Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 07/42/02. The contractual start date was in March 2009. The draft report began editorial review in July 2012 and was accepted for publication in November 2013. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Christian L Althaus received salary support from the Swiss National Science Foundation (PZ00P3_136737). Katherine M E Turner received salary support from the UK National Institute for Health Research Post-Doctoral Fellowship programme (project number: PDF 2009-02-055) and consultancy fees from the Department of Health Office for Sexual Health, University Hospitals Bristol NHS Foundation Trust and National Institute for Health and Care Excellence. Catherine H Mercer received funding from the UK Medical Research Council (grant number: G0601685). Sereina A Herzog received salary support from the Swiss National Science Foundation (grant numbers: PDFMP3_124952, 320030_135654). Jackie A Cassell received grant funding from the National Institute for Health Research Health Technology Assessment programme (project number: 07/42/01) and consultancy fees from the European Centre for Disease Prevention and Control. Peter J White received support from the UK Medical Research Council Centre for Outbreak Analysis and Modelling. Helen Ward received grant funding from the UK Medical Research Council (G0601699) and the Wellcome Trust (090285/Z/09/Z). Nicola Low received funding from the Swiss National Science Foundation (grant numbers: PDFMP3_124952, 320030_135654) and from GlaxoSmithKline to chair a scientific advisory board meeting about chlamydia vaccines in September 2010. She is on the editorial board of the Cochrane Sexually Transmitted Infections Review Group and co-author of a systematic review of partner notification strategies, which will be published in the Cochrane Database of Systematic Reviews.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Althaus et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Partner notification is essential to the comprehensive case management of people with sexually transmitted infections (STIs). The World Health Organization (WHO) defines partner notification as ‘public health services in which sexual partners of individuals with STD [sexually transmitted diseases, sic] are notified, informed of their exposure and offered treatment and support services’. 1 The aims of STI control, including partner notification, are to reduce morbidity and mortality, prevent human immunodeficiency virus (HIV) infection, prevent serious complications such as tubal infertility, and to reduce adverse outcomes of pregnancy. 2 The WHO definition refers only to STIs that can be cured by antibiotic treatment: syphilis, caused by Treponema pallidum; gonorrhoea, caused by Neisseria gonorrhoeae; chlamydia, caused by Chlamydia trachomatis; and trichomonas, caused by Trichomonas vaginalis. Likewise, this monograph does not cover partner notification for HIV infection or other viral STIs.
The Health Technology Assessment (HTA) programme asked, ‘What is the clinical and cost-effectiveness of providing treatment for the partner(s) of people with an STI without testing them for the STI first?’ The project presented in this monograph addresses the question by investigating the effectiveness and cost-effectiveness of both traditional and new technologies for managing the sexual partners of people with STIs. We define traditional methods as those in which the sexual partners have to attend a health service setting to be assessed before treatment and new methods as those that have been developed to facilitate rapid access to antibiotic treatment for sexual partners without them having to be assessed in a health-service setting. This introduction describes the theoretical mechanism of action of partner notification, defines different partner notification technologies, summarises outcomes and methods used to measure the effectiveness and cost-effectiveness of partner notification and defines the infections and populations covered by the included studies. Finally, we present our specific objectives and describe the way they are addressed in the monograph.
The terminology of partner notification differs between countries. We use terminology recognised in the UK, where ‘partner notification’ and ‘contact tracing’ are synonymous. The term ‘notification’ is ambiguous, so we would like to distinguish the term ‘partner notification’ from ‘disease notification’ for surveillance. ‘Disease notification’ requires that details of cases of statutorily notifiable infections be sent to national authorities. ‘Partner notification’ refers only to the process by which a sexual partner is informed (notified) that they have been in contact with a STI. Partner notification is a confidential process: details of the index cases are known only to the health professionals treating them and are not divulged either to sexual partners or to disease notification systems. Partner notification can be considered as a public good, which benefits the wider society and not just an individual, and should be available to all. There is, however, a tension between protecting the welfare of the population and of exposed partners, and protecting the liberty and right to privacy of the person infected with a STI. 3 Both the practice of and research about partner notification offer additional challenges because they require inquiry into private behaviour and because persons with STIs are often stigmatised. Although the authors approach partner notification in an objective and non-judgemental way, the societal and cultural factors that are likely to affect its implementation and impact must be acknowledged, even though they are difficult to measure.
How partner notification works
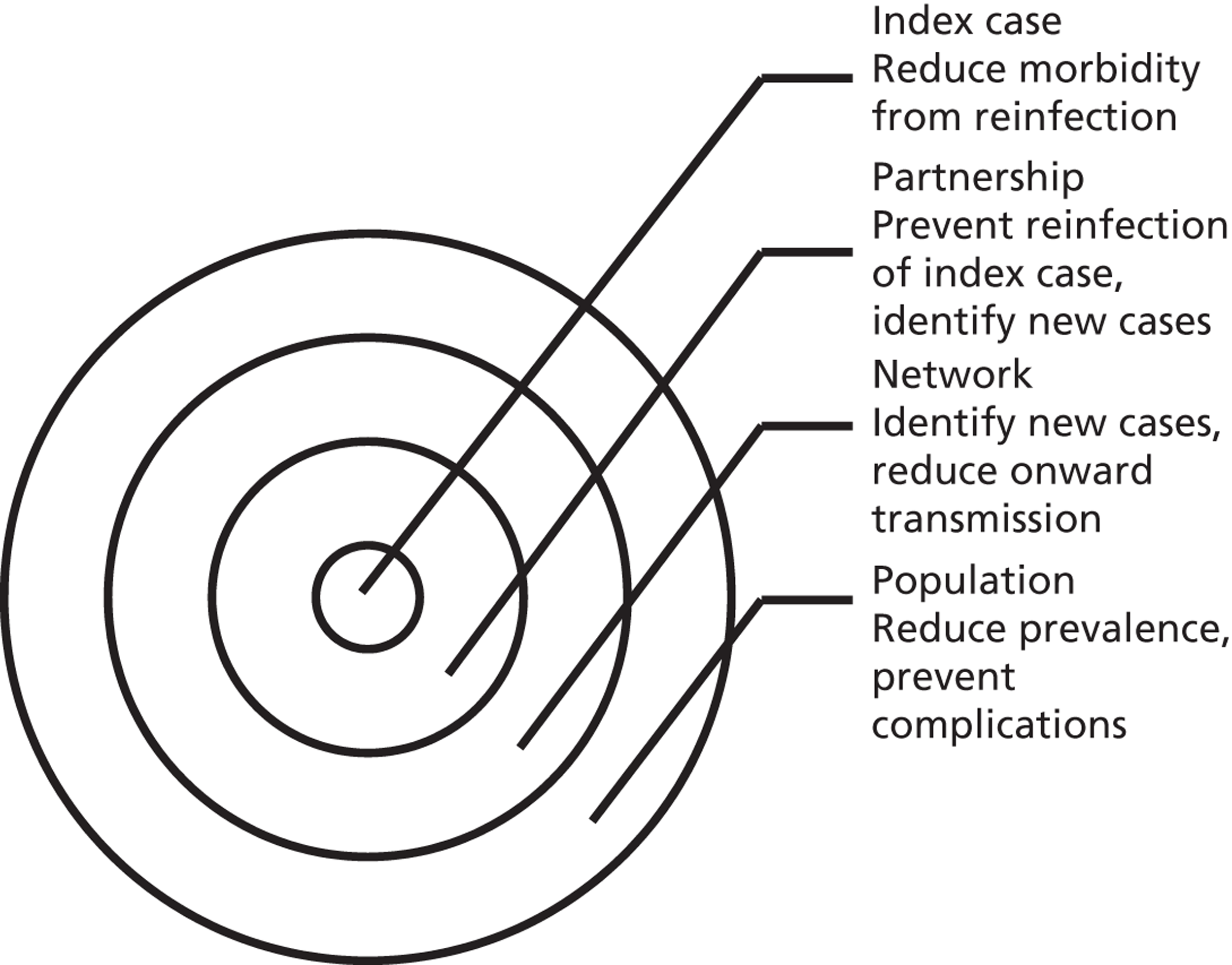
Partner notification is a multilevel process. Its goals and outcomes vary depending on the target level (Figure 1)4 and on the sexual behaviour of the index case and type of sexual partnerships that he or she has (Figure 2). 5 Partner notification contributes to STI control by reducing the duration of the infectious period at three levels.
FIGURE 2.
Mechanisms of action of partner notification in three different partnership types. Partner 1: ongoing regular partnership. Partner notification leads to identification of Partner 1; treatment prevents reinfection between index case and Partner 1; Partner 2: terminated partnership. Partner 2 was the source of index case infection. Partner notification identifies Partner 2 as a new index case; treatment prevents transmission to Partner 2.1; Partner 3: ongoing regular partnership of Partner 3 with index case and casual partnership with Partner 3.1. Partner notification prevents reinfection; partner notification identifies Partner 3 and Partner 3.1 as new index cases; treatment prevents transmission to Partner 3.1.1.
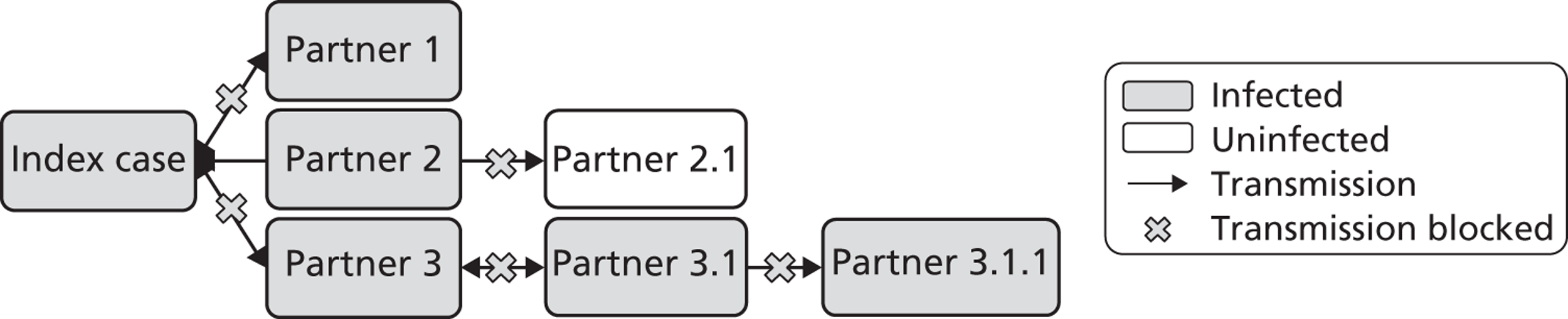
First, partner notification provides epidemiological treatment to sexual partner(s) of an index case (e.g. Partners 1, 2 and 3 in Figure 2), which means giving antibiotics before the outcome of any diagnostic tests is known. If diagnostic tests are positive then new index cases are identified (e.g. Partners 2 and 3 in Figure 2). Their infectious duration is shortened because these cases are likely to have been asymptomatic and could have continued to transmit infection unknowingly. Case finding through partner notification is very efficient, particularly for infections such as gonorrhoea, which are rare in the general population; 70–80% of partners of index cases with gonorrhoea are infected. 6 For chlamydia, 60–70% of partners of index cases are infected. 6,7 Infected sexual partners can receive treatment, which might reduce their probability of developing complications of infection. In turn, partner notification with the new index case can prevent transmission of infection to future partners (e.g. Partners 2.1 and 3.1.1 in Figure 2).
Second, partner notification aims to prevent reinfection of the person who has been treated (the index case) by untreated sexual partner(s) (e.g. Partner 1 in Figure 2). In principle, in an ongoing sexual partnership, reinfection between index case and partner will be prevented if both are treated within a short span of time and do not have unprotected sexual intercourse until antibiotic treatment has eradicated the infection in both. Prevention of reinfection shortens the total infectious period within a partnership and means that the index case is no longer infectious when they leave the partnership.
Third, in a sexual network, partner notification is intended to interrupt chains of transmission. If the uptake is high enough, the average duration of infection at the population level will be reduced and this should reduce the prevalence of the STI.
The effects of partner notification can vary according to the type of sexual partnership in which the infection occurred. The second National Survey of Sexual Attitudes and Lifestyles (Natsal-2) in the UK shows that individuals with regular or live-in partners have fewer casual (usually short-term) sexual partners than individuals who report having only casual partnerships. 8 Treating the regular sexual partner of an individual with chlamydia prevents reinfection within the partnership, but treating their casual partner(s) could prevent both reinfection and onward transmission. 9
Definitions of partner notification technologies
This monograph assesses the health technologies known collectively as ‘partner notification’. The sexual partners of index cases can be reached with a range of methods, each of which can be considered as a separate technology (Table 1). Different partner notification technologies have developed over time in response to changes in the epidemiology of STIs; changes in the organisation of, and resources available for, sexual health services; and advances in technologies for testing, treating and communicating with patients. 3 In the traditional model of sexual health care, sexual partners identified through partner notification have to attend a health-service setting to be assessed clinically before antibiotic treatment can be dispensed or prescribed. Clinical guidelines from the British Association for Sexual Health and HIV recommend that sexual partners of index cases with chlamydia, gonorrhoea, trichomonas or non-specific urethritis should be offered epidemiological treatment and testing for other STIs. 10,11 New guidelines state that epidemiological treatment could be offered to primary syphilis contacts, and that testing for syphilis can be considered for men who have sex with men in outreach settings. 12
| Term | Definition |
|---|---|
| Partner notification (synonym: contact tracing) | The process by which a sexual partner is informed (notified) that they have been in contact with a STI and is offered treatment and support services |
| Index case | The person with a diagnosed STI |
| Patient referral | The index case (patient) takes the responsibility for notifying their sexual partner(s) and telling them that they need to be treated (referral to health services) |
| Simple patient referral | Advice to the index case from a health professional that sexual partners need to be treated and that the patient should inform them and tell them to go to their own doctor or to a specialist clinic |
| Enhanced patient referral | Patient referral with the addition of one or more of:
|
| Epidemiological treatment | Antibiotic treatment given to the sexual partner when they first attend, before knowing whether or not they are infected. The antibiotics are for the same STI that the index case has as well as any others for which the probability of infection is deemed to be sufficiently high |
| APT | Facilitated access to antibiotic treatment for sexual partners to reduce the time between index case diagnosis and partner treatment. It involves a consultation by telephone or with a pharmacist to assess eligibility of the partner for treatment, but a face-to-face consultation with a physician is not required. An adaptation of EPT (see below) that complies with UK prescribing regulations |
| EPT | Facilitated access to antibiotic treatment (as antibiotics or a prescription) for sexual partners to reduce the time between index case diagnosis and partner treatment. No consultation with a health professional is required. Developed in the USA, not used in UK |
| Provider referral | A health-care professional (provider) takes responsibility for notifying the sexual partner(s) of the index case |
| Contract referral | The index case agrees to notify partners within a specified time period. If he or she has not done so, the health adviser will do provider referral. This sometimes involves a written agreement (contract) between the patient and the provider |
New technologies have been developed to allow partners to receive treatment without a face-to-face assessment in a health-service setting. These methods, in fact, simply formalise the reality of existing practice in many places. 13 If an index case has more than one partner, different partner notification technologies might be appropriate for different partners. The main features of partner notification technologies and their advantages and disadvantages for STI control are outlined below (Table 2).
| Partner notification approach | Advantages | Disadvantages |
|---|---|---|
| Partner attends health services for assessment | ||
| Simple patient referral; enhanced patient referral; contract referral; provider referral | Face-to-face consultation for advice about contacting partners and avoiding reinfection or new infection | Inconvenient for partner(s), who might not attend |
| Testing for HIV and other STIs can be done | Delay between notification and attendance allows time for onward transmission | |
| Epidemiological treatment on day of attendance | ||
| Enhancements using additional written information, home-sampling for partners, websites, etc., can improve effectiveness | ||
| Partner does not attend health services for assessment | ||
| APT; EPT | Treatment reaches partners who would not attend health service | No testing for HIV and other STIs |
| Reduced delay between notification and attendance of partner | No face-to-face consultation for advice and information | |
| Treatment package can include condoms, additional written information, etc. | ||
Partner notification technologies requiring partner assessment at health services
In the UK, patient referral is the most commonly used partner notification technology for curable STIs. 14 When the number of cases of gonorrhoea increased markedly in the 1960s and 1970s, patient referral was introduced as an efficient way of bringing sexual partners to treatment. 15 Before this, contact tracing field staff usually notified the partners of people who had either syphilis or gonorrhoea (provider referral). Patient referral was then extended as a method of partner notification for chlamydia, which has since become the most commonly diagnosed STI in the UK.
A major advantage of patient referral, and other methods for which the partner has to attend the clinic, is that tests for other STIs, including HIV, can be done and additional prevention education can be offered at the same visit. The methods used to enable patient referral are now very diverse and can be separated into simple and enhanced categories, according to the intensity of the intervention (see Table 1).
Enhancements to improve the effectiveness of simple patient referral include additional written information, verbal advice and counselling, home-sampling kits for partners, and text messaging or websites to facilitate communication. 16–19
A health-care professional (the provider) can notify partners if the index patient does not want to or cannot do it him or herself. In practice, contract referral is often not seen as a separate method but as an extension of patient referral (if partners have not been notified by the time that a follow-up visit occurs), or as a prelude to provider referral if the index case wants to try first to inform a partner on his or her own. In the UK, advisers in sexual health (health advisers) are the specialist staff for partner notification and they usually work in genitourinary medicine (sexual health, STI) clinics. If the index case agrees that provider referral is acceptable for a particular partner, the health adviser then contacts the partner by telephone, post, e-mail, text message or, less frequently, by home visit. These approaches are most appropriate in instances when attendance of the partner can be confirmed, for example where a single clinic serves a geographic area. They are often used for partner notification for people with syphilis and blood-borne virus infections, including hepatitis B and C.
Partner notification technologies not requiring partner assessment at health services
Accelerated partner therapy (APT) is a new partner notification technology in the UK to provide quicker access to antibiotic treatment for sexual partners to reduce the time between index case diagnosis and partner treatment. 20 APT is based on expedited partner therapy (EPT), which was developed in the USA to increase efficient use of partner notification resources for the most common STIs, chlamydia and gonorrhoea. 21 EPT and APT can be considered as a specific type of enhanced patient referral;22 the enhancement is the provision of antibiotics or prescriptions for index cases to give to partners.
Expedited partner therapy removes the requirement for consultation with a health professional by allowing the index patient to give medication directly as a treatment package (patient-delivered partner therapy) or as a prescription to their partner(s). 21 In the UK, two models that comply with prescribing regulations have been developed: in APT Hotline a health adviser or senior nurse practitioner assesses the eligibility of the partner for treatment by telephone; in APT Pharmacy the sexual partner attends a community pharmacy and is assessed by a pharmacist. 20
The spread of undiagnosed and untreated STIs is the main concern regarding APT and EPT. 21 When antibiotics are provided without a clinic-based consultation, the sexual partner is not tested for STIs at the time of treatment and will not be advised on how to reduce the risk of future infection. If an index case diagnosed with chlamydia gives antibiotics for chlamydia alone to his or her sexual partner(s) and no tests for STIs are done, partners who are co-infected with gonorrhoea or HIV will not have these infections diagnosed. There is then a risk that untreated gonococcal and HIV infections could cause outbreaks, which would have been detected and contained if the partners had been tested. APT consultations therefore involve an ‘assertive invitation’20 to encourage partners to take antibiotics and to undergo testing for STIs, including HIV. Missed opportunities for testing and sexual health counselling might, however, be outweighed by the benefit of interrupting transmission of the index-case STI by reaching sexual partners who would otherwise have remained untreated. 23
Reported diagnoses of curable sexually transmitted infections in the UK
Chlamydia is the most commonly curable STI diagnosed in England, followed by non-specific genital infection, gonorrhoea and syphilis (Table 3). 24 The distributions of cases by diagnosis, age and route of acquisition are assumed to be similar in Northern Ireland, Scotland and Wales. Non-specific genital infection is a clinical diagnosis, which includes cervicitis in women and urethritis in men in whom a specific microbiological diagnosis, such as C. trachomatis or N. gonorrhoeae infection, has been excluded.
| Infection | Setting | Total cases diagnosed, N | 15- to 24-year-olds, n (% of total) | Acquired heterosexually, n (% of total) |
|---|---|---|---|---|
| Chlamydia | All settings | 186,196 | 157,594 (84.6) | Not reported |
| GUM clinics | 100,660 | 62,058 (61.7) | 93,177 (92.6) | |
| Community settings | 85,536 | 85,536 (100) | Not reported | |
| Gonorrhoea | GUM clinics | 20,965 | 9074 (43.3) | 13,478 (64.3) |
| Non-specific genital infection | GUM clinics | 61,931 | Not reported | Not reported |
| Syphilis | GUM clinics | 2915 | 435 (14.9) | 728 (25.0) |
Chlamydia infections have been the most commonly reported STI since 1988, when the term was first introduced as a diagnostic category. In 1988, 30,145 cases of chlamydia and 17,062 cases of gonorrhoea were reported from UK genitourinary medicine clinics. Numbers of diagnosed chlamydia cases now include cases diagnosed in community settings (see Table 3), most of which are from the National Chlamydia Screening Programme (NCSP), which was rolled out across primary care trusts (PCTs) in England from 2003 to 2007.
Co-infection with chlamydia and gonorrhoea is common, but is not reported in routine surveillance data. Observational studies in different countries,6,25,26 and different settings in the same country,26,27 show that 30–40% of people with gonorrhoea are also infected with chlamydia and that 5–15% of those with chlamydia are also infected with gonorrhoea. The co-infection positivity rates are much higher than for either infection alone. Chlamydia is most commonly diagnosed in heterosexual women and men. Conversely, two-thirds of gonorrhoea cases and three-quarters of syphilis cases are diagnosed in men who have sex with men. The differences in the distribution of these STIs reflect the interplay between the biological characteristics of the pathogens and the sexual behaviour and sexual networks of the host.
Measuring the effectiveness and cost-effectiveness of partner notification
Methods for measuring the effectiveness of health technologies can be broadly split into those that collect primary data in empirical studies and those that synthesise existing data. This project used research methods that synthesise the findings of existing data through systematic reviews of the results of primary studies, reanalysis of data from primary studies, and modelling studies. The methods used to measure the effectiveness and cost-effectiveness of partner notification depend, like the goals, on the outcomes for the level at which the technology is targeted. Table 4 lists the measures of effectiveness and cost-effectiveness of partner notification used in this monograph.
| Measure | Level | Type | Definition |
|---|---|---|---|
| Effectiveness | |||
| Reinfection of index case | Individual | Primary | Repeated detection of STI in index case at follow-up with evidence of reinfection from an untreated partner. Measured as a rate per 100 index cases or per 100 person-years |
| Complications of STI | Individual | Primary | E.g. incidence of PID per 100 index cases or per 100 person-years |
| Partners treated | Partner | Intermediate | Number of partners of an index case who received treatment for the same STI. Measured as partners per index case verified to have been treated, OR partners per index case reported by index case to have been treated, OR percentage of index cases with at least one partner treated |
| Cases of secondary transmission | Network | Intermediate | The number of transmissions that might have occurred from index cases’ partners to their sexual partners. Measured as AROT or NNTIT |
| Population prevalence | Population | Primary | Prevalence of STI measured in a representative sample of the general population. Measured as prevalence rate per 100 persons at risk |
| Cost-effectiveness | |||
| Cost per case | Individual | Intermediate | Cost per natural unit of outcome of detecting a new case |
| Cost per secondary case | Network | Intermediate | Cost per natural unit of outcome of preventing transmission to a secondary partner |
| Cost per QALY | Population | Primary | Measure of cost-effectiveness in units that can be compared directly across different interventions and conditions. Requires appropriate valuation of health states associated with the STI |
Clinical effectiveness outcomes
Direct, objective outcomes of the primary end points of partner notification are the preferred measures of effectiveness. Primary end points are often not collected; however, because they are less frequent and more difficult to collect than intermediate outcomes. Reinfection of the index case by an untreated partner is now the preferred outcome of a failure of partner notification. The availability of nucleic acid amplification tests for diagnosis on non-invasively collected specimens has facilitated this. In practice, ‘reinfection’ is measured as repeated detection of infection at follow-up, and misclassification of the source of the repeated infection can occur. Without the additional use of highly discriminatory gene sequencing methods, repeated infection owing to antibiotic treatment failure or infection from a new partner cannot be excluded. 28 In addition, results can be biased if there is differential loss to follow-up between intervention arms.
Biological outcomes of pelvic inflammatory disease (PID) in women and population STI prevalence are difficult to attribute specifically to the impact of a partner notification intervention. To our knowledge, a reduction in the population prevalence of gonorrhoea and chlamydia is the stated primary outcome of one trial of a cluster-randomised trial of EPT in Washington (state), USA, but results have not yet been published. 29 Mathematical modelling studies are therefore the only way to estimate the impact of partner notification on transmission at the population level (see Mathematical modelling for decision-making about sexually transmitted infection control).
Intermediate outcome measures of the clinical effectiveness of partner notification include the number of partners of an index case that have been treated. In the UK, the number of partners treated per index case is the outcome used to monitor partner notification outcomes in health service evaluations, such as clinical audit. 30 Intermediate outcomes are often used as surrogates for successful partner notification but the relationship between the number of partners treated per index case and prevention of reinfection is not known. First, a record that a partner received treatment does not guarantee that medication was taken at all (unless directly observed). Second, the treatment has to be present in high enough concentrations to interrupt replication. Third, sexual partners have to avoid unprotected sexual intercourse for long enough to allow the treatment to work. For the sexual partner, the prevention of onward infection transmission is an important outcome. This can be defined as the number of transmissions that might have occurred from index cases’ partners to their sexual partners. 9 Secondary transmission is difficult to measure empirically in clinical studies because information about subsequent partners is often not collected and, if a secondary partner is infected, the direction of transmission is not known. It can, however, be estimated in modelling studies. In this monograph (see Chapter 2, Estimating the likely public health impact of partner notification for a clinical service: an evidence-based algorithm) we examine two new intermediate measures of the impact of partner notification, based on the concept of the ‘number needed to treat’: the absolute reduction in onward transmission (AROT) and its corollary, the number needed to interrupt transmission (NNTIT). 9
Cost-effectiveness outcomes
The UK National Institute for Health and Care Excellence (NICE) prefers the cost per quality-adjusted life-year (QALY) as the primary outcome for cost-effectiveness. The challenges in measuring clinical effectiveness in partner notification studies therefore also affect the estimation of cost-effectiveness. If the results of economic evaluations are to be presented in terms of cost per QALY, a valuation exercise is necessary. Economic evaluations of STI control interventions such as chlamydia screening programmes include the costs of preventing cases of PID, chronic pelvic pain, ectopic pregnancy and tubal factor infertility. These outcomes are therefore also relevant to evaluating partner notification as a health technology. Measuring health-related quality of life (HRQL) for patients during an episode of chlamydia infection presents several challenges. First, the asymptomatic nature of many STIs means that infections are often undiagnosed, and thus there is no apparent detrimental impact on quality of life. Second, instruments for measuring quality of life usually assume that a health state is chronic or permanent. This is not the case for acute episodes of STIs and for some sequelae such as PID and ectopic pregnancy. Infertility caused by a STI can be considered a permanent health state if a woman intended to start a family but a temporary health state if she decides not to have children. Cost per major outcome averted is often presented when utility weights cannot be determined. Presentation in natural units, however, cannot be directly compared with the cost-effectiveness of other interventions.
Intermediate economic outcomes of partner notification include the cost per new infection treated or of secondary cases prevented. 31 They are intermediate outcomes because they do not give a direct indication of the final outcome. These outcomes can be estimated in natural units (cost per case of chlamydia infection averted), but not in terms of health utilities. In addition, the cost per secondary transmission prevented cannot be measured directly and has to be estimated using transmission models of the infection.
Mathematical modelling for decision-making about sexually transmitted infection control
Mathematical models have emerged as a powerful tool for estimating and interpreting the potential impact of a variety of STI control interventions. 32 Mathematical models are needed to estimate the impact of partner notification because the indirect effects of STI transmission resulting from interactions between individuals that transmit infection need to be taken into account appropriately. 33 As noted above, empirical studies, including randomised controlled trials, are rarely able to collect data about population level changes in STI prevalence. In observational studies, it is difficult to attribute changes in the prevalence of a STI to any one particular intervention.
There is a wide range of different types of mathematical models that can be used to explain and understand health-care systems and interventions. To study the effects of an intervention that is expected to reduce the transmission of infection in a population, we need ‘dynamic models’ that can account for changes in the force of infection, which describes the rate of infection per susceptible individual. Models that cannot take changes in the force of infection into account are called ‘static’ or ‘constant force of infection’ models. 34 Static models can be appropriate if the intervention is studied over a time horizon that is too short to affect the force of infection, or if the proportion of the population affected is too small.
Dynamic transmission models of STIs describe the transmission of infections between individuals and can take into account the complex feedback mechanisms of interventions. However, the results of such models depend heavily on the assumptions made during model development. Most models of STIs belong to one of two types, both of which have advantages and disadvantages, and both of which have been used extensively. Deterministic, population-based models group people according to sex, infection status or sexual behaviour characteristics. Notably, the individuals within one group cannot be distinguished, and it is possible to track infections only within subpopulations of the total population. Stochastic, individual-based models of STIs are characterised by the explicit representation of all individuals as single entities. Individuals can also share sexual behaviour parameters with other individuals, but stochastic effects can result in diverse outcomes. An important aspect of such models is that they allow the representation of the entire sexual contact network, which means that one can, for example, trace the previous partners of infected index cases. A disadvantage of individual-based models is that they are often very difficult to parameterise because many assumptions have to be made about factors for which there are few or no empirical data. Also, since stochastic simulations can be computationally expensive, analysis of such models is usually much more laborious than for deterministic models. In this report, we use both deterministic, population-based models and stochastic, individual-based models, depending on the research question.
Before developing new mathematical models to examine the effects of partner notification, there are specific challenges to overcome. Many dynamic models of the transmission of C. trachomatis and N. gonorrhoeae have been developed, using both deterministic35–37 and individual-based38–40 approaches. There is, however, a need for more certainty about the underlying disease parameters for individual STIs. For example, three individual-based models have all been internally validated to generate realistic age-specific patterns of chlamydia prevalence. In a direct comparison, predictions of the impact of the same screening and partner notification intervention were dramatically different. 41 There are also examples of contrasting predictions from deterministic models that conclude either that screening at high levels of uptake can be very effective35 or that the effects might be counteracted by a loss of immunity against chlamydia in the population. 36 These challenges in interpreting model findings illustrate the importance of studying the impact of different assumptions about critical parameters on model results, and, if necessary, obtaining improved parameter estimates.
Lastly, evaluating the impact of APT requires the dynamics of both C. trachomatis and N. gonorrhoeae to be incorporated into the same model, so that the effects of treating a co-infected individual for one STI but not the other can be explored.
Objectives
Specific objectives of the project were:
-
to compare the effectiveness of different partner notification approaches to providing testing and treatment for the partners of people with curable STIs by
-
systematic reviews and analysis of secondary data to obtain estimates of outcome measures
-
mathematical modelling to estimate impact. The modelling studies considered chlamydia and gonorrhoea transmission in general heterosexual populations
-
-
to determine the cost-effectiveness of different partner notification approaches to providing testing and treatment for the partners of people with curable STIs
-
to provide research recommendations for primary research.
This monograph comprises four sections. The studies focus on partner notification technologies for chlamydia and gonorrhoea in heterosexual populations (a) because these account for the majority of curable STIs in the UK and (b) in order to be able to limit the development of mathematical models to the sexual behaviour network of one type of population and of STIs requiring similar model structures. Gonorrhoea is much less common than chlamydia but it is geographically concentrated in a small number of inner-city urban areas,42 in which rates of diagnosed infection are disproportionately high in black Caribbean minority ethnic groups. 43 The impact of partner notification in a population at high risk of gonorrhoea is therefore also considered. Although originally planned, mathematical modelling studies of the impact of partner notification technologies for syphilis and trichomonas or among men who have sex with men were not included in this project owing to lack of time. The four sections are organised as follows.
Clinical effectiveness of partner notification
The studies in this section address objective (i) by examining the effects of both traditional and new partner notification technologies on different measures of clinical effectiveness. First, we compare primary outcomes of the clinical effectiveness of new technologies (EPT or APT) with traditional partner notification methods (simple and enhanced patient referral) for chlamydia, gonorrhoea and trichomonas, based on a systematic review and meta-analysis of randomised controlled trials. 44 Second, we reanalyse clinical audit data to estimate current levels of numbers of partners treated (intermediate outcome) of partner notification for chlamydia as achieved in UK genitourinary medicine clinics. 45 Third, we estimate the impact on secondary transmission of traditional methods of partner notification for different types of sexual partner seen in UK genitourinary medicine clinics, using static modelling. 9
Mathematical modelling of the impact of partner notification technologies
Studies in this section address objective (ii). First, we describe the modelling studies that we conducted to address uncertainties in estimates of the duration46 and transmissibility47 of C. trachomatis and to resolve differences between the results of previous individual-based models of C. trachomatis transmission. 48 We focus initially on chlamydia because it is the STI for which partner notification is now most often done. The roll-out of the NCSP in England means that partner notification has to be modelled as part of a screening intervention. We then describe how we developed new individual-based models to examine the impact of traditional partner notification methods, depending on whether the outcome is measured at the individual level or the population level49 (Table 5). Finally, we examine the effects of traditional (patient referral) and new (APT) technologies among heterosexuals, using a deterministic model. We developed a new model that allows the investigations of single infections with either chlamydia or gonorrhoea and co-infections to be examined, so that the effect of providing treatment for one STI without testing for the other could be explored.
| Section | Technology | Target STI | Strategy | Scenarios | Population | Outcomes |
|---|---|---|---|---|---|---|
| Chapter 3, Individual- and population-level effects of partner notification for C. trachomatis | Traditional | Chlamydia | Test partners, but treat without waiting for result | Different look-back periods; different numbers of partners | General heterosexual | Percentage of infected partners; population prevalence |
| Chapter 3, The effects of traditional and new partner notification technologies for C. trachomatis and N. gonorrhoeae | Traditional | Chlamydia | Test partners, but treat without waiting for result | Different level of successful partner notification | General and high-risk heterosexual | Population prevalence; reinfection of index case |
| APT | Chlamydia | Treat partners without testing | ||||
| Traditional | Chlamydia + gonorrhoea | Test partners, but treat without waiting for result | Different levels of successful partner notification; different levels of STI screening; different delays to partner treatment | General and high-risk heterosexual | Population prevalence; gonorrhoea outbreak frequency; reinfection of index case | |
| APT | Chlamydia + gonorrhoea | Treat partners without testing |
Cost-effectiveness of partner notification
Studies in this section address objective 2. First, we examine the evidence available for obtaining QALYs for female reproductive tract outcomes of bacterial STIs for use in cost-effectiveness studies based on the primary population level outcomes of partner notification. Second, we used a static model to examine the intermediate outcomes of cost per case and per secondary case for current traditional partner notification technologies. 31
Discussion and conclusions
This section synthesises the findings from the component studies and provides implications for health care and recommendations for further research.
Chapter 2 Clinical effectiveness of partner notification
This section of the monograph reports the findings of both secondary data analysis and static modelling to estimate the outcomes of traditional and new partner notification technologies. The first study summarises the available evidence in a systematic review and meta-analysis of randomised controlled trials of partner notification interventions for all STIs that are treatable with antibiotics, identified from 1 January 1966 up to 31 August 2012. The review provides data about the primary outcome of reinfection of the index case and selected intermediate outcomes for a range of partner notification technologies. The second study provides clinical context by showing the levels of numbers of partners treated per index case with chlamydia achieved in UK genitourinary medicine clinics in 2007. The data were collected for a national audit and use the published audit outcome, which is an intermediate outcome of partner notification. The specific partner notification technology is not defined, but includes any traditional method chosen by the health adviser, doctor or nurse seeing the index case. The third study uses a static model to estimate the potential public health impact of traditional partner notification technologies used in genitourinary medicine clinics on preventing chlamydia transmission, depending on the type of sexual partnership. The study examines two new intermediate outcome measures: the AROT and NNTIT for different partnership types, using audit data from one UK genitourinary medicine clinic in 2011. APT was not in use at the time of the audit and modelling studies.
Clinical effectiveness of partner notification technologies: systematic review
Introduction
Randomised controlled trials are the least biased study design for measuring the causal effect of an intervention on an outcome. Systematic reviews of randomised controlled trials about a specific research question use explicit protocols to identify, collate and synthesise evidence. This allows results to be combined statistically, where appropriate, and reasons for heterogeneous results between different trials to be explored. Previous systematic reviews of the relative effectiveness of different partner notification strategies for curable STIs did not find strong evidence for the superiority of simple patient referral, provider referral or contract referral, based on the intermediate outcomes that have been measured. 50 EPT has been developed as a new partner notification technology during the era of evidence-based medicine and has been evaluated in several large randomised controlled trials with repeat infection as a primary outcome. 16,51–53 EPT involves the use of antibiotic treatment packages or prescriptions as enhancements to patient referral for infected patients with curable STIs. For a comprehensive view of the role of EPT as a new partner notification strategy, it is relevant to describe comparisons between other forms of enhanced patient referral and simple patient referral, provider referral and contract referral. The most up-to-date evidence of the effectiveness of these technologies comes from the Cochrane Sexually Transmitted Infections Collaborative Review Group’s 2012 update of its systematic review of strategies for partner notification. 44 This chapter reports the findings relevant to the STIs, partner notification technologies and outcomes considered in this monograph. 44
Objective
To report the findings of randomised controlled trials comparing at least one method of enhanced patient referral with another partner notification technology in adults with curable STIs, evaluated using a biological outcome.
Methods
The protocol and methods are available in full from the Cochrane Database of Systematic Reviews. 44 In summary, the populations studied were patients with a diagnosis of chlamydia, gonorrhoea, non-specific genital infection, trichomonas, PID, syphilis or co-infection with any of these STIs. The interventions were strategies that aimed to enhance the effectiveness of patient referral using methods including health education and counselling, health education materials (such as pamphlets, posters, and video and audio productions), and patient assistance strategies directed at facilitating patient referral (such as referral cards, incentives, reminders, and video and audio presentations). EPT (including APT) was considered as a separate type of enhanced patient referral. Eligible comparisons were any other partner notification technology. The primary outcome was reinfection of the index case, measured as repeated detection at a follow-up visit. Secondary outcomes were the numbers of partners per index case who were notified, presented for care or treated and harms of partner notification.
Search methods and selection of studies
The search of MEDLINE, EMBASE and Cochrane Central Register of Controlled Trials updated the original search (1966 to July 2001) to 29 January 2012 (see Appendix 2) and was supplemented by hand-searching of conference abstracts, review of bibliographies of included studies and previous reviews and contact with authors and experts in the field. There were no language restrictions. Selection of studies, extraction of data and assessment of the risk of bias (using the Cochrane Collaboration tool) were all done by two independent reviewers. Disagreements were resolved by discussion, or by the decision of a third independent reviewer.
Data synthesis
The treatment effect for the primary outcome was the risk ratio [RR, with 95% confidence intervals (CIs)] comparing reinfection in index cases using an enhanced patient referral strategy with reinfection in index cases using the alternative strategy. The secondary outcome was the number of partners treated per index case. The mean difference (MD, with 95% CIs) between comparison groups was calculated. Heterogeneity was assessed using the I2-statistic. Meta-analysis was performed, where appropriate, using a random-effects model to obtain the average effect size. Where heterogeneity was considered too great to pool results, reasons for heterogeneity were explored by examining stratum-specific effects in predefined subgroups.
Results
The full review in The Cochrane Library reports the results of the search strategy in detail. 44 Twenty-one randomised controlled trials16–19,50–65 reported on eight comparisons of a method of enhanced patient referral compared with an alternative technology in patients with curable STIs (Table 6). The updated search (5 January 2011 to 29 January 2012) identified 15 of these trials (9393 participants)16–19,51–57,59,61,64,65 and six (5331 participants)50,58,60,62,63 were included in the original review. Most trials (12) were conducted in the USA,16,17,50–54,56,58,60–62 three were done in the UK,19,55,64 two in Denmark,63,65 and one each in Australia,18 South Africa,50 Uganda57 and Zimbabwe. 59 There were no randomised controlled trials that evaluated the new UK partner notification technology of APT and no trials with reinfection as an outcome for syphilis, PID or for any STIs in men who have sex with men. There are no published results available for the cluster-randomised trial of EPT for chlamydia and gonorrhoea. 29
| Comparison, studies (total participants); first author, year | Country | Participants | Infections | Reinfection outcome | Follow-up test |
|---|---|---|---|---|---|
| EPT vs. simple patient referral, eight studies (n = 6537) | |||||
| Cameron 200919 | Scotland | W | Chlamydia | Yes | 3 months |
| Golden 200551 | USA | W, M | Chlamydia/gonorrhoea | Yes | 10–18 weeks |
| Kerani 201156 | USA | MSM | Chlamydia/gonorrhoea | No | NA |
| Kissinger 200516 | USA | M | Chlamydia/gonorrhoea | Yes | 2–8 weeks |
| Kissinger 200653 | USA | W | Trichomonas | Yes | 2–8 weeks |
| Nuwaha 200157 | Uganda | W, M | STI syndrome | No | NA |
| Schillinger 200352 | USA | W | Chlamydia | Yes | ≥ 3 weeks |
| Schwebke 201054 | USA | W | Trichomonas | Yes | 1 and 3 months |
| EPT vs. enhanced patient referral, four studies (n = 1253) | |||||
| Cameron 200919 | Scotland | W | Chlamydia | Yes | 3 months |
| Kerani 201156 | USA | MSM | Chlamydia/gonorrhoea | No | NA |
| Kissinger 200516 | USA | M | Chlamydia/gonorrhoea | Yes | 2–8 weeks |
| Kissinger 200653 | USA | W | Trichomonas | Yes | 2–8 weeks |
| EPT and enhanced patient referral vs. EPT or enhanced patient referral or simple patient referral, one study (n = 75) | |||||
| Kerani 201156 | USA | MSM | Chlamydia/gonorrhoea | No | NA |
| EPT vs. contract referral, one study (n = 324) | |||||
| Schwebke 201054 | USA | W | Trichomonas | Yes | 1 and 3 months |
| Enhanced vs. simple patient referral, 16 studies (n = 7642) | |||||
| Andersen 199863 | Denmark | W | Chlamydia | No | NA |
| Apoola 200964 | England | W | Chlamydia | No | NA |
| Cleveland 2001a | USA | W, M | Gonorrhoea | No | NA |
| Cameron 200919 | Scotland | W | Chlamydia | Yes | 3 months |
| Ellison 2001b | South Africa | W, M | STI syndrome | No | NA |
| Kerani 201156 | USA | MSM | Chlamydia/gonorrhoea | No | NA |
| Katz 198858 | USA | M | NGU | No | NA |
| Kissinger 200516 | USA | M | Chlamydia/gonorrhoea | Yes | 2–8 weeks |
| Kissinger 200653 | USA | W | Trichomonas | Yes | 2–8 weeks |
| Low 200655 | England | W, M | Chlamydia | Yes | 6 weeks |
| Moyo 200259 | Zimbabwe | W, M | STI syndrome | No | NA |
| Ostergaard 200365 | Denmark | W, M | Chlamydia | No | NA |
| Solomon 198860 | USA | M | Gonorrhoea | No | NA |
| Tomnay 200618 | Australia | W, M | Chlamydia/NGU | Yes | 2–12 weeks |
| Trent 201061 | USA | W | PID | No | NA |
| Wilson 200917 | USA | W, M | Chlamydia/gonorrhoea | Yes | 6 months |
| Enhanced vs. other enhanced patient referral, two studies (n = 1336) | |||||
| Ellison 2001b | South Africa | W, M | STI syndrome | No | NA |
| Montesinos 199062 | USA | W, M | Gonorrhoea/NGU | No | NA |
| Enhanced patient referral vs. provider referral, one study (n = 461) | |||||
| Katz 198858 | USA | M | NGU | No | NA |
| Enhanced patient referral vs. contract referral, one study (n = 1266) | |||||
| Cleveland 2001a | USA | W, M | Gonorrhoea | No | NA |
The results reported in the rest of this section are restricted to nine randomised controlled trials in which reinfection of the index case was either a primary or a secondary outcome. 16–19,51–55 Intermediate outcomes in these trials, including the number of partners treated, are also reported.
The trials could be grouped into three comparisons: EPT versus simple patient referral,16,19,51–54 EPT versus enhanced patient referral,16,19,53 and enhanced patient referral versus simple patient referral. 16,18,19,53,55 The methods used to enhance patient referral were EPT;16,19,51–54 use of booklets with tear-out information slips for index cases to give to their partner(s);16,53 additional counselling sessions;54 patient referral by a nurse at the time of receiving results at a general practitioners’ surgery rather than referral to a genitourinary medicine clinic; and use of a website. 18
The numbers of partners treated per index case randomised varied across trials and interventions (Table 7). This outcome was not reported in four17,18,52,53 of the nine trials. The median was 0.57 partners per index case (range 0.28–1.14). There was no clear relationship between the numbers of partners treated and the percentage of index cases with infection detected at the follow-up visit.
| Study and year | Infections | Interventions | n | Partners treated per index case randomiseda |
|---|---|---|---|---|
| bCameron 200919 | Chlamydia | EPT | 110 | 0.47 |
| Enhanced patient referral | 110 | 0.46 | ||
| Simple patient referral | 110 | 0.42 | ||
| Golden 200551 | Chlamydia/gonorrhoea | EPT | 1375 | 0.59 |
| Simple patient referral | 1376 | 0.53 | ||
| Kissinger 200516 | Chlamydia/gonorrhoea | EPT | 344 | 1.14 |
| Enhanced patient referral | 348 | 0.92 | ||
| Simple patient referral | 285 | 0.71 | ||
| Kissinger 200653 | Trichomonas | EPT | 154 | Not reported |
| Enhanced patient referral | 154 | |||
| Simple patient referral | 155 | |||
| Low 200655 | Chlamydia | Enhanced patient referral | 68 | 0.57 |
| Simple patient referral | 72 | 0.74 | ||
| Schillinger 200352 | Chlamydia | EPT | 887 | Not reported |
| Simple patient referral | 900 | |||
| Schwebke 201054 | Trichomonas | EPT | 162 | 0.79 |
| Contract referral | 162 | 0.56 | ||
| Simple patient referral | 160 | 0.28 | ||
| Tomnay 200618 | Chlamydia | Enhanced patient referral | 73 | Not reported |
| Simple patient referral | 32 | |||
| Wilson 200917 | Chlamydia/gonorrhoea | Enhanced patient referral | 304 | Not reported |
| Simple patient referral | 296 |
There was a risk of bias in at least one domain in all included studies. Random sequence generation was adequate in six16–19,53,55 of the nine trials that reported reinfection as an outcome. In three trials,51,52,54 random sequence generation was unclear. The method for allocation concealment was adequate in three trials. 18,52,55 In five trials,16,19,51,53,54 the method of allocation concealment was not sufficiently specified and in one17 the method could have introduced a high risk of bias; Wilson et al. 17 reported that participants were assigned study identification numbers sequentially as they enrolled in the study. Blinding of participants and personnel was not possible in any of the trials. Explicit blinding of laboratory personnel was reported in only one trial. 19 The attrition rate was > 20% in four16,19,51,54 of the seven trials16,19,51,54,56,59,61 in which repeat infection was the primary outcome and > 50% in both trials18,55 where repeat infection was measured as a secondary outcome.
Expedited partner therapy versus simple patient referral
Six studies (n = 6018) compared the rate of index patient reinfection EPT with simple patient referral among patients with chlamydia,19,52 trichomoniasis,53,54 or gonorrhoea or chlamydia. 16,51 The treatment pack for partners in all trials included antibiotics, written information about the infection, a telephone number for the study nurse and drug safety information. In addition, Cameron et al. 19 included information about genitourinary medicine clinics as alternative possibilities for seeking testing and treatment, Golden et al. 51 included condoms, and Schillinger et al. 52 included instructions for the index case to inform his or her partner about exposure to a STI and to abstain from sexual intercourse for 7 days after treatment.
The pooled results of trials for all infections showed that index patients in the EPT group had a 29% lower risk of being reinfected compared with index patients in the simple patient referral group (RR 0.71, 95% CI 0.56 to 0.89; heterogeneity p = 0.15, I2 = 39%) (Figure 3).
FIGURE 3.
Forest plot of randomised controlled trials of EPT vs. simple patient referral, by infection and overall. PR, patient referral. Source: Reproduced from Ferreira A, Young T, Mathews C, Zunza M, Low N. Strategies for partner notification for sexually transmitted infections, including HIV. Cochrane Database Syst Rev 2013; 10:CD002843 http://dx.doi.org/10.1002/14651858/CD002843.pub2 with permission from John Wiley and Sons. 44 Copyright © 2013 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
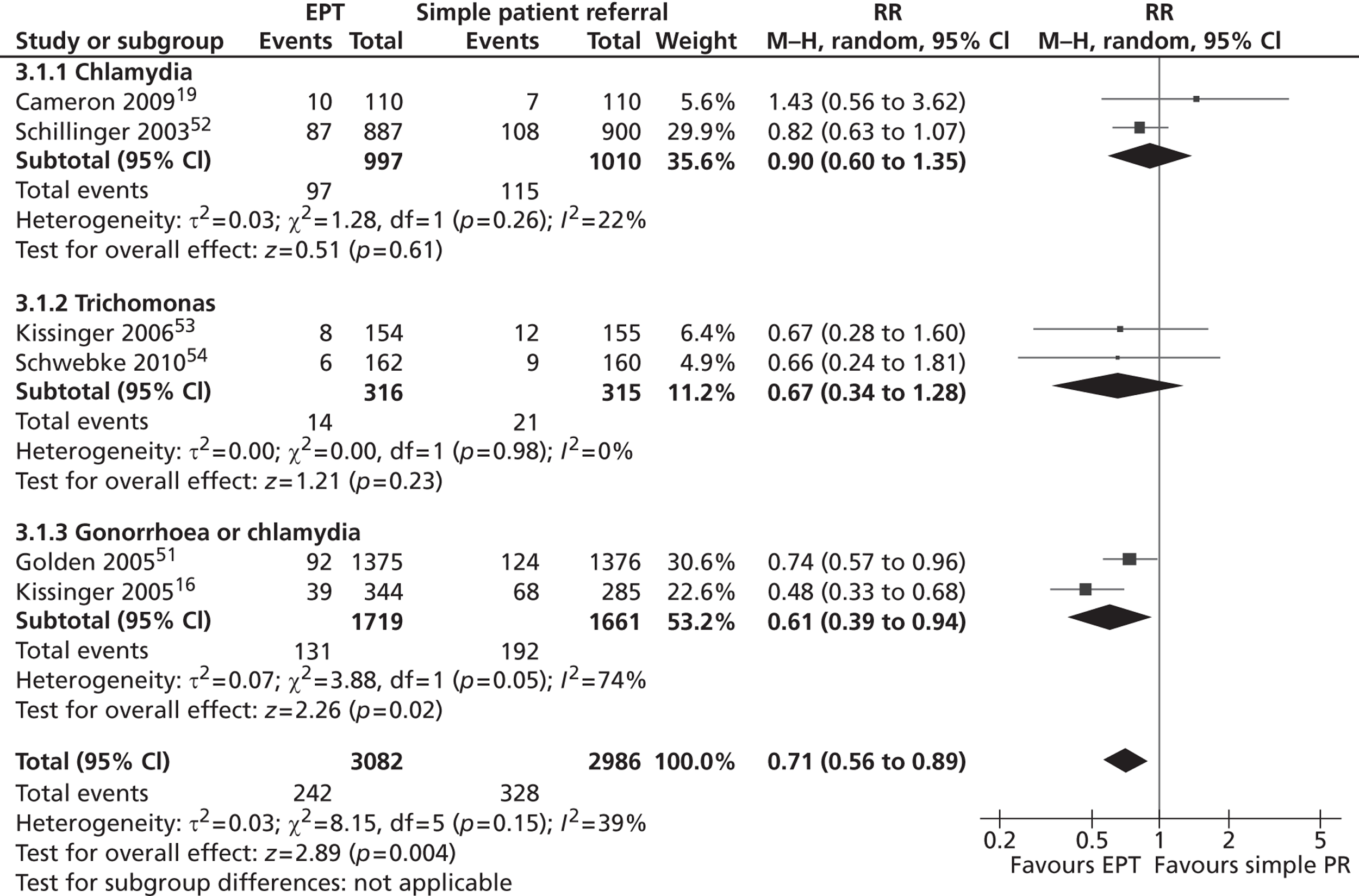
There was a modest level of between-trial heterogeneity, despite marked differences in the infections studied and follow-up testing interval. The size of the treatment effect appeared smaller in trials that included only women with chlamydia (RR 0.90, 95% CI 0.60 to 1.35) than in trials that included patients with either gonorrhoea or chlamydia (RR 0.61, 95% CI 0.39 to 0.94).
There were inconsistent findings about the effects of EPT interventions on intermediate outcomes. Three studies16,19,51 (n = 3600) assessed the number of partners notified. In one study,16 slightly more partners of index patients in the EPT group were notified (MD 0.45, 95% CI 0.28 to 0.62). In the other two studies19,51 the differences included the null effect (Golden et al. :51 MD –0.05, 95% CI –0.12 to 0.01; and Cameron et al. :19 MD 0.13, 95% CI –0.06 to 0.32).
Three16,51,54 studies assessed the number of partners treated. The studies showed results in the same direction but were very heterogeneous (heterogeneity p < 0.001, I2 = 95%). In two16,54 of the three trials, there was a moderate difference favouring EPT (Kissinger et al. :16 MD 0.43, 95% CI 0.28 to 0.58; and Schwebke et al. :54 MD 0.51, 95% CI 0.35 to 0.67). The difference between groups was very small in the fourth trial (MD 0.06, 95% CI 0.01 to 0.12). 51
Expedited partner therapy versus enhanced patient referral
There were three trials16,19,53 that compared the effect on reinfection in the index case of EPT with enhanced patient referral among women with chlamydia19 or trichomonas,53 and men with chlamydial or gonococcal urethritis. 16 There was no evidence of a difference in the reinfection rate between the two groups (RR 0.96, 95% CI 0.6 to 1.53; heterogeneity p = 0.22, I2 = 33%) (Figure 4).
FIGURE 4.
Forest plot of randomised controlled trials of EPT vs. enhanced patient referral. PR, patient referral. Reproduced from Ferreira A, Young T, Mathews C, Zunza M, Low N. Strategies for partner notification for sexually transmitted infections, including HIV. Cochrane Database Syst Rev 2013; 10:CD002843 http://dx.doi.org/10.1002/14651858/CD002843.pub2 with permission from John Wiley and Sons. 44 Copyright © 2013 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
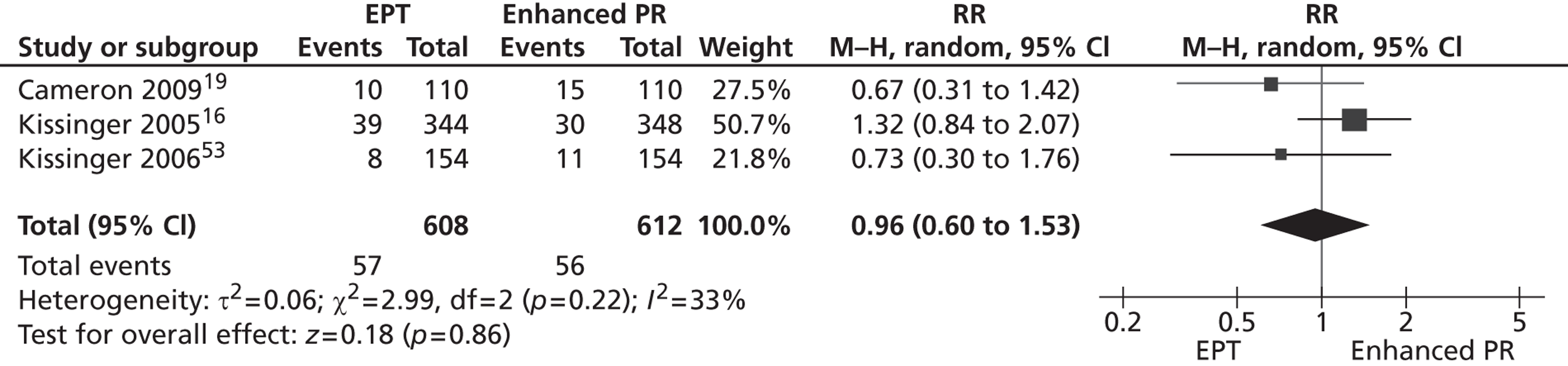
There was no consistent evidence of differences between the strategies in intermediate outcomes. Cameron et al. 19 (n = 220) found no evidence of a difference between groups in the number of partners presenting for care (MD 0.01, 95% CI – 0.02 to 0.03). Another study16 (n = 692) found a small increase in the number of partners treated per index patient randomised to the EPT group compared with the enhanced patient referral group (MD 0.22, 95% CI 0.21 to 0.23).
Enhanced patient referral versus simple patient referral
Six studies16–19,53,55 examined reinfection of the index case as an outcome in comparisons of different types of enhanced patient referral versus simple patient referral among patients with chlamydia,18,19,55 gonorrhoea or chlamydia,16,17 or trichomoniasis. 53 There were six different types of comparisons. Enhanced patient referral interventions included an additional counselling session;17 a postal testing kit for the partners to use;19 patient referral by a nurse versus patient referral by a health adviser;55 an information booklet to be given to the partner;16,53 and a disease-specific website that the partners were advised to access. 18
All six studies16–19,53,55 (n = 2007) assessed the index patient reinfection rate. In one comparison the index patients had a 51% lower risk of being reinfected in the enhanced patient referral (additional counselling) compared with simple patient referral (RR 0.49, 95% CI 0.27 to 0.89). 17 In five studies16,18,19,53,55 there was no evidence of a difference between simple patient referral and enhanced patient referral (Table 8). There were no consistent differences in intermediate outcomes.
| First author, year | Enhanced patient referral method vs. comparator | Studies, n | Participants, n | RR (95% CI) | I2; p-value |
|---|---|---|---|---|---|
| Cameron 200919 | Postal test kit vs. simple patient referral | 1 | 220 | 2.14 (0.91 to 5.05) | NA |
| Kissinger 2005,16 Kissinger 200653 | Information booklet vs. simple patient referral | 2 | 942 | 0.55 (0.22 to 1.33) | 76%; 0.04 |
| aLow 200655 | Nurse conducted vs. health adviser conducted | 1 | 140 | 0.35 (0.01 to 8.51) | NA |
| aTomnay 200618 | Disease-specific website vs. simple patient referral | 1 | 105 | 3.12 (0.17 to 58.73) | NA |
| Wilson 200917 | Additional counselling vs. simple patient referral | 1 | 600 | 0.49 (0.27 to 0.89) | NA |
Discussion
The updated Cochrane systematic review shows that three additional randomised controlled trials comparing EPT with simple patient referral and two trials comparing EPT with enhanced patient referral have been published since an earlier review of EPT trials. 22 When data for all curable STIs are pooled, EPT results in a lower risk of reinfection in the index case when compared with simple patient referral, but not when compared with enhanced patient referral methods.
The strengths of this systematic review are the rigorous and reproducible methods used to search and appraise the literature. It is unlikely that the review missed trials that would change the conclusions. The weaknesses of the review relate mainly to the small number of studies with differences between interventions and reporting. This limits the ability to investigate differences in effectiveness for individual STIs and for specific types of enhanced patient referral. It was also not possible to draw firm conclusions about the relative effectiveness of EPT, enhanced patient referral and simple patient referral. Although EPT was superior to simple patient referral, and there was no statistical evidence of a difference between EPT and enhanced patient referral, there were too few comparable trials to estimate the direct comparison between enhanced and simple patient referral. A meta-analysis of indirect comparison would be useful to investigate this further. A further limitation results from measurement error in the primary outcome. Repeated detection of infection at a follow-up visit includes reinfection from an untreated partner but also persistent infection resulting from treatment failure and newly acquired infections. Misclassification should be non-differential, however.
The findings from the review of trials using primary biological outcomes cannot be extrapolated to syphilis or viral STIs and cannot be generalised to men who have sex with men. EPT has not been recommended as a partner notification method for men who have sex with men. 21
There were no randomised controlled trials evaluating the effectiveness of APT. Cameron et al. 19 evaluated an EPT intervention in Scotland by giving the index cases antibiotics to give to their partners. They noted that this was possible only in a research setting. APT has been described as a new model of EPT,21 but it is not clear whether or not the results of trials evaluating EPT, as developed in the USA, can be extrapolated to APT. The requirement for an assessment by a health-care professional in the APT Hotline and APT Pharmacy models might affect the potential uptake when compared with EPT. The finding that EPT can reduce the risk of reinfection in the index case in comparison with simple patient referral, but not enhanced patient referral, confirms that of our earlier systematic review. 22 It is not known whether or not there was a specific effect of EPT, for example on reducing the time to partner treatment, because information about treatment delays was not reported in any of the included trials. In a non-randomised evaluation of APT in the UK, Estcourt et al. 20 found that the numbers of days between the diagnosis of the index case and treatment of the sexual partner was slightly shorter (median 1 day) when APT was used than for routine partner notification by patient referral (median 3 days; p = 0.11). These results cannot necessarily be extrapolated to the randomised controlled trials in the Cochrane review. First, the routine partner notification described by Estcourt et al. 20 would be classified in the Cochrane review as enhanced patient referral because the consultation with a health adviser was supported with condoms, written information about the infection and advice for the partner to seek treatment. Second, the need for a partner assessment by telephone or pharmacist in the APT protocol might increase time to treatment, although the median time to treatment for EPT could only be reduced to 0 days.
The effects of treatment delays on the effects of APT compared with traditional patient referral are investigated in a modelling study (see Chapter 3, The effects of traditional and new partner notification technologies for C. trachomatis and N. gonorrhoeae).
Partner notification outcomes for chlamydia in UK genitourinary medicine clinics
Substantial portions of the following section are reproduced from Variation in partner notification outcomes for chlamydia in UK genitourinary medicine clinics: multilevel study, Sex Transm Infect Herzog SA, McClean H, Carne CA, Low N, vol. 87, pp. 420–5, 2011, with permission from BMJ Publishing Group Ltd. 45
Introduction
Data about the outcomes of partner notification, as achieved in routine clinical practice, can be used to inform input parameters for the uptake of partner notification in mathematical modelling studies. The levels measured in randomised controlled trials represent those achievable under controlled conditions and vary according to setting, infection, and type of partner notification technology (see Table 7). The British Association for Sexual Health and HIV carried out a national audit of case note documentation of chlamydial infection management in UK genitourinary medicine clinics in 2007. 66–68 These data allow auditable outcomes to be analysed and sources of variation to be examined. Biological outcomes, such as the rate of repeated detection, cannot be measured because repeat testing after treatment of positive cases is not included in treatment guidelines. 10 Similar audit data have previously shown marked differences in partner notification outcomes in genitourinary medicine clinics inside and outside Greater London. 30 This chapter reports data published by Herzog et al. 45
Objectives
To compare different ways of measuring outcomes for partner notification with published standards; to examine variability between clinics; and to examine factors at the individual and clinic levels that contribute to variation in partner notification outcomes.
Methods
We analysed data extracted from case notes of patients seen between 2 January 2007 and 31 March 2007 and responses about clinic-level policies relating to partner notification. Descriptions of the audit methods and responses have been published. 66–68
Individual-level characteristics
We considered four characteristics: sex/sexual orientation of the index case (‘male heterosexual’, ‘men who have sex with men’ and ‘female’, which included all women irrespective of sexual orientation); age group (≤ 18, 19–24, 25–34 or ≥ 35 years); ethnic group (‘white’, ‘black African’, ‘black Caribbean/black other’, ‘other’ and ‘not documented’); and documentation of index case symptoms at baseline, including urethral discharge, dysuria, post-coital or intermenstrual bleeding, lower abdominal pain, vaginal discharge, rectal symptoms, pharyngeal symptoms or chlamydial conjunctivitis.
Clinic-level characteristics
We used data about (1) the number of chlamydia episodes reported by each clinic during the 3-month data collection period as an approximation of workload and (2) the type of health professional providing partner notification advice (‘health adviser only’, ‘health adviser, doctor or nurse’ or ‘any health professional’).
Outcome variables
There were three possible measures of partner notification outcome: the number of partners per index case who were tested for chlamydia (abbreviated as ‘tested’); the number of partners per index case with a positive chlamydia test (‘tested positive’); and the number of partners per index case treated (‘treated’). The primary outcome was the number of partners per index case tested for chlamydia, as verified by a health-care worker or, if information about verification was missing, as reported by the patient.
Statistical analysis
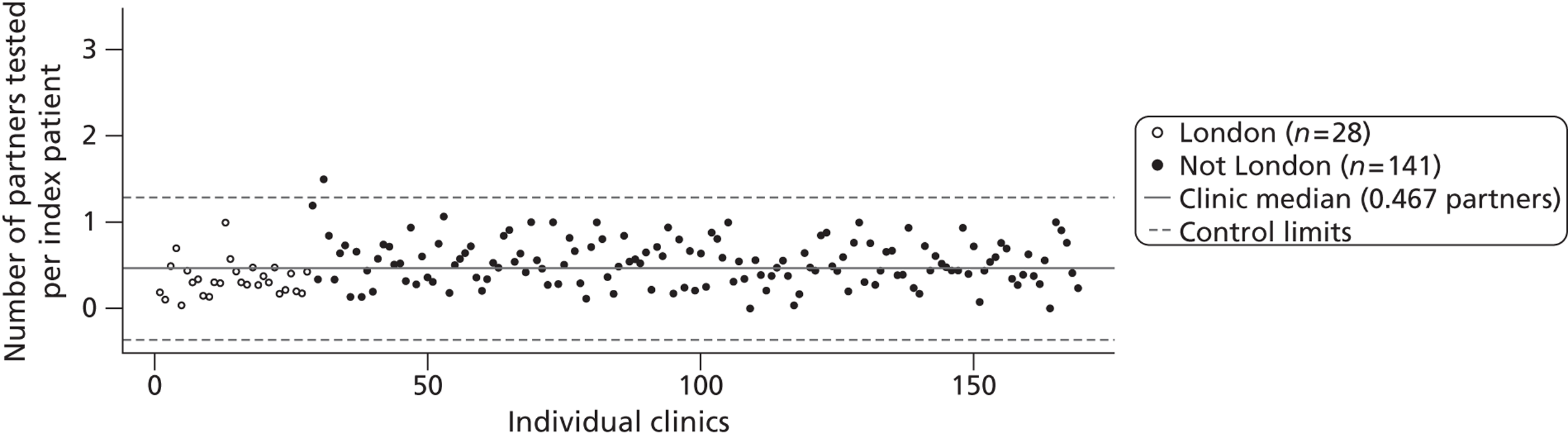
To describe the variability in partner notification outcomes between clinics we used a method similar to that previously described to construct Shewhart control charts. 30,69 First, for all index cases in each clinic, we calculated the mean number of partners tested per index case. We used the ‘median absolute deviation’ (MAD) method70 to calculate the median across all clinics and the control limits. The control limits are approximately the 99% CIs. The MAD is defined as the median of the absolute deviations. The standard deviation (SD) is calculated as 1.48 × MAD and control limits are MAD ± 3 × SD. Differences in outcomes between centres that fall within the control limits are said to result from common causes that might be expected to occur within the health care system. Data points lying outside the control limits are said to result from special causes and such unintended variation should be controlled. 69 We then examined the influence of missing data about numbers of sexual contacts. For each outcome we estimated the median and control limits for the number of partners per index case, assuming that if the data were missing then the number of partners was zero, and excluding all index cases with missing data. We displayed results for clinics inside and outside Greater London separately.
We used a hierarchical logistic regression model71 to examine factors associated with partner notification outcomes at the individual and clinic levels. We recoded the outcome as either zero or one or more partners tested per index case and estimated the odds ratio (OR, with 95% CIs), which takes into account the variation in outcomes between clinics. Data from patients with missing outcome data were excluded.
Results
We merged data on 5032 individuals with chlamydia in 193 clinics with clinic-level data reported by 177 genitourinary medicine clinics. The merged data set consisted of 4616 individuals in 169 genitourinary medicine clinics; this excluded 23 clinics with 415 index cases for which there was no information about clinic policies, and one clinic with only one index case for whom there were no data for any outcome of interest. The demographic characteristics of excluded index cases were comparable with those of included patients (results not shown). The percentages of patients for whom there was no information about the outcome of partner notification were partners tested for chlamydia, 41%; partners with a positive test for chlamydia, 43%; and partners treated for chlamydia, 32%.
Variability between clinics in partner notification outcomes
Figure 5 shows the mean number of partners tested for chlamydia per index case for each clinic, when health-care worker-verified data were supplemented with patient-reported values. The median across all clinics was 0.47 when missing values were assumed to be 0 (0.30 for Greater London genitourinary medicine clinics, 0.52 for all other clinics). No genitourinary medicine clinics were below the lower control limit but most Greater London clinics were below the median. Only one clinic was above the upper control limit, suggesting that most variation resulted from common causes. When index cases with missing data were excluded from analysis, the median number of patients tested for chlamydia increased to 0.92 per index case (1.00 in Greater London, 0.89 in all other clinics).
FIGURE 5.
Control chart for partners tested for chlamydia per index case. Combined outcome was defined as outcome verified by a health-care worker or, if data were missing, as reported by the patient. All cases were included and those with missing data were coded as having zero partners tested.
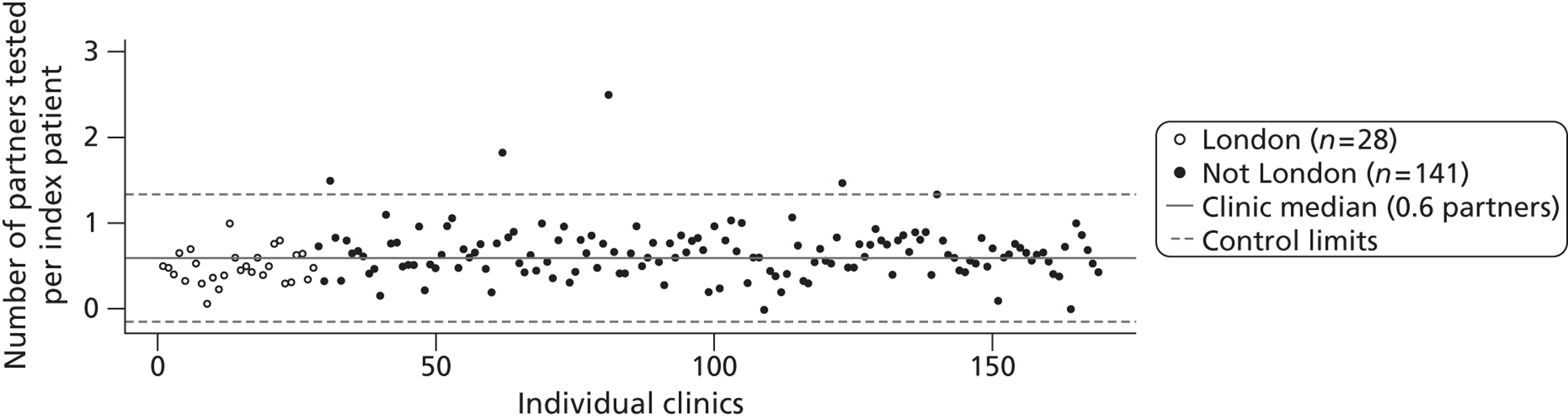
The other measures of partner notification outcome were the number of partners per index case with a positive chlamydia test (Figure 6, median 0.37 assuming missing values as 0 partners, and 0.75 excluding missing values) and the number of partners per index case treated for chlamydia (Figure 7) (median 0.60 and 0.95).
FIGURE 6.
Control chart for partners tested positive for chlamydia per index case. Combined outcome was defined as outcome verified by a health-care worker or, if data were missing, as reported by the patient. All cases were included and those with missing data were coded as having zero partners tested.
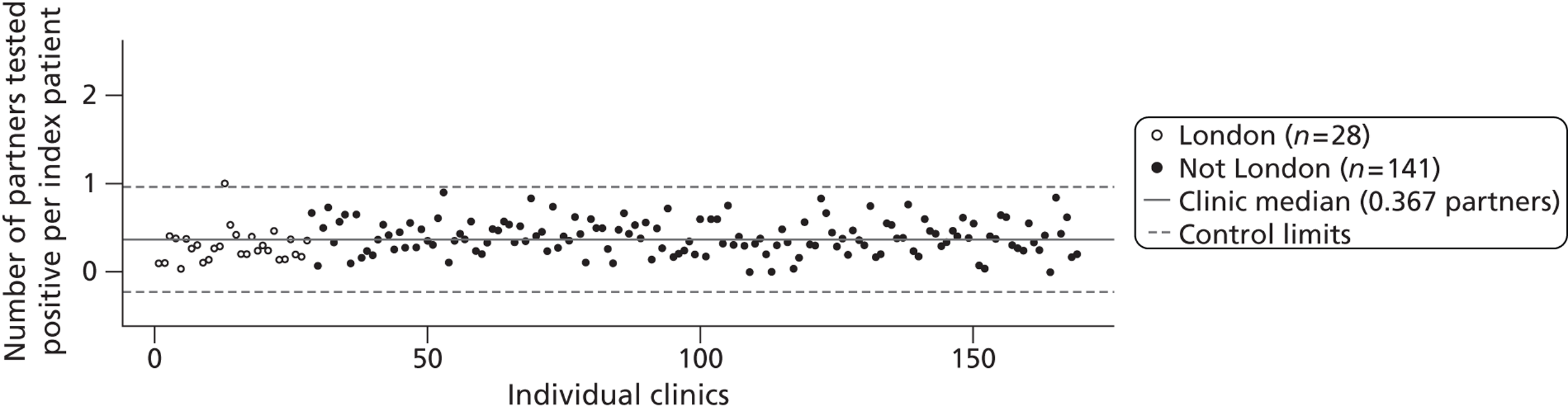
FIGURE 7.
Control chart for partners treated for chlamydia per index case. Combined outcome was defined as outcome verified by a health-care worker or, if data were missing, as reported by the patient. All cases were included and those with missing data were coded as having zero partners tested.
Factors associated with partner notification outcomes
Table 9 shows associations between individual- and clinic-level characteristics and the number of partners tested for chlamydia per index case, taking into account the variability between clinics.
| Characteristic | Partners per index casea | OR (95% CI) | p-valueb | |||
|---|---|---|---|---|---|---|
| 0 (n = 697) | ≥ 1 (n = 1835) | Missing (n = 2084) | Univariable | Multivariable | ||
| Sex/sexual orientation | ||||||
| Male heterosexual | 331 | 907 | 962 | 1 | 1 | 0.009 |
| Men who have sex with men | 24 | 29 | 100 | 0.28 (0.14 to 0.56) | 0.34 (0.17 to 0.68) | |
| Female all | 342 | 899 | 997 | 1.04 (0.85 to 1.28) | 1.03 (0.83 to 1.28) | |
| Missing | NA | NA | 25 | |||
| Age group, years | ||||||
| ≤ 18 | 128 | 337 | 348 | 1 | 1 | 0.135 |
| 19–24 | 334 | 950 | 998 | 1.10 (0.83 to 1.45) | 1.08 (0.82 to 1.43) | |
| 25–34 | 174 | 446 | 537 | 0.95 (0.70 to 1.30) | 0.97 (0.70 to 1.34) | |
| ≥ 35 | 61 | 102 | 192 | 0.55 (0.35 to 0.85) | 0.66 (0.41 to 1.05) | |
| Missing | NA | NA | 9 | |||
| Ethnic group | ||||||
| White | 584 | 1491 | 1562 | 1 | 1 | 0.075 |
| Black African | 10 | 42 | 58 | 1.36 (0.62 to 3.00) | 1.41 (0.63 to 3.14) | |
| Black Caribbean/black other | 33 | 86 | 146 | 0.91 (0.54 to 1.54) | 0.90 (0.53 to 1.52) | |
| Other | 33 | 82 | 126 | 0.76 (0.46 to 1.25) | 0.84 (0.51 to 1.39) | |
| Not documented | 37 | 134 | 192 | 2.30 (1.27 to 4.17) | 2.15 (1.18 to 3.93) | |
| Symptoms at presentation | ||||||
| No | 366 | 1131 | 1119 | 1 | 1 | < 0.001 |
| Yes | 331 | 704 | 965 | 0.61 (0.49 to 0.74) | 0.62 (0.50 to 0.76) | |
| Chlamydia episodes in 2007, quartilec | ||||||
| 0–248 | 216 | 379 | 325 | 1 | 1 | 0.031 |
| 261–451 | 140 | 484 | 486 | 2.34 (1.20 to 4.54) | 2.56 (1.29 to 5.11) | |
| 455–716 | 192 | 462 | 478 | 1.72 (0.89 to 3.32) | 1.96 (0.98 to 3.90) | |
| 720–2179 | 149 | 510 | 507 | 2.23 (1.15 to 4.32) | 2.47 (1.23 to 4.96) | |
| Person giving partner notification adviced | ||||||
| Health adviser, doctor or nurse | 537 | 1318 | 1423 | 1 | 1 | 0.434 |
| Health adviser only | 77 | 253 | 323 | 1.12 (0.55 to 2.29) | 1.14 (0.55 to 2.35) | |
| Any health professional | 83 | 264 | 278 | 1.41 (0.69 to 2.85) | 1.60 (0.78 to 3.30) | |
There was no information for the clinic-level variables for 16 clinics (163 index patients); results for individual-level variables in the remaining clinics were the same as those obtained from the full merged data set. Compared with heterosexual males, men who have sex with men were less likely to have at least one partner tested in both univariable and multivariable analysis. There was no difference in numbers of partners tested between women and men. There was no strong evidence of associations between the numbers of partners tested and the age or ethnic group of index cases.
Index cases who were symptomatic at presentation were less likely to report having one or more partners tested for chlamydia than asymptomatic cases in both univariable and multivariable analysis. There was no statistical evidence of an association between the number of partners tested for chlamydia and the health professional giving the partner notification advice. As the number of chlamydia cases diagnosed by clinics increased, the odds of at least one partner being treated increased (p for trend 0.031) when cases with missing outcome data were not included in the model.
The patterns of associations for the other partner notification outcomes were similar to that observed for the number of partners tested for chlamydia, but there was no association with numbers of chlamydia cases diagnosed. In addition, for the number of partners with a positive chlamydia test, women were less likely than heterosexual men to have a partner with a positive chlamydia test (adjusted OR 0.77, 95% CI 0.63 to 0.94).
Discussion
This study showed marked variation between genitourinary medicine clinics in outcomes of partner notification for chlamydia, most resulting from common causes. Men who have sex with men were less likely than heterosexual men and symptomatic patients less likely than asymptomatic patients to have had at least one partner tested for chlamydia. In clinics diagnosing greater numbers of chlamydia cases the odds of recording at least one partner tested for chlamydia were higher than in smaller clinics. Findings were similar with outcomes of the number of partners with a positive chlamydia test and number of partners treated for chlamydia.
The main strength of this study was that it included information from about 5000 patients with chlamydia in genitourinary medicine clinics across the UK. The main limitation of the study was related to the high level of missing values for numbers of contacts, which resulted in a twofold difference in calculated values for the outcome. Differences between index cases with and without information about the outcomes of partner notification mean that the observed outcomes are likely to be biased but it is not possible to state the direction or degree. A further limitation was that the method of partner notification for each index case was not recorded. We assume that the majority of partners were notified following patient referral by the index case him or herself.
In this study, we were able to investigate individual- and clinic-level factors influencing common causes69 of the variation in partner notification outcomes. Men who have sex with men with gonorrhoea have previously been observed to have fewer partners tested or treated, despite reporting higher numbers of partners than heterosexual men. 72 Symptomatic patients also had poorer partner notification outcomes than asymptomatic patients. This might be related to the differing look-back periods of 1 month for symptomatic patients and 6 months for asymptomatic patients (as recommended by the Society for Sexual Health Advisers73). Additionally, symptomatic and asymptomatic cases may differ in their relationship to, and ability to contact, sexual partners, although data were not collected about these factors. The observed association between numbers of chlamydia cases diagnosed and partner notification outcomes was difficult to interpret. This finding might reflect biases for which we could not control in the analysis, for example if patients in larger clinics reported more partners, or as a result of missing data, which was more common in large clinics. There was a strong influence of missing information about partner notification outcomes on the denominator used to calculate the mean for each clinic, which then influenced the estimates of the overall median: 0.47 partners tested for chlamydia per index case when those with missing data were coded as having no partners tested and 0.92 when missing values were excluded.
There was wide variation in partner notification outcomes across clinics, from 0 to 1.5 partners per index case tested for chlamydia. The median number of partners per index case reported to have been treated in this audit was similar to the median of the values observed in randomised controlled trials of a variety of partner notification technologies (see Table 7). The extent to which unintended variation in clinical outcomes can and should be eliminated has been debated. 69,74,75 If clinics took further measures to improve their performance this would improve both the consistency and the quality of clinical care. The data provided by this audit can be used in mathematical modelling studies as baseline values representing current practice for patient referral. In practice, the completeness of recording needs to improve so that levels of partner notification outcomes can be measured more accurately in future. Further research is needed to identify auditable measures that are associated with successful partner notification that prevents repeated chlamydial infection in index cases.
Estimating the likely public health impact of partner notification for a clinical service: an evidence-based algorithm
Introduction
The previous two sections present clinical outcome measures of partner notification, focusing on the incidence of reinfection in index cases and the number of sexual partners treated per index case. These outcomes are not usually reported according to the type of sexual partnership between an index case and their partner(s). Partnership type, however, is likely to determine whether index cases operate as ‘spread’ networks or ‘dead–end’ networks for STI transmission. 5 The impact of partner notification can therefore differ according to which partner types are sought. Dynamic mathematical models (see Chapter 3) are used to examine the potential epidemiological impact of partner notification at the population level, but they are too complicated to be used by individual clinical services planning or assessing the impact of partner notification activity on local transmission patterns. Here, we use a simple algorithm that clinicians and public health teams can use, without the need for onerous data collection or computational methods. 9
Objective
To estimate the likely short-term impact of partner notification activity on preventing STI transmission according to partner type, using data that are routinely collected in genitourinary medicine clinics.
Methods
The authors developed an algorithm to estimate the numbers of cases of chlamydia transmitted to the sexual partners of index cases and the number of secondary chlamydia transmissions from the partners of the index cases. They then estimated the impact of partner notification on preventing chlamydia transmission. The development of the methods used has been described in detail. 9 Here we applied the methods using audit data collected for this project about patients diagnosed with chlamydia study at one genitourinary medicine clinic in England, and estimates of C. trachomatis transmissibility that were developed for this study (see Modelling the transmission dynamics of C. trachomatis).
Assumptions about index cases
We stratified a sample of heterosexual index cases diagnosed with chlamydia by sex and age group, reflecting how partner numbers and partnership type vary by these characteristics. 8 The data came from a retrospective review over a 3-month period of partner notification records in one genitourinary clinic in England. For each index case, we recorded the numbers of different types of heterosexual partnerships reported in the year before their interview with a health adviser. Partnership types were labelled as live-in, regular, casual or ex-regular/live-in. In the published study describing the development of the method,9 data about the distribution of partnership types were obtained indirectly from respondents to the Natsal-2 survey who reported attending a genitourinary medicine clinic in the 5 years prior to interview as the group most closely comparable with the clinic population. 76 The number of chlamydia positives in the Natsal-2 data set was too small to stratify by sex and age group. 77
Estimating the number of primary transmissions
We used an estimate of the per-partnership probability of chlamydia transmission47 (see Modelling the transmission dynamics of C. trachomatis) and applied this to the data about partnership numbers to estimate the likely number of transmissions from index case to their partner(s) assuming no partner notification effort. As condoms are sometimes used, typically more often in casual and regular partnerships than in live-in partnerships and by younger rather than older people,78,79 we assumed that among those < 25 years old the probability of chlamydia transmission was lower, at 0.42 per casual partnership, 0.56 per regular partnership (and per ex-regular/live-in partnership) and 0.69 per live-in partnership, whereas among those aged ≥ 25 years, we assume that these probabilities were 20% higher, at approximately 0.50, 0.67 and 0.83, respectively.
Estimating the number of secondary transmissions
We next estimated the number of secondary transmissions, that is, the number of transmissions that may occur from the chlamydia-positive partners of index cases to their sexual partners, and thus the number of new chlamydia cases that are potentially preventable. Clinical services are unlikely to have aggregate data on the number of partners index cases’ partners have had, so we estimated this using the median number of new partners per year reported in Natsal-2. We stratified these medians by sex, age and partnership type, and assumed assortative mixing (e.g. casual partners have sex with casual partners). Having obtained an estimate of the number of partners that the infected partners of index cases are likely to have had, we multiplied this by the assumed probabilities of chlamydia transmission (described above), to estimate the number of secondary or onward transmissions.
Quantifying the impact of partner notification definitions and outcome measures
To quantify the impact of partner notification, we propose that the commonly used epidemiological measures ‘number needed to treat’ and the ‘absolute risk reduction’ can be usefully adapted to the context of partner notification. In medicine, the clinical effectiveness and cost-effectiveness of a medication are often assessed using the number needed to treat. This states the number of individuals with a specified condition who will need to receive a given therapy for a specified period in order to prevent the occurrence of one specified outcome of the condition. 80 The number needed to treat is the reciprocal of the absolute risk reduction, defined as the difference in risk of an outcome between the treated and untreated groups. Here we propose that the NNTIT is the reciprocal of the AROT. The AROT is the reduction in the number of onward transmissions achieved through successful partner notification in a population of index cases, expressed per partnership, for example a reduction from 0.8 to 0.6 onward transmissions per partnership. The corresponding NNTIT is the number of partners who must successfully receive partner notification in order to prevent one new transmission. In this example, the reduction of 0.2 per partnership would generate a NNTIT of 1/0.2 = 5.
We define partner notification success as the delivery of treatment to a partner that will eliminate infectivity if it is present, however this may be achieved (e.g. patient referral or provider referral). Partner notification may involve various tasks such as identifying, contacting, testing and treating partners. (By our definition, partner notification success is not simply a question of drug delivery and dosing, as it would thus exclude, for example, treatment taken while sexually active with an as-yet-untreated index case.)
Results
Estimating numbers of primary transmissions
The 203 index cases (112 men and 91 women) reported a total of 346 partners in the past year (Figure 8). Using the per-partnership probabilities of transmission described above, we estimate that a total of 190 primary chlamydia transmissions occurred between the index cases and their 346 partners.
FIGURE 8.
Frequency distribution of partners over the past year, by partnership type, sex and age group of index case. Numbers in parentheses correspond to the number of index cases in each group.
Estimated numbers of secondary transmissions
We assume no onward or secondary transmission within live-in partnerships where primary transmission occurred because live-in partners are assumed to have a median of 0 new partners per year (Table 10).
| Age group (years) | Primary chlamydia transmissions | Secondary chlamydia transmissions | |||||
|---|---|---|---|---|---|---|---|
| Number of partnerships at risk | Assumed p (transmission) | Estimated number of transmissions | Number of partnerships at risk | Assumed p (transmission) | Estimated number of transmissions | ||
| Male index cases, by partnership type | |||||||
| Live-in | < 25 | 0 | 0.69 | 0 | 0 | 0.69 | 0 |
| ≥ 25 | 5 | 0.83 | 4 | 0 | 0.83 | 0 | |
| Regular | < 25 | 36 | 0.56 | 20 | 20 | 0.56 | 11 |
| ≥ 25 | 28 | 0.67 | 19 | 19 | 0.67 | 12 | |
| Casual | < 25 | 61 | 0.42 | 25 | 76 | 0.42 | 32 |
| ≥ 25 | 60 | 0.50 | 30 | 60 | 0.50 | 30 | |
| Ex-regular/live-in | < 25 | 12 | 0.56 | 7 | 20 | 0.56 | 11 |
| ≥ 25 | 14 | 0.67 | 9 | 19 | 0.67 | 12 | |
| All partnershipsa,b | < 25 | 109 | NA | 52 | 116 | NA | 52 |
| ≥ 25 | 107 | NA | 62 | 97 | NA | 55 | |
| Female index cases, by partnership type | |||||||
| Live-in | < 25 | 8 | 0.69 | 6 | 0 | 0.69 | 0 |
| ≥ 25 | 4 | 0.83 | 3 | 0 | 0.83 | 0 | |
| Regular | < 25 | 36 | 0.56 | 20 | 40 | 0.56 | 22 |
| ≥ 25 | 16 | 0.67 | 11 | 11 | 0.67 | 7 | |
| Casual | < 25 | 26 | 0.42 | 11 | 43 | 0.42 | 18 |
| ≥ 25 | 10 | 0.50 | 5 | 15 | 0.50 | 7 | |
| Ex-regular/live-in | < 25 | 19 | 0.56 | 11 | 42 | 0.56 | 23 |
| ≥ 25 | 11 | 0.67 | 7 | 22 | 0.67 | 15 | |
| All partnerships | < 25 | 89 | NA | 48 | 125 | NA | 64 |
| ≥ 25 | 41 | NA | 26 | 48 | NA | 29 | |
In contrast, the median number of new regular partners per year is assumed to be one (except among male partners of index cases aged < 25 years, for whom the median was two partners). As expected, the number of new partners is highest for casual partners of index cases, ranging from two to four partners. Multiplying the number of infected partners of index cases by these likely numbers of new partners and then by the probability of transmission gives an estimated 200 secondary chlamydia transmissions between the index cases’ infected partners and their estimated 387 partners. Thus, together with the 188 primary transmissions, the 203 index cases are estimated to generate a total of 388 new chlamydia cases. Given these results, if partner notification was successful only with live-in partners (i.e. only these partners were identified, tested and treated, as a result of either provider referral or patient referral) then, regardless of age group and sex, only 3.4% (13/388) of all primary and secondary transmissions generated would be treated or prevented, as the greatest proportion of transmissions stems from casual and regular partners.
Quantifying the potential impact of partner notification
We now use the AROT and NNTIT to compare the potential impact of different partner notification intensities by partnership type (Table 11). Given the assumptions described above, successful partner notification needs to be achieved with more than one partner per index case overall in order to prevent one onward (secondary) transmission, as the NNTITs are greater than one for each sex/age group. However, when the NNTIT is considered by partnership type, then a smaller number of partner notification successes for casual and ex-regular/live-in partners will prevent one onward transmission, relative to with regular partners. Thus, for example, among male index cases aged < 25 years, the NNTIT is 1.92 for casual partners, which means that 1.92 casual partners need to be successfully identified and treated via partner notification in order to prevent one onward transmission, whereas among regular partners, successful partner notification would need to occur with 3.25 regular partners of these index cases to have the same impact.
| AROT | NNTIT | |||
|---|---|---|---|---|
| Index case age group (years) | < 25 years | ≥ 25 years | < 25 years | ≥ 25 years |
| Male index cases, by partnership type | ||||
| Live-in | 0 | 0 | NA | NA |
| Regular | 0.31 | 0.44 | 3.25 | 2.25 |
| Casual | 0.52 | 0.50 | 1.92 | 2.00 |
| Ex-regular/live-in | 0.92 | 0.89 | 1.08 | 1.13 |
| All partnershipsa,b | 0.49 | 0.51 | 2.02 | 1.95 |
| Female index cases, by partnership type | ||||
| Live-in | 0 | 0 | NA | NA |
| Regular | 0.62 | 0.44 | 1.62 | 2.25 |
| Casual | 0.69 | 0.75 | 1.44 | 1.34 |
| Ex-regular/live-in | 1.23 | 1.33 | 0.81 | 0.75 |
| All partnershipsa,b | 0.71 | 0.71 | 1.40 | 1.40 |
There are also differences between sexes and between age groups, illustrating the relative intensities of effort required with different population groups. For example, the NNTIT for men aged < 25 years is 3.25 regular partners in contrast to a NNTIT of 1.62 regular partners among women aged < 25 years, i.e. half the number of regular partners of women < 25 years versus the number of regular partners of men < 25 years would need to be successfully identified and treated via partner notification to prevent one onward transmission.
As behavioural parameters will vary between populations (e.g. by ethnicity), and as transmission parameters are hard to measure and therefore uncertain, we undertook sensitivity analyses to check the robustness of these patterns. In all scenarios, the number of partner notification successes required with ex-regular/live-in partners is considerably less than among casual partners and, in turn, regular partners and then live-in partners.
Discussion
This paper has described an evidence-based algorithm that clinicians and public health teams can use to estimate the potential impact of their service’s partner notification effort on preventing STI transmission. Additionally, we have shown how the potential impact of partner notification can be quantified for different patient groups by using two new epidemiological measures: the AROT and the NNTIT.
The strength of this study is that routinely collected data can be used to adapt the algorithm to different settings and populations. In contrast to the published paper,9 we used data on actual partner numbers as reported in health adviser interviews, rather than estimates from Natsal-2. However, index cases reported their partners by type, which was subjective, with women more likely than men, for example, to regard a partnership as regular rather than casual. 81 There are limitations inherent to the static deterministic modelling approach used here. The algorithm makes a number of assumptions about the transmission of chlamydia to secondary partners. Furthermore, we assumed that these potentially infectious encounters were all susceptible to chlamydia (i.e. not already infected or immune), which is highly unlikely in contacts who are closely linked to a case. It is not possible to infer whether or not the estimates of onward transmission derived here are optimistic, as counting further generations of cases would increase the estimate, whereas pre-existing infection, chance effects, and network structure would tend to decrease the effect. The estimates of onward transmission given here should, therefore, be taken as broadly indicative rather than precise. The transmission dynamic models used in Chapter 3 overcome these limitations because they have partnerships explicitly modelled and take into account infection status.
There are additional limitations to the study. We treat age crudely, stratifying into two age groups, though using 25 years old as a threshold does correspond to age groups currently used for UK surveillance. There were insufficient data in Natsal-2 to allow stratification by ethnicity and sexual orientation, which are also associated with both partner numbers and the probability of STI acquisition, though other data sources could also be harnessed. Furthermore, we assumed assortative mixing by behaviour and partnership type, owing to the difficulty of obtaining data on partners’ current behaviours and partnerships. This might be a reasonable assumption given the extent of assortative mixing in the population. 82 Although there is uncertainty and variability surrounding the parameters used in our algorithm, sensitivity analyses undertaken to explore the vulnerability of the algorithm to uncertainty were reassuring.
As provider referral (vs. patient-led partner notification) is required more often with casual and ex-partners than with regular partners (Gill Bell, Sheffield Teaching Hospitals NHS Foundation Trust, 2012, personal communication), realising the potential public health benefits suggested by the NNTIT has cost implications for services. However, as casual and ex-partners are likely to have greater numbers of partners themselves, the potential for preventing onward transmission is greater, yielding greater public health benefit.
Services should collect data from their index cases on the number and type(s) of partners, as well as the partner notification method(s) required (patient vs. provider referral). These data, when combined with cost data on partner notification, are key for demonstrating the cost–benefit of provider referral in preventing onward transmission in a local population.
Our analyses show that future debate and research about partner notification provision need to develop a partnership-orientated focus regarding which index cases should be offered more intensive support for public health benefit. The existing emphasis on different strategies for partner notification (e.g. patient, provider) has a service-orientated focus regarding the types of partner notification services offered. This now needs to be explicitly linked to strategic decisions about what index cases should be offered, according to their different kinds of partnership history. Although clinicians recognise that a ‘one size’ approach to partner notification does not fit all types of sexual partner, outcome measures must also reflect the public health importance of partnership type. Patient-centric measures conceal huge variations in transmission potential by partner type. Clinical services should routinely adopt partner-centric outcome data, and audit their partner notification data by partner type. This will allow them to explore the implications of different approaches to targeting partner notification activity, and assess the public health outcomes of the service.
Our partner notification algorithm has a role to play in providing epidemiological evidence aimed at assessing and justifying the public health value of more expensive and challenging partner notification activity with casual and former partners, which is provided to a variable extent. 83 The algorithm can be adapted to other STIs, such as gonorrhoea, and other infections where partner notification is needed, such as hepatitis B and tuberculosis. Without a better understanding of how to harness the relative transmission prevention potential of different partnership types, evidence-based partner notification practice cannot progress.
Chapter 3 Transmission modelling and the impact of partner notification
We planned to adapt two individual-based models of C. trachomatis transmission that were developed in the UK39,40 to investigate partner notification technologies. In the introduction to this report (see Chapter 1), we outlined how predictions from these and from a third model developed in the Netherlands84 resulted in widely differing conclusions about the effects of the same hypothetical screening and partner notification intervention to prevent C. trachomatis. 41 We use the first part of this section to resolve some of the uncertainties in the modelling of chlamydia transmission in general and to compare the three models in detail. These studies were needed to determine the design of subsequent models. In the second part of this section we investigate the impact of current recommendations for traditional partner notification technologies for chlamydia with outcomes at the individual level (case-finding) and population level (prevalence) (see Table 5). In the third part of this section we investigate a range of partner notification scenarios for treating partners with or without testing for the STI first.
Modelling the transmission dynamics of Chlamydia trachomatis
Introduction
We need to understand the transmission dynamics of STIs if we are to make accurate quantitative, as well as qualitative, predictions about the impact of partner notification and other preventative interventions. Sexual partnership dynamics and the values for infection parameters are key determinants that affect the spread of STIs. In this chapter, we investigate the effect of different assumptions about disease-specific parameters for C. trachomatis on the predicted impact of interventions using a basic transmission model. We then describe the derivation of improved estimates of the infectious duration and the transmissibility of chlamydia. Finally, we present the results of a detailed comparison of the three individual-based models, which allows us to draw more robust conclusions about the effect of screening and partner notification interventions on chlamydia transmission. We end with a brief summary of the insights that we have gained into chlamydia transmission models, and a rationale for the model choice in subsequent chapters.
Infectious duration of chlamydia
Different models of chlamydia transmission use widely different disease-specific parameters. We devised a deterministic chlamydia transmission model with a SEIRS (susceptible-exposed-infected-recovered-susceptible) structure. For simplicity, we assumed a closed population of young adults and a homogeneous population where both sexes become infected and pass through the infected stages at equal rates. The full details of the model can be found in Althaus et al. 46
To understand what parameters are most important for predicting the impact of an intervention we performed a univariate sensitivity analysis of disease-specific parameters, namely the duration of the asymptomatic and symptomatic periods, the duration of temporary immunity and the fraction of infections that become symptomatic. The pre-intervention prevalence of chlamydia in the population was assumed to be 5%. This corresponds roughly to the prevalence observed in sexually active young adults. 77
We investigated the impact of a screening intervention rather than partner notification because the simplicity of the model does not allow identification and tracking of current and previous sex partners of infected index cases. We then calculated the expected prevalence after the introduction of two different screening scenarios: (1) an organised screening programme with a screening rate of 0.25 per year implemented for 10 years and (2) opportunistic screening at a rate of 0.05 per year, in which the new steady-state prevalence is shown after long-term implementation.
Uncertainties in the duration of asymptomatic period and of temporary immunity resulted in large differences in the predicted impact of a screening programme (Figure 9a and b). In contrast, varying the fraction of infections becoming symptomatic and the duration of the symptomatic period within the range of previously used parameter estimates caused little change in the predicted effect of interventions.
FIGURE 9.
Prevalence of chlamydia after the introduction of a screening programme. Dotted line, baseline prevalence in the absence of a screening programme; dashed line, long-term prevalence if the population receives screening at a rate of 0.05 per year; solid line, prevalence after screening the population at a rate of 0.25 per year for 10 years; grey area, parameter range; black dots, baseline scenario. (a) Chlamydia prevalence as a function of the duration of the asymptomatic period. Most estimates on the duration of the asymptomatic period are within 200–400 days; and (b) chlamydia prevalence as a function of the duration of temporary immunity. Reprinted from Epidemics, vol. 2, Althaus CL, Heijne JCM, Roellin A, Low N, Transmission dynamics of Chlamydia trachomatis affect the impact of screening programme, pp. 123–31, 2010, with permission from Elsevier. 46
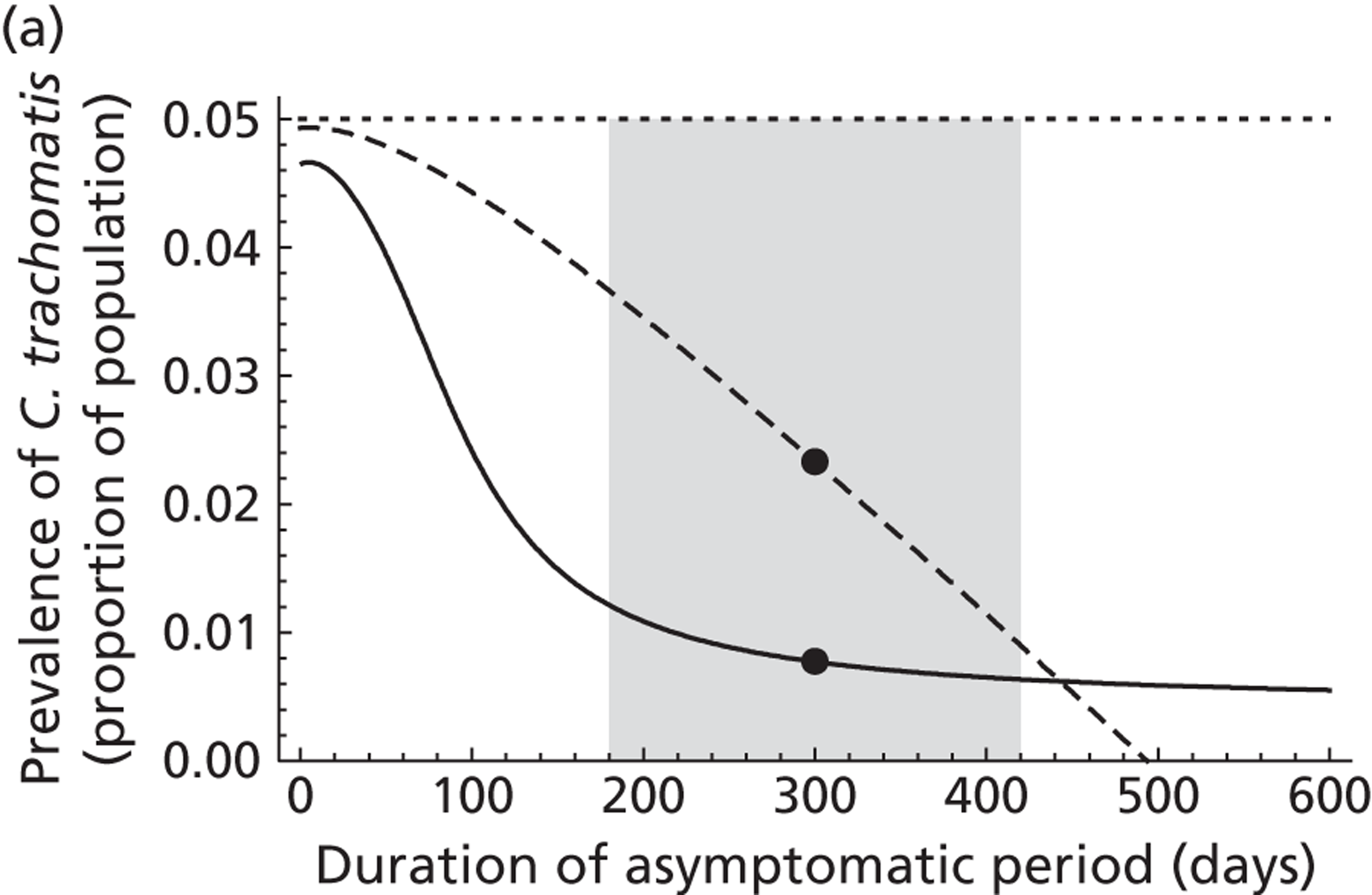
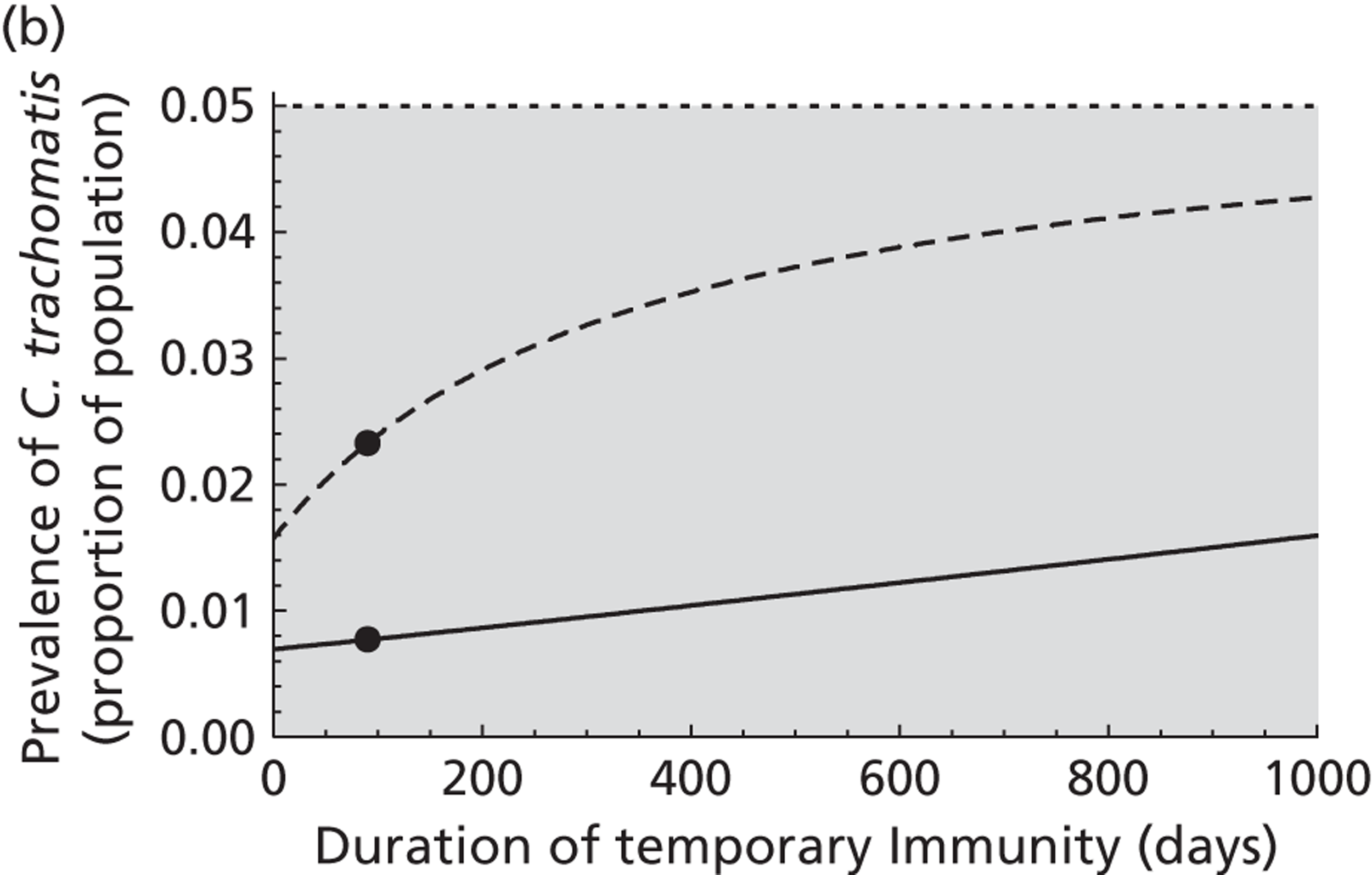
The long-term outcome of a screening programme appeared to be most sensitive to the duration of the asymptomatic period. Figure 9a (grey area) shows the range of values for the asymptomatic period that have been used in previous mathematical models of chlamydia transmission (180–420 days). The long-term impact of screening at a low rate (dotted line) is much more pronounced if the asymptomatic period is at the upper bound of the range.
The duration of temporary immunity also affected the predicted impact of screening. Increasing the duration of temporary immunity substantially decreased the impact of screening (see Figure 9b). Here, screening and treating asymptomatically infected people prevents the development of temporary immunity and renders them immediately susceptible. This somewhat counterbalances the otherwise strong impact of screening. The grey area, which covers the range of values used in previous modelling studies, shows the uncertainty about the existence and duration of immunity after a chlamydia infection.
To obtain a robust estimate of the duration of asymptomatic chlamydia infection in women, we fitted a mathematical model to data from a study that followed a large number of asymptomatic chlamydia-infected women and showed that the infection can persist for several years (Figure 10). 85 The model describes the clearance of chlamydia infections in all women who were infected at the beginning of the study. We also took into account the possibility that women could have cleared their infection naturally and become reinfected from their (presumed) untreated partners. Full details of the model and the parameter estimation have been published. 46
FIGURE 10.
Persistence of chlamydia in asymptomatically infected women. Data from Molano et al. 85 and reprinted from Epidemics, vol. 2, Althaus CL, Heijne JCM, Roellin A, Low N, Transmission dynamics of Chlamydia trachomatis affect the impact of screening programme, pp. 123–31, 2010, with permission from Elsevier. 46
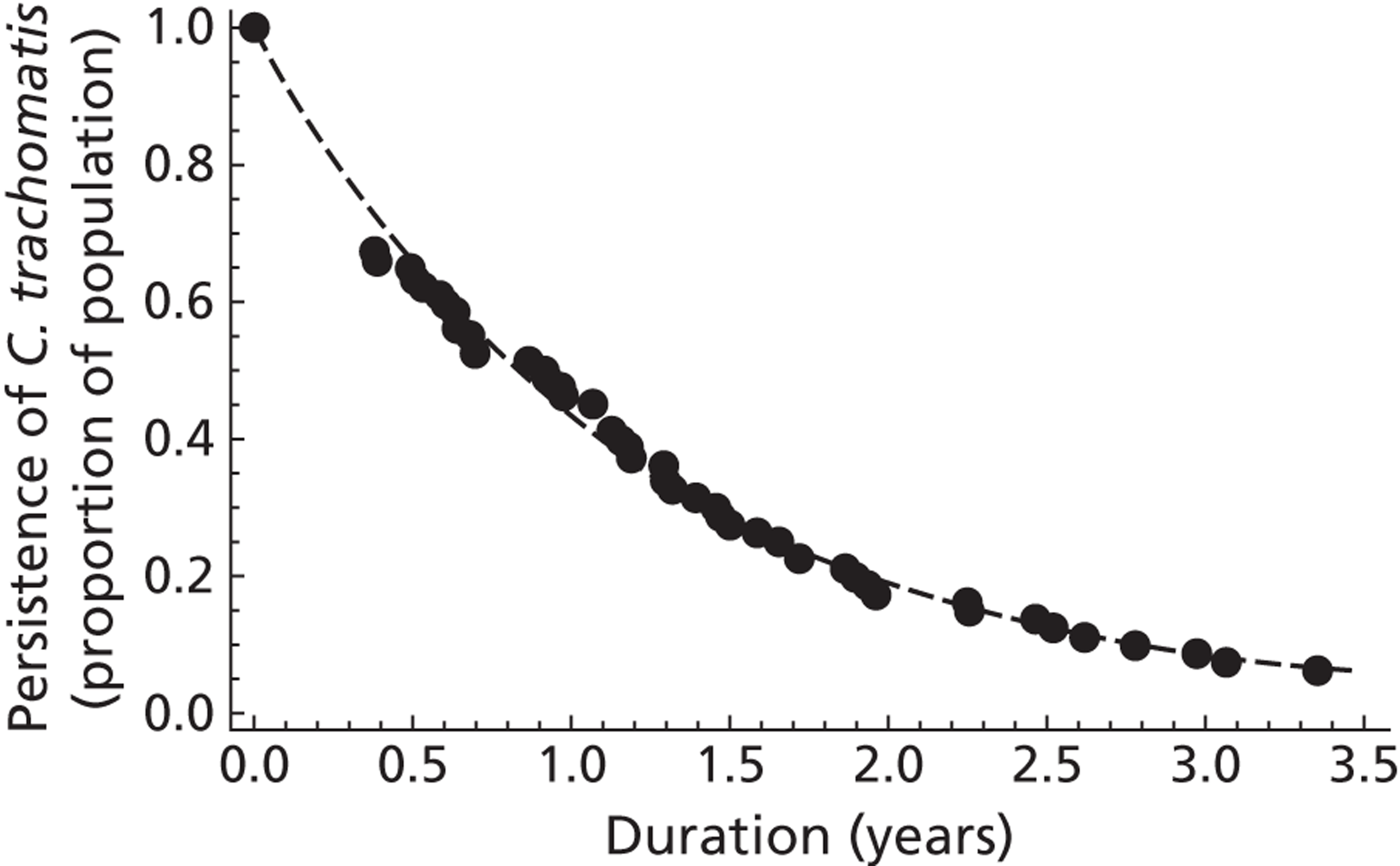
The estimated reinfection rate is low (0.01 per year, 95% CI – 0.01 to 0.03 per year), which indicates that the data are mainly described by natural clearance. With an estimated clearance rate of 0.84 per year (95% CI 0.82 to 0.87 per year), we obtain a mean duration of the asymptomatic period of 433 days (95% CI 420 to 447 days).
Transmissibility of chlamydia
The transmissibility of C. trachomatis through sexual intercourse cannot be observed in empirical studies. Estimates of the transmissibility of chlamydia from epidemiological studies have usually been based on data from couples with and without chlamydia infection and the proportions of concordant and discordant pairs. 6,7,86 Katz86 proposed an original approach for analysing such data using information about heterosexual couples attending a STI clinic in Indianapolis, IN, USA. The expected numbers of concordant and discordant couples before transmission takes place can be calculated if it is assumed that all couples in the population with at least one infected individual have the same probability of observation, and that sexual partnership formation is independent of infection status. After sexual partnerships have formed, transmission can happen in discordant partnerships, resulting in a higher proportion of couples in which both partners are positive.
Katz86 estimated the probabilities that transmission occurred within a couple at 0.395 from men to women and 0.323 from women to men. But there are two major problems with this approach. First, the estimated transmission probabilities do not represent the per-partnership transmission probability because infection status is observed during the partnership and not at the end. The probabilities estimated by Katz are often called per-partnership transmission probabilities, which imply that the partnership has been observed until it has ended. Second, they do not take into account the natural history of chlamydia infection, where spontaneous clearance and reinfection within sexual partnerships can occur. 87 These complexities need to be considered because different assumptions about infectious duration46 and reinfection in sexual partnerships88 can affect the prevalence of chlamydia.
To obtain new estimates of the transmissibility of chlamydia we used data from a cross-sectional partnership study that has frequently been cited as the source of estimates for chlamydia transmissibility. 7 We described the transmission of chlamydia using the pair model framework, which has been used for several STIs. 88–91 The full details of the model and the parameter estimation procedure have been published. 47 In brief, we used maximum likelihood estimation92 to fit the model to the data from the study by Quinn et al. 7 The study contains information about chlamydia infection status and sexual activity in 494 heterosexual couples. The study reported 53 concordant chlamydia positive, 48 discordant and 393 concordant negative couples. We generated 1000 parameter combinations to account for uncertainties in the duration of sexual partnerships and infections. Sexual behaviour parameters were taken from the same study. We obtained model estimates of the per partnership transmission probability for different values of the number of partners during the last 6 months (Figure 11a).
Higher number of partners resulted in lower estimates of the per partnership transmission probability. However, partner numbers of three or more during the last 6 months resulted in poor fits to the data. We therefore consider two partners during the last 6 months as our baseline scenario, for which the median of the estimated per-partnership transmission probability is 55.5% [interquartile range (IQR) 49.2–62.5%]. The estimates of the per-sex act transmission probability seemed to be less affected by the assumed number of partners in the last 6 months (Figure 11b). Most values were around 10% with the median of the baseline scenario at 9.5% (IQR 6.0–16.7%).
FIGURE 11.
Estimated chlamydia transmission probabilities for different values of the number of partners in the last 6 months. (a) Per-partnership transmission probability of chlamydia; and (b) per-sex act transmission probability of chlamydia. Each box plot represents estimates from 1000 different parameter combinations. The baseline scenario, where it is assumed that individuals in a partnership at steady-state have on average of two heterosexual partners during the previous 6 months, is in grey. It is assumed that individuals have one episode of heterosexual intercourse every 5 days. Reprinted with permission from Althaus CL, Heijne JC, Low N, Towards more robust estimates of the transmissibility of Chlamydia trachomatis. Sex Transm Dis, vol. 39, issue 5, pp. 402–4, 2012. 47
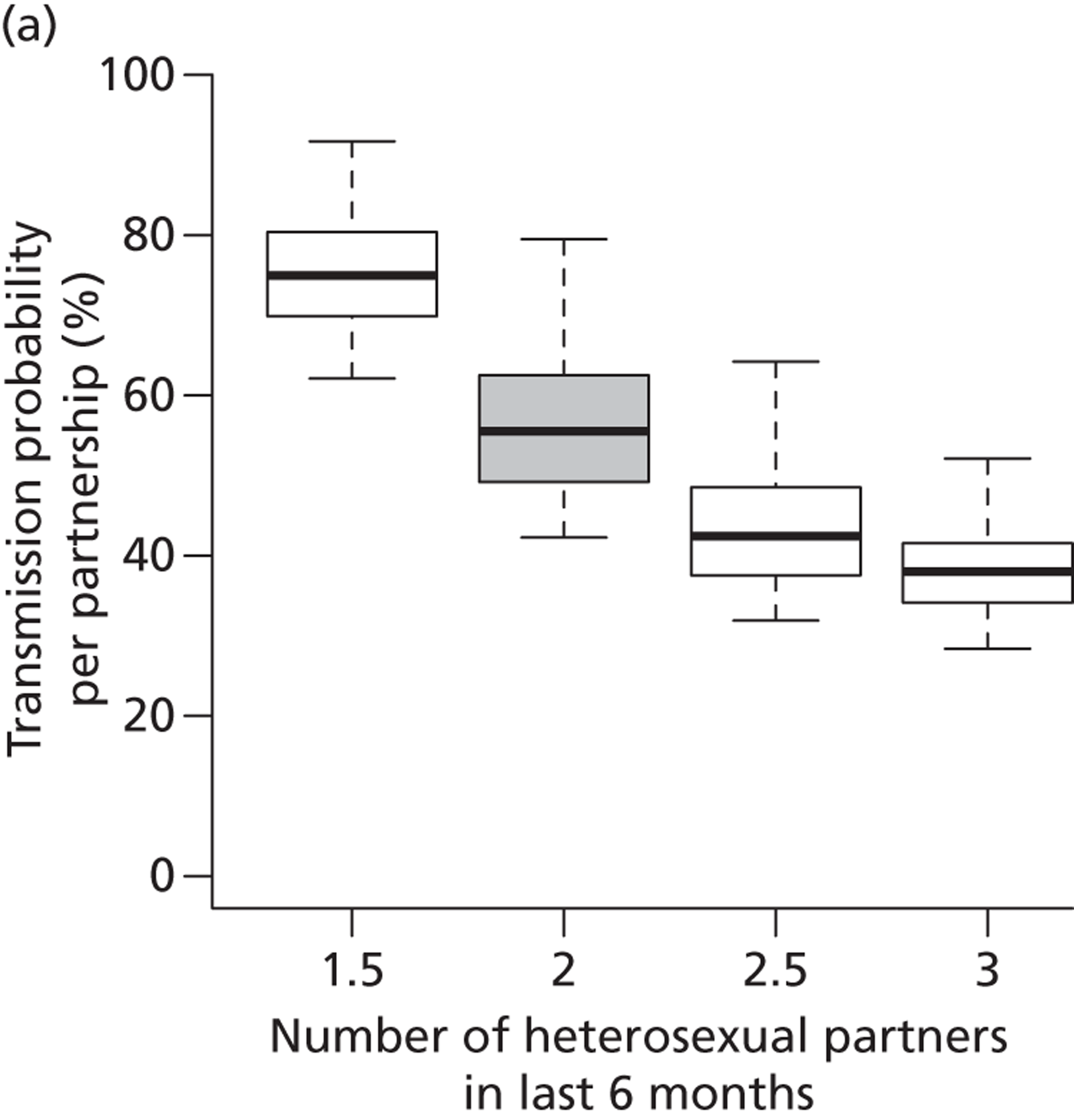
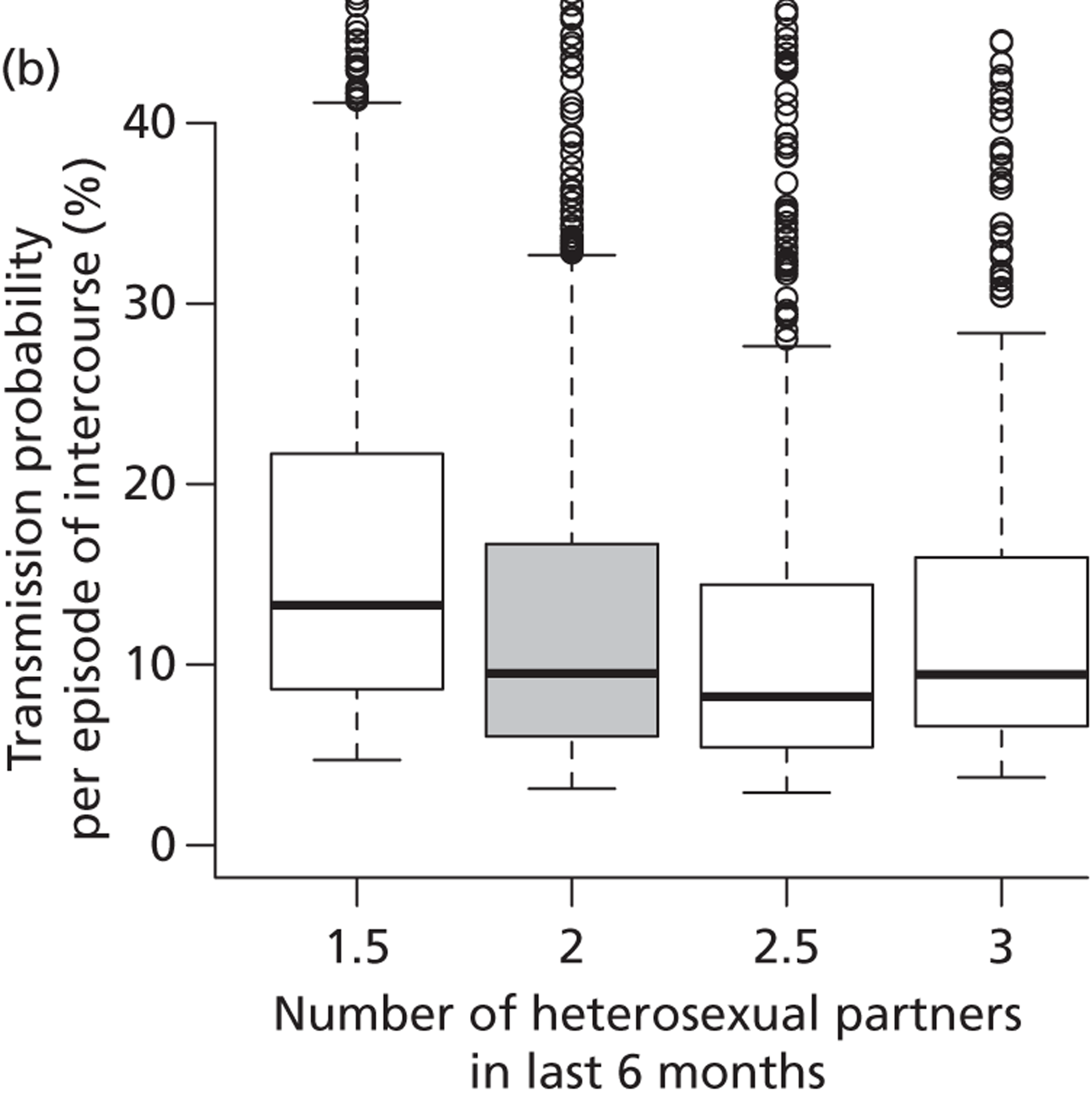
Our estimated range of chlamydia transmission probabilities in heterosexual partnerships is higher than the baseline values reported by Katz. 86 This is expected because we report the probability of transmission taking place by the end of a partnership and we assume that chlamydia can be cleared spontaneously followed by reinfection. Our estimate is, however, lower than the percentages of concordantly infected couples (70% of female partners of infected men and 68% of male partners of infected women). These percentages are often interpreted as the per-partnership transmission probabilities,84,93 but they are not because the direction of transmission cannot be reliably determined. 87 This discrepancy illustrates the importance of taking the natural history of chlamydia infection and the dynamics of sexual partnership formation into account when estimating transmissibility from data of chlamydia positivity in couples. The refined estimates of the per-sex act transmission probability for chlamydia are consistent with those obtained or used in other modelling studies. 35,94,95 In two of the three individual-based models of chlamydia transmission that we had compared, the per-sex act transmission probabilities were also within the same range as our estimate, but the probability in the third model (3.75%) was lower.
Comparison between three individual-based models
We evaluated further the three individual-based models of chlamydia transmission that showed conflicting results about the impact of the same screening intervention. 41 The names of the models are the same as those used in the first comparison: the ClaSS (Chlamydia Screening Studies project) model and Turner model were both developed to examine the effects of different models of chlamydia screening in the UK; the Kretzschmar model was developed in the Netherlands. The comparison has been published in full. 48
First, we compared the sexual partnership dynamics of the models to population-based data from Natsal-2 about the durations of sexual partnerships, the length of gaps and overlaps between partnership and the numbers of partners in the last year. We showed that although all models capture some aspects of the sexual partnership dynamics reasonably, they fail in others. Overall, the Kretzschmar model performed best.
We then investigated the spread of chlamydia in the simulated populations with Natsal-2 (Figure 12). In previous studies, the three models used different assumptions about the duration of infection and the proportion of symptomatic and asymptomatic cases. 41 To better compare their results, we used a harmonised set of chlamydia infection parameters, based on a thorough evaluation of the parameter ranges in the literature and our new estimate of the duration of the asymptomatic period.
FIGURE 12.
Asymmetrical distributions of chlamydia infections among individuals according to level of sexual activity. (a) Natsal-2;8 (b) Kretzschmar model;38 (c) Turner model;40 and (d) ClaSS model. 39 The width of each bar represents the proportion of people who have had a given number of sexual partners within the last year (the legend shows the distribution of sexual partner numbers). The height of the bar indicates the prevalence of chlamydia in each group. Note that the prevalence in the group with highest activity (> 5 partners) is 20% in the Kretzschmar model and 34% in the ClaSS model. Overall prevalence for women and men aged 18–44 years is given by the dashed line and is approximately the same in all graphs (1.7%). Reproduced with permission from Althaus CL, Turner KM, Schmid BV, Heijne JC, Kretzschmar M, Low N, Transmission of Chlamydia trachomatis through sexual partnerships: a comparison between three individual-based models and empirical data, J R Soc Interface 2012; 9:136–46. 48
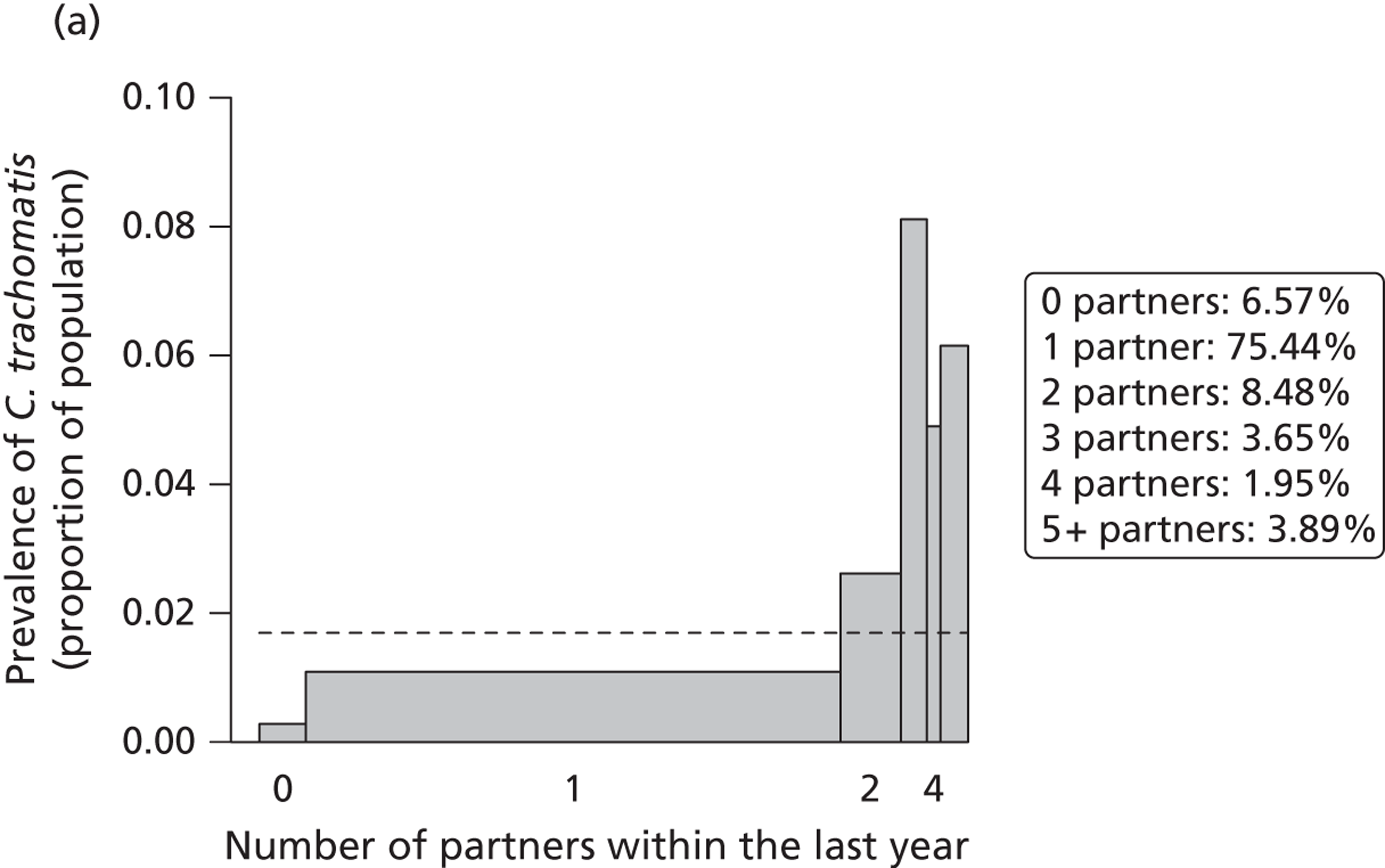
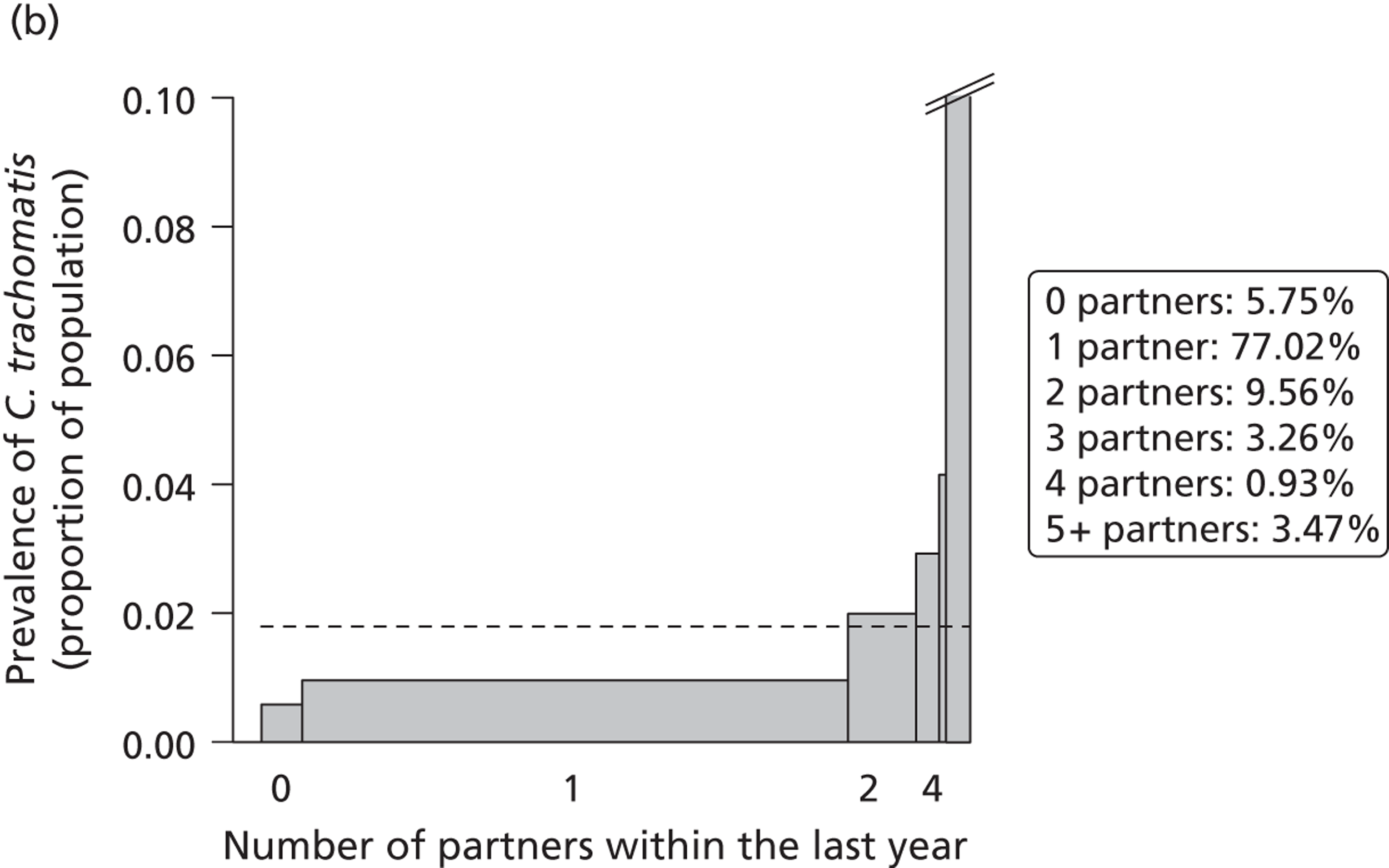
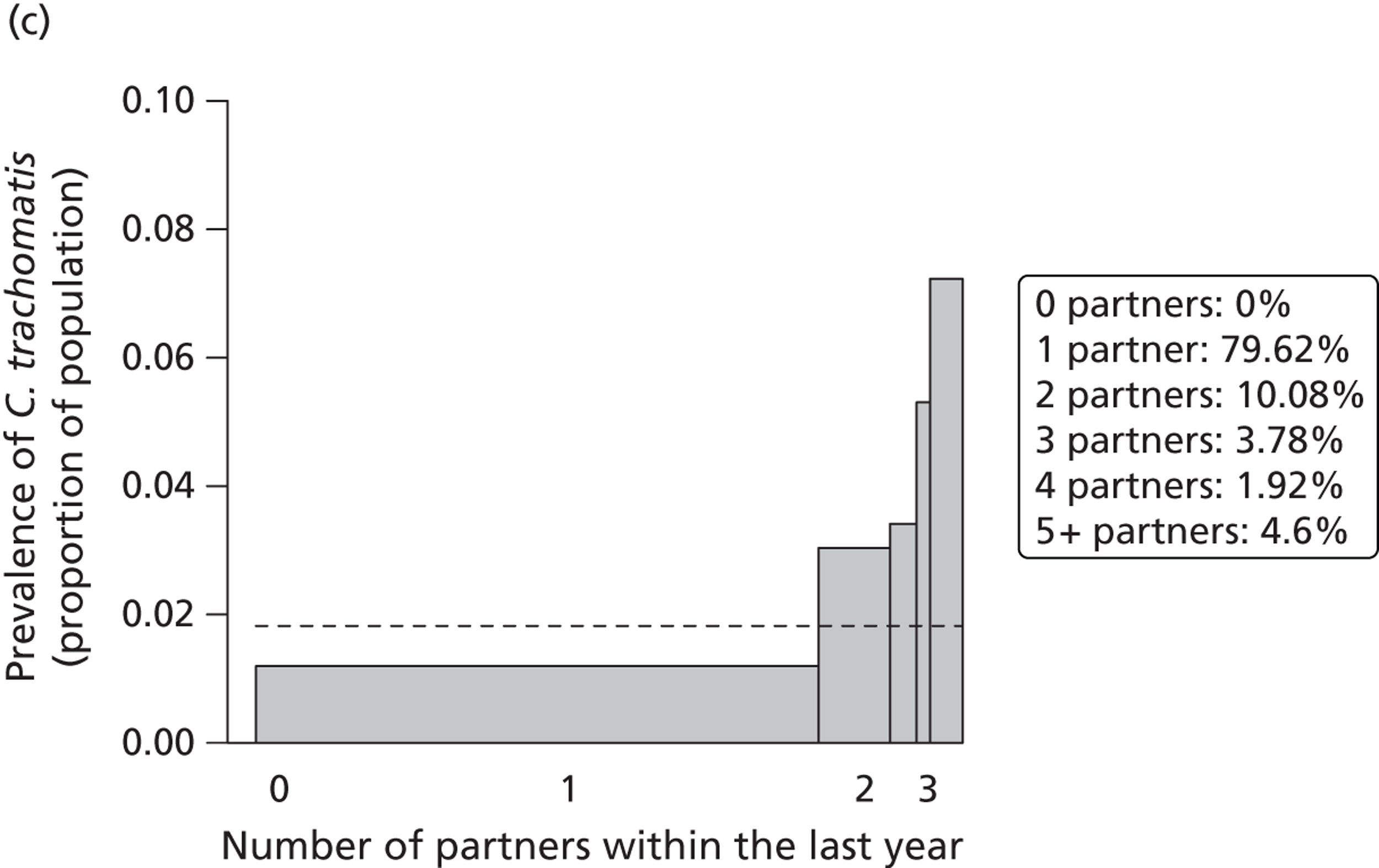
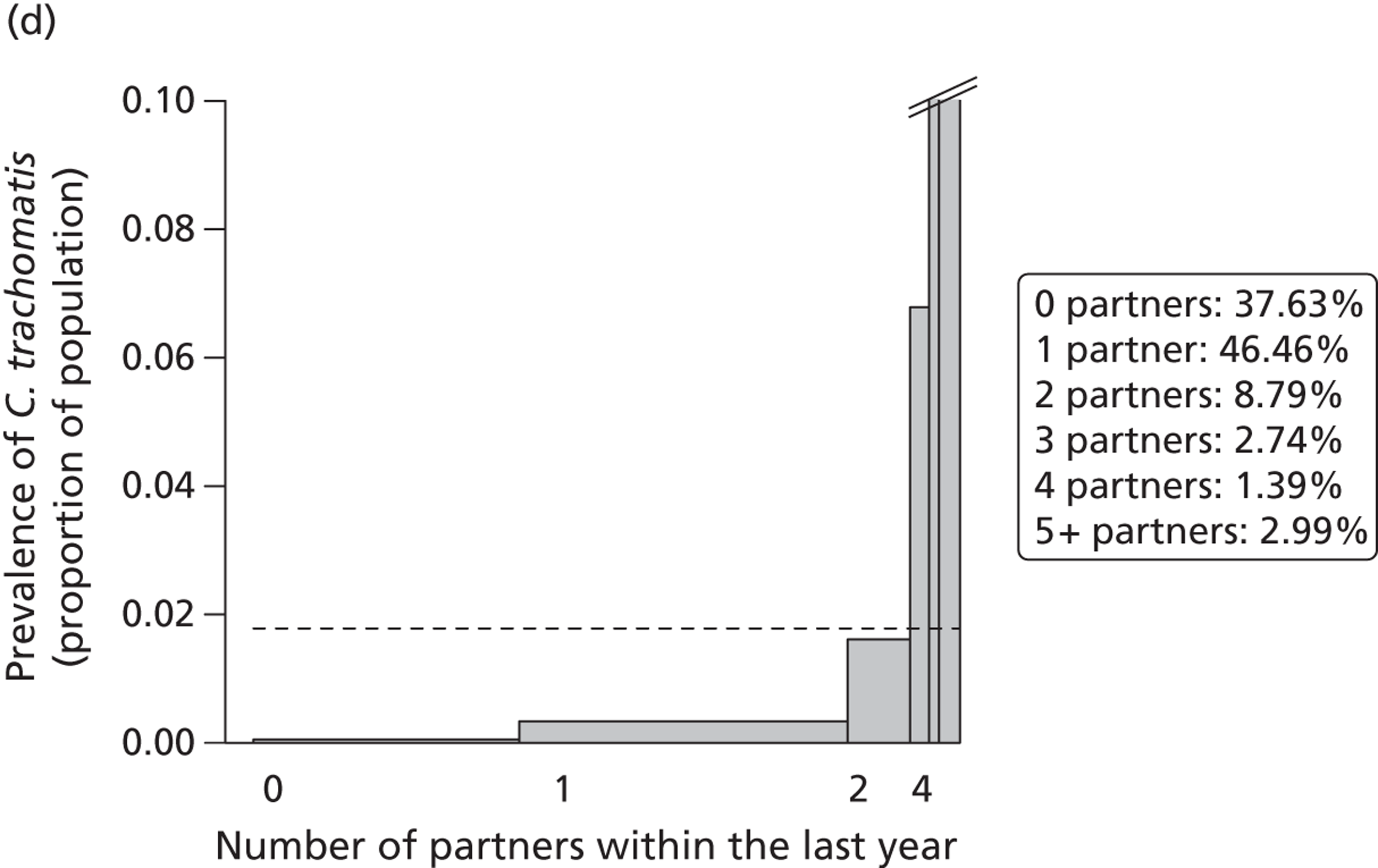
The per-sex act transmission probabilities required to reproduce the estimated prevalence from Natsal-2 are lower than the estimates we obtained (see Transmissibility of chlamydia). This might be the result of unrealistic assumptions about the sexual partnership dynamics in the three models. However, it could also derive from our assumption of a relatively high frequency of sex acts during the first 2 weeks of a sexual partnership, which would balance the lower transmission probabilities per sex act.
We compared the model predictions with Natsal-2 data according to the distribution of chlamydia infections according to numbers of sexual partners and the fraction of infections in individuals with frequent partner change rates. As expected, the prevalence of chlamydia increases with an increasing number of heterosexual partners within the last year (see Figure 12). Again, the Kretzschmar model captures the overall distribution well and bears a striking resemblance to the proportion of people in each ‘risk category’ (shown in inset legend for each panel). The prevalence in the group of individuals with five or more partners within the last year is, however, overestimated. The Turner model captures the overall distribution of chlamydia infections in 18- to 44-year-olds but the figure excludes 16- to 17-year-olds (because they were not included in Natsal-2) in whom chlamydia prevalence in the model was very high. Furthermore, the mode results in an unrealistic outcome where no individual remains without a partner throughout the 1-year period. In contrast to the other two models, chlamydia infections are too heavily concentrated among ‘high-risk’ individuals in the ClaSS model. Furthermore, it becomes apparent that sexual partnership dynamics are not captured well because too many individuals remain single throughout the 1-year period.
Finally, we implemented a standardised screening and partner notification intervention and compared the predicted impact after 5 and 10 years of intervention (Figure 13).
FIGURE 13.
Impact of a standardised screening programme on the prevalence of chlamydia. The proportion of eligible people receiving screening at least once a year is 20%, and 40% of the current or most recent partners of an index case are successfully notified and treated. Means of 100 simulations runs are shown together with the SD. Reproduced with permission from Althaus CL, Turner KM, Schmid BV, Heijne JC, Kretzschmar M, Low N, Transmission of Chlamydia trachomatis through sexual partnerships: a comparison between three individual-based models and empirical data, J R Soc Interface 2012;9:136–46. 48
The harmonisation of disease-specific parameters resulted in more similar results for the Kretzschmar and the ClaSS models. However, the Turner model still predicted a much stronger impact of the same intervention. Part of this discrepancy can be explained by an overestimation of partner change rates among young adults in the Turner model. The unrealistically high rates cause a large proportion of chlamydia transmission to occur in exactly those individuals who become eligible for screening, rendering the intervention more powerful.
Discussion
We re-estimated the average duration of asymptomatic chlamydia infection in women at 433 (95% CI 420 to 447) days. We also obtained new estimates of chlamydia transmissibility and found that the transmission probability per episode of heterosexual intercourse is 9.5% (IQR 6.0–16.7%). The transmission probability per heterosexual partnership was estimated at 55.5% (IQR 49.2–62.5%). We applied this parameter set to three individual-based models of chlamydia transmission and obtained similar predictions of the impact of a screening and partner notification intervention in two of the models. Part of the discrepancies between the models was explained by their use of different assumptions about the infectious duration of chlamydia.
To fully evaluate the role of immunity on the impact of chlamydia prevention, we need further insights about the possibility of temporary immunity to C. trachomatis in humans and the timing of its development. The development of immunity could interfere with the effect of screening on reducing the prevalence of chlamydia. Whether or not natural clearance of asymptomatic infection is followed by a period of temporary (or partial) immunity is still open to debate. 96 We found that long duration of temporary immunity can drastically diminish the effect of screening. If temporary immunity develops only in asymptomatic individuals who clear the infection naturally, screening and treatment might directly interfere with establishing immunity, causing a diminished effect of screening. 36,96,97
The type and structure of models that describe the spread of STIs should depend on the question that one wants to address. 98 The duration and transmissibility of chlamydia infection were investigated with relatively simple deterministic, population-based models. These models do not allow the identification and tracking of current and previous sex partners of infected index cases, which is needed for detailed investigation of the effects of partner notification. The three models that we investigated differed in their ability to realistically describe the sexual partnership dynamics. Overall, the Kretzschmar model performed best in terms of describing the sexual partnership dynamics and the distribution of chlamydia within the population. The Turner model generated a good description of the distribution of chlamydia in the age range investigated, but probably overestimated the effect of chlamydia screening due to an unrealistically high prevalence in the youngest age class. The ClaSS model predictions of the impact of chlamydia screening were similar to those of the Kretzschmar model after harmonisation of parameters, but the distribution of chlamydia was too highly concentrated among those with a high number of sexual partners.
Based on these studies, we decided not use either the Turner or the ClaSS models to study the effects of partner notification interventions. We developed new individual-based models, which were simple enough to allow interpretation of the findings, but which possessed the complexity necessary to construct the sexual network and track individual partnerships.
Individual- and population-level effects of partner notification for Chlamydia trachomatis
Substantial portions of the following section are reproduced with permission from Althaus CL, Heijne JCM, Herzog SA, Roellin A, Low N. Individual and population level effects of partner notification for Chlamydia trachomatis. PLOS ONE 2012; 7:e51438. 49
Introduction
Partner notification is known to be an efficient method of case finding. There is, however, no consensus about the most appropriate or efficient window of time for past partner notification (‘look-back period’). The UK National Guideline for the Management of Genital Tract Infection with Chlamydia trachomatis recommends notifying partners of an asymptomatic index case within a period of 6 months. 10 The same is standard in Sweden, but recommendations from the Swedish National Board of Health and Welfare are changing, based on a recent study that found that extending partner notification periods could improve the identification of new chlamydia cases. 99 This is also supported by a study from the USA, where it was observed that a partner notification period of 6 months or more would help to identify more infected cases. 100 But the Sexually Transmitted Diseases Treatment Guidelines from the US Centers for Disease Control and Prevention recommend notifying partners with whom the index case has had sexual contact within the previous 60 days, or the most recent partner if no sexual contact occurred in this period. 101
Objectives
To investigate the impact of two partner notification strategies – notifying partners in order of the most recent date of sexual intercourse before the end of a partnership, or notifying all partners during a specified look-back period – on outcomes at the level of individuals and the population.
Methods
Individual-based modelling framework
We used a stochastic, individual-based (or agent-based) modelling framework that can simulate the transmission of an arbitrary STI in a sexual partnership network of any level of complexity. 49 Rstisim (R package for STI simulation) is written in C++ and can be downloaded at www.stat.nus.edu.sg/∼staar/rstisim as a package for the R software environment for statistical computing (The R Foundation for Statistical Computing, Vienna, Austria; www.R-project.org).
Modelling chlamydia transmission
To investigate the transmission of chlamydia in a heterosexual partnership network we devised a model with concurrent partnerships in a homogeneous population. 49 The structure of the model described below is implemented into Rstisim where event times (partnership formation and dissolution, sex acts and clearance of chlamydia) are drawn from exponential distributions around mean values (Table 12). If not otherwise indicated, we assume a total population size n = 20,000, equally divided into females and males. Sex difference is indicated by the subscript i and 1 – i for i = 0, 1.
| Parameters | Value |
|---|---|
| Assumed parameters | |
| Mean number of new heterosexual partnerships per individual, n | 1.04 per year |
| Mean number of total heterosexual partnerships per individual, t | 1.70 per year |
| Level of concurrency, c | 8% |
| Frequency of sex acts, f | 1 per week |
| Mean duration of infection | 1 year |
| Prevalence of chlamydia | 3% |
| Derived parameters | |
| Pair formation rate, ρ | 1.36 per year |
| Relative probability of accepting a partnership if already in a pair, α | 0.28 |
| Mean duration of partnership, 1/σ | 0.65 years |
Our model is based on the pair model framework,89 in which singles Xi initiate partnerships, P, at a pair formation rate ρ. This framework is extended to account for individuals who have two sexual partnerships at the same time (concurrency). 102 We assume that singles X and individuals who are in a P can accept another partnership with a probability α (relative to the probability that a single X accepts). Such an event can result in a triple T. Triples can then be elongated to form chains of contacts. We define the level of concurrency at cross-section, c, as the ratio of individuals that have more than one partnership to all individuals in a partnership.
The average duration of a partnership is given by 1/σ. Every partnership begins with an initial sex act where chlamydia can be transmitted at rate π. In an ongoing partnership, the frequency of sex acts is given by f and the transmission probability per sex acts is again π. The frequency of sex acts per partnership is constant, that is, individuals who have two concurrent partnerships have twice as many sex acts per unit of time compared with individuals who are in only one partnership.
Sexual behaviour data and parameter derivation
To parameterise the heterosexual partnership dynamics, we use data from Natsal-2. 103 We adjust the pair formation rate, ρ, so that the model exhibits the same number of realised partnerships as in 16- to 25-year-old women and men in Natsal-2 (see Table 12). Assuming the sexual partnership dynamics has approached steady state, the pair formation rate is given by
where n and t are the numbers of new and total heterosexual partners in the last year, respectively, and c is the level of concurrency. The partnership dissolution rate is given by σ = n/(t – n). Note that t – n corresponds to the proportion of individuals in a partnership. Finally, we varied the level of concurrency between 0% and 100% and found that c = 8% provides the best match between the simulations and data from Natsal-2.
Chlamydia prevalence rates for 18- to 24-year-olds in Natsal-2 were 3.0% in women and 2.7% in men in the UK as a whole. 77 Data from Natsal-2 were weighted to adjust for unequal selection probabilities and to correct for the age and sex profile in the population, and mean values were taken for women and men together. We adjusted the transmission probability per sex act so that the steady state prevalence of chlamydia is 3% in the model.
Sexual network
Figure 14 shows the sexual partnership dynamics of the model where chains of contacts can occur at cross-section. Over the course of 1 year, the sexual partnership network shows closely connected groups or bigger circular structures.
FIGURE 14.
Sexual partnership networks from the individual-based model. Different sexes are indicated by filled and empty circles. (a) At cross-section, individuals can be single, form a sexual partnership or have two concurrent sexual partnerships; and (b) large connected components emerge during a period of 1 year. For illustrative purposes, the population size was limited to 100, resulting in higher connected networks compared with larger population sizes.
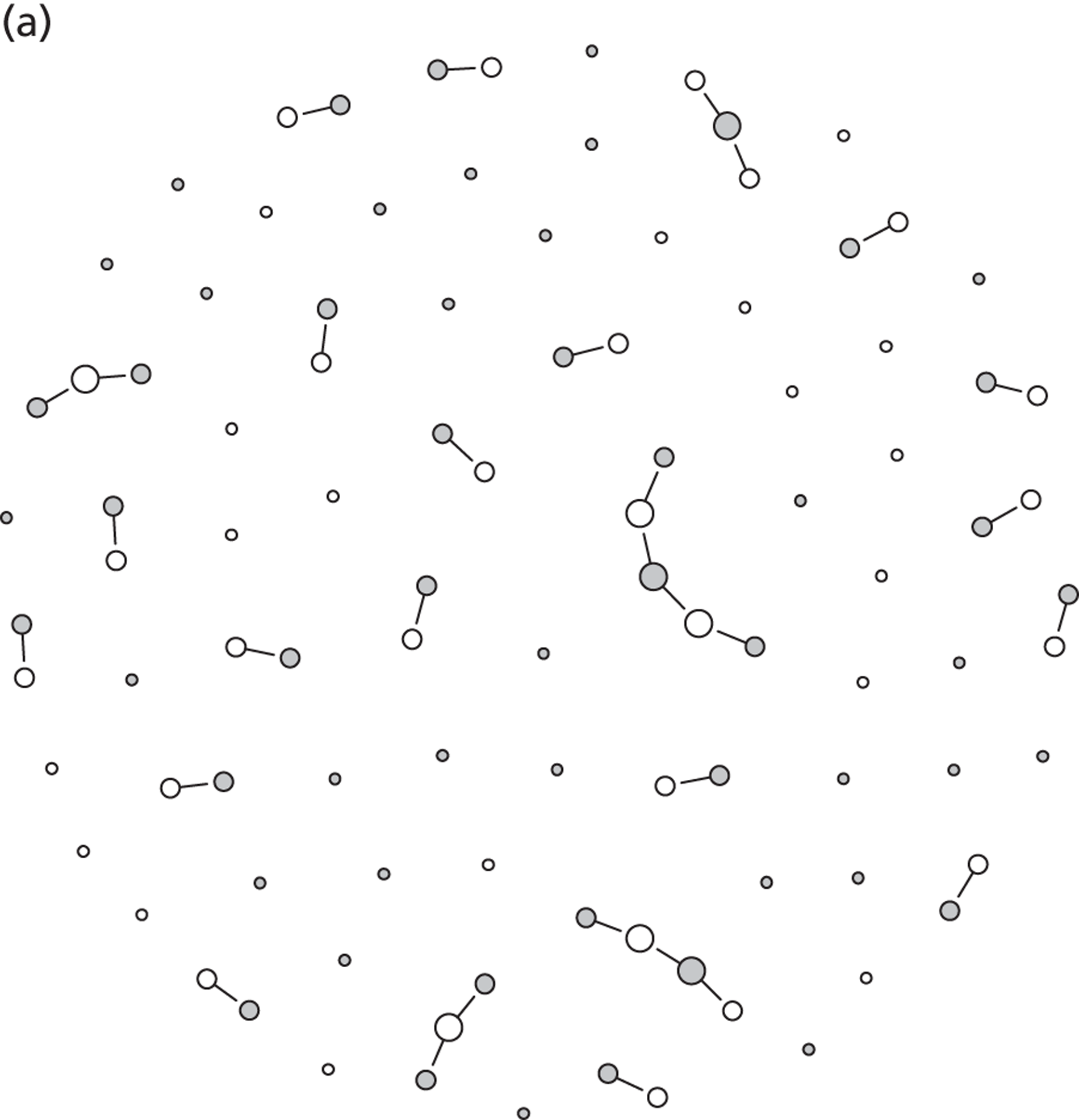
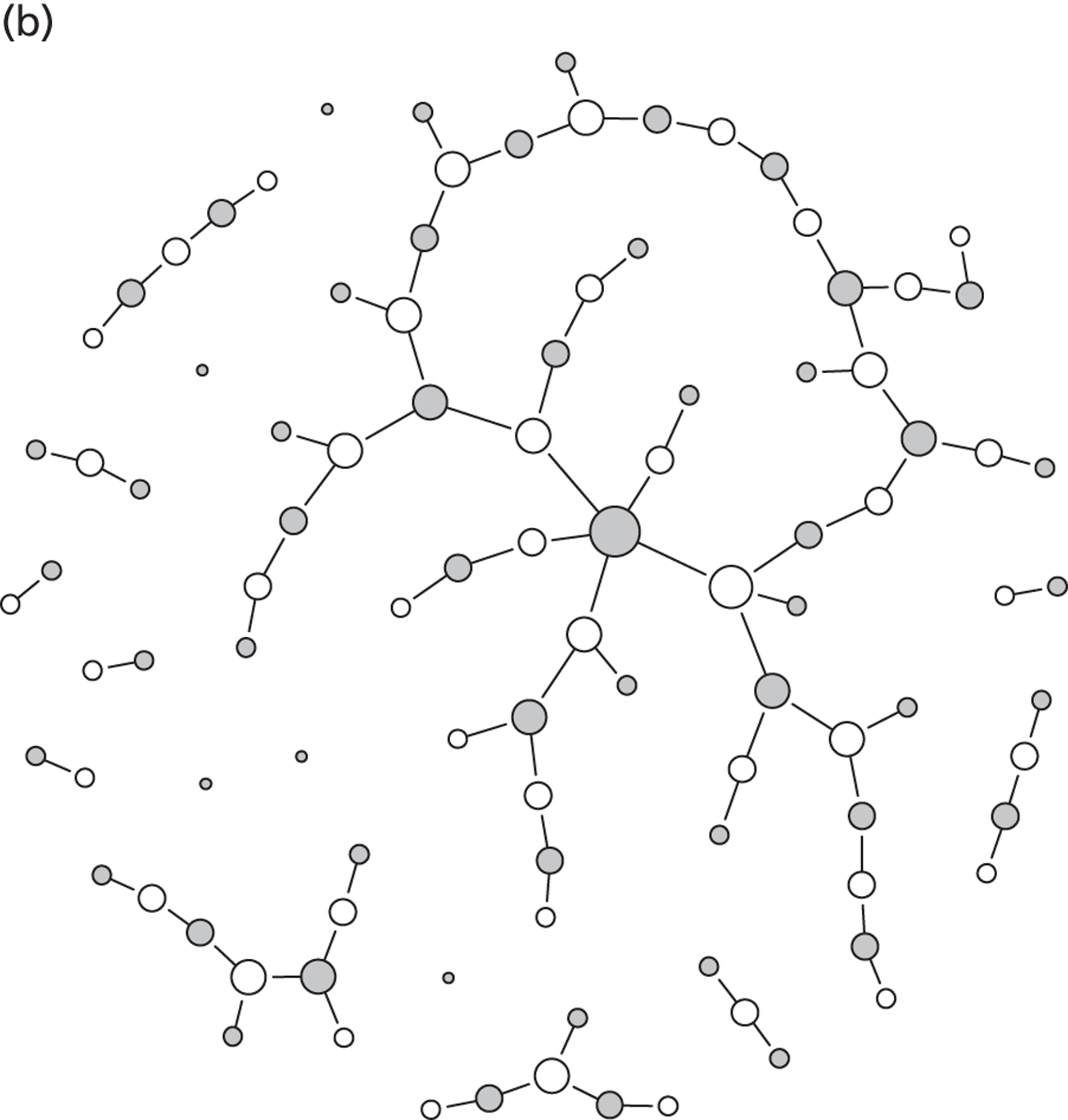
Partner notification strategies
Using our individual-based modelling framework, we can follow an individual’s history of current and previous partners. Figure 15 shows the complex partnership dynamics in which a new partnership can replace a previous one, or where short episodes of concurrency can occur.
FIGURE 15.
Partnership histories and partner notification strategies. The scheme shows an example of an individual’s history of three sexual partnerships. The partnerships can either be separated by a period of being single (partnership 1 and 3) or be concurrent (partnership 1 and 2). One strategy is to notify partners of an index case in order of their recency, e.g. the first, second or third most recent partner(s). Another strategy is to notify all partners from a certain time period, e.g. the partners from partnership 1 and 2 only. Reprinted with permission from Althaus CL, Heijne JCM, Herzog SA, Roellin A, Low N, Individual and population level effects of partner notification for Chlamydia trachomatis, PLOS ONE 2012; 7:e51438. 49
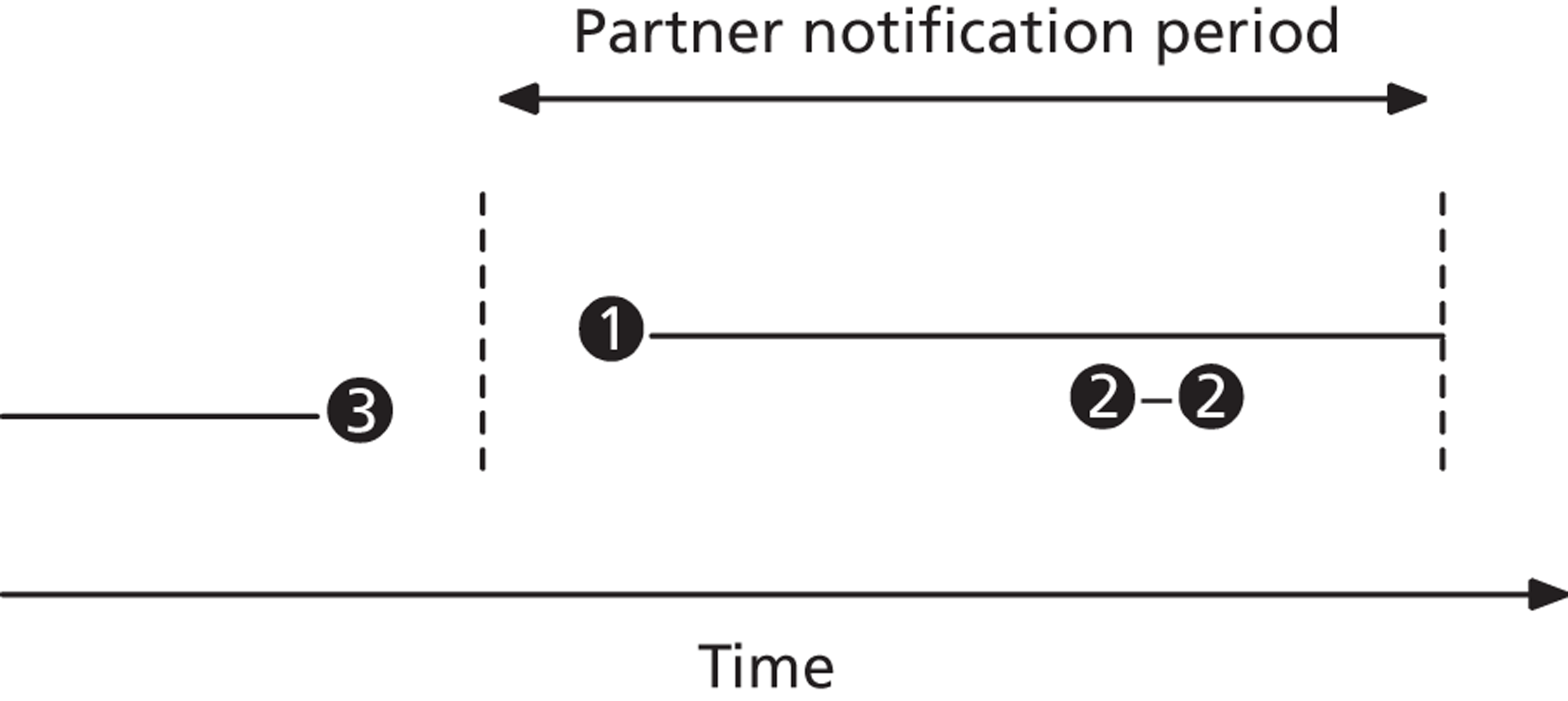
We explore two strategies in which partners of an index case can be notified. One strategy is to notify the partners in order of their recency (by the time since their partnership ended). The other strategy is to notify all partners within a certain look-back period. For each strategy, each notified partner is tested and successfully treated with a probability of 50% (approximated from results; see Partner notification outcomes for chlamydia in UK genitourinary medicine clinics). 45 In practice, a partner who tests positive could become a new index case. We did not include this feature because it made the model more complicated but did not affect the results.
Results
Individual-level effect of partner notification
At the level of individuals, partner notification identifies new index cases. It is therefore important to know how many of an index case’s partners are infected. A Swedish study99 found that the proportion of chlamydia-positive partners decreases as time since last sexual intercourse increases. In the simulations, everyone who is chlamydia positive at cross-section is defined as an index case. This corresponds to infected individuals who would be detected through random screening or after developing symptoms, assuming that symptoms occur uniformly throughout the course of infection. We can then go through all current and previous partners of an index case and ‘test’ whether or not they are infected. When we used the same time periods as in the Carré et al. study,99 chlamydia positivity in the model was similar to the data (Figure 16).
FIGURE 16.
Simulated and empirical data of chlamydia positivity in partners of index cases. Black crosses correspond to the proportion (with 95% CI) of positive partners out of those with a positive test. 99 The black circles represent simulated data from the model. In the simulations, the steady-state prevalence of chlamydia is 3%. Means of 100 simulation runs are shown. Standard errors are small and omitted for better visibility. Reprinted with permission from Althaus CL, Heijne JCM, Herzog SA, Roellin A, Low N, Individual and population level effects of partner notification for Chlamydia trachomatis, PLOS ONE 2012; 7:e51438. 49
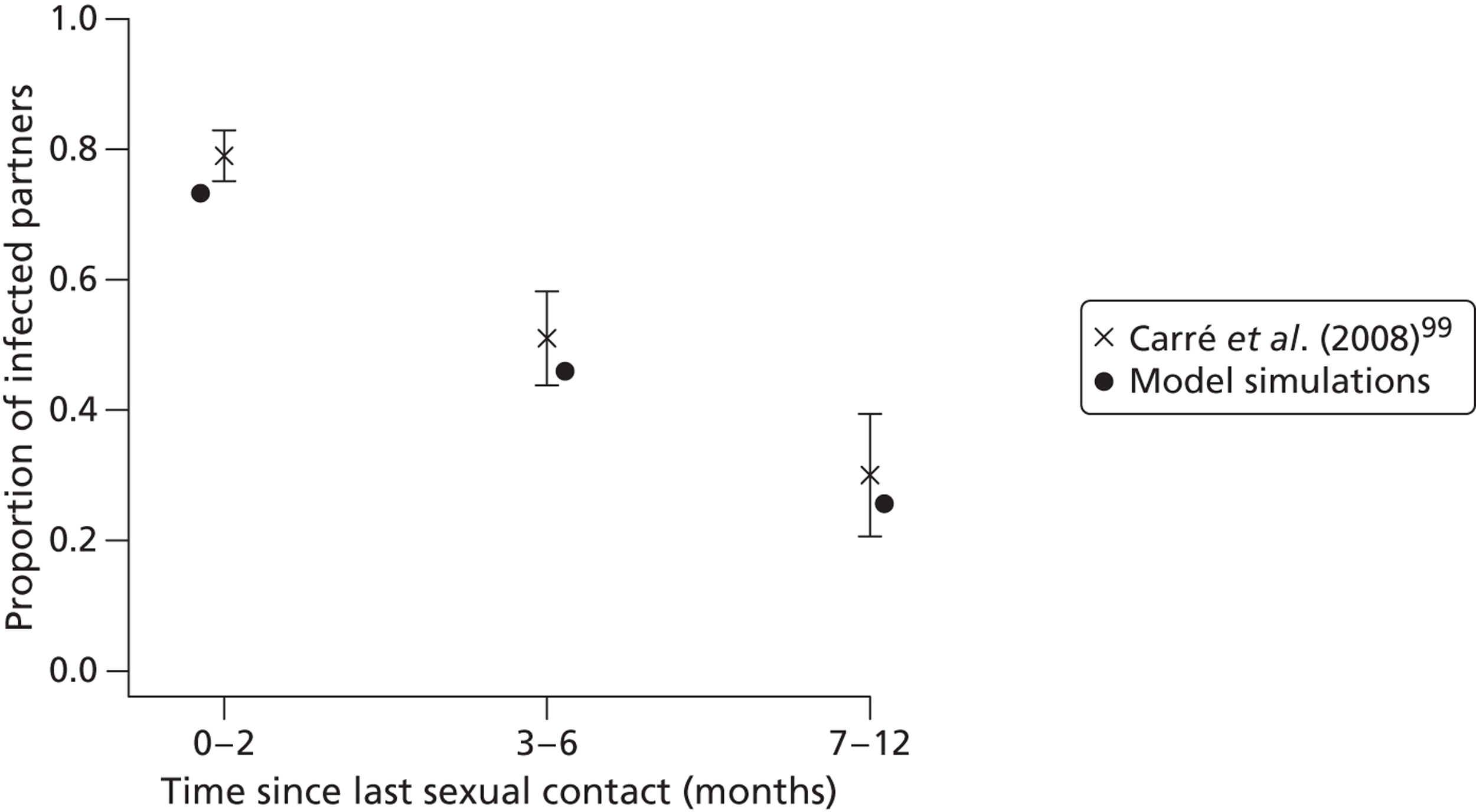
The proportions of infected cases were examined in more detail in the individual-based modelling framework. First, we investigated in order of recency the proportion of infected partners (Figure 17a). The model gave consistent results: 67.5% of the most recent partners of an index case were infected with chlamydia. The less recent a partnership or contact, the lower the probability that the partner is infected. The proportion of infected individuals, up to the third most recent partner, was still substantially higher than the population prevalence.
We could also group the partners of an index case in a look-back period since the partnership has ended in greater detail than that shown by Carré et al. 99 (Figure 17b). We found that as far back as 18 months, a substantial proportion of partners (> 10%) were infected with chlamydia. This suggests that extending partner notification periods beyond 1 year yields more new index cases for individual case management than would be found through random screening.
FIGURE 17.
Proportion of chlamydia-positive partners of index cases. (a) The proportion of infected partners in order of their recency; and (b) the proportion of infected partners grouped into different look-back periods in order of their break-up date. Steady-state chlamydia prevalence is 3% (dashed line). For each strategy, means of 100 simulation runs are shown. Standard errors are small and omitted for better visibility. Reprinted with permission from Althaus CL, Heijne JCM, Herzog SA, Roellin A, Low N, Individual and population level effects of partner notification for Chlamydia trachomatis, PLOS ONE 2012; 7:e51438. 49
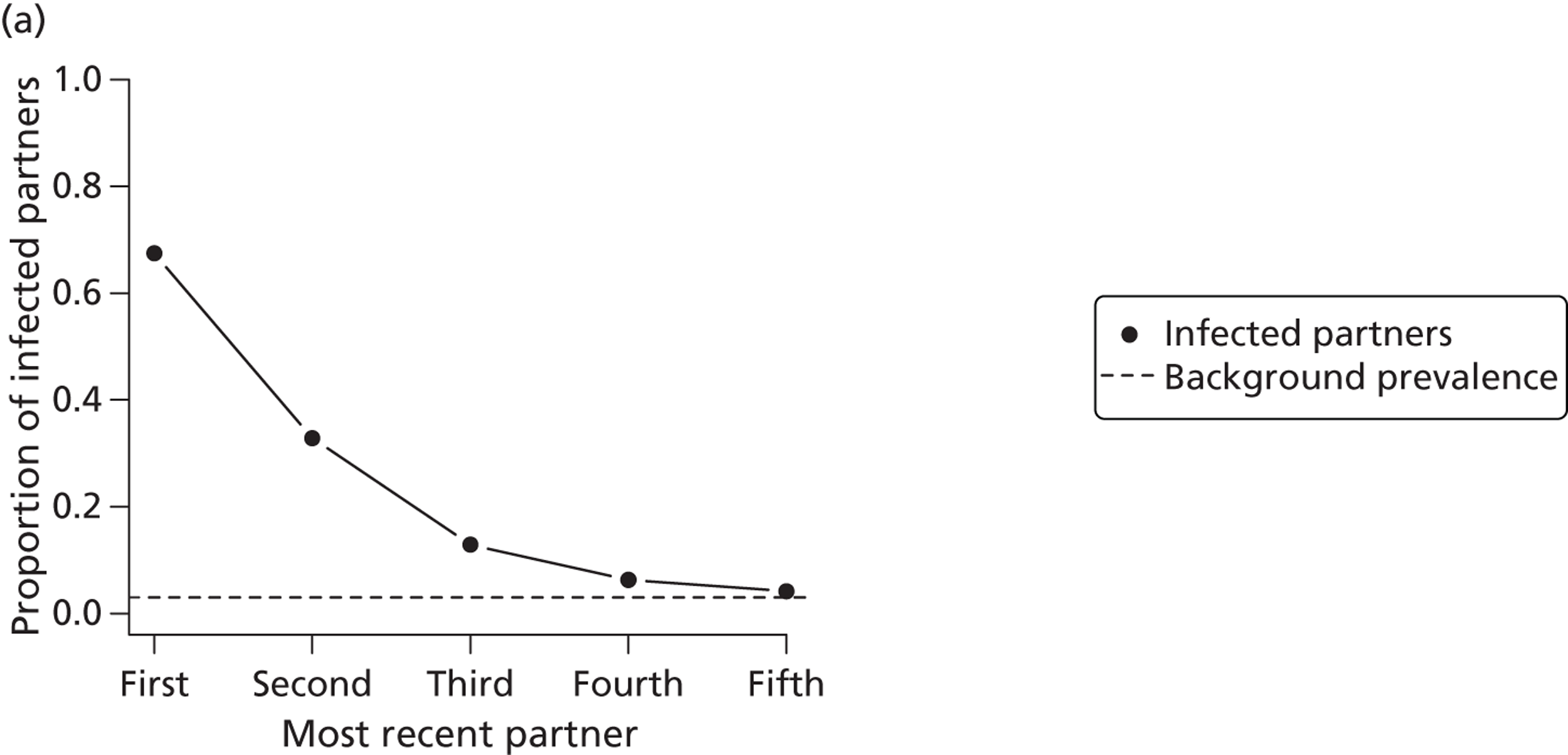
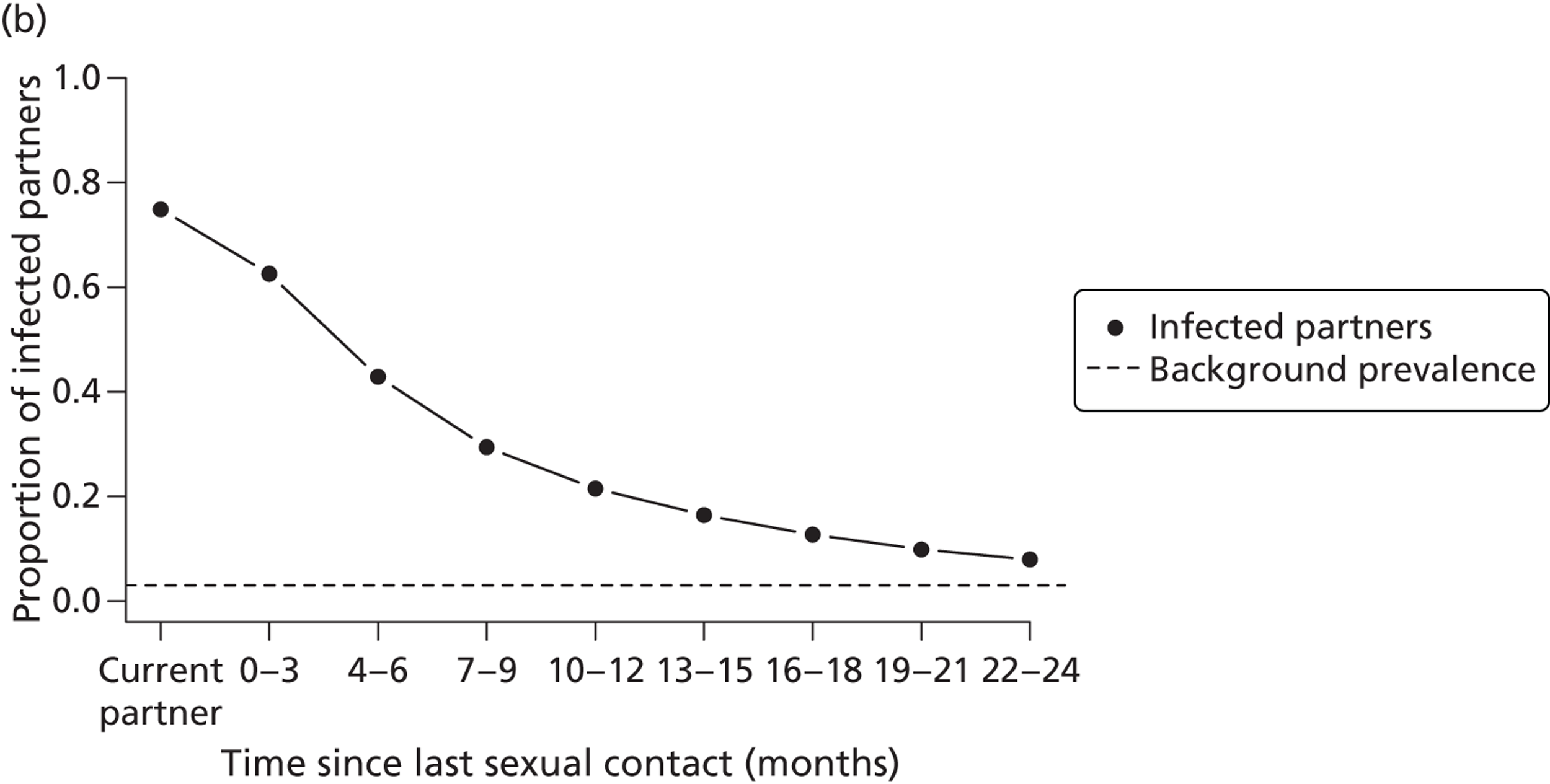
Population-level effect of partner notification
At the population level, partner notification can prevent onward transmission of chlamydia and reduce the overall prevalence of the infection. Here, we investigated the effects of the different partner notification strategies if they were implemented as part of a population-wide screening programme. After the simulations approached the steady-state prevalence of 3%, we introduced random screening of the whole population of young adults. Every woman and man received screening at a rate of 0.1 per year, that is, every 10 years on average. If partner notification is performed, each notified partner is tested and successfully treated with a probability of 50% (see Chapter 2, Clinical effectiveness of partner notification technologies: systematic review). 45
After 5 years of screening, assuming that there is no partner notification, the prevalence of chlamydia was reduced to about 70% (Figure 18). It can be clearly seen that the strongest effect of partner notification stems from notifying only the current partner. This suggests that notification of current partners, or the most recent partners if the index case is single, is sufficient to achieve most of the additional reduction in prevalence at the population level.
FIGURE 18.
Population level effect of partner notification. Graphs show the reduction in the prevalence of chlamydia after screening for 5 years at a rate of 0.1 or 0.25 per year. (a), (c) and (e) show prevalence for different numbers of notified partners; (b), (d) and (f) show prevalence for different partner notification periods. The probability, p, that each notified partner will be tested and successfully treated is set to 10% (a and b), 50% (c and d) or 90% (e and f). For each strategy, means of 100 simulation runs are shown. Standard errors are small and omitted for better visibility.
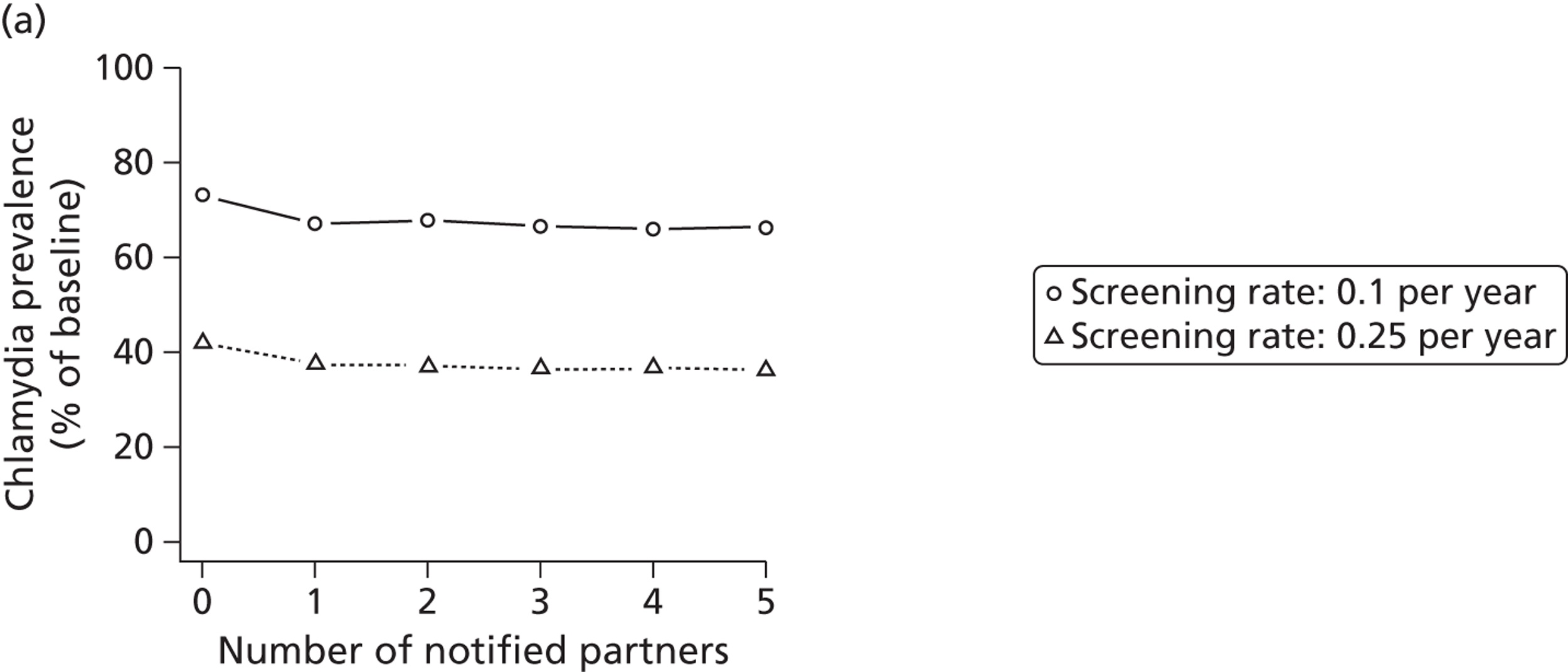
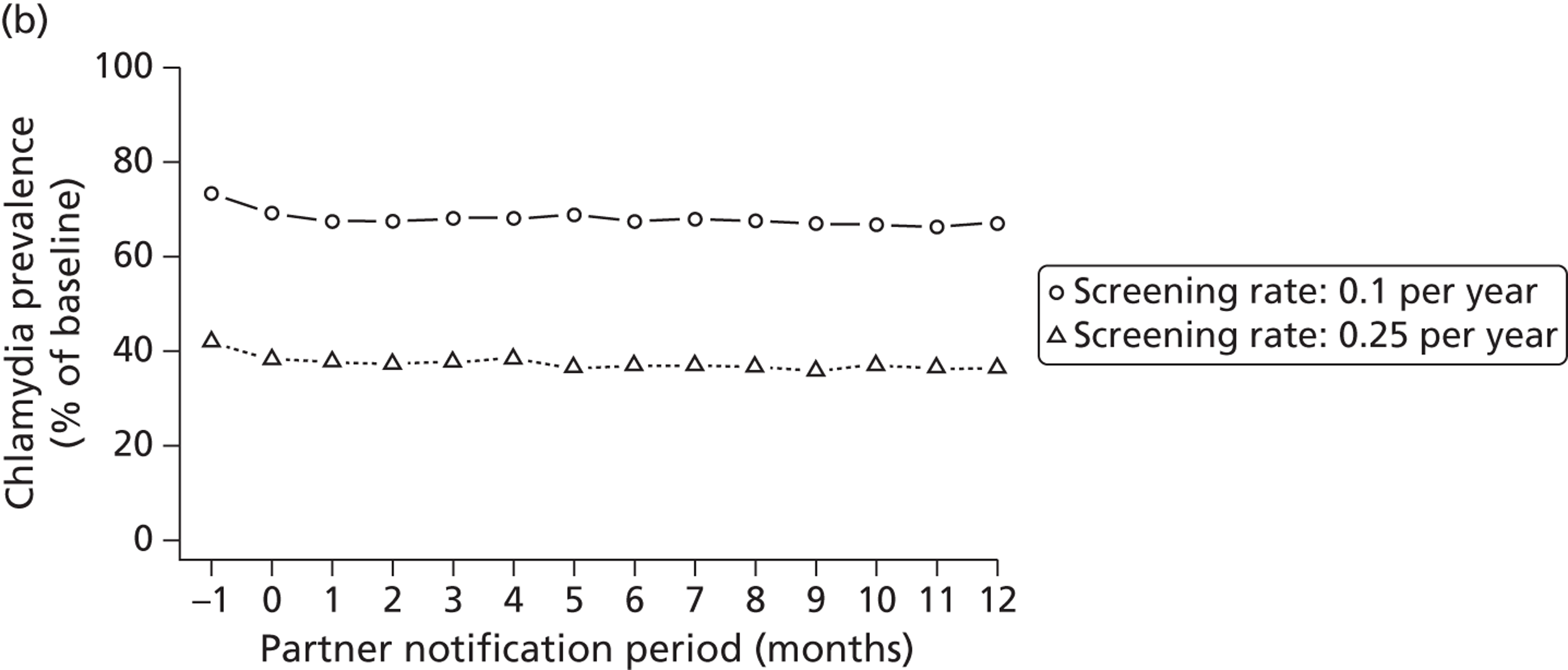
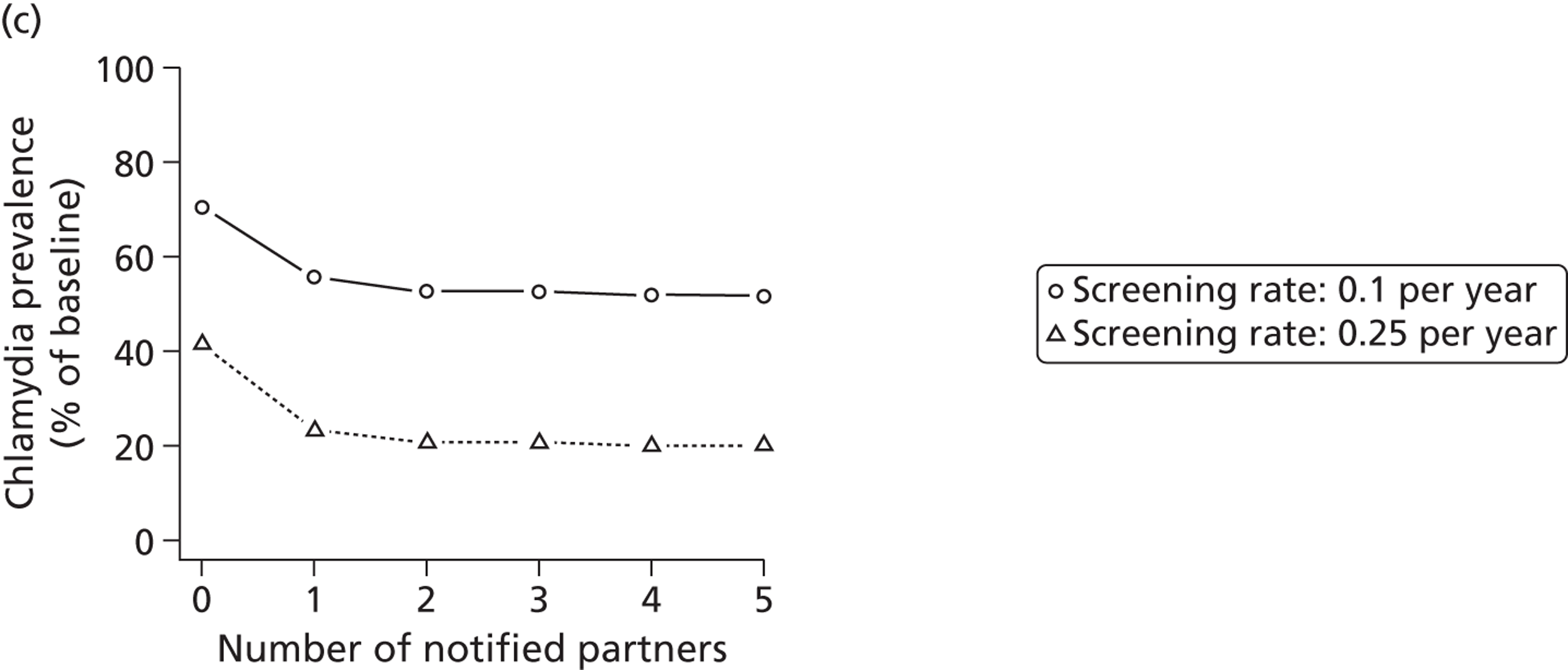
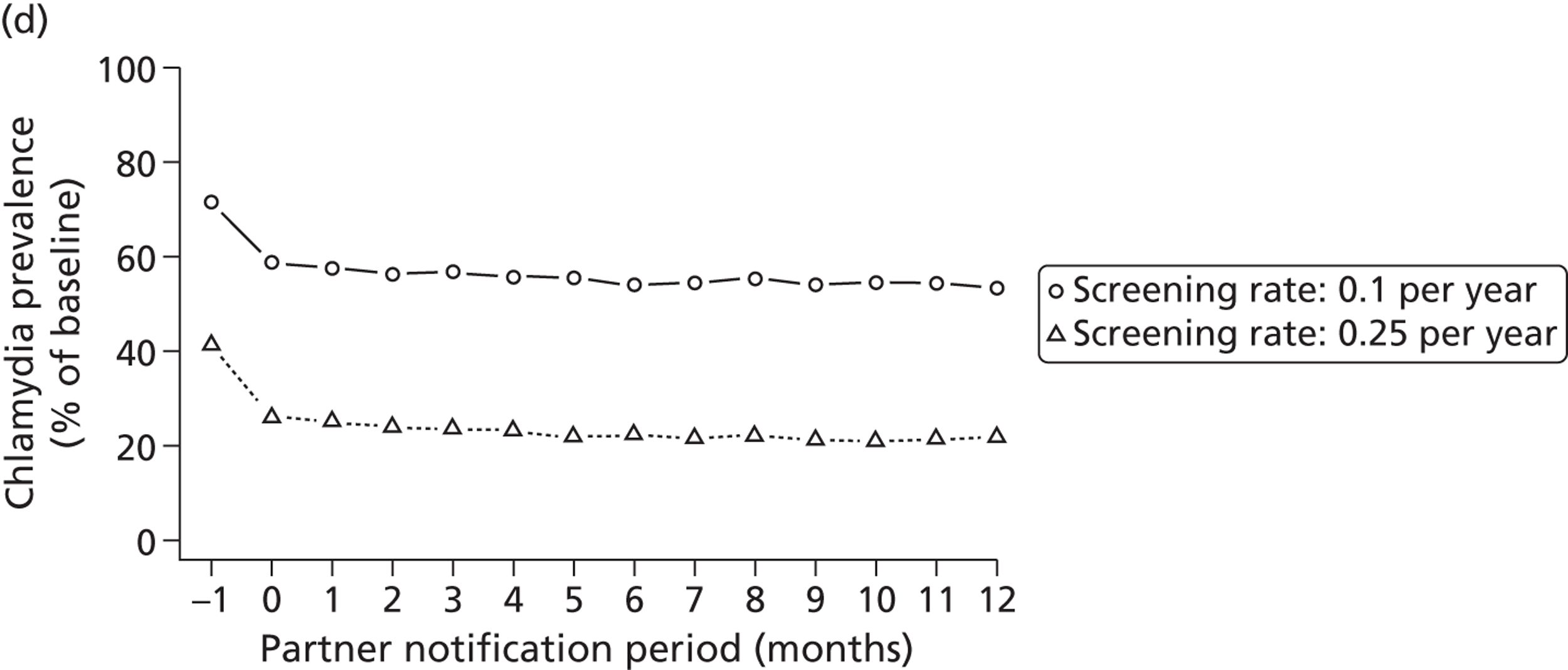
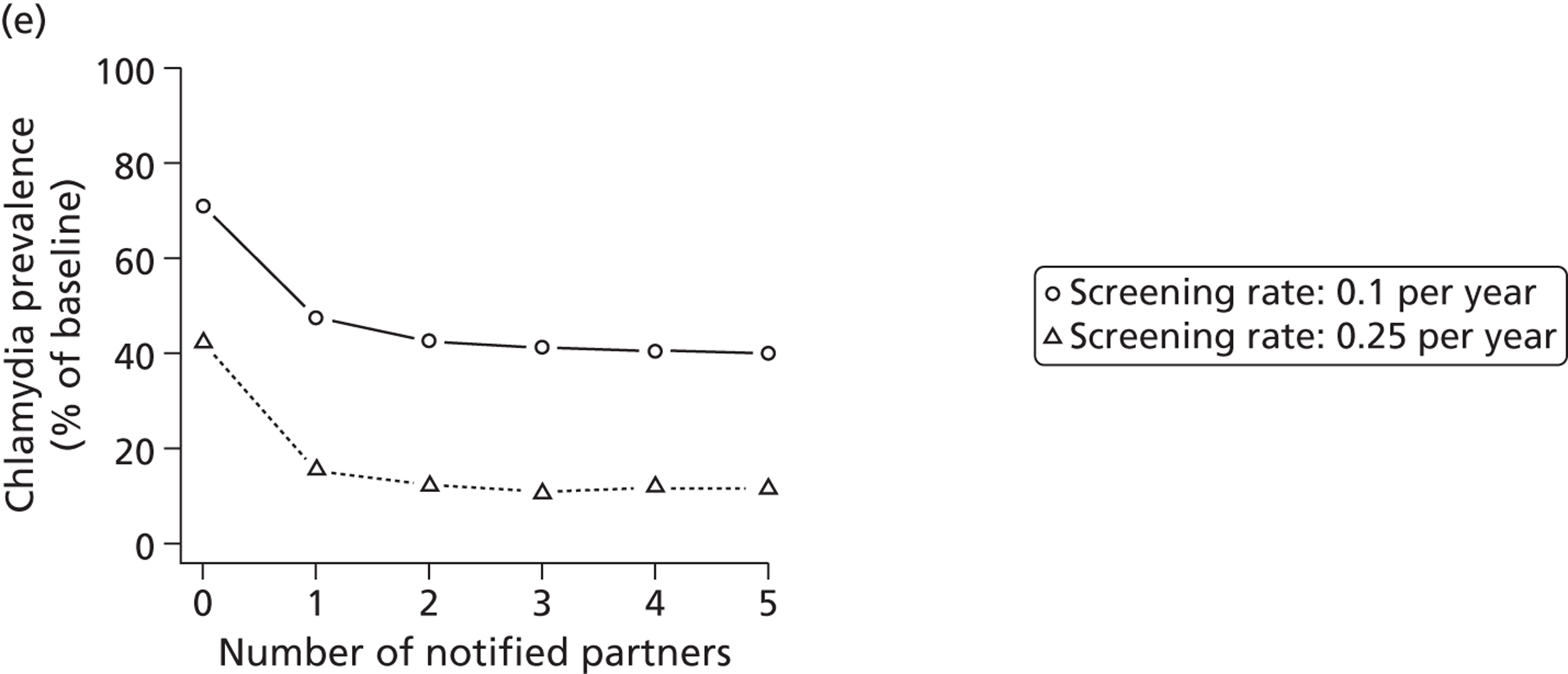
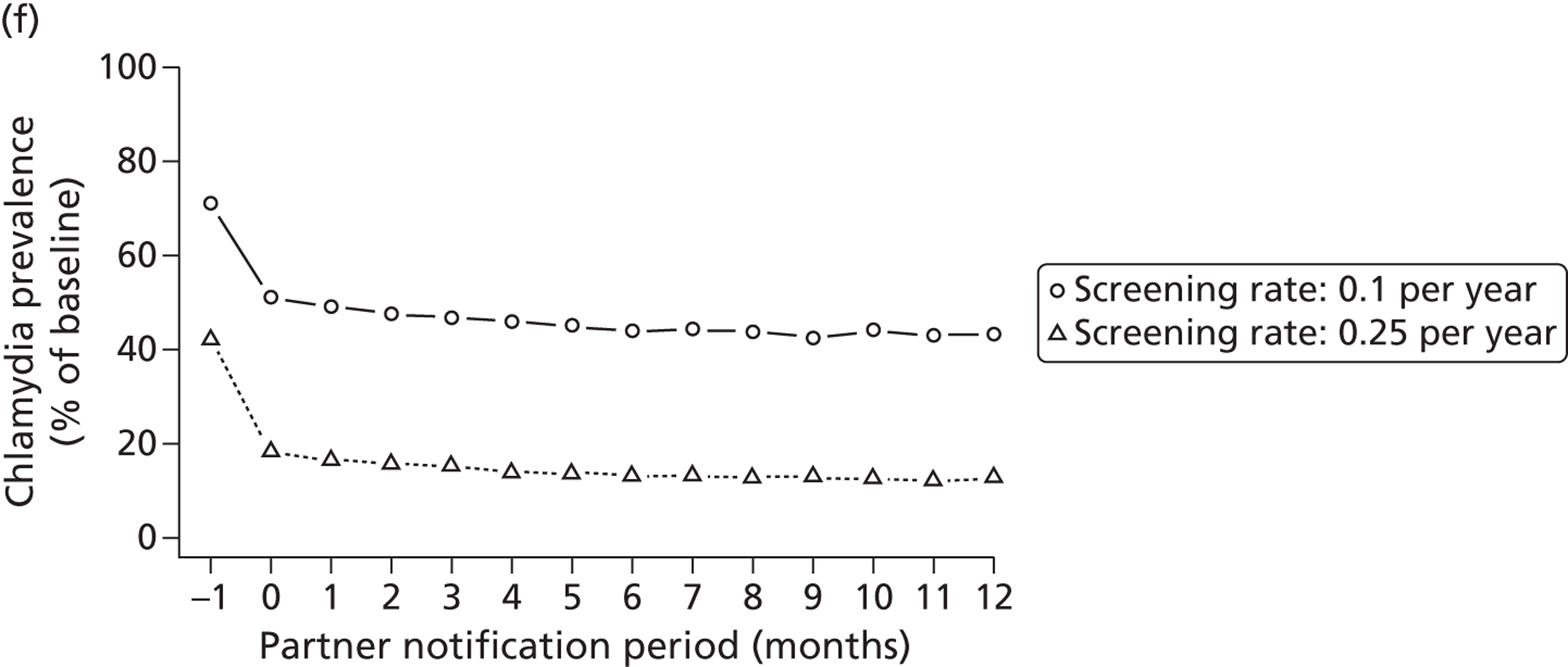
Sensitivity analyses
The results shown are from a model that assumes a homogeneous and closed heterosexual population. Despite its simplicity, this model provides a good description of the chlamydia positivity in partners of index cases (see Figure 16). Our general conclusions about the effects of partner notification at the level of individuals and the population were robust to different assumptions about the infectious duration (see Modelling the transmission dynamics of C. trachomatis), with a shorter infectious duration in men and in different screening and partner notification scenarios. 49
Discussion
Our model simulations show that, while extending partner notification periods beyond 1 year helps to find new index cases, notifying the current or most recent partner has the greatest effect on reducing transmission in a general heterosexual population of young adults.
We made a number of simplifying assumptions in our model. First, we treat the population of 16- to 25-year-olds as a closed population that mixes homogeneously. This assumption probably resulted in realistic chlamydia transmission dynamics in our study because it was restricted to the age group that drives chlamydia transmission in the wider population. We have previously shown that this kind of model results in a realistic distribution of chlamydia infection according to numbers of recent sexual partners,46 and provides conclusions in line with more detailed models that include age and risk stratification. 35,48 However, differences in sexual activity between women and men, and potentially higher concurrent partnerships in men compared with women, are additional complexities that could be considered in future studies.
Our simulation study examined a general strategy for partner notification, which is similar to traditional technologies where the partner attends a health service setting and receives both testing and treatment. Our findings suggest that the choice of partner notification strategy for chlamydia in a general heterosexual population of young adults depends on the public health context in which it is applied. At the individual level, our results suggest that tracing as many as three partners from the preceding 18 months can be helpful in finding new index cases. This is in line with recent recommendations for partner notification periods in Sweden, based on the findings of Carré et al. 99 At the population level, notification of current partners of an index case should be a priority. But if previous partners are chlamydia positive, they are likely to have been infected for a long time and thus notifying them will do little to limit onward transmission in a general heterosexual population with limited levels of concurrent partnerships. In all likelihood, they will have already transmitted the infection to others before clearing it spontaneously or becoming symptomatic and seeking care. If screening is targeted at high-risk individuals, notifying previous partners of index cases will likely have a stronger effect on limiting onward transmission because of their higher partner change rates (see Estimating the likely public health impact of partner notification for a clinical service: an evidence-based algorithm). 9
Few modelling studies have examined the effects of partner notification for chlamydia. Our findings are consistent with those of Kretzschmar et al. ,84,104 who also considered a dynamic sexual partnership network but restricted partner notification to current partners only. In contrast, Armbruster and Brandeau105 found that increasing contact tracing capacity resulted in a substantial reduction of chlamydia prevalence, although with diminishing returns. They investigated the effects of partner notification for index cases who seek treatment for symptoms and assumed a static sexual network with a relatively high prevalence of chlamydia. In our study using a dynamic sexual network, we found that previous partners of an index case do not contribute to reinfection, which might explain our finding that notifying previous partners had little additional effect on reducing chlamydia transmission.
In future modelling studies, different strategies for screening and partner notification in groups with frequent partner change rates could be investigated in models that incorporate heterogeneity in sexual behaviour. The effects of different partner strategies for other bacterial STIs should also be examined. In the following section, we investigate testing and treatment strategies for both chlamydia and gonorrhoea. The impact of partner notification on syphilis would require a different model structure and should be examined in a population of men who have sex with men, or heterosexuals with higher average levels of sexual behaviour. In this study, we have shown that notifying three or more partners from the preceding 18 months is expected to yield substantial numbers of new chlamydia cases. In contrast, the successful treatment of current partners is most important for preventing reinfection of index cases and reducing further transmission of chlamydia at the population level. In the next section we therefore focus on the effects of partner notification for the current or most recent sexual partner.
The effects of traditional and new partner notification technologies for Chlamydia trachomatis and Neisseria gonorrhoeae
Introduction
The second most commonly diagnosed bacterial STI after chlamydia is gonorrhoea (see Table 3). In the previous chapters, we investigated the effects of traditional technologies for partner notification for index cases with chlamydia only. We have assumed that notified partners will attend a health service setting and be tested for other STIs. New partner notification technologies, such as APT, which allow partners to collect treatment without attending a health service setting, might leave partners with undiagnosed STIs. It is therefore important to study the potential effects of cotesting and cotreatment for chlamydia and gonorrhoea.
An important observation for studying combined interventions against both chlamydia and gonorrhoea is the high rate of chlamydia infection in those who are infected with gonorrhoea. This is a consistent finding in studies of different populations and with different study designs. For example, Nsuami et al. 27 found that 41.4% of female and 46.7% of male students tested positive for both gonorrhoea and chlamydia in an urban school district in the USA where STI testing was offered systematically every year. In the US National Health and Nutrition Examination Survey, a nationally representative population-based study, the chlamydia co-infection rate was 45.7% in 14- to 39-year-old civilians with gonorrhoea infections. 26 Similar levels of co-infection are found in genitourinary medicine clinic attenders. 25 These rates are much higher than the population prevalence of chlamydia and even exceed the positivity rates of chlamydia in high-risk populations.
It is important to investigate potential mechanisms for chlamydia and gonorrhoea co-infection on the transmission dynamics in mathematical models. One hypothesis is that biological interactions between the two organisms account for the high rates of co-infection. If such interactions exist, then understanding them would enable us to improve guidelines for testing and treating a population for chlamydia and gonorrhoea. Classical models, where chlamydia and gonorrhoea transmission does not depend on the presence of another organism, generally fail to account for high co-infection rates. 106
Objectives
(1) To develop a dynamic transmission model of chlamydia and gonorrhoea infection in a heterosexual population and (2) to use the model to investigate the effects of APT and traditional partner notification technologies on STI prevalence, the frequency of gonorrhoea outbreaks and STI reinfection rates.
Methods
We examined epidemiological data to inform the development of a mathematical model in which the presence of one organism can increase the susceptibility and the transmissibility of the other. The deterministic, population-based model was then implemented in an individual-based manner using Rstisim and we studied basic aspects of the effects of different testing and treatment strategies in separate models that either assumed that chlamydia and gonorrhoea are transmitted independently or included an interaction between the organisms.
Co-infection with chlamydia and gonorrhoea
We found two recent studies that give some support to the hypothesis that there is a biological interaction between chlamydia and gonorrhoea infections. In one study, increased gonorrhoea organism loads were found in women who were co-infected with chlamydia. 107 The authors hypothesised that this could increase the chances of gonorrhoea transmission. In another, laboratory study, mice were infected with Chlamydia muridarum and gonorrhoea. The authors found that chlamydia-induced host alterations of the immune response could enhance gonoccocal infection. 108 Studying infections at the individual level is important to propose different mechanisms for interactions between the two infections. However, it is important to find out if the interaction hypothesis is supported by epidemiological data about co-infection.
Epidemiological data about co-infection
One of the most detailed studies on co-infections with chlamydia and gonorrhoea was done by Lycke et al. 6 The study comprised women and men attending two outpatient STI clinics in Goteborg, Sweden. The authors tested 155 women with 156 male sex partners and 211 men with 237 female partners for chlamydia and gonorrhoea. Index patients were infected with at least one of the organisms. The data in this study were used to calculate the risk of infection in sexual partners of the index patient. The observed levels of concordant infections suggest a lower risk of transmission of chlamydia than of gonorrhoea.
We then calculated the relative risk of being infected with one organism in partners who were infected with the other organism, compared with those who were not infected with the other organism (Table 13). There was statistical evidence that the risk of gonorrhoea infection was higher in partners who were chlamydia positive than those who were chlamydia negative in both women and men. The relative risk of chlamydia infection in partners who were gonorrhoea positive compared with those who were gonorrhoea negative was of a similar magnitude, but CIs included 1. These data are consistent with the hypothesis that the transmission of one infection is increased when the other infection is already present in the susceptible person (increased susceptibility), although there might be other explanations that could give rise to the observed pattern of co-infection.
| Having gonorrhoea in partners who are chlamydia positive compared with those who are chlamydia negative | |||
|---|---|---|---|
| Gonorrhoea positive, n | Gonorrhoea negative, n | Relative risk (95% CI) | |
| Female partner of gonorrhoea-positive male index case | |||
| Chlamydia positive | 38 | 8 | 1.27 (1.02 to 1.58) |
| Chlamydia negative | 43 | 23 | |
| Male partner of gonorrhoea-positive female index case | |||
| Chlamydia positive | 18 | 1 | 1.28 (1.07 to 1.52) |
| Chlamydia negative | 52 | 18 | |
| Having chlamydia in partners who are gonorrhoea positive compared with those who are gonorrhoea negative | |||
| Chlamydia positive, n | Chlamydia negative, n | Relative risk (95% CI) | |
| Female partner of chlamydia-positive index case | |||
| Gonorrhoea positive | 21 | 19 | 1.18 (0.83 to 1.66) |
| Gonorrhoea negative | 63 | 78 | |
| Male partner of chlamydia-positive index case | |||
| Gonorrhoea positive | 13 | 33 | 1.38 (0.73 to 2.60) |
| Gonorrhoea negative | 16 | 62 | |
Transmission dynamic model
We describe the transmission of chlamydia and gonorrhoea in a heterosexual population of 16- to 29-year-old women and men in Britain. Gonorrhoea transmission is disproportionately concentrated in a small group of individuals with high levels of sexual partner change. Therefore, we cannot assume a homogeneous population as in the previous chapters where we considered chlamydia transmission alone. Instead, we follow the structure from Hethcote and Yorke109 and Garnett et al. 37 and assume four different sexual activity classes. The sexual partner change rates for each risk class (ci) are parameterised with sexual behaviour data from Natsal-2. Maximum likelihood estimation is used to obtain the proportion of individuals in each sexual activity class, Ni, together with the corresponding partner change rate, ci. 92 Data for women and men are pooled (i.e. we assume the same sexual behaviour for both sexes). Assuming that the realised number of heterosexual partners during 1 year for individuals of risk class i follows a Poisson distribution with mean ci, the proportion of individuals in each sexual activity class, starting from the lowest activity, is 80.5%, 17.8%, 1.5% and 0.2%. The corresponding heterosexual partner change rates are 0.3, 2.35, 10.17 and 34.78 per year. Sexual mixing between individuals of different sexual activity classes can be varied between proportionate and fully assortative through a mixing coefficient, ε. 37 We chose ε = 0.1, i.e. close to proportionate (random) mixing, which ensures that the pattern of chlamydia and gonorrhoea infections among individuals with different number of sex partners matches observed data. 37,48
The infectious duration of gonorrhoea has not been established. Many gonorrhoea infections are symptomatic and short, but some last for several months. 37 We made the simplifying assumption that the average duration of gonorrhoea infection in women and men is 3 months. Whether or not spontaneous resolution of chlamydia infection is followed by a period of immunity is a matter of debate. 96,110 Assuming no development of immunity resulted in unrealistically high chlamydia prevalence rates in individuals with high sexual activity. Since this is not consistent with population-based data from Natsal-2,48,77 we assumed that spontaneous clearance of chlamydia infection is followed by an average duration of immunity of 1 year. This resulted in a distribution of chlamydia infections that closely resembles the one presented in Figure 12. We also assume 1 month of immunity after spontaneous resolution of gonorrhoea infection.
Per-partnership transmission probabilities are adjusted so that the endemic prevalence of chlamydia and gonorrhoea are 3%77 and 0.5%,26 respectively. The prevalence of gonorrhoea was estimated from the US National Health and Nutrition Examination Survey since there are no national population-based data for the UK. In the model variant where we assume that the two infections transmit independently, the per-partnership transmission probabilities for chlamydia and gonorrhoea are 38% and 62.5%, respectively. The assumed transmission probability for chlamydia is lower than that estimated in Modelling the transmission dynamics of C. trachomatis because we explicitly include individuals with high levels of sexual partner change, who are expected to have lower numbers of sex acts per partnership. In the ‘interaction model’ variant, the transmission probabilities for one organism are increased by a certain factor if the other organism is present in the person who transmits (transmissibility), or in the person who is at risk of the infection (susceptibility). The baseline transmission probabilities are then reduced slightly so that all models result in the same endemic prevalence of chlamydia and gonorrhoea.
Women and men stay in the population after 14 years on average and, on leaving, are replaced by new individuals. The newly arriving individuals in the two lowest risk classes are considered to start their sexually active life on entry to the model and are fully susceptible to both infections. In contrast, the newly arriving individuals in the two highest risk classes (1.7% of all individuals entering the population) are considered to represent imported cases who are co-infected with chlamydia and gonorrhoea. This assumption is necessary to prevent permanent extinction of gonorrhoea infections in the stochastic, individual-based implementation of the model.
The full model structure can be represented in the form of ordinary differential equations. A deterministic, population-based modelling approach does not, however, allow partner notification to be implemented or the stochastic effects of outbreaks to be investigated. We therefore implemented the full model structure in an individual-based modelling framework using Rstisim (see Individual- and population-level effects of partner notification for C. trachomatis), which allows individuals and partnerships be tracked so that partner notification can be implemented as an integral part of managing chlamydia and gonorrhoea infections.
Reinfection model
The probability of reinfection of index cases by their (as-yet) untreated partners can be calculated as follows:
where pp is the probability that the index case is in an ongoing sexual partnership and pi is the probability that the partner is infected. After the index case is successfully treated, she or he will engage in new sex acts with a frequency f where the infection can transmit at a per-sex act transmission probability β. Transmission can occur until the partner is in treatment after an average delay of 1/δ or the sexual partnership dissolves at rate σ.
Results
Co-infection with chlamydia and gonorrhoea
Figure 19 shows the predicted percentages of the model population infected with chlamydia and gonorrhoea. If chlamydia and gonorrhoea transmit independently (0% increase in susceptibility and transmissibility), 25.2% of those infected with gonorrhoea are also infected with chlamydia and the prevalence of gonorrhoea in those infected with chlamydia is 4.2%. These percentages are lower than those observed in the empirical studies. 26,27 The rates of co-infection increase, particularly for co-infection with chlamydia in those who are infected with gonorrhoea, when we increase the susceptibility and transmissibility in the model. If the risk of transmitting and acquiring one infection when the other infection is also present is increased by 50%, the predicted co-infection rate increases to 36.8% and 6.2%, respectively, and are more consistent with the observed data. 26,27
FIGURE 19.
Rates of co-infection with chlamydia and gonorrhoea. Increasing the susceptibility and transmissibility of one organism if the other organism is present results in an increase in co-infection rates, particularly the rate of chlamydia positivity in those who are infected with gonorrhoea.
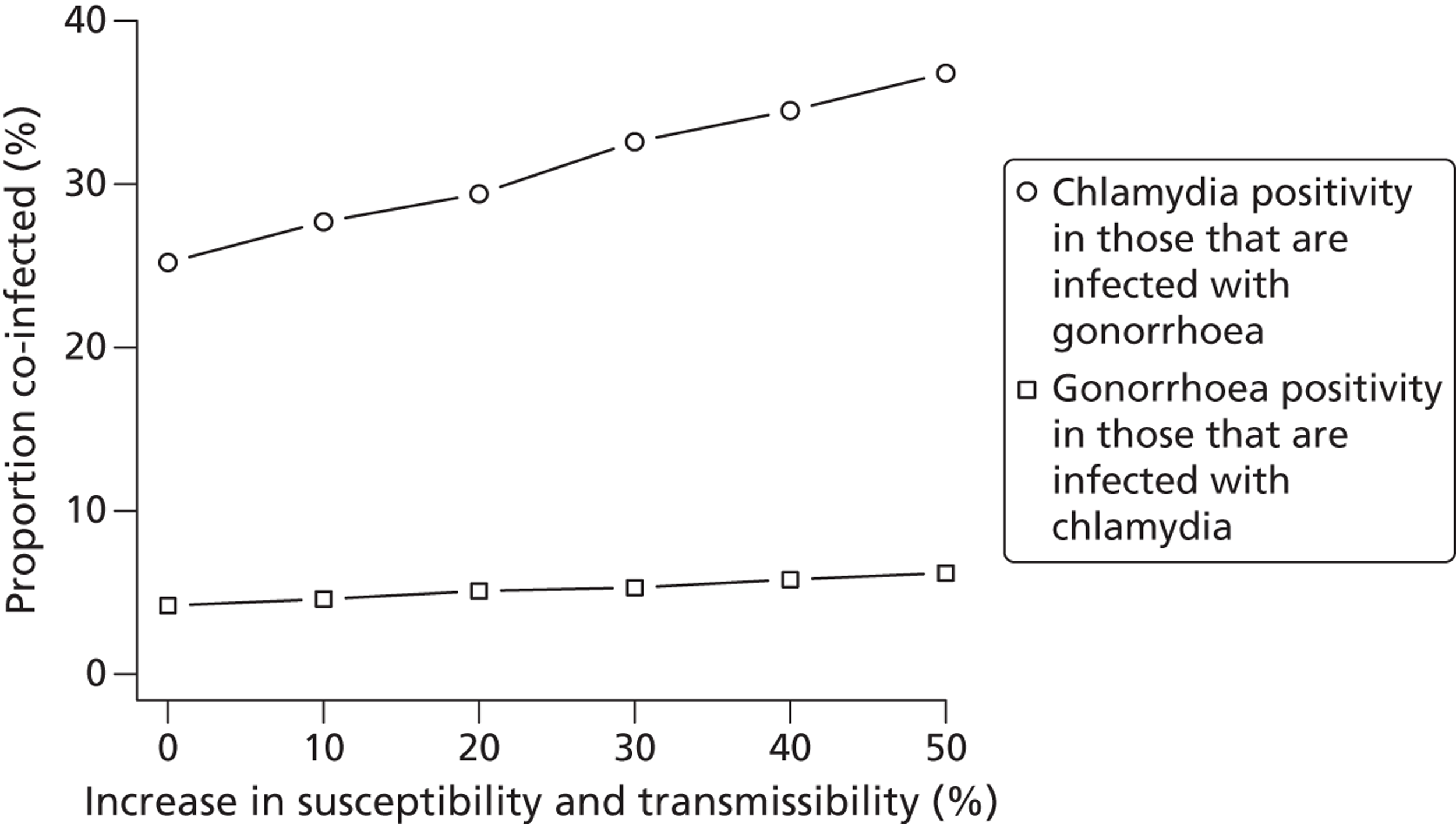
We consider two scenarios for investigating the effects of cotreatment for chlamydia and gonorrhoea: a scenario with no interaction between the two infections and a scenario in which the presence of the other organism enhances the transmissibility and the susceptibility of an infection by 50%.
Screening and partner notification for chlamydia and gonorrhoea
We investigated the effects of testing and treating index patients and their partners for chlamydia and gonorrhoea (Table 14). Partner notification takes place as an integral part of a screening strategy, which detects index patients. The combined intervention results in a reduction in the prevalence of chlamydia and gonorrhoea. The new steady-state prevalence is usually reached about 5 years after starting the intervention.
| Scenario | Treated successfully (%) | |||
|---|---|---|---|---|
| Index patient | Most recent partner | |||
| Chlamydia | Gonorrhoea | Chlamydia | Gonorrhoea | |
| 1: Current practice for chlamydia | 70 | 0 | 30 | 0 |
| 2: APT for chlamydia | 90 | 0 | 60 | 0 |
| 3: Routine testing for chlamydia and gonorrhoea | 70 | 70 | 30 | 30 |
| 4: APT for chlamydia and gonorrhoea | 90 | 90 | 60 | 60 |
The probability of successful treatment is estimated as the product of antibiotic treatment efficacy and the probability that the patient becomes reinfected by an untreated partner. APT is assumed to increase the probability of successful treatment by increasing the probability that the partner receives treatment, reducing the time to treatment of the partner and reducing the risk of reinfection in the index case and partner. Scenarios 1 and 2 (see Table 14) involve screening for chlamydia only with the addition of standard patient referral (scenario 1) or APT (scenario 2). In scenarios 3 (routine testing of partners for chlamydia and gonorrhoea) and 4 (APT for chlamydia and gonorrhoea), all individuals who receive screening are tested for chlamydia and gonorrhoea and, if positive, treated. If the test result yields at least one positive test, the most recent partners are also treated for chlamydia and gonorrhoea.
Figures 20a–d show the effects of the scenarios in the models with and without interactions and in the population overall and those in the highest risk class. With a screening interval of 5 years (screening rate 0.2 per year), scenario 1 (screening plus standard patient referral) can reduce the prevalence of chlamydia in the general population by about one-third (see Figure 20a). This is consistent with the expected effect of chlamydia screening (see Modelling the transmission dynamics of C. trachomatis and Individual- and population-level effects of partner notification for C. trachomatis). Scenario 2 (screening plus APT) results in slightly lower chlamydia prevalence than scenario 1. Interestingly, in the model where the infections co-interact, we observe a small reduction in gonorrhoea prevalence, although treatment (with or without testing) is performed only for chlamydia (see Figure 20c and d). Hence, interactions between chlamydia and gonorrhoea could potentially help to indirectly reduce the prevalence of gonorrhoea in a population that is tested and treated for chlamydia.
FIGURE 20.
Effects of screening and partner notification for chlamydia (a and b) and gonorrhoea (c and d), assuming that the average screening interval for all individuals is 5 years. The prevalence rates of four different scenarios are contrasted with those in absence of any intervention. Strategy 1, routine testing for chlamydia; strategy 2, APT for chlamydia; strategy 3, routine testing for chlamydia and gonorrhoea; strategy 4, APT for chlamydia and gonorrhoea. Overall (a and c): general population of 16- to 29-year-olds. High risk (b and d): individuals with 10 new heterosexual partners per year (1.5% of the general population).
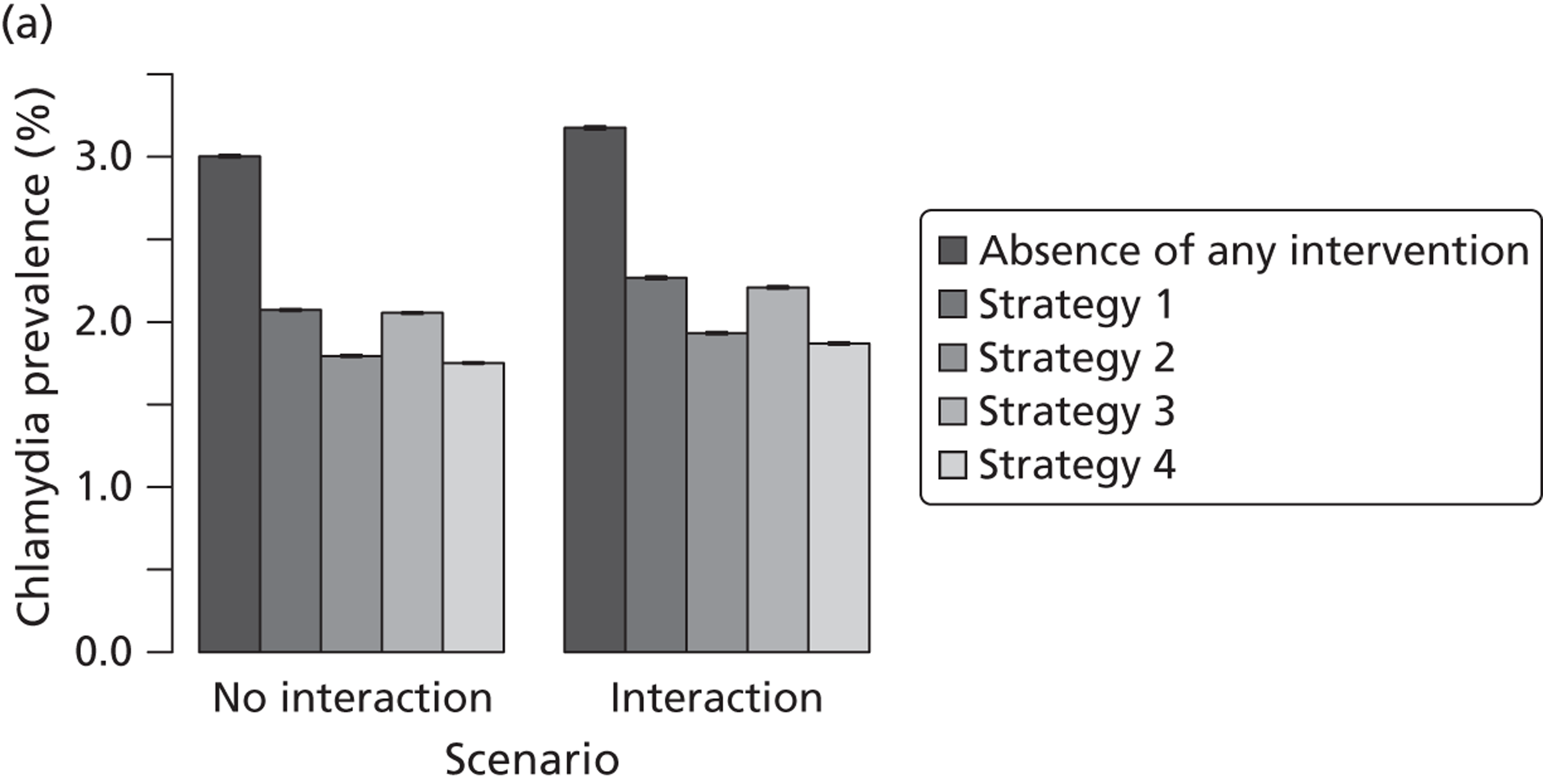
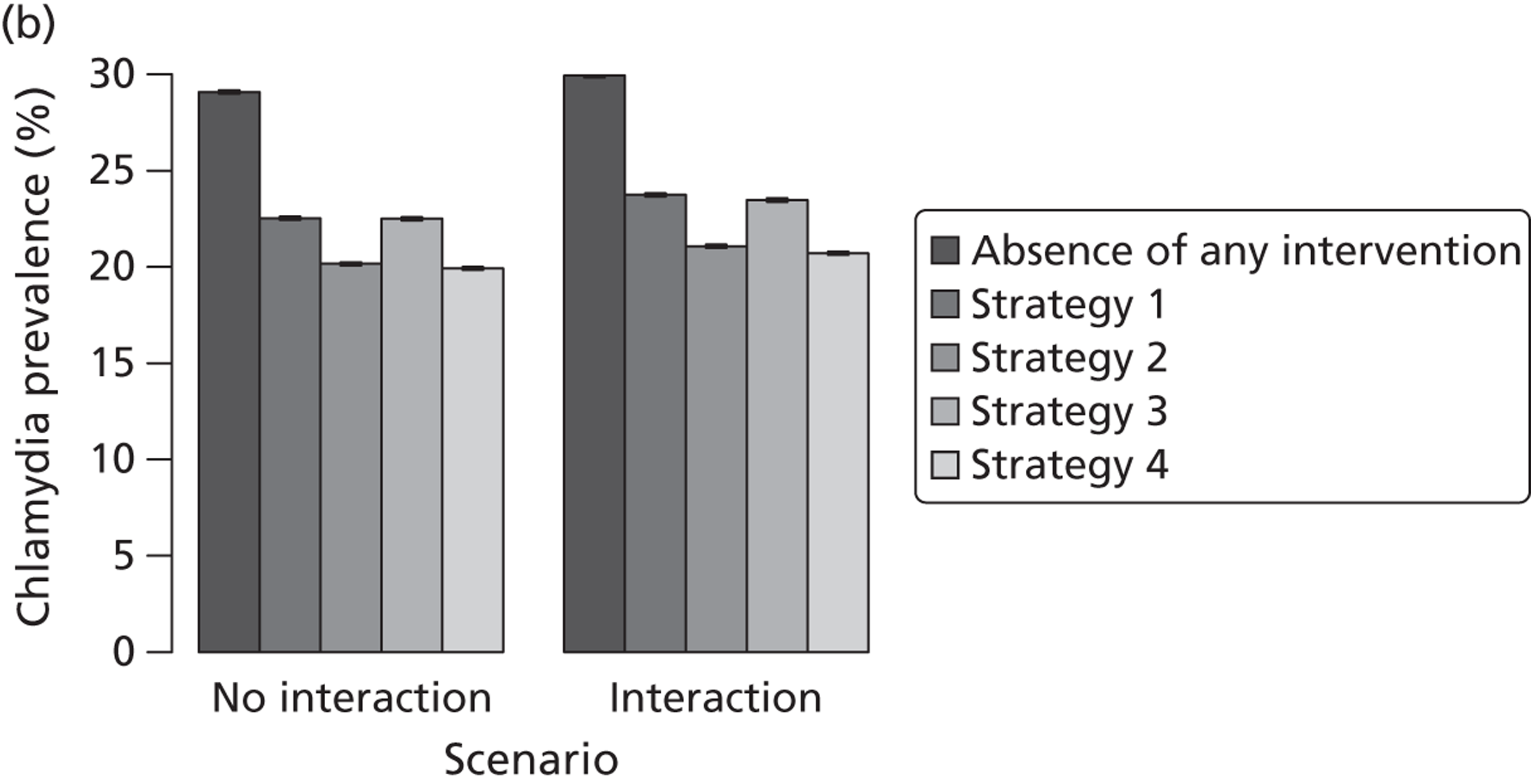
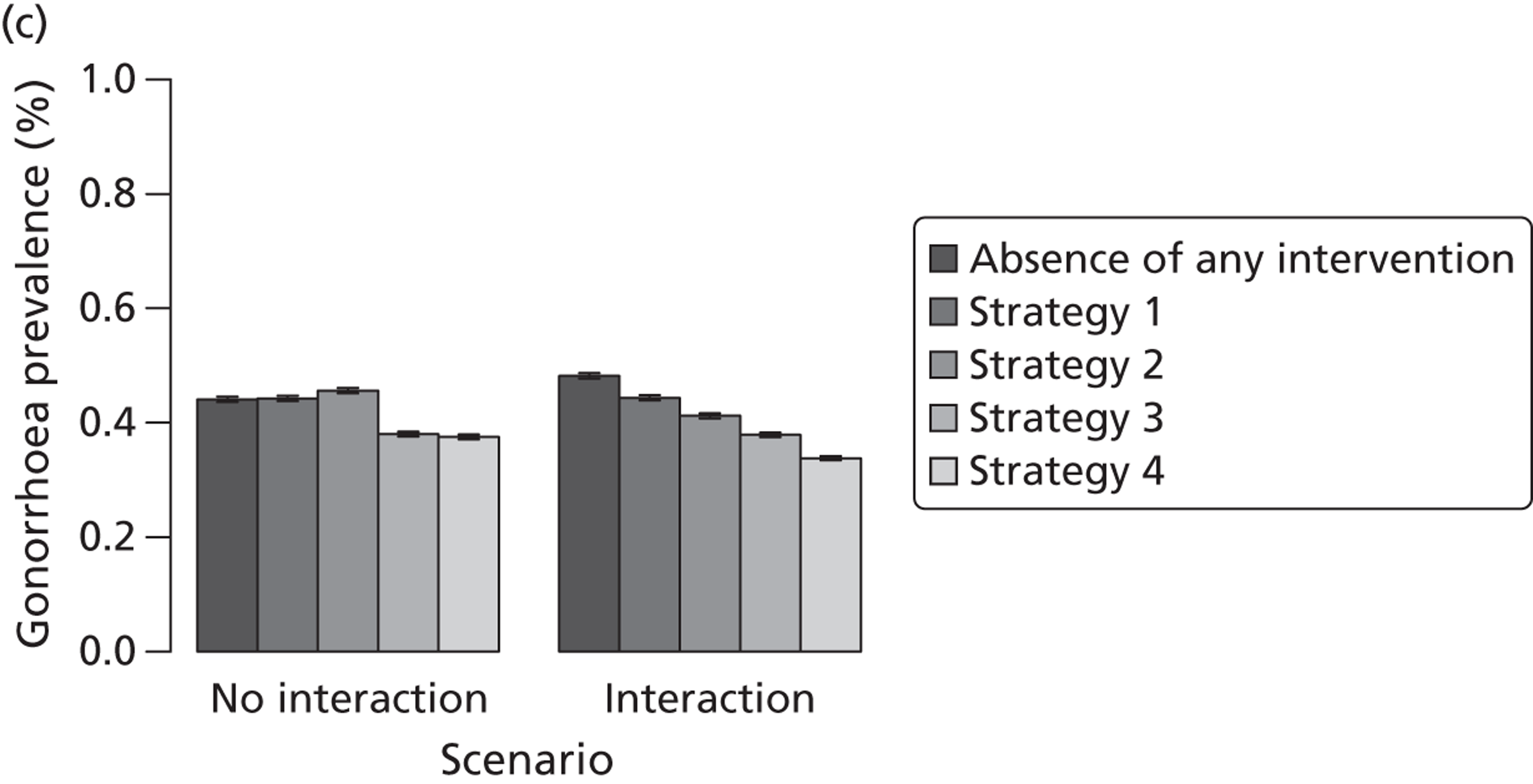
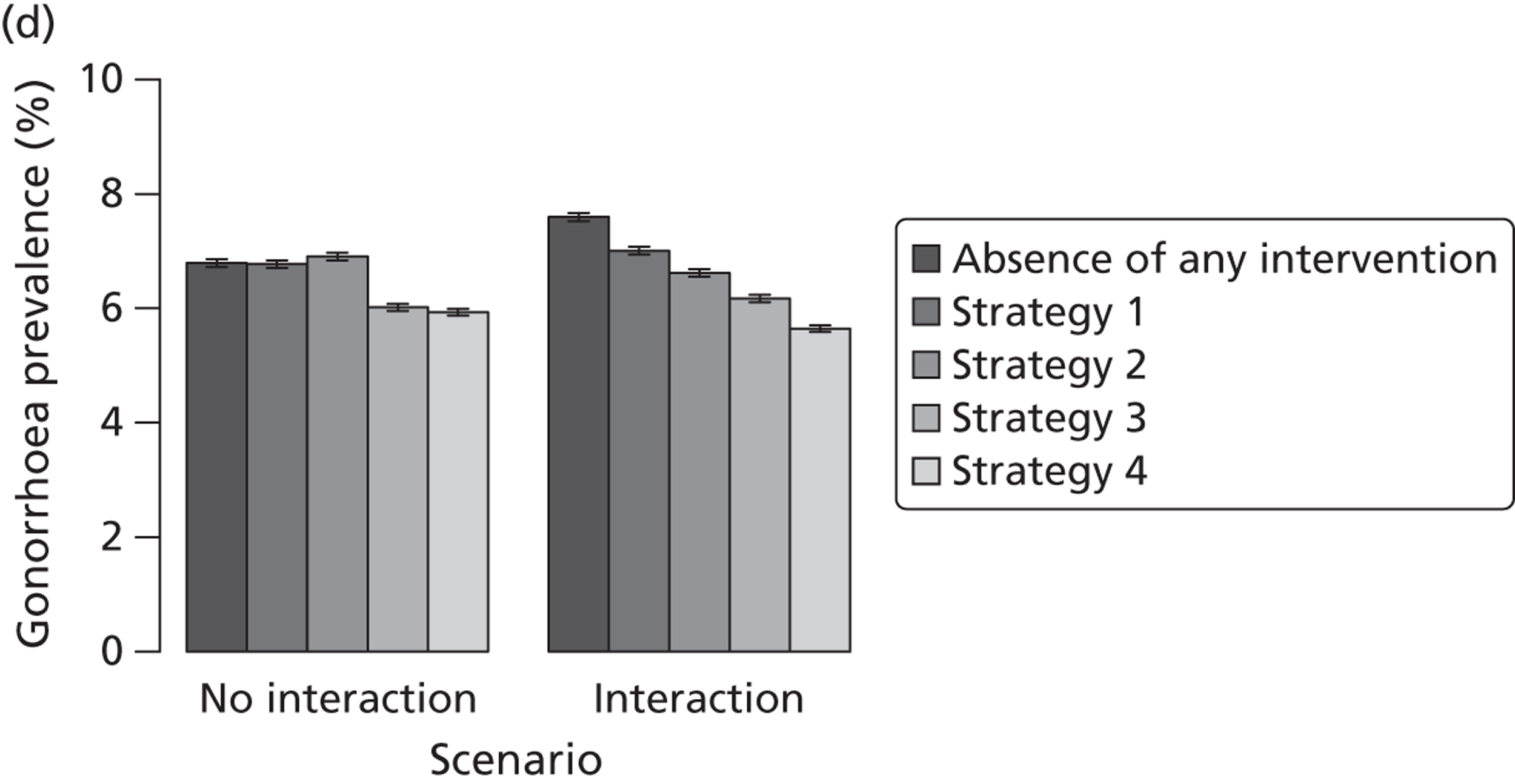
Routine testing for chlamydia and gonorrhoea (scenarios 3 and 4) further reduces the prevalence of gonorrhoea (see Figure 20c and d). Cotesting for gonorrhoea has a negligible impact on reducing the prevalence of chlamydia in the model where we assume interactions between the two infections (see Figure 20a and b), due to the low prevalence of gonorrhoea. APT (scenarios 2 and 4) always results in lower prevalence rates of chlamydia and gonorrhoea compared with standard patient referral (scenarios 1 and 3), particularly for chlamydia but also for gonorrhoea in the case where the two infections interact. This exemplifies the improved clinical effectiveness of APT over standard patient referral for bacterial STIs. Figure 21 shows the results of the same scenarios, assuming that individuals receive screening at intervals of 2 years. The pattern of results is the same but effects are stronger.
FIGURE 21.
Effects of screening and partner notification for chlamydia (a and b) and gonorrhoea (c and d), assuming that the average screening interval for all individuals is 2 years. The prevalence rates of four different scenarios are contrasted with those in absence of any intervention. Strategy 1, routine testing for chlamydia; strategy 2, APT for chlamydia; strategy 3, routine testing for chlamydia and gonorrhoea; strategy 4, APT for chlamydia and gonorrhoea. Overall (a and c): general population of 16- to 29-year-olds. High risk (b and d): individuals with 10 new heterosexual partners per year (1.5% of the general population).
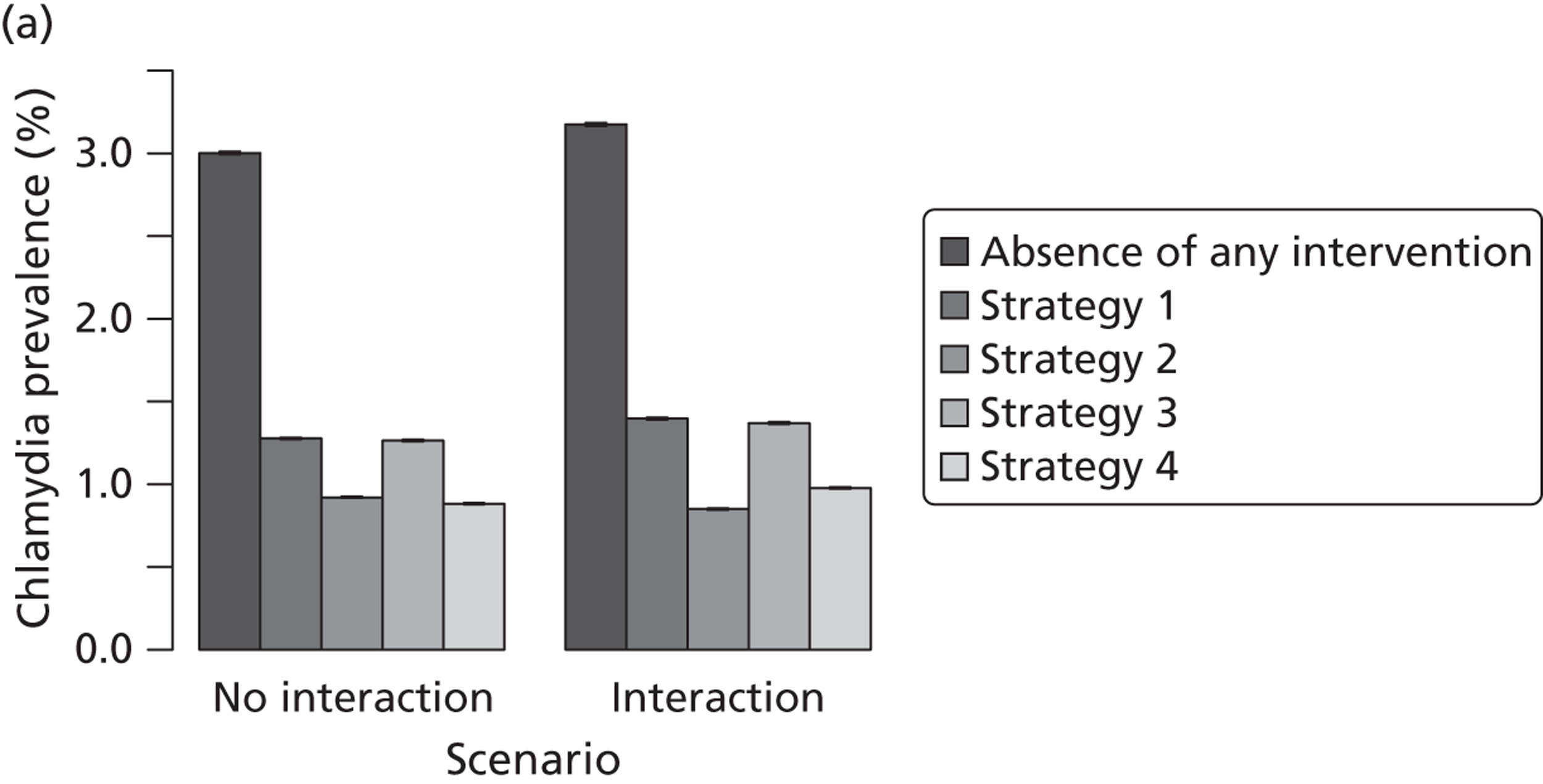
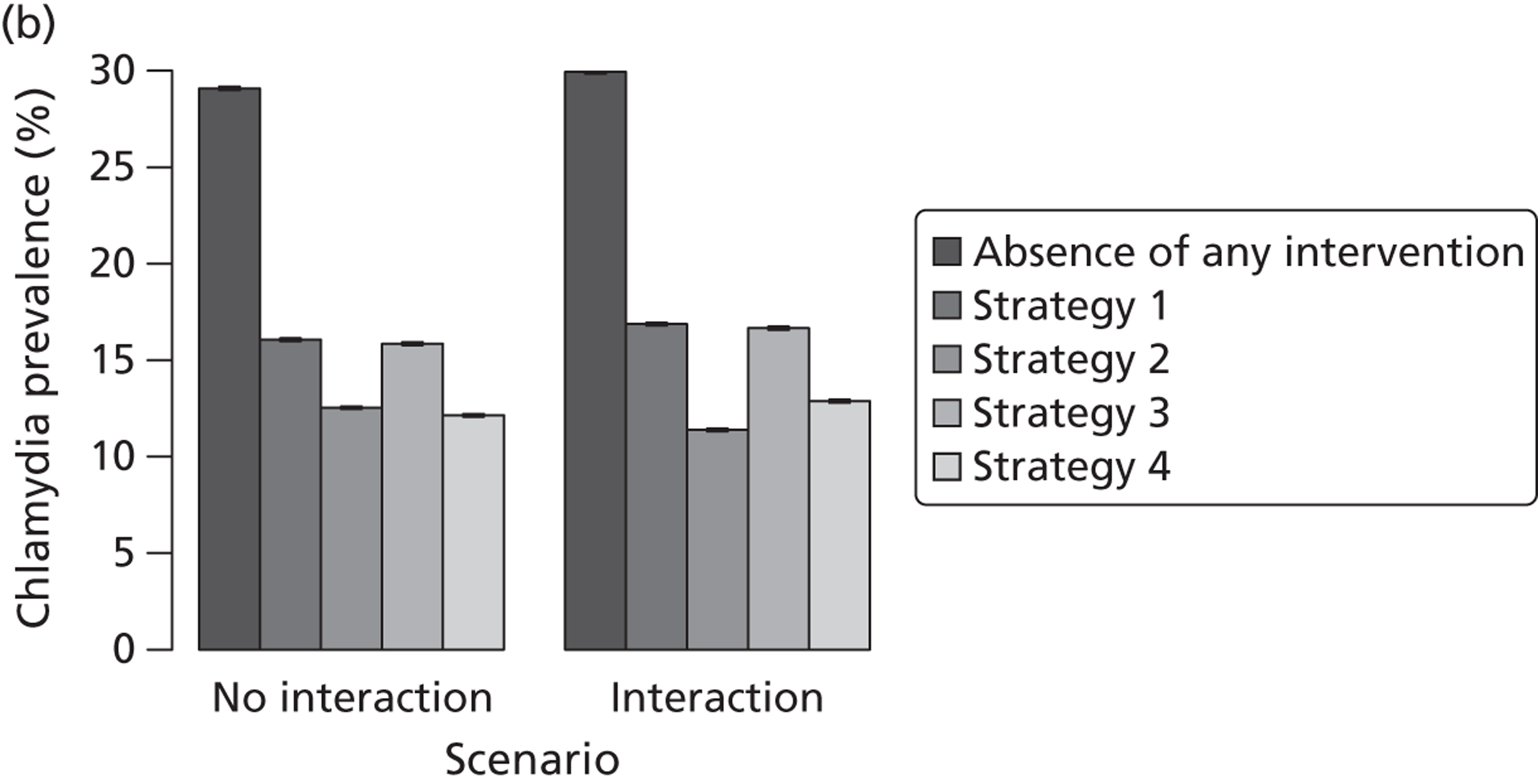
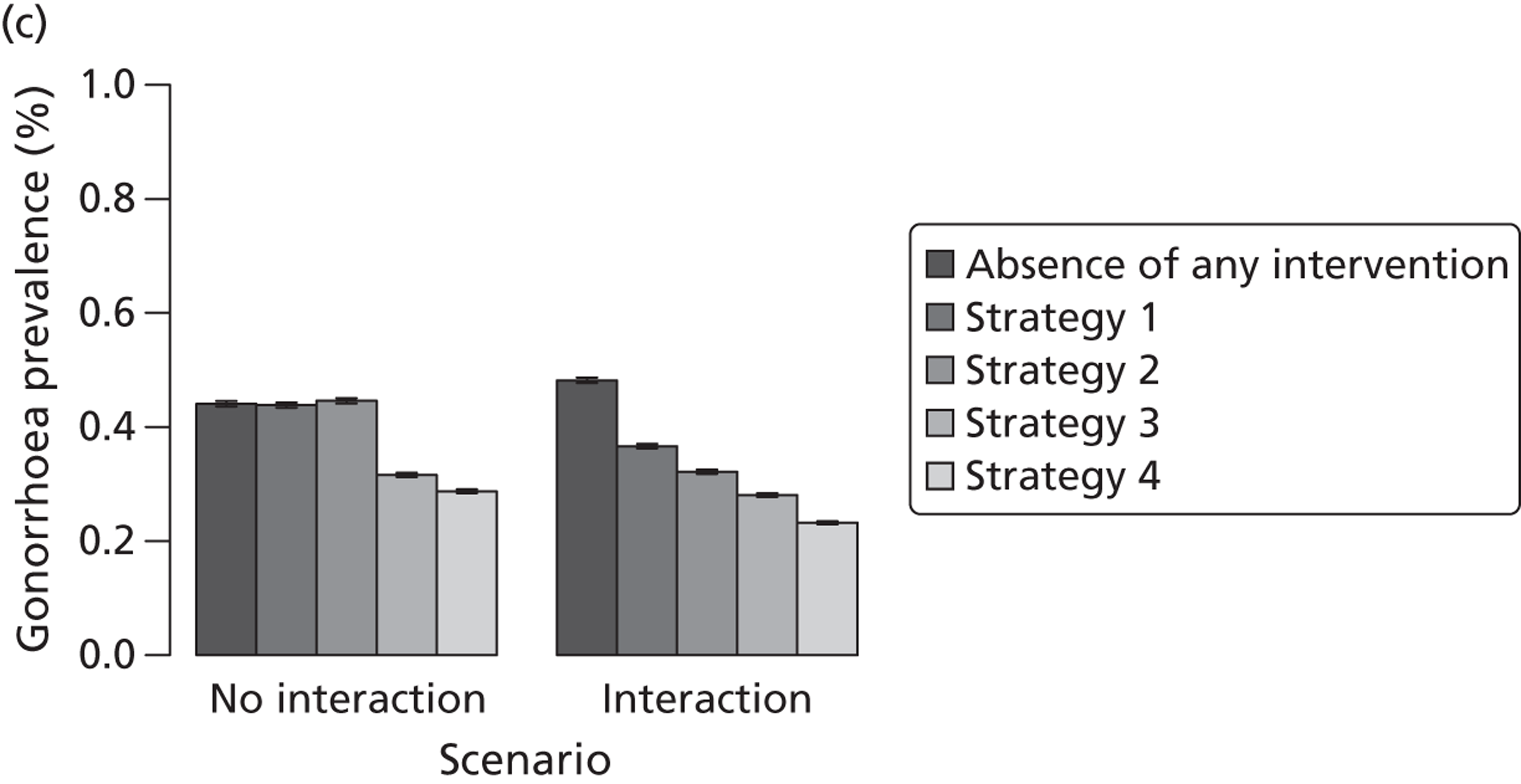
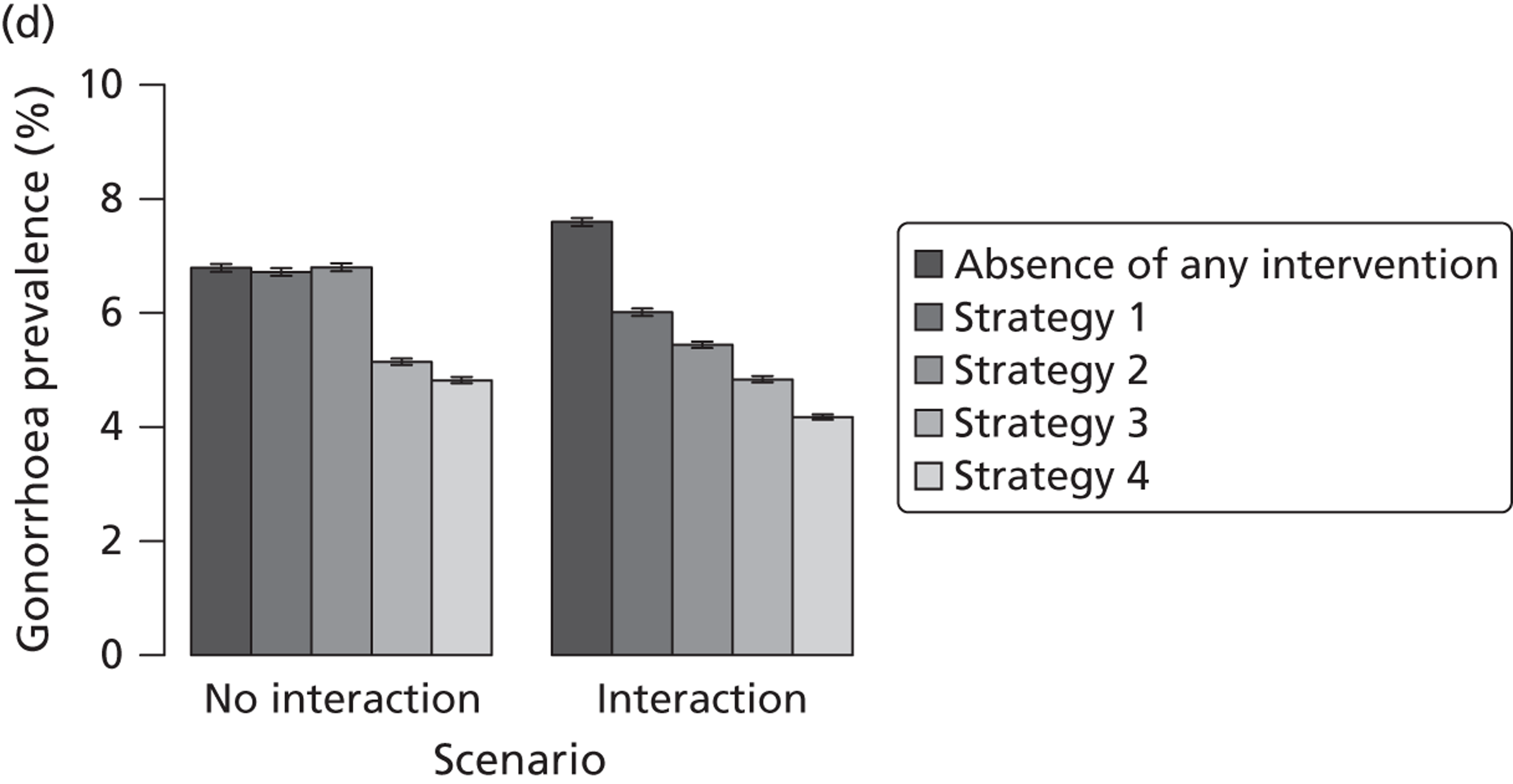
It is surprising that the prevalence of gonorrhoea cannot be lowered through widespread screening at intervals of 2 years (screening rate 0.5 per year). This goes counter to the usual observation that for STIs with very low endemic prevalence rates, only minor interventions can result in extinction. In our model, however, about one imported case per month is co-infected with chlamydia and gonorrhoea (in a total population of 10,000 individuals), which keeps gonorrhoea infections at an endemic level.
Gonorrhoea prevalence can be subjected to strong fluctuations with occasional outbreaks in a population. Partner notification strategies that provide treatment without STI testing might facilitate outbreaks. We calculated the yearly prevalence of gonorrhoea in the total population, and defined an outbreak when the prevalence exceeded 0.5%, which was the baseline prevalence in the model population (Figure 22). The frequency of outbreaks increased very slightly, compared with no intervention, only in the model that assumed no interaction between infections. In the model that incorporated an interaction, both APT and standard partner notification strategies resulted in a reduced frequency of gonorrhoea outbreaks. The greatest reduction in gonorrhoea outbreaks was found in the model in which the two infections interact and in which individuals are tested for chlamydia and gonorrhoea using APT (scenario 4).
FIGURE 22.
Outbreak frequency of gonorrhoea. (a) 5-year screening interval; (b) 2-year screening interval. Gonorrhoea prevalence is measured once a year and an outbreak is defined when the observed annual prevalence in the general population exceeds 0.5%. The prevalence rates of four different scenarios are contrasted to those in absence of any intervention. Strategy 1, routine testing for chlamydia; strategy 2, APT for chlamydia; strategy 3, routine testing for chlamydia and gonorrhoea; strategy 4, APT for chlamydia and gonorrhoea. Overall: general population of 16- to 29-year-olds. High risk: individuals with 10 new heterosexual partners per year (1.5% of the general population).
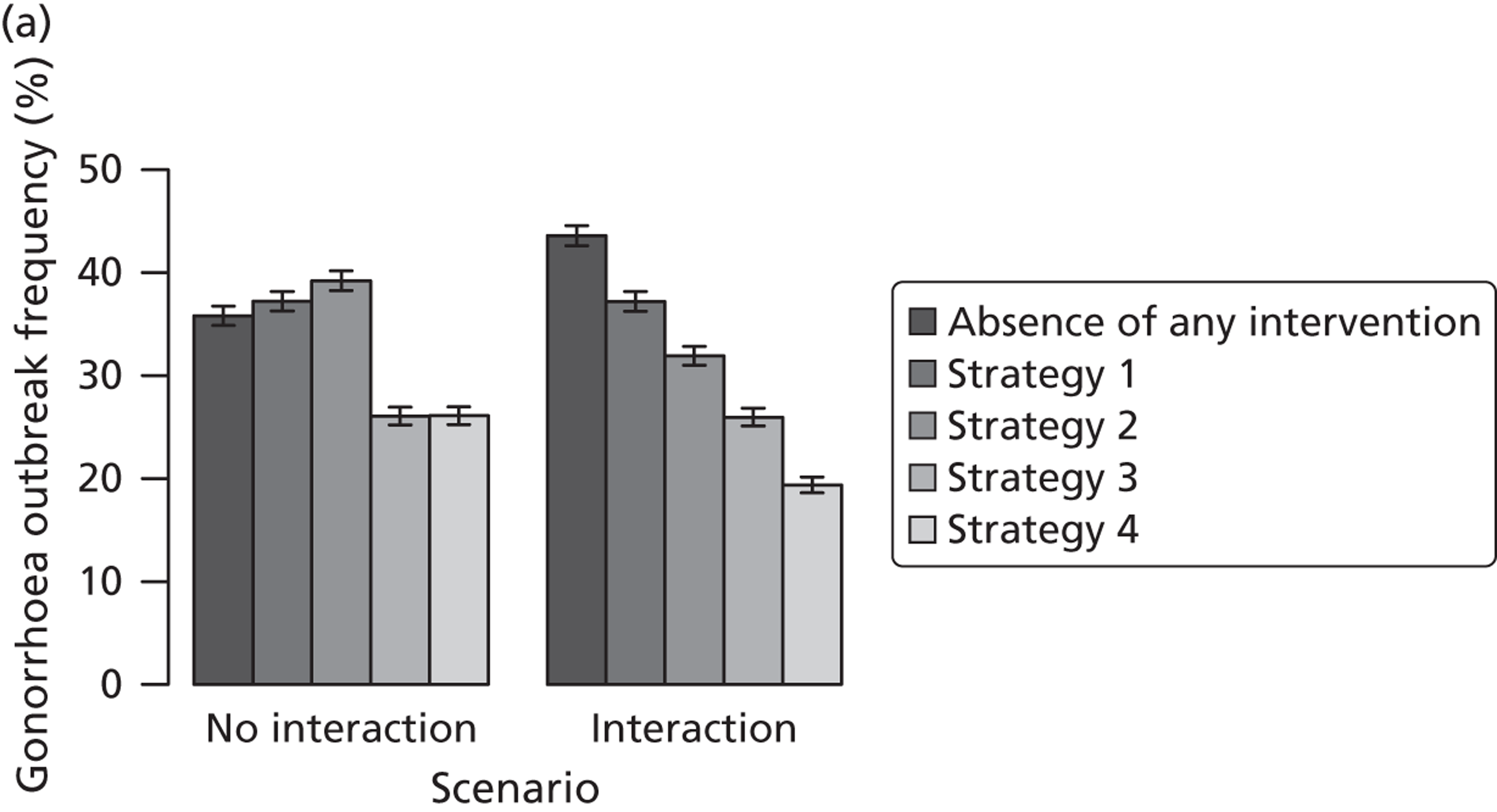
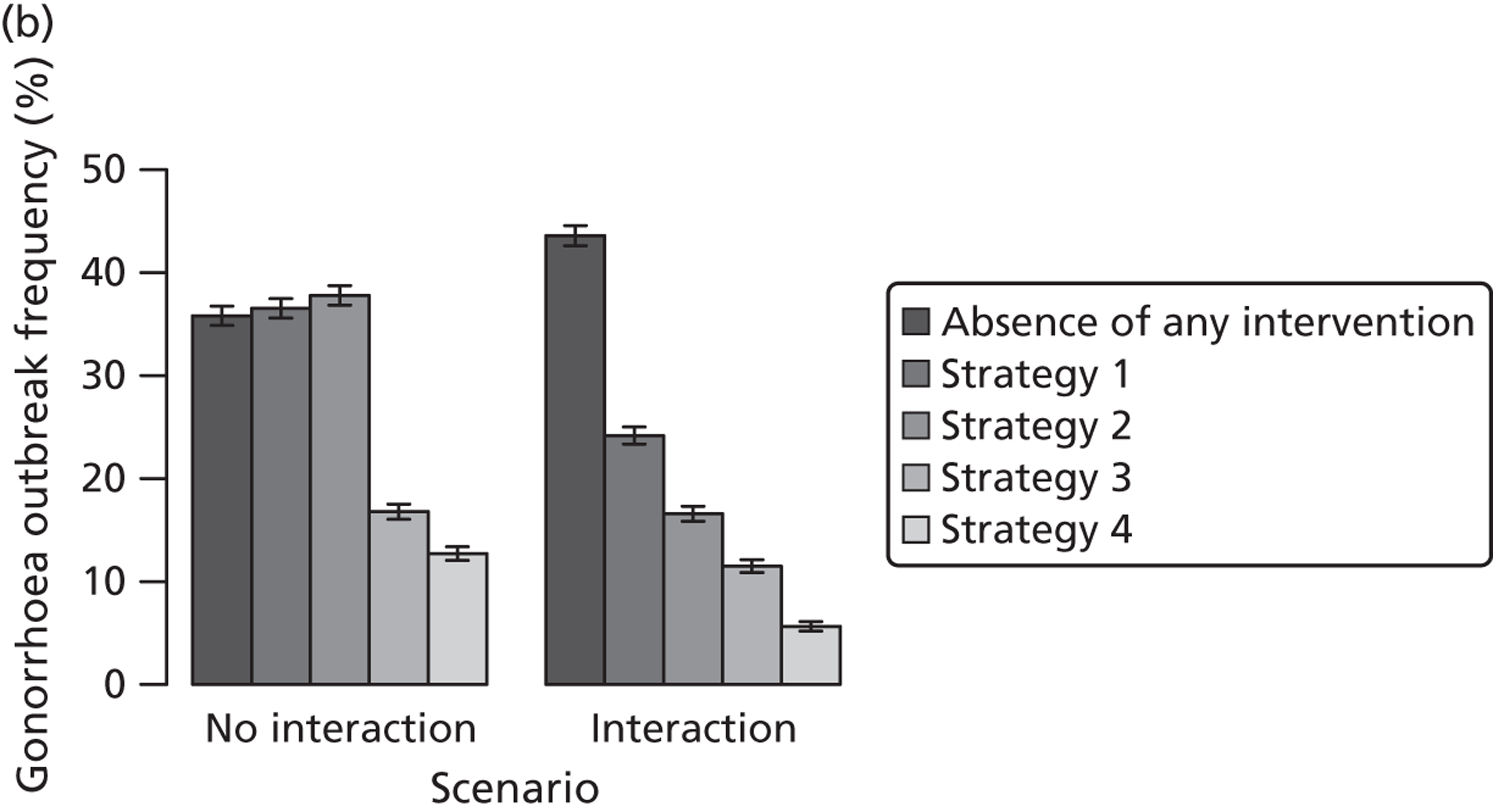
Reinfection of index cases
The transmission dynamic model assumes that sexual partnerships are instantaneous events. Therefore, we cannot directly investigate the outcome of reinfection of treated index cases by their untreated partners. Instead, we use a simplified model that investigates rates of reinfection with chlamydia and gonorrhoea only. We generated 1000 parameter sets by randomly drawing from the following distributions: the probability that index cases are in an ongoing partnership is uniformly distributed between 0 and 1; the probability that partners are infected with chlamydia is uniformly distributed between 60% and 70%;7 the probability that partners are infected with gonorrhoea is uniformly distributed between 70% and 80%;6 the frequency of sex acts is uniformly distributed between once a day and once a week; the per-sex act transmission probability is uniformly distributed between 6% and 16.7%;48 the per-sex act transmission probability for gonorrhoea is assumed to be twice that of chlamydia; and the duration of sexual partnerships is uniformly distributed between 1 week and 6 months. 48
We calculated the reinfection rates for chlamydia and gonorrhoea for different delays of partner referral (Figure 23). Reducing the delay between index case and partner treatment from 14 days111 to 1 or 3 days20 substantially reduces the rates of reinfection. For chlamydia, the median reinfection rates from the 1000 parameter combinations are 7.6%, 2.3% and 0.8% for decreasing delays of partner referral. For gonorrhoea, the corresponding rates are 14.6%, 5.4% and 2.1%.
FIGURE 23.
Reinfection rates of treated index cases by their untreated partners. (a) Chlamydia; and (b) gonorrhoea. The figures show box plots of 1000 different parameter combinations.
Discussion
There is some evidence from epidemiological and animal studies suggesting an interaction between chlamydia and gonorrhoea infections. 6,107,108 Observed co-infection rates cannot be obtained in mathematical models that assume that gonorrhoea and chlamydia are transmitted independently. The results from a model show that a potential interaction between the two organisms can influence the effect of testing and treating index cases and their partners for chlamydia and gonorrhoea. Overall, we found that cotesting and cotreatment for gonorrhoea can lower its prevalence in the general population as well as in individuals with high sexual activity. However, the level of reduction is not as pronounced as for chlamydia, which is partly explained by the ongoing import of new infected cases. In terms of partner notification, APT reduced infection prevalence more than standard patient referral. In addition, shortening the delay to partner treatment reduced rates of reinfection of treated index cases from their untreated partners.
To our knowledge, this study presents the first mathematical model of chlamydia and gonorrhoea infection that describes the observed co-infection rates. While biological co-interactions between chlamydia and gonorrhoea are a plausible mechanism, there might be other explanations that could explain the high rates of co-infection. For example, the specific group of individuals who are co-infected with chlamydia and gonorrhoea could be a subpopulation that has both frequent sex partner change rates and low usage of condoms. Chlamydia infections might be able to reach a very high prevalence in this kind of population. Also, highly assortative mixing by certain behavioural or demographic characteristics could increase rates of co-infection. These and other mechanisms should be further investigated with mathematical models to better understand the observed co-infection rates of chlamydia and gonorrhoea but also of other STIs such as syphilis and HIV.
We kept our modelling approach deliberately simple so that we could study general principles of the effect of interactions between chlamydia and gonorrhoea on the impact of interventions. First, we considered a general heterosexual population of 16- to 29-year-olds. Gonorrhoea infections and co-infection rates are higher in men who have sex with men than in the general heterosexual population, but should be studied in a separate model. Second, we ignored age structure in the level of sexual activity, but we believe that the four risk groups describe the heterogeneity in sexual activity and the transmission of chlamydia and gonorrhoea reasonably well. The model could be developed further by adding different ethnic or social groups among whom chlamydia and gonorrhoea rates vary. 43 Third, we ignored differences in disease-specific parameters among women and men; biological interactions between the two organisms could differ between the two sexes. These additional complexities will be important to consider in future modelling studies of the nature of chlamydia and gonorrhoea co-infection.
The findings from this study suggest that, if there is a strong biological interaction between chlamydia and gonorrhoea, treating chlamydia infections should help to reduce the overall prevalence of gonorrhoea. Although an association between trends of chlamydia prevalence and gonorrhoea prevalence has not been observed empirically, it might be possible to investigate whether or not gonorrhoea incidence rates have declined in populations that were regularly tested for chlamydia. The model results suggest that APT should be favoured over standard patient referral because it results in lower infection prevalence and can substantially reduce reinfection of index cases from their untreated partners. However, future work is required to obtain better estimates of the probabilities of successful treatment of index cases and their partners, given a specific partner notification technology. Furthermore, the risks of not testing for HIV in partners treated via APT should be explored in modelling studies. Finally, we found that a strategy of dual testing and treating in the general heterosexual population had a modest impact on reducing the prevalence of gonorrhoea. The model did not investigate the numbers of false positive test results, however, which might counteract the benefits, particularly in low prevalence settings.
Chapter 4 Economic evaluation of partner notification
The previous chapters present studies about the clinical effectiveness of partner notification technologies. In this chapter we present two studies examining different aspects about the economic evaluation of partner notification.
Outcomes for the economic evaluation of partner notification technologies: systematic review
Introduction
This section outlines the challenges of measuring the cost-effectiveness of partner notification using the outcome of cost per QALY. In a systematic review of economic evaluations of interventions to prevent chlamydia infections,112 we identified three studies published up to 2004 that evaluated partner notification. One presented the outcome as major outcomes averted113 and two reported short-term outcomes. 58,114 None reported cost per QALY.
Objective
To describe alternative approaches that have been used to assess and value the quality of life associated with the reproductive sequelae of chlamydia infection in women.
Methods
We conducted a systematic review of published literature. We followed a two-stage process for screening the results of electronic database searches, as in the earlier review. 112
Search methods
Six electronic databases were searched from 1980 up to 31 December 2011 [EMBASE; MEDLINE; Web of Knowledge ISI Proceedings; NHS Economic Evaluation Database (NHS EED); Database of Abstracts of Reviews of Effects (DARE); and HTA]. We also searched the reference lists of potentially relevant papers. Keywords included Chlamydia trachomatis, gonorrhoea, PID, cervicitis, ectopic pregnancy, economic evaluation, cost–utility analysis, cost-effectiveness analysis and quality of life. The search strategy is included in Appendix 3.
Inclusion and exclusion criteria
Participants were men and/or women undergoing any form of intervention to prevent chlamydia. Primary studies were those that measured HRQL for patients with chlamydia and associated sequelae, PID, ectopic pregnancy, infertility, chronic pelvic pain and epididymitis. Papers were excluded if they were not written in English.
Selection of studies
One investigator (PA) reviewed the titles and abstracts and a subset of these were checked independently against the inclusion criteria (TER). 115 One reviewer (PA) categorised studies according to the type of economic evaluation, based on their titles and abstracts. The full texts of potentially relevant studies were read and those that measured HRQL for patients with chlamydia and its associated sequelae were retained.
Data were extracted about study characteristics, study participant characteristics, health states examined, instruments used and results.
Data synthesis
Data were tabulated and the findings of individual studies compared narratively.
Results
The electronic database search identified 3534 published studies. From these, 1178 studies were duplicates. Titles and abstracts were reviewed for the remaining 2356 studies. Hand searching of bibliographies from published literature identified one additional study. Seventeen studies were considered potentially relevant. Of these, eight studies were excluded because they did not discuss the instruments and techniques available for eliciting the value of health states for avoiding chlamydia. No studies were excluded on the grounds of language. Figure 24 shows the flow of papers retrieved and included.
FIGURE 24.
Flow chart of included studies.
Characteristics of included studies
The nine included publications116–124 reported on eight separate studies measuring HRQL for chlamydia-associated sequelae in women. Ness et al. 124 reported the study design for the PID Evaluation and Clinical Health (PEACH) study, for which findings among women with and without chronic pelvic pain were reported by Haggerty et al. 117,122 There were no studies about epididymitis. There were no studies from Europe; only one study was done outside the USA. 121 Table 15 shows the characteristics of studies and participants.
| First author, year | Country | Perspective | Age (years) | Participant characteristics | Number |
|---|---|---|---|---|---|
| Haggerty 2003117 | USA | Patient | 14–37 | Pelvic pain after PID lasting ≥ 6 months | Pelvic pain, n = 202 |
| No pelvic pain, n = 345 | |||||
| Haggerty 2005122 | USA | Patient | 14–37 | PID | n = 780 |
| IoM 1999116 | USA | Society | Not reporteda | IoM committee membersa | Not reporteda |
| Kuppermann 2007119 | USA | Society | 31–54 | Non-cancerous pelvic problems in last 12 months | n = 1493 |
| Pelvic pain only | n = 272 | ||||
| Mathias 1996120 | USA | Patient | 18–50 | Pelvic pain lasting ≥ 6 months | Pelvic pain, n = 773 |
| No pelvic pain, n unclear | |||||
| Romão 2009121 | Brazil | Patient | 18–45 | Pelvic pain ≥ 6 months requiring treatment | Pelvic pain, n = 52 |
| No pelvic pain, n = 54 | |||||
| Smith 2008118 | USA | Patient | > 18 | History of PID | PID, n = 56 |
| No PID, n = 150 | |||||
| Trent 2011123 | USA | Society | 12–19 | Healthy adolescents | n = 134 |
| > 18 | Parents of healthy adolescents | n = 121 |
Instruments used for health-related quality-of-life measurement and health state valuation
Several different generic multi-attribute instruments, questionnaires or parts of questionnaires were used to measure HRQL (Table 16). Generic instruments have two components: a system to describe health or the impact on the quality of life and an algorithm to assign values to the descriptive system. 125 Health state descriptive systems are made up of a number of multilevel dimensions that uniquely describe health states. The scales used are transformed to scales that range from 0 to 100, with 100 denoting optimal functioning.
| First author, year | Complication | Instrument | Findings | Comment |
|---|---|---|---|---|
| Haggerty 2003117 | Chronic pelvic pain after PID | SF-36 | SF-36 physical health mean 65.1 (SD 13.4) | 5 days after enrolment with PID |
| Mean 75.8 (SD 15.7) | 32 months after PID | |||
| SF-36 mental health mean 60.8 (SD 15.0) | 5 days after enrolment with PID | |||
| Mean 68.1 (SD 16.6) | 32 months after PID | |||
| Haggerty 2005122 | PID | SF-36 | SF-36 physical health median 70 | At baseline PID diagnosis |
| SF-36 mental health median 68 | ||||
| IoM 1999116 | PID | HUI2 | QALY weight 0.46–0.83 | Different weights for scenarios from inpatient treatment with surgery to outpatient treatment only; duration 2 days to 4 weeks |
| Chronic pelvic pain | QALY weight 0.60 | 5-year lag after PID; duration remaining lifetime | ||
| Ectopic pregnancy | QALY weight 0.58 | 5-year lag after PID; duration 4 weeks | ||
| Infertility | QALY weight 0.82 | 5-year lag after PID; duration remaining lifetime | ||
| Kuppermann 2007119 | Pelvic pain only | SF-36, SF-12, questionnaire, TTO | SF-12 physical health mean 45 (SE 1) | Additional results presented for groups with other causes of non-cancerous pelvic pain |
| SF-12 mental health mean 43 (SE 1) | ||||
| Current health utility 0.83 (SE 0.01) | ||||
| Mathia 1996120 | Chronic pelvic pain | Questionnaire | General health mean 70.5 | Questionnaire based on a component of the MOS long form |
| Romão 2009121 | Chronic pelvic pain | Questionnaire | Physical health median, with anxiety 43 | Additional results for social and environmental domains and in women with and without depression |
| Median, no anxiety 57 | ||||
| Psychological health median, with anxiety 46 | ||||
| Median, no anxiety 71 | ||||
| Smith 2008118 | Ectopic pregnancy | SF-12, VAS, TTO | With PID VAS mean 0.55 (SD 0.20) | Additional results for women without PID, TTO in women with and without PID for all complications, and SF-12 in women with and without PID |
| Pelvic pain | With PID VAS mean 0.45 (SD 0.22) | |||
| Infertility | With PID VAS mean 0.53 (SD 0.29) | |||
| Trent 2011123 | Ectopic pregnancy | VAS, TTO | Adolescents VAS mean 55 (SD 25.4); parents VAS mean 73 (SD 23.8) | Additional results for TTO |
| Chronic pain | Adolescents VAS mean 48 (SD 25.4); parents VAS mean 61 (SD 23.8) | |||
| Infertility | Adolescents VAS mean 59 (SD 23.6); parents VAS mean 68 (SD 27.1) |
For HRQL measurement, four publications from three studies used only generic instruments: the PEACH study117,122 used the Medical Outcomes Study (MOS) Short Form questionnaire-36 items (SF-36); Smith et al. 118 used the Short Form questionnaire-12 items (SF-12); and the USA Institute of Medicine (IoM) study116 used the Health Utilities Index Mark 2 (HUI2) instrument. Kuppermann et al. 119 used a combination of generic instruments and questionnaires in different groups enrolled in their study (MOS SF-12, SF-36, MOS health distress scale, MOS sexual problems scale and a pelvic problem impact questionnaire). Mathias et al. 120 used a questionnaire including selected subscales from the MOS quality-of-life scales and questions about pelvic pain. Ramão et al. 121 administered questionnaires alone [WHO quality-of-life score and Hospital Anxiety and Depression Scale (HADS)].
Four studies used valuation techniques to elicit health state utilities. Kupperman et al. 119 used the time trade-off (TTO) metric to value women’s current health state with non-cancerous pelvic problems; Ramão et al. 121 used visual analogue scales (VASs) to assess intensity of pelvic pain; Smith et al. 118 and Trent et al. 123 used both VAS and TTO methods to value the same five PID-associated health states (see Table 16).
Only one study provided QALY weights. The study was commissioned by the IoM to develop an analytical framework for priority setting of vaccine research and development. 116 To estimate utilities, the experts incorporated quality-of-life weights from the Canadian National Population Health Survey and further estimated utilities based on expert opinion using the HUI2126 instrument for women who experienced chlamydia infection, its complications and the time spent in each state. The committee applied the resulting QALY weights to the estimated the number of adults they expected to be infected with chlamydia in that year. Of note, the HUI2 was developed to measure the global burden of childhood cancer and further adverse events as a result of cancer and its treatment. 126
Findings and assumptions about health states are not necessarily consistent between studies, especially when study designs differ. For example, in the cross-sectional IoM study,116 expert valuations resulted in a QALY weight of 0.60 for pelvic pain, lasting the rest of a woman’s life (see Table 16). In a cross-sectional study, Smith et al. 118 obtained similarly low utility values when asking women with and without PID to value a hypothetical scenario of chronic pelvic pain using VAS. They also assumed that chronic pelvic pain was a long-term health state. Haggerty et al. 117 found in a longitudinal study, however, that HRQL scores on the SF-36 improve over time in women both with and without pelvic pain after PID.
Valuations of health states also differed depending on the method used and the study population. Smith et al. 118 and Trent et al. 123 both found lower utility valuations with TTO than with VAS. Trent et al. 123 also found that adolescents, compared with parents, had significantly lower mean valuations for each health state.
Discussion
This systematic review found only one study that provides QALY weights for reproductive tract complications of chlamydia infection. No studies measuring HRQL or health state valuations among women in the UK or in the rest of Europe were found.
The main strength of this study is the comprehensive literature search. We do not think that our search strategy missed major studies. Nevertheless, the only study that reported QALY weights was published in a book chapter and was identified from reference lists of other studies and not from an electronic database search. Weaknesses of the study relate to difficulties in drawing conclusions from the small number of studies retrieved.
To our knowledge, this is the first systematic review to give a critical appraisal of methods used to elicit HRQL information for the economic evaluation of interventions for chlamydia prevention. The studies included in this review demonstrate many of the methodological debates about health state valuation. 125 We reviewed studies that collected data from patients, healthy adults and experts. Torrance et al. 127 suggest that judgements made by experts may be a starting point and are quick to collect. Experts might, however, have different views from those of patients. A major weakness of the IoM study was the lack of detail describing the elicitation process and the size, make-up and selection process of the expert panel, and so the potential for bias in the results cannot be assessed. Current NICE guidelines128 state that HRQL should be elicited directly from patients with the condition and the utilities should be based on public preferences using the European Quality of Life-5 Dimensions.
Partner notification works by reducing the duration of a STI, which is already a temporary health state and might be asymptomatic. Valuing temporary health states is challenging, particularly when most HRQL instruments have been designed to measure health states resulting from chronic or terminal diseases such as arthritis and cancer. This review did not identify any studies explicitly valuing the temporary health state of chlamydia infection in the absence of complications, although the two-stage approach used by Smith et al. 118 appears to have implicitly addressed this. Although STIs do cause physical and emotional damage, complications typically occur in the future and can also be temporary. Costs and benefits in the future are valued less than those occurring in the present, and so preventative interventions for curable STIs are less likely to appear cost-effective than those for ongoing chronic conditions.
Chronic pelvic pain might be easier to assess using currently available instruments. The valuations that we reviewed suggested a substantial detrimental impact on quality of life. 116 Scenarios describing chronic pelvic pain suggest, however, that it is usually very severe and long lasting. Evidence from longitudinal studies117 and from TTO studies118 suggests, however, that both the duration and the severity might have been overestimated. An additional challenge for estimating QALYs is that the fraction of chronic pelvic pain attributable to chlamydia and other STIs is unknown.
This review has implications for future research. HRQL data for chlamydia and its complications should be collected in the UK using appropriate instruments and study populations. Better QALY estimates for outcomes associated with chlamydia are also needed. The methods used to derive the IoM estimates are not transparent and do not meet current NICE guidelines. Based on the results of this systematic review, it was not possible to draw a firm conclusion about the most appropriate techniques for measuring HRQL and for valuing outcomes associated with chlamydia. We did not conduct a formal cost-effectiveness evaluation of partner notification because of the unresolved methodological challenges and the absence of appropriate evidence.
Costs and cost-effectiveness of different strategies for chlamydia screening and partner notification: an economic and mathematical modelling study
Introduction
Partner notification for chlamydia in community health service settings should take place as often as in genitourinary medicine clinics in England because of the large number of cases diagnosed through the NCSP. The standards for partner notification outcomes in the NCSP are the same as in UK genitourinary medicine clinics (see Clinical effectiveness of partner notification technologies: systematic review): 0.6 partners per index confirmed treated (0.4 in inner cities). 45 The number of partners treated per index, however, has fallen from a high of > 0.55 in 2006–7 to just under 0.4 in 2010–11. There is no standard protocol for providing partner notification services in community settings such as general practice and community Contraceptive And Sexual Health (CASH) services (also known as family planning clinics). Different areas operate with very different models of partner notification, ranging from minimal involvement of health-care professionals to active follow-up of index cases and their partners in order to confirm that partners have been treated. Chlamydia Screening Offices (CSOs), which are part of NCSP infrastructure in some areas, can provide more intensive support.
We have shown that chlamydia positivity rates of sexual partners of index cases with chlamydia are 5 to 10 times higher than in the general population (see Individual- and population-level effects of partner notification for C. trachomatis). Partner notification is therefore efficient for case finding. The UK National Audit Office published estimates of the cost of a chlamydia-screening test in 2009, using NCSP data from PCTs; these were updated in the chlamydia cost-guidance initiative. The National Audit Office reported an average cost per screen of £45 in 2009, although a wide range of costs was reported in different PCTs. 129 There were no published data on the cost of partner notification.
The coverage of chlamydia screening in all settings in 2011–12 was > 30% in women in all PCTs but < 20% in men. 130 Improving overall screening coverage will therefore depend on increasing the percentage of young men being screened. Given the renewed focus of the NCSP on effective management of positive individuals and their partners, monitored by the diagnostic rate indicator,130 information about the relative costs and cost-effectiveness of expanding screening coverage and partner notification services in the NCSP is needed.
Objectives
To improve estimates of the relative costs of opportunistic chlamydia screening and partner notification in the context of the English NCSP.
Methods
We used a simple spreadsheet model to investigate the costs of screening and partner notification for chlamydia. The spreadsheet was developed to help local areas explore their own situation within the context of the national picture and help them plan service provision for chlamydia screening and partner notification activities, based on NCSP data for 2008–9. 31 The model is freely available (www.bmj.com/content/342/bmj.c7250?tab=related#datasupp). The spreadsheet model 2008–9 estimates cost per individual tested; cost per positive diagnosis; total cost of screening; number screened; number infected; and sex ratio of those tested and treated.
For this project, we updated the spreadsheet using NCSP data for 2010–11. We then examined a range of scenarios, based on parameter estimates derived from other studies in this project (see Partner notification outcomes for chlamydia in UK genitourinary medicine clinics; Estimating the likely public health impact of partner notification for a clinical service: an evidence-based algorithm; and Modelling the transmission dynamics of C. trachomatis). For each scenario we compared the cost-effectiveness of two interventions using the cost per positive chlamydia case diagnosed: increased partner notification efficacy and increased coverage of primary chlamydia screening in men.
Summary of methods to estimate partner notification costs for programme areas
The costs of partner notification for chlamydia were estimated from an analysis of data collected for the costing guidance initiative of the NCSP. 131 In brief, staff from CSO teams and NCSP service providers in seven PCTs in England took part in semi-structured interviews in 2008–9 and provided data about partner notification outcomes in their areas. Three of these areas were selected as reflecting partner notification activities at low, medium and high intensity of clinical effort. Where data were missing or unavailable, assumptions were based on feedback from staff. In the modelled settings, partner notification was delivered by the co-ordinating CSO team and by the individual screening/treatment venues.
Costs from both sites were estimated and included in the model. To capture the costs of delivering partner notification in genitourinary medicine clinics (in practice, many positive clients were referred to for treatment and partner notification), a proportion of the national genitourinary medicine tariff was taken (estimated to be 33% of the treatment visit). 131 This was also done for general practice and pharmacy locally enhanced service payments if they were explicitly used for treatment as per their agreement. If a screening test was delivered through a CASH service, this was based on the block contract. National costs of delivering screening (marketing, co-ordination, etc.) were excluded.
The time taken for particular activities included direct and indirect clinical time (i.e. cost per minute) multiplied by the number of minutes for a particular activity. Consumable unit costs were also included (telephone, fax, etc.). The indirect and overhead costs of running CSOs as part of local NCSP infrastructure were also included in the total costs. Costs were estimated from the health-care provider perspective. The model uses a bottom-up approach to estimate the total cost of screening and partner notification activities. The costs for four steps were estimated for CSO involvement: initial contact with positive index case; provider referral; partners calling the CSO; and following up index cases and partners and for involvement of the screening venue. The costs are given in Table 17. The cost of partner notification per index case in the high intensity setting was highest (£27) and was applied in all scenarios as a maximum.
| Low intensity | Medium intensity | High intensity | ||||
|---|---|---|---|---|---|---|
| Cost (£) | Proportion of positives (%) | Cost (£) | Proportion of positives (%) | Cost (£) | Proportion of positives (%) | |
| CSO | ||||||
| Initial contact with positive index client | 2.76 | 100 | 2.16 | 100 | 3.17 | 100 |
| Provider referral | NA | NA | 16.64 | 4 | 19.73 | 5 |
| Partners call in | NA | NA | 12.67 | 10 | NA | NA |
| Follow-up positives | NA | NA | NA | NA | 7.85 | 100 |
| Follow-up partners | NA | NA | NA | NA | 27.37 | 5 |
| Other sites | ||||||
| CASH | 4.67 | 75 | 9.62 | 30 | 10.84 | 24 |
| General practice | 10.42 | 3 | 6.94 | 5 | 6.94 | 9 |
| Pharmacy | NA | NA | 2.50 | 1 | NA | 2 |
| Genitourinary medicine | 45.67 | 5 | 45.67 | 1 | 45.67 | 14 |
| Brook young people’s clinics | NA | NA | 15.85 | 33 | 11.56 | 36 |
| Othera | NA | 4 | NA | NA | NA | 11 |
| No partner notification | NA | 13 | NA | 30 | NA | 4 |
| Total cost per partner per index case | 9.01 | 12.90 | 26.96b | |||
Our definition of effective partner notification is the number of partners appropriately treated (i.e. treated presumptively, or tested negative, or known to have been treated already) per index case. We chose this definition to match the NCSP definition as closely as possible. The partner notification technologies used in practice were a mixture of patient referral, enhanced patient referral and provider referral, but the specific arrangements varied between clinics.
Summary of results 2008–9
The published paper presented data for 2008–9. 31 In 2008–9, the cost of screening was estimated at about £46.3M in total, and £506 per infection treated. The model results suggest that increasing partner notification efficacy from the baseline 0.4 partners per index case to 0.8 partners would cost £3.3M at a cost per infection diagnosed of £449. In contrast, increasing male screening coverage from the baseline value of 8% to 24% (to match female coverage) would cost an extra £22.9M and increase the cost per infection treated to £528. Increasing screening coverage to 24% in men would cost over six times as much as increasing partner notification to 0.8 but treat only twice as many additional infections.
Updated data for costing tool
The costing tool was updated to include a calculation of overall coverage of screening and the diagnosis rate per 100,000 target population. This is a new indicator, developed at the Health Protection Agency (now Public Health England) for monitoring NCSP performance, and is likely to become one of the quality outcome indicators. The diagnosis rate is calculated as (number of diagnoses/total target population) × 100,000; overall coverage is calculated as number of screening tests done/total target population.
Update of results for 2010–11
In 2010–11 the reported coverage of chlamydia screening in NCSP settings in England was 25% (35% including tests in genitourinary medicine clinics) compared with 16% in 2008–9 (Table 18). 132 The percentage of positive tests in all non-genitourinary medicine clinic settings was 7.3% in 2008–9 in both sexes. 133 The chlamydia positivity in 2010–11 was 4.6% in men (PCT range 1.5–13.1%) and 5.6% in women (PCT range 2.8–9.4%). The number of partners notified per index was 0.4 and 89% of positive index cases received documented treatment.
| Assumptions 2010–11a | Women | Men | Total |
|---|---|---|---|
| Target population (aged 15–24 years) | 3,442,300 | 3,442,300 | 6,884,600 |
| Coverage | 33% | 17% | 25% |
| Number screened | 1,129,035 | 597,465 | 1,726,500 |
| Number diagnosed | 63,226 | 27,483 | 90,709 |
| Number identified by partner notification who receive appropriate care (partner notification efficacy 0.4) | 10,993 | 25,290 | 36,283 |
| Number identified by partner notification who are infected | 7146 | 16,439 | 23,584 |
| Cost of screening and partner notification combined, £M | 50.5 | 29.0 | 79.5 |
| Cost per positive, £ | 718.1 | 659.4 | 695.6 |
| Positivity (combined partner notification and screen) | 6.2% | 7.1% | 6.5% |
| Proportion of prevalent infections treated (assuming 5% prevalence in 15- to 24-year-olds) | – | – | 33.2% |
| Percentage of total budget used for partner notification | – | – | 5.2% |
| Ratio women to men tested | – | – | 1.83 |
| Ratio women to men infected and treated | – | – | 1.60 |
Table 18 shows the baseline costs, estimated in 2008–9, applied to NCSP coverage and positivity data in 2010–11, assuming 100% index treatment. The total estimated cost would have been £696 per infection treated (including those treated through partner notification). This rise in the cost per positive reflects the higher number of tests but lower positivity observed in 2010–11 compared with 2008–9. We optimistically assumed that all index cases were treated, even if they were not documented; however, if the reported failure of treatment in 11% of cases represents positives who did not receive their results and treatment, this means that almost 10,000 index cases (plus their partners) did not receive treatment and the screening effort was totally wasted. This would increase the cost per positive identified to £782.
Scenario analysis
Table 19 shows additional scenarios, using a range of estimates for partner notification efficacy (number of partners treated per index; values used 0.4, 0.6, 0.9, 1.2); percentage of partners infected (0.5, 0.65, 0.7); and screening coverage [proportion of target population screened in year (men 8%, women 24%; both 20%, both 30%)].
| Scenario number | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter combination | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| PN efficacya | 1.2 | 0.9 | 0.9 | 0.6 | 0.4 | 0.4 | 0.6 | 0.6 | 0.4 |
| Partner positivity | 50% | 50% | 65% | 65% | 65% | 70% | 70% | 70% | 70% |
| Screening coverage | 20% | 20% | 20% | 20% | 20% | 20% | 20% | 16%b | 30% |
| Total cases identified through PN | 92,273 | 69,205 | 69,205 | 46,137 | 30,758 | 30,758 | 46,137 | 36,582 | 46,137 |
| Total infected and treated (screening plus PN) | 123,031 | 111,497 | 121,877 | 106,883 | 96,887 | 98,425 | 109,190 | 86,576 | 147,637 |
| Total cost of screening, £M | 36.9 | 36.9 | 36.9 | 36.9 | 36.9 | 36.9 | 36.9 | 29.5 | 55.4 |
| Total cost of PN, £M | 10.5 | 7.9 | 7.9 | 5.3 | 3.5 | 3.5 | 5.3 | 4.2 | 5.3 |
| Total cost of PN plus screening, £M | 47.4 | 44.8 | 44.8 | 42.2 | 40.4 | 40.4 | 42.3 | 33.7 | 60.6 |
| Cost per infection treated, £ | 385.43 | 401.71 | 367.50 | 394.45 | 417.05 | 410.53 | 386.11 | 389.14 | 410.53 |
| Proportion of prevalent infections treated | 40.0% | 36.3% | 39.6% | 34.8% | 31.5% | 32.0% | 35.5% | 28.2% | 48.0% |
| Diagnosis rate (screening only) | 1250 | 1250 | 1250 | 1250 | 1250 | 1250 | 1250 | 991 | 1875 |
| Diagnosis rate (PN plus screening) | 2000 | 1813 | 1982 | 1738 | 1575 | 1600 | 1775 | 1408 | 2401 |
Any combination of parameters can be fed into the model: a small selection of parameter combinations are presented here as examples. In scenarios 1–7 the underlying screening coverage remains constant, and as the partner notification efficacy or partner positivity increase, the total number of infections treated increases. Hence, the total diagnosis rate increases and the cost per positive decreases. Increasing either partner notification efficacy or partner positivity reduces the cost per positive case identified. As partner positivity has been shown in modelling studies to be insensitive to the underlying prevalence, efforts to improve partner notification efficacy are likely to result in greater cost-effectiveness (i.e. reduced cost per positive case identified regardless of other infection dynamics).
In general this model suggests that it is more cost-efficient to increase partner notification for chlamydia positive patients rather than screening greater numbers of low-risk individuals. For example, increasing the mean partner notification efficacy from 0.4 to 0.9 (scenarios 5 and 3) would be expected to increase the overall diagnosis rate from 1575 to 1982: a 26% increase.
Discussion
The spreadsheet model suggests that increasing the efficacy of partner notification costs less per new positive case diagnosed than increasing the coverage of chlamydia screening in men or in both sexes. The conclusions are unchanged when using updated data from the NCSP for 2010–11 and applying a range of parameter estimates for different components of the model.
A limitation of this simple static model is that it is not possible to estimate long-term cost-effectiveness within this model framework; that would require a transmission dynamic model. We took a conservative approach to determining the relative cost-effectiveness of partner notification by using the highest estimated cost of providing partner notification services. We also intentionally excluded the effects of partner notification on preventing reinfection in treated index cases. Including lower costs of partner notification and the costs averted by preventing reinfection would make partner notification more cost-effective. We took the cost perspective of the health service and restricted ourselves to consideration of the costs of screening and partner notification, and excluded patient costs, the costs of reinfection and the cost of complications arising from the initial infection.
Of note, as long as the positivity of those screened opportunistically remains proportionately much lower than that of partners of infected persons (e.g. ∼5% screen positivity vs. ∼30–60% partner positivity), investing in effective partner notification is a good use of resources. A modelling study has shown that the proportion of partners of an index case who are infected with chlamydia is consistently about 30%. This proportion is insensitive to the underlying population or network prevalence. In contrast, the positivity in those screened is sensitive to the underlying prevalence in the population, changing in line with changes to underlying prevalence.
As the number of index cases is small compared with the number screened, the cost of partner notification is a fraction of the total cost of screening. This remains true even if we make pessimistic assumptions about the cost of partner notification. In fact, partner notification can probably be done effectively at very low cost if most notifications are managed through patient referral (with appropriate motivation and support) plus effective use of web and text-messaging technologies to provide results and information to patients and their partners as well as efficient monitoring and follow-up of outcomes.
There are, however, implications and challenges for services implementing the NCSP. There can be confusion about the definition of partner notification outcome measures and denominator populations. Better information and support in providing these data would improve the quality of monitoring outcomes of partner notification in the NCSP.
In Chapter 3 (see Individual- and population-level effects of partner notification for C. trachomatis), we showed that the most recent partner is the most likely to be infected and the probability declines with time since end of partnership. In addition, we have shown that infected casual or irregular partners are more likely to transmit the infection to other people (see Chapter 2, Estimating the likely public health impact of partner notification for a clinical service: an evidence-based algorithm). 9 Therefore, even if benefit to the index case of treating past partners is limited, the wider population gains more benefit. In this model, we do not distinguish between types of partnership, but it seems likely that increasing the effectiveness of partner notification will result in a reduction in partner positivity as more non-regular partners are included.
Improving the diagnosis rate will be a key indicator of programme performance and monitoring. We demonstrate that significant improvements can be made to this outcome measure through improved case management and partner management.
Chapter 5 Discussion and conclusions
This monograph presents the results of systematic reviews, secondary data analysis, and static and dynamic modelling studies, which address different aspects of the clinical effectiveness and cost-effectiveness of traditional and new partner notification technologies for curable STIs. Here we summarise and synthesise the findings of these studies and give recommendations for future research.
Summary of findings
A systematic review and meta-analysis of randomised controlled trials of the effectiveness of different partner notification technologies (see Chapter 2, Clinical effectiveness of partner notification technologies: systematic review) provide strong evidence that EPT is more effective than simple patient referral in reducing reinfection in the index cases with curable STIs (RR 0.71, 95% CI 0.56 to 0.89) and there was no evidence that EPT was better than enhanced patient referral (RR 0.96, 95% CI 0.60 to 1.53). 44 Findings about the effect of different technologies on intermediate outcomes of partner notification were inconsistent. The evidence was insufficient to determine which method of enhanced patient referral was most effective in particular settings, or to compare the impact on other outcomes including longer-term sequelae in index patients or partners, or on population transmission. There was insufficient evidence to assess whether or not EPT has the potential unintended consequence of reducing HIV and STI testing and counselling in partners. APT has not yet been evaluated in randomised controlled trials.
In routine practice, traditional partner notification technologies practised in specialist genitourinary medicine clinics result in few partners being documented as being tested and treated. 45 A secondary analysis of UK clinical audit data (see Chapter 2, Partner notification outcomes for chlamydia in UK genitourinary medicine clinics) showed a median of 0.47 partners tested for chlamydia per index case and 0.60 partners treated per index case. Partner notification outcomes were lower in London than elsewhere (median number tested per index case 0.3 vs. 0.52) and in men who have sex with men compared with heterosexual men (adjusted OR 0.34, 95% CI 0.17 to 0.68) but improved as clinic volume (numbers of cases of chlamydia diagnosed) increased (p for trend 0.031). These levels of intermediate outcomes of partner notification are low, but are comparable with levels achieved in the randomised controlled trials that we reviewed. 44
In the absence of empirical data about the effects of partner notification on preventing secondary cases of chlamydia, we used a published algorithm,9 which we adapted using routinely available data and parameter estimates developed for this project (see Chapter 2, Estimating the likely public health impact of partner notification for a clinical service: an evidence-based algorithm). Two new indicators, the AROT and its reciprocal, the NNTIT, helped to assess the public health impact of traditional partner notification strategies targeted at different types of sexual partnerships. The NNTIT was lower for casual than regular partners, indicating that fewer casual than regular partners need to be traced and treated to prevent a secondary case. Although this simple algorithm has a number of limitations, it provides a basic tool, available online, to support public health-based decision-making at local clinic level.
In Chapter 3 (see Modelling the transmission dynamics of C. trachomatis), we reviewed uncertainties in parameter estimates in mathematical models of C. trachomatis transmission and predictions of the impact of screening and partner notification interventions. We used existing data in simple deterministic models to improve estimates of the mean duration of untreated chlamydia infection (433 days, 95% CI 420 to 447 days)46 and the per-partnership transmission probability (55.5%, per-sex act probability 9.5%). 47 Detailed investigation of three individual-based models of C. trachomatis transmission showed that differences in sexual partnership dynamics and in infection parameter estimates partly explained the differences in model predictions of preventative interventions. 48
Based on our findings in Chapter 3 (see Modelling the transmission dynamics of C. trachomatis), we developed a new individual-based model to investigate the effects of partner notification technologies on case finding and on population prevalence (see Individual- and population-level effects of partner notification for C. trachomatis). The model was of a general heterosexual population with homogeneous mixing and concurrent sexual partnerships. 49 Partner notification was examined within the context of ongoing chlamydia screening. The model was parameterised using sexual behaviour data from Natsal-2 and calibrated to a steady state chlamydia prevalence of 3%. The model predicted that 68% of current partners of index cases would be infected and that contact tracing periods of up to 18 months would identify > 10% positivity in notified partners. At a chlamydia screening rate of 0.1 per year without partner notification, chlamydia prevalence is reduced to about 70% of the baseline value after 5 years. If each partner is successfully treated with a probability of 50%, notification of the current partner is sufficient to achieve most of the additional reduction (to about 60% of the baseline) in population prevalence. The reduction in prevalence was greater at higher levels of screening uptake and partner notification success.
Also in Chapter 3 (see The effects of traditional and new partner notification technologies for C. trachomatis and N. gonorrhoeae), we developed a dual infection model of both C. trachomatis infection and N. gonorrhoeae infection to investigate the consequences of different partner notification strategies on infection prevalence, reinfection of index cases and missed STI diagnoses. Partner notification strategies that give treatment for chlamydia without testing for gonorrhoea might miss cases of gonorrhoea and lead to outbreaks in high-risk areas. Epidemiological data suggest an interaction between chlamydia and gonorrhoea. To reproduce observed patterns of co-infection, the model had to assume that being infected with either chlamydia or gonorrhoea increased susceptibility to the other. In the model, cotesting and treatment reduced the prevalence of both infections. The effect of APT compared with standard patient referral was minor in reducing the prevalence of both infections at the population level, although in shortening the time to treatment reinfection, rates were substantially reduced. We did not explore the impact on HIV infection.
In Chapter 4 (see Outcomes for the economic evaluation of partner notification technologies: systematic review), we examined aspects of the economic evaluation of partner notification. Our systematic review of HRQL studies for chlamydia infection found few robust and validated tools. The only published QALY estimates were judged to be of insufficient quality to be used in cost-effectiveness studies of partner notification. Based on this finding and the challenges in demonstrating an effect of partner notification on reducing chlamydia prevalence or reproductive tract damage primary end points, we decided not to evaluate cost-effectiveness using QALYs.
We measured costs of chlamydia screening and traditional partner notification, cost per individual tested and cost per positive diagnosis in the context of the NCSP in England (see Chapter 4, Costs and cost-effectiveness of different strategies for chlamydia screening and partner notification: an economic and mathematical modelling study). 31 We used a static model, implemented in a simple spreadsheet, to assess two different interventions. We found that doubling the efficacy of partner notification (from 0.4 to 0.8 partners per index case) would reduce the costs per infection diagnosed for a limited additional investment. In contrast, increasing the screening coverage of men to the same level as for women would require an investment of six times more money but lead to only twice as many additional infections being treated.
Synthesis of findings
Partner notification is an integral part of the management and control of curable STIs. Randomised controlled trials show that EPT strategies can reduce reinfection in index cases with curable STIs and there was no statistical evidence of a difference in index case reinfection rates between EPT and enhanced patient referral. APT has not yet been evaluated in randomised controlled trials. In a mathematical model, an APT-like intervention that reduces the time to partner treatment from 14 days to 1 or 3 days substantially reduces the risk of reinfection of an index case with chlamydia or gonorrhoea.
Partner notification is used as a method for identifying new STI cases: 60–70% of individuals attending STI clinics as partners of index cases with chlamydia are themselves infected, and 70–80% of those attending as partners of index cases with gonorrhoea are themselves infected. Our individual-based mathematical model of chlamydia transmission predicted this level of chlamydia infection well. When implementing partner notification in the model, a look-back period of up to 18 months continued to identify sexual partners with a > 10% probability of being infected, and so current UK guidelines with a recommended look-back period of 6 months provide a reasonable balance of identifying new cases with the realistic chance that they can be traced. As an addition to a population screening programme, the findings from our static modelling of outcomes and costs suggest that partner notification is more cost-effective, in terms of restricted outcomes, for case finding than increasing screening coverage. Our systematic review found insufficient evidence to conduct a full cost-effectiveness analysis about QALYs for the reproductive tract complications of chlamydia.
Partner notification in genitourinary medicine clinics has the potential to interrupt chlamydia transmission if efforts are directed to casual and ex-regular partners of index cases (about two-thirds of partners in our study) who are likely to contribute to spread networks for STI transmission. 5 The NNTIT, estimated using our algorithm, is low compared with that for regular partners. These results were derived from a static model. The benefits of this model are that the data required to populate the spreadsheet can be easily obtained and entered. The disadvantage is that there are strong assumptions about the numbers of transmissions to secondary cases, which can only be fully taken into account in a dynamic model. The effect on prevalence in the general population, however, is likely to be modest. In our individual-based model, treating the current partner reduces chlamydia prevalence but treating previous partners does not reduce population prevalence further in the presence of ongoing chlamydia screening at a rate of at least 0.1 per year. This finding reflects the parameterisation using general population sexual behaviour data from Natsal-2 data; about two-thirds of people are in an ongoing (assumed equivalent to ‘regular’) partnership and levels of concurrent partnerships are low.
Accelerated partner therapy has the potential to reduce the population prevalence of chlamydia and gonorrhoea more than traditional methods requiring attendance at a health service setting. This conclusion from a dynamic modelling study assumes that APT both reduces the probability of reinfection in the index case and increases the number of partners treated. In our mathematical model, APT did not increase the risk of gonorrhoea outbreaks if partners were treated for the same STI as index cases. The risk of gonorrhoea outbreaks was lower with APT when partners were also tested for STI. In all models, effects were stronger when rates of STI screening were higher and when a biological interaction between chlamydia and gonorrhoea was assumed. The effects of APT on gonorrhoea infections are relevant only in areas where the prevalence if gonorrhoea is high. The harms resulting from the low positive predictive value of testing for gonorrhoea in the general population in low prevalence settings might outweigh the benefits of the modest predicted reduction in gonorrhoea prevalence. The benefits of APT on reducing the prevalence of curable STIs might be outweighed if HIV infections, which would have been diagnosed in partners notified by traditional technologies, are missed. In practice, very few partners reached by APT take up the offer of additional STI testing. 20 This is particularly worrying because curable urethral and cervical STIs are cofactors for HIV acquisition and transmission. 134
The cost-effectiveness of APT has not been determined. We found insufficient evidence about HRQL and QALYs for chlamydia infection and its consequences to examine the cost per QALY of introducing APT compared with traditional partner notification technologies. A cost–consequence analysis of the first exploratory trial of APT in England showed the potential for APT to be cost–effective. The cost per partner treated was slightly higher but reached more partners for APT Hotline [£54.42 (95% CI £43.83 to £65.21) per partner, to treat 35% of contactable partners] and APT Pharmacy [£53.29 (95% CI £42.85 to £63.73) per partner, to treat 34% of contactable partners] compared with routine partner notification [£45.89 (95% CI £36.90 to £54.88 per partner) to treat 11% of contactable partners]. 135 Our analysis of the cost-effectiveness of traditional partner notification methods also suggests that there are potential gains of investing in partner notification. Using a restricted outcome, we show that partner notification costs less per additional new chlamydia infection detected than increasing the coverage of screening in the NCSP.
Traditional partner notification methods work best in genitourinary medicine clinics outside London and those with a larger case load, and are less effective for men who have sex with men. EPT is not recommended for men who have sex with men because of a lack of evidence of effectiveness. One small randomised controlled trial examined different combinations of partner notification technologies on intermediate outcomes in men who have sex with men. Rates of testing for HIV and syphilis were lower in partners of men who received EPT than in partners of men who received traditional partner notification. 56 The risk of missing HIV infections in men who have sex with men is considered to outweigh the benefits of reaching sexual partners, many of whom are reported to be untraceable using traditional partner notification methods.
Finally, the studies in this project offer advances in the mathematical modelling of bacterial STIs and maybe also in basic science. We have provided refined parameter estimates for the transmissibility and duration of asymptomatic chlamydia infection, which can be used in future studies. By investigating inconsistencies in model predictions from different individual-based models of chlamydia transmission, we showed the importance of representing key aspects of sexual partnership dynamics accurately. We also avoided using mathematical models for investigating the effects of partner notification technologies that could have given misleading results. Our development of a model of chlamydia and gonorrhoea co-infection found that an interaction to increase the susceptibility to chlamydia in the presence of gonorrhoea and vice versa was necessary to achieve levels of single and co-infections that are consistently observed in epidemiological studies.
Implications for health care
-
A range of enhanced patient referral methods is available. Genitourinary medicine clinics have staff with the skills and resources for conducting enhanced patient referral. Patients with curable STIs in primary care and community sexual health services should also be able to receive enhanced patient referral as part of their management. Support from health advisers in genitourinary medicine clinics and training for staff in primary and community health-care services might need to be strengthened.
-
The findings of two studies in this monograph emphasise the importance of sexual history taking. First, sexual histories need to cover look-back periods that identify previous partners because our mathematical model predicted high percentages of sexual partners infected with chlamydia as far back as 18 months or three previous partners. Second, it is important to find out the type of sexual partnerships of index cases. The need for health adviser support for notifying casual partners should be considered because of the potential gains in interrupting transmission.
-
The analysis of audit data shows that the outcomes of partner notification in genitourinary medicine clinics remain modest. The studies in this monograph show evidence of the gains of improving outcomes. The economic evaluation suggests that relative costs (per case identified) of increasing the success of partner notification are less than the costs of increasing the coverage of chlamydia screening.
-
APT is a technology that is still being developed in the UK. The implications for routine partner notification practice will not become clear until a formal evaluation in a randomised controlled trial with biological outcomes has been conducted.
-
Although we examined the risk of gonorrhoea outbreaks as an unintended consequence of APT, this technology also has implications for the underdiagnosis and undertreatment of other STIs, which we did not consider. First, there are missed opportunities for diagnosing HIV infection and the importance of STI testing needs to be explained to partners receiving APT. Second, if an index case has chlamydia and gonorrhoea but either he or she was not tested for gonorrhoea or the test gave a false-negative result, the APT antibiotic will be a single 1-g dose of azithromycin. If a partner is infected with N. gonorrhoeae (with or without chlamydia co-infection), macrolide resistance could be encouraged if given to a partner who is co-infected and does not receive adequate treatment for gonorrhoea. Third, treatment for uncomplicated chlamydia and/or gonorrhoea will not be adequate treatment if a female partner has PID.
-
The findings about the effects and impact of partner notification technologies cannot be generalised to men who have sex with men, as there is limited trial evidence and because the mathematical modelling studies modelled a general heterosexual population. However, the audit showed that partner notification outcomes were worse in men who have sex with men. We show findings for a population of high-risk individuals within a general population; these should be interpreted cautiously when applied to specific populations at high risk or in high-prevalence areas.
Recommendations for research
-
A randomised controlled trial of the effects of APT compared with traditional partner notification technologies should be conducted, with follow-up measuring biological end points beyond 3 months. Determining whether or not the magnitude of benefit found in trials of EPT can be generalised to APT is a priority.
-
Randomised trials should include interventions to increase rates of testing for other STIs and HIV in partners notified by APT.
-
Modelling studies of the effectiveness and cost-effectiveness of APT should be conducted alongside a clinical trial. This should build on the dynamic models for single and dual infections developed within this project.
-
-
Randomised trials to identify effective partner notification technologies for men who have sex with men should be conducted for both bacterial STIs and HIV.
-
Studies to collect HRQL data, including the development of appropriate tools, should be commissioned so that QALYs for temporary and permanent health states associated with bacterial STIs, which use methods preferred by NICE, can be determined. This is a priority so that robust cost-effectiveness analyses of APT and of other interventions to prevent curable STIs and their consequences can be conducted.
-
Standard sets of disease-specific parameters for bacterial STIs should be developed to help researchers compare the performance of mathematical models and to help policy makers to interpret their outputs. Further research to develop these for gonorrhoea, trichomonas and syphilis is needed.
-
Basic science studies are needed to investigate the possible mechanisms for a biological interaction between the susceptibility to C. trachomatis and N. gonorrhoeae. Additional modelling studies of STI co-infections would be valuable.
Acknowledgements
Contributions of authors
All co-authors made contributions to meetings and discussions during the conduct of the project, read and commented on the draft monograph, and approved the final version. Specific contributions are as follows:
Christian L Althaus developed the mathematical models and led the modelling studies in Chapter 3, and the resulting publications. 46–49 He wrote the first draft of the monograph and contributed to revising it.
Katherine ME Turner developed the mathematical model and led the study reported in Costs and cost-effectiveness of different strategies for chlamydia screening and partner notification: an economic and mathematical modelling study. She wrote the manuscript for the study on which this section is based31 and contributed to revision of the draft report.
Catherine H Mercer developed the algorithm and led the study reported in section Estimating the likely public health impact of partner notification for a clinical service: an evidence-based algorithm, and the publication on which it was based. 9 She wrote the first draft of this section of the draft report and contributed to revisions in the rest of the report.
Peter Auguste and Tracy E Roberts conducted the study reported in Outcomes for the economic evaluation of partner notification technologies: systematic review. Peter Auguste wrote the first draft of this section of the report and Tracy Roberts revised it and contributed to revision of the rest of the report.
Gill Bell collected data for the study reported in Estimating the likely public health impact of partner notification for a clinical service: an evidence-based algorithm and contributed to revising the report.
Sereina A Herzog conducted the statistical analysis of partner notification outcomes from UK genitourinary medicine clinic data,45 wrote the first draft of Partner notification outcomes for chlamydia in UK genitourinary medicine clinics and contributed to revising the report.
Jackie A Cassell contributed to the study reported in Estimating the likely public health impact of partner notification for a clinical service: an evidence-based algorithm and the publication on which it was based. 9 She contributed to drafting the discussion and revising the report.
W John Edmunds contributed to the discussion of the static and dynamic modelling studies.
Peter J White contributed to the discussion of the static and dynamic modelling studies and contributed to revising the report.
Helen Ward wrote the first draft of the discussion and contributed to revising the report.
Nicola Low was a co-investigator on the studies reported in Clinical effectiveness of partner notification technologies: systematic review; Partner notification outcomes for chlamydia in UK genitourinary medicine clinics; Modelling the transmission dynamics of C. trachomatis; Individual- and population-level effects of partner notification for C. trachomatis; The effects of traditional and new partner notification technologies for C. trachomatis and N. gonorrhoeae; and Outcomes for the economic evaluation of partner notification technologies: systematic review. She contributed to the drafting and revision of the report and is guarantor for the project as a whole.
Contributions of people not included as co-authors
Pelham Barton, Geoff Garnett and Caroline Trotter were involved in obtaining funding for this research and contributed to meetings and discussions at earlier stages of the project.
Clinical effectiveness of partner notification technologies: systematic review
Adel Ferreira, Taryn Young, Catherine Mathews and Moleen Zunza are co-authors of the Cochrane Collaboration systematic review of strategies for partner notification. 44
Partner notification outcomes for chlamydia in UK genitourinary medicine clinics
Hugo McClean and Chris A Carne are co-authors of the analysis of clinical audit data about partner notification outcomes for chlamydia. 45
Estimating the likely public health impact of partner notification for a clinical service: an evidence-based algorithm
Catherine R H Aicken, M Gary Brook and Claudia S Estcourt are co-authors on the evidence-based algorithm for estimating the public health impact of partner notification. 9
Modelling the transmission dynamics of Chlamydia trachomatis
Janneke C M Heijne and Adrian Roellin are co-authors on the articles describing the estimate of C. trachomatis infectious duration46 and investigating the individual and population-level effects of partner notification. 49
Individual- and population-level effects of partner notification for Chlamydia trachomatis
Janneke C M Heijne is a co-author on the article estimating the transmissibility of C. trachomatis. 47
Outcomes for the economic evaluation of partner notification technologies: systematic review
C Pswarayi contributed to the systematic review of quality-of-life studies. 136
Costs and cost-effectiveness of different strategies for chlamydia screening and partner notification: an economic and mathematical modelling study
Elisabeth Adams, Arabella Grant, John Macleod, Jan Clarke and Paddy Horner are co-authors to the study of the costs and cost-effectiveness of chlamydia screening and partner notification strategies. 31
Editorial assistance
Kali Tal edited several of the chapters for language, style and consistency.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
Publications
Althaus C, Heijne CM, Roellin A, Low N. Transmission dynamics of Chlamydia trachomatis affect the impact of screening programmes. Epidemics 2010;2:123–31.
Herzog S, McClean H, Carne C, Low N. Variation in partner notification outcomes for chlamydia in UK genitouinary medicine clinics: multi-level study. Sex Transm Infect 2011;87:420–5.
Turner K, Adams E, Macleod J, Bell G, Clarke J, Horner P. Costs and cost effectiveness of different strategies for chlamydia screening and partner notification: an economic and mathematical modelling study. BMJ 2011;342:c7250.
Althaus CL, Turner KME, Schmid BV, Heijne JCM, Kretzschmar M, Low N. Transmission of Chlamydia trachomatis through sexual partnerships: a comparison between three individual-based models and empirical data. J R Soc Interface 2012;9:136–46.
References
- Sexually Transmitted Diseases: Policies and Principles for Prevention and Care. Geneva: UNAIDS, UNAIDS Best Practice Collection; 1999.
- World Health Organization . Global Strategy for the Prevention and Control of Sexually Transmitted Infections: 2006–2015. 2007. http://whqlibdoc.who.int/publications/2007/9789241563475_eng.pdf (accessed 2 November 2012).
- Golden MR, Faxelid E, Low N, Holmes KK, Sparling PF, Stamm WE, et al. Sexually Transmitted Diseases. New York, NY: McGraw-Hill; 2008.
- Low N, Broutet N, Adu-Sarkodie Y, Barton P, Hossain M, Hawkes S. Global control of sexually transmitted infections. Lancet 2006;368:2001-16. http://dx.doi.org/10.1016/S0140-6736(06)69482-8.
- Wasserheit JN, Aral SO. The dynamic topology of sexually transmitted disease epidemics: implications for prevention strategies. J Infect Dis 1996;174:S201-13. http://dx.doi.org/10.1093/infdis/174.Supplement_2.S201.
- Lycke E, Lowhagen GB, Hallhagen G, Johannisson G, Ramstedt K. The risk of transmission of genital Chlamydia trachomatis infection is less than that of genital Neisseria gonorrhoeae infection. Sex Transm Dis 1980;7:6-10. http://dx.doi.org/10.1097/00007435-198001000-00002.
- Quinn TC, Gaydos C, Shepherd M, Bobo L, Hook EW, Viscidi R, et al. Epidemiologic and microbiologic correlates of Chlamydia trachomatis infection in sexual partnerships. JAMA 1996;276:1737-42. http://dx.doi.org/10.1001/jama.276.21.1737.
- Johnson AM, Mercer CH, Erens B, Copas AJ, McManus S, Wellings K, et al. Sexual behaviour in Britain: partnerships, practices and HIV risk behaviours. Lancet 2001;358:1835-42. http://dx.doi.org/10.1016/S0140-6736(01)06883-0.
- Mercer CH, Aicken CR, Brook MG, Estcourt CS, Cassell JA. Estimating the likely public health impact of partner notification for a clinical service: an evidence-based algorithm. Am J Public Health 2011;101:2117-23. http://dx.doi.org/10.2105/AJPH.2011.300211.
- British Association of Sexual Health and HIV Clinical Effectiveness Group . 2006 UK National Guideline for the Management of Genital Tract Infection With Chlamydia Trachomatis n.d. www.bashh.org/documents/65.pdf (accessed 2 November 2012).
- Bignell C, FitzGerald M. UK national guideline for the management of gonorrhoea in adults, 2011. Int J STD AIDS 2011;22:541-7. http://dx.doi.org/10.1258/ijsa.2011.011267.
- British Association of Sexual Health and HIV Clinical Effectiveness Group . UK National Guidelines on the Management of Syphilis 2008 2008. www.bashh.org/documents/1879.pdf (accessed 2 November 2012).
- Golden MR, Whittington WL, Gorbach PM, Coronado N, Boyd MA, Holmes KK. Partner notification for chlamydial infections among private sector clinicians in Seattle-King County: a clinician and patient survey. Sex Transm Dis 1999;26:543-7. http://dx.doi.org/10.1097/00007435-199910000-00011.
- Arthur G, Lowndes CM, Blackham J, Fenton KA. Divergent approaches to partner notification for sexually transmitted infections across the European union. Sex Transm Dis 2005;32:734-41. http://dx.doi.org/10.1097/01.olq.0000175376.62297.73.
- Potterat JJ, Rothenberg R. The case-finding effectiveness of self-referral system for gonorrhea: a preliminary report. Am J Public Health 1977;67:174-6. http://dx.doi.org/10.2105/AJPH.67.2.174.
- Kissinger P, Mohammed H, Richardson-Alston G, Leichliter JS, Taylor SN, Martin DH, et al. Patient-delivered partner treatment for male urethritis: a randomized, controlled trial. Clin Infect Dis 2005;41:623-9. http://dx.doi.org/10.1086/432476.
- Wilson TE, Hogben M, Malka ES, Liddon N, McCormack WM, Rubin SR, et al. A randomized controlled trial for reducing risks for sexually transmitted infections through enhanced patient-based partner notification. Am J Public Health 2009;99:S104-10. http://dx.doi.org/10.2105/AJPH.2007.112128.
- Tomnay JE, Pitts MK, Kuo TC, Fairley CK. Does the Internet assist clients to carry out contact tracing? A randomized controlled trial using web-based information. Int J STD AIDS 2006;17:391-4. http://dx.doi.org/10.1258/095646206777323391.
- Cameron ST, Glasier A, Scott G, Young H, Melvin L, Johnstone A, et al. Novel interventions to reduce re-infection in women with chlamydia: a randomized controlled trial. Hum Reprod 2009;24:888-95. http://dx.doi.org/10.1093/humrep/den475.
- Estcourt C, Sutcliffe L, Cassell J, Mercer CH, Copas A, James L, et al. Can we improve partner notification rates through expedited partner therapy in the UK? Findings from an exploratory trial of Accelerated Partner Therapy (APT). Sex Transm Infect 2012;88:21-6. http://dx.doi.org/10.1136/sti.2010.047258.
- Dombrowski JC, Golden MR. Accelerated partner therapy: a promising new partner treatment option. Sex Transm Infect 2012;88:2-3. http://dx.doi.org/10.1136/sextrans-2011-050377.
- Trelle S, Shang A, Nartey L, Cassell JA, Low N. Improved effectiveness of partner notification for patients with sexually transmitted infections: systematic review. BMJ 2007;334:354-7. http://dx.doi.org/10.1136/bmj.39079.460741.7C.
- Expedited Partner Therapy in the Management of Sexually Transmitted Diseases: Review and Guidance. Atlanta, GA: US Department of Health and Human Services; 2006.
- Health Protection Agency (now Public Health England) . STI Annual Data Tables 2012. www.hpa.org.uk/webw/HPAweb&Page&HPAwebAutoListName/Page/1201094610372#2_STI_data_tables_2011 (accessed 20 June 2012).
- Creighton S, Tenant-Flowers M, Taylor CB, Miller R, Low N. Co-infection with gonorrhoea and chlamydia: how much is there and what does it mean?. Int J STD AIDS 2003;14:109-13. http://dx.doi.org/10.1258/095646203321156872.
- Datta SD, Sternberg M, Johnson RE, Berman S, Papp JR, McQuillan G, et al. Gonorrhea and chlamydia in the United States among persons 14 to 39 years of age, 1999 to 2002. Ann Intern Med 2007;147:89-96. http://dx.doi.org/10.7326/0003-4819-147-2-200707170-00007.
- Nsuami M, Cammarata CL, Brooks BN, Taylor SN, Martin DH. Chlamydia and gonorrhea co-occurrence in a high school population. Sex Transm Dis 2004;31:424-7. http://dx.doi.org/10.1097/01.OLQ.0000130535.96576.D3.
- Batteiger BE, Tu W, Ofner S, Van Der Pol B, Stothard DR, Orr DP, et al. Repeated Chlamydia trachomatis genital infections in adolescent women. J Infect Dis 2010;201:42-51.
- Golden M. Washington State Community Expedited Partner Treatment (EPT) Trial n.d. http://clinicaltrials.gov/ct2/show/NCT01665690 (accessed 1 November 2012).
- Low N, Welch J, Radcliffe K. Developing national outcome standards for the management of gonorrhoea and genital chlamydia in genitourinary medicine clinics. Sex Transm Infect 2004;80:223-9. http://dx.doi.org/10.1136/sti.2003.005165.
- Turner K, Adams E, Grant A, Macleod J, Bell G, Clarke J, et al. Costs and cost effectiveness of different strategies for chlamydia screening and partner notification: an economic and mathematical modelling study. BMJ 2011;342. http://dx.doi.org/10.1136/bmj.c7250.
- Hallett TB, White PJ, Garnett GP. Appropriate evaluation of HIV prevention interventions: from experiment to full-scale implementation. Sex Transm Infect 2007;83:i55-60. http://dx.doi.org/10.1136/sti.2006.023663.
- Barton P, Bryan S, Robinson S. Modelling in the economic evaluation of health care: selecting the appropriate approach. J Health Serv Res Policy 2004;9:110-18. http://dx.doi.org/10.1258/135581904322987535.
- Edmunds WJ, Medley GF, Nokes DJ. Evaluating the cost-effectiveness of vaccination programmes: a dynamic perspective. Stat Med 1999;18:3263-82. http://dx.doi.org/10.1002/(SICI)1097-0258(19991215)18:23<3263::AID-SIM315>3.0.CO;2-3.
- Regan DG, Wilson DP, Hocking JS. Coverage is the key for effective screening of Chlamydia trachomatis in Australia. J Infect Dis 2008;198:349-58.
- Brunham RC, Pourbohloul B, Mak S, White R, Rekart ML. The unexpected impact of a Chlamydia trachomatis infection control program on susceptibility to reinfection. J Infect Dis 2005;192:1836-44.
- Garnett GP, Mertz KJ, Finelli L, Levine WC, St Louis ME. The transmission dynamics of gonorrhoea: modelling the reported behaviour of infected patients from Newark, NJ. Phil Trans R Soc Lond B 1999;354:787-97. http://dx.doi.org/10.1098/rstb.1999.0431.
- Kretzschmar M, Van Duynhoven YTHP, Severijnen AJ. Modeling prevention strategies for gonorrhea and chlamydia using stochastic network simulations. Am J Epidemiol 1996;144:306-17. http://dx.doi.org/10.1093/oxfordjournals.aje.a008926.
- Low N, McCarthy A, Macleod J, Salisbury C, Campbell R, Roberts TE, et al. Epidemiological, social, diagnostic and economic evaluation of population screening for genital chlamydial infection. Health Technol Assess 2007;11:97-114.
- Turner KM, Adams EJ, Gay N, Ghani AC, Mercer C, Edmunds WJ. Developing a realistic sexual network model of chlamydia transmission in Britain. Theor Biol Med Model 2006;3.
- Kretzschmar M, Turner KM, Barton PM, Edmunds WJ, Low N. Predicting the population impact of chlamydia screening programmes: comparative mathematical modelling study. Sex Transm Infect 2009;85:359-66. http://dx.doi.org/10.1136/sti.2009.036251.
- Winter AJ, Sriskandabalan P, Wade AA, Cummins C, Barker P. Sociodemography of genital Chlamydia trachomatis in Coventry, UK, 1992–6. Sex Transm Infect 2000;76:103-9. http://dx.doi.org/10.1136/sti.76.2.103.
- Low N, Sterne JA, Barlow D. Inequalities in rates of gonorrhoea and chlamydia between Black ethnic groups in South East London: cross-sectional study. Sex Transm Infect 2001;77:15-20. http://dx.doi.org/10.1136/sti.77.1.15.
- Ferreira A, Young T, Mathews C, Zunza M, Low N. Strategies for partner notification for sexually transmitted infections, including HIV. Cochrane Database Syst Rev 2013;10. http://dx.doi.org/10.1002/14651858.CD002843.pub2.
- Herzog SA, McClean H, Carne CA, Low N. Variation in partner notification outcomes for chlamydia in UK genitourinary medicine clinics: multilevel study. Sex Transm Infect 2011;87:420-5. http://dx.doi.org/10.1136/sti.2011.049320.
- Althaus CL, Heijne JCM, Roellin A, Low N. Transmission dynamics of Chlamydia trachomatis affect the impact of screening programmes. Epidemics 2010;2:123-31. http://dx.doi.org/10.1016/j.epidem.2010.04.002.
- Althaus CL, Heijne JC, Low N. Towards more robust estimates of the transmissibility of Chlamydia trachomatis. Sex Transm Dis 2012;39:402-4. http://dx.doi.org/10.1097/OLQ.0b013e318248a550.
- Althaus CL, Turner KM, Schmid BV, Heijne JC, Kretzschmar M, Low N. Transmission of Chlamydia trachomatis through sexual partnerships: a comparison between three individual-based models and empirical data. J R Soc Interface 2012;9:136-46. http://dx.doi.org/10.1098/rsif.2011.0131.
- Althaus CL, Heijne JCM, Herzog SA, Roellin A, Low N. Individual and population level effects of partner notification for Chlamydia trachomatis. PLOS ONE 2012;7. http://dx.doi.org/10.1371/journal.pone.0051438.
- Mathews C, Coetzee N, Zwarenstein M, Lombard C, Guttmacher S, Oxman A, et al. Strategies for partner notification for sexually transmitted diseases. Cochrane Database Syst Rev 2001;4. http://dx.doi.org/10.1002/14651858.CD002843.
- Golden MR, Whittington WL, Handsfield HH, Hughes JP, Stamm WE, Hogben M, et al. Effect of expedited treatment of sex partners on recurrent or persistent gonorrhea or chlamydial infection. N Engl J Med 2005;352:676-85. http://dx.doi.org/10.1056/NEJMoa041681.
- Schillinger JA, Kissinger P, Calvet H, Whittington WL, Ransom RL, Sternberg MR, et al. Patient-delivered partner treatment with azithromycin to prevent repeated Chlamydia trachomatis infection among women: a randomized, controlled trial. Sex Transm Dis 2003;30:49-56. http://dx.doi.org/10.1097/00007435-200301000-00011.
- Kissinger P, Schmidt N, Mohammed H, Leichliter JS, Gift TL, Meadors B, et al. Patient-delivered partner treatment for Trichomonas vaginalis infection: a randomized controlled trial. Sex Transm Dis 2006;33:445-50. http://dx.doi.org/10.1097/01.olq.0000204511.84485.4c.
- Schwebke JR, Desmond RA. A randomized controlled trial of partner notification methods for prevention of trichomoniasis in women. Sex Transm Dis 2010;37:392-6. http://dx.doi.org/10.1097/OLQ.0b013e3181dd1691.
- Low N, McCarthy A, Roberts TE, Huengsberg M, Sanford E, Sterne JAC, et al. Partner notification for chlamydia in primary care: randomised controlled trial and analysis of resource use. BMJ 2006;332:14-9. http://dx.doi.org/10.1136/bmj.38678.405370.7C.
- Kerani RP, Fleming M, DeYoung B, Golden MR. A randomized, controlled trial of inSPOT and patient-delivered partner therapy for gonorrhea and chlamydial infection among men who have sex with men. Sex Transm Dis 2011;38:941-6. http://dx.doi.org/10.1097/OLQ.0b013e318223fcbc.
- Nuwaha F, Kambugu F, Nsubuga PS, Hojer B, Faxelid E. Efficacy of patient-delivered partner medication in the treatment of sexual partners in Uganda. Sex Transm Dis 2001;28:105-10. http://dx.doi.org/10.1097/00007435-200102000-00008.
- Katz BP, Danos CS, Quinn TS, Caine V, Jones RB. Efficiency and cost-effectiveness of field follow-up for patients with Chlamydia trachomatis infection in a sexually transmitted diseases clinic. Sex Transm Dis 1988;15:11-6.
- Moyo W, Chirenje ZM, Mandel J, Schwarcz SK, Klausner J, Rutherford G, et al. Impact of a single session of counseling on partner referral for sexually transmitted disease treatment, Harare, Zimbabwe. AIDS Behav 2002;6:237-43.
- Solomon MZ, DeJong W. The impact of a clinic-based educational videotape on knowledge and treatment behavior of men with gonorrhea. Sex Transm Dis 1988;15:127-32. http://dx.doi.org/10.1097/00007435-198807000-00001.
- Trent M, Chung SE, Burke M, Walker A, Ellen JM. Results of a randomized controlled trial of a brief behavioral intervention for pelvic inflammatory disease in adolescents. J Pediatr Adolesc Gynecol 2010;23:96-101. http://dx.doi.org/10.1016/j.jpag.2009.06.005.
- Montesinos L, Frisch LE, Greene BF, Hamilton M. An analysis of and intervention in the sexual transmission of disease. J Appl Behavr Anal 1990;23:275-84. http://dx.doi.org/10.1901/jaba.1990.23-275.
- Andersen B, Ostergaard L, Moller JK, Olesen F. Home sampling versus conventional contact tracing for detecting Chlamydia trachomatis infection in male partners of infected women: randomised study. BMJ 1998;316:350-1. http://dx.doi.org/10.1136/bmj.316.7128.350.
- Apoola A, Beardsley J. Does the addition of a urine testing kit to use of contact slips increase the partner notification rates for genital chlamydial infection?. Int J STD AIDS 2009;20:775-7. http://dx.doi.org/10.1258/ijsa.2009.009196.
- Ostergaard L, Andersen B, Moller JK, Olesen F, Worm AM. Managing partners of people diagnosed with Chlamydia trachomatis: a comparison of two partner testing methods. Sex Transm Infect 2003;79:358-61. http://dx.doi.org/10.1136/sti.79.5.358.
- Carne C, McClean H, Bhaduri S, Bunting P, Fernandes A, Dhar J, et al. UK National Audit of chlamydial infection management in sexual health clinics. Clinic policies audit. Int J STD AIDS 2008;19:477-9. http://dx.doi.org/10.1258/ijsa.2008.008139.
- McClean H, Carne C, Bunting P, Bhaduri S, Fernandes A, Dhar J, et al. UK National Audit of chlamydial infection management in sexual health clinics. Case notes audit: information-giving, partner notification and follow-up. Int J STD AIDS 2008;19:473-6. http://dx.doi.org/10.1258/ijsa.2008.008138.
- McClean H, Carne C, Bunting P, Bhaduri S, Fernandes A, Dhar J, et al. UK National Audit of chlamydial infection management in sexual health clinics. Case notes audit: demography, diagnosis and treatment. Int J STD AIDS 2008;19:469-72. http://dx.doi.org/10.1258/ijsa.2008.008137.
- Berwick DM. Controlling variation in health care: a consultation from Walter Shewhart. Med Care 1991;29:1212-25. http://dx.doi.org/10.1097/00005650-199112000-00004.
- Hoaglin DC. Understanding Robust and Explanatory Data Analysis. New York, NY: Wiley & Sons; 2000.
- Twisk JWR. Applied Multilevel Analysis: A Practical Guide. Cambridge: Cambridge University Press; 2006.
- Rogstad KE, Clementson C, Ahmed-Jushuf IH. Contact tracing for gonorrhoea in homosexual and heterosexual men. Int J STD AIDS 1999;10:536-8. http://dx.doi.org/10.1258/0956462991914636.
- Society for Sexual Health Advisers . The SSHA Manual for Sexual Health Advisers n.d. www.ssha.info/resources/manual-for-sexual-health-advisers/2004 (accessed 20 December 2010).
- Richards S. Should the NHS strive to eradicate all unexplained variation? Yes. BMJ 2009;339:1172-3. http://dx.doi.org/10.1136/bmj.b4811.
- Lilford RJ. Should the NHS strive to eradicate all unexplained variation? No. BMJ 2009;339:1172-3. http://dx.doi.org/10.1136/bmj.b4809.
- Fenton KA, Mercer CH, Johnson AM, Byron CL, McManus S, Erens B, et al. Reported sexually transmitted disease clinic attendance and sexually transmitted infections in Britain: prevalence, risk factors, and proportionate population burden. J Infect Dis 2005;191:S127-38. http://dx.doi.org/10.1086/425286.
- Fenton KA, Korovessis C, Johnson AM, McCadden A, McManus S, Wellings K, et al. Sexual behaviour in Britain: reported sexually transmitted infections and prevalent genital Chlamydia trachomatis infection. Lancet 2001;358:1851-4. http://dx.doi.org/10.1016/S0140-6736(01)06886-6.
- Mercer CH, Copas AJ, Sonnenberg P, Johnson AM, McManus S, Erens B, et al. Who has sex with whom? Characteristics of heterosexual partnerships reported in a national probability survey and implications for STI risk. Int J Epidemiol 2009;38:206-14. http://dx.doi.org/10.1093/ije/dyn216.
- Cassell JA, Mercer CH, Imrie J, Copas AJ, Johnson AM. Who uses condoms with whom? Evidence from national probability sample surveys. Sex Transm Infect 2006;82:467-73. http://dx.doi.org/10.1136/sti.2005.019117.
- Last JM. A Dictionary of Epidemiology. Oxford: Oxford University Press; 2001.
- Howard MM, Fortenberry JD, Blythe MJ, Zimet GD, Orr DP. Patterns of sexual partnerships among adolescent females. J Adolesc Health 1999;24:300-3. http://dx.doi.org/10.1016/S1054-139X(98)00145-1.
- Garnett GP, Hughes JP, Anderson RM, Stoner BP, Aral SO, Whittington WL, et al. Sexual mixing patterns of patients attending sexually transmitted diseases clinics. Sex Transm Dis 1996;23:248-57. http://dx.doi.org/10.1097/00007435-199605000-00015.
- Bell G, Ward H, Day S, Ghani AC, Goan U, Claydon E, et al. Partner notification for gonorrhoea: a comparative study with a provincial and a metropolitan UK clinic. Sex Transm Infect 1998;74:409-14. http://dx.doi.org/10.1136/sti.74.6.409.
- Kretzschmar M, Welte R, van Den HA, Postma MJ. Comparative model-based analysis of screening programs for chlamydia trachomatis infections. Am J Epidemiol 2001;153:90-101. http://dx.doi.org/10.1093/aje/153.1.90.
- Molano M, Meijer CJ, Weiderpass E, Arslan A, Posso H, Franceschi S, et al. The natural course of Chlamydia trachomatis infection in asymptomatic Colombian women: a 5-year follow-up study. J Infect Dis 2005;191:907-16.
- Katz BP. Estimating transmission probabilities for chlamydial infection. Stat Med 1992;11:565-77. http://dx.doi.org/10.1002/sim.4780110502.
- Garnett GP, Bowden FJ. Epidemiology and control and curable sexually transmitted diseases: opportunities and problems. Sex Transm Dis 2000;27:588-99. http://dx.doi.org/10.1097/00007435-200011000-00007.
- Heijne JCM, Althaus CL, Herzog SA, Kretzschmar M, Low N. The role of reinfection and partner notification in the efficacy of Chlamydia screening programs. J Infect Dis 2011;203:372-7. http://dx.doi.org/10.1093/infdis/jiq050.
- Dietz K, Hadeler KP. Epidemiological models for sexually transmitted diseases. J Math Biol 1988;26:1-25. http://dx.doi.org/10.1007/BF00280169.
- Kretzschmar M, Jager JC, Reinking DP, Van Zessen G, Brouwers H. The basic reproduction ratio R0 for a sexually transmitted disease in a pair formation model with two types of pairs. Math Biosci 1994;124:181-205. http://dx.doi.org/10.1016/0025-5564(94)90042-6.
- Powers KA, Ghani AC, Miller WC, Hoffman IF, Pettifor AE, Kamanga G, et al. The role of acute and early HIV infection in the spread of HIV and implications for transmission prevention strategies in Lilongwe, Malawi: a modelling study. Lancet 2011;378:256-68. http://dx.doi.org/10.1016/S0140-6736(11)60842-8.
- Bolker BM. Ecological Models and Data in R. Princeton, NJ: Princeton University Press; 2008.
- Welte R, Postma M, Leidl R, Kretzschmar M. Costs and effects of chlamydial screening: dynamic versus static modeling. Sex Transm Dis 2005;32:474-83. http://dx.doi.org/10.1097/01.olq.0000161181.48687.cf.
- Johnson LF, Alkema L, Dorrington RE. A Bayesian approach to uncertainty analysis of sexually transmitted infection models. Sex Transm Infect 2010;86:169-74. http://dx.doi.org/10.1136/sti.2009.037341.
- Tu W, Ghosh P, Katz BP. A stochastic model for assessing Chlamydia trachomatis transmission risk using longitudinal observational data. J R Stat Soc Ser A Stat Soc 2011;174:975-89. http://dx.doi.org/10.1111/j.1467-985X.2011.00691.x.
- Brunham RC, Rey-Ladino J. Immunology of chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat Rev Immunol 2005;5:149-61. http://dx.doi.org/10.1038/nri1551.
- Brunham RC, Rekart ML. The arrested immunity hypothesis and the epidemiology of chlamydia control. Sex Transm Dis 2008;35:53-4. http://dx.doi.org/10.1097/OLQ.0b013e31815e41a3.
- Regan DG, Wilson DP. Modelling sexually transmitted infections: less is usually more for informing public health policy. Trans R Soc Trop Med Hyg 2008;102:207-8. http://dx.doi.org/10.1016/j.trstmh.2007.08.009.
- Carré H, Boman J, Osterlund A, Garden B, Nylander E. Improved contact tracing for Chlamydia trachomatis with experienced tracers, tracing for one year back in time and interviewing by phone in remote areas. Sex Transm Infect 2008;84:239-42. http://dx.doi.org/10.1136/sti.2007.028068.
- Zimmerman-Rogers H, Potterat JJ, Muth SQ, Bonney MS, Green DL, Taylor JE, et al. Establishing efficient partner notification periods for patients with chlamydia. Sex Transm Dis 1999;26:49-54. http://dx.doi.org/10.1097/00007435-199901000-00008.
- Centers for Disease Control and Prevention . Sexually Transmitted Diseases Treatment Guidelines, 2010. MMWR 2010;59:44-5. www.cdc.gov/std/treatment/2010/STD-Treatment-2010-RR5912.pdf.
- Dietz K, Tudor D, Jewell NP, Kietz K, Farewell VT. AIDS Epidemiology. Boston, MA: Birkhäuser; 1992.
- Johnson AM, Mercer CH, Erens B, Copas AJ, McManus S, Wellings K, et al. Sexual behaviour in Britain: partnerships, practices, and HIV risk behaviours. Lancet 2001;358:1835-42. http://dx.doi.org/10.1016/S0140-6736(01)06883-0.
- Kretzschmar M, Satterwhite C, Leichliter J, Berman S. Effects of screening and partner notification on chlamydia positivity in the United States: a modeling study. Sex Transm Dis 2012;39:325-31. http://dx.doi.org/10.1097/OLQ.0b013e31824e52c2.
- Armbruster B, Brandeau ML. Contact tracing to control infectious disease: when enough is enough. Health Care Manag Sci 2007;10:341-55. http://dx.doi.org/10.1007/s10729-007-9027-6.
- Turner KME. Mathematical Models of Gonorrhoea and Chlamydia: Biology, Behaviour and Interventions 2004.
- Stupiansky NW, Van der Pol B, Williams JA, Weaver B, Taylor SE, Fortenberry JD. The natural history of incident gonococcal infection in adolescent women. Sex Transm Dis 2011;38:750-4. http://dx.doi.org/10.1097/OLQ.0b013e31820ff9a4.
- Vonck RA, Darville T, O’Connell CM, Jerse AE. Chlamydial infection increases gonococcal colonization in a novel murine coinfection model. Infect Immun 2011;79:1566-77. http://dx.doi.org/10.1128/IAI.01155-10.
- Hethcote HW, Yorke JA. Gonorrhea transmission dynamics and control. Lecture 1984;56:1-53.
- Batteiger BE, Xu F, Johnson RE, Rekart ML. Protective immunity to Chlamydia trachomatis genital infection: evidence from human studies. J Infect Dis 2010;201:S178-89.
- Brook MG, Rusere L, Coppin-Browne L, McDonagh S, McSorley J. A prospective study of the effectiveness of electronic patient records in rapid-cycle assessment of treatment and partner notification outcomes for patients with genital chlamydia and gonorrhoea infection. Sex Transm Infect 2011;87:152-5. http://dx.doi.org/10.1136/sti.2010.042440.
- Roberts TE, Robinson S, Barton P, Bryan S, Low N. Screening for Chlamydia trachomatis: a systematic review of the economic evaluations and modelling. Sex Transm Infect 2006;82:193-200. http://dx.doi.org/10.1136/sti.2005.017517.
- Postma MJ, Welte R, Van den Hoek JAR, Van Doornum GJJ, Jager HC, Coutinho RA. Cost-effectiveness of partner pharmacotherapy in screening women for asymptomatic infection with Chlamydia trachomatis. Value Health 2001;4:266-75. http://dx.doi.org/10.1046/j.1524-4733.2001.43009.x.
- Howell MR, Kassler WJ, Haddix A. Partner notification to prevent pelvic inflammatory disease in women. Cost-effectiveness of two strategies. Sex Transm Dis 1997;24:287-92. http://dx.doi.org/10.1097/00007435-199705000-00010.
- Roberts T, Henderson J, Mugford M, Bricker L, Neilson J, Garcia J. Antenatal ultrasound screening for fetal abnormalities: a systematic review of cost and cost effectiveness studies. BJOG 2002;109:44-56. http://dx.doi.org/10.1016/S1470-0328(02)00223-9.
- Vaccines for the 21st Century. A Tool for Decision making. Washington, DC: National Academy Press; 1999.
- Haggerty CL, Schulz R, Ness RB. Lower quality of life among women with chronic pelvic pain after pelvic inflammatory disease. Obstet Gynecol 2003;102:934-9. http://dx.doi.org/10.1016/S0029-7844(03)00695-1.
- Smith KJ, Tsevat J, Ness RB, Wiesenfeld HC, Roberts MS. Quality of life utilities for pelvic inflammatory disease health states. Sex Transm Dis 2008;35:307-11. http://dx.doi.org/10.1097/OLQ.0b013e31815b07dd.
- Kuppermann M, Learman LA, Schembri M, Gregorich S, Jacoby A, Jackson RA, et al. Effect of noncancerous pelvic problems on health-related quality of life and sexual functioning. Obstet Gynecol 2007;110:633-42. http://dx.doi.org/10.1097/01.AOG.0000279153.56275.b5.
- Mathias SD, Kuppermann M, Liberman RF, Lipschutz RC, Steege JF. Chronic pelvic pain: prevalence, health-related quality of life, and economic correlates. Obstet Gynecol 1996;87:321-7. http://dx.doi.org/10.1016/0029-7844(95)00458-0.
- Romão AP, Gorayeb R, Romao GS, Poli-Neto OB, dos Reis FJ, Rosa-e-Silva JC, et al. High levels of anxiety and depression have a negative effect on quality of life of women with chronic pelvic pain. Int J Clin Pract 2009;63:707-11. http://dx.doi.org/10.1111/j.1742-1241.2009.02034.x.
- Haggerty CL, Peipert JF, Weitzen S, Hendrix SL, Holley RL, Nelson DB, et al. Predictors of chronic pelvic pain in an urban population of women with symptoms and signs of pelvic inflammatory disease. Sex Transm Dis 2005;32:293-9. http://dx.doi.org/10.1097/01.olq.0000162361.69041.a5.
- Trent M, Lehmann HP, Qian Q, Thompson CB, Ellen JM, Frick KD. Adolescent and parental utilities for the health states associated with pelvic inflammatory disease. Sex Transm Infect 2011;87:583-7. http://dx.doi.org/10.1136/sextrans-2011-050187.
- Ness RB, Soper DE, Peipert J, Sondheimer SJ, Holley RL, Sweet RL, et al. Design of the PID Evaluation and Clinical Health (PEACH) Study. Control Clin Trials 1998;19:499-514. http://dx.doi.org/10.1016/S0197-2456(98)00022-1.
- Brazier J, Ratcliffe J, Salomon JA, Tsuchiya A. Measuring and Valuing Health Benefits for Economic Evaluation. Oxford: Oxford University Press; 2007.
- Horsman J, Furlong W, Feeny D, Torrance G. The Health Utilities Index (HUI): concepts, measurement properties and applications. Health Qual Life Outcomes 2003;1.
- Torrance GW. Measurement of health state utilities for economic appraisal: a review. J Health Econ 1986;5:1-30.
- Guide to the Methods of Technology Appraisal. London: National Institute for Health and Care Excellence; 2004.
- Young People’s Sexual Health: The National Chlamydia Screening Programme. London: The Stationery Office; 2009.
- National Chlamydia Screening Programme . Data Tables. England April 2011–March 2012 2012. www.chlamydiascreening.nhs.uk/ps/resources/data-tables/CTD-Q1-4-2011_2012.pdf (accessed 6 September 2013).
- National Chlamydia Screening Programme . Guidance for the Commissioners on the Cost of Providing Chlamydia Screening in Primary Care and the Community: A Review of Costs in Practice Across England in 2009 2009. www.chlamydiascreening.nhs.uk/ps/resources/guidelines/NCSP_costing_guidance_Dec09.pdf (accessed 25 June 2012).
- National Chlamydia Screening Programme . NHS Vital Signs 2010 11. Primary Care Trust (PCT) and Strategic Health Authority (SHA) Specific Tables. 1st April 2010 to 31st March 2011 2011. www.chlamydiascreening.nhs.uk/ps/resources/data-tables/VSI_by_PCT_Q1-4_April_2010-March_2011.pdf (accessed 28 June 2011).
- National Chlamydia Screening Programme . NHS Vital Signs 2008 09. Primary Care Trust (PCT) and Strategic Health Authority (SHA) Specific Tables. 1st April 2008 to 31st March 2009 2009. http://web.archive.org/web/20120430015941/http://www.chlamydiascreening.nhs.uk/ps/assets/pdfs/data/VSI_PCT/Vital_Signs%20_PCT_%20April%2008_Mar%2009_Masked.pdf (accessed 28 June 2011).
- Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect 1999;75:3-17. http://dx.doi.org/10.1136/sti.75.1.3.
- Roberts TE, Tsourapas A, Sutcliffe L, Cassell J, Estcourt C. Is Accelerated Partner Therapy (APT) a cost-effective alternative to routine patient referral partner notification in the UK? Preliminary cost-consequence analysis of an exploratory trial. Sex Transm Infect 2012;88:16-20. http://dx.doi.org/10.1136/sextrans-2011-050176.
- Jackson JL, Auguste P, Low N, Roberts TE. Valuing the health states associated with Chlamydia trachomatis infections and their sequelae: a systematic review of economic evaluations and primary studies. Value Health 2014.
Appendix 1 Protocol
This protocol includes activities that were planned up to September 2010. Changes from the planned activities after that date are highlighted in italics.
Research objectives (amended September 2010)
The overall aim of this study is to provide information for public health planning about the comparative disease control potential and cost-effectiveness of different strategies for treating and testing the sexual partners of people with sexually transmitted infections. This will be achieved through the following specific objectives:
-
To provide the best available data about model parameters rapidly and efficiently through the use of existing and planned datasets;
-
To compare the clinical effectiveness of different approaches to providing treatment and testing for the partners of people with curable sexually transmitted infections by using individual-based mathematical models.
-
To determine the cost-effectiveness of different approaches to providing treatment and testing for the partners of people with curable sexually transmitted infections, compared with recommended practice;
-
To provide research recommendations for primary research.
Research methods (amended September 2010)
We propose an efficient research plan that will capitalise on the existing models developed by members of this team and described above. 1–3 We will apply these models to the problem of new partner treatment technologies, using data already collected by this group. Specific new data items will be collected by developing novel data collection tools as part of a newly funded MRC study (PI, Mercer).
Table 1 summarises the infections, settings and scenarios to be studied during this project. The choice is based on the incidence of infection in the general population or specific subgroups, clinical severity and the public health consequences of failing to identify undiagnosed infection.
| Infection | Transmission route | Setting for index case | Scenarios of partner management | Actual project models |
|---|---|---|---|---|
| Current recommendation 1 | GUM, primary care | Treat epidemiologically and test for index and co-infection4;5 | ||
| Chlamydia | Heterosexual | GUM, primary care | Treat without testing for index or co-infection | Modelled in general and high risk populations |
| Test for index and co-infection before treating | ||||
| Gonorrhoea | Heterosexual | GUM, primary care | Treat without testing for index or co-infection | Modelled in general and high risk populations |
| Test for index and co-infection before treating | ||||
| MSM | GUM | Treat without testing for index or co-infection | Not done, owing to lack of time | |
In the case of heterosexuals, these diseases will each be explored in two demographic populations representing important settings for the control of sexually transmitted infections in the UK: (a) a mixed ethnicity urban setting; (b) a largely white mixed rural and urban settings. Differences in prevalence and incidence, risk and transmission of sexually transmitted infections in sexual networks in these populations, which have the potential to affect the outcomes of partner testing technologies at the population level, will then be able to be explored. In the project these populations were modelled as general and high risk populations.
We will begin by collating secondary data to provide the best available information to inform epidemiological and economic modelling. Two workstreams will then work in parallel: (a) baseline model comparisons and programming; (b) collection of parameter data from ongoing studies. The main modelling work involves an iterative process of comparison, programming and re-parameterisation for each infection and partner management strategy to obtain the most robust range of estimates. The scenarios below will be compared between models, for the infections described above, singly and (where appropriate) in combination. In the project, model comparison was used for the early stages only. After that, specific models were developed to address each research question. The economic evaluation will take place when cost data, health state valuations, and model outputs are available, but will inform all elements of new data collection. An economic evaluation using health state valuations could not be done, owing to inadequate data quality.
Literature reviews and other secondary data
NICE rapid review of partner notification
We will use published and unpublished data collected as part of our rapid review of partner notification methods (Cassell and Low),6 which contributed to the development of the NICE guidelines for preventing sexually transmitted infections. The review includes data about: numbers of sexual partners notified and treated per index case, according to the index infection, sexual orientation, ethnic group, and setting. We will update the searches from August 2006 to December 2007 using published recommendations,7 and extract data in duplicate. In the project we used the Cochrane Collaboration Sexually Transmitted Infections Review Group review of partner notification strategies, which was being updated during the project period.
Epidemiological and economic literature reviews
We will conduct rapid searches of Medline, Embase and The Cochrane Library from January 1990 to December 2007 based on recognised techniques,7 for information about: the prevalence of concurrent sexually transmitted infections according to route of transmission, ethnic group, primary care and genitourinary medicine clinic setting, probability of being tested for index and co-infections, quality of life living with complications of chlamydia or with HIV; and costs of treatment for complications of sexually transmitted infections. Owing to time constraints, the project included only a systematic review of studies of quality of life for reproductive tract complications of chlamydia in women.
Surveillance data
We will use the Avon System for Surveillance of Sexually Transmitted Infections (ASSIST) (Cassell and Low)8 to determine the distribution of sexually transmitted infections according to clinical setting of diagnosis. This database contains information from 2000 to 2004 about all tests for sexually transmitted infections in the former Avon Health Authority area and we have comparable data from Brent Primary Care Trust in London. These UK settings represent metropolitan, urban and rural areas, and areas of ethnic heterogeneity. This data source was not required.
Patient Access to care for Sexually Transmitted Infections (PATSI)
This completed study, funded by the MRC and led by Cassell (with Garnett, Mercer, White), collected data about consultations from eight genitourinary medicine clinics in England, including patient preferences about clinical setting, delays in care-seeking, risk behaviour during care-seeking, and types of care offered by GPs. This data source was not required.
Public health decision support tool for population-specific sexual health service planning
This newly funded MRC study, led by Mercer (with Cassell, Ward, White, Low) will collect and analyse new survey and clinical data about modifiable aspects of sexual health service provision in genitourinary medicine and primary care settings that affect the control of sexually transmitted infections. The project begins in October 2008, so the timing of data collection and analysis fits well with this project. We will collect data from patients and providers about existing partner testing and treatment practices and outcomes. Further data from the analysis of large primary care databases up to 2005, such as the General Practice Research Database (GPRD) are also available (from Cassell and Mercer).
Mathematical modelling
We will use three existing UK-developed models of chlamydia and/or gonorrhoea transmission. Our modelling approach takes into account uncertainty in a) parameter estimates and b) model structure in existing and is informed by methods described by Brisson and Edmunds9 and Koopman. 10 We are currently pioneering this technique in our ongoing work to improve the value of modelling studies for public health decision-making about chlamydia screening. 11 Given the variability in outputs (Figure 1), the use of multiple models will improve the robustness of the inferences that can be drawn by allowing differences between models to be explained.
Model comparison: We will begin by comparing in detail the structures, baseline assumptions and predictions of the existing models using features common to all models, for example, predicted chlamydia prevalence in heterosexuals by age and sex following epidemiological treatment and testing for 100%, 50% and 20% of current partners. This will allow a ‘diagnosis’ of parameter and model differences that are likely to result in differences in outputs. Each model offers different advantages and disadvantages, which are relevant to the project’s objectives. For example, the ClaSS project model models the incidence of infertility dynamically over time, which is important for estimating the long term impact of partner testing and treatment technologies. The first round of model comparison found that none of the available individual based models was suitable, and that improved estimates were needed for some parameters. Models specific to each study question were then developed. The Imperial College and HPA models have already been developed to consider both chlamydia and gonorrhoea, expediting investigation of the effects of co-infection with two key infections. Each team will then program their model to give a population that includes the possibility of studying multiple infections, hetero and homosexual partnerships, ethnic heterogeneity, genitourinary medicine and primary care treatment settings, partnership network structure and complications. The Imperial College model could not be developed further, owing to changes in staff.
Clinical outcomes: The main modelling work will focus on determining the most plausible internally and externally consistent estimates of the outcomes important of public health decision making: total and age and sex specific transmissions and complications prevented and, for the urban mixed population, outcomes in both black and white ethnic groups. For each selected infection and partner testing scenario, we will follow a similar process to determine the impact on both the index infection and co-infections with respect to the effects of, a) seeking care for one infection and having others diagnosed, b) co-treatment, and c) lost opportunities to diagnose and treat other infections. For example, for chlamydia we would examine outcomes for, a) base case (epidemiological treatment with testing for index and co-infections), b) the index patient’s current partner treated for chlamydia on the same day but not tested for co-infections, c) current partner being tested for chlamydia but not treated until results are known. We will conduct an iterative process of model comparison, diagnosis, re-parameterisation and programming. At each stage differences between model outputs will be discussed, and reasons explored. We will test alternative assumptions about parameter values, for example, the uptake of different strategies, in sensitivity analyses. We will explore differences due to model structure by modifying one model to reproduce the structure of the one (or ones) from which it differs. If final model outputs converge to produce similar predictions, the final output will be the average from all three models. If final outputs remain different, with no available empirical data to help determine the most plausible outputs, we will present a low, middle and upper estimate. The numbers of infections and complications averted will be used in the economic evaluation. Models specific to each study question were developed. Complications were not modelled, owing to lack of time.
Economic evaluation and health state valuation
We will use published sources to estimate the costs of antibiotic treatment, face-to-face consultation, telephone consultation, hospital and/or outpatient treatment for tubal infertility, ectopic pregnancy, pelvic inflammatory disease, HIV infection at 2009 Sterling prices. 1;12;13
Cost-effectiveness analysis
The cost-effectiveness analysis will be from the health service perspective using current NICE recommendations for the discounting of future costs and outcomes. 14 We will use model data for the numbers of infections averted, cases of infertility and HIV averted by adopting different partner testing strategies. Uncertainty will be explored using appropriate sensitivity analysis given the nature of the models. Incremental cost effectiveness ratios, comparing costs of one intervention with the current standard of care, will be presented in pounds Sterling per major outcome averted and per QALY. A cost-effectiveness analysis using cost per QALY could not be done, owing to inadequate data quality. Economic evaluations were done using intermediate outcomes.
Ethical arrangements
Mercer is responsible for obtaining ethical committee approval for her project.
References
- Low N, McCarthy A, Macleod J, Salisbury C, Campbell R, Roberts TE, et al. Epidemiological, social, diagnostic and economic evaluation of population screening for genital chlamydial infection. Health Technol Assess 2007;11:1-184.
- Turner KM, Adams EJ, Gay N, Ghani AC, Mercer C, Edmunds WJ. Developing a realistic sexual network model of chlamydia transmission in Britain. Theor Biol Med Model 2006;3.
- White PJ, Golden MR, Turner KM, Mercer CH, Ward H, Garnett GP. Can patient delivered partner therapy help us regain control of sexually transmitted infections in the UK?. Sex Transm Infect 2006;82.
- British Association of Sexual Health and HIV Clinical Effectiveness Group . 2006 UK national guideline for the management of genital tract infection with Chlamydia trachomatis n.d. URL: http://www.bashh.org/guidelines (accessed 2012 Nov. 2).
- Bignell C, FitzGerald M. UK national guideline for the management of gonorrhoea in adults, 2011. Int J STD AIDS 2011;22:541-7.
- Trelle S, Shang A, Nartey L, Cassell JA, Low N. Revised rapid review of effectiveness - partner notification 2006. URL: http://guidance.nice.org.uk/pageaspx?o=371771.
- Methods manual, Version 1: December 2005. London: National Institute for Clinical Excellence; 2005.
- Slater W, Sadler K, Cassell JA, Horner P, Low N. What can be gained from comprehensive disaggregate surveillance? The Avon Surveillance System for Sexually Transmitted Infections. Sex Transm Infect 2007. URL: doi:10.1136/sti.2006.023440.
- Brisson M, Edmunds WJ. Impact of model, methodological, and parameter uncertainty in the economic analysis of vaccination programs. Med Decision Making 2006;26:434-46.
- Koopman JS, Jacquez G, Chick SE. New data and tools for integrating discrete and continuous population modeling strategies. Ann NY Acad Sci 2001;954:268-94.
- Kretzschmar M, Turner KM, Barton PM, Edmunds WJ, Low N. Predicting the population impact of chlamydia screening programmes: comparative mathematical modelling study. Sex Transm Infect 2009;85:359-66.
- Curtis L, Netten A. Units Costs of Health and Social Care 2003.
- Low N, McCarthy A, Roberts TE, Huengsberg M, Sanford E, Sterne JAC, et al. Partner notification for chlamydia in primary care: randomised controlled trial and analysis of resource use. BMJ 2006;332:14-9.
- Guide to the Methods of Technology Appraisal. London: National Institute for Clinical Excellence; 2004.
Appendix 2 Cochrane Central Register of Controlled Trials search strategy44
Reproduced from Ferreira A, Young T, Mathews C, Zunza M, Low N. Strategies for partner notification for sexually transmitted infections, including HIV. Cochrane Database Syst Rev 2013; 10:CD002843 http://dx.doi.org/10.1002/14651858/CD002843.pub2 with permission from John Wiley and Sons. 44 Copyright © 2013 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Database: The Cochrane Library 2011, Issue 1 (2001–12)
Date: 22 March 2011, 29 January 2012 and 31 August 2012
| ID | Search |
|---|---|
| #1 | MeSH descriptor Sexually Transmitted Diseases explode all trees |
| #2 | MeSH descriptor Herpes Genitalis, this term only |
| #3 | MeSH descriptor Chancroid, this term only |
| #4 | MeSH descriptor Chlamydia trachomatis, this term only |
| #5 | MeSH descriptor Gonorrhea, this term only |
| #6 | MeSH descriptor Syphilis, this term only |
| #7 | MeSH descriptor Lymphogranuloma Venereum, this term only |
| #8 | MeSH descriptor Granuloma Inguinale, this term only |
| #9 | MeSH descriptor Calymmatobacterium, this term only |
| #10 | MeSH descriptor Treponema pallidum, this term only |
| #11 | MeSH descriptor Condylomata Acuminata, this term only |
| #12 | MeSH descriptor Human papillomavirus 6 explode all trees |
| #13 | MeSH descriptor Hepatitis B explode all trees |
| #14 | MeSH descriptor Trichomonas Vaginitis, this term only |
| #15 | (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14) |
| #16 | sexually transmitted disease*:ti,ab,kw OR sexually transmissible disease*:ti,ab,kw OR sexually transmitted infection*:ti,ab,kw OR sexually transmissible infection*:ti,ab,kw OR sexually transmitted infectious disease*:ti,ab,kw OR sexually transmissible infectious disease*:ti,ab,kw OR sexually transmitted disorder*:ti,ab,kw OR sexually transmissible disorder*:ti,ab,kw OR STI:ti,ab,kw OR STIs:ti,ab,kw OR STD:ti,ab,kw OR STIs:ti,ab,kw OR venereal disease*:ti,ab,kw OR venereal infection*:ti,ab,kw OR venereal disorder*:ti,ab,kw OR genital herpes:ti,ab,kw OR herpes genitalis:ti,ab,kw OR genital infection*:ti,ab,kw OR genital disorder*:ti,ab,kw |
| #17 | herpes simplex:ti,ab,kw OR herpes virus:ti,ab,kw OR HSV-1:ti,ab,kw OR HSV-2:ti,ab,kw OR chancroid*:ti,ab,kw OR haemophilus ducreyi:ti,ab,kw OR chlamydia infection*:ti,ab,kw OR chlamydia trachomatis:ti,ab,kw OR gonorrhoea*:ti,ab,kw OR gonorrhea*:ti,ab,kw OR syphilis:ti,ab,kw OR syphillis:ti,ab,kw OR condylomata lata:ti,ab,kw OR chancre*:ti,ab,kw OR lymphogranuloma venereum:ti,ab,kw OR granuloma inguinale:ti,ab,kw OR donovania:ti,ab,kw OR donovanosis:ti,ab,kw OR calymmatobacterium granulomatis:ti,ab,kw OR klebsiella granulomatis:ti,ab,kw OR klebsiella granulomatis:ti,ab,kw OR treponema pallidum:ti,ab,kw OR genital wart*:ti,ab,kw OR venereal wart*:ti,ab,kw OR hpv-6:ti,ab,kw OR hpv-11:ti,ab,kw OR hpv6:ti,ab,kw OR human papillomavirus:ti,ab,kw OR hepatitis b:ti,ab,kw OR trichomonas vaginitis:ti,ab,kw OR genital ulcer*:ti,ab,kw OR anogenital ulcer*:ti,ab,kw OR anorectal ulcer*:ti,ab,kw OR anorectal ulcer*:ti,ab,kw OR penile ulcer*:ti,ab,kw |
| #18 | (#15 OR #16 OR #17) |
| #19 | MeSH descriptor HIV Infections explode all trees |
| #20 | MeSH descriptor HIV explode all trees |
| #21 | hiv OR hiv-1* OR hiv-2* OR hiv1 OR hiv2 OR HIV INFECT* OR HUMAN IMMUNODEFICIENCY VIRUS OR HUMAN IMMUNEDEFICIENCY VIRUS OR HUMAN IMMUNE-DEFICIENCY VIRUS OR HUMAN IMMUNO-DEFICIENCY VIRUS OR HUMAN IMMUN* DEFICIENCY VIRUS OR ACQUIRED IMMUNODEFICIENCY SYNDROME OR ACQUIRED IMMUNEDEFICIENCY SYNDROME OR ACQUIRED IMMUNO-DEFICIENCY SYNDROME OR ACQUIRED IMMUNE-DEFICIENCY SYNDROME OR ACQUIRED IMMUN* DEFICIENCY SYNDROME |
| #22 | MeSH descriptor Lymphoma, AIDS-Related, this term only |
| #23 | (#19 OR #20 OR #21 OR #22) |
| #24 | (#18 OR #23) |
| #25 | MeSH descriptor Contact Tracing, this term only |
| #26 | partner notification:ti,ab,kw OR partner notifications:ti,ab,kw OR contact tracing:ti,ab,kw OR expedited partner:ti,ab,kw OR patient delivered:ti,ab,kw OR referral:ti,ab,kw OR referrals:ti,ab,kw OR partner tracing:ti,ab,kw |
| #27 | (#25 OR #26) |
| #28 | (#24 AND #27) |
| #29 | (#24 AND #27), from 2001 to 2011 |
Appendix 3 Search strategy
Databases searched were MEDLINE, EMBASE, ISI Web of Knowledge, NHS EED, DARE and HTA, from 1980 to 31 December 2011.
| # | Term |
|---|---|
| #1 | Chlamydia.mp. or exp CHLAMYDIA/ or CHLAMYDIA TRACHOMATIS/ |
| #2 | Gonorrhea.mp. or exp GONORRHEA/ |
| #3 | Pelvic inflammatory disease.mp. or exp pelvic inflammatory disease/ |
| #4 | PID.mp. or exp pelvic inflammatory disease/ |
| #5 | Cervicitis.mp. or exp uterine cervicitis/ |
| #6 | Chronic pelvic pain.mp. |
| #7 | Ectopic pregnancy.mp. or exp ectopic pregnancy/ |
| #8 | Epididymitis.mp. |
| #9 | Economic evaluation.mp. or economic evaluation/ |
| #10 | Cost-utility analysis.mp. or "cost utility analysis"/ |
| #11 | Cost-effectiveness analysis.mp. or "cost effectiveness analysis"/ |
| #12 | Quality of life.mp. or "quality of life"/ |
| #13 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 |
| #14 | 9 or 10 or 11 |
| #15 | 13 and 14 |
| #16 | 14 and 15 |
| #17 | 15 or 16 |
Categories used for study selection
-
Strictly primary research study (original data collected for the study) for costs and outcomes, which reports on costs or utilisation of care, quality of life of individuals with chlamydia, and includes a formal economic evaluation.
-
Combination of primary and secondary research studies, which reports on the costs or utilisation of care but HRQL of life is not based on primary research, and includes a formal economic evaluation.
-
Study discusses economic aspects of care, contains useful primary and secondary cost or utilisation data but not an economic evaluation.
-
Study is not a full economic evaluation but has primary data on outcome valuation (instruments and techniques for health state valuation for eliciting the value for avoiding chlamydia).
-
Study discusses economic aspects of policies for care and does not fit into categories A to D.
-
Study has no relevance to the instruments and techniques available for eliciting the value of the health state for avoiding chlamydia.
Studies categorised as A, B, C or D were considered potentially relevant and were reviewed in full. Studies in categories E and F were excluded.
List of abbreviations
- APT
- accelerated partner therapy
- AROT
- absolute reduction in onward transmission
- CASH
- Contraceptive And Sexual Health (service)
- CI
- confidence interval
- ClaSS
- Chlamydia Screening Studies project
- CSO
- Chlamydia Screening Office
- DARE
- Database of Abstracts of Reviews of Effects
- EPT
- expedited partner therapy
- HADS
- Hospital Anxiety and Depression Scale
- HIV
- human immunodeficiency virus
- HRQL
- health-related quality of life
- HTA
- Health Technology Assessment
- HUI2
- Health Utilities Index Mark 2
- IoM
- Institute of Medicine
- IQR
- interquartile range
- MAD
- median absolute deviation
- MD
- mean difference
- MOS
- Medical Outcomes Study
- Natsal-2
- second National Survey of Sexual Attitudes and Lifestyles
- NCSP
- National Chlamydia Screening Programme
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute of Health and Care Excellence
- NNTIT
- the number of partners that need to be notified to interrupt one secondary transmission
- OR
- odds ratio
- PCT
- primary care trust
- PEACH
- PID Evaluation and Clinical Health
- PID
- pelvic inflammatory disease
- QALY
- quality-adjusted life-year
- RR
- risk ratio
- SD
- standard deviation
- SF-12
- Short Form questionnaire-12 items
- SF-36
- Short Form questionnaire-36 items
- STD
- sexually transmitted disease
- STI
- sexually transmitted infection
- TTO
- time trade-off
- VAS
- visual analogue scale
- WHO
- World Health Organization