Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/116/01. The contractual start date was in January 2011. The draft report began editorial review in July 2012 and was accepted for publication in December 2012. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Simpson et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Peripheral arterial occlusive disease (PAD) is a cause of major morbidity in the UK. Disease in the arteries to the legs causes a reduction in the circulation and can present clinically as intermittent claudication (IC; pain on walking), which can severely impair lifestyle. More severe disease may present as critical ischaemia with rest pain, ulceration or gangrene in the lower extremities.
In recent years, there has been a rapid increase in the use of endovascular treatment, particularly percutaneous transluminal balloon angioplasty (PTA). In this procedure, a device is inserted through a small puncture under local anaesthetic and a narrowed or blocked area of artery is opened up by the inflation of balloons. There is a high demand for PTA for PAD, with in excess of 20,000 procedures per annum in England (based on data for 2010–11). 1 Revascularisation strategy is individual to the patient, and treatment by vascular specialists, or within specialised vascular centres, is recommended by the European Society of Cardiology (ESC) guidelines2 and the Vascular Society of Great Britain and Ireland (VSGBI). 3
There have also been rapid technological developments aimed at improving the short- and long-term results of this treatment. Such developments include the use of stents, drug-eluting stents (DESs), drug-eluting balloons, cryotherapy, atherectomy and drug treatments. Many of these techniques have been developed for use in the coronary circulation and extended to the peripheral circulation or may be evaluated in the peripheral circulation with a view to using similar methods in the coronary circulation.
The purpose of this report was to evaluate the range of additional technologies that are available and identify the clinical situations in which they are most likely to be of benefit, or those technologies for which further research studies are justified.
When considering the introduction of new technologies, there are a number of considerations regarding the clinical situation that may be relevant to the applicability and outcome of particular techniques and may therefore be important in defining subgroups that are important in the consideration of the new technologies. These are particularly the clinical stage or symptomatic presentation of the condition being treated, the anatomical distribution of disease and the place of the endovascular procedure in the treatment pathway.
Clinical presentation
The majority of patients with PAD will present with symptoms of IC (pain in the muscle of the leg brought about by walking). This may vary in severity from mild pain that occurs only after considerable exercise or when going uphill, to severe pains that stop activity after only a few paces. It may also affect one or both legs.
More severe PAD may result in insufficient blood supply to the legs, even at rest. In these circumstances, the patient may develop rest pain, particularly nocturnal pain when the legs are elevated in bed and, in the more advanced stages, tissue loss, ulceration and gangrene. The severity of the symptoms of PAD may be classified using a variety of scales, the most common being the Fontaine or Rutherford classifications. These may be used in research settings, although they are consistently used in routine clinical practice. The classifications divide up patients depending upon the severity of the condition based upon IC and critical limb ischaemia (CLI) and then further subdivide them. The Fontaine classification uses subdivisions based upon pain-free walking distance, whereas the Rutherford classification uses the results of the treadmill exercise test and ankle–brachial pressure index (ABPI) measurements.
In addition, PAD is associated with other forms of arterial disease, particularly ischaemic heart disease and cerebrovascular disease. In many patients with generalised atherosclerosis, there is some degree of asymptomatic PAD, and mild degrees of IC are quite common in the general population: the Edinburgh Artery Study reported a prevalence of 4.5% [95% confidence interval (CI) 3.5% to 5.5%] in people aged 55–74 years. 4 Those with IC may go on to develop worsening symptoms, although it is quite common for symptoms to remain static for many years and only a small proportion, probably around 5–10% over 5 years,5 will go on to develop critical ischaemia, about a quarter of whom may eventually require amputation.
As the clinical presentation has a significant bearing on outcome and particularly the risk of reocclusion following an endovascular procedure, this is an important aspect to be taken into consideration when evaluating new technologies.
Anatomical distribution
Both IC and CLI may be the result of a reduction in blood flow due to narrowing or occlusion of the arteries to the lower limb at any level. From the point of view of management, the levels of arterial disease are often divided into aortoiliac, that is affecting anywhere in the aorta or common and external iliac arteries, and infrainguinal, those arteries below the inguinal ligament. Disease below the inguinal ligament is also often further subdivided into femoropopliteal disease, that is disease in the femoral arteries and popliteal artery above or below the knee and infrageniculate or distal disease, referring to those vessels below the popliteal artery (anterior and posterior tibial and peroneal arteries).
Owing to the differences in arterial calibre and blood flow, the natural history and outcomes of treatments may be expected to differ among the different anatomical sites. The position, size and accessibility of different vessels may also give rise to particular technical challenges. There are many other ways in which the anatomical distribution of disease may be important in determining treatment; these include:
-
whether there is a partial or complete occlusion of a vessel
-
the length of any area of disease that requires treatment
-
the accessibility of the diseased area of artery
-
the eccentricity of any residual lumen
-
the presence or absence of calcification.
The presence or absence of disease either proximal or distal to the area being treated is also a major determinant of the potential success of any procedure. It is therefore important to consider all these issues when evaluating a new technology, particularly as some technologies may be especially useful for dealing with a specific clinical situation, such as when there is calcification or a very eccentric lumen.
Treatment pathway
Many of the new technologies that are considered in this report have been evaluated primarily in relatively simple, short stenotic or occluded areas of a single vessel. However, in practice, PAD is a chronic condition in which there are often multiple areas of disease, and the patient may undergo a series of different treatments over many years. Endovascular treatments may be used for multiple areas of disease as an adjunct to other interventions. This may be either simultaneous or as part of a planned series of procedures for disease at different sites. They may also be used for the retreatment of areas that have previously been treated by endovascular means or in the treatment of stenosis in arterial bypass grafts.
Although these are relevant areas in which some of the technologies considered in this report may be used, these situations are often specifically excluded or simply not represented in the clinical trials.
Limitations of current techniques
Percutaneous transluminal angioplasty has been widely adopted and is a common and useful procedure in the management of peripheral arterial disease; however, it has certain limitations and potential risks that may be addressed by some of the new technologies considered in this report.
The site and extent of disease may determine whether or not endovascular treatments are possible. Longer occlusions of small distal arteries are increasingly difficult to treat and have poor outcomes. However, there is no absolute criterion to determine suitability, as is demonstrated by the variability of clinicians’ readiness to randomise patients in some trials. 6
When endovascular treatment is attempted, there may be failure or complications at any stage of the procedure:
-
There may be failure to gain access to the site of the disease.
-
It may prove impossible to cross the occluded segment with the device used for treatment.
-
It may prove impossible to reopen the vessel sufficiently to obtain a suitable lumen.
-
Procedural complications may occur, including bleeding at the puncture site, embolisation of material from the diseased segment of artery, dissection, perforation or immediate reocclusion.
-
After a successful initial procedure, there is a risk of late restenosis and reocclusion causing recurrence of symptoms.
New techniques associated with angioplasty may address any of these potential difficulties in carrying out the procedure. The technologies that are considered in this report are primarily concerned with either increasing the effectiveness of the initial recanalisation or preventing late restenosis. For example, stents, laser and atherectomy devices are intended to improve the immediate result, whereas DESs, drug-coated balloons (DCBs) and radiotherapy are unlikely to affect the immediate anatomical result but are aimed at reducing the rate of subsequent restenosis and reocclusion.
In addition to these there are other technologies that have not been considered in this report, such as developments in catheters and guide-wire technology, which may improve access and closure devices, which may reduce the risk of the complication of postprocedure bleeding.
Chapter 2 Definition of decision problem
Purpose of assessment
This report aimed to answer the following research questions:
What are the clinical effectiveness and cost-effectiveness of additional techniques designed to improve the results of endovascular treatment (standard transluminal balloon angioplasty) for PAD?
For which of these techniques is further primary research likely to lead to information that will improve the clinical effectiveness and cost-effectiveness of care for this condition?
Place of the intervention in the treatment pathway
The techniques under consideration in this assessment were those that are used either as a replacement for or in conjunction with conventional balloon angioplasty. In general, treatments were considered that occupy the same place as balloon angioplasty in the treatment pathway for PAD.
Included interventions
This assessment is of new endovascular techniques that may be used to either supplement or replace existing endovascular procedures to improve the circulation of the lower limb in cases of PAD. The following interventions were included.
Absorbable stents
This is a type of stent that is bio-absorbable. 7
Self-expanding stents
This is a type of bare-metal stent (BMS) that expands when implanted.
Balloon-expandable stents
This is a type of BMS that requires expansion with a balloon.
Drug-eluting stents
There are a number of designs of metal stents that are coated with drugs that are gradually released and may reduce the rate of restenosis. These include stents that release cytotoxic or immunosuppressant drugs. These have been quite widely used in the coronary circulation and various configurations are now available that are suitable for use in the peripheral circulation.
Stent-graft
Stents may be covered with graft material, usually ePTFE (expanded polytetrafluoroethylene), to produce stent-grafts. Large stent-grafts are now commonly used for treating aneurysms and smaller-diameter versions are available for use in the peripheral arteries. Such devices may be inserted by a percutaneous route or may be used as a part of surgical procedures.
Atherectomy
Whereas conventional balloon angioplasty or stenting does not remove the occluding material but opens up and stretches the lumen of the vessel, atherectomy is a technique that attempts to remove some of the occluding material. There are a number of proprietary devices for this technique, including the Simpson catheter, the Rotablator® (Boston Scientific Corporation, Natick, MA, USA) and the SilverHawk™ (ev3 Endovascular Inc., Plymouth, MN, USA) atherectomy device. Again, these may be divided into subgroups depending upon the mechanism of action, with available devices being either ‘rotational’, removing material in a concentric fashion, or ‘directional’ in nature, removing material from one aspect of the arterial wall.
Cutting balloon
The cutting balloon (CB) is a device that combines a conventional angioplasty balloon with small blades that cut the atheroma at the time of dilatation.
Cryoplasty
This is a method that combines transluminal angioplasty using a balloon with the cryotherapy by cooling the vessel wall. The technique uses inflation of the balloon with a cooling mixture rather than the standard use of contrast medium.
Radiation
Radiation therapy has been used to try and reduce restenosis following angioplasty. This may be carried out through different techniques. Endovascular brachytherapy (EVBT) uses small radioactive probes that can be inserted through an endovascular route. External beam radiotherapy (EBRT) applies radiation from outside the body.
Drug-coated balloon
A recent development has been the use of balloons coated in drugs similar to those used for DESs in order to deliver the agent at the time of angioplasty. Paclitaxel-coated balloons have been used elsewhere and have recently become available in the UK.
Laser angioplasty
There was a considerable body of research published in the late 1980s regarding the use of lasers to unblock arteries. The majority of devices that were used at that time have subsequently been withdrawn. However, there are some devices still available that use excimer lasers as part of an atherectomy procedure to ablate occluding material.
Excluded interventions
Pharmacological interventions
The separate effects of pharmacological measures aimed at altering patency were not specifically considered, except when the use of a particular agent was required as an integral part of a new endovascular technique.
Combined surgical procedures
Some new techniques, such as remote femoral endarterectomy, require a combined surgical and endovascular approach. Many of the others may also be combined with surgical procedures and, in some cases, may be used for different indications in patients who would not necessarily be amenable to conventional endovascular techniques.
Other techniques
There are a number of other new endovascular techniques that may be used as an adjunct to angioplasty. These include closure devices, devices to protect from embolisation and techniques for thrombolysis or thrombectomy. These will be considered only when they are a component of one of the other techniques referred to above.
Interventions above the inguinal ligament (aortoiliac segment)
The outcome of endovascular treatment is also known to be heavily influenced by the site and distribution of arterial occlusive disease. Aortoiliac disease affects the larger vessels above the inguinal ligament. Conventional angioplasty, with or without the use of stents, has been common practice in this area for some years and clinical results are generally good, with lower rates of restenosis or reocclusion. In view of this, the potential advantages of new techniques to improve outcomes are likely to be very much smaller in absolute terms, with very large clinical studies being required to demonstrate significant clinical benefit. The current assessment will therefore focus on disease below the inguinal ligament.
Relevant comparators
The comparator was conventional PTA. Bail-out stenting was included as a possible comparator for any of the interventions, BMSs were considered as a comparator for DESs and sham radiation was included as a possible comparator for radiation interventions.
Population
The population was participants with symptomatic PAD undergoing endovascular treatment for disease distal to the inguinal ligament. Patients with either IC or CLI were included.
Methods for assessment
Review stage 1
A comprehensive search was undertaken to systematically identify clinical effectiveness and cost-effectiveness literature concerning endovascular techniques to supplement or replace balloon angioplasty in the infrainguinal arterial circulation. Systematic reviews were conducted and reported in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement. 8
The clinical effectiveness review methods and results are reported in Chapter 3 and Appendices 1–4. The clinical effectiveness review is registered as Prospero registration number CRD42012002014 (www.crd.york.ac.uk/prospero/index.asp).
The cost-effectiveness review is reported in Chapter 4.
Review stage 2
Where utility data were unavailable from studies identified in review stage 1, literature reviews were conducted to provide data to populate the economic model. This comprised data on the utilities associated with health states relating to the natural history of treated and untreated PAD. The results of this review are reported in Chapter 4.
Development of a health economic model
A new economic evaluation of the cost-effectiveness of technologies for the management of PAD was developed. The model is reported in Chapter 4.
Chapter 3 Systematic review of the clinical effectiveness of enhancements to angioplasty
Methods
Identification of studies
A comprehensive search was undertaken to systematically identify clinical effectiveness literature concerning enhancement to angioplasty in adults with PAD. The search involved combining terms for the population (PAD) with terms for the interventions and then combining these terms with filters designed to retrieve systematic reviews, randomised control trials (RCTs) and economic evaluations as appropriate. The search strategy comprised the following main elements:
-
searching of electronic databases
-
contact with experts in the field
-
scrutiny of bibliographies of retrieved papers.
The preliminary list of interventions included the following: BMSs, DESs, drug-eluting balloons, stent-grafts, cryotherapy, brachytherapy, external beam radiation, CBs and atherectomy. Following consultation with experts and scoping searches, the search terms of scoring balloons and ultrasonic angioplasty were added.
The following electronic databases were searched from inception for published and unpublished research evidence:
-
MEDLINE (Ovid) 1950–present
-
MEDLINE In-Process & Other Non-Indexed Citations (Ovid) (for latest publications)
-
EMBASE (Ovid) 1980–present
-
The Cochrane Library including the Cochrane Systematic Reviews Database, Cochrane Controlled Trials Register, Database of Abstracts of Reviews of Effects (DARE), Health Technology Assessment (HTA) and NHS Economic Evaluation Database (NHS EED) databases 1991–present
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCO) 1982–present
-
Science Citation Index (via ISI Web of Science) 1900–present
-
Social Science Citation Index (via ISI Web of Science) 1956–present
-
Conference Proceedings Citation Index-Science (CPCI-S) (via ISI Web of Science) 1990–present
-
UK Clinical Research Network (UKCRN) Portfolio Database
-
Current Controlled Trials
-
ClinicalTrials.gov.
Other online searches included the US Food and Drug Administration’s website, the European Medicines Agency’s website and relevant conference proceedings. These included the proceedings of the VSGBI, the European Society of Vascular and Endovascular Surgery, the British Society of Interventional Radiology, the Cardiovascular and Interventional Radiological Society of Europe, the Society of Interventional Radiology and the Society for Vascular Surgery.
Searches for clinical effectiveness studies were performed by an information specialist (AC) in May 2011. References were collected in a database, and duplicates removed.
Searches for cost-effectiveness were conducted in May 2011 and are discussed in Chapter 4. Additional focused searches were conducted on MEDLINE to find literature on the natural history of PAD and literature on restenosis and quality of life (QoL) in October 2011. Published data were used, and trial authors were not contacted. Bibliographies of included studies were searched for potential additional trials.
The search strategy for MEDLINE is provided in Appendix 1.
Inclusion criteria
Population
The population was participants with symptomatic PAD undergoing endovascular treatment for disease distal to the inguinal ligament. Patients with either IC or CLI were included.
Interventions
Interventions were techniques used as an adjunct to, or as a replacement for, balloon angioplasty in the peripheral circulation.
These were as follows: absorbable stents, self-expanding stents (SESs), balloon-expandable stents (BESs), DESs, stent-graft, atherectomy, CB, cryoplasty, radiation by EVBT or EVRT, DCB and laser angioplasty.
Comparator
The comparator was conventional PTA. Bail-out stenting was included as a possible comparator for any of the interventions, BMSs were considered as a comparator for DESs, and sham radiation was included as a possible comparator for radiation interventions.
Outcomes
Reported outcomes included patency or restenosis measures, need for reintervention, disease-specific and generic measures of QoL, clinical status, exercise tolerance or walking distance, pain (patient-reported pain scores and analgesic use), limb salvage, complications and adverse events. Cost outcomes are discussed in Chapter 4.
Study design
Initially, RCTs were searched. As data were available from these, other study types from further down the accepted hierarchy of evidence were not sought. Meta-analyses and systematic reviews of RCTs were sought to identify RCTs that met the inclusion criteria of this review.
Exclusion criteria
Interventions
Pharmacological interventions, combined surgical procedures and devices that have been withdrawn, such as older laser angioplasty devices, were not considered, as well as interventions above the inguinal ligament (aortoiliac segment).
Publication types
Studies that were published only in languages other than English, studies based on animal models, and preclinical and biological studies were excluded, as were narrative reviews, editorials and opinion pieces. Reports published as meeting abstracts were excluded only when insufficient details were reported to allow inclusion.
Study selection was made by one reviewer and checked by another, based on the above inclusion and exclusion criteria. Citations were sifted by title and abstract, and those remaining after abstract sift were sifted by full papers. Studies excluded at full-paper screening were placed in Appendix 2.
Data extraction, critical appraisal and synthesis
Data were extracted by one reviewer and checked by another, using a standardised form. The forms are shown in Appendix 3. Data were extracted with no blinding to authors or journal. Quality was assessed according to criteria based on NHS Centre for Reviews and Dissemination (CRD) Report No. 4. 9 Quality assessment forms are shown in Appendix 4. Prespecified outcomes were tabulated and discussed within a descriptive synthesis. For some interventions, meta-analyses were precluded as a result of differences in outcomes. For example, definitions of patency varied across trials and there were also differences in populations, interventions and length of follow-up. When appropriate, meta-analyses were undertaken using fixed- and random-effects methods. Meta-analyses were carried out using Review Manager 5.1 (The Nordic Cochrane Centre, Copenhagen, Denmark). The Mantel–Haenszel methods have been shown to be more reliable than other methods when there are relatively few studies with small sample sizes, so these were employed, with both fixed and random effects, as recommended by the Cochrane Collaboration. 10
Results
Quantity and quality of studies
Study selection
The search of electronic databases yielded 9501 article citations with duplicates removed. Additional searching yielded one reference and two conference presentations. The sifting process is shown in Figure 1, a flow diagram adapted from PRISMA recommendations. 8 Title sifting excluded 8175 citations. There were 1329 abstracts sifted. In total, 95 references were full-text screened. Appendix 2 shows 34 studies that were excluded at the full-paper sifting stage with reasons for exclusions.
There were 40 RCTs accepted into the review, published in 61 references, comprising 53 articles from peer-reviewed journals with additional data in eight conference presentations (Table 1). Following literature searches, the Zilver PTX25–27 trial published an additional paper,71 which confirmed the results included from abstracts.
| Trial (trial name, author, date) | Sample size | Intervention | Comparator | Follow-up | Outcomes reported |
|---|---|---|---|---|---|
| AMS INSIGHT, Bosiers et al. 200911 | 117 CLI | AMS | PTA | 6 months | Patency, late lumen loss, complications |
| Dick et al. 200912 | 73 (of whom 69 IC, 4 CLI) | SES | PTA | 12 months | Restenosis, walking capacity, complications |
| VascuCoil, Greenberg et al. 200413 | 266 ‘symptomatic leg ischaemia’ | SES (IntraCoil®, Sulzer/IntraTherapeutics, St. Paul, MN, USA) | PTA | 9 months | TLR, complications |
| FAST, Krankenberg et al. 200714 | 244 (of whom 226 IC, 7 CLI, 11 data unavailable) | SES (nitinol) | PTA | 12 months | Restenosis, TLR, Rutherford category, walking capacity, complications |
| RESILIENT, Laird et al. 201015 | 206 IC | SES | PTA | 12 months | Restenosis, TLR/TVR, walking capacity, QoL, complications |
| ABSOLUTE, Schillinger et al. 2006,16 2007,17 Sabeti et al. 200718 | 104 (of whom 91 IC, 13 CLI) | SES (nitinol) | PTA | 24 months | Restenosis, reintervention, Rutherford category, walking capacity, QoL, complications |
| Becquemin et al. 200319 | 227 (of whom 180 IC, 47 CLI) | BES (Palmaz®, Cordis, a Johnson & Johnson interventional systems company) | PTA | 12 months | Restenosis, complications |
| Cejna et al. 200120 | 141 (154 limbs of which 108 IC, 46 CLI) | BES (Palmaz) | PTA | 24 months | Patency, complications |
| Grimm et al. 200121 | 53 IC | BES (Palmaz) | PTA | 24 months | Patency, need for reintervention, walking capacity, complications |
| Rand et al. 200622 | 51 CLI | BES (Carbostent™, Sorin, Biomedica, Italy) | PTA | 6 months | Patency, complications |
| Vroegindeweij et al. 199723 | 51 IC | BES (Palmaz) | PTA | 12 months | Patency, complications |
| Zdanowski et al. 199924 | 32 CLI | BES (tantalum) | PTA | 12 months | Restenosis, need for reintervention, complications |
| Zilver PTX, Dake et al. 2010,25 Ansell 2011,26 Dake et al. 200827 | 479 (Rutherford category 2 or above) | DES (paclitaxel) | PTA (with potential second randomisation to DES or BMS) | 12 months | Patency, complications |
| SIROCCO, Duda et al. 2002,28 2005,29 200630 | 93 (of whom 46 Rutherford category 1 or 2, 47 Rutherford category 3 or 4) | DES (sirolimus) | SES | 24 months | Restenosis, TLR/TVR, complications |
| Rastan et al. 201131 | 161 (of whom 86 IC, 75 CLI) | DES (sirolimus) | BMS (placebo coated) | 12 months | Patency, TLR, Rutherford category, complications |
| Saxon et al. 2003,32 200833 | 197 (of whom 175 IC, 21 CLI, 1 unknown) | Stent-graft (nitinol covered) | PTA | 12 months | Patency, Rutherford category, complications |
| Nakamura et al. 199534 | 39 IC | Atherectomy (transcutaneous extraction catheter) | PTA | 6 months | Patency, complications |
| Vroegindeweij et al. 1992,35 1995,36 Tielbeck et al. 199637 | 73 IC | Atherectomy (directional) | PTA | 24 months | Patency, Rutherford category, complications |
| Amighi et al. 200838 | 43 (of whom 35 IC, 8 CLI) | CB | PTA | 6 months | Restenosis, symptoms, walking capacity, complications |
| Dick et al. 200839 | 39 (of whom 30 IC, 9 CLI) | CB | PTA | 6 months | Restenosis, need for reintervention, walking capacity, complications |
| Jahnke et al. 201040 | 86 (of whom 66 IC, 20 CLI) | Cryoplasty | PTA | 9 months | Patency, symptoms, complications |
| Spiliopoulos et al. 201041 | 50 (60 limbs included, of which 36 IC, 24 CLI) | Cryoplasty | PTA | 36 months | Patency, TLR, complications |
| Gallino et al. 2004,42 Bonvini et al. 200343 (results of Diehm et al. 200544 and Zehnder et al. 200345) | 156 IC (in two arms relevant to this review, from four-arm trial) | Radiation (EVBT) plus PTA | PTA and placebo drug | 36 months | Patency, need for reintervention, Rutherford category, complications |
| Zehnder et al. 200345 (results of Diehm et al. 200544 and Gallino et al. 200442/Bonvini et al. 200343) | 100 (of whom 92 IC, 8 CLI) | Radiation (EVBT) plus PTA | PTA and placebo drug | 36 months | Restenosis, need for reintervention, Rutherford category |
| Hagenaars et al. 200246 | 24 (of whom 12 IC, 12 CLI) | Radiation (EVBT) plus PTA | PTA | 6 months | Restenosis, late lumen loss |
| Krueger et al. 2002,47 200448 | 30 (unclear how many IC/CLI; all Fontaine 2a-3) | Radiation (EVBT) plus PTA | PTA plus sham radiation | 24 months | Restenosis, need for reintervention, walking capacity |
| Vienna-2, Wolfram et al. 2006,49 Minar et al. 2000,50 Wolfram et al. 200551 | 113 (of whom 88 IC, 25 CLI) | Radiation (EVBT) plus PTA | PTA | 60 months | Restenosis, TLR/TVR |
| Vienna-3, Pokrajac et al. 2005,52 2000,53 Wolfram et al. 200551 | 96 (of whom 77 IC, 19 CLI) | Radiation (EVBT) plus PTA | PTA plus sham radiation | 12, 24 months | Restenosis, TLR/TVR, complications |
| VARA, van Tongeren et al. 200554 | 60 (of whom 52 IC, 8 CLI) | Radiation (EVBT) plus PTA | PTA | 12 months | Restenosis, need for reintervention, Rutherford category, complications |
| Wyttenbach et al. 2007,55 200456 | 20 (unclear how many IC/CLI, but all Rutherford category 3 or above) | Radiation (EVBT) plus PTA | PTA | 3, 24 months | Late lumen loss |
| Fritz et al. 200457 | 95 (of whom 94 IC, 1 CLI) | Radiation (external beam) plus PTA | PTA plus sham radiation | 12 months | Restenosis, Fontaine stage |
| Therasse et al. 200558 | 99 (of whom 27 IC, 72 CLI) | Radiation (external beam, three doses) plus PTA | PTA plus sham radiation | 12 months | Restenosis, need for reintervention |
| LEVANT I, Scheinert et al. 201059,60 | 101 (of whom 94 IC, 7 CLI) | DCB (paclitaxel) | PTA with uncoated balloon | 6 months | Late lumen loss, TLR |
| THUNDER, Tepe et al. 200861–63 | 102 (in two relevant arms of three-arm trial), Rutherford categories 1–5 | DCB (paclitaxel) | PTA with uncoated balloon | 24 months | Restenosis, late lumen loss, TLR, Rutherford category, complications |
| FemPac, Werk et al. 200864 | 87 (of whom 82 IC, 5 CLI) | DCB (paclitaxel) | PTA with uncoated balloon | 24 months | Restenosis, TLR, Rutherford category, complications |
| Belli et al. 199165,66 | 68 (of whom 48 IC, 20 CLI) | Laser angioplasty (thermal) | PTA | 12 months | Symptoms, complications |
| Fisher et al. 199667 | 82 (of whom 76 IC, 6 CLI) | Laser angioplasty (hot-tip) | PTA | 24 months | Restenosis |
| Lammer et al. 199268 | 116 (of whom 84 IC, 32 CLI) | (1) Laser angioplasty (pulsed XeCI); or (2) laser angioplasty (Nd:YAG, thermal) | PTA | 12 months | Patency, complications |
| Spies et al. 199069 | 25 IC | Laser angioplasty (Nd:YAG, thermal) | PTA | 2 weeks | Complications |
| Tobis et al. 199170 | 40 (of whom 35 IC, 5 CLI) | Laser angioplasty | PTA | 12 months | Patency, complications |
There was one RCT of absorbable metal stents (AMSs), five RCTs of SESs and six RCTs of BESs. There were three trials of DESs, of which one concerned paclitaxel and two sirolimus. There was one trial of stent-graft, two of atherectomy, two of CB and two of cryoplasty. Of the 10 RCTs of radiation, eight employed EVBT and two employed EBRT. Three RCTs of DCB were included and five RCTs of laser angioplasty. Trials of stents, stent-graft, CB, cryoplasty and DCB versus PTA allowed bail-out stenting in the PTA group, when deemed medically necessary. Bail-out atherectomy was permitted in one atherectomy trial (Vroegindeweij et al. 36), and, of the radiation trials, the comparator PTA group had oral placebo in two RCTs (Gallino et al. , 42 Zehnder et al. 45) and sham radiation in four RCTs (Krueger et al. ,47,48 Vienna-3,51–53 Fritz et al. ,57 Therasse et al. 58).
Further study details are shown in Appendix 3.
Critical appraisal
Appendix 4 shows the quality assessment for the included studies. Method of allocation concealment was considered adequate in 11 of the trials [AMS INSIGHT (bio-absorbable metal stent investigation in chronic limb ischaemia treatment),11 Becquemin et al. ,19 Grimm et al. ,21 Rand et al. ,22 Vroegindeweij et al. ,23,35,36 Rastan et al. ,31 Tielbeck et al. ,36 Amighi et al. ,38 Dick et al. ,39 VARA (VAscular RAdiotherapy trial),54 FemPac (Femoral Paclitaxel trial)64]. Both the method used to generate allocation sequences and the method of allocation concealment were considered adequate in seven of these trials (AMS INSIGHT,18 Becquemin et al. ,19 Grimm et al. ,21 Rastan et al. ,31 Amighi et al. ,38 Dick et al. ,39 VARA,54 FemPac64). For other trials, reporting of randomisation methods was unclear.
There was blinding for assessors in at least one of the study outcomes in 20 trials [Dick et al. ,12 FAST (Femoral Artery Stenting Trial),14 ABSOLUTE (randomized balloon angioplasty vs. stenting with nitinol stents in the superficial femoral artery),16–18 Becquemin et al. ,19 Rand et al. ,22 SIROCCO (SIROlimus-Coated COrdis self-expandable stent trial),28–30 Rastan et al. ,31 Amighi et al. ,38 Spiliopoulous et al. ,41 Diehm et al. 44 analysis of Gallino et al. 42 and Zehnder et al. 45 trials, Krueger et al. ,47,48 Vienna-3,51–53 Wyttenbach et al. ,55,56 Fritz et al. ,57 Therasse et al. ,58 THUNDER (local taxane with short exposure for reduction of restenosis in distal arteries),61–63 FemPac,64 Lammer et al. 68]. Blinding of clinicians to the endovascular techniques used in these studies would have been difficult or impossible. One trial (FemPac64) mentioned that the blinding of clinicians was attempted, but the difference in appearance of DCB and uncoated balloons meant that clinicians were likely to know which intervention was being used. There was explicit blinding of patients in eight trials (SIROCCO,28–30 Rastan et al. ,31 Krueger et al. ,47,48 Vienna-3,51–53 Fritz et al. ,57 Therasse et al. ,58 THUNDER,61–63 FemPac64).
Intervention and control groups were largely comparable at baseline in all trials. Some trials reported one variable that was not equal across treatment groups at baseline [AMS INSIGHT,11 Dick et al. ,12 RESILIENT (randomised study comparing the Edwards self-expanding LifeStent with angioplasty alone in lesions involving the superficial femoral artery and/or proximal popliteal artery),15 Zilver PTX,25–27 SIROCCO,28–30 Rastan et al. ,31 Nakamura et al. ,34 Vroegindeweij’s group,35–37 THUNDER,60–62 Fisher et al. 67]. When studies measured more outcomes than they reported, this was because of future expected reports [LEVANT I (the Lutonix paclitaxel-coated balloon for the prevention of femoropopliteal restenosis trial),59,60 Spies et al. 69].
Only one trial had an imbalance in dropouts between treatment groups (Hagenaars et al. 46). An analysis of patients in their allocated groups according to the intention-to-treat (ITT) principle was available for all trials, although for two trials (Gallino et al. , 42 Zehnder et al. 45) this was only available for the combined analysis of these two trials (Diehm et al. 44).
Clinical effectiveness results
Results are presented according to the 11 included interventions (see Appendix 5).
Absorbable metal stent
One RCT identified compared AMS with PTA (AMS INSIGHT11) in CLI patients. The AMS INSIGHT11 trial provided patency data on 94 lesions at 6-month follow-up (Tables 2 and 3). AMS fared significantly worse than PTA (p = 0.013) in terms of restenosis measured by core-lab quantitative vessel analysis (QVA). A patency measure including major amputation or target lesion revascularisation (TLR) as failure showed no significant difference between treatment groups. For adverse events, a measure including major amputation or death did not find any significant difference between groups at 1-month follow-up (Table 4).
| Study | Follow-up | Definition of restenosis/patency | PTA lesions analysed (n) | PTA lesions with restenosis (%) | AMS lesions analysed (n) | AMS lesions with restenosis (%) | Comparative statistic |
|---|---|---|---|---|---|---|---|
| AMS INSIGHT (Bosiers et al. 200911) | 6 months | Patency was defined as the absence of a haemodynamically significant restenosis (> 50%) documented by digital subtraction angiography and confirmed by the core-lab QVA | 50 | 42a | 44 | 68.2a | p = 0.013 |
| Primary patency rates determined by colour-flow Doppler ultrasound and defined as the absence of a haemodynamically significant restenosis (> 50%), derived from the ratio of the PSV at the lesion segment to that at the proximal part, a major amputation or a TLR | 50 | 11.9a | 44 | 19.8a | p = 0.270 |
| Study | Follow-up | Definition of late lumen loss | PTA lesions analysed (n) | PTA size (mm; mean ± SD) | AMS lesions analysed (n) | AMS size (mm; mean ± SD) | Comparative statistic |
|---|---|---|---|---|---|---|---|
| AMS INSIGHT11 | 6 months | Difference between the in-stent MLD post procedure and the MLD at follow-up measured with angiography | 50 | 0.7 ± 0.7 | 44 | 1.4 ± 0.8 | p < 0.0001 |
| Study | Follow-up | Definition of complication | PTA analysed (n) | PTA patients with complications (%) | AMS analysed (n) | AMS patients with complications (%) | Comparative statistic |
|---|---|---|---|---|---|---|---|
| AMS INSIGHT11 | 1 month | Major amputation and/or death within 30 days of intervention | 57 | 5.3 | 60 | 5 | p = 1.0 |
Self-expanding stent
Five RCTs compared SESs with PTA. The populations comprised mostly IC patients, but also some CLI patients.
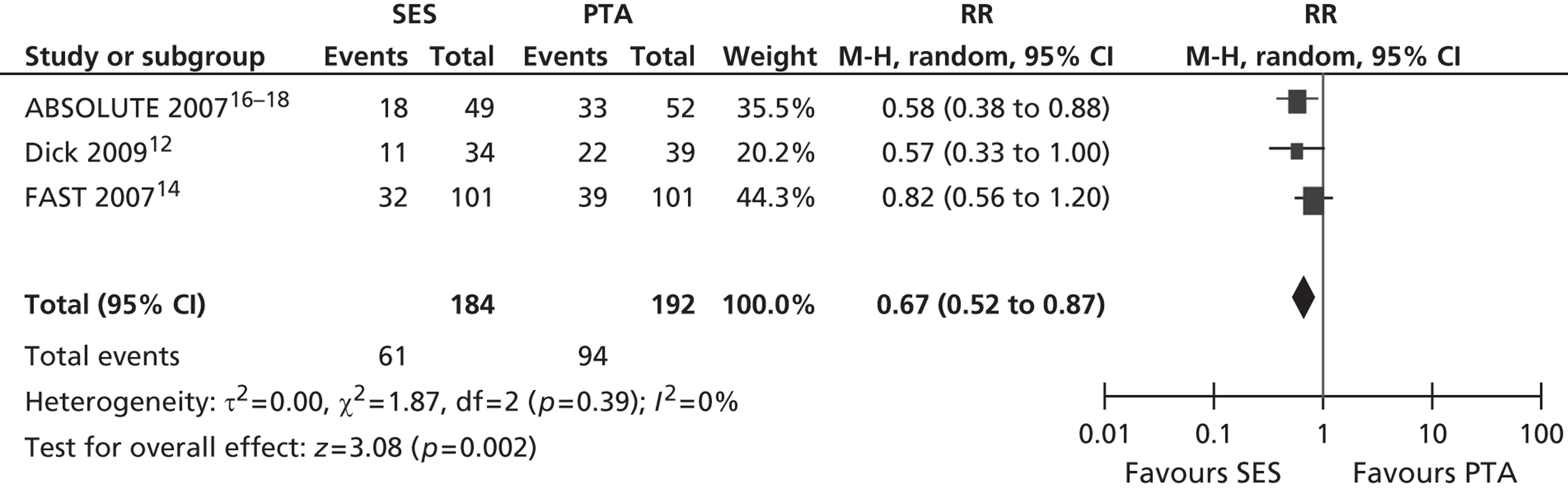
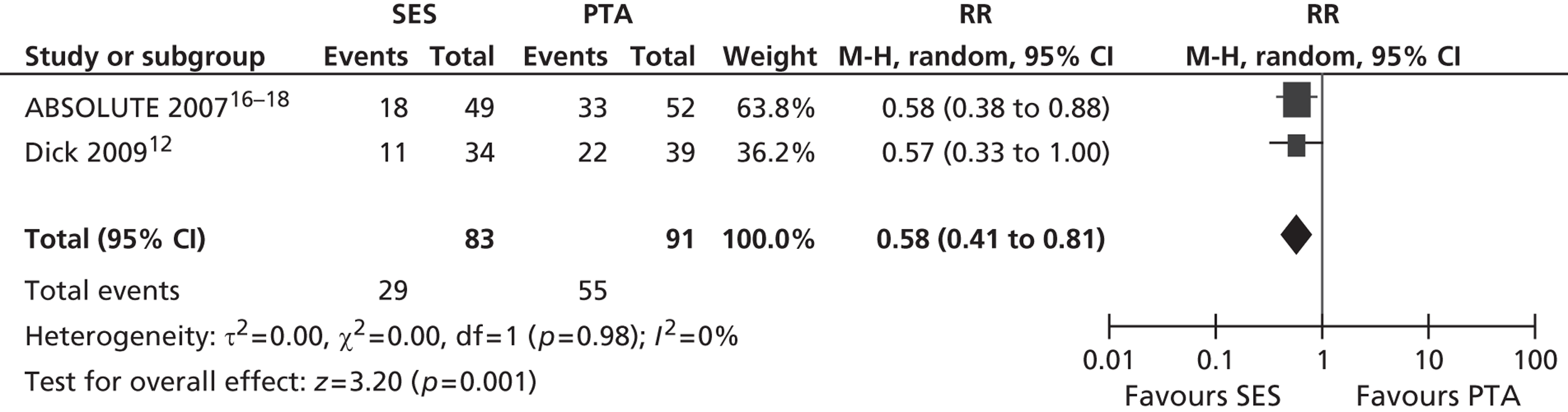
Three RCTs (Dick et al. ,12 RESILIENT,15 ABSOLUTE16–18) showed an advantage for SES over PTA in terms of restenosis (Table 5). Of these, one study (ABSOLUTE16–18) had only a trend favouring SES at 6 months but significant results at 1 and 2 years, whereas the other studies reached and maintained significance at 3–6 months (Dick et al. 12) and 6–12 months (RESILIENT15). One RCT found no significant difference between groups at 1-year follow-up (FAST14). Meta-analysis (Figures 2–7) for restenosis at 6 months using the studies ABSOLUTE16–18 and Dick et al. 12 produced a relative risk (RR) for SES with reference to PTA of 0.49 with a 95% CI of 0.32 to 0.76 by fixed-effect analysis. By random-effect analysis, the RR was 0.50 (95% CI 0.32 to 0.77). Both analyses significantly favoured SES over PTA (p = 0.002). Restenosis at 12 months, using the studies ABSOLUTE,16–18 Dick et al. 12 and FAST14, produced a RR of 0.68 (95% CI 0.53 to 0.87) by fixed-effect analysis (p = 0.003). By random-effect analysis, the RR was 0.67 (95% CI 0.52 to 0.87), significantly favouring SES over PTA (p = 0.002).
| Study | Follow-up | Definition of restenosis/patency | PTA analysed (n) | PTA patients with restenosis (%) | SES analysed (n) | SES patients with restenosis (%) | Comparative statistic |
|---|---|---|---|---|---|---|---|
| Dick et al. 200912 | 6 months | Restenosis defined as a > 50% lumen diameter reduction at the most narrow site within the limits of the treated segment plus the adjacent 10 mm proximal and distal to the treated segment by computed tomography angiography | 39 | 50.0 | 34 | 18.2 | p = 0.006 |
| 3 months | Secondary end point restenosis measured by ultrasound binary restenosis > 50% by duplex ultrasonography defined as PSV of at least 2.4 | 39 | 18.9 | 34 | 2.9 | p = 0.033 | |
| 6 months | a/a | 39 | 55.6 | 34 | 21.9 | p = 0.005 | |
| 12 months | a/a | 39 | 61.1 | 34 | 34.4 | p = 0.028 | |
| FAST14 | 12 months | The primary study end point was binary restenosis, defined as a PVR proximal ≥ 2.4 on duplex ultrasound | 101 | 38.6 | 101 | 31.7 | p = 0.377 |
| RESILIENT15 | 6 months | Restenosis was defined as a loss of primary patency, i.e. PSVR ≥ 2.5, suggesting > 50% reduction in luminal diameter | 63 | 52.6a | 121 | 5.8a | p < 0.0001 |
| 12 months | a/a | 59 | 63.3a | 112 | 18.7a | p < 0.0001 | |
| ABSOLUTE16–18 | 6 months | Restenosis was defined as > 50% restenosis measured by duplex ultrasound | 53 | 45.0 | 51 | 25.0 | p = 0.06 |
| 12 months | a/a | 53 | 63.0 | 51 | 37.0 | p = 0.01 | |
| 24 months | a/a | 52 | 69.2 | 46 | 45.7 | p = 0.031 |
Of the four RCTs that reported a need for reintervention, three showed no significant difference between groups [VascuCoil (intracoil femoropopliteal stent trial),13 FAST,14 ABSOLUTE16–18] (Table 6). One study found an advantage for SES over PTA, with fewer SES participants needing TLR/target vessel revascularisation (TVR) at 6–12 months following the procedure (RESILIENT15). Rutherford category was studied by two RCTs, neither of which found a significant difference between SES and PTA treatment groups (FAST,14 ABSOLUTE16–18) (Table 7).
| Study | Follow-up | Definition of reintervention | PTA analysed (n) | PTA patients undergoing reintervention (%) | SES analysed (n) | SES patients undergoing reintervention (%) | Comparative statistic |
|---|---|---|---|---|---|---|---|
| VascuCoil13 | 9 months | TLR | 131 | 1.5 | 135 | 0.7 | Reported as NS only |
| FAST14 | 12 months | TLR | 115 | 18.3 | 114 | 14.9 | p = 0.595 |
| RESILIENT15 | 6 months | TLR/TVR | 63 | 47.4 | 121 | 1.5 | p < 0.0001 |
| 12 months | TLR/TVR | 59 | 44.9 | 112 | 12.7 | p < 0.0001 | |
| ABSOLUTE16–18 | 12 months | Need for ipsilateral reintervention within 12 months; PTA, stent implantation or bypass surgery | 53 | 31.0 | 51 | 28.6 | NS (PTA p = 0.45, stent p = 0.99, bypass p = 0.22) |
| 24 months | a/a | 52 | 53.8 | 46 | 37.0 | p = 0.14 |
| Study | Follow-up | Definition of pain | PTA analysed (n) | PTA outcome | SES analysed (n) | SES outcome | Comparative statistic |
|---|---|---|---|---|---|---|---|
| FAST14 | 12 months | Rutherford category improvement | 75 | 91% of patients improved | 61 | 89% of patients improved | Reported as NS between groups |
| ABSOLUTE16–18 | 24 months | Rutherford category | 52 | 4.2% CLI | 46 | 4.4% CLI | p = 0.74 |
Treadmill protocols were used by two studies (FAST,14 ABSOLUTE16–18) to assess walking capacity (Table 8) and both found a significant advantage for SES over PTA at 6–12 months. ABSOLUTE16–18 found that by 24 months the difference between treatment groups was no longer significant. Maximum walking capacity, as reported by the patients, was reported as significantly better with SES than PTA in one study (Dick et al. 12). One study (RESILIENT15) found no significant difference between groups, as measured by the walking impairment questionnaire, as both groups improved significantly from baseline. RESILIENT15 reported that the PTA group reported more claudication pain at 12 months (p = 0.009).
| Study | Follow-up | Definition of walking capacity | PTA analysed (n) | PTA outcome | SES analysed (n) | SES outcome | Comparative statistic |
|---|---|---|---|---|---|---|---|
| Dick et al. 200912 | 6 months | Maximum walking capacity (m) (mean) (as reported by patient) | 39 | 600 | 34 | 800 | p = 0.042 |
| 12 months | a/a | 39 | 550 | 34 | 800 | p = 0.002 | |
| FAST14 | 12 months | Absolute walking distance (median) (treadmill test 2 mph on a 12% incline) | 52 | 185 | 20 | 150 | p = 0.0283 |
| RESILIENT15 | 12 months | Improvement from baseline as defined by the walking impairment questionnaire | 59 | 29.4 ± 37.4 | 112 | 25.6 ± 34.6 | NS |
| ABSOLUTE16–18 | 6 months | Maximal treadmill walking capacity (m) (median) (3.2 km/h, 12-degree slope) | 53 | 270 | 51 | 362 | p = 0.041 |
| 12 months | a/a | 53 | 267 | 51 | 387 | p = 0.040 | |
| 24 months | a/a | 52 | 196 | 46 | 302 | p = 0.12 |
The two RCTs investigating QoL (RESILIENT,15 ABSOLUTE16–18) found no significant differences between treatment groups SES and PTA on measures of Short Form questionnaire-8 items (SF-8) or Short Form questionnaire-36 items (SF-36) by ITT analysis (Table 9). There were no significant differences between treatment groups SES and PTA in terms of complications, in any of the five included RCTs (Dick et al. ,12 VascuCoil,13 FAST,14 RESILIENT,15 ABSOLUTE16–18) (Table 10).
| Study | Follow-up | Definition of QoL | PTA analysed (n) | PTA outcome | SES analysed (n) | SES outcome | Comparative statistic |
|---|---|---|---|---|---|---|---|
| RESILIENT15 | 12 months | Improvement from baseline defined by SF-8 | 59 | 5.9 ± 11.2 | 112 | 5.7 ± 11.2 | Statistically significant changes within groups, but not between group |
| ABSOLUTE16–18 | 12 months | SF-36 physical component summary [median (IQR)] | 53 | 37 (27–49) | 51 | 35 (30–48) | p = 0.9 |
| 12 months | SF-36 mental component summary [median (IQR)] | 53 | 51 (35–58) | 51 | 54 (45–59) | p = 0.1 |
| Study | Follow-up | Definition of complication | PTA analysed (n) | PTA patients with complications (%) | SES analysed (n) | SES patients with complications (%) | Comparative statistic |
|---|---|---|---|---|---|---|---|
| Dick et al. 200912 | 1 day (perioperative) | Presence of small pseudoaneurysm at the puncture site | 39 | 2.6 | 34 | 0 | NS |
| VascuCoil13 | 9 months | Death | 131 | 0.8 | 135 | 0 | Reported as NS only |
| Myocardial infarction | 131 | 0 | 135 | 0 | Reported as NS only | ||
| Amputation | 131 | 0.5 | 135 | 0 | Reported as NS only | ||
| Major bleeding | 131 | 0.8 | 135 | 0.7 | Reported as NS only | ||
| Abrupt closure | 131 | 1.5 | 135 | 0 | Reported as NS only | ||
| Renal failure | 131 | 0.5 | 135 | 0 | Reported as NS only | ||
| Major vascular complications | 131 | 4.6 | 135 | 3 | Reported as NS only | ||
| FAST14 | 12 months | Stent fracture | n/a | n/a | 83 | 12 | n/a |
| Perioperative | Procedural complications | 121 | 4 | 123 | 7 | ||
| RESILIENT15 | 6 months | MACE: death within 30 days, stroke, myocardial infarction, significant distal embolisation, emergent surgical revascularisation of target limb, thrombosis and worsening Rutherford category | 63 | 7.2 | 121 | 6.9 | p = 0.95 |
| 12 months | MACE; a/a | 59 | 13.4 | 112 | 14.2 | p = 0.88 | |
| Amputation | 59 | 2 | 112 | 0 | |||
| ABSOLUTE16–18 | 6 months | Stent fracture | 17 | 0 | 51 | 2 | p = 0.99 |
| 12 months | a/a | 17 | 0 | 49 | 2 | p = 0.99 | |
| 6 months | Amputation | 47 | 0 | 51 | 0 | ||
| 12 months | a/a | 47 | 0 | 51 | 0 | ||
| 6 months | Death | 47 | 0 | 51 | 0 | ||
| 12 months | a/a | 47 | 0 | 51 | 2 | p = 0.99 |
Meta-analyses
Restenosis at 6 months: using the studies ABSOLUTE16–18 and Dick et al. ,12 there was no substantial heterogeneity between studies. Fixed- and random-effect analyses gave similar results (see Figures 2 and 3).
FIGURE 2.
Forest plot of comparison: 1 SES vs. PTA, restenosis 6 months fixed two studies.
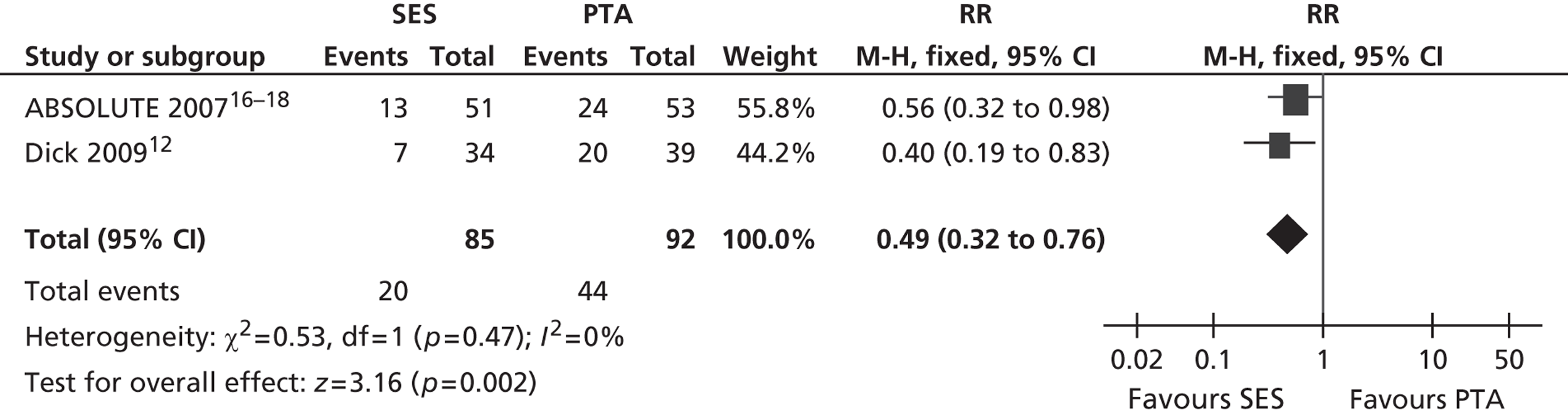
FIGURE 3.
Forest plot of comparison: 1 SES vs. PTA, restenosis 6 months random two studies.
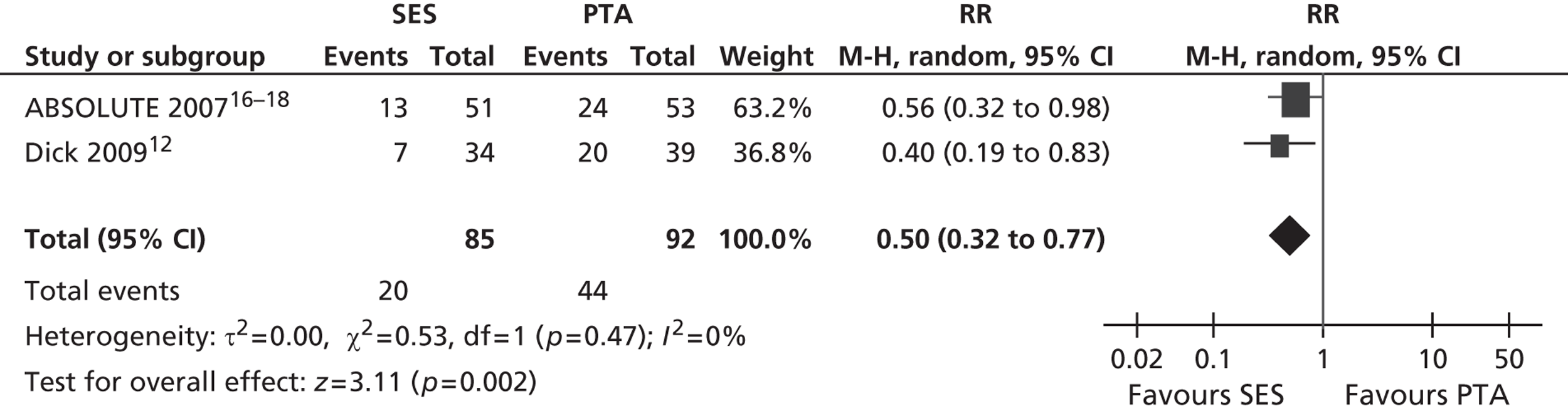
Restenosis at 12 months: using the studies ABSOLUTE,16–18 Dick et al. 12 and FAST,14 there was no significant heterogeneity among studies. The overall effect was similar for fixed- and random-effect analyses (see Figures 4 and 5).
FIGURE 4.
Forest plot of comparison: 1 SES vs. PTA, restenosis 12 months fixed three studies.
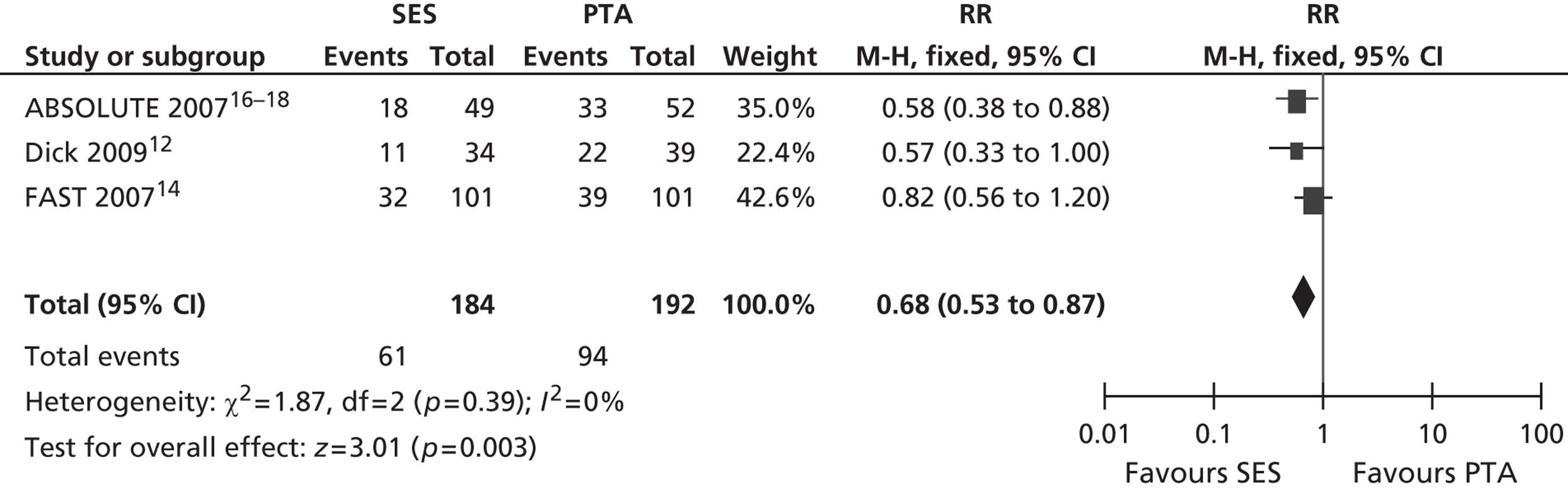
FIGURE 5.
Forest plot of comparison: 1 SES vs. PTA, restenosis 12 months random three studies.
Restenosis at 12 months – using the studies ABSOLUTE16–18 and Dick 2009,12 which had been used for the 6-month restenosis analyses – gave non-significant heterogeneity. Overall effect was similar for fixed- and random-effect analyses (see Figures 6 and 7).
FIGURE 6.
Forest plot of comparison: 1 SES vs. PTA, restenosis 12 months fixed two studies.
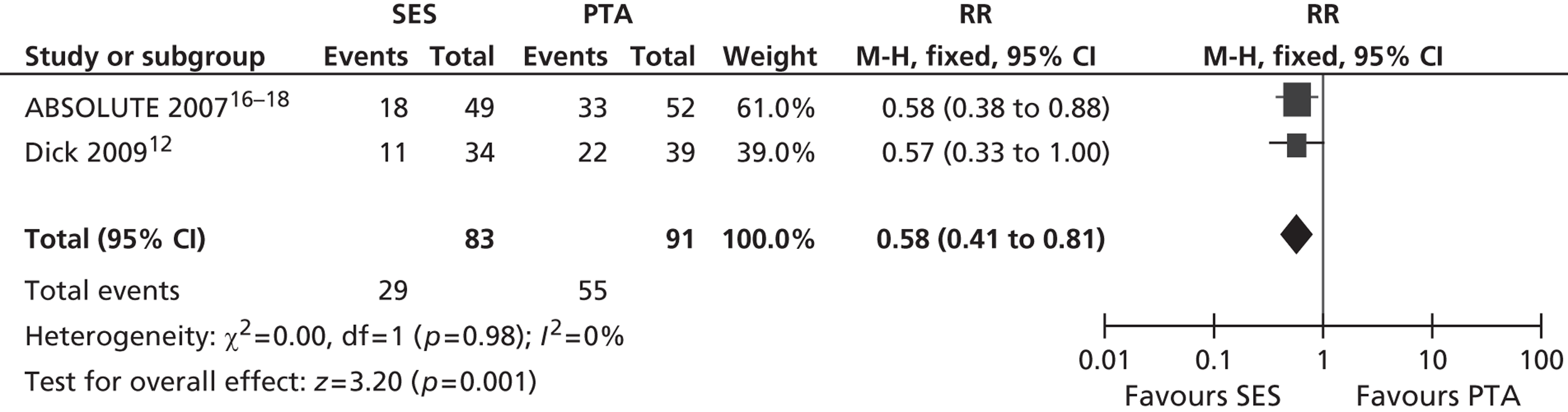
FIGURE 7.
Forest plot of comparison: 1 SES vs. PTA, restenosis 12 months random two studies.
Balloon-expandable stent
Six RCTs compared BESs with PTA.
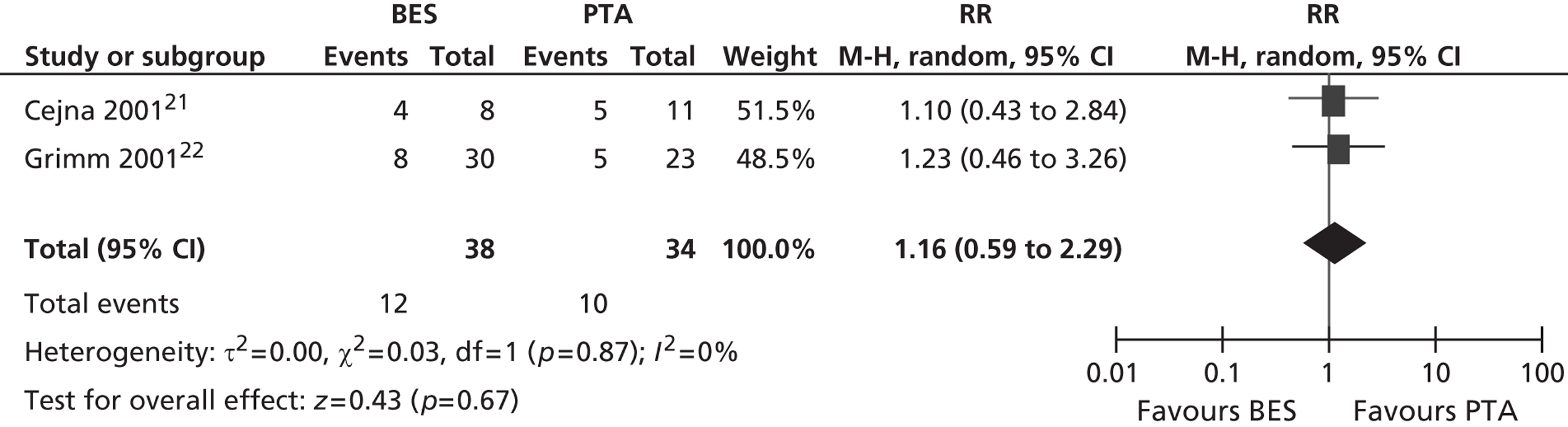
All six included RCTs reported restenosis, and four of these studies, of which two had only IC patients and two had approximately twice as many IC as CLI patients (Becquemin et al. ,19 Cejna et al. ,20 Grimm et al. ,21 Vroegindeweij et al. 23), found no significant difference between BES and PTA (Table 11). One study of CLI patients (Rand et al. 22) reported a significant advantage for BES over PTA, whereas one study of CLI patients (Zdanowski et al. 24) reported that PTA had an advantage over BES. Meta-analyses for restenosis at 6 months, using the studies of Cejna et al. 20 and Rand et al. ,22 gave a RR of 0.49 (95% CI 0.24 to 1.02) for both fixed- and random-effect analyses, with a non-significant trend favouring BES (p = 0.06) (Figures 8–11). Restenosis at 12 months, using the studies Becquemin et al. ,19 Cejna et al. ,20 Grimm et al. ,21 Vroegindeweij et al. 23 and Zdanowski et al. ,24 gave a non-significant treatment group difference by fixed-effect (RR 1.19; 95% CI 0.85 to 1.68; p = 0.31) and random-effect analyses (RR 1.18; 95% CI 0.85 to 1.66; p = 0.34). Restenosis at 24 months, using the studies of Cejna et al. 20 and Grimm et al. ,21 gave a non-significant treatment group difference by fixed-effect (RR 1.17; 95% CI 0.59 to 2.35; p = 0.65) and random-effect analyses (RR 1.16; 95% CI 0.59 to 2.29; p = 0.67).
| Study | Follow-up | Definition of restenosis/patency | PTA analysed (n) | PTA patients with restenosis (%) | BES analysed (n) | BES patients with restenosis (%) | Comparative statistic |
|---|---|---|---|---|---|---|---|
| Becquemin et al. 200319 | 12 months | Presence of > 50% stenosis at 1-year angiographic follow-up | 65 | 32.3a | 75 | 34.7a | p = 0.85 |
| Cejna et al. 200120 | 1 month | Presence of ≥ 70% stenosis as defined by angiography | 42 limbs | 16a | 38 limbs | 8a | |
| 6 months | a/a | 29 limbs | 27a | 25 limbs | 16a | ||
| 12 months | a/a | 16 limbs | 37a | 17 limbs | 37a | ||
| 24 months | a/a | 11 limbs | 47a | 8 limbs | 47a | p = 0.09 | |
| Grimm et al. 200121 | 12 months | Primary patency, narrowing ≤ 20% | 23 | 15.8a | 30 | 25a | p > 0.41 |
| 24 months | a/a | 23 | 22.8a | 30 | 27.6a | p > 0.41 | |
| 39 months | a/a | 23 | 30.4a | 30 | 26.7a | p > 0.41 | |
| Rand et al. 200622 | 6 months | Stenosis > 70% as defined by angiography; critical | 20 (32 lesions) | 38.9 (lesions)a | 17 (25 lesions) | 16.3 (lesions)a | p = 0.02 |
| 6 months | Stenosis > 50% as defined by angiography; subcritical | 20 (32 lesions) | 54.4 (lesions)a | 17 (25 lesions) | 20.3 (lesions)a | p = 0.02 | |
| Vroegindeweij et al. 199723 | 12 months | Primary patency was determined by colour-flow duplex surveillance. All lesions that recurred during follow-up within the same treated arterial segment are considered restenoses. Progression of disease in untreated arterial segments is considered as new lesions. These lesions are not considered for the analysis of patency | 27 | 26a | 24 | 38a | p = 0.22 |
| Zdanowski et al. 199924 | 12 months | Restenosis was defined if the inner diameter was decreased by > 50% compared with the state immediately after stenting defined by angiography | 8 | 25 | 12 | 50 | p = 0.033 |
Neither of the two studies (Grimm et al. ,21 Zdanowski et al. 24) that reported a need for reintervention found a significant difference between BES and PTA treatment groups (Table 12). One study (Grimm et al. 21) investigated walking distance, and found similar results between groups. Although the PTA group had a slightly larger increase in walking distance, no statistic for the difference between groups was reported (Table 13). All six included RCTs reported complications (Table 14), and none of the studies showed a significant difference between groups for BES and PTA.
| Study | Follow-up | Definition of reintervention | PTA analysed (n) | PTA patients undergoing reintervention (%) | BES analysed (n) | BES patients undergoing reintervention (%) | Comparative statistic |
|---|---|---|---|---|---|---|---|
| Grimm et al. 200121 | Within 12 months | Need for second angioplasty | 23 | 30 | 30 | 27 | p = 0.3 |
| Zdanowski et al. 199924 | Within 7 months | Underwent femorodistal bypass | 17 | 11.8 | 15 | 13.3 |
| Study | Follow-up | Definition of walking capacity | PTA analysed (n) | PTA outcome | BES analysed (n) | BES outcome |
|---|---|---|---|---|---|---|
| Grimm et al. 200121 | Within 29 months | Change in mean walking distance (m) | 23 | 316.4 | 30 | 217.1 |
| Study | Follow-up | Definition of complication | PTA analysed (n) | PTA patients with complications (%) | BES analysed (n) | BES patients with complications (%) | Comparative statistic |
|---|---|---|---|---|---|---|---|
| Becquemin et al. 200319 | Perioperative | Perioperative complications | 112 | 4.9 | 115 | 8.6 | p = 0.2 |
| 1 month | Death | 86 | 0 | 89 | 0 | ||
| 12 months | Death | 112 | 14 | 115 | 11 | ||
| 1 month | Minor complications at the puncture site | 112 | 6.3 | 115 | 6.1 | ||
| 1 month | Major amputation | 112 | 0.9 | 115 | 0 | ||
| 1 month | Minor amputation | 112 | 4 | 115 | 1.7 | p = 0.73 | |
| 12 months | Number of failed procedures (death or > 50% stenosis) | 86 | 33% | 89 | 34% | p = 0.9 | |
| Cejna et al. 200120 | 1 month | Major complications: defined as causing a change in the level of care, surgery or prolonged stay in the hospital or death | 77 limbs | 2.6 | 77 limbs | 1.3 | p = 1.0 |
| 1 month | Procedure-related complications | 77 limbs | 1.3 | 77 limbs | 3.9 | ||
| 1 month | Minor amputations | 77 limbs | 5.2 (digital amputations) | 77 limbs | 2.6 (crural amputations) | ||
| 1 month | Peripheral embolism < 30 days post intervention | 77 limbs | 3.9 | 77 limbs | 5.2 | (Any minor complications at 1 month, p = 0.55) | |
| Grimm et al. 200121 | 1 month | Major complications: events requiring therapy and prolonged hospitalisation (> 24 hours) and/or an unplanned increase in the level of care or permanent adverse sequelae or death | 23 | 0 | 30 | 0 | |
| Rand et al. 200622 | 1 month | Major amputation | 53 lesions | 0 | 42 lesions | 2.4 | |
| 1 month | Minor amputation | 53 lesions | 1.8 | 42 lesions | 2.4 | ||
| Vroegindeweij et al. 199723 | Within 1 month | Occurrence of embolus | 27 | 0 | 24 | 4.2 | |
| 1 month | Occurrence of thrombus | 27 | 3.7 | 24 | 0 | ||
| Zdanowski et al. 199924 | Perioperative | Major complications: myocardial infarction, bleeding, emboli | 17 | 23.5% | 15 | 6.7 |
Meta-analyses
Restenosis at 12 months: using the studies of Becquemin et al. ,19 Cejna et al. ,20 Grimm et al. ,21 Vroegindeweij et al. 23 and Zdanowski et al. ,24 there was no significant heterogeneity. The overall effect was similar for fixed- and random-effect analyses (see Figures 8 and 9).
FIGURE 8.
Forest plot of comparison: 2 BES vs. PTA, restenosis at 12 months fixed.
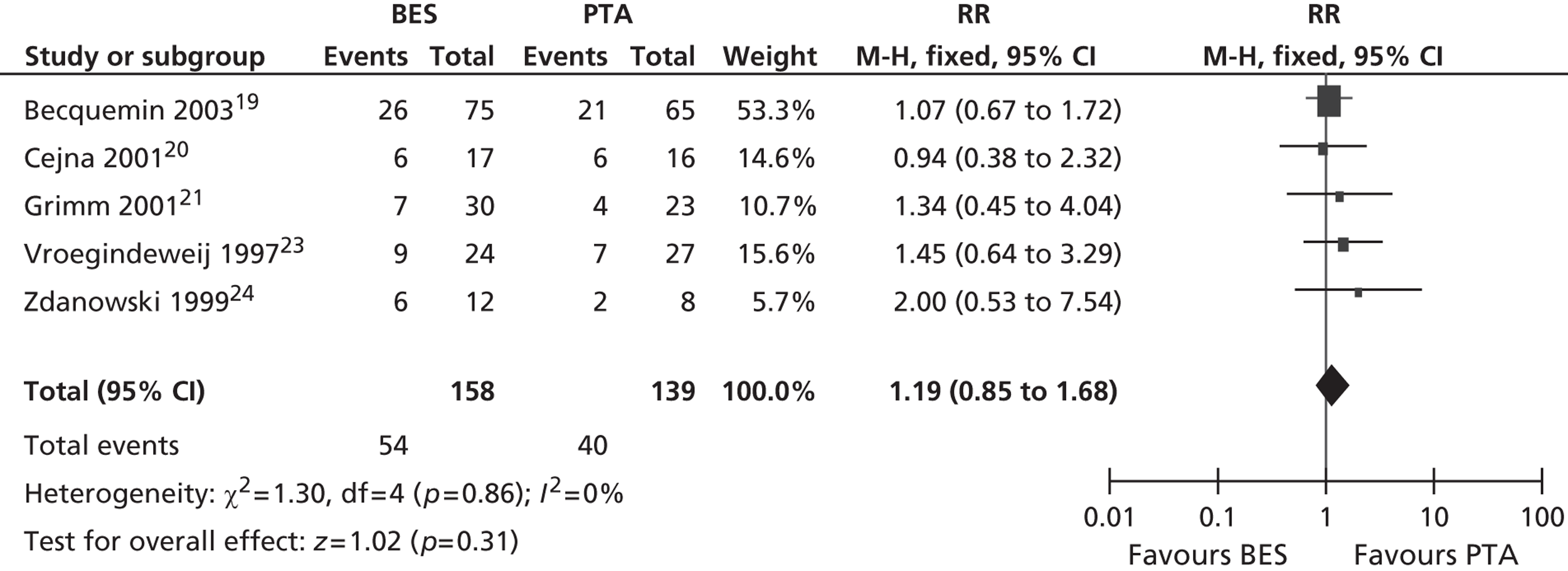
FIGURE 9.
Forest plot of comparison: 2 BES vs. PTA, restenosis at 12 months random.
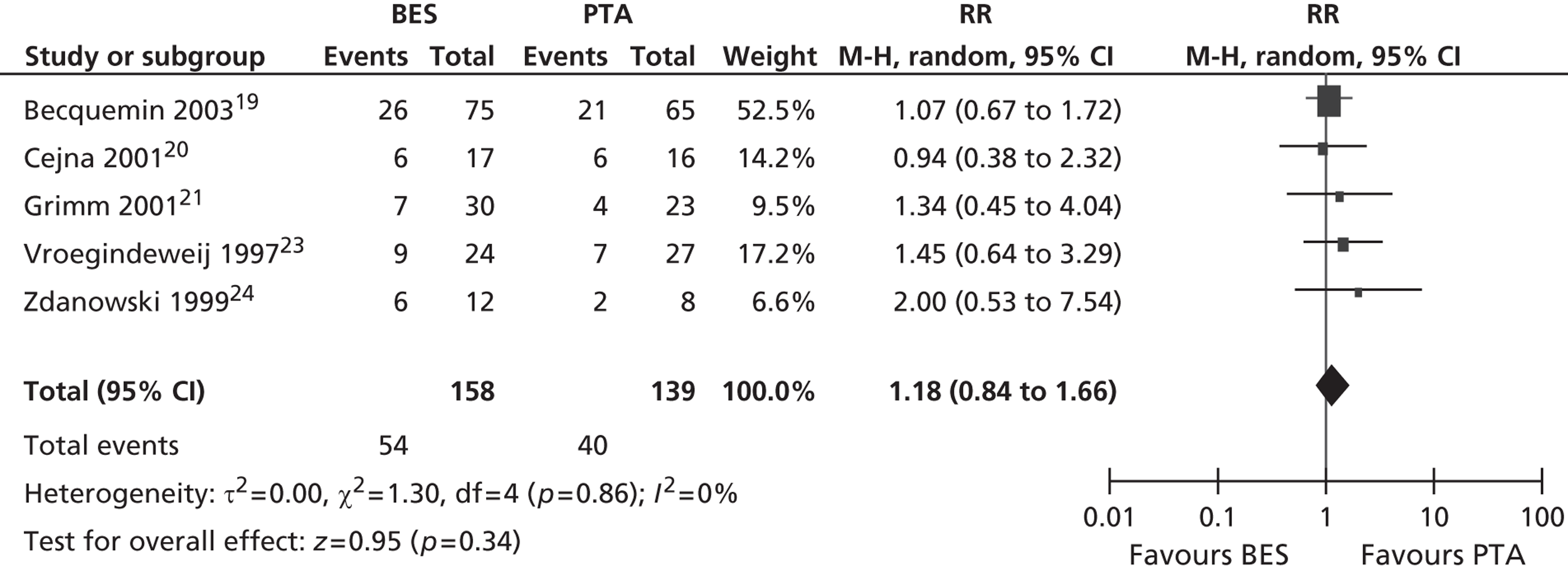
Restenosis at 24 months: using the studies of Cejna et al. 20 and Grimm et al. ,21 there was no significant heterogeneity. The overall effect was similar for fixed- and random-effect analyses (see Figures 10 and 11).
FIGURE 10.
Forest plot of comparison: 2 BES vs. PTA, restenosis at 24 months fixed.
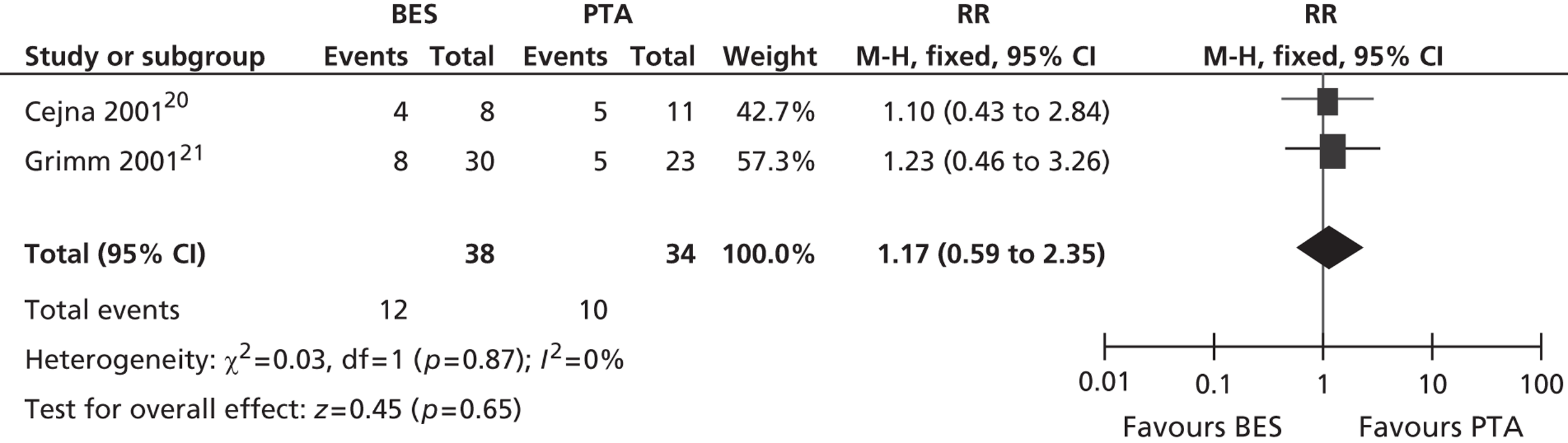
FIGURE 11.
Forest plot of comparison: 2 BES vs. PTA, restenosis at 24 months random.
Drug-eluting stent
Three RCTs of DESs were included. One RCT compared paclitaxel-eluting stents with PTA, with participants in the PTA arm having the potential to be further randomised to DES or BMS. 25 One RCT compared sirolimus-eluting stents with SESs. 30 One RCT compared sirolimus-eluting stents with stents coated with placebo. 31
The RCT of paclitaxel-eluting stents (Zilver® PTX®, Cook Medical, Bloomington, IN, USA) reported a significant advantage for DES over PTA for restenosis at 12 months (Table 15), and also for survival free of amputation, TLR or worsening of Rutherford category (Table 16). Of the two RCTs of sirolimus-eluting stents, one study found no treatment effect for DES and BMS for restenosis (SIROCCO28–30), and the other found a significant advantage of DES over BMS for luminal narrowing (Rastan et al. 31) (Table 17). Neither of these studies found significant differences between groups in terms of the need for reintervention (Table 18).
| Study | Follow-up | Definition of restenosis/patency | PTA analysed (n) | PTA patients with restenosis (%) | Paclitaxel-eluting stent analysed (n) | Paclitaxel-eluting stent patients with restenosis (%) | Comparative statistic |
|---|---|---|---|---|---|---|---|
| aZilver PTX25–27 | 12 months | Patency: duplex ultrasonography, patent = PSVR < 2.0 (or angiography, if available; patent = diameter stenosis < 50%). Group randomised to PTA, with second randomisation to stents | 251 lesions (n = 236) | 67.2a | 247 lesions (n = 235) | 16.9a | p = 0.01 |
| Patency: Duplex ultrasonography, patent = PSVR < 2.0 (or angiography if available, patent = diameter stenosis < 50%). Patients receiving only PTA not undergoing second randomisation | 126 lesions (on treatment, PTA alone) | 34.7a (PTA alone) | a/a | a/a | p < 0.01 |
| Study | Follow-up | Definition of adverse events | PTA analysed (n) | PTA patient survival (%) | Paclitaxel-eluting stent analysed (n) | Paclitaxel-eluting stent patient survival (%) | Comparative statistic |
|---|---|---|---|---|---|---|---|
| Zilver PTX25–27 | 12 months | Event-free survival; freedom from death, amputation, TLR, worsening Rutherford classification | 236 | 82.6 | 235 | 90.4 | p < 0.01 |
| Study | Follow-up | Definition of restenosis/patency | BMS analysed (n) | BMS patients with restenosis [% (95% CI)] | Sirolimus-eluting stent analysed (n) | Sirolimus-eluting stent patients with restenosis [% (95% CI)] | Comparative statistic |
|---|---|---|---|---|---|---|---|
| SIROCCO28–30 | 6 months | Restenosis defined as > 50% stenosis as determined by duplex ultrasonography | 42 | 4.8 (0.6 to 16.2) | 44 | 4.5 (0.6 to 16.2) | NS |
| 9 months | a/a | 42 | 7.1 (1.5 to 19.5) | 36 | 11.1 (3.1 to 26.1) | ||
| 12 months | a/a | 38 | 18.4 (7.7 to 34.3) | 39 | 12.8 (4.3 to 27.4) | ||
| 24 months | a/a | 35 | 22.9 (10.4 to 40.1) | 38 | 21.1 (9.6 to 37.3) | p = 1.0 | |
| Rastan et al. 201131 | 6 months | Luminal narrowing of ≥ 50% detected with duplex ultrasound if not appropriate with angiography | 67 | 31.3a | 64 | 14.1a | p = 0.02 |
| 12 months | a/a | 63 | 44.4a | 62 | 19.4a | p = 0.004 |
| Study | Follow-up | Definition of reintervention | BMS analysed (n) | BMS patients undergoing reintervention (%) | Sirolimus-eluting stent analysed (n) | Sirolimus-eluting stent patients undergoing reintervention (%) | Comparative statistic |
|---|---|---|---|---|---|---|---|
| SIROCCO28–30 | 24 months | TLR | 46 SES | 13 SES | 47 | 6 | p = 0.30 |
| 24 months | TVR | 46 SES | 22 SES | 47 | 13 | p = 0.33 | |
| Rastan et al. 201131 | 12 months | Target limb reintervention | 63 | 17.5 | 62 | 9.7 | p = 0.29 |
One study (Rastan et al. 31) found a significant advantage for SES over BMS for improving Rutherford category (Table 19), although this advantage appeared at 12 months and was not seen 6 months post intervention. The two RCTs of sirolimus-eluting stents found no significant differences between groups for adverse events (Table 20).
| Study | Follow-up | Definition of clinical status | BMS analysed (n) | BMS outcome | Sirolimus-eluting stent analysed (n) | Sirolimus-eluting stent outcome | Comparative statistic |
|---|---|---|---|---|---|---|---|
| Rastan et al. 201131 | 6 months | Change in Rutherford–Becker classification [median (IQR)] | 67 | –1 (–2 to 0) | 64 | –2 (–3 to –1) | p = 0.12 |
| 12 months | a/a | 62 | –1 (–2 to 0) | 63 | –2 (–3 to –1) | p = 0.004 |
| Study | Follow-up | Definition of complication | BMS analysed (n) | BMS patients with complications (%) | Sirolimus-eluting stent in analysis (n) | Sirolimus-eluting stent patients with complications (%) | Comparative statistic |
|---|---|---|---|---|---|---|---|
| SIROCCO28–30 | 24 months | Serious adverse event related to procedure (death or prolonged hospitalisation) | 25 | 4 | 40 | 15 | a |
| 18 months | Device-related adverse events and minor complications (related to stent fractures) | 25 | 36 | 40 | 20 | p = 0.245 | |
| Rastan et al. 201131 | 12 months | Death | 63 | 13.9 | 62 | 17.1 | p = 0.66 |
| Major amputation | 63 | 3.2 | 62 | 1.6 | |||
| Minor amputation | 63 | 3.2 | 62 | 1.6 |
Stent-graft
One RCT was identified that compared stent-graft with PTA (Saxon et al. 32,33). IC and CLI patients were included, with most having IC. This RCT reported significantly superior results for stent-graft compared with PTA in terms of restenosis, after up to 24 months follow-up (Table 21). This RCT also reported significantly superior results for stent-graft compared with PTA in terms of clinical status (Tables 22 and 23). Complications were similar between treatment groups, although there was a borderline significant effect of increased rates of thigh pain for stent-graft compared with PTA (Table 24).
| Study | Follow-up | Definition of restenosis/patency | PTA analysed (n) | PTA patients with restenosis (%) | Stent-graft analysed (n) | Stent-graft patients with restenosis (%) | Comparative statistic |
|---|---|---|---|---|---|---|---|
| Saxon et al. 2003,32 200833 | 6 months; n = 28 from report of single-centre study | > 50% stenosis on duplex ultrasound | 12 | 58a | 15 | 7a | p = 0.002 |
| 24 months; n = 28 from report of single-centre study | > 50% stenosis on duplex ultrasound | 12 | 75a | 15 | 13a | p = 0.002 | |
| 12 monthsb | No TVR; no evidence of restenosis or occlusion within treated vessel from Doppler ultrasound (where target lesion not identified, vessel patency from SFA to popliteal artery was applied); angiography demonstrating < 30% residual diameter stenosis | 100 | 60a | 97 | 35a | p = 0.0003 |
| Study | Follow-up | Definition of clinical status | PTA analysed (n) | PTA patients with clinical success (%) | Stent-graft analysed (n) | Stent-graft patients with clinical success (%) | Comparative statistic |
|---|---|---|---|---|---|---|---|
| Saxon et al. 2003,32 200833 | 12 months | Clinical success rate via Rutherford–Becker classification. Where change in clinical status was ‘improved’ = +3 to +1, ‘no change’ = 0, ‘worse’ = –1 to –3 | 100 | 69 | 97 | 84 | p = 0.025 |
| Study | Follow-up | Definition of clinical status | PTA analysed (n) | PTA outcome | Stent-graft analysed (n) | Stent-graft outcome | Comparative statistic |
|---|---|---|---|---|---|---|---|
| Saxon et al. 2003,32 200833 | 24 months; n = 28 from report of single-centre study | Mean clinical status via Rutherford–Becker classification | 100 | 1.9 (95% CI 1.02 to 2.78) | 97 | 2.8 (95% CI 2.46 to 3.14) | p = 0.08 |
| Study | Follow-up | Definition of complication | PTA (n) | PTA patients with complications (%) | Stent-graft (n) | Stent-graft patients with complications (%) | Comparative statistic |
|---|---|---|---|---|---|---|---|
| Saxon et al. 2003,32 200833 | 1 month | Major adverse event | 100 | 5 | 97 | 11.3 | Reported as NS |
| 12 months | a/a | 100 | 16 | 97 | 9.3 | Reported as NS | |
| 1 month | Minor adverse event: haematoma | 100 | 7 | 97 | 13.4 | p = 0.161 | |
| 1 month | Minor adverse event: thigh pain | 100 | 3 | 97 | 10.3 | p = 0.047 |
Atherectomy
Two RCTs comparing atherectomy with PTA in IC patients were included. One RCT (Nakamura et al. 34) found no significant difference in restenosis rates between atherectomy and PTA at 6 months (Table 25). One RCT (Vroegindeweij et al. ,35,36 Tielbeek et al. 37) found an advantage for PTA over atherectomy for restenosis at 1-year follow-up, although this no longer reached significance at 2-year follow-up.
One RCT (Vroegindeweij et al. ,35,36 Tielbeek et al. 37) found no significant difference in clinical status between atherectomy and PTA, with both groups showing improvement after 1 month, and some continuation of improvement after 12 months (Table 26). Between-group statistics were not reported for complications (Table 27), but neither study (Nakamura et al. ,34 Vroegindeweij et al. ,35,36 Tielbeek et al. 37) suggests significant differences between atherectomy and PTA.
| Study | Follow-up | Definition of restenosis/patency | PTA analysed (n) | PTA patients with restenosis (%) | Atherectomy analysed (n) | Atherectomy patients with restenosis (%) | Comparative statistic |
|---|---|---|---|---|---|---|---|
| Nakamura 199534 | 6 months | Patency was defined as improvement in clinical symptoms as well as sustained improvement in the ABPI | 10 | 50a | 2.7-mm TEC, n = 13; 4.0-mm TEC, n = 8 | With 2.7-mm TEC: 54a. With 4.0-mm TEC: 62a | p = 0.16 |
| Vroegindeweij’s group 199535–37 | 12 months | PSV index = ratio of PSV stenosis to PSV artery. PSV index ≤ 0.5 indicates ≥ 50% diameter reduction. Assessed by colour-flow duplex scanning | 14 | 23a | 16 | 75a | p = 0.017 |
| 24 months | PSVR ≥ 2.5 assessed by colour-flow duplex scanning | 35 | 66a | 38 | 44a | p = 0.07 | |
| Angiographically determined diameter reduction ≥ 50% | 35 | 33a | 38 | 56a | p = 0.06 |
| Study | Follow-up | Definition of clinical status | PTA analysed (n) | PTA outcome (%) | Atherectomy analysed (n) | Atherectomy outcome (%) | Comparative statistic |
|---|---|---|---|---|---|---|---|
| Vroegindeweij’s group 199535–37 | 1 month | Improvement defined by the Society for Vascular Surgery/International Society for Cardiovascular Surgery criteria | 35 | 97 | 38 | 89 | Reported as NS |
| 12 months | Maintenance of clinical category according to Society for Vascular Surgery/International Society for Cardiovascular Surgery criteria | 14 | 74 | 16 | 57 | p = 0.52 |
| Study | Follow-up | Definition of complication | PTA analysed (n) | PTA patients with complications (%) | Atherectomy analysed (n) | Atherectomy patients with complications (%) |
|---|---|---|---|---|---|---|
| Nakamura 199534 | Perioperative | Minor procedural complication | 13 | 23.1 | 2.7-mm TEC, n = 13; 4.0-mm or 4.7-mm TEC, n = 13 | 2.7-mm TEC, 0; 4.0-mm or 4.7-mm TEC, 38.5 |
| Vroegindeweij’s group 199535–37 | Perioperative | Minor procedure-related complications; dissections | 35 | 14.3 | 38 | 0 |
| Major procedure-related complications | 35 | 2.9 | 38 | 7.9 |
Cutting balloon
Two RCTs were identified that compared CB with PTA, with mostly IC, but some CLI, patients. All patients in the trial of Dick et al. 39 had prior stents and the study investigated femoropopliteal in-stent restenosis, whereas the study of Amighi et al. 38 looked at short de novo superficial femoral artery lesions. One RCT (Amighi et al. 38) showed a borderline significant trend favouring PTA over CB for restenosis. The other RCT (Dick et al. 39) found no significant difference in restenosis between CB and PTA (Table 28).
| Study | Follow-up | Definition of restenosis/patency | PTA analysed (n) | PTA patients with restenosis (%) | CB analysed (n) | CB patients with restenosis (%) | Comparative statistic |
|---|---|---|---|---|---|---|---|
| Amighi et al. 200838 | 6 months | > 50% restenosis of the treated vessel segment determined by duplex ultrasound | 22 | 32 | 21 | 62 | p = 0.048 |
| Dick et al. 200839 | 1 month | a/a | 22 | 27 | 17 | 12 | p = 0.42 |
| 3 months | a/a | 22 | 41 | 17 | 47 | p = 0.75 | |
| 6 months | a/a | 22 | 73 (95% CI 54 to 92) | 17 | 65 (95% CI 42 to 88) | p = 0.73 |
One study (Dick et al. 39) showed similar rates of need for reintervention for CB and PTA groups (Table 29). One RCT (Amighi et al. 38) showed a trend favouring PTA over CB for rates of asymptomatic patients (Tables 30 and 31). Both studies (Amighi et al. 38 and Dick et al. 39) showed similar levels of complications between CB and PTA groups (Table 32).
| Study | Follow-up | Definition of reintervention | PTA analysed (n) | PTA patients undergoing reintervention (%) | CB analysed (n) | CB patients undergoing reintervention (%) |
|---|---|---|---|---|---|---|
| Dick et al. 200839 | 6 months | Ipsilateral reintervention with repeat balloon angioplasty or bypass surgery | 22 | 36.4 | 17 | 41 |
| Study | Follow-up | Definition of clinical status | PTA analysed (n) | PTA outcome (%) | CB analysed (n) | CB outcome (%) | Comparative statistic |
|---|---|---|---|---|---|---|---|
| Amighi et al. 200838 | 6 months | Clinically asymptomatic | 22 | 73 | 21 | 38 | p = 0.059 |
| Study | Follow-up | Definition of walking capacity | PTA analysed (n) | PTA outcome | CB analysed (n) | CB outcome | Comparative statistic |
|---|---|---|---|---|---|---|---|
| Amighi et al. 200838 | 6 months | Pain-free walking distance (m) [median (IQR)] | 22 | > 1000 (200 to > 1000) | 21 | 600 (100 to > 1000) | p = 0.17 |
| Dick et al. 200839 | 6 months | Maximum walking capacity on the treadmill (m) | 22 | 103 | 17 | 117 | p = 0.97 |
| Study | Follow-up | Definition of complication | PTA analysed (n) | PTA patients with complications (%) | CB analysed (n) | CB patients with complications (%) | Comparative statistic |
|---|---|---|---|---|---|---|---|
| Amighi et al. 200838 | 6 months | Minor procedure-related complications: peripheral embolism or pseudoaneurysm | 22 | 4.5 | 21 | 9.5 | |
| Dick et al. 200839 | Perioperative | Major complications: access site complications requiring surgical intervention, bleeding complications, amputation, macroembolism, death | 22 | 0 | 17 | 0 | |
| Minor complications: spontaneously resolving | 22 | 18 | 17 | 18 | p = 0.99 |
Cryoplasty
Two RCTs were included that compared cryoplasty with PTA in IC and CLI patients. Neither RCT (Jahnke et al. ,40 Spiliopoulos et al. 41) found a significant treatment group effect between cryoplasty and PTA for restenosis (Table 33).
| Study | Follow-up | Definition of restenosis/patency | PTA analysed (n) | PTA patients with restenosis (%) | Cryoplasty analysed (n) | Cryoplasty patients with restenosis (%) | Comparative statistic |
|---|---|---|---|---|---|---|---|
| Jahnke et al. 201040 | 3 months | > 2.5-fold increase in PSVR across the treated segment indicative of > 50% luminal narrowing | 37 | 9.2a | 31 | 3.2a | |
| 6 months | a/a | 33 | 20.2a | 27 | 17.1a | ||
| 9 months | a/a | 23 | 33.3a | 23 | 20.7a | p = 0.14 | |
| Spiliopoulos et al. 201041 | 12 months | Binary in-lesion restenosis > 50% | 31 limbs | 32.4a | 29 limbs | 33.4a | |
| 24 months | a/a | 31 limbs | 45.4a | 29 limbs | 40.8a | ||
| 36 months | a/a | 31 limbs | 45.4a | 29 limbs | 40.8a | p = 0.894 |
One study (Spiliopoulos et al. 41) found a significant advantage for PTA over cryoplasty, in terms of fewer patients needing reintervention (Table 34). One study (Jahnke et al. 40) showed similar levels of improvement in clinical status for cryoplasty and PTA (Table 35). Both studies (Jahnke et al. ,40 Spiliopoulos et al. 41) showed similar levels of complications between cryoplasty and PTA groups (Table 36).
| Study | Follow-up | Definition of reintervention | PTA analysed (n) | PTA patients undergoing reintervention (%) | Cryoplasty analysed (n) | Cryoplasty patients undergoing reintervention (%) | Comparative statistic |
|---|---|---|---|---|---|---|---|
| Spiliopoulos et al. 201041 | 36 months | TLR | 31 limbs | 52.3 | 29 limbs | 66.5 | p < 0.04 |
| Study | Follow-up | Definition of clinical status | PTA analysed (n) | PTA mean score | Cryoplasty analysed (n) | Cryoplasty mean score | Comparative statistic |
|---|---|---|---|---|---|---|---|
| Jahnke et al. 201040 | 9 months | Improvement defined by the Society for Vascular Surgery/International Society for Cardiovascular Surgery criteria for lower-limb ischaemia ranging from –3 (markedly worse) to +3 (markedly improved) | 23 | 2.43 ± 1.16 | 23 | 2.73 ± 0.55 | Only within-group analysis offered |
| Study | Follow-up | Definition of complication | PTA analysed (n) | PTA patients with complications (%) | Cryoplasty analysed (n) | Cryoplasty patients with complications (%) | Comparative statistic |
|---|---|---|---|---|---|---|---|
| Jahnke et al. 201040 | Perioperative | Major complication: distal embolisation, side branch perforation | 46 | 2.7 | 40 | 5 | |
| Minor complication: groin haematoma | 46 | 2.7 | 40 | 2.5 | |||
| Spiliopoulos et al. 201041 | Perioperative | Minor puncture-site-related complications | 31 limbs | 3.2 | 29 limbs | 3.5 | p = 0.4 |
| Major puncture-site-related complications | 31 limbs | 0 | 29 limbs | 0 | NS | ||
| Procedure-related adverse events | 31 limbs | 0 | 29 limbs | 0 | NS | ||
| Minor amputation | 31 limbs | 9.7 | 29 limbs | 6.9 | p = 0.3 |
Radiation
In this review, 10 RCTs were included that compared radiation with PTA in majority IC and CLI patients. Of these, eight employed EVBT, and two used EBRT.
Endovascular brachytherapy studies
For restenosis (Table 37), three studies (Zehnder et al. ,45 Krueger et al. ,47,48 Vienna-351–53) showed a significant advantage for EVBT over PTA, although, for one of these studies (Krueger et al. 47,48), the advantage at 6 months was not maintained at 2 years, and two studies (Gallino et al. ,42 Hagenaars et al. 46) showed a trend favouring EVBT (Gallino et al. 42 trial significance value not calculated between two arms presented here, as it was part of a four-arm trial). Two studies (Vienna-2,49–51 VARA54), and one combined analysis with long-term follow-up of two included studies (Diehm et al. 44 analysis of Gallino et al. 42 and Zehnder et al. 45 trials), found no significant difference between EVBT and PTA (Table 38). Meta-analyses of restenosis at 6 months using VARA54 and Vienna-249–51 trials (Figures 12–15) gave a RR of 0.93 (95% CI 0.62 to 1.39; p = 0.72) by fixed-effect analysis. By random-effect analysis, the RR was 1.00 (95% CI 0.70 to 1.44; p = 1.00). At 12-month follow-up, restenosis rates based on the meta-analyses of Diehm et al. ,44 VARA54 and Vienna-351–53 had a RR of 0.63 (95% CI 0.48 to 0.83) by both fixed-effect (p = 0.001) and random-effect (p = 0.0008) analyses, significantly favouring EVBT over PTA.
| Study | Follow-up | Definition of restenosis/patency | PTA analysed (n) | PTA patients with restenosis (%) | Radiation analysed (n) | Radiation patients with restenosis (%) | Comparative statistic |
|---|---|---|---|---|---|---|---|
| Gallino et al. 2004,42 Bonvini et al. 2003,43 Diehm et al. 200544 | 6 months | > 50% restenosis measured by duplex ultrasound | 84 | 42a | 81 | 17a | |
| Zehnder et al. 2003,45 Diehm et al. 200546 | 12 months | > 50% recurrent obstruction defined by duplex ultrasound | 56 | 42 | 44 | 23 | p < 0.028 |
| Gallino et al. 2004,42 Zehnder et al. 2003,45 Diehm et al. 200544 | 12 months | 50% or more diameter reduction by digital subtraction angiography | 75 | 29.3a | 72 | 17.3a | p = 0.16 |
| 24 months | a/a | 75 | 36.9a | 72 | 35.7a | p = 0.16 | |
| 36 months | a/a | 75 | 52.9a | 72 | 35.7a | p = 0.16 | |
| Hagenaars et al. 200246 | 6 months | > 50% diameter stenosis defined by angiography | 16 | 31.3 | 8 | 0 | p = 0.08 |
| Krueger et al. 2002, 200447,48 | 6 months | > 50% diameter reduction within the former stenotic section defined by angiography | 15 | 46.7 | 15 | 0 | p = 0.006 |
| 12 months | a/a | 15 | 33.3 | 15 | 0 | p = 0.042 | |
| 24 months | a/a | 15 | 33.3 | 15 | 13.3 | p = 0.39 | |
| Vienna-249–51 | 6 months | Angiographically verified stenosis of > 50% narrowing of the luminal diameter within the recanalised segment compared with the diameters of normal segments. In a patient who only underwent duplex ultrasound a PSVR ≥ 2.4 was used to indicate restenosis | 29 | 69 | 15 | 73.4 | |
| 60 months | a/a | 37 | 32.4 | 37 | 43.2 | ||
| Vienna-351–53 | 12 months | > 50% reduction of arterial lumen determined angiographically or, when patients refused, with duplex ultrasound. PSVR > 2.4 indicated 50% restenosis | 67 | 67.1 | 67 | 41.7 | p < 0.05 |
| VARA54 | 6 months | ≥ 50% restenosis of the treated segment defined by duplex ultrasound | 29 | 31 | 23 | 22 | p = 0.45 |
| 12 months | a/a | 27 | 44 | 23 | 35 | p = 0.49 |
| Study | Follow-up | Definition of late lumen loss | PTA analysed (n) | PTA lumen | Radiation analysed (n) | Radiation lumen | Comparative statistic |
|---|---|---|---|---|---|---|---|
| Hagenaars et al. 200246 | 6 months | Change in lumen area from immediately post procedure to 6-month follow-up (mm2) | 16 | mean –1.6 mm (SD 5.1) | 8 | mean 4.3 mm (SD 6.8) | p = 0.03 |
| Wyttenbach et al. 2004, 200755,56 | 24 hours | Lumen area gain (%) from baseline detected via cross-sectional MRI | 10 | 86% | 10 | 67% | Reported as NS |
| 3 months | a/a | 10 | 40% | 10 | 106% | p = 0.026 | |
| 24 months | a/a | 10 | 30% | 10 | 82% | p = 0.047 |
FIGURE 12.
Forest plot of comparison: 4 EVBT vs. PTA, restenosis at 6 months fixed two studies.
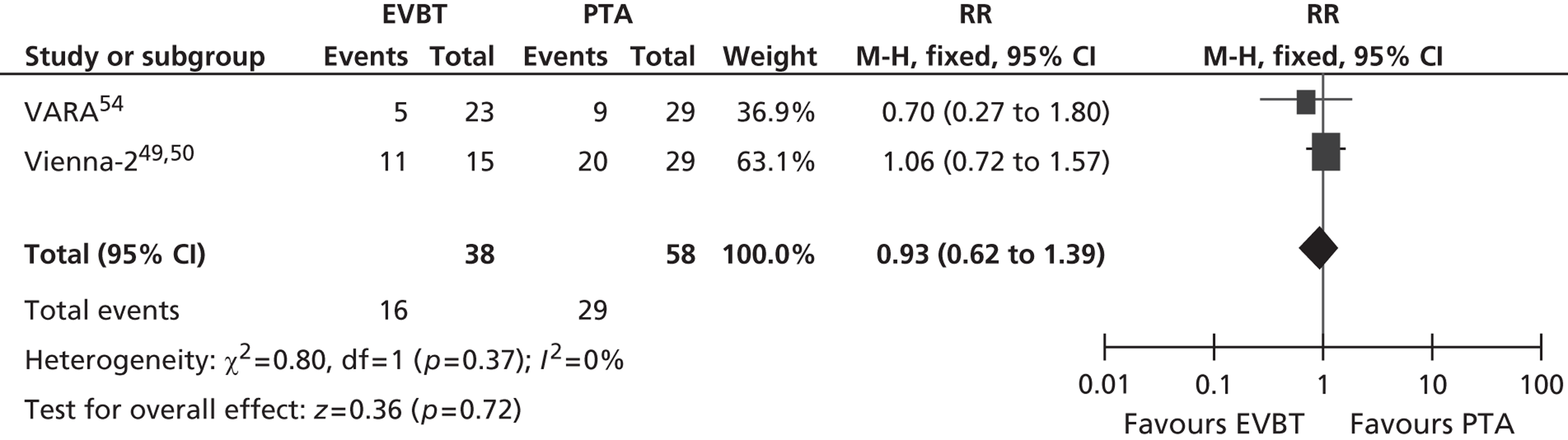
FIGURE 13.
Forest plot of comparison: 4 EVBT vs. PTA, restenosis at 6 months random two studies.
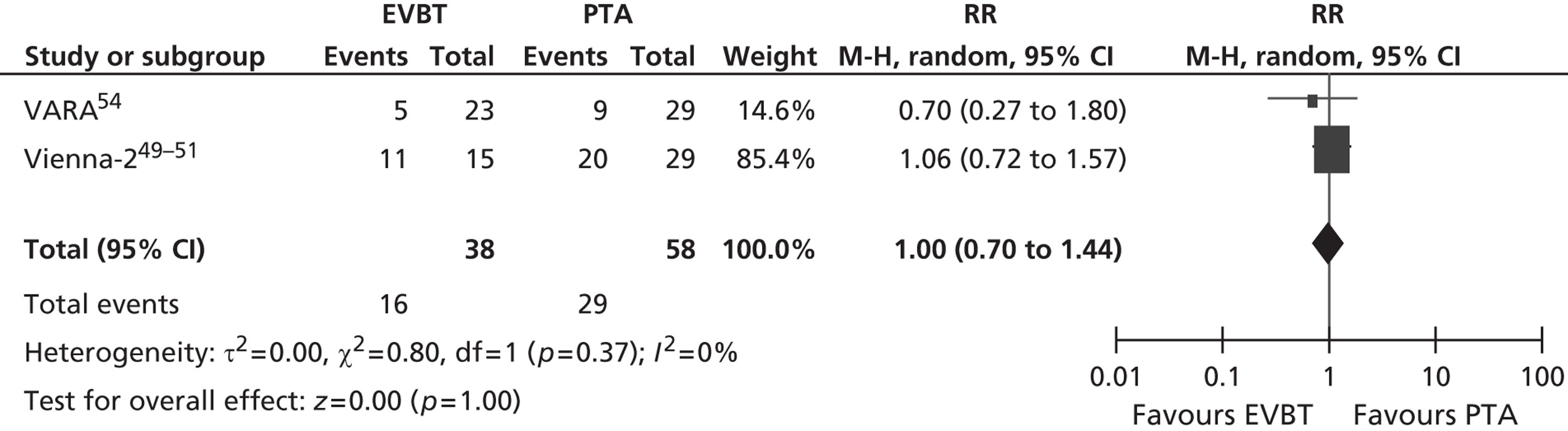
FIGURE 14.
Forest plot of comparison: 4 EVBT vs. PTA, restenosis at 12 months fixed three studies.
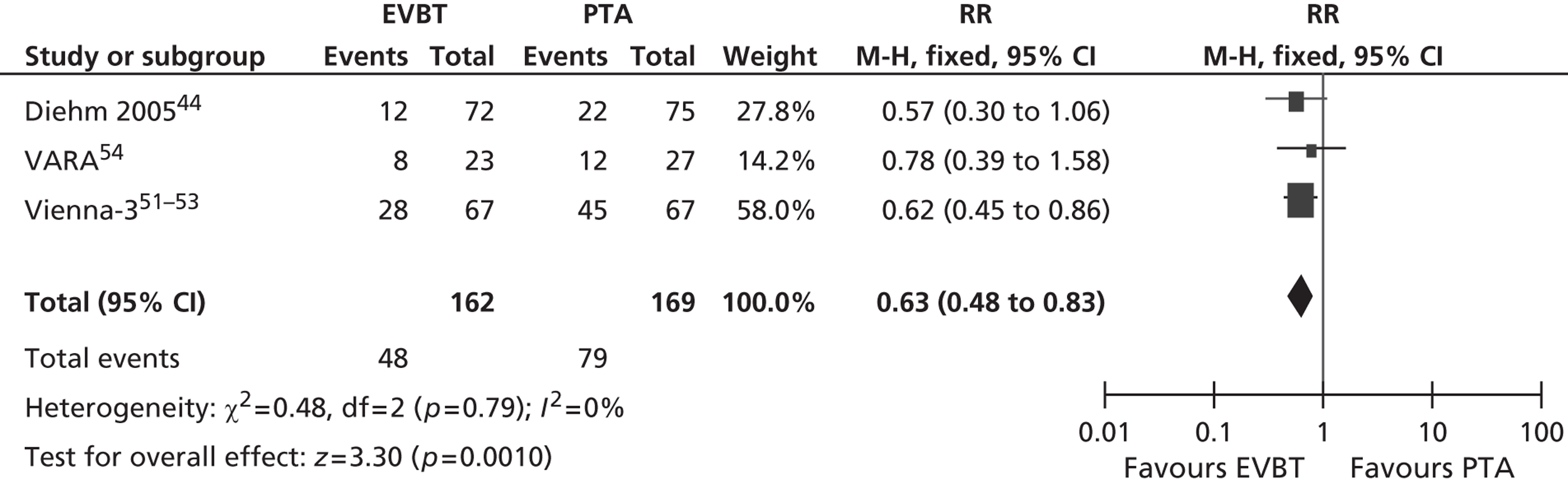
FIGURE 15.
Forest plot of comparison: 4 EVBT vs. PTA, restenosis at 12 months random three studies.
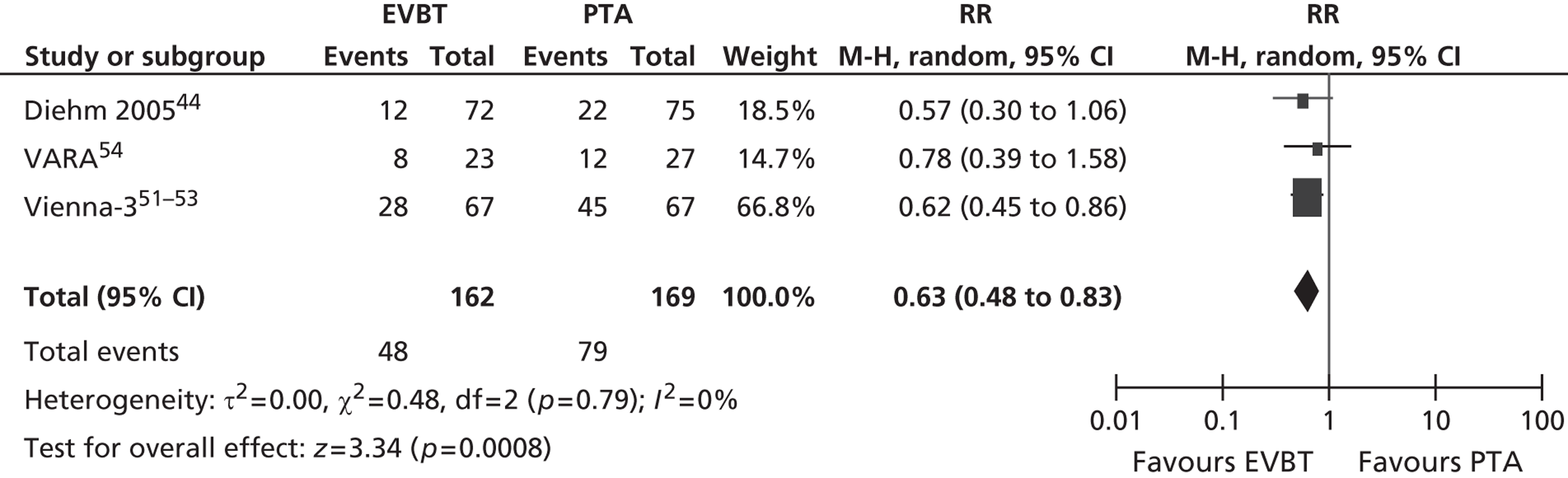
Need for reintervention rates were not significantly different between EVBT and PTA (Gallino et al. ,42 Krueger et al. ,47,48 Vienna-2,49–51 Vienna-3,51–53 VARA,54 and the combined Gallino et al. 42/Zehnder et al. 45 analysis reported by Diehm et al. 44) (Table 39). One RCT (VARA54) and one combined analysis with long-term follow-up of two included studies (Diehm et al. 44 analysis of Gallino et al. 42 and Zehnder et al. 45 trials) found no significant difference between EVBT and PTA in terms of clinical improvement (Table 40).
| Study | Follow-up | Definition of reintervention | PTA analysed (n) | PTA patients undergoing reintervention (%) | Radiation analysed (n) | Radiation patients undergoing reintervention (%) |
|---|---|---|---|---|---|---|
| Gallino et al. 2004,42 Bonvini et al. 2003,43 Diehm et al. 200544 | 6 months | Revascularisation needed | 75 | 11 | 69 | 6 |
| Zehnder et al. 200345 | 12 months | Repeat dilatation or surgery | 56 | 23.2 | 44 | 6.8 |
| Krueger et al. 2002, 200447,48 | 6 months | TLR | 15 | 0 | 15 | 0 |
| 12 months | a/a | 15 | 0 | 15 | 0 | |
| 24 months | a/a | 15 | 13.3 | 15 | 6.7 | |
| 6 months | TVR | 15 | 0 | 15 | 6.7 | |
| 12 months | a/a | 15 | 0 | 15 | 13.3 | |
| 24 months | a/a | 15 | 13.3 | 15 | 26.7 | |
| Vienna-249–51 | 60 months | TLR | 51 | 64.7 | 51 | 62.7 |
| 60 months | TVR | 51 | 72.5 | 51 | 70.6 | |
| Vienna-351–53 | 12 months | TLR | 46 | 30.4 | 50 | 10 |
| 12 months | TVR | 46 | 0 | 50 | 4 | |
| 12 months | Bypass surgery | 46 | 0 | 50 | 2 | |
| VARA54 | 12 months | Mandatory TLR; PTA or bypass surgery | 29 | 21 | 22 | 18 |
| Study | Follow-up | Definition of clinical status | PTA analysed (n) | PTA outcome (95% CI) | Radiation analysed (n) | Radiation outcome (95% CI) | Comparative statistic |
|---|---|---|---|---|---|---|---|
| Gallino et al. 2004,42 Zehnder et al. 2003,45 Diehm et al. 200544 | 12 months | Sustained clinical improvement was defined as survival without repeat revascularisation and with an ABPI > 0.1 and/or an upwards categorical shift in clinical symptoms according to the Rutherford classification | 75 | 84.3 (72.7 to 91.3) | 72 | 82.4 (71.1 to 89.6) | p = 0.26 by log-rank (cumulative rates) |
| 24 months | a/a | 34 | 82.1 (69.8 to 89.8) | 37 | 69.8 (56.5 to 79.7) | p = 0.26 by log-rank (cumulative rates) | |
| 36 months | a/a | 25 | 76.4 (62 to 86) | 25 | 67.5 (53.9 to 77.9) | p = 0.26 by log-rank (cumulative rates) | |
| VARA54 | 6 months | Change in Rutherford classification (median) | 27 | 2 | 23 | 2 | p = 0.75 |
| 12 months | a/a | 27 | 2 | 23 | 2 | p = 0.39 |
The RCT (Krueger et al. 47,48) reporting walking capacity found no significant differences between groups for EVBT and PTA on measures of pain-free walking distance or total walking distance up to 12 months post intervention (Table 41), with similar results up to 24 months. The patient-reported leg pain scores (Krueger et al. 47,48) were also similar between EVBT and PTA following intervention, although there was a borderline significant trend (p = 0.05) at 12 months favouring EVBT over radiation. Reported complications (Table 42) were similar for EVBT and PTA (Gallino et al. ,42 Vienna-3,51–53 VARA54).
| Study | Follow-up | Definition of walking capacity | PTA analysed (n) | PTA outcome | Radiation analysed (n) | Radiation outcome | Comparative statistic |
|---|---|---|---|---|---|---|---|
| Krueger et al. 2002, 200447,48 | 1 month | Pain-free walking distance (m) (mean) (treadmill 3 km/h, slope 12 degrees) | 15 | 288.1 ± 193.9 | 15 | 308 ± 191.2 | p = 0.68 |
| 6 months | a/a | 15 | 307.0 ± 170.2 | 15 | 339.4 ± 185.3 | ||
| 12 months | a/a | 15 | 297.9 ± 205.8 | 15 | 329.2 ± 185.5 | p = 0.72 | |
| 1 month | Total walking distance (m) (mean) (treadmill 3 km/h, slope 12 degrees) | 15 | 321.1 ± 176.0 | 15 | 344.5 ± 171.7 | p = 0.72 | |
| 6 months | a/a | 15 | 345.4 ± 174.8 | 15 | 395.9 ± 140.1 | ||
| 12 months | a/a | 15 | 357.8 ± 170.4 | 15 | 393.0 ± 143.0 | p = 0.59 | |
| 1 month | Walking distance leg pain scores at interview (max. 35) | 15 | 25.7 ± 5.9 | 15 | 28.4 ± 4.5 | p = 0.18 | |
| 6 months | a/a | 15 | 25.7 ± 6.2 | 15 | 27.3 ± 5.3 | p = 0.28 | |
| 12 months | a/a | 15 | 22.2 ± 8.1 | 15 | 26.1 ± 3.7 | p = 0.05 |
| Study | Follow-up | Definition of complication | PTA analysed (n) | PTA patients with complications (%) | Radiation analysed (n) | Radiation patients with complications (%) |
|---|---|---|---|---|---|---|
| Gallino et al. 2004,42 Bonvini et al. 200343 | 6 months | Late acute thrombotic occlusion | 75 | 0 | 69 | 4.3 |
| Vienna-351–53 | 12 months | Amputation | 46 | 2.2 | 50 | 0 |
| VARA54 | Perioperative | Vessel thrombosis/early occlusion | 33 | 3 | 27 | 3.7 |
External beam radiotherapy studies
Two RCTs (Fritz et al. ,57 Therasse et al. 58) found no significant treatment group effect for restenosis rates between EBRT and PTA (Table 43). One of these studies (Therasse et al. 58) reported a treatment group effect for minimum lumen diameter, which was significantly larger in the 14 Gy dose EBRT group than in the PTA group for the dilated zone (p = 0.0039) and the irradiated zone (p = 0.037).
There was no significant treatment effect for the need for reintervention (Table 44) between EBRT and PTA at 18 months post intervention (Therasse et al. 58). Clinical change reported by one RCT (Fritz et al. 57) showed similar improvement in Fontaine stage in EBRT and PTA groups (Table 45). One RCT (Therasse et al. 58) reported that there were no major complications in either EBRT or PTA treatment groups.
| Study | Follow-up | Definition of restenosis/patency | PTA analysed (n) | PTA patients with restenosis (%) | Radiation analysed (n) | Radiation patients with restenosis (%) | Comparative statistic |
|---|---|---|---|---|---|---|---|
| Fritz et al. 200457 | 12 months | Restenosis was assumed if the ABPI was < 0.8 and only a max. of 0.2 > the value before PTA. If the ABPI was not meaningful, then the peak velocity ratio determined by duplex ultrasound or the resulting stenosis using a nomogram and the clinical stage according to Fontaine. > 50% restenosis was regarded as significant | 48 | 33.3 | 46 | 45.7 | p = 0.292 |
| Therasse et al. 200558 | 12 months | > 50% reduction of lumen diameter within the dilated segment determined angiographically | 22 | 50 | 7 Gy, n = 23; 10.5 Gy, n = 23; 14 Gy, n = 20 | 7 Gy, 65; 10.5 Gy, 48; 14 Gy, 25 | p = 0.072 |
| > 50% reduction of lumen diameter within the irradiated zone determined angiographically | 22 | 50 | 7 Gy, n = 23; 10.5 Gy, n = 23; 14 Gy, n = 20 | 7 Gy, 65; 10.5 Gy, 48; 14 Gy, 30 | p = 0.15 |
| Study | Follow-up | Definition of reintervention | PTA analysed (n) | PTA patients undergoing reintervention (%) | Radiation analysed (n) | Radiation patients undergoing reintervention (%) | Comparative statistic |
|---|---|---|---|---|---|---|---|
| Therasse et al. 200558 | 18 months | Repeat PTA or surgery | 24 | 25 | 25 | in 14 Gy group: 12 | p = 0.24 |
| Study | Follow-up | Definition of clinical status | PTA analysed (n) | PTA outcome | Radiation analysed (n) | Radiation outcome |
|---|---|---|---|---|---|---|
| Fritz et al. 200457 | 12 months | Mean change in Fontaine classification | 48 | –0.8 | 47 | –0.6 |
Meta-analyses
Restenosis at 6 months: a meta-analysis using the VARA54 and Vienna-249–51 studies gave non-significant heterogeneity. The overall effect was similar for fixed- and random-effect analyses.
Restenosis at 12 months: using the trials Diehm et al. 2005,44 VARA54 and Vienna-3,51–53 there was no significant heterogeneity. The overall effect was similar for fixed- and random-effect analyses.
Drug-coated balloon
Three RCTs were identified that compared DCB angioplasty with conventional (uncoated balloon) PTA. For all studies, the type of drug utilised was paclitaxel. Most of the patients across the studies had IC, although some had CLI.
Two studies (THUNDER,61–63 FemPac64) reported a significant advantage for DCB over PTA for restenosis rates (Table 46). When meta-analysed for restenosis at 6-month follow-up, these studies gave an RR of 0.40 (95% CI 0.23 to 0.69; p = 0.001), by both fixed- and random-effect analyses. Late lumen loss (LEVANT I59,60) and postintervention lumen diameter difference (THUNDER61–63) showed a significant treatment effect favouring DCB over PTA (Table 47).
| Study | Follow-up | Definition of restenosis/patency | PTA analysed (n) | PTA patients with restenosis (%) | DCB analysed (n) | DCB patients with restenosis (%) | Comparative statistic |
|---|---|---|---|---|---|---|---|
| THUNDER61–63 | 6 months | Angiographically determined ≥ 50% stenosis of the diameter of the reference-vessel segment | 48 | 44 | 41 | 17 | p = 0.01 |
| 12 months | a/a | 36 | 50 | 33 | 24 | NR | |
| FemPac64 | 6 months | Angiographically determined ≥ 50% stenosis in the treated lesion | 34 | 47 | 31 | 19 | p = 0.035 |
| Study | Follow-up | Definition of late lumen loss | PTA analysed (n) | PTA late lumen loss (mm; mean) | DCB analysed (n) | DCB late lumen loss (mm; mean) | Comparative statistic |
|---|---|---|---|---|---|---|---|
| LEVANT I59,60 | 6 months | Late lumen loss (mm) | 35 | 1.09 | 39 | 0.46 | p = 0.016 |
| THUNDER61–63 | 6 months | The difference between the minimum lumen diameters after dilatation and at the 6-month follow-up | 54 | 1.7 ± 1.8 | 48 | 0.4 ± 1.2 | p < 0.001 |
Need for reintervention rates were lower in DCB than in PTA treatment groups (Table 48), significantly favouring DCB over PTA in two RCTs (THUNDER,61–63 FemPac64); the significance level was not reported in the other study (LEVANT I59,60) for TLR. Rates of TVR were also lower in the DCB than in the PTA group (LEVANT I59,60). TLR at 6-month follow-up, by meta-analysis of FemPac,64 LEVANT I59,60 and THUNDER61–63 trials, which showed some heterogeneity (Figures 16–21), produced a RR of 0.26 (95% CI 0.10 to 0.68; p = 0.006) by random-effect analysis. This significantly favoured DCB over PTA, which was also the case at 24-month follow-up using the FemPac64 and THUNDER61–63 trials (RR 0.27; 95% CI 0.16 to 0.47; p < 0.00001).
| Study | Follow-up | Definition of reintervention | PTA analysed (n) | PTA patients undergoing reintervention (%) | DCB analysed (n) | DCB patients undergoing reintervention (%) | Comparative statistic |
|---|---|---|---|---|---|---|---|
| LEVANT I59,60 | 6 months | TLR | 47 | 22 | 41 | 13 | NR |
| THUNDER61–63 | 6 months | a/a | 54 | 37 | 48 | 4 | p < 0.001 |
| 12 months | a/a | 54 | 48 | 48 | 10 | ||
| 24 months | a/a | 54 | 52 | 48 | 15 | p < 0.001 | |
| FemPac64 | 6 months | a/a | 42 | 33 | 45 | 7 | p = 0.0024 |
| 24 months | a/a | 42 | 50 | 45 | 13 | p = 0.001 |
FIGURE 16.
Forest plot of comparison: 5 DCB vs. PTA, restenosis at 6 months fixed.
FIGURE 17.
Forest plot of comparison: 5 DCB vs. PTA, restenosis at 6 months random.
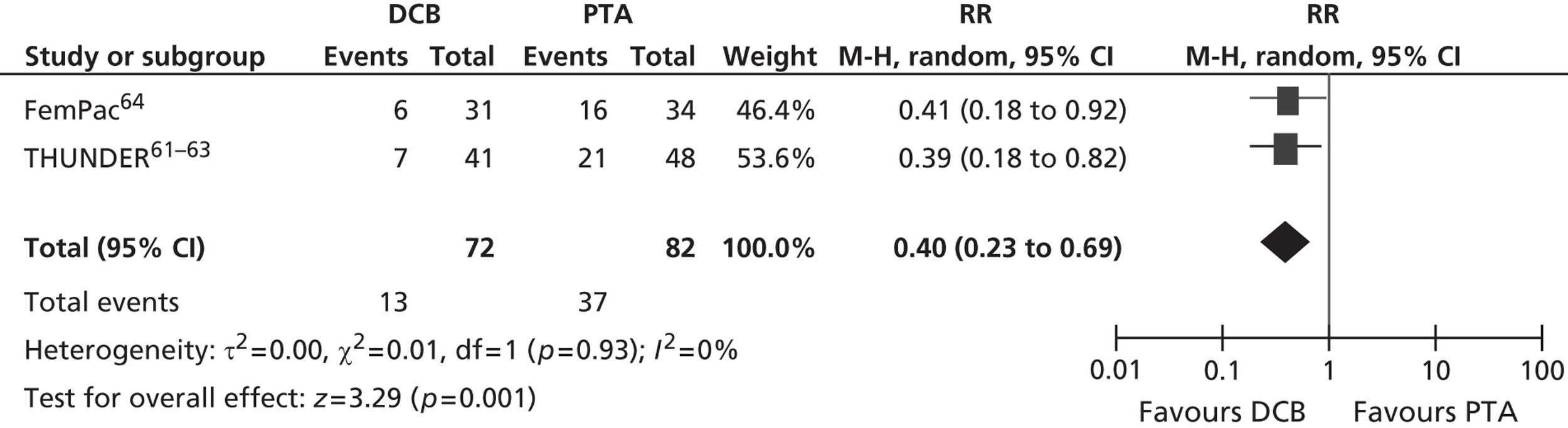
FIGURE 18.
Forest plot of comparison: 5 DCB vs. PTA, TLR at 6 months fixed.
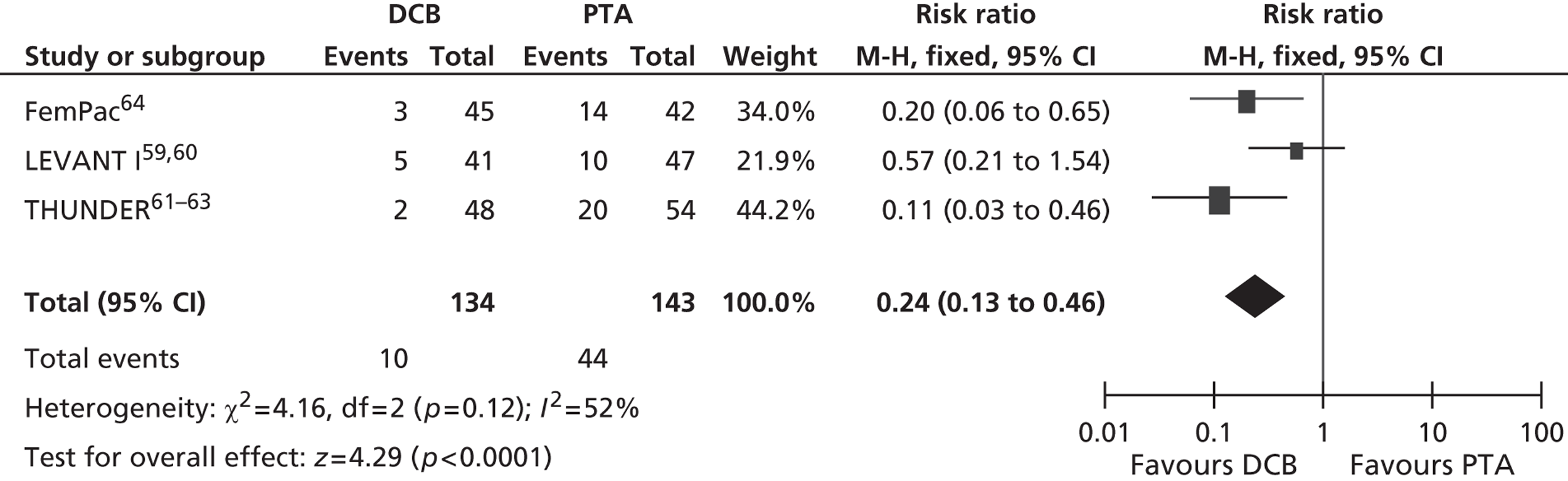
FIGURE 19.
Forest plot of comparison: 5 DCB vs. PTA, TLR at 6 months random.
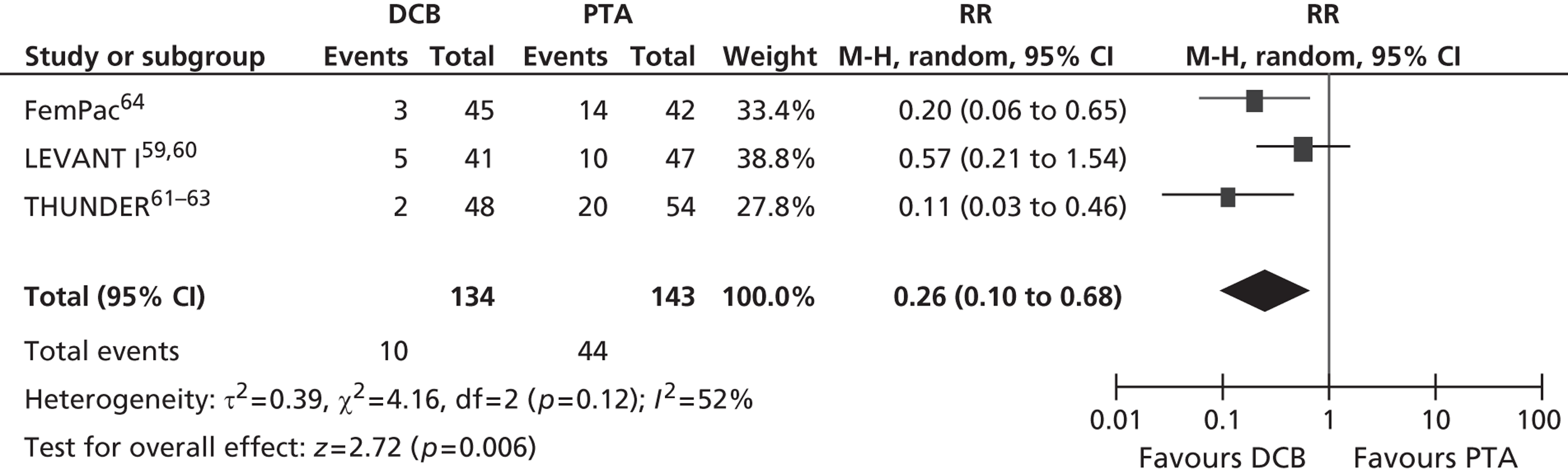
FIGURE 20.
Forest plot of comparison: 5 DCB vs. PTA, TLR at 24 months fixed.
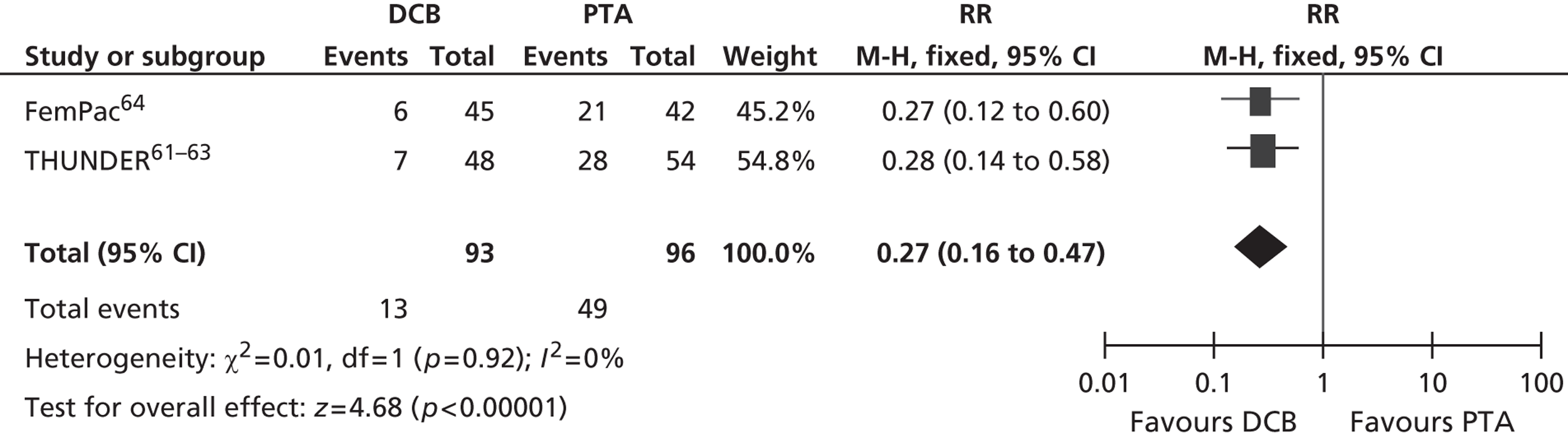
FIGURE 21.
Forest plot of comparison: 5 DCB vs. PTA, TLR at 24 months random.
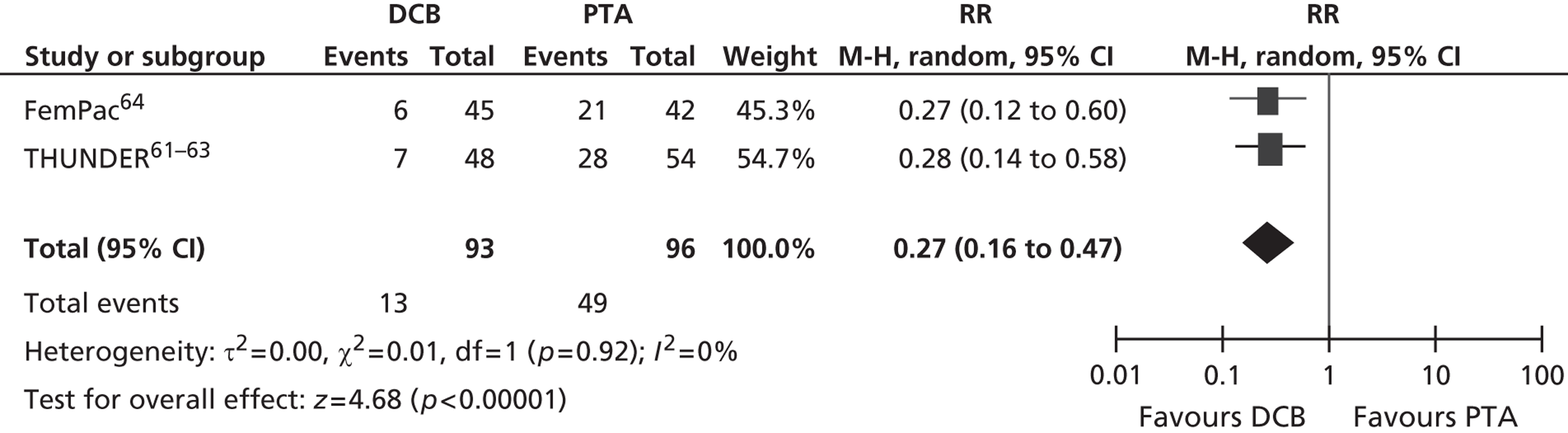
Two studies reported Rutherford category (Table 49). One study found no treatment group effect (THUNDER61–63), and one study (FemPac64) reported a borderline significant group difference, with more patients improving in the DCB group than in the PTA group at 6 months. However, in the latter study, by 18–24 months post intervention there was no significant difference between the groups, with the PTA group remaining stable and the improvement lessening in the DCB group, although both groups still improved from pre intervention. Complications and adverse events (Table 50) showed no significant treatment effects between DCB and PTA groups (LEVANT I,59,60 THUNDER,61–63 FemPac64).
| Study | Follow-up | Definition of clinical status | PTA analysed (n) | Clinical status of PTA patients | DCB analysed (n) | Clinical status of DCB patients | Comparative statistic |
|---|---|---|---|---|---|---|---|
| THUNDER61–63 | 6 months | Change in Rutherford category from baseline to follow-up (mean) | 54 | –1.9 | 48 | –2.3 | Reported as NS |
| FemPac64 | 6 months | Improvement in Rutherford category from baseline to follow-up | 42 | 36% of patients improved | 45 | 58% of patients improved | 0.045 |
| 18–24 months | Improvement in Rutherford category from baseline to follow-up | 42 | 36% of patients improved | 35 | 35% of patients improved | 0.98 |
| Study | Follow-up | Definition of complication | PTA analysed (n) | PTA patients with complications (%) | DCB analysed (n) | DCB patients with complications (%) | Comparative statistic |
|---|---|---|---|---|---|---|---|
| LEVANT I59,60 | 1 month | Adverse device effects | 52 | NR | 49 | NR | NS |
| THUNDER61–63 | < 2 weeks | Embolic complication or thrombosis | 54 | 5.6 | 48 | 4.2 | |
| 6 months | Amputation | 54 | 0 | 48 | 4.2 | p = 0.22 | |
| 6 months | Death | 54 | 2 | 48 | 4.2 | p = 0.59 | |
| FemPac64 | 6 months | Major amputation of target leg, excluding toes | 42 | 2 | 45 | 0 | p = 0.48 |
| 18–24 months | Major amputation of target leg, excluding toes | 42 | 0 | 45 | 0 | p = 1.0 | |
| 6 months | Death | 42 | 0 | 45 | 2 | p = 1.0 | |
| 18–24 months | Death | 42 | 7 | 45 | 13 | p = 0.49 | |
| Perioperative | Adverse events: PE, skin rash, allergic reaction, temporary serum creatinine increase | 42 | 2 | 45 | 2 |
Meta-analyses
Restenosis at 6 months: using the trials FemPac64 and THUNDER,61–63 there was no significant heterogeneity. The overall effect was similar for fixed- and random-effect analyses.
TLR at 6 months: there was some heterogeneity across the three included trials, although this did not reach significance.
TLR at 24 months: non-significant heterogeneity was found using FemPac64 and THUNDER61–63 trials. The overall effect was similar for fixed- and random-effect analyses.
Laser angioplasty
Five RCTs were included that compared laser angioplasty with PTA. Restenosis at 12-month follow-up was reported by one trial (Lammer et al. 68), which found no significant treatment effect between laser and PTA (Table 51).
| Study | Follow-up | Definition of restenosis/patency | PTA analysed (n) | PTA patients with restenosis (%) | Laser analysed (n) | Laser patients with restenosis (%) | Comparative statistic |
|---|---|---|---|---|---|---|---|
| Lammer et al. 199268 | 12 months | Angiographic reobstruction was defined as an increase in diameter stenosis > 30%, an immediate post-PTA diameter stenosis of < 50% increasing to > 70% at follow-up, an increase in stenosis severity to ≤ 10% of predilation obstruction and a loss of > 50% of the gain in luminal diameter achieved by PTA | Unclear (77 across all groups) | 50* | Unclear (77 across all groups) | Pulsed, 55; continuous, 64 | NS |
One study reported clinical success (Belli et al. 65,66) measured by symptoms and peripheral pulses, and found a borderline significant trend favouring PTA over laser angioplasty (Table 52). Procedural complications were similar in laser and PTA groups (Belli et al. ,65,66 Lammer et al. ,68 Spies et al. ,69 Tobis et al. 70), with the exception of dissection, which was significantly more frequent with laser angioplasty than with PTA (Table 53).
| Study | Follow-up | Definition of clinical status | PTA analysed (n) | Clinical status of PTA patients (%) | Laser analysed (n) | Clinical status of laser patients (%) |
|---|---|---|---|---|---|---|
| Belli et al. 199165,66 | 1 month | Clinical success was defined as relief of symptoms and improved peripheral pulses | 34 | 82 | 34 | 79 |
| 3 months | a/a | 34 | 72 | 34 | 56 | |
| 6 months | a/a | 26 | 56 | 30 | 42 | |
| 12 months | a/a | 24 | 47 | 26 | 39 |
| Study | Follow-up | Definition of complication | PTA analysed (n) | PTA patients with complications (%) | Laser analysed (n) | Laser patients with complications (%) |
|---|---|---|---|---|---|---|
| Belli et al. 199165,66 | Perioperative | Small embolus | 34 | 5.9 | 34 | 2.9 |
| Spasm | 34 | 5.9 | 34 | 5.9 | ||
| Lammer et al. 199268 | Perioperative | Embolus | 39 | 7.7 | Pulsed, n = 37; continuous, n = 40 | Pulsed, 0; continuous, 5 |
| Dissection | 39 | 15.4 | Pulsed, n = 37; continuous, n = 40 | Pulsed, 35.1; continuous, 20 | ||
| Perforation | 39 | 7.7 | Pulsed, n = 37; continuous, n = 40 | Pulsed, 5.4; continuous, 5 | ||
| Spasm | 39 | 2.6 | Pulsed, n = 37; continuous, n = 40 | Pulsed, 0; continuous, 0 | ||
| Spies et al. 199069 | Perioperative | Embolus | 13 | 0 | 14 procedures | 7.1 |
| Tobis et al. 199170 | Perioperative | Procedural complication; arterial wall perforation | 20 | 5 | 20 | 15 |
Discussion
Data were available from RCTs for the technologies AMSs, SESs, BESs, DES, stent-graft, atherectomy, CB, cryoplasty, radiation by EVBT or EBRT, DCBs and laser angioplasty. The trials of atherectomy and laser angioplasty were older than those of other technologies. An ITT analysis was available from all trials, and treatment groups within trials were comparable at baseline. Of the 40 RCTs included, 20 included blinding for assessors for at least one of the study outcomes. Method of allocation concealment was considered adequate in 11 of the trials, with unclear reporting in the others. Most trials had small sample sizes and short durations.
Most trials reported measures of restenosis in terms of rates of restenosis or patency, although there was some variation of definitions of patency or restenosis, making direct comparison difficult. Direct comparison was also limited by differences in lesion types between trials. Most trials reported complications or adverse events for the procedures. Some trials reported the need for reintervention and clinical symptoms, and a few trials reported walking capacity or QoL. Not all outcomes were reported for all technologies. Most trials had a majority of IC participants with few CLI participants. Most of the trials recruited participants requiring angioplasty to the superficial femoral arteries or femoropopliteal arteries.
There was evidence of a significant benefit to reducing restenosis rates for SES, stent-graft, EVBT and DCB compared with PTA and for DES compared with BMS. In addition, significantly lower rates of the need for reintervention were reported for DCB, as well as a significant benefit in clinical stage for stent-graft, and a significant benefit to walking capacity at up to 1-year follow-up for SES compared with PTA. PTA was reported as having a significant advantage over AMS for restenosis rates, and over cryoplasty in terms of the need for reintervention.
No significant differences for restenosis rates between technologies and PTA were reported for BES, atherectomy, CB, cryoplasty, EBRT and laser angioplasty. There were also similar results between treatment groups in terms of the need for reintervention for AMS, SES, BES, DES, CB, EVBT and EBRT and in terms of measures of clinical symptoms for SES, DES, atherectomy, cryoplasty, EVBT, EBRT and DCB. Walking capacity did not differ significantly between PTA and BES or EVBT; nor was QoL found to differ significantly between SES and PTA. None of the studies reported significant differences between groups for procedural complications.
A Cochrane review of RCTs regarding stents for IC found no significant advantage for stents over PTA. 72 However, as this was restricted to trials of IC alone, the Cochrane review included only two RCTs of BESs72 (Grimm et al. 21 and Vroegindeweij et al. 23), meaning the lack of positive findings for BESs in this report concurs with the findings of the Cochrane review. 72 Another Cochrane review looked at RCT regarding stents for superficial femoral artery lesions73 and reported a small but statistically significant improvement in patency at 6 months, but non-significant improvement at 12 and 24 months. This Cochrane review included six RCTs of BESs (Becquemin et al. ,19 Cejna et al. ,20 Grenacher 2004,74 Grimm et al. ,21 Vroegindeweij et al. ,23 Zdanowski et al. 24) and two RCTs (FAST,14 ABSOLUTE16–18) of SESs. 73 A systematic review of stents in femoropopliteal lesions75 found a non-significant trend favouring stents for restenosis rates; however, this was based on combining SES, BES and stent-graft trials.
The positive finding for SESs in this report concurs with ESC guidelines,2 which recommend primary nitinol stenting as the first-line intervention for intermediate length, superficial femoral artery lesions. ESC guidelines2 recommend that, for infrapopliteal arteries, stents are used where PTA has been suboptimal, although they refer to favourable outcomes for DES based on evidence from a non-randomised study. ESC guidelines also suggest that, owing to difficulties in producing RCTs for the rapidly developing endovascular treatment options, IC and CLI patients undergoing angioplasty should be entered into a clinical surveillance programme. 2
Chapter 4 Assessment of cost-effectiveness
Systematic review of existing cost-effectiveness evidence
Searches
A systematic literature search was undertaken to identify economic evaluations of techniques used as an adjunct to, or as a replacement for, PTA in people with PAD.
The methods of the search strategy used (including inclusion and exclusion criteria) and databases searched are the same as those for the assessment of clinical effectiveness, as described in Methods in Chapter 3. Key details are reproduced in Table 54.
| Study design | Cost–consequence analysis, cost–benefit analysis, cost-effectiveness analysis or cost–utility analysis |
| Population | Patients with PAD (any type) |
| Comparator | PTA |
| Interventions | BMSs, DESs, stent-grafts, atherectomy, cryoplasty, radiation therapy, CB, DEB, laser angioplasty |
| Outcome | Cost-effectiveness |
Results
The literature searches identified 1306 potentially relevant citations. Of these, only 102 appeared to relate to an economic evaluation comparing the use of PTA with an alternative in the treatment of PAD. In total, 16 full papers were screened, only one of which (Sculpher et al. 76) met the inclusion criteria. The other 15 studies were excluded for being abstracts (five), being in a foreign language (two), relating to an excluded population (coronary; one) or having only an excluded comparator (seven). Figure 22 shows the summary of the study selection and exclusion employed. The studies accepted were evaluated using both the Drummond–Jefferson quality assessment criteria and CHEC-list criteria (details of this evaluation are presented in Appendix 6).
FIGURE 22.
Summary of economic evaluation selection and exclusion.
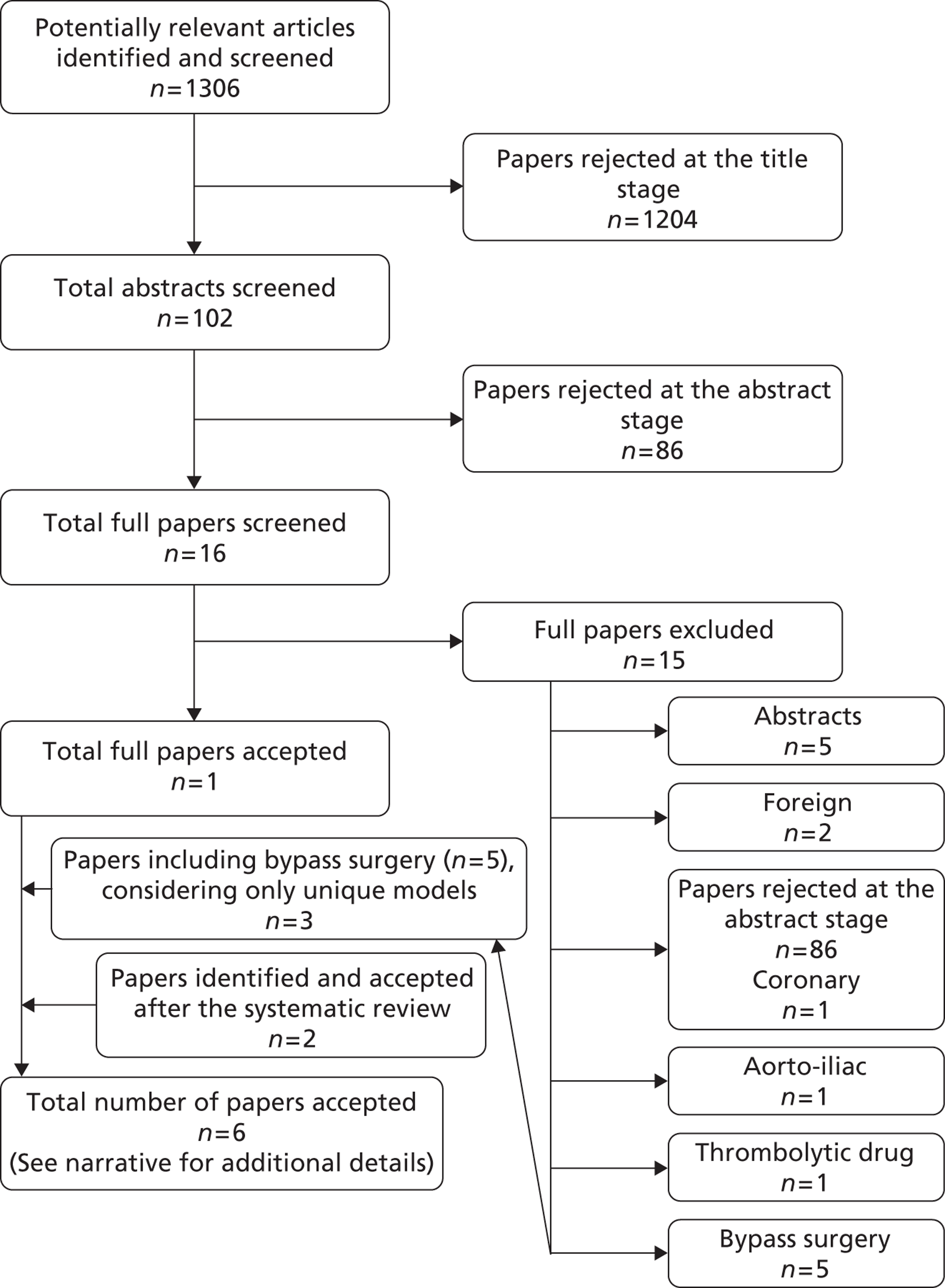
As only one study was identified, the inclusion criteria were relaxed to also include bypass surgery (BS) as an intervention. This intervention was included because it was decided that BS should be considered as a possible second-line treatment (following failure of the initial treatment). The only other second-line treatment considered was PTA. This identified a further five economic evaluations (Hunink et al. ,77 de Vries et al. ,78 Holler et al. ,79 Muradin and Myriam Hunink,80 Visser et al. 81). In addition, two further economic evaluations were manually identified: the BASIL trial (Forbes et al. 82) and the National Institute for Health and Care Excellence (NICE) cost-effectiveness analysis (CEA),83 which is part of the draft NICE guidelines on lower limb peripheral arterial disease (released for consultation). Neither of these evaluations was available at the time of the original systematic review. The research team were aware of the pending NICE guidelines; when they were released for consultation, they were used to identify the journal article by Forbes et al. 82
In two instances, two economic evaluations were generated based on the same underlying model. In the first instance, Muradin and Hunink80 use the model of Hunink et al. 77 to look at the cost-effective price required for a hypothetical new endovascular device. In the second instance, Visser et al. 81 extended the economic evaluation of de Vries et al. 78 to include diagnostic imaging. In both instances the extended evaluations are not of relevance to this study and thus only the original evaluation is considered.
In total, six existing economic evaluations were used to inform this economic evaluation; they are briefly summarised below.
Hunink et al.77
-
Indication: IC and CLI.
-
Lesion type: stenosis and occlusion.
-
Site: femoropopliteal.
-
Comparator: PTA.
-
Interventions: BS or no treatment.
-
Costs: 1990 US dollars.
-
Health utilities: Torrance multiattribute scale.
(1990 US dollars presented in Hunink et al. 77 These values were updated to 1999 US dollars in Muradin and Hunink. 80)
Together the comparator and interventions constitute three treatments. Six specific treatment strategies were compared. Initial PTA could be followed by any of the three treatments. Initial BS could be followed only by NT or graft revision. No treatment completed the strategies under consideration. A maximum of two treatments per patient were modelled.
The authors used a patient-level ‘multistate transition model’ using a lifetime horizon programmed in Borland C (Borland, Scotts Valley, CA, USA). The perspective was that of the health-care system. Input and results were disaggregated by lesion type and graft material. CLI was subdivided into ‘rest pain’ and ‘necrosis’.
Costs for repeat procedures are assumed to be equal to the initial procedure cost. Annual follow-up costs are also provided, depending on whether or not the patient maintained patency, or if they had an amputation.
Quality of life was based on the Torrance multiattribute scale as valued by two vascular surgeons, two interventional radiologists and an internist. Utility values are based on the patient’s indication, and are also altered if the patient receives successful treatment or if the patient receives an amputation. These states are further divided depending on whether or not major morbidity (see below) is present. Procedure-specific decrements are also applied.
Initial success and patency rates are based on a previous systematic review. Disease progression was not modelled. Operative mortality rates were based on 26 studies, and depended on the type of operation and whether or not the patient was ‘high risk’ – defined as being aged 65 years and over with CLI and/or documented coronary artery disease. Procedure-related complications were modelled as the development of non-fatal systemic morbidity (which includes major cardiopulmonary, renal or cerebrovascular complications). Long-term mortality was modelled as an excess per cent, based on the ABPI (2% above the annual risk for individuals with ABPI > 0.3, 12% above the annual risk for individuals with ABPI ≤ 0.3). For a sensitivity analysis, a RR of 3.1 is used, regardless of indication.
Based on an incremental cost-effectiveness ratio (ICER) threshold of US$50,000 per quality-adjusted life-year (QALY), the authors come to the following conclusions:
-
Initial PTA is recommended for all patients with stenoses, and claudicants with occlusive lesions.
-
Initial BS is recommended for patients with both CLI and occlusions.
The authors only presented selected results. QALYs gained range from 2.7 to 7.4 for stenosis and 2.6 to 7.0 for occlusions. Costs range from US$15,000 to US$43,000 for stenosis and US$24,000 to US$51,000 for occlusions.
A variety of univariate sensitivity analyses were performed, with most of the parameters varied according to observed ranges within the literature. Multiway sensitivity analyses considered ‘optimistic’ and ‘pessimistic’ scenarios. Results were found to be most sensitive to procedural mortality and morbidity rates.
Sculpher et al.76
-
Indication: IC and CLI.
-
Lesion type: occlusions.
-
Site: not stated.
-
Comparator: PTA.
-
Interventions: PTA with laser-assisted PTA on acute failure.
-
Costs: 1993/94 UK pounds.
-
Health utilities: European Quality of Life-5 Dimensions (EQ-5D) used; SF-36 values also available.
(Intervention uses data from a study on disease in the femoropopliteal arteries.)
For this cost–utility analysis, a two-part model was used, with a decision tree for initial revascularisation outcomes; these outcomes are then used as the starting health states in a Markov model that employed a lifetime horizon. Only the decision tree explicitly modelled the effects of the intervention. With the exception of death, all probabilities in the decision tree were taken from a single RCT (Lammer et al. 68). This RCT also showed that primary laser-assisted PTA was dominated by primary PTA, so this intervention was not considered.
If the initial operation (with or without laser assistance) failed, then patients may have BS and/or an amputation. There does not seem to be a limit on the number of BS operations that a patient may receive; in addition, patients with IC are able to receive repeat PTA (with or without laser assistance). Bilateral disease is not considered.
A crucial limitation concerning the cost-effectiveness data is that the cost-effectiveness of the laser when used as a secondary intervention (on immediate failure) is based on only seven patients. Long-term cost-effectiveness is based on a Markov model with a cycle length of 1 month, with a time horizon of 25 years. Disease progression was not modelled. General mortality is based on a Gompertz function, adjusted for an increased RR owing to having PAD (RR = 2 for IC and 3 for CLI). All other transition probabilities are independent of time and based on a mixture of published studies, an audit of patients’ notes at John Radcliffe Hospital in Oxford (where a co-author worked) or the clinical judgement of one of the co-authors. The paper does not state which probabilities came from which source. Procedure-related mortality is dependent on indication (IC or CLI) for BS but not for PTA. The secondary use of the laser is assumed not to result in any procedure-related deaths. Procedure-related complications are not modelled.
Utility values were elicited for four health states (IC, CLI and amputation above/below the knee) using both the time trade-off (TTO) method and the EuroQol visual analogue scale (EQ-VAS). Values for successfully treated patients were assumed to equal one. Two samples were used during elicitation: one of 36 health-care professionals (with a 100% response rate), and a random sample of the public (size not stated). As the values elicited were very similar for the two samples, only the results from the latter are used. In the base case, TTO values were used, with EQ-VAS values used in a sensitivity analysis; this did not have a noticeable impact on the results.
Costs are broken down into one-off costs based on procedure type (with an additional cost of angiography for any procedures during the Markov model) and monthly costs based on health state (cured, IC, CLI, amputee). For one-off costs, a breakdown of inpatient and outpatient costs is presented.
For each indication, the numbers in each health state and the numbers receiving a repeat operation (PTA or BS) are presented in 5-yearly increments.
For IC, the secondary use of a laser increases life-years from 6.78 to 6.79 and QALYs from 5.78 to 5.87, while increasing cost from £3669 to £3929. This gives an ICER of £3040 per QALY.
For CLI, the secondary use of a laser increases life-years from 5.44 to 5.46 and QALYs from 4.40 to 4.46, while increasing cost from £8716 to £8823. This gives an ICER of £1180 per QALY.
Sensitivity analyses showed that the following uncertainties had the greatest effect on results:
-
The proportion of patients ‘cured’ following a successful operation (assumed = 100%).
-
Annual utilisation of the laser (affecting its cost per operation).
-
The proportion of patients in whom CLI recurs after reocclusion (assumed = 100%).
-
Patency rates following PTA among CLI patients.
-
The effectiveness of the laser.
de Vries et al.78
-
Indication: IC.
-
Lesion type: stenosis and occlusion.
-
Site: above tibia.
-
Comparator: PTA.
-
Interventions: exercise and bypass surgery.
-
Costs: 1995 US dollars.
-
Health utilities: EQ-5D.
The authors compared five different treatment strategies involving sequences of exercise and PTA. BS was also included as an option in some of the strategies when PTA was deemed to be unsuitable. Exercise is an excluded intervention in our research, so results for this are not discussed here. The study presented an in-depth breakdown of outcomes for PTA and BS, broken down by site and lesion type, which are discussed here.
The authors presented results at both the aortoiliac level and the femoropopliteal level; the latter are of interest for this report. Rates of procedural mortality and systemic complications are taken from Hunink et al. ,77 as previously described. In addition, de Vries et al. 78 also include rates for angiographic investigations. For an amputation, mortality rates are presented separately for patients below and above the age of 75 years; rates for systemic complications were assumed not to vary with age.
As regards patency data, only 2-year results are provided. These are broken down by indication and intervention (PTA or BS). For BS, there is a further subdivision by graft type and, for PTA, there is a further subdivision by lesion type. The values used for the femoropopliteal level are taken from Hunink et al. 77
Health utility values for amputation and CLI are taken from Sculpher et al. 76 Values for IC and asymptomatic disease are taken from two other studies. Utility values associated with systemic complications are based on reported values for myocardial infarction survivors. Costs are taken from a mixture of published studies and the Medicare database. They are different from the costs used in any of the previous economic evaluations.
Holler et al.79
-
Indication: CLI.
-
Lesion type: occlusions. (Not entirely clear.)
-
Site: not stated.
-
Comparator: PTA.
-
Interventions: BS, prostaglandin E1 (PGEI) or no treatment.
-
Costs: euros, year not stated.
-
Health utilities: EQ-5D.
This study looked at treatment strategies, with patients able to experience a maximum of two treatments. As PGE1 is an excluded intervention, only the information provided for PTA and BS are considered.
Cost-effectiveness data were based on a systematic review of German- and English-language literature. Values from studies were weighted by their sample size and the median value was taken. The probability of staying within the same health state (CLI or IC) is calculated based on the logical constraint that transition probabilities must sum to one. For patients with IC, the probability of dying was assumed to be the same as that for a 70-year-old German male (taken from life tables). Mortality rates vary depending on the initial treatment, unless a patient receives an amputation, in which case the probability of mortality is independent of initial treatment. It is assumed that patients with IC do not have an amputation.
Cost data are based on a survey of a patient sample (time period and setting not stated), which included 147 patients with IC, 92 with CLI and 40 who had had an amputation. Treatment costs are applied on a yearly basis, and are different for the two indications. The cost of an amputation is independent of the initial treatment.
Data on QoL are based on the EQ-5D questionnaire given to a sample of 280 patients with PAD. Separate values are given depending on initial treatment and indication. As with cost, the value for having had an amputation is independent of the initial treatment.
The BASIL trial (Forbes et al.82)
-
Indication: Severe ischaemia. (CLI, but without the restriction that ABPI < 50 mmHg.)
-
Lesion type: stenosis and occlusion.
-
Site: infrainguinal.
-
Comparator: PTA.
-
Interventions: BS.
-
Costs: 2006/07 US dollars.
-
Health utilities: EQ-5D used; SF-36 values also available.
This is the only economic evaluation that was conducted alongside a clinical trial (ISRCTN 45398889). Detailed 12-month outcomes for the BASIL trial have been published (Bradbury et al. 84). Data from further follow-up have been presented in a number of publications (Forbes et al. ,82 Bradbury et al. 85–88).
The authors note that their inclusion criteria are different from the technical definition of CLI, but it was felt by our clinical expert (JAM) that they reflect CLI as defined in every-day practice, and thus the results of the BASIL trial are assumed for this evaluation to apply to CLI patients.
Between August 1999 and June 2004, the BASIL trial randomised 452 patients to a treatment strategy of either PTA first or BS first. There were a small number of crossovers; the economic evaluation uses an ITT analysis.
All of the data used in the model come from the BASIL trial. QoL was measured using the Vascular Quality of Life Questionnaire, the generic SF-36 health survey and EQ-5D. The EQ-5D is used within the economic evaluation. Cost data are based on hospital-related activity only. Both costs and utilities are discounted at 3.5% per annum.
Statistical regression methods were used to calculate incremental costs and incremental QALYs, with non-parametric bootstrapping used to assess uncertainty. As the costs data exhibited a heavy skew, the results from three different regression methods were reported. These are reproduced in Table 55, along with the corresponding ICERs. Although the results are not presented in UK pounds, it is clear that BS would not be considered cost-effective by decision-makers such as NICE using any of the three methods given current, and historic, exchange rates.
| BS vs. PTAa | Least squares | Robust regression | Median regression |
|---|---|---|---|
| Incremental costs | 5521 | 9132 | 11,507 |
| Incremental QALYs | 0.03 | 0.03 | 0.03 |
| Incremental cost per QALY | 184,492 | 304,400 | 383,567 |
The National Institute for Health and Care Excellence cost-effectiveness analysis83
-
Indication: IC.
-
Lesion type: stenosis and occlusion.
-
Site: iliac/femoropopliteal. (Analysed separately; only the latter is considered here.)
-
Comparator: PTA (with selective stenting).
-
Interventions: PTA (with primary stenting), unsupervised exercise, supervised exercise, BS.
-
Costs: 2009/10 UK pounds.
-
Health utilities: EQ-5D.
This economic evaluation considered two-stage treatment strategies. BS is considered only as a second-line treatment (giving four different first-line treatments). Neither PTA with primary stenting nor unsupervised exercise is considered as a second-line treatment (giving three different second-line treatments), resulting in (3 × 4) 12 different treatment strategies. A 13th strategy of PTA with selective stenting and supervised exercise (and no secondary treatment) is also evaluated.
A Markov model is employed using 3-monthly cycles. The analysis takes the perspectives of the NHS and personal social services. Both costs and QALYs are discounted at 3.5% per year.
Procedural costs (for PTA, BS and amputation) were taken from 2009/10 NHS Reference Costs. 89 For PTA and BS, the proportion of procedures that were elective or non-elective was based on expert opinion, with slight differences between the initial and repeat procedures. Ongoing costs were modelled only for patients who had undergone an amputation. Costs incurred in the first year were different from those incurred in follow-up years; both were based on a mixture of expert opinion and the 2010 Personal Social Services Research Unit. 90
Quality of life data for patients with IC were based on the values reported by the studies included in the evaluation. Only reports of EQ-5D or SF-36 (when sufficient data were available for them to be mapped to EQ-5D) were included, the final values used being the average of the included values. Data for patients with CLI or an amputation were taken from Sculpher et al. 76
It is assumed that PTA does not affect subsequent rates of mortality or morbidity and that repeat procedures have the same effectiveness as the initial procedure. Failure was taken to include both a loss of patency and symptom deterioration requiring reintervention. Perioperative complications, amputations and deaths were taken from an audit reported by the Royal College of Surgeon’s of England. 91 For patients with IC, there were no amputations or deaths. Based on expert opinion, these probabilities were felt to be non-zero, and therefore values of 0.5 amputations and 0.5 deaths were added to the numerator (and subtracted from the denominator) of the audit. Rates of failure and the amount of patients needing a reintervention are based on expert opinion and are modelled as fixed (time-invariant) amounts. The requirement for reintervention is assumed to vary depending on lesion type (stenosis or occlusion); the prevalence of lesions among patients with IC is based on expert opinion.
Progression to CLI was assumed to be independent of treatment strategy, with a 3-month probability of 0.1% (based on a value of 2% over 5 years). It was assumed that 25% of patients with CLI will receive an amputation as a primary intervention and that 25% will die each year (modelled by 3-month probabilities of 6.9% and 3.9%, respectively).
For patients with IC and femoropopliteal disease, the NICE CEA concluded that there were only four treatment strategies that were neither dominated nor extendedly dominated. These are detailed in Table 56.
| Strategy | Total cost (£) | Incremental cost (£) | Total QALYs | Incremental QALYs | Cost-effectiveness (£) |
|---|---|---|---|---|---|
| UE | SE | 4059 | Baseline | 4.374 | Baseline | Baseline |
| SE | SE | 4276 | 217 | 4.466 | 0.092 | 2362 |
| SE | PTA | 5378 | 1102 | 4.534 | 0.069 | 16,024 |
| PTA | PTA | 6603 | 1225 | 4.572 | 0.037 | 32,898 |
Summary
There are currently no economic evaluations that include all of the relevant interventions considered in this report. There is only one economic evaluation (Sculpher et al. 76) that includes any of the relevant interventions, but this includes only a subgroup of the relevant population. A de novo economic evaluation is therefore required.
Independent economic assessment
Methods
This section provides details of a model developed by the assessment team and used to evaluate the cost-effectiveness of enhancements to angioplasty in the treatment of PAD.
Model description
A discrete-event simulation model (DESM) was developed in Simul8© 17.0 (Simul8 Corporation, Boston, MA, USA) to determine the cost-effectiveness of each enhancement compared with conventional angioplasty alone. A DESM was used in preference to a state-transition model primarily because of the large number of patient characteristics that required tracking over time. A DESM also more appropriately models time to event based on stochastic distributions.
Patient population
The population considered was patients with symptomatic PAD suitable for endovascular treatment for disease distal to the inguinal ligament. A lifetime horizon was used.
The patient population was subdivided into those with IC and those with CLI. The clinical classifications of these subgroups are presented in Table 57.
| Clinical stage (indication) | Fontaine classification | Rutherford classification | Classification used in this evaluation | |
|---|---|---|---|---|
| Grade | Category | |||
| Asymptomatic | Stage I | 0 | 0 | Asymptomatic |
| Mild claudication | Stage II | I | 1 | IC |
| Moderate claudication | 2 | |||
| Severe claudication | 3 | |||
| Ischaemic rest pain | Stage III | II | 4 | CLI |
| Minor tissue loss | Stage IV | III | 5 | |
| Major tissue loss | 6 | |||
Differences in anatomical features were not explicitly modelled. These include features such as proximity to bifurcations, stenosis versus complete occlusions and length of occlusion. These differences were not considered because of a lack of available evidence for the comparator and interventions.
With two exceptions, the effectiveness of all interventions was evaluated in the femoropopliteal arteries. The exceptions were BMSs, which were evaluated in both the femoropopliteal and infrapopliteal arteries, and sirolimus-eluting stents, which were evaluated in the infrapopliteal arteries. As base-case data (for PTA) were available only for the femoropopliteal arteries, the results of evaluations considering the infrapopliteal arteries should be viewed as exploratory.
Interventions and comparators
The base-case analysis considers patients receiving conventional PTA with secondary bare-metal stenting if immediate (acute) failure occurs. Acute failure is defined as either failure of the operation or restenosis within 30 days of the operation. The interventions considered for this research are listed and described in Clinical effectiveness results in Chapter 3 on clinical effectiveness. Based on the results of the clinical effectiveness research, it was decided that there would be little value in including some of the interventions in the economic evaluation, as they were likely to be dominated by either the base case or a comparator (as they were less effective and likely to be more costly). Explicit costs for these comparators were not calculated; instead, it was noted that, because they are all enhancements to PTA, they will be more expensive than PTA. Hence, the following interventions were immediately excluded in the assessment of cost-effectiveness (the sections describing their clinical effectiveness can be found in Clinical effectiveness results in Chapter 3):
-
AMSs
-
atherectomy
-
EBRT
-
laser angioplasty.
No distinction was made between SESs and BESs, as (in general) use of the former has replaced use of the latter. As with the NICE CEA, the use of either of these stents is referred to as use of BMSs. CBs were not included in the economic evaluation, as they were recalled by their manufacturer because of a potential shaft separation of the catheter during operation (www.fda.gov/MedicalDevices/Safety/RecallsCorrectionsRemovals/ListofRecalls/ucm062951.htm referenced in White and Grey92).
The two patient populations (IC and CLI) are analysed separately. Owing to a lack of evidence, the treatment effect of each intervention is assumed to be the same for the two patient populations. It should be noted that in most trials the majority of participants have IC. Natural history data for the two patient populations (for example, patency rates for the comparator and time to amputation) vary. The effectiveness of BS (modelled as a second-line treatment) also varies by patient population.
Each intervention may be used as the initial treatment instead of (or with) PTA, with secondary stenting if required. In addition, the use of conventional PTA with secondary DESs (paclitaxel) was also reported in one study (Dake et al. 71). Because of this, paclitaxel-eluting stents were included as two interventions: one for their use as the initial treatment (no secondary stenting was required, so no distinction is made for this intervention) and one for their use only on acute failure.
To summarise, the base-case comparator and included interventions in the femoropopliteal arteries are:
-
PTA with secondary BMSs (base case)
-
primary BMSs
-
PTA using a DCB
-
primary DESs (paclitaxel)
-
PTA with secondary DESs (paclitaxel)
-
stent-graft
-
cryoplasty
-
EVBT.
In the infrapopliteal arteries they are:
-
PTA with secondary BMSs (base case)
-
primary BMSs
-
primary DESs (sirolimus).
Outcomes
The main model outcome is the incremental cost per QALY gained. A secondary outcome of incremental cost per life-year gained is also presented.
Model structure
The structure of the decision model is presented in Figure 23. Events are modelled such that each event triggers changes in a patient’s health state. Patients can enter the model with either one leg or two; if the patient has two legs, the status of both legs is modelled. For simplicity, on receiving an amputation (to either leg), the only possible events for a patient are procedure-related death or general mortality. Patients can enter the model with either IC or CLI; these two groups are modelled and analysed separately.
FIGURE 23.
Diagram of the structure of the decision model.
For illustrative purposes, Figure 23 has been depicted with three groups of events, entitled ‘30-day outcomes’, ‘While patent’ and ‘On loss of patency’. On entering a group, the time to each event (or the probability that it occurs) is calculated, with the event occurring first being the next simulated event.
For example, upon entering the ‘On loss of patency’ group, the time to develop contralateral symptoms and experience disease progression and time to general mortality are all calculated. The probabilities of requiring and receiving a reintervention are also calculated and compared with random numbers (drawn from the uniform distribution on [0,1]) to see if they occur. If a patient were modelled as receiving a reoperation, this is given a time to event of 1 week. The event with the shortest time to occurrence then becomes the next simulated event.
In the following discussion, a reoperation refers to receiving PTA or BS only; it does not include receiving an amputation. For the purposes of brevity, the term ‘reoperation’ is also used to include the situation in which an individual receives an operation on a contra-lateral limb while the first limb remains asymptomatic.
The structure of the model from the perspective of a patient is presented in Figure 24, which shows the health states modelled. Patients enter the model with either IC or CLI. It is assumed that after a successful operation patients move into the asymptomatic health state, where they remain until they either die or suffer a loss of patency (failure). If a failure occurs, then, as with Sculpher et al. 76 and Hunink et al. ,77 it is assumed that the patient returns to their health status prior to the operation.
FIGURE 24.
Diagram of the health states modelled.
As with Sculpher et al. 76 and Hunink et al. 77 it is assumed that spontaneous improvement from CLI to IC (in the absence of an operation) does not occur. It should be noted that, if a patient’s operation fails (at any time), then they return to their health state prior to the operation, not their health state when entering the model. This affects IC patients; if they progress to CLI, then it is not possible for them to enter the IC health state again.
Patients may also develop contralateral symptoms (PAD in their other leg), so, for example, a patient with CLI in one leg may also develop IC in their other leg. As the status of each leg is tracked separately, Figure 24 actually represents the health states (and permissible transitions) for each leg.
Patients enter the model when undergoing their initial endovascular operation (which varies depending on the intervention or comparator considered). During the 30 days following an operation (the perioperative period), the following events may occur:
-
mortality attributable to the intervention or comparator
-
mortality not attributable to the intervention or comparator (general mortality)
-
acute failure (loss of patency)
-
success; defined as none of the above.
In addition, there is a probability of the patient developing a complication during the perioperative period. This is assumed to result in a (ongoing) utility decrement and cost, but it does not affect subsequent transitions, so it is not modelled as a separate event. Once a patient develops a complication, it is assumed that they remain with this complication for life.
General mortality is taken from life tables. 93 Mortality attributable to the intervention or comparator is not removed from the life tables, as the numbers are small. The life tables are adjusted to reflect an increased RR of dying due to having PAD. Separate RRs are modelled for IC and CLI. It is assumed that this excess risk remains even if a patient experiences a successful operation, as it is based on the patient’s disease prior to the operation.
If the initial operation is a success, then the patient moves into the asymptomatic PAD health state, and postprocedural events-while-patent are modelled:
-
Late failure (loss of patency).
-
Develop contralateral symptoms: these may be either IC or CLI and are influenced by the patient’s disease prior to their last operation.
-
Amputation: this is the risk of amputation owing to progression of disease in the limb (not owing to the result of loss of patency at the treated site).
-
General mortality.
If a patient suffers a failure (loss of patency) at any time point, then the following events are possible:
-
A reintervention (PTA, BS or amputation) is required and received.
-
The patient’s disease progresses to CLI. (This is applicable only for patients with IC.)
-
The patient develops contralateral symptoms (as previously described).
-
General mortality.
There are three situations for which a patient may require a reoperation:
-
After loss of patency, a proportion of patients are modelled as experiencing the (immediate) return of symptoms. If symptoms do return, then the patient requires a reintervention.
-
The patient develops contralateral symptoms.
-
The patient experiences disease progression (to CLI).
It should be noted that loss of patency on its own is not sufficient to require a reoperation; the patient must also experience a return of symptoms. In contrast, the data used to inform the transition probabilities for developing contralateral disease or disease progression both imply that a reoperation will be required as a result of the event.
If patency is lost, the probability of experiencing a return of symptoms is modelled as an immediate event and is independent of the type of operation received. It does, however, depend on the patient’s health state prior to the operation. If symptoms do return, then the patient moves into their health state prior to the operation. If symptoms do not return (but patency is lost), then patients with prior IC remain in the asymptomatic health state, but patients with prior CLI return to the CLI health state. This is because there is evidence to suggest that alternative forms of therapy (exercise and pharmacotherapy) are effective in improving the QoL of patients with IC but not patients with CLI. 83,94
There are three situations for which a patient may require a reoperation but not receive it (for further details, see the section ‘Probability of reintervention following failure’):
-
The patient dies before the operation is received.
-
The assumed maximum number of permissible reoperations (two) has already been reached.
-
The patient’s lesion is not suitable for reoperation.
Patients may receive an amputation at any time, with higher rates of amputation observed for patients with CLI. It is assumed that the low risk of amputation for those with IC is a result of the progression of disease, rather than being directly attributable to restenosis at the site of the original lesion causing IC. The model does not distinguish between below-knee and above-knee amputations but uses average costs and utilities for amputation based upon the proportions in the BASIL Trial. 84
Time horizon, perspective and discounting
The time horizon of the model was 100 years to ensure that all differences in costs and benefits are captured within the model. The analysis takes the perspectives of the NHS and personal social services. Both costs and QALYs were discounted at a rate of 3.5% per year.
Assessment of cost-effectiveness
The main results are an estimate of the lifetime costs and total QALYs of each intervention and the comparator, and the ICERs, presented as cost per QALY gained and cost per life-year gained. Results are reported separately for the IC and CLI populations. In incremental analyses, one intervention may be dominated or extendedly dominated by another. Dominance is defined to occur when an intervention is less effective and more expensive than another intervention. Extended dominance is defined to occur when the ICER for a given treatment alternative is higher than that of the next most effective intervention. 95 To estimate costs and QALYs, 1000 probabilistic sensitivity analysis runs were implemented. A cost-effectiveness acceptability curve (CEAC) and a cost-effectiveness plane are included to give a measure of the uncertainty incorporated into the model. To explore the sensitivity of the model results to parameter values and assumptions, a range of univariate sensitivity analyses were performed. These sensitivity analyses are:
-
exploring the sensitivity of the results to different starting ages
-
no amputation-related costs
-
assume no intervention effect except for lower reintervention rates
-
results for the infrapopliteal arteries.
Estimate of base-case model parameters
Details of the parameters used, their distributions and their sources are discussed on the following pages and summarised in Tables 58–61. Additional details are provided in Appendix 7. For the probabilistic sensitivity analyses, all parameters were independently sampled.
| Parameter | Value | Range used in probabilistic sensitivity analysis | Source |
|---|---|---|---|
| Starting age | 66 | Fixed | Bergqvist et al. 199896 |
| Mortality: RR (compared to general population) | 3.1 | Fixed | Criqui et al. 199297 |
| PTA failure | |||
| Perioperative period | 6.6% | Beta(42,592) | Hunink et al. 199498 |
| Year 1 | 20.7% | Beta(115,443) | Hunink et al. 199498 |
| After year 1 | Weibull (1.415, 17.923) | Only varying beta parameter: Beta(5.35,1.65) – scaled to be between 9.97 and 20.38 | Hunink et al. 199498 |
| Complications during PTAa | 0.51% | Beta(4.29,841)a | Axisa et al. 200291 |
| 30-day mortality following PTA | 0.2%b | Log-normal[ln(0.2),1.30] | Hunink et al. 199577 |
| BS failure | |||
| Perioperative period | Fixed probability (0%) | Beta(0.5,1194.5) | Hunink et al. 199498 |
| Post-perioperative period | Weibull (0.612, 59.607) | Only varying beta parameter: Beta(5.35,1.65) – scaled to be between 33.17 and 67.77d | Hunink et al. 199498 |
| 30-day mortality following BS | 0.8%b | Log-normal(ln[0.8],0.70) | Hunink et al. 199577 |
| Probability of requiring reintervention following failurec | 28.27% | Beta(62.44,160.56) | NICE CEA 201283 |
| Probability of not being suitable for a reintervention | 5% | Beta(5,95)d | de Vries et al. 200278 |
| Time to amputation | Exponential (400) | Exponential parameter varied by ± 20%d | TASC-II,99 ACC/AHA100 |
| Annual rate of progression to CLI | Exponential (28.65) | Exponential parameter varied by ± 20%d | Sculpher et al. 199676 |
| Parameter | Value | Range used in probabilistic sensitivity analysis | Source |
|---|---|---|---|
| Starting age | 74 | Fixed | Bergqvist et al. 199896 |
| Mortality: RR (compared to patients with IC) | 2 | Fixed | Norgren et al. 200799 |
| PTA failure | |||
| Perioperative period | 23.9% | Beta(88,281) | Hunink et al. 199498 |
| Year 1 | 59.5% | Beta(193,131) | Hunink et al. 199498 |
| After year 1 | Weibull (1.369, 6.871) | Only varying beta parameter: Beta(0.75,6.25) – scaled to be between 6.12 and 13.12 | Hunink et al. 199498 |
| Complications during PTA | 6.75% | Beta(16,221) | Bradbury et al. 200584 |
| 30-day mortality following PTA | 3.2% | Log-normal(ln[3.2],1) | Hunink et al. 199577 |
| BS failure | |||
| Perioperative period | Fixed probability (0%) | Beta(0.5,1194.5) | Hunink et al. 199498 |
| Post-perioperative period | Weibull (0.608, 21.101) | Only varying beta parameter: Beta(0.75,6.25) – scaled to be between 18.81 and 40.30c | Hunink et al. 199498 |
| 30-day mortality following BS | 4.7% | Log-normal(ln[4.7],0.71) | Hunink et al. 199577 |
| Probability of requiring reintervention following failurea | 72.7% | Beta(72,27) | Bradbury et al., 200584 Hunink et al. 199498 |
| Probability of not being suitable for a reinterventionb | 1.55% | Beta(7,445) | Bradbury et al. 200584 |
| Time to amputation | |||
| Within first 2 years | Weibull (0.536, 7.239) | Only varying beta parameter: Beta(0.75,6.25) – scaled to be between 6.45 and 13.82c | Bradbury et al. 201086 |
| After 2 years | Exponential (4.86) | Exponential parameter varied by ± 20%c | Bradbury et al. 201086 |
| Parameter | Value | Range used in probabilistic sensitivity analysis | Source |
|---|---|---|---|
| RR of complications (BS vs. PTA) | 1.80 | Log-normal(ln[1.80],0.09) | Bradbury et al. 200584 |
| Mortality during amputation | |||
| Age < 75 | 9.8% | Normal(9.8,0.011) | de Vries et al. 200278 |
| Age ≥ 75 | 14.7% | Normal(14.7,0.017) | de Vries et al. 200278 |
| Annual rate of developing contralateral disease | Exponential (16.42) | Exponential parameter varied by ± 20%a | de Vries et al. 199878 |
| Probability that contralateral disease is CLI | Patient has IC: 10% | Beta(15,135) | de Vries et al. 200278 |
| Patient has CLI: 67% | Beta(257,126) | de Vries et al. 200278 | |
| Parameter | Value | Range used in probabilistic sensitivity analysis | Source |
|---|---|---|---|
| QoL data | |||
| IC (requiring intervention) | 0.70 | Normal(0.70,0.23/280) | Sculpher et al. 199676 |
| CLI (any) | 0.35 | Normal(0.35,0.23/280) | Sculpher et al. 199676 |
| Above-knee amputation | 0.20 | Normal(0.20,0.22/280) | Sculpher et al. 199676 |
| Below-knee amputation | 0.61 | Normal(0.61,0.20/280) | Sculpher et al. 199676 |
| Proportion of amputations above knee | 31.7% | Normal(0.70,0.23/280) | Bradbury et al. 200584 |
| Asymptomatica | Age-matched UK population norms | Fixed | Ara and Brazier 2010101 |
| Systemic complicationb | 0.72 | Log-normal(ln[0.72],0.10) | de Vries et al (2002)78 |
| Costs data | |||
| PTA – no complications | 3661 | Normal(3661,581) | NICE CEA 201283 |
| PTA – with complications | 9367 | Normal(9367,3079) | NICE CEA 201283 |
| BS – no complications | 5988 | Normal(5988,665) | NICE CEA 201283 |
| BS – with complications | 7139 | Normal(7139,882) | NICE CEA 201283 |
| Amputation – operation | 9224 | Normal(9224,923) | NICE CEA 201283 |
| Angiography – no complications | 2169 | Normal(2169,380) | NHS reference costs 2009/1089 |
| Angiography – with complications | 6270 | Normal(6270,1205) | NHS reference costs 2009/1089 |
| Monthly costs – IC | 102 | Normal(305,40.61)/3 | cSculpher et al. 199676 |
| Monthly costs – CLI | 321 | Normal(305,40.61) + Normal(14.56,1.13) × 13/12 | cSculpher et al. 199676 |
| Monthly costs – amputee | 1958.5 | Gamma(400,50.756) | NICE CEA 201283 |
| Monthly costs – complication | 141 | Fixed | NICE CEA 201283 |
Starting age
The starting age was based on data reported from the Swedish Vascular Registry,96 which was the only identified source that gave stratified estimates by indication (IC or CLI). The average (mean) age of a patient with IC receiving PTA was 66 years; for patients with CLI, the average age was 74 years. For comparison, where economic evaluations use (or state) their starting ages, for IC they range from 60 (de Vries et al. 78) to 67 (NICE CEA83). Neither Sculpher et al. 76 nor Hunink et al. 77 stratify their starting age by indication, both use a value of 65 years. Starting/average ages for CLI patients are not stated in the Holler et al. 79 and BASIL trials. 82
Starting age was not varied in probabilistic sensitivity analysis; the sensitivity of the base-case results to starting age was explored in a sensitivity analysis. For this, the variation reported in the Swedish Vascular Registry data96 was used to estimate a plausible range of ages. For patients with IC, a standard deviation of 10.4 about the mean of 66 was reported. For patients with CLI, a standard deviation of 9.3 about the mean of 74 was reported.
General mortality and excess risk
Patients with PAD were assumed to be at greater risk of general mortality than patients without PAD. In the majority of economic evaluations, this increased mortality was modelled as a RR, although the values employed, or suggested by the literature, can vary substantially. Further details are provided in Appendix 7.
Relative risks for IC (compared with the general population) vary from 1.6 to 4. The value of 3.1 quoted by Criqui et al. 97 is used for the base case; this source was also used by Hunink et al. 77 and in the NICE CEA. 83 It is also very similar to the value of 3.14 used by de Vries et al. 78
To ensure that patients with CLI do not have a lower probability of death than patients with IC, the RR of mortality for CLI is compared to that for IC. Two economic evaluations (de Vries et al. 78 and Hunink et al. 77) assume that this RR is 0; other values reported imply that the RR may be as high as 2.8. For the base case, a RR of 2 is used [based on data presented in the TASC-II (Trans-Atlantic Inter-Society Consensus II) guidelines (Norgren et al. 99)].
The RRs were included in the model by modifying 2009/10 UK life tables. 93
For the majority of the economic evaluations, it is not clear whether a successful operation reduces or removes any of this excess risk. The NICE CEA83 assumes that it does not reduce the risk at all, a view shared by our clinical expert (JAM). Hence, RRs after an operation (including amputation) remain the same as before the operation. It should be noted that this is a potential limitation; for example, for patients with CLI, successful treatment should remove some of the effects related to ischaemia of the limb.
Percutaneous transluminal balloon angioplasty failure
The meta-analysis of Hunink et al. 98 is employed in this evaluation; it was also used in four of the six economic evaluations. Of the alternatives, the NICE CEA83 bases its value on expert opinion and uses a fixed annual rate, whereas the BASIL trial82 reports failures only at 1 and 3 years. Further details of the Hunink et al. 98 meta-analysis are presented in Appendix 7.
Failure is defined as loss of patency; estimated failure rates over time are reproduced in Figure 25. To improve fit, failure during the perioperative period is modelled as a probability, as is failure during the first year (conditional on not failing during the perioperative period). Failure after the first year is modelled using a Weibull distribution (conditional on not failing during the first year).
FIGURE 25.
Percutaneous transluminal balloon angioplasty; cumulative failure rates over time for the two patient populations. Based on the meta-analysis of Hunink et al. 98
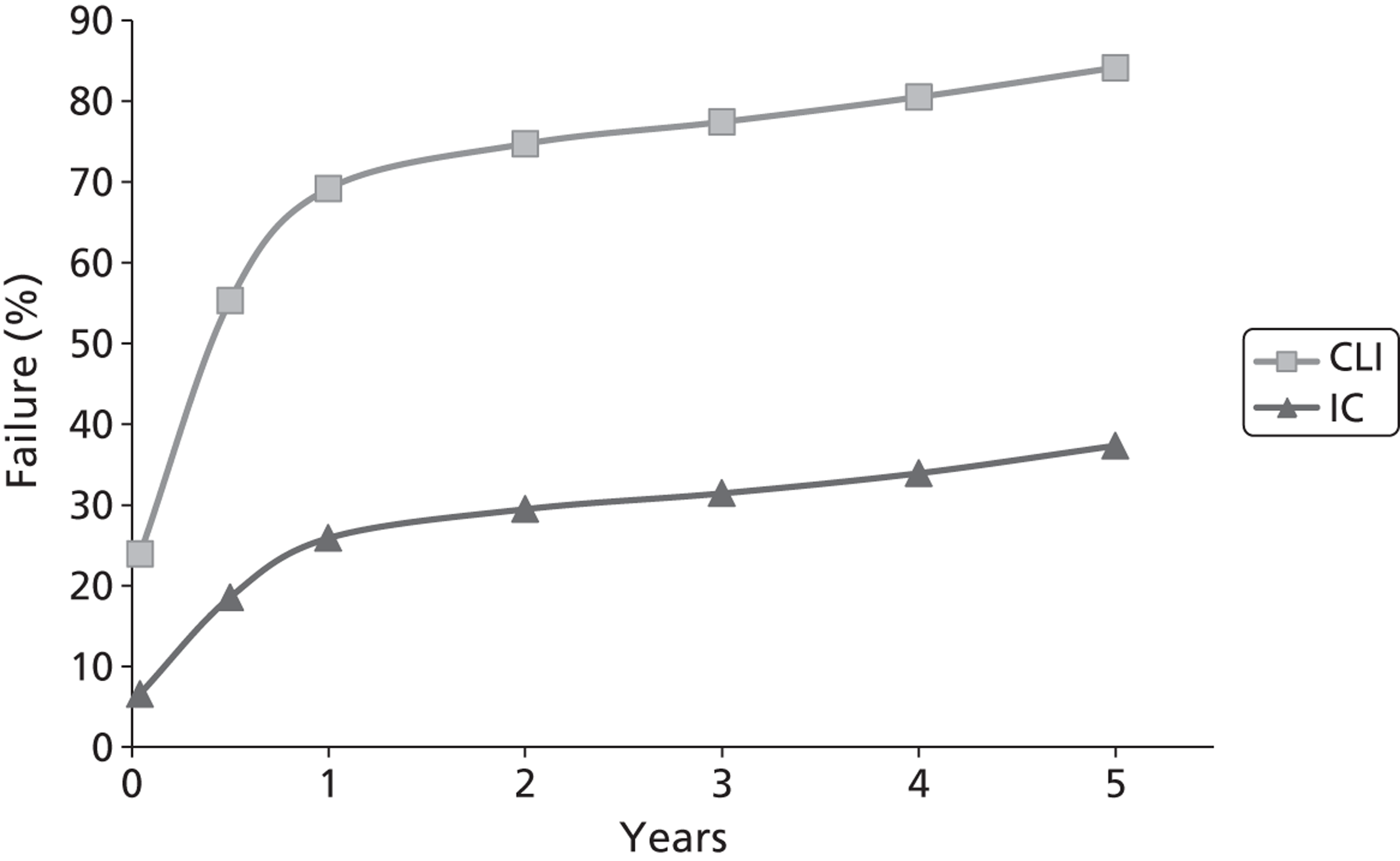
It should be noted that, if PTA fails during the perioperative period, then it is assumed that BS is always required and received. This assumption was employed in the economic evaluation of Hunink et al. ,77 and is used to reflect the fact that failures during the perioperative period usually indicate that repeat PTA would not be feasible (but it may be for longer-term failure).
30-day mortality following percutaneous transluminal balloon angioplasty or bypass surgery
Hunink et al. 77 provide the only economic evaluation to stratify mortality rates by patient status; this stratification is used within the model. Patients are deemed to be at ‘high risk’ of mortality if they are aged over 65, if they have a complication (as defined below) or if they have CLI; otherwise, they are at ‘low risk’ of mortality. High-risk patients have a probability of 30-day mortality following PTA of 3.2% and following BS of 4.7%. For low-risk patients, these values are 0.2% and 0.8%, respectively. It should be noted that, as the starting age of patients with IC is 65 years, all patients in the base case start with a high risk of mortality. The range of alternatives reported by Hunink et al. 77 is used to model uncertainty in these probabilities.
Complications during percutaneous transluminal balloon angioplasty or bypass surgery
A complication is defined as a non-fatal systemic complication (such as stroke, myocardial infarction and renal failure). For PTA, the BASIL trial (Bradbury et al. 84) is used to estimate the probability of a complication for the CLI population (6.75%), whereas the Royal College of Surgeon’s audit (Axisa et al. 91) is used for the IC population. These two sources are used because they reflect observed rates of complications. Axisa et al. 91 do not break down the complications by IC or CLI status, but the number of operations is broken down. This information is used with data from the BASIL trial84 to estimate a rate for IC (0.51%). For more details, see Appendix 7.
The number of complications reported by these two studies is reproduced in Table 62.
| Bradbury et al. 200584 (PTA and CLI) | Axis et al. 200291 (PTA and IC and CLI) |
|---|---|
| Sample: 237 | Sample: 717 |
| Stroke/TIA: 3 (1.3%) | Stroke/TIA: 1 (0.1%) |
| Angina: 5 (2.1%) | Renal failure: 5 (0.7%) |
| Myocardial infarction: 8 (3.4%) | Myocardial infarction: 5 (0.7%) |
| Bronchopneumonia: 6 (0.8%) |
Estimates of complications during BS were modelled using a RR of 1.80, taken from the BASIL trial. 84 Although these data only relate to patients with CLI, they were used as it was felt that they provided the most plausible estimates. See Appendix 7 for more details.
Bypass surgery failure
This is modelled using the same meta-analysis as was used to model PTA failure. 98 This source was also used in four of the six economic evaluations. Of the alternatives, the NICE CEA83 did not model BS failure, and the BASIL trial84 reports failures only at 1 and 3 years.
As with PTA, life table estimates of patency for patients with IC and stenosis were presented. For BS, there was no statistically significant difference in patency by lesion type (hazard ratio not reported), so only the RR associated with having CLI was employed. The results are presented in Figure 26.
FIGURE 26.
Bypass surgery; cumulative failure rates over time for the two patient populations. Based on the meta-analysis of Hunink et al. 98
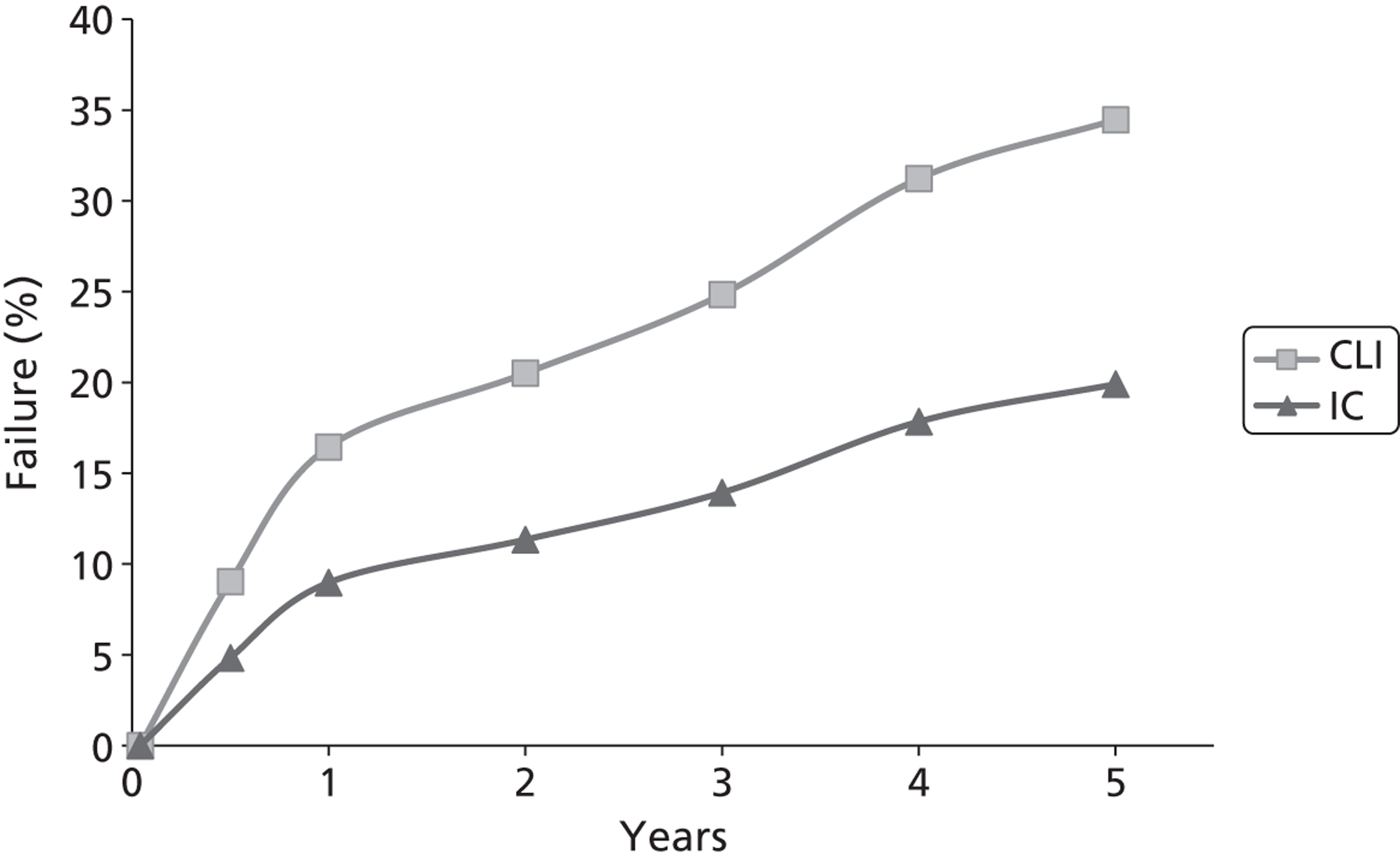
The meta-analysis98 also found that there was no difference between above-knee and below-knee operations, but that the type of graft material used affected the operation (with separate life tables presented for different graft types). Values for saphenous vein bypass are used in this analysis for two reasons:
-
This type of operation was the most frequent in the BASIL trial (76%; 136/179). 84
-
The NICE guidelines for PAD recommend using this type of graft when possible. 83
It should be noted that BS has a modelled perioperative failure rate of 0%.
Probability of reintervention following failure
It is assumed that, following failure during the perioperative period, a reintervention always occurs and it is always BS. If a patient experiences late failure, then three criteria must be met for a reintervention to take place:
-
Symptoms must return. For patients with IC, the values from the NICE CEA83 are used. It is assumed that 17.3% of patients with IC have an occlusion, as opposed to the 20% assumed by the NICE CEA83 (for more details, see Appendix 7: Percutaneous transluminal balloon angioplasty failure). This gives a probability of 28.3% that symptoms will return.
For patients with CLI there are no direct data on the probability of symptoms returning following failure. The BASIL trial84 details the number of patients whose symptoms return, but not the number who lost patency. Instead, the failure rates used in this model are applied to the BASIL data, giving a probability of 72.7% that symptoms will return.
-
The individual must be eligible for a reintervention. Patients may be ineligible either because they have already received the maximum allowable number of interventions or because they are deemed to be physically ineligible. Based on discussions with our clinical expert (JAM), the maximum number of interventions (including the initial operation) was set at three; this is also the same number as was used by de Vries et al. 78
Probabilities for being physically ineligible were taken from the only available evidence. de Vries et al. 78 assume that 5% of individuals with IC will be unsuitable for a reintervention. For patients with CLI, the most relevant data come from the BASIL trial,84 which states that, of 452 patients randomised to receive either PTA or BS, 14 did not receive any form of treatment. Removing the seven patients who did not receive a treatment because they died, the proportion ineligible is 1.55%.
-
The individual must not die before the planned reintervention occurs. Based on discussions with our clinical expert (JAM), it was assumed that the average time of reintervention following the return of symptoms was 1 week for patients with CLI and 1 month for patients with IC.
Type of reintervention and effectiveness
It is assumed that reinterventions are either PTA or BS. As previously mentioned, acute failure is always followed by a reintervention of BS. For the base-case analysis, reinterventions following late failure are always PTA; in a scenario analysis, this is changed to be always BS.
The BASIL trial86 is the only economic evaluation to compare the effectiveness of interventions when used as either the initial (first-line) or a follow-up (post-first-line) intervention. There was no evidence to suggest that there is any difference in patency rates between first-line and post-first-line interventions. This applied to both PTA and BS; for further details, see Appendix 7.
Disease progression
This is assumed to occur only following failure. Data from the analysis of Sculpher et al. 76 are used, as, of the evaluations that model disease progression, their assumptions regarding failure and disease progression are the closest to those used in this model. For further details, see Appendix 7.
Developing contralateral symptoms
Data were taken from an article by de Vries et al. ,100 as used in the economic evaluation of de Vries et al. 78 This is the only evaluation to consider contralateral symptoms. Values for contralateral symptoms requiring a reintervention are used, and adjusted to account for the fact that only 87% of contralateral symptoms (following infrainguinal disease) will also be in the infrainguinal arteries. de Vries et al. 78 state that, for patients with previous CLI, 67% of the contralateral symptoms are CLI (the rest being IC), whereas, for patients with previous IC, 10% of the symptoms are CLI. These values are used in this analysis. The development of contralateral symptoms is independent of the patient’s patency status.
Transition probabilities for a patient with CLI or IC are independent of how the disease was developed.
Amputations
The handling of amputation varies markedly across the economic evaluations. For this analysis, there are two key questions regarding the modelling of amputations.
Do patients with IC receive an amputation?
Five of the economic evaluations considered this; two (de Vries et al. ,78 Holler et al. 79) assumed that it does not happen. Hunink et al. 77 applied an annual rate following failure, the NICE CEA83 applied a fixed probability during PTA and Sculpher et al. 76 applied separate rates depending on whether or not patency was maintained.
In this evaluation, patients with IC are modelled as receiving amputations; this is in agreement with expert opinion (JAM), the majority of the economic evaluations and TASC-II guidance, which states that ‘The concept that all patients who require an amputation have steadily progressed through increasingly severe claudication to rest pain, ulcers and, ultimately, amputation is incorrect’.
What is the relationship between patency and amputation?
Hunink et al. 77 assume that amputations only occur following failure. Sculpher et al. 76 stratify their rates by whether or not patency was maintained. It is unclear how De Vries et al. handled this. 78 The BASIL trial84 did not explore this relationship. Both the NICE CEA83 and Holler et al. 79 apply fixed probabilities irrespective of patency status for CLI patients (their handling of IC patients has been previously described).
Clinical guidelines2 indicate that amputation may be a result of either failure (if reintervention is not possible) or infection or gangrene, irrespective of patency.
As there is no direct evidence that any of the interventions reduce amputation rates, time to amputation is modelled independently of patency status. For patients with CLI, time to amputation is based on data reported by the BASIL trial. 86 For patients with IC, an exponential distribution is used, based on values reported in clinical guidelines: TASC-II guidance99 states that after 5 years 1% to 3.3% of patients with IC will experience an amputation, whereas ACC/AHA guidelines102 say that only 2% of claudicants will ever require amputation. With the fitted distribution, 1.2% of claudicants receive an amputation after 5 years, with this value increasing to 2.4% after 10 years.
Amputation-related mortality
Only three economic evaluations provide data on procedural mortality (Hunink et al. ,77 de Vries et al. ,78 NICE CEA83). There are no reported differences in rates between patients with IC and patients with CLI. Hunink et al. 77 use a value of 11.5%, and the NICE CEA83 uses a value of 12.9%. de Vries et al. 78 stratify their rates by age, with a rate of 9.8% among patients below the age of 75 years, and 14.7% above; these values are used in the model.
The NICE CEA83 is the only evaluation to model a change in the rates of general mortality following an amputation. Whereas the annual mortality rate of patients with CLI is 25%, patients experience a mortality rate of 35% in the first year following an amputation, followed by an annual rate of 19%. For this model, it is assumed that there is no difference in general mortality rates following an amputation.
Quality of life
There was wide variation in the QoL values employed in the existing economic evaluations. A detailed discussion of these is presented in Appendix 7. The values of Hunink et al. 77 are not used, as they were based on the abbreviated form of the Torrance multiattribute scale. All other evaluations used the EQ-5D.
Baseline (pre-treatment) values are taken from the analysis of Sculpher et al. 76 It is assumed that following patency failure an individual’s QoL returns to his or her pre-treatment value.
For IC, the value elicited by Sculpher et al. 76 (0.70) is nearly identical to those elicited by de Vries et al. 78 (0.71) and Holler et al. 79 (0.70). The value used by the NICE CEA83 is much lower (0.57 – this is the average of two studies); Spronk et al. 103 provide a value similar to that of Sculpher et al. 76 (12-month value: 0.77), whereas the value provided by Greenhalgh et al. 104 is much lower (12-month value: 0.48) – this is the only study to not directly measure EQ-5D (values are mapped from SF-36).
There is much variation in the reported values for CLI. The BASIL trial82 (0.26) reports the most recent data, and directly elicits its values from CLI patients. However, there were high levels of comorbid cardiovascular disease in these patients. As the effect of cardiovascular disease is separately modelled, use of this data may not be appropriate. Sculpher et al. 76 elicited their value (0.35) from the general public, and thus the effect of comorbid disease should be less pronounced. This value was also used by de Vries et al. 78 and in the NICE CEA. 83 Holler et al. 79 elicited their value (0.60) from CLI patients; it is noted that this value is over twice that reported by the BASIL trial. 82
Quality of life following an amputation is taken from the analysis of Sculpher et al.,76 who separate their values by above-knee (0.20) and below-knee amputations (0.61). The proportions of these are taken from the BASIL trial (out of 41 amputations, 13 were above the knee and 28 were below the knee84). Both de Vries et al. 78 and the NICE CEA83 use the values of Sculpher et al. 76 The only other evaluation to report EQ-5D values following an amputation is that of Holler et al. 79 (0.52), although it does not state the proportion of above- and below-knee amputations.
The effect of systemic complications is assumed to have a multiplicative decrement on QoL. Only the NICE CEA83 and de Vries et al. 78 report the effects of these. The NICE CEA83 reports the effect of both myocardial infarction and stroke, with separate values for the first and subsequent years. Values following the first year are based on the arbitrary assumption that they are half that of the first year. de Vries et al. 78 only report the effect of myocardial infarction, assuming a constant effect.
The utility decrement reported by de Vries et al. 78 is used. This is primarily to keep the model simple, as there are no data to suggest that any of the interventions alters the probability of experiencing a systemic complication. In addition:
Costs
The NICE CEA83 values costs using the same perspective and time frame (2009/10 NHS reference costs89) as this economic evaluation, so costs are taken from this with the following exceptions:
-
The NICE CEA83 assumes no long-term costs for patients with IC or CLI. In contrast, Hunink et al. 77, Sculpher et al. 76 and Holler et al. 79 all assume that there are costs. Of these, Sculpher et al. 76 is the only evaluation to base their costs on assumed resource use. The costs of these are updated using 2009/10 costs89 and used in the model. For further details see Appendix 7.
-
The long-term costs for patients following an amputation are 20.3% higher in the first year than in subsequent years in the NICE CEA. 83 To keep this model simple only the long-term costs are employed (these are applied at all years).
-
The NICE CEA83 uses a slight increase in the cost of repeat PTA procedures (less than 1%) due to an (assumed) increased number of non-elective admissions. In this evaluation all repeat PTAs cost the same as the initial PTA, with the weight given to non-elective admissions based on their observed frequency of occurrence in the NHS reference costs data.
-
The cost of systemic complications is divided into myocardial infarction and stroke, with an increased cost in the first 3 months in the NICE CEA. 83 Only the costs for myocardial infarction are used in this evaluation for the reason described in the QoL section. As the presence of complications results in an increased procedural cost, the increased cost in the first 3 months is not included, as this may lead to double counting.
Data for interventions
Interventions are assumed to affect only the transition probabilities for acute failure, late failure and the return of symptoms following failure (loss of patency). The effects of interventions are assessed for two different sites: femoropopliteal (Table 63) and infrapopliteal (Table 64) arteries. In Tables 63 and 64 interventions are ranked by their procedural cost. For both sites, the base case is PTA with bailout stenting. The effect of each intervention is assumed to be the same for patients with IC and CLI.
| Intervention | RR | Cost, no complications (£) | |||
|---|---|---|---|---|---|
| Acute failure | Late failure | Return of symptoms | |||
| X(f) | Base case (PTA with bail-out BMSs) | 1 | 1 | 1 | 3837 |
| A | PTA, no bail-out stenting | 2 | 1 | 1 | 3661 |
| B | PTA with bail-out paclitaxel-eluting stents | 1 | 0.82 | 0.66 | 3949 |
| C | Paclitaxel-coated balloon | 1 | 0.40 | 0.68 | 4071 |
| D | BMSs | 1 | 0.58 | 1 | 4316 |
| E | Paclitaxel-eluting stent | 1 | 0.53 | 1 | 4525 |
| F | EVBT | 1 | 0.63 | 1 | 6171 |
| G | Stent-graft | 1 | 0.58 | 1 | 6561 |
| H | Cryoplasty | 0.35 | 2.2 | 1 | 7367 |
| Intervention | RR | Cost, no complications (£) | |||
|---|---|---|---|---|---|
| Acute failure | Late failure | Return of symptoms | |||
| X(i) | Base case (PTA with bail-out stenting) | 1 | 1 | 1 | 3837 |
| α | BMSs | 1 | 0.42 | 1 | 4316 |
| β | Sirolimus-eluting stent | 1 | 0.18 | 1 | 4732 |
From the preceding tables it can be seen that interventions D to G are dominated by intervention C. However, as a result of the uncertainty in assuming mid-point values for the estimates of effectiveness, there is still a possibility that they may represent cost-effective options for the treatment of PAD. Therefore, they are retained in the analysis.
The effects of complications
There were no data available that showed how clinical effectiveness, QoL or costs were affected by the presence of a complication. Hence, these are assumed to have the same effect on interventions as they have on PTA, namely they:
-
do not alter subsequent transition probabilities
-
have a multiplicative decrement on QoL
-
increase procedural costs by 91.7%. (In comparison the costs for BS increase by 44.2%.)
Data on costs
Costs data were derived from two main sources. The cost of interventions involving stents (A, B, D, E, α and β) is based on the base-case cost of PTA with bail-out stents (£3348), adjusted for the cost of a stent (£900 for DESs and £500 for BMSs) and their frequency of use (two per patient when used as the primary procedure, 0.324 per patient when used as a bail-out procedure). These data are taken from the NICE CEA. 83 It should be noted that both types of DES are assumed to have the same procedural cost; however, as the two are applied to different sites, it is not possible to compare the two. It is assumed that the cost of C (paclitaxel-coated balloon) is equal to the base case plus the incremental cost of drug coating (taken to be the difference in costs between a DES and a BMS).
Data for the remaining interventions (EVBT, stent-grafts and cryoplasty) were taken from the literature. 105–107 Instead of adjusting quoted costs to 2009/10 UK pounds, the costs were compared to the quoted costs for the base case and the excess cost applied as a ratio to the base-case cost employed in this evaluation.
Data on clinical effectiveness
With two exceptions, the data on clinical effectiveness come from studies previously discussed in the assessment of clinical effectiveness (see Chapter 3, Results) and, therefore, will not be discussed further here. For each intervention, if multiple studies were available, these were meta-analysed, with the results presented in Results in Chapter 3. Sometimes there were multiple time points with data that could be used to inform clinical effectiveness data. In all instances, the data were judged to be consistent over time, and thus only one result was used, typically the 12-month values. For example, results of the meta-analysis of TLR for DCBs were available for 6 months and 12 months, with RRs of 0.24 and 0.27, respectively – the latter value is used in this evaluation.
Differences in late failure rates are conditional on any differences in acute failure and any differences in the return of symptoms are conditional on any differences in failure. For example, for DCBs a TLR RR of 0.27 is used as a proxy value for the RR of the return of symptoms. As DCBs have a RR for late failure of 0.40, the RR for return of symptoms is calculated as 0.27/0.40 = 0.68.
Data on the clinical effectiveness (late failure and return of symptoms) of cryoplasty were taken from the trial of Spiliopoulos et al. 45 Although this study is included in the assessment of clinical effectiveness, the results used here are adjusted for other variables (as reported in table 5 of the paper of Spiliopoulos et al. 45), and therefore differ from the unadjusted results reported in Results in Chapter 3 of this evaluation.
Data on paclitaxel-eluting stents are taken from a 2012 publication by Dake et al.,71 published after a systematic review of clinical effectiveness was undertaken.
Sources of evidence on each interventions used in the modelling are summarised in Table 65.
| Intervention | RR | Source | |
|---|---|---|---|
| A | PTA, no bail-out stenting | Acute failure: 2.00 | Cejna et al. 200120 |
| B | PTA with bail-out paclitaxel-eluting stents | Late failure: 0.82 | Dake et al. 201171 |
| Return of symptoms: 0.66 | |||
| C | Paclitaxel-coated balloon | Late failure: 0.40 | Meta-analysis of THUNDER61–63 and FemPac64 RCTs; value for 12 months (see Chapter 3, Results) |
| Return of symptoms: 0.68 | |||
| D | BMSs | Late failure: 0.58 | Meta-analysis of ABSOLUTE16–18 and Dick et al.12 RCTs; value for 12 months (see Chapter 3, Results) |
| E | Paclitaxel-eluting stent | Late failure: 0.53 | Dake et al. 201171 |
| F | EVBT | Late failure: 0.63 | Meta-analysis of Vienna-3,51–53 VARA54 and Dick et al. RCTs; value for 12 months (see Chapter 3, Results) |
| G | Stent-graft | Late failure: 0.58 | Saxon et al. 200332 (12-month results) |
| H | Cryoplasty | Acute failure: 0.35 | Jahnke et al. 201040 |
| Late failure: 2.20 | Spiliopoulos et al.41 (Table 5) | ||
| α | BMSs | Late failure: 0.43 | Rand et al.22 |
| β | Sirolimus-eluting stent | Late failure: 0.18 | Rastan et al. 2010108 (12-month results) |
Results
Cost–utility analysis: base case
Intermittent claudication: femoropopliteal arteries
Total costs and total QALYs for each intervention are displayed in Table 66, which is sorted by ascending price. Because the options are mutually exclusive, ICERs are presented based on a fully incremental analysis.
| Intervention | Costs (£) | QALYs | Incremental analysis | |
|---|---|---|---|---|
| C | Paclitaxel-coated balloon | 12,668 | 6.120 | – |
| B | PTA with bail-out paclitaxel-eluting stents | 13,032 | 6.081 | Dominated by C |
| X(f) | PTA with bail-out BMSs | 14,637 | 5.956 | Dominated by C |
| A | PTA, no bail-out stenting | 14,787 | 5.931 | Dominated by C |
| D | BMSs | 15,030 | 5.989 | Dominated by C |
| E | Paclitaxel-eluting stent | 15,692 | 5.993 | Dominated by C |
| F | EVBT | 15,891 | 5.984 | Dominated by C |
| G | Stent-graft | 16,171 | 5.989 | Dominated by C |
| H | Cryoplasty | 17,578 | 5.934 | Dominated by C |
Intervention C, paclitaxel (drug)-coated balloons, is both less expensive and more clinically effective than all of the other options and, therefore, it dominates them. Interventions C and B both dominate the comparator, whereas interventions A and H are dominated by it. The ICERs for the remaining interventions (vs. assumed standard care) are: D (£11,979), E (£28,701), F (£4150) and G (£46,318).
Any decisions regarding which interventions to adopt or fund would be based on the point estimates presented in Table 66. It is also important to look at uncertainty in the decision to adopt. These uncertainties are explored in the following sections. As part of the estimate of uncertainty, probabilistic sensitivity analysis was performed, with 1000 runs.
Figure 27 presents the incremental CEAC for the interventions and assumed standard care. This shows the probability of each procedure being cost-effective at various levels of willingness to pay (maximum acceptable ICER). Thresholds from £0 to £100,000 were tested. Of all the procedures, use of a DCB has the highest probability of being most cost-effective, and use of bailout DESs has the second highest probability of being most cost-effective for all willingness-to-pay thresholds. The probability of any of the other interventions being cost-effective is never greater than 1%. The actual probabilities for each procedure are presented in Table 67 for selected willingness-to-pay thresholds of £20,000, £30,000 and £50,000.
FIGURE 27.
Incremental cost-effectiveness acceptability curve for the base-case model results (all but two of the interventions have probabilities ≈ 0 for all willingness-to-pay thresholds).
| Threshold (£) | Intervention | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C | B | X(f) | E | D | A | G | F | H | |
| 20,000 | 61.8 | 37.0 | 0.6 | 0.3 | 0.2 | 0.1 | 0.0 | 0.0 | 0.0 |
| 30,000 | 61.9 | 36.8 | 0.4 | 0.4 | 0.3 | 0.1 | 0.1 | 0.0 | 0.0 |
| 50,000 | 62.6 | 35.5 | 0.2 | 0.8 | 0.7 | 0.0 | 0.1 | 0.1 | 0.0 |
Based on the values presented in Table 67, interventions C and B have the highest probability of being the most cost-effective. The cost-effectiveness plane for these interventions is presented in Figure 28, which shows the incremental clinical effectiveness and incremental costs of these interventions versus the comparator.
The cost-effectiveness plane shows that both of the presented interventions fall in all four quadrants, suggesting that there is a non-zero probability that each intervention could be dominated by the comparator (represented by points falling in the top-left quadrant).
FIGURE 28.
Cost-effectiveness plane showing incremental clinical effectiveness and costs of selected interventions vs. the comparator (base case).
Additional details of the two interventions B and C, along with the comparator, are presented in Tables 68 and 69. These show the main drivers for the observed differences in clinical and cost-effectiveness outcomes.
| Type of procedure | Average costs per patient (£) | ||
|---|---|---|---|
| Comparator | Bail-out DESs | DCB | |
| All proceduresa | 8361 | 7851 | 7656 |
| First procedure | 3348 | 3461 | 3580 |
| Follow-up procedures | 4816 | 4198 | 3885 |
| Amputations | 198 | 192 | 191 |
| Amputees | 2401 | 2403 | 2295 |
| IC | 801 | 502 | 411 |
| CLI | 124 | 109 | 84 |
| Health state | Average values per patient | ||
|---|---|---|---|
| Comparator | Bail-out DESs | DCB | |
| Life-years gained | 7.80 | 7.78 | 7.78 |
| QALYs | 5.96 | 6.07 | 6.10 |
| Asymptomatic | 5.15 | 5.52 | 5.59 |
| IC | 0.73 | 0.47 | 0.44 |
| Amputees | 0.04 | 0.04 | 0.04 |
| CLI | 0.03 | 0.03 | 0.02 |
Table 68 shows that, although interventions B and C are both more expensive than the comparator, a large component of their cost saving comes from avoiding repeat procedures (by prolonging patency). These interventions also save costs by keeping patients out of the IC health state for longer.
Table 69 shows that, although there is no difference in extension to life offered by the interventions, they keep patients out of the IC health state for longer, resulting in greater QoL.
For both tables, differences in amputation outcomes are minimal, as expected given the assumption that all of the interventions are assumed to have no impact on time to amputation. As amputations are associated with large costs and decrements to QoL, if there is an effect of interventions on reducing these, the cost savings and increases in QoL shown here are likely to be even greater.
An analysis of the expected value of perfect information (EVPI) based on the method described in Claxton and Posnett109 was undertaken and the results are shown in Figure 29.
FIGURE 29.
Results of EVPI.
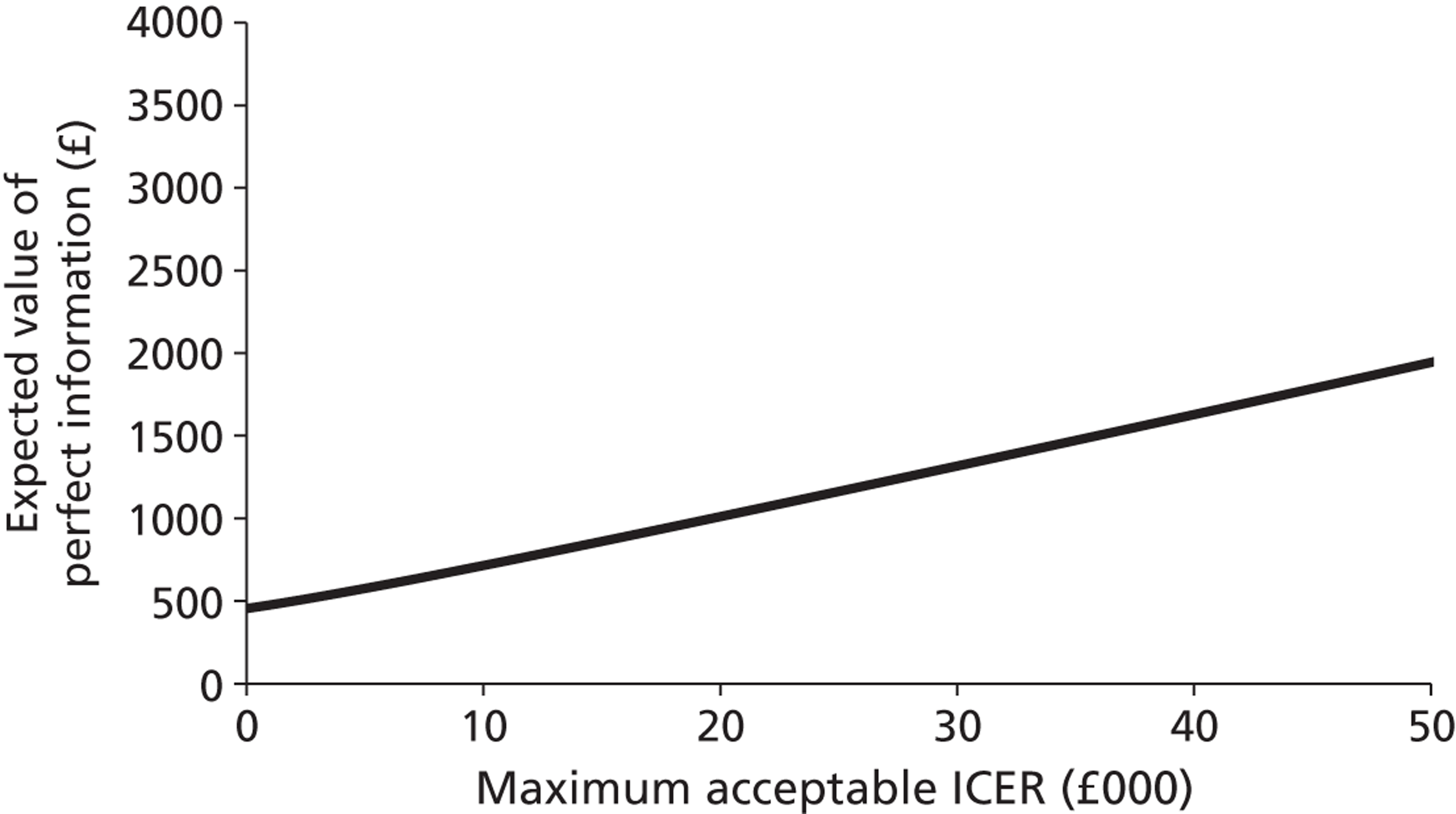
Figure 29 may be interpreted as showing that there is uncertainty in which treatment is more efficacious, with the result that EVPI increases as willingness to pay increases. Often one treatment is more efficacious and, thus, EVPI reaches a maximum; its value decreases as willingness to pay increases and the more efficacious treatment is adopted. In this situation, the decision of which treatment is the most cost-effective does not appear to be dependent upon willingness to pay (the maximum acceptable ICER to a decision-maker). Instead, the most cost-effective treatment is dependent on the clinical effectiveness of the various treatments, and the uncertainty about these treatment effects. This is shown by the CEAC of Figure 27, as the two treatments with non-negligible probabilities of cost-effectiveness have essentially flat curves. Because of this, increasing the maximum acceptable ICER will lead to an increase in the EVPI, as shown in Figure 29. This is in contrast to more commonly seen figures in which there is a trade-off between the cost of an intervention and its efficacy.
It is estimated that about 7% of persons aged ≥ 60 years have IC. 2 Applying this value to 2010 mid-year population estimates for the UK,1 and assuming that the information from this report will be of benefit for a 10-year horizon, gives a multiplier for the EVPI values of 9,847,740.
Critical limb ischaemia: femoropopliteal arteries
Total costs and total QALYs for each intervention are displayed in Table 70, which is sorted by ascending price. Because the options are mutually exclusive, ICERs are presented based on a fully incremental analysis.
| Intervention | Costs (£) | QALYs | Incremental analysis | |
|---|---|---|---|---|
| C | Paclitaxel-coated balloons | 49,890 | 3.402 | – |
| B | PTA with bail-out paclitaxel-eluting stents | 52,335 | 3.297 | Dominated by C |
| D | BMSs | 54,775 | 3.144 | Dominated by C |
| E | Paclitaxel-eluting stent | 55,012 | 3.157 | Dominated by C |
| X(f) | PTA with bail-out BMSs | 55,199 | 3.047 | Dominated by C |
| G | Stent-graft | 55,852 | 3.144 | Dominated by C |
| F | EVBT | 55,928 | 3.134 | Dominated by C |
| A | PTA, no bail-out stenting | 56,539 | 2.988 | Dominated by C |
| H | Cryoplasty | 58,097 | 3.003 | Dominated by C |
As with the results for patients with IC, intervention C, paclitaxel (drug)-coated balloons, is both less expensive and more clinically effective than all of the other options and, therefore, it dominates them. Interventions A and H are again dominated by the comparator, being both more expensive and less clinically effective.
Procedures which include some form of drug and/or the primary use of stents (interventions B to E) are both less expensive and more effective than the comparator of PTA with bailout stenting.
Endovascular procedures that have a different mechanism of action from the comparator (interventions F, G and H) are all more expensive. Only intervention H (cryoplasty) is also less effective. However, the majority of the excluded interventions (atherectomy, EBRT and laser) similarly have a different mechanism of action from the base case, but were excluded because they were known to be both more expensive and less effective. The ICERs for interventions G and F (vs. the comparator) are £6681 (G) and £8341 (F).
Any decisions regarding which interventions to adopt or fund would be based on the point estimates presented in Table 70. It is also important to look at uncertainty in the decision to adopt. These uncertainties are explored in the following sections. As part of the estimate of uncertainty, probabilistic sensitivity analysis was performed, with 1000 runs.
Figure 30 presents the incremental CEAC for the interventions and comparator. This shows the probability of each procedure being cost-effective at various levels of willingness to pay (maximum acceptable ICER). Thresholds from £0 to £100,000 were tested. Of all the procedures, use of a DCB has the highest probability of being most cost-effective and use of bailout DESs has the second highest probability of being most cost-effective for all willingness-to-pay thresholds. The probability of any of the other interventions being cost-effective is never greater than 0.5%. The actual probabilities for each procedure are presented in Table 71 for selected willingness-to-pay thresholds of £20,000, £30,000 and £50,000.
FIGURE 30.
Incremental cost-effectiveness acceptability curve for the base-case model results (all but two of the interventions have probabilities ≈ 0 for all willingness-to-pay thresholds).
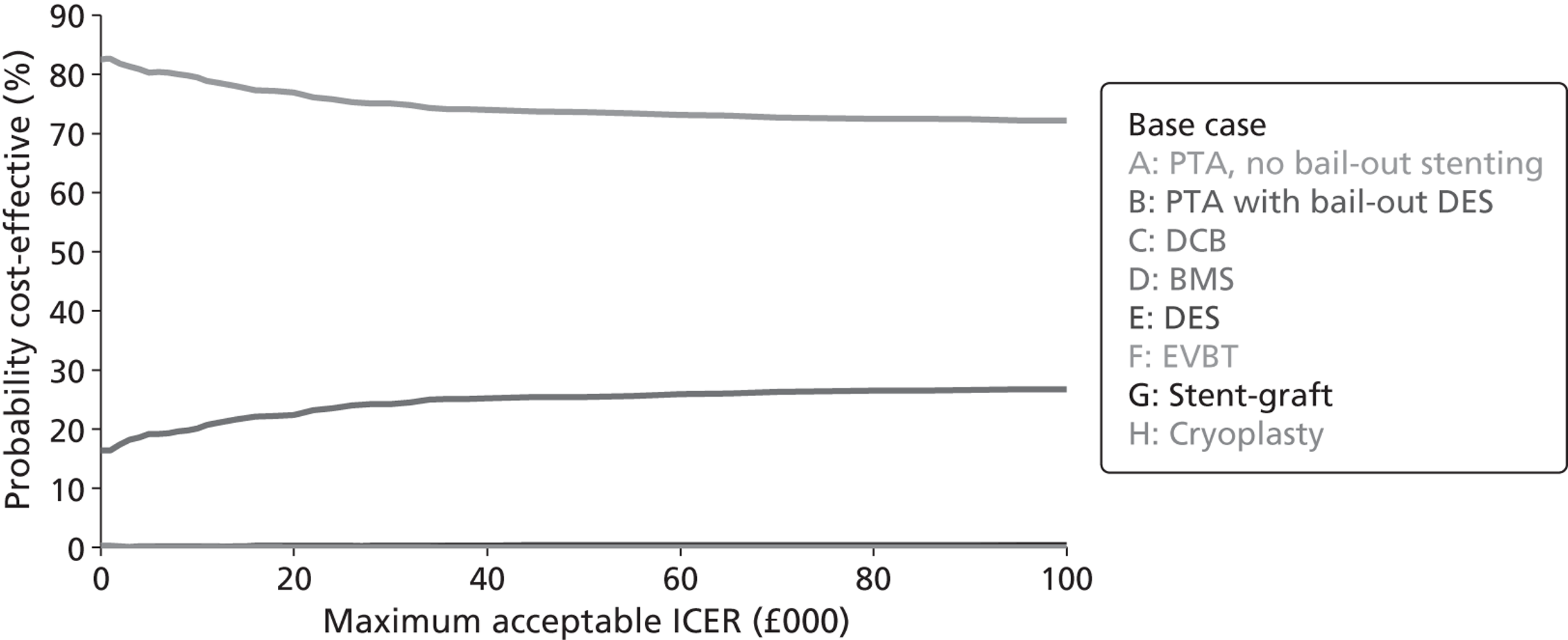
| Threshold (£) | Intervention | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C | B | E | D | F | G | X(f) | A | H | |
| 20,000 | 76.9 | 22.4 | 0.2 | 0.3 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 |
| 30,000 | 75.1 | 24.2 | 0.3 | 0.2 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 |
| 50,000 | 73.6 | 25.4 | 0.4 | 0.3 | 0.1 | 0.2 | 0.0 | 0.0 | 0.0 |
Based on the values presented in Table 71, interventions C and B have the highest probability of being the most cost-effective. The cost-effectiveness plane for these interventions is presented in Figure 31, which shows the incremental clinical effectiveness and incremental costs of these interventions versus the comparator.
FIGURE 31.
Cost-effectiveness plane showing incremental clinical effectiveness and costs of selected interventions vs. the comparator (base case).
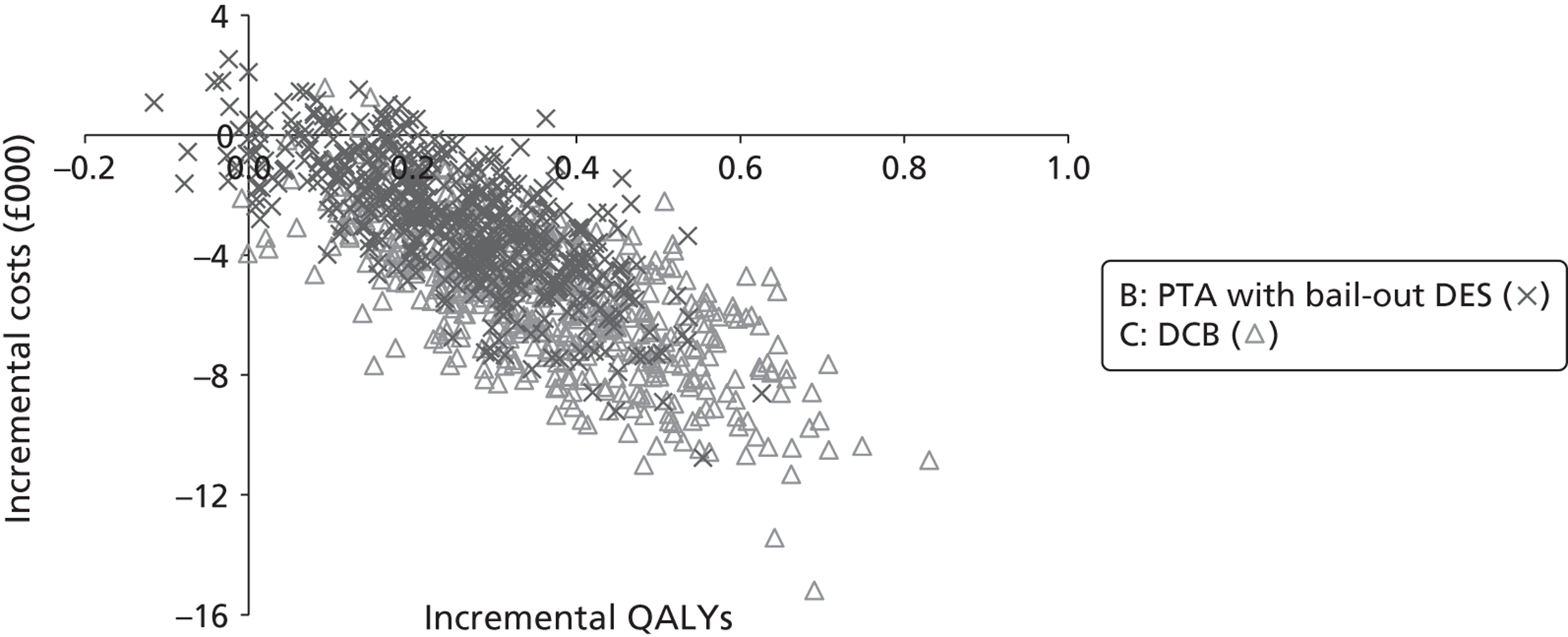
The cost-effectiveness plane shows that, for some realisations of intervention B (but not intervention C), results fall in the top-left quadrants, suggesting that there is a non-zero probability that it could be dominated by the comparator.
Additional details of the two interventions B and C, along with the comparator, are presented in Tables 72 and 73. These show the main drivers for the observed differences in cost-effectiveness outcomes.
| Type of procedure | Average costs per patient | ||
|---|---|---|---|
| Comparator (£) | Bail-out DESs (£) | DCB (£) | |
| All proceduresa | 14,949 | 13,685 | 12,432 |
| First procedure | 3348 | 3461 | 3580 |
| Follow-up procedures | 8320 | 6877 | 5511 |
| Amputations | 3282 | 3347 | 3341 |
| Amputees | 32,478 | 33,731 | 32,600 |
| IC | 87 | 69 | 23 |
| CLI | 1262 | 808 | 668 |
| Health state | Average values per patient | ||
|---|---|---|---|
| Comparator | Bail-out DESs | DCB | |
| Life-years gained | 5.17 | 5.25 | 5.20 |
| QALYs | 2.99 | 3.24 | 3.32 |
| Asymptomatic | 2.20 | 2.50 | 2.65 |
| IC | 0.03 | 0.02 | 0.01 |
| Amputees | 0.50 | 0.53 | 0.53 |
| CLI | 0.27 | 0.18 | 0.13 |
Table 72 shows that, although interventions B and C are both more expensive than the comparator, a large component of their cost saving comes from avoiding repeat procedures (by prolonging patency). These interventions also save costs by keeping patients out of the CLI health state for longer.
Whereas the costs for amputees are of similar magnitude for the comparator and both interventions, the increased costs for intervention B (owing to natural variation) are almost the same as the decreased procedural costs (owing to intervention effect). Therefore, setting amputation costs to zero will result in even greater cost savings for intervention B relative to the comparator. Setting amputation costs to zero will not make intervention B cheaper than intervention C however.
Table 73 shows that the main driver for differences in QoL is keeping patients out of the CLI health state and in the asymptotic health state for longer.
An analysis of the EVPI based on the method described in Claxton and Posnett109 was undertaken and the results are shown in Figure 32.
FIGURE 32.
Results of EVPI analysis.
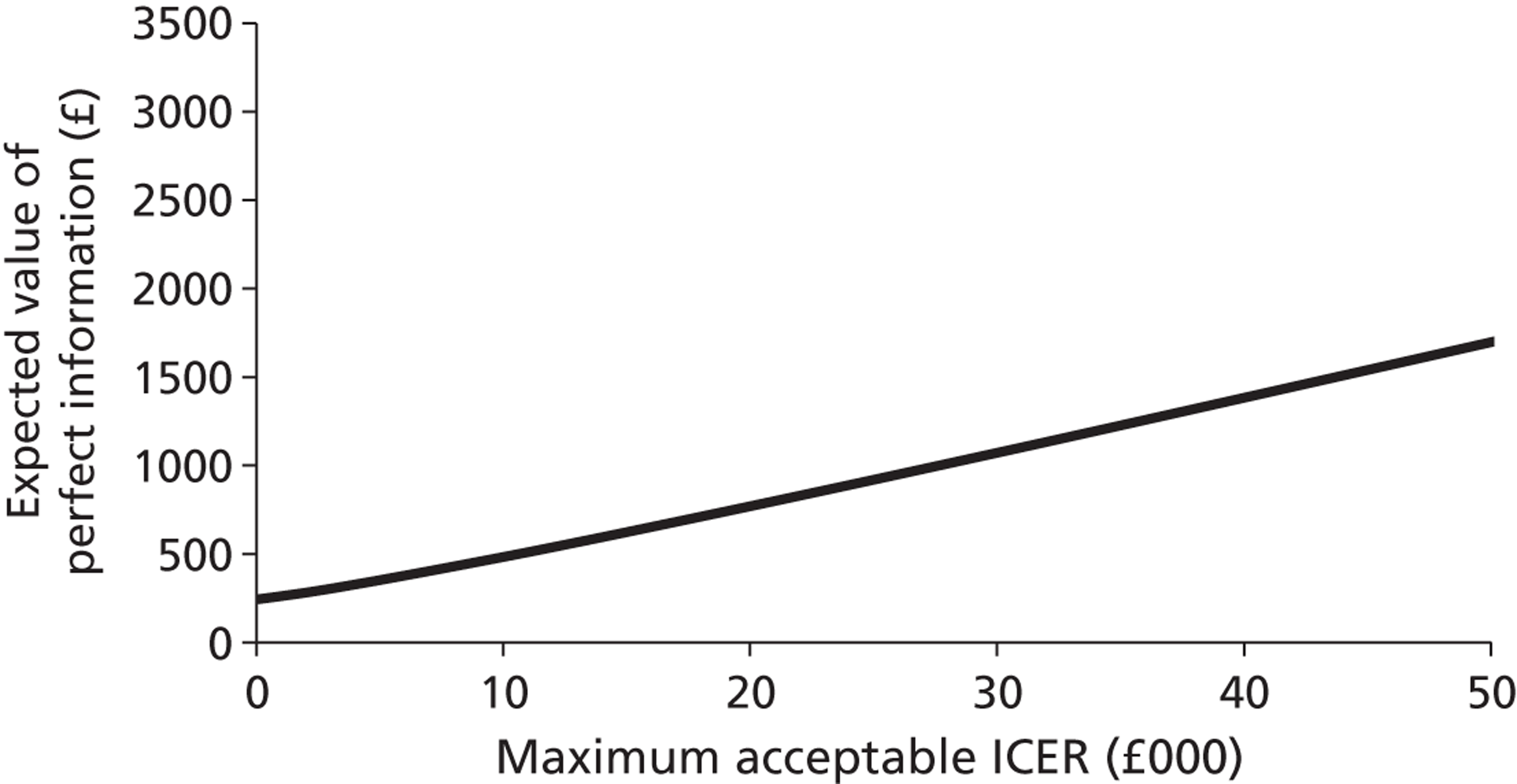
The results of the EVPI analysis for patients with CLI are very similar to the results of the analysis for patients with IC. Again, there is an indication of some uncertainty over the results, with EVPI increasing as willingness to pay increases. In this situation, the decision of which treatment is the most cost-effective does not appear to be dependent upon willingness to pay (the maximum acceptable ICER to a decision-maker). Instead, the most cost-effective treatment is dependent on the inherent effectiveness of the various treatments, and the uncertainty about these treatment effects. This is shown by the CEAC of Figure 30, as the two treatments with non-negligible probabilities of cost-effectiveness have essentially flat curves. Because of this, increasing the maximum acceptable ICER will lead to an increase in the EVPI, as shown in Figure 31. This is in contrast to more commonly seen figures in which there is a trade-off between the cost of an intervention and its efficacy.
It is estimated that about 0.4% of persons aged ≥ 60 years have CLI. 2 Applying this value to 2010 mid-year population estimates for the UK,1 and assuming that the information from this report will be of benefit for a 10-year horizon, gives a multiplier for the EVPI values of 562,728.
Scenario analysis 1: varying age
In the base-case analysis, the starting age for patients was 66 for those with IC and 74 for those with CLI. These values were not varied within probabilistic sensitivity analyses; instead, the sensitivity of the conclusions reached in the base case to starting age are explored here.
Incremental costs and incremental QALYs for each intervention relative to the comparator are shown in Tables 74–77. As many interventions either dominate or are dominated by the comparator, ICERs are not presented.
| Age (years) | Intervention | |||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | |
| 45–49 | –£621 | –£1516 | –£2795 | –£413 | £398 | £781 | £738 | £3331 |
| 50–54 | –£419 | –£1251 | –£2500 | –£244 | £518 | £945 | £907 | £2993 |
| 55–59 | –£548 | –£1220 | –£2181 | –£203 | £581 | £950 | £947 | £2759 |
| 60–64 | –£465 | –£994 | –£1684 | £27 | £893 | £1117 | £1178 | £3027 |
| 65–69 | –£152 | –£762 | –£1166 | £314 | £1027 | £1276 | £1464 | £2682 |
| 70–74 | –£171 | –£643 | –£847 | £443 | £1136 | £1446 | £1593 | £2672 |
| 75–79 | –£66 | –£467 | –£585 | £587 | £1330 | £1531 | £1737 | £2728 |
| 80–84 | –£130 | –£403 | –£462 | £591 | £1276 | £1541 | £1741 | £2640 |
| 85–89 | –£73 | –£231 | –£327 | £687 | £1369 | £1608 | £1837 | £2638 |
| Age (years) | Intervention | |||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | |
| 45–49 | –0.114 | 0.367 | 0.527 | 0.027 | 0.055 | 0.007 | 0.027 | –0.039 |
| 50–54 | –0.137 | 0.305 | 0.421 | 0.002 | 0.063 | –0.026 | 0.002 | –0.040 |
| 55–59 | –0.114 | 0.230 | 0.311 | 0.038 | 0.041 | 0.002 | 0.038 | 0.001 |
| 60–64 | –0.104 | 0.173 | 0.215 | 0.024 | 0.032 | 0.006 | 0.024 | –0.007 |
| 65–69 | –0.027 | 0.089 | 0.122 | 0.012 | 0.023 | 0.008 | 0.012 | –0.029 |
| 70–74 | –0.010 | 0.038 | 0.069 | –0.005 | 0.011 | 0.011 | –0.005 | –0.038 |
| 75–79 | –0.005 | 0.023 | 0.038 | 0.008 | 0.012 | 0.015 | 0.008 | –0.022 |
| 80–84 | –0.006 | 0.006 | 0.021 | 0.005 | 0.012 | 0.005 | 0.005 | –0.006 |
| 85–89 | –0.011 | –0.005 | 0.006 | –0.002 | 0.004 | 0.005 | –0.002 | –0.007 |
| Age (years) | Intervention | |||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | |
| 45–49 | £2441 | –£927 | –£4753 | –£1395 | £1138 | £1826 | –£245 | £5905 |
| 50–54 | £2178 | –£1591 | –£4586 | –£1172 | £601 | £1487 | –£22 | £4707 |
| 55–59 | £2700 | –£1393 | –£4119 | –£641 | £935 | £1897 | £509 | £4560 |
| 60–64 | £3040 | –£603 | –£3508 | –£365 | £1421 | £2160 | £785 | £4276 |
| 65–69 | £2902 | –£642 | –£3163 | £25 | £996 | £2089 | £1175 | £4115 |
| 70–74 | £2027 | –£1114 | –£3109 | –£28 | £790 | £1717 | £1123 | £3540 |
| 75–79 | £1853 | –£933 | –£2410 | £387 | £899 | £1623 | £1538 | £3574 |
| 80–84 | £1388 | –£895 | –£1979 | £261 | £862 | £1337 | £1411 | £3058 |
| 85–89 | £1197 | –£798 | –£1485 | £505 | £1093 | £1442 | £1655 | £2612 |
| Age (years) | Intervention | |||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | |
| 45–49 | –0.159 | 0.573 | 0.847 | 0.110 | 0.150 | 0.088 | 0.110 | –0.072 |
| 50–54 | –0.118 | 0.514 | 0.730 | 0.124 | 0.168 | 0.121 | 0.124 | –0.057 |
| 55–59 | –0.109 | 0.407 | 0.583 | 0.114 | 0.152 | 0.118 | 0.114 | –0.076 |
| 60–64 | –0.099 | 0.336 | 0.446 | 0.084 | 0.140 | 0.111 | 0.084 | –0.072 |
| 65–69 | –0.087 | 0.245 | 0.324 | 0.057 | 0.087 | 0.084 | 0.057 | –0.079 |
| 70–74 | –0.080 | 0.153 | 0.213 | 0.032 | 0.060 | 0.060 | 0.032 | –0.070 |
| 75–79 | –0.052 | 0.098 | 0.127 | 0.024 | 0.035 | 0.052 | 0.024 | –0.031 |
| 80–84 | –0.029 | 0.053 | 0.064 | 0.013 | 0.026 | 0.036 | 0.013 | –0.014 |
| 85–89 | –0.018 | 0.016 | 0.022 | 0.005 | 0.009 | 0.014 | 0.005 | –0.006 |
No intervention alters life-years gained; their effect on costs is to avoid repeat reinterventions and their effect on QALYs is to keep patients in the asymptomatic health state for longer. For QALYs, effectiveness (or lack of it) shows a mostly smooth relationship with age, with effects becoming more (less) pronounced at younger (older) ages. This pattern can be seen for both patient populations.
A similar pattern can be seen for costs, although it is less pronounced – particularly for patients with CLI. This is likely to be a result of the effect of amputations, which are both very costly and independent of the intervention used.
The tables show that interventions B and C dominate the comparator for all ages and for both patient populations. For patients with IC, this effect becomes very small after the age of (about) 80; for patients with CLI, the effect becomes very small after the age of (about) 85.
Scenario analysis 2: no amputation costs
The average costs of the interventions considered range from £3837 (A) to £7367 (H), whereas monthly costs for IC are £15 and for CLI are £52. In contrast, an amputation procedure costs an average of £9733, with follow-up monthly costs just under £2000.
The high costs associated with receiving an amputation mean that any variations in the rates of its occurrence are likely to overwhelm any intervention effects (with respect to costs). Convergence tests were performed to ensure that natural variation is unlikely to be an issue, and it is assumed that interventions do not affect time to amputation (as there is not enough evidence to suggest otherwise). Care was taken to minimise any indirect effects of interventions on amputation rates. For example, patent patients cannot experience ipsilateral disease progression and, hence, interventions prolonging patency also result in lower rates of progression to CLI. As patients with CLI have a shorter time to amputation, this could result in an indirect intervention effect on amputation rates. To avoid this, time to amputation is set based on a patient’s characteristics at model entry, and is not changed upon disease progression (the same applies to time to general mortality).
There remains in the model one indirect intervention effect on amputation rates. Patency avoids the need for reinterventions and thus avoids the slight mortality risk associated with a reintervention. Because of this, more effective interventions will (on average) keep patients alive for a slightly longer time, meaning that patients are slightly more likely to receive an amputation.
This scenario analysis looks at the impacts on the base-case results when amputation-related costs are removed. Costs and QALYs for the comparator and each intervention are shown for each patient population in Table 78. As with the base-case, results are ordered by ascending price.
| IC | CLI | ||||||
|---|---|---|---|---|---|---|---|
| Intervention | Costs | QALYs | Intervention | Costs (£) | QALYs | ||
| C | Paclitaxel-coated balloons | 7920 | 6.120 | C | Paclitaxel-coated balloons | 16,433 | 3.402 |
| B | PTA with bail-out paclitaxel-eluting stents | 8117 | 6.081 | B | PTA with bail-out paclitaxel-eluting stents | 18,283 | 3.297 |
| A | PTA, no bail-out stenting | 8302 | 5.931 | D | BMSs | 18,348 | 3.144 |
| X(f) | PTA with bail-out BMSs | 8368 | 5.956 | X(f) | PTA with bail-out BMSs | 18,619 | 3.047 |
| D | BMSs | 8928 | 5.989 | E | Paclitaxel-eluting stent | 18,816 | 3.157 |
| E | Paclitaxel-eluting stent | 9607 | 5.993 | A | PTA, no bail-out stenting | 18,913 | 2.988 |
| F | EVBT | 9756 | 5.984 | F | EVBT | 19,505 | 3.134 |
| G | Stent-graft | 10,077 | 5.989 | G | Stent-graft | 19,589 | 3.144 |
| H | Cryoplasty | 12,314 | 5.934 | H | Cryoplasty | 22,030 | 3.003 |
There are some slight differences from the base-case results in the order of interventions ranked near the middle. For example, for patients with IC, in the base case intervention A was £150 more expensive than the comparator, but, in this analysis, it is now £67 cheaper.
The main conclusions of the base case – that intervention C dominates all others and intervention B also dominates the comparator – remain unchanged. This is to be expected, given that interventions are not assumed to effect amputation rates.
Scenario analysis 3: reduced clinical benefit owing to patency and cost-minimisation approach
The effects of interventions on prolonging patency are taken from the literature. However, there is some concern over the link between patency and clinical outcomes, such as the need for reinterventions and the effect on QoL. The available literature linking these two is very sparse, and the NICE CEA83 did not include differences in patency because of a lack of evidence.
In the base-case analysis, prolonging patency has the following effects:
-
It improves QOL by stopping the return of symptoms (either IC or CLI).
-
It saves future costs by preventing the need for a reintervention.
-
It stops patients with IC experiencing ipsilateral disease progression.
It is worth noting that each of these effects is diluted:
-
After losing patency, not all individuals will experience a return of symptoms.
-
After losing patency, not all individuals will require a reintervention.
-
Patients with IC can experience contralateral disease progression at any point.
This sensitivity analysis looks at the impact on the base-case results if assumptions C and A are removed. Assumption C was removed by setting time to ipsilateral progression at model entry, using an exponential distribution with a parameter of 249.5 (this is based on the rate of disease progression modelled in the NICE CEA). Results from this are shown in Table 79.
| IC | CLI | ||||||
|---|---|---|---|---|---|---|---|
| Intervention | Costs (£) | QALYs | Intervention | Costs (£) | QALYs | ||
| C | Paclitaxel-coated balloons | 10,776 | 6.127 | C | Paclitaxel-coated balloons | 48,939 | 3.320 |
| B | PTA with bail-out paclitaxel-eluting stents | 10,877 | 6.094 | B | PTA with bail-out paclitaxel-eluting stents | 49,147 | 3.197 |
| A | PTA, no bail-out stenting | 11,688 | 6.124 | D | BMSs | 50,535 | 3.096 |
| D | BMSs | 12,129 | 6.072 | E | Paclitaxel-eluting stent | 51,129 | 3.098 |
| X(f) | PTA with bail-out BMSs | 12,369 | 6.052 | F | EVBT | 51,275 | 3.060 |
| F | EVBT | 12,952 | 6.109 | X(f) | PTA with bail-out BMSs | 51,286 | 2.975 |
| E | Paclitaxel-eluting stent | 13,002 | 6.080 | G | Stent-graft | 51,685 | 3.096 |
| G | Stent-graft | 13,279 | 6.072 | A | PTA, no bail-out stenting | 52,811 | 2.922 |
| H | Cryoplasty | 14,710 | 6.070 | H | Cryoplasty | 54,464 | 2.938 |
Relative to the base-case results, costs are slightly reduced and there are some slight differences in the ordering of the interventions near the middle. However, the main conclusions – that intervention C dominates all others and intervention B also dominates the comparator – remain unchanged.
If assumption A is removed, the effect would be that all interventions have the same QALYs. The only differences would then be in cost, with the cheapest intervention being chosen. Again, this would lead to intervention C being chosen, with intervention B the second cheapest (this conclusion holds for both the base case and the removal of assumption C.
Scenario analysis 4: results for the infrapopliteal arteries
The base case uses an underlying ‘natural’ history (time to patency for the comparator) model for PAD in the femoropopliteal arteries. This natural history is then affected by the interventions, with different underlying natural histories for the two patient populations (IC and CLI).
Data for each intervention considered in the base case were taken from studies that were identified in the systematic review and that looked at the role of the intervention in the femoropopliteal arteries, popliteal artery or superficial femoral artery.
Data for sirolimus-eluting stents were only available for the infrapopliteal arteries. After discussions with our clinical expert (JAM), it was felt that these should be analysed separately, as the underlying natural history was likely to be very different for this anatomical area.
This scenario analysis looks at the results for sirolimus-eluting stents versus the comparator. It should be stressed that these results should be seen as exploratory in nature, as data for the comparator are based on outcomes observed in the femoropopliteal arteries.
Only one study was found that considered the cost-effectiveness of sirolimus-eluting stents (Rastan et al. 108). However, the comparator in this study was BMSs. One study identified in the systematic review considered the cost-effectiveness of BMSs in the infrapopliteal arteries (Rand et al. 22); the cost-effectiveness of sirolimus-eluting stents relative to the comparator used in this analysis is indirectly estimated using the results of Rand et al. 22 BMSs are also included as an additional intervention (using only cost-effectiveness data from Rand et al. 22). As the Rand et al. 22 study only considers patients with CLI, only results for this patient population are considered. Results for this scenario analysis are shown in Table 80.
| Intervention | Costs (£) | QALYs | |
|---|---|---|---|
| α | BMSs | 48,604 | 3.520 |
| X(f) | PTA with bail-out BMSs | 49,890 | 3.402 |
| β | Sirolimus-eluting stents | 51,020 | 3.750 |
Assuming the same natural history model as observed in femoropopliteal arteries, the use of BMSs in infrapopliteal arteries dominates the comparator. Relative to BMSs, the use of sirolimus-eluting stents generates 0.23 additional QALYs at an additional cost of £2416, giving an ICER of £10,571.
Chapter 5 Discussion
The review identified a large number of studies covering most of the technologies that have been included in the scope. However, many trials were small and the populations, clinical indications and nature of the lesions varied among studies. Although the review aimed to consider a range of potential outcome measures, very little evidence was found regarding disease-specific or generic measures of QoL and clinical outcomes such as walking distance or limb loss. For these outcomes and for complications and adverse events, there were no significant differences reported between any of the new technologies and PTA. This may reflect the limited outcome data collected in the trials and that the trials were not sufficiently large to be powered for identification of such outcomes. In addition, nearly all comparisons were with PTA, meaning that it was not possible to conduct a network meta-analysis. The only exceptions are the studies that looked at DESs; these included a comparison with BMSs. However, the studies considered different drugs and, therefore, including these in a network meta-analysis would not have been useful.
The main outcomes reported in the majority of trials were measures of patency or restenosis and the need for reintervention. Based upon this specific outcome, one technology, AMSs, was reported as being significantly worse than PTA and six others: BESs, atherectomy, CB, cryoplasty, EBRT and laser angioplasty showed no significant differences from PTA. There was, however, a group of technologies for which there was evidence of a significant benefit in reducing restenosis rates. These technologies were SESs, stent-grafts, EVBT and DCBs. Studies of DESs versus BMSs also demonstrated an advantage in terms of restenosis rates for DESs.
The health economic analysis considered the effects of eight interventions (PTA with no bail-out stenting, PTA with bail-out paclitaxel-eluting stents, paclitaxel-coated balloons, primary BMSs, primary paclitaxel-eluting stents, EVBT, stent-grafts and cryoplasty) in the femoropopliteal arteries, along with the comparator (PTA with bail-out BMSs). Two interventions (primary BMSs and primary sirolimus-eluting stents) were also considered in the infrapopliteal arteries, although the results for these can be interpreted only as an exploratory sensitivity analysis as data for both the comparator and natural history of PAD were based on the femoropopliteal arteries.
Results for the base-case analysis suggest that the use of paclitaxel-coated balloons dominates both the comparator and all other interventions. Taking account of the uncertainty in this result, of the other interventions, only the use of bail-out paclitaxel-eluting stents (which also dominate the comparator and all other comparators except for paclitaxel-coated balloons) is likely to be the most cost-effective intervention (all other interventions had probabilities of being the most cost-effective that were always less than 1% for willingness-to-pay values between £0 and £100,000).
Exploratory results for the infrapopliteal arteries suggest that the use of BMSs will be cost saving relative to the comparator, and it will also improve QoL for patients. Relative to BMSs, the use of sirolimus-eluting stents is associated with an ICER of £10,571.
A particular strength of this analysis was its consideration of a large number of interventions for peripheral arterial disease. Comparing all of the interventions in a single economic evaluation reduces uncertainty in the recommendations, as all of the alternatives are evaluated in a consistent manner. The use of discrete-event simulation is also a strength. Previous studies mainly used Markov models; the use of discrete-event simulation meant that, for this analysis, a large number of patient characteristics (such as both ipsilateral and bilateral disease progression) could be tracked over time, while still keeping the model relatively transparent.
The main weakness of this study is the lack of evidence and data. Many of the trials identified by the clinical systematic review were small, meaning that some potentially important intervention effects were not detected due to a lack of power. For example, many trials measured differences in adverse events or mortality, but none found a significant difference. Moreover, trials varied in the patient populations, particularly as regards the anatomical distribution and extent of disease and the clinical indications for intervention. In the absence of these data, the modelling required a number of assumptions, which adds to the uncertainty around the results.
This analysis modelled the effect of the interventions by their impact on patency; the lack of evidence linking patency and clinical outcomes, such as claudication distance, QoL and reintervention, is a limitation to the current analysis. It appears to be common for research in this field to use patency as a surrogate for clinical effectiveness; however, this link was not accepted for most interventions in the recent NICE guidance on peripheral arterial disease. 81 There are considerable concerns about the validity of this assumption. Whereas it seems plausible that this relationship may hold for interventions that have very similar mechanical effects, for example two identical stents, with and without drug coating, it is less clear that the degree of restenosis within a stent will have the same clinical implications as a similar degree of stenosis in an area treated by balloon alone or atherectomy.
A further problem with the assumption that clinical outcomes can be implied from patency rates is that there was little evidence on which to base assumptions regarding the costs and clinical effectiveness of retreatment. As the options for retreatment and the outcomes may vary among different primary treatments, it is possible restenosis will have differing implications for downstream costs and outcomes. In the absence of evidence on this, the model assumes a relationship between patency and retreatment that is independent of the primary procedure.
In addition, the relationships between patency of the index lesion and clinical outcomes may not be constant over time, as assumed in the analysis. For example, late adverse outcomes of PAD in patients with claudication will often relate to progressive disease at sites other that the site of initial treatment. This is partly accommodated by modelling contralateral disease progression, which patients may experience at any time (i.e. independently of patency status). In a scenario analysis in which ipsilateral disease progression was also assumed to be independent of patency, the main conclusions of the base-case analysis were unchanged. In addition, the effects of stents (either BMSs or DESs) on the target vessel are very different from those of other interventions such as stent-grafts and cryoplasty. Because of this, the nature of the relationship between vessel diameter (patency) and clinical outcomes may vary for different interventions.
As the use of paclitaxel-coated balloons is less expensive than the comparator, the results of this study still support its use even if it is assumed that there is no link between patency and QoL. This decision remains if the more pessimistic scenario of no link between patency and ipsilateral disease progression is included. However, it does assume that prolonged patency will lead to cost savings as a result of fewer reinterventions. This is based on relatively little direct evidence, although the frequency with which patency is measured in trials of endovascular treatments for PAD suggests that it is an important consideration when deciding on whether or not to perform a reintervention. In addition, it is noted that in the model failed patency will not immediately require a reintervention; on average, 26.9% of patients with IC and 71.6% of patients with CLI will receive a reintervention following failure. The model also assumes that, while patency relates to the rate of reintervention, the nature of reinterventions, and thus their cost and outcome, is independent of the initial intervention. There was no data identified that would confirm or refute this assumption.
After the acute (30 days following operation) period, the effects of each intervention on patency are assumed to be constant over time; in other words, they are assumed to follow a proportional hazards model. While there were no data to suggest that the proportional hazards assumption would not hold, this was mostly because of a lack of data on the clinical effectiveness of interventions over time. It is plausible that the modelled benefits (in particular, for the two most cost-effective interventions: paclitaxel-coated balloons and bail-out paclitaxel-eluting stents) reduce over time, as the effect of the drug may be expected to be most effective immediately after the initial treatment. The possible effects of this on the base-case results have not been explored.
Relative to the costs of the interventions, costs related to amputation are very large. For example, the cost of an amputation is between 32% and 154% greater than the intervention costs, and the yearly cost of being an amputee is between 219% and 513% greater. Because of this, any differences in amputation rates due to intervention are likely to overwhelm differences in any other costs. None of the trials reviewed showed any effect on amputation rates, but they had not been powered to demonstrate such an effect. Thus, assumptions about the relationship between amputation and patency have the potential to drive the results of modelling. In the base-case analysis, the only effect of interventions on amputations was due to higher/lower rates of reinterventions, which result in sooner/later deaths (owing to procedural-related deaths) and, therefore, a slight decrease/increase in the potential for progressing to amputation. It is noted that this will disfavour more clinically effective treatments. When amputation-related costs were removed from the base case, the interpretations of cost-effectiveness remained unchanged.
The main uncertainties about the results presented are the assumed associations between patency and clinical outcomes. While scenario analyses have showed that the base-case results remain fairly robust to changes in these assumptions, further study into these associations would allow for more accurate modelling of the potential cost-effectiveness of the interventions, in particular for paclitaxel-coated balloons and paclitaxel-eluting stents.
Chapter 6 Conclusions
Implications for practice
Despite many studies being identified, there remains uncertainty in the results of the report. Clinically, there was evidence of a significant benefit to reducing restenosis rates for SESs, stent-graft, EVBT and DEB compared with PTA and for DESs compared with BMSs. If it is assumed that patency translates into beneficial long-term clinical outcomes, then DCBs and bailout DESs are most likely to be the cost-effective enhancements to PTA. Of these, the use of DCBs resulted in the lowest lifetime costs and greatest improvement in QoL of all the interventions, hence dominating them.
Current NICE guidance recommends PTA with bailout BMSs. 83 The NICE guidance does not consider many of the interventions considered in this report, and hence this report does not call for changes to the NICE advice for practice, but suggests areas for further research. Research into these areas is important, as a key component of the economic evaluation is the assumption that prolonged patency was associated with improved clinical outcomes; this assumption was not used in the NICE guidelines.
Recommendations for future research
A RCT comparing current recommended practice (PTA with bail-out BMSs) with DCBs and bailout DESs could assess long-term follow-up and cost-effectiveness. In addition to patency, the inclusion of health-related QoL measures EQ-5D and maximum walking distance would be useful.
Our study also indicates that, of the interventions considered, AMSs, atherectomy, EBRT, laser angioplasty, EVBT, stent-grafts and cryoplasty are all unlikely to warrant further investigation.
Acknowledgements
The authors would like to thank the clinical experts who provided suggestions for interventions to be included in the project: Sumaira MacDonald, Consultant Vascular Radiologist, Freeman Hospital, Newcastle Upon Tyne; David Kessel, Consultant Vascular Radiologist, Leeds Teaching Hospitals, Leeds; and Trevor Cleveland, Consultant Vascular Radiologist, Sheffield Teaching Hospitals, Sheffield. The authors would also like to thank the Project Administrator, Kathleen Wilson.
Contributions of authors
Jonathan A Michaels was the principal investigator and was involved in designing the project. Anna J Cantrell conducted the literature searches. Emma L Simpson and Chris Littlewood conducted the clinical effectiveness review. Benjamin Kearns and Matthew D Stevenson conducted the clinical effectiveness modelling.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Office for National Statistics . Population Estimates for UK, England and Wales, Scotland and Northern Ireland, Mid-2010 2012. www.ons.gov.uk/ons/publications/re-reference-tables.html?edition=tcm%3A77–231847 (accessed July 2012).
- Tendera M, Aboyans V, Bartelink ML, Baumgartner I, Clement D, Collet JP, et al. ESC guidelines on the diagnosis and treatment of peripheral artery diseases. Eur Heart J 2011;32:2851-906.
- The Provision of Services for Patients with Vascular Disease. Edinburgh: VSGBI; 2012.
- Fowkes FGR, Housley E, Cawood EHH, MacIntyre CCA, Ruckley CV, Prescott RJ. Edinburgh Artery Study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. Int J Epidemiol 1991;20:384-92. http://dx.doi.org/10.1093/ije/20.2.384.
- Trans-Atlantic Inter-society Consensus . Trans-Atlantic Inter-society Consensus document II on peripheral arterial disease – TASC II. Acta Chir Belg 2007;107:S1-72.
- Bradbury AW, Bell J, Lee AJ, Prescott RJ, Gillespie I, Stansby G, et al. Bypass or angioplasty for severe limb ischaemia? A Delphi Consensus Study. Eur J Vasc Endovasc Surg 2002;24:411-16. http://dx.doi.org/10.1053/ejvs.2002.1709.
- Peeters P, Keirse K, Verbist J, Deloose K, Bosiers M. Are bio-absorbable stents the future of SFA treatment?. J Cardiovasc Surg 2010;51:121-4.
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:65-94.
- Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. York: University of York; 2009.
- Cochrane Collaboration . Cochrane Handbook for Systematic Reviews of Interventions n.d. www.cochrane-handbook.org/ (accessed April 2012).
- Bosiers M, Peeters P, D’Archambeau O, Hendriks J, Pilger E, Duber C, et al. AMS INSIGHT – absorbable metal stent implantation for treatment of below-the-knee critical limb ischemia: 6-month analysis. Cardiovasc Intervent Radiol 2009;32:424-35.
- Dick P, Wallner H, Sabeti S, Loewe C, Mlekusch W, Lammer J, et al. Balloon angioplasty versus stenting with nitinol stents in intermediate length superficial femoral artery lesions. Catheter Cardiovasc Interv 2009;74:1090-5. http://dx.doi.org/10.1002/ccd.22128.
- Greenberg D, Rosenfield K, Garcia LA, Berezin RH, Lavelle T, Fogleman S, et al. In-hospital costs of self-expanding nitinol stent implantation versus balloon angioplasty in the femoropopliteal artery (the VascuCoil Trial). J Vasc Interv Radiol 2004;15:1065-9. http://dx.doi.org/10.1097/01.RVI.0000136293.18041.88.
- Krankenberg H, Schluter M, Steinkamp HJ, Burgelin K, Scheinert D, Schulte KL, et al. Nitinol stent implantation versus percutaneous transluminal angioplasty in superficial femoral artery lesions up to 10 cm in length: the femoral artery stenting trial (FAST). Circulation 2007;116:285-92. http://dx.doi.org/10.1161/CIRCULATIONAHA.107.689141.
- Laird JR, Katzen BT, Scheinert D, Lammer J, Carpenter J, Buchbinder M, et al. Nitinol stent implantation versus balloon angioplasty for lesions in the superficial femoral artery and proximal popliteal artery: twelve-month results from the RESILIENT randomized trial. Circ Cardiovasc Interv 2010;3:267-76. http://dx.doi.org/10.1161/CIRCINTERVENTIONS.109.903468.
- Schillinger M, Sabeti S, Loewe C, Dick P, Amighi J, Mlekusch W, et al. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Eng J Med 2006;354:1879-88. http://dx.doi.org/10.1056/NEJMoa051303.
- Schillinger M, Sabeti S, Dick P, Amighi J, Mlekusch W, Schlager O, et al. Sustained benefit at 2 years of primary femoropopliteal stenting compared with balloon angioplasty with optional stenting. Circulation 2007;115:2745-9. http://dx.doi.org/10.1161/CIRCULATIONAHA.107.688341.
- Sabeti S, Czerwenka-Wenkstetten A, Dick P, Schlager O, Amighi J, Mlekusch I, et al. Quality of life after balloon angioplasty versus stent implantation in the superficial femoral artery: findings from a randomized controlled trial. J Endovasc Ther 2007;14:431-7. http://dx.doi.org/10.1583/1545-1550(2007)14[431:QOLABA]2.0.CO;2.
- Becquemin JP, Favre JP, Marzelle J, Nemoz C, Corsin C, Leizorovicz A. Systematic versus selective stent placement after superficial femoral artery balloon angioplasty: a multicenter prospective randomized study. J Vasc Surg 2003;37:487-94. http://dx.doi.org/10.1067/mva.2003.155.
- Cejna M, Thurnher S, Illiasch H, Horvath W, Waldenberger P, Hornik K, et al. PTA versus Palmaz stent placement in femoropopliteal artery obstructions: a multicenter prospective randomized study. J Vasc Interv Radiol 2001;12:23-31. http://dx.doi.org/10.1016/S1051-0443(07)61397-9.
- Grimm J, Muller-Hulsbeck S, Jahnke T, Hilbert C, Brossmann J, Heller M. Randomized study to compare PTA alone versus PTA with Palmaz stent placement for femoropopliteal lesions. J Vasc Interv Radiol 2001;12:935-42. http://dx.doi.org/10.1016/S1051-0443(07)61572-3.
- Rand T, Basile A, Cejna M, Fleischmann D, Funovics M, Gschwendtner M, et al. PTA versus carbofilm-coated stents in infrapopliteal arteries: pilot study. Cardiovasc Interv Radiol 2006;29:29-38. http://dx.doi.org/10.1007/s00270-005-0276-9.
- Vroegindeweij D, Vos LD, Tielbeek AV, Buth J, vd Bosch HC. Balloon angioplasty combined with primary stenting versus balloon angioplasty alone in femoropopliteal obstructions: a comparative randomized study. Cardiovasc Interv Radiol 1997;20:420-5. http://dx.doi.org/10.1007/s002709900186.
- Zdanowski Z, Albrechtsson U, Lundin A, Jonung T, Ribbe E, Thorne J, et al. Percutaneous transluminal angioplasty with or without stenting for femoropopliteal occlusions? A randomized controlled study. Int Angiol 1999;18:251-5.
- Dake M, Ansel G, Jaff M, Ohki T, Saxon R, Smouse HB, et al. Zilver PTX: a prospective, randomized trial of the polymer-free paditaxel-eluting stent compared to balloon angioplasty with provisional bare metal steating in patients with superficial femoral artery disease. Journal of the American College of Cardiology Conference, 22nd Annual Symposium of the Transcatheter Cardiovascular Therapeutics, Washington DC, 21–25 September 2010. J Am Coll Cardiol 2010;56.
- Ansel G. Zilver PTX randomized trial of paclitaxel-eluting stents for femoropopliteal artery disease: 24-month update n.d.
- Dake M, Ansel G, Ragheb A. Interim report on the Zilver PTX clinical trial n.d.
- Duda SH, Pusich B, Richter G, Landwehr P, Oliva VL, Tielbeek A, et al. Sirolimus-eluting stents for the treatment of obstructive superficial femoral artery disease: six-month results. Circulation 2002;106:1505-9. http://dx.doi.org/10.1161/01.CIR.0000029746.10018.36.
- Duda SH, Bosiers M, Lammer J, Scheinert D, Zeller T, Tielbeek A, et al. Sirolimus-eluting versus bare nitinol stent for obstructive superficial femoral artery disease: the SIROCCO II trial. J Vasc Interv Radiol 2005;16:331-8. http://dx.doi.org/10.1097/01.RVI.0000151260.74519.CA.
- Duda SH, Bosiers M, Lammer J, Scheinert D, Zeller T, Oliva V, et al. Drug-eluting and bare nitinol stents for the treatment of atherosclerotic lesions in the superficial femoral artery: long-term results from the SIROCCO trial. J Endovasc Ther 2006;13:701-10. http://dx.doi.org/10.1583/05-1704.1.
- Rastan A, Tepe G, Krankenberg H, Zahorsky R, Beschorner U, Noory E, et al. Sirolimus-eluting stents vs. bare-metal stents for treatment of focal lesions in infrapopliteal arteries: a double-blind, multi-centre, randomized clinical trial. Eur Heart J 2011;32:2274-81. http://dx.doi.org/10.1093/eurheartj/ehr144.
- Saxon RR, Coffman JM, Gooding JM, Natuzzi E, Ponec DJ. Long-term results of ePTFE stent-graft versus angioplasty in the femoropopliteal artery: single center experience from a prospective, randomized trial. J Vasc Interv Radiol 2003;14:303-11. http://dx.doi.org/10.1097/01.RVI.0000058425.01661.d0.
- Saxon RR, Dake MD, Volgelzang RL, Katzen BT, Becker GJ. Randomized, multicenter study comparing expanded polytetrafluoroethylene-covered endoprosthesis placement with percutaneous transluminal angioplasty in the treatment of superficial femoral artery occlusive disease. J Vasc Interv Radiol 2008;19:823-32. http://dx.doi.org/10.1016/j.jvir.2008.02.008.
- Nakamura S, Conroy RM, Gordon IL, Deutsch LS, Maheswaran B, Antone CS, et al. A randomized trial of transcutaneous extraction atherectomy in femoral arteries: intravascular ultrasound observations. J Clin Ultrasound 1995;23:461-71. http://dx.doi.org/10.1002/jcu.1870230802.
- Vroegindeweij D, Kemper FJ, Tielbeek AV, Buth J, Landman G. Recurrence of stenoses following balloon angioplasty and Simpson atherectomy of the femoro-popliteal segment. A randomised comparative 1-year follow-up study using colour flow duplex. Eur J Vasc Surg 1992;6:164-71. http://dx.doi.org/10.1016/S0950-821X(05)80235-X.
- Vroegindeweij D, Tielbeek AV, Buth J, Schol FP, Hop WC, Landman GH. Directional atherectomy versus balloon angioplasty in segmental femoropopliteal artery disease: two-year follow-up with color-flow duplex scanning. J Vasc Surg 1995;21:255-68. http://dx.doi.org/10.1016/S0741-5214(95)70267-9.
- Tielbeek AV, Vroegindeweij D, Buth J, Landman GH. Comparison of balloon angioplasty and Simpson atherectomy for lesions in the femoropopliteal artery: angiographic and clinical results of a prospective randomized trial. J Vasc Interv Radiol 1996;7:837-44. http://dx.doi.org/10.1016/S1051-0443(96)70857-6.
- Amighi J, Schillinger M, Dick P, Schlager O, Sabeti S, Mlekusch W, et al. De novo superficial femoropopliteal artery lesions: peripheral cutting balloon angioplasty and restenosis rates – randomized controlled trial. Radiology 2008;247:267-72. http://dx.doi.org/10.1148/radiol.2471070749.
- Dick P, Sabeti S, Mlekusch W, Schlager O, Amighi J, Haumer M, et al. Conventional balloon angioplasty versus peripheral cutting balloon angioplasty for treatment of femoropopliteal artery in-stent restenosis: initial experience. Radiology 2008;248:297-302. http://dx.doi.org/10.1148/radiol.2481071159.
- Jahnke T, Mueller-Huelsbeck S, Charalambous N, Trentmann J, Jamili A, Huemme TH, et al. Prospective, randomized single-center trial to compare cryoplasty versus conventional angioplasty in the popliteal artery: midterm results of the COLD study. J Vasc Interv Radiol 2010;21:186-94. http://dx.doi.org/10.1016/j.jvir.2009.10.021.
- Spiliopoulos S, Katsanos K, Karnabatidis D, Diamantopoulos A, Kagadis GC, Christeas N, et al. Cryoplasty versus conventional balloon angioplasty of the femoropopliteal artery in diabetic patients: long-term results from a prospective randomized single-center controlled trial. Cardiovasc Interv Radiol 2010;33:929-38. http://dx.doi.org/10.1007/s00270-010-9915-x.
- Gallino A, Do DD, Alerci M, Baumgartner I, Cozzi L, Segatto JM, et al. Effects of probucol versus aspirin and versus brachytherapy on restenosis after femoropopliteal angioplasty: the PAB randomized multicenter trial. J Endovasc Ther 2004;11:595-604. http://dx.doi.org/10.1583/04-1269MR.1.
- Bonvini R, Baumgartner I, Do DD, Alerci M, Segatto JM, Tutta P, et al. Late acute thrombotic occlusion after endovascular brachytherapy and stenting of femoropopliteal arteries. J Am Coll Cardiol 2003;41:409-12. http://dx.doi.org/10.1016/S0735-1097(02)02684-0.
- Diehm N, Silvestro A, Do DD, Greiner R, Triller J, Mahler F, et al. Endovascular brachytherapy after femoropopliteal balloon angioplasty fails to show robust clinical benefit over time. J Endovasc Ther 2005;12:723-30. http://dx.doi.org/10.1583/05-1583MR.1.
- Zehnder T, von Briel C, Baumgartner I, Triller J, Greiner R, Mahler F, et al. Endovascular brachytherapy after percutaneous transluminal angioplasty of recurrent femoropopliteal obstructions. J Endovasc Ther 2003;10:304-11. http://dx.doi.org/10.1583/1545-1550(2003)010<0304:EBAPTA>2.0.CO;2.
- Hagenaars T, If AP, van Sambeek MR, Coen VL, van Tongeren RB, Gescher FM, et al. Gamma radiation induces positive vascular remodeling after balloon angioplasty: a prospective, randomized intravascular ultrasound scan study. J Vasc Surg 2002;36:318-24. http://dx.doi.org/10.1067/mva.2002.124373.
- Krueger K, Landwehr P, Bendel M, Nolte M, Stuetzer H, Bongartz R, et al. Endovascular gamma irradiation of femoropopliteal de novo stenoses immediately after PTA: interim results of prospective randomized controlled trial. Radiology 2002;224:519-28. http://dx.doi.org/10.1148/radiol.2242010882.
- Krueger K, Zaehringer M, Bendel M, Stuetzer H, Strohe D, Nolte M, et al. De novo femoropopliteal stenoses: endovascular gamma irradiation following angioplasty – angiographic and clinical follow-up in a prospective randomized controlled trial. Radiology 2004;231:546-54. http://dx.doi.org/10.1148/radiol.2312030421.
- Wolfram RM, Budinsky AC, Pokrajac B, Potter R, Minar E. Endovascular brachytherapy for prophylaxis of restenosis after femoropopliteal angioplasty: five-year follow-up – prospective randomized study. Radiology 2006;240:878-84. http://dx.doi.org/10.1148/radiol.2403050883.
- Minar E, Pokrajac B, Maca T, Ahmadi R, Fellner C, Mittlbock M, et al. Endovascular brachytherapy for prophylaxis of restenosis after femoropopliteal angioplasty: results of a prospective randomized study. Circulation 2000;102:2694-9. http://dx.doi.org/10.1161/01.CIR.102.22.2694.
- Wolfram RM, Budinsky AC, Pokrajac B, Potter R, Minar E. Endovascular brachytherapy: restenosis in de novo versus recurrent lesions of femoropopliteal artery – the Vienna experience. Radiology 2005;236:338-42. http://dx.doi.org/10.1148/radiol.2361040084.
- Pokrajac B, Potter R, Wolfram RM, Budinsky AC, Kirisits C, Lileg B, et al. Endovascular brachytherapy prevents restenosis after femoropopliteal angioplasty: results of the Vienna-3 randomised multicenter study. Radiother Oncol 2005;74:3-9. http://dx.doi.org/10.1016/j.radonc.2004.08.015.
- Pokrajac B, Potter R, Maca T, Fellner C, Mittlbock M, Ahmadi R, et al. Intraarterial 192Ir high-dose-rate brachytherapy for prophylaxis of restenosis after femoropopliteal percutaneous transluminal angioplasty: the prospective randomized Vienna-2-trial radiotherapy parameters and risk factors analysis. Int J Radiat Oncol Biol Phys 2000;48:923-31. http://dx.doi.org/10.1016/S0360-3016(00)00716-1.
- van Tongeren RB, van Sambeek MR, van OH, Coen VL, Schmitz PI, Gescher FM, et al. Endovascular brachytherapy for the prevention of restenosis after femoropopliteal angioplasty. Results of the VARA Trial. J Cardiovasc Surg 2005;46:437-43.
- Wyttenbach R, Corti R, Alerci M, Cozzi L, Di VM, Segatto JM, et al. Effects of percutaneous transluminal angioplasty and endovascular brachytherapy on vascular remodeling of human femoropopliteal artery: 2 years follow-up by noninvasive magnetic resonance imaging. Eur J Vasc Endovasc Surg 2007;34:416-23. http://dx.doi.org/10.1016/j.ejvs.2007.05.017.
- Wyttenbach R, Gallino A, Alerci M, Mahler F, Cozzi L, Di VM, et al. Effects of percutaneous transluminal angioplasty and endovascular brachytherapy on vascular remodeling of human femoropopliteal artery by noninvasive magnetic resonance imaging. Circulation 2004;110:1156-61. http://dx.doi.org/10.1161/01.CIR.0000140672.70862.5B.
- Fritz P, Stein U, Hasslacher C, Zierhut D, Wannenmacher M, Pritsch M. External beam radiotherapy fails to prevent restenosis after iliac or femoropopliteal percutaneous transluminal angioplasty: results of a prospective randomized double-blind study. Int J Radiat Oncol Biol Phys 2004;59:815-21. http://dx.doi.org/10.1016/j.ijrobp.2003.11.036.
- Therasse E, Donath D, Lesperance J, Tardif JC, Guertin MC, Oliva VL, et al. External beam radiation to prevent restenosis after superficial femoral artery balloon angioplasty. Circulation 2005;111:3310-15. http://dx.doi.org/10.1161/CIRCULATIONAHA.104.502179.
- Scheinert D, Zeller T, Duda S, Ricke J, Bosiers M. Six month follow-up from a prospective, randomized trial comparing a paclitaxel-coated balloon catheter to a noncoated balloon catheter in patients with femoropopliteal disease n.d.
- Scheinert D, Zeller T, Duda S, Krankenberg H, Ricke J, Bosiers M. A prospective, multicenter, single blind, randomized, controlled trial comparing the Lutonix catheter vs. standard balloon angioplasty for treatment of femoropopliteal arteries with and without stenting (LEVANT I) n.d.
- Tepe G, Zeller T, Albrecht T, Heller S, Schwarzwalder U, Beregi JP, et al. Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg. N Eng J Med 2008;358:689-99. http://dx.doi.org/10.1056/NEJMoa0706356.
- Tepe G, Zeller T, Albrecht T, Speck U. Drug-coated balloons for the prevention of restenosis in peripheral arteries. Angiographic and clinical two-year follow-up of the THUNDER trial. Am J Cardiol 2008;102.
- Tepe G, Zeller T, Albrecht T, Speck U. Persistent benefit of drug coated balloons for prevention of restenosis in peripheral arteries: angiographic follow-up after 6 and 12 months n.d.
- Werk M, Langner S, Reinkensmeier B, Boettcher HF, Tepe G, Dietz U, et al. Inhibition of restenosis in femoropopliteal arteries: paclitaxel-coated versus uncoated balloon: femoral paclitaxel randomized pilot trial. Circulation 2008;118:1358-65. http://dx.doi.org/10.1161/CIRCULATIONAHA.107.735985.
- Belli AM, Cumberland DC, Procter AE, Welsh CL. Follow-up of conventional angioplasty versus laser thermal angioplasty for total femoropopliteal artery occlusions: results of a randomized trial. J Vasc Interv Radiol 1991;2:485-8. http://dx.doi.org/10.1016/S1051-0443(91)72229-X.
- Belli AM, Cumberland DC, Procter AE, Welsh CL. Total peripheral artery occlusions: conventional versus laser thermal recanalization with a hybrid probe in percutaneous angioplasty – results of a randomized trial. Radiology 1991;181:57-60.
- Fisher CM, Fletcher JP, May J, White GH, Lord RS, Crozier J, et al. No additional benefit from laser in balloon angioplasty of the superficial femoral artery. Eur J Vasc Endovasc Surg 1996;11:349-52. http://dx.doi.org/10.1016/S1078-5884(96)80084-0.
- Lammer J, Pilger E, Decrinis M, Quehenberger F, Klein GE, Stark G. Pulsed excimer laser versus continuous-wave Nd:YAG laser versus conventional angioplasty of peripheral arterial occlusions: prospective, controlled, randomised trial. Lancet 1992;340:1183-8.
- Spies JB, LeQuire MH, Brantley SD, Williams JE, Beckett WC, Mills JL. Comparison of balloon angioplasty and laser thermal angioplasty in the treatment of femoropopliteal atherosclerotic disease: initial results of a prospective randomized trial. Work in progress. J Vasc Interv Radiol 1990;1:39-42. http://dx.doi.org/10.1016/S1051-0443(90)72500-6.
- Tobis JM, Conroy R, Deutsch LS, Gordon I, Honye J, Andrews J, et al. Laser-assisted versus mechanical recanalization of femoral arterial occlusions. Am J Cardiol 1991;68:1079-86. http://dx.doi.org/10.1016/0002-9149(91)90499-B.
- Dake MD, Ansel G, Jaff M, Ohki T, Saxon R, Smouse HB, et al. Paclitaxel-eluting stents show superiority to balloon angioplasty and bare metal stents in femoropopliteal disease: twelve month Zilver PTX randomized study results. Circulation 2011;4:495-504. http://dx.doi.org/10.1161/CIRCINTERVENTIONS.111.962324.
- Bachoo P, Thorpe PA, Maxwell H, Welch K. Endovascular stents for intermittent claudication. Cochrane Database Syst Rev 2010;1. http://dx.doi.org/10.1002/14651858.CD003228.
- Twine CP, Coulston J, Shandall A, McLain AD. Angioplasty versus stenting for superficial femoral artery lesions. Cochrane Database Syst Rev 2009;2. http://dx.doi.org/10.1002/14651858.CD006767.
- Grenacher L, Saam T, Geier A, Muller-Hulsbeck S, Cejna M, Kauffmann GW, et al. [PTA versus Palmaz stent placement in femoropopliteal artery stenoses: results of a multicenter prospective randomized study (REFSA)]. Fortschr Röntgenstr 2004;176:1302-10.
- Kasapis C, Henke PK, Chetcuti SJ, Koenig GC, Rectenwald JE, Krishnamurthy VN, et al. Routine stent implantation vs. percutaneous transluminal angioplasty in femoropopliteal artery disease: a meta-analysis of randomized controlled trials. Eur Heart J 2009;30:44-55. http://dx.doi.org/10.1093/eurheartj/ehn514.
- Sculpher M, Michaels J, McKenna M, Minor J. A cost-utility analysis of laser-assisted angioplasty for peripheral arterial occlusions. Int J Technol Assess Health Care 1996;12:104-25. http://dx.doi.org/10.1017/S0266462300009430.
- Hunink MG, Wong JB, Donaldson MC, Meyerovitz MF, de Vries J, Harrington DP. Revascularization for femoropopliteal disease. A decision and cost-effectiveness analysis. JAMA 1995;274:165-71.
- de Vries SO, Visser K, de Vries JA, Wong JB, Donaldson MC, Hunink MG. Intermittent claudication: cost-effectiveness of revascularization versus exercise therapy. Radiology 2002;222:25-36.
- Holler D, Claes C, Von Der SJM. Cost–utility analysis of treating severe peripheral arterial occlusive disease. Int J Angiol 2006;15:25-33. http://dx.doi.org/10.1007/s00547-006-2073-y.
- Muradin GS, Myriam Hunink MG. Cost and patency rate targets for the development of endovascular devices to treat femoropopliteal arterial disease. Radiology 2001;218:464-9.
- Visser K, de Vries SO, Kitslaar PJ, van Engelshoven JM, Hunink MG. Cost-effectiveness of diagnostic imaging work-up and treatment for patients with intermittent claudication in the Netherlands. Eur J Vasc Endovasc Surg 2003;25:213-23. http://dx.doi.org/10.1053/ejvs.2002.1838.
- Forbes JF, Adam DJ, Bell J, Fowkes FG, Gillespie I, Raab GM, et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: health-related quality of life outcomes, resource utilization, and cost-effectiveness analysis. J Vasc Surg 2010;51:43-51. http://dx.doi.org/10.1016/j.jvs.2010.01.076.
- National Institute for Health and Care Excellence . Lower Limb Peripheral Arterial Disease: Diagnosis and Management. (Clinical Guideline 147) 2012. http://guidance.nice.org.uk/CG147 (accessed 27 January 2014).
- Bradbury AW, Ruckley CV, Fowkes FGR, Forbes JF, Gillespie I, Adam DJ, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet 2005;366:1925-34.
- Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FG, Gillespie I, et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: an intention-to-treat analysis of amputation-free and overall survival in patients randomized to a bypass surgery-first or a balloon angioplasty-first revascularization strategy. J Vasc Surg 2010;51:5-17. http://dx.doi.org/10.1016/j.jvs.2010.01.073.
- Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FG, Gillespie I, et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: analysis of amputation free and overall survival by treatment received. J Vasc Surg 2010;51:18-31. http://dx.doi.org/10.1016/j.jvs.2010.01.074.
- Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FG, Gillespie I, et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: a survival prediction model to facilitate clinical decision making. J Vasc Surg 2010;51:52-68. http://dx.doi.org/10.1016/j.jvs.2010.01.077.
- Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FG, Gillespie I, et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: A description of the severity and extent of disease using the Bollinger angiogram scoring method and the TransAtlantic Inter-Society Consensus II classification. J Vasc Surg 2010;51:32-4. http://dx.doi.org/10.1016/j.jvs.2010.01.075.
- Department of Health 2012. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_131140 (accessed October 2013).
- Personal Social Services Research Unit . Unit Costs of Health and Social Care 2010 2012. www.pssru.ac.uk/project-pages/unit-costs/2010/index.php (accessed October 2013).
- Axisa B, Fishwick G, Bolia A, Thompson MM, London NJM, Bell PRF, et al. Complications following peripheral angioplasty. Ann Royal Coll Surg Eng 2002;84:39-42.
- White CJ, Gray WA. Endovascular therapies for peripheral arterial disease – an evidence-based review. Circulation 2007;116:2203-15. http://dx.doi.org/10.1161/CIRCULATIONAHA.106.621391.
- Government Actuary’s Department . Life Tables Index 2012. www.gad.gov.uk/Demography%20Data/Life%20Tables/ (accessed October 2013).
- Brass EP, Anthony R, Dormandy J, Hiatt WR, Jiao J, Nakanishi A, et al. Parenteral therapy with lipo-ecraprost, a lipid-based formulation of a PGE1 analog, does not alter six-month outcomes in patients with critical leg ischemia. J Vasc Surg 2006;43:752-9. http://dx.doi.org/10.1016/j.jvs.2005.11.041.
- Briggs A, Sculpher M, Claxton K. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Bergqvist D, Troeng J, Elfstrom B, Hedberg K, Ljungstrom G, Norgren L, et al. Auditing surgical outcome: ten years with the Swedish vascular registry – Swedvasc. Eur J Surg 1998;164:3-32.
- Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Eng J Med 1992;326:381-6. http://dx.doi.org/10.1056/NEJM199202063260605.
- Hunink MG, Wong JB, Donaldson MC, Meyerovitz MF, Harrington DP. Patency results of percutaneous and surgical revascularization for femoropopliteal arterial disease. Med Decis Making 1994;14:71-8. http://dx.doi.org/10.1177/0272989X9401400109.
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGR, et al. Inter-society consensus for the management of peripheral arterial disease. Int Angiol 2007;26:81-157.
- de Vries SO, Magruder MC, Hunink MGM. Contralateral symptoms after unilateral intervention for peripheral occlusive disease. J Vasc Surg 1998;27:414-21. http://dx.doi.org/10.1016/S0741-5214(98)70315-5.
- Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health 2010;13:509-18. http://dx.doi.org/10.1111/j.1524-4733.2010.00700.x.
- Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, et al. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): Executive summary – A collaborative report from the American association for vascular surgery/society for vascular surgery, society for cardiovascular angiography and interventions, society for vascular medicine and biology, society of interventional radiology, and the ACC/AHA task force on practice guidelines (Writing committee to develop guidelines for the management of patients with peripheral arterial disease). Circulation 2006;113:1474-547.
- Spronk S, Bosch JL, den Hoed PT, Veen HF, Pattynama PM, Hunink MG. Cost-effectiveness of endovascular revascularization compared to supervised hospital-based exercise training in patients with intermittent claudication: a randomized controlled trial. J Vasc Surg 2008;48:1472-80. http://dx.doi.org/10.1016/j.jvs.2008.06.016.
- Greenhalgh RM, Belch JJ, Brown LC, Gaines PA, Gao L, Reise JA, et al. The adjuvant benefit of angioplasty in patients with mild to moderate intermittent claudication (MIMIC) managed by supervised exercise, smoking cessation advice and best medical therapy: results from two randomised trials for stenotic femoropopliteal and aortoiliac arterial disease. Eur J Vasc Endovasc Surg 2008;36:680-8.
- Gorenoi V, Dintsios CM, Schonermark MP, Hagen A. Intravascular brachytherapy for peripheral vascular disease. GMS Health Technol Assess 2008;4.
- Salman M, Asif A. Stent graft for nephrologists: concerns and consensus. Clin J Am Soc Nephrol 2010;5:1347-52. http://dx.doi.org/10.2215/CJN.02380310.
- Schmieder GC, Carroll M, Panneton JM. Poor outcomes with cryoplasty for lower extremity arterial occlusive disease. J Vasc Surg 2010;52:362-8. http://dx.doi.org/10.1016/j.jvs.2010.03.012.
- Rastan A, Tepe G, Krankenberg H, Zahorsky R, Schwarzwalder U, Noory E, et al. Sirolimus-eluting stents versus bare-metal stents for treatment of infrapopliteal arteries: A double blind, multi-centre randomised clinical trial n.d.:xv-xvi.
- Claxton K, Possati G. An economic approach to clinical trial design and research priority-setting. Health Econ 1996;5:513-24. http://dx.doi.org/10.1002/(SICI)1099-1050(199611)5:6<513::AID-HEC237>3.0.CO;2-9.
- Agostoni P, Vermeersch P, Semeraro O, Verheye S, Van LG, Van den Heuvel P, et al. Intravascular ultrasound comparison of sirolimus-eluting stent versus bare metal stent implantation in diseased saphenous vein grafts (from the RRISC [reduction of restenosis in saphenous vein grafts with cypher sirolimus-eluting stent] trial). Am J Cardiol 2007;100:52-8. http://dx.doi.org/10.1016/j.amjcard.2007.02.052.
- Ahn SS, Eton D, Yeatman LR, Deutsch LS, Moore WS. Intraoperative peripheral rotary atherectomy: early and late clinical results. Ann Vasc Surg 1992;6:272-80. http://dx.doi.org/10.1007/BF02000274.
- Aldea GS, Gaudiani JA, Shapira OM, O’Gara P, Bao Y, Lazar HL, et al. Comparison of risk profile and outcomes in patients undergoing surgical and catheter-based revascularization. J Card Surg 1998;13:81-9.
- Allaqaband S, Kirvaitis R, Jan F, Bajwa T. Endovascular treatment of peripheral vascular disease. Curr. Probl Cardiol 2009;34:359-476. http://dx.doi.org/10.1016/j.cpcardiol.2009.05.001.
- Aoki J, Mintz GS, Weissman NJ, Mann JT, Cannon L, Greenberg J, et al. Chronic arterial responses to overlapping paclitaxel-eluting stents: insights from serial intravascular ultrasound analyses in the TAXUS-V and -VI trials. JACC Cardiovasc Interv 2008;1:161-7.
- Carreira JM, Reyes R, Gude F, Gorriz E, Gallardo L, Pardo MD, et al. Long-term follow-up of Symphony nitinol stents in iliac arteriosclerosis obliterans. Minim Invasive Ther Allied Technol 2008;17:34-42. http://dx.doi.org/10.1080/13645700701800343.
- Dalainas I, Nano G, Kashyap A, Anand KP, Kashyap S, Golledge J, et al. Balloon angioplasty or nitinol stents for peripheral-artery disease. N Eng J Med 2006;355:521-4.
- Das TS. Percutaneous peripheral revascularisation with excimer laser: equipment, technique and results. Lasers Med Sci 2001;16:101-7. http://dx.doi.org/10.1007/PL00011338.
- Diehm N, Baumgartner I, Juni P. Local paclitaxel delivery in peripheral vascular disease. N Engl J Med 2008;358:689-99.
- Dieter RS, Nanjundappa A, Lopez JJ. Drug-eluting stents for critical limb ischemia. J Am Coll Cardiol 2010;56. http://dx.doi.org/10.1016/j.jacc.2010.05.038.
- Fleisher HL, Thompson BW, McCowan TC, Ferris EJ, Reifsteck JE, Barnes RW. Human percutaneous laser angioplasty. Patient selection criteria and early results. Am J Surg 1987;154:666-70.
- Gaines PA, Schulte KL, Muller-Hulsbeck S, Seelen J, Maleux G, van Overhagen H, et al. A multicentre evaluation of the Medtronic AVE Flexible Iliac Bridge Stent in the iliac arteries (the first study). Eur J Vasc Endovasc Surg 2005;29:124-30. http://dx.doi.org/10.1016/j.ejvs.2004.12.004.
- Hall P, Gaglione A, Giordano M, Colombo A. Peripheral angioplasty and interventions. Int Angiol 1993;12:32-9.
- Hartnell GG, Jones AM, Murphy P. Do hydrophilic guidewires affect the technical success rates of percutaneous angioplasty?. Angiology 1995;46:229-34. http://dx.doi.org/10.1177/000331979504600306.
- Hassan AK, Bergheanu SC, Stijnen T, van der Hoeven BL, Snoep JD, Plevier JW, et al. Late stent malapposition risk is higher after drug-eluting stent compared with bare-metal stent implantation and associates with late stent thrombosis. Eur Heart J 2010;31:1172-80. http://dx.doi.org/10.1093/eurheartj/ehn553.
- Kaneda H, Ako J, Terashima M, Waseda K, Yock PG, Fitzgerald PJ. Distribution pattern of neointimal hyperplasia following sirolimus-eluting stent implantation assessed by 3-dimensional intravascular ultrasound. Int J Cardiol 2009;135:243-5. http://dx.doi.org/10.1016/j.ijcard.2008.01.054.
- Killewich LA. Improving functional status and quality of life in elderly patients with peripheral arterial disease. J Am Coll Surg 2006;202:345-55. http://dx.doi.org/10.1016/j.jamcollsurg.2005.09.026.
- Jahnke T, Voshage G, Muller-Hulsbeck S, Grimm J, Heller M, Brossmann J. Endovascular placement of self-expanding nitinol coil stents for the treatment of femoropopliteal obstructive disease. J Vasc Interv Radiol 2002;13:257-66. http://dx.doi.org/10.1016/S1051-0443(07)61718-7.
- Jahnke T, Andresen R, Muller-Hulsbeck S, Schafer FK, Voshage G, Heller M, et al. Hemobahn stent-grafts for treatment of femoropopliteal arterial obstructions: midterm results of a prospective trial. J Vasc Interv Radiol 2003;14:41-5. http://dx.doi.org/10.1097/01.RVI.0000052290.26939.cb.
- Jeans WD, Murphy P, Hughes AO, Horrocks M, Baird RN. Randomized trial of laser-assisted passage through occluded femoro-popliteal arteries. Br J Radiol 1990;63:19-21. http://dx.doi.org/10.1259/0007-1285-63-745-19.
- Lammer J, Dake MD, Bleyn J, Katzen BT, Cejna M, Piquet P, et al. Peripheral arterial obstruction: prospective study of treatment with a transluminally placed self-expanding stent-graft. International Trial Study Group. Radiology 2000;217:95-104.
- London NJ, Bolia A, Bell PR. Subintimal angioplasty for femoropopliteal artery occlusion. Lancet 1993;341. http://dx.doi.org/10.1016/0140-6736(93)90098-2.
- Nicholson T. Percutaneous transluminal angioplasty and enclosed thrombolysis versus percutaneous transluminal angioplasty in the treatment of femoropopliteal occlusions: results of a prospective randomized trial. Cardiovasc Interv Radiol 1998;21:470-4. http://dx.doi.org/10.1007/s002709900306.
- Randon C, Jacobs B, De Ryck F, Vermassen F. Angioplasty or primary stenting for infrapopliteal lesions: results of a prospective randomized trial. Cardiovasc Interv Radiol 2010;33:260-9. http://dx.doi.org/10.1007/s00270-009-9765-6.
- Roubin GS. Part II: endovascular therapy: revascularization/primary therapy. J Interv Cardiol 1997;10:481-3. http://dx.doi.org/10.1111/j.1540-8183.1997.tb00078.x.
- Sen SS, Khandker RK, Roth DA, Thomas J. A one-year comparison of cost and outcomes of angioplasty in stent and nonstent patients. Am J Ther 2005;12:210-17.
- Sgura FA, Di Mario C, Liistro F, Montorfano M, Colombo A, Grube E. The lunar stent characteristics and clinical results. Herz 2002;27:514-17.
- Tanabe K, Serruys PW, Degertekin M, Guagliumi G, Grube E, Chan C, et al. Chronic arterial responses to polymer-controlled paclitaxel-eluting stents: comparison with bare metal stents by serial intravascular ultrasound analyses: data from the randomized TAXUS-II trial. Circulation 2004;109:196-200. http://dx.doi.org/10.1161/01.CIR.0000109137.51122.49.
- Tay K, Taneja M, Irani F, Teo T, Khoo L, Burgmans M, et al. ‘Angioplasty first’ approach for limb salvage in patients with critical limb ischemia. J Vasc Interv Radiol 2011;22. http://dx.doi.org/10.1016/j.jvir.2011.01.094.
- Turco MA, Buchbinder M, Popma JJ, Weissman NJ, Mann T, Doucet S, et al. Pivotal, randomized U.S. study of the Symbiottrade mark covered stent system in patients with saphenous vein graft disease: eight-month angiographic and clinical results from the Symbiot III trial. Catheter Cardiovasc Interv 2006;68:379-88.
- Whyman MR, Fowkes FG, Kerracher EM, Gillespie IN, Lee AJ, Housley E, et al. Is intermittent claudication improved by percutaneous transluminal angioplasty? A randomized controlled trial. J Vasc Surg 1997;26:551-7. http://dx.doi.org/10.1016/S0741-5214(97)70052-1.
- Wolfram RM, Budinsky AC, Pokrajac B, Potter R, Minar E. Vascular brachytherapy with 192Ir after femoropopliteal stent implantation in high-risk patients: twelve-month follow-up results from the Vienna-5 trial. Radiology 2005;236:343-51. http://dx.doi.org/10.1148/radiol.2361040696.
- Zabakis P, Kardamakis DM, Siablis D, Kalogeropoulou C, Karnabatidis D, Malatara G, et al. External beam radiation therapy reduces the rate of re-stenosis in patients treated with femoral stenting: results of a randomised study. Radiother Oncol 2005;74:11-6. http://dx.doi.org/10.1016/j.radonc.2004.09.010.
- Drummond M, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. BMJ 1996;313:275-83. http://dx.doi.org/10.1136/bmj.313.7052.275.
- Evers S, Goossens M, De Vet H, Van Tulder M, Ament A. Criteria list for assessment of methodological quality of economic evaluations: Consensus on Health Economic Criteria. Int J Technol Assess Health Care 2005;21:240-5.
- Dormandy J, Mahla E, Ascani A, Balsano F, de Leeuw PW, Blombery P, et al. Fate of patients with chronic leg ischaemia. J Cardiovasc Surg 1989;30:50-7.
- Squires H, Simpson E, Meng Y, Harnan S, Stevens JW, Wong R, et al. A systematic review and economic evaluation of cilostazol, naftidrofuryl oxalate, pentoxifylline and inositol nicotinate for the treatment of intermittent claudication in people with peripheral arterial disease. Health Technol Assess 2011;15.
- Jelnes R, Gaardsting O, Hougaard-Jensen K, Baekgaard N, Tonnesen KH, Schroeder T. Fate in intermittent claudication: outcome and risk factors. BMJ 1986;293:1137-40. http://dx.doi.org/10.1136/bmj.293.6555.1137.
- Levy PJ. Epidemiology and pathophysiology of peripheral arterial disease. Clin Cornerstone 2002;4:1-15. http://dx.doi.org/10.1016/S1098-3597(02)90012-8.
- Johnston KW. Femoral and popliteal arteries: reanalysis of results of balloon angioplasty. Radiology 1992;183:767-71.
- Capek P, McLean GK, Berkowitz HD. Femoropopliteal angioplasty: factors influencing long-term success. Circulation 1991;83:I70-80.
- Hunink MG, Donaldson MC, Meyerovitz MF, Polak JF, Whittemore AD, Kandarpa K, et al. Risks and benefits of femoropopliteal percutaneous balloon angioplasty. J Vasc Surg 1993;17:183-92.
- Jørgensen B, Henriksen LO, Karle A, Sager P, Holstein PE, Tonnesen KH. Percutaneous transluminal angioplasty of iliac and femoral arteries in severe lower-limb ischemia. Acta Chir Scand 1988;154:647-52.
- Henriksen LO, Jørgensen B, Holstein PE, Tonnesen KH, Karle A, Sager P. Percutaneous transluminal angioplasty of infrarenal arteries in intermittent claudication. Acta Chir Scand 1988;154:573-6.
- Walden R, Siegel Y, Rubinstein ZJ, Morag B, Bass A, Adar R. Percutaneous transluminal angioplasty. A suggested method for analysis of clinical, arteriographic, and hemodynamic factors affecting the results of treatment. J Vasc Surg 1986;3:583-90. http://dx.doi.org/10.1067/mva.1986.avs0030583.
- Jeans WD, Armstrong S, Cole SE, Horrocks M, Baird RN. Fate of patients undergoing transluminal angioplasty for lower-limb ischemia. Radiology 1990;177:559-64.
- Krepel VM, van Andel GJ, van Erp WF, Breslau PJ. Percutaneous transluminal angioplasty of the femoropopliteal artery: initial and long-term results. Radiology 1985;156:325-8.
- Samson RH, Sprayregen S, Veith FJ, Scher LA, Gupta SK, Ascer E. Management of angioplasty complications, unsuccessful procedures and early and late failures. Ann Surg 1984;199:234-40. http://dx.doi.org/10.1097/00000658-198402000-00017.
- Murray RR, Hewes RC, White RI, Mitchell SE, Auster M, Chang R, et al. Long-segment femoropopliteal stenoses: is angioplasty a boon or a bust?. Radiology 1987;162:473-6.
- Dumville JC, Lee AJ, Smith FB, Fowkes FGR. The health-related quality of life of people with peripheral arterial disease in the community: the Edinburgh Artery Study. Br J Gen Pract 2004;1:826-31.
- Sprengers RW, Teraa M, Moll FL, de Wit GA, van der Graaf Y, Verhaar MC. Quality of life in patients with no-option critical limb ischemia underlines the need for new effective treatment. J Vasc Surg 2010;52:843-9. http://dx.doi.org/10.1016/j.jvs.2010.04.057.
- Sullivan PW, Slejko JF, Sculpher MJ, Ghushchyan V. Catalogue of EQ-5D Scores for the United Kingdom. Med Decis Making 2011;31:800-4. http://dx.doi.org/10.1177/0272989X11401031.
Appendix 1 Search strategy
Databases searched
| Database | Host/system | Date searched | No. of hits |
|---|---|---|---|
| CENTRAL/CCTR | Cochrane Library | 24 May 2011 | 2205 |
| CDSR | Cochrane Library | 24 May 2011 | 35 |
| CINAHL 1982– | EBSCO | 1074 | |
| Citation Indexes (Science and Social Sciences) | Web of Science | RCTs 2000; systematic reviews 203; economics evaluations 703 | |
| DARE | Cochrane Library | 24 May 2011 | 100 |
| EMBASE 1980– | Ovid | RCTs 4428; systematic reviews 453; economics evaluations 761 | |
| MEDLINE 1966– | Ovid | 24 May 2011 | RCTs 1311; systematic reviews 74; economics evaluations 181 |
| NHS EED | Cochrane Library | 123 | |
| NHS HTA | Cochrane Library | 24 May 2011 | 49 |
| MEDLINE In-Process & Other Non-Indexed Citations | Ovid | 24 May 2011 | RCTs 10; systematic reviews 3; economics evaluations 4 |
Other sources searched
| Other source | Date searched |
|---|---|
| ClinicalTrials.gov (http://clinicaltrials.gov/) | May 2011 |
| Current Controlled Trials (www.controlled-trials.com/) | May 2011 |
| EMEA (www.ema.europa.eu/ema/index.jsp?curl=/pages/home/Home_Page.jsp&jsenabled=true) | May 2011 |
| FDA (www.fda.gov/) | May 2011 |
| National Research Register Archive (www.nihr.ac.uk/Pages/NRRArchiveSearch.aspx) | May 2011 |
| NIHR Clinical Research Network Portfolio Database (http://public.ukcrn.org.uk/search/) | May 2011 |
Relevant conference proceedings, as determined by the project team, were searched May 2011.
Proceedings of the VSGBI, the European Society of Vascular and Endovascular Surgery, the British Society of Interventional Radiology, the Cardiovascular and Interventional Radiological Society of Europe, the Society for Interventional Radiology and the Society for Vascular Surgery.
MEDLINE search strategy
Population and intervention terms
-
Peripheral Arterial Disease/
-
peripheral arter$ occlusive disease$.tw.
-
peripheral occlusive arter$ disease$.tw.
-
paod.tw.
-
peripheral arter$ disease$.tw.
-
Arterial Occlusive Diseases/
-
Peripheral Vascular Diseases/
-
peripheral vascular disease$.tw.
-
pad.tw.
-
pvd.tw.
-
Ankle Brachial Index/
-
critical limb isch?emia.tw.
-
limb salvage.tw.
-
Limb Salvage/
-
Intermittent Claudication/
-
claudicat$.tw.
-
Constriction, Pathologic/
-
femoral artery/ or popliteal artery/ or tibial arteries/
-
17 and 18
-
(narrow$ or obstruct$ or harden$ or steno$ or resteno$ or constric$ or occlus$).tw.
-
femoral arter$.tw.
-
leg arter$.tw.
-
peripheral arter$.tw.
-
popliteal.tw.
-
infrapopliteal.tw.
-
femoropopliteal.tw.
-
or/21–26
-
20 and 27
-
Atherosclerosis/
-
Arteriosclerosis/
-
atheroma/
-
atherosclero$.tw.
-
(arteriosclero$ or athereosclero$ or atheroma$).tw.
-
or/29–33
-
27 and 34
-
18 and 20
-
17 and 27
-
18 and 34
-
femoral atheroma$.tw.
-
angiotome.tw.
-
or/1–16,19,28,35–40
-
endovascular procedures/ or angioplasty, balloon/ or angioplasty, balloon, laser-assisted/ or angioplasty, laser/ or atherectomy/ or catheterization, peripheral/
-
stents/ or drug-eluting stents/
-
stent$.tw.
-
drug eluting.tw.
-
stent graft$.tw.
-
sirolimus paclitaxel.tw.
-
paclitaxel-eluting stent$.tw.
-
sirolimus-eluting stent$.tw.
-
nitinol.tw.
-
palmaz.tw.
-
viabahn.tw.
-
pulsar-18.tw.
-
lifestent.tw.
-
protege.tw.
-
absolute.tw.
-
xpert.tw.
-
zilver.tw.
-
haemobahn.tw.
-
turbo elite.tw.
-
atherectomy.tw.
-
silverhawk.tw.
-
turbohawk.tw.
-
wholey.tw.
-
hi-torque.tw.
-
loc.tw.
-
tad.tw.
-
atherocath.tw.
-
transluminal extraction catheter.tw.
-
tec.tw.
-
predator 360 pad system$.tw.
-
dimondback 360 pad system$.tw.
-
dimondback.tw.
-
pad system$.tw.
-
balloon$.tw.
-
cutting balloon$.tw.
-
scoring balloon$.tw.
-
high pressure balloon$.tw.
-
drug-eluting balloon$.tw.
-
cryoplasty.tw.
-
polarcath.tw.
-
paccocath.tw.
-
dior.tw.
-
genie.tw.
-
advance 18 ptx.tw.
-
advance 18.tw.
-
laser angioplasty.tw.
-
spectranetics.tw.
-
radiotherapy/ or brachytherapy/
-
radiotherap$.tw.
-
brachytherap$.tw.
-
ultraso$.tw.
-
radioisotopes.tw.
-
or/42–93
-
41 and 94
Terms 1–41 were terms for the population and terms 42–94 were terms for the different interventions. These terms were combined together to find relevant literature and then combined with filters designed to retrieve systematic reviews, RCTs and economic evaluations, as appropriate. The filters for MEDLINE are provided below.
Randomised controlled trial filter
-
randomized controlled trial.pt.
-
controlled clinical trial.pt.
-
randomized controlled trials/
-
random allocation/
-
double blind method/
-
single blind method/
-
clinical trial.pt.
-
exp Clinical Trial/
-
(clin$ adj25 trial$).ti,ab.
-
((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab.
-
placebos/
-
placebos.ti,ab.
-
random.ti,ab.
-
research design/
-
or/1–14
Systematic review filter
-
Meta analysis/
-
Meta analys$.tw.
-
Metaanaly$.tw.
-
exp Literature review/
-
(systematic adj (review or overview)).tw.
-
or/1–5
-
Commentary.pt.
-
Letter.pt.
-
Editorial.pt.
-
Animals/
-
or/7–10
-
6 not 11
Economic evaluations filter
-
Economics/
-
"costs and cost analysis"/
-
Cost allocation/
-
Cost-benefit analysis/
-
Cost control/
-
cost savings/
-
Cost of illness/
-
Cost sharing/
-
"deductibles and coinsurance"/
-
Health care costs/
-
Direct service costs/
-
Drug costs/
-
Employer health costs/
-
Hospital costs/
-
Health expenditures/
-
Capital expenditures/
-
Value of life/
-
exp economics, hospital/
-
exp economics, medical/
-
Economics, nursing/
-
Economics, pharmaceutical/
-
exp "fees and charges"/
-
exp budgets/
-
(low adj cost).mp.
-
(high adj cost).mp.
-
(health?care adj cost$).mp.
-
(fiscal or funding or financial or finance).tw.
-
(cost adj estimate$).mp.
-
(cost adj variable).mp.
-
(unit adj cost$).mp.
-
(economic$ or pharmacoeconomic$ or price$ or pricing).tw.
-
or/1–31
Appendix 2 Excluded studies
| Reference | Reason for exclusion |
|---|---|
| Agostoni et al. 2007110 | Population not PAD |
| Ahn et al. 1992111 | Study design not RCT |
| Aldea et al. 1998112 | Population not PAD |
| Allaqaband et al. 2009113 | Study design not RCT |
| Aoki et al. 2008114 | Population not PAD |
| Carreira et al. 2008115 | Study design not RCT and population aortoiliac |
| Dalainas et al. 2006116 | Study design not RCT |
| Das 2001117 | Study design not RCT |
| Diehm et al. 2008118 | Study design not RCT |
| Dieter et al. 2010119 | Study design not RCT |
| Fleisher et al. 1987120 | Study design not RCT |
| Gaines et al. 2005121 | Population aortoiliac |
| Hall et al. 1993122 | Study design not RCT |
| Hartnell et al. 1995123 | Intervention access device |
| Hassan et al. 2010124 | Population not PAD |
| Kaneda et al. 2009125 | Population not PAD |
| Killewich 2006126 | Study design not RCT |
| Jahnke et al. 2002127 | Study design not RCT |
| Jahnke et al. 2003128 | Study design not RCT |
| Jeans et al. 1990129 | Intervention access device, and study design allocation to groups not random |
| Lammer et al. 2000130 | Study design not RCT |
| London et al. 1993131 | Study design not RCT |
| Nicholson 1998132 | Intervention pharmacological, thrombolysis |
| Randon et al. 2010133 | Interventions combined with other interventions that were not part of randomised allocation; no separate data for individual interventions |
| Roubin et al. 1997134 | Population not PAD |
| Sen et al. 2005135 | Population not PAD |
| Sgura et al. 2002136 | Population not PAD |
| Tanabe et al. 2004137 | Population not PAD |
| Tay et al. 2011138 | Study design not RCT |
| Turco et al. 2006139 | Population not PAD |
| Whyman et al. 1997140 | Comparator medical treatment only |
| Wolfram et al. 2005141 | Comparator combined PTA plus stent plus sham irradiation; intervention combined stent plus radiation |
| Zabakis et al. 2005142 | Comparator combined PTA plus stent; intervention combined stent plus radiation |
Appendix 3 Data extraction of included studies
Absorbable metal stent
Study details
| Trial | Objective | Study design | Publication type | Language of publication | Sources of funding | Country of corresponding author | Intervention site(s) | Length of follow-up |
|---|---|---|---|---|---|---|---|---|
| AMS INSIGHT11 | To investigate the safety of AMSs in the infrapopliteal arteries based on 1- and 6-month clinical follow-up and efficacy based on 6-month angiographic patency; and to prove the superiority of the AMS stent over PTA alone for infrapopliteal indications | RCT, prospective, multicentre | Full report in peer-reviewed journal | English | The sponsor, BIOTRONIK AG, funded the total study costs and was responsible for the study administration and monitoring of the study | Belgium | Belgium, the Netherlands, Austria and Germany | Outcomes reported at 1 and 6 months; study follow-up 12 months |
Interventions
| Trial | Focus of interventions (comparisons) | T1: intervention group | T2: control group |
|---|---|---|---|
| AMS INSIGHT11 | AMSs vs. PTA | AMS: after measurement and then selection of a suitable balloon length, the lesion was pre-dilated with the Pleon Explorer (BIOTRONIK AG, Switzerland) balloon under angiographic control. Pre-dilatation was mandatory in this study. After dilatation, the stenosed area was treated by one AMS implant. Post-dilatation was allowed at the discretion of the physician, for cases in which angiographic control revealed suboptimal apposition of the AMS to the vessel wall or flow-limiting residual stenosis | PTA: the lesion was dilated with the Pleon Explorer balloon under angiographic control. In cases in which the residual stenosis after procedure was estimated to be > 50%, balloon inflation was repeated and prolonged. If the stenosis persisted to be > 50% or a flow-limiting dissection occurred, the patient underwent implantation of the AMS study stent and ended up in the crossover group |
Population inclusion
| Trial | Target population | Inclusion/exclusion criteria | Recruitment |
|---|---|---|---|
| AMS INSIGHT11 | The study population consisted of patients with symptomatic CLI (Rutherford categories 4 and 5). They were eligible if they had de novo stenotic (> 50%) or occlusive atherosclerotic disease of the infrapopliteal arteries and presented with a reference vessel diameter of between 3.0 and 3.5 mm and a lesion length of < 15 mm (i.e. less than one stent length) | Inclusion criteria:
|
2005–7 |
Sample size
| Trial | Numbers randomised | Number of participants in T1 (AMS) | Number of participants in T2 (PTA) | Power calculation (a priori sample calculation) | Attrition/loss to follow-up | Number followed up from each condition |
|---|---|---|---|---|---|---|
| AMS INSIGHT11 | 117 patients with 149 lesions | 60 (74 lesions) | 57 (of whom 7 crossed over to AMS) (75 lesions) | The sample size calculation for this study was based on the hypothesis of a superior efficacy of the first-generation AMS system in maintaining a patent vessel lumen at 6 months vs. PTA alone. The following were assumed at 6 months: a patency rate of 50% in the PTA arm and a clinical relevance effect of 25% in the AMS arm. With acceptance of a 10% dropout rate, a crossover rate of 30% in the PTA arm, a two-sided significance level of 0.05, and 80% statistical power, a total of 117 patients were required | Clinical follow-up at 6 months was assessed in 41 of 57 (71.9%) and 39 of 60 (65.0%) initially enrolled PTA and AMS patients, respectively. The number of patients who refused the 6-month angiogram was relatively high in both groups. Reasons for declination were diverse: patient renunciation to repeat angiography (16 patients), patient death (9 patients), major amputation (7 patients), health issues making the angiographic control problematic (5 patients) and difficulties analysing angiograms at the core lab (3 patients). One patient randomised for stenting (1/60) with a double lesion (2/74) underwent implantation of a non-study stent (SES) because of severe tortuosity of the iliac artery. Therefore, this patient is not considered in the on-treatment analysis | 100% at 1-month follow-up. 6-month QVA results (regular or delayed follow-up or clinically indicated visits) were available for 50 PTA lesions (40 patients, 70%) and 44 AMS lesions (37 patients, 62%) |
Baseline characteristics
| Trial | Age | Gender | Classification of PAD | Presence of cardiovascular risk factors |
|---|---|---|---|---|
| AMS INSIGHT11 | The mean age of patients enrolled in the study was 73.1 ± 8.5 (range, 53–91) and 74.7 ± 7.8 (55–87) years in the PTA and AMS groups, respectively | The baseline characteristics of the randomised patients are statistically not different in the two treatment groups except for gender (p = 0.04) (71.9% male PTA, 51.7% male AMS). | Rutherford category 4, 28.1% PTA and 26.7% AMS; category 5, 71.9% PTA and 73.3% AMS | Nicotine abuse was noted in 26 (45.6%) and 24 (40.0%) patients in the PTA and AMS groups, respectively. Comorbidities were arterial hypertension in 51 (89.5%) and 51 (85.0%), hyperlipidaemia in 35 (61.4%) and 32 (53.3%), and diabetes mellitus in 39 (68.4%) and 43 (71.7%) patients in the PTA and AMS groups, respectively |
Outcomes
| Trial | Complications including amputation | Patency measures |
|---|---|---|
| AMS INSIGHT11 | The primary safety end point of the AMS INSIGHT was defined as the absence of clinical complications at 1 month post procedure. Complications were defined as major amputations or any cause of death. Major amputations were defined as amputations at or above the ankle. Secondary end point limb salvage was defined as lack of major amputations at the different prescheduled follow-up visits until 12 months after index intervention | The primary efficacy end point of this study was to analyse and compare the 6-month angiographic patency rate after PTA alone or PTA followed by AMS implantation in patients with stenotic or occlusive atherosclerotic disease of the infrapopliteal arteries. Patency was defined as the absence of a haemodynamically significant restenosis (> 50%), documented by digital subtraction angiography and confirmed by the core-lab QVA. The secondary end point was the primary patency rates at each visit as determined by colour-flow Doppler ultrasound and defined as either the absence of a haemodynamically significant restenosis (> 50%) derived from the ratio of the PSV at the lesion segment to that at the proximal part, a major amputation, or a TLR |
Results
| Trial | Results | Complications |
|---|---|---|
| AMS INSIGHT11 | Patency: the study’s primary efficacy end point was the 6-month angiographic patency rate. Six-month QVA results available for 50 PTA lesions (40 patients) and 44 AMS lesions (37 patients); ITT 58.0% (29/50 lesions) for the PTA, and 31.8% (14/44 lesions) for the AMS group (p = 0.013). Secondary end point was colour-flow Doppler ultrasound patency, Kaplan–Meier estimation of the primary patency rate, 6-month primary patency, ITT 88.1% for PTA only and 80.2% for AMS implantation (p = 0.270). Kaplan–Meier analysis of the QVA measurements resulted in an ITT-based primary patency of 61.2% after PTA and 47.2% after AMS (p = 0.180). Limb salvage (see also adverse events), according to the Kaplan–Meier estimation: 6-month cumulative patient limb salvage rates were calculated on an ITT basis as 92.4% PTA and 87.6% AMS (p = 0.434) Revascularisation: considering the ITT analysis, the incidence of TLR at 6 months was 16.0% (12/75) in the PTA group and 31.1% (23/74) in the AMS group (p = 0.052), where, for PTA, 66.7% (8/12) and, for AMS, 78.3% (18/23) of lesion revascularisations were clinically indicated |
The primary safety end point, i.e. absence of major amputation and/or death within 30 days after index intervention, was not significantly different between the AMS study group and the PTA control group. At 1 month, major amputation was undertaken in four patients: two in the PTA group (2/57) and two in the AMS arm (2/60). 1 of 57 PTA patients and 1 of 60 AMS patients died before the 1-month follow-up. The ITT analysis of the complication rate within 30 days yielded values of 5.3% (3/57) and 5.0% (3/60) in patients randomised for PTA alone and PTA followed by AMS implantation, respectively (p = 1.0) |
Self-expanding stent
Study details
| Trial | Objective | Study design | Publication type | Language of publication | Sources of funding | Country of corresponding author | Intervention site(s) | Length of follow-up |
|---|---|---|---|---|---|---|---|---|
| Dick et al. 200912 | To investigate whether primary nitinol stenting is associated with a morphological and clinical benefit when compared with PTA with optional stenting in intermediate-length lesions | RCT, prospective, multicentre | Full report in peer-reviewed journal | English | NR | Austria | Austria | Outcomes at 3, 6 and 12 months |
| VascuCoil13 | To estimate and compare hospital costs associated with PTA and stent placement for patients with symptomatic peripheral arterial disease; the authors performed a prospective economic evaluation in conjunction with the Intracoil femoropopliteal stent trial (VascuCoil) | RCT, prospective, multicentre | Full report in peer-reviewed journal | English | Supported in part by a grant from IntraTherapeutics, St Paul, MN, USA, the funding agreement ensured the authors’ independence | USA | USA | Outcomes at 30 days and 9 months |
| FAST14 | Designed to investigate the impact of nitinol stenting of SFA lesions, with a maximum length of 10 cm, on restenosis and clinical outcomes at 1 year | RCT, prospective, multicentre | Full report in peer-reviewed journal | English | Sponsored by C.R. Bard Inc., Murray Hill, NJ, USA | Germany | 11 European centres, Germany, Austria, Belgium, Switzerland | 12 months |
| RESILIENT15 | To compare a new, flexible nitinol stent to PTA for the treatment of obstructive lesions of the SFA and the proximal popliteal artery in patients with IC | RCT, prospective, multicentre | Full report in peer-reviewed journal | English | From ClinicalTrials.gov; sponsored by C.R. Bard CardioVascular Research Foundation, Korea | USA | 24 centres in the United States and Europe (Germany, Austria) | 12 months |
| ABSOLUTE16–18 | To determine whether primary implantation of a self-expanding nitinol (nickel titanium) stent yielded anatomical and clinical benefits superior to those afforded by PTA with optional secondary stenting | RCT, prospective, single centre | Full report in peer-reviewed journal | English | Supported by the Medical University of Vienna and the Vienna General Hospital (The authors have no commercial, proprietary or financial interest in any products or companies) | Austria | Austria | Outcomes at 6 months, 12 months and 24 months |
Interventions
| Trial | Focus of interventions (comparisons) | T1: intervention group | T2: control group |
|---|---|---|---|
| Dick et al. 200912 | Primary nitinol stenting is associated with a morphological and clinical benefit when compared with PTA with optional stenting | Stent group: self-expandable nitinol stents (Astron, Biotronik GmbH, Berlin, Germany) with a nominal diameter of 6 mm were used. Pre-dilatation with undersized balloons was performed restrictively in patients with very tight stenosis or heavily calcified lesions that did not allow primary passage with the stent introducer device. Stents were implanted to extend 10 mm proximal and distal to the margins of the target lesion. Multiple stents were overlapped for 10 mm. Post-dilatation after stenting was performed strictly within the stented segment with up to 10% oversizing of the post-dilatation balloon | PTA group: the minimal time for each balloon inflation was 2 minutes at 10–12 atm. After dilatation of the entire target segment, biplane control angiograms were obtained. In cases with a suboptimal primary result, defined as a residual stenosis > 30% or presence of a flow-limiting dissection in the worst angiographic view, a second prolonged balloon dilatation (> 2 minutes) of the target segment was performed. In patients with a persistent suboptimal result after the second balloon dilatation, secondary stenting was performed |
| VascuCoil13 | PTA vs. IntraCoil stent | IntraCoil stent | PTA alone |
| FAST14 | Nitinol stenting vs. PTA | Direct implantation without lesion pre-dilatation was preferably performed. In tight stenoses and totally occluded lesions that precluded stent advancement, angioplasty with a 3-mm-diameter balloon was done to enable stent placement. The stent dimensions were chosen such that the nominal diameter exceeded the reference vessel diameter by 1 mm and the length exceeded the lesion length by 5–10 mm proximal and distal. The intention was to cover the entire lesion with a single stent. Protocol-mandated post-dilatation utilised a balloon shorter than the stent. Technical success was defined on-site as a residual diameter stenosis < 30% by visual estimate. Deployment of a second study stent abutting the index stent was allowed in cases in which the latter was positioned incorrectly or a dissection extended beyond the stent margins | An over-the-wire PTA balloon was advanced into the lesion. Its nominal diameter had to be roughly the same as the reference vessel diameter, and its length had to match the lesion length, with a maximum proximal and distal balloon overhang of 5 mm. The balloon was gradually inflated until the lesion diameter appeared to be visually identical to the reference vessel diameter. When vessel recoil after balloon deflation was taken into account, the procedure was regarded as technically successful by the investigator if the residual diameter stenosis was estimated at < 50% (later validated off-site by independent ultrasound analysis). In cases in which this end point was not reached or a flow-limiting dissection occurred, balloon inflation was repeated once for at least 5 minutes. If technical failure persisted after repeat angioplasty, the patient underwent implantation of the study stent |
Population inclusion
| Trial | Target Population | Inclusion/exclusion criteria | Recruitment |
|---|---|---|---|
| Dick et al. 200912 | The clinical criterion for study entry was symptomatic PAD with either severe IC (Rutherford category 3) or chronic CLI with rest pain (Rutherford category 4) or ischaemic ulcers (Rutherford category 5) | The clinical criterion for study entry was symptomatic PAD with either severe IC (Rutherford category 3) or chronic CLI with rest pain (Rutherford category 4) or ischaemic ulcers (Rutherford category 5). Anatomical inclusion criteria, based upon findings on biplane digital subtraction angiography at the time of intervention, were a > 50% stenosis or occlusion of the SFA with a target lesion length between 30 and 200 mm, and at least one patent (< 50% stenosis) tibioperoneal run-off vessel. Exclusion criteria were acute CLI, previous BS or stenting of the SFA, untreated inflow disease of the ipsilateral pelvic arteries (> 50% stenosis or occlusion) and known intolerance of study medications or contrast agent | Consecutive patients; year NR |
| VascuCoil13 | Patients with stenotic or occluded superficial femoral or popliteal arteries | Eligible patients were candidates for PTA with symptomatic leg ischaemia, requiring treatment of superficial femoral or popliteal vessel with an occluded lesion length of at least 12 cm or stenotic lesion length of at least 15 cm, and located proximal to the bifurcation of the tibial artery | Between May 1997 and December 1999 |
| FAST14 | A single SFA lesion and CLI | Patients were eligible for enrolment if they were ≥ 21 years and had a de novo SFA lesion located at least 1 cm from the SFA origin with a length between 1 and 10 cm. Target lesion diameter stenosis had to be ≥ 70% by visual estimate. The popliteal artery as well as one of the infrapopliteal (below-the-knee) vessels had to be continuously patent for sustained distal runoff. Clinically, the patients had to suffer from CLI of at least Rutherford category 2 (moderate claudication). Major exclusion criteria were a target lesion that required pretreatment with adjunctive devices such as lasers or debulking catheters; a target lesion that extended into the popliteal artery; previous stent implantation in the targeted SFA; multiple lesions exceeding a total length of 10 cm; acute or subacute (≤ 4 weeks) thrombotic occlusion; an untreated ipsilateral iliac artery stenosis; ongoing dialysis treatment; and treatment with oral anticoagulants other than antiplatelet agents | 2004–2005 |
| RESILIENT15 | Patients with obstructive lesions of the SFA, proximal popliteal artery or both | Patients eligible for inclusion in the study were aged ≥ 18 years; had symptoms of IC (Rutherford categories 1–3); were candidates for angioplasty or stenting; had de novo stenotic, occlusive, or restenotic lesions in the SFA, proximal popliteal artery, or both; and had at least one patent infrapopliteal runoff vessel to the foot. The treatment area in the SFA and popliteal artery extended from 1 cm below the origin of the profunda femoris artery to approx. 3 cm above the intercondylar notch of the femur. Target lesions were examined angiographically to verify stenosis or restenosis ≥ 50% and a total lesion length of ≤ 150 mm. More than one lesion in the target vessel could be treated as long as the total length of the lesions did not exceed 150 mm. To allow for proper stent sizing, the reference vessel diameter was required to be between 4 and 6.5 mm. If a restenosed or reoccluded lesion was treated, the previous intervention must have occurred > 6 months before the study procedure and must not have included stenting. If a patient had multiple lesions in the SFA and popliteal arteries of both limbs (i.e. bilateral disease), only one limb could be enrolled in the study. Exclusion criteria included a sensitivity to contrast media that was not amenable to pretreatment with steroids, antihistamines or both; known allergies to study medications or materials; renal failure (serum creatinine > 2.0 mg/dl) or hepatic insufficiency; previous BS of the target limb; extensive peripheral vascular disease that precluded safe insertion of an introducer sheath; aneurysmal disease in the vessel segment to be treated; thrombus in the area to be treated that could not be resolved; or angiographic evidence of poor inflow that was inadequate to support vascular bypass or patients who were receiving dialysis or immunosuppressive therapy | Between December 2004 and August 2006 |
| ABSOLUTE16–18 | Patients who had severe claudication or CLI due to stenosis or occlusion of the SFA | The clinical criteria for study entry were symptomatic peripheral artery disease with severe IC (Rutherford category 3), chronic CLI with pain while the patient was at rest (Rutherford category 4), or chronic CLI with ischaemic ulcers (Rutherford category 5). The anatomical inclusion criteria, based on biplane digital subtraction angiography performed at the time of intervention, were stenosis of > 50% or occlusion of the ipsilateral SFA, a target lesion length of > 30 mm, and at least one patent (< 50% stenosed) tibioperoneal runoff vessel. The exclusion criteria were acute CLI, previous BS or stenting of the SFA, untreated inflow disease of the ipsilateral pelvic arteries (> 50% stenosis or occlusion) and known intolerance to study medications or contrast agents | From June 2003 through August 2004, consecutive patients. A total of 252 patients were screened for participation in the study. Of these, 143 did not meet the inclusion criteria |
Sample size
| Trial | Numbers included in the study | Number of participants in T1 | Number of participants in T2 | Power calculation (a priori sample calculation) | Number (%) followed up from each condition (or attrition) |
|---|---|---|---|---|---|
| Dick et al. 200912 | 73 | 34 nitinol stent | 39 PTA (of whom 10 had stenting) | A sample size of 70–80 patients was estimated necessary assuming a 6-month restenosis rate of 60% in the PTA group vs. 25% in the nitinol stent group. A two-sided p-value of 0.05 was considered statistically significant and a power of 80% was required with a 10% maximum dropout rate | Complete follow-up data could be obtained for 71 of 73 patients (97%) at 3 months, and in 68 of 73 patients (93%) at 6 and 12 months, respectively. Follow-up data were not available in two patients at 3 months (one died and one refused re-evaluation) and in five patients at 6 and 12 months (three died, two refused re-evaluation) |
| VascuCoil13 | 266 | 135 stent (177 lesions) | 131 PTA (175 lesions) | ||
| FAST14 | 244 | 123 stent | 121 PTA (13 of whom crossed over to stent) | The sample size calculation for this trial was based on the assumptions of 12-month binary restenosis rates of 45% in the PTA arm and 25% in the stent arm (an absolute difference of 20%). With acceptance of a 15% lost to follow-up rate, a two-sided significance level of 0.05, and 80% statistical power, a total of 244 patients had to be enrolled | Clinical follow-up at 12 months was assessed in 115 PTA group patients (95%) and 114 stent group patients (93%). The change in the patients’ clinical and haemodynamic statuses, in terms of absolute walking distance, ABPI at rest and Rutherford category, was assessed in a subset of 61 stent group patients (50%) and 75 PTA group patients (62%) who were able to undergo treadmill testing both at baseline and at 12 months |
| RESILIENT15 | 206 | 134 stent | 72 PTA [of whom 29 (40.3%) underwent a secondary bail-out stenting procedure because of an inadequate PTA result] | A minimum sample size of 206 patients was needed to detect a 14% difference in the TVR and TLR rate at 6 months post procedure with a statistical power of 80% (one-sided simple log-rank test with a significance level of 0.05). The 14% difference was based on calculated TVR rates of 26% for the control group and 12% for the test group at 6 months post procedure. A crossover rate of ≤ 16% was assumed for the RESILIENT15 trial. A dropout rate of 7% (2% death; 5% lost to follow-up) was assumed for the log-rank-test-based sample size calculation | Follow-up data were available on 87% of patients at 12 months (87.5% for the stent group and 86.8% for the angioplasty group). Seven patients died, nine patients withdrew consent to be evaluated, and three patients were lost to follow-up |
| ABSOLUTE16–18 | 104 | 51 stent | 53 PTA [of whom 17 (32%) underwent secondary stenting] | We estimated that 100–110 patients would need to be enrolled for the study to have a statistical power of 80% to detect an absolute difference in restenosis rates of 25%, given 6-month rates of restenosis of 50% in the angioplasty group and 25% in the stent group and a maximal dropout rate of 10% | Complete follow-up data were obtained from all 104 patients at 3 and 6 months. Data were not available for three patients at 12 months (one died and two declined to be re-evaluated) |
Baseline characteristics
| Trial | Age (years) [mean (SD)] | Gender | Classification of PAD | Number of patients who have undergone previous revascularisation procedures [n (%)] | Presence of cardiovascular risk factors [n (%)] | Level of exercise tolerance | Other relevant information |
|---|---|---|---|---|---|---|---|
| Dick et al. 200912 | Stent 69 (9), PTA 69 (10) | Stent 74% male; PTA 64% male | Clinical stage of PAD (Rutherford): category 3 (IC), stent n = 31 (91%), PTA 38 (97%); category 4 (ischaemic rest pain), stent 1 (3%), PTA 0; category 5 (ischaemic ulcers) stent 2 (6%), PTA 1 (3%) |
|
Maximum walking distance (m): stent mean 131 (SD 188), PTA 103 (92). Walking distance was assumed “0” in patients with CLI and ischaemic rest pain or ischaemic ulcers | Mean lesion length (SD) (mm): stent group 82 (67); PTA group 65 (46) | |
| VascuCoil13 | Stent 66.8 (10.6); PTA 68.1 (10.2) | Stent, 67.4% male; PTA, 63.4% male |
|
Total occlusion: stent 22.7%, PTA 16.8% | |||
| FAST14 | 66 (10) | 168 men (69%), 76 women | Rutherford category of PAD; data available from 119 stent and 114 PTA patients:
|
Prior peripheral vascular intervention: stent 42 (34.1), PTA 49 (40.5) |
|
Absolute walking distance (m) [median (IQR)]: stent 110 (68–163) (n = 97), PTA 100 (60–150) (n = 99) | In both treatment groups the mean lesion length was 45 mm |
| RESILIENT15 | Stent 68 (10), PTA 66 (9) | Stent, 95 men (70.9%); PTA, 48 men (66.7%) | Rutherford category:
|
Patients with restenosis: stent 4 (2.6), PTA 2 (2.5) |
|
Mean lesion length (SD) (mm): stent group 70.5 (44.3); PTA group 64.4 (40.7) | |
| ABSOLUTE16–18 | Stent 65 (10), PTA 68 (10) | Stent, 30 men (59%); PTA, 25 men (47%) | Rutherford category of PAD [n (%)]: category 3 – stent 45 (88), PTA 46 (87); category 4 – stent 1 (2), PTA 2 (4); category 5 – stent 5 (10), PTA 5 (9) |
|
Mean lesion length (SD) (mm): stent group 132 (71); PTA group 127 (55) |
Outcomes
| Trial | QoL (disease specific or generic) | Exercise tolerance/walking distance | Pain/clinical status | Complications including amputation | Patency measures | Need for reintervention or recurrence rate |
|---|---|---|---|---|---|---|
| Dick et al. 200912 | Maximum walking capacity as reported by the patient (3, 6 and 12 months) | Amputation and death (until 12 months) | The primary study end point was the occurrence of restenosis in the treated segment within 6 months post intervention by CTA. Restenosis was defined as a > 50% lumen diameter reduction at the most narrow site within the limits of the treated segment plus the adjacent 10 mm proximal and distal to the treated segment. Secondary end point was restenosis measured by ultrasound binary restenosis of > 50% by duplex ultrasound defined as PSV of at least 2.4 | |||
| VascuCoil13 | Death, myocardial infarctions, amputation, adverse events | TLR | ||||
| FAST14 | Absolute walking distance | Rutherford category | Complications | The primary study end point was binary restenosis, defined as a proximal PVR ≥ 2.4 on duplex ultrasound | TLRs were performed only if two conditions were met: (1) the patient complained of recurrent claudication, and (2) on-site duplex ultrasound revealed target lesion restenosis | |
| RESILIENT15 | Short Form 8 Question Health Survey | Walking Impairment Questionnaire | Adverse events composite measure (MACE) | Radiographs of the stented limbs were taken 6 and 12 months post procedure and assessed for stent fractures by the angiographic core laboratory | Survival from TLR/TVR | |
| ABSOLUTE16–18 | SF-36 | Maximal walking capacity on the treadmill | Rutherford category of PAD | Complications: amputation by 6 or 12 months; and death by 6 or 12 months | The primary study end point was the rate of binary restenosis (stenosis of ≥ 50% of the luminal diameter) in the treated segment 6 months after intervention, as determined by CTA or digital subtraction angiography Restenosis was defined as a reduction in the luminal diameter of > 50% according to the worst angiographic view at the narrowest site within the treated segment plus the 10-mm segments proximal and distal to the treated segment. The anatomical end points were restenosis of > 50%, as determined by duplex ultrasound at 3, 6 and 12 months; the angiographic degree of restenosis (the per cent reduction in diameter at 6 months); and the occurrence of stent fractures, as determined by biplane radiography at 6 and 12 months |
Need for ipsilateral PTA/stent/BS |
Results
| Trial | Results | Complications |
|---|---|---|
| Dick et al. 200912 | Restenosis: at 6 months the angiographic binary restenosis rate by CTA was 21.9% in the stent group vs. 55.6% in the PTA group (p = 0.005) as analysed by ITT. By ultrasound, restenosis rates in the stent and PTA groups at 3, 6 and 12 months were 2.9% vs. 18.9% (p = 0.033), 18.2% vs. 50.0% (p = 0.006) and 34.4% vs. 61.1% (p = 0.028), respectively. Walking: Patients in the stent group reported a significantly higher maximum walking capacity than those in the PTA group at 6 months (average 800 m vs. 600 m, p = 0.002) and at 12 months (average 800 m vs. 550 m, p = 0.042) | In the PTA group, one small pseudoaneurysm at the puncture site was observed at day 1 after the intervention. This minor complication was resolved by prolonged ultrasound-guided compression without clinical sequelae. No major complication was encountered in either treatment group |
| VascuCoil13 | Incidence of TLR (9 months): stent 0.7%, PTA 1.5%. Incidence of amputation: stent 0.0%, PTA 0.8% | Death (unclear if 30 days or 9 months): stent 0.0%, PTA 0.8%. Myocardial infarctions: stent 0.0%, PTA 0.0%. Major bleeding complications: stent 0.7%, PT 0.8%. Renal failure: stent 0.0%, PTA 0.8%. Major vascular complications: stent 3.0%, PTA 4.6%. Abrupt closure: stent 0.0%, PTA 1.5%. Subacute closure: stent 0.7%, PTA 1.5%. Abrupt and subacute closure were non-significant between groups |
| FAST14 | Limb salvage: lower-limb amputations because of pre-existing gangrene had to be performed in two stent group patients (1.8%). Walking: at 12 months, PTA and stent group patients were able to maximally walk a median of 185 m and 150 m, respectively, on the treadmill, which corresponded to a statistically significant difference in median walking distance improvement (52 vs. 20 m, respectively; ANCOVA p = 0.028). Restenosis: duplex ultrasound recordings at 12 months were available from 101 PTA group patients (83%) and 101 stent group patients (82%). Intention-to-treat analysis yielded binary restenosis rates of 38.6% (39 patients) in the stent group and 31.7% (32 patients) in the PTA group (absolute treatment difference, 6.9%; 95% CI, 19.7% to 6.2%; p = 0.377). Revascularisation: the cumulative incidence of TLRs at 12 months was 18.3% (21 patients) in the PTA group and 14.9% (17 patients) in the stent group (absolute treatment difference, 3.4%; 95% CI, 13.0% to 6.4%; p = 0.595). Disease state: an improvement by ≥ 1 Rutherford category of peripheral arterial disease was observed at 12 months in a total of 122 patients (90%), with no statistically significant difference between treatment modalities | Mortality: There was one death (of a carcinoma) at 11.6 months in the PTA group (0.9%), and four deaths (3.5%) occurred at a median of 8.0 months (IQR, 4.9–9.1 months) in the stent group. The cause of death in the latter patients was a carcinoma, multiple organ failure and severe three-vessel coronary artery disease; the cause remains unknown in one patient. Stent integrity at 12 months was assessed in 83 of 101 patients; stent fractures were detected in 10 of 83 patients. Procedural complications: stent n = 8 (7%), PTA n = 5 (4%) |
| RESILIENT15 | Reintervention: freedom from TLR at 6 months post procedure was significantly better for the stent group than for the angioplasty group (98.5% vs. 52.6%; p = 0.0001) and remained significantly better for the stent group (87.3% vs. 45.1%; p = 0.0001) at 12 months. Patency: primary patency, a combination of ultrasound-confirmed patency and absence of TLR, was significantly better for the stent group than for the angioplasty group at 6 months and 12 months post procedure (p = 0.0001). The 6-month primary patency rate for the stent group was 94.2% compared with 47.4% for the angioplasty group, whereas the 12-month primary patency rate was 81.3% for the stent group vs. 36.7% for the angioplasty group. QoL: both treatment groups demonstrated a significant improvement in all QOL measures (i.e. both SF-8 Question Heath Survey and Walking Impairment Questionnaire) at 6 and 12 months compared with baseline. The baseline SF-8 Question Heath Survey physical score was 41.0 (SD 10.5) in the angioplasty group and 41.4 (SD 9.2) in the stent group. At 12 months, the Short Form 8 Question Heath Survey scores had increased similarly in both groups [5.9 (SD 11.2) vs. 5.7 (SD 11.2); p < 0.0001 vs. baseline]. Walking distance: the 12-month walking distance score was 22.3 (SD 23.2) in the angioplasty group and 22.8 (SD 24.2) in the stent group. At 12 months, walking distance scores had increased similarly in both groups [29.4 (SD 37.4) vs. 25.6 (SD 34.6); p < 0.0001 vs. baseline]. Patients in the angioplasty group reported more claudication pain at 12 months than patients in the stent group (Walking Impairment Questionnaire evaluation, p = 0.009), but there were no other significant differences in QOL measures between treatment groups (t-test p > 0.05) | No patients in either arm of the study died within 30 days of the procedure. There was no statistically significant difference between the MACE rates for the treatment groups Freedom from MACE at 6 months for the stent group was 93.1% and for the angioplasty group 92.8% (p = 0.95). At 12 months, freedom from MACE was 85.8% for the stent group and 86.6% for the angioplasty group (p = 0.88). There were two unplanned amputations reported in the angioplasty group over 12 months. Both were minor, below-the-level-of-the ankle (single-toe) amputations. No amputations were reported in the stent group |
| ABSOLUTE16–18 | Restenosis: at 6 months, the rate of restenosis on angiography was 24% in the stent group and 43% in the angioplasty group, according to the ITT (p = 0.05). At 6 months, the rate of restenosis on duplex ultrasonography was 25% in the stent group and 45% in the angioplasty group (p = 0.06). At 12 months, the restenosis rate on duplex ultrasonography was 37% in the stent group and 63% in the angioplasty group (p = 0.01). Multivariable analysis adjusted for age, sex, presence or absence of diabetes, smoking status, stage of PAD and lesion length confirmed that, as compared with patients who underwent angioplasty, patients who underwent stenting had a reduced risk of restenosis at 6 months (adjusted RR, 0.45; 95% CI, 0.20 to 0.94) and 12 months (adjusted RR, 0.40; 95% CI, 0.19 to 0.80). There was no significant interaction between treatment assignment and the risk of restenosis according to the stage of PAD or the length of the lesion, indicating that the benefit of stenting did not vary according to these strata. Restenosis rates at 2 years were 45.7% (21 of 46) vs. 69.2% (36 of 52) in favour of primary stenting over balloon angioplasty with optional secondary stenting by an ITT analysis (p = 0.031). Reintervention rates at 1 year tended to be lower after primary stenting [17 of 46 (37.0%) vs. 28 of 52 (53.8%); p = 0.14). At 2 years, reintervention rates tended to be lower after stenting than after balloon angioplasty, but this also was not statistically significant [26 of 63 (41.3%) vs. 19 of 35 (54.3%); p = 0.30). Walking distance: patients in the stent group were able to walk significantly further on a treadmill than those in the angioplasty group at 6 months (average distance, 363 vs. 270 m; p = 0.04) and 12 months (average distance, 387 vs. 267 m; p = 0.04). Clinical worsening was rare in both groups. Clinically, Rutherford categories of PAD at 2 years were not significantly different between the two groups. Stent vs. balloon QoL, analysed according to the ITT: no significant difference for any parameter of QoL at any time interval between the balloon angioplasty and stent groups when comparing the 51 patients with primary stenting vs. the 53 patients with balloon angioplasty and optional secondary stenting in 17 patients (role-emotional was lower for PTA than stent with borderline significance level; p = 0.04) |
Balloon-expandable stent
Study details
| Trial | Objective | Study design | Publication type | Language of publication | Sources of funding | Country of corresponding author | Intervention site(s) | Length of follow-up |
|---|---|---|---|---|---|---|---|---|
| Becquemin et al. 200319 | To compare results of systematic or selective stenting of the superficial femoral artery after balloon angioplasty in patients with lesions < 7 cm and disabling claudication or lower limb critical ischaemia | RCT, prospective, multicentre | Full report in peer-reviewed journal | English | Supported by grants from Cordis, a Johnson & Johnson company, Miami Lakes, FL, USA; Lafon; Aventis; and Societe Francaise de Chirurgie Vasculaire | France | France | Outcomes at 1 and 4 years. Median follow-up was 2.43 years (SE 0.08), ranging from 8 days to 4 years |
| Cejna et al. 200120 | To evaluate if stent placement is superior to PTA in the treatment of chronic symptoms in short femoropopliteal arterial lesions | RCT, prospective, multicentre | Full report in peer-reviewed journal | English | Supported by the Ludwig Boltzmann Institute for Radiological Tumour Diagnosis and Johnson & Johnson Interventional Systems, Warren, NJ, USA | Austria | Four hospitals in Austria | Outcomes at 6, 12 and 36 months. Mean follow-up time was 352 days (range, 1–1252 days) for PTA group and 353 days (range, 1–1215 days) for the stent placement group |
| Grimm et al. 200121 | To evaluate whether PTA combined with Palmaz stent placement provides long-term advantages compared with PTA alone after 34 months of follow-up in the femoropopliteal region | RCT, prospective, single centre | Full report in peer-reviewed journal | English | NR | Germany | Germany | Maximum study follow-up 39 months; mean stent 29.1 months, PTA 33.8 months |
| Rand et al. 200622 | To determine the primary success and short-term patency of stent application as a primary treatment modality for high-grade lesions of the infrapopliteal arteries compared with treatment with PTA in CLI | RCT, prospective, multicentre (pilot study) | Full report in peer-reviewed journal | English | This study was supported by the Ludwig Boltzmann Institute for Radiological Tumour Diagnosis and the Ludwig Boltzmann Institute of Interdisciplinary Vascular Research | Austria | Austria. 44 patients were consecutively investigated and randomised at one centre to treatment of lesions by either PTA or stent application. Seven patients were enrolled from two other centres. (It is unclear whether the other centres were in Austria or the USA) | Outcomes at 6 months |
| Vroegindeweij et al. 199723 | To evaluate whether balloon angioplasty combined with stenting of symptomatic femoropopliteal disease would provide better results than balloon angioplasty alone | RCT, prospective | Full report in peer-reviewed journal | English | NR | Netherlands | Netherlands | Outcomes at 1 year reported (survival curves up to 18 months). Median of 14.1 months (range 0–31 months) in patients with PTA and 13.4 months (range 0–27 months) in stent patients |
| Zdanowski et al. 199924 | To investigate the 1-year outcome of PTA and stenting and PTA alone for femoropopliteal occlusions | RCT, prospective | Full report in peer-reviewed journal | English | NR | Sweden | Sweden | The follow-up included clinical examination, measurement of ABPI and control angiography at 12 months or earlier when necessary (20 patients) |
Interventions
| Trial | Focus of interventions (comparisons) | T1: intervention group | T2: control group |
|---|---|---|---|
| Becquemin et al. 200319 | BESs vs. PTA (with selective stent) | Balloon expandable Palmaz stents (Cordis or Johnson & Johnson interventional systems) of various sizes. In the group of patients allocated to undergo primary stenting, the stent was placed either before or after dilatation of the lesion. Two stents were placed in lesions > 5cm | Lesions were approached through an ipsilateral femoral puncture. With angiographic guidance, a 0.89-mm Terumo (Leuven, Belgium) guide wire was passed through the lesion. A balloon dilating catheter was placed in the lesion and inflated to 8–12 atm. Non-compliant balloon catheters (Ultra-thin™, Meditech, Boston Scientific, Boston, MA, USA) were used in 82% of patients, and Olbert balloon catheters (Cordis) were used in 18% of patients. Half a milligram per kilogram of body weight of standard heparin was administered before dilatation. In the group of patients randomised to undergo balloon angioplasty, if results were suboptimal as demonstrated on the control angiogram, i.e. residual stenosis > 30% or dissection, the balloon was inflated one more time in an attempt to model the lesions. According to the results, the physician had the choice of retracting the balloon catheter without any further intervention or placing a stent |
| Cejna et al. 200120 | PTA vs. PTA followed by implantation of balloon expandable Palmaz stents | BESs: the Palmaz stent (P294 or P394, Johnson & Johnson Interventional Systems, Warren, NJ, USA) was mounted on an Olbert balloon (4–6 mm diameter, 4 cm length). The stent was pressed under high-pressure conditions against the stenotic lesion for 30 seconds. In long lesions (4–5 cm in length), a second stent was placed overlapping the first by at least 5 mm | PTA: all interventions were performed with use of digital subtraction angiographic equipment. After antegrade puncture of the common femoral artery by means of the Seldinger technique, a 7-F introducer sheath was inserted into the superficial femoral artery. 5000 units of heparin were administered intra-arterially. The lesion was crossed with the use of a Bentson guide wire (Boston Scientific/Meditech, Natick, MA, USA) or a Terumo wire (Radifocus®, Terumo Europe, Leuven, Belgium) with use of road mapping. Once the lesion was crossed, balloon dilatation was performed for 30 seconds with use of a Gruentzig-type balloon (2 or 4 cm length) 4–6 mm in diameter under high pressure (8–12 atm) (Smash balloon™, Glidex balloon, Boston Scientific/Meditech). Biplane angiography was performed to evaluate technical success |
| Grimm et al. 200121 | Balloon expandable Palmaz stent vs. PTA | Stent: A balloon expandable Palmaz stent (P294, Cordis, Roden, The Netherlands) made from stainless steel (alloy 316L) was used. The thickness of its struts was 0.14 mm and its length, if not expanded, was 29 mm. Expanding the stent to a diameter of 5 or 6 mm leads to a reduction of its length to 28.7 or 27.8 mm, respectively. After a 7-F sheath (Super Arrow, Flex, 65 cm, Arrow, Reading, PA, USA) was placed via antegrade puncture of the common femoral artery, the femoropopliteal lesion was passed with a hydrophilic guide wire (0.81 mm, curved tip; Terumo, Tokyo, Japan) and a multipurpose catheter (5-F, 0.89-mm interior diameter, open tip; Cordis). The sheath was flushed continuously with heparinised (1 IU/ml) saline. Each lesion was dilated with a balloon catheter (5 or 6 mm in diameter, 20 or 40 mm in length, depending on the vessel diameter proximal and location of the lesion; Meditech/Boston Scientific, Watertown, MA, USA). After removing the catheter, the sheath was placed distal to the lesion and the stent (4 cm in length) was mounted on the appropriate balloon catheter and placed within the lesion inside the covering sheath. After cautiously retracting the sheath, the stent was deployed by inflating the balloon | PTA (angioplasty as for intervention without stent) |
| Rand et al. 200622 | PTA vs. carbofilm-coated (and balloon expandable) stents in infrapopliteal arteries | The Carbostent is a balloon expandable, stainless steel tubular stent with innovative multicellular design and a carbon coating. Stent applications were performed using a 0.36-mm guide wire (HI-Torque, Spartacore™ 14, Guidant Corporation, Santa Clara, CA, USA) and Carbostents with a diameter range of 2.0–4 mm and a length of 15–25 mm. Primary stenting was performed. Adjunct therapy for the stent group consisted of clopidogrel (Plavix), administered as a bolus of 300 mg on the day of the procedure and 75 mg per day orally for 4 weeks, and acetylsalicylic acid (ASA, ThromboAss) medication permanently | PTA: an ipsilateral, femoral antegrade puncture technique was primarily used (4-Fr or 5-Fr haemostatic introducer; Cook introducer set, William Cook, Europe and Ultimum, St. Jude Medical Diagnostic Division, Minnetonka, MN, USA). Contralateral femoral access was used only if the antegrade access was unsuitable. After arterial cannulation with an introducer sheath, 5000 units of heparin were administered intra-arterially. The lesions were assessed visually by the interventional radiologist and the balloon diameter was selected to equal the diameter of the artery. Lesions were routinely treated with a 5-Fr conventional balloon angioplasty catheter and guide wire. Postinterventional anticoagulation therapy for the PTA group consisted of low-molecular-weight heparin (Enoxoparin 2 × 40 mg) for 3 days and acetylsalicylic acid (ASA; ThromboAss, 100 mg per day permanently) |
| Vroegindeweij et al. 199723 | Stent: Palmaz stents were placed at angiographically identified lesions and expanded by balloon angioplasty. The lesions were not pre-dilated before stent placement. We attempted to cover the entire diseased section of the vessel with one stent. The length of the stents ranged from 20–40 mm. Heparin was continued for ≥ 48 hours and until the anticoagulation therapy was within the therapeutic level, according to the international normalised ratio. After the procedure, all patients started on oral warfarin (Coumadin). Anticoagulation treatment was continued during the first 3 months, then the treatment was changed to aspirin 80 mg/day indefinitely | PTA: standard technique (described in another publication) | |
| Zdanowski et al. 199924 | To investigate the 1-year outcome of PTA and stenting and PTA alone for femoropopliteal occlusions | Strecker stent: 6-mm stents (length 40 mm or 80 mm) were implanted with an overlap of about 5 mm. Size and number of stents chosen to fully cover dilatation of vessel | PTA: common femoral artery was punctured and the superficial femoral artery catheterised with a 5-F or 6-F straight catheter. Occlusion passed with straight stiff or Terumo guide wire, catheter changed to 8-F introducer and an Olbert balloon with 6 mm diameter |
Population inclusion
| Trial | Target population | Inclusion/exclusion criteria | Recruitment |
|---|---|---|---|
| Becquemin et al. 200319 | Patients with lesions < 7 cm and disabling claudication or lower limb critical ischaemia | Patients of either sex with severe claudication or limb-threatening ischaemia [stage IIb, III or IV (SVS-ISCVS)] and who had stenosis or occlusion of the superficial femoral artery, as demonstrated on a pre-treatment angiogram, were eligible. Inclusion criteria included inflow vessels free of significant lesion; single superficial femoral artery lesion located between 1 cm from the origin of the superficial artery and 5 cm proximal to the projection of the knee joint on anteroposterior angiographic views; lesion length between 1 and 7 cm; and sufficient outflow, with at least one patent leg artery. Exclusion criteria included pregnancy, acute ischaemia, previous endovascular or open surgery in the treated superficial femoral or popliteal artery, allergy to iodine, haemorrhagic diathesis, hypercoagulation and enrolment in an ongoing trial. For each patient, only one leg was included in the trial | June 1995 and December 1997. In 24 of 251 eligible patients, the guide wire could not be placed through the lesion |
| Cejna et al. 200120 | Included were patients aged 40–85 years, with a history of claudication (SVS-ISCVS categories 1–3) or chronic CLI (SVS-ISCVS categories 4–5) | The inclusion criteria allowed up to three lesions (stenosis and/or occlusions), ≤ 5 cm in length, located in the superficial femoral artery or in the above-knee segment of the popliteal artery At least one run-off vessel had to be patent at angiography. Excluded were pregnant women, or patients with an acute onset of symptoms (with an angiographic appearance resembling an acute thromboembolism). Furthermore, patients who had previous vascular surgery in the treated segments, with an untreated obstruction of the inflow vessels (e.g. iliac and common femoral arteries), or patients who were unable or unwilling to participate in follow-up examinations and drug therapy, were also excluded from the study |
February 1994 and April 1997. Of 838 limbs treated for femoropopliteal obstruction between February 1994 and April 1997, 523 fulfilled the anatomical inclusion criteria |
| Grimm et al. 200121 | Claudication in the femoropopliteal region, occlusion or severe stenosis of the superficial femoral artery including the P1 segment of the popliteal artery | Inclusion criteria: The lesion had to be situated at least 1 cm distal from the femoral bifurcation in the superficial femoral artery and could include the P1 segment (proximal third part, above the knee joint space) of the popliteal artery. The P2 segment (middle part of the popliteal artery at the height of the knee joint space) had to be free of disease at the time of the study. The length of the stenosis could not exceed 5 cm; the percentage of stenosis had to be > 70%. At least two patent vessels in the lower limb had to provide sufficient run-off. To ensure proper placement of the stent, the vessel diameter had to be between 4 and 8 mm. Significant stenoses in the iliac or popliteal vessels had to be treated before stent placement Exclusion criteria: lesions > 5 cm in length requiring more than two stents, multifocal disease or complete obstruction (that could not be passed with the guide wire) of the superficial femoral artery, haemodynamically relevant stenoses in the lower limb previously untreated, occlusion of more than two arteries in the lower limb, lesions distal to the P1 segment or including the femoral bifurcation, thrombus within the superficial femoral artery and existing contraindications for vascular surgery or anticoagulation |
|
| Rand et al. 200622 | Chronic CLI stages III and IV of the Fontaine classification | Inclusion criteria: (1) patients suffering from chronic CLI stages III and IV of the Fontaine classification; (2) patients with isolated stenosis > 70% or occlusion of the tibial arteries; (3) patients with up to three lesions; and (4) lesions that were ≤ 3 cm with a cumulative lesion length of ≤ 9 cm, including the tibiofibular trunk, anterior and posterior tibial arteries, and peroneal artery Exclusion criteria: patients with a significant inflow obstruction at the pelvic or superficial femoral artery level, patients with evidence of a systemic coagulopathy in whom anticoagulant and antiplatelet treatment was contraindicated, patients with previously implanted stents in the target lesion, patients with total occlusion in the target vessel following the target lesion, patients without distal run-off, patients with inflammatory vascular disease, patients with peptic ulcer or gastric/intestinal bleeding in the previous 6 months and patients with a clinically assessed intolerance to contrast medium |
Patients were enrolled during a period of 16 months |
| Vroegindeweij et al. 199723 | Patients with femoropopliteal obstructive disease | Inclusion criteria: (1) lesions confined to the femoropopliteal artery, excluding below-knee lesions; (2) lesions eligible for balloon angioplasty alone and balloon angioplasty combined with stenting, which excluded all patients with multisegmental disease and with no run-off; and (3) maximal length of the lesion 5 cm. No patients had undergone any previous endovascular or operative interventions in the ipsilateral femoral artery. Only patients who would be able to comply with the frequent follow-up study visits required by the colour-flow duplex surveillance protocol were selected | Between January 1993 and December 1995 |
| Zdanowski et al. 199924 | Patients with femoropopliteal occlusions or who had CLI | Patients with femoropopliteal occlusions or who had CLI | During 3 years |
Sample size
| Trial | Numbers randomised | Number of participants in T1 (stent) | Number of participants in T2 (PTA) | Power calculation | Number of patients followed up (or attrition) |
|---|---|---|---|---|---|
| Becquemin et al. 200319 | 227 | 115 (systematic stent) | 112 (PTA with selective stenting) [of whom, in the PTA group, 15 patients (13%) required stent placement because of unsatisfactory results after angioplasty alone] | NR | At 1 year, 81 patients (80%) in the angioplasty only group and 83 patients (75.5%) in the angioplasty plus stent group had, respectively, 65 and 75 angiograms available for evaluation |
| Cejna et al. 200120 | 141 patients (154 limbs) | 77 limbs | 77 limbs (10 patients after PTA had secondary stent placement because of primary technical failures) | The clinical estimate was that stent placement might raise the 1-year patency rate from 60% with PTA to 80%. Thus, 148 lesions were calculated to be necessary for a power of 80% (α-error, β-error; p < 0.05) | Angiographic follow-up within 12 months was available in 91 of 154 limbs (59.1%) (45 limbs in the PTA group, 46 limbs in the stent placement group). 111 limbs (55 limbs in the PTA group vs. 56 in the stent placement group) had angiographic follow-up within 24 months |
| Grimm et al. 200121 | 53 | 30 | 23 | NR | Six patients were lost to follow-up (and six deaths) |
| Rand et al. 200622 | 95 lesions in 51 patients | 42 lesions in 24 patients | 53 lesions in 27 patients (one lesion secondary stenting) | NR | 37 patients underwent a follow-up study in which 57 lesions had been treated by PTA (32 procedures in 20 patients) or stent application (25 procedures in 17 patients) Of the 51 patients, 2 patients died, 3 patients underwent amputation, 1 patient underwent major heart surgery, which did not allow further follow-up, and 8 patients were lost to follow-up |
| Vroegindeweij et al. 199723 | 51 | 24 [four patients (8%) had a crossover from the randomised technique (stent) to the opposite treatment] | 27 | NR | Unclear |
| Zdanowski et al. 199924 | 32 | 15 | 17 | NR | All patients available for analyses of technical success and complications; 20 patients available for angiography (8 PTA, 12 stent) Angiography refused by seven patients in PTA group (47%) and two patients in the stent group (14%) because of clinical improvement |
Baseline characteristics
| Trial | Age (mean, years) | Gender | Classification of PAD | Number of patients who have undergone previous revascularisation procedures [n (%)] | Presence of cardiovascular risk factors [n (%)] | Level of exercise tolerance | Other relevant information |
|---|---|---|---|---|---|---|---|
| Becquemin et al. 200319 | PTA 66.42 (SD 11.7), stent 66.54 (SD 11.15) | Angioplasty group, 66 (59%) men; angioplasty plus stent group, 76 (66%) men (p = 0.265) | PTA group: 89 patients (79%) had claudication, 7 patients (6.25%) had rest pain and 16 patients (14.29%) had gangrene or ulcer. Stent group: 91 patients (79%) had claudication, 7 patients (6.09%) had rest pain, and 17 patients (14.78%) had gangrene or ulcer | Previous vascular surgery (contralateral limb, aortoiliac segment, carotid artery): PTA 36 (32), stent 24 (20) |
|
Mean lesion length in the two groups: PTA 25.11 mm (SD 17.8 mm) (range, 20–70 mm), stent 25.36 mm (SD 18 mm) (range 30–70 mm) | |
| Cejna et al. 200120 | PTA 65.5 (range 39.2–83), stent 68.6 (range 39.2–87) | PTA group, 46 (59.8%) men; stent group 49 (63.6%) men | SVS-ISCVS categories: mild and moderate – PTA 13 (16.9%), stent 11 (14.3%); severe claudication – PTA 45 (58.4%), stent 39 (50.6%); ischaemic rest pain – PTA 7 (9.0%), stent 11 (14.2%); minor tissue loss – PTA 12 (15.6%), stent 16 (20.8%) |
|
In the PTA group, lesion length 2 cm was found in 46 patients, compared with 38 patients in the stent placement group. The average lesion length for the PTA group was 2.2 cm (SD 1.2 cm), compared to 2.6 cm (SD 1.4 cm) in the stent placement group (non-significant between groups) | ||
| Grimm et al. 200121 | Palmaz stent group 71 (SD 10), PTA group 68 (SD 8) | Stent group, 22 men and 8 women; PTA group, 10 men and 13 women | Fontaine classification: stent, 2.6 (SD 0.5); PTA, 2.8 (SD 0.4). Rutherford classification: stent, 2.4 (SD 0.7); PTA, 2.1 (SD 0.7) | Preoperative claudication distance (m): stent, 166.4 (SD 140.1); PTA 150.3 (SD 160.5) | |||
| Rand et al. 200622 | 72.0 (range 47–80) (across both groups) | Fontaine III: PTA 8, stent 4. Fontaine IV: PTA 19, stent 20 |
|
Occlusion in four PTA patients and two stent patients | |||
| Vroegindeweij et al. 199723 | PTA 64 (range 41–82), stent 65 (range 46–78) | PTA group, 19 men; stent group, 17 men | 22 patients randomised for balloon angioplasty alone had mild to moderate IC (class I1–2) and five patients had severe claudication (class I3). 20 patients randomised for primary stenting had mild to moderate IC (class I1–2) and four patients had severe claudication (class I3) |
|
Occlusion in five PTA and four stent patients | ||
| Zdanowski et al. 199924 | Median age: stent 72, PTA 71 | Stent group, 10 men and 5 women; PTA group, 4 men and 13 women | Across groups, all patients had CLI, 66% had tissue loss, 19% had rest pain and 15% had disabling claudication. The median ABPI was 0.45. The occlusion was confined to the superficial femoral artery in 30 cases and to the popliteal artery in two cases (both in PTA group). The median length of the occlusions was 7.3 cm |
|
Outcomes
| Trial | Exercise tolerance/walking distance | Complications including amputation | Patency measures | Need for reintervention or recurrence rate |
|---|---|---|---|---|
| Becquemin et al. 200319 | Survival; occurrence of clinical disorders including cardiac events, transient ischaemic attack, stroke, deep venous thrombosis or pulmonary embolism, pulmonary or renal complications and miscellaneous life-threatening complications; occurrence, according to time of follow-up, of vascular events in the treated leg including acute ischaemia, worsening of clinical stage, trash foot and need for another vascular procedure or major amputation; and number of failed procedures at 1 year, defined as > 50% restenosis or death | The primary end point was the presence of > 50% stenosis at 1-year postoperative angiography | ||
| Cejna et al. 200120 | Primary technical success rate, complication rate. Clinical success was defined by an improvement in the SVS-ISCVS category. Reobstruction at follow-up was defined either as occlusion or stenosis of ≥ 70% within the treated area, as defined by angiography | The primary end point was the 12-month primary patency rate. Technical success was defined as a successful PTA or stent placement procedure with maximal 30% residual stenosis of vessel lumen diameter, as defined by biplane angiography | ||
| Grimm et al. 200121 | Claudication distance | Major complications | Primary patency rates, secondary patency rates | Reintervention |
| Rand et al. 200622 | Major amputation | The primary end point was the angiographic patency rate of treated lesions. Evaluation of the primary patency rate referred to lesion reocclusion, which was defined as stenosis of > 70% (threshold 1: critical stenosis) or > 50% (threshold 2: subcritical stenosis) | ||
| Vroegindeweij et al. 199723 | Complications | Late anatomical success or primary patency was determined by colour-flow duplex surveillance. All lesions that recurred during follow-up within the same treated arterial segment were considered restenoses. Progression of disease in untreated arterial segments was considered as new lesions. These lesions were not considered for the analysis of patency. Symptoms due to new lesions in an untreated segment were considered not to be a clinical failure Patency rates were determined by the life table method, restenosis or occlusion being the end point. Only primary patency was considered; the success of reinterventions was not part of this analysis Technical success was defined as a residual stenosis of < 30% diameter reduction on the completion arteriogram by visual estimation on two projections taken at right angles. Clinical and haemodynamic outcomes were classified according to the SVS/ISCVS criteria |
||
| Zdanowski et al. 199924 | Major complications | Restenosis was defined as a decrease by > 50% of the inner diameter compared with the state immediately after stenting. Clinical improvement required claudication distance to improve by ≥ 50%, resolution of rest pain or healing ulcers | Need for reintervention |
Results
| Trial | Results | Complications |
|---|---|---|
| Becquemin et al. 200319 | The number of failed procedures at 1 year (death or > 50% stenosis) was as follows: PTA 29 of 86 (33%) and stent 30 of 89 (34%) (non-significant p = 0.9). At 1 year, 21 procedures (32%) in the PTA group and 26 (34%) in the stent group fulfilled the criteria for failure (p = 0.85). Total occlusion of the treated site was noted in seven patients (11%) and 12 patients (16%), respectively (p = 0.3). The differences were not statistically different. In 23 patients for whom no angiograms were available, a duplex scan was available at 1 year. 2 of 13 patients in the PTA group and 1 of 10 patients in the stent group had > 50% stenosis of the treated artery | Perioperative complications in the PTA and stent groups occurred, respectively, in 5 patients (4.9%) and 10 patients (8.6%) (p = 0.2) and included thrombosis [two patients (1.7%) vs. two patients (1.7%)], embolism [two patients (1.7%) vs. five patients (4%)], arterial rupture [one patient (0.9%) vs. 0 patients] and introducer site problems, defined as difficulty in puncturing the artery or in placing the introducer sheath or guide wire [0 patients vs. three patients (2.6%)]. Additional procedures to treat complications were performed on 20 PTA and 10 stent group patients. There were no statistically significant differences between the two groups (p = 0.341). Mortality (4 years): 29 patients died during follow-up, 16 (14%) in the PTA group and 13 (11%) in the stent group. Cumulative survival rate free of vascular events: there were more events in the PTA plus stent group (p = 0.017). Major amputation: one in each group |
| Cejna et al. 200120 | Patency: the cumulative 1- and 2-year angiographic primary patency rates were 63% and 53%, respectively, for both groups. The secondary 1- and 2-year angiographic patency rates were 86% and 74% in the PTA group vs. 79% and 73% in the stent group (p = 0.5). The cumulative primary angiographic patency rates in the PTA vs. stent placement groups were 84%, 73%, 63% and 53% vs. 92%, 84%, 63% and 53% after 30, 180, 360 and 720 days, respectively (p = 0.09). Secondary patency measure: secondary angiographic patency rates were 100%, 94%, 86% and 74% for the PTA group and 95%, 93%, 79% and 73% for the stent placement group after 30, 180, 360 and 720 days, respectively (non-significant, p = 0.43). Reintervention: in the stent placement group, seven patients underwent femoropopliteal bypass graft surgery after angiographically demonstrated reocclusion, compared with four patients in the PTA group. In one patient of the PTA group, a popliteopedal bypass had to be created. 12 patients in the PTA group had a second intervention in the treated limb, in three cases because of development of a new stenosis (unrelated to the prior intervention site), compared with 21 patients in the stent placement group (six new stenosis). Clinical: there was no difference between groups of treatment – haemodynamic/clinical success at 1 and 2 years in the PTA group was 72% and 65% vs. 77% and 65% in the stent group (p = 0.26) | There were 12 primary failures in the PTA group, resulting in a technical success rate of 84.4%, and the technical success rate of ‘secondary’ stent implantation (i.e. in PTA group) was 100%. In the stent placement group, only one primary failure was observed (technical success rate 98.7%), after incorrect crimping of a stent on the balloon. Major complications or death occurred in four (2.6%) of the PTA group compared with 2 (1.3%) of the stent group. Within 30 days of intervention, three early stent thromboses were observed (3.9%) compared with one early PTA thrombosis (1.3%). During the 36-month follow-up period, seven patients died in the PTA group compared with 12 patients in the stent placement group |
| Grimm et al. 200121 | Walking distance: the mean walking distance increased in the PTA group from 150.3 m (SD 160.0 m) to 466.7 m (SD 461.9 m) (p = 0.18), and in the Palmaz group from 166.4 m (SD 140.0 m) to 383.5 m (SD 237.5 m) (p = 0.04). Reintervention: a second intervention was necessary in seven patients in the PTA group after 11 months and eight patients in the Palmaz stent group after 7 months, but this difference was not significant (p = 0.3). Stenosis: after dilatation or stent placement, respectively, the remaining stenosis percentage was 19.5% (SD 9.9%) in the PTA group and only 2.6% (SD 7.0%) in the Palmaz stent group. This difference of 17% is highly significant (p = 0.0001) and independent from the initial degree of stenosis because no correlation could be found between the degree of stenosis before and after intervention in both groups. Patency: after 12 months, the primary patency rates were 75% in the Palmaz stent group and 84.2% in the PTA group; after 24 months, they were 72.4% in the Palmaz stent group and 77.2% in the PTA group; after 39 months, they were 73.3% in the Palmaz stent group and 69.6% in the PTA group. There was no significant difference at any time (p > 0.41). Secondary patency rates at 12 months were 90% in the Palmaz stent group and 100% in the PTA group; after 24 months, they were 90% in the Palmaz stent group and 90.9% in the PTA group; after 39 months, they were 92.8% in the Palmaz stent group and 91.3% in the PTA group, again with no significant difference at any time (p > 0.7). To exclude a bias in favour of PTA (caused by the higher number of occlusions in the Palmaz stent group; 13 vs. 3), the subgroup of patients with a non-occlusive stenosis were compared, but, again, no significance between the patency rates in the Palmaz stent and PTA groups at 12 (p = 0.83), 24 (p = 0.81) and 39 (p = 0.77) months was found | (Six patients died during the follow-up period; all deaths were unrelated to the procedure and occurred > 30 days after the procedure) |
| Rand et al. 200622 | Patency: for the stent group the cumulative primary patency at 6 months was 83.7% at the 70% restenosis threshold, and 79.7% at the 50% restenosis threshold. For PTA, the primary patency at 6 months was 61.1% at the 70% restenosis threshold and 45.6% at the 50% restenosis threshold. Both results were statistically significant (p = 0.02). Total reocclusion was observed in two lesions (one PTA, one stent). Primary technical success: in one patient, stent application failed because the stent could not pass through a heavily calcified stenosis. In one lesion, PTA alone ended with a high-grade dissection and was unsatisfactory. This lesion was treated by secondary stenting | Amputation: one major amputation and one minor amputation were performed on patients in the stent group. One minor amputation was performed in a patient undergoing PTA. The comparison of cumulative limb salvage in the two groups using the Kaplan–Meier method revealed no significant difference between them |
| Vroegindeweij et al. 199723 | Patency: the cumulative 1-year patency, determined by restenosis or occlusion in the overall group (ITT), was 69% (SE 9%) for all patients, 74% (SE 8%) in the PTA group and 62% (SE 9%) in the patients randomised to stent (p = 0.22). This difference did not reach statistical significance. Overall 19 (37%) of the patients developed a PSVR of ≥ 2.5 in an initially treated segment: eight balloon angioplasty patients after a mean follow-up of 7 months (range 1–18 months) and 11 stent patients after 6 months (range 0–15 months). Total occlusion occurred in two (7%) PTA patients and five (21%) stent patients. In eight patients (30%) treated by PTA and in nine patients (43%) treated by stent, a clinical deterioration occurred after 1 year of follow-up. When analysed by life table analysis, the cumulative rate of maintained improvement (class +1 or more according to the SVS/ISCVS criteria) after 1 year of follow-up was 80% (SE 9%) in all patients, 85% (SE 7%) in the balloon angioplasty group and 74% (SE 9%) in the stent group (non-significant, p = 0.25) | In one patient treated by stent an embolus occurred 10 days after stent placement, which was successfully managed with streptokinase. In one PTA patient a thrombosis occurred which was also successfully managed with thrombolysis |
| Zdanowski et al. 199924 | Clinical: the rate of clinical improvement was 71% after PTA and stent and 60% after PTA alone (p = 0.17). Restenosis: angiographic reocclusions were seen in 33% and 75% in the stent and PTA groups, respectively (p = 0.17), while the rate of restenosis was significantly higher in the stent group (50% vs. 25%) (p = 0.033) | No technical failure and no limb loss. In the PTA group, one patient had a myocardial infarction and three patients needed arteriography owing to bleeding. In the stent group, one patient required arteriography and embolectomy. The 1-year mortality was 6% (two patients, group not specified)and there were no amputations. Four patients (two in each group) were operated on with a femorodistal bypass |
Drug-eluting stent
Study details
| Trial | Objective | Study design | Publication type | Language of publication | Sources of funding | Country of corresponding author | Intervention site(s) | Length of follow-up |
|---|---|---|---|---|---|---|---|---|
| Zilver PTX25,26 | RCT, prospective, multicentre | Dake – slides online (not peer reviewed)/Ansel – abstract only | English | Cook Medical | USA | USA, Japan, Germany. 55 sites | Outcomes at 12 months (ongoing follow-up through 5 years) | |
| SIROCCO28–30 | To review clinical outcomes of patients with CLI and TASC type C lesions treated with sirolimus-eluting vs. bare SMART (Cordis) nitinol SESs | RCT, prospective, multicentre. SIROCCO trial conducted in two phases: phase one, Duda 200228 publication; phase two, Duda 200529 publication; and complete results presented in Duda 200630 publication | Full report in peer-reviewed journal, three publications | English | The study was sponsored by Cordis Corporation, a Johnson & Johnson company | Germany | Germany, Austria, Belgium, Canada, the Netherlands, Australia, the USA, France | Mean 24 months |
| Rastan et al. 201131 | The rationale of this double-blinded randomised study was to prove the concept of using sirolimus-eluting stents to improve primary patency rates after interventional therapy of focal lesions of infrapopliteal arteries | RCT, prospective, multicentre | Full report in peer-reviewed journal | English | NR | Germany | Germany | Outcomes at 6 months and 12 months |
Interventions
| Trial | Focus of interventions (comparisons) | T1: intervention group | T2: control group |
|---|---|---|---|
| Zilver PTX25,26 | Paclitaxel-eluting stent vs. PTA (or BMS) | Zilver paclitaxel-eluting stent (PTX): paclitaxel 3 µg/mm2 dose density, no polymer or binder | PTA: if suboptimal PTA (> 30% residual stenosis), then secondary randomisation to BMS or PTX |
| SIROCCO28–30 | Sirolimus-eluting vs. bare SMART nitinol SESs | Sirolimus-eluting stent implantation procedure (for both groups): six or seven 80-mm stents implanted through a 7-F introducer sheath. A maximum of three stents were implanted in SIROCCO I and two stents in the SIROCCO II study. Patients not already on aspirin were to receive a 300-mg loading dose the day before the procedure; all received intra-arterial heparin boluses (3000–5000 units) at the time of the procedure, followed by a 750- to 1000-U/h infusion, as necessary. Overnight (24-hour) treatment with heparin was also permitted. After the procedure, either ticlopidine or clopidogrel was recommended for 4 weeks in addition to aspirin, which was continued for at least 12 months | Bare SMART nitinol SESs (Cordis) (implantation procedure as for T1) |
| Rastan et al. 201131 | Sirolimus-eluting stents vs. BMSs | A polymer-free sirolimus-eluting stent (Yukon™, Transluminal, Hechingen, Germany) was used. The polymer-free sirolimus-eluting stent was coated with a 2% sirolimus-containing solution | The BMS was coated with ethanol (placebo) |
Population inclusion
| Trial | Target population | Inclusion/exclusion criteria | Recruitment |
|---|---|---|---|
| Zilver PTX25,26 | SFA, symptomatic disease of the above-the-knee femoropopliteal artery | Inclusion criteria: Rutherford category 2 or greater, proximal 1 cm below bifurcation, distal medial femoral epicondyle, reference vessel diameter 4–9 mm | |
| SIROCCO28–30 | Symptomatic peripheral artery disease classified as Rutherford categories 1 (mild claudication) to 4 (rest pain) | Eligible patients were ≥ 30 years of age with symptomatic PAD classified as Rutherford categories 1 (mild claudication) to 4 (rest pain). All had obstructive (≥ 70%) de novo or restenotic lesions in the native SFA. The reference vessel diameter was 4–6 mm. The stenotic lesions varied in length from 7 to 20 cm in the first phase of the study and from 7 to 14.5 cm in the second phase. The occlusions varied in length from 4 to 20 cm in the first phase of the study and from 4 to 14.5 cm in the second phase. All lesions treated in SIROCCO I and II trials were classified as TASC type C. Signed informed consent Exclusion criteria included poor aortoiliac or common femoral inflow; uraemia; aneurysm in the target vessels; tandem lesions; previously stented lesions; ischaemic tissue loss; deep venous thrombosis; pregnancy; hepatic insufficiency; end-stage renal failure requiring dialysis; immunosuppressant therapy; recent haemorrhagic stroke (within the past 3 months); severe calcification that was deemed resistant to stenting; vessel tortuosity; revascularisation involving the same limb within 30 days; total occlusions of the iliac artery on the same side; requirement for stent in the popliteal artery; allergies to aspirin, heparin, sirolimus, nitinol, anticoagulants, antiplatelet therapy or contrast media; known or suspected active infection; presence of an aortic, iliac or femoral vascular prosthesis; and a life expectancy of < 2 years. Female patients of childbearing potential had a documented negative pregnancy test within 3 days prior to randomisation |
Phase one February to July 2001, phase two August to December 2001 |
| Rastan et al. 201131 | Focal lesions of infrapopliteal arteries | Patients were eligible for the study if they were ≥ 21 years old, were not pregnant and suffered from PAD with a Rutherford–Becker class (RC) of 3–5. Patients with lifestyle-limiting claudication, RC 2, could also be included after successful intervention of TASC A (single stenosis < 3 cm of the SFA or popliteal artery) femoropopliteal lesions to improve run-off status. Angiographic eligibility criteria were the presence of a single primary target lesion in a native infrapopliteal artery that was 2.5–3.5 mm in diameter and that did not exceed 45 mm in length to assure complete lesion coverage by the treatment with a maximum of two stents with a stent length of 25 mm; diameter stenosis of > 70%, as estimated by duplex-ultrasound and visually on angiography. Major exclusion criteria were a visible thrombus within the target lesion, known systemic coagulopathy, Buerger’s disease, acute limb ischaemia and life expectancy of < 1 year, or an intolerance to aspirin, clopidogrel and heparin | Between April 2006 and April 2008 |
Sample size
| Trial | Number of patients included in the study | Number of patients in T1 | Number of patients in T2 | Power calculation (a priori sample calculation) | Number of patients followed up from each condition (or attrition) |
|---|---|---|---|---|---|
| Zilver PTX25,26 | 479 | PTX, 241 from first randomisation; data presented from 238 | PTA 238, of whom 120 unsuccessful and 61 allocated to PTX and 59 to BMS (analysed in PTA group); data presented from 236 | Safety data from 236 PTA (of whom some had stents at second randomisation) and 235 PTX patients. Patency data from 251 lesions in PTA group, and 247 lesions in PTX group | |
| SIROCCO28–30 | 93 (36 from phase one, 57 from phase two of trial) | Sirolimus-eluting stent, 47 | Bare metal SES, 46 | Planned sample size: 74 patients, which would provide 90% statistical power to detect 0.8-mm difference between groups at 6 months assuming SD of 1.0 mm in each group | DES: at 6 months 42/47, at 2 months 35/47. BMS: at 6 months 44/46, at 24 months 38/46 |
| Rastan et al. 201131 | 161 | SES, 82 | BMS, 79 | Based on the published data, a patency rate of 50% was assumed with BMS. The study was designed to have a power of 95% to detect an elevation of the patency rate by the SES to 75% with a two-sided p < 0.05. Considering a dropout rate of 30%, total sample size of 155 patients | 62 (76.5%) patients in the SES group and 63 (79.7%) patients in the BMS group completed 1-year follow-up. Owing to inappropriate duplex-ultrasound or TLR, angiography was performed in 55 (44%) patients 25 (15.5%) patients died, 8 (4.9%) patients were lost during the follow-up period, and 3 (1.9%) patients could only be contacted by telephone because of care dependency |
Baseline characteristics
| Trial | Age (mean, years) | Gender | Classification of PAD | Number of patients who have undergone previous revascularisation procedures | Presence of cardiovascular risk factors |
|---|---|---|---|---|---|
| Zilver PTX25,26 | PTA 68 (SD 11), PTX 68 (SD 10) | PTA 64% male, PTX 66% male |
|
||
| SIROCCO28–30 | 47 patients [mean age 66.3 (SD 9.1), range 50–84] received the sirolimus-eluting SMART stent and 46 patients [mean age 65.9 (SD 10.8), range 38–83) received a bare SMART nitinol stent | 47 patients (31 men; 66%) received the sirolimus-eluting SMART stent and 46 patients (36 men; 78%) received a bare SMART nitinol stent |
|
|
|
| Rastan et al. 201131 | SES, 73.4 (SD 8); BMS, 72.3 (SD 9) | SES 67.9% male, BMS 64.9% male | CLI: SES 51.2%, BMS 41.8% |
|
Outcomes
| Trial | Clinical status | Complications including amputation | Patency measures | Need for reintervention or recurrence rate |
|---|---|---|---|---|
| Zilver PTX25,26 | Primary safety end point: 12-month event-free survival – freedom from death, amputation, TLR or worsening Rutherford category (by two classes or to class 5 or 6), per-protocol cohort, Kaplan–Meier p-values from log-rank test | Primary effectiveness end point: 12-month primary patency duplex ultrasonography, patent = PSVR < 2.0 (or angiography if available, patent = diameter stenosis < 50%), intent-to-treat cohort, Kaplan–Meier p-values from log-rank test | ||
| SIROCCO28–30 | Adverse events | Primary end point was in-stent mean lumen diameter stenosis at 6 months as determined by QA. The in-lesion segment was defined as the in-stent segment plus 5 mm proximal and distal to the stent. Restenosis as determined by QA (> 50% stenosis) was defined as haemodynamic failure of the stented lesion (increase in PSV > 100% by duplex in the stenotic segment when compared with a reference segment proximal to the stenosis or absence of a Doppler signal) or incidence of serious adverse events (death or prolonged hospitalisation) | TLR/TVR | |
| Rastan et al. 201131 | Rutherford–Becker classification | Death, major and minor amputations, TLR including need for surgical revascularisation and myocardial infarction were defined as major adverse events | The main study end point was primary patency rate after 1 year, defined as freedom from in-stent restenosis (luminal narrowing of < 50%) detected with duplex ultrasonography or angiography if appropriate. The definition of 50% restenosis was based on a PSVR (PSV within the stent divided by PSV ≥ 1 cm proximal of the stent in a healthy vessel segment) > 2.4. The presence of a significant restenosis was confirmed by intra-arterial angiography during clinically driven TLR in all cases Secondary end points included primary patency rate after 6 months and secondary patency rate, defined as patency following successful TLR after 12 months |
Target limb reintervention |
Results
| Trial | Results | Complications |
|---|---|---|
| Zilver PTX25,26 | Primary patency (PSVR < 2.0) at 12 months: PTX group 83.1%, PTA group (of 126 patients who actually had PTA alone) 65.3% (p < 0.01; significantly lower than PTX); PTA group of 125 lesions with stent implantation (bare metal or PTX) 32.8% (significantly lower than those randomised to PTX, p < 0.01); for the 62 lesions from patients randomised to PTA then BMS 67% patency at 12 months (significantly lower than those randomised to PTX, p < 0.01) – this group had a reported restenosis rate of 33% at 12 months, whereas the PTX restenosis rate was 12.9% (49% reduction). At 24 months, the patency rate of PTX vs. BMS was 81.2% vs. 62.7% (p < 0.01). Author notes relatively high acute PTA failure rate, and, for lesions < 14 cm, no in-stent restenosis | PTX and BMSs: 0.9% stent fracture rate over 12 months. Safety analysis – event-free survival at 12 months: PTX 90.4%, PTA 82.6% (p < 0.01) |
| SIROCCO28–30 | Restenosis: at 24 months, the cumulative in-stent restenosis rates according to duplex ultrasound were 4.7%, 9.0%, 15.6% and 21.9%, respectively, at 6, 9, 18 and 24 months. The rates did not differ significantly between the treatment groups (duplex ultrasound restenosis rates and 95% CI): at 6 months DES 4.8%, 0.6% to 16.2% (n = 42), and BMS 4.5%, 0.6% to 15.5% (n = 44); at 9 months DES 7.1%, 1.5% to 19.5% (n = 42), and BMS 11.1%, 3.1% to 26.1% (n = 36); at 18 months DES 18.4%, 7.7% to 34.3% (n = 38), and BMS 12.8%, 4.3% to 27.4% (n = 39); at 24 months DES 22.9%, 10.4% to 40.1% (n = 35), and BMS 21.1%, 9.6% to 37.3% (n = 38); at 24 months TVR DES n = 6 (13%) and BMS n = 10 (22%), TLR DES n = 3 (6%), BMS n = 6 (13%). In both groups at 24 months, no amputations were performed as a complication of the stent procedure. Both groups of patients showed an improvement in Rutherford classification immediately after implantation of the stent, which was sustained over the 24-month follow-up | Seven patients died owing to stroke (n = 1), lung emboli (n = 1), cancer (n = 1), cardiac disease (n = 2) and natural causes (n = 2) in the sirolimus-eluting group, whereas only two patients died in the BMS group (complications of coronary BS and progressive cardiac failure). Stent fractures (defined as one broken strut) were detected by the independent angiographic and radiographic core laboratory ≤ 18 months post procedure in eight patients in the BMS group and 9 in the sirolimus stent group (p = 0.245) |
| Rastan et al. 201131 | Restenosis: the rates of ≥ 50% target lesion restenosis after 1 year were 19.4% (n = 2) for the SES group and 44.4% (n = 28) for the BMS group. Patency: the 1-year primary patency rates were 80.6% (n = 50) and 55.6% (n = 35; p = 0.004), and 6-month primary patency rates were 85.9% (n = 55) and 68.7% (n = 46; p = 0.02), respectively. The secondary 1-year patency rates were 91.9% (n = 57) for the SES group and 71.4% (n = 45; p = 0.005) for the BMS group. The BMS hazard ratio for restenosis was 3.2 (95% CI 1.5 to 6.7; p = 0.003) compared with SES after 1 year. The risk of restenosis associated with BMS prevailed after adjustment for diabetes mellitus, smoking status and body mass index. The corresponding adjusted hazard ratio was 3.0 (95% CI 1.4 to 6.4; p = 0.005). No significant interaction could be observed between stent type and stage of disease (CLI or IC). Clinical: the median (IQR) Rutherford category decreased from 4 (3–5) in the SES group and 3 (3–5) in the BMS group (p = 0.40) at baseline to 1 (1–3) and 2 (1–3; p = 0.37) at 6 months and 2 (0.75–3) and 2 (1–3; p = 0.01) at 1 year, respectively. Moreover, the median (IQR) change in Rutherford category in the SES and BMS groups was –2 (–3 to –1) and –1 (–2 to 0; p = 0.12) at 6 months and –2 (–3 to –1) and –1 (–2 to 0) at 1 year, respectively (p = 0.004). TLR: TLR was performed in 6 patients (9.7%) in the SES group and in 11 patients (17.5%) in the BMS group (p = 0.29) | Owing to study stent dislocation in one (1.2%) patient of the SES group and two (2.5%) patients of the BMS group, three stents had to be implanted to cover the target lesion. Adverse events: a total of 51 (31.5%) adverse events occurred, 22 (27.1%) in the SES group, and 29 (36.7%) in the BMS group. 14 patients (17.1%) in the SES group and 11 patients (13.9%, p = 0.66) in the BMS group died during the follow-up period: eight patients (5%) died because of major cardiac events (myocardial infarction, heart failure); five patients (3.1%) died as a result of gastrointestinal and pulmonary infections; and one patient (0.6%) had lung cancer. In 11 patients (6.8%) the cause of death remained uncertain. Limb salvage: owing to insufficiently controlled wound infection despite adequate antibiotic treatment, one lower-leg major amputation and one minor toe amputation of the target limb in the SES group (3.2%), and two lower-leg major amputations and two minor toe amputations in the BMS group (6.4%), were documented. Hence, the limb salvage rate was 98.4% in the SES group and 96.8% in the BMS group after 12 months (p = 0.61) |
Stent graft
Study details
| Trial | Objective | Study design | Publication type | Language of publication | Sources of funding | Country of corresponding author | Intervention site(s) | Length of follow-up |
|---|---|---|---|---|---|---|---|---|
| Saxon et al. 2003, 200832,33 | To compare the safety and effectiveness of the Viabahn® endoprosthesis (W. L. Gore, Flagstaff, AZ, USA) (ePTFE-covered stent) with those of PTA alone in the treatment of symptomatic peripheral arterial disease affecting the SFA | RCT, prospective, multicentre | Full report in peer-reviewed journal | English | NR (single-centre reference implies Gore and associates) | USA | USA, 25 centres | Outcomes at 12 months |
Interventions
| Trial | Focus of interventions (comparisons) | T1: intervention group | T2: control group |
|---|---|---|---|
| Saxon et al. 2003, 200832,33 | Viabahn endoprosthesis (ePTFE-covered stent graft) vs. PTS | Viabahn endoprosthesis stent graft placement (ePTFE/nitinol SES graft): stent grafts were oversized 5–20% relative to native vessel diameter, placed preferably from a retrograde over-the-bifurcation approach, but also from antegrade approach. An angioplasty balloon with diameter equal to that of stent graft was inflated throughout entire length of device. Antiplatelet therapy at discretion of operator (aspirin, occasional ticlopidine) | PTA: patients with ≥ 30% residual stenosis after PTA could have uncovered stent as ‘bail-out’ (no crossover to ePTFE stent graft). In general, PTA was performed with 6-F sheaths. Patients in both groups given heparin during procedure |
Population inclusion
| Trial | Target population | Inclusion/exclusion criteria | Recruitment |
|---|---|---|---|
| Saxon et al. 2003, 200832,33 | Patients with symptomatic SFA PAD | Inclusion criteria: De novo or restenotic atherosclerotic or occlusive lesion of SFA up to 13 cm in length, chronic lifestyle-altering claudication or chronic lower limb ischaemia. Exclusion criteria: prior, planned or concurrent limb BS, intolerance to antiplatelet therapy, bleeding disorders, renal failure, bacteraemia, lesion within 0.5 cm of profunda femoris artery origin, prior stent implantation in target lesion, fewer than one continuously patent run-off infrapopliteal artery with stenosis of ≤ 50% diameter | From 1998 to 1999 |
Sample size
| Trial | Number included in the study | Number of patients in T1 | Number of patients in T2 | Power calculation (a priori sample calculation) | Attrition | Number followed up from each condition |
|---|---|---|---|---|---|---|
| Saxon et al. 2003, 200832,33 | 197 | Stent graft, 97 | PTA, 100 | Originally designed to enrol a maximum of 415 patients and was statistically powered to show 15% increase in patency in the stent graft group | At 1 year patency results for 69/100 PTA and 78/97 stent graft patients |
Baseline characteristics
| Trial | Age (mean, years) | Gender (M/F) | Classification of PAD | Presence of cardiovascular risk factors (% of patients) |
|---|---|---|---|---|
| Saxon et al. 2003, 200832,33 | PTA 67 (range 40–84), stent graft 67 (46–88) | PTA 70/30, stent graft 80/17 | PTA: 88% claudication; 12% CLI. Stent graft: 91% claudication; 9% CLI |
|
Outcomes
| Trial | Pain/clinical status | Complications including amputation | Patency measures |
|---|---|---|---|
| Saxon et al. 2003, 200832,33 | Rutherford–Becker classification | Major and minor adverse events | Primary outcome was primary patency at 12 months, which was defined as technical success without interrupted blood flow and no procedures performed (any major adverse events within 30 days led to a loss of primary patency), and > 50% stenosis on duplex ultrasound. Redefined during study to: no TVR, no evidence of restenosis or occlusion within treated vessel from Doppler ultrasound, where target lesion not identified vessel patency from SFA to popliteal artery was applied, angiography demonstrating < 30% residual diameter stenosis. Technical success defined as treatment success with no major adverse events within 30 days and improvement in limb pressure indexes of ≥ 0.15 relative to pre treatment. Redefined during study to: successful completion of randomised treatment with no rescue procedure on day of treatment and angiography demonstrating < 30% residual diameter stenosis |
Results
| Trial | Results | Complications |
|---|---|---|
| Saxon et al. 2003, 200832,33 | Technical success: the stent graft group had a significantly higher technical success rate (95% vs. 66%, p < 0.0001). Subgroup analysis non-significant for lesions < 3cm in length. Patency: the stent graft group had a significantly higher 1-year primary vessel patency rate at duplex ultrasonography (65% vs. 40%, p = 0.0003). A patency benefit was seen for lesions ≥ 3 cm in length. Clinical: at 12 months, chronic limb ischaemia status was 15% further improved for the stent graft group (p = 0.003) | There were no significant differences between treatment groups with regard to the occurrence of early or late major adverse events. 21 major adverse events for PTA group, and 20 in the stent graft group. Thigh pain in 10 cases in stent graft group and 3 in PTA group (p = 0.047); pain was transient and resolved within 2 months |
Atherectomy
Study details
| Trial | Objective | Study design | Publication type | Language of publication | Sources of funding | Country of corresponding author | Intervention site(s) | Length of follow-up |
|---|---|---|---|---|---|---|---|---|
| Nakamura et al. 199534 | To test the hypothesis that, in occlusions of the superficial femoral artery, removal of atherosclerotic plaque would result in a higher long-term patency rate than that resulting from balloon dilatation alone. A secondary hypothesis was that long-term patency would be proportional to the amount of plaque removed | RCT, prospective, single centre | Full report in peer-reviewed journal | English | Supported in part by an NIH grant | USA | USA | Outcomes at 6 months |
| Vroegindeweij et al. 1992, 1995,35,36 Tielbeek et al. 199637 | To evaluate whether directional atherectomy would provide better results than conventional balloon angioplasty in symptomatic femoropopliteal disease | RCT, prospective, single centre | Full report in peer-reviewed journal | English | NR | Netherlands | Netherlands | Outcomes at 2 years (median follow-up duration was 13 months) |
Interventions
| Trial | Focus of interventions (comparisons) | T1: intervention group | T2: control group |
|---|---|---|---|
| Nakamura et al. 199534 | Atherectomy (TEC) vs. PTA, with 2 groups of TEC (2.7 or 4.0/4.7 mm) | Two groups of atherectomy: (1) a 2.7-mm or (2) a larger (4.0 or 4.7 mm) TEC atherectomy device followed by PTA. TEC: after successful recanalisation, guide wire inserted into femoral artery, a 2.7-mm atherectomy cutter was inserted and the rotating cutter was slowly advanced under fluoroscopic control. For patients in the large TEC group, TEC atherectomy was then performed with a 4- or 4.7-mm cutter. For both groups, the patients then had balloon dilatation with a 6- or 7-mm-diameter catheter | PTA: common femoral artery punctured in antegrade direction, 7.5-F sheath, heparin administered, 8-F introducing sheath. Balloon angioplasty performed using a balloon catheter 6 or 7 mm in diameter by 10 mm in length |
| Vroegindeweij et al. 1992, 1995,35,36 Tielbeek et al. 199637 | DA vs. PTA | DA: the DA device consists of a hollow cutting cylinder with a window on one side and a balloon on the opposite side. Inflation of the balloon pushes the window against the diseased arterial wall, and obstructing plaque protrudes into the cylinder. A high-speed rotating cutter shaves off the plaque and pushes it into a collection chamber. An introducer sheath is advanced in an antegrade fashion through an arterial puncture in the common femoral artery either percutaneously (in the angiography suite) or via a ‘cut down’ approach (in the operating room). The patient receives 5000 IU of heparin intra-arterially, and, under fluoroscopic guidance, a 6-F to 8-F atherectomy catheter (Simpson’s Atherocath™, Devices for Vascular Intervention, Inc., Redwood City, CA) is advanced distally. The size of the atherectomy catheter was chosen so that the working diameter, with the balloon inflated, was equal to slightly greater than a normal adjacent artery segment | PTA: introducing a 5-F non-compliant balloon catheter via a 6-F sheath, balloon length 2 cm except two cases of 4 cm, balloon diameter 5–7 mm. Use of only the technique selected by randomisation was attempted, although crossover was permitted if an acceptable result could be obtained only by the opposite technique or by combined techniques |
Population inclusion
| Trial | Target population | Inclusion/exclusion criteria | Recruitment |
|---|---|---|---|
| Nakamura et al. 199534 | Patients with occluded superficial femoral arteries | Inclusion criteria: symptoms of claudication, evidence of peripheral vascular disease by diminished pulses and decreased ABPI, angiographic evidence of complete occlusion of an SFA. Exclusion criteria: prior peripheral bypass, insufficient distal run-off vessels | |
| Vroegindeweij et al. 1992, 1995,35,36 Tielbeek et al. 199637 | All patients had segmental lesions of the femoropopliteal arteries | Eligible patients included those with IC of ≥ 3 months duration and obstructive lesions of the femoropopliteal arteries that appeared suitable for either atherectomy or balloon angioplasty, that is, lesions with a maximum length of 5 cm. This restriction was because atherectomy is applicable only in discrete stenoses or short occlusions. Therefore, any patient with a diffusely diseased femoropopliteal artery with a stenosis extending > 5 cm or an occlusion > 2 cm in length was not considered a good candidate for the trial and was relegated to an obligatory balloon dilatation. Only de novo lesions were admitted, and any previous ipsilateral femoropopliteal endovascular or operative intervention was considered an exclusion criterion, irrespective of whether this treatment had concerned a different segment from the one considered for intervention at the time of the study. Only patients were selected who would be able to comply with the frequent follow-up visits required by the involved colour-flow duplex surveillance protocol | From January 1990 until May 1993; 187 patients undergoing endovascular treatment; 114 did not meet inclusion criteria or refused to participate |
Sample size
| Trial | Numbers included in the study | Number of patients in T1 | Number of patients in T2 | Power calculation (a priori sample calculation) | Number followed up from each condition (or attrition) |
|---|---|---|---|---|---|
| Nakamura et al. 199534 | 39 | TEC 2.7 mm, 13; TEC 4.0/4.7 mm, 13 | PTA, 13 | NR | 6 months patency available from those with procedural success: PTA, 10/13; TEC 2.7 mm, 13/13; TEC 4.0/4.7 mm, 8/13 |
| Vroegindeweij et al. 1992, 1995,35,36 Tielbeek et al. 199637 | 73 | DA, 38 | PTA, 35 | NR | Follow-up ended because of death in three and because of surgical intervention for severe claudication or conversion to the stage of critical ischaemia in three patients. 19 patients had repeat endovascular treatment, and two of the patients were lost to follow-up |
Baseline characteristics
| Trial | Age (mean, years) | Gender (male) | Classification of PAD [n (%)] | Presence of cardiovascular risk factors | Other relevant information |
|---|---|---|---|---|---|
| Nakamura et al. 199534 | PTA, 61 (SD 0.1); TEC 2.7 mm, 64 (SD 6); TEC 4.0/4.7 mm 70 (SD 6) | PTA 13/13; TEC 2.7 mm, 12/13; TEC 4.0/4.7 mm, 13/13 |
|
The mean occlusion length was 19.4 cm (SD 11.7 cm) | |
| Vroegindeweij et al. 1992, 1995,35,36 Tielbeek et al. 199637 | 64 (range 49–77) in patients treated with atherectomy and 64 (range–80) in the PTA group | DA, 28 (74%); PTA, 27 (77%) | Mild to moderate claudication: DA 26 (68), PTA 27 (77) Severe claudication: 12 (32), PTA 8 (23) |
|
Occlusion: DA 3%, PTA 6%. ‘It should be noted that the patients in this study comprised a primarily favourable group, with only IC and with lesions less than 5 cm in length’ |
Outcomes
| Trial | Clinical status | Complications including amputation | Patency measures |
|---|---|---|---|
| Nakamura et al. 199534 | Procedural complications | Improvement in clinical symptoms as well as sustained improvement in ABPI | |
| Vroegindeweij et al. 1992, 1995,35,36 Tielbeek et al. 199637 | Clinical and haemodynamic outcome was classified according to Society for Vascular Surgery/International Society for Cardiovascular Surgery criteria on a scale from –1 to –3 for deterioration of symptoms and ABPI: 0 for unchanged symptoms, +1 for either a categorical improvement of clinical classification of claudication or increase of ABPI > 0.10, +2 for at least a single category improvement of claudication combined with ABPI increase of > 0.10 and +3 for markedly improved symptoms combined with an ABPI > 0.90 | Procedural complications | Primary patency ended if a restenosis with ≥ 50% diameter reduction developed Late anatomical success or patency was determined by colour-flow duplex surveillance. As a baseline characteristic, the severest lesion is considered the index lesion. All lesions that recurred during follow-up within the same arterial segment are considered restenoses. Lesions in different segments that are treated at the same time are associate lesions, and their recurrences also are defined as restenoses. The severest of the restenoses is the lesion whose velocity values are used for the patency analysis. When studied as a dichotomous variable, a PSV ratio greater than 2.5 was the criterion for restenosis. Progression of disease in non-treated arterial segments is defined as new lesions. These lesions are not considered for the analysis of late patency. The rate of restenosis or occlusion was assessed by use of colour-flow duplex scanning. Restenosis was defined on the basis of a PSVR of ≥ 2.5, and occlusion of the treated segment was diagnosed if flow signals were absent, that is, loss of patency |
Results
| Trial | Results | Complications |
|---|---|---|
| Nakamura et al. 199534 | Across groups, the mean lumen area increased from 4.7 to 15.1 mm2, primarily because of balloon dilatation, but the mean atheroma area of 19.8 mm2 did not change with either size of TEC device. Although the initial procedure success rate was high (79%), the 6-month patency was only 45%. There was no difference in 6-month patency between the groups; at 6 months, the percentages of patients still patent were as follows: PTA, 50%; TEC 2.7 mm, 46%; TEC 4.0/4.7 mm, 38% (p = 0.16) | PTA: three perforations due to guide wire manipulation (no haematoma formation). TEC 4.0/4.7 mm: one perforation and two cases of distal embolisation with 4.7-mm device (4.0 mm used for all further patients) |
| Vroegindeweij et al. 1992, 1995,35,36 Tielbeek et al. 199637 | The patency rate at 2 years of treated segments was 34% in the atherectomy group and 56% in PTA patients (non-significant, p = 0.07). In patients with lesions > 2 cm, the 1-year patency rate of atherectomy was significantly lower than that of balloon angioplasty (p = 0.03). Stenosis: residual stenoses (≥ 30% diameter reduction) resulted in five patients (13%) undergoing atherectomy and three patients (9%) undergoing balloon angioplasty. Clinical: at 1 month, clinical and haemodynamic improvement by Society for Vascular Surgery/International Society for Cardiovascular Surgery criteria for lower-limb ischaemia was observed in 34 patients (89%) treated with atherectomy and in 34 (97%) treated with balloon angioplasty. By life table analysis, the cumulative rate of clinical and haemodynamic success at 2 years was 52% in patients treated with atherectomy and 87% in patients treated with balloon angioplasty (p = 0.06) | DA: one small dissection, one large dissection, one failure to pass guide wire, one thrombosis/embolisation. PTA: five small dissections. Residual stenoses of ≥ 30% diameter reduction were seen in five patients treated with atherectomy and three treated with PTA. However, in none of these cases was the residual stenosis > 50% diameter reduction. Immediate operative intervention was not required in any patient |
Cutting balloon
Study details
| Trial | Objective | Study design | Publication type | Language of publication | Sources of funding | Country of corresponding author | Intervention site(s) | Length of follow-up |
|---|---|---|---|---|---|---|---|---|
| Amighi et al. 200838 | To prospectively determine, in a RCT, whether CBA yields superior morphological and clinical outcomes at 6 months compared with the 6-month outcomes after conventional PTA in patients with short de novo SFA lesions | RCT, prospective, two centres | Full report in peer-reviewed journal | English | (From ClinicalTrials.gov. Sponsored by Medical University of Vienna, Vienna) | Austria | Austria, 2 centres | Outcomes at 6 months |
| Dick et al. 200839 | To prospectively determine whether CBA, when compared with conventional balloon angioplasty, improves morphological and clinical outcomes in patients with femoropopliteal in-stent restenosis | RCT, prospective, single centre | Full report in peer-reviewed journal | English | NR | Austria | Austria | Outcomes at 1, 3 and 6 months |
Interventions
| Trial | Focus of interventions (comparisons) | T1: intervention group | T2: control group |
|---|---|---|---|
| Amighi et al. 200838 | CBA vs. PTA | CBA: the diameter of the balloon for PTA or CBA corresponded to the proximal non-diseased vessel area in a 1 : 1 ratio. The CBs were inflated slowly to a pressure of up to 8 atm according to manufacturer (Boston Scientific, Natick, MA) recommendations. 0.018-inch peripheral CBs (5–6 mm in diameter, 10 or 20 mm in length) were used over a standard 0.018-inch guide wire. For 4-mm lesions, 0.014-inch CBs (15 mm in length) were used over a standard 0.014-inch guide wire | PTA: experienced staff interventionists with 6–15 years’ experience in peripheral vascular intervention performed PTA by following a standardised protocol involving an antegrade or over-the-bifurcation approach with use of 5- to 7-F sheaths. Heparin (5000 IU) was routinely administered intra-arterially. The diameter of the balloon for PTA or CBA corresponded to the proximal non-diseased vessel area in a 1 : 1 ratio. The regular balloons were inflated to 8–10 atm for ≤ 2 minutes. As a bail-out procedure, self-expandable nitinol stent implantation was performed in patients who had > 30% residual stenosis after repeated angioplasty or because of flow-limiting dissection or elastic recoil in the worst angiographic view |
| Dick et al. 200839 | PCBA vs. PTA | PCBA: PCBA was performed by using a peripheral CB (Boston Scientific). The balloon diameter in both groups corresponded to the proximal non-diseased vessel area. Bail-out stenting using self-expanding nitinol stents was performed in patients with a residual stenosis of > 30% or flow-limiting dissection. All patients continuously received 100 mg of aspirin daily, in addition to 75 mg of clopidogrel daily for 1 month after intervention | PTA: interventions were performed percutaneously by one of three experienced interventionists from an over-the-bifurcation approach. After insertion of a 7-F sheath, 5000 IU of heparin was administered intra-arterially. Bail-out stenting using self-expanding nitinol stents was performed in patients with a residual stenosis of > 30% or flow-limiting dissection. All patients continuously received 100 mg of aspirin daily, in addition to 75 mg of clopidogrel daily for 1 month after intervention |
Population inclusion
| Trial | Target population | Inclusion/exclusion criteria | Recruitment |
|---|---|---|---|
| Amighi et al. 200838 | Patients with short (≤ 5cm) de novo SFA lesions | Patients with SFA lesions ≤ 5 cm in length who were referred for endovascular treatment of the SFA owing to IC or chronic CLI. Inclusion criteria: the clinical criterion for study entry was symptomatic PAD with severe IC (Fontaine stage IIb) or chronic CLI (Fontaine stage III or IV). The anatomical inclusion criterion was a single SFA target lesion – specifically, a SFA with > 50% stenosis or occlusion – ≤ 5 cm in length. Exclusion criteria were previous BS or stent placement at the ipsilateral lower limb; history of intolerance to antiplatelet therapy, heparin or contrast media; bleeding diathesis; active systemic bacterial infection; and severely impaired renal function (serum creatinine level > 2.5 mg/dl) | From August 2004 to June 2006; 45 recruited; two patients (one treated with CBA and one treated with PTA) had to be excluded because of their withdrawal from follow-up examinations |
| Dick et al. 200839 | Femoropopliteal in-stent restenosis (angiographic stenosis of > 50% of the vessel lumen diameter) | Entry criteria included symptomatic PAD with IC or CLI related to a recurrent stenosis in a previously stented segment of ≤ 20 cm in length. Only patients with a restenosis of a self-expanding nitinol stent (Absolute/Dynalink, Abbott Vascular, Abbott Park, IL, USA; Protege, EV3, Paris, France; Sentinol®, Boston Scientific, Galway, Ireland; or SMART CONTROL®, Cordis, Miami Lakes, FL, USA) implanted at our institution or others were eligible. Exclusion criteria were a history of intolerance to antiplatelet therapy, an adverse reaction to heparin, bleeding diathesis, a creatinine level of > 2.5 mg/dl, haemodialysis, active bacterial infection, allergy to contrast media, pregnancy; patients with stent fractures were not included in the study, as treatment of fractured stents frequently requires repeat stenting of the lesion. Patients with acute stent thrombosis were also not eligible, as these patients were treated with thrombolysis prior to angioplasty | Consecutive patients with femoropopliteal in-stent restenosis (angiographic stenosis of > 50% of the vessel lumen diameter) were enrolled from November 2004 to March 2007 – 40 enrolled, one lost to follow-up |
Sample size
| Trial | Number included in the study | Number of patients in T1 | Number of patients in T2 | Power calculation (a priori sample calculation) | Number followed up from each condition (or attrition) |
|---|---|---|---|---|---|
| Amighi et al. 200838 | 43 | CBA 21 (of whom four had secondary stent placement) | PTA 22 (of whom four had secondary stent placement) | Estimated that a sample size of 40–50 patients would be necessary to demonstrate any superiority of CBA over PTA. On the basis of data in the literature, expected restenosis rates of 40% in the PTA group (literature-reported restenosis rates of 35–45% in patients with short lesions) and 10–20% in the CBA group (estimated) | 6-month outcomes for 22/23 enrolled for PTA, and 21/22 for CBA |
| Dick et al. 200839 | 39 (40, 1 lost to follow-up) | PCBA 17 | PTA 22 | NR | One patient lost to follow-up, group not specified |
Baseline characteristics
| Trial | Age (years) | Gender (male) | Classification of PAD [n (%)] | Presence of cardiovascular risk factors [n (%)] | Level of exercise tolerance [median (IQR)] | Other relevant information |
|---|---|---|---|---|---|---|
| Amighi et al. 200838 | Median PTA, 71.4 (IQR 60.8–76.6); median CBA, 67.4 (62–75.6) | PTA 14 (64%); CBA 12 (57%) |
|
|
Pain-free walking distance (m): PTA 100 (0–200), CBA 100 (10–150) | Occlusion in 23% of PTA and 29% of CBA group. Across groups, mean length of the treated segments was 2.5 cm, and the mean degree of stenosis was 90%. Four (18%) patients in the PTA group vs. one (5%) patient in the CBA group (p = 0.17) underwent secondary stent placement owing to flow-limiting dissection or residual stenosis |
| Dick et al. 200839 | Mean PCBA, 70 (SD 10); mean PTA, 66 (SD 10) | PCBA 65%; PTA 55% | Clinical (Rutherford) classification of PAD:
|
|
Maximum walking distance on treadmill (m): PCBA 42 (23–100), PTA 55 (10–92) | Average lesion length was 80 mm (SD 68). Average length of the treated segments was 85 mm (SD 70), with no significant difference between the two groups. Chronic occlusion: PCBA 12%, PTA 9% |
Outcomes
| Trial | Exercise tolerance/walking distance | Pain/clinical status | Complications including amputation | Patency measures | Need for reintervention or recurrence rate |
|---|---|---|---|---|---|
| Amighi et al. 200838 | Patient-reported pain-free walking distance | Clinical stage of PAD | Complications, adverse events | The primary study end point was the occurrence of a duplex-ultrasonography-assessed relevant (> 50%) restenosis in the treated vessel segment(s) 6 months after treatment. Restenosis was defined according to haemodynamic criteria as a > 50% reduction in vessel diameter at the level of the previously treated lesion. A focal increase in PSV of ≥ 140% (corresponding to a PSVR of ≥ 2.4) was considered to be indicative of > 50% stenosis at that site | |
| Dick et al. 200839 | Maximum walking capacity on the treadmill (no further details of treadmill protocol) | Complications were classified as either major or minor. Major complications were access site complications requiring surgical interventions, bleeding complications with a decrease of serum haemoglobin of > 2 g/dl, amputation, macroembolism with the need for further revascularisation and any death before discharge. Minor complications were those that resolved spontaneously (e.g. superficial haematoma and groin pain owing to nerve injury) | The primary study end point was the occurrence of a > 50% restenosis at the treated segment at 6 months after intervention, as determined by duplex ultrasonography | Reintervention at the site of the treated segment or BS was also defined as a restenosis and loss of primary patency |
Results
| Trial | Results | Complications |
|---|---|---|
| Amighi et al. 200838 | Restenosis: 6-month restenosis rate was 32% (seven patients) in the PTA group vs. 62% (13 patients) in the CBA group (p = 0.048). Clinical: 16 (73%) PTA group patients vs. 8 (38%) CBA group patients were asymptomatic at follow-up (p = 0.059). Walking distance: there was no significant difference for pain-free walking distance (median > 1000 m vs. 600 m for PTA vs. CBA group, respectively; p = 0.17) between the two groups. Pain-free walking distance (m) [median (IQR)]: PTA 1000 (200 to > 1000), CBA 600 (100 to > 1000) (non-significant, p = 0.17) | One patient randomly assigned to undergo CBA had the minor complication of peripheral embolism of the tibioperoneal trunk, which was successfully resolved with thrombus aspiration during the intervention without clinical sequelae. No patient died during the follow-up period. Three patients (group not specified) – all with CLI—underwent minor amputations (toe to distal forefoot) within 14 days of angioplasty |
| Dick et al. 200839 | Maximum walking capacity at 6 months, on the treadmill: PCBA 117 m vs. PTA 103 m (non-significant, p = 0.97). Restenosis: restenosis rates at 6 months were 65% (11 of 17; 95% CI 42% to 88%) after PCBA vs. 73% (16 of 22; 95% CI 54% to 92%) after PTA (non-significant, p = 0.73). Earlier restenosis rates in the PCBA vs. CBA groups were 12% (2 of 17; 95% CI 3% to 27%) vs. 27% (6 of 22; 95% CI 8% to 46%) at 1 month (p = 0.42); and 47% (8 of 17; 95% CI 23% to 71%) vs. 41% (9 of 22; 95% CI 20% to 62%) at 3 months (p = 0.75). Clinical: comparable outcomes between PCBA and CBA were observed until 6 months after intervention. Deterioration at 6 months: 1% PCBA, 3% PTA | Technical success could be achieved in all patients. No major complications were observed. Bail-out stenting was done infrequently in both groups (12% PCBA, 5% PTA). No amputations and no deaths at 6 months. Thrombosis and/or reocclusions at 6 months: PCBA 6%, PTA 23%. Ipsilateral reinterventions by 6 months: PCBA 41%, PTA 36% |
Cryoplasty
Study details
| Trial | Objective | Study design | Publication type | Language of publication | Sources of funding | Country of corresponding author | Intervention site(s) | Length of follow-up |
|---|---|---|---|---|---|---|---|---|
| Jahnke et al. 201040 | To evaluate safety and efficacy of cryoplasty vs. conventional angioplasty for focal popliteal arterial occlusive disease | RCT, prospective, single centre | Full report in peer-reviewed journal | English | NR | Germany | Germany | Outcomes reported at 9 months |
| Spiliopoulos et al. 201041 | To investigate the immediate and long-term results of cryoplasty vs. conventional balloon angioplasty in the femoropopliteal artery of diabetic patients | RCT, prospective, single centre | Full report in peer-reviewed journal | English | NR | Greece | Greece | Follow-up visits at 6 months, 1 year and annually thereafter. The mean angiographic follow-up period was 23.5 months (SD 1.9 months) for the cryoplasty group vs. 25.3 months (SD 2.0 months) in the PTA group (p = 0.6), whereas the mean clinical follow-up period was 32 months (SD 9 months) in the cryoplasty group vs. 32 months (SD 2 months) in the PTA group (p = 0.7), with no significant differences in patient compliance between the two groups |
Interventions
| Trial | Focus of interventions (comparisons) | T1: intervention group | T2: control group |
|---|---|---|---|
| Jahnke et al. 201040 | Cryoplasty vs. PTA | Cryoplasty: from an ipsilateral antegrade puncture of the femoral artery, placement of 7-F sheath (Terumo, Tokyo, Japan) lesions were recanalised with guide wire and 5-F catheter (Berenstein, Cordis, Roden, the Netherlands). Correct intraluminal position verified with contrast medium, guide wire replaced with 0.9-mm Radifocus Glidewire, heparin administered. Cryoplasty balloon sizes chosen to be the same size as reference vessel diameter, and allowed to exceed luminal diameter of nearest normal appearing vessel by 20%, thus balloon-to-vessel ratios of 1.5 : 1 to 1.25 : 1. PolarCath® Peripheral Dilatation System (Boston Scientific, Natick, MA, USA) used. In the event of residual stenosis, conventional balloon angioplasty was performed; if persistent failure with > 30% residual stenosis, then a SES was implanted | PTA: from an ipsilateral antegrade or retrograde crossover approach, placement of 5- or 6-F sheath lesions were recanalised with guide wire and 5-F catheter. Correct intraluminal position verified with contrast medium, heparin administered, angioplasty with Sterling Balloon (Boston Scientific). Balloon sizes chosen to be the same size as reference vessel diameter, and allowed to exceed luminal diameter of nearest normal appearing vessel by 20%, thus balloon-to-vessel ratios of 1.5 : 1 to 1.25 : 1. In the event of residual stenosis, the balloon of next greatest diameter used or device inflated again for 3–5 minutes. If persistent failure with > 30% residual stenosis, then SES was implanted |
| Spiliopoulos et al. 201041 | Cryoplasty vs. PTA | Cryoplasty: cryoplasty therapy was performed with the use of the PolarCath Peripheral Dilatation System, which includes an over-the-wire, double-lumen dilatation balloon catheter manufactured of Pebax® (Atochem Inc., PA, USA) and an inflation system consisting of a microprocessor unit and a nitrous oxide cartridge. The cryoplasty catheter is formed by three layers (inner, middle and outer), and its fluoroscopic visibility is attained by radio-opaque markers placed in the middle layer. Balloon inflation is achieved by a specially designed apparatus that releases liquid nitrous oxide from the specially designed high-pressure cartridge through the catheter lumen and into the lower-pressure balloon chamber, where it changes state from liquid to gas and expands its volume | PTA: conventional balloon angioplasty with commercially available semi-compliant or non-compliant balloon catheters (inflation period 60–120 seconds). In all cases, balloon size was chosen according to reference vessel diameter per visual estimate. Balloon length was chosen to match lesion length, and, if that was not possible, to slightly exceed it, according to routine clinical practice. Stenting was reserved for bail-out in case of elastic recoil, post-dilatation residual stenosis > 30% or severe flow-limiting dissection (type C). An antegrade or retrograde femoral artery access using an appropriately sized sheath (6 F to 7 F) was performed. A bolus dose of unfractionated heparin (3000–5000 IU) was administered immediately after sheath placement, and an infusion rate of 1000 U/h was maintained during the rest of the procedure. Routine endovascular manoeuvres using standard guide wires and catheters were used to cross the SFA and/or the popliteal artery lesion as needed |
Population inclusion
| Trial | Target population | Inclusion/exclusion criteria | Recruitment |
|---|---|---|---|
| Jahnke et al. 201040 | Patients with focal atherosclerotic stenoses and occlusions of the popliteal artery | Inclusion criteria: lifestyle-limiting claudication (Rutherford–Becker 1–3), rest pain or ischaemic skin changes of the feet (Rutherford–Becker 4 or 5) induced by focal atherosclerotic stenoses or occlusions of popliteal artery. Exclusion criteria: haemodynamically relevant lesions (> 50% luminal stenosis) of the arterial in/out-flow, prior stent or graft placement into popliteal artery, lesions induced by former vascular surgery, fresh embolic occlusions, contraindications to contrast media, renal failure, hyperthyroidism, allergic diathesis | Over 2.5 years |
| Spiliopoulos et al. 201041 | Diabetic patients with femoropopliteal arterial occlusive disease | Inclusion criteria: non-insulin-dependent diabetes mellitus or insulin-dependent diabetes mellitus, severe claudication or CLI (Rutherford categories 3–6), stenosis ≥ 70% or occlusion of the SFA and/or the popliteal artery and de novo and in-stent restenotic lesions. Exclusion criteria: diet-controlled diabetes, history of severe contrast allergy or hypersensitivity, intolerance to aspirin and/or clopidogrel, systemic coagulopathy or hypercoagulation disorders, acute limb ischaemia, Buerger’s disease, deep-vein thrombosis, infected tissue loss and absent pedal arch run-off | Between January 2005 and October 2007 |
Sample size
| Trial | Numbers included in the study | Number of patients in T1 | Number of patients in T2 | Power calculation (a priori sample calculation) | Number followed up from each condition (or attrition) |
|---|---|---|---|---|---|
| Jahnke et al. 201040 | 86 | Cryoplasty, 40 (crossover to long-term angioplasty in n = 23, 58%; bail-out stent placement n = 12, 30%) | PTA, 46 (bail-out stent placement n = 18, 39%) | NR | At time of publication, 23/40 cryoplasty and 23/46 PTA patients have reached 9 months follow-up |
| Spiliopoulos et al. 201041 | 50 | Cryoplasty, 24 patients with 31 lesions | PTA, 26 patients with 34 lesions | NR | Only one patient (1 of 24; 4.16%) assigned to the cryoplasty group was lost from angiographic but not from clinical follow-up after 6 months. This was due to an ischaemic stroke |
Baseline characteristics
| Trial | Age (mean, years) | Gender (%male) | Classification of PAD | Presence of cardiovascular risk factors | Other relevant information |
|---|---|---|---|---|---|
| Jahnke et al. 201040 | Across groups 72 (range, 50–94); cryoplasty group 73.6 (SD 9.7); PTA group 70.6 (SD 10.2) | Cryoplasty 43%, PTA 49% |
|
|
Mean lesion length (mm): cryoplasty 35 (SD 28.8), PTA 36.5 (SD 28.5) |
| Spiliopoulos et al. 201041 | Cryoplasty 65.3 (SE 10.4), PTA 70.3 (SE 7.8) | Cryoplasty 87.5%, PTA 84.6% |
|
|
61.3% (19 of 31) of cryoplasty group lesions and 52.9% (18 of 34) of PTA group lesions were de novo lesions. > 70% of the lesions were TASC B and C in both groups. The average lesion length was 11.9 cm (SD 5 cm) in the cryoplasty group and 12.0 cm (SD 6 cm) in the PTA group (p > 0.05) |
Outcomes
| Trial | Pain/clinical status | Complications including amputation | Patency measures | Need for reintervention or recurrence rate |
|---|---|---|---|---|
| Jahnke et al. 201040 | Improvement defined by Society for Vascular Surgery/International Society for Cardiovascular Surgery criteria for lower-limb ischaemia ranging from –3 (markedly worse) to +3 (markedly improved) | Procedural complications | The primary objective was target lesion patency. > 2.5-fold increase in PSVR across the treated segment indicative of > 50% luminal narrowing | |
| Spiliopoulos et al. 201041 | Procedural complications | Primary patency was defined as angiographic visualisation of a non-occluded lesion and no need for any additional repeat interventional procedure within the previously treated lesion. Absent or thread-like blood flow was classified as vascular occlusion. Binary in-lesion restenosis (> 50%) | Freedom from target lesion recanalisation. TLR included any additional recanalisation procedure within the area of the treated femoropopliteal lesion because of clinical deterioration and relapse of symptoms (i.e. clinically driven repeat procedures) |
Results
| Trial | Results | Complications |
|---|---|---|
| Jahnke et al. 201040 | Patency: the mean target lesion patency at 9 months was 79.3% (SD 7.5) for cryoplasty and 66.7% (SD 8.1) for conventional angioplasty (non-significant, p = 0.14). At 6 months, target lesion patency was 82.9% (SD 7.0) for cryoplasty and 79.8% (SD 6.4) for conventional angioplasty (non-significant). Clinical: improvement of clinical stage at 9 months – cryoplasty +2.73 (SD 0.55), PTA +2.43 (SD 1.16) (non-significant, p = 0.29). Optional long-term PTA was performed in 58% of cryoplasty patients. The rate of stent placement for dissection and/or residual stenosis was 30% after cryoplasty (including long-term dilatation) and 39% after conventional angioplasty (p = 0.34) | Initial success was 35% for cryoplasty vs. 54% for conventional angioplasty (p = 0.02). Minor complications: 2.5% cryoplasty, 2.7% PTA. Major complications: 5% cryoplasty, 2.7% PTA |
| Spiliopoulos et al. 201041 | Restenosis: there was a non-significant trend of increased binary restenosis in the cryoplasty group (HR 1.3; 95% CI 0.6 to 2.6; p = 0.45). Reintervention: significantly more repeat intervention events because of recurrent symptoms were required in the cryoplasty group (HR 2.5; 95% CI 1.2 to 5.3; p = 0.01). Patency: primary patency was significantly lower in the cryoplasty group than in the PTA group (HR 2.2; 95% CI 1.1 to 4.3; p = 0.02). Cox model adjusted for insulin-dependent diabetes mellitus, renal disease, smoking, hyperlipidaemia, lesion grade, lesion type (de novo or in-stent restenotic), heavy calcifications, TASC classification and type of treatment (cryoplasty or PTA) | Immediate technical success rate was 58.0% in cryoplasty group vs. 64.0% in PTA group (p = 0.29). According to 3-year Kaplan–Meier estimates, there were no significant differences with regard to patient survival (86.8% in cryoplasty group vs. 87.0% in PTA group; p = 0.54) and limb salvage (95.8% vs. 92.1% in cryoplasty and PTA groups, respectively; p = 0.60). None of the deaths was related to the procedure. Minor amputation rates were similar in the two study arms (6.9% in cryoplasty group vs. 9.7% in PTA group, p = 0.3) |
Radiation
Study details
| Trial | Objective | Study design | Publication type | Language of publication | Sources of funding | Country of corresponding author | Intervention site(s) | Length of follow-up |
|---|---|---|---|---|---|---|---|---|
| Gallino et al. 2004,42 Bovini et al. 2003,43 Diehm et al. 200544 | To evaluate the effect of probucol and/or EVBT on restenosis after PTA of femoropopliteal arteries | RCT | Full report | English | Swiss Heart Foundation | Switzerland | Switzerland | 0-, 3-, 6-, 12- and 24-month follow-up post intervention |
| Diehm et al. 2005,44 Zehnder et al. 200345 | To evaluate the effect of EVBT on restenosis following secondary angioplasty of femoropopliteal segment | RCT | Full report | English | Not disclosed | Switzerland | Switzerland | 1-day and 3-, 6-, 9- and 12-month follow-up post intervention. Annually up to 5 years (Diehm et al.44 combined results of Gallino et al.43 and Zehnder et al.45) |
| Hagenaars et al. 200246 | To evaluate the effect of EVBT on the extent of plaque growth and vascular remodelling after PTA of the femoropopliteal artery | RCT | Full report | English | The Revolving Fund and Interuniversity Cardiology Institute of the Netherlands | Netherlands | Netherlands | 6 months |
| Krueger et al. 2002, 200447,48 | To evaluate whether centred endovascular irradiation after PTA for de novo femoropopliteal stenoses reduces restenosis | RCT | Full report | English | Cologne Fortune | Germany | Germany | 6, 12 and 24 months |
| Vienna-249,50 | To evaluate the efficacy of EVBT for prophylaxis of restenosis after femoropopliteal PTA. | RCT | Full report | English | Not disclosed | Austria | Austria | 1 day, 1, 3, 6, 12, 18, 24 months and 5 years post procedure |
| Vienna-352 | To evaluate the effect of EVBT on restenosis after femoropopliteal angioplasty | RCT | Full report | English | Not disclosed | Austria | Austria | The primary end point of the study was arterial patency of the recanalised segment after 12 months and mean follow-up was 15.7 months |
| VARA54 | To evaluate the efficacy of EVBT for prophylaxis of restenosis after femoropopliteal PTA | RCT | Full report | English | The Professor Michaël-van Vloten Foundation | Netherlands | Belgium/Netherlands | 6 and 12 months. The primary end point was a ≥ 50% restenosis at duplex ultrasound of the treated segment after 12 months |
| Wyttenbach et al. 2004, 200755,56 | To evaluate the short- and long-term effects of PTA on severely stenotic femoropopliteal lesions as well as the effect of brachytherapy on restenosis by means of serial MRI | RCT | Full report | English | Swiss Heart Foundation | Switzerland | Switzerland | 24 hours, 3 and 24 months |
| Fritz et al. 200457 | To evaluate the effect of hypofractionated EBRT as a prophylaxis for restenosis | RCT | Full report | English | Not disclosed | Germany | Germany | 1 day, 3, 6 and 12 months |
| Therasse et al. 200558 | To evaluate whether external beam radiation can prevent stenosis after femoropopliteal PTA | RCT | Full report | English | The study was supported by a grant from the Fonds de la recherche en sante du Quebec | Canada | Canada | The main study end point was the minimum lumen diameter within the dilated vessel segment 1 year after PTA |
Interventions
| Trial | Focus of interventions (comparisons) | T1: intervention group | T2: control group |
|---|---|---|---|
| Gallino et al. 2004,42 Bovini et al. 2003,43 Diehm et al. 200544 | PTA + EVBT vs. PTA + placebo drug. All groups received aspirin 100 mg/day | PTA with ipsilateral antegrade puncture and 6-F introducer sheath (Cordis Europe, Roden, The Netherlands) with 5- or 7-mm balloon catheters (Smash, Schneider Europe, Bulach, Switzerland) + EVBT with gamma irradiation (192iridium, 14 Gy, 5-mm reference depth) | PTA + placebo drug given 1 g/day orally from 1 month before PTA and continued for 6 months post PTA |
| Diehm et al. 2005,44 Zehnder et al. 200345 | PTA + EVBT vs. PTA alone | PTA with ipsilateral antegrade approach to the common femoral artery using a 6-F sheath with 4- to 6-mm balloons. Stents were inserted if residual stenosis > 30% persisted or flow was obstructed. High-dose EVBT (192iridium, 12-Gy reference dose, 5-mm reference depth) without a centring device | PTA as for intervention |
| Hagenaars et al. 200246 | PTA + EVBT vs. PTA alone | ‘Standard’ PTA + EVBT (192iridium, dose of 14 Gy with centring balloon) with an over-the-wire delivery catheter | PTA as for intervention |
| Krueger et al. 2002, 200447,48 | PTA + endovascular irradiation vs. PTA alone | PTA performed according to conventional practice using an ipsilateral or crossover approach with a short 8-F or flexible 8-F sheath, respectively. The balloon diameter was between 5 and 6 mm. EVBT was 192iridium, 14 Gy, centred | PTA as for intervention |
| Vienna-249,50 Wolfram 2006 | PTA + EVBT vs. PTA alone | PTA using an ipsilateral anterograde puncture and 6-F introducer sheath with 5- or 6-mm balloon catheters + EVBT (192iridium, 12-Gy dose, 3 mm from the source axis, uncentred) | PTA as for intervention |
| Vienna-352 | PTA + EVBT vs. PTA + sham irradiation | PTA using an ipsilateral anterograde puncture and 6-F introducer sheath with 4- to 6-mm balloon catheters + EVBT (192iridium, 18-Gy dose, 7-F centring catheter) | PTA as for intervention + sham irradiation, but no further detail about this process was reported |
| VARA54 | PTA + EVBT vs. PTA alone | PTA via an ipsilateral antegrade puncture, 5-F catheter, 5- to 7-mm balloon. EVBT using 192iridium, a dose of 14 Gy | PTA as for intervention |
| Wyttenbach et al. 2004, 200755,56 | PTA + EVBT vs. PTA alone | PTA via an ipsilateral anterograde puncture of the common femoral artery, 6-F introducer sheath, 5- to 6-mm balloon. EVBT using 192iridium, reference dose of 14 Gy, non-centred | PTA as for intervention |
| Fritz et al. 200457 | PTA + EBRT vs. PTA + sham EBRT | PTA using conventional balloon catheter techniques with ipsilateral (femoropopliteal) or retrograde (iliac) puncture with balloon catheters 4–9 mm in diameter using a 6-F introducer sheath + EBRT daily in 3-Gy fractions to a total dose of 21 Gy | PTA as for intervention + sham ERBT |
| Therasse et al. 200558 | PTA + 7-Gy, 10.5-Gy, 14-Gy EBR vs. PTA + 0-Gy EBR | PTA + 7-Gy, 10.5-Gy, 14-Gy EBR (three groups) delivered in a single session 24 hours post PTA | PTA + 0-Gy EBR |
Population inclusion
| Trial | Target population | Inclusion/exclusion criteria | Recruitment |
|---|---|---|---|
| Gallino et al. 2004,42 Bovini et al. 2003,43 Diehm et al. 200544 | Patients with IC of the femoropopliteal arteries | Inclusion criteria: (1) age > 50 years, (2) chronic, moderate to severe IC (Rutherford category 2 or 3), referable to > 50% stenosis or total occlusion. Exclusion criteria: (1) rest pain or CLI, (2) non-atherosclerotic arterial occlusive disease, (3) vascular surgery during the preceding 6 months, (4) uncontrolled arterial hypertension, (5) haemorrhagic diathesis, (6) liver disease, (7) impaired renal function (serum creatinine level > 180 µmol/l), (8) a prolonged corrected QT interval (≥ 480 ms) on electrocardiogram, (9) life expectancy < 6 months, (10) questionable compliance or an insufficient insonation window over the target lesion at duplex ultrasound, (11) patients who were non-compliant (> 20% of unused study drug at 4 weeks follow-up) during the run-in phase before angioplasty | Not reported |
| Diehm et al. 2005,44 Zehnder et al. 200345 | Patients with restenosis or reocclusion after primarily successful femoropopliteal PTA | Inclusion criteria: (1) restenosis > 50% after previously successful femoropopliteal PTA, (2) IC or CLI, (3) age > 50 years, (4) willingness to consent. Exclusion criteria: (1) acute or subacute occlusion of the vessel, (2) non-atherosclerotic occlusive disease, (3) vascular surgery or angioplasty during the preceding 3 months, (4) life expectancy < 6 months, (5) inadequate visualisation of the lesion on duplex images | Patients referred and meeting criteria |
| Hagenaars et al. 200246 | Patients with disabling claudication due to femoropopliteal arterial stenosis | Inclusion criteria: (1) angiographically proven femoropopliteal stenosis (> 50%) or occlusion, (2) lesion length < 10 cm, (3) age 40–85 years, (4) no inflow obstruction or significant stenosis in the iliac artery. Exclusion criteria: (1) impaired renal function, (2) acute ischaemia, (3) pregnancy, (4) life expectancy < 12 months | |
| Krueger et al. 2002, 200447,48 | Patients with de novo femoropopliteal stenosis Fontaine stage 2a to 3 | Inclusion criteria: (1) age > 50 years, (2) femoropopliteal arterial occlusive disease Fontaine stage 2a to 3, (3) de novo stenosis of maximum length 8 cm. Exclusion criteria: (1) patients with untreated stenosis proximal to the region of PTA or with less than one run-off vessel, (2) exposure to endovascular treatments other than PTA, (3) patients with malignant disease | Consecutive patients |
| Vienna-249,50 | Patients with de novo or recurrent femoropopliteal lesions | Inclusion criteria: (1) age > 40 years, (2) history of claudication (Rutherford category 2 or 3) for > 3 months or CLI with pain at rest with or without tissue damage, (3) de novo lesion in the femoropopliteal region with a minimal lesion length of 5 cm or a recurrent lesion (after former PTA) of any length, (4) technical success of the angioplasty procedure, which required angiographic patency with residual stenosis of > 30% diameter reduction, (5) no further stent implantation | Consecutive patients |
| Vienna-352 | Patients with de novo or recurrent femoropopliteal lesions | Inclusion criteria: (1) age > 45 years, (2) history of claudication (Rutherford category ≥ 2), (3) stenosis of ≥ 50%, (4) de novo lesion of ≥ 5 cm or recurrent lesion after prior angioplasty of any length, (5) successful angioplasty of < 30% residual stenosis. Exclusion criteria: (1) stenting and crossover approach, (2) in-stent restenosis, (3) former irradiation of superficial femoropopliteal artery, (4) life expectancy < 12 months, (5) thrombolysis at the time of randomisation | All patients admitted to the trial’s host institutions with femoropopliteal lesions |
| VARA54 | Patients with symptomatic stenotic or totally occluding lesions in the femoropopliteal artery | Inclusion criteria: (1) age between 40 and 80 years, (2) claudication or non-acute CLI (Rutherford category ≥ 2), (3) lesion in the femoropopliteal artery with a maximum length of 10 cm, (4) reference diameter of the segment 4–8 mm, (5) no significant haemodynamic iliac stenosis, (6) written informed consent. Exclusion criteria: (1) after randomisation of the revascularisation was unsuccessful; (2) where the maximum lesion length is 10 cm the dilated segment should not exceed 13 cm | Patients accessing the participating hospitals |
| Wyttenbach et al. 2004, 200755,56 | Patients with severe superficial femoropopliteal artery stenosis classified as Rutherford category ≥ 3 | Patients were not eligible for the study if they had non-atherosclerotic occlusive disease, vascular surgery during the preceding 6 months, uncontrolled hypertension, haemorrhagic diathesis, impaired renal function (creatinine level ≥ 180 mmol/l), a life expectancy of < 6 months or a contraindication for MRI | Consecutive patients |
| Fritz et al. 200457 | Patients who underwent successful PTA for claudication or CLI with Fontaine stage II to IV | Inclusion criteria: (1) age > 50 years, (2) claudication or CLI (Fontaine stage II to IV), (3) ABPI < 0.8 at rest, (4) focal de novo or recurrent lesion in the iliac or femoropopliteal region with a maximal lesion length of 10 cm, (5) PTA success, (6) no stent implantation or surgical intervention after PTA | |
| Therasse et al. 200558 | Patients with symptomatic, lifestyle-limiting vascular insufficiency, either claudication or CLI secondary to a de novo atherosclerotic obstructive lesion of the femoropopliteal artery | Inclusion criteria: (1) stenosis or occlusion of the femoropopliteal artery with a diameter reduction of ≥ 50% and ABPI < 0.9. Exclusion criteria: (1) age < 45 years, (2) women of child bearing age, (3) patients who had received a radiosensitising agent or radiation therapy to the lower limb in the past, (4) previous stent implantation, (5) residual stenosis > 50% after PTA | Patients referred for PTA by their physicians |
Sample size
| Trial | Number included in the study | Number of patients in T1 | Number of patients in T2 | Power calculation (a priori sample calculation) | Attrition | Number followed up from each condition |
|---|---|---|---|---|---|---|
| Gallino et al. 2004,42 Bovini et al. 2003,43 Diehm et al. 200544 | 335 (includes all four treatments arms – only data related to two arms extracted here) | EVBT + placebo drug immediately after femoropopliteal PTA, 81 | Placebo drug, 75 | Assuming a 35% recurrence rate within 6 months, and treatment effect of 20% with α = 0.05 and β = 0.1 and anticipating 10% loss to follow-up, it was estimated that 90 participants per group would be required | PTA + EVBT: 12/81 (14.8%) loss to follow-up. Control/placebo: 9/75 (12%) loss to follow-up. These figures include those excluded from final analysis owing to per protocol requirements (54). The authors report that six were lost to follow-up, but do not specify the arms from which they were lost. Only data for participants who completed the trial satisfactorily were analysed | PTA + EVBT: 69/81 (85.2%). Control/placebo: 66/75 (88.0%) |
| Diehm et al. 2005,44 Zehnder et al. 200345 | 100 (Diehm et al.,44 147) | 51 (Diehm et al.,44 72) | 49 (Diehm et al.,44 75) | Not reported | 12-month follow-up: 56/100. This is qualified as ‘excluding patients with additional interventions’. The group distribution is unclear. Moreover, seven participants who did not receive adequate EVBT were added to the control group. [Diehm et al.:44 at 31.8 months (range 12 days to 77.5 months) 30/72 from T1 (41.7%) and 34/75 from T2 (45.3%) were lost to follow-up.] Participants who did not provide follow-up data appear to have been excluded | For per protocol analysis, T1 44 (86.3%), T2 56 (114.3%; 49 randomised to PTA alone, and 7 originally randomised to EVBT). [Diehm et al.,44 ITT: at 31.8 months (range 12 days to 77.5 months) T1 42/72 (58.3%) and T2 41/75 (54.7%)] |
| Hagenaars et al. 200246 | 38 | 18 | 20 | Not reported | 14/38 were excluded or lost to follow-up: T1 = 10/18, T2 = 4/20. Data concerning those lost to follow-up were excluded from analysis | T1: 8/18 (44.4%). T2: 16/20 (80.0%) |
| Krueger et al. 2002, 200447,48 | 30 | 15 | 15 | The report refers to requiring 40 participants, but no further detail is provided | Minimal: 2/30 (6.7%) at 24-month follow-up | 14/15 (93.3%) followed up in both groups. One participant in the control group refused and one participant in the intervention group died through gastric bleeding 15 months after randomisation |
| Vienna-249,50 | 117 | 60 | 57 | Assuming a 30% absolute difference between treatment arms with α = 0.05 and β = 0.15, it was estimated that 82 patients would be needed | 4/117 were excluded from further follow-up: one refused brachytherapy and three (T1 = 2, T2 = 1) suffered early recurrence within 24 hours. Subsequently, 107 patients were followed up with regards to 6-month patency. Missing data were excluded | T1: 53/60 (88.3%). T2: 54/57 (94.7%). At 5-year follow-up: T1 – 51/60 (85.0%); T2 – 51/57 (89.5%) |
| Vienna-352 | Only detail offered is that 134 were randomised | 67 | 67 | Not reported | 38/134 were excluded for various reasons but maintained in the ITT. No additional attrition is reported. All excluded patients were treated as ‘failures’ | T1: 50/67 (74.6%). T2: 46/67 (68.7%) |
| VARA54 | 60 | 27 | 33 | Assuming an incidence of restenosis of 50% in T2 and 20% in T1 with α = 0.05 and power = 0.8, 38 participants per group were required | 53/60 (88.3%). Missing data were excluded | T1: 23/27 (85.2%). T2: 30/33 (90.9%) |
| Wyttenbach et al. 2004, 200755,56 | 20 | 10 | 10 | Not reported | No apparent loss to follow-up at 3 months. At 24 months, 3/20 (15%) attrition | 100% at 3 months in both groups. At 24 months: T1 – 9/10 (90%); T2 – 8/10 (80%) |
| Fritz et al. 200457 | 100 | 47 | 48 | Unclear whether an a priori calculation was undertaken, but the report states that a sample of 100 patients enables the detection of an absolute reduction in the failure rate from 40% to 15% with α = 0.05 and β = 0.2 | 5/100 dropped out following first follow-up examination and were excluded from the analysis. Reason for attrition is not stated. One patient in the EBRT group had a stroke and was also excluded. Minimal attrition, but participants excluded from the analysis | Unclear because the number randomised to each group prior to first round of dropouts was not reported, but 94/100 followed up with regards to failure |
| Therasse et al. 200558 | 99 | 7 Gy, 24; 10.5 Gy, 26; 14 Gy, 25 | 24 | With power of 0.80 and a two-tailed significance level of 0.05 to detect an effect size of 0.40 between the control group and one of the EBR groups, 19 patients were required per group. To compensate for non-compliant patients and for those lost to follow-up, the sample size was increased to a total of 99 patients | 88/99 (88.9%). Missing data were excluded for most analyses except the rate of restenosis of dilated segments | See attrition |
Baseline characteristics
| Trial | Age (mean, years) | Gender (male) | Classification of PAD | Number of patients who have undergone previous revascularisation procedures | Presence of cardiovascular risk factors [n (%)] | Level of exercise tolerance |
|---|---|---|---|---|---|---|
| Gallino et al. 2004,42 Bovini et al. 2003,43 Diehm et al. 200544 | Rutherford categories 2 and 3 referable to > 50% stenosis or total occlusion of the femoropopliteal arteries | Not reported | EVBT: diabetes mellitus 28 (35%), smoking 21 (26), hypertension 59 (73), hypercholesterolaemia 27 (53). PTA: diabetes mellitus 25 (30), smoking 26 (31), hypertension 50 (59), hypercholesterolaemia 41 (49) | Not reported | ||
| Diehm et al. 2005,44 Zehnder et al. 200345 | T1: 70.8 (± 8.0). T2: 70.7 (±9.0) | T1: 31 (62%). T2: 27 (55%) | IC or chronic limb ischaemia, > 50% restenosis documented by angiography or duplex ultrasound | At least one, but not reported more specifically | T1: diabetes mellitus 12 (23), smoking 21 (41), hypertension 32 (62), dyslipidaemia 27 (53). T2: diabetes mellitus 14 (29), smoking 19 (39), hypertension 29 (59), dyslipidaemia 25 (51) | Not reported |
| Hagenaars et al. 200246 | T1: 60.0 (±9.8). T2: 65.9 (± 9.9) | T1: 6/8 (75%). T2: 11/16 (68.8%) | Fontaine stages II to IV (T1: 6/1/1, respectively) (T2: 6/6/4, respectively) | Not reported | T1: diabetes mellitus 2 (25), smoker 7 (88.8), systemic hypertension 5 (63), hypercholesterolaemia 5 (63). T2: diabetes mellitus 3 (19), smoker 10 (63), systemic hypertension 7 (44), hypercholesterolaemia 4 (25) | Not reported |
| Krueger et al. 2002, 200447,48 | T1: 60.4 (SD 5.7). T2: 61.3 (SD 5.4) | T1: 12/15 (80.0%). T2: 11/15 (73.3%) | Fontaine stage 2a to 3 | None, de novo lesions included only | T1: diabetes mellitus 5 (33.3), smoker 8 (53.3), arterial hypertension 9 (60), hypercholesterolaemia 10 (66.7). T2: diabetes mellitus 4 (26.7%), smoker 9 (60), arterial hypertension 8 (53.3), hypercholesterolaemia 7 (46.7) | Treadmill testing undertaken pre intervention: mean pain-free walking distance for T1/T2 = 92.2m (SD 113.1m)/95.9m (SD 123.2m) (p = 0.83) |
| Vienna-249,50 | 71 (range 43 to 89) for whole group only reported | T1: 29/57 (50.9%). T2: 34/56 (60.7%) | Rutherford category 2 or 3 for > 3 months or CLI with pain at rest with or without tissue damage. Duration of symptoms: T1 = 6 months (±6), T2 = 6 months (±5) | Recurrent stenosis following previous PTA: T1, 27/57 (47.4%); T2, 28/56 (50%) | T1: diabetes mellitus 26 (45.6), smoker 12 (21.1), arterial hypertension 42 (73.7). T2: diabetes mellitus 29 (51.8), smoker 13 (23.2), arterial hypertension 27 (48.2) | Not reported |
| Vienna-352 | Not reported | T1: 34/50 (68.0%). T2: 28/46 (60.9%) | Rutherford category 2, 3, 4 or 5. T1: n = 2/40/2/6. T2: n = 1/34/5/6 | T1: 13/50 (26%) had recurrent lesions with previous angioplasty. T2: 8/46 (17.4%) had recurrent lesions with previous angioplasty | T1: diabetes mellitus 21 (42), smoker 16 (32), arterial hypertension 38 (76). T2: diabetes mellitus 22 (47.8), smoker 7 (15.2), arterial hypertension 37 (80.4) | Not directly reported – Rutherford classification reported |
| VARA54 | T1: 63.2 (range 43 to 76). T2: 64.7 (range 50 to 85) | T1: 18 (63.0%). T2: 22 (67.0%) | Rutherford category ≥ 2. All had de novo stenosis | None | T1: diabetes mellitus 5 (19), smoker 24 (89), hypertension 9 (33), hypercholesterolaemia 10 (37). T2: diabetes mellitus 7 (21), smoker 30 (91), hypertension 14 (42), hypercholesterolaemia 11 (33) | Only the Rutherford classification reported |
| Wyttenbach et al. 2004, 200755,56 | T1: 68.7 ± 6.1. T2: 73.4 ± 6.6 | T1: 7/10 (70%). T2: 7/10 (70%) | Rutherford category 3 or 4. T1: n = 9/1. T2: n = 7/3. Duration: T1, 6.3 months (± 5.5); T2, 5.9 months (± 5.0) | Only de novo lesions included | T1: diabetes mellitus 4 (40), smoker 5 (50), arterial hypertension 6 (60). T2: diabetes mellitus 6 (60), smoker 7 (70), arterial hypertension 6 (60) | Only the Rutherford classification reported |
| Fritz et al. 200457 | T1: 67.6 (± 8.3). T2: 69.3 (± 9.5) | T1: 30 (63.8%). T2: 22 (45.8%) (p = 0.1) | Fontaine stages II to IV | Former PTA: T1, 4/47 (8.5%); T2, 8/48 (16.7%) | T1: diabetes mellitus 17 (36.2), smoker 17 (36.2), arterial hypertension 29 (61.7). T2: diabetes mellitus 20 (41.7), smoker 18 (37.5), arterial hypertension 30 (62.5) | Not reported |
| Therasse et al. 200558 | 63.7 (± 8.7) | 65% | Patients with symptomatic, lifestyle-limiting vascular insufficiency, either claudication or CLI secondary to a de novo atherosclerotic obstructive lesion of the femoropopliteal artery | Not reported | Dyslipidaemia 62%, diabetes mellitus 31%, hypertension 65%, smoker 87% | Not reported |
Outcomes
| Trial | Pain/clinical status | Complications including amputation | Patency measures | Need for reintervention or recurrence rate |
|---|---|---|---|---|
| Gallino et al. 2004,42 Bovini et al. 2003,43 Diehm et al. 200544 | Rutherford classification | Adverse events | > 50% restenosis on duplex ultrasonography | Revascularisation needed |
| Diehm et al. 2005,44 Zehnder et al. 200345 | Rutherford classification | > 50% restenosis on duplex ultrasonography | Repeat dilatation or surgery | |
| Hagenaars et al. 200246 | > 50% restenosis. Change in lumen, vessel and plaque area and plaque dissections through an intravascular ultrasound scan | |||
| Krueger et al. 2002, 200447,48 | Treadmill test for absolute and claudication distances; pain scores at structured interview | > 50% diameter reduction by angiography. Mean absolute change in degree of stenosis; rate of target lesion restenosis | Need for target lesion retreatment, TLR/TVR | |
| Vienna-249,50 | > 50% diameter reduction by angiography, Doppler ultrasound, colour-flow duplex ultrasonography PSV | TVR was defined as further PTA or surgical bypass of the target vessel required because of the presence of > 50% diameter stenosis of the target lesion | ||
| Vienna-352 | Amputation | Restenosis was defined as > 50% reduction of arterial lumen. Arterial patency at 12 months was assessed angiographically or, if patients refused, with duplex ultrasonography. ABPI | TLR was defined as clinically manifested stenosis within intervention length which needed new recanalisation | |
| VARA54 | Rutherford classification | Complications | > 50% diameter reduction by angiography, Doppler ultrasound; PSVR > 2.4 | Defined as need for further PTA or BS |
| Wyttenbach et al. 2004, 200755,56 | Lumen area and total vessel area by MRI | |||
| Fritz et al. 200457 | Fontaine stage | > 50% diameter reduction | ||
| Therasse et al. 200558 | > 50% diameter reduction by angiogram | Repeat lower-limb angioplasty |
Results
| Trial | Results | Complications |
|---|---|---|
| Gallino et al. 2004,42 Bovini et al. 2003,43 Diehm et al. 200544 | After successful PTA, 6-month patency according to the Kaplan–Meier life table method was 83% in the EVBT group and 58% in the control/placebo group | Late occlusion, QTc. Late occlusion occurred exclusively in patients receiving EVBT following stenting and always in concert with elimination of clopidogrel from the antiplatelet regimen. No other major EVBT-associated side effects were detected |
| Diehm et al. 2005,44 Zehnder et al. 200345 | ABPI: within-group improvement reported but no between-group differences. The per protocol life table analysis showed a longer recurrence-free time, 7.0 months (SD 2.2 months), for T1 than for T2, 5.8 months (SD 2.8 months) (p = 0.028). 33/100 needed reintervention owing to recurrent stenosis > 50% before the end of follow-up (T1 = 16, T2 = 17). But, per protocol analysis: T1 = 10 (23%), T2 = 23 (42%) (p = 0.028). [Diehm et al.44 T1: cumulative sustained clinical success rates at 1, 2 and 3 years – 82.4% (95% CI 71.1% to 89.6%), 69.8% (95% CI 56.5% to 79.7%), 67.5% (95% CI 53.9% to 77.9%). T2: cumulative sustained clinical success rates at 1, 2 and 3 years – 84.3% (95% CI 72.7% to 91.3%), 82.1% (95% CI 69.8% to 89.8%), 76.4% (95% CI 62.0% to 86.0%) (p = 0.26). T1: freedom from restenosis at 1, 2 and 3 years – 82.7% (95% CI 67.1% to 91.4%), 64.3% (95% CI 47.2% to 77.2%) and 64.3% (95% CI 47.2% to 77.2%). T2: freedom from restenosis at 1, 2 and 3 years – 70.7% (95% CI 54.3% to 82.2%), 63.1% (95% CI 46.3% to 57.9%) and 47.1% (95% CI 31.0% to 61.7%) (p = 0.16)] | No adverse events reported |
| Hagenaars et al. 200246 | Lumen area change in mm2: T1/T2, +4.3 (± 6.8)/−1.6 (± 5.1) (p = 0.03). Vessel area change in mm2: T1/T2, +6.9 (± 8.7)/+ 0.8 (± 5.5) (p = 0.05). Change in plaque area in mm2: T1/T2, +2.8 (± 6.0)/+2.2 (± 4.0) (p = 0.80) | |
| Krueger et al. 2002, 200447,48 | Mean absolute individual changes in degree of stenosis compared with the degree of stenosis shortly after PTA at 6 months, T1/T2 −10.6% (± 22.3)/39.6% (± 24.6) (p < 0.001); at 12 months, T1/T2 −2.0% (± 34.2)/40.6% (± 32.6) (p = 0.002); at 24 months, T1/T2 7.4% (± 43.2)/37.7% (± 34.5) (p = 0.043). Rate of target lesion restenosis at 6 months, T1/T2 − 0/15 = 0%/7/15 = 46.7% (p = 0.006); at 12 months, T1/T2 − 0/15 = 0%/5/15 = 33.3% (p = 0.042); at 24 months, T1/T2 − 2/15 = 13.3%/5/15 = 33.3% (p = 0.39). Target lesion retreatment at 24 months T1/T2 − 1/15 = 6.6%/2/15 = 13.3%. Target vessel retreatment at 24 months T1/T2 − 4/15 = 26.7%/2/15 = 13.3%. No significant differences in interview or treadmill testing between the groups | One patient developed a lower-limb thromboembolic occlusion during the procedure of brachytherapy |
| Vienna-249,50 | Cumulative patency rates at 12 months: T1/T2 − 63.6%/35.3% (p < 0.005). Recurrence rate after 6 months: T1/T2 −15/53 = 28.3%/29/54 = 53.7% (p < 0.05). The mean ABPI increased from 0.50 (range 0.18 to 0.91) in the PTA group and 0.51 (range 0.1 to 0.92) in the PTA + brachytherapy group before PTA to 0.79 (range 0.40 to 1.13) and 0.85 (range 0.48 to 1.09), respectively, the day after PTA. Follow-up examinations demonstrated mean values of 0.77 (range 0.15 to 1.14) and 0.88 (range 0.47 to 1.20) in the PTA and PTA + brachytherapy groups, respectively, after 3 months and 0.74 (range 0.21 to 1.25) and 0.84 (range 0.27 to 1.25), respectively, after 6 months. (Values for patients with secondary interventions because of recurrence are not included.) TLR was performed during a mean follow-up period of 12 months in 22 patients (in 20 patients by further PTA and in two patients by BS) in the PTA group and in 14 patients (all with PTA) in the PTA + brachytherapy group. At 5-year follow-up, recurrence rate was 72.5% in each group (p > 0.99) but time to recurrence was significantly delayed in the PTA + EVBT group, 17.5 months (± 14.7) vs. 7.4 months (± 6.8) for the PTA alone group (p < 0.05). The mean PVR decreased from 7.3 (range 3.0 to 12.1) in the PTA group and 6.3 (range 2.7 to 11.9) in the PTA + brachytherapy group before PTA to 1.7 (range 1.05 to 2.2) and 1.7 (range 1.0 to 2.15), respectively, the day after PTA. The mean follow-up values were 2.50 (range 1.0 to 10.6) and 1.93 (range 1.0 to 11.8), respectively, after 3 months and 3.05 (range 1.1 to 9.8) and 2.41 (range 1.0 to 9.9), respectively, after 6 months. (Values for patients with secondary interventions because of recurrence are not included. Furthermore, in patients with occlusion, no PVR value can be calculated) | The report suggests that no adverse events were encountered in relation to brachytherapy, but describes two patients (one in each group) who developed small pseudoaneurysms at the puncture site and a further two patients (one in each group) who had haematoma at the puncture site |
| Vienna-352 | The binary restenosis rate was 41.7% (28/67 patients) in brachytherapy cohort and 67.1% (45/67 patients) in placebo cohort (χ2 test, p < 0.05). The cumulative patency rates of the treated segment on intent-to-treat analysis, calculated by the Kaplan–Meier method at 24 months, were 54% in the brachytherapy group and 27% in the placebo group (p < 0.005). PVR improved from mean 6.0 (range 2.5–11.3) to mean 1.8 (range 1.0–2.3) in the placebo group the day after treatment. In the brachytherapy group, PVR decreased from mean 8.0 (range 3.0 to 12.0) to mean 1.8 (range 1.0 to 2.2). At 6 months, mean PVR in the placebo cohort was 1.8 (range 1.1 to 3.0) and at 12 months 2.4 (range 1.1 to 8.6). Mean PVR in the brachytherapy cohort was at 6 months 1.7 (range 1.1 to 4.3) and at 12 months 1.9 (range 1.0 to 4.8). A total of 14 patients in the placebo group and five in the brachytherapy group needed TLR (i.e. recurrence within treated segment) at 12 months. Further, two patients in the brachytherapy group had TVR (recurrence outside the initially treated segment) because of disease progression. No patient in placebo cohort had TVR. BS was necessary in one patient from brachytherapy cohort and amputation in one patient from placebo cohort | Late thrombosis characterised by acute onset of symptoms was not diagnosed in this trial. Two of five patients in one centre treated with brachytherapy developed minor peripheral embolism post intervention |
| VARA54 | At 6 months, the restenosis rate was 9/29 (31%) in the PTA group vs. 5/23 (22%) in the PTA + EVBT group (p = 0.045). At 12 months, the restenosis rate was 12/27 (44%) in the PTA group vs. 8/23 (35%) in the PTA + EVBT group (p = 0.049). After 12 months, 6/29 (21%) in the PTA group and 4/22 (18%) in the PTA + EVBT group required revascularisation (p = 0.82). The alteration of the median Rutherford categories at 6 and 12 months compared with the pre-procedural score was not significantly different between the groups. ABPI and PSVR were not significantly different between groups | In two patients in the PTA + EVBT group a stent was placed owing to severe dissection with partial luminal obstruction. One patient in the PTA + EVBT group suffered from thrombosis of the treated vessel within 24 hours and an early occlusion was also seen in one patient in the PTA alone group |
| Wyttenbach et al. 2004, 200755,56 | At 24 hours, lumen area (86% and 67%), total vessel area (47% and 34%) and vessel wall area (37% and 25%) increased similarly in the PTA and PTA + EVBT groups (respectively) compared with baseline (reported as not significant but no p-value). At 3 months, there was a significant difference in lumen area change between the PTA and PTA + EVBT groups (40% and 106%, respectively; p = 0.026) and in the total vessel area (14% and 39%, respectively; p = 0.018). At 24 months, lumen area gain compared with baseline was + 30% in PTA vs. + 82% in PTA + EVBT (p < 0.047). Total vessel area returned to pre-treatment values in both groups; the difference was not significant | All patients showed severe splitting of the atherosclerotic plaque, resulting in an irregularly shaped lumen. At 3 months, plaque disruption was still present in 50% of the patients treated with PTA + EVBT. Otherwise, there were no procedural or radiation-related complications |
| Fritz et al. 200457 | No statistically significant differences between the groups. The day following the procedure, T1 ABPI increased from 0.59 (SD 0.12) to 0.92 (SD 0.12). T2 ABPI increased from 0.57 (SD 0.14) to 0.92 (SD 0.11). T1 failures 21 (45.7%), T2 failures 16 (33.3%) (p = 0.292) | One patient in the EBRT group had a stroke |
| Therasse et al. 200558 | The minimum lumen diameter in the dilated vessel segments (the primary efficacy end point) was significantly larger in the 14-Gy group (2.91 ± 1.32 mm) than in the placebo group (1.92 ± 1.22 mm, p = 0.0072), the 7-Gy group (1.64 ± 1.05 mm, p < 0.001) and the 10.5-Gy group (1.92 ± 0.95 mm, p = 0.0071). The difference between the 14-Gy and placebo groups was 0.98 mm, with a 95% CI of 0.27 to 1.69 mm. Reinterventions were performed in 6 of 24 (25%) patients in the placebo group (four PTAs and two surgeries) vs. 3 of 25 (12%) patients in the 14-Gy group (one PTA and two surgeries) at 18-months follow-up (p = 0.24) | Two patients in the 14-Gy group had transient thigh pain 2–4 months after EBR. The pain lasted a few months |
Drug-coated balloon
Study details
| Trial | Objective | Study design | Publication type | Language of publication | Sources of funding | Country of corresponding author | Intervention site(s) | Length of follow-up |
|---|---|---|---|---|---|---|---|---|
| LEVANT I59,60 | To evaluate the safety and efficacy of a paclitaxel + excipient-coated balloon vs. an uncoated balloon catheter for the treatment of femoropopliteal disease | RCT | Abstracts + PowerPoint delivered at the Transcatheter Cardiovascular Therapeutics conference 2010 | English | Lutonix, Inc. (Minneapolis, MN, USA) | Germany | Germany | 6 months |
| THUNDER (Tepe et al. 200861–63) | To evaluate the effect of paclitaxel on restenosis after angioplasty of stenotic or occluded superficial femoral or popliteal arteries | RCT (three-arm; one including uncoated balloon with paclitaxel dissolved in the contrast medium, which has been excluded from further data extraction) | One full report, plus abstracts | English | Sponsored by the Bavaria Medizin Technologie, Oberpfaffenhofen, and Schering, Berlin, Germany | Germany | Germany | The primary end point was late lumen loss, defined as the difference between the minimum lumen diameters after dilatation and at the 6-month follow-up |
| FemPac64 | To evaluate the efficacy and safety of PTA balloons (Indena, Milan, Italy) coated with paclitaxel compared with conventional uncoated balloon catheters (Bavaria Medizin Technologie) in a patient population with short femoropopliteal artery occlusion or stenosis | RCT | Full report | English | The authors received balloon catheters for the study from Bavaria Medizin Technologie, Oberpfaffenhofen, Germany, and financial support from Bayer-Schering-Pharma AG, Berlin, Germany | Germany | Germany | The primary end point was late lumen loss at 6 months |
Interventions
| Trial | Focus of interventions (comparisons) | T1: intervention group | T2: control group |
|---|---|---|---|
| LEVANT I59,60 | Paclitaxel-coated balloon vs. uncoated balloon catheter | Paclitaxel + excipient-coated balloon catheter | Uncoated balloon catheter |
| THUNDER61–63 | Standard balloon catheters coated with paclitaxel vs. uncoated balloon without paclitaxel | Balloon dilatation of the target lesion was performed with balloon catheters provided by Bavaria Medizin Technologie. The balloons were coated with paclitaxel at a dose of 3 μg/mm2 balloon surface. To restore the reference diameter of the vessel, the balloons were inflated with a maximum of 12 atm for a standardised inflation time of 1 minute. All study balloons were inflated only once. Additional study balloons were used for lesions exceeding the length of the first balloon. If angiography after the procedure showed residual stenosis of > 30%, inflation with a conventional non-study balloon was repeated for 5 minutes. Nitinol stents were implanted in lesions that had persistent residual stenosis or as clinically needed | Uncoated balloon but, otherwise, as for intervention |
| FemPac64 | Paclitaxel-coated balloon catheters vs. uncoated balloon catheters | Regular commercial PTA balloon catheters produced by Bavaria Medizin Technologie GmbH were used. Balloons were coated with paclitaxel at a dose of 3 μg/mm2 balloon surface | As described for intervention, but uncoated balloons |
Population inclusion
| Trial | Target population | Inclusion/exclusion criteria |
|---|---|---|
| LEVANT I59,60 | Inclusion criteria: (1) Rutherford categories 2–5, (2) > 70% stenosis, (3) lesion length 4–15 cm, (4) reference vessel diameter 4–6 mm. Exclusion criteria: (1) inadequate distal outflow, (2) severe calcification, (3) previous surgery of target lesion, (4) acute/subacute thrombosis | |
| THUNDER61–63 | Patients with stenotic or occluded superficial femoral or popliteal arteries | Eligible patients were between 18 and 95 years of age and had symptomatic PAD (Rutherford categories 1–5). All patients had one or more obstructive lesions, either new lesions or restenoses, ≥ 70% of vessel diameter and ≥ 2 cm in length, in the superficial femoral artery, the popliteal artery or both. If more than one lesion required intervention, only one was treated as the study lesion. Exclusion criteria included poor inflow, absence of a patent crural artery, acute onset of symptoms, pregnancy, life expectancy of < 1 year and contraindications to required medication |
| FemPac64 | Patients with short femoropopliteal artery occlusion or stenosis | Eligible patients had an occlusion or stenosis ≥ 70% diameter of the superficial femoral artery and/or popliteal artery with clinical Rutherford categories 1–5. Study entry criteria also included adult age (18–90 years) and successful guide wire passage of the lesion. The main exclusion criteria were acute symptoms with an indication for thrombolytic therapy or operation, leg-threatening ischaemia, distal outflow over < 1 vessel, manifest hyperthyroidism, renal insufficiency (creatinine > 2.0 mg/dl) and major gastrointestinal bleeding within the last 6 months. Patients with known intolerance to study medications or contrast agents and additional severe disease that may have lead to non-compliance or was associated with reduced life expectancy (< 2 years) also were excluded. Further exclusion criteria were conditions requiring different treatment, serious safety concerns regarding the procedure or doubtful willingness or capability of patients to undergo the 6-month follow-up |
Sample size
| Trial | Numbers included in the study | Number of patients in T1 | Number of patients in T2 | Power calculation (a priori sample calculation) | Attrition | Number followed up from each condition |
|---|---|---|---|---|---|---|
| LEVANT I59,60 | 101 | 49 | 52 | Not reported | With regards to primary study end point at 6 months, 20% were lost in T1 and 31% in T2 (angiographic findings) | T1 at 30 days = 100%, angiographic at 6 months 80%, clinical at 6 months 96%. T2 at 30 days = 92%, angiographic at 6 months 69%, clinical at 6 months 87% |
| THUNDER61–63 | 154 were enrolled into the three-arm RCT. Data relating to the two relevant arms (n = 102) was extracted | 48 | 54 | It was estimated that 45 patients would have to be enrolled in each group to yield a statistical power of 80% for the detection of an absolute difference in late lumen loss of 15% of the reference diameter between study groups at a p-value of < 0.05. These calculations assumed a standard deviation for late lumen loss of 20% of the reference diameter and a 20% loss of patients to angiographic follow-up | It is reported that 128/154 (83%) underwent angiography at 6-month follow-up. No patients were excluded until they reached one of the defined end points | See attrition |
| FemPac64 | 87 | 45 | 42 | To detect a 15% difference in late lumen loss between the equally sized treatment groups, which is considered to be clinically meaningful, e.g. 0.75 mm for a reference diameter of 5 mm at a level of p < 0.05 with a power of 80%, a standard deviation of ± 1.0 mm for late lumen loss was estimated to result in a raw total sample size of 58 patients. Assuming a loss to follow-up of 20%, at least 74 patients were to be enrolled. The ethics committee approved inclusion of up to 90 patients | Across both groups, at 6 months, 74.7% were followed up, and at 18–24 months 31% were followed up. Missing data were excluded | The 6-month follow-up angiography was performed in 31 of 45 (T1) and 34 of 42 (T2) patients |
Baseline characteristics
| Trial | Age (mean, years) | Gender (male) | Classification of PAD | Number of patients who have undergone previous revascularisation procedures | Presence of cardiovascular risk factors | Level of exercise tolerance |
|---|---|---|---|---|---|---|
| LEVANT I59,60 | T1: 67 ± 8. T2: 70 ± 10 | T1: 69%. T2: 58% | Rutherford category 2, 3, 4 or 5: T1, 22%/71%/2%/4%; T2, 21%/71%/4%/4% | Unclear, but participants had not had any previous surgery to the target lesion. T1 presented 11% restenosis and T2 12% | T1: smoker 68%, diabetes mellitus 45%, hypertension 96%, dyslipidaemia 59%. T2: smoker 70%, diabetes mellitus 50%, hypertension 87%, dyslipidaemia 69% | Not reported |
| THUNDER61–63 | T1: 69 ± 8. T2: 68 ± 9 | T1: 31 (65%). T2: 34 (63%) | Rutherford categories 1–5. Mean score at baseline: T1, 3.4 ± 0.8; T2, 3.1 ± 0.8 (p = 0.03) | Not reported | T1: diabetes mellitus 24 (50%), smoker 11 (23%), hyperlipidaemia 33 (69%), hypertension 38 (79%). T2: diabetes mellitus 25 (46%), smoker 12 (22%), hyperlipidaemia 34 (63%), hypertension 45 (83%) | Rutherford classification only reported |
| FemPac64 | T1: median age, 67.3 years. T2: median age, 70.2 years | T1: 27 (60%). T2: 25 (60%) | Rutherford categories 1–4. Rutherford 1, 2, 3 or 4: T1, n = 2/10/31/2; T2, n = 1/7/31/3 | In T1 14/45 (31%) and in T2 10/42 (24%) presented with restenosis following previous PTA | T1: diabetes mellitus 18 (40%), smoker 21 (47%), hypertension 35 (78%), hypercholesterolaemia 26 (58%). T2: diabetes mellitus 23 (55%), smoker 15 (36%), hypertension 34 (81%), hypercholesterolaemia 24 (59%) | Rutherford classification only reported |
Outcomes
| Trial | Pain/clinical status | Complications including amputation | Patency measures | Need for reintervention or recurrence rate |
|---|---|---|---|---|
| LEVANT I59,60 | Late lumen loss at 6 months | TLR | ||
| THUNDER61–63 | Rutherford category | Amputation or death | > 50% restenosis on angiographic evaluation, late lumen loss | Incidence of TLR |
| FemPac64 | Rutherford category | Amputation or death, adverse events | Late lumen loss was defined as the difference between the minimal luminal diameter after the procedure and at 6 months by quantitative angiography. Restenosis rate (defined as incidence of stenosis ≥ 50%) in the treated lesion at the 6-month follow-up angiography | TLR |
Results
| Trial | Results | Complications |
|---|---|---|
| LEVANT I59,60 | Late lumen loss at 6 months: T1 0.46 mm vs. T2 1.09 mm (p = 0.016). TLR: T1 13% vs. T2 22%. 30-day safety was equal between the two groups (no data provided) | Brief report suggesting no reported incidents of acute or late thrombosis in T1 |
| THUNDER61–63 | The mean Rutherford category improved after the intervention from 3.1 ± 0.8 to 1.2 ± 1.5 in the control group, and from 3.4 ± 0.8 to 1.1±1.2 in the group treated with paclitaxel-coated balloons. The primary end point of mean late lumen loss was significantly lower in the group treated with paclitaxel-coated balloons than in the control group (0.4 ± 1.2 mm vs. 1.7 ± 1.8 mm; p < 0.001). The angiographic restenosis rate was significantly lower among patients treated with paclitaxel-coated balloons than among patients in the control group (17% vs. 44%; p = 0.01) at 6 months and (24% vs. 50%) at 12 months. There were no significant differences in the primary patency rate at 6 months between groups. TLR was performed in 20 of 54 patients in the control group (37%), and 2 of 48 patients in the group treated with paclitaxel-coated balloons (4%; p < 0.001). The rate of TLR at 12 months remained low in the group treated with paclitaxel-coated balloons. In this group, 5 of 48 patients (10%) underwent TLR during the first year, as compared with 26 of 54 (48%) in the control group. Only a few additional TLRs were reported between 12 and 24 months, for a total of 28 of 54 in the control group (52%) compared with 7 of 48 in the group treated with paclitaxel-coated balloons (15%; p < 0.001). Amputation of the target leg above the foot at 6 months was 0 in the control group and 2 (4%) in T1 (p = 0.22) | Embolic complications during the procedure or thrombosis ≤ 2 weeks afterwards occurred in three patients in the control group and two patients in the group treated with paclitaxel-coated balloons. No late thrombosis was recorded in any patient. During the period from 2 weeks after the intervention until follow-up angiography, 46% to 58% of patients in the three treatment groups had a serious adverse event (p > 0.05); most events were related to progression of atherosclerosis or underlying disease. In 75 of 80 patients, these events were judged by the investigators to be unrelated to the study medication. By 6 months after the intervention, five patients had died and four had undergone major amputation (above the foot or higher) |
| FemPac64 | The 6-month follow-up angiography showed less late lumen loss in the coated balloon group (0.5 ± 1.1 vs. 1.0 ± 1.1 mm; p = 0.031). The number of TLRs was lower in the paclitaxel-coated balloon group than in the control group (3 of 45 vs. 14 of 42 patients; p = 0.002). Improvement in Rutherford category was greater in the coated balloon group (p = 0.045), whereas the improvements in ABPI were not different. The difference in TLRs between treatment groups was maintained up to > 18 months | During and shortly after the intervention, four adverse events were reported: two events in the paclitaxel-coated balloon group (peripheral embolism, skin rash) and two in the control group (allergic reaction, temporary serum creatinine increase) During the 6-month follow-up period, one patient in the paclitaxel-coated balloon group died as a result of multiple organ failure, which was not related to the study medication or PTA. In one patient in the uncoated balloon group, bilateral below-knee amputation had to be performed within this time period. A comparable number of serious adverse events, including any hospitalisation or prolongation of hospitalisation according to the common definition (serious adverse events), were reported in the treatment groups: 22 patients (48.9%) in the paclitaxel-coated balloon group and 22 patients (52.4%) in the uncoated balloon group. Most of these serious adverse events were due to vascular disorders, including TLR, which was significantly more frequent in the control group (14 of 42, 33%) than in the coated balloon group (3 of 45, 7%) (p = 0.002). The majority of TLRs (10 of 14 in the control group and two of three in the coated balloon group) were stimulated by documented complaints the patients had before control angiography was performed; in the remaining cases, the decision was based on the angiographic result Neither of the two treatment groups showed unexpected adverse events or an unusual frequency of adverse events |
Laser angioplasty
Study details
| Trial | Objective | Study design | Publication type | Language of publication | Sources of funding | Country of corresponding author | Intervention site(s) | Length of follow-up |
|---|---|---|---|---|---|---|---|---|
| Belli et al. 199165,66 | To evaluate the efficacy of laser thermal recanalisation vs. conventional PTA in total occlusions of the femoropopliteal artery | RCT | Full report | English | Not disclosed | England | England | 12 months |
| Fisher et al. 199667 | To evaluate the efficacy of laser-assisted balloon angioplasty compared with conventional balloon angioplasty alone in the treatment of localised disease in the superficial femoral artery | RCT | Full report | English | New South Wales Department of Health, Australia | Australia | Australia | Immediately post intervention and 1, 3 and 6 months. Median duration of follow-up was 350 days; for limbs with treatment success this was 430 days |
| Lammer et al. 199268 | To evaluate the efficacy of pulsed XeCI excimer laser vs. Nd:YAG laser vs. conventional PTA in patients with segmental femoropopliteal artery occlusions | RCT | Full report | English | Not disclosed | Austria | Austria | 12 months |
| Spies et al. 199069 | To evaluate the efficacy of laser thermal angioplasty vs. standard balloon angioplasty in the femoropopliteal artery | RCT | Full report – initial results of a randomised trial | English | Not disclosed | USA | USA | Unclear – reported as initial technical success |
| Tobis et al. 199170 | To evaluate the efficacy of laser-assisted angioplasty vs. standard guide wire and catheter techniques and to see whether there is additional value in using thermal energy during laser intervention | RCT | Full report | English | National Institutes of Health, Bethesda, MD, USA, and from the Office of Naval Research, Arlington, VA, USA | USA | The study reports initial comparative technical success, and describes overall up to 12 months but offers no comparative analysis for these data |
Interventions
| Trial | Focus of interventions (comparisons) | T1: intervention group | T2: control group |
|---|---|---|---|
| Belli et al. 199165,66 | Laser treatment vs. conventional PTA | Laser thermal angioplasty using a 2.5-mm hybrid laser probe (Spectraprobe PLR, Trimedyne, Santa Ana, CA, USA). During the initial study period (October 1988 to May 1989), the laser source was a continuous wave argon laser generator and between June 1989 and May 1990 the source was a continuous wave neodymium:yttrium aluminium garnet (Nd:YAG) generator. In both cases, 10–12 W of laser energy was used to heat the probe. Balloon dilatation was subsequently performed | Conventional treatment included crossing the occlusion with a guide wire of the operators choice before dilatation with a 7-F balloon catheter |
| Fisher et al. 199667 | Laser-assisted balloon angioplasty vs. conventional balloon angioplasty alone | Laser-assisted balloon angioplasty using a Trimedyne argon or Nd:YAG ‘over-the-wire-hot-tip’ laser system | No detail was provided in relation to conventional balloon angioplasty alone |
| Lammer et al. 199268 | Pulsed XeCI laser vs. Nd:YAG laser vs. conventional PTA | Excimer laser-assisted angioplasty: 308-nm XeCL excimer laser (MAX 10, Technolas, Grafeling, Germany) with a pulse width of 60–115 ns and a repetition rate of 20 Hz. A 2.2-mm catheter with 30 fibres, 200 µm in diameter. The energy fluence per pulse at the fibre tip was 45–60 mJ/mm2. Nd:YAG laser-assisted angioplasty: continuous-wave laser (CL 60, Surgical Laser Technologies, Malvern, PA, USA) via a 1.064-nm laser. Exposure time of 0.5–1.0 s and a repetition rate of 0.5 Hz. A 2.2-mm single fibre catheter (600 µm) was used with a ‘sapphire’ contact probe. The energy fluence per pulse at the fibre tip was 35 J/mm2. All procedures were carried out percutaneously through a 7-F introducer sheath. All patients had additional angioplasty with a 4- to 6-mm balloon | Conventional angioplasty: recanalisation via steerable guide wire followed by balloon angioplasty |
| Spies et al. 199069 | Laser thermal angioplasty (Nd:YAG laser, Optilase 1000, Trimedyne) vs. standard balloon angioplasty | Laser thermal angioplasty: a standard catheter and wire were initially used to cross the lesion followed by use of the laser probe (2.5-mm PLR Flex, Trimedyne) over it. Lasing lasted 30–60 seconds at 12–14 W followed by digital subtraction angiography. Then a standard balloon catheter was passed and inflated in the diseased segment in the standard fashion | Standard balloon angioplasty: an angiographic wire was passed through the lesion and angioplasty was performed with use of standard techniques |
| Tobis et al. 199170 | Laser-assisted angioplasty vs. standard guide wire and catheter techniques | Laser-assisted angioplasty: initially the laser probe was used as a cold, mechanical device without turning the laser on. The laser probe was a 1.5-mm-diameter laser probe model PLR-plus. Two different laser generating systems were used: an argon laser (Optilase model 900, Trimedyne) or a KTP-YAG laser model 532 (Laserscope, San Jose, CA, USA). The probe was inserted through a Y connector and passed along through the introducer sheath. Under fluoroscopic guidance, the probe was pushed into the occlusion, without activating the laser, with increasing force subjectively determined by the operator. If successful recanalisation was achieved, balloon dilatation angioplasty was then performed with a 4- to 7-mm-diameter balloon. If recanalisation was unsuccessful with the laser probe as a cold, mechanical device, then the laser was turned on at 10–12 W and gentle pressure was maintained at the level of occlusion for 5–10 seconds | Standard guide wire and catheter: a variety of guide wires were inserted through a 6-F or 7-F plastic catheter. The occlusion was probed under fluoroscopic guidance and the catheter was advanced over the guide wire as it progressed through the occlusion. Balloon angioplasty was undertaken as in intervention |
Population inclusion
| Trial | Target population | Inclusion/exclusion criteria | Recruitment |
|---|---|---|---|
| Belli et al. 199165,66 | Patients with total occlusions of the femoropopliteal artery | Inclusion criteria: (1) total occlusion of the femoropopliteal artery, (2) patients suitable for PTA via an ipsilateral approach. Exclusion criteria: patients in whom PTA was via a contralateral approach | Patients recruited but process is unclear |
| Fisher et al. 199667 | Patients with lower-limb PAOD | Inclusion criteria: patients with isolated occlusions < 3cm or stenoses > 50% in the SFA, and with popliteal and two or three calf-vessel run-offs. Exclusion criteria: patients with iliac or popliteal artery occlusion or significant stenosis | |
| Lammer et al. 199268 | Patients with segmental femoropopliteal artery occlusions | Inclusion criteria: (1) femoropopliteal artery occlusion, (2) suitable for PTA, (3) unsuccessful conservative treatment, (4) symptoms for > 4 months, (5) length of obstruction between 1 and 20 cm, (6) anticoagulation therapy feasible. Exclusion criteria: (1) stenoses without occlusion, (2) acute thrombotic or embolic occlusions, (3) incomplete angiographic demonstration of run-off arteries, (4) cardiac or renal failure, (5) insulin-dependent diabetes mellitus | Consecutive symptomatic patients |
| Spies et al. 199069 | Patients presenting with treatment for IC | Inclusion criteria: (1) patients with IC, normal femoral pulses and either abnormal resting ABPI or a significant drop in ABPI after exercise, (2) no haemodynamically significant iliac stenosis or occlusion, (3) no more than three atherosclerotic lesions in the SFA or popliteal artery, (4) > 50% narrowing of the vessel, (5) maximum lesion length of 10 cm, (5) a lesion at least 2 cm proximal to the tibial trifurcation, (6) at least one continuous run-off vessel | Not reported |
| Tobis et al. 199170 | Patients with symptoms of claudication and angiographic evidence of an occluded SFA | Inclusion criteria: (1) patients with complete occlusions on angiography, (2) at least one patent tibial vessel for run-off. Exclusion criteria: stenotic lesions | Not reported |
Sample size
| Trial | Number included in the study | Number of patients in T1 | Number of patients in T2 | Power calculation (a priori sample calculation) | Attrition | Number followed up from each condition |
|---|---|---|---|---|---|---|
| Belli et al. 199165,66 | 68 | 34 | 34 | Not reported | At 6 months: 12/68 (17.6%). At 12 months: 18/68 (26.5%) | At 6 months: T1, 30/34 (88%); T2, 8/34 (76%). At 12 months: T1, 26/34 (76%); T2, 24/34 (71%) |
| Fisher et al. 199667 | 82 (90 limbs) | Not reported | Not reported | Not reported | Not reported | Not reported |
| Lammer et al. 199268 | 116 | 37 (group 2, 40) | 39 | To demonstrate a difference between 65% and 85% in the primary recanalisation rate at 79% power and α = 0.05, 40 participants would be required in each group | At 3, 6 and 12 months, 103/116 (89%) for clinical data; follow-up angiography within 14 months was available in 80/116 (69%) | See attrition |
| Spies et al. 199069 | 25 patients, 27 procedures | 14 procedures | 13 procedures | Not reported | None | 100% |
| Tobis et al. 199170 | 40 | 20 | 20 | Not reported | None, the primary end point was immediate technical success | 100%. This reduced at later follow-up, but individual group data were not published, only an overall summary |
Baseline characteristics
| Trial | Age (mean, years) | Gender (male) | Classification of PAD | Number of patients who have undergone previous revascularisation procedures | Presence of cardiovascular risk factors | Level of exercise tolerance |
|---|---|---|---|---|---|---|
| Belli et al. 199165,66 | Not reported | T1: 24/34 (70.6%). T2: 21/34 (61.8%) | T1: 24/34 (70.6%) IC; 10/34 (29.4%) rest ischaemia. T2: 24/34 (70.6%) IC; 10/34 (29.4%) rest ischaemia | Not reported | T1: diabetes mellitus 3 (8.8%), current smoker 10 (29.4%). T2: diabetes mellitus 9 (26.5%), current smoker 4 (11.8%) | Not reported |
| Fisher et al. 199667 | For whole group: 69 ± 9 | Not reported adequately | 44 patients were mild claudicants (Fontaine class IIa), 32 severe claudicants (Fontaine IIb), six had either rest pain or tissue loss (Fontaine III or IV) | Not reported | All five patients with diabetes mellitus were randomised to T1; otherwise, the data were inadequately reported | Not reported |
| Lammer et al. 199268 | T1: 68 ± 9.2. T2: 63 ± 10.9. T3: 66 ± 8.6 | T1: 25/37 (67.6%). T2: 30/40 (75.0%). T3: 22/39 (56.4%) | Fontaine stage IIa, IIb, III or IV: T1 n = 9/11/6/11; T2 n = 13/22/1/4; T3 n = 7/22/2/8 | Not reported | T1: diabetes mellitus 7 (18.9%), hyperlipidaemia 11 (29.7%), hypertension 16 (43.2%), smoking 24 (64.9%). T2: diabetes mellitus 9 (22.5%), hyperlipidaemia 18 (45.0%), hypertension 10 (25.0%), smoking 31 (77.5%). T1: diabetes mellitus 13 (33.3%), hyperlipidaemia 10 (25.6%), hypertension 17 (43.6%), smoking 28 (71.8%) | Not reported |
| Spies et al. 199069 | Age range 45 to 75 years | 16/25 (64%) | Two patients had mild claudication, 23 had severe claudication | Not reported | Not reported | Not reported |
| Tobis et al. 199170 | 65 (range 42 to 83) | 36/40 (90%) | All patients had symptoms of claudication, but five patients had pain at rest without gangrene or an active skin ulcer due to vascular insufficiency. Duration range: 3 months to 17 years | Not reported | For the whole group (n = 40): diabetes mellitus 10 (25%), smokers 100% | Not reported |
Outcomes
| Trial | Pain/clinical status | Complications including amputation | Patency measures |
|---|---|---|---|
| Belli et al. 199165,66 | Clinical success was defined as relief of symptoms and improved peripheral pulses | Procedural complications | |
| Fisher et al. 199667 | Treatment failure was defined as restenosis of the original lesion to > 50% diameter stenosis or occlusion | ||
| Lammer et al. 199268 | Procedural complications | Angiographic reobstruction was defined as an increase in diameter stenosis > 30%, an immediate post-PTA diameter stenosis of < 50% increasing to > 70% at follow-up, an increase in stenosis severity to ≤ 10% of pre-dilatation obstruction, and a loss of > 50% of the gain in luminal diameter achieved by PTA | |
| Spies et al. 199069 | Procedural complications | ||
| Tobis et al. 199170 | Procedural complications |
Results
| Trial | Results | Complications |
|---|---|---|
| Belli et al. 199165,66 | Cumulative clinical success (immediately and 1, 3, 6, 12 months): T1 88, 79, 56, 42, 39; T2 88, 82, 72, 56, 47, respectively. Kaplan–Meier analysis: no significant difference between the groups (p = 0.81). Clinical success at 2 weeks according to group to which they were randomised: T1 (n = 29) 85%; T2 (n = 30) 88% (p = 0.67). In T1, three (9%) received both interventions and in T2 six (18%). Technical success was reported as 91% in both groups when analysed according to the group to which they were randomised | In three cases (two conventional group, one laser) a small embolus was detected in the calf vessels. Spasm was induced in four patients (two conventional group, two laser). Haematoma formation, dissection and perforation were not considered significant complications unless they necessitated prolonged hospital stay or operative intervention or worsened the patient’s clinical grade |
| Fisher et al. 199667 | Treatment failed in 40 limbs during follow-up – distribution between groups unclear. Median time to failure was 220 days. 21 limbs underwent repeat intervention | No direct adverse events were reported |
| Lammer et al. 199268 | Primary recanalisation rate by excimer laser (18/37, 49%) was lower than with Nd:YAG laser (31/40, 78%; p < 0.01) or PTA (32/39, 82%; p < 0.003). No significant difference between Nd:YAG and PTA. After excimer laser, there was no residual stenosis in 8/37, < 50% in 9/37 and 50% stenosis in one patient. For Nd:YAG the results are 21/40, 9/40 and 1/40, respectively, and for PTA 25/39, 5/39 and 2/39. Secondary recanalisation: PTA was successful in 13/19 patients in whom excimer laser failed and in 5/9 in whom Nd:YAG laser failed. Laser angioplasty was successful in 4/7 patients in whom PTA failed. At 12-month follow-up one patient had below-the-knee amputation, 13 had femoropopliteal bypass, eight had PTA for recurrent stenosis – individual group data not reported. Life table analysis based on clinical symptoms revealed a 12-month patency rate of 64% for patients treated successfully with excimer laser, 70% for Nd:YAG and 71% PTA. Life table analysis revealed a 12-month patency rate after successful primary recanalisation with excimer laser, Nd:YAG and PTA of 45%, 36% and 50%, respectively | Excimer laser, 15/37: embolus 0, dissection 13, perforation 2, spasm 0. Nd:YAG, 12/40: 2, 8, 2, 0, respectively. PTA 13/39: 3, 6, 3, 1. The number of dissections in the PTA group was significantly lower (p = 0.005) |
| Spies et al. 199069 | Laser: 9/14 initial technical success. Standard balloon angioplasty: 10/13 initial technical success. Of the five laser failures three were subsequently successfully treated with standard balloon angioplasty. Of the three standard balloon failures, none were subsequently successfully treated with laser | One patient in the laser group suffered an embolus and one further patient in the embolus group complained of severe procedural discomfort |
| Tobis et al. 199170 | The primary end point was recanalisation of the occluded segment of the artery with angiographic evidence of direct flow between the proximal and distal lumens. In T1 the success rate was 15/20 (75%), and in T2 it was 19/20 (95%). This difference was reported as not being statistically significant. No patient from T2 required crossover to T1. T1 initially used as a cold, mechanical device resulted in 13/20 (65%) successes with a further two successes when the probe was heated | Perforation of the arterial wall occurred in one patient in T2 and five patients in T1. Other adverse events included development of three arteriovenous fistulas, but it is unclear which groups these developed in. Haematomas developed in a further two patients |
Appendix 4 Quality assessment of included studies
Quality was assessed according to criteria based on NHS CRD Report No. 4. 9
Absorbable metal stent
| Trial name/Study authors and year | Method of randomisation | Was the method used to generate random allocations adequate? | Method of allocation concealment | Was the allocation adequately concealed? | Blinding | Were intervention and control groups comparable? | Were there any unexpected imbalances in dropouts between groups? | Is there any evidence to suggest that the authors measured more outcomes than they reported? | Were effectiveness outcomes analysed in allocated group according to ITT principle? |
|---|---|---|---|---|---|---|---|---|---|
| AMS INSIGHT11 | Computer-generated randomisation list | Adequate | Numbered, sealed envelopes | Adequate | Unblinded | Yes, apart from gender (p = 0.04): 71.9% male PTA, 51.7% male AMS | No | No | Yes |
Self-expanding stent
| Trial name/Study authors and year | Method of randomisation | Was the method used to generate random allocations adequate? | Method of allocation concealment | Was the allocation adequately concealed? | Blinding | Were intervention and control groups comparable? | Were there any unexpected imbalances in dropouts between groups? | Is there any evidence to suggest that the authors measured more outcomes than they reported? | Were effectiveness outcomes analysed in allocated group according to ITT principle? |
|---|---|---|---|---|---|---|---|---|---|
| Dick et al. 200912 | Computer-generated randomisation list | Adequate | Sealed envelopes | Unclear | Outcome assessors blinded. Patients and clinicians unblinded | Yes, apart from the average length of the treated segments, which was 98 ± 54 mm and 71 ± 43 mm in the stent and PTA groups (p = 0.011), respectively | No | No | Yes |
| VascuCoil13 | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | No | No | Yes |
| FAST14 | Unclear | Unclear | Central allocation | Adequate | Unblinded, but blinded assessors for ultrasound analysis | Yes | No | No | Yes |
| RESILIENT15 | Computer-generated randomisation list | Adequate | Unclear | Unclear | Unblinded | Yes, apart from more patients with hypertension in the PTA group | No | No | Yes |
| ABSOLUTE16–18 | Computer-generated randomisation list | Adequate | Sealed envelopes | Unclear | Outcome assessors blinded. Patients and clinicians unblinded | Yes | No | No | Yes |
Balloon-expandable stent
| Trial name/Study authors and year | Method of randomisation | Was the method used to generate random allocations adequate? | Method of allocation concealment | Was the allocation adequately concealed? | Blinding | Were intervention and control groups comparable? | Were there any unexpected imbalances in dropouts between groups? | Is there any evidence to suggest that the authors measured more outcomes than they reported? | Were effectiveness outcomes analysed in allocated group according to ITT principle? |
|---|---|---|---|---|---|---|---|---|---|
| Becquemin et al. 200319 | Computer-generated randomisation list | Adequate | Central allocation | Adequate | Outcome assessors blinded. Patients and clinicians unblinded | Yes | No | No | Yes |
| Cejna et al. 200120 | Unclear | Unclear | Closed envelopes | Unclear | Unblinded | Yes | No | No | Yes |
| Grimm et al. 200121 | Randomisation list | Adequate | Numbered, sealed envelopes | Adequate | Unblinded | Yes | No | No | Yes |
| Rand et al. 200622 | Unclear | Unclear | Numbered, sealed envelopes | Adequate | Outcome assessors blinded. Patients and clinicians unblinded | Yes | No | No | Yes |
| Vroegindeweij et al. 199723 | Unclear | Unclear | Numbered, sealed envelopes | Adequate | Unblinded | Yes | No | No | Yes |
| Zdanowski et al. 199924 | Computer-generated randomisation list | Adequate | Unclear | Unclear | Unblinded | Yes | No | No | Yes |
Drug-eluting stents
| Trial name/Study authors and year | Method of randomisation | Was the method used to generate random allocations adequate? | Method of allocation concealment | Was the allocation adequately concealed? | Blinding | Were intervention and control groups comparable? | Were there any unexpected imbalances in dropouts between groups? | Is there any evidence to suggest that the authors measured more outcomes than they reported? | Were effectiveness outcomes analysed in allocated group according to ITT principle? |
|---|---|---|---|---|---|---|---|---|---|
| Zilver PTX25,26 | Unclear | Unclear | Unclear | Unclear | Unclear | Yes, apart from more patients with hypertension in paclitaxel-eluting stent group (p = 0.02) | No | No | Yes |
| SIROCCO28–30 | Unclear | Unclear | Unclear | Unclear | Outcome assessors and patients blinded. Clinicians unblinded | Yes, apart from more severe calcification for DES group. Calcification (moderate and severe): DES 27 (57%), BMS 16 (35%) (p = 0.03) | No | No | Yes |
| Rastan et al. 201131 | Computer-generated randomisation | Adequate | Central allocation | Adequate | Outcome assessors and patients blinded. Clinicians unblinded | Yes, with the exception of a significantly higher body mass index in the SES group | No | No | Yes |
Stent graft
| Trial name/Study author and year | Method of randomisation | Was the method used to generate random allocations adequate? | Method of allocation concealment | Was the allocation adequately concealed? | Blinding | Were intervention and control groups comparable? | Were there any unexpected imbalances in dropouts between groups? | Is there any evidence to suggest that the authors measured more outcomes than they reported? | Were effectiveness outcomes analysed in allocated group according to ITT principle? |
|---|---|---|---|---|---|---|---|---|---|
| Saxon et al. 2003, 200832,33 | Unclear | Unclear | Unclear | Unclear | Unblinded | Yes | No | No | Yes |
Atherectomy
| Trial name/Study authors and year | Method of randomisation | Was the method used to generate random allocations adequate? | Method of allocation concealment | Was the allocation adequately concealed? | Blinding | Were intervention and control groups comparable? | Were there any unexpected imbalances in dropouts between groups? | Is there any evidence to suggest that the authors measured more outcomes than they reported? | Were effectiveness outcomes analysed in allocated group according to ITT principle? |
|---|---|---|---|---|---|---|---|---|---|
| Nakamura et al. 199534 | Random number table | Adequate | Unclear | Unclear | Unblinded | yes, except mean age older for TEC4mm than other groups | No | No | Yes |
| Vroegindeweij et al. 1992,35 1995,36 Tielbeek et al. 199637 | Unclear | Unclear | Numbered, sealed envelopes | Adequate | Unblinded | Yes, although more patients in directional atherectomy group had hypertension | No | No | Yes |
Cutting balloon
| Trial name/Study authors and year | Method of randomisation | Was the method used to generate random allocations adequate? | Method of allocation concealment | Was the allocation adequately concealed? | Blinding | Were intervention and control groups comparable? | Were there any unexpected imbalances in dropouts between groups? | Is there any evidence to suggest that the authors measured more outcomes than they reported? | Were effectiveness outcomes analysed in allocated group according to ITT principle? |
|---|---|---|---|---|---|---|---|---|---|
| Amighi et al. 200838 | Computer-generated randomisation list | Adequate | Numbered, sealed envelopes | Adequate | Outcome assessors blinded. Patients and clinicians unblinded | Yes | No | No | Yes |
| Dick et al. 200839 | Computer-generated randomisation list | Adequate | Numbered, sealed envelopes | Adequate | Unblinded, but blinded outcome assessors for ultrasound analysis | Yes | No | No | Yes |
Cryoplasty
| Trial name/Study authors and year | Method of randomisation | Was the method used to generate random allocations adequate? | Method of allocation concealment | Was the allocation adequately concealed? | Blinding | Were intervention and control groups comparable? | Were there any unexpected imbalances in dropouts between groups? | Is there any evidence to suggest that the authors measured more outcomes than they reported? | Were effectiveness outcomes analysed in allocated group according to ITT principle? |
|---|---|---|---|---|---|---|---|---|---|
| Jahnke et al. 201040 | Unclear | Unclear | Sealed envelopes | Unclear | Unblinded | Yes | No | No | Yes |
| Spiliopoulos et al. 201041 | Unclear | Unclear | Sealed envelopes | Unclear | Unblinded, but independent angiographic image analysis | Yes | No | No | Yes |
Radiation
| Trial name/Study authors and year | Method of randomisation | Was the method used to generate random allocations adequate? | Method of allocation concealment | Was the allocation adequately concealed? | Blinding | Were intervention and control groups comparable? | Were there any unexpected imbalances in dropouts between groups? | Is there any evidence to suggest that the authors measured more outcomes than they reported? | Were effectiveness outcomes analysed in allocated group according to ITT principle? |
|---|---|---|---|---|---|---|---|---|---|
| Gallino et al. 2004,42 Bonvini et al. 2003,43 Diehm et al. 200544 | Unclear | Unclear | Unclear | Unclear | Unblinded (but blinded outcome assessors for angiographic analysis of Gallino et al.42 and Zehnder et al.45 trials in Diehm et al.44) | Yes | No | No | No (but ITT analysis of Gallino et al.42 and Zehnder et al.45 trials in Diehm et al.44) |
| Diehm et al. 2005,44 Zehnder et al. 200345 | Unclear | Unclear | Unclear | Unclear | Unclear (but blinded outcome assessors for angiographic analysis of Gallino et al.42 and Zehnder et al.45 trials in Diehm et al.44) | Yes | No | No | No (only baseline characteristics analysed in ITT analysis) (but ITT analysis of Gallino et al.42 and Zehnder et al.45 trials in Diehm et al.44) |
| Hagenaars et al. 200246 | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes, more dropouts in radiation group | No | Yes |
| Krueger et al. 2002, 200447,48 | Computer-generated randomisation list | Adequate | Sealed envelopes | Unclear | Outcome assessors and patients blinded. Clinicians unblinded | Yes | No | No | Yes |
| Vienna-249,50 | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | No | No | Yes |
| Vienna-352 | Unclear | Unclear | Unclear | Unclear | Outcome assessors and patients blinded. Clinicians unblinded | Yes | No | No | Yes |
| VARA54 | Computer-generated randomisation list | Adequate | Central allocation | Adequate | Unclear | Yes | No | No | Yes |
| Wyttenbach et al. 2004, 200755,56 | Unclear | Unclear | Unclear | Unclear | Outcome assessors blinded. Patients and clinicians unblinded | Yes | No | No | Yes |
| Fritz et al. 200457 | Unclear | Unclear | Unclear | Unclear | Outcome assessors and patients blinded. Clinicians unblinded | Yes | No | No | Yes |
| Therasse et al. 200558 | Random number table | Adequate | Sealed envelopes | Unclear | Outcome assessors and patients blinded. Clinicians unblinded | Yes | No | No | Yes |
Drug-coated balloon
| Trial name/Study authors and year | Method of randomisation | Was the method used to generate random allocations adequate? | Method of allocation concealment | Was the allocation adequately concealed? | Blinding | Were intervention and control groups comparable? | Were there any unexpected imbalances in dropouts between groups? | Is there any evidence to suggest that the authors measured more outcomes than they reported? | Were effectiveness outcomes analysed in allocated group according to ITT principle? |
|---|---|---|---|---|---|---|---|---|---|
| LEVANT I59,60 | Unclear | Unclear | Unclear | Unclear | Single blind, unclear if outcome assessors or patients blinded | Yes | No | Yes (but for future report) | Yes |
| THUNDER61–63 | Lot-generated random list | Adequate | Unclear | Unclear | Outcome assessors and patients blinded. Clinicians unblinded | Yes, apart from some difference in baseline Rutherford classification | No | No | Yes |
| FemPac64 | Random number list | Adequate | Central allocation | Adequate | Patients blinded, angiographic image assessors blinded (6-month outcome). Blinding of investigators attempted, but unlikely because of difference in appearance of balloons | Yes | No | No | Yes |
Laser
| Trial name/Study authors and year | Method of randomisation | Was the method used to generate random allocations adequate? | Method of allocation concealment | Was the allocation adequately concealed? | Blinding | Were intervention and control groups comparable? | Were there any unexpected imbalances in dropouts between groups? | Is there any evidence to suggest that the authors measured more outcomes than they reported? | Were effectiveness outcomes analysed in allocated group according to ITT principle? |
|---|---|---|---|---|---|---|---|---|---|
| Belli et al. 199165,66 | Unclear | Unclear | Blind selection of a pre-marked card from a box | Unclear | Unblinded | Yes | No | No | Yes |
| Fisher et al. 199667 | Unclear | Unclear | Unclear | Unclear | Unblinded | Yes, except for diabetes mellitus, as all (n = 5) diabetes mellitus patients in laser group | No | No | Yes |
| Lammer et al. 199268 | Unclear | Unclear | Unclear | Unclear | Outcome assessors blinded. Patients and clinicians unblinded | No | No | No | Yes |
| Spies et al. 199069 | Coin toss | Adequate | Unclear | Unclear | Unblinded | Unclear | No | Yes (but for future report) | Only safety data reported, ITT |
| Tobis et al. 199170 | Computer-generated randomisation list | Adequate | Unclear | Unclear | Unblinded | Yes | No | No | Yes |
There were too few studies for each comparison to produce funnel plots. Taking studies with any intervention that provided results for the outcome of restenosis, it appears that there is a spread of results from the larger studies, although overall they slightly favour intervention over PTA alone (Figure 33). The two small studies that favoured intervention were EVBT trials (Hagenaars et al. 200246 and Krueger et al. 2002,47 200448) with very small sample sizes (n = 24 and n = 30, respectively). Given the differing interventions, and that not all studies reported the same outcomes, we cannot draw definite conclusions about the possibility of publication bias.
FIGURE 33.
Funnel plot of studies reporting restenosis.
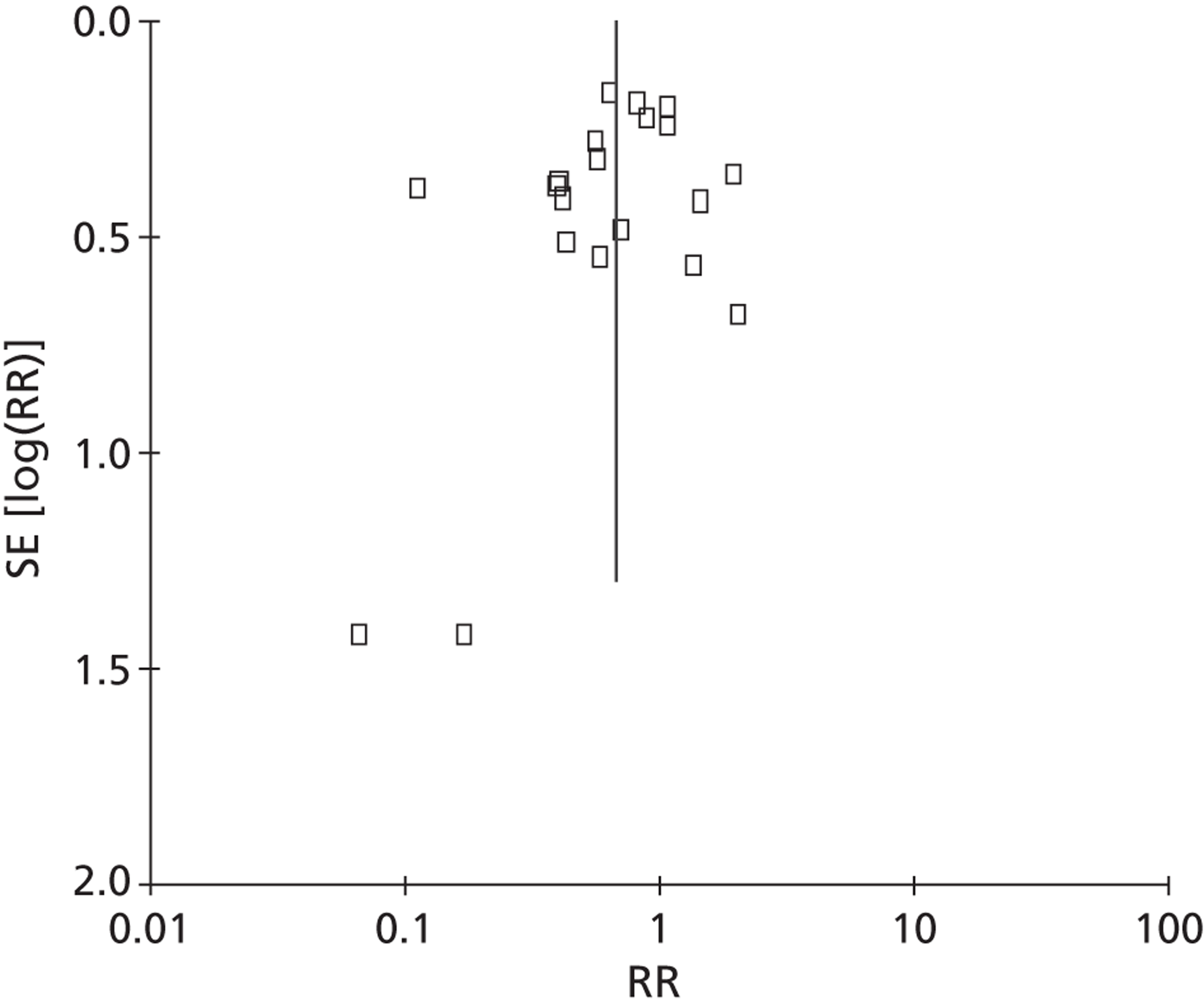
Appendix 5 Summary
| Intervention | Trial (trial name, first author, date) | Sample size | Results (more detailed results shown in Chapter 3, Results) |
|---|---|---|---|
| AMS | AMS INSIGHT, Bosiers 200911 | 117 CLI | Restenosis: AMS significantly worse than PTA (p = 0.013) |
| SES | Dick 200912 | 73 (of whom 69 IC, 4 CLI) | Restenosis: SES significantly better than PTA (p = 0.006) |
| SES | VascuCoil, Greenberg 200413 | 266 ‘symptomatic leg ischaemia’ | TLR: non-significant between treatment groups |
| SES | FAST, Krankenberg 200714 | 244 (of whom 226 IC, 7 CLI, 11 data unavailable) | Restenosis: non-significant between treatment groups. TLR: non-significant between treatment groups |
| SES | RESILIENT, Laird 201015 | 206 IC | Restenosis: SES significantly better than PTA (p < 0.0001). TLR/TVR: SES significantly better than PTA (p < 0.0001) |
| SES | ABSOLUTE, Schillinger 2006,16 2007,17 Sabeti 200718 | 104 (of whom 91 IC, 13 CLI) | Restenosis: SES significantly better than PTA at 12 months (p = 0.01). TLR: non-significant between treatment groups |
| BES | Becquemin 200319 | 227 (of whom 180 IC, 47 CLI) | Restenosis: non-significant between treatment groups |
| BES | Cejna 200120 | 141 (154 limbs, of which 108 IC, 46 CLI) | Restenosis: non-significant between treatment groups |
| BES | Grimm 200121 | 53 IC | Restenosis: non-significant between treatment groups. TLR: non-significant between treatment groups |
| BES | Rand 200622 | 51 CLI | Restenosis: BES significantly better than PTA (p = 0.02) |
| BES | Vroegindeweij 199723 | 51 IC | Restenosis: non-significant between treatment groups |
| BES | Zdanowski 199924 | 32 CLI | Restenosis: PTA significantly better than BES (p = 0.033) |
| DES (paclitaxel) | Zilver PTX, Dake 2008,27 2010,25 Ansell 201126 | 479 Rutherford category 2 or above | Restenosis: DES significantly better than PTA (p < 0.01) |
| DES (sirolimus) | SIROCCO, Duda 2002,28 2005,29 200630 | 93 (of whom 46 Rutherford category 1 or 2, 47 Rutherford category 3 or 4) | Restenosis: non-significant between treatment groups. TLR: non-significant between treatment groups (DES, BMS) |
| DES (sirolimus) | Rastan 201131 | 161 (of whom 86 IC, 75 CLI) | Restenosis: DES significantly better than PTA (p = 0.02). TLR: non-significant between treatment groups (DES, BMS) |
| Stent graft | Saxon 2003,32 200833 | 197 (of whom 175 IC, 21 CLI, 1 unknown) | Restenosis: stent graft significantly better than PTA (p = 0.0003) |
| Atherectomy | Nakamura 199534 | 39 IC | Restenosis: non-significant between treatment groups |
| Atherectomy | Vroegindeweij 1992,35 1995,36 Tielbeck 199637 | 73 IC | Restenosis: non-significant between treatment groups |
| CB | Amighi 200838 | 43 (of whom 35 IC, 8 CLI) | Restenosis: CB significantly better than PTA (p = 0.048) |
| CB | Dick 200839 | 39 (of whom 30 IC, 9 CLI) | Restenosis: non-significant between treatment groups. TLR: non-significant between treatment groups |
| Cryoplasty | Jahnke 201040 | 86 (of whom 66 IC, 20 CLI) | Restenosis: non-significant between treatment groups |
| Cryoplasty | Spiliopoulos 201041 | 50 (60 limbs included, of which 36 IC, 24 CLI) | Restenosis: non-significant between treatment groups. TLR: cryoplasty significantly better than PTA (p < 0.04) |
| Radiation (EVBT) | Diehm 200544 (results of Gallino 200442 and Zehnder 200345) | Gallino 2004:42 n = 156. Zehnder 2003:45 n = 100 | Restenosis: EVBT significantly better than PTA (p = 0.16). TLR: non-significant between treatment groups |
| Radiation (EVBT) | Hagenaars 200246 | 24 (of whom 12 IC, 12 CLI) | Restenosis: EVBT significantly better than PTA (p = 0.08) |
| Radiation (EVBT) | Krueger 2002,47 200448 | 30 (unclear how many IC/CLI, all Fontaine 2a to 3) | Restenosis: EVBT significantly better than PTA (p = 0.006). TLR: non-significant between treatment groups |
| Radiation (EVBT) | Vienna-2, Wolfram 2005,51 2006,49 Minar 200050 | 113 (of whom 88 IC, 25 CLI) | Restenosis: non-significant between treatment groups. TLR: non-significant between treatment groups |
| Radiation (EVBT) | Vienna-3, Pokrajac 2000,53 2005,52 Wolfram 200551 | 96 (of whom 77 IC, 19 CLI) | Restenosis: EVBT significantly better than PTA (p < 0.05). TLR: non-significant between treatment groups |
| Radiation (EVBT) | VARA, van Tongeren 200554 | 60 (of whom 52 IC, 8 CLI) | Restenosis: non-significant between treatment groups. TLR: non-significant between treatment groups |
| Radiation (external beam) | Fritz 200457 | 95 (of whom 94 IC, 1 CLI) | Restenosis: non-significant between treatment groups |
| Radiation (external beam, three doses) | Therasse 200558 | 99 (of whom 27 IC, 72 CLI) | Restenosis: EBRT significantly better than PTA (p = 0.072). TLR: non-significant between treatment groups |
| DCB (paclitaxel) | LEVANT I, Scheinert 201059,60 | 101 (of whom 94 IC, 7 CLI) | TLR: non-significant between treatment groups |
| DCB (paclitaxel) | THUNDER, Tepe 200861–63 | 102 (in two relevant arms of three-arm trial) (Rutherford categories 1–5) | Restenosis: DCB significantly better than PTA (p = 0.01). TLR: DCB significantly better than PTA (p < 0.001) |
| DCB (paclitaxel) | FemPac, Werk 200864 | 87 (of whom 82 IC, 5 CLI) | Restenosis: DCB significantly better than PTA (p = 0.035). TLR: DCB significantly better than PTA (p = 0.0024) |
| Laser angioplasty | Lammer 199268 | 116 (of whom 84 IC, 32 CLI) | Restenosis: non-significant between treatment groups |
Appendix 6 Quality assessment forms (cost-effectiveness systematic review)
| Drummond-adapted criteria (Drummond et al.143) | Hunink et al. 199577 | Sculpher et al.76 | de Vries et al. 200278 | Holler et al. 200679 | BASIL trial (Forbes et al. 201082) | NICE CEA 201283 |
|---|---|---|---|---|---|---|
| (1) Was a well-defined question posed in answerable form? | Yes | Partial | Partial | Yes | Yes | Yes |
| (2) Was a comprehensive description of the competing alternatives given? | Yes | Yes | Yes | Yes | Yes | Yes |
| (3) Was the effectiveness of the programme or services established? | Yes | Partial | Yes | Partial | Partial | Partial |
| (4) Were all the important and relevant costs and consequences for each alternative identified? | Yes | Partial | Partial | No | Yes | Yes |
| (5) Were costs and consequences measured accurately in appropriate physical units? | Yes | Yes | Yes | No | Yes | Yes |
| (6) Were the cost and consequences valued credibly? | Partial | Yes | Yes | Partial | Yes | Yes |
| (7) Were costs and consequences adjusted for differential timing? | Yes | Yes | Yes | No | Yes | Yes |
| (8) Was an incremental analysis of costs and consequences of alternatives performed? | Yes | Yes | Yes | Yes | Yes | Yes |
| (9) Was allowance made for uncertainty in the estimates of costs and consequences? | Partial | Yes | Partial | Partial | Partial | Yes |
| (10) Did the presentation and discussion of study results include all issues of concern to users? | Yes | Yes | Yes | Partial | Yes | Yes |
| Consensus on Health Economic Criteria list (Evers et al.144) | Hunink et al. 199577 | Sculpher et al.76 | de Vries et al. 200278 | Holler et al. 200679 | BASIL trial (Forbes et al. 201082) | NICE CEA 201283 |
|---|---|---|---|---|---|---|
| (1) Is the study population clearly described? | Yes | Partial | Partial | Yes | Yes | Yes |
| (2) Are competing alternatives clearly described? | Yes | Yes | Yes | Yes | Yes | Yes |
| (3) Is a well-defined research question posed in answerable form? | Yes | Partial | Yes | Yes | Yes | Yes |
| (4) Is the economic study design appropriate to the stated objective? | Yes | Yes | Yes | Partial | Yes | Yes |
| (5) Is the chosen time horizon appropriate to include relevant costs and consequences? | Yes | Yes | Yes | No | Partial | Yes |
| (6) Is the actual perspective chosen appropriate? | Yes | Yes | Yes | Unclear | Yes | Yes |
| (7) Are all important and relevant costs for each alternative identified? | Yes | Partial | Partial | Yes | Yes | Yes |
| (8) Are all costs measured appropriately in physical units? | Yes | Yes | Yes | No | Yes | Yes |
| (9) Are costs valued appropriately? | Yes | Yes | Yes | Unclear | Yes | Yes |
| (10) Are all important and relevant outcomes for each alternative identified? | Yes | Yes | Yes | Yes | Yes | Yes |
| (11) Are all outcomes measured appropriately? | Yes | Yes | Yes | Yes | Yes | Yes |
| (12) Are outcomes valued appropriately? | Partial | Yes | Yes | Yes | Yes | Yes |
| (13) Is an incremental analysis of costs and outcomes of alternatives performed? | Yes | Yes | Yes | Yes | Yes | Yes |
| (14) Are all future costs and outcomes discounted appropriately? | Yes | Yes | Yes | No | Yes | Yes |
| (15) Are all important variables, whose values are uncertain, appropriately subjected to sensitivity analysis? | Partial | Yes | Partial | Partial | Partial | Yes |
| (16) Do the conclusions follow from the data reported? | Partial | Yes | Yes | Partial | Yes | Yes |
| (17) Does the study discuss the generalisability of the results to other settings and patient/client groups? | Yes | Yes | Yes | No | Yes | Yes |
| (18) Does the article indicate that there is no potential conflict of interest of study researcher(s) and funder(s)? | Yes | Yes | Yes | No | Yes | Not applicable |
| (19) Are ethical and distributional issues discussed appropriately? | Yes | Yes | Yes | Yes | Yes | Yes |
Appendix 7 Additional details for the base-case model parameters
This section provides additional information about the parameters used in the model developed for the independent economic assessment.
General mortality; excess risk
Holler et al. 79 model general mortality as fixed transition probabilities, depending on indication and initial operation. All the other evaluations model general mortality by applying an additional risk to general mortality rates. Hunink et al. 77 apply an excess mortality risk, stratified by level of ABPI; in a sensitivity analysis, they use a RR of 3.1, as reported by Criqui et al. 97 de Vries et al. 78 apply a RR of mortality of 3.14 for having PAD. Five references are provided for this value (none of them is Criqui et al. ,97 which also only reported the value to one decimal place). It is unclear how the RR of 3.14 was derived. Neither Hunink et al. 77 nor de Vries et al. 78 use separate RRs for patients with CLI, even though their referenced studies are all for patients with IC only. The NICE CEA83 also uses the RR quoted by Criqui et al. 97 for patients with IC (although this is misquoted as 3.14). For CLI, the NICE CEA83 uses an annual mortality rate of 25%, assuming that 70% of the population is male. Applying this proportion to general population life tales gives an annual probability of death of 2.87% for a 74-year-old. This is equivalent to assuming a RR of 8.7 for patients with CLI. TASC II99 suggests that the annual mortality rate is actually 20%; this gives a relative of 7 for patients with CLI.
Sculpher et al. 76 use RRs of 2 for patients with IC and 3 for patients with CLI, based on data presented in Dormandy et al. 145
In a previous HTA report looking at the use of drugs for treating patients with IC, Squires et al. 146 apply a RR of 1.6. TASC II99 present data (see figure A8 in TASC II99) that suggest that the RRs for IC and CLI are about 3 and 6, respectively. Other journal articles have also reported different RRs; the following are all for IC: in addition to the value already quoted from Criqui et al. 97 (3.1), Jelnes et al. 147 say that the value is about 2, whereas Levy148 quotes studies for which the values were about 3 and 4. There is little evidence of mortality rates being affected by lesion type.
For the base case, a RR (compared with the general population) of mortality due to having IC of 3.1 (Criqui et al. 97) is used. It is felt that patients with CLI will have a RR at least equal to that of patients with IC, if not higher. Compared with patients with IC, patients with CLI have a RR of death of 0 (de Vries et al. ,78 Hunink et al. 77), 1.5 (Schulpher et al. 76), 2 (see figure A8 in TASC II99), 2.2 (TASC II99 annual mortality of 20%) or 2.8 (NICE CEA83 annual mortality of 25%). For the base case, the RR of 2 is used; this is equivalent to CLI patients having a RR of mortality of 6.2 compared with the general population.
PTA failure
The meta-analysis used in this evaluation (Hunink et al. 98) uses data from 11 studies. A life table of yearly patency following PTA for 5 years is presented (patency at half a year and immediate technical and clinical failures are also included) for patients with IC and stenosis. The effects of having CLI or occlusions are assumed to act independently and follow a proportional hazards model. Hazard ratios for these two risk factors are presented and were used to derive yearly patency rates depending on indication (IC or CLI) and lesion type (stenosis or occlusion). These data are presented in Table 81.
| Interval (years) | Number at risk | Censored | Failures | Interval patency (%) | Cumulative patency (%) |
|---|---|---|---|---|---|
| 0–0 | 1003 | 71 | 50 | 95 | |
| 0–0.5 | 882 | 72 | 89 | 89 | 95 |
| 0.5–1 | 721 | 49 | 52 | 93 | 85 |
| 1–2 | 620 | 45 | 24 | 96 | 79 |
| 2–3 | 551 | 150 | 11 | 98 | 75 |
| 3–4 | 390 | 60 | 11 | 97 | 74 |
| 4–5 | 319 | 138 | 11 | 96 | 71 |
| 5+ | 170 | 68 |
The authors do not present data on the prevalence of each lesion type for each indication; this was derived using the data presented for the 11 studies in Hunink et al. 98 These data are reproduced in Table 82.
| Study | Size | CLI (%) | Occlusions (%) |
|---|---|---|---|
| (1) Gallino et al.42 | 289 | 39 | 41 |
| (2) Johnston149 | 254 | 20 | 39 |
| (3) Capek et al.150 | 217 | 26 | 32 |
| (4) Hunink et al.151 | 131 | 42 | 10 |
| (5) Jørgenson et al.152 | 58 | 100 | 62 |
| (6) Henriksen et al.153 | 31 | 0 | 42 |
| (7) Walden et al.154 | 23 | 65 | 71 |
| (8) Jeans et al.155 | 190 | 49 | 66 |
| (9) Krepel et al.156 | 164 | 10 | 23 |
| (10) Samson et al.157 | 89 | 90 | 0 |
| (11) Murray et al.158 | 193 | 34 | 40 |
An ordinary least squares regression was performed to judge the association between the proportion of patients with CLI and the proportion with occlusions. None of the studies reported restricting its sample by lesion type, but based on clinical opinion (JAM) the values from study 10 seen highly implausible, so this study is excluded from the analysis. The logit of occlusions (which was taken as the outcome variable) was used, where the logit is defined as follows: ln[Occ/(1 – Occ)], where ‘Occ’ is the proportion with occlusions. The results are presented in Figure 34.
FIGURE 34.
Regression analysis of the association between the proportion of patients with an occlusion and the proportion with CLI. (a) With possible outliers [weighted by sample size (see table for numbers)]; and (b) without (excluding study 4).
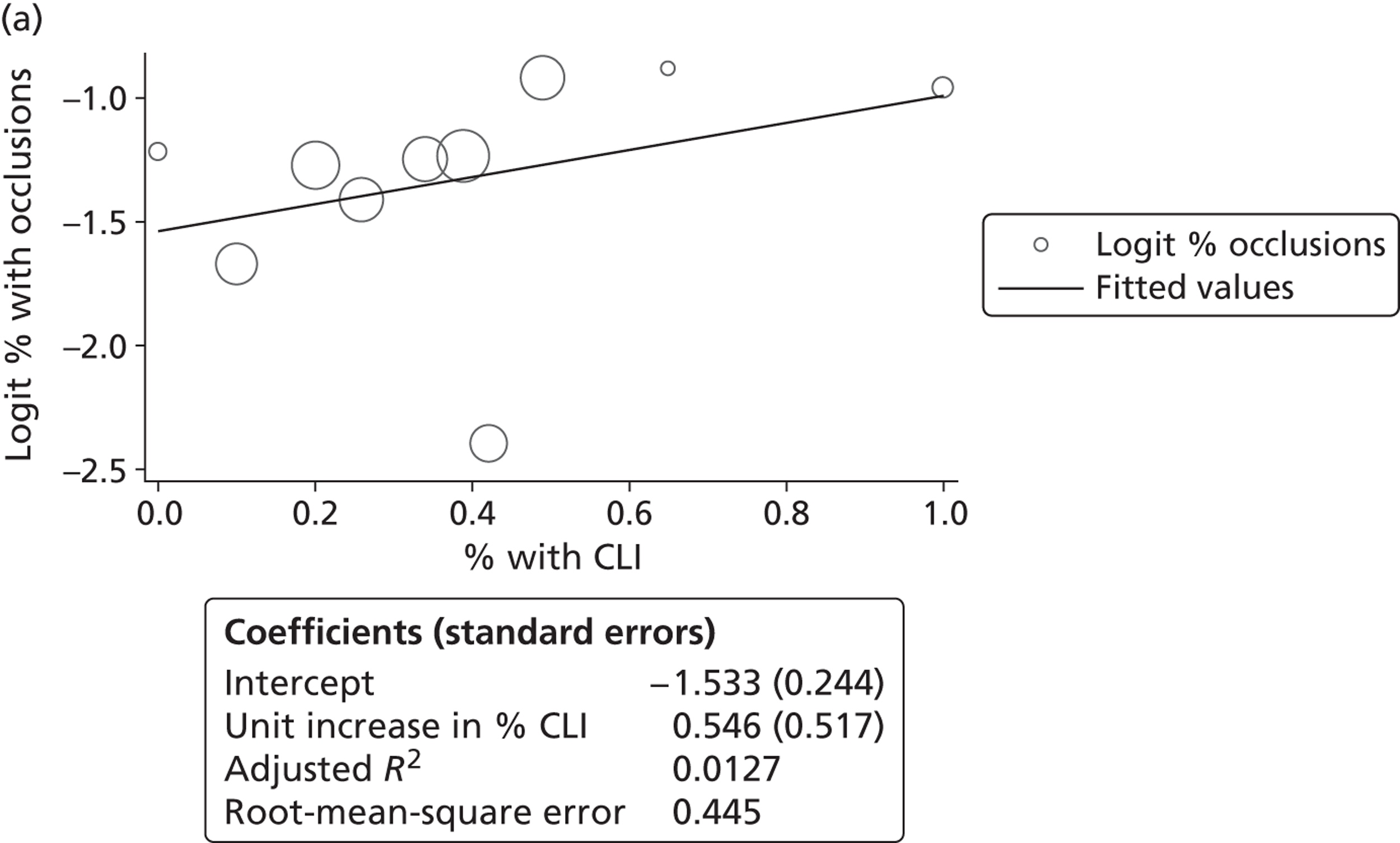
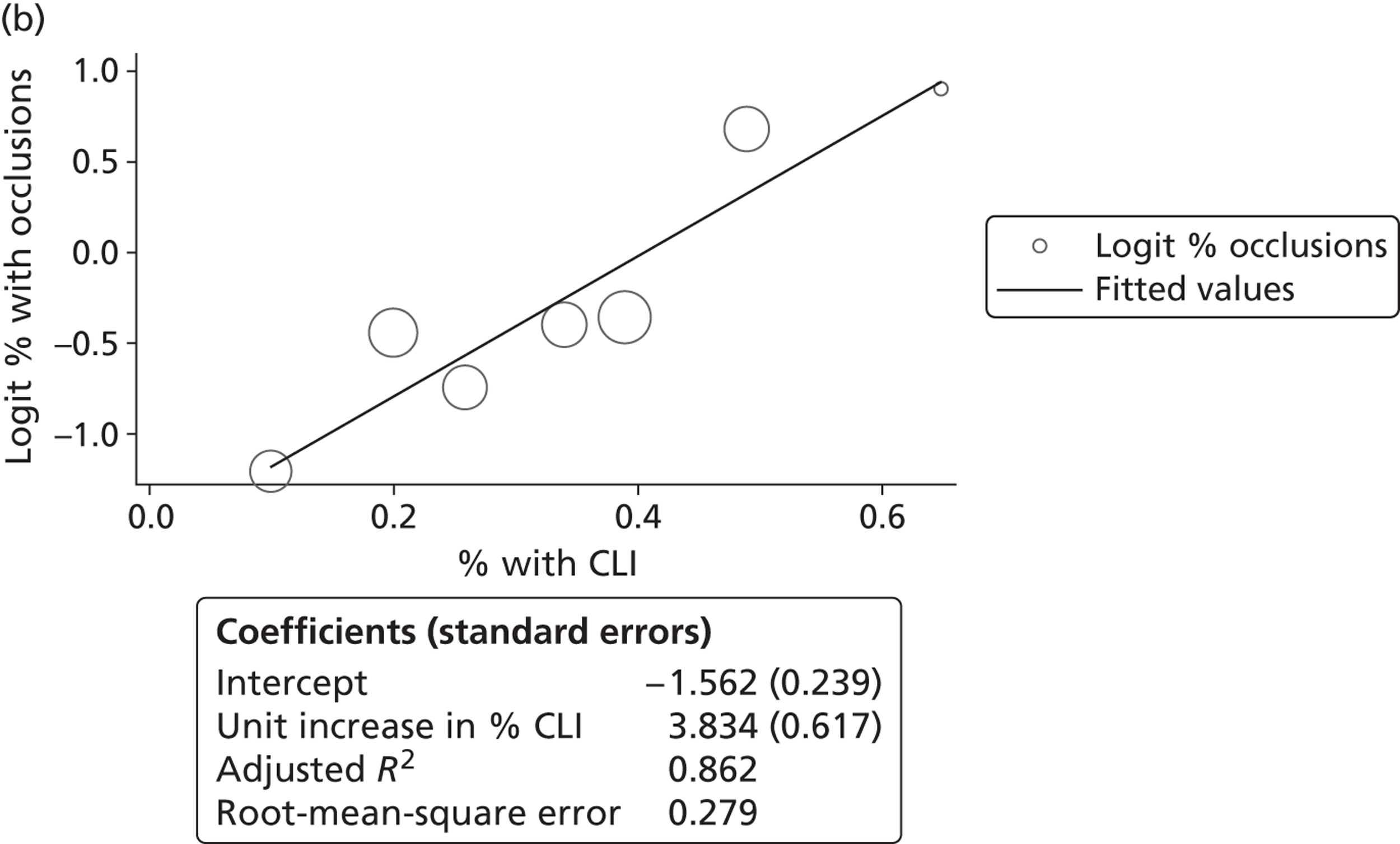
The initial results are shown on the left; they predict that CLI does not have a statistically significant association with the percentage of occlusions. Using this model, it is predicted that 17.8% of claudicants and 27.1% of patients with CLI will have occlusions. The value for CLI was felt by our clinical expert (JAM) to not be plausible. As the studies 4 and 5 were potentially outliers, the analysis was repeated omitting these, giving the results on the right. Using these it is predicted that among patients with IC, about 17.3% will have occlusions, with this value rising to 90.6% in the CLI population. These values are used in the base case.
In comparison, the NICE CEA83 (released after this analysis was performed) assumes that 20% of patients with IC will have occlusions, based on expert opinion. The value of 17.3% for patients with IC estimated in this report is used for consistency with the value used for patients with CLI.
To extrapolate beyond the 5 years presented by Hunink et al. ,77 parametric survival models were fitted to the data (the parametric models were used to predict failure after the first year). Both Weibull and log-Normal models were fitted; the model which resulted in the smallest sum of squared residuals was selected. For both IC and CLI, a Weibull model was selected. Details of the fitted Weibull models are presented in Figure 35.
FIGURE 35.
Weibull models used to predict failure. Conditional failure rates are conditional on surviving beyond year 1. Solid line = observed; dashed line = modelled (Weibull).
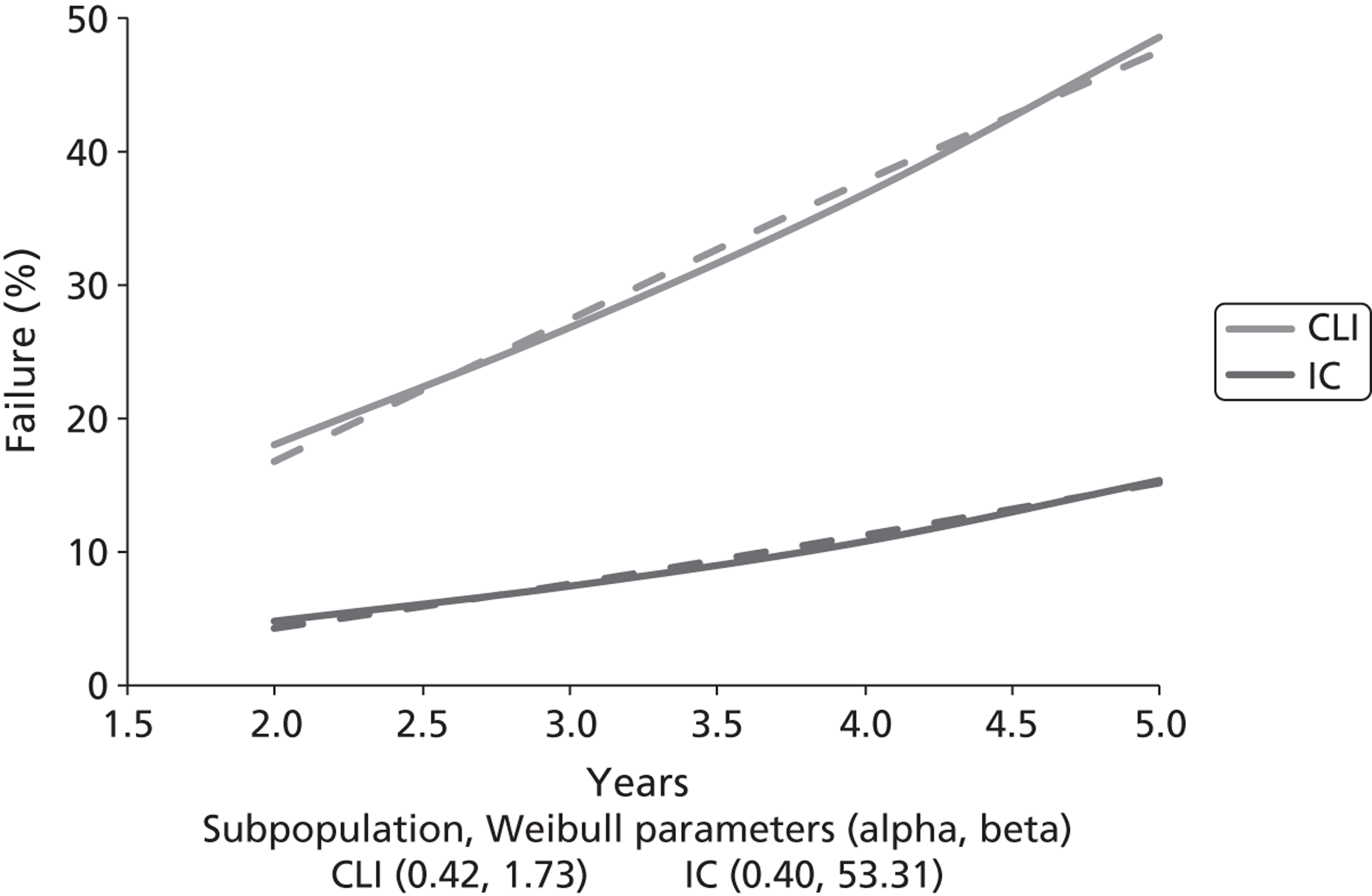
In comparison, in their economic evaluation Hunink et al. 77 state that for extrapolating failure beyond 5 years they use a constant rate (of failure), but this rate is not stated.
Complication during an operation
Hunink et al. ,77 the NICE CEA83 and the BASIL trial82 are the only economic evaluations to explicitly consider complications as a result of an operation. The values used by Hunink et al. 77 are also used by de Vries et al. 78
Both Hunink et al. 77 and the NICE CEA83 define a procedure-related complication as a non-fatal systemic complication (such as stroke, myocardial infarction and renal failure). Hunink et al. 77 use a value of 1.3%; the NICE CEA83 uses a rate of 2.4%.
Alongside their value of 1.3%, Hunink et al. 77 also use a range of 0.2% to 11%. In total, 14 studies are referenced, but it is unclear where the value of 1.3% comes from. The authors state that this value includes ‘major cardiopulmonary, renal or cerebrovascular complications’.
The NICE CEA83 value of 2.4% comes from an audit published by the Royal College of Surgeon’s of England in 2002. 91 This report includes a breakdown of the types of complication experienced; of 717 PTA procedures, 1 (0.14%) was a stroke or transient ischaemic attack, renal failure and myocardial infarction both occurred 5 (0.70%) times and the remaining 6 (0.84%) were bronchopneumonia.
The BASIL trial provides a detailed breakdown of the complications encountered during the perioperative period. 84 Following PTA (237 operations), an angina, myocardial infarction or stroke occurred 16 times, giving a probability of 6.75%. For BS, the probability is 12.18% (24/197).
Hunink et al. 77 use a base-case value of 8.5% for BS, along with a range of 2.7% to 13%. As with PTA, it is unclear where the base-case value comes from.
For BS, the NICE CEA83 apply a RR of 0.60. However, there are weaknesses with this value; it is based on a single study that reports a small number of complications (4/40 for PTA and 3/46 for BS), none of which is a systemic complication (as defined here). This value also contradicts both the Hunink et al. 77 evaluation and the BASIL trial,84 for which BS is associated with higher levels of complication.
The audit reported by the Royal College of Surgeon’s for PTA91 does not break down its results by indication. However, it does give the information that (excluding maintenance operations) 30.3% of PTAs were for CLI, with the remainder for IC. Applying the complication rate observed in the BASIL trial84 to the CLI population results in a complication rate of 0.51% for patients with IC. Applying the RR from the BASIL trial for BS (12.18/6.75 = 1.80) gives a complication rate of 0.92% for patients with IC.
As the BASIL trial84 and the Royal College of Surgeon’s audit91 both use patients in the United Kingdom, and both are relatively recent (since 2000), they are used together to derive the complication rates.
Effectiveness of reintervention
With the exception of the BASIL trial82, all the economic evaluations assumed that subsequent treatments were as effective as the initial treatment.
In the BASIL trial,84 the 12-month success rate for initial PTA was 49.54% (107/216), and for repeat PTA it was 69.23% (9/13). This difference is not tested by the authors, but it is not statistically significant (p = 0.168, two-sided test).
The authors did compare initial BS with BS following a failed PTA. They found that both amputation-free survival and overall survival were both statistically significantly lower in the latter group. However, it is unclear whether these differences are due to the procedures or due to differing patient characteristics. The 12-month success rate for initial BS was 56.41% (110/195), and for BS following failed PTA it was 45.65% (21/46). This difference is not tested by the authors, but it is not statistically significant (p = 0.188, two-sided test). Hence, it is assumed that subsequent PTA reinterventions are as effective, with regards to maintaining patency, as the initial PTA intervention.
Patency following BS is taken from the same meta-analysis used to derive patency following PTA. 98 Values for saphenous vein bypass are used as these were most commonly experienced in the BASIL trial (76%; 136/179). 84 The meta-analysis reported differences in patency by indication (CLI or IC), but not by lesion status (stenosis or occlusion) or by site (above or below knee).
Disease progression
Only Sculpher et al. 76 and de Vries et al. 78 specifically model the progression from IC to CLI after PTA failure. Sculpher et al. 76 use a monthly probability of 0.0029%, giving a yearly probability of 3.43% in the absence of any other events. de Vries et al. 78 use a 5-week probability of 6.2%. It is unclear whether this probability is applied as a one-off or every 5 weeks. If the latter is the case, then it gives a yearly probability of 48.61% in the absence of any other events.
The NICE CEA83 assumes that progression is independent of treatment, and uses a 5-yearly probability of 2%, giving a yearly probability of 0.4%. In addition, a yearly rate of 5.6% is used to model patients with IC whose symptoms deteriorate to the point where they require an operation.
The yearly probability of 3.43% from Sculpher et al. 76 is used because, of the three economic evaluations, the related assumptions employed by Sculpher et al. 76 are the most similar to the assumptions used in this analysis:
-
Sculpher et al. 76 are the only ones to assume that on failure the patient returns to their pre-operation health state, and that progression to CLI varies depending on whether or not the patient is patent. These two assumptions are also employed in this analysis.
-
The NICE CEA83 assumes that progression to CLI is independent of patency.
-
de Vries et al. 78 assume that on failure the patient is still asymptotic.
To model a yearly probability of 3.43%, an exponential distribution (mean: 28.65) is used.
Quality of life
An overview of the use of QoL values in the economic evaluations is presented in Table 83. In addition, two additional papers that were known to the authors are included.
| Study | Method | Health states and values |
|---|---|---|
| Hunink et al.77 |
|
|
| de Vries et al.78 |
|
|
| Holler et al.79 |
|
|
| Sculpher et al.76 |
|
|
| BASIL trial82 |
|
|
| NICE CEA83 |
|
|
| Dumville et al.159 | Report that QoL among patients with asymptomatic PAD is no different from QoL among patients without PAD | |
| Sprengers et al.160 |
|
CLI: 0.34 (0.24 to 0.44) |
In the following discussion, the values presented by Hunink et al. 77 are not used, as it was not possible to map the Torrance multiattribute scale to EQ-5D.
Baseline values
For patients with IC, Holler et al. 79 and Sculpher et al. 76 both elicit values of 0.7, whereas de Vries et al. 78 elicit a value of 0.71. This is considerably higher than the value used in the NICE CEA (0.573). A value of 0.7 is used in the base case, with the NICE CEA value (which is based on the average of the RCTs used in the evaluation) used in a scenario analysis.
There is more variation in the baseline values used for CLI: Sculpher et al. 76 elicit a value of 0.35 (with this value used by de Vries et al. 78 and the NICE CEA83), Holler et al. 79 elicit a value of 0.60 and the BASIL trial82 reports a value of 0.26. In this trial, 75% of patients had tissue loss; the remainder had rest pain.
As the value reported by Sculpher et al. 76 was also observed in the JUVENTAS trial (as reported by Sprengers et al. 160), and appears to reflect a similar decrement (relative to an IC value of 0.7) to that reported by Hunink et al. ,77 this value is used in the base case. In addition, the Sculpher et al. 76 values were elicited by members of the general public, and thus can be assumed to reflect patients with CLI without any comorbidities. In the BASIL trial84 comorbidities were generally high (with the prevalence of angina, previous myocardial infarction and previous stroke all being about 20%); this may be part of the reason for the lower observed EQ-5D values.
Values following successful treatment
Sculpher et al. ,76 de Vries et al. 78 and Hunink et al. 77 all assume that the values for asymptomatic patients are independent of the patients’ prior disease, although the last two evaluations apply a utility decrement for having major morbidity or complications.
Sculpher et al. 76 assume that patients move to full health (EQ-5D = 1), which does not seem plausible. Neither do the values presented by Holler et al. ,79 which suggest that QoL reduces following successful treatment. The NICE CEA83 suggests only moderate gains to QoL. However, there are also some possible problems with these values. They are taken from two sources, the RCTs of Greenhalgh et al. 104 and Spronk et al. 103 The increases in QoL modelled by NICE range from –0.005 to +0.061. In the Spronk et al. 103 RCT the increases observed range from +0.08 to +0.16, whereas in the Greenhalgh et al. 104 RCT they ranged from +0.042 to +0.088.
de Vries et al. 78 use a value of 0.79. This is based on patients with previous IC, and excludes those with severe comorbidities. The average age of the patients was 60. Population norms for the 50–59 and 60–69 years age groups are 0.798 and 0.774, respectively (median values 0.796 for both, Sullivan et al. 161), suggesting that following a successful operation patients’ QoL is comparable to that of the general population. This finding is supported by Dumville et al. 159
The values following treatment with PTA reported in the BASIL trial82 are much lower than those reported by de Vries et al. 78 This will be for two reasons; the BASIL trial82 participants had major comorbidities and the treatment failures are included in the values. For example, at 12 months, a value of 0.56 is reported. This is based on a failure rate (among those still alive) of approximately 19% (about 35/180). Assuming that failures have the pre-treatment value of 0.26, the value for successfully treated patients is about 0.63.
Values following amputation
Hunink et al. ,77 Sculpher et al. 76 and Holler et al. 79 provide three separate estimates of the QoL associated with amputation. Sculpher et al. 76 elicited values based on whether the amputation was above or below the knee, and combined these to obtain an average value for the QoL associated with an amputation by using a ratio of above : below knee amputations of 0.84 : 1. de Vries et al. 78 used the same EQ-5D values and ratio, and the NICE CEA83 used the same ED-5D values, but a ratio of 13 : 12.
The BASIL trial82 does not explicitly report EQ-5D values, but (based on an ITT analysis of all patients) reports that, from 3 months onwards, values are consistently lower by about 0.06 (regardless of initial treatment); this would give a 12-month value of about 0.5 (there was no statistically significant difference in post-treatment values between PTA and BS). Of those alive at 12 months, 28 had a below-knee amputation, and 13 an above-knee amputation (patients who progressed from below to above are only included in the latter count). Applying these proportions to the EQ-5D values reported by Sculpher et al. 76 results in a value of 0.48.
As the QoL following an amputation reported in the BASIL trial82 seems to be similar to that elicited by Sculpher et al. ,76 these values are used. As the ratio of above : below knee amputations used by the NICE CEA83 is not referenced, the values observed in the BASIL trial84 are used, giving a base-case value of 0.49.
It is noted that whereas Sculpher et al. 76 and the BASIL trial82 both report that QoL following an amputation is higher than baseline QoL with CLI, Hunink et al. 77 and Holler et al. 79 report that it is lower (by 21% and 13%, respectively).
The effect of systemic complication
Both de Vries et al. 78 and the NICE CEA83 assume that systemic complications have a multiplicative effect on QoL. The former use a value of 0.72 (based on survivors of myocardial infarction), whereas the latter use values between 0.629 and 0.880, depending on the type and timing of complication. Of the systemic complications observed by Axisa et al. ,91 myocardial infarctions were five times more likely to occur than a stroke, so only the effect of the former are considered in the model.
In the NICE CEA83 it is stated that the effect of myocardial infarction after the first year is based on an arbitrary reduction in the effect of 50% relative to the first year. The (constant) effect of myocardial infarction reported by de Vries et al. 78 is in between the first-year and subsequent-year effects used by the NICE CEA,83 so this effect is used at all time points in the model.
Costs
The NICE CEA83 values costs using the same perspective and time frame as this economic evaluation, so, where possible, costs are based on it.
Procedure-related costs
The costs stated include the subsequent hospital stay. Costs from the BASIL trial82 are not included, as they are not broken down into cost per procedure. Other sources of procedural costs are summarised in Table 84.
| Study (costs detail) | Procedural costs | ||
|---|---|---|---|
| PTA | BS | Amputation | |
| Sculpher et al.76 (1993/94 UK pounds) | 1186 | 2450 | 8106 |
| Hunink et al.77 (1999 US dollarsa) | 10,168 (18,171)a | 20,531 (25,881)a | 34,384 |
| de Vries et al.78 (1998 US dollarsa) | 4170 (13,940)c | 16,490 (26,260)c | 14,420 (7790)b |
| Holler et al.79 (euros, year not stated) | 2328 (3916)a | 5309 (7778)a | 4964 |
| NICE CEA83 (2009/10 UK pounds) | 3661 (9367)c | 5988 (7139)c | 9733 (14,044)c |
Both Hunink et al. 77 and Holler et al. 79 provide separate procedural costs for patients with IC and with CLI. In both of these analyses, it is assumed that the procedural cost is the same regardless of complication. Both the NICE CEA83 and de Vries et al. 78 provide separate procedural costs for whether or not the patient has a complication. This approach is used in the model; it is assumed that the difference in cost between IC and CLI patients modelled by Hunink et al. 77 and Holler et al. 79 is a result of patients with CLI having more complications.
Subsequent operations are assumed to cost the same as the initial operation (unless the patient has developed a complication). This is the same assumption as that used by all of the economic evaluations, apart from the NICE CEA83 for PTA, for which subsequent operations cost either £3695 (no complications) or £9385 (with a complication).
Any reinterventions are also assumed to be preceded by angiography. NHS 2009/10 reference costs89 are used, which price diagnostic angiography at £202 (no complications) and £5101 (with a complication).
Long-term costs
It is assumed that patent patients have no long-term costs. This assumption is also used by the NICE CEA,83 de Vries et al. 78 and Sculpher et al. 76 In contrast, Holler et al. 79 and Hunink et al. 77 do model long-term costs for patent patients.
Long-term costs for each of the health states (other than asymptomatic) are summarised in Table 85. Costs from the BASIL trial82 are not included, as no breakdown (by health state or patency status) is provided.
| Study (costs detail) | Yearly costs | |||
|---|---|---|---|---|
| IC | CLI | Amputee | Systemic complication | |
| Sculpher et al.76 (1993/94 UK pounds) | 180 | 648 | 744 | – |
| Hunink et al.77 (1999 US dollarsa) | 543 | 543 | 48,877 | 7764 |
| de Vries et al.78 (1998 US dollars) | 0 | 0 | 31,920 | 10,780 |
| Holler et al.79 (euros, year not stated) | 1044 | 2721 | 4964 | – |
| NICE CEA83 (2009/10 UK pounds) | 0 | 0 | Year 1: 28,270. After year 1: 23,502 | a |
With the exception of Sculpher et al. ,76 the reported long-term costs are based on empirical (observed) data. Sculpher et al. 76 assume that long-term costs for IC patients take the form of an outpatient appointment once every 3 months. For CLI patients, it is assumed that there is an outpatient appointment once a month and half-an-hour of a ‘Grade F’ (agenda for change 6; assume point 24) nurse’s time used every 2 weeks. This gives annual costs in 2009/10 UK pound of 1220 for IC and 3849 for CLI (it was not possible to update the costs for amputees).
For the base-case analysis, long-term costs for patients with IC or CLI are taken from the updated Sculpher et al. 76 values. After an amputation, a constant value of £23,502 is used for patients (the increased costs in the first year after an amputation are not modelled to keep the model simple).
As with QoL, it is assumed that any systemic complications are myocardial infarctions. Using the values reported by the NICE CEA,83 this costs £5395 in the first year and £1692 in subsequent years. It is assumed that the initial high cost of having myocardial infarction is captured by the increased cost of any intervention (including angiography) due to having a complication. Hence, for the model only the fixed yearly cost of £1692 is used.
Number of runs required for stable results.
Figures 36–39 present the average costs and QALYs by run number for both patient populations considered in this analysis. Numerical results for the standard errors of each estimate are as follows:
Costs: IC, £51.17 (mean: £14,637); CLI, £191.73 (mean: £55,199).
QALYs: IC, 0.0105 (mean: 5.956); CLI, 0.0066 (mean: 3.047).
FIGURE 36.
Average cost by run number for patients with IC.
FIGURE 37.
Average QALY by run number for patients with IC.
FIGURE 38.
Average cost by run number for patients with CLI.
FIGURE 39.
Average QALY by run number for patients with CLI.
Appendix 8 Protocol
ENHANCEMENTS TO ANGIOPLASTY FOR PERIPHERAL ARTERIAL OCCLUSIVE DISEASE (PAOD): SYSTEMATIC REVIEW, COST-EFFECTIVENESS ASSESSMENT AND EXPECTED VALUE OF INFORMATION ANALYSIS.
Decision problem
Purpose of assessment
The planned assessment is to answer the following research questions:
-
What is the clinical and cost effectiveness of additional techniques designed to improve the results of endovascular treatment (standard transluminal balloon angioplasty) for peripheral arterial disease?
-
In which of these techniques is further primary research likely to lead to information that will improve the effectiveness and cost effectiveness of care in this condition?
Definition of interventions
This assessment is of new endovascular techniques that may be used to either supplement or replace existing endovascular procedures to improve the circulation of the lower limb in cases of PAOD.
Place of the intervention in the treatment pathway
The techniques under consideration in this assessment will be those that are either used as a replacement for, or in conjunction with, conventional balloon angioplasty. These cover a variety of different clinical settings and subgroups (see below). In general, treatments will be considered that occupy the same place as balloon angioplasty in the treatment pathway for PAOD. There are however several different potential situations that may need to be considered separately, particularly in relation to the assumptions of an economic model:
A technique intended to be used as a replacement or adjunct in all primary procedures;
A procedure or device that is intended to be used selectively in a subgroup of patients based upon anatomical or radiological features or an inadequate response to the initial balloon procedure; Those procedures intended to be used in cases of restenosis or failure of the primary procedure. The specific place in the pathway will therefore need to be considered individually for each of the technologies, depending upon their intended use and the available evidence.
Excluded interventions
In order for the review to be practicable some limitations will be placed on the interventions and devices that will be considered.
Pharmacological interventions
The separate effects of pharmacological measures aimed at altering patency will not be specifically considered, except where the use of a particular agent is required as an integral part of a new endovascular technique.
Combined surgical procedures
Some new techniques, such as remote femoral endarterectomy, require a combined surgical and endovascular approach. Many of the others may also be combined with surgical procedures and, in some cases, may be used for different indications in patients who would not necessarily be amenable to conventional endovascular techniques. Inclusion would considerably extend the scope of the proposed reviews and require additional modelling.
These will therefore be excluded from the current review.
Other techniques
There are a number of other new endovascular techniques that may be used as an adjunct to angioplasty. These include closure devices, devices to protect from embolisation and techniques for thrombolysis or thrombectomy. These will only be considered where they are a component of one of the other techniques referred to above.
Relevant comparators
There are a large number of potential new technologies, many of which are mutually exclusive alternatives for the endovascular treatment of PAOD. The starting point for the evaluation will be direct comparisons with balloon angioplasty but where several treatments are appropriate to the same clinical subgroups mixed treatment comparisons will be carried out to compare all relevant technologies.
Population and subgroups
There are a number of different subgroups of population that may need to be considered separately within the review and modelling as they may have different clinical and economic implications. Subgroups will be identified where possible, within the published literature.
Modelling will include a consideration of appropriate subgroups as regards clinical presentation, anatomical site, demographic features and comorbidities. Several of these represent potentially important issues that will need to be addressed within the review.
Symptomatic presentation
Patients with PAOD may present either with intermittent claudication (pain on exercise) or with critical ischaemia which includes ulceration, gangrene and ischaemic rest pain. The Trans-Atlantic Inter-Society Consensus (TASC) has standardised the anatomical and symptomatic definitions of vascular disease, including the use of the Rutherford classification, which is often used to categorise the severity of ischaemia. The symptomatic classification has significant implications both for the appropriate treatment modalities and comparators and the likely outcome of treated and untreated disease. It is also closely related to the utilities associated with the relevant health states. It will therefore be necessary to consider separate subgroups within the review, and economic analysis will be based upon these factors.
Anatomical features
The outcome of endovascular treatment is also known to be heavily influenced by the site and distribution of arterial occlusive disease. Aortoiliac disease affects the larger vessels above the inguinal ligament. Conventional angioplasty, with or without the use of stents, has been common practice in this area for some years and clinical results are generally good with a lower rates of restenosis or reocclusion. In view of this, the potential advantages of new techniques to improve outcomes are likely to be very much smaller in absolute terms, with very large clinical studies being required to demonstrate significant clinical benefit. The current assessment will therefore focus on disease below the inguinal ligament.
The assessment will include all infrainguinal disease, but it is recognised that some technologies are used or designed specifically for certain areas within this and, where the evidence allows, subgroups will be considered separately for femoral, popliteal and infrageniculate disease.
Key factors to be addressed
The specific objectives of the review are:
-
To investigate by systematic review the effectiveness and cost effectiveness of endovascular techniques to supplement or replace balloon angioplasty in the infrainguinal arterial circulation (Review 1).
-
To investigate by systematic review the utilities associated with health states relating to the natural history of treated and untreated PAOD (Review 2).
-
To estimate the incremental cost effectiveness of the new technologies identified in Review 1.
-
To assess the potential value and optimum design for further research studies to collect data on areas of uncertainty identified by the above reviews.
Methods for synthesis of evidence
Description of reviews
Review stage 1: A comprehensive search will be undertaken to systematically identify clinical and cost effectiveness literature concerning endovascular techniques to supplement or replace balloon angioplasty in the infrainguinal arterial circulation.
Review stage 2: Where utility data are unavailable from studies identified in review stage 1, literature reviews will be conducted to provide data to populate the economic model. This will comprise data on the utilities associated with health states relating to the natural history of treated and untreated PAOD. This is likely to be necessary as it is expected that most published clinical research in this area will provide surrogate end points such as vessel patency or symptomatic and disease specific end points such as exercise tolerance, symptomatic state or amputation rates.
Identifying and systematic reviewing of clinical effectiveness evidence
Population
The population will be patients with symptomatic PAOD undergoing endovascular treatment for disease distal to the inguinal ligament.
Interventions
Clinical studies that evaluate techniques used as an adjunct to, or as a replacement for balloon angioplasty in the peripheral circulation. The identified procedures include but are not limited to those procedures identified in the inclusion criteria below.
Search strategy
The search strategy for both reviews will comprise the following main elements: searching of electronic databases; contact with experts in the field; scrutiny of bibliographies of retrieved papers. The electronic databases to be searched from inception will include MEDLINE; Medline in Process (for latest publications); EMBASE; Cochrane Database of Systematic
Reviews; Cochrane Controlled Trials Register; CINAHL; NHS EED, DARE, and HTA databases; NIHR Clinical Research Network Portfolio database; NRR (National Research Register) Archive; Web of Science Proceedings; Science Citation Index; Current Controlled Trials; Clinical Trials.gov; FDA website; EMEA website; and relevant conference proceedings. These will include the proceedings of the Vascular Society of Great Britain and Ireland, The European Society of Vascular and Endovascular Surgery, The British Society of Interventional Radiology, Cardiovascular and Interventional Radiological Society of Europe, The Society for Interventional Radiology and the Society for Vascular Surgery.
Searches will not be restricted by publication type, study design, date or language. In addition citations within relevant papers will be checked and hand searching of relevant journals, using the search strategy described by the Cochrane Peripheral Vascular Diseases Group, will be performed (Cochrane Collaboration 2006).
An initial draft search strategy based upon the identified technologies and relevant anatomical sites identified over 5,000 references. Standard methodological filters will be used to limit this to systematic reviews, randomised and controlled trials and cost effectiveness analyses. This is still expected to identify a large number of potentially relevant papers. Further limitation may be required to exclude papers referring to angioplasty at other sites. Limitation by publication date may also be necessary, but is likely to be different for individual technologies based upon expert advice regarding technological developments (see below).
Study selection
In both stages of the review citations will be imported into reference management software and screened for inclusion, based on inclusion/exclusion criteria below. Titles and abstracts will be examined for inclusion by one reviewer. Two reviewers will independently make decisions on inclusion of studies at full text stage and any discrepancies resolved by discussion.
Inclusion criteria
Interventions
Transluminal balloon angioplasty, self-expanding and balloon-expandable stent, drug eluting stent, drug eluting balloon angioplasty, percutaneous stent-graft insertion, laser angioplasty, atherectomy, cryoplasty, cutting balloon angioplasty, brachytherapy and external beam radiotherapy and other techniques used as an adjunct to, or replacement for, balloon angioplasty in the peripheral circulation.
Population
Adult patients with symptomatic PAOD suitable for endovascular treatment for disease distal to the inguinal ligament. Where data allows, patients with critical ischaemia will be considered as a separate group to those with only claudication. Other important subgroups will be identified from the included studies.
Comparator
Conventional balloon angioplasty. Other comparators will be considered if included interventions are specifically designed as alternatives to angioplasty for patients in whom conventional angioplasty has failed or is contraindicated, in which case the comparator will be current standard care as determined by the clinical evidence and expert advice.
Setting
Secondary care
Outcomes
Outcome measures will include: Disease-specific and generic measures of quality of life, exercise tolerance, pain (patient reported pain scores and analgesic use), limb salvage (for patients with critical ischaemia), walking distance (for patients with claudication), patency measures, need for reintervention, complications, costs.
Study types
According to the accepted hierarchy of evidence, randomised controlled trials and meta-analyses from systematic reviews will be searched initially, as they provide the most authoritative forms of evidence. If data are not available from these, other study types will be included.
Exclusion criteria
Interventions: Pharmacological interventions, combined surgical procedures, devices that have been withdrawn, such as older laser angioplasty devices.
Publication types: Studies which are only published in languages other than English; studies based on animal models; preclinical and biological studies; narrative reviews, editorials, opinions; and reports published as meeting abstracts only where insufficient details are reported to allow inclusion.
Data extraction and critical appraisal
Data will be extracted with no blinding to authors or journal. Data will be extracted by one reviewer using a standardised form. A standard proforma will be used and the data checked by a second reviewer. Discrepancies will be resolved by discussion, with involvement of a third reviewer when necessary.
Quality assessment will be subject to the types of studies identified but will be undertaken using appropriate and established tools, for example randomised controlled trials will be assessed according to criteria based on NHS CRD Report No.424
(http://www.york.ac.uk/inst/crd/report4.htm). The purpose of such quality assessment is to provide a narrative account of trial quality for the reader and, where meta-analysis is appropriate, inform potential exclusions from any sensitivity analysis.
Data synthesis
Prespecified outcomes will be tabulated and discussed within a descriptive synthesis. Where statistical synthesis is appropriate, meta-analysis will be conducted using fixed and random effect models, using RevMan software. If sufficient trials are available, a sensitivity analysis will be undertaken to see if the removal of poor quality trials affects the results.
Mixed treatment comparisons
If it is deemed appropriate a mixed treatment comparison will be undertaken to synthesise the direct and indirect evidence in a single network, and to provide an indirect comparison where head-to-head trials are not available.
Methods for synthesising cost effectiveness evidence
Identifying and reviewing published cost effectiveness studies The review above will be used to identify studies of cost effectiveness of balloon angioplasty and the new technologies. An economic search filter will be incorporated into the search strategy to identify relevant studies. Identified economic literature will be critically appraised and quality assessed using the critical appraisal checklist for economic evaluations proposed by Drummond et al (2005). Existing cost effectiveness analyses will also be used to identify sources of evidence to inform structural modelling assumptions and parameter values for the de novo economic model.
Development of a health economic model
A new economic evaluation of the cost effectiveness of technologies for the management of PAOD will be developed. Cost effectiveness modelling will take account of potential benefits and harms of the new treatment and will identify subgroups of patients based upon the anatomical, radiological, symptomatic and other features discussed above where the data allows this.
The primary outcome from the model will be an estimate of the incremental cost per additional quality-adjusted life-year (QALY) gained associated with the use of new technologies to improve outcomes, used alongside or as alternatives for conventional balloon angioplasty of the infrainguinal arteries. A lifetime time horizon will be used in order to reflect the chronic effects of arterial disease and the on-going risk of vessel reocclusion, symptomatic deterioration, amputation and potential mortality. The perspective used will be that of the National Health Services and Personal Social Services. Costs and QALYS will be discounted at 3.5% as recommended in current guidelines. Modelling assumptions will be taken from the literature, supplemented by clinical expert opinion where required.
The ScHARR modelling team have published papers using different modelling techniques (such as discrete event simulation, transition state modelling and meta-modelling). The model structure and software used to construct the model will be determined following data collection in order that the most appropriate technique is used for this particular assessment. The expert advisory group will be consulted at the conceptual stage to ensure that the structure of the model is appropriate to clinical practice.
Ideally, health related quality of life evidence will be available directly from the review of the literature. In the absence of such evidence, the mathematical model may use indirect evidence on quality of life from alternative sources. Quality of life data will be reviewed and used to generate the quality adjustment weights required for the model. In addition to the reviewed literature, national sources (e.g. NHS reference costs, national unit costs, British National Formulary (http://bnf.org)) and manufacturers’ list prices will be used to estimate unit costs for use in the economic model. Where data on resource use associated with the new technologies are not available from the literature, advice will be sought from the expert advisory panel in the first instance. If uncertainty remains regarding the resources required for specific procedures, arrangements will be made for a member of the research team to observe and record the resource use associated with the procedures.
It is anticipated that there may be limited evidence for some of the parameters that will be included in the economic model. Therefore, the uncertainty around the parameter estimates will be modelled to take this into account. The uncertainty in the central value for each required parameter will be represented by a distribution, enabling probabilistic sensitivity analysis to be undertaken. This will allow an assessment of the uncertainty to be made.
Value of information techniques will be undertaken within the work. The expected value of perfect information (EVPI) will be explicitly calculated. EVPI is defined as the maximum investment a decision-maker would be willing to pay to eliminate all uncertainty from the decision problem. It is initially calculated in terms of a defined unit (typically per patient) and then multiplied by the number of people expected to benefit from eliminating all uncertainty to form an estimate of total EVPI. EVPI per person is relatively high where there is large uncertainty in the adoption decision; conversely where there is only a small probability of error and the impact of an incorrect decision is small the EVPI per person will be relatively low.
Depending upon the resources required more complex methodologies (the expected value of partial perfect information (EVPPI) and the expected value of sample information (EVSI)) may be undertaken. EVPPI differs from EVPI as it evaluates the maximum value of removing all uncertainty in one, or a subset of parameters, but it is more computationally expensive as it requires two nested Monte Carlo sampling levels.
EVSI is more advanced methodology for determining the value of information, which explicitly takes into account that uncertainty will not be removed even with large sample sizes. The EVSI methodology simulates the results from the proposed research and synthesises the simulated data with prior knowledge to form a posterior distribution: the larger the trial size the more the posterior distribution resembles the simulated data which is then used in probabilistic sensitivity analyses. The optimal trial size from the options evaluated can then be estimated based on the costs of conducting the trial and the expected net benefit of the sampled information. The application of EVSI is becoming more widespread and case studies employing this methodology have been published.
Glossary
- Dominated (simple)
- When an intervention is less effective and more expensive than its comparator.
- Meta-analysis
- A statistical method whereby the results of a number of studies are pooled to give a combined summary statistic.
- Posterior distribution
- A representation of the knowledge associated with the true value of a population parameter after combining the prior distribution with sample data.
- Prior distribution
- A representation of the knowledge associated with the true value of a population parameter in addition to any sample data.
- Relative risk
- The ratio of the probability of an event occurring in an exposed group relative to a non-exposed or control group.
List of abbreviations
- ABPI
- ankle–brachial pressure index
- ABSOLUTE
- randomized balloon angioplasty versus stenting with nitinol stents in the superficial ankfemoral artery
- AMS
- absorbable metal stent
- AMS INSIGHT
- bio-absorbable metal stent investigation in chronic limb ischaemia treatment
- BES
- balloon-expandable stent
- BMS
- bare-metal stent
- BS
- bypass surgery
- CB
- cutting balloon
- CEA
- cost-effectiveness analysis
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CLI
- critical limb ischaemia
- DCB
- drug-coated balloon
- DES
- drug-eluting stent
- DESM
- discrete-event simulation model
- EBRT
- external beam radiotherapy
- EQ-5D
- European Quality of Life-5 Dimensions
- EQ-VAS
- EuroQol visual analogue scale
- ESC
- European Society of Cardiology
- EVBT
- endovascular brachytherapy
- EVPI
- expected value of perfect information
- FAST
- Femoral Artery Stenting Trial
- FemPac
- Femoral Paclitaxel trial
- IC
- intermittent claudication
- ICER
- incremental cost-effectiveness ratio
- ITT
- intention to treat
- LEVANT I
- the Lutonix paclitaxel-coated balloon for the prevention of femoropopliteal restenosis trial
- MACE
- composite outcome for adverse events including death, stroke, myocardial infarction, revascularisation, embolisation in treated limb, worsening of 1 + Rutherford category
- NICE
- National Institute for Health and Care Excellence
- PAD
- peripheral arterial occlusive disease
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PTA
- percutaneous transluminal balloon angioplasty
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- QVA
- quantitative vessel analysis
- RCT
- randomised controlled trial
- RESILIENT
- randomised study comparing the Edwards self-expanding LifeStent with angioplasty alone in lesions involving the superficial femoral artery and/or proximal popliteal artery
- RR
- relative risk
- SES
- self-expanding stent
- SF-36
- Short Form questionnaire-36 items
- SF-8
- Short Form questionnaire-8 items
- SIROCCO
- SIROlimus-Coated COrdis self-expandable stent trial
- TASC
- Trans-Atlantic Inter-Society Consensus
- THUNDER
- local taxane with short exposure for reduction of restenosis in distal arteries
- TLR
- target lesion revascularisation
- TTO
- time trade-off
- TVR
- target vessel revascularisation
- VARA
- VAscular RAdiotherapy trial
- VascuCoil
- intracoil femoropopliteal stent trial
- VSGBI
- The Vascular Society of Great Britain and Ireland