could be made. The findings of the process evaluation study has provided valuable and insightful data to inform interpretation of the TRACS trial results.
Interpretation of results
The observations conducted on the SRUs in the process evaluation study reflected the completion rate of training records in the main trial – the LSCTC was observed successfully in some SRUs, some of the time in other SRUs, and not at all in one SRU. The key findings of the process evaluation study were that the LSCTC was only one of a number of competing priorities for SRU teams. Members of staff were trained to deliver the LSCTC and then expected to train their teams but training did not reach all staff, particularly nurses. Therapy staff were the most actively involved with training delivery in the observed units. Different disciplines often worked separately with caregivers rather than the MDT working together as a team. Caregiver training was typically delivered very late in the inpatient stay. Staff concern with reducing risk and safe discharge often meant caregiver training involved passive observation and not active engagement and practice. For caregivers, the stroke was a shock, this impacted on their understanding of what had happened and readiness to participate in training. After discharge, caregivers' recall of information and training was very limited.
The LSCTC was implemented using cascade training. Although training did occur in all centres, the staff attending varied, and the time available to cascade the training was relatively brief. In general, nurses received less training, and for a shorter amount of time. Training tools were left on each unit to help support new members of staff, but the process evaluation study suggests that these resources were not well utilised.
Stroke survivors' abilities could change rapidly once at home, so home visits and early supported discharge schemes provided opportunities for in situ training, which was valued. Caregivers perceived the training to be important, but it only addressed one of many areas of adjustment with which to cope.
It may be speculated from process evaluation work that the intervention was more robustly implemented when key members of the MDT took ownership of the LSCTC and training records. This form of working is more similar to that used in the single-centre study, for which the training was completed by a small, highly experienced, team.
Economic evaluation
The economic evaluation from the patient perspective suggests that the intervention and control groups have similar costs and outcomes at all of the assessment points considered. From the caregiver perspective, the intervention group had higher health and social care costs at 6 months (for equivalent outcomes). Costs and outcomes over the longer term and from the societal perspective were similar, and illustrate that increasing the breadth and length of such an evaluation can provide alternative conclusions. However, even 1 year is a relatively short time horizon in the context of a lifetime and it is unclear whether or not our findings would hold over patient and caregiver lifetimes.
The cost findings are in contrast with those from the single-centre study,19,29 which found a statistically significant difference in total patient health and social care costs over 1 year of £4043 in favour of the intervention group, largely due to a lower length of stay (–12 days) for the initial stroke admission. Total patient health and social care costs over 1 year in the present study were £563 lower in the control group (although not statistically significant) and we found no difference in the average length of stroke unit stay for the initial stroke admission (45 and 42 days in the intervention and control groups, respectively), which interestingly was similar to that for the control group in the single-centre study (43 days). It is unclear whether the intervention, or the way it was implemented, simply did not impact on discharge practices in the study stroke units, or whether or not other factors (e.g. care standards) differed over time and place to reduce the potential for earlier discharge that existed in the single-centre study.
With regards to caregiver costs, we cannot discount the possibility that providing caregivers with more structured training increased their use of health and social care resources and their provision of informal care to the patient as an appropriate response to their and the patients' needs. However, if this was the case, any potential benefits of this were not evident in the outcome measures we examined.
Examining cost and outcome differences based on point estimates gave a mixed set of conclusions across the various time point and perspectives but the meaningfulness of these is unclear given the lack of statistically significant differences in costs and outcomes except for higher caregiver health and social care costs at 6 months. There were no differences in patient or caregiver QALYs at 6 months, 12 months or over 1 year, and CEACs based on patient and caregiver QALYs suggested that the intervention group was less likely to be cost-effective compared with the control group. The single-centre study on which this was based19,29 also failed to detect any QALY differences. As we used no additional health-related quality-of-life measure, it is unclear whether there truly were no differences or whether the EQ-5D was unable to detect changes in quality of life. Any such limitation of the EQ-5D may be addressed in future research with the new five-level version (EQ-5D-5L), which has been developed to improve the instrument's sensitivity and to reduce its ceiling effects.
Patient CEACS based on the NEADL score suggested equivalent probabilities of cost-effectiveness for the two groups but caregiver CEACs based on the CBS suggested 53–68% probability of cost-effectiveness for thresholds of £500–2000 per point improvement on the CBS. However, the value of a one point change in this measure and the willingness to pay for it from a policy-making viewpoint is unclear.
The cost of developing the intervention and training ward staff in its use was estimated at an average of £39 per patient/caregiver dyad across the whole intervention group. The average cost was higher at £82 when considering only those known to have received some caregiver training. This is relatively inexpensive in the context of the average cost of an acute stroke bed-day (£294) and the average stroke admission cost in this sample (£13,127 and £12,471 in the intervention group and control group, respectively). We did not include the cost of delivering the intervention to caregivers to avoid double-counting admission costs. However, this exclusion is unlikely to have affected the findings, first because these costs are likely to be small in the context of total care costs and, second, because there was little difference in the amount of time spent with caregivers in each group.
Although the difficulties of reliably assessing informal care inputs is well known, the study suggests that the informal care burden for caregivers of stroke patients is sizeable. Patient care costs were 70% higher in both intervention and control groups at 6 months when informal care costs were added in. Caregiver costs were 21% and 34% higher in the intervention and control groups, respectively, when the costs of the inputs they provided were considered. These care inputs and their costs remained high at 12 months, thus the informal care burden is both significant in size and duration. The impact of patients' strokes on caregivers' own health is further evidenced by the noticeable increase in their use of community- and secondary-based care in the post-stroke period compared with baseline.
There were two main limitations with the economic evaluation, both of which are common to evaluations of this type. First, resource-use data were collected retrospectively by self-report as this was the most efficient way of collecting data for a large sample, across a large geographical area and from a broad perspective incorporating primary health care, secondary health care, social care and informal care. Such data may be subject to recall bias, but a trade-off between reliability and scope was necessary given the trial design. Second, the cost-effectiveness and cost–utility analyses were performed on subsamples of cases with available data for both costs and relevant outcomes. However, overall follow-up rates were very good given the features of the trial (multicentre, older participants, follow-up period 6/12 months after the acute event), and sensitivity analyses that imputed missing costs and QALY data suggested that findings from the incomplete sample analyses were fairly robust.
Overall evidence
A recent Cochrane review has examined the effectiveness of non-pharmacological interventions for caregivers of stroke survivors in reducing caregiver burden or enhancing caregiver well-being. 14 Eight relevant studies were found that assessed information and support interventions, psychoeducational interventions and caregiver training. The combined sample size of the eight studies was 1007. The conclusions of this review was that it is not, at present, possible to determine the usefulness of information and support interventions, or psychoeducational interventions on reducing caregiver burden, or enhancing their psychological well-being or health-related quality of life. The results of the caregiver training programme in the single-centre study looked promising, appearing to reduce caregiver burden, depression, and improve health-related quality of life for caregivers. The purpose of TRACS was to see if this caregiver training programme – the LSCTC, continued to show benefits to caregivers and stroke patients if it was implemented as a part of standard practice in SRUs across the UK. The TRACS trial (n = 928) has almost doubled the sample size of the Cochrane Review, providing conclusive evidence that there is no difference between the LSCTC and usual care with respect to stroke patients' recovery, caregivers' burden, or other physical and psychological outcomes, nor is it cost-effective when compared with usual care. Caregivers need more than just an inpatient structured training programme to improve the patients and their own outcomes.
The findings of the process evaluation study completed in parallel with TRACS suggest that caregiver training while the stroke patient is in hospital may not be the optimal time to complete such training. The LSCTC competes with other priorities for MDT staff, and caregivers are experiencing stress, making any such training less effective at that time point. Caregiver training in the community, by a dedicated experienced team, might be more beneficial for patients and their caregivers.
Of the eight studies reviewed by Legg et al. ,14 three interventions were completed while the stroke patient was still in hospital, one was completed both as an inpatient and continued once at home, two were longer-term community interventions and the location of delivery of two were unclear, although both recruited patients in the acute inpatient setting. The timing/setting of the intervention in these studies did not have any clear influence on the effectiveness of these interventions.
Chapter 7 Conclusions
Implications for clinical practice
The intervention evaluated had reported benefits in a previous single-centre evaluation but these benefits have not been replicated in this large, multicentre trial in which the intervention was evaluated in a range of settings with a greater diversity of patient populations. There was no difference between the LSCTC and usual care with respect to improving stroke patients' recovery, reducing caregivers' burden, or improving other physical and psychological outcomes, nor is it cost-effective when compared with usual care.
Compliance with the intervention varied across stroke units but analysis demonstrated no link between the degree of compliance and associated patient or caregiver outcomes, indicating that a dose effect is unlikely. The LSCTC provided a structured framework for caregiver training. It is possible that the immediate post-stroke period, when potential caregivers are coming to terms with their new situation, may not be the ideal time for the delivery of structured training. The intervention approach might be more relevant if delivered after discharge by community-based teams.
Implications for research
The TRACS trial is the world's largest completed stroke rehabilitation trial. We have demonstrated for the first time that this methodology can feasibly be implemented in stroke rehabilitation research, which represents a major step forward in research methodology for this large client group. The TRACS trial fills some of the gaps in evidence of the recent Cochrane review. Despite the promising results of the single-centre study, the results of TRACS provides conclusive evidence that there is no difference between the LSCTC and usual care on stroke patients' functional independence, caregivers' burden, or other physical and psychological outcomes, nor is it cost-effective when compared with usual care. Caregivers need more than just an inpatient structured training programme to improve the patients and their own outcomes.
Future studies should consider carefully the optimal delivery of any caregiver interventions – whether or not the staff have the time and opportunity to implement the intervention, and when is the optimal timing of the intervention for the caregiver. Caregiver interventions may be more effective in the post-acute phase, once patients are back home and caregiving has become a reality.
Acknowledgements
This project was funded by the MRC and is managed by the NIHR (project number 09/800/10) on behalf of the MRC–NIHR partnership, and will be published in full in the Health Technology Assessment journal. See the NETSCC website for further project information.
We would like to thank all the patients and caregivers who participated in this study, the MDT members on all participating SRUs, the NIHR Stroke Research Network staff involved and the Consumer Research Advisory Group for their invaluable contribution to TRACS. We also thank Renee Romeo, Margaret Heslin and Rachel Breen for their assistance with the trial.
Thank you to all who helped deliver this trial.
TRACS Trial Collaboration
Chief Investigator A Forster.
TRACS Trial Management Group A Forster, J Young, J Dickerson, A Patel, L Kalra, D Smithard, J Nixon, M Knapp, I Holloway, S Anwar and A Farrin.
Trial Steering Committee P Langhorne (Chairperson), A Drummond, K Hood and H Rodgers.
Data Monitoring and Ethics Committee D Wade (Chairperson), A Bowen and J Norrie.
LSCTC Development Team Ann Melbourn, Jayne Steadman and Margreet Wittink.
South East TRACS Coordinator Emily Jay.
Participating SRUs (no. of dyads recruited): Airedale General Hospital (34), S Mawyer, S Williamson and P Garnett; Blackpool Victoria Hospital (27), K Waywell, J Howard, H Goddard, S Preston, J McIlmoyle and M J O'Donnell; Budleigh Salterton Hospital and Crediton Hospital (31) T Ayers, L Barron and A Bowring; Calderdale and Huddersfield NHS Foundation Trust (20), G Seebass, I Shakir, C Button, J Greig, D Nicholson, M Barber, T Smith and P Finn; Camborne Redruth Community Hospital (21), S Coltman, G Courtald and S Connife-Jones; Cumberland Infirmary (24), P Davies, C Hagon and L Pearce; Darent Valley Hospital (30), S Hussein, T Daniels and J Hancock; Dewsbury and District Hospital (33), P Datta, G Bateman and K Mallinder; Ellesmere Port/Countess of Chester Hospitals (32), K Chatterjee, C Kelly, K James and C Child; Fairfield General Hospital (18), K Kawafi, A Bell, S Moulton and C Curley; Harrogate District Hospital (25), S Brotheridge, J Strover, A Norton and A Wray; Salford Royal NHS Foundation Trust (27), S Moss, J Stevens, R Marsh and E Barberan; Kent and Canterbury Hospital (12), H Baht and B Bourne; King's College Hospital (38), L Kalra, E Jay, C Potter, A Davies, A M Murtagh and M Fitzpatrick; Macclesfield District General Hospital (23), C Davison, H Rooney, B Simpson and S Bailey; Mile End Hospital (23), P Gompertz, T Sachs, H Kariuki; Mount Gould Hospital (35), C Gatehouse, B Hyams, C Brown and R Truscott; Newton Abbot Hospital (29), R Allison, N Wedge, S Thomas, A Beck and H Nott; Royal Oldham Hospital (25), E Walker and S Parnell; Pinderfields General Hospital (30), M Carpenter, A Needle, C Holland, V Newton and AM Doran; Queen Elizabeth The Queen Mother Hospital (13), G Guna, J Idris and S Jones; Rochdale Infirmary (26), S Powell, N Thomas, R Namushi and N Saravanan; Rotherham District General Hospital (26), J Okwera, K McNulty and L Strachan; Scarborough General Hospital (16), J Paterson, R Rose, S Jamieson, M Sellers, A Davidson, M Foden and L Beadle; Sheffield Teaching Hospitals NHS Foundation Trust (23), A Jones, R Palmer, S Ross and C Draper; Sussex Rehabilitation Centre, Brighton and Sussex University Hospitals (BSUH) (35), A Harper, J Breeds, G Spurling and K Stephenson; Southport and Ormskirk Hospital NHS Trust (17), H Duff, M Marshall, S Wright, R Lawrence; St Helens & Knowsley NHS Trust (14), V Gowda, S Dealing, R Brown, A Ledger, G Fletcher and R Irving; St Luke’s Hospital (35), C Patterson, L Johnston, J Stevens, D Walshaw, A Adams and S Oxley; St Thomas’ Hospital (19), A Rudd; University Hospital Aintree (35), Helen Martin, E Bacabac and V Sutton; West Cumberland Hospital (32), O Orugun and R Jolly; Weston General Hospital (17), G Saunders and H Dymond; William Harvey Hospital (25), D Smithard and A Reid; Williton Hospital (35), S Glanfield and L Caudwell; York Teaching Hospital NHS Foundation Trust (26), J Coyle, E Iveson, C Rhymes, M Keeling, S Craigie and J Heppel.
Contributions of authors
Anne Forster (Professor of Stroke Rehabilitation): the conception and design of the study, acquisition and interpretation of data and drafting of this paper.
Josie Dickerson (Trial Manager): the acquisition and interpretation of data and drafting of this paper.
John Young (Professor of Elderly Care): the design of the study, interpretation of data and commenting on the draft of this paper.
Anita Patel (Reader, Health Economics): the design of the study, analysis and interpretation of data, and drafting of this paper.
Lalit Kalra (Professor of Stroke Medicine): the conception and design of the study, and commenting on the draft of this paper.
Jane Nixon (Deputy Director, CTRU): the design of the study, acquisition of data and commenting on a draft of this paper.
David Smithard (Stroke Consultant): the design of the study and commenting on the draft of this paper.
Martin Knapp (Professor of Health Economics): the design of the study and commenting on the draft of this paper.
Ivana Holloway (Trial Statistician): the analysis of the data and drafting of this paper.
Shamaila Anwar (Senior Trial Co-ordinator): data acquisition and commenting on a draft of this paper.
Amanda Farrin (Director of Health Sciences Division, CTRU/Principal Statistician): the design of the study, acquisition, analysis and interpretation of data, drafting of this paper and statistical guarantor.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the MRC, NETSCC, the HTA programme, the EME programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme, the EME programme or the Department of Health.
Publication
Forster A, Young J, Nixon J, Kalra L, Smithard D, Patel A, et al. A cluster randomised controlled trial of a structured training programme for caregivers of inpatients after stroke (TRACS Trial). Int J Stroke 2012;7:94–9.
Forster A, Dickerson J, Young J, Patel A, Kalra L, Nixon J, et al. A structured training programme for caregivers of inpatients after stroke (TRACS): a cluster randomised controlled trial and cost–effectiveness analysis [published online ahead of print 18 September 2013]. Lancet http://dx.doi.org/10.1016/S0140-6736(13)61603-7
References
- Rothwell PM, Coull AJ, Giles MF, Howard SC, Silver LE, Bull LM, et al. Change in stroke incidence, mortality, case-fatality, severity, and risk factors in Oxfordshire, UK from 1981 to 2004 (Oxford Vascular Study). Lancet 2004;363:1925-33. http://dx.doi.org/10.1016/S0140-6736(04)16405-2.
- National Sentinel Audit for Stroke. London: RCP; 2010.
- Tooth L, Mckenna K, Barnett A, Prescott C, Murphy S. Caregiver burden, time spent caring and health status in the first 12 months following stroke. Brain Injury 2005;19:963-74. http://dx.doi.org/10.1080/02699050500110785.
- Dewey HM, Thrift AG, Mihalopoulos C, Carter R, Macdonell RAL, McNeil JJ, et al. Informal care for stroke survivors: results from the North East Melbourne Stroke Incidence Study (NEMESIS). Stroke 2002;33:1028-33. http://dx.doi.org/10.1161/01.STR.0000013067.24300.B0.
- Reducing brain damage: faster access to better stroke care. London: NAO; 2005.
- Mackenzie A, Perry L, Lockhart E, Cottee M, Cloud G, Mann H. Family carers of stroke survivors: needs, knowledge, satisfaction and competence in caring. Disabil Rehabil 2007;29:111-21. http://dx.doi.org/10.1080/09638280600731599.
- Murray J, Young J, Forster A, Ashworth R. Developing a primary care-based stroke model: the prevalence of longer-term problems experienced by patients and carers. Br J Gen Pract 2003;53:803-7.
- Evans RL, Bishop DS, Haselkorn JK. Factors predicting satisfactory home care after stroke. Arch Phys Med Rehabil 1991;72:144-7.
- Forsberg-Wärleby G, Möller A, Blomstrand C. Psychological well-being of spouses of stroke patients during the first year after stroke. Clin Rehabil 2004;18:430-7. http://dx.doi.org/10.1191/0269215504cr740oa.
- Visser-Meily A, van Heugten C, Post M, Schepers V, Lindeman E. Intervention studies for caregivers of stroke survivors: a critical review. Patient Educ Couns 2005;56:257-67. http://dx.doi.org/10.1016/j.pec.2004.02.013.
- Kerr SM, Smith LN. Stroke: an exploration of the experience of informal caregiving. Clin Rehabil 2001;15:428-36. http://dx.doi.org/10.1191/026921501678310234.
- McKevitt C, Redfern J, Mold F, Wolfe C. Qualitative studies of stroke: a systematic review. Stroke 2004;35:1499-505. http://dx.doi.org/10.1161/01.STR.0000127532.64840.36.
- Greenwood N, Mackenzie A, Cloud GC, Wilson N. Informal carers of stroke survivors – factors influencing carers: a systematic review of quantitative studies. Disabil Rehabil 2008;30:1329-49. http://dx.doi.org/10.1080/09638280802051721.
- Legg LA, Quinn TJ, Mahmood F, Weir CJ, Tierney J, Stott DJ, et al. Non-pharmacological interventions for caregivers of stroke survivors. Cochrane Database Syst Rev 2011;10. http://dx.doi.org/10.1002/14651858.CD008179.
- Larson J, Franzén-Dahlin Å, Billing E, von Arbin M, Murray V, Wredling R. The impact of a nurse-led support and education programme for spouses of stroke patients: a randomized controlled trial. J Clin Nurs 2005;14:995-1003. http://dx.doi.org/10.1111/j.1365-2702.2005.01206.x.
- Mant J, Carter J, Wade DT, Winner S. Family support for stroke: a randomised controlled trial. Lancet 2000;356:808-13. http://dx.doi.org/10.1016/S0140-6736(00)02655-6.
- Yoo EK, Jeon S, Yang J. The effects of a support group intervention on the burden of primary family caregivers of stroke patients. Taehan Kanho Hakhoe Chi 2007;37:693-702.
- Pierce LL, Steiner VL, Khuder SA, Govoni AL, Horn LJ. The effect of a web-based stroke intervention on carers’ well-being and survivors’ use of healthcare services. Disabil Rehabil 2009;31:1676-84. http://dx.doi.org/10.1080/09638280902751972.
- Kalra L, Evans A, Perez I, Melbourn A, Patel A, Knapp M, et al. Training care givers of stroke patients: randomised controlled trial. BMJ 2004;328:1099-101.
- Draper B, Bowring G, Thompson C, Van Heyst J, Conroy P, Thompson J. Stress in caregivers of aphasic stroke patients: a randomized controlled trial. Clin Rehabil 2007;21:122-30. http://dx.doi.org/10.1177/0269215506071251.
- Grant JS, Elliott TR, Weaver M, Bartolucci AA, Giger JN. Telephone intervention with family caregivers of stroke survivors after rehabilitation. Stroke 2002;33:2060-5. http://dx.doi.org/10.1161/01.STR.0000020711.38824.E3.
- Hartke RJ, King RB. Telephone group intervention for older stroke caregivers. Top Stroke Rehabil 2003;9:65-81.
- Brereton L, Carroll C, Barnston S. Interventions for adult family carers of people who have had a stroke: a systematic review. Clin Rehabil 2007;21:867-84. http://dx.doi.org/10.1177/0269215507078313.
- Eldred C, Sykes C. Psychosocial interventions for carers of survivors of stroke: a systematic review of interventions based on psychological principles and theoretical frameworks. Br J Health Psychol 2008;13:563-81. http://dx.doi.org/10.1348/135910707X236899.
- Smith J, Forster A, Young J, Knapp P, House A, Wright J. Information provision for stroke patients and their caregivers. Cochrane Database Syst Rev 2001;3. http://dx.doi.org/10.1002/14651858.CD001919.
- Smith J, Forster A, Young J. Cochrane Group for information provision after stroke. Cochrane review: information provision for stroke patients and their caregivers. Clin Rehabil 2009;23:195-206. http://dx.doi.org/10.1177/0269215508092820.
- Brereton L, Nolan M. ‘You do know he’s had a stroke, don’t you?’ Preparation for family care-giving – the neglected dimension. J Clin Nurs 2000;9:498-506. http://dx.doi.org/10.1046/j.1365-2702.2000.00396.x.
- Smith LN, Lawrence M, Kerr SM, Langhorne P, Lees KR. Informal carers’ experience of caring for stroke survivors. J Adv Nurs 2004;46:235-44. http://dx.doi.org/10.1111/j.1365-2648.2004.02983.x.
- Patel A, Knapp M, Evans A, Perez I, Kalra L. Training care givers of stroke patients: economic evaluation. BMJ 2004;328:1102-4A. http://dx.doi.org/10.1136/bmj.328.7448.1102.
- Elmstahl S, Malmberg B, Annerstedt L. Caregiver’s burden of patients 3 years after stroke assessed by a novel caregiver burden scale. Arch Phys Med Rehabil 1996;77:177-82. http://dx.doi.org/10.1016/S0003-9993(96)90164-1.
- The EuroQol Group . EuroQol: a new facility for the measurement of health-related quality of life. The EuroQol Group. Health Pol 1990;16:199-208.
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983;67:361-70. http://dx.doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Wade DT, Legh-Smith J, Langton Hewer R. Social activities after stroke: measurement and natural history using the Frenchay Activities Index. Int Rehabil Med 1985;7:176-81. http://dx.doi.org/10.3109/03790798509165991.
- Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J 1965;14:61-5.
- van Swieten J, Koudstaal P, Visser M, Schouten H, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604-7. http://dx.doi.org/10.1161/01.STR.19.5.604.
- Thorpe KE, Zwarenstein M, Oxman AD, Treweek S, Furberg CD, Altman DG, et al. A pragmatic explanatory continuum indicator summary (PRECIS): a tool to help trial designers. Can Med Assoc J 2009;180:E47-57. http://dx.doi.org/10.1503/cmaj.090523.
- National sentinel audit for stroke. London: Royal College of Physicians; 2006.
- National clinical guidelines for stroke. London: Royal College of Physicians; 2004.
- National clinical guidelines for stroke. London: Royal College of Physicians; 2008.
- Mental Capacity Act 2005. London: The Stationery Office; 2005.
- Nouri F, Lincoln N. An extended activities of daily living scale for stroke patients. Clin Rehabil 1987;1:301-5. http://dx.doi.org/10.1177/026921558700100409.
- Lincoln N, Gladman JRF. The extended activities of daily living scale: a further validation. Disabil Rehabil 1992;14:41-3. http://dx.doi.org/10.3109/09638289209166426.
- Krabbe P, Weijnen T, Brooks R, Rabin R, De Charro F. The measurement and valuation of health status using EQ-5D: a European perspective (evidence from the EuroQol BIOMED Reasearch Programme). Dordrecht: Kluwer Academic Publishers; 2003.
- Brooks R. EuroQol: the current state of play. Health Pol 1996;37:53-72. http://dx.doi.org/10.1016/0168-8510(96)00822-6.
- Gompertz P, Pound P, Ebrahim S. A postal version of the Barthel Index. Clin Rehabil 1994;8:233-9. http://dx.doi.org/10.1177/026921559400800308.
- Duncan PW, Bode RK, Min Lai S, Perera S. Glycine Antagonist in neuroprotection Americans Investigators. Rasch analysis of a new stroke-specific outcome scale. Arch Phys Med Rehabil 2003;84:950-63.
- Duncan PW, Lai SM, Bode RK, Perera S, DeRosa J. Stroke Impact Scale–16: a brief assessment of physical function neurology. Neurology 2003;60:291-6.
- Duncan PW, Reker DM, Horner RD, Samsa GP, Hoenig H, LaClair BJ, et al. Performance of a mail-administered version of a stroke-specific outcome measure, the Stroke Impact Scale. Clin Rehabil 2002;16:493-505. http://dx.doi.org/10.1191/0269215502cr510oa.
- Duncan PW, Wallace D, Lai SM, Johnson D, Embretson S, Laster LJ. The stroke impact scale version 2.0; evaluation of reliability, validity, and sensitivity to change. Stroke 1999;30:2131-40. http://dx.doi.org/10.1161/01.STR.30.10.2131.
- Lai SM, Perera S, Duncan PW, Bode RK. Physical and Social Functioning After Stroke: Comparison of the Stroke Impact Scale and Short Form-36. Stroke 2003;34:488-93. http://dx.doi.org/10.1161/01.STR.0000054162.94998.C0.
- Lai SM, Studenski S, Duncan PW, Perera S. Persisting consequences of stroke measured by the Stroke Impact Scale. Stroke 2002;33:1840-4. http://dx.doi.org/10.1161/01.STR.0000019289.15440.F2.
- Carter J, Mant F, Mant J, Wade D, Winner S. Comparison of postal version of the Frenchay activities index with interviewer-administered version for use in people with stroke. Clin Rehabil 1997;11:131-8. http://dx.doi.org/10.1177/026921559701100206.
- Patel A, Knapp M, Perez I, Evans A, Kalra L. Alternative strategies for stroke care: cost-effectiveness and cost-utility analyses from a prospective randomized controlled trial. Stroke 2004;35:196-203. http://dx.doi.org/10.1161/01.STR.0000105390.20430.9F.
- Parker CJ, Gladman JRF, Drummond AER, Dewey ME, Lincoln NB, Barer D, et al. A multicentre randomized controlled trial of leisure therapy and conventional occupational therapy after stroke. Clin Rehabil 2001;15:42-5. http://dx.doi.org/10.1191/026921501666968247.
- Walker MF, Gladman JRF, Lincoln NB, Siemonsma P, Whiteley T. Occupational therapy for stroke patients not admitted to hospital: a randomised controlled trial. Lancet 1999;354:278-80. http://dx.doi.org/10.1016/S0140-6736(98)11128-5.
- Gladman JRF, Lincoln NB, Adams SA. Use of the Extended ADL Scale with Stroke Patients. Age Ageing 1993;22:419-24. http://dx.doi.org/10.1093/ageing/22.6.419.
- Cunliffe AL, Gladman JRF, Husbands SL, Miller P, Dewey ME, Harwood RH. Sooner and healthier: a randomised controlled trial and interview study of an early discharge rehabilitation service for older people. Age Ageing 2004;33:246-52. http://dx.doi.org/10.1093/ageing/afh076.
- Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 2002;52:69-77.
- Brooke P, Bullock R. Validation of a 6 item cognitive impairment test with a view to primary care usage. Int J Geriatr Psychiatry 1999;14:936-40. http://dx.doi.org/10.1002/(SICI)1099-1166(199911)14:11<936::AID-GPS39>3.3.CO;2-T.
- Counsel C, Dennis MS, Lewis S, Warlow C. The FOOD Trial Collaboration, Feed or Ordinary Diet. Performance of a statistical model to predict stroke outcome in the context of a large, simple, randomized, controlled trial of feeding. Stroke 2003;34:127-33.
- Pound P, Gompertz P, Ebrahim S. Patients’ satisfaction with stroke services. Clin Rehabil 1994;8:7-17. http://dx.doi.org/10.1177/026921559400800102.
- Boter H, De Haan RJ, Rinkel GJ. Clinimetric evaluation of a satisfaction-with-stroke-care questionnaire. J Neurol 2003;250:534-41. http://dx.doi.org/10.1007/s00415-003-1031-2.
- Parker C, Dewey M. Assessing research outcomes by postal questionnaire with telephone follow-up. TOTAL Study Group. Trial of Occupational Therapy and Leisure. Int J Epidemiol 2000;29:1065-9. http://dx.doi.org/10.1093/ije/29.6.1065.
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JA, Burney PG. Methods for evaluating area-wide and organisation-based interventions in health and health care: a systematic review. Health Technol Assess 1999;3.
- Campbell MK, Mollison J, Grimshaw JM. Cluster trials in implementation research: estimation of intracluster correlation coefficients and sample size. Stat Med 2001;20:391-9. http://dx.doi.org/10.1002/1097-0258(20010215)20:3<391::AID-SIM800>3.3.CO;2-Q.
- Eldridge SM, Ashby D, Kerry S. Sample size for cluster randomized trials: effect of coefficient of variation of cluster size and analysis method. Int J Epidemiol 2006;35:1292-300. http://dx.doi.org/10.1093/ije/dyl129.
- Department of Health . NHS Trust Reference Cost Schedules (Appendix NSRC01) 2009–10 2011. www.dh.gov.uk/en/Publicationsandstatistics/Publications/publicationspolicyandguidance/DH_123459 (accessed 3 November 2011).
- Koopmanschap MA, van Exel JNA, van den Berg B, Brouwer WBF. An overview of methods and applications to value informal care in economic evaluations of healthcare. Pharmacoeconomics 2008;26:269-80. http://dx.doi.org/10.2165/00019053-200826040-00001.
- Williams A. Economics of coronary artery bypass grafting. Br Med J 1985;291:326-9. http://dx.doi.org/10.1136/bmj.291.6491.326.
- Dolan P, Gudex C, Kind P, Williams A. A social tariff for EuroQol: results from a UK population survey. York: University of York; 1995.
- van Hout BA, Al MJ, Gordon GS, Rutten FF. Costs, effects and C/E-ratios alongside a clinical trial. Health Econ 1994;3:309-19. http://dx.doi.org/10.1002/hec.4730030505.
- Guide to the methods of technology appraisal. London: NICE; 2008.
- McDaid D. Estimating the costs of informal care for people with Alzheimer’s disease: methodological and practical challenges. Int J Geriatr Psychiatry 2001;16:400-5. http://dx.doi.org/10.1002/gps.353.
- Farrin A, Russell I, Torgerson D, Underwood M. Differential recruitment in a cluster randomized trial in primary care: the experience of the UK Back pain, Exercise, Active management and Manipulation (UK BEAM) feasibility study. Clin Trials 2005;2:119-24. http://dx.doi.org/10.1191/1740774505cn073oa.
- National Stroke Strategy. London: DoH; 2007.
- Evans RL, Matlock AL, Bishop DS, Stranahan S, Pederson C. Family intervention after stroke: does counseling or education help?. Stroke 1988;19:1243-9. http://dx.doi.org/10.1161/01.STR.19.10.1243.
- The NHS Information Centre SCS . Personal Social Services Expenditure and Unit Costs England, 2009–10 n.d. www.ic.nhs.uk/webfiles/publications/009_Social_Care/pss0910expfinal/pss0910expfinal_update_070311/Personal_Social_Services_Expenditure_Report%202009_10.pdf (accessed 15 November 2011).
- n.d. www.nice.org.uk/nicemedia/live/13042/54898/54898.pdf (accessed 3 November 2011).
- Curtis L. Unit Costs of Health and Social Care 2010. Canterbury: Personal Social Services Research Unit, University of Kent; 2010.
- NHS Choices – Chiropractor n.d. www.nhs.uk/Conditions/chiropractic/Pages/Introduction.aspx (accessed 15 November 2011).
- NHS Choices – Osteopathy n.d. www.nhs.uk/conditions/Osteopathy/Pages/Introduction.aspx (accessed 15 November 2011).
- Boots Website n.d. www.boots.com/en/GBP10-eye-test_1240962/ (accessed 15 November 2011).
- ONS Annual Survey of Hours and Earnings, 2010 Revised n.d. http://www.ons.gov.uk/ons/publications/re-reference-tables.html?Edition=tcm%3A77–238620 (accessed 23 November 2011).
- Department for Transport . TAG Unit 3.5.6: Values of Time and Operating Costs n.d. www.dft.gov.uk/webtag/documents/expert/unit3.5.6.php#01 (accessed 14 November 2011).
- NHS Direct n.d. www.nhsdirect.nhs.uk/about/∼/media/files/boardpapers/november2010/board%20scorecard%20-%20header%20sheet.ashx (accessed 10 June 2011).
- NHS Employers . Pay Circular (AforC) 1 2009. Pay and Conditions for NHS Staff Covered by the Agenda for Change Agreement n.d. www.nhsemployers.org/Aboutus/Publications/PayCirculars/Documents/pay_circular%20_AfC_%20%201_2009.pdf (accessed 23 November 2011).
- NHS Employers . NHS Terms and Conditions of Service Handbook. Amendment Number 24, Pay Circular (AforC) 3 2011 n.d. www.nhsemployers.org/SiteCollectionDocuments/AfC_tc_of_service_handbook_fb.pdf (accessed 18 August 2011).
- University of Leeds . Academic Pay Scales from August 2009 n.d. www.leeds.ac.uk/hr/progression/documents/SalaryScales.xls (accessed 19 August 2011).
Appendix 1 London Stroke Carers Training Course training record and control time log
Appendix 2 Outline of the London Stroke Carers Training Course training days
Day 1
| Session | Given by | Time (minutes) | Brief description |
|---|---|---|---|
| 1. Introduction | AF | 20 | Introduction to the day |
| Background to the intervention – the problems of carer burden in stroke | |||
| 2. Principles of the training programme | AM | 5 | Background and development of the LSCTC in the single-centre study |
| MW | 5 | Introduction and description of the LSCTC (using training manual) | |
| JS | 5 | Recommended delivery of LSCTC to carers | |
| JS | 15 | Assessing carers competency | |
| JS | 5 | The 14 training components – suggested delivery, suggested resources to aid training and a recommended strategy for checking off the competencies | |
| 3. Workshops 1 and 2 | All | 120 | In local teams – go through competency items 1–14 (done over two sessions) and discuss how to approach the delivery of each item – who will deliver it, and how best to deliver it in your stroke unit |
| Feedback and discussion from all teams | |||
| 4. Discussion | JS | 30 | Questions, comments and discussion of issues arising during the workshops |
| 5. Completing the training records | AF | 20 | Explanation of the training record, and how to complete it |
| 6. Summing up | AF | 10 | Summing up of day and further questions discussed |
Day 2
| Session | Given by | Time (minutes) | Brief description |
|---|---|---|---|
| 1. Cascading of LSCTC to the MDT | All | 30 | Discussion of any issues, questions, tips for cascading the LSCTC to local stroke unit teams |
| 2. Review of training components | JS | 30 | Refresher of each training component, suggested delivery, suggested resources and recommended strategy for checking competency |
| 3. Practical issues of delivering the components | All | 30 | Open discussion of the practical issues of delivering the components and assessing competency |
| 4. Practical issues of using the training record | All | 30 | Open discussion of the training record, recommendations for changes |
| 5. Next steps | AF | 30 | Implementing the LSCTC as a part of standard practice |
| 6. Discussion | All | 30 | Open session for further questions and answers |
Appendix 3 London Stroke Carers Training Course training manual
Appendix 4 Protocol – Version 7.0
A cluster randomised controlled trial of a structured training programme for caregivers of in-patients after stroke
Chief Investigator: Professor Anne Forster, Academic Unit of Elderly Care and Rehabilitation, Bradford Institute for Health Research, Temple Bank House, Bradford Royal Infirmary, Duckworth Lane, Bradford, BD9 6RJ, UK.
Authors: Professor Anne Forster, Professor John Young, Professor Lalit Kalra, Dr David Smithard, Professor Martin Knapp, Dr Anita Patel, Ms Amanda Farrin, Dr Rachel Breen, Dr Josie Monaghan, Dr Shamaila Anwar and Dr Jane Nixon.
Co-ordinating Groups: Academic Unit of Elderly Care and Rehabilitation, Leeds Institute of Health Sciences, University of Leeds, UK Clinical Trials Research Unit, University of Leeds, Leeds, UK. Guy's, King's & St Thomas' School of Medicine and Institute of Psychiatry, Kings College London, London, UK. East Kent Hospitals NHS Trust, Department of Medicine, William Harvey Hospital, Ashford, Kent, UK.
Protocol Version 7.0 (2nd November 2009)
Protocol Grant Number: 470772
MRC Reference Number: G0501807
ISRCTN: 49208824
Leeds (West) Research Ethics Committee Reference Number: 07/Q1205/12
Funded by: (PDF download)
Chief Investigator
Professor Anne Forster Tel: 01274 38 3406
Academic Unit of Elderly Care and Rehabilitation Fax: 01274 382766
Bradford Institute for Health Research E-mail: a.forster@leeds.ac.uk
Temple Bank House
Bradford Royal Infirmary
Duckworth Lane
Bradford
BD9 6RJ
Research Manager
Dr Josie Monaghan Tel: 01274 383408
Academic Unit of Elderly Care and Rehabilitation Fax: 01274 382766
Bradford Institute for Health Research E-mail: j.monaghan@leeds.ac.uk
Temple Bank House
Bradford Royal Infirmary
Duckworth Lane
Bradford
BD9 6RJ
Senior Trial Coordinator
Dr Shamaila Anwar Tel: 0113 343 2653
Trial Coordinator Fax: 0113 343 1471
Clinical Trials Research Unit E-mail: s.t.anwar@leeds.ac.uk
17 Springfield Mount
Leeds
LS2 9NG
Supervising Statistician
Ms Amanda Farrin Tel: 0113 343 8017
Director/Principal Statistician – Health Sciences Fax: 0113 343 1471
Portfolio
Clinical Trials Research Unit E-mail: a.j.farrin@leeds.ac.uk
17 Springfield Mount
Leeds
LS2 9NG
Trial Statistician
Miss Maria Efthymiou Tel: 0113 343 1472
Medical Statistician Fax: 0113 343 1471
Clinical Trials Research Unit E-mail: m.efthymiou@leeds.ac.uk
17 Springfield Mount
Leeds
LS2 9NG
TRIAL SUMMARY
This cluster, randomised, controlled trial is designed to evaluate the clinical and cost-effectiveness of a structured, competency-based training programme for caregivers of stroke patients returning home with stroke-related disabilities. The trial aims to recruit 950–1000 patients and caregivers in 36 stroke rehabilitation units. The intervention developed by Kalra and colleagues is known as the London Stroke Carer Training Course (LSCTC) and comprises a number of carer training sessions, competency assessment and one follow up session after discharge. The multidisciplinary teams (MDTs) in the units randomised to the intervention group will be trained to deliver the LSCTC, whilst those randomised to the control group will continue to provide usual care as per the National Guidelines. The primary outcomes are extended activities of daily living for the patient and caregiver burden measured at six months after recruitment. Secondary outcomes include cost-effectiveness, with final follow-up at twelve months.
FLOWCHART

BACKGROUND
A cluster randomised controlled trial of a structured training programme for caregivers of in-patients after stroke
Stroke remains a major health problem in the 21st Century with incidence rates of 1.65 per 1000 population for first ever strokes [1]. Robust evidence supports the recommendation that patients with moderate or severe symptoms should be referred to hospital with the expectation of admission to a stroke unit [2]. Stroke is a family illness generating considerable personal, financial and societal burdens. After a recommended initial hospital admission [2], up to 80% of patients are discharged home and many will be dependent on informal caregivers, usually family members, to provide assistance with activities of daily living, including bathing, dressing, and toileting [3]. For some, this avoids or delays admission to institutional care and the economic value of the informal care provided is considerable [4]. This burden of care, however, has an important effect on caregivers' physical and psychosocial well-being [5] with up to 48% of caregivers reporting health problems, two-thirds a decline in social life [6] and high self-reported levels of strain. These issues are compounded by families being over-protective and a perception of lack of respite with reluctance to leave the patient alone [3]. With the current emphasis on shorter hospital stays caregivers will play an increasingly important role in the care and continued rehabilitation of patients after stroke. The successful adjustment of patients and their caregivers to the aftermath of stroke is clearly interlinked. Caregivers have an important role in enhancing patients' rehabilitation, and coping strategies that lead to negative experiences are associated with increasing dependence [7]. The caregivers of patients with poor physical and emotional states are likely to have poor emotional outcomes themselves [8]. Effective training of caregivers therefore should not only improve their own health but also the recovery and adjustment of the stroke patient [9]. However, despite the physical, psychological and social consequences of caregiving, its economic benefit to society and its importance in patient recovery, caregivers' central role is often given low priority in the management of stroke [10] and there are missed opportunities for structured skills training [11]. Caregivers identify information and skills training required to implement physical care as the most important pre-discharge needs [12]. Caregiver support is a key component of stroke unit care yet, as currently provided, is not compatible with their expressed needs and their ability to care is not assessed [9, 12].
These deficiencies have recently been addressed in a single centre, randomised controlled trial (RCT) by Kalra et al. [13]. They reported the effectiveness of a purposely designed systematic and structured training programme for caregivers which included assessment in competencies in skills essential for the day-to-day management of disabled stroke survivors (The London Stroke Carer Training Course, LSCTC). The LSCTC was effective in decreasing caregiver burden and in decreasing their anxiety and depression, improving psychological outcomes for patients and reducing overall costs [14]. However, there are important limitations to the generalisability of the trial findings as the LSCTC was tested in a single centre, delivered by a separate specialist team that might be expected to have heightened motivation and expertise, and the patient population was predominantly recruited from a middle class suburban area who might be more responsive to a training and education programme. In addition, having demonstrated benefit for caregivers on a range of domains, it is now important to evaluate the effectiveness of the LSCTC programme on improving patient outcomes. We therefore seek to embed the LSCTC in usual practice and thereby test wider generalisability in settings where the population, health and social care provision are different.
AIMS AND OBJECTIVES
The aim of the TRACS trial is to evaluate the clinical and cost-effectiveness of a structured competency based caregiver-training programme (LSCTC) on improved patient outcomes in patients with a confirmed primary diagnosis of new stroke by comparison to usual practice according to the National Guidelines for Stroke.
Primary Patient Objective:
The primary patient objective of the trial will be to determine whether a structured, competency-based training programme (LSCTC) for caregivers improves physical outcomes for patients after disabling stroke.
Primary Caregiver Objective:
The primary caregiver objective of the trial will be to determine whether a structured, competency-based training programme (LSCTC) for caregivers reduces burden for caregivers of patients after disabling stroke.
Secondary Objectives:
The secondary objectives are:
-
To determine whether the provision of the LSCTC for caregivers improves physical and psychological outcomes for patients after disabling stroke;
-
To determine whether the provision of the LSCTC for caregivers improves physical and psychological outcomes for caregivers of patients after disabling stroke;
-
To assess whether such a training programme is cost-effective based on (a) patient outcomes, from both health/social care and societal perspectives and (b) caregiver outcomes, from a health care perspective.
DESIGN
TRACS has been designed as a pragmatic, multicentre, cluster randomised, controlled trial with blinded follow-up. 950–1000 stroke patients with residual disability and their caregivers will be recruited, where the patient is likely to return home with the support of the caregiver. The unit of randomisation will be the participating stroke rehabilitation units, 18 will deliver the London Stroke Carer Training Course (LSCTC) to all caregivers of in-patients and 18 will continue to deliver usual care as per the National Guidelines for Stroke. Blinded follow-up will be through postal questionnaire at six and twelve months after recruitment.
A cluster randomised trial design has been purposely selected to reduce between-group treatment contamination. Although patient-level randomisation was used in the earlier single centre study [13], the LSCTC was delivered by a small, purposefully trained team (nurse and therapists) and intensive monitoring was required to reduce treatment contamination. However, in routine stroke unit care, the LSCTC intervention will not be delivered by a discrete team but will be incorporated into usual practice by the whole multidisciplinary team (MDT). Patient level randomisation has the consequence therefore that the MDT will need to operate two approaches (usual care and the LSCTC). The risk of contamination will be high as it will not be possible to blind members of the MDT and the new care process is likely to be extended to patients in the usual care group. Monitoring to reduce opportunities for treatment contamination, as undertaken in the single centre study, would be considerably more difficult in the context of a multi centre trial. Randomisation will therefore be at the level of the stroke unit. In order to minimise selection bias, there will be a clear separation between the provision of the intervention by clinical staff and the recruitment and consent of patients and caregivers by the research practitioners.
CENTRE ELIGIBLITY, RANDOMISATION AND PROCESS EVALUATION
CENTRE ELIGIBILITY
Stroke rehabilitation units will be defined according to the definition provided by the Royal College of Physicians of London for the National Sentinel Stroke Audit 2006 [15].
The 5 key criteria are:
-
Consultant physician with responsibility for stroke
-
Formal links with patient and caregiver organisations
-
Multidisciplinary meetings at least weekly to plan patient care
-
Provision of information to patients about stroke
-
Continuing education programmes for staff.
A stroke unit will be defined by the presence of 4/5 of these criteria. Additional criteria will be that a substantial number of patients on the unit will have a diagnosis of stroke, that the unit will be able to deliver the LSCTC and that the majority of patients are discharged to a permanent place of residence.
CENTRE RANDOMISATION
Eligible centres that agree to participate will be registered and randomised to the trial. The Principal Investigator for each centre will provide the following information prior to randomisation:
-
Name of Stroke unit
-
Stroke unit centre code
-
Stroke unit address and telephone number
-
Geographical region
-
Name of Consultant physician with responsibility for stroke
-
Name of main contact
-
Confirmation of willingness to participate
-
Score on the key 12 indicator score of the 2006 National Stroke Audit (NSSA) [15]
-
Average monthly admission figures for past 12 months
-
Number of beds
-
Confirmation that a substantial number of patients on the unit will have a diagnosis of stroke
-
Confirmation of eligibility by confirming at least 4/5 of the following centre eligibility criteria:
-
Consultant physician with responsibility for stroke
-
Formal links with patient and caregiver organisations
-
Multidisciplinary meetings at least weekly to plan patient care
-
Provision of information to patients about stroke
-
Continuing education programmes for staff.
-
Cluster randomisation will be performed centrally at the Clinical Trials Research Unit (CTRU). The eligible stroke rehabilitation units will be randomised on a 1:1 basis to either the intervention group or the control group. The randomisation will be stratified by the following stroke unit co-variates: geographical region and quality of care (as defined by on and above or below the median score on the key 12 indicator score of the 2006 National Stroke Audit (NSSA) [15]). Block randomisation will ensure these important covariates are balanced between the arms of the trial.
Staff Training in Centres Randomised to the Intervention (LSCTC)
Training on the implementation and delivery of the LSCTC will be provided at each of the 18 stroke rehabilitation units randomised to the intervention prior to commencement of patient recruitment. The training will be provided by an LSCTC training team who were part of the LSCTC implementation team in the initial single centre study [13]. The LSCTC training team will not be involved in the research procedures. An initial meeting will discuss the structured competencies based training and practical implementation aspects, re-enforced by illustrative cases and involving sessions of role play. Each unit will be provided with the competencies assessment tool presented in a ‘training manual’ and required to develop an initial case load of patients and caregivers. These practical experiences will be used for discussion at a second centrally based training session which staff from the 18 stroke rehabilitation units will attend four weeks after the first. Subsequent to this, the staff will gradually increase the delivery of the LSCTC until it becomes an integral part of the ward care process. Members of the trial team will visit each site and use the completed caregivers' competencies assessment tool as a basis for discussions on structure and process. A further training session will be arranged if necessary to provide feedback and support, and discuss any problems with LSCTC provision. The LSCTC training team will be available, by telephone, to provide clinical support throughout the intervention period. Stroke units will open to recruitment three to six months after the initial training meeting when the research practitioners are happy that the LSCTC has been implemented correctly.
PROCESS EVALUATION
Process data will be used to inform the health economic quantification and describe the care process that prepares patients and caregivers for discharge in the participating centres (stroke rehabilitation units). Data will be collected at the following time points:
Pre-recruitment
A process questionnaire will be completed by the Research Manager, Principal Investigator and the stroke rehabilitation unit MDT.
Observations of current practice.
Process case report forms (CRFs) to record staff time input into caregiver support will be completed by the MDT.
During the Trial
A process questionnaire will be completed by the trial researchers and the stroke rehabilitation unit MDT.
Observations of current practice will be undertaken by a researcher independent of trial procedures*.
Process case report forms (CRFs) to record staff time input into caregiver support will be completed by the MDT.
Qualitative interviews will be conducted with a purposeful sample of stroke patients and caregivers up to three months post discharge from a participating stroke rehabilitation unit*.
Additional questions regarding caregiver support will be added to the six month follow–up caregiver postal questionnaire pack (see section 0).
When Recruitment has ended
Qualitative interviews will be conducted with a purposeful sample of MDT members to capture their experience of delivering the intervention*.
*Please note that the activities denoted (*) above will be incorporated into a process evaluation sub-study and separate ethical approval will be sought.
PATIENT AND CAREGIVER ELIGIBILITY
INCLUSION CRITERIA:
All patients with the following characteristics are eligible for this trial:
-
Have a confirmed primary diagnosis of new stroke.
-
Are medically stable.
-
Are likely to return home with residual disability at the time of discharge.
-
Have a caregiver available, defined as the main person, other than health, social, or voluntary care provider, helping with activities of daily living and/or advocating on behalf of the patient, is willing and able to provide support to the patient after discharge.
-
Written informed patient consent/a caregiver declaration and caregiver consent will be obtained prior to any trial specific procedures.
Exclusion Criteria:
Unless the patients exhibit the following characteristics:
-
In need of palliative care.
-
If discharge is planned within one week of admission to the current stroke unit.
-
If the patient or caregiver was registered to the trial on a previous admission.
-
Patients involved in other stroke research network adopted studies will also be recruited into this study unless: 1) the patient is recruited into the ActNoW study (which assesses intensive speech therapy versus no speech therapy); 2) the patient is first recruited into another trial involving 6 and 12 months follow up questionnaires (currently only the STICH study).
PATIENT AND CAREGIVER RECRUITMENT AND REGISTRATION
SCREENING
The clinical research team will complete a log of all patients and caregivers screened for eligibility including those who are not registered either because they are ineligible or because they decline participation. Anonymised information will be collected including:
-
the reason not eligible for trial participation or
-
eligible but declined
-
eligible and consented
-
age
-
gender
-
ethnicity
-
relationship of the patient to the caregiver
-
living circumstances (live alone , co-habit or in residential/nursing home)
-
living circumstances (caregiver co-resident or non-resident)
-
length of hospital stay.
RECRUITMENT PROCESS
Recruitment to the trial and baseline assessment will be undertaken by a member of the clinical research team, (independent of the clinical team) who will visit the stroke rehabilitation units at least once a week to liaise with the clinical team, assess patient and caregiver suitability and obtain informed consent from both patients and caregivers to undertake baseline and follow-up assessments. Rates of identification, recruitment and refusals will be monitored for all sites (see Section 8.1) Identification rates will be monitored against past admission rates for each site.
INFORMED CONSENT
A verbal explanation of the trial and Patient and Caregiver Information Sheets will be provided by the clinical research team for the patient and caregiver to consider. These will include detailed information about the rationale, design and personal implications of the study. Following information provision, patients and caregivers will be given sufficient time to consider participation and will be given the opportunity to discuss the trial with their family and healthcare professionals before they are asked whether they would be willing to take part in the trial. Information about the trial will be repeated again if the patients and caregivers require time to consider their participation and the participants (patient and caregiver) will again have the opportunity to ask questions and confer with other members of their family. Consent will then be taken. The right of the patient and caregiver to refuse consent without giving reasons will be respected.
Assenting patients and caregivers will then be invited to provide informed, written consent to complete baseline and follow up assessments. For patients unable to read/sign the consent form due to stroke related disabilities, and for those with problems of comprehension, a caregiver declaration will be sought. For patients unable to consent for themselves, this study complies with the Mental Capacity Act (MCA) 2005. In such cases, the caregiver will act as consultee. The caregiver will be advised to set aside their own views and provide advice on the participation of the patient in the research, taking into consideration the patient's wishes and interests. Research participants will not be required to do anything which is contrary to any advance decisions or statements that have been made by them in relation to their treatment or any other matter. Advance decisions made by the patient about their preferences and wishes will always take precedence.
The caregiver will also be approached to provide consent on their own behalf. Formal assessment of eligibility and informed consent will be undertaken by a member of the clinical research team. The patient and caregiver will remain free to withdraw from the study at any time without giving reasons and without prejudicing any further treatment. The original consent forms will be retained in the investigator site file. A copy of the patient and caregiver consent forms will be given to the patient and caregiver respectively. Further copies will be filed in the patient hospital notes and a fourth set of copies will form part of the central study archive and be returned to the CTRU.
Informed written patient consent/caregiver declaration and caregiver consent for entry into the trial will be obtained prior to patient and caregiver registration. The responsibility for the overall care of the patient remains with the attending clinical teams.
REGISTRATION
Patients and caregivers will be registered with the CTRU following informed consent, confirmation of eligibility and collection of baseline data. Registration will be performed centrally using the CTRU automated 24-hour telephone registration system. Authorisation codes and PINs, provided by the CTRU, will be required to access the registration system.
When eligibility has been confirmed and the necessary details obtained (see Section 0) patients and caregivers will be allocated trial numbers.
DIRECT LINE FOR 24-HOUR REGISTRATION: +44 (0)113 343 4928
TREATMENT
All patients in this study will be treated within an organised stroke service (stroke unit). The clinical research team will be available to all participating stroke units (control and intervention groups) to provide support regarding the research procedures.
USUAL CARE (CONTROL GROUP)
In stroke rehabilitation units randomised to the control group, usual care for caregivers following national guidelines [2] will include:
-
provision of information on stroke and its consequences, prevention, and management options
-
involvement in goal setting for rehabilitation and discharge planning
-
encouragement to attend nursing and therapy activities to learn about patients' abilities and informal instruction on facilitating transfers, mobility and activities of daily living tasks
-
advice on community services, benefits and allowances, including contact information provided for voluntary support services for caregivers.
CAREGIVER TRAINING PROGRAMME (INTERVENTION GROUP)
In stroke rehabilitation units randomised to the intervention group usual care will be augmented by provision of the LSCTC programme incorporated into ward practice (see Section 0). Caregivers will receive sessions of the structured training programme depending on need [12, 13]. Caregivers' competencies will be assessed (and signed off) at the end of training. In addition, one “follow through” session will be provided either through a telephone call or home visit by an appropriate member of the clinical team (member of MDT, community stroke team or other professional who normally completes such a follow up on discharge) to adapt skills learnt to the home environment.
As part of the LSCTC programme, caregivers will receive:
Instruction by appropriate professionals on common stroke related problems and their prevention, management of pressure areas and prevention of bed sores, continence, nutrition, positioning, gait facilitation, and advice on benefits and local services.
“Hands-on” training in lifting and handling techniques, facilitation of mobility and transfers, continence, assistance with personal activities of daily living and communication, tailored to the needs of individual patients.
WITHDRAWAL
In line with usual clinical care, cessation or alteration of regimes at any time will be at the discretion of attending clinical teams, clinicians or the patients and caregivers themselves. Where caregivers or patients wish to withdraw, there will be clarification of whether this is withdrawal from postal or medical records follow up or both.
DATA COLLECTION/ASSESSMENTS
Participating stroke rehabilitation units will be expected to maintain a file of essential trial documentation (Investigator Site File), which will be provided by CTRU, and keep copies of all completed CRFs for the trial. Stroke rehabilitation unit usual practice will be determined by the process evaluation (see section 0).
Patient and caregiver assessments will be undertaken as follows:
-
Registration and Baseline (after consent but prior to registration)
-
Discharge/Home visit
-
6-month follow-up
-
12-month follow-up.
REGISTRATION AND BASELINE DATA
Patients and caregivers who meet the inclusion criteria and provide informed written consent (for baseline assessment and follow-up) will be registered to the trial. The TRACS research practitioners will provide details at registration, including:
-
Patient details including initials and date of birth
-
Caregiver details including initials and date of birth
-
Centre code
-
Name of the research practitioner conducting the registration
-
Confirmation of eligibility
-
Confirmation of written informed consent
The TRACS clinical research team will also record additional baseline information including:
Centre details:
-
Hospital name
-
Centre code
-
Name of the research practitioner conducting the registration.
Patient details:
-
Name
-
Gender
-
Date of birth (age to be calculated*)
-
Ethnicity
-
NHS ID
-
Hospital ID
-
Confirmation of eligibility
-
Modified Rankin score [16]
-
Living circumstances*
-
Relationship of the patient to the caregiver
-
Address and telephone number
-
GP address and telephone number
-
Preferred language
-
Education and employment
-
Name of attending physician
-
Date of patient admission
-
Date of stroke
-
Classification of stroke type
-
Language ability
-
Short Orientation-Memory-Concentration Test (6CIT) [17]
-
Pre-stroke independence*
-
Verbal subsection of the Glasgow Coma Scale [17, 18]
-
Ability to lift both arms off the bed*
-
Ability to walk independently*
-
Barthel Index [19] (pre-stroke activities of daily living)
-
Previous stroke
Caregiver details:
-
Name
-
Gender
-
Date of birth
-
Ethnicity
-
Confirmation of eligibility
-
Modified Rankin score [16]
-
Address and telephone number
-
GP address and telephone number
-
Preferred language
-
Education and employment
The patients will complete the following questionnaires at baseline; details on proxy completion will also be collected:
-
The Nottingham Extended Activities of Daily Living Scale (NEADL) [20, 21] for pre-stroke independence
-
Hospital Anxiety and Depression Scale (HADS) [22] (post stroke mood)
-
EQ-5D [23–25] (post stroke health state)
-
Barthel Index [19, 26] (post-stroke activities of daily living)
-
Stroke Impact Scale [27–30] (post stroke functional ability and health related quality of life)
-
Patient Client Service Receipt Inventory (CSRI) [14, 31] (pre-stroke economic outcome)
The caregivers will complete the following questionnaires at baseline:
-
Frenchay activities index [32, 33] (social restriction)
-
HADS [22] (mood)
-
EQ-5D [23–25] (post-stroke health state)
-
Caregiver Client Service Receipt Inventory (CSRI; pre-stroke economic outcome)
The six factors (highlighted by *) from the Edinburgh stroke case mix adjuster [34, 35] will enable an adjustment for case mix to be made.
Patients and caregivers will be provided with change of address cards to be returned to the CTRU by post if required.
DISCHARGE/HOME VISIT DATA
The TRACS clinical research team will record the following information for patients at discharge:
-
Date of discharge.
-
Destination at discharge.
-
Pre-discharge home visit details.
-
Details of Expected Serious Adverse Events (see Section 0) between the date of consent and date of discharge
-
Documented evidence regarding the patient's care process.
ADDITIONAL DATA COLLECTED FOR THE INTERVENTION GROUP
During the LSCTC intervention period (between the date of consent and date of the home visit) members of the stroke rehabilitation unit MDT will record their inputs into delivering the LSCTC (for the purposes of the economic evaluation) and caregiver compliance with the LSCTC including:
-
number of training sessions
-
time taken
-
competencies sign off
FOLLOW-UP DATA
Patients and caregivers will be followed-up by the CTRU via postal questionnaires at six and twelve months. This will be supported by postal and telephone reminders if questionnaires are not returned within two weeks. If necessary, a ‘masking’ system will be used so that research administration staff can undertake telephone reminders blind to the patients' and caregivers' regional telephone code. All losses to follow-up, through death, withdrawal and loss of contact will be fully reported by the clinical research team. Completion rates will be monitored as agreed by the DMEC, TSC and TMG in the monitoring schedule. If completion falls below an acceptable standard agreed by the DMEC, TSC and TMG, patients and caregivers may be contacted by telephone to complete the primary outcome measures (NEADL and caregiver burden scale).
All patients and caregivers who are registered into the study will be considered as part of the intention to treat population and efforts will be made to follow them up when appropriate.
The CTRU will check with the GP whether the patient and caregiver are alive prior to contact. If the patient has died, no further follow-up will be undertaken. If the caregiver has died, patient follow-up will still be undertaken. In both cases their death will be recorded on the appropriate form and the cause will be established through contact with the GP and reference to electronic or paper health records. When patient and caregiver survival status has been established postal questionnaires will be sent out by the CTRU.
The following questionnaires will be included in the postal packs for patients:
-
NEADL [20, 21]
-
HADS [22] (mood)
-
EQ-5D [23–25] (health state)
-
Barthel Index [19, 26] (activities of daily living)
-
Stroke Impact Scale [27–30] (functional ability and health related quality of life)
-
Patient CSRI [14, 31] (economic outcome).
-
If the patient was aware of receiving / being denied better treatment because of this research (at 12 month follow-up only).
The following questionnaires will be included in the postal packs for caregivers:
-
Caregivers Burden Scale [36]
-
Frenchay activities index (social restriction) [32–33]
-
HADS [22] (mood)
-
EQ-5D [23–25] (health state)
-
Caregiver CSRI (economic outcome)
-
Additional questions relating to:
-
Caregiver support during the intervention period (at six month follow-up only)
-
If the caregiver is still caring for the patient
-
If the caregiver was aware of receiving / being denied an enhanced training package (at 12 month follow-up only)
-
Caregiver's stroke knowledge [37, 38].
-
The TRACS team will record the following information for patients and if necessary caregivers, at six and twelve months post registration using electronic or paper health and patient social care records:
-
Death
-
Hospital re-admissions
-
Institutionalisation
-
Treatment on an emergency outpatient basis.
ASSESSMENT INSTRUMENTS
The assessment instruments have been incorporated into patient and caregiver assessment packs. All instruments have been used extensively in previous stroke research, are sensitive to change, valid and reliable. They have been reviewed by the Consumer Research Advisory Group attached to the Academic Unit of Elderly Care and Rehabilitation who felt that the questions were understandable, relevant and appropriate. Information on who completed the outcome measures will be requested, proxy responses will be allowed. Where the patient is unable to complete the questionnaire due to stroke related disabilities (visual/motor) a friend/relative/carer can complete the questionnaire using the patient's verbal responses. Where the patient is unable to communicate answers and/or understand the questions, a proxy can complete the questionnaire entirely on the patient's behalf. The number of proxy responses will be compared between the two groups.
Nottingham Extended Activities Of Daily Living Scale (NEADL)
Physical and social independence will be measured using the Nottingham Extended ADL Scale (NEADL) [20, 21]. It was designed as a postal questionnaire and assesses aspects of physical and social independence performance across 22 items (score range 0–66) grouped in four categories (mobility, kitchen, domestic and leisure activities). It has been widely used as an outcome measure in rehabilitation trials [39, 40]. It has proven validity, reliability [41] and has demonstrated responsiveness to change and able to discriminate between services [42].
Hospital Anxiety And Depression Scale (HADS)
Both patients and caregivers mood will be assessed using the 14 component Hospital Anxiety and Depression Scale (HADS) [22]. It was initially developed as an instrument to identify anxiety disorders and depression in medical outpatients [22], but has since proven to exhibit wider generalisability [43].
EQ-5D
The non-disease-specific EQ-5D instrument [23–25] will be used to evaluate health-related quality of life for both patients and caregivers via a six component questionnaire. It was developed to yield a fundamental index of health, which can be used to calculate quality-adjusted life-year (QALY) gains, and thus will facilitate the health economic evaluation.
Barthel Index
Patient activities of daily living and mobility will be assessed using the Barthel Index [19, 26]. This instrument will be used to evaluate the patient's disability and level of dependence on their caregiver via assessment of their ability in bathing, transferring from bed to chair, dressing, feeding, mobility, climbing stairs, toilet use, grooming, and bladder and bowl continence.
Stroke Impact Scale
Functional ability and health related quality of life of the patients will be measured using the Stroke Impact Scale [27–30]. This scale consists of eight components measuring strength, memory and thinking, emotion, communication, activities and independent activities of daily living, mobility, hand function, and social participation. It was developed for use as a self reporting questionnaire, which has proven to be reliable, valid and sensitive to change [27, 44]. SIS has also been validated for use as a postal questionnaire [45].
Client Service Receipt Inventory (CSRI)
Data on patient socio-demographics and use of health and other formal care services and informal care will be collected using a Client Service Receipt Inventory validated for use with stroke patients [14, 31]. A reduced form of this instrument will be used with caregivers.
Caregiver Burden Scale
Caregiver burden will be measured using a proven and reliable Caregiver Burden Scale [36]. This 22-item scale will assess various aspects of caregiver burden including general strain, isolation, disappointment, emotional involvement and environment.
Frenchay Activities Index
The social restriction on caregivers will be assessed using the Frenchay Activities Index [32, 33]. Although initially validated to assess the activities of acute stoke patients, this assessment instrument is applicable to caregivers of patients with disabling stroke [13].
Definition of End of Trial
The end of the trial is defined as the date the last twelve month postal questionnaire pack is completed.
SERIOUS ADVERSE EVENTS PROCEDURES
GENERAL DEFINITIONS
An adverse event (AE) is:
-
any unintentional, unfavourable clinical sign or symptom
-
any new illness or disease or the deterioration of existing disease or illness
-
any clinically relevant deterioration in any laboratory assessments or clinical tests.
A serious adverse event (SAE) is defined in general as an untoward event which:
-
is fatal or life threatening
-
requires or prolongs hospitalisation
-
is significantly or permanently disabling or incapacitating
-
constitutes a congenital anomaly or a birth defect or
-
may jeopardise the participant and may require medical or surgical intervention to prevent one of the outcomes listed above.
A SAE occurring to a research participant, where in the opinion of the chief investigator the event is related and unexpected will be reported to the main Research Ethics Committee (REC).
The National Research Ethics Service (NRES) defines related and unexpected SAEs as follows:
-
‘related’ – that is, it resulted from administration of any research procedures; and
-
‘unexpected’ – that is, the type of event is not listed in the protocol as an expected occurrence.
TRACS OPERATIONAL DEFINITIONS
Events such as patient falls and caregiver musculoskeletal injury represent an inherent consequence of an active rehabilitation process and therefore cannot be entirely avoided. Similarly, in this patient population, acute illness resulting in hospitalisation, new medical problems and deterioration of existing medical problems are expected.
Expected AE/SAEs – not reportable
In recognition of this, events fulfilling the definition of an adverse event (except those listed in Section 0) will not be reported in this study.
In addition, Serious Adverse Events will not be reported as follows:
-
Episode of acute illness (e.g. further stroke, cardiovascular event, infection) occurring between the time of consent and date of discharge.
Expected AEs/SAEs – standard reporting
The following Serious Adverse Events are not common but are expected within the patient study population during patient hospitalisation and will be reported by the clinical research team between the date of consent and date of discharge using a standardised discharge CRF.
-
Patient falls with or without fracture will be reported when they occur at any time between the date of consent and date of discharge.
The following AEs and SAEs are expected within the patient study population following discharge from hospital and will be established during follow-up and self-reported by patients within the Client Service Receipt Inventory. The TRACS clinical research team will also collect this data using electronic or paper health and social care records:
-
Death (SAE)
-
Hospital admissions and re-admissions for any reason (SAE)
-
Institutionalisation (AE)
-
Treatment on an emergency outpatient basis (AE).
In addition, caregiver hospitalisation, institutionalisation and death are expected and will be subject to standard reporting using the caregiver postal questionnaire pack at follow-up. If necessary The TRACS clinical research team will also collect this data using electronic or paper health and social care records
As these events are expected within the study population they will not be subject to expedited reporting to the main REC. They will however, be included in the annual safety report provided to the main REC.
Unexpected and related SAEs – expedited reporting
All Related/Unexpected SAEs occurring to either the patient or caregiver from the date of consent up to twelve months post registration must be recorded on the Related/Unexpected Serious Adverse Event Form and faxed to the CTRU within 24 hours of the clinical research staff becoming aware of the event. The original form should also be posted to the CTRU in real time and a copy retained on site.
For each Related/Unexpected SAE the following information will be collected:
-
date of SAE
-
full details in medical terms with a diagnosis, if possible
-
its duration (start and end dates; times, if applicable)
-
action taken
-
outcome.
Any follow-up information should be faxed to CTRU as soon as it is available. Events will be followed up until the event has resolved or a final outcome has been reached.
CTRU Fax number for reporting Related/Unexpected Serious Adverse Events: 0113 343 1471
All Related/Unexpected SAEs will be reviewed by the Chief Investigator and subject to expedited reporting to the Sponsor and the main REC by the CTRU on behalf of the Chief Investigator within 15 days.
Responsibilities of the Chief Investigator, CTRU, Trial Steering Committee (TSC), Data Monitoring and Ethics Committee (DMEC) and Sponsor will be detailed in a study specific Work Instruction.
HEALTH ECONOMICS
Given that health care resources are finite it is important to demonstrate the economic implications of any new intervention. The single centre study found that LSCTC reduced costs, largely as a result of reduced length of stay. It is unclear whether this finding is generalisable to other settings due to variations across stroke rehabilitation units in, for example, discharge policies, availability of beds, staff mix and patient case-mix. Therefore, a comprehensive economic evaluation will assess the cost-effectiveness of the LSCTC in this multi-centre trial, based on both patient and caregiver outcomes. It will be carried out from a health care perspective for the caregiver outcome evaluation, and both a health/social care perspective (health care resources and other formal care agencies) and a societal perspective (health care resources, other formal care agencies and informal care inputs) for the patient outcome evaluation and will follow the familiar stages of an economic evaluation [46]. Briefly, this involves measuring the resources associated with each treatment approach from the chosen analysis perspective(s), estimating the total costs of those resources and then, importantly, linking costs with outcomes. The economic evaluation will be fully integrated into the effectiveness evaluation, with the same criteria adopted for trial eligibility, randomisation and intervention modes.
Data will be collected retrospectively at baseline (for the previous three months) and then at 6 and 12 months (for the time since previous assessment) which will allow estimation of total costs over 1 year (and therefore a comparison with the cost findings from the single centre study). Data on use of health and other formal care resources and informal care will be collected using a Client Service Receipt Inventory (CSRI) validated for use with stroke patients [14, 31]. A reduced version of this instrument, containing questions about use of core health care services, will be used with caregivers. In both cases, it will be completed as a self-complete questionnaire alongside other measures at baseline, 6 months and 12 months. Self-reports of patient and carer inpatient admissions will be verified against hospital records.
Unit costs will be attached to each service or element of support in turn, using the best available estimates of long-run marginal opportunity cost which will include capital and overhead elements. National unit costs will be used where possible to facilitate generalisability of results, with new local estimations calculated where necessary. Informal care costs will be estimated using the opportunity cost method (the value of the opportunities forgone by caregivers as a result of time spent on caregiving). The cost of LSCTC inputs (including staff training) will be incorporated into the evaluation. Average unit costs per session of LSCTC will be calculated and multiplied by the number of sessions received by each caregiver. If necessary, adjustments will be made to unit costs of the stroke rehabilitation units in order to avoid double-counting staff inputs. Unit costs will be combined with resource volumes to obtain a total cost per patient, from each analysis perspective, at each assessment point, for both patient and caregiver evaluations and over the entire period of participation in the trial.
All costs will be reported as mean values with standard deviations. To accommodate a cluster randomisation design, differences in costs between groups will be tested by multi level modelling.
The primary economic analysis will take the form of a cost-effectiveness analysis. This will involve combining and comparing total average costs from each analysis perspective with the primary outcome measures (patient NEADL and Caregiver Burden Scale) in the form of incremental cost-effectiveness ratios (ICERs) to represent additional cost per additional point on the NEADL for the patient evaluation and the Caregiver Burden Scale for the caregiver evaluation.
A secondary economic analysis will examine quality of life outcomes through cost-utility analyses, again from each analysis perspective, exploring cost per quality-adjusted life-year (QALY) gained for both patients and caregivers. Health states will be measured at each assessment point (baseline, 6 months and 12 months) using the EQ-5D. Utility weights from a United Kingdom general population survey [47] will be applied to these health states to calculate quality-adjusted life-years. Quality-adjusted life-year outcomes will be examined in terms of change between post-stroke and each follow-up point, using linear interpolation to calculate the area under the QALY curve. Given the wide range of other outcome domains of interest in the study, a supplementary cost-consequence analysis will additionally present total average costs for each trial arm alongside all outcome measures.
Sensitivity analyses will alter any key assumptions made in the economic analyses (e.g. unit costs of stroke rehabilitation units, LSCTC and informal care) to explore the consequences for the results. Uncertainty around the cost-effectiveness and cost-utility of LSCTC will also be explored using incremental cost-effectiveness planes and cost-effectiveness acceptability curves (CEACs) based on the net benefit approach [48]. The CEAC reveals to the decision-maker the likelihood of LSCTC being cost-effective relative to usual care given different (implicit monetary) values placed on incremental improvements in the NEADL, Caregiver Burden Scale and QALYs. CEACs will be based on bootstrapped (to account for non-normally distributed data) regressions of study group upon net benefits, controlling for clusters.
ENDPOINTS
In common with other stroke rehabilitation trials [39, 40] the primary outcome point is at six months with final follow-up at 12 months to assess whether any intervention effect is sustained.
PRIMARY ENDPOINTS
Patient endpoint
-
Nottingham Extended Activities of Daily Living (NEADL) [20, 21] as completed by the patient at six months.
Caregiver endpoint
-
Caregivers Burden Scale [36] as completed by the caregiver at six months.
SECONDARY ENDPOINTS
Patient endpoints at six months
-
Hospital Anxiety and Depression Scale (HADS) (mood) [22]
-
EQ-5D (health state) [23–25]
-
Barthel Index (activities of daily living) [19, 26]
-
Stroke Impact Scale [27–30] (functional ability and health related quality of life)
-
Death
-
Hospital re-admission
-
Institutionalisation
-
Total costs
Patient endpoints at twelve months
-
Nottingham Extended Activities of Daily Living (NEADL)
-
Hospital Anxiety and Depression Scale (HADS)
-
EQ-5D
-
Barthel Index
-
Stroke Impact Scale
-
Death
-
Institutionalisation
-
Hospital re-admission
-
Total costs
Caregiver endpoints at six months
-
Frenchay activities index [32, 33] (social restriction)
-
HADS
-
EQ-5D
-
Death
-
Hospitalisation
-
Institutionalisation
-
Total costs
Caregiver endpoints at twelve months
-
Caregivers Burden Scale
-
Frenchay activities index (social restriction)
-
HADS
-
EQ-5D
-
Death
-
Hospitalisation
-
Institutionalisation
-
Total costs
STATISTICAL CONSIDERATIONS
SAMPLE SIZE
Thirty-six stroke rehabilitation units each recruiting 25 patients, will result in 450 patients in each group and provide close to 90% power at 5% significance level to detect a clinically relevant difference of six points (as defined in the TOTAL study [39, 49]) on the primary patient outcome, the NEADL (scored 0–66, SD 18). A range of 3–9 points has been taken to be a clinically relevant difference in previous studies. We have taken 6 points as a difference of clinical relevance to the patient and caregiver (patient requiring less help in at least two activities) and also substantive enough to influence commissioners to change service delivery. The sample size takes account of an inflation factor of 1.9 due to clustering (cluster size of 19 after loss to follow-up; Intracluster Correlation Coefficient (ICC) no greater than 0.05 [50]) and 25% loss to follow-up. The assumption that the ICC will be no larger than 0.05 is based on methodological research [51] showing that ICCs for patient outcomes in the community are generally less than 0.05. This sample size of 900 patients will provide more than 85% power at the 5% significance level to detect an effect size of one third in any of the other outcomes. Such an effect size is usually considered moderate. So, for instance, this will ensure more than 85% power to detect a difference of 4.3 points on the Caregiver Burden Scale at six months, assuming the same variability as in the single centre study [13] (i.e. ds of 12.9 at 6 months). Although the differences observed in the single centre study were larger (6.2, 6.7, 8.7 at 3, 6 and 12 months respectively), it is unrealistic to predict that a multi-centre trial would find such large effects. In addition with a sample of 900 the trial will have more than 85% power to detect the annual admission cost difference of £3175 as found in the single centre study [14].
The power of the trial is adversely affected by a higher than expected loss to follow-up (likely to be closer to 30%) and unequal cluster sizes (extent of the imbalance unknown until end of recruitment). Hence, to preserve final power of close to 90%, the trial should aim to recruit between 950 and 1000 patients.
ACCRUAL
Based on a recently completed study [52] involving six stroke units and an ongoing pilot, it is anticipated that 40% of patients/caregivers on a stroke unit will fulfil the entry criteria. Based on recruitment rates in previous studies [53, 54] and the ongoing pilot study, it is conservatively estimated that at least 50% of these will provide informed consent. The average number of patients admitted annually per unit is at least 120, this provides a pool of 4,320 to recruit from and therefore it is estimated that a recruitment rate of 75 patients per month, just over 2 per month for each participating site is more than achievable.
Statistical Analysis
Statistical analysis is the responsibility of the CTRU Statistician. A final statistical analysis plan will be written and reviewed before any final analysis is undertaken. All statistical testing will be performed at a 2-sided 5% significance level.
ANALYSIS POPULATIONS
The Intention to Treat (ITT) population is defined as all patients registered for active follow-up regardless of non-compliance with the intervention. All patients (and the corresponding caregivers) within a stroke unit will be analysed according to the intervention that stroke unit was randomised to. All analyses and data summaries will be carried out using the ITT population.
A per-protocol analysis will be considered if there are a considerable number of protocol violators. This decision will be made jointly by the trial statistician in co-operation with other members of the Trial Management Group on examination of the population. The decision will be made without reference to the endpoint data.
OUTLINE ANALYSIS PLAN
As the trial is cluster randomised, the primary outcome measures, the six month NEADL score and the Caregiver Burden Scale, will each be compared between the intervention and control groups using a two-level hierarchical model, with patients (or caregivers) nested within stroke rehabilitation units. Patient-level covariates, such as patient baseline scores, the Edinburgh stroke case-mix adjuster [35], and the caregiver baseline HADS score, and stroke unit-level covariates, such as the key 12 indicator score will be included in the analysis. Secondary outcomes for patients and caregivers will be analysed using similar multi-level models. Effect sizes and 95% confidence intervals will be reported.
Three of the secondary caregiver outcomes (death, hospitalisation and institutionalisation) will be summarised by treatment group. No formal statistical comparison between the intervention and control groups will be undertaken for these outcomes.
A secondary analysis will explore the relationship between outcome and compliance with the LSCTC (e.g. number of sessions, number of formal competency assessments conducted and ‘signed off’). Process data collected in the control stroke units will be summarised at each time point to ascertain whether care in the control arm has changed over the course of the trial.
The proportion of non-responders will be compared between randomised groups. Missing items within individual outcome measures will be treated according to instructions for that particular measure. Simple imputation will be used if there are no instructions. A sensitivity analysis will be performed using the last available observation of the NEADL for all patients lost to follow-up to assess the impact of missing follow-up data on the analysis of the primary endpoint. Further secondary analyses will utilise a two-stage model incorporating deaths and primary outcomes, if there is a large number of deaths or an imbalance in the death rates between the two arms.
The number of patients reporting a SAE and details of all SAEs will be reported for each treatment group.
All outcomes will be analysed at the end of the trial but recruitment and safety will be monitored at regular intervals. Outcomes will be summarised once during recruitment for monitoring by the Data Monitoring and Ethics Committee (DMEC).
INTERIM ANALYSIS
No formal interim analyses are planned.
PLANNED SUB-GROUP ANALYSES
No sub-group analyses are planned.
DATA MONITORING
DATA MONITORING
Data will be monitored for quality and completeness by the CTRU, using established verification, validation and checking processes. Missing data, except individual data items collected via the postal questionnaires, will be chased until it is received, confirmed as not available, or the trial is at analysis. Reminders will be sent to patients and caregivers if postal questionnaires are not returned on time.
Rates of recruitment and refusals will be monitored for all sites on a monthly basis to check for differential recruitment rates between control and intervention sites. The completed competencies assessment tool for all trial participants in the intervention units will be returned to the CTRU and a summary of this will be included in a standard monitoring report to the Trial Management Group (TMG), Trial Steering Committee (TSC) and Data Monitoring and Ethics Committee (DMEC). This will enable monitoring of the intervention delivery and compliance.
The CTRU, Academic Unit of Elderly Care and Rehabilitation and the University of Leeds (Sponsor) reserve the right to intermittently conduct source data verification on a sample of patients. Source data verification will involve direct access to patient notes at the participating stroke rehabilitation units and the collection of copies of consent forms and other relevant investigation reports. A monitoring schedule including primary end point, compliance and safety data will be defined and agreed by the DMEC, TSC and TMG.
DATA MONITORING AND ETHICS COMMITTEE
An independent DMEC will be established to review the safety and ethics of the trial. Detailed unblinded reports will be prepared by the CTRU for the DMEC during set-up and annually thereafter. SAEs will be summarised by treatment group in a monthly report sent to the DMEC. This will enable monitoring of safety rates between control and intervention sites.
TRIAL STEERING COMMITTEE (TSC)
A TSC will be established to provide overall supervision of the trial, in particular trial progress, adherence to protocol, patient safety, and consideration of new information. The committee will meet once during the set-up period and every six months thereafter for the duration of the trial.
CLINICAL GOVERNANCE ISSUES
To ensure responsibility and accountability for the overall quality of care received by patients during the study period, clinical governance issues pertaining to all aspect of routine management will be brought to the attention of the DMEC and where applicable to individual NHS Trusts.
QUALITY ASSURANCE AND ETHICAL CONSIDERATIONS
QUALITY ASSURANCE
The trial will be conducted in accordance with current MRC Good Clinical Practice (GCP) guidelines, NHS Research Governance Framework and through adherence to CTRU Standard Operating Procedures (SOPs).
ETHICAL CONSIDERATIONS
The trial will be performed in accordance with the recommendations guiding physicians in biomedical research involving human subjects adopted by the 18th World Medical Assembly, Helsinki, Finland, 1964, amended at the 52nd World Medical Association General Assembly, Edinburgh, Scotland, October 2000. Informed written consent will be obtained from the patients and caregivers prior to registration into the study. The right of a patient and caregiver to refuse participation without giving reasons will be respected. The patient and caregiver will remain free to withdraw at any time from the study without giving reasons and without prejudicing the patient's further treatment. The study will be submitted to and approved by a main Research Ethics Committee (REC) and the appropriate REC for each participating stroke unit prior to entering patients and caregivers into the study. The CTRU will provide the main REC with a copy of the final protocol, patient and caregiver information sheets, consent forms and all other relevant study documentation.
CONFIDENTIALITY
All information collected during the course of the trial will be kept strictly confidential. Information will be held securely on paper and electronically at the CTRU. The CTRU will comply with all aspects of the 1998 Data Protection Act and operationally this will include:
-
consent from patients to record personal details including name, date of birth, address and telephone number, NHS ID, hospital ID, GP name and address;
-
consent from caregivers to record personal details including name, date of birth, address and telephone number, GP name and address;
-
appropriate storage, restricted access and disposal arrangements for patient and caregiver personal and clinical details;
-
consent from patients and caregivers for access to their medical records by responsible individuals from the research team or from regulatory authorities, where it is relevant to trial participation;
-
consent from patients and caregivers for the data collected for the trial to be used to evaluate safety and develop new research;
-
patient and caregiver name, address and telephone number will be collected when a patient and caregiver are registered into the trial but all other data collection forms that are transferred to or from the CTRU will be coded with a trial number and will include two patient and caregiver identifiers, usually their initials and date of birth.
If a patient or caregiver withdraws consent from further trial participation their data will remain on file and will be included in the final study analysis.
ARCHIVING
At the end of the trial, data will be securely archived at the CTRU and participating stroke rehabilitation units for a minimum of 5 years. If a patient or caregiver withdraws consent for their data to be used, it will be confidentially destroyed.
STATEMENT OF INDEMNITY
This trial is sponsored by the University of Leeds and the University of Leeds will be liable for negligent harm caused by the design of the trial. The NHS has a duty of care to patients treated, whether or not the patient is taking part in a clinical trial, and the NHS remains liable for clinical negligence and other negligent harm to patients under this duty of care.
STUDY ORGANISATIONAL STRUCTURE
RESPONSIBILITIES
Chief Investigator
As defined by the NHS Research Governance Framework, the Chief Investigator is responsible for the design, management and reporting of the study.
Clinical Trials Research Unit (CTRU)
The CTRU will have responsibility for conduct of the trial in accordance with the Research Governance Framework, MRC GCP standards and CTRU SOPs.
OPERATIONAL STRUCTURE
Trial Management Group (TMG)
The TMG, comprising the Chief Investigator, research manager, CTRU team and co-investigators will be assigned responsibility for the clinical set-up, on-going management, promotion of the trial, and for the interpretation of results. Specifically the TMG will be responsible for (i) protocol completion, (ii) CRF development, (iii) obtaining approval from the main REC and supporting applications for Site-Specific Assessments (SSA), (iv) completing cost estimates and project initiation, (vi) appointing and facilitating the TSC and DMEC, (vii) reporting of serious adverse events, (vii) monitoring of screening, recruitment, consent, treatment and follow-up procedures, safety, data quality and compliance (viii) interpretation of results and contribution to publications.
Clinical Trials Research Unit (CTRU)
The CTRU will provide set-up and monitoring of trial conduct to CTRU SOPs and MRC GCP standards including randomisation design and implementation, patient and caregiver registration, database development and provision, protocol development, CRF design, trial design, monitoring schedule and statistical analysis of clinical endpoints for the trial. In addition the CTRU will support main REC, SSA and R&D submissions and clinical set-up, ongoing management including training, monitoring reports and promotion of the trial. The CTRU will be responsible for the database administrative functions, data management including postal follow-up and telephone reminders, safety reporting, all statistical analyses of clinical endpoints and drafting of publications. The CTRU will have responsibility for the conduct of the study in accordance with the Research Governance Framework and CTRU SOPs.
Clinical Research Team
The Clinical Research Team will comprise the Research Manager, Senior Clinical Research Practitioners and Clinical Research Practitioners organised in a hub and spoke model. The Research Manager will be based at Bradford and will be responsible for the day-to-day running of the trial, centre set-up, liaison with, recruitment and supervision of the other clinical research team members. They will also co-ordinate the centre initiation and LSCTC training.
The Research Practitioners will lead the implementation of the trial across geographical regions to recruit patients and caregivers and undertake data collection.
LSCTC Training Team
The LSCTC training for staff at the stroke rehabilitation units randomised to the intervention group will be provided by an ‘LSCTC training team’, who were part of the LSCTC implementation team in the initial single centre study [13].
Multidisciplinary Teams at Participating Stroke Rehabilitation Units
The intervention (usual care or LSCTC) will be delivered by the MDTs at the participating stroke rehabilitation units.
Health Economics
Anita Patel and Martin Knapp will assist the CTRU in database development and will be responsible for the design of the economic questionnaires, collation of unit costs, and the conduct, interpretation and writing up of the economic evaluation.
Trial Steering Committee (TSC)
The Trial Steering Committee, with an Independent Chair, will provide overall supervision of the trial, in particular trial progress, adherence to protocol, patient safety and consideration of new information. It will include an Independent Chair and not less than two other independent members. The Chief Investigator and other members of the TMG will attend the TSC meetings to present and report progress.
Data Monitoring and Ethics Committee (DMEC)
The DMEC will review the safety and ethics of the trial by reviewing interim data during recruitment.
PUBLICATION POLICY
The success of the trial depends upon the collaboration of all participants. For this reason, credit for the main results will be given to all those who have collaborated in the trial, through authorship and contributor ship. Uniform requirements for authorship for manuscripts submitted to medical journals will guide authorship decisions. These state that authorship credit should be based only on substantial contribution to:
-
conception and design, or acquisition of data, or analysis and interpretation of data;
-
drafting the article or revising it critically for important intellectual content;
-
and final approval of the version to be published;
-
and that all these conditions must be met (www.icmje.org).
In light of this, the Chief Investigator, research manager, Co-Applicants and relevant senior CTRU staff will be named as authors in any publication. In addition, all collaborators will be listed as contributors for the main trial publication, giving details of roles in planning, conducting and reporting the trial.
To maintain the scientific integrity of the trial, data will not be released prior to the end of the trial, either for trial publication or oral presentation purposes, without the permission of the Trial Steering Committee or the Chief Investigator. In addition, individual collaborators must not publish data concerning their patients which is directly relevant to the questions posed in the trial until the main results of the trial have been published.
References
- Rothwell P.M., . Change in stroke incidence, mortality, case-fatality, severity, and risk factors in Oxfordshire, UK from 1981 to 2004 (Oxford Vascular Study). Lancet 2004;363:1925-33.
- Royal College of Physicians . National Clinical Guidelines for Stroke 2004.
- Anderson C.S., Lento J., Stewartwynne E.G. A Population-Based Assessment of the Impact and Burden of Caregiving for Long-Term Stroke Survivors. Stroke 1995;26:843-9.
- Dewey H.M., . Informal care for stroke survivors – Results from the North East Melbourne Stroke Incidence Study (NEMESIS). Stroke 2002;33:1028-33.
- Han B., Haley W.E. Family caregiving for patients with stroke – Review and analysis. Stroke 1999;30:1478-85.
- Murray J., . Developing a primary care-based stroke model: the prevalence of longer-term problems experienced by patients and carers. British Journal of General Practice 2003;53:803-7.
- Evans R.L., D.S. Bishop, Haselkorn J.K. Factors Predicting Satisfactory Home Care after Stroke. Archives of Physical Medicine and Rehabilitation 1991;72:144-7.
- Dennis M., . A quantitative study of the emotional outcome of people caring for stroke survivors. Stroke 1998;29:1867-72.
- Visser-Meily A., . Intervention studies for caregivers of stroke survivors: a critical review. Patient Education and Counseling 2005;56:257-6.
- Kerr S.M., Smith L.N. Stroke: an exploration of the experience of informal caregiving. Clinical Rehabilitation 2001;15:428-36.
- Smith L.N., . Informal carers' experience of caring for stroke survivors. Journal of Advanced Nursing, 2004;46:235-44.
- Brereton L., Nolan M. ‘You do know he's had a stroke, don't you?’ Preparation for family care-giving – the neglected dimension. Journal of Clinical Nursing 2000;9:498-506.
- Kalra L., . Training care givers of stroke patients: randomised controlled trial. British Medical Journal, 2004;328:1099-101.
- Patel A., . Training care givers of stroke patients: economic evaluation. British Medical Journal 2004;328:1102-4.
- Royal College of Physicians . National Sentinel Audit for Stroke. 2004.
- Van Swieten J.C., . Interobserver Agreement for the Assessment of Handicap in Stroke Patients. Stroke 1988;19:604-7.
- Brooke P., Bullock R. Validation of a 6 Item Cognitive Impairment Test With A View To Primary Care Usage. International Journal of Geriatric Psychiatry 1999;14:936-40.
- Teasdale G., Jennett B. Assessment of Coma and Impaired Consciousness – Practical Scale. Lancet 1974;2:81-4.
- Mahoney F.I., Barthel D.W. Functional Evaluation: The Barthel Index. Maryland State Medical Journal 1965;14:61-5.
- Nouri F., Lincoln N. An extended activities of daily living scale for stroke patients. Clinical Rehabilitation 1987;1:301-5.
- Lincoln N., Gladman J.R.F. The Extended Activities of Daily Living Scale: A Further Validation. Disability and Rehabilitation 1992;14:41-3.
- Zigmond A.S., Snaith R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica 1983;67:361-70.
- Group T.E. EuroQol: a new facility for the measurement of health-related quality of life. The EuroQol Group. Health Policy 1990;16:199-208.
- Brooks R. EuroQol: The current state of play. Health Policy 1996;37:53-72.
- Krabbe P., Weijnen T., Brooks R., Rabin R., F. Guidelines for Analysing and Reporting EQ-5D Outcomes. The Netherlands: Kluwer Academic Publishers Dordrecht; 2003.
- Gompertz P., P. Pound, Ebrahim S. A Postal Version of the Barthel Index. Clinical Rehabilitation 1994;8:233-9.
- Lai S.M., . Persisting consequences of stroke measured by the Stroke Impact Scale. Stroke 2002;33:1840-4.
- Duncan P.W., . Glycine Antagonist in Neuroprotection Americans Investigators. Rasch Analysis of a New Stroke-Specific Outcome Scale. Archives of Physical Medicine and Rehabilitation 2003;84:950-63.
- Duncan P.W., . Stroke Impact Scale –16: A Brief Assessment of Physical Function. Neurology 2003;60:291-6.
- Lai S.M., . Physical and Social Functioning After Stroke: Comparison of the Stroke Impact Scale and Short Form-36. Stroke 2003;34:488-93.
- Patel A., . Alternative strategies for stroke care – Cost-effectiveness and cost-utility analyses from a prospective randomized controlled trial. Stroke 2004;35:196-203.
- Wade D.T., Legh-Smith J., Langton Hewer R. Social activities after stroke: measurement and natural history using the Frenchay Activities Index. International Rehabilitation Medicine 1985;7:176-81.
- Carter J., . Comparison of postal version of the Frenchay activities index with interviewer-administered version for use in people with stroke. Clinical Rehabilitation 1997;11:131-8.
- Forster A., . Information provision for stroke patients and their caregivers. Cochrane Database Syst Rev 2001;3.
- Dennis M. Performance of a statistical model to predict stroke outcome in the context of a large, simple, randomized, controlled trial of feeding. Stroke 2003;34:127-33.
- Elmstahl S., Malmberg B., Annerstedt L. Caregiver's burden of patients 3 years after stroke assessed by a novel caregiver burden scale. Archives of Physical Medicine and Rehabilitation 1996;77:177-82.
- Pound P., Gompertz P., Ebrahim S. Patients' satisfaction with stroke services. Clinical Rehabilitation 1994;8:7-17.
- Boter H., de Haan R.J., Rinkel G.J. Clinimetric evaluation of a Satisfaction-with-Stroke-Care questionnaire. Journal of Neurology 2003;250:534-41.
- Parker C.J., . A multicentre randomized controlled trial of leisure therapy and conventional occupational therapy after stroke. Clinical Rehabilitation 2001;15:42-5.
- Walker M.F., . Occupational therapy for stroke patients not admitted to hospital: a randomised controlled trial. Lancet, 1999;354:278-80.
- Gladman J.R.F., Lincoln N.B., Adams S.A. Use of the Extended ADL Scale with Stroke Patients. Age and Ageing 1993;22:419-24.
- Cunliffe A.L., . Sooner and healthier: a randomised controlled trial and interview study of an early discharge rehabilitation service for older people. Age and Ageing 2004;33:246-52.
- Bjelland I., . The validity of the Hospital Anxiety and Depression Scale. An updated literature review. Journal of Psychosomatic Research 2002;52:69-77.
- Duncan P.W., . The stroke impact scale version 2.0 – Evaluation of reliability, validity, and sensitivity to change. Stroke 1999;30:2131-40.
- Duncan P.W., . Performance of a mail-administered version of a stroke-specific outcome measure, the Stroke Impact Scale. Clinical Rehabilitation 2002;16:493-505.
- Drummond M.F., . Oxford: Oxford University Press; 1997.
- Dolan P., . University of York; 1995.
- Fenwick E., Claxton K., Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Economics 2001;10:779-87.
- Parker C., Dewey M. Assessing research outcomes by postal questionnaire with telephone follow-up. TOTAL Study Group. Trial of Occupational Therapy and Leisure. International Journal of Epidemiology 2000;29:1065-9.
- Ukoumunne O.C., . Methods for evaluating area-wide and organisation-based interventions in health and health care: a systematic review. Health Technology Assessment 1999;3:iii-92.
- Campbell M.K., Mollison J., Grimshaw J.M. Cluster trials in implementation research: estimation of intracluster correlation coefficients and sample size. Statistics in Medicine 2001;20:391-9.
- Forster A., Young J. Research networks for stroke rehabilitation: opportunities and barriers. Clinical Medicine 2005;5:42-6.
- Forster A., Young J. Specialist nurse support for patients with stroke in the community: A randomised controlled trial. British Medical Journal 1996;312:1642-6.
- Smith J., Forster A., Young J. A randomized trial to evaluate an education programme for patients and carers after stroke. Clinical Rehabilitation 2004;18:726-3.
APPENDIX A: ABBREVIATIONS
APPENDIX B: TRIAL MANAGEMENT GROUP
The TMG includes those listed as key contacts and the following Co-applicants:
Professor Lalit Kalra
Professor of Stroke Medicine
Guy's, King's & St Thomas' School of Medicine
King's College
Denmark Hill Campus, Bessemer Road
London
SE5 9PJ
Tel: 020 3299 1718
E-mail: lalit.kalra@kcl.ac.uk
Professor Martin Knapp
Professor of Health Economics
Institute of Psychiatry
King's College London
CEMH, Box 24
De Crespigny Park
London
SE5 8AF
Tel: 020 7848 0174
Fax: 020 7701 7600
Email: m.knapp@iop.kcl.ac.uk
Dr Jane Nixon
Deputy Director CTRU
Clinical Trials Research Unit
17 Springfield Mount
Leeds
LS2 9NG
Tel: 0113 343 1488
Fax: 0113 343 1471
Email: j.e.nixon@leeds.ac.uk
Dr Anita Patel
Institute of Psychiatry
King's College London
CEMH, Box 24
De Crespigny Park
London
SE5 8AF
Tel: 020 7848 0589
Fax: 020 7701 7600
Email: a.patel@iop.kcl.ac.uk
Dr David Smithard
Hon. Senior. Lecturer & Consultant Physician
East Kent Hospitals NHS Trust
Department of Medicine
William Harvey Hospital
Kennington Road
Willesborough
Ashford Kent
TN24 0LZ
Tel: 01233 616214
E-mail: david.smithard@ekht.nhs.uk
Professor John Young
Academic Unit of Elderly Care and Rehabilitation
Bradford Institute for Health Research
Temple Bank House
Bradford Royal Infirmary
Duckworth Lane
Bradford
BD9 6RJ
Tel: 01274 38 3406
Fax: 01274 382766
E-mail: john.young@bradfordhospitals.nhs.uk
APPENDIX C: COMMITTEE TERMS OF REFERENCE
TRIAL STEERING COMMITTEE
-
To provide overall supervision of the trial.
-
To monitor and supervise the progress of the trial towards its interim and overall objectives, adherence to protocol and patient accrual within the set time-frame.
-
To review at regular intervals relevant information from other sources (e.g. other related trials), and recommend appropriate action (e.g. changes to trial protocol, stopping or extending the trial).
-
To consider the recommendations of the Data Monitoring and Ethics Committee.
-
To recommend appropriate action in the light of points 1, 2, 3 and 4 to ensure that the rights, safety and well-being of the trial participants are the most important considerations, and prevail over the interests of science and society.
-
In light of 1, 2, 3 and 4 to inform the MRC and relevant Research Boards on the progress of the trial.
-
To advise the MRC on publicity and presentation of all aspects of the trial.
DATA MONITORING AND ETHICS COMMITTEE
-
To determine if interim analyses of trial data should be undertaken.
-
To consider the data from interim data monitoring/analyses plus any additional safety issues for the trial and relevant information from other sources.
-
In the light of 2. (above), and ensuring that ethical considerations are of prime importance, to report (following each DMEC meeting) to the Trial Steering Committee and to recommend on the continuation of the trial.
-
To consider any requests for release of interim trial data and to make recommendations to the TSC on the advisability of this.
-
In the event of further funding being required, to provide to the TSC and MRC appropriate information and advice on the data gathered to date that will not jeopardise the integrity of the study.
APPENDIX D: STUDY ASSESSMENT SCHEDULE
Appendix 5 Unit costs
| Item | Unit | Unit cost (£) 2009–10 prices | Source | Notes |
|---|---|---|---|---|
| Residential and nursing home | ||||
| Residential care home | Night | 74 | The NHS Information Centre SCS77 | |
| Nursing home | Night | 73 | ||
| Inpatient services | ||||
| A – Nervous system | Bed-day | 334 | Department of Health67 | Tab TNEI_L: Weighted mean of codes A |
| B – Eyes and periorbita | Bed-day | 554 | Department of Health67 | Tab TNEI_L: Weighted mean of codes B |
| C – Mouth, head, neck and ears | Bed-day | 468 | Department of Health67 | Tab TNEI_L: Weighted mean of codes C |
| D – Respiratory system | Bed-day | 308 | Department of Health67 | Tab TNEI_L: Weighted mean of codes D |
| E – Cardiac surgery and primary cardiac conditions | Bed-day | 417 | Department of Health67 | Tab TNEI_L: Weighted mean of codes E |
| F – Digestive system | Bed-day | 388 | Department of Health67 | Tab TNEI_L: Weighted mean of codes F |
| G – Hepatobiliary and pancreatic systems | Bed-day | 369 | Department of Health67 | Tab TNEI_L: Weighted mean of codes G |
| H – Musculoskeletal system | Bed-day | 445 | Department of Health67 | Tab TNEI_L: Weighted mean of codes H |
| J – Skin, breast and burns | Bed-day | 370 | Department of Health67 | Tab TNEI_L: Weighted mean of codes J |
| K – Endocrine and metabolic system | Bed-day | 308 | Department of Health67 | Tab TNEI_L: Weighted mean of codes K |
| L – Urinary tract and male reproductive systems | Bed-day | 326 | Department of Health67 | Tab TNEI_L: Weighted mean of codes L |
| M – Female reproductive system and assisted reproduction | Bed-day | 535 | Department of Health67 | Tab TNEI_L: Weighted mean of codes M |
| N – Obstetrics | Bed-day | 755 | Department of Health67 | Tab TNEI_L: Weighted mean of codes N |
| P – Diseases of childhood and neonates | Bed-day | 493 | Department of Health67 | Tab TNEI_L: Weighted mean of codes P |
| Q – Vascular system | Bed-day | 438 | Department of Health67 | Tab TNEI_L: Weighted mean of codes Q |
| S – Haematology, chemotherapy, radiotherapy and specialist palliative care | Bed-day | 404 | Department of Health67 | Tab TNEI_L: Weighted mean of codes S |
| W – Immunology, infectious diseases and other contacts | Bed-day | 327 | Department of Health67 | Tab TNEI_L: Weighted mean of codes W |
| Mental health | Bed-day | 318 | Department of Health67 | Index tab TMHIP unit cost (Mental Health Inpatients). Assumption made that this is per day even though this is not stated |
| Geriatric | Bed-day | 361 | Department of Health67 | Costed as ‘Inpatient stroke rehabilitation’ |
| Accident and emergency | Occurrence | 110 | Department of Health67 | Index tab weighted average for all accident and emergency services unit costs (TAandEMSAD, TAandEMSNA, TAandEMinAD, TAandEMinNA, TAandEWiCAD, TAandEWiCNA, TNon24HRDEPAD, TNon24HRDEPNA) |
| Oncology/cancer | Bed-day | 404 | Department of Health67 | Costed as ‘Inpatient general medical’ |
| Surgery | Bed-day | 404 | Department of Health67 | Costed as ‘Inpatient general medical’ |
| Intensive care | Bed-day | 1229 | Department of Health67 | Index tab TCCSALCCU (Critical Care Services – Adult) unit cost. Assumption made that this is per day even though this is not stated. This assumption is based on a NICE reference which states critical care in the NHS reference costs is per day78 |
| Stroke, acute | Bed-day | 294 | Department of Health67 | Tab TNEI_L: Weighted average of AA22Z (Non-Transient Stroke or Cerebrovascular Accident, Nervous system infections or Encephalopathy) and AA23Z (Haemorrhagic Cerebrovascular Disorders) |
| Stroke, rehabilitation | Bed-day | 361 | Department of Health67 | Tab TREHAB_CSRS_LEVEL_1_BEDDAY_APC: VC04Z (Rehabilitation for stroke) cost. Assumption this is per bed-day. |
| General medical | Bed-day | 404 | Department of Health67 | TNEI_L tab: weighted average of all costs |
| Unknown/other | Bed-day | 404 | Department of Health67 | Costed as ‘Inpatient general medical’ |
| Clinical decision unit | Occurrence | 110 | Department of Health67 | Costed as ‘Inpatient A&E’ |
| Intermediate care | Bed-day | 240 | Curtis 201079 | |
| Inpatient services | ||||
| A – Nervous system | Stay | 2415 | Department of Health67 | Tab TNEI_L: Weighted mean of codes A |
| B – Eyes and periorbita | Stay | 2007 | Department of Health67 | Tab TNEI_L: Weighted mean of codes B |
| C – Mouth, head, neck and ears | Stay | 1879 | Department of Health67 | Tab TNEI_L: Weighted mean of codes C |
| D – Respiratory system | Stay | 1999 | Department of Health67 | Tab TNEI_L: Weighted mean of codes D |
| E – Cardiac surgery and primary cardiac conditions | Stay | 1941 | Department of Health67 | Tab TNEI_L: Weighted mean of codes E |
| F – Digestive system | Stay | 2197 | Department of Health67 | Tab TNEI_L: Weighted mean of codes F |
| G – Hepatobiliary and pancreatic systems | Stay | 2505 | Department of Health67 | Tab TNEI_L: Weighted mean of codes G |
| H – Musculoskeletal system | Stay | 3492 | Department of Health67 | Tab TNEI_L: Weighted mean of codes H |
| J – Skin, breast and burns | Stay | 2495 | Department of Health67 | Tab TNEI_L: Weighted mean of codes J |
| K – Endocrine and metabolic system | Stay | 1794 | Department of Health67 | Tab TNEI_L: Weighted mean of codes K |
| L – Urinary tract and male reproductive systems | Stay | 2276 | Department of Health67 | Tab TNEI_L: Weighted mean of codes L |
| M – Female reproductive system and assisted reproduction | Stay | 1701 | Department of Health67 | Tab TNEI_L: Weighted mean of codes M |
| N – Obstetrics | Stay | 2174 | Department of Health67 | Tab TNEI_L: Weighted mean of codes N |
| P – Diseases of childhood and neonates | Stay | 1534 | Department of Health67 | Tab TNEI_L: Weighted mean of codes P |
| Q – Vascular system | Stay | 3877 | Department of Health67 | Tab TNEI_L: Weighted mean of codes Q |
| S – Haematology, chemotherapy, radiotherapy and specialist palliative care | Stay | 2547 | Department of Health67 | Tab TNEI_L: Weighted mean of codes S |
| W – Immunology, infectious diseases and other contacts | Stay | 1865 | Department of Health67 | Tab TNEI_L: Weighted mean of codes W |
| Oncology/cancer | Stay | 2267 | Department of Health67 | Costed as ‘Inpatient general medical’ |
| Surgery | Stay | 2267 | Department of Health67 | Costed as ‘Inpatient general medical’ |
| Stroke, acute | Stay | 2808 | Department of Health67 | Tab TNEI_L: Weighted average of AA22Z (Non-Transient Stroke or Cerebrovascular Accident, Nervous system infections or Encephalopathy) and AA23Z (Haemorrhagic Cerebrovascular Disorders) |
| General medical | Stay | 2267 | Department of Health67 | TNEI_L tab: weighted average of all costs |
| Unknown/other | Stay | 2267 | Department of Health67 | Costed as ‘Inpatient general medical’ |
| Day hospital/day cases | ||||
| A – Nervous system | Activity | 625 | Department of Health67 | TDC tab: Weighted mean of codes A |
| B – Eyes and periorbita | Activity | 760 | Department of Health67 | TDC tab: Weighted mean of codes B |
| C – Mouth, head, neck and ears | Activity | 736 | Department of Health67 | TDC tab: Weighted mean of codes C |
| D – Respiratory system | Activity | 562 | Department of Health67 | TDC tab: Weighted mean of codes D |
| E – Cardiac surgery and primary cardiac conditions | Activity | 1149 | Department of Health67 | TDC tab: Weighted mean of codes E |
| F – Digestive system | Activity | 578 | Department of Health67 | TDC tab: Weighted mean of codes F |
| G – Hepatobiliary and pancreatic systems | Activity | 845 | Department of Health67 | TDC tab: Weighted mean of codes G |
| H – Musculoskeletal system | Activity | 1058 | Department of Health67 | TDC tab: Weighted mean of codes H |
| J – Skin, breast and burns | Activity | 668 | Department of Health67 | TDC tab: Weighted mean of codes J |
| K – Endocrine and metabolic system | Activity | 368 | Department of Health67 | TDC tab: Weighted mean of codes K |
| L – Urinary tract and male reproductive systems | Activity | 507 | Department of Health67 | TDC tab: Weighted mean of codes L |
| M – Female reproductive system and assisted reproduction | Activity | 703 | Department of Health67 | TDC tab: Weighted mean of codes M |
| N – Obstetrics | Activity | 530 | Department of Health67 | TDC tab: Weighted mean of codes N |
| P – Diseases of childhood and neonates | Activity | 652 | Department of Health67 | TDC tab: Weighted mean of codes P |
| Q – Vascular system | Activity | 785 | Department of Health67 | TDC tab: Weighted mean of codes Q |
| S – Haematology, chemotherapy, radiotherapy and specialist palliative care | Activity | 476 | Department of Health67 | TDC tab: Weighted mean of codes S |
| V – Multiple trauma, emergency medicine and rehabilitation | Activity | 917 | Department of Health67 | TDC tab: Weighted mean of codes V |
| W – Immunology, infectious diseases and other contacts | Activity | 421 | Department of Health67 | TDC tab: Weighted mean of codes W |
| Unknown day hospital | Activity | 673 | Department of Health67 | Index tab: Unit cost for day cases HRG data – (TDC) |
| Outpatient services | ||||
| Accident and emergency | Activity | 113 | Department of Health67 | Total – OPATT Tab: Service code 180 |
| Allergy | Activity | 139 | Department of Health67 | Total – OPATT Tab: Service code 317 |
| Anticoagulant service | Activity | 25 | Department of Health67 | Total – OPATT Tab: Service code 324 |
| Audiology | Activity | 158 | Department of Health67 | Total – OPATT Tab: Service code 840 |
| Stroke rehabilitation day unit | Activity | 167 | Department of Health67 | TDCFRAD tab: Stroke patient – DCF10 |
| Breast surgery | Activity | 119 | Department of Health67 | Total – OPATT Tab: Service code 103 |
| Burns care | Activity | 123 | Department of Health67 | Total – OPATT Tab: Service code 161 |
| Cardiology | Activity | 124 | Department of Health67 | Total – OPATT Tab: Service code 320 |
| Chemical pathology | Activity | 73 | Department of Health67 | Total – OPATT Tab: Service code 822 |
| Clinical genetics | Activity | 588 | Department of Health67 | Total – OPATT Tab: Service code 311 |
| Clinical haematology | Activity | 131 | Department of Health67 | Total – OPATT Tab: Service code 303 |
| Clinical immunology | Activity | 195 | Department of Health67 | Total – OPATT Tab: Service code 316 |
| Obstetrics | Activity | 109 | Department of Health67 | Total – OPATT Tab: Service code 501 |
| Colorectal surgery | Activity | 104 | Department of Health67 | Total – OPATT Tab: Service code 104 |
| Dermatology | Activity | 93 | Department of Health67 | Total – OPATT Tab: Service code 330 |
| Dietetics | Activity | 50 | Department of Health67 | Total – OPATT Tab: Service code 654A, Adult (19 years and over only) |
| Endocrinology | Activity | 136 | Department of Health67 | Total – OPATT Tab: Service code 302 |
| Ear, nose and throat | Activity | 85 | Department of Health67 | Total – OPATT Tab: Service code 120 |
| General medicine | Activity | 142 | Department of Health67 | Total – OPATT Tab: Service code 300 |
| General surgery | Activity | 112 | Department of Health67 | Total – OPATT Tab: Service code 100 |
| Geriatric medicine | Activity | 199 | Department of Health67 | Total – OPATT Tab: Service code 430 |
| Gynaecology | Activity | 112 | Department of Health67 | Total – OPATT Tab: Service code 502 |
| Haemophilia | Activity | 785 | Department of Health67 | Total – OPATT Tab: Service code 309 |
| Hepatology | Activity | 172 | Department of Health67 | Total – OPATT Tab: Service code 306 |
| Maxillofacial surgery | Activity | 102 | Department of Health67 | Total – OPATT Tab: Service code 144 |
| Medical gastroenterology | Activity | 123 | Department of Health67 | Total – OPATT Tab: Service code 301M |
| Medical oncology | Activity | 135 | Department of Health67 | Total – OPATT Tab: Service code 370 |
| Nephrology | Activity | 156 | Department of Health67 | Total – OPATT Tab: Service code 361 |
| Neurology | Activity | 165 | Department of Health67 | Total – OPATT Tab: Service code 400 |
| Neurosurgery | Activity | 162 | Department of Health67 | Total – OPATT Tab: Service code 150 |
| Nuclear medicine | Activity | 136 | Department of Health67 | Total – OPATT Tab: Service code 371 |
| Occupational therapy | Activity | 61 | Department of Health67 | Total – OPATT Tab: Service code 651A, Adult (19 years and over only) |
| Ophthalmology | Activity | 80 | Department of Health67 | Total – OPATT Tab: Service code 130 |
| Oral surgery | Activity | 113 | Department of Health67 | Total – OPATT Tab: Service code 140 |
| Orthodontics | Activity | 109 | Department of Health67 | Total – OPATT Tab: Service code 143 |
| Orthoptics | Activity | 53 | Department of Health67 | Total – OPATT Tab: Service code 655 |
| Pain management | Activity | 124 | Department of Health67 | Total – OPATT Tab: Service code 191 |
| Palliative medicine | Activity | 185 | Department of Health67 | Total – OPATT Tab: Service code 315 |
| Physiotherapy | Activity | 39 | Department of Health67 | Total – OPATT Tab: Service code 650A, Adult (19 years and over only) |
| Plastic surgery | Activity | 86 | Department of Health67 | Total – OPATT Tab: Service code 160 |
| Podiatry | Activity | 39 | Department of Health67 | Total – OPATT Tab: Service code 653 |
| Rehabilitation | Activity | 122 | Department of Health67 | Total – OPATT Tab: Service code 314 |
| Rheumatology | Activity | 137 | Department of Health67 | Total – OPATT Tab: Service code 410 |
| Speech and language therapy | Activity | 80 | Department of Health67 | Total – OPATT Tab: Service code 652A, Adult (19 years and over only) |
| Spinal injuries | Activity | 300 | Department of Health67 | Total – OPATT Tab: Service code 323 |
| Thoracic surgery | Activity | 216 | Department of Health67 | Total – OPATT Tab: Service code 173 |
| Transplantation surgery | Activity | 231 | Department of Health67 | Total – OPATT Tab: Service code 102 |
| Trauma and orthopaedics: non-trauma | Activity | 96 | Department of Health67 | Total – OPATT Tab: Service code 110N, used when type of orthopaedics unspecified |
| Trauma and orthopaedics: trauma | Activity | 99 | Department of Health67 | Total – OPATT Tab: Service code 110T |
| Urology | Activity | 99 | Department of Health67 | Total – OPATT Tab: Service code 101 |
| Dental medicine specialties | Activity | 87 | Department of Health67 | Total – OPATT Tab: Service code 450 |
| Vascular surgery | Activity | 126 | Department of Health67 | Total – OPATT Tab: Vascular surgery – 107 |
| Respiratory | Activity | 145 | Department of Health67 | Total – OPATT Tab: Service code 340 |
| Diabetic medicine | Activity | 127 | Department of Health67 | Total – OPATT Tab: Service code 307 |
| Mental health/psychiatry | Activity | 174 | Department of Health67 | A weighted average of all Mental Health Consultant Services from hospital outpatient settings was calculated. Index tab: variables: TMHCSOPFAF, TMHCSOPFANF, TMHCSOPFUAF, TMHCSOPFUANF, TMHCSOPSSFAF, TMHCSOPSSFANF, TMHCSOPSSFUAF, TMHCSOPSSFUANF |
| Imaging – generic | Activity | 116 | Department of Health67 | Index tab: TDIAGIM_OP unit cost (Diagnostic imaging: outpatient) |
| Patient transport services | Activity | 31 | Department of Health67 | Index tab: TPTS_OP unit cost (Patient transport services) |
| Primary care trust dietician | Activity | 54 | Department of Health67 | TOCS tab (Other community services): N800 unit cost (Community dietician) |
| Elderly day unit | Activity | 181 | Department of Health67 | TDCFRAD tab (Day care facilities: regular attendances): DCF20 (Elderly patient) |
| Stroke generic | Activity | 165 | Department of Health67 | Costed as ‘Outpatient – neurology’ |
| Vascular general | Activity | 142 | Department of Health67 | Costed as ‘Outpatient – general medicine’ |
| Walk-in centre | Activity | 54 | Department of Health67 | Index tab: TAandEWiCNA unit cost (Costed as Accident and emergency services: walk in centres: not leading to admitted) |
| Paramedic service without accident and emergency attendance | Activity | 222 | Department of Health67 | Index tab: TPARB unit cost (Paramedic services: Category B/Amber) |
| Community mental health team | Activity | 45 | Curtis 201079 | Costed as per hour of face-to-face contact divided by 2 to assume 1–2-hour appointments |
| Wheelchair services | Year | 85 | Curtis 201079 | Costed as self propelled chair per year |
| Outpatient procedures | ||||
| Sigmoidoscopy | Procedure | 207 | Department of Health67 | TOPROC tab: Diagnostic or therapeutic rigid sigmoidoscopy 19 years and over: FZ57Z |
| DEXA scan | Scan | 78 | Department of Health67 | TDIAGIM_OP Tab: DEXA Scan – RA15Z |
| CT/CAT scan | Scan | 100 | Department of Health67 | TDIAGIM_OP Tab: Computerised tomography scan, one area, no contrast – RA08Z |
| Colonoscopy | Procedure | 271 | Department of Health67 | TOPROC tab: Therapeutic colonoscopy 19 years and over -FZ53Z |
| Electrocardiogram | Procedure | 127 | Department of Health67 | TOPROC tab: Electrocardiogram monitoring and stress testing – EA47Z |
| Ultrasound | Procedure | 55 | Department of Health67 | TDIAGIM_OP Tab: Ultrasound scan more than 20 minutes – RA24Z |
| Eye test | Procedure | 30 | Department of Health67 | TDADS Tab: Diabetic retinal screening – DA11 |
| MRI | Procedure | 175 | Department of Health67 | TDIAGIM_OP Tab: Magnetic resonance imaging scan, one area, no contrast – RA01Z |
| X-ray | Procedure | 29 | Department of Health67 | Total – OPATT Tab: Direct access plain film – DAPF |
| Blood test | Procedure | 5 | Department of Health67 | TDAPS Tab: Phlebotomy – DAP839 |
| Endoscopy | Procedure | 88 | Department of Health67 | TOPROC tab: Upper genital tract laparoscopic/endoscopic major procedures – MA08Z |
| Community-based services | ||||
| GP surgery, surgery | Visit | 32 | Curtis 201079 | Average surgery consultation lasting 11.7 minutes. Includes direct care staff costs; excludes qualification costs |
| GP, home | Visit | 106 | Curtis 201079 | Average home visit lasting 23.4 minutes. Includes travel time and direct care staff costs; excludes qualification costs |
| GP, telephone consultation | Call | 19 | Curtis 201079 | Average telephone consultation lasting 7.1 minutes. Includes direct care staff costs; excludes qualification costs |
| Practice nurse surgery, surgery | Consultation | 10 | Curtis 201079 | Excludes qualification costs |
| Practice nurse, telephone call | Consultation | 6 | Curtis 201079 | Assumed ratio of time spent on telephone consultation compared with face-to-face consultation is same as for a GP (60.68%). On this basis, a face-to-face nurse consultation of 15.5 minutes at £10, translates to a 9.4-minute telephone consultation at £6.07 |
| Repeat prescription request (without nurse/doctor contact) | Call | 14 | Curtis 201079 | Assumed 5 minutes of GP time i.e. 5 × £2.70 per surgery/clinic minute. Includes direct care staff costs; excludes qualification costs |
| Physiotherapist home, home | Visit | 41 | Curtis 201079 | Average home visit lasting 1 hour. Includes travel time and travel costs; excludes qualification costs |
| Physiotherapist surgery, surgery | Visit | 15 | Curtis 201079 | Excludes qualification costs |
| Physiotherapist visit elsewhere (not private) | Visit | 15 | Curtis 201079 | Excludes qualification costs |
| Occupational therapist, home | Visit | 42 | Curtis 201079 | Average home visit lasting 1 hour. Includes travel time and travel costs; excludes qualification costs |
| Occupational therapist surgery | Visit | 15 | Curtis 201079 | Excludes qualification costs |
| Occupational therapist visit elsewhere (not private) | Visit | 15 | Curtis 201079 | Excludes qualification costs |
| Speech and language therapist, home | Visit | 41 | Curtis 201079 | Average home visit lasting 1 hour. Includes travel time and travel costs; excludes qualification costs |
| Speech and language therapist, surgery | Visit | 15 | Curtis 201079 | Excludes qualification costs |
| Speech and language therapist visit elsewhere (not private) | Visit | 15 | Curtis 201079 | Excludes qualification costs |
| Community or district nurse | Visit | 24 | Curtis 201079 | Community nurse average home visit lasting 20 minutes. Includes travel time and costs; excludes qualification costs |
| Health visitor | Visit | 37 | Curtis 201079 | Health visitor average home visit lasting 20 minutes. Includes travel time and costs; excludes qualification costs |
| Geriatrician | Contact | 49 | Curtis 201079 | Assumed 20-minute contact with medical consultant (£146 per patient-related hour). Excludes qualification costs |
| Psychiatrist | Contact | 129 | Department of Health67 | Index Tab: TMHCSCFUAF unit cost (Mental Health Consultant Services (Community Setting) – Follow-up contact face to face) |
| Psychologist | Contact | 81 | Curtis 201079 | Per hour of client contact. Assumed 1-hour contact |
| Chiropodist | Visit | 11 | Curtis 201079 | Per clinic visit |
| Chiropractor | Contact | 28 | NHS choices – chiropractor80 | Assumed mid-point cost per session from range of £20-£35 per 30-minute appointment |
| Osteopath | Contact | 43 | NHS choices – osteopathy81 | Assumed mid-point cost per session from range of £35-£50 per 30–40 minute contact |
| Dentist | Activity | 90 | Department of Health67 | TOCS tab (Other community services): CN20 unit cost (Community dental services) |
| Optician | Eye test | 19 | Boots website82 | Eye test rate (£20) at Boots Opticians, as at 15 November 2011 deflated to 2010 rate using Consumer Price Index |
| Day hospital | Half day | 91 | Department of Health67 | TDCFRAD tab (Day care facilities: regular attendances): half of DCF20 unit cost (Elderly patient) |
| Social club | Session | 36 | Curtis 201079 | Local authority day care for older people |
| Lunch club | Visit | 12 | Curtis 201079 | Costed as a meal and one-quarter of other running costs of a voluntary day care for older people |
| Drop-in centre | Session | 36 | Curtis 201079 | Local authority day care for older people |
| Meals on wheels | Meal | 6 | Curtis 201079 | Average cost per local authority meal on wheels |
| Frozen meals | Meal | 3 | Curtis 201079 | Assume half the average cost per local authority meal on wheels |
| Home care worker | Hour | 28 | Curtis 201079 | Local authority home care worker per hour of face-to-face contact. Weighted average accounting for different rates for day/evening/weekday/weekends |
| Social worker | Hour | 158 | Curtis 201079 | Per hour of face-to-face contact. Excludes qualification costs |
| Social worker, telephone call | Call | 14 | Curtis 201079 | Assumed 15 minutes of social worker time based on £55 per hour of client related work. Excludes qualification costs |
| Social services day care centre | Visit | 36 | Curtis 201079 | Local authority day care for older people |
| Intermediate care team | Hour | 23 | Curtis 201079 | Assumed the cost of a lower cost professional: clinical support worker nursing (community); per hour spent with a patient |
| Value of time | ||||
| National average wage (opportunity cost) | Hour | 15 | ONS Annual Survey of Hours and Earnings, 2010 Revised 83 | Table 1.5a: hourly pay gross for all employees, UK, 2010. Mean, not affected by absence = £14.60 |
| Leisure time cost (opportunity cost) | Hour | 5 | Department for Transport 201184 | Cost of leisure: £5.42 (£4.46 per hour is the value of non-working time per person in 2002 according to the department of transport then inflated up to 2009) |
| Average opportunity cost | Hour | 10 | ONS Annual Survey of Hours and Earnings, 2010 Revised83 and Department for Transport. 201184 | Assumed an average of the national average wage (£14.60) and the cost of leisure time (5.42 hours) in the absence of information on whether or not the carer would instead have worked |
| Home help cost (replacement cost) | Hour | 28 | Curtis 201079 | Home help cost: £25 weekday; £30 weekday evening; £37 Saturday; £50 Sunday; all costs per hour of face-to-face contact. Weighted average £27.69 |
| Other services | ||||
| Cardiac rehabilitation nurse at home | Hour | 78 | Curtis 201079 | Costed as community nurse specialist. Per hour of client contact (excluding qualification costs) plus travel cost per visit |
| Care on call – weekly visit alarm pendant | Home visit | 9 | Curtis 201079 | Costed as clinical support worker nursing (community, includes travel) |
| NHS Direct | Call | 15 | NHS direct85 | |
Appendix 6 Calculation of London Stroke Carer Training Course training costs
Table 62 summarises costs related to each component of the LSCTC development and staff training. Tables 63–73 provide further details of the calculations of each of the components.
| LSCTC development and staff training | Costs (£, 2009–10 prices) |
|---|---|
| 1. Core training and refresher training: developmenta | |
| Staffing costs, main training | 6662 |
| Staffing costs, refresher training | 1017 |
| Total | 7680 |
| 2. Core training: preparation | |
| Staffing costs | 3554 |
| Total | 3554 |
| 3. Refresher training: preparation | |
| Staffing costs, training team | 753 |
| Total | 753 |
| 4. Core training: delivery | |
| Staffing costs, training team | 10,230 |
| Other costs | 13,086 |
| Total | 23,317 |
| 5. Refresher training: delivery | |
| Staffing costs, training team | 3197 |
| Other costs | 2407 |
| Total | 5603 |
| 6. Local refresher visits: delivery | |
| Staffing costs, training team | 12,513 |
| Other costs | 4080 |
| Total | 16,593 |
| 7. Ward staff time | |
| Attendance at main training | 27,667 |
| Attendance at refresher training | 4626 |
| Local cascaded training, trainers | 1577 |
| Local cascaded training, trainees | 6881 |
| Attendance at local refresher training | 4327 |
| Total | 45,077 |
| Total cost | |
| Total including development costs | 102,577 |
| Total excluding development costs | 94,897 |
| Cost per minute of input to caregivers, including development costsb | 0.60 |
| Cost per minute of input to caregivers, excluding development costsb | 0.56 |
| Salary grade, band and point | Salary, basic86 | Salary, on-costs | aSalary, overheads79 | Salary, total per annum | b,cSalary, total per hour worked87 | Salary, total per minute worked |
|---|---|---|---|---|---|---|
| Training team | ||||||
| Academic Grade 8, point 3788 | 36,715 | 11,015 | 47,730 | 95,459 | 56.39 | 0.94 |
| Academic Grade 6, point 2688 | 26,523 | 7957 | 34,480 | 68,960 | 40.74 | 0.68 |
| Academic Grade 10, point 5288 | 57,201 | 17,160 | 74,361 | 148,723 | 87.86 | 1.46 |
| NHS Band 7, point 35 | 39,273 | 11,782 | 18,855 | 69,909 | 41.30 | 0.69 |
| NHS Band 7, point 31 | 34,410 | 10,323 | 16,520 | 61,253 | 36.19 | 0.60 |
| NHS Band 5, point 20 | 23,345 | 7004 | 11,208 | 41,556 | 24.55 | 0.41 |
| NHS Band 4, point 14 | 19,495 | 5849 | 9359 | 34,703 | 20.50 | 0.34 |
| Ward staff | ||||||
| NHS Band 1, point 2d | 13,588 | 4076 | 6523 | 24,188 | 14.29 | 0.24 |
| NHS Band 2, point 4 | 14,359 | 4308 | 6894 | 25,560 | 15.10 | 0.25 |
| NHS Band 3, point 9 | 16,698 | 5009 | 8017 | 29,724 | 17.56 | 0.29 |
| NHS Band 4, point 14 | 19,495 | 5849 | 9359 | 34,703 | 20.50 | 0.34 |
| NHS Band 5, point 20 | 23,345 | 7004 | 11,208 | 41,556 | 24.55 | 0.41 |
| NHS Band 6, point 26 | 28,816 | 8645 | 13,834 | 51,295 | 30.30 | 0.51 |
| NHS Band 7, point 30 | 33,436 | 10,031 | 16,052 | 59,519 | 35.16 | 0.59 |
| NHS Band 8, point 42 | 53,256 | 15,977 | 25,568 | 94,800 | 56.00 | 0.93 |
| cNHS Senior House Officer80 | 31,900 | 7818 | 6422 | 46,140 | 21.65 | 0.36 |
| cNHS Consultant79 | 120,200 | 31,482 | 42,361 | 194,043 | 108.25 | 1.80 |
| cNHS Social worker79 | 30,633 | 9010 | 19,850 | 59,493 | 39.66 | 0.66 |
| Resource | Activity | Hours per activity | Costs (£, 2009–10 prices) |
|---|---|---|---|
| A. Core training: April to November 2007 | |||
| Academic Grade 8, point 37 | Administration – training manuals, Royal College of Nursing continuing professional development accreditation and talks | 35 | 1974 |
| Academic Grade 8, point 37 | Post-meeting feedback | 5 | 282 |
| Academic Grade 8, point 37 | Post-meeting compiling compact discs | 8 | 451 |
| NHS Band 7, point 35a | Development time | 20 | 818 |
| NHS Band 7, point 35a | Development time | 17 | 706 |
| NHS Band 7, point 35a | Development time | 23 | 929 |
| Academic Grade 10, point 52a | Development time | 17 | 1502 |
| Core training, total development cost | 6662 | ||
| B. Refresher training: August 2009 | |||
| Academic Grade 8, point 37 | Compiling training manual and training compact disc, version 2 | 14 | 789 |
| NHS Band 7, point 35a | Development time | 2 | 74 |
| NHS Band 7, point 35a | Development time | 2 | 74 |
| Academic Grade 10, point 52a | Development time | 1 | 79 |
| Refresher training, total development cost | 1017 | ||
| C. Development, total cost (A+B) | |||
| 7680 | |||
| Resource | Activity | Hours per activity | Costs (£, 2009–10 prices) |
|---|---|---|---|
| A. Core training: April – November 2007 | |||
| Academic Grade 8, point 37 | Arranging Leeds training event × 2 | 10 | 564 |
| Academic Grade 8, point 37 | Arranging Leeds travel and attendance × 2 | 10 | 564 |
| Academic Grade 8, point 37 | Arranging London training event × 2 | 10 | 564 |
| Academic Grade 8, point 37 | Arranging London travel and attendance × 2 | 10 | 564 |
| NHS Band 5, point 20 | Administration – training manuals composition | 35 | 859 |
| NHS Band 7, point 35a | Preparation time | 2.2 | 91 |
| NHS Band 7, point 35a | Preparation time | 1.9 | 78 |
| NHS Band 7, point 35a | Preparation time | 2.5 | 103 |
| Academic Grade 10, point 52a | Preparation time | 1.9 | 167 |
| Core training, total preparation cost | 3554 | ||
| B. Refresher training: August 2009 | |||
| Academic Grade 8, point 37 | Arranging London refresher event | 5 | 282 |
| Academic Grade 8, point 37 | Arranging London travel and attendance | 5 | 282 |
| NHS Band 4, point 14 | Administration – training manuals and compact disc, version 2 | 8 | 164 |
| NHS Band 7, point 35a | Preparation time | 0.2 | 8 |
| NHS Band 7, point 3a | Preparation time | 0.2 | 8 |
| Academic Grade 10, point 52a | Preparation time | 0.1 | 9 |
| Refresher training, total preparation cost | 753 | ||
| C. Preparation, total cost (A + B) | |||
| 4308 | |||
| Resource | Activity | Hours per activity | Costs (£, 2009–10 prices) |
|---|---|---|---|
| A. Core training: April to November 2007 | |||
| Trainers' time | |||
| Academic Grade 8, point 37 | Travel and attendance | 40 | 2256 |
| NHS Band 7, point 35 | Travel and attendance | 36 | 1487 |
| NHS Band 7, point 35 | Travel and attendance | 36 | 1487 |
| NHS Band 7, point 35 | Travel and attendance | 36 | 1487 |
| Academic Grade 10, point 52 | Travel and attendance | 40 | 3514 |
| A1. Total cost, trainers' time | 10,230 | ||
| Non-staff inputsa | |||
| Room hire and catering, Leeds day 1 | 760 | ||
| Room hire and catering, Leeds day 2 | 336 | ||
| Room hire and catering, London day 1 | 753 | ||
| Room hire and catering, London day 2 | 627 | ||
| Travel costs, trainers and attendees, Leeds day 1 | 1423 | ||
| Travel costs, trainers and attendees, Leeds day 2 | 1472 | ||
| Travel costs, trainers and attendees, London day 1 | 1242 | ||
| Travel costs, trainers and attendees, London day 2 | 1051 | ||
| Royal College of Nursing accreditation | 273 | ||
| Filming and production of training compact discs | 4097 | ||
| Copying compact discs | 140 | ||
| Printing of training manual files and paperwork | 284 | ||
| Printing of training records | 629 | ||
| A2. Total cost, non-staff inputsa | 13,086 | ||
| A3. Core training delivery, total cost (A1 + A2) | 23,317 | ||
| B. Refresher training: August 2009 | |||
| Academic Grade 8, point 37 | Travel and attendance | 15 | 846 |
| NHS Band 7, point 35 | Travel and attendance | 9 | 372 |
| NHS Band 7, point 35 | Travel and attendance | 9 | 372 |
| Academic Grade 10, point 52 | Travel and attendance | 15 | 1318 |
| NHS Band 7, point 31 | Travel and attendance | 8 | 290 |
| B1. Total cost, trainers' time | 3197 | ||
| Room hire and catering, London | 232 | ||
| Travel costs, trainers and attendees, London | 2175 | ||
| B2. Total cost, non-staff inputs | 2407 | ||
| B3. Refresher training delivery, total cost (B1 + B2) | 5603 | ||
| C. Delivery, total cost (A1 + A2 + B1 + B2) | |||
| 28,920 | |||
| Centre | Totala | Trainer 1, Academic Grade 8, point 37 | Trainer 2, NHS Band 7, point 31 | Staffing costs (£, 2009–10 prices) | Other costs (£, 2009–10 prices) | Costs (£, 2009–10 prices) |
|---|---|---|---|---|---|---|
| 3 | No. of visits | 2 | ||||
| Total delivery time (minutes) | 75 | |||||
| Total travel time (minutes) | 480 | |||||
| Total travel cost (£) | 160 | |||||
| Total cost (£) | 682 | 522 | 160 | 682 | ||
| 5 | No. of visits | 3 | ||||
| Total delivery time (minutes) | 60 | |||||
| Total travel time (minutes) | 720 | |||||
| Total travel cost (£) | 240 | |||||
| Total cost (£) | 973 | 733 | 240 | 973 | ||
| 1a | No. of visits | 2 | ||||
| Total delivery time (minutes) | 65 | |||||
| Total travel time (minutes) | 480 | |||||
| Total travel cost (£) | 160 | |||||
| Total cost (£) | 672 | 512 | 160 | 672 | ||
| 1b | No. of visits | 3 | ||||
| Total delivery time (minutes) | 45 | |||||
| Total travel time (minutes) | 720 | |||||
| Total travel cost (£) | 240 | |||||
| Total cost (£) | 959 | 719 | 240 | 959 | ||
| 15 | No. of visits | 2 | 2 | |||
| Total delivery time (minutes) | 60 | 90 | ||||
| Total travel time (minutes) | 480 | 480 | ||||
| Total travel cost (£) | 160 | 160 | ||||
| Total cost (£) | 668 | 502 | 850 | 320 | 1170 | |
| 13 | No. of visits | 2 | ||||
| Total delivery time (minutes) | 50 | |||||
| Total travel time (minutes) | 480 | |||||
| Total travel cost (£) | 160 | |||||
| Total cost (£) | 658 | 498 | 160 | 658 | ||
| 18 | No. of visits | 2 | ||||
| Total delivery time (minutes) | 40 | |||||
| Total travel time (minutes) | 480 | |||||
| Total travel cost (£) | 160 | |||||
| Total cost (£) | 649 | 489 | 160 | 649 | ||
| 7 | No. of visits | 2 | ||||
| Total delivery time (minutes) | 120 | |||||
| Total travel time (minutes) | 480 | |||||
| Total travel cost (£) | 160 | |||||
| Total cost (£) | 724 | 564 | 160 | 724 | ||
| 6 | No. of visits | 3 | ||||
| Total delivery time (minutes) | 140 | |||||
| Total travel time (minutes) | 720 | |||||
| Total travel cost (£) | 240 | |||||
| Total cost (£) | 1048 | 808 | 240 | 1048 | ||
| 12 | No. of visits | 2 | 3 | |||
| Total delivery time (minutes) | 105 | 105 | ||||
| Total travel time (minutes) | 480 | 720 | ||||
| Total travel cost (£) | 160 | 240 | ||||
| Total cost (£) | 710 | 735 | 1045 | 400 | 1445 | |
| 11 | No. of visits | 4 | ||||
| Total delivery time (minutes) | 180 | |||||
| Total travel time (minutes) | 960 | |||||
| Total travel cost (£) | 320 | |||||
| Total cost (£) | 1392 | 1072 | 320 | 1392 | ||
| 17 | No. of visits | 1 | 1 | |||
| Total delivery time (minutes) | 60 | 40 | ||||
| Total travel time (minutes) | 240 | 240 | ||||
| Total travel cost (£) | 80 | 80 | ||||
| Total cost (£) | 362 | 248 | 450 | 160 | 610 | |
| 4 | No. of visits | 1 | 1 | |||
| Total delivery time (minutes) | 45 | 30 | ||||
| Total travel time (minutes) | 240 | 240 | ||||
| Total travel cost (£) | 80 | 80 | ||||
| Total cost (£) | 348 | 242 | 430 | 160 | 590 | |
| 16 | No. of visits | 3 | ||||
| Total delivery time (minutes) | 95 | |||||
| Total travel time (minutes) | 720 | |||||
| Total travel cost (£) | 240 | |||||
| Total cost (£) | 1006 | 766 | 240 | 1006 | ||
| 14 | No. of visits | 2 | ||||
| Total delivery time (minutes) | 40 | |||||
| Total travel time (minutes) | 480 | |||||
| Total travel cost (£) | 160 | |||||
| Total cost (£) | 649 | 489 | 160 | 649 | ||
| 8 | No. of visits | 2 | ||||
| Total delivery time (minutes) | 60 | |||||
| Total travel time (minutes) | 480 | |||||
| Total travel cost (£) | 160 | |||||
| Total cost (£) | 668 | 508 | 160 | 668 | ||
| 10 | No. of visits | 4 | ||||
| Total delivery time (minutes) | 105 | |||||
| Total travel time (minutes) | 960 | |||||
| Total travel cost (£) | 320 | |||||
| Total cost (£) | 1321 | 1001 | 320 | 1321 | ||
| 9 | No. of visits | 2 | ||||
| Total delivery time (minutes) | 75 | |||||
| Total travel time (minutes) | 480 | |||||
| Total travel cost (£) | 160 | |||||
| Total cost (£) | 682 | 522 | 160 | 682 | ||
| 2 | No. of visits | 2 | ||||
| Total delivery time (minutes) | 90 | |||||
| Total travel time (minutes) | 480 | |||||
| Total travel cost (£) | 160 | |||||
| Total cost (£) | 696 | 536 | 160 | 696 | ||
| Delivery of local refresher training, total cost | 12,513 | 4080 | 16,593 | |||
| Delivery of local refresher training, average cost per centre | 659 | 215 | 873 | |||
| Centre | Totala,b | NHS Band 5 | NHS Band 6 | NHS Band 7 | NHS Band 8 | NHS consultant | NHS unknownc | Costs (£, 2009–10 prices) |
|---|---|---|---|---|---|---|---|---|
| 3 | n | 2 | 1 | 2 | 3 | |||
| Attendance time (minutes) | 660 | 300 | 660 | 1020 | ||||
| Total travel time (minutes) | 480 | 240 | 480 | 720 | ||||
| Cost (£) | 467 | 275 | 673 | 713 | 2129 | |||
| 5 | n | 1 | 2 | 1 | ||||
| Attendance time (minutes) | 360 | 660 | 360 | |||||
| Total travel time (minutes) | 240 | 480 | 240 | |||||
| Cost (£) | 306 | 673 | 246 | 1225 | ||||
| 1ad | n | 1 | 2.5 | 4 | ||||
| Attendance time (minutes) | 360 | 510 | 1230 | |||||
| Total travel time (minutes) | 240 | 600 | 960 | |||||
| Cost (£) | 246 | 566 | 1292 | 2104 | ||||
| 1bd | n | 1 | 2.5 | 4 | ||||
| Attendance time (minutes) | 360 | 510 | 1230 | |||||
| Total travel time (minutes) | 240 | 600 | 960 | |||||
| Cost (£) | 246 | 566 | 1292 | 2104 | ||||
| 15 | n | 2 | 1 | 1 | ||||
| Attendance time (minutes) | 660 | 360 | 360 | |||||
| Total travel time (minutes) | 480 | 240 | 240 | |||||
| Cost (£) | 467 | 306 | 558 | 1331 | ||||
| 13 | n | 1 | 2 | |||||
| Attendance time (minutes) | 360 | 660 | ||||||
| Total travel time (minutes) | 240 | 480 | ||||||
| Cost (£) | 246 | 1060 | 1306 | |||||
| 18 | n | 1 | 4 | |||||
| Attendance time (minutes) | 360 | 1320 | ||||||
| Total travel time (minutes) | 240 | 960 | ||||||
| Cost (£) | 246 | 1163 | 1409 | |||||
| 7 | n | 2 | 2 | 2 | ||||
| Attendance time (minutes) | 660 | 660 | 660 | |||||
| Total travel time (minutes) | 480 | 480 | 480 | |||||
| Cost (£) | 581 | 673 | 1060 | 2314 | ||||
| 6 | n | 2 | 2 | |||||
| Attendance time (minutes) | 660 | 660 | ||||||
| Total travel time (minutes) | 480 | 480 | ||||||
| Cost (£) | 467 | 673 | 1140 | |||||
| 12 | n | 2 | 1 | 1 | ||||
| Attendance time (minutes) | 660 | 360 | 360 | |||||
| Total travel time (minutes) | 480 | 240 | 240 | |||||
| Cost (£) | 581 | 354 | 1080 | 2015 | ||||
| 11 | n | 3 | ||||||
| Attendance time (minutes) | 960 | |||||||
| Total travel time (minutes) | 720 | |||||||
| Cost (£) | 857 | 857 | ||||||
| 17 | n | 3 | 1 | |||||
| Attendance time (minutes) | 1020 | 300 | ||||||
| Total travel time (minutes) | 720 | 240 | ||||||
| Cost (£) | 887 | 319 | 1206 | |||||
| 4 | n | 2 | 1 | |||||
| Attendance time (minutes) | 660 | 360 | ||||||
| Total travel time (minutes) | 480 | 240 | ||||||
| Cost (£) | 581 | 354 | 935 | |||||
| 16 | n | 1 | ||||||
| Attendance time (minutes) | 360 | |||||||
| Total travel time (minutes) | 240 | |||||||
| Cost (£) | 354 | 354 | ||||||
| 14 | n | 4 | 2 | |||||
| Attendance time (minutes) | 1320 | 720 | ||||||
| Total travel time (minutes) | 960 | 480 | ||||||
| Cost (£) | 1163 | 708 | 1871 | |||||
| 8 | n | 3 | ||||||
| Attendance time (minutes) | 1020 | |||||||
| Total travel time (minutes) | 720 | |||||||
| Cost (£) | 887 | 887 | ||||||
| 10 | n | 3 | 4 | |||||
| Attendance time (minutes) | 660 | 1380 | ||||||
| Total travel time (minutes) | 720 | 960 | ||||||
| Cost (£) | 704 | 1381 | 2084 | |||||
| 9 | n | 2 | 2 | |||||
| Attendance time (minutes) | 660 | 660 | ||||||
| Total travel time (minutes) | 480 | 480 | ||||||
| Cost (£) | 467 | 581 | 1049 | |||||
| 2 | n | 4 | ||||||
| Attendance time (minutes) | 1320 | |||||||
| Total travel time (minutes) | 960 | |||||||
| Cost(£) | 1345 | 1345 | ||||||
| Total cost for attending main training events | 27,667 | |||||||
| Centre | Totala,b | NHS Band 4 | NHS Band 5 | NHS Band 6 | NHS Band 7 | NHS Band 8 | Costs (£, 2009–10 prices) |
|---|---|---|---|---|---|---|---|
| 3 | n | ||||||
| Attendance time (minutes) | |||||||
| Total travel time (minutes) | |||||||
| Cost (£) | 0 | ||||||
| 5 | n | ||||||
| Attendance time (minutes) | |||||||
| Total travel time (minutes) | |||||||
| Cost (£) | 0 | ||||||
| 1a | n | ||||||
| Attendance time (minutes) | |||||||
| Total travel time (minutes) | |||||||
| Cost (£) | 0 | ||||||
| 1b | n | ||||||
| Attendance time (minutes) | |||||||
| Total travel time (minutes) | |||||||
| Cost (£) | 0 | ||||||
| 15 | n | ||||||
| Attendance time (minutes) | |||||||
| Total travel time (minutes) | |||||||
| Cost (£) | 0 | ||||||
| 13 | n | 1 | 1 | ||||
| Attendance time (minutes) | 360 | 360 | |||||
| Total travel time (minutes) | 240 | 240 | |||||
| Cost (£) | 246 | 306 | 552 | ||||
| 18 | n | 2 | |||||
| Attendance time (minutes) | 720 | ||||||
| Total travel time (minutes) | 480 | ||||||
| Cost (£) | 492 | 492 | |||||
| 7 | n | 1 | |||||
| Attendance time (minutes) | 360 | ||||||
| Total travel time (minutes) | 240 | ||||||
| Cost (£) | 354 | 354 | |||||
| 6 | n | 1 | 1 | ||||
| Attendance time (minutes) | 360 | 360 | |||||
| Total travel time (minutes) | 240 | 240 | |||||
| Cost (£) | 246 | 306 | 552 | ||||
| 12 | n | ||||||
| Attendance time (minutes) | |||||||
| Total travel time (minutes) | |||||||
| Cost (£) | 0 | ||||||
| 11 | n | 1 | |||||
| Attendance time (minutes) | 360 | ||||||
| Total travel time (minutes) | 240 | ||||||
| Cost (£) | 306 | 306 | |||||
| 17 | n | ||||||
| Attendance time (minutes) | |||||||
| Total travel time (minutes) | |||||||
| Cost (£) | 0 | ||||||
| 4 | n | ||||||
| Attendance time (minutes) | |||||||
| Total travel time (minutes) | |||||||
| Cost (£) | 0 | ||||||
| 16 | n | ||||||
| Attendance time (minutes) | |||||||
| Total travel time (minutes) | |||||||
| Cost (£) | 0 | ||||||
| 14 | n | 1 | 1 | ||||
| Attendance time (minutes) | 360 | 360 | |||||
| Total travel time (minutes) | 240 | 240 | |||||
| Cost (£) | 246 | 354 | 600 | ||||
| 8 | n | 1 | 1 | ||||
| Attendance time (minutes) | 360 | 360 | |||||
| Total travel time (minutes) | 240 | 240 | |||||
| Cost (£) | 204 | 306 | 510 | ||||
| 10 | n | 1 | 1 | ||||
| Attendance time (minutes) | 360 | 360 | |||||
| Total travel time (minutes) | 240 | 240 | |||||
| Cost (£) | 246 | 354 | 600 | ||||
| 9 | n | 1 | |||||
| Attendance time (minutes) | 360 | ||||||
| Total travel time (minutes) | 240 | ||||||
| Cost (£) | 306 | 306 | |||||
| 2 | n | 1 | |||||
| Attendance time (minutes) | 360 | ||||||
| Total travel time (minutes) | 240 | ||||||
| Cost (£) | 354 | 354 | |||||
| Total cost for attending refresher training event | 4626 | ||||||
| Centre | Total | NHS Band 4 | NHS Band 5 | NHS Band 6 | NHS Band 7 | NHS Band 8 | Researcher Grade 6 | Researcher Grade 8 | Costs (£, 2009–10 prices) |
|---|---|---|---|---|---|---|---|---|---|
| 3 | n | 5 | |||||||
| Attendance time (minutes) | 300 | ||||||||
| Cost (£) | 123 | 123 | |||||||
| 5 | n | ||||||||
| Attendance time (minutes) | |||||||||
| Cost (£) | 0 | ||||||||
| 1a | n | ||||||||
| Attendance time (minutes) | |||||||||
| Cost (£) | 0 | ||||||||
| 1b | n | ||||||||
| Attendance time (minutes) | |||||||||
| Cost (£) | 0 | ||||||||
| 15 | n | ||||||||
| Attendance time (minutes) | |||||||||
| Cost (£) | 0 | ||||||||
| 13 | n | 2 | |||||||
| Attendance time (minutes) | 120 | ||||||||
| Cost (£) | 112 | 112 | |||||||
| 18 | n | 14 | |||||||
| Attendance time (minutes) | 235 | ||||||||
| Cost (£) | 120 | 120 | |||||||
| 7 | n | 1 | 1 | ||||||
| Attendance time (minutes) | 60 | 60 | |||||||
| Cost (£) | 56 | 56 | 112 | ||||||
| 6 | n | 1 | 10 | ||||||
| Attendance time (minutes) | 240 | 480 | |||||||
| Cost (£) | 98 | 283 | 382 | ||||||
| 12 | n | 9 | |||||||
| Attendance time (minutes) | 320 | ||||||||
| Cost (£) | 163 | 163 | |||||||
| 11 | n | 4 | |||||||
| Attendance time (minutes) | 130 | ||||||||
| Cost (£) | 66 | 66 | |||||||
| 17 | n | ||||||||
| Attendance time (minutes) | |||||||||
| Cost (£) | 0 | ||||||||
| 4 | n | ||||||||
| Attendance time (minutes) | |||||||||
| Cost (£) | 0 | ||||||||
| 16 | n | ||||||||
| Attendance time (minutes) | |||||||||
| Cost (£) | 0 | ||||||||
| 14 | n | ||||||||
| Attendance time (minutes) | |||||||||
| Cost (£) | 0 | ||||||||
| 8 | n | 5 | |||||||
| Attendance time (minutes) | 140 | ||||||||
| Cost (£) | 71 | 71 | |||||||
| 10 | n | 4 | |||||||
| Attendance time (minutes) | 220 | ||||||||
| Cost (£) | 150 | 150 | |||||||
| 9 | n | 4 | 4 | ||||||
| Attendance time (minutes) | 160 | 150 | |||||||
| Cost (£) | 66 | 77 | 142 | ||||||
| 2 | n | 7 | |||||||
| Attendance time (minutes) | 230 | ||||||||
| Cost (£) | 136 | 136 | |||||||
| Total cost for delivering cascaded training | 1577 | ||||||||
| Centre | Totala | Student nurse | NHS Band 2 | NHS Band 3 | NHS Band 5 | NHS Band 6 | NHS Band 7 | NHS Band 8 | NHS consultant | NHS Bands 3–8b | NHS Bands 5–7b | NHS unknownb | Costs (£, 2009–10 prices) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | n | 9 | 11 | 16 | 1 | 2 | |||||||
| Attendance time (minutes) | 540 | 660 | 960 | 60 | 120 | ||||||||
| Cost (£) | 157 | 271 | 490 | 35 | 216 | 1168 | |||||||
| 5 | n | ||||||||||||
| Attendance time (minutes) | |||||||||||||
| Cost (£) | 0 | ||||||||||||
| 1a | n | ||||||||||||
| Attendance time (minutes) | |||||||||||||
| Cost (£) | 0 | ||||||||||||
| 1b | n | ||||||||||||
| Attendance time (minutes) | |||||||||||||
| Cost (£) | 0 | ||||||||||||
| 15 | n | ||||||||||||
| Attendance time (minutes) | |||||||||||||
| Cost (£) | 0 | ||||||||||||
| 13 | n | 6 | 2 | 9 | 6 | 4 | 2 | ||||||
| Attendance time (minutes) | 360 | 120 | 540 | 360 | 240 | 120 | |||||||
| Cost (£) | 90 | 35 | 221 | 184 | 142 | 112 | 783 | ||||||
| 18 | n | 14 | 4 | 3 | 2 | ||||||||
| Attendance time (minutes) | 280 | 115 | 45 | 65 | |||||||||
| Cost (£) | 115 | 59 | 27 | 27 | 227 | ||||||||
| 7 | n | 5 | 4 | ||||||||||
| Attendance time (minutes) | 300 | 240 | |||||||||||
| Cost (£) | 123 | 122 | 245 | ||||||||||
| 6 | n | 3 | 1 | 3 | 5 | 3 | 2 | ||||||
| Attendance time (minutes) | 720 | 30 | 540 | 225 | 225 | 135 | |||||||
| Cost (£) | 180 | 9 | 221 | 115 | 133 | 55 | 713 | ||||||
| 12 | n | 2 | 1 | 1 | 30 | 5 | 40 | ||||||
| Attendance time (minutes) | 30 | 15 | 15 | 2700 | 100 | 1200 | |||||||
| Cost (£) | 12 | 8 | 14 | 1593 | 51 | 492 | 2170 | ||||||
| 11 | n | 1 | 1 | 3 | |||||||||
| Attendance time (minutes) | 30 | 40 | 100 | ||||||||||
| Cost (£) | 12 | 20 | 41 | 74 | |||||||||
| 17 | n | ||||||||||||
| Attendance time (minutes) | |||||||||||||
| Cost (£) | 0 | ||||||||||||
| 4 | n | ||||||||||||
| Attendance time (minutes) | |||||||||||||
| Cost (£) | 0 | ||||||||||||
| 16 | n | ||||||||||||
| Attendance time (minutes) | |||||||||||||
| Cost (£) | 0 | ||||||||||||
| 14 | n | ||||||||||||
| Attendance time (minutes) | |||||||||||||
| Cost (£) | 0 | ||||||||||||
| 8 | n | 3 | 5 | 3 | 13 | 2 | 4 | 2 | |||||
| Attendance time (minutes) | 60 | 100 | 140 | 420 | 120 | 200 | 40 | ||||||
| Cost (£) | 14 | 25 | 41 | 172 | 61 | 118 | 16 | 448 | |||||
| 10 | n | 3 | 5 | 3 | |||||||||
| Attendance time (minutes) | 170 | 260 | 180 | ||||||||||
| Cost (£) | 43 | 107 | 92 | 241 | |||||||||
| 9 | n | 38 | |||||||||||
| Attendance time (minutes) | 1475 | ||||||||||||
| Cost (£) | 605 | 605 | |||||||||||
| 2 | n | 8 | 5 | 1 | |||||||||
| Attendance time (minutes) | 255 | 170 | 40 | ||||||||||
| Cost (£) | 105 | 87 | 16 | 208 | |||||||||
| Total cost for receiving cascaded training | 6881 | ||||||||||||
| Centre | Totala | NHS Band 2 | NHS Band 3 | NHS Band 5 | NHS Band 6 | NHS Band 7 | NHS Band 8 | NHS consultant | Senior house officer | Social worker | Cost (£, 2009–10 prices) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | n | 1 | 8 | 1 | 4 | 1 | |||||
| Attendance time (minutes) | 30 | 270 | 45 | 150 | 45 | ||||||
| Cost (£) | 8 | 111 | 23 | 89 | 81 | 311 | |||||
| 5 | n | 2 | 8 | 6 | 1 | 2 | |||||
| Attendance time (minutes) | 30 | 135 | 90 | 15 | 30 | ||||||
| Cost (£) | 12 | 69 | 53 | 27 | 20 | 181 | |||||
| 1a | n | 6 | 2 | 4 | |||||||
| Attendance time (minutes) | 90 | 90 | 130 | ||||||||
| Cost (£) | 37 | 46 | 77 | 160 | |||||||
| 1b | n | 4 | 3 | 2 | 2 | 2 | |||||
| Attendance time (minutes) | 50 | 45 | 25 | 25 | 25 | ||||||
| Cost (£) | 21 | 23 | 15 | 45 | 9 | 112 | |||||
| 15 | n | 2 | 1 | 8 | 2 | 1 | 2 | ||||
| Attendance time (minutes) | 50 | 20 | 200 | 60 | 30 | 60 | |||||
| Cost (£) | 13 | 6 | 82 | 31 | 18 | 56 | 204 | ||||
| 13 | n | 2 | 6 | ||||||||
| Attendance time (minutes) | 40 | 130 | |||||||||
| Cost (£) | 16 | 66 | 83 | ||||||||
| 18 | n | 4 | |||||||||
| Attendance time (minutes) | 80 | ||||||||||
| Cost (£) | 41 | 41 | |||||||||
| 7 | n | 1 | 3 | 2 | 2 | ||||||
| Attendance time (minutes) | 20 | 140 | 100 | 100 | |||||||
| Cost (£) | 5 | 57 | 59 | 93 | 214 | ||||||
| 6 | n | 1 | 7 | 7 | 1 | ||||||
| Attendance time (minutes) | 60 | 320 | 340 | 60 | |||||||
| Cost (£) | 15 | 131 | 201 | 40 | 386 | ||||||
| 12 | n | 2 | 1 | 16 | 7 | 3 | 2 | 1 | |||
| Attendance time (minutes) | 20 | 45 | 705 | 315 | 135 | 90 | 30 | ||||
| Cost (£) | 5 | 13 | 289 | 161 | 80 | 84 | 54 | 685 | |||
| 11 | n | 1 | 9 | ||||||||
| Attendance time (minutes) | 60 | 390 | |||||||||
| Cost (£) | 25 | 199 | 224 | ||||||||
| 17 | n | 1 | 6 | 5 | 1 | 2 | |||||
| Attendance time (minutes) | 40 | 240 | 240 | 40 | 100 | ||||||
| Cost (£) | 10 | 98 | 122 | 24 | 180 | 434 | |||||
| 4 | n | 1 | 6 | ||||||||
| Attendance time (minutes) | 30 | 195 | |||||||||
| Cost (£) | 12 | 99 | 112 | ||||||||
| 16 | n | 8 | 2 | 7 | 2 | ||||||
| Attendance time (minutes) | 260 | 65 | 225 | 65 | |||||||
| Cost (£) | 107 | 33 | 133 | 117 | 390 | ||||||
| 14 | n | 5 | 6 | 2 | 1 | 1 | |||||
| Attendance time (minutes) | 110 | 140 | 40 | 10 | 10 | ||||||
| Cost (£) | 28 | 71 | 24 | 18 | 7 | 147 | |||||
| 8 | n | 1 | 3 | ||||||||
| Attendance time (minutes) | 30 | 90 | |||||||||
| Cost (£) | 12 | 46 | 58 | ||||||||
| 10 | n | 4 | 4 | 5 | 4 | ||||||
| Attendance time (minutes) | 95 | 145 | 125 | 70 | |||||||
| Cost (£) | 24 | 59 | 64 | 41 | 188 | ||||||
| 9 | n | 1 | 3 | 5 | 2 | ||||||
| Attendance time (minutes) | 45 | 135 | 210 | 75 | |||||||
| Cost (£) | 11 | 55 | 107 | 70 | 243 | ||||||
| 2 | n | 1 | 4 | 3 | |||||||
| Attendance time (minutes) | 45 | 180 | 135 | ||||||||
| Cost (£) | 11 | 74 | 69 | 154 | |||||||
| Total cost for receiving further refresher training | 4327 | ||||||||||
| Centre | Total cost per centre (£, 2009–10 prices) |
|---|---|
| 3 | 3731 |
| 5 | 1406 |
| 1a | 2264 |
| 1b | 2216 |
| 15 | 1536 |
| 13 | 2836 |
| 18 | 2288 |
| 7 | 3240 |
| 6 | 3173 |
| 12 | 5034 |
| 11 | 1526 |
| 17 | 1640 |
| 4 | 1047 |
| 16 | 744 |
| 14 | 2618 |
| 8 | 1975 |
| 10 | 3263 |
| 9 | 2345 |
| 2 | 2196 |
| Total cost for ward staff | 45,077 |
| Average ward staff cost per centre | 2372 |
Appendix 7 Literature review search strategy
Note: Searching only for controlled trials.
Added relevant terms:
(community NEAR/2 network*)
(community NEAR/2 support*)
(support* NEAR/2 conversation*)
(patient NEAR/3 feedback)
(patient NEAR/3 education)
| ID | Search | Hits | Edit | Delete |
|---|---|---|---|---|
| #1 | Medical subject heading (MeSH) descriptor Cerebrovascular Disorders, this term only | 1339 | Edit | Delete |
| #2 | MeSH descriptor Basal Ganglia Cerebrovascular Disease explode all trees | 20 | Edit | Delete |
| #3 | MeSH descriptor Brain Ischemia explode all trees | 1862 | Edit | Delete |
| #4 | MeSH descriptor Carotid Artery Diseases explode all trees | 836 | Edit | Delete |
| #5 | MeSH descriptor Stroke, this term only | 3232 | Edit | Delete |
| #6 | MeSH descriptor Brain Infarction explode all trees | 624 | Edit | Delete |
| #7 | MeSH descriptor Cerebrovascular Trauma explode all trees | 19 | Edit | Delete |
| #8 | MeSH descriptor Hypoxia-Ischemia, Brain explode all trees | 87 | Edit | Delete |
| #9 | MeSH descriptor Intracranial Arterial Diseases explode all trees | 772 | Edit | Delete |
| #10 | MeSH descriptor Intracranial Arteriovenous Malformations, this term only | 43 | Edit | Delete |
| #11 | MeSH descriptor Intracranial Embolism and Thrombosis explode all trees | 230 | Edit | Delete |
| #12 | MeSH descriptor Intracranial Hemorrhages explode all trees | 1080 | Edit | Delete |
| #13 | MeSH descriptor Vasospasm, Intracranial, this term only | 84 | Edit | Delete |
| #14 | MeSH descriptor Vertebral Artery Dissection, this term only | 2 | Edit | Delete |
| #15 | MeSH descriptor Aneurysm, Ruptured, this term only | 91 | Edit | Delete |
| #16 | MeSH descriptor Brain Injuries, this term only | 745 | Edit | Delete |
| #17 | MeSH descriptor Brain Injury, Chronic, this term only | 25 | Edit | Delete |
| #18 | MeSH descriptor Carotid Arteries explode all trees | 883 | Edit | Delete |
| #19 | MeSH descriptor Endarterectomy, Carotid, this term only | 424 | Edit | Delete |
| #20 | MeSH descriptor Endarterectomy, this term only | 108 | Edit | Delete |
| #21 | MeSH descriptor Heart Septal Defects, Atrial, this term only | 95 | Edit | Delete |
| #22 | MeSH descriptor Atrial Fibrillation, this term only | 2056 | Edit | Delete |
| #23 | (brain* or cerebr* or cerebell* or cortical or vertebrobasilar or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or MCA or “anterior circulation” or “posterior circulation” or “basal ganglia”) and (ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* or hypox* or vasospasm or obstruction or vasculopathy) | 8321 | Edit | Delete |
| #24 | (“lacunar infarct*” or “cortical infarct*”) | 14 | Edit | Delete |
| #25 | (brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraventricular or infratentorial or supratentorial or “basal gangli*” or subarachnoid or putaminal or putamen or “posterior fossa”) and (haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed*) | 4420 | Edit | Delete |
| #26 | (“vertebral artery dissection” or “cerebral art* disease*”) | 39 | Edit | Delete |
| #27 | (brain or intracranial or “basal ganglia” or lenticulostriate) and (vascular) and (disease* or disorder or accident or injur* or trauma* or insult or event) | 1038 | Edit | Delete |
| #28 | (ischemic or ischaemic or apoplectic) and (event or events or insult or attack*) | 3721 | Edit | Delete |
| #29 | (“cerebral vein” or “cerebral venous” or sinus or sagittal) and (thrombo*) | 262 | Edit | Delete |
| #30 | (CVDST or CVT) | 37 | Edit | Delete |
| #31 | (intracranial or “cerebral art*” or “basilar art*” or “vertebral art*” or vertebrobasilar or “vertebral basilar”) and (stenosis or ischemia or ischaemia or insufficiency or arteriosclero* or atherosclero* or occlus*) | 937 | Edit | Delete |
| #32 | (venous or arteriovenous or “brain vasc*”) and malformation* | 199 | Edit | Delete |
| #33 | (brain or cerebral) and (angioma* or hemangioma* or haemangioma*) | 17 | Edit | Delete |
| #34 | (carotid*) | 3418 | Edit | Delete |
| #35 | (“patent foramen ovale” or PFO) | 112 | Edit | Delete |
| #36 | (atrial or atrium or auricular) and fibrillation | 3557 | Edit | Delete |
| #37 | (“asymptomatic cervical bruit”) | 7 | Edit | Delete |
| #38 | (aphasi* or apraxi* or dysphasi* or dysphagi* or “deglutition disorder*” or “swallow* disorder*” or dysarthri* or hemipleg* or hemipar* or paresis or paretic or hemianop* or hemineglect or spasticity or anomi* or dysnomi* or “acquired brain injur*” or hemiball* ) | 4485 | Edit | Delete |
| #39 | (unilateral or visual or hemispatial or attentional or spatial) and neglect | 313 | Edit | Delete |
| #40 | (brain or cerebral or intracranial or communicating or giant or basilar or “vertebral artery” or berry or saccular or ruptured) and aneurysm* | 1008 | Edit | Delete |
| #41 | MeSH descriptor Aphasia explode all trees | 131 | Edit | Delete |
| #42 | MeSH descriptor Anomia, this term only | 8 | Edit | Delete |
| #43 | MeSH descriptor Hemiplegia, this term only | 377 | Edit | Delete |
| #44 | MeSH descriptor Hemianopsia, this term only | 20 | Edit | Delete |
| #45 | MeSH descriptor Paresis explode all trees | 269 | Edit | Delete |
| #46 | MeSH descriptor Deglutition Disorders, this term only | 404 | Edit | Delete |
| #47 | MeSH descriptor Dysarthria, this term only | 34 | Edit | Delete |
| #48 | MeSH descriptor Pseudobulbar Palsy, this term only | 4 | Edit | Delete |
| #49 | MeSH descriptor Muscle Spasticity, this term only | 429 | Edit | Delete |
| #50 | (stroke or poststroke or post NEXT stroke or cerebrovasc* or “brain vasc*” or “cerebral vasc*” or cva* or apoplex* or “ischemi* attack*” or “ischaemi* attack*” or tia* or “neurologic* deficit*” or SAH or AVM) | 31,157 | Edit | Delete |
| #51 | (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50) | 43,189 | Edit | Delete |
| #52 | (SR-STROKE) | 15,097 | Edit | Delete |
| #53 | (#51 AND NOT #52) | 28,092 | Edit | Delete |
| #54 | MeSH descriptor Caregivers, this term only | 865 | Edit | Delete |
| #55 | MeSH descriptor Friends, this term only | 58 | Edit | Delete |
| #56 | MeSH descriptor Parents explode all trees | 2023 | Edit | Delete |
| #57 | MeSH descriptor Spouses, this term only | 170 | Edit | Delete |
| #58 | MeSH descriptor Visitors to Patients, this term only | 22 | Edit | Delete |
| #59 | MeSH descriptor Home Nursing, this term only | 269 | Edit | Delete |
| #60 | MeSH descriptor Community Networks, this term only | 79 | Edit | Delete |
| #61 | MeSH descriptor Parent-Child Relations explode all trees | 968 | Edit | Delete |
| #62 | MeSH descriptor Interpersonal Relations explode all trees | 3269 | Edit | Delete |
| #63 | MeSH descriptor Family, this term only | 809 | Edit | Delete |
| #64 | MeSH descriptor Family Characteristics explode all trees | 594 | Edit | Delete |
| #65 | MeSH descriptor Family Relations, this term only | 117 | Edit | Delete |
| #66 | MeSH descriptor Intergenerational Relations, this term only | 21 | Edit | Delete |
| #67 | MeSH descriptor Family Therapy, this term only | 573 | Edit | Delete |
| #68 | MeSH descriptor Family Nursing, this term only | 15 | Edit | Delete |
| #69 | MeSH descriptor Family Health, this term only | 301 | Edit | Delete |
| #70 | (carer* or caregiv* or “care giv*” or care NEXT giv*) | 4765 | Edit | Delete |
| #71 | (family or families or spous* or parent or parents or father* or mother* or friend or friends or husband* or wife or wives or partner or partners) | 30,648 | Edit | Delete |
| #72 | (home or communit*) and (caring or care*) or (community NEAR/2 network*) | 16,360 | Edit | Delete |
| #73 | (home NEXT based) | 1559 | Edit | Delete |
| #74 | (community NEXT based) or (community NEAR/2 network*) | 3323 | Edit | Delete |
| #75 | (homebased or communitybased) | 1069 | Edit | Delete |
| #76 | “home nursing” | 367 | Edit | Delete |
| #77 | (non NEXT professional) and (care or nursing) | 78 | Edit | Delete |
| #78 | (nonprofessional or informal or unpaid) and (care or nursing) | 728 | Edit | Delete |
| #79 | “next of kin” or (relatives) | 33,281 | Edit | Delete |
| #80 | (#54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63 OR #64 OR #65 OR #66 OR #67 OR #68 OR #69 OR #70 OR #71 OR #72 OR #73 OR #74 OR #75 OR #76 OR #77 OR #78 OR #79) | 73,994 | Edit | Delete |
| #81 | (#53 AND #80) | 5562 | Edit | Delete |
| #82 | MeSH descriptor Community Networks, this term only | 79 | Edit | Delete |
| #83 | MeSH descriptor Social Support, this term only | 1862 | Edit | Delete |
| #84 | MeSH descriptor Social Isolation, this term only | 117 | Edit | Delete |
| #85 | MeSH descriptor Social Welfare, this term only | 39 | Edit | Delete |
| #86 | MeSH descriptor Patient Education as Topic, this term only | 5338 | Edit | Delete |
| #87 | MeSH descriptor Professional-Family Relations, this term only | 110 | Edit | Delete |
| #88 | MeSH descriptor Altruism, this term only | 29 | Edit | Delete |
| #89 | MeSH descriptor Helping Behavior, this term only | 52 | Edit | Delete |
| #90 | MeSH descriptor Social Adjustment, this term only | 753 | Edit | Delete |
| #91 | MeSH descriptor Adaptation, Psychological, this term only | 2471 | Edit | Delete |
| #92 | MeSH descriptor Stress, Psychological, this term only | 2751 | Edit | Delete |
| #93 | MeSH descriptor Anxiety, this term only | 4079 | Edit | Delete |
| #94 | MeSH descriptor Depression, this term only | 4232 | Edit | Delete |
| #95 | MeSH descriptor Emotions, this term only | 1534 | Edit | Delete |
| #96 | MeSH descriptor Family, this term only with qualifier: PX | 318 | Edit | Delete |
| #97 | MeSH descriptor Respite Care, this term only | 26 | Edit | Delete |
| #98 | MeSH descriptor Day Care, this term only | 262 | Edit | Delete |
| #99 | (attitude* or perception* or belief* or expectation* or satisfaction or emotion* or relationship* or support* or control or adjust* or guid* or information or advi* or help* or train*) and (carer* or caregiv* or “care giv*”) | 4444 | Edit | Delete |
| #100 | (attitude* or perception* or belief* or expectation* or satisfaction or emotion* or relationship* or support* or control or adjust* or guid* or information or advi* or help* or train*) and (care NEXT giv*) | 482 | Edit | Delete |
| #101 | (anxiet* or stress* or fatigue* or resent* or burden* or cope* or coping) | 50,135 | Edit | Delete |
| #102 | (moral*) and (oblig* or duty or duties or responsibilit*) | 75 | Edit | Delete |
| #103 | (social or psychosocial or practical or group*) and (information or advice or help or support or network) | 200,444 | Edit | Delete |
| #104 | “post discharge” or postdischarge | 708 | Edit | Delete |
| #105 | (respite) | 111 | Edit | Delete |
| #106 | MeSH descriptor Quality of Life, this term only | 11,312 | Edit | Delete |
| #107 | (health or problem* or mood*) and (carer* or caregiv* or “care giv*”) | 3292 | Edit | Delete |
| #108 | (health or problem* or mood*) and (care NEXT giv*) | 421 | Edit | Delete |
| #109 | MeSH descriptor Self-Help Groups, this term only | 495 | Edit | Delete |
| #110 | “self help” NEXT group* | 626 | Edit | Delete |
| #111 | (selfhelp NEXT group*) | 28 | Edit | Delete |
| #112 | (self NEXT “help group”) | 86 | Edit | Delete |
| #113 | (community NEAR/2 network*) | 118 | Edit | Delete |
| #114 | (community NEAR/2 support*) | 226 | Edit | Delete |
| #115 | (support* NEAR/2 conversation*) | 3 | Edit | Delete |
| #116 | (patient NEAR/3 feedback) | 259 | Edit | Delete |
| #117 | (patient NEAR/3 education) | 7378 | Edit | Delete |
| #118 | (#82 OR #83 OR #84 OR #85 OR #86 OR #87 OR #88 OR #89 OR #90 OR #91 OR #92 OR #93 OR #94 OR #95 OR #96 OR #97 OR #98 OR #99 OR #100 OR #101 OR #102 OR #103 OR #104 OR #105 OR #106 OR #107 OR #108 OR #109 OR #110 OR #111 OR #112) | 23,4748 | Edit | Delete |
Appendix 8 Appendix to Chapter 4
Completion of London Stroke Carers Training Course training records
| Training components (mandatory) | Registered (N = 450), n (%) |
|---|---|
| Carer has demonstrated understanding of: | |
| 1. Relative having a stroke | |
| Achieved | 232 (51.6) |
| Not achieved | 94 (20.9) |
| Training record not completed | 124 (27.6) |
| 2. What a stroke is | |
| Achieved | 211 (46.9) |
| Not achieved | 115 (25.6) |
| Training record not completed | 124 (27.6) |
| 4. Healthy lifestyle and prevention | |
| Achieved | 220 (48.9) |
| Not achieved | 106 (23.6) |
| Training record not completed | 124 (27.6) |
| 12. Compliance with medication | |
| Achieved | 146 (32.4) |
| Not achieved | 180 (40.0) |
| Training record not completed | 124 (27.6) |
| 13. Post-discharge recommendations and help | |
| Achieved | 199 (44.2) |
| Not achieved | 127 (28.2) |
| Training record not completed | 124 (27.6) |
| 14. Knowledge and skills to home environment | |
| Achieved | 160 (35.6) |
| Not achieved | 166 (36.9) |
| Training record not completed | 124 (27.6) |
| No. of components achieved | Registered (N = 450), n (%) | Cumulative N patients (%) |
|---|---|---|
| 8 | 6 (1.3) | 6 (1.3) |
| 7 | 12 (2.7) | 18 (4.0) |
| 6 | 30 (6.7) | 48 (10.7) |
| 5 | 31 (6.9) | 79 (17.6) |
| 4 | 56 (12.4) | 135 (30.0) |
| 3 | 47 (10.4) | 182 (40.4) |
| 2 | 60 (13.3) | 242 (53.8) |
| 1 | 41 (9.1) | 283 (62.9) |
| 0 | 3 (0.7) | 286 (63.6) |
| Missing | 164 (36.4) | 450 (100.0) |
| Training components (non-mandatory) | Registered (N = 450), n (%) |
|---|---|
| 3. Specific stroke-related problems | |
| Appropriate and achieved | 204 (45.3) |
| Appropriate but not achieved | 5 (1.1) |
| Not completed | 79 (17.6) |
| Not appropriate | 38 (8.4) |
| No training record returned | 124 (27.6) |
| 5. Dietary needs and feeding techniques | |
| Appropriate and achieved | 75 (16.7) |
| Not completed | 20 (4.4) |
| Not appropriate | 231 (51.3) |
| No training record returned | 124 (27.6) |
| 6. Communication with dysphasic relative | |
| Appropriate and achieved | 65 (14.4) |
| Not completed | 13 (2.9) |
| Not appropriate | 248 (55.1) |
| No training record returned | 124 (27.6) |
| 7. Managing washing and dressing | |
| Appropriate and achieved | 167 (37.1) |
| Appropriate but not achieved | 1 (0.2) |
| Not completed | 30 (6.7) |
| Not appropriate | 128 (28.4) |
| No training record returned | 124 (27.6) |
| 8. Limb positioning and skin integrity | |
| Appropriate and achieved | 131 (29.1) |
| Appropriate but not achieved | 1 (0.2) |
| Not completed | 25 (5.6) |
| Not appropriate | 169 (37.6) |
| No training record returned | 124 (27.6) |
| 9. Continence management | |
| Appropriate and achieved | 60 (13.3) |
| Not completed | 17 (3.8) |
| Not appropriate | 249 (55.3) |
| No training record returned | 124 (27.6) |
| 10. Bowel management | |
| Appropriate and achieved | 54 (12.0) |
| Appropriate but not achieved | 1 (0.2) |
| Not completed | 19 (4.2) |
| Not appropriate | 252 (56.0) |
| No training record returned | 124 (27.6) |
| 11. Assisting mobility and safe transfers | |
| Appropriate and achieved | 239 (53.1) |
| Not completed | 26 (5.8) |
| Not appropriate | 61 (13.6) |
| No training record returned | 124 (27.6) |
| Centre | Yes, n (%) | No, n (%) | Not required, n (%) | Confirmed not completed by site, n (%) | Confirmed lost by site, n (%) | Time logs not completed, n (%) | Total, n |
|---|---|---|---|---|---|---|---|
| Control | 211 (44.1) | 4 (0.8) | 174 (36.4) | 78 (16.3) | 9 (1.9) | 2 (0.4) | 478 |
| 19 | 17 (50.0) | 0 (0.0) | 14 (41.2) | 3 (8.8) | 0 (0.0) | 0 (0.0) | 34 |
| 20 | 18 (66.7) | 0 (0.0) | 3 (11.1) | 6 (22.2) | 0 (0.0) | 0 (0.0) | 27 |
| 21 | 9 (29.0) | 0 (0.0) | 19 (61.3) | 3 (9.7) | 0 (0.0) | 0 (0.0) | 31 |
| 22 | 9 (50.0) | 0 (0.0) | 9 (50.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 18 |
| 23 | 14 (56.0) | 3 (12.0) | 7 (28.0) | 0 (0.0) | 0 (0.0) | 1 (4.0) | 25 |
| 24 | 1 (8.3) | 0 (0.0) | 2 (16.7) | 9 (75.0) | 0 (0.0) | 0 (0.0) | 12 |
| 25 | 17 (44.7) | 1 (2.6) | 20 (52.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 38 |
| 26 | 12 (52.2) | 0 (0.0) | 8 (34.8) | 3 (13.0) | 0 (0.0) | 0 (0.0) | 23 |
| 27 | 11 (31.4) | 0 (0.0) | 14 (40.0) | 10 (28.6) | 0 (0.0) | 0 (0.0) | 35 |
| 28 | 7 (30.4) | 0 (0.0) | 5 (21.7) | 11 (47.8) | 0 (0.0) | 0 (0.0) | 23 |
| 29 | 16 (53.3) | 0 (0.0) | 12 (40.0) | 0 (0.0) | 1 (3.3) | 1 (3.3) | 30 |
| 30 | 1 (7.7) | 0 (0.0) | 3 (23.1) | 9 (69.2) | 0 (0.0) | 0 (0.0) | 13 |
| 31 | 12 (46.2) | 0 (0.0) | 9 (34.6) | 5 (19.2) | 0 (0.0) | 0 (0.0) | 26 |
| 32 | 17 (68.0) | 0 (0.0) | 7 (28.0) | 0 (0.0) | 1 (4.0) | 0 (0.0) | 25 |
| 33 | 9 (52.9) | 0 (0.0) | 2 (11.8) | 0 (0.0) | 6 (35.3) | 0 (0.0) | 17 |
| 34 | 11 (32.4) | 0 (0.0) | 14 (41.2) | 9 (26.5) | 0 (0.0) | 0 (0.0) | 34 |
| 35 | 15 (46.9) | 0 (0.0) | 15 (46.9) | 1 (3.1) | 1 (3.1) | 0 (0.0) | 32 |
| 36 | 15 (42.9) | 0 (0.0) | 11 (31.4) | 9 (25.7) | 0 (0.0) | 0 (0.0) | 35 |
FIGURE 19.
Number of training sessions undertaken by carers: intervention. Missing = there is no information about the number of training sessions.
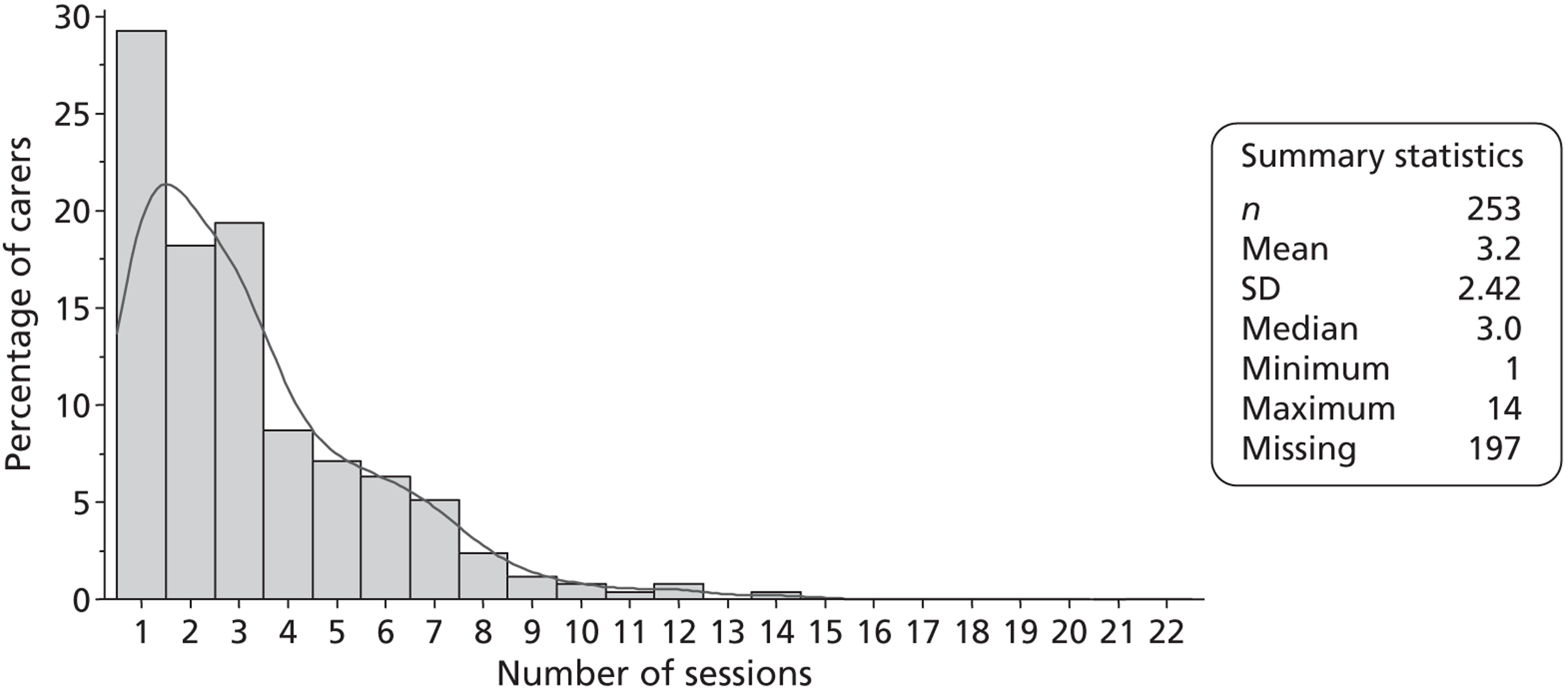
Patient safety
| SRU | No. registered | Patients with falls, n (%) | Patients with SAEs, n (%) |
|---|---|---|---|
| Intervention | 450 | 35 (7.8) | 2 (0.4) |
| 1 | 20 | 1 (5.0) | 0 (0.0) |
| 2 | 21 | 1 (4.8) | 0 (0.0) |
| 3 | 24 | 2 (8.3) | 0 (0.0) |
| 4 | 30 | 2 (6.7) | 0 (0.0) |
| 5 | 32 | 1 (3.1) | 0 (0.0) |
| 6 | 32 | 1 (3.1) | 0 (0.0) |
| 7 | 27 | 0 (0.0) | 0 (0.0) |
| 8 | 23 | 5 (21.7) | 0 (0.0) |
| 9 | 29 | 3 (10.3) | 0 (0.0) |
| 10 | 25 | 1 (4.0) | 1 (4.0) |
| 11 | 16 | 1 (6.3) | 0 (0.0) |
| 12 | 35 | 4 (11.4) | 0 (0.0) |
| 13 | 14 | 2 (14.3) | 0 (0.0) |
| 14 | 35 | 4 (11.4) | 0 (0.0) |
| 15 | 19 | 0 (0.0) | 0 (0.0) |
| 16 | 17 | 3 (17.6) | 1 (5.9) |
| 17 | 25 | 2 (8.0) | 0 (0.0) |
| 18 | 26 | 2 (7.7) | 0 (0.0) |
| Control | 478 | 35 (7.3) | 3 (0.6) |
| 19 | 34 | 2 (5.9) | 0 (0.0) |
| 20 | 27 | 5 (18.5) | 0 (0.0) |
| 21 | 31 | 6 (19.4) | 0 (0.0) |
| 22 | 18 | 3 (16.7) | 0 (0.0) |
| 23 | 25 | 0 (0.0) | 0 (0.0) |
| 24 | 12 | 1 (8.3) | 1 (8.3) |
| 25 | 38 | 2 (5.3) | 0 (0.0) |
| 26 | 23 | 6 (26.1) | 0 (0.0) |
| 27 | 35 | 4 (11.4) | 1 (2.9) |
| 28 | 23 | 0 (0.0) | 0 (0.0) |
| 29 | 30 | 1 (3.3) | 0 (0.0) |
| 30 | 13 | 0 (0.0) | 0 (0.0) |
| 31 | 26 | 0 (0.0) | 0 (0.0) |
| 32 | 25 | 0 (0.0) | 0 (0.0) |
| 33 | 17 | 0 (0.0) | 0 (0.0) |
| 34 | 34 | 1 (2.9) | 0 (0.0) |
| 35 | 32 | 1 (3.1) | 0 (0.0) |
| 36 | 35 | 3 (8.6) | 1 (2.9) |
| SAE criteria | Description of fall | |
|---|---|---|
| 1 | Significantly or permanently disabling or incapacitating | Fainted while in bathroom, low BP 88/46 mmHg |
| 2 | Prolonged hospitalisation | Patient found on floor near bed, patient not attempting to get up, appeared confused. Bruising to arm and face |
| 3 | Prolonged hospitalisation | No description or explanation possible. X-ray showed fracture of distal clavicle |
| 4 | Prolonged hospitalisation | Patient found on floor in bathroom – confirmed with fractured hip. Has had dynamic hip screw |
| 5 | Life threatening | Patient was due to be discharged that day – fell while standing leaning on a table. Fractured neck of femur – acute hospitalisation for repair and surgery. Then on 25 August 2009 was transferred to Minehead hospital for rehabilitation after surgery |
Sensitivity analyses
| Patients' primary outcome at 6 months – adjusted scores | |||||||
|---|---|---|---|---|---|---|---|
| Sensitivity analyses | Intervention, mean (SE), n | Control, mean (SE), n | Difference (SE) | 95% CI of the difference | p-value | Unadjusted | Adjusted |
| ICC | ICC | ||||||
| Patients that died (NEADL = 0) | 24.2 (0.97) 370 | 25.1 (0.96) 384 | –0.9 (1.30) | (–3.5 to 1.8) | 0.507 | 0.016 | 0.027 |
| Proxy responses excluded | 28.2 (1.08) 315 | 28.6 (1.07) 326 | –0.4 (1.45) | (–3.4 to 2.5) | 0.766 | 0.017 | 0.038 |
| Questionnaire | Intervention, mean (SD), n | Control, mean (SD), n | Difference (95% CI) | p-value |
|---|---|---|---|---|
| Patients | 201.6 (14.08) 303 | 202.8 (14.13) 338 | –1.2 (–3.4 to 1.0) | 0.2862 |
| Caregivers | 201.6 (14.28) 304 | 201.6 (13.12) 320 | 0 (–2.1 to 2.2) | 0.9836 |
Additional follow-up data
| Additional questions | Intervention (N = 450), n (%) | Control (N = 478), n (%) | Total (N = 928), n (%) |
|---|---|---|---|
| Do you think your treatment was different because of the research? | |||
| Yes | 34 (7.6) | 42 (8.8) | 76 (8.2) |
| No | 91 (20.2) | 94 (19.7) | 185 (19.9) |
| Not sure | 145 (32.2) | 154 (32.2) | 299 (32.2) |
| Missing | 180 (40.0) | 188 (39.3) | 368 (39.7) |
| If yes, how do you think the treatment you received was different? | |||
| Much better | 14 (41.2) | 17 (40.5) | 31 (40.8) |
| Better | 14 (41.2) | 23 (54.8) | 37 (48.7) |
| Unsure | 5 (14.7) | 2 (4.8) | 7 (9.2) |
| Worse | 1 (2.9) | 0 (0.0) | 1 (1.3) |
| Additional questions | Intervention (N = 450), n (%) | Control (N = 478), n (%) | Total (N = 928), n (%) |
|---|---|---|---|
| Do you feel adequately prepared to care for your relative/friend? | |||
| Yes | 229 (50.9) | 251 (52.5) | 480 (51.7) |
| No | 103 (22.9) | 93 (19.5) | 196 (21.1) |
| Missing | 118 (26.2) | 134 (28.0) | 252 (27.2) |
| Are you still caring for your relative/friend? | |||
| Yes | 296 (65.8) | 323 (67.6) | 619 (66.7) |
| No | 37 (8.2) | 25 (5.2) | 62 (6.7) |
| Missing | 117 (26.0) | 130 (27.2) | 247 (26.6) |
| I have received enough information about stroke recovery and rehabilitation | |||
| Yes | 255 (56.7) | 255 (53.3) | 510 (55.0) |
| No | 72 (16.0) | 84 (17.6) | 156 (16.8) |
| Missing | 123 (27.3) | 139 (29.1) | 262 (28.2) |
| Additional questions | Intervention (N = 450), n (%) | Control (N = 478), n (%) | Total (N = 928), n (%) |
|---|---|---|---|
| Are you still caring for your relative/friend? | |||
| Yes | 266 (59.1) | 284 (59.4) | 550 (59.3) |
| No | 20 (4.4) | 20 (4.2) | 40 (4.3) |
| Missing | 164 (36.4) | 174 (36.4) | 338 (36.4) |
| Do you think your treatment was different because of the research? | |||
| Yes | 28 (6.2) | 42 (8.8) | 70 (7.5) |
| No | 99 (22.0) | 95 (19.9) | 194 (20.9) |
| Not sure | 152 (33.8) | 168 (35.1) | 320 (34.5) |
| Missing | 171 (38.0) | 173 (36.2) | 344 (37.1) |
| If yes how do you think the treatment you received was different? | |||
| Much better | 9 (32.1) | 19 (45.2) | 28 (40.0) |
| Better | 12 (42.9) | 18 (42.9) | 30 (42.9) |
| Unsure | 4 (14.3) | 3 (7.1) | 7 (10.0) |
| Worse | 3 (10.7) | 1 (2.4) | 4 (5.7) |
| Missing | 0 (0.0) | 1 (2.4) | 1 (1.4) |
| Intervention centre no. | Hospital type | Unit type | Total bed no., 2007 | Stroke rehabilitation bed no. | 2006 audit positionb | 2008 audit positionb | Eligibilitya | Community team | Early supported discharge | Significant changes during trial | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2007 | 2009 | 2007 | 2009 | 2007 | 2009 | 2007 | 2009 | |||||||
| 1 | Acute | R | 14 | 14 | 14 | N/A | Mid | 5 | 5 | ✗ | ✗ | ✗ | ✗ | ✗ |
| 2 | Comm | R | 23 | 11 | 23 | Lower | Lower | 5 | 5 | ✗ | ✗ | ✗ | ✗ | ✗ |
| 3 | Acute | R | 20 | 14 | 14 | Upper | Mid | 5 | 5 | ✗ | ✗ | ✗ | ✗ | ✗ |
| 4 | Acute | A + R | 26 | 23 | 23 | Mid | Lower | 5 | 5 | ✗ | ✗ | ✗ | ✓ | ✗ |
| 5 | Acute | A + R | 20 | 20 | 20 | N/A | Lower | 5 | 5 | ✗ | ✗ | ✗ | ✗ | ✗ |
| 6 | Comm | R | 15 | 14 | 14 | Mid | Mid | 4 | 5 | ✓ | ✓ | ✗ | ✗ | ✗ |
| 7 | Acute | R | 18 | 18 | 18 | Upper | Upper | 5 | 5 | ✗ | ✗ | ✗ | ✓ | ✗ |
| 8 | Acute | A + R | 24 | 24 | 18 | Mid | Mid | 5 | 5 | ✗ | ✗ | ✗ | ✗ | ✗ |
| 9 | Comm | R | 15 | 15 | 15 | Upper | Upper | 4 | 4 | ✓ | ✓ | ✗ | ✓ | ✗ |
| 10 | Acute | A + R | 27 | 16 | 14 | Mid | Upper | 5 | 5 | ✗ | ✗ | ✗ | ✗ | ✗ |
| 11 | Acute | A + R | 16 | 11 | 11 | Lower | Mid | 4 | 5 | ✗ | ✗ | ✗ | ✗ | ✓ |
| 12 | Comm c | R | 30 | 30 | 17 | Upper | Upper | 5 | 5 | ✓ | ✓ | ✗ | ✗ | ✓ |
| 13 | Comm | R | 20 | 14 | 14 | Mid | Upper | 5 | 5 | ✗ | ✓ | ✓ | ✓ | ✗ |
| 14 | Acute | R | 24 | 24 | 24 | Mid | Mid | 5 | 5 | ✗ | ✗ | ✗ | ✗ | ✗ |
| 15 | Acute | A + R | 24 | 20 | 19 | Upper | Upper | 5 | 5 | ✓ | ✓ | ✗ | ✓ | ✓ |
| 16 | Acute | R | 30 | 10 | 8 | Mid | Lower | 5 | 5 | ✗ | ✗ | ✗ | ✗ | ✗ |
| 17 | Acute | A + R | 24 | 16 | 16 | Lower | Upper | 5 | 5 | ✗ | ✗ | ✗ | ✗ | ✗ |
| 18 | Acute | R | 19 | 19 | 19 | Lower | Mid | 4 | 4 | ✓ | ✓ | ✗ | ✗ | ✗ |
| Summary [totals or means (SD)] | 13 Acute | 7 A + R | 22 (4.96) | 17 (5.35) | 17 (4.24) | 5 Upper | 7 Upper | 5 | 6 | 4 | 3 | 2 | ||
| 7 Mid | 7 Mid | |||||||||||||
| 5 Comm | 11 R | 4 Lower | 4 Lower | |||||||||||
| 19 | Acute | A + R | 24 | 24 | 24 | Lower | Upper | 4 | 4 | ✗ | ✗ | ✗ | ✗ | ✗ |
| 20 | Acute | A + R | 31 | 16 | 16 | Lower | Lower | 4 | 5 | ✗ | ✓ | ✗ | ✓ | ✗ |
| 21 | Comm | R | 23 | 15 | 15 | N/A | Mid | 4 | 4 | ✗ | ✗ | ✗ | ✗ | ✗ |
| 22 | Acute | R | 25 | 10 | 10 | Upper | Upper | 5 | 5 | ✗ | ✗ | ✗ | ✗ | ✗ |
| 23 | Acute | A + R | 23 | 23 | 23 | Mid | Mid | 4 | 5 | ✗ | ✗ | ✗ | ✗ | ✗ |
| 24 | Acute | A + R | 23 | 17 | 15 | Mid | Upper | 5 | 5 | ✗ | ✗ | ✗ | ✗ | ✗ |
| 25 | Acute | A + R | 20 | 12 | 12 | Upper | Upper | 5 | 5 | ✓ | ✓ | ✓ | ✓ | ✗ |
| 26 | Comm | R | 20 | 20 | 16 | Upper | Upper | 5 | 5 | ✓ | ✓ | ✗ | ✓ | ✗ |
| 27 | Comm | R | 24 | 24 | 19 | Mid | Lower | 5 | 5 | ✗ | ✗ | ✗ | ✗ | ✗ |
| 28 | Acute | A + R | 56 | 34 | 34 | Mid | Mid | 5 | 5 | ✗ | ✗ | ✓ | ✓ | ✗ |
| 29 | Acute | A + R | 26 | 16 | 16 | N/A | Lower | 5 | 5 | ✗ | ✗ | ✗ | ✗ | ✗ |
| 30 | Acute | A + R | 21 | 14 | 14 | Lower | Mid | 5 | 5 | ✗ | ✗ | ✗ | ✗ | ✗ |
| 31 | Acute | R | 15 | 15 | 15 | Upper | Upper | 5 | 5 | ✗ | ✓ | ✗ | ✗ | ✓ |
| 32 | Acute | A + R | 28 | 16 | 16 | Mid | Mid | 5 | 4 | ✗ | ✗ | ✓ | ✓ | ✗ |
| 33 | Acute | A + R | 20 | 12 | 12 | Upper | Upper | 5 | 5 | ✗ | ✗ | ✗ | ✗ | ✓ |
| 34 | Acute | A + R | 29 | 17 | 17 | Upper | Upper | 5 | 5 | ✗ | ✗ | ✗ | ✓ | ✗ |
| 35 | Acute | A + R | 18 | 18 | 14 | Mid | Mid | 5 | 5 | ✗ | ✗ | ✗ | ✗ | ✗ |
| 36 | Comm | R | 20 | 20 | 20 | Mid | Lower | 4 | 5 | ✗ | ✗ | ✗ | ✗ | ✗ |
| Summary [totals or means (SD)] | 14 Acute | 12 A + R | 25 (8.75) | 18 (5.66) | 17 (5.52) | 6 Upper | 8 Upper | 2 | 4 | 3 | 6 | 2 | ||
| 7 Mid | 6 Mid | |||||||||||||
| 4 Comm | 6 R | 4 Lower | 4 Lower | |||||||||||
| Change to process of care | |
|---|---|
| Centre: 11 | |
| Change | Organisational restructure from combined stroke unit to acute unit with community hospital rehabilitation unit |
| Date | January 2009 |
| Description | The stroke unit changed from a combined acute and rehabilitation unit to a primarily acute unit (short stay or short-term rehabilitation only), with a new long-term rehabilitation unit in a separate community hospital in a nearby town |
| Impact | Few patients on the stroke unit were eligible for TRACS owing to the short length of stay, or transfer to another unit rather than discharge home. The new rehabilitation unit did not meet the stroke unit eligibility criteria, so could not be included in TRACS. MDT staff on the stroke unit continued with the LSCTC where possible, but very few patients were eligible for the intervention. Few patients' caregivers were given any training prior to discharge, and few patients and caregivers were eligible for TRACS recruitment |
| Change to organisational structure of the stroke unit, not affecting usual process of care | |
| Centre: 33 | |
| Change | Merging of a primary care trust rehabilitation unit with the acute NHS trust combined stroke unit |
| Date | April 2008 |
| Description | A number of patients admitted to 33 began to be transferred to the second hospital for stroke rehabilitation, as this was now a part of the same NHS trust. This reduced the numbers of eligible TRACS patients at 33, as they were no longer meeting the eligibility criteria of returning home from the participating stroke unit. The second stroke unit met the trial criteria and staff rotated between the two units, had the same training and shared the same stroke care processes. The second centre was included in TRACS as a part of centre 33 |
| Impact | Recruitment was able to continue |
| Centre: 31 | |
| Change | Loss of the acute stroke unit within the hospital |
| Date | October 2007 (pre-recruitment) |
| Description | The acute stroke unit at centre 31 was closed and all strokes admitted to the acute unit at the nearby centre 22. Both units had separate rehabilitation stroke units with independent staff, who were also independent to the acute unit. Following admission to the acute unit, patients were transferred to one of the rehabilitation units at centre 22 or 31 |
| Impact | Organisational issues at the acute unit meant that the transfer system was not working well initially. As a consequence recruitment did not start until June 2008; however, by the end of the study the centre did achieve the recruitment target |
| Centre: 15 | |
| Change | Stroke unit became a hyperacute stroke unit |
| Date | November 2008 to January 2010 |
| Description | Centre 15 was preparing to become a hyperacute stroke unit. As a consequence the focus was more on acute care and the average length of stay on the ward was very short at 12 days – not many stay for > 7 days to be eligible for TRACS. Centre 15 became a hyperacute ward at the end of 2009 resulting in more acute beds (10, compared with four previously) and 19 short-term rehabilitation beds |
| Impact | No significant change in usual involvement of patients and carers as a consequence of this organisational change. Staff continued to rehabilitate patients and involve carers as much as possible within the short time frame. The change did have an impact on recruitment, making it hard to find and recruit eligible patients owing to the acute focus |
| Centre: 12 | |
| Change | Stroke rehabilitation unit moved from a community hospital to an acute hospital |
| Date | January 2009 |
| Description | The move was planned prior to randomisation into TRACS, but the timing was not yet agreed. The move took place in January 2009 when the entire centre moved to an acute hospital. The community hospital had two interlinked wards of 30 elderly stroke beds. The new centre comprised of one elderly rehabilitation ward of 25 beds, 17 of which were dedicated stroke beds |
| Impact | An unforeseen effect of the potential move was a loss of permanent staff at the original site owing to the uncertainty – a lot of the nursing team were bank staff and the physiotherapists and occupational therapists had a high turnover. This centre was an intervention centre and the high staff turnover did affect the implementation of the LSCTC, as training was not cascaded successfully among staff, and low staff moral reduced compliance with the new intervention. A positive effect of the move was a more settled staff team, treating fewer stroke beds, which improved the implementation of, and compliance with, the LSCTC |
Appendix 9 Patient and caregiver resource use
| Resource | Unit | Intervention (N = 450) | Control (N = 478) | ||||
|---|---|---|---|---|---|---|---|
| N users/valid n | Meana | SD | N users/valid n | Meana | SD | ||
| Residential care home | Night | 1/439 | 6.00 | – | 3/472 | 19.50 | 2.12 |
| Nursing home | Night | 1/439 | – | – | 1/474 | 60.00 | – |
| Inpatient services | Bed-day | 42/441 | 13.61 | 21.40 | 67/472 | 10.63 | 14.35 |
| Day hospital/day cases | Activity | 32/440 | 1.19 | 0.47 | 36/469 | 1.06 | 0.23 |
| Accident and emergency | Occurrence | 35/442 | 1.31 | 0.64 | 33/469 | 1.32 | 0.61 |
| Outpatient services | Activity | 111/437 | 1.88 | 1.54 | 126/466 | 2.24 | 2.50 |
| Physiotherapist, hospitalb | Visit | 18/424 | 3.93 | 2.43 | 29/450 | 4.78 | 5.19 |
| Occupational therapist, hospitalb | Visit | 7/423 | 3.83 | 3.54 | 17/451 | 3.67 | 2.35 |
| Speech and language therapist, hospitalb | Visit | 7/424 | 3.00 | 2.16 | 5/448 | 2.00 | 1.00 |
| Community-based services | |||||||
| GP | |||||||
| Surgery visit | Visit | 225/408 | 2.16 | 1.60 | 251/428 | 2.09 | 1.61 |
| Home visit | Visit | 42/372 | 1.48 | 0.82 | 38/379 | 1.73 | 1.70 |
| Telephone call | Call | 44/363 | 1.66 | 1.09 | 40/370 | 1.55 | 0.89 |
| Practice nurse | |||||||
| Surgery visit | Visit | 111/383 | 1.82 | 1.12 | 124/392 | 1.96 | 1.85 |
| Telephone call | Call | 9/359 | 1.75 | 1.04 | 13/366 | 1.80 | 1.10 |
| Physiotherapist | |||||||
| Home visit | Visit | 6/416 | 2.17 | 0.75 | 5/435 | 2.25 | 1.26 |
| Surgery visit | Visit | 9/417 | 2.50 | 2.45 | 7/435 | 7.83 | 15.77 |
| Elsewhere | Visit | 1/416 | – | – | 2/429 | 3.00 | – |
| Occupational therapist | |||||||
| Home visit | Visit | 6/417 | 3.00 | 2.76 | 11/439 | 1.11 | 0.33 |
| Surgery visit | Visit | 1/415 | 1.00 | – | 3/434 | 1.00 | – |
| Elsewhere | Visit | 1/417 | 2.00 | – | 2/433 | – | – |
| Speech and language therapist | |||||||
| Home visit | Visit | 2/416 | 1.00 | – | 0/435 | – | – |
| Surgery visit | Visit | 1/417 | – | – | 0/434 | – | – |
| Elsewhere | Visit | 0/415 | – | – | 0/433 | – | – |
| Social worker | |||||||
| Home visit | Visit | 7/421 | 3.60 | 4.72 | 5/441 | 1.75 | 0.96 |
| Telephone call | Call | 2/420 | 2.00 | – | 7/443 | 2.50 | 2.35 |
| Repeat prescription | Occurrence | 235/393 | 2.48 | 1.24 | 294/417 | 2.39 | 1.80 |
| Community/district nurse | Contact | 26/413 | 6.05 | 11.29 | 29/442 | 3.77 | 4.84 |
| Health visitor | Contact | 1/406 | 1.00 | – | 3/430 | 7.00 | 7.07 |
| Geriatrician | Contact | 2/407 | 1.00 | – | 1/428 | – | – |
| Psychiatrist | Contact | 2/408 | 1.00 | – | 6/429 | 3.00 | 2.16 |
| Psychologist | Contact | 2/407 | 4.00 | 1.41 | 3/428 | 1.00 | 0.00 |
| Chiropodist | Contact | 58/411 | 1.53 | 0.92 | 63/436 | 1.83 | 2.14 |
| Chiropractor | Contact | 3/407 | 3.50 | 0.71 | 2/430 | 4.00 | – |
| Osteopath | Contact | 3/406 | 4.00 | 4.24 | 1/428 | – | – |
| Dentist | Contact | 61/410 | 1.39 | 1.00 | 65/433 | 1.54 | 1.13 |
| Optician | Contact | 62/408 | 1.18 | 0.43 | 79/431 | 1.24 | 0.52 |
| Day hospital | Half-day | 2/421 | 6.00 | – | 8/450 | 2.86 | 2.91 |
| Social club | Half-day | 15/418 | 9.64 | 8.07 | 15/438 | 7.60 | 6.31 |
| Lunch club | Visit | 9/420 | 8.38 | 4.10 | 12/441 | 10.60 | 11.14 |
| Drop-in centre | Visit | 2/418 | 12.00 | 0.00 | 6/435 | 12.67 | 7.02 |
| Meals on wheels | Meal | 4/424 | 60.00 | – | 4/451 | 15.50 | 20.51 |
| Frozen meals | Meal | 8/423 | 32.67 | 49.74 | 7/444 | 52.00 | 27.71 |
| Home help: personal care | Visit | 9/424 | 87.00 | 63.64 | 10/445 | 60.20 | 68.19 |
| Home help: household care | Visit | 8/423 | 15.40 | 4.22 | 10/444 | 16.80 | 10.73 |
| Home help: shopping care | Visit | 4/423 | 8.00 | – | 7/443 | 12.00 | 0.00 |
| Social services day-care centre | Hour | 1/422 | – | – | 5/443 | 17.00 | 26.85 |
| Intermediate care team | Contact | 2/420 | 6.00 | – | 0/443 | – | – |
| Other services | Occurrence | 16/433 | 3.09 | 4.18 | 14/466 | 1.90 | 0.99 |
| Informal care from co-residents | |||||||
| Personal care | Hour | 55/427 | 169.67 | 360.58 | 67/458 | 202.71 | 418.65 |
| Providing transport | Hour | 83/430 | 100.98 | 322.55 | 94/453 | 82.52 | 150.68 |
| Preparing meals | Hour | 110/429 | 155.62 | 258.25 | 127/454 | 124.91 | 111.47 |
| Housework/laundry | Hour | 110/426 | 110.93 | 260.35 | 113/458 | 98.05 | 112.18 |
| DIY | Hour | 53/420 | 127.40 | 446.07 | 68/446 | 39.89 | 47.35 |
| Gardening | Hour | 87/423 | 73.40 | 289.08 | 90/447 | 44.90 | 56.70 |
| Shopping | Hour | 117/429 | 77.08 | 247.10 | 130/453 | 54.29 | 50.74 |
| Outings | Hour | 82/428 | 105.81 | 326.87 | 92/447 | 59.66 | 47.53 |
| Socialising | Hour | 104/427 | 269.72 | 386.63 | 128/451 | 407.34 | 575.97 |
| Help managing finances | Hour | 99/430 | 64.42 | 274.58 | 104/455 | 37.26 | 33.65 |
| Personal care | Hour | 20/426 | 28.09 | 27.70 | 22/446 | 55.27 | 53.55 |
| Providing transport | Hour | 43/421 | 32.36 | 44.06 | 63/443 | 38.16 | 52.31 |
| Preparing meals | Hour | 29/423 | 58.29 | 59.55 | 35/444 | 81.74 | 98.63 |
| Housework/laundry | Hour | 38/423 | 47.53 | 65.11 | 52/443 | 43.00 | 56.16 |
| DIY | Hour | 23/420 | 21.00 | 21.43 | 28/435 | 19.46 | 21.27 |
| Gardening | Hour | 26/421 | 21.19 | 20.77 | 50/437 | 18.68 | 21.03 |
| Shopping | Hour | 46/423 | 32.42 | 32.40 | 57/441 | 27.65 | 26.58 |
| Outings | Hour | 45/423 | 35.80 | 50.03 | 57/441 | 41.42 | 38.85 |
| Socialising | Hour | 53/419 | 113.12 | 196.01 | 69/442 | 139.97 | 342.95 |
| Help managing finances | Hour | 31/422 | 28.94 | 39.35 | 39/443 | 21.83 | 20.26 |
| Resource | Unit | Intervention (N = 450) | Control (N = 478) | ||||
|---|---|---|---|---|---|---|---|
| N users/valid n | Meana | SD | N users/valid n | Meana | SD | ||
| Stroke admission | Bed-days | 448/448 | 44.81 | 32.88 | 478/478 | 42.42 | 29.48 |
| Residential care home | Night | 10/315 | 27.00 | 16.70 | 19/339 | 16.38 | 13.62 |
| Nursing home | Night | 7/313 | 117.00 | 96.42 | 7/327 | 14.50 | 96.42 |
| Inpatient services | Bed-day | 56/319 | 12.40 | 21.82 | 65/338 | 8.41 | 10.15 |
| Day hospital/day cases | Activity | 27/314 | 1.41 | 0.97 | 26/336 | 1.46 | 0.71 |
| Accident and emergency | Occurrence | 52/311 | 1.68 | 1.14 | 63/338 | 1.39 | 0.59 |
| Outpatient services | Activity | 178/308 | 2.98 | 3.82 | 158/328 | 3.33 | 4.76 |
| Physiotherapist, hospitalb | Visit | 97/258 | 9.20 | 16.37 | 114/289 | 8.56 | 7.93 |
| Occupational therapist, hospitalb | Visit | 30/235 | 3.87 | 3.38 | 50/267 | 8.03 | 11.64 |
| Speech and language therapist, hospitalb | Visit | 33/261 | 2.64 | 2.84 | 32/272 | 4.24 | 3.50 |
| Community-based services | |||||||
| GP | |||||||
| Surgery visit | Visit | 177/270 | 3.28 | 2.32 | 202/301 | 3.01 | 2.04 |
| Home visit | Visit | 124/252 | 2.14 | 1.61 | 135/267 | 2.26 | 1.93 |
| Telephone call | Call | 85/230 | 1.82 | 1.11 | 95/247 | 2.31 | 1.93 |
| Practice nurse | |||||||
| Surgery visit | Visit | 116/241 | 3.06 | 3.34 | 116/250 | 2.78 | 3.14 |
| Telephone call | Call | 35/215 | 2.48 | 2.83 | 32/219 | 2.19 | 1.55 |
| Physiotherapist | |||||||
| Home visit | Visit | 159/264 | 8.58 | 8.33 | 158/285 | 10.48 | 11.32 |
| Surgery visit | Visit | 9/231 | 3.00 | 1.41 | 13/252 | 3.40 | 2.63 |
| Elsewhere | Visit | 6/222 | 2.20 | 0.84 | 14/246 | 9.43 | 8.79 |
| Occupational therapist | |||||||
| Home visit | Visit | 164/270 | 7.50 | 8.76 | 144/284 | 6.10 | 8.08 |
| Surgery visit | Visit | 1/222 | – | – | 5/246 | 2.75 | 0.50 |
| Elsewhere | Visit | 5/222 | 1.80 | 1.30 | 10/243 | 3.67 | 2.73 |
| Speech and language therapist | |||||||
| Home visit | Visit | 65/262 | 7.41 | 7.58 | 65/271 | 6.46 | 5.60 |
| Surgery visit | Visit | 1/243 | 4.00 | – | 2/250 | 3.00 | 0.00 |
| Elsewhere | Visit | 6/242 | 2.50 | 1.91 | 12/252 | 5.43 | 2.99 |
| Social worker | |||||||
| Home visit | Visit | 65/274 | 2.38 | 1.52 | 70/297 | 2.32 | 1.77 |
| Telephone call | Call | 31/274 | 3.65 | 2.46 | 49/297 | 2.78 | 1.42 |
| Repeat prescription | Occurrence | 208/264 | 4.62 | 2.72 | 253/288 | 4.90 | 3.22 |
| Community/district nurse | Contact | 112/261 | 4.56 | 4.47 | 108/277 | 6.20 | 7.30 |
| Health visitor | Contact | 25/236 | 2.73 | 1.72 | 19/249 | 2.93 | 2.37 |
| Geriatrician | Contact | 0/231 | – | – | 5/242 | 1.20 | 0.45 |
| Psychiatrist | Contact | 11/235 | 2.55 | 2.70 | 5/244 | 2.25 | 0.50 |
| Psychologist | Contact | 13/233 | 2.58 | 1.83 | 15/247 | 2.36 | 1.60 |
| Chiropodist | Contact | 60/242 | 1.98 | 1.27 | 84/274 | 2.00 | 1.28 |
| Chiropractor | Contact | 3/232 | 3.33 | 1.53 | 1/243 | 2.00 | – |
| Osteopath | Contact | 1/232 | 2.00 | – | 0/242 | – | – |
| Dentist | Contact | 58/243 | 2.04 | 1.43 | 79/261 | 1.74 | 0.94 |
| Optician | Contact | 66/247 | 1.31 | 0.74 | 92/264 | 1.51 | 1.10 |
| Day hospital | Half-day | 15/278 | 4.36 | 2.92 | 20/299 | 5.80 | 6.20 |
| Social club | Half-day | 15/277 | 4.64 | 3.27 | 19/290 | 6.56 | 11.89 |
| Lunch club | Visit | 8/277 | 3.14 | 1.07 | 12/291 | 8.80 | 15.60 |
| Drop-in centre | Visit | 7/275 | 8.83 | 8.26 | 10/294 | 3.13 | 1.96 |
| Meals on wheels | Meal | 5/277 | 7.50 | 6.36 | 10/300 | 16.00 | 19.80 |
| Frozen meals | Meal | 9/278 | 15.00 | 21.66 | 10/296 | 17.00 | 17.97 |
| Home help: personal care | Visit | 71/275 | 87.17 | 134.38 | 70/297 | 80.80 | 116.76 |
| Home help: household care | Visit | 18/273 | 14.20 | 15.05 | 20/293 | 8.75 | 6.40 |
| Home help: shopping care | Visit | 4/271 | 14.50 | 13.44 | 8/292 | – | – |
| Social services day-care centre | Hour | 9/275 | 3.17 | 2.04 | 12/296 | 4.14 | 5.34 |
| Intermediate care team | Contact | 18/259 | 6.44 | 5.03 | 20/282 | 7.10 | 7.93 |
| Other services | Occurrence | 27/308 | 3.29 | 3.27 | 20/327 | 9.69 | 10.64 |
| Informal care from co-residents | |||||||
| Personal care | Hour | 206/302 | 225.67 | 332.01 | 211/317 | 323.65 | 448.95 |
| Providing transport | Hour | 190/286 | 130.18 | 223.37 | 193/304 | 137.68 | 159.34 |
| Preparing meals | Hour | 224/295 | 286.16 | 292.26 | 237/316 | 305.48 | 212.73 |
| Housework/laundry | Hour | 228/299 | 220.26 | 293.08 | 239/313 | 238.28 | 197.90 |
| DIY | Hour | 119/272 | 108.26 | 321.30 | 139/300 | 67.13 | 90.35 |
| Gardening | Hour | 159/284 | 90.90 | 228.14 | 166/304 | 72.52 | 69.04 |
| Shopping | Hour | 225/300 | 108.16 | 202.37 | 231/315 | 132.95 | 116.38 |
| Outings | Hour | 182/288 | 127.10 | 223.29 | 187/310 | 132.41 | 121.74 |
| Socialising | Hour | 213/289 | 980.38 | 1293.44 | 213/307 | 775.17 | 935.81 |
| Help managing finances | Hour | 205/296 | 89.61 | 211.78 | 203/312 | 125.49 | 267.22 |
| Informal care from non-residents | |||||||
| Personal care | Hour | 52/281 | 83.29 | 168.43 | 59/308 | 133.25 | 143.09 |
| Providing transport | Hour | 102/282 | 50.10 | 113.02 | 118/311 | 45.02 | 54.52 |
| Preparing meals | Hour | 53/278 | 28.42 | 27.81 | 57/305 | 95.05 | 130.70 |
| Housework/laundry | Hour | 54/279 | 55.05 | 62.17 | 67/305 | 101.29 | 103.46 |
| DIY | Hour | 56/274 | 26.71 | 30.40 | 48/303 | 36.18 | 58.56 |
| Gardening | Hour | 61/276 | 25.94 | 22.43 | 56/304 | 25.39 | 30.89 |
| Shopping | Hour | 75/280 | 45.26 | 64.18 | 81/306 | 52.03 | 43.76 |
| Outings | Hour | 96/285 | 47.02 | 68.63 | 86/305 | 66.87 | 82.07 |
| Socialising | Hour | 113/281 | 104.76 | 137.88 | 110/300 | 122.31 | 141.10 |
| Help managing finances | Hour | 43/278 | 29.25 | 20.23 | 53/304 | 62.42 | 93.37 |
| Resource | Unit | Intervention (N = 450) | Control (N = 478) | ||||
|---|---|---|---|---|---|---|---|
| N users/valid n | Meana | SD | N users/valid n | Meana | SD | ||
| Residential care home | Night | 17/283 | 22.33 | 26.88 | 25/312 | 23.41 | 29.24 |
| Nursing home | Night | 10/277 | 13.00 | 9.70 | 7/305 | 9.33 | 5.13 |
| Inpatient services | Bed-day | 43/288 | 8.75 | 12.10 | 58/312 | 9.19 | 12.31 |
| Day hospital/day cases | Activity | 31/284 | 1.29 | 0.64 | 28/313 | 1.29 | 0.66 |
| Accident and emergency | Occurrence | 48/284 | 1.58 | 1.45 | 52/311 | 1.56 | 0.92 |
| Outpatient services | Activity | 126/281 | 2.68 | 2.23 | 130/310 | 2.99 | 2.55 |
| Physiotherapist, hospitalb | Visit | 61/244 | 8.90 | 8.28 | 58/284 | 8.49 | 6.78 |
| Occupational therapist, hospitalb | Visit | 23/237 | 9.00 | 8.80 | 17/275 | 5.73 | 7.54 |
| Speech and language therapist, hospitalb | Visit | 18/238 | 7.88 | 12.78 | 20/277 | 4.89 | 3.77 |
| Community-based services | |||||||
| GP | |||||||
| Surgery visit | Visit | 167/246 | 2.96 | 2.26 | 197/276 | 2.67 | 2.32 |
| Home visit | Visit | 86/227 | 2.25 | 1.62 | 86/237 | 2.20 | 1.87 |
| Telephone call | Call | 56/202 | 2.13 | 1.59 | 69/232 | 2.71 | 4.03 |
| Practice nurse | |||||||
| Surgery visit | Visit | 110/223 | 2.61 | 2.91 | 135/248 | 2.50 | 2.94 |
| Telephone call | Call | 17/195 | 2.33 | 1.88 | 22/213 | 2.53 | 2.15 |
| Physiotherapist | |||||||
| Home visit | Visit | 54/232 | 8.09 | 10.55 | 44/265 | 6.32 | 7.62 |
| Surgery visit | Visit | 6/217 | 3.75 | 4.27 | 18/268 | 2.85 | 1.63 |
| Elsewhere | Visit | 6/210 | 4.20 | 4.49 | 9/260 | 13.50 | 8.80 |
| Occupational therapist | |||||||
| Home visit | Visit | 34/230 | 7.77 | 10.08 | 39/267 | 4.00 | 4.32 |
| Surgery visit | Visit | 2/220 | – | – | 9/263 | 2.50 | 1.91 |
| Elsewhere | Visit | 5/218 | 2.75 | 1.50 | 8/259 | 11.00 | 8.55 |
| Speech and language therapist | |||||||
| Home visit | Visit | 31/234 | 7.04 | 7.03 | 32/268 | 4.18 | 4.11 |
| Surgery visit | Visit | 1/224 | 6.00 | – | 6/263 | 4.50 | 3.87 |
| Elsewhere | Visit | 1/223 | 1.00 | – | 8/260 | 7.00 | 6.68 |
| Social worker | |||||||
| Home visit | Visit | 21/251 | 1.63 | 0.62 | 34/287 | 1.91 | 1.00 |
| Telephone call | Call | 10/245 | 2.33 | 1.21 | 24/283 | 2.11 | 1.37 |
| Repeat prescription | Occurrence | 191/234 | 5.25 | 4.19 | 226/265 | 4.98 | 2.67 |
| Community/district nurse | Contact | 67/233 | 5.82 | 8.18 | 82/275 | 5.86 | 9.06 |
| Health visitor | Contact | 6/217 | 6.83 | 6.94 | 12/251 | 2.09 | 1.45 |
| Geriatrician | Contact | 1/212 | – | – | 1/249 | 1.00 | – |
| Psychiatrist | Contact | 6/215 | 1.00 | 0.00 | 9/250 | 1.75 | 1.39 |
| Psychologist | Contact | 6/213 | 1.67 | 0.58 | 7/248 | 1.83 | 1.33 |
| Chiropodist | Contact | 78/241 | 2.30 | 1.38 | 80/273 | 2.24 | 1.50 |
| Chiropractor | Contact | 6/214 | 2.67 | 2.08 | 4/250 | 2.25 | 1.29 |
| Osteopath | Contact | 5/212 | 7.75 | 11.50 | 1/247 | 3.00 | – |
| Dentist | Contact | 84/231 | 1.72 | 1.36 | 73/259 | 1.69 | 1.03 |
| Optician | Contact | 69/223 | 1.49 | 1.04 | 75/261 | 1.27 | 0.56 |
| Day hospital | Half-day | 12/252 | 5.00 | 5.63 | 12/288 | 6.91 | 11.35 |
| Social club | Half-day | 14/249 | 12.38 | 16.18 | 19/286 | 7.85 | 7.82 |
| Lunch club | Visit | 5/243 | 15.60 | 19.65 | 22/285 | 10.38 | 9.79 |
| Drop-in centre | Visit | 6/245 | 8.67 | 9.87 | 13/285 | 7.60 | 9.62 |
| Meals on wheels | Meal | 5/252 | 3.00 | – | 7/292 | 180.00 | – |
| Frozen meals | Meal | 12/253 | 13.50 | 9.98 | 13/286 | 15.57 | 10.69 |
| Home help: personal care | Visit | 42/250 | 92.89 | 84.71 | 42/289 | 164.00 | 175.57 |
| Home help: household care | Visit | 15/250 | 20.75 | 10.87 | 19/287 | 72.40 | 105.67 |
| Home help: shopping care | Visit | 7/248 | – | – | 9/284 | 23.67 | 2.52 |
| Social services day-care centre | Hour | 12/251 | 12.33 | 11.50 | 9/284 | 58.75 | 94.64 |
| Intermediate care team | Contact | 8/243 | 204.00 | 277.19 | 11/273 | 13.80 | 25.83 |
| Other services | Occurrence | 14/283 | 3.00 | 3.20 | 18/311 | 2.62 | 2.50 |
| Informal care from co-residents | |||||||
| Personal care | Hour | 153/263 | 255.63 | 376.21 | 170/285 | 318.51 | 352.46 |
| Providing transport | Hour | 139/253 | 109.69 | 104.41 | 158/281 | 150.18 | 146.17 |
| Preparing meals | Hour | 175/263 | 280.73 | 156.79 | 206/287 | 318.34 | 216.04 |
| Housework/laundry | Hour | 170/258 | 207.32 | 159.25 | 200/280 | 184.82 | 153.80 |
| DIY | Hour | 97/247 | 46.29 | 45.23 | 116/268 | 56.02 | 61.29 |
| Gardening | Hour | 123/250 | 74.41 | 86.83 | 134/273 | 72.78 | 86.83 |
| Shopping | Hour | 174/257 | 101.15 | 85.04 | 183/279 | 115.19 | 92.91 |
| Outings | Hour | 143/251 | 113.41 | 102.39 | 152/277 | 138.42 | 135.28 |
| Socialising | Hour | 163/251 | 645.96 | 694.37 | 165/282 | 865.98 | 1000.19 |
| Help managing finances | Hour | 161/259 | 145.16 | 491.49 | 163/283 | 76.23 | 80.10 |
| Informal care from non-residents | |||||||
| Personal care | Hour | 34/257 | 89.08 | 108.84 | 41/287 | 78.65 | 108.78 |
| Providing transport | Hour | 73/254 | 49.53 | 47.03 | 86/284 | 40.15 | 51.81 |
| Preparing meals | Hour | 39/255 | 70.13 | 58.81 | 50/286 | 90.95 | 136.70 |
| Housework/laundry | Hour | 40/255 | 81.71 | 64.93 | 60/287 | 87.83 | 106.61 |
| DIY | Hour | 43/248 | 53.68 | 108.21 | 46/278 | 36.35 | 48.84 |
| Gardening | Hour | 50/251 | 27.17 | 23.55 | 52/281 | 45.93 | 74.34 |
| Shopping | Hour | 44/256 | 59.25 | 62.26 | 70/287 | 42.57 | 43.15 |
| Outings | Hour | 63/256 | 38.80 | 48.07 | 79/287 | 42.46 | 60.38 |
| Socialising | Hour | 77/252 | 123.95 | 149.35 | 90/286 | 114.18 | 168.16 |
| Help managing finances | Hour | 33/252 | 53.50 | 43.64 | 43/287 | 56.15 | 50.91 |
| Resource | Unit | Intervention (N = 450) | Control (N = 478) | ||||
|---|---|---|---|---|---|---|---|
| N users/valid n | Meana | SD | N users/valid n | Meana | SD | ||
| Inpatient services | Bed-day | 7/440 | 0.06 | 0.66 | 16/470 | 0.36 | 3.66 |
| Day hospital/day cases | Activity | 21/438 | 1.15 | 0.37 | 21/469 | 1.19 | 0.51 |
| Accident and emergency | Occurrence | 13/407 | 1.69 | 1.70 | 23/436 | 1.23 | 0.61 |
| Outpatient services | Activity | 88/440 | 2.31 | 2.03 | 92/470 | 2.05 | 1.90 |
| Community-based services | |||||||
| GP | |||||||
| Surgery visit | Visit | 199/425 | 1.81 | 1.21 | 228/460 | 1.82 | 1.13 |
| Home visit | Visit | 12/389 | 1.33 | 1.00 | 10/421 | 1.00 | 0.00 |
| Telephone call | Call | 25/392 | 1.50 | 0.96 | 33/423 | 1.40 | 0.89 |
| Practice nurse | |||||||
| Surgery visit | Visit | 120/404 | 1.61 | 1.35 | 101/430 | 1.83 | 2.75 |
| Telephone call | Call | 2/388 | 2.00 | 1.41 | 13/420 | 1.45 | 0.69 |
| Physiotherapist | |||||||
| Hospital visit | Visit | 7/404 | 3.71 | 3.20 | 16/436 | 5.54 | 9.51 |
| Home visit | Visit | 2/399 | 2.00 | – | 2/429 | – | – |
| Surgery visit | Visit | 14/402 | 2.57 | 1.95 | 14/431 | 3.67 | 5.23 |
| Elsewhere | Visit | 0/398 | – | – | 4/428 | 4.50 | 2.12 |
| Repeat prescription | Occurrence | 214/415 | 2.26 | 1.15 | 229/443 | 2.26 | 1.11 |
| Community/district nurse | Contact | 7/398 | 1.67 | 0.82 | 5/434 | 2.00 | 1.41 |
| Health visitor | Contact | 2/397 | 1.00 | – | 4/430 | 1.50 | 0.71 |
| Psychiatrist | Contact | 40/404 | 1.41 | 0.66 | 34/433 | 1.38 | 0.56 |
| Psychologist | Contact | 5/397 | 2.00 | 1.41 | 6/431 | 4.67 | 2.31 |
| Chiropodist | Contact | 7/397 | 3.00 | 2.00 | 6/431 | 4.25 | 5.25 |
| Chiropractor | Contact | 4/396 | 4.00 | 1.41 | 4/430 | 1.00 | – |
| Osteopath | Contact | 3/400 | 1.50 | 0.71 | 4/430 | 1.50 | 0.71 |
| Informal care for patient | |||||||
| Personal care | Hour | 72/420 | 116.95 | 172.87 | 105/451 | 82.80 | 106.69 |
| Providing transport | Hour | 141/419 | 58.04 | 98.00 | 158/448 | 57.52 | 71.90 |
| Preparing meals | Hour | 228/426 | 103.89 | 96.37 | 240/455 | 124.19 | 208.76 |
| Housework/laundry | Hour | 229/421 | 77.22 | 113.96 | 254/452 | 87.18 | 113.46 |
| DIY | Hour | 88/412 | 34.65 | 79.32 | 101/442 | 30.35 | 44.21 |
| Gardening | Hour | 159/423 | 32.13 | 63.98 | 158/451 | 33.87 | 35.39 |
| Shopping | Hour | 233/422 | 44.28 | 59.16 | 264/452 | 44.60 | 49.82 |
| Outings | Hour | 140/414 | 59.96 | 86.75 | 163/443 | 65.36 | 83.45 |
| Socialising | Hour | 239/425 | 281.30 | 409.07 | 269/448 | 274.38 | 399.62 |
| Help managing finances | Hour | 175/425 | 26.64 | 56.94 | 193/450 | 35.02 | 45.93 |
| Resource | Unit | Intervention (N = 450) | Control (N = 478) | ||||
|---|---|---|---|---|---|---|---|
| N users/valid n | Meana | SD | N users/valid n | Meana | SD | ||
| Residential care home | Night | 0/319 | – | – | 0/333 | – | – |
| Nursing home | Night | 0/316 | – | – | 0/330 | – | – |
| Inpatient services | Bed-day | 20/320 | 0.36 | 2.29 | 15/335 | 0.20 | 1.33 |
| Day hospital/day cases | Activity | 16/315 | 1.25 | 0.45 | 16/327 | 1.44 | 0.89 |
| Accident and emergency | Occurrence | 20/309 | 1.74 | 1.63 | 24/319 | 1.33 | 0.66 |
| Outpatient services | Activity | 83/315 | 2.96 | 2.64 | 93/326 | 2.73 | 2.16 |
| Community-based services | |||||||
| GP | |||||||
| Surgery visit | Visit | 176/290 | 2.85 | 2.11 | 197/318 | 2.58 | 1.80 |
| Home visit | Visit | 16/238 | 1.77 | 1.69 | 12/261 | 1.27 | 0.47 |
| Telephone call | Call | 37/242 | 2.09 | 1.13 | 47/271 | 1.95 | 1.05 |
| Practice nurse | |||||||
| Surgery visit | Visit | 116/267 | 2.17 | 1.90 | 111/287 | 1.95 | 1.88 |
| Telephone call | Call | 12/234 | 2.55 | 1.97 | 21/262 | 1.87 | 1.36 |
| Physiotherapist | |||||||
| Hospital visit | Visit | 10/256 | 4.78 | 4.79 | 15/280 | 2.31 | 1.38 |
| Home visit | Visit | 4/252 | 7.25 | 8.77 | 6/275 | 8.75 | 6.99 |
| Surgery visit | Visit | 12/256 | 2.64 | 3.26 | 10/277 | 3.00 | 2.26 |
| Elsewhere | Visit | 2/249 | 3.50 | 3.54 | 5/272 | 3.00 | 1.41 |
| Repeat prescription | Occurrence | 152/273 | 4.26 | 2.34 | 178/299 | 4.54 | 2.89 |
| Community/district nurse | Contact | 15/254 | 4.33 | 3.97 | 17/275 | 3.55 | 2.73 |
| Health visitor | Contact | 3/249 | 5.00 | 2.65 | 4/272 | 1.50 | 0.58 |
| Psychiatrist | Contact | 33/259 | 2.00 | 1.41 | 44/283 | 2.15 | 1.56 |
| Psychologist | Contact | 5/246 | 4.20 | 2.86 | 4/273 | 3.00 | 1.83 |
| Chiropodist | Contact | 5/248 | 3.00 | 1.83 | 5/273 | 3.75 | 2.50 |
| Chiropractor | Contact | 1/249 | 2.00 | – | 6/271 | 2.67 | 1.53 |
| Osteopath | Contact | 2/251 | 3.00 | 0.00 | 3/271 | 2.50 | 2.12 |
| Informal care for patient | |||||||
| Personal care | Hour | 238/306 | 221.84 | 331.92 | 243/323 | 370.06 | 600.72 |
| Providing transport | Hour | 201/295 | 140.40 | 219.80 | 201/309 | 144.36 | 171.54 |
| Preparing meals | Hour | 282/310 | 252.51 | 239.41 | 295/330 | 285.67 | 227.19 |
| Housework/laundry | Hour | 278/310 | 181.51 | 201.93 | 295/325 | 218.40 | 222.58 |
| DIY | Hour | 130/285 | 99.12 | 311.09 | 151/290 | 56.34 | 62.17 |
| Gardening | Hour | 181/294 | 81.84 | 209.96 | 196/307 | 63.76 | 71.43 |
| Shopping | Hour | 281/307 | 108.58 | 181.74 | 297/321 | 111.38 | 106.71 |
| Outings | Hour | 205/293 | 113.97 | 204.40 | 214/310 | 123.17 | 131.61 |
| Socialising | Hour | 271/303 | 740.91 | 984.28 | 262/317 | 679.46 | 1023.29 |
| Help managing finances | Hour | 258/309 | 69.55 | 172.02 | 268/317 | 72.44 | 95.80 |
| Resource | Unit | Intervention (N = 450) | Control (N = 478) | ||||
|---|---|---|---|---|---|---|---|
| N users/valid n | Meana | SD | N users/valid n | Meana | SD | ||
| Residential care home | Night | 0/281 | – | – | 2/309 | – | – |
| Nursing home stay | Night | 0/281 | – | – | 0/307 | – | – |
| Inpatient services | Bed-day | 18/282 | 0.27 | 1.90 | 15/306 | 0.27 | 2.85 |
| Day hospital/day cases | Activity | 15/280 | 1.20 | 0.41 | 27/305 | 1.11 | 0.32 |
| Accident and emergency | Occurrence | 17/267 | 1.69 | 1.14 | 16/293 | 1.57 | 1.16 |
| Outpatient services | Activity | 71/275 | 2.89 | 3.04 | 82/303 | 2.42 | 1.66 |
| Community-based services | |||||||
| GP | |||||||
| Surgery visit | Visit | 151/263 | 2.20 | 1.58 | 191/290 | 2.47 | 1.61 |
| Home visit | Visit | 9/216 | 1.89 | 1.05 | 14/236 | 1.43 | 1.09 |
| Telephone call | Call | 24/218 | 2.14 | 2.48 | 34/234 | 1.75 | 0.84 |
| Practice nurse | |||||||
| Surgery visit | Visit | 105/239 | 1.79 | 1.31 | 124/263 | 1.95 | 1.55 |
| Telephone call | Call | 9/214 | 2.13 | 1.89 | 14/228 | 1.69 | 1.18 |
| Physiotherapist | |||||||
| Hospital visit | Visit | 13/233 | 5.00 | 3.65 | 14/254 | 3.85 | 3.91 |
| Home visit | Visit | 2/229 | 3.50 | 0.71 | 1/243 | 1.00 | – |
| Surgery visit | Visit | 3/230 | 4.00 | 4.36 | 7/246 | 2.43 | 1.27 |
| Elsewhere | Visit | 0/229 | – | – | 2/243 | 3.00 | 1.41 |
| Repeat prescription | Occurrence | 139/238 | 3.89 | 1.93 | 164/264 | 3.96 | 2.08 |
| Community/district nurse | Contact | 8/232 | 7.43 | 11.12 | 10/250 | 9.00 | 10.00 |
| Health visitor | Contact | 1/230 | – | – | 4/244 | 2.75 | 1.50 |
| Psychiatrist | Contact | 32/240 | 2.12 | 1.31 | 35/254 | 2.20 | 1.42 |
| Psychologist | Contact | 5/229 | 6.60 | 6.88 | 6/246 | 5.00 | 4.30 |
| Chiropodist | Contact | 5/230 | 5.25 | 2.75 | 1/244 | 6.00 | – |
| Chiropractor | Contact | 2/230 | 4.00 | – | 0/243 | – | – |
| Osteopath | Contact | 1/229 | 2.00 | – | 3/244 | 4.00 | 2.00 |
| Informal care for patient | |||||||
| Personal care | Hour | 187/256 | 243.81 | 346.07 | 207/288 | 278.90 | 398.06 |
| Providing transport | Hour | 173/251 | 107.70 | 106.82 | 188/279 | 150.43 | 148.65 |
| Preparing meals | Hour | 243/265 | 264.64 | 196.99 | 265/296 | 297.91 | 206.24 |
| Housework/laundry | Hour | 229/260 | 194.84 | 180.61 | 257/289 | 191.98 | 179.30 |
| DIY | Hour | 129/241 | 47.46 | 69.61 | 136/268 | 46.54 | 48.18 |
| Gardening | Hour | 155/252 | 69.77 | 86.61 | 182/280 | 77.02 | 136.17 |
| Shopping | Hour | 233/257 | 106.64 | 96.55 | 259/286 | 150.43 | 251.86 |
| Outings | Hour | 181/244 | 103.84 | 102.82 | 196/280 | 146.99 | 148.80 |
| Socialising | Hour | 229/256 | 644.07 | 767.11 | 234/282 | 666.61 | 882.95 |
| Help managing finances | Hour | 222/264 | 61.35 | 87.78 | 225/290 | 84.19 | 107.49 |
List of abbreviations
- 6CIT
- Six-item Cognitive Impairment Test
- ADL
- activities of daily living
- AE
- adverse event
- CBS
- Caregiver Burden Scale
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CSRI
- Client Service Receipt Inventory
- CTRU
- Clinical Trials Research Unit
- DMEC
- Data Monitoring and Ethics Committee
- EQ-5D
- European Quality of Life-5 Dimensions
- FAI
- Frenchay Activities Index
- GP
- general practitioner
- HADS
- Hospital Anxiety and Depression Scale
- HRG
- Healthcare Resource Group
- ICC
- intracluster correlation coefficient
- ICER
- incremental cost-effectiveness ratio
- ITT
- intention to treat
- LSCTC
- London Stroke Carers Training Course
- MDT
- multidisciplinary team
- MeSH
- medical subject heading
- MRC
- Medical Research Council
- NEADL
- Nottingham Extended Activities of Daily Living
- NICE
- National Institute for Health and Care Excellence (formerly National Institute for Health and Clinical Excellence)
- NIHR
- National Institute for Health Research
- NSSA
- National Sentinel Stroke Audit
- PI
- principal investigator
- QALY
- quality-adjusted life-year
- R&D
- research and development
- RCP
- Royal College of Physicians of London
- RCT
- randomised controlled trial
- RUSAE
- related and unexpected serious adverse event
- SAE
- serious adverse event
- SD
- standard deviation
- SE
- standard error
- SIS
- Stroke Impact Scale
- SRN
- Stroke Research Network
- SRU
- stroke rehabilitation unit
- TMG
- Trial Management Group
- TRACS
- Training Caregivers After Stroke
- TSC
- Trial Steering Committee