Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/79/03. The contractual start date was in July 2010. The draft report began editorial review in June 2012 and was accepted for publication in October 2012. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
All authors have completed the unified competing interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author). Dr D Breen has undertaken consultancy for Galil Medical Ltd; Professor D Cunningham’s institution has received grants from Amgen Inc. and Merck & Co. Inc.; Professor G Poston has received payment for lectures from pharmaceutical companies and royalties from books. All other authors declare (1) no financial support for the submitted work from anyone other than their employer; (2) no financial relationships with commercial entities that might have an interest in the submitted work; (3) no spouses, partners, or children with relationships with commercial entities that might have an interest in the submitted work; and (4) no non-financial interests that may be relevant to the submitted work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Loveman et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Aim
This project will evaluate the clinical effectiveness and cost-effectiveness of the different ablative and minimally invasive therapies for treating liver metastases. It will review systematically the evidence on those interventions that are currently available and used to treat liver metastases, including radiofrequency ablation, microwave ablation, cryoablation, ethanol ablation, laser ablation, focused ultrasound, electrolytic ablation, chemoembolisation and radioembolisation. These will be assessed separately and/or in sequence, where possible and appropriate. If the systematic review of cost-effectiveness shows that there are no appropriate good-quality economic evaluations, a new economic model relevant to the UK setting will be developed. Deficiencies in current knowledge will be identified and recommendations for future research generated.
Chapter 2 Background
Description of underlying health problem
Malignant neoplasms are, by definition, those that metastasise, although some do so only rarely. Metastases (also known as secondary cancers or secondaries) are lesions that have separated from the primary cancer and have disseminated to distant sites within the body. 1 Metastases are very common in the late stages of cancer, and tend to grow and multiply rapidly, and disseminated cancer accounts for many deaths due to malignancy. The most common route of tumour spread comes via the blood vessels or the lymphatic system. Malignant cells from the primary tumour proliferate to form new tumours in the secondary site or sites. 1 This is a complex process: for example, cells entering the liver may go down one of several pathways. They may immediately be destroyed by local defence mechanisms, they may enter a state of dormancy and never metastasise, they may initiate a short-lived process of proliferation that is aborted before a metastasis is established or they may actively proliferate to form macrometastases. Success or failure of the cells to produce a metastasis depends on their ability to induce stromal reaction within the liver and to recruit host cells into the developing metastasis. Thus, although metastases are initiated by abnormal cells from the primary cancer, not the host site, host cells play a major part in their formation. Metastases in the liver that have come from breast cancer will be partly made up of abnormal breast cancer cells, but most cells will be derived from the host tissue.
The liver is a common site of metastatic disease. It has a rich blood supply, which provides a suitable environment for growth of metastases, and these metastases typically occur in the tissue close to blood vessels. The liver is the first capillary bed to be encountered by the circulating malignant cells of some primary tumours. 2 The liver has a dual blood supply, from the hepatic arteries and from the portal vein. Supplying approximately three-quarters of the liver’s blood supply, the portal vein carries venous blood drained from the spleen and gastrointestinal tract and its associated organs. The hepatic arteries, stemming directly from the aorta, supply arterial blood to the liver, accounting for the remainder of its blood flow. The liver is thus an anatomically and physiologically obvious site for metastases to occur from tumours of the colon and rectum, stomach, pancreas, biliary tree and small intestine. However, breast cancer, lung cancer and melanoma also have a propensity to metastasise in the liver, but in these cases the mechanism leading to the spread is less well understood. 2 Blood supply is not the whole story, however; other tissues with a very good blood supply do not characteristically develop metastases (e.g. heart, skeletal muscle) and other factors must be involved. Recent evidence from Germany has shown that approximately 59% of liver metastases were from colorectal cancer, 13% from pancreatic cancer, 13% from breast cancer, 6% from gastric cancer, 4% from lung cancer, and 4% from oesophageal cancers. 3
Liver metastases can grow as a mass or by spreading through the tissues. Most liver metastases are multiple: in approximately 80% of cases more than one lobe of the liver is affected. 4 Factors such as age, sex, primary cancer site, histological type and duration of tumour influence the incidence and patterns of liver metastases. 4
Epidemiology
Despite advances in treatments for primary cancers, many deaths from cancer are caused by metastatic burden. Survival rates vary, but survival beyond 5 years of patients with untreated metastatic disease in the liver is rare. 5 In metastatic colorectal cancer the median survival without treatment is 8 months from diagnosis of metastatic disease. 6 Prognosis can vary according to the extent of the disease in the liver, and according to the site of the primary cancer. 7 Those with a limited number of metastases, or disease in one lobe of the liver only, tend to survive longer,5 and those with colorectal liver metastases have a better prognosis than those from most other primary cancers. 7
Large-scale population-based assessments of the epidemiology of liver metastases are limited. This is largely because of the fact that data in cancer registries are collected across primary cancer sites, and therefore to gain full coverage for all people with liver metastases requires considerable resource. 8 Studies available have therefore tended to focus on colorectal liver metastases and as such are discussed here. No recently undertaken studies identified on literature searches have a UK focus.
Leporrier and colleagues9 assessed 1325 people with colorectal carcinoma registered on the Digestive Cancer Registry of Calvados, France, between January 1994 and December 1999. At a mean follow-up of 32 months, 358 (27%) individuals developed hepatic metastases. This equated to a rate of 19.5% [95% confidence interval (CI) 17.5% to 21.4%] at 1 year, 25.0% (95% CI 23.1% to 27.0%) at 2 years and 29.3% (27.3% to 31.2%) at 3 years. These data include both synchronous (occurring at the time or within 6 months of surgical resection of colorectal cancer) and metachronous (occurring subsequently) liver metastases. For incidence data on these subgroups, see Table 1 below. Leporrier and colleagues9 also present data on the characteristics of those with liver metastases and the actuarial survival estimates for the group as a whole, which is not discussed here given the range of different treatments people received.
In a population-based cancer registry from Burgundy, France, Manfredi and colleagues8 studied 13,463 people diagnosed with a large bowel cancer between 1976 and 2000. Age-standardised incidence rates of synchronous liver metastases, and actuarial incidence of metachronous liver metastases, can be seen in Table 1.
| Study | All metastases | Synchronous metastases | Metachronous metastases rate | |
|---|---|---|---|---|
| Leporrier et al.9 | ||||
| Registry study Population: 1325 people with colorectal cancer within the Calvados region of France 1994–1999 |
Actuarial incidence of liver metastases, % (95% CI) | |||
| 1 year | 19.5 (17.5 to 21.4) | Not reported | 4.0 (3.1 to 4.8) | |
| 2 year | 25.0 (23.1 to 27.0) | 8.7 (8.3 to 9.1) | ||
| 3 year | 29.3 (27.3 to 31.2) | 13.5 (12.4 to 14.7) | ||
| Proportion with liver metastases during the study period (%) | ||||
| 27 | 18.9 | 8.1 | ||
| Manfredi et al.8 | ||||
| Registry study Population: 13,463 people with colorectal cancer within the Burgundy region of France 1976–2000 |
Age-standardised incidence rate, per 100,000 | |||
| Males | 7.6 | |||
| Females | 3.7 | |||
| Actuarial incidence of liver metastases (%) | ||||
| 1 year | 4.3 | |||
| 3 year | 12.0 | |||
| 5 year | 14.5a | |||
| Proportion with liver metastases (%) | ||||
| Not reported | 14.5 | 12.8 | ||
Although comparisons between these two studies is difficult owing to the different outcomes reported, these results suggest that rates of synchronous liver metastases range from 14.5% to 18.9% of the total populations. Where data are comparable (e.g. 1-year and 3-year data) similar values of actuarial incidence of metachronous liver metastases can be seen across the two studies. The authors of these studies suggest that these rates are in line with earlier published estimates of the incidence of synchronous and metachronous liver metastases. 10,11
These estimates may be high, however. Recent data from a so far unpublished trial of intensive imaging in colorectal cancer (the UK FACS Trial) show that of 1211 patients with curatively treated primary colorectal cancer, all of whom had full evaluation by CT-CAP (computed tomography – chest/abdomen/pelvis) after surgical resection or adjuvant chemotherapy if given and had a normal carcinoembryonic antigen (CEA) prior to trial entry, only 174 (14.4%) had recurrences, with a median follow up of 54 months. 12 These results are derived from a randomised controlled trial (RCT), where experience has shown that patient treatment is generally better than in ‘the real world’. They show, however, that if patients are fully staged by computed tomography (CT) after treatment of the primary tumour and have a normal CEA, then the relapse rate is low, suggesting that all metastases are synchronous and that those which appear to be metachronous have been missed, or in some cases may not have been looked for.
Cancer statistics in the UK suggest that approximately 39,000 cases of colorectal cancer are diagnosed each year. 13 An estimate of the possible incident cases can be made by using the proportions seen to develop liver metastases in the studies by Leporrier and colleagues9 and Manfredi and colleagues. 8 If between 14% and 19% of people diagnosed with colorectal cancer had liver metastases at the time of diagnosis, it would be expected that there would be in the region of between 5000 and 7000 cases in the UK each year. Estimating the incidence of subsequent liver metastases is more difficult from these data sets as the rates of metachronous liver metastases relate to the proportions seen over the entire study durations in both studies, and 1, 2 and 3 years in Leporrier and colleagues’,9 and 1, 3 and 5 years in Manfredi and colleagues’ studies. 8 Using 1-year rates of 4–4.3% would, however, suggest approximately 1500–1600 new cases per year. Taking these estimates together suggests that there may be somewhere in the region of 7000–9000 incident cases of colorectal liver metastases in the UK each year. Corresponding data are seen when estimating the rate by using the 1-year actuarial incidence (19.5%) of all metastases in the Leporrier and colleagues’ study. 9 An estimate of the likely number of people who will develop liver metastases within 5 years of diagnosis can be made using the data from the Manfredi and colleagues8 study. This suggests that approximately 5000 individuals who did not have liver metastases at diagnosis would be expected to develop liver metastases within 5 years of diagnosis.
These estimates relate only to those with colorectal cancer and only from data within 1 year of colorectal diagnosis, and therefore the number of cases of liver metastases in the UK would be expected to be higher than those shown here.
The prevalence of liver metastases is similarly difficult to establish, with most evidence being based on pathological case series reports rather than population-based studies. As such, reports give varying pictures of the likely number of people with liver metastases. For example, autopsy studies suggest that somewhere between 30% and 70% of people who have died from cancer had liver metastases. Precise estimates for the numbers of people affected by liver metastases are therefore difficult to establish.
The impact of liver metastases
People with liver metastases may show relatively few symptoms and in many cases are symptom free. However, for others, pain, weight loss, nausea and fevers are frequently experienced. A small group of people develop jaundice or abdominal ascites (an accumulation of fluid in the abdominal cavity) that can be uncomfortable and painful. In addition, the psychological impact of having a life-limiting illness is considerable.
In recent years, improvements in survival through cytoreductive therapies have led to an increased interest in the use of local therapies for liver metastases. Local therapies have been shown to be effective in eradicating early-stage primary liver tumours. However, the case for using such therapies in people with liver metastases is less well established. With a higher likelihood of people surviving longer after effective systemic therapy, even if not cured, the eradication of residual metastases via local therapies may improve prognosis and quality of life. 14
The procedure of choice has been surgical resection, with 5-year survival figures ranging from 25% to 39%15,16 for liver metastases from colorectal cancer. However, surgical resection is feasible in only approximately 20–30% of people. 17 Non-surgical alternatives have been developed in recent years to treat some liver metastases that are not suitable for surgical resection, and in some cases to be used as an alternative to surgical resection. Minimally invasive treatments can include various forms of ablative therapies and other targeted treatments.
Current service provision
The current service provision of ablation treatments for colorectal liver metastases in the UK is well regulated within the various cancer plans of the four NHS health-care providers (England, Scotland, Wales and Northern Ireland). Cancer networks covering populations of about 2 million within each provider have designated tertiary specialised hepatobiliary centres to provide and be reimbursed for these treatments, through network-designated Specialist Hepatobiliary Multidisciplinary Teams (SMDTs). Effectively this means 18 such providers in England, three in Scotland, and one each in Wales and Northern Ireland. Elsewhere in Europe, the provision of these services is more haphazard, being similar in Scandinavia and the Netherlands, but fragmented in other health care economies including France and Germany.
The delivery of percutaneous image-guided (radiological) ablation of liver metastases is usually coterminous with the provision of liver surgery, usually on the same hospital site, and certainly within the same organisation. All decisions relating to whether or not to offer ablation therapy to a patient with colorectal liver metastases are made through the hepatobiliary SMDT, although some latitude will be offered to an operating surgeon who, at the time of surgery with the intention of resecting colorectal liver metastases, may reconsider the operative strategy to include surgical resection plus ablation or ablation alone.
Cryotherapy, laser therapy and ethanol injection have largely been abandoned for the treatment of colorectal liver metastases. Radiofrequency ablation, either percutaneous or operative, has been the standard of care for the last decade; however, it is now being rapidly superseded in clinical practice by microwave ablation. Microwave ablation has the advantage that it generates sufficient energy to destroy metastases in only 20% of the time required for similar tumour destruction using radiofrequency ablation, yet has the same capital cost for the energy generator and single use disposable delivery probe as radiofrequency ablation (and as such is considered more cost-effective). Present estimates are that about 2000 patients are being offered ablation therapies for colorectal liver metastases each year in England.
Description of technology under assessment
Ablative therapies
Ablation is the localised destruction of abnormal tissue in situ. 18 Tumour ablation is defined as the direct application of chemical or thermal therapies to a specific focal tumour or tumours in an attempt to achieve eradication or substantial tumour destruction. 19 The objectives of ablative therapy vary from complete ablation of all lesions with curative intent to palliative debulking of the tumour load. A potentially curative ablation aims to extend the area of treatment beyond the margins of the metastasis, whereas palliative debulking aims to achieve maximum tumour necrosis while preserving adequate liver function and patient well-being. 20
Ablative therapies are a separate group of therapies to those that are delivered via a catheter (transcatheter therapies such as embolisation, chemoembolisation and radioembolisation)20,21 (see Other non-invasive therapies).
There are two broad categories of ablative therapy: thermal ablation and chemical ablation. 19,20 Different terminology has been used in the literature to refer to some of these therapies, and so for consistency this report uses recommended22 standard terms where possible, rather than necessarily those used by the studies (Table 2).
| Standard term used in report | Alternative terminology |
|---|---|
| Thermal ablation techniques | |
| Cryoablation19 | |
| Laser ablation | Laser coagulation therapy |
| Laser interstitial tumour therapy | |
| Laser-induced thermotherapy | |
| Laser interstitial photocoagulation19 | |
| Laser-induced interstitial thermotherapy20 | |
| Microwave ablation19 | Percutaneous microwave coagulation therapy |
| Microwave coagulation therapy | |
| Radiofrequency ablation19,20,23 | |
| Ultrasound ablation19 | Focused ultrasound |
| Chemical ablation techniques | |
| Acetic acid ablation19 | Percutaneous acetic acid injection23 |
| Electrolytic ablation24,25 | Electrolysis |
| Ethanol ablation | Percutaneous ethanol instillation/injection |
| Percutaneous alcohol instillation19,20 | |
Thermal ablation techniques
These techniques use a source of thermal energy to destroy a tumour either with heat (e.g. radiofrequency ablation) or cold (e.g. cryoablation). Tumour cells are heated to a temperature that produces coagulative necrosis or lesser degrees of cell death, either by prolonged heating of cells at 50–55 °C or short exposure to temperatures above 60 °C. 20
Each technique uses a different type of applicator to deliver the thermal energy: radiofrequency ablation applicators are monopolar (single) or bipolar (two) electrodes; microwave applicators are radiating antennas; laser applicators are light-emitting fibres; and due to existing convention cryoablation applicators are termed cryoprobes. 19 Thermal ablation procedures may be carried out by open surgery, laparoscopically or percutaneously. 19,26,27
Blood flow affects all of the thermal ablation methods by potentially removing heat before complete tumour ablation is achieved (the heat sink effect refers to cooling by adjacent visible blood vessels) or by prematurely warming tissue and limiting the effects of freezing. Parenchymal perfusion or perfusion-mediated tissue cooling (or heating) also acts to diminish the overall ablation volume achieved. 19 Strategies to overcome this include pharmacologically decreased blood flow, hypotensive anaesthesia, temporary vascular balloon occlusion of a specific vessel, intra-arterial embolisation and chemoembolisation, and a Pringle manoeuvre (temporary hepatic arterial and portal venous occlusion by means of direct compression of the vessels) during laparotomy. 19
Cryoablation
Cryoablation has a longer history than some other local ablative therapies, but there is controversy regarding its use in the liver, including recent guidance suggesting that it be used in the context of clinical trials only. 28 Cryoablation destroys tissue by delivering tissue-lethal freeze–thaw cycles to the tissue via cryoprobes through which a cryogen (liquid nitrogen or, more frequently, argon gas) is circulated. 19,26,29 By insulating the probe shaft and delivery hoses, cooling is limited to the probe tip. Liquid nitrogen and argon gas are capable of producing temperatures of at least –100 °C and cellular death results from direct freezing of tissue between –20 and –40 °C. 26,29 Freezing potentially produces large ablation zones and allows clearer delineation of the margins around the metastases. The procedure has usually required general anaesthesia and laparotomy for probe placement;29 however, cryoprobes small enough to be used percutaneously have been developed in recent years. 20 The efficacy of liver cryoablation is unclear. 28
Complications potentially include infections and haemorrhage, and perioperative mortality from myocardial infarction, pulmonary embolus, respiratory failure and cryoshock syndrome (a systemic response consisting of marked thrombocytopenia leading to coagulopathies, acute respiratory distress syndrome-like syndrome and myoglobinuria). 28
Laser ablation
Laser ablation uses a laser source such as neodymium-doped yttrium aluminium garnet (Nd: YAG)19,29 to deliver high-energy light (wavelength between 800 and 1064 nm) into the tumour. The optical fibres are placed into the tumour through a percutaneously located needle and the emitted photons travel up to 12 mm through soft tissue producing heating and cell death. 20 The terms ‘direct’ or ‘interstitial’ are often reported to clarify that optical fibres are inserted directly into the tissue; however, it has been recommended that ‘laser ablation’ replaces terms such as ‘laser interstitial tumour therapy’, ‘laser coagulation therapy’ and ‘laser interstitial photocoagulation’. 19
To enlarge the area of necrosis multiple fibres can be inserted into the tumour at regularly spaced intervals. This allows up to a 6–7-cm area of necrosis when the fibres are simultaneously energised. Adaptations have also been made to the fibre tip to avoid localised overheating and charring which decreases photon penetration, such as water-cooled fibres. 20,29 Treatment times vary but may exceed 1 hour for a large (6–7 cm diameter) ablation. 29
Smaller tumours show greater success30 and 5-year survival rates of 26% and median survival rates of 22–41 months have been reported across varying tumours. 29,31–35 Complications include pain, segmental infarction, abscess, pleural infusion and tumour seeding. 29
It is thought that this technique has been largely replaced by others, although it may still be used outside the UK.
Microwave ablation
Microwave ablation refers to all electromagnetic methods of inducing tumour destruction by using devices with frequencies from 30 MHz to 30 GHz. 19 As with radiofrequency ablation, microwave ablation involves placement of an antenna directly into the target tumour, under imaging guidance. Thermal coagulation of tissues is caused when microwaves (between 915 MHz and 2.45 GHz) oscillate water molecules and impart tissue heating through inefficiencies in this process. 20,29 Dependent on frequency, and unlike radiofrequency ablation, the zone of active heating around the probe feedpoint is approximately 2 cm either side of the probe. Microwave ablation does not utilise retractable tines and the resulting ablation tends to be slightly more elliptical. 29 However, the treatment sessions are shorter than for radiofrequency ablation with a 2-cm ablation zone produced in 60 seconds with microwave therapy. Multiple overlapping ablations are used to treat larger volumes. 20
Microwave ablation can be undertaken percutaneously, with imaging guidance and confirmation of probe positioning, or through open or laparoscopic surgery. 36
Adverse events include abscess, bleeding, infection, pneumothorax, colonic perforation, fever, pain, tumour seeding, pleural effusion, and (rarely) bile duct injury. Theoretical complications might include deterioration in liver function, and adjacent organ damage to the kidney, lung or heart. 36
At present, most of the evidence for microwave ablation has been published in East Asia and concerns hepatocellular carcinoma rather than metastatic disease. 26 A recent study based on registry data from 18 international centres included 140 participants, 81% of whom were treated with microwave ablation alone. This study suggests short times to achieve tumour ablation and low morbidity and mortality rates. 37 Clinical experts have stated that microwave ablation using 2.45-GHz probes are being used in the UK and appear to produce more rounded ablation zones with fewer issues of ‘reflected power’ and thinner ablation zones than seen with devices at 915 MHz.
Interventional procedure guidance issued in 2011 by the National Institute for Health and Care Excellence (NICE) stated that ‘current evidence on microwave ablation for the treatment of liver metastases raises no major safety concerns. The evidence on efficacy is inadequate in quantity and quality. Therefore this procedure should only be used with special arrangements for clinical governance, consent and audit or research’. 36 The cost-effectiveness of microwave ablation was not discussed in the NICE guideline.
Radiofrequency ablation
The term radiofrequency ablation applies to coagulation induced from all electromagnetic energy sources with frequencies less than 30 MHz. 19 An electrode is inserted into the tumour, usually assisted using some form of image guidance. 19,20 High-frequency alternating current (460 kHz)20,29 is transmitted from the tip of the electrode into the immediate tissue, causing ionic agitation and subsequent frictional heating of localised tissue to 100 °C, resulting in tissue death in an approximately spheroid volume of tissue. 26,29,38 The zone of active heating is, however, within only a few millimetres of the probe tines and tissue destruction is considerably reliant on conductive heating with its vagaries in the in vivo setting. A 2-cm to 5-cm spherical thermal injury can be produced with each ablation, which takes in the region of 20 minutes. 29
The needle tip has to be kept as cool as possible to prevent the adjacent tissue becoming charred, which increases impedance and prevents conduction of current into the tumour beyond the zone of ablation. 20 There are many electrode modifications available, and the type used influences the extent and predictability of ablation. Multitined expandable electrodes have an array of multiple electrode tines that expand from a central needle,19 allowing distribution of current distant from the needle tip. 20 Internally cooled electrodes are perfused with saline or water to prevent overheating of the needle tip. Perfusion electrodes have small apertures at the active tip that allow saline to be infused or injected into the tissue before, during or after ablation. 19
Depending on the size, location and number of tumours, radiofrequency ablation can be undertaken percutaneously, laparoscopically, or through open surgery (laparotomy). 38 The percutaneous approach is the least invasive and minimises morbidity. It is used for a limited number of small tumours usually remote from hollow viscera. ‘Hydrodissection’ techniques can be utilised to displace adjacent thermally sensitive organs, for small recurrences after prior surgical resection and for patients who are not candidates for other approaches for anatomic or clinical reasons. The laparoscopic approach reduces access morbidity and can permit some mobilisation of the liver, and detection of additional hepatic or extrahepatic disease. The open laparotomy approach is the most invasive and can be performed alone or in conjunction with other procedures such as surgical resection. It allows fuller mobilisation of the liver and use of the Pringle manoeuvre, and is most often used for large tumours, larger number of tumour or tumours in difficult locations. 38
A systematic review of complications of intraoperative radiofrequency ablation of liver metastases39 reported overall mortality ranging from 0% to 1.8% in studies of people undergoing radiofrequency ablation without surgical resection, and morbidity rates ranging from 0% to 16%. The review reported rates of wound infection (0–1.8%), biliary complications (0–1.8%), pleural effusion (0–3.6%), liver failure (0–1.8%), vascular complications (0%) and skin burns (0–1.8%). Complications specific to radiofrequency ablation include hepatic abscesses, caused by infection of the necrotic tissue in the ablation site; biliary stenosis, which appear after the fibrous healing of biliary tract thermal damage; vascular thrombosis, caused by thermal endothelial damage; and skin burns, which occur when the dispersion surface is inadequate for the radiofrequency power.
Interventional procedure guidance issued in 2009 by NICE stated that ‘current evidence on the safety and efficacy of radiofrequency ablation for colorectal liver metastases is adequate to support the use of this procedure in people unfit or otherwise unsuitable for surgical resection, or in those who have previously had hepatic surgical resection, provided that normal arrangements are in place for clinical governance, consent and audit’. 40 The cost-effectiveness of radiofrequency ablation was not discussed in the NICE guideline.
Ultrasound ablation (focused ultrasound)
High-frequency sound waves (ultrasound), which use energy levels of 30–100 W, can heat tissue to over 90 °C and ablate liver metastases. Two methods of application of the ultrasound are currently used: extracorporeal (or transcutaneous), and direct, for percutaneous application with a needle-like applicator. 19,41 In the former method, the ultrasound ablation is truly non-invasive. Most reports of the use of ultrasound ablation to date relate to prostate, uterine fibroids and hepatocellular carcinoma, but it can be used in any solid tumour, including metastatic disease in the liver. Although early reports in hepatic solid tumours indicated some promise,42 clinical experts advise that it is not widely used in the UK.
Chemical ablation techniques
Acetic acid ablation
An alternative chemical agent which can be used for chemical ablation is acetic acid19 (alternative descriptions include percutaneous acetic acid injection23). The procedure itself mirrors that of ablation with ethanol. Most reports to date relate to its use in hepatocellular carcinoma rather than liver metastases and clinical experts have indicated that it is not widely used in the UK.
Electrolytic ablation (electrolysis)24,26
Electrolytic ablation is performed by passing a direct current between two platinum electrodes. This creates a change in the pH of the surrounding tissues, with an acidic environment around the anode and an alkaline environment around the cathode. 24,26 Chemical reactions then also cause the creation of toxic products such as chlorine and hydrogen ions26 and these chemical changes result in tissue destruction in the tumour. There is, at the present time, a lack of high-quality published data on the efficacy of electrolytic ablation in liver metastases. 24 Clinical experts have stated that electrolytic ablation is not widely used in the UK.
Ethanol ablation
Percutaneous ethanol injection is the chemical ablative technique with the most extensive clinical experience. Most reports to date relate to its use in hepatocellular carcinoma rather than liver metastases. It is relatively simple to perform, quite inexpensive, and requires minimal equipment. Percutaneous ethanol injection is performed by the injection of absolute alcohol through a needle placed percutaneously directly into a tumour. The necrosis produced by ethanol injection results from cellular dehydration and tissue ischaemia from vascular thrombosis. However, ethanol ablation is not as effective as thermal ablation techniques for the treatment of metastases due to the limitations of adequate physical dispersal. For appropriately sized tumours, ethanol ablation improves survival and compares favourably with surgical resection for patients with hepatocellular carcinoma. 43,44
Other non-invasive therapies
Other forms of minimally invasive treatment involve the combination of various substances to block the circulation through the hepatic artery (i.e. embolisation) and to introduce different chemo- or radio-therapeutic agents to reduce or remove the malignancy. Although not ablative therapies, they are used as cytostatic/cytoreductive interventions for managing liver metastases.
Chemoembolisation
Transarterial chemoembolisation provides a treatment option that is minimally invasive as a cytoreductive therapy. It is an alternative to systemic chemotherapy, surgical resection and non-surgical ablative techniques to treat resectable and non-resectable tumours. This technique aims to selectively target the liver metastases by delivering chemotherapy locally to the tumour via a hepatic arterial catheter combined with embolisation of the blood flow which acts both to prevent washout of the chemotherapeutic agent into the general circulation and to cause selective tumour ischaemia,45 the aim being to improve the pharmacodynamic availability of the delivered drug and reduce systemic toxicity. It is felt that liver metastases are particularly amenable to chemoembolisation given their relative arterialisation against background and the fact that the liver parenchyma receives two independent blood supplies, which serves to minimise normal parenchymal injury. Commonly used chemotherapeutic agents include doxorubicin, cisplatin and mitomycin (Mitomycin C Kyowa®, Kyowa Hakko) which may be used either singly or in combination. The chemotherapeutic agent(s) may also be mixed with ethiodised oil (i.e. lipiodol), a viscous material that was originally used as a contrast agent but which also helps to localise the chemotherapy drugs inside the tumour cells. 45,46 A variety of different agents have been used to achieve embolisation and these agents have different properties. The properties of the particles that make up the embolic agent are an important consideration as these factors determine whether the embolisation will be permanent or transient, and what diameter of capillary will be occluded by the particles and the rate of drug elution from the embolic material. Infusion rate and particle size, shape (spherical or non-spherical) and concentration all affect the way that the particles penetrate the target tissue. 45,46 New controlled-release technologies in the form of drug-eluting beads provide a means to simultaneously deliver embolisation and chemotherapy. Furthermore, drug-eluting beads release the chemotherapeutic agent that they are loaded with in a more sustained manner for a prolonged period of time, thereby enhancing drug delivery to the tumour and reducing systemic toxicity. 45,46 In general, the systemic concentration of the chemotherapy drug being delivered via drug-eluting beads is low, which helps to limit possible systemic side effects.
Treatment may be repeated for the same tumour to enhance the adequacy of treatment and may be repeated in a staged approach in the contralateral lobe, for example, so as to reduce overall hepatotoxicity. A recent non-systematic review46 cites RCTs which showed increased overall survival for treating hepatocellular carcinoma. The evidence reported by this review indicates some benefits when treating hepatic metastases of breast, colorectal, and neuroendocrine primary tumours although none of the studies cited appears to be a RCT in which overall survival is reported. As drug-eluting bead transarterial chemoembolisation is a more recent technology, the evidence base is less mature; nevertheless, a recent systematic review47 identified eight studies. Three of these are case series focusing on liver metastases, which report response rates and procedure associated complications. No survival outcomes are reported.
Radioembolisation
Although radiotherapy is an important part of the treatment of most malignancies, its use in hepatic cancers has been limited due to the low tolerance of the organ to radiation, the risk of radiation hepatitis and damage to adjacent organs. 20 Recent technological developments (e.g. co-axial microcatheters and the development of embolic carrier particles of ∼30 μm) have, however, permitted targeted internal delivery of radiotherapy to metastases while minimising background irradiation, a form of ‘microbrachytherapy’. 48 As such, it relies on careful preparation of the liver to determine vascular anatomy and embolic exclusion of potential extrahepatic collaterals so as to ensure appropriate deployment of the microembolic to the target metastases. To ensure that no particles are able to travel to non-target sites, embolisation with metal coils20,21 of vessels that feed tissue beyond the area to be treated may also be required before treatment begins. The microspheres that act as the carrier particles may be made of glass, for example TheraSpheres® (Nordion, Ottawa, ON, Canada) which are 20–30 μm in diameter,49 or resin, for example SIR-Spheres® (Sirtex Medical Ltd, Sydney, NSW, Australia) which have diameters between 20 and 60 microns. 50 Both of the two commercially available microspheres contain the β-emitter yttrium-90 (90Y), which has a maximum range of emissions in tissue of 11 mm (mean 2.5 mm) and a half-life of 64 hours. 49,50 Providing that the microspheres are correctly directed to the tumour, the short penetration distance means that radiation doses above 120 Gy can be delivered to the tumour with tolerable levels of radiation to the normal liver tissue. 48 Radioembolisation has been used in the treatment of primary and metastatic liver tumours. Unlike local ablation techniques, surgical resection and external beam radiation, the scope of the therapy is not limited by the number, size or location of the tumours within the liver. 20,48 However, there are some contraindications to treatment which include arterial-systemic shunting from liver to lungs and major arterial reflux from the hepatic vasculature to arteries supplying the gastroduodenal region. Radioembolisation is usually delivered with concomitant local or systemic chemotherapy. A recent meta-analysis51 identified two RCTs in which resin microspheres were used to treat colorectal metastases with the encouraging results of a survival benefit for the radioembolisation arm. 51 There is also evidence showing benefits in reducing liver metastases, allowing subsequent surgical resection. It is also thought to increase time to progression, have limited adverse effects, be tolerable and maintain quality of life. This study also included non-RCT data and studies focusing on hepatocellular carcinoma but a conclusion could not be drawn from this larger pool of evidence about whether glass or resin microspheres were more effective. Radioembolisation is relatively easy to deliver and can be undertaken as a day case or with a one-night stay, but requires significant angiographic work-up before administration.
Relevant comparators
Surgical resection of metastases
The aim of surgical resection of liver metastases is to remove all macroscopic disease with clear margins and leave sufficient functioning liver. 17 Five-year survival rates following the procedure range from 25% to 39%. 15,16
Selection criteria for liver resection usually include controlled or controllable primary tumour, no extrahepatic metastases detectable (or extrahepatic disease that can also be completely resected) and surgical resection technically feasible with tumour-free margins. Surgical resection would not be undertaken in people with such widespread hepatic involvement that residual liver function following surgical resection would be inadequate. People should be fit enough to tolerate general anaesthesia, have no major comorbidity and ideally have normal liver function. People with extrahepatic disease that should be considered for liver resection include resectable/ablatable pulmonary metastases; resectable/ablatable isolated extrahepatic sites, for example spleen, adrenal, or resectable local recurrence; and local direct extension of liver metastases to diaphragm/adrenal that can be resected. 52 In the case of liver metastases from colorectal cancer, approximately 20–30% of people may have disease that is potentially resectable. 17 A further 20–30% of people with unresectable liver limited disease might be brought to surgical resection with curative intent using induction systemic chemotherapy. 53 In some people curative surgical resection is aborted during surgery as metastases are unresectable.
Chemotherapy
Chemotherapy provides a clinically effective option for treating liver metastases in people who are not considered suitable for surgical resection. 6,54 As well as having been shown to improve survival itself, chemotherapy has resulted in the downsizing of tumours in cases where it was initially thought not possible to resect due to their location or inadequate hepatic functional reserve. 6,54,55 Where a compromised hepatic function reserve is a concern,56 preoperative chemotherapy may reduce overt tumour volume, so increasing the potential volume of future remnant liver. 57 In some cases the use of chemotherapy has allowed subsequent surgical resection or ablation, improving the chances of long-term survival and the possibility of cure. 58,59 Others have used a two-stage approach to surgical resection with an initial non-curative surgical resection or ablation followed by chemotherapy and further surgical resection to remove any remaining tumour. 59,60 Consideration of such strategies is recommended to be taken by a regional hepatobiliary unit.
Several options for chemotherapy have been recommended by NICE. 61,62 These include oxaliplatin-based regimens and irinotecan-based regimen for people with non-resectable liver metastases. 62 NICE have also recognised the benefits of the addition of therapies that target the epidermal growth factor receptor to conventional cytotoxic chemotherapies, which are thought to improve the chances of surgical resection of previously irresectable or suboptimally resectable tumours considerably, although they are of benefit only in patients who do not have activating mutations on the K-RAS gene. The addition of cetuximab (Erbitux,® Merck Serono) to oxaliplatin-based regimens and irinotecan-based regimens (if unable to tolerate, or contraindications to, oxaliplatin) has also been recommended by NICE for first-line treatment of metastatic colorectal cancer. 61
Best supportive care
Best supportive care (BSC) is often used as the comparator arm in cancer trials; however, it is usually not well defined and may be left open to local interpretation. A Cochrane systematic review of supportive care for gastrointestinal cancer found that the descriptions of supportive care in the included studies were often vague and heterogeneous, making direct comparisons difficult. 63 BSC may include antibiotics to control infections, analgesics (including non-steroidal anti-inflammatory drugs and opioids), antiemetic drugs, transfusions for anaemia, corticosteroids, nutritional support, localised radiation therapy to alleviate symptoms such as pain, and psychosocial support. The European Organisation for Research and Treatment of Cancer (EORTC) Pain and Symptom Control Task Force agreed on the following definition: ‘supportive care for cancer patients is the multi professional attention to the individual’s overall physical, psychosocial, spiritual and cultural needs, and should be available at all stages of the illness, for patients of all ages, and regardless of the current intention of any anti-cancer treatment’. 63 Chemotherapy can be administered as a palliative treatment to people with unresectable metastatic disease. It can prolong both the time to tumour progression and survival. 6
Place of the interventions in the treatment pathway
The clinical pathway for people with liver metastases depends in part upon the primary cancer the person has when the metastases were diagnosed and the extent and location of the metastases. Although liver metastases may originate from several primary sites, most research has been focused on colorectal liver metastases as treatment has been noted to confer survival benefit. As such, the clinical pathway outlined reflects that recommended for people who have liver metastases from colorectal cancers. 17,64
It is recognised that the management of people with liver metastases associated with colorectal cancer should involve a hepatobiliary multidisciplinary team based in a cancer centre with responsibility for colorectal cancer and, where available, for hepatobiliary cancer. Such teams would be likely to include specialist surgeons trained and maintaining a special interest in liver resection, oncologists, diagnostic and interventional radiologists with expertise in hepatobiliary disease, histopathologists and clinical nurse specialists. 17
The detection of liver metastases may occur at the point of first presentation and diagnosis of the primary cancer or subsequently during follow-up. Contrast CT scans of the abdomen are used to detect liver metastases, although their sensitivity and specificity will vary depending on the equipment and contrast enhancement used. Contrast enhanced magnetic resonance imaging (MRI) provides an alternative option for assessing the liver for metastases and can be more effective than CT scans for small volume disease. Ultrasound examination is thought not sufficiently sensitive to identify the presence of liver metastases. CEA levels are thought to be elevated in up to 90% of people with liver metastases, although the point at which these levels rise is less certain. Baseline measures should be undertaken, allowing assessment of local or distant recurrence following initial treatment. The occurrence of isolated liver metastases should be followed up with CT scans of the chest, abdomen and pelvis. Biopsy of the liver should not be performed without discussion with the regional hepatobiliary unit, as there is the opportunity for dissemination of the metastases. Fluorodeoxyglucose positron emission tomography (FDG-PET) may also be used to identify hepatic colorectal metastases and extrahepatic disease that may have a bearing on treatment planning and help with staging of the cancer.
Surgical resection has been considered the primary treatment option for people with liver metastases. The intention is to remove all macroscopic disease with clear (negative) margins, while leaving sufficient functioning liver. Candidates for surgical resection include people with solitary, multiple and bilobar disease that have undergone, or who are able to undergo, radical treatment for the primary cancer. It can be undertaken as a major or a staged process. Staged surgical resection may be undertaken where there is a bulky bilateral lesion and it may be effective in sparing more normal liver than a major surgical resection or in allowing surgical resection of metastases not normally included in a major surgical resection. The selection criteria for people to undergo liver resection and the contraindications are outlined above. Although the surgical resection of colorectal cancer and liver metastases would not normally be undertaken simultaneously, this has occurred, and has been shown to be safe and efficient when undertaken in high-volume centres. Decisions concerning surgical resection and the patient’s suitability for this should be discussed by the surgeon in consultation with a radiologist, an anaesthetist and the patient. Recurrence occurs in 60% to 70% of people and follow-up should be continued for at least 5 years using CT of the chest and liver and blood CEA. 17
Of the people unable to undergo liver resection, those with isolated unresectable metastases and no extrahepatic disease may be considered for ablative therapy. There are several ablative therapies which may be curative for some people and offer the opportunity for improvements in symptoms and quality of life more generally (see Chapter 5, Independent economic evaluation). The relative clinical effectiveness of the different ablative therapies remains unclear and decisions concerning their use should be considered by the surgeon, oncologist and interventional radiologist in consultation with the patient. For those people not suitable for either liver resection or ablative therapy, referral should be made to the clinical and medical oncologists for further management and supportive care.
Chemotherapy also has a role in managing people with liver metastases. People who have extensive liver metastasises may receive systemic chemotherapy, hepatic artery catheter chemotherapy or chemoembolisation. Systemic chemotherapy should be administered to people with extrahepatic disease. It is thought that intra-arterial chemotherapy has consistently higher response rates compared with systemic chemotherapy for people with liver metastases, due to the more direct delivery to the liver. Hepatic artery chemoembolisation has developed to treat unresectable non-disseminated liver tumours, increasing the response rates and decreasing tumour dissemination. Portal vein embolisation may be used as an adjunctive technique to cause liver hypertrophy and extend the scope of liver resection. On occasions, chemotherapy has been shown to reduce tumours to allow surgical resection. Selective internal radiation therapy has been used for people with extensive liver disease without extrahepatic metastases who have failed with 5-fluorouracil (5-FU) and other cytotoxic treatments. However, the data on the effectiveness of 5-FU administered via hepatic artery infusion provide no clear evidence to support its use and combination chemotherapy may be equally or more effective. It is not used commonly in the UK.
Chapter 3 Methods for systematic review of clinical effectiveness and cost-effectiveness
The a priori methods for systematically reviewing the evidence of clinical effectiveness and cost-effectiveness are described in the research protocol (see Appendix 1), which was sent to experts for comment. Although helpful comments were received relating to the general content of the research protocol, there were none that identified specific problems with the methodology of the review. The methods outlined in the protocol are briefly summarised below. The systematic review followed the general principles outlined in the Centre for Reviews and Dissemination (CRD) report ‘Undertaking Systematic Reviews of Research of Effectiveness’ (third edition)65 and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement on the reporting of systematic reviews and meta-analyses. 66
Identification of studies
A comprehensive search strategy was developed, tested and refined by an experienced information scientist. Separate searches were conducted to identify studies of clinical effectiveness, cost-effectiveness, quality of life, and epidemiology. Sources of information and search terms are provided in Appendix 2. Searches were undertaken in March 2011 and updated in September 2011.
A total of 14 electronic resources were searched: 10 databases listing published papers and abstracts and four databases listing ongoing studies. Searches were from 1990 to September 2011 with no language restrictions. The following electronic databases were searched: MEDLINE (Ovid); MEDLINE In-Process & Other Non-Indexed Citations (MEIP); EMBASE; The Cochrane Library including Central Register of Controlled Trials (CENTRAL) and Cochrane Database of Systematic Reviews (CDSR); CRD including Health Technology Assessment (HTA) Database, Database of Abstracts of Reviews of Effects (DARE) and NHS Economic Evaluation Database (NHS EED); Science Citation Index and Conference Proceedings Citation Index (Web of Science); Zetoc, The British Library; National Institute for Health Research Clinical Research Network (NIHR CRN) Portfolio; Current Controlled Trials (CCT); Clinical trials.gov; and World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP).
In addition, professional society websites and conferences were searched for recent abstracts and ongoing studies (see Appendix 2). Bibliographies of included articles were checked for any additional references, and our expert advisory group was contacted to identify additional published and unpublished studies.
Study selection and data extraction
Studies were selected for inclusion in the systematic review of clinical effectiveness through a two-stage process using predefined and explicit criteria. The full literature search results were independently screened by two reviewers to identify all citations that possibly met the inclusion criteria. Full papers of relevant studies were retrieved and assessed by one reviewer and checked by a second reviewer using a standardised eligibility form. As far as possible, full papers or abstracts describing the same study were linked together, with the article reporting key outcomes designated as the primary publication.
Data were extracted by one reviewer using a standard data extraction form (see Appendix 4) and checked by a second reviewer. At each stage, any disagreements between reviewers were resolved by consensus or if necessary by arbitration by a third reviewer.
Titles and abstracts identified by the search strategy for the systematic review of cost-effectiveness were assessed for potential eligibility by two health economists using predetermined inclusion criteria. Full papers were formally assessed for inclusion by one health economist with respect to their potential relevance to the research question.
Quality assessment
The methodological quality and the quality of reporting of the included clinical effectiveness studies were assessed using criteria based on those recommended by the CRD65 (see Appendix 3). Quality criteria were applied by one reviewer and checked by a second reviewer, with any differences in opinion resolved by consensus or by arbitration by a third reviewer.
Quality assessment for the systematic review of cost-effectiveness was based on a checklist for economic evaluation publications67 and guidelines for good practice in decision-analytic modelling in health technology assessment. 68
Inclusion and exclusion criteria
Intervention
All ablative therapies currently used in the UK, either alone or in sequence, including:
-
radiofrequency ablation
-
microwave ablation
-
cryoablation
-
ethanol ablation
-
laser ablation
-
focused ultrasound
-
electrolytic ablation.
Other minimally invasive therapies currently used, specifically:
-
chemoembolisation
-
radioembolisation.
Comparators
-
Surgical resection of metastases.
-
Chemotherapy.
-
BSC (as defined by the included studies and including chemotherapy as part of BSC/palliative care).
Participants
-
People with liver metastases from any solid tumour primary site.
Outcomes
-
Procedure-related morbidity, mortality and hospital stay.
-
Rate of complete tumour ablation.
-
Local recurrence rate.
-
Progression-free survival.
-
Overall survival.
-
Health-related quality of life.
-
Costs and cost-effectiveness.
Design
-
RCTs.
-
Prospective non-randomised comparative studies.
-
Prospective case series studies (sample size > n = 100).
-
Economic evaluations (i.e. costs and consequences), including cost-effectiveness, cost–utility or cost-benefit analyses.
Systematic reviews identified by the search were used as a source for identifying primary studies (see Appendix 1) and summarised (see Chapter 4, Existing systematic reviews).
Studies published as abstracts or conference presentations were included only if sufficient details were presented to allow an appraisal of the methodology and the assessment of results to be undertaken. Only abstracts published from 2006 onwards were eligible for inclusion as it was expected that full publications would be available for abstracts published before this time.
Where there was evidence from different types of study design for a specific intervention, only those studies with the most rigorous designs were included and data extracted.
Studies that have assessed surgical resection of metastases have used several terms describing the intervention and presenting results, including surgery, surgical resection and hepatectomy. Where appropriate, these different terms have been changed to surgical resection to help clarity. The original terms used have been kept in references, data extraction tables, where a specific description or reference is made.
Method of data synthesis
Studies of clinical effectiveness and cost-effectiveness were synthesised through a narrative review with full tabulation of the results of all included studies. It was considered inappropriate to combine the results of the studies in a meta-analysis due to differences in the outcome measures and patient populations. Within the clinical effectiveness section of this report, results are discussed according to the intervention to aid interpretation.
Evidence from case series is generally considered to be very low quality. 69 Thus, limited attention is given to these studies to avoid undue weight being given to their results. The narrative synthesis of case series studies focuses on the key outcomes for the whole study population (or participants eligible for inclusion in the systematic review only, if reported separately) for survival, mortality, response rates and adverse events. Where studies have reported outcomes for various subgroups this has been noted in the data extraction forms (see Appendix 4). If the subgroups were specified a priori and appeared to be statistically powered, they were discussed in the text.
The methods for the economic model are described in Chapter 5 (see Independent economic evaluation).
Chapter 4 Clinical effectiveness
Totality of research available
Searching identified 5381 references after deduplication. The number of references excluded at each stage of the systematic review is shown in Figure 1. References which were retrieved but later excluded are listed in Appendix 5 with reasons for exclusion. Studies were often excluded for more than one reason; the most common reason was study design, including small sample sizes of prospective case series studies (121 studies), followed by irrelevant participants (34 studies), irrelevant intervention (21 studies), and irrelevant comparator (four studies). Fourteen studies reported data only in abstracts and, as they did not provide sufficient information, were excluded. Two studies remain unclear on whether they are prospective or retrospective despite review by three independent reviewers, and were therefore excluded. These are listed in Appendix 5. Seventy-four potentially relevant non-English references were identified by the searches and can be seen in Appendix 6. After examination of the titles and English abstracts (where available) seven of these studies were retrieved for closer inspection. Three studies were excluded at this stage, leaving four studies of possible relevance to the review. In view of the limited time and resources, translation and full screening of the papers was not undertaken. These studies are listed in Appendix 6. Searches identified 46 existing reviews or systematic reviews which were used as a source of references. Finally, five potentially relevant ongoing studies were identified, and these are described in Research in progress later in this chapter.
FIGURE 1.
Flow chart of identification of studies. a, Two studies remain unclear on study design.
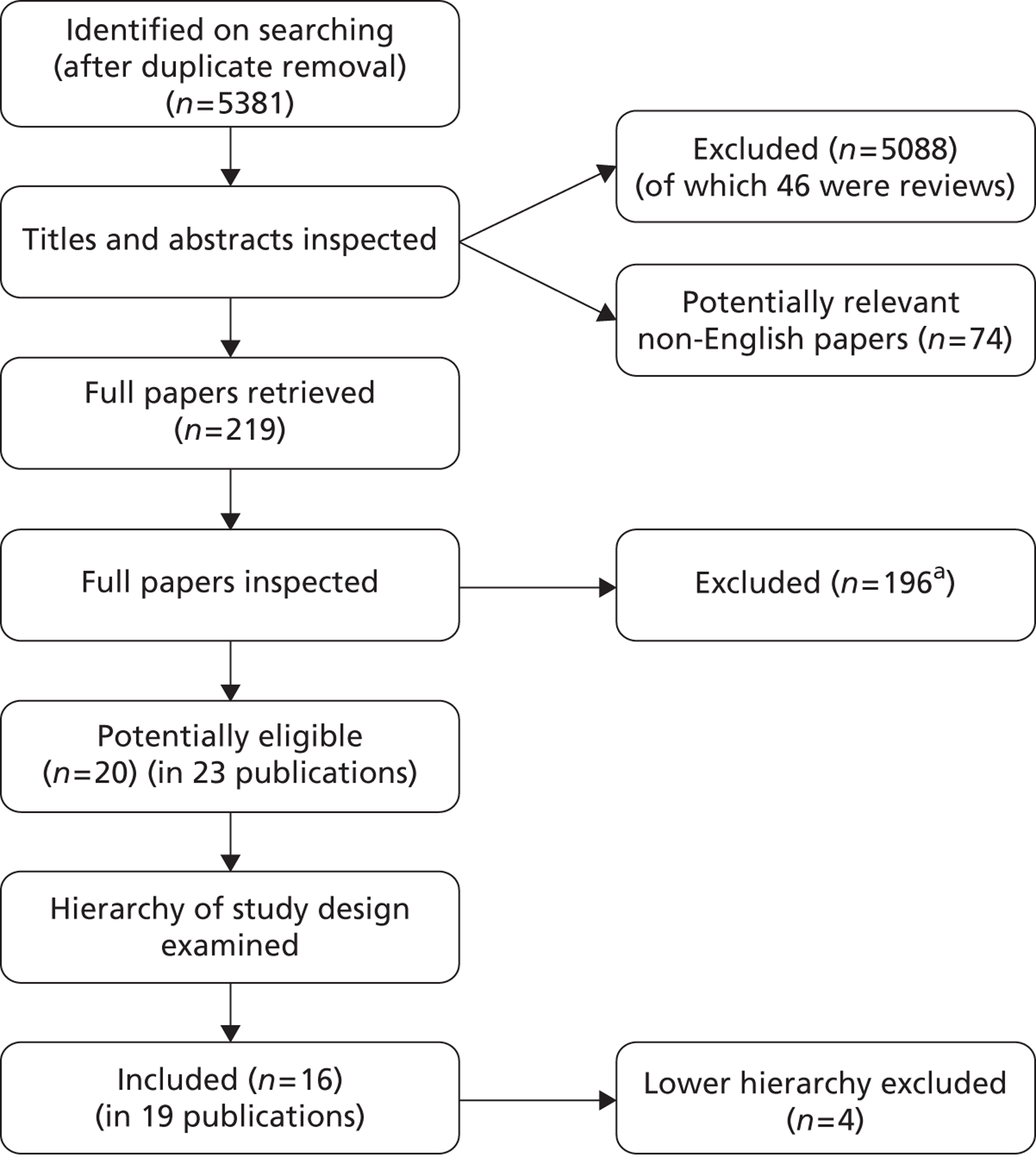
Twenty potentially eligible studies (in 23 publications) were identified. After selecting the highest level of evidence available for each intervention, 16 studies (in 19 publications) were included in the systematic review of clinical effectiveness. The included studies were either RCTs or prospective case series studies. A summary of the highest level of evidence available for each intervention can be seen in Table 3, with further details of these studies being reported in subsequent sections. The remaining four lower evidence studies are listed in Appendix 5.
| First author | Intervention | Comparator | Study design |
|---|---|---|---|
| Ablative technologies | |||
| Mack70 | Laser ablation | None | Case series |
| Mack71 | Laser ablation | None | Case series |
| Shibata72 | MWA | Surgical resection | RCT |
| Kim73 | RFA | (1) Surgical resection | Non-randomised comparison |
| (2) Surgical resection + RFA | |||
| Berber74,75 | RFA | None | Case series |
| Gillams76,77 | RFA | None | Case series |
| Siperstein78 | RFA | None | Case series |
| Solbiati79 | RFA | None | Case series |
| Sorensen80 | RFA | None | Case series |
| Other non-invasive therapies | |||
| Taguchi81 | Chemoembolisation | Chemotherapy | RCT |
| Agarwala82 | Chemoembolisation | Chemotherapy | RCT |
| Vogl83 | Chemoembolisation and laser ablation | None | Case series |
| Vogl84 | Chemoembolisation and laser ablation | None | Case series |
| Gray85 | Radioembolisation | HAC | RCT |
| Hendlisz86 | Radioembolisation | Chemotherapy | RCT |
| Van Hazel87,88 | Radioembolisation | Chemotherapy | RCT |
Laser ablation
Quantity and quality of research
Two prospective case series70,71 describing laser ablation were included (Table 4). The narrative synthesis of these data focuses on the key outcomes for survival, mortality, response rates and adverse events, as described in Chapter 3 (see Method of data synthesis). Subgroups were reported in the studies; however, these do not appear to have been specified a priori or be statistically powered and the results are therefore not discussed here (see Appendix 4 for further details).
| Study | Key inclusion criteriaa | N | Age (years), mean | Sex, M/F | No. of LM, mean | Size of LM (% metastases) | Previous treatment | Extrahepatic disease (%) |
|---|---|---|---|---|---|---|---|---|
| Mack et al., 200170 (Germany); follow-up: NR | Various tumours. Recurrent metastases after partial liver resection; metastases in both liver lobes; contraindications for surgical resection; unresectable lesions. Five or fewer metastases, metastases ≤ 5 cm diameter, no extrahepatic spread; refused surgical resection | Total, N = 705b | NR | NR | NR | NR | NR | 0 |
| Colorectal, n = 393 | 60.8 | NR | NR | NR | NR | 0 | ||
| Breast, n = 127c | NR | NR | NR | NR | NR | 0 | ||
| Mack et al., 200471 (Germany); follow-up: mean 1.8 years | Breast primary. Recurrent metastases after partial liver resection; metastases in both liver lobes; contraindications for surgical resection; non-resectable tumours; refused surgical resection. Five or fewer metastases, metastases ≤ 5 cm diameter, no extrahepatic spread. Bone metastases under control not a contraindication | 232c | 54.4 (SD 9.9) | 0/232 | 2.5 (range 1–13) | < 2 cm: 43.4 | All had chemo prior to or after therapy | 31d |
| 2–3 cm: 33.0 | ||||||||
| 3–4 cm: 13.0 | ||||||||
| 4–5 cm: 10.6 |
Both included case series were from the same institution in Germany, and some of the participants with breast cancer may have been reported in both studies (see Table 4). The 2001 study by Mack and colleagues70 presented combined data from participants with a variety of primary tumours, including hepatocellular carcinoma, which were not eligible for inclusion in this systematic review. However, the authors also presented data separately for participants with colorectal cancer and breast cancer, which are reported here as per our inclusion criteria. However, caution is required as these data are subgroup analyses (see Chapter 3, Method of data synthesis). The 2004 study by Mack and colleagues71 included only women with liver metastases from breast cancer. Other eligibility criteria, such as the number and size of metastases, were similar between studies. The 2001 study70 specified no extrahepatic spread, but in the 2004 study 31% of participants had bone metastases considered to be under control. 71 The mean age of participants was reported only for the colorectal cancer group in the 2001 study,70 and at 60.8 years this was slightly higher than for participants with breast cancer in the 2004 study (54.4 years). 71 Other baseline characteristics were not reported in the 2001 study. 70 Participants in the 2004 study71 all had chemotherapy prior to or after treatment of liver metastases and they had a mean of 2.5 (range 1–13) liver metastases. The studies did not state whether intention of treatment was curative or palliative.
Case series provide very low-quality evidence69 and the quality of reporting of the two included case series70,71 was poor (Table 5). Both studies specified patient selection criteria in advance, but neither study completely described whether or not there were withdrawals and dropouts or reported if or how statistical analysis accounted for missing data. Both studies failed to adequately describe blinding of participants to the research question, although the importance of this is more relevant to subjective outcomes, such as quality of life, than to objective outcomes, such as mortality. It was unclear whether or not authors measured more outcomes than they reported, which can result in reporting bias.
Assessment of effectiveness: laser ablation
Mean survival of participants with liver metastases from colorectal cancer who had been treated with laser ablation was 41.8 months (95% CI 37.3 months to 46.4 months) in the 2001 study by Mack and colleagues,70 although it is not clear whether this is from diagnosis or treatment of liver metastases. Among those with breast cancer liver metastases, mean survival was 51.6 months (95% CI 43.2 months to 60 months). 70 In the 2004 study, mean survival of women with breast cancer was 58.8 months from diagnosis of liver metastases [(95% CI 51.6 months to 64.8 months), median survival 51.6 months (95% CI 40.8 months to 63.6 months)] and 50.4 months (95% CI 43.2 months to 57.6 months) from first treatment with laser ablation. However, the authors of the 2004 series note that the estimated mean survival times are biased owing to the number of censored cases, that is to say if the event had not been noted in the patient chart by the end of the observation period the cases were treated as if the event had been reported at that time. Survival rates ranged from 85%71 to 97%70 at 1 year, and from 30%70 to 41%71 at 5 years (Table 6); however, comparison between studies is difficult due to unclear reporting of, and differences in, the calculation of survival.
| Study details | Estimated overall survival | Mortality | ||||||
|---|---|---|---|---|---|---|---|---|
| Survival calculated from: | Median survival (95% CI), months | 1 year (%) | 2 years (%) | 3 years (%) | 4 years (%) | 5 years (%) | During follow-up | |
| Mack et al., 2001;70 follow-up: NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Colorectal primary, n = 393 | NR | Mean 41.8 (37.3 to 46.4) | 93 | 74 | 50 | NR | 30 | NR |
| Breast cancer primary, n = 127a | NR | Mean 51.6 (43.2 to 60)b | 97 | 75 | 65 | NR | 34 | NR |
| Mack et al., 2004;71 breast cancer primary, n = 232;a follow-up: mean 1.8 years | Diagnosis of LM | 51.6 (40.8 to 63.6);b mean 58.8 (51.6 to 64.8)b | 96 | 80 | 63 | NR | 41 | NR |
| Treatment of LM | Mean 50.4 (43.2 to 57.6)b | 85 | 66 | 51 | NR | 38 | NR | |
The low quality of the evidence from these studies should be taken into consideration when interpreting these results.
Adverse events: laser ablation
Only one study71 reported adverse events from laser ablation (Table 7). No deaths occurred within 30 days of treatment. The most common adverse event was non-symptomatic pleural effusion, which occurred 41 (9.1%) times in 452 treatments sessions. There were 20 (4.4%) events of small non-symptomatic subscapular haematoma. Less frequent events also occurred (see Table 7).
| Study details | Total number of complications | Details |
|---|---|---|
| Mack et al., 2004;71 breast cancer primary, n = 232; length of follow-up: mean 1.8 years; 452 treatment sessions | 68a | 30-day mortality: 0 |
| Pleural effusion: 4 | ||
| Non-symptomatic pleural effusion: 41 | ||
| Liver abscess: 2 | ||
| Injury to bile duct: 1 | ||
| Bronchial biliary fistula: 0 | ||
| Pneumothorax: 0 | ||
| Small non-symptomatic subscapular haematoma: 20 |
Summary of clinical effectiveness: laser ablation
No comparative studies of laser ablation were identified. Two prospective case series, which provide very low-quality evidence, were included from the same institution. One study reported data for people with liver metastases from colorectal cancer and breast cancer separately, and the second study involved people with breast cancer primary tumours only, although the eligibility criteria for the studies were similar. Minimal baseline characteristics were provided for the breast and colorectal subgroups. The studies provided estimates of overall survival for the study population, but comparisons are difficult due to unclear reporting of, and differences in, the calculation of survival. One study provided data on adverse events.
Microwave ablation
Quantity and quality of evidence available
One RCT72 that compared microwave ablation with surgical resection met the inclusion criteria (Table 8). The RCT by Shibata and colleagues72 took place at a single centre in Japan and randomised 40 participants whose primary cancer was colorectal. The intention of treatment with microwave ablation was not explicitly stated but participants were potentially amenable to surgical resection and the aim of the study was to compare the therapeutic efficacy of microwave ablation in comparison with surgical resection. Other key criteria for inclusion in the RCT were that there should be fewer than 10 liver metastases and that the greatest dimension of the largest tumour should be < 80 mm. The length of participant follow-up after the intervention was not reported. For further details of the intervention see Appendix 4.
| Study | Key inclusion criteria | Arm | n | Age (years), mean | Sex (M/F) | No. of LM | Size of LM | Previous treatment | Extrahepatic disease (%) |
|---|---|---|---|---|---|---|---|---|---|
| Shibata et al., 200072 (Japan) follow-up: not stated | Colorectal carcinoma. Potentially amenable to surgical resection. Primary tumour and one or more liver tumours characterised histologically, more than one but fewer than 10 liver metastases, greatest dimension of largest tumour < 80 mm, no periportal lymph node, coeliac lymph node or extrahepatic distant metastases, or ascites, no liver cirrhosis or chronic hepatitis | MWA | Randomised n = 20; studied n = 14 | 61 (SD 10) | 6/8 | Mean 4.1 (SD 2.1) | Largest mean 27 mm (SD 11) | NR | 0a |
| Surgical resection | Randomised n = 20; studied n = 16 | 61 (SD 9) | 10/6 | Mean 3.0 (SD 1.0) | Largest mean 34 mm (SD 17) | NR | 0a |
Although 40 participants were enrolled, only 30 participants were studied and reported on in the trial publication (see paragraph below describing withdrawals). Slightly more than half of these 30 participants were male, although in the microwave ablation arm females outnumbered males. The mean age of participants was 61 years in each trial arm. The microwave ablation participants had a higher mean number of liver metastases (4.1 vs. 3.0) with a slightly smaller mean size of the largest tumour than the participants with surgical resection. Further details on baseline characteristics of the included participants can be seen in Appendix 4.
Many of the methodological details needed to judge methodological validity were not reported (Table 9). The authors reported an adequate method of generating the randomisation sequence, although it was not reported whether or not any method was used to conceal intervention allocation. Without concealed allocation there would be the possibility of selection bias. Shibata and colleagues72 did not report whether or not those assessing patient outcomes were blind to intervention assignment. The outcome of overall survival is not likely to be affected by blinding but there would be a risk of detection bias for the outcomes of disease-free interval and adverse events if outcome assessors were not blind to the intervention received by the patient.
| Study | Random allocation? | Allocation concealment? | Similar groups | Blinding of outcome assessors? | Unexpected imbalances in withdrawals? | Selective outcome reporting? | Intention-to-treat analysis | Missing data accounted for? |
|---|---|---|---|---|---|---|---|---|
| Shibata et al., 200072 | Yes | NR | Yes | NR | No | Unclear | No | NR |
Ten participants were withdrawn from the trial intraoperatively when additional tumours were found either in the liver or elsewhere, which meant that these participants no longer met the inclusion criteria for the trial. The group assignments and withdrawal reasons were provided for each of the 10 participants and these were similar between the two arms of the trial. The paper does not report whether or not any method was used to account for missing data; however, no further withdrawals were reported among the 30 participants who continued in the trial. The study did not undertake an intention-to-treat analysis and, as noted above, other than the 10 participants who were withdrawn intraoperatively, there appeared to be no other missing data to account for. It was not clear whether or not the authors had measured more outcomes than they were reporting on in the trial publication. Overall, therefore, although 10 of the initially randomised participants dropped out of the trial and an intention-to-treat analysis was not conducted, the risk of measurement bias appears low because the reasons for participant dropout are provided and appear balanced between the groups.
Assessment of effectiveness: microwave ablation
Overall survival
Shibata and colleagues72 calculated overall survival (which was one of their two primary outcomes) from the time of treatment to the end of the follow-up period (the length of this was not reported). During follow-up nine of the microwave ablation group and 12 of the surgical resection group died (Table 10). There was no statistically significant difference in the estimated cumulative survival rates (p = 0.83). Mean overall survival in the microwave ablation group was 27 months versus 25 months in the surgical resection group. The estimated 1-, 2- and 3-year survival rates also indicated that therapeutic efficacy was similar between the two treatments (see Table 10).
| Study details | Estimated overall survival calculated from treatment | Mortality | ||||||
|---|---|---|---|---|---|---|---|---|
| Arm | Mean, months | 1 year (%) | 2 years (%) | 3 years (%) | 4 years (%) | 5 years (%) | During follow-up | |
| Shibata et al., 2000;72 colorectal primary, n = 40; follow-up: not stated | MWA (n = 14) | 27 | 71 | 57 | 14 | NR | NR | 9 |
| Surgical resection (n = 16) | 25 | 69 | 56 | 23 | NR | NR | 12 | |
| p-value | NR | NR | NR | NR | NR | NR | NR | |
Response
Response data were not reported by Shibata and colleagues. 72
Disease-free survival
Shibata and colleagues72 reported the outcome of disease-free survival but did not define this (Table 11). There was no statistically significant difference in the mean duration of disease-free survival between microwave ablation and surgical resection groups (11.3 months vs. 13.3 months respectively, p = 0.47).
| Study details | Disease-free interval (months) | |
|---|---|---|
| Arm | Mean (months) | |
| Shibata et al., 2000;72 colorectal primary, n = 40; follow-up: not stated | MWA (n = 14) | 11.3 |
| Surgical resection (n = 16) | 13.3 | |
| p-value | 0.47 | |
Indications of surgical invasiveness
Surgical invasiveness was the second of the study’s primary outcomes. 72 Although there was no statistically significant difference between the two interventions for operation time or the length of hospitalisation there was statistically significantly less intraoperative blood loss in the microwave ablation group compared with the surgical resection group {mean 360 ml [standard deviation (SD) 230 ml] vs. 910 ml [SD 490 ml], p = 0.027}. Furthermore, no participants in the microwave ablation group required a blood transfusion, whereas six participants (38%) required a mean volume of 540 ml of transfused blood in the surgical resection group (Table 12).
| Study details | Indications of surgical invasiveness, mean (SD) unless otherwise stated | |||||
|---|---|---|---|---|---|---|
| Arm | Intraoperative blood loss (ml) | Blood transfusion (ml) | Participants requiring blood transfusion | Operation time (minutes) | Hospitalisation (days) | |
| Shibata et al., 2000;72 colorectal primary, n = 40; follow-up: not stated | MWA (n = 14) | 360 (230) | 0 (0) | n = 0 (0%) | 180 (20) | 20 (7) |
| Surgical resection (n = 16) | 910 (490) | 540 (690) | n = 6 (38%) | 200 (50) | 25 (12) | |
| p-value | 0.027 | 0.08 | 0.035 | 0.20 | 0.23 | |
Adverse events
Adverse events occurred in both trial arms with no statistically significant difference between them (two participants in the microwave ablation group vs. three participants in the surgical resection group, p = 0.87). In the microwave ablation group one participant had a hepatic abscess and one had a bile duct fistula. A bile duct fistula was also a complication for one participant in the surgical resection group, the other adverse events in the surgical resection group being an intestinal obstruction and a wound infection (Table 13).
| Study details | Adverse events, number (%) of participants | Total frequency of complications | |||||
|---|---|---|---|---|---|---|---|
| Arm | Intra- or post-operative deaths | Intestinal obstruction | Bile duct fistula | Hepatic abscess | Wound infection | ||
| Shibata et al., 2000;72 colorectal primary, n = 40; follow-up: not stated | MWA (n = 14) | 0 | 0 | 1 (7) | 1 (7) | 0 | 2 (14.3) |
| Surgical resection (n = 16) | 0 | 1 (6) | 1 (6) | 0 | 1 (6) | 3 (18.8) | |
| p-value | NR | NR | NR | NR | NR | 0.87 | |
Summary
One RCT72 compared microwave ablation with surgical resection. The methodological quality of the RCT appeared reasonable although some details needed to judge methodological quality were not reported. No statistically significant difference in the estimated cumulative survival, disease-free interval or incidence of adverse events was found between the two groups. Measures of surgical invasiveness (blood loss, requirement for and volume of blood transfusion) all showed a statistically significant difference in favour of the microwave ablation group. However, this did not lead to any shortening of the operation time or length of hospital stay as there was no significant difference in these two outcomes between the groups.
Radiofrequency ablation
Quantity and quality of evidence available
One prospective non-randomised comparison study73 and five prospective case series studies describing radiofrequency ablation were included (Tables 14 and 15). The narrative synthesis of the data from the case series studies focuses on the key outcomes for survival, mortality, response rates and adverse events, as described in Chapter 3 (see Method of data synthesis). Subgroups were reported in some of these studies, however these do not appear to have been specified a priori or be statistically powered and the results are therefore not discussed here (see Appendix 4 for further details).
| Study | Key inclusion criteria | Arm | n | Age (years), mean | Sex (M/F) | No. of LM | Size of LM | Previous treatment | Extrahepatic disease (%) |
|---|---|---|---|---|---|---|---|---|---|
| Kim et al., 201173 (Republic of Korea); follow-up: not stated | Colorectal carcinoma. Potentially amenable to surgical resection. No extrahepatic metastases at onset of treatment | RFA | 177 | 60.4 (SD 10.7) | 121/56 | Mean 1.6 (SD 0.9) | Largest mean 2.1 cm (SD 1.0) | NR | 0a |
| Surgical resection | 278 | 57.1 (SD 10.9) | 168/110 | Mean 1.5 (SD 0.8) | Largest mean 2.6 cm (SD 2.0) | NR | 0a | ||
| RFA + surgical resection | 27 | 55.7 (SD 11.1) | 15/12 | Mean 3.1 (SD 1.6) | Largest mean 2.1 cm (SD 1.4) | NR | 0a |
| Study | Key inclusion criteriaa | n | Age (years), mean | Sex (M/F) | No. of LM, mean | Size of LM, mean | Previous treatment | Extrahepatic disease (%) |
|---|---|---|---|---|---|---|---|---|
| Berber et al. 200574,75 (USA); follow-up: 1–52 months | Colorectal primary. Unresectable, minor extrahepatic disease allowed, failure to respond to other treatment, fewer than eight metastases, < 20% total liver volume replaced with tumour | 135 | 62 (SEM 1) | 85/50 | (Ablated) 3.2 (SEM 0.2) | Largest 4.1 (SEM 0.4) | Chemotherapy 80%; liver resection 14% | 30 |
| Gillams and Lees 200976,77 (UK); follow-up: NR | Colorectal primary. Inoperable due to inadequate residual liver volume or inability to achieve margins. Five or fewer tumours of ≤ 5 cm diameter, or fewer than nine tumours with diameter ≤ 4 or 4.5 cm, or a solitary tumour < 7 cm diameter. Extrahepatic disease if stable on treatment. Larger tumours ablated later in study | 309 | 64 (range 24–92) | 198/111 | 4 (range 1–27) | Largest 3.7 (range 0.9–12) | Liver resection 16% | 37 |
| Siperstein et al. 200778 (USA); follow-up: median 24 (range 1–94) months from ablation | Colorectal primary. Not candidates for surgical resection and/or failed chemotherapy, presence of extrahepatic spread, fewer than 12 hepatic lesions with a maximal dominant lesion size of 10 cm | 234 | 62 (SEM 1) | 153/81 | 2.8 (SEM 0.14) | Largest 3.9 (SEM 0.2) | Chemotherapy ‘majority’ | 23.5 |
| Solbiati et al. 200179 (Italy); follow-up: 6–52 months | Colorectal primary. Not candidates for surgical resection due to extrahepatic metastases, prior hepatic metastasectomy, age, disease extent and/or comorbidity, or refused surgical resection | 117 | 64.8 (SD 10.8) | 81/36 | n (%) | Largest 3.2 (SD 1.3) | Chemotherapy 89%;c liver resection 24% | 0b |
| One – 74 (63.2) | ||||||||
| Two – 29 (24.8) | ||||||||
| Three – 9 (7.7) | ||||||||
| Four – 5 (4.3) | ||||||||
| Sorensen et al. 200780 (Denmark); follow-up: mean 23.6 (range 1–92) months | Colorectal primary. Inoperable due to prior surgical resection or comorbidity or refused surgical resection. Four or fewer tumours ≤ 4 cm diameter. No known extrahepatic spread | 102 | 64 (range 33–84) | 61/41 | 3.3 (range 1–17) | All metastases: 2.2 (range 0.5–6.5) | Chemotherapy 25%; liver resection 25%d | 0b |
All participants in the non-randomised comparison study (Kim and colleagues)73 had colorectal liver metastases. The participants were all reported to be eligible for surgical resection except those with comorbidities, difficult anatomical sites of metastases or more than four metastases. All five case series studies included participants with colorectal liver metastases who were not candidates for surgical resection due to various reasons (see Table 15). The study by Berber and colleagues74,75 was associated with two publications which had overlapping recruitment dates. Although the 2008 paper75 reported that intention of treatment was curative, this was not reported in the 2005 paper74 containing data relevant to this systematic review. Intention of treatment was not reported by the other studies. The eligibility criteria, such as the number and size of metastases and the presence of extrahepatic spread, varied between studies. The mean age of participants ranged from 55 to 65 years, and 55% to 70% of the participants were men. Participants had a mean of 1.573 to 476,77 liver metastases, although the number ranged up to 27 metastases in one of the studies. 76,77 In one study that did not report mean number of metastases, most (63%) participants had just one tumour. The mean size of the largest liver tumour was similar between the case series studies reporting this, ranging from 3.279 to 4.1 cm. 74 In the comparison study the mean maximum size of liver tumour ranged from 2.1 to 2.6 cm across the three treatment groups. 73 Some of the participants had undergone previous liver resection in four of the studies,74,76,77,79,80 and chemotherapy for liver metastases was also reported by four of the studies. 74,78–80
The quality of reporting in the non-randomised comparative study was poor, with details lacking to assess many aspects of the quality of the study and subsequent risk of bias (Table 16). This study therefore has an uncertain risk of bias which should be noted when interpreting the results. Case series provide very low-quality evidence69 and the quality of reporting of the five included case series was poor (Table 17). One79 of the five included studies did not appear to specify patient selection criteria in advance, and four74,76–79 of the studies did not completely describe whether or not they had any withdrawals and dropouts. None of the studies reported if or how statistical analysis accounted for missing data. All studies failed to adequately describe blinding of participants to the research question, although the importance of this is more relevant to subjective outcomes, such as quality of life, than to objective outcomes, such as mortality. It was unclear whether or not authors measured more outcomes than they reported, which can result in reporting bias.
| Study | Patient criteria specified a priori? | Allocation concealment? | Similar groups | Blinding of outcome assessors? | Selective outcome reporting? | Withdrawals described? | Unexpected imbalances in withdrawals? | Intention-to-treat analysis | Missing data accounted for? |
|---|---|---|---|---|---|---|---|---|---|
| Kim et al.73 | No | Unclear | No | NR | Unclear | NR | NR | NR | NR |
| Study | Patient criteria specified a priori? | Blinding of participants to research question? | Selective outcome reporting? | Withdrawals described? | Missing data accounted for in analysis? |
|---|---|---|---|---|---|
| Berber et al., 200574 | Yes | NR | Unclear | NR | NR |
| Gillams and Lees 200976,77 | Yes | NR | Unclear | NR | NR |
| Siperstein et al., 200778 | Yes | NR | Unclear | NR | NR |
| Solbiati et al., 200179 | No | NR | Unclear | NR | NR |
| Sorensen et al., 200780 | Yes | NR | Unclear | Yes | NR |
Assessment of effectiveness: radiofrequency ablation
Four studies reported overall survival for all participants and these results are presented below (Table 18). The non-randomised comparison study73 and one case series study76,77 reported survival for subgroups only; these results are not discussed. Two studies74,80 calculated survival from both diagnosis of liver metastases and radiofrequency ablation of liver metastases, one study from ablation only78 and one study did not report how survival was calculated. 79 Median overall survival after diagnosis of liver metastases was reported as 44.6 months (measure of variation not reported)74 and 52 (95% CI 34 to 82) months. 80 In the same studies, median survival after radiofrequency ablation was 28.9 months74 and 32 (95% CI 24 to 45) months. 80 Median survival was 24 months (measure of variance not reported) in the third study reporting survival from treatment. 78 The remaining study reported median survival time of 36 (95% CI 28 to 52) months,79 although it is unclear whether this is from diagnosis or ablation of liver metastases.
| Study details | Estimated overall survival | Mortality | ||||||
|---|---|---|---|---|---|---|---|---|
| Survival calculated from: | Median survival (95% CI), months | 1 year (%) | 2 years (%) | 3 years (%) | 4 years (%) | 5 years (%) | During follow-up | |
| Berber et al., 2005;74 colorectal primary, n = 135; follow-up: 1–52 months | Diagnosis of LM | 44.6 | NR | NR | NR | NR | NR | NR |
| Treatment of LM | 28.9 | NR | NR | NR | NR | NR | NR | |
| Siperstein et al., 2007;78 colorectal primary, n = 234; follow-up: median 24 months (range 1–94 months) from ablation | Treatment of LM | 24 | NR | NR | (Actual) 20.2 | NR | (Actual) 18.4 | 148 (63%) |
| Solbiati et al., 2001;79 colorectal primary, n = 117; follow-up: 6–52 months | NR | 36 (28 to 52) | 93 | 69 | 46 | NR | NR | 36 (31%) |
| Sorensen et al., 2007;80 colorectal primary, n = 102; follow-up: mean 23.6 months (range 1–92 months) | Diagnosis of LM | 52 (34 to 82) | 96 | 79 | 64 | 52 | 44 | 35 (35%) |
| Treatment of LM | 32 (24 to 45) | 87 | 62 | 46 | 26 | NR | ||
Survival rates at 1, 2, 3, 4 and 5 years were not consistently reported between the studies, making comparison difficult (see Table 18). Two studies79,80 reported estimated survival rates, one study78 reported actual survival rates and one study74 did not report survival rates for the whole population. Estimated 1-year survival rates were 93% in the study that did not state the start point for the analysis,79 and 96% (from diagnosis) or 87% (from radiofrequency ablation) in another study. 80 The actual 5-year survival rate was 18.4% (calculated from radiofrequency ablation) in the study by Siperstein and colleagues,78 and the estimated 5-year survival rate was 44% (from diagnosis of liver metastases) in the study by Sorensen and colleagues. 80 The reason for the differences between studies is not clear, but may be due to differences in inclusion criteria and methods of calculating survival.
One study reported recurrence rates. Solbiati and colleagues79 followed participants for 6 months to 52 months and found that 57% developed new metastases, which were detected after an estimated median 12 (95% CI 10 to 18) months. At least one local recurrence was experienced by 54.7% of participants. The estimated local recurrence rate at 18 months for all participants was 44%. One other study76,77 provided data which may be indicative of the local recurrence rate; however, the data were poorly defined and it is therefore difficult to establish the overall local recurrence rate. These data can be found in Appendix 4.
The low quality of the evidence from these studies should be taken into consideration when interpreting these results.
Adverse events: radiofrequency ablation
Three studies76,77,79,80 reported data on adverse events from radiofrequency ablation (Table 19). Twenty-nine (4.7%) major complications occurred in 617 treatment sessions in the study by Gillams and Lees,76,77 comprising one anaesthetic, five systemic and 23 local complications. Sorensen and colleagues80 reported 12 (6.8%) major and seven (4%) minor complications occurring in 176 treatment sessions (see Table 19). Just one (0.4%) severe complication (perforation of right colon) and one (0.4%) ‘other’ complication were reported by Solbiati and colleagues,79 occurring in 229 treatment sessions.
| Study details | Total number of complications | Details |
|---|---|---|
| Gillams and Lees, 2009;76,77 colorectal primary, n = 309; follow-up: NR; 617 treatment sessions | Major complicationsa 29 | Systemic: 5 |
| Anaesthetic: 1 | ||
| Local: 23 [1 pneumothorax, 4 visceral thermal injuries, 6 abscesses, 4 jaundice (2 bile duct injury, 2 inadequate liver reserve), 7 haemorrhagic complications, 1 asymptomatic pseudoaneurysm] | ||
| Solbiati et al., 2001;79 colorectal primary, n = 117; follow-up: 6–52 months; 229 treatment sessions | Severe complications 1 | Perforation of right colon requiring surgical repair |
| Other complications 1 | Small intraperitoneal haemorrhage | |
| Sorensen et al., 2007;80 colorectal primary, n = 102; length of follow-up: mean 23.6 (range 1–92) months; 176 treatment sessions | Major complicationsb 12 | Abscesses requiring drainage: hepatic, n = 2; subcutaneous, n = 1 |
| Perforation of gastrointestinal wall: gastric, n = 2; colonic, n = 1 | ||
| Vascular: pseudoaneurysm of the right hepatic artery, n = 1; thrombosis of inferior caval vein, n = 1 | ||
| Seeding, n = 1 | ||
| Pneumothorax requiring drainage, n = 1 | ||
| Perforation of the diaphragm, n = 1 | ||
| Acalcular cholecystitis, n = 1 | ||
| Minor complications 7 | Hepatic abscesses not requiring drainage, n = 2 | |
| Second-degree skin burn, n = 1 | ||
| Subcapsular liver haematoma, n = 1 | ||
| Subcutaneous haematoma, n = 1 | ||
| Pleural effusion not requiring drainage, n = 1 | ||
| Fistula between RFA necrosis and secondary biliary tract, n = 1 |
Summary of clinical effectiveness: radiofrequency ablation
One non-randomised comparative study and five prospective case series were included, which provide very low-quality evidence. These studies involved participants with liver metastases from colorectal cancer, although eligibility criteria for the studies differed. Four of the studies provided estimates of overall survival for the study population, but comparisons are difficult due to unclear reporting of, and differences in, the calculation of survival. Major or severe complication rates were low.
Chemoembolisation
Quantity and quality of evidence available
Two RCTs81,82 in which the intervention was chemoembolisation met the inclusion criteria (Table 20). The Taguchi and colleagues RCT81 was a Phase III study of intra-arterial chemotherapy with mitomycin C with the addition of degradable starch microspheres to achieve chemoembolisation compared with intra-arterial chemotherapy alone (no embolisation). The other RCT,82 by Agarwala and colleagues, was a Phase I/II study of hepatic artery infusion with cisplatin plus polyvinyl sponge to achieve chemoembolisation or hepatic artery infusion with cisplatin only (no embolisation). Neither study stated the intention of treatment (e.g. curative, palliative) although the aim of Agarwala and colleagues82 was to identify the maximum tolerated dose of cisplatin and consequently statistical comparisons between cisplatin plus polyvinyl sponge and cisplatin alone were not made. Taguchi and colleagues81 included 60 participants with non-resectable liver metastases from a variety of primary tumour sites (colorectal, stomach, gall bladder and pancreas) recruited from 11 hospital clinics in Japan. The primary tumour was an ocular melanoma in all 19 participants enrolled in the single-centre RCT by Agarwala and colleagues82 that took place in the USA. The inclusion criteria of this study did not specify that liver metastases had to be non-resectable. Inclusion criteria also required participants to have a performance status (not defined) as 0–3 in the Taguchi and colleagues’ study81 and an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2 in the Agarwala and colleagues82 study. Neither study reported the length of their follow-up period. For further details of the intervention see Appendix 4.
| Study | Key inclusion criteriaa | Arm | n | Age (years), mean | Sex, (M/F) | No. of LM | Size of LM | Previous treatment | Extrahepatic disease (%) |
|---|---|---|---|---|---|---|---|---|---|
| Agarwala et al., 2004;82 (USA); follow-up: NR | Primary tumour: ocular melanoma. Histologically proven liver metastases, age ≥ 18 years, ECOG performance status 0–2, adequate hepatic, renal and haematological function. No peripheral vascular disease or other contraindication to femoral artery catheterisation | Cisplatin (100 mg/m2) | 3 | 59.7 (range 36–81) | 9/10 | NR | NR | Chemo, n = 2; immuno, n = 3; tamoxifen, n = 1 | NR |
| Cisplatin (100 mg/m2) plus CE | 4 | ||||||||
| Cisplatin (125 mg/m2) | 6 | ||||||||
| Cisplatin (125 mg/m2) plus CE | 6 | ||||||||
| Taguchi et al., 1992;81 (Japan); follow-up: NR | Primary tumour: various including colorectal (n = 25), stomach (n = 15), gall bladder (n = 1), pancreas (n = 1).b Non-resectable, without demonstrable tumours in other organs; resected primary; patent portal vein; performance status 0–3; hepatic artery anatomy suitable for catheterisation | Intra-arterial mitomycin C with DSM | 30 | NR | NR | NR | NR | NR | 0c |
| Intra-arterial mitomycin C without DSM | 30 | NR | NR | NR | NR | NR | 0c |
Participant characteristics were not reported by Taguchi and colleagues;81 however, it was stated that no significant differences existed between the two groups in terms of age, sex, performance status, site, stage of tumours, numbers of tumours, maximal tumour size or preceding treatment. It was inferred from the inclusion criteria that participants in this RCT81 did not have any extrahepatic disease. Agarwala and colleagues82 reported participant characteristics for the overall group but not separately for each arm. The mean age was 59.7 years, with almost equal numbers of male and female participants. Six participants had received previous treatment for liver metastatic disease. Number and size of liver metastases treated and proportion of participants with extrahepatic disease were not reported. Further details on baseline characteristics of the included participants can be seen in Appendix 4.
Methodological details of the RCTs were poorly reported; therefore, most of the aspects of quality that were assessed were judged as unclear (Table 21).
| Study | Random allocation? | Allocation concealment? | Similar groups? | Blinding of outcome assessors? | Unexpected imbalances in withdrawals? | Selective outcome reporting? | Intention-to-treat analysis? | Missing data accounted for? |
|---|---|---|---|---|---|---|---|---|
| Agarwala et al., 200482 | Unclear | Unclear | Unclear | Unclear | No | Unclear | No | NR |
| Taguchi et al., 199281 | Unclear | Unclear | Yes | Unclear | No | Unclear | No | NR |
Although both studies were described as RCTs and Taguchi and colleagues81 stated that randomisation charts had been used, neither study reported the method used to generate the randomisation sequence and neither study reported whether or not intervention allocation had been concealed. Taguchi and colleagues81 stated that there were no significant differences between the groups for key variables at baseline but did not present any data on this, and Agarwala and colleagues82 did not present baseline data separately for each study arm. Consequently, the risk of selection bias in these trials is uncertain.
Neither RCT reported whether or not those assessing patient outcomes were blind to intervention assignment. For the outcomes reported by these RCTs (response, toxicity/adverse events, and overall survival) blinding of outcome assessors would have been feasible. However, as it is unknown whether or not blinding occurred, the risks of detection bias and performance bias are uncertain.
Taguchi and colleagues81 indicated that fewer participants were evaluable for efficacy (42 of the initial 60 participants) and safety outcomes (51 of 60 participants) than had been randomised. The numbers of dropouts seem similar between the groups; however, reasons for participant drop-out are not provided except that 10 withdrawals were due to adverse events. This study did not appear to undertake an intention-to-treat analysis or account for the missing data in any way. These factors indicate an uncertain risk of attrition bias. Agarwala and colleagues82 lost 2 of the 19 participants to follow-up with no other dropouts reported. An intention-to-treat analysis was not undertaken and it is not clear how the missing data were accounted for in the analysis of overall survival, and so the risk of attrition bias is also uncertain in this study. It was not clear from either study whether other outcomes were assessed but not reported on in the published paper (uncertain risk of reporting bias). Overall, the risk of bias in the two RCTs was uncertain because many of the details needed to make a judgement were inadequately reported.
Assessment of effectiveness: chemoembolisation
Overall survival
Taguchi and colleagues81 calculated overall survival in all randomised participants when the majority of the participants in the treatment groups had died (number of deaths not reported). Although participants in the chemoembolisation arm (intra-arterial mitomycin C with degradable starch microspheres) had a longer median survival time than those receiving intra-arterial mitomycin C only, the difference between the groups was not statistically significant (9.7 months vs. 7.6 months, no p-value reported) (Table 22). Agarwala and colleagues82 reported that median overall survival for all treatment groups was 8.5 months with no data presented for the separate arms of this study. Consideration should be given to the uncertainties on key methodological attributes that were identified in the assessment of methodological quality when interpreting these results.
| Study details | Estimated overall survivala | |
|---|---|---|
| Arm | Median, months (95% CI) | |
| Agarwala et al., 2004;82 ocular melanoma primary, n = 19; follow-up: NR | Cisplatin 100 mg/m2 (n = 3) | 8.5 |
| Cisplatin 100 mg/m2 plus PVS (n = 4) | ||
| Cisplatin 125 mg/m2 (n = 6) | ||
| Cisplatin 125 mg/m2 plus PVS (n = 6) | ||
| p-value | NR | |
| Taguchi et al., 1992;81 various primary cancers, n = 60; follow-up: NR | Intra-arterial mitomycin C with DSM (n = 30) | 9.7b |
| Intra-arterial mitomycin C without DSM (n = 30) | 7.6b | |
| p-value | Not significant | |
Response
The two RCTs reported on the response of the tumour to treatment, but used slightly different criteria for classifying response (see Appendix 7). Taguchi and colleagues81 reported that 42 of the 60 enrolled participants could be evaluated for tumour response and found that the chemoembolisation group had a higher response rate (complete response + partial response) than the group receiving intra-arterial mitomycin C only (54.5% vs. 20%, p < 0.05). Results for response were also provided separately according to primary tumour type (Table 23). In the RCT by Agarwala and colleagues,82 none of the participants achieved a complete response, and only 3 of the 17 evaluable participants achieved a partial response. The statistical comparisons carried out are not described in detail but the study authors state that there was no statistically significant difference in the response rates between the participants who received chemoembolisation and those who did not, nor was there any difference between the two chemotherapy dose tiers (p = 0.2769). When interpreting the results of these RCTs,81,82 consideration should be given to the uncertainties on key methodological attributes that were identified in the assessment of methodological quality.
| Study details | Response (n) | Response rate (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Arm | CR | PR | StD | PD | NE | ||||
| Agarwala et al., 2004;82 ocular melanoma primary, n = 19; follow-up: NR | Cisplatin 100 mg/m2 (n = 3) | 0 | 0 | 3 | 0 | 0 | |||
| Cisplatin 100 mg/m2 plus PVS (n = 4) | 0 | 0 | 2 | 1 | 1 | ||||
| Cisplatin 125 mg/m2 (n = 6) | 0 | 1 | 5 | 0 | 0 | ||||
| Cisplatin 125 mg/m2 plus PVS (n = 6) | 0 | 2 | 3 | 0 | 1 | ||||
| p-valuec | |||||||||
| Arm | CR | PR | MR | NC | PD | CR + PR | |||
| Taguchi et al., 1992;81 various primary cancers, n = 60; follow-up: NR | All metastases | Intra-arterial mitomycin C with DSM (n = 22) | 1 | 11 | 0 | 6 | 4 | 54.5 | |
| Intra-arterial mitomycin C without DSM (n = 20) | 0 | 4 | 1a | 7 | 8 | 20 | |||
| p-value | < 0.05 | ||||||||
| Colorectal metastases | Intra-arterial mitomycin C with DSM (n = 10) | 1 | 3 | 5 | 1 | 40 | |||
| Intra-arterial mitomycin C without DSM (n = 15) | 0 | 3 | 6a | 6 | 20 | ||||
| p-value | |||||||||
| Stomach metastases | Intra-arterial mitomycin C with DSM (n = 11) | 0 | 8 | 0 | 3 | 72.7 | |||
| Intra-arterial mitomycin C without DSM (n = 4) | 0 | 1 | 2a | 1 | 25 | ||||
| p-value | |||||||||
| Other metastasesb | Intra-arterial mitomycin C with DSM (n = 1) | 0 | 0 | 1 | 0 | 0 | |||
| Intra-arterial mitomycin C without DSM (n = 1) | 0 | 0 | 0 | 1 | 0 | ||||
| p-value | |||||||||
Adverse events and toxicity
Pain, gastrointestinal disturbances and fever were experienced by a greater proportion of the participants who were treated by Taguchi and colleagues81 with chemoembolisation than by those treated with intra-arterial mitomycin C alone and the difference between the groups was statistically significant for all symptoms (Table 24). Five participants from each arm were withdrawn from the trial because of the adverse events experienced. The participants withdrawn from the group receiving intra-arterial mitomycin C alone were all withdrawn because of adverse results from clinical chemistry analysis. Two participants were also withdrawn for this reason from the chemoembolisation group, and the other three participants withdrawn from this group were withdrawn for subjective reasons (not further defined). In addition to the data reported here, the Taguchi and colleagues study81 reported on hepatocellular carcinoma. These data did not meet the inclusion criteria for the review. Taguchi and colleagues81 report two deaths that occurred during their study, but it is not clear whether these deaths were of participants with liver metastases or hepatocellular carcinoma.
| Study details | Arm | Major adverse events (% participants) | ||
|---|---|---|---|---|
| Pain | Gastrointestinal disturbances | Fever | ||
| Taguchi et al., 1992;81 various primary cancers, n = 60; follow-up: NR | Intra-arterial mitomycin C with DSM (n = unclear) | 59.3 | 48.1 | 40.7 |
| Intra-arterial mitomycin C without DSM (n = unclear) | 16.7 | 12.5 | 8.3 | |
| p-value | < 0.01 | < 0.01 | < 0.01 | |
| Participants withdrawn from trial due to adverse reactions | ||||
| Intra-arterial mitomycin C with DSM (n = 30) | Subjective, 3; clinical chemistry, 2 | |||
| Intra-arterial mitomycin C without DSM (n = 30) | Subjective, 0; clinical chemistry, 5 | |||
| Agarwala et al., 2004;82 ocular melanoma primary, n = 19; follow-up: NR | Toxicity (number of participants) | |||
| Dose limiting | Grade 3 | Grade 4 | ||
| Cisplatin 100 mg/m2 (n = 3) | 0 | 2 | 0 | |
| Cisplatin 100 mg/m2 plus PVS (n = 4) | 0 | 1 | 2 | |
| Cisplatin 125 mg/m2 (n = 6) | 3a | 3 | 3 | |
| Cisplatin 125 mg/m2 plus PVS (n = 6) | 3b | 1 | 4 | |
| All doses | Toxicity (grade as stated) | |||
| Transient alteration in liver function tests (grades 1 and 2) | 13/19 | |||
| Dose reduction as result of altered liver function | 1/19 | |||
| Haematological toxicity | ||||
| Anaemia (grade 1–2) | 2/19 | |||
| Thrombocytopenia (grade 3) | 2/19 | |||
Toxicity was the primary outcome of the study by Agarwala and colleagues,82 which aimed to establish the maximum tolerated dose of cisplatin with and without polyvinyl sponge. Dose-limiting toxicity did not occur at the 100 mg/m2 cisplatin dose tier, but half of the participants who received cisplatin (either with or without polyvinyl sponge) at the 125 mg/m2 dose tier experienced dose-limiting toxicity. A greater number of grade 4 toxicities were also experienced by the participants at the 125 mg/m2 dose tier than participants at the 100 mg/m2 dose tier. Across the whole study population the most common toxicity during treatment was a transient alteration in liver function tests (grade 1 or 2), with only one participant requiring dose reduction because of this. Haematological toxicity was less common than liver toxicity. Two participants developed anaemia (grade 1–2) and two developed thrombocytopenia (grade 3). It is assumed (although not clarified in the paper) that the two occurrences of thrombocytopenia are included in the earlier reporting of grade 3 toxicities. No statistical comparisons were reported between the groups but this may have been due to the small numbers of participants in each study arm of this Phase I/II RCT. The study authors concluded that their results indicated that the maximum tolerated dose of cisplatin for hepatic artery infusion (either with or without polyvinyl sponge) was 125 mg/m2. Agarwala and colleagues82 noted that there were no complications related to the catheterisation procedure.
Summary
Two RCTs81,82 compared chemoembolisation versus chemotherapy alone delivered directly via the hepatic artery but only one compared overall survival. The methodological quality of the RCTs was uncertain because the methodological details needed to judge different aspects of quality were poorly reported. One RCT81 reported a statistically significant difference in response rate that was in favour of chemoembolisation treatment with mitomycin C and degradable starch microspheres. However, there was no statistically significant difference in overall survival. Furthermore, a greater proportion of the chemoembolisation group experienced adverse events (statistically significantly more pain, gastrointestinal disturbances and fever). The chief aim of the other RCT82 was to establish the maximum tolerated dose of cisplatin with and without polyvinyl sponge. Consequently toxicity was the primary outcome of the study and the toxicity outcomes indicated that the maximum tolerated dose of cisplatin was 125 mg/m2. Overall survival was not compared between groups and the RCT did not find a statistically significant difference in response between participants who received chemoembolisation and those receiving chemotherapy alone. However, numbers were small in this study and it may not have been statistically powered.
Chemoembolisation followed by laser ablation
Quantity and quality of research
Two prospective case series studies describing the combination of chemoembolisation and laser ablation were included (Table 25). 83,84 The narrative synthesis of these data focuses on the key outcomes of survival, response rates (where reported) and adverse events, as described in Chapter 3 (see Method of data analysis). Subgroups were reported in these case series studies; however, these do not appear to have been specified a priori or be statistically powered and the results are therefore not discussed here (see Appendix 4 for further details).
| Study | Key inclusion criteriaa | n | Age (years), mean | Sex (M/F) | No. of LM, mean | Size of LM, mean | Previous treatment | Extrahepatic disease (%) |
|---|---|---|---|---|---|---|---|---|
| Vogl et al., 200383 (Germany); follow-up: NR | Various tumours. No more than four unresectable liver metastases, no response to systemic chemotherapy, no extrahepatic spread. Performance status not ‘poor’ and good general health | 82 | 61.8 (range 23.2–88.3) | 43/39 | 1.2 | Not reported | Chemotherapy: all | 0b |
| Vogl et al., 201184 (Germany); follow-up: mean 13.8 (SD 17.1) months, max. 90 months | Breast primary. Five or fewer lesions either in both liver lobes, locally non-resectable, or recurrent after partial liver resection. Contraindicated or refused surgical treatment, had adverse reaction or no response to systemic chemotherapy, good general health | 161 | 57 (SD 1.3) | All female | Not stated | Mean diameter 3.36 (SD 1.94) | Chemotherapy: allc | Unclear |
Both of these studies were undertaken in the same centre and by the same group. The premise of the studies was to assess the use of chemoembolisation to ‘downstage’ liver metastases before laser ablation treatment. The eligibility criteria varied between studies (see Table 25) and there is no evidence that participants overlap. In the study by Vogl and colleagues84 all participants were women with metastatic breast cancer who either were non-resectable, had recurrence after partial liver resection, or were contraindicated or had refused surgical treatment. Participants were required to have five or fewer lesions which were no larger than 5 cm in diameter. In the second study (Vogl and colleagues)83 participants had various primary cancers with unresectable liver metastases that had shown no response to systemic chemotherapy. Participants were required to have no more than four lesions, two of which could have a diameter of 50–80 mm but otherwise should be smaller than 50 mm. Participants with extrahepatic spread were excluded. Across the two included studies the mean age of participants ranged from 57 to 62 years, and in the study with various primary tumours 52% of the participants were men. 83 All participants appear to have received systemic chemotherapy for liver metastases in both studies, although this was not explicitly reported in the more recent study. 84
Case series provide very low-quality evidence69 and the quality of reporting of the two included case series was poor (Table 26). Both included studies specified their patient selection criteria in advance, but both failed to adequately describe blinding of participants to the research question, although the importance of this is more relevant to subjective outcomes, such as quality of life, than to objective outcomes, such as mortality. Neither study described completely whether or not they had any withdrawals and dropouts, and it was also not reported if or how statistical analysis accounted for missing data. Finally, it was unclear whether or not authors measured more outcomes than they reported, which can result in reporting bias.
Results: chemoembolisation followed by laser ablation
Overall survival
In both studies,83,84 survival was calculated from the commencement of the first treatment with chemoembolisation until death or last follow-up examination. In the study which included participants with various primary cancers,83 the median survival was reported to be 26.2 months (Table 27). In the study which included participants with metastatic breast cancer,84 the mean survival was reported to be 32.5 months (see Table 27). Survival rates at 1 2, 3 and 5 years were reported in one study only84 and were 88.8%, 55.9%, 36.6% and 13.7%, respectively.
| Study details | Estimated overall survival | Mortality | ||||||
|---|---|---|---|---|---|---|---|---|
| Survival calculated from: | Median survival (95% CI), months | 1 year (%) | 2 years (%) | 3 years (%) | 4 years (%) | 5 years (%) | During follow-up | |
| Vogl et al., 2003;83 various tumours, n = 82; follow-up: NR | First chemoembolisation treatment | 26.2 (20.3 to 32.9) | NR | NR | NR | NR | NR | NR |
| Vogl et al., 2011;84 breast primary, n = 161; follow-up: mean 13.8 months(SD 17.1 months), max. 90 months | First chemoembolisation treatment | Mean: 32.5 (SD 21.6) | 88.8 | 55.9 | 36.6 | NR | 13.7 | NR |
Response
Vogl and colleagues84 reported on the response of the tumour to treatment as measured by the Response Evaluation in Solid Tumours (RECIST) criteria (see Appendix 7). The study reported the numbers of participants fulfilling the criteria for each category of response and results showed that 62 (38.5%) of participants had a complete response, 8 (5%) a partial response, 20 (12.4%) stable disease and 71 (44.1%) progressive disease. Of those with progressive disease, 64 (90%) underwent subsequent chemoembolisation treatment. Vogl and colleagues84 also reported the mean time to progression as being 8.2 (SD 12.29) months and overall tumour control as 13.1 (SD 15.9) months. The study also reports the proportion of participants with local recurrence of the lesion (see Appendix 4).
Adverse events: chemoembolisation followed by laser ablation
Complications and side effects of treatments were reported in both studies (Table 28). These are reported per participant, and show that adverse events varied, with many events being mild to moderate. Some more marked complications were reported, including in one study where six events required further intervention. 84 No deaths occurred in the 2011 study,84 but one death occurred within 30 days in the earlier Vogl and colleagues 200383 study.
| Study details | Total number of complications | Details of participants (%) |
|---|---|---|
| Vogl et al., 2003;83 various tumours, n = 82; follow-up: NR | ‘No’ to ‘few’ symptoms:a total n, not reported | Abdominal pain: 57.1% |
| Nausea: 82.2% | ||
| Fever:b 89.3% | ||
| Vomiting: 86.4% | ||
| Lethargy: 38.4% | ||
| ‘Moderate’ to ‘marked’ symptoms:a total n, not reported | Abdominal pain: 42.9% | |
| Nausea: 17.8% | ||
| Fever:b 10.7% | ||
| Vomiting: 13.6% | ||
| Lethargy: 61.6% | ||
| Vogl et al., 2011;84 breast primary, n = 161; follow-up: mean 13.8 (SD 17.1) months, max. 90 months | Complications requiring further intervention: six (3.7%) | Four pleural effusions |
| Two biloma | ||
| Side effects of laser ablation: 100 (62.1%)c | 57 (35.4%) reactive pleural effusion (four reported above) | |
| 15 (9.3%) biloma (two reported above) | ||
| 11 (6.8%) subcapsular haematoma | ||
| 17 (10.6%) small basal lung atelectasis | ||
| 0 deaths | ||
| 0 seeding |
The uncertain quality evidence from these studies should be taken into consideration when interpreting the results discussed above.
Summary of clinical effectiveness: chemoembolisation followed by laser ablation
Two case series studies which provide data on participants who had chemoembolisation followed by laser ablation were included. 83,84 By their nature these studies provide very low-quality evidence. Eligibility criteria differed between the two studies, but the general intention of treatment was to reduce tumours by chemoembolisation so that they were suitable for treatment with laser ablation. Data for only the combination treatment are presented here as higher-quality evidence on the effectiveness of chemoembolisation therapy has been included elsewhere in this report. Survival rates were presented but comparisons are difficult owing to different calculations. Major or severe complication rates were low.
Radioembolisation
Quantity and quality of evidence available
Three RCTs85–87 comparing radioembolisation with chemotherapy met the inclusion criteria (Table 29). In two RCTs (Hendlisz and colleagues86 and Van Hazel and colleagues87) radioembolisation plus systemic chemotherapy was compared with systemic chemotherapy only. In the RCT by Grey and colleagues85 radioembolisation with hepatic artery chemotherapy was compared with hepatic artery chemotherapy alone. None of the included RCTs were undertaken in the UK, with two studies being carried out in Australia85,87 and one in Belgium. 86 Sample sizes were small; the largest sample was 70 participants in the Grey and colleagues study of radioembolisation versus hepatic artery chemotherapy. 85 Participant follow-up was not reported in one study. 87 In the other two RCTs follow-up was a minimum of 3.5 years from randomisation in one85 and a median of 25 months in the other study. 86 In this latter study seven participants were alive at the time of analysis with a median follow-up of 10 months. 86 In all three RCTs the primary cancer site was colorectal (specified as colon only in one). 85 The intention for treatment with radioembolisation (i.e. curative, palliative, pre-surgical) was not explicitly stated in any of these studies; however, eligible participants were required to have liver metastases that were unsuitable for surgical resection in all three trials. Other key criteria for trial inclusion were a World Health Organization (WHO) performance status of 0–2 in one RCT,85 and < 3 in one other RCT87 and an ECOG performance status of 0–2 in the remaining RCT. 86
| Study | Key inclusion criteriaa | Arm | N | Age (years), mean | Sex (M/F) | No. of LM | Size of LMb | Previous treatment | Extrahepatic disease (%) |
|---|---|---|---|---|---|---|---|---|---|
| Grey et al., 200185 (Australia); follow-up: minimum of 3.5 years from randomisation | Primary tumour: adenocarcinoma of the colon. Non-resectable metastases limited to the liver and lymph nodes in porta hepatis, WHO performance status 0–2, adequate haematological and hepatic function | Radioembolisation and HAC | n = 36 | 59 | 28/8 | NR | < 25% n = 24; 25%–50% n = 9; > 50% n = 3 | 14% | 0c |
| HAC | n = 34 | 62 | 26/8 | NR | < 25% n = 24; 25%–50% n = 8; > 50% n = 2 | 15% | 0c | ||
| Hendlisz et al., 201086 (Belgium); follow-up: median 24.8 months (range 2–41). Seven participants still alive with median follow-up 10 months | Primary tumour: colorectal. Histologically proven adenocarcinoma of colon or rectum; metastases in liver only, not amenable to curative surgical resection or local ablation and resistant or intolerant to standard chemotherapy; ECOG performance status 0–2, ≥ 18 years of age. Adequate bone marrow, renal, and liver function | Radioembolisation plus systemic chemotherapy | Randomised n = 23; treated n = 21 | Mean not reported; median 62 (range 46–91) | 18/5 | 1 LM, n = 2; 2–4 LM, n = 10; ≥ 5 LM, n = 8; not measurable, n = 1 | Sum of diameters, median 176.5 mm (range 31–324 mm) | Systemic chemotherapy 100% | 0c |
| Systemic chemotherapy only | n = 23 | Mean not reported; median 62 (range 45–80) | 10/11 | 1 LM, n = 1; 2–4 LM, n = 10; ≥ 5 LM, n = 10; not measurable, n = 2 | Sum of diameters, median 216 mm (range 51–416 mm) | Systemic chemotherapy 100% | 0c | ||
| Van Hazel et al., 200487 (Australia); follow up: NR | Primary tumour: colorectal. Histologically proven adenocarcinoma of the colorectum; age ≥ 18 years; unequivocal evidence of non-resectable, non-ablatable liver metastases; adequate haematological, hepatic and renal function; WHO performance status < 3 | Radioembolisation + systemic chemotherapy | n = 11 | 64 | 10/1 | Multiple | < 25% n = 8; > 25% n = 3 | 0%d | 18 |
| Systemic chemotherapy | n = 10 | 65 | 8/2 | Multiple | < 25% n = 7; > 25% n = 3 | 0%d | 30 |
The majority of participants in each of the three RCTs were male, although in one arm of the Hendlisz and colleagues RCT86 the ratio of male to female was approximately 50 : 50. Participant ages appear to be similar across the three included RCTs, with mean or median ages being around 59–65 years. The reporting of the number and size of liver metastases differed between the three studies. In the Grey and colleagues85 RCT the number of liver metastases per participant were not reported. The study reports the number of tumours sized as < 25%, 25–50% or > 50%, which relates to the proportion of liver involvement. In this study the majority of participants were classified as having < 25% involvement. In the Hendlisz and colleagues RCT,86 the number of metastases were presented per participant. In the radioembolisation arm 10 participants had 2–4 metastases and eight participants had five or more metastases. In the chemotherapy-alone arm, there were 10 participants with 2–4 metastases and 10 with five or more metastases. In each arm, only one or two participants had a single metastasis or the number of metastases could not be measured. The median sum of diameters of the metastases was reported to be 176.5 mm in the radioembolisation arm compared with 216 mm in the chemotherapy-alone arm. 86 The third RCT, by Van Hazel and colleagues,87 reports that all participants had multiple liver metastases, but no further details were provided. The size of metastases was reported as either < 25% or > 25% of liver involvement, with the majority of cases meeting the < 25% category.
All participants had received previous chemotherapy treatment for their liver metastases in the Hendlisz and colleagues RCT. 86 In the RCT by Van Hazel and colleagues,87 this was not explicitly reported as a baseline characteristic. It has been assumed that no participants received any treatment for their liver metastases prior to randomisation because the participants would be excluded by the eligibility criteria if they had received prior chemotherapy or radiotherapy. Finally, in the Grey and colleagues RCT85 14% of participants receiving radioembolisation and 15% of participants receiving hepatic artery chemotherapy were reported to have received prior treatment for their liver metastases before study entry.
Extrahepatic disease was apparent in 18% of the participants randomised to the radioembolisation arm, and in 30% of participants randomised to the chemotherapy-only arm of the Van Hazel and colleagues RCT. 87 Participants were ineligible for study entry if they had evidence of extrahepatic metastatic disease in the remaining two studies. 85,86
Further details on baseline characteristics of the included participants can be seen in Appendix 4.
The three RCTs varied on a number of aspects of methodological quality and likelihood of bias (Table 30). Only one RCT87 reported the information needed to assess the adequacy of the randomisation and allocation processes. In this study87 there appeared to be a low risk of selection bias, the methods used to generate random allocations were assessed as adequate, and the allocation was adequately concealed. In the other two RCTs there is an uncertain risk of selection bias. Grey and colleagues85 state that participants were randomised using a blinded envelope batch method, but no further details were provided. In the Hendlisz and colleagues study86 randomisation was reported to use a minimisation technique, but no further details were provided. These studies have therefore been classified as ‘unclear’ or ‘not reported’ on questions relating to randomisation and allocation, which may suggest a possible risk of selection bias. Study groups were reported to be well balanced on clinical criteria in all three studies. From the baseline characteristics shown in Table 29, it would appear that there were some differences between groups in terms of the gender ratio in the Hendlisz and colleagues study,86 and there was an apparent difference in the proportion of participants with extrahepatic disease between arms in the study by Van Hazel and colleagues. 87 These largely similar groups may, however, indicate that the groups were well matched and provide some indication that the randomisation schedules were reasonable (although assessed as unclear based on the studies reporting).
| Study | Random allocation? | Allocation concealment? | Similar groups? | Blinding of outcome assessors? | Unexpected imbalances? | Selective outcome reporting? | Intention-to-treat analysis? | Missing data accounted for? |
|---|---|---|---|---|---|---|---|---|
| Grey et al., 200185 | Unclear | Unclear | Yes | Yes | No | Unclear | Yes, not defined | NR |
| Hendlisz et al., 201086 | Unclear | NR | Yes | No | No | Unclear | No | No |
| Van Hazel et al., 200487 | Yes | Yes | Yes | Yesa | No | Unclear | Yes, definition can be inferred | Yes, methods appropriate |
Blinding of outcome assessors was assessed as being adequate in the Grey and colleagues85 study, only partially adequate in the van Hazel and colleagues87 study (for some outcomes but not others), and not undertaken in the Hendlisz and colleagues study. 86 Therefore, there is some risk of detection bias in two of the included RCTs.
All three RCTs were deemed to be at uncertain risk of reporting bias, as it was not clear whether or not outcomes had been omitted from the study results. There were no unexpected imbalances in dropouts between groups in any of the three included studies, which suggests low risk of attrition bias. Attrition bias may, however, be a risk in the Hendlisz and colleagues study86 as there was no discussion of any analysis by intention to treat, and missing data were not accounted for in the analyses. The study by van Hazel and colleagues87 was assessed to be of low risk of bias on these factors, but it was not clear whether or not missing data were accounted for in the study by Grey and colleagues. 85
The methodological issues mentioned above for these three studies should be taken into account when considering their results, presented subsequently.
Treatment protocols
The treatment doses of yttrium-90 received by the participants in the radioembolisation arms of the three included RCTs were presented in all three included studies, as were the doses of chemotherapy used in both the radioembolisation arms and the chemotherapy arms. In addition, two studies reported data on the number of cycles of chemotherapy received. These data can be seen in Table 31. For dose of yttrium-90 received these were presented as mean doses in two studies,85,87 and median doses in the remaining study. 86 It would appear from the data that the doses used were similar across the three studies. The chemotherapy agents used in the comparator arms of these three studies differed (see Table 31). With regard to the dose of chemotherapy received, one study reported the mean dose,85 one reported the median dose86 and the remaining study reported the dose intensity delivered. It is therefore difficult to compare doses between studies. However, the doses received did not appear to be different between the treatment arms of each individual study. The total number of cycles delivered in the Van Hazel and colleagues RCT87 did, however, appear to be different between the radioembolisation group and the chemotherapy-only group.
| Study details | Treatment dosesa | |||
|---|---|---|---|---|
| Arm | Dose of yttrium-90 received (GBq) | Number cycles chemotherapy | Dose, chemotherapy per patient (mg) | |
| Grey et al., 2001;85 adenocarcinoma of the colon primary, n = 70; follow-up: minimum of 3.5 years from randomisation | Radioembolism + HAC, n = 36 | Meanb (SD): 2.156 (0.324) | Mean: 8.7 (SD 5.6) per patient | Mean: 1863 (SD 1735) |
| HAC, n = 34 | Not applicable | Mean: 8.0 (SD 5.0) per patient | Mean: 1822 (SD 1323) | |
| Hendlisz et al., 2010;86 colorectal primary, n = 46; follow-up: median 24.8 months (range 2–41). Seven participants still alive with a median follow-up of 10 months | Radioembolisation + systemic chemotherapy, n = 21 | Median (range): 1.79 (1.32–2.15); 3 (14%) missing values | NR | Median (range): 14,588 (4740–97,612); 1 missing value |
| Systemic chemotherapy only, n = 23 | Not applicable | NR | Median (range): 17,700 (3240–119,700); 3 (13%) missing values | |
| Van Hazel et al., 2004;87 colorectal primary, n = 21; follow-up: NR | Radioembolisation + systemic chemotherapy, n = 11 | Mean: 2.25 | Total number of cycles:c 89d | Dose intensity: 85.4% |
| Systemic chemotherapy only, n = 10 | Not applicable | Total number of cycles:c 38 | Dose intensity: 92% | |
Hendlisz and colleagues86 report details of additional therapies received by participants in both treatment groups. Nine participants (of 21) in the radioembolisation arm received further therapy, seven received chemotherapeutic agents (either alone or in combination with immunotherapy), one received radiotherapy and in one further therapy was unspecified. In the chemotherapy-only arm, 16 of 23 participants received further therapies; 10 crossed over to receive radioembolisation, and 6 received chemotherapeutic agents (either alone or in combination with immunotherapy).
Assessment of effectiveness: radioembolisation
Overall survival
Estimated median overall survival was reported in all three included RCTs,85–87 with the RCT by Grey and colleagues85 also reporting estimated percentage survival at 1, 2, 3 and 5 years. In the two studies comparing radioembolisation with systemic chemotherapy, participants in the radioembolisation arm had a longer median survival than those receiving chemotherapy alone (Table 32). This difference was statistically significant in only one of these studies [hazard ratio (HR) 0.33; 95% CI 0.12 to 0.91; p = 0.025]. 87 In this study87 overall survival was calculated in all randomised participants when the majority of the participants in the treatment groups had died (with one participant in the radioembolisation group alive). This study was rated on a number of methodological attributes to have a low risk of bias, although the sample size was small and this should be considered when interpreting the results. In the Hendlisz and colleagues study,86 which showed a non-statistically significant trend for longer median survival in the radioembolisation arm compared with the chemotherapy arm, the overall survival was calculated from time of randomisation until death from any cause. Seven participants remained alive at the time of the analysis; these participants had a median follow-up of 10 months, and this may in part account for the shorter duration of estimated survival seen in this study compared with the other two RCTs.
| Study details | Estimated overall survival | Mortality | ||||||
|---|---|---|---|---|---|---|---|---|
| Arm | Median, months (95% CI) | 1 year (%) | 2 years (%) | 3 years (%) | 4 years (%) | 5 years (%) | During follow-up | |
| Grey et al., 2001;85 adenocarcinoma of the colon primary, n = 70; follow-up: minimum of 3.5 years from randomisation | Radioembolism + HAC, n not stated | 17 | 72 | 39 | 17 | NR | 3.5 | 32/36a |
| HAC, n not stated | 15.9 | 68 | 29 | 6 | NR | 0 | 33/34a | |
| p-value | HR 1.41; 95% CI 0.86 to 2.34; p = 0.18 | NSb | NR | |||||
| Hendlisz et al., 2010;86 colorectal primary, n = 46; follow-up: median 24.8 months (range 2–41 months). Seven participants still alive with a median follow-up of10 months | Radioembolisation + systemic chemotherapy, n = 21 | 10.0 | NR | NR | NR | NR | NR | 16/21 |
| Systemic chemotherapy only, n = 23 | 7.3 | NR | NR | NR | NR | NR | 21/23 | |
| p-value | HR 0.92; 95% CI 0.47 to 1.78; p = 0.80c | NR | NR | NR | NR | NR | NR | |
| Van Hazel et al., 2004;87 colorectal primary, n = 21; follow-up: NR | Radioembolisation + systemic chemotherapy, n = 11 | 29.4 (29.4)d | NR | NR | NR | NR | NR | 10e |
| Systemic chemotherapy only, n = 10 | 12.8 (14.1)d | NR | NR | NR | NR | NR | 10e | |
| p-value | HR 0.33; 95% CI 0.12 to 0.91; p = 0.025 (HR 0.39; 95% CI 0.14 to 1.13; p = 0.07)d | NR | NR | NR | NR | NR | NR | |
In the RCT85 comparing radioembolisation with hepatic artery chemotherapy, survival rates were calculated from the time of randomisation to death or last follow-up. In this study the majority of participants had died at the time of follow up (minimum of 3.5 years, at which time 65 of 70 participants had died). Median survival was reported as being 17 months in the radioembolisation group compared with 15.9 months in the hepatic artery chemotherapy group (HR 1.41, 95% CI 0.86 to 2.34, p = 0.18). Survival to 1, 2, 3 and 5 years was achieved by a higher percentage of those in the radioembolisation group than of those receiving hepatic artery chemotherapy alone (see Table 32). These differences were not statistically different between the two groups (p-value not reported). The trial authors state that the HRs suggest that those receiving hepatic artery chemotherapy alone had a 40% higher death rate than those receiving radioembolisation. In addition, the study authors stated that Cox regression suggests those treated with radioembolisation who survive more than 15 months experience a survival advantage compared with those treated with hepatic artery chemotherapy alone (p = 0.06). However, these results should be treated cautiously as it appears that this analysis was not defined a priori.
The results of these studies are not directly comparable, and each has some methodological shortcomings; however, results suggest that there may be a trend for a survival advantage following treatment with radioembolisation.
Response
The three included RCTs reported on the response of the tumour to treatment, but used slightly different criteria for classifying responses seen (for further details, see Appendix 7). In addition, Grey and colleagues85 measured response by two different means, by tumour area and by tumour volume, and reported data for both of these measures, and Van Hazel and colleagues87 reported response data from the ‘first integrated response’ and the ‘best confirmed response’. Grey and colleagues85 found that those in the radioembolisation group had a higher response rate (complete response and partial response) than the group receiving hepatic artery chemotherapy alone [44% vs. 18% (p = 0.01) when measured by tumour area, and 50% vs. 24% (p = 0.03) when measured by tumour volume]. Hendlisz and colleagues86 reported disease control rates as partial response and stable disease (no complete responses occurred). They found that the disease control rate in those in the radioembolisation group was higher than in those in the chemotherapy alone group [86% vs. 35%, p = 0.001 (95% CI for the difference between groups: 0.19 to 0.71)]. Both of these RCTs85,86 were rated as having a number of uncertainties on key methodological attributes which should be considered in the interpretation of these results. Van Hazel and colleagues87 did not report an overall estimate of tumour response rates in their study. 87
As can be seen in Table 33, the studies reported the numbers of participants fulfilling the criteria for each category of response. These were generally seen to be better in the radioembolisation arms compared with the comparator arms, although statistical significance testing was not undertaken in all cases. In the Grey and colleagues RCT85 when response was measured by tumour area, two participants in the radioembolisation arm achieved a complete response and 14 a partial response. In the hepatic artery chemotherapy arm there were no complete responses and six partial responses. In terms of progressive disease, rates were lower in the radioembolisation arm compared with the hepatic artery chemotherapy arm. Rates of participants with no change in their tumour status were, however, the same across both arms. Overall the groups were reported to be statistically significantly different on all measures of response (p = 0.01). Similar patterns of response were seen when measuring this outcome by tumour volume (see Table 33). The authors of this study also reported that one participant in each arm had disease reduced to such an extent that they were subsequently deemed surgically resectable and underwent surgical excision of metastases.
| Study details | Response, n (%) | Response rate (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Arm | CR | PR | NC | PD | NA | CR + PR | ||
| Grey et al., 2001;85 adenocarcinoma of the colon primary, n = 70; follow up: minimum of 3.5 years from randomisation | Measured by tumour area | Radioembolism + HAC, n = 36 | 2 | 14 | 13 | 3 | 4 | 44 |
| HAC, n = 34 | 0 | 6 | 13 | 8 | 7 | 18 | ||
| p-value | Difference between groups p = 0.01 | p = 0.01 | ||||||
| Measured by tumour volume | Radioembolism + HAC, n = 36 | 2 | 16 | 10 | 5 | 3 | 50 | |
| HAC, n = 34 | 1 | 7 | 12 | 9 | 5 | 24 | ||
| p-value | Difference between groups p = 0.03 | p = 0.03 | ||||||
| CR | PRa | StD | PD | NE | Disease control rates (PR + SD), n/N (%) | |||
| Hendlisz et al., 2010;86 colorectal primary, n = 46; follow up: median 24.8 months (range 2–41). Seven participants still alive with a median follow-up of 10 months | Radioembolisation + chemotherapy, n = 21 | 0 | 2 (10) | 16 (76) | 2 (10) | 1 (5) | 18/21 (86) | |
| Chemotherapy only, n = 23 | 0 | 0 (0) | 8 (35) | 14 (61) | 1 (4) | 8/23 (35) | ||
| p-value | NR | 0.22 (95% CI −0.10 to 0.32) | NR | NR | NR | 0.001 (95% CI 0.19 to 0.71) | ||
| CR | PR | StD | PD | |||||
| Van Hazel et al., 2004;87 colorectal primary, n = 21; follow up: NR | First integrated response | Radioembolisation + systemic chemotherapy, n = 11 | 0 | 10 | 1 | 0 | ||
| Systemic chemotherapy only, n = 10 | 0 | 0 | 6 | 4 | ||||
| p-value | < 0.001 | |||||||
| Best confirmed response | Radioembolisation + systemic chemotherapy, n = 11 | 0 | 8 | 3 | 0 | |||
| Systemic chemotherapy only, n = 10 | 0 | 0 | 6 | 4 | ||||
| p-value | < 0.001 | |||||||
In the two studies comparing radioembolisation with systemic chemotherapy, similar patterns of response occurred, although no complete responses were observed in either study. In the RCT by Hendlisz and colleagues86 two participants achieved a partial response in the radioembolisation arm compared with no participants in the chemotherapy-alone arm, but this difference was not statistically significant (95% CI for the difference between groups –0.10 to 0.32, p = 0.22). The number of participants with stable disease was higher in the radioembolisation arm than in the chemotherapy arm, and the number of participants with progressive disease was lower in the radioembolisation arm than in the chemotherapy arm. 86 In the RCT by Van Hazel and colleagues87 there were eight participants whose best confirmed response was a partial response in the radioembolisation group compared with no participants in the chemotherapy-alone group. Best confirmed responses of stable disease and progressive disease were fewer in the participants treated with radioembolisation compared with those treated with chemotherapy (3 vs. 6 stable disease, and 0 vs. 4 progressive disease). Across all categories of response the groups were reported to be statistically significantly different (p = 0.001).
The results on response measures from the three studies are not directly comparable; however, they suggest that radioembolisation treatment leads to better tumour response than systemic chemotherapy or hepatic artery chemotherapy treatment alone.
Time to progression
All three included RCTs reported outcomes related to time to disease progression. Grey and colleagues85 and Hendlisz and colleagues86 reported the median time to progression in the liver. Hendlisz and colleagues86 additionally report the median time to progression (any), which is also reported as median time to progressive disease in the Van Hazel and colleagues’ study. 87 The study by Grey and colleagues85 measured progression by two different means, by tumour area and by tumour volume, and reported data for both of these measures, and Hendlisz and colleagues86 reported progression from ‘all progressions considered as events’ and ‘patients with treatment change censored at the time of change’. The results, seen in Table 34, are therefore not comparable, and for these studies results should also be interpreted in the context of some uncertainties around the potential for bias and/or small sample sizes.
| Study details | Arm | Median time to progression in the liver (months)a | |
|---|---|---|---|
| Grey et al., 2001;85 adenocarcinoma of the colon primary, n = 70; follow up: minimum of 3.5 years from randomisation | By tumour area | Radioembolism + HAC, n = 36 | 15.9 |
| HAC, n = 34 | 9.7 | ||
| p-value | 0.001 | ||
| By tumour volume | Radioembolism + HAC, n = 36 | 12.0 | |
| HAC, n = 34 | 7.6 | ||
| p-value | 0.04 | ||
| Arm | Median time to liver progression (months) | ||
| Hendlisz et al., 2010;86 colorectal primary, n = 46; follow up: median 24.8 months (range 2–41). Seven participants still alive with a median follow-up of 10 months | All progressions considered as events | Radioembolisation + chemotherapy, n = 18b | 5.5 |
| Chemotherapy only, n = 23 | 2.1 | ||
| p-value | HR 0.38; 95% CI 0.20 to 0.72; p = 0.003 | ||
| Participants with treatment change censored at the time of change | Radioembolisation + chemotherapy, n = 18b | 5.6 (15 events) | |
| Chemotherapy only, n = 23 | 2.1 (22 events) | ||
| p-value | HR 0.35; 95% CI 0.18 to 0.69; p = 0.002 | ||
| Arm | Median time to progression (months) | ||
| Radioembolisation + chemotherapy, n = 21 | 4.5 | ||
| Chemotherapy only, n = 23 | 2.1c | ||
| p-value | HR 0.51; 95% CI 0.28 to 0.94; p = 0.03 | ||
| Arm | Median time to progressive disease (months) | ||
| Van Hazel et al., 2004;87 colorectal primary, n = 21; follow up: NR | Radioembolisation + systemic chemotherapy, n = 11 | 18.6 | |
| Systemic chemotherapy only, n = 10 | 3.6 | ||
| p-value | < 0.0005 | ||
Median time to progression in the liver was reported to be statistically significantly different between radioembolisation and hepatic artery chemotherapy in the Grey and colleagues85 trial when measured by either tumour area (15.9 months vs. 9.7 months, respectively, p = 0.001) or tumour volume (12 months vs. 7.6 months, respectively, p = 0.04). Radioembolisation also led to a longer time to progression in the liver than chemotherapy alone in the Hendlisz and colleagues86 trial (all progressions considered an event: 5.5 months vs. 2.1 months for the two groups, respectively, p = 0.003). HRs can be seen in Table 34. Hendlisz and colleagues86 report that local progression was documented in four participants (three radioembolisation; one chemotherapy arm) after an unjustified change in the treatment allocated by randomisation (no further details). Censoring these four participants does not change the median time to liver progression (5.6 months vs. 2.1 months, respectively, p = 0.002).
Median time to progression at any site was also shown to be longer in the radioembolisation group compared with the chemotherapy alone group in the Hendlisz and colleagues86 RCT (4.5 months vs. 2.1 months, respectively, p = 0.03). A similar pattern was seen in the study by Van Hazel and colleagues87 (radioembolisation 18.6 months vs. chemotherapy 3.6 months, p < 0.0005). This study also presents the site of the first disease progression. The most common site was the liver (eight participants in each treatment arm). Other sites that showed progression in the radioembolisation group were the liver and lung combined (n = 1), and the lung (n = 1) (one participant in this arm had died without progression). In the chemotherapy-only group, the site of first progression in the other two participants was the liver and peritoneum combined in one, and bone in one other.
In these three studies radioembolisation led to more favourable results in terms of time before progressive disease than chemotherapy alone.
Quality of life
Two included RCTs assessed quality of life as an outcome. 85,87 No numerical outcome data were reported in either of these two studies. Grey and colleagues85 used a 13-point linear analogue self-assessment scale, which was reported as validated. However, no further details of the measure or the validation were provided. The study reports that there were no significant differences between the treatment groups. Sexual interest/ability deteriorated for both treatment groups over the 18-month period during which the protocol treatments were delivered. For all other measures, there were trends towards improvement in quality-of-life scores over the first 18 months for both treatment groups.
Van Hazel and colleagues87 used the 23-point validated Functional Living Index – Cancer questionnaire. 89 The authors state that, at 3 months, changes from baseline patient-rated quality of life were very similar between both arms (p = 0.96). This was also the case for quality of life rated by the physician using the Spitzer index (p = 0.98). 90 The authors suggest that the lack of variation from baseline may have been because most participants were still receiving chemotherapy during this time. In a more recently published conference abstract,88 Van Hazel and colleagues state that at 3 months the radioembolisation group showed a statistically significant improvement in health-related quality of life compared with the chemotherapy-only group (p = 0.03, 95% CI 1.4 to 27.6), but no statistically significant differences thereafter. This appears to be different from the result presented in the full publication; however, there is limited detail reported in either publication and each may, in fact, be presenting different things. As in the earlier publication, no outcome data were reported in the abstract.
Adverse events and toxicity
The total number of complications and toxicities graded 3 or 4 from the included studies can be seen in Table 35. Two RCTs85,87 used the Union International Control Criteria (UICC) to define adverse events and toxicity, and Hendlisz and colleagues86 used the Common Terminology Criteria for Adverse Events (CTCAE, version 3). In the RCT comparing radioembolisation and hepatic artery chemotherapy with hepatic artery chemotherapy, the total number of complications was the same in each of the two treatment groups. 85 In the two RCTs comparing radioembolisation with systemic chemotherapy, one found a greater number of complications in the chemotherapy arm than in the radioembolisation arm,86 and the other found a greater number of complications in the radioembolisation arm than in the chemotherapy arm. 87 In terms of the number of grade 3 or 4 complications, there appears to be no consistent finding across these three studies.
| Study details | Arm | Total number complications | Details |
|---|---|---|---|
| Grey et al., 2001;85 adenocarcinoma of the colon primary, n = 70; follow-up: minimum of 3.5 years from randomisation | Radioembolism + HAC, n not reported | 23a | Bilirubin, n = 1 (grade 3) |
| Aspartate transaminase, n = 5 (grade 3); n = 2 (grade 4) | |||
| Alkaline phosphatase, n = 14 (grade 3) | |||
| Nausea/vomiting, n = 1 (grade 3) | |||
| HAC, n not reported | 23 | Haemoglobin, n = 1 (grade 3) | |
| Aspartate transaminase, n = 12 (grade 3); n = 2 (grade 4) | |||
| Alkaline phosphatase, n = 5 (grade 3) | |||
| Nausea/vomiting, n = 2 (grade 3) | |||
| Diarrhoea, n = 1 (grade 3) | |||
| Hendlisz et al., 2010;86 colorectal primary, n = 46; follow-up: median 24.8 months (range 2–41). Seven participants still alive with median follow-up 10 months | Radioembolisation + systemic chemotherapy, n = 21 | 1 | Hand–foot syndrome, n = 1 (grade 3) |
| Systemic chemotherapy only, n = 23 | 10 | Stomatitis, n = 1 (grade 3) | |
| Anorexia, n = 1 (grade 3) | |||
| Fatigue, n = 5 (grade 3) | |||
| Dyspnoea, n = 1 (grade 3) | |||
| Pulmonary, n = 1 (grade 3) | |||
| Allergy, n = 1 (grade 3) | |||
| Text states n = 6 participants with grade 3 or 4 toxicity | |||
| p-value | p = 0.10 (between number of participants with grade 3/4 toxicity) | ||
| Van Hazel et al., 2004;87 colorectal primary, n = 21; follow-up: NR | Radioembolisation + systemic chemotherapy, n = 11 | 13 | Granulocytopenia, n = 3 |
| Nausea, vomiting, n = 1 | |||
| Mucositis, n = 4 | |||
| Gastritis, n = 1 | |||
| Diarrhoea, n = 2 | |||
| Cirrhosis, n = 1 | |||
| Liver abscess, n = 1 | |||
| Systemic chemotherapy only, n = 10 | 5 | Nausea, vomiting, n = 1 | |
| Mucositis, n = 1 | |||
| Gastritis, n = 1 | |||
| Diarrhoea, n = 1 | |||
| Anorexia, n = 1 |
The types of complications and toxicities recorded in the three studies can also be seen in Table 35. These are all those graded 3 or 4. One study86 also reported grade 1 and 2 complications and toxicities, which can be seen in Appendix 4. Overall, the results suggest that radioembolisation is well tolerated, with the occurrence of toxicities and adverse events not appearing to be particularly different from those with hepatic artery chemotherapy or systemic chemotherapy. However, the methodological quality and sample sizes of these included studies would indicate a need for caution in the interpretation of these results.
Summary
Three RCTs85–87 compared radioembolisation with chemotherapy delivered either via the hepatic artery or intravenously. The methodological quality of the RCTs was mixed, in many cases because the methodological details needed to judge different aspects of quality were poorly reported. Two of the RCTs85,87 have an uncertain risk of selection bias which should be considered in the interpretation of these results. The two RCTs86,87 that compared radioembolisation with systemic chemotherapy compared overall survival between the groups. Radioembolisation led to longer median survival in both of these studies; however, the difference between groups was statistically significant in only one study. 87 The third RCT85 which compared radioembolisation with hepatic artery chemotherapy reported median overall survival and estimates of survival at 1, 2, 3 and 5 years but the difference between the groups was not statistically significant.
All three RCTs reported tumour response to treatment but used slightly different criteria for classifying response. Only two RCTs85,86 calculated an estimate of overall response and showed that those in the radioembolisation group had statistically significantly better response rates than their respective comparator groups. The third RCT87 did not report an overall estimate of tumour response rate. Time to progressive disease was reported in different ways in the three included RCTs, which all showed favourable results in the radioembolisation groups when compared with their chemotherapy groups. Quality of life was an outcome in two of the included studies; however, no data were presented. Adverse events and toxicity occurred in all trial arms. In one RCT the adverse events were more numerous in the radioembolisation arm,87 but this pattern was not seen in the other two RCTs. 85,86
Research in progress
As stated in Totality of research available, 14 publications were identified in searches but were excluded as they were published as abstracts only. These 14 publications (eight studies) can be seen in Appendix 5. Four of these were reported to be comparative studies (three RCTs, one non-randomised comparison) and the abstracts were published between 2009 and 2010. As such these may be in the process of being prepared for full publication at the time of writing. A summary of these four studies and the results from their primary outcome is provided here for information.
Two studies assessed the use of radiofrequency ablation; one RCT compared radiofrequency ablation with chemotherapy,91–94 and one non-randomised study compared radiofrequency ablation with surgical resection. 95 In a series of abstracts, Ruers and colleagues91–94 report on a RCT which compared overall survival and safety outcomes in participants with up to nine unresectable colorectal liver metastases. Participants were randomised to either systemic chemotherapy and radiofrequency ablation (n = 60) or systemic chemotherapy alone (n = 59). Baseline characteristics were reported to be similar in the two arms. The 30-month overall survival estimates were 61.7% (95% CI 48.21 to 73.93) in the radiofrequency and chemotherapy arm and 57.6% (95% CI 44.0 to 70.39) in the chemotherapy arm. The study authors report that the study design, however, did not allow for comparisons between treatment arms. Some participants in the intervention arm did not receive the chemotherapy and some received surgical resection of their metastases in addition to radiotherapy. Hirata and colleagues95 compared survival and complication rates in participants with colorectal liver metastases having either radiofrequency ablation (n = 14) or surgical resection (n = 35). Minimal baseline characteristics were provided; however, participants were predominantly male and mean age was approximately 63 years. Study authors report no statistically significant differences in 5-year survival based on Kaplan–Meier estimates (34.2% in the radiofrequency group vs. 30% in the surgical resection group).
One RCT assessed the use of radioembolisation compared with chemotherapy in participants with colorectal liver metastases. 96 The primary outcome for the study was time to liver progression; other outcomes included overall survival and adverse events. Minimal baseline characteristics were reported in the abstract but the authors state that they were well balanced in the two groups. Crossovers were permitted after disease progression. Median time to liver progression was reported to be 24 weeks in the radioembolisation arm (n = 21) and 9 weeks in the chemotherapy arm (n = 23) (HR 0.38; 95% CI 0.20 to 0.72, p = 0.003). An additional RCT compared chemoembolisation with systemic chemotherapy in those with colorectal cancer liver metastases. 97,98 Seventy-four participants were randomised and assessed for median survival, progression-free survival, quality of life, response and safety. Median survival, assessed with a median follow-up of 24 months, was 38% in the chemoembolisation group (n = 36) and 18% in the chemotherapy group (n = 39).
In addition to these studies, our literature searches identified five ongoing trials of potential relevance to this review. The FOXFIRE study is a UK-based open-label RCT of chemotherapy alone compared with chemotherapy and radioembolisation. Adults with colorectal liver metastases are eligible for this ongoing study, which has a primary outcome of overall survival. The study aims to recruit 490 participants and is sponsored by the University of Oxford, UK, and funded by Cancer Research UK. A similar RCT is also being undertaken currently in the Middle East and the USA (SIRFLOX).
Another open-label RCT evaluating the efficacy of chemoembolisation in combination with chemotherapy is currently ongoing in Germany (the DEBIRITUX study). This study is recruiting adults with unresectable colorectal liver metastases. The primary outcome for the study is progression-free survival 6 months following first administration of study treatment. The study is sponsored by Martin Luther Universität, Halle-Wittenberg, Germany, and Biocompatibles UK Ltd.
The DEBIRI study is an open-label RCT aiming to assess chemoembolisation for the treatment of unresectable colorectal liver metastases. Participants with at least one measurable liver metastasis (size > 1cm) will be randomised to receive either a combination of chemoembolisation plus chemotherapy or chemotherapy alone. The study is sponsored by the University of Louisville, KY, and Biocompatibles UK Ltd. The study details were last verified on ClinicalTrials.gov in November 2011, when the study status was recorded as currently recruiting participants.
A RCT comparing transhepatic arterial chemotherapy with transcatheter arterial chemoembolisation plus Folfox4 is currently ongoing in China. Participants with unresectable colorectal liver metastases are eligible for this study, which has a primary outcome measure of overall survival. Follow-up is reported to be until 5 years post treatment. The study sponsor is the Fudan University, Shanghai, China. The recruitment status of this study is unknown as the information on ClinicalTrials.gov was last updated in March 2009.
Existing systematic reviews
Forty-six review articles were identified on literature searches; 13 (in 15 publications) were assessed as being systematic reviews. These 13 systematic reviews differed in terms of the interventions, participants, and study designs eligible for inclusion. Many have included retrospective study designs and studies with small sample sizes rather than exclude studies on the basis of quality. The comprehensiveness of the literatures searches and the dates of searches also varied, with the most recently published systematic review searching until January 2010. All 13 systematic reviews have been used as a source of potential references and are briefly described here. No systematic review of these reviews was undertaken.
One recently published systematic review (Pathak and colleagues)99,100 included all ablative therapies used for those with colorectal liver metastases. All study designs were eligible for inclusion, including retrospective studies, if they had a follow-up of at least 1 year and a sample size of at least 10 participants. Searches were undertaken until January 2010 and the reviewers identified 75 studies; 26 cryoablation studies, 13 microwave ablation studies and 36 radiofrequency ablation studies. The majority (50) of these studies were retrospective in design; other studies had combination treatments or had samples of fewer than 100 participants. Four of the included studies are included in the present review. Results were discussed narratively, providing the ranges of estimates of the outcomes across the studies within each ablative type. There was no discussion of the study validity or appropriateness of including all studies together. No comparative data are presented except for one study of microwave ablation (included in the present review); however, the review concludes that ablation offers a survival advantage compared with chemotherapy alone, with acceptable complication rates. It is unclear where the data for this conclusion come from as they do not appear in the publication.
Four systematic reviews (six publications) of radiofrequency ablation of liver metastases have been identified on searches. 38,39,101–104
The Australian Safety and Efficacy Register of New Interventional Procedures group have published three systematic reviews, each with slightly different inclusion criteria. 101,102,104 Participants could include those with colorectal liver metastases or hepatocellular carcinoma, and studies eligible were RCTs, quasi-RCTs, and non-randomised comparative studies. In addition, case series studies (prospective or retrospective) were eligible if they included consecutive participants and had a follow-up of at least 12 months. The dates of searches varied but in the most recent publication the date given was April 2006. Twenty-six studies were included, of which 13 were of participants with colorectal liver metastases. The review conclusion was that there was not enough evidence to determine safety or efficacy of radiofrequency ablation in colorectal liver metastases.
Wong and colleagues38 also systematically reviewed the evidence for the use of radiofrequency ablation in participants with colorectal liver metastases. Again, prospective and retrospective studies were eligible, and these had to have a sample of 10 or more participants and a minimum length of follow-up of greater than 6 months. Searches were completed in April 2007. The review included 43 studies of various treatment modalities (laparotomy, laparoscopy and open). Most of the studies were retrospective or small series studies. The authors point out issues of many of the included studies, such as mixed populations, the risk of selection bias, and inconsistency of reporting of outcomes. They concluded that survival and tumour response to radiofrequency ablation varies widely between the included studies and that more research is needed.
The third systematic review of radiofrequency ablation (Stang and colleagues)103 included studies of radiofrequency ablation alone or combined with surgical resection or compared with surgical resection or chemotherapy. Any study design was eligible for inclusion if there were at least 40 participants and a median follow-up of 18 months or longer. In addition, the studies had to provide estimates of at least 3-year overall survival in those with colorectal liver metastases. Searches were of MEDLINE only up until August 2008. Twenty-one studies were included: nine of radiofrequency ablation as a single therapy, seven of combinations of therapies and five comparative studies. Many of the included studies were retrospective. Conclusions were that radiofrequency ablation offers a complementary option for the treatment of limited colorectal liver metastases, but there were no data to support radiofrequency ablation as an alternative in surgical candidates with resectable colorectal liver metastases. No validity assessment or discussion of the limitations in study designs was provided.
Razafindra and colleagues39 did not explicitly state their inclusion criteria but included those with intraoperative radiofrequency ablation and excluded those with percutaneous radiofrequency ablation, and those with only hepatocellular carcinoma. The review searched until December 2008 and reported only complication rates. Thirty studies were included: most were small samples, most combined intraoperative radiofrequency ablation with surgical resection, 13 included participants with hepatocellular carcinoma, and all but one were retrospective studies. No validity assessment was undertaken. Conclusions were that intraoperative radiofrequency ablation, which has different indications from percutaneous radiofrequency ablation, has no specific complications associated with it, but that combining it with surgical resection may lead to higher morbidity.
Two systematic reviews were identified which assessed radioembolisation. 51,105 The Australian Medical Services Advisory Committee105 reviewed the evidence from any study type in those with non-resectable colorectal liver metastases. Sample sizes were required to be at least 10 participants. Searches were undertaken until February 2001 and 11 studies were included. Two were RCTs and nine were case series studies. Four studies met the inclusion criteria of the present review (two were below the threshold for the hierarchy of evidence for radioembolisation), the others were either small sample, in primary liver cancers or in mixed populations. The review concludes that radioembolisation may be more effective than chemotherapy and that the treatment is reasonably safe. The second systematic review (Vente and colleagues)51 included participants having radioembolisation for primary or secondary liver malignancies. All study types were potentially eligible. The dates of the last search for literature were not reported. The review included 30 articles, 19 on colorectal liver metastases. Of these, two were included in our review; the others either were lower in the hierarchy of evidence or had small sample sizes. The review concludes that radioembolisation is associated with high response rates, but the impact on survival is less certain with the current evidence base.
Carter and Martin47 systematically reviewed the evidence for the use of chemoembolisation for liver tumours (included hepatocellular carcinoma, liver metastases and cholangiocarcinoma). Searches were undertaken until 2008 and eight studies were identified. Of these, only three were in populations with liver metastases. Sample sizes of these three studies were fewer than 40. No overall conclusion for the use of chemoembolisation in liver metastases was provided.
Interventions in the remaining five systematic reviews were not predominantly of ablative or cytoreductive therapies but their respective inclusion criteria have the potential to include studies of these technologies. Bergenfeldt and colleagues106 reviewed liver resection and local ablation (including radiofrequency ablation and laser ablation) in those with breast cancer liver metastases. The review included 32 retrospective studies, of which seven were of local ablation (six radiofrequency ablation; one laser ablation) and concluded that local ablation has a favourable outcome on survival. Smith and McCall107 investigated evidence for radical surgical resection and/or ablation in those with colorectal liver metastases. Forty-six studies were included, seven with primarily participants undergoing ablative technologies (cryoablation, radiofrequency ablation and laser ablation), with or without surgical resection. Six of these were excluded from our review as either retrospective or small sample size, and one met the inclusion criteria of our review. Results showed a range of survival estimates (all ablative therapies were taken together).
Gurusamy and colleagues’ systematic review of liver resection versus radiofrequency ablation or cryoablation in participants with liver metastases from gastro-entero-pancreatic neuroendocrine tumours found no studies that met the review inclusion criteria. 108 Similarly, Gurusamy and colleagues109 found no studies of surgical liver resection with lymphadenectomy versus other potentially curative (radiofrequency ablation) or palliative treatment in participants with colorectal liver metastases, who are found to have hepatic node involvement. Both of these reviews were for the Cochrane database of systematic reviews of effectiveness, and eligible studies would have been required to be RCTs.
Finally, Al-Asfoor and colleagues110 reviewed evidence of liver resection versus cryotherapy or radiofrequency ablation in people who were candidates for liver resection or any other modalities of intervention (i.e. cryosurgery and radiofrequency ablation as treatments for liver metastases) with colorectal liver metastases. One RCT was identified which partially met the inclusion criteria. The study indicated a 5-year and 10-year survival rate of 44% and 19%, respectively, after cryosurgery. This study was excluded from the present review as the intervention differed between participants in the cryosurgery arm (to include 32% of participants having cryoresection).
Chapter 5 Economic analysis
The aim of this chapter is to assess the cost-effectiveness of ablative therapies for liver metastases, provided for treatment with curative or palliative intent. The economic analysis comprises:
-
a systematic literature review of economic evaluations, health-related quality of life (HRQoL) and cost studies of ablative therapies for the treatment of liver metastases
-
the development of a de novo economic model and presentation of cost-effectiveness results.
Systematic review of existing cost-effectiveness evidence
The methods and inclusion criteria considered for this review of economic evaluations are presented in Chapter 3 of this report. Searches for economic evaluations of ablative therapies for liver metastases identified 108 studies that potentially met the inclusion criteria set out in Chapter 3 (see Inclusion and exclusion criteria) of this report. From screening titles and abstracts, 102 studies were excluded and six retrieved for full screening. Four of these studies did not meet the inclusion criteria as they assessed a different population group from that specified in the research protocol111,112 or were found not to be full economic evaluations. 113,114 Hence, two economic evaluations were included in the current review – Abramson and colleagues115 and Gazelle and colleagues116 (Figure 2 shows the flow chart) – and a summary of their characteristics in shown in Table 36.
FIGURE 2.
Flow chart of identification of studies for inclusion in the review of cost-effectiveness.
| Characteristics | Author | |
|---|---|---|
| Abramson et al.115 | Gazelle et al.116 | |
| Publication year | 2000 | 2004 |
| Country | USA | USA |
| Funding source | Harvard Medical School (Department of Radiology, Beth Israel Deaconess Medical Center) | Institute for Technology Assessment and Department of Radiology, Massachusetts General Hospital, Harvard Medical School; Center for Risk Analysis and Department of Health Policy and Management, Harvard School of Public Health; the National Cancer Institute; and the US Department of the Army |
| Study type | Cost-effectiveness analysis | Cost–utility analysis |
| Perspective | Direct costs to the payer | Societal perspective restricted to direct health and productivity costs and benefits |
| Study population | Participants with liver metastases of colorectal carcinoma | Participants with metachronous colorectal carcinoma liver metastases |
| Intervention(s) | HACE vs. palliative care | Percutaneous RFA vs. surgical resection [consisting of surgical resection (or metastasectomy) and laparotomy (for unresectable metastases found at surgery)] |
| Intervention effect | Range of speculative incremental survival estimates (0–24 months) | Mortality: 5% (surgical resection), 1% (laparotomy), 0.3% (RFA) |
| Morbidity: 20% (surgical resection), 4% (laparotomy), 2% (RFA) | ||
| Length of hospital stay: 12 days (surgical resection), 5 days (laparotomy) | ||
| Additional LoS due to complications: | ||
| 3 days (surgical resection, laparotomy, RFA) | ||
| QoL: 70% of pre-surgical value for 1 month; 95% x pre-ablation value for 1 month | ||
| Probability of complete tumour necrosis with RFA: 0.784 (≤ 2.5 cm), 0.472 (> 2.5–4 cm), 0.316 (> 4–10 cm)a | ||
| Currency base | 1998 US$ | 1998 US$ |
| Model type | Simple calculation model | State-transition Monte Carlo decision model |
| Time horizon | 24 months | Lifetime |
| Baseline cohort | Participants with liver metastasis of colorectal carcinoma | Simulated hypothetical cohort of 10,000 participants |
| Base-case results | ICER ranges from $82,385/LY gained with a 3-month survival benefit to $10,747/LY gained with 24 months’ survival benefit | Permitting surgical resection vs. RFA up to six metastases with CT follow-up every 12 months: 3.26 QALY gain, incremental cost of $57,000, and ICER = $16,900 per QALY Permitting surgical resection up to six metastases with CT follow-up every 4 months vs. 12 months: 3.39 QALY gain, incremental cost of $61,000, and ICER = $31,200 per QALY Permitting surgical resection up to six metastases with 4 months’ follow-up vs. RFA up to six metastases with 12 months’ follow-up: ICER = $17,800 per QALY gain |
Although both studies examined participants with liver metastases from colorectal carcinoma, the populations may differ in characteristics that affect their eligibility for the different interventions available. In fact, Abramson and colleagues115 analysed different interventions from the ones studied by Gazelle and colleagues. 116 Abramson and colleagues115 analysed the relative cost-effectiveness of hepatic arterial chemoembolisation (HACE) and palliative care, in terms of US dollars (US$) per life-year gained adopting a third-party payer perspective. Gazelle and colleagues116 conducted a cost–utility analysis of radiofrequency ablation versus surgical resection according to a societal perspective.
Critical appraisal of the studies
The economic evaluations included for this systematic review were assessed against a critical appraisal checklist (Table 37) adapted from checklists by Philips and colleagues,68 Drummond and colleagues,67 and the NICE reference case requirements. 117
Both included economic evaluations concern a health-care system – Medicare, a US social insurance programme designed specifically for citizens aged 65 or older – that is not comparable with the UK NHS, which comprises the entire population. Though the patient groups of these studies are relevant for our review in terms of pathology, they may differ significantly from the population of interest for the UK NHS in other characteristics due to the patient selection inherent in the different health-care systems. Also, even if the settings in which the strategies took place were similar to the UK setting and thus the resource use could be similar, the unit costs are likely to differ substantially from those for the UK.
Modelling approach
Abramson and colleagues115 report the estimation of the incremental cost of hepatic artery chemoembolisation versus palliative care and of the incremental cost-effectiveness ratios (ICERs) using a range of hypothetical incremental survival estimates over 24 months. There is no description of a model structure and its assumptions or any other indication of modelling the natural progression of the disease. This approach is justified by the lack of evidence on the differential in survival benefit between interventions. However, Abramson and colleagues115 do not report their literature review as systematic. The 11.1-month median survival reported by Stangl and colleagues57 for palliative care justified the use of 12 months of survival benefit for the baseline. 115 Yet details on the search conducted to identify this publication, and the reason for its choice and use of an approximate value is not provided. Results of the case series reported by Abramson and colleagues115 substantiated the speculative range of values used for the incremental survival of hepatic artery chemoembolisation versus palliative care (0–24 months). The rationale for a 2-year model and discounting was not discussed. 115 A lifetime horizon would have been more appropriate;117 however, given the advanced stage of these patients’ disease, 2 years may be close to their lifetime. The assumptions made adopting this type of analysis are not listed and the discussion of them is very limited.
Gazelle and colleagues116 adapted an existing Markov state-transition microsimulation model118 to estimate the relative cost-effectiveness of radiofrequency ablation and surgical resection and a variety of imaging and treatment strategies in people with metastatic colorectal cancer. The modelling approach used is appropriate and the model structure and its assumptions are described in detail. For each strategy, a cohort of 10,000 patients with potential for treatment was simulated, having each individual moving from the ‘alive_treat’ health state to the ‘alive no treat’ or to the ‘dead’ absorbing state, in monthly cycles. Patients eligible for treatment stay in the ‘alive_treat’ health state until they die or become untreatable, that is to say when they present more metastases than the treatment threshold or when they reach the maximum number of interventions for the strategy being analysed. While patients are in the ‘alive_treat’ state, the model tracks metastases progression, diagnosis of the existing metastases (previously undetected), the impact of diagnostic tests and of interventions and their complications.
For each cohort, only one imaging and treatment strategy is modelled by specifying parameters such as the treatment threshold (1–6 metastases), follow-up interval (4, 6 or 12 months), and imaging test sensitivity. For each simulated patient, the model tracks up to 15 individual hepatic metastases, specifying and updating the location, size, rate of growth, detection, and removal/ablation of each metastasis. The initial number, location and size of each metastasis are randomly drawn according to the distributions assigned to each parameter. Over time, metastases grow, may be detected with imaging tests (preoperatively or intraoperative), and may be removed. Each time a patient undergoes a diagnostic imaging test, metastases may be independently detected or missed. Tumour detection is based on test sensitivity, assuming that below a certain (definable) size threshold, all metastases are missed. The authors116 assume that no new liver metastases develop over time.
This microsimulation model also accounted for patients’ quality of life, procedure-related adverse events, and the impact of repeating the procedure. Patients’ baseline survival rates varied according to the percentage of liver volume replaced by tumour (LVRT), but procedure-related morbidity and HRQoL were assumed not to vary according to percentage of LVRT. 116
Gazelle and colleagues116 described and justified the assumptions related to each of these parameters and their sources (Appendix 8, data extraction form). However, their data were derived from a comprehensive review of the literature, which was not reported as systematic and based on expert opinion. Limited detail on parameter derivation is provided, as there is no explanation of the method used to pool estimates from several sources, such as the hazard rates according to the percentage of LVRT. Most estimates used by Gazelle and colleagues116 have come from different data sources and may not be representative of the population of interest. Most input estimates used in the model were applied deterministically, apart from the number of metastases per patient and the size of metastases (which were randomly drawn from a population distribution), and some were subject to sensitivity analysis (further details are provided in Appendix 8b).
Estimation of quality-adjusted life-years
Patients’ HRQoL was accounted for only by Gazelle and colleagues,116 as Abramson and colleagues115 refer to a cost-effectiveness analysis in which health benefits are measured in life-years.
Gazelle and colleagues116 derived the HRQoL data used in their model from a review of the literature (not reported as systematic and described with limited detail) and from expert opinion. 116 According to Gazelle and colleagues,116 the limited data available regarding quality of life in patients with metastatic colorectal cancer suggest that the majority of survival is spent with a normal age-adjusted quality of life and that patients’ quality of life seems to decline rapidly at the end of life. Thus, age- and sex-adjusted population utilities reported by Fryback and colleagues119 [who used a preference measurement method, time trade-off (TTO), to measure the utility for current health of the US general adult population] were applied to survival without liver metastases. Based on evidence from studies120,121 conducted with non-preference-based measures, the quality of life in the month prior to death was assumed to be 60% of that for the control subjects, and a decrement was applied to reflect the impact of the interventions (70% and 95% of the pre-intervention period for surgical resection and ablation respectively). 120,121 None of the values assigned to the health states of patients with liver metastases were estimated through the use of a standardised and validated instrument for HRQoL measurement.
Estimation of costs
Abramson and colleagues115 described in detail the unit costs, resource use, and the sources of the estimates used in their analysis – Abramson and colleagues’ case series data, assumptions, or referenced sources. For instance, the probabilities of re-embolisation and of complications were estimated from the case series reported by Abramson and colleagues. 115 The perspective adopted was that of the third-party payer using 1998 Medicare reimbursement rates as a proxy for inpatient hospitalisation, procedural, and outpatient visit costs, and the Health Care Financing Administration Federal Upper Limit prices in 1998 were used for outpatient drug costs. The resource use considered comprises the initial evaluation of the patient (including specialist visits, CT imaging and blood tests), resources related to the hepatic artery chemoembolisation procedure (including a range of inpatient care accounting for several types of eventual complications), follow-up costs, and iatrogenic symptom control. The costs of disease-related symptom control were assumed to be nearly equal for both arms and therefore not included explicitly.
Gazelle and colleagues116 derived cost data from a non-systematic review of the literature and the methods for their derivation were appropriately described. A societal perspective restricted to productivity and direct medical costs and benefits (including postoperative death costs) was adopted, and the unit costs from the Medicare payment schedule were used for both tests and procedures. The cost of both technical and professional resources for each intervention (liver resection, laparotomy and radiofrequency ablation) and the costs of patient care per year (according to LVRT, derived from Taplin and colleagues122) were included. Concerning radiofrequency ablation, it was assumed that up to three liver metastases could be treated in 1 day and that two treatment sessions would be required for patients undergoing treatment for four to six metastases; thus, the costs were adjusted accordingly, and the cost of repeat radiofrequency ablation was assumed to be the same as the cost of initial therapy. Gazelle and colleagues116 also included morbidity costs related to each intervention, deriving them from daily routine care costs among patients undergoing surgical resection. Complications from radiofrequency ablation were assumed to require 1-day hospitalisation and the resource use involved would be similar to laparotomy-related complications. The cost of preoperative diagnostic CT scans (assumed to be performed on an outpatient basis) and intraoperative ultrasonography were also accounted for.
Cost-effectiveness results
Abramson and colleagues115 estimated that a cost-effectiveness threshold of $50,000 per life-year gained would require a survival benefit for hepatic artery chemoembolisation of nearly 5 months more than palliative care, and that the increment of 1 year in survival would be associated with an incremental cost of $21,045. Statistical analysis of these results is not reported; however, deterministic one-way sensitivity analyses are reported for the estimates of benefit, resource use and unit costs of several components of the strategies and it is stated that the results of the model were slightly influenced by changes in the cost of chemoembolisation (varied by ± 25%), the mean number of re-embolisation procedures per patient (1.0 or 2.0) and the schedule of follow-up visits after hepatic artery chemoembolisation (every 2 months or every 6 months). Abramson and colleagues115 also state that the cost-effectiveness of hepatic artery chemoembolisation varies considerably with expected survival benefit, and that practitioners and payers can generate preliminary cost-effectiveness estimates by using their own empirical assessments of the survival benefit of hepatic artery chemoembolisation. The authors115 acknowledge that a limitation of their study is the lack of adjustment for quality of life, and that although hepatic artery chemoembolisation may affect patients’ HRQoL positively due to the palliation of symptoms, there is potential for negative impact on quality of life due to its morbidity and potential for complications.
Gazelle and colleagues’ results116 show that the incremental benefits of surgical resection relative to radiofrequency ablation seem to outweigh the incremental costs of surgical resection regardless of the follow-up frequency. The ICER of a strategy of surgical resection of six or fewer metastases with a 12-month follow-up interval relative to the most effective non-dominated ablation strategy (i.e. ablation of up to six metastases with 12-month follow-up as well) was $16,900 per quality-adjusted life-year (QALY) gained. Increasing the frequency of follow-up for the up to six metastases surgical resection strategy to a 4-month interval yields a similar ICER ($17,800 per QALY gained) relative to the most effective non-dominated ablation strategy. Follow-up frequency had a greater impact on incremental cost-effectiveness when both strategies of surgical resection of up to six metastases were compared – the most effective surgical resection strategy of 4-month follow-up had an ICER of $31,200 per QALY gained relative to the 12-month strategy.
Despite not reporting any statistical analysis for their results or probabilistic sensitivity analysis, Gazelle and colleagues116 conducted sensitivity analyses and scenario analyses on two comparisons that differ from the base-case analysis (which involved comparison of all non-dominated strategies, i.e. all strategies apart from those that were more costly and less efficient) – radiofrequency ablation of up to five metastases with 4-month follow-up versus a no-treat strategy and versus a surgical resection strategy of up to four metastases with 6-month follow-up. The justification for the choice of these comparisons of interest was explained as due to their clinical relevance, but no further rationale was given. Sensitivity analysis was performed with several parameters, such as diagnostic test sensitivity, tumour doubling time, and cost of care. The number of metastases and the maximum tumour size treatable with radiofrequency ablation were the parameters with greater impact on the ICER, which increased from $24,300 to $47,500 per QALY when 10 metastases were considered, and to $88,300 per QALY if radiofrequency ablation is conducted for tumours of up to 10 cm. No sensitivity analysis was performed on quality-of-life estimates, such as the decrements applied to mimic the impact of surgical resection or ablation. The parameters for which sensitivity analysis was performed were also tested under three scenarios of local control rates for radiofrequency ablation: base case, improved radiofrequency ablation and perfect radiofrequency ablation. The ‘perfect radiofrequency ablation’ scenario is intended to simulate the achievement of complete tumour necrosis in all treated tumours, whereas in the ‘improved RFA’ scenario complete tumour necrosis is assumed to be achieved in 100%, 75% and 50% of treated tumours ≤ 2.5 cm, > 2.5 cm and ≤ 4 cm, and > 4 cm but ≤ 10 cm, respectively.
Gazelle and colleagues116 state their results suggest that radiofrequency ablation of larger lesions should be encouraged, even though local control rates diminish as the size of the target tumour increases. It is important to keep in mind that these results were obtained for the comparison of radiofrequency ablation of up to five metastases with 4-month follow-up versus a surgical resection strategy of up to four metastases with 6-month follow-up. Gazelle and colleagues116 also state that across all scenarios, when all possible radiofrequency ablation and surgical resection strategies were considered, more aggressive surgical strategies (i.e., the treatment of patients with more tumours, more frequent post-treatment follow-up regimens, and surgical resection rather than ablation) resulted in more QALYs gained than radiofrequency ablation. In most cases, the ICERs of even the most aggressive surgical strategies were less than $35,000 per QALY gained.
Published economic evaluation – summary of methods
Table 37 below summarises the critical appraisal of the included economic evaluations. 115,116
| Item | Abramson et al.115 | Gazelle et al.116 | |
|---|---|---|---|
| 1 | Is there a clear statement of the decision problem? | Yes | Yes |
| 2 | Is the comparator routinely used in UK NHS? | Yes | Yes |
| 3 | Is the patient group in the study similar to those of interest in UK NHS? | Yes | Yes |
| 4 | Is the health care system comparable with UK? | No | No |
| 5 | Is the setting comparable with the UK? | Yesa | Yesa |
| 6 | Is the perspective of the model clearly stated? | Yes | Yes |
| 7 | Is the study type appropriate? | Nob | Yes |
| 8 | Is the modelling methodology appropriate? | No | Yes |
| 9 | Is the model structure described and does it reflect the disease process? | No | Yes |
| 10 | Are assumptions about model structure listed and justified? | No | Yes |
| 11 | Are the data inputs for the model described and justified? | Yes | Yes |
| 12 | Is the effectiveness of the intervention established based on a systematic review? | No | No |
| 13 | Are health benefits measured in QALYs? | No | Yes |
| 14 | Are health benefits measured using a standardised and validated generic instrument? | No | Noc |
| 15 | Are the resource costs described and justified? | Yes | Yes |
| 16 | Have the costs and outcomes been discounted? | No | Yes |
| 17 | Has uncertainty been assessed? | Yes | Yes |
| 18 | Has the model been validated? | ? | Yes |
None of the published studies addresses the decision problem of this report, as neither study concerns a comparable health care system to the UK NHS, and therefore their resource use, costs and HRQoL estimates are not appropriate for the UK setting. Moreover, none of the studies used effectiveness estimates that had been found through a systematic review of the literature or report HRQoL estimates from a standardised validated preference-based instrument, as per UK guidance for technology appraisal. 117
Abramson and colleagues115 offer limited data useful to the current decision problem as the modelling approach adopted does not reflect the disease process and the estimation of health benefits did not consider patients’ HRQoL, hence a cost–utility analysis (standard method for UK technology appraisal)117 was not performed.
Gazelle and colleagues116 present an appropriate modelling approach for the current decision problem. However, their cost–utility analysis does not follow UK standard methodology117 as the estimation of health benefits and the perspective adopted are inappropriate. HRQoL estimates used by Gazelle and colleagues116 are assumptions based on Earlam and colleagues’ study,121 which had used several non-preference-based instruments to measure the quality of life of patients with colorectal liver metastases. Also, Gazelle and colleagues116 took on a societal perspective where productivity and postoperative death costs are considered contrasting with the NHS and Personal Social Services (PSS) perspective.
Systematic review of health-related quality-of-life evidence
A systematic review of the literature on the HRQoL of patients with liver metastases from any solid tumour primary site was undertaken. The aim was to provide data to populate the economic model with utility values appropriate to conduct a cost–utility analysis and to calculate the benefits of interventions in QALYs. The sources and search terms used are detailed in Appendix 9. Given the prospect that limited HRQoL data would be available, the inclusion criteria were broadened to retrieve any primary or secondary research of any design reporting HRQoL measures for patients with liver metastases at any stage of the disease, irrespective of treatments received and of the HRQoL instrument(s) used.
Results of the health-related quality-of-life systematic review
Searches for HRQoL data identified 535 studies that potentially met the inclusion criteria (Figure 3). From screening titles and abstracts, 501 studies were excluded and 34 retrieved for full screening. Twelve of these studies did not meet the inclusion criteria as they assessed a different population group from that specified in the research protocol111,123–131 or were conference abstracts. 132,133 Also the study by Lorenz and colleagues134 was found irretrievable by the time of completion of the current report. From the updated searches, 12 titles and abstracts were screened and no study was found to meet the inclusion criteria. One of these was a conference abstract by Borrego-Estella and colleagues,135 which was excluded for not providing sufficient details.
FIGURE 3.
Flow chart of identification of studies for inclusion in the HRQoL review. a, The abstracts provided insufficient details of methods and results to allow inclusion in the systematic review.
From the 21 publications found to meet the inclusion criteria, three of them were reviews116,118,136 and 18 reported on 14 primary studies. 14,120,121,137–150
Primary research
The main characteristics of the included primary studies are briefly presented in Table 38 – further details on each study and their results are reported in Appendix 10.
| Author(s), publication year, country | Study design/intervention | Study purpose | Study population | HRQoL instrument(s) and administration time period |
|---|---|---|---|---|
| Allen-Mersh et al.,120 Earlam et al.,121 Earlam et al.,152 Durand-Zaleski et al.142 1994, 1996, 1997, 1998 (UK) | RCT: (1) HAC, n = 51; (2) systemic chemotherapy, n = 34; (3) symptom control, n = 49 | Clinical efficacy including QoL and cost-effectiveness study | Unresectable CRC LM occupying up to 60% of the liver | RSC, SIP, HADS. Completed monthly between randomisation and death |
| Blair et al.,137 2003 (USA) | Prospective cohort study: (1) intrahepatic chemotherapy via the portal vein or hepatic artery, n = 16; compared with 169 non-patient volunteers | To examine QoL after regionally delivered chemotherapy and develop a specific QoL questionnaire | CRC LM, following liver resection | The City of Hope QoL Scale/Cancer Patient scale. Administered at baseline only |
| Blazeby et al.,138 2009 (UK, Germany, France) | Prospective cohort study: (1) surgical resection, n = 263; (2) palliative treatment, n = 93 | Validation study (for the HRQoL instrument) | CRC LM with an expected survival time of at least 4 weeks | EORTC QLQ-C30; EORTC QLQ-LMC21. Completed before and 3 months after treatment |
| Bruns et al.,139 2010 (Germany) | Prospective cohort study: (1) surgical resection, n = 183 (96 completed); compared with general German population norms and subgroups of indication | To assess QoL | Benign lesions, liver primaries and liver metastases (colorectal carcinoma and other primary cancers) | SF-12 health survey: completed post surgical resection, at least 3 months after discharge (range 3–36 months); pain assessment on a 0–10 scale and a liver-specific symptom score |
| Cella et al.,141 2009 (USA) | RCT: (1) sunitinib, n = 375; (2) interferon-alfa, n = 375 | To assess clinical efficacy including QoL and to assess the use of QoL to predict survival | Metastatic renal cell carcinoma | FACT-G, FKSI-DRS, EQ-VAS. Only baseline data presented in the publication |
| Haidet et al.,143 1998 (USA) | Prospective cohort study: (1) no treatment, n = 520 (304 tested for QoL; 322 for functional status) | To assess clinical outcomes and patient preferences at the end of life | CRC LM, with a likely 6-month survival estimated to be approximately 50% | Functional status on modified version of Katz scale; QoL reported on a scale of excellent, very good, good, fair or poor; POMS. Administrated at study entry |
| Kemeny et al.,144 2006 (USA) | RCT: (1) HAC, n = 68; (2) systemic chemotherapy, n = 67 | To assess efficacy including QoL | Unresectable CRC LM occupying < 70% of the liver | RAND-36; MSAS; Medical Outcomes Study Social Support Questionnaire; Medical Outcomes Study Sexual Functioning Scale. Administered before treatment and every 3 months until 18 months |
| Knox et al.,145 2004 (USA) | Retrospective cohort study: (1) surgical resection, n = 13 | To review clinical and functional QoL outcomes following surgical resection of LM | Hepatic carcinoid metastases | KPS. Measured preoperatively, 3 and 6 months postoperatively and annually thereafter |
| Krabbe et al.,146 2004 (the Netherlands) | Prospective cohort study. Exploratory laparotomy followed by: (1) surgical resection (± ablation), n = 48; (2) ablation only, n = 11; (3) no treatment, n = 16 | To compare generic HRQoL measure with disease-specific measure | CRC LM | EQ-5D index and VAS; EORTC QLQ-C30. Administered at baseline, 2 weeks, 3 months and 6 months post intervention |
| Langenhoff et al.,147 2006 (the Netherlands) | Prospective cohort study: (1) surgical resection/ablation, n = 60; (2) no treatment (after exploratory laparotomy), n = 17; (3) no treatment, n = 20 | To assess the impact on HRQoL | CRC LM | EQ-5D index and VAS; EORTC QLQ-C30. Administered at baseline, and 2 weeks, 3 months, 6 months and 12 months post laparotomy |
| Pistevou-Gombaki et al.,148 2003 (Greece) | Prospective cohort study: (1) octreotide chemotherapy, n = 16 | To investigate QoL after chemotherapy for LM | Symptomatic LM from various primary cancers (non-small cell lung, colon, pancreas, prostate, stomach) | EORTC QLQ-C30. Administered at baseline, at time of octreotide administration, 1 week later, and monthly thereafter (up to 7 months) |
| Ruers et al.,14 2006 (the Netherlands) | Prospective cohort study: (1) laparotomy for surgery followed by surgical resection, n = 53; (2) ablation (±surgical resection), n = 29; or (3) chemotherapy only, n = 27 | To assess survival and HRQoL in patients selected for laparotomy | CRC LM | EQ-VAS; EORTC QLQ-C30 physical functioning scale. Administered preoperatively, 2–3 weeks post laparotomy, and then every 3 months until 1 year post laparotomy |
| Wiering et al.,149,151 2010, 2011 (the Netherlands) | RCT and prospective cohort study: (1) surgical resection with PET imaging, n = 70; (2) surgical resection without PET imaging, n = 7 | To assess the added value of PET imaging including HRQoL in those undergoing surgical resection | CRC LM (up to 4 resectable metastases, no extrahepatic metastases) | EQ-5D index and VAS. Completed at baseline, at week 3 and week 6 post surgical resection and then every 3 months for the next 3 years |
| Wietzke-Braun et al.,150 2004 (Germany) | Prospective cohort study: (1) laser ablation, n = 45 | To investigate clinical outcomes and QoL after laser ablation for LM | CRC LM (in palliative patients with progressive disease undergoing second- or third-line chemotherapies) | EORTC QLQ-C30. Administered at baseline, 1 week, 1 month and 6 months post intervention |
Study purpose and design
Six of the 14 included studies14,120,121,140–142,144,145,149–151 are intervention studies encompassing the assessment of patients’ HRQoL, and six studies137–139,146–148,151 were aimed specifically at studying the quality of life of patients with liver metastases. One study143 was designed to investigate the clinical outcomes and patient preferences at the end of life. Four RCTs are reported in eight included publications;120,121,140–142,144,149,151 four publications report results from the same RCT. 120,121,140,142 One retrospective cohort study145 and nine prospective cohort studies14,137–139,143,146–148,150 were also included.
Populations
Ten studies14,120,121,137,138,140,142–144,146,147,149–151 involved only participants with colorectal liver metastases. One study139 also enrolled participants with primary liver tumours and benign hepatic lesions, whereas another study148 included patients with liver metastases from other primary tumours (non-small cell lung, pancreas, prostate, and stomach) besides those arising in the colorectum. There was one study on patients with renal liver metastases,141 and another where patients’ primary tumours were not specified. 145
The included studies differed in their participant inclusion criteria and therefore in participants’ prognostic factors. Patients were enrolled in different stages of the disease (resectable,149,151 unresectable,120,121,140,142 unablatable,14 or resected),137 differing as well on the number or extent of metastases. 120,121,140,142,144,149,151
Interventions and comparators
No comparative evidence of the ablative interventions included in the review and the relevant comparators were found. One before-and-after study was found of relevance to the present review – laser ablation. 150 Comparative evidence is available for non-included interventions involving relevant comparators: chemotherapy (hepatic artery chemotherapy and systemic) and BSC,120,121,140–142,144 surgical resection with and without positron emission tomography (PET) imaging,149,151 surgical resection and BSC,138 and surgical resection and/or ablation and BSC following exploratory laparotomy. 14,146,147
Health-related quality-of-life instruments
From the 14 primary studies found on HRQoL of patients with liver metastases, only three report the use of a preference-based measure – the generic instrument European Quality of Life-5 Dimensions (EQ-5D). 146,147,149,151 All 14 studies report the use of a non-preference-based instrument, either generic139,143–145,149,151 or condition-specific,137,138,148,150 or the use of both. 14,120,121,140–142,146,147 No publications involving a condition-specific preference-based measure were found.
The following generic non-preference-based instruments were used among the studies included: EQ visual analogue scale (VAS),14,141,146,147,149,151 Short Form questionnaire-12 items (SF-12),139 Karnofsky Performance Scale index,121,138,142,145 Sickness Impact Profile (SIP),121,140,142 Hospital Anxiety and Depression Scale (HADS),120,121,140,142 Rand 36-item health status profile (RAND-36), Memorial Symptom Assessment Scale, Medical Outcomes Study Sexual Functioning Scale,144 functional scale by Katz, quality of life (QoL) Excellent–Poor scale and Profile of Mood States (POMS). 143
Nine of the 14 studies using non-preference-based measures used condition-specific measures. These were the European Organisation for Research and Treatment of Cancer quality-of-life questionnaire (EORTC QLQ-C30),14,138,146–148,150 its liver metastases-specific questionnaire module EORTC QLQ-LMC21,138 the Functional Assessment of Cancer Therapy – General scale (FACT-G) and its kidney-specific supplement the Functional Assessment of Cancer Therapy – Kidney Symptom Index – Disease-Related Symptoms Subscale (FKSI-DRS),141 the City of Hope QoL Scale/Cancer Patient Survey,137 and the Rotterdam Symptom Checklist (RSC). 120,121,140,142
One study using both condition-specific (EORTC QLQ-C30) and generic (EQ-VAS) non-preference-based measures reports similar profiles of quality-of-life variation over time for both measures. 153 Although some evidence suggests that both preference- and non-preference-based instruments capture the variation of people’s quality of life over time,146 results for patients with unresectable liver metastases suggest that non-preference-based (generic and condition-specific) and preference-based measures are sensitive to different aspects of quality of life over time. 147 For resectable/ablatable patients, the condition-specific measure appeared to be more sensitive to variations in patients’ quality of life. 147 Despite the slight discrepancies in results from studies with few participants, the EQ-5D seems to capture appropriately the quality of life of patients with liver metastases, as its pattern of scores over time was similar to those obtained with the non-preference-based EQ-5D VAS (generic) and EORTC QLQ-C30 (cancer-specific) measures. 146,147
Preference-based measures
Three studies129,146,149,151 reported utility values for people with liver metastases. Each individual study investigated the HRQoL of fewer than 100 patients with colorectal liver metastases. Two studies149,151 report results from one RCT151 to assess the added value of PET imaging that subsequently led to a prospective cohort study of the total population,149 whereas the other two publications146,147 are prospective cohort studies aimed specifically at the study of HRQoL in patients with liver metastases. Table 39 shows a summary of the utility values reported in the primary research included in this review.
| Study health states | Utility values, mean (SD) |
|---|---|
| Wiering et al.151 | |
| Resected | |
| Disease free | |
| Overalla | 0.78 (0.23) |
| Baseline | 0.82 (0.02) |
| 2 weeks | 0.65 (0.05) |
| 3, 6 and 12 monthsb,c | 0.80 (0.02), 0.83 (0.02), 0.83 (0.03) |
| 24, 27 and 36 monthsb,c | 0.81 (0.04), 0.83 (0.03), 0.76 (0.05) |
| Non-curative | |
| Overalla | 0.67 (0.31) |
| Baseline | 0.78 (0.05) |
| 3, 6 and 12 monthsb,c | 0.67 (0.07), 0.60 (0.11), 0.64 (0.10) |
| Recurrence | |
| Overalla | 0.74 (0.25) |
| 24, 27 and 36 monthsb,c | 0.76 (0.04), 0.76 (0.04), 0.57 (0.07) |
| Recurrence treated with surgical resection | |
| Overalla | 0.82 (0.17) |
| 12, 18 and 21 months | 0.85 (0.02), 0.85 (0.02), 0.84 (0.03) |
| 30 and 36 months | 0.85 (0.03), 0.74 (0.08) |
| Recurrence treated with chemotherapy | |
| Overalla | 0.68 (0.28) |
| 12, 18 and 21 months | 0.75 (0.04), 0.68 (0.05), 0.70 (0.05) |
| 30 and 36 months | 0.70 (0.05), 0.45 (0.10) |
| Langenhoff et al.147 | |
| Inoperablec | |
| Baseline | 0.70 (0.07) |
| 2 weeks | 0.80 (0.02) |
| 3 and 6 months | 0.78 (0.02), 0.67 (0.11) |
| Resected or ablatedc,d | |
| Baseline | 0.86 (0.02) |
| 2 weeks | 0.70 (0.02) |
| 3 and 6 months | 0.78 (0.02), 0.85 (0.01) |
| Unresectable or unablatablec,d | |
| Baseline | 0.77 (0.06) |
| 2 weeks | 0.59 (0.09) |
| 3 and 6 months | 0.76 (0.04), 0.83 (0.02) |
| Krabbe et al.146 | |
| Overall baseline utilityd | 0.84 (0.12) |
| Resected (± ablated)c,d | |
| Baseline | 0.85 (0.02) |
| 2 weeks | 0.68 (0.03) |
| 3 and 6 months | 0.74 (0.02), 0.83 (0.03) |
| Ablatedc,d | |
| Baseline | 0.90 (0.02) |
| 2 weeks | 0.81 (0.03) |
| 3 and 6 months | 0.90 (0.03), 0.90 (0.02) |
| Unresectable or unablatablec,d | |
| Baseline | 0.79 (0.05) |
| 2 weeks | 0.60 (0.08) |
| 3 and 6 months | 0.77 (0.07), 0.83 (0.03) |
Wiering and colleagues149,151 provide EQ-5D utilities for resectable patients at baseline and after surgical resection over 3 years in different health states: disease-free, non-curative, and recurrence (treated with surgical resection or chemotherapy). Patients in the different health states had baseline scores crudely close to 0.8 and a clear decrease 3 weeks after surgical resection,151 suggesting that patients with liver metastases have similar HRQoL to that of the general population at that age. The Health Survey for England (1996) reports the mean (± standard error) utilities for people living in private households in England as 0.79 (± 0.006) for 55–64-year-olds and 0.78 (± 0.006) for 65- to 74-year-olds. 154
Two other studies146,147 provide EQ-5D index scores over time for patients undergoing exploratory laparotomy. The design of these studies146,147 does not allow the differentiation between surgical resection and ablation in their impact on patients’ quality of life. Results for patients with unresectable liver metastases (n = 20) suggest that non-preference-based (generic and condition-specific) and preference-based measures are sensitive to different aspects of quality of life over time, as non-preference-based scores presented deterioration of HRQoL at 6 months after enrolment, whereas EQ-5D utilities improved slightly 2 weeks after enrolment but became comparable with baseline scores at 3 and 6 months. 147 These results should be considered with caution given that this is a prospective study of a small cohort.
Estimates for the unresectable/unablatable group reported by Langenhoff and colleagues147 are similar to those obtained by Krabbe and colleagues. 146
Effect of liver metastases on patients’ quality of life
Some evidence from generic non-preference-based measures suggests that liver metastases do not have much impact on patients’ overall quality of life. 121,137,142,143 This may be a result of the lack of sensitivity of the instruments to the health domains affected by liver metastases. Condition-specific measure EORTC QLQ-LMC21 symptom scales and single items indicate the main symptoms affecting patients with liver metastases. There is evidence that patients with liver metastases on palliative care report significant deterioration over time of symptoms, such as taste problems, dry mouth, sore mouth, and peripheral neuropathy. 138 Patients with liver metastases show variation in quality of life according to the severity of the disease (as different baseline EQ-5D and EORTC scores are shown in studies reporting results by resectable/ablatable/unresectable subgroups),14,138,146,147 and to the recurrence of liver metastases. 151
No significant difference in functional improvement was shown between patients with ≥ 90% or < 90% total liver tumour volume resected. 145 Contrastingly, Earlam and colleagues121 showed a positive correlation between percentage of hepatic replacement and RCS, SIP and HADS scores. There is also evidence that the quality of life of patients with liver metastases is influenced by the percentage of hepatic replacement,121 and by the percentage of tumour removed (non-curative surgery). 151 These results suggest that Karnofsky Performance Scale145 is not sensitive to variations on quality of life of patients with liver metastases according to hepatic volume replaced by tumour.
The quality of life of patients on symptom control seems to deteriorate late in the disease course (a significant decrement of quality of life is only seen 5 months to 1 month before death) and patients receiving symptom control spent approximately 24% of their survival with normal RSC, SIP and HAD scores. 120,121 Unablatable patients had no clear variation of quality of life (EQ-VAS and EORTC QLQ-C30 scores) at 2 weeks and 3 months after enrolment, showing deterioration only at 6 months. However, improvement of the EQ-5D index scores was seen 2 weeks after enrolment and these scores were comparable with baseline at 3 and 6 months. 147 Unresectable patients had worse EORTC QLQ-LMC21 symptoms scores at 3 months (taste problems, dry mouth, sore mouth and peripheral neuropathy). 138
The quality of life of patients with liver metastases appears to vary according to the stage of the disease and prognostic factors, such as number and size of liver metastases (surrogate indicators: hepatic volume replaced by tumour, eligibility for surgical resection/ablation), and seems to deteriorate slowly over time, weakening severely at the end of life.
Secondary research
The three reviews which met the inclusion criteria were economic evaluations using utility values to calculate QALYs (Table 40). 116,118,136 Two of these were conducted by Gazelle and colleagues,116,118 who performed a cost–utility analysis to estimate the relative cost-effectiveness of radiofrequency ablation and surgical resection (addressed also in Systematic review of existing cost-effectiveness evidence) through the adaptation of the model they had previously used to study the cost-effectiveness of surgical resection compared with no treatment. 118 Gazelle and colleagues used the same rationale and HRQoL data sources for the estimation of QALYs in both economic evaluations, based on published evidence119–121 and expert opinion. From the Earlam and colleagues121 and Allen-Mersh and colleagues120 studies (both included in the current review), Gazelle and colleagues116 inferred that the majority of survival of patients with colorectal cancer liver metastases is spent with a normal age-adjusted quality of life. Thus, age- and sex-adjusted population utilities119 were applied to survival without liver metastases (following surgical resection/ablation). A decrement was applied to reflect the impact of the interventions (70%116,118 and 95%116 of the pre-intervention period for surgical resection and ablation, respectively). The quality of life of patients with liver metastases in the month prior to death was also assumed to be 60% of that for the control subjects. No sensitivity analyses were conducted on these estimates.
| Study | Health states | Utility values | Source |
|---|---|---|---|
| Gazelle et al.,116,118 2003, 2004 (USA) | With LM (pre intervention) | Age- and sex-adjusted population utilities | Fryback et al.119 |
| Without LM (post intervention) | |||
| After surgical resection (first month) | 70% of pre-intervention utility | Expert opinion based on Earlam et al.121 and Allen-Mersh120 | |
| After RFA (first month) | 95% of pre-intervention utility | ||
| With LM (month prior to death) | 60% of pre-intervention utility | ||
| Karuna et al.,136 2008 (USA) | Post resection without morbidity/mortality | 0.60, 0.74, 0.80a,b | Langenhoff et al.147 |
| Post laparoscopy, then laparotomy without surgical resection | 0.54, 0.67, 0.72a,b | ||
| Post resection with morbidity | 0.57, 0.71, 0.78a | Langenhoff et al.,147 Poon et al.155 | |
| Post-laparoscopy unresectable disease | 0.76, 0.72, 0.72a |
Karuna and colleagues136 conducted a cost–utility analysis of laparoscopy versus laparotomy for initial surgical assessment and treatment of potentially resectable colorectal liver metastases. The utility weights used for the estimation of QALYs were derived from the HRQoL study by Langenhoff and colleagues147 (included in the current review) and a HRQoL study in hepatocellular carcinoma patients155 (a different population, hence not included in this review). Patients with immediate post-operative complications were assigned a small decrement (0.02) in utility to account for the additional loss of quality of life. Patients’ quality of life was also assumed to be constant after 6 months post intervention, which was supported by recent evidence that showed that quality of life is stable from 6 months post intervention until immediately prior to death. 119,147,155
Summary and conclusions
The current review did not identify any comparative studies for the ablative therapies and the relevant comparators included in the current study. No evidence of the impact on patients’ HRQoL was found for most of these interventions. Only a before and after study was found related to one of the included ablative therapies and this reported no statistical significant difference before and 1 week, 1 month and 6 months after initiation of laser ablation in people with progressive disease undertaking second- and third-line chemotherapy. 150
Published research involving the comparators included in this study is very limited as it consists of studies not involving comparisons of interest to our review120,121,140,142,144,146–149,151,153 and those assessing HRQoL for a short follow-up period (3 or 6 months)138,146,147 or only at baseline. 141 Part of the evidence identified consists of prospective cohort studies where the impact of the intervention is assessed through comparison of the cohort results before and after the intervention, and therefore without a control group. 137,139,148,150 Several studies have enrolled fewer than 100 participants. 137,145–151 Therefore, evidence of the impact of ablative therapies is lacking.
Additionally, most of the evidence available has not been measured using a preference-based instrument or non-preference-based measures that can be mapped to derive utilities. 120,121,137,139–145
Moreover, there is little evidence of the variability of HRQoL of patients with liver metastases according to the severity of the disease (indicated by prognostic factors such as the number and size of metastases, or by surrogate indicators such as the proportion of total liver tumour volume resected or residual liver volume), and the rate of tumour growth.
Implications for future cost-effectiveness modelling
Modelling options are restricted by the little evidence available on HRQoL of patients with liver metastases. The evidence available suggests near-normal quality-of-life levels at baseline, substantial decrement due to the intervention followed by a quick recovery to baseline values138,146,147,149,151,153 and serious deterioration of quality of life at the end of life,120,121,140,142 supporting the rationale taken on in previously published economic evaluations. 116,118,136 Evidence also suggests that patients’ eligibility to the interventions varies according to disease severity (resectable, ablatable, inoperable). 138,146,147 The quality of life of patients treated by surgical resection or ablation does not deteriorate as much as that for patients found to be unresectable/unablatable after exploratory laparotomy, and this latter group takes longer to regain its baseline quality of life. 147 Ablatable patients presented better scores than resectable/ablatable ones over 6 months, while those found to be unresectable at laparotomy reported worse scores over 6 months for EQ-VAS, EORTC QLQ-C30, and EQ-5D index. 146
Moreover, statistically significant differences in quality of life over time were found between groups in different health states (disease free, non-curative, and recurrence) indicating different quality of life for progression-free survival and post-progression survival of resected patients and the impact of non-curative interventions. 151 Also, the RSC physical score was shown to be a predictor of survival for patients on symptom control,121 and patients’ RAND-36 physical functioning was shown to vary over time as a function of survival. 144
Implications for the independent survival model
In order to capture the most significant variations of HRQoL of patients with liver metastases over time, the independent survival-based model (further described in Independent economic evaluation) includes a number of health states. The impact of the interventions is captured by the resultant life expectancy in each of the different utility-weighted health states.
The comparisons being analysed in the section of the report headed Independent economic evaluation (regarding radioembolisation relative to hepatic arterial infusion, hepatic arterial infusion alone, microwave ablation, radiofrequency ablation, and surgical resection) involve different subgroups of patients. The comparison of radioembolisation associated to hepatic arterial infusion with hepatic arterial infusion alone investigated unablatable patients (non-eligible to any sort of surgical or local ablative intervention);85 hence, the utility values reported for the inoperable group by Langenhoff and colleagues147 were assumed to be generalisable to these patients. Both Shibata and colleagues72 and Kim and colleagues73 enrolled resectable patients to compare an ablative intervention with surgical resection. Although patients’ inclusion criteria (such as the maximum number of metastases) differ among studies, Wiering and colleagues’151 utility estimates seem generalisable to the surgical resection arms of the clinical effectiveness studies. 72,73 Similarly, utilities from Krabbe and colleagues’146 ablatable group were used as estimates for the impact of the ablative therapies on quality of life and for ablated patients with stable disease.
The following assumptions underlie the choice of the estimates presented in Table 41:
-
Interventions’ impact measured 2 weeks after intervention is assumed to remain for a whole month as it is applied to the first cycle of the model.
-
Given the lack of specific estimates, all ablative therapies are assumed to have the same impact on patients’ quality of life.
-
Ablated patients are assumed to reach stable disease at 6 months.
-
Utility weight for resected patients with progressive disease assumed to be the same for ablated patients in this health state, given the lack of particular evidence. This is thought to be a conservative approach, as ablatable patients presented better scores than resectable/ablatable ones over 6 months for EQ-VAS, EORTC QLQ-C30, and EQ-5D index. 146
-
The same utility weight was assigned to all patients with terminal disease irrespective of their initial disease severity and the interventions they had undertaken. This is the estimate from the final measurement by Wiering and colleagues151 for their recurrence group with most advanced disease.
| Utility values | Health states, mean (SD) | ||||
|---|---|---|---|---|---|
| Interventions | Baseline | Intervention impact | Stable | Progressive | Terminal |
| HAC | 0.70 (0.07)a | 0.80 (0.02)b | 0.78 (0.02)c | 0.67 (0.11)d | 0.45 (0.10)l |
| RE + HAC | 0.70 (0.07)a | 0.80 (0.02)b | 0.78 (0.02)c | 0.67 (0.11)d | 0.45 (0.10)l |
| MWA | 0.90 (0.02)e | 0.81 (0.03)f | 0.90 (0.02)g | 0.74 (0.25)k | 0.45 (0.10)l |
| RFA | 0.90 (0.02)e | 0.81 (0.03)f | 0.90 (0.02)g | 0.74 (0.25)k | 0.45 (0.10)l |
| Surgical resection | 0.82 (0.02)h | 0.65 (0.05)i | 0.78 (0.23)j | 0.74 (0.25)k | 0.45 (0.10)l |
Conclusions
There is a lack of randomised comparative studies concerning ablative therapies and the relevant comparators using a standardised, validated preference-based HRQoL instrument, such as the EQ-5D (recommended for measuring and valuing health benefits for health technology assessment in the UK). 117 No evidence was found on condition-specific preference-based measures (expected to offer greater resolution of patients’ HRQoL than a generic instrument such as the EQ-5D) either. At the time of writing, the EORTC QLQ-LMC21 is the only instrument specific to liver metastases available; however, this is a non-preference-based tool and no publications on its mapping into EQ-5D have been found.
Independent economic evaluation
A survival-based model was developed to estimate the cost-effectiveness of ablative therapies, or other non-invasive therapies, in cohorts of adult patients with surgically resectable or unresectable liver metastases. Due to data limitations identified in the clinical effectiveness review and discussed later in this section of this report (primarily a lack of comparative studies, or limitations on the reporting of survival outcomes in those comparative studies that were identified) the analysis does not include all therapies identified (in Inclusion and exclusion criteria) as relevant to this review. The analysis for ablative therapies was limited to comparisons of:
-
microwave ablation versus surgical resection in surgically resectable patients
-
radiofrequency ablation versus surgical resection in subgroups of surgically resectable patients.
The analysis for other non-invasive therapies was limited to a comparison of:
-
radioembolisation (combined with hepatic arterial chemotherapy) versus hepatic arterial chemotherapy alone for surgically unresectable patients.
The clinical effectiveness evidence used to populate the model was derived from studies with varying designs (RCT72,85 and prospective cohort73) and varying risk of bias. The limitations of the evidence base need to be borne in mind when interpreting the results of the modelled analyses.
Methods for economic analysis
Model type and rationale for model structure
Figure 4 presents a schematic of the basic survival model which, in its simplest form, contains three states – stable disease (i.e. patients’ state at entry to the trial), progressive disease and death. 156,157 Movements between these states are usually permitted only in the progressive direction. This approach has been adopted to model the cost-effectiveness of ablative therapies for liver metastases.
FIGURE 4.
Schematic of the survival model adopted for the cost-effectiveness model. OS, mean overall survival; OS-TTP, mean survival duration with progressive disease; TTP, mean time to progression.
An advantage of this approach is that the model is based on relevant final outcomes (survival weighted by quality of life) rather than using surrogate measures, such as tumour response or complete/partial resection for which additional evidence would be required to establish the link to final outcomes. Interpretation of this basic model in the context of metastatic disease is slightly more complex than in the usual application, where therapy is directed at the primary tumour. In this case patients’ primary tumour is assumed to be stable and the intention of treatment is to remove the metastases (in the case of treatment with curative intent) or to reduce the size of the metastases, improving quality of life and offering some increase in life expectancy, in the case of treatment with palliative intent. Therefore, the stable disease state may include quite different patients – those whose metastases have been completely removed and those with remaining metastases. Quality of life for this group of patients is weighted to take account of those metastases that are removed (equivalent to a complete response on standard chemotherapy outcome scales) and those with remaining metastases (see later in this section). Including both groups of patients in a single ‘stable’ health state in the survival model, rather than adopting a state transition model (explicitly modelling response/removal of metastases), is a pragmatic decision based on the fact that studies have not reported survival, duration of response or time to disease progression for these patient groups separately.
Patients enter the model with a stable primary tumour, with liver metastases and receive treatment with an ablative therapy (with curative or palliative intent depending on the patient population in the included study) or the relevant comparator treatment. Patients may experience disease progression or may die without experiencing documented disease progression.
The model was developed in Microsoft Excel (Microsoft Corporation, Redmond, WA, USA). Two versions of the model were developed independently, using the same input parameters, and tested against each other to ensure internal consistency. It is fully probabilistic to take into account parameter imprecision. In addition, deterministic sensitivity analysis was used to explore different scenarios and assumptions in the model.
The base-case analysis compares the mean overall survival with ablative therapy (OS¯AT) with the mean overall survival for comparator (OS¯C). he estimate of life-years gained with the ablative therapy, in the base case, was calculated as:
To estimate the QALY gain associated with the ablative therapy (QALYGAT), response-specific utilities [US for stable (which may be a weighted sum of the utility in patients whose metastases are removed and those successfully treated but with residual metastases) and UP for disease progression, respectively], derived in our review of quality-of-life studies (see Systematic review of health-related quality-of-life evidence), were applied to the mean overall survival estimates, taking into account survival before disease progression (mean time to progression, TTP¯AT) and post-progression survival (OS¯AT−TTP¯AT). The quality-adjusted life expectancy gain was therefore calculated as:
Baseline cohort
The population in the base-case analysis are adult patients with liver metastases. Patients receiving ablative therapies, other non-invasive therapies or comparator treatments are assumed to have a stable primary tumour and may be undergoing treatment of curative or of palliative intent.
Perspective and time horizon
The perspective of the cost-effectiveness analysis is that of the NHS and PSS. A lifetime time horizon has been adopted for the model.
Discounting
Both costs and outcomes were discounted using a 3.5% discounting rate, as currently recommended by the UK Treasury for public sector appraisal. 158
Assessment of uncertainty
The purpose of this analysis is to test the robustness of the cost-effectiveness results to variation in structural assumptions and parameter inputs. Deterministic analyses were used to address particular areas of uncertainty in the model. The uncertainties around the probability, resource use and cost estimates that were expected, a priori, to have a disproportionate impact on the study results were investigated by applying ranges around the point estimates used in the base-case analysis. Scenario analysis was used to address the uncertainty associated with the choice of data source adopted for parameter values in the base case and some aspects of the chosen structure of the model.
Parameter uncertainty was addressed using probabilistic sensitivity analysis (PSA). Probability distributions were assigned to describe uncertainty around the point estimates used in the base-case analysis. Variables included in the PSA, the sampling distribution and the parameterisation of the sampling distribution are reported in Appendix 11. Each PSA is run for 1000 iterations.
Data sources used in model
Figure 5 reports the ablative therapies and the number of studies identified in the clinical effectiveness review. However, not all these studies provide robust data for the model or reported all relevant outcomes in sufficient detail to be included in the economic model.
FIGURE 5.
Studies of ablative therapies providing sufficient data to model.
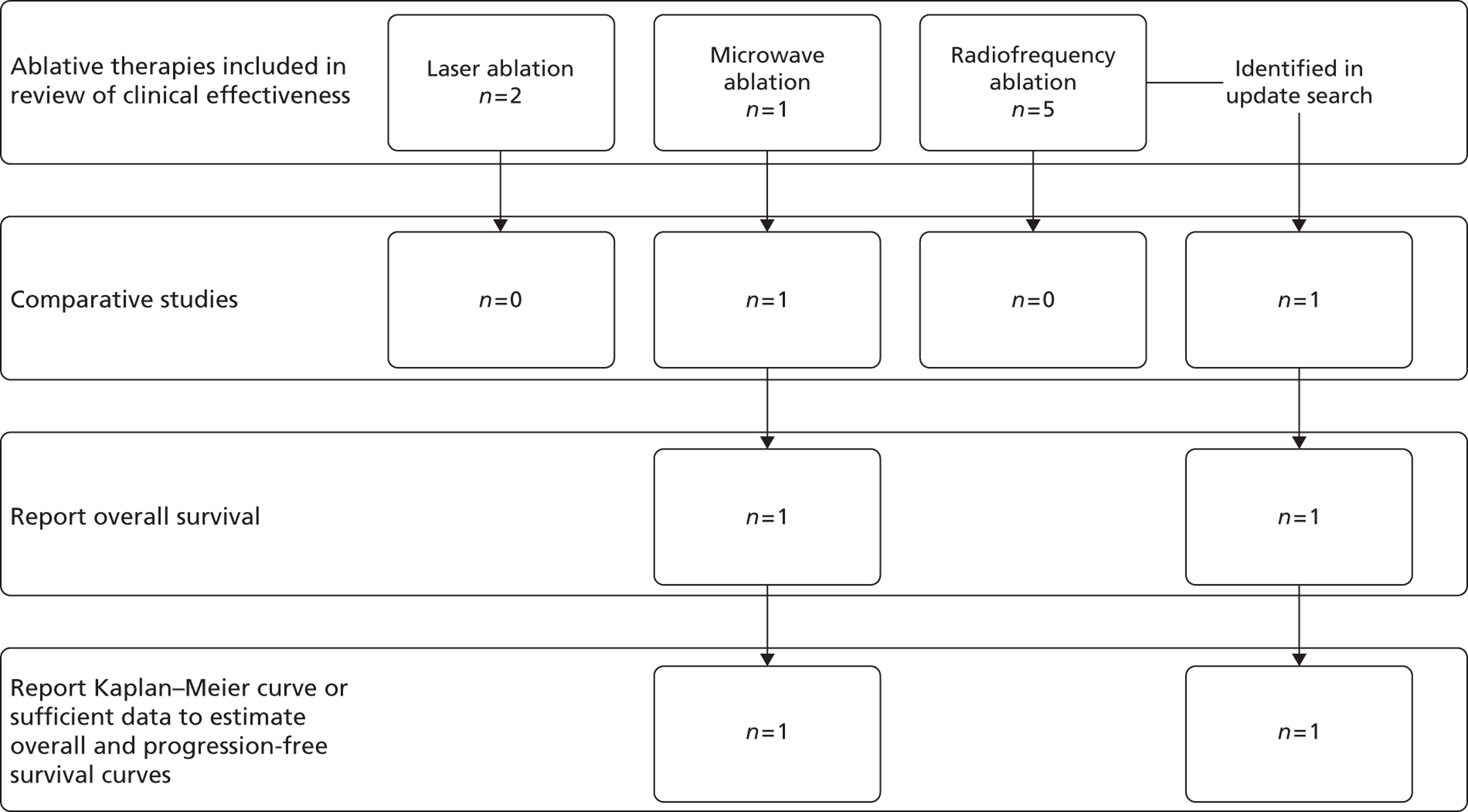
One ablative therapy (laser ablation) was excluded from the model due to a lack of comparative studies. Radiofrequency ablation was also initially excluded, due to a lack of comparative studies. However, one non-randomised comparative study was subsequently identified in update searches,73 which compared radiofrequency ablation with surgical resection and which reported overall survival and disease-free survival for three subgroups of patients with liver metastases from colorectal cancer. The identified subgroups were patients with solitary liver metastases of < 3 cm; patients with solitary liver metastases ≥ 3 cm; and patients with multiple liver metastases. This was not a randomised study and patients undergoing each treatment may not be directly comparable [e.g. mean age of patients undergoing surgical resection was younger (57.1 years) than that of those undergoing radiofrequency ablation (60.4 years, reported p-value on difference of 0.001)] and also appear to differ in terms of synchronicity (9.6% of surgically resected patients had synchronous metastases and 90.4% had metachronous metastases, whereas 77% of patients undergoing radiofrequency ablation had synchronous metastases and the remaining 23% had metachronous metastases). The results from the modelled analysis based on this study should therefore be treated with caution.
Figure 6 lists the other non-invasive therapies and the number of studies identified in the clinical effectiveness review. While comparative studies of chemoembolisation were identified and included in the systematic review of clinical effectiveness, the included studies did not report sufficient detail on relevant outcomes (overall survival and time to progression) to be included in the model. Two studies involving radioembolisation also reported insufficient detail on overall survival and time to progression to be included in the model.
FIGURE 6.
Studies of other non-invasive therapies providing sufficient data to model.
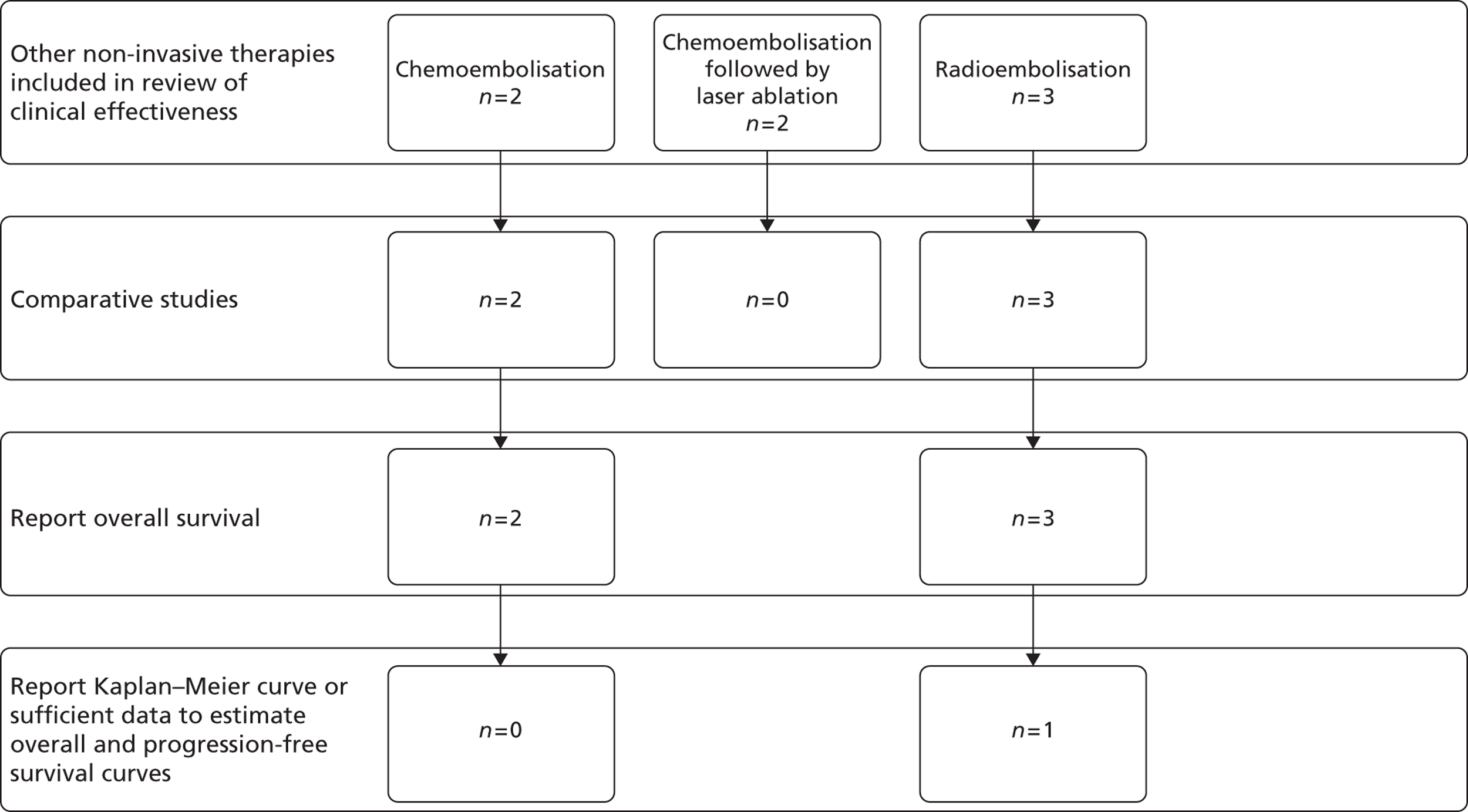
As a result three comparisons are included in the model – two ablative therapies compared with surgical resection (microwave ablation compared with surgical resection based on the RCT reported by Shibata and colleagues72 and radiofrequency ablation compared with surgical resection based on the cohort study reported by Kim and colleagues73) and one other non-invasive therapy (radioembolisation in conjunction with hepatic artery chemotherapy compared with hepatic artery chemotherapy alone, based on data reported in the RCT by Grey and colleagues85). Each of these comparisons is based on a single study.
The following sections describe the data sources used to populate the model and any additional analyses required to prepare the data for use in the model. There are three main subsections describing the derivation of data for clinical effectiveness, quality of life and resource and cost. Where relevant these subsections are further subdivided; for example, the resource and cost section is broken down to describe costs applied at different phases of the patient care pathway, characterised as treatment/monitoring costs and disease management costs.
The first of the following subsections reports on the clinical effectiveness data included in the model under the headings of overall survival, progression-free survival and adverse events. The derivation of data used in the model is described under these headings for each of the therapies included in each analysis. The second subsection describes the quality-of-life data (utilities associated with treatment and with disease state) applied in the model, based on the review of quality-of-life studies reported earlier (see Systematic review of health-related quality-of-life evidence). The final subsection reports the data assumptions applied in the model for costing interventions and patient management – reported under headings of treatment costs (including on-treatment management and post-discharge follow-up for surgical resection); post-treatment monitoring (prior to disease progression); post-progression/palliative care; and adverse events (if relevant).
Clinical effectiveness
Statistical models were fitted to data derived from survival plots reported in the included studies. Three survival functions (exponential, Weibull and log-logistic) were fitted in each case and the function offering the best fit was selected for the survival model. The three survival functions have different assumptions regarding the hazard function: the exponential function assumes constant hazards over time; the Weibull function assumes that hazard either increases or decreases monotonically with time; and the log-logistic function has a hazard function with a single peak, thus the hazard may increase until time t and decrease thereafter.
The exponential model has a very simple form and can be derived easily with minimal reported data (e.g. from median survival),159 which may explain its popularity in many health economic models. However, the constant hazard property may not be appropriate in many clinical settings. In contrast, the log-logistic function, while having the flexibility to capture monotonically declining rates as well as those that initially increase and subsequently decline, can give rise to implausibly high maximum survival durations. As a rule, the simplest form that is consistent with the observed data will be selected, which will, in many cases, be the Weibull function.
Overall survival – microwave ablation compared with surgical resection
Overall survival curves for patients undergoing surgical resection and microwave ablation, reported by Shibata and colleagues,72 were scanned using Engauge software (Engauge Digitizer: http://digitizer.sourceforge.net) and imported into Microsoft Excel. At the end of follow-up all patients with complete follow-up in the trial had died [12 in the surgical resection arm (seven with hepatic failure) and nine in the microwave ablation arm (six with hepatic failure)], with four patients in the surgical resection arm and five in the microwave ablation arm censored at between 10 and 30 months of follow-up.
The extrapolated overall survival curves for the surgical resection arm, using different survival functions, are given in Figure 7 along with Kaplan–Meier survival estimates from the trial report. The Weibull and log-logistic survival functions appear to give the closest fit to the overall survival curves.
FIGURE 7.
Kaplan–Meier survival estimates from the Shibata and colleagues72 trial showing Weibull and alternative model fit.
The survival model was fitted to the survival plots for both interventions simultaneously, using linear regression, with surgical resection as the base intervention and estimating a HR for microwave ablation, relative to surgical resection – full details on the method for transforming data for use in the linear regression and for estimating the survival models are reported in Appendix 12.
Table 42 reports a comparison of observed survival at 1, 2 and 3 years against model predictions.
| Year | Trial report | Weibull model | Exponential model | Log-logistic model | ||||
|---|---|---|---|---|---|---|---|---|
| MWA | Surgical resection | MWA | Surgical resection | MWA | Surgical resection | MWA | Surgical resection | |
| 1 | 71% | 69% | 82% | 83% | 48% | 40% | 80% | 78% |
| 2 | 57% | 56% | 43% | 47% | 23% | 16% | 34% | 31% |
| 3 | 14% | 23% | 14% | 18% | 11% | 8% | 13% | 12% |
Data in Table 42 suggest that, while the exponential survival function is a particularly poor fit for the observed data, none of the survival functions provides good predictions of survival for the reported time periods. Both the Weibull and log-logistic functions overestimate early survival (up to 1 year) and underestimate later survival (from 2 years). The exponential and log-logistic functions estimate lower survival with surgical resection at each reported time point, while the observed data has a higher survival with surgical resection at 3 years.
Mean survival reported by Shibata and colleagues72 and from each of the modelled survival functions (extrapolated to a maximum duration of 49 months for comparison with the area under the reported Kaplan–Meier curve) are reported in Table 43.
| Treatment arm | Mean overall survival (months) | |||
|---|---|---|---|---|
| Trial report | Weibull | Exponential | Log-logistic | |
| Surgical resection | 24.7 | 24.1 | 12.7 | 21.1 |
| MWA | 24.8 | 22.9 | 15.4 | 21.7 |
Mean overall survival for the surgical resection arm estimated using the Weibull survival function is similar to that estimated from the Kaplan–Meier curve and the value reported in the trial publication (24.7 estimated from the Kaplan–Meier curve, 24.1 using the Weibull survival function and 25 months reported in the trial publication). However, while mean survival estimated from the Kaplan–Meier curve for microwave ablation is the same as for surgical resection (and in the trial publication is higher, at 27 months), mean overall survival for microwave ablation, estimated using the Weibull survival function (22.9 months), is lower than for surgical resection. The model using the exponential function appears to substantially underestimate overall survival for both surgical resection and microwave ablation (with lower estimated survival for surgical resection compared with microwave ablation). The log-logistic survival function also underestimates overall survival for both surgical resection and microwave ablation (with lower estimated survival for surgical resection than for microwave ablation), though the differences compared with the reported trial values are not as great.
The Weibull model for the base-case analysis was adopted and the alternative survival functions in structural sensitivity analyses were included.
Overall survival – radiofrequency ablation compared with surgical resection
Overall survival curves reported by Kim and colleagues73 were scanned using Engauge software and imported into Microsoft Excel. The study reports survival curves for three groups of patients undergoing surgical resection or radiofrequency ablation: those with solitary liver metastases with tumour size of < 3 cm, those with solitary liver metastases of ≥ 3 cm and patients with multiple metastases. At the end of follow-up (maximum of 72 months) the survival curves show a proportion of patients were still alive (Table 44 shows 5-year survival reported in the study). The final portions of the survival curves were extrapolated using a regression analysis for each patient population separately. The survival model was fitted to data derived from the survival plots for both interventions simultaneously with surgical resection as the base intervention and estimating a HR for radiofrequency ablation, relative to surgical resection.
Radiofrequency ablation compared with surgical resection for solitary metastases < 3 cm
The extrapolated overall survival curves, for surgical resection, are given in Figure 8 along with Kaplan–Meier survival estimates from the trial report. The Weibull and log-logistic survival functions appear to fit the overall survival curves reasonably well, although all of the modelled functions give lower predictions than the Kaplan–Meier curves for survival over 5 years. The Weibull and log-logistic functions appear to overestimate median survival [trial report = 59.6 months (this is not reported in the study, but has been estimated from the Kaplan–Meier curve), Weibull = 52.9 months, log-logistic = 54.5 months] while the exponential provides a closer estimate for median overall survival (61.2 months).
FIGURE 8.
Kaplan–Meier survival estimates for resected patients with solitary metastases (< 3 cm) from the Kim and colleagues73 study showing alternative model fit.
Table 45 reports a comparison of observed survival at 1, 2, 3 and 5 years against model predictions.
| Year | Study report | Exponential model | Weibull model | Log-logistic model | ||||
|---|---|---|---|---|---|---|---|---|
| Resect | RFA | Resect | RFA | Resect | RFA | Resect | RFA | |
| 1 | 95% | 95% | 87% | 87% | 95% | 95% | 95% | 95% |
| 2 | 81% | 85% | 76% | 75% | 84% | 83% | 83% | 83% |
| 3 | 67% | 63% | 67% | 65% | 70% | 69% | 69% | 69% |
| 5 | 51.2% | 51.1% | 51% | 49% | 42% | 42% | 45% | 45% |
Data in Table 45 suggest that the exponential survival function is a poor fit to the observed data over the first 24 months, while the Weibull and log-logistic functions both provide a reasonable fit for early survival (up to 3 years). Both functions seem to underestimate later survival with surgical resection and RFA. In contrast the exponential function appears to give very close predictions for 3- and 5-year survival for surgical resection.
Mean survivals derived from the study by Kim and colleagues73 and from each of the modelled survival functions (extrapolated to a maximum duration of 72 months for comparison with the area under the reported Kaplan–Meier curve) are reported in Table 46.
| Treatment arm | Mean overall survival (months) | |||
|---|---|---|---|---|
| Study report | Exponential | Weibull | Log-logistic | |
| Surgical resection | 49.7 | 49.2 | 49.5 | 50.2 |
| RFA | 49.5 | 48.5 | 49.5 | 49.9 |
| Difference | 0.2 | 0.7 | 0.0 | 0.3 |
The exponential and Weibull give close predictions for mean overall survival, although the Weibull function indicates no difference in survival between surgical resection and RFA, while the values read from the Kaplan–Meier curve suggest that survival is slightly higher with surgical resection. The log-logistic function slightly overestimates overall survival compared with the values read from the Kaplan–Meier curve and suggests a slightly greater survival difference.
The Weibull model was adopted for the base-case analysis and the alternative survival functions in structural sensitivity analyses were included.
Radiofrequency ablation compared with surgical resection for solitary metastases ≥ 3 cm
The extrapolated overall survival curves, for surgical resection, using different survival functions, are given in Figure 9 along with Kaplan–Meier survival estimates from the trial report. The Weibull and log-logistic survival functions appear to fit the overall survival curves reasonably well, although all of the modelled functions give lower predictions than the Kaplan–Meier curves for survival over 5 years (48% from the Kaplan–Meier curve vs. 43% for the exponential, 42% for Weibull and 43% for the log-logistic model). Each of the modelled functions appears to overestimate median survival [trial report = 45.6 months (this is not reported in the study, but has been estimated from the Kaplan–Meier curve), exponential = 49.5 months, Weibull = 50.6 months, log-logistic = 50.2 months].
FIGURE 9.
Kaplan–Meier survival estimates for resected patients with solitary metastases (≥ 3 cm) from the Kim and colleagues73 study showing alternative model fit.
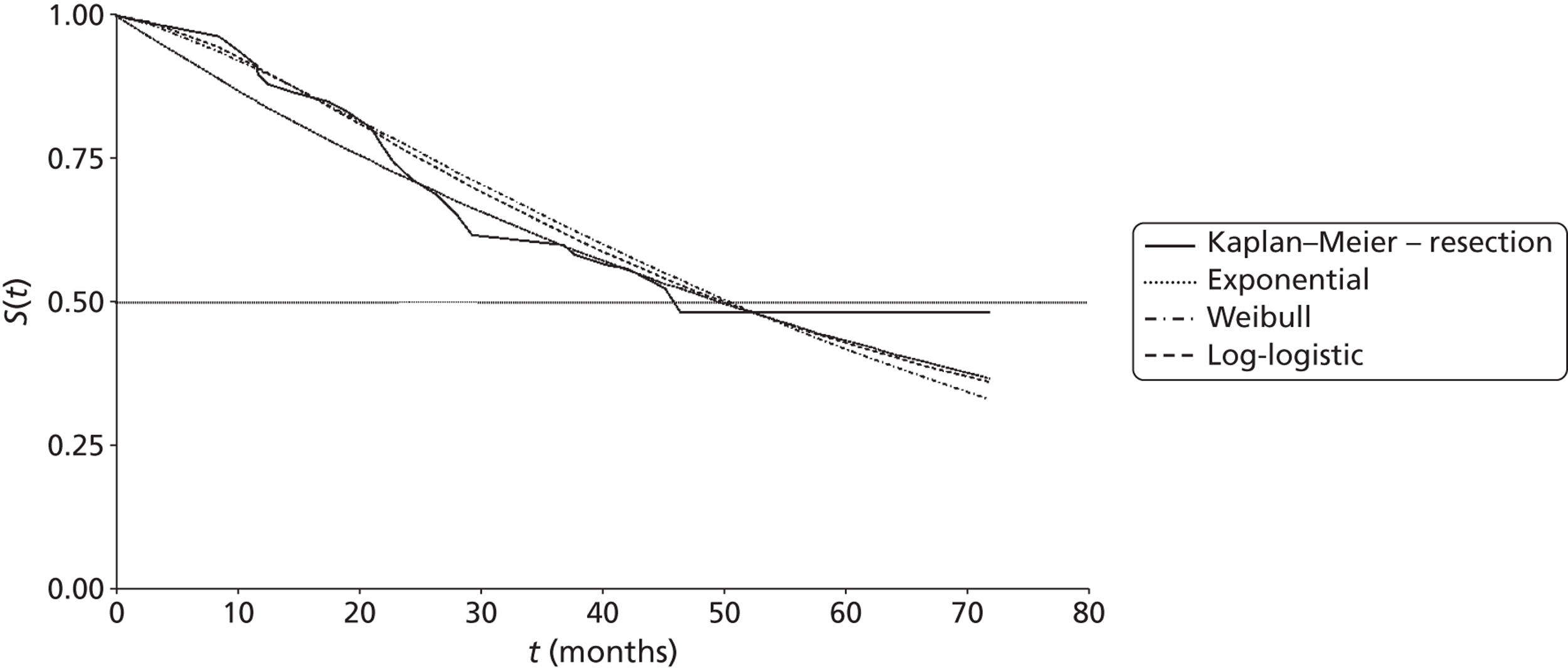
Table 47 reports a comparison of observed survival at 1, 2, 3 and 5 years against model predictions.
| Year | Trial report | Exponential model | Weibull model | Log-logistic model | ||||
|---|---|---|---|---|---|---|---|---|
| Resect | RFA | Resect | RFA | Resect | RFA | Resect | RFA | |
| 1 | 89% | 77% | 86% | 86% | 90% | 82% | 91% | 79% |
| 2 | 72% | 56% | 74% | 74% | 77% | 60% | 76% | 56% |
| 3 | 60% | 34% | 63% | 64% | 64% | 42% | 63% | 40% |
| 5 | 48% | 34% | 47% | 47% | 42% | 18% | 43% | 23% |
Data in Table 47 suggest that the exponential survival function underestimates early (up to 1 year) and late (5 years) survival, while the Weibull and log-logistic functions both provide a reasonable fit for early survival (up to 2 years). Both functions seem to overestimate survival at 3 years and to underestimate survival at 5 years.
Mean survival derived from the study by Kim and colleagues73 and from each of the modelled survival functions (extrapolated to a maximum duration of 72 months for comparison with the area under the reported Kaplan Meier curve) is reported in Table 48.
| Treatment arm | Mean overall survival (months) | |||
|---|---|---|---|---|
| Trial report | Exponential | Weibull | Log-logistic | |
| Surgical resection | 47.4 | 47.2 | 47.2 | 47.2 |
| RFA | 32.9 | 47.4 | 34.2 | 34.5 |
| Difference | 14.5 | 0.2 | 13.0 | 12.7 |
Mean overall survival for surgical resection estimated using the Weibull and log-logistic survival functions is similar to that reported from the trial (47.2 months for the modelled functions compared with 47.4 months from the Kaplan–Meier curve). However, both functions estimate higher mean survival with radiofrequency ablation (34.2 and 34.5 months for Weibull and log-logistic functions, respectively) compared with the area under the Kaplan–Meier curve (32.9 months). The model using the exponential function appears to provide a particularly poor fit for both surgical resection and radiofrequency ablation.
The Weibull model was adopted for the base-case analysis and the alternative survival functions in structural sensitivity analyses were included.
Overall survival – radioembolisation plus hepatic artery chemotherapy compared with hepatic artery chemotherapy alone
Overall survival curves reported by Grey and colleagues85 were scanned using Engauge software and imported into Microsoft Excel. At the end of follow-up four patients included in the trial were still alive (three in the radioembolisation plus hepatic artery chemotherapy arm and one in the control arm). The final portions of the survival curves were extrapolated using a regression analysis.
The extrapolated overall survival curves, for radioembolisation plus hepatic artery chemotherapy, using different survival functions, are given in Figure 10 along with Kaplan–Meier survival estimates from the trial report. The Weibull and log-logistic survival functions appear to fit the overall survival curves reasonably well, with the log-logistic survival function providing the closest fit for median survival (trial report = 17 months, log-logistic = 18 months, Weibull = 20 months, exponential = 15.6 months).
FIGURE 10.
Kaplan–Meier overall survival estimates for radioembolisation plus hepatic artery chemotherapy from the Grey and colleagues85 trial showing alternative model fits. HAC, hepatic artery chemotherapy.
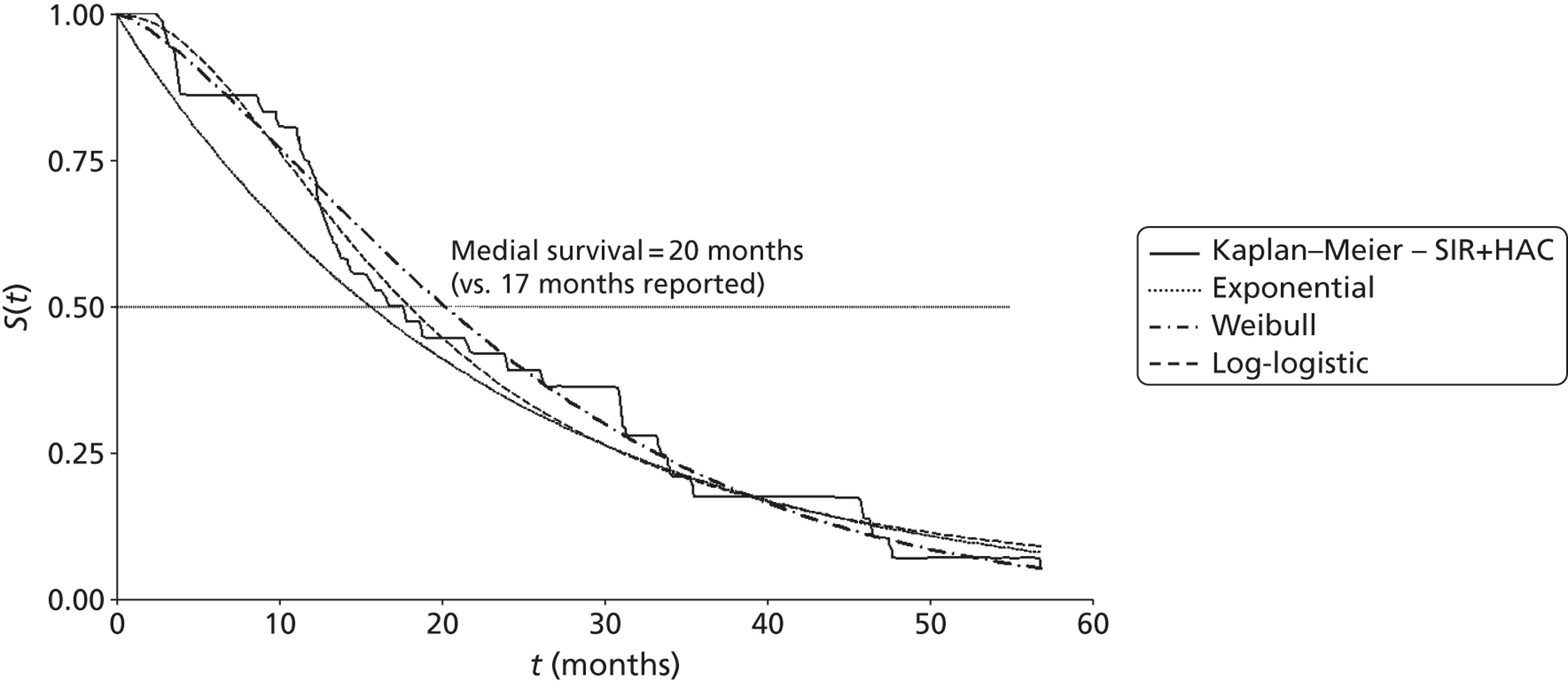
Table 49 reports a comparison of observed survival at 1, 2, 3 and 5 years against model predictions.
| Year | Trial report | Weibull model | Exponential model | Log-logistic model | ||||
|---|---|---|---|---|---|---|---|---|
| HAC | RE + HAC | HAC | RE + HAC | HAC | RE + HAC | HAC | RE + HAC | |
| 1 | 68% | 72% | 62% | 72% | 47% | 59% | 61% | 71% |
| 2 | 29% | 39% | 29% | 41% | 22% | 34% | 34% | 36% |
| 3 | 6% | 17% | 11% | 21% | 10% | 20% | 13% | 19% |
| 5 | 0% | 4% | 1% | 4% | 2% | 7% | 5% | 7% |
Data in Table 49 suggest that the exponential survival function is a poor fit to the observed data, while the Weibull and log-logistic functions both provide a reasonable fit for early survival (up to 2 years). Both functions seem to overestimate survival with hepatic artery chemotherapy at 3 years, with the log-logistic also overestimating survival for radioembolisation plus hepatic artery chemotherapy and hepatic artery chemotherapy alone. In contrast, the Weibull function appears to give reasonable predictions of 5-year survival for both treatment arms.
Mean survival reported by Grey and colleagues85 and from each of the modelled survival functions (extrapolated to a maximum duration of 57 months for comparison with the area under the reported Kaplan–Meier curve) is reported in Table 50.
| Treatment arm | Mean overall survival (months) | |||
|---|---|---|---|---|
| Trial report | Weibull | Exponential | Log-logistic | |
| RE + HAC | 23.5 | 23.3 | 20.7 | 22.7 |
| HAC | 18.4 | 18.6 | 15.5 | 19.0 |
| Difference | 5.1 | 4.7 | 5.2 | 3.7 |
Mean overall survival for radioembolisation plus hepatic artery chemotherapy and for hepatic artery chemotherapy alone estimated using the Weibull survival function is very similar to that reported from the trial (23.3 compared with 23.5 months and 18.6 compared 18.4 months, for radioembolisation plus hepatic artery chemotherapy and for hepatic artery chemotherapy alone, respectively). The model using the exponential function appears to underestimate overall survival for both radioembolisation plus hepatic artery chemotherapy and for hepatic artery chemotherapy alone, and to overestimate the survival difference when compared with the trial report. In contrast, while the log-logistic survival function gives the closest fit for median survival it appears to underestimate mean survival for radioembolisation plus hepatic artery chemotherapy while overestimating for hepatic artery chemotherapy alone, hence underestimating the survival difference compared with the trial report.
The Weibull model was adopted for the base-case analysis and the alternative survival functions in structural sensitivity analyses were included.
Progression-free survival – microwave ablation compared with surgical resection
The trial report by Shibata and colleagues72 does not report Kaplan–Meier curves for progression-free survival, or the proportion of patients remaining progression free at given time points. The only reported information on time to disease progression reported in the trial publication is the mean disease-free interval – no measures of variability (SDs or CIs) are reported. These mean values (Table 51) are used in the model as estimates of progression-free survival – in the absence of any further indication an exponential form for the survival function was assumed.
| Treatment arm | Mean disease-free interval (months) |
|---|---|
| Surgical resection | 13.3 |
| MWA | 11.3 |
Progression-free survival – radiofrequency ablation compared with surgical resection
Radiofrequency ablation compared with surgical resection for solitary metastases < 3 cm
The extrapolated progression-free survival functions for surgical resection are given in Figure 11 along with Kaplan–Meier estimates from the study publication. The Weibull and log-logistic survival functions appear to fit reasonably well for up to 12 months, but overestimate survival from 12 to 30 months. All the parametric functions appear to overestimate median survival with the exponential function (at approximately 30 months) giving the greatest discrepancy with the Kaplan–Meier estimate.
FIGURE 11.
Kaplan–Meier progression-free survival estimates for resected patients with solitary liver metastases (< 3 cm) from the Kim and colleagues73 study showing alternative model fit.
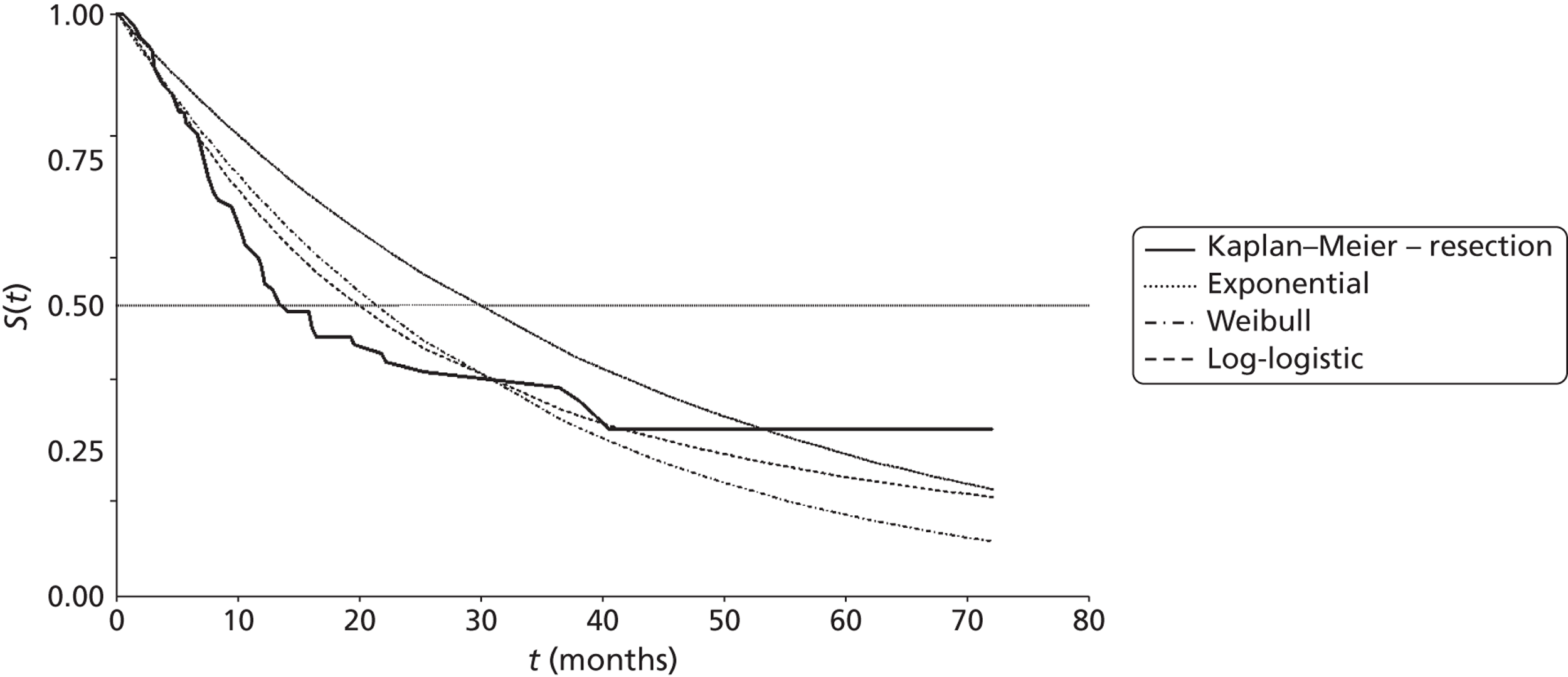
Mean times to progression from each of the modelled survival functions (truncated at 72 months for comparison with the area under the reported Kaplan–Meier curve) are reported in Table 52.
| Treatment arm | Mean progression-free survival (months) | |||
|---|---|---|---|---|
| Study report | Exponential | Weibull | Log-logistic | |
| Surgical resection | 30.0 | 34.3 | 27.8 | 29.2 |
| RFA | 31.6 | 34.4 | 29.0 | 30.5 |
| Difference | 1.6 | 0.1 | 1.2 | 1.3 |
Both the Weibull and log-logistic functions appear to underestimate mean progression-free survival, and to slightly underestimate the difference between surgical resection and radiofrequency ablation. The exponential function shows the greatest discrepancy with the values observed in the study.
The Weibull model was adopted for the base-case analysis and the alternative survival functions in structural sensitivity analyses were included.
Radiofrequency ablation compared with surgical resection for solitary metastases ≥ 3 cm
The extrapolated progression-free survival curves, for surgical resection, using different survival functions, are given in Figure 12 along with Kaplan–Meier survival estimates from the trial report. The Weibull and log-logistic survival functions appear to fit the progression-free survival curves reasonably well, although all the modelled functions give lower predictions than the Kaplan–Meier curves for survival over 5 years. Each of the modelled functions appears to overestimate progression-free survival [trial report = 24.9 months (this is not reported in the study, but has been estimated from the Kaplan–Meier curve), exponential = 37.1 months, Weibull = 32 months, log-logistic = 30 months].
FIGURE 12.
Kaplan–Meier progression-free survival estimates for surgical resection in patients with solitary metastases (≥ 3 cm) from the Kim and colleagues73 study showing alternative model fit.
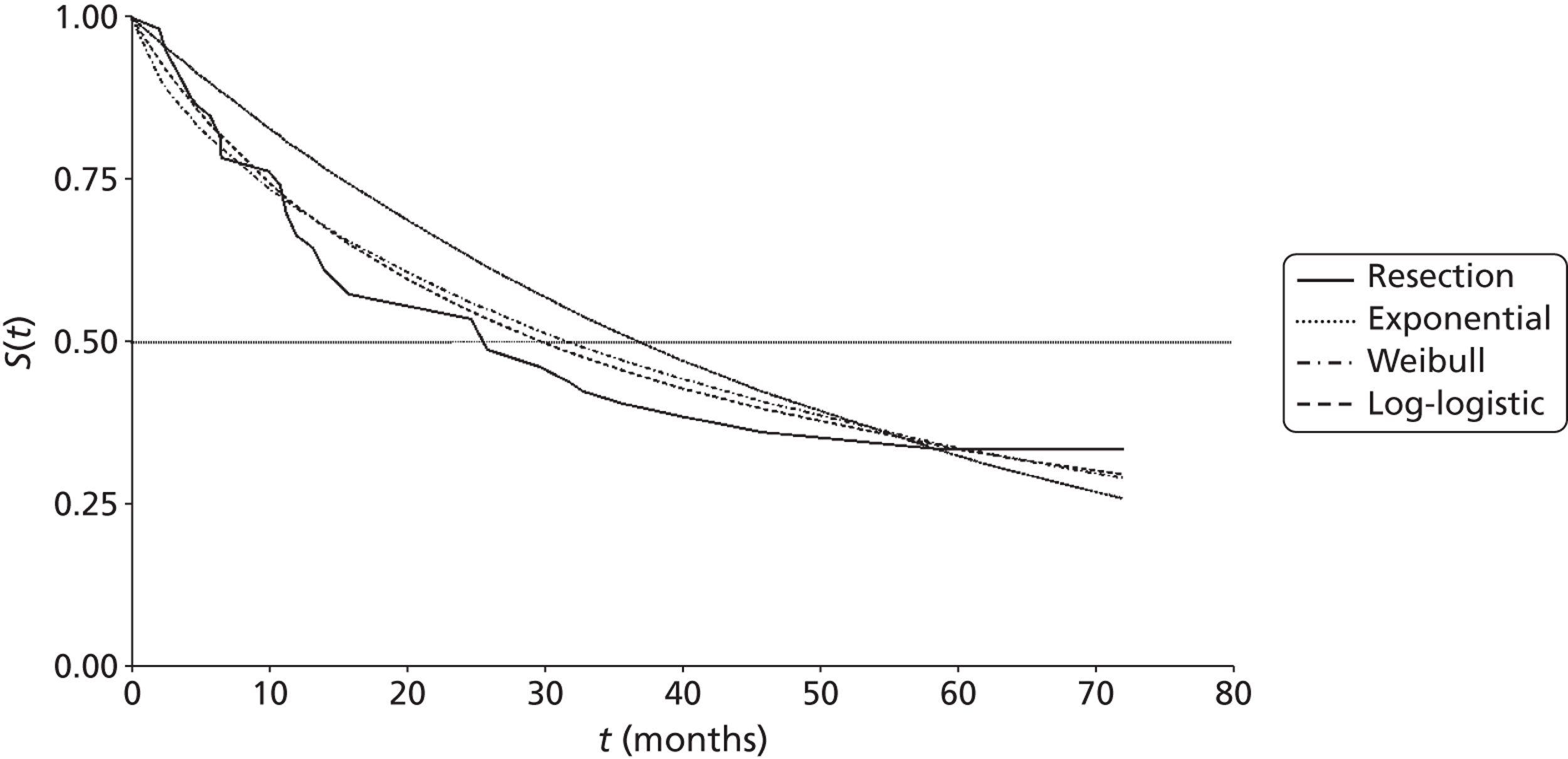
Mean progression-free survival derived from the study by Kim and colleagues73 and from each of the modelled survival functions (extrapolated to a maximum duration of 72 months for comparison with the area under the reported Kaplan–Meier curve) is reported in Table 53.
| Treatment arm | Mean overall survival (months) | |||
|---|---|---|---|---|
| Study report | Exponential | Weibull | Log-logistic | |
| Surgical resection | 35.5 | 39.1 | 37.0 | 36.7 |
| RFA | 20.1 | 39.3 | 17.1 | 18.5 |
| Difference | 15.1 | 0.2 | 19.9 | 18.3 |
The Weibull model was adopted for the base-case analysis and the alternative survival functions in structural sensitivity analyses were included.
Progression-free survival – radioembolisation plus hepatic artery chemotherapy compared with hepatic artery chemotherapy alone
Kaplan–Meier estimates for time to disease progression (by tumour area and by tumour volume) reported by Grey and colleagues85 were scanned using Engauge software and imported into Microsoft Excel. The extrapolated survival functions for time to disease progression for radioembolisation plus hepatic artery chemotherapy are given in Figure 13 along with Kaplan–Meier survival estimates from the trial report. The Weibull and log-logistic survival functions appear to fit reasonably well, with both survival functions providing close fits for median time to disease progression (trial report = 15.9 months, Weibull = 15.7 months, log-logistic = 15.4 months, exponential = 14.9 months).
FIGURE 13.
Kaplan–Meier time to progression estimates from the Grey and colleagues85 trial showing Weibull and alternative model fit. HAC, hepatic artery chemotherapy; RE, radioembolisation.
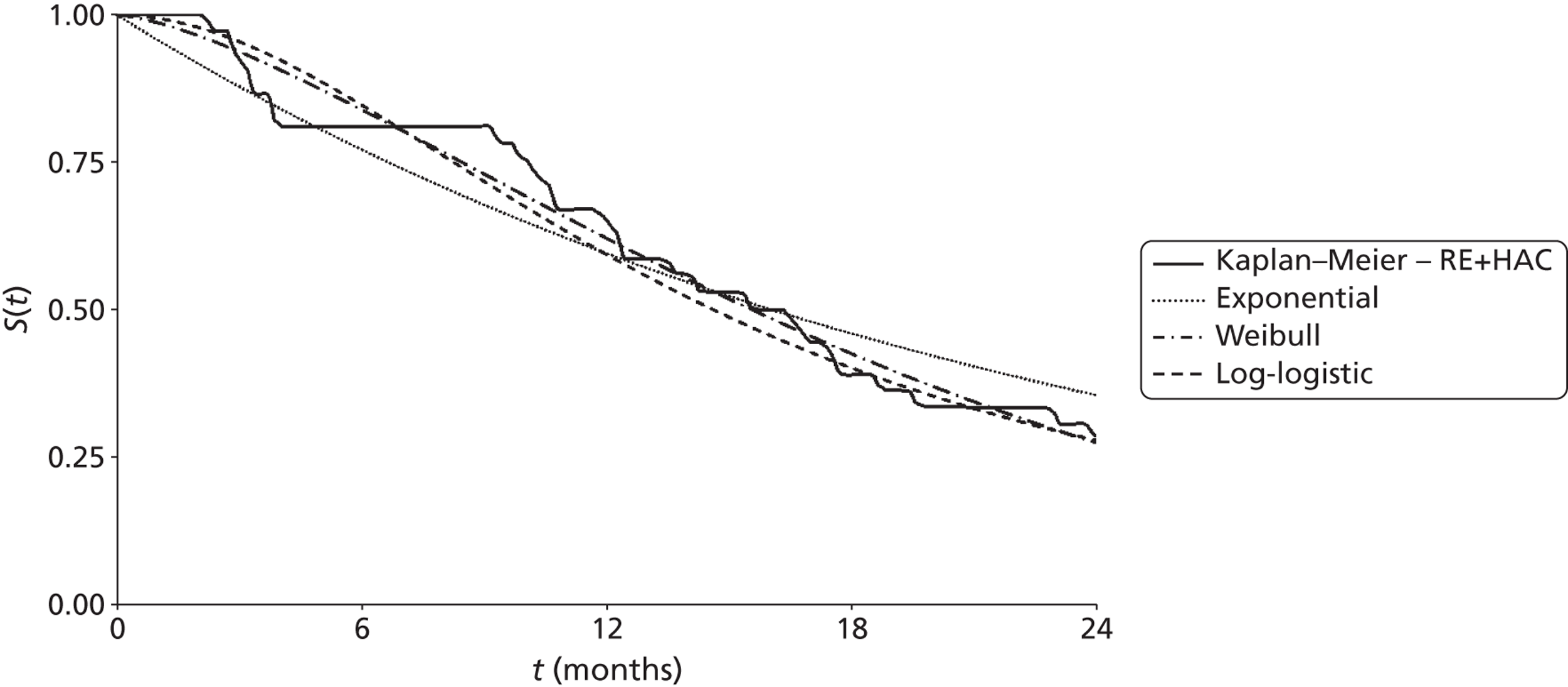
Mean times to progression from each of the modelled survival functions (truncated at 24 months for comparison to the area under the reported Kaplan–Meier curve) are reported in Table 54.
| Treatment arm | Mean progression-free survival (months) | |||
|---|---|---|---|---|
| Trial report | Weibull | Exponential | Log-logistic | |
| RE + HAC | 15.2 | 15.2 | 14.9 | 14.9 |
| HAC | 9.8 | 9.8 | 11.6 | 9.1 |
| Difference | 5.4 | 5.3 | 3.3 | 5.8 |
Mean progression-free survival for radioembolisation plus hepatic artery chemotherapy and for hepatic artery chemotherapy alone estimated using the Weibull survival function is the same as the value estimated from the Kaplan–Meier curves reported from the trial (15.2 months compared with 15.2 months and 9.8 months compared with 9.8 months, for radioembolisation plus hepatic artery chemotherapy and for hepatic artery chemotherapy alone, respectively), with similar estimates of the difference. The model using the exponential function appears to underestimate progression-free survival for radioembolisation plus hepatic artery chemotherapy but overestimate for hepatic artery chemotherapy alone, leading to an underestimate of the difference in progression-free survival when compared with the trial report and the Weibull survival model. In contrast, while the log-logistic survival function appears to give a similar fit to the Weibull function (see Figure 13), it underestimates progression-free survival for both radioembolisation plus hepatic artery chemotherapy and for hepatic artery chemotherapy alone, and appears to overestimate the survival difference compared with the trial report and the Weibull survival model.
The Weibull model was adopted for the base-case analysis and the alternative survival functions in structural sensitivity analyses were included.
Adverse events – microwave ablation compared with surgical resection
Adverse events for patients in the study by Shibata and colleagues72 are reported in Table 13 in the clinical effectiveness section of this report (see Chapter 4, Microwave ablation).
Adverse events – radiofrequency ablation compared with surgical resection
The study by Kim and colleagues73 reports that there was no treatment-related mortality in the radiofrequency ablation or surgical resection groups, but that treatment-related morbidity was statistically significantly higher in the surgical resection group [11/177 (6.2%) of the radiofrequency ablation group compared with 59/278 (21.2%) of the surgical resection group, p < 0.001]. The number and proportion of patients in each group experiencing treatment-related morbidity, by cause, is reported in Table 55.
| Adverse event | RFA, n = 177 | Surgical resection, n = 278 |
|---|---|---|
| Bleeding (transfusion) | 2 (1.1) | 13 (4.7) |
| Abscess | 8 (4.5) | 17 (6.1) |
| Wound infection | 0 (0.0) | 10 (3.6) |
| Transient respiratory failure | 1 (0.6) | 18 (2.9) |
| Ileus | 0 (0.0) | 11 (4.0) |
| Total | 11 (6.2) | 59 (21.2) |
Adverse events – radioembolisation plus hepatic artery chemotherapy compared with hepatic artery chemotherapy alone
Adverse events for patients in the study by Grey and colleagues85 are reported in Table 35 in the clinical effectiveness section of this report (see Chapter 4, Radioembolisation).
Quality of life
Utility weights currently applied in the model are reported in Table 56 and were derived from our systematic review of quality-of-life studies in patients with liver metastases (see Systematic review of health-related quality-of-life evidence). The utility value for stable disease (with or without liver metastases) is based on the baseline value reported by Wiering and colleagues. 151 The effect of treatment on quality of life is based on the proportionate reduction for treated patients at 2 weeks reported for ablated patients by Krabbe and colleagues146 (mean utility reduced from 0.90 to 0.81 at 2 weeks, a 10% reduction) and by Wiering and colleagues151 for surgical patients (mean utility reduced from 0.82 to 0.65 at 2 weeks, a 21% reduction). The reduction in quality of life resulting from surgical resection is applied for 6 months, based on profiles reported by Wiering and colleagues151 and Krabbe and colleagues,146 while the quality-of-life reduction resulting from ablation is applied for 3 months, based on profiles reported by Krabbe and colleagues. 146 A relative weighting of 0.75 for disease progression is applied in the model, based on an estimate reported by Tappenden and colleagues,160 derived from Petrou and Campbell161 which was identified in a systematic review of studies reporting utility values for health states relevant for patients with metastatic colorectal cancer (not specific to people with liver metastases).
| Health state in model | Utility weight | Source |
|---|---|---|
| Stable disease, with residual metastases | 0.82 | Wiering et al.151 |
| Up to 6 months post surgical resection | 0.65a | Wiering et al.,151 Krabbe et al.146 |
| Up to 3 months post ablation | 0.74b | Krabbe et al.146 |
| Disease progression | 0.62c | Tappenden et al.,160 Petrou and Campbell161 |
| Terminal (month before death in progressive disease) | 0.45 | Wiering et al.151 |
Resource use and cost
The groups of health care costs included in the base-case health economic model are treatment costs [including cost of interventions, costs of intervention administration, on-treatment monitoring and costs of post-discharge follow-up (where relevant)], post-treatment monitoring and palliative care costs.
Treatment costs: microwave ablation compared with surgical resection
In the trial reported by Shibata and colleagues,72 microwave ablation was performed after laparotomy and consisted of a series of 10–30 second coagulation periods, followed by a 10-second coagulation-free period, for each tumour (up to total coagulation period of between 2 and 20 minutes). The average reported operation time was 180 minutes, with total hospitalisation period of 20 days (SD 7 days). Five of the 14 patients undergoing microwave ablation for liver metastases (36%) in the trial were reported to receive a second round of treatment. Microwave ablation procedure costs can be seen in Table 57.
| Item | Cost (£) |
|---|---|
| MWA procedurea | 3866 |
| Additional bed-daysb | 4436 |
| Total | 8302 |
It was assumed that all patients admitted for microwave ablation would require follow-up as outpatients 1 month after discharge from hospital.
The average total cost for patients undergoing microwave ablation, including outpatient follow-up at 1 month post discharge and allowing for repeat procedures in 36% of patients, was estimated as £11,613.
In the trial reported by Shibata and colleagues,72 hepatectomy (referred to here as surgical resection) was defined as lobectomy, segmentectomy, subsegmentectomy and/or wedge resection depending on the number, location and size of tumours. The trial report does not indicate the relative frequency of each of these procedures. It was assumed that all procedures were performed as wedge resection (HRG code GA05B, Hepatobiliary Procedures category 5). The impact of this assumption is explored in sensitivity analyses applying lower unit costs for less resource intensive procedures, and higher unit costs for more resource intensive procedures.
It was assumed that all patients admitted for surgical resection would require follow-up as outpatients 1 month after discharge from hospital, as for those undergoing microwave ablation (Table 58). The average total cost for patients undergoing surgical resection, including outpatient follow-up at 1 month post discharge, was estimated as £12,173 (Table 59).
| Item | Frequency of use | Unit cost (£) |
|---|---|---|
| Outpatient attendancea | Once, at 1 month post discharge | 128.13 |
| Full blood countb | Once, at 1 month post discharge | 0.50 |
| Liver function testsb | Once, at 1 month post discharge | 0.38 |
| CEAb | Once, at 1 month post discharge | 1.74 |
| Abdominal CTc | Once, at 1 month post discharge | 100.65 |
| Total | 236.81 |
| Item | Cost (£) |
|---|---|
| Surgical resection procedurea | 6629 |
| Additional bed-daysb | 5308 |
| Total | 11,937 |
Treatment costs: radiofrequency ablation compared with surgical resection
In the trial reported by Kim and colleagues,73 radiofrequency ablation was performed percutaneously under local anaesthetic using ultrasound guidance to ensure a 1-cm ablation margin around the tumour. A single electrode, with 3-cm exposed tip, was used for small tumours and a triple-cluster electrode with 2.5-cm exposed tip was used for larger tumours, at the discretion of the radiologist. Radiofrequency current was emitted for 10–15 minutes with the generator set to deliver maximum power impedance in impedance control mode. Destruction of liver metastases was confirmed by follow-up CT and ultrasound the next day. Mean duration of hospitalisation was 4.2 days (SD = 2.8 days, with a range of 1–32 days). The study does not report whether or not patients underwent repeat treatment with radiofrequency ablation.
It was assumed that all patients undergoing radiofrequency ablation would require follow-up as outpatients 1 month after discharge from hospital.
The average total cost for patients undergoing microwave ablation was estimated as £3628 (Table 60).
| Item | Cost (£) |
|---|---|
| RFA procedurea | 3391 |
| Additional bed-daysb | 0 |
| Total | 3391 |
Of the patients undergoing surgical resection in the study reported by Kim and colleagues,73 5% (4/278) underwent lobectomy, 15% (42/278) segmentectomy and 80% (222/278) subsegmentectomy. Surgical resection of a liver segment and wedge resection of the liver are both classified under ‘Hepatobiliary procedures – category 5’ (a relatively resource intensive grouping) for NHS Reference Costs, while hemi-hepatectomy is classified under ‘Hepatobiliary procedures – category 6’ (a more resource-intensive grouping). It was assumed that the majority of procedures would be classified as category 5 – equivalent to segmentectomy or subsegmentectomy – and the relevant NHS Reference Costs have been applied (Table 61). The impact of this assumption is explored in sensitivity analyses applying lower unit costs, for less resource-intensive procedures, and higher unit costs, for more resource-intensive procedures. The mean duration of hospitalisation for resected patients was 13.4 days (SD = 4.5, with a range from 7 to 42 days).
| Item | Cost (£) |
|---|---|
| Surgical resection procedurea | 6629 |
| Additional bed-daysb | 2118 |
| Total | 8747 |
It was assumed that all patients undergoing surgical resection would require follow-up as outpatients 1 month after discharge from hospital, as for those undergoing radiofrequency ablation (Table 62). The average total cost for patients undergoing surgical resection, including outpatient follow-up at 1 month post discharge, was estimated as £8983.
| Item | Frequency of use | Unit cost (£) |
|---|---|---|
| Outpatient attendancea | Once, at 1 month post discharge | 128.13 |
| Full blood countb | Once, at 1 month post discharge | 0.50 |
| Liver function testsb | Once, at 1 month post discharge | 0.38 |
| CEAb | Once, at 1 month post discharge | 1.74 |
| Abdominal CTc | Once, at 1 month post discharge | 100.65 |
| Total | 236.81 | |
Treatment costs: radioembolisation plus hepatic artery chemotherapy compared with hepatic artery chemotherapy alone
In the trial reported by Grey and colleagues,85 hepatic artery chemotherapy consisted of the administration of floxuridine (FUDR) at 0.3 mg/kg of body weight per day (in 12-day cycles). The chemotherapy was administered via a hepatic artery port inserted during a laparotomy procedure. The laparotomy was also used to confirm that patients did not have existing, non-excisable, extrahepatic disease. Chemotherapy was discontinued on evidence of tumour progression within the liver, extrahepatic disease requiring change to systemic chemotherapy, unacceptable toxicity, at patient request or at a maximum of 18 cycles. As the laparotomy procedure and hepatic artery chemotherapy were common treatments in both trial arms, and hepatic artery chemotherapy dosing appears to have been similar in both arms (mean number of cycles was 8.0 and 8.7, amount of protocol chemotherapy per patient 1822 and 1863 for hepatic artery chemotherapy and radioembolisation plus hepatic artery chemotherapy, respectively), this cost is not included in the model as it would not influence the calculation of incremental cost for radioembolisation plus hepatic artery chemotherapy.
Radioembolisation, in the trial reported by Grey and colleagues,85 consisted of the administration of SIR-Spheres,® at a dosage dependent on the size of tumour. Patients with a tumour < 25% of liver volume received a dose equivalent to 2 GBq, those with tumour volume of 25–50% received equivalent to 2.5 GBq and those with tumours > 50% of liver volume received equivalent to 3 GBq. The trial protocol allowed for dose reduction in patients who experienced greater than 10% lung–liver breakthrough (reducing the yttrium-90 by 2% for every 1% of lung–liver breakthrough in excess of 10%). Estimating the average dose, based on the distribution of tumour size across patients in the radioembolisation plus hepatic artery chemotherapy arm of trial, yields an average dose of 2.21 GBq (Table 63). This differs slightly from the mean delivered dose of 2.156 GBq (SD = 0.324) in the trial report, due to five patients receiving less and one patient receiving more than the protocol-specified dose (although these dose variations do not appear to be due to lung–liver breakthrough as the trial reported that no patient experienced a greater than 10% breakthrough).
| Tumour size (percentage of liver involved with tumour) | Number of patients (%) | Dose |
|---|---|---|
| < 25% | 24 (67) | 2.0 GBq |
| 25–50% | 9 (25) | 2.5 GBq |
| > 50% | 3 (8) | 3.0 GBq |
| Average for patient group | 2.2 GBq | |
Average costs for radioembolisation using SIR-Spheres® are reported in Table 64.
| Item | Cost (£) |
|---|---|
| Average consumables costs for SIR (parts 1 and 2) | 2861 |
| SIR-Spheres® yttrium-90 therapy | 8050 |
| Total cost | 10,911 |
Administration and on-treatment monitoring
All patients eligible for treatment with hepatic artery chemotherapy or radioembolisation plus hepatic artery chemotherapy undergo laparotomy by a surgical oncologist to confirm non-resectable status of metastases, to look for evidence of intra-abdominal spread of the tumour and insertion of a permanent hepatic artery catheter connected to a subcutaneous access port (for delivery of hepatic artery chemotherapy and for insertion of SIR-Spheres® for radioembolisation). As indicated, this procedure is common to both intervention and comparator and is not included in the costing.
Patients undergoing radioembolisation require a nuclear medicine scan to determine the amount of SIR-Spheres® that would pass through the liver and lodge in the lungs [macroaggregated albumin (MAA) shunt study and breakthrough study] and will be admitted as inpatients for the radioembolisation procedure (Table 65 shows estimated costs).
| Item | Cost (£) |
|---|---|
| MAA shunt studya | 500 |
| Breakthrough studya | 500 |
| Radioembolisation procedureb | 2305 |
| Total | 3305 |
Patients are assumed to attend as outpatients every 4 weeks for on-treatment monitoring while continuing to receive hepatic artery chemotherapy. The full package of on-treatment monitoring is listed in Table 66 and consists of an outpatient visit (with full blood count, liver function tests and serum CEA test) every 4 weeks as well as an abdominal CT every 3 months (to identify disease progression) and a alpha-fetoprotein test every 3 months.
| Item | Frequency of use | Unit cost (£) |
|---|---|---|
| Outpatient attendancea | Every 4 weeks | 98.25 |
| Full blood countb | Every 4 weeks | 0.50 |
| Liver function testsb | Every 4 weeks | 0.38 |
| CEA testb | Every 4 weeks | 1.74 |
| Abdominal CTc | Every 3 months | 100.65 |
| Alpha-fetoprotein testb | Every 3 months | 1.82 |
Follow-up costs: microwave ablation compared with surgical resection
Following the end of treatment, and prior to disease progression, patients are assumed to attend as outpatients (with full blood count, liver function tests and serum CEA test) and to have an abdominal CT (Table 67).
| Item | Frequency of use | Unit cost (£) |
|---|---|---|
| Outpatient attendancea | Every 3 months | 128.13 |
| Full blood countb | Every 3 months | 0.50 |
| Liver function testsb | Every 3 months | 0.38 |
| CEAb | Every 3 months | 1.74 |
| Abdominal CTc | Every 3 months | 100.65 |
Follow-up costs: radiofrequency ablation compared with surgical resection
Following the end of treatment, and prior to disease progression, patients are assumed to attend as outpatients every 3 months (with full blood count, liver function tests and serum CEA test) and to have an abdominal CT (Table 68).
| Item | Frequency of use | Unit cost (£) |
|---|---|---|
| Outpatient attendancea | Every 3 months | 128.13 |
| Full blood countb | Every 3 months | 0.50 |
| Liver function testsb | Every 3 months | 0.38 |
| CEAb | Every 3 months | 1.74 |
| Abdominal CTc | Every 3 months | 100.65 |
Follow-up costs: radioembolisation plus hepatic artery chemotherapy compared with hepatic artery chemotherapy alone
Following the end of treatment, and prior to disease progression, patients are assumed to attend as outpatients every 3 months (with full blood count, liver function tests and serum CEA test) and to have an abdominal CT (Table 69).
| Item | Frequency of use | Unit cost (£) |
|---|---|---|
| Outpatient attendancea | Every 3 months | 98.25 |
| Full blood countb | Every 3 months | 0.50 |
| Liver function testsb | Every 3 months | 0.38 |
| CEAb | Every 3 months | 1.74 |
| Abdominal CTc | Every 3 months | 100.65 |
Palliative care costs
Palliative care costs in the model are estimated at £600 per month and are based on those adopted by Tappenden and colleagues,160 derived from Remak and Brazil. 163 These costs are applied for the remaining life expectancy of patients who experience disease progression, irrespective of their initial treatment.
Results of independent economic analysis
Microwave ablation compared with surgical resection
This section reports cost-effectiveness results for a cohort of patients with resectable liver metastases undergoing either microwave ablation or surgical resection, based on the trial report by Shibata and colleagues. 72 Discounted costs (identifying the contribution of procedure costs, surgical follow-up, monitoring prior to disease progression and palliative care) are presented alongside the life expectancy and quality-adjusted life expectancy for patients in the cohort. The results are presented as incremental cost per life-year gained and incremental cost per QALY gained.
Costs and outcomes modelled for cohorts of patients receiving treatment with microwave ablation or surgical resection are presented in Table 70. Costs and health outcomes in the table have been discounted at 3.5%.
| Treatment | Costs (£) | Life-years | Incremental cost per life-year gained (£) | QALYs | Incremental cost per QALY gained (£) |
|---|---|---|---|---|---|
| Surgical resection | 20,152 | 1.94 | 1.38 | ||
| MWA | 19,825 | 1.84 | 3293 | 1.29 | 3664 |
The estimated gain in discounted life expectancy associated with microwave ablation is negative – microwave ablation is associated with a reduction of 0.10 years (5.2 weeks) when compared with surgical resection. The equivalent undiscounted values are 0.11 years (5.7 weeks). The estimated reduction in discounted QALYs, associated with microwave ablation, is 0.09. The equivalent undiscounted value is 0.10 QALYs.
The incremental cost associated with microwave ablation is also negative (£327), indicating that treatment of liver metastases with microwave ablation is slightly lower cost than treatment with surgical resection. Table 71 reports a breakdown of treatment costs, by phase of treatment, for each cohort.
| Phase of treatment | MWA (£) | Surgical resection (£) | |
|---|---|---|---|
| Active treatment | Procedure cost | 11,291 | 11,937 |
| Surgical follow-up | 322 | 237 | |
| Non-progressive disease monitoring | 1487 | 1606 | |
| Palliative care | 6726 | 6373 | |
| Total | 19,825 | 20,152 | |
In this analysis, microwave ablation as a treatment for patients with resectable liver is associated with both reduced outcomes (in terms of life expectancy and quality-adjusted life expectancy) and reduced costs, resulting in an ICER of £3664 per QALY gained. However, it should be noted that this positive ICER is derived from negative incremental cost and incremental QALY values – in this analysis microwave ablation is associated with reduced cost but also poorer outcome than surgical resection, requiring a judgement on whether or not the savings can justify the poorer outcome.
Deterministic sensitivity analysis
A sensitivity analysis was conducted to consider the effect of uncertainty around the model structure and for variation in certain key parameters that were expected, a priori, to be influential on the cost-effectiveness results. The method adopted in most cases was univariate sensitivity analysis; that is, varying one parameter at a time, leaving all other variables unchanged. This is to highlight the impact, if any, of each selected parameter alone on the cost-effectiveness results. In some situations (such as the analysis of alternative parametric forms for the survival function, or the analysis using the upper confidence limits for all parameters in survival model) a set of related parameters are varied simultaneously. The effects of uncertainty in multiple parameters were addressed using PSA, which is reported later in the section.
Table 72 reports the results of the sensitivity analysis. Except for the sensitivity analysis with respect to time horizon, all analyses were conducted using a 5-year time horizon. The table is divided to distinguish between analyses undertaken due to uncertainties over structural assumptions in the model, methodological uncertainties (in this case related to the discount rates applied in the model) and uncertainty over parameter values. Where unit costs have been taken from NHS Reference Costs, the upper and lower quartiles have been used in the sensitivity analysis. In all other cases unit costs have been varied by plus or minus 20%.
| Incremental cost (£) | Life-years gained | QALYs gained | ICER (£ per QALY gained) | |
|---|---|---|---|---|
| Base case | –327 | –0.10 | –0.09 | 3664 |
| Structural assumptions | ||||
| Truncate survival at maximum follow-up for trial | –325 | –0.09 | –0.08 | 3866 |
| Extrapolate overall survival up to 10 years | –328 | –0.10 | –0.09 | 3634 |
| Exponential overall survival modela | 1869 | 0.22 | 0.11 | 17,007 |
| Log-logistic overall survival modela | 767 | 0.05 | 0.00 | 648,069 |
| Methodological assumptions | ||||
| Discount rates (0% for both costs and outcomes) | –329 | –0.11 | –0.10 | 3409 |
| Discount (6% for costs and 1.5% for outcomes) | –326 | –0.10 | –0.09 | 3496 |
| Parameter uncertainty | ||||
| Lower 95% CI for treatment effect | –5239 | –0.78 | –0.50 | 10,526 |
| Upper 95% CI for treatment effect | 3194 | 0.44 | 0.24 | 13,035 |
| Lower 95% CI for all parameters in overall survival model | –819 | –0.17 | –0.13 | 6348 |
| Upper 95% CI for all parameters in overall survival model | –175 | 0.12 | 0.09 | –1979 |
| Exclude palliative care costs | –680 | –0.10 | –0.09 | 7618 |
| Upper limit for utility values | –327 | –0.10 | –0.08 | 4116 |
| Lower limit for utility values | –327 | –0.10 | –0.11 | 2951 |
| Procedure cost: lower quartile | 534 | –0.10 | –0.09 | –5976 |
| Procedure cost: upper quartile | 1008 | –0.10 | –0.09 | –11,292 |
| Cost of palliative care reduced by 20% | –398 | –0.10 | –0.09 | 4455 |
| Cost of palliative care increased by 20% | –257 | –0.10 | –0.09 | 2873 |
| Cost of outpatient visit for monitoring: lower quartile | –312 | –0.10 | –0.09 | 3494 |
| Cost of outpatient visit for monitoring: upper quartile | –343 | –0.10 | –0.09 | 3842 |
The cost-effectiveness results appear to be generally within the range of £2800 to £5000. However, it should be noted that the majority of these positive ICERs are the result of negative incremental cost and incremental QALY values – that is to say, in this analysis microwave ablation is associated with reduced cost but also poorer outcome than surgical resection. In this context the conventional interpretation of cost-effectiveness as being below a given acceptable threshold is reversed. Rather than balancing any increased cost against gains in outcome (as would be typical for interpreting cost-effectiveness results) the decision framework in this case would be to consider whether or not the size of savings (indicated by the lower incremental cost) can justify the reduced outcome.
Among the structural sensitivity analyses the results appear to be most sensitive to assumptions over the functional form for the survival functions. In terms of parameter inputs, the results appear to be most sensitive to variation in utility estimates applied in the model, variation in values of parameters in the survival functions (for treatment effect on overall survival and all parameters in the overall survival function), variation in procedure costs, and the cost of palliative care.
Time horizon for the model appears to have a limited impact on the cost-effectiveness estimates. Truncating survival at the maximum duration observed for each arm reported in the RCT by Shibata and colleagues72 leaves both incremental cost and incremental QALYs almost unchanged, while increasing the maximum survival duration to 10 years leads to a slight increase in incremental cost and incremental QALYs. Adopting alternative parametric forms for the overall survival functions leads to large changes in the incremental cost and incremental QALYs, and hence the ICER. Adopting an exponential form for the overall survival function results in positive incremental cost of £1869 and also results in a positive QALY gain, unlike the base-case analysis. Adopting a log-logistic form also results in a positive incremental cost, but produces a QALY gain close to zero and results in a high-value ICER. However, it should be borne in mind that these functional forms did not fit the observed data as closely as did the Weibull form adopted in the base case.
Varying the discount rates applied has comparatively little effect. Zero discount rates for costs and outcomes result in a slight increase in incremental cost and a slight increase in incremental QALYs compared with baseline values. Applying discount rates of 6% for costs and 1.5% for outcomes leads to a slight reduction in incremental cost and leaves incremental QALYs almost unchanged.
Varying the value of the treatment effect parameter in the overall survival model, between its upper and lower confidence limits, has a large effect on both incremental cost and incremental outcome. At the lower limit of the treatment effect parameter, incremental costs increase to –£5239 and incremental QALYs increase to –0.50, causing the ICER to increase to £10,526. At the upper limit of the treatment effect parameter, the incremental cost and incremental QALYs become positive, the QALY gain changing from a small negative number (–0.09) to 0.24 and incremental cost rising to approximately £3200. At the lower limit of all the parameters in the overall survival model the proportionate increase in incremental cost is greater than the proportionate increase in incremental QALYs, leading to an increase in ICER. At the upper limit for all the parameters in the overall survival model the incremental cost remains negative while the incremental QALYs becomes positive – in this case microwave ablation would be dominant (providing better outcomes at lower cost).
Variation in procedure costs (between the upper and lower quartiles reported for the NHS Reference Costs) appears to have a large influence on the results. Incremental costs are positive at the upper and lower limits for procedure cost so that, given that outcomes are slightly poorer for microwave ablation than for surgical resection, microwave ablation is dominated (providing poorer outcomes at higher cost) in this analysis. The cost-effectiveness results also appear to be sensitive to variation in palliative care costs. If palliative care costs are reduced, or removed, incremental cost increases up to a maximum of –£680 (when palliative care costs are removed from the model).
Probabilistic analysis
In a PSA, where the parameters of the survival models (both overall and progression-free survival), health state utility values, treatment cost, cost of outpatient attendances and patient monitoring, as well as costs of managing adverse events and palliative care were sampled probabilistically, microwave ablation is associated with reduced costs in 56% of simulations (with a range from –£10,866 to £8606) and lower QALYs in 68% of simulations (with a range of –0.80 to 0.54) when compared with surgical resection (Figure 14 shows this and also shows the 95% confidence ellipse).
FIGURE 14.
Cost-effectiveness plane – incremental cost and incremental QALYs for microwave ablation compared with surgical resection.
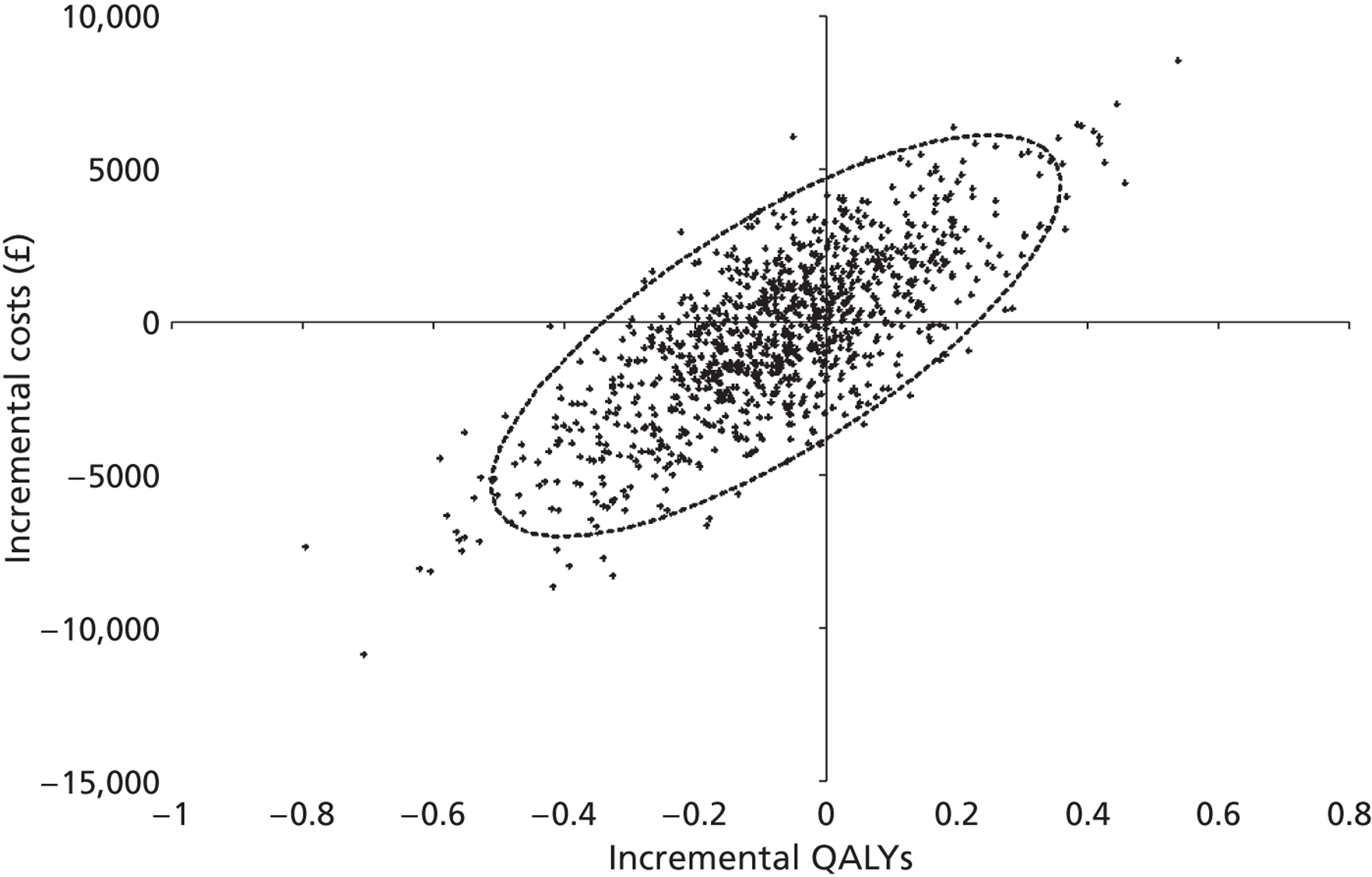
The probabilistic analysis generated cost and QALY estimates for each intervention that were greater than those for the base-case analysis (see Table 71 for the base-case analysis). However, the mean incremental cost and mean QALY gain were similar (–£327 and –0.09 QALYs compared with –£377 and –0.07 QALYs for the deterministic base case and PSA respectively). Table 73 reports the mean costs and outcomes from the probabilistic analysis (including the 2.5th and 97.5th percentiles to give an indication of the range of the simulated values) and the ICER for microwave ablation compared with surgical resection, based on the mean values generated in the probabilistic analysis.
| Procedure | Discounted costs (£) | Discounted QALYs | ICER (£) | ||||
|---|---|---|---|---|---|---|---|
| Mean | 2.5th percentile | 97.5th percentile | Mean | 2.5th percentile | 97.5th percentile | ||
| Surgical resection | 21,578 | 13,298 | 35,722 | 1.44 | 0.59 | 2.61 | |
| MWA | 21,160 | 13,091 | 35,529 | 1.37 | 0.61 | 2.55 | 5428 |
In addition to graphing the incremental cost and incremental QALYs for microwave ablation, a cost-effectiveness acceptability curve was derived, representing the proportion of simulations where microwave ablation is cost-effective for a range of willingness-to-pay thresholds, up to £100,000 (Figure 15). In this analysis microwave ablation had a probability of being cost-effective of 31% at a willingness-to-pay threshold of £20,000 per QALY and 30% at a willingness-to-pay threshold of £30,000 per QALY.
FIGURE 15.
Cost-effectiveness acceptability curve for microwave ablation.
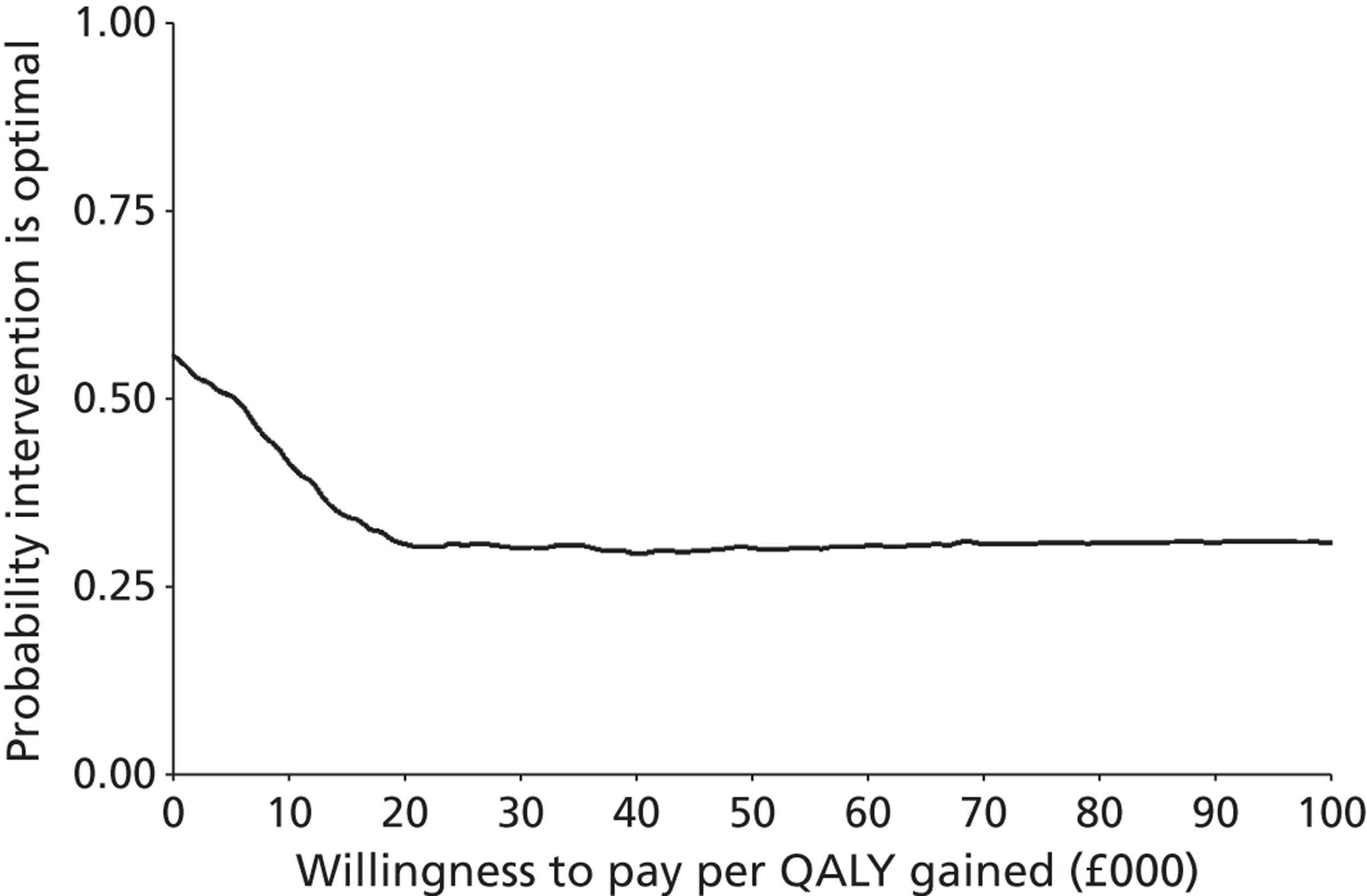
Radiofrequency ablation compared with surgical resection
This section reports cost-effectiveness results for a cohort of patients with resectable liver metastases (small or large solitary liver metastases) undergoing either radiofrequency ablation or surgical resection, based on data reported by Kim and colleagues. 73 The analyses are conducted separately for each patient population, those with:
-
solitary metastases < 3 cm
-
solitary metastases ≥ 3 cm.
Discounted costs (identifying the contribution of procedure costs, surgical follow-up, monitoring prior to disease progression and palliative care) are presented alongside the life expectancy and quality-adjusted life expectancy for patients in the cohort. The results are presented as incremental cost per life-year gained and incremental cost per QALY gained.
Radiofrequency ablation compared with surgical resection for solitary metastases < 3 cm
Costs and outcomes modelled for cohorts of patients receiving treatment of radiofrequency ablation or surgical resection are presented in Table 74. Costs and health outcomes in the table have been discounted at 3.5%.
| Treatment | Costs (£) | Life-years | Incremental cost per life-year gained (£) | QALYs | Incremental cost per QALY gained (£) |
|---|---|---|---|---|---|
| Surgical resection | 25,612 | 4.28 | Not applicablea | 2.99 | |
| RFA | 19,322 | 4.28 | 3.05 | –98,998 |
The incremental cost associated with radiofrequency ablation, compared with surgical resection, is negative (£6290) indicating that costs of treatment for small solitary liver metastases with radiofrequency ablation are lower than for surgical resection. Table 75 reports a breakdown of treatment costs, by phase of treatment, for each cohort.
| Phase of treatment | RFA (£) | Surgical resection (£) | |
|---|---|---|---|
| Active treatment | Procedure cost | 3391 | 8747 |
| Surgical follow-up | 237 | 237 | |
| Adverse event costs | 50 | 251 | |
| Non-progressive disease monitoring | 2344 | 2232 | |
| Palliative care | 13,301 | 14,145 | |
| Total | 19,322 | 25,612 | |
There is no difference in discounted life expectancy between surgical resection and RFA for patients with solitary liver metastases of < 3 cm. This reflects the non-significant differences in overall survival shown in Table 44 (under Clinical effectiveness: overall survival earlier in this section) – the HR for overall survival for RFA compared with surgical resection estimated in the linear regression used to derive the survival model was 1.00 indicating no difference. However, as surgical resection is associated with significant reduction in quality of life for up to 6 months post-operatively, surgical resection is associated with lower QALY outcome than radiofrequency ablation. The estimated gain in discounted QALYs associated with radiofrequency ablation is 0.06.
In this analysis, radiofrequency ablation as a treatment for patients with (surgically resectable) small solitary liver metastases is associated with similar outcomes (in terms of life expectancy and quality-adjusted life expectancy) and reduced costs (compared with surgical resection), resulting in an ICER of –£266,767 per QALY gained. In conventional terms this would indicate that radiofrequency ablation dominates surgical resection for the treatment of (surgically resectable) small solitary liver metastases.
Deterministic sensitivity analysis
Table 76 reports the results of the sensitivity analysis. Except for the sensitivity analysis with respect to time horizon, all analyses were conducted using a 10-year time horizon. The table is divided to distinguish between analyses undertaken due to uncertainties over structural assumptions in the model, methodological uncertainties (in this case related to the discount rates applied in the model) and uncertainty over parameter values. Where unit costs have been taken from NHS Reference Costs, the upper and lower quartiles have been used in the sensitivity analysis. In all other cases unit costs have been varied by ± 20%.
| Incremental cost (£) | Life-years gained | QALYs gained | ICER (£ per QALY gained) | |
|---|---|---|---|---|
| Base case | –6290 | 0.00 | 0.06 | –98,998 |
| Structural assumptions | ||||
| Truncate survival at maximum follow-up for trial | –6127 | 0.00 | 0.06 | –105,296 |
| Extrapolate overall survival up to 15 years | –6342 | 0.00 | 0.07 | –97,208 |
| Exponential overall survival and progression-free survival model | –8385 | –0.09 | 0.05 | –158,041 |
| Log-logistic survival and progression-free survival model | –6618 | –0.03 | 0.05 | –142,645 |
| Methodological assumptions | ||||
| Discount rates (0% for both costs and outcomes) | –6407 | 0.00 | 0.07 | –95,021 |
| Discount (6% for costs and 1.5% for outcomes) | –6220 | 0.00 | 0.07 | –94,716 |
| Parameter uncertainty | ||||
| Lower 95% CI for treatment effect | –4506 | 0.25 | 0.21 | –21,275 |
| Upper 95% CI for treatment effect | –8046 | –0.24 | –0.08 | 97,599 |
| Lower 95% CI for all parameters in survival model | –4729 | 0.22 | 0.19 | –24,517 |
| Upper 95% CI for all parameters in overall survival model | –7541 | –0.17 | –0.04 | 185,312 |
| Lower 95% CI for all parameters in progression-free survival model | –8907 | 0.00 | 0.15 | –60,483 |
| Upper 95% CI for all parameters in progression-free survival model | –4772 | 0.00 | 0.01 | –327,301 |
| Exclude palliative care costs | –5446 | 0.00 | 0.06 | –85,709 |
| Upper limit for utility values | –6290 | 0.00 | 0.00 | –41,012,309 |
| Lower limit for utility values | –6290 | 0.00 | 0.20 | –30,988 |
| Procedure cost: lower quartile | –5805 | 0.00 | 0.06 | –91,365 |
| Procedure cost: upper quartile | –7175 | 0.00 | 0.06 | –112,926 |
| Cost of palliative care reduced by 20% | –6121 | 0.00 | 0.06 | –96,340 |
| Cost of palliative care increased by 20% | –6459 | 0.00 | 0.06 | –101,656 |
| Cost of outpatient visit for monitoring: lower quartile | –6307 | 0.00 | 0.06 | –99,254 |
| Cost of outpatient visit for monitoring: upper quartile | –6273 | 0.00 | 0.06 | –98,730 |
The cost-effectiveness results appear to be generally robust to variation in parameters included in the deterministic sensitivity analysis, with the majority of ICERs (for radiofrequency ablation compared with surgical resection) remaining negative. In this case negative ICERs indicate reduced costs for radiofrequency ablation compared with surgical resection and small gains in QALY outcomes. In general there are no differences in expected survival (no life-years gained or lost) between radiofrequency ablation and surgical resection, except in the structural sensitivity analysis adopting alternative forms for the survival function and the parameter sensitivity analysis on parameter values in the survival function. Variation in values of parameters in the overall survival function would be expected to have some impact on life expectancy estimates, whereas variation in values of parameters in the progression-free survival function would be expected to impact on the QALY estimates (given the difference in utility for stable and progressive disease states).
Among the structural sensitivity analyses the results appear to be most sensitive to assumptions over the functional form for the survival functions. The exponential and log-logistic functions are associated with slightly poorer survival with radiofrequency ablation, hence a slightly reduced QALY gain compared with the base case. However, the cost reduction, with RFA compared with surgical resection, is also greater when using the exponential and log-logistic functions of the survival function. In terms of parameter inputs, the results appear to be most sensitive to variation in values of parameters in the survival functions (for treatment effect on overall survival and all parameters in the overall survival function) and variation in the utility estimates applied in the model. The results appear to be relatively insensitive to variation in costs, time horizon and discount rates.
Probabilistic analysis
In a PSA, where the parameters of the survival models (both overall and progression-free survival), health state utility values, treatment cost, cost of outpatient attendances and patient monitoring, as well as costs of managing adverse events and palliative care were sampled probabilistically, radiofrequency ablation is associated with reduced costs (with a range from –£11,189 to –£1369) in all simulations and increased QALYs for the majority of simulations when compared with surgical resection (Figure 16 shows this and also shows the 95% confidence ellipse). However, radiofrequency ablation is associated with lower QALYs in 23% of simulations (with an overall range of –0.21 to 0.07).
FIGURE 16.
Cost-effectiveness plane – incremental cost and incremental QALYs for RFA and surgical resection for (resectable) liver metastases < 3 cm.
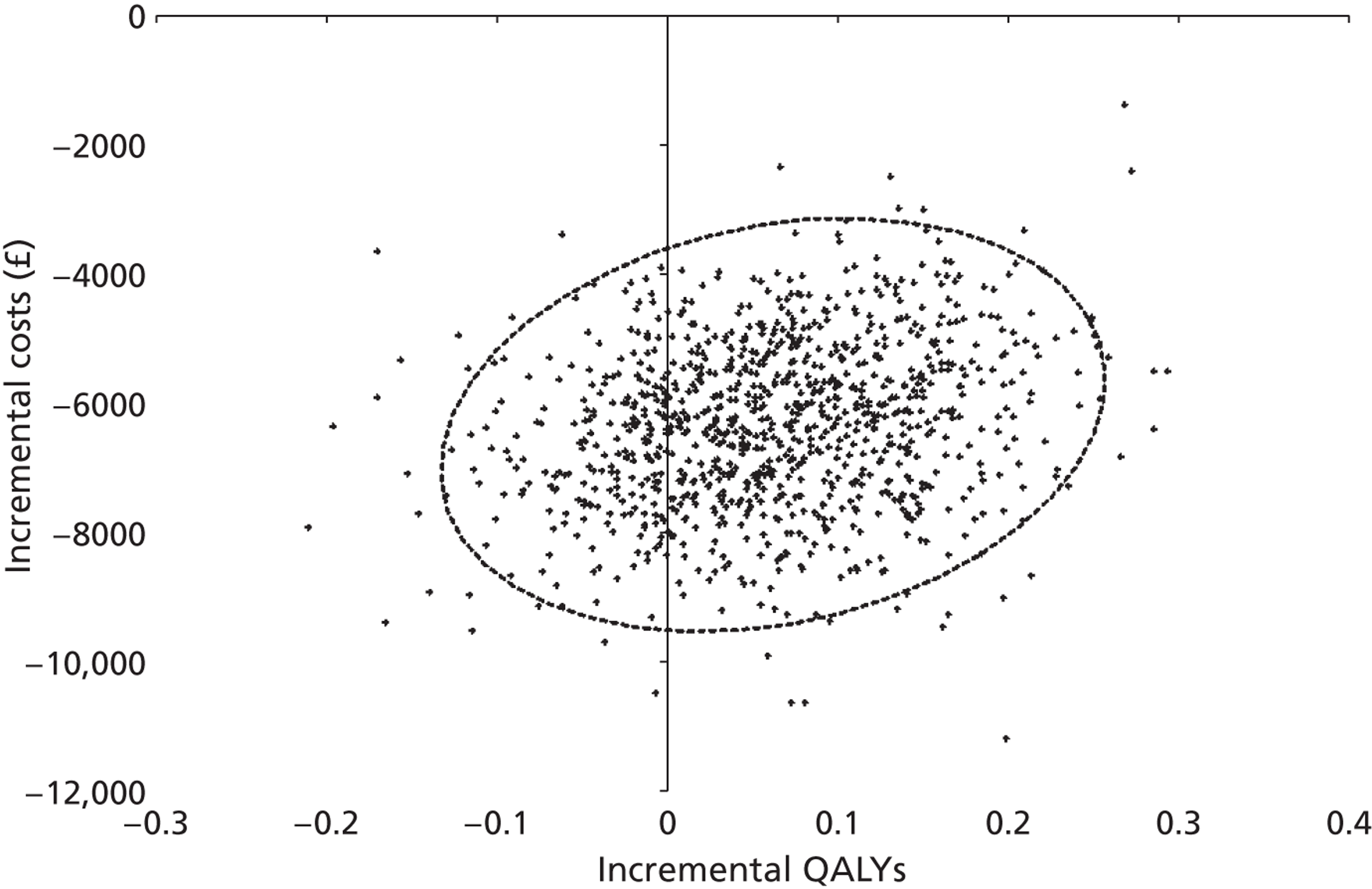
The probabilistic analysis generated cost and QALY estimates for each intervention that were similar to those for the base-case analysis (see Table 74 for the base-case analysis). Table 77 reports the mean costs and outcomes from the probabilistic analysis (including the 2.5th and 97.5th percentiles to give an indication of the range of the simulated values) and the ICER for radiofrequency ablation compared with surgical resection, based on the mean values generated in the probabilistic analysis.
| Procedure | Discounted costs (£) | Discounted QALYs | ICER (£) | ||||
|---|---|---|---|---|---|---|---|
| Mean | 2.5th percentile | 97.5th percentile | Mean | 2.5th percentile | 97.5th percentile | ||
| Surgical resection | 25,521 | 16,749 | 35,217 | 3.00 | 2.32 | 3.82 | |
| RFA | 19,192 | 9,767 | 28,751 | 3.07 | 2.36 | 3.85 | –102,415 |
In addition to graphing the incremental cost and incremental QALYs for radiofrequency ablation, a cost-effectiveness acceptability curve was derived, representing the proportion of simulations where radiofrequency ablation is cost-effective for a range of willingness-to-pay thresholds, up to £100,000 (Figure 17). In this analysis radiofrequency ablation had a probability of being cost-effective of 100% at a willingness-to-pay threshold of £20,000 per QALY and 100% at a willingness-to-pay threshold of £30,000 per QALY.
FIGURE 17.
Cost-effectiveness acceptability curve for radiofrequency ablation for (resectable) liver metastases < 3 cm.
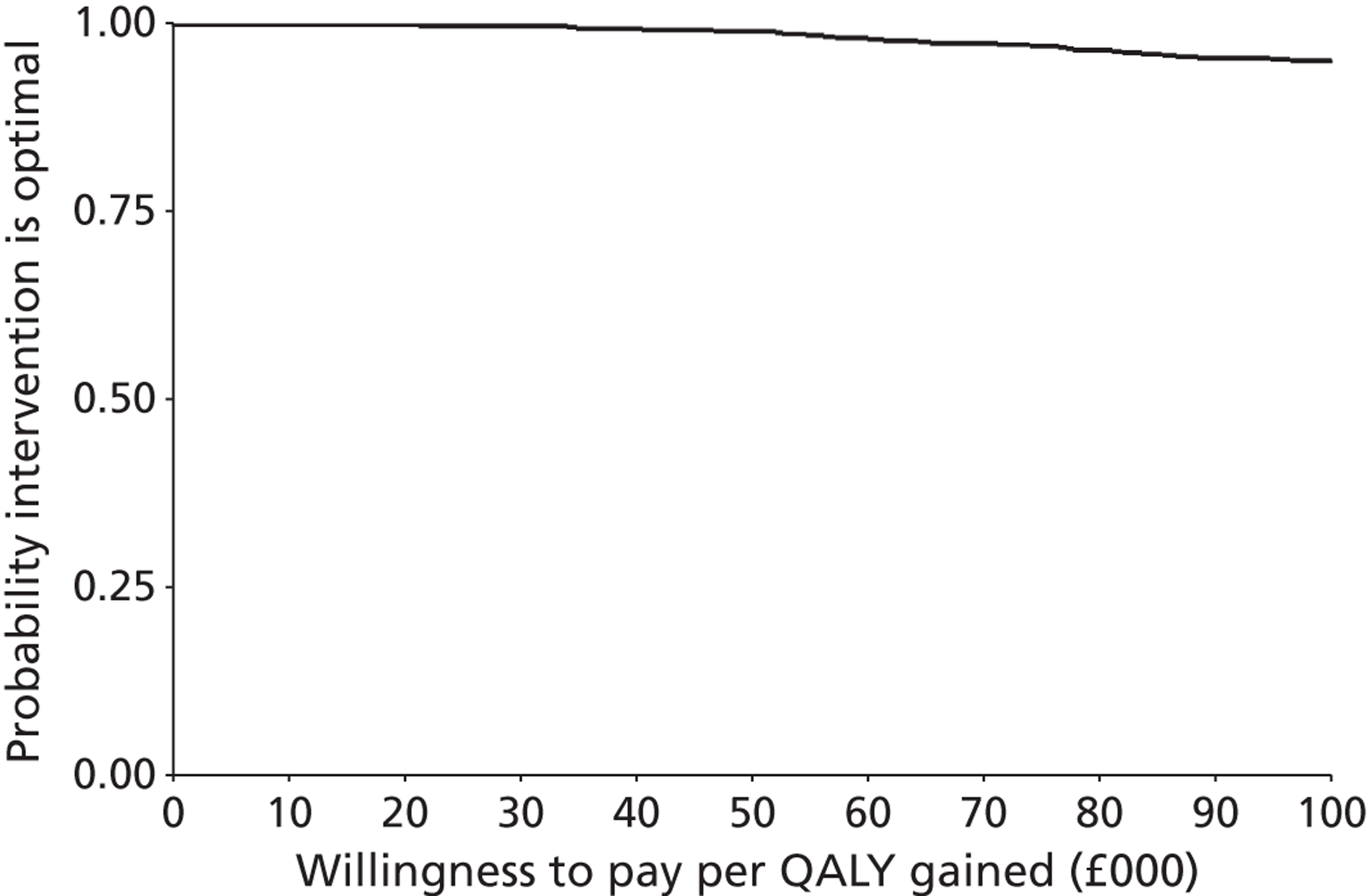
Radiofrequency ablation compared with surgical resection for solitary metastases ≥ 3 cm
Costs and outcomes modelled for cohorts of patients receiving treatment radiofrequency ablation or surgical resection are presented in Table 78. Costs and health outcomes in the table have been discounted at 3.5%.
| Treatment | Costs (£) | Life-years | Incremental cost per life-year gained (£) | QALYs | Incremental cost per QALY gained (£) |
|---|---|---|---|---|---|
| Surgical resection | 18,527 | 4.24 | 3.24 | ||
| RFA | 15,050 | 2.81 | 2241 | 1.97 | 2538 |
Radiofrequency ablation for patients with solitary liver metastases of ≥ 3 cm is associated with significantly poorer outcome – reduced life expectancy and poorer QALY outcomes – than for surgical resection. The incremental cost associated with radiofrequency ablation, compared with surgical resection, is negative (£3207) indicating that costs of treatment for larger solitary liver metastases with radiofrequency ablation are lower than for surgical resection. Table 79 reports a breakdown of treatment costs, by phase of treatment, for each cohort.
| Phase of treatment | RFA (£) | Surgical resection (£) | |
|---|---|---|---|
| Active treatment | Procedure cost | 3391 | 8747 |
| Surgical follow-up | 237 | 237 | |
| Adverse event costs | 50 | 251 | |
| Non-progressive disease monitoring | 1392 | 3312 | |
| Palliative care | 9981 | 5711 | |
| Total | 15,050 | 18,257 | |
In this analysis, radiofrequency ablation as a treatment for patients with (surgically resectable) larger solitary liver metastases is associated with poorer outcomes (in terms of life expectancy and quality-adjusted life expectancy) and reduced costs (compared with surgical resection), resulting in an ICER of £2538 per QALY gained. This positive ICER is the result of negative incremental cost and incremental QALY values – that is to say, in this analysis RFA is associated with reduced cost but also poorer outcome than surgical resection, requiring a judgement on whether or not the savings can justify the reduced outcome.
Deterministic sensitivity analysis
Table 80 reports the results of the sensitivity analysis. Except for the sensitivity analysis with respect to time horizon, all analyses were conducted using a 10-year time horizon. The table is divided to distinguish between analyses undertaken due to uncertainties over structural assumptions in the model, methodological uncertainties (in this case related to the discount rates applied in the model) and uncertainty over parameter values. Where unit costs have been taken from NHS Reference Costs, the upper and lower quartiles have been used in the sensitivity analysis. In all other cases unit costs have been varied by plus or minus 20%.
| Incremental cost (£) | Life-years gained | QALYs gained | ICER (£ per QALY gained) | |
|---|---|---|---|---|
| Base case | –3207 | –1.43 | –1.26 | 2538 |
| Structural assumptions | ||||
| Truncate survival at maximum follow-up for trial | –3069 | –0.95 | –0.87 | 3534 |
| Extrapolate overall survival up to 10 years | –3373 | –1.61 | –1.41 | 2399 |
| Exponential overall survival and progression-free survival model | –2790 | –0.84 | –0.79 | 3543 |
| Log-logistic overall survival and progression-free survival model | –3655 | –1.37 | –1.20 | 3057 |
| Methodological assumptions | ||||
| Discount rates (0% for both costs and outcomes) | –3130 | –1.71 | –1.50 | 2093 |
| Discount (6% for costs and 1.5% for outcomes) | –3265 | –1.58 | –1.39 | 2349 |
| Parameter uncertainty | ||||
| Lower 95% CI for treatment effect | –750 | –1.09 | –1.06 | 709 |
| Upper 95% CI for treatment effect | –5414 | –1.74 | –1.45 | 3725 |
| Lower 95% CI for all parameters in the overall survival model | 114 | –1.03 | –1.04 | –110 |
| Upper 95% CI for all parameters in the overall survival model | –5673 | –1.11 | –0.92 | 6184 |
| Lower 95% CI for all parameters in progression-free survival model | –6374 | –1.43 | –1.16 | 5484 |
| Upper 95% CI for all parameters in progression-free survival model | –7329 | –1.43 | –1.13 | 6474 |
| Exclude palliative care costs | –7477 | –1.43 | –1.26 | 5918 |
| Upper limit for utility values | –3207 | –1.43 | –1.14 | 2801 |
| Lower limit for utility values | –3207 | –1.43 | –1.52 | 2104 |
| Procedure cost: lower quartile | –2722 | –1.43 | –1.26 | 2154 |
| Procedure cost: upper quartile | –4092 | –1.43 | –1.26 | 3238 |
| Cost of palliative care reduced by 20% | –4061 | –1.43 | –1.26 | 3214 |
| Cost of palliative care increased by 20% | –2353 | –1.43 | –1.26 | 1862 |
| Cost of outpatient visit for monitoring: lower quartile | –2926 | –1.43 | –1.26 | 2316 |
| Cost of outpatient visit for monitoring: upper quartile | –3501 | –1.43 | –1.26 | 2771 |
The cost-effectiveness results appear to be generally robust to variation in parameters included in the deterministic sensitivity analysis, with the majority of ICERs (for radiofrequency ablation compared with surgical resection) varying between £2000 and £4000. However, it should be noted that these positive ICERs are the result of negative incremental cost and incremental QALY values – that is to say, in this analysis radiofrequency ablation is associated with reduced cost but also poorer outcome than surgical resection. As noted earlier, the decision framework in this situation involves a judgement on whether or not the size of savings (indicated by the lower incremental cost) can justify the reduced outcome.
The results appear relatively insensitive to structural or methodological assumptions. Adopting the exponential overall survival and progression-free survival model reduces the difference in life expectancy and the QALY loss for radiofrequency ablation compared with surgical resection, but also reduces the cost reduction. Adopting the log-logistic overall survival and progression-free survival model gives results closer to the base case. However, it should be borne in mind that these functional forms did not fit the observed data as closely as did the Weibull form adopted in the base case.
In terms of parameter inputs, the results appear to be most sensitive to variation in values of parameters in the survival functions (for treatment effect on overall survival and all parameters in the overall survival function). The results appear to be relatively insensitive to variation in costs other than palliative care costs, where reduction in palliative care costs is associated with an increase in the difference in costs between radiofrequency ablation and surgical resection. However, even at the extreme value (where palliative care costs are excluded from the model), the cost reduction with RFA does not appear to be large enough to make the associated QALY loss, when compared with surgical resection, acceptable for patients with larger solitary liver metastases.
Probabilistic analysis
In a PSA, where the parameters of the survival models (both overall and progression-free survival), health state utility values, treatment cost, cost of outpatient attendances and patient monitoring, as well as costs of managing adverse events and palliative care were sampled probabilistically, radiofrequency ablation for patients with solitary liver metastases ≥ 3 cm is associated with reduced costs for the majority (97%) of simulations (with an overall range from –£10,721 to £4591) and reduced QALYs for all of the simulations (with a range of –1.63 to –0.63) when compared with surgical resection (Figure 18 shows this and also shows the 95% confidence ellipse).
FIGURE 18.
Cost-effectiveness plane – incremental cost and incremental QALYs for radiofrequency ablation and surgical resection for (resectable) liver metastases ≥ 3 cm.
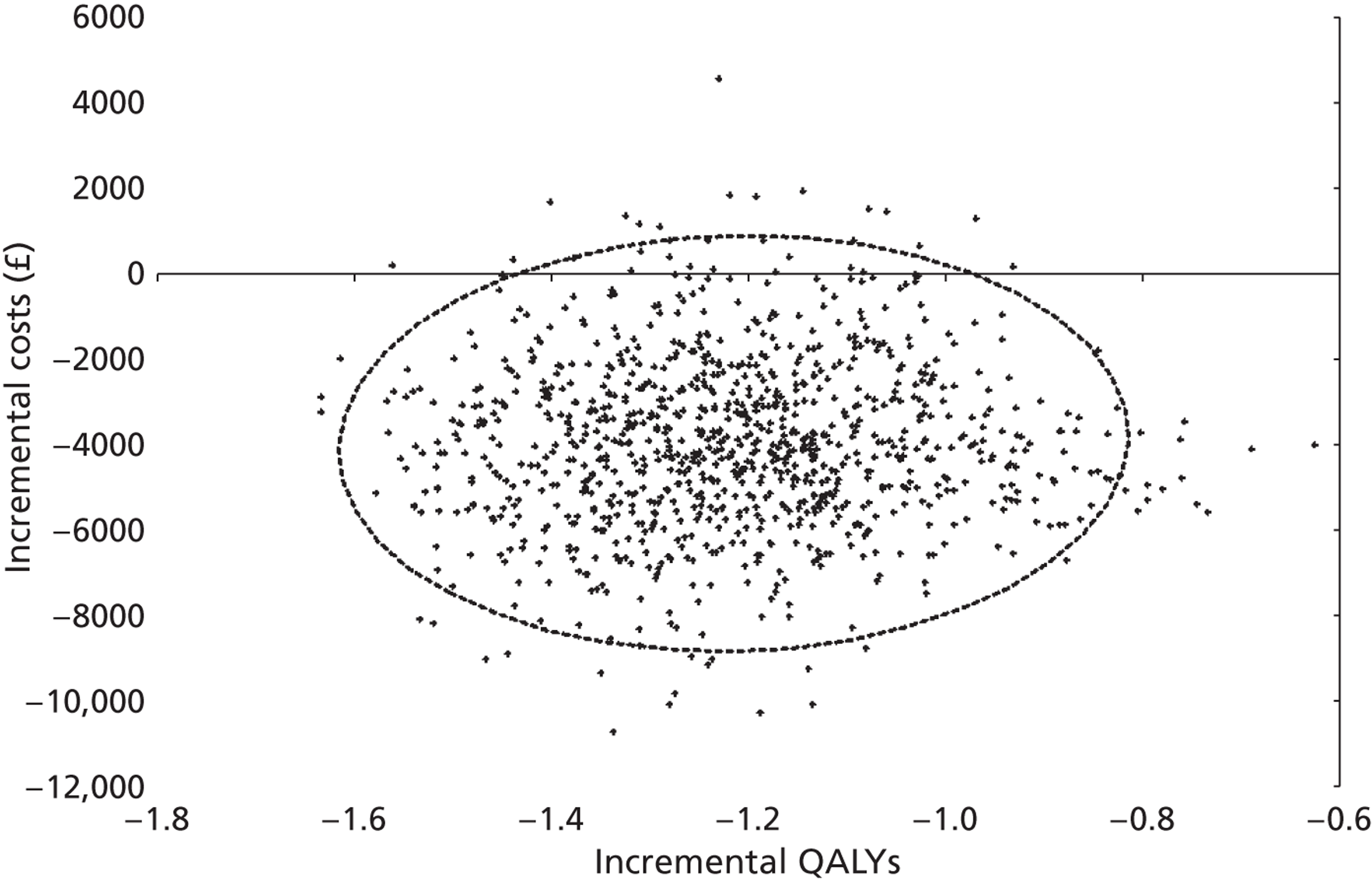
The probabilistic analysis generated cost and QALY estimates for each intervention that were similar to those for the base-case analysis (see Table 78 for the base-case analysis). Table 81 reports the mean costs and outcomes from the probabilistic analysis (including the 2.5th and 97.5th percentiles to give an indication of the range of the simulated values) and the ICER for radiofrequency ablation compared with surgical resection, based on the mean values generated in the probabilistic analysis.
| Procedure | Discounted costs (£) | Discounted QALYs | ICER (£) | ||||
|---|---|---|---|---|---|---|---|
| Mean | 2.5th percentile | 97.5th percentile | Mean | 2.5th percentile | 97.5th percentile | ||
| Surgical resection | 19,267 | 13,464 | 29,456 | 3.23 | 2.36 | 4.11 | |
| RFA | 15,307 | 8306 | 25,301 | 2.02 | 1.35 | 2.82 | 3259 |
In addition to graphing the incremental cost and incremental QALYs for radiofrequency ablation, a cost-effectiveness acceptability curve was derived, representing the proportion of simulations where radiofrequency ablation is cost-effective for a range of willingness-to-pay thresholds, up to £100,000 (Figure 19). In this analysis, radiofrequency ablation had a probability of being cost-effective of 0% at all willingness-to-pay thresholds of above £9000 per QALY.
FIGURE 19.
Cost-effectiveness acceptability curve for radiofrequency ablation for (resectable) liver metastases ≥ 3 cm.
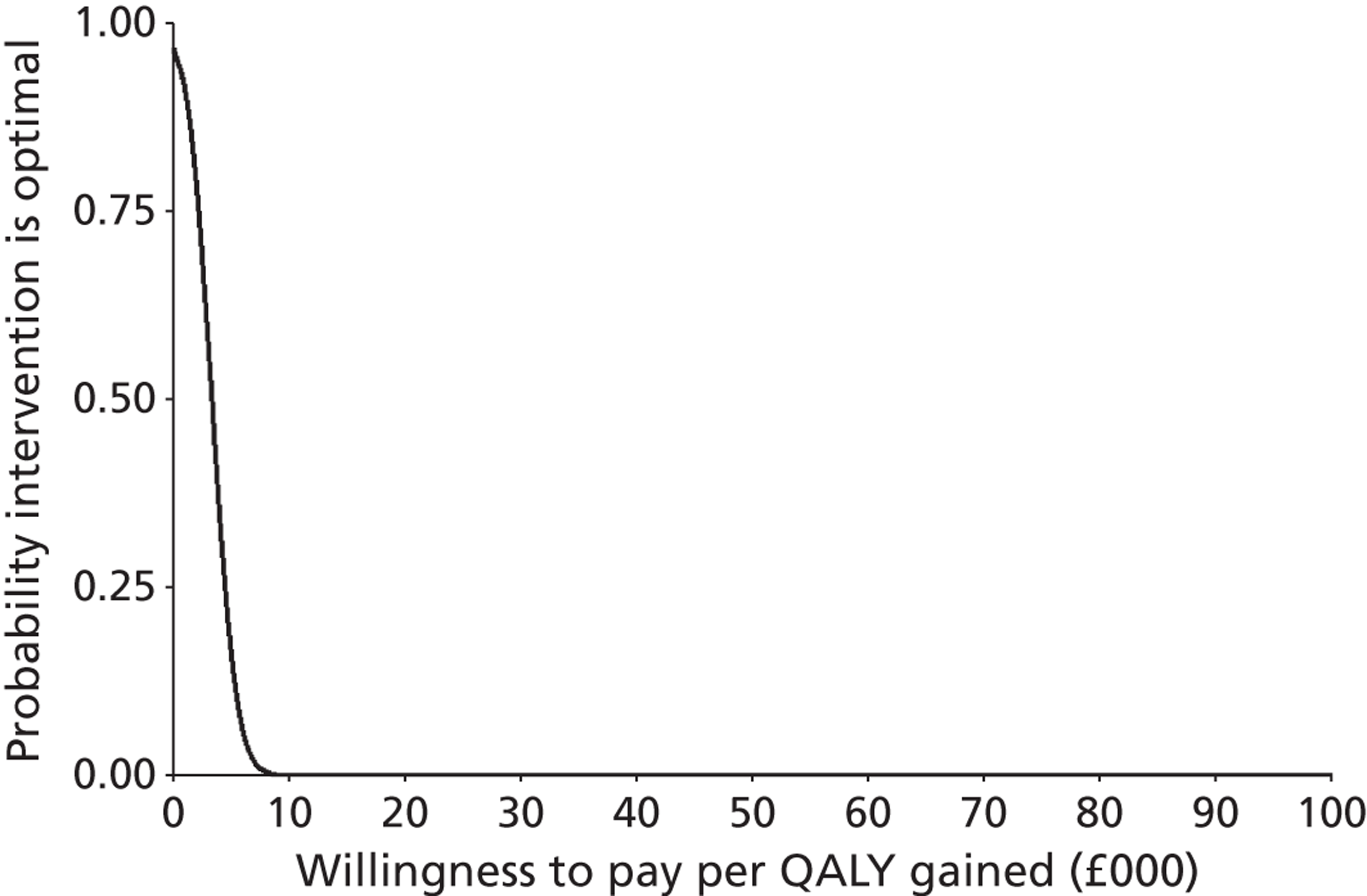
Radioembolisation plus hepatic artery chemotherapy compared with hepatic artery chemotherapy alone
This section reports cost-effectiveness results for a cohort of patients with non-resectable liver metastases from adenocarcinoma of the large bowel receiving either radioembolisation plus hepatic artery chemotherapy or hepatic artery chemotherapy alone, based on the trial report by Grey and colleagues. 85 Discounted costs (identifying the contribution of radioembolisation consumables, administration of treatment and monitoring while on treatment, management of adverse events, monitoring prior to disease progression and palliative care) are presented alongside the life expectancy and quality-adjusted life expectancy for patients in the cohort. The results are presented as incremental cost per life-year gained and incremental cost per QALY gained.
Costs and outcomes modelled for cohorts of patients receiving treatment with radioembolisation plus hepatic artery chemotherapy and hepatic artery chemotherapy alone are presented in Table 82. Costs and health outcomes in the table have been discounted at 3.5%.
| Treatment | Costs (£) | Life-years | Incremental cost per life-year gained (£) | QALYs | Incremental cost per QALY gained (£) |
|---|---|---|---|---|---|
| HAC | 6010 | 1.49 | 1.06 | ||
| RE + HAC | 18,955 | 1.86 | 35,225 | 1.41 | 37,303 |
The estimated gain in discounted life expectancy, associated with the addition of radioembolisation to hepatic artery chemotherapy, is 0.37 years (19 weeks). The equivalent undiscounted values are 0.40 years (21 weeks). The estimated gain in discounted QALYs, associated with the addition of radioembolisation to hepatic artery chemotherapy, is 0.35. The equivalent undiscounted value is 0.37 QALYs.
The incremental cost, associated with the addition of radioembolisation to hepatic artery chemotherapy, is £12,945. Table 83 reports a breakdown of treatment costs, by phase of treatment, for each cohort. For patients receiving hepatic artery chemotherapy alone, the costs fall under the heading of monitoring (both on-treatment monitoring and post-treatment monitoring for disease progression) and palliative care, with palliative care constituting the largest component of total costs (80% total costs) for this cohort. In contrast, for patients receiving treatment with radioembolisation plus hepatic artery chemotherapy, while palliative care remains a costly phase of care, this has reduced to 15% of total costs for this cohort. Active treatment with radioembolisation (including radioembolisation administration and on-treatment monitoring in addition to the costs of the intervention itself) represents 81% of total costs for this cohort, with radioembolisation consumables constituting 71% of total treatment costs. Monitoring for disease progression in patients following cessation of treatment makes a relatively small contribution to total costs in this cohort of patients (4% of total costs).
| Phase of treatment | RE + HAC (£) | HAC (£) | |
|---|---|---|---|
| Active treatment | Radioembolisation consumables | 10,911 | |
| Radioembolisation administration | 3305 | ||
| On-treatment monitoring | 1122 | 267 | |
| Non-progressive disease monitoring | 686 | 909 | |
| Palliative care | 2930 | 4834 | |
| Total | 18,955 | 6010 | |
Radioembolisation, in addition to hepatic artery chemotherapy, as a treatment for patients with non-resectable liver metastases from adenocarcinoma of the large bowel, is associated with both improved outcomes (in terms of life expectancy and quality-adjusted life expectancy) and increased costs. QALY outcomes have increased by approximately 33% while costs have approximately trebled, yielding an ICER for the addition of radioembolisation to hepatic artery chemotherapy of £37,303 per QALY gained.
Deterministic sensitivity analysis
A sensitivity analysis was conducted to consider the effect of uncertainty around the model structure and for variation in certain key parameters that were expected, a priori, to be influential on the cost-effectiveness results. The method adopted in most cases was univariate sensitivity analysis; that is, varying one parameter at a time, leaving all other variables unchanged. This is to highlight the impact, if any, of each selected parameter alone on the cost-effectiveness results. In some situations (such as the analysis of alternative parametric forms for the survival function, or the analysis using the upper confidence limits for all parameters in survival model) a set of related parameters are varied simultaneously. The effects of uncertainty in multiple parameters were addressed using PSA, which is reported later in the section.
Table 84 reports the results of the sensitivity analysis. Except for the sensitivity analysis with respect to time horizon, all analyses were conducted using a 5-year time horizon. The table is divided to distinguish between analyses undertaken due to uncertainties over structural assumptions in the model, methodological uncertainties (in this case related to the discount rates applied in the model) and uncertainty over parameter values. Where unit costs have been taken from NHS Reference Costs, the upper and lower quartiles have been used in the sensitivity analysis. In all other cases unit costs have been varied by plus or minus 20%.
| Incremental cost (£) | Life-years gained | QALYs gained | ICER (£ per QALY gained) | |
|---|---|---|---|---|
| Base case | 12,945 | 0.37 | 0.35 | 37,303 |
| Structural assumptions | ||||
| Truncate survival at maximum follow-up for trial | 12,907 | 0.36 | 0.34 | 37,697 |
| Extrapolate overall survival up to 10 years | 13,118 | 0.37 | 0.37 | 35,935 |
| Exponential overall survival and progression-free survival model | 13,938 | 0.41 | 0.38 | 36,782 |
| Log-logistic overall survival and progression-free survival model | 11,826 | 0.29 | 0.32 | 37,198 |
| Methodological assumptions | ||||
| Discount rates (0% for both costs and outcomes) | 12,930 | 0.40 | 0.37 | 34,562 |
| Discount (6% for costs and 1.5% for outcomes) | 12,958 | 0.39 | 0.36 | 35,778 |
| Parameter uncertainty | ||||
| Lower 95% CI for treatment effect | 16,085 | 0.80 | 0.61 | 26,436 |
| Upper 95% CI for treatment effect | 9023 | –0.18 | 0.02 | 423,802 |
| Lower 95% CI for all parameters in survival model | 16,871 | 0.91 | 0.67 | 25,067 |
| Upper 95% CI for all parameters in survival model | 9143 | –0.16 | 0.03 | 294,208 |
| Lower 95% CI for all parameters in progression-free survival model | 12,449 | 0.37 | 0.36 | 34,377 |
| Upper 95% CI for all parameters in progression-free survival model | 13,447 | 0.37 | 0.33 | 40,536 |
| Exclude palliative care costs | 14,848 | 0.37 | 0.35 | 42,788 |
| Upper limit for utility values | 12,945 | 0.37 | 0.29 | 44,032 |
| Lower limit for utility values | 12,945 | 0.37 | 0.46 | 27,917 |
| Procedure cost: lower quartile | 11,830 | 0.37 | 0.46 | 34,090 |
| Procedure cost: upper quartile | 13,565 | 0.37 | 0.46 | 39,089 |
| Cost of palliative care reduced by 20% | 13,946 | 0.37 | 0.35 | 40,186 |
| Cost of palliative care increased by 20% | 12,564 | 0.37 | 0.35 | 36,206 |
| Cost of outpatient visit for monitoring: lower quartile | 12,891 | 0.37 | 0.35 | 37,147 |
| Cost of outpatient visit for monitoring: upper quartile | 13,018 | 0.37 | 0.35 | 37,515 |
The cost-effectiveness results appear to be generally robust to variation in the parameters included in the deterministic sensitivity analysis, with ICERs varying between approximately £34,000 and £40,000 per QALY gained. Among the structural sensitivity analyses, the results appear to be most sensitive to assumptions over the functional form for the survival functions. In terms of parameter inputs, the results appear to be most sensitive to variation in utility estimates applied in the model, variation in values of parameters in the survival functions (for treatment effect on overall survival and all parameters in the overall survival function) and to the cost of palliative care.
Time horizon for the model appears to have a limited impact on the cost-effectiveness estimates. Truncating survival at the maximum duration observed for each arm reported in the RCT by Grey and colleagues85 reduces the QALY gain by 0.005 and costs by £38. The proportionate reduction in outcome (1.3%) is greater than the proportionate reduction in costs (0.3%), hence the ICER increases, but only by a small amount. Increasing the maximum survival duration to 10 years has the opposite effect – a slight increase in QALY gain and a slight increase in costs, with the proportionate change in QALYs being greater than the proportionate increase in costs, leading to a small reduction in the ICER. Adopting an alternative (log-logistic) parametric form for the overall survival and progression-free survival functions has more effect, resulting in an 8.4% reduction in QALY gain, a 8.6% reduction in cost, and a small reduction in the ICER to £37,198. In contrast, with the exponential form of the overall survival and progression-free survival functions, the QALY gain increases by 9.2% and incremental cost by 7.7%, leading to a slightly larger reduction in the ICER (to £36,782).
Varying the discount rates applied has comparatively little effect. Zero discount rates for costs and outcomes result in a slight reduction in incremental cost and a slight increase in incremental QALYs compared with baseline values. Applying discount rates of 6% for costs and 1.5% for outcomes leads to a slight increase in incremental cost and incremental QALYs. The resulting ICER is slightly lower than in the base case.
Varying the value of the treatment effect parameter in the overall survival model, between its upper and lower confidence limits, has a greater effect on outcomes than on cost. In the model, variation in survival (unless it is assumed to be associated with variation in progression-free survival) has an impact only on the duration of post-progression survival, and therefore will affect only the estimate of palliative care costs. A similar situation applies to QALY outcomes where it is assumed that all gains or losses of life expectancy associated with variation in the treatment effect parameter are weighted by post-progression utility values. This explains why the proportionate variation in QALY gains is less than the variation in life-years gained.
The cost-effectiveness results are less variable if all parameters in the survival models are included (at the 95% confidence limits) in the sensitivity analysis, rather than just the treatment effect estimated in the overall survival model, with ICERs varying between approximately £25,000 and £294,200 per QALY gained. Variation in the parameters of the progression-free survival model had less impact on the results.
The next greatest variation in cost-effectiveness results, associated with parameter inputs, is related to deterioration in quality of life related to disease progression. Assuming no quality-of-life impact from disease progression (the upper limit of the relative effect, i.e. 1) leads to a reduction of 0.05 (15%) in the QALY gain associated with radioembolisation plus hepatic artery chemotherapy. As a result the ICER increases to £44,032 per QALY gained. In contrast, assuming a much greater reduction in quality of life due to disease progression (using the lower limit of the relative effect, i.e. 0.20) leads to an increase of 0.12 (34%) in the QALY gain associated with radioembolisation plus hepatic artery chemotherapy, with the ICER reducing to £27,917 per QALY gained.
In terms of cost parameters, the model results appear to be most sensitive to variation in the cost of palliative care.
Probabilistic analysis
In a PSA, where the parameters of the survival models (both overall and progression-free survival), health state utility values, treatment cost, cost of outpatient attendances and patient monitoring, as well as costs of managing adverse events and palliative care were sampled probabilistically, radioembolisation plus hepatic artery chemotherapy is associated with increased costs (from £469 to £21,241) in all simulations and increased QALYs for the majority of simulations when compared with hepatic artery chemotherapy alone (Figure 20 shows this and also shows the 95% confidence ellipse). However, radioembolisation plus hepatic artery chemotherapy is associated with lower QALYs in 8% of simulations (with a range of –0.44 to 0.88).
FIGURE 20.
Cost-effectiveness plane – incremental cost and incremental QALYs for radioembolisation plus hepatic artery chemotherapy compared with hepatic artery chemotherapy alone.
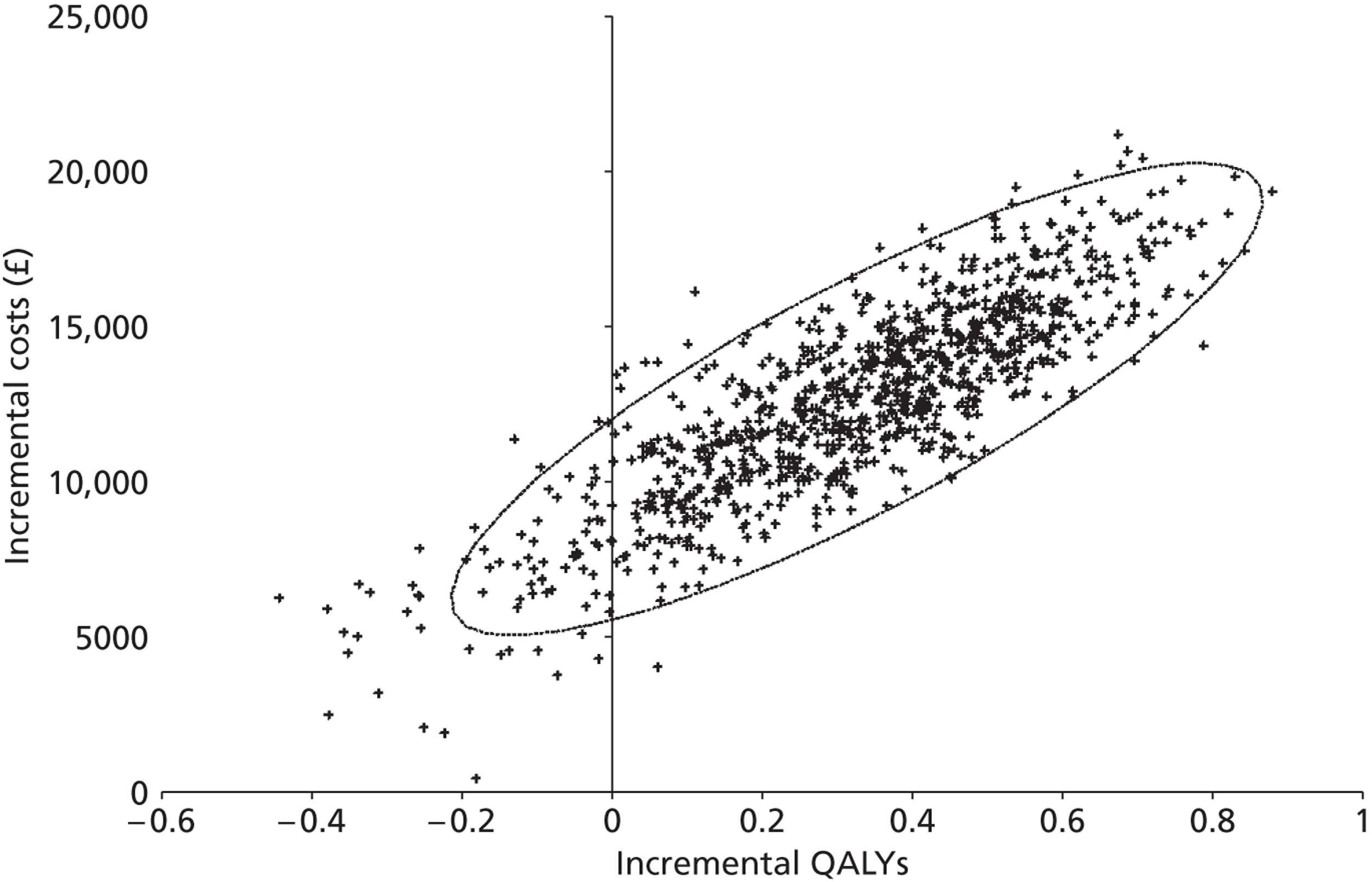
The probabilistic analysis generated cost and QALY estimates for each intervention that were similar to those for the base-case analysis (see Table 82 for the base-case analysis). Table 85 reports the mean costs and outcomes from the probabilistic analysis (including the 2.5th and 97.5th percentiles to give an indication of the range of the simulated values) and the ICER for radioembolisation plus hepatic artery chemotherapy compared with hepatic artery chemotherapy alone, based on the mean values generated in the probabilistic analysis.
| Procedure | Discounted costs (£) | Discounted QALYs | ICER (£) | ||||
|---|---|---|---|---|---|---|---|
| Mean | 2.5th percentile | 97.5th percentile | Mean | 2.5th percentile | 97.5th percentile | ||
| HAC | 6262 | 1624 | 12,880 | 1.08 | 0.69 | 1.58 | |
| RE + HAC | 18,954 | 15,676 | 22,734 | 1.41 | 1.28 | 1.55 | 38,944 |
In addition to graphing the incremental cost and incremental QALYs for radioembolisation plus hepatic artery chemotherapy, a cost-effectiveness acceptability curve was derived, representing the proportion of simulations where radioembolisation plus hepatic artery chemotherapy is cost-effective for a range of willingness-to-pay thresholds, up to £100,000 (Figure 21). In this analysis radioembolisation plus hepatic artery chemotherapy had a probability of being cost-effective of 0.1% at a willingness-to-pay threshold of £20,000 per QALY, 26% at a willingness-to-pay threshold of £30,000 per QALY and 68% at a willingness-to-pay threshold of £50,000 per QALY.
FIGURE 21.
Cost-effectiveness acceptability curve for radioembolisation plus hepatic artery chemotherapy.
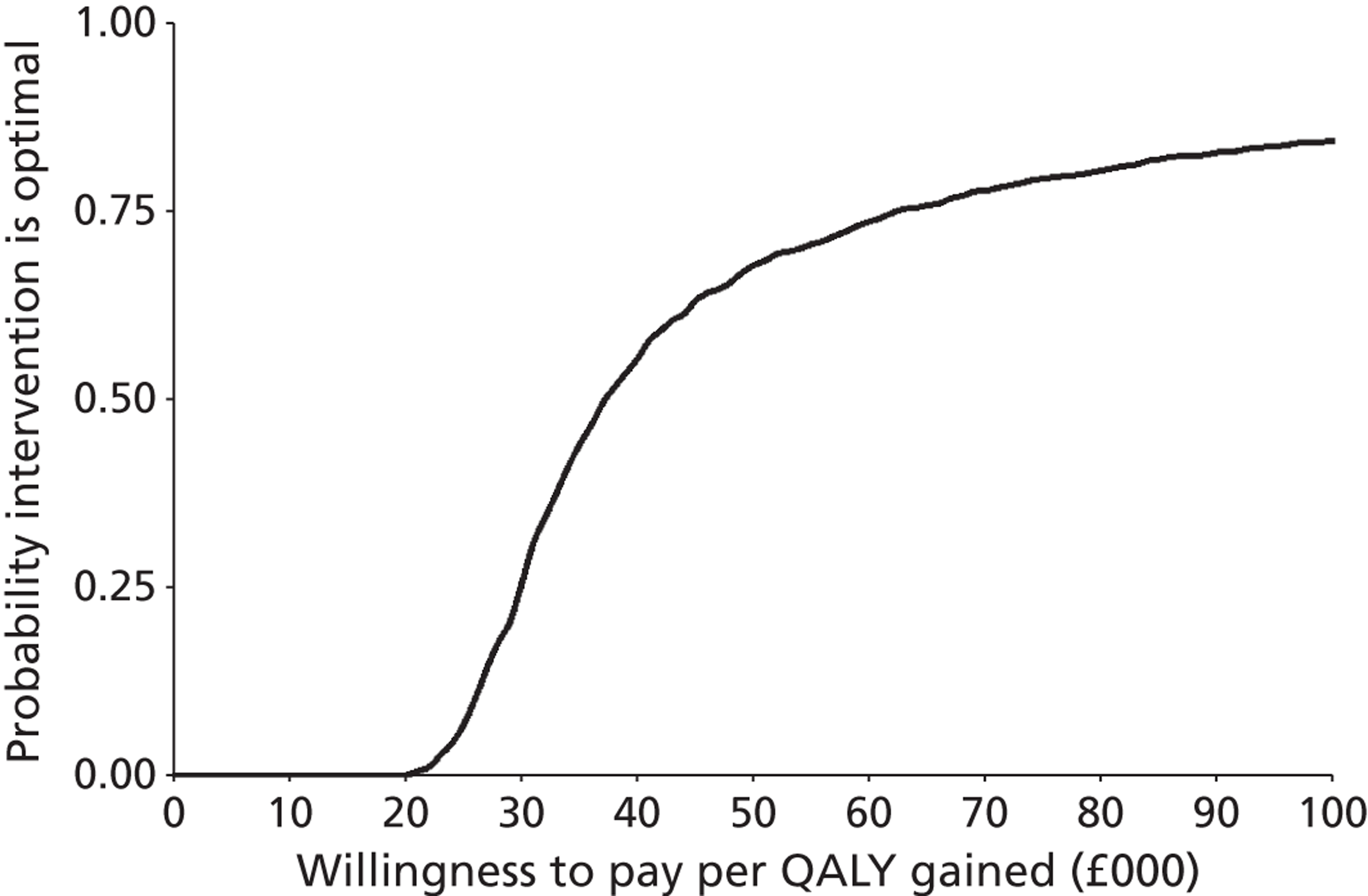
Summary of economic analysis
There is a very limited evidence base for conducting economic analysis of ablative therapies and other non-invasive therapies for the treatment of patients with surgically resectable, or unresectable, liver metastases. There is a limited number of comparative studies reporting final outcomes for ablative therapies or other non-invasive therapies, although the comparator (in some cases) or patterns of care may be of limited relevance to current UK clinical practice, the study designs may be prone to bias or study publications may not include sufficient detail to allow robust economic analysis.
Three comparative studies were identified that reported sufficient data to model two ablative therapies (microwave ablation and radiofrequency ablation) and one non-invasive therapy (radioembolisation). Ablative or other non-invasive therapies may be less costly than surgical resection, as a result of shorter lengths of hospital stay and reduced complication rates. The analysis suggests that, where ablative therapies or other non-invasive therapies can achieve similar overall and progression-free survival, they may result in improved outcomes for patients (in terms of QALY gains) by avoiding short- to medium-term reduction in quality of life associated with surgical resection. In this situation, ablative or other non-invasive therapies may dominate surgical resection (by providing a QALY gain at reduced cost) or may offer a cost-saving option (by providing equivalent outcomes at reduced cost). In contrast, in situations where ablative or other non-invasive therapies are associated with inferior survival, the lower short to medium quality-of-life reduction associated with these therapies is not sufficient to yield improved overall outcomes compared with surgical resection. If ablative or other non-invasive therapies are associated with a QALY loss, the potential cost savings, compared with surgical resection, are unlikely to be sufficient to make such treatment acceptable, in cost-effectiveness terms.
The results of the economic analysis are subject to a high degree of uncertainty, given the limited data available and the comparatively small size of the majority of included studies. Additional – higher quality – research on outcomes for patients with liver metastases, treated with ablative or other non-invasive therapies and comparator treatments, is required for robust cost-effectiveness modelling to be undertaken.
Chapter 6 Discussion
Statement of principal findings
Clinical effectiveness
Ablative therapies
Nine studies assessing ablative therapies for treating liver metastases were included in the systematic review. 70–80 One RCT compared microwave ablation with surgical resection,72 two case series assessed laser ablation70,71 and one non-randomised comparison73 and five cases series assessed radiofrequency ablation. 74–80 Although the people included in the RCT assessing microwave ablation72 and the non-randomised comparison assessing radiofrequency ablation73 were amenable to surgical resection, those undergoing either laser surgery or radiofrequency ablation in the seven case series were not considered to be candidates for surgical resection. 70,71,74–80 The studies tended to focus on people diagnosed with colorectal carcinoma as the primary cancer,72–80 although one study included women with metastatic breast cancer71 and another study assessed people with primary cancers from several sites. 70 People included in most studies were similar in terms of age (mean age ranged from 55 to 65 years), sex (male 55–70%), previous treatment (received chemotherapy and/or liver resection), and extent of metastases (mean number of metastases 1.5–4; mean size of metastases 2–4 cm). 72–80 One case series differed, focusing on younger women with breast cancer as the primary site (mean age 54.4 years)71 and another provided limited details about the participants. 70 Although the methodological quality of the RCT was considered moderate,72 the quality of reporting of the methodology used in the non-randomised comparison73 and the case series was poor and, as a consequence, their methodological quality was uncertain. 70,73–80 All studies were at risk of bias.
The RCT comparing microwave ablation with surgical resection found no statistically significant difference between the interventions on estimated cumulative survival rates (p = 0.83). Limited differences were also reported in mean overall survival (27 months vs. 25 months respectively), survival at 1, 2 or 3 years (1 year 71% vs. 69%, 2 years 57% vs. 56%, 3 years 14% vs. 23%), mean disease-free survival (11.3 months vs. 13.3 months, p = 0.47) or adverse events. 72 Microwave ablation did result in statistically significant benefits in terms of measures of surgical invasiveness compared with surgical resection, with people experiencing lower blood loss and having less need for blood transfusions. In contrast, there was no statistically significant difference in operation times or days the participants stayed in hospital.
The case series studies assessing the effects of laser-induced70,71 and radiofrequency ablative therapies74,75,78–80 reported median or mean survival, survival to 5 years’ follow-up and mortality. Laser-induced ablation resulted in a mean survival of between 50.4 to 58.8 months for women with metastatic breast cancer and 41.8 months for people with metastatic colorectal cancer. 70,71 Five-year survival varied from 34% to 41% for women with metastatic breast cancer and 30% for people with metastatic colorectal cancer. 70,71 People with metastatic colorectal cancer who underwent radiofrequency ablation had median survival from diagnosis ranging from 44.6 months to 52 months and from treatment from 24 months to 32 months. 74,75,78–80 Three-year survival from treatment ranged from 20% to 46% and 5-year survival was reported as 18%. 78–80 A non-randomised comparison study73 and a case series76,77 assessing radiofrequency ablation reported survival for subgroups only; these results were not discussed.
Mortality among people with liver metastases following colorectal cancer who had undergone radiofrequency ablation ranged from 31% to 63%, reflecting in part the difference in the follow-up period of the studies. 78–80 Adverse events were reported and, although major or severe events were evident, rates were thought to be relatively low. 71,76,77,79,80
Other minimally invasive techniques
Five RCTs assessing the clinical effectiveness of other minimally invasive techniques were included in the systematic review. 81,82,85–88 Three RCTs compared the use of radioembolisation plus either hepatic artery chemotherapy or systemic chemotherapy with chemotherapy alone85–88 and two RCTs compared the use of different types of chemotherapy with and without embolisation (i.e. degradable starch microspheres or polyvinyl sponge). 81,82 People included in the three RCTs assessing the use of radioembolisation had metastatic colorectal cancer and were not amenable to curative surgical resection, local ablation and, in some instances, to standard chemotherapy. 85–88 The two RCTs comparing chemoembolisation with chemotherapy alone included people with ocular melanoma or various tumours as the primary cancer site. 81,82 Although people recruited to one RCT assessing chemoembolisation were not candidates for surgical resection,81 it was unclear if this was so in the other RCT. 82 In two RCTs assessing radioembolisation, the people randomised to the different arms appeared similar in terms of age (mean age 59–65 years), sex (male 76% to 91%) and the number, size and extent of liver metastases. 85,87,88 The third RCT of radioembolisation had arms that differed in terms of the sex of the participants and the size of the liver metastases. 86 Limited information was presented on the characteristics of the participants in the two RCTs assessing chemoembolisation. 81,82 Although the methodological quality of the five RCTs varied, many lacked information on key methodological criteria making the quality uncertain and the studies prone to a risk of bias.
The three RCTs of radioembolisation plus chemotherapy and the two RCTs of chemoembolisation showed some benefit when compared with chemotherapy alone on measures of survival, response, time to progression and adverse events, although differences were not always statistically significant. 81,82,85–88 Estimated overall survival for people receiving radioembolisation plus systemic chemotherapy was longer, significantly so in one RCT (p = 0.025),87,88 than that experienced by people receiving systemic chemotherapy alone (10 vs. 7.3 months;86 29.4 vs. 12.8 months). 87,89 Survival rates up to 5 years’ follow-up were higher for people receiving radioembolisation plus chemotherapy than for those receiving chemotherapy alone (1 year 72% vs. 68%, 2 years 39% vs. 29%, 3 years 17% vs. 6%, 5 years 3.5% vs. 0%), though differences were not statistically significant. 85 Estimated overall survival was reported in one RCT comparing chemotherapy with and without embolisation, finding a non-statistically significant benefit for chemoembolisation (9.7 months vs. 7.6 months). 81
Radioembolisation and chemoembolisation were shown to improve tumour response compared with chemotherapy alone. Radioembolisation plus chemotherapy led to statistically significant benefit on all categories of response compared with chemotherapy alone (p < 0.05) in two RCTs. 85,87,88 It also resulted in statistically significant improvement on response rates in one RCT (complete plus partial response: 44% vs. 18%, p = 0.01)85 and disease control rates (partial response plus stable disease: 86% vs. 35%, p = 0.001)86 in another RCT. Improvements in tumour response were shown for chemoembolisation compared with chemotherapy alone on the different categories of response and a summary response measure (complete plus partial response: 54.5% vs. 20%, p < 0.05). 81 Time to disease progression was assessed in the three RCTs comparing radioembolisation plus chemotherapy with chemotherapy alone. 85–88 Despite using different measures, it was evident that people receiving radioembolisation plus chemotherapy had significantly longer median times to disease progression than those receiving chemotherapy only (p < 0.05).
Adverse events and toxicity were reported by all five RCTs comparing radioembolisation or chemoembolisation with chemotherapy alone. 81,82,85–88 Although the three RCTs comparing radioembolisation plus chemotherapy with chemotherapy presented differing findings, overall it appeared that both interventions were reasonably well tolerated. 85–88 In contrast, one RCT reported significantly (p < 0.01) higher rates of pain, gastrointestinal disturbance and fever following chemoembolisation compared with chemotherapy. 81 Another RCT comparing chemoembolisation with chemotherapy assessed toxicity associated with different doses, finding limited difference between the comparators. 82
Combination therapies
Two prospective case series assessed the use of chemoembolisation to ‘downstage’ liver metastases prior to laser thermotherapy. 83,84 Although the participants included in the studies differed in the site of their primary cancers, specifically women with breast cancer84 and people with various primary tumours,83 both groups were of similar age (mean 61.8 years83 vs. 57 years84), considered to have unresectable liver metastases and had had no response to previous systemic chemotherapy for liver metastases. The quality of reporting of the methodology used in the case series was poor and, as a result, their methodological quality was uncertain. All studies were at risk of bias.
Survival from first chemoembolisation treatment ranged from a median survival of 26.2 months for those with various primary tumours83 to a mean survival of 32.5 months for women with metastatic breast cancer. 84 Survival rates at 1, 3 and 5 years for women with metastatic breast cancer were 88.8%, 36.6% and 13.7% respectively. 84 Tumour response for women with metastatic breast cancer showed that 38.5% had complete response, 5% partial response, 12.4% stable disease and 44.1% progressive disease. 84 Time to progression was reported as 8.2 months and overall tumour control 13.1 months for women with metastatic breast cancer. 84 Adverse events varied between the studies, with the majority being considered mild to moderate. 83,84 The case series of women with metastatic breast cancer reported that six people had complications requiring further intervention. 84
Summary
The search strategy identified 16 studies for inclusion in the systematic review. Five RCTs provided comparative evidence on the clinical effectiveness of the addition of radioembolisation or chemoembolisation to chemotherapy when compared with chemotherapy alone. All five RCTS did not clearly state the intention for treatment (i.e. pre-surgical, curative or palliative); however, none was in people thought to be amenable to surgical resection. Although these interventions resulted in statistically significant improvements in tumour response and time to disease progression relative to their comparators, benefits in terms of survival were equivocal. Radioembolisation and chemoembolisation were thought to be as well tolerated as their comparators in terms of adverse events, although one study reported significant pain, gastrointestinal disturbance and fever following chemoembolisation. The evidence provided by the nine studies on the clinical effectiveness of ablative therapies was limited. Only one RCT was identified that assessed an ablative therapy, specifically comparing microwave ablation with surgical resection. It found no statistically significant difference between the interventions on measures of survival. Benefits following microwave ablation were shown in terms of surgical invasiveness (i.e. intraoperative blood loss and blood transfusions). A non-randomised comparison study assessing radiofrequency ablation reported survival for subgroups only and the results were not reported. All the other studies assessing ablative therapies, whether separately or in combination with other non-invasive therapies, which presented results for relevant groups, were case series. These studies focused on people who were considered not to be candidates for surgical resection. Differences in the studies preclude any sensible comparison of the relative benefits of the different ablative therapies other than against no treatment. Unsurprisingly, when compared with the natural history of the condition, ablative therapies provide improved survival (mean survival 8 months vs. 26 to 60 months respectively; 5-year survival 0% vs. 13% to 41%, respectively). Assessment of the adverse events reported by the case series indicates that the different ablative therapies have limited serious adverse events.
Cost-effectiveness
Systematic review of published economic evaluations
Two full economic evaluations of ablative therapies or other non-invasive therapies for the treatment of liver metastases were included in the systematic review. 115,116 One of the economic evaluations compared radiofrequency ablation (with a range of treatment thresholds, retreatment and follow-up options), surgical resection (with a range of treatment thresholds and follow-up options) with no treatment in a population of people with surgically resectable or unresectable liver metastases. 116 In this study resectability was defined only in terms of the total number of metastases (with treatment threshold varying from 1 to 6). The other economic evaluation compared hepatic artery chemoembolisation with palliative care for people with unresectable liver metastases. 115 Both studies were conducted in the USA and both were modelling studies, deriving estimates of effectiveness (overall survival115 or quality-adjusted survival116) from assumption115 or from microsimulation based on the surrogate measure of proportion of liver replaced by tumour.
Abramson and colleagues115 present a simple spreadsheet model which estimated costs, based on assumption and (for complication rates) on a small, unpublished case series of 21 people in one institution. The incremental costs of hepatic artery chemoembolisation, above standard palliative care, were estimated over a range of potential survival benefits – from 0 to 24 months. Cost-effectiveness was estimated by dividing the estimated incremental cost by the assumed survival benefit. The study is explicit in its assumptions and clear on the absence of empirical data to support the assumed survival benefits. The absence of comparative studies demonstrating a survival benefit for hepatic artery chemoembolisation means that no firm conclusions can be drawn from the study – the study is further weakened by the absence of any adjustment for quality of life in estimating the benefits of the technology.
Gazelle and colleagues116 used microsimulation in a state transition model to estimate the effectiveness of radiofrequency ablation and surgical resection in reducing (or eliminating) the proportion of liver replaced by tumour and thereby reducing patients’ risk of death. Model parameters (such as number, size and rate of growth of liver metastases, risk of death associated with increasing proportion of liver volume replaced by tumour and effectiveness of radiofrequency ablation in achieving tumour necrosis) were derived from a range of studies. Simulated patients had a mean number of six metastases, up to a maximum of 15, with the number, size and location determined by random draws from probability distributions. Patients undergoing surgical resection were allowed one reoperation, whereas those undergoing radiofrequency ablation were permitted a larger number of repeat procedures. The key determinant of patient survival in the model was the proportion of liver volume replaced by tumour, although this was not modelled as having any impact on quality of life – age- and sex-specific utility values for the general population were applied to patients with liver metastases. Patients whose liver metastases were successfully resected or ablated were assumed to face general population age- and sex-specific mortality risks. Gazelle and colleagues116 modelled a number of treatment thresholds (1–6 metastases) and follow-up intervals (4, 6 and 12 months) yielding a large number of potential strategies. Strategies involving low treatment thresholds (fewer than three metastases for radiofrequency ablation and fewer than six for surgical resection) were dominated by strategies with higher thresholds, leading the authors to conclude that more aggressive strategies – particularly for surgical resection – were likely to be more cost-effective. Radiofrequency ablation was generally associated with lower QALY outcomes than surgical resection – for example, at a treatment threshold of six metastases and follow-up at 12 months, quality-adjusted life expectancy for a 65-year-old man with liver metastases undergoing radiofrequency ablation was 1.36 and the corresponding figure for surgical resection was 3.39.
Both of the identified economic evaluations have limited relevance to the NHS as both were conducted in the USA, and while both attempt to overcome the absence of comparative evidence to support their analyses the credibility of their results depends on an evaluation of the assumptions regarding the survival advantage with hepatic artery chemoembolisation, over palliative care, in the study by Abramson and colleagues115 and the key assumption on quality of life and adequacy of the proportion of liver volume replaced by tumour to predict mortality in the study by Gazelle and colleagues. 116
Southampton Health Technology Assessments Centre economic evaluation
A survival model was developed to estimate the cost of ablative therapies or other non-invasive therapies in cohorts of adult patients with surgically resectable, or unresectable, liver metastases. Limitations in the evidence base (lack of comparative studies or limitations in reporting survival outcomes) meant that not all identified therapies were included in the model.
The clinical effectiveness data used to populate the model were derived from studies with varying designs and risk of bias. Some of the comparisons in the model use data from studies with small numbers of participants. The limitations of the evidence base need to be borne in mind when interpreting the results of the economic evaluation. The model includes separate comparisons of two ablative therapies with surgical resection (microwave ablation compared with surgical resection and radiofrequency ablation compared with surgical resection) and one other non-invasive therapy (radioembolisation in conjunction with hepatic artery chemotherapy compared with hepatic artery chemotherapy alone). Each of these comparisons is based on a single study.
Clinical effectiveness data in the model were based on overall survival and progression-free survival functions estimated using linear regression on data extracted from survival plots reported in included studies. Three parametric survival functions were fit to the observed data and the best-fitting function was selected on the basis of a visual inspection of the model fit and comparison of predictions of median survival period (1, 2, 3 or 5 years) with values reported in included studies. The exponential function generally gave a poor fit to the observed data, while the Weibull and log-logistic functions typically provided reasonable fits to the observed data. Where the Weibull and log-logistic appeared to provide equally good fit to the observed data, the Weibull function was selected as it was less prone to give large maximum survival durations.
Health state utilities, for stable disease and disease progression, derived in our review of published quality-of-life studies were applied in the model. Additional quality-of-life adjustments were incorporated into the model in recognition of a short- to medium-term impact of treatment on quality of life.
Resource use estimates were developed based on treatment intensity (number of treatments and length of stay), on-treatment management and post-discharge monitoring reported in included studies. Unit costs were derived from NHS Reference Costs – where these were inadequate unit costs were sourced from a local NHS provider (University Hospital Southampton NHS Foundation Trust).
Microwave ablation compared with surgical resection
In the analysis comparing microwave ablation with surgical resection, total costs for surgical resection were £20,152 with outcomes of 1.94 life-years and 1.38 QALYs, while for microwave ablation the total costs were £19,825 and outcomes were 1.84 life-years and 1.29 QALYs. Microwave ablation is associated with a reduction of 0.10 years (5.2 weeks), which translates to a QALY reduction of 0.09. The incremental cost associated with microwave ablation is also negative (£327) – treatment cost of liver metastases with microwave ablation is slightly lower than treatment with surgical resection – resulting in an ICER of £3664 per QALY gained. It should be noted that this positive ICER is derived from negative incremental cost and incremental QALY values – that is to say, in this analysis microwave ablation is associated with reduced cost but also poorer outcome than surgical resection so that the decision framework in this case would be to consider whether or not the extent of savings (indicated by the negative incremental cost) can justify the reduced outcome.
In deterministic sensitivity analyses the ICER generally remained within the range of £2800 to £5000, where microwave ablation was associated with a small reduction in QALY and slightly lower costs. Among the structural sensitivity analyses, the results appear to be most sensitive to assumptions over the functional form for the survival functions. In terms of parameter inputs, the results appear to be most sensitive to variation in utility estimates applied in the model, variation in values of parameters in the survival functions (for treatment effect on overall survival and all parameters in the overall survival function), variation in procedure costs and to the cost of palliative care.
In a PSA microwave ablation is associated with reduced costs in around half of all simulations and lower QALYs in 68% of simulations when compared with surgical resection. Microwave ablation had a probability of being cost-effective of 31% at a willingness-to-pay threshold of £20,000 per QALY and 30% at a willingness-to-pay threshold of £30,000 per QALY.
Radiofrequency ablation compared with surgical resection for solitary metastases < 3 cm
In the analysis comparing radiofrequency ablation with surgical resection for solitary metastases < 3 cm, total costs for surgical resection were £25,612 with outcomes of 4.28 life-years and 2.99 QALYs, while for radiofrequency ablation the total costs were £19,322 and outcomes were 4.28 life-years and 3.05 QALYs. The incremental cost for radiofrequency ablation compared with surgical resection is negative (£6290) – a reduction of around 25% in total costs. There is no difference in discounted life expectancy between surgical resection and radiofrequency ablation for patients with solitary liver metastases of < 3 cm. However, as surgical resection is associated with significant reduction in quality of life for up to 6 months post-operatively, surgical resection is associated with lower QALY outcome than radiofrequency ablation. The estimated gain in discounted QALYs associated with radiofrequency ablation is 0.06. The ICER is –£266,767 per QALY gained. In conventional terms this would indicate that radiofrequency ablation dominates surgical resection for the treatment of (surgically resectable) small solitary liver metastases.
The cost-effectiveness results appear to be generally robust to variation in parameters included in the deterministic sensitivity analysis, with the majority of ICERs (for radiofrequency ablation compared with surgical resection) remaining negative. In this case negative ICERs indicate reduced costs for radiofrequency ablation compared with surgical resection and small gains in QALY outcomes. In general there are no differences in expected survival (no life-years gained or lost) between radiofrequency ablation and surgical resection, except in the structural sensitivity analysis adopting alternative forms for the survival function and the parameter sensitivity analysis on parameter values in the survival function. The exponential and log-logistic functions are associated with slightly poorer survival with radiofrequency ablation, hence a slightly reduced QALY gain compared with the base case. However, the cost reduction, with radiofrequency ablation compared with surgical resection, is also greater when using the exponential and log-logistic functions of the survival function. In terms of parameter inputs, the results appear to be most sensitive to variation in values of parameters in the survival functions (for treatment effect on overall survival and all parameters in the overall survival function) and variation in the utility estimates applied in the model. The results appear to be relatively insensitive to variation in costs, time horizon and discount rates.
In a PSA radiofrequency ablation is associated with reduced costs in all simulations and increased QALYs for the majority of simulations when compared with surgical resection. Radiofrequency ablation had a probability of being cost-effective of 100% at willingness-to-pay thresholds of £20,000 and £30,000 per QALY gained.
Radiofrequency ablation compared with surgical resection for solitary metastases ≥ 3 cm
Total costs for surgical resection were £18,527 with outcomes of 4.24 life-years and 3.24 QALYs, while for radiofrequency ablation the total costs were £15,050 and outcomes were 2.81 life-years and 1.97 QALYs. The incremental cost for radiofrequency ablation compared with surgical resection is negative (£3207) – a reduction of around 17% in total costs. However, radiofrequency is also associated with significantly poorer outcome – reduced life expectancy and poorer QALY outcomes – than for surgical resection resulting in an ICER of £2538 per QALY gained. However, it should be noted that these positive ICERs are the result of negative incremental cost and incremental QALY values – that is to say, in this analysis radiofrequency ablation is associated with reduced cost but also poorer outcome than surgical resection. In this context the conventional interpretation of cost-effectiveness as being below a given acceptable threshold is reversed. Rather than balancing any increased cost against gains in outcome (as would be typical for interpreting cost-effectiveness results) the decision framework in this case would be to consider whether or not the size of savings (indicated by the lower incremental cost) can justify the reduced outcome.
The cost-effectiveness results appear to be generally robust to variation in parameters included in the deterministic sensitivity analysis, with the majority of ICERs varying between £2000 and £4000. The results appear relatively insensitive to structural or methodological assumptions. In terms of parameter inputs, the results were most sensitive to variation in values of parameters in the survival functions (for treatment effect on overall survival and all parameters in the overall survival function). The results were relatively insensitive to variation in costs other than palliative care costs, where reduction in palliative care costs is associated with an increase in the difference in costs between radiofrequency ablation and surgical resection. However, even at the extreme value (where palliative care costs are excluded from the model) the cost reduction with radiofrequency ablation does not appear to be large enough to make the associated QALY loss, when compared with surgical resection, acceptable for patients with larger solitary liver metastases.
In a PSA, radiofrequency ablation is associated with reduced costs for the vast majority of simulations and reduced QALYs for all of the simulations when compared with surgical resection. Radiofrequency ablation had a probability of being cost-effective of 0% at willingness-to-pay thresholds of £20,000 and £30,000 per QALY gained.
Radioembolisation plus hepatic artery chemotherapy compared with hepatic artery chemotherapy alone
Total costs of hepatic artery chemotherapy alone were £6010 with outcomes of 1.49 life-years and 1.06 QALYs, while for radioembolisation plus hepatic artery chemotherapy the total costs were £18,955 and outcomes were 1.86 life-years and 1.41 QALYs. The incremental cost for radioembolisation plus hepatic artery chemotherapy compared with hepatic artery chemotherapy alone is £12,945 – an increase of around 215% in total costs. Radioembolisation plus hepatic artery chemotherapy is also associated with improved outcomes (0.35 QALY gain) resulting in an ICER of £37,303 per QALY gained.
The cost-effectiveness results appear to be generally robust to variation in the parameters included in the deterministic sensitivity analysis, with ICERs varying between approximately £34,000 and £40,000 per QALY gained. Among the structural sensitivity analyses, the results appear to be most sensitive to assumptions over the functional form for the survival functions. In terms of parameter inputs, the results appear to be most sensitive to variation in utility estimates applied in the model, variation in values of parameters in the survival functions (for treatment effect on overall survival and all parameters in the overall survival function) and to the cost of palliative care.
In a PSA, radioembolisation plus hepatic artery chemotherapy is associated with increased costs in all simulations and increased QALYs for the majority of simulations when compared with hepatic artery chemotherapy alone. In this analysis radioembolisation plus hepatic artery chemotherapy had a probability of being cost-effective of 0.1% at a willingness-to-pay threshold of £20,000 per QALY, 26% at a willingness-to-pay threshold of £30,000 per QALY and 68% at a willingness-to-pay threshold of £50,000 per QALY.
Summary of Southampton Health Technology Assessments Centre economic evaluation
There is a very limited evidence base for conducting economic analysis of ablative therapies and other non-invasive therapies for the treatment of patients with surgically resectable, or unresectable, liver metastases. There is a limited number of comparative studies reporting final outcomes for ablative therapies or other non-invasive therapies, although the comparator (in some cases) or patterns of care may be of limited relevance to current UK clinical practice, the study designs may be prone to bias or study publications may not include sufficient detail to allow robust economic analysis.
Three comparative studies were identified that reported sufficient data to model two ablative therapies (microwave ablation and radiofrequency ablation) and one non-invasive therapy (radioembolisation). Ablative or other non-invasive therapies may be less costly than surgical resection, as a result of shorter lengths of hospital stay and reduced complication rates. The analysis suggests that, where ablative therapies or other non-invasive therapies can achieve similar survival to surgery, they may result in improved outcomes for patients (in terms of QALY gains) by avoiding short- to medium-term reduction in quality of life associated with surgical resection. In this situation, ablative or other non-invasive therapies may dominate surgical resection (by providing a QALY gain at reduced cost) or may offer a cost-saving option (by providing equivalent outcomes at reduced cost). In contrast, where ablative or other non-invasive therapies provide inferior survival, the lower short-term quality-of-life reduction associated with such therapies is not sufficient to yield improved overall outcomes compared with surgical resection. If ablative or other non-invasive therapies are associated with a QALY loss, the potential cost savings, compared with surgical resection, are unlikely to be sufficient to make such treatment acceptable, in cost-effectiveness terms.
Strengths and limitations of the assessment
The evidence synthesis has the following strengths:
-
It is independent of vested interests.
-
It has been undertaken following the principles for conducting systematic reviews and economic evaluations. The methods were set out in a research protocol (see Appendix 1), which defined the research question, study selection criteria, quality assessment criteria, data extraction process and the process by which the methods will be employed at different stages in the systematic review and economic evaluations.
-
An advisory group has informed the evidence synthesis from its initiation. The research protocol and a copy of the draft final report were sent to the advisory group for review and comment.
-
The systematic review brings together the evidence for the clinical effectiveness and cost-effectiveness of ablative and other minimally invasive therapies for treating people with liver metastases. This evidence has been critically appraised and presented in a consistent and transparent manner.
-
A new economic model has been developed following recognised guidelines and systematic searches have been conducted to identify data to populate the different parameters. The main results have been summarised and presented.
In contrast, the evidence synthesis has certain limitations:
-
Despite conducting a wide-ranging and systematic search of the literature, it was evident that the evidence assessing the clinical effectiveness and cost-effectiveness of ablative and other minimally invasive therapies was limited. Although a large number of case series studies were identified, they tended to be retrospective, involve small numbers of participants and present limited details of their methods and results. As a consequence, the evidence synthesis focused on experimental studies and prospective case series involving 100 participants or more. Unfortunately, these studies were often poorly reported in terms of their methods and results.
-
Although non-English-language studies were identified in the searches, translation and full screening of the papers was not undertaken. These studies are listed in Appendix 6.
-
Authors of primary studies were not contacted to request additional information. Although this may have provided helpful data to clarify study selection, assessment of the quality of studies and additional outcome data, it was felt that the considerable information that could be requested would not be feasible within the time constraints of the project.
-
With limited comparative studies identified by the evidence synthesis, the interventions included may not be those currently relevant to current management in the NHS. The six RCTs included in the evidence synthesis compared ablative and other minimally invasive therapies with surgical resection, hepatic arterial chemotherapy and local or systemic chemotherapy.
-
The lack of high-quality comparative evidence on the clinical effectiveness of the different ablative and other minimally invasive techniques limited the opportunities for developing an economic evaluation. As a consequence, the models were limited to a comparison of radioembolisation plus hepatic artery chemotherapy with hepatic artery chemotherapy, radiofrequency ablation compared with surgical resection and microwave ablation compared with surgical resection. These may not be the most appropriate comparisons.
Other relevant factors
-
Given the heterogeneous nature of the studies included in the systematic review of clinical effectiveness, synthesis of the results was difficult. With few comparative studies, differences in participants, outcomes assessed and the interventions used meant that meta-analysis was considered inappropriate. As a result, studies were synthesised narratively with limited conclusions drawn about the clinical effectiveness of the different interventions.
-
It is thought that ablative and other minimally invasive therapies are a possible treatment option for people with liver metastases who currently (i) would undergo surgical resection with curative intent, (ii) are not candidates for surgical resection but would receive chemotherapy or (iii) are receiving palliative treatment with chemotherapy. Unfortunately, the limited extent of the evidence has meant that the majority of the evidence has focused on people who are not candidates for surgical resection.
-
Outcomes reported by the studies assessing clinical effectiveness varied. Although survival was reported by studies, it differed in terms of the measure reported (i.e. mean or median, estimated or actual, overall or disease-free survival), period covered (i.e. from diagnosis or treatment) and the groups of participants included (i.e. all people or subgroups). Measures of recurrence, response, time to progression, quality of life and surgical invasiveness are reported by a limited number of studies, limiting the extent of comparisons that can be made.
Chapter 7 Conclusions
Implications for service provision
Clearly, although much has been defined by this analysis, much remains unresolved and worthy of further investigation (see Suggested research priorities, below). Bearing in mind the increasing incidence of primary hepatocellular carcinoma, which is also treated by surgical resection and liver ablation therapies, and the widening indications for repeated and multiple ablation treatments of colorectal cancer metastases in the liver and at other sites (most notably lung), there may be an increasing unmet demand for the different treatment options assessed in this report. There are only finite numbers of trained hepatobiliary surgeons and interventional radiologists experienced and competent to deliver these treatments, and so it is imperative to anticipate these demands and match them to training and accreditation in these disciplines. Inevitably, the extent and nature of the development of the service will depend on further clarification of the needs for treatment and the comparative effectiveness of the options available for the different patient groups. As this is new and previously undelivered activity, any increase in provision will have a significant financial impact on the demands for NHS resources and time, at a time of increasing austerity within the health care budget.
These are technically challenging and complex technologies, frequently integrated into complex long-term multidisciplinary treatment strategies, and should not be considered as stand-alone ‘one-off’ interventions administered in isolation. Given the specialist nature of these services, it seems appropriate for the provision of ablative therapies to continue through the current cancer network-determined SMDTs and service providers at the designated tertiary hepatobiliary centres across the NHS. The development of the service will need continued review to ensure that the extent and quality of provision is appropriate to the needs identified.
Suggested research priorities
The systematic review has shown limited good-quality evidence assessing the clinical effectiveness and the cost-effectiveness of the different ablative and other minimally invasive therapies for treating liver metastases. As a consequence, it has proved difficult to differentiate between the different therapies in their ability to improve survival, time to progression, response to treatment, recurrence rates, quality of life, and adverse events. Although comparative evidence has shown some benefit from radioembolisation and chemoembolisation when compared with different forms of chemotherapy on measures of survival, response and quality of life, results were not unequivocal. Similarly, the experimental and observational evidence on the ablative therapies provides limited evidence to judge the effectiveness of these technologies relative to other options. Studies focused on people who were not deemed suitable candidates for surgical resection. The studies did not consider other groups, such as people suitable for surgical resection or people with disease progression following conventional management. Furthermore, there is little upon which to base a judgement of the effectiveness of ablative therapies on metastases arising from different primary cancers, despite it being thought that different cancers, and even different subtypes of the same cancer, have different patterns of metastasis. Lastly, as with all emerging technologies, analysis of published literature can report only work that has been completed, and will under-report ongoing research and that which is still in its planning stages.
The lack of evidence on the benefits and costs of the different ablative and minimally invasive therapies rendered the economic evaluation limited in its scope and speculative, with many uncertainties remaining. Although results for the comparison of radioembolisation plus hepatic artery chemotherapy with hepatic artery chemotherapy (£37,303 per QALY) and microwave ablation compared with surgical resection (£4681 per QALY) are presented, it is unclear whether or not these are entirely relevant to the NHS. The limited evidence base and the assumptions required mean that the analysis should be considered illustrative. It does not provide evidence for recommendations for future practice.
The evidence synthesis has revealed the limited evidence available to assess the clinical effectiveness and cost-effectiveness of the different ablative and other minimally invasive technologies. It will be important to undertake further primary research to provide comparative evidence on the different interventions that provide alternative treatment options for people at various stages in the management of their liver metastases.
Given the lack of an ideal preference-based measure for this population and the limited evidence for mapping the cancer-specific, non-preference based EORTC QLQ-C30 into the generic preference-based EQ-5D, both tools (EQ-5D and EORTC QLQ-C30, supplemented with EORTC QLQ-LMC21, preferably) should be used in future studies. Using both tools would enable capturing clinically relevant outcomes as well as those relevant to subsequent economic evaluations in a UK context.
Further evidence on the variation of quality of life of patients with liver metastases along disease progression and according to different prognostic factors (such as number and size of metastases or hepatic volume replaced by tumour, and percentage of tumour removed) could enable the assessment of the cost-effectiveness of ablative therapies for relevant patient subgroups, provided that effectiveness estimates for those subgroups were available as well.
Although studies could include those who would undergo such procedures as part of their palliative care, it would appear more beneficial to focus on people who are receiving treatment with curative intent. This would include people who are and are not suitable for surgical resection. With most studies included in this evidence synthesis being non-comparative studies of people who are not candidates for surgical resection and only one comparative study of a microwave ablation compared with surgical resection, both treatments require further research. Criteria for suitability for inclusion in a trial would also include number of lesions identified. A local recurrence rate of 6–7% following surgical resection will stay the same regardless of whether there is one lesion or three separate lesions (provided, of course, that all three are in one lobe of the liver), whereas for radiofrequency ablation there would be a degree of multiplication of risk of local recurrence by the number of lesions treated.
Several interventions could be included in any study undertaken, but they should be those that are considered most relevant to the NHS in the UK. Of the different ablative and minimally invasive techniques considered by this evidence synthesis, radiofrequency ablation, microwave ablation, chemoembolisation and radioembolisation appear to have received most research and to be considered relevant. Inevitably, opinions may differ as to which of these interventions or the other options available should be prioritised for further research. Although all four could be studied, comparative experimental evidence assessing chemoembolisation and radioembolisation has been published, and there are ongoing studies of chemoembolisation (e.g. the FOXFIRE trial). However, limited evidence is available on radiofrequency, microwave or other forms of ablative therapy. Ideally, given the uncertainty that remains as regards their effectiveness in treating people who are and are not candidates for surgical resection, both groups should be included and the comparator technologies should include surgical resection and chemotherapy. However, pragmatic thought should be applied to issues arising from study design, including the ability of a trial to recruit subjects.
Liver resection is an effective treatment for localised colorectal liver metastases. 15 Liver resection is also now a very safe procedure with perioperative mortalities of around 1%, and indeed in patients with limited disease (those also most suitable for ablation) the risks may be even lower. The advent of laparoscopic resection further increases the acceptability of surgical resection over other modalities. It thus becomes difficult to argue ethically that a trial of liver resection versus an alternative technique is needed in most patients, and it would be difficult in patients with a certain level of fitness to recruit any to a trial without concealing some of the facts regarding liver resection. Certainly this is the situation in colorectal cancer, although the situation in other cancers (e.g. breast) may be different.
There is a group of patients, however, in whom the risk and benefits are less clear. These patients are elderly or they have significant comorbidities which pose significant risk from a surgical procedure. This is a group in which there is equipoise regarding treatment and many such patients may already be offered ablation rather than surgical resection.
Summary
Any study should assess the clinical effectiveness and cost-effectiveness of the different techniques, assessing measures of survival, response, recurrence, quality of life, adverse events, and costs. Outcomes should be reported separately for the different groups of participants. A RCT would provide the most appropriate design for undertaking the evaluation and should include a full economic evaluation, but the group to be randomised needs careful selection.
Acknowledgements
We would like to thank members of our advisory group panel who provided expert advice and comments on the protocol and/or draft of this report:
Professor O James Garden, Regius Professor of Clinical Surgery, Clinical Surgery, University of Edinburgh, Royal Infirmary, Edinburgh, UK.
Dr Gail ter Haar, Reader in Physics, Division of Radiotherapy and Imaging, Royal Marsden, Sutton, UK.
Dr Paul Tappenden, Senior Research Fellow, HEDS, ScHARR, University of Sheffield, Sheffield, UK.
We are also grateful to Karen Welch, Information Specialist (SHTAC, University of Southampton), for conducting the searches and Dr Jonathan Shepherd, Principal Research Fellow (SHTAC, University of Southampton) for reviewing a draft of this report.
Contribution of authors
Emma Loveman (Senior Research Fellow) managed the systematic review of clinical effectiveness, developed the research protocol, assisted in the development of the search strategy, assessed studies for inclusion, extracted data from and quality assessed included studies, synthesised evidence, and drafted and edited the final report.
Jeremy Jones (Principal Research Fellow) developed the original research grant application, developed the research protocol, assisted in the development of the search strategy, assessed studies for inclusion for the economic evaluation, extracted data from and quality assessed included studies for the economic evaluation, undertook the economic analyses, and drafted and edited the final report.
Andrew J Clegg (Professor/Director of SHTAC) developed the original research grant application, project managed the research project, assisted in the development of the search strategy, developed the research protocol, assessed studies for inclusion, extracted data from and quality assessed included studies, synthesised evidence, and drafted and edited the final report.
Joanna Picot (Research Fellow) developed the research protocol, assisted in the development of the search strategy, assessed studies for inclusion, extracted data from and quality assessed included studies, synthesised evidence, and drafted the report.
Jillian Colquitt (Senior Research Fellow) developed the research protocol, assisted in the development of the search strategy, assessed studies for inclusion, extracted data from and quality assessed included studies, synthesised evidence, and drafted the report.
Diana Mendes (Research Fellow) developed the research protocol, assisted in the development of the search strategy, assessed studies for inclusion, extracted data from and quality assessed included studies for the economic evaluation, undertook the economic analyses, and drafted the report.
David Breen (Consultant Radiologist) developed the original research grant application, developed the research protocol, and drafted and edited the final report.
Emily Moore (Operations Director, University of Southampton Clinical Trials Unit) developed the research protocol and drafted the final report.
Steve George (Reader in Public Health) developed the original research grant application, developed the research protocol, and drafted and edited the final report.
Graeme Poston (Professor of Surgery) developed the original original research grant application, developed the research protocol, and drafted and edited the final report research grant application, developed the research protocol, and drafted and edited the final report.
David Cunningham (Professor of Oncology) developed the original research grant application, developed the research protocol, and drafted and edited the final report.
Theo Ruers (Professor of Oncology) developed the original research grant application, developed the research protocol, and drafted and edited the final report.
John Primrose (Professor of Surgery) developed the original research grant application, developed the research protocol, and drafted and edited the final report.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Poste G, Fidler IJ. The pathogenesis of cancer metastasis. Nature 1980;283:139-46. http://dx.doi.org/10.1038/283139a0.
- Pritchard KI. Liver metastases: can our understanding of their biology and prognostic value contribute to a strategy for optimum therapeutic management?. Eur J Cancer 1997;33:S11-14. http://dx.doi.org/10.1016/S0959-8049(97)90003-4.
- Kasper HU, Drebber U, Dries V, Dienes HP. Liver metastases: incidence and histogenesis. Z Gastroenterol 2005;43:1149-57.
- Khan AN, MacDonald S, Pankhania A, Sherlock D. Liver Metastases n.d. http://emedicine.medscape.com/article/369936-overview (accessed 7 February 2011).
- Stangl R, Altendorf-Hofmann A, Charnley RM, Scheele J. Factors influencing the natural history of colorectal liver metastases. Lancet 1994;343:1405-10. http://dx.doi.org/10.1016/S0140-6736(94)92529-1.
- Simmonds PC. Palliative chemotherapy for advanced colorectal cancer: systematic review and meta-analysis. Colorectal Cancer Collaborative Group. BMJ 2000;321:531-5.
- Warrell DA. Oxford Textbook of Medicine. Oxford: Oxford University Press; 2003.
- Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg 2006;244:254-9. http://dx.doi.org/10.1097/01.sla.0000217629.94941.cf.
- Leporrier J, Maurel J, Chiche L, Bara S, Segol P, Launoy G. A population-based study of the incidence, management and prognosis of hepatic metastases from colorectal cancer. Br J Surg 2006;93:465-74. http://dx.doi.org/10.1002/bjs.5278.
- Kune GA, Kune S, Field B, White R, Brough W, Schellenberger R, et al. Survival in patients with large-bowel cancer. A population-based investigation from the Melbourne Colorectal Cancer Study. Dis Colon Rectum 1990;33:938-46. http://dx.doi.org/10.1007/BF02139103.
- Alley PG, McNee RK. Colorectal cancer in Auckland 1981–1982: patients with liver metastases. N Z Med J 1985;98:697-9.
- Primrose JN, Fuller A, Rose P, Perera-Salazar R, Mellor J, Corkhill A, et al. Follow-up after colorectal cancer surgery: preliminary observational findings from the UK FACS trial. ASCO Meeting Abstracts 2011;29.
- Bowel (colorectal) cancer UK incidence statistics. London: Cancer Research UK; 2011.
- Ruers TJ, Joosten JJ, Wiering B, Langenhoff BS, Dekker HM, Wobbes T, et al. Comparison between local ablative therapy and chemotherapy for non-resectable colorectal liver metastases: a prospective study. Ann Surg Oncol 2007;14:1161-9. http://dx.doi.org/10.1245/s10434-006-9312-5.
- Simmonds PC, Primrose JN, Colquitt JL, Garden OJ, Poston GJ, Rees M. Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br J Cancer 2006;94:982-99. http://dx.doi.org/10.1038/sj.bjc.6603033.
- Kanas GP, Taylor A, Primrose JN, Langeberg WJ, Kelsh MA, Mowat FS, et al. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Br J Cancer 2012;4:283-301.
- Garden OJ, Rees M, Poston GJ, Mirza D, Saunders M, Ledermann J, et al. Guidelines for resection of colorectal cancer liver metastases. Gut 2006;55:iii1-8. http://dx.doi.org/10.1136/gut.2006.098053.
- Oxford English Dictionary. Oxford: Oxford University Press; 2011.
- Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD, Dupuy DE, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. Radiology 2005;235:728-39. http://dx.doi.org/10.1148/radiol.2353042205.
- Evans J. Ablative and catheter-delivered therapies for colorectal liver metastases (CRLM). Eur J Surg Oncol 2007;33:S64-75. http://dx.doi.org/10.1016/j.ejso.2007.09.027.
- Brown DB, Gould JE, Gervais DA, Goldberg SN, Murthy R, Millward SF, et al. Transcatheter therapy for hepatic malignancy: standardization of terminology and reporting criteria. J Vasc Interv Radiol 2007;18:1469-78. http://dx.doi.org/10.1016/j.jvir.2007.08.027.
- Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD, Dupuy DE, et al. Image-guided tumor ablation: Standardization of terminology and reporting criteria. J Vasc Interv Radiol 2005;16:765-78. http://dx.doi.org/10.1148/radiol.2353042205.
- Cho YK, Kim JK, Kim MY, Rhim H, Han JK. Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies. Hepatology 2009;49:453-9. http://dx.doi.org/10.1002/hep.22648.
- Electrolytic Ablation of Tumours. North Adelaide, SA: ASERNIP-S; 2002.
- Gravante G, Ong SL, Metcalfe MS, Bhardwaj N, Maddern GJ, Lloyd DM, et al. Experimental application of electrolysis in the treatment of liver and pancreatic tumours: principles, preclinical and clinical observations and future perspectives. Surg Oncol 2011;20:106-20. http://dx.doi.org/10.1016/j.suronc.2009.12.002.
- Bhardwaj N, Strickland AD, Ahmad F, Dennison AR, Lloyd DM. Liver ablation techniques: a review. Surg Endosc 2010;24:254-65. http://dx.doi.org/10.1007/s00464-009-0590-4.
- Leen E, Horgan PG. Radiofrequency ablation of colorectal liver metastases. Surg Oncol 2007;16:47-51. http://dx.doi.org/10.1016/j.suronc.2007.04.004.
- Cryotherapy for the treatment of liver metastases: IPG369. London: National Institute for Health and Care Excellence; 2010.
- Dodd GD, Soulen MC, Kane RA, Livraghi T, Lees WR, Yamashita Y, et al. Minimally invasive treatment of malignant hepatic tumors: at the threshold of a major breakthrough. Radiographics 2000;20:9-27.
- Vogl TJ, Muller PK, Hammerstingl R, Weinhold N, Mack MG, Philipp C, et al. Malignant liver tumors treated with MR imaging-guided laser-induced thermotherapy: technique and prospective results. Radiology 1995;196:257-65.
- Gillams AR, Lees WR. Survival after percutaneous, image-guided, thermal ablation of hepatic metastases from colorectal cancer. Dis Colon Rectum 2000;43:656-61. http://dx.doi.org/10.1007/BF02235582.
- Pacella CM, Valle D, Bizzarri G, Pacella S, Brunetti M, Maritati R, et al. Percutaneous laser ablation in patients with isolated unresectable liver metastases from colorectal cancer: results of a phase II study. Acta Oncol 2006;45:77-83. http://dx.doi.org/10.1080/02841860500438029.
- Snoeren N, Voest EE, Bergman AM, Dalesio O, Verheul HM, Tollenaar RA, et al. A randomized two arm phase III study in patients post radical resection of liver metastases of colorectal cancer to investigate bevacizumab in combination with capecitabine plus oxaliplatin (CAPOX) vs CAPOX alone as adjuvant treatment. BMC Cancer 2010;10. http://dx.doi.org/10.1186/1471-2407-10-545.
- Vogl TJ, Mack MG, Eichler K, Zangos S, Naguib NN, Gruber-Rouh T. Chemoperfusion and embolization in the treatment of liver metastases. Rofo 2011;183:12-23.
- Vogl TJ, Mack MG, Muller PK, Straub R, Engelmann K, Eichler K. Interventional MR: interstitial therapy. Eur Radiol 1999;9:1479-87. http://dx.doi.org/10.1007/s003300050874.
- Microwave ablation for the treatment of liver metastases: IPG406. London: National Institute for Health and Care Excellence; 2011.
- Lloyd DM, Lau KN, Welsh F, Lee KF, Sherlock DJ, Choti MA, et al. International multicentre prospective study on microwave ablation of liver tumours: preliminary results. HPB 2011;13:579-85. http://dx.doi.org/10.1111/j.1477-2574.2011.00338.x.
- Wong SL, Mangu PB, Choti MA, Crocenzi TS, Dodd GD, . American Society of Clinical Oncology 2009 clinical evidence review on radiofrequency ablation of hepatic metastases from colorectal cancer. J Clin Oncol 2010;28:493-508. http://dx.doi.org/10.1200/JCO.2009.23.4450.
- Razafindratsira T, Isambert M, Evrard S. Complications of intraoperative radiofrequency ablation of liver metastases. HPB 2011;13:15-23. http://dx.doi.org/10.1111/j.1477-2574.2010.00243.x.
- Radiofrequency ablation for the treatment of colorectal metastases in the liver – guidance: IPG092. London: National Institute for Health and Care Excellence; 2004.
- ter Haar G, Coussios C. High intensity focused ultrasound: physical principles and devices. Int J Hyperthermia 2007;23:89-104. http://dx.doi.org/10.1080/02656730601186138.
- Illing RO, Kennedy JE, Wu F, ter Haar GR, Protheroe AS, Friend PJ, et al. The safety and feasibility of extracorporeal high-intensity focused ultrasound (HIFU) for the treatment of liver and kidney tumours in a Western population. Br J Cancer 2005;93:890-5. http://dx.doi.org/10.1038/sj.bjc.6602803.
- Castells A, Bruix J, Bru C, Fuster J, Vilana R, Navasa M, et al. Treatment of small hepatocellular carcinoma in cirrhotic patients: a cohort study comparing surgical resection and percutaneous ethanol injection. Hepatology 1993;18:1121-6. http://dx.doi.org/10.1016/0270-9139(93)90467-2.
- Livraghi T, Bolondi L, Buscarini L, Cottone M, Mazziotti A, Morabito A, et al. No treatment, resection and ethanol injection in hepatocellular carcinoma: a retrospective analysis of survival in 391 patients with cirrhosis. Italian Cooperative HCC Study Group. J Hepatol 1995;22:522-6.
- Tam KY, Leung KC, Wang YX. Chemoembolization agents for cancer treatment. Eur J Pharm Sci 2011;44:1-10. http://dx.doi.org/10.1016/j.ejps.2011.06.013.
- Liapi E, Geschwind JF. Transcatheter arterial chemoembolization for liver cancer: is it time to distinguish conventional from drug-eluting chemoembolization?. Cardiovasc Intervent Radiol 2011;34:37-49. http://dx.doi.org/10.1007/s00270-010-0012-y.
- Carter S, Martin Ii RC. Drug-eluting bead therapy in primary and metastatic disease of the liver. HPB (Oxford) 2009;11:541-50. http://dx.doi.org/10.1111/j.1477-2574.2009.00071.x.
- Nicolay NH, Berry DP, Sharma RA. Liver metastases from colorectal cancer: radioembolization with systemic therapy. Nat Rev Clin Oncol 2009;6:687-97. http://dx.doi.org/10.1038/nrclinonc.2009.165.
- TheraSphere. A Patient’s Guide. Mont Saint-Guibert: Nordion; 2011.
- Sirtex Medical Limited . SIRTeX SIR-Spheres Microspheres 2010.
- Vente MA, Wondergem M, van der Tweel I, van den Bosch MA, Zonnenberg BA, Lam MG, et al. Yttrium-90 microsphere radioembolization for the treatment of liver malignancies: a structured meta-analysis. Eur Radiol 2009;19:951-9. http://dx.doi.org/10.1007/s00330-008-1211-7.
- Cabanillas M, Perez-Perez L, Sanchez-Aguilar D, Fernandez-Redondo V, Toribio J. Acrokeratosis paraneoplastica with bullous lesions associated with esophageal squamous cell carcinoma. Actas Dermosifiliogr 2006;97:196-9.
- Folprecht G, Grothey A, Alberts S, Raab HR, Kohne CH. Neoadjuvant treatment of unresectable colorectal liver metastases: correlation between tumour response and resection rates. Ann Oncol 2005;16:1311-19. http://dx.doi.org/10.1093/annonc/mdi246.
- Jonker DJ, Maroun JA, Kocha W. Survival benefit of chemotherapy in metastatic colorectal cancer: a meta-analysis of randomized controlled trials. Br J Cancer 2000;82:1789-94. http://dx.doi.org/10.1054/bjoc.1999.1254.
- Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet 2008;371:1007-16. http://dx.doi.org/10.1016/S0140-6736(08)60455-9.
- Fahy BN, Aloia TA, Jones SL, Bass BL, Fischer CP. Chemotherapy within 30 days prior to liver resection does not increase postoperative morbidity or mortality. HPB 2009;11:645-55. http://dx.doi.org/10.1111/j.1477-2574.2009.00107.x.
- Farges O, Belghiti J, Kianmanesh R, Regimbeau JM, Santoro R, Vilgrain V, et al. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg 2003;237:208-17. http://dx.doi.org/10.1097/01.SLA.0000048447.16651.7B.
- Adam R, Hagopian EJ, Linhares M, Krissat J, Savier E, Azoulay D, et al. A comparison of percutaneous cryosurgery and percutaneous radiofrequency for unresectable hepatic malignancies. Arch Surg 2002;137:1332-9. http://dx.doi.org/10.1001/archsurg.137.12.1332.
- Adam R, Laurent A, Azoulay D, Castaing D, Bismuth H. Two-stage hepatectomy: a planned strategy to treat irresectable liver tumors. Ann Surg 2000;232:777-85. http://dx.doi.org/10.1097/00000658-200012000-00006.
- Neeleman N, Andersson R. Repeated liver resection for recurrent liver cancer. Br J Surg 1996;83:893-901. http://dx.doi.org/10.1002/bjs.1800830705.
- Cetuximab for the first-line treatment of metastatic colorectal cancer: TA176. London: National Institute for Health and Care Excellence; 2009.
- Colorectal cancer: the diagnosis and management of colorectal cancer: CG131. London: National Institute for Health and Care Excellence; 2011.
- Ahmed N, Ahmedzai S, Vora V, Hillam S, Paz S. Supportive care for patients with gastrointestinal cancer. Cochrane Database Syst Rev 2004;3. http://dx.doi.org/10.1002/14651858.CD003445.
- Liu LX, Zhang WH, Jiang HC. Current treatment for liver metastases from colorectal cancer. World J Gastroenterol 2003;9:193-200.
- Systematic reviews: CRD’s guidance for undertaking reviews in health care. York: CRC, University of York; 2009.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6. http://dx.doi.org/10.1371/journal.pmed.1000097.
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ 1996;313:275-83. http://dx.doi.org/10.1136/bmj.313.7052.275.
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8. http://dx.doi.org/10.2165/00019053-200624040-00006.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2009.
- Mack MG, Straub R, Eichler K, Engelmann K, Zangos S, Roggan A, et al. Percutaneous MR imaging-guided laser-induced thermotherapy of hepatic metastases. Abdom Imaging 2001;26:369-74. http://dx.doi.org/10.1007/s002610000197.
- Mack MG, Straub R, Eichler K, Sollner O, Lehnert T, Vogl TJ. Breast cancer metastases in liver: laser-induced interstitial thermotherapy – local tumor control rate and survival data. Radiology 2004;233:400-9. http://dx.doi.org/10.1148/radiol.2332030454.
- Shibata T, Niinobu T, Ogata N, Takami M. Microwave coagulation therapy for multiple hepatic metastases from colorectal carcinoma. Cancer 2000;89:276-84. http://dx.doi.org/10.1002/1097-0142(20000715)89:2<276::AID-CNCR11>3.3.CO;2-S.
- Kim KH, Yoon YS, Yu CS, Kim TW, Kim HJ, Kim PN, et al. Comparative analysis of radiofrequency ablation and surgical resection for colorectal liver metastases. J Korean Surg Soc 2011;81:25-34. http://dx.doi.org/10.4174/jkss.2011.81.1.25.
- Berber E, Pelley R, Siperstein AE. Predictors of survival after radiofrequency thermal ablation of colorectal cancer metastases to the liver: a prospective study. J Clin Oncol 2005;23:1358-64. http://dx.doi.org/10.1200/JCO.2005.12.039.
- Berber E, Siperstein A. Local recurrence after laparoscopic radiofrequency ablation of liver tumors: an analysis of 1032 tumors. Ann Surg Oncol 2008;15:2757-64. http://dx.doi.org/10.1245/s10434-008-0043-7.
- Gillams AR, Lees WR. Radio-frequency ablation of colorectal liver metastases in 167 patients. Eur Radiol 2004;14:2261-7. http://dx.doi.org/10.1007/s00330-004-2416-z.
- Gillams AR, Lees WR. Five-year survival in 309 patients with colorectal liver metastases treated with radiofrequency ablation. Eur Radiol 2009;19:1206-13. http://dx.doi.org/10.1007/s00330-008-1258-5.
- Siperstein AE, Berber E, Ballem N, Parikh RT. Survival after radiofrequency ablation of colorectal liver metastases: 10-year experience. Ann Surg 2007;246:559-65. http://dx.doi.org/10.1097/SLA.0b013e318155a7b6.
- Solbiati L, Livraghi T, Goldberg SN, Ierace T, Meloni F, Dellanoce M, et al. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology 2001;221:159-66. http://dx.doi.org/10.1148/radiol.2211001624.
- Sorensen SM, Mortensen FV, Nielsen DT. Radiofrequency ablation of colorectal liver metastases: long-term survival. Acta Radiol 2007;48:253-8. http://dx.doi.org/10.1080/02841850601161539.
- Taguchi T, Ogawa N, Bunke B, Nilsson B. The use of degradable starch microspheres (Spherex) with intra-arterial chemotherapy for the treatment of primary and secondary liver tumours – results of a phase III clinical trial. Regional Cancer Treatment 1992;4:161-5.
- Agarwala SS, Panikkar R, Kirkwood JM. Phase I/II randomized trial of intrahepatic arterial infusion chemotherapy with cisplatin and chemoembolization with cisplatin and polyvinyl sponge in patients with ocular melanoma metastatic to the liver. Melanoma Res 2004;14:217-22. http://dx.doi.org/10.1097/01.cmr.0000129377.22141.ea.
- Vogl TJ, Mack MG, Balzer JO, Engelmann K, Straub R, Eichler K, et al. Liver metastases: neoadjuvant downsizing with transarterial chemoembolization before laser-induced thermotherapy. Radiology 2003;229:457-64. http://dx.doi.org/10.1148/radiol.2292021329.
- Vogl TJ, Naguib NN, Nour-Eldin NE, Mack MG, Zangos S, Abskharon JE, et al. Repeated chemoembolization followed by laser-induced thermotherapy for liver metastasis of breast cancer. AJR Am J Roentgenol 2011;196:W66-72. http://dx.doi.org/10.2214/AJR.09.3836.
- Gray B, Van Hazel G, Hope M, Burton M, Moroz P, Anderson J, et al. Randomised trial of SIR-Spheres plus chemotherapy vs. chemotherapy alone for treating patients with liver metastases from primary large bowel cancer. Ann Oncol 2001;12:1711-20.
- Hendlisz A, Van den Eynde M, Peeters M, Maleux G, Lambert B, Vannoote J, et al. Phase III trial comparing protracted intravenous fluorouracil infusion alone or with yttrium-90 resin microspheres radioembolization for liver-limited metastatic colorectal cancer refractory to standard chemotherapy. J Clin Oncol 2010;28:3687-94. http://dx.doi.org/10.1200/JCO.2010.28.5643.
- Van Hazel G, Blackwell A, Anderson J, Price D, Moroz P, Bower G, et al. Randomised phase 2 trial of SIR-Spheres plus fluorouracil/leucovorin chemotherapy versus fluorouracil/leucovorin chemotherapy alone in advanced colorectal cancer. J Surg Oncol 2004;88:78-85. http://dx.doi.org/10.1002/jso.20141.
- Van Hazel GA, Turner D, Gebski V. Impact of Y-90 Resin Microspheres on Health-Related Quality of Life (HRQoL) in Patients With Colorectal Cancer (CRC) Liver Metastases Receiving First-Line Chemotherapy 2009. http://meetinglibrary.asco.org/content/10692-63.
- Schipper H, Clinch J, McMurray A, Levitt M. Measuring the quality of life of cancer patients: the Functional Living Index-Cancer: development and validation. J Clin Oncol 1984;2:472-83.
- Spitzer WO, Dobson AJ, Hall J, Chesterman E, Levi J, Shepherd R, et al. Measuring the quality of life of cancer patients: a concise QL-index for use by physicians. J Chronic Dis 1981;34:585-97.
- Ruers T, Van Coevorden F, Pierie J, Rinkes IB, Punt C, Lederman J, et al. Radiofrequency ablation (RFA) combined with chemotherapy for unresectable colorectal liver metastases (CRC LM): interim results of a randomised phase II study of the EORTC-NCRI CCSG-ALM Intergroup 40004 (CLOCC). Ann Oncol 2010;21:I41-I2.
- Ruers T, Van Coevorden F, Pierie J, Borel-Rinkes I, Punt C, Ledermann J, et al. Radiofrequency ablation combined with chemotherapy for unresectable CRC liver metastases: interim results of a randomised phase II study (CLOCC) (EORTC-NCRI CCSG-ALM). Ann Oncol 2009;20.
- Ruers T, Van Coevorden F, Pierie J, Borel-Rinkes I, Punt C, Ledermann J, et al. Radiofrequency ablation (RFA) combined with chemotherapy for unresectable colorectal liver metastases (CRC LM): Interim results of a randomised phase II study of the EORTC-NCRI CCSG-ALM Intergroup 40004 (CLOCC). Ann Surg Oncol 2009;16.
- Ruers T, Punt CJ, Van Coevorden F, Borel-Rinkes I, Ledermann J, Poston GJ, et al. Final results of the EORTC intergroup randomized study 40004 (CLOCC) evaluating the benefit of radiofrequency ablation (RFA) combined with chemotherapy for unresectable colorectal liver metastases (CRC LM). J Clin Oncol 2010;28.
- Hirata M, Suzuki N, Miura Y, Katayama A, Tanaka Y, Tanaka K, et al. Effect of radio frequency ablation therapy for liver metastasis from colorectal cancer n.d.
- Van Den Eynde M, Hendlisz A, Peeters M, Defreyne L, Maleux G, Vannoote J, et al. Prospective randomized study comparing hepatic intra-arterial injection of Yttrium-90 resin-microspheres (HAI-Y90) with protracted IV 5FU (5FU CI) versus 5FU CI alone for patients with liver-limited metastatic colorectal cancer (LMCRC) refractory to standard chemotherapy (CT). J Clin Oncol 2009;27.
- Fiorentini G, Aliberti C, Montagnani F, Tilli M, Mambrini A, Benea G. Trans-arterial chemoembolization of metastatic colorectal carcinoma (MCRC) to the liver adopting polyvinyl alcohol microspheres (PAM) loaded with irinotecan compared with Folfiri (CT): evaluation at two years of a phase III clinical trial. Ann Oncol 2010;21.
- Fiorentini G, Aliberti C, Montagnani F, Tilli M, Mambrini A, Giannessi P, et al. First evaluation of phase III trial of tace adopting polyvinyl-alcohol microspheres (PAM) irinotecan (IRI) loaded vs Folfiri (CT) for non operable colorectal cancer (CRC) liver metastases. Ann Oncol 2009;20.
- Jones R, Tang J, Pathak S, Parmar C, Fenwick S, Malik H, et al. Systematic review of ablation therapies for the treatment of unresectable colorectal liver metastases n.d.
- Pathak S, Jones R, Tang JM, Parmar C, Fenwick S, Malik H, et al. Ablative therapies for colorectal liver metastases: a systematic review. Colorectal Dis 2011;13:e252-65. http://dx.doi.org/10.1111/j.1463-1318.2011.02695.x.
- Radiofrequency Ablation of Liver Tumours (Update and Re-appraisal): A Systematic Review. Report no. North Adelaide, SA: ASERNIP-S; 2006.
- Radiofrequency Ablation of Liver Tumours. Canberra, ACT: Commonwealth Department of Health and Ageing; 2003.
- Stang A, Fischbach R, Teichmann W, Bokemeyer C, Braumann D. A systematic review on the clinical benefit and role of radiofrequency ablation as treatment of colorectal liver metastases. Eur J Cancer 2009;45:1748-56. http://dx.doi.org/10.1016/j.ejca.2009.03.012.
- Sutherland LM, Williams JA, Padbury RT, Gotley DC, Stokes B, Maddern GJ. Radiofrequency ablation of liver tumors: a systematic review. Arch Surg 2006;141:181-90. http://dx.doi.org/10.1001/archsurg.141.2.181.
- Selective Internal Radiation Therapy for Hepatic Metastases using SIR-Spheres. Canberra, ACT: Commonwealth Department of Health and Ageing; 2002.
- Bergenfeldt M, Jensen BV, Skjoldbye B, Nielsen D. Liver resection and local ablation of breast cancer liver metastases – a systematic review. Eur J Surg Oncol 2011;37:549-57. http://dx.doi.org/10.1016/j.ejso.2011.04.013.
- Smith MD, McCall JL. Systematic review of tumour number and outcome after radical treatment of colorectal liver metastases. Br J Surg 2009;96:1101-13. http://dx.doi.org/10.1002/bjs.6735.
- Gurusamy KS, Ramamoorthy R, Sharma D, Davidson BR. Liver resection versus other treatments for neuroendocrine tumours in patients with resectable liver metastases. Cochrane Database Syst Rev 2009;2. http://dx.doi.org/10.1002/14651858.CD007060.pub2.
- Gurusamy KS, Ramamoorthy R, Imber C, Davidson BR. Surgical resection versus non-surgical treatment for hepatic node positive patients with colorectal liver metastases. Cochrane Database Syst Rev 2010;1. http://dx.doi.org/10.1002/14651858.CD006797.
- Al-Asfoor A, Fedorowicz Z, Lodge M. Resection versus no intervention or other surgical interventions for colorectal cancer liver metastases. Cochrane Database Syst Rev 2008;1. http://dx.doi.org/10.1002/14651858.CD006039.pub3.
- McKay A, Kutnikoff T, Taylor M. A cost–utility analysis of treatments for malignant liver tumours: a pilot project. HPB (Oxford) 2007;9:42-51. http://dx.doi.org/10.1080/13651820600994541.
- Shetty SK, Rosen MP, Raptopoulos V, Goldberg SN. Cost-effectiveness of percutaneous radiofrequency ablation for malignant hepatic neoplasms. J Vasc Interv Radiol 2001;12:823-33. http://dx.doi.org/10.1016/S1051-0443(07)61507-3.
- Bonastre J, De Baere T, Elias D, Evrard S, Rouanet P, Bazin C, et al. Cost of radiofrequency ablation in the treatment of hepatic malignancies. Gastroenterol Clin Biol 2007;31:828-35. http://dx.doi.org/10.1016/S0399-8320(07)73973-8.
- Grundmann RT. Liver metastases of colorectal carcinoma – may the DRG-system influence the operative procedure?. Zentralbl Chir 2007;132:287-92.
- Abramson RG, Rosen MP, Perry LJ, Brophy DP, Raeburn SL, Stuart KE. Cost-effectiveness of hepatic arterial chemoembolization for colorectal liver metastases refractory to systemic chemotherapy. Radiology 2000;216:485-91.
- Gazelle GS, McMahon PM, Beinfeld MT, Halpern EF, Weinstein MC. Metastatic colorectal carcinoma: cost-effectiveness of percutaneous radiofrequency ablation versus that of hepatic resection. Radiology 2004;233:729-39. http://dx.doi.org/10.1148/radiol.2333032052.
- Guide to the methods of technology appraisal. London: National Institute for Health and Care Excellence; 2008.
- Gazelle GS, Hunink MG, Kuntz KM, McMahon PM, Halpern EF, Beinfeld M, et al. Cost-effectiveness of hepatic metastasectomy in patients with metastatic colorectal carcinoma: a state-transition Monte Carlo decision analysis. Ann Surg 2003;237:544-55. http://dx.doi.org/10.1097/01.SLA.0000059989.55280.33.
- Fryback DG, Dasbach EJ, Klein R, Klein BE, Dorn N, Peterson K, et al. The Beaver Dam Health Outcomes Study: initial catalog of health-state quality factors. Med Decis Making 1993;13:89-102. http://dx.doi.org/10.1177/0272989X9301300202.
- Allen-Mersh TG, Earlam S, Fordy C, Abrams K, Houghton J. Quality of life and survival with continuous hepatic-artery floxuridine infusion for colorectal liver metastases. Lancet 1994;344:1255-60. http://dx.doi.org/10.1016/S0140-6736(94)90750-1.
- Earlam S, Glover C, Fordy C, Burke D, Allen-Mersh TG. Relation between tumor size, quality of life, and survival in patients with colorectal liver metastases. J Clin Oncol 1996;14:171-5.
- Taplin SH, Barlow W, Urban N, Mandelson MT, Timlin DJ, Ichikawa L, et al. Stage, age, comorbidity, and direct costs of colon, prostate, and breast cancer care. J Natl Cancer Inst 1995;87:417-26. http://dx.doi.org/10.1093/jnci/87.6.417.
- Burgdorf SK. Dendritic cell vaccination of patients with metastatic colorectal cancer. Dan Med Bull 2010;57.
- Chau I, Norman AR, Cunningham D, Waters JS, Oates J, Ross PJ. Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer – pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J Clin Oncol 2004;22:2395-403. http://dx.doi.org/10.1200/JCO.2004.08.154.
- Coates A, Thomson D, McLeod GR, Hersey P, Gill PG, Olver IN, et al. Prognostic value of quality of life scores in a trial of chemotherapy with or without interferon in patients with metastatic malignant melanoma. Eur J Cancer 1993;29A:1731-4. http://dx.doi.org/10.1016/0959-8049(93)90115-V.
- Conroy T, Uwer L, Deblock M. Health-related quality-of-life assessment in gastrointestinal cancer: are results relevant for clinical practice?. Curr Opin Oncol 2007;19:401-6. http://dx.doi.org/10.1097/CCO.0b013e32816f7704.
- Eid S, Stromberg AJ, Ames S, Ellis S, McMasters KM, Martin RC. Assessment of symptom experience in patients undergoing hepatic resection or ablation. Cancer 2006;107:2715-22. http://dx.doi.org/10.1016/j.jamcollsurg.2005.06.149.
- Kavadas V, Blazeby JM, Conroy T, Sezer O, Holzner B, Koller M, et al. Development of an EORTC disease-specific quality of life questionnaire for use in patients with liver metastases from colorectal cancer. Eur J Cancer 2003;39:1259-63. http://dx.doi.org/10.1016/S0959-8049(03)00236-3.
- Lloyd Jones M, Hummel S, Bansback N, Orr B, Seymour M. A rapid and systematic review of the evidence for the clinical effectiveness and cost-effectiveness of irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer. Health Technol Assess 2001;5.
- Méndez Romero A, Wunderink W, van Os RM, Nowak PJCM, Heijmen BJM, Nuyttens JJ, et al. Quality of life after stereotactic body radiation therapy for primary and metastatic liver tumors. Int J Radiat Oncol Biol Phys 2008;70:1447-52. http://dx.doi.org/10.1016/j.ijrobp.2007.08.058.
- Scheithauer W, Rosen H, Kornek GV, Sebesta C, Depisch D. Randomised comparison of combination chemotherapy plus supportive care with supportive care alone in patients with metastatic colorectal cancer. BMJ 1993;306:752-5. http://dx.doi.org/10.1136/bmj.306.6880.752.
- Adams R, Wilson RH, Seymour MT, Meade AM, Madi A, Cassidy J, et al. Intermittent versus continuous oxaliplatin-fluoropyrimidine (Ox-Fp) chemotherapy (CT) in first-line treatment of patients (pts) with advanced colorectal cancer (aCRC): Predictive factors (PF), quality of life (QL), and final efficacy results from the MRC COIN trial. J Clin Oncol 2010;28.
- Foss A, Andersen MH, Hagness M, Vidnes T, Finstad ED, Wahl AK, et al. Patient survival and quality of life following liver transplantation for colorectal liver metastases. Liver Transplantation 2010;16:S189-90. http://dx.doi.org/10.1097/00007890-201007272-01582.
- Lorenz M, Hanisch E. Quality of life and survival after continuous intrahepatic floxuridine infusions in liver metastases of colorectal carcinomas. Z Gastroenterol 1995;33:679-80.
- Borrego-Estella V, Montero-Martin J, Serrablo A. Quality of life of patients with liver metastases colorectal cancer n.d.
- Karuna ST, Thirlby R, Biehl T, Veenstra D. Cost-effectiveness of laparoscopy versus laparotomy for initial surgical evaluation and treatment of potentially resectable hepatic colorectal metastases: a decision analysis. J Surg Oncol 2008;97:396-403. http://dx.doi.org/10.1016/j.jamcollsurg.2007.06.177.
- Blair SL, Grant M, Chu DZ, Cullinane C, Dean G, Schwarz RE, et al. Quality of life in patients with colorectal metastasis and intrahepatic chemotherapy. Ann Surg Oncol 2003;10:144-9. http://dx.doi.org/10.1245/ASO.2003.03.060.
- Blazeby JM, Fayers P, Conroy T, Sezer O, Ramage J, Rees M. Validation of the European Organization for Research and Treatment of Cancer QLQ-LMC21 questionnaire for assessment of patient-reported outcomes during treatment of colorectal liver metastases. Br J Surg 2009;96:291-8. http://dx.doi.org/10.1002/bjs.6471.
- Bruns H, Kratschmer K, Hinz U, Brechtel A, Keller M, Buchler MW, et al. Quality of life after curative liver resection: a single center analysis. World J Gastroenterol 2010;16:2388-95. http://dx.doi.org/10.3748/wjg.v16.i19.2388.
- Carrera G, Garcia-Albeniz X, Ayuso JR, Aparicio J, Castells A, Codony-Servat J, et al. Design and endpoints of clinical and translational trials in advanced colorectal cancer. a proposal from GROUP Espanol Multidisciplinar en Cancer Digestivo (GEMCAD). Rev Recent Clin Trials 2011;6:158-70. http://dx.doi.org/10.2174/157488711795177868.
- Cella D, Cappelleri JC, Bushmakin A, Charbonneau C, Li JZ, Kim ST, et al. Quality of life predicts progression-free survival in patients with metastatic renal cell carcinoma treated with sunitinib versus interferon alfa. J Oncol Pract 2009;5:66-70. http://dx.doi.org/10.1200/JOP.0922004.
- Durand-Zaleski I, Earlam S, Fordy C, Davies M, Allen-Mersh TG. Cost-effectiveness of systemic and regional chemotherapy for the treatment of patients with unresectable colorectal liver metastases. Cancer 1998;83:882-8. http://dx.doi.org/10.1002/(SICI)1097-0142(19980901)83:5<882::AID-CNCR12>3.3.CO;2-S.
- Haidet P, Hamel MB, Davis RB, Wenger N, Reding D, Kussin PS, et al. Outcomes, preferences for resuscitation, and physician-patient communication among patients with metastatic colorectal cancer. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. Am J Med 1998;105:222-9.
- Kemeny NE, Niedzwiecki D, Hollis DR, Lenz HJ, Warren RS, Naughton MJ, et al. Hepatic arterial infusion versus systemic therapy for hepatic metastases from colorectal cancer: a randomized trial of efficacy, quality of life, and molecular markers (CALGB 9481). J Clin Oncol 2006;24:1395-403. http://dx.doi.org/10.1200/JCO.2005.03.8166.
- Knox CD, Feurer ID, Wise PE, Lamps LW, Kelly Wright J, Chari RS, et al. Survival and functional quality of life after resection for hepatic carcinoid metastasis. J Gastrointest Surg 2004;8:653-9. http://dx.doi.org/10.1016/j.gassur.2004.04.003.
- Krabbe PF, Peerenboom L, Langenhoff BS, Ruers TJ. Responsiveness of the generic EQ-5D summary measure compared to the disease-specific EORTC QLQ C-30. Qual Life Res 2004;13:1247-53. http://dx.doi.org/10.1023/B:QURE.0000037498.00754.b8.
- Langenhoff BS, Krabbe PF, Peerenboom L, Wobbes T, Ruers TJ. Quality of life after surgical treatment of colorectal liver metastases. Br J Surg 2006;93:1007-14. http://dx.doi.org/10.1002/bjs.5387.
- Pistevou-Gombaki K, Eleftheriadis N, Plataniotis GA, Sofroniadis I, Kouloulias VE. Octreotide for palliative treatment of hepatic metastases from non-neuroendocrine primary tumours: evaluation of quality of life using the EORTC QLQ-C30 questionnaire. Palliat Med 2003;17:257-62. http://dx.doi.org/10.1191/0269216303pm698oa.
- Wiering B, Oyen WJ, Adang EM, van der Sijp JR, Roumen RM, de Jong KP, et al. Long-term global quality of life in patients treated for colorectal liver metastases. Br J Surg 2011;98:565-71. http://dx.doi.org/10.1002/bjs.7365.
- Wietzke-Braun P, Schindler C, Raddatz D, Braun F, Armbrust T, Nolte W, et al. Quality of life and outcome of ultrasound-guided laser interstitial thermo-therapy for non-resectable liver metastases of colorectal cancer. Eur J Gastroenterol Hepatol 2004;16:389-95. http://dx.doi.org/10.1097/00042737-200404000-00004.
- Wiering B, Adang EM, van der Sijp JR, Roumen RM, de Jong KP, Comans EF, et al. Added value of positron emission tomography imaging in the surgical treatment of colorectal liver metastases. Nucl Med Commun 2010;31:938-44. http://dx.doi.org/10.1097/MNM.0b013e32833fa9ba.
- Earlam S, Glover C, Davies M, Fordy C, Allen-Mersh TG. Effect of regional and systematic fluorinated pyrimidine chemotherapy on quality of life in colorectal live metastasis patients. J Clin Oncol 1997;15:2022-9.
- Chan JA, Kulke MH. Emerging therapies for the treatment of patients with advanced neuroendocrine tumors. Expert Opin Emerg Drugs 2007;12:253-70. http://dx.doi.org/10.1517/14728214.12.2.253.
- Health Survey for England 1996. London: Department of Health; 1998.
- Poon RT, Fan ST, Yu WC, Lam BK, Chan FY, Wong J. A prospective longitudinal study of quality of life after resection of hepatocellular carcinoma. Arch Surg 2001;136:693-9. http://dx.doi.org/10.1001/archsurg.136.6.693.
- Loveman E, Jones J, Hartwell D, Bird A, Harris P, Welch K, et al. The clinical effectiveness and cost-effectiveness of topotecan for small cell lung cancer: a systematic review and economic evaluation. Health Technol Assess 2010;14.
- Tappenden P, Chilcott J, Ward S, Eggington S, Hind D, Hummel S. Methodological issues in the economic analysis of cancer treatments. Eur J Cancer 2006;42:2867-75. http://dx.doi.org/10.1016/j.ejca.2006.08.010.
- The Green Book: Appraisal and Evaluation in Central Government. London: The Stationery Office; 2003.
- Beck JR, Pauker SG, Gottlieb JE, Klein K, Kassirer JP. A convenient approximation of life expectancy (the “DEALE”). II. Use in medical decision-making. Am J Med 1982;73:889-97.
- Tappenden P, Jones R, Paisley S, Carroll C. Systematic review and economic evaluation of bevacizumab and cetuximab for the treatment of metastatic colorectal cancer. Health Technol Assess 2007;11.
- Petrou S, Campbell N. Stabilisation in colorectal cancer. Int J Palliat Nurs 1997;3:275-80.
- NHS Reference Costs 2009–2010. London: Department of Health; 2011.
- Remak E, Brazil L. Cost of managing women presenting with stage IV breast cancer in the United Kingdom. Br J Cancer 2004;91:77-83. http://dx.doi.org/10.1038/sj.bjc.6601890.
- NHS Reference Costs 2008–2009. London: Department of Health; 2010.
- Curtis L. Unit Costs of Health and Social Care 2007. Canterbury: PSSRU, University of Kent; 2007.
- Coyle D, Lee M, Donaldson C, Mugford M, Vale L. Evidence-based Health Economics: From Effectiveness to Efficiency in Ssystematic Rreviews. London: BMJ Books; 2002.
- Heusner TA, Hamami ME, Ertle J, Hahn S, Poeppel T, Hilgard P, et al. Angiography-based C-arm CT for the assessment of extrahepatic shunting before radioembolization. Rofo 2010;182:603-8. http://dx.doi.org/10.1055/s-0029-1245192.
- Briggs A, Claxton K, Sculpher M. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2008.
Appendix 1 Protocol methods
Planned investigation
Research aim
The research will undertake an evidence synthesis to assess the clinical and cost effectiveness of ablative therapies for treating liver metastases.
Objectives
-
To conduct a systematic review of the evidence on the clinical and cost effectiveness of ablative therapies for liver metastases.
-
To adapt an existing or construct a de novo economic model to estimate the cost effectiveness of different approaches to treating liver metastases.
-
To identify deficiencies in current knowledge and to generate recommendations for future research.
Methods for synthesis of evidence of clinical effectiveness
A systematic review of the evidence for clinical effectiveness will be undertaken following the general principles outlined in Centre for Reviews and Dissemination (CRD) report ‘Undertaking Systematic Reviews of Research on Effectiveness’ (Third edition)65 and the PRISMA statement on the reporting of systematic reviews and meta-analyses. 66
Search strategy
A systematic search will be conducted to identify relevant studies assessing the use of ablative therapies for treating liver metastases. Several sources will be used including electronic databases, bibliographies of articles, grey literature and consultation with experts. A comprehensive database of relevant published and unpublished articles will be constructed using the Reference Manager software package. The searches carried out will include:
-
General health and biomedical databases: MEDLINE (Ovid), EMBASE (Ovid), and the Science Citation Index.
-
Specialist electronic databases: The Cochrane Library (Wiley), NHS Centre for Reviews and Dissemination HTA database.
-
Research in progress: Current controlled trials meta Register, ISRCTN database, WHO ICTRP Portal and ClinicalTrials.gov.
-
WWW: Subject specific internet sites or relevant associations (e.g. ASGBI).
-
Grey literature, specialist abstract and conference proceeding resources (The British Library’s ZETOC and ISI Proceedings).
-
Checking of reference lists of included studies.
-
Hand searching of journals relevant to the topic (previous 2 years only).
-
Consultation with experts in the field.
All databases will be searched from 1990 to the current date. Searches will be conducted with no language restriction, with non-English-language articles placed in a separate database. Articles in French, Spanish, Italian or German that have an abstract in English will be considered in the selection process along with English language articles, if resources are available.
Study Selection
Studies will be selected for inclusion in the systematic review through a two-stage process using predefined and explicit criteria (see Table 86). The full literature search results will be screened by two reviewers to identify all citations that may meet the inclusion criteria. Full manuscripts of all selected citations will be retrieved and assessed by two reviewers against the selection criteria. These criteria will be piloted on a sample of papers. Any disagreements over study inclusion will be resolved by consensus or if necessary by arbitration involving a third reviewer.
| Intervention | All ablative therapies currently used in the UK, including: Radio-frequency ablation (RFA); Microwave Ablation; Cryoablation; Ethanol Ablation; Laser Ablation; Focused Ultrasound; Electrolytic Ablation. Other minimally-invasive therapies currently used, specifically: Chemoembolisation; Radioembolisation. These will be assessed separately and/or in sequence, where possible and appropriate. |
| Comparators | Surgical resection of metastases; Chemotherapy; BSC (as defined by the included studies and including chemotherapy as part of BSC/palliative care). |
| Population | People with liver metastases from any solid tumour primary site |
| Outcomes | Procedure-related morbidity, mortality and hospital stay; Rate of complete tumour ablation; Local recurrence rate; Progression-free survival; Overall survival; Health related quality of life; Costs and cost effectiveness. |
| Study Design | Randomised controlled trials; Prospective non-randomised comparative studies; Prospective case series studies (sample size > n = 100); Economic evaluations. Systematic reviews identified by the search will be used as a source for identifying primary studies. Studies published as abstracts or conference presentations will only be included if sufficient details are presented to allow an appraisal of the methodology and the assessment of results to be undertaken. Only abstracts published from 2006 onwards will be eligible for inclusion. Where there is evidence from different types of study design for a specific intervention, only those studies with the most rigorous designs will be included and data extracted. |
Studies including patients with primary hepatocellular carcinoma, cholangio-carcinoma, lymphomas, focal nodular hyperplasias and liver cell adenomas will be excluded. Case control studies and qualitative studies will also not be included.
Data extraction
The extraction of studies’ characteristics, methods and findings will be conducted by one reviewer, and checked by a second reviewer, using a standardised pre-piloted data extraction form. Any disagreements between reviewers will be resolved by discussion or, if necessary, by arbitration by a third reviewer.
Quality assessment
The methodological quality of all included studies will be appraised using formal quality assessment criteria. Studies assessing clinical effectiveness will be assessed using criteria recommended by CRD65 and Cochrane Collaboration. 69 Cost effectiveness studies will be assessed using an adapted set of criteria based on those recommended by Drummond and colleagues67 and Philips and colleagues. 68 Two reviewers will assess all studies included in the review for quality with any disagreements between reviewers being resolved by discussion with a third reviewer.
Data synthesis
The results of included studies will be tabulated and summarised in a narrative review. The methods of data synthesis will be determined by the nature of the studies identified through searches and included in the review. Quantitative synthesis of results will be considered if there are several high-quality studies of the same design, but specific details are not possible until the data has been obtained. Sources of heterogeneity will be investigated using appropriate methods.
Methods for synthesis of evidence of cost effectiveness
The cost effectiveness of ablative therapies for liver metastases will be assessed through a systematic review and, where appropriate, the development of an economic model. The systematic review of cost effectiveness will focus on full economic evaluations (i.e. costs and consequences) and will follow the methods outlined in section 5.3. The inclusion criteria will be the same as for the clinical effectiveness review apart from study design (cost effectiveness, cost utility or cost benefit analyses). The quality of the included economic evaluations will be assessed using a critical appraisal checklist based upon that proposed by Drummond and colleagues67 and Philips and colleagues. 68 The data from any included studies will be tabulated and discussed in a narrative review. Published studies carried out from the UK NHS and Personal Social Services (PSS) perspective will be examined in more detail. Any economic models identified will be considered for further development for the current evaluation. If no relevant high quality economic evaluations are identified, a de novo economic model will be developed.
The type and structure of any economic model will be informed by several sources including previous models identified in the systematic review of cost effectiveness, evidence on the epidemiology, natural history of the condition and through discussions with relevant clinical experts. The model structure will be validated through discussion with expert advisors. The model will compare those interventions considered effective in the systematic review of clinical effectiveness (see Table 1) with the standard comparators of surgical resection of metastases and, where this is not appropriate, either chemotherapy or best supportive care. The analysis will be from an NHS and PSS perspective and adopt a lifetime horizon. Future costs and benefits will be discounted at an annual rate of 3.5%. The economic evaluation will adhere to best practice for developing economic models as outlined in the current NICE methodological guidance117 and by Philips and colleagues. 68
Data to populate the model parameters will be derived from best available evidence and will originate from several sources. The systematic reviews of clinical and cost effectiveness will be used to provide data inputs for the measures of clinical effectiveness and will also provide information on adverse events and complications associated with included interventions. Where suitable, studies included in the systematic reviews of clinical and cost effectiveness will be used to provide data on resources use, costs and quality of life. Additional targeted searches will be conducted to identify other relevant studies reporting resource use, quality of life or utility values, and cost data for relevant patient groups. Data on unit costs will be obtained from published sources where available (e.g. NHS Reference Costs,164 Unit Costs for Health and Social Care165) and/or developed from a collaboration with the costing unit at Southampton University Hospitals NHS Trust. Costs will be inflated to current prices as necessary. Where data are limited, possibly on quality of life and resource use, judgements will be made in consultation with clinical and other experts as to the input values for these parameters. In all instances, the model description will clearly indicate the place in the evidence hierarchy for data entering the model,166 particularly as evidence of effectiveness may come from studies with a range of designs.
The modelled population will be defined based on the published evidence about the characteristics of UK population of patients with liver metastases of any solid tumour primary site, and the populations for which good quality clinical effectiveness evidence is available. Where evidence is available patient subgroups will be analysed separately. The results of the economic model will be presented as the incremental cost effectiveness of each ablative therapy in comparison with the other ablative therapies and the specified comparators (i.e. surgical resection of metastases, chemotherapy or best supportive care, where appropriate). Cost effectiveness will be estimated in terms of incremental cost per quality-adjusted life-year (QALY) gained. Uncertainty will be investigated through deterministic and probabilistic sensitivity analysis and scenario analysis. Deterministic sensitivity analysis will be used to address particular areas of uncertainty in the model relating to the model structure, methodological assumptions and parameters around which there is considerable uncertainty or which may be expected, a priori, to have a disproportionate effect on study results. Parameter uncertainty will be more generally addressed using probabilistic sensitivity analysis, with probability distributions assigned to point estimates used in the base-case analysis. Results will be presented using appropriate methods including plots in the cost effectiveness plane, cost effectiveness acceptability curves and, if relevant, the cost effectiveness acceptability frontier.
Quality assurance of the economic model will be undertaken through several different stages. The structure of the model will be discussed with expert clinical advisors to assess clinical relevance and plausibility. The model will be subjected to logic checks to assure internal consistency. Validation will also include dual development (development in two packages, for example MS Excel and TreeAge, using identical parameter inputs). External validation will be based on comparison of model outputs with available clinical data and against outputs from similar models.
Advisory group
An advisory group to inform the project will be recruited. The advisory group will be representative of potential users of the review from different professional backgrounds and opinions. These will include academics and clinicians, and a health economist/methodologist. A patient group representative will also be recruited to the advisory group. The advisory group provide expert advice to support the project, provide comments on a version of the protocol and of the final report, as well as advising on the identification of relevant evidence. All members of the advisory group will be asked to register competing interests and to keep the details of the report confidential.
Competing interests of authors
None declared.
Appendix 2 Search strategy
All databases searched for the systematic review are presented below. Searches were updated on 13 September 2011.
| Database searched | Clinical effectiveness searches | Cost-effectiveness and quality-of-life searches |
|---|---|---|
| Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library) | All available years | All available years |
| Cochrane Database of Systematic Reviews (CDSR, The Cochrane Library) | All available years | All available years |
| Database of Abstracts of Reviews of Effects (DARE, Centre for Reviews and Dissemination) | All available years | All available years |
| EMBASE | 1990–2011 | 1990–2011 |
| Health Technology Assessment Database (Centre for Reviews and Dissemination) | All available years | All available years |
| MEDLINE (Ovid) | 1990–2011 | 1990–2011 |
| MEDLINE In-Process & Other Non-Indexed Citations (MEIP) | Searched 12 April 2011 | |
| National Health Service Economic Evaluation Database (NHS EED, CRD) | All available years | All available years |
| Web of Knowledge (Science Citation Index and Conference Proceedings) | 1990–2011 | 1990–2011 |
| Zetoc, The British Library | 1990–2011 |
| Searched for ongoing trials |
|---|
| National Institute for Health Research Clinical Research Network (NIHR CRN Portfolio, formally UKCRN website) |
| Current Controlled Trials (CCT) |
| Clinical trials.gov |
| WHO International Clinical Trials Registry Platform (ICTRP) |
The MEDLINE search strategy (presented below) for the systematic review of clinical effectiveness was adjusted as necessary for other electronic databases for both clinical effectiveness and cost-effectiveness (including quality-of-life information) searches. Search strategies for the systematic review are available from the authors on request. Citations identified by the searches were added to a Reference Manager (Thomson ResearchSoft, San Francisco, CA, USA) database.
MEDLINE search strategy
-
exp Liver Neoplasms/sc [Secondary] (22,058)
-
exp Neoplasm Metastasis/ (133,433)
-
exp liver neoplasms/ (106,857)
-
2 and 3 (9386)
-
(liver adj5 (secondar* or metasta* or micro-metasta* or micrometasta*)).tw. (21,489)
-
(hepat* adj5 (secondar* or metasta* or micro-metasta* or micrometasta*)).tw. (12,239)
-
1 or 4 or 5 or 6 (40,827)
-
exp catheter ablation/ or exp ablation techniques/ (75,978)
-
exp Laser Therapy/ (43,645)
-
Cryosurgery/ (10,032)
-
Electrocoagulation/ (9522)
-
Electrolysis/ (1849)
-
Microwaves/ (10,483)
-
High-Intensity Focused Ultrasound Ablation/ (110)
-
Chemoembolization, therapeutic/ (2561)
-
electrochemotherapy/ (158)
-
(chemoemboli?ation or radioemboli?ation).tw. (2897)
-
("chemo-emboli*" or "radio-emboli*").tw. (223)
-
(ablat* or photoablat* or cautery or electrocautery or electrolysis or electrolytic or diathermy or laser* or microwave* or radiofrequency or "radio frequency" or cryosurg* or cryoablat* or cryotherap* or thermoablati* or "thermo destruc*" or "thermo coag*" or "thermal coag*" or "thermometry" or "transarterial emboli*" or "transarterial emboli*" or electrocoagulation* or "bead emboli*").tw. (192,928)
-
(RFA or HIFU or IGA or "DEBs" or SIRT or TACE or PAAI or T or PEIT).tw. (44,547)
-
"drug eluting bead*".tw. (50)
-
(focus* adj5 ultrasound).tw. (1939)
-
(microsphere* adj2 inject*).tw. (1193)
-
(ethanol adj2 inject*).tw. (2467)
-
("acetic acid" adj2 inject*).tw. (261)
-
acetic acid/ad, tu (363)
-
or/8-26 (263,845)
-
7 and 27 (3215)
-
limit 28 to yr="1990 -Current" (3034)
-
limit 29 to humans (2877)
-
(editorial or comment or letter).pt. (1,052,888)
-
30 not 31 (2766)
Reference lists
The reference lists of retrieved articles were examined for additional studies.
Other searches
The experts advisory group were contacted in order to obtain information about additional references and any ongoing studies.
British Societies and Conferences (sources checked on 26 July 2011)
American Society of Clinical Oncology (ASCO).
Association of Surgeons of Great Britain and Ireland (ASGBI).
Society of Academic and Research Surgery (SARS).
UK Clinical Research Collaboration (UKCRC).
Journal of One Day Surgery.
Appendix 3 Quality assessment
Quality assessment for randomised controlled trials (answer yes/no/not reported/unclear)
| 1. Was the method used to generate random allocations adequate? |
| 2. Was the allocation adequately concealed? |
| 3. Were the groups similar at the outset of the study in terms of prognostic factors, e.g. severity of disease/size, number of tumours, performance status, and extrahepatic disease? |
| 4. Were outcome assessors blinded to the treatment allocation? |
| 5. (i) Were there any unexpected imbalances in dropouts between groups? (ii) If so, were they explained or adjusted for? |
| 6. Is there any evidence to suggest that the authors measured more outcomes than they reported? |
| 7. (i) Did the analysis include an intention-to-treat analysis? (ii) If so, was this defined? |
| 8. (i) Did the analysis account for missing data? (ii) If so, were the methods appropriate? |
Note: only answer part (ii) of a question if the answer to part (i) is yes.
Quality assessment for non-randomised comparisons (answer yes/no/not reported/unclear)
| 1. Were the patient selection criteria specified a priori? |
| 2. Was the allocation to study groups adequately concealed? |
| 3. Were the groups comparable on key prognostic factors? |
| 4. Were outcome assessors blinded to the treatment allocation? |
| 5. Is there any evidence to suggest that the authors measured more outcomes than they reported? |
| 6. Were withdrawals and dropouts completely described? |
| 7. (i) Were there any unexpected imbalances in dropouts between groups? (ii) If so, were they explained or adjusted for? |
| 8. Did the analyses include an intention-to-treat analysis? |
Quality assessment for case series studies (answer yes/no/not reported/unclear)
| 1. Were the patient selection criteria specified a priori? |
| 2. Was the participant blinded to the research question? |
| 3. Is there any evidence to suggest that the authors measured more outcomes than they reported? |
| 4. Were withdrawals and dropouts completely described? |
| 5 (i) Did the analysis account for missing data? (ii) If so, were the methods appropriate? |
Note: only answer part (ii) of a question if the answer to part (i) is yes.
Appendix 4 Data extraction tables
Laser ablation
Mack et al., 200170
| Study and intervention details | Participants | Outcome measures |
|---|---|---|
| Country: Germany Funding: not reported Intervention: laser ablation Number of centres: one |
Indication for treatment: liver metastases from a mix of primary tumour types Intention of treatment: not stated Number of participants: overall 705 (outcomes not data extracted as contains 5% hepatocellular carcinoma, 20% other primary tumours without separate outcome data). Data extracted: 393 participants with colorectal cancer primary; 127 participants with breast cancer primary Sample attrition/dropout: not reported Inclusion criteria: patients who developed metastases in remaining liver after hepatectomy; patients who had metastases in both liver lobes; patients with contraindications for surgery or unresectable lesions, patients refusing hepatic surgery Exclusion criteria: patients with more than five metastases, metastases larger than 5 cm in greatest diameter, extrahepatic tumour spread |
Outcomes: rate of complications and side effects; local tumour control rate, overall survival. Only overall survival data extracted for the groups of interest (see below) Method of assessing outcomes: survival by Kaplan–Meier, no further details reported Length of follow-up: not reported |
| Participant characteristics | Laser ablation (n = 705 overall;a 393 colorectal cancer primary; 127 breast cancer primary) | |
| Age (years), mean (range) | Overall: 59 (24–89)a | |
| Colorectal primary: 60.8 (range not reported) | ||
| Breast cancer primary – age not reported | ||
| Male/female, n (%) | Overall 385/320 (55/45)a | |
| Primary cancer site | Colorectal cancer 57%, 393 participantsb | |
| Breast cancer 18%, 127 participants | ||
| Hepatocellular carcinoma 5%, number not reported | ||
| Other tumours 20%, number not reported | ||
| Number of liver metastases | Not reported per patient | |
| Size of metastases | Not reported per patient | |
| Previous treatments for liver metastases | Not reported | |
Results
| Outcomes | Laser ablation |
|---|---|
| Comments: reported for the overall group and not data extracted: rate of complications and side effects; local tumour control rate, overall survival. States that survival did not differ significantly (p > 0.05) between male and female patients or between patients with colorectal metastases and those with metastases from other tumour types | |
| Patients with unresectable colorectal liver metastases (n = 393) | |
| Overall survival, mean (95% CI) months | 41.8 (37.3 to 46.4) |
| 1-year survival rate | 93% |
| 2-year survival rate | 74% |
| 3-year survival rate | 50% |
| 5-year survival rate | 30% |
| Overall survival of patients with one or two initial liver metastases, mean (95% CI) | 50.4 months (44.4 to 56.4) |
| Overall survival of patients with three or more initial liver metastases, mean (95% CI) | 34.8 months (31.2 to 38.4) |
| Comments: differences between overall survival of patients with one or two initial metastases versus those with three or more initial metastases were assessed with the log-rank test and the Tarone–Ware test and were shown to be not statistically significant Kaplan–Meier plot of overall survival presented but not data extracted |
|
| Patients with breast cancer liver metastases (n = 127) | |
| Overall survival, mean (95% CI) years | 4.3 (3.6 to 5.0) |
| 1-year survival rate | 97% |
| 2-year survival rate | 75% |
| 3-year survival rate | 65% |
| 5-year survival rate | 34% |
| Comments: there may be some overlap between these breast cancer liver metastases patients and those reported in another paper by the same author (Mack et al., 200471) Kaplan–Meier plots of overall survival presented but not data extracted |
|
| General comments | |
| Generalisability: difficult to determine due to very limited baseline characteristics Outcome measures: for the groups of interest only overall survival was reported Inter-centre variability: not applicable, only one centre Conflict of interests: not reported |
|
Quality assessment for case series studies (answer yes/no/not reported/unclear)
| 1. Were the patient selection criteria specified a priori? | Yes |
| 2. Was the participant blinded to the research question? | Not reported |
| 3. Is there any evidence to suggest that the authors measured more outcomes than they reported? | Unclear |
| 4. Were withdrawals and dropouts completely described? | Not reported |
| 5. (i) Did the analysis account for missing data? (ii) If so, were the methods appropriate? | Not reported |
Mack et al., 200471
| Study and intervention details | Participants | Outcome measures |
|---|---|---|
| Country: Germany Funding: not stated Intervention: laser ablation NB: Ten patients treated with non-irrigated laser application system, the remainder (n = 222) with an internally cooled laser applicator system with the first 38 of these patients treated less aggressively than the remaining 184 Number of centres: one |
Indication for treatment: breast cancer liver metastases Intention of treatment: not stated Number of participants: 232 Sample attrition/dropout: not reported Inclusion criteria: recurrent liver metastases after partial liver resection (n = 19, 8.2%); metastases in both liver lobes (n = 105, 45.2%); locally non-resectable tumours (n = 44, 19%); general contraindications for surgery (n = 6, 2.6%); refusal of surgical resection (n = 58, 25%) Exclusion criteria: more than five tumours, tumour larger than 5 cm at greatest diameter, known extrahepatic spread (lymph node metastases resected at the time of primary breast cancer resection were not considered extrahepatic spread). Bone metastases that were under control (by systemic treatments and/or radiation therapy) were not a contraindication to laser ablation |
Outcomes: ablation volume, adverse events, local tumour control rate, overall survival Method of assessing outcomes: local tumour control – evaluated only for metastases treated with the irrigated power laser applications system with at least a 6-month follow-up. Enhanced and unenhanced magnetic resonance images obtained before and after laser treatment were compared with each other and those obtained at 3, 6 and 12 months’ follow-up. Areas that did not enhance with contrast medium were considered to represent necrotic tissue. Recurrent tumour at ablation site defined as volume of lesion increased compared with that at the examination 3 months earlier and parts of the lesions showing a bulge consisting of solid material with contrast enhancement Tumour volume and volume of necrosis calculated using the three greatest dimensions of the tumour (x, y and z) as [(4π/3)(x/2)(y/2)(z/2)] All evaluations performed by two radiologists and decision made by consensus Length of follow-up: mean follow-up after the first treatment was 1.8 years (maximum 7.7 years, median 1.6 years) |
| Participant characteristics | Laser ablation (n = 232) | |
| Age (years), mean (SD) years | 54.4 (9.9) range 27–79 | |
| Male/female | 100% female | |
| Primary cancer site | Breast | |
| Number of liver metastases, mean (range) | 2.5 (1–13) | |
| Size of metastases, n/N metastases (% of metastases) | ||
| < 2 cm | 251/578 (43.4) | |
| 2–3 cm diameter | 191/578 (33.0) | |
| 3–4 cm diameter | 75/578 (13.0) | |
| 4–5 cm diameter | 61/578 (10.6) | |
| Previous treatments for liver metastases | States all patients receiving chemotherapeutic regimens of choice prior to or after laser treatment | |
| Bone metastases that were considered to be under control | 72/232 (31%) | |
| Comments: it is not clear whether or not the maximum number of liver metastases (n = 13) is above the threshold of five (as per exclusion criteria) because of additional metastases found during the first round of treatment that had not been visible at the time of inclusion, or whether or not the number of liver metastases includes new metastases which were detected during follow-up after the first round of treatment Baseline data reported but not extracted: tumour location by liver segment; percentage known bone metastases considered under control; proportion of metasynchronous and synchronous metastases; mean time between diagnosis of primary tumour and diagnosis of metastases |
||
Results
| Outcomes | Laser ablation (n = 232) |
|---|---|
| Mean survival from date of diagnosis of metastases | 4.9 years (95% CI 4.3 to 5.4) |
| Median survival from date of diagnosis of metastases | 4.3 years (95% CI 3.4 to 5.3) |
| 1-year survival rate metastases diagnosis | 96% (198 patients) |
| 2-year survival rate metastases diagnosis | 80% (133 patients) |
| 3-year survival rate metastases diagnosis | 63% (68 patients) |
| 5-year survival rate metastases diagnosis | 41% (23 patients) |
| Mean survival after first laser treatment | 4.2 years (95% CI 3.6 to 4.8) |
| 1-year survival rate after first laser treatment | 85% (154 patients) |
| 2-year survival rate after first laser treatment | 66% (86 patients) |
| 3-year survival rate after first laser treatment | 51% (34 patients) |
| 5-year survival rate after first laser treatment | 38% (12 patients) |
| Comments: states that estimated mean survival times are biased due to the number of censored cases. If the event had not been noted in the patient chart by the end of the observation period the cases were treated as if the event had been noted at that time. The proportion of participants surviving at 1, 2, 3, and 5 years is also reported but has not been data extracted. Kaplan–Meier plots for survival from data of metastases diagnosis and survival after first laser treatment are presented in the paper but have not been data extracted Subgroup analyses of survival by number of metastases, patient treatment group, indications for laser therapy, lymph node stage, metasynchronous or synchronous metastases, timing of development of metastases after diagnosis of primary tumour, size of metastases, and presence or absence of controlled bone metastases are presented but have not been data extracted Local tumour recurrence rate at 6 months (n/N metastases) reported according to size of metastases. Data are also provided for recurrence at 3 months; it is not clear in the paper whether or not these 6-month data include the recurrences noted at 3 months’ follow-up |
|
| Incomplete ablation, n metastases | 2 |
| Adverse events, n/N treatment sessions | |
| Pleural effusion | 4/452 |
| Non-symptomatic pleural effusion | 41/452 |
| Liver abscess | 2/452 |
| Injury to bile duct | 1/452 |
| Bronchial biliary fistula | None |
| Thirty-day mortality | None |
| Pneumothorax | None |
| Small non-symptomatic subscapular haematoma | 20/452 |
| Comments: note that a treatment session could include the treatment of more than one metastasis | |
| Number of treatment sessions per patient, mean (range) | 2.0 (1.0–8.0) |
| Comments: data on number of laser applicators per metastasis, number of laser applicators per patient, and number of treatment rounds not data extracted (all treatment sessions necessary to ablate all metastases visible were summarised as one treatment round) | |
| General comments | |
|
|
Quality assessment for case series studies (answer yes/no/not reported/unclear)
| 1. Were the patient selection criteria specified a priori? | Yes |
| 2. Was the participant blinded to the research question? | Not reported |
| 3. Is there any evidence to suggest that the authors measured more outcomes than they reported? | Unclear |
| 4. Were withdrawals and dropouts completely described? | Not reported |
| 5. (i) Did the analysis account for missing data? (ii) If so, were the methods appropriate? | Not reported |
Microwave ablation
Shibata et al., 200072
| Study details | Participants | Outcome measures | |
|---|---|---|---|
| Country: Japan Design: RCT Number of centres: one Funding: not reported Intervention: 1. microwave ablation; 2. hepatic resection |
Indication for treatment: multiple metastatic colorectal carcinoma in liver Intention of treatment (curative/palliative/pre-resection/not stated): not stated Number of participants: Number randomised: (1) microwave, n = 20; (2) hepatectomy, n = 20 Number studied: (1) microwave, n = 14; (2) hepatectomy, n = 16 Sample attrition/dropout: 10 excluded intraoperatively Inclusion criteria: participants were potentially amenable to hepatic resection Primary colorectal carcinoma confirmed histologically, multiple but < 10 metastatic tumours in liver, at least one liver tumour characterised histologically by ultrasound-guided needle biopsy, largest tumour had greatest dimension < 80 mm by contrast-enhanced CT, no evidence of periportal lymph node, coeliac lymph node or extrahepatic distant metastases, or ascites, no liver cirrhosis or chronic hepatitis |
Primary outcomes: survival rate; surgical invasiveness Secondary outcomes: adverse events Method of assessing outcomes: serum CEA was measured 4 weeks before and 4 weeks after treatment. CT, ultrasonography and chest radiography performed every 3 months Length of follow-up: not reported |
|
| Participant characteristics | Microwave ablation (n = 14) | Hepatectomy (n = 16) | p-value |
| Age, years, mean (SD) | 61 (10) | 61 (9) | p = 1.0 |
| Male/female | 6/8 | 10/6 | |
| Primary cancer site | Colorectal 100% | Colorectal 100% | |
| Number of liver metastases, mean (SD) | 4.1 (2.1) | 3.0 (1.0) | p = 0.10 |
| Distribution of liver metastases | |||
| Size of metastases: greatest dimension of largest liver tumour, mm, mean (SD) | 27 (11) | 34 (17) | p = 0.21 |
| Previous treatments for primary cancer | NR | NR | |
| Time from primary treatment | NR | NR | |
| Previous treatment for liver metastases | NR | NR | |
| Time from previous liver metastases treatment | NR | NR | |
| Comorbidities (including other metastases) | NR | NR | |
Results
| Outcomes | Microwave ablation (n = 14) | Hepatectomy (n = 16) | p-value |
|---|---|---|---|
| Cumulative survival rate | p = 0.83 | ||
| Mean duration of survival, months | 27 | 25 | |
| Estimated survival, % | |||
| 1 year | 71 | 69 | |
| 2 year | 57 | 56 | |
| 3 yeara | 14 | 23 | |
| Deaths during follow-up | 9 | 12 | |
| Disease-free interval (months), mean | 11.3 | 13.3 | p = 0.47 |
| a The statistical power calculated for the 3-year survival rates with a one-sided significance level of 0.05 was 0.65 Legend to figure 3 indicates that survival was measured from time of treatment As 9 out of 14 patients in the microwave group died during follow-up and 12 out of 16 died in the hepatectomy group there may be an error in figure 3 of the paper (Kaplan–Meier chart of overall survival). Although the figure is a small size it appears to show tick marks, the typical indicator for censored data, e.g. due to participants still being alive at the time of analysis. However, there seem to be four tick marks on the microwave survival line (when five might be expected) and conversely five tick marks on the hepatectomy line (when four might be expected). Furthermore, checking the plot using digitising software also suggests the survival plot lines have been mislabelled |
|||
| Adverse events | |||
| Intraoperative or postoperative deaths | 0 | 0 | |
| Intestinal obstruction | 0 | 1 (6%) | |
| Bile duct fistula | 1 (7%) | 1 (6%) | |
| Hepatic abscess | 1 (7%) | 0 | |
| Wound infection | 0 | 1 (6%) | |
| Total frequency of complications | 2 (14.3%) | 3 (18.8%) | p = 0.87 |
| Indications of surgical invasiveness | |||
| Intraoperative blood loss (ml), mean (SD) | 360 (230) | 910 (490) | p = 0.027 |
| Blood transfusion (ml), mean (SD) | 0 (0) | 540 (690) | p = 0.08 |
| No. of patients requiring blood transfusion (%) | 0 (0) | 6 (38) | p = 0.035 |
| Operation time (minimum), mean (SD) | 180 (20) | 200 (50) | p = 0.2 |
| Period of hospitalisation (days), means (SD) | 20 (7) | 25 (12) | p = 0.23 |
| Intervention details | |||
|
|||
| Details of post-intervention treatments | |||
|
|||
| Methodological comments | |||
|
|||
| General comments | |||
|
|||
Quality assessment for randomised controlled trials (answer yes/no/not reported/unclear)
| 1. Was the method used to generate random allocations adequate? | Yes |
| 2. Was the allocation adequately concealed? | Not reported |
| 3. Were the groups similar at the outset of the study in terms of prognostic factors, e.g. severity of disease/size, number of tumours, performance status, extrahepatic disease | Yes |
| 4. Were outcome assessors blinded to the treatment allocation? | Not reported |
| 5. (i) Were there any unexpected imbalances in dropouts between groups? (ii) If so, were they explained or adjusted for? | No |
| 6. Is there any evidence to suggest that the authors measured more outcomes than they reported? | Unclear |
| 7. (i) Did the analysis include an intention-to-treat analysis? (ii) If so, was this defined? | No |
| 8. (i) Did the analysis account for missing data? (ii) If so, were the methods appropriate? | Not reported |
Radiofrequency ablation
Non-randomised comparison study
Kim et al., 201173
| Study and intervention details | Participants | Outcome measures | |
|---|---|---|---|
| Country: Republic of Korea Funding: not stated Intervention: radiofrequency ablation Comparators: (1) hepatic resection; (2) combination radiofrequency ablation and resection Number of centres: one |
Indication for treatment: colorectal liver metastases Intention of treatment: not explicitly stated Number of participants: 482: RFA 177; resection 278; combination 27 Sample attrition/dropout: not stated Inclusion criteria: not stated as such. All patients were initially considered for resection, except for those with surgery-prohibitive comorbidities, difficult anatomical site, and more than four metastases: these were considered for RFA. No details of how the other participants were allocated to groups Exclusion criteria: extrahepatic metastases excluded at onset of treatment |
Outcomes: recurrence, disease-free survival, overall survival Method of assessing outcomes: not stated Length of follow-up: states carried out every 3–6 months, unclear on duration |
|
| Participant characteristics | RFA (n = 177) | Resection (n = 278) | RFA + resection (n = 27) |
| Age (years), mean (SD) | 60.4 (10.7) | 57.1 (10.9) | 55.7 (11.1) |
| Male/female (%) | 121/56 (68.4/31.6) | 168/110 (60.4/39.6) | 15/12 (55.6/44.4) |
| Primary cancer site | Colorectal | Colorectal | Colorectal |
| Number of liver metastases, mean (SD) | 1.6 (0.9) | 1.5 (0.8) | 3.1 (1.6) |
| Size of metastases, maximum, mean (SD) | 2.1 (1.0) | 2.6 (2.0) | 2.1 (1.4) |
| Previous treatments for liver metastases | Not stated | Not stated | Not stated |
| Comments: the RFA group had more participants with metachronous liver metastases than the resection group (RFA 90.4%; resection 23.1%; combination group 0%) | |||
Results
| Outcomes | RFA (n = 177) | Resection (n = 278) | RFA + resection (n = 27) |
|---|---|---|---|
| Survival | Not reported | Not reported | Not reported |
| Mortality (treatment related) | 0 | 0 | 0 |
| Comments: presents data for subgroups with solitary metastases < 3 cm and ≥ 3 cm but not extracted as not a pre-defined subgroup. Also presents univariate and multivariate analyses of factors associated with overall survival and disease-free survival (age, sex, type of treatment, number of metastases, maximum tumour size, synchronicity, location and chemotherapy after treatment), but not extracted here. Also reports overall survival and disease-free survival rates in a subgroup who had multiple liver metastases across the three treatments (presented graphically), but not extracted here as not a predefined subgroup | |||
| General comments | |||
|
|||
Quality assessment for non-randomised comparisons (answer yes/no/not reported/unclear)
| 1. Were the patient selection criteria specified a priori? | No |
| 2. Was the allocation to study groups adequately concealed? | Unclear |
| 3. Were the groups comparable on key prognostic factors? | No |
| 4. Were outcome assessors blinded to the treatment allocation? | Not reported |
| 5. Is there any evidence to suggest that the authors measured more outcomes than they reported? | Unclear |
| 6. Were withdrawals and dropouts completely described? | Not reported |
| 7. (i) Were there any unexpected imbalances in dropouts between groups? (ii) If so, were they explained or adjusted for? | Not reported |
| 8. Did the analyses include an intention-to-treat analysis? | Not reported |
| 9. (i) Did the analysis account for missing data? (ii) If so, were the methods appropriate? | Not reported |
Case series studies
Berber et al., 2005,74 200875
| Study and intervention details | Participants | Outcome measures |
|---|---|---|
| Country: USA Funding: not reported Intervention: radiofrequency ablation (laparoscopic) Number of centres: one |
Indication for treatment: colorectal liver metastases Intention of treatment: not stated in 2005 paper.74 States curative intent in 2008 paper75 Number of participants: 135 Sample attrition/dropout: not stated Inclusion criteria: unresectable liver disease, predominant liver disease (minor extrahepatic disease allowed), enlarging metastases, worsening symptoms, failure to respond to other treatments, fewer than eight metastases on preoperative CT scan (if identified during treatment to have more not excluded), < 20% total liver volume replaced with tumour, normal biliary duct diameters Exclusion criteria: not stated |
Outcomes: survival, progression-free survival Method of assessing outcomes: not stated Length of follow-up: between 1 and 52 months depending on how long since RFA procedure at the time of the analysis |
| Participant characteristics | Radiofrequency ablation (n = 135) | |
| Age (years), mean (SEM) | 62 (1) | |
| Male/female (%) | 85/50 (63/37) | |
| Primary cancer site | Colorectal | |
| Number of liver metastases ablated, mean (SEM) | 3.2 (0.2) | |
| Largest liver metastases, size, mean (SEM) | 4.1 (0.4) | |
| Previous chemotherapy for liver metastases | 80% | |
| Previous liver resection | 14% | |
| Extrahepatic disease | 40 (30%) | |
| Comments: none | ||
Results
| Outcomes | Radiofrequency ablation (n = 135) |
|---|---|
| Median Kaplan–Meier survival post radiofrequency ablation | 28.9 months |
| Median Kaplan–Meier survival after diagnosis liver metastases | 44.6 months |
| Comments: purpose of study was to identify predictors of survival and therefore presents data for a range of subgroups of potential relevance to survival, and a number of prognostic factors. Report sex, age, primary tumour, node status, location of liver metastases, type of metastases, number of metastases, largest size of metastases, extrahepatic disease, pre-RFA chemotherapy and carcinoembryonic agent. Data not extracted Also present median progression-free survival for those participants who did not have extrahepatic disease at time of the ablation, not extracted here Also reports cause of death and site of initial recurrence of disease Kaplan–Meier curves presented in paper The recruitment dates of the 2005 and 2008 publications overlap but do not have the same starting point The 2008 publication76 aimed to identify factors that predict local recurrence following radiofrequency ablation. Primary and metastatic liver tumours were included. Data are presented per tumour and the paper does not report the number of participants with colorectal tumours or any baseline data Presents Kaplan–Meier curve (analysed on a per-lesion basis) of local tumour control separately for colorectal metastases Local recurrence was identified in at least one liver tumour in 46% of patients with colorectal cancer No other relevant data to extract |
|
| General comments | |
|
|
Quality assessment for case series studies (answer yes/no/not reported/unclear)
| 1. Were the patient selection criteria specified a priori? | Yes |
| 2. Was the participant blinded to the research question? | Not reported |
| 3. Is there any evidence to suggest that the authors measured more outcomes than they reported? | Unclear |
| 4. Were withdrawals and dropouts completely described? | Not reported |
| 5. (i) Did the analysis account for missing data? (ii) If so, were the methods appropriate? | Not reported |
Gillams and Lees, 2009,77 200476
| Study and intervention details | Participants | Outcome measures |
|---|---|---|
| Country: UK Funding: not reported Intervention: radiofrequency ablation Number of centres: one |
Indication for treatment: colorectal liver metastases Intention of treatment: not stated Number of participants: n = 309 Sample attrition/dropout: not stated. Some baseline characteristics not known for some patients, numbers reported Inclusion criteria: all patients treated with radiofrequency ablation since 1997. Acceptance criteria: inoperable due to number or distribution of tumours resulting in inadequate residual liver volume, inability to achieve margins because of tumour location adjacent to the vena cava and hepatic confluence, or presence of extrahepatic disease or concomitant morbidity. Five or fewer tumours of 5 cm or less in diameter, or up to nine tumours with a maximum diameter of 4 or 4.5 cm, or a solitary tumour less than 7 cm in diameter. Extrahepatic disease if stable on treatment. Ablation of larger tumours performed later in study period due to evolving ablation technology. More extensive disease sometimes treated due to progression between referral and ablation and cross-sectional imaging underestimating extent of disease |
Outcomes: survival; complications Method of assessing outcomes: CT scan prior to discharge (baseline for comparison) and at 3-month intervals Complications assessed using Society of Interventional Radiology guidelines Length of follow-up: not reported in the 2009 publication |
| Participant characteristics | Radiofrequency ablation (n = 309) | |
| Age (years), mean (range) | 64 (24–92) | |
| Male/female | 198/111 | |
| Primary cancer site | Left colon: 118/285 (41%) | |
| Right colon: 51/285 (18%) | ||
| Rectum: 95/285 (33%) | ||
| Multiple: 12/285 (4%) | ||
| Unspecified colonic: 9/285 (3%) | ||
| Number of liver metastases, mean (median, range) | 4 (3, 1–27) | |
| Size of largest metastasis (cm), mean (median, range) | 3.7 (3.5, 0.9–12) | |
| Previous treatments for liver metastases | Liver resection: 48/309 (16%) | |
| Extrahepatic disease | 115/309 (37%) | |
| Data on chemotherapy received incomplete and no data extracted | ||
Results
| Outcomes | Radiofrequency ablation (n = 309) |
|---|---|
| Overall survival | Not reported for whole group. Univariate and multivariate survival analysis presented for a number of variables |
| Procedure related mortality | 0 |
| Number of major complications (requiring intervention or hospital stay beyond 72 hours) | 29 (617 treatment sessions) |
| Systemic | 5 |
| Anaesthetic | 1 |
| Local | 23 (3.7%) [one pneumothorax, four visceral thermal injuries, six abscesses, four jaundice (two bile duct injuries, two inadequate liver reserve), seven haemorrhagic complications and one asymptomatic pseudoaneurysm] |
| Developed new liver metastases | 72 (50%), of whom 23 had recurrence adjacent to ablated lesions |
| Developed new sites or progression of extrahepatic metastases | 71 (49%), of whom 19 had local liver recurrence |
| Developed local liver recurrence without evidence of disease elsewhere | 30 |
| Survival curves presented for those with and without extrahepatic disease, and comparing different liver tumour volumes in patients without extrahepatic disease. Overall survival presented for 167 patients reported the 2004 paper76 | |
| General comments | |
|
|
Quality assessment for case series studies (answer yes/no/not reported/unclear)
| 1. Were the patient selection criteria specified a priori? | Yes |
| 2. Was the participant blinded to the research question? | Not reported |
| 3. Is there any evidence to suggest that the authors measured more outcomes than they reported? | Unclear |
| 4. Were withdrawals and dropouts completely described? | Not reported |
| 5 (i) Did the analysis account for missing data? (ii) If so, were the methods appropriate? | Not reported |
Siperstein et al., 200778
| Study and intervention details | Participants | Outcome measures |
|---|---|---|
| Country: USA Funding: not reported Intervention: radiofrequency ablation Number of centres: two |
Indication for treatment: colorectal liver metastases Intention of treatment: not stated Number of participants: 234 Sample attrition/dropout: not reported Inclusion criteria: not candidates for resection and/or failed chemotherapy, presence of extrahepatic spread, up to 12 hepatic lesions with a maximal dominant lesion size of 10 cm Exclusion criteria: not reported |
Outcomes: progression of ablated disease; evidence of new hepatic disease or extrahepatic disease, overall survival Method of assessing outcomes: data collected 1 week post ablation, at 1 month, and then every 3 months. CT scans and laboratory data used to identify the presence or recurrence of metastatic disease Survival calculated from treatment of liver metastases Length of follow-up: median 24 months (range 1 to 94) from time of ablation to death or data analysis |
| Participant characteristics | Radiofrequency ablation (n = 234) | |
| Age (years), mean (SEM) | 62 (1) | |
| Male/female, n (%) | 153/81 (65/35) | |
| Primary cancer site | Colorectal | |
| Number of liver metastases, mean (SEM) | 2.8 (0.14), range 1–12 | |
| Largest tumour size, mean (SEM), cm | 3.9 (0.2), range 1.1–10.2 | |
| Previous chemotherapy for liver metastases | ‘Majority’ | |
| Extrahepatic disease, n/N (%) | 55/234 (23.5) | |
| Comments: indicates that chemotherapy received by participants differed across the course of the 10-year study | ||
Results
| Outcomes | Radiofrequency ablation (n = 234) |
|---|---|
| Actuarial median survival | 24 months |
| Actual 3-year survival | 20.2% |
| Actual 5-year survival | 18.4% |
| Deaths during study period | 148/234 |
| Comments: survival in a range of subgroups also presented to assess factors affecting long-term survival (number of lesions, lesion size, chorioembryonic antigen levels, presence of extrahepatic disease, stage of disease at presentation) with survival curves presented. Also present median progression-free survival for those participants who did not have extrahepatic disease at time of the ablation, not extracted here | |
| General comments | |
|
|
Quality assessment for case series studies (answer yes/no/not reported/unclear)
| 1. Were the patient selection criteria specified a priori? | Yes |
| 2. Was the participant blinded to the research question? | Not reported |
| 3. Is there any evidence to suggest that the authors measured more outcomes than they reported? | Unclear |
| 4. Were withdrawals and dropouts completely described? | Not reported |
| 5. (i) Did the analysis account for missing data? (ii) If so, were the methods appropriate? | Not reported |
Solbiati et al., 200179
| Study and intervention details | Participants | Outcome measures |
|---|---|---|
| Country: Italy Funding: not reported Intervention: radiofrequency ablation Number of centres: two |
Indication for treatment: colorectal liver metastases Intention of treatment: not stated Number of participants: 117 Sample attrition/dropout: of 117 patients, status determinable in 96 (82%) at 12 months; 64 (55%) at 24 months; 45 (38%) at 36 months; and 38 (32%) at 48 months Inclusion criteria: patients not considered for surgical metastasectomy due to extrahepatic metastases, prior hepatic metastasectomy, age, disease extent and/or comorbidity; refusal to consent to surgery Exclusion criteria: not stated |
Outcomes: time to death, time to new metastases, time to tumour recurrence, complications Method of assessing outcomes: post-treatment CT scans obtained 7–14 days following ablation, with additional follow-up scans at 3–4 months following therapy and thereafter at 4- to 6-month intervals. Each image was compared with baseline studies. Scans were interpreted by a total of four investigators: two from each institution. No more than two were involved in the treatment of any one patient. A consensus of the readers was used to judge treatment effectiveness Technical success defined as no detectable tumour 7–14 days after treatment Length of follow-up: 6 to 52 months |
| Participant characteristics | Radiofrequency ablation (n = 117) | |
| Age (years), mean (SD) | 64.8 (10.8) | |
| Male/female, n (%) | 81/36 (69.2/30.8) | |
| Primary cancer site | Colorectum | |
| Number of liver metastases, n (%) | ||
| One | 74 (63.2) | |
| Two | 29 (24.8) | |
| Three | 9 (7.7) | |
| Four | 5 (4.3) | |
| Diameter of metastases (cm), mean (SD); median (range) | 2.8 (1.2); 2.6 (0.6–9.6) | |
| Largest metastasis (cm), mean (SD); median (range) | 3.2 (1.3); 3.0 (0.7–9.6) | |
| Previous liver resection, n (%) | 24 | |
| Systemic chemotherapy only prior to ablation, n (%) | 20 (17) | |
| Systemic chemotherapy prior to and following ablation, n (%) | 84 (72) | |
| No systemic chemotherapy, n (%) | 13 (11) | |
Results
| Outcomes | Radiofrequency ablation (n = 117) |
|---|---|
| Technical success, n/N (%) tumours treated | 176/179 (98) |
| Incomplete initial therapy, n patients (%) | 3 (2.6) |
| Deaths, n (%) | 36 (31) |
| Estimated median survival, months | 36 (95% CI 28 to 52 months) |
| Estimated 1-year survival | 93% |
| Estimated 2-year survival | 69% |
| Estimated 3-year survival | 46% |
| New metastases developed during follow up, n/N (%) patients | 67/117 (57) |
| Median time to detection of new metastases, months | 12 (95% CI 10 to 18 months) |
| Local recurrence, n/N (%) lesions | 70/179 (39.1) |
| Estimated local recurrence rate at 18 months for all patients | 44% |
| Patients with at least one local recurrence, n (%) | 64 (54.7) |
| Comments: time to death is reported by number of metastases but has not been data-extracted. Median time to local recurrence not estimable due to small number of events. Time to new metastases also reported by number of metastases but not data extracted. Data on retreatments not data extracted. Kaplan–Meier curves presented | |
| Severe complications | n = 1 (perforation of right colon, required surgical repair) |
| Other complications | n = 1 (small intraperitoneal haemorrhage, stabilised without treatment) |
| Comments: 229 treatment sessions | |
| General comments | |
|
|
Quality assessment for case series studies (answer yes/no/not reported/unclear)
| 1. Were the patient selection criteria specified a priori? | No |
| 2. Was the participant blinded to the research question? | Not reported |
| 3. Is there any evidence to suggest that the authors measured more outcomes than they reported? | Unclear |
| 4. Were withdrawals and dropouts completely described? | Not reported |
| 5. (i) Did the analysis account for missing data? (ii) If so, were the methods appropriate? | Not reported |
Sorensen et al., 200780
| Study and intervention details | Participants | Outcome measures |
|---|---|---|
| Country: Denmark Funding: not reported Intervention: radiofrequency ablation Number of centres: one |
Indication for treatment: colorectal liver metastases. Intention of treatment: not stated Number of participants: 102 consecutive patients Sample attrition/dropout: two participants excluded from follow-up evaluation. One RFA was to reduce tumour prior to surgery, one resulted in a fistula and the whole liver segment was resected Inclusion criteria: inoperability due to prior hepatic resection or comorbidity (n = 100), or refused surgery (n = 2). Four or fewer tumours with a maximum diameter of 4 cm (based on contrast-enhanced CT diagnosis) Exclusion criteria: known extrahepatic spread, uncontrolled coagulopathy, or tumours situated less than 1 cm from the gall bladder, central biliary structures, or bowel |
Outcomes: survival; complications Method of assessing outcomes: survival estimated using Kaplan–Meier method and defined as the time between diagnosis of liver metastases and death or the end of the follow-up period, or the time between the first RFA treatment and death or the end of the follow-up period Complications were classified in accordance with the definitions of the Society for Cardiovascular and Interventional Radiology (reference provided). Complications threatening life, leading to substantial morbidity and disability or requiring prolonged hospitalisation were considered major complications. All others were considered minor complications Length of follow-up: mean follow-up was 23.6 months (range 1–92 months) |
| Participant characteristics | Radiofrequency ablation (n = 102) | |
| Age (years), mean (range) | 64 (33–84) | |
| Male/female | 61/41 | |
| Primary cancer site | Rectum 35 (34%) | |
| Colon 67 (66%) | ||
| Number of liver metastases, mean (range) per participant | 3.3 (1–17) | |
| Size of metastases, mean (range) | 2.2 (0.5–6.5) | |
| Previous treatments for metastases | 26 participants (25%) were down-staged with chemotherapy prior to RFA 25 (25%) had undergone resection of liver metastases prior to RFA | |
| Presentation of liver metastases | Synchronous: 27 (26%) = diagnosed < 12 months following primary resection | |
| Metachronous: 75 (74%) | ||
| Comments: RFA was performed with or without resection. Six participants (6%) received chemotherapy during RFA treatment and one (1%) was treated with a combination of stereotactic radiation therapy and RFA | ||
Results
| Outcomes | Radiofrequency ablation (n = 102) |
|---|---|
| Median survival, from diagnosis | 52 months (95% CI 34 to 82) |
| Estimated survival from diagnosis | |
| 1-year survival | 96% |
| 2-year survival | 79% |
| 3-year survival | 64% |
| 4-year survival | 52% |
| 5-year survival | 44% |
| Median survival from first RFA treatment | 32 months (95% CI 24 to 45) |
| Estimated survival from first RFA treatment | |
| 1-year survival | 87% |
| 2-year survival | 62% |
| 3-year survival | 46% |
| 4-year survival | 26% |
| Mortality rate during follow-up | 35 (35%) |
| Comments | |
| Major complications | Total number of major complications: n = 12 |
| Abscesses requiring drainage: hepatic n = 2, subcutaneous n = 1 | |
| Perforation of gastrointestinal wall: gastric n = 2, colonic n = 1 | |
| Vascular: pseudoaneurysm of the right hepatic artery n = 1, thrombosis of inferior caval vein n = 1 | |
| Seeding n = 1 | |
| Pneumothorax requiring drainage n = 1 | |
| Perforation of the diaphragm n = 1 | |
| Acalcular cholecystitis n = 1 | |
| Minor complications | Total number of minor complications, n = 7 |
| Hepatic abscesses not requiring drainage, n = 2 | |
| Second-degree skin burn, n = 1 | |
| Subcapsular liver haematoma, n = 1 | |
| Subcutaneous haematoma, n = 1 | |
| Pleural effusion not requiring drainage, n = 1 | |
| Fistula between RFA necrosis and secondary biliary tract, n = 1 | |
| Comments: complication rates based on number of treatments (n = 176) | |
| General comments | |
|
|
Quality assessment for case series studies (answer yes/no/not reported/unclear)
| 1. Were the patient selection criteria specified a priori? | Yes |
| 2. Was the participant blinded to the research question? | Not reported |
| 3. Is there any evidence to suggest that the authors measured more outcomes than they reported? | Unclear |
| 4. Were withdrawals and dropouts completely described? | Yes |
| 5. (i) Did the analysis account for missing data? (ii) If so, were the methods appropriate? | Not reported |
Chemoembolisation
Agarwala et al., 200482
| Study and intervention details | Participants | Outcome measures |
|---|---|---|
| Country: USA Design: RCT (dose escalation trial) Number of centres: one Funding: not reported Intervention: (1) chemotherapy (100 mg/m2); (2) chemotherapy (100 mg/m2) plus chemoembolisation; (3) chemotherapy (125 mg/m2); (4) chemotherapy (125 mg/m2) plus chemoembolisation See below for intervention details |
Indication for treatment: ocular melanoma liver metastases Intention of treatment (curative/palliative/pre-resection/not stated): not stated Number of participants: randomised n = 19; assessable for response n = 17 (1) cisplatin 100 mg/m2, n = 3 (2) cisplatin 100 mg/m2 plus polyvinyl sponge, n = 4 (3) cisplatin 125 mg/m2 n = 6 (4) cisalpin 125 mg/m2 plus polyvinyl sponge, n = 6 Sample attrition/dropout: n = 2 Complete history and physical examination performed before study entry. Laboratory studies listed Inclusion criteria: history of ocular melanoma, histologically proven liver metastases measured by CT or ultrasound. Age at least 18 years, ECOG performance status of 0–2, adequate hepatic (bilirubin < 3 × normal), renal (creatinine < 2.0 mg%, creatinine clearance > 50 ml/min) and haematological (white blood cell count > 4000/mm3, platelet count > 100,000/mm3) function Exclusion criteria: peripheral vascular disease or any other contraindication to femoral artery catheterisation. Pregnancy (use of acceptable form of contraception for duration of study required). Any prior systemic antineoplastic therapy had to be discontinued at least 4 weeks (6 weeks for nitrosoureas) before study |
Primary outcomes: toxicity (establishing maximum tolerated dose) Secondary outcomes: response; median survival Method of assessing outcomes: baseline tumour evaluations obtained by CT scans no longer than 6 weeks before first treatment. Daily blood tests during therapy (hospitalised for treatment). After discharge, pre-treatment blood studies repeated weekly for 4 weeks Response assessed 4 weeks after treatment by abdominal CT scan Tumour response assessed via standard response criteria (no reference given) Complete response defined as complete radiographic disappearance of evident hepatic disease for at least 4 weeks Partial response defined as a 50% or greater decrease in the sum of the products of the perpendicular diameters of all measurable hepatic lesions lasting at least 4 weeks without increase in size of existing lesions or appearance of new lesions Stable disease defined as less than 25% increase or less than 50% decrease in all measurable lesions Progressive disease defined as a greater than 25% increase in liver lesions or appearance of any new lesions in liver or elsewhere Toxicities graded according to National Cancer Institute Common Toxicity Criteria ‘Dose-limiting toxicity’ defined as occurrence of grade 3 or higher toxicities in 2 of 6 patients treated at any dose tier. Death from any cause on study was considered to be a dose limiting toxicity ‘Maximum tolerated dose, determined to be the tier below “dose-limiting toxicity”’ Length of follow-up: not stated |
| Participant characteristics | Total (n = 19) | |
| Age (years), mean (range) | 59.7 (36–81) | |
| Male/female | 9/10 | |
| Primary cancer site | Ocular melanoma 100% | |
| Number of liver metastases | Not stated | |
| Distribution of liver metastases | Not stated | |
| Size of metastases | Not stated | |
| Previous treatments for primary cancer | Enucleation, n = 18 | |
| Local radiation, n = 7 | ||
| Time from primary treatment | Not stated | |
| Previous treatment for liver metastases | Chemotherapy, n = 2 | |
| Immunotherapy, n = 3 | ||
| Tamoxifen, n = 1 | ||
| Time from previous liver mets treatment | Not stated | |
| Comorbidities (including other metastases) | Not stated | |
| ECOG performance status of 0 or 1 | 17 | |
Results
| Outcomes | Total (not clear if n = 19 or n = 17) | |||
|---|---|---|---|---|
| Median survival | 8.5 months | |||
| Kaplan–Meier survival curve with 95% CIs presented | ||||
| Clinical response | Cisplatin 100 mg/m2 (n = 3) | Cisplatin 100 mg/m2 plus polyvinyl sponge (n = 4) | Cisplatin 125 mg/m2 (n = 6) | Cisplatin 125 mg/m2 plus polyvinyl sponge (n = 6) |
| Complete response | 0 | 0 | 0 | 0 |
| Partial response | 0 | 0 | 1 | 2 |
| Stable disease | 3 | 2 | 5 | 3 |
| Progressive disease | 0 | 1 | 0 | 0 |
| Not evaluable | 0 | 1 | 0 | 1 |
| States no statistically significant difference in the response rates between patients who did or did not receive polyvinyl sponge or between the two dose tiers (p = 0.2769) | ||||
| Toxicity | Cisplatin 100 mg/m2 (n = 3) | Cisplatin 100 mg/m2 plus polyvinyl sponge (n = 4) | Cisplatin 125 mg/m2 (n = 6) | Cisplatin 125 mg/m2 plus polyvinyl sponge (n = 6) |
| Dose limiting toxicity | 0 | 0 | 3 (renal 2, haematological 1) | 3 (hepatic 1, haematological 1, death from progressive tumour) |
| Grade 3 toxicity | 2 | 1 | 3 | 1 |
| Grade 4 toxicity | 0 | 2 | 3 | 4 |
| 125 mg/m2 was determined to be the maximum tolerated dose. Twelve of 19 participants went on to receive cycle 2. One patient each received five, six and seven cycles of therapy | ||||
| Total (n = 19) | ||||
| Transient alteration in liver function tests (grade 1 and 2) | 13 of 19 | |||
| Dose reduction as result of altered liver function | 1 of 19 | |||
| Haematological toxicity | ||||
| Anaemia (grade 1–2) | 2 | |||
| Thrombocytopenia (grade 3) | 2 | |||
| No complications related to catheterisation procedure | ||||
| Intervention details | ||||
| Hospitalised for treatment Intrahepatic arterial infusions chemotherapy with cisplatin and chemoembolisation with polyvinyl sponge: polyvinyl sponge administered at a fixed dose of 15 ml and mixed with increasing doses of cisplatin. Total dose of cisplatin administered in two portions during cycle 1. All patients first received cisplatin 50 mg mixed with 5 ml of polyvinyl sponge suspension (10 mg/ml of 150 nm polyvinyl solution premixed with water-based radio-opaque contrast material) in bolus aliquots over an average of 30 minutes, followed by the balance of the total dose of cisplatin administered as a 1 mg/ml solution into the same hepatic artery over 30 minutes. This was followed by administration of sufficient volume of polyvinyl sponge suspension (without cisplatin) to achieve slowing of arterial blood flow to tumour as identified by fluoroscopy. Additional cycles of chemoembolisation were administered to same hepatic arterial branch pending re-evaluation by arteriography. Cycle 2 administered same as cycle 1. If 50 mg cisplatin with 5-ml polyvinyl sponge could not be completely administered due to slowing of blood flow from previous treatment, polyvinyl sponge administration was stopped and cisplatin solution administered. If arterial blood flow was allowed, the remaining mixture was administered. If as a result of previous treatment no amount if cisplatin/polyvinyl sponge could be administered without completely occluding the artery, the treatment order was reversed, i.e. cisplatin solution over 30 minutes followed cisplatin 50 mg with 5-ml polyvinyl sponge suspension. Cycle 3 and 4 administered as cycle 1 and 2, but previously untreated metastases were targeted Intrahepatic arterial infusions chemotherapy with cisplatin alone: prepared as an admixture of cisplatin powder reconstituted with sterile water to yield volume of 150 ml consistent with solubility. Mixture administered as a1-mg/ml solution (total dose 15 ml) infused over 30 minutes into hepatic artery branch supplying tumour Cisplatin dose escalation: planned in five tiers of three patients each (100 mg/m2, 125 mg/m2, 156 mg/m2, 195 mg/m2, and 245 mg/m2), reflecting a 25% increase in dosage per tier. If three patients treated at a particular dose tier of a given treatment arm did not experience any grade 3 or higher toxicity, then the cisplatin dosage was escalated by 25% for the next patient. If one of these did experience a grade 3 or higher toxicity, another three patients were treated at that same dose and treatment arm. Patients not entered onto higher dose tier until at least 4 weeks after last patient treated on previous dose tier Patients who demonstrated stable or responding disease were eligible to receive additional cycles of therapy at the same dose (no intrapatient dose escalation was permitted). Therapy was repeated for up to a maximum of 10 cycles unless a dose-limiting toxicity intervened Pre and concomitant medications: to decrease risk of renal tubular injury from cisplatin: prior to treatment patients hydrated overnight with 0.5 normal saline and 20 mEq KCl/l at rate of 125 ml per hour. This was increased to 250 ml per hour during and after treatment to maintain urine output of 100 ml per hour. To prevent nausea and vomiting: patients premedicated with ondansetron 10 mg intravenously every 4 hours for four doses, and a single dose of lorazepam 1 mg intravenous with the first ondansetron dose. Narcotic analgesics for pain control as required |
||||
| Methodological comments | ||||
|
||||
| General comments | ||||
|
||||
Quality assessment for randomised controlled trials (answer yes/no/not reported/unclear)
| 1. Was the method used to generate random allocations adequate? | Unclear |
| 2. Was the allocation adequately concealed? | Unclear |
| 3. Were the groups similar at the outset of the study in terms of prognostic factors, e.g. severity of disease/size, number of tumours, performance status, extrahepatic disease | Unclear |
| 4. Were outcome assessors blinded to the treatment allocation? | Unclear |
| 5. (i) Were there any unexpected imbalances in dropouts between groups? (ii) If so, were they explained or adjusted for? | No |
| 6. Is there any evidence to suggest that the authors measured more outcomes than they reported? | Unclear |
| 7. (i) Did the analysis include an intention-to-treat analysis? (ii) If so, was this defined? | No |
| 8. (i) Did the analysis account for missing data? (ii) If so, were the methods appropriate? | Not reported |
Taguchi et al., 199281
| Study and intervention details | Participants | Outcome measures | |
|---|---|---|---|
| Country: Japan Design: RCT Number of centres: 11 hospital clinics Funding: not stated Intervention: (1) intra-arterial mitomycin C with degradable starch microspheres (DSM); (2) intra-arterial mitomycin C without DSM See below for intervention details |
Indication for treatment: metastatic liver cancer Intention of treatment (curative/palliative/pre-resection/not stated): not stated Number of participants: (1) intra-arterial mitomycin C with DSM, n = 30; (2) intra-arterial mitomycin C without DSM, n = 30 Sample attrition/dropout: not stated; however, 18 (intervention 1, n = 8; intervention 2, n = 10) and 9 patients were not evaluable for efficacy and safety respectively. No reasons provided. Five in each group withdrawn due to adverse events Inclusion criteria: non-resectable liver cancer without demonstrable malignancies in other organs; a resected primary; a patent portal vein; performance status of 0–3; a hepatic artery anatomy suitable for catheterisation Exclusion criteria: patients had received chemotherapy, radiotherapy or immunotherapy in preceding month, were suffering from severe cardiac or pulmonary disease, had a serum bilirubin count more than twice the normal level, had a white blood count below 3000/mm3 or a platelet count below 50,000/mm3 |
Primary and secondary outcomes: tumour response; adverse events; overall survival Method of assessing outcomes: Complete response defined as disappearance of all measurable lesions, evaluable lesions as well as non-quantifiable malignant lesions, and no appearance of new lesions, determined by two observations not less than 4 weeks apart Partial response defined by decrease of 50% or more in lesions that can be measured in two directions without deterioration of any evaluable or other lesion, and no appearance of new lesions, determined by two observations not less than 4 weeks apart No change defined by decrease of 25% in lesions that can be measured in two directions, less than 30% in lesions that can be measured in one direction, to an increase of less than 25% in such lesions without deterioration in other lesions, with no appearances of new lesions, determined by two observations not less than 4 weeks apart Progressive disease defined as an increase of 25% or more in the product of the sum of diameters of measurable lesions, or deterioration in other lesions or appearance of new lesions Minimal response defined as a condition between no change and partial response Adverse events noted by patient or clinician were rated on a scale 0–4 by the clinician and followed to normalisation. Clinical blood profile undertaken at start and two weekly, including blood cell count, blood analyses, urinalyses, tumour markers Length of follow-up: not stated |
|
| Participant characteristics | Intra-arterial mitomycin C with DSM | Intra-arterial mitomycin C without DSM | p-value |
| Age (years) | Not stated | Not stated | Not stated |
| Male/female | Not stated | Not stated | Not stated |
| Primary cancer site | Not stated | ||
| Colorectal | 10/22 | 15/20 | |
| Stomach | 11/22 | 4/20 | |
| Gall bladder | 1/22 | 0/20 | |
| Pancreas | 0 | 1/20 | |
| Number of liver metastases | Not stated | Not stated | Not stated |
| Distribution of liver metastases | Not stated | Not stated | Not stated |
| Size of metastases | Not stated | Not stated | Not stated |
| Previous treatments for primary cancer | Not stated | Not stated | Not stated |
| Time from primary treatment | Not stated | Not stated | Not stated |
| Previous treatment for liver metastases | Not stated | Not stated | Not stated |
| Time from previous liver mets treatment | Not stated | Not stated | Not stated |
| Comorbidities (including other metastases) | Not stated | Not stated | Not stated |
| Comments: patient characteristics are not reported separately for the different indications or treatment groups. Study states that no significant differences existed in age, sex, performance status, site, stage of tumours, numbers of tumours or maximal tumour size and preceding treatment between patients | |||
Results
| Outcomes | Intra-arterial mitomycin C with DSM (n = 22) | Intra-arterial mitomycin C without DSM (n = 20) | p-value |
|---|---|---|---|
| Tumour response rates | |||
| Complete response | 1 | 0 | Not stated |
| Partial response | 11 | 4 | Not stated |
| Minimal response | 0 | 1 | Not stated |
| No change | 6 | 7 | Not stated |
| Progressive disease | 4 | 8 | Not stated |
| Total | 22 | 20 | Not stated |
| Response rate, % (CR + PR) | 54.5 | 20 | p < 0.05 |
| Comments | |||
| Tumour response rates by primary tumour site | |||
| Colorectum | |||
| Complete response | 1 | 0 | Not stated |
| Partial response | 3 | 3 | Not stated |
| No change | 5 | 6 | Not stated |
| Progressive disease | 1 | 6 | Not stated |
| Total | 10 | 15 | Not stated |
| Response rate, % (CR + PR) | 40 | 20 | Not stated |
| Comments | |||
| Stomach | |||
| Complete response | 0 | 0 | Not stated |
| Partial response | 8 | 1 | Not stated |
| No change | 0 | 2 | Not stated |
| Progressive disease | 3 | 1 | Not stated |
| Total | 11 | 4 | Not stated |
| Response rate, % (CR + PR) | 72.7 | 25 | Not stated |
| Comments | |||
| Others (gall bladder and pancreas) | |||
| Complete response | 0 | 0 | Not stated |
| Partial response | 0 | 0 | Not stated |
| No change | 1 | 0 | Not stated |
| Progressive disease | 0 | 1 | Not stated |
| Total | 1 | 1 | Not stated |
| Response rate, % (CR + PR) | 0 | 0 | Not stated |
| Comments | |||
| Patients withdrawn from trial due to adverse reactions | Intra-arterial mitomycin C with DSM (n = 30) | Intra-arterial mitomycin C without DSM (n = 30) | |
| Subjective | 3 | 0 | Not stated |
| Clinical chemistry | 3 | 5 | Not stated |
| Comments | |||
| Major adverse events (% patients) | Intra-arterial mitomycin C with DSM (n = unclear) | Intra-arterial mitomycin C without DSM (n = unclear) | |
| Pain | 59.3 | 16.7 | p < 0.01 |
| Gastrointestinal disturbances | 48.1 | 12.5 | p < 0.01 |
| Fever | 40.7 | 8.3 | p < 0.01 |
| Comments: two patients died; however, it is unclear whether they were from the patients with hepatocellular carcinoma or metastatic liver cancer. One died from a high dose of mitomycin C and in the other the cause remains uncertain | |||
| Other outcomes | |||
| n = 30 | n = 30 | ||
| Median survival times (days) | 294 | 231 | Not significant |
| Comments: haematological measures were reported; however, these have not been extracted. Overall, 21% (9/44) patients died of extrahepatic malignant disease | |||
| Intervention details | |||
| The study included people with hepatocellular carcinoma or metastatic liver cancer; only information for people with metastatic liver cancer was extracted Intra-arterial mitomycin C (< 8 mg/m2 body surface area), with DSM every second week. Mean DSM dose levels were 569.8 mg ± 173.6 mg Intra-arterial mitomycin C without DSM Treatment was administered through a catheter and port implanted. Prophylactic cholecystectomy was performed on most patients. Dose of DSM was individually established for each patient by angiography prior to first administration and was achieved through incremental injections of 300 mg DSM 1 minute apart. When arterial occlusion was achieved without back flow or pain, the appropriate dose was identified. If severe pain occurred, dosage was reduced by 150 mg subsequently. Treatment was given over a 17-week period and comprised three sessions. Each session consisted of three treatment occasions every second week making a total of nine treatments. Treatment was terminated if no response to therapy after the first session (three treatments) – classified as a progression of disease |
|||
| Methodological comments | |||
|
|||
| General comments | |||
|
|||
Quality assessment for randomised controlled trials (answer yes/no/not reported/unclear)
| 1. Was the method used to generate random allocations adequate? | Unclear |
| 2. Was the allocation adequately concealed? | Unclear |
| 3. Were the groups similar at the outset of the study in terms of prognostic factors ,e.g. severity of disease/size, number of tumours, performance status, extrahepatic disease | Yes |
| 4. Were outcome assessors blinded to the treatment allocation? | Unclear |
| 5. (i) Were there any unexpected imbalances in dropouts between groups? (ii) If so, were they explained or adjusted for? | No |
| 6. Is there any evidence to suggest that the authors measured more outcomes than they reported? | Unclear |
| 7. (i) Did the analysis include an intention-to-treat analysis? (ii) If so, was this defined? | No |
| 8. (i) Did the analysis account for missing data? (ii) If so, were the methods appropriate? | Not reported |
Chemoembolisation combined with laser ablation
Vogl et al., 201184
| Study and intervention details | Participants | Outcome measures |
|---|---|---|
| Country: Germany Funding: not stated Intervention: chemoembolisation followed by laser ablation NB: twenty-one participants were eligible for laser ablation at baseline, and only underwent one or two sessions of chemoembolisation first. The other participants had at least three sessions Number of centres: not stated |
Indication for treatment: breast cancer Intention of treatment: not stated Number of participants: 314 chemoembolisation, of whom 161 underwent laser ablation (reported here) Sample attrition/dropout: not stated Inclusion criteria: metastatic lesions of breast cancer, either in both liver lobes, locally non-resectable, or recurrent after partial liver resection. Those with existing contraindication to surgery, refused surgical treatment, had adverse reactions or no response to systemic chemotherapy also included. Five or fewer lesions, none larger than 5 cm in diameter Exclusion criteria: poor general condition (Karnofsky rating < 70%); presence of ascites, partial or complete thrombosis of the portal vein, poor hepatic synthesis (serum albumin < 2 mg/dl), high serum bilirubin (> 3 mg/dl), renal insufficiency (serum creatinine > 2 mg/dl) and respiratory or cardiovascular failure |
Outcomes: survival, response, time to progression, local tumour control rate, complications and adverse events Method of assessing outcomes: survival period defined as starting with the first chemoembolisation treatment and ending with death or last follow-up examination. Survival rate measured with the Kaplan–Meier method Response to combination treatment was evaluated using the Response Evaluation in Solid Tumours (RECIST) criteria Time to progression and the period for local tumour control rate were defined as starting as of completion of the last laser treatment and ending with progression or local recurrence. For those without progression or local recurrence, the end point was the last follow-up examination of the study. Time to progression included development of de novo metastasis and local intrahepatic recurrence. The local tumour control rate referred only to local recurrence in previously ablated areas Complications and adverse events obtained from imaging studies and from clinical and laboratory data, blood test results, ultrasound tests Length of follow-up: a mean of 13.8 (SD 17.1) months, maximum 90 months |
| Participant characteristics | Chemoembolisation + laser ablation (n = 161) | |
| Age (years), mean | 57 (SD 1.3), range 31–83 | |
| Male/female | All female | |
| Primary cancer site | Breast | |
| Number of liver metastases | Not stated | |
| Size of metastases | Mean diameter 3.36 (SD 1.94), range 0.6–12.5 cm | |
| Previous treatments for liver metastases | All had systemic chemotherapy, unclear if for metastatic disease | |
| Mitomycin C alone: 53 | ||
| Mitomycin C and gemcitabine: 108 | ||
| Comments | ||
Results
| Outcomes | Chemoembolisation + laser ablation (n = 161) |
|---|---|
| Mean survival period | 32.5 (SD 21.6) months, range 5–101 months |
| Estimated survival | |
| 1 year | 88.8% |
| 2 years | 55.9% |
| 3 years | 36.6% |
| 5 years | 13.7% |
| Comments: paper reports mean survival period for those who did or did not have progressive disease, not reported here. Also reports survival by those treated with mitomycin alone or mitomycin with gemcitabine, not reported here | |
| Response | |
| Complete | 62 (38.5%) |
| Partial | 8 (5%) |
| Stable disease | 20 (12.4%) |
| Progressive disease | 71 (44.1%) |
| Comments: 64 of those with progressive disease underwent additional chemoembolisation treatment | |
| Overall local tumour control | 13.1 (SD 15.9) months |
| Mean time to progression | 8.2 (SD 12.29) months, range 0–69 months |
| Local recurrence | 3 (1.9%) |
| Complications requiring further intervention (aspiration or drainage) | 6 (3.7%) |
| 4 pleural effusions | |
| 2 biloma | |
| Side effects of laser ablation | 57 (35.4%) reactive pleural effusion (4 reported above) |
| 15 (9.3%) biloma (2 reported above) | |
| 11 (6.8%) subcapsular haematoma | |
| 17 (10.6%) small basal lung atelectasis | |
| 0 deaths | |
| 0 seeding | |
| Comments | |
| General comments | |
|
|
Quality assessment for case series studies (answer yes/no/not reported/unclear)
| 1. Were the patient selection criteria specified a priori? | Yes |
| 2. Was the participant blinded to the research question? | Not reported |
| 3. Is there any evidence to suggest that the authors measured more outcomes than they reported? | Unclear |
| 4. Were withdrawals and dropouts completely described? | Not reported |
| 5. (i) Did the analysis account for missing data? (ii) If so, were the methods appropriate? | Not reported |
Vogl et al., 200383
| Study and intervention details | Participants | Outcome measures |
|---|---|---|
| Country: Germany Funding: not stated Intervention: chemoembolisation followed by laser ablation. Also reports case series data on those just receiving chemoembolisation, not reported here Number of centres: not stated |
Indication for treatment: various primary cancers (see below). Patients had systemic chemotherapy and either developed progressive disease or had not responded; surgical resection of primary tumour, unresectable synchronous or metachronous liver metastases Intention of treatment: not stated Number of participants: 162 chemoembolisation, of which, 82 underwent laser ablation (reported here) Sample attrition/dropout: not stated Inclusion criteria: unresectable liver metastases that showed no response to systemic chemotherapy, as seen at contrast material-enhanced MRI, no more than four metastases, and no extra-hepatic spread. Two of the four metastases were allowed to have a diameter between 50 and 80 mm, but the other had to be smaller than 50 mm. Those between 50 and 80 mm were targeted with chemoembolisation Exclusion criteria: poor performance status (Karnofsky status ≤ 70%), nutritional impairment, presence of neoplastic ascites, high serum total bilirubin (> 3 mg/dl), poor hepatic synthesis (serum albumin < 2.0 mg/dl), renal failure (serum creatinine > 2 mg/dl), partial or complete thrombosis of the portal vein, cardiovascular or respiratory failure, poor mental state, unable to provide consent |
Outcomes: treatment success, survival, side effects and complications Method of assessing outcomes: tumour volume calculated from magnetic resonance images. Treatment success defined as achievement of shrinkage of target lesions to a diameter of less than 50 mm so that local ablation was possible. Stable disease defined as no substantial change in size during the chemoembolisation treatment. Progressive disease defined as an increase in size of a target lesion during chemoembolisation or newly developing lesions in the liver (these outcomes were used to determine which participants would receive laser treatment) Survival calculated from commencement of the first chemoembolisation treatment by using Kaplan–Meier method Complications and side effects by questionnaire where symptoms were rated as ‘none’, score 1; ‘few’, score 2; ‘moderate’, score 3; ‘marked’, score 4 Length of follow-up: not stated |
| Participant characteristics | Chemoembolisation + laser ablation (n = 82) | |
| Age (years) | Total group only reported, n = 162. Mean age 61.8 (range 23.2–88.3) | |
| Male/female | 43/39 | |
| Primary cancer site | Colorectal: 62; breast: 14; other: 6 | |
| Number of liver metastases | Average of 1.2 lesions per participant | |
| Size of metastases | Unclear, only those > 50 mm or larger recorded | |
| Previous treatments for liver metastases | All had systemic chemotherapy | |
| Comments: a mean decrease in tumour size of 35% (SD 14) was estimated at follow-up after chemoembolisation, so allowing follow-up with laser ablation | ||
Results
| Outcomes | Chemoembolisation + laser ablation (n = 82) | |
|---|---|---|
| Cumulative survival after first treatment | 24.9 months (median, 26.2; 95% CI 20.3 to 32.9) | |
| Comments: paper also reports survival rates by categories of tumour load (umber of lesions, vascularisation, and diameter) but unclear whether this is for the total group or the combined group | ||
| Complications | No to few symptoms | Moderate to marked symptoms |
| Abdominal pain | 57.1% | 42.9% |
| Nausea | 82.2% | 17.8% |
| Fevera | 89.3% | 10.7% |
| Fevera | 86.4% | 13.6% |
| Lethargy | 38.4% | 61.6% |
| Comments: no major complications such as bleeding or abscess were observed immediately after chemoembolisation, no major complications occurred during and immediately after laser treatment. In seven (8.5%) minor complications such as pain, pleural effusion or subcapsular haematoma were noted One major complication (1.2%) was observed within 30 days of the laser treatment; a 73-year-old patient died, most likely as a result of sepsis a Fever was indicated with a temperature of > 38.5 °C |
||
| General comments | ||
|
||
Quality assessment for case series studies (answer yes/no/not reported/unclear)
| 1. Were the patient selection criteria specified a priori? | Yes |
| 2. Was the participant blinded to the research question? | Not reported |
| 3. Is there any evidence to suggest that the authors measured more outcomes than they reported? | Unclear |
| 4. Were withdrawals and dropouts completely described? | Not reported |
| 5. (i) Did the analysis account for missing data? (ii) If so, were the methods appropriate? | Not reported |
Radioembolisation
Grey et al., 200185
| Study details | Participants | Outcome measures | |
|---|---|---|---|
| Country: Australia Design: RCT Number of centres: one Funding: not stated Intervention (1) radioembolisation (SIRT) and hepatic artery chemotherapy; (2) hepatic artery chemotherapy See below for intervention details |
Indication for treatment: non-resectable liver metastases from primary adenocarcinoma of the colon Intention of treatment: not stated Number of participants: (1) radioembolisation, n = 36; (2) hepatic artery chemotherapy (HAC), n = 34. Total n = 74 but n = 70 eligible for trial entry Sample attrition/dropout: four participants deemed ineligible due to the presence of unconfirmed disseminated cancer at the time of randomisation. One participant in the SIRT group died before any protocol treatment could be administered Inclusion criteria: non-resectable metastases limited to the liver and lymph nodes in the porta hepatis, WHO performance status 0–2, adequate haematological and hepatic function (not defined). Previous systemic chemotherapy for metastases eligible All participants underwent a full laparotomy to confirm the non-resectable status of the metastases, ensure no intra-abdominal spread of the tumour, and to insert a hepatic artery catheter and port. Catheter positioned to ensure perfusion of the whole liver Exclusion criteria: distant metastases, previous radiotherapy to the liver, evidence of cirrhosis or ascites, those in whom the liver metastases could be treated by local ablation (surgical resection or cryotherapy) |
Primary outcomes: initially median survival, changed subsequently to time to disease progression and treatment response when regulator changed their criteria Secondary outcomes: quality of life, toxicity Method of assessing outcomes: definitions of outcomes reported here Partial response (PR): objectively measured decrease in tumour size (area and volume, see below), by 50% or more on two successive CT scans not less than 3 months apart after randomisation and before evidence of progressive disease in the liver and before any non-protocol treatments had been given Complete response (CR): the disappearance of all tumour on two successive CT scans not less than 3 months apart after randomisation and before evidence of progressive disease in the liver and before any non-protocol treatments had been given Progressive disease in the liver (PD): same three objective measures that determine response to treatment and defined as (i) an increase on any occasion in cross-sectional tumour area, or tumour volume, by 25% or more over the nadir as measured on serial CT scans, (ii) the development of new lesions in the liver or (iii) an increase in the serum CEA (details not extracted, see below) No change (NC): either a decrease in tumour area, volume, CEA that is less than that required for a PR, or an increase that is less than that required for PD Not assessable (NA): for any of the response criteria was attributed to those who had either (i) no follow-up CT scans due to rapid deterioration after randomisation, or (ii) unmeasureable index lesions for estimating cross-sectional tumour areas Definitions of response based on CEA (carcinoembryonic antigen) were also provided and used in the study but have not been data extracted Quality of life was recorded at 3-monthly intervals using a validated 13-point linear analogue Self Assessment Scale (recommended by cited reference), 11 measures in the questionnaire but no further details Toxicity was recorded using standard UICC (Union for International Cancer Control) criteria Survival time from randomisation to death or last follow-up if still alive Length of follow-up: minimum of 3.5 years from randomisation |
|
| Participant characteristics | Radioembolisation + HAC (n = 36) | HAC (n = 34) | p-value |
| Age (years), mean | 59 | 62 | Not reported |
| Male/female | 28/8 | 26/8 | Not reported |
| Primary cancer site | Not reported | ||
| Colon/rectum | 29/7 | 31/3 | |
| Involved lymph nodes | 24 | 24 | |
| Poorly differentiated | 5 | 5 | |
| Number of liver metastases | Not reported | Not reported | |
| Distribution of liver metastases | Bi-lobar | Bi-lobar | |
| Size of metastases | Not reported | ||
| < 25% | 24 | 24 | |
| 25%–50% | 9 | 8 | |
| > 50% | 3 | 2 | |
| Previous treatments for primary cancer | |||
| Complete surgical resection, n | 36 | 34 | |
| Systemic chemotherapy | n not reported | n not reported | |
| Time from primary treatment, mean/median days | 137/56 (therefore ≤ 12 months) | 135/57 (therefore ≤ 12 months) | |
| Previous treatment for liver metastases, n participants | 5 (14%) | 5 (15%) | Not reported |
| Time from previous liver mets treatment | Not reported | Not reported | |
| Comorbidities (including other metastases) | Not reported | Not reported | |
| Comments: only 35/36 radioembolisation and HAC participants received treatment as one died before any treatment could be started | |||
Results
| Outcomes | Radioembolisation + HAC (n = 36) | HAC (n = 34) | p-value |
|---|---|---|---|
| Response measured by | |||
| Tumour area | CR = 2 | CR = 0 | Difference between groups p = 0.01 |
| PR = 14 | PR = 6 | ||
| NC = 13 | NC = 13 | ||
| PD = 3 | PD = 8 | ||
| NA = 4 | NA = 7 | ||
| CR or PR: 44% | CR or PR: 18% | p = 0.01 | |
| Tumour volume | CR = 2 | CR = 1 | Difference between groups p = 0.03 |
| PR = 16 | PR = 7 | ||
| NC = 10 | NC = 12 | ||
| PD = 5 | PD = 9 | ||
| NA = 3 | NA = 5 | ||
| CR or PR: 50% | CR or PR: 24% | p = 0.03 | |
| Comments: states one participant in each arm had disease reduced to such an extent that they were subsequently deemed surgically resectable and underwent surgical excision of metastases Also states that by all measures of response (volumes, areas and CEA) more participants in the HAC-alone arm had Progressive Disease as their best response to treatment. Response measured by CEA not data extracted |
|||
| Median time to progression in the liver | Radioembolisation + HAC (n = 36) | HAC (n = 34) | p-value |
| By tumour area | 15.9 months | 9.7 months | p = 0.001 |
| By tumour volume | 12.0 months | 7.6 months | p = 0.04 |
| Comments: time to progression presented in graphical form, only data are available in the abstract. Time to progression by CEA not data extracted | |||
| Survival rates per year | Radioembolisation + HAC (n not reported) | HAC (n not reported) | p-value |
| Median overall survival, months (mean OS also reported but not extracted) | 17 | 15.9 | HR 1.41, 95% CI 0.86 to 2.34, p = 0.18 |
| 1 year | 72% | 68% | NS (see comment) |
| 2 years | 39% | 29% | |
| 3 years | 17% | 6% | |
| 5 years | 3.5% | 0% | |
| Comments: states shows a non-significant trend towards increased survival for those treated with SIRT compared with those treated with HAC alone. The hazard ratios suggest those receiving HAC alone have approximately a 40% higher death rate than those receiving SIRT Cox regression suggests those treated with SIRT who survive more than 15 months experience a survival advantage compared with those treated with HAC alone (p = 0.06). This is not evident for those surviving less than 15 months |
|||
| Radioembolisation + HAC (n = 36) | HAC (n = 34) | p-value | |
| Mortality | n = 32 deaths | n = 33 deaths | |
| States 65 participants have died, four remain alive, three in radioembolisation and one in HAC groups. Reviewers assume that the one participant who died prior to treatment starting is not counted here as n = 69 Also reports the cause of death specifically related to disease progression of the liver metastases. Not reported here, as no details as to how this was ascertained. Extrahepatic metastases caused or contributed to some deaths |
|||
| Radioembolisation + HAC (n not reported) | HAC (n not reported) | p-value | |
| Complications | 1 (pancreatitis) | ||
| Toxicities | |||
| Haemoglobin grade 3 | – | 1 | |
| Bilirubin grade 3 | 1 | – | |
| Aspartate aminotransferase grade 3 | 5 | 12 | |
| Aspartate aminotransferase grade 4 | 2 | 2 | |
| Alkaline phosphatase grade 3 | 14 | 5 | |
| Nausea/vomiting grade 3 | 1 | 2 | |
| Diarrhoea grade 3 | – | 1 | |
| Total number grade 3 and 4 events | 23 | 23 | |
| Comments: states there was no difference in rates of any grade 3 and 4 toxicity events between the two treatment arms (based on total number of toxicity events being 23 in each arm) States there were more grade 1 and 2 toxicity events for those receiving SIRT for assessments of liver function tests (300 vs. 207 events) and nausea and diarrhoea (16 vs. 11 events) |
|||
| Quality of life | Radioembolisation + HAC (n = 36) | HAC (n = 34) | p-value |
| Comments: no data presented, states that only sexual interest/ability deteriorated over the 18-month period during which the protocol treatments were delivered. For all other measures, there were trends towards improvement in QoL scores over the first 18 months for both treatment groups. There were no significant differences between the treatment groups | |||
| Mean dose (SD) of yttrium-90 received (GBq) | 2.156 (0.324) | Not applicable | |
| Comments: states five participants received less and one participant more than the designated protocol amount of ytttrium-90 activity, but all received within 90% of the 2–3 GBq required by the protocol. The three ineligible patients in this treatment arm received a mean of 2.276 (SD 0.231) of GBq of yttrium-90 activity | |||
| Mean (SD) dose of floxuridine received (mg), per patient, | 1863 (1735) | 1822 (1323) | |
| Mean (SD) number of cycles of floxuridine | 8.7 (5.6) | 8.0 (5.0) | |
| Intervention details | |||
| 1) Radioembolisation and hepatic artery chemotherapy group | |||
| Hepatic artery chemotherapy with floxuridine (dose as noted below for group 2) plus single injection of SIR-Spheres® (Sirtex Medical Ltd), radioactive yttrium-90 microspheres administered as SIRT. SIR-Spheres® administered within 4 weeks of the insertion of the access port (except in one where it was administered at the same time as the placement of the access port) Nuclear medicine scan to determine the amount of SIR-Spheres® that would pass through the liver and lodge in the lungs (required to be < 10% lung break through) otherwise dose reduction required 50 μg angiotensin-2 injected over 30-second period, then 30 seconds later the SIR-Spheres® were injected over a period of several minutes. The quantity of SIR-Spheres® varied depending on the size of the tumour: < 25% = 2 GBq, 25–50% = 2.5 GBq, > 50% = 3 GBq of yttrium-90 activity. Per protocol this would be reduced if the lung–liver break through percentage was > 10% (by 2% for each 1% over) but no participant required this adjustment |
|||
| 2) Hepatic artery chemotherapy group | |||
| Hepatic artery chemotherapy with floxuridine only. Twelve-day cycles of continuous infusion at 0.3 mg/kg body weight per day, repeated at 4-weekly intervals, for 18 cycles or until there was evidence of either tumour progression in the liver, the development of extrahepatic metastases necessitation a change to systemic treatment, unacceptable toxicity, port failure, or at a participant’s request Details of post-intervention treatments: many participants received non-protocol chemotherapy (mainly 5-flurouracil plus folinic acid and mitomycin-C systemically or via the port, or floxuridine into the port). The total amount used was higher for those in the HAC-only arm (5-FU, 36% more, floxuridine 59% more) |
|||
| Methodological comments | |||
|
|||
| General comments | |||
|
|||
Quality assessment for randomised controlled trials (answer yes/no/not reported/unclear)
| 1. Was the method used to generate random allocations adequate? | Unclear |
| 2. Was the allocation adequately concealed? | Unclear |
| 3. Were the groups similar at the outset of the study in terms of prognostic factors, e.g. severity of disease, size, number of tumours, performance status, extrahepatic disease? | Yes |
| 4. Were outcome assessors blinded to the treatment allocation? | Yes |
| 5. (i) Were there any unexpected imbalances in dropouts between groups? (ii) If so, were they explained or adjusted for? | No |
| 6. Is there any evidence to suggest that the authors measured more outcomes than they reported? | Unclear |
| 7. (i) Did the analysis include an intention-to-treat analysis? (ii) If so, was this defined? | Yes No |
| 8. (i) Did the analysis account for missing data? (ii) If so, were the methods appropriate? | Not reported |
Hendlisz et al., 201086
| Study and intervention details | Participants | Outcome measures | |
|---|---|---|---|
| Country: Belgium Design: RCT Number of centres: three Funding: support from 2 of the authors and Sirtex Medical Ltd. provided the microspheres Intervention: (1) radioembolisation plus systemic chemotherapy; (2) systemic chemotherapy only See below for intervention details |
Indication for treatment: metastases of colorectal cancer (mCRC) limited to liver, all other evidence-based treatments failed Intention of treatment: not stated Number of participants: n = 46. (1) radioembolisation plus systemic chemotherapy, n = 23 but two not treated (see below); (2) systemic chemotherapy only, n = 23 Sample attrition/dropout: two patients in the radioembolisation plus systemic chemotherapy arm were found to be ineligible Inclusion criteria: histologically proven adenocarcinoma of colon or rectum, metastases in liver only, not amenable to curative surgery or local ablation and resistant or intolerant to standard chemotherapy [fluorouracil (FU), oxaliplatin, and irinotecan]. If chemotherapy stopped due to intolerance, documented progressive disease was required. ECOG performance status of 0 to 2, ≥ 18 years of age. Adequate bone marrow function (absolute neutrophil count ≥ 1000/µL, platelet count ≥ 100,000/µL), renal function [creatinine < 1.5 × upper limit of normal limit (ULN) or creatinine clearance > 50 ml per minute], and liver function [defined by direct bilirubin < 1.0 × ULN; aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase levels each < 5 × ULN]. Able to give informed consent Exclusion criteria: pre-existing hepatic disease (cirrhosis > Child–Pugh B, liver abscess, hepatic sarcoidosis or tuberculosis, sclerosing cholangitis); extrahepatic disease; clinically significant ascites; > 20% arteriovenous shunting from liver to lungs observed on the technetium-99m-labelled macroaggregated albumin (99mTc-MAA) scan; hepatic arterial anatomy that would not allow safe administration of yttrium-90 (90Y) microspheres, partial or total thrombosis or hepatic artery or main portal vein, prior hepatic arterial infusion with FU, floxuridine (FUDR), or other chemotherapeutic agent(s) or transarterial embolisation procedures; prior external-beam irradiation of the liver; severe chronic or acute disease, concomitant or previous malignancies within 5 years other than basal cell or squamous cell carcinoma of the skin or cervix; pregnant or breastfeeding women, women refusing to take adequate pregnancy prevention measures |
Primary outcomes: time to liver progression (TTLP) Secondary outcomes: overall survival, time to progression overall (TTP), adverse events Method of assessing outcomes: physical examination and blood tests every 3 weeks, CT scans of chest, abdomen and pelvis repeated every 6 weeks until disease progression Disease progression in the liver and objective tumour response based on Response Evaluation Criteria in Solid Tumours (RECIST, version 1.0) and documented by CT or MRI. Radiologic tumour assessment could be repeated early on the basis of clinical need or suspicion of disease progression at the investigators’ discretion TTLP and time to tumour progression (TTP) were calculated as time elapsed between randomisation and first documented progression in the liver or first documented progression at any site, death or date of last observation (in patients lost to follow-up) Overall survival defined as time elapsed between randomisation and death from any cause Adverse events classified and coded for severity using Common Terminology Criteria for Adverse Events (CTCAE, version 3.0) Length of follow-up: median (range) 24.8 (2 to 41) months at time of database closure (for the seven patients still alive, median follow-up was 10 months) |
|
| Participant characteristics | Radioembolisation + chemotherapy (n = 21) | Chemotherapy only (n = 23) | p-value |
| Age (years), median (range) | 62 (46–91) | 62 (45–80) | |
| Male/female, n (%) | 18/5 (78/22) | 10/11 (48/52) | |
| Primary cancer site | Colorectal | Colorectal | |
| Primary cancer type, n (%) | |||
| Adenocarcinoma | 21 (100) | 22 (96) | |
| Adenocarcinoma mucinous | 0 | 1 (4) | |
| Number of liver metastases measured, n (%) | |||
| 1 lesion | 2 (10) | 1 (4) | |
| 2–4 lesions | 10 (48) | 10 (44) | |
| ≥ 5 lesions | 8 (38) | 10 (44) | |
| Not measurable | 1 (5) | 2 (9) | |
| Distribution of liver metastases | Not reported | Not reported | |
| Size of metastases | |||
| Sum of lesions diameters (mm), median (range) | 176.5 (31–324), 1 missing value | 216 (51–416), two missing values | |
| Previous treatments for primary cancer | Not reported, see below for chemotherapy | Not reported, see below for chemotherapy | |
| Time from primary treatment | Not reported | Not reported | |
| Time since diagnosis, months, median (range) | 22 (7–52), 1 missing value | 22 (12–44), no missing values | |
| Previous treatment for liver metastases | Not reported | Not reported | |
| Time from previous liver mets treatment | Not reported, but see detail re chemo below which assumed to be for metastases | Not reported, but see detail re chemotherapy below which is assumed to be for metastases | |
| Time since last chemotherapy (weeks), median (range) | 8 (2–57), 2 missing values | 14 (2–60), no missing values | |
| Last chemotherapy regimen received before study entry, n (%) | |||
| Irinotecan based | 13 (62) | 20 (87) | |
| Oxaliplatin based | 4 (19) | 2 (9) | |
| Other based | 4 (19) | 1 (4) | |
| Comorbidities | |||
| Presence of non-target lesions, n (%) | 5 (24), 1 missing value | 6 (26), one missing value | |
| ECOG performance status, n (%) | |||
| 0 | 15 (71) | 17 (74) | |
| 1 | 5 (24) | 5 (22) | |
| 2 | 1 (5) | 1 (4) | |
| Comments: the two participants in the radioembolisation plus chemotherapy group who were found to be ineligible are not included, hence, n = 21. If lesions were not measurable this was mainly due to extensive and confluent lesions | |||
Results
| Outcomes | Radioembolisation + chemotherapy (n = 21) | Chemotherapy only (n = 23) | p-value |
|---|---|---|---|
| Partial response, n (%) | 2 (10) | 0 (0) | 0.22 (95% CI for difference between arms − 0.10 to 0.32) |
| Stable disease, n (%) | 16 (76) | 8 (35) | Not reported |
| Progressive disease, n (%) | 2 (10) | 14 (61) | Not reported |
| Non-evaluable, n (%) | 1 (5) | 1 (4) | Not reported |
| Disease control rates (partial response + stable disease), n/N (%) | 18/21 (86) | 8/23 (35) | 0.001 (95% CI for difference between arms 0.19 to 0.71) |
| Comments: data are the best response for target lesions according to RECIST criteria. Overall response rates are equivalent to the partial response rates as no complete responses occurred | |||
| Outcomes | Radioembolisation + chemotherapy (n = 21) | Chemotherapy only (n = 23) | p-value |
| Liver progression documented, n | 18 | 23 | |
| TTLP (months), median | |||
| All progressions considered as events | 5.5 | 2.1 | HR 0.38; 95% CI 0.20 to 0.72; p = 0.003 |
| Patients with treatment change censored at the time of change | 5.6 (15 events) | 2.1 (22 events) | HR 0.35; 95% CI 0.18 to 0.69; p = 0.002 |
| TTP (months), median | 4.5 | 2.1 | HR 0.51; 95% CI 0.28 to 0.94; p = 0.03 |
| Overall survival (months), median | 10.0 (five patients alive at time of analysis) | 7.3 (two patients alive at time of analysis) | HR 0.92; 95% CI 0.47 to 1.78; p = 0.80 |
| Comments: all patients in the chemotherapy-only arm experienced disease progression first in the liver. For these patients, TTLP equals TTP. Three patients in the radioembolisation arm were without documented progression and were censored at 4.3, 6.6, and 26 months. A Kaplan–Meier plot of TTLP and TTP is presented in the paper. Local progression was documented in four patients (three radioembolisation + chemo; one chemotherapy-only arm) after an unjustified change in the treatment allocated by randomisation (not further described). Censoring these four patients does not change the median TTLP. One patient in the radioembolisation + chemotherapy arm had sufficient downsizing of their liver metastasis to receive a right hepatectomy. The rapid cross-over of 70% of patients in the chemotherapy-only arm to receive further therapy (see below) may have confounded the survival data | |||
| Radioembolisation + chemotherapy (n = 21) | Chemotherapy only (n = 23) | p-value | |
| Received further therapy | n = 9 | n = 16 | |
| Crossed over to radioembolisation monotherapy | Not applicable | n = 10 | |
| Cetuximab combined with chemotherapy | n = 3 | n = 5 | |
| Chemotherapy alone | n = 4 | n = 1 | |
| Palliative brain radiotherapy | n = 1 | ||
| Unspecified treatment | n = 1 | ||
| Comments: chemotherapy received as further therapy is not described | |||
| Radioembolisation + chemotherapy (n = 21) | Chemotherapy only (n = 23) | p-value | |
| Toxicity analysis | n = 21 | n = 22, see below | |
| Grade 3 or 4 toxicity recorded | n = 1 | n = 6 | 0.10 |
| Gastrointestinal | |||
| Stomatitis | n = 1 Grade 1 | n = 1 Grade 1 | |
| n = 1 Grade 2 | n = 1 Grade 3 | ||
| Diarrhoea | n = 1 Grade 1 | ||
| Nausea | n = 4 Grade 1 | ||
| n = 1 Grade 2 | |||
| Vomiting | n = 2 Grade 2 | n = 2 Grade 2 | |
| Constipation | n = 3 Grade 1 | ||
| Anorexia | n = 4 Grade 1 | n = 4 Grade 1 | |
| n = 1 Grade 2 | n = 2 Grade 2 | ||
| n = 1 Grade 3 | |||
| Gastrointestinal | n = 1 Grade 2 | ||
| Pain | |||
| Abdominal pain | n = 3 Grade 1 | n = 2 Grade 1 | |
| n = 1 Grade 2 | n = 1 Grade 2 | ||
| Myalgia | n = 2 Grade 1 | n = 1 Grade 1 | |
| Other pain | n = 1 Grade 1 | ||
| Constitutional | |||
| Fatigue | n = 4 Grade 1 | n = 2 Grade 1 | |
| n = 4 Grade 2 | n = 4 Grade 2 | ||
| n = 5 Grade 3 | |||
| Fever | n = 2 Grade 1 | n = 1 Grade 1 | |
| n = 1 Grade 2 | n = 2 Grade 2 | ||
| Dermatology/skin | |||
| Skin | n = 2 Grade 2 | ||
| Hand–foot syndrome | n = 1 Grade 3 | n = 2 Grade 2 | |
| Pulmonary | |||
| Dyspnoea | n = 1 Grade 2 | ||
| n = 1 Grade 3 | |||
| Pulmonary | n = 1 Grade 3 | ||
| Neurology | |||
| Neurosensorial | n = 2 Grade 1 | ||
| Cognitive disturbance | n = 1 Grade 2 | n = 1 Grade 2 | |
| Cardiac arrhythmia | n = 1 Grade 1 | ||
| Allergy/immunology | |||
| Allergy | n = 1 Grade 3 | ||
| Other toxicity | n = 1 Grade 1 thrombocytopenia | n = 1 Grade 2 ascites | |
| n = 1 Grade 2 stomach ulcer, ascites | |||
| Comments: states two participants in the chemotherapy arm were never treated and so were not evaluated for toxicity; however, gives n = 22 for the chemotherapy arm whereas n = 21 would be expected if two patients were excluded from this analysis Events listed by CTCAE (version 3.0) grade. Discussion notes that the probable reason for more patients in the chemotherapy arm experiencing grade 3 toxicities was the lower efficacy and more rapidly progressive disease in this arm – with adverse events essentially indistinguishable from those due to disease progression. Also the FU dose intensity was not significantly higher in the chemotherapy-only arm which may partly explain some differences |
|||
| Treatment received | Radioembolisation + chemotherapy (n = 21) | Chemotherapy only (n = 23) | |
| 90Y activity administered (GBq), median (range) | 1.79 (1.32–2.15), 3 (14%) missing values | Not applicable | |
| FU dose per patient (mg), median (range) | 14,588 (4740–97,612), 1 missing value | 17,700 (3240–119,700), 3 (13%) missing values | |
| Intervention details | |||
| Radioembolisation plus systemic chemotherapy: radioembolisation (dose details below) plus intravenous FU 225 mg/m2 for 14 days followed by 1 week of rest. Thereafter protracted intravenous (PIV) FU 300 mg/m2 for 14 days every 3 weeks until documented hepatic progression. Therapy was organised within 14 days of excluding lung shunting. All patients were treated only once Administered activity of 90Y-microspheres for radioembolisation: described as stepwise administration. Dose calculated according to manufacturer’s instructions based on body surface area (BSA) and extent of tumour involvement using the equation below:Activity injected (GBq)=(BSA−0.2)+(tumour volumetotal liver volume)For hepatopulmonary shunting of 10% to 15% activity was decreased by 20%, for shunting of 15% to 20% activity decreased by 40%. Radioembolisation contraindicated if shunting > 20% Systemic chemotherapy: PIV infusion of FU 300 mg/m2 for days 1 to 14 every 3 weeks until progression. For ethical reasons, patients with documented progression were permitted to cross-over to receive radioembolisation at the investigators’ discretion Chemotherapy (presumably in both study arms) continued until disease progression, unacceptable toxicity, or withdrawal of consent |
|||
| Methodological comments | |||
|
|||
| General comments | |||
|
|||
Quality assessment for randomised controlled trials (answer yes/no/not reported/unclear)
| 1. Was the method used to generate random allocations adequate? | Unclear |
| 2. Was the allocation adequately concealed? | Not reported |
| 3. Were the groups similar at the outset of the study in terms of prognostic factors, e.g. severity of disease/size, number of tumours, performance status, extrahepatic disease | Yes |
| 4. Were outcome assessors blinded to the treatment allocation? | No |
| 5. (i) Were there any unexpected imbalances in dropouts between groups? (ii) If so, were they explained or adjusted for? | No |
| 6. Is there any evidence to suggest that the authors measured more outcomes than they reported? | Unclear |
| 7. (i) Did the analysis include an intention-to-treat analysis? (ii) If so, was this defined? | No |
| 8. (i) Did the analysis account for missing data? (ii) If so, were the methods appropriate? | No |
Van Hazel et al., 200487,88
| Study and intervention details | Participants | Outcome measures | |
|---|---|---|---|
| Country: Australia Design: RCT Number of centres: three Funding: not stated Intervention: (1) radioembolisation (SIRT) + systemic chemotherapy; (2) systemic chemotherapy See below for intervention details |
Indication for treatment: multiple bilobar colorectal liver metastases with or without extrahepatic metastases Intention of treatment: not stated Number of participants: (1) SIRT + chemotherapy n = 11; (2) chemotherapy only n = 10 Sample attrition/dropout: two deaths in the chemotherapy-only arm occurred before any protocol treatment could be given Inclusion criteria: adenocarcinoma of the colorectum (histologically proven). Age 18 or over. Unequivocal CT scan evidence of liver metastases not treatable by resection or any locally ablative technique. Adequate haematological, hepatic and renal function (no definitions provided). WHO performance status < 3 Exclusion criteria: receipt of chemotherapy or radiotherapy for the liver metastases. Central nervous system metastases. Evidence of cirrhosis, ascites or portal hypertension |
Primary outcomes: response rate and toxicity Secondary outcomes: time to progressive disease, survival, quality of life Method of assessing outcomes: three-monthly clinical evaluation and quality-of-life assessment, 3-monthly CT scans of the abdomen and either chest radiography or CT scan of the chest. Monthly serological tests of haematological, liver and renal function and CEA Patients lost to follow-up or dying before any follow up scans had been performed were regarded as having progressed in the liver at the time of death Response: determined using Response Evaluation Criteria In Solid Tumours (RECIST) criteria (reference provided) Patients with complete response or partial response on CT scan had a second confirmatory CT scan at not less than 4 weeks after the initial scan that showed the response. Progressive disease is a measure that treatment is no longer effective Toxicity: recorded on all patients using standard Union for International Cancer Control recommendations (no reference provided) for grading of acute and subacute toxicity criteria Quality of life: measured at randomisation and then 3-monthly using the validated 23-point Functional Living Index – Cancer questionnaire (reference provided). Clinicians completed an assessment of patients’ well-being at the same intervals using the Spitzer index (reference provided) Length of follow-up: not stated |
|
| Participant characteristics | SIRT + systemic chemotherapy (n=11) | Systemic chemotherapy only (n=10) | p-value |
| Age (years) | 64 | 65 | |
| Male/female | 10/1 | 8/2 | |
| Primary cancer site | Adenocarcinoma of colorectum | Adenocarcinoma of colorectum | |
| Number of liver metastases | Multiple (no number reported) | Multiple (no number reported) | |
| Distribution of liver metastases | Multiple bilobar | Multiple bilobar | |
| Size of metastases | |||
| < 25% | 8 | 7 | |
| > 25% | 3 | 3 | |
| Previous treatments for primary cancer | Not reported | Not reported | |
| Time from primary treatment | Not reported | Not reported | |
| Previous treatment for liver metastases | None | None | |
| Time from previous liver mets treatment | NA | NA | |
| Comorbidities – extrahepatic metastases | 2 (2 lung) (18%) | 3 (2 lung, 1 peritoneal cavity) (30%) | |
| Histological differentiation of primary bowel cancer | Poor (n = 1) | Poor (n = 2) | |
| Moderate (n = 10) | Moderate (n = 6) | ||
| Well (n = 0) | Well (n = 2) | ||
Results
| Outcomes | SIRT + systemic chemotherapy (n = 11) | Systemic chemotherapy only (n = 10) | p-value |
|---|---|---|---|
| Mortality (time interval not specified) | 10 | 10 | |
| First integrated response | p < 0.001 | ||
| Complete response | 0 | 0 | |
| Partial response | 10 | 0 | |
| Stable disease | 1 | 6 | |
| Progressive disease | 0 | 4 | |
| Best confirmed response | p < 0.001 | ||
| Complete response | 0 | 0 | |
| Partial response | 8 | 0 | |
| Stable disease | 3 | 6 | |
| Progressive disease | 0 | 4 | |
| Comments: states p < 0.001 for comparison between groups below the table containing these data but not clear exactly what the comparison(s) is (are). There was no difference in response rate between patients receiving the standard 2.5-GBq yttrium-90 activity or the individualised dose | |||
| Median time to progressive disease (PD), months | 18.6 | 3.6 | < 0.0005 |
| Site of first disease progression | |||
| Liver | 8 | 8 | |
| Liver and peritoneum combined | 1 | ||
| Bone | 1 | ||
| Liver and lung | 1 | ||
| Lung | 1 | ||
| Died from chemotherapy-related sepsis without progression | 1 | ||
| Outcomes | SIRT + systemic chemotherapy (n = 11) | Systemic chemotherapy only (n = 10) | p-value |
| Median survival, months | 29.4 | 12.8 | HR 0.33; 95% CI 0.12 to 0.91; p = 0.025 |
| Median survival excluding two patients in the chemotherapy group who did not receive treatment, months | 29.4 | 14.1 | HR 0.39; 95% CI 0.14 to 1.13; p = 0.07 |
| Comments: at the time of analysis one SIRT + chemotherapy patient remained alive; all others had died. Kaplan–Meier survival curve is available in the paper | |||
| SIRT + systemic chemotherapy (n = 11) | Systemic chemotherapy only (n = 10) | p-value | |
| Treatment related death | 1 (sepsis, due to chemotherapy induced neutropenia) | ||
| Liver abscess | 1 | ||
| Radiation induced liver cirrhosis | 1 | ||
| Transient abdominal pain at time of injection | 4 | ||
| Number of grade 3 and 4 toxicity events experienced during protocol | SIRT + systemic chemotherapy (n = 11) | Systemic chemotherapy only (n = 10) | p-value |
| Granulocytopenia | 3 | 0 | |
| Nausea, vomiting | 1 | 1 | |
| Mucositis | 4 | 1 | |
| Gastritis | 1 | 1 | |
| Diarrhoea | 2 | 1 | |
| Anorexia | 0 | 1 | |
| Cirrhosis | 1a | 0 | |
| Liver abscess | 1a | 0 | |
| Liver function tests | 0 | 0 | |
| Total number of events | 13 | 5 | |
| Comments: a It is assumed that these events are the same as those mentioned in the section above |
|||
| Quality of life | SIRT + systemic chemotherapy (n = 11) | Systemic chemotherapy only (n = 10) | p-value |
| Comments: no numerical outcome data reported. However, paper states that changes from baseline patient-rated quality of life for the first 3 months of treatment were almost identical in both arms (p = 0.96). This was also the case for physician-rated quality of life (p = 0.98). After 3 months the number of quality-of-life assessments in the chemotherapy-only arm diminished due to disease progression. The lack of variation from baseline during the first 3 months may have been because most patients were still receiving chemotherapy during this time. Abstract88 states that at 3 months the SIRT group showed statistically significant improvement in HRQoL compared with the chemotherapy-only group (p = 0.03, 95% CI 1.4 to 27.6), but was not statistically significantly different thereafter. This appears to be different from the information provided in the main publication | |||
| SIRT + systemic chemotherapy (n = 11) | Systemic chemotherapy only (n = 10) | p-value | |
| Total number of chemotherapy cycles | 89 | 38 | |
| Fluorouracil dose intensity (%) | 85.4 | 92 | |
| Mean SIR-Spheres dose | 2.25 GBq | NA | |
| Comments: apart from the two early deaths in the chemotherapy-only arm (received no chemotherapy) the reason number of chemotherapy cycles was greater in the SIRT + chemotherapy group was because most patients had a prolonged response and therefore continued to receive on-going treatment. The slightly higher dose intensity in the chemotherapy only group indicates that the lower response rate for patients in the control arm was not due to less intensive chemotherapy. The latter six patients whose yttrium-90 dose was individualised to BSA received from 1.5 to 2.1 GBq; the initial five received a standard amount of 2.5 GBq yttrium-90 activity | |||
| Intervention details | |||
| Single treatment with SIRT using SIR-spheres + standard regimen of systemic fluorouracil/folinic acid chemotherapy Arterial anatomy of liver was assessed by transfemoral hepatic angiogram to plan subsequent administration of SIR-Spheres. Technetium-99 labelled macro-aggregated albumin was injected into the hepatic artery during the angiogram to estimate ‘percentage lung breakthrough’. If lung breakthrough was > 13% yttrium-90 activity administered was reduced Single dose of SIR-Spheres administered on the third or fourth day of the second cycle of chemotherapy. If more than one hepatic artery supplied blood to the liver the total dose of SIR-Spheres was divided into separate aliquots depending on the estimated volume of tumour being supplied by each feeding artery A single bolus of 25 µg Antiotensin-2 was pulsed into the hepatic artery 30 seconds before administering the SIR-Spheres First five patients received 2.5 GBq of yttrium-90 activity Subsequent six patients dose was calculated as: Dose of SIR-Spheres in GBq=(BSAa− 0.2)+% tumor involvement100(a, BSA = body surface area measured in square metres) Change in dosing due to evidence of radiation hepatitis in one of the first five patients who was very small Patients treated with SIRT were generally kept in hospital overnight and discharged home the following day Chemotherapy received by participants in both trial arms: fluorouracil/folinic acid 5-fluorouracil 425 mg/m2 per day plus folinic acid 20 mg/m2 per day for 5 consecutive days and repeated at 4-weekly intervals. Chemotherapy cycles continued until evidence of unacceptable toxicity, patient request or disease progression Details of post-intervention treatments: cancer specific treatment could include non-protocol chemotherapy. All non-protocol cancer specific treatment recorded for all patients. Other supportive, but not cancer specific treatment was allowed for patient management |
|||
| Methodological comments | |||
|
|||
| General comments | |||
|
|||
Quality assessment for randomised controlled trials (answer yes/no/not reported/unclear)
| 1. Was the method used to generate random allocations adequate? | Yes |
| 2. Was the allocation adequately concealed? | Yes |
| 3. Were the groups similar at the outset of the study in terms of prognostic factors, e.g. severity of disease/size, number of tumours, performance status, extrahepatic disease | Yes |
| 4. Were outcome assessors blinded to the treatment allocation? | Yes for outcomes reliant on CT scan data, e.g. response. Not reported for toxicity or quality of life |
| 5. (i) Were there any unexpected imbalances in dropouts between groups? (ii) If so, were they explained or adjusted for? | No |
| 6. Is there any evidence to suggest that the authors measured more outcomes than they reported? | Unclear |
| 7. (i) Did the analysis include an intention-to-treat analysis? | Yes |
| (ii) If so, was this defined? | Yes (can be inferred) |
| 8. (i) Did the analysis account for missing data? | Yes |
| (ii) If so, were the methods appropriate? | Yes |
Appendix 5 Excluded studies
Lower hierarchy studies
1. Sato KT, Lewandowski RJ, Mulcahy MF, Atassi B, Ryu RK, Gates VL, et al. Unresectable chemorefractory liver metastases: radioembolization with 90Y microspheres – safety, efficacy, and survival. Radiology 2008;247:507–15.
2. Stubbs RS, O’Brien I, Correia MM. Selective internal radiation therapy with 90Y microspheres for colorectal liver metastases: single-centre experience with 100 patients. A N Z J Surg 2006;76:696–703.
3. Vogl TJ, Gruber T, Balzer JO, Eichler K, Hammerstingl R, Zangos S. Repeated transarterial chemoembolization in the treatment of liver metastases of colorectal cancer: prospective study. Radiology 2009;250:281–9.
4. Vogl TJ, Naguib NN, Nour-Eldin NE, Eichler K, Zangos S, Gruber-Rouh T. Transarterial chemoembolization (TACE) with mitomycin C and gemcitabine for liver metastases in breast cancer. Eur Radiol 2010;20:173–80.
Studies that remain unclear on whether prospective or retrospective and therefore excluded
1. Chen MH, Wei Y, Yan K, Gao W, Dai Y, Huo L, et al. Treatment strategy to optimize radiofrequency ablation for liver malignancies. J Vasc Interv Radiol 2006;17:671–83.
2. Vogl TJ, Straub R, Eichler K, Sollner O, Mack MG. Colorectal carcinoma metastases in liver: laser-induced interstitial thermotherapy – local tumor control rate and survival data. Radiology 2004;230:450–8.
Foreign-language studies of potential relevance
1. Kondo M, Itani K, Yoshikawa T, Tanaka Y, Watanabe N, Hiraoka M, et al. [A prospective randomized clinical trial comparing intra-arterial chemotherapy alone and when combined with hyperthermia for metastatic liver cancer.] Gan to Kagaku Ryoho 1995:22:1807–11.
2. Shibata T, Shimano T, Kitada M, Niinobu T, Fukushima T, Hata S, et al. [Assessment of colorectal cancer patients exhibiting bilobular multiple hepatic metastases.] Gan to Kagaku Ryoho 2000;27:1842–5.
3. Vogl T, Mack M, Straub R, Zangos S, Woitaschek D, Eichler K, et al. [Thermal ablation of liver metastases. Current status and prospects.] Radiologe 2001;41:49–55.
4. Vogl TJ, Muller PK, Mack MG, Straub R, Engelmann K, Neuhaus P. [Therapeutic options in non-resectable liver metastases. Percutaneous radiological interventions.] Chirurg 1999;70:133–40.
Excluded studies
Excluded on design
1. Abdalla EK, Vauthey J-N, Ellis LM, Ellis V, Pollock R, Broglio KR, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg 2004;239:818–27.
2. Abdeldayem HM, Salama IA, El-Deen TAE, Touny AO, Osman MA, ElAbd O, et al. Role of radiofrequency ablation in the treatment of colorectal liver metastasis. J Hepatol 2008;48:355.
3. Abitabile P, Hartl U, Lange J, Maurer CA. Radiofrequency ablation permits an effective treatment for colorectal liver metastasis. Eur J Surg Oncol 2007;33:67–71.
4. Akyildiz HY, Mitchell J, Milas M, Siperstein A, Berber E. Laparoscopic radiofrequency thermal ablation of neuroendocrine hepatic metastases: long-term follow-up. Surgery 2010;148:1288–93.
5. Albert M, Kiefer MV, Sun W, Haller D, Fraker DL, Tuite CM, et al. Chemoembolization of colorectal liver metastases with cisplatin, doxorubicin, mitomycin C, ethiodol, and polyvinyl alcohol. Cancer 2011;117:343–52.
6. Aliberti C, Marzola M, Carandina I, Ballardini P, Lelli G, Benea G. First report of treatment of hepatic metastases from colorectal carcinoma in irinotecan-refractory patients by tace with Dc Beads microspheres preloaded with irinotecan 200 mgr plus systemic cetuximab. Ann Oncol 2009;20:76.
7. Aloia TA, Vauthey JN, Loyer EM, Ribero D, Pawlik TM, Wei SH, et al. Solitary colorectal liver metastasis: resection determines outcome. Arch Surg 2006;141:460–6.
8. Aprile G, Sponza M, Vit A, Iaiza E, De Pauli F, Pascoletti G, et al. A single institution experience in percutaneous laser-induced thermoablation (PLIT) for non-resectable colorectal liver metastases: Correlation between maximum diameter of the dominant lesion and clinical outcome. Ann Oncol 2006;17:XI43.
9. Berber E, Ari E, Herceg N, Siperstein A. Laparoscopic radiofrequency thermal ablation for unusual hepatic tumors: operative indications and outcomes. Surg Endosc 2005;19:1613–7.
10. Berber E, Tsinberg M, Tellioglu G, Simpfendorfer CH, Siperstein AE. Resection versus laparoscopic radiofrequency thermal ablation of solitary colorectal liver metastasis. J Gastrointestinal Surg 2008;12:1967–72.
11. Berber E, Tsinberg M, Simpfendorfer CH, Siperstein A. Resection versus laparoscopic radiofrequency thermal ablation of solitary colorectal liver metastasis. Gastroenterology 2008;134:A852.
12. Boutros C, Yi B, Somasundar P, Espat NJ. Feasibility study of same setting laparoscopic bipolar radiofrequency ablation and laparoscopic colectomy for synchronous colorectal liver metastasis as a bridge to hepatic resection. Gastroenterology 2009;136:A915.
13. Bower M, Metzger T, Robbins K, Tomalty D, Valek V, Boudny J, et al. Surgical downstaging and neo-adjuvant therapy in metastatic colorectal carcinoma with irinotecan drug-eluting beads: a multi-institutional study. HPB 2010;12:31–6.
14. Cassera MA, Hammill C, Wolf R, Swanstrom LL, Hansen PD. Radiofrequency ablation as regional management of breast cancer liver metastases. Ann Surg Oncol 2010;17:S94.
15. Chen M-H, Yang W, Yan K, Zou M-W, Solbiati L, Liu J-B, et al. Large liver tumors: protocol for radiofrequency ablation and its clinical application in 110 patients – mathematic model, overlapping mode, and electrode placement process. Radiology 2004;232:260–71.
16. Chen M-H, Yang W, Yan K, Gao W, Dai Y, Wang Y-B, et al. Treatment efficacy of radiofrequency ablation of 338 patients with hepatic malignant tumor and the relevant complications. World J Gastroenterol 2005;11:6395–401.
17. Christante D, Pommier S, Givi B, Pommier R. Hepatic artery chemoinfusion with chemoembolization for neuroendocrine cancer with progressive hepatic metastases despite octreotide therapy. Surgery 2008;144:885–93.
18. Christophi C, Nikfarjam M, Malcontenti-Wilson C, Muralidharan V. Long-term survival of patients with unresectable colorectal liver metastases treated by percutaneous interstitial laser thermotherapy. World J Surg 2004;28:987–94.
19. Cianni R, Urigo C, Notarianni E, Saltarelli A, D’Agostini A, Iozzino M, et al. Radioembolisation using yttrium 90 (Y-90) in patients affected by unresectable hepatic metastases. Radiologia Medica 2010;115:619–33.
20. Cianni R, Urigo C, Notarianni E, Saltarelli A, Salvatori R, Pasqualini V, et al. Selective internal radiation therapy with SIR-spheres for the treatment of unresectable colorectal hepatic metastases. Cardiovasc Intervent Radiol 2009;32:1179–86.
21. Cosimelli M, Golfieri R, Cagol PP, Carpanese L, Sciuto R, Maini CL, et al. Multi-centre phase II clinical trial of yttrium-90 resin microspheres alone in unresectable, chemotherapy refractory colorectal liver metastases. Br J Cancer 2010;103:324–31.
22. Curley SA. Outcomes after surgical treatment of colorectal cancer liver metastases. Semin Oncol 2005;32:(Suppl. 11).
23. Danesi R, Di PA. Chemoembolization is effective as second-line therapy in patients with colorectal carcinoma metastatic to the liver. Clinical Colorectal Cancer 2002;2:180–1.
24. Dayem H, Essam-el-deen T, Touny A, Osman M, ElAbd O, El-waraky M, et al. Role of radiofrequency ablation in the treatment of colorectal liver metastases. Ann Oncol 2008;19:49–50.
25. De Baere T. Radiofrequency of liver metastasis: clinical results. Cardiovasc Intervent Radiol 2006;29(Suppl. 1):128–9.
26. Elvin A, Skogseid B, Hellman P. Radiofrequency ablation of neuroendocrine liver metastases. Abdom Imaging 2005;30:427–34.
27. Eriksson J, Stalberg P, Nilsson A, Krause J, Lundberg C, Skogseid B, et al. Surgery and radiofrequency ablation for treatment of liver metastases from midgut and foregut carcinoids and endocrine pancreatic tumors. World J Surg 2008;32:93–8.
28. Evrard S, Becouarn Y, Fonck M, Brunet R, Mathoulin-Pelissier S, Picot V. Surgical treatment of liver metastases by radiofrequency ablation, resection, or in combination. Eur J Surg Oncol 2004;30:399–406.
29. Faynsod M, Wagman LD, Longmate J, Carroll M, Leong LA. Improved hepatic toxicity profile of portal vein adjuvant hepatic infusional chemotherapy. J Clin Oncol 2005;23:4876–80.
30. Fiorentini G, Del CA, De SM, Guadagni S, Mambrini A, D’Alessandro M, et al. Complete response of colorectal liver metastases after intra-arterial chemotherapy. Tumori 2008;94:489–92.
31. Fiorentini G, Aliberti C, Turrisi G, Rossi S, Dentico P, Benea G. Trans arterial chemoembolization (TACE) of liver metastases (LM) from colorectal cancer (CRC) adopting irinotecan-eluting beads: results of a phase II clinical study. Ann Oncol 2007;18:7.
32. Gedaly R, Toledano A, Millan G, Essenfeld H, Zambrano VJ. Treatment of liver metastases from a solid pseudopapillary tumor of the pancreas. J Hepatobiliary Pancreat Surg 2006;13:587–90.
33. Gervais DA, Arellano RS, Mueller PR. Percutaneous radiofrequency ablation of ovarian cancer metastasis to the liver: indications, outcomes, and role in patient management. AJR 2006;187:746–50.
34. Gibbs P, Lipton L, Price D, Bower GD, Field K, Dowling R, et al. A Phase Ii trial of radioembolization and systemic chemotherapy in patients with liver metastases from primary cancer of the pancreas. Ann Oncol 2010;21:263.
35. Gibbs P, Stubbs RS, Nick N, Shannon JA, Eliadis P, Tapner MJ, et al. Durable disease-free survival without resection of target lesions following radioembolisation with yttrium-90 resin microspheres in patients with colorectal liver metastases. Ann Oncol 2010;21:I50.
36. Gillams A, Cassoni A, Conway G, Lees W. Radiofrequency ablation of neuroendocrine liver metastases: the Middlesex experience. Abdom Imaging 2005;30:435–41.
37. Gillams AR, Lees WR. Radiofrequency ablation of colorectal liver metastases. Abdom Imaging 2005;30:419–26.
38. Gillams AR, Lees WR. Survival after percutaneous, image-guided, thermal ablation of hepatic metastases from colorectal cancer. Dis Colon Rectum 2000;43:656–61.
39. Giovannini M, Seitz J-F. Ultrasound-guided percutaneous alcohol injection of small liver metastases: Results in 40 patients. Cancer 1994;73:294–7.
40. Gonsalves CF, Eschelman DJ, Sullivan KL, Anne PR, Doyle L, Sato T. Selective internal radiation therapy (SIRT) as salvage therapy for uveal melanoma (UM) hepatic metastases. J Clin Oncol 2009;27:9066.
41. Gruenberger T, Morris DL. Staged treatment of bilobar hepatic metastases from colorectal cancer. Eur J Surg 2002;168:516–18.
42. Hager ED, Dziambor H, Hohmann D, Gallenbeck D, Stephan M, Popa C. Deep hyperthermia with radiofrequencies in patients with liver metastases from colorectal cancer. Anticancer Res 1999;19:3403–8.
43. Henn AR, Levine EA, McNulty W, Zagoria RJ. Original report – percutaneous radiofrequency ablation of hepatic metastases for symptomatic relief of neuroendocrine syndromes. Am J Roentgenol 2003;181:1005–10.
44. Hosoda K, Yasumura T, Yagawa A, Sueki R, Kaji S, Maruyama T, et al. Efficacy of radiofrequency ablation in patients with colorectal hepatic metastases. Gastroenterology 2006;130:A499.
45. Iannitti DA, Dupuy DE, Mayo-Smith WW, Murphy B. Hepatic radiofrequency ablation. Arch Surg 2002;137:422–6.
46. Isenberg J, Fischbach R, Kruger I, Keller HW. Treatment of liver metastases from colorectal cancer. Anticancer Res 1996;16:1291–5.
47. Jaiswal R, Sewkani A, Vyas S, Sharma S, Naik S, Purohit D, et al. Results of 5 years’ experience of liver secondaries treated by radiofrequency ablation. Am J Gastroenterol 2007;102:276.
48. Jakobs TF, Hoffmann RT, Schrader A, Stemmler HJ, Trumm C, Lubienski A, et al. CT-guided radiofrequency ablation in patients with hepatic metastases from breast cancer. Cardiovasc Intervent Radiol 2009;32:38–46.
49. Johnson LB, Krebs TL, Van ED, Plotkin JS, Njoku M, Wong JJ, et al. Cytoablative therapy with combined resection and cryosurgery for limited bilobar hepatic colorectal metastases. Am J Surg 1997;174:610–13.
50. Joosten J, Jager G, Oyen W, Wobbes T, Ruers T. Cryosurgery and radiofrequency ablation for unresectable colorectal liver metastases. Eur J Surg Oncol 2005;31:1152–9.
51. Kamat PP, Gupta S, Ensor JE, Murthy R, Ahrar K, Madoff DC, et al. Hepatic arterial embolization and chemoembolization in the management of patients with large-volume liver metastases. Cardiovasc Intervent Radiol 2008;31:299–307.
52. Kennedy AS, Warner R, McNeillie P, Dezarn WA, Coldwell D, Nutting C, et al. Radioembolization for neuroendocrine hepatic metastases. Cancer Invest 2007;25:55.
53. Kennedy AS, Coldwell D, Nutting C, Murthy R, Wertman DE Jr, Loehr SP, et al. Resin 90Y-microsphere brachytherapy for unresectable colorectal liver metastases: modern USA experience. Int J Radiat Oncol Biol Phys 2006;65:412–25.
54. Lang EK, Brown CL Jr. Colorectal metastases to the liver: selective chemoembolization. Radiology 1993;189:417–22.
55. Leblanc F, Fonck M, Brunet R, Becouarn Y, Mathoulin-Pelissier S, Evrard S. Comparison of hepatic recurrences after resection or intraoperative radiofrequency ablation indicated by size and topographical characteristics of the metastases. Eur J Surg Oncol 2008;34:185–90.
56. Lehmann ED, Kachura JR, Ho CS, Beecroft JR, Tomlinson GA, Wei AC. Radiofrequency ablation of hepatic colorectal carcinoma metastases: patient survival, local metastasis progression-free survival, and factors for failure of treatment effectiveness. Am J Roentgenol 2007;188:A56.
57. Li XP, Meng ZQ, Guo WJ, Li J. Treatment for liver metastases from breast cancer: results and prognostic factors. World J Gastroenterol 2005;11:3782–7.
58. Liao Z, You X, Qiu M. Transarterial chemoembolization combined with systemic chemotherapy in the treatment of liver metastases of colorectal cancer. Ann Oncol 2010;21:94.
59. Livraghi T, Solbiati L, Meloni F, Lerace T, Goldberg SN, Gazelle GS. Percutaneous radiofrequency ablation of liver metastases in potential candidates for resection: the ”test-of-time approach“. Cancer 2003;97:3027–35.
60. Lopez-Ben S, Figueras J. Radiofrequency ablation of colorectal liver metastases: long-term survival? Acta Radiol 2008;49:19–20.
61. Lu Q, Zhao A, Shen L. Preoperative transcatheter arterial chemoembolization (TACE) and chemotherapy for hepatic colorectal metastases: Impact on hepatic histology and postoperative outcome. J Clin Oncol 2009;27:e15090.
62. Maire F, Lombard-Bohas C, O’Toole D, Vullierme MP, Rebours V, Pelletier AL, et al. Hepatic arterial embolization versus chemoembolization in patients with liver metastases of a digestive neuroendocrine tumors. Gastroenterology 2011;140:S875.
63. Mandair D, Bridgestock H, Shah T, Mangat K, Oliff S, Mahon B, et al. Effectiveness of transarterial embolization of liver neuroendocrine metastases. Neuroendocrinology 2010;92:45.
64. Martin R, Scoggins C, Tomalty D, Shreeder M, Metzger T, Tatum C, et al. Treatment of chemo naive colorectal liver metastasis by using irinotecan drug eluting bead with concomitant systemic fluorouracil and oxaliplatin. Ann Oncol 2010;21:23.
65. Martin RC, Robbins K, Tomalty D, O’Hara R, Bosnjakovic P, Padr R, et al. Transarterial chemoembolisation (TACE) using irinotecan-loaded beads for the treatment of unresectable metastases to the liver in patients with colorectal cancer: an interim report. World J Surg Oncol 2009;7:80.
66. Martin RCG, Scoggins CR, McMasters KM. Safety and efficacy of microwave ablation of hepatic tumors: a prospective review of a 5-year experience. Ann Surg Oncol 2010;17:171–8.
67. Mazzaglia PJ, Berber E, Milas M, Siperstein AE. Laparoscopic radiofrequency ablation of neuroendocrine liver metastases: a 10-year experience evaluating predictors of survival. Surgery 2007;142:10–9.
68. Michl M, Paprottka P, Hoffmann R, Laubender R, Haug A, Bartenstein P, et al. Selective internal radiation therapy (SIRT) for treatment of pretreated pancreatic cancer patients with metastatic liver disease. Ann Oncol 2010;21:58–9.
69. Mulcahy MF, Lewandowski RJ, Ibrahim SM, Sato KT, Ryu RK, Atassi B, et al. Radioembolization of colorectal hepatic metastases using yttrium-90 microspheres. Cancer 2009;115:1849–58.
70. Nasoni S, Pacella CM, Bizzarri G, Papini E, Rossi Z, Petrolati A. Percutaneous laser ablation for palliative treatment of neuroendocrine liver metastases. J Hepatol 2009;50:S294–5.
71. Navarra G, Ayav A, Weber JC, Jensen SL, Smadga C, Nicholls J, et al. Short- and-long term results of intraoperative radiofrequency ablation of liver metastases. Int J Colorect Dis 2005;20:521–8.
72. Nazir S, Lau K, Sindram D, Martinie J, Asarian A, Iannitti D. Microwave ablation (MWA) of colorectal liver metastases: a single institution experience. Am J Gastroenterol 2010;105:S106.
73. Niu R, Yan TD, Zhu JC, Black D, Chu F, Morris DL. Recurrence and survival outcomes after hepatic resection with or without cryotherapy for liver metastases from colorectal carcinoma. Ann Surg Oncol 2007;14:2078–87.
74. Nouso K, Uematsu S, Shiraga K, Okamoto R, Iwado S, Kanzaki H, et al. Application of radiofrequency ablation therapy for the treatment of metastatic liver cancers. Gastroenterology 2007;132:A203.
75. Pacella CM, Valle D, Bizzarri G, Pacella S, Brunetti M, Maritati R, et al. Percutaneous laser ablation in patients with isolated unresectable liver metastases from colorectal cancer: results of a phase II study. Acta Oncol 2006;45:77–83.
76. Paganini AM, Rotundo A, Barchetti L, Lezoche E. Cryosurgical ablation of hepatic colorectal metastases. Surg Oncol 2007;16:S137–40.
77. Pajkos G, Szentpetery L, Kristo K, Izso J. Combined treatment of colorectal cancer liver metastases: intrahepatical chemoembolisation and systemic chemotherapy. Regional Cancer Treat 1998;9:202–7.
78. Park I, Kim H, Yu C, Kim J, Choi P, Jung S, et al. Radiofrequency ablation for metachronous liver metastasis from colorectal cancer after curative surgery. Dis Colon Rectum 2007;50:773.
79. Park IJ, Kim HC, Yu CS, Kim PN, Won HJ, Kim JC. Radiofrequency ablation for metachronous liver metastasis from colorectal cancer after curative surgery. Ann Surg Oncol 2008;15:227–32.
80. Pech M, Wieners G, Freund T, Dudeck O, Fischbach F, Ricke J, et al. MR-guided interstitial laser thermotherapy of colorectal liver metastases: efficiency, safety and patient survival. Eur J Med Res 2007;12:161–8.
81. Perrone S, Silva F, Dambrosio M, Boioli F, Tagliaferri B, Gribaudi G, et al. Intralesional treatment of liver metastases from colorectal carcinoma: percutaneous ethanol injection (PEI) and percutaneous intralesional chemotherapy (PIC). Regional Cancer Treat 1996;9:71–8.
82. Rhee TK, Lewandowski RJ, Liu DM, Mulcahy MF, Takahashi G, Hansen PD, et al. 90Y Radioembolization for metastatic neuroendocrine liver tumors: preliminary results from a multi-institutional experience. Ann Surg 2008;247:1029–35.
83. Ricke J, Ruhl R, Seidensticker M, Dudeck O, Pech M, Amthauer H. Extensive liver-dominant colorectal (CRC) metastases failing multiple lines of systemic chemotherapy treated by 90y radioembolisation: a matched-pair analysis. Ann Oncol 2009;20:17.
84. Rougier P, Pelage J, Beauchet A, Lagrange C, Lepere C, Bachet J, et al. Transarterial chemoembolization (TACE) in liver metastases of neuroendocrine tumors using streptozocin and tris-acryl microspheres (Embozar). Ann Oncol 2008;19:62.
85. Ruehl R, Seidensticker M, Denecke T, Pethe A, Grosser O, Amthauer H, et al. Survival and response after selective internal radiation therapy (SIRT) for extensive, therapy refractory liver metastases secondary to colorectal carcinoma. Ann Oncol 2008;19:24.
86. Saigusa Y, Teratani T, Takeuchi S. Radiofrequency ablation for liver metastasis of colorectal cancer. Gastroenterology 2011;140:S596.
87. Salman HS, Cynamon J, Jagust M, Bakal C, Rozenblit A, Kaleya R, et al. Randomized phase II trial of embolization therapy versus chemoembolization therapy in previously treated patients with colorectal carcinoma metastatic to the liver. Clin Colorect Cancer 2002;2:173–9.
88. Sanz-Altamira PM, Spence LD, Huberman MS, Posner MR, Steele G Jr, Perry LJ, et al. Selective chemoembolization in the management of hepatic metastases in refractory colorectal carcinoma: a phase II trial. Dis Colon Rectum 1997;40:770–5.
89. Saxena A, Chua TC, Bester L, Kokandi A, Morris DL. Factors predicting response and survival after yttrium-90 radioembolization of unresectable neuroendocrine tumor liver metastases: a critical appraisal of 48 cases. Ann Surg 2010;251:910–6.
90. Saxena A, Morris DL, Chua TC, Bester L. Factors predicting response and survival after yttrium-90 radioembolization of unresectable neuroendocrine tumor liver metastases: a critical appraisal of 48 cases. Ann Surg Oncol 2010;17:S76.
91. Schuder G, Pistorius G, Schneider G, Feifel G. Preliminary experience with percutaneous cryotherapy of liver tumours. Br J Surg 1998;85:1210–1.
92. Seidensticker M, Ruhl R, Kraus P, Denecke T, Pethe A, Pech M, et al. Radioembolization with 90Y-resin microspheres as a salvage treatment for refractory liver-dominant colorectal metastases: a matched-pair analysis. EJC Supplements 2009;7:343.
93. Seifert JK, Morris DL. Cryotherapy of the resection edge after liver resection for colorectal cancer metastases. A N Z J Surg 1998;68:725–8.
94. Seifert JK, Morris DL. Repeat hepatic cryotherapy for recurrent metastases from colorectal cancer. Surgery 1999;125:233–5.
95. Singh V, Mishra P, Sharma S, Naik S, Purohit D, Sewkani A, et al. Results of 6 years experience of liver secondaries treated by radiofrequency ablation. J Hepatol 2008;48:160–1.
96. Sofocleous CT, Nascimento RG, Gonen M, Theodoulou M, Covey AM, Brody LA, et al. Clinical observations. Radiofrequency ablation in the management of liver metastases from breast cancer. Am J Roentgenol 2007;189:883–9.
97. Solbiati L, Lerace T, Tonolini M, Osti V, Cova L. Radiofrequency thermal ablation of hepatic metastases. Eur J Ultrasound 2001;13:149–58.
98. Stubbs RS, Cannan RJ, Mitchell AW. Selective internal radiation therapy with 90yttrium microspheres for extensive colorectal liver metastases. J Gastrointest Surg 2001;5:294–302.
99. Talamonti M, Vauthey PLR, Thirlby RC, Posner MC. Results of a single-center experience with resection and ablation for sarcoma metastatic to the liver. Arch Surg 2006;141:543–4.
100. Tarazov PG, Polysalov VN, Prozorovskij KV, Grishchenkova IV, Rozengauz EV. Ischemic complications of transcatheter arterial chemoembolization in liver malignancies. Acta Radiol 2000;41:156–60.
101. Terraz S, Constantin C, Majno PE, Spahr L, Mentha G, Becker CD. Image-guided multipolar radiofrequency ablation of liver tumours: initial clinical results. Eur Radiol 2007;17:2253–61.
102. Tie J, Yip D, Dowling R, Lichtenstein M, Tapner MJ, Gibbs P. Radioembolization and systemic chemotherapy in patients with hepatic metastases from primary colorectal cancer. Ann Oncol 2010;21:221–2.
103. Tommasi L, Buonadonna A, Torrisi E, De Marchi F, Talamini R, Frustaci S. Radiofrequency ablation of liver metastases from colon-rectal cancer: a single institute experience. Ann Oncol 2010;21:75–6.
104. van Duijnhoven FH, Jansen MC, Junggeburt JM, van HR, Rijken AM, van CF, et al. Factors influencing the local failure rate of radiofrequency ablation of colorectal liver metastases. Ann Surg Oncol 2006;13:651–8.
105. Veltri A, Sacchetto P, Tosetti I, Pagano E, Fava C, Gandini G. Radiofrequency ablation of colorectal liver metastases: small size favorably predicts technique effectiveness and survival. Cardiovasc Intervent Radiol 2008;31:948–56.
106. Vit A, Aprile G, Giovannoni M, Sponza M, Laiza E, Ferretti G, et al. Selective internal radiotherapy (SIRT) for unresectable liver metastases from heavily pretreated colorectal cancer (CRC) patients. A single institution experience. Ann Oncol 2007;18:VII87–8.
107. Vogl TJ, Eichler K, Zangos S, Mack MG. Interstitial laser therapy of liver tumors. Medical Laser Application 2005;20:115–8.
108. Vogl TJ, Mack MG, Straub R, Roggan A, Felix R. Magnetic resonance imaging-guided abdominal interventional radiology: laser-induced thermotherapy of liver metastases. Endoscopy 1997;29:577–83.
109. Vogl TJ, Mack MG, Straub R, Eichler K, Engelmann K, Zangos S, et al. MR guided laser-induced thermotherapy (LITT) of malignant liver and soft tissue tumours. Medical Laser Application 2001;16:91–102.
110. Vogl TJ, Mack MG, Scholz W-R, Muller P, Weinhold N, Philipp C, et al. MR imaging-guided laser-induced thermotherapy. Minimally Invasive Therapy Allied Technologies 1996;5:243–8.
111. Vogl TJ, Naguib NN, Eichler K, Lehnert T, Ackermann H, Mack MG. Volumetric evaluation of liver metastases after thermal ablation: long-term results following MR-guided laser-induced thermotherapy. Radiology 2008;249:865–71.
112. Walker RJE, Toumpanakis C, Marelli L, Yu D, Davies N, Woodward N, et al. Transarterial embolization/chemoembolization for hepatic NET metastases: recent efficacy and toxicity data. Regul Pept 2010;164:6.
113. Wallace JR, Christians KK, Pitt HA, Quebbeman EJ. Cryotherapy extends the indications for treatment of colorectal liver metastases. Surgery 1999;126:766–74.
114. Wallace S, Carrasco CH, Charnsangavej C, Richli WR, Wright K, Gianturco C. Hepatic-artery infusion and chemoembolization in the management of liver metastases. Cardiovasc Intervent Radiol 1990;13:153–60.
115. Weaver ML, Atkinson D, Zemel R. Hepatic cryosurgery in the treatment of unresectable metastases. Surg Oncol 1995;4:231–6.
116. Weaver ML, Atkinson D, Zemel R. Hepatic cryosurgery in treating colorectal metastases. Cancer 1995;76:210–4.
117. Weaver ML, Ashton JG, Zemel R. Treatment of colorectal liver metastases by cryotherapy. Semin Surg Oncol 1998;14:163–70.
118. Wietzke-Braun P, Schindler C, Raddatz D, Braun F, Armbrust T, Nolte W, et al. Quality of life and outcome of ultrasound-guided laser interstitial thermo-therapy for non-resectable liver metastases of colorectal cancer. Eur J Gastroenterol Hepatol 2004;16:389–95.
119. You YT, Changchien CR, Huang JS, Ng KK. Combining systemic chemotherapy with chemoembolization in the treatment of unresectable hepatic metastases from colorectal cancer. Int J Colorect Dis 2006;21:33–7.
120. Zarva A, Mohnike K, Ruhl R, Damm R, Ulrich G, Seidensticker M, et al. Repeated radioembolisations in patients with advanced primary and secondary liver tumors and progressive disease after first SIRT: safety and effectiveness. Eur J Nucl Med Mol Imaging 2010;37:S425.
121. Zharkov VV, Dudarau VS, Kokhniuk VT, Akinfeyeu VV, Rebeko IV, Dorosh DD. Preoperative transcatheter hepatic artery chemoembolization (TACE) in colorectal cancer (CRC) liver metastases patients. Ann Oncol 2006;17.
Excluded on participants
1. Berber E, Herceg NL, Casto KJ, Siperstein AE. Laparoscopic radiofrequency ablation of hepatic tumors: prospective clinical evaluation of ablation size comparing two treatment algorithms. Surg Endosc 2004;18:390–6.
2. Berber E, Siperstein AE. Perioperative outcome after laparoscopic radiofrequency ablation of liver tumors: an analysis of 521 cases. Surg Endosc 2007;21:613–18.
3. Clasen S, Boss A, Schmidt D, Schraml C, Fritz J, Schick F, et al. MR-guided radiofrequency ablation in a 0.2-T open MR system: technical success and technique effectiveness in 100 liver tumors. J Magn Reson Imaging 2007;26:1043–52.
4. Curley SA, Marra P, Beaty K, Ellis LM, Vauthey JN, Abdalla EK, et al. Early and late complications after radiofrequency ablation of malignant liver tumors in 608 patients. Ann Surg 2004;239:450–8.
5. Curley SA, Izzo F, Delrio P, Ellis LM, Granchi J, Vallone P, et al. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg 1999;230:1–8.
6. de Baere T, Deschamps F, Briggs P, Dromain C, Boige V, Hechelhammer L, et al. Hepatic malignancies: Percutaneous radiofrequency ablation during percutaneous portal or hepatic vein occlusion. Radiology 2008;248:1056–66.
7. Ducreux M, Malka D, Boige V, de Baere T, Elias D. Local regional chemotherapy and chemoembolization of primary and secondary liver tumors. Ann Oncol 2006;17:29.
8. Elias D, Baton O, Sideris L, Matsuhisa T, Pocard M, Lasser P. Local recurrences after intraoperative radiofrequency ablation of liver metastases: a comparative study with anatomic and wedge resections. Ann Surg Oncol 2004;11:500–5.
9. Geller DA, Tsung A, Marsh JW, Dvorchik I, Gamblin TC, Carr BI. Outcome of 1000 liver cancer patients evaluated at the UPMC Liver Cancer Center. J Gastrointest Surg 2006;10:63–8.
10. Giorgio A, Tarantino L, de SG, Coppola C, Ferraioli G. Complications after percutaneous saline-enhanced radiofrequency ablation of liver tumors: 3-year experience with 336 patients at a single center. AJR 2005;184:207–11.
11. Havlik R, Vojacek P, Habib N. [Radiofrequency thermal ablation in liver tumours.] Acta Univer Palack Olom Facult Med 2000;143:84–5.
12. Herfarth KK, Debus J, Wannenmacher M. Stereotactic radiation therapy of liver metastases: update of the initial phase-I/II trial. Front Radiat Ther Oncol 2004;38:100–5.
13. Hildebrand P, Leibecke T, Kleemann M, Mirow L, Birth M, Bruch HP, et al. Influence of operator experience in radiofrequency ablation of malignant liver tumours on treatment outcome. Eur J Surg Oncol 2006;32:430–4.
14. Hildebrand P, Kleemann M, Roblick UJ, Mirow L, Birth M, Leibecke T, et al. Radiofrequency-ablation of unresectable primary and secondary liver tumors: results in 88 patients. Langenbecks Arch Surg 2006;391:118–23.
15. Hisa N, Ohkuma K, Fujikura Y, Nakatsuka S, Takeda T, Kobayashi S, et al. Percutaneous ethanol injection therapy for hepatic tumors. Results and technical considerations. Semin Intervent Radiol 1993;10:27–34.
16. Iannitti DA, Martin RCG, Simon CJ, Hope WW, Newcomb WL, McMasters KM, et al. Hepatic tumor ablation with clustered microwave antennae: the US Phase II Trial. HPB 2007;9:120–4.
17. Ibrahim SM, Lewandowski RJ, Ryu RK, Sato KT, Gates VL, Mulcahy MF, et al. Radiographic response to yttrium-90 radioembolization in anterior versus posterior liver segments. Cardiovasc Intervent Radiol 2008;31:1124–32.
18. Jakobs TF, Hoffmann RT, Tatsch K, Reiser MF. Radioembolization using (yttrium)-Y-90 microspheres in primary and metastatic liver cancer. Anticancer Res 2008;28:299.
19. Jansen MC, van Duijnhoven FH, van HR, Rijken A, van CF, van der Sijp J, et al. Adverse effects of radiofrequency ablation of liver tumours in the Netherlands. Br J Surg 2005;92:1248–54.
20. Kosari K, Gomes M, Hunter D, Hess DJ, Greeno E, Sielaff TD. Local, intrahepatic, and systemic recurrence patterns after radiofrequency ablation of hepatic malignancies. J Gastrointest Surg 2002;6:255–63.
21. Martin RCG, Howard J, Tomalty D, Robbins K, Padr R, Bosnjakovic PM, et al. Toxicity of irinotecan-eluting beads in the treatment of hepatic malignancies: Results of a multi-institutional registry. Cardiovasc Intervent Radiol 2010;33:960–6.
22. Morris DL, Ross WB, Iqbal J, McCall JL, King J, Clingan PR. Cryoablation of hepatic malignancy: an evaluation of tumour marker data and survival in 110 patients. GI Cancer 1996;1:247–51.
23. Paulet E, Aube C, Pessaux P, Lebigot J, Lhermitte E, Oberti F, et al. Factors limiting complete tumor ablation by radiofrequency ablation. Cardiovasc Intervent Radiol 2008;31:107–15.
24. Pearson AS, Izzo F, Fleming RY, Ellis LM, Delrio P, Roh MS, et al. Intraoperative radiofrequency ablation or cryoablation for hepatic malignancies. Am J Surg 1999;178:592–9.
25. Penka I, Kaplan Z, Sefr R, Sirotek L, Eber Z, Ondrak M. Use of radiofrequency ablation in the treatment of malignant liver lesions. Hepatogastroenterology 2008;55:562–7.
26. Sartori S, Tombesi P, Macario F, Nielsen I, Tassinari D, Catellani M, et al. Subcapsular liver tumors treated with percutaneous radiofrequency ablation: a prospective comparison with nonsubcapsular liver tumors for safety and effectiveness. Radiology 2008;248:670–9.
27. Shankar S, vanSonnenberg E, Morrison PR, Tuncali K, Silverman SG. Combined radiofrequency and alcohol injection for percutaneous hepatic tumor ablation. AJR 2004;183:1425–9.
28. Vogl TJ, Mack MG, Muller PK, Straub R, Engelmann K, Eichler K. Interventional MR: interstitial therapy. Eur Radiol 1999;9:1479–87.
29. Vogl TJ, Eichler K, Straub R, Engelmann K, Zangos S, Woitaschek D, et al. Laser-induced thermotherapy of malignant liver tumors: general principals, equipment(s), procedure(s) – side effects, complications and results. Eur J Ultrasound 2001;13:117–27.
30. Vogl TJ, Straub R, Eichler K, Woitaschek D, Mack MG. Malignant liver tumors treated with MR imaging-guided laser-induced thermotherapy: experience with complications in 899 patients (2,520 lesions). Radiology 2002;225:367–77.
31. Vogl TJ, Straub R, Zangos S, Mack MG, Eichler K. MR-guided laser-induced thermotherapy (LITT) of liver tumours: experimental and clinical data. Int J of Hyperthermia 2004;20:713–24.
32. Wong SL, Edwards MJ, Chao C, Simpson D, McMasters KM. Radiofrequency ablation for unresectable hepatic tumors. Am J Surg 2001;182:552–7.
33. Wood TF, Rose DM, Chung M, Allegra DP, Foshag LJ, Bilchik AJ. Radiofrequency ablation of 231 unresectable hepatic tumors: indications, limitations, and complications. Ann Surg Oncol 2000;7:593–600.
34. Yasuda M, Kondo M, Kokura S, Naito Y, Yoshida N, Yoshikawa T. Chemoembolization with degradable starch microspheres combined with concurrent local hyperthermia for unresectable or metastatic liver tumors is clinically useful. Ann Oncol 2006;17:136–7.
Excluded on interventions
1. Azoulay D, Castaing D, Smail A, Adam R, Cailliez V, Laurent A, et al. Resection of nonresectable liver metastases from colorectal cancer after percutaneous portal vein embolization. Ann Surg 2000;231:480–6.
2. Bageacu S, Kaczmarek D, Lacroix M, Dubois J, Forest J, Porcheron J. Cryosurgery for resectable and unresectable hepatic metastases from colorectal cancer. Eur J Surg Oncol 2007;33:590–6.
3. Bathe OF, Dowden S, Sutherland F, Dixon E, Butts C, Bigam D, et al. Phase II study of neoadjuvant 5-FU + leucovorin + CPT-11 in patients with resectable liver metastases from colorectal adenocarcinoma. BMC Cancer 2004;4:32.
4. Boige V, Malka D, Elias D, Castaing M, De BT, Goere D, et al. Hepatic arterial infusion of oxaliplatin and intravenous LV5FU2 in unresectable liver metastases from colorectal cancer after systemic chemotherapy failure. Ann Surg Oncol 2008;15:219–26.
5. Elias D, Baton O, Sideris L, Boige V, Malka D, Liberale G, et al. Hepatectomy plus intraoperative radiofrequency ablation and chemotherapy to treat technically unresectable multiple colorectal liver metastases. J Surg Oncol 2005;90:36–42.
6. Gray B, Van HG, Buck M, Paton G, Burton M, Anderson J. Treatment of colorectal liver metastases with SIR-spheres plus chemotherapy. GI Cancer 2000;3:249–57.
7. Ho AS, Picus J, Darcy MD, Tan B, Gould JE, Pilgram TK, et al. Long-term outcome after chemoembolization and embolization of hepatic metastatic lesions from neuroendocrine tumors. AJR 2007;188:1201–7.
8. Hoyer M, Roed H, Hansen AT, Ohlhuis L, Petersen J, Nellemann H, et al. Phase II study on stereotactic body radiotherapy of colorectal metastases. Acta Oncol 2006;45:823–30.
9. Korpan NN. Hepatic cryosurgery for liver metastases. Long-term follow-up. Ann Surg 1997;225:193–201.
10. Navarra G, Musolino C, Lucchese L, Zumbo A, Rinaldi F, Bartolotta G. Resection for colorectal liver metastases using heat coagulative ablation. Ann Oncol 2006;17:VII149.
11. Onik GM, Atkinson D, Zemel R, Weaver ML. Cryosurgery of liver cancer. Semin Surg Oncol 1993;9:309–17.
12. Pawlik TM, Izzo F, Cohen DS, Morris JS, Curley SA. Combined resection and radiofrequency ablation for advanced hepatic malignancies: results in 172 patients. Ann Surg Oncol 2003;10:1059–69.
13. Pawlik TM, Abdalla EK, Ellis LM, Vauthey JN, Curley SA. Debunking dogma: surgery for four or more colorectal liver metastases is justified. J Gastrointest Surg 2006;10:240–8.
14. Ricke J, Mohnike K, Pech M, Seidensticker M, Ruhl R, Wieners G, et al. Local response and impact on survival after local ablation of liver metastases from colorectal carcinoma by computed tomography-guided high-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys 2010;78:479–85.
15. Ritz JP, Lehmann KS, Zurbuchen U, Wacker F, Brehm F, Isbert C, et al. Improving laser-induced thermotherapy of liver metastases – effects of arterial microembolization and complete blood flow occlusion. Eur J Surg Oncol 2007;33:608–15.
16. Ruers T, Joosten J, Wiering B, Wobbes V, Dekker H, Oyen W, et al. Comparison between local ablative therapy and chemotherapy for non-resectable colorectal liver metastases: a prospective study. Ann Oncol 2007;18:VII85.
17. Ruers T, Joosten J, Wiering B, Dekker H, Oyen W, Wobbes T, et al. Non-randomised comparison between local ablative therapy and chemotherapy for non-resectable colorectal liver metastases; A prospective study. Ann Oncol 2006;17:85.
18. Ruers TJ, Joosten JJ, Wiering B, Langenhoff BS, Dekker HM, Wobbes T, et al. Comparison between local ablative therapy and chemotherapy for non-resectable colorectal liver metastases: a prospective study. Ann Surg Oncol 2007;14:1161–9.
19. Seifert JK, Achenbach T, Heintz A, Bottger TC, Junginger T. Cryotherapy for liver metastases. Int J Colorect Dis 2000;15:161–6.
20. Seifert JK, Springer A, Baier P, Junginger T. Liver resection or cryotherapy for colorectal liver metastases: a prospective case control study. Int J Colorect Dis 2005;20:507–20.
21. Zangos S, Mack MG, Balzer JO, Engelmann K, Straub R, Eichler K, et al. Neoadjuvant transarterial chemoembolization (TACE) before percutaneous MR-guided laser-induced thermotherapy (LITT): results in large-sized primary and secondary liver tumors. Med Laser Appl 2004;19:98–108.
Excluded on comparators
1. Martinelli DJ, Wadler S, Bakal CW, Cynamon J, Rozenblit A, Haynes H, et al. Utility of embolization or chemoembolization as second-line treatment in patients with advanced or recurrent colorectal carcinoma. Cancer 1994;74:1706–12.
2. Ruutiainen AT, Soulen MC, Tuite CM, Clark TW, Mondschein JI, Stavropoulos SW, et al. Chemoembolization and bland embolization of neuroendocrine tumor metastases to the liver. J Vasc Intervent Radiol 2007;18:847–55.
3. Scaife CL, Curley SA, Izzo F, Marra P, Delrio P, Daniele B, et al. Feasibility of adjuvant hepatic arterial infusion of chemotherapy after radiofrequency ablation with or without resection in patients with hepatic metastases from colorectal cancer. Ann Surg Oncol 2003;10:348–54.
4. van der Pool AE, Lalmahomed ZS, de Wilt JH, Eggermont AM, Ijzermans JM, Verhoef C. Local treatment for recurrent colorectal hepatic metastases after partial hepatectomy. J Gastrointest Surg 2009;13:890–5.
Excluded as abstracts
1. Aliberti C, Benea G, Tilli M, Fiorentini G. Transarterial chemoembolization (TACE) of liver metastases (LM) from colorectal carcinoma (CRC) adopting drug eluting beads preloaded with irinotecan (DEBIRI): results of a phase II study on 82 patients. J Clin Oncol 2009;27:4092.
2. Fiorentini G, Aliberti C, Montagnani F, Tilli M, Mambrini A, Giannessi P, et al. First evaluation of phase III trial of TACE adopting polyvinil-alcohol microspheres (PAM) irinotecan (IRI) loaded vs folfiri (CT) for non operable colorectal cancer (CRC) liver metastases. Ann Oncol 2009;20:14.
3. Fiorentini G, Aliberti C, Montagnani F, Tilli M, Mambrini A, Benea G. Trans-arterial chemoembolization of metastatic colorectal carcinoma (mCRC) to the liver adopting polyvinyl alcohol microspheres (pam) loaded with irinotecan compared with folfiri (ct): evaluation at two years of a phase III clinical trial. Ann Oncol 2010;21:191.
4. Hirata M, Suzuki N, Miura Y, Katayama A, Tanaka Y, Tanaka K, et al. Effect of radio frequency ablation therapy for liver metastasis from colorectal cancer. Eur J Surg Oncol 2008;34:1071.
5. Koike Y, Yoshida H, Shima S, Kogure H, Omata M, Kawase T. Percutaneous radio-frequency ablation improves the prognosis of the patients with unresectable hepatic metastasis from colorectal cancer. Gastroenterology 2008;134:A365–6.
6. Koike Y, Shiina S, Kajiwara H, Gore E, Ohira K, Goto O, et al. Percutaneous radio-frequency ablation inproves the prognosis of the patients with unresectable hepatic metastasis from colorectal cancer. Gastroenterology 2006;130:A468.
7. Koike Y, Shiina S, Yoshida H, Watanabe K, Kawase T, Omata M. Radio-frequency ablation for unresectable liver metastases from colo-rectal cancer. Gastroenterology 2007;132:A203.
8. Rebeko I, Zharkov VV, Kokhnyuk VV, Dudarev V, Akinfeyev V, Dorosh D. Colorectal (CR) resectable liver metastases: long-term patient survival after preoperative transarterial chemoembolization (Tace) with oxaliplatin following hepatic resection (HR). Ann Oncol 2010;21:220.
9. Ruers T, Punt C, Van Coevorden F, Borel R, I, Lederman JA, Poston GJ, et al. Final results of the EORTC intergroup randomized study 40004 (CLOCC) evaluating the benefit of radiotherapy ablation (RFA) combined with chemotherapy for unresectable colorectal liver metastases (CRC LM). J Clin Oncol 2010;28:3526.
10. Ruers T, Van Coevorden F, Pierie J, Rinkes IB, Punt C, Ledermann J, et al. Radiofrequency ablation (RFA) combined with chemotherapy for unresectable colorectal liver metastases (CRC LM): interim results of a randomised phase II study of the EORTC-NCRI CCSG-ALM Intergroup 40004 (CLOCC). Ann Surg Oncol 2009;16:3.
11. Ruers T, Van Coevorden F, Pierie J, Rinkes IB, Punt C, Lederman J, et al. Radiofrequency ablation (RFA) combined with chemotherapy for unresectable colorectal liver metastases (CRC LM): interim results of a randomised phase II study of the EORTC-NCRI CCSG-ALM intergroup 40004 (CLOCC). Ann Oncol 2010;21:I41–2.
12. Ruers T, Van Coevorden F, Pierie J, Borel-Rinkes I, Punt K, Ledermann J, et al. Radiofrequency ablation combined with chemotherapy for unresectable CRC liver metastases: interim results of a randomised phase II study (CLOCC) (EORTC-NCRI CCSG-ALM). Ann Oncol 2009;20:13.
13. Van den EM, Hendlisz A, Peeters M, Defreyne L, Maleux G, Vannoote J, et al. Prospective randomized study comparing hepatic intra-arterial injection of Yttrium-90 resin-microspheres (HAI-Y90) with protracted IV 5FU (5FU CI) versus 5FU CI alone for patients with liver-limited metastatic colorectal cancer (LMCRC) refractory to standard chemotherapy (CT). J Clin Oncol: ASCO annual meeting proceedings 2009;27:191.
14. Vogl TJ, Mack MG, Straub R, Roggan A, Felix R. Percutaneous MRI-guided laser-induced thermotherapy for hepatic metastases for colorectal cancer. Lancet 1997;350:29.
Appendix 6 Foreign-language studies
1. Novomlinsky VV, Glukhov AA, Chvikalov ES, Shamaeva TE, Ostroushko AP. [Experience of application radiofrequent thermoablation in patients with metastases of the colorectal cancer to the liver.] Proktologia –Warszawa 2008;9:100.
2. Murakami H, Ogata Y, Uchida S, Sasatomi T, Murakami N, Yamaguchi K, et al. [Therapeutic results of hepatic resection using thermal ablation for unresectable colorectal liver metastases.] Jpn J Cancer Chemother 2009;36:2039–41.
3. Tsuchiya M, Watanabe M, Otsuka Y, Yamazaki K, Tamura A, Ishii J, et al. [Transarterial chemoembolization with irinotecan (CPT-11) and degradable starch microspheres (DSM) in patients with liver metastases from colorectal cancer.] Jpn J Cancer Chemother 2007;34:2038–40.
4. Alvarez CB, Fernandez JM, Magro de la PA, Bustos FA, Calvo AG, Vicent JG, et al. [Efficacy and safety of radiofrequency ablation of malignant liver tumours: a systematic review.] Report No. 43. Agencia de Evaluacion de Tecnologias Sanitarias 2004.
5. Augustovski F, Pichon Riviere A, Alcaraz A, Bardach A, Ferrante D, Garcia Marti S, et al. Usefulness of radiofrequency ablation of liver tumors. Informe de respuesta rapida no. 54. 2005.
6. Beppu T, Doi K, Ishiko T, Hirota M, Egami H, Ogawa M. [Efficacy of local ablation therapy for liver metastasis from colorectal cancer – radiofrequency ablation and microwave coagulation therapy.] Nippon Geka Gakkai Zasshi 2001;102:390–7.
7. Beppu T, Ogawa M, Matsuda T, Ohara C, Hirota M, Shimada S, et al. [Efficacy of microwave coagulation therapy (MCT) in patients with liver tumors.] Gan to Kagaku Ryoho 1998;25:1358–61.
8. Blusse vO-A, Fioole B, Jansen MC, van Duijnhoven FH, van HR, Rijken AM, et al. [Radiofrequency ablation of colorectal metastases to the liver: results since the first application in the Netherlands.] Ned Tijdschr Geneeskd 2008;152:880–6.
9. Brufani C, Bizzarri G, Papini E, Guglielmi R, Petrucci L, Rinaldi R, et al. [Laser thermal ablation (LTA) in the palliative treatment of liver metastases from adrenocortical carcinoma.] Giornate Endocrinologiche Pisane 2004;27:51.
10. Chen M-H, Yang W, Yan K, Gao W, Dai Y, Wang Y-B, et al. [Standard treatment of liver malignancies with radiofrequency ablation.] National Medical Journal of China 2005;85:1741–6.
11. Chen MH, Yan K, Yang W, Gao W, Dai Y, Wang YB, et al. [Efficacy of radiofrequency ablation of 343 patients with hepatic tumor and the relevant complications.] Beijing da Xue Xue Bao 2005;37:292–6.
12. Chen MH, Yang W, Yan K, Zou MW, Dai Y, Gao W, et al. [Mathematical protocol for radiofrequency ablation of liver tumors and its clinical application.] Chung-Hua i Hsueh Tsa Chih 2004;84:203–8.
13. Chen Q-L, Ye Q, Gu W-Z, Zhang J-X, Tong Q-G, Xi S-F. [Evaluation of transcatherter arterial chemoembolization combined with radiofrequency capacitive heating on clinical therapeutic effect of metastatic hepatic carcinoma.] J Intervent Radiol 2006;15:407–9.
14. Chiou Y-Y, Chou Y-H, Chiang J-H, Wang H-K, Chang C-Y. [Percutaneous ultrasound-guided radiofrequency ablation of colorectal liver metastases.] Chin J Radiol 2005;30:153–8.
15. Crucitti A, Danza FM, Antinori A, La GA, Antonacci V, Giustacchini P, et al. [Thermal ablation by radiofrequency of hepatic metastasis of colorectal cancer: short-term results.] Suppl Tumori 2005;4:S34–Jun.
16. Eickmeyer F, Schwarzmaier HJ, Muller FP, Nakic Z, Yang Q, Fiedler V. [Survival after laser-induced interstitial thermotherapy of colorectal liver metastases – a comparison of first clinical experiences with current therapy results.] Rofo: Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin 2008;180:35–41.
17. Eisele RM, Schumacher G, Neuhaus P. [Local recurrence following hepatic radiofrequency ablation: diagnosis and treatment.] Strahlenther Onkol 2008;184:598–604.
18. Frich L. [Local ablation of colorectal liver metastases – a systematic review.] Tidsskr Nor Laegeforen 2008;128:54–6.
19. Gaspari AL, Rossi P, Danza FM. [Treatment of liver metastasis with radiofrequency.] Suppl Tumori 2002;1:S41–5.
20. Genov I, Grigorov N, Mitova R, Golemanov B, Dinkov L, Donov M. [Radiofrequency ablation of hepatic metastases: clinical results.] Khirurgiia 2005;16–20.
21. Granov AM, Tarazov PG, Granov DA. [Chemoembolization of the hepatic artery in the treatment of primary and metastatic liver cancer.] Vestn Rentgenol Radiol 1996;34–8.
22. Herfarth KK, Debus J. [Stereotactic radiation therapy for liver metastases.] Chirurg 2005;76:564–9.
23. Hidaka H, Kokubu S, Ono K. [Radiofrequency ablation.] Gan to Kagaku Ryoho 2001;28:1955–61.
24. Inoue T,.Kudo M. [Radio frequency ablation for metastatic liver cancer.] Gan to Kagaku Ryoho 2009;36:1253–5.
25. Ji D-H, Wang F, Liu Y-S, Li C, LI F, Zhao L-J. [Radio-frequency ablation of liver tumor.] J Dalian Med Univer 2007;29:136–8.
26. Koike Y. [Impact of radio-frequency ablation for unresectable hepatic metastases.] Nippon Shokakibyo Gakkai Zasshi 2009;106:1421–7.
27. Kondo M, Itani K, Yoshikawa T, Tanaka Y, Watanabe N, Hiraoka M, et al. [A prospective randomized clinical trial comparing intra-arterial chemotherapy alone and when combined with hyperthermia for metastatic liver cancer.] Gan to Kagaku Ryoho 1995;22:1807–11.
28. Kormann J, Ockert D, Bunk A. [Radiofrequency ablation of liver tumours.] Zentralbl Chir 2001;126:576–85.
29. Li H, Zeng H, Yang L. [Influence factors in hepatic artery infusion chemotherapy and embolization of liver metastases from alimentary tract cancer.] Chung Hua Kan Tsang Ping Tsa Chih 1999;7:142–3.
30. Liang P, Dong BW, Yu XL, Yu DJ, Feng L, Gao YY, et al. [Evaluation of long-term therapeutic effects of ultrasound-guided percutaneous microwave ablation of liver metastases.] Chung-Hua i Hsueh Tsa Chih 2006;86:806–10.
31. Liang P, Dong BW, Yu XL, Yang YR, Yu DJ, Wang Y, et al. [Ultrasound-guided percutaneous microwave coagulation therapy for hepatic metastases.] Chung-Hua Chung Liu Tsa Chih 2004;26:301–4.
32. Liang Y-C, Yang J-Z, Lin J, Li Z-Y, Ke X. [Transcatheter arterial chemoembolization combined with CT-guided percutaneous intratumor ethanol injection for liver metastases.] Chin J Intervent Imaging Ther 2007;4:49–51.
33. Michl M, Hoffmann RT, Laubender R, Haug A, Bartenstein P, Stemmler HJ, et al. [Radioembolisation (SIRT): Treatment of hepatic metastasis in breast cancer patients.] Onkologie 2010;33:55.
34. Paganini AM, Feliciotti F, Guerrieri M, Sarnari J, Lezoche E. [Cryoablation and thermal ablation with radiofrequency in the treatment of neoplasms of the liver.] Suppl Tumori 2002;1:S7–14.
35. Pech M, Wieners G, Kryza R, Dudeck O, Seidensticker M, Mohnike K, et al. [CT-guided brachytherapy (CTGB) versus interstitial laser ablation (ILT) of colorectal liver metastases: an intraindividual matched-pair analysis.] Strahlenther Onkol 2008;184:302–6.
36. Peter M, Toth J, Peter M, Jr., Sapy P, Andras C. [Treatment of malignant liver tumors with radio-frequency ablation (RFA).] Orvosi Hetilap 2002;143:2229–34.
37. Pichler L, Anzbock W, Schiessel R, Hruby W. [High-frequency thermoablation of malignant tumors of the liver: An effective therapeutic alternative Eine wirksame therapiealternative: Hochfrequenz-Thermoablation maligner Raumforderungen der Leber.] Wien Klinische Wochenschrift 2000;112:18–21.
38. Polikarpov AA, Gasanov IS, Tarazov PG, Generalov MI. [Transcatheter treatment of liver metastases from breast cancer.] Vopr Onkol 2008;54:90–4.
39. Saitsu H, Takami Y, Saku M. [Surgical treatment for multiple colorectal metastases: efficacy of ablation therapy.] Nippon Geka Gakkai Zasshi 2006;133–7.
40. Sasaki Y, Imaoka S, Masutani S, Nagano H, Ohashi I, Kameyama M, et al. [Chemoembolization for liver metastasis from colorectal cancer.] Jpn J Cancer Chemother 1990;17:1661–4.
41. Seifert JK, Heintz A, Junginger T. [Cryotherapy for primary and secondary liver tumours.] Zentralbl Chir 2002;127:275–81.
42. Shao C-W, Tian J-M, Zuo C-J, Zhao Q, Lu T-Z. [CT-guided percutaneous ethanol injection for hepatic metastasis.] J Intervent Radiol 2007;16:171–3.
43. Shibata T, Shimano T, Kitada M, Niinobu T, Fukushima Y, Hata S, et al. [Assessment of colorectal cancer patients exhibiting bilobular multiple hepatic metastases.] Gan to Kagaku Ryoho 2000;27:1842–5.
44. Shibata T, Niinobu T, Shimano T, Kitada M, Tsukahara Y, Ikeda K, et al. [Microwave coagulation therapy under laparotomic ischemia for multiple liver metastases of colorectal cancer.] Gan to Kagaku Ryoho 1999;26:1760–3.
45. Stang A, Keles H, von SC, Hentschke S, Malzfeldt E, Teichmann W, et al. [Percutanous and intraoperative ultrasound-guided radiofrequency ablation of hepatic tumours.] Ultraschall Med 2007;28:181–8.
46. Tarazov PG. [Arterial chemoembolization for metastatic colorectal cancer in the liver.] Vopr Onkol 2000;46:561–6.
47. Tarazov PG, Granov DA, Polikarpov AA, Polisalov VN, Borovik VV. [Combined hepatic-artery and portal-vein chemoembolization for colorectal cancer metastatic to the liver.] Vopr Onkol 2002;48:83–7.
48. Tarazov PG, Granov DA, Polikarpov AA. [Interventional radiological treatment of non-colorectal liver metastases.] Vestn Rentgenol Radiol 1999;22–7.
49. Tobinaga S, Nanashima A, Araki M, Hidaka S, Kunizaki M, Takeshita H, et al. [Evaluation of local coagulation therapy with hepatectomy for liver metastases.] Gan to Kagaku Ryoho 2009;36:2042–4.
50. Treska V, Liska V, Skalicky T, Sutnar A, Smid D, Narsanska A, et al. [Liver metastases of other than colorectal origin.] Rozhl Chir 2010;89:202–7.
51. Treska V, Skalicky T, Sutnar A, Liska V. [Surgical management of the colorectal carcinoma liver metastases.] Rozhl Chir 2009;88:69–74.
52. Treska V, Skalicky T, Liska V, Kormunda S, Sutnar A, Ferda J, et al. [Extension of resection capability of liver metastasis from colorectal cancer by two stage surgery.] Ceska a Slovenska Gastroenterologie a Hepatologie 2007;61:254–9.
53. Uetsuji S, Yamamura M, Yamamichi K, Okuda Y, Komada Y, Kwon AH, et al. [A trial of extension of indication for advanced hepatocellular carcinoma. Hepatectomy combining with operative microwave tissue coagulation therapy or ethanol injection therapy for intrahepatic metastasis.] J Jpn Soc Cancer Ther 1992;27:999–1004.
54. Urtasun F, Tejedor M, Valerdi JJ, Barberena J. [Intraarterial chemotherapy in the treatment of liver metastases secondary to colorectal carcinoma Quimioterapia intraarterial en metastasis hepaticas secundarias a carcinoma colorrectal.] Radiologia 1999;41:591–6.
55. Vekic B, Jakovljevic M, Cvetanovic M, Zivic R, Zivanovic J, Jovanovic Z, et al. [Hepatic resection and radiofrequency ablation as a treatment for multiple liver metastases from colorectal carcinoma.] Proktologia-Warszawa 2008;9:141.
56. Vogl T, Mack M, Straub R, Zangos S, Woitaschek D, Eichler K, et al. [Thermal ablation of liver metastases. Current status and prospects.] Radiologe 2001;41:49–55.
57. Vogl T, Eichler K, Zangos S, Naguib N, Jost A. [Liver metastases of breast cancer: Neoadjuvant Chemoembolisation (TACE) before MR controlled Thermoablation (LITT).] Onkologie 2010;33:32.
58. Vogl TJ, Straub R, Eichler K, Woitaschek D, Mack MG. [Modern alternatives to resection of metastases – MR-guided laser-induced thermotherapy (LITT) and other local ablative techniques.] Ther Umsch 2001;58:718–25.
59. Vogl TJ, Weinhold N, Muller P, Mack M, Scholz W, Philipp C, et al. [MR-controlled laser-induced thermotherapy (LITT) of liver metastases: clinical evaluation.] Rontgenpraxis 1996;49:161–8.
60. Vogl TJ, Mack M, Straub R, Eichler K, Engelmann K, Roggan A, et al. [Percutaneous interstitial thermotherapy of malignant liver tumors.] Rofo: Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin 2000;172:12–22.
61. Vogl TJ, Mack MG, Straub R, Zangos S, Engelmann K, Eichler K. [Percutaneous laser ablation of malignant liver tumors.] Zentralbl Chir 2001;126:571–5.
62. Vogl TJ, Muller PK, Mack MG, Straub R, Engelmann K, Neuhaus P. [Therapeutic options in non-resectable liver metastases. Percutaneous radiological interventions.] Chirurg 1999;70:133–40.
63. Vogl TJ, Zangos S, Balzer JO, Thalhammer A, Mack MG. [Transarterial chemoembolization of liver metastases: Indication, technique, results.] Rofo: Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin 2002;174:675–83.
64. Vogl TJ, Mack MG, Straub R, Engelmann K, Zangos S, Eichler K, et al. [Interventional laser-induced thermotherapy of hepatic metastases from breast cancer. Method and clinical outcome.] Gynakologe 1999;32:666–74.
65. Vogl TJ, Lee C, Nour-Eldin NE, Farshid P, Mack MG, Balzer J. [Interventional tumor destruction by thermal procedures Interventionelle tumordestruktion durch thermische verfahren.] Onkologe 2010;16:1086–94.
66. Vogl TJ, Straub R, Eichler K, Mack MG. [Minimal invasive therapy of metastases with MR-guides laser-induced thermotherapy.] Biolog Med 2003;32:157–60.
67. Vogl TJ, Mack MG, Straub R, Muller P, Eichler K, Engelmann K, et al. [MR-guided laser-induced thermotherapy of malignant liver lesions: Technique and results.] Onkologie 1998;21:412–9.
68. Vogl T, Mack MG, Straub R, Zangos S, Engelmann K, Eichler K. [Percutaneous laser ablation of malignant liver tumors Perkutane laserablation von malignen lebertumoren.] Zentralbl Chir 2001;126:571–5.
69. Vogl T, Zangos S, Balzer JO, Thalhammer A, Mack MG. [Transarterial chemoembolization of liver metastases: Indication, technique, results.] Rofo Fortschritte auf dem Gebiet der Rontgenstrahlen und der Bildgebenden Verfahren 2002;174:675–83.
70. Vyslouzil K, Klementa I, Stary L, Zboril P, Skalicky P, Dlouhy M, et al. [Radiofrequency ablation of colorectal liver metastases.] Zentralbl Chir 2009;134:145–8.
71. Wang X, Chen X-F. [Transcatheter hepatic arterial thermo-chemotherapy and thermo-lipiodol embolization for the treatment of hepatic metastases from colorectal carcinoma.] J Intervent Radiol 2009;18:737–9.
72. Wybranski C, Mohnike K, Ricke J. [Minimally invasive tumor ablation in the liver.] Anasthesiol Intensivmed Notfallmed Schmerzther 2009;44:670–7.
73. Yayoi E, Furukawa J, Sekimoto M, Kinuta M, Tateishi H, Maruyama H, et al. [A comparison of intra-arterial chemoembolization and infusion chemotherapy for liver metastases of breast cancer.] Gan to Kagaku Ryoho 1995;22:1519–22.
74. Zeng H, Li H, Shi Z. [Infusion chemotherapy and chemoembolization of liver metastases from cancer of the alimentary tract.] Chung-Hua Chung Liu Tsa Chih 2000;22:422–4.
Appendix 7 Response criteria used in studies in the clinical effectiveness review
| Extended WHO criteria (used by Taguchi and colleagues)81 | ‘Standard response criteria’ (used by Agarwala and colleagues)82 | Grey and colleagues85 provided the definitions used, no published criteria referenced | RECIST (v. 1.0) (used by Hendlisz and colleagues,86 Van Hazel and colleagues,87 and Vogl84) | |
|---|---|---|---|---|
| Complete response (CR) | Disappearance of all measurable lesions, evaluable lesions as well as non-quantifiable malignant lesions, and no appearance of new lesions, determined by two observations not less than 4 weeks apart | Complete radiographic disappearance of evident hepatic disease for at least 4 weeks | Disappearance of all tumour on two successive CT scans not less than 3 months apart after randomisation and before evidence of progressive disease in the liver and before any non-protocol treatments had been given | Disappearance of all target lesions |
| Partial response (PR) | Decrease of 50% or more in lesions that can be measured in two directions without deterioration of any evaluable or other lesion, and no appearance of new lesions, determined by two observations not less than 4 weeks apart | A 50% or greater decrease in the sum of the products of the perpendicular diameters of all measurable hepatic lesions lasting at least 4 weeks without increasing in size of existing lesions or the appearance of new lesions | Objectively measured decrease in tumour size by 50% or more on two successive CT scans not less than 3 months apart after randomisation and before evidence of progressive disease in the liver and before any non-protocol treatments had been given | At least a 30% decrease in the sum of the longest diameter of target lesions, taking as reference the baseline sum longest diameter |
| Minimal response (MR) | Defined to be a condition between NC below and PR above | |||
| No change (NC) | Decrease of 25% in lesions that can be measured in two directions, less than 30% in lesions that can be measured in one direction, to an increase of less than 25% in such lesions without deterioration in other lesions, with no appearances of new lesions, determined by two observations not less than 4 weeks apart | Either a decrease in tumour area or volume, or a CEA that is less than that required for a PR, or an increase that is less than that required for PD | ||
| Stable disease (SD) | A less than 25% increase or less than 50% decrease in all measurable lesions | Neither sufficient shrinkage to qualify for partial response nor sufficient increase to qualify for progressive disease, taking as reference the smallest sum longest diameter since the treatment started | ||
| Progressive disease (PD) | Increase of 25% of more in the product of the sum of diameters of measurable lesions, or deterioration in other lesions or appearance of new lesions | A greater than 25% increase in liver lesions or the appearance of any new lesions within the liver or elsewhere | PD in the liver: (i) an increase on any occasion in cross-sectional tumour area, or tumour volume, by 25% or more over the nadir as measured on serial CT scans, (ii) the development of new lesions in the liver or (iii) an increase in the serum CEA by 25% or more over the nadir for those patients with an elevated CEA at the time of randomisation | At least a 20% increase in the sum of the longest diameter of target lesions, taking as reference the smallest sum longest diameter recorded since the treatment started or the appearance of one or more new lesions |
| Other | Not assessable (NA): (i) no follow-up CT scans due to rapid deterioration after randomisation, or (ii) unmeasureable index lesions for estimating cross-sectional tumour areas |
Appendix 8 Data extractions from included economic evaluations
APPENDIX 8A: DATA EXTRACTION FROM ABRAMSON,115 2000
Study characteristics
Reference
Abramson, 2000115
Health technology
HACE
Interventions and comparators
What interventions/strategies were included?
HACE and palliative care
Was a no treatment/supportive care strategy included?
Palliative care – symptom control
Describe interventions/strategies
HACE – 5-FU, mitomycin, and ethiodised oil followed by embolisation with use of absorbable gelatin sponge (Gelfoam; Pharmacia & Upjohn, Peapack, NJ)
For patients with metastatic lesions confined to one hepatic lobe, a single embolisation procedure was planned. All patients were eligible for re-embolisation if the original lesion or lesions grew or if new lesions developed. The only contraindication for re-embolisation was portal venous thrombosis. In patients with metastatic lesions in both the right and left hepatic lobes, the lobe with the greatest metastatic burden was embolised first. If the portal vein remained patent, lesions in the alternate lobe were embolised during a second session. Specific lesions were considered for re-embolisation only if they failed to respond to the initial treatment. Survival times were noted from the date of first chemoembolisation
Palliative care – symptom control
Research question
What are the stated objectives of the evaluation?
To calculate the cost-effectiveness of HACE for the treatment of colorectal liver metastases (CLM) that are refractory to systemic chemotherapy
Study type: cost-effectiveness/cost–utility/cost–benefit analysis?
Cost-effectiveness analysis
Study population
What definition was used for [condition]? What are the characteristics of the baseline cohort for the evaluation?
All patients had a diagnosis of colorectal carcinoma that was metastatic to the liver, as confirmed at surgery and/or biopsy, and that had been deemed to be unresponsive to systemic chemotherapy
A series of 21 patients who underwent HACE at the teaching hospital of Harvard Medical School from April 1996 to December 1998
Institutional setting: where is/are the intervention(s) being evaluated usually provided?
Hospital
Country/currency
Has a country setting been provided for the evaluation? What currency are costs expressed in and does the publication give the base year to which those costs relate?
USA, 1998 US dollars ($)
Funding source
Harvard Medical School (Department of Radiology, Beth Israel Deaconess Medical Center)
Analytical perspective
What is the perspective adopted for the evaluation (health service, health and personal social services, third-party payer, societal (i.e. including costs borne by individuals and lost productivity)?
Only direct costs to the payer are considered
Effectiveness
Were the effectiveness data derived from a single study, a review/synthesis of previous studies or expert opinion? Give the definition of treatment effect used in the evaluation. Give the size of the treatment effect used in the evaluation.
| Survival for the HACE strategy was estimated from the authors’ 21 case series; however, these data were not used in the CEA. Moreover, the authors found no RCT of HACE for the treatment of CLM, i.e. no data on the survival benefit of HACE compared with palliative care A baseline survival expectation of 12 months was used for the palliative care strategy. Stangl et al.’sa observed that patients with CLM who are treated with chemotherapy have a median survival time of 11.1 months Moreover, survival benefit of HACE was defined as additional survival beyond the expected baseline survival for patients receiving only palliative care. To calculate cost-effectiveness over a range of potential survival benefits, the denominator was maintained as an independent variable and varied from 0 to 24 months Of the 21 patients in the authors’ series, seven died and 14 were still alive as of March 1999. The seven patients who died each underwent a mean of 1.57 additional re-embolisation procedure. Survival times from the first chemoembolisation procedure ranged from 83 to 1097 days (2.8–36.6 months; mean, 17.4 months; median, 21.1 months) As Stangl and colleagues’ median survival time overstates the survival time of patients who have already failed systemic chemotherapy, the authors conducted sensitivity analyses with baseline survival expectations of 6 and 3 months |
Intervention costs
Were the cost data derived from a single (observational) study, a review/synthesis of previous studies or expert opinion? Were the methods for deriving these data adequately described (give sources if using data from other published studies)? List the direct intervention costs and other direct costs used in the evaluation – include resource estimates (and sources for these estimates, if appropriate) as well as sources for unit costs used.
| Input parameters derived from one of the following three sources: the authors’ internal data series, abstraction from referenced sources, or reasonable assumption Unit costs: Direct costs to the payer were calculated by using 1998 Medicare reimbursementa as a proxy for inpatient hospitalisation, procedural, and outpatient visit costs Health Care Financing Administration (HCFA) Federal Upper Limit prices in 1998b were used for outpatient drug costs; if HCFA prices were unavailable, average wholesale prices for generic equivalents were substituted Resource use: Probabilities of re-embolisation were estimated by using the mean from our patient series. Schedules for follow-up visits and dosing regimens for analgesic medications were both modelled on assumption |
Indicate the source for individual cost values (if appropriate).
Indirect costs (costs due to lost productivity, unpaid inputs to patient care)
Were indirect costs included?
Indirect costs were not included
Health state valuations/utilities (if study uses quality-of-life adjustments to outcomes)
Were the utility data derived from a single (observational) study, a review/synthesis of previous studies or expert opinion? Were the methods for deriving these data adequately described (give sources if using data from other published studies)?
Not applicable
List the utility values used in the evaluation.
Not applicable
Indicate the source for individual utility values (if appropriate).
Modelling
If a model was used, describe the type of model used (e.g. Markov state transition model, discrete event simulation). Was this a newly developed model or was it adapted from a previously reported model? If an adaptation, give the source of the original. What was the purpose of the model (i.e. why was a model required in this evaluation)? What are the main components of the model (e.g. health states within a Markov model)? Are sources for assumptions over model structure (e.g. allowable transitions) reported – list them if reported.
A computer spreadsheet (Excel 97; Microsoft Corporation, Redmond, WA, USA) was constructed to calculate the marginal direct cost of HACE compared with palliative care. Marginal cost-effectiveness was calculated from marginal direct cost by varying the survival benefit of HACE compared with palliative care from 0 to 24 months
A break-even analysis was performed to determine the survival benefit at which HACE would be considered to be cost-effective according to benchmarks obtained from the authors’ literature review
Extract transition probabilities for [natural history/disease progression] model and show sources (or refer to table in text).
Not applicable
What is the model time horizon?
24 months
What, if any, discount rates have been applied in the model? Same rate for costs and outcomes?
Discounting was not performed due to the short time horizon
Results/analysis
What measure(s) of benefit were reported in the evaluation?
Life-years
Provide a summary of the clinical outcome/benefits estimated for each intervention/strategy assessed in the evaluation.
Baseline mean survival for palliative care – 12 months
Speculative incremental survival for the intervention arm ranging from 0 to 24 months
Provide a summary of the costs estimated for each intervention/strategy assessed in the evaluation.
Abramson et al. table 1: Parameters for calculation of marginal direct cost of HACE vs. palliative care
Synthesis of costs and benefits – are the costs and outcomes reported together (e.g. as cost-effectiveness ratios)? If so, provide a summary of the results.
| Abramson et al. table 3: Marginal cost and cost-effectiveness of HACE vs. palliative care as a function of survival benefit |
| The authors also state that a cost-effectiveness standard of $50,000 per life-year gained requires a survival benefit of nearly 5 months more than baseline |
Give results of any statistical analysis of the results of the evaluation.
Not reported
Was any sensitivity analysis performed – if yes, what type(s) [i.e. deterministic (one-way, two-way, etc.) or probabilistic]?
Deterministic one-way sensitivity analyses to the estimates of benefit, resource use and unit costs of several components of the strategies
What scenarios were tested in the sensitivity analysis? How do these relate to structural uncertainty (testing assumptions over model structure such as relationships between health states), methodological uncertainty (such as choices of discount rate or inclusion of indirect costs) or parameter uncertainty (assumptions over values of parameters in the model, such as costs, quality of life or disease progression rates)?
The sensitivity analyses carried out tested the model robustness to parameter uncertainty
Give a summary of the results of the sensitivity analysis – did they differ substantially from the base-case analysis? If so, what were the suggested causes?
The authors stated that results of the sensitivity analyses performed show the robustness of the model results to variation in key input parameters. The results of the model did not change substantially with 25% increases or decreases in the cost of non-enhanced CT examination and the cost of measuring the CEA level. Neither an increase or decrease in the post-chemoembolisation medication regimen nor a decrease the baseline survival expectation (i.e. survival for patients receiving palliative care only) to 6 and 3 months substantially alter the results of the model. The results of the model were somewhat influenced by changes in the cost of chemoembolisation, the mean number of re-embolisation procedures per patient and the schedule of follow-up visits after HACE
The authors report the range of marginal cost of HACE vs. palliative care and the survival benefit for three different thresholds of CE (US$20,000, US$50,000 and US$100,000) as shown in Abramson et al. table 4: Sensitivity analyses
Conclusions/implications
Give a brief summary of the author’s conclusions from their analysis.
The authors state that their results demonstrate that the cost-effectiveness of HACE varies considerably with expected survival benefit
What are the implications of the evaluation for practice?
The authors state that practitioners and payers can generate preliminary CE estimates by using their own empirical assessments of the survival benefit of HACE
The authors acknowledge that one of their study limitations is the lack of adjustment for quality of life. Though HACE may affect patients’ HRQoL positively due to the palliation of symptoms, there is potential for negative impact on QoL due to its morbidity and potential for complications
Southampton Health Technology Assessments Centre commentary
Selection of comparators:
Appropriate
Validity of estimate of measure of benefit:
Validity of estimate of costs
| Measure of effectiveness of HACE estimated from a 21-case series. However, it was not included in the CEA due to lack of comparator group. An estimate of incremental benefit was not found by the authors. HRQoL was not included in the analysis |
The authors did not find an estimate of incremental benefit costs, thus unlikely to reflect the current UK clinical practice
APPENDIX 8B: DATA EXTRACTION FROM GAZELLE,116 2004
Study characteristics
Reference
Gazelle et al. 2004116
Health technology
Percutaneous Radiofrequency Ablation (RFA); hepatic metastasectomy
Interventions and comparators
What interventions/strategies were included?
No treatment, percutaneous radiofrequency ablation, surgical resection (metastasectomy) and laparotomy
Was a no-treatment/supportive care strategy included?
Yes
Describe interventions/strategies
No details were provided regarding the no-treatment strategy (e.g. whether any monitoring is performed, chemotherapy or other palliative care)
Strategies for radiofrequency ablation differed in the maximum number of lesions that could be treated, the maximum number of repeat ablations allowed, and the frequency of follow-up imaging
Resection strategies differed in approach (surgical resection/metastasectomy or laparotomy), maximum number of lesions or segments removed, and frequency of follow-up imaging. According to Gazelle et al. (2003), surgical therapy may consist of resection (or metastasectomy) for resectable metastases or laparotomy for unresectable metastases found at surgery
Research question
What are the stated objectives of the evaluation?
To evaluate the relative cost-effectiveness of radiofrequency ablation and hepatic resection and compare the outcomes, cost, and cost-effectiveness of a variety of treatment and follow-up strategies
Study type: cost-effectiveness/cost–utility/cost–benefit analysis?
Cost–utility analysis
Study population
What definition was used for [condition]? What are the characteristics of the baseline cohort for the evaluation?
Patients with metachronous liver metastases from colorectal carcinoma (CRC)
Institutional setting: Where is/are the intervention(s) being evaluated usually provided?
Secondary care
Country/currency
Has a country setting been provided for the evaluation? What currency are costs expressed in and does the publication give the base year to which those costs relate?
USA/1998 US dollars ($)
Funding source
Institute for Technology Assessment and Department of Radiology, Massachusetts General Hospital, Harvard Medical School; Centre for Risk Analysis and Department of Health Policy and Management, Harvard School of Public Health; the National Cancer Institute; and the US Department of the Army
Analytical perspective
What is the perspective adopted for the evaluation (health service, health and personal social services, third-party payer, societal (i.e. including costs borne by individuals and lost productivity)?
Societal perspective (restricted to productivity and direct medical costs and benefits). Costs from the Medicare and Medicaid health care subsystems
Effectiveness
Were the effectiveness data derived from a single study, a review/synthesis of previous studies or expert opinion? Give the definition of treatment effect used in the evaluation. Give the size of the treatment effect used in the evaluation.
| Effectiveness data were derived from a comprehensive review of the English-language literature, by using PubMed and reviewing the bibliographies of the articles identified, or from expert opinion The study assessed the impact of the intervention on mortality, morbidity, length of stay, quality of life, and probability of complete tumour necrosis (with RFA) Mortality: 5% (resection),a 1% (laparotomy),b 0.3% (RFA)c Morbidity: 20% (resection),a 4% (laparotomy),b 2% (RFA)c Length of hospital stay: 12 days (resection), 5 days (laparotomy)d Additional LoS due to complications: 3 days (resection, laparotomy, RFA)d Probability of complete tumour necrosis with RFA: 0.784 (tumour size ≤ 2.5 cm), 0.472 (> 2.5–4 cm), 0.316 (> 4–10 cm)e |
Intervention costs
Were the cost data derived from a single (observational) study, a review/synthesis of previous studies or expert opinion? Were the methods for deriving these data adequately described (give sources if using data from other published studies)? List the direct intervention costs and other direct costs used in the evaluation – include resource estimates (and sources for these estimates, if appropriate) as well as sources for unit costs used.
| Cost data were derived from a review of the literature and the methods for their derivation were adequately described. Cost data for tests and procedures were estimated from the Medicare payment schedule, and the costs of patient care per year according to the LVRT were derived from Taplin and colleagues’ study (1995) The authors assumed that up to three liver metastases could be treated with ablation on 1 day and that two treatment sessions would be required for patients undergoing treatment for 4–6 metastases. The costs were adjusted accordingly assuming the cost of repeat radiofrequency ablation to be the same as the cost of initial therapy Morbidity costs were derived from per diem routine care (non-intensive care unit) costs among patients undergoing hepatic resection at their institution (Massachusetts General Hospital) in the fiscal year of 1998. Complications from radiofrequency ablation were assumed to require a 1-day hospitalisation and the resource use involved would be similar to laparotomy-related complications |
| Gazelle et al. 2004, table 2: Direct costs |
Indicate the source for individual cost values (if appropriate).
Indirect costs (costs due to lost productivity, unpaid inputs to patient care)
Were indirect costs included?
| Productivity costs were based on the daily wage rate of the US Bureau of Labor Statistics | |||||||||||||||
| TABLE 87 Indirect costs (Gazelle et al. 2004, table 2) Test or procedureBase-case estimate (year 1998 dollars)Values used in sensitivity analysisSourceDaily wage ratec96NAUS BLSBLS, Bureau of Labor Statistics; NA, not applicable.c The daily wage rate is calculated by multiplying the average hourly wage rate by 7.5 hours. |
Test or procedure | Base-case estimate (year 1998 dollars) | Values used in sensitivity analysis | Source | Daily wage ratec | 96 | NA | US BLS | BLS, Bureau of Labor Statistics; NA, not applicable. c The daily wage rate is calculated by multiplying the average hourly wage rate by 7.5 hours. |
||||||
| Test or procedure | Base-case estimate (year 1998 dollars) | Values used in sensitivity analysis | Source | ||||||||||||
| Daily wage ratec | 96 | NA | US BLS | ||||||||||||
| BLS, Bureau of Labor Statistics; NA, not applicable. c The daily wage rate is calculated by multiplying the average hourly wage rate by 7.5 hours. |
|||||||||||||||
Health state valuations/utilities (if study uses quality-of-life adjustments to outcomes)
Were the utility data derived from a single (observational) study, a review/synthesis of previous studies or expert opinion? Were the methods for deriving these data adequately described (give sources if using data from other published studies)?
Utility data were derived from a review of the literature and based on expert opinion. There is limited detail on the methods for deriving these data. Age- and sex-adjusted population values to survival without liver metastases were derived from Fryback et al. (1993)
List the utility values used in the evaluation
| QoL after ablation = 0.95 × preablation value for 1 month (Solbiati et al. 2001) QoL after surgery = 0.7 × presurgical value for 1 month QoL one month prior to death = 0.6 × presurgical/ablation value [Assumptions based on Earlam et al (1996) who had used Rotterdam Symptom Checklist (RSC), Hospital Anxiety and Depression Scale (HADS), and Sickness Impact Profile (SIP) in 50 patients with colorectal liver metastases (CLM)] |
Indicate the source for individual cost values (if appropriate).
Modelling
If a model was used, describe the type of model used (e.g. Markov state transition model, discrete event simulation). Was this a newly developed model or was it adapted from a previously reported model? If an adaptation, give the source of the original. What was the purpose of the model (i.e. why was a model required in this evaluation)? What are the main components of the model (e.g. health states within a Markov model)? Are sources for assumptions over model structure (e.g. allowable transitions) reported – list them if reported.
Markov state transition model adapted from previously published model to evaluate the cost-effectiveness of surgical removal of metastases in patients with liver metastases from CRC (Gazelle et al. 2003). The main health states are ‘alive_treat’, ‘alive_notreat’, and ‘dead’, where patients on ‘alive_treat’ are potential candidates to intervention who move to the ‘alive_notreat’ when found untreatable (to have more metastases than the threshold for treatment) or reached the maximum allowed number of interventions for the strategy under consideration. The model simulates cohorts of 10,000 hypothetical patients, one at a time, from initial presentation until death, while tracking disease progression, diagnostic tests, procedures, complications, survival, and costsStructural assumptions
|
Extract transition probabilities for [natural history/disease progression] model and show sources (or refer to table in text).
| The authors refer to several reports that had described the natural history of patients with untreated metastases and have consistently identified the extent of liver replacement by the tumour as the single most important determinant of survival – Bengtsson et al. (1981), Finan et al. (1985), Goslin et al. (1982), Lahr et al. (1983), Wagner et al. (1984), Wood et al. (1976), Boey et al. (1981), Stangl et al. (1994) and Scheele et al. (1990) The authors also report that as the number, size, and growth of all metastases were explicitly modelled, it was possible to calculate percentage LVRT in each patient and to revise it with each cycle of the model. State-to-state transition probabilities for patients with liver metastases were calculated from the corresponding estimated median survivals assuming constant hazard rates |
||||||||||||||||||||||||||||||||||||||||||||||||||||
| TABLE 88 Parameter estimates used to calculate transition probabilities (from Gazelle et al. 2004, table 1) ParameterBase-case estimateValues used in sensitivity analysisSourceNo. of metastases per patient∼ Poisson [6]∼ Poisson [10]See materials and methodsSize of metastases (cm)∼ Gamma [0.8333,3]aNASee materials and methodsTumour volume doubling time (days)15578 and 233Finan et al. (1985)Normal liver volume (cm3)1225NAUrata et al. (1995)Sensitivity of CT0.850.75See materials and methods and Wernecke et al. (1991), Knol et al. (1993), Valls et al. (1998), Matsui et al. (1987), Clarke et al. (1989), Heiken et al. (1989), Zerhouni et al. (1996) and Parker et al. (1989)Detection threshold at CT (cm)0.51.0Detection threshold at intraoperative US (cm)0.30.5Machi et al. (1987) and Onik et al. (1986)Hazard rate when no metastases2 × that of population (age and sex specific)1.5 and 2 × that of base-case estimate1988 US life tablesHazard rate when < 25% LVRT0.74270.5 and 1.5 × that of base-case estimateBengtsson et al. (1981), Finan et al. (1985) and Stangl et al. (1994)Hazard rate when ≥ 25% LVRT1.3203NAMedian survival when < 25% LVRT (months)11.5nrMedian survival when ≥ 25% LVRT(months)6.3nrNA, not applicable; nr, not reported. |
Parameter | Base-case estimate | Values used in sensitivity analysis | Source | No. of metastases per patient | ∼ Poisson [6] | ∼ Poisson [10] | See materials and methods | Size of metastases (cm) | ∼ Gamma [0.8333,3]a | NA | See materials and methods | Tumour volume doubling time (days) | 155 | 78 and 233 | Finan et al. (1985) | Normal liver volume (cm3) | 1225 | NA | Urata et al. (1995) | Sensitivity of CT | 0.85 | 0.75 | See materials and methods and Wernecke et al. (1991), Knol et al. (1993), Valls et al. (1998), Matsui et al. (1987), Clarke et al. (1989), Heiken et al. (1989), Zerhouni et al. (1996) and Parker et al. (1989) | Detection threshold at CT (cm) | 0.5 | 1.0 | Detection threshold at intraoperative US (cm) | 0.3 | 0.5 | Machi et al. (1987) and Onik et al. (1986) | Hazard rate when no metastases | 2 × that of population (age and sex specific) | 1.5 and 2 × that of base-case estimate | 1988 US life tables | Hazard rate when < 25% LVRT | 0.7427 | 0.5 and 1.5 × that of base-case estimate | Bengtsson et al. (1981), Finan et al. (1985) and Stangl et al. (1994) | Hazard rate when ≥ 25% LVRT | 1.3203 | NA | Median survival when < 25% LVRT (months) | 11.5 | nr | Median survival when ≥ 25% LVRT(months) | 6.3 | nr | NA, not applicable; nr, not reported. | |||
| Parameter | Base-case estimate | Values used in sensitivity analysis | Source | |||||||||||||||||||||||||||||||||||||||||||||||||
| No. of metastases per patient | ∼ Poisson [6] | ∼ Poisson [10] | See materials and methods | |||||||||||||||||||||||||||||||||||||||||||||||||
| Size of metastases (cm) | ∼ Gamma [0.8333,3]a | NA | See materials and methods | |||||||||||||||||||||||||||||||||||||||||||||||||
| Tumour volume doubling time (days) | 155 | 78 and 233 | Finan et al. (1985) | |||||||||||||||||||||||||||||||||||||||||||||||||
| Normal liver volume (cm3) | 1225 | NA | Urata et al. (1995) | |||||||||||||||||||||||||||||||||||||||||||||||||
| Sensitivity of CT | 0.85 | 0.75 | See materials and methods and Wernecke et al. (1991), Knol et al. (1993), Valls et al. (1998), Matsui et al. (1987), Clarke et al. (1989), Heiken et al. (1989), Zerhouni et al. (1996) and Parker et al. (1989) | |||||||||||||||||||||||||||||||||||||||||||||||||
| Detection threshold at CT (cm) | 0.5 | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Detection threshold at intraoperative US (cm) | 0.3 | 0.5 | Machi et al. (1987) and Onik et al. (1986) | |||||||||||||||||||||||||||||||||||||||||||||||||
| Hazard rate when no metastases | 2 × that of population (age and sex specific) | 1.5 and 2 × that of base-case estimate | 1988 US life tables | |||||||||||||||||||||||||||||||||||||||||||||||||
| Hazard rate when < 25% LVRT | 0.7427 | 0.5 and 1.5 × that of base-case estimate | Bengtsson et al. (1981), Finan et al. (1985) and Stangl et al. (1994) | |||||||||||||||||||||||||||||||||||||||||||||||||
| Hazard rate when ≥ 25% LVRT | 1.3203 | NA | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Median survival when < 25% LVRT (months) | 11.5 | nr | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Median survival when ≥ 25% LVRT(months) | 6.3 | nr | ||||||||||||||||||||||||||||||||||||||||||||||||||
| NA, not applicable; nr, not reported. | ||||||||||||||||||||||||||||||||||||||||||||||||||||
What is the model time horizon?
Lifetime
What, if any, discount rates have been applied in the model? Same rate for costs and outcomes?
Costs and QALYs were discounted at a real annual rate of 3%. Discount rates of 0% and 5% were considered in sensitivity analyses
Results/analysis
What measure(s) of benefit were reported in the evaluation?
Quality-adjusted life-years (QALYs)
Provide a summary of the clinical outcome/benefits estimated for each intervention/strategy assessed in the evaluation.
| The expected benefits of the dominant strategies from the base-case analysis are presented in Table 89 below. | ||||||||||||||||||||||||||||
| TABLE 89 Results of base-case analysis: resection vs. ablation – benefits (from Gazelle et al. 2004, table 3) TreatmentInterval for follow-up testing and treatment (months)Maximum no. of metastases treatedEffectiveness per patient (QALY)RFA1231.1327RFA1251.3255RFA1261.3575Resection1263.2634Resection463.3923RFA, radiofrequency ablation. |
Treatment | Interval for follow-up testing and treatment (months) | Maximum no. of metastases treated | Effectiveness per patient (QALY) | RFA | 12 | 3 | 1.1327 | RFA | 12 | 5 | 1.3255 | RFA | 12 | 6 | 1.3575 | Resection | 12 | 6 | 3.2634 | Resection | 4 | 6 | 3.3923 | RFA, radiofrequency ablation. | |||
| Treatment | Interval for follow-up testing and treatment (months) | Maximum no. of metastases treated | Effectiveness per patient (QALY) | |||||||||||||||||||||||||
| RFA | 12 | 3 | 1.1327 | |||||||||||||||||||||||||
| RFA | 12 | 5 | 1.3255 | |||||||||||||||||||||||||
| RFA | 12 | 6 | 1.3575 | |||||||||||||||||||||||||
| Resection | 12 | 6 | 3.2634 | |||||||||||||||||||||||||
| Resection | 4 | 6 | 3.3923 | |||||||||||||||||||||||||
| RFA, radiofrequency ablation. | ||||||||||||||||||||||||||||
Provide a summary of the costs estimated for each intervention/strategy assessed in the evaluation.
| The estimated costs for the dominant strategies from the base-case analysis are presented in Table 90 below. | ||||||||||||||||||||||||||||
| TABLE 90 Results of base-case analysis: resection vs. ablation – costs (from Gazelle et al. 2004, table 3) TreatmentInterval for follow-up testing and treatment (months)Maximum no. of metastases treatedCost per patient (year 1998 dollars)aRFA12324,700RFA12524,800RFA12624,800Resection12657,000Resection4661,000RFA, radiofrequency ablation.a Rounded to nearest $100. |
Treatment | Interval for follow-up testing and treatment (months) | Maximum no. of metastases treated | Cost per patient (year 1998 dollars)a | RFA | 12 | 3 | 24,700 | RFA | 12 | 5 | 24,800 | RFA | 12 | 6 | 24,800 | Resection | 12 | 6 | 57,000 | Resection | 4 | 6 | 61,000 | RFA, radiofrequency ablation. a Rounded to nearest $100. |
|||
| Treatment | Interval for follow-up testing and treatment (months) | Maximum no. of metastases treated | Cost per patient (year 1998 dollars)a | |||||||||||||||||||||||||
| RFA | 12 | 3 | 24,700 | |||||||||||||||||||||||||
| RFA | 12 | 5 | 24,800 | |||||||||||||||||||||||||
| RFA | 12 | 6 | 24,800 | |||||||||||||||||||||||||
| Resection | 12 | 6 | 57,000 | |||||||||||||||||||||||||
| Resection | 4 | 6 | 61,000 | |||||||||||||||||||||||||
| RFA, radiofrequency ablation. a Rounded to nearest $100. |
||||||||||||||||||||||||||||
Synthesis of costs and benefits – are the costs and outcomes reported together (e.g. as cost-effectiveness ratios)? If so, provide a summary of the results.
| The estimated ICER for the dominant strategies from the base-case analysis are presented in Table 91 below. | ||||||||||||||||||||||||||||||||
| TABLE 91 Results of base-case analysis: resection versus ablation – ICER (from Gazelle et al. 2004, table 3) TreatmentInterval for follow-up testing and treatment (months)Maximum no. of metastases treatedICER (dollars per QALY)aRFA123. . .RFA125300RFA1261,300Resection12616,900Resection4631,200RFA, radiofrequency ablation.a Rounded to nearest $100; ICERs may not equal the ratios of costs per QALY owing to rounding.The ICER of a resection strategy of a 4-month follow-up interval and resection of six or fewer metastases relative to the most effective non-dominated ablation strategy (i.e. ablation of up to six metastases with 12-month follow-up) was $17,800 per QALY |
Treatment | Interval for follow-up testing and treatment (months) | Maximum no. of metastases treated | ICER (dollars per QALY)a | RFA | 12 | 3 | . . . | RFA | 12 | 5 | 300 | RFA | 12 | 6 | 1,300 | Resection | 12 | 6 | 16,900 | Resection | 4 | 6 | 31,200 | RFA, radiofrequency ablation. a Rounded to nearest $100; ICERs may not equal the ratios of costs per QALY owing to rounding. |
The ICER of a resection strategy of a 4-month follow-up interval and resection of six or fewer metastases relative to the most effective non-dominated ablation strategy (i.e. ablation of up to six metastases with 12-month follow-up) was $17,800 per QALY | ||||||
| Treatment | Interval for follow-up testing and treatment (months) | Maximum no. of metastases treated | ICER (dollars per QALY)a | |||||||||||||||||||||||||||||
| RFA | 12 | 3 | . . . | |||||||||||||||||||||||||||||
| RFA | 12 | 5 | 300 | |||||||||||||||||||||||||||||
| RFA | 12 | 6 | 1,300 | |||||||||||||||||||||||||||||
| Resection | 12 | 6 | 16,900 | |||||||||||||||||||||||||||||
| Resection | 4 | 6 | 31,200 | |||||||||||||||||||||||||||||
| RFA, radiofrequency ablation. a Rounded to nearest $100; ICERs may not equal the ratios of costs per QALY owing to rounding. |
||||||||||||||||||||||||||||||||
| The ICER of a resection strategy of a 4-month follow-up interval and resection of six or fewer metastases relative to the most effective non-dominated ablation strategy (i.e. ablation of up to six metastases with 12-month follow-up) was $17,800 per QALY | ||||||||||||||||||||||||||||||||
Give results of any statistical analysis of the results of the evaluation.
Not reported
Was any sensitivity analysis performed – if yes, what type(s) [i.e. deterministic (one-way, two-way, etc.) or probabilistic]?
| Yes, deterministic one-way and two-way sensitivity analyses were performed In all comparisons, RFA refers to a strategy of treating up to five metastases with a 4-month follow-up interval, and resection refers to a strategy of resection of up to four metastases with a 6-month follow-up interval For the base-case analysis, patients with one or more tumours larger than 5 cm in diameter were not considered candidates for ablation. Different thresholds for treatment candidacy were evaluated in sensitivity analyses Model assumptions tested: women aged 65 years, RFA for tumours ≤ 7 cm and ≤ 10 cm, CT sensitivity of 0.75, CT sensitivity of 0.75 and RFA for tumours ≤ 10 cm, intraoperative US sensitivity of 0.90, number of metastases = 8 and = 10, mortality hazard (with no metastases) decreased by 50%, tumour doubling time doubled to 310 days and halved to 78 days, cost of RF ablation doubled and halved, cost of care halved, discount rate of 5% and 0% |
What scenarios were tested in the sensitivity analysis? How do these relate to structural uncertainty (testing assumptions over model structure such as relationships between health states), methodological uncertainty (such as choices of discount rate or inclusion of indirect costs) or parameter uncertainty (assumptions over values of parameters in the model, such as costs, quality of life or disease progression rates)?
| Yes, the above-mentioned assumptions over parameter values and methodological choices were tested under three scenarios of local control rates for RFA were considered: base case, improved RFA and perfect RFA In the ‘perfect RF’ scenario, complete tumour necrosis was achieved in all treated tumours, whereas in the ‘improved RF’ one complete tumour necrosis was achieved in 100%, 75%, and 50% of treated tumours 2.5 cm or smaller, larger than 2.5 cm but smaller than or equal to 4 cm, and larger than 4 cm but smaller than or equal to 10 cm, respectively |
Give a summary of the results of the sensitivity analysis – did they differ substantially from the base-case analysis. If so, what were the suggested causes?
| The results were sensitive to changes in the number of metastases present (i.e., the assumed population distribution), the size threshold above which lesions were considered untreatable with RFA, and the cost of RFA. More generally, any changes in model parameters that increased the effectiveness of RF resulted in an increase in the ICER for resection relative to RFA In all cases, the ICER of RFA (up to five metastases with 4-month follow-up interval) versus the no-treat strategy was less than $5000 per QALY, and, with very few exceptions, the ICER of resection (up to 4 metastases with 6-month follow-up interval) versus RFA was less than $30,000 per QALY For the base-case scenario, the ICER of resection vs. RFA ranged from $21,200 to $88,300 per QALY (from non-discounting costs or QALYs to performing RFA for tumours smaller than or equal to 10 cm). The authors state that these results suggest that RFA of larger lesions should be encouraged, even though local control rates diminish as the size of the target tumour increases Across all scenarios, when all possible RFA and resection strategies were considered (results not shown), more aggressive surgical strategies were superior (i.e. resulted in more QALYs gained) to RFA. In most cases, the ICERs of even the most aggressive surgical strategies were less than $35,000 per QALY. An additional finding was that some of the less aggressive RFA strategies (e.g. treatment of one to two metastases) were both less expensive and more effective than the no-treat strategy Further results are reported in Gazelle et al. 2004 table 4 |
Conclusions/implications
Give a brief summary of the authors’ conclusions from their analysis.
The authors conclude that both RFA and hepatic resection are relatively cost-effective management strategies for patients with limited hepatic metastases from CRC. In some instances, particularly with more restrictive selective criteria for treatment candidacy (i.e. those based on the number of lesions detected), ablation was found to be cost-saving relative to no treatment. However, throughout the analysis, more aggressive strategies (i.e. the treatment of patients with more tumours, more frequent post-treatment follow-up regimens, and surgery rather than ablation) were superior to more conservative strategies
What are the implications of the evaluation for practice?
The authors suggest that physicians performing ablation and resection should be encouraged to select patients for therapy on the basis of technical feasibility rather than numerical thresholds and to pursue repeat treatment when new lesions are identified after initial therapy
SHTAC commentary
Selection of comparators:
Appropriate
Validity of estimate of measure of benefit:
Unclear; non-compliant with NICE Reference Case
Validity of estimate of costs:
Appropriate; non-compliant with NICE Reference Case
Appendix 9 Search strategy for review of quality-of-life studies
MEDLINE (Ovid)
-
*quality of life/
-
value of life/
-
quality adjusted life year/
-
quality adjusted life.ti,ab.
-
(qaly$ or qald$ or qale$ or qtime$).ti,ab.
-
disability adjusted life.ti,ab.
-
daly*.ti,ab.
-
(sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirty six or short form thirtysix or short form thirty six).ti,ab.
-
(sf6 or sf 6 or SF 6D or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).ti,ab.
-
(sf12 or sf 12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).ti,ab.
-
(sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen).ti,ab.
-
(sf20 or sf 20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).ti,ab.
-
(euroqol or "euro qol" or "eq5d" or "eq 5d").ti,ab.
-
(hql or hqol or "h qol" or hrqol or "hr qol").ti,ab.
-
("hye" or "hyes").ti,ab.
-
health* year* equivalent*.ti,ab.
-
(hui or hui1 or hui2 or hui3).ti,ab.
-
disutil*.ti,ab.
-
rosser.ti,ab.
-
quality of well being.ti,ab.
-
quality of wellbeing.ti,ab.
-
qwb.ti,ab.
-
willingness to pay.ti,ab.
-
standard gamble*.ti,ab.
-
time trade off.ti,ab.
-
time tradeoff.ti,ab.
-
tto.ti,ab.
-
(index adj2 well being).mp.
-
(quality adj2 well being).mp.
-
qwb.tw.
-
(health adj3 util* adj ind*).mp.
-
((multiattribute* or multi attribute) adj3 (health ind* or theor* or health state* or util* or analys*)).mp.
-
quality adjusted life year*.mp.
-
qualy.tw.
-
health status indicator*.ti,ab.
-
"health related quality of living".ti,ab.
-
"health related quality of life".ti,ab.
-
(patient* adj2 (preference* or satisfaction or acceptance)).ti,ab.
-
(health adj ("state" or "status" or "states")).ti,ab.
-
rating scale*.mp.
-
linear scale*.mp.
-
visual analog*.mp.
-
(categor* adj scale*).mp.
-
(FACT-G or "EORTC QLQ-C-30" or FLIC or QLI-CV).tw.
-
"functional assessment of cancer therapy".tw.
-
("model of end stage liver disease" or MELD).tw.
-
"functional living index cancer".tw.
-
(LDQOL or "liver disease quality of life").tw.
-
or/1-48
-
exp Liver Neoplasms/sc
-
exp Neoplasm Metastasis/
-
exp Liver Neoplasms/
-
51 and 52
-
(liver adj5 (secondar* or metasta* or micro-metasta* or micrometasta*)).tw.
-
(hepat* adj5 (secondar* or metasta* or micro-metasta* or micrometasta*)).tw.
-
50 or 53 or 54 or 55
-
49 and 56
Appendix 10 Further details and results from studies included in review of quality-of-life studies
Allen-Mersh et al.,120 1994; Earlam et al.,121 1996; Earlam et al.,140,152 1997; Durand-Zaleski et al.,142 1998
| Country Study design Study type/purpose |
Intervention(s), n Study population Indication Age Gender Comparator population |
QoL instrument(s) used Methodology of collecting QoL data Time period where HRQoL instruments administered |
Results |
|---|---|---|---|
| UK RCT and cost-effectiveness study (participants in group 2 were taken from a separate RCT) To assess efficacy including QoL and to compare costs and benefits of treatments |
|
Physical symptoms were assessed by the Rotterdam Symptom Checklist (RSC) and the Sickness Impact Profile (SIP) Mood assessed by Hospital Anxiety and Depression Scale (HADS) and the RSC Completed monthly for a median of 91% (74–100) of months between randomisation and death (for groups 1 and 3, unclear for group 2) |
Limited data presented on quality-of-life results QoL scores for a typical control patient presented graphically in Allen-Mersh,120 but no mean score provided Earlam et al.121 report correlations between quality of life and survival, and QoL and tumour growth but no data provided for overall scores only individual questions/domains Earlam et al.152 state no significant differences were seen on QoL scores between symptom control patients and HAC or chemotherapy patients (some short-term differences in HAD observed). No data presented. A significant increase in RSC physical score was seen in the chemotherapy versus symptom control group (p < 0.05). No data presented Earlam et al.152 also report statistically significant differences between individual items on the RSC physical scale (not extracted here), and changes over time (not extracted here) Survival with normal QoL scores (median, IQR, days) were:
|
Blair et al.,137 2003
| USA Prospective cohort study including qualitative analysis To examine QoL after regionally delivered chemotherapy and develop a specific quality-of-life questionnaire |
|
In-depth semi-structured interviews to identify specific concerns related to 6 domains of QoL The City of Hope QoL Scale/Cancer Patient scale Self-report scale consisting of 40 items, each on a scale of 0 (worse) to 10 (best), with items representing 4 domains of physical, social, spiritual and psychological well-being Administered at baseline only Compared with 169 non-patient norms (volunteers) |
Overall QoL score was decreased, 6.25 ± 2.44 in patients (overall score in volunteers not presented) Physical well-being scores were similar between patients and volunteers (7.74 ± 1.77 vs. 7.63 ± 2.33) Psychological, social and spiritual well-being scores were statistically significantly lower in the patient sample (mean ± SD):
|
Blazeby et al.,138 2009
| UK, Germany, France Prospective cohort study Validation study (for the HRQoL instrument) |
|
EORTC QLQ-C30 (a generic questionnaire assessing aspects of health) EORTC QLQ-LMC21 (a 21-item questionnaire specifically developed for patients with colorectal liver metastases) Self-report questionnaire. Completed before and 3 months after treatment The 21 items are grouped into five scales: fatigue, nutrition, pain, social and emotional problems Adherence 100% at baseline and 88.5% at follow-up |
Results relate to the reliability and validity of the instrument, and subsequent remodelling of the questionnaire itself Some statistically significant differences were observed on individual items between the two groups, and in all participants before and after treatment No overall score of QoL was provided |
Bruns et al.,139 2010
| Germany Prospective cohort study To assess QoL |
Median age 63.4 (IQR 54.5–70.5) years Gender (male/female): 48/48 Compared with general German population norms (n, demographics unknown) and subgroups of indication for surgery (metastases, primary liver cancer, benign liver disease) |
SF-12 health survey, an abbreviated version of the SF-36, which has summary measures of physical and mental health status, derived from subscales for physical function, physical role, pain, general health, vitality, social function, emotional role and mental health Self-report questionnaire completed post surgery, at least 3 months after discharge (range 3–36 months) Also conducted pain assessment on a 0–10 scale and a liver-specific symptom score (including fever, dyspepsia, heartburn, lack of appetite, nausea, vomiting, night sweat and exhaustion) 68% (128 patients) response rate or which 75% (96) were complete |
Median overall SF-12 scores for the physical health status and mental health status, stratified for demographic items, type of resection, indication and overall morbidity: physical: 46.7 (IQR 34.2–53.9); mental: 54.1 (IQR 42.8–58.2) (all indications) SF-12 data on the mental health scale was significantly higher (p < 0.05) for the subgroup who had liver resection for metastases [55.9 (47.5–58.8)] compared with primary liver cancer [49.6 (36.5–55.1)] or benign liver disease [49.2 (37.7–56.3)]. On the SF-12 physical health scale the scores for the three subgroups were: 43.5 (33.4–54.9) for benign liver disease; 41.6 (32.9–53.1) for primary liver cancer; and 50.6 (39.0–54.2) for liver metastases Also reports data on the SF-12 by type of resection (major, minor), age, and gender, but not extracted here Also reports data on the relationship between subjective impairment and QoL score, and the distribution of liver-specific symptom scores, not extracted here SF-12 scores vs. the general population presented by age. Patients younger than 40–51 years who underwent liver resection showed significantly lower SF-12 scores (physical and mental health status) compared with their age-matched normal group (p < 0.05) (all indications) |
Cella et al.,141 2009
| USA RCT RCT to assess effectiveness of treatments, including QoL. This particular study aimed to assess the use of QOL to predict survival |
|
Functional Assessment of Cancer Therapy-General scale (FACT-G), a 27-item scale ranging from 0 (worse) to 108 (best) QoL; Functional Assessment of Cancer Therapy-Kidney Symptom Index-Disease-Related Symptoms subscale (FKSI-DRS), a 9 item scale ranging from 0 (most severe) to 36 (no symptoms; EQ-VAS (0–100; worse to best) Self-reported questionnaires completed at day 1 and 28 of each cycle of treatment, and at the end of treatment or on withdrawal from the study. Only baseline data presented in the publication |
FACT-G:
|
Haidet et al.,143 1998
| USA Prospective cohort study To assess clinical outcomes and patient preferences at the end of life |
|
Chart review and interviews by trained interviewers to include functional status and QoL Functional status measured using a modified version of the activities of daily living scale by Katz, with higher scores indicating a greater number of dependencies QoL reported on a scale of excellent, very good, good, fair, or poor Timing of administration at study entry |
QoL (during the 2 weeks before administration) good to excellent: 62% (188/304 tested) QoL rated as good to excellent in those interviewed at 2 months (n = 209) and 6 months (n = 140) was 73% and 68%, respectively. By category this was: Quality of life (%)2 months6 monthsExcellent14 (30/209)16 (22/140)Very good26 (55/209)23 (32/140)Good32 (67/209)29 (41/140)Fair or poor27 (57/209)32 (45/140) Independent in activities of daily living (during the 2 weeks before administration): 83% (268/322). Median number of dependencies before study entry was 0 Of interviewed survivors at 2 months (n = 210) and 6 months (n = 142) there was a median number of disabilities of 0 at both times. By number of dependencies this was: 2 months6 monthsFunctional status, % No dependency82 (172/210)88 (125/142) 1 dependency8 (17/210)2 (3/142) ≥ 2 dependencies10 (2/210)10 (14/14) Of those unavailable for interview but had surrogate responses at 2 months (n = 65) and 6 months (n = 38), the median number of disabilities was 1 at both times (IQR 0–5 at 2 months, 0–3 at 6 months) |
Quality of life (%) | 2 months | 6 months | Excellent | 14 (30/209) | 16 (22/140) | Very good | 26 (55/209) | 23 (32/140) | Good | 32 (67/209) | 29 (41/140) | Fair or poor | 27 (57/209) | 32 (45/140) | 2 months | 6 months | Functional status, % | No dependency | 82 (172/210) | 88 (125/142) | 1 dependency | 8 (17/210) | 2 (3/142) | ≥ 2 dependencies | 10 (2/210) | 10 (14/14) | |||
| Quality of life (%) | 2 months | 6 months | |||||||||||||||||||||||||||||||
| Excellent | 14 (30/209) | 16 (22/140) | |||||||||||||||||||||||||||||||
| Very good | 26 (55/209) | 23 (32/140) | |||||||||||||||||||||||||||||||
| Good | 32 (67/209) | 29 (41/140) | |||||||||||||||||||||||||||||||
| Fair or poor | 27 (57/209) | 32 (45/140) | |||||||||||||||||||||||||||||||
| 2 months | 6 months | ||||||||||||||||||||||||||||||||
| Functional status, % | |||||||||||||||||||||||||||||||||
| No dependency | 82 (172/210) | 88 (125/142) | |||||||||||||||||||||||||||||||
| 1 dependency | 8 (17/210) | 2 (3/142) | |||||||||||||||||||||||||||||||
| ≥ 2 dependencies | 10 (2/210) | 10 (14/14) | |||||||||||||||||||||||||||||||
Kemeny et al.,144 2006
| USA RCT To assess efficacy of hepatic artery infusion chemotherapy, including QoL |
|
Rand 36-item Health Status Profile; Memorial Symptom Assessment Scale; Medical Outcomes Study Social Support Questionnaire; Medical Outcomes Study Sexual Functioning Scale Self-report measures administered before treatment and every 3 months until 18 months 63 (47%) completed the entire study QoL assessments (baseline, 3, 6, 9, 12, 15 and 18 months) |
Limited data presented on QoL The physical functioning of the HAC group was better than the systemic chemotherapy group at 3 months (p < 0.038) and 6 months (p < 0.024) No differences between treatments were found in social functioning, role functioning-emotional, or general health perceptions Across the rest of the follow-up period, treatment arm differences among the four QoL outcomes were not evident, except for physical functioning at the 12-month assessment. The relationship between treatment and time varied significantly as a function of the time of dropout (shown graphically for the RAND-36) |
Knox et al.,145 2004
| USA Retrospective cohort study To review clinical and functional QoL outcomes following resection of liver metastases |
|
Functional performance assessed by the Karnofsky function score which the authors state measures functional QoL. This ranges from 0 to 100 (higher scores relate to better function) and was assigned by study investigators preoperatively, 3 and 6 months postoperatively and annually thereafter | Average functional QOL improved by the postoperative month 3 from 75 ± 5 to 88 ± 5 (p < 0.01) Assessed by subgroup (amount of tumour resected ≥ 90% vs. < 90%) but this did not reach a statistically significant group × time interaction effect (p = 0.26) Overall longitudinal functional QoL in 10 participants who survived until at least month 54 presented graphically. This showed a statistically significant improvement (p < 0.05) by month 3, which was sustained through to month 54 |
Krabbe et al.,146 2004
| Netherlands Prospective cohort study To compare generic HRQoL measure with disease-specific measure NB: possible overlap of participants with Langerhoff et al. |
Exploratory laparotomy were split into three groups:
|
EQ-5D VAS and index; EORTC QLQ C-30 Self-report questionnaires administered at baseline (1 day before intervention), 2 weeks post intervention, 3 and 6 months post intervention |
Mean scores on the EQ-5D index, EQ-5D VAS and EORTC QLQ C-30 are presented graphically by time and subgroup Mean scores of total EQ-5D index and EQ-5D VAS are presented for the four data collection points for all participants (not expressed by group) Mean scores of the individual items and domains of the EQ-5D are presented for each time point for all participants (not expressed by group) Mean scores of the individual domains of the EORTC QLQ C-30 are presented for the four data collection points for all participants (not expressed by group) Effect sizes (calculated as baseline value – post-assessment value/standard deviation of the baseline value) for the EQ-5D domains, EQ-5D index, EQ-5D VAS compared with EORTC QLQ C-30 presented (to test the measures against one another) for all participants (not by group) |
Langenhoff et al.,147 2006
| Netherlands Prospective cohort study To assess the impact of HRQoL |
|
EQ-5D VAS and index; EORTC QLQ C-30 Self-report questionnaires administered at baseline, and 2 weeks, 3 months, 6 months and 12 months post laparotomy |
Mean scores of the individual items of the EORTC QLQ C-30 are presented for each of the three groups at baseline, and 2 weeks, 3 and 6 months (not reported at 12 months) Mean scores of the four domains of the EORTC QLQ C-30 are presented graphically for the three groups Mean scores of the EQ-5D VAS and EQ-5D index are presented graphically for the three groups Effect sizes for the EQ-5D (calculated as baseline value – post assessment value/standard deviation of the baseline value): EQ-5D VAS 0–0.5 months0–3 months0–6 monthsGroup 11.050.280.05Group 21.301.070.40Group 30.020.270.51 EQ-5D index 0–0.5 months0–3 months0–6 monthsGroup 11.210.440.25Group 20.730.140.42Group 30.430.240.13 |
0–0.5 months | 0–3 months | 0–6 months | Group 1 | 1.05 | 0.28 | 0.05 | Group 2 | 1.30 | 1.07 | 0.40 | Group 3 | 0.02 | 0.27 | 0.51 | 0–0.5 months | 0–3 months | 0–6 months | Group 1 | 1.21 | 0.44 | 0.25 | Group 2 | 0.73 | 0.14 | 0.42 | Group 3 | 0.43 | 0.24 | 0.13 | ||
| 0–0.5 months | 0–3 months | 0–6 months | |||||||||||||||||||||||||||||||||
| Group 1 | 1.05 | 0.28 | 0.05 | ||||||||||||||||||||||||||||||||
| Group 2 | 1.30 | 1.07 | 0.40 | ||||||||||||||||||||||||||||||||
| Group 3 | 0.02 | 0.27 | 0.51 | ||||||||||||||||||||||||||||||||
| 0–0.5 months | 0–3 months | 0–6 months | |||||||||||||||||||||||||||||||||
| Group 1 | 1.21 | 0.44 | 0.25 | ||||||||||||||||||||||||||||||||
| Group 2 | 0.73 | 0.14 | 0.42 | ||||||||||||||||||||||||||||||||
| Group 3 | 0.43 | 0.24 | 0.13 |
Pistevou-Gombaki et al.,148 2003
| Greece Prospective cohort study To investigate QoL after chemotherapy for liver metastases. |
|
EORTC QLQ C-30 Self-report questionnaire administered at baseline, at time of octreotide administration, 1 week later, and monthly thereafter (up to 7 months) |
Mean scores of the individual items and domains of the EORTC QLQ C-30 are presented for baseline and 1 month post chemotherapy. All the functioning scales and pain, fatigue, insomnia, appetite loss were statistically significantly improved (p < 0.05) Mean score for global QoL at baseline and 1 month also statistically significant improvement: 4.2 (SD 8.0) vs. 23.9 (SD 11.7) respectively, p = 0.001 Results also presented for individual items of the EORTC QLQ C-30 Results also presented for subgroups according to primary site (not extracted here) |
Ruers et al.,14 2006
| Netherlands Prospective cohort study To assess survival and HRQoL in a consecutive cohort of patients selected for laparotomy |
|
EQ-5D VAS; EORTC QLQ C-30 physical functioning scale EQ-5D scores used to compute QALYs QALYs expressed as quality-adjusted days Self-report questionnaires administered preoperatively, 2–3 weeks post laparotomy, and then every 3 months until 1 year post laparotomy |
Baseline HRQoL scores on both measures were similar All groups showed a decline in HRQoL 3 weeks post laparotomy At 3 months those in the resection and ablation/resection groups returned to baseline During the first year QALYs (days in full health) were:
|
Wiering et al.,149,151 2010 and 2011
| Netherlands RCT leading to a prospective cohort study of the total population To assess the added value of positron emission tomography imaging including HRQoL in those undergoing hepatic resection NB: some minor discrepancies between these two publications; however, likely to be the same participants as the dates of study and treatments appear to be the same |
|
EQ-5D index (society based) and visual analogue scale (patient perspective) Q-TWIST – an expression of HRQoL for four distinct clinical health states: death, disease free, non-curative, recurrence, based on the EQ-5D measure Self-reported questionnaire (EQ-5D) completed at baseline, at week 3 and week 6 post surgery and then every 3 months for the next 3 years Overall response rate was 90.8% after 3 years |
Wiering et al.151 HRQoL, mean (SD), max/min, EQ-5D index values at:
Statistically significant effects were observed between the disease-free and non-curative groups from week 12 onwards (p < 0.05), and the disease-free and recurrence groups at the end of follow-up (144 weeks, p = 0.024) Results by time presented graphically Wiering et al.149 Results of EQ-5D by time and intervention group presented graphically based on intention to treat population except two participants who did not return their questionnaires in the control group |
Wietzke-Braun et al.,150 2004
| Germany Prospective cohort study To investigate clinical outcomes and quality of life after laser ablation for liver metastases |
|
EORTC QLQ C-30 Self-report questionnaire administered at baseline, 1 week post intervention, 1 month and 6 months post intervention |
Mean scores of general symptoms for the 18 patients who survived until 6 months presented for each data collection point graphically The variable pain before and 1 week after laser ablation was seen to be statistically significantly different (p < 0.05), and before and 6 months after treatment (p < 0.05). Authors suggest this may not be clinically relevant, however For general symptoms, only constipation reached statistical significance (p < 0.05) in a multivariate analysis Mean scores for functioning scales and global QoL presented graphically. No statistically significant changes were observed in any of these domains before and after laser ablation |
Appendix 11 Variables included in probabilistic sensitivity analysis, sampling distributions and parameterisation of sampling distributions
Overall survival
Correlation between parameters in the overall survival regression is handled using the Cholesky decomposition method. 168 The Cholesky decomposition of the variance–covariance matrix for the regression used to fit the Weibull survival function would be:
| ln(t) | treat | ln(λ) | |
|---|---|---|---|
| ln(t) | C [1,1] | ||
| treat | C [2,1] | C [2,2] | |
| ln(λ) | C [3,1] | C [3,2] | C [3,3] |
In each simulation three draws are taken from standard normal distributions (mean = 0, SD = 1), labelled here as z1, z2, z3. Three new variables (Tz1, Tz2 and Tz3) are defined by multiplying elements of the Cholesky decomposition matrix (C) by the values draw from standard normal distributions (z1, z2, z3). Identifying elements of the Cholesky decomposition as C [i,j], as above, then:
For each simulation the sampled values of the parameter estimates are therefore:
The same approach was used to handle correlation between parameters in the model used to estimate time to progression.
Variables included in probabilistic sensitivity analysis, sampling distributions and parameterisation of sampling distributions
| Mean | Standard error | Distribution | Parameters | |
|---|---|---|---|---|
| Health state utility | ||||
| Stable disease | 0.82 | 0.01 | Beta | α = 1209.50, β = 265.50 |
| Stable disease – up to 6 months post surgery | 0.65 | 0.01 | Beta | α = 1478.10, β = 795.90 |
| Stable disease – up to 3 months post ablation | 0.74 | 0.01 | Beta | α = 1423.02, β = 499.98 |
| Proportionate reduction due to disease progression | 0.75 | 0.08 | Beta | α = 23.26, β = 7.75 |
| Procedure costs | ||||
| Microwave ablation | 8302 | 847.16 | Gamma | α = 96.0365, β = 86.4463 |
| Surgical resection | 11,937 | 1218.08 | Gamma | α = 96.0365, β = 124.2965 |
| Microwave ablation with adverse event | 9550 | 974.51 | Gamma | α = 96.0365, β = 99.4414 |
| Surgical resection with adverse event | 13,240 | 1351.05 | Gamma | α = 96.0365, β = 137.8643 |
| Radiofrequency ablation | 3391 | 346.03 | Gamma | α = 96.0365, β = 35.3095 |
| Surgical resection | 8747 | 892.57 | Gamma | α = 96.0365, β = 91.0800 |
| Radiofrequency ablation with adverse event | 3900 | 397.97 | Gamma | α = 96.0365, β = 40.6096 |
| Surgical resection with adverse event | 9930 | 1013.28 | Gamma | α = 96.0365, β = 103.3982 |
| Radioembolisation consumables | 10,911 | 1113.39 | Gamma | α = 96.0365, β = 113.6131 |
| Radioembolisation procedure | 3305 | 337.25 | Gamma | α = 96.0365, β = 34.4140 |
| On-treatment monitoring – HACE | 203 | 20.75 | Gamma | α = 96.0365, β = 2.1173 |
| Follow-up costs | ||||
| Post-discharge follow-up | 237 | 24.16 | Gamma | α = 96.0365, β = 2.4658 |
| Post-treatment follow-up | 231 | 23.61 | Gamma | α = 96.0365, β = 2.4095 |
| Adverse events | ||||
| Microwave ablation complications | 14.3% | 0.0935 | Beta | α = 1.8571, β = 11.1429 |
| Surgical resection | 18.8% | 0.0976 | Beta | α = 2.8125, β = 12.1875 |
| Radiofrequency ablation complications | 6.2% | 0.0181 | Beta | α = 10.9379, β = 165.0621 |
| Surgical resection complications | 21.2% | 0.0245 | Beta | α = 58.7878, β = 218.2122 |
Appendix 12 Survival model methodology
As described in the main body of the text, the survival models adopted for this report were developed using linear regression to estimate the parameters of linear transformations of the observed Kaplan–Meier estimates for overall survival and progression-free survival reported in included studies. Three parametric survival functions were estimated – exponential, Weibull and log-logistic survival functions – which were compared for goodness of fit with the observed overall survival and progression-free survival functions by visual inspection.
For an exponential distribution the survival function is given by
Taking the log of both sides gives
which is a linear function and can be fit using least squares methods to estimate λ.
For a Weibull distribution the survival function is given by
with scale parameter λ and shape γ. Taking the log of both sides gives
Taking the log of both sides again gives
which is a linear function and can be fit using least squares methods to provide estimates of log(λ) and γ.
Similarly, the log-logistic survival function, given by
can be transformed to the linear function
This can be fit using least squares methods to provide estimates of log(λ) and β.
General method for extracting data from published survival curves
Figures presenting overall and survival and progression-free survival curves were scanned from the original publications and imported into Engauge software. The process of extracting data from a chart usually begins with the user identifying key reference points on the chart (e.g. indicating the location of the origin and maximum points for the x- and y-axes). Engauge software will indicate what appear to be data points in the imported image (shown in green in the figure below, using the example of the overall survival chart from Shibata and colleagues)72 or the user can select individual data points to be extracted using the mouse. Points along the Kaplan–Meier were selected by hand as shown in the figure below, and the raw data (without any interpolation) were extracted to a text file and imported into Microsoft Excel.
FIGURE 22.
Extracting survival data from scanned images using Engauge.
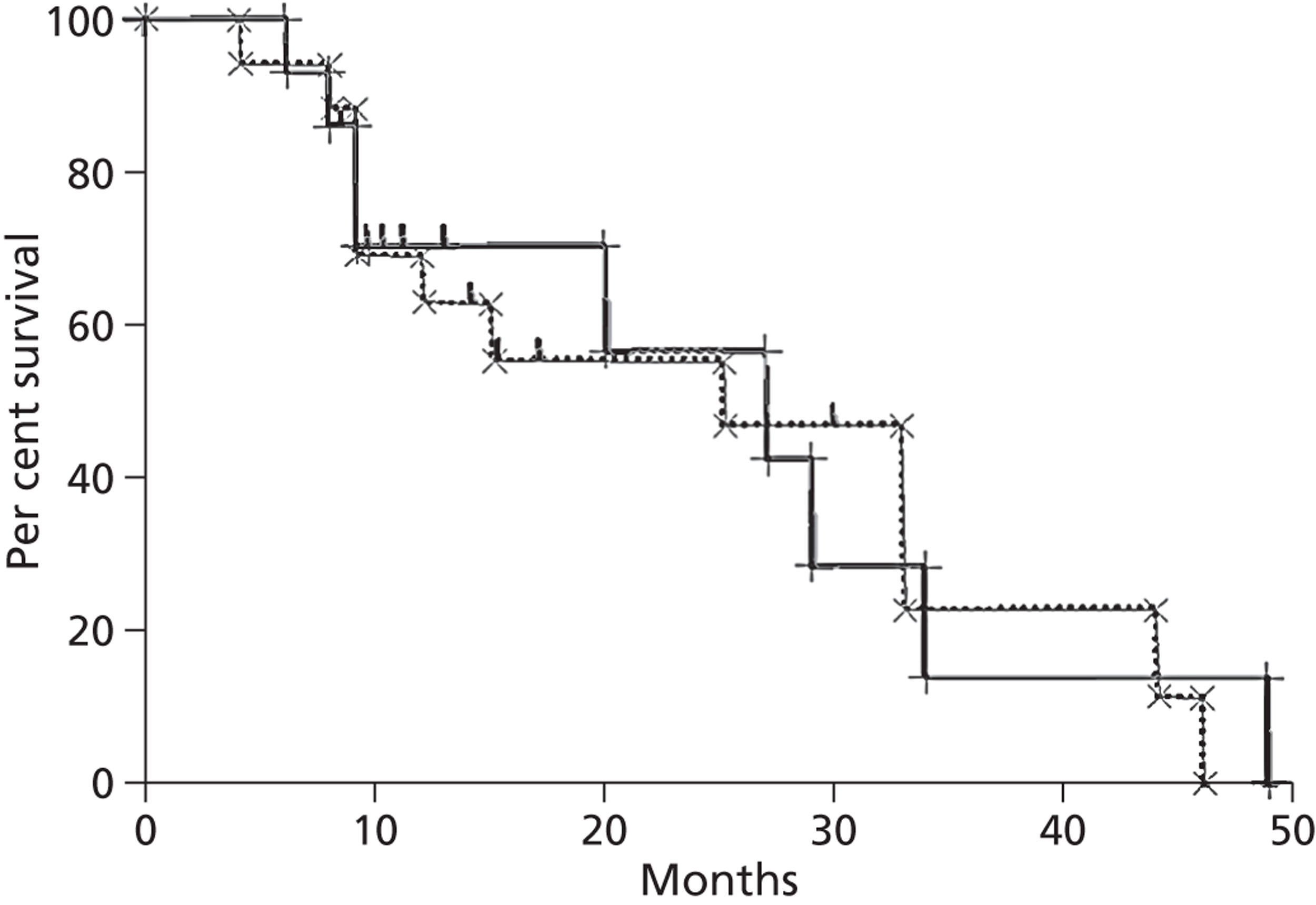
Overall survival
The following tables report the parameter estimates for linear regressions for the exponential, Weibull and log-logistic survival functions. In each of these an additional parameter (treat) was included in the regression – this was a dummy (0,1) variable that indicated whether the observed survival data were for the intervention (treat = 1) or the comparator (treat = 0).
| Exponential | Weibull | Log-logistic | |||
|---|---|---|---|---|---|
| Parameter | Coefficient | Parameter | Coefficient | Parameter | Coefficient |
| λ | –0.05409 | ln(λ) | –6.70614 | ln(λ) | –8.78953 |
| treat | –0.00821 | γ | 2.05724 | γ | 2.98013 |
| treat | –0.11424 | treat | 0.09976 | ||
| Exponential | Weibull | Log-logistic | |||
|---|---|---|---|---|---|
| Parameter | Coefficient | Parameter | Coefficient | Parameter | Coefficient |
| λ | –0.01133 | ln(λ) | –7.19176 | ln(λ) | –7.84791 |
| treat | 0.00044 | γ | 1.71996 | γ | 1.96272 |
| treat | –0.00010 | treat | 0.02289 | ||
| Exponential | Weibull | Log-logistic | |||
|---|---|---|---|---|---|
| Parameter | Coefficient | Parameter | Coefficient | Parameter | Coefficient |
| λ | –0.01270 | ln(λ) | –5.57716 | ln(λ) | –6.33878 |
| treat | –0.01397 | γ | 1.32759 | γ | 1.62057 |
| treat | 0.67780 | treat | 0.94135 | ||
| Exponential | Weibull | Log-logistic | |||
|---|---|---|---|---|---|
| Parameter | Coefficient | Parameter | Coefficient | Parameter | Coefficient |
| λ | –0.04441 | ln(λ) | –4.56813 | ln(λ) | –6.22661 |
| treat | Not estimateda | γ | 1.39754 | γ | 2.14318 |
| treat | Not estimateda | treat | 0.45931 | ||
FIGURE 23.
Transformed Kaplan–Meier overall survival curves for microwave ablation and surgical resection derived from Shibata and colleagues72 plus linear fit. (a) Exponential model; (b) Weibull model; and (c) log-logistic model.
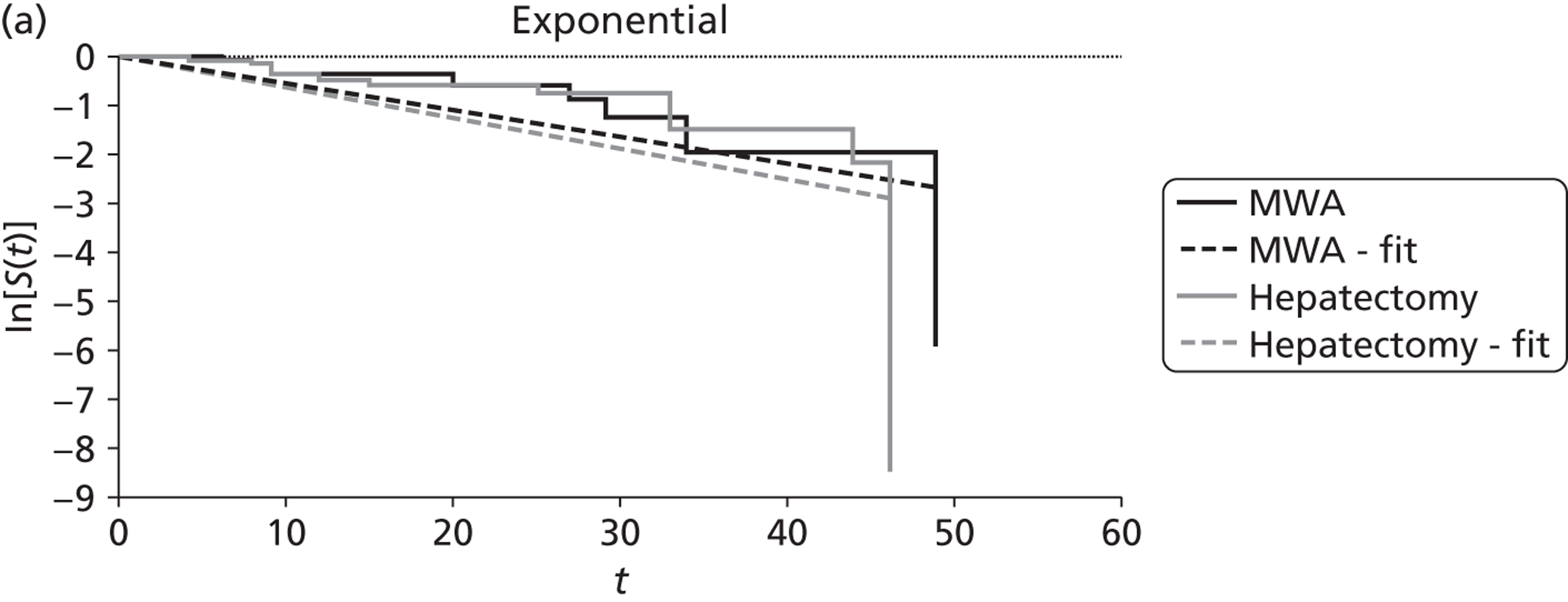
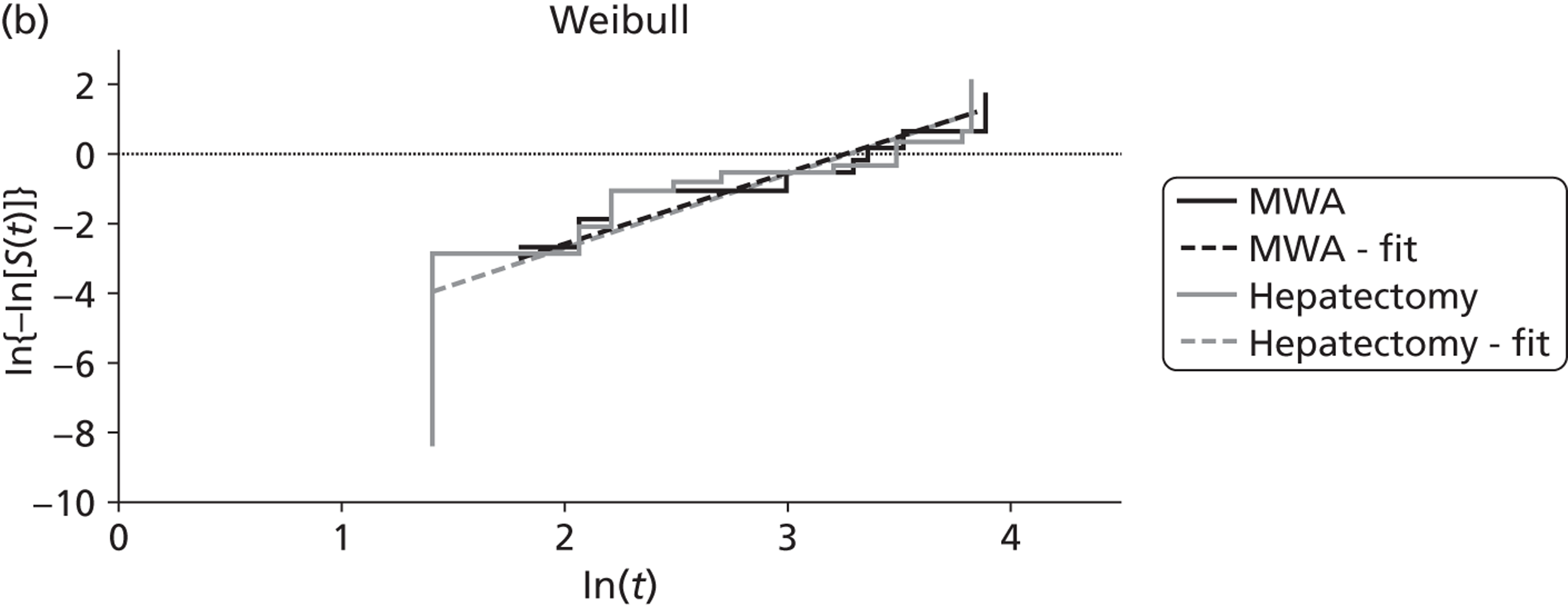
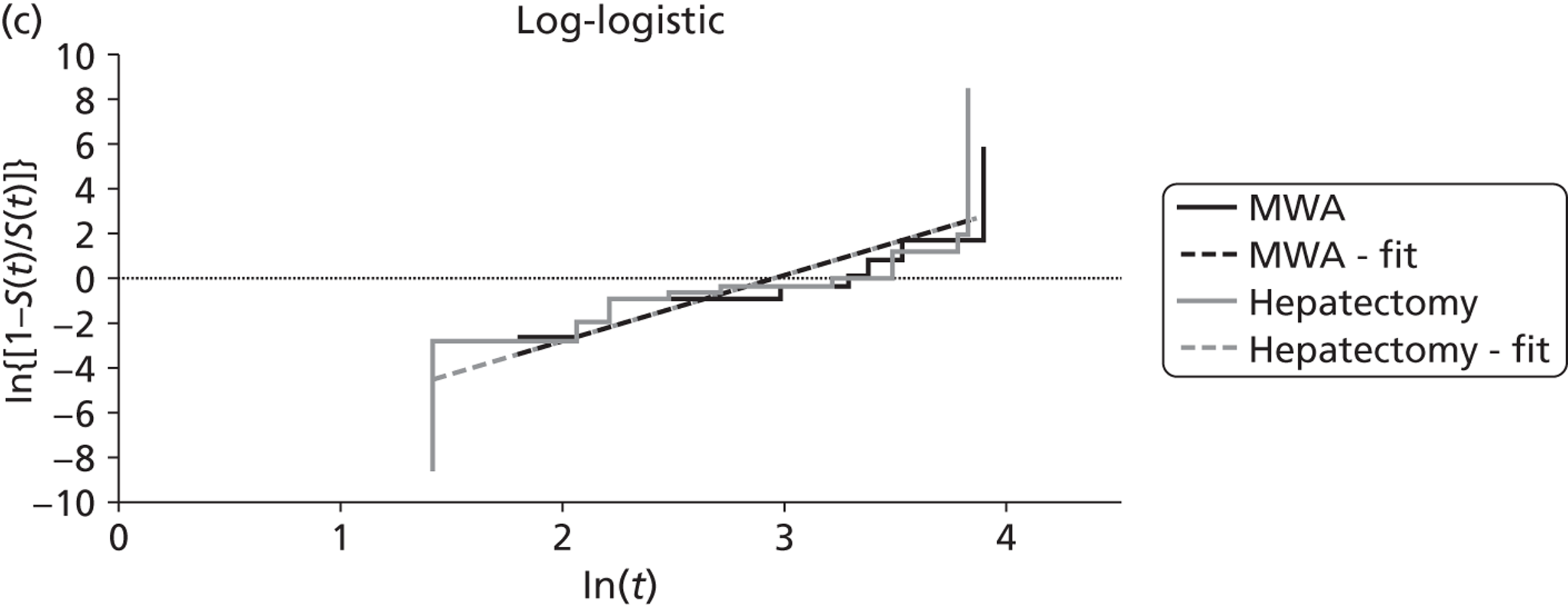
FIGURE 24.
Transformed Kaplan–Meier overall survival curves for radiofrequency ablation and surgery, for small (< 3 cm) metastases, derived from Kim and colleagues73 plus linear fit. (a) Exponential model; (b) Weibull model; and (c) log-logistic model.
FIGURE 25.
Transformed Kaplan–Meier overall survival curves for radiofrequency ablation and surgery, for large (≥ 3 cm) metastases, derived from Kim and colleagues73 plus linear fit. (a) Exponential model; (b) Weibull model; and (c) log-logistic model.
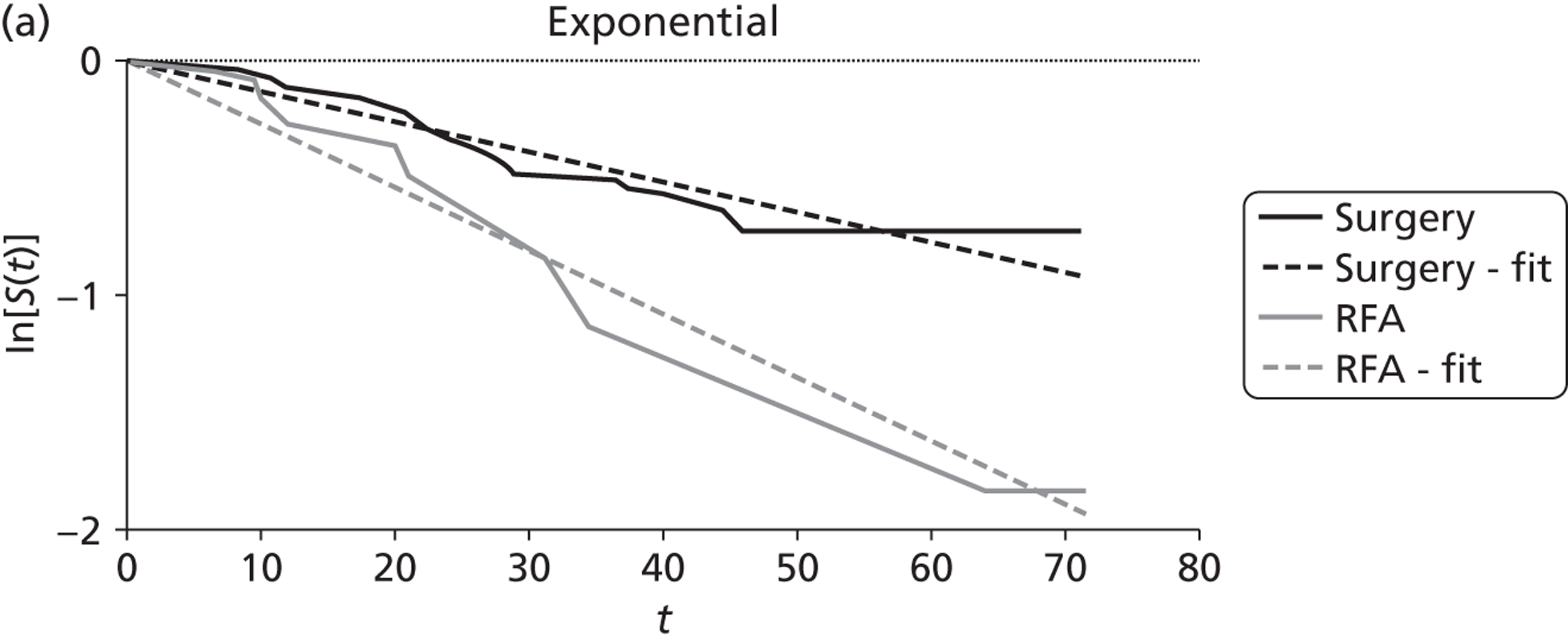
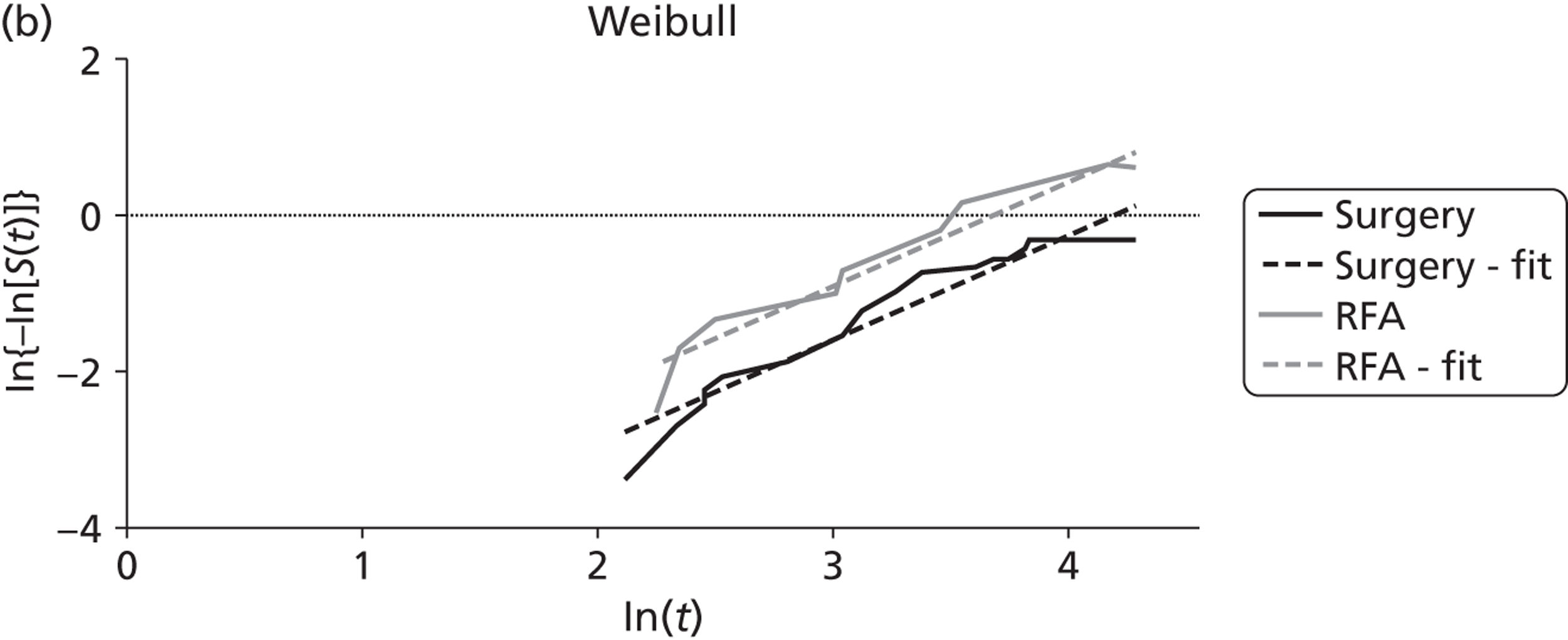
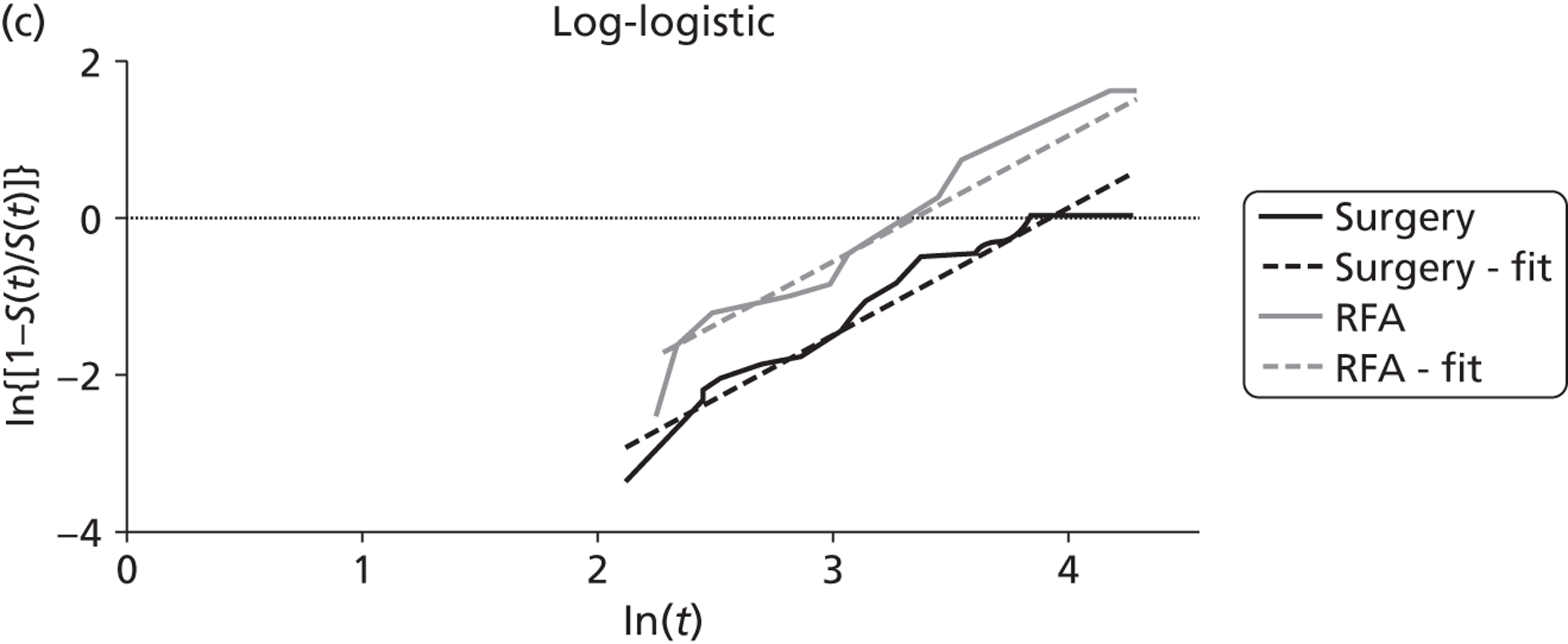
FIGURE 26.
Transformed Kaplan–Meier overall survival curves for radioembolisation plus hepatic artery chemotherapy and hepatic artery chemotherapy alone derived from Grey and colleagues85 plus linear fit. (a) Exponential model; (b) Weibull model; and (c) log-logistic model.
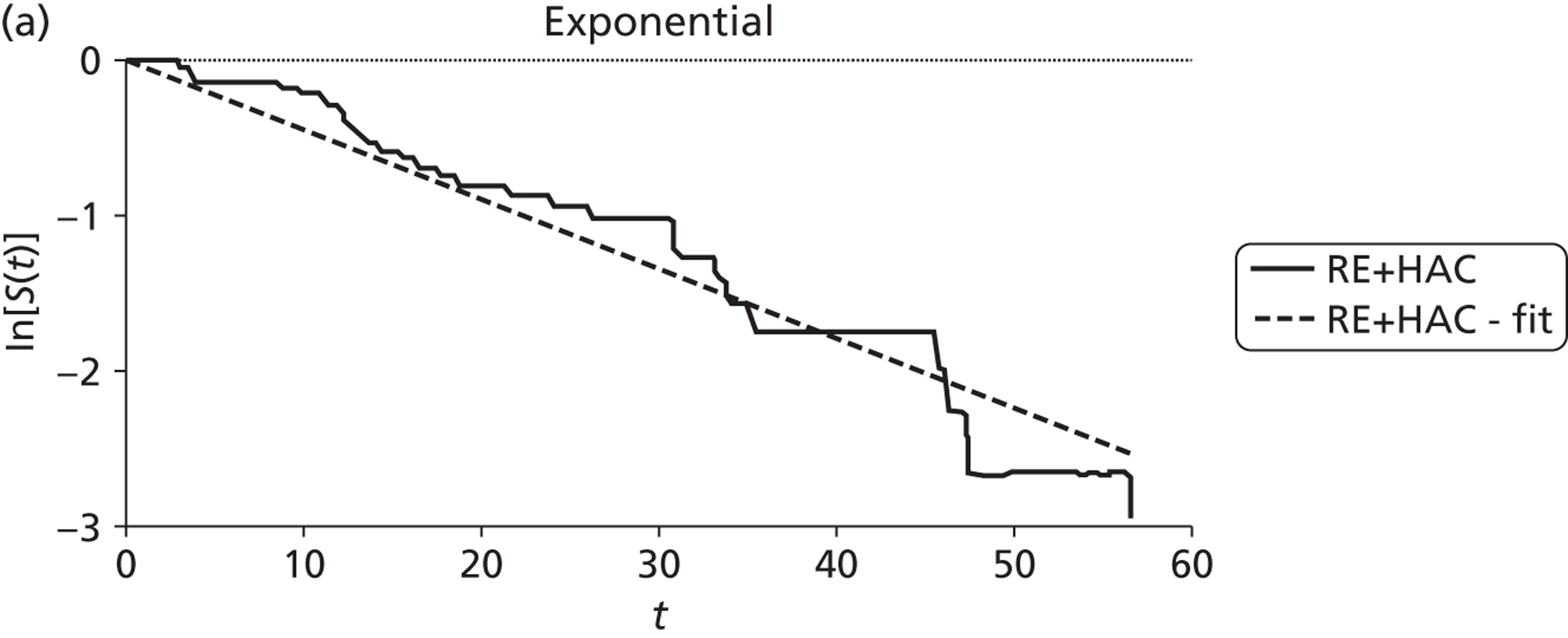
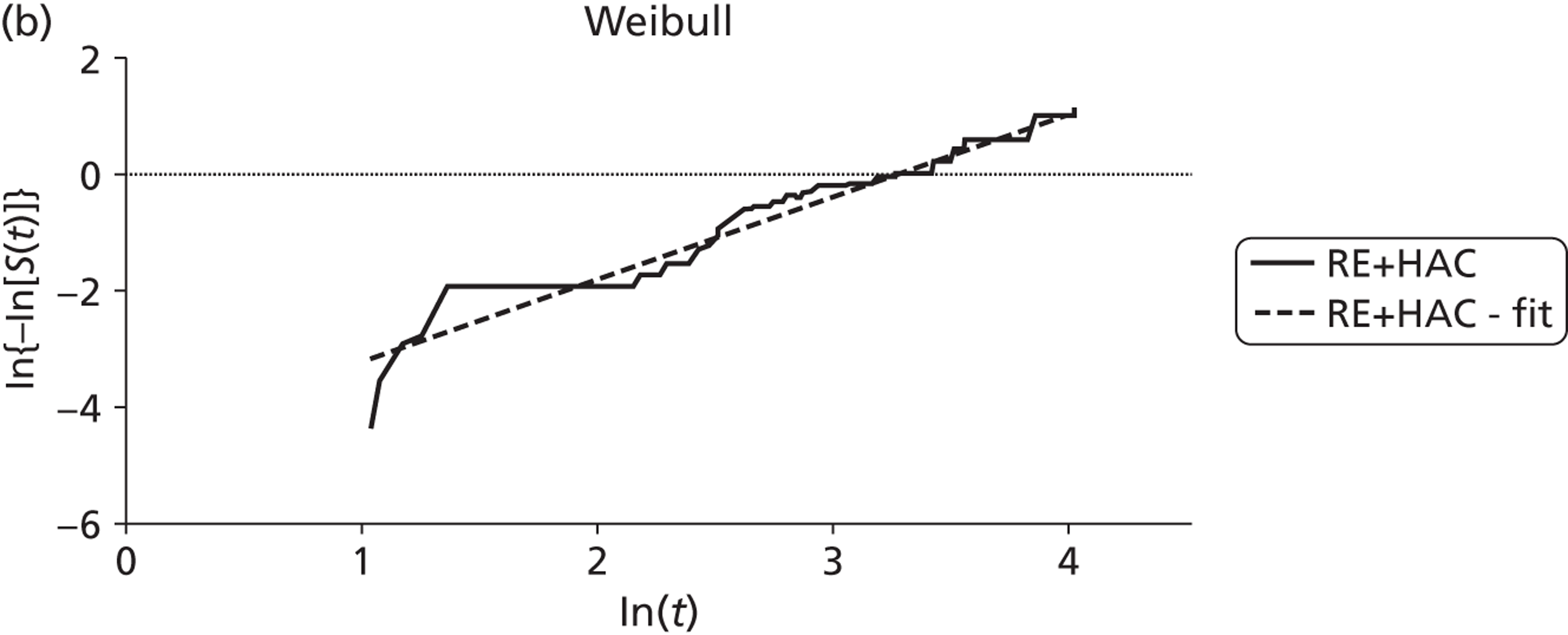
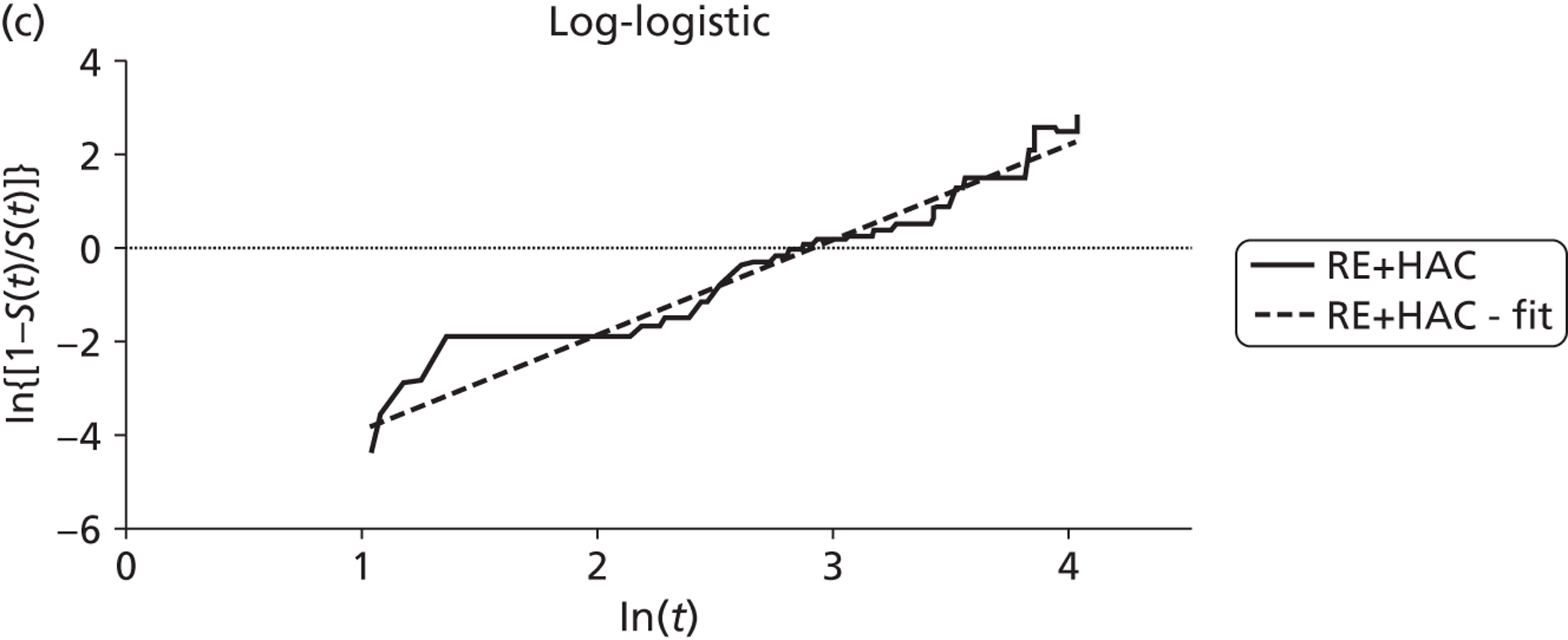
Progression-free survival
The following tables report the parameter estimates for linear regressions for the exponential, Weibull and log-logistic survival functions. As before an additional parameter (treat) was included in the regression – this was a dummy (0,1) variable that indicated whether the observed survival data were for the intervention (treat = 1) or the comparator (treat = 0).
| Exponential | Weibull | Log-logistic | |||
|---|---|---|---|---|---|
| Parameter | Coefficient | Parameter | Coefficient | Parameter | Coefficient |
| λ | –0.02394 | ln(λ) | –3.22771 | ln(λ) | –3.43363 |
| treat | –0.00281 | γ | 0.92444 | γ | 1.14061 |
| treat | 0.05773 | treat | 0.03730 | ||
| Exponential | Weibull | Log-logistic | |||
|---|---|---|---|---|---|
| Parameter | Coefficient | Parameter | Coefficient | Parameter | Coefficient |
| λ | –0.01915 | ln(λ) | –2.81730 | ln(λ) | –3.09404 |
| treat | –0.01085 | γ | 0.70733 | γ | 0.91478 |
| treat | 0.89781 | treat | 1.22393 | ||
| Exponential | Weibull | Log-logistic | |||
|---|---|---|---|---|---|
| Parameter | Coefficient | Parameter | Coefficient | Parameter | Coefficient |
| λ | –0.04640 | ln(λ) | –4.32603 | ln(λ) | –5.16223 |
| treat | –0.07796 | γ | 1.44306 | γ | 1.92574 |
| treat | 0.85427 | treat | 1.40902 | ||
FIGURE 27.
Transformed progression-free survival curves for radiofrequency ablation and surgery, for small (< 3 cm) metastases, derived from Kim and colleagues73 plus linear fit. (a) Exponential model; (b) Weibull model; and (c) log-logistic model.
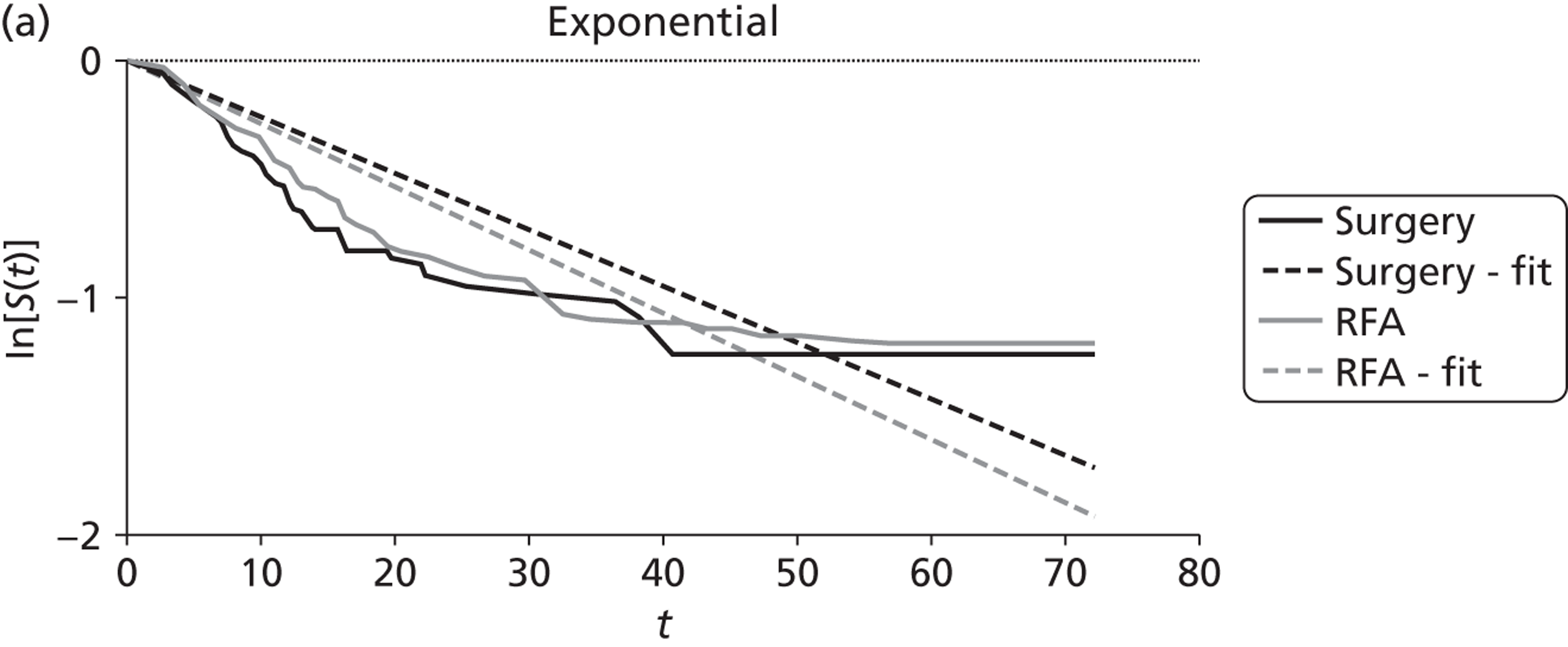
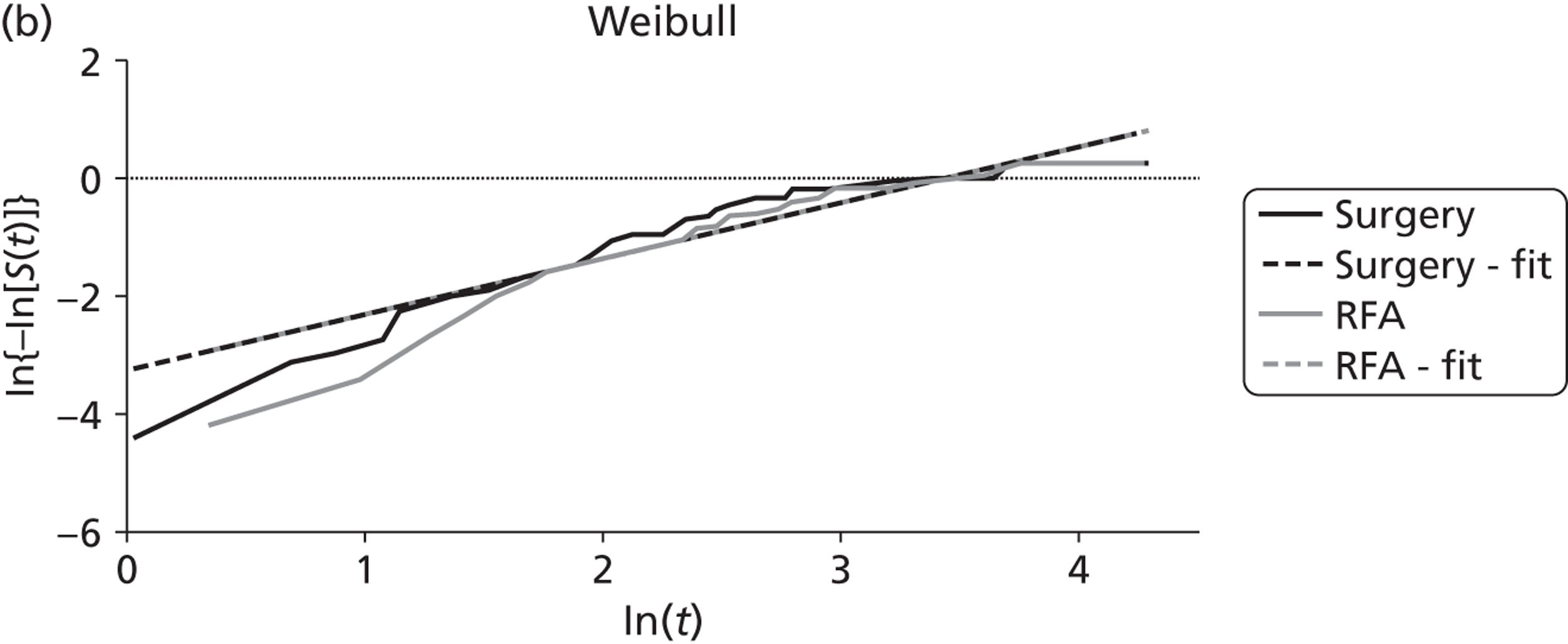
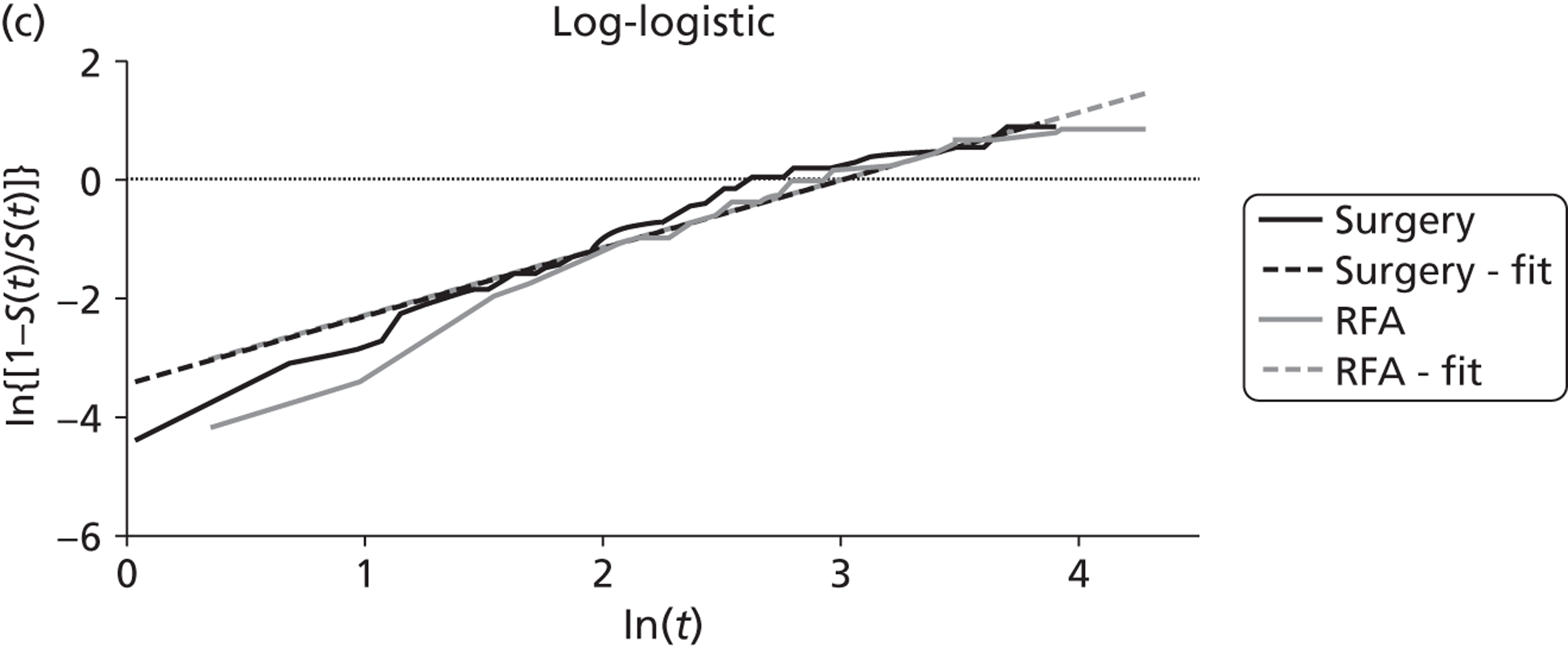
FIGURE 28.
Transformed progression-free survival curves for radiofrequency ablation and surgery, for large (≥ 3 cm) metastases, derived from Kim and colleagues73 plus linear fit. (a) Exponential model; (b) Weibull model; and (c) log-logistic model.
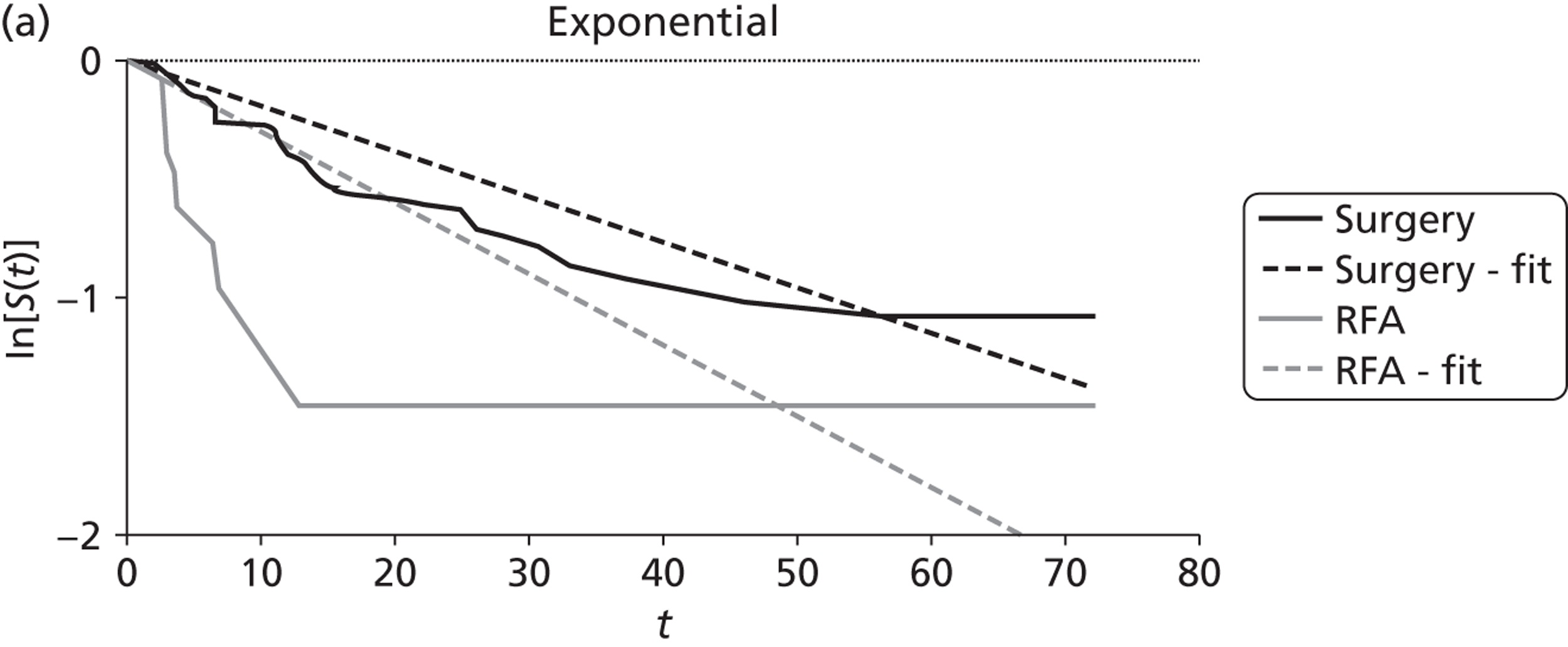
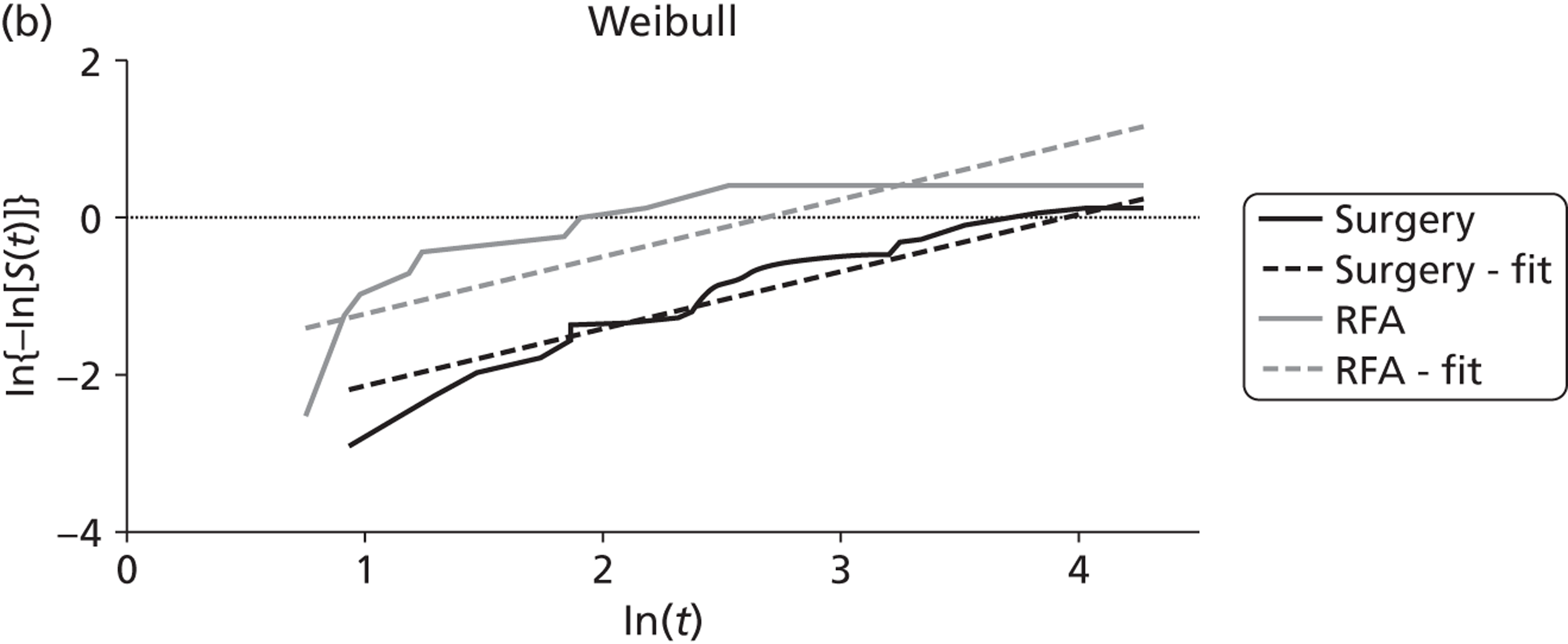
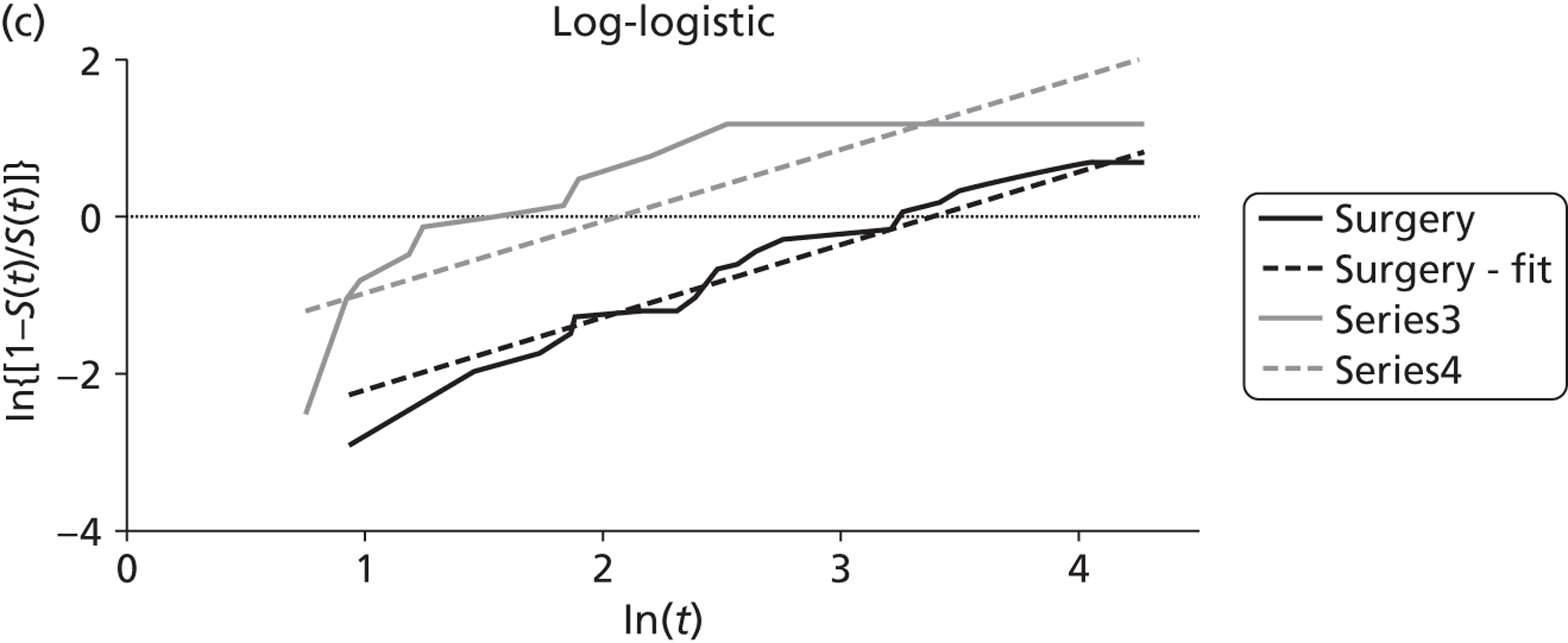
FIGURE 29.
Transformed progression-free survival curves for radioembolisation plus hepatic artery chemotherapy and hepatic artery chemotherapy alone derived from Grey and colleagues85 plus linear fit. (a) Exponential model; (b) Weibull model; and (c) log-logistic model.
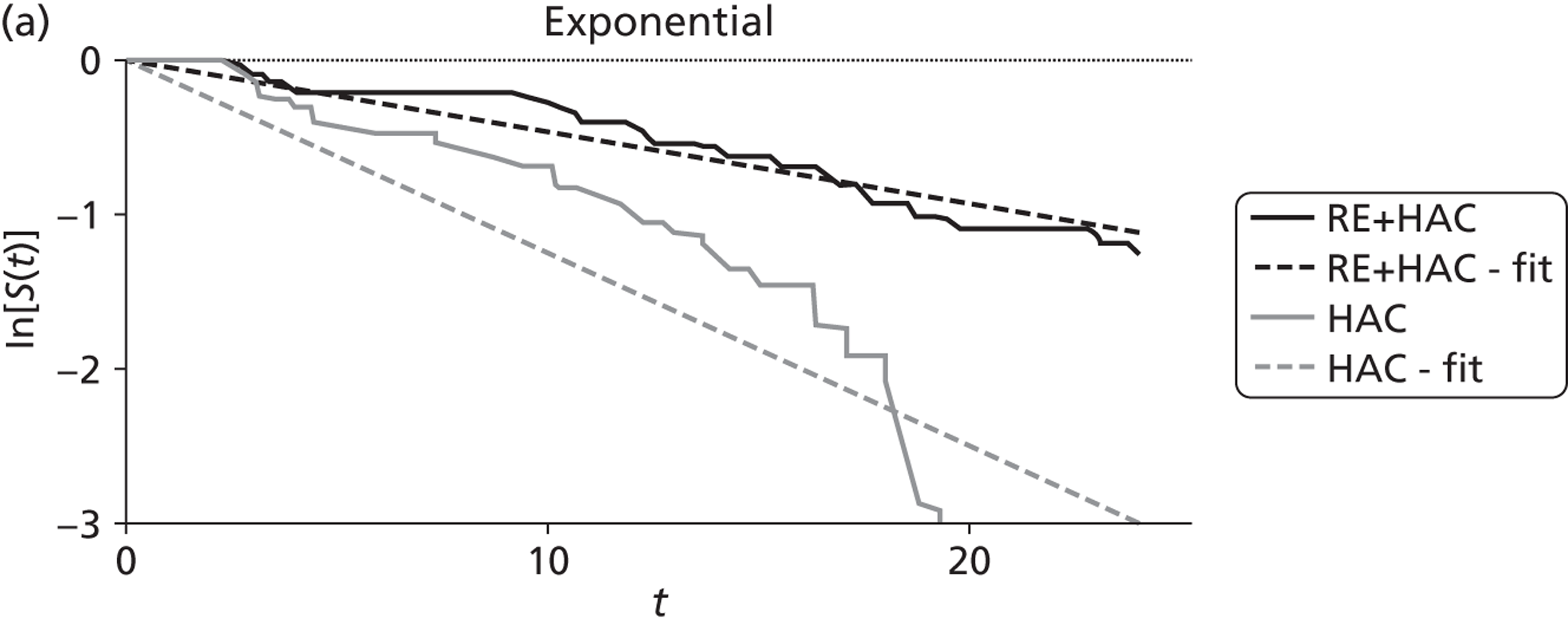
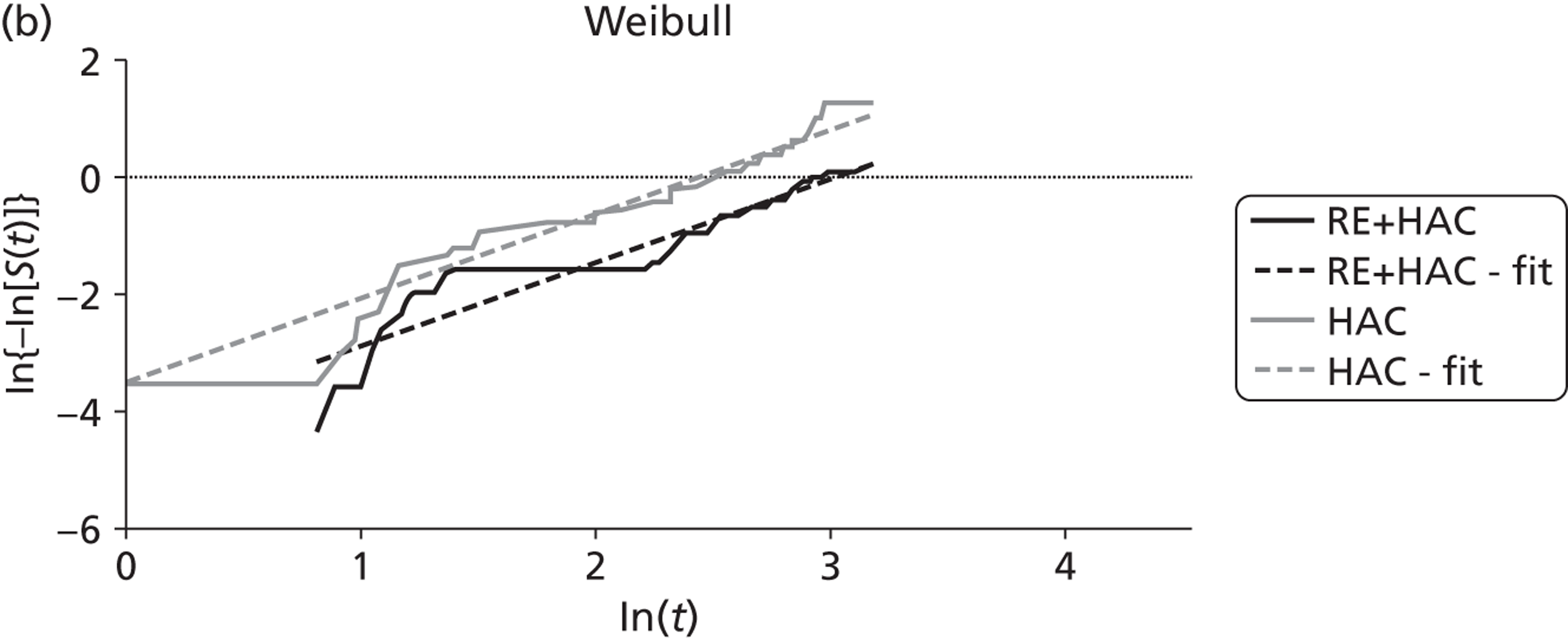
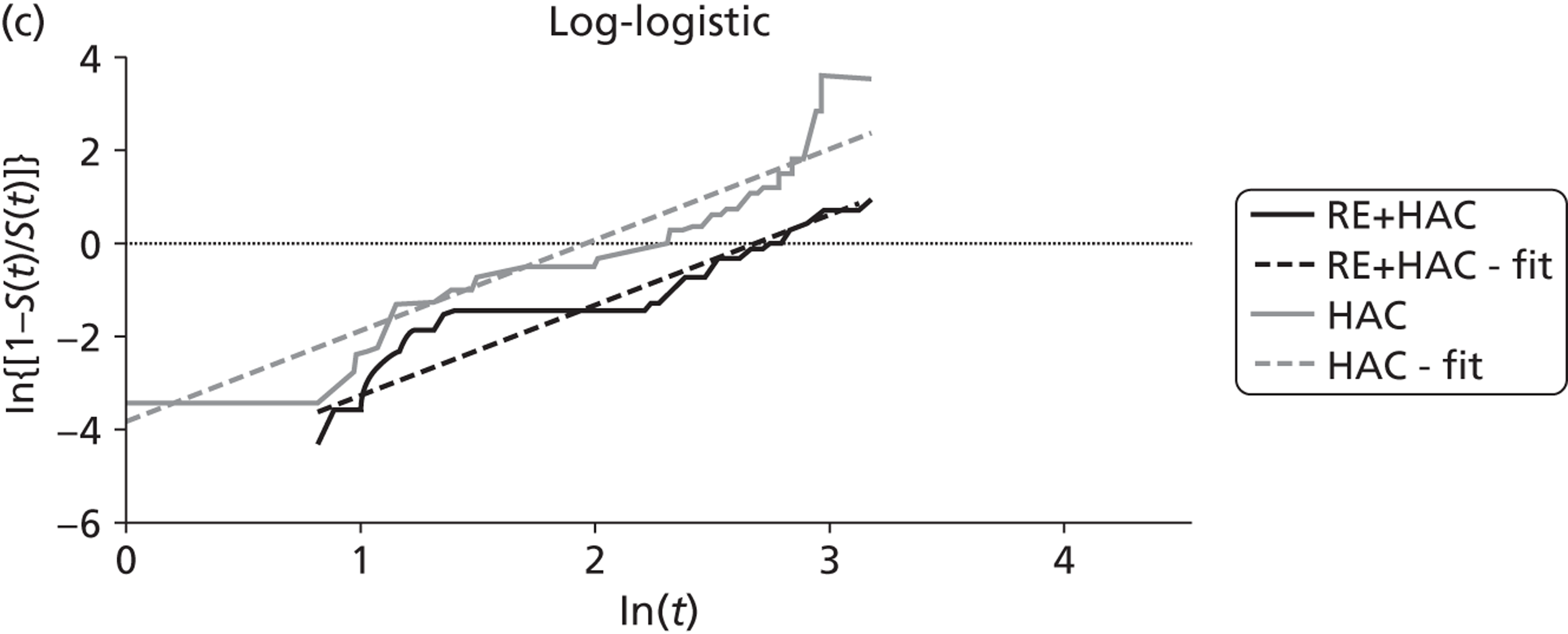
List of abbreviations
- 5-FU
- 5-fluorouracil
- BSC
- best supportive care
- CEA
- carcinoembryonic antigen
- CI
- confidence interval
- CRD
- Centre for Reviews and Dissemination
- CT
- computed tomography
- ECOG
- Eastern Co-operative Oncology Group
- EORTC
- European Organisation for Research and Treatment of Cancer
- EQ-5D
- European Quality of Life-5 Dimensions
- HACE
- hepatic artery chemoembolisation
- HADS
- Hospital Anxiety and Depression Scale
- HR
- hazard ratio
- HRQoL
- health-related quality of life
- ICER
- incremental cost-effectiveness ratio
- LVRT
- liver volume replaced by tumour
- MAA
- macroaggregated albumin
- MRI
- magnetic resonance imaging
- NICE
- National Institute for Health and Care Excellence
- PSA
- probabilistic sensitivity analysis
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- RAND-36
- Rand 36-item health status profile
- RCT
- randomised controlled trial
- RECIST
- response evaluation criteria in solid tumours
- RSC
- Rotterdam Symptom Checklist
- SD
- standard deviation
- SIP
- Sickness Impact Profile
- SMDT
- Specialist Hepatobiliary Multidisciplinary Teams
- VAS
- visual analogue scale
- WHO
- World Health Organization