Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 07/01/44. The contractual start date was in September 2008. The draft report began editorial review in October 2012 and was accepted for publication in February 2013. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Dr Hemming carried out paid peer-review work for statistical review, as statistical editor, of papers submitted to BJOG and she is the parent of a baby born with a lower urinary tract obstruction. Before the start of this project Dr Daniels was a co-investigator on a project grant from Wellbeing of Women to initiate the PLUTO trial. Professor Denny has received royalties for three academic textbooks on health sociology. Professor Khan provides expert reports in medical negligence cases for which a fee is paid by instructing solicitors. In addition, Professor Khan has received grants from public bodies and pharmaceutical companies in the UK and EU. Money was paid to Professor Khan and his institution from Ferring Pharmaceuticals and various universities and societies and he has received honoraria for speaking at meetings. Professor Khan has also received royalties for his books from publishers Hodder Arnold and Huber and payment for advice on medial research, and he and his institution have received sponsorship from Ferring Pharmaceuticals, Leo-Pharma, Alere, Ethicon, Hologic, Viforpharma and Preglem/Quintiles for organising educational meetings. Before the start of this project Professor Khan was a co-investigator on a project grant from Wellbeing of Women to initiate the PLUTO trial. Before the start of this project Professor Kilby received a project grant from Wellbeing of Women to initiate the PLUTO trial. He also received a $750 honorarium for air travel to a debate in the USA on the role of fetal vesicoamniotic shunting at the Society of Maternal and Fetal Medicine, San Francisco, CA, USA in February 2013. He will be the Visiting Professor at the University of Delft and Leiden in June 2013. This will be to lead a joint meeting between fetal medicine subspecialists and paediatric urologists on congenital bladder neck obstructions. He will be paid travelling expenses and subsistence only. Professor Kilby also provides expert witness statements for medical negligence claims for which a fee is paid by instructing solicitors. All other authors declared no competing interests.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2013. This work was produced by Morris et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Fetal bladder obstruction and its treatment
Congenital anomalies of the genitourinary tract are identified using prenatal ultrasound in between 1 : 250 and 1 : 1000 pregnancies (the rates being dependant on the inclusion of terminations of pregnancy, prenatal and postnatal acquisition). 1 Chronic lower urinary tract obstruction (LUTO), also known as fetal bladder outlet obstruction, will predispose the fetus to abnormal renal development and function, and this risk continues to ‘track’ into childhood. If there is severe prenatal renal impairment, the condition is commonly associated with significant oligohydramnios (reduced liquor volume). Such an association, when present at mid-gestation (between 16 and 24 weeks), is associated with pulmonary hypoplasia in a high proportion of pregnancies, resulting in a high perinatal mortality and morbidity risk for the fetus. 2–4 Chronic oligohydramnios may be associated with positional postural anomalies such as talipes. The diagnosis, when made prenatally, often occurs at 20 weeks of gestation when the majority of pregnant women have a routine detailed fetal anomaly scan. Bladder drainage by serial vesicocentesis (insertion of a fine needle to drain the fetal bladder at regular intervals) and continuous drainage into the amniotic cavity by vesicoamniotic shunting (VAS) have been used to relieve fetal LUTO (bypassing the urethral blockage). These techniques attempt to reduce or avoid renal parenchymal damage and chronic oligohydramnios that may adversely affect pulmonary development. 5–7 These ‘treatment’ procedures may be associated with theoretical maternal morbidity (mainly in terms of infection risk) and fetal morbidity (infection or bleeding). They also carry a risk of causing miscarriage (2–5%) and the risk of serial drainages is often cumulative making VAS insertion, at least in theory, preferable. Prenatal VAS insertion, in current practice, can be justified only if safety and effectiveness is demonstrated with reliability [as indicated in the National Institute for Health and Care Excellence (NICE) interventional procedures guidance of 2006 (IPG 2028)].
Definition of lower urinary tract obstruction
Lower urinary tract obstruction may be a consequence of a range of fetal pathological processes. The two most common are the congenital malformations posterior urethral valves (PUVs), accounting for approximately half of cases presenting with ultrasound features of LUTO,9 and urethral atresia. 10 The affected fetus is typically male. When ultrasound demonstrates a female fetus with LUTO, this is often indicative of a more complex, morbid pathology such as cloacal plate anomalies, including megacystis microcolon syndrome (dysfunctional smooth muscle in the bladder and distal bowel). LUTO is a congenital anomaly with high mortality and morbidity. It is potentially associated with cystic renal dysplasia and abnormal renal (glomerular and tubular) function. Progressive renal dysfunction may be associated with significant oligohydramnios. Chronic oligohydramnios predisposes the fetus to pulmonary hypoplasia and positional limb abnormalities. 1
Accurate prenatal detection of LUTO is possible using ultrasound. Typical ultrasound features in the fetus are megacystis (enlarged bladder) with bilateral hydronephrosis (dilatation of the ureters) with or without renal parenchymal cystic change. Such ultrasound features are commonly associated with oligohydramnios. The prenatal sign on ultrasound of renal echogenicity and oligohydramnios with megacystis are predictive of a urethral obstructive aetiology in up to 87% of cases. 11 Such prenatal ultrasound findings are of limited value in differentiating PUVs from other causes of LUTO11,12 and thus the final diagnosis is often not known until postnatally. For the purposes of trial entry LUTO was thus defined using the ultrasound criterion ‘evidence of isolated bladder outflow obstruction from ultrasound imaging, male fetus’.
Evidence of the need for a randomised controlled trial
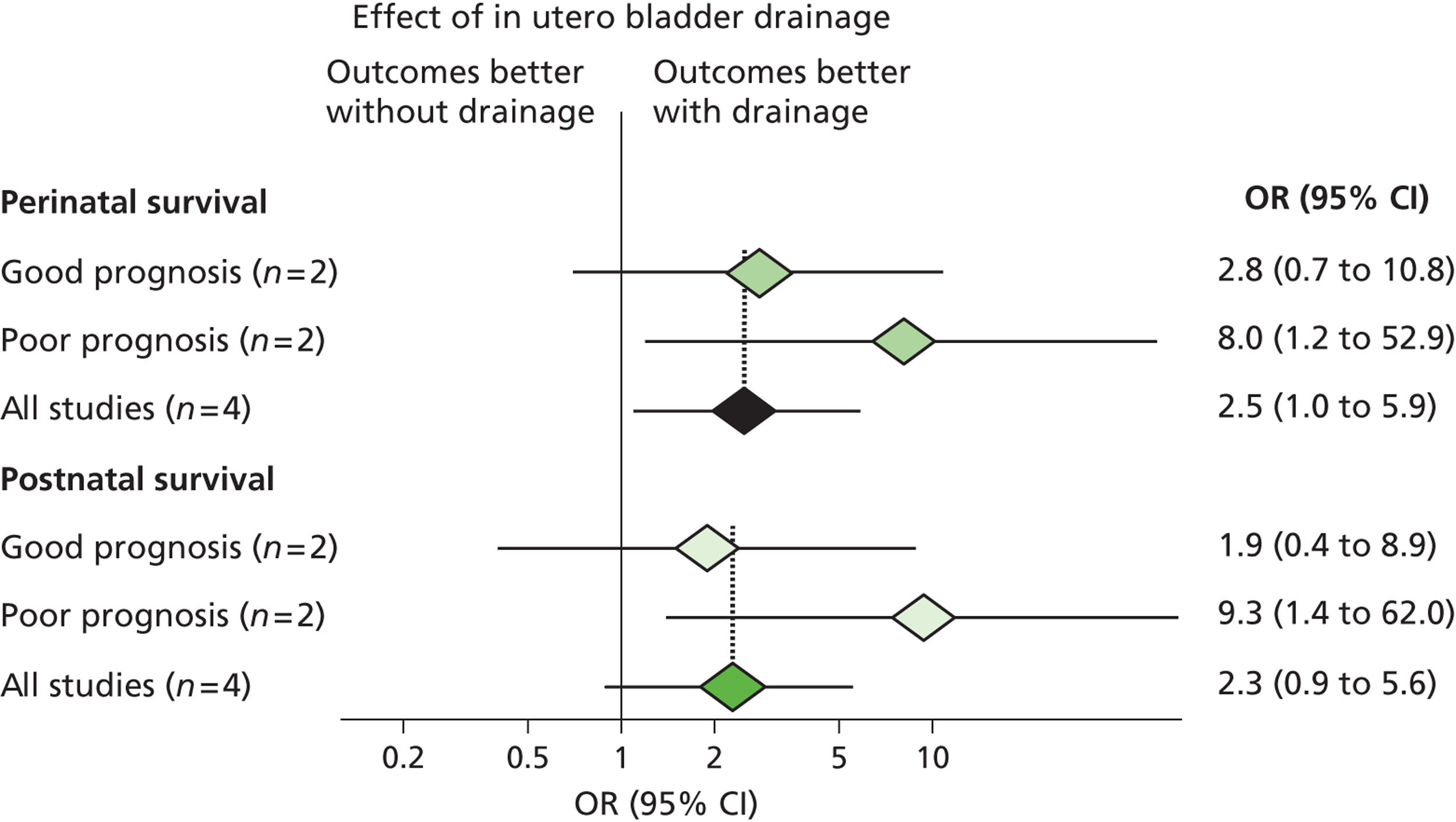
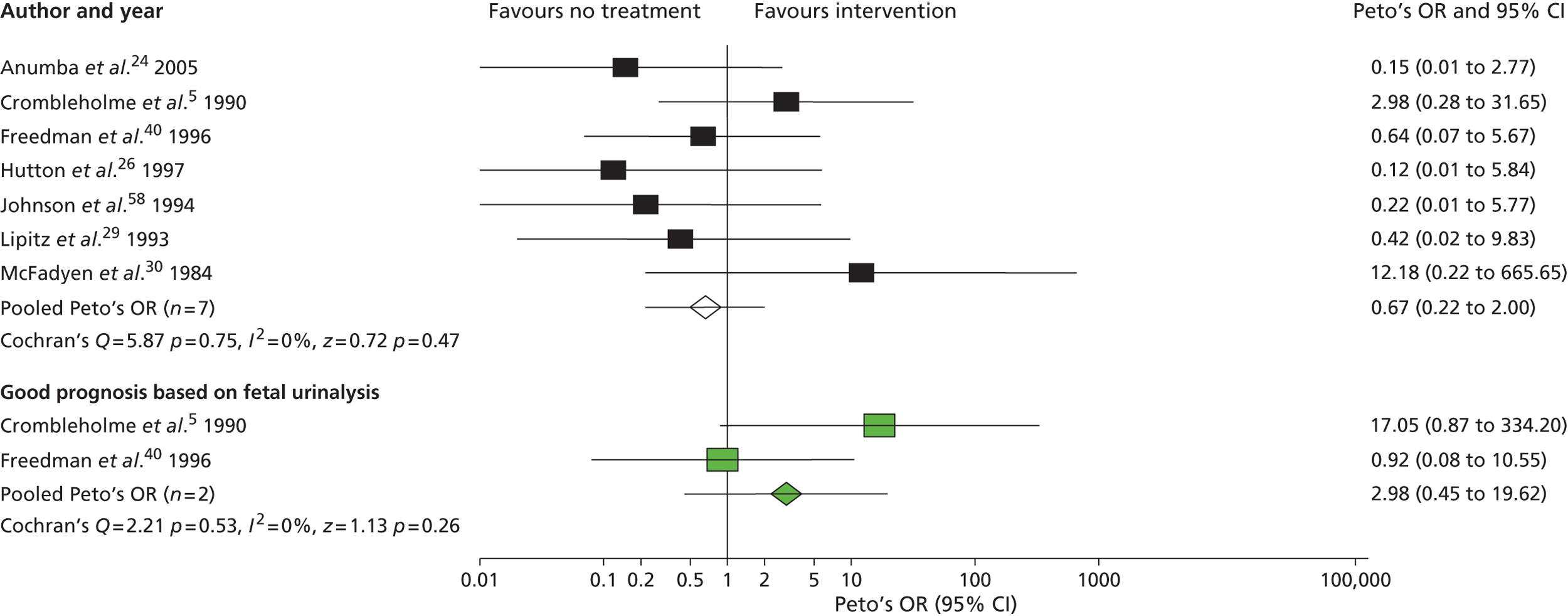
In 2003 members of our group published a systematic review evaluating the effectiveness of bladder drainage (VAS or vesicocentesis) for the management of LUTO. 13 Of the 16 studies deemed suitable for inclusion in the review, there was not a single randomised controlled trial (RCT). Within studies, authors divided the population into good and poor prognostic groups based on ultrasound features and fetal urinalysis. The conclusion of the systematic review was that the quality of the evidence was poor, with variability in study design and follow-up and the potential for bias in observational studies, raising concerns about the validity of the results ( Figure 1 ). In the four studies included in the meta-analysis ( Figure 2 ), the pooled perinatal survival rate in the conservative management group was 13/33 (39%), with a pooled odds ratio (OR) for perinatal survival of 2.53 [95% confidence interval (CI) 1.08 to 5.93], equivalent to an improvement in survival of 23 percentage points with bladder drainage (i.e. 39% survival compared with 62% survival). Observational evidence therefore suggested that bladder drainage was of potential benefit in improving survival in fetuses with LUTO, but the observational nature of the studies left a significant possibility that the estimate was biased through patient selection. This justified the need to proceed with a RCT. The results of this systematic review were also used to inform the sample size calculation for the RCT.
FIGURE 1.
Bar chart summarising the quality of evidence of papers included in the systematic review of the effectiveness of prenatal bladder drainage in LUTO. 13
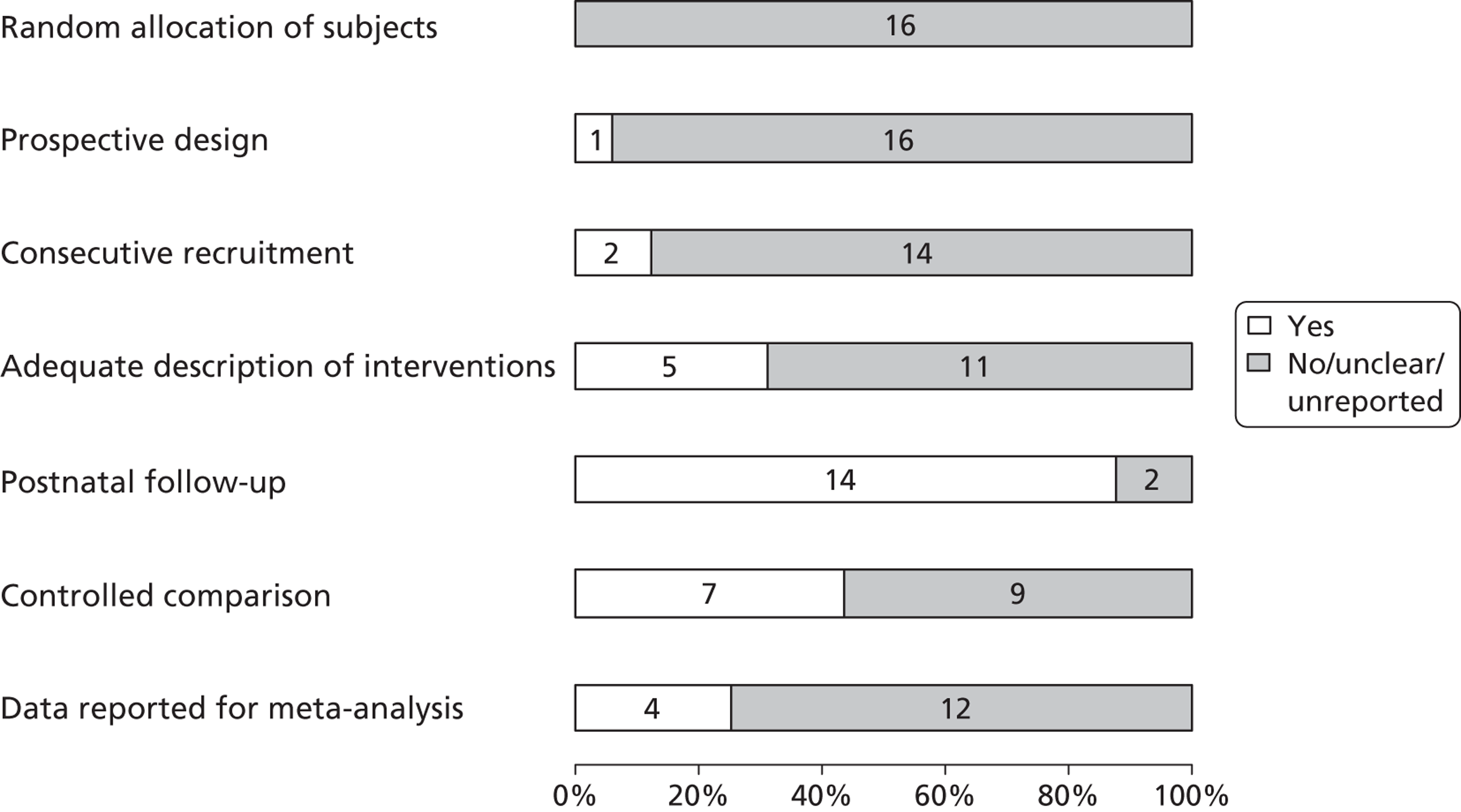
FIGURE 2.
Forest plot showing the meta-analysis from the systematic review of the effectiveness of prenatal bladder drainage in LUTO. 13
On the basis of this systematic review, in 2005 the charity Wellbeing of Women (WoW) provided funding to set up a multicentre RCT and initiate recruitment and neonatal follow-up. Randomisation of patients commenced at Birmingham and Liverpool Women’s Hospitals in September 2005. At the time of applying for funding from the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme, the WoW funds had been used to appoint a research midwife and a trial co-ordinator (both part-time) and set up a secure web-based database, hosted by the Birmingham Clinical Trials Unit.
At the commencement of the HTA funding in September 2008, 12 patients had been randomised and 10 included in the prospective registry. Fifteen UK fetal medicine centres had full ethics and local research and development (R&D) approval. During this time we also published several papers14–18 to raise awareness of the trial. These resulted in enquiries from major international institutions wishing to collaborate. The purpose of HTA funding was to extend recruitment for 5 years until September 2013 and to extend recruitment to international centres. The HTA programme also funded long-term paediatric follow-up until 5 years of age (for the assessment of cognitive development, bladder function and control, renal function and the need for transplantation, and quality of life), an evaluation of clinicians’ prior beliefs of effectiveness, an evaluation of patient acceptability and an economic evaluation. It also allowed the resource for updating the evidence on the effectiveness of antenatal intervention in LUTO, the accuracy of ultrasound features at diagnosis for predicting outcome and the accuracy of fetal urinary analysis to predict postnatal renal outcome. Summaries of these systematic reviews are presented in the following sections.
Systematic reviews performed during the PLUTO study
Systematic review of the effectiveness of antenatal interventions in lower urinary tract obstruction
We have undertaken a systematic review of the effectiveness of antenatal intervention (VAS, vesicocentesis, fetal cystoscopy, open procedures), building on a previously published systematic review,13 using methodological advances in search strategies, quality assessment and statistical analysis. 19,20 Authors of papers in the original review were contacted to ensure that the correct and most complete data were obtained. The objective was to systematically review the literature to evaluate the effectiveness of antenatal interventions in improving perinatal survival and postnatal renal function in congenital LUTO. (This systematic review has been published in full21 and we summarise the methods and results here only.)
Extensive electronic searches (from database inception to 2009) were performed using medical subject headings (MeSH) and keywords, without restrictions. Reference lists of included studies were checked and all authors were contacted. Studies were selected according to the following criteria:
-
population – fetuses with ultrasonographic evidence of LUTO (enlarged bladder, bilateral hydronephrosis, keyhole sign)
-
intervention – bladder drainage by vesicocentesis, VAS, fetoscopic surgery (e.g. cystoscopy and ablation of valves, open fetal bladder surgery)
-
outcome – perinatal mortality, measurement of renal function in survivors (e.g. serum creatinine, need for dialysis/transplantation), other morbidity indicators (e.g. need for ventilation)
-
study design – RCTs, controlled and uncontrolled observational studies; case reports and case series with less than five cases were excluded.
Data on study design and quality and the results were extracted to construct 2 × 2 tables. The reporting of the study was judged according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement, incorporating assessment of aspects of study quality. 22 We performed meta-analysis to explore the effect of antenatal interventions on outcome according to predefined subgroups. The effect of any intervention compared with no treatment was computed for each outcome: overall survival, survival excluding voluntary termination of pregnancy (TOP), perinatal survival [excluding voluntary TOP and intrauterine death (IUD)] and survival with normal postnatal renal function. Subgroup analyses were performed according to predicted fetal prognosis (according to antenatal ultrasound features or fetal urinalysis), and comparing treatments with one another. Forest plots were constructed for each group. We inspected for heterogeneity visually and statistically, calculating the Cochran’s Q statistic and the I 2 value. 19 An I 2 value > 50% was felt to demonstrate significant heterogeneity between studies. 23 In the event, low levels of heterogeneity resulted in fixed-effects models being used throughout using Peto’s method.
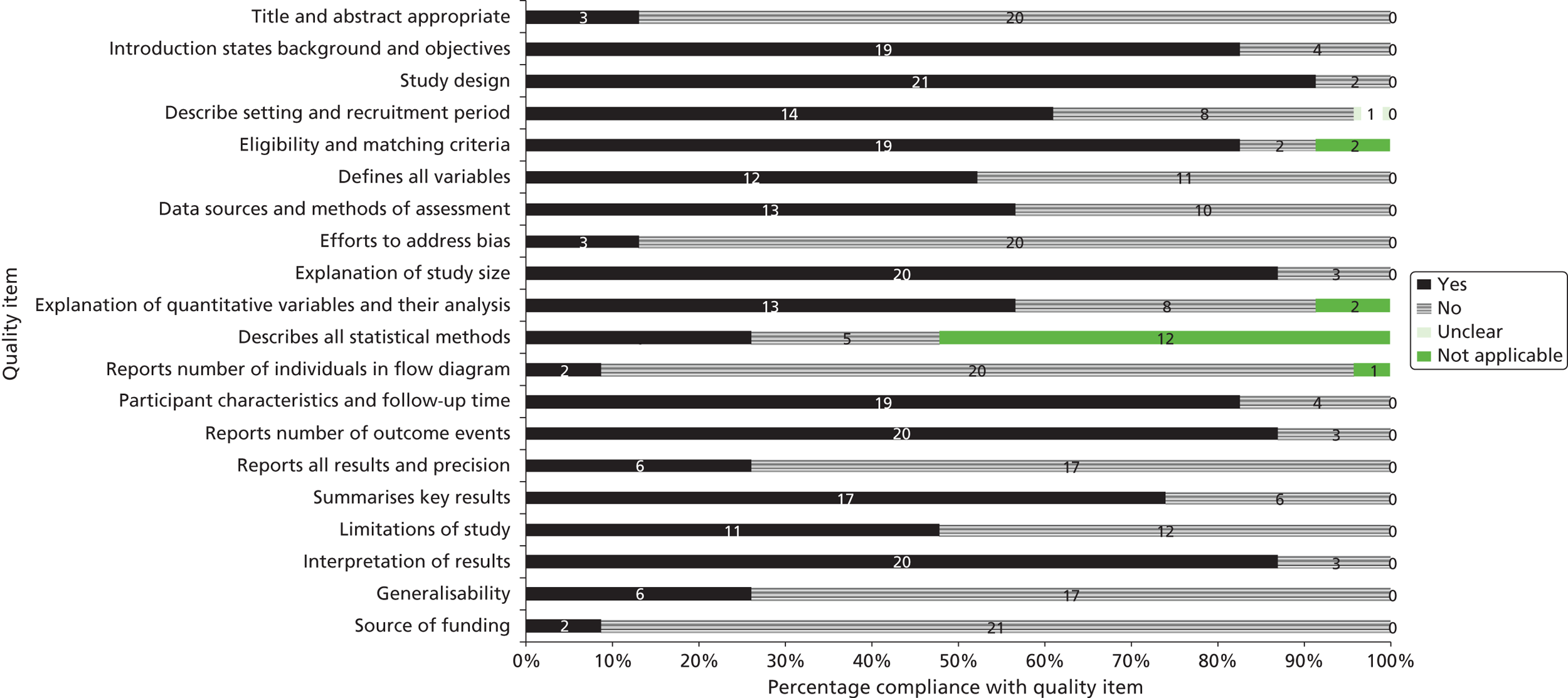
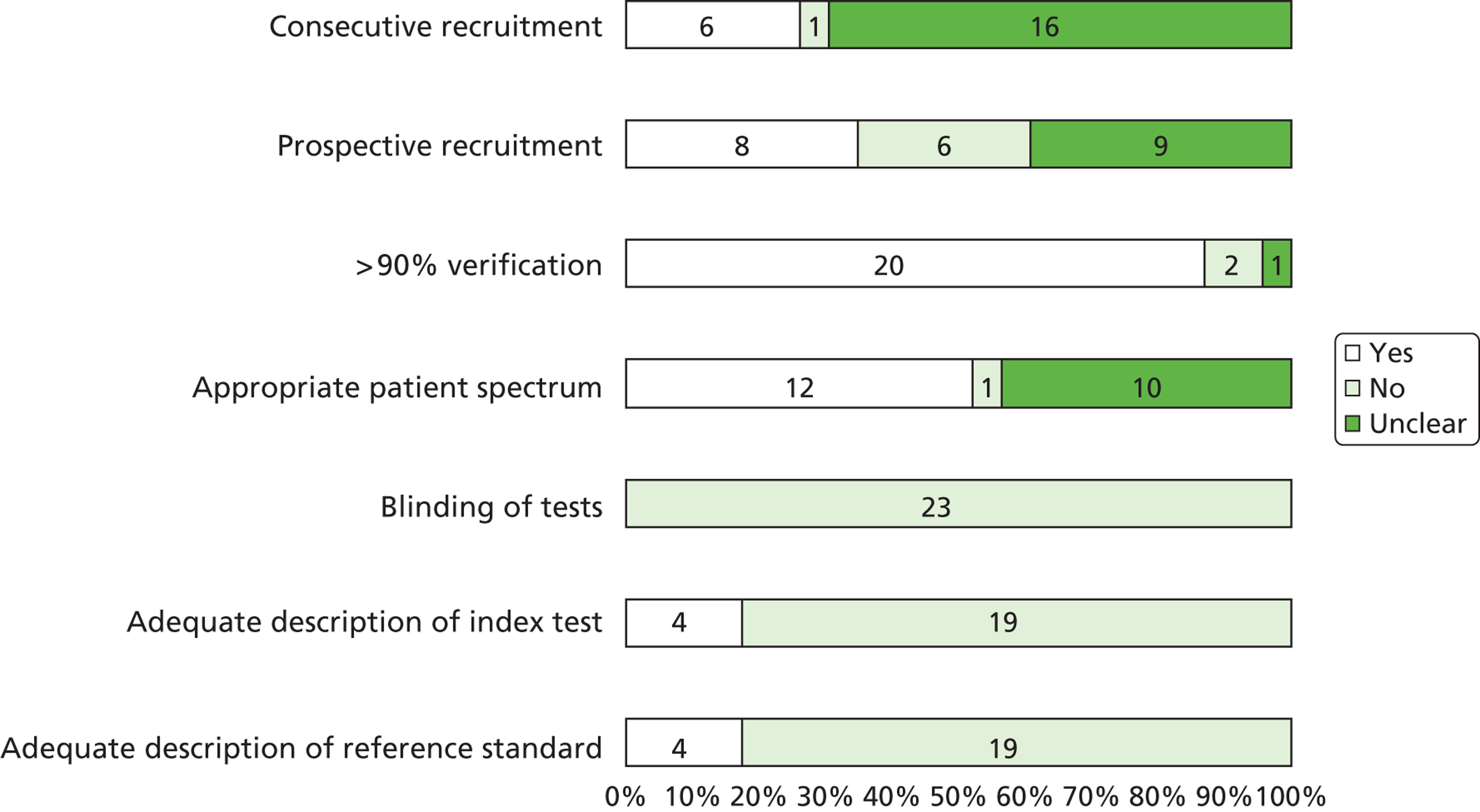
A total of 20 articles5,6,9,24–40 including 369 fetuses were eligible for inclusion in the review. All fetuses included had ultrasound features suggestive of LUTO (i.e. enlarged fetal bladder with dilated proximal urethra with or without associated hydronephrosis). The reported gestational age at diagnosis ranged from 13 to 38 weeks. Of the 369 fetuses, 261 (71%) received an antenatal intervention intended to relieve the obstruction. In the majority of cases (87%) this was a percutaneous VAS. Nine fetuses underwent an open procedure (by maternal laparotomy and hysterotomy) including open shunt insertion, bladder marsupialisation or cutaneous ureterostomy; 26 underwent fetal cystoscopy and 14 of these had ablation of PUVs using laser fulguration, urethral stent or hydroablation. Vesicocentesis was considered a diagnostic or therapeutic procedure depending on the technique used. Fetuses were classified as having a good or poor predicted prognosis by the study authors based on fetal urinalysis results prior to intervention. The overall study quality was variable ( Figure 3 ). There were no eligible RCTs; included studies represented a combination of prospective and retrospective cohort studies. Over 80% of studies complied with the STROBE statement elements, describing study design, explanation of study size, participant eligibility criteria, patient characteristics and follow-up, and number of outcome events. 22 However, few studies made efforts to address bias and most were poor in their reporting of the technique used for the intervention, the overall results and the precision of the findings.
FIGURE 3.
Bar chart summarising the quality of evidence of papers included in the systematic review of the effectiveness of antenatal intervention in LUTO. Numbers in the bars are the numbers of studies. Reproduced from Morris RK, Malin GL, Khan KS, Kilby MD. Systematic review of the effectiveness of antenatal intervention for the treatment of congenital lower urinary tract obstruction. BJOG 2010;117:382–9021 © 2010 The Authors Journal compilation © RCOG 2010 BJOG An International Journal of Obstetrics and Gynaecology, with permission from John Wiley & Sons, Inc.
Meta-analysis of non-randomised observational studies ( Figure 4 ) demonstrated that prenatal bladder drainage for LUTO may improve perinatal survival (OR 3.82, 95% CI 2.14 to 6.84), particularly in those with a poor predicted outcome (identified by fetal urinalysis) (OR 26.19, 95% CI 4.39 to 156.25). However, the observational data suggested that survivors had a high residual risk of poor postnatal renal function ( Figure 5 ). VAS compared with no treatment had an OR of 3.86 (95% CI 2.00 to 7.45) for perinatal survival and an OR of 0.50 (95% CI 0.13 to 1.90) for survival with normal renal function. The conclusions of the systematic review were that the quality of evidence was poor, with heterogeneity in study design and follow-up, and there was the potential for bias in observational studies raising concerns about the validity of the results. 41
FIGURE 4.
Effect of antenatal intervention compared with no treatment on perinatal survival (including voluntary TOP) stratified by predicted prognosis. Reproduced from Morris RK, Malin GL, Khan KS, Kilby MD. Systematic review of the effectiveness of antenatal intervention for the treatment of congenital lower urinary tract obstruction. BJOG 2010;117:382–9021 © 2010 The Authors Journal compilation © RCOG 2010 BJOG An International Journal of Obstetrics and Gynaecology, with permission from John Wiley & Sons, Inc.
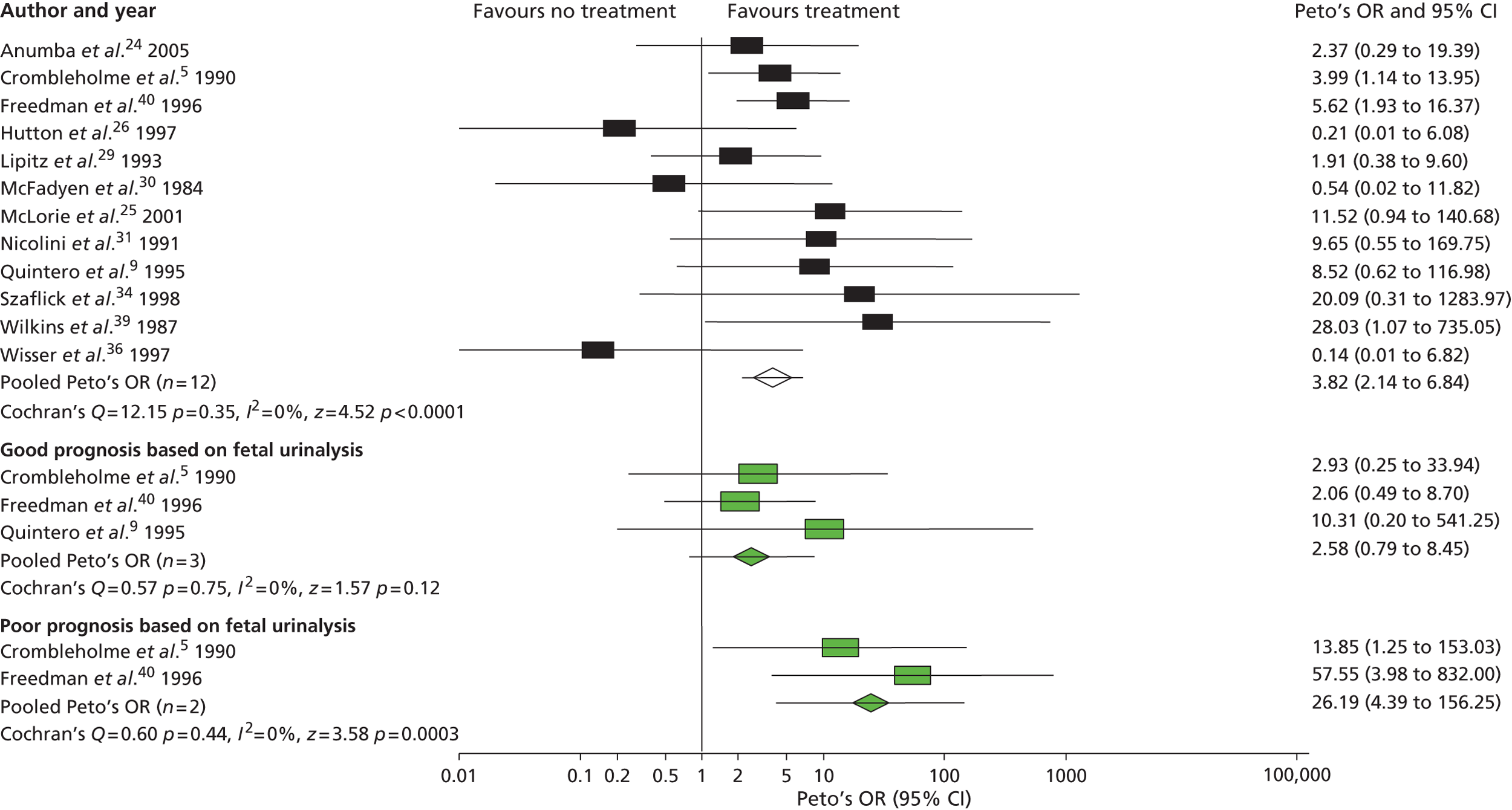
FIGURE 5.
Effect of antenatal intervention compared with no treatment on postnatal survival with normal renal function (excluding IUDs and voluntary TOP) stratified according to predicted prognosis. Reproduced from Morris RK, Malin GL, Khan KS, Kilby MD. Systematic review of the effectiveness of antenatal intervention for the treatment of congenital lower urinary tract obstruction. BJOG 2010;117:382–9021 © 2010 The Authors Journal compilation © RCOG 2010 BJOG An International Journal of Obstetrics and Gynaecology, with permission from John Wiley & Sons, Inc.
The observational evidence thus still justified the need to proceed with a RCT of this rare congenital malformation including long-term follow-up for renal function.
Systematic review of the accuracy of fetal urinalysis as a predictor of postnatal renal function
We systematically reviewed14 the evidence that obtaining a fetal urine sample before therapy by vesicocentesis and urinary analysis to measure sodium, calcium and β2-microglobulin concentrations may be helpful in identifying those babies most at risk of postnatal kidney damage. 42
The systematic review was conducted according to a protocol designed using widely recommended methods. 43–46 We searched MEDLINE (1966–2006), EMBASE (1980–2006), The Cochrane Library (2006, issue 2), the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (from inception to 2006), Medion, System for Information on Grey Literature in Europe (SIGLE), the Index of Scientific and Technical Proceedings, SciSearch®, the National Research Register and the Medical Conferences Register for relevant citations. In MEDLINE the search consisted of a combination of MeSH (e.g. urethral obstruction, hydronephrosis, fetal diseases) ‘or’ keywords (e.g. enlarged bladder, congenital urinary tract obstruction, posterior urethral valves) for disease. These were combined using ‘and’ with MeSH and keywords for intervention (e.g. shunting) ‘or’ investigation (e.g. ultrasound and fetal urine analysis).
Papers were included if they satisfied the following criteria:
-
population – fetuses with ultrasound evidence of congenital urinary tract obstruction
-
index test – any test on fetal urine
-
reference standard – any reference standard looking at postnatal renal function or renal dysplasia in non-survivors
-
study design – test accuracy studies that allowed generation of 2 × 2 tables to compute indices of test accuracy; case series with less than eight cases were excluded.
All articles meeting the selection criteria were also assessed for methodological quality using items from the Quality Assessment of Diagnostic Accuracy Studies (QUADAS) tool. 47,48 A study was considered to be of good quality if it utilised a prospective design with consecutive recruitment, included full verification of the test result with a reference standard and had an adequate description of both the index test and the reference standard, thus reducing bias. 43,44,48–50 Sensitivity, specificity and likelihood ratios (LRs) were generated and pooled using meta-analytical methods.
A total of 23 articles5,24,29,39,40,42,51–67 met the selection criteria. All of the studies were observational studies and of poor quality ( Figure 6 ).
FIGURE 6.
Quality of studies included in the review of fetal urinalysis in fetuses with obstructive uropathy to predict poor postnatal renal function. Stack bar chart with numbers inside bars indicating the numbers of studies. Reproduced from Morris RK, Quinlan-Jones E, Kilby MD, Khan KS. Systematic review of accuracy of fetal urine analysis to predict poor postnatal renal function in cases of congenital urinary tract obstruction. Prenatal Diagnosis 2007;27:900–1114 Copyright © 2007 John Wiley & Sons, Ltd, with permission from John Wiley & Sons, Inc.
The two most accurate tests were calcium > 95th centile for gestation (LR+ 6.65, 95% CI 0.23 to 190.96; LR− 0.19, 95% CI 0.05–0.74) and sodium > 95th centile for gestation (LR+ 4.46, 95% CI 1.71 to 11.6; LR− 0.39, 95% CI 0.17 to 0.88). β2-microglobulin was found to be less accurate (LR+ 2.92, 95% CI 1.28 to 6.69; LR− 0.53, 95% CI 0.24 to 1.17). However, there was no individual urinary analyte or threshold that could be shown to be of particular clinical value ( Table 1 ). The conclusion was that the current evidence demonstrated that none of the analytes of fetal urine investigated so far can be shown to yield clinically significant accuracy to predict poor postnatal renal function.
| Index test | Threshold | No. of studies | LR+ (95% CI) | LR− (95% CI) |
|---|---|---|---|---|
| Sodium | > 95th centile | 3 | 4.46 (1.71 to 11.6) | 0.39 (0.17 to 0.88) |
| Sodium | > 100 mEq/l or 100 mmol/l | 3 | 3.13 (0.78 to 12.58) | 0.37 (0.12 to 1.12) |
| Sodium | > 100 mg/dl | 3 | 3.33 (1.84 to 6.02) | 0.44 (0.19 to 1.01) |
| β2-microglobulin | > 2/2.5 mg/dl | 4 | 3.50 (0.37 to 33.5) | 0.46 (0.19 to 1.13) |
| β2-microglobulin | > 10 mg/dl | 2 | 4.61 (0.65 to 32.68) | 0.52 (0.24 to 1.13) |
| β2-microglobulin | ≥ 13 mg/dl | 3 | 2.92 (1.28 to 6.69) | 0.53 (0.24 to 1.17) |
| Calcium | > 95th centile | 2 | 6.65 (0.23 to 190.96) | 0.19 (0.05 to 0.74) |
| Calcium | > 0.95 mmol/l or > 1.25 mmol/l | 3 | 3.44 (1.78 to 6.65) | 0.43 (0.26 to 0.69) |
| Osmolality | > 200 mOsm/l or > 210 mOsm/l | 4 | 3.41 (1.88 to 6.19) | 0.33 (0.14 to 0.77) |
| Chloride | > 90 mmol/l or > 90 mEq/l | 3 | 3.09 (0.57 to 16.71) | 0.46 (0.15 to 1.42) |
Systematic review of the accuracy of antenatal diagnosis and ultrasound markers in predicting postnatal outcome
Data relating to the natural history of LUTO are difficult to identify. However, recent registry data from Finland68 have presented outcomes in babies with PUVs (n = 46), with 23 diagnosed prenatally (with no intervention) and 23 diagnosed postnatally (with apparently ‘normal’ ultrasound appearances in the majority). This cohort study is unique, in that long-term follow-up over 10 years was performed (mean 12.5 years, range 5.5–20.1 years). Despite postnatal treatment, 13% of this cohort developed chronic renal failure in childhood and adolescence with 17% developing end-stage renal disease. These rates are lower than those reported in the literature for antenatally diagnosed LUTO, possibly reflecting a different pathogenesis. There was no significant difference in the proportion of patients with primary or acquired renal dysplasia between those prenatally diagnosed and those postnatally diagnosed. There was also no difference in long-term renal outcome between those patients with a prenatal diagnosis and those with a postnatal diagnosis, with no difference in the mean age of advancing to end-stage renal disease. The mean age of achieving continence in both prenatal and postnatal diagnostic groups was not significantly different at 5 years of age. Such data indicate that the severity of the ultrasound appearances (in the postnatally diagnosed group at least two prenatal ultrasound examinations had been performed and demonstrated no anomaly) in LUTO have little bearing on the eventual long-term paediatric outcome. A limited amount of literature has reported on the effects of gestational age of < 24 weeks at diagnosis,5 associated prenatal ultrasound features of macrocystic/microcystic renal abnormality (indicative of dysplastic change)6,7 and significant oligohydramnios,69 demonstrating good predictive accuracy for poor outcome (but with considerable heterogeneity of results). No consensus exists at present as to the best prenatal ultrasound sign (or combination of signs) to predict postnatal renal function.
During the study period our group performed and published a systematic review of the literature on antenatal ultrasound to predict postnatal renal function in LUTO. 70 We searched MEDLINE (1966–April 2008), EMBASE (1980–April 2008), The Cochrane Library (2008, issue 4), CINAHL (from inception to 2008), Medion, SIGLE, the Index of Scientific and Technical Proceedings; SciSearch, the National Research Register and the Medical Conferences Register for relevant citations. In MEDLINE the search consisted of a combination of MeSH (e.g. urethral obstruction, hydronephrosis, fetal diseases) ‘or’ keywords (e.g. enlarged bladder, congenital urinary tract obstruction, posterior urethral valves) for disease. These were combined using ‘and’ with MeSH and keywords for intervention (e.g. shunting) ‘or’ investigation (e.g. ultrasound and fetal urine analysis).
Papers were assessed for inclusion using the following criteria:
-
population – fetuses with ultrasound evidence of congenital LUTO
-
diagnostic measure – any prenatal ultrasound parameter
-
outcome measure – any outcome measure looking at postnatal renal function or renal dysplasia in non-survivors
-
study design – RCTs or observational studies that allowed generation of 2 × 2 tables (true positives, false positives, false negatives and true negatives) to compute indices of test accuracy; case series with less than five cases were excluded.
Data were used to construct 2 × 2 tables of test accuracy using the antenatal ultrasound parameter reported in the paper and the authors’ definition of a positive or negative test and comparing these results to the postnatal renal function or outcome for each individual patient. All articles meeting the selection criteria were also assessed for quality using items from validated tools. 47,48,71 A study was considered to be of good quality if it utilised a prospective design with consecutive recruitment, included full verification of the diagnostic measure with the outcome measure and included an adequate description of both of these measures, thus reducing bias. 43,44,72 Sensitivity, specificity and LRs were generated.
Using the 2 × 2 tables we computed sensitivity (true positive rate), specificity (true negative rate) and the LRs73 (the ratio of the probability of the specific test result in people who do have the disease to the probability in people who do not) and 95% CIs for individual studies. Subgroup analysis with pooling of LRs was performed only when there were at least two studies with similar characteristics within that group. All statistical analyses were performed using Meta-DiSc 1.3 software (see www.hrc.es/investigacion/metadisc.html)74 with 0.5 being added to all cells in 2 × 2 tables and using a random-effects model. A p-value of < 0.05 was used throughout for statistical significance and the chi-squared test was used as a statistical test of heterogeneity. When there was still significant statistical heterogeneity, summary receiver operating characteristic curves were drawn and the area under the curve presented as the summary measure of accuracy.
The final data set included 13 articles,11,12,24,26,29,53,54,75–80 with a total of 215 women and 33 2 × 2 tables. All included studies were observational. The populations of included studies all consisted of fetuses with antenatally suspected LUTO that was subsequently confirmed postnatally. The thresholds used for the diagnostic measure varied between the studies as did the outcome measure. The papers all described investigation of a small number of subjects with imprecise results and did not report characteristics of patients or data collection. Meta-analysis was performed using subgroups with the same diagnostic measure and an outcome measure assessing renal function in survivors to minimise clinical heterogeneity ( Table 2 ). The ultrasound parameter that showed the best predictive value for postnatal renal function in survivors was renal cortical appearance, with a sensitivity of 0.57 (95% CI 0.37 to 0.76), a specificity of 0.84 (95% CI 0.71 to 0.94) and an area under the curve of 0.78.
| Diagnostic measure | Sensitivity (95% CI) | Specificity (95% CI) | χ2 test and p-value | Area under receiver operating characteristic curve |
|---|---|---|---|---|
| Oligohydramnios | 0.63 (0.51 to 0.74) | 0.76 (0.65 to 0.85) | 19.67, p = 0.02 | 0.74 |
| Renal cortical appearance | 0.57 (0.37 to 0.76) | 0.84 (0.71 to 0.94) | 10.29, p = 0.04 | 0.78 |
| Gestation of < 24 weeks at diagnosis | 0.48 (0.26 to 0.70) | 0.82 (0.66 to 0.92) | 3.88, p = 0.14 | 0.68 |
We concluded that measurement of amniotic fluid volume and the appearance of the renal cortex at diagnosis showed promising predictive accuracy for poor postnatal renal function (see Table 2 ).
Evidence on long-term outcomes and assessment measures in children affected by lower urinary tract obstruction
There is published evidence to suggest that, as well as the long-term complication of end-stage renal failure, children affected by LUTO are at risk of bladder dysfunction and incontinence (many requiring reconstructive surgery). In addition, many children have poor growth velocities and eventually male infertility (often associated with chronic renal impairment). 81–83 A recently published small cohort study83 suggested that children with LUTO in whom VAS is performed antenatally are expected to have normal cognitive abilities and to achieve acceptable continence with medical and surgical care with similar quality-of-life scores to those of a healthy child. Factors reported to be associated with poor long-term outcome in PUV include (1) prenatal detection and, at postnatal investigation, (2) bilateral vesicoureteric reflux, (3) poor detrusor muscle function, (4) delayed achievement of urinary continence, (5) recurrent urinary tract infections and (6) persistent elevation of serum creatinine concentrations after PUV ablation. 7,9,10,69
Our group has performed literature searches and directly contacted experts in the field to determine the best diagnostic tools available for assessment of long-term disability and micturition function in children. To the best of our knowledge there are no questionnaires specifically designed to assess these outcomes in children affected by LUTO. However, the Pediatric Quality of Life Inventory (PedsQL) 4.0 questionnaire84 has been used to assess quality of life in many different chronic conditions; it is of proven reliability and validity in the age group included in this trial. For assessment of cognitive function the Parent Report of Children’s Abilities (PARCA) tool has been validated in children up to age 2 years,85 including children born preterm. 86 For assessment of micturition we found no suitable validated questionnaires reported in the literature and thus in collaboration with paediatric urologists and nephrologists we designed a specific questionnaire for the PLUTO trial.
Evidence of experts’ prior beliefs for the effectiveness of vesicoamniotic shunting and evidence for Bayesian analysis
Published examples of collecting Bayesian priors are sparse in obstetrics and particularly in relation to treatments in the subspecialty of fetal medicine. There are no previous publications relating to clinicians’ prior beliefs for the effectiveness of VAS.
The Bayesian approach to analysis provides several important benefits. These include the ability to interpret CIs as providing intervals that contain the true effect with some particular probability, say a 95% probability, and importantly the provision of estimates of probabilities of benefits greater than some clinically important effect size. An additional important feature of Bayesian methods is the incorporation of evidence additional to the conventional data. 87,88 This additional evidence is called a prior distribution and summarises information from external sources.
This external evidence might take the form of subjective opinions or down-weighted less rigorous, or indirectly related, results from previous studies. 89,90 Opinion-based prior distributions might, for example, be based on sceptical opinions, perhaps reflecting the belief that the intervention might be less effective in routine practice (compared with the clinical trial setting), or on optimistic opinions, reflecting the belief that the intervention might be more beneficial in practice (compared with the clinical trial setting), perhaps because of more flexible dosing titration for example. 91,92 Or opinion-based prior distributions might, for example, represent the current beliefs of those considered experts within the particular field.
The use of Bayesian methods in clinical trials, although far from novel, is not conventional. Recently Bayesian methods have begun to feature in both the design and the analysis of trials. The use of Bayesian informative priors is viewed by many as controversial but may be particularly important in the study of rare diseases for which recruitment to trials can be difficult.
Evidence for cost-effectiveness
There are no studies of the cost-effectiveness of interventions for LUTO.
Evidence for patient acceptability
The involvement of pregnant women in RCTs raises a number of practical and ethical issues. A literature search revealed that there were few studies93,94 examining women’s reasons for declining to participate in trials and the factors that may affect consent in pregnancy. We found no studies that specifically looked at trials of a surgical intervention in pregnancy. Factors that appear to negatively influence participation in trials during pregnancy are the existence of a placebo arm93,94 and a belief by mothers that they are not entitled to place their fetus at risk. 93 Positive influences include the potential or perceived benefit to the fetus. 94,95
The need for a large simple trial of vesicoamniotic shunting compared with conservative management for lower urinary tract obstruction
On the basis of published evidence and evidence from specialist advisors, in 2006 NICE produced an interventional procedures guidance for fetal VAS (IPG 2028). The guidance states that the ‘current evidence on safety and efficacy of fetal vesico-amniotic shunting for LUTO does not appear adequate for this procedure to be used without special arrangements for consent and for audit or research’. The guidance thus recommends randomisation to PLUTO: ‘Clinicians are encouraged to enter patients into this trial or the associated registry’ (section 1.1). Thus, from the beginning of the HTA-funded trial, the national recommendation to clinicians was to use VAS only within the PLUTO trial.
Chapter 2 Objectives
The PLUTO study addressed the following objectives:
-
primary objective:
-
to determine if intrauterine VAS for fetal bladder outflow obstruction improves perinatal and neonatal mortality and long-term renal function compared with conservative non-interventional care
-
-
secondary objectives:
-
to determine if placement of a VAS in LUTO improves short-term morbidity
-
to determine the long-term effects of VAS with respect to (1) the development of chronic renal failure and need for dialysis or transplantation, (2) the development of incontinence (bladder dysfunction) and (3) disability-free life-years (incorporating assessment of cognitive development, quality of life, micturition and general health)
-
to determine if improvement is related to prognostic assessment at diagnosis and, if possible, to derive a prognostic risk index
-
to maintain a prospective registry of patients with LUTO as part of a comprehensive cohort design
-
to ascertain influences on the decision-making of women with respect to opting for TOP or randomisation (patient acceptability of trial) and to determine acceptability of the intervention to parents
-
to determine clinicians’ prior beliefs about the effectiveness of shunting and to analyse trial results from a Bayesian perspective
-
to determine the cost-effectiveness of VAS compared with conservative management
-
to determine the epidemiology of this condition.
-
Outline of the report
This report presents the work completed as part of the PLUTO study. It commences with the epidemiological study (see Chapter 3 ). We then report the methods for the RCT and the registry in Chapter 4 and their findings in Chapters 5 and 6 , respectively, along with our conclusions. In Chapter 6 we also present a logistic regression analysis for the RCT and registry to look at predictors of survival. In Chapter 7 we present the Bayesian analysis and in Chapter 8 the economic analysis. The patient acceptability study is presented in Chapter 9 . In Chapter 10 we discuss the overall findings of all parts of the PLUTO study and present our recommendations for future research and implications for practice.
When previously published work from the PLUTO study is discussed this is acknowledged with the appropriate citation; large duplications of previously published material are indicated with a note after the corresponding text. A list of publications arising from the PLUTO study can be found in the acknowledgements section.
Chapter 3 A population-based epidemiological study
Introduction
As discussed in Chapter 1 , LUTO is a rare condition of varying aetiology. Unfortunately, no national register exists for LUTO as it does for other conditions such as cleft lip and palate and Down syndrome. However, cases of LUTO are notified to the individual regional congenital anomaly registers, which cover 49% of births in England, 52% of births in Ireland, 24% of births in Scotland and 100% of births in Wales. Of the regional registries, the West Midlands Congenital Anomaly Register (WMCAR) has the second largest number of annual births and the largest number of notifications annually.
To date, the largest published population-based registry study from the northern region of England has demonstrated that LUTO has an incidence of 2.2 per 10,000 total births. 24 In total, 113 cases were registered between 1984 and 1997 with the underlying pathology identified by postnatal investigation or autopsy. PUV was noted in 64% (1.4/10,000 births), urethral atresia in 29% (0.7/10,000 births) and ‘prune belly syndrome’ (deficient abdominal wall musculature, undescended testes and urinary tract abnormalities) in 4%; in the remaining 4% the pathology was unclassified. 24 However, there have been significant improvements in prenatal ultrasound affecting the potential detection of LUTO and changes in the management of this condition since the publication of data from these historical cohorts.
During the period of WoW funding (2005–8) the trial group noticed that a smaller number of cases were being reported to the trial office than would have been expected from historical incidence rates. It was unclear whether this was because of under-reporting by clinicians or a change in the epidemiology of the condition. Thus, as part of the HTA-funded PLUTO study we proposed a retrospective epidemiological study of two parts. The first was to use the WMCAR and the second was to use all population-based registries that were part of the British Isles Network of Congenital Anomaly Registers (BINOCAR).
Objectives
Study within West Midlands:
-
to perform a population-based study using the WMCAR to ascertain the incidence of LUTO
-
to ascertain the notification of cases to the PLUTO trial
-
to look at prenatal diagnosis of the condition and outcomes for the West Midlands population using the fetal medicine unit ultrasound and pathology data.
Study within BINOCAR:
-
to perform a population-based study using the BINOCAR (England and Wales) to ascertain the incidence of LUTO and look for trends over time and regional differences.
The study within the West Midlands commenced in 2008 and was completed in 2010. During this period there were consultations with the BINOCAR group to gain access to other registries. Unfortunately, because of difficulties with funding the searches of these individual registries, this part of the study was not possible. BINOCAR do publish yearly data for prevalence and the 2010 report gave a prevalence for PUV+/− prune belly syndrome of 1.2 per 10,000 total births (95% CI 0.8 to 1.7), of which 23 cases were live births and nine were TOPs. 96 The prevalence rate was not affected by exclusion of chromosomal anomalies. Trends in prevalence from 2006 to 2010 show an average percentage decrease per year of −8.3% (95% CI −2.3% to −17.9%), with the highest prevalence being seen in 2006. However, it must be noted that these rates include cases with multiple anomalies and pre/postnatal diagnoses. BINOCAR also publish TOP rates per major anomaly group and in 2010 urinary anomalies had a TOP rate of 5.3 per 10,000 total births (95% CI 4.5 to 6.3); only chromosomal, nervous system and congenital heart defects had higher TOP rates. 96
Our group thus performed a population-based epidemiological study using data from the WMCAR of cases of LUTO from a cohort of births between 1995 and 2007 (13-year period). The trends in incidence, associated anomalies, clinical outcomes according to time of diagnosis and sensitivity of prenatal diagnosis by ultrasound are described. This work has been previously published and is reproduced from Malin GL, Tonks A, Morris RK, Gardosi J, Kilby MD. Congenital lower urinary tract obstruction: a population based epidemiology study. BJOG 2012;119:1455–64,97 © 2012 The Authors BJOG An International Journal of Obstetrics and Gynaecology © 2012 RCOG, with permission from John Wiley & Sons, Inc.
Methods
The study sample comprised births to West Midlands residents between 1995 and 2007. All outcomes of affected pregnancies were included, whether registered births (live and stillborn), TOPs or spontaneous fetal losses at all gestations. Cases were identified from the WMCAR, which covers a birth population of 10.2% of the total number of births for England and Wales. 98 Cases were identified using a list of International Classification of Diseases, 10th Edition (ICD-10) codes and keywords in diagnoses or text fields (see Appendix 1 ). Only cases with a final confirmed diagnosis of LUTO (PUVs, urethral atresia, urethral stenosis, prune belly syndrome and evidence of LUTO but exact pathology not determined), verified by pathology report, radiology or surgical report, were included in the final cohort. Cases of cloacal dysgenesis/urorectal septum malformation sequence (n = 14) were excluded from detailed analysis. Such pathologies are complex and the fetal and perinatal outcome poor, not only because of underlying urogenital malformation but also because of coexistent pathologies and morbidities.
To ensure high ascertainment levels, cross-validation was carried out with cases identified from other fetal and paediatric services. These included post-mortem reports (from the regional perinatal pathology service), ultrasound scans from the regional fetal medicine department and referrals (surgical and radiology) to Birmingham Children’s Hospital (surgical database and radiology database). Searches of these databases used the same keywords (see Appendix 1 ). For cases identified from antenatal databases this relied on the characteristic features as described in Appendix 1 or on the practitioner stating a diagnosis of suspected LUTO. Survival data were obtained from the WMCAR database. For all survivors, attempts were made to obtain outcome data regarding surgical procedures, renal function, including the most recent status of renal impairment (i.e. normal, mild, moderate, severe or end stage as defined by the primary clinician according to treatment required and serum creatinine) and nadir creatinine levels. Data regarding these outcomes were abstracted from the individual hospital records at Birmingham Children’s Hospital and cross-referenced to those at the West Midlands Perinatal Institute. These data were analysed using Fisher’s exact test to look for differences in outcome for prenatally and postnatally diagnosed cases.
Lower urinary tract obstruction cases were subdivided into two groups: isolated malformations of the bladder neck and those with coexisting structural/chromosomal anomalies. A subgroup of cases eligible for VAS (male, singleton, isolated) was also identified. False-positive cases (suspected prenatally not confirmed postnatally) and false-negative cases (diagnosed postnatally with no suspicion prenatally) were identified and analysed separately. Annual denominator data on total births by maternal age were provided by the Office for National Statistics (ONS). 99
Missing data
In 67 cases it could not be determined whether a prenatal diagnosis had been made. However, it was confirmed that no referral was made to the regional fetal medicine centre or local ultrasound centres nor was a prenatal notification sent to the WMCAR. Therefore, within the analysis of prenatal detection rates it was assumed that this group had no prenatal diagnosis. There were four cases (all isolated LUTO cases) in which the fetal sex could not be determined as this information was not included in the prenatal ultrasound report and all were terminated or delivered spontaneously before 20 weeks’ gestation and sex could not be determined macroscopically. These were included in the ‘isolated, non-female subgroup’ as they were likely to be male fetuses (however, we did not have cytogenetic confirmation of this fact).
Prevalence/statistical analyses
Birth prevalence is the preferred measurement when estimating the frequency of congenital anomalies. The calculation of birth incidence requires a precise definition of the denominator,100 including the number of all early spontaneous losses, and these data are not routinely recorded, thus leading to an underestimation of the denominator and consequent overestimation of the prevalence. The prevalence ratio at birth of the congenital anomaly of interest is therefore the number of affected births divided by the total number of live births and stillbirths. In studies in which mid-trimester loss data and TOP data are collected for specific anomalies, the denominator can be adjusted by including TOP data for the population at risk. However, there are no estimates of the total number of mid-trimester losses available routinely for population data. The denominator used is therefore a slight underestimate but it is the best possible denominator available. Numerator and denominator data were linked by maternal postcode at delivery to small-area deprivation scores based on the Index of Multiple Deprivation (IMD) 2004 (see http://data.gov.uk/dataset/imd_2004). Incidence rates were calculated for quintiles of deprivation.
We also present the prevalence of LUTO by maternal ethnic group, as defined at antenatal booking. The lack of population-based data on maternal ethnic group for the study period necessitates the use of estimates for denominator data. The distribution of all births by maternal ethnic group was generated by standardising the total number of births during the study using an ethnic distribution derived from census data. The ethnic distribution applied to the denominator was that of the population aged 0–4 years born in the UK derived from the 2001 census. 101 A sensitivity analysis was undertaken on the denominator to test significant findings.
A chi-squared test was used to examine the association between prevalence and maternal age and to examine secular trends. ORs and confidence limits were calculated using Microsoft Excel 2003 (Microsoft Corporation, Redmond, WA, USA) and trends were calculated within Epi lnfo version 6 (Centers for Disease Control and Prevention, Atlanta, GA, USA).
Results
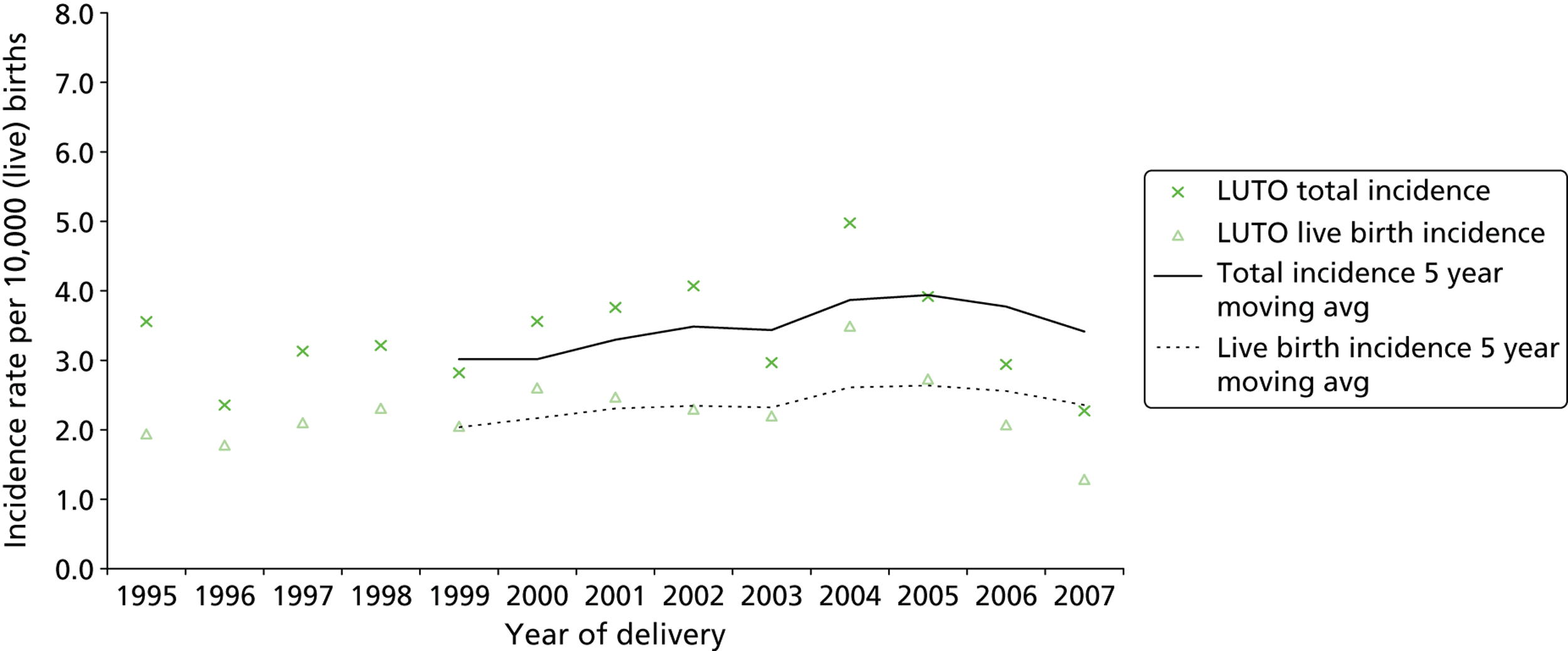
During the study period (1995–2007) there were 851,419 live births and stillbirths to West Midlands residents, including 284 identified and confirmed LUTO cases, giving a total incidence of 3.34 (95% CI 2.95 to 3.72) per 10,000 total births ( Figure 7 ). The incidence did not change significantly over time (χ2 = 0.175, p = 0.68). The cohort comprised 270 singleton pregnancies, 13 twin pregnancies and a triplet pregnancy. All LUTO cases occurring in multiple pregnancies involved a single affected fetus. Of the affected cases, 66.9% (n = 190) resulted in a live birth, giving a live birth incidence of 2.24 (95% CI 1.93 to 2.56) per 10,000 live births (1/4455). There was no significant association between the incidence of LUTO and maternal age (χ2 = 1.35, p = 0.245). The median maternal age for all cases was 27 years [interquartile range (IQR) 24 to 32 years].
FIGURE 7.
Lower urinary tract obstruction cases: total and live birth incidence with trend 1995–2007. Reproduced from Malin GL, Tonks A, Morris RK, Gardosi J, Kilby MD. Congenital lower urinary tract obstruction: a population based epidemiology study. BJOG 2012;119:1455–64,97 © 2012 The Authors BJOG An International Journal of Obstetrics and Gynaecology © 2012 RCOG, with permission from John Wiley & Sons, Inc.
Lower urinary tract obstruction incidence was significantly associated with deprivation quintile [χ2 = 6.80 (linear trend), p < 0.01] ( Table 3 ). Within the West Midlands only 43% of mothers are resident in the three least deprived quintiles combined (quintiles 1–3); the incidence of LUTO in the remaining two most deprived quintiles combined (quintiles 4 and 5) was significantly higher (OR 1.56, 95% CI 1.22 to 2.00).
| Deprivation (IMD 2004) | No. of cases of LUTO | Total no. of births | Incidence per 10,000 total births (95% CI) |
|---|---|---|---|
| Quintiles 1–3 | 92 | 363,911 | 2.5 (2.0 to 3.0) |
| Quintile 4 | 70 | 166,441 | 4.2 (3.2 to 5.2) |
| Quintile 5 | 122 | 320,931 | 3.8 (3.1 to 4.5) |
| Quintiles 4 and 5 | 192 | 487,372 | 3.9 (3.4 to 4.5) |
| Not known | 0 | 130 | |
| All areas | 63 | 284 | 3.3 (2.9 to 3.7) |
Table 4 shows the incidence of LUTO by maternal ethnic group. Compared with white European mothers (reference group), all ethnic groups had an elevated LUTO incidence, which reached significance in all minority groups except for Chinese/other/mixed. For the black and minority ethnic (BME) groups combined, the incidence was 6.3 per 10,000 compared with 2.6 per 10,000 (OR 2.38, 95% CI 1.87 to 3.03).
| Maternal ethnic group | No. of cases of LUTO | Total no. of births | Incidence per 10,000 total births (95% CI) | OR (95% CI) |
|---|---|---|---|---|
| British European | 181 | 687,087 | 2.6 (2.3 to 3.0) | Reference |
| Black African/Caribbean/Other | 21 | 16,943 | 12.4 (7.1 to 17.7) | 4.7 (3.00 to 7.40) |
| Indian | 17 | 33,573 | 5.1 (2.7 to 7.5) | 1.9 (1.17 to 3.20) |
| Pakistani | 32 | 54,834 | 5.8 (3.8 to 7.9) | 2.2 (1.52 to 3.20) |
| Bangladeshi | 7 | 11,656 | 6.0 (1.6 to 10.5) | 2.3 (1.07 to 4.90) |
| Chinese/Other/Mixed | 19 | 47,327 | 4.0 (2.2 to 5.8) | 1.5 (0.95 to 2.40) |
| Not known | 7 | – | ||
| BME | 103 | 164,332 | 6.3 (5.1 to 7.5) | 2.4 (1.87 to 3.03) |
| Total | 284 | 851,413 | 3.3 (2.9 to 3.7) |
Aetiology of lower urinary tract obstruction
Table 5 shows the incidence of the different causes of LUTO. The most common underlying aetiology of LUTO was PUVs (63.0%).
| LUTO subtype | Complex (no. of cases) | Isolated (no. of cases) | Total (no. of cases) | % of total no. of LUTO cases | Incidence per 10,000 total births (95% CI) |
|---|---|---|---|---|---|
| PUVs | 19 | 160 | 179 | 63.0 | 2.10 (1.79 to 2.41) |
| Urethral atresia | 18 | 10 | 28 | 9.9 | 0.33 (0.21 to 0.45) |
| Urethral stenosis | 4 | 16 | 20 | 7.0 | 0.23 (0.13 to 0.34) |
| Prune belly syndrome | 5 | 2 | 7 | 2.5 | 0.08 (0.02 to 0.14) |
| Unspecified/specified other | 17 | 33 | 50 | 17.6 | 0.59 (0.42 to 0.75) |
| Total | 63 | 221 | 284 | 100.0 | 3.34 (2.95 to 3.72) |
Outcomes and survival
The outcomes of cases of LUTO are presented in Table 6 , separately for isolated and complex cases. TOP was undertaken in 24.6% of cases. In the complex and isolated groups, the proportion of pregnancies ending in TOP was 41.3% and 19.9% respectively. However, this will be influenced by prenatal detection rates and parental choice. For cases resulting in a live birth or stillbirth, the perinatal mortality rate was 458/1000 births for complex cases and 120/1000 births for isolated cases.
| Pregnancy outcome | Complex cases | Isolated cases | Total cases | |||
|---|---|---|---|---|---|---|
| No. of cases | % | No. of cases | % | No. of cases | % | |
| TOP | 26 | 41.3 | 44 | 19.9 | 70 | 24.6 |
| Spontaneous fetal loss | 5 | 7.9 | 8 | 3.6 | 13 | 4.6 |
| Spontaneous stillbirth | 8 | 12.7 | 3 | 1.4 | 11 | 3.9 |
| Infant deatha | 11 | 17.5 | 20 | 9.0 | 31 | 10.9 |
| Alive at 1 year | 13 | 20.6 | 146 | 66.1 | 159 | 56.0 |
| Total | 63 | 100.0 | 221 | 100.0 | 284 | 100.0 |
Table 7 demonstrates perinatal and infant mortality rates according to pathological diagnosis. Of those that survived, 67 had normal renal function (64 PUV, three urethral stenosis), 16 had mild renal impairment (all PUV), eight had chronic renal failure (all PUV) and five had end-stage renal failure (all PUV).
| LUTO subtype | No. of isolated casesa | PNMR (95% CI) | IMR (95% CI) |
|---|---|---|---|
| PUVs | 160 | 74 (32 to 116) | 68 (28 to 109) |
| Urethral atresia | 10 | 0 | 0 |
| Urethral stenosis | 16 | 429 (62 to 795) | 333 (0 to 711) |
| Prune belly syndrome | 2 | ||
| Unspecified/specified other | 33 | 571 (312 to 831) | 615 (351 to 880) |
| Total | 221 | 129 (78 to 179) | 120 (71 to 170) |
Pathology
The post-mortem rate in babies who died was 73.6% (92/125). For all outcomes (deaths and survivors) a diagnosis was confirmed by pathology (post-mortem or surgical) in 32.4% of cases (92/284).
Chromosomal anomaly
In total, 100 cases had a karyotype available and a chromosomal abnormality was noted in 16 cases ( Table 8 ), all classified in the complex group, which represents a prevalence of chromosomal anomaly of 5.6% for all LUTO cases or 16.0% for cases with a known karyotype. For complex LUTO cases, chromosome analysis was undertaken and was successful in 82.5% of cases and abnormal in 25.4%.
| Karyotype | n | % of complex cases with known karyotype |
|---|---|---|
| Whole chromosome abnormality | ||
| Trisomy 18 | 5 | 9.6 |
| Trisomy 21 | 4 | 7.7 |
| Trisomy 13/other | 3 | 5.8 |
| Other rearrangements | 4 | 7.7 |
| Apparently normal karyotype | 36 | 69.2 |
| No karyotype available | ||
| Failed cytogenetics | 2 | 3.8 |
| No karyotype requested/parents declined | 9 | 17.3 |
| Total no. of complex cases | 63 | |
| Total no. of complex cases with known karyotype | 52 | |
Prenatal detection
Table 9 demonstrates that prenatal diagnosis of LUTO by ultrasound was made in 50.7% of all cases. This rate was higher in complex cases (57.1%) than in isolated cases (48.9%), which would be expected as there was additional pathology. In a further 10.6% of cases coexistent anomalies of the renal tract were suspected and in 4.9% of cases there were abnormal ultrasound features including anomalies of other systems. In total, 66.2% (188/284) of LUTO cases had an abnormal prenatal ultrasound finding and in 76.6% (144/188) of diagnoses this was reported specifically as LUTO. The proportion of cases with any ultrasound anomaly (LUTO, renal, other) was higher in complex cases (87.3%) than in isolated LUTO cases (60.2%).
| Pregnancy outcome | Complex cases | Isolated cases | Total cases | |||
|---|---|---|---|---|---|---|
| No. of cases | % | No. of cases | % | No. of cases | % | |
| Prenatal diagnosis: renal system | ||||||
| LUTO | 36 | 57.1 | 108 | 48.9 | 144 | 50.7 |
| Hydronephrosis | 2 | 3.2 | 14 | 6.3 | 16 | 5.6 |
| Other urinary tract | 10 | 15.9 | 4 | 1.9 | 14 | 5.0 |
| Prenatal diagnosis: other | ||||||
| Anomaly other | 5 | 7.9 | 7 | 3.1 | 12 | 4.2 |
| Prenatal diagnosis no details | 2 | 3.2 | 0 | 0.0 | 2 | 0.7 |
| No prenatal notification/diagnosis | ||||||
| No prenatal diagnosis | 2 | 3.2 | 27 | 12.2 | 29 | 10.2 |
| Unknown prenatal diagnosis | 6 | 9.5 | 61 | 27.6 | 67 | 23.6 |
| Total | 63 | 100.0 | 221 | 100.0 | 284 | 100.0 |
Over the period of the study there was no significant change in the proportion of cases with a prenatal diagnosis of any ultrasound anomaly (LUTO/renal/other system) (χ2 = 2.63, p = 0.105). However, the proportion of cases with a specific prenatal diagnosis of LUTO did increase over time (χ2 = 5.68, p = 0.017) ( Figure 8 ).
FIGURE 8.
Proportion of LUTO cases with a prenatal diagnosis 1995–2007. Reproduced from Malin GL, Tonks A, Morris RK, Gardosi J, Kilby MD. Congenital lower urinary tract obstruction: a population based epidemiology study. BJOG 2012;119:1455–64,97 © 2012 The Authors BJOG An International Journal of Obstetrics and Gynaecology © 2012 RCOG, with permission from John Wiley & Sons, Inc.
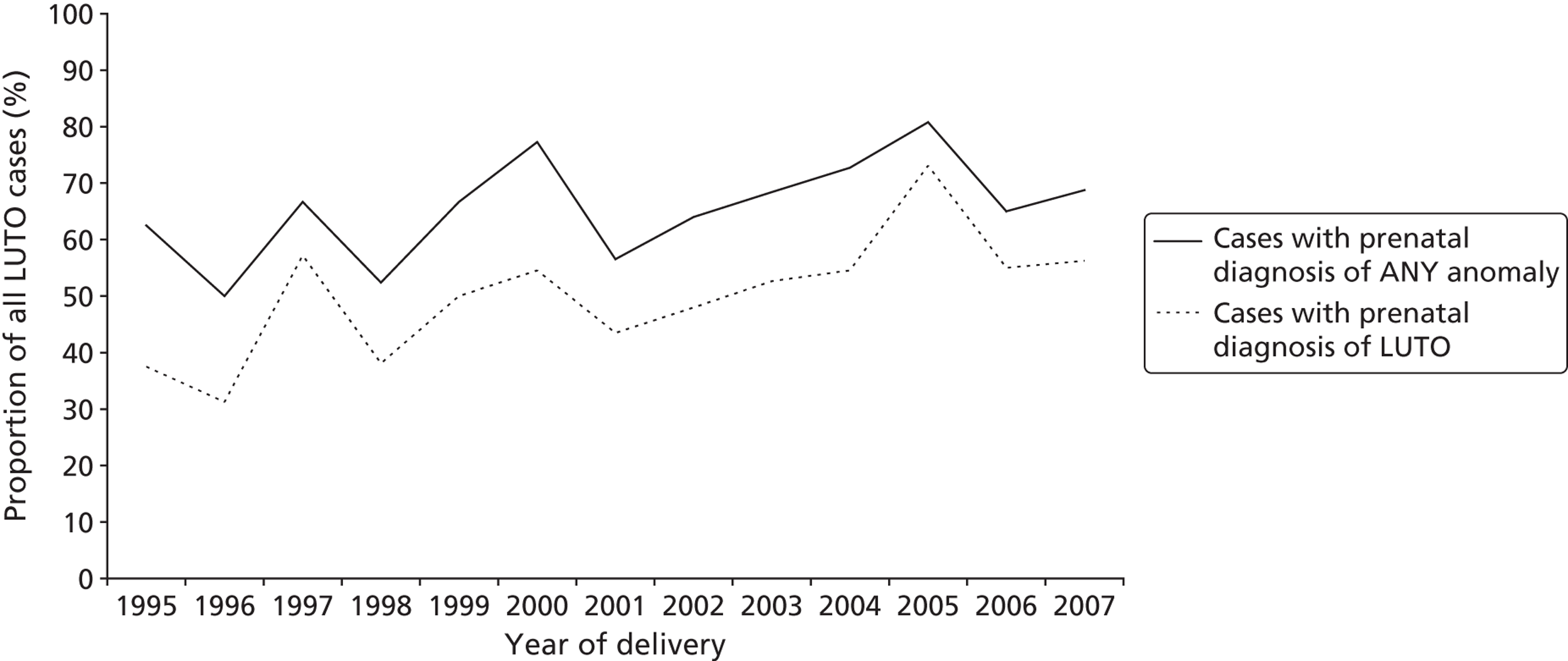
Table 10 presents pregnancy and infant outcomes according to gestation of diagnosis. Postnatally detected cases were more likely than prenatally detected cases to survive to 1 year of age (91% vs. 50% for isolated cases and 62.5% vs. 15% for complex cases, p < 0.001). There was no significant difference between postnatally detected cases and prenatally detected cases in the proportion of live-born babies who eventually developed end-stage renal failure.
| Outcome | Prenatal diagnosis (n = 188), n (%) | Postnatal diagnosis (n = 96), n (%) | Prenatal vs. postnatal p-value | ||||
|---|---|---|---|---|---|---|---|
| Isolated cases (n = 133) | Complex cases (n = 55) | Isolated cases (n = 88) | Complex cases (n = 8) | Total cases | Isolated cases | Complex cases | |
| Survivala | |||||||
| TOP | 44 (33) | 26 (47) | 0 (0) | 0 | – | – | – |
| Spontaneous fetal lossb | 9 (6.8) | 13 (23.6) | 2 (2.3) | 0 | – | – | – |
| Infant deathc | 14 (10.5) | 8 (14.5) | 6 (6.8) | 3 (37.5) | NS | NS | NS |
| Alive beyond 1 year | 66 (50) | 8 (15) | 80 (91) | 5 (62.5) | < 0.001 | < 0.001 | 0.007 |
| Surgery | |||||||
| Primary resection of valvesd | 39/42 | 1/4 | 49/50 | 2/2 | NS | NS | NS |
| Repeat valve ablation required | 1 (2) | 0 | 0 | 0 | – | – | – |
| Time from birth to valve resectione | |||||||
| In first week | 19 (45) | 0 | 3 (6) | 0 | < 0.001 | < 0.001 | – |
| In first month | 12 (29) | 0 | 18 (36) | 0 | NS | NS | NS |
| 2–5 months | 10 (24) | 0 | 11 (22) | 2 (100) | NS | NS | NS |
| 6–12 months | 1 (2) | 2 (50) | 8 (16) | 0 | NS | NS | NS |
| After 1 year of age | 0 | 0 | 10 (20) | 0 | 0.001 | 0.004 | 0.002 |
| No. of additional surgical procedures performedf | 8 | 2 | 1 | 0 | |||
| Renal functiong | |||||||
| Normal renal function | 33 (69) | 3 (60) | 34 (71) | 0 | NS | NS | NS |
| Mild renal impairment | 8 (17) | 1 (20) | 8 (17) | 0 | NS | NS | NS |
| Chronic renal failure | 5 (10) | 1 (20) | 3 (6) | 1 (50) | NS | NS | NS |
| End-stage renal failure | 2 (4) | 0 | 3 (6) | 1 (50) | NS | NS | NS |
| Nadir creatinine (μmol/l)h | 40 (34–51) | 39 (34–47) | |||||
False-positive prenatal diagnoses
There were 53 cases with an antenatal ultrasound suspicion of LUTO that were later excluded from the study cohort ( Table 11 ). This represents 26.9% of the prenatal diagnoses of LUTO (53/197). Of these 53 cases, 38 had bladder signs (25 enlarged bladder, 21 thick-walled bladder, eight keyhole sign), 18 had hydronephrosis (15 bilateral), 10 had oligohydramnios (reduced) or anhydramnios (complete absence of liquor) and 15 had an abnormal appearance of the renal cortex (eight bilateral echogenicity, four unilateral echogenicity, two unilateral macrocysts and one bilateral macrocysts). The most common final postnatal diagnoses in these false-positive cases were ultrasound finding associated with significant renal reflux (24.5%), cloacal dystrophy (18.9%) and hydronephrosis (11.3%). In 10 cases (20.8%) there was no renal/bladder anomaly at delivery and in five of these the appearance of LUTO on ultrasound was seen to resolve during the antenatal period (see Table 11 ).
| False-positive cases: final diagnosis | n | % of total |
|---|---|---|
| Reflux | 13 | 24.5 |
| Cloacal dystrophy | 10 | 18.9 |
| Hydronephrosis | 6 | 11.3 |
| Ureteric obstruction/ureterocele | 3 | 5.7 |
| Bladder malformation/exstrophy | 2 | 3.8 |
| Other urogenital | 8 | 15.1 |
| Normal at delivery | 11 | 20.8 |
| Total no. of complex cases – known karyotype | 53 |
Cases eligible for antenatal (in utero) vesicoamniotic shunting
When the cohort is restricted to isolated non-female singleton fetuses, there were 211 cases of LUTO (74.3% of all LUTO cases), a total incidence of 2.48 (95% CI 2.14 to 2.81) per 10,000 total births. However, the prenatal diagnosis rate of LUTO within this group was 46.9% (99/211), further reducing the proportion that could be referred for VAS. In this antenatally treated group, two fetuses underwent vesicocentesis, with both pregnancies ending in TOP. A total of eight fetuses were treated with VAS, of whom five had PUV, one had urethral atresia, one had urethral stenosis and one had unspecified LUTO. In the PUV group the outcome was one TOP, one IUD and one neonatal death, with two fetuses surviving, one with mild renal impairment and the other with renal failure. The baby with urethral stenosis survived with normal renal function and the baby with unspecified LUTO was a neonatal death.
In the apparently ‘isolated’ cohort with a specific prenatal diagnosis of LUTO, 33% of parents opted for TOP. Of those that survived to birth, primary resection of valves was performed in 92% with a diagnosis of PUVs at cystoscopy (29% of the total isolated LUTO cohort). For 45% this was within the first week postnatally and for 29% this was within the first month. Of these, 2% (a single case) required repeat cystoscopic resection and 50% were alive at 1 year. Of these cases, 69% had documented ‘normal renal function’ with 10% being classified as having chronic renal failure and 4% end-stage renal failure.
Discussion
Principal findings
This cohort of pregnancies complicated by LUTO is the largest reported series in the UK. The study design examines a single, large, multiple-source, population-based congenital anomaly register that uses active case finding ensuring that ascertainment is high and incidence rates are reliable and unaffected by information bias.
The incidence of LUTO was reported as 3.34 (95% CI 2.95 to 3.72) per 10,000 total births (1 in 2994). LUTO incidence was found to be significantly associated with maternal ethnic group and deprivation, being highest in the most deprived quintile. This may explain the higher incidence seen in the West Midlands compared with other published series, such as that from NorCAS (a population-based register in the northern region) (2.2 per 10,000 total births 1984–97). 24 As there has been no change in incidence over time it is unlikely that the difference seen is due to the use of a later cohort in our study, and similarly it is unlikely to be due to any differences in the maternal age profile of the two regions. Both WMCAR and NorCAS are part of the BINOCAR, a network of UK regional anomaly registers that use the same methodology for data collection, generating good-quality data with high ascertainment rates,15,16,102,103 and so variations between registry area data are not likely to be the result of ascertainment bias. There is no longer national coverage for anomaly data for England; these data are available only at regional level. Regional register data have been shown to be better than previous national data. 103 It is recognised that as early spontaneous pregnancy losses are not recorded by regional anomaly registers this leads to an underestimate of the denominator and thus an overestimate of the incidence of LUTO. We have produced stratified incidence rates for ethnic group and deprivation that can be applied to other areas to estimate incidence.
As with other population-based series, these data from the WMCAR indicated that, of the 83% of complex prenatal cases for which fetal karyotyping was known, 5.6% were abnormal. In ‘isolated’ LUTO the most prevalent postnatally confirmed diagnosis was PUVs (72%) followed by urethral atresia/stenosis in 12% of cases. In 14% of cases the underlying pathology was not clearly delineated.
These data indicate that overall perinatal and infant survival are significantly worse if the congenital bladder neck obstruction is ‘complex’ (associated with other anomalies) and also if the diagnosis is made antenatally. In this series from the West Midlands (1995–2007) the prenatal detection rate using routine ultrasound screening was 66%. This is not significantly different from detection rates quoted for the northern region for a cohort between 1984 and 1997. 24 This indicates that, despite national recommendations for screening during a detailed ultrasound scan at 20 weeks from the Royal College of Obstetricians and Gynaecologists (see www.rcog.org.uk/womens-health/clinical-guidance/ultrasound-screening) and improved ultrasound equipment and ultrasonographer training, a significant proportion of babies with LUTO were not detected prenatally. This may be because a less severe phenotype is not amenable to early detection. Also, for the first time in a population-based study it was documented that in 26.9% of prenatal diagnoses there was a false-positive diagnosis of LUTO. The majority of these cases were associated with structural anomalies of the urinary tract (i.e. ureterocele or cloacal dystrophy). However, significantly, in 5.9% of cases the ultrasound appearances were secondary to reflux nephropathy and were not distinguished from obstructive renal disease antenatally. Our data demonstrated a significant trend towards an increase in the number of prenatally diagnosed cases with a specific prenatal diagnosis. This could be because of a multitude of factors including improved ultrasound equipment, improved training and improved documentation/reporting. Our cohort study indicated that in the West Midlands it was not common for prenatal assessment of liquor volume and assessment of renal parenchymal appearance to be supplemented by fetal urinalysis. This is in all likelihood because evaluation of this diagnostic test has indicated that it has little value over assessment of renal appearance and liquor volume. 14,70
It was also interesting to note the number of antenatally identified babies with LUTO who were potentially amenable to ‘in utero’ therapy. Of the prenatally suspected, isolated, singleton male LUTO cases, 2% underwent serial vesicocentesis and 8% underwent VAS. The number having an intervention is relatively small indicating that any prenatal intervention aimed at influencing the pathogenesis of this condition will influence relatively low numbers of babies in absolute terms. It is important to realise that this number will have been influenced by the significant proportion of parents who would have opted for TOP rather than intervention and by the potential for not all eligible fetuses to have been referred to the tertiary centre for consideration for intervention. These are local influences and may not be representative of the situation internationally. There were no cases in which fetal cystoscopy was performed. There is very little published evidence on the effectiveness of fetal cystoscopy as a diagnostic and therapeutic intervention for LUTO and the quality of this evidence is poor;21,104 thus, we do not feel that this is unusual or a limitation of our cohort.
Conclusion
In conclusion, this chapter report the diagnosis and management of a large population-based series of fetal LUTO in a region where prenatal assessment and potential treatment were available. It highlights the continuing limitations of prenatal ultrasound screening (even within the last 5 years) for both detection of LUTO and indeed correctly identifying obstructive uropathy (with a 5% association of reflux in the isolated LUTO cohort). This may be because of the subtlety of prenatal diagnosis in some cases but also because of the overlap between ultrasound appearances between obstructive pathologies and those secondary to reflux. 105 Long-term outcomes were worse in those fetuses identified prenatally and with coexistent anomalies. Parental choice of TOP was not insignificant in this cohort (and indeed in those pregnancies with apparently isolated LUTO). Mortality and morbidity are high in this condition with a significant number of babies having chronic renal impairment in infancy. These data will be useful in the prospective counselling of women with babies who have a pre- and postnatal diagnosis of this condition.
We will discuss the findings of this epidemiological study performed within the West Midlands in relation to the predicted incidence of LUTO in the UK in our concluding remarks. In addition, these data are of interest in defining both the total number of prenatal cases being potentially identified using ultrasound and the choices of women/health-care professions in managing such anomalies.
Chapter 4 Methods of the randomised controlled trial and the registry
Objectives
The PLUTO trial and registry had the following objectives:
-
primary objective:
-
to determine if intrauterine VAS for fetal bladder outflow obstruction improves perinatal and neonatal mortality and long-term renal function compared with conservative non-interventional care
-
-
secondary objective:
-
to determine if placement of a VAS in fetuses with LUTO improves short-term morbidity
-
to determine the long-term effects of VAS with respect to (1) the development of chronic renal failure and need for dialysis or transplantation, (2) the development of incontinence (bladder dysfunction) and (3) disability-free life-years (incorporating assessment of cognitive development, quality of life, micturition and general health)
-
to determine if improvement is related to prognostic assessment at diagnosis and, if possible, to derive a prognostic risk index
-
to maintain a prospective registry of patients with LUTO as part of a comprehensive cohort design.
-
Study design
A multicentre RCT with parallel registry ( Figure 9 ). Both the RCT and registry had the same setting, structure and design, including outcome measures. The RCT design was used to provide an unbiased (free from selection or confounding bias) assessment of the primary objective, however given the rarity of the condition if was felt important to capture information on all cases, hence the registry was developed in cases where the specialist or parent had a strong preference. Blinding of intervention was not considered ethical or feasible.
FIGURE 9.
The PLUTO trial study design: Consolidated Standards of Reporting Trials (CONSORT) diagram.
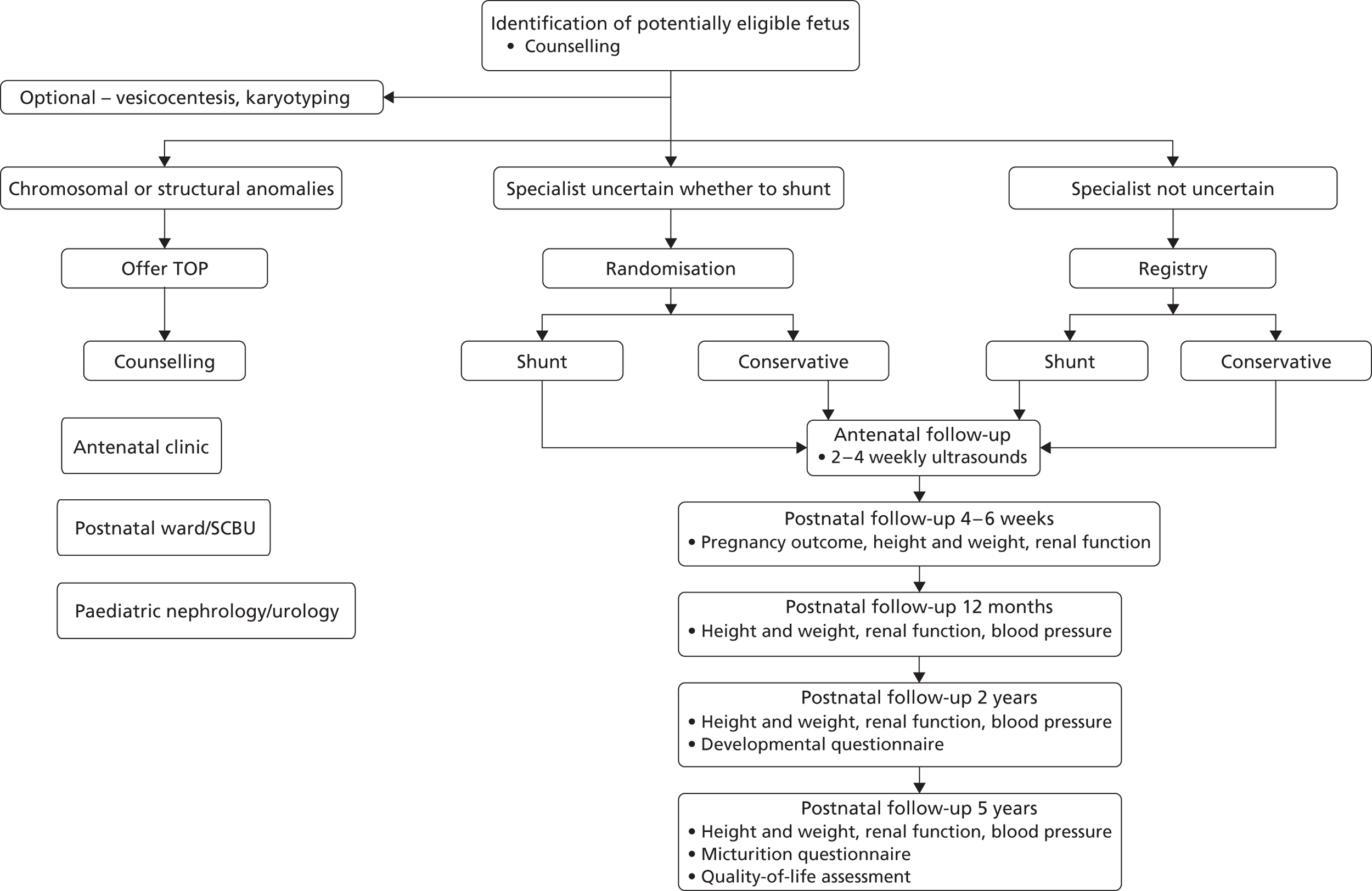
Randomised controlled trial
Inclusion criteria
Mother
-
Written informed consent given.
-
Singleton pregnancy.
Fetus
-
Evidence of isolated bladder outflow obstruction from ultrasound imaging.
-
Male fetus.
Exclusion criteria
-
Additional major structural or chromosomal anomaly.
Female fetuses were excluded from the trial as they are likely to have a more complex aetiology with a very poor prognosis. There were no eligibility criteria relating to gestation or liquor volume. There is some evidence in the literature that gestation and liquor volume at diagnosis may determine prognosis in these fetuses; this evidence is, however, limited. 4,69 Mothers of fetuses that were identified as being eligible for inclusion into the study before 16 weeks’ gestation were counselled regarding the trial but not randomised until 16 weeks’ gestation to allow VAS to be performed if allocated to this arm.
Registry
Women whose fetus was eligible to be randomised into the PLUTO trial but who did not give consent to randomisation, women for whom the fetal medicine specialist had a strong preference for either shunting or conservative management or women with a multiple pregnancy were eligible to take part in the registry (see Figure 9 ). Consent to allow recording of follow-up information was still required in these cases.
Inclusion criteria
Mother
-
Written informed consent given.
-
Singleton or multiple pregnancy.
Fetus
-
Evidence of isolated bladder outflow obstruction from ultrasound imaging.
-
Male fetus.
Exclusion criteria
-
Additional major structural or chromosomal anomaly.
Setting of the study
All fetal medicine centres within England, Scotland and the Republic of Ireland agreed to take part within the study and received local research ethics approval and trust R&D approval, either as a centre for randomisation and intervention or as a referring centre. Referral centres identified eligible women and counselled them regarding entry into the trial with referral to Birmingham for consent, randomisation and VAS when appropriate. The full list of centres and the principal investigators can be found in Appendix 2 . This includes 24 randomising centres, three referring centres and three paediatric follow-up centres.
Setting for the randomised controlled trial
The setting for the RCT was six fetal medicine centres in the UK [Birmingham Women’s Hospital NHS Foundation Trust, Liverpool Women’s NHS Foundation Trust, Nottingham University Hospitals NHS Trust, Forth Park Hospital (Fife), Royal Victoria Infirmary (Newcastle), St George’s Healthcare NHS Trust (London)] and one in the Netherlands (Leiden University Medical Centre, receiving referrals from all hospitals within the Netherlands).
Setting for the registry
The setting for the registry was nine fetal medicine centres, eight within the UK [Birmingham Women’s Hospital NHS Foundation Trust, Liverpool Women’s NHS Foundation Trust, St George’s Healthcare NHS Trust (London), St Michael’s Hospital (Bristol), Princess Anne Hospital (Southampton), King’s College Hospital (London), Queen Mother’s Hospital (Glasgow), St Mary’s Hospital (Manchester)] and one in the Netherlands (Leiden University Medical Centre, receiving referrals from all hospitals within the Netherlands).
Screening and consent for the randomised controlled trial and registry
All women were offered a high-resolution scan at about 18–22 weeks of gestation and were counselled about the possibility of fetal abnormalities. 106 The diagnosis of fetal LUTO was made prospectively on the basis of the ultrasound appearances of the fetal bladder and kidneys and the amniotic fluid volume. Some clinicians used fetal vesicocentesis with analysis of urinary analytes (see Chapter 1 ) in an attempt to further aid diagnostic accuracy and inform prognosis. The diagnosis was explained carefully to the parents (often over several days) and the possibility of participation in the PLUTO trial was proposed. A written participant information sheet (see Appendix 3 ) and consent form (see Appendix 4 ) were provided along with supporting literature for those receiving a diagnosis of fetal abnormality. Support and counselling were provided according to local practice and a contact telephone number for a specialist midwife counsellor was provided. In all cases, because of the natural history of the condition, the fetal medicine subspecialist felt that it was appropriate to discuss the option of TOP. The PLUTO trial was not discussed further with those mothers who opted for termination but they were approached at their follow-up counselling appointment about participation in the qualitative research study (see Chapter 9 ). An anonymised register of TOPs (including gestational age, fetal medicine centre) was kept within the site file.
At the next visit, typically up to 7 days following vesicocentesis or at the follow-up scan, the mother was invited to consent to participation. Only fetal medicine subspecialists experienced in fetal bladder shunt insertion performed and consented for the procedure. All participants provided written informed consent.
Randomisation
Eligible consenting participants were allocated to placement of a VAS or observation until delivery (conservative management) by a telephone or internet randomisation service organised by the Birmingham Clinical Trials Unit. Allocation was concealed until a participant’s baseline details had been provided. Minimisation was used to ensure balance of treatment allocation overall and so that the following variables could be used in the prespecified subgroup analyses: gestational age at diagnosis, age of mother at diagnosis, liquor volume by maximum pool depth (MPD). The data collected at randomisation are detailed in Appendix 5 .
Interventions: randomised controlled trial and registry
Protocol for vesicoamniotic shunting
Once a prospective diagnosis was made and consent obtained (with randomisation), the patient was usually offered sedation (maternal oral lorazepam 2–4 mg) and prophylactic antibiotics (cefalexin 500 mg orally) given 2 hours before the procedure. The fetus was monitored continuously by high-quality and high-resolution, real-time two-dimensional ultrasound [Siemens S2000 machine (Siemens Healthcare, Munich, Germany) or equivalent] and the fetal bladder visualised. Under sterile, minimal-touch technique, a vesicoamniotic pigtail catheter was percutaneously inserted [using either the King’s College/Rocket introducer (Rocket Medical, Washington, UK) or the Harrison Shunting set (Cook Medical, Bloomington, IN, USA) (operator preference)]. Optimal placement of the shunt (the distal end in the fetal bladder and the proximal end in the amniotic cavity) and fetal viability were confirmed immediately and at several hours post procedure. Follow-up ultrasound scans were arranged at the clinician’s discretion but usually no less frequently than every 2 weeks. All investigators were accredited and had advance training in fetal medicine [recognised by the Royal College of Obstetrics and Gynaecology (RCOG) or international equivalent]. Investigators had previous experience and demonstrable competence in ultrasound-guided VAS insertion. It was recognised that not all centres were able to perform VAS for patients randomised to the intervention. In such cases the procedure was performed at the Fetal Medicine Centre, Birmingham Women’s Hospital NHS Foundation Trust.
Women were counselled and received a written information sheet outlining the risks of the procedure: miscarriage, prelabour rupture of membranes and premature labour, chorioamnionitis and shunt blockage or migration.
Conservative management
The patient and fetus were scanned regularly at the clinician’s discretion, commonly no less frequently than every 2 weeks.
Registration
Not all patients eligible for the RCT consented to be randomised. Those women who did not agree to be randomised were then asked if they would provide consent for their details to be included in a register of LUTO cases. If a decision was made to enter a patient onto the registry this was ideally done prospectively, but retrospective registration, ideally before delivery, was accepted. All parents entered onto the registry were counselled in the same way as in the RCT, were provided with the patient information leaflet and were consented as in the RCT.
Entry onto the registry was via the internet or by telephone registration when internet access was not available, as for randomisation; all information on the randomisation form was required and recorded.
Register of terminations of pregnancy
All centres were contacted on a monthly basis to ask for anonymous details of women who had opted for TOP after a diagnosis of LUTO and counselling. This was to allow a more accurate assessment of the prevalence of disease and influences on women’s decision-making (see Chapter 9 ). Ethical approval was obtained from the Nottingham Multicentre Research Ethics Committee (MREC). Consent was not required as anonymised data only were collected.
Study management
Independent trial steering committee
The trial steering committee (TSC) provided independent supervision for the trial, providing advice to the chief and co-investigators and the sponsor on all aspects of the trial and affording protection for patients by ensuring that the trial was conducted according to the Medical Research Council (MRC) Guidelines for Good Clinical Practice in Clinical Trials. 107 The TSC consisted of the following independent members: Professor M Whittle (Emeritus Chair in Fetal Medicine, University of Birmingham), Professor David James (Professor of Obstetrics and Gynaecology, University of Nottingham), Professor D Field (Neonatal Paediatrician, University Hospitals of Leicester, Leicester University), Professor Y Ville (Fetal Medicine, Hôpital Necker, Paris), lay representative Mrs Sarah Caranchi (midwife and mother of baby requiring in utero treatment), Professor Adrian Woolf (Paediatric Nephrologist, University College London, London/Manchester) and Mr Nicholas Madden (Paediatric Urologist, St Mary’s Hospital London).
Data monitoring and ethics committee
During the period of recruitment to the study, interim analyses of major end points were supplied, in strict confidence. The data monitoring and ethics committee’s (DMEC) role was to advise the chair of the TSC if, in their view, any of the randomised comparisons in the trial have provided both (1) proof beyond reasonable doubt that for all, or for some, pregnancies either policy is definitely indicated or definitely contraindicated in terms of a net difference in the major end points and (2) evidence that might reasonably be expected to influence the patient management of many clinicians who are already aware of the other main trial results. The TSC, the investigators and all of the central administrative staff (except the statisticians who supply the confidential analyses) remained unaware of the interim results. The DMEC consisted of the following independent members: Dr C Cummins (Paediatric Epidemiologist, University of Birmingham and Birmingham Children’s Foundation Trust), Professor J Thornton (Professor of Obstetrics and Gynaecology, University of Nottingham) and Professor J Neilson (Professor of Obstetrics and Gynaecology, University of Liverpool).
Ethical considerations
The study was conducted according to the principles of the 1998 MRC Guidelines for Good Clinical Practice in Clinical Trials 107 and the appropriate NHS research governance frameworks. PLUTO did not fall within the scope of the European Directive on Clinical Trials (2001/20/EU). The University of Birmingham was the sponsor.
The RCT and registry obtained ethical approval from the Nottingham MREC (COREC Ref. 04/Q2404/89), which determined that the trial design respects the rights, safety and well-being of the participants. Every potential UK centre also obtained local research ethics committee (LREC) and trust R&D approval. All UK centres were required to sign an investigator’s agreement with the University of Birmingham, detailing their commitment to accrual, compliance, good clinical practice, confidentiality and publication. The sponsor ensured that all researchers not employed by a NHS organisation held a NHS honorary contract for that organisation if required by current guidelines.
International centres were asked to apply for ethics committee approval according to their own system, providing the PLUTO trial office with written evidence of approval. One local principal investigator in each country was asked to act as a national co-ordinator and monitor the centres in that country for compliance with good clinical practice and any relevant local regulations.
Assessments and outcome measures: randomised controlled trial and registry
All antenatal and perinatal outcome measures were obtained as per standard clinical practice for diagnosis, monitoring and treatment of prenatal bladder outflow obstruction. Long-term follow-up of all children born with this condition is also routine practice but for the trial this was undertaken by a paediatric nephrologist or urologist, who may be at a different hospital from where the shunt was placed.
Primary outcome measure
The primary objective was to determine whether in utero shunting improves perinatal and neonatal mortality (IUD at ≥ 24 weeks or death from any reason within 28 days of delivery); thus, the primary outcome measure was survival rate at 28 days. It should be noted that the planned primary outcome within the protocol was perinatal mortality at 4–6 weeks. However, all babies within the study had their follow-up completed by 4 weeks and thus this outcome is referred to as the 28-day outcome.
Other outcome measures
Antenatal assessment
To confirm the diagnosis of bladder obstruction and assess renal function a number of anatomical and biochemical markers were recorded at diagnosis and at each 2- to 4-weekly antenatal visit. A high-resolution ultrasound assessment of the pregnancy was performed at each antenatal visit with hard-copy or video images archived in the antenatal notes. The following measurements were obtained from the ultrasound:
-
bladder wall thickness (mm)
-
renal pelvic dilatation measured in transverse section and if > 10 mm bilaterally (mm)
-
longitudinal renal length (mm)
-
presence/absence of renal cysts (yes/no)
-
amniotic fluid volume – this was reported as the maximum vertical pool depth (cm) and the fifth centile for gestational age was used108
-
VAS in situ (yes/no)
-
VAS complications (migration/blockage/need for reinsertion, premature rupture of membranes)
-
treatment failures (IUD/TOP because of lack of success or compliance)
-
serious adverse events including pregnancy loss before 24 weeks, premature rupture of membranes, chorioamnionitis, damage to maternal uterus/organs, damage to fetal organs, migration of stent
-
optional assessments including vesicocentesis and fetal urinalysis.
Outcomes at birth
-
Pregnancy outcome (live birth rate).
-
Interval between randomisation/registration and delivery.
-
Gestational age at delivery.
-
Mode of delivery.
-
Birthweight (kg), birthweight centile for gestational age [UK World Health Organization (WHO) Boys Neonatal and Infant Close Monitoring Chart (23 weeks’ gestation to 2 years corrected age)].
-
Admission to neonatal intensive care unit (NICU) and length of stay.
-
Ventilatory/oxygen support required.
-
Oxygen dependency at discharge.
-
Surgery/medical management for renal impairment.
-
Adverse events including pregnancy loss before 24 weeks, preterm rupture of membranes, preterm labour (< 37 weeks).
Perinatal and neonatal outcomes at 28 days
-
Serum creatinine (μmol/l).
-
Classification of renal function.*
-
Weight of infant and weight centile [UK WHO boys growth charts for weight and length (0–1 years)].
-
Hospital inpatient status.
-
Management of renal impairment (none, medical, surgical, dialysis, transplant).
-
Documentation of any surgery.
The following outcomes were optional and dependent on the centre’s ability to perform the necessary tests:
-
renal ultrasound measurements – bladder wall thickness (mm), renal pelvic dilatation measured in transverse section (mm), ureteric dilatation (mm)
-
micturating cystourethrogram (MCUG) – to demonstrate reflux
-
markers of tubular damage – the urinary albumin-to-creatinine ratio and cystatin C concentration were measured to determine whether the neonate was in chronic renal failure. 109
One-year outcomes
-
Survival rate.
-
Serum creatinine (μmol/l).
-
Classification of renal function.*
-
Need for any surgery.
-
Number of admissions to hospital and length (days).
-
Need for renal dialysis/transplantation.
-
Height (m) and weight (kg) and height and weight centile for corrected age [UK WHO boys growth charts for weight and length (0–1 years)].
-
Renal ultrasound measurements, MCUG and markers of tubular damage were again optional and dependent on the centre’s ability to perform the necessary tests and on the consent of the parent. Measurements were the same as at 28 days.
Planned 2-year outcomes
-
Serum creatinine (μmol/l).
-
Classification of renal function.*
-
Need for any surgery.
-
Number of admissions to hospital and length (days).
-
Need for renal dialysis/transplantation.
-
Height (m) and weight (kg) and height and weight centile for corrected age [UK WHO boys growth charts for weight and length (1–5 years)].
-
Developmental questionnaire (PARCA, completed by parents). 85,86
*Renal function was classified as:
-
normal renal function – serum creatinine < 50 μmol/l
-
mild renal impairment – serum creatinine ≥ 50 μmol/l not requiring medical or surgical management
-
moderate renal impairment – serum creatinine ≥ 50 μmol/l and requiring medical management
-
end-stage renal failure – need for dialysis or renal transplant.
This classification was reached following a review of the literature and the normal ranges for serum creatinine in the neonatal period and infancy110,111 and after discussion with experts within the field.
Planned 5-year outcomes
Follow-up and outcome measures as at 1 year with the addition of:
-
the micturition questionnaire (developed specifically for this study by experts within the field)
-
a quality-of-life assessment (PedsQL questionnaire). 84
It was planned to use the scores from these measures and the PARCA questionnaire to assess overall disability as a composite outcome measure. This outcome measure consisted of six clinical domains: bladder function, physical function, psychological function, social function, cognitive development and general health (incorporating renal function, blood pressure, height/weight centiles). In each of these domains it was planned to assess the child’s disability status as normal, impaired, mildly disabled, moderately disabled or severely disabled. In the statistical analysis it was planned to assess each of these domains individually and as a composite measure, disability free at the end of 1 year. A child’s overall disability status for the composite measure was defined by the highest degree of disability across any of the six domains.
Statistical considerations
Sample size
The sample size for the RCT was based around a meta-analysis of observational studies published at the time of designing the trial and performed some years earlier by members of the group. 13 This systematic review is presented in Chapter 1 . In the four studies included, the pooled survival rate in the conservative management group was 13/33 or 0.394, with a pooled OR for perinatal survival of 2.53 (95% CI 1.08 to 5.93), equivalent to an improvement in survival of 23 percentage points with bladder drainage (e.g. 39% survival compared with 62% survival). At 80% power and α = 0.05, we calculated that 75 pregnancies in each group (150 pregnancies in total) would be sufficient to detect such a difference. Ultimately, the trial managed to recruit 31 pregnant women (see Chapter 5 , Results).
Analysis
Relative risks (RRs) and associated 95% CIs (using standard normal approximation methods) were calculated for the primary measure of survival rate at 28 days, with statistical significance assessed using two-sided Fisher’s exact test (when there is some slight inconsistency between quoted estimates of p-values and CIs, i.e. the CI crosses 1 but with p < 0.05, this is because of the impact of small numbers having different impact on the different methods used to calculate the CIs and p-values). In the first instance analysis was performed on an intention-to-treat (ITT) basis (according to group assignment, regardless of actual intervention received), although an analysis comparing the groups according to the intervention received (as treated) was also performed to investigate the impact of a relatively high number of group crossovers. IUDs and TOPs were included in the analysis and classed as a death in the first instance, although a sensitivity analysis was performed excluding non-treatment-related TOP [for both the ITT and ‘as treated’ (AT) analyses].
Analysis of live birth survival rates and survival to 1 year was performed as per the primary outcome. Baseline characteristics of registry mothers and fetuses were compared using two-sided Fisher’s exact tests for categorical data and the Mann–Whitney U-test otherwise. Formal statistical analysis was not attempted for the other outcome measures because of the limited size of the sample recruited and low rate of survival. Summary statistics [means and standard deviations (SDs) or medians and IQRs] or individual values are presented for these outcomes when appropriate. Again, because of the limited size of our sample, subgroup analysis or covariate adjustment was not appropriate for the analysis of survival rates and so was not attempted (although adjustments were attempted when we combined both randomised and registry data as we had a bigger sample – see Chapter 6 ).
A logistic regression analysis was performed on the combined data sets of randomised and registry data with survival to 28 days as the outcome, with the aim of trying to show whether the predefined subgroups of gestational age, liquor volume and maternal age were associated with survival. We also used this analysis to examine whether apparent differences in effect on survival between groups in the randomised and registry data were statistically important (by examining the method of entry by intervention interaction parameter). Covariates were initially considered individually and then included in a multivariable analysis if statistically important (a conservative level of p < 0.1 was used here). A sensitivity analysis including all covariates in the multivariable analysis regardless of statistical significance made little difference to point estimates and so the results are not presented. Treatment group and a variable indicating whether the fetus was randomised or registered were also included in these analyses. Unadjusted and adjusted ORs along with 95% CIs and associated p-values are presented.
The final protocol for the PLUTO study is available on the HTA website (www.hta.ac.uk/project/1732.asp) and in Appendix 6 .
Chapter 5 Findings of the randomised controlled trial
Recruitment and participants
In total, 31 women agreed to be randomised from seven centres between October 2006 and October 2010 ( Table 12 and Figure 10 ).
| Centre | Recruitment, n (%) |
|---|---|
| Birmingham Women’s Hospital, UK | 15 (48) |
| Liverpool Women’s Hospital, UK | 7 (23) |
| University Medical Centre, Leiden, Netherlands | 5 (16) |
| Nottingham City Hospital, UK | 1 (3) |
| Forth Park Hospital, Fife, UK | 1 (3) |
| Royal Victoria Hospital, Newcastle, UK | 1 (3) |
| St George’s Hospital, London, UK | 1 (3) |
FIGURE 10.
Recruitment graph for randomised participants.
Barriers to recruitment
-
International barriers – There were delays in the international centres being able to recruit. By June 2009 no international centres had approval. Of the two international centres, that in the Netherlands eventually randomised five participants although the centre in Dublin did not randomise anyone. The trial team also noted the barriers that they had encountered in getting international centres involved (namely problems with the sponsor, the University of Birmingham, having to pay an individual insurance premium for the trial of £30,000 per annum for each international centre). This eventually led to the University of Birmingham deciding that no further international centres could be recruited.
-
Delays in approval for UK centres – There were delays in getting UK centres recruiting because of lengthy approval processes partly because some R&D offices were more complex in their paperwork requirements than others. Three centres also negotiated changes to the site agreement with the university to make it better suited to their trust. These negotiations were complex and caused delays.
-
High proportion of women opting for TOP – More women than expected were opting for TOP rather than continuing to delivery after their fetus was diagnosed with LUTO. The reasons for this were explored as part of the patient acceptability study (see Chapter 9 ). By June 2009, 20 women had been randomised, 21 had been entered onto the registry and 41 had opted for TOP.
-
Lower prevalence of LUTO – It appeared that the prevalence of isolated LUTO might be less than that quoted in the literature; this was determined in the epidemiological study (see Chapter 3 ).
-
High proportion of women being entered on the registry – Of those women who did agree to join the study, more than anticipated were being placed on the registry rather than being entered into the RCT. The tendency to use the register was felt to be a result of the fetal medicine specialists having firm preferences for when and how to treat. It was felt that this operational lack of equipoise [despite the assurances of support for the trial in the assessment of clinicians’ prior beliefs (see Chapter 7 )] would ultimately make it impossible to deliver the required number of participants.
A recommendation was thus made that there should be no absolute target for recruitment at the 24-month stage but that a confidential report should be sent on behalf of the TSC (after consultation with the DMEC) to the HTA programme for its views on projected recruitment and whether funding should be continued in September 2010. On receipt of this report the HTA decided to perform a monitoring visit in May 2010.
Amendments to the PLUTO study
The funder and the TSC decided to halt recruitment into the PLUTO trial in December 2010 as it was agreed that there was no realistic possibility of recruiting sufficient participants.
In October 2011 a final closure plan (a revised proposal for the PLUTO study and financial plan) was agreed (see Appendix 7 ). In summary, this was a 12-month plan with the following components:
-
completion of follow-up to the primary end point for all women within the randomised and registry arms of the study
-
a limited economic analysis and decision-analytic modelling would be performed
-
qualitative research would continue in its present form
-
a HTA monograph would be prepared.
This was amended in June 2012 after the HTA programme was informed that long-term follow-up to 1 year was now available for all RCT and registry participants and thus 1-year outcomes could be presented.
Thus, the following secondary objectives from the original protocol could not be met and completion of long-term follow-up to 2 and 5 years of age was not funded:
-
to determine the long-term effects of VAS with respect to (1) the development of chronic renal failure and need for dialysis or transplantation, (2) the development of incontinence (bladder dysfunction) and (3) disability-free life-years (incorporating assessment of cognitive development, quality of life, micturition and general health)
-
to derive a prognostic risk index.
Baseline characteristics of participants
The baseline characteristics of those randomised appeared well balanced ( Table 13 ). The median age of mothers in the trial was 28 years (IQR 23 to 33) and the vast majority of fetuses were of < 24 weeks’ gestation at the time of LUTO diagnosis (27/31, 87%). The majority of the fetuses had a liquor volume less than the fifth centile (19/31, 61%).
| Characteristic | VAS, n (%) | Conservative management, n (%) |
|---|---|---|
| No. randomised | 16 | 15 |
| Maternal age in years, median (IQR) | 27 (23 to 33) | 28 (26 to 33) |
| Maternal age (years)a | ||
| < 20 | 2 (13) | 0 |
| 20–35 | 12 (75) | 12 (80) |
| > 35 | 2 (13) | 3 (20) |
| Ethnicity | ||
| White | 13 (81) | 13 (87) |
| Asian | 2 (13) | 1 (7) |
| Black | 1 (6) | 1 (7) |
| Gestational age (days), median (IQR) | 142 (112 to 154) | 150 (133 to 154) |
| Gestational age (weeks)a | ||
| < 24 | 13 (81) | 14 (93) |
| ≥ 24 weeks | 3 (19) | 1 (7) |
| Liquor volume MPD (cm), median (IQR) | 1.6 (0 to 2.9) | 1.0 (0.2 to 2.9) |
| Liquor volumea,b | ||
| < 5th centile | 10 (63) | 9 (60) |
| ≥ 5th centile | 6 (38) | 6 (40) |
| Renal pelvis dilatation left (mm), median (IQR) | 7.4 (5.0 to 14) | 7.3 (5.0 to 9.0) |
| Renal pelvis dilatation right (mm), median (IQR) | 8 (4.8 to 10) | 8.6 (5.0 to 11) |
| Renal pelvis dilatation AP > 90th centilec | ||
| Bilateral | 13 (81) | 12 (80) |
| Unilateral | 2 (13) | 0 |
| Neither | 0 | 1 (7) |
| NR | 1 (6) | 2 (13) |
| Renal pelvis severe hydronephrosis > 1.5 cmd | ||
| Bilateral | 1 (6) | 0 |
| Unilateral | 1 (6) | 1 (7) |
| Neither | 13 (81) | 13 (87) |
| NR | 1 (6) | 1 (7) |
| Macrocystic renal appearance | ||
| Bilateral | 0 | 1 (7) |
| Unilateral | 2 (13) | 4 (27) |
| Neither | 14 (88) | 10 (67) |
| Renal ‘echogenicity’ | ||
| Bilateral | 2 (13) | 4 (27) |
| Unilateral | 3 (19) | 3 (20) |
| Neither | 7 (44) | 5 (33) |
| NR | 4 (25) | 3 (20) |
| Bladder wall thickness > 3 mm | ||
| Yes | 6 (38) | 9 (60) |
| No | 8 (50) | 5 (33) |
| NR | 2 (13) | 1 (7) |
Seven fetuses had vesicocentesis and fetal urinalysis before VAS insertion (n = 5 good prognosis; n = 2 poor prognosis). 58 Four fetuses in the VAS arm and five in the conservative arm were karyotyped; all were normal males (46, XY).
Compliance with allocation
In total, 3/16 (19%) of those randomised to VAS did not receive the intervention and 2/15 (13%) randomised to conservative management ended up receiving a VAS. Of the three cases that were randomised to VAS but did not receive it, one patient withdrew consent after randomisation (for treatment but not follow-up), one patient was found to have a contraindication to VAS after randomisation and in the final patient the clinician determined that inserting a VAS was inappropriate because of poor fetal condition. In both of the cases in which the patient was randomised to conservative management but received a VAS this was because of deterioration in the clinical picture (anhydramnios) and the clinician determining that VAS was appropriate. In three cases (one in the VAS group and two in the conservative management group) parents opted for TOP between 18 and 25 weeks because of perceived poor prognosis by health-care professionals and parents. These decisions were unrelated to treatment allocation.
Survival outcomes (including primary outcome of survival to 28 days)
There were 12 live births in each arm. Within the VAS arm there was one IUD at 16 weeks following VAS insertion and three TOPs [two treatment related following spontaneous rupture of membranes (SROM) after shunt insertion and one as a result of patient choice] ( Figure 11 ). Within the conservative arm there were three pregnancy losses, one IUD at 18 weeks and two TOPs at 18 and 24 weeks as a result of patient choice.
FIGURE 11.
Consolidated Standards of Reporting Trials (CONSORT) flow chart. a, SROM within 28 days of shunt insertion at 17 and 22 weeks; b, parental decision at 18–25 weeks; c, following SROM at 16 weeks; d, because of pulmonary hypoplasia; e, renal function definitions: normal renal function (serum creatinine < 50 μmol/l), mild renal impairment (serum creatinine ≥ 50 μmol/l not requiring medical treatment), moderate renal impairment (serum creatinine ≥ 50 μmol/l and requiring medical treatment), end-stage renal failure (need for transplant or dialysis). NND, neonatal death. Reprinted from The Lancet, vol. 382, Morris RK, Malin GL, Quinlan-Jones E, Middleton LJ, Hemming K, Burke D, et al. Percutaneous vesicoamniotic shunting versus conservative management for fetal lower urinary tract obstruction (PLUTO): a randomised trial, pp. 1496–506, 2013,114 with permission from Elsevier.
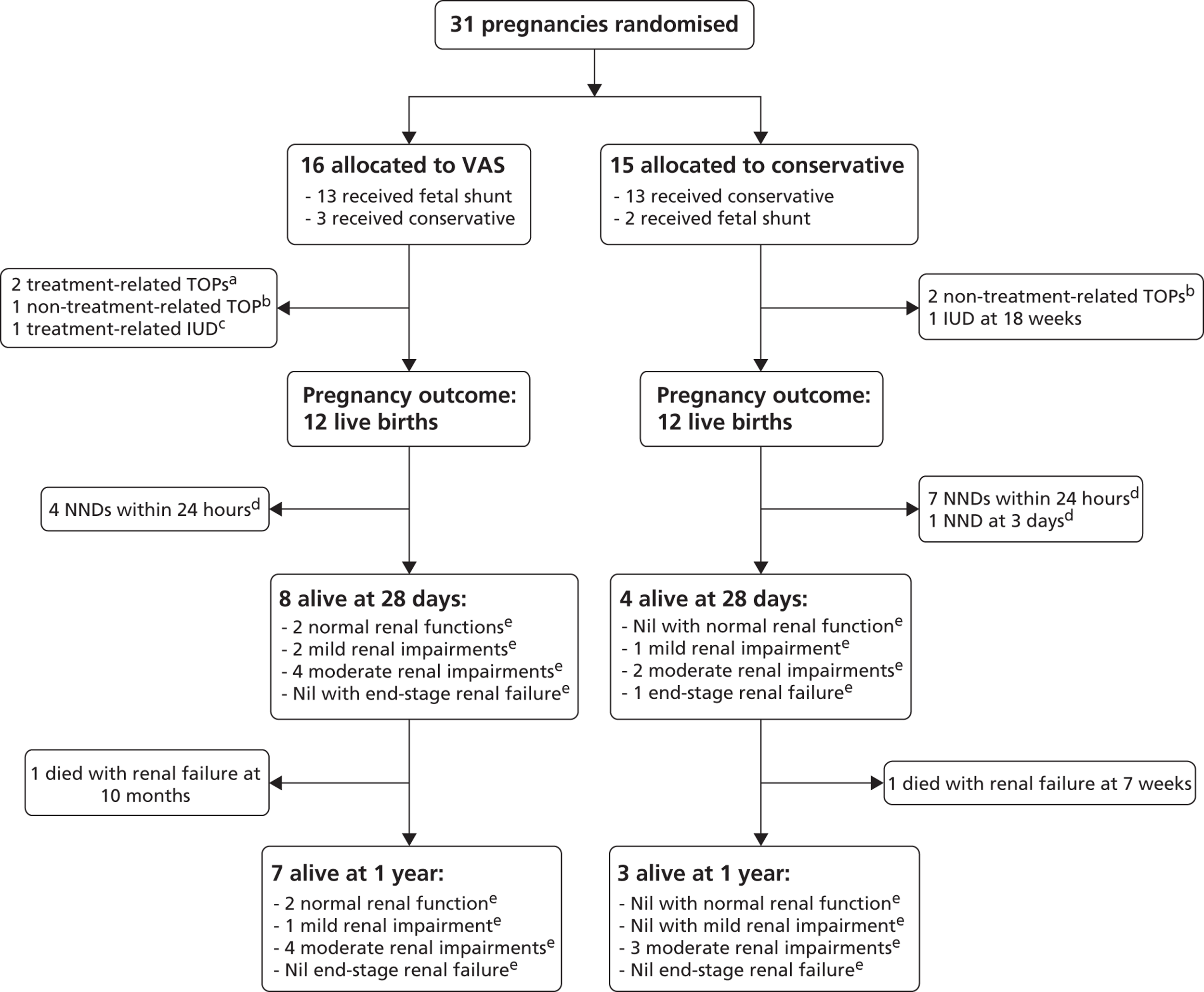
In total, 8/16 (50%) of the babies randomised to VAS survived to 28 days compared with 4/15 (27%) of those randomised to conservative management (ITT RR 1.88, 95% CI 0.71 to 4.96, p = 0.27) ( Table 14 ). All 12 neonatal deaths were as a result of pulmonary hypoplasia; 11 occurred within the first 24 hours following birth with the other at 3 days (all within 1 week).
| Time | ITT | ATa | ||||||
|---|---|---|---|---|---|---|---|---|
| VAS, n/N (%) | Conservative management, n/N (%) | RR (95% CI) | p-valueb | VAS, n/N (%) | Conservative management, n/N (%) | RR (95% CI) | p-valueb | |
| Live births | 12/16 (75) | 12/15 (80) | 0.94 (0.64 to 1.37) | > 0.99 | 11/15 (73) | 13/16 (81) | 0.90 (0.61 to 1.33) | 0.69 |
| Survival at 28 days | 8/16 (50) | 4/15 (27) | 1.88 (0.71 to 4.96) | 0.27 | 9/15 (60) | 3/16 (19) | 3.20 (1.06 to 9.62) | 0.03 |
| Survival at 1 year | 7/16 (44) | 3/15 (20) | 2.19 (0.69 to 6.94) | 0.25 | 8/15 (53) | 2/16 (13) | 4.27 (1.07 to 17.0) | 0.02 |
In the analysis of treatment received, 9/15 (60%) of those given a VAS survived to 28 days compared with 3/16 (19%) in the conservative management group (AT RR 3.20, 95% CI 1.06 to 9.62, p = 0.03) (see Table 14 ). Sensitivity analysis excluding non-treatment-related TOPs resulted in some slight changes to point estimates only ( Table 15 ).
| Time | ITT | ATb | ||||||
|---|---|---|---|---|---|---|---|---|
| VAS, n/N (%) | Conservative management, n/N (%) | RR (95% CI) | p-valuec | VAS, n/N (%) | Conservative management, n/N (%) | RR (95% CI) | p-valuec | |
| Live births | 12/15 (80) | 12/13 (92) | 0.87 (0.64 to 1.17) | 0.60 | 11/14 (79) | 13/14 (93) | 0.85 (0.62 to 1.15) | 0.60 |
| Survival to 28 days | 8/15 (53) | 4/13 (31) | 1.73 (0.68 to 4.45) | 0.28 | 9/14 (64) | 3/14 (21) | 3.00 (1.02 to 8.80) | 0.05 |
| Survival to 1 year | 7/15 (47) | 3/13 (23) | 2.02 (0.65 to 6.26) | 0.25 | 8/14 (57) | 2/14 (14) | 4.00 (1.03 to 15.6) | 0.05 |
One baby in each arm subsequently died from renal failure before age 1 year (see Figure 11 ). RR estimates at 1 year and p-values were similar to those seen at 28 days (see Tables 14 and 15 ).
Other outcomes
Birth and follow-up outcomes are difficult to interpret because of the small number of survivors and because of the imbalance in survival between groups; formal statistical comparisons have been omitted for these reasons. Individual values are provided for continuous measurements ( Table 16 ).
| Outcome | VAS, n (%) | Conservative management, n (%) |
|---|---|---|
| Live births | n = 12 | n = 12 |
| Randomisation to delivery (days), median (IQR) | 93 (69 to 118) | 104 (94 to 112) |
| Gestational age at delivery (days), median (IQR) | 249 (234 to 263) | 255 (242 to 262) |
| Preterm labour < 37 weeks | 7 (58) | 8 (67) |
| Vaginal delivery | 8 (67) | 7 (58)a |
| Birth weight (kg), mean (SD) | 2.8 (0.5) | 2.8 (0.4) |
| Birth weight < 10th centile | 5 (42) | 4 (33) |
| Admitted to NICU or children’s hospital | 10 (83) | 10 (83) |
| Required ventilation | 6 (55)b | 7 (70%)c |
| Required treatment for renal impairment | 4 (36)b | 3 (30%)c |
| Perinatal (at around 28 days) | n = 8 | n = 4 |
| Required surgery in perinatal period | 5 (63) | 3 (75) |
| Still an inpatient | 3 (43)b | 2 (50) |
| Serum creatinine (μmol/l) (individual values) | 29, 29, 88, 96, 105, 108, 119, 342 | 70, 126, 449, 620 |
| Renal functiond | ||
| Normal | 2 (25) | 0 |
| Mild impairment | 2 (25) | 1 (25) |
| Moderate impairment | 4 (50) | 2 (50) |
| End-stage renal failure | 0 | 1 (25) |
| 1 year | n = 7 | n = 3 |
| Required surgery from perinatal period to 1 year | 6 (86) | 0 |
| No. of days in hospital (individual values) | 0, 1, 3, 20, 25, 84, 102 | 22, 39, NR |
| Weight < 10th centile | 4 (67)b | 2 (100)b |
| Serum creatinine (μmol/l) (individual values) | 34, 37, 58, 64, 81, 88, 226 | 60, 60, 501 |
| Renal functiond | ||
| Normal | 2 (29) | 0 |
| Mild impairment | 1 (14) | 0 |
| Moderate impairment | 4 (57) | 3 (100) |
| End-stage renal failure | 0 | 0 |
| 2 years | n = 7 | n = 3 |
| Required surgery 1–2 years | 4 (57) | 1 (33) |
| No. of days in hospital (individual values) | 0, 1, 5, 19, 30, 37, 116 | 23, 37, 40 |
| Weight < 10th centile | 3 (60)c | 2 (67) |
| Serum creatinine (μmol/l) (individual values) | 65, 34, 87, 227, 60, 74, NR | 502, 61, 72 |
| Renal functiond | ||
| Normal | 2 | 0 |
| Mild | 0 | 0 |
| Moderate | 5 | 2 (67) |
| End-stage renal failure | 0 | 1 (33)e |
| Cognitive impairment | 1 significant | None reported abnormal |
Most live births were preterm (< 37 weeks) (7/12 VAS arm; 8/12 conservative management arm) and were admitted to a NICU or a children’s hospital (10/12 in both groups). A number required ventilation (6/11 VAS arm; 7/10 conservative management arm) or immediate treatment for renal impairment (4/11 VAS arm; 3/10 conservative management arm). Of those babies who survived to 28 days, only two (6% of those randomised) did not have any renal impairment at this stage. Both of these babies were in the group receiving VAS and were also the same two babies without impairment at the 1-year follow-up. The same result was seen for survival with normal renal function at 28 days or at 1 year when the data were analysed by treatment received (both 2/15 VAS arm; 0/16 conservative management arm).
A pathological diagnosis was made in all perinatal survivors (n = 12) and in three of the neonatal deaths (one in the VAS arm, two in the conservative management arm) a post-mortem was performed. The diagnoses were nine cases of PUV (five in the VAS arm, four in the conservative management arm), five cases of urethral atresia (four in the VAS arm, one in the conservative management arm) and one case of urethral syrinx causing obstruction (conservative management arm).
All babies with PUV underwent urethral valve resection in the perinatal period and all those with urethral atresia had a vesicostomy. One baby required both valve resection and a vesicostomy in the perinatal period. Six of the 10 survivors (all in the VAS group) required some sort of renal surgery at 1 year (see Table 16 ). At 1 year of age in the VAS group, three babies required a orchiopexy and one a vesicostomy and two underwent repeat valve resection, one underwent a nephrostomy and one underwent a nephrectomy.
Vesicoamniotic shunt complications
Vesicoamniotic shunting complications are described in Table 17 [as a proportion of the total number who received the VAS intervention (n = 15)]. Total pregnancy losses totalled 4/15 (27%), although one of these was not thought to be treatment related.
| Complication | Proportion affected (six fetuses/babies affected in total), n/N (%) | Outcome |
|---|---|---|
| SROM following VAS insertion | 3/15 (20) | IUD at 16 weeks’ gestation (n = 1); pregnancy affected by chorioamnionitis and opted for TOP at 17–22 weeks’ gestation (n = 2) |
| Dislodged | 3/15 (20) | SROM and TOP at 17 weeks’ gestation (also included above) (n = 1); subsequent urinary ascites and NND because of pulmonary hypoplasia (n = 1); shunt reinserted – subsequent preterm labour at 26 weeks’ gestation, alive at 28 days (n = 1) |
| Blocked | 1/15 (7) | Opted for TOP at 19 weeks’ gestation (not treatment related – fetus bradycardic) (n = 1) |
| Total pregnancy losses | 4/15 (27) | As described above: three following SROM and one following blockage |
Discussion
Principal findings
The PLUTO trial was stopped early because of poor recruitment over time. Although this is a limitation, results are available for all babies for outcomes to 1 year of age. The results demonstrate that prognosis in both arms (conservative management and VAS) is poor, with only two babies surviving to 1 year of age with no renal impairment. This finding again reinforces the natural pathogenesis of this fetal disease as one of severe and significant mortality and morbidity independent of treatment. In this randomised study all perinatal survivors had pathology confirmed as being secondary to congenital bladder outflow obstruction (PUVs and atresia).
There was a high proportion of pregnancy losses in both arms and pregnancy outcomes (live births) show no significant difference between the two arms. There appears to be a higher survival rate at 28 days (perinatal and neonatal survival) with VAS but this result is not statistically significant. All neonatal deaths were secondary to pulmonary hypoplasia and the possible reduction in perinatal mortality observed with VAS is probably secondary to prevention or amelioration of oligohydramnios at the critical time of lung development (canalicular phase between 16 and 24 weeks’ gestation).
Clinical outcomes at 1 year demonstrate an overall poor prognosis (in both groups) but again with a possible improved survival (but not statistically significant) with VAS. The likelihood of surviving with normal renal function is minimal in both groups, suggesting that the damage to the renal parenchyma has already occurred at the time of diagnosis and is irreversible. There was no difference in baseline characteristics nor other outcomes between the arms of the trial (e.g. gestational age at delivery, birthweight/small for gestational age and admission to NICU).
Another principal finding of the PLUTO trial was the high proportion of women who did not receive the allocated treatment (crossover). This was because of either clinician choice or changing clinical features as the pregnancy progressed. As this proportion was so high it was felt appropriate to perform an analysis according to treatment received. This analysis demonstrated larger differences in outcomes between the treatment arms of the trial. For survival at 28 days and at 1 year, the outcomes favoured VAS and the differences were statistically significant. There was no difference in survival with normal renal function at 28 days or 1 year between the analysis according to treatment received and the ITT analysis. However, it is conceded that there may have been confounding by selection bias.
Finally, the PLUTO trial demonstrated that, in the context of a RCT of an intervention for a rare fetal disease, relatively few women (or their families) were willing to consider randomisation of treatment and opted instead for either entry onto the registry (where they or their health professional could choose treatment) or, more commonly, TOP. This is investigated further in our qualitative research into patient acceptability (see Chapter 9 ).
Strengths and limitations of the study
The strengths of this study lie in its design. This was a RCT that was multicentre and international. The trial had clear objectives with well-defined (designated a priori) primary and secondary end points. The trial was registered and the protocol published. 16 There were no significant changes to this protocol during the lifetime of the study with the exception of those requested by the primary funder in the light of poor recruitment (i.e. removal of follow-up to 5 years of age). These are clearly documented in Appendix 7 .
A major limitation of this trial is the low number of subjects recruited, making the study underpowered. For this reason the trial was sufficiently powered to detect only very large differences in the primary outcome. The reasons for poor recruitment have already been discussed and are explored further in Chapters 3 , 7 and 9 . One of the major barriers to recruitment was the high proportion of women entered onto the registry rather than being randomised. This was often because the clinician was apparently not in ‘equipoise’ as to the benefits of the intervention (despite the findings of a published systematic review of the literature13) and is in contrast to the expert opinions elicited at the start of the study (see Chapter 7 ). This indicated a divergence of opinion among specialists and established clinical equipoise.
A strength in this respect is that all patients completed follow-up and there were no missing data for the primary and secondary end points. All end points were assessed independently by paediatric neonatologists and nephrologists as part of routine practice. The primary analysis reported is an ITT analysis with no exclusions.
Blinding of fetal medicine specialists, parents and end point assessors was not possible because of the nature of the intervention. As the primary end point was survival the primary outcome would not have been affected by this lack of blinding. However, this may have had an impact on the number of women who ‘crossed over’ from the conservative management arm to the VAS arm because of a change in the assessment of the condition as the pregnancy progressed. This selection bias will have been a confounder and the amount or direction of effect this may have had cannot be assessed. Thus, the number of women crossing over from the conservative management arm to the VAS arm is a limitation with regard to the study’s conclusions.
Strengths and limitations in relation to other studies
This is the only RCT of the effectiveness of VAS in LUTO. We therefore cannot make direct comparisons between our results and previously published results of a RCT/prospective study in terms of strengths and limitations. Although the numbers recruited are small (in the context of the prospective power calculation for the RCT), in relation to previously published cohort studies they are comparable. These retrospective cohort studies and their results have been rigorously assessed by our group as part of the systematic reviews discussed in Chapter 1 . The results from these systematic reviews indicated an OR of 3.86 (95% CI 2.00 to 7.45) for perinatal survival with VAS and an OR of 0.50 (95% CI 0.13 to 1.90) for survival with normal renal function. The conclusion of the observational evidence is that VAS improves perinatal survival but the effect on long-term renal function is unclear. The outcome measures used for renal function in these observational studies were also heterogeneous. The results of the PLUTO RCT are consistent with the findings of the systematic review summarising the observational evidence for perinatal survival and suggest that the chance of survival with normal renal function is very low regardless of whether VAS is performed or not.
Recommendations for future practice
As the findings of the PLUTO trial were limited by the small numbers recruited and thus the RCT was underpowered, any recommendations must be interpreted in this light. However, the results of the RCT and the observational evidence are consistent and thus the body of objective evidence suggests that VAS improves overall perinatal survival compared with conservative management but that the long-term prognosis for these babies into infant life is poor (with high rates of mortality and morbidity). Parents should be counselled about the risks of pregnancy loss with or without VAS insertion. The NICE interventional procedures guidance (IPG 2028) should be updated to reflect this new evidence.
Recommendations for future research
As the PLUTO trial was a multicentre international trial and did not recruit the required number of participants to achieve statistical power, it is unlikely that a further RCT would be feasible. It is imperative that the babies recruited into the PLUTO trial are prospectively followed up throughout childhood to determine the effects of VAS on outcomes such as renal function, incontinence, cognitive development and quality of life. However, the number of children surviving to this age (2–5 years) is likely to be small. There should be further research to try and overcome the barriers to recruitment identified within this study, namely in relation to the methodology of RCTs in rare diseases (especially relating to pregnancy). Improvements have already been made in the last few years in the UK relating to achieving ethics and R&D approval for studies recruiting at multiple sites. These changes greatly shortened the time that recruitment sites took to recruit their first patient in the latter period of the PLUTO study. However, the study was limited in its recruitment ability because of the difficulties in obtaining indemnity and thus sponsorship for international centres. This is something that the international academic community, higher education institutions and funders must work hard to resolve so that research questions can be adequately addressed in the study of rare diseases.
Chapter 6 Findings of the registry and termination of pregnancy register
Recruitment and participants
In total, 45 women from nine centres entered the PLUTO registry between August 2006 and December 2010. The majority (35/45, 78%) opted for conservative management ( Table 18 and Figure 12 ).
| Centre | Recruitment, n (%) | Conservative management, n | VAS, n |
|---|---|---|---|
| Birmingham Women’s Hospital, UK | 23 (51) | 21 | 2 |
| St Michael’s Hospital, Bristol, UK | 6 (13) | 4 | 2 |
| Princess Anne Hospital, Southampton, UK | 5 (11) | 4 | 1 |
| St George’s Hospital, London, UK | 3 (7) | 2 | 1 |
| Liverpool Women’s Hospital, UK | 2 (4) | 2 | 0 |
| University Medical Centre, Leiden, Netherlands | 2 (4) | 1 | 1 |
| King’s College Hospital, London, UK | 2 (4) | 0 | 2 |
| Queen Mother’s Hospital, Glasgow, UK | 1 (2) | 1 | 0 |
| St Marys Hospital, Manchester, UK | 1 (2) | 0 | 1 |
| Total | 45 | 35 | 10 |
FIGURE 12.
Randomised and registry recruitment to the PLUTO study.
Baseline characteristics of registry participants
The baseline characteristics of those registered can be seen in Table 19 . The average age of mothers was 31 years (p = 0.82 between groups). The proportion of fetuses with a liquor volume less than the fifth centile was higher among those opting for VAS than among those opting for conservative management (70% vs. 34%, p = 0.07). The proportion of fetuses for whom age at diagnosis was < 24 weeks also appeared higher in the VAS group (80% vs. 51%), but this was not statistically significant (p = 0.15). There were no other statistically significant differences between the groups but we cannot rule out that this was because of the small sample size.
| Characteristic | VAS, n (%) | Conservative management, n (%) | p-value |
|---|---|---|---|
| No. registered | 10 | 35 | |
| Maternal age (years), median (IQR) | 31 (28 to 38) | 31 (27 to 33) | 0.82 |
| Maternal age (years) | |||
| < 20 | 1 (10) | 1 (3) | |
| 20–35 | 6 (60) | 29 (83) | 0.20 |
| > 35 | 3 (30) | 5 (14) | |
| Ethnicity | |||
| White | 7 (70) | 28 (80) | 0.62 |
| Asian | 2 (20) | 4 (11) | |
| Black | 1 (10) | 2 (6) | |
| Not given | – | 1 (3) | |
| Gestational age (days), median (IQR) | 154 (125 to 157) | 157 (141 to 222) | 0.27 |
| Gestational age (weeks) | |||
| < 24 | 8 (80) | 18 (51) | 0.15 |
| ≥ 24 | 2 (20) | 17 (49) | |
| Liquor volume MPD (cm), median (IQR) | 1.5 (0 to 3.3) | 3.2 (1.0 to 5.0) | 0.07 |
| Liquor volume | |||
| < 5th centilea | 7 (70) | 12 (34) | 0.07 |
| ≥ 5th centilea | 3 (30) | 23 (66) | |
| Renal pelvis dilatation left (mm), median, (IQR) | 7.0 (4.0 to 12)b | 8.2 (6.5 to 11)c | 0.54 |
| Renal pelvis dilatation right (mm), median (IQR) | 7.1 (5.0 to 21)b | 7.5 (5.8 to 14)c | > 0.99 |
| Renal pelvis dilatation AP > 90th centiled | |||
| Bilateral | 5 (50) | 19 (54) | > 0.99 |
| Unilateral | 1 (10) | 3 (9) | |
| Neither | 1 (10) | 3 (9) | |
| NR | 3 (30) | 10 (29) | |
| Renal pelvis severe hydronephrosis > 1.5cme | |||
| Bilateral | 1 (10) | 4 (11) | > 0.99 |
| Unilateral | 1 (10) | 2 (6) | |
| Neither | 5 (50) | 19 (54) | |
| NR | 3 (30) | 10 (29) | |
| Macrocystic renal appearance | |||
| Bilateral | 2 (20) | 4 (11) | 0.33 |
| Unilateral | 1 (10) | 3 (9) | |
| Neither | 4 (40) | 27 (77) | |
| NR | 3 (30) | 1 (3) | |
| Renal ‘echogenicity’ | |||
| Bilateral | 5 (50) | 10 (29) | 0.37 |
| Unilateral | 0 | 3 (9) | |
| Neither | 1 (10) | 9 (26) | |
| NR | 4 (40) | 13 (37) | |
| Bladder wall thickness > 3 mm | |||
| Yes | 3 (30) | 12 (34) | |
| No | 3 (30) | 11 (31) | > 0.99 |
| NR | 4 (40) | 12 (34) |
Compliance with intervention selection
In four cases (one in the VAS group and three in the conservative management group) parents opted for TOP between 19 and 23 weeks. This was because the overall prognosis for the fetus was felt to be poor. These decisions were considered unrelated to intervention choice.
Survival outcomes (including primary outcome of survival to 28 days)
There were seven live births in the VAS group (out of 10, 70%) and 30 in the conservative group (out of 35, 86%); this followed one treatment-related TOP (following SROM at 21 weeks after VAS insertion) and one treatment-associated IUD (2 days after VAS insertion) in the VAS group ( Figure 13 ).
FIGURE 13.
Non-randomised registry participant flow chart. a, SROM within 28 days of shunt insertion at 17 and 22 weeks; b, TOP at 23 weeks after two shunt insertions: fetus still anhydramnios, considered treatment failure; c, miscarriage 2 days after shunt insertion at 22 weeks; d, TOP at 19, 19 and 21 weeks (parental decision); e, IUD at 18 weeks and 31 weeks; the latter was an intrapartum stillbirth (fetus was actually female); f, because of pulmonary hypoplasia; g, renal function definitions: normal renal function: serum creatinine < 50 μmol/l; mild renal impairment: serum creatinine ≥ 50 μmol/l not requiring medical treatment; moderate renal impairment: serum creatinine ≥ 50 μmol/l and requiring medical treatment; end-stage renal failure: need for transplant or dialysis. NND, neonatal death.
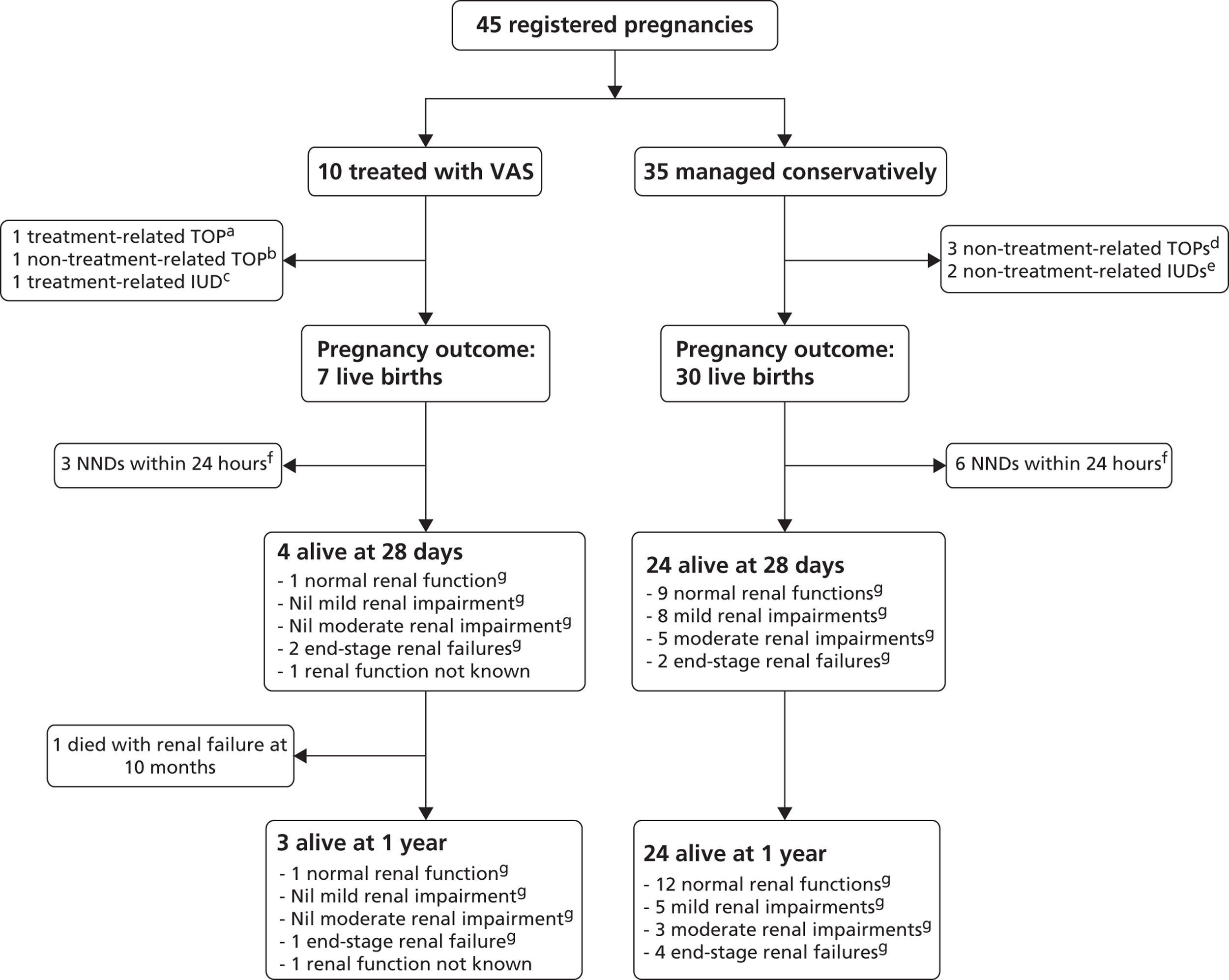
Of the babies treated by VAS, 4/10 (40%) survived to 28 days; this compares with 24/35 (69%) of those conservatively managed (RR 0.58, 95% CI 0.26 to 1.29, p = 0.14) ( Table 20 ). All nine neonatal deaths (three in the VAS group, six in the conservative management group) were associated with pulmonary hypoplasia with all deaths occurring within the first 24 hours of the neonatal period. Results from the sensitivity analysis (excluding non-treatment-related TOPs, Table 21 ) showed only a slight difference in point estimates (RRs, CIs) and tests for statistical significance.
| Time | VAS, n/N (%) | Conservative management, n/N (%) | RR (95% CI) | p-valuea |
|---|---|---|---|---|
| Live births | 7/10 (70) | 30/35 (86) | 0.82 (0.53 to 1.25) | 0.35 |
| Survival to 28 days | 4/10 (40) | 24/35 (69) | 0.58 (0.26 to 1.29) | 0.14 |
| Survival to 1 year | 3/10 (30) | 24/35 (69) | 0.44 (0.17 to 1.16) | 0.06 |
| Time | VAS, n/N (%) | Conservative management, n/N (%) | RR (95% CI) | p-valueb |
|---|---|---|---|---|
| Live births | 7/9 (78) | 30/32 (94) | 0.83 (0.58 to 1.19) | 0.20 |
| Survival to 28 days | 4/9 (44) | 24/32 (75) | 0.59 (0.28 to 1.26) | 0.11 |
| Survival to 1 year | 3/9 (33) | 24/32 (75) | 0.44 (0.17 to 1.14) | 0.04 |
One baby died between 28 days and 1 year (at 10 months); this baby was treated by insertion of a VAS and died of chronic renal failure (see Figure 13 ). RR estimates at 1 year were borderline statistically significant in favour of conservative management (see Tables 20 and 21 ).
Other outcomes
The results of the birth and follow-up outcomes can be seen in Table 22 . As per the results of the RCT, formal statistical comparisons have been omitted because of the small number of survivors, particularly in the VAS group in which only 10 women were initially registered and only four babies survived to 28 days. Individual values are again provided for continuous measurements.
| Outcome | VAS, n (%) | Conservative management, n (%) |
|---|---|---|
| Live births | n = 7 | n = 30 |
| Registration to delivery interval (days), median (IQR) | 98 (15 to 116) | 77 (44 to 129) |
| Gestational age at delivery (days), median (IQR) | 241 (211 to 255) | 267 (260 to 278) |
| Preterm labour < 37 weeks | 6 (86) | 7 (23) |
| Vaginal delivery | 3 (50)a | 18 (62)a,b |
| Birthweight (kg), mean (SD) | 2.4 (0.9)a | 3.3 (0.8)c |
| Birthweight < 10th centile | 1 (17)a | 4 (14)c |
| Admitted to NICU or children’s hospital | 3 (75)d | 21 (70) |
| Required ventilation | 2 (33)a | 6 (21)a |
| Required treatment for renal impairment | 1 (17)a | 10 (34)a |
| Perinatal (at around 28 days) | n = 4 | n = 24 |
| Required surgery in perinatal period | 3 (75)a | 17 (71) |
| Still an inpatient | 3 (73)b | 10 (42) |
| Serum creatinine (μmol/l) (individual values) | 24, 280, 456, NR | 32, 32, 36, 38, 39, 49, 59, 64, 66, 74, 84, 86, 99, 103, 104, 110, 115, 259, 262, 429, NR, NR, NR, NR |
| Renal functione | ||
| Normal | 1 (25) | 9 (38) |
| Mild impairment | 0 | 8 (33) |
| Moderate impairment | 0 | 5 (21) |
| End-stage renal failure | 2 (50) | 2 (8) |
| Not known | 1 (25) | 0 |
| 1 year | n = 3 | n = 24 |
| Required surgery from perinatal period to 1 year | 1 (33)a | 9 (38)a |
| No. of days in hospital (individual values) | 4, 365, NR | 0, 1, 3, 3, 3, 6, 7, 11, 13 14, 18, 20, 27, 122, NR, NR, NR, NR, NR, NR, NR, NR, NR, NR |
| Infant weight < 10th centile | 1 (50)a | 10 (43)a |
| Serum creatinine (μmol/l) (individual values) | 19, 132, NR | 16, 21, 23, 23, 27, 28, 42, 54, 57, 65, 70, 79, 110, 219, 237, 241, 465, 709, NR, NR, NR, NR, NR, NR |
| Renal functione | ||
| Normal | 1 (33) | 12 (50) |
| Mild impairment | 0 | 5 (21) |
| Moderate impairment | 0 | 3 (13) |
| End-stage renal failure | 1 (33) | 4 (17) |
| Not known | 1 (33) | 0 |
Of those born alive, 6/7 (86%) babies in the VAS group were born preterm (< 37 weeks) compared with only 7/30 (23%) in the conservative management group. Most were admitted to a NICU or a children’s hospital (3/4 VAS group; 21/30 conservative management group). A number required ventilation (2/6 VAS group; 6/29 conservative management group) or immediate treatment for renal impairment (1/6 VAS group; 10/29 conservative management group); these proportions appeared to be lower than in the RCT. Only one of the 10 babies initially treated by VAS did not have any renal impairment by the 28-day and 1-year follow-up (although one baby was known to be alive but its renal function status was unknown) compared with nine babies in the conservative group at 28 days. By the 1-year follow-up 12 babies in the conservative arm had no renal impairment; 19 had required renal surgery by this stage.
Of the perinatal survivors all had a pathological diagnosis except for one in the VAS group (alive and well at 1 year, no renal investigations performed as well). In the group receiving VAS with a known diagnosis (n = 3), there were two cases of PUV (one alive with end-stage renal failure at 28 days, died at 10 months of age, and one alive and well at 1 year) and one case of urethral atresia (alive with end-stage renal failure at 28 days and 1 year).
In the conservative group the pathological diagnoses was known for all but two babies (22/24). The two cases in which there was no pathological diagnosis were babies that were well postnatally and thus did not undergo any further investigation. They were both alive with normal renal function at 1 year. Of those for whom a pathological diagnosis was known, there were 16 cases of PUV. At 28 days these were classified as four alive and normal renal function, six alive with mild renal impairment, four alive with moderate renal impairment and two alive with end-stage renal failure; at 1 year of age they were all still alive and classified as six normal renal function, four mild renal impairment, three moderate renal impairment and three end-stage renal failure. There was one case of urethral atresia who had moderate renal impairment at 28 days and end-stage renal failure at 1 year. For two babies there was no renal tract abnormality detected; one of these underwent no further follow-up and the other had normal renal function at 1 year. There were three cases of bilateral vesicoureteric reflux; two had normal renal function at 1 year and the other had mild renal impairment. Thus, in the group electing to have conservative management, of those with a known postnatal diagnosis there were five cases of a false-positive antenatal diagnosis (5/22, 24%) (two with a normal renal tract and three cases of reflux).
All perinatal survivors in the VAS group underwent surgery (one bilateral open nephrostomy; one PUV ablation, circumcision and right inguinal hernia repair; and one vesicostomy). In the conservative group 19 survivors underwent surgery. In the group with PUV six underwent PUV ablation and circumcision, five underwent PUV ablation only, one underwent PUV ablation and vesicostomy, one underwent vesicostomy and insertion of a peritoneal dialysis catheter, two underwent PUV ablation and insertion of a peritoneal dialysis catheter and one had a suprapubic catheter and ablation of PUV and required a hernia repair and sling repair to the urethra in the first year of life. In two of the cases with vesicoureteric reflux, circumcision was required.
Vesicoamniotic shunt complications
As described in Table 23 , the risk of total pregnancy loss was 3/10 (30%) (one of these was not considered to be a serious adverse event related to the intervention but was considered to be a treatment failure as the fetus had persistent anhydramnios and thus the parents opted to terminate the pregnancy).
| Complication | Proportion affected (seven fetuses/babies affected in total), n/N (%) | Outcome |
|---|---|---|
| SROM following VAS insertion | 1/10 (10) | Opted for TOP at 21 weeks |
| Miscarriage following VAS | 1/10 (10) | 2 days after VAS insertion at 22 weeks |
| Dislodgement of VAS | 4/10 (40) | One case of SROM and TOP as described above, one case alive at 1 year with end-stage renal failure, one case underwent reinsertion of VAS before TOP at 23 weeks, one died at delivery |
| Blockage of VAS | 2/10 (20) | One case died within 24 hours of birth, one preterm labour at 33 weeks |
| Total pregnancy losses associated with VAS insertion | 3/10 (30) | Two cases as described above plus a further TOP at 23 weeks after two VAS insertions (see dislodgement). This was considered a treatment failure (fetus still had anhydramnios) as opposed to an adverse event |
Combined logistic regression analysis of randomised and registry data focusing on predictors of survival to 28 days
Background
We have combined data from the randomised and registry data sets to explore whether the difference in survival to 28 days between treatment groups in the randomised and registry data (the RCT appeared to favour VAS whereas the registry favoured conservative management) is related to the demographics of the randomised and registry participants. We also examined whether the predefined subgroups of gestational age, liquor volume and maternal age were indeed associated with survival to 28 days. Evidence for the importance of these variables in urinary tract obstruction is thus far limited, as discussed in Chapter 1 . The combined data set gives us a larger group to make statistical inferences from (n = 40 survivors from 76 participants in total).
Results
Difference in effect between the randomised and registry data sets
The RCT appeared to favour VAS in terms of survival to 28 days [ITT RR 1.88, 95% CI 0.71 to 4.96 (50% vs. 27%, p = 0.27); AT RR 3.20, 95% CI 1.06 to 9.62 (60% vs. 19%, p = 0.03)] whereas the registry favoured conservative management [RR 0.58, 95% CI 0.26 to 1.29 (40% vs. 69%, p = 0.14)] (see Chapters 5 and 6 ). This apparent difference in treatment effect was tested by including an interaction parameter (method of entry by intervention, in which method of entry is either RCT or registry) along with method of entry and intervention parameters in a logistic regression model with survival to 28 days as the outcome. This interaction parameter was statistically significant regardless of whether we used allocated treatment (p = 0.040) or received treatment (p = 0.006) (i.e. the probability of seeing these differing treatment effects between the RCT and the registry was low enough for us to be fairly convinced that this was not due to the play of chance alone), thus showing that the difference between the results of the RCT and the results of the registry could not be explained as a chance finding.
Composition of the randomised and registry data sets
A higher proportion of those patients who entered the study registry with conservative management had a liquor volume greater than the fifth centile than those who were registered to VAS (p = 0.07 by Fisher’s exact test) or who were randomised (p = 0.05) ( Table 24 ). A higher proportion of this group also had a gestational age at diagnosis of ≥ 24 weeks (p = 0.15 and p = 0.003 compared with those registered with VAS and those randomised respectively). There was no evidence of a difference between the groups in terms of the mother’s age at diagnosis (p = 0.2 and p = 0.8 for the same comparisons).
| Variable | Randomised, n/N (%) | Registered VAS, n/N (%) | Registered conservative management, n/N (%) |
|---|---|---|---|
| Liquor volume centile (MPD): > 5th centile | 12/31 (39) | 3/10 (30) | 23/35 (66) |
| Gestational age at diagnosis: ≥ 24 weeks | 4/31 (13) | 2/10 (20) | 17/35 (49) |
| Mother’s age at diagnosis: > 35 years | 5/31 (16) | 3/10 (30) | 5/35 (14) |
Univariable and multivariable logistic regression analyses over both randomised and registry data sets
There appeared to be a higher 28-day survival rate for those women who entered the study registry than for those who were randomised (62% vs. 39%, p = 0.046) ( Table 25 ). Of the other variables those fetuses diagnosed at ≥ 24 weeks’ gestation had a higher survival rate than those diagnosed at < 24 weeks (87% vs. 38%, p < 0.001). Similarly, those with a liquor volume greater than the fifth centile had a higher survival rate than those with a liquor volume less than or equal to the fifth centile (74% vs. 32%, p < 0.001). There was no evidence that survival was dependent on mother’s age, although we cannot rule this out because the sample may be too small to detect an association.
| Variable | Survival to 28 days by group | Unadjusted OR (95% CI) | p-value |
|---|---|---|---|
| Study entry | Registered 28/45 (62%), randomised 12/31 (39%) | 2.6 (1.0 to 6.7) | 0.046 |
| Treatment allocated | VAS 12/26 (46%), conservative management 28/50 (56%) | 0.7 (0.3 to 1.7) | 0.42 |
| Treatment received | VAS 13/25 (52%), conservative management 27/51 (53%) | 1.0 (0.4 to 2.5) | 0.94 |
| Gestational age at diagnosis | ≥ 24 weeks 20/23 (87%), < 24 weeks 20/53 (38%) | 11.0 (2.9 to 41.8) | < 0.001 |
| Gestational age at diagnosis as a continuous variable (weeks) | – | 1.2 (1.1 to 1.4) | 0.001 |
| Liquor volume centile (MPD) | > 5th centile 28/38 (74%), ≤ 5th centile 12/38 (32%) | 6.1 (2.2 to 16.4) | < 0.001 |
| Liquor volume as a continuous variable (MPD in cm) | – | 2.0 (1.4 to 2.9) | < 0.001 |
| Mother’s age at diagnosis | < 20 years 3/4 (75%), 20–35 years 31/59 (53%), > 35 years 6/13 (46%) | 3.5 (0.3 to 43.2),a 1.3 (0.4 to 4.3),b reference | 0.62 |
| Mother’s age at diagnosis as a continuous variable (years) | – | 1.0 (0.9 to 1.1) | 0.99 |
When we include the statistically important variables (p < 0.1) from the univariable analysis together in a multivariable analysis [along with method of entry, treatment (both allocated and received separately) and a method of entry by treatment interaction variable], gestational age ≥ 24 weeks and liquor volume greater than the fifth centile are still strongly associated with increased survival to 28 days. The entry by treatment interaction term is borderline significant if treatment received is used ( Tables 26 and 27 ), indicating that there is still some evidence of differing intervention effect between those randomised and those registered even after statistical adjustment for the other variables.
| Variable | Comparison | Adjusted OR (95% CI) | p-value |
|---|---|---|---|
| Allocation by entry (p-value for interaction = 0.31) | Registered: VAS vs. conservative management | 0.8 (0.1 to 5.1) | 0.84 |
| Randomised: VAS vs. conservative management | 2.9 (0.5 to 16.4) | 0.22 | |
| Gestational age at diagnosis (weeks) | ≥ 24 vs. < 24 | 11.2 (2.4 to 51.6) | 0.002 |
| Liquor volume centile (MPD) | > 5th vs. ≤ 5th | 7.1 (2.1 to 23.6) | 0.002 |
| Variable | Comparison | Adjusted OR (95% CI) | p-value |
|---|---|---|---|
| Received by entry (p-value for interaction = 0.056) | Registered: VAS vs. conservative management | 0.9 (0.1 to 5.4) | 0.87 |
| Randomised: VAS vs. conservative management | 10.9 (1.6 to 76.3) | 0.016 | |
| Gestational age at diagnosis (weeks) | ≥ 24 vs. < 24 | 12.9 (2.7 to 61.6) | 0.001 |
| Liquor volume centile (MPD) | > 5th vs. ≤ 5th | 7.7 (2.2 to 27.4) | 0.002 |
The apparent difference between treatment effects in the randomised study and the registry, and the importance of gestational age/liquor volume are very likely to be related. The demographics of those opting for conservative management in the registry (see Table 24 ) are distinct from the demographics of those randomised, with a higher proportion of those in the registry having a liquor volume greater than the fifth percentile and a gestational age at diagnosis of ≥ 24 weeks. As we have shown, these two variables seem to be strongly associated with better survival to 28 days.
The logistic regression analysis was performed excluding five false positives (all in the registry group) and, although this did reduce the overall proportion who survived in the registry set by a small amount (from 62% to 58%), it did not alter our overall conclusions that (1) there was some evidence that the treatment effect differed between the randomised and registry sets in terms of survival and (2) later gestational age at diagnosis and higher liquor volume were highly association with increased chance of survival.
Termination of pregnancy register
At the time of closure of the PLUTO trial to recruitment (December 2010), 68 TOPs had been reported to the trial office. It is recognised that this figure is likely to be an underestimate of the total number of TOPs as many centres did not return their data as this was a voluntary register or women underwent TOP without referral to a PLUTO centre. International centres were also not included in this registry. However, this number of TOPs is significant, accounting for 47% (68/144) of the number of cases seen over the period October 2006–October 2010. It can be seen that if many more of these women had opted for randomisation then the number recruited to the trial would have been nearer to that originally projected.
The reasons for the high number of parents opting for TOP are explored further in the patient acceptability study (see Chapter 9 ).
Discussion
Principal findings
A high proportion of women and clinicians opted to select their management and thus entered the registry. In addition, a much higher than anticipated number of parents underwent TOP as the only option for management. Although not statistically significant, survival at 28 days and at 1 year appeared higher in the conservative arm. Exclusion of non-treatment-related TOPs gave an improved survival to 1 year in the conservative arm that was statistically significant. There was a higher proportion of babies born preterm in the VAS group; thus, there was a shorter interval from registration to delivery and a younger gestational age at delivery in this group. Longer-term outcomes demonstrated a higher proportion of babies within the conservative arm surviving with normal renal function at both 28 days and 1 year, although the outlook was still poor. High proportions of babies required surgery in either the perinatal period or the first year of life in both arms.
Comparing the results of the registry with those of the RCT there appears to be no difference in live birth rates for either VAS or conservative management, highlighting again the poor prognosis with pulmonary hypoplasia. The main difference is seen in the conservative group of the registry, with higher survival to 28 days and 1 year. The chance of surviving with normal renal function is also higher in the group registered for conservative management than among those randomised.
However, there were clinically important differences between fetuses in the VAS arm and fetuses in the conservative arm within the registry. There was a statistically significant higher proportion of babies with a liquor volume less than the fifth centile in the VAS group and they were also more likely to have their diagnosis made earlier in the pregnancy (at < 24 weeks). There was also a difference in the pathological diagnoses made postnatally in these groups, in that all babies with a pathological diagnosis in the VAS group were confirmed to have had LUTO. However, in the conservative group there was a high proportion of babies with a false-positive antenatal diagnosis, which was comparable to that identified in the epidemiological study (see Chapter 3 ). These factors should be seen as confounders, likely to influence the results for both survival and renal impairment and explain the difference in outcomes seen between the RCT and the registry.
The exploratory analysis of the combined PLUTO study data set out to ascertain if apparent differences in effect on 28-day survival between VAS and conservative management in the two data sets (randomised and registry) were statistically important and furthermore to investigate possible reasons for these differences. We also set out to provide some stronger evidence for or against the importance of gestational age, liquor volume and maternal age as influential prognostic factors for survival.
We found that there was statistically important heterogeneity between the survival rate estimates in the randomised and registry data sets (randomised set favoured VAS whereas the registry set favoured conservative management). The composition of the randomised and registry sets clearly differed, with those registered for conservative management comprising a greater proportion of fetuses with a liquor volume greater than the fifth centile and with a gestational age at diagnosis of ≥ 24 weeks. Liquor volume greater than the fifth centile and gestational age at diagnosis of ≥ 24 weeks appear to be associated with increased chance of survival to 28 days.
It is possible that the number of TOPs in the registry was a national underestimate. The denominator (nationally) is unknown with the most complete data arising from the ONS. 115 In 2010 there were 726,879 births in England and Wales and 196,109 TOPs. 116 Of these TOPs, only 9% were performed at > 13 weeks and < 0.1% (n = 147) at > 24 weeks. Of those TOPs performed in 2010 under clause E (termination up to term pregnancy when there is a substantial risk that if the child is born it would suffer from such physical or mental abnormalities as to be seriously handicapped), 100 were performed for urinary tract abnormalities (ICD-10 codes Q60–64). Similar rates were reported for 2011: 196,082 TOPs, 9% at < 13 weeks, < 0.1% at > 24 weeks (n = 146) and, of those carried out under clause E, 107 were performed for urinary tract abnormalities. However, these national data are available for England and Wales only and the ICD-10 codes Q60–64 include all urinary tract abnormalities (e.g. renal agenesis, cystic kidney disease, congenital obstruction of the renal pelvis). BINOCAR reported similar data for 2010 with urinary anomalies having a TOP rate of 5.3 per 10,000 total births (95% CI 4.5 to 6.3). 96
Strengths and limitations of the study
The major limitation of this part of the study is that it was a non-randomised treatment allocation study. However, data collection was prospective and was carried out according to a protocol designed and published a priori as for the RCT. The data collected was identical to that collected in the RCT, as were the outcome measures.
A strength of this study is again the high number of cases with complete follow-up. There were no cases with missing data for the primary end point of survival and only one case in the VAS group with missing data for renal function. As for the RCT, all end points were assessed independently by paediatric neonatologists and nephrologists as part of routine practice.
The logistic regression analysis provides the strongest evidence to date that liquor volume and gestational age at diagnosis are associated with survival to 28 days in fetuses with a confirmed diagnosis of LUTO. The data come from a high-quality RCT and registry with excellent follow-up of all babies. The main limitation is the relatively small data set (even with trial and registry data combined), which means that we are limited in the number of variables that we can examine for possible association with survival to 28 days. The small data set also means that we cannot rule out a possible association of maternal age with survival.
Strengths and limitations in relation to other studies
As discussed in Chapter 1 there have been other observational studies comparing VAS with conservative management. The numbers recruited to this registry are comparable to those in the previously published cohorts. However, these other cohorts were retrospective and did not have the high rate of follow-up and length of follow-up involved in this registry, nor was a diagnosis of LUTO sought in all patients. The outcome measures used for renal function in these observational studies were also heterogeneous. Our registry results are in contrast to the results of our systematic review, which indicated an OR of 3.86 (95% CI 2.00 to 7.45) for perinatal survival with VAS. The reason for the apparent better outcomes in the registry conservative management group may be because of the confounders identified above, which have already been discussed.
Conclusion
Those women entered onto the registry and receiving VAS had similar baseline characteristics and outcomes to those in the VAS arm of the RCT, with a similar but relatively poor prognosis. The conservatively managed group in the registry appeared to be different from the conservatively managed group in the RCT in terms of baseline characteristics (later age of gestation at diagnosis and a higher proportion with a liquor volume greater than the fifth centile) and had a better prognosis for survival. There were a significant number of cases of false-positive antenatal diagnosis within this conservatively managed group, with final postnatal diagnoses being conditions associated with a better prognosis than LUTO. A high proportion of parents opted for TOP.
Normal liquor volume (greater than the fifth centile) and gestational age at diagnosis of ≥ 24 weeks seem to be associated with an increased probability of survival at 28 days in fetuses with a confirmed diagnosis of LUTO. Non-randomised comparisons of survival between groups in the registry are likely to be confounded with the effect of these parameters and are likely to have contributed to the observed statistical heterogeneity between the randomised and registry data sets.
Chapter 7 Bayesian analysis of the randomised controlled trial
Introduction
Bayesian statistical inferences allow an inductive and philosophical approach to statistics. 87,89,117 This is in contrast to the conventional approach to statistics, which, from what is known as a frequentist perspective, provides a deductive approach. There are several important benefits that can be obtained from conducting a Bayesian analysis. These include the ability to interpret CIs as providing intervals that contain the true effect with some particular probability, say a 95% probability; shifting the focus of interpretation away from dichotomising whether the data suggest the intervention works or not, that is, by not claiming a ‘significant’ or ‘null result’, and instead providing estimates of probabilities of benefits greater than some clinically important effect size, and so switching focus to how clinically important the intervention might be; and, finally, allowing for the inclusion of evidence additional to the conventional data. 88,118 This additional evidence is called a prior distribution and summarises information from external sources. External evidence could perhaps be subjective opinions. 89,90 Opinion-based prior distributions can either reflect sceptical opinions, or optimistic opinions, or reflect beliefs from clinicians. 91,92
The incorporation of external information often results in an increase in the effective sample size, which leads to an increase in the precision with which the treatment effect parameter is estimated. For studies that are very large, this increase in effective sample size is often relatively minimal and the trial data dominate. However, in RCTs with small sample sizes, such as the PLUTO trial, increases in effective sample sizes brought about by the inclusion of expert opinions have the possibility of altering clinical conclusions. Although this of course must be balanced against the use of opinions and not evidence in the conventional sense from randomised trial data.
Our objective here was to provide a Bayesian analysis of the PLUTO trial incorporating opinions from experts in the field. We also contrast this with a Bayesian analysis in which uninformative (flat) priors and default enthusiastic and sceptical priors are adopted. We restrict analysis here to the primary outcome of the PLUTO trial, that of perinatal survival, and also a survival analysis from conception to a minimum of 1 year of follow-up. This analysis is particularly beneficial as the PLUTO trial failed to recruit to the required sample size, but was nevertheless preplanned.
The prior elicitation of clinicians’ beliefs has been previously published. 119
Methods
We consider two outcomes here. The first is survival to 28 days (perinatal mortality), which is a binary outcome and the primary outcome of the PLUTO trial. We also consider the survival analysis with follow-up from conception to a minimum of 1 year of age. We consider a number of Bayesian priors: informative priors elicited from experts in the field, enthusiastic and sceptical priors and an uninformative prior.
Analysis methods differ for these two outcomes both because one is a binary outcome and the other is a survival outcome, and because Bayesian informative priors were elicited only for the outcome of survival to 28 days. These two outcomes are therefore analysed separately using appropriate methods. For the outcome of survival to 28 days we present posterior distributions for all informative (elicited) priors, the enthusiastic and sceptical priors and the uninformative prior. For the survival analysis we present posterior distributions only for the enthusiastic, sceptical and uninformative priors. For reasons that will become evident the sceptical and enthusiastic priors differ slightly between the two outcomes.
Prior elicitation
We selected as experts consultant members of the British Maternal and Fetal Medicine Society, the British Association for Paediatric Nephrology and the British Association of Paediatric Urologists. These expert groups are henceforth referred to as fetal medicine experts, paediatric nephrologists and paediatric urologists respectively.
We developed a questionnaire that was used to elicit opinions. An electronic copy of the elicitation form and a worked example illustrating the method (see Appendix 8 ) was e-mailed to all experts, with an additional e-mail reminder being sent to those who did not respond. The e-mails were sent over the period February 2007–November 2008. 119
The questionnaire sought to elicit opinions on both perinatal survival under conventional treatment and survival using intrauterine VAS. To this end each expert was asked to specify their beliefs for three components:
-
the most likely percentage mortality under conservative treatment (i.e. control) at 6 weeks
-
the most likely change in mortality under intrauterine VAS
-
the likelihood (or distribution) of change in mortality for each 5-point interval between a 40% relative increase in mortality and a 40% relative decrease in mortality (total of 17 point values).
The primary outcome for the PLUTO study was survival to 28 days. We assume throughout that elicited beliefs for outcomes at 6 weeks are equivalent to those at 28 days.
To elicit these distributions for change (no. 3 above) each expert was asked to use the graph illustrated in Figure 14 to record their beliefs. Experts were told that each vertical line represents a possible true benefit/harm from intrauterine VAS compared with the control treatment. Experts were asked to mark a horizontal line through each vertical line to show how likely it is that this level of benefit/harm is the true level. Experts were provided with illustrative examples.
FIGURE 14.
Graphical figure used to elicit distributions of beliefs from experts used in part 3.
Summarising beliefs elicited for outcomes at 28 days
The elicited beliefs for perinatal mortality under conservative management (no. 1 above) are summarised using medians and IQRs and are graphically displayed in a histogram for all experts combined across specialties and for experts stratified by specialty. Elicited beliefs for change in mortality under VAS (no. 2 above) are similarly summarised.
In part 3 of the elicitation process, for the elicited beliefs for change in perinatal mortality under intrauterine VAS, we first measured the height of each mark made on the graph in mm (see Figure 14 ). The distributions of change for each expert were then computed by dividing the height of each mark (at 5-point intervals between −40 to 40) by the sum of the heights for that individual, henceforth referred to as standardised height. These then formed the expert’s beliefs for the distribution of change in perinatal mortality under intrauterine VAS. The distributions of change are graphically displayed for each expert independently, grouped by specialty.
The individual expert’s distributions for change in perinatal mortality were then pooled across experts, by weighting by standardised height of marks. These pooled distributions are displayed graphically and summarised using medians and IQRs, again by specialty and over all specialties.
Obtaining informative prior distributions from elicited beliefs
The elicited beliefs for perinatal mortality under conservative management and expected change in perinatal mortality using intrauterine VAS were then transformed into ORs for survival (intrauterine VAS compared with conservative treatment) along with corresponding distributions for each OR. It was necessary to parameterise prior distributions on the OR scale as statistical models fitted to the trial data, to obtain posterior distributions, were paramaterised using logistic regression. Outputs from the logistic regression were subsequently transformed into RRs to be consistent with the frequentist analysis (see Obtaining posterior distributions for the outcome of perinatal mortality). This process was implemented according to the following steps:
-
Transforming changes in mortality into ORs – The first stage was to convert each expert’s distribution of changes in mortality into a distribution of ORs for the effect of VAS on mortality. From the value elicited to be the expert’s belief for perinatal mortality under conservative treatment, and for each 5-point value (between a 40% increase and a 40% decrease) in the change in mortality, we calculated a corresponding OR (intrauterine VAS vs. conservative management). For example, an expert might believe that the perinatal mortality rate under conservative treatment is 50%. They might then give various weights to between a 40% increase and a 40% decrease in mortality using intrauterine VAS. A scenario including a 40% increase in mortality using intrauterine VAS and 50% mortality under conservative treatment would equate to a mortality rate of 70% [50% × (1 + 0.4)] in the intervention arm, equating to an OR for survival of 0.43 [(0.3/(1 − 0.3))/(0.5/(1 − 0.5))]. Similarly, a scenario including a 40% decrease in mortality using intrauterine VAS and 50% mortality under conservative treatment would equate to a mortality rate of 30% [50% × (1 − 0.4)] under VAS, equating to an OR for survival of 2.33 [(0.7/(1 − 0.7))/(0.5/(1 − 0.5))]. The standardised height for each OR value directly corresponded to the standardised height assigned to the change in mortality value.
-
Pooling over experts – The individual expert’s distributions for ORs were combined to produce a pooled distribution for the OR. For each value of OR, the overall standardised height was computed as the mean of the standardised heights for that OR value from the individual OR distributions of the experts. These pooled distributions for ORs then formed the basis for the prior distributions of likely effectiveness of intrauterine VAS, but, to aid assumptions of normality, these ORs were then transformed to log-ORs (we use natural logs throughout).
-
Summarising resulting prior distributions – These prior distributions for ORs for survival are graphically displayed using histograms for all experts combined and are summarised using medians and IQRs (weighted by standardised height). Prior distributions for log-ORs are similarly summarised but we also present the means and SDs (again weighted by standardised height) and superimpose normal densities on histograms.
-
Evaluating assumptions of normality – We then formally evaluated assumptions of normality for the log-OR through quantile normal plots, by specialty and for all specialties combined. Assuming normality we then formed four informative priors for the log-OR of survival: a fetal medicine prior, a paediatric nephrology prior, a paediatric urology prior and an all experts prior.
Experts who did not specify a value for perinatal mortality under conservative management could not contribute to this estimation of ORs or log-ORs. Furthermore, some experts specified relative changes in perinatal mortality under intrauterine VAS, which, when paired with their specified value for mortality under conservative management, resulted in mortality using intrauterine VAS of either < 0% or > 100%. Any such out-of-range values were excluded.
In summary, we therefore elicited for each expert their belief about the percentage perinatal mortality under conservative management and their belief for the distribution of change in mortality under intrauterine VAS across the range from a 40% increase to a 40% decrease in relative mortality (in 5-point changes). From these elicited values we then formed prior distributions for beliefs for the log-OR for survival.
Default prior distributions
We also constructed three additional default priors: an uninformative prior, a sceptical prior and an enthusiastic prior. To construct default priors we followed methods as outlined by others. 117
For the binary outcome of perinatal survival all priors were for log-ORs and were assumed to be normally distributed. The enthusiastic prior was centred at the alternative hypothesis that PLUTO was designed to test. PLUTO was powered to detect a change in survival at 28 days from 39% under conservative management to 62% using intrauterine VAS (i.e. an absolute increase of 23%), equating to an OR (for survival) of 2.55 (log-OR = 0.94). The SD for this prior was found by finding the value for the SD for which the enthusiastic prior has a small probability (5%) that the treatment effect is negative (i.e. favours conservative management). Therefore, the enthusiastic prior was centred at log-OR = 0.94 and a SD of 0.57 (0.94/1.645).
The sceptical prior was constructed similarly but was centred at the value of no difference (and so harmful effects possible) and with a 5% probability that the treatment effect was greater than a log-OR of 0.94. This prior was therefore N(0, SD 0.57) for the log-OR.
The uninformative prior, sometimes called a uniform or vague prior, was centred at the value of no effect (log-OR = 0) with very large variance (100). 120
For the outcome survival to 1 year, all priors for hazard ratios (HRs) were for the log-scale and were assumed to be normally distributed.
The enthusiastic prior was centred at the alternative hypothesis that PLUTO was designed to test. The results from a meta-analysis13 informed the calculation of the sample size for the PLUTO trial. The original meta-analysis computed the pooled OR; however, the prior for a HR is best approximated by the distribution for a RR. The pooled RR was calculated using the summary data in the paper and by rerunning the meta-analysis, which gives an estimate for the RR of 1.55 (log-RR = 0.44). The SD for this prior was found by finding the value for the SD for which the enthusiastic prior has a small probability (5%) that the treatment effect is negative (i.e. favours conservative management). Therefore, the enthusiastic prior was centred at log-RR = 0.437 and a SD of 0.27 (0.44/1.645). An estimate of the distribution for the RR can be used as a prior for the HR by assuming that the RR is constant and instantaneous for the whole time period.
The sceptical prior was constructed similarly but was centred at the value of no difference (and so harmful effects possible) and with a 5% probability that the treatment effect was greater than a log-RR of 0.44. This prior was therefore N(0, SD 0.27) for the log-RR.
The uninformative prior was centred at the value of no effect (log-RR = 0) with very large variance (100). 3,121
Combining prior distributions with PLUTO trial data to obtain posterior distributions
The primary outcome for the PLUTO trial was survival to 28 days and the prior distributions were elicited for this outcome. Because beliefs for the outcome of survival to 28 days are likely to be quite different from beliefs for the other outcomes, we chose not to combine these prior distributions with data for outcomes other than survival to 28 days.
The sensitivity of TOPs to the treatment was considered; however, the results showed little sensitivity. Therefore, the results will be presented assuming that the TOPs that were not a result of treatment failure were, in the survival analysis (survival at 1 year), censored at the time of termination; within the binary outcome (survival to 28 days) analysis TOPs were assumed to be deaths (a sensitivity analysis was considered under the ‘frequentist’ analysis and the results were not found to be sensitive).
We report unadjusted treatment effects only. We chose not to report adjusted effects because balance was achieved on the three important prognostic variables (as a minimisation algorithm was used on gestational age at diagnosis in days, liquor volume in cm and age of mother in years). Other covariates were not fully recorded for every observation and are not considered further.
We report results from both the ITT analysis and the AT analysis.
Obtaining posterior distributions for the outcome of perinatal mortality
For the binary outcome of survival to 28 days we used logistic regression models to model the outcome (survival at 28 days) as a function of the treatment (conservative management or VAS) and so, consequently, prior distributions for the treatment effect are on the log-OR scale. However, the treatment effect measure prespecified in the trial protocol was the RR and so this is also the treatment effect measure reported here. We computed the RR using a transformation from the OR, obtained from the logistic regression model, to a RR using the formula risk = odds/(1 + odds).
We used logistic regression to model the outcome (survival at 28 days):
where the probability, p, of the outcome of survival at 28 days is:
From these estimated log-odds (α0 for the control group and α0 + α1 for the treatment group) we computed risks [risk = odds/(1+ odds)] and RRs.
Bayesian analyses are valued for their ability to provide posterior probability estimates of effect sizes being greater (or less) than some clinically meaningful value, sometimes referred to as Bayesian p-values. The PLUTO trial was designed to detect an OR for survival of 2.5, informed by a meta-analysis of non-randomised studies. Repeating this meta-analysis, on the RR scale this OR of 2.5 is equivalent to a RR of 1.55. We therefore additionally calculated the posterior probability that the RR is > 1.55.
Finally, to aid clinical interpretability we also computed the number needed to treat (NNT). Credible intervals (CrIs) and CIs for NNTs that cross the null value include estimates of both harms and benefits of an intervention. We present them as being bounded by a negative NNT (indicating the NNT for one additional person to be harmed) and a positive NNT (indicating the NNT for one additional person to benefit). Note that unlike other CrIs and CIs, more extreme values (i.e. higher than the positive upper limit and lower than the negative lower limit) are included in the interval.
Obtaining posterior distributions for the outcome of survival to 1 year
For the outcome of survival to 1 year we use Cox proportional hazards regression models to model the hazard of death as a function of the treatment (conservative management or VAS).
Mothers entered the study on the date of randomisation and were followed up until death of the fetus or TOP or the end of the study. Patients were censored because of loss to follow-up or if the fetus had not died before the end of the study, which was 1 January 2012. The survival time is measured as the length of time in the study in weeks from the date of conception. The presumed conception date is estimated from the estimated date of delivery minus 267 days (date of the last menstrual period) plus 14 days.
We used a Cox proportional hazards regression model to model the difference in survival over time between the group who received VAS and the group who received conservative management. The Cox proportional hazards regression model specifies the hazard for individual i as:
where λ 0(t) is the baseline hazard function that is left unspecified and β 1 X i1 + . . . + β k X ik is a linear function of a set of k fixed covariates. 122
The variable X 1 denotes the intervention group thus X 1 = 1 for those subjects who were randomised to receive the control and X 1 = 0 for those who were randomised to receive the treatment. Therefore, the hazard rates for those in the control and treatment groups are:
Of note, the hazard rate comparison is conservative care compared with treatment. This is so that the effect estimate on the HR scale will be consistent with RR estimates in so far as treatment effects > 1 will favour the intervention.
As for the binary outcome, we computed the posterior probability estimate of the effect size being greater (or less) than some clinically meaningful value. We used the value of 1.55 as a clinically meaningful difference.
Kaplan–Meier plots were produced for categorical variables to show unadjusted differences in the probability of survival over time between the infants randomised to receive VAS and the infants randomised to receive conservative management.
The Cox regression model assumes that the HR for a variable is constant over time. If this assumption does not hold this implies that the HR changes over time and so time-dependent effects need to be incorporated in the model. This assumption was investigated with the visual inspection of log-cumulative hazard plots in which log(−log) survival curves for two or more subgroups are plotted against time (or log-time). If the hazard rates are proportional the resulting lines should be approximately parallel.
The hazard rates for the treatment groups were not found to be proportional as the curves cross in the log-cumulative hazard plots. To deal with this the survival data were split at the time point when the lines cross and a Cox proportional hazards model was fitted with a time-dependent effect, which results in the estimation of two HRs for the treatment effect. The two HRs estimate the difference in 1-year mortality rates between the two groups in the two time periods. The proportional hazards assumption was held within each of the two distinct time periods. For those infants whose survival time was less than the time that the curves cross, their data remains the same. For each infant who has survived beyond the time that the curves cross, two separate records of data for each period were created. The first line of data includes their survival time from conception to the crossing point. Therefore, the survival time for each individual who survived beyond the time that the curves cross was the time that the curves cross, for example if the curves cross at 35 weeks the survival time is calculated as 35 weeks for each infant. The second line of data includes the survival time from the crossing point until the end of each infant’s survival time. The survival time here was calculated from 0 to the end of their survival time. A new binary variable, split_time, was created for the subjects to allow for the interaction between the time period and the intervention variable. For the first time interval split_time = 0 and for the second time interval split_time = 1. This results in the Cox proportional hazards regression model:
Here, exp{β 1} is the HR estimate of the difference in mortality rates for up to 1 year between the intervention groups when split_time = 0, that is, the mortality ratio from conception to the time that the curves cross. Exp{β 2} is the change in the HR estimate when split_time = 1 and therefore exp{β 1 + β 2} is the HR estimate for the difference in mortality rates between the intervention groups when split_time = 1, that is, the mortality ratio from the time that the curves cross to the end of follow-up.
The proportional hazards assumption was difficult to assess in this study as there are very little data. Therefore, results are calculated under two assumptions. Results are given assuming that the hazard rates are proportional from conception to the end of follow-up and therefore the estimate of a HR is an average of the treatment effect over time. The results are calculated under the assumption of a time-dependent treatment effect as described above.
Implementation
All Bayesian analyses were performed using the WinBUGS software version 14 (MRC Biostatistics Unit, Cambridge, UK) with 200,000 iterations after allowing for a 10,000 iteration burn-in and checking for convergence using several common measures. Summary estimates provided are medians and 95% CrIs, which can be interpreted as one would like to interpret a conventional CI, that is, as there being 95% probability that the true value of the parameter lies between the upper and lower bounds. For parameters other than the treatment effect we used standard uninformative priors: normal distribution centred at 0 and with variance 100. 3,121 The WinBUGS code for the analysis of prenatal mortality is provided in Appendix 9 and that for the survival analysis is provided in Appendix 10 .
Results
Elicitation results
The questionnaire survey was emailed to 248 experts and 59 replies were received, seven of which were too incomplete to be included, leaving 52 partially completed forms. These 248 experts included 132 fetal medicine experts (of whom 37 replied), 56 paediatric nephrologists (of whom 16 replied) and 60 paediatric urologists (of whom six replied). The overall response rate was 24% (59/248).
Two experts did not provide a belief for perinatal mortality under conservative management and so could not contribute to the estimate of the OR for survival (intrauterine VAS compared with conservative management). Furthermore, some values were given that implied mortality outside of the range 0–100 and these were excluded from the analysis. These affected 46 values out of the 884 (52 × 17) values provided by all experts.
Elicited beliefs for perinatal mortality under conservative management
In total, 50 of the 52 experts provided their opinion on the percentage perinatal mortality rate under conservative management. These beliefs were elicited from 35 fetal medicine experts, 10 paediatric nephrologists and five paediatric urologists. The percentage mortality rates under conservative management elicited from the 50 experts are presented in histograms in Figure 15 . There is considerable variability in the elicited percentage mortality rates both within and between specialties.
FIGURE 15.
Histograms of experts’ elicited beliefs for percentage perinatal mortality rate at 28 days when using conservative management.
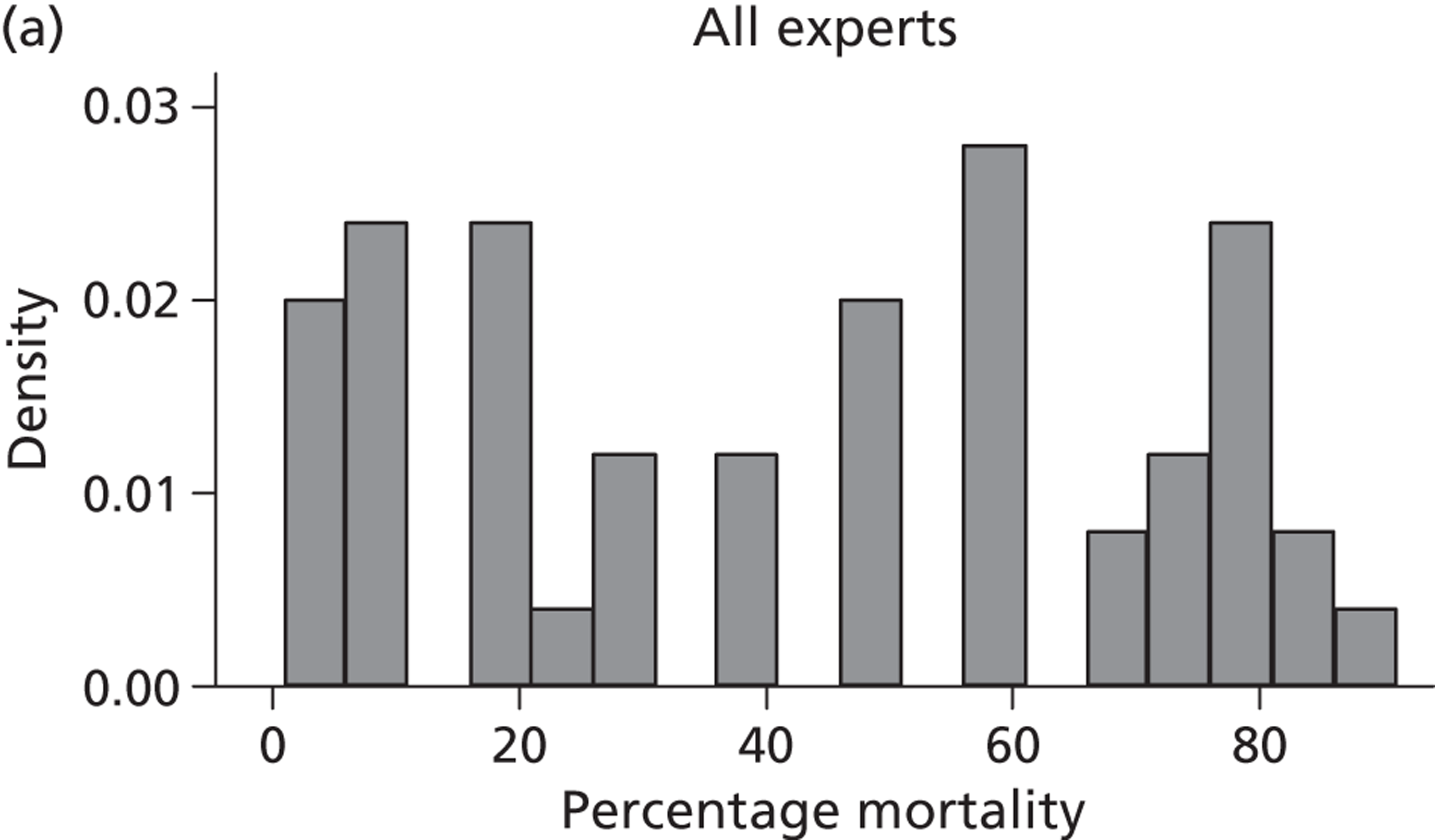
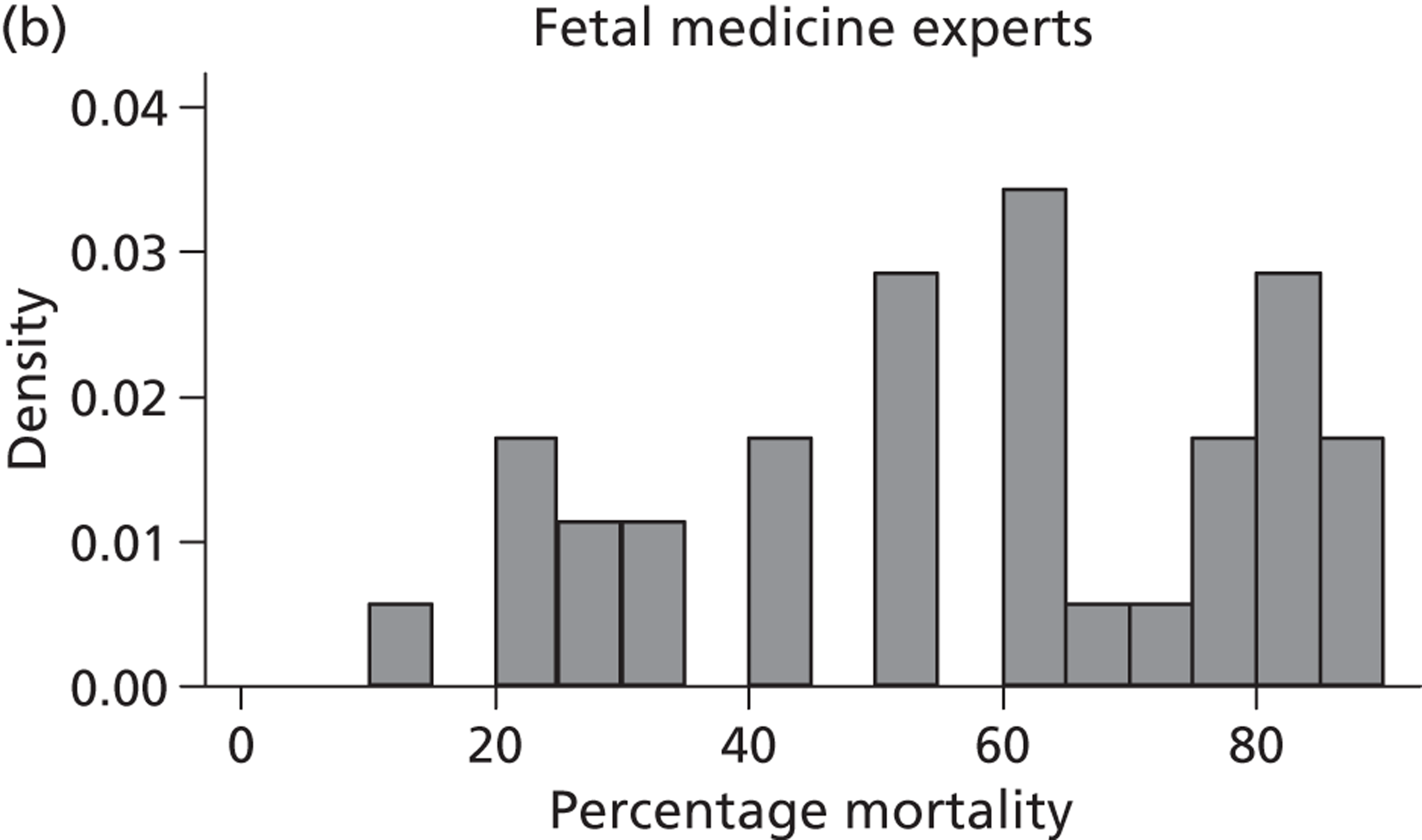
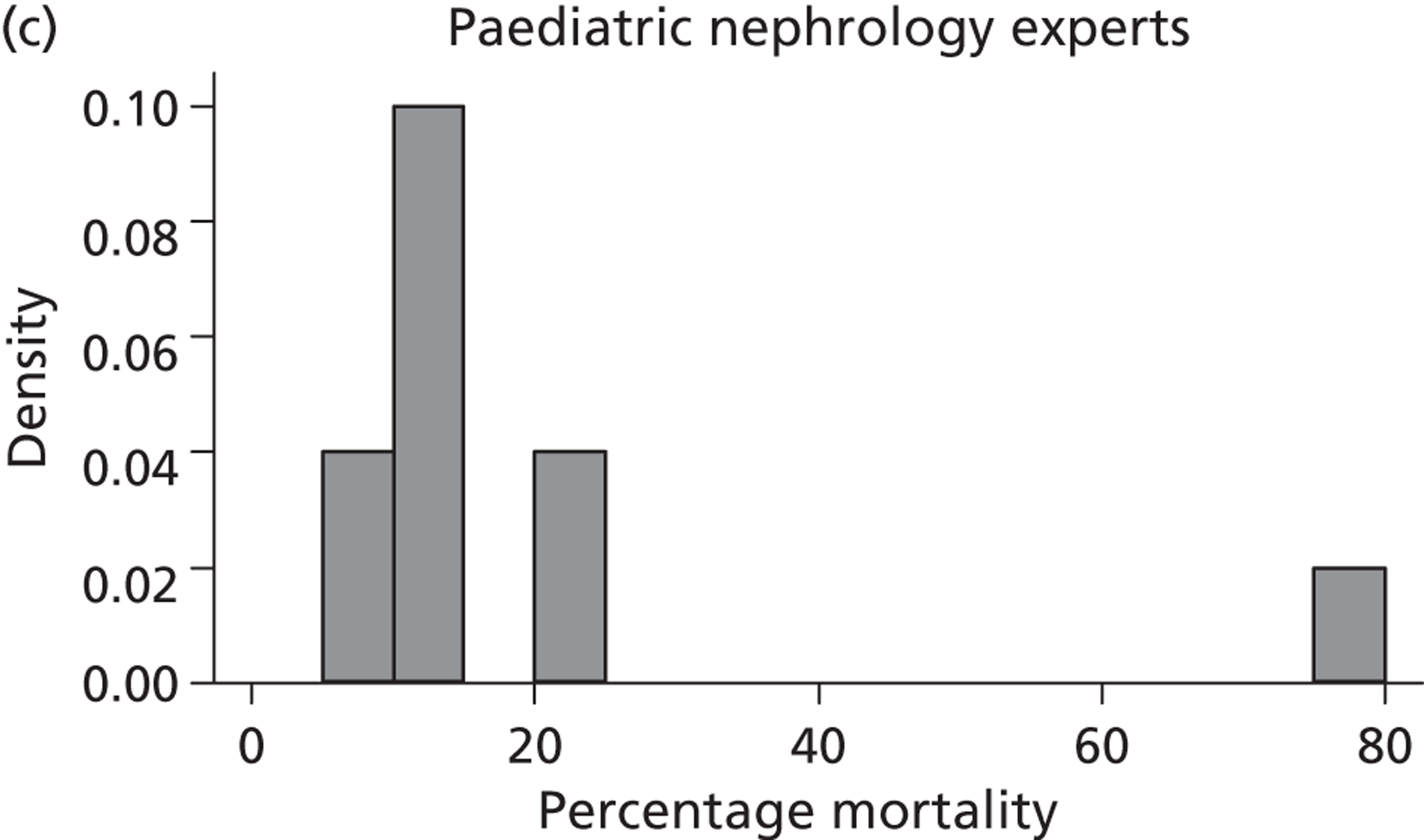
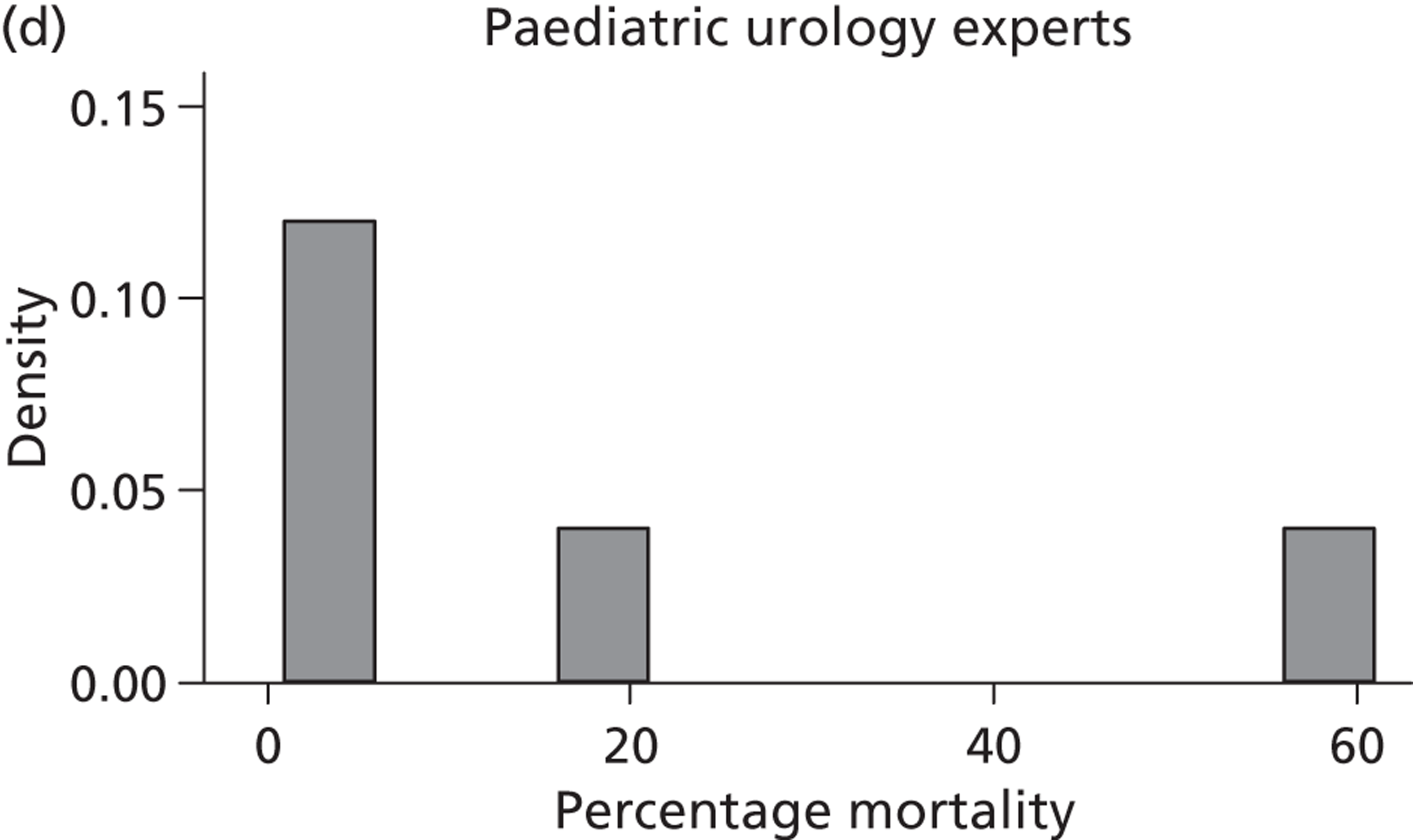
The fetal medicine experts, on average, showed less optimism for a favourable outcome under conservative management ( Table 28 ), with an elicited median perinatal mortality rate of 60% (IQR 40% to 75%). Paediatric nephrologists and urologists generally believed that perinatal mortality under conservative management would be much lower: the median mortality rate under conservative management elicited from the paediatric nephrologists was 10% (IQR 10% to 20%) and that elicited from the paediatric urologists was 5% (IQR 1% to 20%) (see Table 28 ).
| Specialty | ||||
|---|---|---|---|---|
| Fetal medicine | Paediatric nephrology | Paediatric urology | All | |
| No. of experts | 35 | 12 | 5 | 52 |
| Perinatal mortality with conservative management | ||||
| No. of beliefs elicited | 35 | 10 | 5 | 50 |
| Percentage mortality, median (IQR) | 60 (40 to 75) | 10 (10 to 20) | 5 (1 to 20) | 50 (20 to 70) |
| Change in mortality with VAS | ||||
| No. of beliefs elicited | 35 | 12 | 5 | 52 |
| Percentage decrease, median (IQR)a | 10 (0 to 20) | 5 (−5 to 10) | 5 (0 to 15) | 10 (0 to 20) |
| Perinatal mortality with VAS | ||||
| No. of beliefs elicited | 35 | 10 | 5 | 50 |
| Percentage mortality, median (IQR)a | 51 (33 to 68) | 10 (7 to 18) | 5 (1 to 18) | 40 (15 to 63) |
| Summary of comparative measures | ||||
| No. of experts contributing | 35 | 10 | 5 | 50 |
| OR for survival, median (IQR)a | 1.28 (1.00 to 1.63) | 1.07 (1.00 to 1.19) | 1.11 (1.00 to 1.28) | 1.22 (1.00 to 1.50) |
| Log-OR for survival, median (IQR)a | 0.25 (0.00 to 0.49) | 0.06 (0.00 to 0.17) | 0.11 (0.00 to 0.25) | 0.20 (0.00 to 0.41) |
| Log-OR for survival, mean (SD)a | 0.26 (0.50) | 0.07 (0.20) | 0.13 (0.20) | 0.21 (0.44) |
| OR (95% CI) | 1.30 (0.48 to 3.36) | 1.07 (0.72 to 1.59) | 1.14 (0.77 to 1.69) | 1.23 (0.52 to 2.92) |
| Probability OR > 2.55 | 0.09 | < 0.01 | < 0.01 | 0.05 |
Elicited beliefs for change in perinatal mortality using intrauterine vesicoamniotic shunting
A total of 52 experts provided information on their beliefs for a change in perinatal mortality with intrauterine VAS. These distributions are displayed in Figures 16 and 17 . There is considerable variation between these distributions, again both within and between specialties. Some experts provided distributions for change similar to normal distributions. For example, expert 3 believed that the most likely value for change would be a 20% decrease in perinatal mortality and that this was symmetrically distributed between a 0% change and a 40% decrease. On the other hand, other experts believed that the likelihood of change increased with an increasing reduction in mortality. So, for example, expert 29, although believing that the change might be anywhere between a 40% increase and a 40% decrease, gave steadily increasing weights to more favourable changes. Several experts (e.g. expert 9) placed all of their weight at a single point for change, suggesting no uncertainty in their beliefs, perhaps suggesting a lack of understanding of the question.
FIGURE 16.
Elicited beliefs for percentage change in perinatal mortality when using intrauterine VAS compared with conservative management: fetal medicine specialists (experts 1–35).
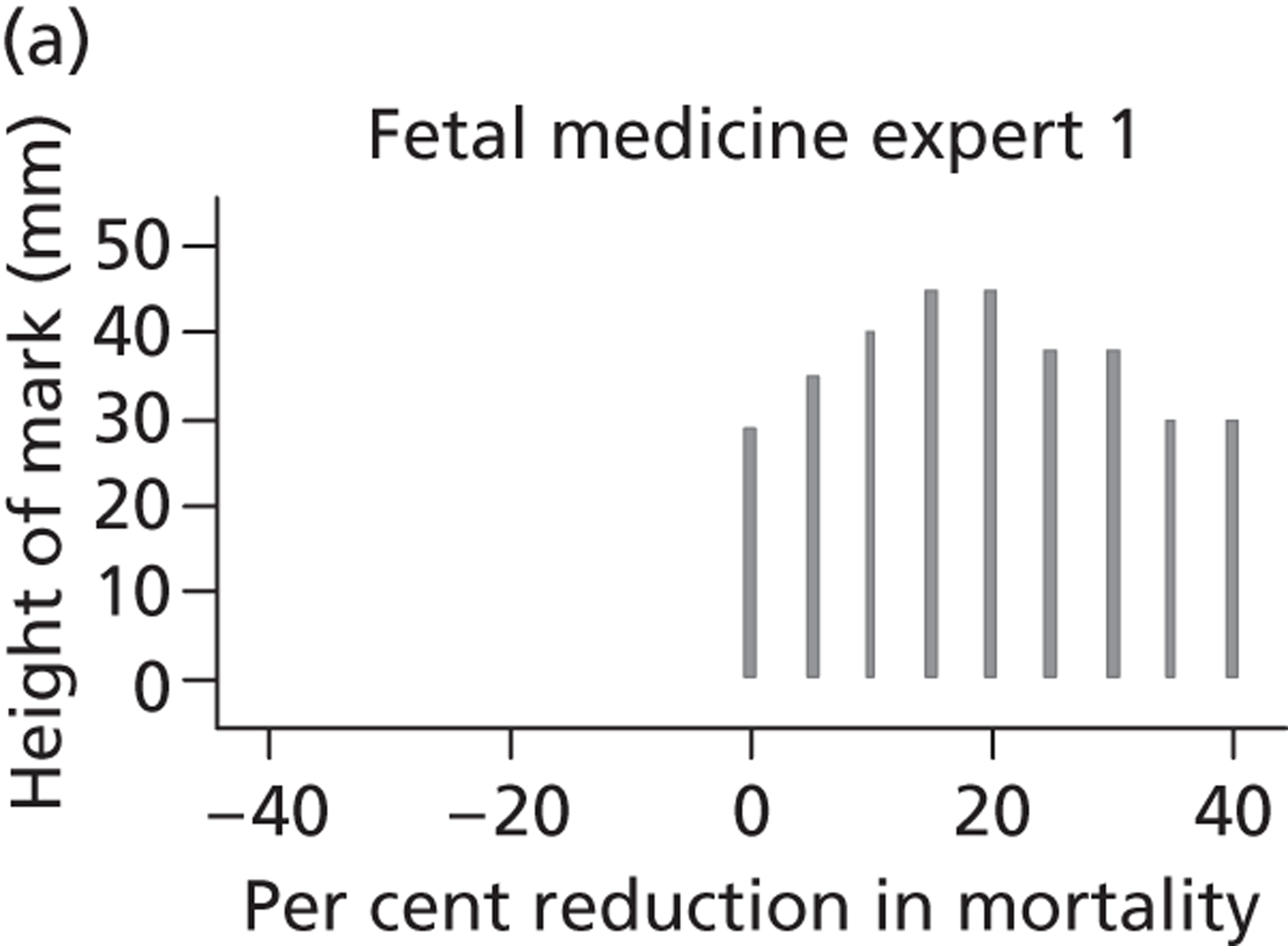
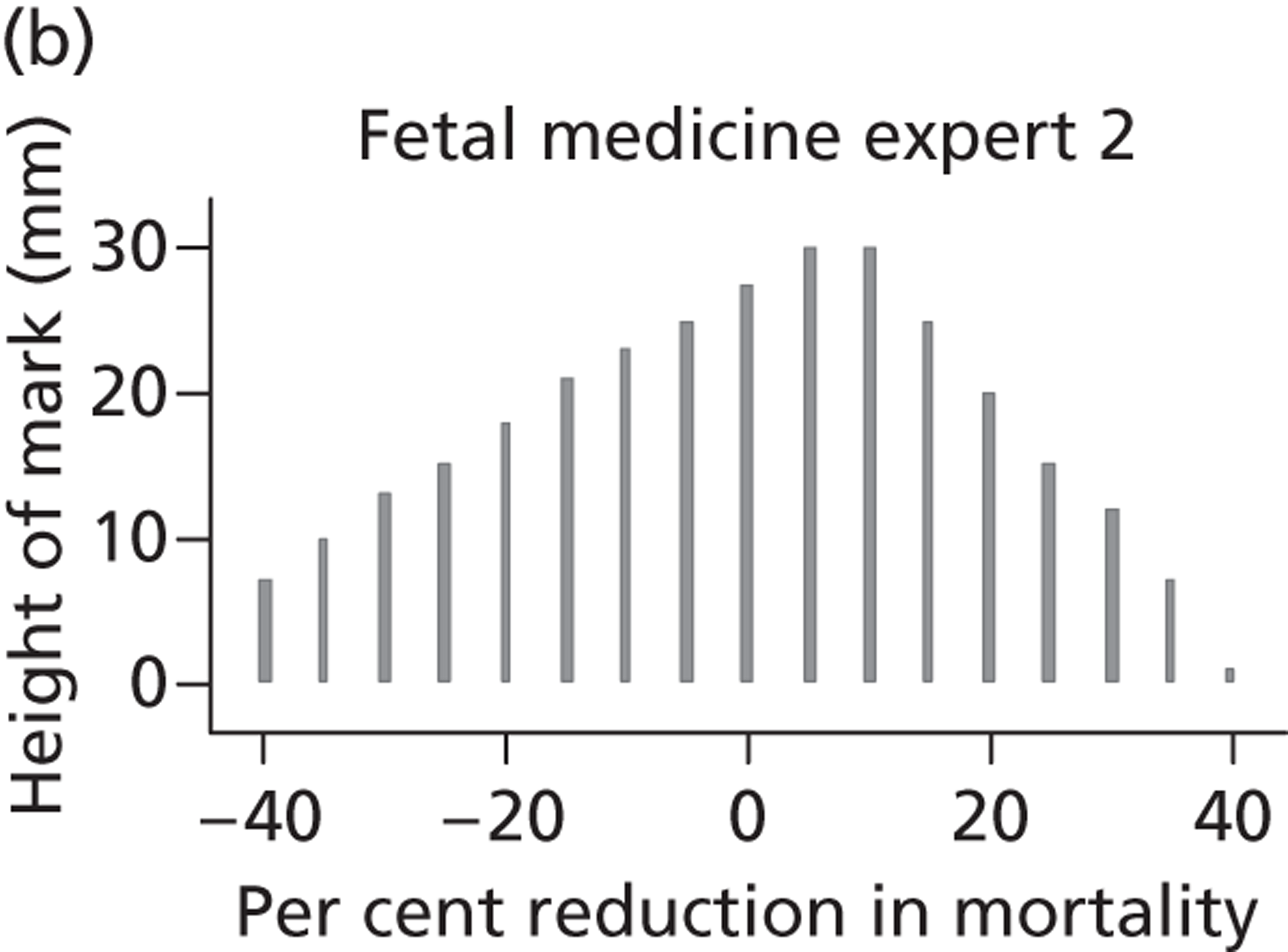
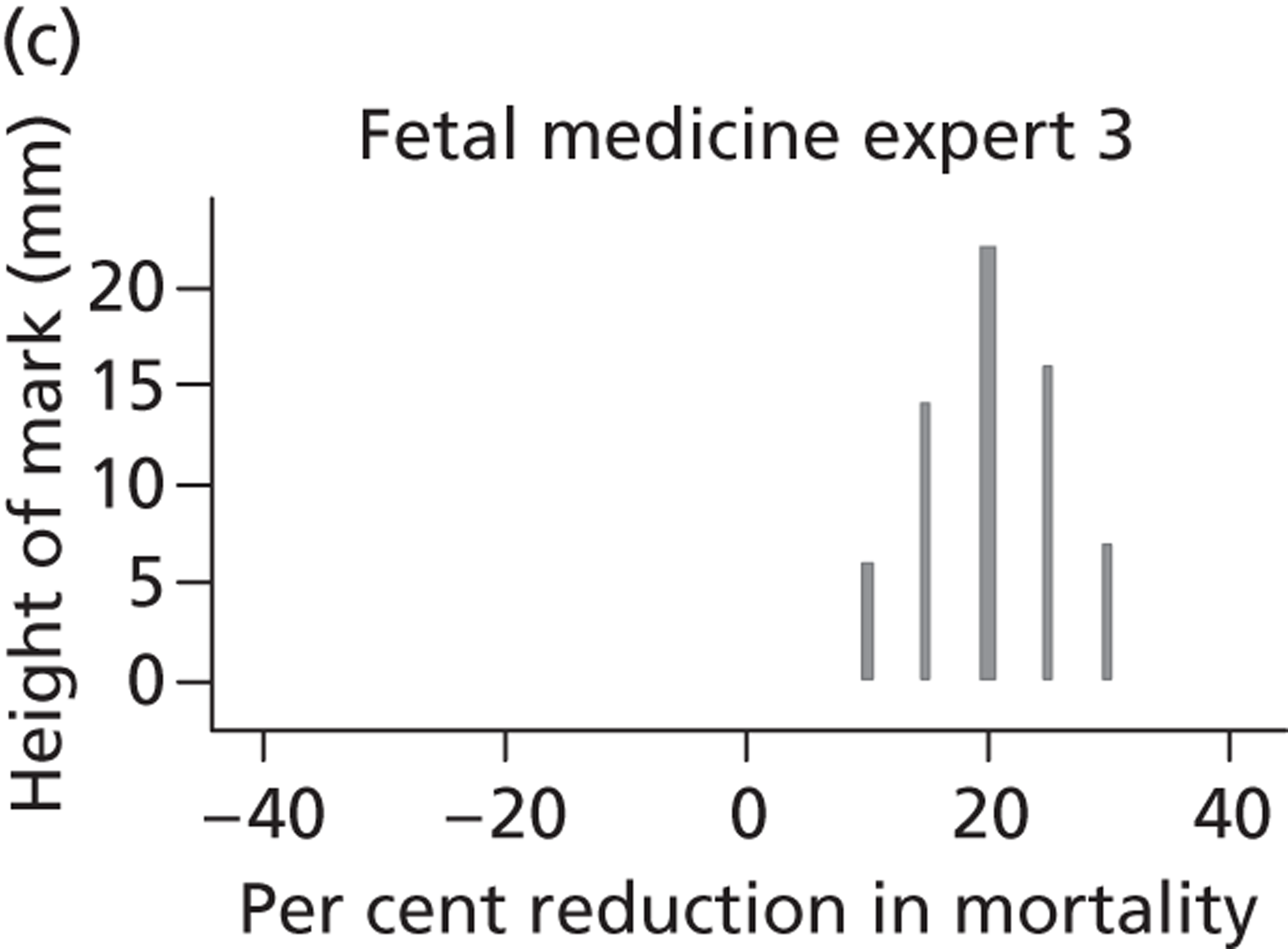
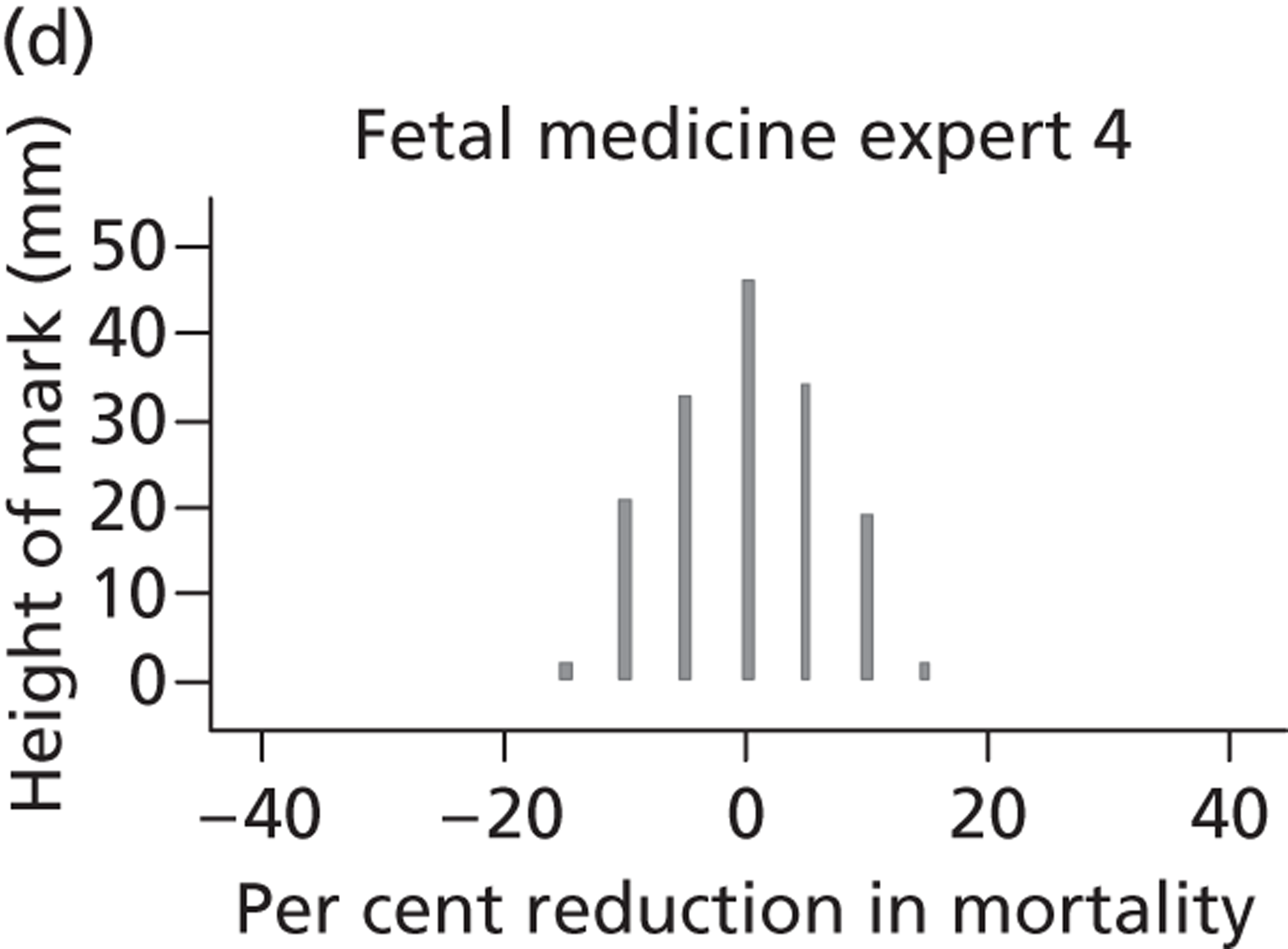
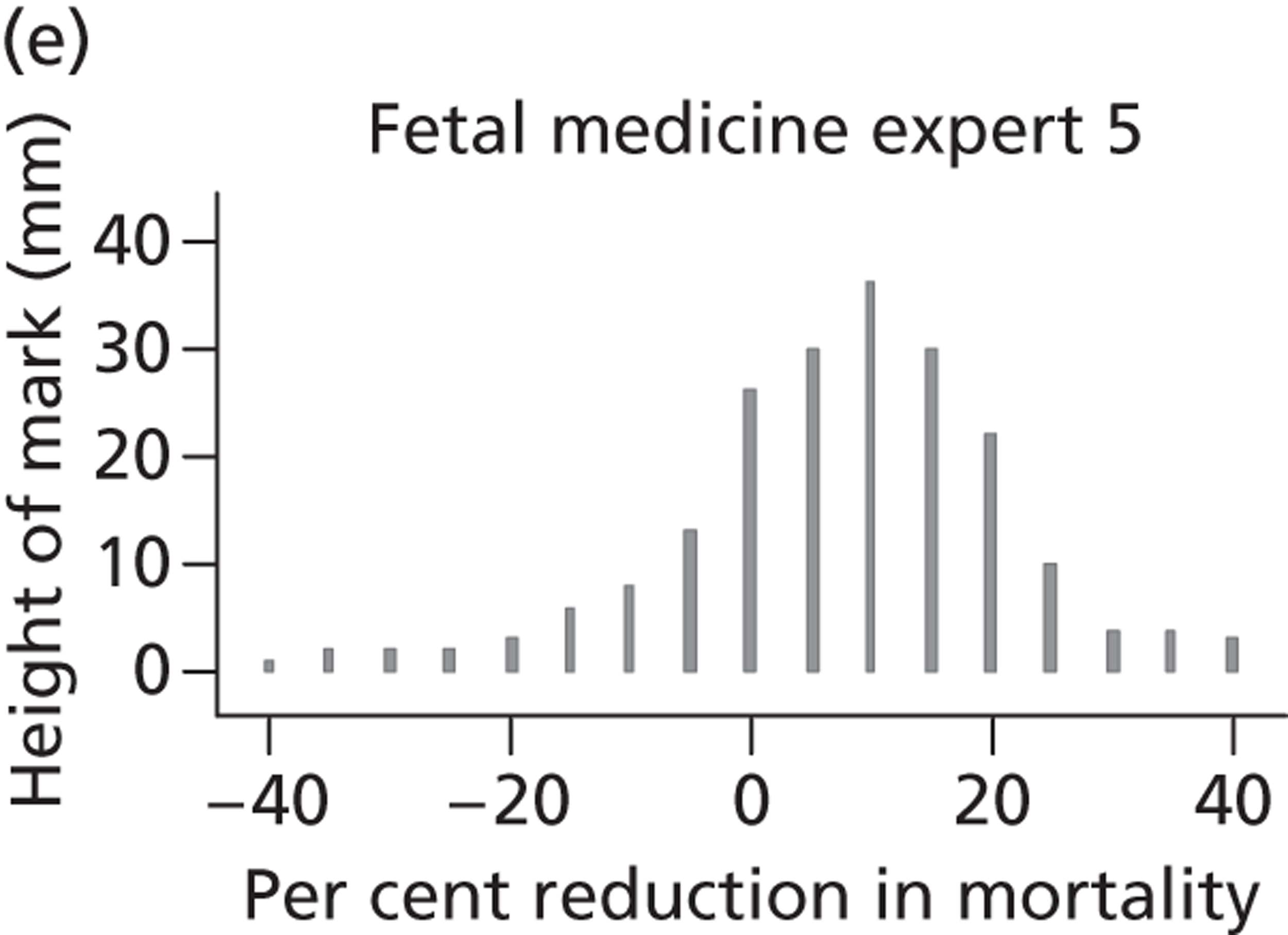
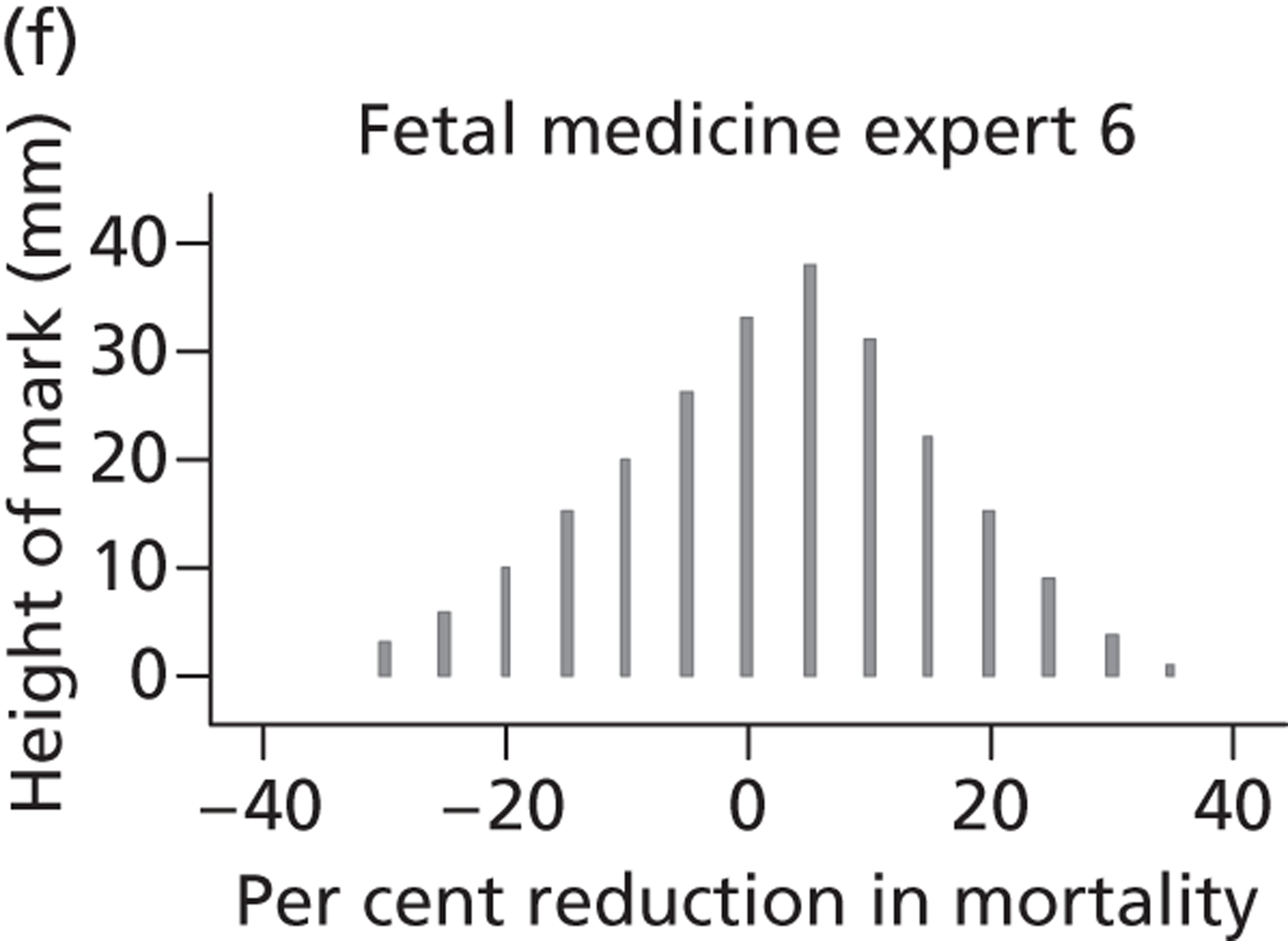
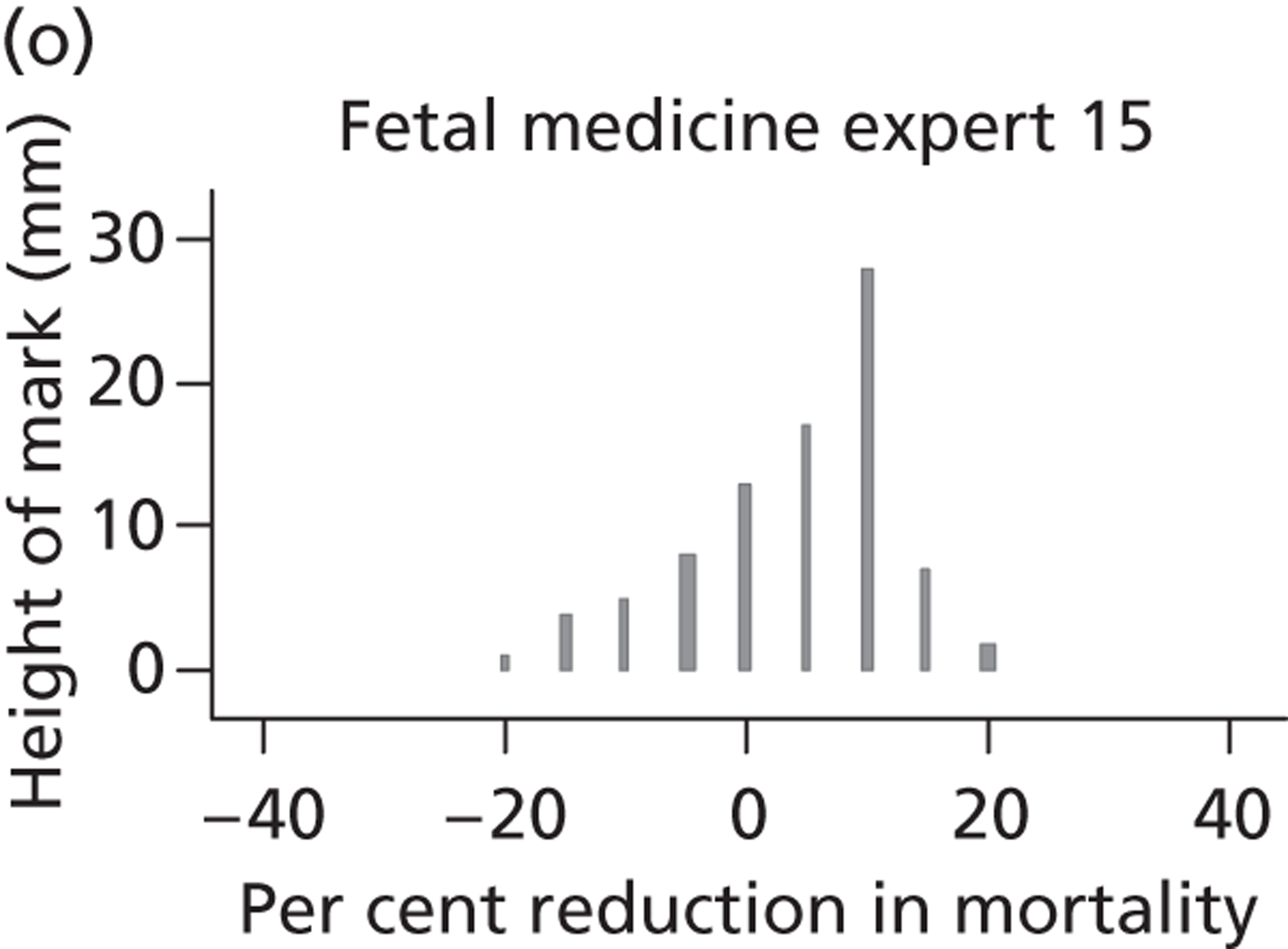
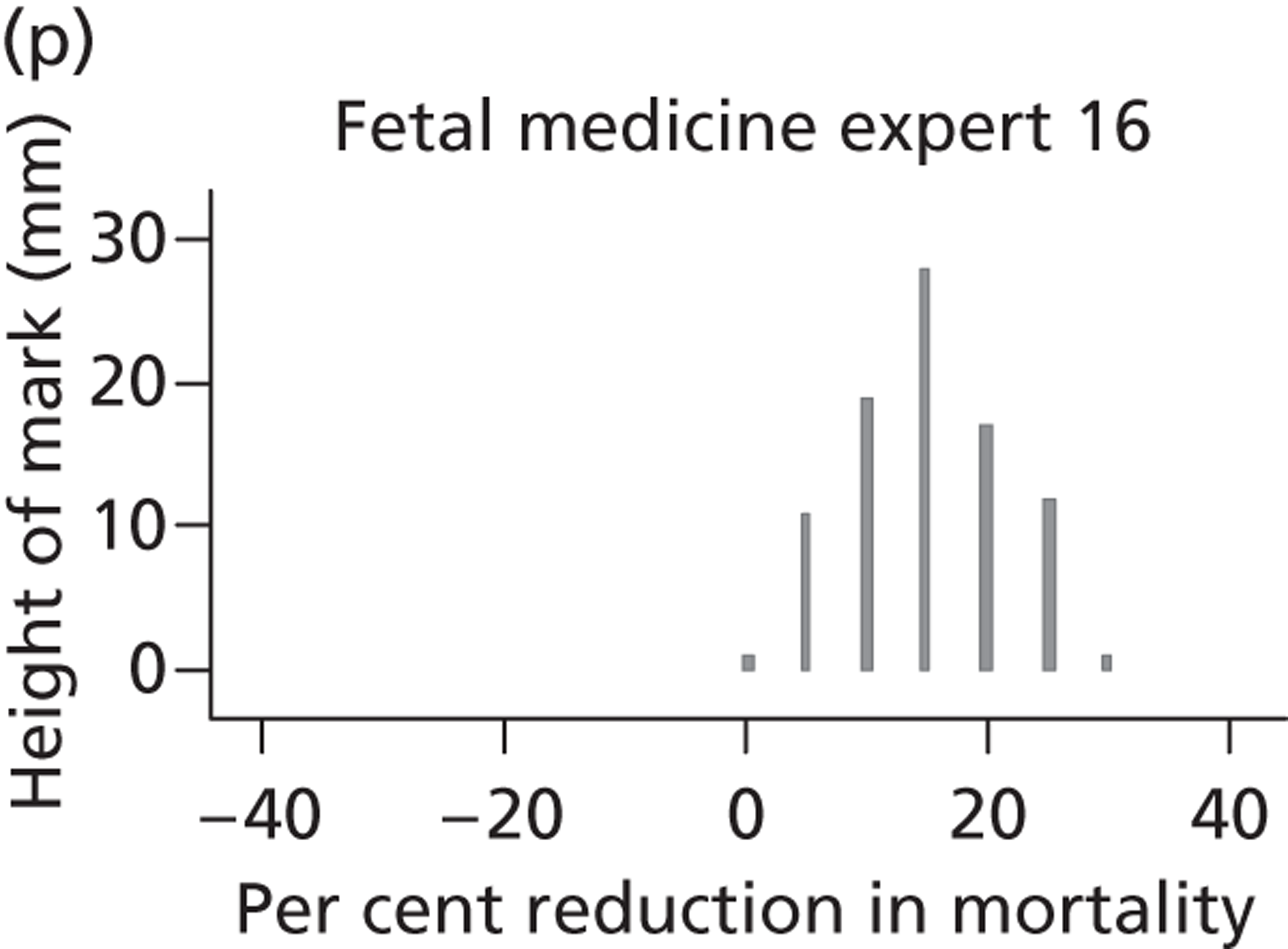
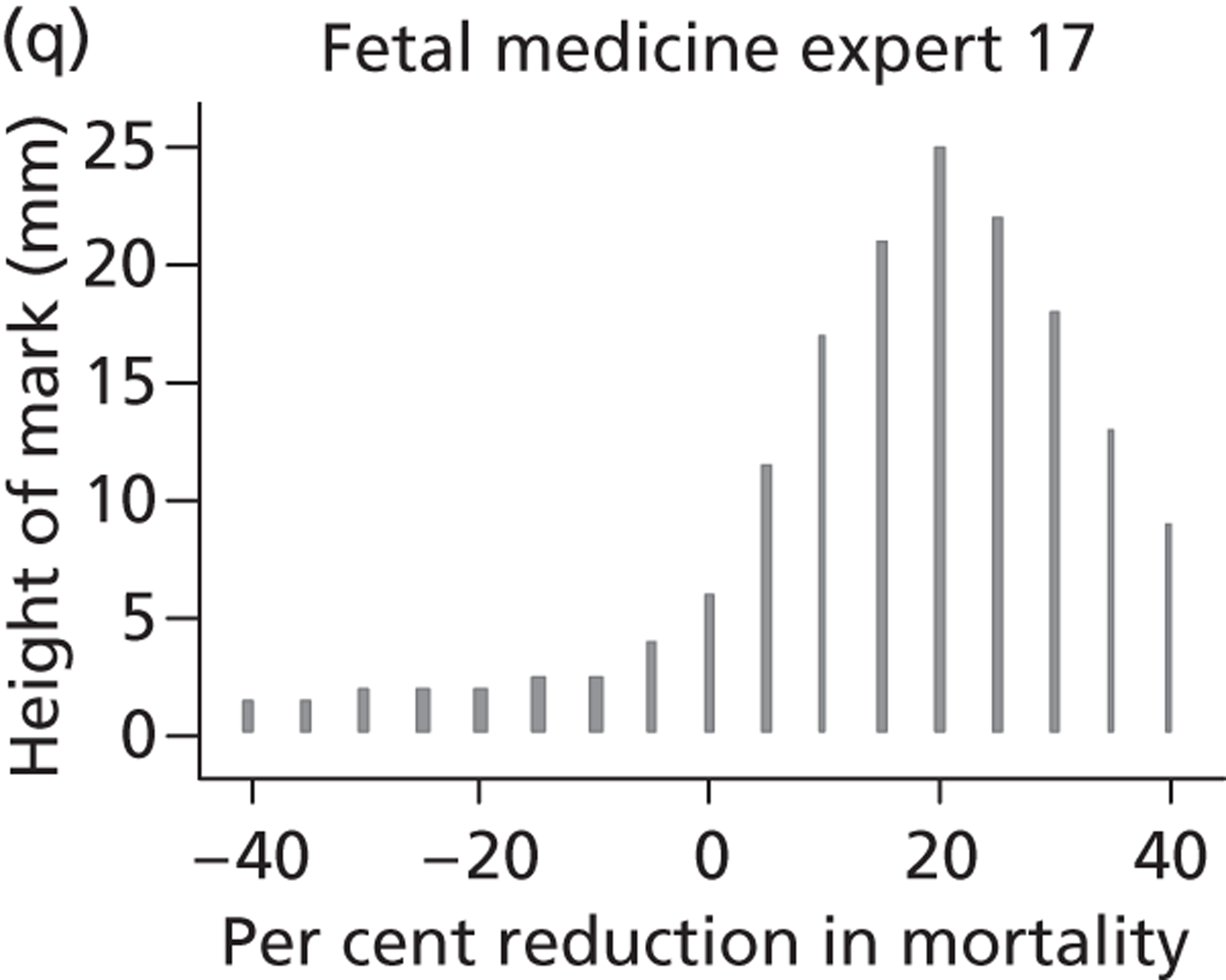
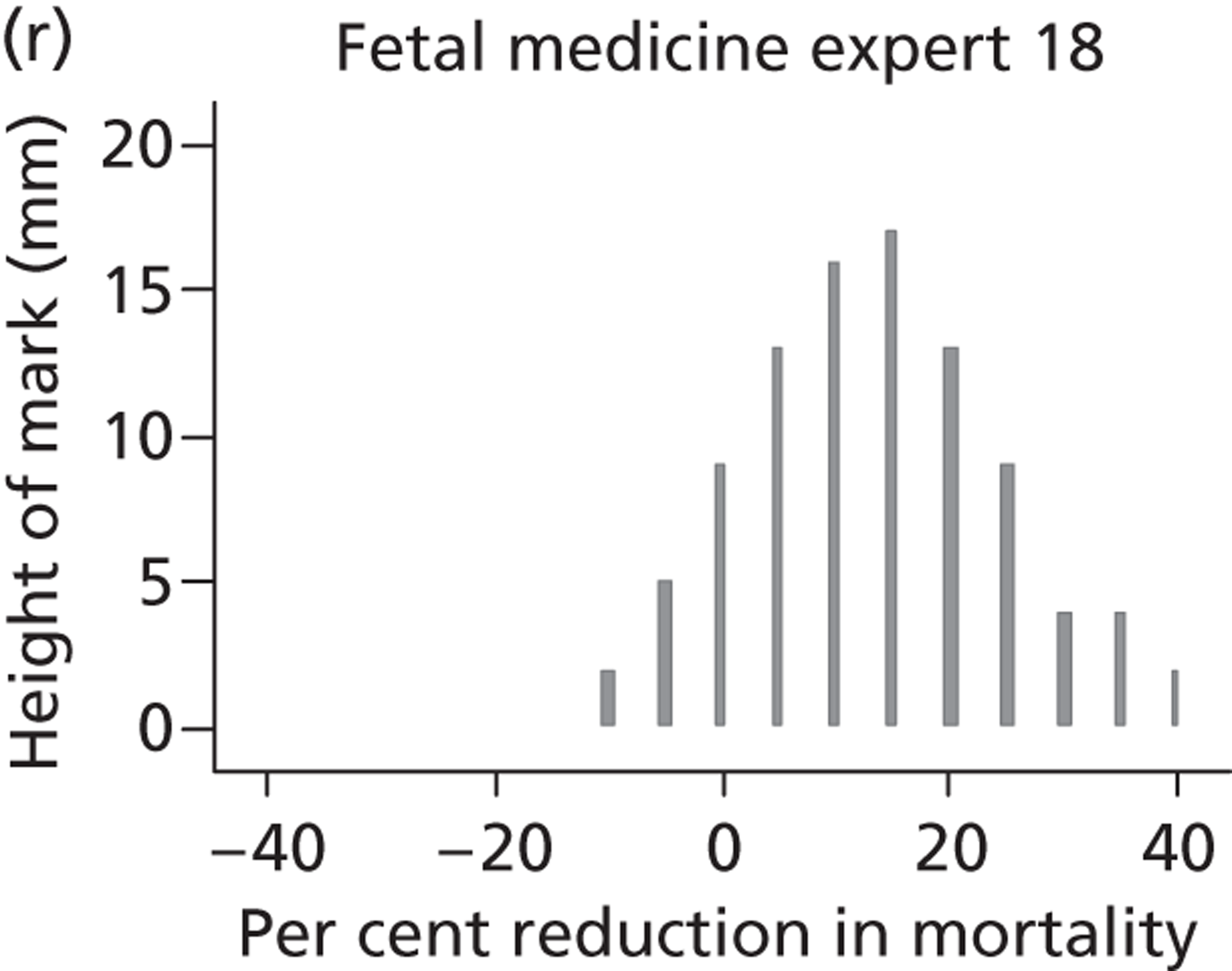
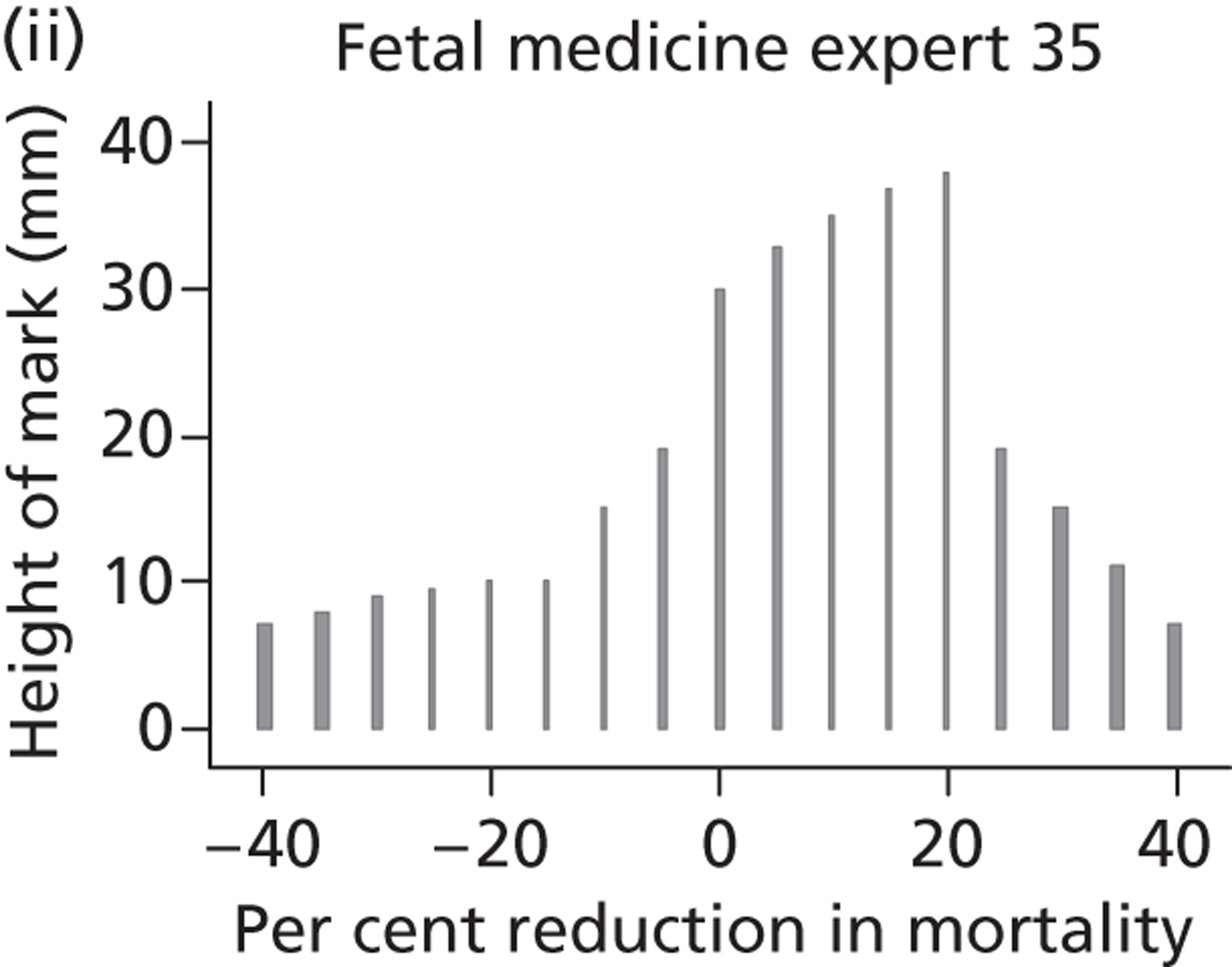
FIGURE 17.
Elicited beliefs for percentage change in perinatal mortality when using intrauterine VAS compared with conservative management: paediatric nephrologists specialists (experts 36–52).
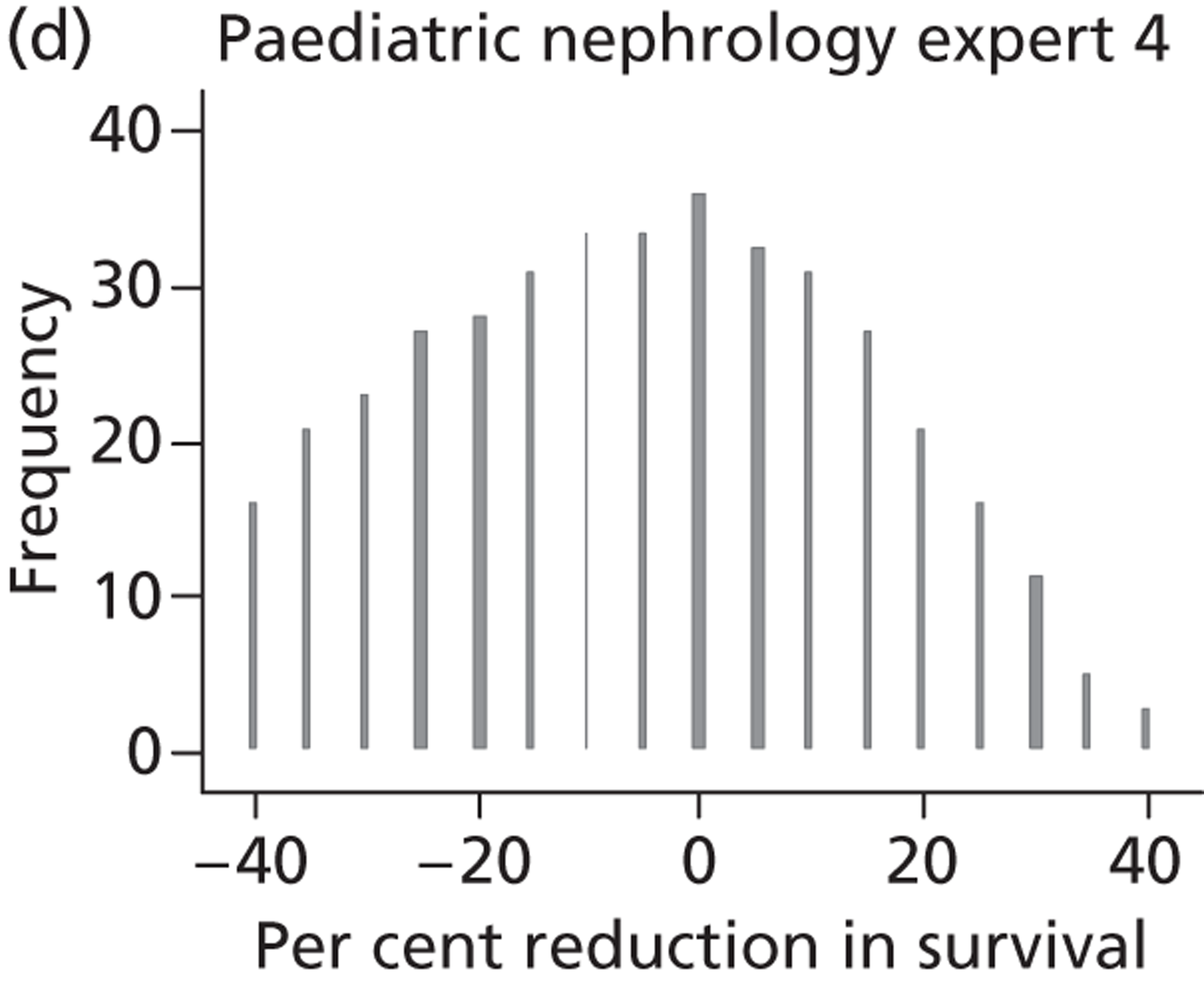
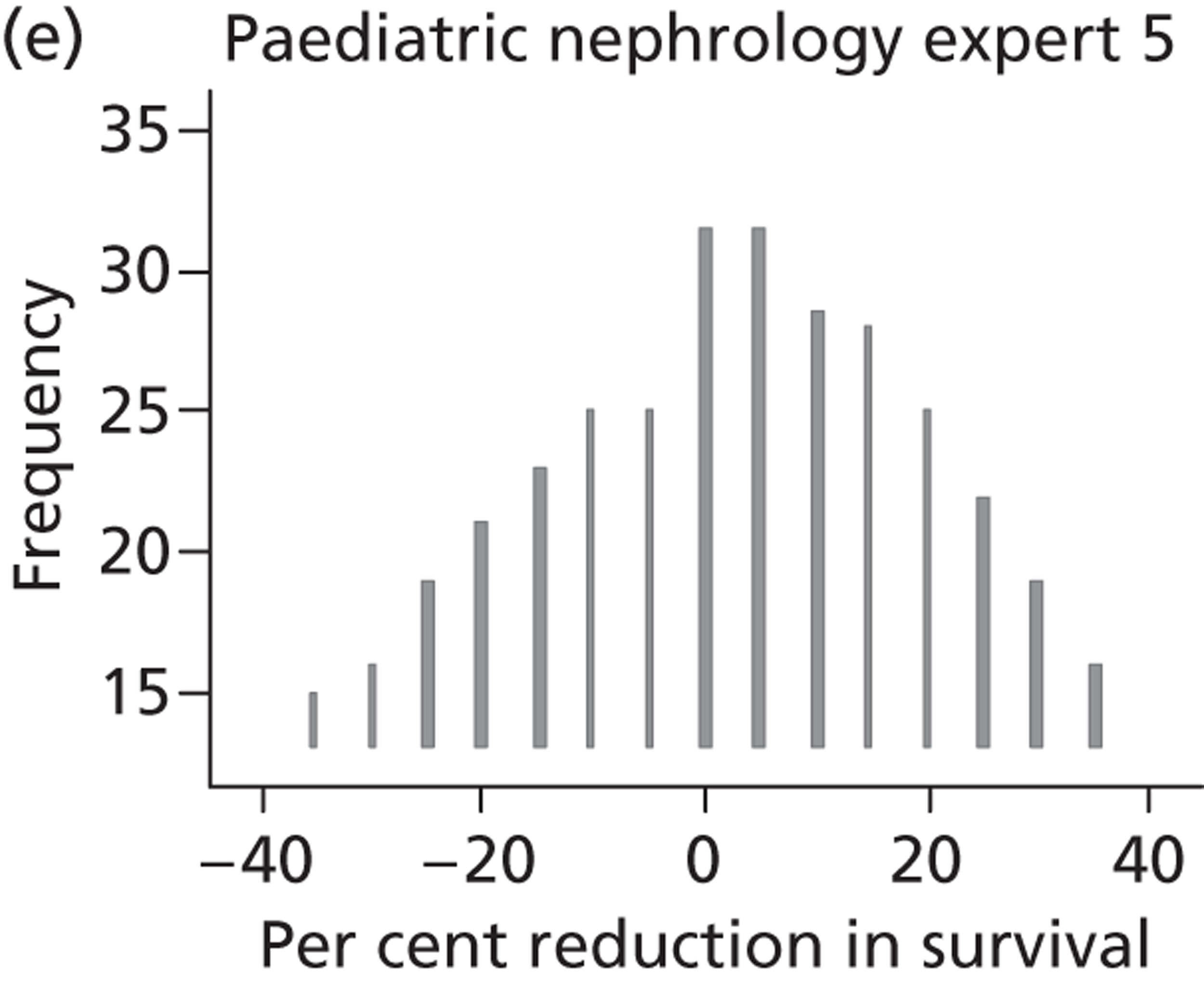
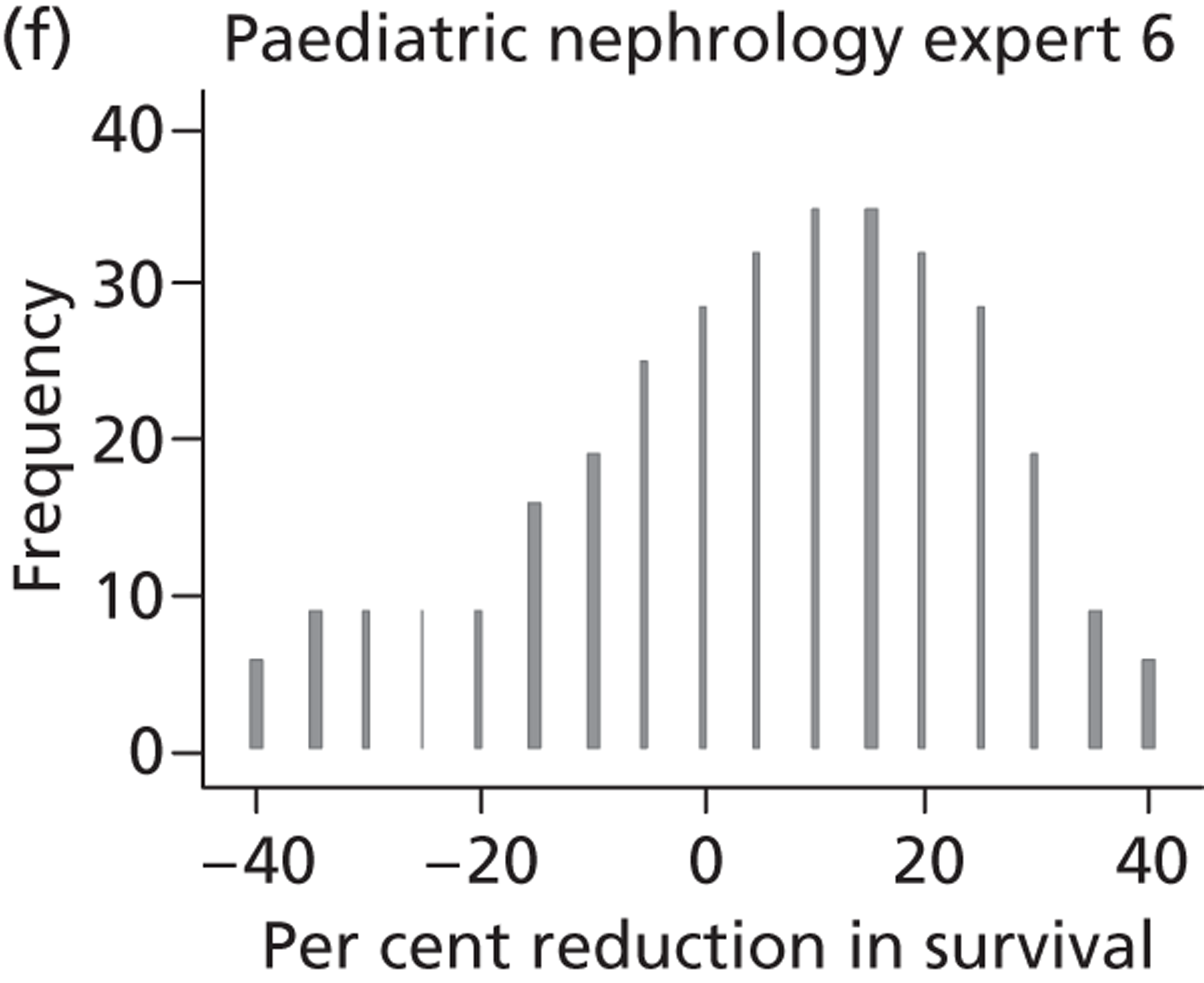
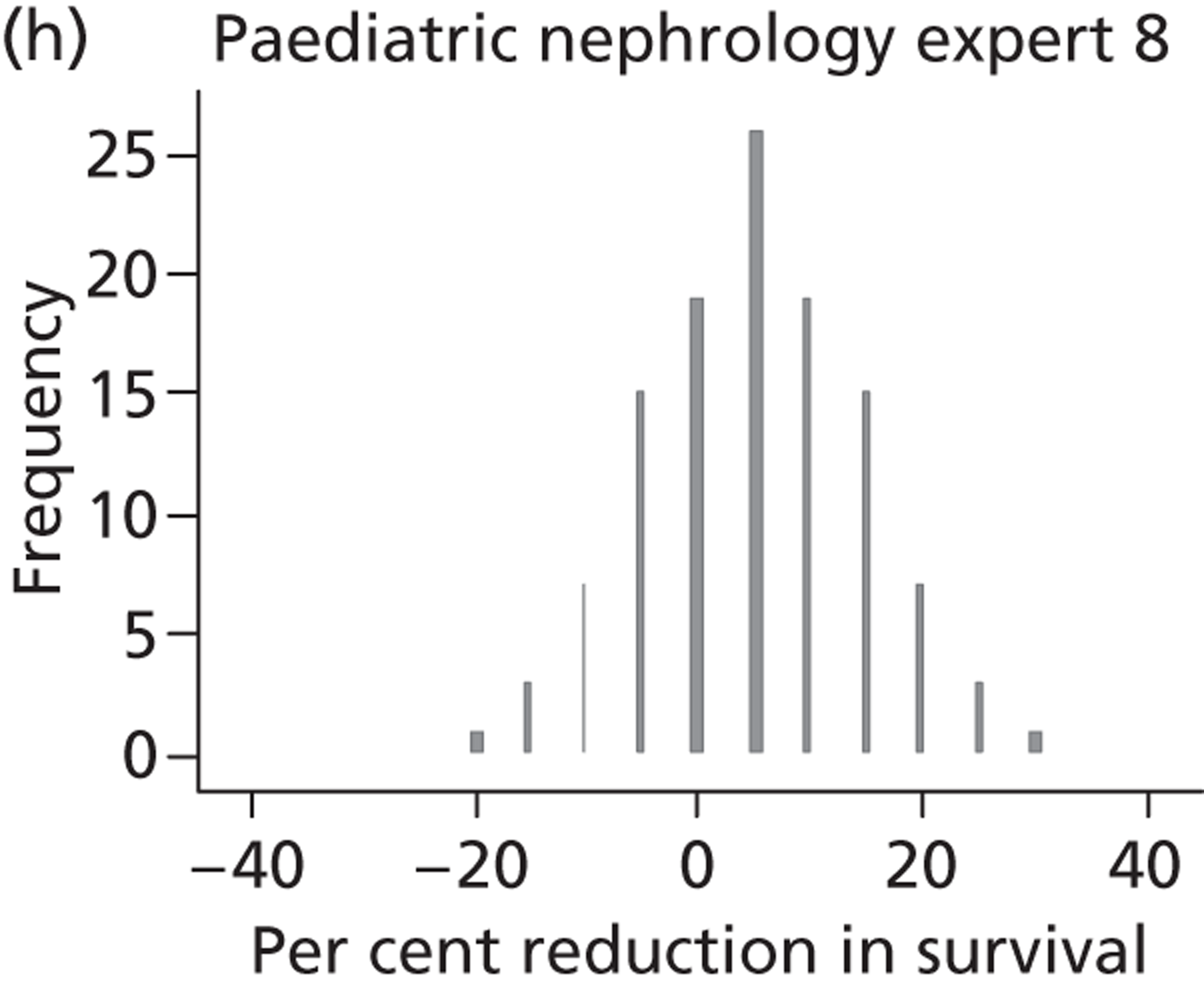
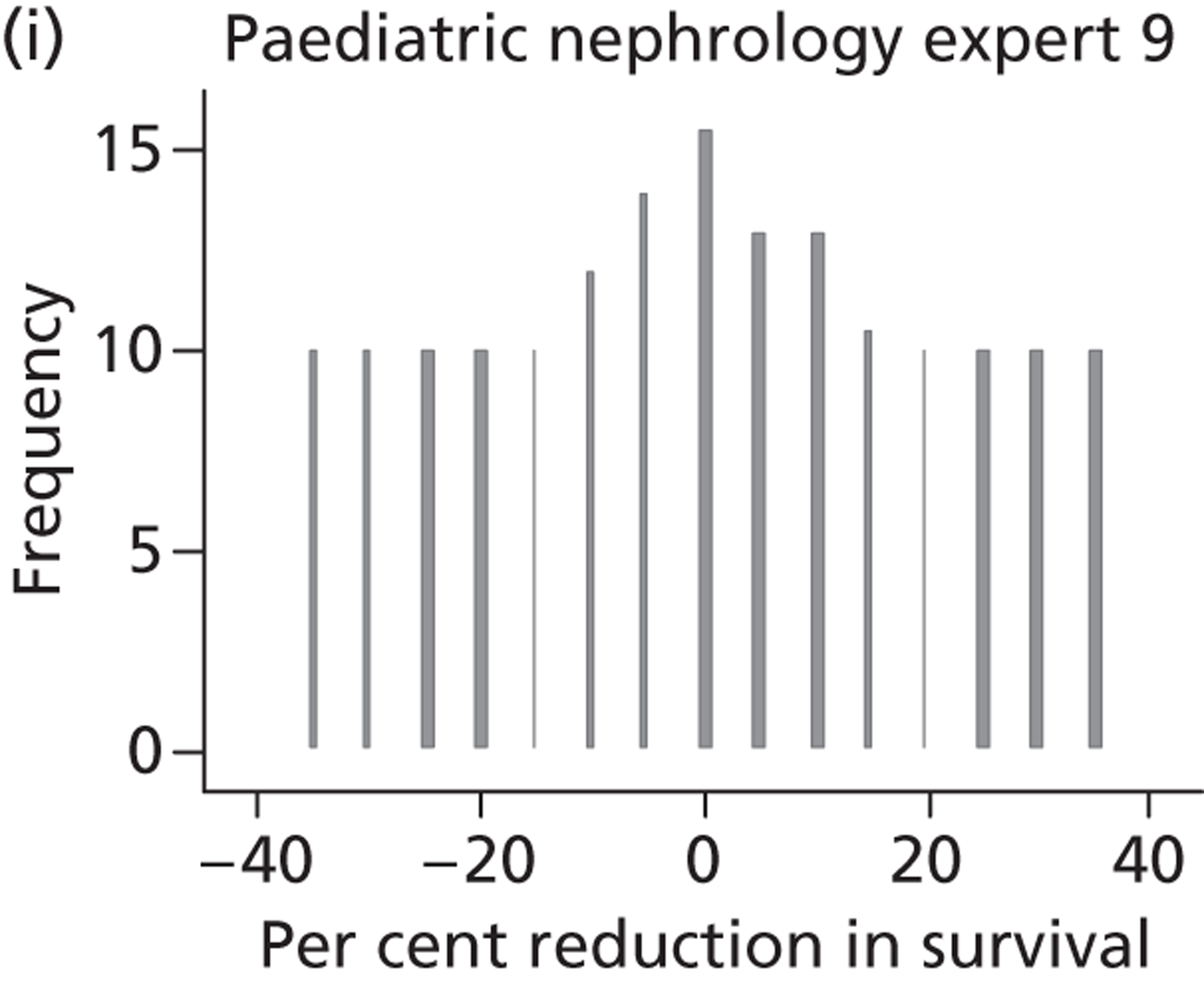
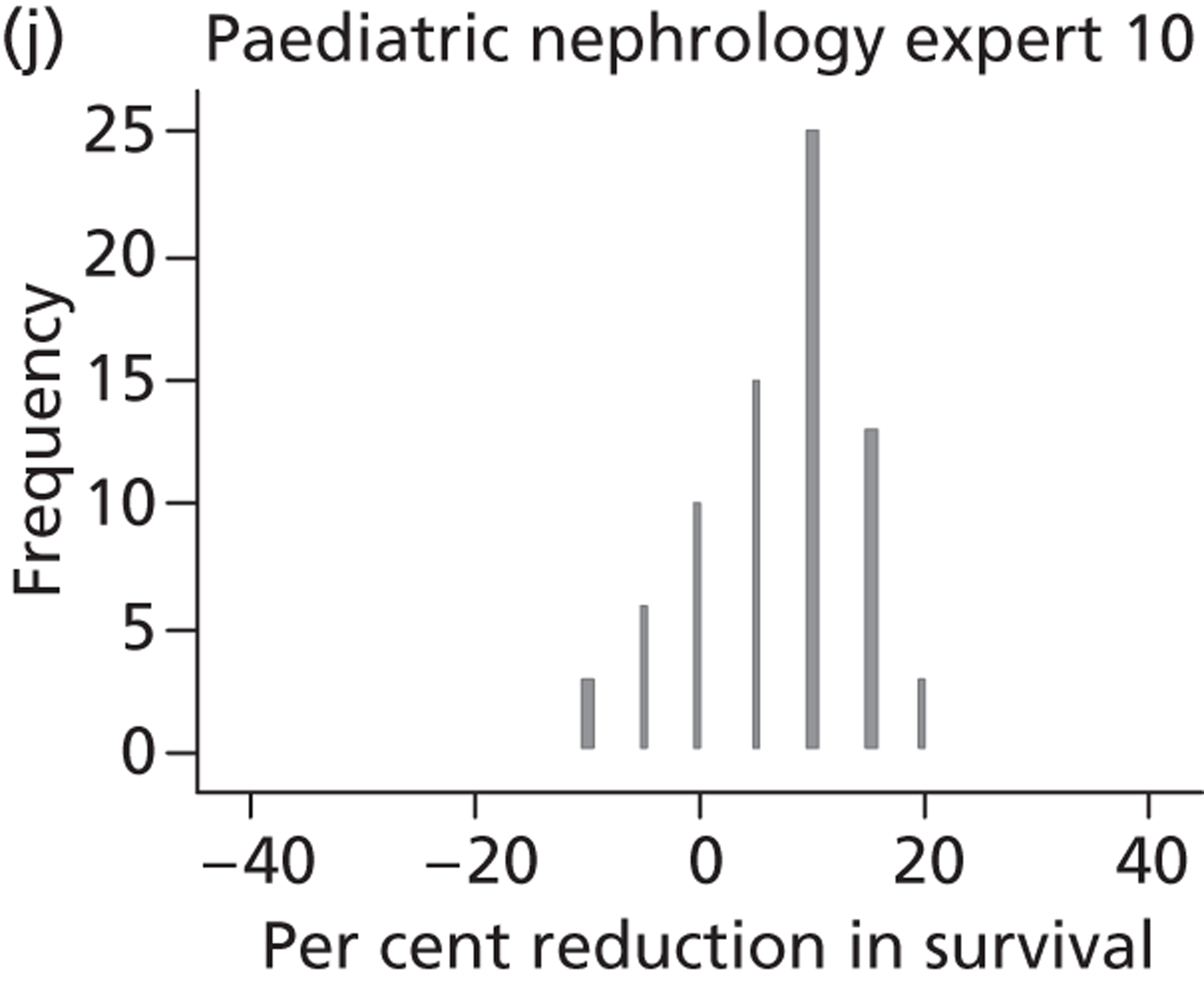
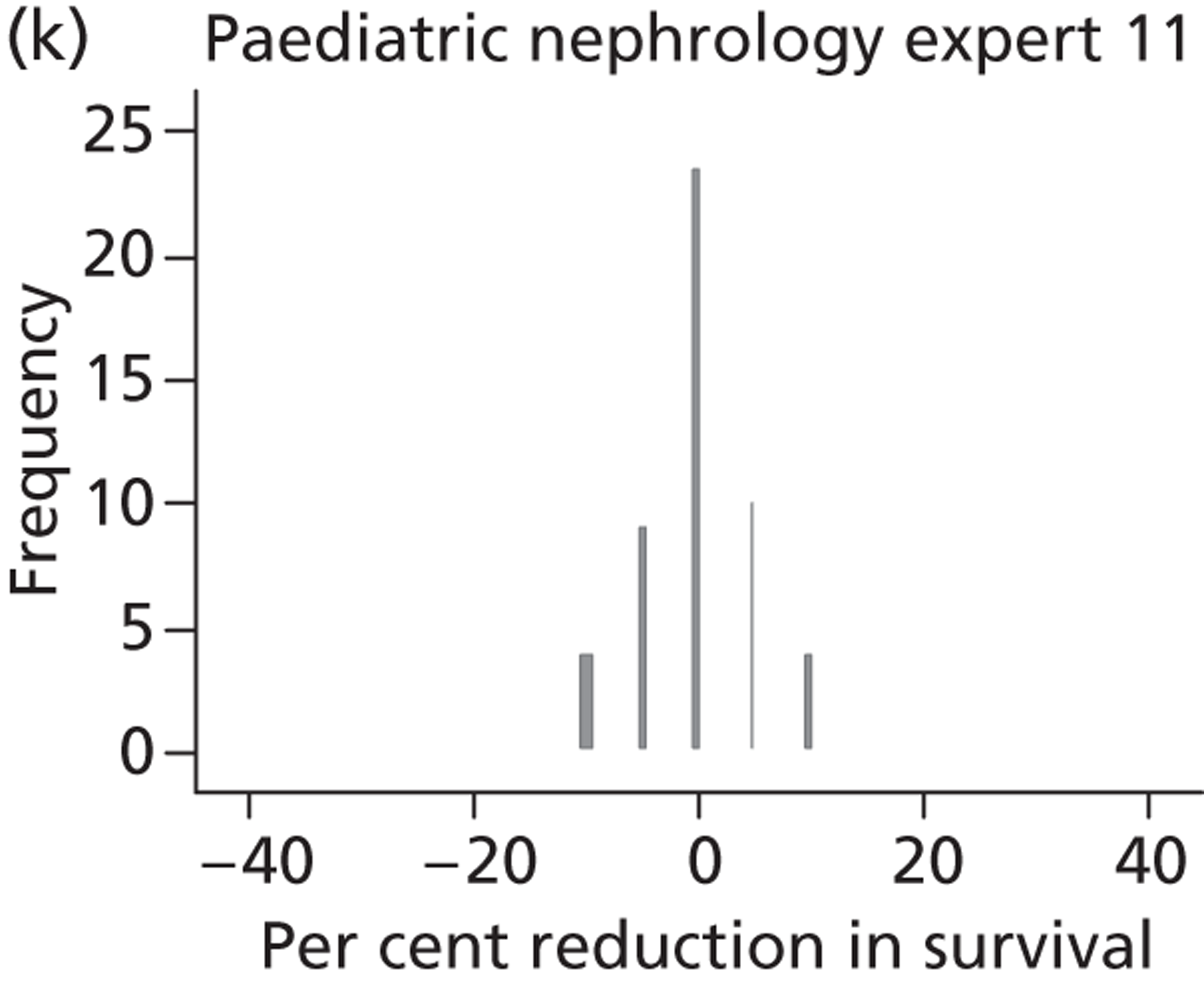
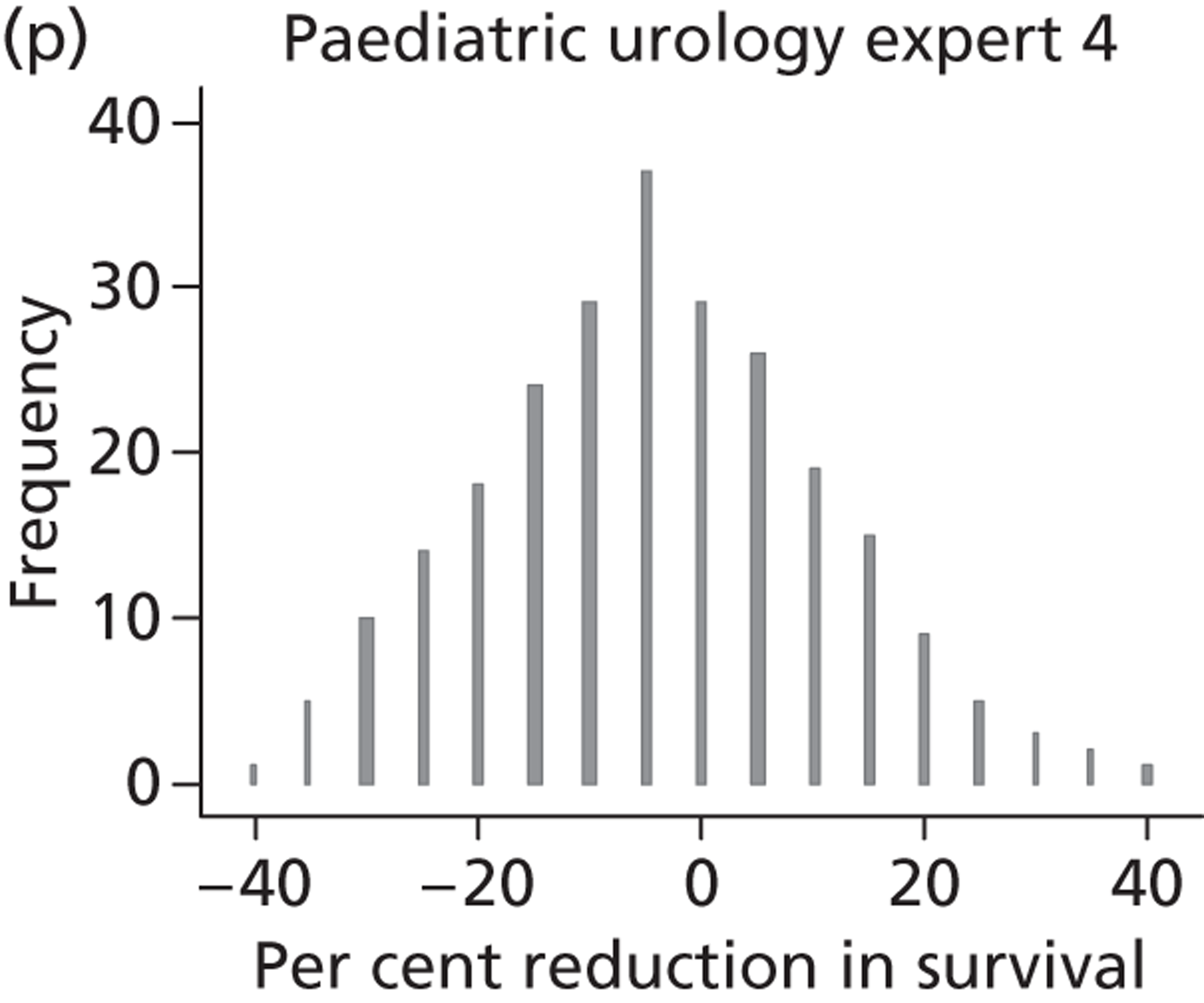
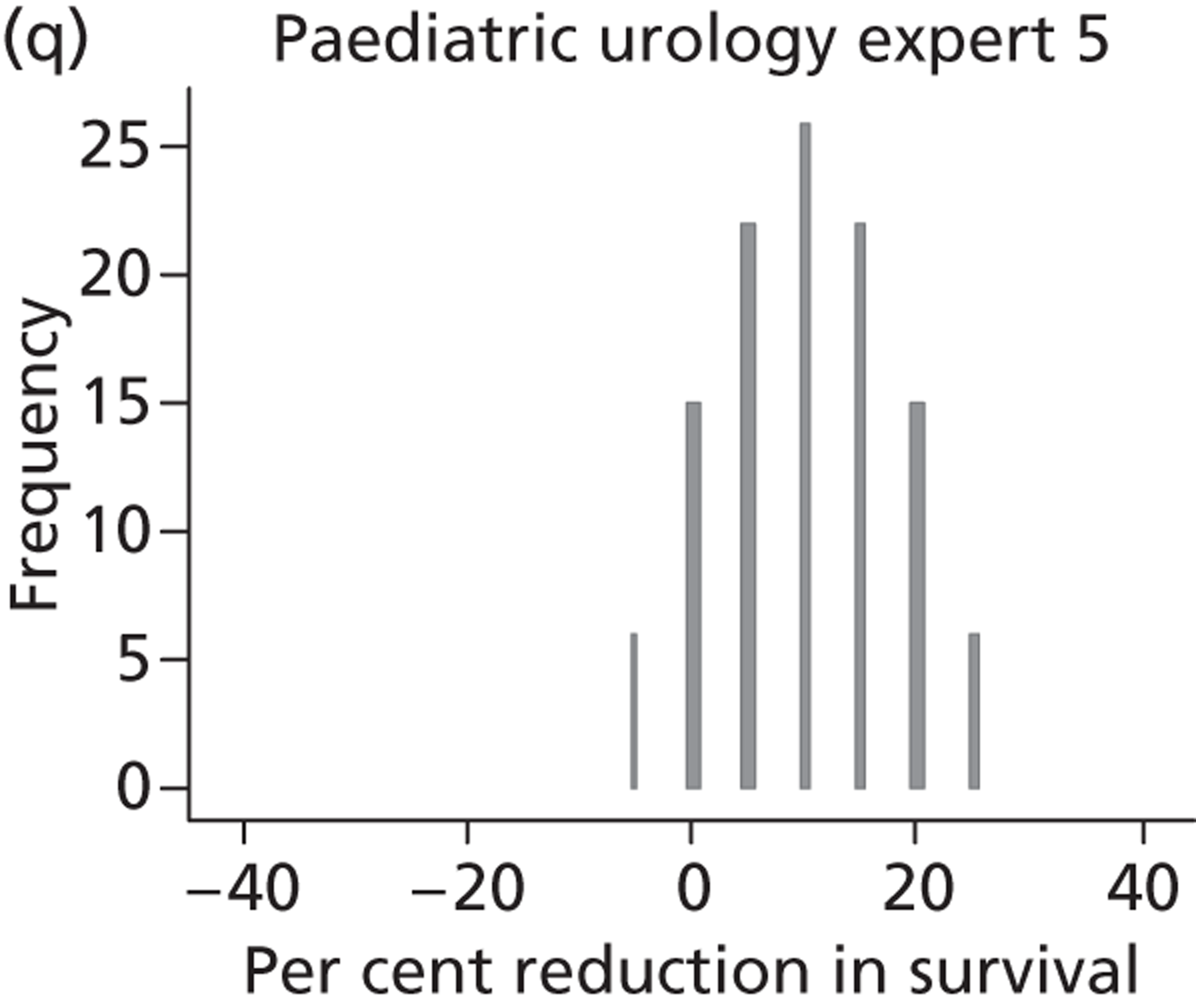
The pooled (over experts) distributions for change (in perinatal mortality using intrauterine VAS compared with conservative management) were reasonably similar between specialties and were reasonably normally distributed for fetal medicine and all experts combined ( Figure 18 ). All three specialties, on average, placed more weight on small values for change, giving preference to intrauterine VAS, but all were reasonably uncertain in their beliefs to provide non-zero density across the range of changes given. Summary statistics are provided in Table 28 . The fetal medicine experts believed that the median percentage decrease in mortality would be 10% (IQR 0% to 20%) whereas the paediatric nephrologists believed that the median decrease would be smaller (5%, IQR −5% to 10%), as did the paediatric urologists (5%, IQR 0% to 15%).
FIGURE 18.
Histogram of pooled elicited beliefs for percentage change in perinatal mortality when using intrauterine VAS compared with conservative management.
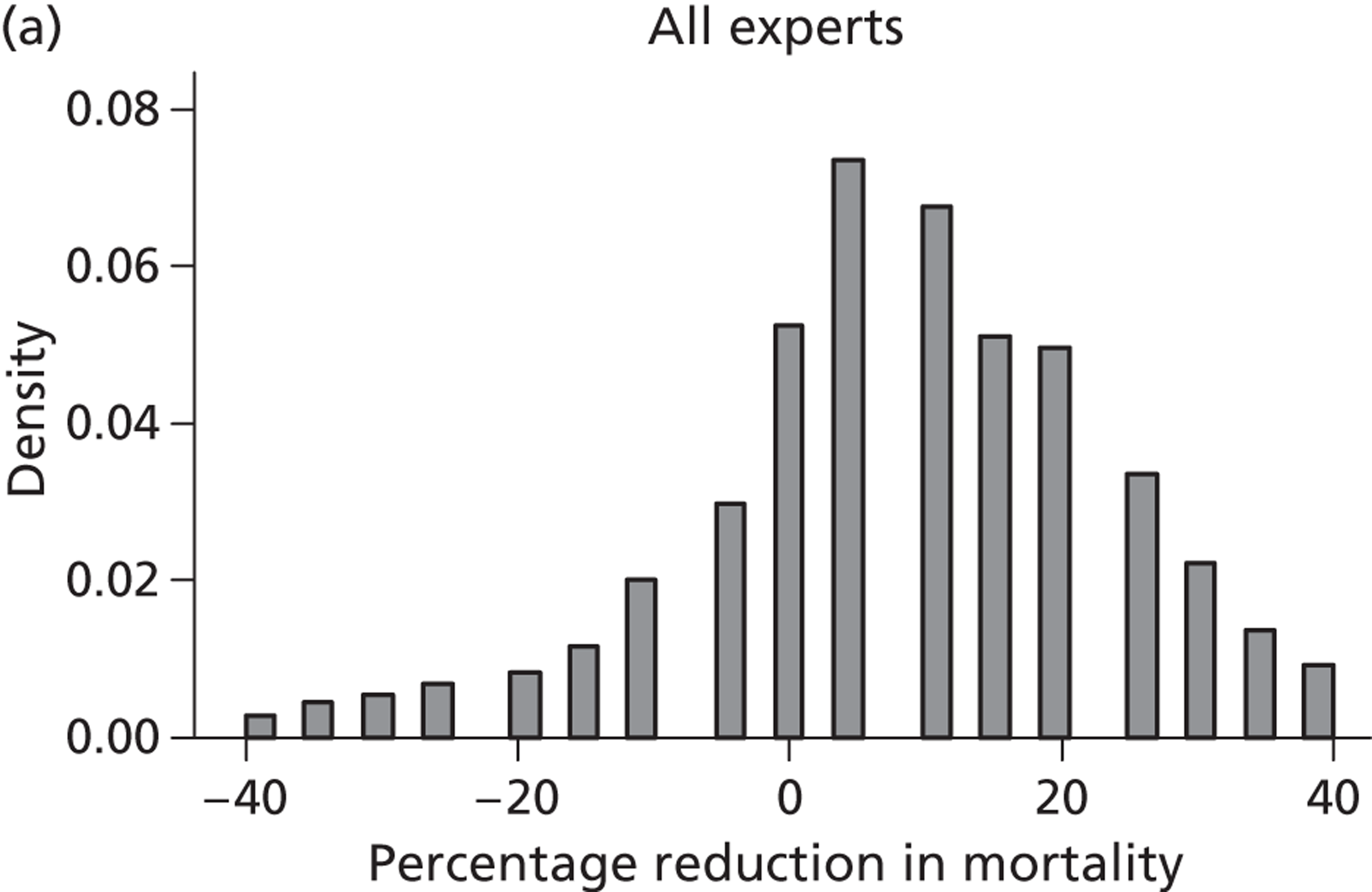
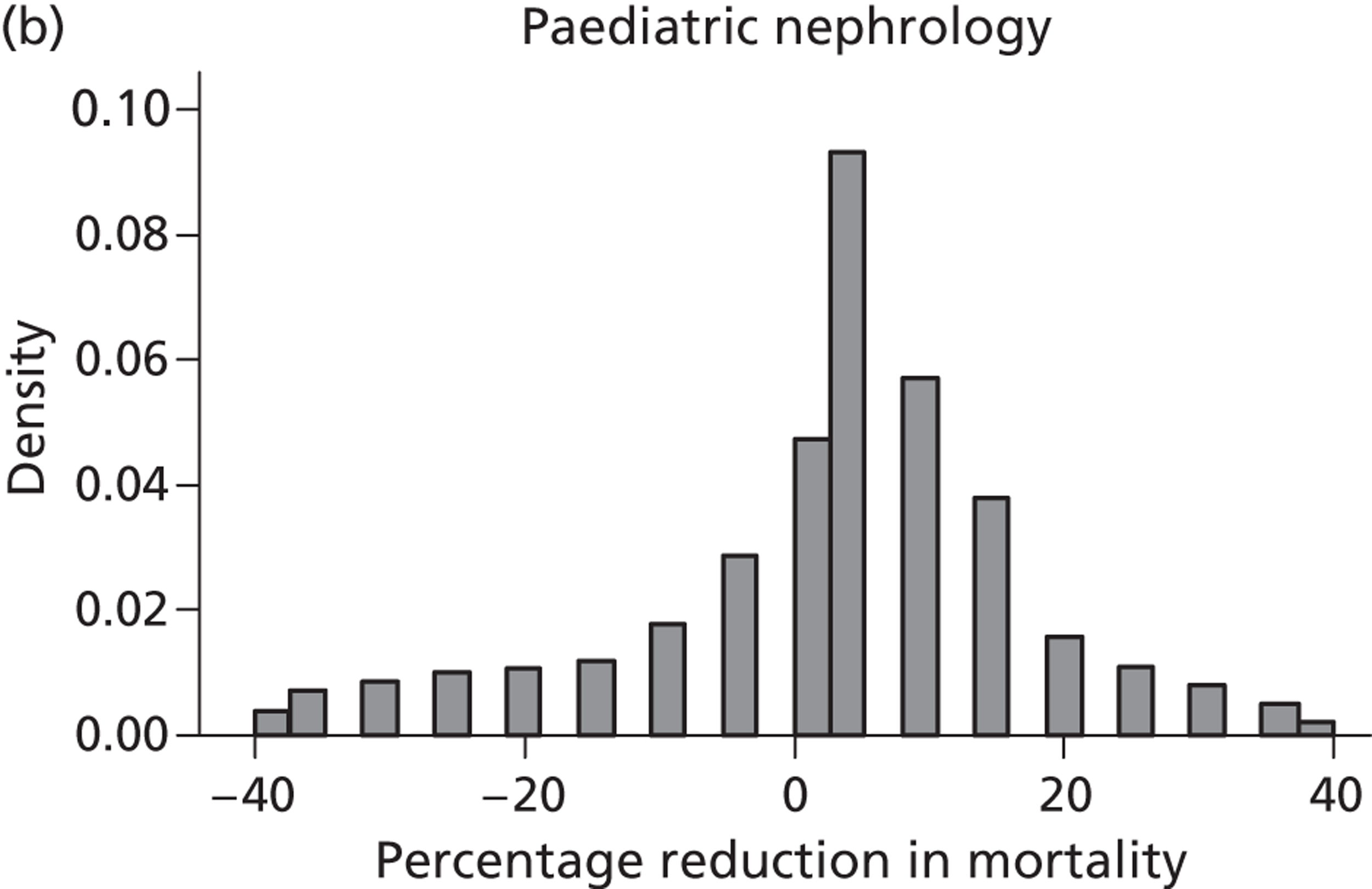
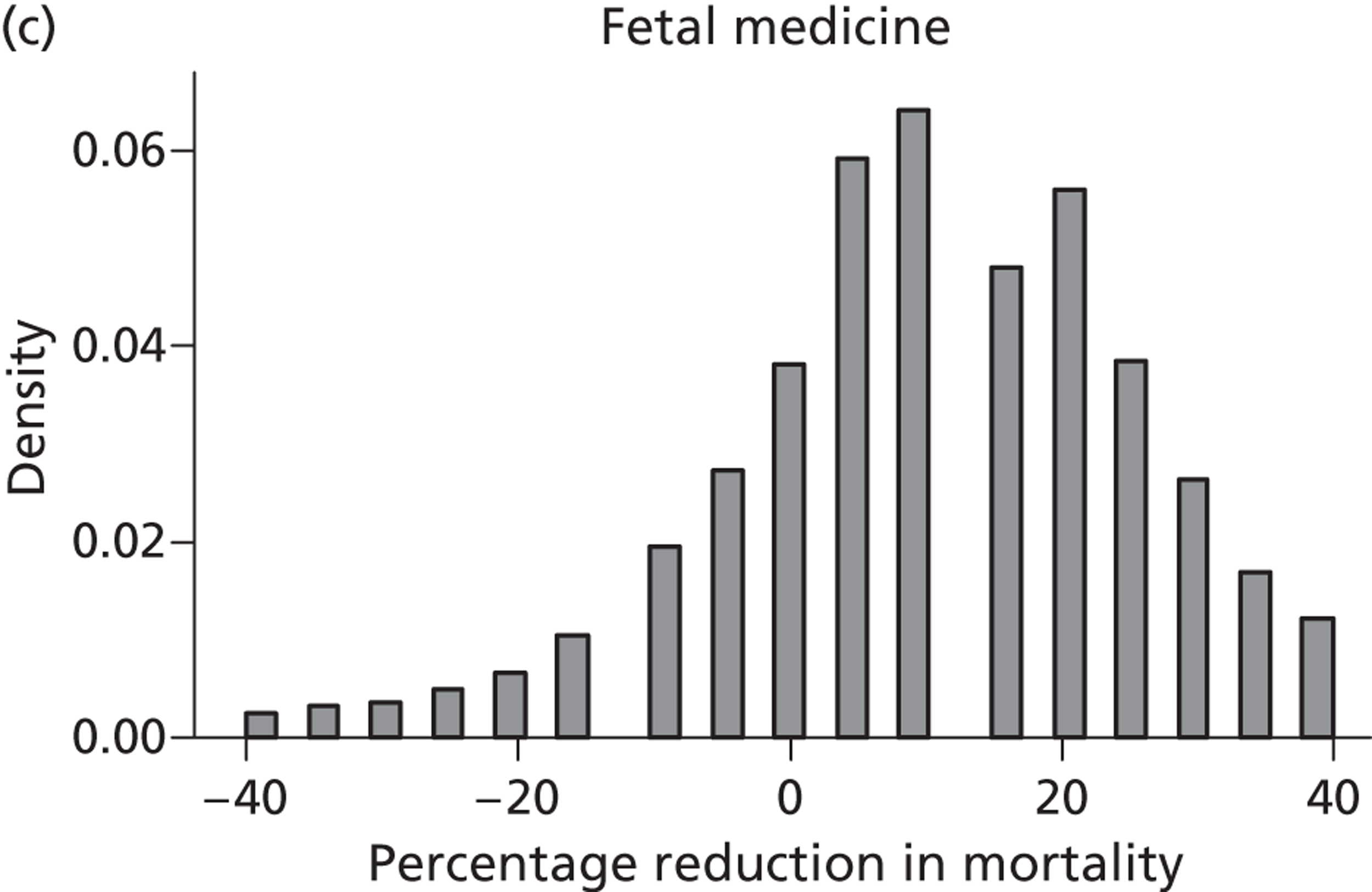
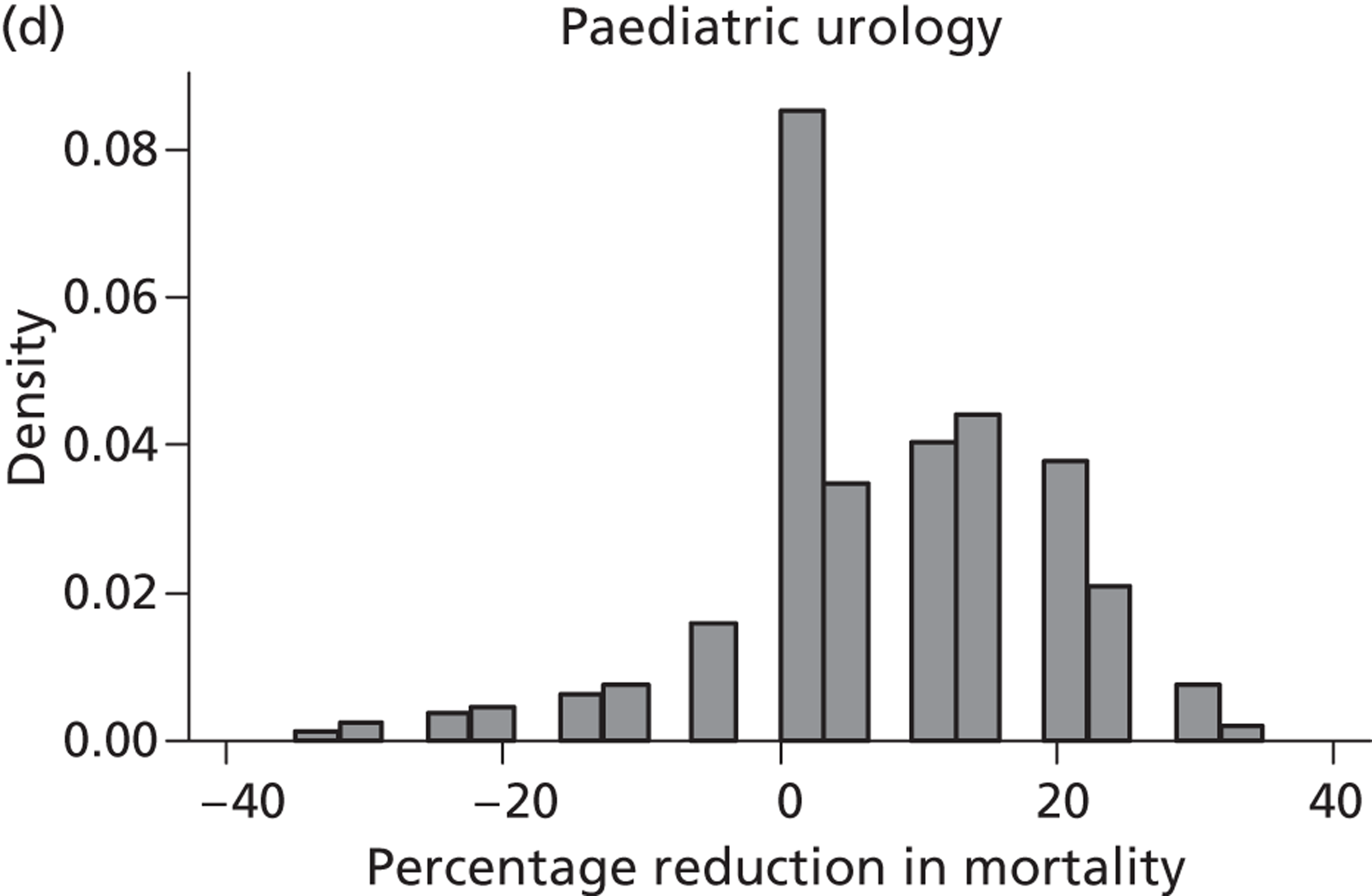
Pooled elicited beliefs for mortality using intrauterine vesicoamniotic shunting
From both the beliefs elicited for perinatal mortality under conventional treatment and the beliefs elicited for change in mortality with intrauterine VAS we elicited beliefs for mortality under intrauterine VAS. Two experts did not provide a belief of mortality under conservative management and so could not contribute to the estimate of mortality under intrauterine VAS.
Again, there was a wide variation in beliefs between the three specialty groups (see Table 28 ). The fetal medicine experts were the most pessimistic, giving a median perinatal mortality rate with VAS of 51% (IQR 33% to 68%). The paediatric nephrologists and the paediatric urologists were much more optimistic, with a median mortality rate of 10% (IQR 7% to 18%) for the paediatric nephrologists and a median mortality rate of 5% (IQR 1% to 18%) for the paediatric urologists.
Pooled elicited prior distributions for odds ratios
From these elicited beliefs for perinatal mortality under conservative management and change in mortality with VAS along with corresponding distributions, pooled prior distributions were obtained for ORs (VAS vs. conservative management). The paediatric nephrology and paediatric urology experts were more certain in their beliefs and less optimistic for a benefit of intrauterine VAS than the fetal medicine experts: the elicited median OR for survival from experts in fetal medicine was 1.28 (IQR 1.00 to 1.63) compared with 1.07 (IQR 1.00 to 1.19) for paediatric nephrology experts and 1.11 (IQR 1.00 to 1.28) for paediatric urology experts (see Table 28 ).
These elicited ORs for (perinatal) survival were transformed onto a log-scale. Histograms of the distributions for log-ORs pooled over experts (weighted by standardised height) by speciality appear reasonably normally distributed ( Figure 19 ). The distribution of log-ORs for fetal medicine experts is centred slightly above 0, suggesting a slight preference towards the belief that intrauterine VAS will be beneficial, with a mean value of 0.26 and SD of 0.5 (see Table 28 ). The distributions for both paediatric specialties are narrower and centred slightly closer to 0 than that of the fetal medicine experts (paediatric nephrologists: mean 0.07, SD 0.20; paediatric urologists: mean 0.13, SD 0.20).
FIGURE 19.
Elicited pooled log-ORs (overlaid with normal distribution) by specialty and for all specialties combined for odds of perinatal survival with intrauterine VAS compared with conservative management.
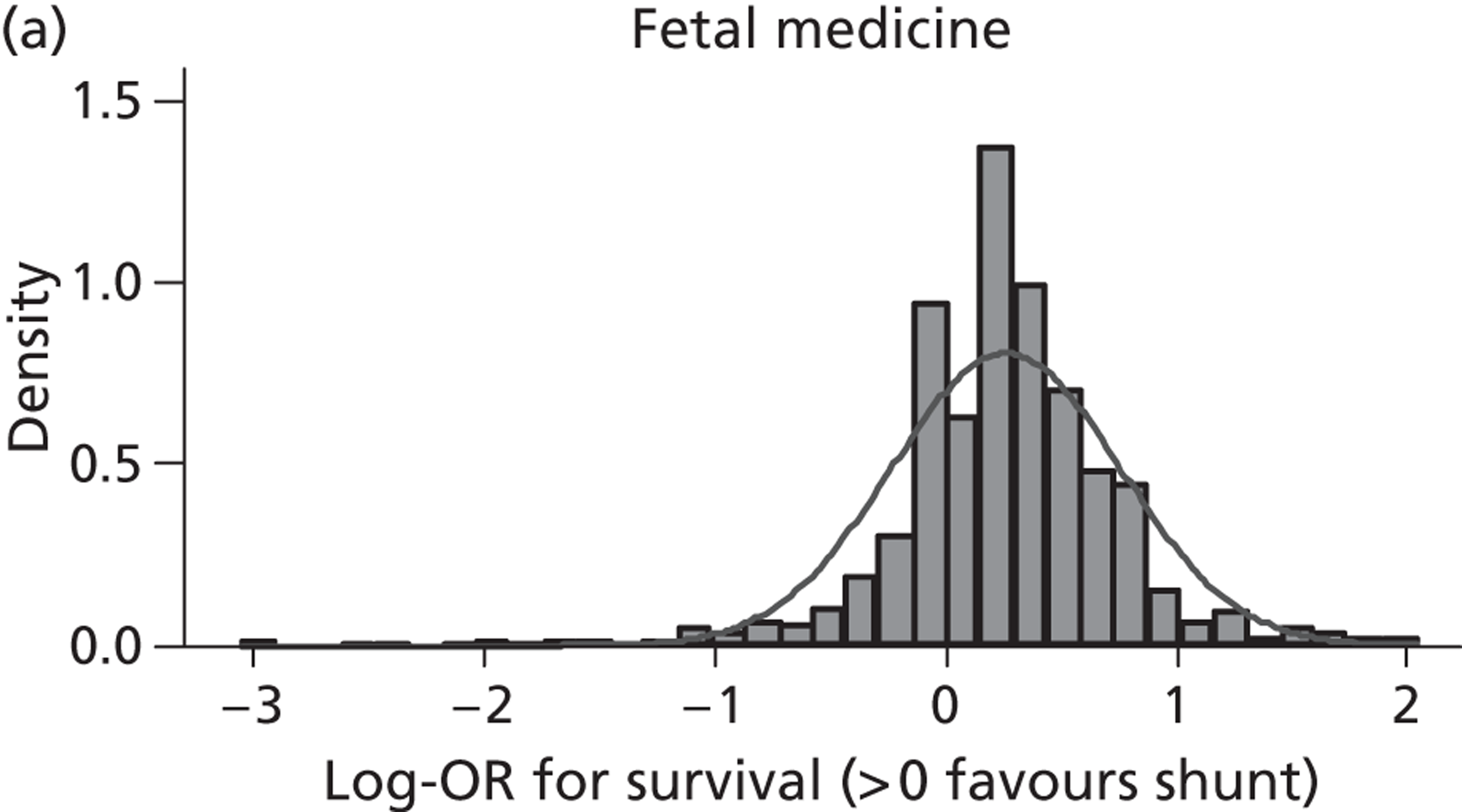
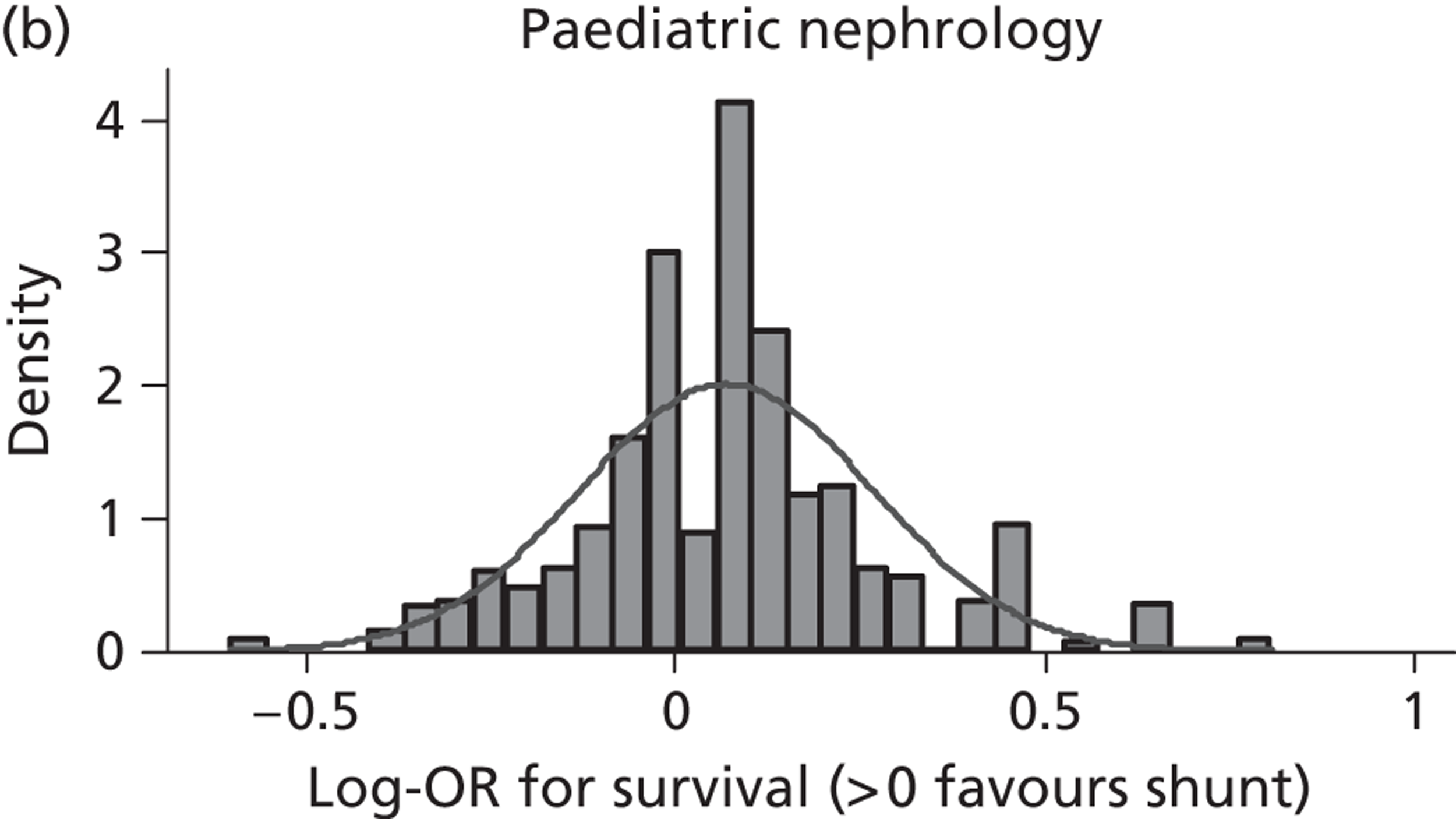
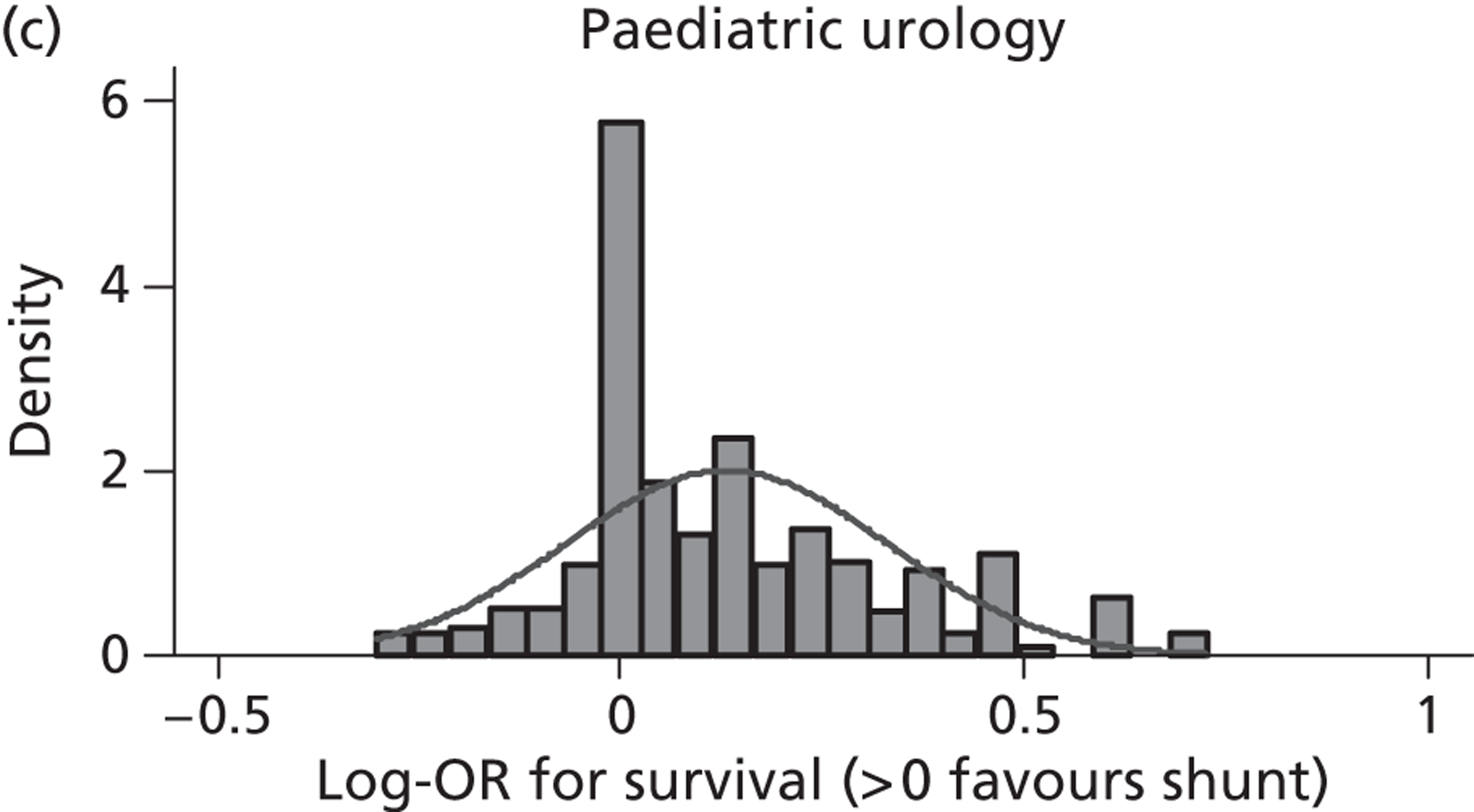
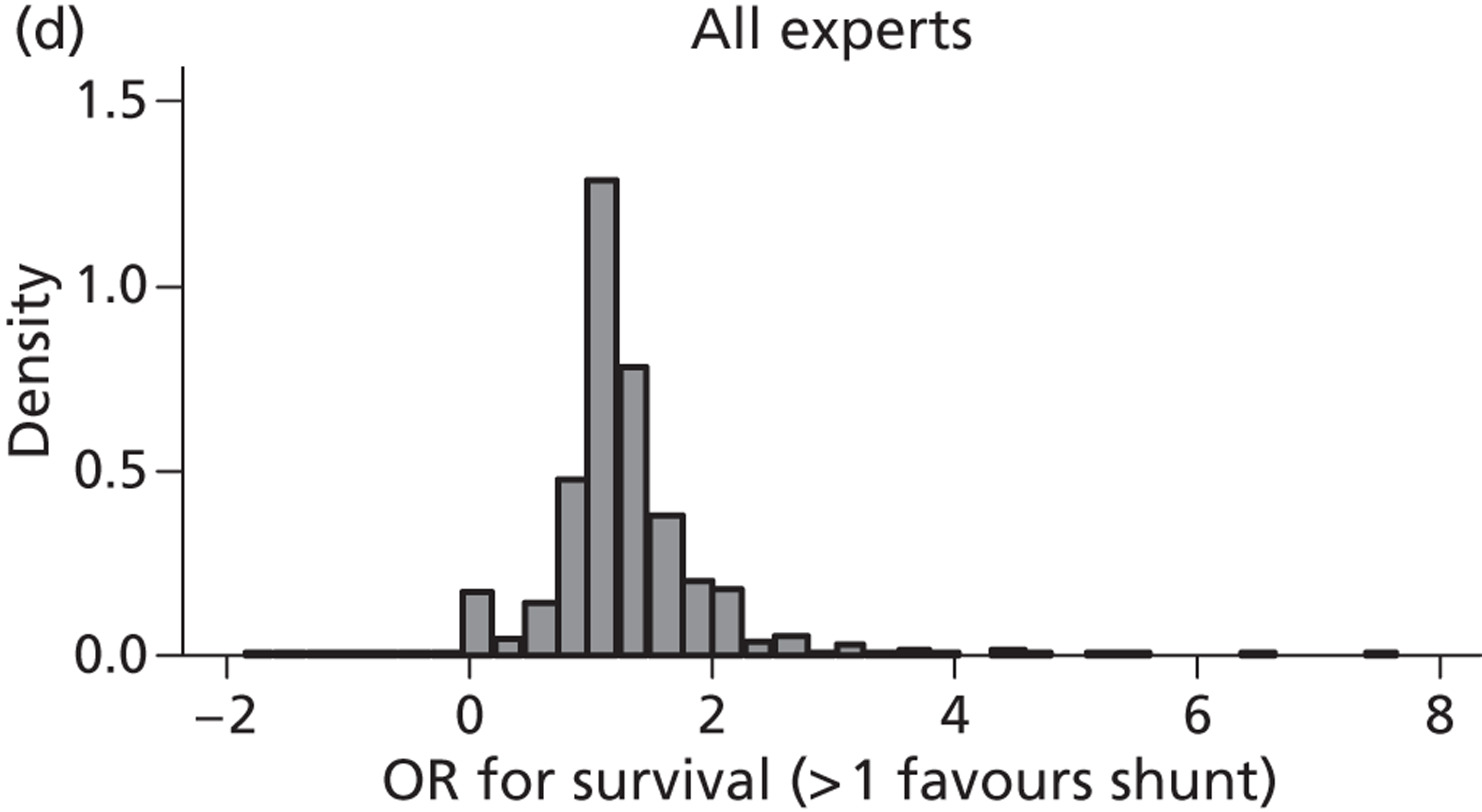
Although demonstrating some departure from normality ( Figure 20 ), these data were deemed sufficiently normal and so the log-OR approximated by a normal distribution with mean and SD values taken to be those elicited.
FIGURE 20.
Quantile normal plot for log-OR of perinatal survival by specialty and for all specialties combined.
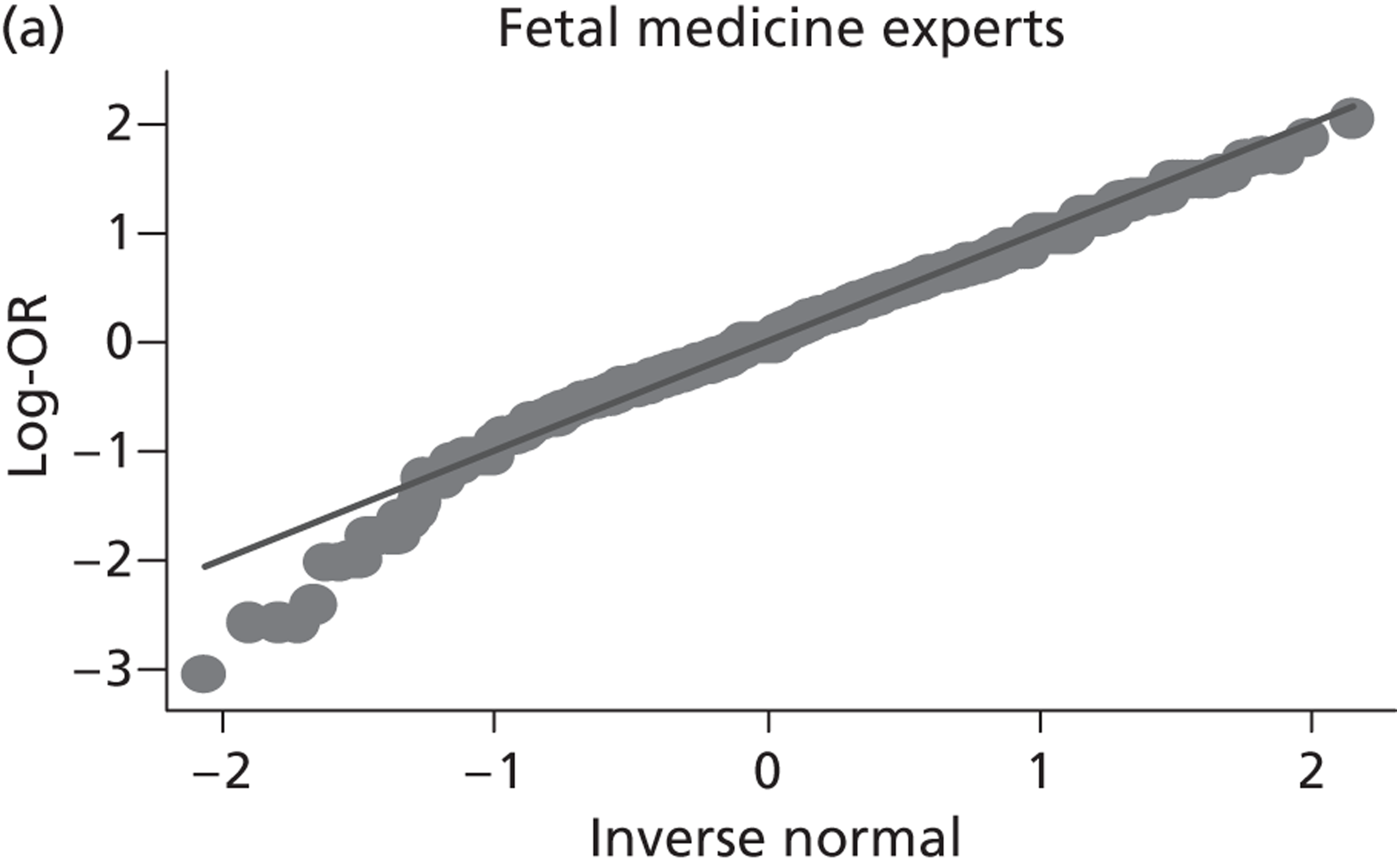
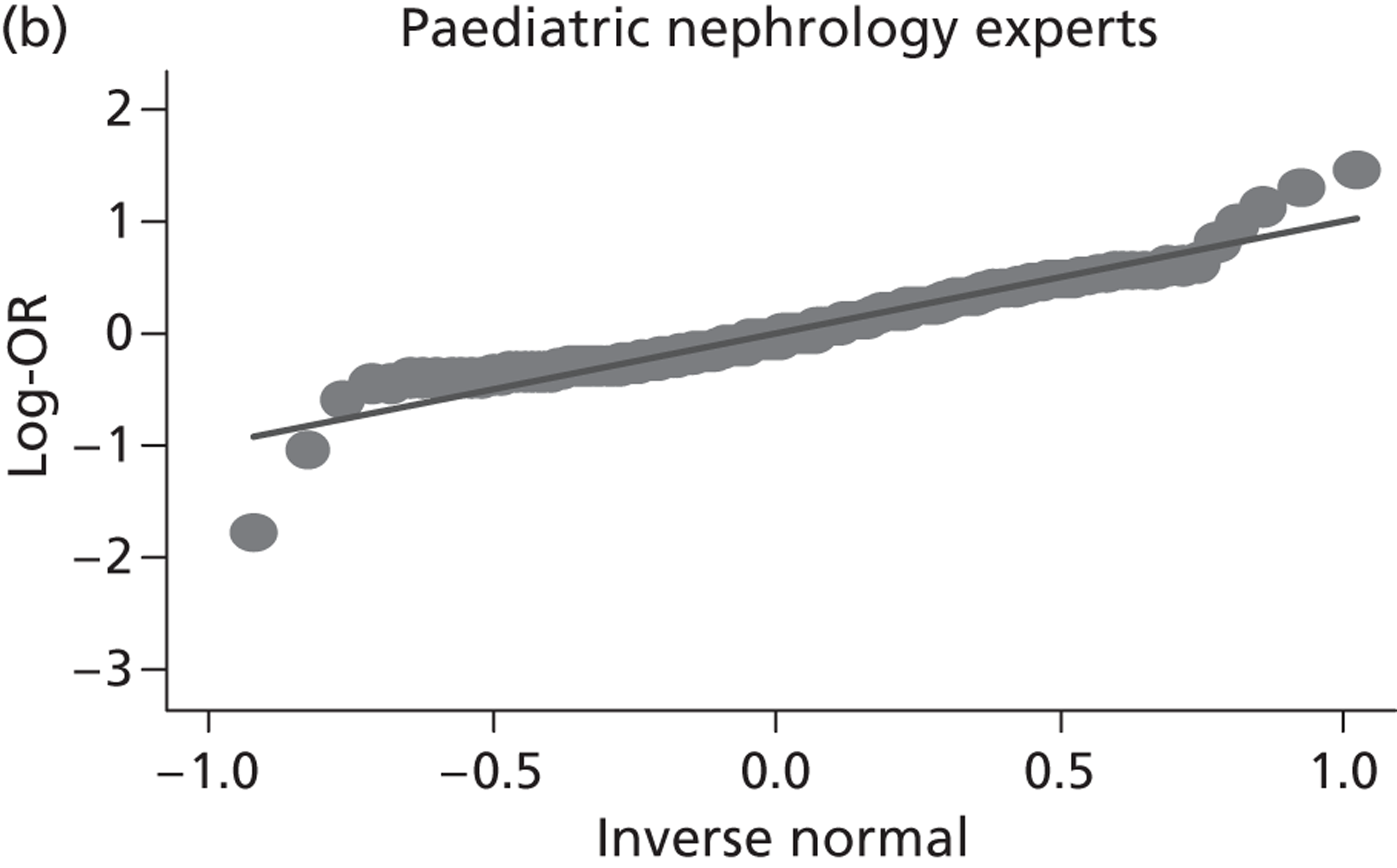
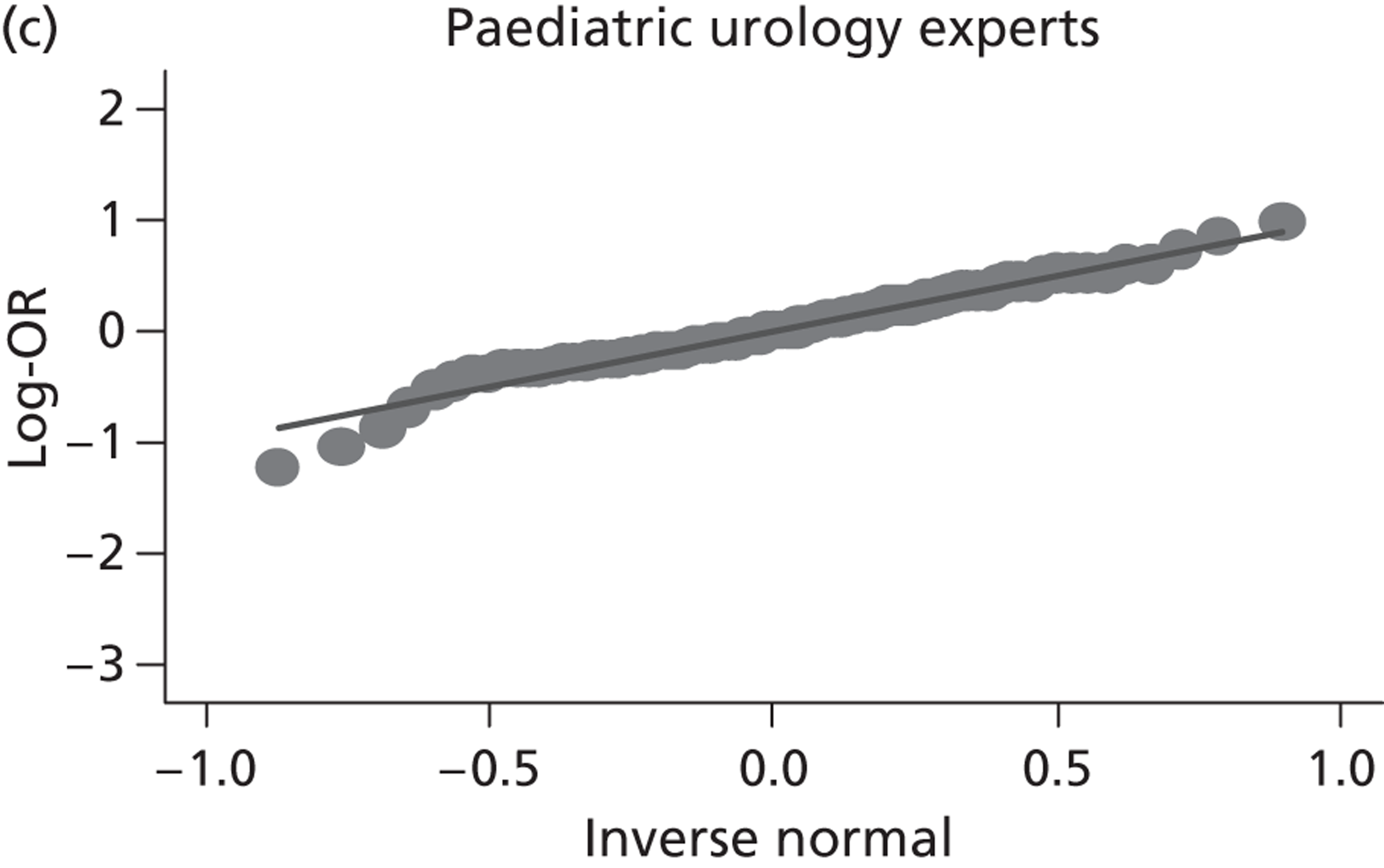
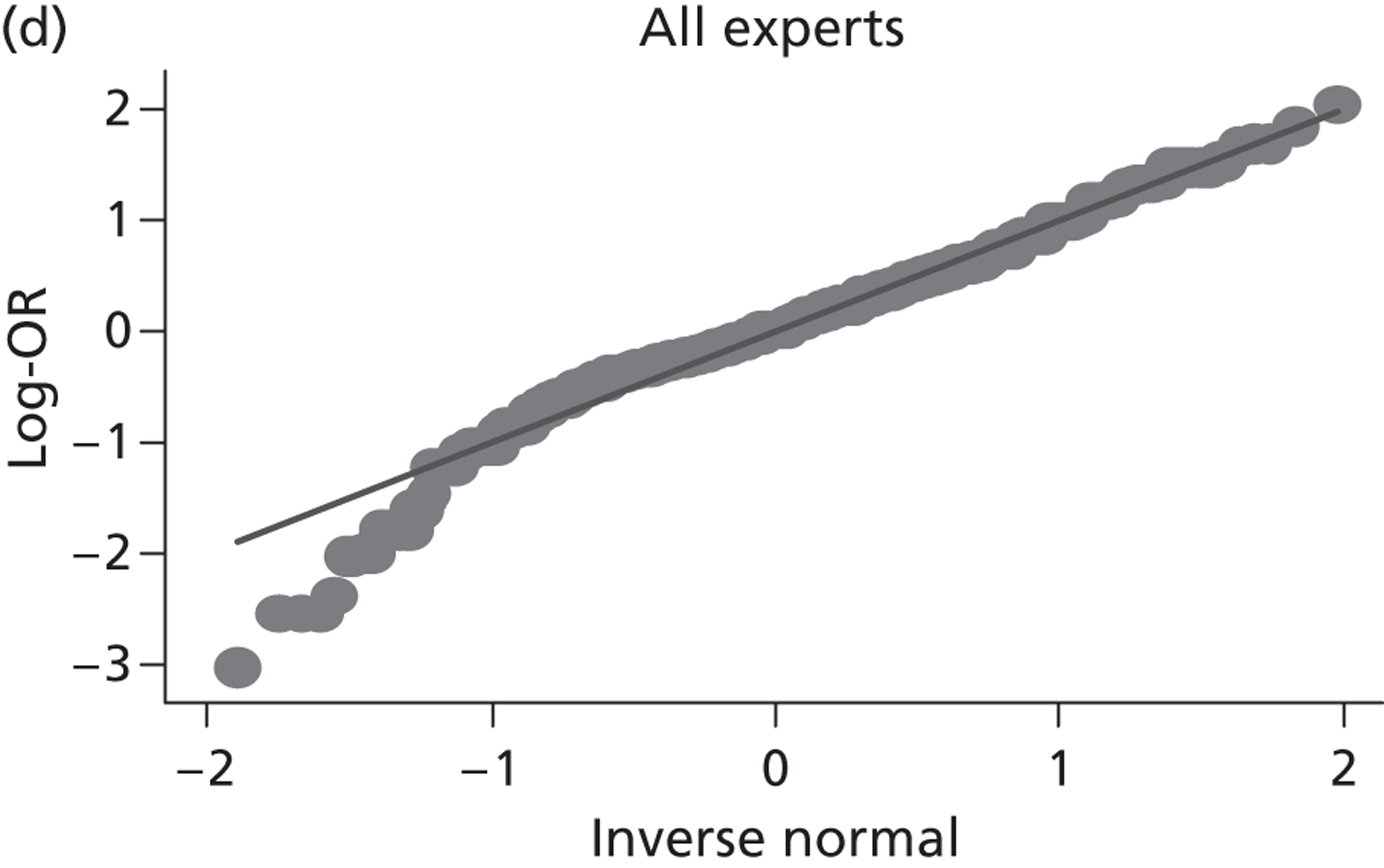
Summary of elicited priors
From the above elicitation process we therefore arrived at four informative priors. These informative priors were for log-ORs (for perinatal survival) and were assumed to be normally distributed with means and SDs as estimated from the process outlined above (values in Table 28 ). Therefore, the fetal medicine prior for the log-OR was taken to be N(mean 0.26, SD 0.50), the paediatric nephrology prior was taken to be N(mean 0.07, SD 0.20), the paediatric urology prior was taken to be N(mean 0.13, SD 0.20) and the all experts prior was taken to be N(mean 0.21, SD 0.44). These densities are displayed in Figure 21 . Consistent with the results above, the prior for the fetal medicine experts is wider and centred very slightly at a value more favourable to intrauterine VAS than the prior for either the paediatric urologists or the paediatric nephrologists. Because the majority of the experts (35 out of 52) were fetal medicine experts the combined overall expert prior was very similar to that of the fetal medicine experts.
FIGURE 21.
Elicited priors by specialty group for log-OR and OR of perinatal survival with VAS compared with conservative management. (a) Informative priors: log-OR scale; and (b) informative priors: OR scale.
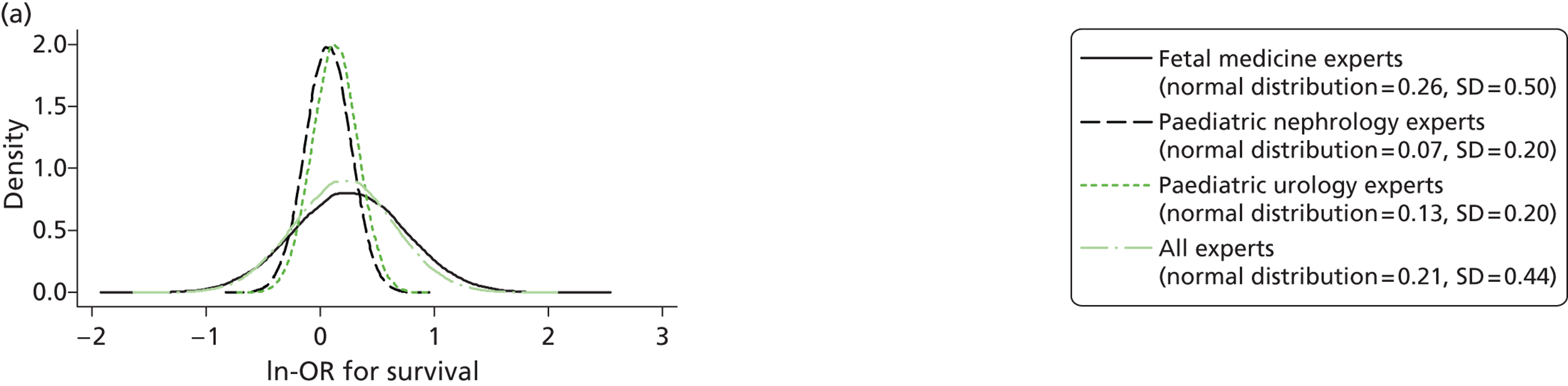
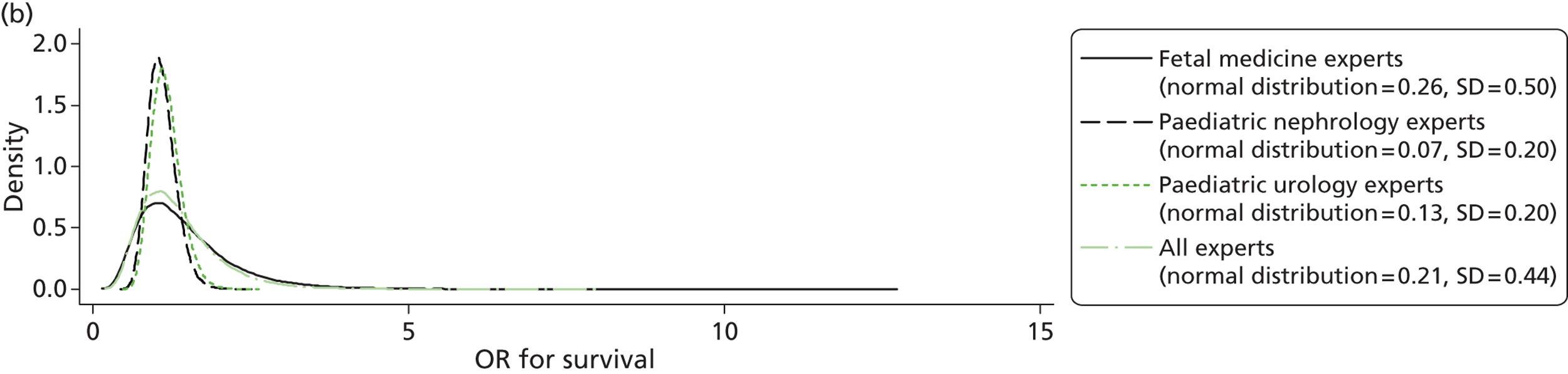
Experts were collectively very sceptical about the ability of VAS to provide a treatment effect as large as that anticipated by the trial (OR 2.55), and from the elicited opinions we estimated that the clinicians believed that the probability that the treatment effect would be this large was only 5% (see Table 28 ).
Default prior distributions
The elicited informative priors were then compared with three default priors, an enthusiastic prior centred at a log-OR of 0.94 and including a small possibility (about 5%) of harm (SD 0.57), a sceptical prior, centred at the value of no difference (log-OR 0) and also with a SD of 0.57, and finally an uninformative prior, once again centred at 0 but with a very large SD (variance 1002), to essentially provide uniform distributions over the range of plausible log-ORs. The sceptical and enthusiastic prior densities for outcomes at 28 days are displayed in Figure 22 .
FIGURE 22.
Sceptical and enthusiastic priors for the outcome of perinatal survival. (a) Default priors: log-OR scale; and (b) default priors: OR scale.
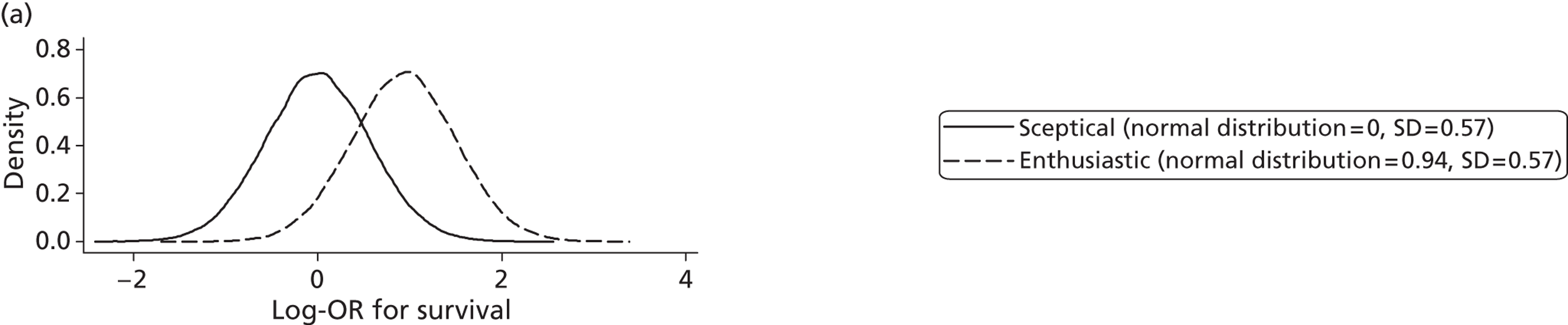
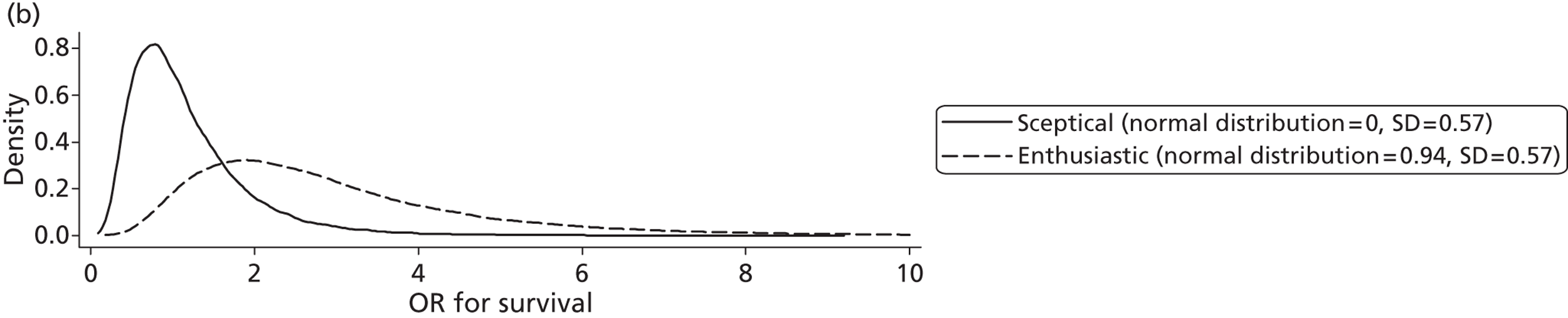
For the outcome survival to one year, the default priors were slightly different: an enthusiastic prior is formed with a centre at a log-RR of 0.44 and including a small possibility (about 5%) of harm (SD 0.27); a sceptical prior is centred at the value of no difference (log-RR 0) and also with a SD of 0.27; and finally a non-informative prior centred at 0 but with a very large SD (variance 1002). The sceptical and enthusiastic prior densities for the outcomes at one year are displayed in Figure 23 .
FIGURE 23.
Sceptical and enthusiastic priors for the outcome of survival to 1 year. (a) Sceptical and enthusiastic prior distributions on the log-HR scale; and (b) sceptical and enthusiastic prior distributions on the HR scale.
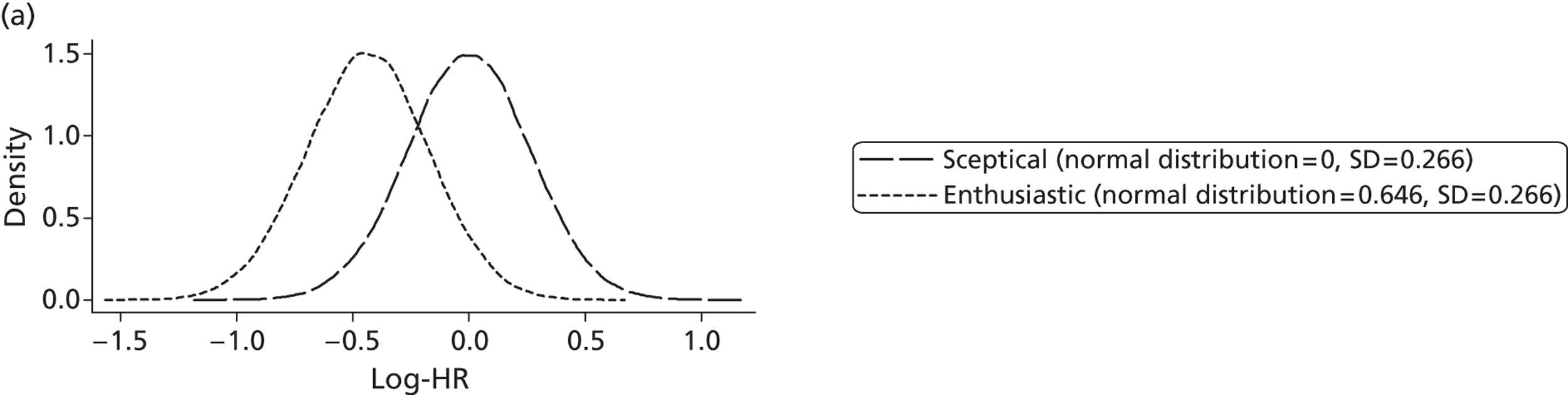
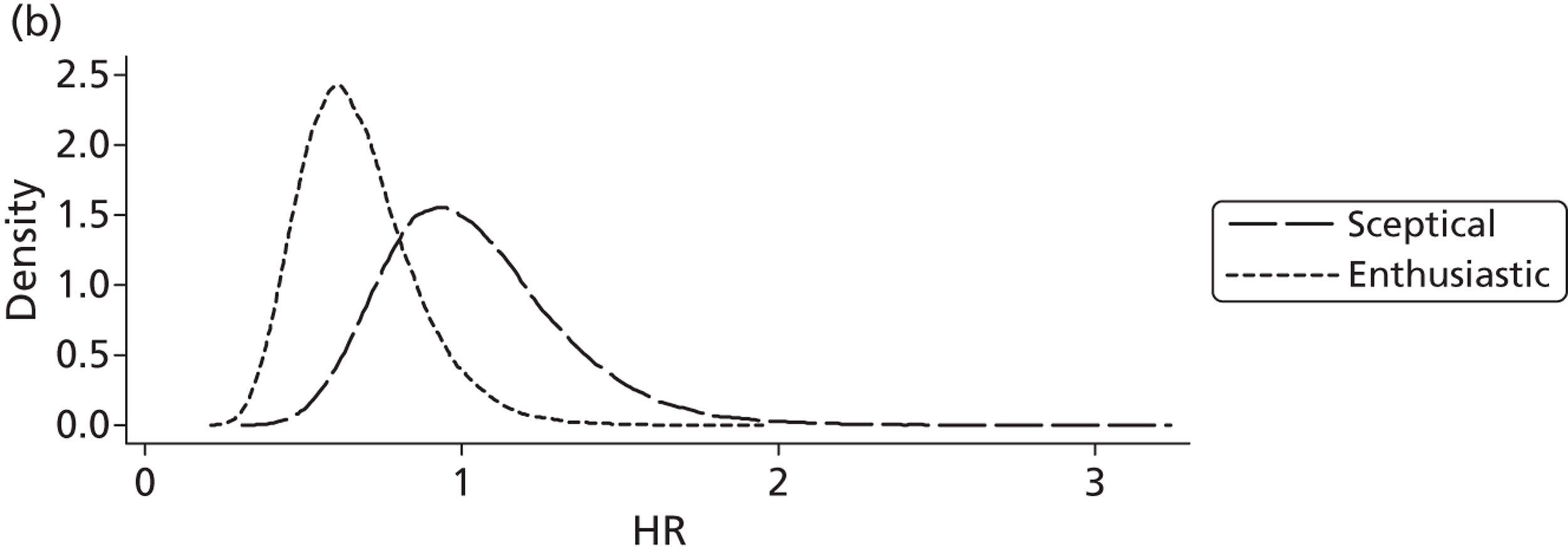
PLUTO trial results for the outcome of perinatal survival
As reported in Chapter 5 , 15 participants were randomised to the conservative management arm and 16 to the intrauterine VAS arm of the PLUTO RCT. Of the 15 infants randomised to conservative management, four (27%) were alive at 28 days. Of the 16 randomised to the intrauterine VAS arm, eight (50%) were alive at 28 days ( Table 29 ).
| Outcome | Conservative management | VAS |
|---|---|---|
| No. randomised | 15 | 16 |
| No. received allocation | 13 | 13 |
| ITT analysis | ||
| n | 15 | 16 |
| Survival to 28 days, n (%) | 4 (27) | 8 (50) |
| AT analysis | ||
| n | 16 | 15 |
| Survival to 28 days, n (%) | 3 (19) | 9 (60) |
In the event, two patients randomised to conservative management actually received intrauterine VAS and three patients randomised to the intrauterine VAS arm actually had conservative management. Therefore, 16 patients received conservative management and 15 patients received intrauterine VAS; these form the AT analysis (see Table 29 ). Of the 15 patients who received intrauterine VAS nine (60%) were alive at 28 days and, of the 16 patients who received conservative management, three (19%) were alive at 28 days.
Posterior distributions for the outcome of perinatal survival
Table 30 presents both the estimated RR along with the 95% CrI and the associated Bayesian p-value (probability that the RR is > 1.55, which is the effect size that the trial was designed to detect) and the NNT for each of the four informative prior distributions (fetal medicine, paediatric urologists, paediatric nephrologists and all experts) and the three additional prior distributions (uninformative, enthusiastic and sceptical).
| RR (95% CrI) | Probability RR > 1.55 | NNT (95% CrI) | |
|---|---|---|---|
| ITT analysis | |||
| Uninformative prior | 1.93 (0.77 to 6.25) | 0.67 | 4 (−31 to 38) |
| Enthusiastic prior | 1.82 (1.05 to 3.52) | 0.71 | 5 (2 to 24) |
| Sceptical prior | 1.25 (0.73 to 2.27) | 0.23 | 7 (−103 to 118) |
| All experts prior | 1.31 (0.84 to 2.18) | 0.25 | 8 (−81 to 96) |
| Paediatric nephrologists prior | 1.08 (0.86 to 1.38) | 0.01 | 17 (−285 to 289) |
| Paediatric urologists prior | 1.12 (0.89 to 1.44) | 0.01 | 16 (−214 to 237) |
| Fetal medicine experts prior | 1.35 (0.82 to 2.34) | 0.30 | 7 (−76 to 91) |
| AT analysis | |||
| Uninformative prior | 3.41 (1.28 to 14.16) | 0.94 | 2 (1 to 9) |
| Enthusiastic prior | 2.16 (1.25 to 4.26) | 0.87 | 4 (2 to 11) |
| Sceptical prior | 1.48 (0.87 to 2.73) | 0.44 | 6 (−45 to 62) |
| All experts prior | 1.48 (0.95 to 2.47) | 0.43 | 6 (−31 to 50) |
| Paediatric nephrologists prior | 1.12 (0.89 to 1.43) | 0.01 | 16 (−221 to 238) |
| Paediatric urologists prior | 1.15 (0.92 to 1.48) | 0.01 | 15 (−154 to 181) |
| Fetal medicine experts prior | 1.56 (0.95 to 2.74) | 0.50 | 6 (−24 to 44) |
Under the ITT analysis and the uninformative prior, the estimated RR for survival is 1.93 (95% CrI 0.77 to 6.25) with a probability of a clinically important benefit (RR > 1.55) of 0.67 and a NNT of 4 (95% CrI −31 to 38). Perhaps surprisingly, the median estimated RR under the enthusiastic prior is a little closer to the null (RR 1.82), although the CrI includes only values that favour the intervention (95% CrI 1.05 to 3.52); the probability of a clinically important effect increases to 0.71; and the 95% CrI for the NNT includes only beneficial effects (NNT 5, 95% CrI 2 to 24). As expected, the sceptical prior pulls the RR even closer towards the null (RR 1.25) and the 95% CrI does not exclude values that favour the conventional treatment (95% CrI 0.73 to 2.27); and the probability of a clinically important effect reduces to 0.23 and the 95% CrI for the NNT is very wide (NNT 7, 95% CrI −103 to 118).
When including the elicited priors the probability that the RR is > 1.55 decreases; the point estimates for the RRs are generally closer to the null and the 95% CrIs include the possibility of either treatment being optimal; and the estimated NNTs, although all positive, have 95% CrIs that are wide and include both beneficial and harmful effects. The posterior estimates using the fetal medicine and all expert priors are similar (RR 1.31, 95% CrI 0.84 to 2.18 for all experts; RR 1.35, 95% CrI 0.82 to 2.34 for fetal medicine experts). Both the paediatric nephrology and paediatric urology posteriors show less optimism for the intervention with the probability that the RR is > 1.55 being just 0.01 for both and the RRs being close to the null and 95% CrI intervals crossing the null but showing increased certainty (RR 1.08, 95% CrI 0.86 to 1.38 for paediatric nephrologists; RR 1.12, 95% CrI 0.89 to 1.44 for paediatric urologists).
Under the AT analysis all estimates of RRs show greater preference for the intervention and the probabilities that the RR is > 1.55 are increased (compared with the ITT analysis), although 95% CrIs are wider, indicating less certainty, and include both positive and harmful effects, as do the 95% CrIs for the NNTs.
A plot of the posterior distributions for the ORs under the seven prior distributions for the ITT analysis is shown in Figure 24 and for the as-treated analysis is shown in Figure 25 . Figure 26 displays the individual prior distributions overlaid with their updated posterior distributions for the ITT analysis only.
FIGURE 24.
Posterior distributions for ORs (perinatal survival) under the ITT analysis.
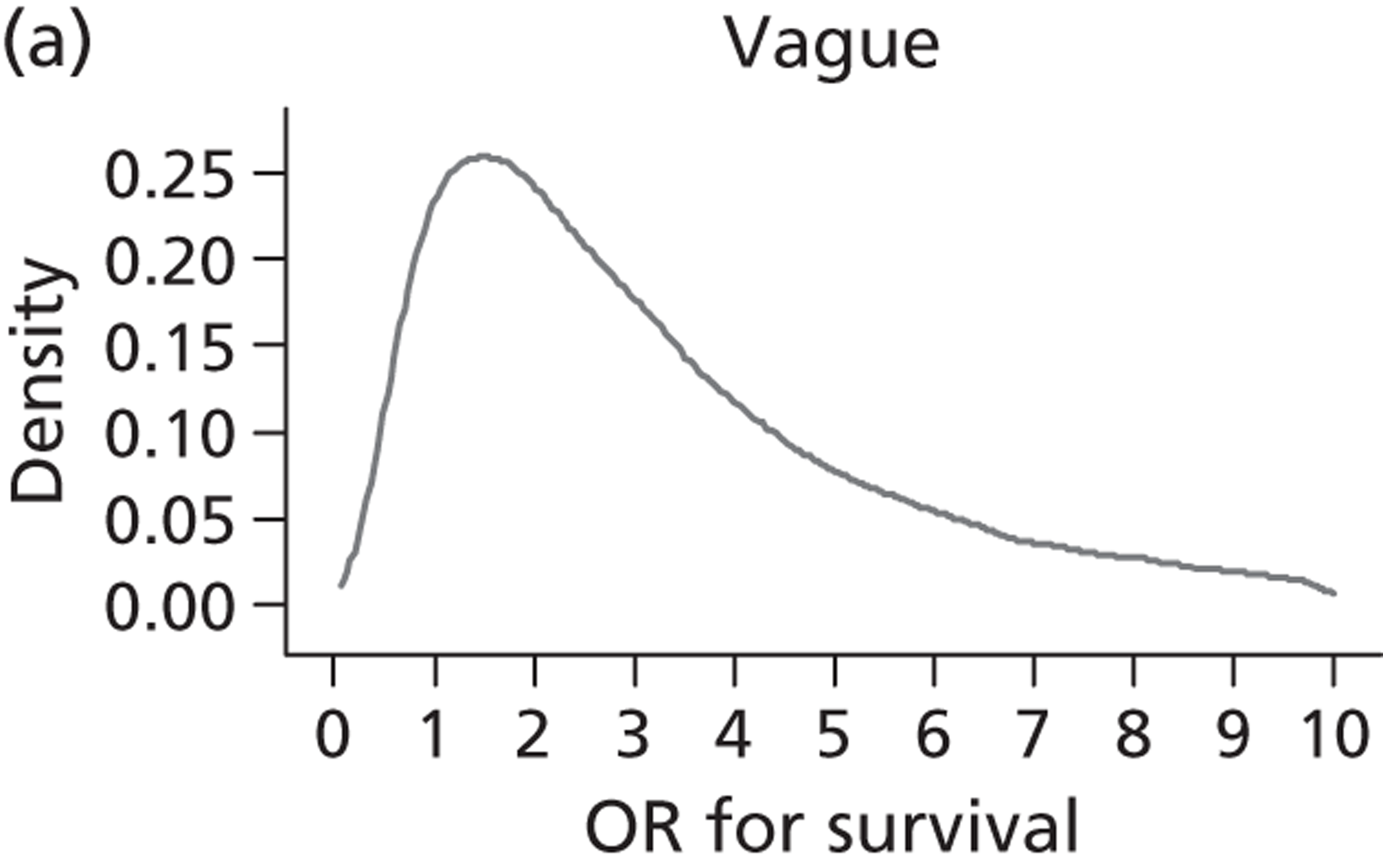
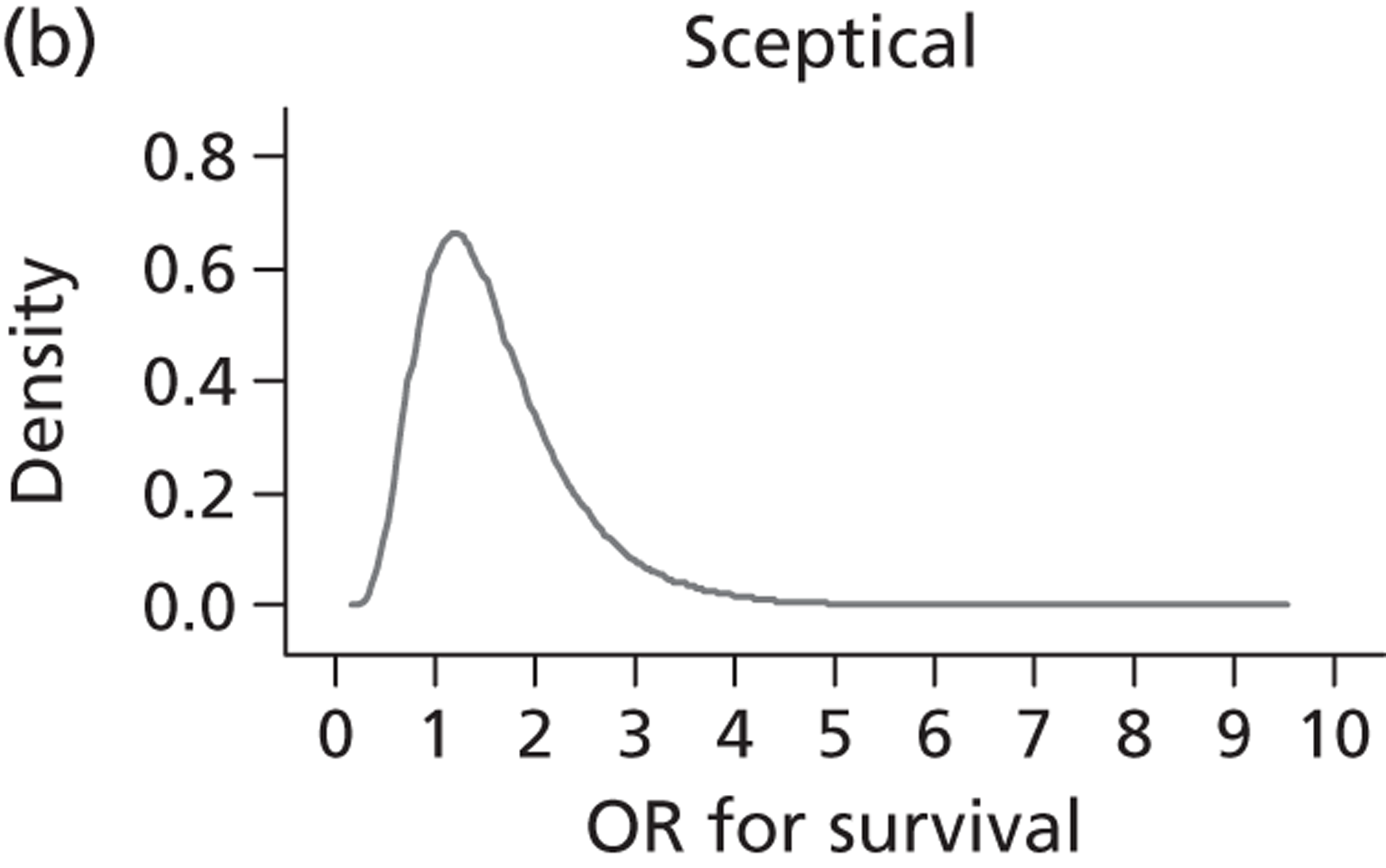
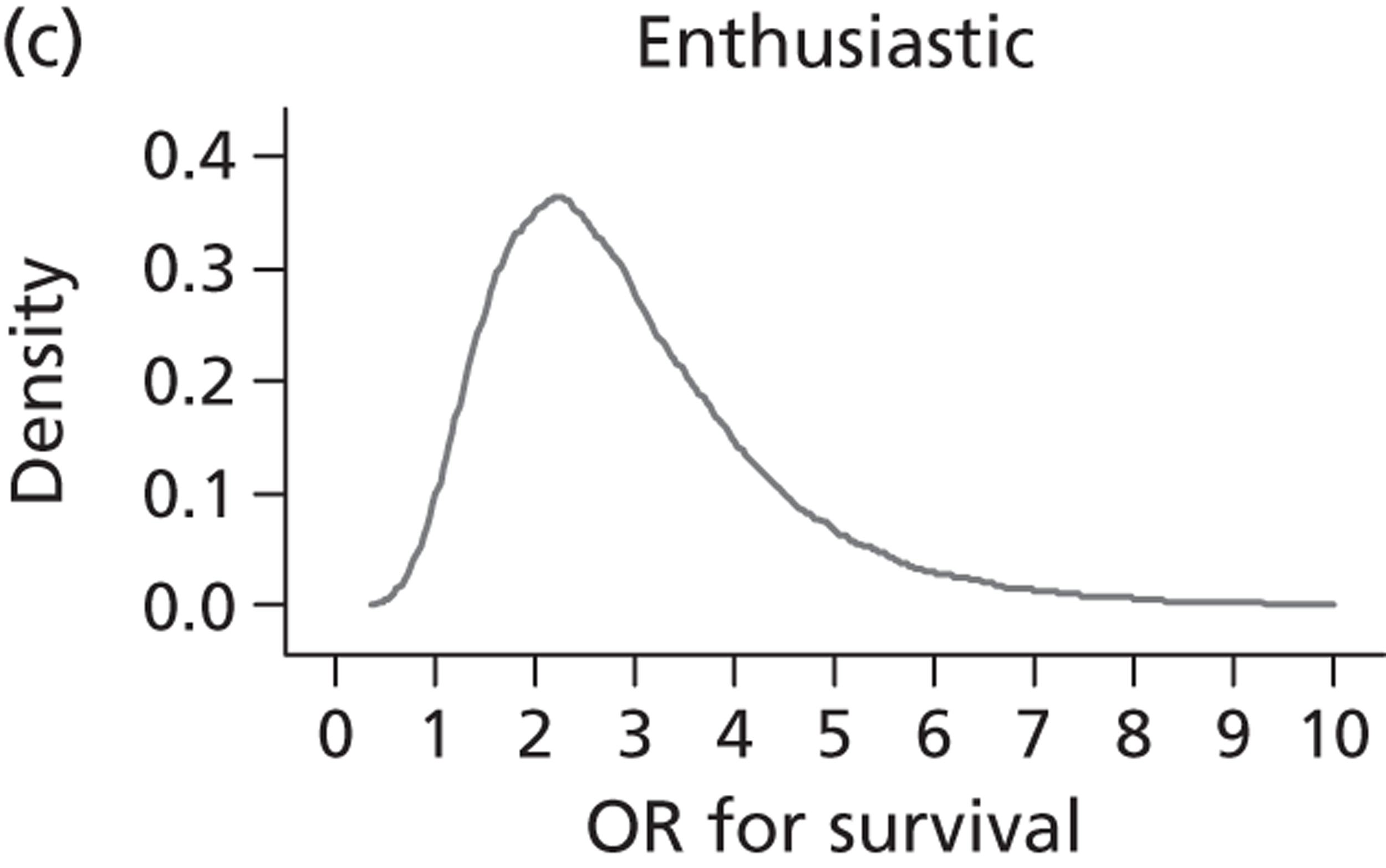
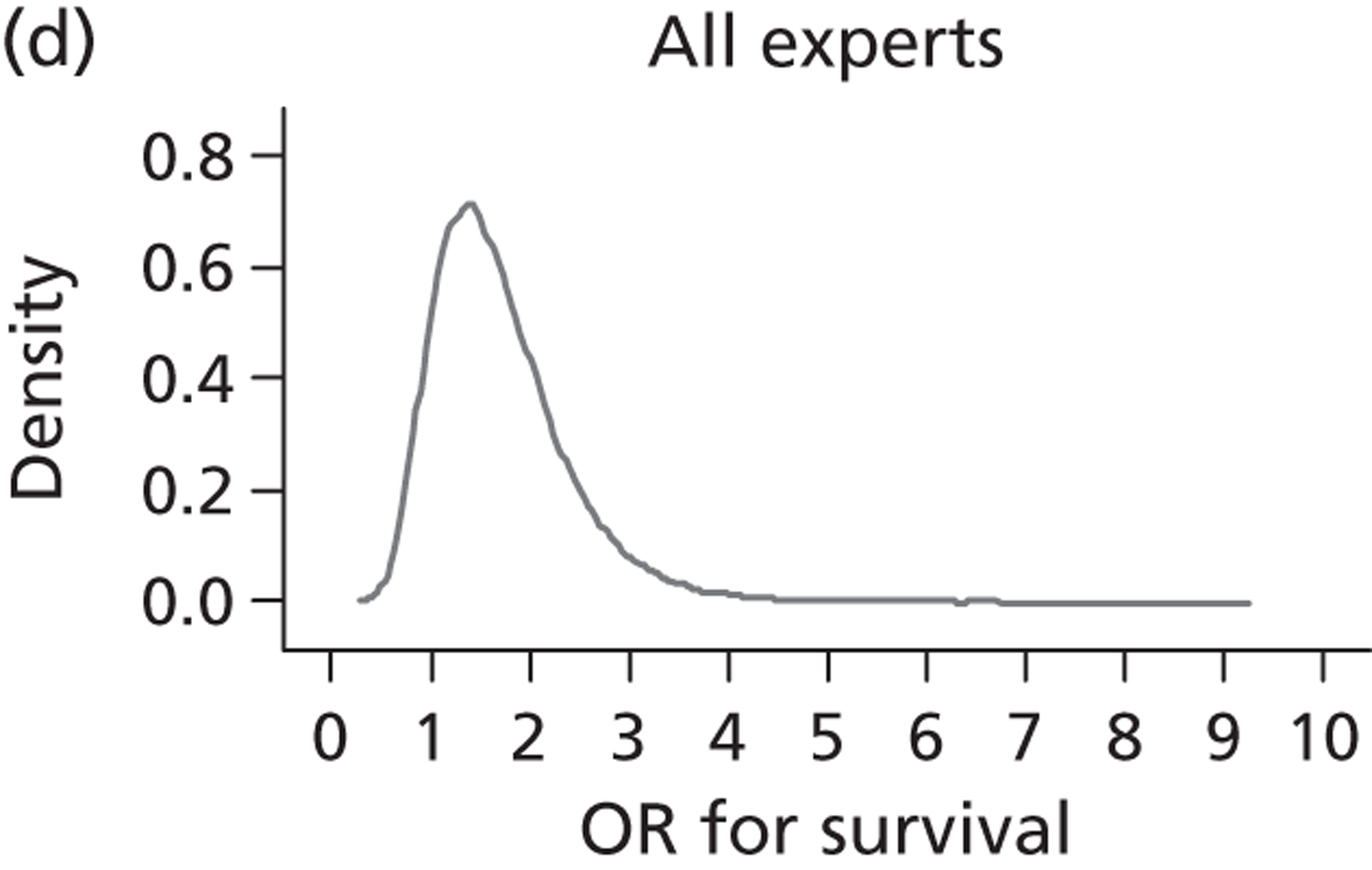
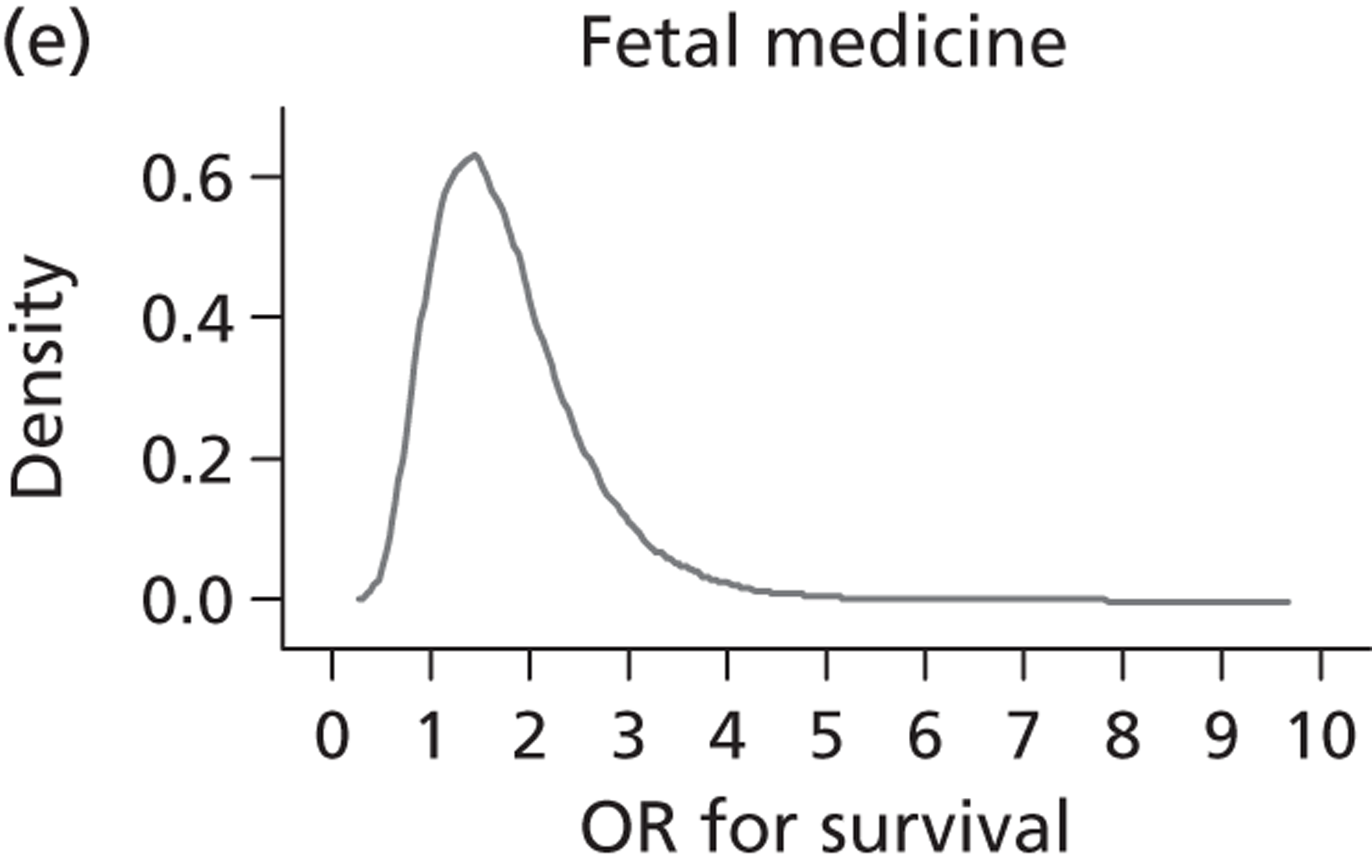
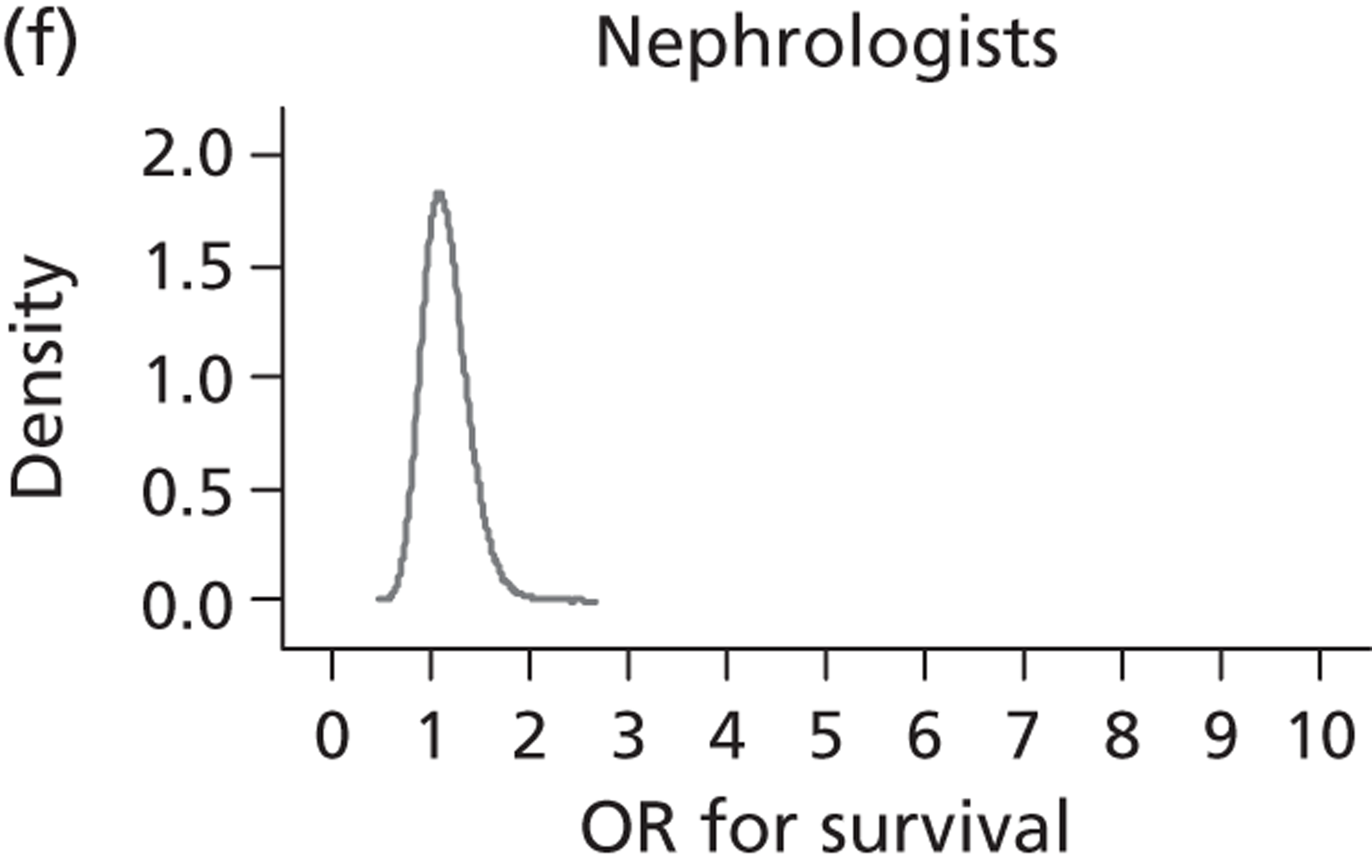
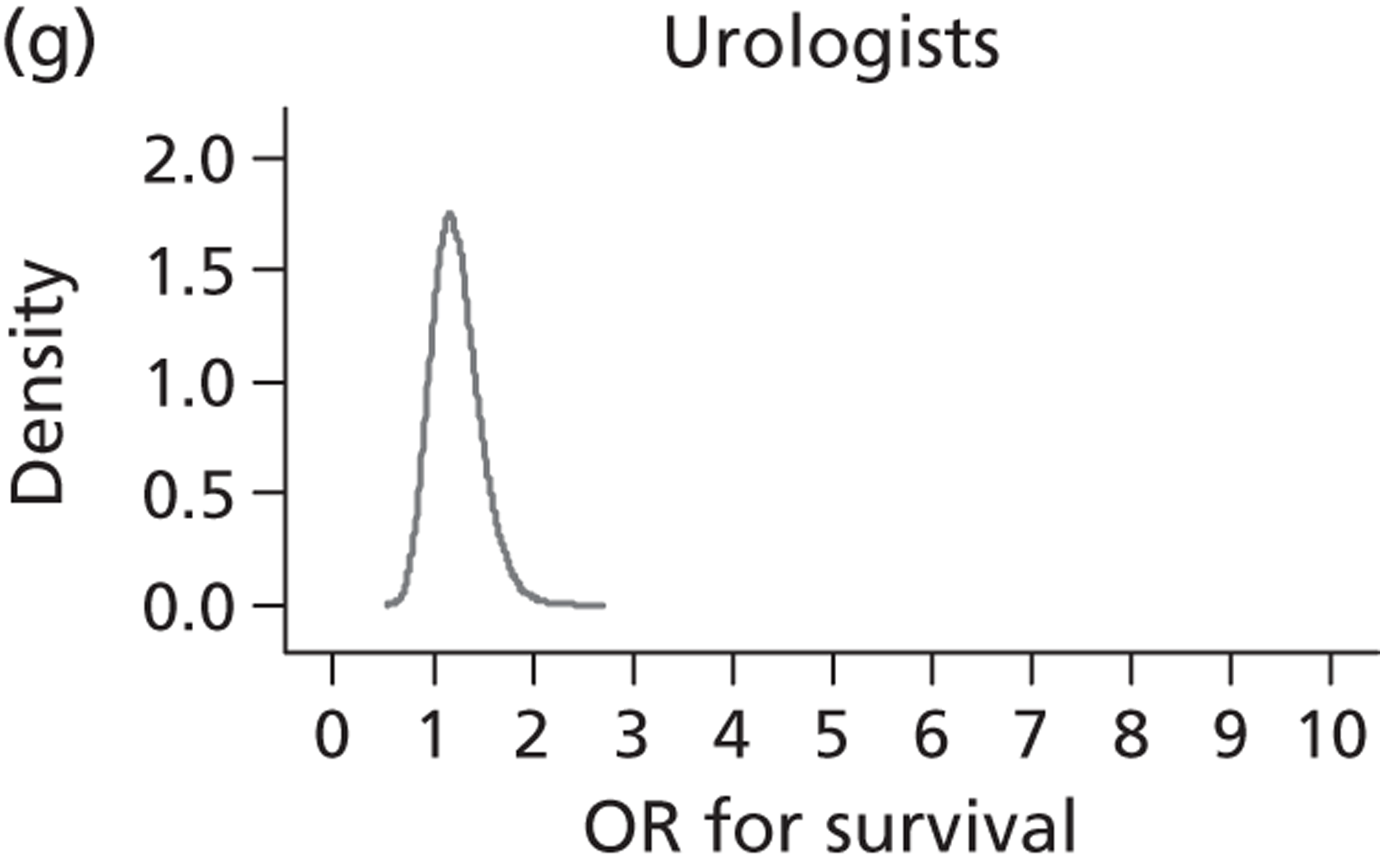
FIGURE 25.
Posterior distributions for ORs (perinatal survival) under the AT analysis.
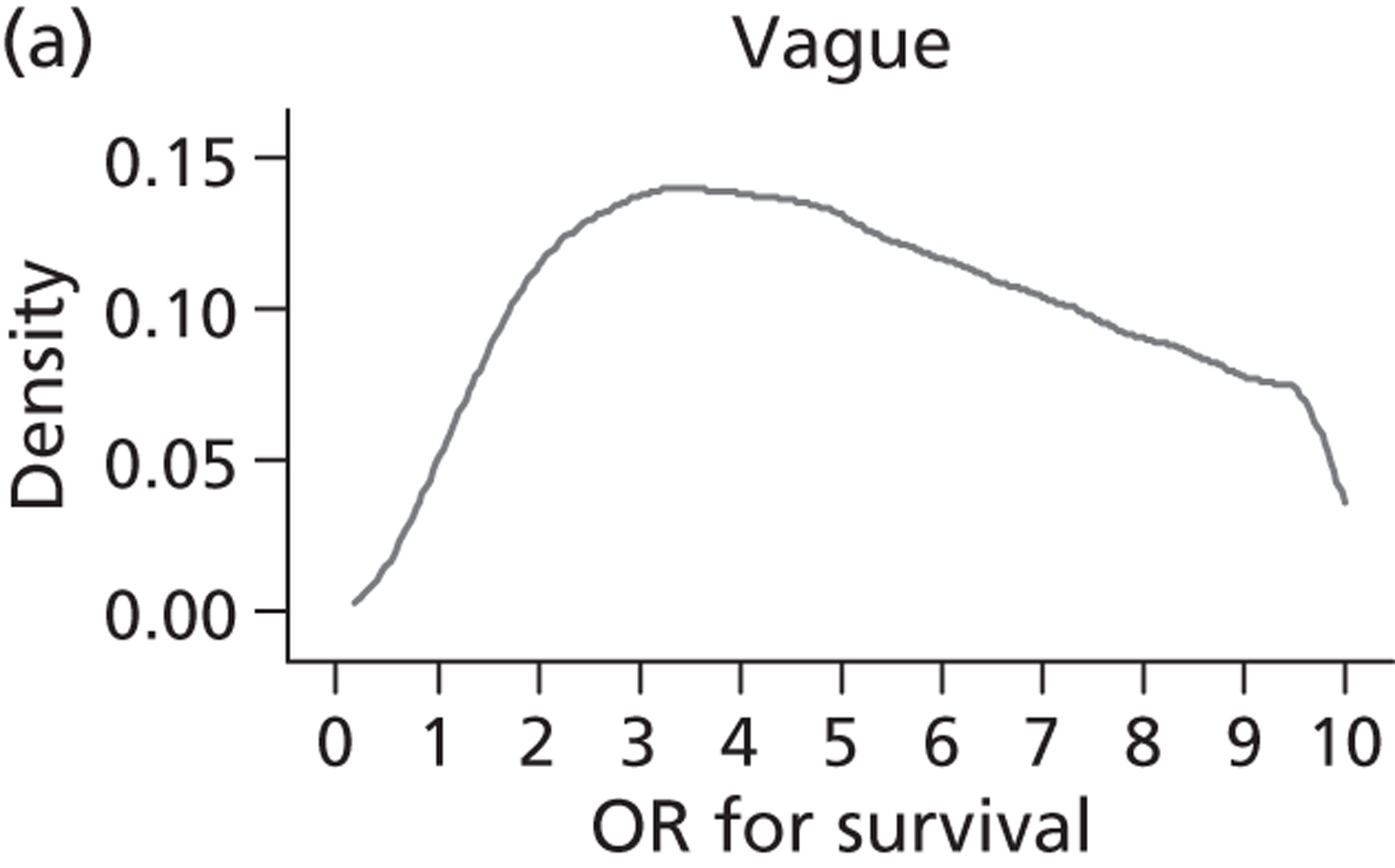
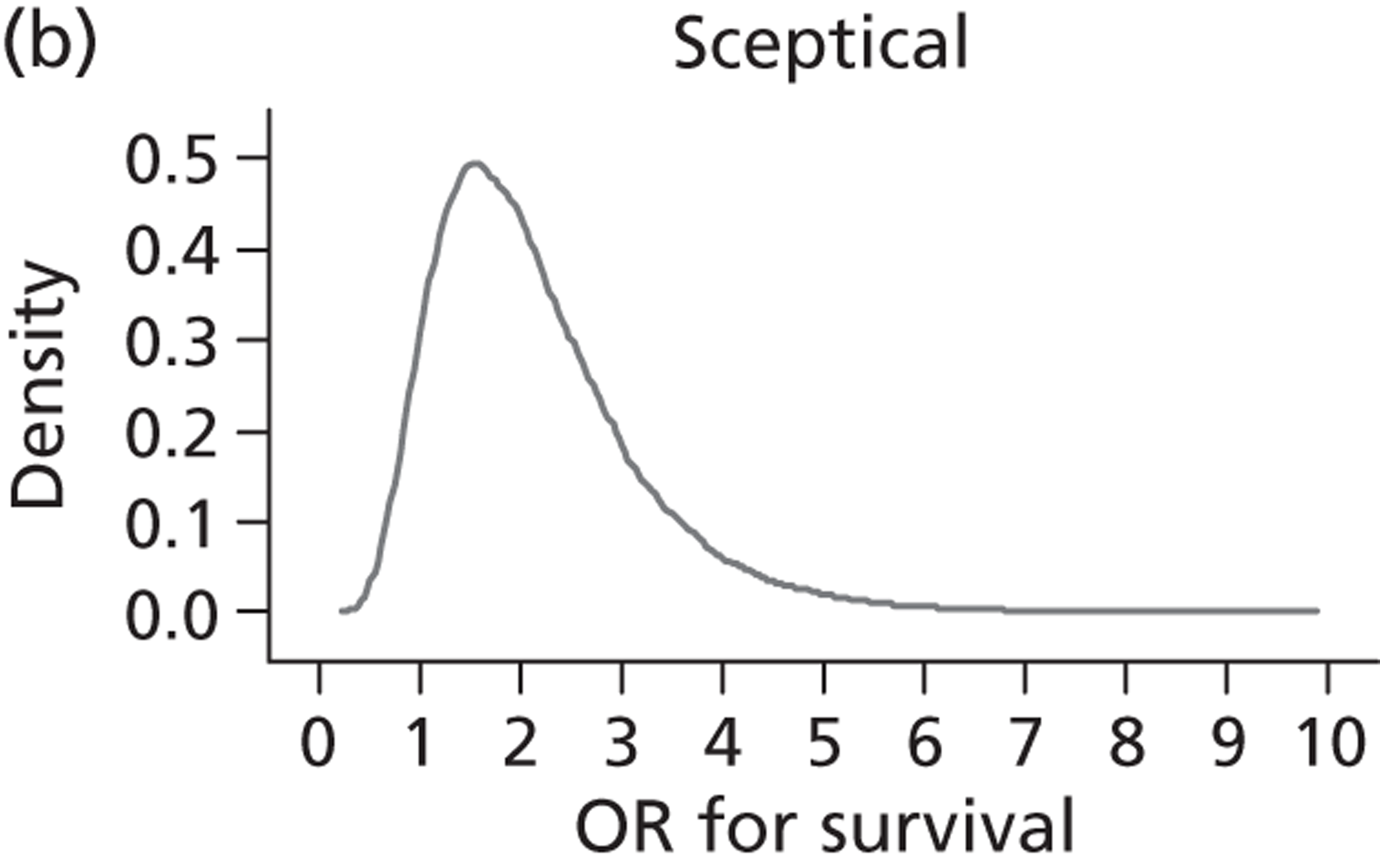
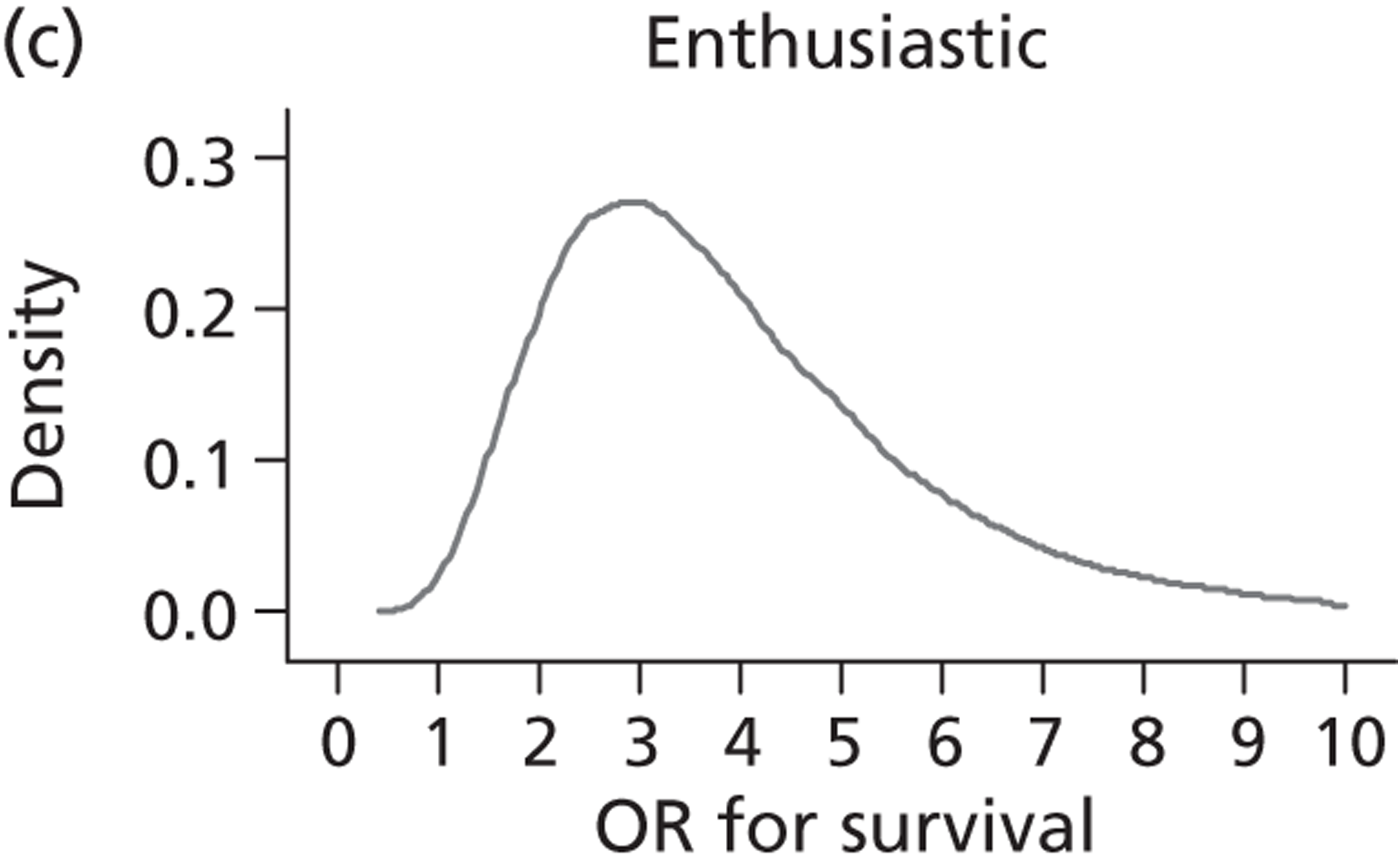
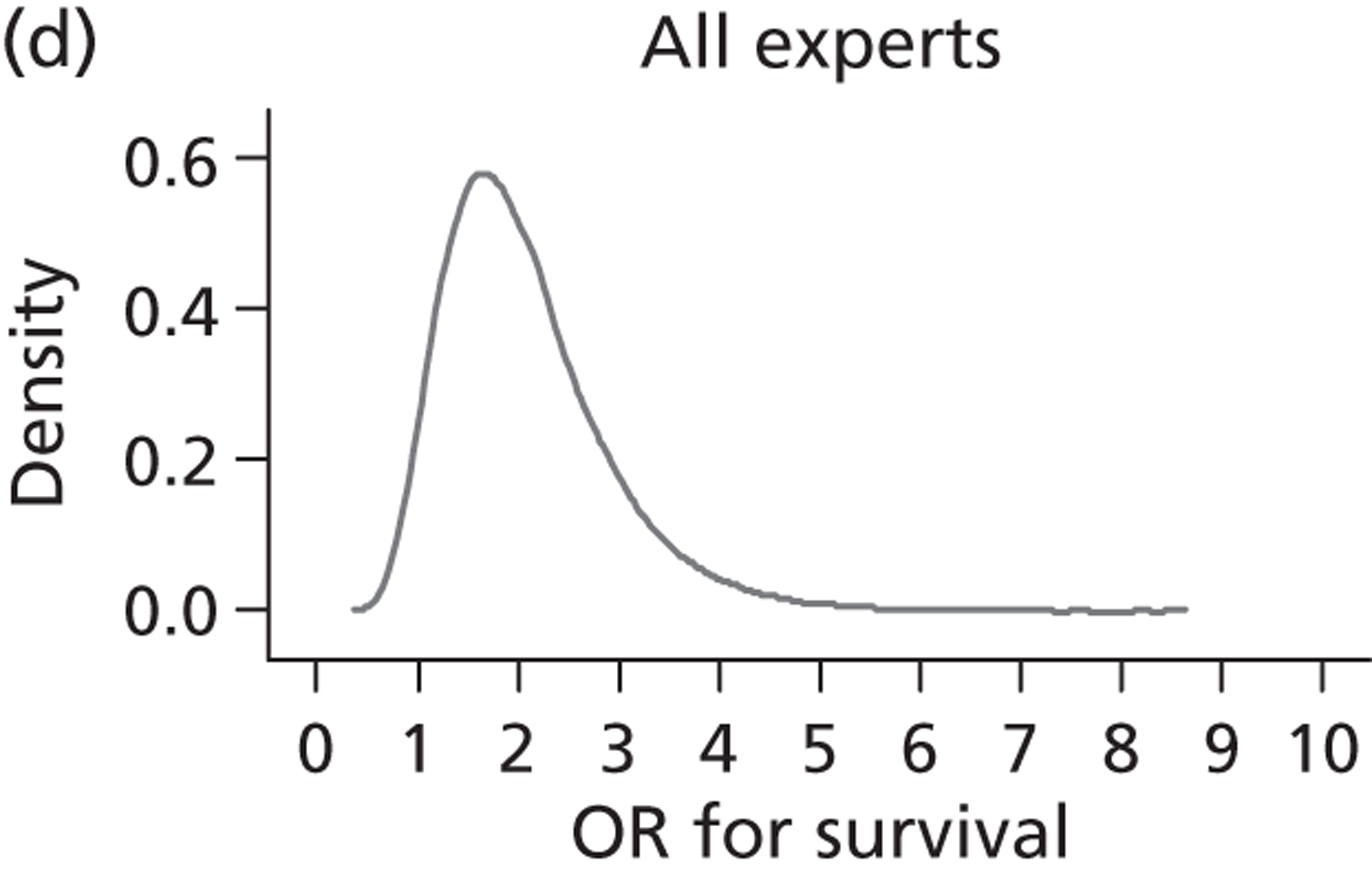
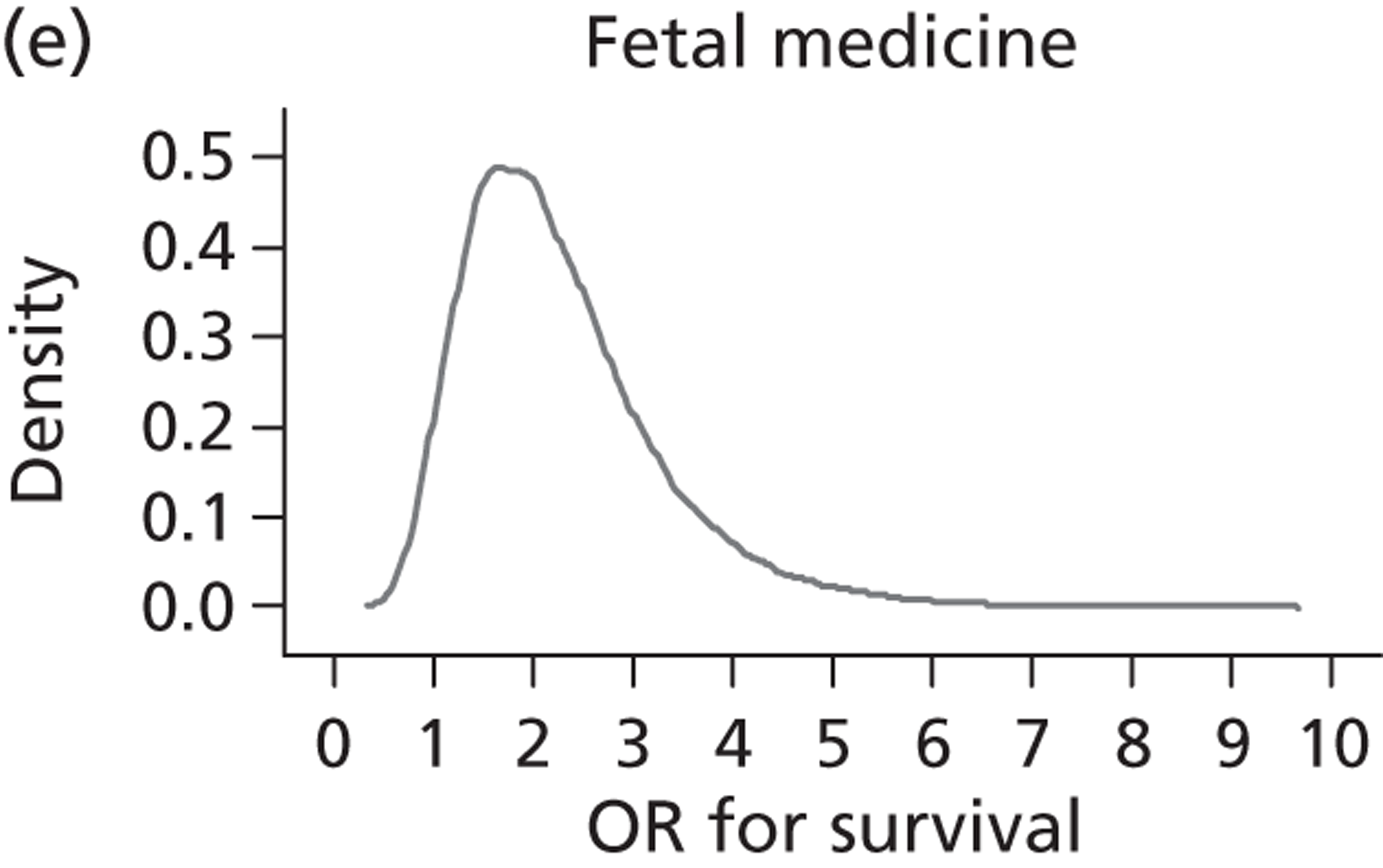
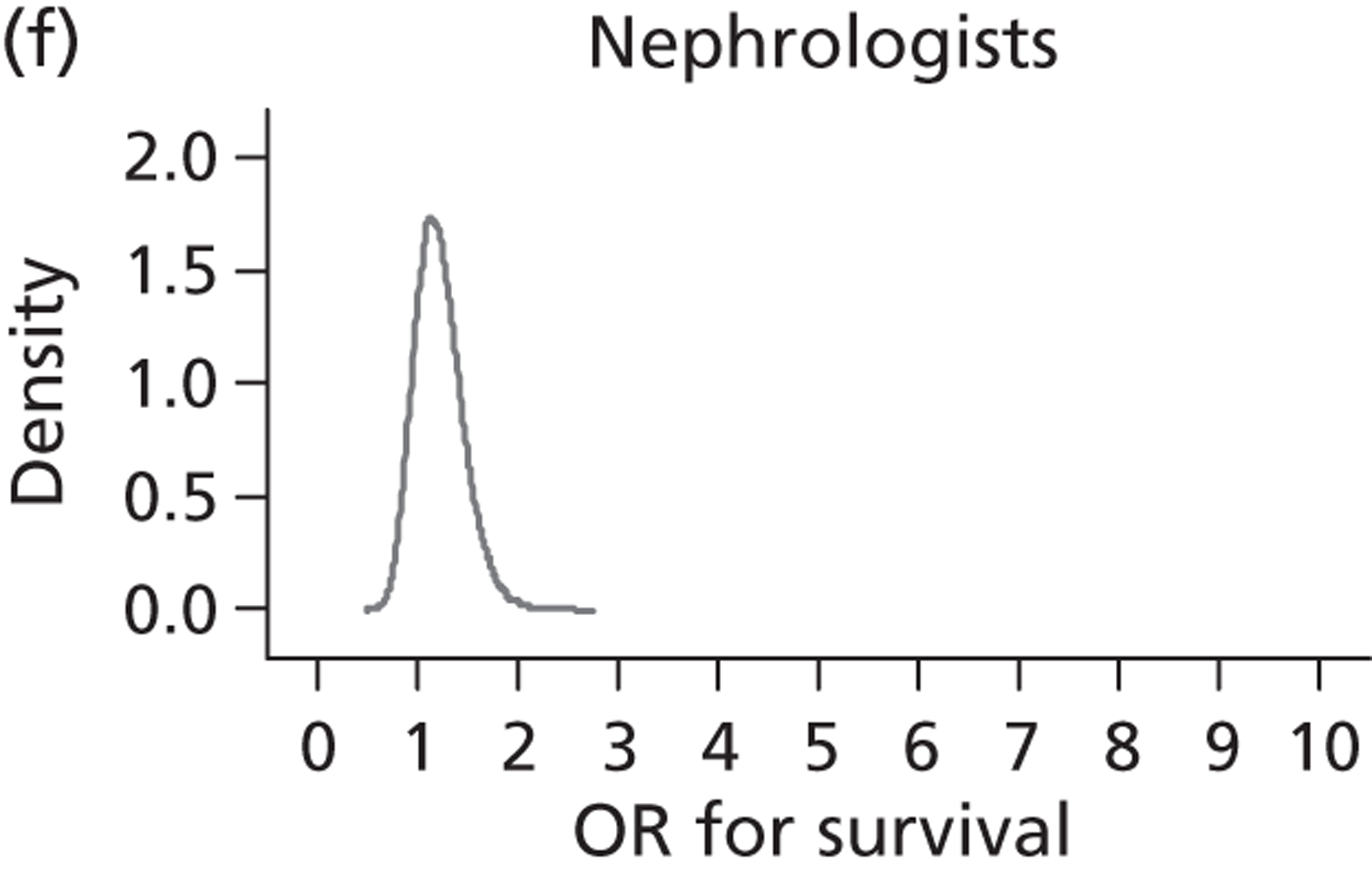
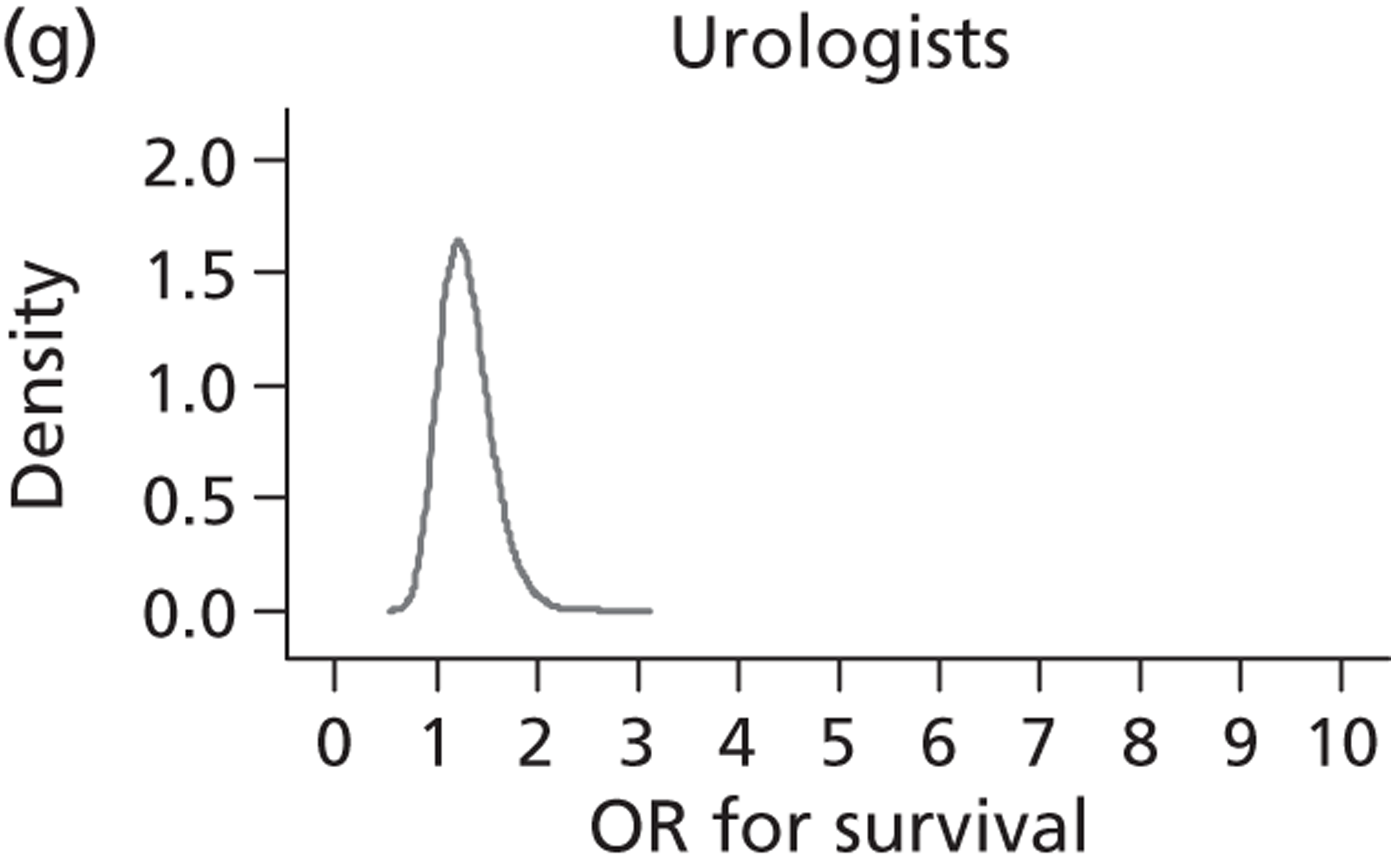
FIGURE 26.
Prior and posterior distributions (ITT) overlaid (log-OR scale). Solid line, prior; dashed line, posterior.
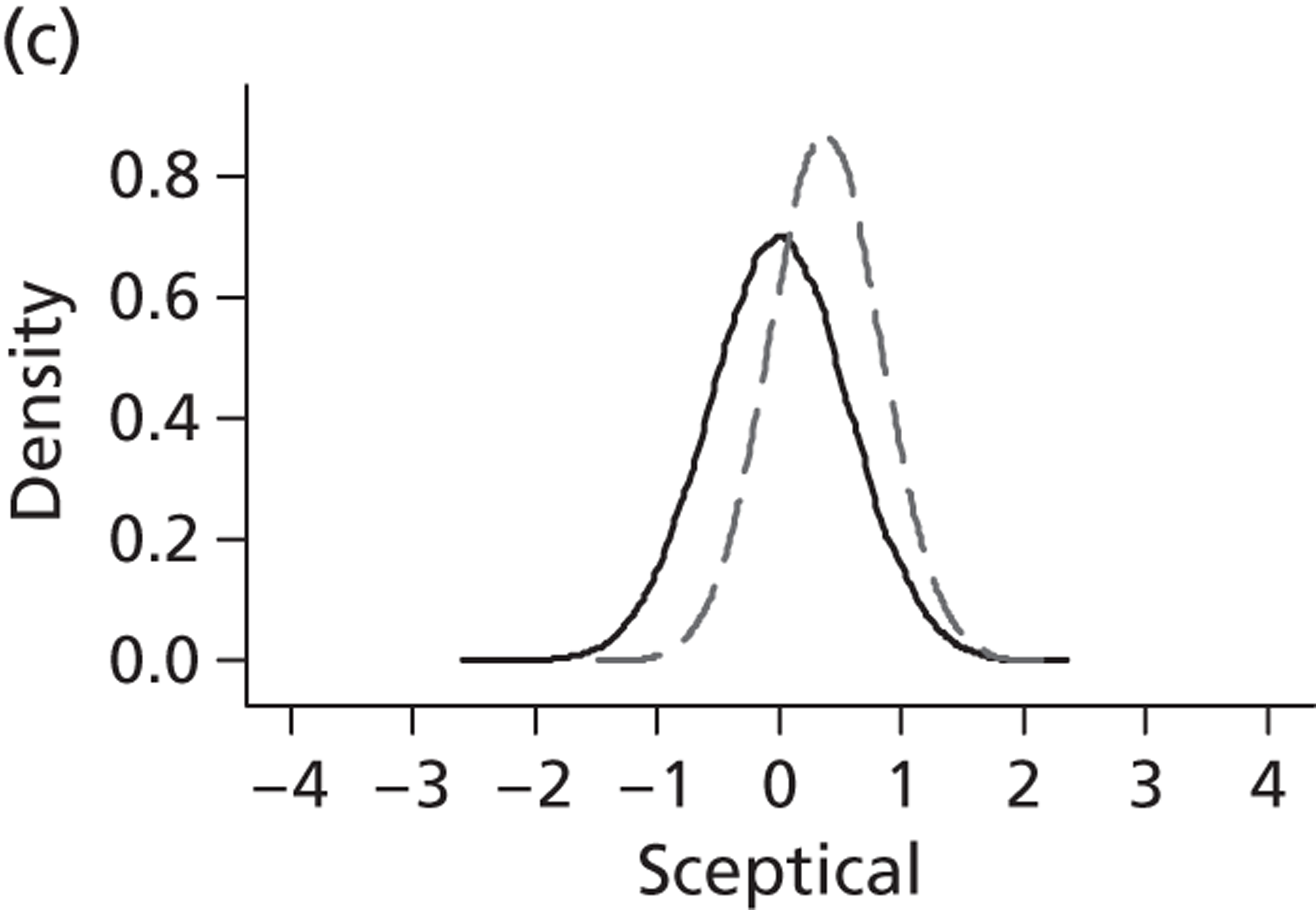
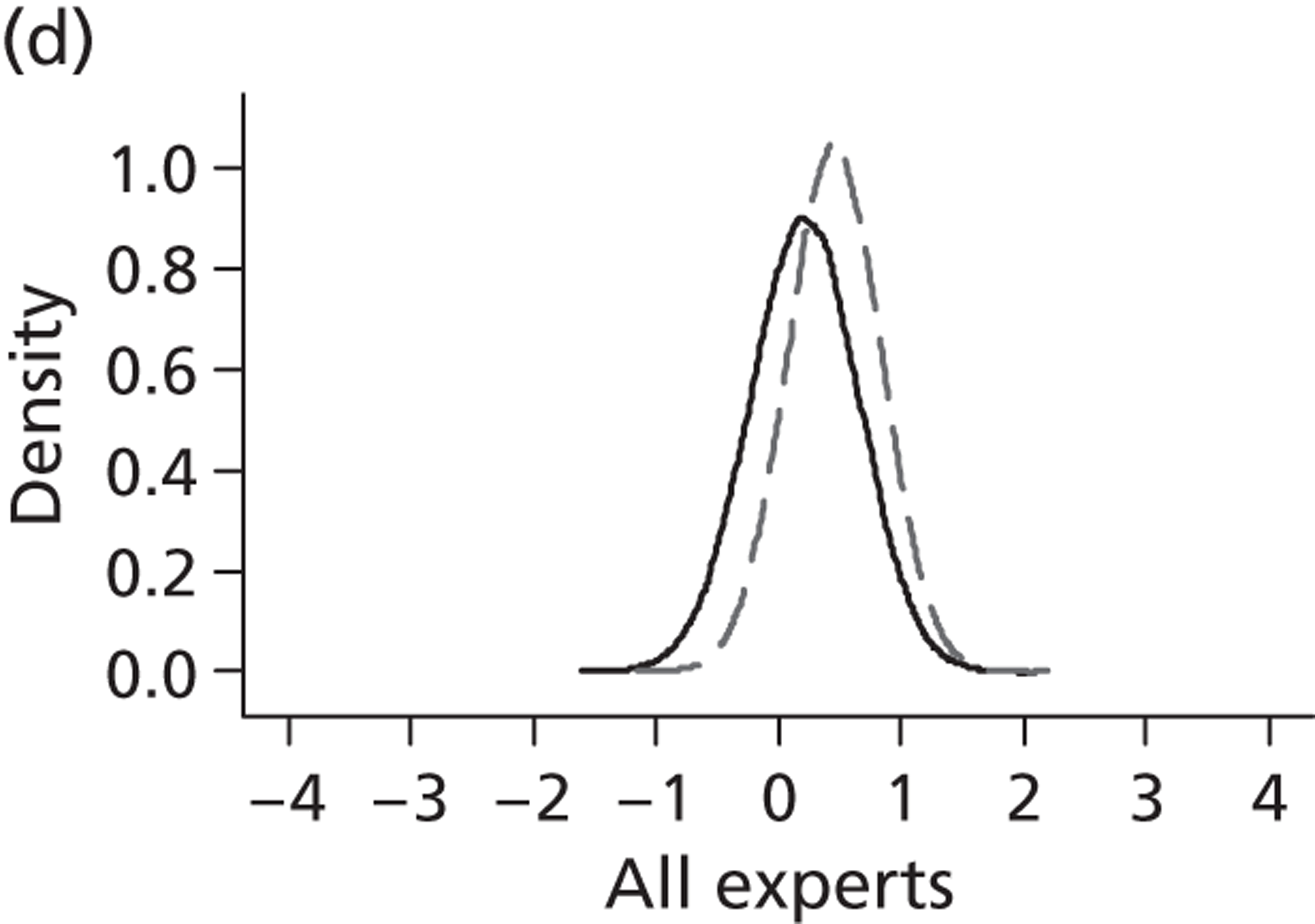
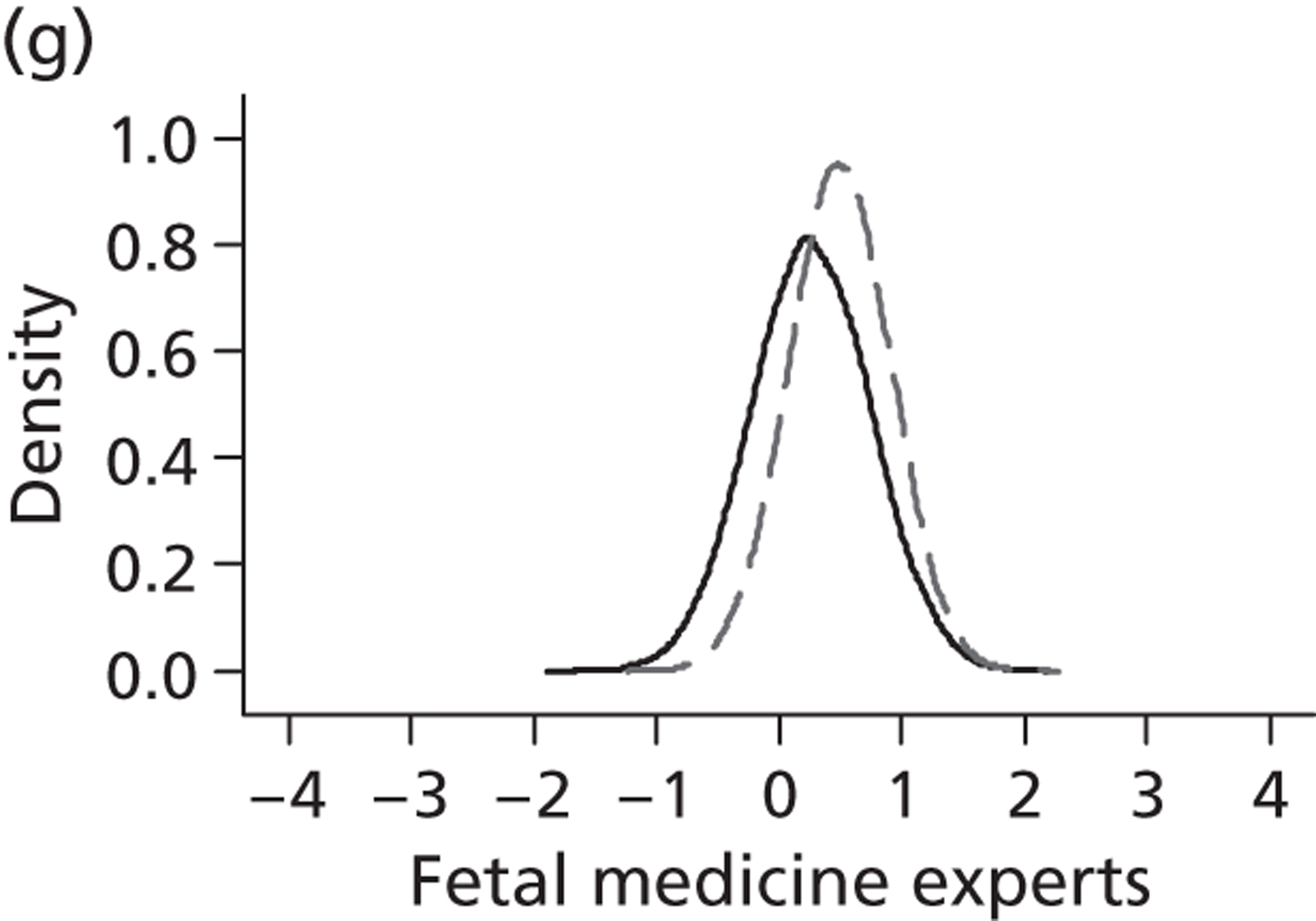
Survival analysis
Fifteen participants were randomised to the conservative management arm and 16 to the intrauterine VAS arm ( Table 31 ). Of the 15 infants randomised to conservative management, three (20%) were alive at the end of follow-up; in two cases (13%) the pregnancy had been terminated and 10 (67%) had died. These two TOPs were analysed as censored observations. Of the 16 infants randomised to the intrauterine VAS arm, seven (44%) were alive at the end of the study, in three cases (19%) the pregnancy had been terminated and six (38%) had died. One of the three TOPs was analysed as a censored observation as it was not considered to be as a result of treatment failure. The remaining two TOPs were a result of SROM within 28 days of receiving the shunt and were therefore analysed as failure of the treatment.
| Outcome | VAS, n (%) | Conservative management, n (%) | Overall, n (%) |
|---|---|---|---|
| ITT analysis | |||
| End point | |||
| Death | 6 (37.5) | 10 (66.7) | 16 (51.6) |
| TOP | 3 (18.8) | 2 (13.3) | 5 (16.1) |
| Censored | 7 (43.8) | 3 (20.0) | 10 (32.3) |
| AT analysis | |||
| End point | |||
| Death | 4 (26.7) | 12 (75.0) | 16 (51.6) |
| TOP | 3 (20.0) | 2 (12.5) | 5 (16.1) |
| Censored | 8 (53.3) | 2 (12.5) | 10 (32.3) |
In the event, two patients randomised to conservative management actually received intrauterine VAS and three patients randomised to intrauterine VAS arm actually received conservative management. Therefore, 16 patients received conservative management and 15 patients received intrauterine VAS; these form the AT analysis (see Table 31 ). Of the 15 patients who received intrauterine VAS eight (53%) were alive at the end of the study and, of the 16 patients who received conservative management, two (13%) were alive at the end of the study.
Assessing the proportional hazards assumption
The Kaplan–Meier unadjusted survival curves for the treatment arms are shown in Figure 27 . A time point of 0 corresponds to the time of conception. The probability of survival is lower for those subjects who received a VAS than for those who received conservative management for approximately the first 36.5 weeks from conception. After this time the Kaplan–Meier curves cross and the probability of survival is higher for those who received a VAS than for those who received conservative management. The time point that the survival curves cross approximately corresponds to the time of birth of the infants. Therefore, this plot suggests that the infants who received a VAS have a lower probability of survival before birth than the infants who received conservative management. However, the infants who received a VAS have a higher probability of survival than those in the conservative management arm if they survive the antenatal period.
FIGURE 27.
Kaplan–Meier plots for survival estimates by treatment arm.
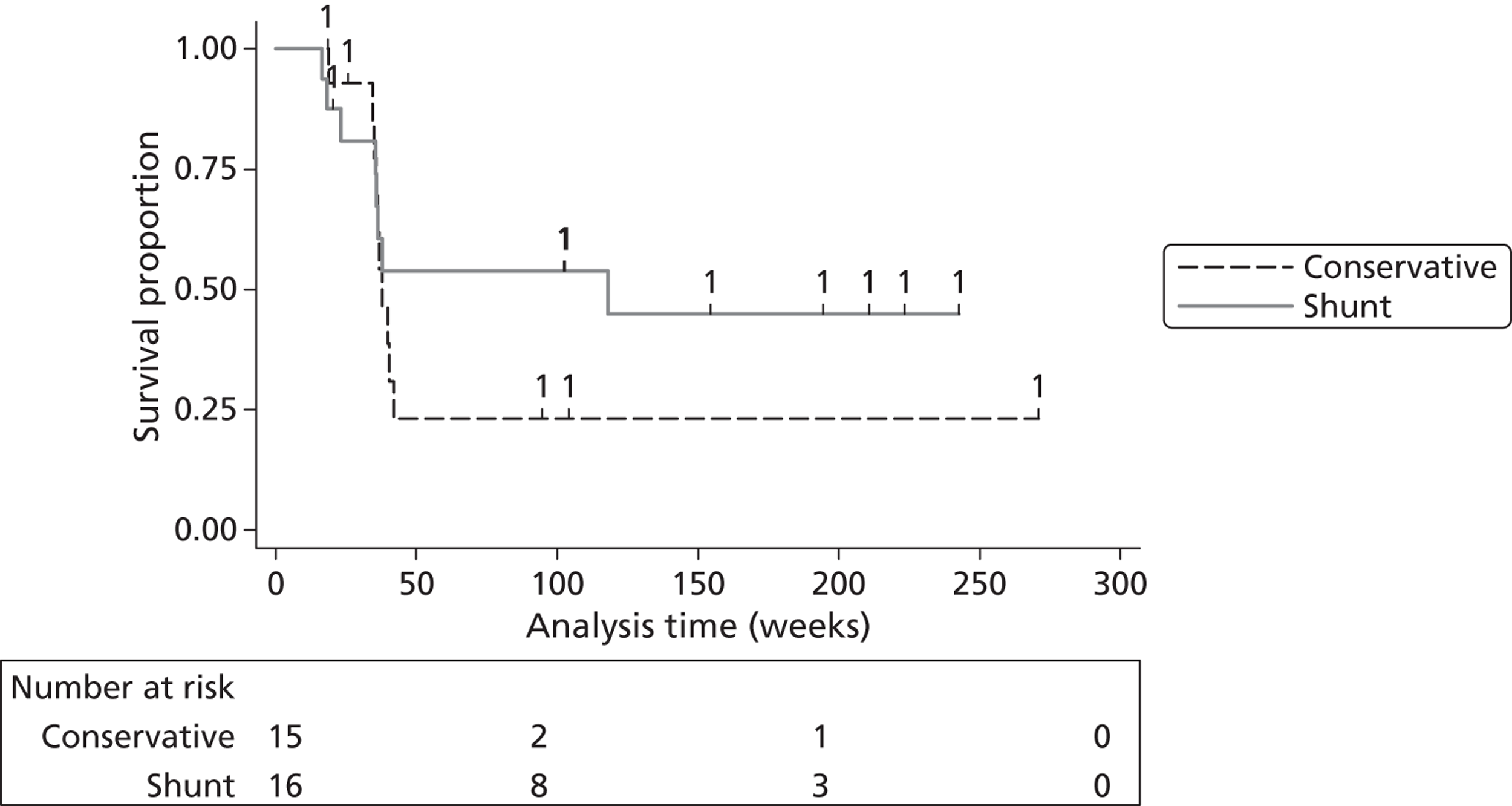
The log-cumulative hazards plot of the treatment variable is displayed in Figure 28 , which was used to assess the proportional hazards assumption. The lines cross suggesting that the assumption is violated. This is again suggestive of a harmful effect for the VAS arm for the time period of conception to approximately 36.5 weeks and a beneficial effect for the VAS arm in the time period from 36.5 weeks onwards. Therefore, the data were split at the time point of 36.5 weeks and the treatment effect was estimated in the form of two HRs.
FIGURE 28.
Testing the assumption of proportional hazards: ‘log-log’ plot.
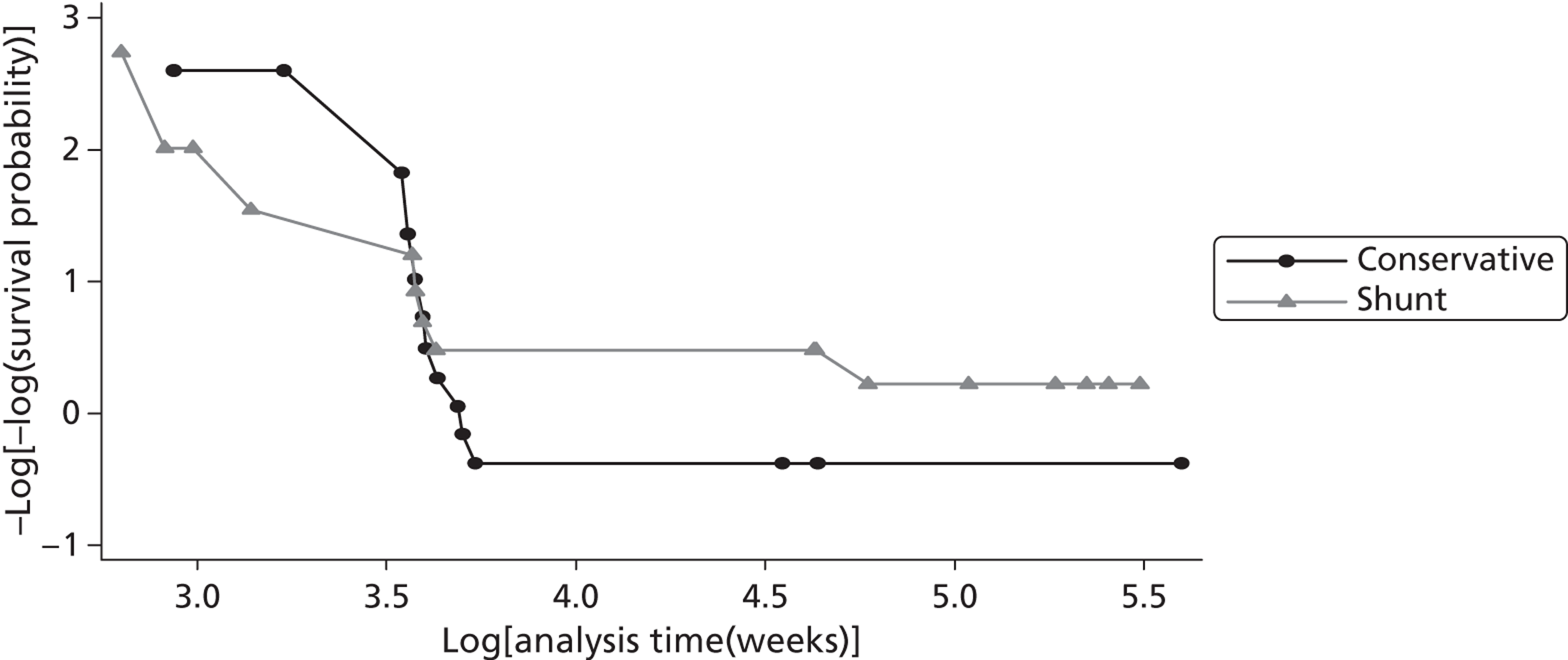
Posterior distributions for hazard ratios
Table 32 presents the results under the first assumption that the hazard rates are proportional from conception to the end of follow-up and so the HR is an estimate of the average (over the period from conception to 1 year of age) difference in mortality rates between the two groups. Table 32 presents the estimated HRs with 95% CrIs and the Bayesian posterior probabilities that the HR is > 1.55 for the three default prior distributions (uninformative, enthusiastic and sceptical).
| HR (95% CrI) | Probability HR > 1.55 | |
|---|---|---|
| ITT analysis | ||
| Uninformative prior | 1.63 (0.63 to 4.43) | 0.54 |
| Enthusiastic prior | 1.56 (0.99 to 2.47) | 0.52 |
| Sceptical prior | 1.12 (0.71 to 1.75) | 0.08 |
| AT analysis | ||
| Uninformative prior | 2.87 (1.06 to 8.63) | 0.88 |
| Enthusiastic prior | 1.76 (1.12 to 2.78) | 0.71 |
| Sceptical prior | 1.26 (0.80 to 1.99) | 0.19 |
For clarity, it is reiterated that the HR comparison is conservative care compared with VAS. This is so that the effect estimate on the HR scale will be consistent with RR estimates in so far as treatment effects > 1 will favour the intervention.
Under the ITT analysis and uninformative prior, the estimated HR is 1.63 (95% CrI 0.63 to 4.43) with a probability that the HR is > 1.55 estimated to be 0.54 (i.e. 54%). Perhaps surprisingly, the median estimated HR under the enthusiastic prior is a little closer to the null (HR 1.56); however, the CrI (95% CrI 0.99 to 2.47) is narrower than that for the uninformative results. Under the enthusiastic prior the probability that the HR is > 1.55 (favours treatment) is 0.52. As expected, the sceptical prior pulls the HR even closer towards the null and the 95% CrI includes values that favour the conventional treatment (HR 1.12, 95% CrI 0.71 to 1.75). The probability that the HR is > 1.55 reduces to 0.08.
Under the AT analysis all estimates of the HR show a greater preference for the intervention and the probabilities that the HR is > 1.55 are increased (compared with the ITT analysis). However, the 95% CrIs are wider indicating less certainty and the 95% CrI for the sceptical prior still includes both positive and harmful effects.
A plot of the posterior distributions under the three prior assumptions for the ITT analysis is shown in Figure 29 . Figure 30 shows the prior distributions overlaid with the updated posterior distributions.
FIGURE 29.
Posterior distributions for the HRs under the ITT analysis assuming proportional hazard rates.
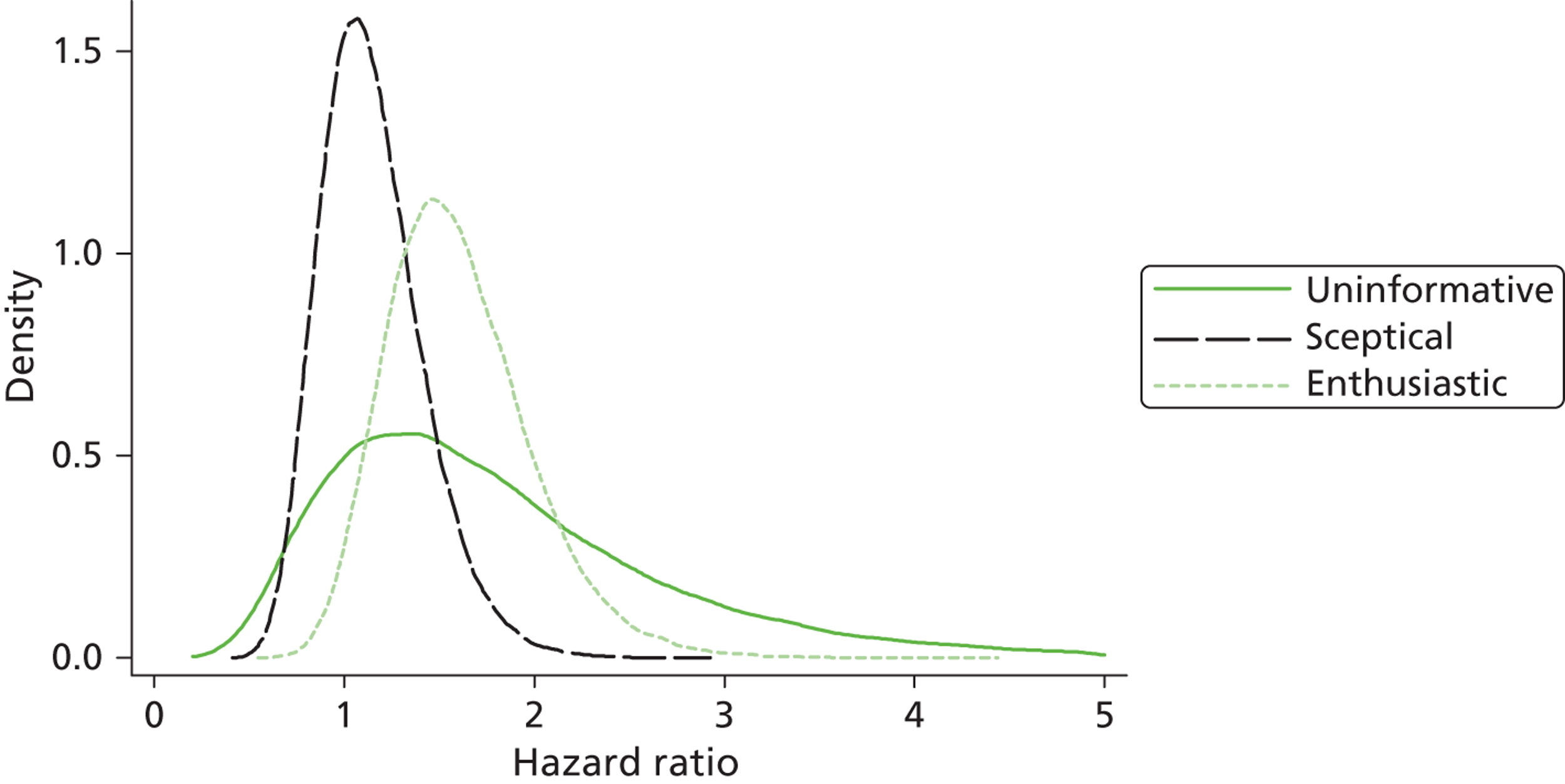
FIGURE 30.
Prior and posterior distributions for HRs (ITT) overlaid, assuming proportional hazard rates. (a) Posterior distribution; (b) posterior distribution (dashed line) and sceptical prior distribution (solid line); (c) posterior distribution (dashed line) and enthusiastic prior distribution (solid line).
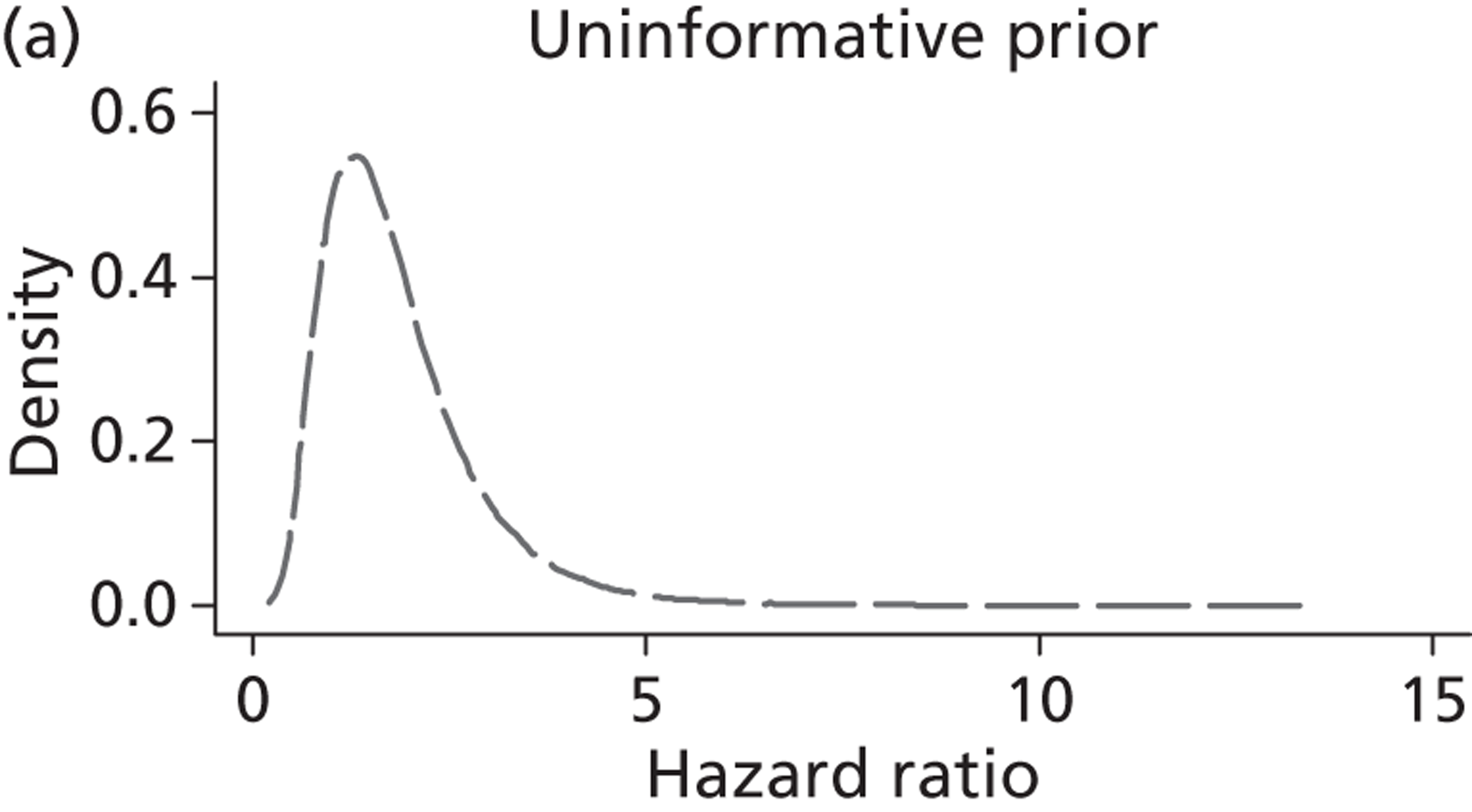
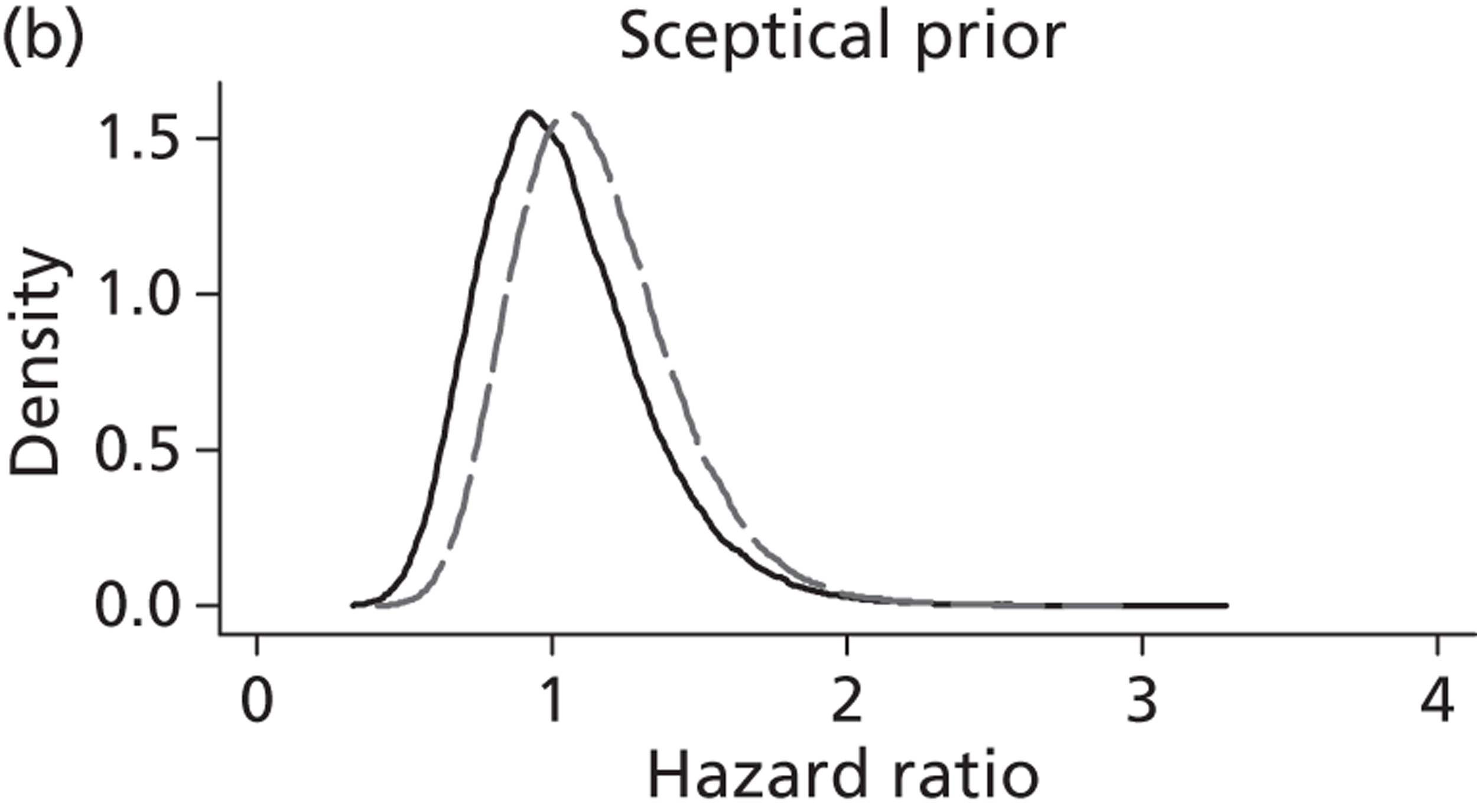
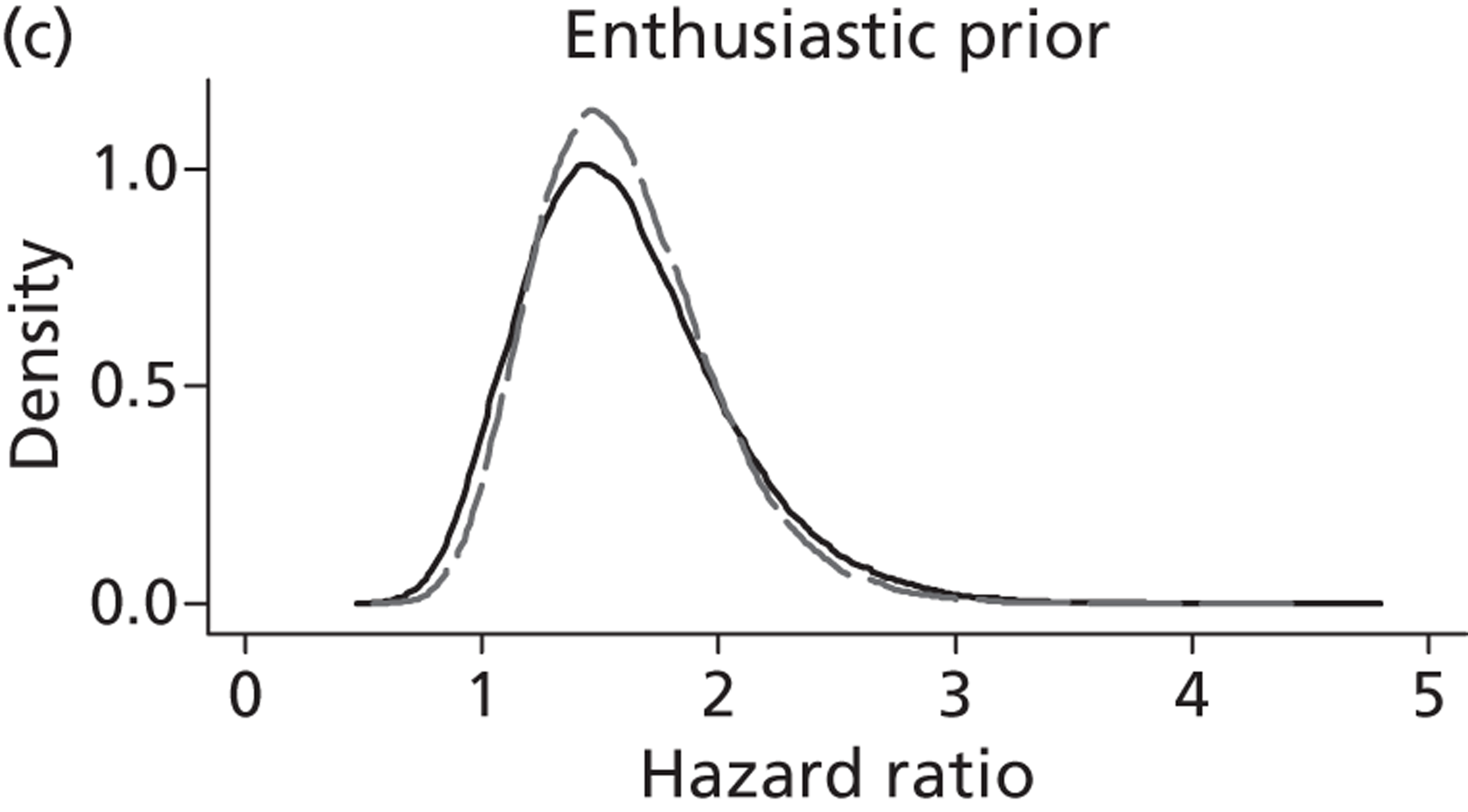
Table 33 presents the results under the assumption of a time-dependent treatment effect. Two HRs estimate the difference in mortality rates between the two intervention groups during two time periods. HR1 is the estimate of the treatment effect between conception and 36.5 weeks and HR2 is the estimate of the treatment effect between 36.5 weeks and the end of follow-up. Table 33 includes the median estimate of both HRs with their 95% CrIs and the probability that the HRs are > 1.55. Once again, a HR > 1 favours treatment.
| HR1a (95% CrI) | Probability HR1 > 1.55 | HR2b (95% CrI) | Probability HR2 > 1.55 | |
|---|---|---|---|---|
| ITT analysis | ||||
| Uninformative prior | 0.90 (0.25 to 3.04) | 0.19 | 4.73 (0.96 to 37.13) | 0.91 |
| Enthusiastic prior | 1.42 (0.88 to 2.29) | 0.37 | 1.72 (1.05 to 2.80) | 0.66 |
| Sceptical prior | 0.99 (0.62 to 1.59) | 0.03 | 1.16 (0.71 to 1.90) | 0.13 |
| AT analysis | ||||
| Uninformative prior | 1.75 (0.51 to 6.84) | 0.58 | 6.17 (1.25 to 49.68) | 0.95 |
| Enthusiastic prior | 1.57 (0.98 to 2.54) | 0.53 | 1.77 (1.09 to 2.88) | 0.71 |
| Sceptical prior | 1.09 (0.68 to 1.76) | 0.07 | 1.19 (0.73 to 1.96) | 0.15 |
Under the ITT analysis, and for all prior assumptions, the estimate for HR2 suggests that the intervention has a higher survival rate in proportion to conservative management, compared with the estimate for HR1. The probability that the HR is > 1.55 is greater for the second time period than the first time period.
Under the uninformative prior the estimate of HR1 is 0.90 (95% CrI 0.25 to 3.04) and the probability of 0.19 (19%) that this HR is > 1.55. The estimate of HR1 under the enthusiastic prior is closer to the null (1.42, 95% CrI 0.88 to 2.29) with a probability of 0.37 that this HR is > 1.55. Under the sceptical prior the estimate of HR1 is even closer to the null at 0.99 (95% CrI 0.62 to 1.59) with a probability of 0.03 that HR1 is > 1.55.
Under the uninformative prior the estimate of HR2 is 4.73 (95% CrI 0.96 to 37.13) and the probability that this HR is > 1.55 is 0.91. Under the enthusiastic prior the estimate of HR2 is closer to the null (1.72); however, the CrI is narrower (95% CrI 1.05 to 2.80) and the probability that this HR2 is > 1.55 is 0.66. For the sceptical prior the estimate of HR2 is even closer to the null (1.16, 95% CrI 0.71 to 1.90) with a probability of 0.13 that HR2 is > 1.55.
Under the AT analysis (see Table 33 ) all of the estimates of the HRs show a greater preference for the intervention for both time periods and the probabilities that both HRs are > 1.55 are increased (compared with the ITT analysis). Again, the 95% CrIs are wider indicating less certainty.
A plot of the posterior distributions for HR1 under the three default prior distributions and the ITT analysis is shown in Figure 31 . Figure 32 displays the individual prior distributions overlaid with their updated posterior distributions for the estimate of HR1. Figures 33 and 34 are the corresponding plots for the estimate of HR2.
FIGURE 31.
Posterior distributions for HR1 (period conception to 36.5 weeks) under the ITT analysis.
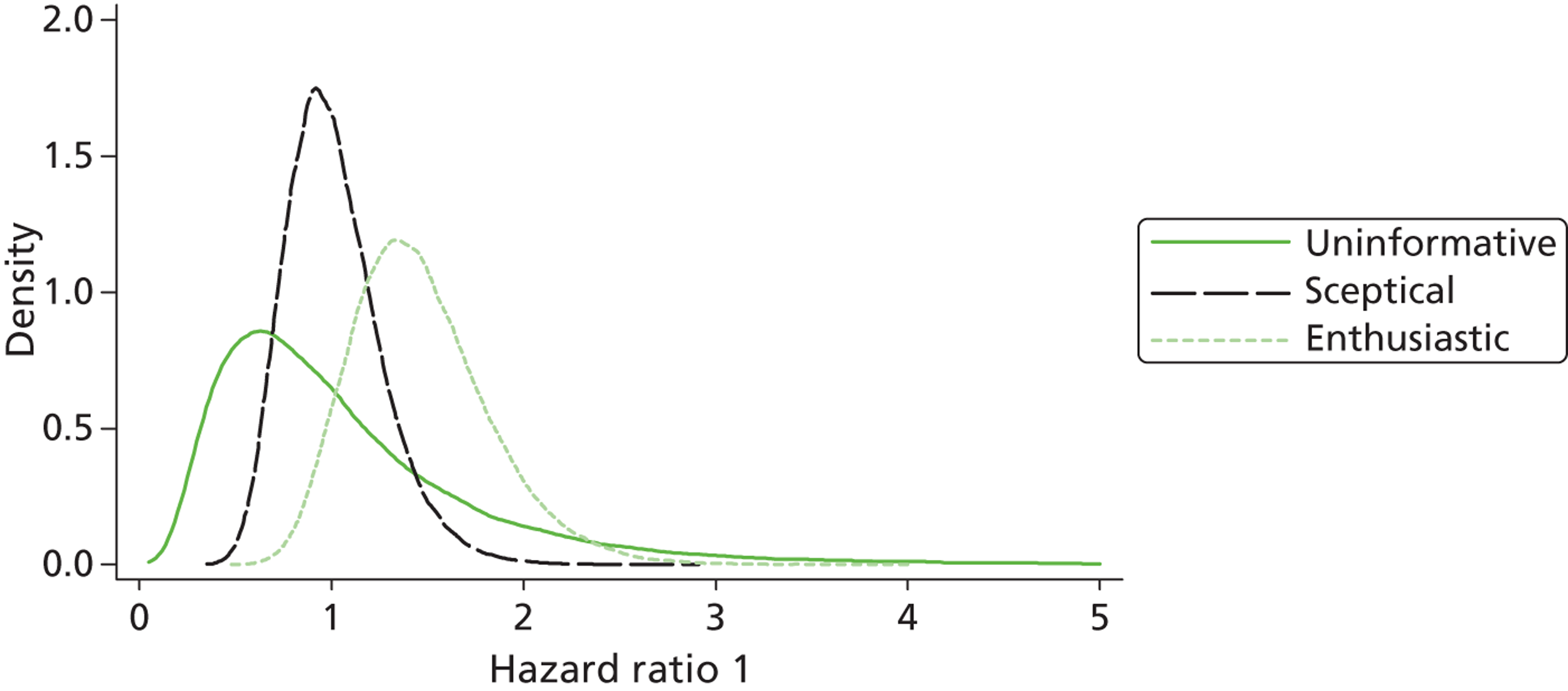
FIGURE 32.
Prior and posterior distributions for HR1 (period conception to 36.5 weeks) (ITT) overlaid. (a) Posterior distribution; (b) posterior distribution (dashed line), sceptical prior distribution (solid line); and (c) posterior distribution (dashed line), enthusiastic prior distribution (solid line).
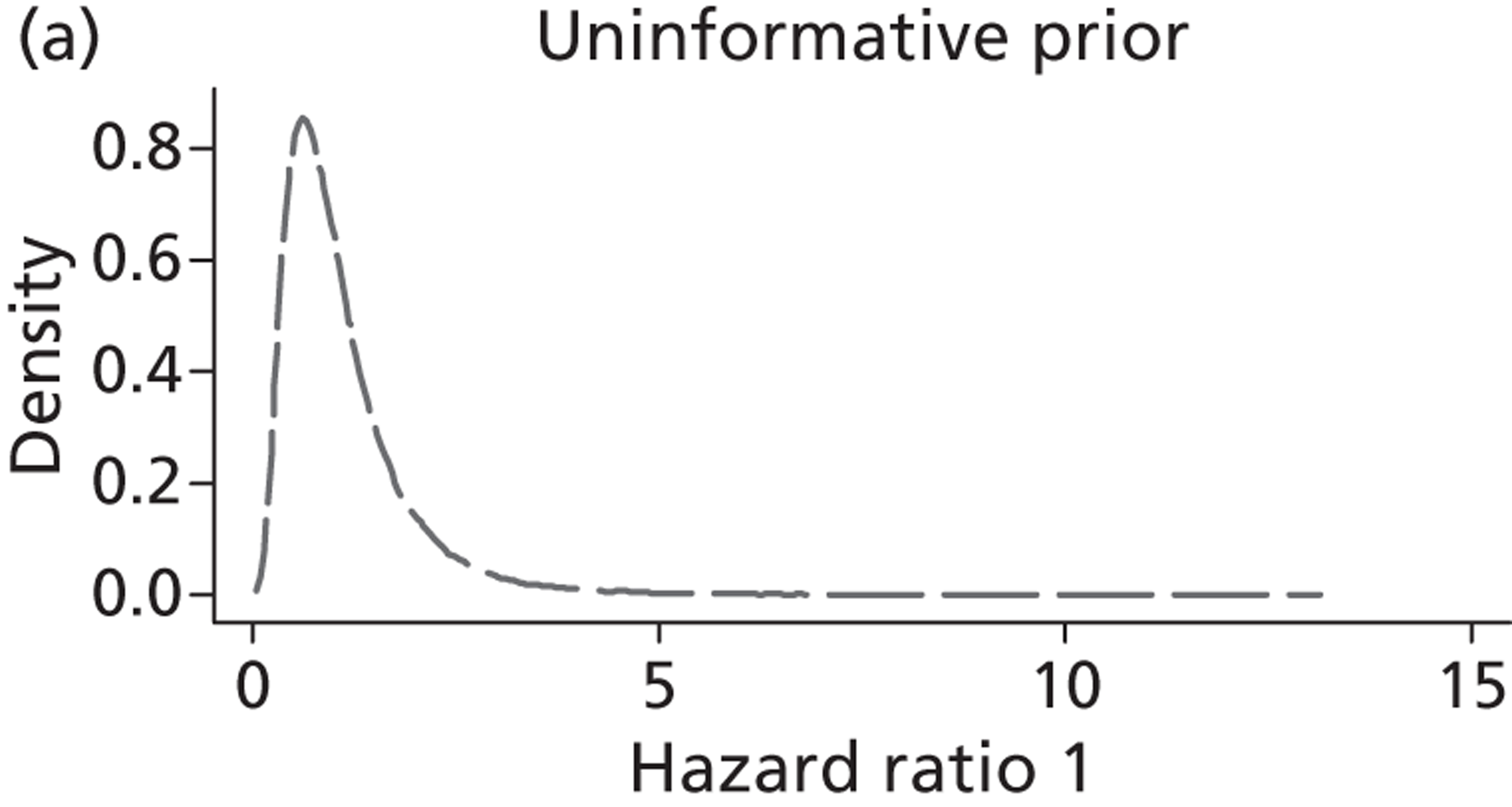
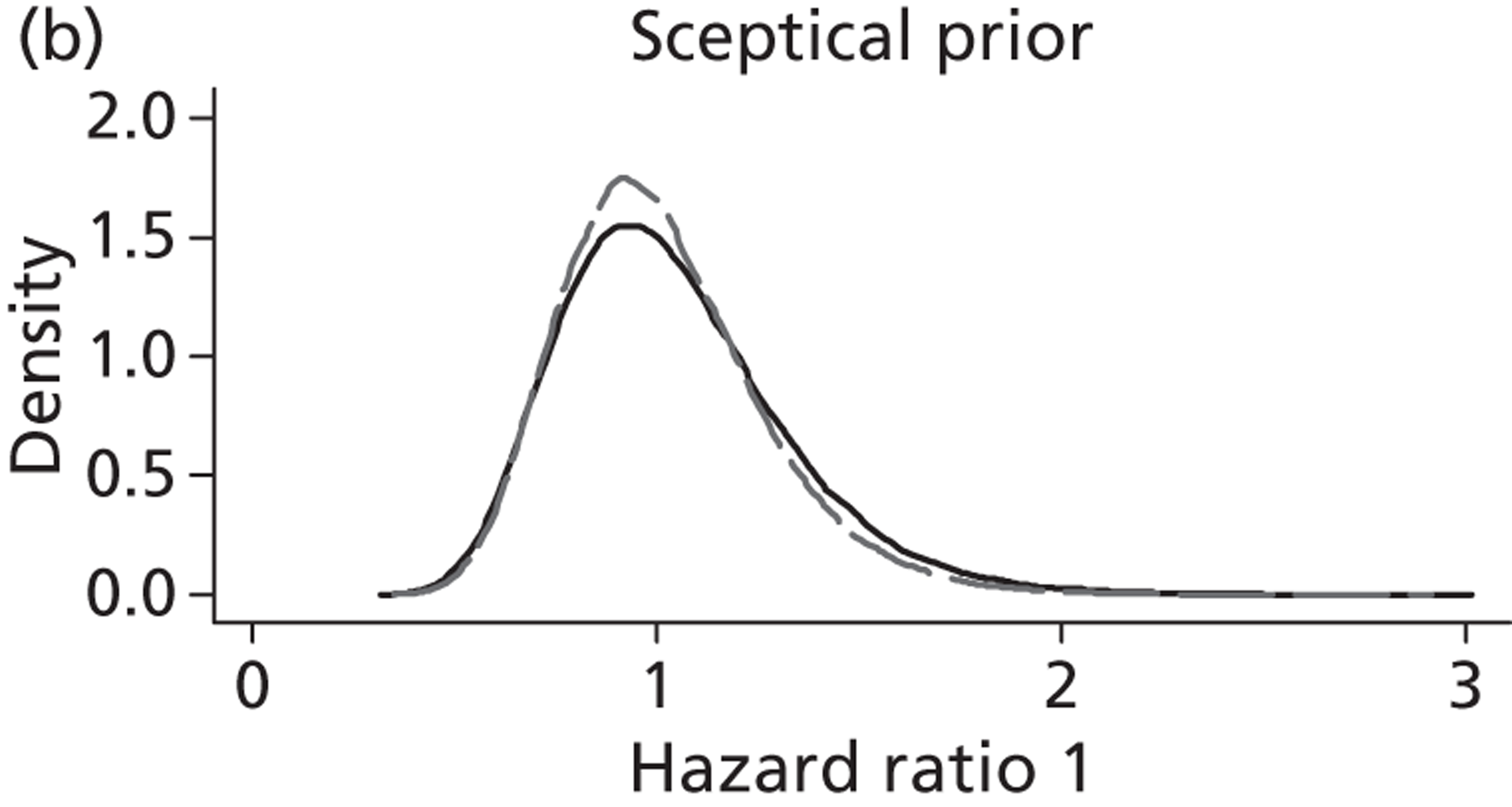
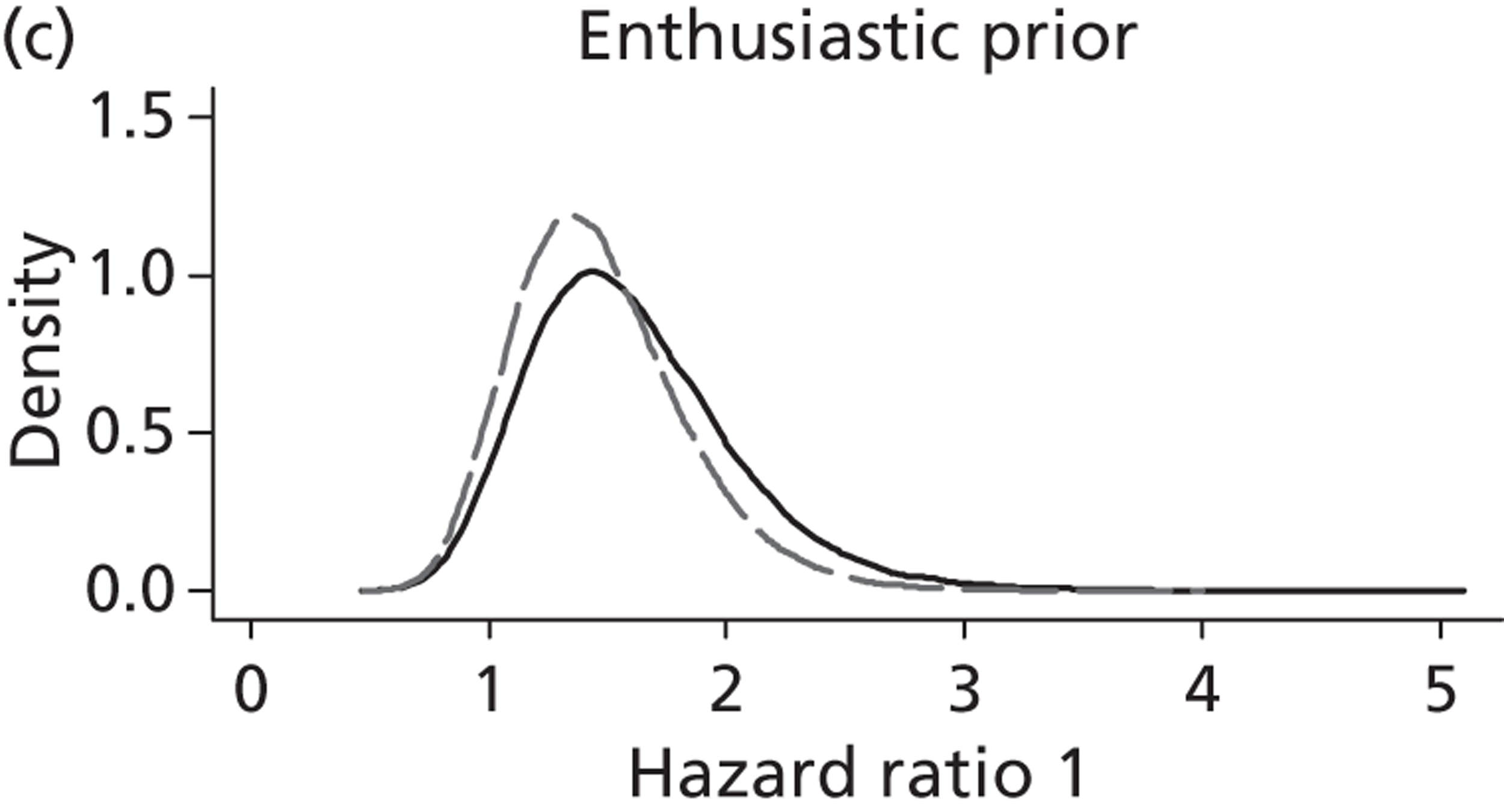
FIGURE 33.
Posterior distributions for HR2 (period 36.5 weeks to the end of follow-up) under the ITT analysis.
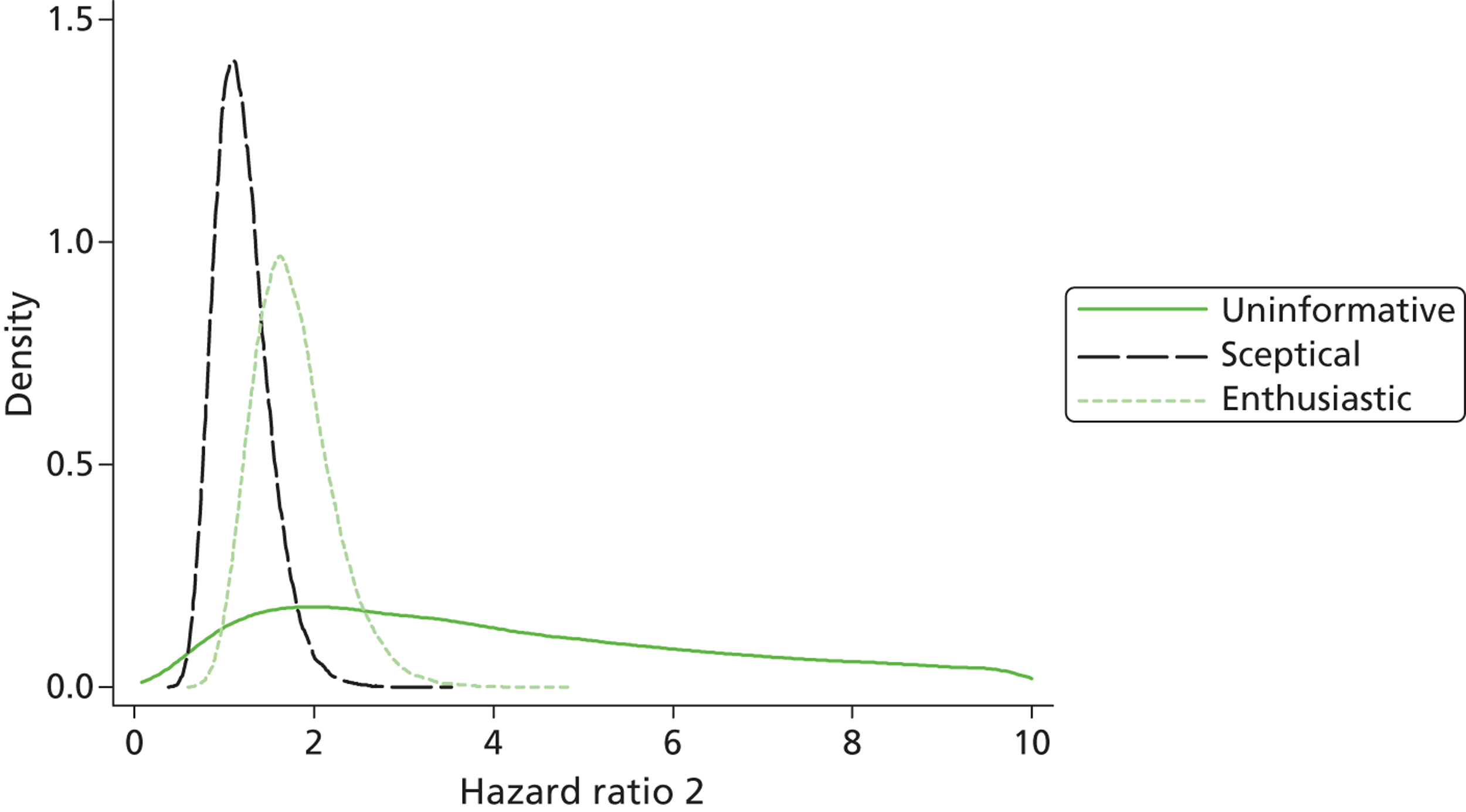
FIGURE 34.
Prior and posterior distributions for HR2 (period 36.5 weeks to the end of follow up) (ITT) overlaid. (a) Posterior distribution; (b) posterior distribution (dashed line), sceptical prior distribution (solid line); and (c) posterior distribution (dashed line), enthusiastic prior distribution (solid line).
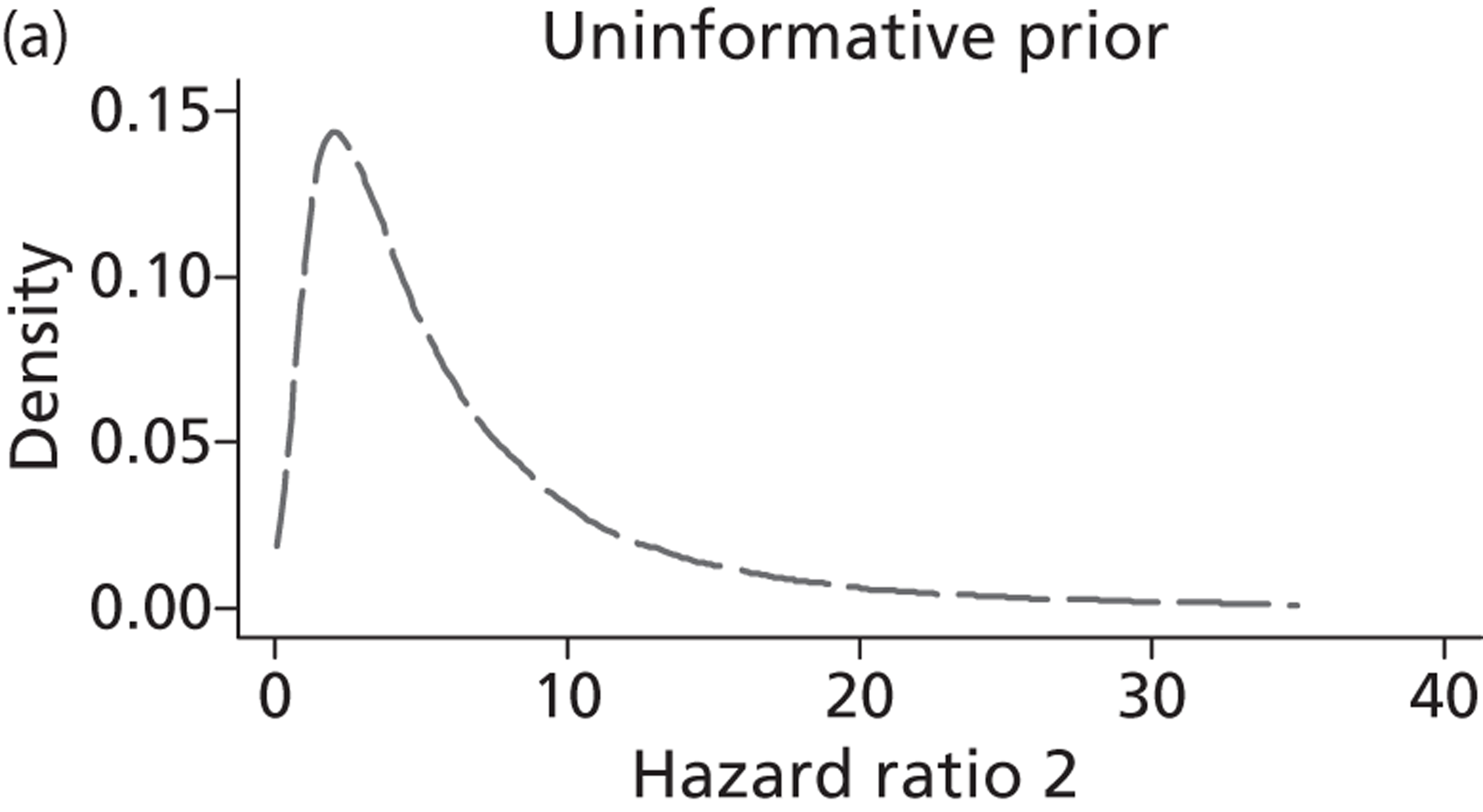
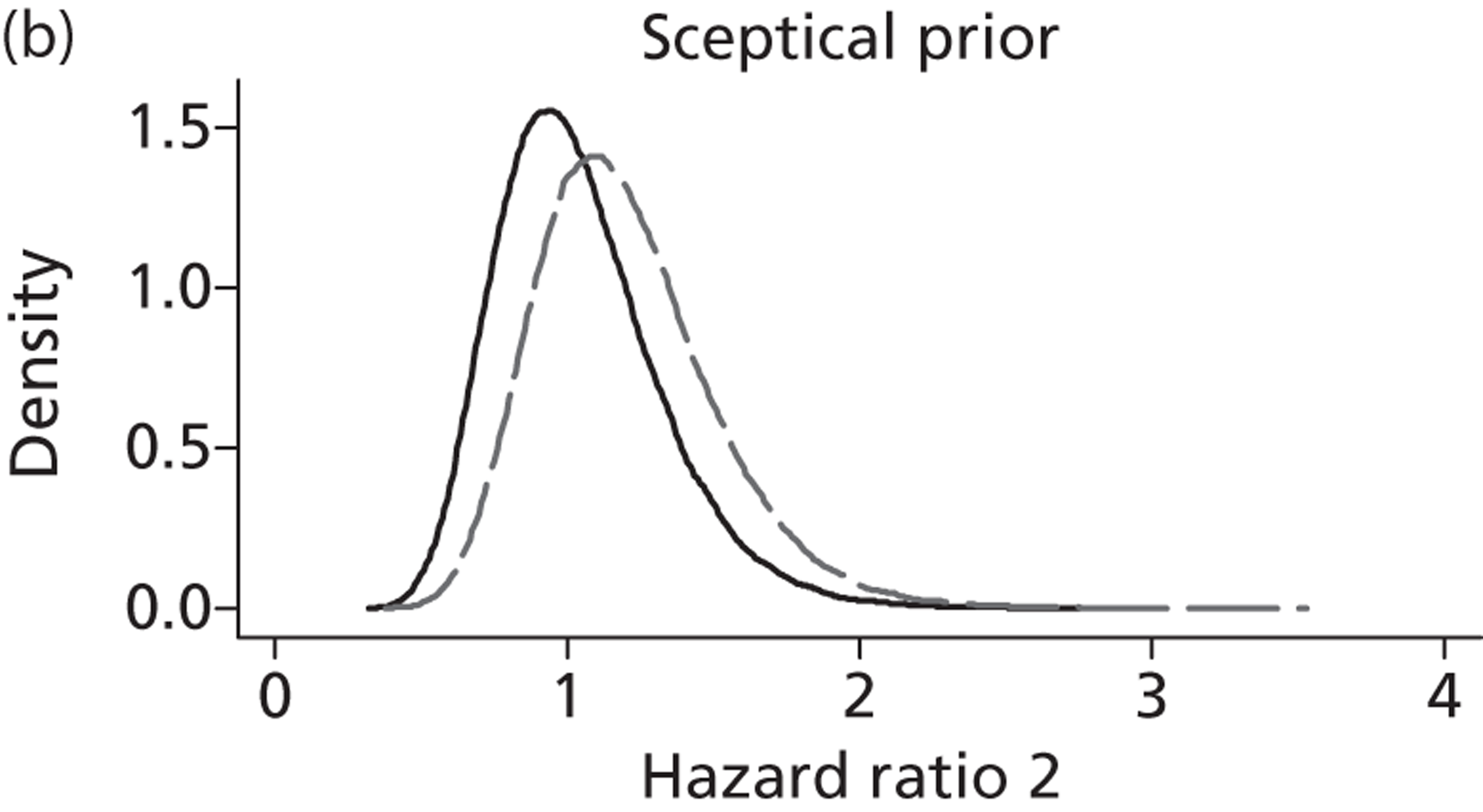
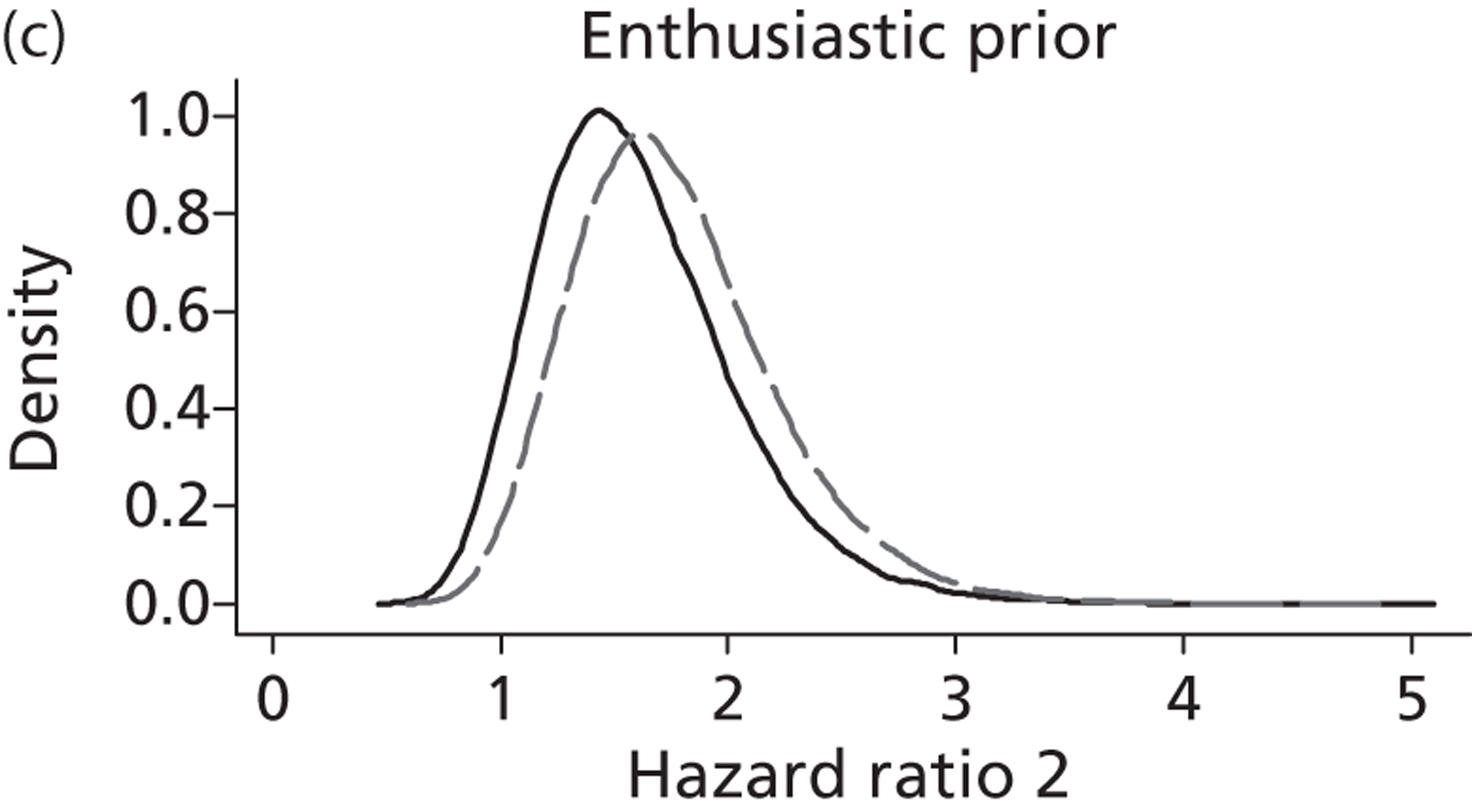
Discussion
The PLUTO trial of conservative management compared with intrauterine VAS in babies with LUTO was designed to detect a change in perinatal survival (4 weeks) from 39% under conservative management to 62% under shunting, equating to a RR (for survival) of 1.55. The PLUTO trial needed to recruit 150 singleton pregnancies but unfortunately failed to meet recruitment targets and the trial closed after recruiting just 31 pregnancies. Fortuitously, before the trial commenced the triallists had sought opinions from consultants in the specialties of fetal medicine, paediatric urology and paediatric nephrology. These opinions were elicited in such a way as to enable the construction of informative Bayesian prior distributions, which when combined with the PLUTO trial data on the 31 pregnancies provide Bayesian posterior probabilities that the treatment effect (RR) is greater than the 1.55 value that the study was initially designed to detect.
To this end we compared the elicited prior distributions from the three groups of experts with what are commonly referred to as off-the-shelf priors (an uninformative prior, a sceptical prior and an enthusiastic prior) and also compared an ITT analysis with an AT analysis. For the ITT analysis only under the enthusiastic prior did we find convincing evidence that VAS is beneficial (NNT 5, 95% CrI 2 to 24), with a probability of 71% that the effect of VAS is clinically important (i.e. RR > 1.55). Under all other priors we found that it was not possible to rule out harmful effects of VAS. Under the AT analysis, which does not preserve the randomisation, both the enthusiastic prior and the uninformative prior suggested that the intervention was beneficial and again under all other prior distributions it was not possible to rule out harmful effects.
Beliefs about the likely impact on survival of intrauterine VAS were elicited from a range of experts including fetal medicine experts, paediatric urologists and paediatric nephrologists. We observed that beliefs differed between these groups. For example, fetal medicine experts were much more pessimistic about outcome under conservative management than the other two specialty groups. This is possibly explained by the fact that fetal medicine experts see all infants with fetal bladder neck obstruction whereas paediatric urologists and nephrologists see only those who survive the perinatal period and who therefore have a better prognosis.
The elicited prior distributions from all groups of experts were more pessimistic than the observed trial data. The PLUTO trial failed to recruit the required number of participants and this is likely to be because of reluctance from both parents and clinicians. The finding that the expert clinicians who took part in the Bayesian elicitation exercise were quite pessimistic suggests that many clinicians may not have referred patients for inclusion in the PLUTO trial because of preconceived opinions that the intervention was not beneficial.
We included an enthusiastic prior, representing an opinion that intrauterine VAS would lead to an increase in survival. This enthusiastic prior was centred at the OR that the PLUTO trial was powered to detect (2.55, equivalent to a RR of 1.55), giving a small probability (5%) that the intervention would be harmful (i.e. that survival under conservative treatment would be better). Although we observed that the posterior distribution incorporating this enthusiastic prior had a narrower CrI than that of the uninformative prior, it suggested that the treatment effect might be smaller (RR 1.93, 95% CrI 0.77 to 6.25 under the uninformative prior and RR 1.82, 95% CrI 1.05 to 3.52 under the enthusiastic prior), although the probability that the RR is > 1.55 (the treatment effect that the PLUTO trial was designed to detect) was higher under the enthusiastic prior (0.71) than under the uninformative prior (0.67).
Furthermore, we also carried out a similar Bayesian survival analysis for a minimum follow-up of 1 year. This also allowed the computation of the posterior probability that the HR is > 1.55. We compared an uninformative prior, a sceptical prior and an enthusiastic prior and also compared an ITT analysis with an AT analysis. This analysis was parameterised as a conservative management compared with treatment comparison so that the effect estimate on the HR scale was consistent with RR estimates in so far as treatment effects > 1 favoured the intervention.
For the ITT analysis, and under the assumption that the HR is an average estimate of the treatment effect from conception to the end of follow-up, the only prior distribution that resulted in convincing evidence that VAS is beneficial was the enthusiastic prior (HR 1.56, 95% CrI 0.99 to 2.46). Under the uninformative and sceptical priors we found that it was not possible to rule out harmful effects of VAS. Under the AT analysis, which does not preserve the randomisation, both the enthusiastic prior and the uninformative prior suggested that the intervention was beneficial but, again, under the sceptical prior distribution it was not possible to rule out harmful effects.
For both the ITT analysis and the AT analysis and under the assumption that there is a time-dependent treatment effect, the results for all prior distributions include harmful effects of VAS from conception to 36.5 weeks. The probability that the HR is > 1.55 is low for all three prior distributions under the ITT analysis with a probability of just 0.03 for the sceptical prior.
For the ITT analysis from 36.5 weeks onwards, only the enthusiastic prior gave convincing evidence that the intervention is beneficial (HR2 1.71, 95% CrI 1.05 to 2.79). For both the uninformative prior and the sceptical prior the results show that there may be harmful effects of the intervention. Under the AT analysis both the enthusiastic prior and the uninformative prior suggested that the intervention was beneficial but, again, under the sceptical prior distribution it was not possible to rule out harmful effects.
The inclusion of the enthusiastic prior (ln HR ∼ N(0.44, 0.27) meant that, although we observed that the posterior distribution had a narrower CrI than that of the non-informative prior, the treatment effect might be smaller. For example, under the proportional hazards assumption the HR estimate (95% CrI) is 1.63 (0.63 to 4.43) for the non-informative prior and 1.56 (0.99 to 2.46) for the enthusiastic prior.
Limitations
A Bayesian analysis is heavily reliant on the choice of prior distributions, especially when the amounts of data available are small, as in the PLUTO trial. Although a large number of opinions were sought, the priors will be valid only if the questionnaires were properly interpreted and we were successful in eliciting opinions. Indeed, with hindsight, one of the questions, although intended to be on a relative scale, may have been interpreted as being on an absolute scale. Furthermore, opinions were elicited on expected outcomes under conservative management and expected change under VAS. Analysis methods therefore required substantial manipulation of these opinions into pooled prior distributions for analysis within the posterior modelling, and so analysis is further reliant on the appropriateness of our pooling techniques and in particular on the assumptions of normality invoked.
Of note, some of the values published here for the pooled expert beliefs do not quite correspond to earlier published values. 119 This is because of a small mistake in the formula used in the earlier analysis. In addition, the questionnaires referred a little ambiguously to the outcome being both perinatal mortality (28 days) and 6 weeks. It is not expected that elicited beliefs were sensitive to this anomaly.
The analysis had to be restricted to an unadjusted perinatal and survival analysis because of the very small sample size. It is likely that there will be other factors related to survival other than the intervention variable; however, these could not be included. The variables that could have been considered for the analysis had missing data, which, if incorporated, would have reduced the number of subjects included in the analysis and further reduced the power. Therefore, any results shown must be interpreted with a high degree of caution.
For perinatal survival, the RR estimated from this Bayesian (ITT) analysis using the uninformative prior is similar to but has a wider CrI than the frequentist counterpart (RR 1.93, 95% CrI 0.77 to 6.25 vs. RR 1.88, 95% CI 0.71 to 4.96). This is somewhat contrary to the Bayesian paradigm, that of increasing the effective sample size by including additional ‘data’, but we are not the first to observe such a phenomenon. 123,124
Finally, the primary outcome stated within the protocol for the PLUTO study was survival at 28 days. In concordance with this protocol all analysis subsequently treated the outcome event as survival (as opposed to death). However, in a survival analysis the natural outcome is death. So that the effect estimate on the HR scale was consistent with RR estimates (in so far as treatment effects > 1 will favour the intervention), the survival analysis had to take the somewhat unusual parameterisation of being a control versus treatment comparison.
Conclusion
In conclusion, the PLUTO trial failed to recruit its target sample size and the results from the analysis of the RCT data were therefore inconclusive. Fortuitously, before the trial had commenced the triallists had sought opinions from consultants in the specialties of fetal medicine, paediatric urology and paediatric nephrology. These opinions were elicited in such a way as to enable the construction of informative Bayesian prior distributions, which, when combined with the PLUTO trial data on the 31 pregnancies, provide Bayesian posterior probabilities that the treatment effect (RR) is greater than the 1.55 value that the study was initially designed to detect. This was complemented with Bayesian analysis based on what are commonly referred to as off-the-shelf priors (an uninformative prior, a sceptical prior and an enthusiastic prior).
Importantly, we found that our conclusions were dependant on the choice of prior. Under the ITT assumption and using an elicited prior, the posterior probability that the RR is > 1.55 (a clinical and prespecified difference) is 25%. Results from the other priors were equally or less optimistic and only under the enthusiastic prior did we find evidence that VAS is beneficial and even then the probability that the RR is > 1.55 is still only 71%. Under all other priors we found that it was not possible to rule out harmful effects of VAS.
Finally, we also observed that the clinicians from whom we elicited opinions were highly sceptical about VAS but also quite variable in their opinions. This has several important implications. First, it is likely that it contributed to the failure of the trial to recruit the number of participants required. Therefore, although not having intentionally set out to explain the low recruitment rate, eliciting Bayesian priors may also have provided an explanation for the failure of the study. Second, because the clinicians were sceptical and the number of patients recruited to the study small, the estimated treatment effect obtained from these Bayesian analyses are pulled towards the null.
Chapter 8 Economic analysis
Introduction
This chapter reports the model-based economic evaluation carried out alongside the PLUTO trial. The primary objective of the study was to determine whether intrauterine VAS for fetal bladder outflow obstruction improves perinatal and neonatal mortality and renal function compared with conservative non-interventional care.
For reasons explained in Chapter 5 , recruitment targets for the RCT were not met and the trial had to be terminated early. As a result, the economic analysis was revised to accommodate the lack of long-term data. The modified protocol is provided in Appendix 7 .
The aim of the economic evaluation is to inform current treatment policy in this clinical area given the available data and to inform possible future research funding decisions. For the purposes of this evaluation we compared individuals who were randomised into the VAS arm of the trial with those who were randomised into the conservative arm of the trial. Data from the registry were not included in the economic analysis.
The economic evaluation adopted the perspective of the NHS and took the form of a cost-effectiveness analysis and estimated results based on a number of outcomes, which were survival at 28 days and 1 year as well as a composite measure, disability free at the end of 1 year, which refers to patients who survived to 1 year without renal or any other morbidity.
This work has been previously published in Diwakar L, Morris RK, Barton P, Middleton LM, Kilby MD, Roberts TE. Evaluation of the cost effectiveness of vesico-amniotic shunting in the management of congenital lower urinary tract obstruction (based on data from the PLUTO trial). PLOS ONE. 2013; (in press). 125
Methods
Model structure
A model-based economic analysis was carried out based on the data from the PLUTO trial. A decision tree model was felt to be most appropriate for this analysis given the small number of participants and the short time frame of the study. The model was constructed in TreeAge Pro 2012 (TreeAge Software Inc., Williamstown, MA, USA) by the authors. The structure of the model was informed by clinical input with regards to presentation of the condition and treatment pathways as well, as by systematic reviews published in this disease area. 13,21 In this model we examine the antenatal, perinatal and postnatal progress of the affected fetus within the trial following randomisation to VAS ( Figure 35 ) or conservative management ( Figure 36 ). Pathways experienced by the patients within the trial over a period of 1 year are represented. Data collection at 2 years was not complete at the time of writing this report and hence was not included in the analysis. Health states used in the model are defined in Table 34 .
FIGURE 35.
The VAS pathway.
FIGURE 36.
The conservative management pathway.
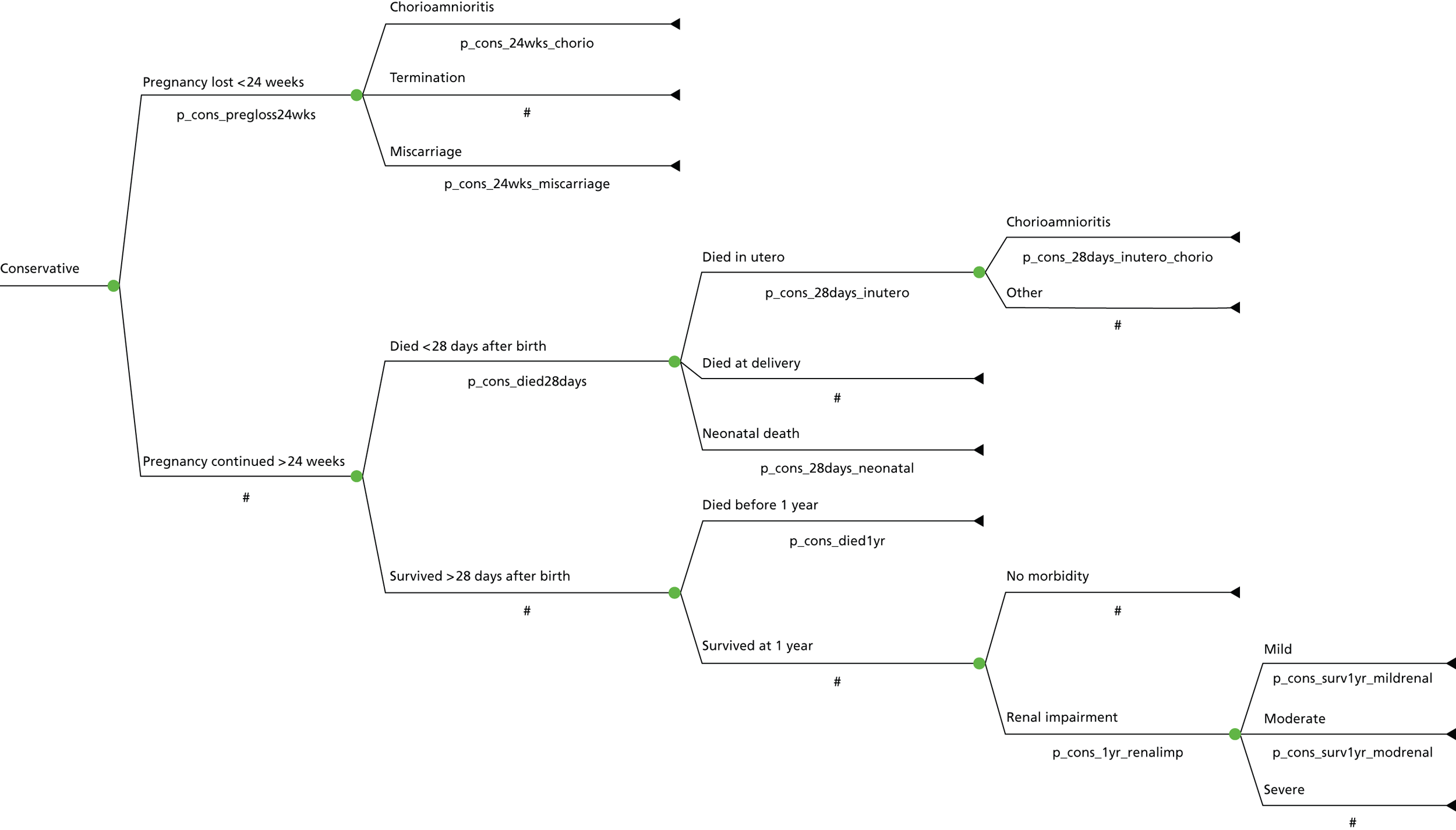
| Health state | Definition |
|---|---|
| VAS | All fetuses with LUTO who received intrauterine VAS |
| Conservative | All fetuses with LUTO who did not receive the intervention |
| Miscarriage | Pregnancy loss at < 24 weeks as a result of unexplained causes |
| Died in utero | Death of fetus as a result of unexplained reasons after 24 weeks of gestation |
| Died at delivery | Died within 24 hours of birth |
| Neonatal death | Death between 24 hours and 28 days of birth |
| No morbidity | No known ongoing, chronic pathology related to LUTO or its repair |
| Mild renal impairment | Serum creatinine > 50 µmol/l; no signs or symptoms of renal disease |
| Moderate renal impairment | Serum creatinine > 50 µmol/l; medical management of renal disease |
| Severe renal impairment | Serum creatinine > 50 µmol/l; patient on dialysis ± transplant being considered |
Data inputs for the model
Clinical
The clinical data used in the model have been discussed in Chapter 5 . Complications that were noted within the trial as a result of VAS placement included chorioamnionitis (associated with SROM) and dislodgement of shunt requiring reinsertion. Probabilities for the decision tree model were derived directly from the trial data. Beta distribution was applied for the decision tree model and, when multinomial data were available, Dirichlet distribution was used.
For example, of the 15 patients randomised to the conservative arm of the model, three could not continue their pregnancy beyond 24 weeks whereas 12 did. Because only two possibilities occur at this node (binomial), the probabilities were assigned a beta distribution and were calculated as 0.2 and 0.8 respectively. If, on the other hand, more than two outcomes are possible at a node (multinomial), a Dirichlet distribution was preferred.
Table 35 provides the probability data for patients randomised to the conservative arm (n = 15). The probabilities applied to those patients who were randomised to the VAS arm and developed shunt-related complications are listed in Table 36 and the probabilities applied to those patients who were randomised to the VAS arm but did not develop complications from shunt insertion are included in Table 37 .
| Parameter (distribution)b | ITT analysis (n = 15) | Per-protocol analysis (n = 13) | Uniform prior analysis |
|---|---|---|---|
| Pregnancy lost at < 24 weeks (beta) | 0.2 (3,15) | 0.08 (1,13) | 0.24 (4,17) |
|
|
|
|
|
|
|
|
|
|
|
|
| Pregnancy continued beyond 24 weeks (beta) | 0.8 (12,15) | 0.92 (12,13) | 0.76 (13,17) |
| Died at < 28 days after birth (beta) | 0.67 (8,12) | 0.67 (8,12) | 0.64 (9,14) |
| Died in utero at > 24 weeks (Dirichlet) | 0 (0,8) | 0 (0,8) | 0.09 (5,14) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Survived for > 28 days after birth | 0.33 (4,12) | 0.33 (4,12) | 0.36 (5,14) |
|
|
|
|
| Survived at 1 year (beta) |
|
|
|
|
|
|
|
| Renal impairment | 1 (3,3) | 1 (3,3) | 0.8 (4,5) |
|
|
|
|
|
|
|
|
|
|
|
|
| Parameter (distribution)b | ITT analysis (n = 16) | Per-protocol analysis (n = 15) | Uniform prior analysis |
|---|---|---|---|
| Complications (beta) | 0.25 (4,16) | 0.27 (4,15) | 0.28 (5,18) |
| Pregnancy lost at < 24 weeks (beta) | 0.75 (3,4) | 0.75 (3,4) | 0.67 (4,6) |
| Chorioamnionitis (Dirichlet) | 0.67 (2,3) | 0.67 (2,3) | 0.5 (3,6) |
| Termination (Dirichlet) | 0 (0,3) | 0 (0,3) | 0.17 (1,6) |
| Miscarriage (Dirichlet) | 0.33 (1,3) | 0.33 (1,3) | 0.33 (2,6) |
| Pregnancy continued beyond 24 weeks (beta) | 0.25 (1,4) | 0.25 (1,4) | 0.33 (2,6) |
| Died at < 28 days after birth (beta) | 0 (0,1) | 0 (0,1) | 0.33 (1,3) |
| Died in utero at > 24 weeks (Dirichlet) | 0 (0,0) | 0 (0,0) | 0.33 (1,3) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Survived for > 28 days after birth | 1 (1,1) | 1 (1,1) | 0.67 (2,3) |
|
|
|
|
| Survived at 1 year (beta) |
|
|
|
|
|
|
|
| Renal impairment | 1 (1,1) | 1 (1,1) | 0.67 (2,3) |
|
|
|
|
|
|
|
|
|
|
|
|
| Parameter (distribution)b | ITT analysis (n = 16) | Per-protocol analysis (n = 15) | Uniform prior analysis |
|---|---|---|---|
| No complications (beta) | 0.75 (12,16) | 0.73 (11,15) | 0.72 (13,18) |
| Pregnancy lost at < 24 weeks (beta) | 0.08 (1,12) | 0 (0,11) | 0.14 (2,14) |
|
|
|
|
|
|
|
|
| Pregnancy continued beyond 24 weeks (beta) | 0.92 (11,12) | 1 (11,11) | 0.86 (12,14) |
| Died at < 28 days after birth (beta) | 0.36 (4,11) | 0.36 (4,11) | 0.38 (5,13) |
| Died in utero at > 24 weeks (Dirichlet) | 0 (0,4) | 0 (0,4) | 0.14 (1,7) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Survived for > 28 days after birth | 0.64 (7,11) | 0.64 (7,11) | 0.61 (8,13) |
|
|
|
|
|
|
|
|
|
|
|
|
| Renal impairment | 0.67 (4,6) | 0.67 (4,6) | 0.62 (5,8) |
|
|
|
|
|
|
|
|
|
|
|
|
Resource use and costs
Resource use data associated with admission into hospital including antenatal scans, NICU admission, procedures performed and morbidity such as renal impairment were collected prospectively from all participating centres during the study. Complications related to VAS placement such as IUD or chorioamnionitis were also captured within the trial. Costs associated with resource use were obtained from standard sources such as Curtis126 and UK Healthcare Resource Group (HRG) cost data. 127 When exact costs for procedures were not available, comparable costs were derived from these sources with the approval of the clinician investigators. Costs of investigations were obtained from local NHS laboratories. The cost of VAS placement was derived from the bottom up by estimating the resource use and costing these individually ( Table 38 ).
| Description | Unit cost (£) | Source |
|---|---|---|
| Cost of the VAS | 154.30 | Birmingham Women’s Hospital |
| Cost of scan (including consultant time) | 445.50 | Birmingham Women’s Hospital |
| Counselling (30 minutes of consultant time) | 80.50 | Curtis126 |
| VAS insertion | ||
| 30 minutes of consultant time | 80.50 | Curtis126 |
| 30 minutes of assistant time (senior nurse) | 61 | |
| Total | 821.80 | |
All costs in the model are in 2011 UK pounds. Hospital and Community Health Services (HCHS) pay and price indices were used to inflate costs where appropriate. 126 The costs incurred during VAS placement are discussed in Table 38 . Detailed scanning is generally performed in women with suspected fetal abnormalities. This cost is not included in the NHS reference costs and was obtained from the Birmingham Women’s Hospital (which was the largest recruiting centre for the trial). Because these costs were not obtained from standardised sources, we carried out deterministic sensitivity analyses by doubling and halving the costs (DSA1 and DSA2, discussed later in this chapter).
The costs in Tables 39 – 41 are based on NHS reference costs. Table 39 details the costs of delivery and medical TOP whereas Table 40 provides the costs associated with hospitalisation of neonates. Procedures carried out during the course of the trial are costed in Table 41 . If exact procedures were not listed in the reference costs then the closest plausible cost was input after discussion with the clinicians.
| Description | Unit cost (£) | Source |
|---|---|---|
| Normal vaginal delivery without cc | 1236 | Normal delivery no cc (NZ01F)b |
| Normal vaginal delivery with cc | 1906 | Normal delivery with cc (NZ01E)b |
| Elective caesarean section | 2378 | Caesarean section no cc (NZ03D)b |
| Emergency caesarean section | 3236 | Caesarean section with cc (NZ03C)b |
| Medical TOP | 431 | (MA18Z)b |
| Description | Cost (£)a | Sourceb |
|---|---|---|
| NICU | £1117 | PbR 2008–9 cost code: XA01Z128 |
| High-dependency unit (HDU) | £785 | PbR 2008–9 cost code: XA02Z128 |
| Special care baby unit (SCBU) | £490 | PbR 2008–9 cost code: XA03Z128 |
| Neonatal care transportation | £857 | PbR 2008–9 cost code: XA06Z128 |
| Neonatal critical care – normal care | £460 | PbR 2008–9 cost code: XA05Z128 |
| Description | Cost (£) | Source |
|---|---|---|
| Cystoscopic resection of PUV | 1380 | Bladder major procedure (LB13B)a |
| Nephrectomy | 4430 | Kidney major open procedure (LB02D)a |
| Nephrostomy | 2282 | Percutaneous nephrostomy no cc (LB01B)a |
| Vesicostomy | 803 | Bladder intermediate procedure (LB14D)a |
| Vesicostomy closure | 703 | Bladder minor procedure (LB15D)a |
| Peritoneal dialysis catheter insertion | 1508 | Peritoneal dialysis-related procedure, no cc (LA05B)a |
| Orchipexy | 1105 | Testes open procedure (LB34C)a |
| Circumcision | 740 | Penile minor procedure (LB32C)a |
| Cystoscopy | 803 | Bladder intermediate procedure (LB14D)a |
| Suprapubic catheter insertion (and replacement) | 703 | Bladder minor procedure (LB15D)a |
| VAS removal | 703 | Bladder minor procedure (LB15D)a |
| Amnioinfusions | 445 | Detailed scan including counselling timeb |
| MCUG | 340 | Dynamic studies of the urinary tract (LB42Z)a |
| Peritoneal dialysis | 67 | Cost per day of continuous ambulatory peritoneal dialysis for children 2008/9 (RD3C),128 inflated to 2010–11 prices using the PSSRU inflation indices.126 |
Table 42 details the costs of the laboratory tests used within the trial. These were obtained from local NHS laboratories or from a previous publication6 (in the case of post-mortem costs).
| Description | Cost (£) | Source |
|---|---|---|
| Serum creatinine | 3 | Local laboratory (Birmingham Heartlands Hospital) |
| Creatinine clearance | 5 | Local laboratory (Birmingham Heartlands Hospital) |
| β2-microglobulin | 3 | Local laboratory (Birmingham Heartlands Hospital) |
| Urea and electrolytes | 3 | Local laboratory (Birmingham Heartlands Hospital) |
| Urinary calcium | 4 | Local laboratory (Birmingham Heartlands Hospital) |
| Urinary sodium | 4 | Local laboratory (Birmingham Heartlands Hospital) |
| Full blood count | 2 | Local laboratory (Birmingham Heartlands Hospital) |
| Karyotyping | 197 | QF-PCR trisomy test + sex chromosome (Birmingham Women’s Hospital) |
| Post-mortem examination | 1440 | From Roberts,129 inflated to 2010/11 prices using the PSSRU inflation indices.126 |
Table 43 presents the average resource use for each patient within each of the decision tree pathways within the PLUTO trial. As an example, each of the four patients in the VAS with complications arm had 1.5 antenatal ultrasound scans (as three out of four patients in this arm had their pregnancy terminated). An average of 6.5 scans were performed on each of the 12 patients who underwent VAS without complications, whereas the 15 patients in the conservative arm had an average of 4.4 scans each.
| Resource | VAS (complications) (n = 4) | VAS (no complications) (n = 12) | Conservative management (n = 15) |
|---|---|---|---|
| Antenatal ultrasound scan | 1.5 | 6.5 | 4.4 |
| VAS | 1.25 | 1.25 | 0.13 |
| Karyotype testing | 0 | 0.25 | 0.33 |
| Antenatal urinalysis | 0.25 | 0.50 | 0 |
| Post-mortem examination | 0 | 0.083 | 0.27 |
| Delivery | |||
| Normal vaginal | 0.75 | 0.58 | 0.47 |
| Vaginal breech | 0 | 0 | 0.067 |
| Vaginal TOP | 0.25 | 0.083 | 0.133 |
| Caesarean section | 0 | 0.33 | 0.33 |
| Admission days (up to 60 days) | 15 | 12.67 | 8.4 |
| NICU | 15 | 11.5 | 4 |
| High-dependency unit (HDU) | 0 | 0.17 | 0 |
| Ward | 0 | 1 | 4.4 |
| Surgeries | |||
| CRb | 0 | 0.17 | 0.067 |
| Shunt removal | 0 | 0 | 0.067 |
| Vesicostomy | 0.25 | 0.17 | 0.067 |
| Nephrostomy | 0.25 | 0.083 | 0 |
| Procedures | |||
| MCUG | 0.25 | 0 | 0.067 |
| Cystoscopy | 0.5 | 0.17 | 0 |
| Video urodynamic study | 0 | 0 | 0.067 |
| Other | 1.25c | 0.42d | 0 |
| Peritoneal dialysis (person-years) | 0 | 0.028 | 0 |
| Laboratory tests | |||
| Serum creatinine | 0 | 0.5 | 0.2 |
| Sodium | 0.25 | 0 | 0.067 |
| Urinary sodium | 0 | 0 | 0.067 |
| Urinary calcium | 0.25 | 0 | 0.067 |
| Creatinine clearance | 0 | 0.083 | 0.067 |
| Renal ultrasound scan | 0.25 | 0.5 | 0.29 |
| Admission days (60 days–1 year) | 25.5 | 11.1 | 4.07 |
Outcomes
The survival outcomes explored in this analysis are summarised in Table 14 . In addition to survival to 28 days and 1 year we considered a composite measure, namely remaining disability free at the end of 1 year. This measure incorporated all children who survived up to 1 year of life without any renal or other chronic morbidity.
There is currently no validated method available for reliably estimating quality-adjusted life-years (QALYs) in children aged < 2 years. A cost–utility analysis could not therefore be performed. Although a neurological development assessment using a validated PARCA questionnaire was initially planned in the study protocol, this was not available for all patients at the time of writing this report and therefore was not included.
Assumptions
The following assumptions hold true for all of the analyses performed:
-
Data relating to drug use were not collected within the trial and were assumed to be minimal. These costs are therefore not included in the analysis.
-
All centres (UK and international) were assumed to have similar expertise and protocols for management of these patients.
Analysis
The results are reported as incremental cost per outcome of interest, that is, as cost per infant who survived to 28 days, who survived to 1 year or who remained disability free at the end of 1 year.
Base-case analysis
We carried out three alternative analyses for the base case based on the trial data.
Method 1: intention-to-treat analysis
In this method patients are compared in the groups to which they were initially randomised. 130 This method of analysis enables estimation of benefit in a real-life situation following policy change rather than when treatment is provided exactly as planned in the trial. Not using ITT analysis can often exaggerate the benefits of a given intervention and it is widely acknowledged as the least biased of the analysis options. 131
All 31 patients recruited into the RCT were included in this analysis.
Method 2: excluding termination of pregnancy patients from the intention-to-treat data set
Within the trial, three expectant mothers chose to terminate the pregnancy after they were randomised into the trial. This represented about 10% of all patients randomised into the trial and therefore there was a possibility that this could significantly impact on the findings of the economic analysis. To consider this issue, a separate per-protocol analysis was carried out excluding these patients.
Thus, one patient from the VAS arm and two patients from the conservative arm of the trial were excluded, bringing the total number of individuals captured within the analysis to 28. It should be noted that protocol violation because of incorrect treatment allocation (i.e. patients who were randomised into the conservative arm but who received a VAS and vice versa) is not considered in this analysis but is addressed in a separate deterministic sensitivity analysis (DSA).
Method 3: uniform prior analysis
Given the small numbers of patients recruited into the trial, the outcomes and costs that were observed in the trial may not be truly representative of real-life scenarios. This was especially true for some of the pathways created within the decision tree model for which there were no data available.
As PLUTO is the largest clinical trial for this condition to date, it was not possible to obtain other systematically collected data on this rare condition. We carried out a pragmatic analysis employing Bayesian principles. In this analysis we assumed that all patients had a uniform prior possibility of going down any of the designated branches from a given node. To these uniform priors we added the trial data to obtain posterior probabilities, which were used to populate the decision tree. For example, in the conservative arm, the data for the three possibilities following pregnancy loss at < 24 weeks were no cases of chorioamnionitis, two cases of termination and one case of miscarriage in the ITT data set. Assuming a uniform prior, the posterior distribution is equivalent to one case of chorioamnionitis, three cases of termination and two cases of miscarriage. These three possibilities were then assigned a Dirichlet distribution within the model. This method has been previously suggested by Briggs et al. 132
The cost data for the uniform prior analysis were obtained from the trial. As LUTO is a rare condition (see Chapters 1 and 3 ) and as the pregnancies associated with this condition cannot be considered ‘normal’, normal pathways of care cannot apply to these patients. The closest group to the hypothetical cohort of babies that were considered in the uniform prior analysis, therefore, would be those included in the trial.
To calculate costs for the uniform prior analysis, a few further assumptions were made:
-
Apart from costs arising directly from complications, the costs incurred in the VAS arm with and without complications were assumed to be the same. For example, costs incurred for moderate renal failure by a patient in the VAS with no complications arm were assumed to be similar to those for a patient in the VAS with complications arm except for the costs directly incurred because of complications.
-
Prenatal costs incurred in the VAS and conservative arms were assumed to be similar (apart from direct costs resulting from VAS placement and complications, if any). Differences in outcomes (and hence in costs) were considered only after birth.
Sensitivity analyses
Deterministic and probabilistic sensitivity analyses were used to assess the uncertainty associated with the input parameters for all three methods of analysis.
Deterministic sensitivity analysis
Deterministic sensitivity analysis aims to estimate the effect of changing a single parameter on the overall incremental cost-effectiveness ratio (ICER) obtained. The point estimates used for all of the other model parameters remain unchanged. Within the current analysis, the following deterministic sensitivity analyses were considered.
-
Changing the cost of VAS insertion. The VAS used in this trial is not currently available as standard within the NHS. Therefore, there are no HRG tariffs to inform the cost of the intervention. For the purposes of the base-case analysis we estimated the cost of the procedure at £821.80 by costing each of the individual components of resource use, that is, bottom-up costing (see Table 38 ). As the costs of VAS placement may vary between individual centres, we investigated the effect of different VAS costs on the overall cost-effectiveness of the intervention. In the absence of definite data regarding cost variability, we arbitrarily doubled and halved the VAS costs in two separate sensitivity analyses (DSA1 and DSA2).
-
Normalising costs by adjusting extreme values. Within the trial, three patients had significantly higher costs than the average for the group (numbers 1015, 1020 and 1021). Assuming that this finding was totally by chance, we decided to explore the effect of omitting these outlying costs from our analysis. We therefore carried out a sensitivity analysis substituting these extreme costs with average costs for the group.
-
Analysis as per treatment option delivered. Five individuals within the trial did not receive the treatment that they were randomised to. Although randomisation was respected during the base-case analysis, we felt that given the small number of participants within the trial it was important to explore whether the changes to treatment in these patients (who represent about 16% of the total number of participants) significantly affected the overall result.
Probabilistic sensitivity analysis
A probabilistic sensitivity analysis (PSA) was carried out for the base-case analysis within all three analyses to explore the effects on the ICERs of the uncertainty in the model input data.
For PSA each model parameter was assigned a normal, beta or Dirichlet distribution reflecting the extent of its variation and the possible pathways within the decision tree (see Tables 35 – 37 ). Cost-effectiveness results were calculated by simultaneously selecting random values from each distribution. The process was repeated 20,000 times in a Monte Carlo simulation of the model to give an indication of how variation in the model parameters leads to variation in the ICERs for a given combination of a treatment and outcome pairing.
Results
Base-case analysis
The results of the analyses are presented in Tables 44 – 46 . For the base case, in the ITT analysis the average cost incurred within the VAS arm was £20,851 for 1.38 survivors at 28 days. The average cost incurred in the conservative arm was £9868 for 0.67 survivors at 28 days, resulting in an ICER for 28-day survival of £15,506 per survivor in the VAS arm. The ICER indicates that, within the ITT analysis, for every additional child who survived up to 28 days (and 1 year) a cost of £15,506 (and £15,415) was incurred. Similar results were obtained in the method 2 analysis and uniform prior analysis (method 3). The ICER results for being disability free at the end of 1 year were substantially higher for all analyses as very few patients survived to 1 year without any morbidity.
| Intervention | Total cost (£) | Survival (28 days), n | Survival (1 year), n | Disability free (1 year), n | ICER (28-day survival) (£) | ICER (1-year survival) (£) | ICER (disability free at 1 year) (£) |
|---|---|---|---|---|---|---|---|
| Base case | |||||||
| VAS | 20,851 | 1.38 | 1.31 | 0.25 | 15,506 | 15,415 | 43,932 |
| Nil (conservative) | 9868 | 0.67 | 0.6 | 0 | |||
| PSA | |||||||
| VAS | 20,901 | 1.38 | 1.31 | 0.25 | 15,482 | 15,407 | 44,145 |
| Nil (conservative) | 9853 | 0.67 | 0.6 | 0 | |||
| DSA1 (doubled VAS costs) | |||||||
| VAS | 21,582 | 1.38 | 1.31 | 0.25 | 16,382 | 16,287 | 46,417 |
| Nil (conservative) | 9978 | 0.67 | 0.6 | 0 | |||
| DSA2 (halved VAS costs) | |||||||
| VAS | 20,492 | 1.38 | 1.31 | 0.25 | 15,076 | 14,988 | 42,715 |
| Nil (conservative) | 9813 | 0.67 | 0.6 | 0 | |||
| DSA3 (adjusted outlier costs) | |||||||
| VAS | 9212 | 1.38 | 1.31 | 0.25 | −926 | −920 | −2623 |
| Nil (conservative) | 9868 | 0.67 | 0.6 | 0 | |||
| DSA4 (as per treatment received) | |||||||
| VAS | 22,827 | 2.07 | 2.00 | 0.27 | 8052 | 8071 | 52,965 |
| Nil (conservative) | 8703 | 0.31 | 0.25 | 0 | |||
| Intervention | Total cost (£) | Survival (28 days), n | Survival (1 year), n | Disability free (1 year), n | ICER (28-day survival) (£) | ICER (1-year survival) (£) | ICER (disability free at 1 year) (£) |
|---|---|---|---|---|---|---|---|
| Base case | |||||||
| VAS | 21,970 | 1.47 | 1.4 | 0.27 | 15,499 | 15,275 | 40,536 |
| Nil (conservative) | 11,161 | 0.77 | 0.69 | 0 | |||
| PSA | |||||||
| VAS | 21,993 | 1.47 | 1.4 | 0.27 | 15,510 | 15,321 | 40,465 |
| Nil (conservative) | 11,147 | 0.77 | 0.69 | 0 | |||
| DSA1 (doubled VAS costs) | |||||||
| VAS | 22,683 | 1.47 | 1.4 | 0.27 | 16,339 | 16,103 | 42,734 |
| Nil (conservative) | 11,287 | 0.77 | 0.69 | 0 | |||
| DSA2 (halved VAS costs) | |||||||
| VAS | 21,614 | 1.47 | 1.4 | 0.27 | 15,080 | 14,988 | 42,715 |
| Nil (conservative) | 11,097 | 0.77 | 0.69 | 0 | |||
| DSA3 (adjusted outlier costs) | |||||||
| VAS | 9555 | 1.47 | 1.4 | 0.27 | −2301 | −2268 | −6019 |
| Nil (conservative) | 11,161 | 0.77 | 0.69 | 0 | |||
| DSA4 (as per treatment received) | |||||||
| VAS | 24,167 | 2.21 | 2.14 | 0.29 | 7770 | 7770 | 50,506 |
| Nil (conservative) | 9736 | 0.36 | 0.29 | 0 | |||
| Intervention | Total cost (£) | Survival (28 days), n | Survival (1 year), n | Disability free (1 year), n | ICER (28-day survival) (£) | ICER (1-year survival) (£) | ICER (disability free at 1 year) (£) |
|---|---|---|---|---|---|---|---|
| Base case | |||||||
| VAS | 20,617 | 1.29 | 1.1 | 0.35 | 15,327 | 15,518 | 31,426 |
| Nil (conservative) | 10,855 | 0.66 | 0.47 | 0.04 | |||
| PSA | |||||||
| VAS | 21,993 | 1.29 | 1.1 | 0.35 | 15,364 | 15,588 | 31,615 |
| Nil (conservative) | 11,147 | 0.61 | 0.42 | 0.06 | |||
| DSA1 (doubled VAS costs) | |||||||
| VAS | 21,316 | 1.29 | 1.1 | 0.35 | 16,428 | 16,617 | 39,330 |
| Nil (conservative) | 10,055 | 0.61 | 0.42 | 0.06 | |||
| DSA2 (halved VAS costs) | |||||||
| VAS | 20,268 | 1.29 | 1.1 | 0.35 | 15,056 | 15,230 | 36,041 |
| Nil (conservative) | 9947 | 0.61 | 0.42 | 0.06 | |||
| DSA3 (adjusted outlier costs) | |||||||
| VAS | 8684 | 1.29 | 1.1 | 0.35 | 252 | 255 | 604 |
| Nil (conservative) | 8511 | 0.61 | 0.42 | 0.06 | |||
| DSA4 (as per treatment received) | |||||||
| VAS | 22,094 | 1.75 | 1.55 | 0.37 | 9347 | 9596 | 36,917 |
| Nil (conservative) | 9476 | 0.4 | 0.24 | 0.03 | |||
Deterministic sensitivity analysis
-
DSA1 and DSA2 (changing VAS costs). As there was some uncertainty associated with the cost of VAS, we explored the effect on the ICER of changing the VAS cost. Accordingly, VAS costs were doubled (DSA1) or halved (DSA2) and the analysis was repeated. This did not result in significant changes in the ICER values. Thus, the results of the base-case analysis were not sensitive to changes in VAS cost.
-
DSA3 (normalising the high costs of outliers). When the high costs incurred by a few of the patients within the trial (patients 1015, 1020 and 1023, all within the VAS arm) were substituted with the average cost incurred by all other patients in that arm, the overall costs within the VAS arm reduced significantly and the VAS arm dominated over the conservative arm in the ITT and per-protocol analysis. The savings were even higher in the method 2 analysis. Thus, patients receiving VAS incurred lower costs overall whilst accruing more benefits. Although costs in the uniform prior analysis also reduced significantly, dominance was not reached.
-
DSA4 (as per treatment received). Two patients who were randomised to the conservative arm (patients 1027 and 1029) received a VAS and three patients randomised to the VAS arm (patients 1013, 1016 and 1028) did not receive a VAS. When the analysis was repeated as per the treatment received, the incremental costs increased slightly (£14,124 vs. £10,983 in the base-case analysis) and the gains in effectiveness were higher (1.7 vs. 0.71 in the base case), resulting in substantially lower ICERs for survival at 28 days and 1 year. The ICER for those who remained disability free at the end of 1 year did not change significantly however.
Probabilistic sensitivity analysis
Probabilistic analysis using 20,000 simulations as per the Monte Carlo principle did not result in a significant change to the base-case ICERs. The scatter plot representing survival at 28 days (for the ITT analysis) is shown in Figure 37 . The ellipse in this figure represents 95% of all data points and most of these lie in the upper right quadrant of the cost-effectiveness plane. This illustrates that VAS placement is very likely to be more effective whilst being more expensive for this outcome.
FIGURE 37.
Incremental cost-effectiveness scatter plot of VAS vs. conservative for the base-case ITT analysis for survival at 28 days. Note: the dotted lines represent the ‘status quo’ with respect to cost and effectiveness.
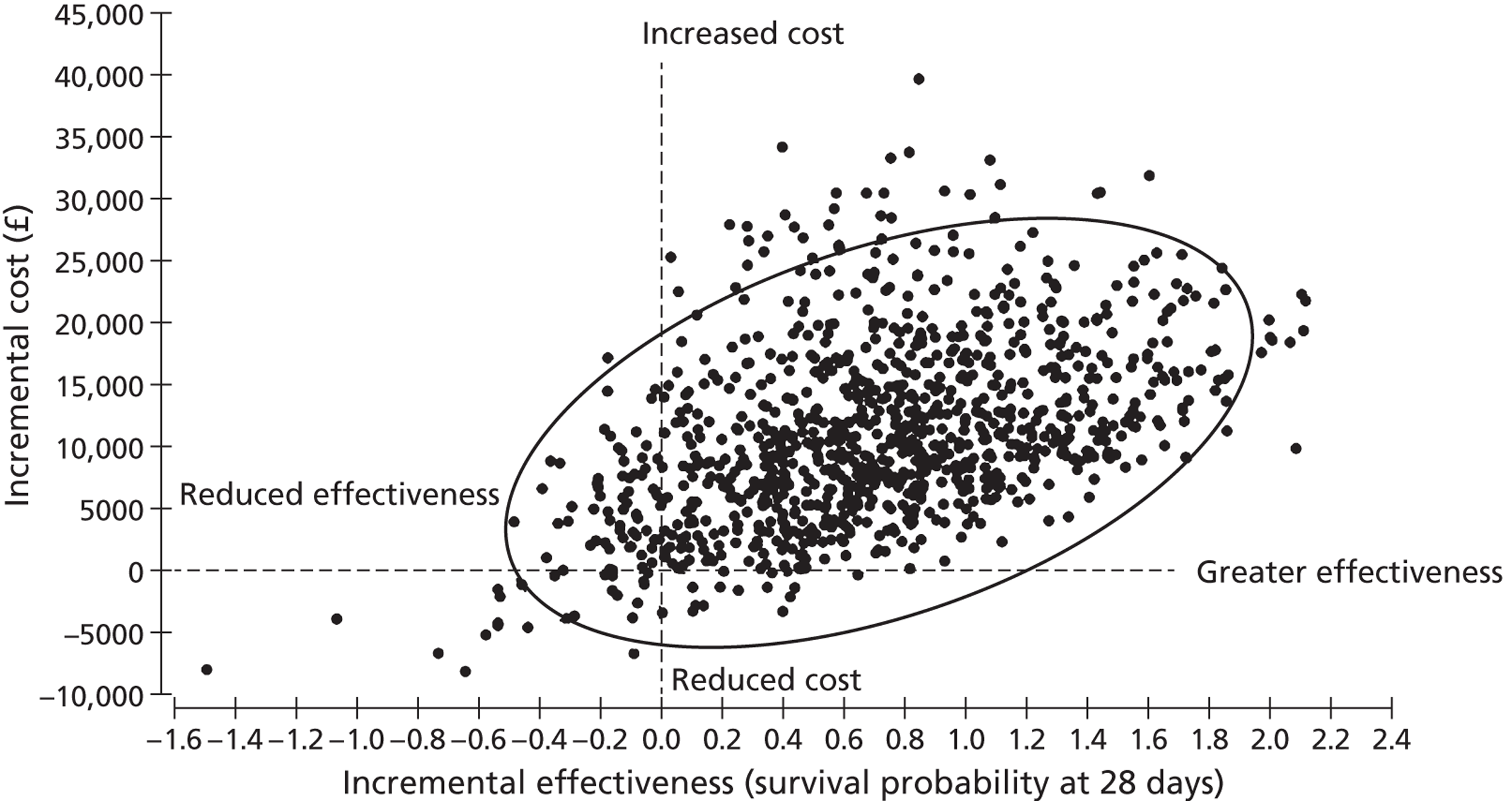
Figures 38 – 40 represent the probability of VAS being cost-effective for a given outcome of interest at various willingness-to-pay (WTP) thresholds. Figure 38 represents the cost-effectiveness acceptability curve for survival at 28 days using ITT data, which suggests that, at an arbitrary WTP threshold of £40,000, the probability of VAS being the acceptable treatment modality for achieving survival at 28 days is > 80%. However, if the WTP threshold is low at £10,000, the acceptability of VAS falls to < 30%. Similarly, Figures 39 and 40 represent the cost-effectiveness acceptability curves for survival at 1 year and disability-free survival at 1 year, respectively, using ITT data. Thus, at a WTP of £40,000 per survivor at 1 year, the probability that VAS is cost-effective is > 80%, whereas at a WTP of £40,000 per 1 disability-free year there is a < 50% chance that VAS is cost-effective. It should be noted that the WTP threshold of £40,000 is arbitrary and relates to the cost per infant surviving to 28 days or 1 year or achieving 1 disability-free year and is not comparable to NICE’s preferred cost-effectiveness threshold of £20,000 per QALY.
FIGURE 38.
Cost-effectiveness acceptability curve representing the likelihood of the acceptability of VAS for survival at 28 days at various WTP thresholds (ITT analysis).
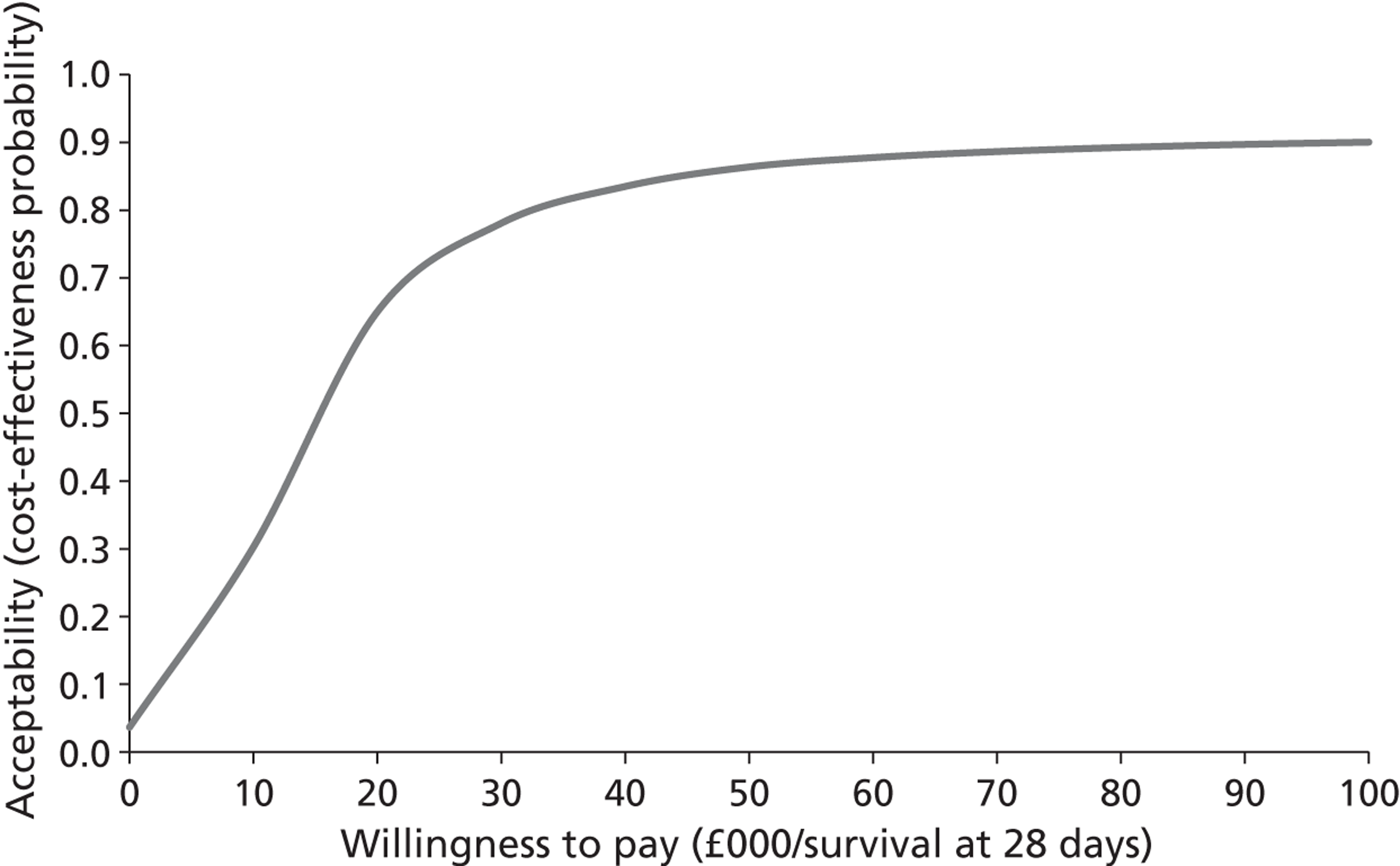
FIGURE 39.
Cost-effectiveness acceptability curve representing the likelihood of the acceptability of VAS for survival at 1 year at various WTP thresholds (ITT analysis). Reproduced from Diwakar L, Morris RK, Barton P, Middleton LM, Kilby MD, Roberts TE. Evaluation of the cost effectiveness of vesico-amniotic shunting in the management of congenital lower urinary tract obstruction (based on data from the PLUTO trial). PLOS ONE. 2013; (in press). 125
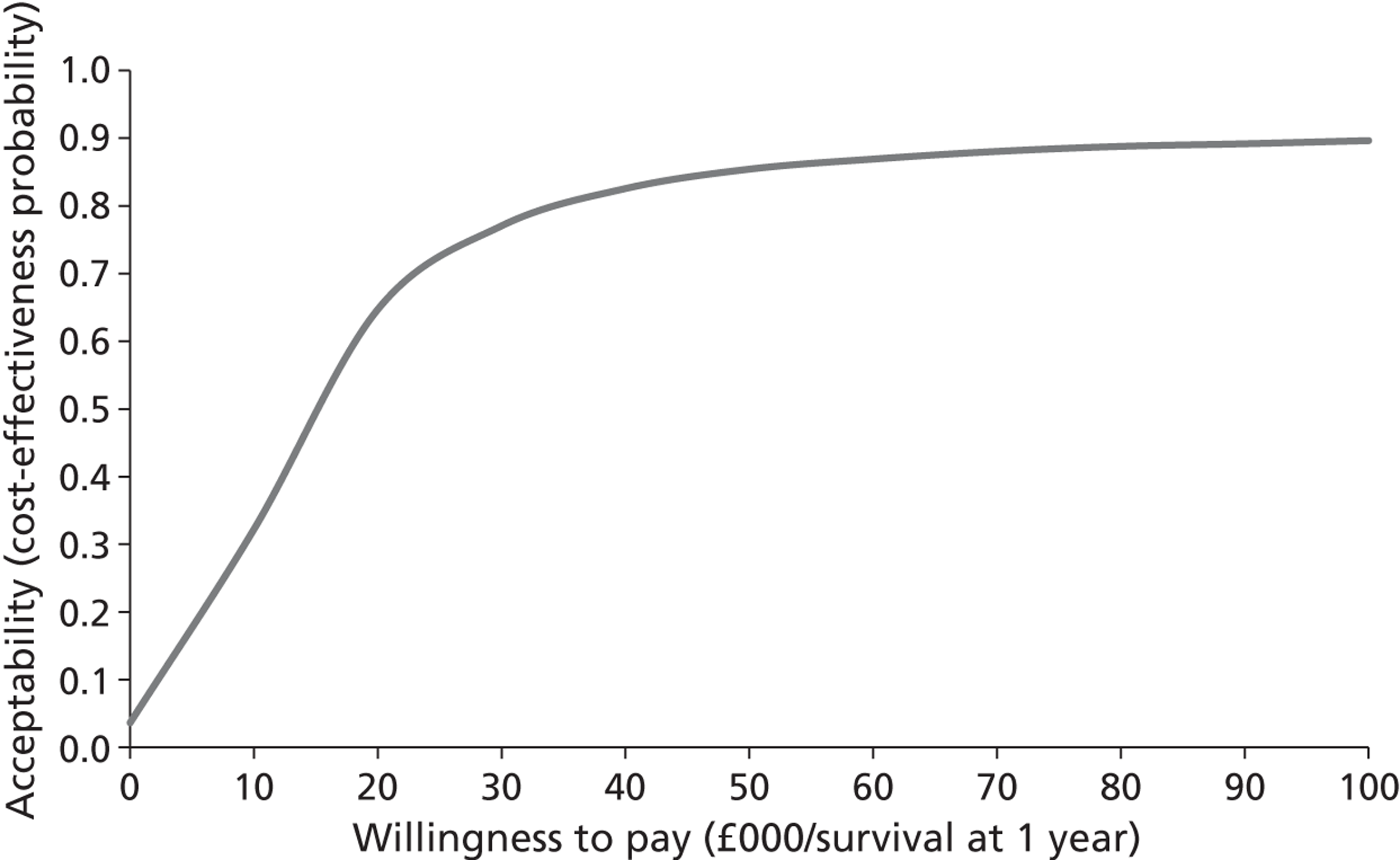
FIGURE 40.
Cost-effectiveness acceptability curve representing the likelihood of the acceptability of VAS for disability-free survival at 1 year at various WTP thresholds (ITT analysis). Reproduced from Diwakar L, Morris RK, Barton P, Middleton LM, Kilby MD, Roberts TE. Evaluation of the cost effectiveness of vesico-amniotic shunting in the management of congenital lower urinary tract obstruction (based on data from the PLUTO trial). PLOS ONE. 2013; (in press). 125
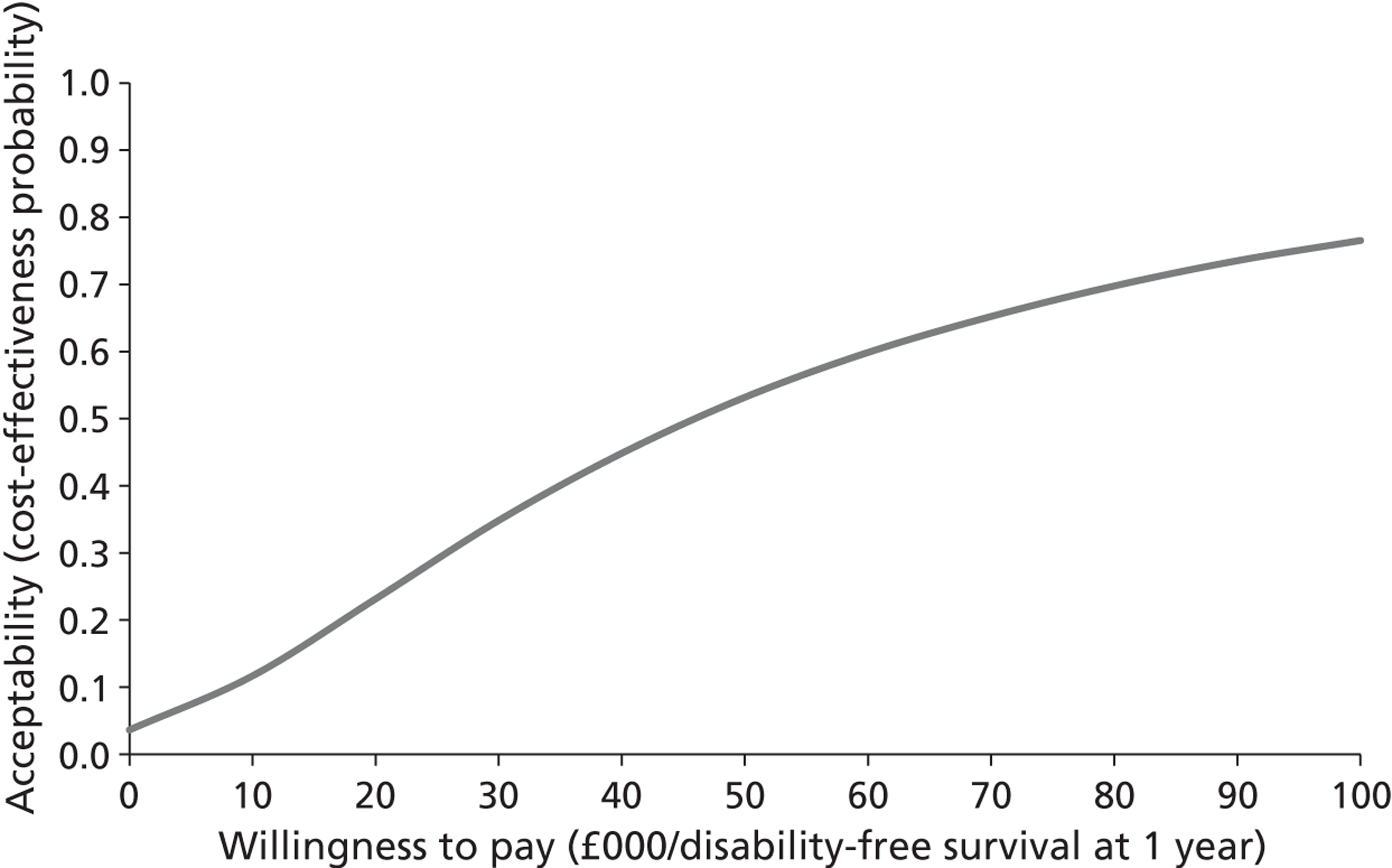
Figure 41 shows the value (per patient) of resolving the uncertainties in data obtained in this trial to obtain ‘perfect information’. This parameter, known as the expected value of perfect information (EVPI), reflects uncertainties in both costs and outcomes to calculate what additional amount it is worth paying at each WTP threshold to obtain perfect information. For example, at a WTP threshold equivalent to the ICER obtained by PSA (£15,482 for survival at 28 days using the ITT analysis), it is worth just over £3000 per patient to obtain perfect information. The ‘per-patient’ figure must then be multiplied by an estimate of the number of patients who will benefit from the results of a future study to reduce the uncertainty in the results. The result of this multiplication provides an upper limit on the amount that it would be worth spending on such a future study.
FIGURE 41.
A representation of the EVPI for 28-day survival using the ITT analysis.
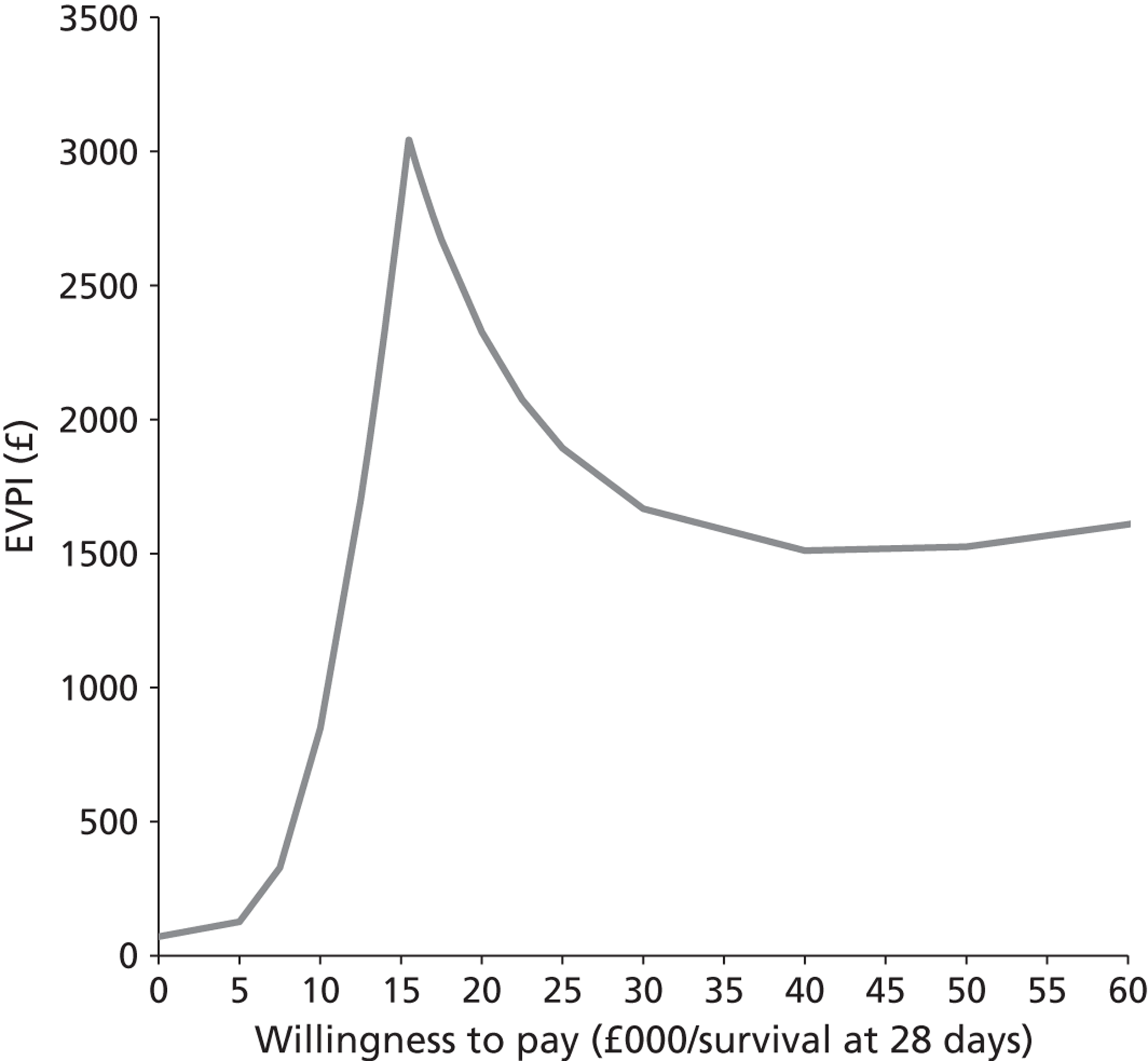
Discussion
Principal findings
Percutaneous VAS, although being more expensive, might lead to improved perinatal and 1-year survival in fetuses with LUTO. It does not appear to reduce long-term renal morbidity (at 1 year of age) in these infants as there was very little difference in the probability of being disability free at 1 year between the VAS arm and the conservative management arm.
The results were not sensitive to changes in the cost of the VAS. When the analysis was repeated according to treatment received (and not as per randomisation), the costs in the VAS arm reduced relative to the base-case costs, rendering the VAS less expensive for the same survival outcomes. Given the small number of patients within the trial, it may be argued that the uniform prior analysis is the most appropriate for this study as it considers all possible outcomes for the patients. Nevertheless, as ITT analysis is generally accepted as the least biased method of analysing data from RCTs, the conclusions in this report are based on the results of the ITT analysis. However, as demonstrated in Tables 44 – 46 , the ICER values for the base-case for survival at 28 days and 1 year in the uniform prior analysis do not vary significantly from those of the ITT analysis or indeed from the analysis excluding patients who underwent TOP.
We also found that some patients who received a VAS utilised a disproportionate amount of resources within the first year of life. Normalising these high costs improved the cost-effectiveness of VAS, even conferring dominance in the ITT analysis and analysis excluding TOP patients (method 2). Although for the purposes of this analysis we assumed that the high cost incurred by a few patients in this trial was entirely coincidental, it is unlikely to be the case. It is remarkable that all of the patients with a high overall cost (> £50,000) were within the VAS group. The high costs incurred were almost entirely due to prolonged NICU admission immediately after birth in these infants (data not shown). It is possible that these infants would have succumbed to LUTO in the absence of VAS (the infants with ‘poor prognosis’ on antenatal scans70). These children continued to have higher admission rates in the first year (beyond the neonatal period) and are responsible for the higher costs in the VAS arm relative to the conservative arm. Ironically, these are the patients who are deemed to benefit the most from VAS placement (in terms of survival). 13,21
The National Institute for Health and Care Excellence recommends that cost-effectiveness be expressed in terms of cost per QALY. 133 Cost–utility analysis enables comparison of costs across disease areas and is considered the cornerstone for health policy decisions within the UK. However, as discussed previously, QALYs or other utility measures could not be obtained in this trial. Nevertheless, we could extrapolate the ICER results obtained to surmise that an extra 0.77 QALYs would need to be obtained in the VAS arm compared with the conservative arm to make the intervention cost-effective at 1 year (assuming a cost–utility threshold of £20,000 per QALY). From the data obtained from this trial and other observational evidence, it appears unlikely that VAS would result in such significant QALY gains in these infants.
Strengths and limitations of the study
The main strength of this economic analysis is that it is the first analysis that is based on a RCT in this clinical area. The data are directly derived from the PLUTO study, which is the largest RCT (albeit small in absolute terms) in this clinical area to date. This analysis benefits from collaborative work between clinicians, nurses, statisticians and health economists. Furthermore, resource use data were collected prospectively within the trial and represent actual UK NHS costs incurred by the participants.
One major drawback of the study was the small sample size, which introduces uncertainties in the analysis. However, given that LUTO is a very uncommon condition, it may not be possible to carry out larger studies in this disease area in the future. To improve the robustness of our findings we carried out various different analyses for the base case and also incorporated extensive deterministic and probability sensitivity analyses.
Another drawback is that these findings apply only to those babies who were included in the randomised arms of the PLUTO trial. All those babies for whom the clinicians felt sure of the advantages (or futility) of VAS were included in the registry arm of the trial and were not considered in this analysis. Thus, the overall cost-effectiveness of VAS may have been under- or overstated in this analysis.
Further, it can be argued that the longer-term effects of LUTO (e.g. cognition, continence, infertility and need for dialysis or renal transplantation) will have a bearing on the overall effectiveness of VAS in the management of these patients. Indeed, the actual costs of renal morbidity do not become apparent until the child is aged > 1 year as dialysis is not favoured in infants. Resource use data from the longer-term management of these children will, therefore, be instrumental in judging the cost-effectiveness of the intervention. It was not possible to include such data in the current economic evaluation as no reliable data are currently available and the participants within the PLUTO trial are still very young.
Strengths and limitations in relation to other studies
Despite the difficulties with recruitment, the PLUTO trial is the largest RCT to study the effectiveness of VAS for the management of LUTO and the first to prospectively estimate the cost-effectiveness of the intervention.
Some researchers who have studied these patients prospectively have reported significant renal morbidity (notably end-stage renal failure requiring dialysis or transplantation)68 and others have found acceptable quality-of-life scores in a majority in the long term. 83 Such data, which will be useful in understanding the overall cost-effectiveness of VAS, are not currently available from the PLUTO trial and hence were not included in this analysis.
Meaning of this study
The results of this analysis suggest that VAS is costlier than the conservative option for the management of LUTO and there is no statistically significant increase in survival in these infants after VAS. Most infants who survived to 28 days managed to survive to 1 year in this study.
However, taking into account the uncertainty in the data, the ICER could be much higher than the base-case estimate. It is possible that VAS is both more costly and less effective than conventional treatment.
Using survival alone as the outcome of interest in these patients will be misleading as most of the survivors are left with significant renal morbidity. Therefore, the effectiveness of the intervention can be overstated if only survival is considered in the analysis. It is worth mentioning that a small study of children with LUTO (aged 1–14 years, mean 5.83 years) who received intrauterine VAS reported quality-of-life scores that were comparable to those of the general healthy population. 83 Thus, longer-term follow-up of patients recruited into the PLUTO trial may provide greater clarity regarding VAS effectiveness in this group of patients.
Unanswered questions and future research
To obtain a better understanding of the outcomes of VAS in LUTO, a larger study needs to be carried out. However, this may be difficult given the infrequent occurrence of this condition. The long-term effects of VAS on these patients need to be determined to establish the true clinical effectiveness and cost-effectiveness of the intervention. As reliable longer-term data are not currently available for VAS, we did not attempt to extend our model beyond 1 year in this analysis. However, within the PLUTO trial, longer-term follow-up of patients in the registry as well as in the randomisation arms is planned and these data can be input into the model when available.
Chapter 9 Patient acceptability and experience of the PLUTO study
Introduction
It is becoming increasingly common to utilise qualitative research within RCTs to explore in some depth the expectations, motivations and experience of participants. Qualitative data provide an additional dimension to quantitative results, allowing people to focus on the issues of importance to them and to explain how they make sense of events within the context of their lives. The growth of qualitative studies within the area of experimental medicine is hugely important, particularly in trials undertaken within sensitive settings such as maternal and fetal medicine, where participants or their proxies may be considered to be vulnerable. 134 The purpose of this qualitative study is to gain an insight into the perceptions and experiences of pregnant women asked to participate in the PLUTO trial. This seems both timely and important given the obvious uncertainty relating to the efficacy of the VAS procedure.
This work has been previously published and is reprinted from Midwifery, Denny E, Quinlan-Jones E, Bibila S, Kilby MD. The experience of pregnant women with a diagnosis of fetal Lower Urinary Tract Obstruction (LUTO), 2013, [in press and published online ahead of print November 2013]135 doi: 10.1016/j.midw.2013.10.023 with permission from Elsevier.
Methods
Aim of the study
To gain an insight into the experiences and perceptions of pregnant women asked to participate in an interventional fetal medicine trial requiring an invasive procedure.
Objectives
-
To critically explore pregnant women’s views and perceptions of the PLUTO trial.
-
To critically examine influences on the decision by women to participate or to decline to participate in the PLUTO trial, with particular regard to the impact of the information provided to them.
-
To ascertain influences on the decision-making process of women opting for TOP following an antenatal diagnosis of fetal LUTO.
A phenomenological research approach was adopted in this study as this was considered to be consistent with the purpose of the research. By following a phenomenological approach to inquiry it was anticipated that greater insights into how pregnant women in this particular situation make sense of their experiences would be obtained.
Sample
Purposeful sampling is an appropriate technique in phenomenology136 as it permits the selection of interviewees whose experiences enable an understanding of the phenomenon in question. 137 All women who were invited to participate in the PLUTO trial were also approached to take part in this qualitative study, regardless of whether they decided to enter the trial or not. A separate information leaflet was given and a consent form signed for this study.
In qualitative research a priori predictions of sample size can be difficult but observation of the basic qualitative rule of data saturation is the norm, whereby researchers continue to sample until no new or relevant data appear to be emerging in any given category and the relationships between the categories are well established. 138 In this case it was anticipated that to achieve saturation women would be recruited in similar numbers from amongst the randomised and registry patients and from amongst those requesting TOP. As the PLUTO trial was concluded early, recruitment for the qualitative study was prematurely halted and so data saturation was not achieved.
The sample comprised six women who had received an antenatal diagnosis of fetal LUTO between 12 and 29 weeks of gestation. Women interviewed were both nulliparous and multiparous and aged between 25 and 41 years. Five women who took part in this research defined themselves as white British and one described herself as black African of sub-Saharan origin. Of the six women interviewed in this study two were randomised in the PLUTO trial, three were registered and one had opted for TOP following her diagnosis. Interpreters were not required in this study as all of the women spoke English to a sufficient standard, and in only one instance was the woman’s partner present.
Methods
The phenomenological approach to data collection used in this study was consistent with the intention to gather participants’ experiences in a way that was internally meaningful and set within the context of their lives. 139 Data were collected using in-depth conversations between the researcher and the women as coparticipants. 140 An interview guide was used to ensure data collection on relevant topics (see Appendix 11 ) but participants were free to raise any issue of importance or concern to them. 141
Interviews were conducted at a time and in a place that were convenient to the participant and lasted between 35 and 50 minutes. All were tape-recorded with permission and transcribed verbatim. A form of study verification referred to as ‘member checking’ was conducted by providing the participants with a typed copy of their interview transcript to read through; they were asked to indicate whether they considered it to be an accurate reflection of what they had said during their interview. All but one interviewee responded to this request for further verification and all who responded considered the content of their interview transcript to have been accurate and fair.
A reflexive diary was also maintained by the researcher to record contextual details and reflections on the research process,95 including emerging impressions and views. 142 Limited demographic data including current age, parity, gestation at diagnosis and whether the pregnancy was singleton or multiple were collected from participants at the beginning of the interview.
Data analysis
Phenomenological data analysis involves a disciplined process designed to ensure that the details of experiences intimately contribute to an articulation of a level of generality that is helpful to one’s interests. 143 In consideration of this perception the data analysis process described by Burnard144 was adopted in this study. This began with the verbatim transcription of the interview tapes followed by intensive reading and rereading of the transcripts by the researcher to enable a search to be made for regularities, contradictions, patterns and themes within the data. Notes on general themes were made throughout the reading and rereading process to serve as memory joggers and to record ideas that the researcher had whilst working with the transcripts. The aim at this initial stage of the analysis was to become immersed in the data in an attempt to become more fully aware of the ‘life world’ of the interview participants. The transcripts were then reread and summarised again as whole documents. Individually the transcripts were then coded and further analysed to develop themes. The two main themes identified were antenatal diagnosis and participation in the study and emotional impact of diagnosis and sources of support.
A second researcher reviewed the original transcripts and the emerging themes as a form of researcher validation.
Results
Women were approached to take part in the study at a time of considerable anxiety and it is therefore unsurprising that the experience of participation in a trial was frequently entwined in their memory with the experience of receiving an abnormal fetal diagnosis during pregnancy. All of the participants in this study, however, spoke openly about the topics addressed during their interview and did not decline to answer any of the questions put to them. Despite the relatively small sample size, the interview data collected were considered to be particularly rich and interesting in content.
The presentation of the findings will be consistent with the study objectives:
-
antenatal diagnosis and participation in the PLUTO trial
-
influences on the decision by women to participate or to decline to participate in the PLUTO trial
-
influences on the decision-making process of women opting for TOP following an antenatal diagnosis of fetal LUTO.
Although this allows for clarity it is acknowledged that separating out different strands of experience creates a false distinction, and that people’s emotions and actions are interlinked and cannot easily be reduced to simple categories.
Antenatal diagnosis and participation in the PLUTO trial
Three of the women interviewed in this study highlighted the personal significance of visualising the fetal abnormality on ultrasound scan, particularly in terms of the obvious severity of the condition diagnosed. For these women the experience of seeing the abnormal ultrasound image represented a major turning point in their pregnancy, a time when they realised that things might not go well. Despite this visual evidence of an abnormality some women recalled sensing a difficulty in accepting the actual diagnosis and found themselves to be in a state of denial for some time. This they related to having previously achieved a successful and healthy pregnancy as well as the expectation that nothing would go wrong with this pregnancy:
The consultant did a scan and at that point it was clear to see that there was more of an issue because rather than being just a little dot on the scan, the baby’s bladder looked like a little water balloon inside it and even we could see that this was not good. So that was the big time for me when I realised that this was not good.
Mother 1, RCT, conservative management
Literally as she put the scan on me I could see that there was a problem because I couldn’t see anything hardly, I could just see a big black blob.
Mother 4, RCT, intervention
Most women reported the predominant emotions that they experienced following their antenatal diagnosis as being worry, distress and fear. For some the worry continued throughout their pregnancy whereas for others it lessened as time went on although it did not disappear entirely:
It was very upsetting for me to be honest and I worried about how things would be sorted out. I was worried and upset and it was hard for me to deal with especially being my first pregnancy, I didn’t expect anything to go wrong.
Mother 5, registry, conservative management
Emotions-wise I think worry was the main thing. Just worry that things were not going to go well. I think you worry for everyone involved and you worry for the little one inside, what stage he would get to, what would happen if he reached full term, were we making the right decision for him. We didn’t want to give up on him too soon.
Mother 1, RCT, conservative management
Some women described feeling a ‘sense of loss’ or ‘devastation’ after being told of their baby’s condition. Another common recollection for women was the fear that they might lose their baby, which for some was combined with a heartfelt desire to continue their pregnancy:
I guess for me it felt like I had lost something, like a death, that is the best way I can describe it. There was a sense of loss of this baby, I had chosen to have this baby and I wanted this baby, I didn’t want to start all over again.
Mother 4, RCT, Intervention
I was really emotional and I thought I would lose my baby. At the time I just kept thinking ‘is he in pain’ and things like that.
Mother 3, registry, conservative management
I am quite a logical person but in that situation I could not have even pictured a place in my head that I would have felt comfortable saying ‘please end this pregnancy’. There was just an overwhelming urge to continue and to think that everything just might be ok or I would never know.
Mother 1, RCT, conservative management
These extracts illustrate a common theme amongst women who participated in this study – what they described as a ‘rollercoaster of emotions’ following their antenatal diagnosis. Some even suggested that at times the emotions that they experienced seemed to conflict and proved challenging to resolve on a personal level. So, for example, the woman quoted below describes feeling anger at being out of control when first receiving a diagnosis, but a later sense of relief at having decisions taken out of her hands:
That is when the conversations started about the trial and that is when I had feelings of anger. I think this came mainly because of feeling out of control, not having any control of what might happen, and not just us but the doctors not having any control over what was then going to happen. It felt very unfair, that was probably the most difficult time.
When we went for the scan and realised that the baby’s heart was no longer going, we both felt a sense of relief, we didn’t want to make that decision [to terminate the pregnancy] and it got took out of our hands so that was ok because nature had decided and it was not meant to be and we could live with that an awful lot better.
Mother 1, RCT, conservative management
Partners and significant others were also affected by strong emotions on receiving an antenatal diagnosis of LUTO: ‘My partner definitely, it has caused quite a lot of extra ripples for him. He was extremely depressed and still is quite low but getting through it. I think it’s just sadness now really’ (mother 6, TOP, conservative management). However, the feelings of partners were sometimes at variance with the emotions of the woman, in terms of dealing with the diagnosis and getting through the following months:
I think he has just blocked himself off from hearing anything negative and I think that if anything was to happen when the baby is born, I would be more prepared than he would and I think that is when we have a problem.
Mother 4, RCT, intervention
My partner is supporting me but he mentioned that we should perhaps consider a termination and I found that very difficult to accept from him.
Mother 2, registry, conservative management
One woman reported that she worried deeply throughout the pregnancy that her husband had not grasped the potential gravity of the situation and remained somewhat in denial of the fetal diagnosis:
He thinks that everything will be fine and he cannot understand why we are having a baby but we are not preparing for a baby so I think sometimes the person I need to speak to I can’t because he won’t listen to me or he won’t have it any other way.
Mother 4, RCT, intervention
Other family members were also affected by a diagnosis of LUTO and it was felt that this could lead to them experiencing increased levels of anxiety during subsequent pregnancies:
When I lost the baby in the end I think that my family were extremely sad. I don’t know if they would be more wary if I became pregnant again but that would be interesting to see, how people react then.
Mother 6, TOP, conservative management
Three women described feeling a specific need to use the internet to find out further information about LUTO following their antenatal diagnosis, such as its causes, treatments and effects:
The internet is a wonderful thing and you can get so much information, I know we certainly tend to seek out more information to try and understand what has happened or what could happen.
Mother 1, RCT, conservative management
I am a bit of a web hound anyway so I found it very easy to go and find the right information but if you had been pointed to the website and Google and were told go and have a look and you were not really sure what you were doing maybe that would have been difficult.
Mother 6, TOP, conservative management
In contrast, some women reported a lack of reliable information on the internet, which created a sense of confusion and frustration within them as conflict arose between their personal experiences and the experiences of others in managing this particular complication in pregnancy:
To have seen stuff out there and I cannot say whether that is right or wrong, and then to be sitting in a room getting told we cannot say whether it [a treatment] works or not and all it comes down to is whether a computer says yes or no, was very, very hard.
Mother 1, RCT, conservative management
Commonly, women reported that they felt themselves to be in ‘safe hands’ following their diagnosis and appreciated the particular reassurance provided by regular ultrasound scanning. They reported that in general they were satisfied with the standard of care that they received during their pregnancy and felt relieved that the condition was detected antenatally, thus allowing them the opportunity to prepare for the delivery and the potential postnatal course:
Going for the scans put my mind at ease. I hadn’t been looking forward to going for them but I am glad I have had the opportunity to go every 4 weeks and keep an eye on his kidneys and stuff.
Mother 3, registry, conservative management
However, for one mother who reported that each scan seemed to reveal more problems, this was more worrying:
It is actually the scans that frighten me because I think what is going to be coming up next because one minute it is the kidneys and the bladder, then they say the head hasn’t grown much, then it’s the fluid around him and I am thinking every time I go for a scan everything like seems to be going wrong.
Mother 5, registry, conservative management
The obvious value and genuine reliance that some women placed on this form of medical intervention during pregnancy was evident within the interviews, and some women suggested that regular scanning assisted to some degree with bonding with their baby. This contrasts with the ‘turning point’ that women described feeling on undergoing their first scan. Certainly none of the women interviewed appeared to have any safety issues with regard to receiving multiple and sometimes lengthy ultrasound scans during pregnancy. Possibly the perceived benefit that regular ultrasound scanning provided to them both on a personal level and with regard to their fetus outweighed any risks that they may have associated with the actual procedure and its potential cumulative effect in pregnancy.
In general, women interviewed were highly satisfied with the standard of the fetal medicine care that they received and reported a sense of relief that their pregnancy complications had been detected before their baby’s delivery:
We were getting the best advice and that is as much as you can ask for really, to know that the people you are dealing with are the right people and we totally, totally felt that.
Mother 1, RCT, conservative management
I was pleased with the treatment I was given and I was pleased it was picked up so soon and obviously having the extra scans done put my mind at ease.
Mother 3, registry, conservative management
Influences on the decision by women to participate or to decline to participate in the PLUTO trial
Various influences were presented by women to account for their personal reasons for participation in the study. A common perception among the women interviewed involved them deriving some personal benefit from taking part, either for this or subsequent pregnancies. In addition, women frequently reported that they were encouraged to participate for more altruistic reasons. Indeed, altruism is commonly reported by women as a reason for research participation during pregnancy:145,146
It was more that if I did go on to have another child I would know what had possibly caused the problem and I felt that [taking part] would help other mothers, pregnancies and other people’s daughter.
Mother 5, registry, conservative management
Me and my husband wanted to go into the study and I felt happy to do that because at the end of the day it is just to help somebody.
Mother 3, registry, conservative management
It was also reported by some women that participation in the study was considered to be their only perceived real option at the time. In addition, women often viewed the opportunity to participate in the study as being able to offer their baby a ‘chance’, a lifeline as one mother described it:
I really didn’t feel that there was any other option, we were just going along with ‘let’s see how this goes’,
Mother 1, RCT, conservative management
I suppose for both of us really it was just that we wanted the baby and we wanted a chance so the trial for us was still a chance. Although you don’t know, it seems to make sense to us that it [the treatment] is something that could help so why not just do it. When you have been through something so dire, to have a little bit of hope is like being thrown a lifeline.
Mother 4, RCT, Intervention
Further reasons reported to influence participation related to women’s perceptions of the extent of existing ‘damage’ to the baby, particularly in earlier gestation, or of the treatment procedure itself:
It was the results of the scan really when professor described the damage that was already there and knowing that even with the trial intervening the damage was already done.
Mother 6, TOP, conservative management
The actual treatment procedure really scared me and I didn’t want to be randomised in case I was the one chosen to have the shunt put in.
Mother 2, registry, conservative management
Personal faith also represented an influential factor relating to study participation but this was raised by only one woman: ‘I am praying a lot and hoping that the baby will be ok but as a Christian I believe that we shouldn’t interfere with nature’ (Mother 2, registry, conservative management).
In general, the women felt that they had fully understood the concept of the study when it was introduced to them. Most also considered it beneficial to have had the study explained to them by someone closely involved in the project, that is, a midwife linked directly to the research:
I think having someone to explain it to you first would be good and then have information to go away and think about.
Mother 1, RCT, conservative management
I was happy with the way I was told, I had plenty of leaflets about the study and you [research midwife] explained everything to me anyway, so I totally understood.
Mother 5, registry, conservative management
It was suggested by some women that receiving more information might have been helpful when making their decision whether to participate in terms of assisting them to make a more informed choice: ‘I think the way they did it (explained about the study) with us was fine but I think maybe if there is anything where there are case studies or statistics that you can give then that might help’ (mother 6, TOP, conservative management). The concept of informed choice and patient consent is a particularly complex and debated issue and it could certainly be argued that within the context of professional–patient interactions the facilitation of truly informed decision-making and consent is questionable given the subtle power that health professionals naturally exert over their patients.
Some women in the study felt that they did not require any additional time beyond the minimum of 24 hours as encouraged for ethical purposes to make a decision to participate and considered this to have been a hindrance to the recruitment process: ‘I just wanted desperately to be picked and I wanted them to do the shunt that day. I didn’t want to wait, I didn’t want to wait any more than what I had already’ (mother 4, RCT, intervention).
However, some women reported that they would have particularly appreciated being able to communicate with someone who had personal experience of this diagnosis in pregnancy, suggesting that this might have been helpful when making a decision whether or not to participate in the study. The reasons why some women expressed this preference were not altogether clear during the interviews but their need for this type of specific peer support can be surmised. For example, conflicting internet information available to some women in this study may have caused them to doubt what they were being told by professionals involved in their care and encouraged them to want to seek additional reassurance and support from others with direct experience of a similar situation in pregnancy. Similarly, some women may have particularly appreciated the potentially unbiased manner with which a peer with shared experience could have offered them focused support and information, in other words, they could have told them about the reality of the situation:147
We would like to have been told about others that had gone through the same thing as us as this would have been helpful.
Mother 3, registry, conservative management
I just would feel better if I could talk to somebody who has had a baby with it or been through the same.
Mother 4, RCT, intervention
Influences on women opting for termination of pregnancy
Although a number of women decided to terminate their pregnancy following diagnosis of LUTO, only one agreed to be interviewed. It is worth giving some space to her story while recognising that it is individual and cannot be held up as representative of women approached to take part in the trial who subsequently opted for termination.
The woman concerned and her husband received an initial diagnosis of LUTO at a district hospital and were referred to a fetal medicine centre:
You keep like this tiny bit of your brain in saying that we might get to [fetal medicine centre] and it’s a miracle and actually there is nothing really damaged and that something can be done which is a possibility and we know that now but at the same time you are trying to prepare yourself for the worst.
I think that felt like the longest week and the hardest and most horrific week of my life . . . it was a whole process of having to come to terms with the fact that your baby looks healthy and is acting healthy but isn’t and that you are ultimately likely to be facing a terrible decision and I think it was the deep concern, worry and fear about that is a distinct memory for me in that period.
Later in the interview this woman spoke of her feelings during the week between receiving a definitive diagnosis and prognosis and the termination of her pregnancy.
Fear was obviously the first thing and sadness obviously because as soon as something like that happens, maybe not everybody does but we both thought the worst and that this is likely to end badly and it was distinct sadness and grieving even before the decision had been made and I felt that we generally were grieving even until the point where we were offered the trial and told about the damage that was there and made that decision, which may seem premature to do so but we had a very clear idea of what was wrong and that it was almost as if we went into another stage of grieving. We both felt we were in a bit of cloud for days, depressed let’s say, didn’t want to really speak to anybody particularly.
Deciding on a termination was something that was agreed between this woman and her husband before they knew the prognosis, as they both had similar views on severe disability. It was this that influenced their decision-making:
Different people have different views on disability and all those different things and there is a level of disability that you can live with. I think we had both discussed this because of my age and what would do because you have to take these things this seriously because it can happen and I think we both said to ourselves if there is anything seriously wrong it will bring suffering to the child and make their life less than we would want it to be and we would have to make a tough choice and we were both of the same mind. So as soon as this happened I think we both knew what decision we would make unless we can reverse this and it would be fine. You know what I mean? Or if it was a minor ongoing complaint that needed treatment that would have been fine but it is severity of it. It was grief for a healthy baby, grief for what we had planned if I am honest. It is the healthy child. We had worried about all sorts of things, who was going to give up their job, are we going to be able to go out on Friday night and then this happened and you say none of that matters, what mattered was that it was going to be a healthy child so I think it was probably that more than anything.
Mother 6, TOP, conservative management
She later expressed the view that had they been told at the fetal medicine department that the baby’s kidneys were in a better condition than expected and that some treatment could be offered then they would have continued with the pregnancy. A more favourable prognosis would also have encouraged this couple to take part in the trial, but because of the amount of damage already done to the kidneys they felt that it was too late for them.
Discussion
Summary of findings
The study findings demonstrated that various factors appear to be influential to women when deciding whether to take part in research during pregnancy, which, for the purposes of simplified illustration, were reported in a manner consistent with the objectives of the study.
In summary, the majority of women interviewed in this study described the significant personal impact of visualising the fetal anomaly on ultrasound scan, with some remembering this as a particular ‘turning point’ in their pregnancy when it first became apparent that things might not go as they had expected. Some women also experienced difficulty in initially accepting the diagnosis and found themselves to be in a state of denial for some time. Some women attributed this denial to not expecting anything to go wrong with their pregnancy. The emotional impact for partners and significant others following diagnosis was also highlighted by some women in this study, who suggested that reactions had included sadness and depression and increased anxiety levels during this and potentially even future pregnancies.
The need for more detailed information about the condition and its implications during pregnancy and following delivery was highlighted by a number of women interviewed in this study, with many turning to internet sources for additional knowledge and support. Using the internet in this way was reported by some women to have been hugely beneficial, which is consistent with the findings of a global study on the influence of the internet on decision-making in pregnancy,148 which concluded that its impact on all aspects of pregnancy was highly visible, with information-seeking being the key theme. In this study some women also suggested that the information that they found on the internet led to feelings of confusion and frustration, particularly in terms of the apparent differences in the way in which the condition is managed in the UK and elsewhere.
Most women in this study described feeling in ‘safe hands’ following their diagnosis and found particular reassurance in the regular ultrasound scanning that they subsequently received, although this was not the case for one woman, who observed a worsening problem on subsequent scans. Some suggested that this regular scanning assisted to some degree with bonding with their baby and was especially valuable to them in terms of providing immediate reassurance that their baby was still alive. Most women interviewed reported that in general they were satisfied with the standard of care that they received during their pregnancy and felt relieved that the condition was detected antenatally, thus allowing them the opportunity to prepare for the delivery and the potential postnatal course.
In terms of the influences on participation in this study, personal benefit and altruism were reported as the most common reasons by women interviewed. This is consistent with the literature. Typically, women will agree to be recruited into clinical research when the benefit to the fetus is considered probable,146 and the mother’s/parent’s duty to their (sometimes unborn) child will usually be given a higher priority than consideration for the mothers own health. 145 Women undoubtedly feel an inherent responsibility for the child that they carry to the extent that they often modify their habits and behaviours during pregnancy. 146 As such, pregnancy may impair their ability as women to make ‘free’ choices, making them feel bound to accept interventions that might benefit the unborn child or causing them to decline treatment for themselves because of potential teratogenic fears.
Enrolment into this trial was described by one mother as being ‘her only real option’ in terms of being able to give her baby a chance. Personal faith, fear of the actual procedure and the knowledge that existing ‘damage’ to the baby could not be corrected were other reasons cited as influential in non-participation in this study.
Support from a clinical trial co-ordinator or research nurse with responsibility for trial recruitment has been found to be positively associated with recruitment to research studies. 149 In general, women interviewed for this study felt that they understood the concept of the study when it was introduced to them but reported that it had helped to have the details of the research explained to them by someone closely involved in the study,95 in this case a research midwife. It was acknowledged that the communication style of the researcher responsible for recruitment was particularly important to women considering participation in this study, with women citing that an open, honest and detailed discussion was important to enable them to make an informed choice with regard to participation in the research.
Strengths and limitations of the qualitative study
The strength of the study lies in the rich data obtained on a poorly understood phenomenon. The use of a phenomenological approach resulted in an in-depth understanding of participants’ experiences that were internally meaningful.
The limitation, as with all qualitative research, is that findings are not generalisable outside of the study population. In particular, the one woman who opted for TOP was not representative of either the trial population or the population of women opting for TOP, most of whom did not enter the trial. However, as the aim of the study was to gain insight into experience rather than to extrapolate to a wider population, her story merited inclusion. Moreover, as participation in an interventional fetal medicine trial is an under-researched area, it is not possible to obtain additional verification of findings by embedding them within existing literature. A further limitation is that because of low recruitment numbers it was not possible to achieve data saturation, and therefore it is not known whether there are additional important issues to uncover. Similarly, as the study was terminated early, it is not known how women’s perceptions about inclusion in the RCT would have altered with time. Women’s experiences of the trial were bound up with their experiences of receiving a fetal diagnosis during pregnancy, and the outcome of that pregnancy, in particular a negative outcome, may have influenced their ultimate views on the management of LUTO.
Consideration of the ways in which the participants’ perceptions of the interviewer influenced the interview interactions may have particular significance with regard to this study. Interviews were conducted by the dedicated research midwife involved in the PLUTO trial, who was required to maintain an effective professional relationship with all trial participants. It is possible that some women involved in the trial may have considered that this created some ‘distance’ between the midwife as a ‘professional’ and themselves as ‘patients’. Some participants may have assumed that the midwife would act purely in the best interests of them and their babies and therefore would not discuss involvement in research that might carry risks. 95 However, this patient–professional relationship could also lead to participants responding to questions in a way that they deemed socially or culturally acceptable, or expected.
Implications for practice
As data saturation was not reached within this study, further research with women who are asked to participate in both interventional and non-interventional clinical trials during pregnancy would provide additional data on the expectations of women/couples and their motivations for participating.
However, the findings of the study do raise issues that have implications for practice. First, they have demonstrated some of the influences on decision-making that women report when considering their potential involvement in research during pregnancy. Knowledge of the factors that appear to influence decision-making with regard to participation may be used to tailor future research designs to meet the needs of pregnant women and address the issues of importance to them around this difficult time. In addition, the requirement of women for more appropriate information sharing regarding their particular fetal anomaly seem to be a particularly important finding of this research and one that all clinicians involved in the recruitment of pregnant women to research studies should endeavour to meet.
The importance of ongoing focused discussion with regard to entry into clinical research is also a consideration for clinicians and research staff recruiting to clinical trials. Pregnant women may need to speak to professionals from different disciplines and specialties as well as dedicated research staff as they consider their potential involvement in research and may also require numerous consultations to take place before they feel able to decide whether to agree to participation.
It seems that an individualised approach to recruitment of pregnant women to research might be beneficial not only to ensure that recruitment targets are facilitated but also to enable the individual informational needs of women to be met. An individualised approach to recruitment of pregnant women into research studies will of necessity be mediated by both time restrictions and the availability of various clinical experts, but this could be facilitated by the use of dedicated research staff such as midwives who can focus on support during the entire research process. Indeed, the ability of women to be able to discuss the implications relating to their potential involvement in research in pregnancy was reported to be very helpful by the participants in this study.
Finally, although the experience of receiving a fetal abnormality diagnosis such as LUTO in pregnancy does not appear to deter women from embarking on a subsequent pregnancy, it should be remembered that the emotional impact that receiving this type of diagnosis infers is significant to women and their partners alike. The frequent visualisation of the fetal abnormality on ultrasound scan seems to provoke mixed emotions in families and serves as a reminder of the need for professionals to be highly sensitive during ultrasound scan assessments and during the discussions that follow them, and to be both observant and reactive to the needs of women undergoing regular ultrasound scan assessments in this respect.
Chapter 10 Discussion
Congenital anomalies of the genitourinary tract are identified using prenatal ultrasound in between 1 : 250 and 1 : 1000 pregnancies (the rates being dependant on the inclusion of terminations of pregnancy and prenatal and postnatal acquisition). 1 Chronic LUTO, also known as fetal bladder outlet obstruction, will predispose the fetus to abnormal renal development and function and this risk continues into childhood. If there is severe prenatal renal impairment, the condition is commonly associated with significant oligohydramnios (reduced liquor volume). Such an association when present at mid-gestation (between 16 and 24 weeks) is associated with pulmonary hypoplasia in a high proportion of pregnancies, resulting in high perinatal mortality and morbidity rates for the fetus. 2–4 Chronic oligohydramnios may be associated with positional postural anomalies such as talipes. When made prenatally the diagnosis occurs most commonly at 20 weeks of gestation when the majority of pregnant women have a routine detailed fetal anomaly scan. Bladder drainage by serial vesicocentesis (insertion of a fine needle to drain the fetal bladder at regular intervals) and continuous drainage into the amniotic cavity by in utero VAS have been used to relieve fetal LUTO (bypassing the urethral blockage) in an attempt to reduce or avoid renal parenchymal damage and chronic oligohydramnios that may adversely affect pulmonary development. 5–7 These ‘treatment’ procedures may be associated with theoretical maternal morbidity (in terms of infection risk) and fetal morbidity (infection or bleeding). They also carry a risk of miscarriage (2–5%) and the risk of serial drainages is often cumulative, making VAS insertion, at least in theory, preferable.
Evidence of clinical effectiveness and cost-effectiveness
The PLUTO study aimed to evaluate the clinical effectiveness, cost-effectiveness and acceptability of intrauterine VAS to treat fetal bladder outflow obstruction. Although we aimed to undertake an appropriately powered RCT to assess clinical effectiveness, an inability to recruit to the study led to its early termination, with only 31 participants (out of a planned 150) recruited during the 4-year study period from October 2006 to October 2010. The study is thus underpowered to detect the hypothesised treatment effect. The results in this report are based on the 12-month follow-up of the 31 participants. The 24-month follow-up has also now been completed and published. 114 Follow-up is planned up to 5 years of age.
The primary outcome in the trial was survival to 28 days. Those who were randomised to VAS were observed to have a twofold higher 28-day survival rate (50%) than those allocated to conservative management (27%). This difference was in fact larger than that hypothesised in the sample size calculation, but is not statistically significant (p = 0.27). Thus, it is not possible to formally conclude benefit or rule out the possibility of VAS having a harmful effect. The 95% CI for the overall effect ranged from a relative harmful increase in mortality of 29% to a relative increase in survival of 396%.
We wished to explore the degree to which the uncertainty that exists concerning the value of VAS obtained from the PLUTO trial can be lessened by including opinions from clinical experts. Opinions on the value of VAS were elicited from experts119 who reported that they believed that, on average, VAS would lead to a relative increase in the odds of perinatal survival of 23%, but with a 95% range of views, from a decrease in survival of 48% to an increase in survival of 192%. Differences were noted in the opinions of experts from paediatric nephrology, paediatric urology and fetal medicine, with fetal medicine specialists being the most optimistic. When combined with the study data in a Bayesian analysis, the average estimated increase in survival rose from 23% to 31%, and the 95% interval was constricted to lie between a decrease in risk of 16% and an increase in risk of 118%. The trial has had value in focusing the range of values that can be considered as likely estimates of effect, but it has not ruled out concerns that VAS may have a harmful effect. We also estimated the probability that VAS had a large clinically important effect (a relative increase in survival of ≥ 55%). Although the experts considered the probability of this to be 5%, combining evidence from the trial raised this probability to 25%.
Follow-up data were available up to 12 months. The direction and magnitude of the difference in mortality remained across this period, with survival at 12 months being 44% in the VAS arm and 20% in the conservative treatment arm, but the difference again was not statistically significant (p = 0.25). Detailed analysis showed the antenatal survival rate to be lower in the VAS arm than in the conventional care arm before delivery (potentially because of procedural mortality) but higher in the VAS arm than in the conventional care arm after delivery. However, many of these babies had abnormal renal function, with the rate of survival to 12 months with normal renal function being 13% (2/16) in the VAS arm and 0% (0/15) in the conservative treatment arm. Thus, the chance of healthy survival was low in all groups. The results of the RCT are consistent with findings of retrospective, observational studies suggesting that VAS improves perinatal and 1-year survival but that the chance of surviving with normal renal function is poor regardless of intervention.
The health economic analysis used a model-based economic evaluation to explore the relative cost-effectiveness of VAS compared with standard conservative management. A decision tree was constructed and data inputs on resource use and outcomes were derived from the RCT. Unit costs from routine sources were applied to resource use. The model adopted a time horizon of 1 year and took the perspective of the NHS.
The average health-care cost for VAS was £21,000 whereas that for conservative management was £9900. Only a small proportion of this increase related to the cost of the VAS procedure (estimated as £820); additional costs occurred mainly through additional surgery and intensive care costs. The incremental cost per additional survivor at 28 days was estimated as £15,500 and per survivor at 1 year as £15,400. Taking into account the poor health of many of those who did survive, the incremental cost per disability-free survival at the end of 1 year for VAS was much higher at about £43,900. However, taking into account the uncertainty in the data, the ICER could be much higher than the point estimate suggests and it is possible that VAS is both more costly and less effective than conventional treatment.
It was noted that about one in six women did not comply with their randomised allocation (crossovers occurring in both directions). When trial data were reanalysed on the basis of the treatment received rather than the treatment allocated, differences between groups in perinatal and 1-year survival became larger and of borderline statistical significance. The validity of such an analysis is questionable, though, as decisions to switch treatment were likely to have been made with knowledge of prognosis, and thus this risks introducing selection bias. An analysis of prognostic markers in the combined RCT and registry data indicated that liquor volume and gestational age at diagnosis were prognostic of outcome, confirming the potential for selection bias to occur through treatment crossovers. Use of the AT data made little difference to the results of the cost-effectiveness analysis.
Data collected in the registry of patients not randomised were in contrast to those from the RCT and other observational evidence, suggesting a poorer survival to 28 days with VAS (RR 0.58, 95% CI 0.26 to 1.29) and poorer survival at 1 year with VAS (RR 0.44, 95% CI 0.17 to 1.16). At 1 year of age, 50% (12/24) of those in the conservative group had normal renal function compared with only 33% (1/3) in the VAS group. This difference in findings was explained by confounding. Those women who entered the study registry and had conservative management were more likely to have a normal liquor volume at diagnosis (greater than the fifth centile) than those receiving VAS (p = 0.07) or those randomised (p = 0.05). They were also more likely to have been diagnosed at > 24 weeks’ gestation than those randomised (p = 0.003). These variables were strongly associated with improved survival to 28 days in a multivariable logistic regression analysis. There was also, significantly, a high false-positive antenatal diagnosis rate of 24% in the registry conservative management group (comparable with that identified in the epidemiological study); the eventual postnatal diagnosis in this group was either normal renal tract or vesicoureteric reflux (conditions with a much better prognosis). Exclusion of these false positives from the logistic regression analysis did not alter our conclusion that gestational age at diagnosis and liquor volume were associated with survival.
Why was it hard to recruit to the trial?
The barriers to recruitment were identified as being fourfold. First, there was a difficulty in getting the number of UK and international centres required to meet the recruitment targets. In the UK this was because of a delay in centres gaining ethics and local R&D approval, although eventually all UK fetal medicine centres were approved. Barriers to international recruitment arose through issues related to obtaining adequate research governance arrangements and insurance indemnity.
Second, the prevalence of disease was lower than anticipated from the literature. Our epidemiological study estimated the incidence of LUTO as 3.34 (95% CI 2.95 to 3.72) per 10,000 total births (1 in 2994). A lower proportion of cases was identified antenatally (66%) than previously documented, further limiting the number of cases suitable for intervention antenatally.
Third, a high proportion of women and clinicians expressed a preference for a particular intervention, often for conservative management. In total, 45 women were entered onto the registry of whom the majority (78%) underwent conservative management. The reasons for entry onto the registry were reluctance by women to be randomised or to have an invasive procedure and clinician preference for a particular treatment. Two further pieces of evidence are of relevance. First, the elicited beliefs of the expert clinicians were quite pessimistic concerning the likely benefit of VAS, suggesting that many clinicians may not have referred patients for inclusion to the PLUTO trial because of preconceived opinions that the intervention was not beneficial. Second, the study of acceptability indicated that women often had strong reasons for non-participation, particularly around fear of the shunting procedure, personal faith and perceived extent of the condition.
Finally, both in the epidemiological study and in the study centres, a large proportion of women elected for TOP. Over the period of recruitment there were 68 TOPs notified to the trial office. It is likely that this is an underestimate of the total number of TOPs performed for this condition. Our register did not receive returns from all centres and did not include the international centres. Although robust data for the number of TOPs performed annually for LUTO does not exist, in both reports from the national congenital anomaly register (BINOCAR)96 and the ONS,116 urinary tract anomalies are associated with a high termination rate compared with other anomalies.
Implications for practice
The findings of the PLUTO trial are limited by two main factors: the small numbers recruited, meaning that the study did not have adequate statistical power, and follow-up only until 1 year of age (currently), thus limiting the conclusions that can be made regarding the long-term effects of VAS on renal function. Any recommendations must be interpreted in this light. The results of the RCT and observational evidence21 are consistent and thus the body of objective evidence suggests that VAS improves overall perinatal survival compared with conservative management but that the long-term prognosis for these babies into infant life is poor (with high rates of mortality and morbidity). The health economic analysis suggests that the costs associated with this small gain in disability-free survival at the end of 1 year are high and thus VAS is unlikely to be judged cost-effective. Much depends on the long-term survival of those who have reached 1 year; should they continue to have poor health and prognosis the value of VAS will be seriously questioned.
Parents should be counselled about the risks of pregnancy loss with or without VAS insertion and the likely health state of those who do survive. The NICE interventional procedures guidance (IPG 2028) should be updated to reflect this evidence.
Recommendations for research
As the PLUTO trial was a multicentre, international trial and it did not recruit to the required number to achieve statistical power, it is unlikely that a further RCT would be feasible. It is imperative that the babies recruited into the PLUTO trial are prospectively followed up throughout childhood to determine the effects of VAS on outcomes such as renal function, incontinence, cognitive development and quality of life. Ideally, the registry would continue as a national registry, such as those that exist for Down syndrome and cleft palate, with collection of outcomes in LUTO babies to assess whether prognosis improves. However, the numbers of children surviving to this age (2–5 years) is likely to be small.
There should be further research to try and overcome the barriers to recruitment identified within this study, namely in relation to the methodology of RCTs in rare diseases (especially relating to pregnancy). The study was limited in its recruitment ability because of the difficulties in obtaining indemnity and thus sponsorship for international centres. This is something that the international academic community, higher education institutions and funders must work hard to resolve so that research questions can be adequately resolved in the study of rare diseases.
The factors that appear to influence decision-making with regard to participation in an RCT may be used to tailor future research designs to meet the needs of pregnant women and address the issues of importance to them around this difficult time.
Acknowledgements
We thank the members of the trial steering committee for their assistance throughout the project: Professor Martin Whittle (Emeritus Chair, Fetal Medicine, University of Birmingham, and chair of the committee until the end of 2009), Professor David James (Professor of Obstetrics and Gynaecology, University of Nottingham, chair from 2010 onwards), Professor D Field (Neonatal Paediatrician, University Hospitals of Leicester, Leicester University), Professor Y Ville (Fetal Medicine, Hôpital Necker, Paris), lay representative Mrs Sarah Caranchi (midwife and mother of baby requiring in utero treatment), Professor Adrian Woolf (Paediatric Nephrologist, University College London/Manchester) and Mr Nicholas Madden (Paediatric Urologist, St Mary’s Hospital, London).
We thank the members of the data monitoring committee for their assistance throughout the project: Dr C Cummins (Paediatric Epidemiologist, University of Birmingham and Birmingham Children’s Hospital, Chair), Professor J Thornton (Professor of Obstetrics and Gynaecology, University of Nottingham) and Professor J Neilson (Professor of Obstetrics and Gynaecology, University of Liverpool).
The PLUTO trial was co-ordinated by the Birmingham Clinical Trials Unit and we acknowledge the hard work of all of the staff involved in the study, especially the trial co-ordinators, Leanne Fulcher, Laura Magill, Elizabeth Lawson and Edward Tyler, who designed and developed the trial database.
We thank all of the principal investigators and the many sites (see Appendix 2 ) and the fetal medicine midwives at the centres.
We thank Robyn Caley, Catherine Franklin and Chris Blount of WMCAR for their work in the collection, validation, clinical coding and data processing of all notifications for the epidemiological study. Ms Ann Tonks and Professor Jason Gardosi of WMCAR and the West Midlands Perinatal Institute were involved in the epidemiology study (data collection and analysis and authors of the article on which Chapter 3 is based). We also thank the Perinatal Institute’s regional network of ultrasonographers, midwives, obstetricians and paediatricians for supplying clinical information on anomaly cases, and Liam McCarthy for his help with retrieving data from the Birmingham Children’s Hospital NHS Foundation Trust.
We acknowledge the contribution of Dr Celia Brown and Professor Richard Lilford to Chapter 7 in performing the original data collection and analysis of Bayesian priors.
We thank Wellbeing of Women and the Hannah Eliza Guy Charity (Birmingham Children’s Hospital charity) who funded the original commencement of the trial at Birmingham Women’s Hospital NHS Foundation Trust in 2005. During the lifetime of this work Dr Morris was also funded by a MRC/RCOG Clinical Research Training Fellowship and a NIHR Clinical Lectureship. Dr Malin was funded by a Mary Crosse Fellowship (Birmingham Women’s Hospital NHS Foundation Trust Research and Development). Danielle Burke was funded by the MRC Midlands Hub for Trials Methodology Research at the University of Birmingham (MRC grant ID G0800808).
We thank the parents who took part in the trial and registry. Without their participation none of this work would have been possible.
Contribution of authors
Dr R Katie Morris (Clinical Lecturer in Maternal Fetal Medicine) was a research fellow for the trial (2005–12), managed the trial and was involved in data collection and analysis for the systematic reviews and the epidemiological study, data collection and analysis of randomised and registry data and data collection for the Bayesian priors. She was responsible for writing Chapters 1 , 2 , 3 and 10 and cowrote Chapters 4 , 5 and 6 . She took overall responsibility for writing the report.
Dr Gemma L Malin (Clinical Lecturer in Obstetrics and Gynaecology) was a research fellow for the trial (July 2008–May 2010), participated in management of the trial and was involved in data collection and analysis for the systematic reviews and the epidemiological study and data collection of randomised and registry data. She participated in writing Chapter 3 .
Miss Liz Quinlan-Jones (Research Midwife) was the co-ordinating midwife for the trial, was involved in data collection and analysis for the systematic review of fetal urine accuracy and data collection and analysis of the randomised and registry data, undertook the patient acceptability study and cowrote Chapter 9 .
Mr Lee J Middleton (Statistician) contributed to the design of the randomised and registry studies in which he also performed the statistical analysis. He contributed to the writing of Chapters 1 , 4 , 5 and 6 .
Dr Lavanya Diwakar (Research Fellow in Health Economics) performed the health economics analysis and wrote Chapter 8 .
Dr Karla Hemming (Senior Lecturer in Public Health, Epidemiology and Biostatistics) undertook and wrote the Bayesian analysis of binary outcomes. She analysed the elicited prior information and cowrote Chapter 7 .
Miss Danielle Burke (PhD student) performed the Bayesian analysis of survival to 1 year and was responsible for writing the results of this analysis for Chapter 7 .
Ms Jane Daniels (Deputy Director, Birmingham Clinical Trials Unit) was involved in the trial design and management and edited the final report.
Professor Elaine Denny (Professor of Health Sociology) was the supervisor for the qualitative work and was involved in qualitative data analysis and cowrote Chapter 9 .
Dr Pelham Barton (Reader in Health Economics) supervised the modelling for the health economics research and cowrote Chapter 8 .
Professor Tracy E Roberts (Professor of Health Economics) supervised the health economics research and cowrote Chapter 8 .
Professor Khalid S Khan (Professor of Obstetrics, Gynaecology and Clinical Epidemiology) was involved in the study design and management. He edited the final report.
Professor Jon J Deeks (Professor of Biostatistics and Director of the Birmingham Clinical Trials Unit) supervised the RCT, registry and Bayesian analysis. He co-wrote Chapters 4 , 5 , 6 and 10 and edited the final report.
Professor Mark D Kilby (Professor of Maternal Fetal Medicine) was the chief investigator for the study and thus was involved in the study design and management, analysis of the results and writing of the report.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
Publications
Lissauer D, Morris RK, Kilby MD. Fetal lower urinary tract obstruction. Semin Fetal Neonatal Med 2007;12:464–70.
Morris RK, Khan KS, Kilby MD. Vesicoamniotic shunting for fetal lower urinary tract obstruction: an overview. Arch Dis Child Fetal Neonatal Ed 2007;92:F166–8.
Morris RK, Quinlan-Jones E, Kilby MD, Khan KS. Systematic review of accuracy of fetal urine analysis to predict poor postnatal renal function in cases of congenital urinary tract obstruction. Prenat Diagn 2007;27:900–11.
PLUTO Collaborative Study Group, Kilby M, Khan K, Morris K, Daniels J, Gray R, et al. PLUTO trial protocol: percutaneous shunting for lower urinary tract obstruction randomised controlled trial. BJOG 2007;114:904–5.
Morris RK, Kilby MD. Congenital lower urinary tract obstruction. Best Pract Res Clin Obstet Gynaecol 2008;22:97–122.
Morris RK, Malin GL, Kilby MD, Khan KS. Accuracy of antenatal ultrasound to predict renal outcome in fetal lower urinary tract obstruction. Arch Dis Child Fetal Neonatal Ed 2008;93(Suppl. 1):Fa49.
Malin GL, Morris RK, Khan KS, Kilby MD. Antenatal intervention in the management of lower urinary tract obstruction: a systematic review of effectiveness with meta-analysis. Arch Dis Child Fetal Neonatal Ed 2009;94(Suppl. 1):Fa18.
Malin GL, Morris RK, Khan KS, Kilby MD. Systematic review of accuracy of prenatal ultrasound to predict postnatal renal function in cases of congenital lower urinary tract obstruction (LUTO). Reprod Sci 2009;16(Suppl. 3):139A.
Morris RK, Kilby MD. An overview of the literature on congenital lower urinary tract obstruction and introduction to the PLUTO trial: percutaneous shunting in lower urinary tract obstruction. Aust N Z J Obstet Gynecol 2009;49:6–10.
Morris RK, Malin GL, Khan KS, KIlby MD. Antenatal ultrasound to predict postnatal renal function in congenital lower urinary tract obstruction: systematic review of test accuracy. BJOG 2009;116:1290–9.
Brown C, Morris RK, Daniels J, Khan KS, Lilford RJ, Kilby MD. Effectiveness of percutaneous vesico-amniotic shunting in congenital lower urinary tract obstruction: divergence in prior beliefs among specialist groups. Eur J Obstet Gynaecol Reprod Biol 2010;152:25–9.
Morris RK, Malin GL, Khan KS, Kilby MD. Systematic review of the effectiveness of antenatal intervention for the treatment of congenital lower urinary tract obstruction. BJOG 2010;117:382–90.
Morris RK, Kilby MD. Long-term renal and neurodevelopmental outcome in infants with LUTO, with and without fetal intervention. Early Hum Dev 2011;87:607–10.
Morris RK, Malin GL, Kilby MD, the PLUTO Collaborative Group. The PLUTO trial: percutaneous shunting in lower urinary tract obstruction. Arch Dis Child Fetal Neonatal Ed 2011;96(Suppl. 1):Fa13.
Morris RK, Ruano R, Kilby MD. Effectiveness of fetal cystoscopy as a diagnostic and therapeutic intervention for lower urinary tract obstruction: a systematic review. Ultrasound Obstet Gynecol 2011:37:629–37.
Malin GL, Tonks AM, Morris RK, Gardosi J, Kilby MD. Congenital lower urinary tract obstruction: a population based epidemiology study [published online ahead of print 24 August 2012]. BJOG 2012. doi:10.1111/j.1471–0528.2012.03476.x.
Morris RK, Malin GL, Quinlan-Jones E, Middleton LJ, Hemming K, Burke D, et al. , for the Percutaneous vesicoamniotic shunting in Lower Urinary Tract Obstruction (PLUTO) Collaborative Group. Percutaneous vesicoamniotic shunting versus conservative management for fetal lower urinary tract obstruction (PLUTO): a randomised trial. Lancet 2013;382:1496–506. doi:10.1016/S0140-6736(13)60992-7.
Diwakar L, Morris RK, Barton P, Middleton LM, Kilby MD, Roberts TE. Evaluation of the cost effectiveness of vesico-amniotic shunting in the management of congenital lower urinary tract obstruction (based on data from the PLUTO trial). PLOS ONE. 2013; (in press).
Denny E, Quinlan-Jones E, Bibila S, Kilby MD. The experience of pregnant women with a diagnosis of fetal Lower Urinary Tract Obstruction (LUTO). [in press and published online ahead of print November 2013]Midwifery 2013. doi: 10.1016/j.midw.2013.10.023
References
- Merrill DC, Weiner CP, Fisk NM, Moise KJ. Fetal Therapy: Invasive and Transplacental. Cambridge: Cambridge University Press; 1997.
- Nakayama DK, Harrison MR, de Lomimier AA. Prognosis of posterior urethral valaves presenting at birth. J Pediatr Surg 1986;21:43-5.
- Barker AP, Cave MM, Thomas DF, Lilford RJ, Irving HC, Arthur RJ, et al. Fetal pelvi-ureteric junction obstruction: predictors of outcome. Br J Urol 1995;76:649-52. http://dx.doi.org/10.1111/j.1464-410X.1995.tb07796.x.
- Hutton KA, Thomas DF, Arthur RJ, Irving HC, Smith SE. Prenatally detected posterior urethral valves: is gestational age at detection a predictor of outcome?. J Urol 1994;152:698-701.
- Crombleholme TM, Harrison MR, Golbus MS, Longaker MT, Langer JC, Callen PW, et al. Fetal intervention in obstructive uropathy: prognostic indicators and efficacy of intervention. Am J Obstet Gynecol 1990;162:1239-44.
- Manning FA, Harrison MR, Rodeck C. Catheter shunts for fetal hydronephrosis and hydrocephalus. Report of the International Fetal Surgery Registry. N Engl J Med 1986;315:336-40. http://dx.doi.org/10.1056/NEJM198607313150532.
- Elder JS, Duckett JW, Snyder HM. Intervention for fetal obstructive uropathy: has it been effective?. Lancet 1987;2:1007-10.
- Fetal Vesico-amniotic Shunt for Lower Urinary Tract Outflow Obstruction. London: NICE; 2006.
- Quintero RA, Johnson MP, Romero R, Smith C, Arias F, Guevara-Zuloaga F, et al. In-utero percutaneous cystoscopy in the management of fetal lower obstructive uropathy. Lancet 1995;346:537-40. http://dx.doi.org/10.1016/S0140-6736(95)91381-5.
- Steinhardt G, Hogan W, Wood E, Weber T, Lynch R. Long-term survival in an infant with urethral atresia. J Urol 1990;143:336-7.
- Kaefer M, Peters CA, Retik AB, Benacerraf BB. Increased renal echogenicity: a sonographic sign for differentiating between obstructive and nonobstructive etiologies of in utero bladder distension. J Urol 1997;158:1026-9. http://dx.doi.org/10.1016/S0022-5347(01)64380-5.
- Abbott JF, Levine D, Wapner R. Posterior urethral valves: inaccuracy of prenatal diagnosis. Fetal Diagn Ther 1998;13:179-83. http://dx.doi.org/10.1159/000020834.
- Clark TJ, Martin WL, Divakaran TG, Whittle MJ, Kilby MD, Khan KS. Prenatal bladder drainage in the management of fetal lower urinary tract obstruction: a systematic review and meta-analysis. Obstet Gynecol 2003;102:367-82. http://dx.doi.org/10.1016/S0029-7844(03)00577-5.
- Morris RK, Quinlan-Jones E, Kilby M, Khan KS. Systematic review of accuracy of fetal urine analysis to predict poor postnatal renal function in cases of congenital urinary tract obstruction. Prenat Diagn 2007;27:900-11. http://dx.doi.org/10.1002/pd.1810.
- Morris R, Khan K, Kilby M, Studd J, Tan S, Chervenak F. Progress in Obstetrics and Gynaecology 17. London: Elsevier; 2006.
- Kilby M, Khan K, Morris K, Daniels J, Gray R, . PLUTO Collaborative Study Group . PLUTO trial protocol: percutaneous shunting for lower urinary tract obstruction randomised controlled trial. BJOG 2007;114:904-5.
- Morris RK, Khan KS, Kilby M. Vesico-amniotic shunting for lower urinary tract obstruction: an overview. Arch Dis Child Fetal Neonatal Ed 2007;92:166-8.
- Lissauer D, Morris RK, Kilby MD. Fetal lower urinary tract obstruction. Semin Fetal Neonatal Med 2007;12:464-70. http://dx.doi.org/10.1016/j.siny.2007.06.005.
- Cochrane Handbook for Systematic Reviews of Interventions. Oxford: Wiley-Blackwell; 2008.
- Akers J, Aguiar-Ibanez R, Baba-Akbari Sari A, Beynon S, Booth A. CRD’s guidance for undertaking reviews in health care. York: Centre for Reviews and Dissemination, University of York; 2009.
- Morris RK, Malin GL, Khan KS, Kilby MD. Systematic review of the effectiveness of antenatal intervention for the treatment of congenital lower urinary tract obstruction. BJOG 2010;117:382-90. http://dx.doi.org/10.1111/j.1471-0528.2010.02500.x.
- von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. Strenghtening the reporting of observational studies in epidemiology (STROBE): guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344-9.
- Higgins JP, Thompson SG, Deeks JJ. Measuring inconsistencies in meta-analyses. BMJ 2003;327:557-60. http://dx.doi.org/10.1136/bmj.327.7414.557.
- Anumba DO, Scott JE, Plant ND, Robson SC. Diagnosis and outcome of fetal lower urinary tract obstruction in the northern region of England. Prenat Diagn 2005;25:7-13. http://dx.doi.org/10.1002/pd.1074.
- McLorie G, Farhat W, Khoury A, Geary D, Ryan G. Outcome analysis of vesicoamniotic shunting in a comprehensive population. J Urol 2001;166:1036-40. http://dx.doi.org/10.1016/S0022-5347(05)65913-7.
- Hutton KA, Thomas DF, Davies BW. Prenatally detected posterior urethral valves: qualitative assessment of second trimester scans and prediction of outcome. J Urol 1997;158:1022-5. http://dx.doi.org/10.1016/S0022-5347(01)64379-9.
- Bierkens AF, Feitz WF, Nijhuis JG, de Wildt MJ, Flos MS, de Vries JD. Early urethral obstruction sequence: a lethal entity?. Fetal Diagn Ther 1996;11:137-45. http://dx.doi.org/10.1159/000264293.
- Holmes N, Harrison MR, Baskin LS. Fetal surgery for posterior urethral valves: long-term postnatal outcomes. Pediatrics 2001;108. http://dx.doi.org/10.1542/peds.108.1.e7.
- Lipitz S, Ryan G, Samuell C, Haeusler MC, Robson SC, Dhillon HK, et al. Fetal urine analysis for the assessment of renal function in obstructive uropathy. Am J Obstet Gynecol 1993;168:174-9. http://dx.doi.org/10.1016/S0002-9378(12)90909-6.
- McFadyen IR, Wigglesworth JS, Dillon MJ. Fetal urinary tract obstruction: is active intervention before delivery indicated?. Br J Obstet Gynaecol 1983;90:342-9. http://dx.doi.org/10.1111/j.1471-0528.1983.tb08921.x.
- Nicolini U, Tannirandorn Y, Vaughan J, Fisk NM, Nicolaidis P, Rodeck CH. Further predictors of renal dysplasia in fetal obstructive uropathy: bladder pressure and biochemistry of ‘fresh’ urine. Prenat Diagn 1991;11:159-66. http://dx.doi.org/10.1002/pd.1970110305.
- Salam MA. Posterior urethral valve: outcome of antenatal intervention. Int J Urol 2006;13:1317-22. http://dx.doi.org/10.1111/j.1442-2042.2006.01555.x.
- Shimada K, Hosokawa S, Tohda A, Matsumoto F, Suzuki M, Morimoto Y. Follow-up of children after fetal treatment for obstructive uropathy. Int J Urol 1998;5:312-16. http://dx.doi.org/10.1111/j.1442-2042.1998.tb00357.x.
- Szaflik K, Kozarzewski M, Adamczewski D. Fetal bladder catheterization in severe obstructive uropathy before the 24th week of pregnancy. Fetal Diagn Ther 1998;13:133-5. http://dx.doi.org/10.1159/000020823.
- Welsh A, Agarwal S, Kumar S, Smith RP, Fisk NM. Fetal cystoscopy in the management of fetal obstructive uropathy: experience in a single European centre. Prenat Diagn 2003;23:1033-41. http://dx.doi.org/10.1002/pd.717.
- Wisser J, Kurmanavicius J, Lauper U, Zimmermann R, Huch R, Huch A. Successful treatment of fetal megavesica in the first half of pregnancy. Am J Obstet Gynecol 1997;177:685-9. http://dx.doi.org/10.1016/S0002-9378(97)70165-0.
- Won HS, Kim SK, Shim JY, Lee PR, Kim A. Vesicoamniotic shunting using a double-basket catheter appears effective in treating fetal bladder outlet obstruction. Acta Obstet Gynecol Scand 2006;85:879-84. http://dx.doi.org/10.1080/00016340500449923.
- Bernaschek G, Deutinger J, Hansmann M, Bald R, Holzgreve W, Bollman R. Feto-amniotic shunting – report of the experience of four European centres. Prenat Diagn 1994;14:821-33. http://dx.doi.org/10.1002/pd.1970140910.
- Wilkins IA, Chitkara U, Lynch L, Goldberg JD, Mehalek KE, Berkowitz RL. The nonpredictive value of fetal urinary electrolytes: preliminary report of outcomes and correlations with pathologic diagnosis. Am J Obstet Gynecol 1987;157:694-8. http://dx.doi.org/10.1016/S0002-9378(87)80031-5.
- Freedman AL, Bukowski TP, Smith CA, Evans MI, Johnson MP, Gonzalez R. Fetal therapy for obstructive uropathy: diagnosis specific outcomes. J Urol 1996;156:720-3. http://dx.doi.org/10.1016/S0022-5347(01)65795-1.
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008-12. http://dx.doi.org/10.1001/jama.283.15.2008.
- Freedman AL, Bukowski TP, Smith CA, Evans MI, Berry SM, Gonzalez R, et al. Use of urinary beta-2-microglobulin to predict severe renal damage in fetal obstructive uropathy. Fetal Diagn Ther 1997;12:1-6. http://dx.doi.org/10.1159/000264415.
- Irwig L, Tosteson AN, Gatsonis C, Lau J, Colditz G, Chalmers TC, et al. Guidelines for meta-analyses evaluating diagnostic tests. Ann Intern Med 1994;120:667-76. http://dx.doi.org/10.7326/0003-4819-120-8-199404150-00008.
- Khan KS, Dinnes J, Kleijnen J. Systematic reviews to evaluate diagnostic tests. Eur J Obstet Gynecol Reprod Biol 2001;95:6-11. http://dx.doi.org/10.1016/S0301-2115(00)00463-2.
- Deeks JJ, Bossuyt PM, Gatsonis C. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 2013. http://srdta.cochrane.org/ (accessed 7 July 2006).
- Deeks JJ. Systematic reviews in health care: systematic reviews of diagnostic and screening tests. BMJ 2001;323:157-62. http://dx.doi.org/10.1136/bmj.323.7305.157.
- Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3.
- Whiting PF, Weswood ME, Rutjes AW, Reitsma JB, Bossuyt PN, Kleijnen J. Evaluation of QUADAS, a tool for the quality assessment of diagnostic accuracy studies. BMC Med Res Methodol 2006;6.
- Lijmer JG, Mol BW, Heisterkamp S, Bonsel GJ, Prins MH, van der Meulen JH, et al. Empirical evidence of design-related bias in studies of diagnostic tests. JAMA 1999;282:1061-6. http://dx.doi.org/10.1001/jama.282.11.1061.
- Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. BMJ 2003;326:41-4. http://dx.doi.org/10.1136/bmj.326.7379.41.
- Bunduki V, Saldanha LB, Sadek L, Miguelez J, Miyadahira S, Zugaib M. Fetal renal biopsies in obstructive uropathy: feasibility and clinical correlations – preliminary results. Prenat Diagn 1998;18:101-9. http://dx.doi.org/10.1002/(SICI)1097-0223(199802)18:2<101::AID-PD234>3.0.CO;2-4.
- Bussieres L, Laborde K, Souberbielle JC, Muller F, Dommergues M, Sachs C. Fetal urinary insulin-like growth factor I and binding protein 3 in bilateral obstructive uropathies. Prenat Diagn 1995;15:1047-55. http://dx.doi.org/10.1002/pd.1970151110.
- Daikha-Dahmane F, Dommergues M, Muller F, Narcy F, Lacoste M, Beziau A, et al. Development of human fetal kidney in obstructive uropathy: correlations with ultrasonography and urine biochemistry. Kidney Int 1997;52:21-32. http://dx.doi.org/10.1038/ki.1997.299.
- El-Ghoneimi A, Desgrippes A, Luton D, Macher MA, Guibourdenche J, Garel C, et al. Outcome of posterior urethral valves: to what extent is it improved by prenatal diagnosis?. J Urol 1999;162:849-53. http://dx.doi.org/10.1097/00005392-199909010-00076.
- Eugene M, Muller F, Dommergues M, Le Moyec L, Dumez Y. Evaluation of postnatal renal function in fetuses with bilateral obstructive uropathies by proton nuclear magnetic resonance spectroscopy. Am J Obstet Gynecol 1994;170:595-602. http://dx.doi.org/10.1016/S0002-9378(94)70235-7.
- Grannum P. Assessment of fetal renal reserve in low level obstructive uropathy. Lancet 1989;1:281-2. http://dx.doi.org/10.1016/S0140-6736(89)91296-8.
- Guez S, Assael BM, Melzi ML, Tassis B, Nicolini U. Shortcomings in predicting postnatal renal function using prenatal urine biochemistry in fetuses with congenital hydronephrosis. J Pediatr Surg 1996;31:1401-4. http://dx.doi.org/10.1016/S0022-3468(96)90838-6.
- Johnson MP, Bukowski TP, Reitleman C, Isada NB, Pryde PG, Evans MI. In utero surgical treatment of fetal obstructive uropathy: a new comprehensive approach to identify appropriate candidates for vesicoamniotic shunt therapy. Am J Obstet Gynecol 1994;170:1770-6.
- Johnson MP, Corsi P, Bradfield W, Hume RF, Smith C, Flake AW, et al. Sequential urinalysis improves evaluation of fetal renal function in obstructive uropathy. Am J Obstet Gynecol 1995;173:59-65. http://dx.doi.org/10.1016/0002-9378(95)90170-1.
- Mandelbrot L, Dumez Y, Muller F, Dommerques M. Prenatal prediction of renal function in fetal obstructive uropathies. J Perinat Med 1991;19:283-7.
- Muller F, Dommergues M, Mandelbrot L, Aubry MC, Nihoul-Fekete C, Dumez Y. Fetal urinary biochemistry predicts postnatal renal function in children with bilateral obstructive uropathies. Obstet Gynecol 1993;82:813-20.
- Muller F, Bernard M-A, Benkirane A, Ngo S, Lortat-Jacob S, Oury J, et al. Fetal urine cystatin C as a predictor of postnatal renal function in bilateral uropathies. Clin Chem 1999;45:2292-3.
- Nicolaides KH, Cheng HH, Snijders RJ, Moniz CF. Fetal urine biochemistry in the assessment of obstructive uropathy. Am J Obstet Gynecol 1992;166:932-7. http://dx.doi.org/10.1016/0002-9378(92)91367-J.
- Nicolini U, Fisk NM, Rodeck CH, Beacham J. Fetal urine biochemistry: an index of renal maturation and dysfunction. Br J Obstet Gynaecol 1992;99:46-50. http://dx.doi.org/10.1111/j.1471-0528.1992.tb14391.x.
- Qureshi F, Jacques SM, Seifman B, Quintero R, Evans MI, Smith C, et al. In utero fetal urine analysis and renal histology correlate with the outcome in fetal obstructive uropathies. Fetal Diagn Ther 1996;11:306-12. http://dx.doi.org/10.1159/000264329.
- Reuss A, Wladimiroff JW, Pijpers L, Provoost AP. Fetal urinary electrolytes in bladder outlet obstruction. Fetal Ther 1987;2:148-53. http://dx.doi.org/10.1159/000263309.
- Miguelez J, Bunduki V, Yoshizaki C, Santos Rodrigues Sadek L, Koch V. Fetal obstructive uropathy: is urine sampling useful for prenatal counselling?. Prenat Diagn 2006;26:81-4. http://dx.doi.org/10.1002/pd.1360.
- Ylinen E, Ala-Houhala M, Wikstrom S. Prognostic factors of posterior urethral valves and the role of antenatal detection. Pediatr Nephrol 2004;19:874-9. http://dx.doi.org/10.1007/s00467-004-1474-4.
- Oliveira EA, Rabelo EA, Pereira AK, Diniz JS, Cabral AC, Leite HV, et al. Prognostic factors in prenatally-detected posterior urethral valves: a multivariate analysis. Pediatr Surg Int 2002;18:662-7.
- Morris RK, Malin GL, Khan KS, Kilby MD. Antenatal ultrasound to predict postnatal renal function in congenital lower urinary tract obstruction: systematic review of test accuracy. BJOG 2009;116:1290-9. http://dx.doi.org/10.1111/j.1471-0528.2009.02194.x.
- Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Ann Intern Med 2003;138:40-4. http://dx.doi.org/10.7326/0003-4819-138-1-200301070-00010.
- Lijmer JG, Mol BW, Heisterkamp S, Bonsel GJ, Prins MH, van der Meulen JH, et al. Empirical evidence of design-related bias in studies of diagnostic tests. JAMA 1999;282:1061-6. http://dx.doi.org/10.1001/jama.282.11.1061.
- Honest H, Khan KS. Reporting of measures of accuracy in systematic reviews of diagnostic literature. BMC Health Serv Res 2002;2.
- Zamora J, Abraira V, Muriel A, Khan KS, Coomarasamy A. Meta-disc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol 2006;12.
- Oliveira EA, Diniz JS, Cabral AC, Pereira AK, Leite HV, Colosimo EA, et al. Predictive factors of fetal urethral obstruction: a multivariate analysis. Fetal Diagn Ther 2000;15:180-6. http://dx.doi.org/10.1159/000021002.
- Coplen D, Hare J, Zderic S, Canning D, Snyder H. 10 year experience with pre-natal intervention for hydronephrosis. J Urol 1996;153:1142-5.
- Jee LD, Rickwood AM, Turnock RR. Posterior urethral valves. Does prenatal diagnosis influence prognosis?. Br J Urol 1993;72:830-3. http://dx.doi.org/10.1111/j.1464-410X.1993.tb16277.x.
- Reinberg Y, De Castano IGR. Prognosis for patients with prenatally diagnosed posterior urethral valves. J Urol 1992;148:125-6.
- Zaccara A, Giorlandino C, Mobili L, Brizzi C, Bilancioni E, Capolupo I, et al. Amniotic fluid index and fetal bladder outlet obstruction. Do we really need more?. J Urol 2005;174:1657-60. http://dx.doi.org/10.1097/01.ju.0000179538.85893.cb.
- Glick PL, Harrison MR, Golbus MS, Adzick NS, Filly RA, Callen PW, et al. Management of the fetus with congenital hydronephrosis II: prognostic criteria and selection for treatment. J Pediatr Surg 1985;20:376-87. http://dx.doi.org/10.1016/S0022-3468(85)80223-2.
- Freedman AL, Johnson MP, Smith CA, Gonzalez R, Evans MI. Long-term outcome in children after antenatal intervention for obstructive uropathies. Lancet 1999;354:374-7. http://dx.doi.org/10.1016/S0140-6736(98)11006-1.
- Holmdahl G, Sillén U. Boys with posterior urethral valves: outcome concerning renal function, bladder function and paternity at ages 31 to 44 years. J Urol 2005;174:1031-4. http://dx.doi.org/10.1097/01.ju.0000170233.87210.4f.
- Biard J-M, Johnson MP, Carr MC, Wilson RD, Hedrick HL, Pavlock C, et al. Long-term outcomes in children treated by prenatal vesicoamniotic shunting for lower urinary tract obstruction. Obstet Gynecol 2005;106:503-8. http://dx.doi.org/10.1097/01.AOG.0000171117.38929.eb.
- Varni J, Seid M, Kurtin P. PedsQL 4.0: reliability and validity of the pediatric quality of life inventory version 4.0 generic core scales in healthy and patient populations. Med Care 2001;39:800-12. http://dx.doi.org/10.1097/00005650-200108000-00006.
- Saudino K, Dale P, Oliver B, Petrill SA, Richardson V, Rutter M, et al. The validity of parent based assessment of the cognitive abilities of 2 year olds. Br J Dev Psychol 1998;16:349-63. http://dx.doi.org/10.1111/j.2044-835X.1998.tb00757.x.
- Johnson S, Marlow N, Wolke D, Davidson L, Marston L, O’Hare A, et al. Validation of a parent report measure of cognitive development in very preterm infants. Dev Med Child Neurol 2004;46:389-97. http://dx.doi.org/10.1017/S0012162204000635.
- O’Hagan A, Stevens JW. Bayesian methods for design and analysis of cost-effectiveness trials in the evaluation of health care technologies. Stat Method Med Res 2003;11:469-90. http://dx.doi.org/10.1191/0962280202sm305ra.
- Johnson SR, Tomlinson GA, Hawker GA, Granton JT, Feldman BM. Methods to elicit beliefs for Bayesian priors: a systematic review. J Clin Epidemiol 2010;63:335-69. http://dx.doi.org/10.1016/j.jclinepi.2009.06.003.
- Spiegelhalter DJ, Myles JP, Jones DR, Abrams KR. Bayesian methods in health technology assessment: a review. Health Technol Assess 2000;4.
- Turner RM, Spiegelhalter DJ, Smith GC, Thompson SG. Bias modelling in evidence synthesis. J R Stat Soc Ser A Stat Soc 2009;172:21-47. http://dx.doi.org/10.1111/j.1467-985X.2008.00547.x.
- Lilford RJ, Braunholtz D. The statistical basis of public policy: a paradigm shift is overdue. BMJ 1996;313:603-7. http://dx.doi.org/10.1136/bmj.313.7057.603.
- Braunholtz D, Lilford RJ. Bayesian statistics may inform public policy better than significant odds ratios. BMJ 1997;314. http://dx.doi.org/10.1136/bmj.314.7088.1202a.
- Mohanna K, Tunna K. Withholding consent to participate in clinical trials: decisions of pregnant women. BJOG 1999;106:892-7. http://dx.doi.org/10.1111/j.1471-0528.1999.tb08426.x.
- Rodger MA, Makropoulos D, Walker M, Keely E, Karovitch A, Wells PS. Participation of pregnant women in clinical trials: will they participate and why?. Am J Perinatol 2003;20:69-76. http://dx.doi.org/10.1055/s-2003-38318.
- Kenyon S, Dixon-Woods M, Jackson CJ, Windridge K, Pitchforth E. Participating in a trial in a critical situation: a qualitative study in pregnancy. Qual Saf Health Care 2006;15:98-101. http://dx.doi.org/10.1136/qshc.2005.015636.
- Springett A, Morris JK. Congenital Anomaly Statistics 2010: England and Wales. London: British Isles Network of Congenital Anomaly Registers; 2012.
- Malin GL, Tonks A, Morris RK, Gardosi J, Kilby MD. Congenital lower urinary tract obstruction: a population based epidemiology study. BJOG 2012;119:1455-64. http://dx.doi.org/10.1111/j.1471-0528.2012.03476.x.
- 2007 Mortality Statistics. Series DH3 No. 40. London: ONS; 2009.
- Birth Statistics: Review of the National Statistician on BIrths and Patterns of Family Building in England and Wales. London: ONS; 2010.
- Hennekens CH, Buring JE. Epidemiology in Medicine. Boston, MA: Little Brown; 1987.
- Office for National Statistics . 2001 Census, Output Area Boundaries n.d. http://www.ons.gov.uk/ons/guide-method/census/census-2001/data-and-products/output-geography/output-area-production/index.html (accessed 28 November 2009).
- Savva GM, Morris JK. Ascertainment and accuracy of Down’s syndrome cases reported in congenital anomaly registers in England and Wales. Arch Dis Child Fetal Neonatal Ed 2009;94:F23-7.
- Boyd PA, Armstrong B, Dolk H, Botting B, Pattenden S, Abramsky L, et al. Congenital anomaly surveillance in England – ascertainment differences in the national system. BMJ 2005;1.
- Morris RK, Ruano R, Kilby MD. The effectiveness of fetal cystoscopy as a diagnostic and therapeutic intervention for lower urinary tract obstruction: a systematic review. Ultrasound Obstet Gynaecol 2011;37:629-37. http://dx.doi.org/10.1002/uog.8981.
- Broadley P, McHugo J, Morgan I, Whittle MJ, Kilby MD. The 4 year outcome following the demonstration of bilateral renal pelvic dilatation on pre-natal renal ultrasound. B J Radiol 1999;72:265-70.
- Public Health England . Fetal Anomaly Screening Policy n.d. http://fetalanomaly.screening.nhs.uk/standardsandpolicies (accessed 2010).
- Medical Research Council . Guidelines for Good Clinical Practice in Clinical Trials 1998. www.mrc.ac.uk/Utilities/Documentrecord/index.htm?d=MRC002416 (accessed October 2013).
- Magann EF, Sanderson M, Martin JN, Chauhan S. The amniotic fluid index, single deepest pocket, and two diameter pocket in normal human pregnancy. Am J Obstet Gynecol 2000;182:1581-8. http://dx.doi.org/10.1067/mob.2000.107325.
- Harmoinen A, Ylinen E, Ala-Houhala M, Jana M, Kaila M, Kouri T. Reference intervals for for cystatin C in pre- and full term infants and children. Pediatr Nephrol 2000;15:105-8. http://dx.doi.org/10.1007/s004670000421.
- Savory DJ. Reference ranges for serum creatinine in infants, children and adolescents. Ann Clin Biochem 1990;27:99-101.
- Finney H, Newman D, Thakkar H, Fell J, Price C. Reference ranges for plasma cystatin C and creatinine measurements in premature infants, neonates and older children. Arch Dis Child 2000;82:71-5. http://dx.doi.org/10.1136/adc.82.1.71.
- Chitty LS, Altman DG. Charts of fetal size: kidney and renal pelvis measurements. Prenat Diagn 2003;23:891-7. http://dx.doi.org/10.1002/pd.693.
- Fernbach SK, Maizels M, Conway JJ. Ultrasound grading of hydronephrosis: introduction to the system used by the Society for Fetal Urology. Paediatr Radiol 1993;23:478-80. http://dx.doi.org/10.1007/BF02012459.
- Morris RK, Malin GL, Quinlan-Jones E, Middleton LJ, Hemming K, Burke D, et al. Percutaneous vesicoamniotic shunting in Lower Urinary Tract Obstruction (PLUTO) Collaborative Group . Percutaneous vesicoamniotic shunting versus conservative management for fetal lower urinary tract obstruction (PLUTO): a randomised trial. Lancet 2013;382:1496-506. doi:10.1016/S0140-6736(13)60992-7.
- Office for National Statistics . Live Births in England and Wales, 2010 n.d. www.ons.gov.uk (accessed 2011).
- Office for National Statistics . Abortion Statistics, England and Wales: 2010 n.d. www.dh.gov.uk (accessed 2012).
- Spiegelhalter DJ. Incorporating Bayesian ideas into health-care evaluation. Stat Sci 2004;19:156-74. http://dx.doi.org/10.1214/088342304000000080.
- O’Hagan A, Buck CE, Daneshkhah A, Eiser JR, Garthwaite PH, Jenkinson DJ, et al. Uncertain Judgements: Eliciting Experts’ Probabilities. Chichester: Wiley; 2006.
- Brown C, Morris RK, Daniels J, Khan KS, Lilford R, Kilby MD. Effectiveness of percutaneous vesico-amniotic shunting in congenital lower urinary tract obstruction: a divergence of prior beliefs among specialist groups. Eur J Obstet Gynecol Reprod Biol 2010;152:25-9. http://dx.doi.org/10.1016/j.ejogrb.2010.04.019.
- Lambert PC, Sutton AJ, Burton PR, Abrams KR, Jones DR. How vague is vague? A simulation study of the impact of the use of vague prior distributions in MCMC using WinBUGS. Stat Med 2005;24:2401-28. http://dx.doi.org/10.1002/sim.2112.
- Lunn DJ, Thomas A, Best N, Spiegelhalter DJ. WinBUGS – a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput 2000;10:325-37.
- Ibrahim J, Chen M, Sinha D. Bayesian Survival Analysis. New York, NY: Springer-Verlag; 2001.
- Sutton AJ, Abrams KR. Bayesian methods in meta-analysis and evidence synthesis. Stat Meth Med Res 2001;10:277-303. http://dx.doi.org/10.1191/096228001678227794.
- Ma J, Thabane L, Kaczorowski J, Chambers L, Dolovich L, . Comparison of Bayesian and classical methods in the analysis of cluster randomised controlled trials with a binary outcome: the Community Hypertension Assessment Trial (CHAT). BMC Med Res Methodol 2009;9.
- Diwakar L, Morris RK, Barton P, Middleton LM, Kilby MD, Roberts TE AJ, Abrams KR, Sutton AJ, Abrams KR. Evaluation of the cost effectiveness of vesico-amniotic shunting in the management of congenital lower urinary tract obstruction (based on data from the PLUTO trial). PLOS ONE 2003.
- Curtis L. Unit Costs of Health and Social Care 2011. Canterbury: Personal Social Services Research Unit, University of Kent; 2011.
- Confirmation of Payment by Results (PbR) Arrangements for 2011–12. London: Department of Health; 2011.
- Confirmation of Payment by Results (PbR) Arrangements for 2008–9. London: Department of Health; 2008.
- Roberts TE. Economic evaluation and randomised controlled trial of extracorporeal membrane oxygenation: UK collaborative trial. The Extracorporeal Membrane Oxygenation Economics Working Group. BMJ 1998;317:911-15. http://dx.doi.org/10.1136/bmj.317.7163.911.
- Sally H, Fiona C. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ 1999;319:670-4.
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomised controlled trials. The CONSORT statement. JAMA 1996;276:637-9.
- Briggs A, Claxton K, Sculpher M. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Updated Guide to the Methods of Technology Appraisal. London: NICE; 2008.
- Snowden C, Elbourne D, Garcia J, Lavender T, Edwards G, Alfirevic Z. Demystifying Qualitative Research in Pregnancy and Childbirth. Salisbury: Quay Books; 2001.
- Denny E, Quinlan-Jones E, Bibila S, Kilby MD. Midwifery. 2013.
- Streubert HJ, Carpenter DR. Qualitative Research in Nursing: Advancing the Humanistic Imperative. Philadelphia, PA: Lippincott; 1999.
- Robinson A. Principles and Practice of Research in Midwifery. Edinburgh: Balliere Tindall; 2000.
- Strauss A, Corbin J. Basics of Qualitative Research. London: Sage; 1990.
- Holloway I, Todres L, Holloway I. Qualitative Research In Health Care. Maidenhead: Open University Press; 2005.
- Dykes F, Lavender T, Edwards G, Alfirevic Z. Demystifying Qualitative Research in Pregnancy and Childbirth. Salisbury: Quay Books; 2004.
- Graham RH, Mason K, Rankin J, Robson SC. The role of feticide in the context of late termination of pregnancy: a qualitative study of health professionals’ and parents’ views. Prenat Diagn 2009;29:875-81. http://dx.doi.org/10.1002/pd.2297.
- Park A, Matthews M. Women’s decisions about maternal serum screening testing: a qualitative study exploring what they learn and the role prenatal care providers play. Women Birth 2009;22:73-8. http://dx.doi.org/10.1016/j.wombi.2009.01.005.
- Todres L, Holloway I. Qualitative Research in Health Care. Maidenhead: Open University Press; 2005.
- Burnard P. A method of analysing interview transcripts in qualitative research. Nurse Educ Today 1991;11:461-6. http://dx.doi.org/10.1016/0260-6917(91)90009-Y.
- Tooher RL, Middleton PF, Crowther A. A thematic analysis of factors influencing recruitment to maternal and perinatal trials. BMC Pregnancy Childbirth 2008;8. http://dx.doi.org/10.1186/1471-2393-8-36.
- Smyth RMD, Duley L, Jacoby A, Elbourne D. Women’s experiences of participating in the Magpie Trial: a postal survey in the United Kingdom. Birth 2009;36:220-9. http://dx.doi.org/10.1111/j.1523-536X.2009.00326.x.
- Law M, King S, Stewart D, King G. The perceived effects of parent-led support groups for parents of children with disabilities. Phys Occup Ther Pediatr 2001;21:29-48. http://dx.doi.org/10.1300/J006v21n02_03.
- Lagan BM, Sinclair M, Kernohan WG. What is the impact of the internet on decision-making in pregnancy? A global study. Birth 2011;38:336-45. http://dx.doi.org/10.1111/j.1523-536X.2011.00488.x.
- McDonald A, Knight R, Campbell M, Entwhistle V, Grant A, Cook J, et al. What influences recruitment to randomised controlled trials? A review of trials funded by two UK funded agencies. Trials 2006;7.
Appendix 1 Search terms for the epidemiological study
International Classification of Diseases, 10th Edition, code search
Q64.2: Congenital posterior urethral valves
Q64.3: Other atresia and stenosis of urethra and bladder neck
Q64.8: Other specified congenital malformations of urinary system
Q64.9: Congenital malformation of urinary system, unspecified
Q79.4: Prune belly syndrome
Keyword search terms for lower urinary tract obstruction
-
[Posterior urethral valves or PUV or urethral atresia or bladder neck obstruction or bladder outlet obstruction or obstructive uropathy or congenital urinary tract obstruction or congenital lower urinary tract obstruction or LUTO or Prune Belly syndrome or Megacystis microcolon syndrome or MMIHS or urogenital abnormality or urogenital tract malformation]
-
[Enlarged bladder or large bladder or big bladder or megacystis or keyhole sign or oligohydramnios or reduced liquor or anyhdramnios or cystic kidneys or microcystic or macrocystic or hydronephrosis or dilated ureters or dilated renal pelvis or renal dysplasia]
-
[Vesico-amniotic shunting or vesicoamniotic shunting or vesico-amniotic drainage or vesicoamniotic drainage or pig-tail catheter or bladder shunt or intrauterine shunt]
1 = diagnosis
2 = ultrasound features
3 = treatment
Appendix 2 List of all centres (randomised and registry) and principal investigators
| Study centre | Principal investigator |
|---|---|
| Birmingham Women’s Hospital NHS Foundation Trust | Professor Mark Kilby |
| Liverpool Women’s NHS Foundation Trust | Professor Zarko Alfirevic |
| Queen Charlotte’s Hospital, London | Dr Sailesh Kumar |
| Queen Mother’s Hospital, Glasgow | Professor Alan Cameron |
| Nottingham City Hospital | Dr Pamela Loughna |
| Princess Anne Hospital, Southampton | Dr Karen Brackley |
| St Michael’s Hospital, Bristol | Professor Peter Soothill |
| Leeds General Infirmary | Mr Gerald Mason |
| Royal Hallamshire Hospital, Sheffield | Mr Dilly Anumba |
| Forth Park Hospital, Fife (referring centre) | Mr Graham Tydeman |
| Royal Victoria Infirmary, Newcastle | Professor SC Robson |
| King’s College Hospital, London | Dr Sarah Bower |
| St Mary’s Hospital, Manchester | Dr Philip Bullen |
| Rosie Hospital, Cambridge | Mr Jeremy Brockelsby |
| St George’s Hospital, London | Dr Basky Thilaganathan |
| St James’s Hospital, Leeds | Dr Tracey Glanville |
| University Hospitals of Leicester NHS Trust | Dr Manjiri Khare |
| Guys and St Thomas Hospitals, London | Mr Darryl Maxwell |
| Chelsea and Westminster Hospital, London | Dr Makrina Savvidou |
| National Maternity Hospital, Dublin | Professor Fionnuala Mcauliffe |
| University Medical Centre, Leiden, the Netherlands | Professor Dick Opekes |
| John Radcliffe Hospital, Oxford | Mr Lawrence Impey |
| Heartlands Hospital, Birmingham (referring) | Mr Neil Shah |
| Birmingham City Hospital (referring) | Mr Neil Shah |
| Royal Liverpool Children’s Hospital, Alder Hey (paediatric follow-up) | Mr Simon Kenny |
| Birmingham Children’s Hospital Foundation Trust | Mr Liam McCarthy |
| Bristol Royal Hospital for Children | Dr Jane Tizard |
| Norfolk and Norwich University Hospital | Mr Richard Smith |
| Queen’s Medical Centre, Nottingham | Mr George Bugg |
| Royal Jubilee Maternity Hospital, Belfast | Mr Stephen Ong |
Appendix 3 Participant information sheet for the randomised controlled trial and registry
Participant information sheet for the randomised controlled trial and registry (PDF download)
Appendix 4 Consent form for the randomised controlled trial and registry
Consent form for the randomised controlled trial and registry (PDF download)
Appendix 5 Randomisation and registry notepad
Appendix 6 The PLUTO study protocol
Protocol summary
Congenital lower urinary tract (often bladder outflow) obstruction (LUTO) is usually identifiable using ultrasound. Its natural history is associated with a high prevalence of chronic renal impairment in infancy and childhood. If associated with oligohydramnios of early-onset there is a significant risk of pulmonary hypoplasia, with perinatal mortality of up to 50% within the early neonatal period (first week of life). Even if oligohydramnios has not yet developed, there is a significant risk of renal impairment, secondary to obstruction. In utero, percutaneous vesicoamniotic shunting bypasses the congenital urethral obstruction to potentially improve fetal outcome. To date, there are no large prospective studies assessing the risks and benefits of this intervention to either mother or baby.
We propose a multi-centre randomised controlled (RCT) trial of 150 singleton pregnancies with ultrasound evidence of LUTO to evaluate the safety and effectiveness of in utero shunting compared to conservative management. The sample size will allow reliable (80% power, α = 0.05) detection of an improvement in survival of 23 percentage points (i.e. 39% survival versus 62% survival). Babies who survive will also be followed up for effects upon renal and bladder function and cognitive development. This trial will provide important information to guide future clinical practice and research. In addition, it will provide information to counsel women whose babies have prenatally diagnosed bladder outflow obstruction.
In those pregnancies not entered into the RCT PLUTO trial, we propose that they are entered onto a register, so that outcome may be tracked.
Eligibility for the RCT will primarily be based on the ‘Uncertainty Principle’. This is that if the fetal medicine specialist is uncertain as to whether shunting is the most appropriate option, then the pregnancy (and fetus) is eligible to be randomised. The minority of pregnancies where the clinician is certain that ‘shunting’ is clinically indicated or contraindicated or where the women declines randomisation will be entered on to a prospective registry. Data from trial and registry babies will be used to construct a prognostic risk index.
The incidence of prenatally diagnosed LUTO is 1 in 3000, necessitating a collaborative multicentre trial to obtain the required sample size of 150 pregnancies in a timely manner. The results of the Study will be published on behalf of the ‘PLUTO Collaborative Study Group’.
FIGURE 1.
Pluto Trial Algorithm (Consort diagram).
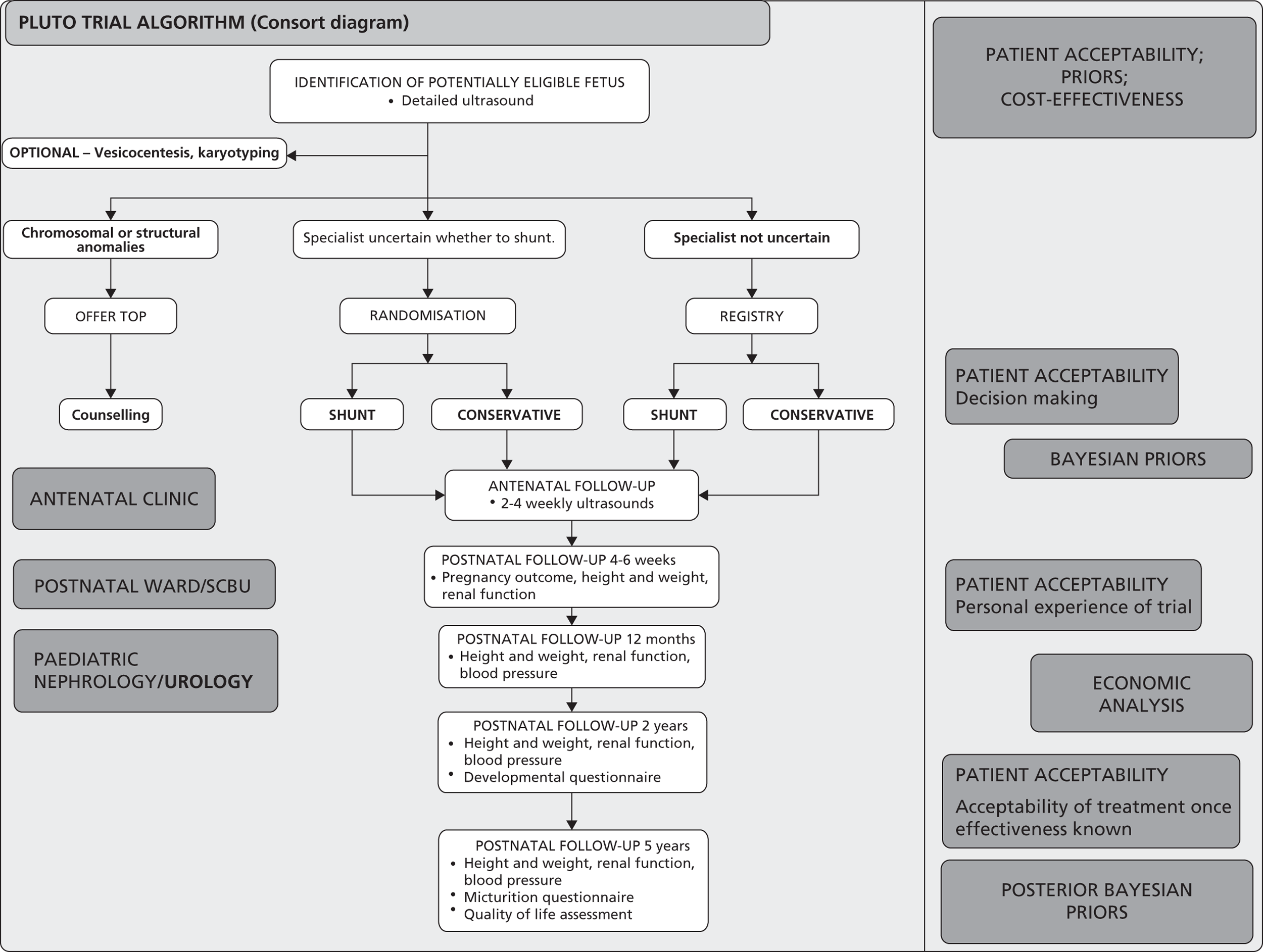
PLUTO Trial Management Committee
|
Principal Investigators
Professor Mark Kilby Consultant in Fetal Medicine Professor Khalid Khan Consultant in Obstetrics and Gynaecology Dr Katie Morris, Clinical Research Fellow Birmingham Women’s Hospital |
Clinical Trial Design and Management
Jane Daniels Unit Coordinator Lee Middleton Statistician Professor Richard Gray Unit Co-Director University of Birmingham Clinical Trials Unit |
|
Paediatric Nephrology
Dr Mark Taylor Dr Liam McCarthy Consultant Paediatric Nephrologist. Mr K. Parashah Consultant Paediatric Urologist. Birmingham Children’s Hospital |
Midwifery
Elizabeth Quinlan-Jones. Coordinating Research Midwife Birmingham Women’s Hospital |
Independent Trial Steering Committee
-
Chair: Professor Martin Whittle, Consultant in Fetal Medicine, Birmingham Women’s Hospital
-
Dr Yves Ville, Fetal Medicine Specialist, University of Paris-Ouest
-
Mrs Sarah Carranchi, Lay Representative
-
Professor David Field, Consultant Paediatrician, Leicester Royal Infirmary
-
Professor Adrian Woolf, Consultant Paediatric Nephrologist, Great Ormond Street Hospital
-
Mr Nicholas Madden, Consultant Paediatric Urologist, Chelsea and Westminster and St Mary’s Hospital
Data Monitoring and Ethics Committee
For interim analysis and response to specific concerns:
-
Chair: Dr Carole Cummins, Statistician, Birmingham Children’s Hospital
-
Professor Jim Thornton, Consultant in Obstetrics and Gynaecology, Nottingham City Hospital
-
Professor Jim Neilson, Consultant in Obstetrics and Gynaecology, Liverpool Women’s Hospital
PLUTO Trials Office
The University of Birmingham Clinical Trials Unit,
Division of Medical Sciences,
Robert Aitkin Institute,
University of Birmingham,
Edgbaston,
Birmingham, B15 2TT.
Telephone: 0121 415 9100 (Voicemail outside office hours)
Fax: 0121 415 9135/9136
E-mail: bctu@bham.ac.uk
Co-ordination: Ms Elizabeth Quinlan-Jones (Midwife) and Dr K Morris.
Trial Administraton: Leanne Fulcher
Statistics: Lee Middleton
Computing: Edward Tyler
Clinical queries should be directed during office hours to an appropriate member of the Management Committee. Other queries should be directed to the PLUTO Trials Office.
https://www.trials.bham.ac.uk/PLUTO or TELEPHONE: 0800 953 0274
Version Number
Protocol version 5.0 dated 11/08/09 (changes to follow-up assessments and amendment of measurements)
Protocol version 4.0 dated 21/04/08 (changes to protocol to coincide with start of HTA funding)
Protocol version 3.0 dated 05/06/07 (removal of gestational age limit as entry criteria)
Protocol version 2.02 dated 25/07/06 (change to follow up forms, version 1.2; addition of TOP register)
Protocol version 2.01 dated 08/05/06 (change to consent form, version 2)
Protocol version 2.0 dated 28/10/05 (amendments to add registry, urinary assessments made optional)
Previous version 1.0 dated 07/08/2004 (initial submission to MREC prior to grant award)
ISRCTN
ISRCTN53328556
Funding Body
Wellbeing of Women September 2005–August 2008.
Health Technology Assessment Programme June 2008 onwards.
Sponsor and Sponsor Roles
The University of Birmingham is the sponsor.
Professor Mark Kilby is the Chief Investigator.
The University of Birmingham is responsible for obtaining necessary approvals and for safety reporting, the Trial Management Committee is jointly responsible for overseeing good clinical practice and the Investigators are responsible for obtaining informed consent and care of the participants.
1. BACKGROUND
Fetal Bladder Obstruction and its Treatment
Congenital anomalies of the genitourinary tract are identified using prenatal ultrasound in 1 : 250 to 1 : 1000 pregnancies1. Chronic lower urinary tract obstruction (e.g. fetal bladder outlet obstruction) can result in abnormal renal development and function. This in turn leads to chronic renal impairment associated with oligohydramnios, pulmonary hypoplasia and postural anomalies resulting in a high perinatal mortality and morbidity. 2–4 The diagnosis of this condition is often made at 20 weeks of gestation when the majority of pregnant women have a detailed scan. Bladder drainage by serial vesicocentesis (insertion of a fine needle to drain the fetal bladder at regular intervals) and continuous drainage into the amniotic cavity by vesico-amniotic shunting (insertion of a catheter) have been used to relieve fetal urinary obstruction in an attempt to avoid renal parenchymal damage and chronic oligohydramnios that may adversely affect pulmonary development. 5–7 These procedures may be associated with maternal morbidity (mainly in terms of infection risk) and fetal morbidity (infection or bleeding). They also carry a risk of causing miscarriage (1–2%) and the risk of serial drainages is often cumulative making shunt insertion preferable. Their use in current practice can only be justified if safety and effectiveness is demonstrated with reliability.
Evidence for vesico-amniotic shunting as a treatment
We performed a systematic review with meta-analysis of studies of fetal bladder shunting. 8 Of the 16 studies deemed suitable for inclusion, there was not a single RCT. The conclusions of the systematic review were drawn with caution, as the quality of evidence was poor, with variability in study design, follow up, and the potential for bias in observational studies raising concerns about validity of results ( Figure 2 ).
FIGURE 2.
Bar chart summarising quality of evidence of papers included in systematic review of effectiveness of prenatal bladder drainage in lower urinary tract obstruction.
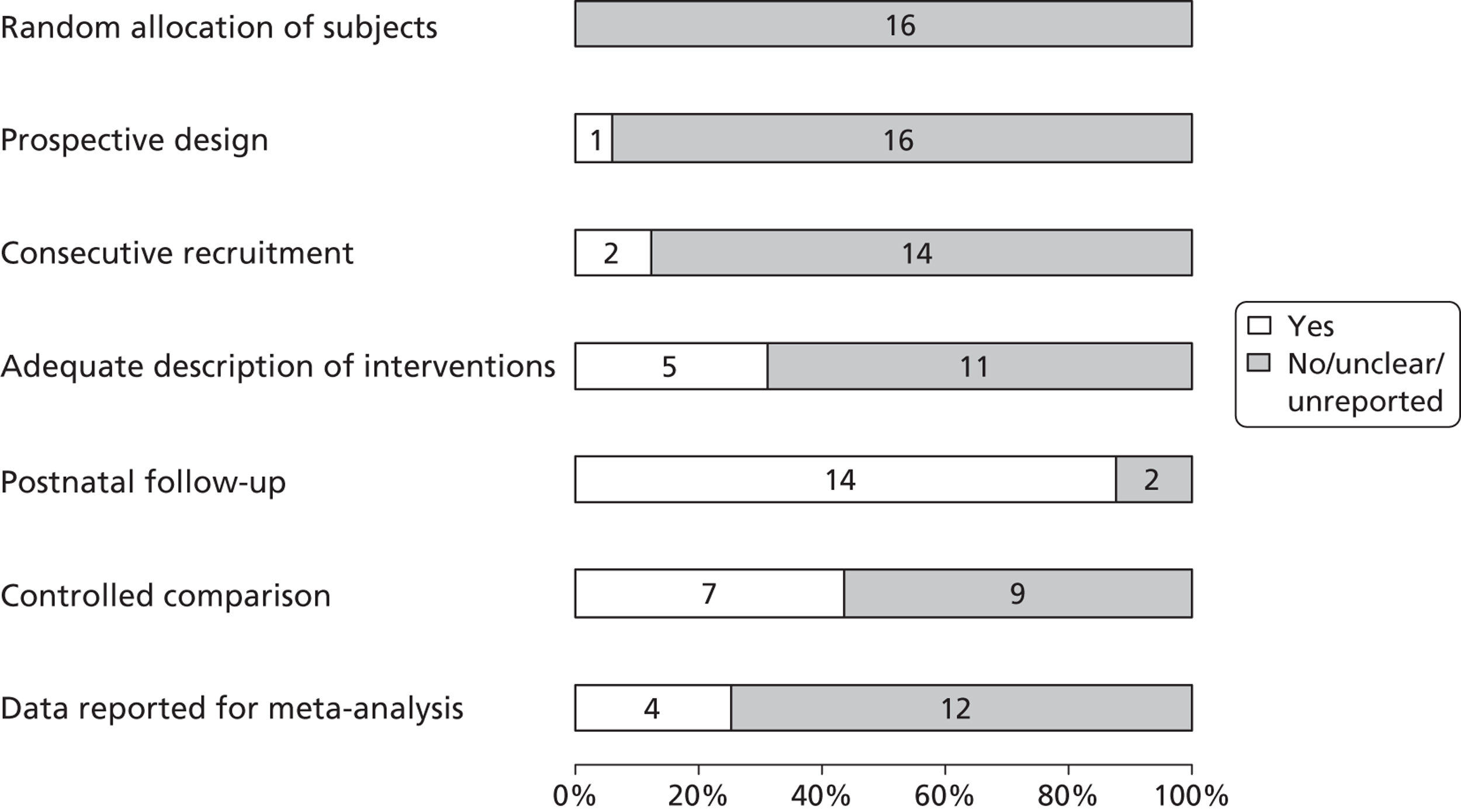
Meta-analysis of non-randomised observational studies ( Figure 3 ) demonstrated that prenatal bladder drainage for LUTO might improve perinatal survival in fetuses, particularly those with poor predicted outcome, which justifies the need to proceed with an RCT. A similar conclusion was reached 15 years previously, but there has been an absence of good research in the intervening period7. As fetal bladder outlet obstruction is a relatively uncommon condition, a multi-centre RCT is required to assess the short and long-term effects of this intervention. 9 This is a crucial step in establishing whether this procedure has a place in future fetal medicine practice. 10
FIGURE 3.
Forest Plot showing meta-analysis of results from the systematic review of effectiveness of prenatal bladder drainage in lower urinary tract obstruction.
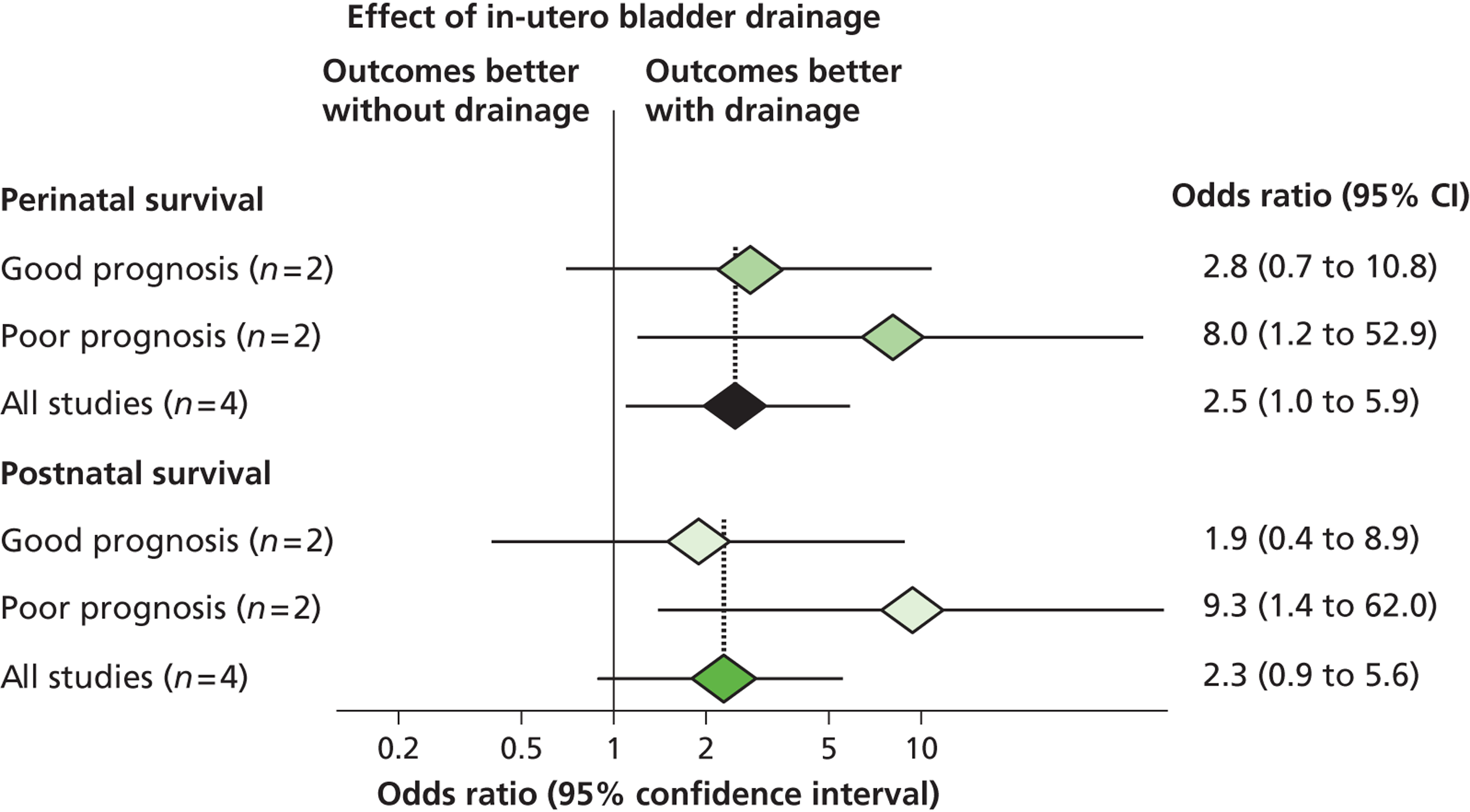
Evidence for fetal urinalysis as a predictor of postnatal renal function
We performed a systematic review and meta-analysis of studies informing the literature on accuracy of fetal urinalysis to predict poor postnatal renal function. 11 There were 23 articles that met the selection criteria. All of the studies were observational studies and of poor quality ( Figure 4 ).
FIGURE 4.
Quality of studies included in review of fetal urinalysis in fetuses with obstructive uropathy to predict poor postnatal renal function. Stack bar chart used with numbers inside bars indicating number of studies.
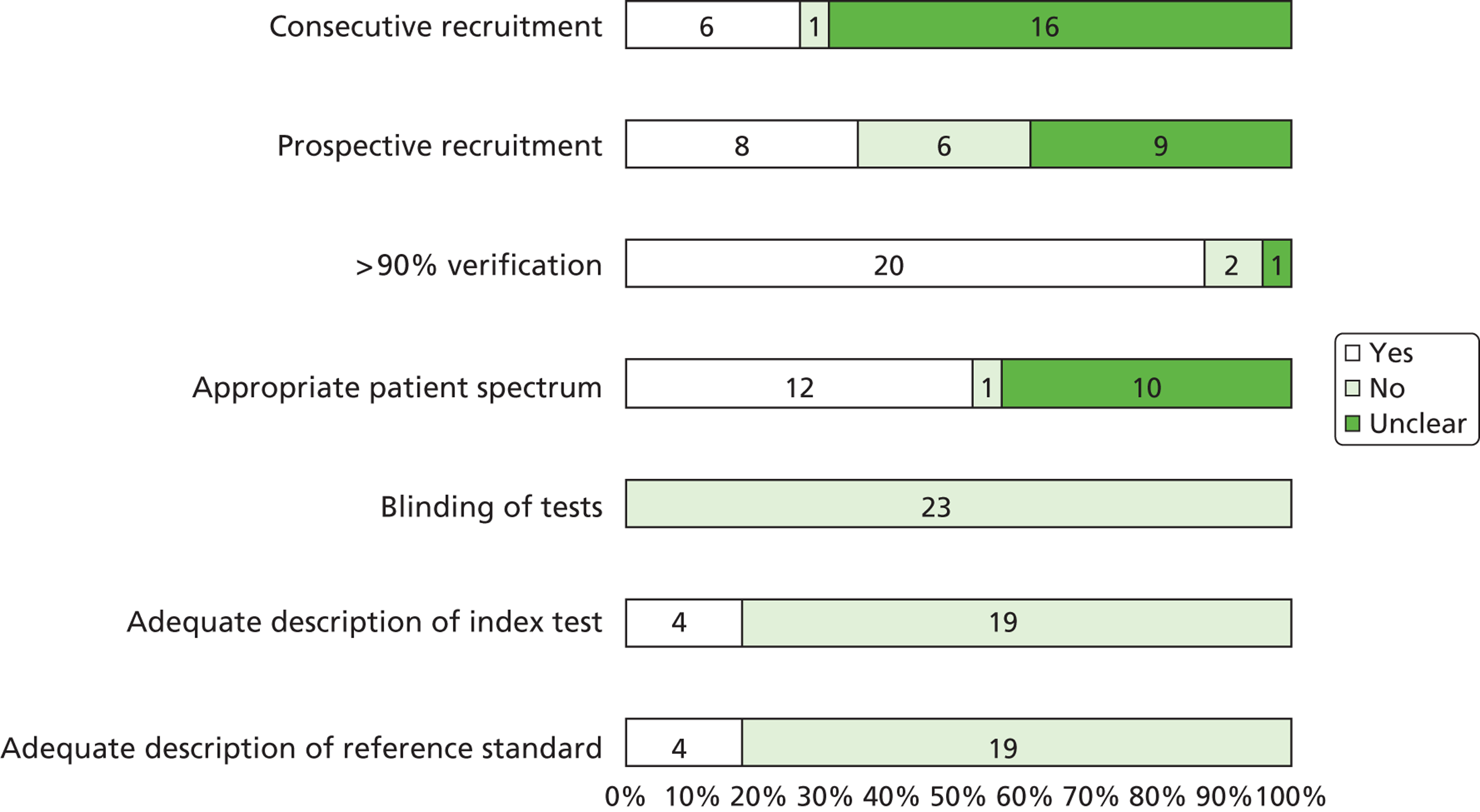
There was no individual analyte or threshold that could be shown to be of particular clinical value ( Table 1 ).
| Index Test | Threshold | No of studies | LR + (95% CI) | LR – (95% CI) |
|---|---|---|---|---|
| Sodium | > 95th centile | 3 | 4.46 (1.71–11.6) | 0.39 (0.17–0.88) |
| Sodium | > 100 mEq/l or 100 mmol/l | 3 | 3.13 (0.78–12.58) | 0.37 (0.12–1.12) |
| Sodium | > 100 mg/dl | 3 | 3.33 (1.84–6.02) | 0.44 (0.19–1.01) |
| Beta 2 microglobulin | > 2/2.5 mg/dl | 4 | 3.50 (0.37–33.5) | 0.46 (0.19–1.13) |
| Beta 2 microglobulin | > 10 mg/dl | 2 | 4.61 (0.65–32.68) | 0.52 (0.24–1.13) |
| Beta 2 microglobulin | ≥ 13 mg/dl | 3 | 2.92 (1.28–6.69) | 0.53 (0.24–1.17) |
| Calcium | > 95th centile | 2 | 6.65 (0.23–190.96) | 0.19 (0.05–0.74) |
| Calcium > 0.95 mmol/l or > 1.25 mmol/l | > 0.95 mmol/l or > 1.25 mmol/l | 3 | 3.44 (1.78–6.65) | 0.43 (0.26–0.69) |
| Osmolality | > 200 mOsm/l or > 210 mOsm/l | 4 | 3.41 (1.88–6.19) | 0.33 (0.14–0.77) |
| Chloride | > 90 mmol/l or > 90 mEq/l | 3 | 3.09 (0.57–16.71) | 0.46 (0.15–1.42) |
Evidence for antenatal diagnosis and ultrasound markers to predict postnatal outcome
Data relating to the natural history of LUTO is difficult to identify. However, a recent study from Finland has presented outcomes in babies with posterior urethral valves (n = 46) where 23 were diagnosed prenatally (with no intervention) and 23 postnatally (with apparently ‘normal’ ultrasound appearances in the majority). 11 This study is unique, in that long-term follow up over 10 years was performed (mean 12.5 years; range 5.5–20.1 years). Despite postnatal treatment, 13% ended up developing chronic renal failure with 17% ending the study with end-stage renal disease. There was no significant difference in the proportion of patients with primary or acquired renal dysplasia in those prenatally or postnatally diagnosed. There was no difference in long-term renal outcome in patients with prenatal or postnatal diagnosis with no difference in the mean age of advancing to end-stage renal disease. In addition, the mean age to achieving continence in both prenatal and postnatal diagnostic groups was not significantly different. Such data indicate that the severity of the ultrasound appearances (in the postnatally diagnosed group at least two prenatal ultrasound examinations had been performed) in LUTO have little bearing on the eventual paediatric outcome. Papers have reported on gestational age at diagnosis < 24 weeks,5 macrocystic/microcystic renal appearance6,7 and oligohydramnios8,9 showing good predictive accuracy but with varying results. No consensus exists at present however, as to the best ultrasound marker or combination of markers antenatally to predict postnatal renal function.
Evidence for long term outcomes and assessment measures in children affected by LUTO
There is published evidence to suggest that as well as long-term complications of end-stage renal failure, children affected by LUTO are at risk of bladder dysfunction (many involving reconstructive surgery), poor growth and male infertility if uraemic. 12–14 A recently published small cohort study however suggested that children with LUTO that are shunted antenatally are expected to have normal cognitive abilities and achieve acceptable continence with medical and surgical care with similar quality of life scores to a healthy child. 14 Factors reported to be associated with long-term outcome in PUV include antenatal detection, and on postnatal investigation bilateral vesicoureteral reflux, poor detrusor function and delayed achievement of urinary continence, recurrent urinary tract infections and persistent elevation of serum creatinine concentrations after valve ablation. 7,21–24
We have performed literature searches and contacted experts to determine the best tools available for assessment of disability and micturition function in children. To the best of our knowledge, there are no questionnaires specifically designed to assess these modalities in children affected by LUTO. However, the PedsQL 4.0 questionnaire has been used to assess quality of life in many different chronic conditions, it is of proven reliability and validity in the age group included in this trial. 15 For assessment of cognitive function the Parent Report of Children’s Abilities (PARCA) tool has been validated in children up to age 2,16 including children born preterm. 17 For assessment of micturition we have found no suitable validated questionnaires reported in the literature and thus in collaboration with Paediatric urologists and nephrologists will design a specific questionnaire for the PLUTO trial.
Evidence for patient acceptability, cost-effectiveness and Bayesian priors
The involvement of pregnant women in randomised clinical trials raises a number of practical and ethical issues. A literature search revealed that there were few studies18,19 examining women’s reasons for declining to participate in trials and the factors that may affect consent in pregnancy and non specifically looking at trials of a surgical intervention in pregnancy. There are no studies of cost-effectiveness of interventions for LUTO. Published examples of collecting Bayesian priors are sparse in obstetrics and none relate to fetal medicine.
The need for a large simple trial of shunt versus no shunt for congenital bladder outflow obstruction
On the basis of published evidence and evidence from Specialist Advisors in 2006, NICE have produced an Interventional Procedure Guidance for fetal vesico-amniotic shunting. The guidance states that the ‘current evidence on safety and efficacy of fetal vesico-amniotic shunting for LUTO does not appear adequate for this procedure to be used without special arrangements for consent and for audit or research.’ The guidance thus recommends randomisation to PLUTO ‘Clinicians are encouraged to enter patients into this trial or the associated registry…’. 20
Feasibility of undertaking the trial
An informal survey of all national and selected international fetal medicine units revealed that the majority of the centres that undertook this procedure were doing so using poorly defined, non-objective case selection criteria. All centres expressed a willingness to randomize to this study and to date 15 UK centres have obtained ethics and local NHS R&D approval to randomize to the study. We also have several international centres who have expressed an interest in being part of the study. Fetal medicine, as a specialty, needs to develop a strong evidence base for interventions. The Eurofetus study evaluating treatment of twin-twin transfusion therapy has shown that multi-centre RCTs evaluating in-utero therapy are feasible21. This has engendered enthusiasm among practitioners for such studies and our project is timely in that it now provides them an opportunity to participate in a serious, large multicentred trial of an intervention for which robust evidence currently does not exist. We have the support of the British Maternal and Fetal Medicine Society, British Association of Paediatric Surgeons and British Association of Paediatric Nephrologists. The support of these societies and co-operation of their members is very important to the success of a RCT where the condition is rare. To our knowledge, there is no other randomised controlled trial in progress or proposed to address this topic worldwide. The UK fetal medicine sub-specialist community and its official society are fully committed to recruitment to the trial.
2. PLANNED INVESTIGATION
Research Objectives
A high quality HTA (Health Technology Assessment) with the following objectives:
Primary Objective
-
To determine if intrauterine vesico-amniotic shunting for fetal bladder outflow obstruction, compared to conservative, non-interventional care improves perinatal and neonatal mortality and renal function.
Secondary Objective
-
To determine if shunting for fetal bladder outflow obstruction improves short-term morbidity.
-
To determine the long-term effects of shunting with respect to: a) Development of chronic renal failure, need for dialysis or transplantation. b) Development of incontinence (bladder dysfunction). c) Disability free life year (incorporating assessment of cognitive development, quality of life, micturition and general health).
-
To determine if improvement is related to prognostic assessment at diagnosis, and if possible, derive a prognostic risk index.
-
To maintain a prospective non-randomised registry of patients with lower urinary tract obstruction as part of a comprehensive cohort design.
-
To ascertain influences on the decision making of women with respect to opting for termination of pregnancy, randomisation (patient acceptability of trial) and to determine acceptability of the intervention to parents.
-
To determine clinicians’ prior beliefs about the effectiveness of shunting and to analyse trial results from a Bayesian perspective.
-
To determine the cost-effectiveness of vesico-amniotic shunting compared to conservative management.
-
To determine the local and national epidemiology of this condition.
Trial Design
A randomised controlled trial (RCT) embedded within a comprehensive cohort design ( Figure 1 ).
Following an initial ultrasound diagnosis of fetal bladder outflow obstruction, eligibility and baseline characteristics will be confirmed including standard assessments of renal function, a detailed ultrasound examination to exclude other co-existing anomalies and ideally fetal karyotyping. Eligible mothers will receive an explanation of the diagnosis, prognosis, and treatment options for the condition and rationale for the trial. If the mother consents to participation, the fetus will be randomised to receive either a fetal vesico-amniotic shunt or continue with conservative management without a shunt. A minimum of 150 pregnancies will be randomised from both the UK and international centres. The primary outcome measures are perinatal mortality and serum creatinine at 6 weeks of age. Secondary outcome measures include bladder and renal function, termination and miscarriage rates and resource usage. Initial follow-up of secondary outcomes will continue to one year of age. Long-term follow-up of continence and assessment of childhood development and quality of life is planned at five years. Alongside the trial assessment of Bayesian priors, patient acceptability studies and economic analysis will be performed.
In order to obtain the large number of pregnancies necessary for the reliable evaluation of surgical intervention for congenital bladder outflow obstruction, the trial will need the participation of the majority of centres capable of performing the shunt procedure. To make this practicable, the trial procedures need to be kept simple, with the minimal extra workload placed on participating clinicians, beyond that required to treat the mother and baby. This will be achieved by simple entry procedures, the use of standard local diagnosis and monitoring regimens (with few additional hospital visits or tests to be performed above those done as part of standard care), minimizing documentation and using secure web based data collection methods. This information will be supplemented by the use of national mortality records to ensure long-term follow-up. Regular newsletters will keep collaborators informed of trial progress, and regular meetings will be held to report progress of the trial and to address any problems encountered in the conduct of the study.
Eligible patients that refuse consent for randomisation or cases where the clinician is not uncertain about the best treatment should be entered onto the registry (Section 4).
3. ELIGIBILITY AND RANDOMISATION
Inclusion Criteria
Mother
-
Written informed consent given
-
Able to understand information provided (use of interpreter may be required)
-
Singleton pregnancy.
Fetus
-
Evidence of isolated bladder outflow obstruction from ultrasound imaging
-
Male fetus.
Exclusion Criteria
-
Additional major structural or chromosomal anomaly.
There are no eligibility criteria relating to gestation nor liquor volume. There is some evidence in the literature that gestation and liquor volume at diagnosis may determine prognosis in these fetuses, this evidence is however limited. 4,22 We wish to capture all male fetuses with the condition and allow sub-group analysis to be performed based on these criteria. Female fetuses are excluded from the trial as they are likely to have a more complex aetiology with a poorer prognosis. We recognize that some fetuses may be identified prior to the 20 week detailed scan, as there are no gestational criteria, these fetuses will still be eligible to enter the trial. It should however be recognized that vesioc-amniotic shunting can not normally be performed until at least 16 weeks and thus fetuses identified earlier will not be able to be randomised until this time.
Eligibility and randomisation based on ‘uncertainity’
The PLUTO trial adopts a pragmatic approach and eligibility is based not on rigid entry criteria but on the ‘uncertainty principle’. That is, if the clinical team is substantially uncertain which treatment (shunt versus no shunt) should be offered that fetus is eligible to be randomised. If, on the other hand, the clinical team considers, for any reason, that there is a definite indication for or contraindication against shunting, then the fetus is not eligible for randomisation. In these circumstances randomisation is both scientifically and ethically preferable to the uninformative alternative of treating such cases in an ad hoc way outside of the study as recommended in the NICE guidance. Eligibility based on uncertainty has been used in many previous surgical trials (e.g., the MRC’s Carotid Endartectomy Trial and Parkinson’s Disease Surgery Trial) and has been shown to simplify trial procedures and to facilitate large-scale recruitment of an appropriately heterogeneous range of pregnancies.
3.1.1 The choice of questions to be asked
-
Should observation with ultrasound be utilised? Are the ultrasound features of LUTO mild and presenting in the third trimester and associated with normal liquor volume for gestation.
Ideally these patients should still be randomised as we need to know if shunting helps or not in these cases. However, if entered onto the registry then this will give important prognostic information.
-
Am I sure that the fetus with LUTO will benefit from vesico-amniotic shunting? Are the ultrasound features of LUTO marked and I am sure that the benefits of insertion of the shunt outweigh the risk.
There is no obvious evidence that the ‘severity’ of ultrasound features indicates accurately the appropriateness and potential efficacy of PLUTO and thus all patients should be approached about randomisation regardless of these features. If necessary at a later date, these patients may cross-over arms in the trial e.g. if they are allocated to non-shunting at a time unspecific afterwards, they may be crossed over into shunting.
-
Am I uncertain? There are significant ultrasound features of LUTO in the first or second trimester. These may or may not be associated with oligohydramnios (maximum vertical pool depth < 5th centile for gestation) or impaired renal sodium handling (urinary Na+).
As with reference from our paediatric urological colleagues, these may well be the patients most likely to benefit from PLUTO. Some may consider that when cystic renal disease and oligohydraminos are present then the opportunity for intervention has been missed. When liquor volume is still normal then renal function may be protected. It is important to consider that the risk of shunting may be small compared to the benefit. The trial will answer this question.
3.1.2 Risks and Benefits of shunting
Insertion of a fine siliastic catheter percutaneously into the fetal bladder in the presence of LUTO would logically appear to be efficacious, as it would bypass the urethral obstruction underlying this congenital anomaly ( Figure 5 ). This would protect the fetal kidneys from an ‘increased backpressure’ leading to bilateral hydronephosis and renal parenchymal damage (cystic change). This may have benefit in cases where oligohydramnios has occured, allowing amniotic fluid (fetal urine) to accumulate around the fetus allowing optimal pulmonary develop (critically between 16–24 weeks). In cases where there is normal or borderline normal liquor volume it may allow the obstruction to be relieved prior to significant renal damage.
FIGURE 5.
Insertion of vesico-amniotic shunt.
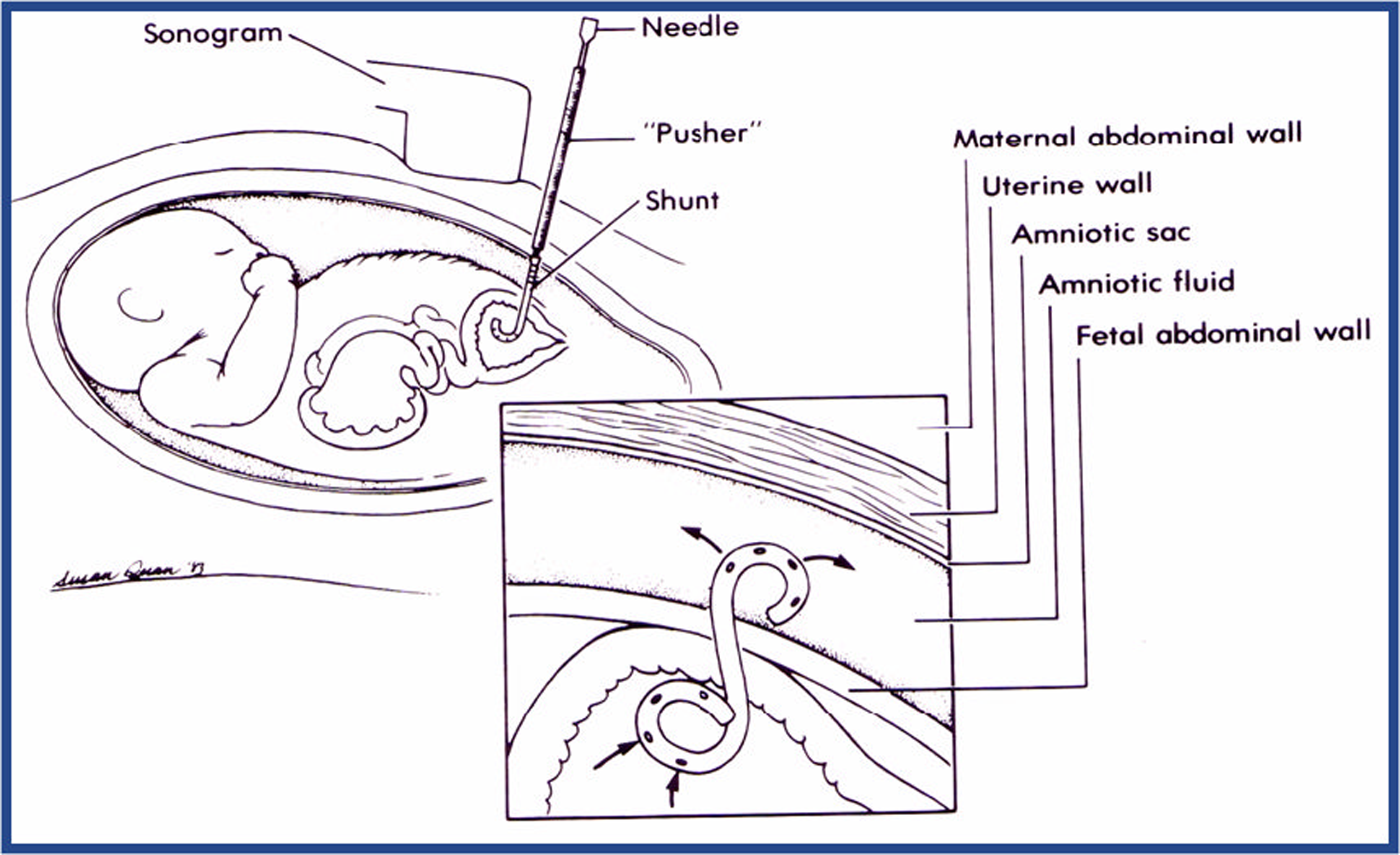
We recognise that not all centres will feel competent to perform vesico-amniotic shunting. If this is the case, we can perform the procedure at the Fetal Medicine Centre, Birmingham Women’s Hospital if the patient is randomised to intervention.
However, any procedure involving in utero manipulation may lead to miscarriage, pre-labour rupture of membrane and premature labour. This may in itself increase perinatal morbidity and mortality. In addition, percutenaous insertion of a fine needle and catheter into the fetal bladder through the anterior abdominal wall may introduce infection, cause bleeding or cause hernia to form. The effects of chronic suprapubic catheterisation are unknown in the fetus and the effects on long term bladder and kidney function are not accurately known.
Definition of Lower Urinary Tract Obstruction (LUTO)
LUTO can be a consequence of a range of pathological processes. The most common anomaly is posterior urethral valves (PUV) accounting for approximately half of cases presenting with ultrasound features of LUTO. 23 Urethral obstruction is another common cause of urethral atresia. 24 The affected fetus is typically male; females with LUTO often demonstrate more complex, morbid pathologies such as cloacal plate anomalies, including megacystis microcolon syndrome (dysfunctional smooth muscle in bladder and distal bowel). LUTO is a disease of high mortality and morbidity. It is associated with cystic renal dysplasia and abnormal renal (glomerular and tubular) function. Progressive renal dysfunction may lead to severe oligohydramnios, predisposing the fetus to pulmonary hypoplasia and positional limb abnormalities. 25
Accurate detection of LUTO is possible via ultrasound, typical ultrasound features are megacystis with bilateral hydronephrosis with or without renal parenchymal change and oligohydramnios. The association of increased echogenicity and oligohydramnios with megacystis being predictive of an obstructive aetiology in approximately 87% of cases. 26 It is however of limited value in differentiating PUV from other causes of LUTO26,27 and this the final diagnosis is often not known until postnatally. For the purposes of trial entry LUTO is thus defined via the ultrasound criteria ‘Evidence of isolated bladder outflow obstruction from ultrasound imaging, male fetus’.
Screening and Recruitment of Potential Participants
All women, including minors deemed to have ‘Gillick competence’, referred for a high-resolution scan at about 18–24 weeks of gestation will be counselled as to the possibility of fetal abnormalities. Diagnosis of bladder outflow obstruction will be made on the basis of ultrasound, fetal vesicocentesis (bladder drainage and measure of urinary Na+ [optional]) and amniotic fluid volume. The diagnosis will be explained carefully to the parents and the possibility of participation in the trial will be proposed. A written participant information sheet (Appendix A) and consent (Appendix B) form will be provided, along with any other supporting literature for those receiving a diagnostic of fetal abnormality. Support and counselling should be provided according to local practice and a contact telephone number for a specialist midwife counsellor provided. In some cases, the fetal medicine sub specialist will discuss termination of the pregnancy. Those mothers who opt for termination will not have the trial discussed further but will be approached at their follow up counselling appointment about participation in the qualitative research study (see Section 8). An anonoymised register of termination of pregnancies (gestation, Fetal medicine centre) will be kept.
At the next visit, typically 3–7 days following vesicocentesis or for follow up scan, the mother will be invited to consent to participation. Only Fetal Medicine Sub-specialists and ultrasonographers experienced in fetal bladder shunt insertion will perform the procedure, consent for the procedure and countersign consent forms. Professional interpreters will be used for women who speak little or no English and will countersign consent forms. A copy of the consent form will be given to the mother; one will be kept in the hospital’s ultrasound notes, one for the baby’s notes and the top copy sent to the Trial Office. Once the mother has been discharged from obstetric care, all trial forms in the ultrasound notes should be transferred to the study site file. The mother’s GP should be notified, with her consent, a proforma for the GP letter will be provided in the investigator’s folder (Appendix C).
Ineligible Pregnancies
The clinical alternatives for ineligible pregnancies (those with multiple abnormalities), including termination, will be discussed with the parents as in routine practice. Women whose fetus is eligible to be randomised into PLUTO but who do not give consent to randomisation or for women where the fetal medicine specialist has a strong preference for either shunting or conservative management or there is a multiple pregnancy will be treated by the Fetal Medicine Specialist according to best clinical practice and his/her best judgment. Given that there is no reliable evidence for the effectiveness of shunt in bladder outflow obstruction and the risks of the procedure, the current standard of practice should be not to shunt and manage conservatively. This alternative should be explained to the parents as part of the consent process. In all cases of pregnancy ineligible for randomisation the women should be counselled and consented to take part in the non-randomised registry (see Section 4.0). Women that opt for termination of pregnancy should be notified to the trial office, an anonymised registry of terminations will be kept and thus no consent is required.
Randomisation
Internet randomisation will be organised by the University of Birmingham Clinical Trials Unit. Randomisation will allocate consenting eligible participants to either intervention (placement of a vesico-amniotic shunt) or observation until delivery. All required baseline data must be provided before an allocation is given. Telephone randomisation will be provided as backup in case the internet randomisation is unavailable.
Minimisation will be used to ensure balance of treatment allocation overall and by the following variables to be used in the pre-specified sub-group analyses; gestational age at diagnosis, age of mother at diagnosis, liquor volume by AFI. Due to serious risk at foreknowledge, the randomisation will not be minimized by fetal medicine specialist. Randomisation notepads (Appendix D) will be provided to investigators and may be used to collate the necessary information prior to randomisation. Mothers are registered and randomised into the trial at the website https://www.trials.bham.ac.uk/PLUTO or via telephone to BCTU. Telephone randomisation is Monday–Friday 0900–1700 on 0800 9530274.
4. NON-RANDOMISED REGISTRY
4.1 The importance of the registry
The registry arm of the study allows us to establish a comprehensive cohort design with data collection on non-randomised patients. It is a very important part of this study where randomisation is based on clinicians’ uncertainty and a parent’s wishes must be taken into account. As stated previously, this is a rare condition and thus it is important to capture information about all cases. The registry will provide important additional information about outcomes, safety and adverse effects not obtainable from RCT data. These data will be very useful in decision-analytic modelling.
4.2 Eligibility criteria for the registry
4.2.1 Inclusion criteria
Mother
-
Written informed consent given
-
Able to understand information provided (use of interpreter may be required)
-
Singleton or multiple pregnancy
Fetus
-
Evidence of isolated bladder outflow obstruction from ultrasound imaging
-
Male fetus
4.2.2 Exclusion criteria
-
Additional major structural or chromosomal anomaly.
As discussed previously, the registry is designed for those parents that do not consent to the process of randomisation or where either parent or clinician has a strong preference for one form of treatment. As with the RCT, there are no eligibility criteria relating to gestation or liquor volume.
4.3 Entry onto registry
Ideally all patients if eligible should be randomised. If a decision is made to enter onto the registry this should ideally be done prospectively, at the time of diagnosis but retrospective registration during the pregnancy but prior to delivery will be accepted. All parents entering on the registry must be counselled in the same way as those for the RCT, provided with the patient information leaflet and consented in the same manner.
Entry onto the registry will be via telephone or internet registration as for randomisation (see Section 3.7); all information on the randomisation notepad will be required.
4.4 Follow-up of registry participants
The antenatal and post-natal follow-up for registry participants will be identical to those that are randomised (see Section 6).
5. TREATMENT ALLOCATIONS
Trial Treatment
The vesico-amniotic shunt will remain in place until removed by the attending Paediatric team following delivery.
5.1.1 Vesico-amniotic shunting
After ultrasound diagnosis, appropriate counselling and consent the insertion of the vesicoamniotic shunt can be performed. In general terms, the patient is given some sedation (usually maternal oral lorazepam 2–4 mg) and prophylactic antibiotics (cephalexin 500 mg orally) two hours prior to the procedure. The patient is scanned and the fetal bladder visualised. Under sterile, minimal touch technique, the percutaneous placement of the vesicoamniotic pigtail catheter is performed under ultrasound guidance using either the King’s College/Rocket introducer or the Harrison Shunting set (operator preference). Correct placement of the shunt and viability should be noted post-procedure. Follow up scans should be arranged at the clinicians’ discretion but usually are performed no less frequently than every four weeks.
5.1.2 Monitoring of the shunt
Ultrasound scans throughout the pregnancy will confirm that the shunt is in situ, and if required, further procedures will be performed to replace the shunt. The patient and fetus should be scanned regularly at the clinician’s discretion, commonly no less frequently than every four weeks.
5.1.3 Conservative Management
The patient and fetus should be scanned regularly at the clinician’s discretion, commonly no less frequently than every four weeks.
Withdrawal of treatment or protocol violation
The shunting procedure will be performed as soon after randomisation as possible, to reduce the possibility of a change of mind regarding treatment. Once the shunt is in place, it cannot be removed until delivery. In some instances with conservative management, shunting may become definitely indicated after randomisation in the clinician’s judgment, due to worsening prognosis. In this circumstance, the insertion of a shunt should be reported to the Trial Office and will be considered as a treatment failure. The participant is not withdrawn from the study and will have continued data collection to allow intention to treat analysis.
Other management at discretion of local doctors
Investigators will agree a standard care protocol and compliance with this will be monitored throughout the antenatal period to determine if performance bias will influence results. Inevitably, some mothers will request termination of pregnancy after randomisation; this event should be reported immediately to the Trial Office. Intrauterine death after randomisation or termination of pregnancy (TOP) due to lack of success of treatment will be considered as treatment failures.
6. FOLLOW-UP AND OUTCOME MEASURES
Format
All ante- and perinatal outcome measures are obtained as part of the standard clinical practice for diagnosis, monitoring and treatment of bladder outflow obstruction. The patient and fetus should be scanned regularly at the clinician’s discretion, commonly no less frequently than every four weeks. A table of planned antenatal assessments is shown in Table 2 and Table 3 . Long-term follow-up of all children born with this condition is also routine practice but will be undertaken by Paediatric nephrologists or urologists, who may be at a different hospital from the where the shunt was placed. An online data entry system, with appropriate security, will allow clinicians to enter outcome data directly. Parents will be consented at the time of delivery for long-term follow-up at five years and NHS numbers collected for both mother and child, essential for tracking the location of the participants in this trial. All babies will be registered with regional and national congenital anomaly registers.
| Assessment | Pre-Randomisation | Post Procedure | Antenatal USS Assessment (Ideally fortnightly) | +21wk (depends on DOB) | BIRTH | Perinatal 4–6wk |
|---|---|---|---|---|---|---|
| PRENATAL | ||||||
| Fetal Ultrasound | ♦ | ♦ | ♦ | ♦ | ||
| -mortality | ||||||
| -amniotic fluid vol | ||||||
| -hydronephrosis | ||||||
| Surgery | ♦ | |||||
| -shunt in situ | ♦ | ♦ | ♦ | |||
| POSTNATAL | ||||||
| Serum creatinine/creatinine clearance | ♦ | ♦ | ||||
| Markers of tubular damage | ♦ | |||||
| Degree of reflex on MCUG (optional) | ♦ | |||||
| Renal ultrasound | ♦ | ♦ | ||||
| Resource usage | ♦ | ♦ | ||||
| NICU use | ♦ | ♦ | ||||
| Oxygen dependency at discharge | ♦ | |||||
| Birth weight/centile | ♦ | ♦ | ||||
| Surgery | ♦ | |||||
| Mortality | ♦ | |||||
| Assessment | Postnatal | Infant | |
|---|---|---|---|
| 1yr | 2yr | 5yr | |
| POSTNATAL | |||
| Serum creatinine/creatinine clearance | ♦ | ♦ | ♦ |
| Markers of tubular damage | ♦ | ♦ | |
| Degree of reflex on MCUG (optional) | ♦ | ♦ | |
| Renal ultrasound | ♦ | ♦ | |
| Blood pressure, height and weight | ♦ | ♦ | |
| Admissions to hospital | ♦ | ♦ | ♦ |
| Number of urinary tract infections | ♦ | ♦ | |
| Need for dialysis/transplantation | ♦ | ♦ | ♦ |
| Surgery | ♦ | ♦ | |
| Resource usage | ♦ | ♦ | |
| Bladder function | ♦ | ||
| Developmental questionnaire | ♦ | ||
| Quality of life assessment | ♦ | ||
| Mortality | ♦ | ♦ | ♦ |
Antenatal anatomical markers
To confirm diagnosis of bladder obstruction and assess renal function, a number of anatomical and biochemical markers will be recorded at diagnosis and at each 2–4 weekly antenatal visit. These will enable a prognostic risk index to be constructed from the emerging data, which will be used to identify sub-groups of pregnancies that are most likely to benefit from shunting.
A high-resolution ultrasound assessment of the pregnancy must be performed at each antenatal visit with hard-copy or video images archived in the antenatal notes. The following measurements should be obtained from the ultrasound:
-
Bladder wall thickened
-
Renal pelvic dilatation measured in transverse section and > 10 mm bilaterally (mm)
-
Longitudinal renal length (mm)
-
Presence/absence of renal cysts
Amniotic fluid volume must be reported as the maximum pool depth (cm) and the 5th centile for gestational age will be used16 ( Figure 6 ).
FIGURE 6.
Maximum pool depth chart for liquor volume. This figure was published in High Risk Pregnancy, James DK, Steer PJ, Weiner CP, Gonik D, Chapter 13 Hydramnios and Oligohydramnios, pp. 273, Copyright Elsevier (2006), with credit to the American Journal of Obstetrics and Gynecology, vol. 182, Magann EF, Sanderson M, Martin JN, Chauhan S, The amniotic fluid index, single deepest pocket, and two-diameter pocket in normal human pregnancy, pp. 1581–1588, 2000, with permission from Elsevier.
Antenatal biochemical markers
When fetal urine is obtained, biochemical analysis of urine should be recorded; fetal urinary calcium (mmol/L), fetal urinary sodium (mmol/L) and fetal β2-microglobulin (mmol/L).
These investigations are ‘optional’ and if performed should be recorded.
Primary Outcome Measures
6.1.1 Perinatal and Neonatal Mortality
The effectiveness of shunting will be determined by its effect on perinatal mortality and early neonatal mortality: intrauterine death ≥ 24 weeks or death from any reason within 28 days of delivery. The date and cause of death and any relevant pathological details will be recorded. Perinatal death need not be reported as a serious adverse event as high mortality is an unfortunate consequence of bladder outflow obstruction. Miscarriage prior to < 24 weeks must be reported as a serious adverse event.
Secondary Outcome Measures
6.1.3 During antenatal period
-
Shunt migration or blockage – this is an accepted complication of vesico-amniotic shunting and thus will be recorded for the purposes of economic analysis only.
-
Treatment failures – intra-uterine death, termination of pregnancy due to lack of success of treatment or lack of compliance with treatment (e.g. shunting in the conservative management arm).
6.1.4 Outcomes at birth
Secondary outcomes recorded on the pregnancy outcome form, completed by the midwife/neonatal nurse at discharge will include:
-
Outcome of pregnancy – will be collected for all randomised women to compare rates of termination as well as miscarriage, perinatal mortality, neonatal mortality and survival.
-
Admission to special care baby unit, length of stay (SCBU) and ventilatory support required
-
Oxygen dependency at discharge
-
Serum creatinine/creatinine clearance (if available)
-
Renal ultrasound (if performed)
-
Birth weight/centile for gestation
6.1.5 Outcomes at 4–6 weeks
At 4–6 weeks, follow-up by Paediatric nephrologists/urologists the following data will be collected in addition to serum creatinine/creatinine clearance:
-
Renal ultrasound – bladder wall thickness (mm) and texture, renal pelvic dilatation measured in transverse section and > 10 mm bilaterally (mm), ureteric dilatation (mm)
-
Weight on centile chart
-
Micturating cysto-urethrogram (MCUG) (to demonstrate reflux, due to its invasive nature will be performed at clinician’s discretion)
-
Diagnosis of posterior urethral valves, Prune Belly or other anomaly by cystoscopy and/or MCUG
-
Markers of tubular damage – the urinary albumin: creatinine ratio and c-cystatin concentration will be measured to determine if the neonatal is in chronic renal failure30
-
Documentation of any surgery.
6.1.6 First Year Outcomes
At the age of 12 months, follow-up by Paediatric nephrologists/urologists the following data will be collected:
-
Serum creatinine
-
Urinary sodium, calcium and β2 microglobulin
-
Creatinine clearance as an estimate of GFR (CrCl is derived by the Schwartz equation 0.45 × length (cm)/serum creatinine (μmol/L)). 31
-
Renal ultrasound and other forms of renal imaging, as at birth
-
Markers of tubular damage, as at 4–6 weeks
-
Need for renal dialysis/transplant
-
Admissions to hospital number and length
-
Number of acute renal tract infections (sub-classified as lower urinary tract infection or ascending infection)
-
Blood pressure
-
Height (m) and weight (kg)
-
Documentation of any surgery
6.1.7 Two Year Outcomes
At the age of 2 years, follow-up by Paediatric nephrologists will be performed and the following data will be collected:
6.1.8 Five Year Outcomes
At the age of 5 years, follow-up by Paediatric nephrologists will collect data as at the one year follow up. In addition the following data will be collected via patient and parent questionnaire:
-
Micturition questionnaire (developed specifically for this study by experts in the field).
-
Quality of life assessment (PedsQL15).
The scores from these and the PARCA questionnaire will be used to assess overall disability as a composite outcome measure. This outcome measure will consist of 6 clinical domains: bladder function, physical function, psychological function, social function, cognitive development and general health (incorporating renal function, blood pressure, height/weight centiles). In each of these domains the child’s disability status will be assessed as normal, impaired, mildly disabled, moderately disabled or severely disabled. In the statistical analysis each of these domains will be assessed individually and as a composite measure, disability free life year. A child’s overall disability status for the composite measure will be defined by the highest degree of disability across any of the 6 domains.
Serious Adverse Events
All serious adverse events must be specifically reported to the Trial Office using the SAE Form (Appendix E) within one week of the start of the event. Further details may be requested, including post-mortem reports. For the purposes of this study, adverse events are those following randomisation or registration that are fatal, life-threatening, disabling or require hospitalisation to either the mother or the fetus. This includes, but is not limited to the following events for example:
-
Miscarriage (pregnancy loss prior to 24 weeks)
-
Premature rupture at membranes (amniorrhexis prior to onset of labour)
-
Preterm labour (delivery before 37 weeks)
-
Maternal in utero infection (maternal persistent pyrexia, offensive liquor, fetal compromise, uterine tenderness)
-
Damage to maternal vasculature, uterus or other abdominal organs
-
Damage to fetal organs, such as bladder or bowel
-
Migration of stent outside of the uterine cavity requiring a surgical procedure to remove it into bladder or abdomen resulting in re-stenting, recovery or delivery
-
Adverse drug reactions to anaesthetic or antibiotic.
Perinatal mortality should be reported on the Pregnancy Outcome Form. All serious adverse events will be sent immediately to the Chair of the Data Monitoring and Ethics Committee and the multi-centre Research Ethics Committee by the Trial Office. The local Principal Investigator is responsible for complying with any Trust adverse event reporting process. As some of these events are considered to be ‘not rare’ following an invasive procedure in the antenatal period (e.g. miscarriage, premature rupture of membranes, preterm labour, chorioamnionitis) all adverse events reported will be considered by the Committees on an individual basis.
Data Management and Validation
6.1.9 Confidentiality of personal data
PLUTO will collect personal data and sensitive information about the participants either directly or from their clinical team. Participants will be informed about the transfer of this information to the PLUTO trial office at the University of Birmingham Clinical Trials Unit (BCTU) and will be asked to consent to this. The data will be entered onto a secure computer database, either by BCTU staff or directly via a secure internet connection. Any data to be processed outside the BCTU will be anonymised. All personal information obtained for the study will be held securely and treated as (strictly) confidential. All staff, at each hospital or the BCTU, share the same duty of care to prevent unauthorised disclosure of personal information. No data that could be used to identify an individual will be published.
6.1.10 Long-term storage of data
In line with MRC guidelines, all data will be stored for up to 20 years after the last participant has reached the 5 year follow-up to allow adequate time for review, reappraisal or further research, and to allow any queries or concerns about the data, conduct or conclusions of the study to be resolved. Limited data on the participants and records of any adverse events may be kept for longer if so recommended by an independent advisory board.
6.1.11 Withdrawal from follow-up
Withdrawal from follow-up is the decision of the mother. However, withdrawn participants can bias clinical trial results and reduce the power of the study to detect important differences, so parents should be encouraged allow data collection by the fetal medicine specialist or Paediatric nephrologists or to complete any follow-up questionnaires. If the reason for withdrawal is known, it should be communicated to the PLUTO Trial Office. To reduce loss to follow-up, we shall record both the mother and child’s NHS number, which allows us to track changes of address or name.
6.1.12 Definition of the End of Trial
The trial will have two phases ( Figure 7 ) based on reaching pre-specified recruitment targets.
-
Phase 1: Recruitment of women into the RCT commences. An interim analysis of key end points will be performed by June 2010. If a recruitment target of n = 80 is not substantively reached then we will halt accrual but will continue to follow up those woman and babies already recruited. The end of the follow up phase of the trial will be when the last baby recruited completes their 5 year follow-up in 2015.
-
Phase 2: If the above target is met, we will continue recruitment to the target sample size over a further 3 years to June 2013. The end of the follow-up phase of the trial will be when the last baby recruited completes their 5 year follow up in 2018.
FIGURE 7.
Project Timetable and Milestones.
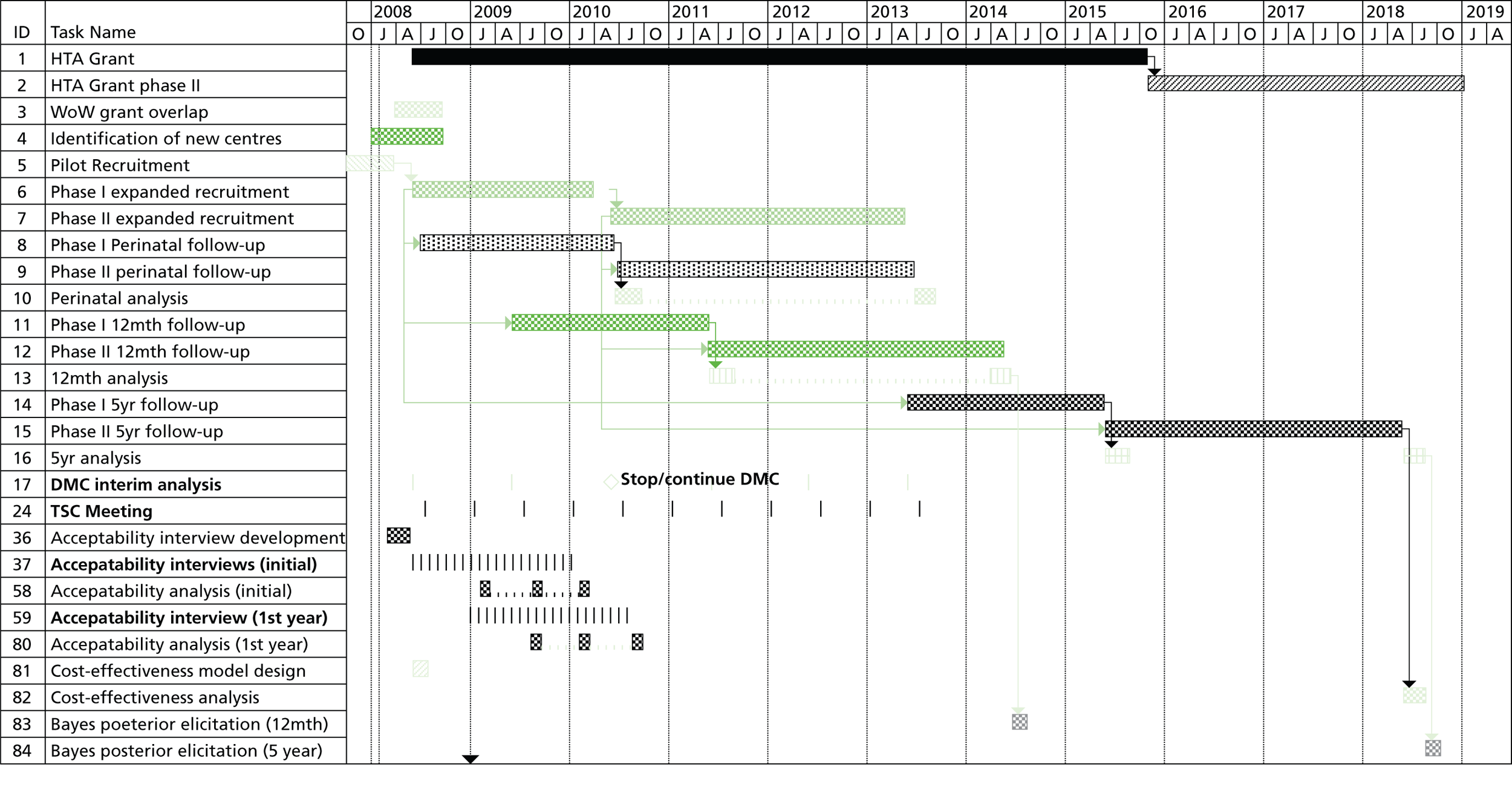
7. ACCRUAL AND ANALYSIS
Sample size
The meta-analysis of observational studies provides the basis for the sample size calculations for the PLUTO trial. 8 However, in the absence of randomised controlled trials, and limited information on the survival rate in conservatively managed pregnancies, precise sample size calculations are problematic, so we propose a minimum and an increased target sample size. We will seek the approval of the DMC for any revised sample size based on actual trial data, at the end of Phase I of recruitment. In the four studies in the meta-analysis ( Figure 3 ), the pooled survival rate in the conservative management group was 13/33 or 0.394, with a pooled odds ratio (OR) for perinatal survival of 2.53 (95% CI 1.08–5.93; excluding voluntary termination of pregnancies), equivalent to an improvement in survival of 23 percentage points (e.g. 39% survival compared with 62% survival). At 80% power and α = 0.05, we will need 75 pregnancies in each group, 150 pregnancies in total, to detect such a difference. Considering continuous outcome measures such as serum creatinine or disability free life year, 150 pregnancies will be sufficient to detect a 0.47 standardised effect size, which is considered to be a large difference32. Observational studies are prone to exaggeration of treatment effect, due to selection bias; therefore we aim to recruit beyond 150 pregnancies if possible, and if advised by the DMC, to 200 pregnancies. This would allow smaller differences to be detected, as shown in Table 4 .
| Power | 150 pregnancies | 200 pregnancies | ||
|---|---|---|---|---|
| Difference in Survival (percentage points) | Standardised difference | Difference in Survival (percentage points) | Standardised difference | |
| 80% | 23 (e.g. 39% to 62%) | 0.47 | 20 (e.g. 39% to 59%) | 0.40 |
| 90% | 27 (e.g. 39% to 66%) | 0.54 | 23 (e.g. 39% to 62%) | 0.47 |
7.1 Projected accrual and attrition rates
Congenital anomalies of the genitourinary tract are identified using prenatal ultrasound in 1 : 250 to 1 : 1000 pregnancies. With a relatively uncommon condition such as this, a collaborative effort is required involving all Consultants in Fetal Medicine who are capable of performing the shunt procedure. A survey of Fetal medicine centres (national and international) was performed in 2007, the total estimated number of cases seen at these centres was 180 per annum. Assuming 30% of women approached consent to take part, we estimate we will be able to recruit 54 pregnancies per year, so would require 3.7 years to reach the target of 200 pregnancies. If the consent rate were higher, a higher total could be achieved, improving the power of the study. From previous experience of organising and participating in other studies of fetal intervention, the estimated 30% consent rate is conservative. We also feel that as there is no gestational limit for randomisation we will improve the rate of recruitment to the trial as later in pregnancy termination rates are fewer. Loss to follow-up for the primary outcome measures is not anticipated in the short perinatal period. The babies in the trial should be prospectively registered with regional and national anomaly registers. Of the registered cases scanned at Birmingham Women’s Hospital in 2003/2004, 97% were followed up in the Paediatric renal clinic.
Statistical Analysis of RCT
7.1.1 Interim analyses
Interim analyses will be undertaken at twelve-monthly intervals, to be reviewed by an independent Data Monitoring and Ethics Committee (DMC) who will advise the Trial Steering Committee (TSC) of any clear evidence that one or other approach is preferable or if there is an unacceptable level of serious adverse events. We intend to carry out Phase 1 analysis in June 2010 to provide the DMC with evidence on effectiveness. At this time we will also be able to present to the DMC evidence from the qualitative research, as to why patients choose TOP or not to be randomised, information from the registry, and information on clinicians’ prior beliefs. This evidence will help inform the DMC in their decision making when looking at accrual. A review of the trial at two years will be performed by the DMC looking at effectiveness and level of recruitment. The DMC will report to the independent TSC who will provide the HTA with a confidential report at this stage to allow them to make a decision about providing funding for phase 2.
7.1.2 Statistical analysis
Analyses will be undertaken using a chi-squared test to examine survival proportions with corresponding risk ratios and 95% confidence intervals presented. Serum creatinine levels at 6 weeks will be considered by analysis of covariance (adjusting for measurement at 28 days), following a log transformation to stabilise the variance. Adjusted means will be analysed using a t-test, with estimates of differences presented with 95% confidence intervals. Similar standard methods will be used to analyse any secondary outcome measures. Where possible, multi-level modelling techniques will also be used to examine treatment effects over time for any continuous repeated measurements, thus maximising available power. For the randomisation part of the study analysis will be performed on an intention to treat basis.
7.1.3 Sub-group analysis
Within the randomisations, there will be sub-group analysis by gestational age at diagnosis (< 24, ≥ 24 weeks), liquor volume (≤ 5th, > 5th centile) and age of mother at diagnosis (< 20, 20–35, > 35 years). A retrospective stratification and sub-group analysis by fetal medicine specialist will also be performed to examine the effect of expertise and technique. Because of the serious dangers of misinterpretation, all subgroup analysis will be interpreted cautiously. The power for subgroup analyses is considerably less than that for the primary outcome analyses. Pre-specifying subgroup analyses is therefore important because if sub-group effects are observed these are much more plausible if in the anticipated direction.
Derivation of a Prognostic Risk Index
There is a clear need to identify the sorts of fetuses which are at greatest risk of mortality and morbidity from LUTO. Currently measures of prognosis are largely based on subjective clinical opinion. After discussion with other experts, it is considered that a prognostic index score at diagnosis (based on renal function as assessed by ultrasound appearances and biochemical urine analysis) is most likely to prove clinically useful in predicting which babies derive net benefit from intervention. Data collected at randomisation will be used to produce a prognostic index, using logistic regression. This index can then be validated against data from other trials, or against a future prospective cohort.
8. PATIENT ACCEPTABILITY
As LUTO is a rare condition and RCTs in fetal medicine uncommon, it is imperative that women’s views on the trial are assessed. In the event of poor recruitment we will be able to make recommendations for how this might be improved for both PLUTO and future RCTs in fetal medicine. It is also important to determine the acceptability of the intervention to parents, particularly in the case of an intervention requiring an invasive procedure, as these views will affect implementation of the technology in clinical practice and can be incorporated into decision-model analysis.
The patient acceptability study has the following aims:
-
To examine women’s views about PLUTO, to ascertain the influences on their decision making of the information given.
-
To determine the acceptability of the intervention.
All women who are approached regarding PLUTO will be invited to participate in the acceptability study including women that enter the registry or opt for termination of pregnancy. The research will be performed in three stages and consist of semi-structured interviews and questionnaires. The first stage of the study will consist of a semi-structured interview with questions focusing on the information given to the woman and partner at the time of diagnosis and the influences on her decision making. Women who have had a termination of pregnancy will take part in the first stage only. The second stage will occur within the first year after birth. Women will again be interviewed using a semi-structured interview with questions focusing on their individual experience of the trial and intervention once their baby has been born. The final stage will occur after the final long-term results of the study have been analysed and participants informed. A postal questionnaire will be sent to all women who agreed to participate in the patient acceptability study. Questions will address their views on the treatment and trial with the knowledge of the results of the trial to see whether and how these have changed.
This study has a separate protocol and ethics approval from Nottingham Research Ethics Committee.
9. ELICITATION OF BAYESIAN PRIOR AND POSTERIOR EXPERT BELIEFS
In this study we aim to employ Bayesian analysis to augment the traditional inferences generated from the frequentist approach. This will be particularly valuable should the study be closed early at the end of Phase 1 allowing more complete interpretation of the information accrued in order to better inform clinical practice. The data collected during the survey will be considered using a Bayesian framework33 for the following purposes:
-
Summarising pre-trial expert opinion and identifying whether the pooled opinions of specialists in fetal medicine and paediatric nephrology differ.
-
Examination of the power of the study (with n = 200) using experts’ required ‘clinically significant’ effect size, rather than the point estimate of effectiveness from the systematic review. 34,35
-
Summarise post-trial expert opinion (posterior beliefs) on the effectiveness of shunting.
Methods
A questionnaire survey to elicit Bayesian prior beliefs of effectiveness has already been distributed to approximately 400 ‘experts’ (Consultants) in the fields of fetal medicine and paediatric nephrology. The questionnaire asks respondents to provide a summary of their belief as to the effectiveness of shunting, and also to represent their belief on a frequency density diagram. We are also interested in identifying experts’ current practice, the effect size that would need to be identified in the trial to convince the experts to change their practice and the effect of shunting on various morbidities. The questionnaire was designed taking into account current recommendations regarding the methodology for eliciting priors. 36,37 A questionnaire-based survey will be used, rather than an interview approach. The questionnaire has been distributed via email (to members of the British Maternal and Fetal Medicine Society and British Association of Paediatric Nephrologists), post (to Principal Investigators) and by placing questionnaires, instruction sheets and return envelopes into delegate packs at British Renal Society Annual Conference. This multifaceted strategy is intended to maximise the sample size, but makes calculation of a response rate very difficult. We are still receiving responses to the questionnaire and are aiming to obtain at least 200 responses, which will make this the largest elicitation of prior beliefs to date.
A second questionnaire survey will be distributed to all respondents to the initial survey following final analysis of the trial results in 2018 (or 2013 if the trial closes early). This second survey will aim to elicit experts’ posterior beliefs as to the effectiveness of shunting. The results of this survey will be used to assess whether clinicians actually update their beliefs as predicted by Bayes Rule. This analysis will also be useful in predicting the likely effect of the trial results on clinical practice. For example, even if the trial results are positive, an initially sceptical clinician may not consider the evidence to be strong enough to convince them to change their practice. 38 There is evidence that such personal conservatism acts as a barrier to change,39,40 but the nature of the empirical relationship between the strength of a prior belief and the level of evidence required to change this belief is unclear. 33,41
10. HEALTH ECONOMICS
Perspective and cost data collection
If intrauterine vesico-amniotic shunting for fetal bladder outflow obstruction, compared to conservative, non-interventional care improves perinatal mortality and renal function then it is likely that important cost implications will be seen for the health care sector. For example, surviving infants may require more inpatients stays to neonatal and Paediatric intensive care, additional ongoing ultrasound scans or more ongoing additional support treatments such as renal dialysis or transplantation, compared to standard care. The economic evaluation will take the perspective of the NHS and a wider societal perspective by considering the costs to the family of a child disabled by LUTO.
Resource use data will be collected from all participating centres to estimate the costs associated with vesico-amniotic shunting in fetuses and resource use associated with their subsequent care. We shall therefore prospectively collect data on NHS resource use for a sub-sample of the study. The main resources to be monitored for an NHS perspective include:
-
The additional time for the appropriate counselling and the insertion of the vesicoamniotic shunt and consultation/explanation, compared to current practice (principally midwife and Obstetrician time)
-
The equipment and resources associated with the insertion of the vesicoamniotic shunt and knock on costs associated with additional ultrasound scans
-
Neonatal surgery if required
-
Admissions and length of stay in neonatal intensive care
-
Dialysis and transplantation.
Information on unit costs or prices will then be required to attach to each resource item in order that an overall cost per infant can be calculated. As far as possible, cost data, such as cost of ultrasound scan, or midwife time etc. to carry out the test etc., will be collected from routine sources, including Netten et al. 42 and hospital finance departments. 42 Many cost data are already available in recently published sources. A study to investigate the costs of different levels of neonatal intensive care has already been carried out43 and other cost studies with relevant costs and costs associated with pre term delivery are available to supplement these. 44–46
In order to consider the costs to the family of a child disabled by lower urinary tract obstruction, we will seek information from the parents during the interviews that will be carried out within one year, and after the study. We will adapt to make appropriate, standard questionnaires which have been used previously by the applicants to ascertain the private costs to families associated with other study interventions. We are currently using such a questionnaire in the NCCHTA funded Screening for Haemoglobinopathies study (Shift Trial) being carried out at Guy’s Hospital. Similar questionnaires were also used in the Chlamydia Screening Studies (ClaSS) project47. Information required includes whether or not the carer has taken time off paid employment, or incurred out of pocket expenses associated with travel etc. in order to look after the child e.g. time off work and travel costs associated with taking them to their outpatients appointments or to undergo dialysis etc. Acquiring such information will allow us to extend the study perspective from the NHS perspective to a wider societal perspective.
Economic analysis
There will be two components to the analysis: a within study analysis and a model-based analysis. A model-based analysis will be carried out and will allow projection of costs and benefits beyond the trial follow up period. Studies by Biard14 and Holmdahl et al. 13 have looked at long-term outcomes of patients treated with shunts or not, respectively. A review of published studies will be updated towards the end of the study to ensure that any additional data that becomes available can be used to inform the model. A retrospective epidemiological study using local congenital anomaly registers and British Isles Network of Congenital Anomaly Registers (BINOCAR letter of support available from Chair Elizabeth Draper) will be performed to enable us to populate the model with precise information on incidence rates and occurrence of events. The model will consider treatment over the total duration of follow up and will include consideration of medical and/or surgical treatments provided in the longer term. Since the model extends beyond the outcome of the study and given the potentially relatively long time horizons being considered in these analyses, many of the costs (and benefits) will be incurred (and experienced) in future years. Using discounting, adjustments will be made to reflect this differential timing. The base-case analysis will follow NICE recommendations for public sector projects.
10.1.1 Within study analysis
This will use data collected within the study. Estimates of costs and benefits will therefore relate only to the period of follow-up, and no predictions for costs and benefits beyond the study will be made. Data from the follow up assessment carried out at one year, two years and five years will be available from the study. Given that at present there is no consensus regarding the methodology for developing QALYs in children a cost utility analysis will not be attempted. Standardised neuro developmental assessments will be performed on all infants at age two years using a validated questionnaire e.g. PARCA. 16 Outcome status will be defined across 6 clinical domains including cognitive ability and general health. A child’s overall disability status will be defined by the highest degree of disability in any of the defined clinical domains. The outcome will be expressed in terms of ‘cost per additional disability-free life year gained’ following the methods used by Petrou. 48 The data available for this analysis will be patient-specific resource use and costs. Given the skewness inherent in most cost data and the concern of economic analyses with mean costs, we shall use a bootstrapping approach in order to calculate confidence intervals around the difference in mean costs. 49,50 An incremental economic analysis will be conducted. The base-case analysis will be framed in terms of cost-consequences, reporting data in a disaggregated manner on the incremental cost and the important consequences.
10.1.2 Presentation of results and sensitivity analysis
The results of these economic analyses will be presented using cost-effectiveness acceptability curves to reflect sampling variation and uncertainties in the appropriate threshold cost-effectiveness value. We shall also use both simple and probabilistic sensitivity analyses to explore the robustness of these results to plausible variations in key assumptions and variations in the analytical methods used, and to consider the broader issue of the generalisability of the results.
11. EPIDEMIOLOGY STUDY
11.1 Need for an epidemiology study
As discussed previously LUTO is a rare condition of varying aetiology. Accurate data on incidence rates and outcomes that is population based is thus lacking with the most recent report being from the Northern Region in 2005. 51 There have also been many improvements in the antenatal detection of LUTO and changes in its management since the publication of the data from historical cohorts.
Over the three year period that PLUTO has been recruiting in the UK a much smaller number of cases has been reported to the trial office than would be expected from historical incidence rates. This may be due to under reporting or it may be due to a change in the epidemiology of this condition.
A retrospective epidemiology study will this be performed with two parts. The first using the West Midlands Congenital Anomaly Register only and the second using all population based registries that are a part of BINOCAR (British Isles Network of Congenital Anomaly Registers).
11.2 Objectives of Epidemiology Study:
Study within West Midlands:
-
To perform a population based study using the West Midlands Congenital Anomaly Register to ascertain the incidence of LUTO.
-
To ascertain the notification of cases to the PLUTO trial.
-
Via using comparative Fetal Medicine Unit ultrasound data and pathology data, to look at prenatal diagnosis of the condition and outcome for the West Midlands population.
Study within BINOCAR:
-
To perform a population based study using the BINOCAR registries (England and Wales) to ascertain incidence of LUTO, look for trends over time and regional differences.
The incidence data will also be used to populate the economic model with precise information on incidence rates and occurrence of events.
11.3 Methods
Data collection will be retrospective using congenital anomaly registers. Only population based registers will be used (excludes North West Thames and Wessex). There will be no limit on time period, registers will be asked to submit as much data as they have. Cases will be retrieved based on ICD-10 codes and all data will be anonymised. The exact data set to be retrieved will be finalized after discussion with the involved registers but is likely to include diagnosis, maternal age, associated anomalies diagnosed, outcome.
In the West Midlands this study will be extended to look at antenatal diagnosis and ultrasound features and postmortem data. The radiology and fetal medicine databases at the Fetal Medicine Unit of Birmingham Women’s Hospital and the database of the pathology department will be searched using diagnosis and text fields to identify all cases of LUTO seen over the lifetime of the congenital anomaly register.
12. DATA ACCESS, QUALITY ASSURANCE AND INDEPENDENT MONITORING
In-house Data Quality Assurance
The study will adopt a centralised approach to monitoring data quality and compliance and will aim to prevent data entry errors at source, rather than retrospectively identify problems.
12.1.1 Data quality control
A computer database has been constructed specifically for the study data and includes range and logic checks to prevent erroneous data entry. Online data entry systems will be made available to local investigators if resources, which will reduce the occurrence of transcription errors. Double data entry of paper data collection forms will be periodically undertaken on a small sub-sample. Source data verification will only be employed if there is reason to believe data quality has been compromised, and then only in a sub-set of hospitals.
12.1.2 Statistical monitoring throughout the trial
The trial statistician will regularly check the balance of allocations by the stratification variables. Complete data collection will be facilitated by in-house computer data management and reminder systems.
Independent Trial Steering Committee (TSC)
The TSC provides independent supervision for the trial, providing advice to the Chief and Co-Investigators and the Sponsor on all aspects of the trial and affording protection for patients by ensuring the trial is conducted according to the MRC Guidelines for Good Clinical Practice in Clinical Trials. If the Chief and Co-Investigators are unable to resolve any concern satisfactorily, Principal Investigators, and all others associated with the study, may write through the Trial Office to the chairman of the TSC, drawing attention to any concerns they may have about the possibility of particular side-effects, or of particular categories of pregnancies requiring special study, or about any other matters thought relevant. The TSC consists of the following independent members Profs M Whittle & D Field, Dr Y Ville, lay representative, Mrs. Sarah Caranchi (midwife and mother of baby requiring in-utero treatment), Prof Adrian Woolf and Mr Nicholas Madden.
Data Monitoring and Ethics Committee (DMC): determining when clear answers have emerged
If shunting for congenital bladder outflow obstruction really is substantially better or worse than conservative management with respect to the primary endpoints, then this may become apparent before the target recruitment has been reached. Alternatively, new evidence might emerge from other sources that shunting is definitely more, or less, effective than observation. During the period of recruitment to the study, interim analyses of major endpoints will be supplied, in strict confidence, to the DMC along with updates on results of other related studies, and any other analyses that the DMC may request. The DMC will advise the chair of the TSC if, in their view, any of the randomised comparisons in the trial have provided both (a) proof beyond reasonable doubt that for all, or for some, pregnancies either policy is definitely indicated or definitely contraindicated in terms of a net difference in the major endpoints, and (b) evidence that might reasonably be expected to influence the patient management of many clinicians who are already aware of the other main trial results. The TSC can then decide whether to close or modify any part of the trial. Unless this happens, however, the TSC, the investigators and all of the central administrative staff (except the statisticians who supply the confidential analyses) will remain unaware of the interim results. The Committee will have the opportunity to consider both classical and Bayesian analysis. The DMC consists of the following independent members Dr C Cummins, Prof J Thornton and Prof J Neilson.
A joint meeting of the TSC/DMC was held in April 2007. In June 2010 the TSC and DMC will evaluate if Phase 1 has been successful.
13. ORGANISATION AND RESPONSIBILITIES
To ensure the smooth running of the trial and to minimise the overall procedural workload, it is proposed that each participating centre should designate individuals who would be chiefly responsible for local co-ordination of clinical and administrative aspects of the trial. All investigators are responsible for ensuring that any research they undertake follows the agreed protocol, for helping care professionals to ensure that participants receive appropriate care while involved in research, for protecting the integrity and confidentiality of clinical and other records and data generated by the research, and for reporting any failures in these respects, adverse events or suspected misconduct through the appropriate systems.
Investigator eligibility
All investigators must have maternal-fetal sub-specialty recognised by the Royal College of Obstetrics and Gynaecology or international equivalent. Investigators would be expected to have previous experience and demonstrable competence in ultrasound guided shunt insertion. Potential investigators lacking experience are encouraged to visit other centres for training. In addition, centres will be expected to provide a full counselling service with dedicated specialist midwifes. Interpreters should be available for those who are unable to fully understand the information and consent in English.
Referral Centres
Not every UK centre has a fetal medicine consultant capable of performing the shunt procedure but will refer complex obstetric cases to larger tertiary centres. In order to maximise recruitment, these hospitals are encourage to participate as referral centres and recruit participants by one of two routes:
-
Refer mother to tertiary centre at diagnosis but without discussing the PLUTO Trial. The tertiary centre would introduce the trial to the mother, consent her to registry or randomisation, provide clinical care as per normal arrangements and ultimately discharge the mother back to the care of the referring obstetrician. The referring centre would be a ‘Participant Identification Centre’ and would not require site specific assessment, although the Trial Office will need to know who the referring consultants are for that centre.
-
The referral centre diagnoses LUTO and introduces the PLUTO Trial to the mother. If the obstetrician is uncertain and considers randomisation appropriate, it must be explained to the mother that if allocated shunting, she will be referred to the tertiary hospital for the procedure but if allocated conservative management, she will remain under local care. The referral will be as per local arrangements. In this situation, site specific assessment will be required and a local principal investigator will need to be identified. A participant information sheet specifically for referral centres should be provided to women in this category.
Similar referral models should be considered outside the UK.
Local Co-ordinator at each centre
Each recruiting and referring centre should nominate a Consultant in Fetal Medicine to act as the local Principal Investigator and bear responsibility for the conduct of research at their centre. Close collaboration between all clinical teams is particularly important in PLUTO in order that women for whom PLUTO is an option can be identified sufficiently early for entry. The responsibilities of the local Principal Investigator will be to ensure that all medical and midwifery staff involved in the care of women with babies diagnosed with bladder outflow obstruction are well informed about the study and trained in trial procedures, including obtaining informed consent. The local Principal Investigator should liaise with the Trial Coordinator on logistic and administrative matters connected with the trial.
Midwife Co-ordinator at each centre
Each participating centre should also designate one midwife as local Midwife Coordinator. This person would be responsible for ensuring that all eligible women are considered for the study, that women are provided with study information sheets, receive appropriate counselling and have an opportunity to discuss the study if required. The midwife may be responsible for collecting the pre- and perinatal obstetric data. Again, this person would be sent updates and newsletters, and would be invited to training and progress meetings.
Nephrology Follow-up Coordinator
The follow-up of infants born with lower urinary tract obstruction is undertaken by a Paediatric nephrologist, who may be at a different hospital. As most specialist fetal medicine units will have established links, a nephrology coordinator should be identified. This person will be contacted for the long term assessments for all randomised infants and must also report any late adverse events and mortality. Occasionally, an infant may be transferred out of the area, so the fetal medicine specialist must inform whoever is providing nephrology care about the PLUTO Trial and the follow-up assessment and notify the Trial Office of who to contact for follow-up.
The PLUTO Trial Office
The Trial Office at the University of Birmingham Clinical Trials Unit (BCTU) is responsible for providing all trial materials, including the trial folders containing printed materials and the update slides. These will be supplied to each collaborating centre, after relevant ethics and Trust approval has been obtained. Additional supplies of any printed material can be obtained on request. The Trial Office also provides the central randomisation service and is responsible for collection and checking of data (including reports of serious adverse events thought to be due to trial treatment), for reporting of serious and unexpected adverse events to the sponsor and/or regulatory authorities and for analyses. The Trial Office will help resolve any local problems that may be encountered in trial participation.
Continuity of trial
It is recognized that if recruitment is successful the PLUTO trial and HTA funding will be running for 11 years. This can cause problems with continuity. The chief investigator (MDK) and expert supervisors (KSK, SB, RG, RJL) involved in this trial are permanent members of staff of the University of Birmingham and have experience of successfully completing trials run over long periods of time. The research staff involved in the different departments are often constant features of the departments even though the project that they are principally working on may change.
Research Governance
The conduct of the trial will be according to the principles of MRC Guidelines for Good Clinical Practice in Clinical Trials (1998) and the appropriate NHS Research Governance Frameworks. PLUTO does not fall within the scope of the European Directive on Clinical Trials 2001/20/EU. The University of Birmingham is sponsor for the PLUTO trial.
13.1.1 Ethical and Research Governance Approval
The Trial has a favourable ethical opinion from Nottingham Research Ethics Committee (MREC) approval (COREC Ref 04/Q2404/89), determining that the trial design respects the rights, safety and wellbeing of the participants. Every potential UK centre has already obtained LREC (Local Research Ethics Committee) and Trust R&D approval. All UK centres will be required to sign an Investigator’s Agreement with the University of Birmingham, detailing their commitment to accrual, compliance, Good Clinical Practice, confidentiality and publication. Deviations from the agreement will be monitored and the TSC will decide whether any action needs to be taken, e.g. suspension of centre. The Trial Office will ensure researchers not employed by an NHS organisation hold an NHS honorary contract for that organisation if required by current guidelines.
New international centres will be asked to apply for ethics committee approval according to their own systems, providing the PLUTO Trial Office with written evidence of approval. One local Principal Investigator in each country will be asked to act as a national coordinator and monitor the centres in their country for compliance with good clinical practice and any relevant local regulations.
Funding and Cost implications
The initial research costs of the trial were funded by a grant from WoW awarded to the University of Birmingham and the trial is now funded by an award from the HTA. The trial has been designed to minimise extra ‘service support’ costs for participating hospitals, with no extra visits to hospital and no extra tests. Additional costs associated with the trial, e.g. gaining consent, for consultant or midwife to explain the questionnaires to mothers, etc., are estimated at £207. These service costs should be met by accessing the Trust’s transitional funding budget or via the Comprehensive Research Network. For international, centres we propose a per patient payment of £150, payable when all perinatal data are received, to offset research and support costs in countries where there is no equivalent to the UKCRC support system.
Indemnity for centres in the UK
If there is clinical or other negligent harm during a clinical trial, the NHS Trust is responsible because of its duty of care to the person harmed. NHS Trusts will accept full financial liability for the negligence under Directive HSG (96)48. This Directive does not provide for no-fault compensation although the Research Governance Framework does not require no-fault compensation. Although public bodies, including the NHS, cannot agree in advance to pay compensation for non-negligent harm, they are able to consider an ex-gratia payment in the case of a claim. This would not include harm arising from a defect in a medicinal product or shunt, for which the manufacturer is liable under the Consumer Protection Act 1987. International centres will be asked to demonstrate the extent of their indemnity for clinical negligence.
Hence the University’s liability for the PLUTO trial is limited to claims against them for non-negligent liability, for example in relation to matters such as trial design. The University of Birmingham has insurance to cover this risk but believes that it is unlikely that any such claim would be successful as there are a number of layers of protection to ensure that reasonable care is taken to ensure that trials are scientifically and ethically sound.
The trial management team are currently discussing the options for sponsorship for International centres with the University of Birmingham.
Ancillary Studies
It is requested that any proposals for formal additional studies of the effects of the trial treatments on some patients (e.g. special investigations in selected hospitals) be referred to the Trial Management Committee for consideration. In general, it would be preferable for the trial to be kept as simple as possible, and add-on studies will need to be fully justified.
14. REPORTS AND PUBLICATIONS
Reports
At the end of the study an HTA monograph will be produced to include the assessment of the effectiveness of vesico-amniotic shunting, the acceptability of the intervention to patients, clinicians’ prior and posterior beliefs about the intervention and economic analysis. If the trial is stopped after phase 1, either due to poor recruitment or to demonstration of an effect a full HTA monograph will still be able to be produced.
Publication
A meeting will be held after the end of the study to allow discussion of the main results among the collaborators prior to publication. The success of the study depends entirely on the wholehearted collaboration of a large number of fetal medicine specialists, midwives and others. For this reason, chief credit for the main results will be given not to the committees or central organisers but to all those who have collaborated in the study. Centres will be permitted to publish data obtained from participants in the PLUTO Trial that use trial outcome measures but do not relate to the trial randomised evaluation and hypothesis. Joint acknowledgement will be given to all funders on any publications.
15. References
- Merrill DC, Weiner CP, Fisk NM, Moise KJ. Fetal Therapy: Invasive and Transplacental. Cambridge University Press; 1997.
- Nakayama DK, Harrison MR, de Lomimier AA. Prognosis of posterior urethral valaves presenting at birth. Journal of Pediatric Surgery 1986;21:43-5.
- Barker AP, Cave MM, Thomas DF, . Fetal pelvi-ureteric junction obstruction: predictors of outcome. British Journal of Urology 1995;76:649-52.
- Hutton KA, Thomas DF, Arthur RJ, Irving HC, Smith SE. Prenatally detected posterior urethral valves: is gestational age at detection a predictor of outcome?. Journal of Urology 1994;152:698-701.
- Crombleholme TM, Harrison MR, Golbus MS, . Fetal intervention in obstructive uropathy: prognostic indicators and efficacy of intervention. Obstetrics &Amp; Gynecology 1990;162:1239-44.
- Manning FA, Harrison MR, Rodeck C. Catheter shunts for fetal hydronephrosis and hydrocephalus. Report of the International Fetal Surgery Registry. N Engl J Med 1986;315:336-40.
- Elder JS, Duckett JW, Snyder HM. Intervention for fetal obstructive uropathy: has it been effective?. Lancet 1987;2:1007-10.
- Clark TJ, Martin WL, Divakaran TG, Whittle MJ, Kilby MD, Khan KS. Prenatal bladder drainage in the management of fetal lower urinary tract obstruction: a systematic review and meta-analysis. Obstetrics &Amp; Gynecology 2003;102:367-82.
- Kilby MD, Somerset DA, Khan KS. Potential for correction of fetal obstructive uropathy: time for a randomised, controlled trial?. Ultrasound in Obstetrics &Amp; Gynecology 2004;23:527-30.
- Whittle M. Does fetal bladder drainage have a role? PLUTO – a trial long overdue. Br J Obstet Gynaecol 2007;114:794-5.
- Morris RK, Quinlan-Jones E, Kilby M, Khan KS. Systematic review of accuracy of fetal urine analysis to predict poor postnatal renal function in cases of congenital urinary tract obstruction. Prenatal Diagnosis 2007.
- Freedman AL, Johnson MP, Smith CA, Gonzalez R, Evans MI. Long-term outcome in children after antenatal intervention for obstructive uropathies. Lancet 1999;354:374-7.
- Holmdahl, Gundela, Sillen, Ulla . Boys with posterior urethral valves: Outcome concerning renal function, bladder function and paternity at ages 31 to 44 years. Journal of Urology 2005;174:1031-4.
- Biard J-M, Johnson MP, Carr MC, . Long-term outcomes in children treated by prenatal vesicoamniotic shunting for lower urinary tract obstruction. Obstetrics &Amp; 2005.
- Varni J, Seid M, Kurtin P. PedsQL4.0: Reliability and Validity of the Pediatric Quality of Life Inventory Version 4.0 Generic Core Scales in Healthy and Patient Populations. Medical Care 2001;39:800-12.
- Saudino K, Dale P, Oliver B, . The validity of parent-based assessment of the cognitive abilities of 2 year olds. Br J Dev Psychol 1998;16:349-63.
- Johnson S, Marlow N, Wolke D, . Validation of a parent report measure of cognitive development in very preterm infants. Developmental Medicine &Amp; Child Neurology 2004;46:389-97.
- Mohanna K, Tunna K. Withholding consent to partiiapte in clinical trials: decisions of pregnant women. Br J Obstet Gynaecol 1999;106:892-7.
- Rodger M, Makropoulos D, Walker M, Keely E, Karovitch A, Wells P. Participation of pregnant women in clinical trials: will they participate and why?. American Journal of Perinatology 2003;20:69-76.
- Fetal vesico-amniotic shunting for lower urinary tract outflow obstruction. National Institute for Clinical Excellence; 2006.
- Senat MY, Deprest J, Boulvain M, Paupe A, Wimer N, Ville Y. Endoscopic laser surgery versus serial amnioreduction for severe twin-twin transfusion syndrome. New England Journal of Medicine 2004;351:136-44.
- Oliveira EA, Rabelo EA, Pereira AK, . Prognostic factors in prenatally-detected posterior urethral valves: a multivariate analysis. Pediatric Surgery International 2002;18:662-7.
- Quintero RA, Johnson MP, Romero R, . In-utero percutaneous cystoscopy in the management of fetal lower obstructive uropathy. Lancet 1995;346:537-40.
- Steinhardt G, Hogan W, Wood E, Weber T, Lynch R. Long-term survival in an infant with urethral atresia. Journal of Urology 1990;143:336-7.
- Merrill DC, Weiner C. Fetal medicine. Curr Opin Obstet Gynecol 1992;4:273-9.
- Kaefer M, Peters CA, Retik AB, Benacerraf BB. Increased renal echogenicity: a sonographic sign for differentiating between obstructive and nonobstructive etiologies of in utero bladder distension. Journal of Urology 1997;158:1026-9.
- Abbott JF, Levine D, Wapner R. Posterior urethral valves: inaccuracy of prenatal diagnosis. &Amp; Therapy 1998;13:179-83.
- Savory DJ. Reference ranges for serumc reatinine in infants, children and adolescents. Annals Clinical Biochemistry 1990;27:99-101.
- Schwartz GJ, HAycock GB, Spitzer A. Plasma creatinine and urea concentration in children: normal values for age and sex. Journal of Pediatrics 1976;88:828-30.
- Harmoinen A, Ylinen E, Ala-Houhala M, Jana M, Kaila M, Kouri T. Reference intervals for cystatin C in pre- and full term infants and children. Pediatric Nephrology 2000;15:105-8.
- Schwartz GJ, HAycock GB, Edelmann CM, Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 1976;58:259-63.
- Cohen J. Statistical power analysis for the behavioural sciences. New York: Academic Press; 1977.
- Spiegelhalter D, Freedman L, Parmar M. Bayesian approaches to randomised trials. Journal of the Royal Statistical Society Series A 1994;57:357-416.
- Spiegelhalter D, Freedman L. A predicitve approach to selecting the size of a clinical trial, based on subjective clinical opinion. Statistics in Medicine 1986;5:1-13.
- Girling A, Lilford R, Braunholtz D, Gillet W. Sample size calculations for trials that inform individual treatment decisions: a ‘true choice’ approach. Clinical Trials 2007;4:15-24.
- O’Hagan A, Buck C, Daneshkhah A, . Uncertain Judgements: Eliciting experts’ probabilities. Chichester: John Wiley & Sons Ltd; 2006.
- Chaloner K, Rhame F. Quantifying and documenting prior beliefs in clinical trials. Statistics in Medicine 2001;20:581-600.
- Fayers P, Cuschieri A, Fielding J, Craven J, Uscinska B, Freedman L. sample size calculation for clinical trials: the impact of clinician beliefs. British Journal of Cancer 2000;82:219-31.
- Scott I, Heyworth R, Fairweatherm P. The use of evidence-based medicine in the practice of consultant physicians: results of a questionnaire survey. Australia and New Zealand Journal of Medicine 2000;30:319-26.
- Jones P, Johanson R, Baldwin K, Lilford R, Jones P. Changing belief in obstetrics: impact of two multicentre randomised controlled trials. Lancet 1998;352:1988-9.
- Knipschild P. Changing belief in iridology after an empirical study. BMJ 1989;299:491-2.
- Netten A, Knight J. Annuitizing the human capital investment costs of health service professionals. Health Economics 1999;8:245-5.
- O’Neill C, Malek M, Mugford M, . A cost analysis of neonatal care in the UK: results from a multicentre study. ECSURF Study Group. Journal of Public Health Medicine 2000;22:108-15.
- Petrou S, Mugford M, McIntosh N. Current Topic in Neonatology. London: WB Saunders; 2003.
- Petrou S, Mugford M. Economic issues in the follow-up of neonates. Seminars in Neonatology 2000;5:159-6.
- Sach T, Petrou S, Davidson L. The long-term costs of preterm birth: results of a systematic review. Child: Care, Health and Development 2003.
- Robinson S, Roberts T, Barton P, Bryan S, Macleod J, McCarthy A, et al. Healthcare and patient costs of a proactive chlamydia screening programme: the Chlamydia Screening Studies project. Sexually Transmitted Infections 2007;83:276-81.
- Petrou S, Edwards L. Cost effectiveness analysis of neonatal extracorporeal membrane oxygenation based on four year results from the UK Collaborative ECMO Trial. Archives of Disease in Childhood Fetal and Neonatal Edition 2004;89:263-8.
- Briggs A, Gray A. The distribution of health care costs and their statistical analysis for economic evaluation. J Health Serv Res Policy 1998;3:233-45.
- Thompson S, Barber J. How should cost data in pragmatic randomised trials be analysed?. BMJ 2000;320:1197-200.
- Anumba DO, Scott JE, Plant ND, Robson SC. Diagnosis and outcome of fetal lower urinary tract obstruction in the northern region of England. Prenatal Diagnosis 2005;25:7-13.
Appendix A Mothers’ information sheet
See Appendix 3 .
Appendix B Mother consent form
Appendix C GP letter
Appendix D Registry and randomisation notepad
Appendix E Serious adverse event form
Appendix F PLUTO trial and registry schema
Appendix 7 Revised proposal for the PLUTO study
Final agreed closure plan for PLUTO trial HTA project: 07/01/44 October 2011
The following plan has been prepared following consultation with the following:
Birmingham Clinical Trials Unit – Professor Richard Gray, Ms Jane Daniels, Mr Lee Middleton
Trial Group – Professor Mark Kilby, Professor Khalid Khan, Dr Katie Morris
Health economics and modelling – Professor Tracy Roberts, Dr Pelham Barton
Qualitative research – Professor Elaine Denny
The proposal has been reviewed and approved by the TSC and DMC.
1. Background
The PLUTO trial has been funded by the HTA since September 2008. Following progress reports and a monitoring visit in May 2010, the decision was reached to close the PLUTO trial after a meeting with the TSC chair, PI and HTA on 3rd November 2010, due to poor recruitment and lack of equipoise among clinicians.
2. Follow-up to primary end-point
There are 31 women within the randomised arm of the PLUTO project. The last women recruited have an expected date of delivery of 10/3/2011. Thus the last date that the primary end-point of perinatal mortality could be reached is 21/4/2011 (assuming delivery at term+14 and 6 weeks postnatally) to record end-point. Of the women in the randomised arm, 28 have already reached the primary end-point with completion of data collection to this point for 27.
There are 43 women within the trial registry of which 39 have reached the primary end-point with data collection complete on 30. There are 68 women who opted for termination of pregnancy on whom there will be no further data collected.
3. Plans for orderly closure of PLUTO
Figure 1 provides the proposed plan for the orderly closure of PLUTO. This is a 12 month plan with the following components:
-
Completion of follow-up to the primary end point for all women within the randomised and registry arms of the study by September 2011.
-
Statistical analysis of results complete by end of September 2011.
-
Economic analysis and decision analytic modelling (DAM) is performed as detailed in Section 6 and complete by March 2012.
-
Qualitative research continues in present form as detailed in Section 7 with analysis complete by February 2012.
-
An HTA monograph is prepared with planned submission by end of June 2012.
FIGURE 42.
Plan for orderly closure of PLUTO.
4. Plan for statistical analysis
Analysis will proceed as per the statistical analysis section of the protocol (7.2.2) for the primary measures of perinatal/neonatal mortality and serum creatinine levels at 4–6 weeks. Inevitably, given the small numbers randomised (31 babies) the study will lack power to detect anything less than very large differences, but estimates of differences with appropriate confidence intervals will still be presented as they represent an improvement on any other available evidence. Secondary outcomes up to and including 4–6 weeks will also be analysed and presented.
As a sensitivity analysis only, data from both the randomised and registered sections of the trial (currently 75 babies in total) will be combined and assessed together as above with appropriate tests of heterogeneity completed to check for difference between the two sections of the study. We are mindful of the possibility of bias from the registered section of the study, in particular selection bias, so any results from this sensitivity will be interpreted cautiously.
Given the small numbers available any subgroup analysis or derivation of a prognostic risk index will not be feasible or appropriate and so will not be attempted. Bayesian analysis will still be completed as per the protocol following the dissemination of the above trial results in 2011. Experts will be re-surveyed to ascertain posterior beliefs in light of the trial results, though again we need to bear in mind the limited amount of data available for this analysis.
Any data collected will be made available to other academic groups if any other randomised evidence emerges so individual patient data meta-analysis can be completed.
5. Health economics and decision analytic modelling
5.1 Perspective and cost data collection
In a revised approach to the economic evaluation – the following section on perspective and data collection would remain the same – the cost data collection as outlined below is still relevant, required and possible in spite of the lower than expected recruitment numbers.
If intrauterine vesico-amniotic shunting for fetal bladder outflow obstruction, compared to conservative, non-interventional care improves perinatal mortality and renal function then it is likely that important cost implications will be seen for the health care sector. For example, surviving infants may require more inpatients stays to neonatal and Paediatric intensive care, additional ongoing ultrasound scans or more ongoing additional support treatments such as renal dialysis or transplantation, compared to standard care. The economic evaluation will take the perspective of the NHS.
Resource use data will be collected from all participating centres to estimate the costs associated with vesico-amniotic shunting in fetuses and resource use associated with their subsequent care. We shall therefore prospectively collect data on NHS resource use for a sub-sample of the study. The main resources to be monitored include:
(1) the additional time for the appropriate counselling and the insertion of the vesicoamniotic shunt and consultation/explanation, compared to current practice (principally midwife and Obstetrician time),
(2) the equipment and resources associated with the insertion of the vesicoamniotic shunt and knock on costs associated with additional ultrasound scans,
(3) Neonatal surgery if required
(4) Admissions and length of stay in neonatal intensive care
(5) Dialysis and transplantation.
Information on unit costs or prices will then be required to attach to each resource item in order that an overall cost per infant can be calculated. As far as possible, cost data, such as cost of ultrasound scan, or midwife time etc. to carry out the test etc., will be collected from routine sources, including Netten et al. 1 and hospital finance departments. 1 Many cost data are already available in recently published sources. A study to investigate the costs of different levels of neonatal intensive care has already been carried out2 and other cost studies with relevant costs and costs associated with pre term delivery are available to supplement these. 3–5
5.2 Economic analysis
An economic analysis is proposed for three different outcomes, outcome 1 is the simple analysis with outcomes 2 and 3 being part of the extended analysis proposal:
A. Outcome: Perinatal survival at 4–6 weeks: Additional cost per additional survivor at 4–6 weeks
First, we will analyse the disaggregated data in terms of costs and outcomes associated with survivors in both arms of the trial at 4–6 weeks. The initial assessment will take the form of a cost–consequence assessment – if neither arm of the trial is shown to be dominant in terms of reduced cost and improved perinatal survival we will estimate a cost effectiveness ratio in terms of additional costs per additional survivor at 4–6 weeks.
We will assess this outcome in two ways in two different analyses: first we will assess the outcome including data collected in the trial only; second, in addition to the data from the trial we will supplement the trial outcome data in terms of perinatal survival at 4–6 weeks with secondary sources from systematic reviews which have already been carried out (by other members of the team). 3
However, it must be emphasised that such a result is likely to be misleading since at 4–6 weeks the value of the surviving infant is not the same. Survivors in the shunt arm of the trial may be biased to perinatal survival but with an outlook of a very poor quality of life that cannot be assessed in infants of this age.
B. Outcomes: Survival and disability free life year gained at 1 year: Cost per survivor with or without disability at 1 year
An economic evaluation will be carried based on outcome data at one year of age which relates to serum creatinine (renal function),6,7 renal ultrasound, markers of tubular damage, need for dialysis/transplant, admissions to hospital, blood pressure, height and weight. Thus the composite measure ‘disability free life year gained’ will be used as described in the original proposal for the 5 year end-point. This composite measure will be determined from the 1 year outcomes, it must be noted however that while this will allow an assessment of morbidity and disability at 1 year this will not be comparable with any 5 year outcomes as the latter will include the domains of cognitive development and micturition/continence which can only be assessed at 2 and 5 years respectively.
Based on an outcome cost per survivor at 1 years with or without disability we will estimate a cost-effectiveness ratio using data on the proportions of infants in each arm of the trial at one year. However, the registry which contains data of infants not randomised but who have either received conservative management or shunting, will also have the same 1 year outcome data. This data will be used to inform the conditional probabilities of these infants surviving to five years and allow extrapolation of the model to this end-point if required. We would stress that we do not believe it appropriate to pool the data from the registry with trial infants to inform this outcome because the patient recorded on the registry are not randomised to their allocation and so will introduce bias.
Clearly this result will be subject to the same bias as explained for the outcome at 4–6 weeks – as we will not have full data on quality of life of these survivors at 5 years – but the implication that they have had renal failure (or not) or would require a transplant is of some value.
Presentation of results and sensitivity analysis
The results of these economic analyses will be presented using cost-effectiveness acceptability curves to reflect sampling variation and uncertainties in the appropriate threshold cost-effectiveness value. We shall also use both simple and probabilistic sensitivity analyses to explore the robustness of these results to plausible variations in key assumptions and variations in the analytical methods used, and to consider the broader issue of the generalisability of the results.
5.3 Decision analytic model
In addition to the ‘direct’ economic analysis, we propose a model-based analysis including value of information. We will build a model to allow projections of short-term trial results to longer-term outcomes. This model can be populated by a combination of data from the trial and from other sources including registry data. We acknowledge that the population following a given clinical pathway in the registry data may not be representative of the population that might follow an equivalent pathway in a trial. Accordingly, we would analyse a range of scenarios to take into account possible bias in the registry data. The adjustment made for bias in the registry data would be varied across its plausible range.
Substantial deterministic and probabilistic sensitivity analysis will be carried out to test the robustness of the model to various assumptions and to the uncertainty in the data used to populate the model. This would also allow us to carry out a value of information analysis. The most commonly seen form of value of information analysis is expected value of perfect information (EVPI) analysis. EVPI attempts to measure the total value of resolving all the uncertainty in a model12. This then places an upper bound on the amount it is worth spending on future research for the given decision problem. EVPI analysis would not be the most helpful type of analysis to carry out in this case. More useful will be expected value of sample information (EVSI) analysis. EVSI analysis would allow us to estimate the expected value of reducing the uncertainty from a trial of a realistic size given the small number of possible recruits for any trial. This would enable an estimate to be made of whether it is worthwhile to continue recruitment.
5.4 Costs of economic analysis
A formal health economic analysis requires appropriate funding to ensure that the data that informs it is robust, this includes data on costs of interventions, outcomes as well as the effectiveness data. Thus however ‘simple’ the analysis there will ideally be time required for a researcher to obtain this data. Bringing the planned economic analysis forward presents challenges in planning of work load and in being able to employ appropriate staff within short time scales. Ideally to perform the simple analysis we would require 3 months of a health economics researcher, this however is not a post that can realistically be advertised and appointed to. Thus we would propose for the simple analysis that this is performed by Dr Tracy Roberts (TR), Dr Pelham Barton (PB) and Dr Katie Morris in the time that they have allocated to the trial already. The proviso to this is that if it is felt that the resultant analysis is inferior in any way the investigators reserve the right not to publish this analysis.
To perform the extended economic analysis and DAM would require the same input from TR and PB but also the funding of a health economics researcher at 0.5WTE for 12 months. This will ensure that the analysis is informed by robust data and as described earlier the most information is obtained from the data available to either inform clinical practice or direct future research.
6. Qualitative research
The patient acceptability study has the following aims:
-
To examine women’s views about PLUTO, to ascertain the influences on their decision making of the information given.
-
To determine the acceptability of the intervention.
All women who are approached regarding PLUTO have been invited to participate in the acceptability study including women that enter the registry or opt for termination of pregnancy. This research is being performed in two stages consisting of semi-structured interviews. The first stage of the study consists of a semi-structured interview with questions focusing on the information given to the woman and partner at the time of diagnosis and the influences on her decision making. Women who have had a termination of pregnancy take part in this first stage only. The second stage occurs within the first year after birth. Women are again interviewed using a semi-structured interview with questions focusing on their individual experience of the trial and intervention once their baby has been born. This final stage of the study is necessary to truly assess the acceptability of the intervention as long term outcomes need to be known.
This study has a separate protocol and ethics approval from Nottingham Research Ethics Committee.
To date women have only been recruited to the first stage of the study (n = 7) – interviews focusing on the information given to them at diagnosis and the influences on their decision making. Graphic data have been collected to date around the following themes:
-
Experience of diagnosis
-
Emotions experienced
-
Trial participation
-
Sources of support
Data collection for the second phase will now commence with all women needs to continue with women in all groups (i.e. those having termination, those who have shunting and those who have conservative management) to allow rigorous analysis of findings using a constant comparison method.
The qualitative research can continue within its existing budget using the research midwife and Professor Elaine Denny.
7. Costs of orderly closure
7.1 12 month plan
The budget profile has been restructured to end on 31/12/2011. For the period 01/09/11–31/12/11, the majority of budget lines have been reduced pro rata to a quarter of the original amount for year 4. The exceptions are as follows:
-
Lee Middleton (trial statistician) 0.8fte for three months
-
Trial administrator to finish on 31/08/11.
-
Health economic research fellow brought forward from 2018 to 2011 at the same grade
-
Per patient payments – reduced overall budget from £16,000 to £2,000 due to lower than anticipated international recruitment.
This brings the overall total required to £442,341 (£572,031 at full economic cost), reduced from £1,024,815 (£1,278,282 at full economic cost).
8. Reference List
- Netten A, Knight J. Annuitizing the human capital investment costs of health service professionals. Health Economics 1999;8:245-5.
- O’Neill C, Malek M, Mugford M, . A cost analysis of neonatal care in the UK: results from a multicentre study. ECSURF Study Group. Journal of Public Health Medicine 2000;22:108-15.
- Petrou S, Mugford M, McIntosh N. Current Topic in Neonatology. London: WB Saunders; 2003.
- Petrou S, Mugford M. Economic issues in the follow-up of neonates. Seminars in Neonatology 2000;5:159-6.
- Sach T, Petrou S, Davidson L. The long-term costs of preterm birth: results of a systematic review. Child: Care, Health and Development 2003.
- Savory DJ. Reference ranges for serum creatinine in infants, children and adolescents. Annals Clinical Biochemistry 1990;27:99-101.
- Schwartz GJ, HAycock GB, Spitzer A. Plasma creatinine and urea concentration in children: normal values for age and sex. Journal of Pediatrics 1976;88:828-30.
- Saudino K, Dale P, Oliver B, . The validity of parent-based assessment of the cognitive abilities of 2 year olds. Br J Dev Psychol 1998;16:349-63.
- Petrou S, Edwards L. Cost effectiveness analysis of neonatal extracorporeal membrane oxygenation based on four year results from the UK Collaborative ECMO Trial. Archives of Disease in Childhood Fetal and Neonatal Edition 2004;89:263-8.
- Briggs A, Gray A. The distribution of health care costs and their statistical analysis for economic evaluation. J Health Serv Res Policy 1998;3:233-45.
- Thompson S, Barber J. How should cost data in pragmatic randomised trials be analysed?. BMJ 2000;320:1197-200.
- Briggs A, Claxton K, Sculpher M. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
Appendix 8 PLUTO trial Bayesian priors clinician questionnaire
PLUTO trial Bayesian priors clinician questionnaire (PDF download)
Appendix 9 WinBUGS code for perinatal survival
Appendix 10 WinBUGS code for survival analysis for proportional hazards assumption and uninformative prior distribution
Appendix 11 Participation in an interventional trial requiring an invasive procedure during pregnancy: influences on women’s decision-making
This work has been previously published and is reprinted from Midwifery, Denny E, Quinlan-Jones E, Bibila S, Kilby MD. The experience of pregnant women with a diagnosis of fetal Lower Urinary Tract Obstruction (LUTO), 2013, [in press and published online ahead of print November 2013]135 doi: 10.1016/j.midw.2013.10.023 with permission from Elsevier.
Interview schedule/topic guide
The following key questions will be used to trigger conversations with participants. Extra probing with follow-up questions will be determined by the initial responses given by participants and therefore the structure of each interview undertaken will vary.
The opportunity will be given to the respondent to ask any questions that she may have regarding the interview process.
Opening statement:
I am very interested in hearing about what happened around the time you were told that your baby was affected by lower urinary tract obstruction (LUTO). By this I mean the time from when you received the diagnosis until the time you made your decision to end your pregnancy or agree to participate in the PLUTO trial.
Before we begin this interview, which will last about forty minutes, can I just confirm some information with you?
-
Your age
-
Your parity
-
Gestation in weeks when the diagnosis of LUTO was first made
-
Whether this was a singleton or multiple pregnancy
Can you tell me about what happened around the time you were told that your baby was affected by LUTO?
Follow-up any specific information to clarify participant’s description of events where necessary.
You have told me what happened, were you told of this information in a way that you would have expected?
Clarify differences between participant’s expectations and what happened in reality
Can you remember how you felt when you first found out that there was a problem with your baby?
Clarify participant’s initial thoughts and feelings following diagnosis.
Do you remember if these initial feelings changed at all over time?
Clarify how these feelings may or may not have altered.
Were you able to speak to anyone other than people at the hospital about how you were feeling at the time?
Clarify who they were able to speak with and how this may have helped or hindered them in coming to terms with their baby’s diagnosis.
Have you been able to talk to anyone since about your experiences at the time?
Clarify who and what were the circumstances of the discussion.
Looking back, can you describe what impact receiving this diagnosis has had on you and those close to you?
Clarify what impact their interactions at the time have had on them and on those closely involved and how these interactions may have affected how they might feel in the future regarding their experience as a whole.
Do you have any suggestions about ways that the hospital staff could help women when telling them about this diagnosis in pregnancy?
Clarify what in their opinion would have helped them understand the experience more fully.
What do you remember being told about the PLUTO trial?
Clarify whether on reflection they felt able to fully comprehend the information being given to them at the time.
Did you discuss the opportunity to participate in the PLUTO trial with anyone other than people at the hospital?
Clarify whether others were possibly influential in helping them to decide whether or not to participate in the study.
What factors were particularly influential to your decision-making regarding whether or not to take part in the PLUTO trial?
Clarify what personally influenced them in making their decision whether or not to participate.
Can you make any suggestions about how women could be better approached to take part in trials during pregnancy where a problem has been found with the baby?
Clarify what they feel are particularly important issues during this type of interaction.
Is there anything else that you would like to tell me about your experience of being told that your baby had this condition?
Thank you very much for your help with this research study.
List of abbreviations
- AT
- as treated
- BCTU
- Birmingham Clinical Trials Unit
- BINOCAR
- British Isles Network of Congenital Anomaly Registers
- BME
- black and minority ethnic
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- Crl
- credible interval
- DMEC
- data monitoring and ethics committee
- DSA
- deterministic sensitivity analysis
- EVPI
- expected value of perfect information
- HR
- hazard ratio
- HRG
- Healthcare Resource Group
- HTA
- Health Technology Assessment
- ICD-10
- International Classification of Diseases, 10th Edition
- ICER
- incremental cost-effectiveness ratio
- IMD
- Index of Multiple Deprivation
- IPG
- interventional procedures guidance
- IQR
- interquartile range
- ITT
- intention to treat
- IUD
- intrauterine death
- LR
- likelihood ratio
- LUTO
- lower urinary tract obstruction
- MCUG
- micturating cystourethrogram
- MeSH
- medical subject headings
- MPD
- maximum pool depth
- MRC
- Medical Research Council
- MREC
- multicentre research ethics committee
- NICE
- National Institute for Health and Care Excellence
- NICU
- neonatal intensive care unit
- NIHR
- National Institute for Health Research
- NNT
- number needed to treat
- ONS
- Office for National Statistics
- OR
- odds ratio
- PARCA
- Parent Report of Children’s Abilities
- PedsQL
- Pediatric Quality of Life Inventory
- PLUTO
- Percutaneous shunting in Lower Urinary Tract Obstruction
- PSA
- probabilistic sensitivity analysis
- PUV
- posterior urethral valve
- QALY
- quality-adjusted life-year
- R&D
- research and development
- RCOG
- Royal College of Obstetrics and Gynaecology
- RCT
- randomised controlled trial
- RR
- relative risk
- SD
- standard deviation
- SIGLE
- System for Information on Grey Literature in Europe
- SROM
- spontaneous rupture of membranes
- STROBE
- Strengthening the Reporting of Observational Studies in Epidemiology
- TOP
- termination of pregnancy
- TSC
- trial steering committee
- VAS
- vesicoamniotic shunting
- WHO
- World Health Organization
- WMCAR
- West Midlands Congenital Anomaly Register
- WoW
- Wellbeing of Women
- WTP
- willingness to pay