Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 07/25/02. The contractual start date was in February 2009. The draft report began editorial review in November 2012 and was accepted for publication in May 2013. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Jeff Breckon has delivered consultancy and training services on behalf of Sheffield Hallam University relating to motivational interviewing in health care.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Goyder et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Regular physical activity is associated with reductions in all-cause mortality, risk of cardiovascular disease and some types of cancer. 1–3 Frequent exercise has a role in both the prevention and the management of hypertension, type 2 diabetes and obesity as well as a variety of mental health conditions. 4–6 However, most of the UK population do not attain levels of physical activity sufficient to confer such benefits. 7 For the last 10 years, a key Department of Health policy objective has been encouraging the population to undertake a total of at least 30 minutes of at least moderate intensity physical activity on 5 or more days of the week. 8
Brief interventions delivered in primary care can increase physical activity levels and are recommended as effective and cost-effective interventions by the National Institute for Health and Care Excellence (NICE). 9,10 However, the evidence base largely consists of studies with short-term follow-up post intervention and that use self-reported increases in physical activity by trial participants as a primary outcome. Maintenance of recommended physical activity levels is understood to be essential to achieve the reported health benefits. As a result, NICE identified that further research was warranted on the long-term sustainability of such treatment effects. 10 At the time, the few studies that had followed participants over the long term suggested that approximately half of those who initiate a physical activity programme relapse and return to their previous sedentary lifestyle within 6 months. 11
In the last 6 years, since the publication of the original NICE guidance in March 2006 recommending the use of brief interventions in primary care to encourage physical activity,10 a number of additional primary studies and relevant systematic reviews have been published. These recent reviews of effectiveness and cost-effectiveness evidence are further discussed in Chapter 8 (see Other recent evidence for the effectiveness and cost-effectiveness of ‘brief’ and ‘booster’ interventions for increasing and sustaining physical activity). The availability of further evidence on the use of brief interventions has led to the initiation of a programme to update the original NICE guidance and this new guidance was published in May 2013. 12
In the meantime, the Sheffield physical activity booster trial was funded and undertaken to address one of the major gaps in the evidence base identified by the original NICE guidance. 10
The booster trial was designed to evaluate the effectiveness and cost-effectiveness of motivational interviewing (MI) ‘booster’ interventions to help previously sedentary people maintain recently increased physical activity levels acquired following a brief intervention. MI13 is one of the behaviour change interventions recommended by NICE for health promotion. 14
The brief intervention involved provision of an interactive DVD based on a MI approach that is directive, is person centred and replicates the style of other successful behaviour change programmes and was underpinned by the theoretical construct of self-determination theory. 15,16 All interventions were delivered by trained facilitators whose competence was independently assessed using a treatment fidelity framework to ensure consistent delivery. 17,18
Chapter 2 Methods
Methods for the main trial
This report is concordant with the extension of the Consolidated Standards of Reporting Trials (CONSORT) statement to improve the reporting of pragmatic trials. 19 An internal pilot trial, conducted between May 2009 and March 2010 and focusing on feasibility outcomes, is reported elsewhere. 20 The final protocol can be found in Appendix 1 and a table of changes made to the protocol over the course of the project is presented in Appendix 2.
Participants
To identify potentially eligible study candidates we worked with NHS Sheffield (Sheffield Primary Care Trust), the health service organisation responsible for commissioning health services for the local population (until April 2013). Between May 2009 and June 2011 NHS Sheffield sent letters with postage-paid reply cards to 70,388 people inviting them to enrol in a programme to help them become more physically active. Six general practices were also given a total of 305 marked recruitment packs to distribute. These packs were identical to those sent from NHS Sheffield with the exception that the covering letter was not personalised. An unknown number of packs were also given to four community centres to be distributed by health trainers and health champions. Mail-outs and responses were allocated to output areas (OAs) according to the National Statistics Postcode Look-up table [November 2010 version; see http://geoconvert.mimas.ac.uk/help/documentation/10nov/nspd-version-notes-november-2010.pdf (accessed 21 November 2013)]. The response rate for each OA was calculated as the total number of allocated responses divided by the total number of allocated mail-outs. We performed a post hoc analysis to investigate whether response rate was related to population transience. Transience rate was calculated using information obtained from the 2001 census (small area statistics, Table CAS008), specifically the outgoing population divided by the outgoing population plus the static population. 21 An unweighted Spearman’s rank correlation test between response rate and transience was performed in R version 2.15.0. (R Foundation for Statistical Computing, Vienna, Austria; see www.R-project.org/).
The physical activity programme involved a ‘brief intervention’ combining an interactive DVD and area-specific written information about local facilities and opportunities for physical activity. The DVD was developed by a team at Sheffield Hallam University using MI principles, which are consistent with NHS guidance on physical activity and behaviour change interventions. 10,14 The content and development of this DVD are provided more fully in Appendix 1.
Research assistants (RAs) telephoned respondents and administered the Scottish Physical Activity Questionnaire (SPAQ). 22 Those eligible to receive the brief intervention (DVD and information sheet) were (1) residents of the 55 most economically deprived neighbourhoods in the city of Sheffield (out of 100), (2) those aged 40–64 years and (3) those not achieving the recommended activity level (30 minutes of moderate activity on at least 5 days) assessed using the SPAQ and wishing to have support to become more active. RAs telephoned those sent a DVD 3 months later to assess their eligibility for participation in the trial. Eligible candidates (4) had increased their physical activity level by at least 30 minutes of moderate or vigorous activity per week (assessed using the SPAQ) over the 3-month brief intervention (DVD) period and (5) were capable of giving written informed consent for trial participation. Individuals with chronic conditions who could benefit from physical activity were not excluded unless their condition significantly impaired their ability to exercise.
The validated SPAQ contains a series of questions assessing physical activity behaviour over the previous 7 days and also asks whether or not this level of weekly activity is typical. When observed weekly activity was reported as atypical, SPAQ asks participants to clarify what constitutes a more typical week in terms of an increased or reduced number of minutes of physical activity. Before and after the brief intervention period the RAs were asked to interpret the observed minutes of physical activity (as assessed using SPAQ) and to include any additional minutes of activity that were felt to be typical for study candidates who reported that the previous week had been atypical.
Interventions
Candidates who were assessed as eligible during the telephone assessment described in the previous section were invited to attend a baseline assessment at a community venue. Those who consented were randomly allocated (see Randomisation and blinding) to one of three groups:
-
a ‘full booster’ group receiving two face-to-face physical activity consultations provided in a MI style, 1 and 2 months after randomisation
-
a ‘mini booster’ group receiving two telephone-based physical activity consultations provided in a MI style, 1 and 2 months after randomisation
-
a control group who received no intervention after randomisation.
Both booster interventions are fully described in the study protocol, which can be found in Appendix 1. The full booster involved two face-to-face consultations, intended to last between 20 and 30 minutes, which took place in community venues. The consultations replicated a brief MI method designed for time-limited consultations in medical settings and which had already been successfully employed to change health-related behaviours. 23,24 During the full booster consultations, strategies were worked through at a pace dictated by the participant and the menu used to structure information exchange without being prescriptive.
The mini booster involved two physical activity MI consultations delivered by telephone, each intended to last approximately 20 minutes. The telephone consultations followed a script of known efficacy that has been implemented in previous physical activity promotion studies delivered by members of this research team. 25,26 It is thought that telephone counselling can provide an alternative to face-to-face contact that is relatively inexpensive in time and financial terms. 14 In previous studies in adult populations, telephone-based approaches have increased physical activity participation at 6 months compared with no telephone support and to a greater extent than standard reading materials. 27,28
Objectives
The primary objective of the main study, a parallel-group randomised controlled trial (RCT), was to determine if physical activity assessed using an Actiheart device (CamNtech Ltd, Cambridge, UK) 3 months after randomisation (6 months after a brief intervention) increases in participants allocated to two intervention groups (receiving two booster physical activity consultations, delivered in a MI style, either by telephone or face to face) compared with participants allocated to a control group (receiving no further contact after the baseline assessment). Secondary objectives were to:
-
determine whether physical activity 9 months after randomisation (12 months after the brief intervention) is significantly increased in participants allocated to the two intervention groups compared with participants allocated to the control group
-
compare physiological measures of fitness (12-minute walk test29) and self-reported physical activity (SPAQ instrument) between allocated groups
-
compare health-related quality of life (HRQoL), resource use (including health and social care contacts) and economic costs between allocated groups
-
investigate whether the impact of the intervention may be modified by gender, ethnicity or the types of physical activity undertaken (including use of community facilities for physical activity)
-
undertake a process evaluation to identify, using both quantitative and qualitative methods, psychosocial and environmental factors that may mediate or modify the effectiveness of the intervention.
Outcomes
Table 1 shows the timing of assessments and interventions, all made at community venues. The primary end point was the level of physical activity measured at 3 months post randomisation using Actiheart [specifically, the mean total energy expenditure (TEE) in kcal per day over a 1-week period]. Secondary end points were:
-
objective measures of physical activity including:
-
TEE in kcal per day from 7-day accelerometry and heart rate monitoring using Actiheart (at 9 months)
-
physical activity counts (PACs) per week
-
minutes of moderate/vigorous physical activity per day
-
meeting the current physical activity recommendation of at least 30 minutes per day (continuous or in bouts of at least 10 minutes of at least moderate intensity) for at least 5 days a week (yes or no)
-
-
self-reported moderate or strenuous physical activity using the SPAQ, which records type and duration of activities in the previous week
-
HRQoL using the Sheffield version of the 16-item Short Form health survey instrument (SF-12v2 plus 4)
-
self-reported use of community facilities for physical activity
-
self-reported health and social care contacts (see Methods for the health economic analysis for the analysis plan)
-
self-determination using the Behavioural Regulation in Exercise Questionnaire (BREQ-2)30
-
body weight and height [to allow calculation of body mass index (BMI)]
-
physiological measure of fitness (12-minute walk test).
| Assessment/intervention | Minus 3 months | Minus 2 months | Minus 1 month | ∼ Minus 1 week | Baseline | 1 month | 2 months | 3 months | 9 months |
|---|---|---|---|---|---|---|---|---|---|
| Brief intervention screening checklist | ✓ | ||||||||
| SPAQ | ✓ | ✓ | ✓ | ✓ | |||||
| Brief intervention questionnaire 1 | ✓ | ||||||||
| DVD (if eligible) | ✓ | ||||||||
| DVD usage assessment/advice | ✓ | ✓ | |||||||
| Booster trial screening checklist | ✓ | ||||||||
| Participant information sheet | ✓ | ✓ | |||||||
| Participant consent form | ✓ | ||||||||
| BREQ-2 | ✓ | ✓ | ✓ | ||||||
| Booster trial questionnaire 2 | ✓ | ✓ | ✓ | ||||||
| Questionnaire 3 (SF-12v2 plus 4) | ✓ | ✓ | ✓ | ||||||
| Height and weight | ✓ | ✓ | ✓ | ||||||
| Randomisation | ✓ | ||||||||
| Booster intervention (booster groups only) | ✓ | ✓ | |||||||
| 12-minute walk test | ✓ | ✓ | |||||||
| 7-day accelerometry | ✓ | ✓ |
The primary outcome was measured at 3 months post randomisation whereas the secondary outcomes were measured at 3 and 9 months post randomisation.
A decision was made to abandon the analysis of self-reported physical activity based on a questionnaire adopted from the HTA-funded Exercise Evaluation Randomised Trial (EXERT) trial. 31 This was because completion errors remained high even after repeat training of those administering it. The decision to abandon the use of this form was made in consultation with the trial steering committee and the HTA programme manager and the EXERT team and is fully documented elsewhere. 32
Sample size
The sample size was originally based on a primary outcome that was subsequently superseded by the use of Actiheart, that is, a physical activity measure based on the mean physical activity levels from the 7-day accelerometric assessment (recorded as counts per week) at 3 months post randomisation (6 months after initial contact). Before progression to the main trial, an internal pilot was undertaken to estimate the variability of the outcomes and the minimum clinically important difference (MCID) based on one-third of the standard deviation (SD) of the primary outcome from the observed data and power estimation (conditional on the initial proposed sample size of 600 subjects). From the feasibility phase, the estimated effect size based on one-third of the SD was 34,464.7 PACs per week and 101.5 kcal per day TEE. 32 When re-estimating the sample size using data from an internal pilot study the revised sample size estimate either stays the same or increases (it cannot be less than the original estimate). 33,34
Assuming a mean difference in TEE of 101.5 kcal per day between the intervention group and the control group as the smallest difference of clinical and practical importance that is worth detecting, then with 450 subjects (300 intervention, 150 control) the trial was originally determined to have 92% power to detect this mean difference or greater between the ‘booster’ arm and the control arm (assuming a SD of 304.6 kcal per day) as statistically significant at the 5% (two-sided) significance level using a two independent samples t-test. With 300 subjects in the booster intervention (150 mini booster, 150 full booster) the trial would also have had approximately 82% power to detect a similar mean difference in TEE of 101.5 kcal per day between the two booster arms as statistically significant at the 5% (two-sided) significance level using a two independent samples t-test. Assuming an approximate 25–35% loss to follow-up by 3 months post randomisation, we proposed to recruit and randomise 200 subjects per intervention group to give a total sample size of 600 participants, giving the study power of between 87% and 92% to detect a mean difference in TEE of 101.5 kcal per day.
Randomisation and blinding
A Sheffield Clinical Trials Research Unit (CTRU) statistician, not on the trial team, used a simple randomisation procedure to generate the randomisation sequence, with each participant having a one-third probability of being allocated to one of the three intervention arms. We used a block size of 200 with no stratification. Eligible participants were randomised to one of the three arms using a central web-based randomisation service delivered by Sheffield CTRU after patient eligibility and informed consent were confirmed by a RA. Participants and outcome assessors were not blind to treatment allocation because of the practical nature of the intervention. However, the primary outcome was objectively assessed using the Actiheart device. Most other outcomes were self-reported. Study statisticians and the principal investigator were blinded to the treatment allocation codes until after the final analysis.
Statistical methods
Analysis population
The intention-to-treat (ITT) data set included all participants who were randomised according to randomised treatment assignment (ignoring anything that happened after randomisation, including non-compliance, protocol deviations and withdrawals); participants also had to have a valid 3-month post-randomisation Actiheart accelerometry measurement of physical activity.
A valid Actiheart accelerometry measurement was defined as having at least 4 complete days (of the 7 days) of measurements of physical activity. We used the ‘auto-fill’ option on the Actiheart to minimise the amount of missing data. The ‘auto-fill’ option fills the gaps, of up to 2 hours, with the average value calculated from the recorded portion of the same day. 35 If the device identified > 2 continuous hours of missing data then, even using the ‘auto-fill’ option, this was classified as an incomplete day. When data were obviously missing because the participant had taken the accelerometer off to sleep, imputation of ‘sleeping’ values was employed by using the mean values during sleeping times. The decision about which data were missing as a result of ‘sleeping’ was made by study team members blind to the treatment allocation. After discussion with the trial management group and steering committee, incomplete days were classified as having > 1000 minutes (16.7 hours) of lost or missing activity per day as measured by the Actiheart. Although the manufacturers assert that there should be no issue with missing data, few studies report the majority of participants returning complete data sets. A brief review of published studies shows that a ‘complete day’ of Actiheart data is typically considered to be 500–600 minutes of recording. 36,37 Although many studies do not report the thresholds used, in the absence of advice from the manufacturers or definitive studies to determine the optimal threshold, researchers must determine an appropriate cut point to maximise both data validity and participant inclusion.
In addition to the ITT set, two other data sets were analysed as part of the sensitivity analysis. A complete cases data set was a subset of the ITT data set that included randomised participants with all 7 complete days of physical activity measurements at 3 months post randomisation. A per-protocol data set was defined as including those who received the intended two booster sessions (either face to face or by telephone) among participants in the booster intervention arm. Participants who did not receive the booster sessions as intended were excluded from this data set. Additional data sets were also analysed for the primary outcome as part of the sensitivity analysis assuming different missing mechanisms using regression and multiple imputation approaches.
Handling incomplete days or missing daily counts measurements
Exploratory analysis of potential risk factors associated with not having evaluable data (at least 4 complete days of Actiheart data) was undertaken using logistic regression. Spaghetti plots stratified by intervention arm were also used to explore the missing pattern of the primary end point with respect to the measurements during days of the week. To achieve 80% reliability with respect to activity counts and time spent in moderate to vigorous activity in adults, at least 3–4 days (of the 7 days) of activity monitoring are required. 38,39 In this regard, the primary outcome measure (mean TEE in kcal per day) was calculated as the mean TEE over 7 days among those with at least 4 complete days of evaluable Actiheart data.
The primary method to deal with missing data on the secondary outcome, activity counts per week, was to scale up complete observed daily measurements to 7 days using the following formula:
where PAC7i is the new standardised physical activity measurement for 7 days for participant i. The number of PACs per day was calculated by multiplying PACs per minute by 1440 (24 hours in a day multiplied by 60 minutes in an hour). This approach was used for patients who have at least 4 complete days measured after imputation using ‘auto-fill’ and ‘sleeping’ time as described earlier.
Statistical analysis
Multiple logistic and linear regression was used to compare the baseline characteristics [i.e. gender, ethnicity, employment status, age, BMI, weight, height, SF-12v2 plus 4 physical component summary score (PCS), SF-12v2 plus 4 mental component summary score (MCS), Relative Autonomy Index (RAI) of the BREQ-2, and SPAQ change scores] of the completers (≥ 4 days of valid Actiheart data at 3 months post randomisation) and non-completers (< 4 days). An interaction term was included in the regression model to see whether the characteristics of the completers and non-completers were different between the booster and the control groups. The purpose was to explore whether the missing data mechanism was related to the intervention or whether there are observed characteristics that might predict whether or not a randomised participant would have valid and complete Actiheart data at 3 months post randomisation.
For sensitivity analysis, multiple imputation was used to obtain a complete data set for the primary outcome by filling incomplete daily measurements. Twenty multiple imputation data sets were created and we imputed, at most, 3 incomplete days of the week per participant (among those with at least 4 complete days). The multiple imputation model took into account participants’ baseline characteristics (such as age, gender, weight, height, HRQoL and BMI) and longitudinal time sequence as well as total physical activity at baseline and 3 months before randomisation. In addition, for participants with at least 1 complete day of the outcome measure, multiple imputation was also used to impute the missing daily measurements as part of further sensitivity analysis using the same multiple imputation model as described above but imputing at most 6 incomplete days of the week per participant.
Baseline characteristics
The baseline demographic characteristics and physical measurements were summarised and assessed for comparability between the booster and the control arms. 40–42 Age, weight (mass), height (stature), BMI, SPAQ change score, BREQ-2 RAI dimension score and SF-12v2 plus 4 PCS and MCS scores were presented on a continuous scale. For these continuous variables, summary statistics such as the minimum, maximum, mean, SD, median and interquartile range (IQR) were presented. Numbers of observations used with number and percentages in each category are presented for categorical variables (e.g. gender, marital status, ethnicity and stage of change). Summary statistics are presented by treatment group and assessed for comparability. No statistical significance testing has been carried out to test baseline imbalances between the intervention arms but any noted differences are descriptively reported. 43,44
Data completeness
Reporting of data completeness is an integral part of clinical trial reporting. Hence, summaries of data completeness are shown on a CONSORT flow chart from participants’ enrolment, during follow-up and at the end of the trial. Data completeness is based on the primary outcome (mean TEE in kcal per day) and having a valid measurement at 3 and 9 months post randomisation.
Effectiveness analyses
The primary aim was to compare the intervention group (full or mini booster) with the control group (no booster). The primary comparison was between the mean physical activity levels from the accelerometer (mean TEE in kcal per day) in the two ‘booster’ arms combined compared with the mean physical activity levels in the control arm at 3 months post randomisation. This difference in means between the intervention group and the control group was compared using a two independent samples t-test; a 95% confidence interval (CI) for estimated mean difference between the groups was also calculated and reported with its associated p-value. The research hypothesis was that the booster intervention groups will have greater levels of physical activity than the control group. In all of the analyses the control group was treated as the reference for comparisons.
An adjusted analysis using multiple regression was also conducted to estimate the effect of the intervention adjusted for baseline covariates (such as age, gender, HRQoL, BMI and SPAQ at baseline and 3 months before randomisation). The ordinary least squares adjusted regression coefficient for the intervention effect was presented and reported with its associated 95% CI and p-value.
A secondary objective of the study was to compare the effect of the two interventions (full booster vs. mini booster) at 3 months post randomisation using the primary outcome, mean TEE per day. Therefore, we repeated the above analysis to compare the effects of the full and mini booster interventions.
The analysis outlined above for the primary outcome was also repeated for the main secondary outcome, PACs per week at 3 months post randomisation, and for the TEE and PAC outcomes at 9 months post randomisation.
Analysis of secondary outcomes
The following continuous secondary outcome measures were assessed at 3 and 9 months post randomisation:
-
PCS, MCS and Short Form questionnaire-6 Dimensions (SF-6D) scores from the SF-12v2 plus 4
-
average minutes per day spent on moderate activity [3–6 metabolic equivalents of task (METs)]
-
average minutes per day spent on vigorous activity (> 6 METs)
-
average minutes per day spent on moderate and vigorous activity (≥ 3 METS)
-
BREQ-2 dimensions (amotivation, external regulation, introjected regulation, identified regulation, intrinsic regulation, RAI)
-
BMI
-
distance walked (in minutes) during a 12-minute walk test.
Mean outcomes were compared between the combined booster groups and the control group using two analysis of covariance (ANCOVA) multiple regression models: a simple model that adjusted for the baseline value of the outcome only and a more complex model that adjusted for several covariates including age, gender, BMI, total minutes of physical activity at 3 months and 1 week before randomisation and the baseline outcome measurement. The mean differences in outcomes between the groups and their associated 95% CI and p-value from the two models were reported.
Secondary binary categorical outcomes were the number and proportion maintaining (or increasing) their weekly duration of physical activity (based on the self-reported SPAQ) and the number and proportion meeting the current recommendations of at least 30 minutes of moderate physical activity (MET level ≥ 3) on at least 5 days of the week. We compared these outcomes between the booster groups and the control group at 3 and 9 months post randomisation using a continuity-corrected chi-squared test; 95% CIs for the estimated differences in proportions between the booster groups and the control group were also calculated. 45
Gender, ethnicity and access to community facilities (self-reported use of community facilities vs. no use of community facilities) were predefined as subgroups that we wished to test for evidence of effectiveness in an exploratory analysis. An additional post hoc exploratory subgroup evaluation was undertaken to assess the impact of the timing of the initial mail-out (summer/spring vs. winter/autumn). The exploratory subgroup analysis used multiple linear regression with the primary outcome, the mean TEE (per day) levels from the Actiheart at 3 months post randomisation, as the response. We used an statistical test for interaction between the randomised intervention group and the subgroup to directly examine the evidence for the treatment effect of the combined booster groups varying between subgroups. 43,46,47 Subgroup analyses were performed regardless of the statistical significance of the overall intervention effect (booster vs. control). The model below was used to assess the interaction:
A graphical plot of mean profile subgroups with intervention group was used to display the interaction effect. 48
Methods for the process evaluation
The aim of the process evaluation was to (1) assess how acceptable and appropriate participants found the intervention and (2) identify psychosocial and environmental factors that may modify the effectiveness of the intervention. The study contained two components: (1) a survey by postal questionnaire, incorporating closed and open questions, and (2) a semistructured interview conducted individually face to face or over the telephone, depending on the preference of each participant.
Our methodological and theoretical approach is that adopted by Snape and Spencer,49 characterised by a subtle realism, interpretivism and pragmatism: we understand our subject matter through participants’ contextually situated perspectives; we strive for neutrality and objectivity during data collection and analysis and we attempt to be as transparent as possible as we move beyond the data during interpretation to serve the needs of policy-makers.
Survey
Survey questionnaires were sent to participants before their 3-month research assessment and they were asked to completed the questionnaire before the assessment and return it in a sealed envelope. If the survey was not completed participants were asked if they would be willing to complete it at the 3-month research assessment, after which the participant sealed it in an envelope and handed it back to the RA. Because of delays in regulatory approvals, questionnaires were sent out from April 2010 only and the first 47 randomised participants were not invited to complete it.
The survey questionnaire asked participants about the type and location of physical activity that they had undertaken during the previous 3 months, reasons for staying physically active, factors that influenced their physical activity behaviour and social support from significant others. The versions of the questionnaire sent to participants in the full and mini booster arms of the trial also contained questions on the intervention received. Participants were asked why they chose to participate; their preferred format for such an intervention; their expectations of the intervention and the extent to which these were met and whether they found the intervention easy, convenient, non-judgemental and non-confrontational. Participants were also asked their opinion about the amount of contact time with the project worker, the extent to which they felt encouraged to set their own goals for physical activity and the extent to which they felt that the intervention had helped them to resolve their barriers to physical activity, expand their knowledge of physical activity, increase their awareness of local facilities and opportunities and increase their confidence to stay active. Finally, participants were asked whether they had become more physically active than they were before participating and what had helped them achieve this. The questionnaire sent to those in the full booster arm of the trial can be found in Appendix 4. The questionnaires distributed to the mini booster and control arms are available from the team on request.
In-depth interviews
Those receiving a booster intervention who also responded to the survey questionnaire (see Survey) were given the option of participating in an in-depth interview. Because of the poor response there was no scope for purposive sampling; as a result, we interviewed a sample comprising all of the 26 people who volunteered. We did not elicit reasons for declining a research interview. Three RAs performed the interviews: Andrew Hutchison PhD (male) and Kimberly Horspool MSc and Sue Kesterton MSc (both female). KH and SK had both studied qualitative research techniques as part of their MSc but were novice interviewers. AH was more experienced having conducted a number of qualitative research interviews as part of his doctoral research. None of the interviewers delivered the intervention to the interviewees but interviewers may have been involved in collecting baseline data for the RCT component from some interviewees. Interviewees would have known that interviewers were on the research team and were from Sheffield Hallam University and may have associated them with exercise science and delivery of the intervention. The interviewers were asked to withhold their own opinions and to make it clear that this interview was separate from the intervention motivational interviews. No field notes were taken and no repeat interviews were undertaken.
Semistructured interviews lasted between 9 and 32 minutes (median 21 minutes) and were conducted over the telephone or face to face in a quiet room at a community venue, according to each participant’s choice. For most interviews no one was present except for the participant and the researcher. In one case a participant chose to conduct the interview on a mobile phone and, for part of the time, in a public place.
A topic guide was provided to interviewers (see Appendix 5); this was not pilot tested. This guide included questions on participants’ levels, choice and prioritisation of physical activity as well as the benefits and costs associated with staying physically active. It also included questions on participants’ experiences of the booster sessions and why they had or had not helped them to stay active and why participants felt that the booster sessions were or were not a good way to give them the support that they needed.
Interviews were digitally recorded and transcribed verbatim. Transcripts were not returned to participants for comment or correction. Daniel Hind conducted the initial data analysis in NVivo version 10 (QSR International, Southport, UK) using a constant comparative method to identify themes. We used a ‘framework’ approach to analysis in which a priori and emergent themes were identified using the following stages: familiarisation, identifying a thematic framework, indexing, mapping and interpretation (charting was not undertaken). 50 For instance, a theme of a priori interest was the perceived effectiveness of the booster sessions; subthemes within this category were derived inductively from familiarisation with the transcripts. 50,51 The results were used to explore insights into the mechanisms that may have contributed towards the quantitative findings and to identify any other emerging issues or factors that may have influenced the uptake of the boosters and which had not previously been documented. 52 Data saturation was achieved53 with no substantively new themes emerging in the last 10 interview transcripts. Participants were not asked to provide feedback on the themes.
Having indexed transcripts using our own thematic framework, we undertook a rapid review of the literature to find existing frameworks to evaluate dimensions of (1) prior conditions experienced by the participants; (2) barriers to and facilitators of adoption of new behaviours or technologies; and (3) the acceptability/appropriateness of the interventions. For prior conditions (see Chapter 4, Prior conditions) we used dimensions described by Rogers (p. 172). 54 We adopted, with modifications, the dimensions of the Motivators of and Barriers to Health-Smart Behaviors Inventory, developed by Tucker and colleagues55 (see Chapter 4, Barriers to physical activity and Physical activity: motivators). Our dimensions of intervention acceptability are based on those described by Nastasi and Hitchcock56 (see Chapter 4, Motivational interviewing: perceived effectiveness, Motivational interviewing: consistency with perspectives or world views, Motivational interviewing: perceived feasibility and Motivational interviewing: perceived importance).
Methods for the fidelity study
Background
Although a small number of studies assessing the efficacy of physical activity counselling have reported the content, frequency and duration of training of those delivering the physical activity counselling intervention, the majority do not, and it is not uncommon for most clinical trials to fail to even report the content of the counselling intervention. 57,58
Although physical activity counselling based on MI has been rolled out across the UK through the Let’s Get Moving education programme,59 it remains unclear whether those delivering the training and those delivering the intervention to patients are doing so according to the approach intended. This failure to embed assessments of competence has raised questions over the value of short-term workshops with little or no ongoing supervision and professional practice reflection. It is clear that programmes such as this offer a potentially valuable additional education framework but few studies are currently being published that have clearly assessed the fidelity of those delivering the intervention.
It has therefore been suggested that behavioural interventions should clearly report (1) the content of or elements of the intervention, (2) the characteristics of those delivering the intervention, (3) the characteristics of the recipients, (4) the setting [e.g. Physical Activity Referral Scheme (PARS)], (5) the mode of delivery (e.g. face to face or by telephone), (6) the intensity and contact time (e.g. number of sessions) and (7) participant adherence to delivery protocols. 60 The booster trial embedded these principles into its design along with treatment fidelity frameworks intended to provide standardisation of the behavioural intervention without losing innovation and flexibility within the two intervention arms. 17,18 Furthermore, the action planning and maintenance phases in both experimental arms used behaviour change techniques, as recommended by Michie and colleagues,60 which are thought to enhance self-regulation towards change. The assessment of the existing competence and subsequent training of those delivering the MI interventions was evidence based and built on recent reviews of training in MI. 61
Motivational interviewing content and delivery
The MI component was delivered by RAs trained by a member of the Motivational Interviewing Network of Trainers and followed existing frameworks for MI in physical activity contexts. 62,63 The key phases and content of the intervention are provided in Table 2 and follow the phases of MI. 64 The relational aspect (or ‘spirit’) of MI is pivotal to the approach and emphasises participant ‘autonomy’ as opposed to ‘imposing authority’; ‘evocation’ rather than ‘education’; and ‘collaboration’ instead of ‘confrontation’. Once underpinned with the relational approach, the technical skills of MI were delivered, which included open-ended questions, affirmations, reflective listening and summarising. 13 Those delivering the intervention received 6 days of formal training over the first 12 months of the study in addition to follow-up supervision for the remainder of the study using audio recordings for reflective feedback.
| MI content | MI phase |
|---|---|
| Opening exchange/agenda setting | Engagement |
| Decisional balance | Focusing |
| Importance of change (agreed target behaviour) | Evoking |
| Readiness to change (agreed target behaviour) | |
| Action planning | Planning |
| Maintenance phase | Maintenance |
Treatment fidelity assessment and methods
To ensure that the criteria for treatment fidelity were met, those delivering the MI physical activity booster interventions (RAs) were assessed for their competence in delivering MI before and throughout the intervention period. The assessment of practitioner competence used was the Motivational Interviewing Treatment Integrity (MITI) assessment. 65 Minimum practitioner levels will be based on the levels of ‘competence’ as stated in the MITI coding system. To account for practitioner competence ‘drift’ (post training), follow-up reviews of practitioner MI competence were carried out at appropriate intervals (approximately every 9 months).
The MITI assessment was used to measure the interventionist application of key facets of MI, which included ‘global ratings’ of evocation, collaboration, autonomy/support, direction and empathy. In addition, ‘behaviour counts’ were recorded, which included giving information, MI adherent behaviours (e.g. asking permission, affirming, emphasising personal control), MI non-adherent behaviours (e.g. advising, confronting, directing), open compared with closed questions and simple and complex reflections. The calculations for MITI were based on existing standards,65 as seen in Table 3.
| Clinician behaviour count or summary score thresholds | Beginner proficiency | Competency |
|---|---|---|
| Global clinician ratings (average) | 3.5 | 4.0 |
| Reflection to question ratio (R : Q) | 1 | 2 |
| Per cent open questions (% OQ) | 50 | 70 |
| Per cent complex reflections (% CR) | 40 | 50 |
| Per cent MI adherent (% MIA) | 90 | 100 |
The relationship between motivational interviewing fidelity and levels of physical activity: statistical methods
We employed analysis of variance (ANOVA) to test the null hypothesis that physical activity measured by mean TEE at 3 months was the same across all of the RAs who delivered the MI intervention. We plotted the means of mean TEE with their associated 95% CIs stratified by the RA who delivered the MI intervention to show how physical activity varies across RAs ranked by their global proficiency ratings. A further ANOVA model was fitted with RAs with the same global proficiency rating grouped together. We dropped from the analysis RAs who delivered very few sessions. In addition, for the few sessions in which MI was delivered by two RAs, we allocated the session to the RA who delivered more sessions.
Methods for the geographical information systems study
Aim
The aim of this substudy was to explore whether access to green space and leisure facilities influenced the effectiveness of the intervention. We used network distance analysis to produce a set of variables for each of the 282 trial participants, which represents his or her pedestrian access to municipal green space and any relevant leisure facilities.
Network distance analysis
Euclidean (straight line) distance is the simplest measure of distance between two points. However, in a city it is rarely possible to follow a straight line between two points. Moreover, rivers, railway lines and sometimes roads force pedestrians to take routes that may deviate considerably from a straight line. To gauge the realistic walking distance between two points in a city, it is necessary to build a network representation of the pedestrian-accessible routes within the city and surrounding area. The shortest route between any two points on the network can then be calculated mathematically.
The most labour-intensive step is creating the network. The Integrated Transport Network™ (ITN) in OS MasterMap® [Ordnance Survey, Southampton, UK; see www.ordnancesurvey.co.uk/oswebsite/products/os-mastermap/itn-layer/index.html (accessed 10 October 2012)], can be used for this purpose, but unfortunately it is not possible to download a single portion of the ITN covering all of Sheffield and the surrounding area. Instead, we downloaded the data for this area from OpenStreetMap (see www.openstreetmap.org). In a certain respect, OpenStreetMap is actually more useful to us than the ITN; it is created (in part) by local volunteers, walking on foot and recording data with global positioning system (GPS) devices, and therefore provides information on pedestrian access that is more detailed than a typical OS map.
Roads and footpaths, etc. in OpenStreetMap are stored in a simple vector format that makes it relatively easy to extract the information required to build a network. The only problem is that the labelling of pedestrian accessibility for roads (especially trunk roads) is not consistent and is occasionally inaccurate. It was therefore necessary to manually exclude the following sections of road:
-
A61 Dronfield bypass
-
Sheffield Parkway
-
Mosborough Parkway
-
A57 (section linking Mosborough Parkway to the M1)
-
A616 Stocksbridge bypass
-
Park Square roundabout
-
Tinsley roundabout and Tinsley viaduct.
Crossing points for non-pedestrian roads, for example bridges and underpasses, are well detailed in OpenStreetMap.
Although the shortest distance between points within the network can be calculated very accurately, there is the potential for error when calculating the network distance between features that do not lie within the network. Unfortunately, the centroids of postcode areas, and the polygons representing municipal green space boundaries, do not lie within the network. Here it is necessary to spatially ‘join’ these features to an appropriate point on the network. This is best done manually; however, because of the large amount of work that this would require it was necessary to automate the joining process, as follows.
Each postcode centroid was linked to the closest point on the network and the Euclidean distance between the centroid and the network point added to the network distance.
Each green space polygon was linked to all network points falling inside it, with the minimum network distance to any of these points deemed to be the minimum network distance to the polygon itself. As pedestrian entry points to green space are well detailed in OpenStreetMap, this means that the network distance mostly takes the entry points into account.
The major limitation of the automated joining process is that sometimes the network point that the feature is linked to is not always the most appropriate. For example, a postcode area may include adjacent houses that face onto different streets so that an address may be attributed to a network point on a street from which there is actually no access to the property. Similarly, for green space, points on the network outside the green space polygon may sometimes be closer to the actual entry points than those inside the polygon. This can result in network distance errors of ≥ 100 m, in some cases substantially more if the difference in network distance between the automated choice of point and the optimum choice of point is large. Fortunately, very large anomalies appear only in rural areas where the road network is sparse and therefore were not a significant concern in this analysis.
Green space measures
All municipal green spaces in Sheffield were considered, excluding certain types of land use inappropriate for exercise. Sheffield City Council classifies these spaces as ‘city’, ‘district’ or ‘local’ according to their catchment areas. We used ‘city’ as a proxy for high-quality space and ‘district’ as a proxy for medium-quality space in terms of attractiveness. Local spaces were too ubiquitous for a meaningful spatial analysis. Of municipal green spaces belonging to other authorities, only Rother Valley Country Park was included (designated high quality).
Digitised boundary data for municipal green spaces in Sheffield were provided by the Sheffield City Council Parks and Countryside team. As well as boundary information, these data included a classification of the usage and catchment area categorisation of each space. These are explained fully in a Sheffield City Council Parks and Countryside report66 but are summarised here as follows:
-
Usage type:
-
parks
-
gardens
-
sports sites (e.g. tennis courts)
-
playing fields
-
playgrounds
-
playground/open space
-
woodlands
-
moorland/heathland
-
open spaces
-
allotments
-
churchyards/cemeteries
-
other site types – specified:
-
golf courses
-
farms
-
show grounds
-
depots
-
ancillary sites to other leisure facilities (e.g. car parks).
-
-
-
Catchment area categorisation:
-
city wide
-
district (up to 1.3 km)
-
local (up to 0.4 km).
-
We decided to disregard the following usage types: playgrounds (because our participants are middle-aged), allotments (as nearby allotments are relevant only to the minority of local residents who rent them), churchyards/cemeteries (less likely to be appropriate places for exercise), farms, depots and ancillary sites.
Inclusion of the catchment area categorisation information is more problematic than inclusion of usage type as it is not clear on what research, if any, it is based. However, disregarding the categorisation is equally problematic as ‘local’ municipal green space is ubiquitous. For example, our preliminary study considered all municipal green spaces in Sheffield of the appropriate usage type and the size of a football pitch or greater and found very few postcodes in the city that were further than 1 km from such a space, with roughly 50% falling within 500 m and roughly 30% falling within 300 m. This tallies with the findings of Barbosa and colleagues. 67 Also, most distances > 500 m fell in the most affluent parts of the city, which were not included in the booster trial (this also tallies with the findings of Barbosa and collegaues67). Because of random errors inherent in our network distance measurements (see Network distance analysis), such small network distances would contain an unacceptable amount of uncertainty. It was also felt that it was important for us to take some account of the attractiveness of municipal green spaces.
Therefore, we decided to make use of the catchment area categorisation as a proxy for the quality/attractiveness of the space, using an ordinal scale in which citywide represents the highest quality, district represents medium quality and local represents the lowest quality. Disregarding all green spaces with unsuitable usage types (see earlier) we measured the shortest network distance to:
-
high-quality municipal green spaces of appropriate types
-
high- or medium-quality municipal green spaces of appropriate types.
We excluded Sheffield’s three municipal golf courses; these have citywide catchment areas but this is clearly based on their attraction as a leisure facility (as defined in leisure facility measures) offering golf, rather than as a green space, and the booster participant questionnaire responses indicated that very few participants played golf.
One potential problem with using the green space data for Sheffield is that many of the booster participants live on the edge of the city and could be using municipal green spaces belonging to surrounding authorities (Barnsley Metropolitan Borough Council, Rotherham Metropolitan Borough Council, Derbyshire County Council or North East Derbyshire District Council). This was a matter of sufficient concern for us to request green space boundary data from these authorities. However, because of the green belt around Sheffield, there are actually few important municipal green spaces belonging to these authorities that are close to the borders of Sheffield. The exception to this is Rotherham, with the two conurbations forming a continuous urban area. However, this area is heavily industrialised and there are few important municipal green spaces. One very important exception is Rother Valley Country Park, which borders south-east Sheffield; a boundary polygon for this park was added manually to the set of green spaces having a citywide catchment area.
All green space measures in this study are shortest distance measures. So far we have decided not to use a gravity model for green space as the need to include an arbitrary distance decay parameter would be a potential source of bias. However, this remains an option for future work. Regarding the more sophisticated floating catchment area gravity model, this may actually be inappropriate for green space: unlike a capacity-constrained service such as a health practitioner, a green space that is heavily used (and thus full of people) may be more attractive and more likely to be perceived as appropriate for recreational exercise or as a walking route than one that is less well used.
Leisure facility measures
Leisure facilities, as distinct from green spaces, are defined here as places where a physical activity is facilitated by some kind of organisation, usually in return for payment. For example, we define a tennis club as a leisure facility but an unsupervised tennis court within a park as part of a green space. Similarly, a publicly accessible playing field is considered a green space rather than a leisure facility, unless it is part of a sports club. Data on leisure facilities are easier to collect than data on green spaces because leisure facilities can be treated as point locations. Even though these facilities may cover a large area, there is usually an office where people must go to pay, and this is typically also the building to which the address of the facility relates. Therefore, noting the limitations of geocoding accuracy explained in Network distance analysis, it is credible to use the postcode centroid of the facility’s address as a point location.
The chief problems in collecting data on leisure facilities were twofold:
-
deciding which types of leisure facility were relevant to the booster participants
-
ensuring that all leisure facilities of these types, located within a reasonable distance of the study area, were included.
To address the first point we had the benefit of the questionnaires completed by the booster participants (see Outcomes and Survey). These data suggest that the main physical activities relevant to the participants were walking, gardening, swimming and gym-based activities. Walking relates to municipal green space rather than leisure facilities and gardening relates to neither, so we considered only leisure facilities offering a gym and/or swimming.
To address the second point we used the Sport England online ‘active places’ database,68 which provides an authoritative list of sports facilities, with each facility listed as offering one or more types of activity. Two of these types of facilities, ‘health and fitness suite’ and ‘swimming pool’, correspond directly to gyms and swimming respectively. The postcode of each facility is also included on the database, as is an indicator of how the public can access the facility (those open only to sports clubs or community associations, rather than individuals, were excluded). For each facility postcode we calculated the network distance to every other postcode within Sheffield, and for each Sheffield postcode we recorded the shortest network distance to:
-
a unisex gym
-
a female-only gym
-
a swimming pool.
As with green space, we did not implement any of the more sophisticated ‘gravity’ models for leisure facilities. The basic gravity model is additive, meaning that having three gyms a 500-m network distance from one’s home would be scored three times better than having one gym 500 m away. This is clearly inappropriate as gyms are often paid for on a membership basis, and it is unlikely that a participant would join several gyms. Although swimming may be offered on a pay-per-session basis more frequently than gym facilities, it is still questionable whether having three local pools is exactly three times better than having one local pool.
Here, the floating catchment area gravity model is potentially more useful than the basic gravity model as it links the usefulness of local facilities to local demand. 69 A gym may be less desirable if it is frequently crowded and one has to wait to use particular exercise machines, and the same may also apply to lane swimming in pools. In this context having multiple local facilities can be beneficial, as greater provision of facilities means that they are less likely to be crowded. Unfortunately, the problem with interpreting this model is determining local demand; although the size of the local population can be easily obtained from census data, people often choose leisure facilities close to where they work rather than where they live, so using population data could be misleading, particularly in the city centre.
Given these issues, and for consistency in the analysis methods, we have used straightforward minimum distance analysis.
With leisure facilities there is also the issue of affordability. Some facilities are expensive and may not be realistically accessible to the more deprived communities targeted by the booster recruitment strategy. However, pricing information is not included in the Sport England database and it was not possible within the timescale of this project to obtain pricing information separately for each facility. Restricting the analysis to municipal facilities was considered but in Sheffield some privately run leisure facilities are less expensive than municipal ones. It is therefore important to recognise that affordability may be an additional barrier to accessing local leisure facilities, even when they are geographically close, which this analysis does not address.
Statistical methods
We constructed crude scatter plots of mean TEE per day (kcal) at 3 months against potential moderators generated from the geographical information systems (GIS) analysis and other continuous baseline potential moderators to explore any univariable linear relationships. In addition, we stratified these plots by intervention arm to explore whether the univariable linear relationships were consistent within the intervention arms. Univariable linear regression models were fitted with amount of physical activity measured by mean TEE per day (kcal) as the response and potential moderator as an explanatory variable to explore whether a potential moderator was a predictor of physical activity at 3 months.
To explore the moderation effect, we fitted multivariable linear regression models on the same response variable with intervention group, potential moderator and interaction between treatment group and potential moderator as explanatory variables (as given by Equation 3). Plots of the fitted regression line stratified by booster treatment group were constructed to explore any potential interactions. In addition, hypothesis testing on the interaction term was also undertaken using Equation 3 to test the moderation effect of GIS variables on physical activity. Variables were classified as being moderators of physical activity if they had a significant interaction effect with treatment at 3 months.
Methods for the health economic analysis
Introduction
As part of the trial data collection, participants answered questions on their use of NHS facilities at 3 months and 9 months post randomisation. Participants also completed the SF-12v2 plus 4 HRQoL questionnaire, allowing SF-6D HRQoL scores at 3 and 9 months to be produced. 70 Together with an estimate of the intervention cost, such data allow a simple estimate of the effect of the intervention on mean HRQoL and average cost to be produced by comparing costs and utility scores at the end of the intervention with those before the intervention.
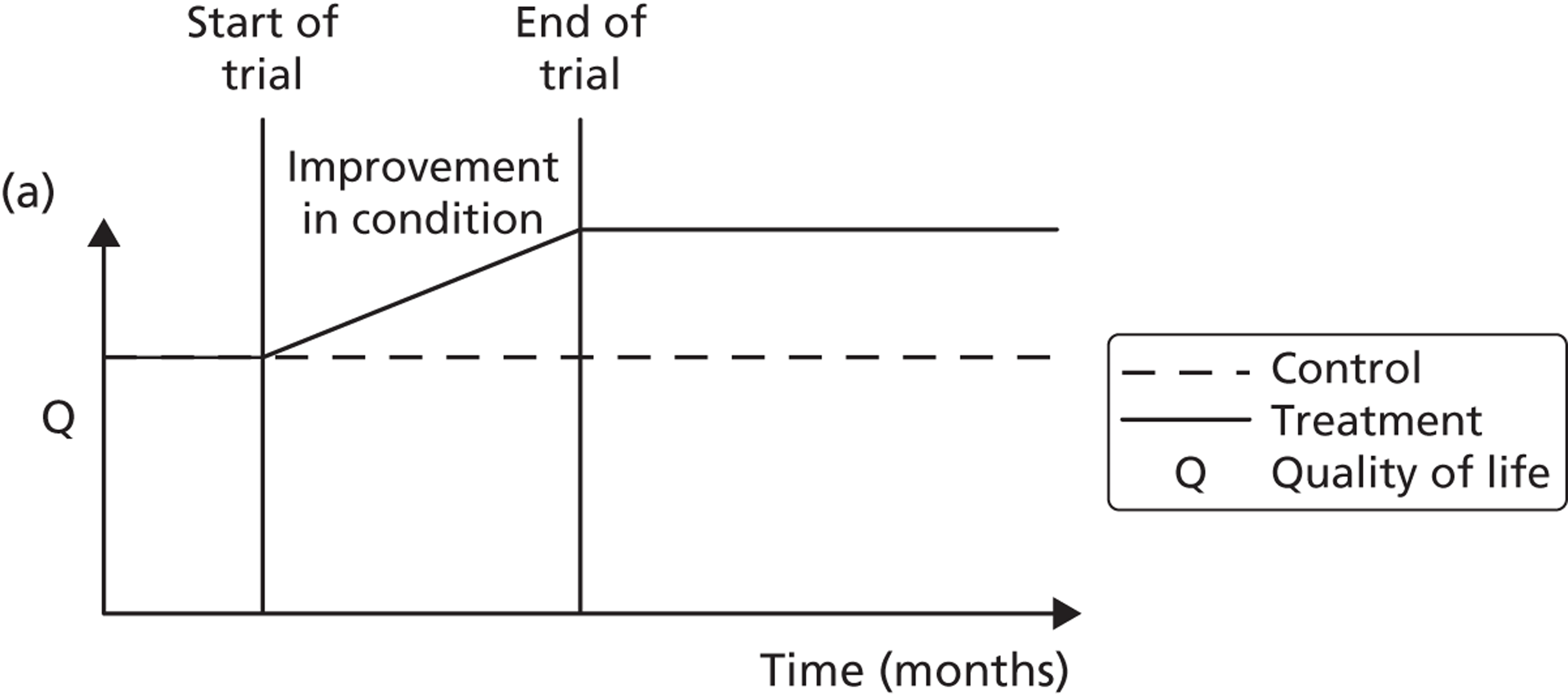
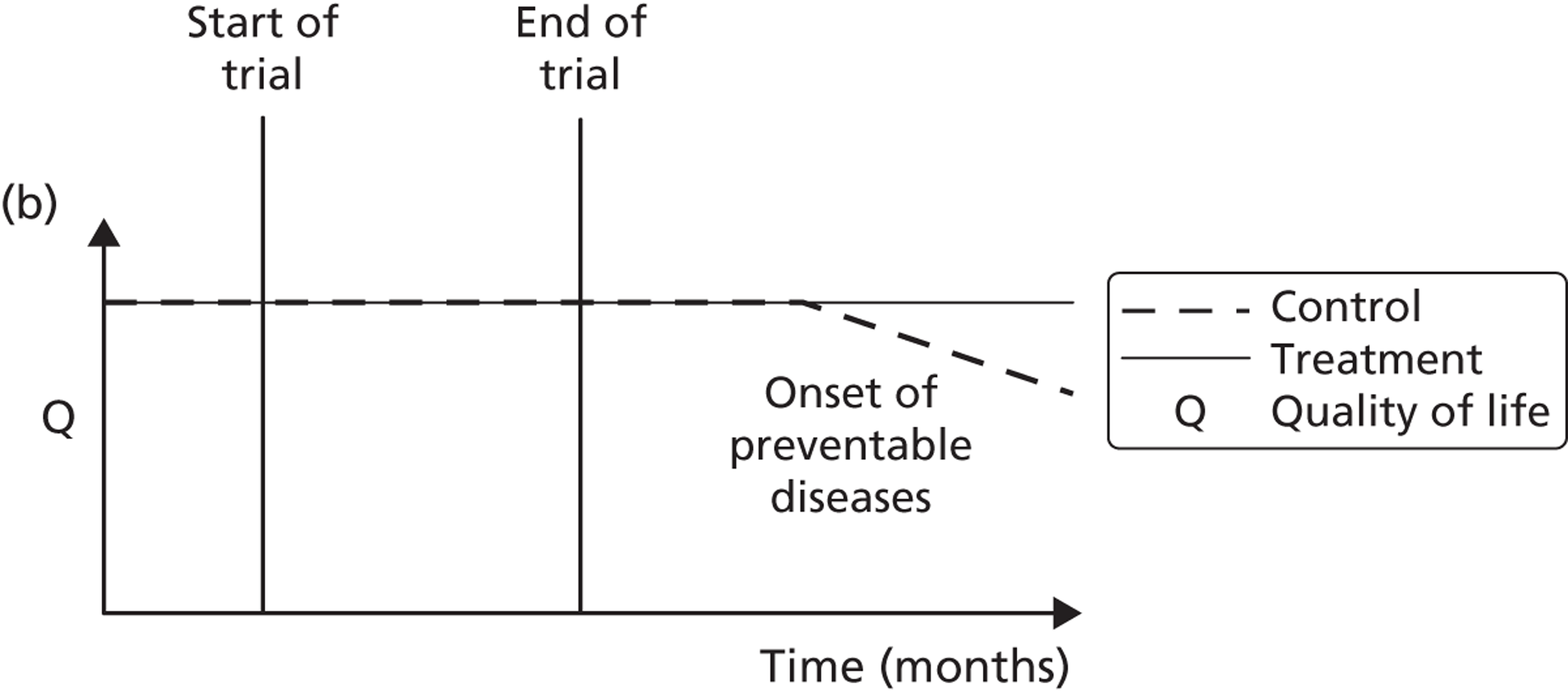
A potential problem with using this approach in the case of the booster intervention is illustrated in Figure 1. Figure 1a illustrates a typical pharmaceutical RCT. The patient population is recruited on the basis of suffering from a particular disease, which has a progressive effect on their HRQoL. The intervention reduces disease progression and so a difference in HRQoL emerges between the intervention arm (dashed line) and the control arm (solid line) by the end of the trial. Figure 1b indicates the potential effect on HRQoL of a lifestyle intervention. Both the control arm and the intervention arm are generally in good health initially but because of low levels of physical activity both are at an increased risk of developing a range of diseases and conditions related to sedentary behaviour. As the intervention is not intended to modify a disease but instead modify a lifestyle factor that predisposes people over the long term to increased morbidity and mortality risks, a straightforward comparison of before-and-after levels of resource use and HRQoL may be misleading as it may have been too soon for the mediating effect of physical activity on health to have become apparent.
FIGURE 1.
Timing of HRQoL benefits. (a) Curative intervention; and (b) preventative public health intervention.
For this reason, the cost-effectiveness of the interventions was assessed using two distinct modelling approaches. Alongside a short-term model comparing participants’ self-reported HRQoL and NHS resource use at 3 and 9 months, a long-term epidemiological model was also developed in which differences in the primary physical activity measure recorded in the trial, TEE, were mapped onto effects on mortality reported in epidemiological literature. This approach better allows the complex mediating effects of physical activity on health to be formally represented but also increases the dependence of the modelling results on strong assumptions about this physical activity–health relationship.
In the rest of this section overviews of the methods used to produce the two models are provided.
Cost-effectiveness modelling overview
Two types of cost-effectiveness model were developed, which used different approaches and sources of data to estimate the health effect, in quality-adjusted life-years (QALYs), of the interventions. Both types of model are described in more detail below.
Short-term questionnaire-based model
A short-term cost-effectiveness model has been constructed that incorporates trial-based estimates of the effect of the two interventions – mini booster and full booster – on participants’ use of NHS resources during the trial period. It also uses trial-based estimates of the effect of the interventions on participant utility using responses from participants who completed the SF-12v2 plus 4 HRQoL questionnaire at baseline and 9 months. 70 Approximate costs of the interventions are also incorporated in the model alongside the estimates of the effect of the interventions on resource use.
Resource use data collected and used
Data were collected at randomisation and at the end of the trial on participant use of NHS facilities. This included all face-to-face and telephone consultations with general practitioners (GPs) and other primary care staff, attendance at hospital accident and emergency (A&E) facilities, use of hospital outpatient facilities, number of hospital day cases and number and duration of hospital stays. With these data it is possible to produce an estimate of the effect of the intervention on resource use from an NHS perspective. Uncertainty in the true frequency with which each type of NHS resource was accessed was represented using a Dirichlet distribution with a non-informative prior of 0.5 added to each cell count.
For each trial arm – control, mini booster and full booster – the number of times that each participant had been to A&E, used hospital outpatient facilities, been a hospital day case and stayed overnight in hospital was recorded at both randomisation and up to 9 months later. Along with the reason for each resource use event, the number of times that type of resource was accessed for that reason was also recorded. For example, some participants may stay 2 nights in a hospital for a particular reason, others may have three hospital day appointments for another reason. What is of interest from a resource use perspective is the number of resource use units – such as nights in hospitals, day appointments, A&E admissions – rather than number of events. For this reason the number of resource use units of each resource use type was calculated by multiplying the number of resource units represented by an event by the frequency of each event. Costs for each type of resource use unit were estimated from a standard source71 to produce estimates of the cost of each type of resource use at the start and end of the trial and in each trial arm. These were divided by the number of participants in each arm to produce an estimated cost per participant.
Estimating costs from a societal perspective
These data were also used to provide estimates of the effect of different health conditions from a broader, societal perspective, which are presented as separate analyses. From this broader perspective, each use of a NHS facility was assumed to incur an additional cost. This additional cost was calculated as the national minimum wage for older people of working age multiplied by an assumed amount of time that it would take a patient to make use of each particular service type. The number of working hours foregone in making use of each type of NHS resource is shown in Table 4.
| Resource type | Assumed working hours foregone |
|---|---|
| Visit to A&E | 15 (2 days) |
| Hospital day case | 7.5 (1 day) |
| Hospital outpatient | 7.5 (1 day) |
| Hospital overnight stay | 7.5 (1 day) |
| GP telephone consultation | 1 |
| Visit to GP surgery | 3.75 (half a day) |
Although a significant proportion of the patient population was close to, or older than, retirement age and so may not be expected to be in full-time employment, the same opportunity costs were applied to all patients. This is to account for the societal value of services that people who are not in employment can be assumed to provide to friends and family, such as looking after children, home maintenance, looking after pets and so on. Each of these services can be bought and so has a market value at or above minimum wage. These simplifying assumptions are more likely to overestimate than underestimate the true societal costs and so could be considered an upper range of the cost-effectiveness of the interventions from a societal perspective.
Estimating health-related quality of life
Responses from participants who completed the SF-12v2 plus 4 questionnaire were used to derive an SF-6D utility score. 70 Differences in differences between an intervention arm and the control arm are used to produce an estimate of the incremental effect of each intervention on participant utility over this period.
Deterministic results
A version of the model is presented that provides a single best estimate of the mean incremental cost and mean incremental QALY gain for both the mini booster and full booster interventions. These best estimates combine resource use and utility values estimated from trial data with approximate intervention costs elicited from report authors involved in conducting the interventions. These estimates are somewhat approximate and are intended more to be illustrative than authoritative. The effect of the high level of uncertainty in these estimates on decision uncertainty is explored through probabilistic sensitivity analysis (PSA).
Probabilistic sensitivity analysis
Within PSA, instead of a single best estimate of the incremental cost and QALY gain of each intervention being used, the effect of uncertainty around these values was assessed by drawing 1000 plausible values from joint distributions of the mean costs and mean QALYs of each intervention. These are shown on scatterplots, with different plotting symbols representing estimates from different arms.
Cost-effectiveness acceptability frontiers
Cost-effectiveness acceptability frontiers (CEAFs) were used to translate the joint incremental cost and incremental QALY estimates (‘scatter’) produced from the PSA into an indication of decision uncertainty. This involved calculating the incremental cost-effectiveness ratio (ICER) implied by each joint estimate of incremental cost and incremental QALYs and identifying the proportion of ICERs that are below a given willingness-to-pay threshold (λ). The horizontal axis of a CEAF indicates how the proportion of ICER estimates below the threshold varies across different values of λ. Within this analysis, the range of λ values considered was between £0 and £50,000 per QALY. CEAFs differ from cost-effectiveness acceptability curves in that only the option estimated to be optimal, that is, with the greatest net mean benefit, is plotted.
Long-term epidemiological model
As stated previously, interpretation of the short-term cost-effectiveness model can be problematic as the intervention is preventative rather than curative and the participants are not selected from a population characterised by a particular disease. Because of this, it may be more valid to consider the effect of the intervention over a much longer time horizon than the trial duration and to assume that any potential QALY benefits of the intervention are mediated through the clinically measurable health benefits of increased physical activity. This is the rationale for presenting a long-term epidemiologically based model alongside the short-term questionnaire-based model described in the previous section. Unfortunately, adopting this approach requires making a number of strong assumptions about how the physical activity measures recorded within the trial translate into an impact on population health. The long-term model was an individual sampling model constructed in the R statistical programming language (version 2.14.2). The approach taken to develop this model, together with the assumptions made, are described in the following section.
Populating a hypothetical cohort of individuals
As the population who participated and were eligible for the intervention were drawn from a fairly general working age population, an individual sampling model was constructed to represent variability in terms of age and gender of the population at baseline. After discussion amongst project members, it was decided that the age distribution of the hypothetical population considered in the long-term model should be drawn from the age distribution of the booster trial participants rather than the population eligible for the trial. This is because trial participants did not appear to be uniformly drawn from the population eligible to participate but were disproportionately drawn from the upper end of the age range eligible to participate. For simplicity, it was assumed that trial participants were drawn equally from both genders.
Hypothetical population size
Within these analyses, the size of the simulated cohort was selected to be 500,000 individuals. Increasing the number of individuals sampled in the simulation will lead to greater stability in estimates of mean effect but will substantially increase the computing time that models need to run.
Defining and simulating the ongoing mortality hazard in the simulated population
Once the hypothetical cohort of individuals was constructed, the aim was to simulate their clinical experiences over their lifetime. Beginning at their initial age, the probability that they die in the following year was estimated using Office for National Statistics (ONS) life table data. 72 If they survive the following year, their age is increased by 1 year and the probability of them dying in the following year is updated to reflect their new age. The mathematical model involves applying this process iteratively for each of the individuals in the cohort until death, producing a series of simulated initial ages and ages at death. Table 5 provides a simple illustration of this:
| Person number | Initial age (years) | Gender | Age at death (years) |
|---|---|---|---|
| 1 | 51 | Male | 82 |
| 2 | 45 | Male | 75 |
| 3 | 59 | Female | 84 |
| 4 | 48 | Female | 90 |
| 5 | 52 | Male | 86 |
Calculating quality-adjusted life-years
The purpose of simulating each individual 1 year at a time is to estimate the accrual of QALYs over the life course. Recent work by the School of Health and Related Research (ScHARR) at the University of Sheffield has indicated that the QALYs associated with living another year differ according to the age and gender of the patient and to reflect this it has produced a regression equation that allows age- and gender-adjusted QALYs to be calculated. 73 This equation has been used within the mathematical model to produce more accurate estimates. Each additional year lived by a simulated individual therefore results in an increment to the number of QALYs accrued by that individual but this amount differs each year according to this equation.
In adjusting QALYs according to the age and gender of an individual, the different levels of morbidity that typically affect people at different ages and which differentially affect men compared with women are implicitly accounted for and so the model incorporates morbidity effects alongside mortality effects. For simplicity, however, it was assumed that different levels of physical activity do not have a mediating effect on the degree of morbidity that an individual experiences relative to other people of the same age and gender.
Discounting
As is standard practice in UK-based health-care economic modelling, QALYs gained are discounted at a rate of 3.5% per annum. 74
Assumptions about the longevity of the effect of the intervention
An intention of the long-term model was to represent a plausible decline in physical activity levels in the years following the trial, returning to baseline levels for participants in each of the three trial arms after 2 years. As baseline levels of physical activity were not recorded using Actiheart, three alternative scenarios using the long-term model were used. One scenario used the 9-month activity levels relative to the 3-month activity levels and two other scenarios compared differences between arms at 3 months and 9 months post randomisation. The implications of these different assumptions about the appropriate comparators are discussed below, see Problems with inferring causal effects from the data.
Assumptions about the causal relationship between physical activity and mortality hazards
A number of sources of epidemiological research exist which suggest that a monotone relationship exists between how physically active people of working age are and their all-cause mortality rates. 75–80 However, no source could be identified that related directly to the population eligible for the intervention (those aged between 40 and 64 years inclusive), those who participated in the intervention (who tended to be drawn disproportionately from the older ages within the pool of those eligible) or the particular physical activity measures recorded by the Actiheart system.
Because of this a number of assumptions had to be made to relate the epidemiological results to the outcomes reported in the booster trial. The main epidemiological source used to inform the assumed relationship between physical activity measures and adjusted mortality risks was a US-based study published in the New England Journal of Medicine (NEJM) in 2002. 81 In this study the peak exercise capacity, as measured in METs, for older men of working age (mean age 59 years) was assessed through standardised treadmill-based testing. The paper presents a figure showing the relationship between relative risk (RR) of death and quintile of exercise capacity. The risk ratio for the highest exercise capacity quintile was chosen as the reference category. These data are presented in Table 6, as well as values adjusted to have the third quintile as the reference category.
| Quintile | Risk ratioa | Centralised risk ratiob |
|---|---|---|
| 1 (lowest) | 4.5 | 2.5 |
| 2 | 2.5 | 1.4 |
| 3 | 1.8 | 1.0 |
| 4 | 1.4 | 0.8 |
| 5 (highest) | 1.0 | 0.6 |
Although initially it was assumed that the risk ratio to MET relationship could be mapped directly from the NEJM results to the Actiheart data, as both the data in the NEJM article and the booster trial data were categorised according to METs, it was recognised that this was not appropriate. This is because the contexts in which these METs were recorded were not equivalent. In the case of the NEJM article, METs were of physical exercise capacity and involved trying to identify an upper physical limit to patients’ physical performance over a short period of time. In the case of the Actiheart data the METs were of typical activity over a period of 1 week. Instead, an indirect approach to mapping the relationship was used. This involved making the additional assumption that the relationship between quintiles held, even if the unit of measurement was different.
The outcome measure used from the booster trial to estimate the relationship between exercise quintile and RR was mean daily TEE, which was recorded using the Actiheart device at both 3 months and 9 months. The median TEE score from both time periods combined was identified and all scores recorded were converted to a proportion of this median score. The central proportional TEE score within each of the five quintiles was calculated by sorting all values from lowest to highest and identifying the values 10%, 30%, 50%, 70% and 90% of the way across this distribution, to identify proportional TEE values associated with each quintile. The relationship between TEE (as a proportion of the median TEE) and exercise quintile is presented in Table 7. This shows that someone whose TEE was approximately 80% of the median is in the lowest physical activity quintile and someone whose TTE was approximately 30% more than the median was in the highest quintile. Participants’ TEE levels at 3 months and 9 months are also presented graphically in Figure 2.
| Quintile | Mean TEE per day (kcal) | TEE as proportion of the median |
|---|---|---|
| 1 (lowest) | 1703 | 0.77 |
| 2 | 1998 | 0.90 |
| 3 | 2224 | 1.00 |
| 4 | 2434 | 1.09 |
| 5 (highest) | 2839 | 1.28 |
FIGURE 2.
Relationship between mean daily TEE at 3 months and mean daily TEE at 9 months by intervention group. Grey lines indicate the centre of each quintile.
Data from Tables 6 and 7 were combined to produce a regression equation mapping proportional TEE against RR of death. A good fit was found by assuming a power law relationship between these variables. The data and mapping equation identified are shown in Figure 3. The best fit equation identified was y = 1.056x−2.951, where y is RR and x is TEE as a proportion of the median.
FIGURE 3.
Mapping equation between assumed RR of death and TEE as a proportion of the median.
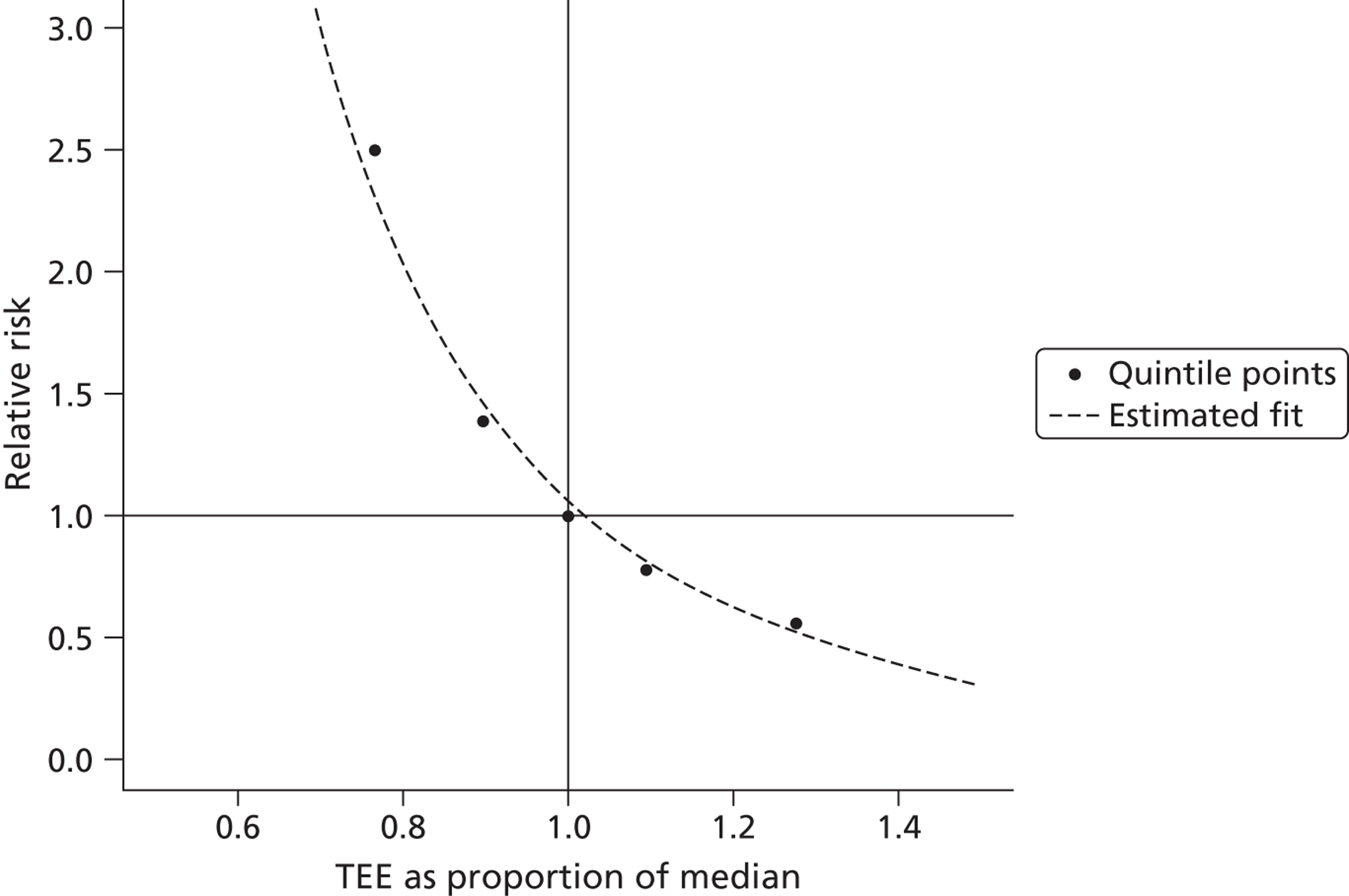
Using Actiheart data to adjust mortality risks in the simulated cohort
By using the power law mapping equation shown in Figure 3, an estimated RR for each proportional TEE value determined from the Actiheart data can be produced. The procedure for this within the mathematical model involved first duplicating the initial simulated cohort of individuals produced using the ONS population data and then applying TEE scores drawn from the final observations in treatment arms combined to one of these cohorts and TEE scores drawn from the final observations in the control arm to the other of these cohorts. TEE scores were sampled with replacement from each respective arm. The RR associated with the particular proportional TEE assigned to that individual was then assumed to apply to him or her. An illustration of this is provided in Table 8.
| ‘Control’ cohort | ‘Treatment’ cohort | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Person number | Initial age (years) | Gender | pTEE | RR | Person number | Initial age (years) | Gender | pTEE | RR |
| 1 | 51 | Male | 1.1 | 0.80 | 1 | 51 | Male | 0.9 | 1.45 |
| 2 | 45 | Male | 0.9 | 1.45 | 2 | 45 | Male | 1.1 | 0.80 |
| 3 | 59 | Female | 0.9 | 1.45 | 3 | 59 | Female | 1.3 | 0.49 |
| 4 | 48 | Female | 0.8 | 2.05 | 4 | 48 | Female | 1.0 | 1.06 |
| 5 | 52 | Male | 1.0 | 1.06 | 5 | 52 | Male | 1.7 | 0.22 |
The risk of death in the following year for each individual is then calculated by finding the estimated probability of death in the following year using life table data adjusted for age and gender and then multiplying that risk by that individual’s estimated RR. Within the mathematical model the positive effect of the treatment can operate only through this life table adjustment, although as described above other positive clinical relationships between physical activity and mortality risk could also be incorporated in later additions of the model.
Problems with inferring causal effects from the data
There are two fundamental challenges to the validity of the long-term model: first, the main physical activity outcome recorded in the booster trial, TEE, is a surrogate or proxy from the perspective of the long-term model, in which the outcome is QALYs; second, it is difficult to judge what is the counterfactual or baseline, that is, what would have happened to those participants who were assigned to the intervention arms of the trial if they were assigned to the control arm.
The previous section has shown the degree of potential model dependence on a number of assumptions that had to be made to link TEE with mortality. Some of these assumptions are strong, such as the assumption that quintiles of exercise capacity in one population correspond directly to quintiles of physical activity in another population. The validity of such assumptions therefore has a knock-on effect on the validity of the model outputs.
The issue of estimating the counterfactual is a fundamental challenge in the case of the booster trial for a number of reasons:
-
Actiheart measurements including TEE and PACs were not recorded at the start of the trial (‘baseline’). Instead, the first measures were recorded at 3 months post intervention. Additionally, subjective measures of physical activity such as the SPAQ were known to have only very slight correlation with the objective measures so cannot be reliably used to impute the objective scores at baseline. 82
-
The level of attrition within the trial was high, meaning that the sample of participants who completed the trial may not be representative of those who started the trial, and so there is a significant potential bias issue.
-
The number of participants who had valid and complete Actiheart measures at 3 months and 9 months post randomisation is significantly smaller than the number who completed the booster trial.
Because of the high level of attrition and low number of participants with complete Actiheart data, it may be wrong to assume that the population mix in each arm is similar at 3 months and 9 months after the start of the trial, because of patterns of selective attrition. This means that it may be problematic to assume that differences in the outcome measures between the control arm and the treatment arm observed at 3 months and 9 months are due to the treatment effect.
For this reason, a differences-in-differences approach was used. This involves comparing change over time in the outcome in the treatment group with change over time in the outcome in the control group. As no TEE measurements were recorded at baseline, the 3-month scores were used as proxies for the baseline levels and the 9-month scores were assumed to be the longer-term post-intervention levels.
It may be argued that using the 3-month post-intervention scores as the baseline scores is a very strong assumption because a significant treatment effect may be present at 3 months post intervention. In theory, a highly effective intervention could appear to be less effective than a less effective option because the more effective intervention is most effective at 3 months whereas the less effective option is more effective at 9 months than at 3 months. This is illustrated in Figure 4, in which the more effective intervention is indicated by the solid line and the less effective intervention by the dashed line. Because of this potential problem with using the 3-month scores as the baseline scores in the differences-in-differences approach, separate analyses using a simple differences approach were also carried out. These analyses involved comparing the TEE levels at 3 months in the intervention arms with the TEE levels at 3 months in the control group, and the 9-month TEE levels in the intervention arms with the 9-month TEE levels in the control group.
FIGURE 4.
Illustration of a scenario in which it would be wrong to attribute differences in differences (9 months vs. 3 months) between the treatment arm and the control arm to a treatment effect.
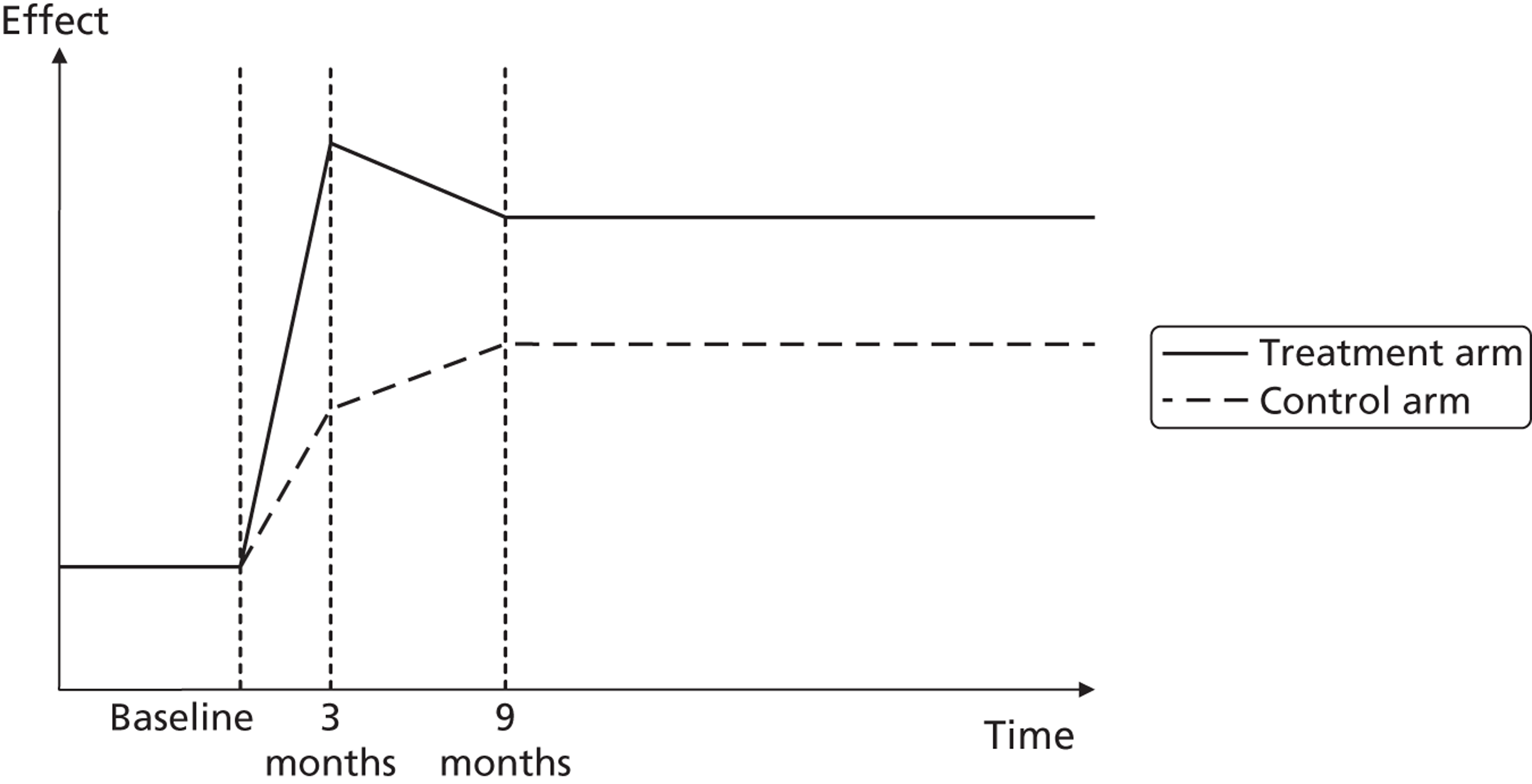
The fundamental problem with the simple differences approach is that differences between groups at either 3 months or 9 months could be due to differences between the groups that were present at randomisation, rather than differences as a result of the intervention. This is illustrated in Figure 5 in which the intervention had no effect on the physical activity levels of either arm (the solid or dashed lines), but comparing scores in arms at either 3 months or 9 months would suggest a treatment effect. In this study, although the participants were randomly assigned to each of the three arms and so on average can be assumed to have similar baseline characteristics at randomisation, because of the degree of attrition in the study the composition of the three arms cannot be assumed to be the same at 3 months or 9 months because the attrition may have been selective. For example, participants who were least inclined towards additional physical activity may be more likely to leave the treatment arms but may stay in the control arm.
FIGURE 5.
Illustration of a scenario in which it would be wrong to attribute differences between the treatment arm and the control arm at 3 months or 9 months to a treatment effect.
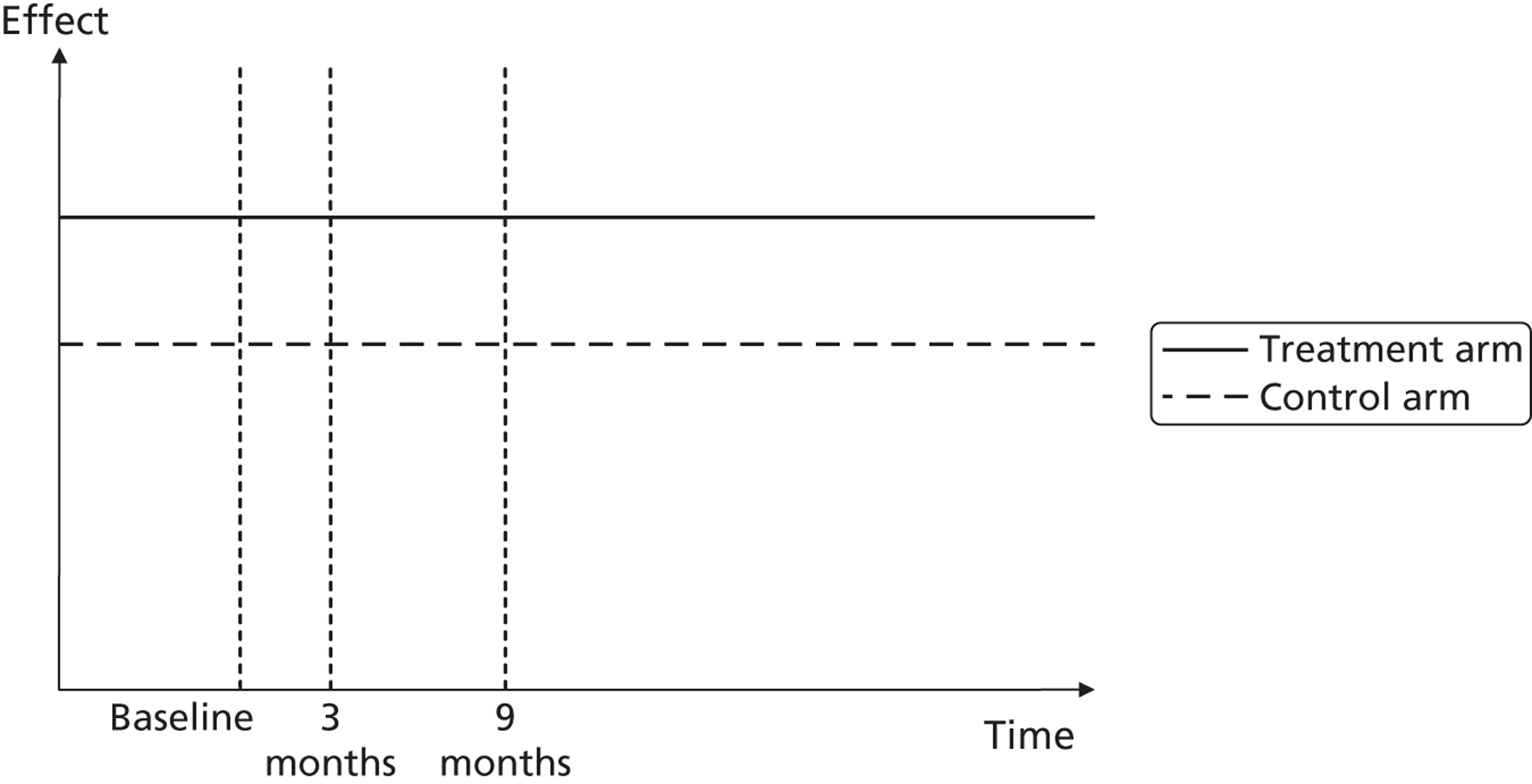
Estimating the mean incremental treatment effect on patient utilities using a differences-in-differences approach
In the differences-in-differences approach, four out of six hypothetical groups with the same initial age and gender profiles were compared. The six groups are:
-
FT – the ‘treated’ full booster arm. All individuals’ scores were sampled with replacement from the scores of participants who were assigned to the full booster group and provided valid Actiheart data at 9 months and 3 months. The RRs associated with the individuals’ 9-month scores were used for the first 2 years, then the RRs associated with individuals’ scores at 3 months were used thereafter. This represents the assumption that any treatment effect of the intervention is unlikely to last much longer than the duration of the trial.
-
FU – the ‘untreated’ full booster arm. The same as for FT but with the 3-month RRs used throughout.
-
MT – the ‘treated’ mini booster arm. The same as FT but drawing from participants in the mini booster arm rather than the full booster arm.
-
MU – the ‘untreated’ mini booster arm. The same as FU but drawing from participants in the mini booster arm rather than the full booster arm.
-
CT – the ‘treated’ control arm. As with FT and MT but using 9-month control group scores for 2 years instead. Using scores from this group helps to control for trends over time and any effect on physical activity of being part of the trial even if not assigned to one of the treatment arms.
-
CU – the ‘untreated’ control arm. As with FU and MU.
The utility effect of the intervention was therefore estimated as (MT – MU) – (CT – CU) for the mini booster group and (FT – FU) – (CT – CU) for the full booster group.
Estimating the mean incremental treatment effect on patient utilities using a simple differences approach
Using notation similar to that presented above, the six groups are:
-
F3 – scores at 3 months in the full booster arm
-
F9 – scores at 9 months in the full booster arm
-
M3 – scores at 3 months in the mini booster arm
-
M9 – scores at 9 months in the mini booster arm
-
C3 – scores at 3 months in the control arm
-
C9 – scores at 9 months in the control arm.
The utility effects of the interventions were therefore estimated as F9 – C9 (full booster) and M9 – C9 (mini booster) using 9-month data and F3 – C3 (full booster) and M3 – C3 (mini booster) using 3-month data.
Primary and secondary models
Because both the simple differences approach and the differences-in-differences approach require that problematic assumptions be made, neither the simple difference models nor the differences-in-differences models should be considered preferable to each other. Instead, they should all be considered equal primary models.
In addition to these primary analyses, a secondary model was also produced. This model involved assuming that the changes in physical activity levels recorded between 3 months and 9 months in each arm were equally distributed. This means that a mean increase in physical activity between 3 and 9 months will translate into a mean increase in survival and longevity. Because the primary long-term models are individual-level models and make use of a separate single observation from the trial and involve mapping changes in physical activity to changes in annual risks, this does not necessarily occur in these models. The approach used in this ‘value-added’ model is described in more detail in the following section.
The ‘value-added’ model
If F9i defines the level of activity recorded in the individual in the full booster arm at 9 months and F3i defines the level of activity recorded in that same individual at 3 months, then in the differences-in-differences model the physical activity level change in each individual at the end of 9 months is calculated as F9i – F3i and so is different for each individual. In the ‘value-added’ model, however, an equal increase is assumed for all participants in each arm. This increase, ΔF, is the mean difference between 9-month and 3-month scores observed in individuals in the full booster group. The increases in the control group, ΔC, and the mini booster group, ΔM, are calculated similarly.
‘Value added’ means that the direction of effect in terms of estimated effect on mean utility should always be in the same direction as the estimated effect on mean activity level and so may appear to produce more plausible estimates when trial results indicate a mean increase in physical activity but the economic model indicates a mean decrease in estimated utility. The cost of doing this, however, is to not make use of some available individual-level data relating level of physical activity at 3 months to level of physical activity at 9 months.
Additional assumptions about the physical activity–mortality relationship
As assuming that RRs related to physical activity levels applied indefinitely produces implausible estimates of the mortality effects of the intervention (such as indicating that a substantial proportion of those in the highest exercise quintile will live to > 140 years), and as the available results are for middle-aged individuals, it was additionally assumed that the relationship between physical activity and mortality rate stopped when participants reached 70 years of age and that uniform risks applied from that age onwards.
For simplicity, and because of a paucity of data in those above this age, it was also assumed that no individual would live longer than 99 years.
Additional scenario analyses
Assessing the effect of baseline physical activity on additional benefits of additional physical activity
As the relationship between exercise quintile and mortality RR appears to follow a power law, it seems important to consider how estimates of cost-effectiveness vary as a function of baseline levels of physical activity. This is because a given increase in physical activity for people already relatively active is likely to provide less additional health benefit than the same increase in physical activity in a very sedentary population. As a supplementary analysis, the long-term model was therefore used to estimate the effect of an intervention that increased people’s physical activity from the first to the second quintile, the second to the third quintile, and so on.
Chapter 3 Results of the main trial
All tables relating to results from the main trial can be found in Appendix 6.
Recruitment of trial participants
Figure 6 provides a detailed flow chart showing participant recruitment and follow-up during the study. Between May 2009 and June 2011 a total of 70,388 postal invitation letters with prepaid reply cards were sent to residents aged 40–64 years in our study neighbourhoods inviting them to enrol in a programme to help them become more physically active. Response and randomisation rates from the six mail-outs are detailed in Appendix 6, Table 16. Mail-out 6 was sent in June 2011 in a final attempt to boost recruitment but with the expectation that 9-month follow-up results would not be available. The trial was closed to recruitment in November 2011, the last opportunity within the funding envelope to recruit and follow participants up for 3 months (the primary outcome assessment). No marked reply cards distributed by GPs were returned; the team have no information on whether those distributed through community sources were returned but it is assumed that most if not all respondents were approached through the primary care trust mail-out (see Chapter 2, Methods for the main trial).
FIGURE 6.
Participant flow. a, 10 withdrew consent (one disappointed at allocation, two found it too time intensive, two because of ill health, five for other reasons), one did not attend assessment, nine were lost to follow-up (uncontactable); b, nine withdrew consent (three found it too time intensive, one because of ill health, five for other reasons), four did not attend assessment, 16 were lost to follow-up (uncontactable); c, 14 withdrew consent (10 found it too time intensive, one because of ill health, three for other reasons), one died, three did not attend assessment, 14 were lost to follow-up (uncontactable); d, one withdrew consent (because of ill health), three did not attend assessment, five were lost to follow-up, 12 were randomised with insufficient time for 9-month follow-up; e, two withdrew consent (too time intensive), one did not attend assessment, six were lost to follow-up, 11 were randomised with insufficient time for 9-month follow-up; and f, two withdrew consent (one found it too time intensive, one for other reasons), two did not attend assessment, three were lost to follow-up, 13 were randomised with insufficient time for 9-month follow-up.
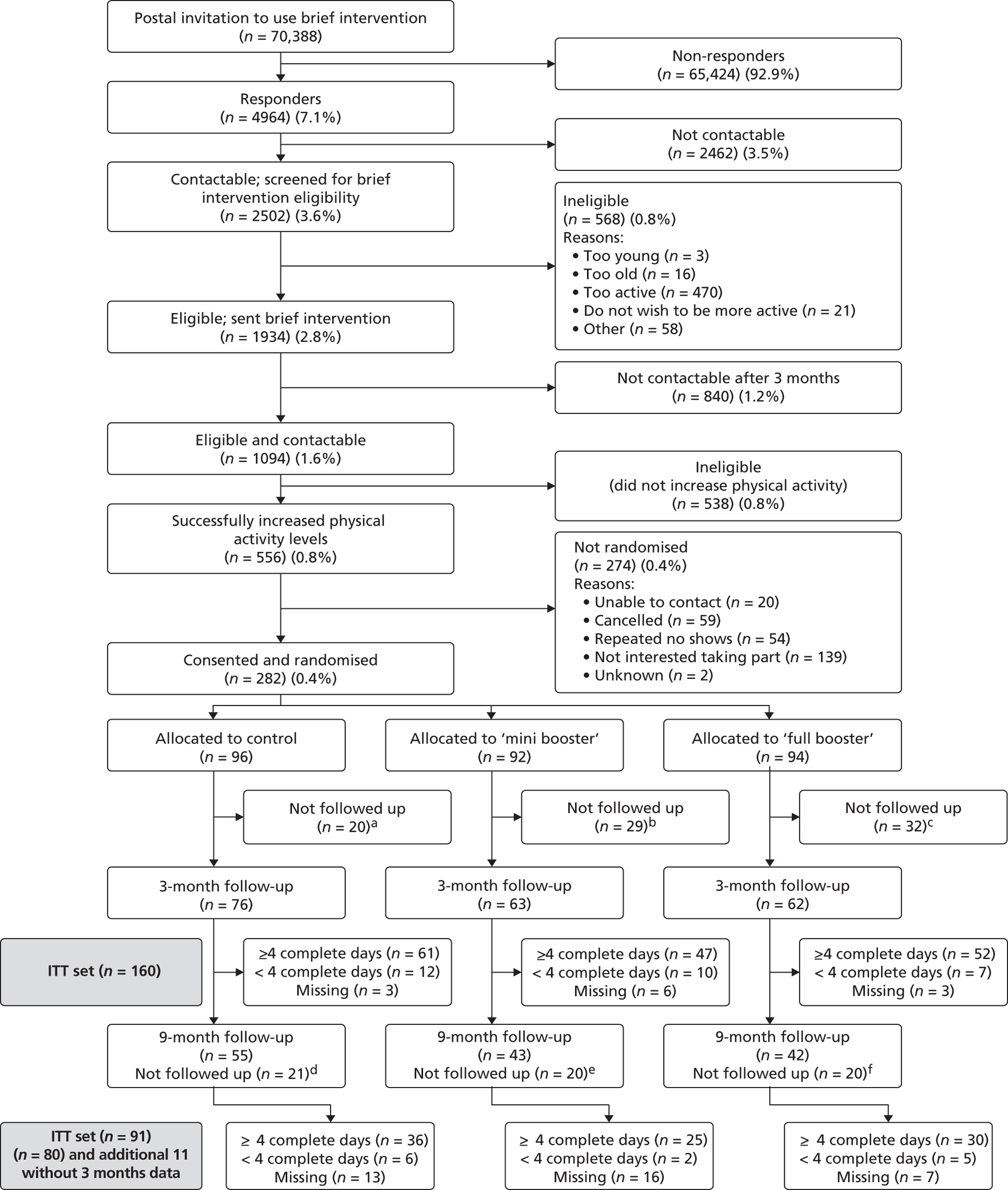
Of the invitation letters sent, only 4964 (7.1%) replies were received indicating an interest in taking part in the trial. Of these potential participants, 2502 (50.4%) were contactable and were screened for eligibility for the brief intervention. A total of 568 (22.7%) of those screened for the brief intervention were ineligible to take part for various reasons (three were too young, 16 were too old, 470 were too active and already achieving the current recommended activity levels of at least 30 minutes of at least moderate activity on at least 5 days a week, 21 did not wish to be more active and 58 were ineligible for other reasons).
A total of 1934 participants wishing to have support to become more active and meeting the inclusion criteria and eligible for the brief intervention were sent a DVD focused on increasing physical activity in general. Three months after receiving the brief intervention, 1094 (56.6%) were contactable and their physical activity levels were reassessed.
Of these, 556 (50.8%) successfully increased their physical activity levels and were eligible to be randomised into the booster intervention trial, of whom 282 consented and were randomised into the three arms of the trial in a ratio of 1 : 1 : 1 (control = 96, mini booster = 92 and full booster = 94).
Retention of participants during follow-up and evaluable data
Participants were followed up between August 2009 and February 2012. Of the 282 participants who were randomised, 201 (71%) were followed up and assessed at 3 months after baseline (control = 76, mini booster = 63 and full booster = 62). However, evaluable data on the primary outcome, mean daily TEE, which was defined as data from those with at least 4 complete days (lost not more than 1000 minutes in a day) of Actiheart measurements, were available for only 160 (57%) of the randomised participants, which translated to an attrition rate of 43%. Therefore, 160 participants were available for the ITT analysis on the primary outcome.
At 9 months post randomisation 50% (140/282) of the participants had an assessment, of whom 91 had evaluable primary outcome data at 9 months of follow-up [a 9-month response rate of 32% (91/282)]; 80 had evaluable data at both 3 and 9 months and the remaining 11 had evaluable data only at 9 months.
Figures 7 and 8 show the crude distributions of evaluable primary outcome data at 3 and 9 months of follow-up respectively.
FIGURE 7.
Crude distribution of the number of complete days (evaluable data) at 3 months.
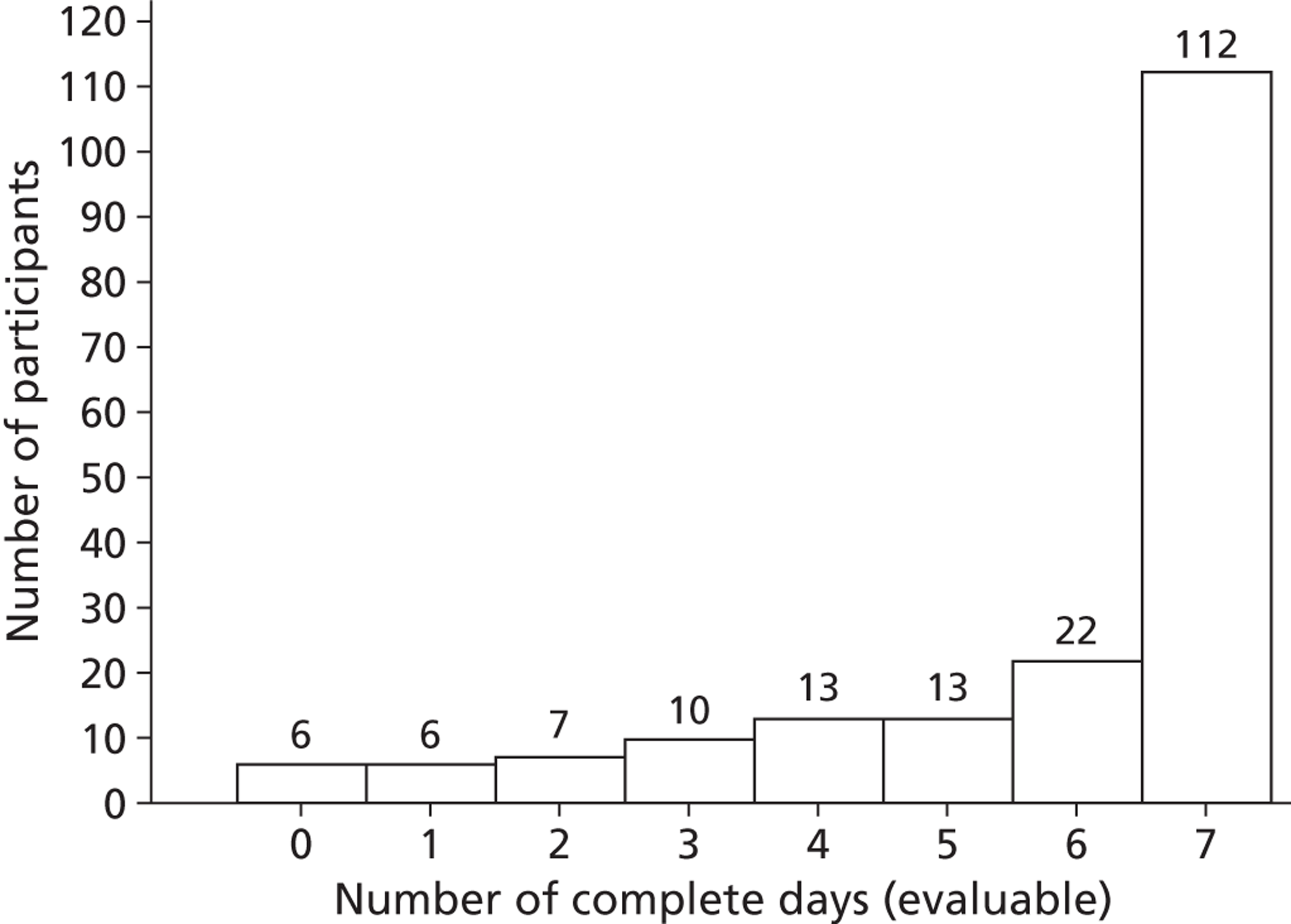
FIGURE 8.
Crude distribution of the number of complete days (evaluable data) at 9 months.
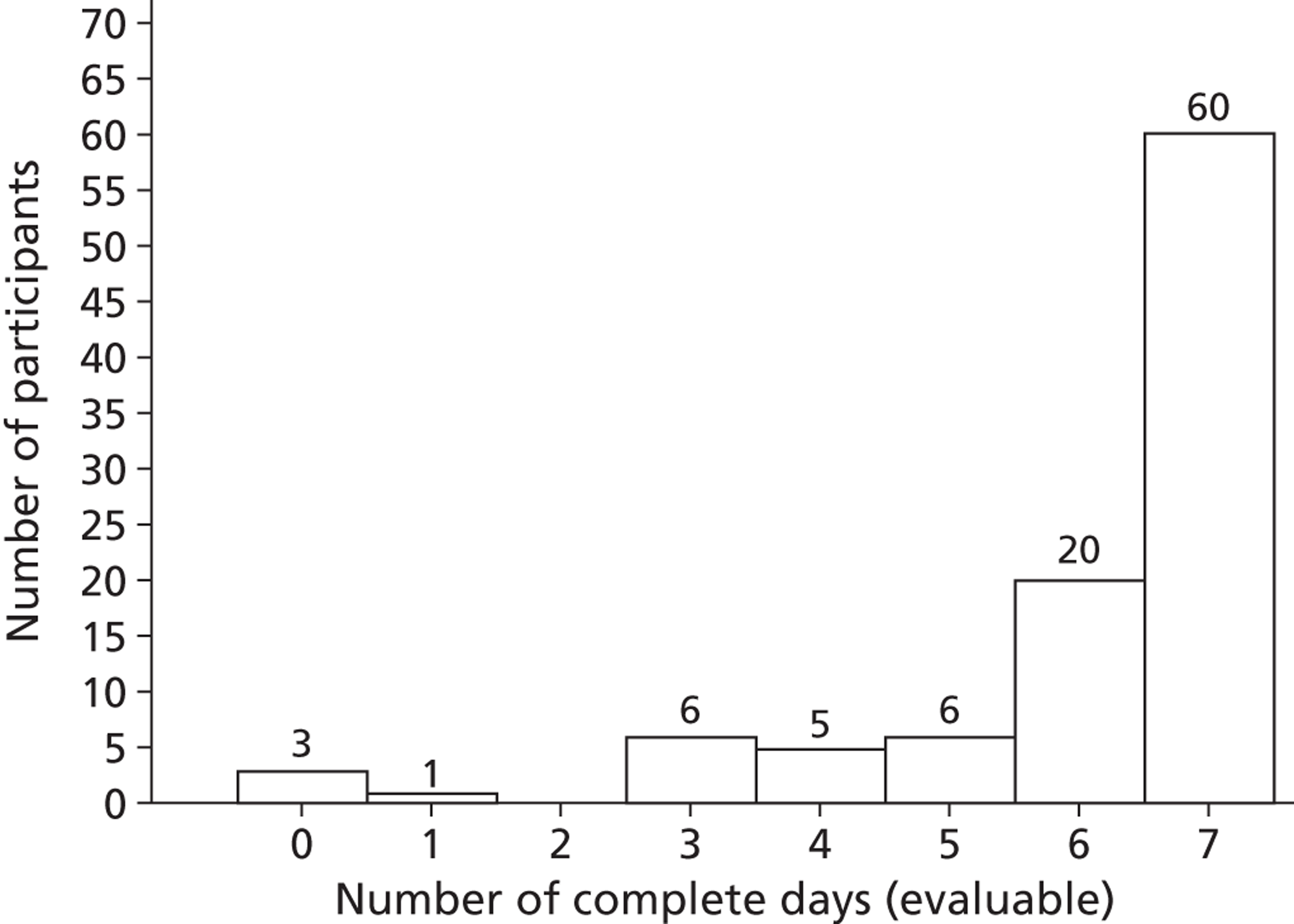
Intervention received per protocol
Of the 186 participants who were randomised to the mini booster and full booster interventions, 73% (136/186) received the intervention as per protocol [77% (71/92) in the mini booster arm and 69% (65/94) in the full booster arm].
Baseline characteristics of participants
The baseline characteristics of the randomised and ITT participants are shown in Appendix 6, Tables 17 and 18, respectively. The distributions of participants’ characteristics were fairly similar between the control and intervention arms in both sets of data. There were 28 participants among those randomised who did not show an increase of at least 30 minutes of physical activity based on the SPAQ at brief intervention screening and pretrial screening. Of these, 23 reported atypical activity at either brief intervention screening or pretrial screening (see Chapter 2, Participants). However, the remaining five reported typical activity on both assessments and reported an increase of less than the required amount of at least 30 minutes but were randomised into the study in violation of the protocol (see Chapter 8, Recruitment and retention). Four of these participants contributed 3-month Actiheart data and were retained in the ITT analysis but excluded from a post hoc sensitivity analysis to assess their likely impact on the treatment effect (see Effectiveness of the booster intervention at 3 months).
Figures 9 and 10 show Index of Multiple Deprivation (IMD) 201083 overall scores and income scores, respectively, for booster participants based on the lower super output area (LSOA) corresponding to their postcode. High scores indicate high levels of deprivation.
FIGURE 9.
Index of Multiple Deprivation 2010 overall scores for randomised participants.
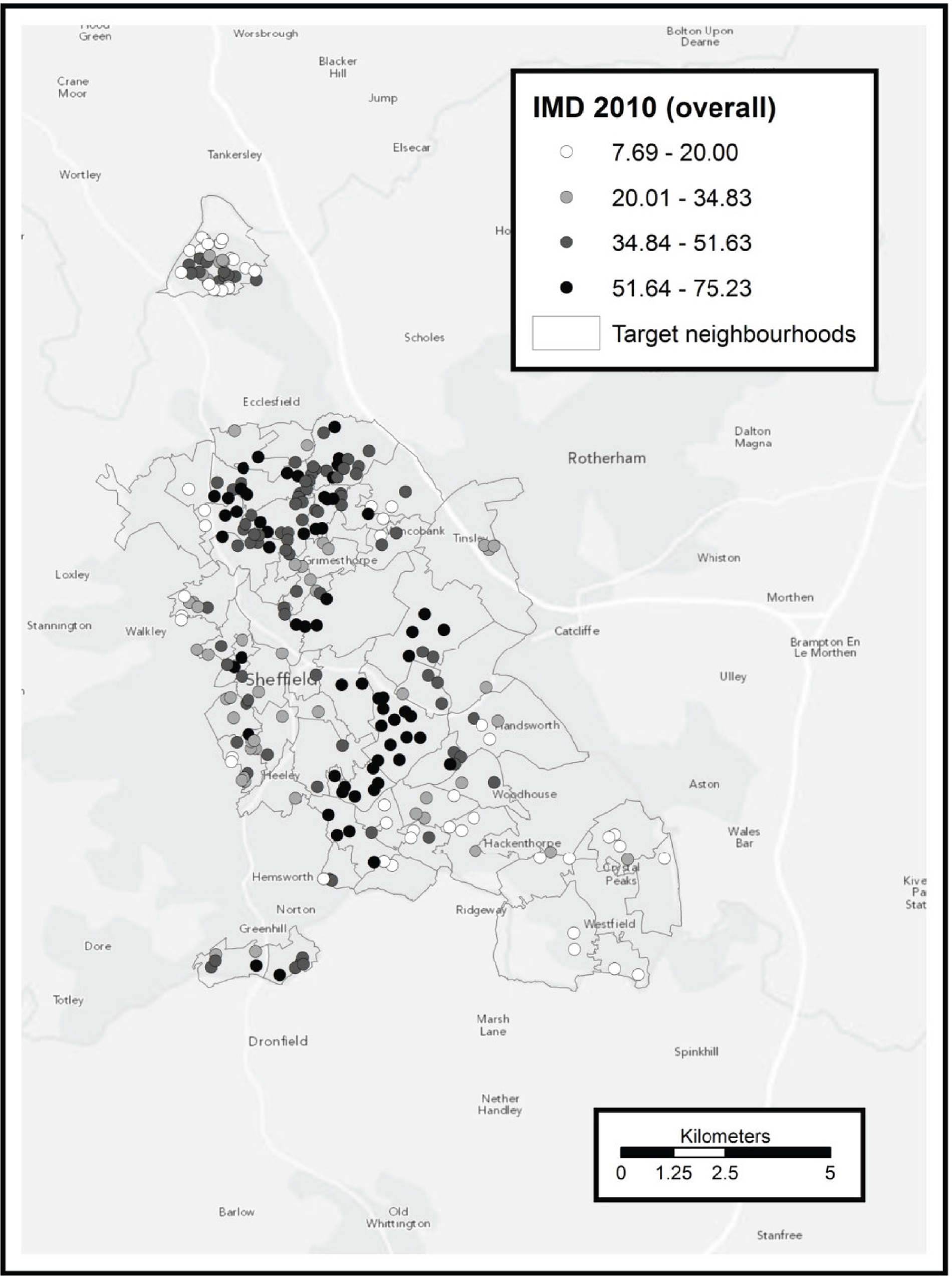
FIGURE 10.
Index of Multiple Deprivation 2010 income scores for randomised participants.
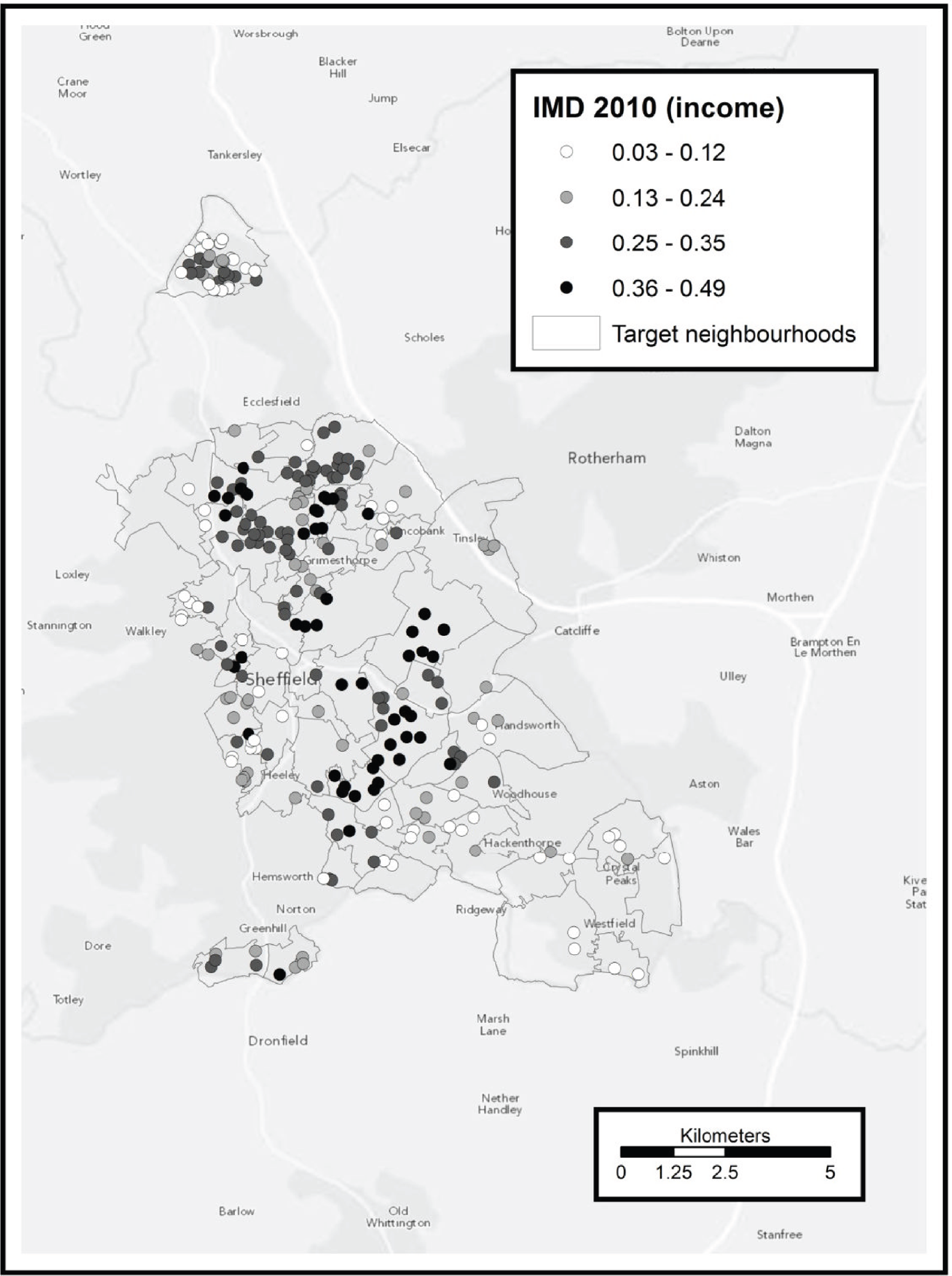
Figure 11 shows the results of a preliminary geospatial cluster analysis using SaTScan™ version 9.1 [see www.satscan.org/ (accessed December 2013)]. Clusters of high response rates were present in High Green and the Longley area of the city. Clusters of low response rates were present in the north and central-south parts of the city. In a post hoc analysis we aggregated the number of mail-outs and the number of response rates at LSOA level. A negative Spearman rank correlation of −0.22 was found between the two factors. This indicates that people living in LSOAs with higher levels of deprivation were less likely to respond to the initial mail-out (see Chapter 8, Recruitment and retention, for discussion).
FIGURE 11.
Map showing response rate by neighbourhood to booster mail-outs. Mail-outs from April 2009 to November 2010 together with clusters of high/low response rates identified by SaTScan.
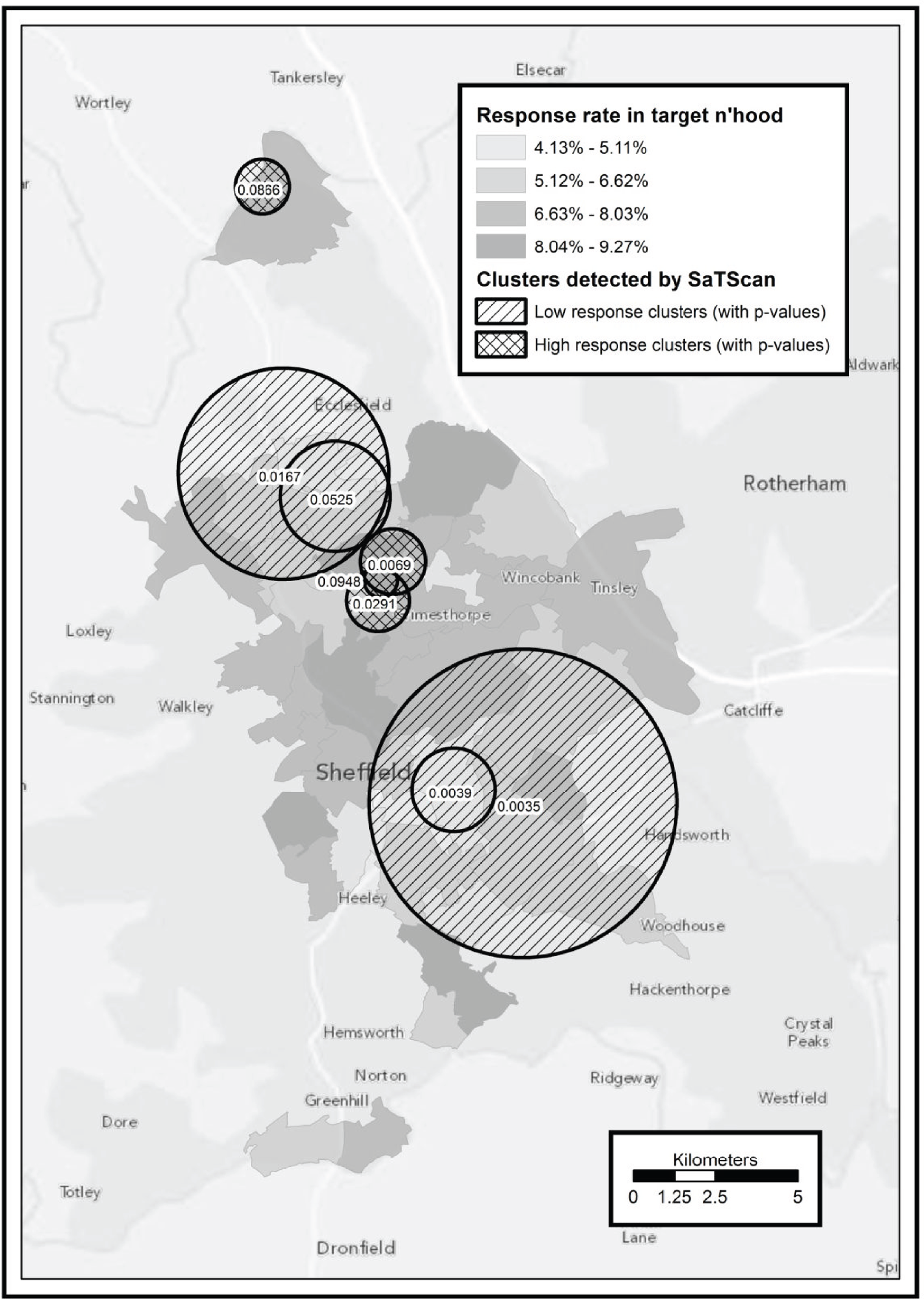
Response rate was not found to be related to population transience, with the Spearman rank correlation test between response rate and transience reporting a very weak correlation. The unweighted correlation was −0.005 and the weighted correlation was 0.030. With n = 687, the correlation must have a magnitude > 0.075 to be significant at the 5% level.
Factors associated with having evaluable data at 3 months of follow-up
The descriptive statistics of completers (with evaluable data) and non-completers (for any other reason) are shown in detail stratified by intervention group in Appendix 6, Table 19. Non-completers had higher values than completers for BMI (mean 31.5 kg/m2 and 29.4 kg/m2 respectively; mean difference −2.0 kg/m2, 95% CI −3.4 to −0.6 kg/m2; p = 0.005; see Appendix 6, Table 21) and weight (mean 89.4 kg and 82.0 kg respectively; mean difference −7.4 kg, 95% CI −11.7 to −3.1 kg; p = 0.001; see Appendix 6, Table 21). However, there was no evidence of an interaction between the intervention and the completers group. This means that, even though the overall distribution of completers and non-completers was slightly different with respect to weight, BMI, PCS scores and RAI between the booster group and the control group, their within-group distributions were similar and in the same direction across these treatment groups (see Appendix 6, Tables 19 and 21). There was some evidence to suggest an interaction between gender and the completers group with a high proportion of male non-completers in the booster group [51 (58.6%)] compared with completers [42 (42.4%)] (interaction p = 0.034; see Appendix 6, Table 20). In addition, the odds of having evaluable data were slightly lower in the mini and full booster intervention arms although this was not statistically significant at the 5% significance level. There was no sufficient evidence to indicate an association between having complete evaluable data at 3 months and other variables (see Appendix 6, Tables 19–21).
Appendix 6, Table 18 shows that the baseline characteristics of the ITT sample (n = 160) were broadly similar despite the 43% (122/282) attrition rate from the original randomised sample.
Distribution of the primary outcome at 3 months of follow-up
Figures 12 and 13 show the distribution of mean TEE per day (kcal) stratified by intervention arm, which was fairly normally distributed. The pooled SD of the mean TEE per day at 3 months was 417.4 kcal.
FIGURE 12.
Box plot of mean TEE per day (kcal) at 3 months stratified by intervention arm (n = 160).
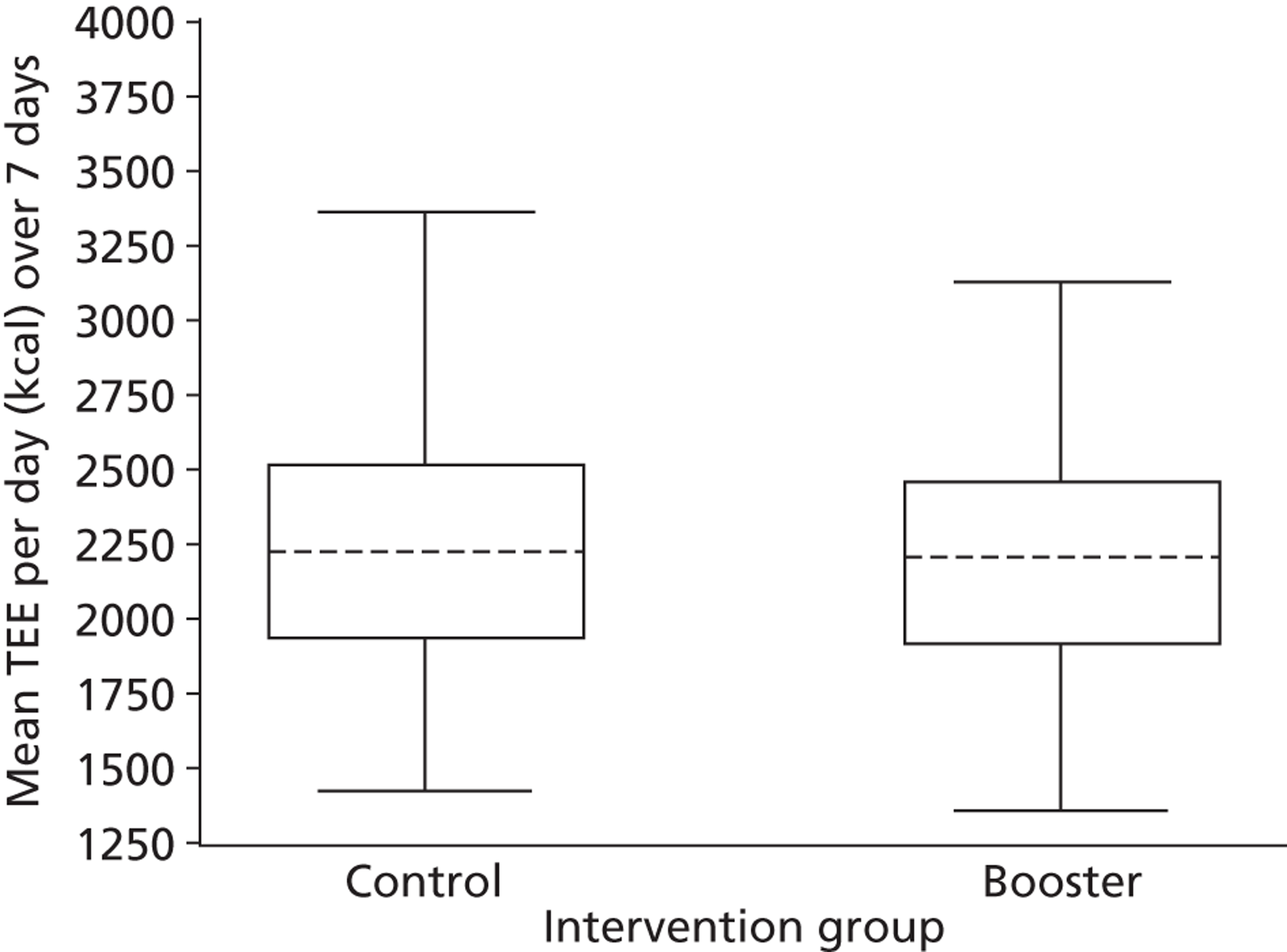
FIGURE 13.
Histogram showing distribution of mean TEE per day (kcal) at 3 months stratified by intervention arm (n = 160).
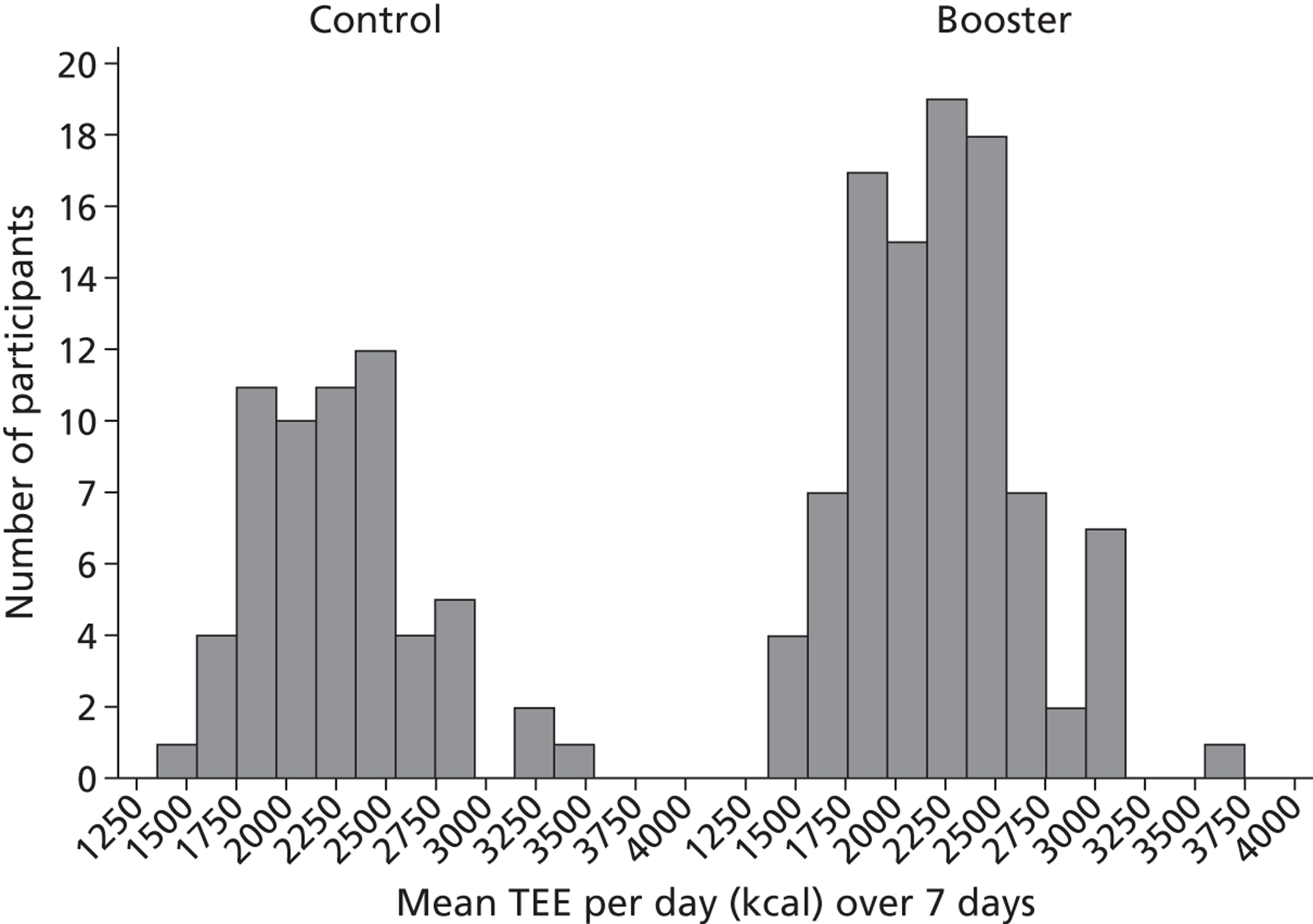
Effectiveness of the booster intervention at 3 months
At 3 months the observed mean TEE per day was 2266 kcal and 2227 kcal in the control and booster intervention arms respectively (see Appendix 6, Table 22), which translated to a mean difference of −39 kcal (95% CI −173 to 95 kcal; p = 0.6) in favour of the control arm and less than the expected MCID of 102 kcal in favour of the booster intervention. After adjusting for age, gender, BMI, total minutes of physical activity at brief intervention and pretrial screening and HRQoL based on SF-12v2 plus 4 total scores (see Appendix 6, Table 22), the adjusted mean difference in mean TEE per day was similar (–40 kcal, 95% CI −117 to 37 kcal; p = 0.3) and still in favour of the control group. The wide CIs around the observed treatment effect indicate the uncertainty in the effectiveness estimates but the results are not statistically significant, potentially of some clinical or practical importance as the CIs include the MCID of 102 kcal but the point estimates clearly show that the control group had the better outcomes.
A forest plot (Figure 14) summarises the treatment effect and its direction, which is in favour of the control arm in five analysis sets (including the primary analysis) under different assumptions except using imputations including all participants with at least 1 complete day of evaluable data, which was slightly in favour of the intervention. However, this observed effect was very small, only 20% of the expected MCID of 102 kcals, and diminishes after adjusting for age, gender, BMI, total minutes of physical activity at brief intervention and pretrial screening and SF-12v2 plus 4 total scores (see Appendix 6, Table 21). Details of sensitivity analysis on the primary analysis in Appendix 6, Table 22 are shown in Appendix 6, Table 21 and graphically displayed in Figure 14.
FIGURE 14.
Different analysis sets: clinical effectiveness at 3 months. MI, multiple imputation; RI, regression imputation.
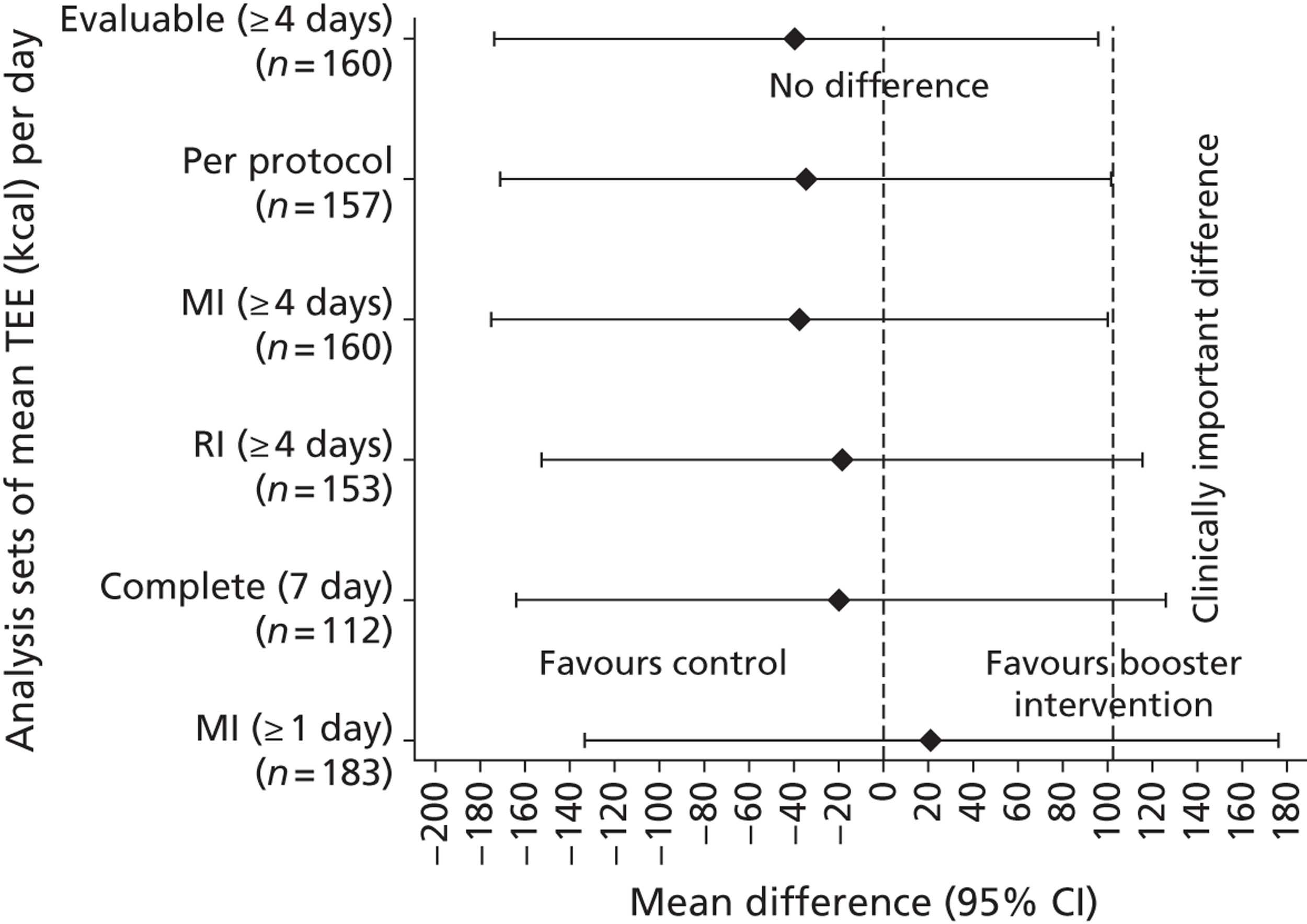
Figure 15 shows the distribution of mean daily TEE for the cohort of participants at 3 and 9 months. The treatment effect (mean difference) excluding the four protocol violators, described in Baseline characteristics of participants, was −51.2 kcal (95% CI −185.7 to 83.3 kcal; p = 0.453) in favour of the control group.
FIGURE 15.
Mean daily TEE per day (kcal) over time for participants with valid 3- and 9-month post-randomisation outcome data (n = 80).
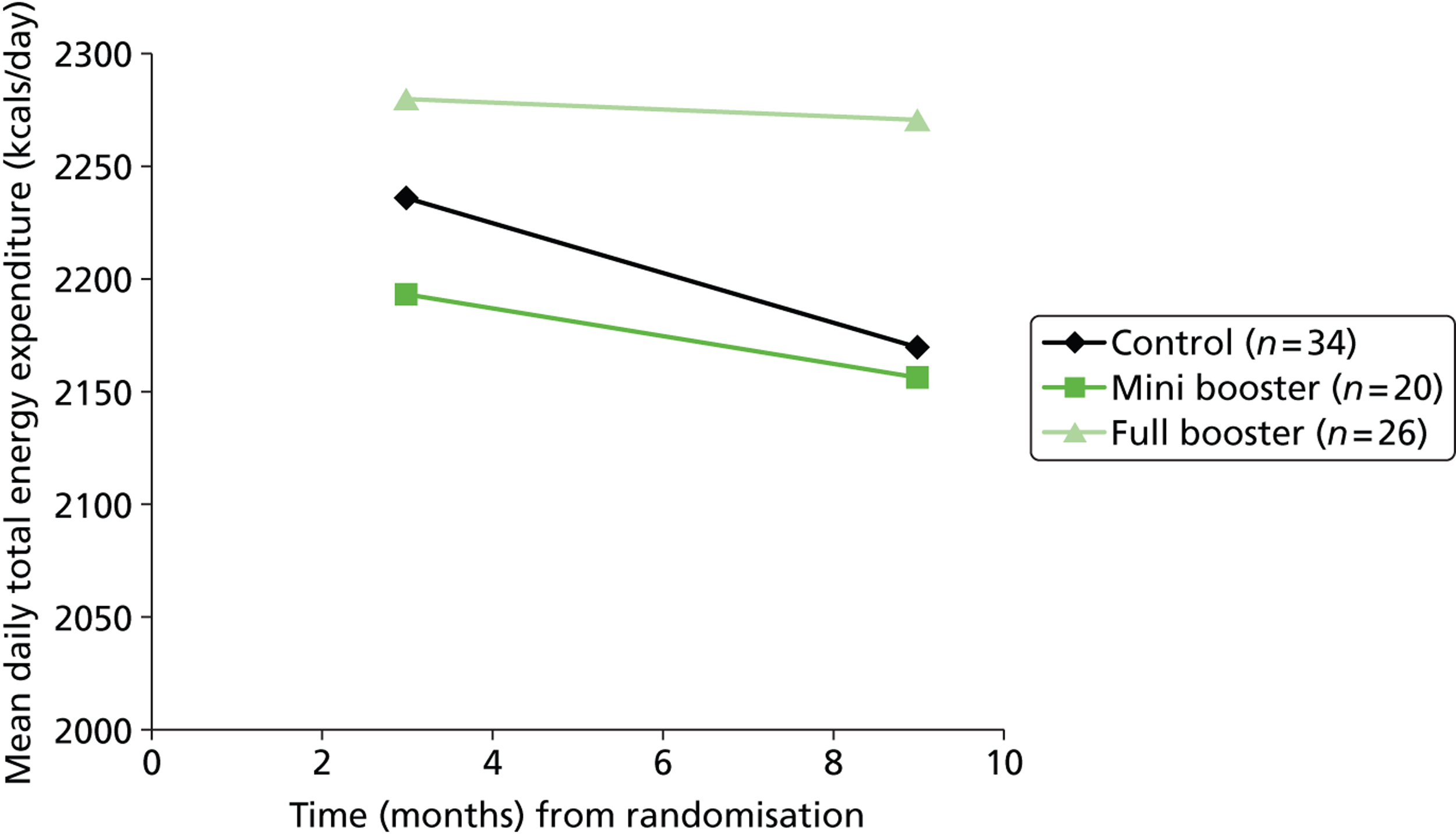
Effectiveness of the mini booster intervention compared with the full booster intervention at 3 months
At 3 months post randomisation we observed a mean TEE per day of 2168 kcal and 2280 kcal in the mini booster (n = 47) and full booster (n = 52) intervention groups, respectively, which translated to a mean difference of 112 kcal (95% CI −57 to 280 kcal; p = 0.2) in favour of the full booster group. After adjusting for age, gender, BMI, total minutes of physical activity at brief intervention and pretrial screening and HRQoL based on SF-12v2 plus 4 total scores, the mean difference was 56 kcal (95% CI −38 to 149 kcal; p = 0.4). Again, the wide CIs around the observed treatment effect indicate the uncertainty in the effectiveness estimates and the results are not statistically significant. However, as the CIs do include the MCID of 102 kcal, and the point estimates clearly show that the full booster group had the better outcomes at 3 months, we cannot rule out a significant difference between the mini booster group and the full booster group.
Secondary outcomes at 3 and 9 months
Appendix 6, Table 24 shows the effectiveness of the full booster (face-to-face) intervention compared with the control. We observed a small increase in physical activity of 14 kcal in favour of the full booster intervention at 3 months although this is not statistically significant and far from being clinically important. The wide CI around this estimated effect indicates huge uncertainty and is compatible with no difference in effectiveness between the control and the full booster intervention. The direction of the effect changed in favour of the control group after adjusting for potential confounders (see Appendix 6, Table 24).
As shown in Appendix 6, Table 25, we observed a decrease in physical activity of approximately 98 kcal in the mini booster intervention group compared with the control group at 3 months, although this was not statistically significant and the uncertainty around the estimated effect was also large. The direction of the effect was consistent even after adjusting for age, gender, BMI, total minutes of physical activity at 3 months and 1 week before randomisation, and HRQoL (SF-12v2 plus 4 total scores). Similarly, the differences at 9 months in TEE are not statistically or clinically significant, as illustrated by Figures 15 and 16.
FIGURE 16.
Mean TEE per day (kcal) at 9 months stratified by intervention arm (n = 91).
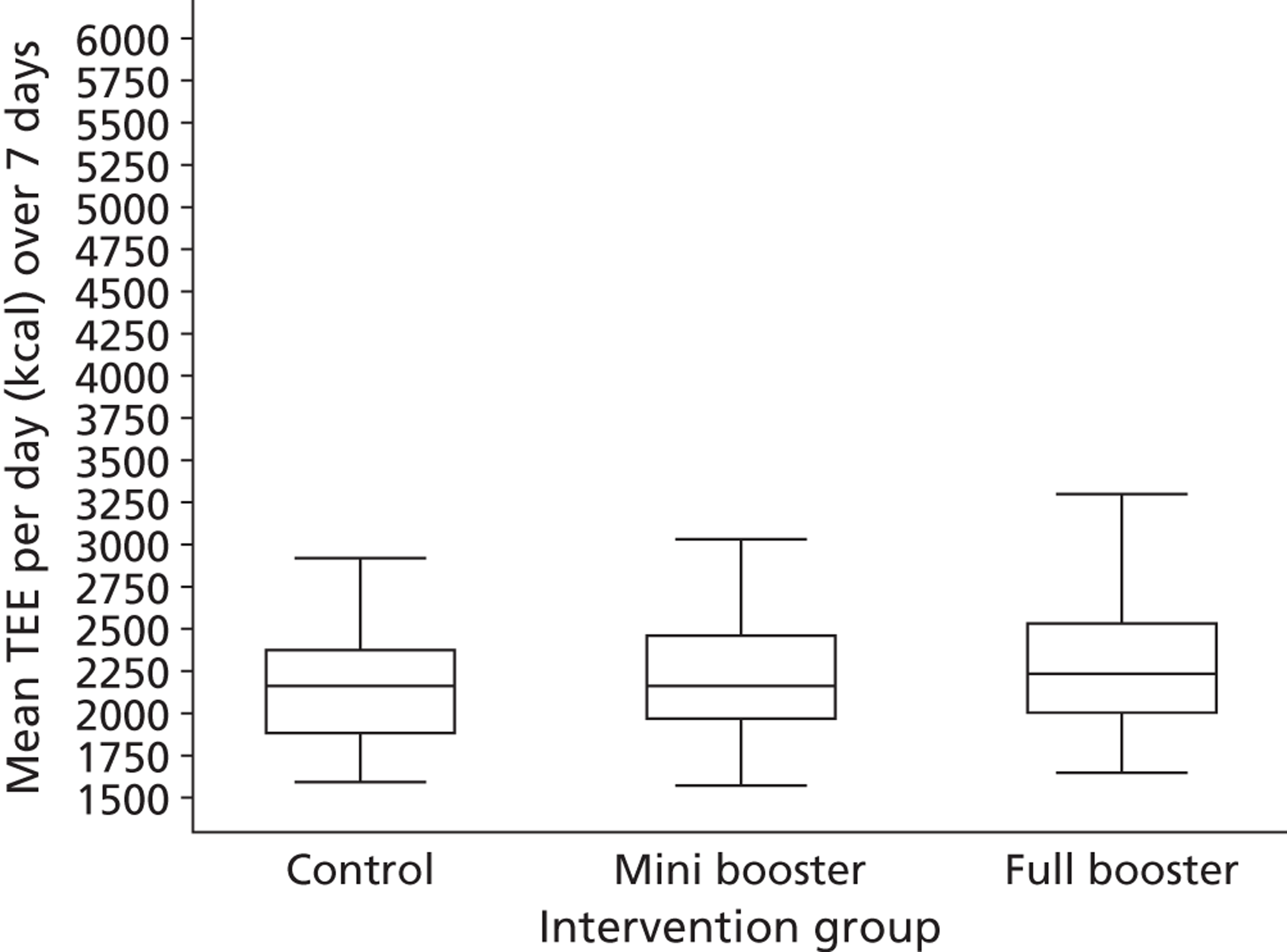
For the other continuous secondary outcomes – PACs (see Appendix 6, Tables 30 and 32), PCS, MCS and SF-6D scores from the SF-12v2 plus 4 (see Appendix 6, Tables 34 and 35), average minutes per day spent on moderate activity (3–6 METs), average minutes per day spent on vigorous activity (> 6 METs) and average minutes per day spent on moderate and vigorous activity (≥ 3 METS) (see Appendix 6, Tables 36 and 37) and BMI (see Appendix 6, Tables 44 and 45) – we found no reliable statistical evidence of a difference in outcomes between the booster group and the control group at 3 and 9 months post randomisation. There was insufficient evidence to support differences in self-reported levels of physical activity between the booster group and the control group at 3 and 9 months. However, based on the ITT set (see Appendix 6, Table 46), participants in the booster group reported increased physical activity of around 49 minutes and 74 minutes over a 1-week period at 3 and 9 months, respectively, although this was not statistically significant at the 5% significance level. In addition, there was a very weak correlation to support a positive linear association between the objective measure (based on accelerometry) and the self-reported measure of physical activity (based on the SPAQ) at 3 months of follow-up (Pearson correlation coefficient = 0.03, 95% CI −0.13 to 0.19; p = 0.686), as shown in Figure 17. Some studies elsewhere reported similar results of a weak association between objective and subjective measures of physical activity. 82 In light of the very weak association between the objective and the self-reported measures of physical activity, we urge that results based on the self-reported SPAQ measure are interpreted with caution (see Figure 17).
FIGURE 17.
Scatter plot and regression line showing the correlation between mean TEE per day and self-reported minutes of physical activity (based on the SPAQ) at 3 months. Correlation coefficient = 0.03; p-value = 0.686; n = 156. Dots represent mean values for individuals for whom data were available; the dotted line represents the regression line.
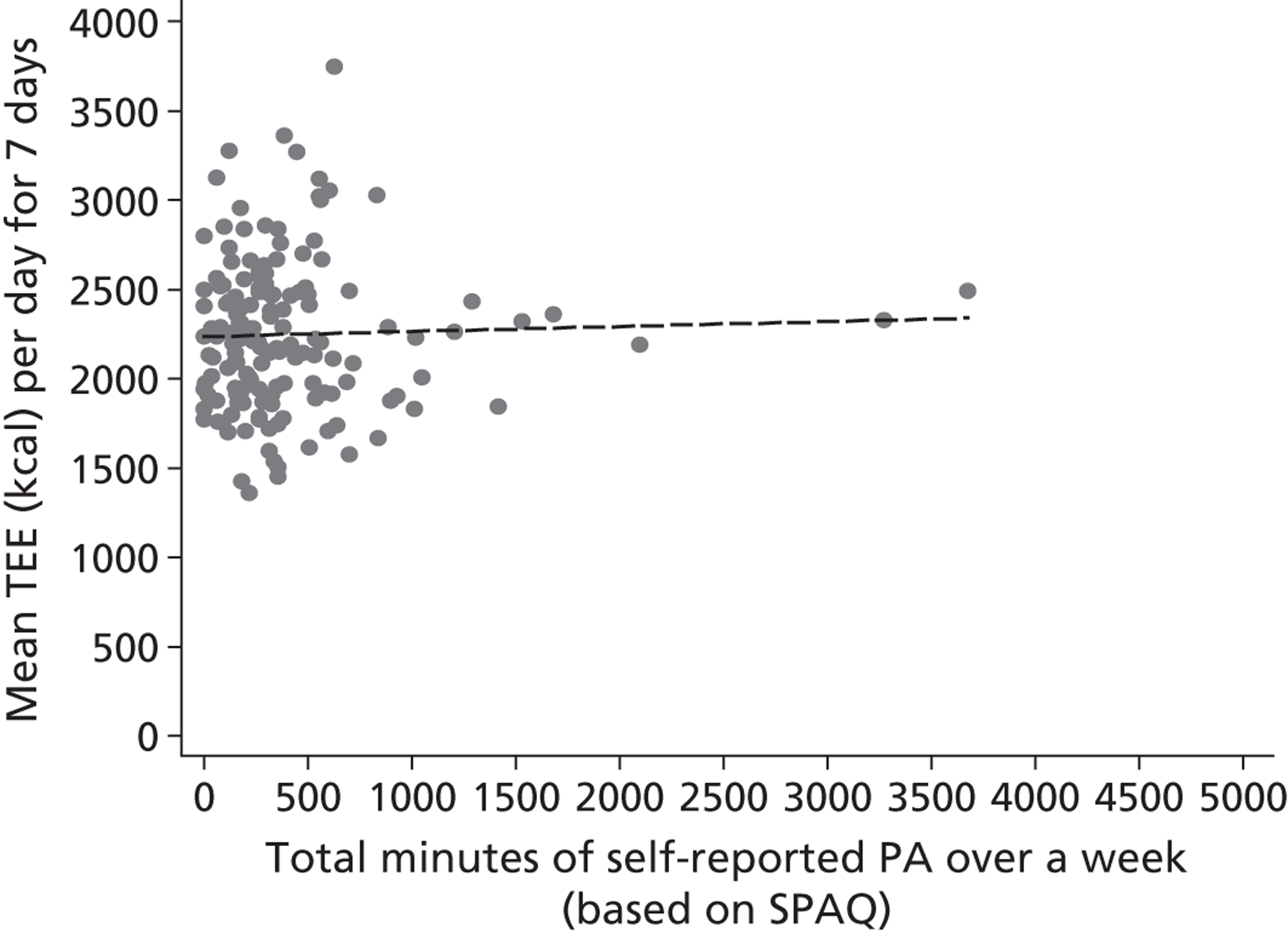
Similarly, for the following binary categorical outcomes, we found no reliable statistical evidence of a difference in outcomes between the booster group and the control group at 3 and 9 months post randomisation: the number and proportion maintaining (or increasing) their weekly duration of physical activity (based on the self-reported SPAQ; see Appendix 6, Tables 38 and 39) and the number and proportion meeting the current recommendations of at least 30 minutes of at least moderate physical activity (MET level ≥ 3) on at least 5 days of the week (see Appendix 6, Table 50). Figure 18 shows a clustered bar chart of the distribution of the number of days with at least 30 minutes of at least moderate physical activity.
FIGURE 18.
Crude distribution of the number of days with at least 30 minutes of at least moderate physical activity based on the Actiheart device (n = 189).
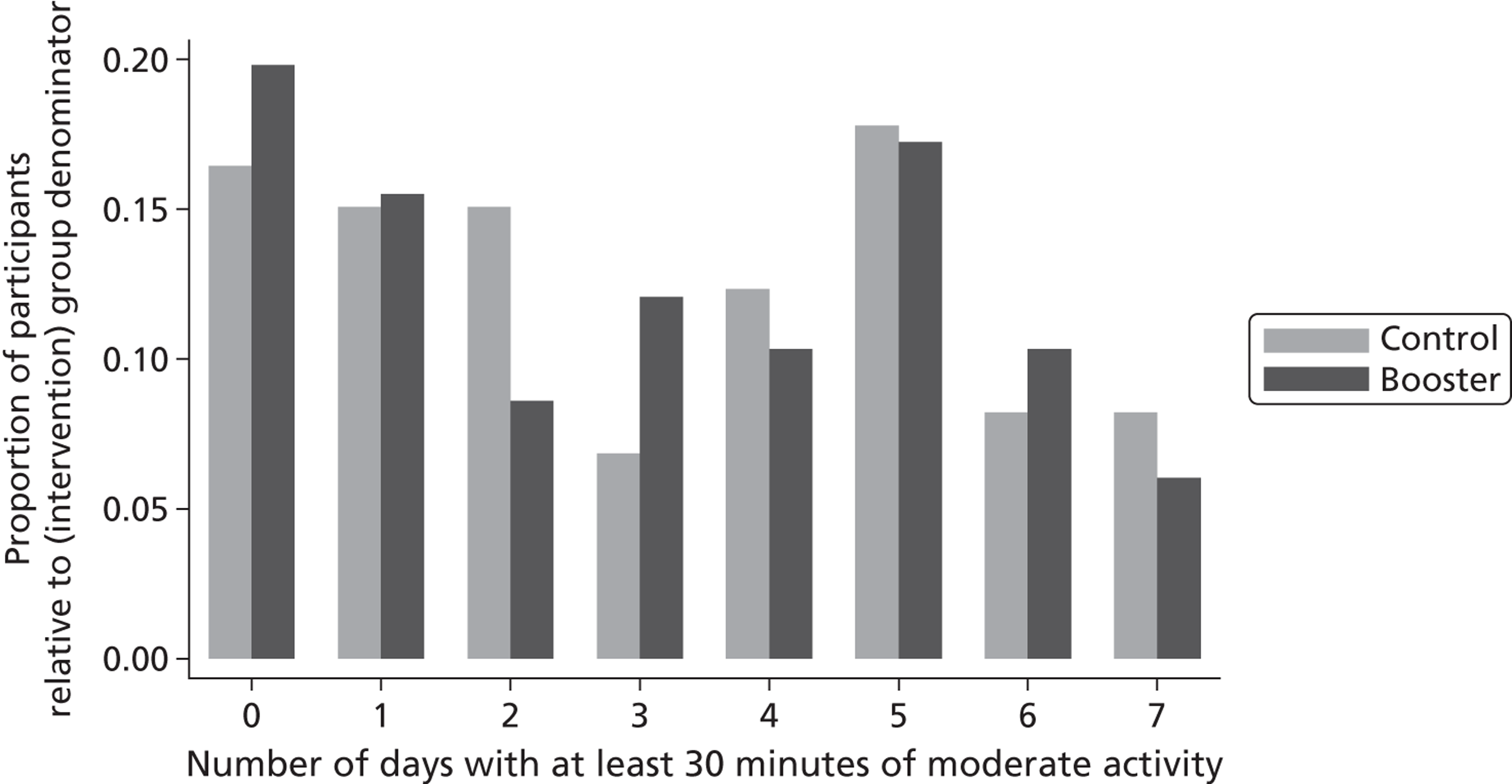
Only for the distance walked (in metres) during a 12-minute walk test (see Appendix 6, Tables 48 and 49) and the BREQ-2 dimensions (introjected regulation, identified regulation and intrinsic regulation; see Appendix 6, Table 40) did we find any reliable or borderline statistical evidence of a difference in outcomes between the booster group and the control group at 3 and 9 months post randomisation at the 5% significance level.
Subgroup analyses
Gender, ethnicity and access to community facilities (self-reported use vs. no use of community facilities) were predefined as subgroups that we wished to test for evidence of effectiveness in an exploratory analysis. As the majority of participants with valid 3-month primary outcome data were white British [89% (143/160)], we could not test for an ethnicity subgroup effect. We found no reliable statistical evidence of any (gender or access to community facilities) subgroup effects or interactions between the booster group and control group at 3 and 9 months post randomisation (Figures 19–24 and see Appendix 6, Tables 51–54). In addition, there was no reliable statistical evidence to suggest that the timing of the initial mail-out had an impact on the response of participants at 3 months (see Appendix 6, Tables 55 and 56). However, there was some indication (with respect to direction of the effect) that participants who were approached to take part in the summer or the spring were performing slightly better in the full booster arm than their counterparts who were approached in the winter or the autumn, although there was huge uncertainty in the estimated effect (see Appendix 6, Table 55).
FIGURE 19.
Subgroup evaluation for the primary outcome at 3 months stratified by gender (n = 160).
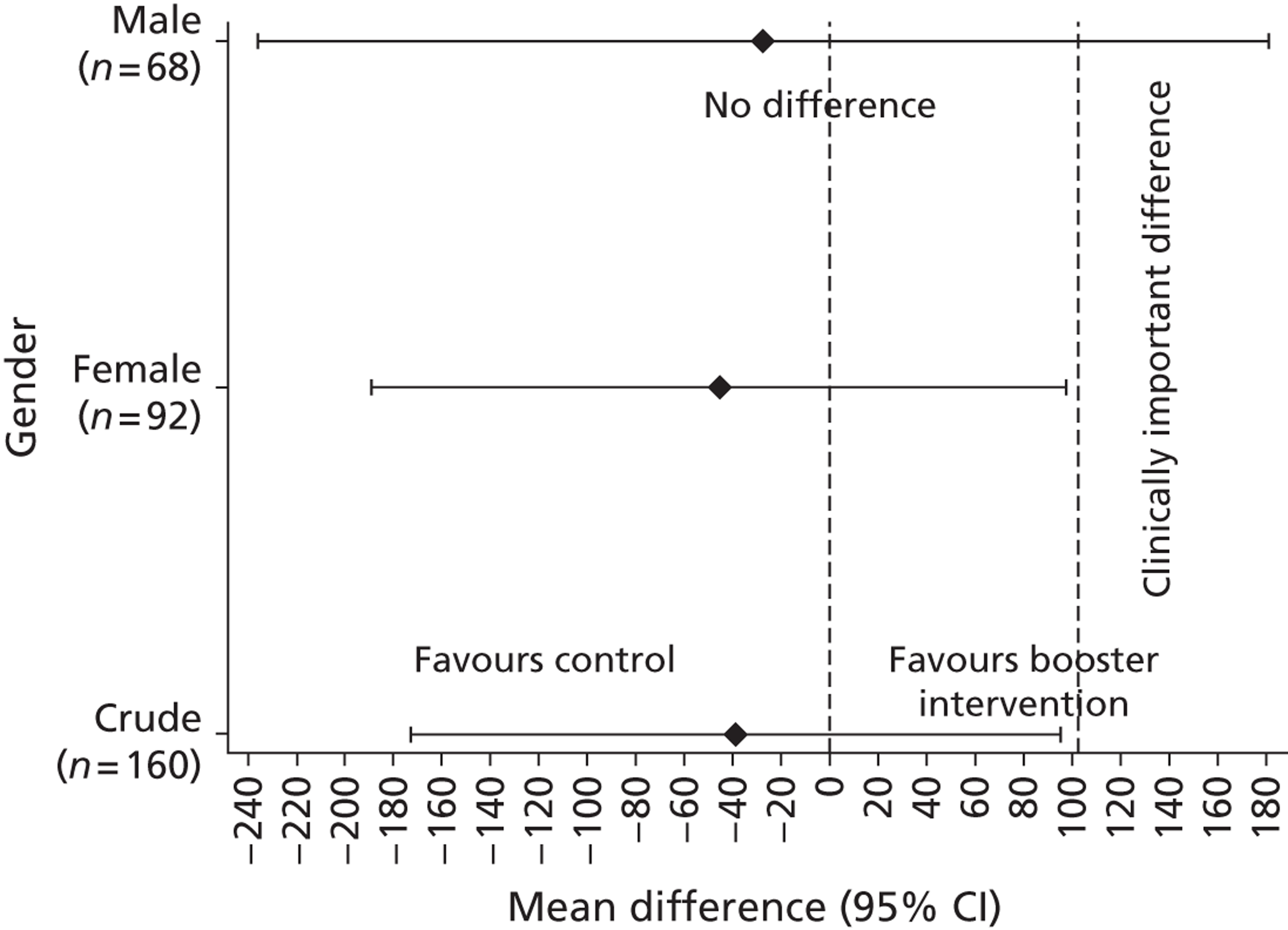
FIGURE 20.
Interaction effect of the intervention and gender at 3 months. Interaction = –18.2 (95% CI −260.6 to 224.3; p = 0.882).
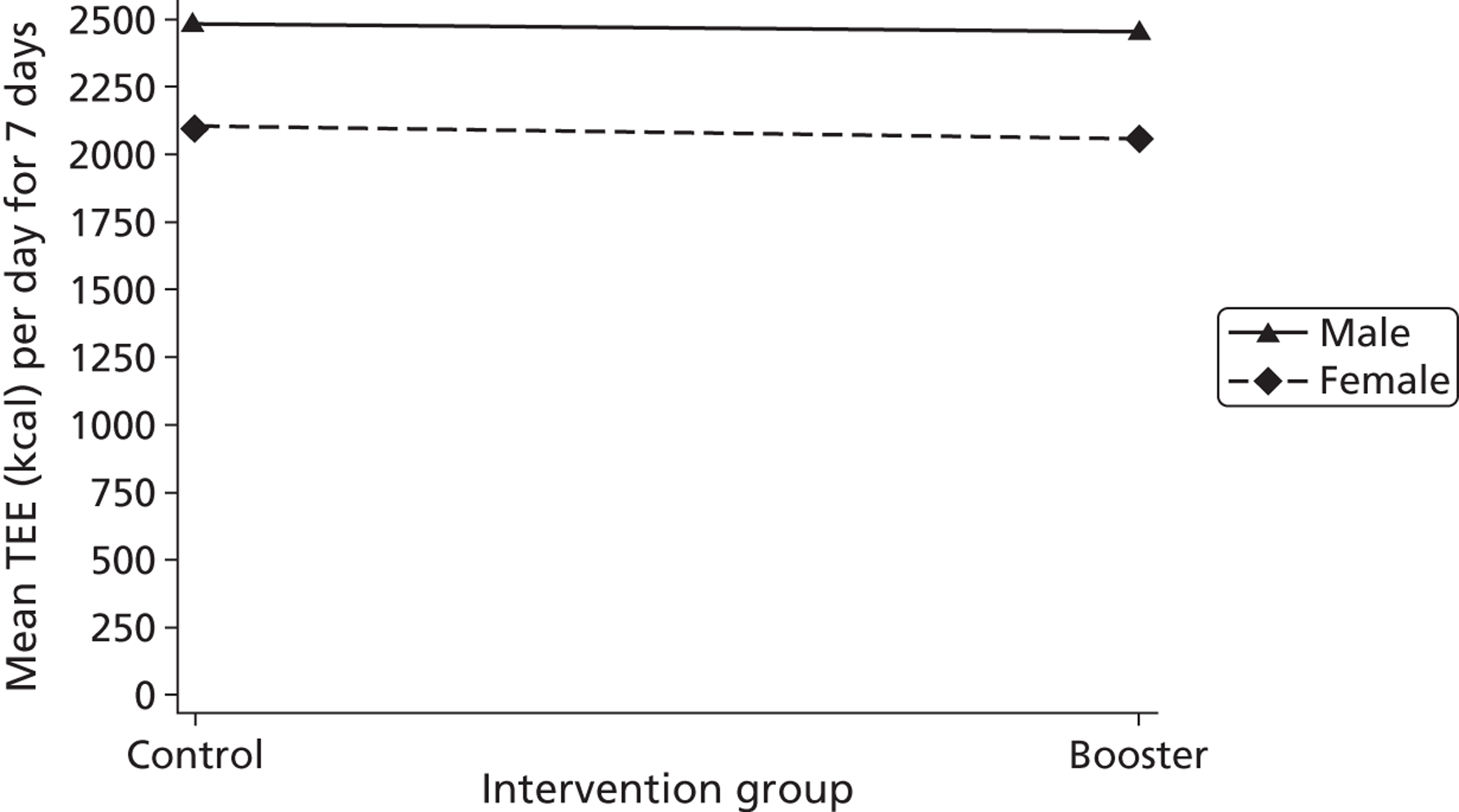
FIGURE 21.
Interaction effect of the intervention and gender at 9 months. Interaction = 49.2 (95% CI −262.6 to 361.0; p = 0.755).
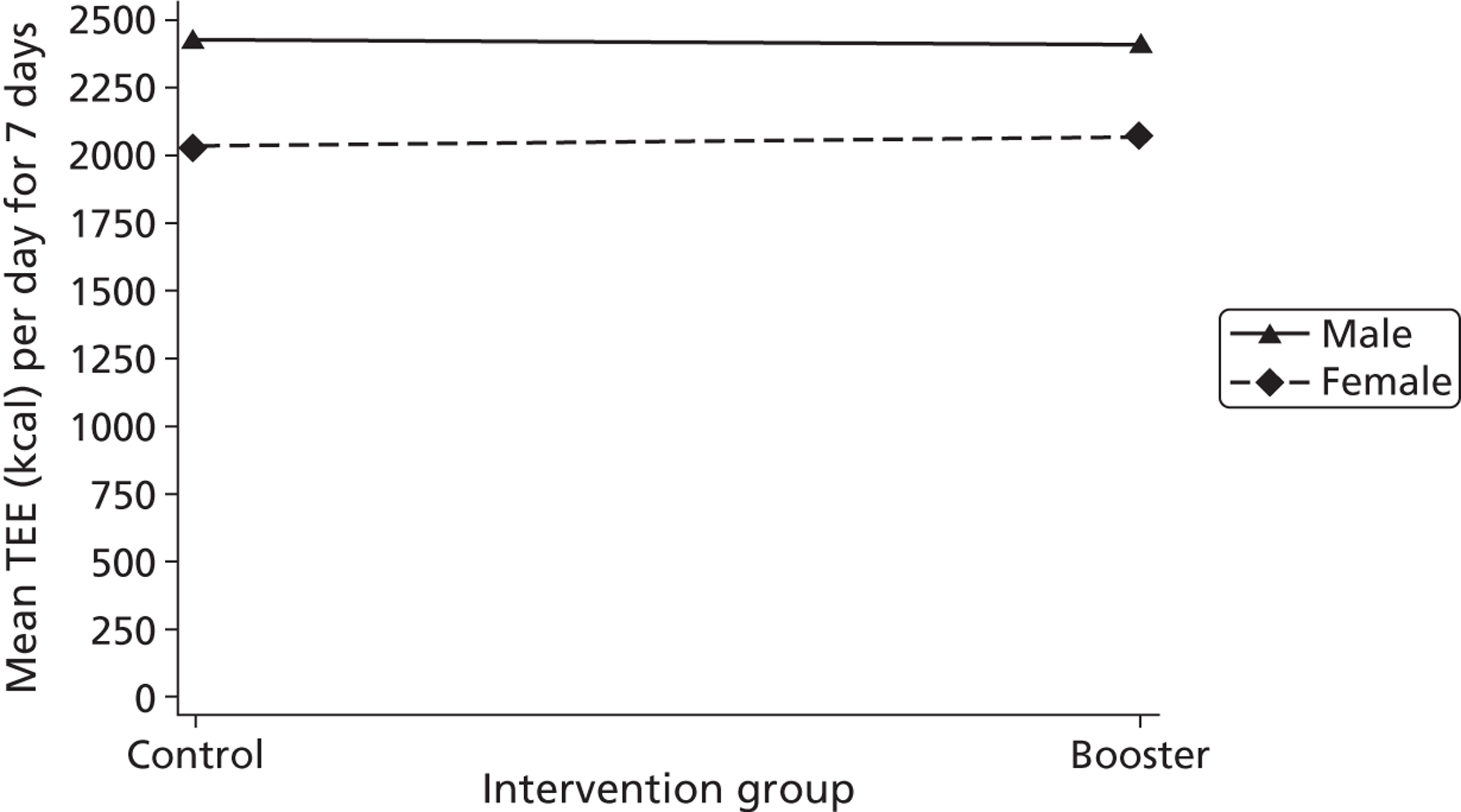
FIGURE 22.
Forest plot of the effect of the intervention stratified by use of community facilities in the last month at 3 months.
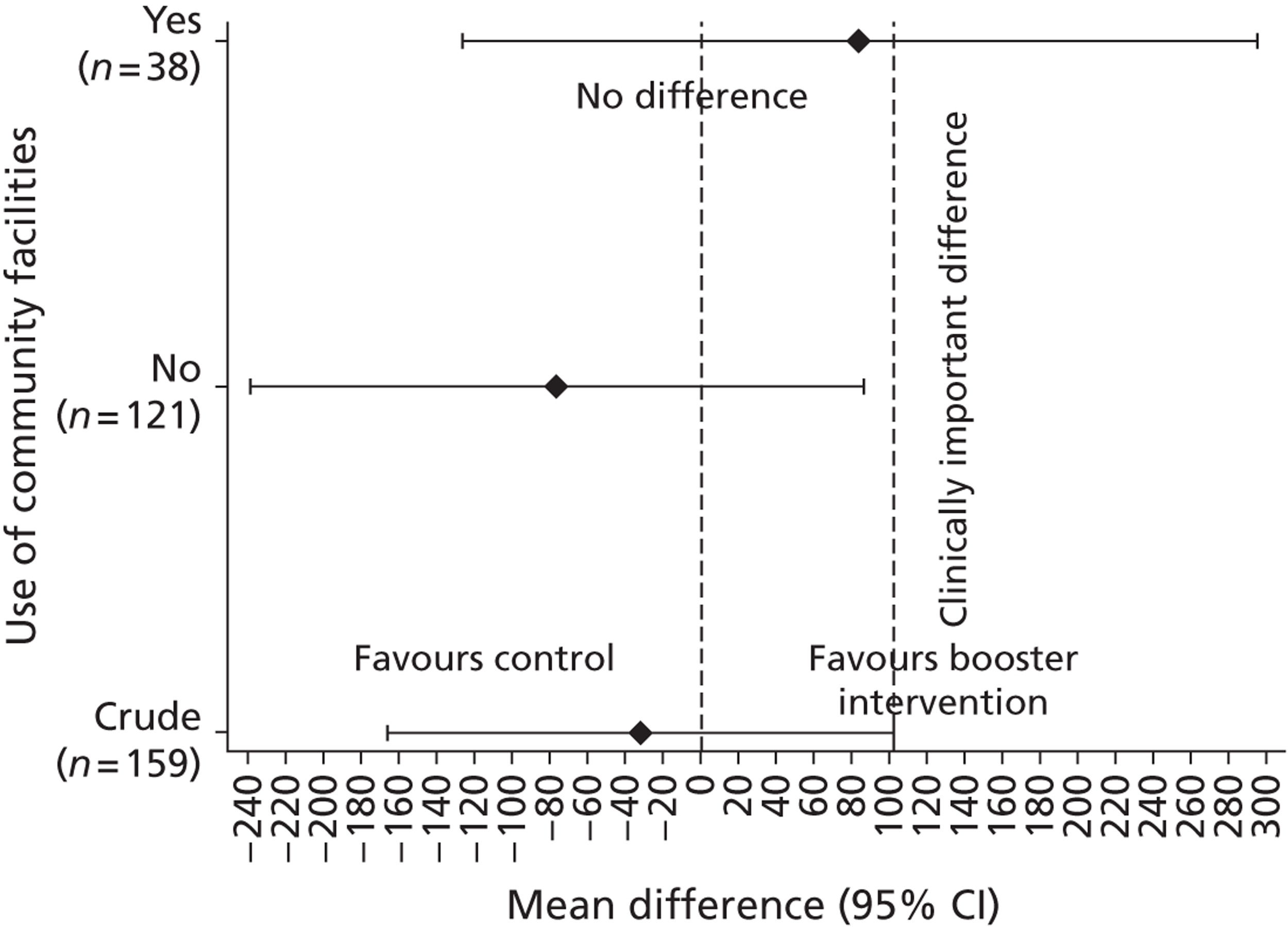
FIGURE 23.
Interaction plot of the intervention and use of community facilities in the last month at 3 months. Interaction = −160.2 (95% CI −469.1 to 148.7; p = 0.307).
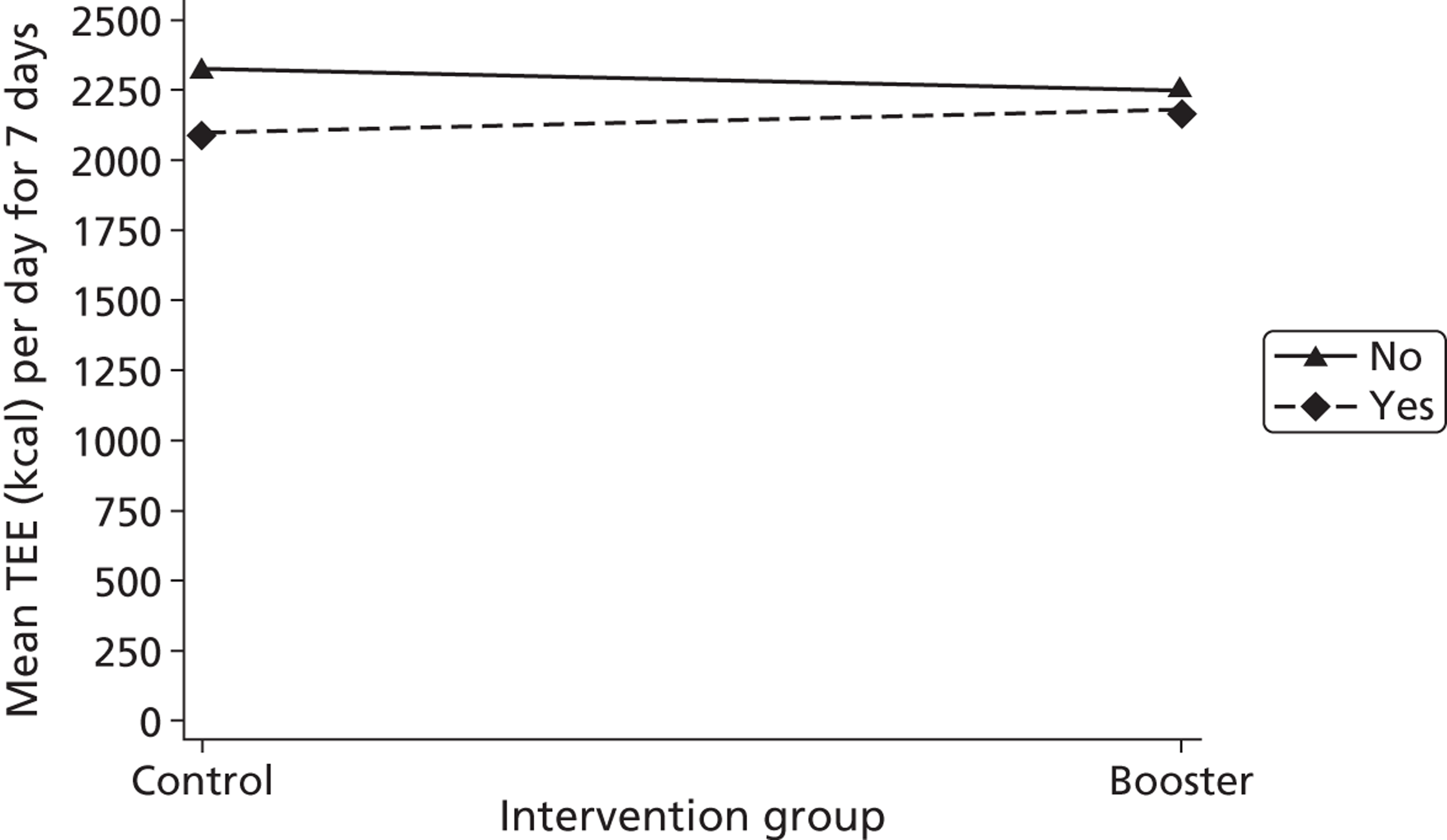
FIGURE 24.
Interaction plot of the intervention and use of community facilities in the last month at 9 months. Interaction = 100.3 (95% CI −264.8 to 465.3; p = 0.586).
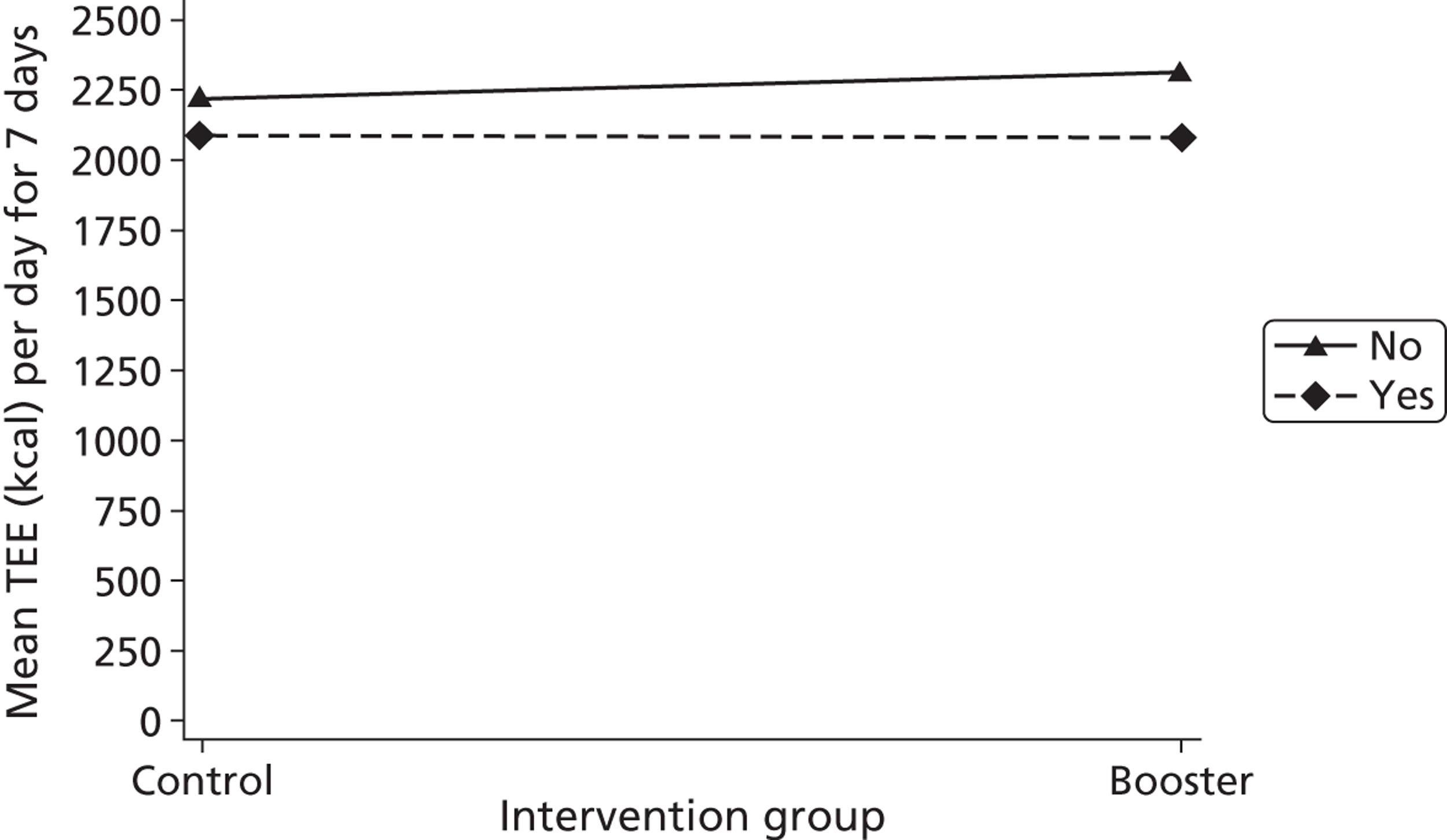
Chapter 4 Results of the process evaluation
All tables relating to the process evaluation can be found in Appendix 7.
Results of the survey
The sample
In total, 239 people were sent and 75 returned the survey questionnaire. The baseline characteristics of the survey population are compared with the baseline characteristics of trial participants who did not take part in the survey in Appendix 7, Table 57. The only variable that shows a difference between groups worth highlighting was the change in SPAQ score between −3 months and baseline, with non-responders reporting larger changes than survey responders. Formal significance testing for baseline comparability was not performed.
Closed questions
The responses to the process evaluation closed questions are presented in Appendix 7, Table 58. Over the 3 months before they were surveyed, 64 respondents (84.2%) reported taking part in recreational/leisure activities (e.g. gardening, cycling), 10 (13.2%) in competitive sports/exercise, 26 (34.2%) in structured exercise (e.g. an exercise class) and 54 (71.1%) in active commuting (e.g. walking/cycling to work). In total, 37 (48.7%) respondents reported undertaking physical activity at home, 41 (53.9%) in a local open space (e.g. a park), 34 (44.7%) in a facility (e.g. a gym, pool, community centre or track), 52 (68.4%) as part of their daily activities (e.g. in work, shopping, walking the dog or commuting) and 11 (14.5%) in other places. The most common reasons given for staying physically active were to improve health and get fitter or to stay fit, with 59 respondents (77.6%) reporting that this was very much the case. The most commonly reported influence on being able to perform their chosen activity was a respondent’s own health (n = 56, 73.7%). In total, 34 respondents (44.7%) reported that they did physical activity with other people, of whom most (n = 29, 38.2%) said that they found this fairly or very useful. The most commonly reported partners for physical activity were a spouse (n = 19, 25.0%), friend (n = 15, 19.7%) or other family member (n = 10, 13.2%). If further physical activity ‘booster’ advice had been available in the future, more people would have preferred it to be delivered face to face (n = 29, 58.0%) than over the telephone (n = 13, 26.0%) or by written advice (n = 12, 24.0%).
Overall, 43 (86.0%) of those who received physical activity ‘booster’ advice said that it met their expectations, with more in the face-to-face full booster group reporting so (n = 24, 96.0%) than in the telephone mini booster group (n = 19, 76.0%). The majority (n = 38, 76.0%) agreed or strongly agreed that the ‘booster’ counselling/advice sessions fitted easily into their daily schedule, with no obvious difference between the mini booster (n = 19, 76%) and full booster (n = 19, 76%) participants. The majority of respondents who were full booster recipients (n = 21, 84.0%) agreed or strongly agreed that the ‘booster’ counselling/advice sessions had been conducted at a convenient location.
In total, 41 respondents (82.0%) who received booster interventions agreed or strongly agreed that the project worker was non-judgemental and 39 (78.0%) agreed or strongly agreed that the booster counselling/advice sessions were non-confrontational in nature. A total of 44 participants (88.0%) agreed or strongly agreed that, throughout the ‘booster’ counselling/advice sessions, the project worker understood what they were saying. Overall, 22 respondents (44.0%) said that they spoke more than the project worker and 21 (42.0%) spoke roughly the same amount as the project worker. In total, 45 respondents (90.0% of those receiving the intervention) said that the amount of contact time with the project worker was about right. The majority (n = 42, 84.0%) agreed or strongly agreed that they were encouraged to set their own goals for physical activity and 31 (62.0%) agreed or strongly agreed that, as a result of the ‘booster’ counselling/advice sessions, they had been able to resolve their barriers towards physical activity. Overall, 33 (66.0%) agreed or strongly agreed that, as a result of the ‘booster’ counselling/advice sessions, they now knew more about the benefits of physical activity. The same number agreed or strongly agreed that, as a result of the ‘booster’ counselling/advice sessions, they now knew more about the risks associated with living an inactive lifestyle. In total, 32 (64.0%) agreed that, as a result of the ‘booster’ counselling/advice sessions, they were now more aware of available physical activity facilities and opportunities and 41 (82.0%) agreed or strongly agreed that, as a result of the ‘booster’ counselling/advice sessions, their confidence to stay active had increased.
Open questions
Recreational/leisure activities over the previous 3 months
Common recreational/leisure activities reported were gardening (n = 38), walking (including walking the dog) (n = 24) and swimming (n = 10). Housework/DIY (n = 7), gym-based activities (n = 5), cycling (n = 5), rambling/hillwalking (n = 4), running (n = 3), football (n = 2), badminton (n = 2), Wii/Wii Fit (n = 2) and yoga (n = 2) were also reported. The following activities were listed by one respondent each: Zumba, tai chi, diving, camping, bowling, snooker and golf. Some people reported more than one activity. Few people reported activities relating to competitive sports and there was significant overlap with activities classified as recreational/leisure activities. Football, walking/rambling, gym-based activity, badminton and golf were all included in both categories.
Common forms of structured exercise reported were gym-based exercise (n = 8), dance-based exercise (including at home) (n = 7), low-impact exercise classes (n = 6), cardiovascular exercise classes (n = 5), home-based exercise (not including dance/dance videos) (n = 4) and swimming (n = 2). Some people reported more than one activity in this category.
The most commonly reported form of active commuting was walking (n = 33). Walking activities that did not involve commuting (n = 9) or in which it was unclear whether or not they formed part of a commute (n = 10) were also reported. One participant reported cycling and one responded ‘yes’ without any further details. Some people reported more than one activity in this category.
Location of activities
Commonly reported facilities used included a leisure centre (including a gym and/or pool) (n = 28), a rural location (n = 6), a community centre (n = 2), a football facility (n = 2), a physiotherapy centre (n = 2) and shops (n = 2). The following locations were listed by one respondent: dancing, local area, school lessons, tennis courts, local neighbourhood, friend’s home and allotment. Some people reported the use of more than one location.
Reasons for staying physically active
Several people stated that a reason for staying physically active was that a health professional recommended that they should do so. This included a doctor (n = 4), consultant (n = 2), nurse (n = 1), dietitian (n = 1) and the booster research team (n = 1). Under ‘other’, one person reported that the decision to stay physically active was prompted by a wellness advisor at work.
Reasons for taking part in the project
Using thematic analysis, we grouped the reasons that people gave for participating in the project into the following categories: self-improvement, altruism and curiosity. Responses concerning the wish for self-improvement were the most numerous and thematically diverse: respondents wished to improve or maintain their health or well-being, weight level, medical prognosis, fitness level, motivation to do physical activity, performance or practice in particular physical activities or their knowledge about what activities and levels of activities were best for them as individuals. A minority of respondents also discussed their wish to challenge or better understand themselves. Responses that we categorised as ‘curious’ included comments to the effect that the project sounded interesting, respondents wanted to know more or to see what was ‘on offer’ and respondents were looking for new ideas or wanted to try something new. Responses classified as ‘altruistic’ conveyed a desire to help with the research, to advance science and to help other people in the future.
Prior expectations of the ‘booster’ physical activity sessions
Participant reports of prior expectations of the booster sessions overlapped considerably with reports of the reasons for participating in the study, perhaps indicating that they were not making a clear distinction between the contacts made by RAs for the purposes of research study data collection and the contacts made by RAs as part of the intervention (see The importance of monitoring and feedback). Although many of the answers categorised in the previous section under self-improvement, altruism and curiosity were reiterated, there were responses that were conceptually distinct. For instance, respondents reported hoping that the intervention would increase their confidence. A number of respondents reflected on needing encouragement to set, achieve and maintain goals, targets or standards. Respondents also wanted information about their own fitness levels (see The importance of monitoring and feedback) and this included advice on physical activity that would meet or suit their existing medical needs (see Previous conditions, Barriers to physical activity and Physical activity: motivators on medical health issues).
What helped people become more physically active than they were before the study?
Participants from all three groups listed similar factors contributing to an increase in their physical activity levels since the onset of the study. The largest number of reasons given related to the booster intervention. Some respondents felt that the booster intervention had given them help, advice and support and confidence. A number of respondents reported the role played by their newly increased awareness of the health benefits of physical activity, including weight loss, and the dangers of being inactive. A number mentioned the motivation or encouragement provided by the RAs, including that provided by a desire to achieve goals and targets set with the counsellor. Participants reported responding to prompts to think about an active daily routine, discussing the need and different ways to keep fit, as well as an increased awareness about their own activity levels. Aside from the engagement with the RAs, respondents reported other, wide-ranging, extrinsic factors affecting changes in physical activity levels including the adoption of flexible working hours, having appropriate footwear, the receipt of a medical diagnosis and support from family or friends. A few reported more intrinsic reasons, with some crediting themselves for becoming more proactive. These individuals talked about their self-motivation, their own desire to improve their fitness or reduce their weight.
Results of the in-depth interviews
The sample
In total, 26 people participated in the in-depth interviews between December 2010 and February 2012. The baseline characteristics of the interview population are compared with the baseline characteristics of the trial participants who did not take part in the interviews in Appendix 7, Table 59. Participants in full-time employment were under-represented (15% in the interview population compared with 35% of those not interviewed). As with the survey population, increases in self-reported physical activity in the 3 months before baseline were lower for those interviewed than for those not interviewed.
Actiheart data were not available for six interviewees. Of the remaining 20, 17 contributed 7 days of data, one contributed 4 days of data and two contributed 3 days of data (see Appendix 7, Table 60). We have no evidence that any of the participants met the national guidance of at least 30 minutes of moderate/vigorous exercise on at least 5 days per week, although it is highly likely that one participant would have done so as he did > 5 hours of at least moderate activity on each of the 3 days over which he contributed data. Two people met the target of 30 minutes of moderate/vigorous exercise on 3 days; two people met the target on 2 days; three people met the target on 1 day; and the remaining 13 people did not meet the target on any day for which they provided accelerometry data.
Participants noted that they preferred the following physical activities: walking, swimming, cycling, gardening, walking the school run, football, running, yoga, gym, badminton, physical jobs, playing on the Wii and dancing. Some participants also declared a strong dislike for the gym and one declared a strong dislike for jogging.
Previous conditions
Previous practice
Two distinct groups of people seemed to emerge as salient in the sample. First were people with positive past experiences of physical activity whose commitment to an active lifestyle had been disrupted by the time that they were contacted by the trial team. This disruption frequently involved medical conditions, environmental factors, such as a change in working patterns, or a change in priorities, such as new commitments brought on by family (see also Barriers to physical activity and Physical activity: motivators):
Yeah, but I did do quite a bit of walking in my working life. I’ve cut back on the work and eventually retired . . . and suddenly it hits you that yes, all you’re doing is sitting round all day.
Co-habiting male, 65 years
Because I suffer from vertigo and I kept going dizzy – it’s the Raynaud’s [syndrome] – I’d stopped cycling for five years.
Married male, 65 years
I’d more or less retired from running because I’ve been doing sport all my life and I just thought, coming up to 50, I thought, ‘Give it up’ because I wasn’t, because we’ve got twins and . . . we were up early in the morning and you were breaking your sleep pattern . . . So I just thought, I’ll give up the running and just look after the kids.
Divorced male, 49 years
Second were those who did not discuss previous positive experiences and for whom the uptake of physical activity was related to morbid introspection brought on by the birth of children, grandchildren or ageing (see also Physical activity: motivators):
Well I’ve been going to the gym for 10 years and I can’t say I really enjoyed any of that time. I’ve never been a particularly sporty person all through my life but I got to about 52 and I thought well if you don’t get you know . . . I was doing a lot of driving and a lot of sitting behind a desk, so I thought if you don’t do something now, it’s never going to happen because it’s all downhill from 60 so . . .
Married male, 64 years
My main motivation is the fact I’ve stopped smoking and well, my girls . . . At the end of the day it is as much for me as it is for them . . . I’m sure they don’t want to lose their dad when they’ve just turned 20 do they?
Married male, 48 years
Many people in the sample, but especially those with previous positive experiences of physical activity, were revising their expectations of what they were capable of after injury, the onset of a chronic physical condition or just the ageing process more generally. This was clearly difficult for many and the subject of intense rumination:
I did used to be a very, very good squash player, played up at [. . .] many times against the masters . . . the first operation stopped me playing squash . . . the same surgeon, promised me that it would get me back doing everything . . . and it didn’t, you know. I could have done with [the motivational interviewing] then, you know, saying, ‘Don’t do this; move onto something else’.
Co-habiting male, 65 years
I’m not doing what I did some 12 months before . . . I know now I’m not going to be able to return to that level.
Divorced male, 55 years
Yes. Yes. I realise I can’t be what I was, so I’ll never be doing a marathon again, but at least . . . every five miles is a marathon to me now, between start and finished.
Married male, 65 years
Felt needs or problems
Many participants simply wanted ‘to feel fitter’. Of these, a number were looking for advice on tailoring a physical activity regimen to their lifestyle: ‘I didn’t want to join any gyms or anything . . . I wanted some other options and something that if I didn’t fancy going one week I didn’t have to’ (married female, 52 years).
Many were motivated by goals or perceived benefits which they hoped that increased physical activity levels would deliver (see Physical activity: motivators). For some, these anticipated benefits were urgent because of changing life circumstances:
I wanted to build my strength up, My husband became ill and I was having to do a lot of . . . physical work and I was finding it very difficult because I suffer with arthritis myself. So I needed to lose weight . . . to build my muscle up as well.
Married female, 64 years
Long-term conditions
At least half of those interviewed had a chronic condition that affected their attitude to physical activity in one way or another (see Barriers to physical activity and Physical activity: motivators), including traumatic stress; high blood pressure and high cholesterol; arthritis and depression; obesity, stress and depression; depression; asthma plus a family history of diabetes, mental illness and obesity; breast cancer, high cholesterol and pre-diabetes; a history of total knee surgery; arthritis and knee problems; chronic lung disease; chemotherapy-related neuropathy; shoulder injury; hypertension, diabetes and a history of heavy drinking; Raynaud syndrome and a history of transient ischaemic attacks.
Social norms
Although not prevalent, there were at least three instances of participants reporting that physical activity was not socially normative in their community:
I think so yeah. I think some of it is being on your own, if you know what I mean? If you are on your own and your friends don’t do exercise it doesn’t encourage you much.
Married female, 52 years
And that’s the thing; a lot of pressures get put on people that they can’t, because their peers will say: ‘Oh you don’t want to be doing that, you want to be coming down the pub and having a few beers like we normally do’ . . . then they feel pressurised to actually say, ‘Well I’d better not do that because I’ll upset them’.
Divorced male, 55 years
People have said to me, ‘Oh . . . you’ve done Sheffield round walk – which is about 16 mile – you must be daft!’
Married male, 65 years
Barriers to physical activity
Preferred alternatives
At least 10 people explicitly mentioned that they had preferred alternatives to physical activity, including college classes and work; watching television; looking after their children and going to work and running their own business. A number of people also mentioned ‘personal’ or ‘family situations’ that had disrupted or prevented the uptake of physical activity routines:
I find it more difficult to keep motivated, cos I don’t really like exercise. I could . . . I’m one of these people that could just sit and watch telly all day and enjoy it.
Divorced female, 65 years
Yeah . . . my personal life has been a bit different in these last 12 months or so, I think that’s, you know, made more of an impact on my fitness . . . I can’t get down to the gym, I won’t have time, coz I run my own business, you see, I have my own shop.
Widowed male, 60 years
Cos I do still do some part time work – I look after a lady with dementia – so I’m not free every day by any means. So the main thing is, sort of fitting it in around . . . Once a week is fine. Fitting in twice or three times can be difficult sometimes to find that bit of free time.
Single female, 62 years
Medical health issues
At least 10 interviewees had chronic health issues (see Physical activity: motivators) and a number of other more acute episodes were also declared, including muscle strains and trauma leading to sepsis; knee injury; muscle pain – non-specific; back pain; foot pain; severe depression and pain in the side and leg. Those with acute injuries often described difficulty maintaining their existing programme of exercise: ‘I was doing an hour’s session with a personal trainer. She gave me a programme to do. But the trouble is I was finding with these injuries . . . I couldn’t do all of it’ (married male, 64 years).
Those who have had surgical treatment or aggressive treatment for a chronic disease often reported constraints placed on them by caregivers so as not to aggravate their condition:
I’m not allowed to . . . as much as I hated running on me own, it always had to be done for stamina, didn’t it? . . . After you’ve had total knee surgery . . . you can’t go pounding on the road and you’ve got to be careful, she says to me, cos I had it done at such a young age, they said, you know, 50, you’ve got to look after it cos it’ll last 25 years if you want, but they just said to me, ‘you know if there’s any problems, you’ll have to have remedial surgery’.
Married male, 58 years
Oh yeah, I was always active . . . Well, I was working as well before I went off on the sick, so everything fell – because of the illness [cancer] and whatnot, everything sort of stopped for a while.
Married male, 57 years
Psychosocial conditions such as depression and heavy drinking presented participants with barriers to starting physical activity that were more related to apathy, lethargy or procrastination:
Yeah, because my doctor recommended the exercise for severe depression, it’s the depression . . . vicious circle . . . it’s the depression that stops the motivation, you know, I can sort of very easily think, ‘Can’t be bothered’ or ‘Let’s not bother’.
Single female, 62 years
Environmental support
Earlier we introduced the idea that maintaining physical activity levels might be difficult when it was not socially normative (see Previous conditions, Social norms). Some people also found their family to be a barrier. Societal and personal budgetary constraints were mentioned by a number of people. Some had experienced cherished physical activity programmes being cancelled because of local authority cuts: ‘They’ve got a group, a dad’s group, and I do the 5-a-side, but that’s kind of stopped due to lack of funding’ (married male, 48 years).
Others saw their own financial situation as constraining their options:
Well, I do want . . . I’d like a lifestyle that is more active, but . . . until I earn more money [laughs] . . . But at the moment, I’ve got very little coming in and very little being spent . . . membership for gyms costs a fortune, I can’t even entertain the idea really.
Divorced male, 50 years
I mean, this is the other thing about my lifestyle, because of the severe restrictions on income, I have to spend most of my time at home so I’m not wearing down my shoes too often . . . And that’s the thing: it’s very easy, you know, when you’re on your own, on a low income, it’s very easy to get depressed and get down. You can spend a lot of time wasting a lot of time just not doing anything, you can just sit down, feel absolutely miserable.
Single male, 51 years
Those who did work often found that a constraint, especially when shift work prevented access to facilities and establishment of a routine (see also Barriers to physical activity, Preferred alternatives). Threats to personal safety were not a common concern, except among those who had aspired to cycle:
I thought, ‘God, to ride a bike on these streets is going to be terrifying’.
Single female, 47 years
I don’t feel confident riding on the roads . . . so I’ve decided I’m not doing cycling.
Divorced female, 65 years
Roads are a nightmare in the morning on a bike, you know, when there’s lots of cars on the road.
Married male, 58 years
A number of people cited bad weather as a barrier to physical activity. For some, this was specifically related to chronic conditions or the side effects of aggressive medical treatment. For most, it was simply a self-evident fact that cold, wet weather was not appropriate for exercise:
Yeah, so, what I need is one of those cycle machines in my flat during the winter time, during the grotty weather, then I’ll have no excuse at all!
Single male, 51 years
I am a summer cyclist because, when it’s cold and wet and you’re on a bike . . . we havn’t got the weather, have we?
Married male, 58 years
The problem I have, in England of course we have the weather in the winter . . . come spring, like now, I’m increasing my energy and my exercise regime.
Co-habiting male, 61 years
Self-consciousness
Self-consciousness was not a widely cited problem; the following two instances were noted:
There’s a gym outside, I think she called it a green gym, it’s the one at Millhouses Park . . . I think my problem is, I’m not really a great fan of gyms . . . I suppose I’m just too self-conscious about these things.
Single male, 51 years
The fact that I am getting older and I feel more embarrassed about going to the swimming baths and things, or a gym, [deterred me] whereas it never used to bother me in the past.
Single female, 56 years
Physical activity: motivators
General commitment and priorities
As many as 15 interviewees expressed views that could be characterised as commitment to physical activity. For about one-third of these, the commitment seemed intrinsic or part of their identity:
To anybody that doesn’t walk and they’re quite capable . . . I find that as alien as what they find me doing them sort of things, not doing anything when you‘re quite capable that you could do something.
Married male, 65 years
Everybody should be involved in sport.
Divorced male, 49 years
Yeah, like I say I’ve always either gone to a gym . . . for, god knows how long . . . I’ve never been idle about it.
Widowed male, 60 years
For the majority, especially those without previous positive experiences of physical activity, it seemed extrinsic – a response to received understandings about the need to ward off infirmity, to stay in a condition to live independently or to discharge one’s responsibilities and to be a role model to one’s family:
Staying alive [is my main goal]. I’ve got two babies. Two and a half and three and a half, you know, it would be nice to see them have kids. You know, so pretty much that singularly would be my main goal I would say.
Married male, 48 years
It would be nice if [my children] could enjoy sport the way I enjoyed sport growing up.
Divorced male, 49 years
I have to be as self-reliant as I can.
Single male, 51 years
I lied to [my children] as much as I wasn’t involved in any sports activities with them when they were young, and they’ve taken it up themselves and enjoying it, I have now found in sport activities something that’s helping me to stay healthy and I’m getting more benefits out of it now at this age.
Single female, 47 years
I have experience of people in the family who’ve not been active and how quickly they’ve deteriorated, health wise and they just couldn’t get about any more, because they weren’t prepared to push their selves and that sort of scared me into thinking, ‘I’m not going to get like that’.
Married female, 62 years
Goals or benefits
Most interviewees (at least 20) described their motivation to maintain physical activity levels in terms of anticipated benefits or goals, including wanting to feel fitter and not so lethargic in the long term; knowing that exercise will make you feel good during and afterwards; for social contact; being a role model; warding off illness, disease or infirmity and living longer; dealing with stress or depression; gaining in self-confidence; wanting to lose weight for ‘vanity’, a holiday, a wedding outfit or health purposes; wanting to remain independent and wanting to try other activities previously thought too taxing:
It’s like if you’re a runner you never feel like going out for a run do you? But then when you’re out there, as soon as you’ve even got your trainers on, you’re feeling better, do you know what I mean? . . . Yeah you feel better when you have done it, you feel a bit sluggish when you go and when you come back you feel more energetic. I mean you feel a bit tired, but it’s a nice feeling.
Married female, 52 years
The thing is that, it’s a way I’ve had to deal with stresses throughout my life, if I had a particularly stressful time, I usually get out for a nice brisk walk up a hill or two. I’ve been on benefits for a very, very long time so I’ve, the only way to handle such stress is actually to do lots of exercise.
Single male, 51 years
The cycling has a sort of almost immediate effect, when I’ve done sort of 20 minutes/half an hour on the bike. You can actually sort of feel a bit of mood lift. It’s strange.
Single female, 62 years
You live longer and I think you just feel better if you do even if you don’t want to do it, you just feel better, look better.
Divorced female, 65 years
I’ve got to build the body up, you know. My wife laughs at me . . . when she sees the bingo wings and whatnot.
Married male, 57 years
Personal preference
Only two people explicitly made the point that they actively preferred physical activity to other activities and embraced it as part of their identity:
Your body is made to work, not just to sit and watch television like my brother . . . There is other things to do than watch television all day.
Married male, 65 years
To anybody that doesn’t walk and they’re quite capable . . . if they’ve got an injury or disabled etc., I find that as alien as what they find me doing them sort of things.
Married male, 65 years
Medical health issues
Although illness and injury could often be a barrier to uptake or maintenance of physical activity, it was also cited in response to questions about motivation by at least 20 interviewees. Those with mental health problems were driven by their perception that physical activity could alleviate symptoms of stress, anxiety or depression, either somatically (‘I really do find that there’s an immediate lift’) or psychologically (‘it takes your mind off it’). In at least one case a person with depression had been prescribed exercise for the management of depression. Prescribed exercise was much more common among those with chronic physical conditions. Most of those with a long-term physical condition (see Previous conditions) talked about clinic visits when asked about what motivated them to become or stay active. However, nobody explicitly claimed that doctors had recommended exercise to them, although conversations such as the following often suggested that this was the case:
[My cholesterol] went up to 5.7 and the doctor said we do prevention now . . . and she’s put me on to 10 mg of Eprinel now . . . because she said my blood pressure were a bit higher than it had been so that’s a big motivation to keep fit.
Single male, 54 years
Environmental and social support
For some, having support and advice from someone who was not a friend or family member, such as a supportive personal trainer, was critical (see also Motivational interviewing: perceived importance). Some people reported getting support from their families, particularly children or grandchildren, or neighbours. For others, the experience of exercising with other people, with whom one could talk, was motivating, with buddying up for swimming, joining classes and cycling, walking or running in groups or clubs all being mentioned:
I suppose if you get somebody who motivates you as well, that goes with you or join a group or something, that’s when it’s enjoyable more I think.
Married female, 52 years
[You] buddy up with someone . . . So that you get a bit of talk. I’ve made friends I’ve had from walking and it’s made a difference.
Single female, 56 years
Sociable sentiments were not ubiquitous with some people reporting choosing physical activities or times and locations to avoid their family and friends. Ready access to facilities was also important for some. One person reported being encouraged to make regular use of the exercise bike and step machine in their GP’s summerhouse. Easy access to countryside where it was pleasurable to walk was encouraging for a number of people.
Motivational interviewing: perceived effectiveness
Only three people said that they thought that the booster sessions had no impact on them. All expressed the sentiment that they continued to do what they would have done anyway. Two of these achieved 0 and 5 minutes of moderate levels of activity during 7 Actiheart days. The other did not contribute Actiheart data.
nothing that was said over the phone or through interview made the slightest bit of difference.
Married male, 48 years
Seven people expressed ambivalence about whether the booster sessions were effective, through convoluted, contradictory, evasive or indirect answers:
Well it’s ticking boxes, yes . . . there’s things I mentioned to [motivational interviewer] which I haven’t quite got round to doing yet . . . I don’t know if I can get down to it. I think my problem is, I’m not really a great fan of gyms.
Single male, 51 years
Of these seven, three contributed no Actiheart data and three achieved 0, 3 and 9 minutes of moderate/vigorous activity over 7 Actiheart days. The last had 3 days where they met (43 minutes) or almost met (27 and 28 minutes) the national target of 30 minutes of moderate/vigorous activity a day.
In total, 15 people said that they thought that the booster sessions had been effective. Of these, 11 met the national target of 30 minutes of moderate/vigorous activity on at least 1 day. Three exhibited fairly sedentary activity levels on all days.
Motivational interviewing: consistency with perspectives or world views
At least four people, were, at best, ambivalent about MI. At least two explicitly said that they had hoped for an exercise programme or some kind of physical activity provision:
I would have expected more of a, shall we say, physical challenge? . . . I thought there would have been more physical activity to be measured and said, ‘Well, yeah, you did this when you came on the programme, you were able to do this by the time you’d got to the end of the programme’.
Co-habiting male, 65 years
Two disliked the autonomy-supported communication style used in MI (see Chapter 8, Views on physical activity and motivational interviewing ‘boosters’, for discussion):
Just two people chatting really, you know, I’m trying to give her some answers and she’s doing the best to give me some advice . . . it’s just like same old same old.
Married male, 48 years
I wanted to be told what the opinion was of my state of fitness. I want some results, you know, and being told off or being told that, ‘Well, it’s what you actually do in your day to day work keeps you reasonably fit, if necessary, or . . .’ . . . I wanted somebody to say, ‘Well, we’ve done your blood pressure’ or ‘We’ve measured you, we’ve weighed you and so we think’, blah blah blah.
Divorced male, 50 years
However, at least 10 people expressed enthusiasm for the autonomy-supported communication style. Common themes that emerged were that people liked the question-and-answer format and the informality. They found it businesslike and characterised the approach as encouraging, supportive and to do with fact finding rather than being ‘pushy’ or judgemental:
Well I was encouraged, but not pushed . . . So it’s obviously a lot better than virtually being told, what about this or no sorry, do this or this or this. So it was open and it enabled me to do it at the pace I wanted it to.
Widowed female, 65 years
At least 10 people made comments to the effect that they found the advice appropriate and flexible to their context. Clients frequently made statements to the effect that MI was thought-provoking or that it helped them focus on the importance of and barriers to physical activity. It gave them ideas that they could not have come up with themselves and pushed them to think about how much exercise they actually did. For many, the assistance in setting realistic goals was critical:
We set little goals for me to achieve . . . sort of saying, build your exercise up from 15 minutes to 30, 40 and I managed to get up to an hour a day on some days . . . he sort of set me targets for me to achieve, not too big, and goals and I found that because I knew that I was meeting him again . . . I made myself do them. I enjoyed the exercise once I got into it . . . I didn’t want to go and say, oh I didn’t achieve what we’d set out because the goals weren’t big goals and I think that’s the key, not to set a goal too big that it’s impossible to reach, so you think well I’m never going to do it, so I’ll not bother. You know, you can give up if the goal is too far ahead . . . I knew that I could achieve it. If I thought it was impossible to do, then human nature being as it is, I thought well I’m never going to do that, so I’m not even going to try.
Married female, 52 years, achieving recommended activity levels on 3/7 Actiheart days
It’s gradually making the changes . . . it’s about not drastic changes, it’s about taking one step at a time and gradually make the changes towards setting the goals that you want . . . make the changes to achieve the goals that you want to set yourself. So it’s helping people to achieve realistic goals.
Single female, 47 years
Motivational interviewing: perceived feasibility
For the vast majority of participants, the timing, duration and periodicity of booster sessions was highly convenient. Complaints were infrequent but focused on continuity of care (‘I don’t think I’ve seen the same person twice have I?’) and the need for more sessions. Suspecting that lack of funding might explain the limited provision (two sessions), one participant suggested that introducing clients into a peer-support (‘self-help’) group would be better than the current ‘abrupt’ end to the programme.
Seven out of 13 people interviewed who received MI by telephone believed that face-to-face booster sessions would have been preferable. One man with his own business and children found it difficult to protect time either at work or at home:
because I work for myself, you know, if someone walks in, you know, I’ve got to see to them straight away . . . so it’s easier for me to make a time [for a face-to-face meeting] and, you know, I’ll be there . . . at 6 o’clock, when I get home, it’s like ‘Daddy, Daddy’ and . . . I’m being followed into every room, can’t go anywhere!
Divorced male, 49 years
Those receiving face-to-face MI often said that they thought that the physical presence of the person made the MI more effective (see Motivational interviewing: perceived importance and The importance of monitoring and feedback):
Actually seeing the individuals who come and talk to you, so it’s like a one-to-one communication, the physical contact I think is very important . . . What difference would it make to me – somebody just over the telephone, ticking a box? Why can’t they just meet me?
Single female, 47 years
Motivational interviewing: perceived importance
A majority of people expressed the view that they felt that MI was important in some way, for instance because it met some acknowledged need. Sixteen people used language to the effect that the MI gave them encouragement, motivation, ‘a push’, impetus, a boost to their confidence or similar. For some, it helped them keep motivated in a situation of social isolation or disliking exercise or preferring alternatives:
Because I live on my own, I find it more difficult to keep motivated, cos I don’t really like exercise. I could . . . I’m one of these people that could just sit and watch telly all day and enjoy it.
Divorced female, 65 years
Many people said that they needed help with ideas for alternative physical activity strategies or help with overcoming barriers or thinking through life changes, especially retirement. People frequently claimed that the sessions helped them ‘focus’ or reinforced knowledge or goals:
I did start to try and look for ways and means of filing my time in, after I’d retired. Most of my immediate family were worried that I wasn’t going to survive this winter with not being able to go to work, so I was looking to broaden my horizons.
Co-habiting male, 65 years
At least four people mentioned that they enjoyed the social contact of the booster session. Sometimes this was because they were able to engage in a depth of conversation about an issue that was important to them that they did not feel able to do (or would not be productive) with a friend or family member:
Well it’s good to have a chat with someone. I don’t get much opportunity at home . . . I mean, it’s a welcome break to my day, having a conversation along those lines. It’s a sort of a change . . . having to think about something different.
Single male, 51 years
Because your family and your friends know you too well and they have got a preconceived idea of you, whereas [the motivational interviewer] hadn’t, ya know, it was just me.
Married female, 62 years
In summary, participants claimed that the MI met a number of needs, including providing encouragement and the opportunity to think laterally about problems and solutions with a professional removed from their normal social context. However, as the next section discusses, interviewees often framed these views in terms of altering their behaviour because of an awareness that they were being observed:
It’s little things that you can talk through which you probably wouldn’t have thought of and it seems to be important, but who would you talk to about it? Like, how do I fit in the jobs that I’ve got and fit in what I want to do, and is the diet I’ve got important and should I be doing this, and things like, ‘Oh for goodness sake, I can’t be bothered with it’, but because I’ve had to think about it, because I’m going to talk to someone about it and then . . . It appears to have worked very well.
Married female, 65 years; our emphasis
The importance of monitoring and feedback
Around half (n = 13) of the participants expressed enthusiasm not for the motivational interview per se but for the opportunity to be monitored and have feedback on their performance. For some, this involved the expected pleasure of receiving objective feedback from the researcher or the Actiheart on their level of activity over a week or during particular activities, as well as how that compared with national guidance or other people:
to be honest, one of the reasons I participated in this is because I did want some feedback. I wanted to be told what the opinion was of my state of fitness.
Divorced male, 50 years
I just wish I’d been able to carry on with the monitor, just to see what the heart monitor showed through my normal week . . . I was quite disappointed I couldn’t just keep it on.
Single female, 62 years
I wanted to be able to see that result, to see what kind of exercise I did over that time, how much calorie did I burn, you know, things like that. I would love to have something like that. I think that generate a greater interest to go out and do something.
Single female, 47 years
Others reported that arranging to meet a researcher in 3 months’ time, for the research study primary outcome follow-up, motivated them to increase their physical activity. This may indicate possible confusion between research and intervention procedures and possible contamination of the intervention by the research procedures (see Strength and weaknesses of the study). Some thought that they would feel ashamed to admit it if they had not achieved their goals. Others actively looked forward to reporting achievements that they had made:
the fact that you know you’re going to a session . . . you think I’ve got to do something because she’s going to say, ‘what have you done since last time?’ If you sit there and say, ‘well, actually, nothing’ . . . [it] increases the guilt value [laughs].
Married male, 64 years
It’s sort of saying, I realise that I remember you saying I’d do this and I haven’t done that yet, sort of thing, and I’m always disappointed with myself when I say things and I don’t do them.
Single male, 51 years
The very fact that I actually told [the motivational interviewer] I was going to go swimming meant I had to do it. There was no pressure on me to do it from her but the very fact that I actually told somebody I was going to do it meant I had to do it . . . because I’d actually told her I was going to do it, it made me want to do it.
Married female, 56 years
in the nicest possible way, I was having to report to somebody and . . . I know me: that is what I need. I don’t mean that I was being told off or anything like that . . . Just to have to report that, yes I’m doing this, or I’ve started doing that and getting a bit of praise. I think we all like praise don’t we, deep down, and that helped.
Widowed female, 65 years
you keep that pressure on by saying, ‘Right, you’ve got an appointment in 4 weeks’ time and we’re going to put this on, and blah-di-blah’, so you know, people think, ‘Ah, I must do it, I must do it’.
Widowed female, 65 years
Of these, a number of people expressed how important it was that the person holding them to account was a relative stranger rather than a friend:
It could’ve been a friend, but it’s not as easy with a friend to do it . . . And it kept my mind focused, just the fact that I was talking to someone, someone who obviously knew what they were talking about as opposed to, I mentioned a friend before, where a friend wouldn’t know the same, wouldn’t have the same knowledge.
Widowed female, 65 years
Yeah, yes, cos day-to-day people, they’re with you all day, they’re either bored of it or they can see it for themselves, or you know, you might catch them when they’re not particularly interested. You know, so it’s not quite so easy, whereas with somebody else who is a stranger as well, I quite liked that, rather than, sort of, if it hadn’t gone well, it’s not, you know, somebody judgemental.
Married female, 53 years
The prevalence of optimistic self-assessment
We have already noted earlier that self-reported physical activity from the SPAQ correlated poorly with objective physical activity (see Figure 17) and the low numbers of people meeting physical activity targets of 30 minutes of moderate to vigorous physical activity on any particular day (see Appendix 6, Table 50). However, some interviewees made very explicit and detailed claims to the effect that they were very physically active when the Actiheart data suggested otherwise:
Well don’t forget, my work involves me running around after a tower crane up buildings that were anything from 6 to 18 floors up that had no lifts in them, and you know so that’s kind of pretty physical all by itself.
Married male, 48 years, who met physical activity guidance on 1/7 Actiheart days; median (range) minutes per day of moderate activity = 0 (0–40)
Chapter 5 Results of the fidelity assessment
Assessment of interventionist motivational interviewing experience and competence
Those delivering the intervention (RAs) typically had approximately 3 years’ experience of delivering physical activity interventions; in addition, two had formal psychological or counselling workshop training prior to this trial. However, all RAs had experience of physical activity programming training in a range of settings. This was predominantly in local authority, gym, health or university contexts and did not focus on health behaviour change. All of the RAs had an educational background in sport and exercise science, psychology, physical activity and wellness at undergraduate and postgraduate level.
At the initial MI training sessions an evaluation was made of the competence of the RAs in delivering the technical and relational components of MI. All of the RAs were able to competently describe and demonstrate the level 1 skills of MI, which focused on a higher ratio of open to closed questions, increased client talk time, simple reflections and summarising. In addition, the RAs were able to identify the components and characteristics of the ‘spirit’ of MI. By the end of the first training block (2 days equivalent) the group could identify the use of affirmation compared with praise although found it more difficult to apply this consistently in practice and still tended to fall into a ‘righting reflex’ of problem-solving rather than eliciting opportunities and resources from the client.
At the first formal assessment, independently coded using MITI (see Appendix 7, Table 61; first assessment), the RAs were consistently using level 2 MI skills, which included reflective listening, directional open questions (e.g. optimism for change, intention to change, advantages and disadvantages of change) and tools such as decisional balance, readiness and confidence rulers and action planning. This level of proficiency was in line with the clinician behaviour scores reported in Table 3.
The global rating scores (evocation, collaboration, autonomy/support, direction and empathy) across both assessment period 1 (9 months from first training) and assessment period 2 (18 months from first training) were consistent and the mean scores reflected a level of competence in all RAs with respect to ‘direction’ and a level of proficiency/competence in respect to ‘evocation’ and ‘empathy’. The per cent MI adherent clinician behaviours were all at 100% at phase 1 and/or 2.
With regard to behaviour counts (e.g. per cent complex reflections, per cent open questions and reflection to question ratio), the per cent open questions increased across all RAs from baseline, the reflection to question ratio increased from phase 1 to phase 2 across all RAs and the open questions to complex reflections ratio was higher than anticipated across all RAs.
Independent assessment of interventionist delivery (Motivational Interviewing Treatment Integrity assessment)
The MITI coded sessions were independently assessed by a qualified MI coder and a number of aspects were highlighted as positive and effective in addition to there being areas for enhancement. Although ‘direction’ scored as proficient on the global ratings scale, feedback indicated a need for greater recognition of individual participants’ level of readiness, values and the strength of change talk. 84 This was highlighted as requiring a greater use of more challenging complex reflections and a greater use of strategies to elicit and strengthen change talk. The RAs scored highly for levels of empathy, however, and similarly demonstrated a high level of client engagement, which is a common aspect of MI ‘spirit’. Although the use of ‘direction’ across all RAs was consistently high, most RAs did not sufficiently demonstrate empathy and autonomy support, which are global MI measures.
The relationship between motivational interviewing fidelity and levels of physical activity
There is moderate evidence to suggest that MI fidelity is associated with physical activity as measured by mean TEE per day (kcal) at 3 months (p = 0.027), that is, the level of physical activity of participants at follow-up was associated with the overall fidelity of delivery of the RA who delivered their MI intervention.
Figure 25 shows the means of mean TEE per day at 3 months with their associated 95% CIs stratified by RA ranked by their global fidelity rating. It should be noted that RA1, RA2 and RA3 had a similar global proficiency rating of 3.5. Grouping these RAs together showed a stronger association between MI fidelity and mean TEE per day at 3 months (p = 0.003). As observed from Figure 25, RAs with a higher global proficiency rating are associated with higher physical activity levels at 3 months. These results should be interpreted with caution, however, given that some RAs delivered a lower number of sessions, as observed by huge uncertainty around their estimated means. Moreover, the sessions were brief and it was not possible to demonstrate all MI processes in some RA sessions that were coded. RA6 does not appear in Figure 25 because she delivered very few sessions and was subject to MITI recording only over a short period after initial training.
FIGURE 25.
Means of mean TEE per day (kcal) stratified by RA who delivered the MI sessions.
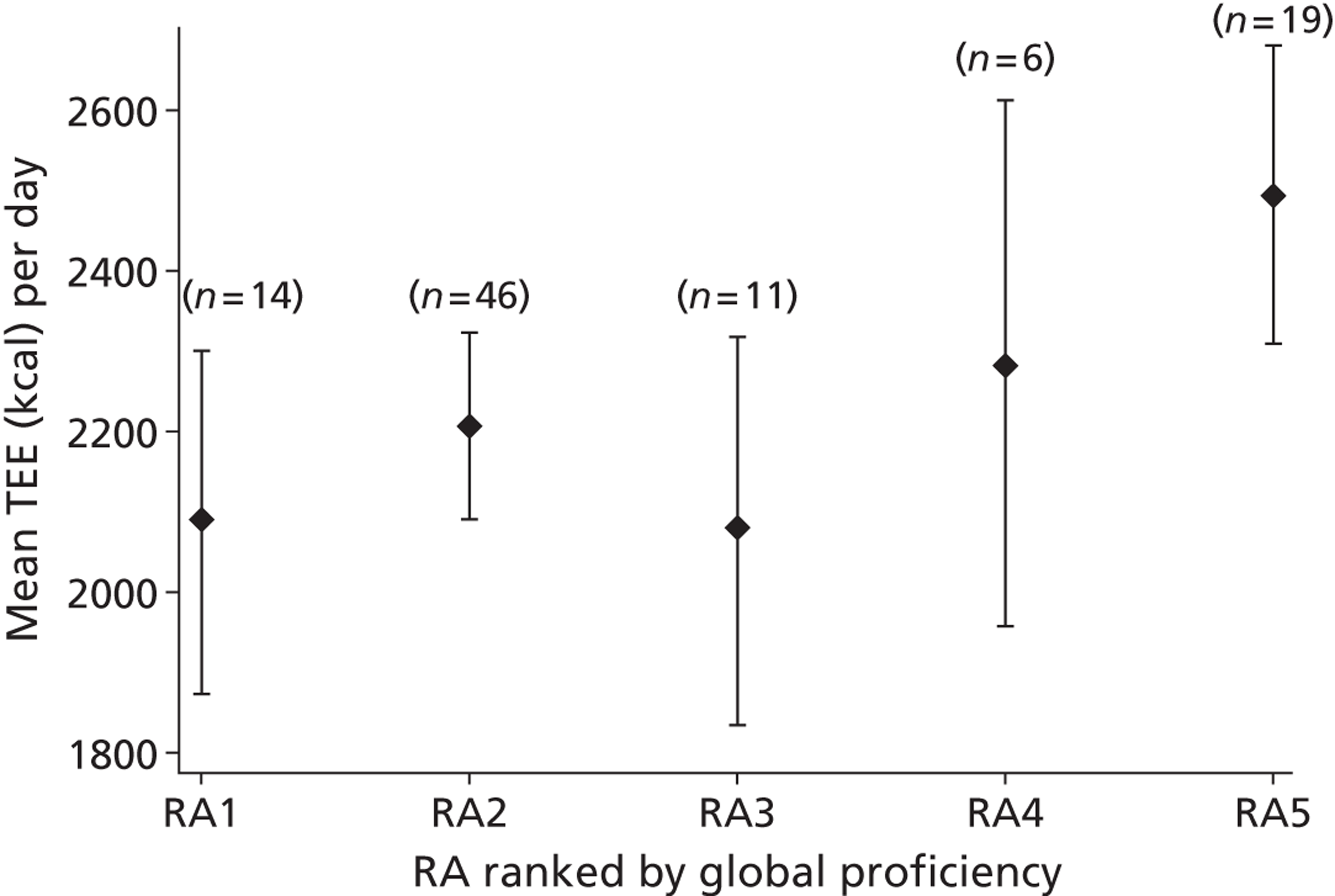
Chapter 6 Results of the geographical information systems analysis
Distance from municipal green spaces
Chapter 2 (see Green space measures) described the subdivision of municipal green spaces based on their catchment area classification (as determined by Sheffield City Council) and certain other criteria. This led to two sets of polygons, one representing the highest-quality green spaces and the other representing medium- and high-quality green spaces. We calculated the shortest network distance from the home postcode centroid of each participant to the nearest polygon in both sets. This resulted in two variables for each participant, which are shown in Figures 26 and 27, respectively, using quantile colour-coded circles placed at each of the randomised participants’ home postcode centroids.
FIGURE 26.
Shortest pedestrian network distance to high-quality green space (randomised participants only), as determined using a citywide catchment area.
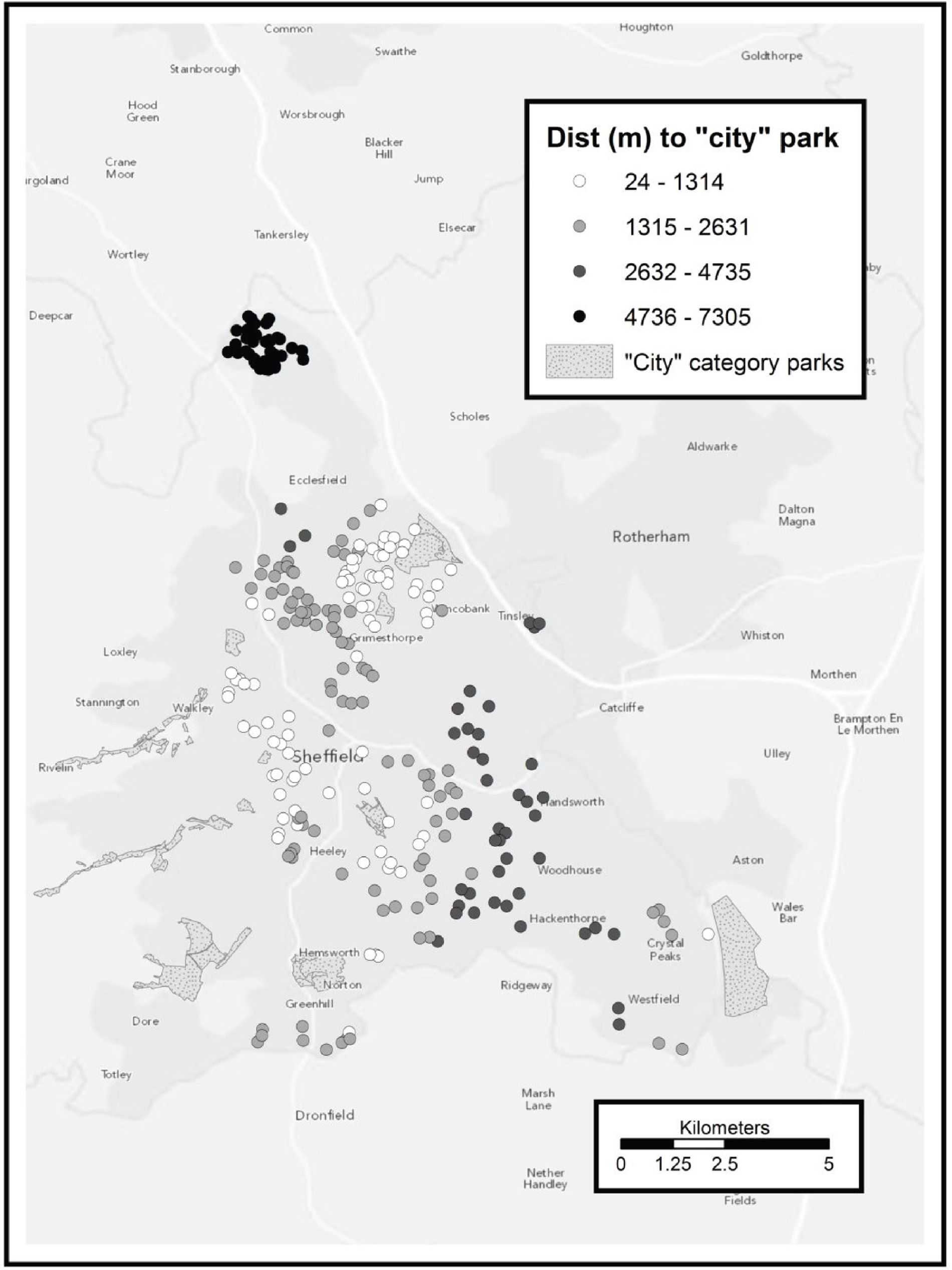
FIGURE 27.
Shortest pedestrian network distance to medium or high-quality green space (randomised participants only), as determined using a district or citywide catchment area.
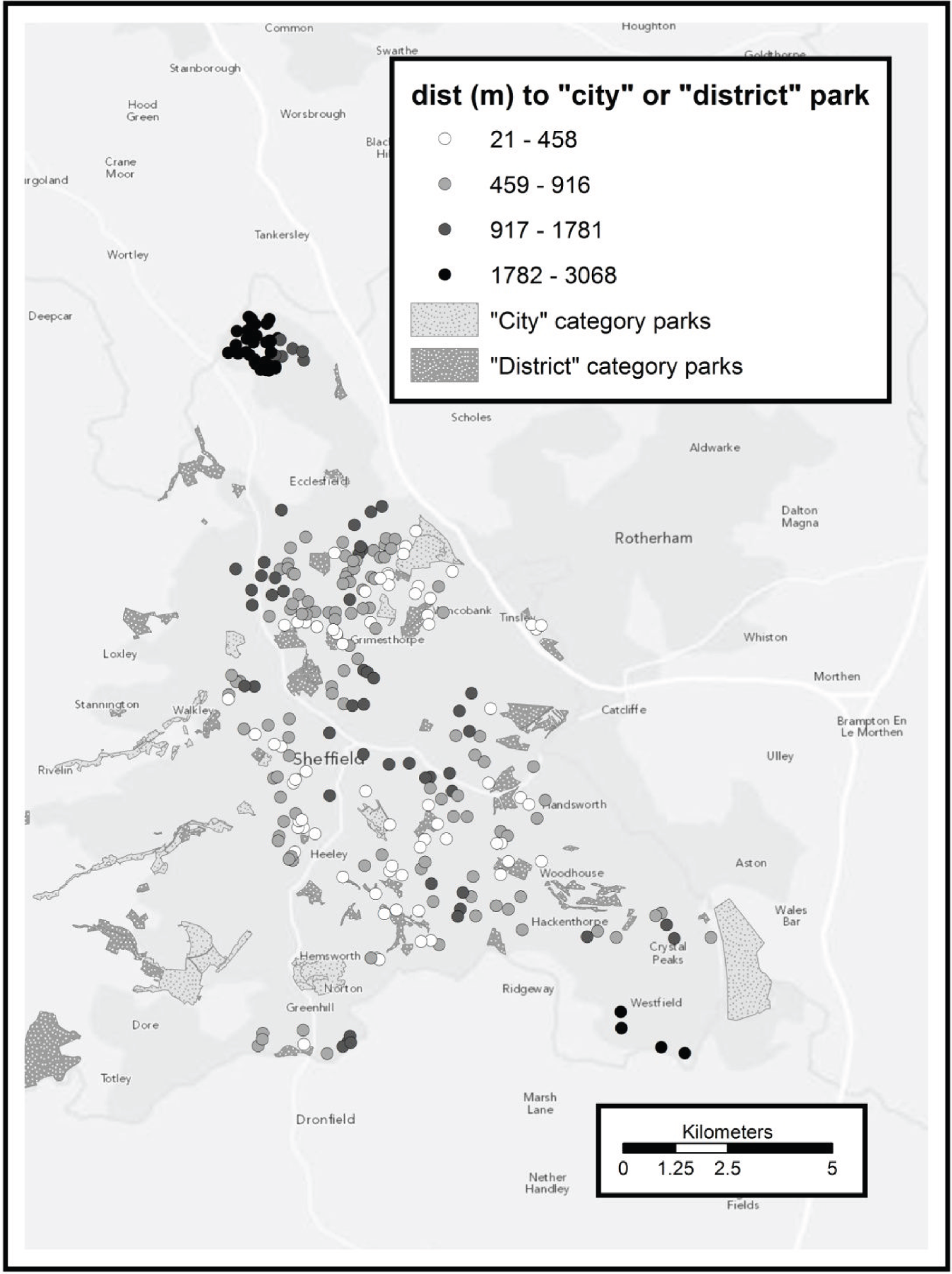
The most striking feature of Figures 26 and 27 is that participants in High Green (the cluster of points in the upper part of the map) live much further from these municipal green spaces than other participants located more centrally within Sheffield, despite being surrounded by open countryside. This is an issue that has been encountered in other studies linking green space and exercise. 85,86
Although there is a case for including all forms of green space in an analysis of access to spaces for physical activity, it is also likely that municipal green space is, or is perceived to be, more accessible than the green space that is available in the countryside. More specifically, municipal spaces typically offer a ‘tame’ version of nature that may be more attractive to those unused to active outdoor pursuits, with better-marked paths and routes and not requiring any specific clothing or footwear such as walking boots.
Regarding the differences between Figure 26 and Figure 27, it is noticeable that, aside from Rother Valley Country Park (the large park in the south-east corner of both maps), there are no high-quality green spaces in the east or south-east of the city, despite these areas being well provided with medium-quality green space. This aside, the two sets of data do not differ strongly.
Regarding the measuring of green space, it is worth noting that the greenery comes in many forms, not all of which can be measured by land usage. For instance, many public roads in the Victorian areas of Sheffield are lined with mature deciduous trees, lending these areas a ‘green’ feel, even though these do not count as municipal green spaces. However, it is beyond the scope of this study to address this issue.
Distance from leisure facilities
Chapter 4 (see Leisure facility measures) described the identification of gyms and swimming pools in the local area. Based on survey and interview data from the booster participants, these facilities were felt to be the most relevant to the study. Figures 28 and 29 show the location of gyms and swimming pools, respectively, in Sheffield and the surrounding area, together with the network distance to the nearest facility for each of the randomised participants. Note that as some gyms are female only, the set of locations used for calculating network distance was different for male and female participants. In Figure 28, female-only gyms are represented by a black star, with a white star for unisex gyms.
FIGURE 28.
Shortest pedestrian network distance to a gym (randomised participants only). This was dependent on the gender of the participant as some gyms are female only.
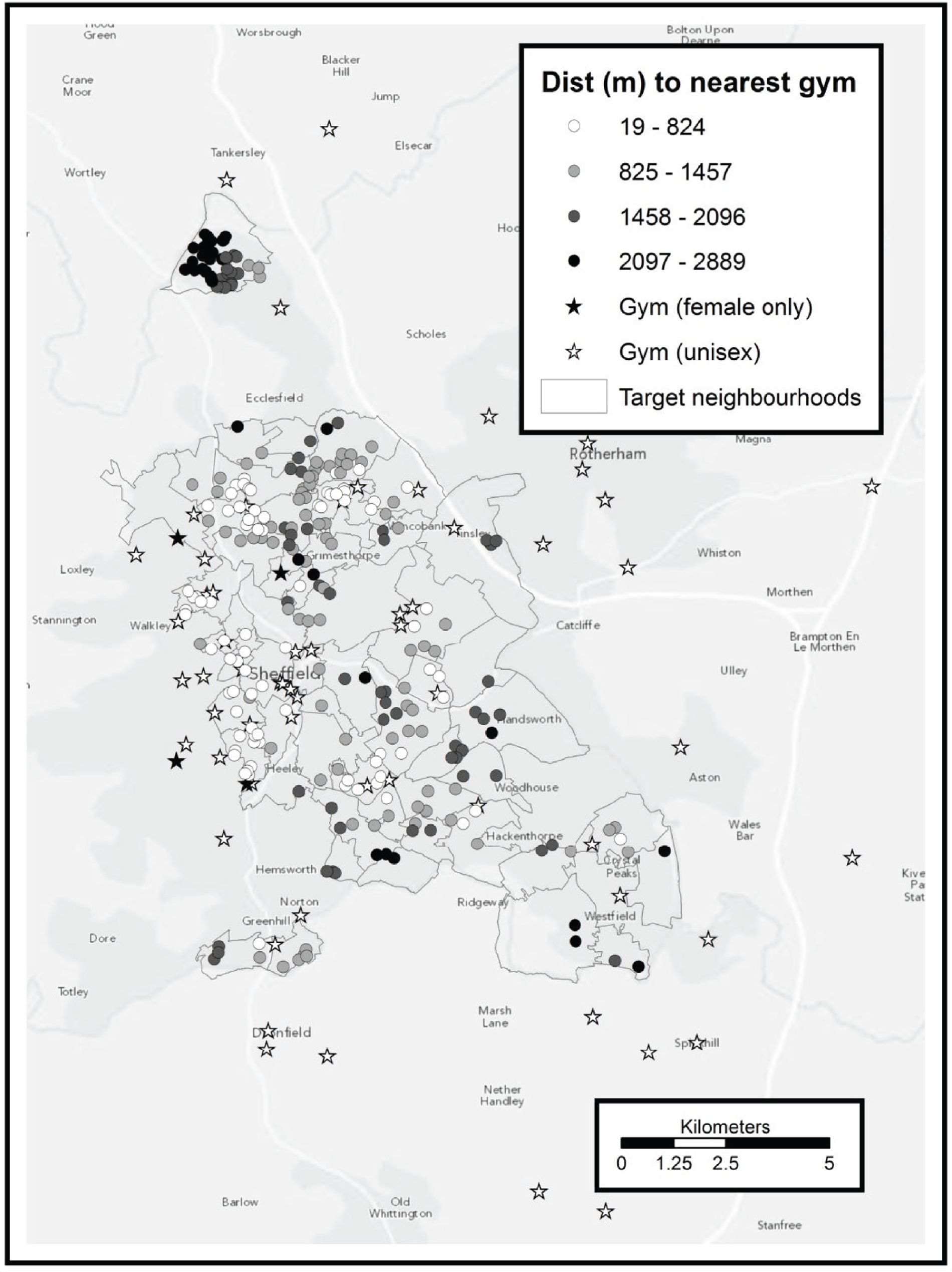
FIGURE 29.
Shortest pedestrian network distance to a swimming pool (randomised participants only).
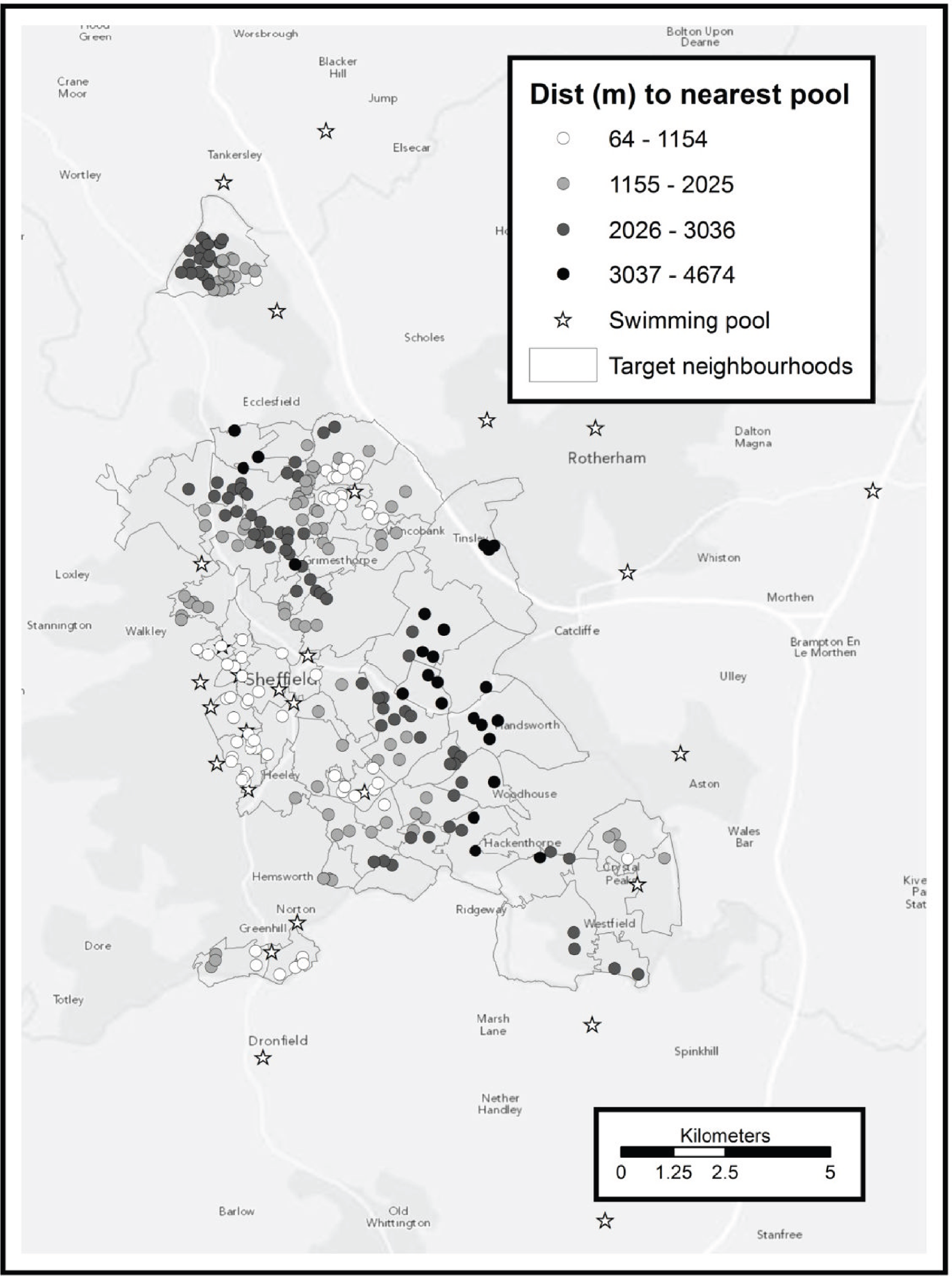
Regarding Figure 28, as with Figures 26 and 27, there is a noticeable difference in the results for participants in High Green (the cluster of points in the upper part of the map). This is especially true of the northern-most part of High Green, despite a gym being present at Tankersley Manor (the star just above High Green on the map). This is because there is a high-speed trunk road (the A616 Stocksbridge bypass) between the two, which is not suitable for pedestrians; the relatively high network distances here reflect a detour across fields to reach a footbridge over the road.
The presence of female-only gyms does not have a large effect on the results, as these are mostly in areas where other gyms are present.
In contrast to Figure 28, Figure 29 shows a marked absence of swimming pools in the east and south-east of the city, except for the far south-east. Figure 29 also shows an apparent ‘corridor’ of mid-grey points (indicating a relatively high network distance to the nearest swimming pool) in the north-west of the city; however, this is an artefact resulting from the participant locations happening to fall in a line.
Association between trial outcomes and proximity to green space and leisure facilities
The main purpose of mapping proximity to both green spaces and leisure facilities was to explore whether geographical access to opportunities for physical activity was a predictor of activity levels in trial participants. However, there was no statistical association observed between TEE at 3 months and proximity to the above variables in trial participants and we found no evidence therefore to support the hypothesis that geographical access to either municipal green space or swimming pools and gyms was an independent predictor or moderator of physical activity. As the booster intervention was not shown to be effective (a necessary condition for mediation analysis) and use of community facilities by participants (a planned subgroup analysis) was not a moderator of intervention effect, we did not further explore the hypothesis that geographical access or any other variables could be mediators of the impact of a booster intervention.
Chapter 7 Results of the health economic analysis
Scenarios modelled
As discussed in Chapter 2, a range of sources of structural uncertainty exist when attempting to infer the long-term clinical and cost-effectiveness implications of the booster trial results, based largely on the Actiheart data (as the primary outcome assessing differences in physical activity between trial groups). For this reason it was decided to consider two different types of model and a number of scenarios within each model type:
-
short-term model [see Short-term (directly elicited) model]:
-
perspective: NHS, societal
-
comparison: baseline compared with 3 months; baseline compared with 9 months; 3 months compared with 9 months
-
data: all available data; completers only
-
-
long-term model [see Long-term (epidemiological) model]
-
primary
-
individual-level differences-in-differences approach
-
3-month differences only
-
9-month differences only
-
-
secondary
-
‘value-added’ differences-in-differences approach
-
quintile effects.
-
-
A total of 12 separate short-term model scenarios are considered, involving all combinations of the three variables listed above. Each of these scenarios is numbered as shown in Table 9.
| Scenario no. | Perspective | Time comparison | Data used |
|---|---|---|---|
| 1 | NHS | Baseline to 3 months | All |
| 2 | Societal | Baseline to 3 months | All |
| 3 | NHS | Baseline to 9 months | All |
| 4 | Societal | Baseline to 9 months | All |
| 5 | NHS | 3 months to 9 months | All |
| 6 | Societal | 3 months to 9 months | All |
| 7 | NHS | Baseline to 3 months | Completers |
| 8 | Societal | Baseline to 3 months | Completers |
| 9 | NHS | Baseline to 9 months | Completers |
| 10 | Societal | Baseline to 9 months | Completers |
| 11 | NHS | 3 months to 9 months | Completers |
| 12 | Societal | 3 months to 9 months | Completers |
Short-term (directly elicited) model
Resource use analysis
An analysis of the NHS resources consumed by participants in each of the trial arms indicated that participants in the intervention arms had greater increases in NHS resource consumption at 9 months compared with baseline than participants in the control arm, as indicated in Table 10.
| Differences in differences between control arm and . . . | Mean value (per participant) (bootstrapped 95% CI) (£) |
|---|---|
| Mini booster arm | 26.68 (26.59 to 27.12) |
| Full booster arm | 190.91 (190.56 to 191.25) |
Cost of the intervention
Estimates of the cost of the intervention were arrived at taking into account a range of factors including number of sessions per completer; number of completer sessions per RA; numbers of completers at 3 and 9 months in the mini and full booster intervention arms; numbers of participants in the mini and full booster arms; estimates of the duration of sessions in the mini and full booster arms; RA training and monitoring costs; venue hire (full booster) and telephone call (mini booster) costs; and the ratio of participants who completed a full or mini booster course to those assigned to the course.
Following discussion with RAs, the average duration of a mini booster session was estimated to be 20 minutes and the average duration of a full booster session was estimated to be 30 minutes. The cost per minute of a mini booster session was estimated to be lower than the cost per minute of a full booster session as the latter required venue hire and RA travel time. Rates of attrition were similar in both intervention arms at 3 months but were higher in the mini booster arm at 9 months. Because of this the average total cost per completer (someone who attended two booster sessions and provided valid Actiheart data) was estimated to be slightly higher in the mini booster arm at 9 months than in the full booster arm at 9 months. Because of the high level of uncertainty in estimating the cost of the intervention, eight different intervention cost estimates were produced for both the mini booster arm and the full booster arm using different plausible assumptions. The assumptions made to produce these estimates are shown in Appendix 7, Table 62; 2011 prices were assumed.
The range of estimates appeared to follow a log-normal distribution with a mean (SD) cost per completer of £216 (£88) for the mini booster and £205 (£76) for the full booster. Because of the similarity of these numbers the booster intervention was assumed to cost approximately the same irrespective of whether it was the full booster or the mini booster. The two pairs of eight estimates were combined to produce a mean (SD) cost per completer of £211 (£91), also assuming a log-normal distribution. Within the deterministic analyses the mean estimate of £211 was used. Within PSA 1000 values were drawn from an appropriately parameterised log-normal distribution.
Health-related quality of life data used
The trial outcome data used to populate the short-term model are patient-estimated utilities based on SF-12v2 plus 4 questionnaire data recorded at baseline and at the end of the trial. These data are presented in Figure 30 for participants who provided valid 9-month Actiheart data (‘completers’).
FIGURE 30.
Estimated utility scores reported at 3 and 9 months by allocation group (trial completers only).
Within scatter plots of this form, an overall trend over time would be indicated by the scatter deviating from the diagonal line: above the line in the case of an upwards trend and below the line in the case of a downwards trend. A difference between groups would be apparent if the three sets of scatter, indicated by the three plotting symbols, tended to cluster in different places. It is seen from this plot that there does not appear to be either a trend over time or a difference between groups.
Table 11 shows the mean (SD) change in HRQoL from baseline to 9 months by allocation group. Differences-in-differences estimates, comparing the control group with either the mini booster group or the full booster group, are also presented. These differences are very close to zero in all cases but very slightly favour the control group over either of the intervention groups.
| Group | Mean (SD) change in HRQoL from 3 to 9 months | Differences in differences compared with the control group | |
|---|---|---|---|
| Mean | Bootstrapped mean 95% CIs | ||
| Control | 0.018 (0.010) | ||
| Mini booster | 0.001 (0.015) | −0.016 | −0.018 to −0.016 |
| Full booster | 0.016 (0.014) | −0.002 | −0.002 to −0.000 |
This approach was adopted for each of the analyses.
Simple threshold analysis
Given an estimated cost of the intervention of £211 per participant, and assuming a willingness-to-pay threshold of £20,000 per QALY, the intervention would have to provide an additional 0.01055 QALYs to be considered cost-effective.
Short-term scenarios
Figures 31–42 present scatter plots, CEAFs and tables summarising mean scores and ICERs for each of the 12 scenarios described in Table 9.
FIGURE 31.
Scenario 1. (a) Scatter plot of difference in costs (£) against differences in QALYs; (b) CEAF; and (c) mean costs, QALYs (to two decimal places) and ICERs.
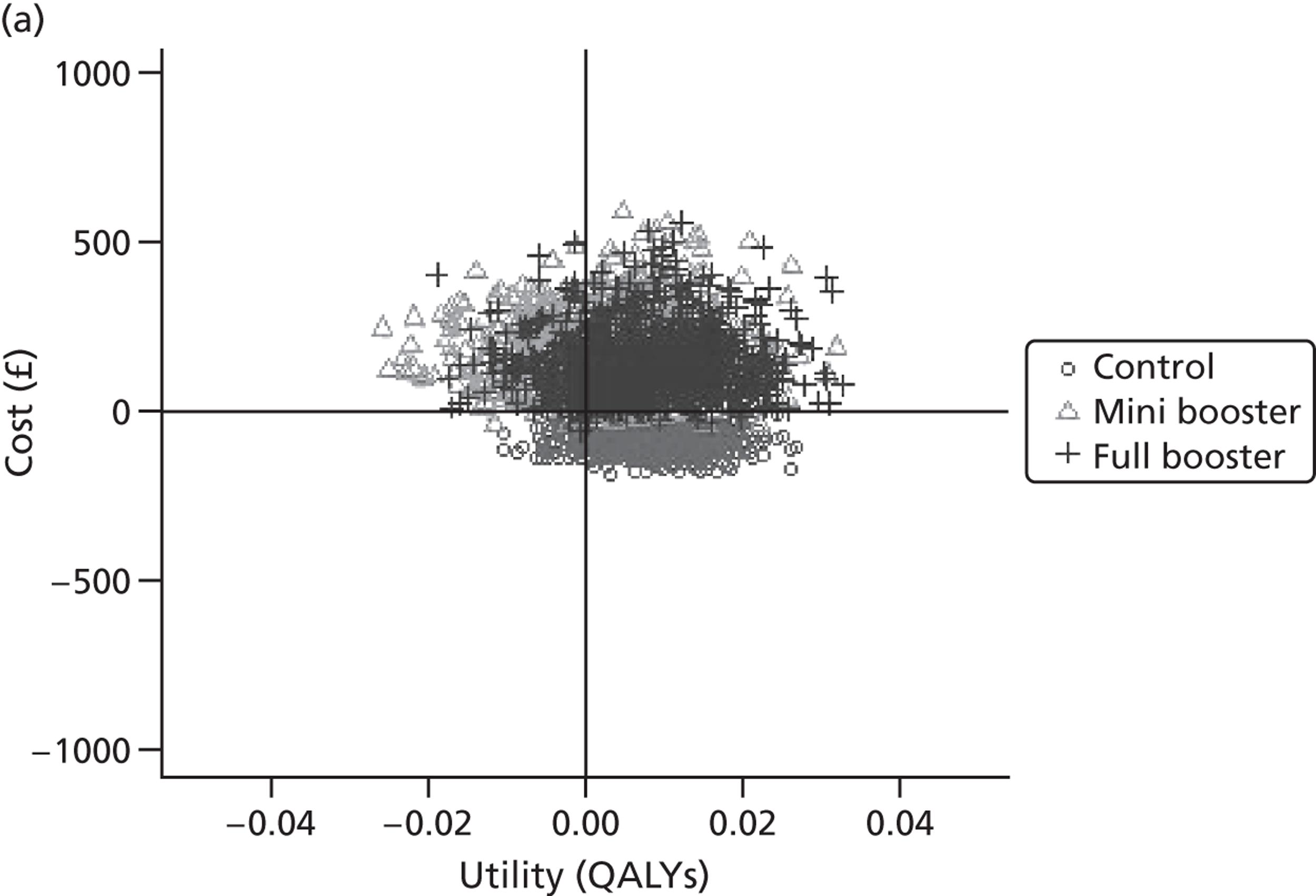
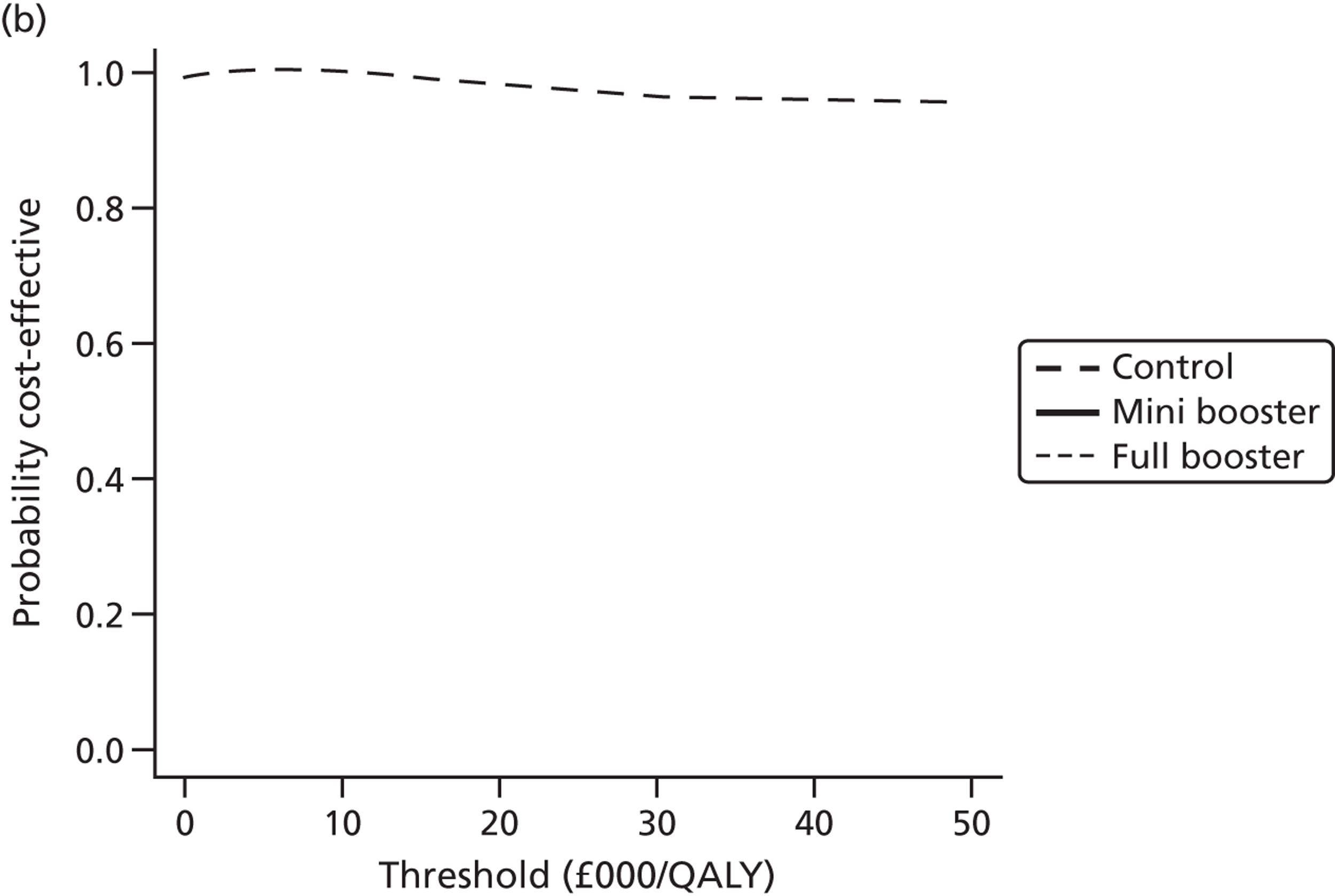
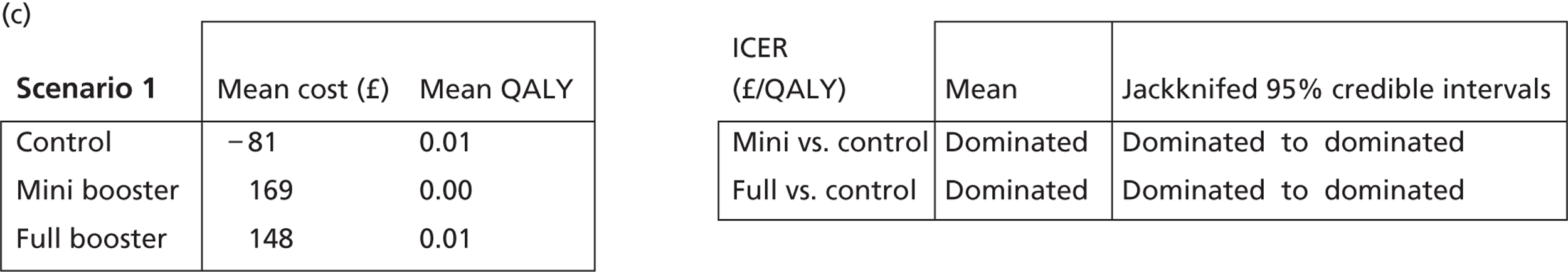
FIGURE 32.
Scenario 2. (a) Scatter plot of difference in costs (£) against differences in QALYs; (b) CEAF; and (c) mean costs, QALYs (to two decimal places) and ICERs.
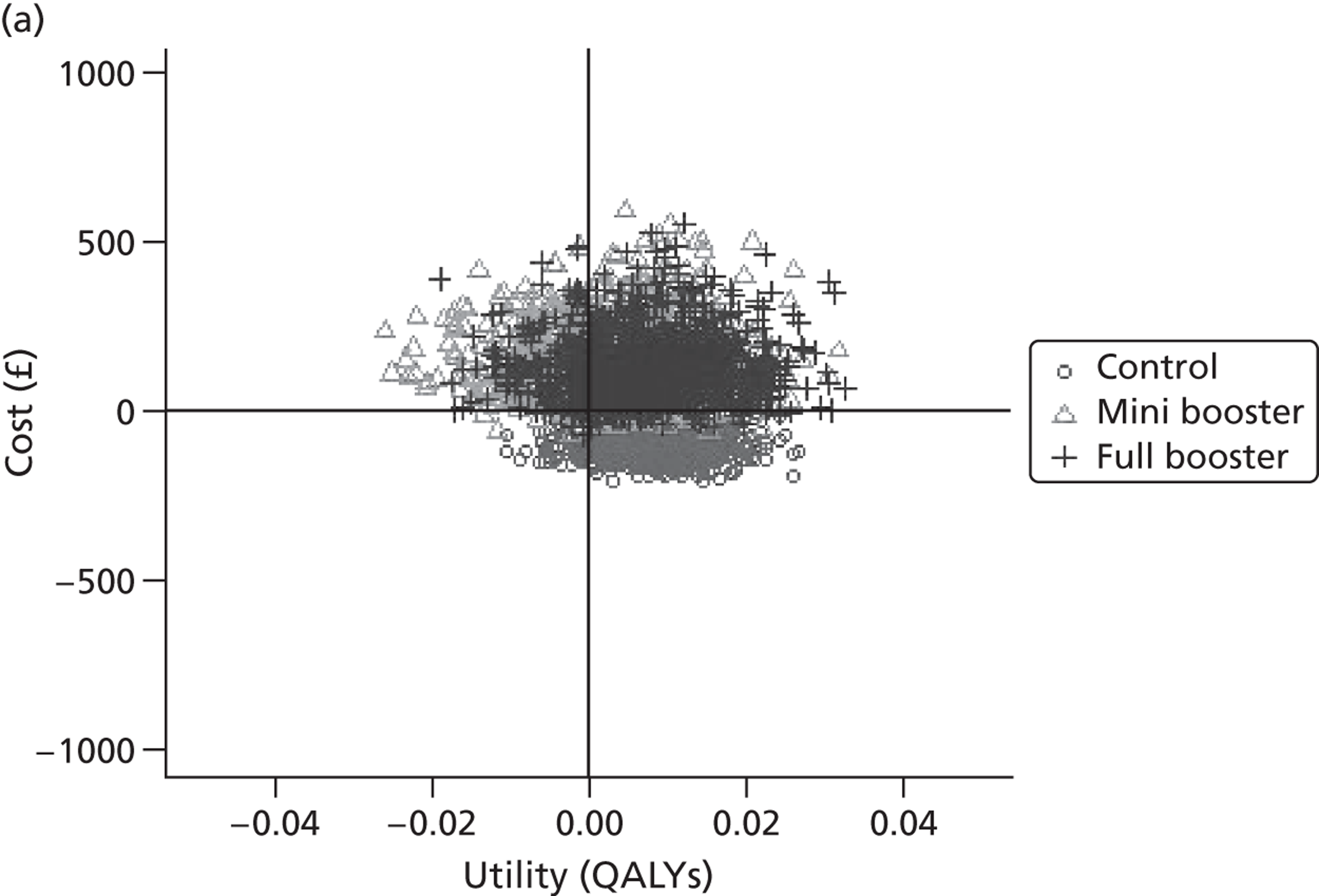
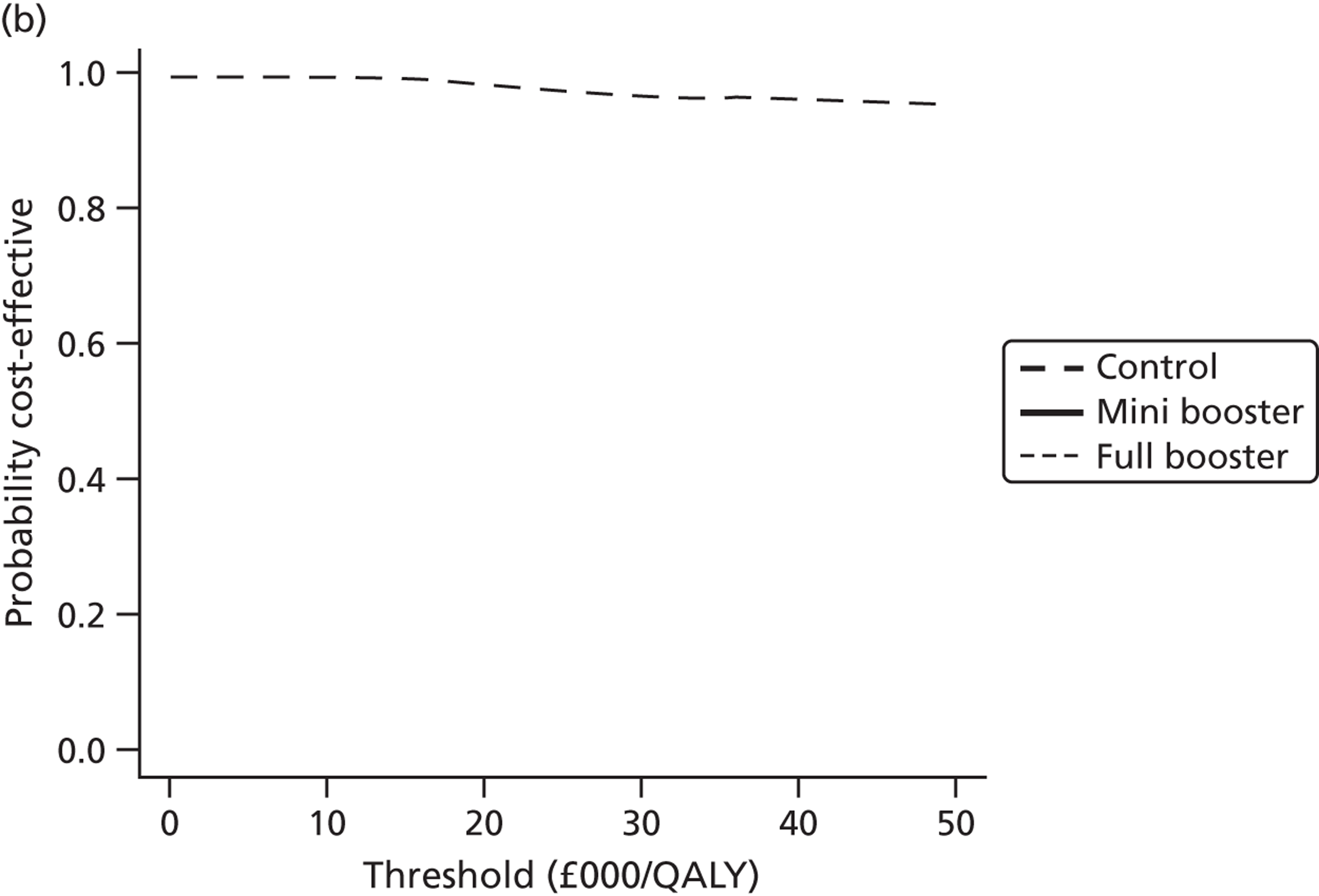
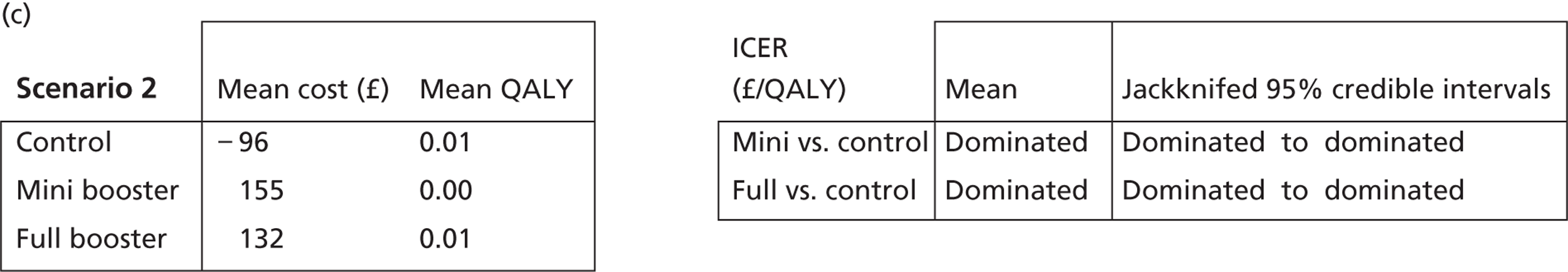
FIGURE 33.
Scenario 3. (a) Scatter plot of difference in costs (£) against differences in QALYs; (b) CEAF; and (c) mean costs, QALYs (to two decimal places) and ICERs.
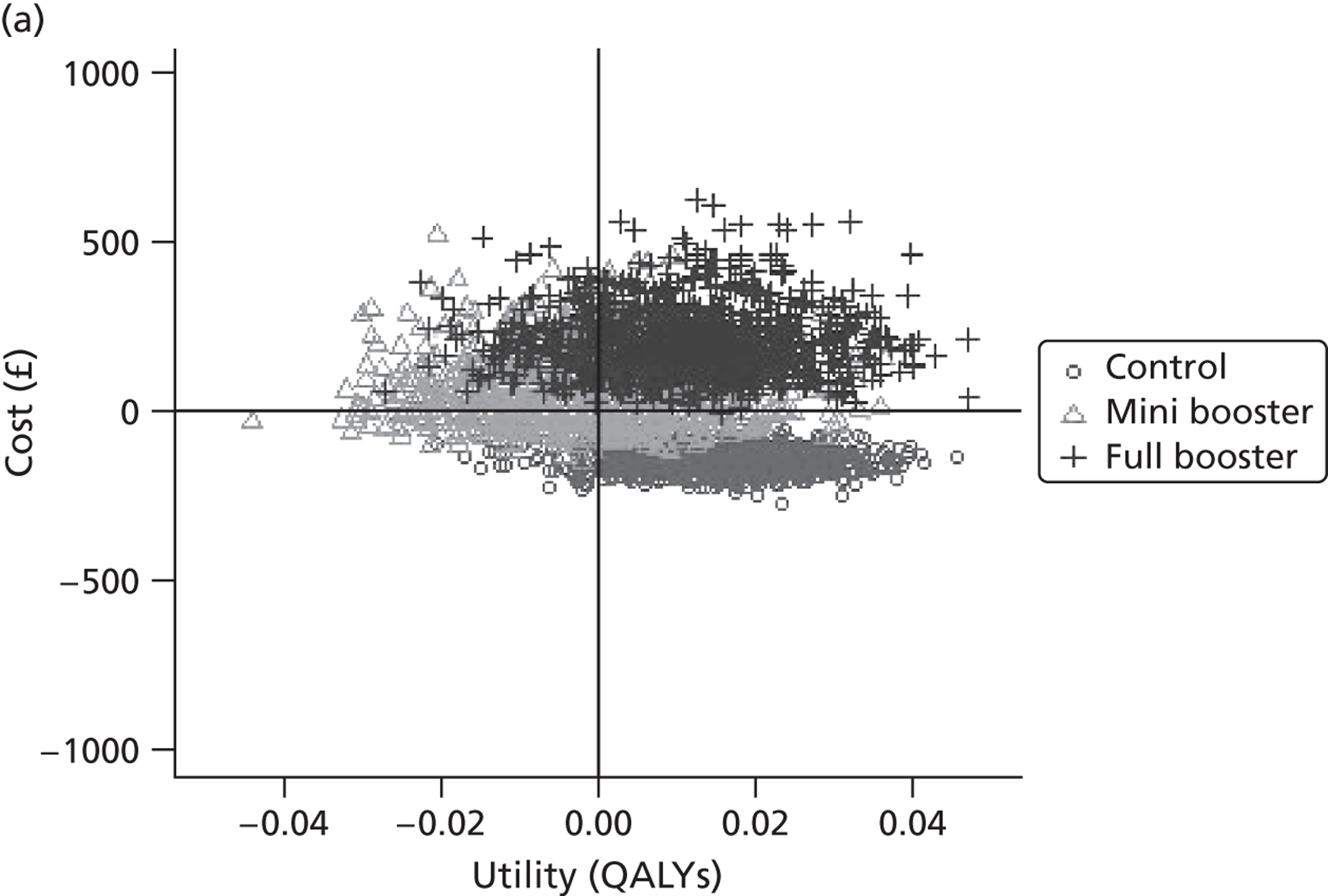
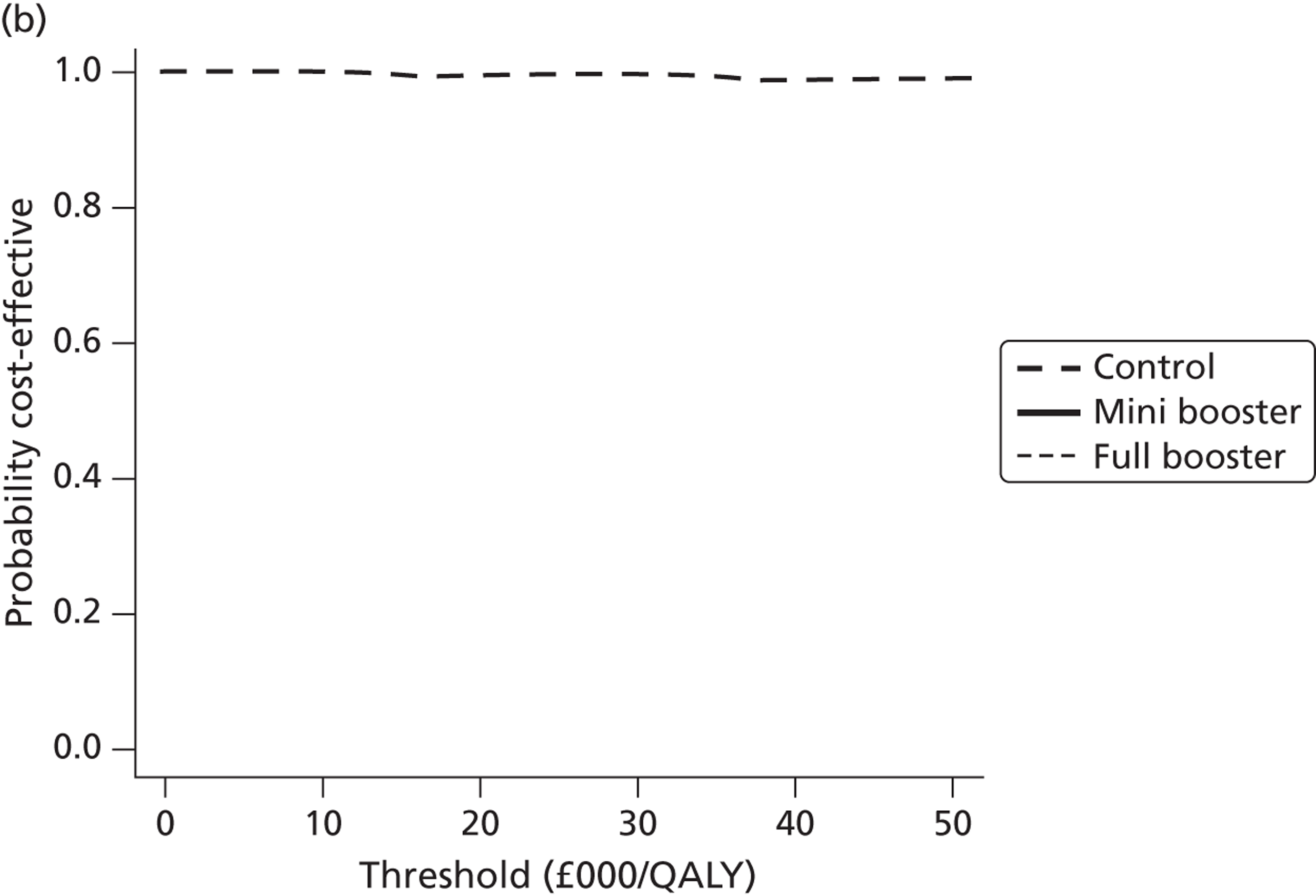
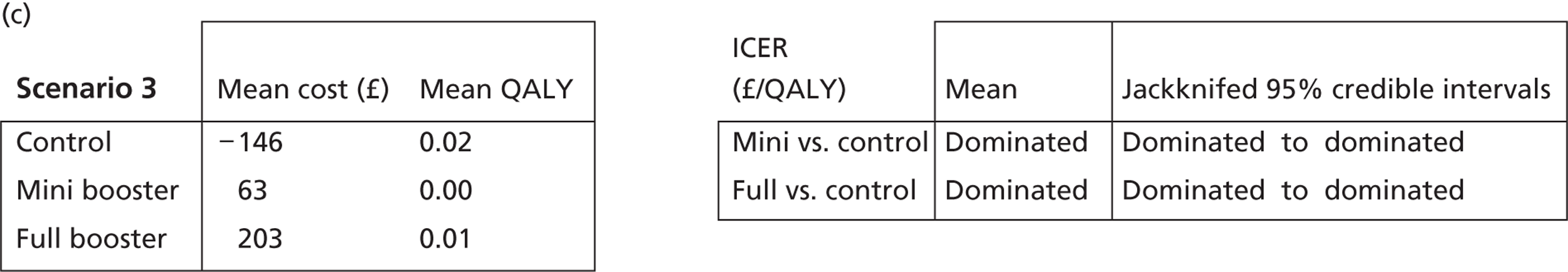
FIGURE 34.
Scenario 4. (a) Scatter plot of difference in costs (£) against differences in QALYs; (b) CEAF; and (c) mean costs, QALYs (to two decimal places) and ICERs.
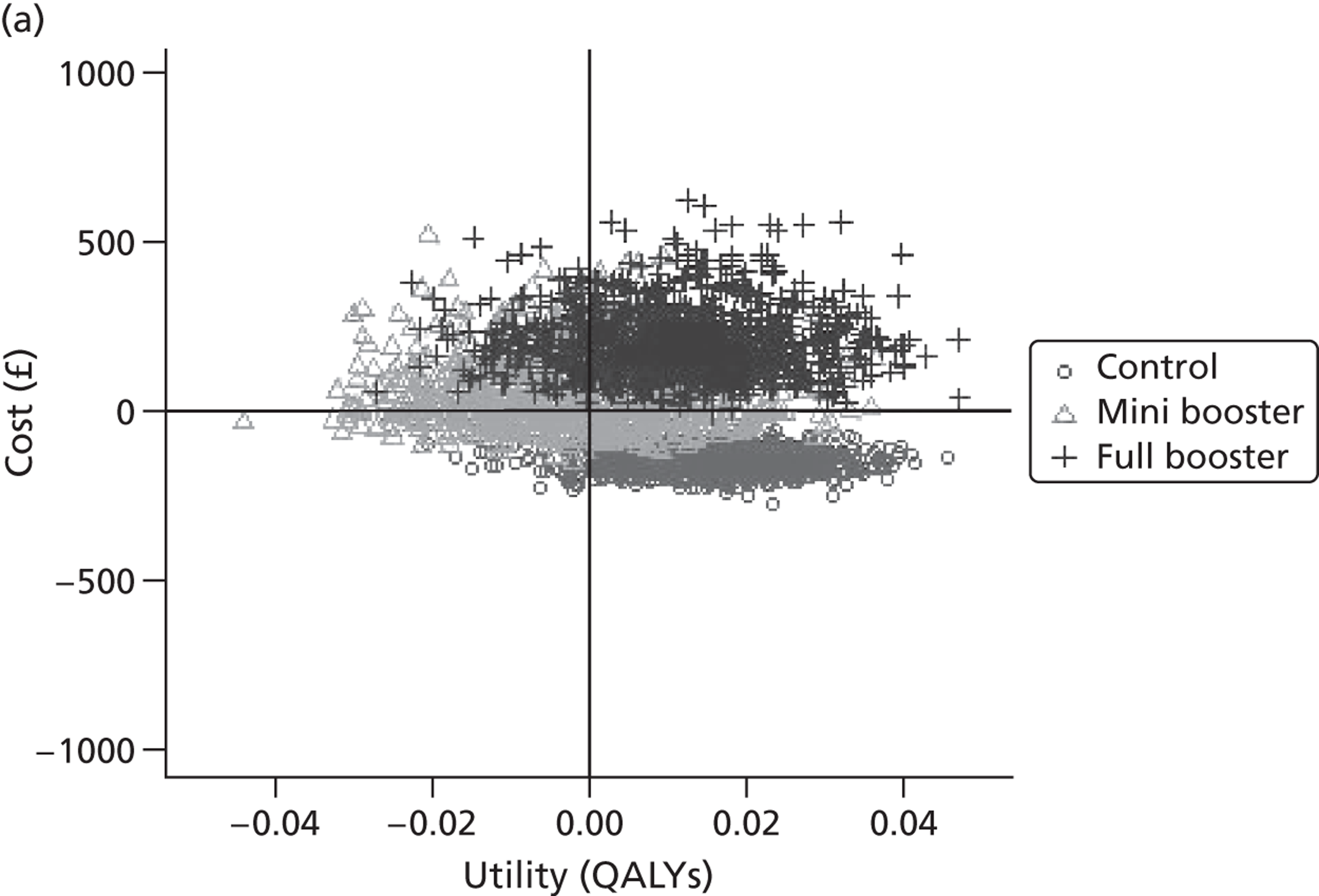
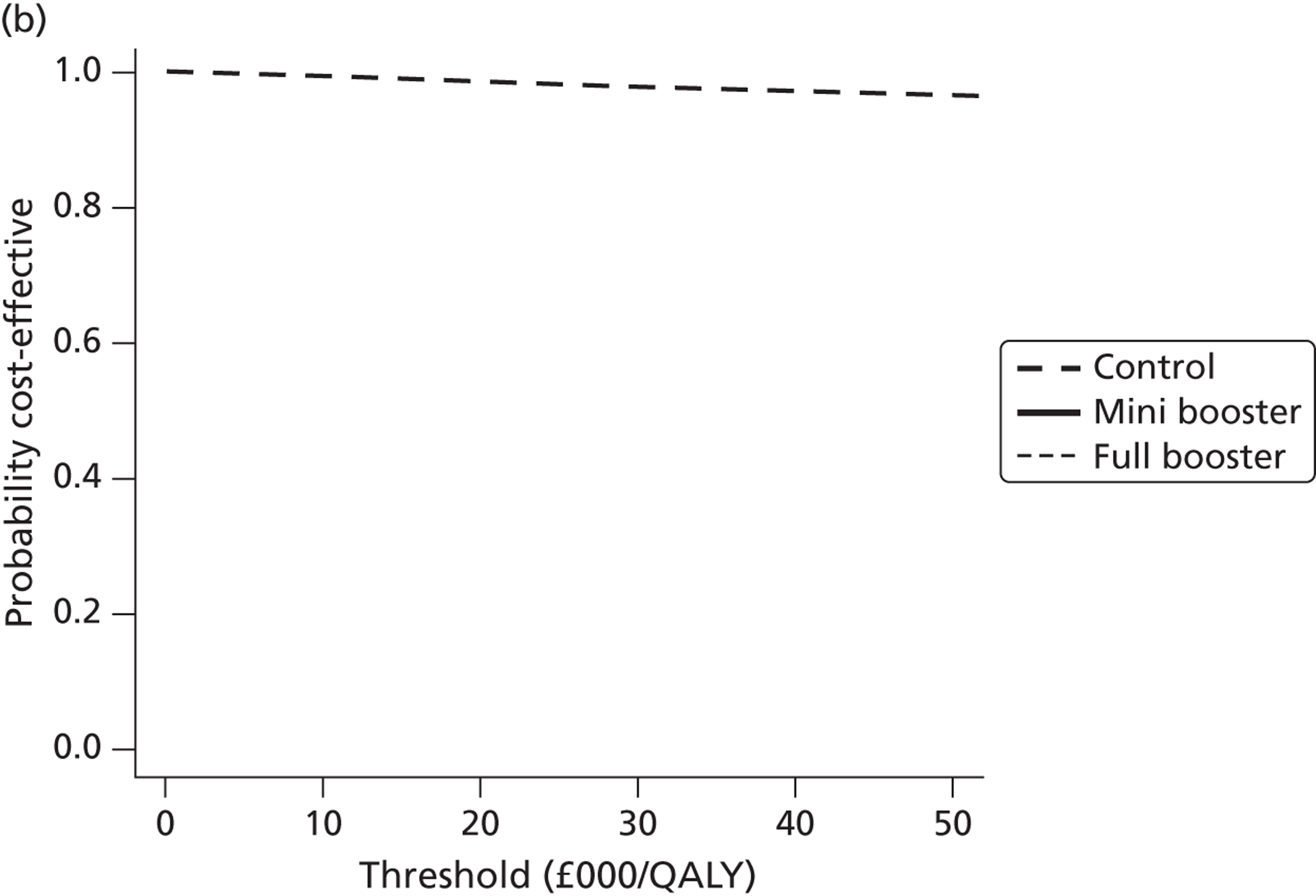
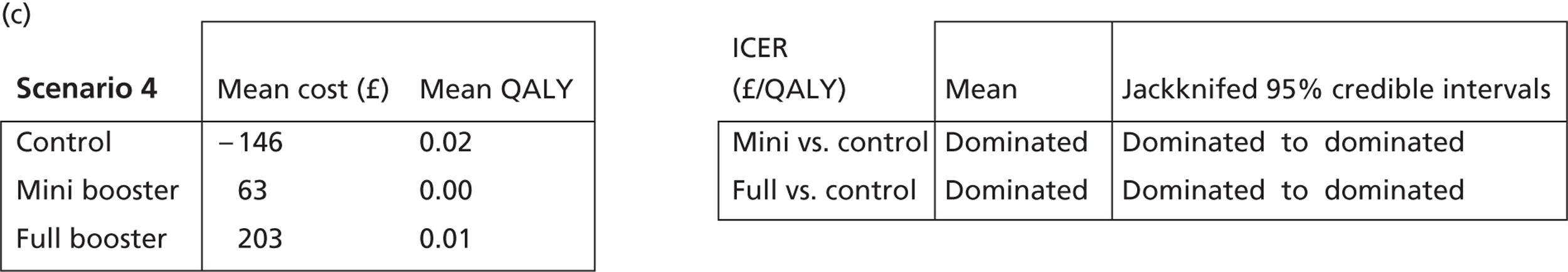
FIGURE 35.
Scenario 5. (a) Scatter plot of difference in costs (£) against differences in QALYs; (b) CEAF; and (c) mean costs, QALYs (to two decimal places) and ICERs.
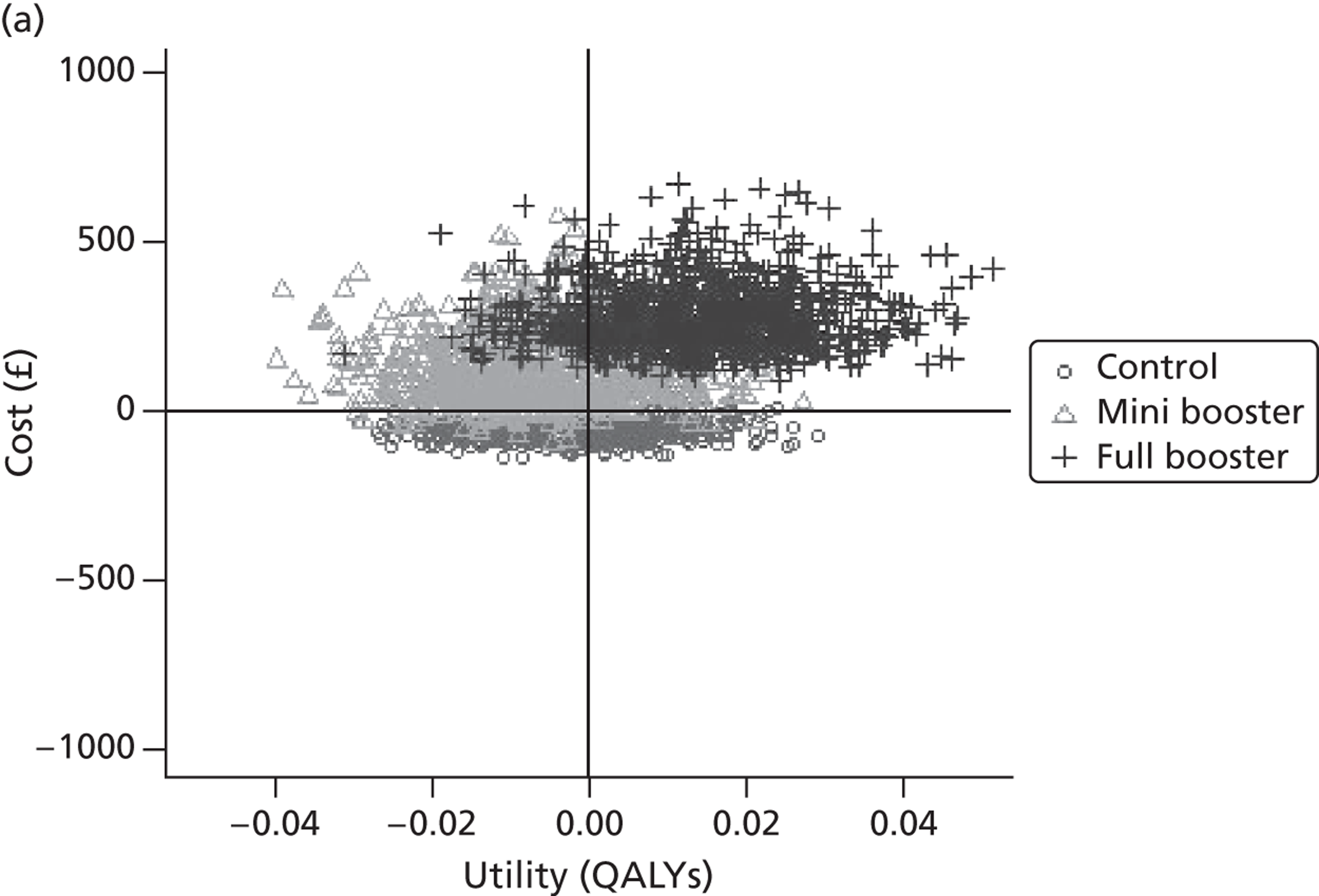
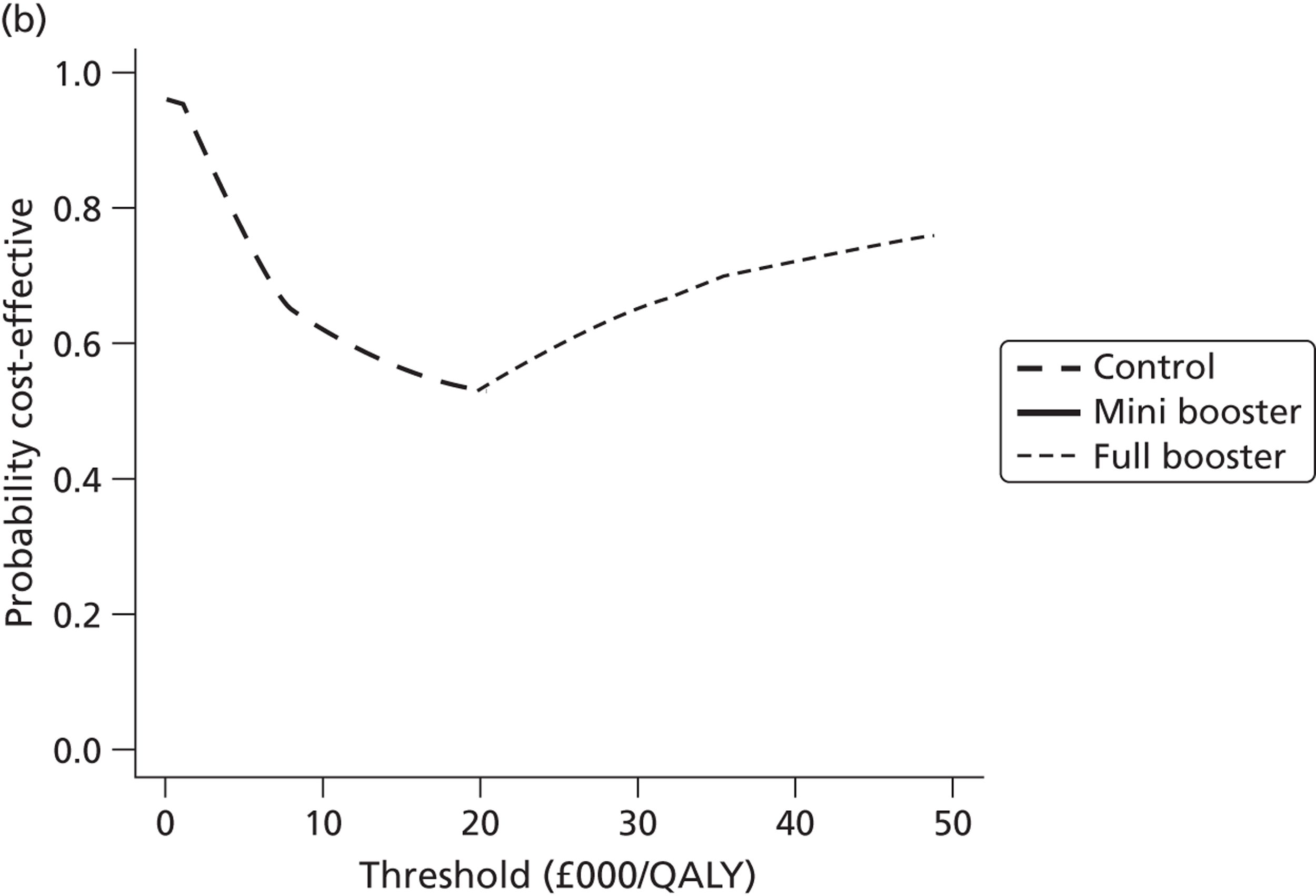
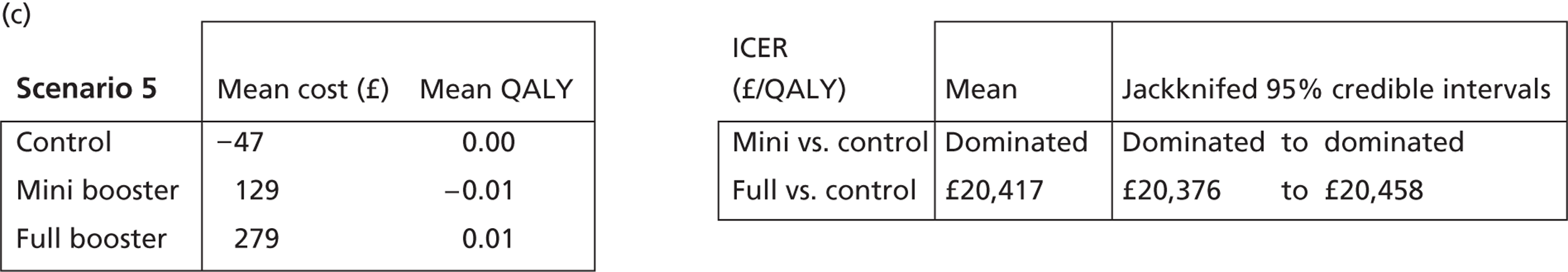
FIGURE 36.
Scenario 6. (a) Scatter plot of difference in costs (£) against differences in QALYs; (b) CEAF; and (c) mean costs, QALYs (to two decimal places) and ICERs.
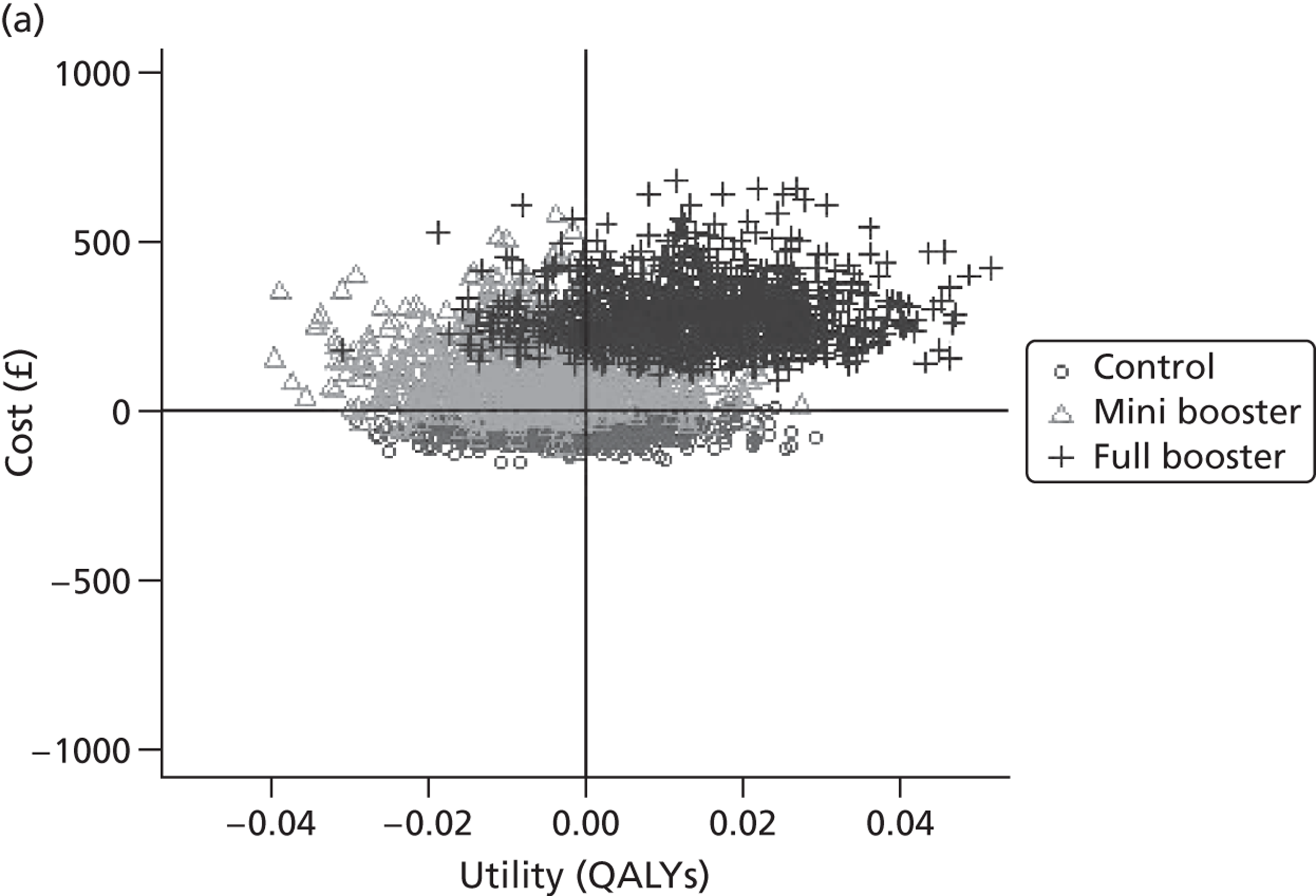
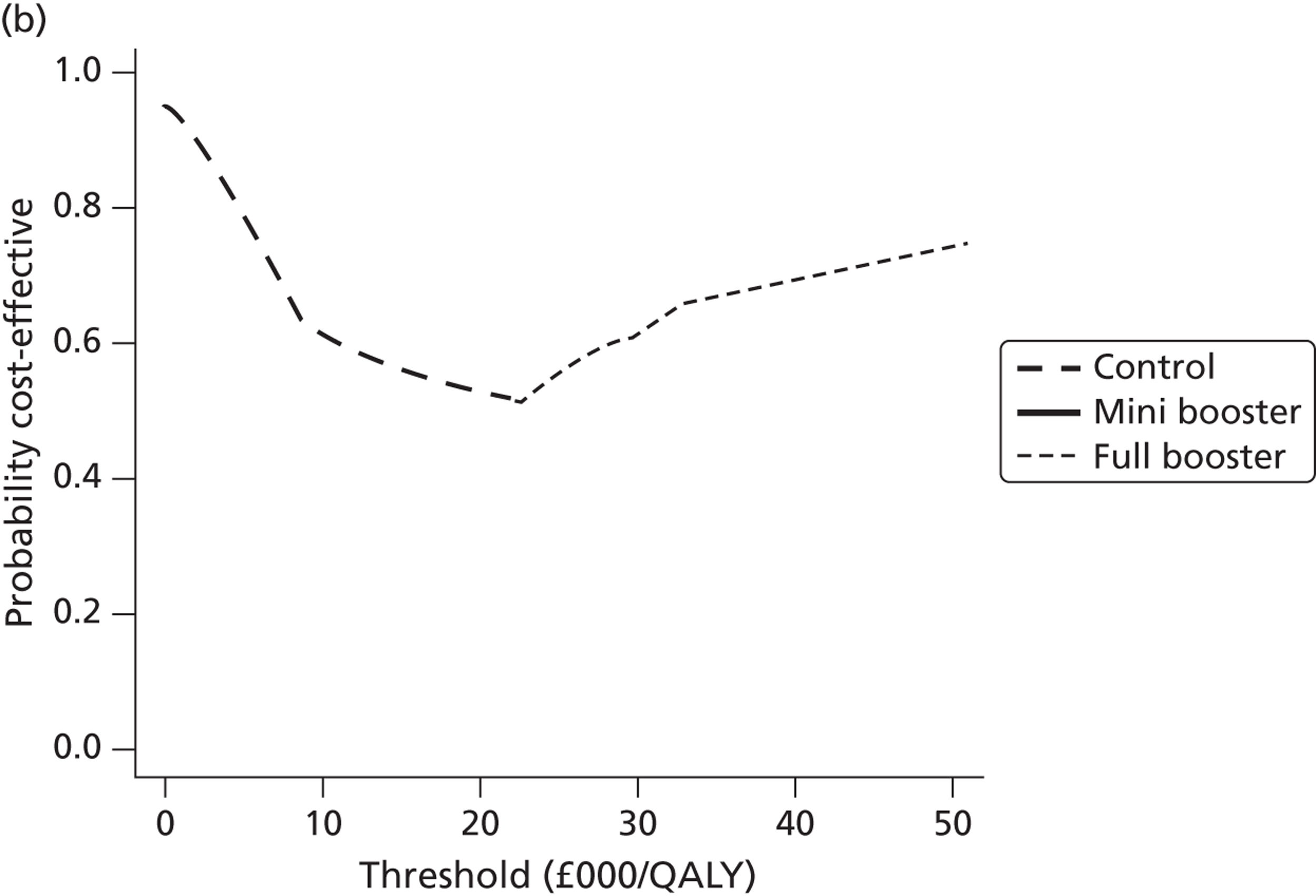
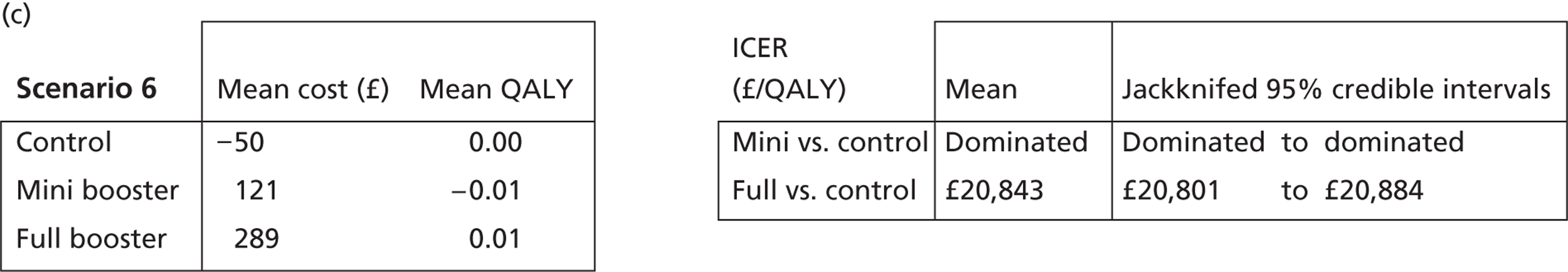
FIGURE 37.
Scenario 7. (a) Scatter plot of difference in costs (£) against differences in QALYs; (b) CEAF; and (c) mean costs, QALYs (to two decimal places) and ICERs.
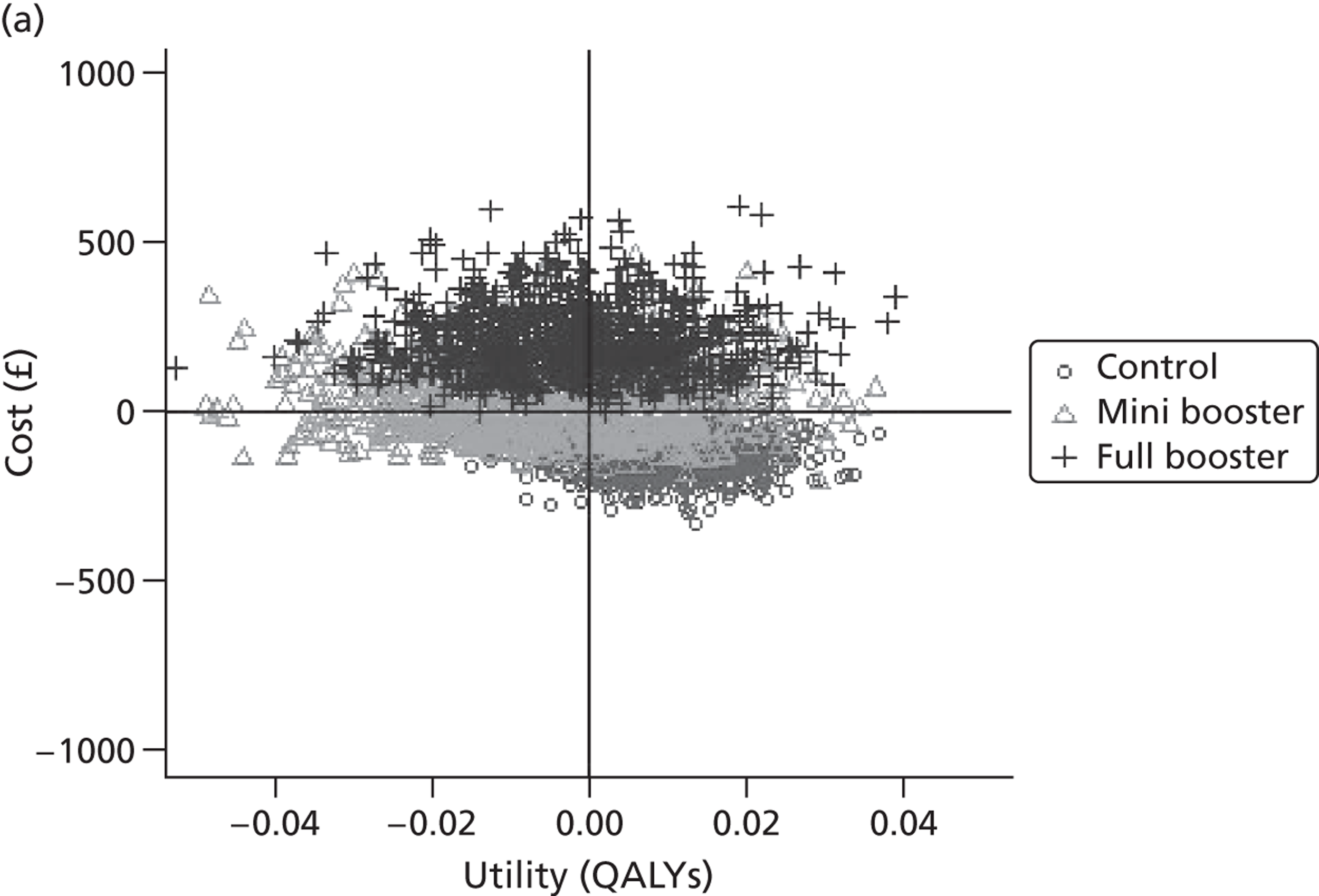
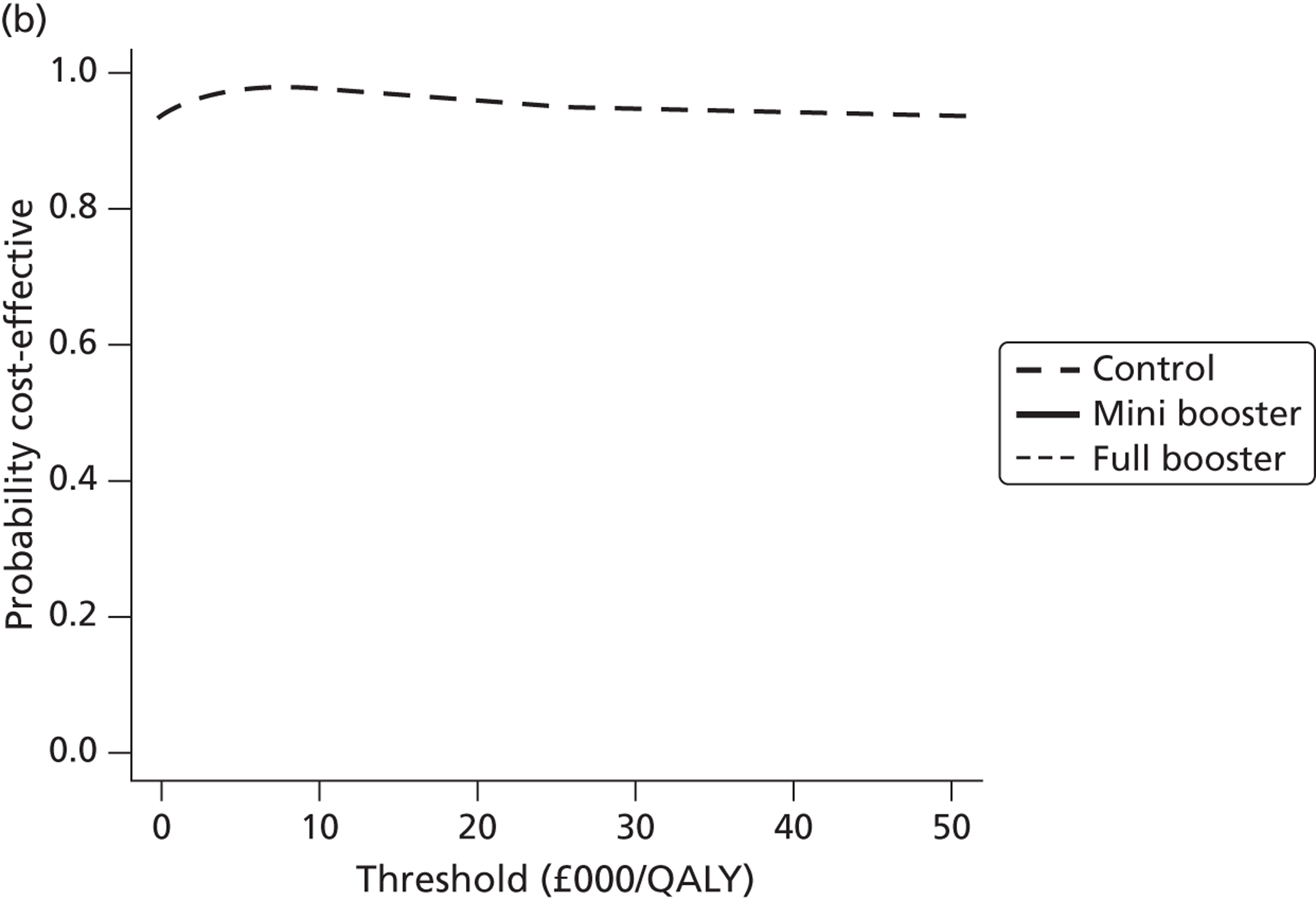
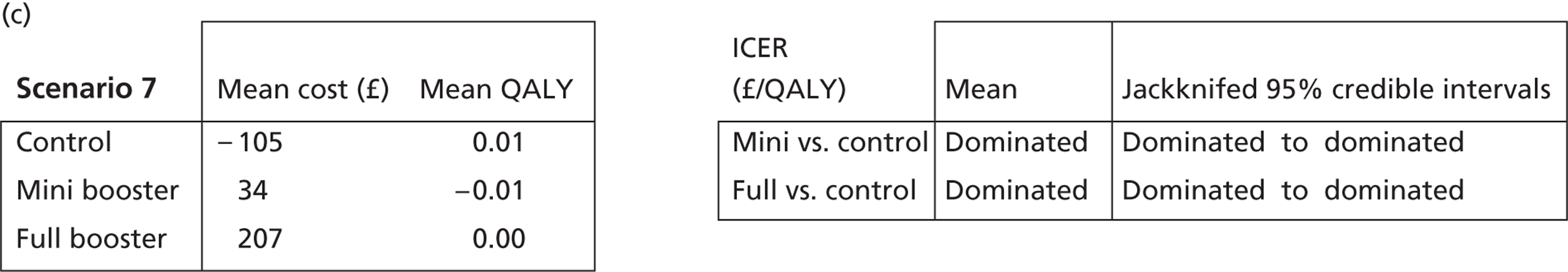
FIGURE 38.
Scenario 8. (a) Scatter plot of difference in costs (£) against differences in QALYs; (b) CEAF; and (c) mean costs, QALYs (to two decimal places) and ICERs.
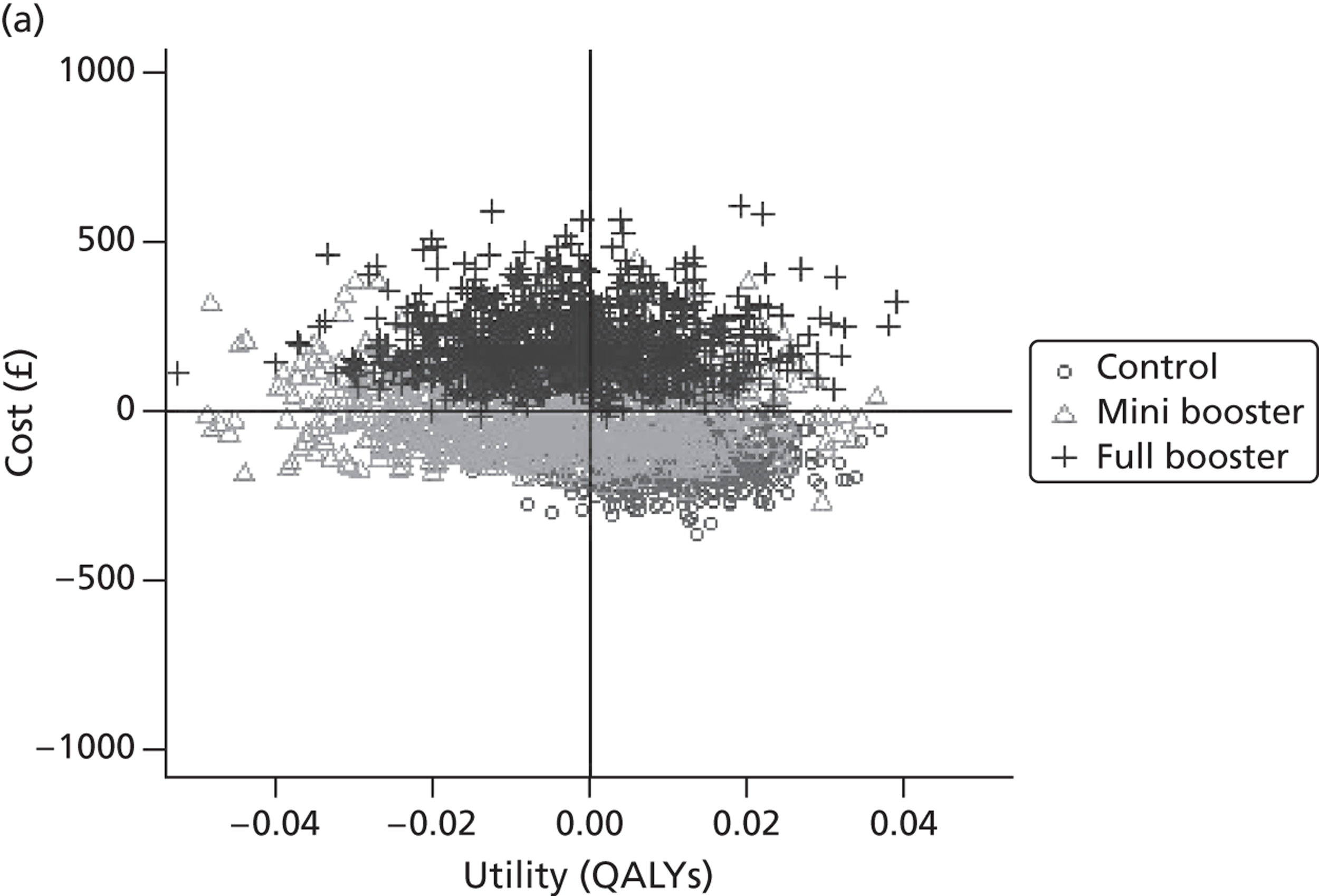
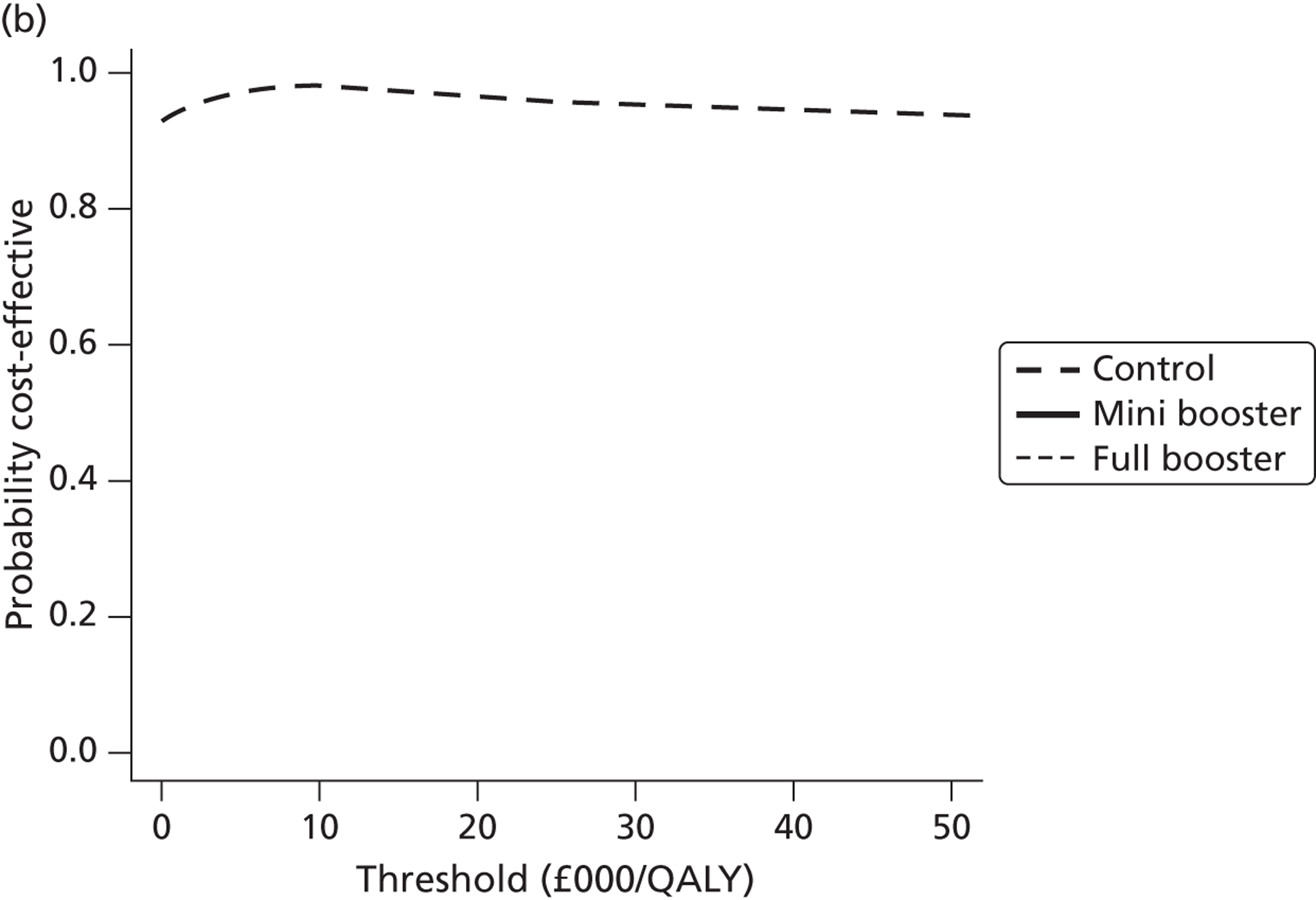
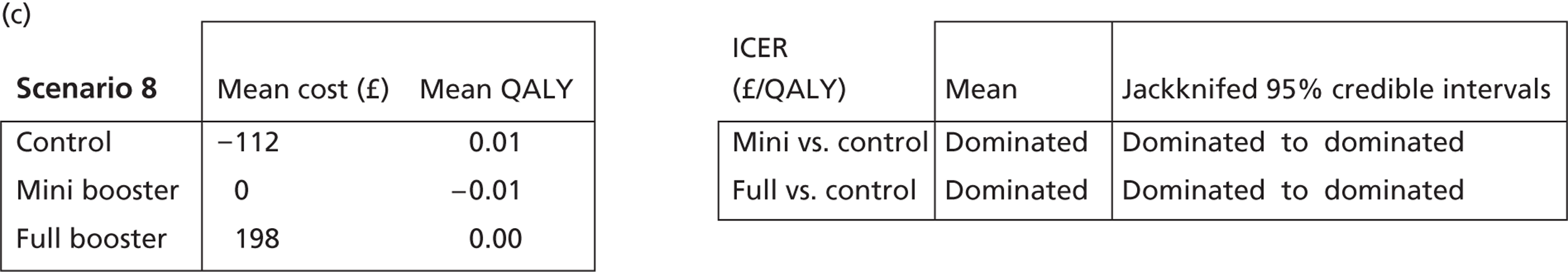
FIGURE 39.
Scenario 9. (a) Scatter plot of difference in costs (£) against differences in QALYs; (b) CEAF; and (c) mean costs, QALYs (to two decimal places) and ICERs.
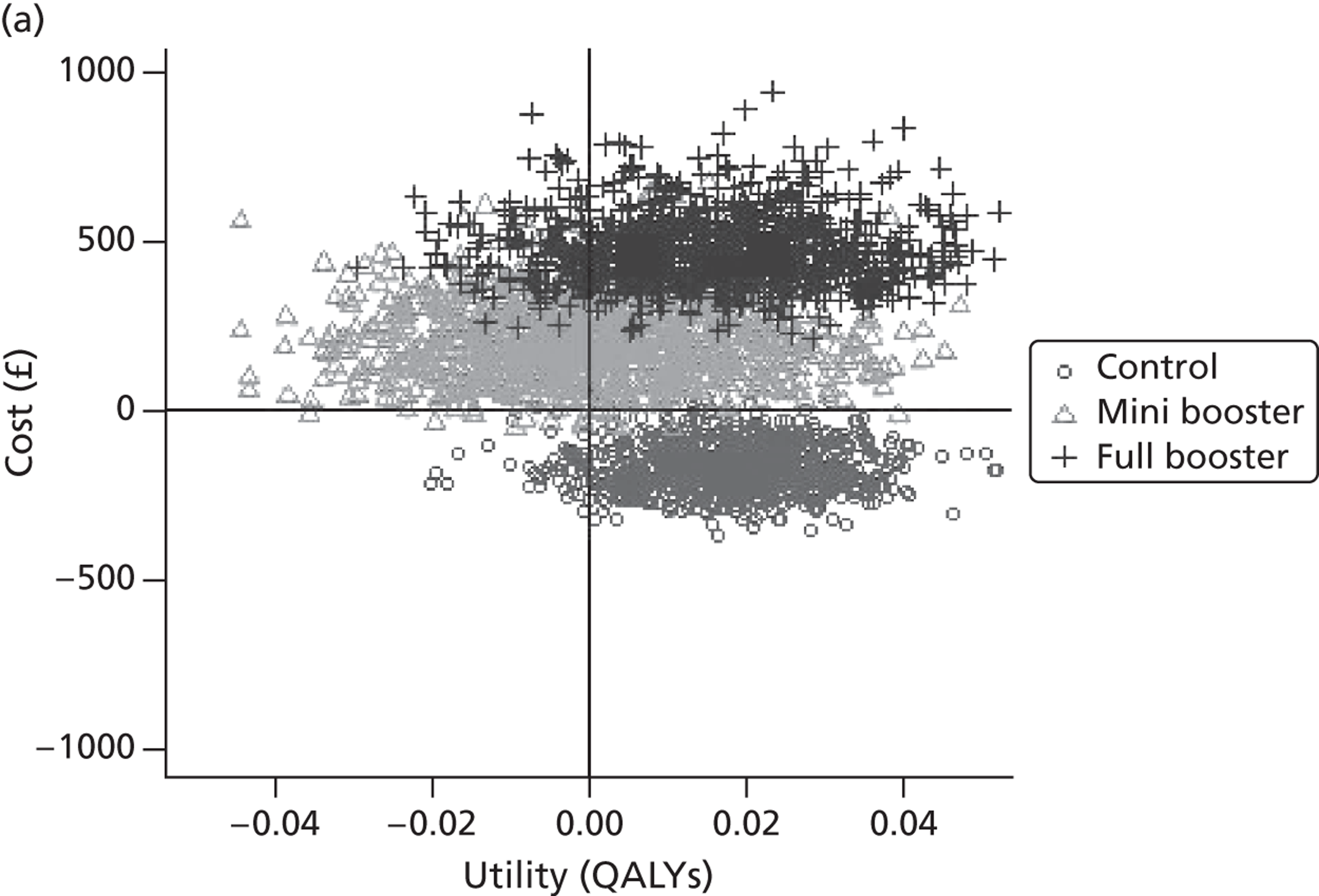
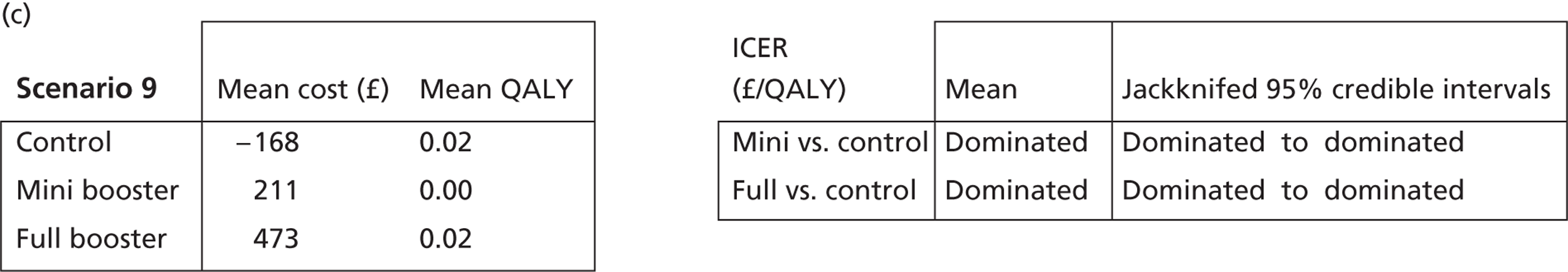
FIGURE 40.
Scenario 10. (a) Scatter plot of difference in costs (£) against differences in QALYs; (b) CEAF; and (c) mean costs, QALYs (to two decimal places) and ICERs.
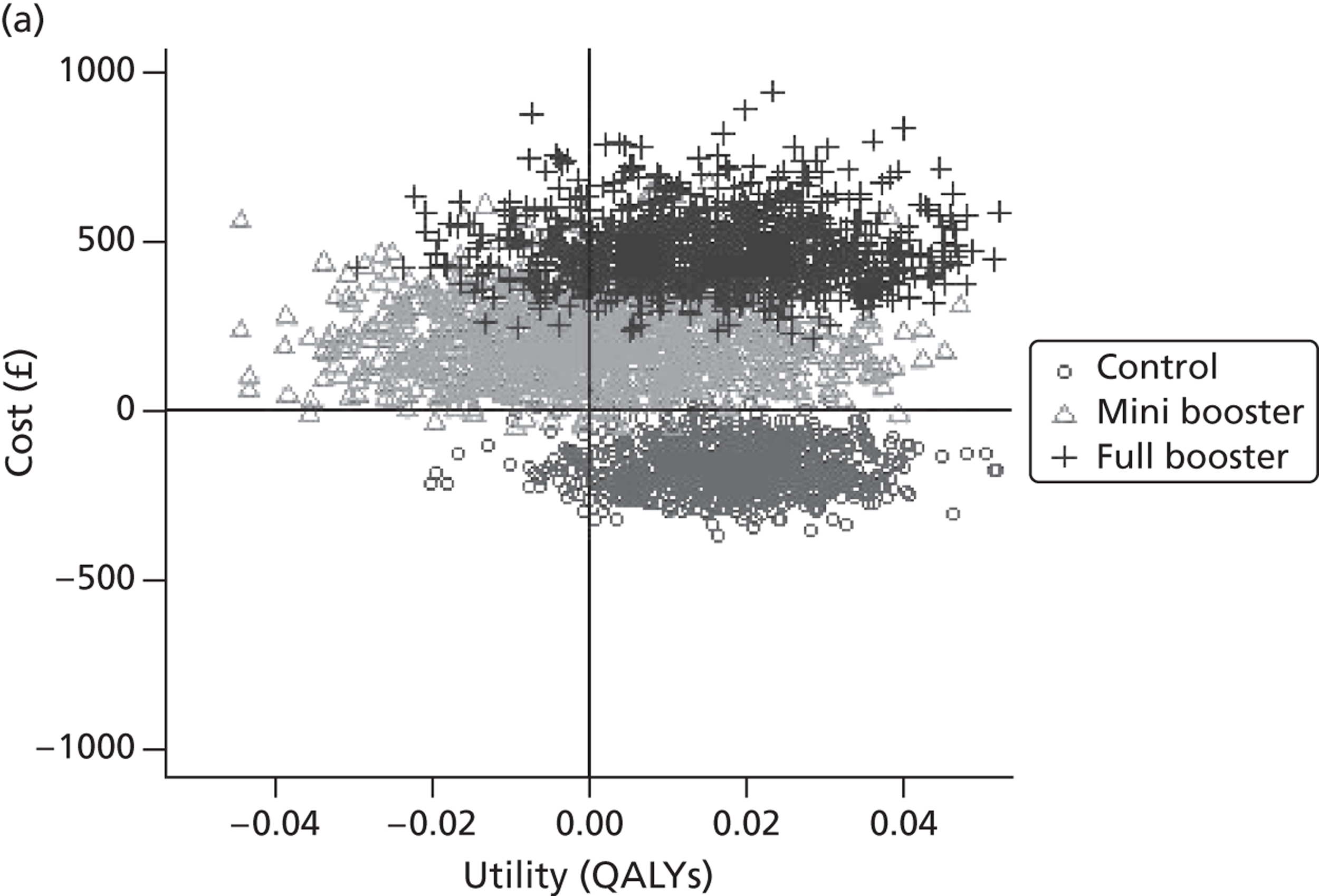
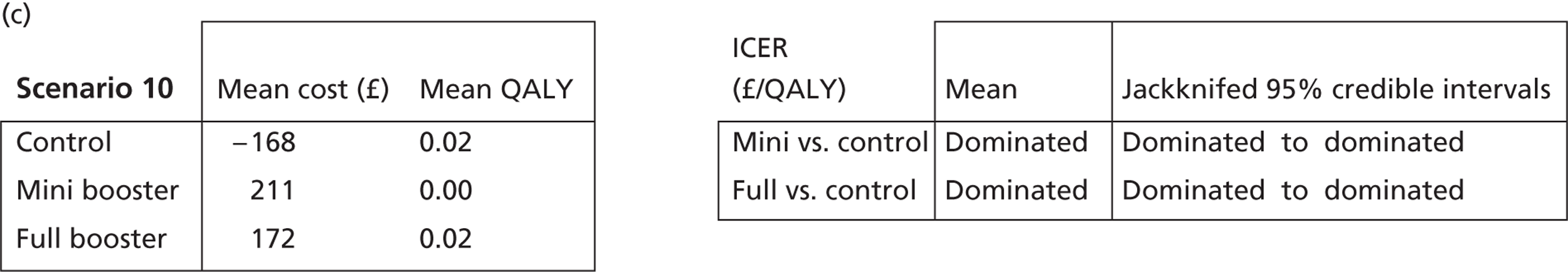
FIGURE 41.
Scenario 11. (a) Scatter plot of difference in costs (£) against differences in QALYs; (b) CEAF; and (c) mean costs, QALYs (to two decimal places) and ICERs.
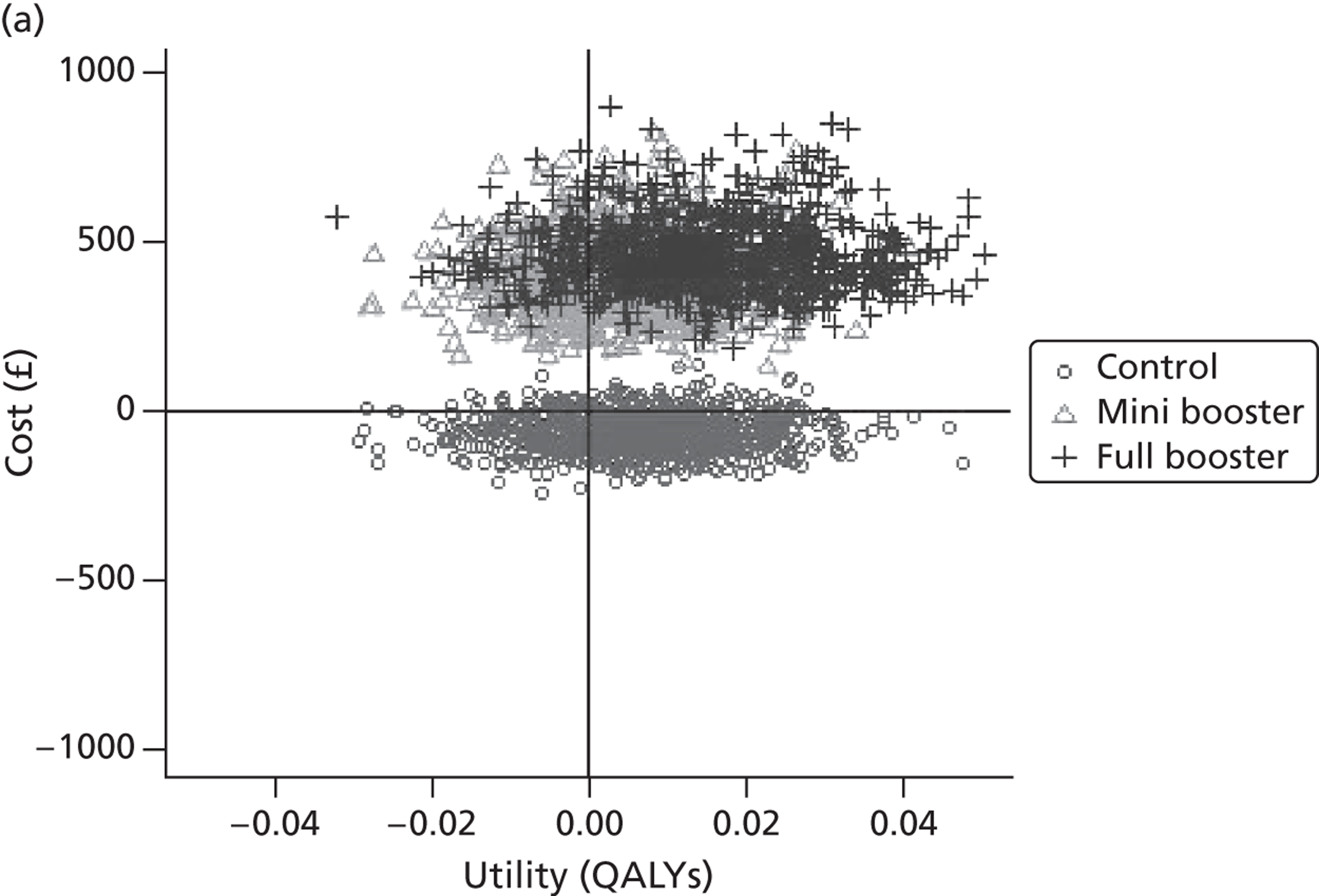
FIGURE 42.
Scenario 12. (a) Scatter plot of difference in costs (£) against differences in QALYs; (b) CEAF; and (c) mean costs, QALYs (to two decimal places) and ICERs.
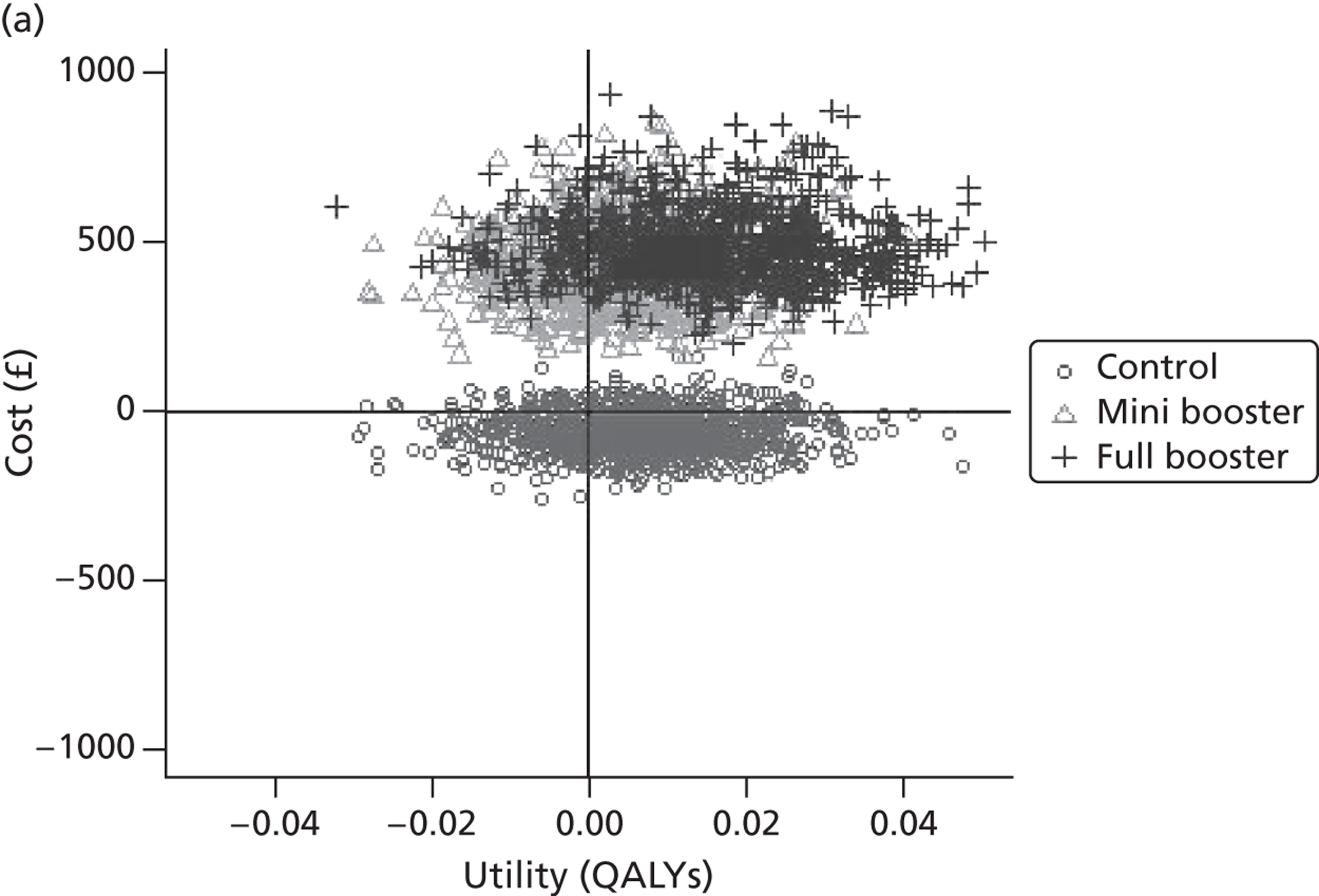
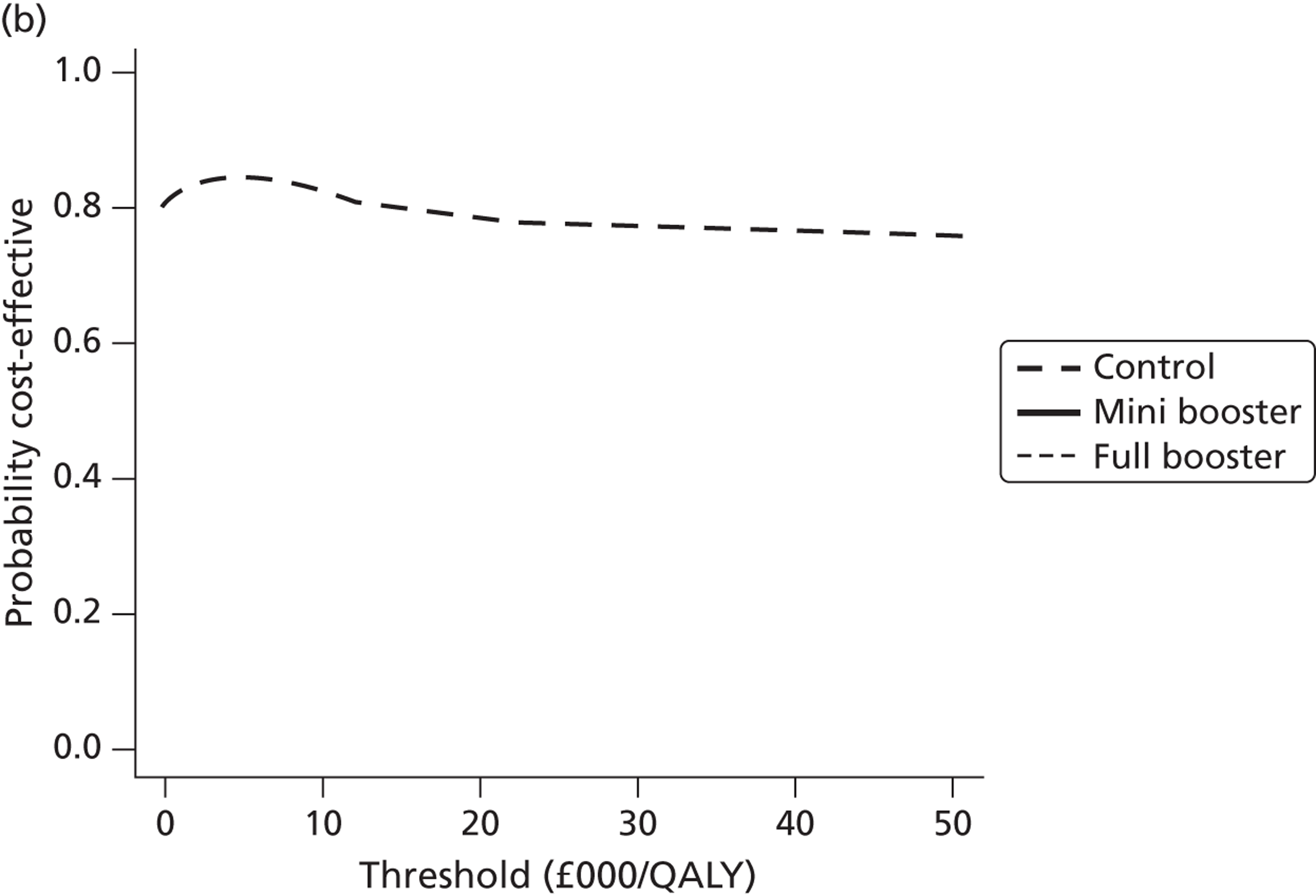
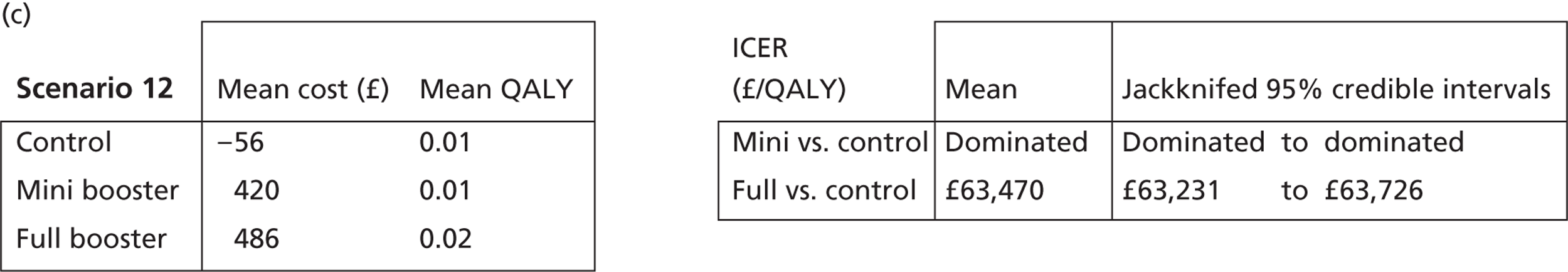
Summary of the short-term model results
The results of the separate scenarios for the short-term, questionnaire-based cost-effectiveness model suggest that the estimated cost-effectiveness of the intervention is subject to a high degree of structural uncertainty and depends on factors such as which data are used and which time periods are compared. In the majority of the scenarios, the control intervention, no booster, appears both the optimal choice and the option with the highest probability of cost-effectiveness at all willingness-to-pay thresholds considered. However, if the assumptions involved in scenarios 5 and 6 are made, there is an indication that the full booster may be the optimal choice when assuming a willingness-to-pay threshold of ≥ £20,000.
Looking at the estimated mean costs and mean QALYs for each arm in each scenario, it is apparent that the differences in costs and QALYs are marginal for all scenarios. For QALYs, the differences in mean values between arms are typically around or < 0.01 QALYs. The cost of the booster intervention is just one of a number of costs to the NHS and society that the participant population incurred and, compared with the costs of other NHS resources accessed by the participants over the trial period, it is not large. As the mean costs and QALYs observed in all arms are very similar, and the ICER is a ratio of two numbers, even slight decreases in costs or increases in QALYs could lead to very different indications of the cost-effectiveness of either intervention and so all results presented are potentially very dependent on statistical noise.
As previously discussed, the approaches taken to estimate the causal effect of the intervention on mean resource use and mean utility should be considered cautiously. This is partly because of the small sample sizes involved but mainly because the population considered does not as a rule suffer from any particular NHS resource-consuming and quality of life-reducing disease that the intervention is specifically designed to treat. Because of this, changes in HRQoL or NHS resource consumption over the relatively short time horizon of the trial are unlikely to be directly related to the effect of the intervention. As the intervention is more preventative than curative, the effect of the intervention on NHS resource consumption and HRQoL is likely to be relatively indirect, mediated by the effect of increased physical activity on lifelong morbidity and mortality risks, and to operate over a much longer time horizon. The long-term model results presented in the following section attempt to take these factors into account.
Long-term (epidemiological) model
Individual sampling model results
In the long-term model, 9-month and 3-month mortality RRs associated with individuals within each intervention arm are based on TEE scores sampled directly from individual participant trial data. Because of the power law mapping equation used to associate individual TEE scores with mortality RRs, and slight differences in the age and gender distribution of the trial arms, the mean incremental effects of the intervention on HRQoL may differ from the effects on TEE. This is particularly likely to be the case when the distribution of baseline levels of physical activity differs by group, as the mapping equation assumes that the most sedentary individuals have much higher mortality RRs than slightly less sedentary individuals.
As discussed earlier, a number of separate primary scenarios were considered to investigate the impact of structural uncertainty on the model results. These are shown in Table 12, which shows mean incremental life-years and QALYs in each arm, and Table 13, which shows, to two decimal places, estimated incremental differences in effectiveness in the intervention arms compared with the control arm in each of the scenarios.
| Arm | Long-term physical activity scenarios assumeda | Extra years lived | QALYs accrued | ||
|---|---|---|---|---|---|
| Mean | SE | Mean | SE | ||
| Control | Scenario A | 26.73 | 0.02 | 12.75 | 0.01 |
| Scenario B | 26.73 | 0.02 | 12.75 | 0.01 | |
| Scenario C | 26.90 | 0.02 | 12.81 | 0.01 | |
| Mini booster | Scenario A | 26.71 | 0.02 | 12.73 | 0.01 |
| Scenario B | 26.82 | 0.02 | 12.78 | 0.01 | |
| Scenario C | 26.14 | 0.02 | 12.52 | 0.01 | |
| Full booster | Scenario A | 26.58 | 0.02 | 12.69 | 0.01 |
| Scenario B | 26.67 | 0.02 | 12.72 | 0.01 | |
| Scenario C | 26.18 | 0.02 | 12.53 | 0.01 | |
| Scenario | Comparison | Extra years lived | QALYs accrued | ||
|---|---|---|---|---|---|
| Mean | SE | Mean | SE | ||
| Individual-level differences in differences | Control vs. mini | −0.10 | 0.03 | −0.05 | 0.01 |
| Control vs. full | −0.09 | 0.03 | −0.04 | 0.01 | |
| 3-month differences | Control vs. mini | −0.09 | 0.02 | 0.03 | 0.01 |
| Control vs. full | 0.06 | 0.02 | −0.02 | 0.01 | |
| 9-month differences | Control vs. mini | 0.76 | 0.02 | −0.29 | 0.01 |
| Control vs. full | 0.72 | 0.02 | −0.27 | 0.01 | |
As Table 12 indicates, all estimates for the number of additional life-years lived and QALYs accumulated are very similar, with all values differing by < 1 life-year and < 0.5 QALYs. As Table 13 indicates, this in turn leads to very small estimates for incremental differences, of < 1 life-year and < 0.33 of a QALY. Because of the stochastic qualities of the models, and the effect of initial ages on QALYs and annual QALY increments, the direction of the incremental differences in life-years and QALYs is different for some of the scenarios, highlighting how marginal the differences between the trial arms were and the resulting influence on random variation of the estimated results.
Secondary analyses
In addition to the main long-term model results, two supplementary analyses were also conducted. In the first series of analyses, the levels of physical activity of all participants were set to fixed values. These fixed values were varied from the first (lowest) quintile to the fourth quintile observed in the trial. The mean additional utility that resulted from shifting up by one quintile was estimated for each of these baseline levels of physical activity so that the relationship between additional physical activity and baseline activity could be explored. The second series of analyses adopted a similar approach but used a level of physical activity gain based on the mean differences-in-differences estimates for TEE in the full booster group compared with the control group.
Scenarios assuming gains of one quintile
Given the power law relationship that appears to exist linking physical activity with mortality RRs, it is important to consider the effect of the baseline level of physical activity in estimating the cost-effectiveness of an intervention. Given that the least physical active quintile has the highest mortality risk, it can be assumed that a given improvement in physical activity is likely to be disproportionately effective in terms of reduced mortality in this population compared with less sedentary baseline populations. Within the scenario analysis it was assumed for simplicity that the intervention led to an improvement in physical activity of one quintile for 2 years. The effect of this temporary increase in physical activity was modelled when assuming that the entire population was initially in the first (most sedentary) quintile, the second quintile, the third quintile and then the fourth quintile. The results of this analysis are shown in Table 14 and Figure 43. The maximum acceptable intervention cost for each of these quintiles is presented, assuming a standard willingness-to-pay threshold of £20,000 per QALY.
| Quintiles moved between | Mean utility gain | SE | Maximum acceptable intervention cost (£) |
|---|---|---|---|
| 1 (most sedentary) to 2 | 0.122 | 0.0119 | 2430.70 |
| 2 to 3 | 0.046 | 0.0102 | 914.36 |
| 3 to 4 | 0.043 | 0.0094 | 853.83 |
| 4 to 5 (most physically active) | 0.032 | 0.0088 | 649.66 |
FIGURE 43.
Shift in physical activity quintile.
‘Value-added’ model
Analyses of available data comparing mean daily TEE levels at 3 months and 9 months, and in the control arm, mini booster arm and full booster arm, suggest that those in the control arm used on average 66.64 kcal less per day at the end of the trial than at 3 months. In comparison, those in the mini booster arm used 36.37 kcal less per day at the end of the trial than at 3 months and those in the full booster arm used 8.85 kcal less at the end of the trial than at 3 months. This indicates a difference of 30.27 kcal favouring the mini booster over the control and a difference of 57.78 kcal favouring the full booster over the control. These differences are very small but positive. Because of the non-linear relationship between TEE gain and baseline TEE level, the main economic model, which used individual-level data from all participants, produced utility estimates that very slightly favour the control arm over either of the booster interventions. Within this additional series of analyses, the implications of assuming that the mean relative gains of the intervention groups relative to the control group were applied equally to all participants was explored. To do this it was noted that 30.27 kcal is equal to 1.36% of the 3-month median TEE score and 57.78 kcal is equal to 2.53% of the 3-month median TEE score. Just as in the previous series of analyses an intervention was assumed to lead to a 20 percentage point increase in activity for 2 years relative to no intervention (i.e. shift activity levels from the first to the second quintile, the second to the third quintile, and so on), so in this series of analyses the full booster was assumed to result in a 2.53 percentage point increase and the mini booster was assumed to result in a 1.36 percentage point increase.
With these assumptions it appears that the full booster may be cost-effective, assuming a willingness-to-pay threshold of £20,000 per QALY, if the intervention costs < £332 per participant (95% credible interval dominated to £725 per participant), as shown in Table 15. However, the clinical differences between the control arm and the mini booster arm appear so marginal that the mini booster and control arms appear largely indistinguishable in terms of QALYs and so the central estimate suggests that the mini booster is ruled out by simple dominance compared with the control (although the 95% credible intervals of the maximum acceptable intervention cost vary from not acceptable/dominated to £299 per participant).
| Scenario | Extra years lived, mean (SE) | QALYs accrued, mean (SE) |
|---|---|---|
| Control | ||
| Untreated | 26.70 (0.0175) | 12.74 (0.00610) |
| Treated | 26.74 (0.0175) | 12.75 (0.00608) |
| Mini booster | ||
| Untreated | 26.83 (0.0174) | 12.79 (0.00605) |
| Treated | 26.86 (0.0174) | 12.80 (0.00605) |
| Full booster | ||
| Untreated | 26.70 (0.0175) | 12.74 (0.00610) |
| Treated | 26.77 (0.0174) | 12.77 (0.00605) |
| Differences-in-differences | ||
| Control vs. mini booster | −0.008 (0.0291) | −0.0047 (0.0100) |
| Control vs. full booster | 0.033 (0.0292) | 0.0166 (0.0100) |
Chapter 8 Discussion
Effectiveness of motivational interviewing ‘boosters’
Overall, despite the low recruitment rate and significant loss to follow-up in the trial cohort, it is possible to draw some useful, and relatively robust, conclusions from the results of this randomised trial. The statistical analysis and economic modelling suggest that the interventions are unlikely to be effective or cost-effective whilst the process evaluation suggests some potential explanations for both the poor uptake and the lack of overall effectiveness in this relatively deprived and middle-aged population.
We found no evidence of a difference in TEE between the booster groups and the control group and the point estimates suggested that the combined booster group was less active than the control group. Even allowing for sampling uncertainty because of the small sample size, the confidence limits do not cross 101.5 kcal, the clinically important difference on which the trial was re-powered following the internal pilot/feasibility phase. This effect size represents about 4% of a typical energy expenditure of 2400 kcal per day. 87,88 In the comparison of the two forms of booster, we observed a difference in TEE per day of 112 kcal favouring the full (face-to-face) booster, although this result was not statistically significant and of borderline clinical significance.
Although there may be major concerns about the generalisability of the findings from such a small group of trial completers relative to the population targeted, the 160 (60%) participants who contributed data to the primary end point do still represent the target population: they are resident in deprived areas; aged between 40 and 64 years (if skewed towards the upper end of the range) and mostly highly sedentary at baseline (with a minority who have previous positive experiences of physical activity). If a similar intervention was offered outside the context of a trial, we have no reason to suppose that those taking up the offer of ‘booster’ interventions would differ significantly from those who volunteered to participate in this trial and complied with the intervention.
Views on physical activity and motivational interviewing ‘boosters’
A wide range of leisure activities were reported in the open-ended survey responses, with few sports and competitive activities being reported. Locations also varied, encompassing facilities, domestic contexts, the local area and rural locations. Reasons for taking part in the study were also varied, including wanting to help with the research; being advised by medical staff to increase activity; wanting to improve or gain knowledge of health and well-being, physical activity levels and fitness; and to gain personal benefit.
Medical health issues were frequently cited as both barriers to and motivators for being physically active. Musculoskeletal injuries were a prevalent barrier whereas chronic physical conditions were often a direct cause of people wanting to become more active than previously. Social support was important with many people saying that they found exercising with other people encouraging and motivating oneself in isolation difficult. Friends and strangers from outside of the home were sometimes seen as better support than family, who were often perceived as a barrier, especially in younger participants who had young children.
Most people found some benefit from the MI although some people would have liked something more action oriented (see Chapter 4, Motivational interviewing: consistency with perspectives or world views). The setting for delivery was generally considered convenient and many people talked about MI meeting some unmet need, particularly an occasion to talk about goals and barriers relating to physical activity with an informed and non-judgemental person (in contrast to family and sometimes friends). The term ‘counsellor’ was used by several participants to describe the MI practitioners, reflecting a perception of the relationship as impartial and supportive.
A prevalent theme was that people were keen to receive feedback (both verbal and accelerometry) on their performance and strove to meet goals so as not to disappoint the MI practitioners. Monitoring and normative feedback is an important part of ongoing motivation to maintain behaviour change.
More participants who received a booster intervention said that a face-to-face (full) booster would be preferable to a telephone (mini) booster, which was seen as less easy to prioritise. This is corroborated in the literature: specifically in MI, a face-to-face intervention is identified as an effective way of developing client engagement. 89 This, together with accurate empathy, is consistently highlighted as an important factor in supporting health behaviour change and, as technology attempts to substitute this level of contact with potentially cheaper and more convenient platforms such as smartphone apps, the telephone, DVDs and online alternatives, trade-offs between cost and efficacy might be expected.
Self-reported moderate to vigorous physical activity often appeared to be at odds with the objective data, both for individuals, as illustrated in Chapter 4 (see The prevalence of optimistic self-assessment), and for the participant population as a whole, for whom the correlation between self-report (SPAQ) and objective (Actiheart) data at 3 months was almost non-existent. This incongruence is common in the measurement of health behaviours and is widely reported in the literature. 90 In terms of physical activity, participants typically over-report moderate and vigorous activities and under-report low-intensity activities. 91 Concerns are often raised about participants altering their behaviour in response to the knowledge that they are being monitored, the ‘Hawthorne effect’. 92 Reactivity of this kind causes a change in actual behaviour, rather than a discrepancy between different methods of measurement. Over-reporting of activity may occur out of a desire to please the researcher and meet with perceived expectations, that is, social desirability bias, whereas it has been suggested that the under-reporting of lower-intensity activities, such as walking, may be due to a simple lack of awareness of undertaking them as many instances occur as part of everyday living rather than as planned activities. 93
There are some parallels between the findings of the process evaluation and the findings of other qualitative research examining physical activity in older adults. For instance, engaging in a lifelong habit of being physically active was a motivator for physical activity in a systematic review of physical activity during the transition to retirement, and relates to previous practice and also commitment to physical activity found in the current study. 94 Some of the barriers to physical activity identified in the current study, that is, preferred alternatives and lack of social support (for certain types of activity), were also identified in the review by Barnett and colleagues. 94 In both pieces of research physical activity was adopted in response to felt needs or problems and had social benefits that could be motivational.
The importance of the social context as both a barrier and a facilitator was also identified in a recent systematic review of physical activity perceptions among older adults of South Asian origin. 95 Specifically, ‘lack of time’ (encompassed by ‘preferred alternatives’ in the booster study) was a barrier that related to people’s obligations to others; taking time out from these obligations to exercise could be perceived as culturally inappropriate behaviour.
Monitoring and feedback have also been found to be desirable in a process evaluation of a primary care-based walking intervention for older adults. Mutrie and colleagues96 found that pedometers were perceived as easy to use and were popular as a motivational tool. Unlike the booster study, however, they found good correspondence between reported and measured increases in physical activity levels.
In a process evaluation of telephone health mentoring for chronic obstructive pulmonary disease patients (mean age of 65 years) in Australia, Walters and colleagues97 found that the experience of engaging in telephone health mentoring was valuable to most people and enabled them to reassess their activities of daily living, including physical activity. There was a perceived motivational element, with some people finding that the health mentoring served as a useful reminder to perform the relevant health behaviours. As in the booster study, some participants found the anticipation of speaking to someone about their health behaviour generally, and physical activity in particular, a motivator.
In another study98 the anticipation of mental and physical health benefits motivated the initiation of physical activity in South Asians aged 60–70 years and motivation for adherence also came from a drive to maintain good physical and mental health, which were among the reasons given by booster study participants for maintaining physical activity. In addition, Horne and colleagues98 found social support from family, friends, peers and statutory and voluntary workers to be important in motivating people to initiate and maintain physical activity, often through the impact of the social environment on their confidence. The self-confidence in their own physical ability gained from mastering a specific activity also served to motivate maintenance of physical activity among some people, and similarly confidence was given as a motivator for maintenance in the current study. Another recent study99 exploring the views of older Slavic immigrants in the USA about physical activity found that motivators included the desire to be active and go for walks and the anticipated benefits for existing medical health issues (including pain, poor sleep and use of medication). Anticipated benefits also included greater independence as well as social support, as physical activity was seen to provide a way of engaging with other family members, especially grandchildren. Some similar barriers to those in the current study were also reported, namely lack of desire, limitations imposed by medical health issues such as tiredness, pain and specific chronic conditions, and environmental issues such as safety concerns, bad weather and heavy traffic.
Geographical information systems analysis
The primary aim of the GIS analysis was to produce a set of variables for each of the 282 participants in the booster study that represented their pedestrian access to municipal green space and relevant leisure facilities (gyms and swimming pools). We found wide variations in pedestrian access across Sheffield, most notably with regard to proximity to swimming pools and the highest-quality green space. However, there was no statistical association observed in trial participants between mean TEE per day at 3 months and proximity to the above variables.
It is unlikely that proximity to either green space or leisure facilities was an important determinant of activity levels in our participants, which equally suggests that lack of access to green space and facilities was not a significant barrier to activity in trial participants. This is consistent with the qualitative evidence from the in-depth interviews, which suggests a range of other pertinent barriers.
Cost-effectiveness of motivational interviewing ‘boosters’
Because of the complex processes by which changing levels of physical activity lead to changes in HRQoL, two qualitatively different approaches to estimating the cost-effectiveness of the interventions were adopted. One of these approaches utilised resource use and SF-6D utility scores elicited directly from participants at 3 months and 9 months following the intervention to produce cost-effectiveness estimates over the short term. The other approach combined data on changes in levels of physical activity recorded using the Actiheart system with epidemiological data linking exercise capacity to relative mortality risks to produce individual-level simulations estimating long-term HRQoL effects resulting from the effect of changing levels of physical activity on participant mortality rates.
Despite adopting qualitatively different approaches and methodologies, both main models indicated that both the mini booster and the full booster appear to be ruled out by simple dominance and so do not appear cost-effective at any willingness-to-pay threshold. An additional variation of the long-term model, however, which incorporated data on physical activity differences in differences between arms in a different way from the main analysis, indicated that the full booster intervention may be cost-effective assuming a willingness-to-pay threshold of £20,000 per QALY as long as the cost of the intervention is below approximately £300 per participant. The additional assumption made in this model, however, is that all participants who receive the intervention increase their physical activity levels by equal amounts, which may be a particularly strong assumption given that it may be those participants who are already comparatively physically active who increase their physical activity levels most, and the least physically active who increase their physical activity levels the least. Given that it appears that the greatest health benefits for a given increase in physical activity are in people who were initially the most sedentary, patterns of selective take-up of additional physical activity may mean that the health benefits of a mean increase in physical activity may be less than initially assumed. 81
Other recent evidence for the effectiveness and cost-effectiveness of ‘brief’ and ‘booster’ interventions for increasing and sustaining physical activity
While the booster trial has been ongoing a number of other trials and non-randomised evaluations of physical activity interventions have reported, but none with a specific focus on maintenance. A team at ScHARR have recently conducted a review of the evidence for the effectiveness of brief interventions in promoting physical activity in primary care100 and this comprehensive systematic review, together with an associated review of the cost-effectiveness evidence, has informed an update of the NICE guidance on this topic,12 which is currently out to consultation (http://www.nice.org.uk). In the NICE-commissioned review the current evidence base for brief interventions was synthesised and it was found that, although there is evidence (based mainly on self-reported outcomes) that brief interventions are effective at increasing physical activity, there is not consistent evidence to suggest a ‘dose–response’ effect with more intensive or repeated interventions having a greater impact than a simple brief intervention (defined by the review inclusion criteria as one lasting up to 30 minutes).
Three other recent systematic reviews101–103 provide evidence that interventions in primary care, including brief interventions and telephone interventions, may be effective and/or cost-effective. The review by Orrow and colleagues103 also mainly synthesised trials with self-reported activity as the primary outcome, but included all primary care interventions with at least 12 months’ follow-up. This review found evidence for an impact of a range of interventions on self-reported activity, but similarly reported that there was no robust evidence that more intensive interventions (such as exercise on referral) were more effective than a simple brief intervention. A systematic review by Goode and colleagues102 included eight studies evaluating maintenance of physical activity behaviour change up to 12 months after a telephone intervention. The majority of telephone interventions involved > 12 contacts and took place over a period of > 6 months, with many interventions involving significantly more frequent and longer contacts than the mini booster intervention in our trial. The review defined successful maintenance as between-group differences in at least 50% of reported outcomes in favour of the telephone intervention group at least 3 months after completion of the intervention. Of the eight studies reporting on the maintenance of behaviour change, two reported a maintenance effect for at least 50% of outcomes over 3 months without the intervention. Garrett and colleagues101 reviewed cost-effectiveness studies of physical activity interventions in primary care and found that interventions involving advice (in person, by telephone or by mail) were more cost-effective than supervised exercise interventions. This review did not examine the marginal benefit of additional interventions to maintain behaviour change as the included studies all compared a specific intervention with usual practice or no intervention.
Considering the findings from the booster trial and the implications of the cost-effectiveness modelling in the context of these reviews suggests that simple, brief interventions targeting sedentary populations are likely to be more cost-effective than more expensive and intensive interventions, including ‘boosters’. A major caveat in assuming the generalisability of the effectiveness of brief interventions is that the majority of the evidence of effectiveness comes from self-reported outcomes in unblinded studies. The booster trial is one of the few trials with objectively measured energy expenditure (or physical activity) as the primary outcome measure and this confirmed the poor correlation between objectively measured activity levels and self-reported activity levels in trial participants. Future cost-effective modelling should also take account of the baseline physical activity levels (and fitness) of participants who achieve an increase in activity levels, given the exponential relationship between baseline risk and impact on mortality.
Strengths and weaknesses of the study
Recruitment and retention
The principal shortcoming of this study was its failure to recruit 600 participants and retain 450 within the allotted time and budget. This is a common experience as only 31% of Medical Research Council- and HTA-funded trials that recruited participants between 1994 and 2002 met their recruitment target on time and within budget, with 45% closing to recruitment having reached < 80% of their target and 54% requiring a time extension. 104 Furthermore, there is considerable evidence that recruitment to trials evaluating preventative interventions is considerably more difficult than recruitment to those evaluating therapeutic interventions, with typical rates of those randomised as a proportion of those screened cited of 1–5% and 20–27%, respectively, according to one overview. 105 High levels of deprivation are known to predict poor consent rates in trials evaluating behavioural interventions for sedentary people. 106,107 It may be particularly difficult to recruit healthy participants in deprived communities for an intervention that may have significant costs to participants, including both the costs of behaviour change and the time and travel costs of attending for the intervention as well as recruitment, baseline data collection and follow-up. We were also recruiting participants from an age group (40–65 years) in which many people have both work and family responsibilities, which may make it particularly likely that perceived costs will outweigh the perceived benefits of participation.
Our study needed to recruit patients who had already received and responded to a brief intervention but was hampered by the absence of access to patients already being given a brief intervention in the local primary care setting whom we could recruit. For this reason the study team had to devise and distribute a brief intervention (see Chapter 2, Participants) and deliver it in partnership with the local primary care authorities but outside of the routine NHS care setting. The principal method of approaching participants was through personalised letters, electronically signed by the local director of public health, inviting people to contact the study team. This method generated 4964 responses from the 70,388 letters sent, a response rate of 7.1%. It is possible that we could have generated more responses by recruiting GPs to carry out the mail-outs. However, this method would have been much more expensive and there is little evidence that it would have been more effective. The Food and Immunity Trial (FIT), another South Yorkshire-based public health study, recruited people aged 65–85 years using mail-outs from general practices. 108 Its response rate was 528 out of 7482 (7.1%), identical to our own. The FIT study team used a wide range of other methods to recruit participants but GP mail-outs accounted for 90% of consented participants. It is worth noting that there is no good-quality evidence for the superiority of direct face-to-face recruitment compared with mail-outs in this population. 109 Even had there been it is unlikely that we would have had the reach or the resources required to reach full accrual using face-to-face accrual. We attempted to recruit through a range of other stakeholders, including GPs, health trainers and health champions, but this was not successful (see Chapter 3, Recruitmant of trial participants). This suggests that direct recruitment through primary care and community networks may not have improved recruitment yields. Previous research comparing targeted mail-outs with recruitment through general practices has found that mail-outs are more effective and cost-effective, even when there are strong collaborative links with GPs. 110
The brief intervention devised by Sheffield Hallam University reached 1934 (2.7%) of the 70,388 people who were invited to apply for it, although a further 568 who contacted and were screened by us were excluded from using the brief intervention, mostly because they were already physically active (see Chapter 3, Recruitmant of trial participants). Of those who received the brief intervention, 840 (43%) were not contactable at 3 months, 538 (28%) were contactable but had not increased their self-reported physical activity levels sufficiently to be eligible for the trial and 274 (14%) who were eligible could not be randomised (half of whom actively refused consent; the other half did not attend consent appointments). The concept of the ‘funnel effect’, in which a pool of potential patients who are contacted about entering a clinical trial becomes progressively smaller as it passes through successive screens and the informed consent process, is relevant here. 105 It is widely believed that the lower the percentage recruitment yield, the more questionable it is to generalise from the study findings to the target population. 111 We would assert that the results of this study are generalisable. Although we found it difficult to identify participants who had benefited from receipt of the brief intervention (the key eligibility criterion), we randomised 51% (282/556) of those we were able to identify (see Figure 6). The issue is the willingness to take up and respond to a brief intervention of those who could benefit, as supported by the published evidence. Of the four published RCTs evaluating brief interventions delivered to UK populations,112–115 none reported statistically significant improvements in self-reported physical activity. For the same reason it is fair to say that the MI boosters cannot be seen as public health interventions, because they cannot be rolled out at a community level. Rather, individuals will identify whether the offered intervention is appropriate and acceptable to them, and only a minority of sedentary individuals are likely to take such an intervention up.
Impact of area deprivation on recruitment
We aimed to recruit people from deprived neighbourhoods. We knew from the outset that there were pockets of affluence within the predominantly deprived areas that we surveyed. In a few areas people from LSOAs that were less deprived were recruited to the study (see Figure 9). However, the majority of the study participants were from relatively deprived LSOAs. Out of 147 LSOAs targeted, 102 were in the bottom 20% of national IMD 2010 overall scores.
The contact details for each recipient were confirmed as current by the NHS in the week before each mail-out. Despite this we received 1117 letters (1.6%) returned to sender because of the addressee being unknown at that address. There were undoubtedly more letters that were not returned to us, suggesting that a sizable minority of invitations were not received and reflecting the inaccuracy of the primary care databases that depend on patients reliably informing their current general practice when they move. Registers will be less accurate for more transient populations and this population characteristic may have had an impact on recruitment in more deprived neighbourhoods. We tested the hypothesis that the impact might be more severe in areas with transient populations but an exploratory GIS analysis using a simple linear model found no evidence that response rate was correlated with indicators of population transience (see Chapter 2, Participants, and Chapter 3, Baseline characteristics of participants). However, as noted in Chapter 3, there was a statistically significant difference in the response rates from different neighbourhoods, with fewer responses from more deprived LSOAs. It is important to stress that none of the areas approached was affluent in national terms and that this finding indicates relative resistance to recruitment from very deprived communities compared with only moderately deprived communities.
Of the randomised participants, 165 out of 282 were living in LSOAs in the bottom 20% of national IMD 2010 overall scores. This may indicate that, although we surveyed neighbourhoods that are normally understood to be deprived, the study population from these areas is likely to have been relatively more affluent. This is most visible in Figure 10 in which the plot for High Green, the geographical outlier to the north of the city, shows a ‘halo’ of participants from relatively affluent LSOAs.
Operationalisation of eligibility criteria
Four protocol violators (2.5% of the ITT population) were included in the ITT analysis (see Chapter 3, Baseline characteristics of participants). These participants did not meet the eligibility criterion of increasing self-reported physical activity by 30 minutes per week over the previous 3 months and did not signal, in the final SPAQ question, that the amount of physical activity that week was atypical. Academic opinion is divided on the utility of post-randomisation exclusions. For some, ‘the only safe way to deal with [protocol violations] is to keep all randomized patients in the trials’ (p. 464). 116 Others advise excluding ‘ineligible patients from analysis, provided that the eligibility criteria are absolutely clear and objective’ (p. 176). 117 Guideline E9 of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) also makes provision for the exclusion of participants if a ‘major’ eligibility criterion was violated. 118 Although ICH E9 fails to define ‘major’, commentators have suggested that it might include criteria that, the violation of which, produce ‘a less homogeneous set of patients (a possible loss of power)’ (p. 885). 119 With the benefit of hindsight we can see that the SPAQ was a far from objective screening tool: the reports of physical activity provided by those to whom it was administered correlated poorly with objective measures of physical activity (using the Actiheart device) during follow-up. Further, the use by RAs of negative answers to the final question (‘Was the last week typical of the amount of physical activity in minutes you usually do?’) to provide a basis for exemption from the eligibility criterion and an argument for randomisation was probably inconsistently and subjectively applied across randomised and non-randomised participants. For this reason it is unlikely that the presence of these four individuals seriously affects the homogeneity of the analysis set. A post hoc sensitivity analysis also suggests that the treatment effect was unaffected by their presence (see Chapter 3, Effectiveness of the booster intervention at 3 months). Although the concurrent use of the SPAQ as a screening tool is clearly problematic, it is not clear what the alternative might have been, given the need to screen 2502 unconsented members of the public at 3 months before randomisation to randomise just 282. The SPAQ also has a level of external validity that accelerometry might not, in that it, or self-report measures like it, are in use by the kind of local services to which our findings were intended to be generalisable.
Impact of the use of the Actiheart system on the collection of primary outcome data
The trial design team anticipated a participant attrition rate of up to 25%. In practice, the trial lost 81 participants (29%) to follow-up at the primary outcome assessment time point of 3 months (see Figure 6), despite deploying many of the preventative measures described by Brueton and colleagues. 120 Although this was disappointing, the loss of a further 18% of participants from the main analysis because of missing Actiheart data was entirely unexpected. The majority of early Actiheart studies were focused on the validation of the device for use in young people and healthy adults in controlled laboratory conditions. There are more recent studies in which Actiheart has been used in hospital settings, free-living environments and community settings. 121–123 However, few provide comments on its acceptability or suggest the kind of poor compliance observed in this study. The best available evidence on its acceptability comes from a recent study comparing instruments for the objective assessment of physical activity frequency and intensity. 122 In this study of Native Hawaiian participants, the Actiheart device was deemed more comfortable than ActiTrainer (an accelerometer and a Polar heart rate monitor; ActiGraph, Pensacola, FL, USA) and was worn for 6.5 days out of 7 for an average 12 hours per day. However, only 17 people wore the Actiheart in this study and two participants (12%) reported skin irritation. Although only a few people in our study reported irritation from the electrodes, many found that the electrodes did not adhere for the full week. As a result of this, and after trying several different brands of electrodes in addition to the ones recommended by the manufacturer, all participants were issued with spare electrodes so that they could replace them during the week as necessary. It is also interesting to note that the manufacturer of Actiheart, CamNtech, now offer a belt as an alternative to electrodes for holding the Actiheart in place. 124 As far as we are aware, this study is one of the first studies to suggest problems with the acceptability of the device in community settings and with repeated measures, with most published studies having asked participants to use the device on one occasion only. Our relatively large sample and limited involvement in placing/replacing the device means that this study provides a novel insight into the use of the Actiheart as a device for community-based assessment of physical activity.
The most problematic characteristic of accelerometer data is that activity is not measured over a uniform period each day and therefore determining the minimum number of monitoring days with valid accelerometry measurements is always a challenge in practice, especially in pragmatic trials. 38 Ideally, at least 7 days of physical activity monitoring using the Actiheart device is recommended to achieve estimates of physical activity in adults of approximately 90% reliability. 39 However, in practice, there is a balance between achieving the required degree of reliability in physical activity estimates and the number of days with valid accelerometry data required to optimise retention of study participants in the ITT population. To achieve 80% reliability with respect to activity counts and time spent in moderate to vigorous activity in adults, at least 3–4 days (of the 7 days) of activity monitoring are required. 39,125 In this regard, in this study, participants were required to have carried the Actiheart monitor for at least 4 days (of the 7 days) and have a minimum of approximately 7 hours (not lost > 1000 minutes) of accelerometry measurements each day.
This meant that a significant number of participants, despite completing the study, did not provide primary outcome data at 3 and 9 months and it is impossible to rule out information bias being introduced because of differences in physical activity levels between those who successfully used and returned the Actiheart monitor and those who did not.
Potential impact of trial participation on physical activity levels independent of the trial intervention
The phenomenon of behaviour modification caused by the act of being observed (the Hawthorne effect126) has been reported in some studies127,128 but not in others. 129
A specific concern that influenced the design of the booster trial was the perceived risk that the objective measurement of baseline activity (Actiheart) would have an effect on all participants’ physical activity levels because of their awareness of being monitored, potentially reducing the likelihood of observing an intervention effect in those also receiving booster interventions over and above this. In other words, the very act of measuring participants’ baseline activity levels, although an element of the research data collection process and not part of the intervention, might in itself act as an intervention, thus reducing the potential to have a ‘true’ control group with no intervention.
In theory we might expect this type of Hawthorne effect, but at least expect it to be similar between the booster group and the control group if Actiheart devices are administered in all arms. Therefore, we would not expect the estimated treatment effect, the difference in 3-month post-randomisation energy expenditure, to be affected by the Hawthorne effect in this scenario, mitigating any potential concern about an intervention effect masking the measurable impact of the face-to-face or telephone MI sessions.
However, it is possible that a Hawthorne effect did still occur during the study, as several participants reported in interviews that the expectation of seeing a researcher for their 3-month research study outcome assessment provided a motivation for staying physically active (see Chapter 4, The importance of monitoring and feedback). Although the same RA was never used for both the intervention and the research procedures, visual cues (such as Sheffield Hallam University Centre for Sports and Exercise Science logos on polo shirts) might have blurred the distinction in the minds of participants.
Lack of objective measurement of physical activity at baseline
The main rationale for not collecting baseline Actiheart data in our trial was to reduce the possibility of encountering the Hawthorne effect as discussed in the previous section. However, we acknowledge that baseline measurements could have had three potential advantages in this trial. First, the availability and utilisation of baseline Actiheart data would have improved the precision around the estimated treatment effect (the difference in outcomes between the booster group and the control group). Second, in hindsight, with a baseline assessment of energy expenditure we would have predicted problems with the Actiheart devices at an earlier stage of our study. We could have then developed strategies to minimise attrition and improve retention of participants with valid Actiheart data at 3 months. Third, baseline measurements of activity levels might have provided additional information about whether any differences between groups at follow-up were due to differential retention in the three arms, rather than intervention effects (which is always a potential source of bias when participants cannot be blinded to their intervention group).
Strengths and limitations of the process evaluation
Neither the process evaluation survey nor the topic guide for the interviews was piloted, although the response to questionnaire closed items was generally good. Another weakness was that, because of sickness and maternity leave, interviews were conducted by Sheffield Hallam University researchers who also delivered the intervention. However, no participant was interviewed by the same person who delivered their intervention and the analysis was conducted independently by University of Sheffield researchers not involved in the delivery of the intervention.
The use of qualitative research allowed for a nuanced interpretation of the quantitative elements and raised several supplementary questions that we were able to address through additional analyses, for instance the importance of relative autonomy in participants’ responses to the intervention.
Strengths and limitations of the trial intervention
The booster trial applied a humanistic counselling approach that attempted to action plan based on participant readiness, values and perceived resources. The approach was manualised and fidelity calibration of those delivering the intervention was attempted. This was a clear strength of the study intervention, which is rarely seen in physical activity counselling settings. 18 The theoretical approach (self-determination theory15) underpinned the cognitive and behavioural intervention in line with the treatment fidelity framework. This ensured a consistent rubric, based on empirical evidence, providing justification for the content and delivery of the counselling intervention (MI). Measurement of the cognitive effect of factors such as exercise motivation (measured using the BREQ-226) on behavioural adaptations to physical activity (measured using accelerometers) also provides evidence for the effectiveness of an MI approach. In the process evaluation the use of empathy and engagement was reported by the participants throughout, which is a fundamental part of MI. 89 These concepts are clearly distinct from a traditional medical model in which advice giving, warning and confronting with evidence are often found to generate the opposite result.
The MI counselling intervention, measurement of behavioural change using objective instruments (accelerometer) and measurement of epidemiological change (physical markers such as BMI) were again a strength of the trial in attempting to clearly assess and report the link between cognitive, behavioural and epidemiological interventions and outcomes. Although objective measures of behaviour change are difficult to achieve, this is likely to be improved as technology enables smaller and less intrusive instrumentation. Correlating the cognitive (or affect) and behavioural change pre–post allows inference of the change in cognitive affect (motivation) mediating a corresponding change in behaviour. Moreover, process evaluations can provide greater explanations of the reasons for this change. 130
The identification of the target behaviour (e.g. physical activity, diet or other lifestyle behaviours such as smoking) is fundamental to MI and yet is challenging to achieve and manage in a brief session (approximately 30 minutes) such as that delivered in the trial. Moreover, the duration of the full (face-to-face) and mini (telephone) booster sessions was more akin to the duration of a brief MI intervention, whereas longer and more frequent sessions are often found to be significant in other health settings. 131 The duration of the sessions was usually dictated by the participant, although the RAs’ limited use of deeper reflections and extended exploration of issues and participant values, as reflected in the MITI scores, could also have contributed to sessions being briefer than might be optimal. Multifaceted behaviours often emerged, which are rarely discrete, and combinations such as diet, alcohol, physical activity, smoking and medication adherence are challenging to manage in such a short period. Therefore, a longer period of time would be useful to explore the agenda, target behaviour and carry out action planning as a result. Lundahl and colleagues131 and Hettema and colleagues132 have suggested that MI interventions including up to six repeated sessions are the most significant and, although briefer interventions have been found to show positive results, these are more reliant on interventionist effects and influence. In the current trial the duration of the individual intervention sessions limited the opportunity to fully explore client values within the level of MI skillfulness of the RAs.
There was limited opportunity to explore maintenance and relapse prevention with participants within the mini and full booster MI sessions primarily because of the limited time available for the interaction. It is clear that action planning in physical activity contexts rarely embeds maintenance strategies (which include relapse prevention) and these need to be considered in future trials and community-based interventions. Although the action plan did attempt to account for setbacks and managing relapse this was not perhaps explicit enough and specific ‘active’ compared with ‘passive’ follow-up sessions would support this. 60 In addition to the value of specific cognitive–behavioural ‘maintenance’ strategies, a number of components were delivered (e.g. monitoring and normative feedback) although these were not made explicit enough in the intervention.
More generally, the complexity of the intervention could not be managed sufficiently in the short time available. ‘Adoption’ behaviours such as physical activity and diet change involve multifaceted lifestyle adaptations over a long duration and the current trial had a limited intervention duration with what could be described as a ‘passive’ follow-up. There was also limited opportunity for the use of explicit maintenance strategies that would have embedded cognitive–behavioural content such as adapting change goals, active follow-up and normative feedback. Future studies should make more explicit the use of maintenance strategies based on client readiness and changes in levels of motivation and opportunity for change.
Motivational interviewing training and assessment of practitioner competence
The current trial also demonstrated the value of detailed documentation and reporting of both the MI training and assessment of MI competence, which identified both strengths and limitations in these key factors that influence intervention delivery and effectiveness.
The trial applied a theoretical underpinning for the intervention in line with treatment fidelity, which was based on empirical studies and contemporary evidence at the time of conception. 16 Since that time the evidence on the link between MI and self-determination theory has become even more robust, which has justified its use. 64 This study is one of the first to apply a treatment fidelity framework with a clear description of the design (theoretical underpinning using self-determination theory), training (MI component and assessment of competence), delivery (to the participant, recorded and reviewed), receipt (by the participant) and enactment (behavioural change measured using objective and self-report methods) in a physical activity counselling intervention.
A significant strength of this study was the application of a validated and reliable assessment tool to indicate the competence of those delivering the intervention. This sat comfortably within the treatment fidelity framework and was a robust approach rarely seen in previous physical activity counselling trials. 17,18 The treatment fidelity framework itself provided a sound basis for the design and delivery of the intervention with an appropriate theoretical framework (self-determination theory16,64) and facilitated a clear link between the cognitive intervention and the outcome measure using BREQ-2. 15,30 No other MI studies in physical activity settings have attempted this level of assessment and it provides a number of lessons for future studies that are likely to train and assess those delivering the intervention in situ (health professionals already positioned in health-care settings as opposed to additional RAs).
One key implication of the trial has been the importance of the assessment of the baseline ability of the RA before the start of a trial as well as the need for ongoing assessments and self-reflection to account for ‘drift’ in counselling competence. Although the RAs were trained in foundation practitioner skills, it was not possible to optimise the level of competence through specific ‘real-world’ reflective practice in the run up to intervention delivery to trial participants because the RAs were heavily involved in the recruitment phase of the study. Practitioners in an existing service might have been less likely to experience this lag period in skill development (although the risk of skill drift might have been greater).
Other studies in health contexts, such as studies on medication adherence and management and prevention of transmission of human immunodeficiency virus, have assessed the competence of practitioners at interview stage and filtered out those who do not meet strict criteria. This was not possible in this study as the practitioners had to be experienced in both physical activity programming and lifestyle change, which meant that the chance of them also being experienced MI practitioners was limited. In the current austerity climate there has been a move towards training existing practitioners in health services as opposed to attaching practitioners from outside this setting. Although this will increase the ecological validity and context-specific nature of the training, existing practitioners are likely to have a broader range of abilities.
Although a number of studies have emerged in recent years assessing the efficacy of MI in promoting physical activity behaviour change, few have clearly reported the content, frequency and duration of the MI intervention; the heuristic value of this trial is therefore the clear reporting for future adaptations.
Those delivering the mini and full booster (RAs) were not existing health professionals, which may challenge the ecological validity of the intervention and the relevance of the direct inferences for health-care settings. Although the use of RAs allows for bespoke training and focused attention on the trial, their awareness of specific care pathways was limited.
The amount of time required for ensuring MI fidelity and applying self-reflection (and accounting for practitioner competence ‘drift’) was a challenge in the current trial as the RAs were also heavily involved in the recruitment process and the lead-in time between training and delivery to participants was short. As a result the RAs trained ‘on the job’ and had little opportunity for group supervision and full-day workshops followed by reflective practice. However, one could argue that this is likely to be similar for any other practitioner delivering physical activity MI sessions alongside or as part of their full-time job.
Although the use of a treatment fidelity framework provided an outline for all stages of the trial, there is a risk of a ‘pure’ application of such a framework, constraining the adaptability and innovation often seen in a health-care setting. 133 The value of internal validity and process awareness of training, for example, is unquestionable, although they may be seen as a ‘dictate for provider adherence to a set of therapist behaviours rather than as adherence to the delivery of the “active” treatment component’ (p. 454). 133 Leventhal and Friedman133 suggest that ‘such manualised approaches can discourage functional analysis of the complexities of individual cases’ (p. 454).
Strengths and limitations of the geographical information systems analysis
The main strengths of the GIS analysis are the use of network distance rather than straight line distance and the use of the Sports England database for comprehensive coverage of local leisure facilities. Unlike some other studies in this field, which use Euclidean distance as a proximity measure (including a well-cited study by Maas and colleagues86), we have calculated walking distance using network information extracted from OpenStreetMap, which provides extensive coverage of pedestrian access routes.
The main weaknesses are the use of automated geocoding (rather than more precise but labour-intensive manual geocoding) and the use of a ‘distance to nearest’ measure rather than a more sophisticated ‘gravity’ distance model. A more sophisticated ‘gravity’ measure (such as that used in the study by Dai134) can take into account multiple facilities in the local area and it is possible that it could be used in future work; however, the required assumptions meant that it was not suitable for all of the facilities that we studied in this trial.
Strengths and limitations of the health economic modelling
Because of considerable structural uncertainty and difficulties in translating from the measures recorded in the booster trial to QALYs and costs, two alternative models were developed and within each model a wide range of scenarios was considered. The majority of these scenarios indicated that both the full booster intervention and the mini booster intervention appeared to be ruled out by simple dominance, although a small number of scenarios provide weak indications that the full booster may be the optimal choice assuming willingness-to-pay thresholds of approximately £20,000 per QALY or more. All scenarios were reflections of the evidence of the trial, indicating that in substantive terms there were only marginal differences between trial arms.
Although the short-term model has the benefit of being heavily based on evidence collected during the trial, it remains an open matter whether a lifestyle intervention such as the booster intervention is likely to produce a clinically significant effect in terms of either participant HRQoL or use of NHS resources over the duration considered. This is because the intervention is primarily preventative rather than curative and participants were not selected on the basis of suffering from a particular disease adversely affecting their HRQoL and NHS resource use. Because of this it may not be plausible to assume that increasing physical activity now will lead to systematic changes in HRQoL or resource use in the short term. Instead, positive health effects of the intervention may emerge over a much longer time horizon, reducing the risk of a range of diseases developing and so improving health outcomes indirectly.
It was because of this concern that the long-term model was developed to be considered alongside the short-term model. In this modelling approach, the HRQoL gains from increased physical activity result from the mediating effect of physical activity on relative mortality risks, meaning that a cohort of more physically active people should be expected to live for slightly longer on average than an otherwise identical cohort of slightly less physically active people and so experience slightly higher average HRQoL. The model implicitly incorporates the effect of morbidity on HRQoL, by using age- and gender-specific mean HRQoL scores, but does not incorporate an additional mediating effect of physical activity on either the onset or the severity of non-fatal diseases. As such, it is similar in its approach to the economic model developed by Brennan and colleagues. 135 The Brennan and colleagues’ model uses work by Andersen and colleagues75 to map physical activity levels onto relative mortality risks, whereas this model uses work by Myers and colleagues for the same purpose. 81 Both mapping exercises indicate qualitatively similar relationships between physical activity and relative mortality risk, suggesting that the greatest benefit of a given increase in physical activity is likely to be in people who are the most sedentary and that the marginal benefit of additional physical activity is less in people who are already relatively physically active.
The report by Brennan and colleagues135 contains a review of existing papers on the cost-effectiveness of physical activity interventions, as well as a brief review of existing model frameworks. Readers are directed to this publication for further information.
The economic model in this report does not yet directly consider the relationship between physical activity levels and morbidity risks. Morbidity is indirectly handled through the changing mortality risk ratios and also by adjusting HRQoL scores for age and gender (e.g. because of differences in disease burden at different ages). However, specific disease states are not considered in the model. A further iteration of the model could consider the additional cost and utility implications of different diseases by modelling annual probabilities of developing particular conditions and combining these with RR modifiers related to physical exercise levels. In theory, a physical activity intervention could then appear cost saving if the additional cost of the intervention is outweighed by the additional reduction in NHS resource use costs as a result of a reduced burden of disease. An important example of such a disease would be type 2 diabetes mellitus, whose onset is known to be linked to lifestyle factors and whose treatment represents a significant and increasing annual cost to the NHS. 136
Given the complex array of mediators and moderators involved in translating changes in physical activity into changes in mortality and morbidity risks, HRQoL and NHS resource use over the short, medium and long term, it is important that economic models are based on a solid clinical and epidemiological foundation. Models should incorporate current expert opinion on how physical activity interventions lead to sustained changes in lifestyle behaviour and through this to changes in health-related utility and costs, as well as the range of other mediating and moderating factors that need to be taken into consideration so that the model represents the patient experience with sufficient accuracy. The paths of causal influence linking individual- and ecological-level factors to short-term and long-term mortality and morbidity risks should be established through extensive consultation with clinical experts and used to establish flexible public health models that can be used to consistently model a wide range of interventions affecting different parts of the aetiological pathways. This process of attempting to understand the clinical and epidemiological consensus in understanding causal processes could be used to form directed acyclic graphs and influence diagrams, which can be used as the basis for economic models. 137 When it is identified that consensus between clinical experts does not exist with regard to certain causal pathways, the effect of different structural assumptions could be explored by constructing separate models that reflect alternative clusters of opinion.
Implications for practice and commissioning
The uptake of both the brief intervention and the booster intervention suggests that the potential impact on population-level change of these types of recruitment approaches and interventions is limited. The overall findings suggest that neither the mini booster nor the full booster is likely to be an appropriate population-level approach to promote physical activity in middle-aged populations in deprived areas. Better integration in local primary care services, such as GP clinics and IAPT (Improving Access to Psychological Therapies) programmes, might yield improved take-up and results, although even with better integration these types of interventions are unlikely to enable sufficient throughput of participants to have the reach of a true public health intervention.
The qualitative research component identified large numbers of people who wished to maintain physical activity levels in the hope of managing chronic disease symptoms. In particular, those with mental health issues (depression and anxiety) expressed a belief that physical activity would provide symptom relief, although the same people were often exercising alone in private spaces, were unenthusiastic about doing so and reported problems maintaining exercise levels. The social element of exercise may mediate some of the mental health benefits and those making exercise referrals should be aware of this. The needs of people with long-term injuries are different again; these people are typically seeking highly tailored exercise advice to suit their physical abilities. Referrers and advisors should be sensitive to these diverse needs.
The duration of both the mini booster and the full booster was approximately 30 minutes, which is more akin to a ‘brief’ MI session. Longer MI sessions have been found to be more effective and therefore a greater response might be expected from a higher dose, similar to the findings in contexts in which MI is more commonly applied, such as addictions settings and behaviour adoption (e.g. smoking and alcohol). 97 Although the current trial attempted to provide an ecologically valid intervention period, delivering a bespoke, person-centred intervention in this time frame that explored and set the client agenda, managed resistance and ambivalence and set appropriate goals with effective relapse prevention strategies was challenging, and delivering a greater number of sessions would be a realistic mechanism for managing this in the future. It is unlikely that delivering a greater number of sessions would be possible within existing care pathways, although the Let’s Get Moving programme59 proposes more than two sessions.
Face-to-face contact was more acceptable than telephone contact. More evidence is needed on relative costs and effectiveness as the results of this trial suggest that neither intervention is cost-effective at conventional levels of statistical significance and willingness-to-pay threholds. At present, PARSs are being decommissioned by a large number of primary care providers and their local council partners. PARSs provide a face-to-face physical activity promotion intervention that typically consists of up to 12 practical sessions, although not all of these are delivered on a one-to-one basis. Currently, the preferred option for physical activity promotion within the NHS is Let’s Get Moving, which involves multiple one-to-one contacts regarding physical activity that are either integrated into existing health-care contacts or delivered by the primary health-care team as standalone appointments. 59 This is arguably a cheaper way of delivering an intervention. It does, however, rely on individuals initiating contact with a member of their health-care team who is trained to provide the intervention.
Some people seemed to like the MI approach but others seemed to want, or had expected, a more didactic delivery approach. On the surface this seems counterintuitive and is at odds with the premise that MI can foster intrinsic motivation, which is linked to longer-term maintenance of activity, whereas more didactic advice-giving approaches would provide extrinsic motivation for being active. Many people we interviewed appeared to be extrinsically motivated, with few expressing thoughts about exercising for enjoyment, despite using a maintenance intervention designed to foster intrinsic motivation. This may be related to participants’ stage of readiness to be regularly active. Those in a less advanced stage such as contemplation or preparation (or even action) may be becoming active for extrinsic reasons such as to lose weight or improve health; a true appreciation of and desire for physical activity for its own sake may not come until individuals have been active for some time and entered the maintenance stage of behaviour change. By definition, the participants in this study have become active only in the 3 months preceding the delivery of the booster intervention and are therefore still in the action stage. Thus, although there is a focus on MI and related interventions that emphasise building intrinsic motivation for physical activity in the field of exercise psychology, it is possible that many people who have only just started to become more active may prefer to be told what they should be doing or participate in an exercise class. It is also possible that other differences aside from motivational style may influence people’s preference for a certain delivery approach. Whatever the reason, the implication here is that delivery of physical activity promotion should involve some form of assessment to ascertain each person’s preferred approach and then a relevant style of delivery should be selected from a toolkit of approaches. 138,139
The monitoring and feedback elements of the intervention were identified as important motivators by participants. However, this may be another indicator of the prevalence of extrinsic motivation amongst these participants, and psychological theories of behaviour change suggest that extrinsically (rather than intrinsically) motivated behaviour change might impact negatively on the sustainability of the treatment effect.
The role of a professional has traditionally been seen as one of diagnosis and prescription for both pharmacological and behavioural interventions. In recent years, this has given way to a more inclusive person-centred approach, which challenges the individual, with empathy, to take a more active role in their behaviour change. The focus of more health practitioner sessions, also described as a ‘directive approach’, can resolve people’s resistance to change. 140 However, the process evaluation suggested that some people have concerns about the client centeredness of MI consultations (see Chapter 4, Motivational interviewing: consistency with perspectives or world views). As in other studies involving low socioeconomic status groups, some of our participants said that they had hoped for more paternalistic and didactic communication approaches. 63 Although autonomy-supported communication is understood to enhance intrinsic motivation (whereas paternalistic approaches diminish it), it has been suggested that MI can be adapted to suit client communication preferences. 63–65 Therefore, if the motivational interviewer becomes aware that the client would prefer a more didactic approach, the consultation can be directed towards that need. This ‘way of being’ with the client is a key part of skilful MI and a flexible and adaptive approach is an important part of challenging with empathy in a bespoke manner.
The process evaluation also suggested that two key motivators for adherence to physical activity targets are the anticipated shame, when face to face with a professional monitor, if one does not achieve the agreed objectives, and the pleasure of receiving feedback on one’s performance (see Chapter 4, The importance of monitoring and feedback). Different methods of feedback are the subject of the Feedback, Awareness and Behaviour (FAB) study (ISRCTN92551397; funded by the National Institute for Health Research programme). 141 The FAB team aims to randomly allocate 500 people aged 30–55 years to either a control group (no feedback) or one of three types of personalised physical activity feedback (‘simple’, ‘visualised’ or ‘contextualised’) and complete repeat measures of self-rated physical activity and psychosocial correlates.
One concern for physical activity promotion and ongoing patient behaviour change support is that there is no clear pathway for physical activity promotion at the moment in the UK. Current provision is limited to GP advice to individual patients to become active or, at best, referral to a PARS. Although other community-based interventions such as ‘green gyms’ and walking programmes do exist, these are rarely integrated into the care pathway. The traditional approach to physical activity promotion delivered in health-care settings is one of information exchange, which can often be seen as provision of unsolicited advice. In 2009, the Department of Health launched Let’s Get Moving59 to be delivered by health-care professionals and complement the PARS. Let’s Get Moving is physical activity counselling intervention based on MI principles and designed to be delivered by health-care professionals within usual care settings. Early research questioned the feasibility of using Let’s Get Moving in a primary care setting. 82,142 Materials have recently been revised and were relaunched in March 2012. Aside from referral to a PARS and Let’s Get Moving, there are some nurse practitioners who do deliver diet and exercise counselling within primary care. This provision, however, is variable by nurse and practice.
It is therefore essential that local strategies for physical activity promotion and local commissioning by clinical commissioning groups and local authorities is in future informed by the wider evidence base in relation to the broad range of potential community and individual interventions. As resources are limited and the potential for population benefits large, a systematic approach, prioritising the most cost-effective interventions and targeting the groups with the greatest baseline risk and ability to benefit from supportive interventions, is required.
Implications for future research
The process evaluation suggested that some participants were confused about the initiation and maintenance components and also between data collection and delivery of the intervention. They often seemed to perceive that the data collection sessions were part of the intervention and a source of support and there is therefore a need to be aware of a Hawthorne effect, which will influence participant behaviour and therefore both self-reported and objectively measured primary outcomes. Future research should ensure that clear goals, and the agenda for specific sessions, are discussed and agreed with the participants. When baseline information is required this has a different role (unless part of normative feedback) and should be treated accordingly in the session. Therefore, future interventions should clearly define (and report) the content of the intervention so that aspects such as agenda setting, exploring pros and cons of change and action planning or information exchange are systematic and meaningful.
Because some participants seemed to like the MI approach whereas others seemed to expect or prefer a more didactic approach, future research could investigate the impact of people’s intervention style preferences (i.e. didactic information giving vs. MI) on the effectiveness of a physical activity maintenance intervention. A suitable comparison might be to examine preferred style compared with not preferred style (compared with the control).
There is a clear need to ensure that counselling interventions such as MI are delivered as intended and that the content and approach are accurately reported. Recent studies have explored the potential use of integrating MI and cognitive–behavioural therapy to form physical activity interventions and unless a clear description of the components of interventions are clearly stated it is difficult for practitioners to adapt and embed such approaches when they are shown to be efficacious in research settings. Until studies have demonstrated that an intervention is consistently delivered and internal validity is gained, it is not possible to infer accurately its likely efficacy in increasingly varied contexts.
Our research suggests that correlation between activity questionnaires and accelerometer data is poor (see Chapter 3, Effectiveness of the mini booster intervention compared with the full booster intervention at 3 months, and Chapter 4, The prevalence of optimistic self-assessment), a phenomenon that has been noted previously in the peer-reviewed literature. 143 This might be an indication of social desirability bias, the tendency to answer questions in a fashion that will be viewed positively by others. Social desirability has been found to bias self-reports of diet and physical activity and some research teams recommend methods to measure and control for social desirability bias when self-report questionnaires are used. 144,145
In Chapter 4 (see The prevalence of optimistic self-assessment) we reviewed claims of large amounts of moderate to vigorous physical activity when 7-day accelerometry data showed low levels of physical activity across the week. Nicaise and colleagues143 make the point that, although low-income Latinas often over-report the amount of moderate to vigorous activity carried out (compared with accelerometry results), they do substantial amounts of light-intensity activity. Healy and colleagues146 have suggested that light-intensity physical activity is associated with improved glycaemic control that is independent of the amount of moderate and vigorous physical activity carried out. Researchers and policy-makers may need to adjust unreasonable expectations of those who are constrained by motivational, physical, social or environmental factors; for such people, reducing sedentary behaviour and increasing low-intensity activity may be both appropriate and clinically important.
Chapter 9 Conclusions
Although some individuals do find a community-based, brief MI ‘booster’ intervention supportive, the low levels of recruitment and retention in this trial and the lack of impact on objectively measured physical activity levels in those with adequate outcome data suggest that it is unlikely to represent a clinically effective or cost-effective intervention for the maintenance of recently acquired physical activity increases in deprived, middle-aged, urban populations.
The gap between the size of the sedentary population who could achieve significant long-term health benefits from relatively small but sustained increases in physical activity levels and the numbers taking up the offers of both the initial brief intervention and the subsequent ‘booster’ interventions suggests that this type of MI-based approach will be appropriate only for a minority of the sedentary population who could, in principle, benefit from being more physically active. Other approaches that require less proactive (and potentially time-consuming) engagement from individuals, including environmental interventions to encourage active travel and recreational activity, may be particularly important for deprived communities and middle-aged populations who are less likely to prioritise their own physical fitness and well-being over other demands on their time and resources.
Many types of physical activity interventions can exacerbate health inequalities because they are more likely to be taken up, and subsequent behaviour change achieved and maintained, by those who are already active and those in better health. The booster study was therefore explicitly designed to target recruitment in communities that were known to have poorer health outcomes. The lessons learnt in undertaking this trial should inform both the design of future physical activity intervention trials and the development of more effective interventions that not only are feasible and affordable but also will have sufficient reach to have an impact in the most deprived and most sedentary populations who could benefit most from sustained increases in their physical activity levels.
Acknowledgements
We gratefully acknowledge support and advice from the following: Professor Jon Nicholl and Professor John Brazier, University of Sheffield; Dr Jeremy Wight, NHS Sheffield and Sheffield City Council; Mr Paul Billington, Sheffield City Council; Ms Sarah Nickson, Sheffield City Council; Ms Dawn Lockley, NHS Sheffield; Ruth Granger, NHS Sheffield; Elaine Brookes, Sheffield Hallam University; Dr Alan Batterham, University of Teesside; Dr Nick Taub, University of Leicester; Professor Edward Winter, Sheffield Hallam University; and Dr Michael Gordon, Gleadless Medical Centre, for their contribution to the conception and design of the study; Harriet Farr, Anna Myers and Hanna Leahy at the Centre for Sport and Exercise Science, Sheffield Hallam University, for participant screening, data collection and intervention delivery; Mike Bradburn, David White, John Martindale, Tim Chater, Amanda Loban, Karen Beck, Sheffield CTRU, for support and advice on statistical, data management and administrative concerns; Tony Isaacs and Julia Critchley for advice on the EXERT questionnaire; Sarah Nickson at Activity Sheffield; Jeremy Wight, Dawn Lockley, Shazia Idris, Paula Mackintosh, Fatima Musa, Stella Mekonnen, Joanna Rutter, Kath Horner, Sam Davey, Sarah Hepworth, Lesley Lockwood, Ellie Brown, Chetna Patel, Karen Harrison, Lyn Brandon, Tahira Faiz, Elise Gilwhite, Carole Circuit, Gina Harris, Margot Jackson, Chris Nield, Elaine Muscoft, Gary McCulloch, Permjeet Dhoot, Elaine Goddard, Gareth Johnston and Anne Richardson at Sheffield Primary Care Trust; Guy Weston at Southey & Owlerton Area Regeneration Trust; Colin Havard and the team at Sharrow Shipshape, London Road; Miriam Densham at the Zest Centre, Sheffield; and Nigel West, Community Health Champions Network Coordinator, Sheffield Well-being Consortium, for support in recruitment.
We offer special thanks to the members of our two oversight committees, the trial steering committee (TSC) and the data monitoring and ethics committee (DMEC): Alan Batterham (Chair of the TSC, Professor in Exercise Science, Teesside University), Edward Winter (Professor of the Physiology of Exercise, Sheffield Hallam University), Michael Gordon (GP, Gleadless Medical Centre), Nick Taub (Research Fellow in Medical Statistics, University of Leicester), Joy Adamson (Chair of the DMEC, Deputy Director York Clinical Trials Unit), Rebecca Playle (Lecturer in Medical Statistics, Cardiff University), Tim Anstiss (Medical Director, Academy for Health Coaching), Melvyn Hillsdon (Associate Professor, Sport and Health Sciences, University of Exeter), Nick Cavill (Research Associate, National Obesity Observatory).
Finally, we would like to offer thanks to Helen Wakefield for her support in the preparation of this report.
The views expressed in this report are those of the authors and not necessarily those of the National Institute for Health Research Health Technology Assessment programme. Any errors are the responsibility of the authors.
Contributions of authors
Elizabeth Goyder, Stephen J Walters, Jeff Breckon, Helen Crank, Robert Copeland and Cindy Cooper made substantial contributions to the conception and design of the main study.
Daniel Hind, Emma Everson-Hock, Elizabeth Goyder, Jeff Breckon, Helen Crank, Robert Copeland and Karen Collins made substantial contributions to the conception and design of the process evaluation (qualitative substudy).
Nicolas Latimer and Jonathan Minton made substantial contributions to the conception and design of the health economic analysis.
Emma Scott, Kimberly Horspool, Liam Humphreys, Andrew Hutchison, Sue Kesterton, Peter Swaile and Rebecca Wood made substantial contributions to the acquisition of data.
Munyaradzi Dimairo, Stephen J Walters, Daniel Hind, Emma Everson-Hock, Jeff Breckon, Jonathan Minton and Simon Read made substantial contributions to the analysis. All of the above made substantial contributions to the interpretation of the data.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
Publications
Hind D, Scott EJ, Copeland R, Breckon JD, Crank H, Walters SJ, et al. A randomised controlled trial and cost-effectiveness evaluation of ‘booster’ interventions to sustain increases in physical activity in middle-aged adults in deprived urban neighbourhoods. BMC Public Health 2010;10:3.
Scott E, Dimairo M, Hind D, Goyder E, Copeland RJ, Breckon JD, et al. ‘Booster’ interventions to sustain increases in physical activity in middle-aged adults in deprived urban neighbourhoods: internal pilot and feasibility study. BMC Public Health 2011;11:129.
References
- Paffenbarger RS, Hyde R, Wing AL, Hsieh CC. Physical activity, all-cause mortality, and longevity of college alumni. N Engl J Med 1986;314:605-13. http://dx.doi.org/10.1056/NEJM198603063141003.
- Coronary Heart Disease Statistics. London: British Heart Foundation; 2003.
- Food, Nutrition, Physical Activity and the Prevention of Cancer: a Global Perspective. Washington DC: American Institute for Cancer Research; 2007.
- Taylor A, Biddle SJH, Fox KR, Boutcher SH. Physical Activity and Psychological Well-Being. New York, NY: Routledge; 2000.
- Shin Y. The effects of a walking exercise program on physical function and emotional state of elderly Korean women. Public Health Nurs 1999;16:146-54. http://dx.doi.org/10.1046/j.1525-1446.1999.00146.x.
- Brosse AL, Sheets ES, Lett HS, Blumenthal JA. Exercise and the treatment of clinical depression in adults: recent findings and future directions. Sports Med 2002;32:741-60. http://dx.doi.org/10.2165/00007256-200232120-00001.
- Fox K, Rickards L. Sport and Leisure: Results from the Sport and Leisure Module of the 2002 General Household Survey. London: The Stationery Office; 2004.
- At Least Five a Week: Evidence on the Impact of Physical Activity and its Relationship with Health – A Report from the Chief Medical Officer. London: Department of Health; 2004.
- Hillsdon M, Foster C, Cavill N, Crombie H, Naidoo B. The Effectiveness of Public Health Interventions for Increasing Physical Activity among Adults: a Review of Reviews. London: Health Development Agency; 2005.
- Public health intervention guidance no. 2. London: NICE; 2006.
- Dishman RK, Bouchard C, Shephard RJ, Stephens T, Sutton JR, McPherson BD. Exercise Fitness and Health: a Consensus of Current Knowledge. Champaign, IL: Human Kinetics; 1990.
- Public health intervention guidance no. 44. London: NICE; 2013.
- Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. New York, NY: Guilford; 2002.
- NICE public health guidance 6. London: NICE; 2007.
- Deci E, Ryan R. The support of autonomy and the control of behavior. J Pers Soc Psychol 1987;53:1024-37. http://dx.doi.org/10.1037//0022-3514.53.6.1024.
- Markland D, Ryan RM, Tobin V, Rollnick S. Motivational interviewing and self-determination theory. J Soc Clin Psychol 2005;24:811-31. http://dx.doi.org/10.1521/jscp.2005.24.6.811.
- Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, et al. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH behavior change consortium. Health Psychol 2004;23:443-51. http://dx.doi.org/10.1037/0278-6133.23.5.443.
- Breckon JD, Johnston LH, Hutchison A. Physical activity counseling content and competency: a systematic review. J Phys Activity Health 2008;5:398-417.
- Merrick Z, Shaun T, Joel JG, Douglas GA, Sean T, Brian H, et al. Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ 2008;337.
- Hind D, Scott E, Copeland R, Breckon J, Crank H, Walters S, et al. A randomised controlled trial and cost-effectiveness evaluation of ‘booster’ interventions to sustain increases in physical activity in middle-aged adults in deprived urban neighbourhoods. BMC Public Health 2010;10. http://dx.doi.org/10.1186/1471-2458-10-3.
- Office for National Statistics . Census Area Statistics 2001. Table 8: Net Migration Within the UK for People Aged 20–64. n.d. www.statistics.gov.uk/hub/index.html (accessed 10 October 2001).
- Lowther M, Mutrie N, Loughlan C, McFarlane C. Development of a Scottish physical activity questionnaire: a tool for use in physical activity interventions. Br J Sports Med 1999;33:244-9. http://dx.doi.org/10.1136/bjsm.33.4.244.
- Britt E, Blampied NM, Hudson SM. Motivational interviewing: a review. Aus Psychol 2003;38:193-201. http://dx.doi.org/10.1080/00050060310001707207.
- Rollnick S, Heather N, Bell A. Negotiating behaviour change in medical settings: the development of brief motivational interviewing. J Mental Health 1992;1:25-37. http://dx.doi.org/10.3109/09638239209034509.
- Havenar JM, Breckon J. Adapted motivational interviewing for increasing physical activity: a 12 month clinical trial. Med Sci Sports Exerc 2008;40. http://dx.doi.org/10.1249/01.mss.0000323436.20338.2c.
- Brodie D, Inoue A. Motivational interviewing to promote physical activity for people with chronic heart failure. J Adv Nurs 2005;50:518-27. http://dx.doi.org/10.1111/j.1365-2648.2005.03422.x.
- Ball K, Salmon J, Leslie E, Owen N, King AC. Piloting the feasibility and effectiveness of print- and telephone-mediated interventions for promoting the adoption of physical activity in Australian adults. J Sci Med Sport 2005;8:134-42. http://dx.doi.org/10.1016/S1440-2440(05)80004-0.
- Green BB, McAfee T, Hindmarsh M, Madsen L, Caplow M, Buist D. Effectiveness of telephone support in increasing physical activity levels in primary care patients. Am J Prev Med 2002;22:177-83. http://dx.doi.org/10.1016/S0749-3797(01)00428-7.
- McGavin CR, Gupta SP, Mchardy GJR. Twelve-minute walking test for assessing disability in chronic bronchitis. Br Med J 1976;1:822-3. http://dx.doi.org/10.1136/bmj.1.6013.822.
- Mullan E, Markland D, Ingledew DK. A graded conceptualisation of self-determination in the regulation of exercise behaviour: development of a measure using confirmatory factor analytic procedures. Pers Indiv Differ 1997;23:745-52. http://dx.doi.org/10.1016/S0191-8869(97)00107-4.
- Isaacs AJ, Critchley JA, See Tai S, Buckingham K, Westley D, Harridge SDR, et al. Exercise Evaluation Randomised Trial (EXERT): a randomised trial comparing GP referral for leisure centre-based exercise, community-based walking and advice only. Health Technol Assess 2007;11.
- Scott E, Dimairo M, Hind D, Goyder E, Copeland R, Breckon J, et al. ‘Booster’ interventions to sustain increases in physical activity in middle-aged adults in deprived urban neighbourhoods: internal pilot and feasibility study. BMC Public Health 2011;11. http://dx.doi.org/10.1186/1471-2458-11-129.
- Birkett MA, Day SJ. Internal pilot studies for estimating sample size. Stat Med 1994;13:2455-63. http://dx.doi.org/10.1002/sim.4780132309.
- Wittes J, Brittain E. The role of internal pilot studies in increasing the efficiency of clinical trials. Stat Med 1990;9:65-71. http://dx.doi.org/10.1002/sim.4780090113.
- The Actiheart User Manual (v.4.0.35). Cambridge: Cambridge Neurotechnology Ltd; 2004.
- Corder K, van Sluijs EMF, Steele RM, Stephen AM, Dunn V, Bamber D, et al. Breakfast consumption and physical activity in British adolescents. Br J Nutr 2011;105:316-21. http://dx.doi.org/10.1017/S0007114510003272.
- Ridgway CL, Brage S, Sharp SJ, Corder K, Westgate KL, van Sluijs EM, et al. Does birth weight influence physical activity in youth? A combined analysis of four studies using objectively measured physical activity. PLOS ONE 2011;6. http://dx.doi.org/10.1371/journal.pone.0016125.
- Catellier DJ, Hannan PJ, Murray DM, Addy CL, Conway TL, Yang S, et al. Imputation of missing data when measuring physical activity by accelerometry. Med Sci Sports Exerc 2005;37:S555-62. http://dx.doi.org/10.1249/01.mss.0000185651.59486.4e.
- Matthews CE, Ainsworth BE, Thompson RW, Bassett DR. Sources of variance in daily physical activity levels as measured by an accelerometer. Med Sci Sports Exerc 2005;34:1376-81. http://dx.doi.org/10.1097/00005768-200208000-00021.
- Structure and Content of Clinical Study Reports. E3.; 1995.
- Burgess DC, Gebski VJ, Keech AC. Baseline data in clinical trials. Med J Aust 2003;179:105-7.
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Clin Oral Invest 2003;7:2-7.
- Pocock SJ, Assmann SE, Enos LE, Kasten LE. Subgroup analysis, covariate adjustment and baseline comparisons in clinical trial reporting: current practice and problems. Stat Med 2002;21:2917-30.
- Senn S. Testing for baseline balance in clinical trials. Stat Med 1994;13:1715-26. http://dx.doi.org/10.1002/sim.4780131703.
- An Introduction to Categorical Data Analysis. Hoboken, NJ: Wiley-Interscience; 2007.
- Assmann SF, Pocock SJ, Enos LE, Kasten LE. Subgroup analysis and other (mis)uses of baseline data in clinical trials. Lancet 2000;355:1064-9. http://dx.doi.org/10.1016/S0140-6736(00)02039-0.
- Brookes ST, Whitley E, Peters TJ, Mulheran PA, Egger M, Davey SG. Subgroup analyses in randomised controlled trials: quantifying the risks of false-positives and false-negatives. Health Technol Assess 2001;5.
- Walters SJ. Quality of Life Outcomes in Clinical Trials and Health Care Evaluation: a Practical Guide to Analysis and Interpretation. Chichester: Wiley; 2009.
- Snape D, Spencer L, Ritchie J, Lewis J. Qualitative Research Practice – a Guide for Social Science Students and Researchers. London: Sage; 2003.
- Ritchie J, Spencer L, O’Connor W, Ritchie J, Lewis J. Qualitative Research Practice: a Guide for Social Science Students and Researchers. London: Sage; 2003.
- Pope N, Zeibland S, Mays N, Pope C, Mays N. Qualitative Research in Health Care. Oxford: Blackwell; 2006.
- O’Cathain A, Thomas K, Pope C, Mays N. Qualitative Research in Health Care. Oxford: Blackwell; 2006.
- Glaser BG, Strauss A. The Discovery of Grounded Theory: Strategies for Qualitative Research. Chicago, IL: Aldine; 1967.
- Rogers EM. Diffusion of Innovations. London: Free Press; 2003.
- Tucker CM, Rice KG, Hou W, Kaye LB, Nolan SE, Grandoit DJ, et al. Development of the motivators of and barriers to health-smart behaviors inventory. Psychol Assess 2011;23:487-503. http://dx.doi.org/10.1037/a0022299.
- Nastasi BK, Hitchcock J. Challenges of evaluating multilevel interventions. Am J Community Psychol 2009;43:360-76. http://dx.doi.org/10.1007/s10464-009-9239-7.
- Bolognesi M, Nigg C, Massarini M, Lippke S. Reducing obesity indicators through brief physical activity counseling (pace) in Italian primary care settings. Ann Behav Med 2006;31:179-85. http://dx.doi.org/10.1207/s15324796abm3102_10.
- Naar-King S, Earnshaw P, Breckon JD. Towards a universal maintenance intervention: integrating cognitive-behavioral treatment with motivational interviewing for maintenance of behavior change. J Cogn Psychother 2013;27:126-37.
- Let’s Get Moving – A New Physical Activity Care Pathway for the NHS: Commissioning Guidance. London: Department of Health; 2009.
- Michie S, Abraham C, Whittington C, McAteer J, Gupta S. Effective techniques in healthy eating and physical activity interventions: a meta-regression. Health Psychol 2009;28:690-701. http://dx.doi.org/10.1037/a0016136.
- Madson MB, Loignon AC, Lane C. Training in motivational interviewing: a systematic review. J Subst Abuse Treat 2009;36:101-9. http://dx.doi.org/10.1016/j.jsat.2008.05.005.
- Brodie DA, Inoue A, Shaw DG. Motivational interviewing to change quality of life for people with chronic heart failure: a randomised controlled trial. Int J Nurs Stud 2008;45:489-500. http://dx.doi.org/10.1016/j.ijnurstu.2006.11.009.
- Hardcastle S, Taylor A, Bailey M, Castle R. A randomised controlled trial on the effectiveness of a primary health care based counselling intervention on physical activity, diet and CHD risk factors. Patient Educ Couns 2008;70:31-9. http://dx.doi.org/10.1016/j.pec.2007.09.014.
- Miller W, Rollnick S. Meeting in the middle: motivational interviewing and self-determination theory. Int J Behav Nutr Phys Activity 2012;9. http://dx.doi.org/10.1186/1479-5868-9-25.
- Moyers TB, Martin T, Manual JK, Miller WR, Ernst D. Revised Global Scales: Motivational Interviewing Treatment Integrity 3.0 (MITI 3.0). Albuquerque, NM: University of New Mexico, Center on Alcoholism, Substance Abuse and Addictions; 2007.
- Sheffield Site Categorisation Strategy. Sheffield: Sheffield City Council; 2000.
- Barbosa O, Tratalos JA, Armsworth PR, Davies RG, Fuller RA, Johnson P, et al. Who benefits from access to green space? A case study from Sheffield, UK. Landscape Urban Plan 2007;83:187-95. http://dx.doi.org/10.1016/j.landurbplan.2007.04.004.
- Active Places database . Sport England 2012. https://spogo.co.uk/ (accessed 6 December 2013).
- Luo W, Wang F. Measures of spatial accessibility to health care in a GIS environment: synthesis and a case study in the Chicago region. Environ Plan B 2003;30:865-84. http://dx.doi.org/10.1068/b29120.
- Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care 2004;42:851-9. http://dx.doi.org/10.1097/01.mlr.0000135827.18610.0d.
- Curtis L. Unit Costs of Health and Social Care 2010 2010.
- Interim Life Tables: Summaries UK Interim Life Tables, 1980–82 to 2008–10. London: ONS; 2012.
- Ara R, Brazier J. Populating an economic model with health state utility values: moving toward better practice. Value Health 2010;13:509-18. http://dx.doi.org/10.1111/j.1524-4733.2010.00700.x.
- Guide to the Methods of Technology Appraisal. London: NICE; 2008.
- Andersen LB, Schnohr P, Schroll M, Hein HO. All-cause mortality associated with physical activity during leisure time, work, sports, and cycling to work. Arch Intern Med 2000;160:1621-8. http://dx.doi.org/10.1001/archinte.160.11.1621.
- Kiely DK, Wolf PA, Cupples LA, Beiser AS, Kannel WB. Physical activity and stroke risk – the Framingham study. Am J Epidemiol 1994;140:608-20.
- Koba S, Tanaka H, Maruyama C, Tada N, Birou S, Teramoto T, et al. Physical activity in the Japan population: association with blood lipid levels and effects in reducing cardiovascular and all-cause mortality. J Atheroscler Thromb 2011;18:833-45. http://dx.doi.org/10.5551/jat.8094.
- Kokkinos P, Sheriff H, Kheirbek R. Physical inactivity and mortality risk. Cardiol Res Pract 2011;2011. http://dx.doi.org/10.4061/2011/924945.
- Lauer MS, Miller TD. The exercise treadmill test: estimating cardiovascular prognosis. Cleve Clin J Med 2008;75:424-30. http://dx.doi.org/10.3949/ccjm.75.6.424.
- Lee IM, Hsieh CC, Paffenbarger RS. Exercise intensity and longevity in men. JAMA 1995;273:1179-84. http://dx.doi.org/10.1001/jama.273.15.1179.
- Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 2002;346:793-801. http://dx.doi.org/10.1056/NEJMoa011858.
- Affuso O, Stevens J, Catellier D, McMurray R, Ward D, Lytle L, et al. Validity of self-reported leisure-time sedentary behavior in adolescents. J Negat Results Biomed 2011;10. http://dx.doi.org/10.1186/1477-5751-10-2.
- Department for Communities and Local Government . Indices of Deprivation 2010 n.d. www.gov.uk/government/uploads/system/uploads/attachment_data/file/6872/1871524.xls (accessed 30 April 2012).
- Amrhein PC, Miller WR, Yahne CE, Palmer M, Fulcher L. Client commitment language during motivational interviewing predicts drug use outcomes. J Consult Clin Psychol 2003;71:862-78. http://dx.doi.org/10.1037/0022-006X.71.5.862.
- Hillsdon M, Panter J, Foster C, Jones A. The relationship between access and quality of urban green space with population physical activity. Public Health 2006;120:1127-32. http://dx.doi.org/10.1016/j.puhe.2006.10.007.
- Maas J, Verheij RA, Groenewegen PP, de Vries S, Spreeuwenberg P. Green space, urbanity, and health: how strong is the relation?. J Epidemiol Community Health 2006;60:587-92. http://dx.doi.org/10.1136/jech.2005.043125.
- Hurren C, Ashwell M. The Scientific Basis of Nutrition Education: A Synopsis of Dietary Reference Values. London: Health Education Authority; 1994.
- Ravussin E, Lillioja S, Anderson TE, Christin LF, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest 1986;78:1568-78. http://dx.doi.org/10.1172/JCI112749.
- Miller WR, Rose GS. Toward a theory of motivational interviewing. Am Psychol 2009;64:527-37. http://dx.doi.org/10.1037/a0016830.
- Bassett DR, Cureton AL, Ainsworth BE. Measurement of daily walking distance –questionnaire versus pedometer. Med Sci Sports Exerc 2000;32:1018-23. http://dx.doi.org/10.1097/00005768-200005000-00021.
- Sallis JF, Saelens BE. Assessment of physical activity by self-report: status, limitations, and future directions. Res Q Exerc Sport 2000;71:S1-14.
- Marshall G. The Concise Oxford Dictionary of Sociology. Oxford: Oxford University Press; 1994.
- Scott EJ, Eves FF, French DP, Hopp R. The theory of planned behaviour predicts self-reports of walking, but does not predict step count. Br J Health Psychol 2007;12:601-20. http://dx.doi.org/10.1348/135910706X160335.
- Barnett I, Guell C, Ogilvie D. The experience of physical activity and the transition to retirement: a systematic review and integrative synthesis of qualitative and quantitative evidence. Int J Behav Nutr Phys Activity 2012;9. http://dx.doi.org/10.1186/1479-5868-9-97.
- Horne M, Tierney S. What are the barriers and facilitators to exercise and physical activity uptake and adherence among South Asian older adults: a systematic review of qualitative studies. Prev Med 2012;55:276-84. http://dx.doi.org/10.1016/j.ypmed.2012.07.016.
- Mutrie N, Doolin O, Fitzsimons CF, Grant PM, Granat M, Grealy M, et al. Increasing older adults walking through primary care: results of a pilot randomized controlled trial. Family Pract 2012;29:633-42. http://dx.doi.org/10.1093/fampra/cms038.
- Walters JA, Cameron-Tucker H, Courtney-Pratt H, Nelson M, Robinson A, Scott J, et al. Supporting health behaviour change in chronic obstructive pulmonary disease with telephone health-mentoring: insights from a qualitative study. BMC Fam Pract 2012;13. http://dx.doi.org/10.1186/1471-2296-13-55.
- Horne M, Skelton DA, Speed S, Todd C. Attitudes and beliefs to the uptake and maintenance of physical activity among community-dwelling South Asians aged 60–70 years: a qualitative study. Public Health 2012;126:417-23. http://dx.doi.org/10.1016/j.puhe.2012.02.002.
- Purath J, Van Son C, Corbett CF. Physical activity: exploring views of older Russian-speaking slavic immigrants. Nurs Res Pract 2011;2011. http://dx.doi.org/10.1155/2011/507829.
- Campbell F, Blank L, Messina J, Day M, Buckley Woods H, Payne N, et al. Physical Activity: Brief Advice for Adults in Primary Care. NICE Public Health Programme 2013. www.nice.org.uk/guidance/index.jsp?action=download&o=64825 (accessed 7 December 2013).
- Garrett S, Elley CR, Rose SB, O’Dea D, Lawton BA, Dowell AC. Are physical activity interventions in primary care and the community cost-effective? A systematic review of the evidence. Br J Gen Pract 2011;61:e125-33. http://dx.doi.org/10.3399/bjgp11X561249.
- Goode AD, Reeves MM, Eakin EG. Telephone-delivered interventions for physical activity and dietary behavior change: an updated systematic review. Am J Prev Med 2012;42:81-8.
- Orrow G, Kinmonth A-L, Sanderson S, Sutton S. Effectiveness of physical activity promotion based in primary care: systematic review and meta-analysis of randomised controlled trials. BMJ 2012;344. http://dx.doi.org/10.1136/bmj.e1389.
- McDonald A, Knight R, Campbell M, Entwistle V, Grant A, Cook J, et al. What influences recruitment to randomised controlled trials? A review of trials funded by two UK funding agencies. Trials 2006;7.
- Spilker B, Cramer JA, Spilker B, Cramer JA. Patient Recruitment in Clinical Trials. New York, NY: Raven; 1991.
- Chinn DJ, White M, Howel D, Harland JOE, Drinkwater CK. Factors associated with non-participation in a physical activity promotion trial. Public Health 2006;120:309-19. http://dx.doi.org/10.1016/j.puhe.2005.11.003.
- Sniehotta FF, Dombrowski SU, Avenell A, Johnston M, McDonald S, Murchie P, et al. Randomised controlled feasibility trial of an evidence-informed behavioural intervention for obese adults with additional risk factors. PLOS ONE 2011;6. http://dx.doi.org/10.1371/journal.pone.0023040.
- Forster S, Jones L, Saxton J, Flower D, Foulds G, Powers H, et al. Recruiting older people to a randomised controlled dietary intervention trial – how hard can it be?. BMC Med Res Methodol 2010;10. http://dx.doi.org/10.1186/1471-2288-10-17.
- Treweek S, Pitkethly M, Cook J, Kjeldstrøm M, Taskila T, Johansen M, et al. Strategies to improve recruitment to randomised controlled trials. Cochrane Database Syst Rev 2010;4. http://dx.doi.org/10.1002/14651858.MR000013.pub4.
- Sanders K, Stuart A, Merriman E, Read M, Kotowicz M, Young D, et al. Trials and tribulations of recruiting 2,000 older women onto a clinical trial investigating falls and fractures: Vital D study. BMC Med Res Methodol 2009;9. http://dx.doi.org/10.1186/1471-2288-9-78.
- Spilker B, Cramer JA, Spilker B, Cramer JA. Patient Recruitment in Clinical Trials. New York, NY: Raven; 1991.
- Harland J, White M, Drinkwater C, Chinn D, Farr L, Howel D. The Newcastle exercise project: a randomised controlled trial of methods to promote physical activity in primary care. BMJ 1999;319:828-32. http://dx.doi.org/10.1136/bmj.319.7213.828.
- Hillsdon M, Thorogood M, White I, Foster C. Advising people to take more exercise is ineffective: a randomized controlled trial of physical activity promotion in primary care. Int J Epidemiol 2002;31:808-15. http://dx.doi.org/10.1093/ije/31.4.808.
- Little PF, Dorward MF, Gralton S, Hammerton LF, Pillinger JF, White PF, et al. A randomised controlled trial of three pragmatic approaches to initiate increased physical activity in sedentary patients with risk factors for cardiovascular disease. Br J Gen Pract 2004;54:189-95.
- Naylor PJ, Simmonds GF, Riddoch CF, Velleman GF, Turton P. Comparison of stage-matched and unmatched interventions to promote exercise behaviour in the primary care setting. Health Educ Res 1999;14:653-66. http://dx.doi.org/10.1093/her/14.5.653.
- Altman DG. Practical Statistics for Medical Research. London: Chapman & Hall; 1991.
- Pocock S. Clinical Trials: A Practical Approach. Chichester: Wiley; 1983.
- European Medicines Agency . Statistical Principles for Clinical Trials. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline E9 n.d. www.emea.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002928.pdf (accessed 4 October 2012).
- Lange S. The all randomized/full analysis set (ICH E9) may patients be excluded from the analysis?. Drug Inform J 2001;35:881-91. http://dx.doi.org/10.1177/009286150103500327.
- Brueton V, Tierney J, Stenning S, Nazareth I, Meredith S, Harding S, et al. Strategies to reduce attrition in randomised trials. Trials 2011;12. http://dx.doi.org/10.1186/1745-6215-12-S1-A128.
- Chen JIE, Davis SL, Davis KG, Pan W, Daraiseh NM. Physiological and behavioural response patterns at work among hospital nurses. J Nurs Manag 2011;19:57-68. http://dx.doi.org/10.1111/j.1365-2834.2010.01210.x.
- Moy KL, Sallis JF, Tanjasiri SP. Culturally-specific physical activity measures for Native Hawaiian and Pacific Islanders. Hawaii Med J 2010;69:21-4.
- Thompson D, Batterham AM, Markovitch D, Dixon NC, Lund AJS, Walhin JP. Confusion and conflict in assessing the physical activity status of middle-aged men. PLOS ONE 2009;4. http://dx.doi.org/10.1371/journal.pone.0004337.
- CamNtech . Actiheart. n.d. www.camntech.com/products/actiheart/actiheart-overview (accessed November 2013).
- Ward DS, Evenson KR, Vaughn AF, Rodgers AB, Troiano RP. Accelerometer use in physical activity: best practices and research recommendations. Med Sci Sports Exerc 2005;37:S582-8. http://dx.doi.org/10.1249/01.mss.0000185292.71933.91.
- Wickstrom GF, Bendix T. The ‘Hawthorne effect’ – what did the original Hawthorne studies actually show?. Scand J Work Environ Health 2000;26:363-7.
- Motl RW, McAuley E, Dlugonski D. Reactivity in baseline accelerometer data from a physical activity behavioral intervention. Health Psychol 2012;31:172-5. http://dx.doi.org/10.1037/a0025965.
- Motl RW, Dlugonski D. Increasing physical activity in multiple sclerosis using a behavioral intervention. Behav Med 2011;37:125-31. http://dx.doi.org/10.1080/08964289.2011.636769.
- Behrens TK, Dinger MK. Motion sensor reactivity in physically active young adults. Res Q Exerc Sport 2007;78:1-8. http://dx.doi.org/10.1080/02701367.2007.10599397.
- Peter C, Paul D, Sally M, Susan M, Irwin N, Mark P. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337.
- Lundahl BW, Kunz C, Brownell C, Tollefson D, Burke BL. A meta-analysis of motivational interviewing: twenty-five years of empirical studies. Res Soc Work Pract 2010;20:137-60. http://dx.doi.org/10.1177/1049731509347850.
- Hettema J, Steele J, Miller WR. Motivational interviewing. Annu Rev Clin Psychol 2005;1:91-111. http://dx.doi.org/10.1146/annurev.clinpsy.1.102803.143833.
- Leventhal HF, Friedman MA, . (2004). Health Psychol 2004;23:452-6. http://dx.doi.org/10.1037/0278-6133.23.5.452.
- Dai D. Racial/ethnic and socioeconomic disparities in urban green space accessibility: where to intervene?. Landscape Urban Plan 2011;102:234-44. http://dx.doi.org/10.1016/j.landurbplan.2011.05.002.
- Brennan A, Blake L, Hill-McManus D, Payne N, Buckley Woods H, Blank L. Walking and Cycling: Local Measures to Promote Walking and Cycling As Forms of Travel or Recreation. NICE Public Health Programme 2012. http://publications.nice.org.uk/walking-and-cycling-local-measures-to-promote-walking-and-cycling-as-forms-of-travel-or-recreation-ph41/appendix-c-the-evidence#economic-modelling-2 (accessed 1 October 2012).
- Gillett M, Dallosso HM, Dixon S, Brennan A, Carey ME, Campbell MJ, et al. Delivering the diabetes education and self management for ongoing and newly diagnosed (DESMOND) programme for people with newly diagnosed type 2 diabetes: cost effectiveness analysis. BMJ 2010;341. http://dx.doi.org/10.1136/bmj.c4093.
- Fleischer NL, Roux AVD. Using directed acyclic graphs to guide analyses of neighbourhood health effects: an introduction. J Epidemiol Community Health 2008;62:842-6. http://dx.doi.org/10.1136/jech.2007.067371.
- Abraham C, Michie S. A taxonomy of behavior change techniques used in interventions. Health Psychol 2008;27:379-87. http://dx.doi.org/10.1037/0278-6133.27.3.379.
- Michie S, Ashford S, Sniehotta FF, Dombrowski SU, Bishop A, French DP. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: the CALO-RE taxonomy. Psychol Health 2011;26:1479-98. http://dx.doi.org/10.1080/08870446.2010.540664.
- Rollnick S, Butler CC, McCambridge J, Kinnersley P, Elwyn G, Resnicow K. Consultations about changing behaviour. BMJ 2005;331:961-3. http://dx.doi.org/10.1136/bmj.331.7522.961.
- Watkinson C, van Sluijs E, Sutton S, Marteau T, Griffin S. Randomised controlled trial of the effects of physical activity feedback on awareness and behaviour in UK adults: the FAB study protocol [ISRCTN92551397]. BMC Public Health 2010;10. http://dx.doi.org/10.1186/1471-2458-10-144.
- Bull F, Milton K. A process evaluation of a ‘physical activity pathway’ in the primary care setting. BMC Public Health 2010;10. http://dx.doi.org/10.1186/1471-2458-10-463.
- Nicaise V, Marshall S, Ainsworth BE. Domain-specific physical activity and self-report bias among low-income Latinas living in San Diego County. J Phys Act Health 2011;8:881-90.
- Adams SA, Matthews CE, Ebbeling CB, Moore CG, Cunningham JE, Fulton J, et al. The effect of social desirability and social approval on self-reports of physical activity. Am J Epidemiol 2005;161:389-98. http://dx.doi.org/10.1093/aje/kwi054.
- Klesges LM, Baranowski T, Beech B, Cullen K, Murray DM, Rochon J, et al. Social desirability bias in self-reported dietary, physical activity and weight concerns measures in 8- to 10-year-old African-American girls: results from the Girls Health Enrichment Multisite Studies (GEMS). Prev Med 2004;38:S78-87. http://dx.doi.org/10.1016/j.ypmed.2003.07.003.
- Healy GN, Dunstan DW, Salmon J, Cerin E, Shaw JE, Zimmet PZ, et al. Objectively measured light-intensity physical activity is independently associated with 2-h plasma glucose. Diabetes Care 2007;30:1384-9. http://dx.doi.org/10.2337/dc07-0114.
- Prochaska JO, Diclemente CC. Stages and processes of self-change of smoking – toward an integrative model of change. J Consult Clin Psychol 1983;51:390-5.
Appendix 1 Protocol

Booster Study
A randomised controlled trial and cost-effectiveness evaluation of ‘booster’ interventions to sustain increases in physical activity in middle-aged adults in deprived urban neighbourhoods.
RESEARCH PROTOCOL (Version 05) 09 March 2011
University of Sheffield (Sponsor) 120243
Sheffield CTRU J07-012
HTA 07/25/02
IRAS 08/H1308/270
Authorised by: Liddy Goyder
Sheffield Clinical Trials Research Unit (CTRU)
A randomised controlled trial and cost-effectiveness evaluation of ‘booster’ interventions to sustain increases in physical activity in middle-aged adults in deprived urban neighbourhoods.
Booster Activity Trial
This document describes a clinical trial, and provides information about procedures for entering participants. The protocol is not intended for use as a guide to the treatment of other patients. Amendments may be necessary; these will be circulated to known participants in the trial.
Abbreviations
A&E Accident and Emergency
ANCOVA Analysis of Covariance
BMI Body Mass Index
BREQ-2 Behavioural Regulation in Exercise Questionnaire
CONSORT Consolidated Standards of Reporting Trials
CI Confidence Interval
CTRU Clinical Trials Research Unit
DMEC Data Monitoring and Ethics Committee
EXERT Exercise Evaluation Randomised Trial
GCP Good Clinical Practice
HRQoL Health Related Quality of Life
HTA (National Institutes for Health Research) Health Technology Assessment programme
IMD Index of Multiple Deprivation
MI Motivational Interviewing
NICE National Institute for Health and Clinical Excellence
QALY Quality-Adjusted Life Year
RA Research Assistant
ScHARR School of Health And Related Research
SD Standard Deviation
SF-12v2 plus 4 16-item Short Form Health Survey of the Medical Outcomes Study
SF-6D Short Form Health Survey – 6 Dimensions
SMART Specific, Measurable, Achievable, Realistic, Time-related goals
SPAQ Scottish Physical Activity Questionnaire
TMG Trial Management Committee
TSC Trial Steering Committee
TTM Trans-Theoretical Model
General information
Sponsor
Gill Wells, Research Development Manager, the University of Sheffield, New Spring House, 231 Glossop Road, Sheffield, S10 2GW.
Persons authorised to sign the protocol & amendments
Elizabeth Goyder, Director of the Section of Public Health and Reader in Public Health Medicine, ScHARR, University of Sheffield, Regent Court, 30 Regent Street, Sheffield, S1 4DA. Tel: (+44)(0)114 222 0783; Fax: (+44)(0)114 222 0791; e-mail : e.goyder@sheffield.ac.uk.
Partner institutions
Sheffield Primary Care Trust
Jeremy Wight, Director of Public Health, Public Health, Sheffield PCT, Don Valley House, Savile Street, Sheffield S4 7UQ. Tel: 0114 226 4555; Fax: 0114 226 4542; e-mail: jeremy.wight@sheffieldpct.nhs.uk.
Sheffield Hallam University
Jeff Breckon, Senior Lecturer, Centre for Sport and Exercise Science, School of Health and Wellbeing, Sheffield Hallam University, Collegiate Crescent Campus, Sheffield, S10 2BP. Tel: 0114 225 4353; Fax: 0114 225 4341; e-mail: j.breckon@sheffield.ac.uk.
Sheffield City Council
Paul Billington, Head of Sport and Physical Activity, Sport and Physical Activity, Chair of Active Sheffield, Sheffield City Council, 2–10 Carbrook Hall Road, Sheffield, S9 2DB. Tel: 0114 273 4775; Fax: 0114 273 5504; e-mail: paul.billington@sheffield.ac.uk.
Trial Manager
Daniel Hind, Research Fellow, Clinical Trials Research Unit, University of Sheffield, Regent Court, 30 Regent Court, Sheffield, S1 4DA. Tel: 0114 222 0707; Fax: 0114 222 0870; e-mail: d.hind@sheffield.ac.uk.
Trial Statistician
Stephen Walters, Reader in Medical Statistics, ScHARR, University of Sheffield, Regent Court, 30 Regent Court, Sheffield, S1 4DA. 0114 222 0730; Fax: 0114 222 0870; e-mail: s.j.walters@sheffield.ac.uk.
Trial Steering Committee
| Dr Alan Batterham (Chair) Head of Centre, Centre for Food, Physical Activity and Obesity, School of Health & Social Care, University of Teesside, Middlesbrough, TS1 3BA Tel: 01642 342 771 Fax: 01642 384 105 e-mail: a.batterham@tees.ac.uk |
Mr David White (Secretary) Administrator, Clinical Trials Research Unit, University of Sheffield, Regent Court, 30 Regent Court, Sheffield, S1 4DA Tel: 0114 222 0807 Fax: 0114 222 0870 e-mail: d.a.white@sheffield.ac.uk |
| Dr Nick Taub (Statistician) Leicester University, Trent RDSU, Department of Epidemiology and Public Health, 22–28 Princess Road West, Leicester, LE1 6TP Tel: 0116 252 5416 Fax: 0116 252 3272 e-mail: nat2@leicester.ac.uk |
Prof Edward M Winter Professor of the Physiology of Exercise, The Centre for Sport and Exercise Science, Sheffield Hallam University, Collegiate Hall, Collegiate Crescent Campus, Sheffield, S10 2BP Tel: 0114 225 4333 Fax: 0114 225 4341 email: e.m.winter@shu.ac.uk |
| Dr Michael Gordon Honorary Senior Research Fellow, ScHARR, University of Sheffield, Regent Court, 30 Regent Court, Sheffield, S1 4DA Tel: Fax: e-mail: michael.gordon@sheffield.ac.uk |
Trial Management Committee
| Elizabeth Goyder (Chair) Director of the Section of Public Health and Reader in Public Health Medicine, ScHARR, University of Sheffield, Regent Court, 30 Regent Street, Sheffield, S1 4DA Tel: (+44)(0)114 222 0783 Fax: (+44)(0)114 222 0791 e-mail : e.goyder@sheffield.ac.uk |
|
| Danny Hind (Study manager) Research Fellow, ScHARR, University of Sheffield, Regent Court, 30 Regent Street, Sheffield, S1 4DA Tel: (+44)(0)114 222 0707 Fax: (+44)(0)114 222 0870 e-mail: d.hind@sheffield.ac.uk. |
Stephen Walters (Statistician) Professor of Medical Statistics, ScHARR, University of Sheffield, Regent Court, 30 Regent Street, Sheffield, S1 4DA Tel: (+44)(0)114 222 0730 Fax: (+44)(0)114 222 0870 e-mail: s.j.walters@sheffield.ac.uk |
| Helen Crank Research Fellow, Clinical trials, Centre for Sport and Exercise Science, Faculty of Health and Wellbeing, Sheffield Hallam University, Collegiate Crescent Campus Sheffield, S10 2BP Tel: (+44)(0)114 225 5572 Fax: (+44)(0)114 225 4341 e-mail: h.crank@shu.ac.uk |
Robert Copeland Senior Exercise Scientist, Physical activity measurement, Centre for Sport and Exercise Science, Faculty of Health and Wellbeing, Sheffield Hallam University, Collegiate Crescent Campus, Sheffield, S10 2BP Tel: (+44)(0)114 225 5635 Fax: (+44)(0)114 225 4341 e-mail: r.j.copeland@shu.ac.uk |
| Dawn Lockley Health Improvement Principal, Sheffield Primary Care Trust, 722 Prince of Wales Road, Sheffield, S9 4EU Tel: 0114 3051052 e-mail: Dawn.Lockley@sheffieldpct.nhs.uk |
Sarah Nickson Service Manager, Activity Sheffield, Sheffield City Council, 2–10 Carbrook Hall Road, Sheffield, S9 2DB Tel: (+44)(0)114 273 4775 Fax: (+44)(0)114 273 5504 e-mail: sarah.nickson@sheffield.gov.uk |
| Jeff Breckon Senior Lecturer, Exercise Psychology, Centre for Sport and Exercise Science, Faculty of Health and Wellbeing, Sheffield Hallam University, Collegiate Crescent Campus Sheffield, S10 2BP Tel: (+44)(0)114 225 4353 Fax: (+44)(0)114 225 4341 e.mail: j.breckon@shu.ac.uk |
Emma Scott Research Fellow, ScHARR, University of Sheffield, Regent Court, 30 Regent Street, Sheffield, S1 4DA Tel: (+44)(0)114 222 5200 Fax: (+44)(0)114 222 0870 e.mail: e.scott@sheffield.ac.uk |
Trial Summary
This study assesses whether it is worth providing further support, 3 months after giving initial advice, to those who have managed to do more physical activity. All participants will initially be given an interactive DVD, supported by advice from a trained facilitator. The facilitator will provide two telephone follow ups at one month intervals. Only those that have increased their physical activity at this point will remain in the study. These participants will receive a ‘mini booster’, a ‘full booster’ or no booster. The ‘mini booster’ consists of a two telephone calls one month apart to discuss physical activity and usage of the DVD. A ‘full booster’ consists of a face-to-face meeting with the facilitator at the same intervals. The purpose of these booster sessions is to help the individual to maintain their increase in physical activity. We will measure the differences in physical activity, quality of life and costs, associated with the booster interventions, 3 months and 9 months from randomisation. The research will be carried out in 20 of the most deprived neighbourhoods in Sheffield. These locations have large, ethnically diverse populations, high levels of economic deprivation, low levels of physical activity, poorer health and shorter life expectancy. Participants will be recruited through general practices and community groups, as well as by postal invitation to ensure the participation of minority ethnic groups and those with lower levels of literacy. Sheffield City Council and Primary Care Trust fund a range of facilities and activities to promote physical activity and variations in access to these between neighbourhoods will make it possible to examine whether the effectiveness of the intervention is modified by access to community facilities.
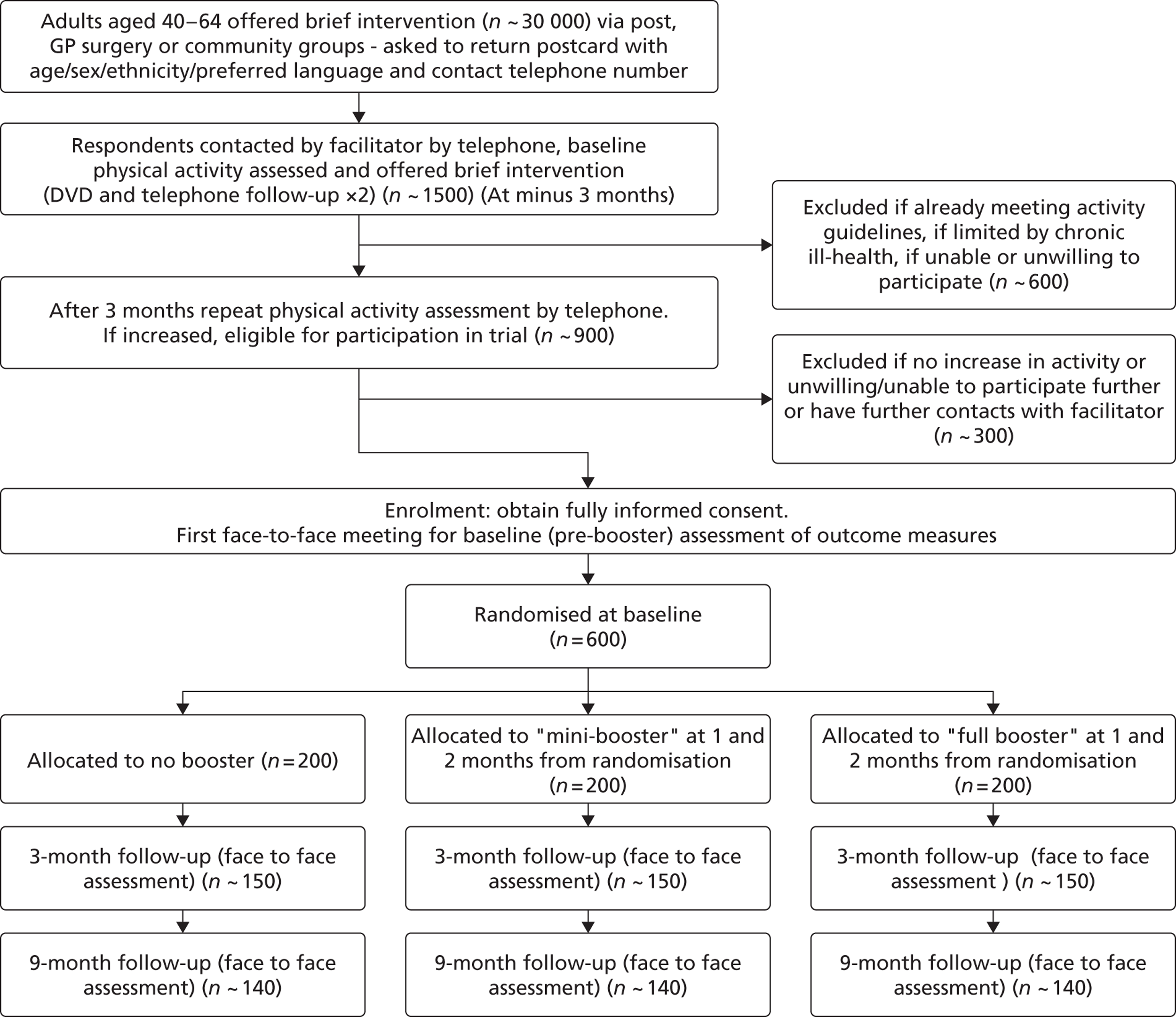
Introduction
Rationale
There are a number of published systematic reviews of evidence for interventions that increase physical activity. 1–5 More recently the evidence base for brief interventions in primary care has been reviewed. 6 This review identified a sufficient evidence base for NICE to recommend the use of brief interventions to promote physical activity but also identified specific evidence gaps that this trial will be able to address, particularly in relation to the value of follow up beyond three months, for the longer term maintenance of physical activity.
Searches of the National Research Register and ClinicalTrials.gov for research in progress confirm that although there are a number of physical activity intervention trials in progress in specific patient groups and in older age groups or in children, there are few trials including ‘healthy’ middle-aged participants and no other trials specifically examining the value of further intervention after an initially successful ‘brief intervention’.
Investigational interventions
The trial will compare a ‘mini booster’ of two telephone physical activity consultations and a ‘full booster’ of two face-to-face physical activity consultations, offered four and five months after an initial brief intervention, to a standardised three month brief intervention alone. The purpose of these booster sessions is to help participants to sustain their physical activity levels and prevent relapse. The brief intervention will involve provision of an interactive DVD based on a MI approach that is directive, client-centred and replicates the style of other successful behaviour change programmes. 7,8 All interventions, including the initial brief intervention, will be delivered by trained facilitators (employed as research assistants and trained by the research team) to ensure consistent delivery.
Theoretical underpinning of interventions
Meta-analytical and systematic reviews of physical activity and behaviour change9,10 suggest that the transtheoretical model (TTM)11 is the most commonly adopted theoretical framework for promoting physical activity. The TTM has demonstrated effectiveness as an approach to increasing exercise adoption and adherence in adults. 10,12–14 The TTM describes how people modify problem behaviours or acquire positive new ones. 11,15,16 The TTM determines behaviour change as a process rather than a single event and offers practical suggestions for how individuals can change behaviour. The TTM consists of the following constructs: stages of change (describes when people change), processes of change (outlines techniques for helping people to change), decisional balance (weighing up the pro’s and con’s of change) and self-efficacy (increasing one’s confidence to change behaviour). 11 The TTM offers practitioners a common, validated framework for guiding participants through periods of change and proposes strategies for maintaining positive behaviours. We will also adopt a client centred approach to all interventions based upon the style of motivational interviewing.
Motivational Interviewing and its use in promoting physical activity
Motivational interviewing (MI) is a directive, client-centred counselling style for eliciting behaviour change by helping clients to explore and resolve ambivalence. 17 Motivational interviewing has been used in many settings and ethnic groups and meta-analysis suggests effect sizes from motivational interviewing-based interventions are larger in ethnic minority populations. 18 A MI approach has been shown to impact positively on lifestyle and health outcomes including physical activity behaviours in adults. 18–21 MI has been applied in a number of formats including technology-based, such as internet and video,7,8,22,23 telephone24 and face-to-face consultations. 19 An example of a technology-based intervention adopting an MI approach is The Drinker’s Check-up. 7,8,23 The Drinker’s Check-up offers a comprehensive assessment of the client’s drinking and related behaviours. A key element of the programme is providing feedback that matches the user’s individual circumstances, motivational readiness and confidence for changing their behaviour.
Justification of use of interactive DVD:
The use of video format in the promotion of physical activity has been shown to increase self-reported physical activity,25–27 positively influence user engagement and self-efficacy and yield health benefits in low-income populations. 28,29 Survey data reveals that at least 80% of adults aged 35–64 own a DVD player. 30 Furthermore, 84% of households classified as ‘hard pressed’ (which includes: inner city adversity, high rise hardship, burdened singles, struggling families, ACORN classification, 2007) own a DVD player. The DVD clearly represents an opportunity to reach a wide audience, at relatively low cost, using a medium that is familiar and accessible. We will ensure participants without home access to a DVD player have community access by arranging that DVD players are provided and accessible in community venues including neighbourhood centres, libraries and GP surgeries. The content of the DVD has already been developed based on existing materials already used for face-to-face interventions and the first phase of the trial will include translation, production, and piloting of the DVD. This potentially offers a very cost-effective way to promote change and utilises technology that will already be familiar to most participants. Practical support with using the DVD will also be available from local library staff in libraries where the DVD can be played on public access computers.
Content of the Brief Intervention (Interactive DVD and telephone follow up)
Consistent with NICE guidance on physical activity interventions, the brief intervention will aim to help middle age adults consider, initiate and maintain physical activity behaviours. 6 The DVD represents an interactive tool that is based on the style of motivational interviewing and the principles of the TTM. The DVD offers individuals the opportunity to choose information on the following: the benefits (social, health, environmental) of physical activity; current physical activity recommendations; things to consider before starting; different types of physical activity; building confidence and efficacy to become physically active; myths and misconceptions about physical activity; staying motivated; sign-posting of opportunities to be physically active in Sheffield/South Yorkshire and example case studies.
Compliance with NICE guidance and UK practice
A. NICE Physical Activity Guidance (March 2006)6
‘identify inactive adults and advise to aim for 30 minutes of moderate activity on 5 days of the week (or more)’. The DVD will allow individuals to assess their own physical activity relative to current physical activity recommendations that will be provided on the DVD. Information will be provided that will help individuals to understand what is meant by ‘active’ and ‘moderate intensity’.
‘When providing physical activity advice, primary care practitioners should take into account the individual’s needs, preferences and circumstances. They should follow them up at appropriate intervals over a 3 to 6 month period’. The DVD offers trainers a tool to support individuals in making healthier lifestyle choices regarding positive change in physical activity. The DVD is theoretically underpinned by the Trans-theoretical model of behaviour change and the Theory of Planned Behaviour – which both place emphasis upon the individual considering their perceived behavioural control, subjective norms and attitudes when initiating behaviour change – such as increasing physical activity. The DVD asks the individual to reflect upon their individuals needs, preferences and circumstances and this is done within an evidence-based stage matched approach – meaning that greater emphasis will be placed on those factors (such as circumstances and needs) that mediate behaviour change at the early stages of change adoption (pre-contemplation) and factors that mediate sustaining the new behaviour such as reinforcement management that will be more salient for those individuals who have already decided to take action but need help to implement change.
‘Practitioners should agree goals with them’. Goal setting, what it means and examples of goal setting for physical activity will be given in the DVD. This will put the potentially abstract theory of goal setting into a real life context. Appropriate language will be used to facilitate understanding and relevance.
‘They should also provide written information about the benefits of activity and the local opportunities to be active’. The DVD will provide a comprehensive list of physical activity opportunities within the Sheffield area with contact details. These will include activities that have already been found to be popular with minority ethnic groups eg. dance activities.
‘Local policy makers, commissioners and managers, together with primary care practitioners, should pay particular attention to the needs of hard to reach and disadvantaged communities, including minority ethnic groups, when developing service infrastructures to promote physical activity. ’ The information given within the DVD will have been discussed with local ethnic minority community groups to ensure it is culturally and contextually appropriate. The local strategic partnership will be consulted about current service provision for disadvantaged and ethnic minority groups.
B. NICE Behaviour Change Guidance (October 2007)31
‘Employ a range of behaviour change methods and approaches, according to the best available evidence. These concepts could be used to structure and inform interventions.’ Action planning, implementation-intention action plans, promoting user autonomy, providing tips and practical strategies to develop physical activity self-efficacy, adopting a client centred approach, information giving, expert based advice, peer modelling, engaging in reflective tasks, contingency planning are all features of the DVD intervention that comply with the list of concepts that are advocated by NICE to structure behaviour change interventions. Through engagement with the DVD, individuals will be able to devise their own personal physical activity plan, understand the benefits of physical activity, be given information regarding safe physical activity, how to monitor intensity, myths about activity, information re the difference between activity for health and fitness.
‘It should be taken into account behaviour is embedded in social, cultural & material circumstances. ’ The complexity of an individual’s personal circumstances, their socioeconomic and cultural context and their interactions with health behaviours will be considered in the content of the DVD and in ensuring associated advice is realistic and culturally appropriate.
Compliance with pragmatic practice
The brief intervention will be delivered as part of the local strategy for promoting physical activity in more deprived neighbourhoods within the Enhanced Public Health Programme which is based on a comprehensive needs assessment and targets specific neighbourhoods with poorer health outcomes. The use of DVDs is in line with the strategic approach and methods developed locally by Active Sheffield and supported by the partnership of bodies that deliver support for increasing physical activity across Sheffield. Use of DVDs is already being introduced in other areas with anecdotal success but without systematic evaluation. Health First, the specialist health promotion agency for Lambeth, Southwark and Lewisham, has recently produced a DVD designed to promote physical activity. This DVD includes similar content to that in our proposed intervention, tailored for a local population. The DVD ‘Choosing Physical Activity’ is in the public domain and can be viewed using http://video.google.co.uk.
The main reasons for not using existing community or primary care practitioners to deliver an initial brief intervention were both to ensure fidelity to the intervention protocol (so an ineffective intervention can be distinguished from inadequate delivery of an effective intervention) and also because in practice, if the intervention is effective and introduced into practice, it will need to be delivered consistently and efficiently across a large population with capacity to benefit by a range of health trainers and other community staff as well as primary care staff. The proposed DVD has the potential to reach a wide targeted audience at minimal cost compared to a one to one based intervention with a health professional.
Content of the Full Booster:
The full booster will comprise two 20–30 minute face-to-face physical activity consultations that aim to promote and sustain change in physical activity status. The full booster sessions will take place in community venues. The consultation will be underpinned by the principle of the TTM15 and replicate a brief version of motivational interviewing based on a method designed for time limited consultations in medical settings. 32 Such an approach has been successfully employed to change health-related behaviours previously. 33 This approach also mirrors that adopted by the health trainer initiative which provides a current model of face-to-face promotion of healthy behaviours. For the Full booster, a menu of six strategies has been developed32 to guide the 30-minute consultation. Each strategy is suitable for participants who are in the maintenance stage of motivational readiness for physical activity behaviour change. They are:
-
Assessment of motivation and confidence for maintaining physical activity
-
Increasing knowledge of the benefits of physical activity; awareness of the risks of a sedentary lifestyle; increasing awareness of physical activity opportunities; increasing awareness of the current recommendations for physical activity
-
Increasing confidence to be physically active – self-efficacy
-
Goal setting and tracking using SMART (specific, measurable, achievable, realistic, time-related) goal principles
-
Strategies for staying motivated
-
Relapse prevention strategies
-
During the full booster consultations, strategies will be worked through at a pace dictated by the participant and the menu used to structure information exchange without being prescriptive.
-
Content of the Mini Booster:
Although face-to-face interventions have been found to be efficacious in promoting physical activity,33 many of the barriers associated with this approach, including time and financial costs, highlight the need for pragmatic alternatives that are both relatively cheap to deliver and may make it easier for the participant to access the intervention. The Mini Booster will consist of two 20-minute telephone based physical activity consultations. The telephone consultation will follow the same menu of six behaviour change strategies as the Full Booster (outlined above) and aim to promote and sustain change in physical activity status. A number of studies using telephone support for physical activity in older adults have been carried out. 3,34 A telephone based approach has been effective in increasing physical activity participation at six months compared to no telephone support35 and has also been shown to increase physical activity participation to a greater extent than standard reading materials in adult populations. 34 The telephone consultations will follow a script of known efficacy that has been implemented in previous physical activity promotion studies delivered by members of this research team.
Quality assurance:
The proposed interventions will adhere to an intervention fidelity framework based on the Behaviour Change Consortium. 36 This framework provides quality assurance parameters based on the intervention design, training, delivery, receipt and enactment. Further detail of this can be found elsewhere. 36 A recent review has highlighted inconsistent delivery and levels of competence of physical activity interventionists reporting to deliver a physical activity counselling components37 and it has been suggested that the effectiveness of behaviour change counselling is predicted by the length, the intensity, the content of interventions and the competence of the deliverer. 38 The sessions will therefore be delivered by a team of four research assistants trained in MI and behaviour change techniques and assessed to ensure their competency. A framework will be developed for each session to ensure consistency of advice across sessions and between participants. All research assistants (RAs) will be trained (by JB, HC and RC) using a training package and a detailed manual to ensure standardised delivery of the booster interventions. All booster interventions will be audio-recorded. A random selection of 5% of all booster consultations (20 telephone and 20 face-to-face) will be reviewed and assessed by an independent clinical psychologist (LJ) using a pre-determined check list. The RAs will be provided with individual feedback if required, to ensure intervention fidelity is maintained.
Population
Men and women aged between 40 and 64 who have increased their physical activity by at least 30 minutes per day.
This trial will be conducted in compliance with the protocol, GCP and regulatory requirements.
Aims and objectives
The overall aim is to measure the effectiveness and cost effectiveness of ‘mini’ and ‘full’ booster sessions, as an adjunct to a brief intervention, in sustaining physical activity in middle-aged adults.
Primary objective
To determine whether physical activity measured by accelerometry three months after randomisation (six months after a brief intervention) is significantly increased in participants allocated to two intervention groups (receiving two booster physical activity consultations, delivered in a motivational interviewing style, either by telephone or face-to-face) compared to participants allocated to a control group (receiving no further contact after the baseline assessment).
Secondary objectives
-
To determine whether physical activity nine months after randomisation (12 months after the brief intervention) is significantly increased in participants allocated to the two intervention groups compared to participants allocated to the control group.
-
To compare physiological measures of fitness (12 minute walk test) and self-reported physical activity (SPAQ instrument) between allocated groups.
-
To compare health related quality of life, resource use (including health and social care contacts) and economic costs between allocated groups.
-
To investigate whether the impact of the intervention may be modified by gender, ethnicity or the types of physical activity undertaken (including use of community facilities for physical activity).
-
To undertake a process evaluation to identify, using both quantitative and qualitative methods, psychosocial and environmental factors that may mediate or modify the effectiveness of the intervention.
Trial Design
Design
This is a three-arm, parallel group, randomised controlled trial with a feasibility study.
Feasibility study
In the first year a pilot trial will be undertaken to assess the feasibility of both trial recruitment plans and the proposed interventions39,40. A total of 3000 mailshots will be sent to the patients of a general practice situated in a ‘typical’ deprived ward (Manor Ward), at least 150 will receive the brief intervention and 60 randomised to the three trial arms. This will allow outcome measurement in 15–20 individuals in each study arm to estimate a mean and standard deviation for the primary outcome, total energy expenditure per day (averaged over a 7-day accelerometry assessment period in each group using the Actiheart Device). The main risks to trial success identified by reviewers that the feasibility trial will test are:
-
Recruitment targets for the brief intervention will not be met;
-
The brief intervention will not be effective enough to generate sufficient individuals eligible for the trial;
-
Insufficient eligible individuals will consent to participate in the trial.
These three issues in combination will determine whether the trial recruitment rate is adequate.
The success criteria for the feasibility study will therefore be:
-
At least 60 patients recruited to the pilot trial and 45 having 3 month follow-up measurements including accelerometry completed on the basis of an initial mailshot to 3000 individuals. (We will not use community recruitment at this stage since it may represent a more limited pool for recruitment that we can use to booster participants from ‘hard-to-reach’ groups as required in the main trial)
-
At least 70% of those randomised to booster interventions actually receiving the interventions per protocol
-
On the basis of the pilot primary outcome (accelerometry) data collected, the sample size for the main trial will be re-calculated. The trial will not proceed if the revised sample size calculation suggests a total sample size > 600 will be required. Assuming the protocol and intervention remain unchanged, the participants recruited during the feasibility phase will be included in the full trial population.
Main trial
The trial participants will be recruited from the 20 most deprived neighbourhoods of the city of Sheffield, based on Index of Multiple Deprivation (IMD) 2004 and health indicators. Average life expectancy is six years lower in lowest versus highest quintile of IMD (based on data from www.neighbourhoodstatistics.gov.uk). Up to 30 000 middle-aged residents, (aged 40 to 64 years at recruitment), will be invited to participate in a brief intervention to help them get more physically active. Up to 1500 residents who respond to the invitation will receive an initial assessment telephone call to determine their physical activity status. Eligible participants (i.e. those not already meeting current recommendations of 30 minutes moderate activity, 5 times a week) will then receive an interactive DVD and supporting written materials through the post. Follow up telephone contacts will be made one month and two months from initial contact. Active Sheffield, the organisation responsible for promoting physical activity in Sheffield, will ensure participants have access to a range of community facilities for exercise and will provide regularly updated information on current provision.
After three months, the Study Introduction letter will be sent to all DVD recipients and a further telephone assessment will establish whether they are eligible to participate in the booster trial (i.e. have increased their activity by at least 30 minutes per week and are willing to have further assessment and follow up). Eligible participants will then be invited to attend a baseline assessment appointment at a community venue and they will be randomly allocated to one of three groups:
-
a control group who will be assessed at randomisation, after three months and after nine months and receive no additional intervention between those assessments;
-
a ‘mini booster’ group also receiving an intervention comprising two telephone-based physical activity consultations, delivered in a motivational interviewing style, at one month and two months from randomisation;
-
a ‘full booster’ group also receiving an intervention comprising two face-to-face physical activity consultations, delivered in a motivational interviewing style, at one month and two months from randomisation.
Written consent to trial participation will be obtained at the start of the trial baseline assessment meeting. All randomised participants will be assessed at baseline, 3 months and 9 months from randomisation (i.e. 3 months, 6 months and 12 months from the initial contact for the brief intervention). Where possible, staff conducting assessments will not know participants’ group allocation and participants will be asked not to tell them.
Endpoints
-
Objective measure of physical activity including:
-
– Total Energy Expenditure (TEE), in Kcal per day, from seven-day accelerometry and heart rate monitoring using Actiheart
-
– Physical activity counts (PAC) per week;
-
– Minutes of moderate/vigorous physical activity per day;
-
– Meeting the current physical activity recommendation of at least 30 min per day (continuous or in bouts of at least 10 min] of at least moderate intensity) for at least 5 days a week (yes or no).
-
-
Self-reported moderate or strenuous physical activity using the Scottish Physical Activity Questionnaire (SPAQ) which records type and duration of activities in the previous week;
-
Health-related quality of life using the Sheffield Version of the 16-item Short Form health survey instrument (SF-12v2 plus 4);
-
Self-reported use of community facilities for physical activity;
-
Self-reported health and social care contacts;
-
Psychological measures of motivation, intentions, attitudes, beliefs, social influences and self-efficacy towards physical activity, measured using the Theory of Planned Behaviour. 41 Exercise stages of change42, and self-determination will be assessed using Behavioural Regulation in Exercise Questionnaire43 and questions used in the HTA-funded Exercise Evaluation Randomised Trial (EXERT). 44 This will allow comparison with results from other physical activity trials including EXERT.
-
Body weight and height (to allow calculation of BMI)
-
Physiological measures of fitness (12 minute walk test)45
Design measures to avoid bias
The allocation schedule will be concealed through the use of a centralised web-based randomisation service. The randomisation sequence is computer-generated. Data analysts will be blind to treatment allocation, but the study manager, participants (who are also outcome assessors) will not be blinded. Analysis will be by intention-to-treat. Where individuals are lost to follow-up or data is missing, imputation methods will be employed, which will be described in the statistical analysis plan.
Randomisation codes and allocation concealment
The randomisation schedule is generated prior to the study by the Clinical Trials Unit Randomisation Service. On identification of an eligible volunteer, the study manager or data manager will randomise and inform the patient and their general practitioner on the treatment allocation.
Selection and withdrawal of participants
Inclusion criteria for brief intervention
-
Residents of the 20 most deprived neighbourhoods in the city of Sheffield
-
Aged 40 to 64 years
-
Not achieving the current recommended activity level (30 minutes of moderate activity on at least 5 days) assessed using the SPAQ46 and wishing to have support to become more active
Additional inclusion criteria for booster trial:
-
Have increased their physical activity level by at least 30 minutes of moderate or vigorous activity per week (assessed using the SPAQ) since initial assessment of activity level
-
Capacity to give written informed consent to trial participation
Exclusion criteria
Individuals with chronic conditions who can benefit from physical activity will not be excluded unless their condition significantly impairs their ability to exercise. They will be asked to consult their GP if they have a condition that increases their risk of adverse events during exercise (i.e. chronic cardiovascular or pulmonary disease).
Criteria for withdrawal from trial treatment
Participants may withdraw from active participation in the study on request. If a participant experiences chest pain or severe breathlessness during the 12-minute walk test, then the researcher will advise the GP directly and immediately, and will also advise the participant to make an appointment with their GP at their earliest convenience.
If analysis of Actiheart (accelerometer) readings suggests pre-existing arrhythmias, this information will be shared with the participant and their GP.
Subjects removed from active participation will not be replaced and, with their consent, will be followed up for all outcome information.
Randomisation and enrolment
We will use a remote web-based randomisation service. Eligible participants will be randomised to one of the three arms by the study manager, after receiving the consent form, via a centralised telephone randomisation service provided through the Clinical Trials Research Unit (CTRU).
Assessments and procedures
Procedures required at screening or before randomisation
Letter 1. Up to 30 000 middle-aged residents will be sent a letter and business response envelope inviting them to enroll in a programme to help them get more physically active.
Scottish Physical Activity questionnaire. The research team will send this out to potential participants with Letter 1. It will be administered face-to-face by a member of the research team at screening and sent out again by post on two subsequent occasions to participants (3 months and 9 months after randomisation).
Brief intervention
Interactive DVD and supporting written materials, delivered through the post. The research team will send this out to potential participants who respond to Letter 1.
Procedures required at initial follow-up
Follow up telephone contacts, one month and two months from initial contact to assess DVD usage and offer advice on physical activity. A member of the research team will contact the individual at home by telephone.
Procedures required at screening
After three months, the Study Introduction letter will be sent to all DVD recipients and eligibility to participate in the booster trial will be assessed by telephone. Eligible participants will be invited to attend a baseline assessment.
Procedures required before randomisation
Participant information sheet sent to potential participant at home. The research team will send this out to potential participants who are eligible and willing to participate in the trial (see above).
Visit to a community venue for informed consent, baseline assessment and randomisation. A member of the research team will meet and consent the individual, take baseline assessments and randomise them. A member of the research team will administer:
-
Scottish Physical Activity questionnaire;
-
Short-Form 12v2 plus 4 questionnaire, plus one wellbeing question;
-
Behavioural Regulation in Exercise Questionnaire (BREQ-2);
-
Exercise Evaluation Randomised Trial (EXERT) questionnaire ;
-
Personal information questionnaire (Questionnaire 1).
-
Questions about use of community facilities for physical activity, and about health and social care service contacts; and,
-
Measurement of weight and height.
Procedures required at three month follow-up
A member of the research team will meet the individual, take baseline assessments and randomise them. A member of the research team will administer:
-
Scottish Physical Activity questionnaire;
-
Short-Form 12v2 plus 4 questionnaire, plus one wellbeing question;
-
Behavioural Regulation in Exercise Questionnaire (BREQ-2);
-
Exercise Evaluation Randomised Trial (EXERT) questionnaire’
-
Questions about use of community facilities for physical activity, and about health and social care service contacts;
-
Measurement of weight and height;
-
Twelve-minute walk test; and,
-
Seven-day accelerometry using Actiheart.
Procedures required at nine month follow-up
A member of the research team will meet the individual, take baseline assessments and randomise them. A member of the research team will administer:
-
Scottish Physical Activity questionnaire;
-
Short-Form 12v2 plus 4 questionnaire, plus one wellbeing question;
-
Behavioural Regulation in Exercise Questionnaire (BREQ-2);
-
Exercise Evaluation Randomised Trial (EXERT) questionnaire ;
-
Questions about use of community facilities for physical activity, and about health and social care service contacts;
-
Measurement of weight and height;
-
Twelve-minute walk test; and,
-
Seven-day accelerometry using Actiheart.
List procedures for attempted follow-up of patients ‘lost to follow-up’
Patients will be considered lost-to-follow-up if they fail to respond to questionnaires, one reminder letter and two telephone calls. There are no procedures for further follow-up.
Procedures required when closing a trial (premature or planned)
At the point at which all questionnaires have been collected (or participants have failed to respond despite reminders) and all data have been entered and cleaned, the management group will approve closure of the database. Further details will be presented in the data management protocol.
Procedures required to record serious adverse events
At each follow-up, participants will be asked if they have experienced any event or illness which:
-
has required unscheduled hospitalisation; or,
-
has resulted in persistent or significant disability/incapacity.
The details of serious adverse events will be confirmed with the participant’s general practitioner before classification.
It is the Chief Investigator’s responsibility:
-
To follow the procedure outlined in the study protocol for the reporting of SAEs;
-
To assess each event for causality and AE category;
-
To provide the Dean of ScHARR and the University Research Office (in their capacity as representatives of the sponsor) with details of all SAEs identified within agreed timeframes;
-
To notify the Trial Steering Committee and Data Monitoring and Ethics Committee of any SAEs; and,
-
To submit the annual safety report to the REC.
Statistics
Number of patients to be enrolled
600.
Reason for choice of sample size
The original sample size calculation assumed that physical activity would be measured using a simple hip-mounted accelerometer. It was also assumed that a mean difference of 400,000 PAC per week between the intervention and control groups at three months was the smallest clinically and practically important difference and that the SD of this outcome was 1.2 million counts/per week. Hence with 450 participants (300 intervention: 150 control), the main trial was determined to have 90% power to detect this mean difference or greater between the intervention and control arms as statistically significant at the 5% (two-sided) level using a two independent samples t-test. Assuming an approximate 25% loss to follow-up by three-months, it was proposed to recruit and randomise 200 participants per group giving total sample size of 600. The Actiheart accelerometer measures physical activity counts per week on a different scale of magnitude to a simple hip mounted accelerometer with a considerably lower mean and standard deviation.
When re-estimating the sample size using data from an internal pilot study the revised sample size estimate either stays the same or increases (it cannot be less than the original estimate). The original sample size calculation of 450 subjects with valid outcome data at 3 months post-randomisation, was based on detecting a standardised effect size of 0.33 or a mean difference of one-third of a standard deviation in the outcome measures between the intervention and control groups. This equates to an estimated mean difference between the Booster and Control groups, based on the observed standard deviation from the feasibility stage of 34,465 PAC per week and 102 kcal per day for TEE.
To have a 90% power of detecting a mean difference of 102 kcal in mean TEE per day is between the groups would require 426 participants in total with evaluable data (control = 142; intervention = 254). Similarly, the total required sample size under the above conditions to detect a mean difference of a 34,465 PAC per week is 429. Therefore since the re-estimated sample size, of around 430 participants, is lower than the original estimate of 450 participants the trial will proceed with the original sample size estimate of 450 participants with evaluable data.
Statistical criteria to terminate the trial
There are no statistical criteria for stopping the trial early; as the intervention is considered low risk, there is no DMEC and decisions to stop the trial will be made on safety grounds by the Trial Steering Committee.
Procedure for accounting for missing data
The primary analysis will be an ITT analysis with participants with complete accelerometry data at three months post-randomisation. A sensitivity analysis will be undertaken to impute missing accelerometry data using baseline and follow-up data from the group of patients with valid accelerometry data at three months post-randomisation. As this is an ITT analysis, withdrawals and protocol violations will be analysed in their groups as randomised.
Analysis of primary objective
As the trial is a parallel group RCT data will be reported according to the revised CONSORT statement. 47 The statistical analyses will be performed on an intention-to-treat basis. All statistical exploratory tests will be two-tailed with alpha = 0.05. Baseline demographic variables (age, gender), physical measurements (e.g. weight, height, BMI), and health-related quality of life data (SF-36) will be summarised with appropriate summary statistics, tabulated and assessed for comparability between the treatment groups. For example, categorical variables (e.g. gender, the number and percentage who are male and female will be reported). For continuous variables, e.g. age, depending on the distribution of the data, if it is symmetric, the data will be summarised with a mean and standard deviation; if it has a non-symmetric distribution it will be summarised with a median and inter-quartile range.
The primary aim is to compare the intervention (Full or Mini Booster) versus control treatment (No booster). Secondary aims are to compare the two interventions (Full versus Mini booster). The primary comparison will be between the mean physical activity levels from the Actiheart accelerometer (average Total Energy Expenditure per day in Kcal, from a seven-day assessment) in the two ‘booster’ arms combined compared with the mean physical activity levels in the control arm at 6 months follow-up (3 months post-randomisation). This difference in means, between the intervention and control groups, will be compared using a two independent samples t-test and a 95% confidence interval for estimated mean difference between the groups will also be calculated. In the event of differences between the Booster and Control groups with respect to baseline demographic, physical, and health-related quality of life and accelerometer measurements, multiple regression will be used to adjust the treatment effect for these variables. The ordinary least squares adjusted regression coefficient estimate for the treatment group parameter along with its 95% confidence interval (CI) will then be reported.
The research hypothesis is that the booster interventions will have greater levels of physical activity than the control. The statistical and null hypothesis is that there are no differences between the intervention and control groups at follow up. The alternative hypothesis is that there is a difference in physical activity levels between the intervention and control groups at follow up.
Secondary aims are to compare the effect of the two interventions (Full versus Mini booster). This will be done using the same methods as for the primary endpoint as described above. Interim analyses will not be required. An exploratory sub-group analysis using multiple linear regression, with the primary outcome the mean physical activity levels from the Actiheart accelerometer (average Total Energy Expenditure per day in Kcal, from a seven-day assessment) at 6 months (3 months post-randomisation), will look for an interaction between treatment group (Booster or control) and sub-groups defined by gender, ethnicity and access to community facilities (self reported use versus no use of community facilities).
Analysis of secondary outcomes
Analyses will identify any significant different between groups for each outcome measure, at three months and nine months from randomisation:
-
Objective measures of physical activity from the Actiheart:
-
– Physical activity counts (PAC) per week;
-
– Minutes of moderate/vigorous physical activity per day;
-
– Meeting the current physical activity recommendation of at least 30 min per day (continuous or in bouts of at least 10 min of at least moderate intensity) for at least 5 days a week (yes or no).
-
-
Physiological measures of fitness (12 minute walk test) and types of physical activity (self report) and change in self-reported physical activity levels
-
Change in health-related quality of life measured by changes in SF-12v2 plus 4 (converted to SF-6D)
-
Health and social care contacts
-
Changes in psychological measures of motivation, intention and stages of change, and self-efficacy
Secondary categorical outcomes such as the proportions maintaining (or increasing) their weekly duration of physical activity in the two ‘booster’ arms combined compared with the proportion in the control arm at 6 months follow-up (3 months post-randomisation), will be compared between the intervention and control groups, using a continuity corrected chi squared test and a 95% confidence interval for estimated differences in proportions will also be calculated. In the event of differences between the groups with respect to baseline demographic, physical, and health-related quality of life measurements, multiple logistic regression will be used to adjust the treatment effect for these variables. The maximum likelihood estimated regression coefficient for the treatment group parameter (odds ratio) along with its 95% confidence interval (CI) will then be reported.
Secondary outcomes such as HRQoL (SF-12v2 plus 4 dimension scores) and distance walked on 12 minute walk test, at six month follow-up, will be assumed to be continuous outcomes. A two independent samples t-test will be used to compare mean outcomes between the Booster and control groups in this parameter. A 95% confidence interval (CI) for the mean difference in this parameter between the groups will also be calculated. In the event of differences between the Booster and Control groups with respect to baseline demographic, physical, and health-related quality of life measurements, multiple regression or analysis of covariance (ANCOVA) will be used to adjust the treatment effect for these variables. The ordinary least squares adjusted regression coefficient estimate for the treatment group parameter along with its 95% confidence interval (CI) will then be reported. Twelve month outcomes will be analysed in a similar way. We shall also compare the effect of the two interventions (Full versus Mini booster) on these secondary outcomes at 3 and 9 months post-randomisation, using the same methods as described above.
Economic analysis
The basic design of the health economic component of the study will be to estimate the incremental cost effectiveness of the mini-booster and full booster interventions compared to no booster. It will include an estimation of the cost effectiveness of the intervention from a NHS perspective in terms of their incremental cost per quality adjusted life year (QALY) and a broader societal assessment of efficiency that includes costs for other Government agencies and productivity (inside and outside the home). It uses similar methods to those used in the successfully completed evaluation of a community exercise programme. 48
There will be two components to the costing. The interventions will be costed, as well as the consequences for the use of health and social services in general. The costs of the booster consultations will be assessed in a micro costing study. The costs will include enrolment of participants, training and time of facilitators, travel and telephone calls. Actual cost data will be collected for consumables and facilitator time will be costed using national grades. Despite being a highly pragmatic trial, there are some features of the programme which are specific to the research study and it will be necessary to adjust for these in order to make the results generalisable. Care will also be taken to compare costs assuming a routine level of throughput, rather than that achieved in the trial. Any research related costs will be excluded.
The consequences for use of health and social services will use resource data collected from participants. Use of primary, secondary, community and social services will be obtained using a self-completed resource questionnaire administered to participants at each assessment at baseline, three months and nine months. Resources will be costed using the best available national estimates. Where appropriate, national unit costs will be used. 49
SF-12v2 plus 4 data will be converted into health state utility values using the SF-6D preference-based algorithm. 50 The area under the curve between assessments will be used to provide an overall estimate of the QALY difference between the intervention arms and the control arm after adjusting for significant baseline variables. 51 Given cost and benefit data will only be collected for nine months, the on-going costs and health benefits will not be discounted, though start-up costs, including training costs, will be annuitised over a five year period. The sensitivity of the results to possible uncertainties in key parameters will be explored by a full sensitivity analysis, including a probabilistic sensitivity analysis.
Trial supervision
Details of the composition of the Trial Steering Committee (TSC) and Trial Management Group (TMG) are given at the front of this protocol. Sheffield CTRU standard operating procedures Gov001 and Gov002 apply. Sheffield CTRU trials require an independent Chair for a TSC.
There is no interim analysis. The responsibility of the TSC is to evaluate serious adverse events and make decisions about the continuation or discontinuation of the trial on safety grounds. In the event that any women consented into the trial are, or become pregnant, the safety of these individuals will also be monitored by the TSC.
There is no Endpoint Review Committee.
Data handling and record keeping
Data from the study will be stored in accordance with the Archiving Standard Operating Procedure (Shef/CTRU/DM002) for at least 5 years following completion. It will be stored in a commercial archive in Sheffield, which will protect the data from damage by fire, water, etc. The data will be packed into boxes and labelled with a number, the study title/reference no., the sponsor, the investigator and date until which it is to be archived. Named individuals will be responsible for archiving the data and for retrieving data from the archives. It will be necessary for the named individuals to go to the commercial archive to physically retrieve the data. Access will be restricted to the investigator and regulatory authorities. Details of what is kept in the archive will be logged on a register. These details will be the same as is detailed on the archive box labels. When data is removed from the archive, this is also logged on a register by one of the named individuals. Electronic data will be stored in an ‘archive’ area of the secure CTRU server for a minimum of five years to ensure that access is future-proofed against changes in technology. Electronic data may also be stored (e.g. on a compact disc) with the paper files.
The detailed data management and data quality issues will be set out in a data management and monitoring protocol in conjunction with the CTRU database manager.
Publication
Dissemination will be undertaken through peer reviewed scientific journals and clinical and academic conferences.
Ethics approval
The protocol will be approved by North Sheffield Research Ethics Committee.
Indemnity/Compensation/Insurance
The University of Sheffield has in place insurance against liabilities for which it may be legally liable and this cover includes any such liabilities arising out of this research project.
References
- Hillsdon M, Foster C, Cavill N, Crombie H, Naidoo B. The Effectiveness of Public Health Interventions for Increasing Physical Activity among Adults: a Review of Reviews. London: Health Development Agency; 2005.
- Eaton CB, Menard LM. A systematic review of physical activity promotion in primary care office settings. Br J Sports Med 1998;32:11-6.
- Humpel N, Marshall AL, Iverson D, Leslie E, Owen N. Trial of print and telephone delivered interventions to influence walking. Prev Med 2004;39:635-41.
- Kahn EB, Ramsey LT, Brownson RC, Heath GW, Howze EH, Powell KE, et al. The effectiveness of interventions to increase physical activity – a systematic review. Am J Prev Med 2002;22:73-108.
- Lawlor DA, Hanratty B. The effect of physical activity advice given in routine primary care consultations: a systematic review. J Public Health Med 2001;23:219-26.
- Four Commonly Used Methods to Increase Physical Activity: Brief Interventions in Primary Care, Exercise Referral Schemes, Pedometers and Community-Based Exercise Programmes for Walking and Cycling. London: NICE; 2006.
- Miller WR, Sovereign RG, Miller WR, Nathan PE, Marlatt GA. Addictive Behaviors: Prevention and Early Intervention. Leiden, the Netherlans: Swets & Zeitlinger; 1989.
- Schippers GM, Brokken LCHM, Otten J. Doorlichting Voorlichting Alcoholgebruik (Drinker’s Check-up). Nijmegen: Bureau Beta; 1994.
- Marcus BH, Forsyth LH. Physical Activity Intervention Series: Motivating People to be Physically Active. Champaign, IL: Human Kinetics; 2003.
- Marshall SJ, Biddle SJH. The transtheoretical model of behavior change: a meta-analysis of applications to physical activity and exercise. Ann Behav Med 2001;23:229-46.
- Prochaska JO, Diclemente CC. Stages and processes of self-change of smoking – toward an integrative model of change. J Consult Clin Psychol 1983;51:390-5.
- Nigg CR, Courneya KS. Transtheoretical model: examining adolescent exercise behavior. J Adolescent Health 1998;22:214-24.
- Prochaska JO, Marcus BH, Dishman RK. Advances in Exercise Adherence. Champaign, IL: Human Kinetics; 1994.
- Woods C, Mutrie N, Scott M. Physical activity intervention: a transtheoretical model-based intervention designed to help sedentary young adults become active. Health Educ Res 2002;17:451-60.
- Prochaska JO, Diclemente CC, Norcross JC. In search of how people change – applications to addictive behaviors. Am Psychol 1992;47:1102-14.
- Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. Am J Health Promot 1997;12:38-4.
- Rollnick S, Miller WR. What is motivational interviewing?. Behav Cogn Psychol 1995;23:325-34.
- Hettema J, Steele J, Miller WR. Motivational interviewing. Annu Rev Clin Psychol 2005;1:91-111.
- Bennett JA, Lyons KS, Winters-Stone K, Nail LM, Scherer J. Motivational interviewing to increase physical activity in long-term cancer survivors – a randomized controlled trial. Nurs Res 2007;56:18-27.
- Berg-Smith SM, Stevens VJ, Brown KM, Van Horn L, Gernhofer N, Peters E, et al. A brief motivational intervention to improve dietary adherence in adolescents. Health Educ Res 1999;14:399-410.
- Knight KM, McGowan L, Dickens C, Bundy C. A systematic review of motivational interviewing in physical health care settings. Br J Health Psychol 2006;11:319-32.
- Atkinson NL, Gold RS. The promise and challenge of eHealth interventions. Am J Health Behav 2002;26:494-503.
- Hester RK, Squires DD, Delaney HD. The Drinker’s Check-up: 12-month outcomes of a controlled clinical trial of a stand-alone software program for problem drinkers. J Subst Abuse Treat 2005;28:159-69.
- Stotts AL, Diclemente CC, Dolan-Mullen P. One-to-one – a motivational intervention for resistant pregnant smokers. Addict Behav 2002;27:275-92.
- Pinto BM, Friedman R, Marcus BH, Kelley H, Tennstedt S, Gillman MW. Effects of a computer-based, telephone-counseling system on physical activity. Am J Prev Med 2002;23:113-20.
- Tate DF, Wing RR, Winett RA. Using Internet technology to deliver a behavioral weight loss program. JAMA 2001;285:1172-7.
- Tate DF, Jackvony EH, Wing RR. Effects of Internet behavioral counseling on weight loss in adults at risk for type 2 diabetes – a randomized trial. JAMA 2003;289:1833-6.
- Campbell MK, Devellis BM, Strecher VJ, Ammerman AS, Devellis RF, Sandler RS. Improving dietary behavior – the effectiveness of tailored messages in primary-care settings. Am J Public Health 1994;84:783-7.
- Skinner CS, Campbell MK, Rimer BK, Curry S, Prochaska JO. How effective is tailored print communication?. Ann Behav Med 1999;21:290-8.
- Mintel . DVD Players – UK – May 2007 2007. URL: http://oxygen.mintel.com/display/220132/ (accessed November 2013).
- Behaviour Change at Population, Community and Individual Levels. London: NICE; 2007.
- Rollnick S, Heather N, Bell A. Negotiating behaviour change in medical settings: the development of brief motivational interviewing. J Mental Health 1992;1:25-37.
- Britt E, Blampied NM, Hudson SM. Motivational interviewing: a review. Aus Psychol 2003;38:193-201.
- Ball K, Salmon J, Leslie E, Owen N, King AC. Piloting the feasibility and effectiveness of print- and telephone-mediated interventions for promoting the adoption of physical activity in Australian adults. J Sci Med Sport 2005;8:134-42.
- Green BB, McAfee T, Hindmarsh M, Madsen L, Caplow M, Buist D. Effectiveness of telephone support in increasing physical activity levels in primary care patients. Am J Prev Med 2002;22:177-83.
- Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, et al. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH behavior change consortium. Health Psychol 2004;23:443-51.
- Breckon JD, Johnston LH, Hutchison A. Physical activity counseling content and competency: a systematic review. J Phys Activity Health 2008;5:398-417.
- Tulloch H, Fortier M, Hogg W. Physical activity counseling in primary care: who has and who should be counseling?. Patient Educ Couns 2006;64:6-20.
- Birkett MA, Day SJ. Internal pilot studies for estimating sample size. Stat Med 1994;13:2455-63.
- Wittes J, Brittain E. The role of internal pilot studies in increasing the efficiency of clinical trials. Stat Med 1990;9:65-71.
- Ajzen I. Perceived behavioral control, self-efficacy, locus of control, and the theory of planned behavior. J Appl Soc Psychol 2002;32:665-83.
- Marcus BH, Selby VC, Niaura RS, Rossi JS. Self-efficacy and the stages of exercise behavior change. Res Q Exerc Sport 1992;63:60-6.
- Mullan E, Markland D, Ingledew DK. A graded conceptualisation of self-determination in the regulation of exercise behaviour: development of a measure using confirmatory factor analytic procedures. Pers Indiv Differ 1997;23:745-52.
- Isaacs AJ, Critchley JA, See Tai S, Buckingham K, Westley D, Harridge SDR, et al. Exercise Evaluation Randomised Trial (EXERT): a randomised trial comparing GP referral for leisure centre-based exercise, community-based walking and advice only. Health Technol Assess 2007;11.
- McGavin CR, Gupta SP, Mchardy GJR. Twelve-minute walking test for assessing disability in chronic bronchitis. Br Med J 1976;1:822-3.
- Lowther M, Mutrie N, Loughlan C, McFarlane C. Development of a Scottish physical activity questionnaire: a tool for use in physical activity interventions. Br J Sports Med 1999;33:244-9.
- Moher D, Schulz KF, Altman DG, Consort G. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet 2001;357:1191-4.
- Munro JF, Nicholl JP, Brazier JE, Davey R, Cochrane T. Cost effectiveness of a community based exercise programme in over 65 year olds: cluster randomised trial. J Epidemiol Community Health 2004;58:1004-10.
- Netten A, Curtis L. Unit Costs of Health and Social Care 2005. Canterbury: PSSRU, University of Kent; 2005.
- Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care 2004;42:851-9.
- Matthews JNS, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ 1990;300:230-5.
Appendix 2 Changes to protocol
| Change to Protocol | Progress Report | Date | Approved by |
|---|---|---|---|
| Protocol Version 2 (3rd Feb 2009), changes for accuracy, clarification and internal consistency were never approved by the REC | Not reported | – | Sheffield REC |
| Protocol Version 3 (25 Jun 09) Sheffield Research Ethics Committee approved a protocol amendment on 14 July 2009. The amendment related to the collection of data during the brief intervention (pre-randomisation or screening) phase of the study | 01 | 03 Aug 2009 | Sheffield REC |
| Protocol Version 04 (13 Jan 2011). This change involved the way the primary outcome was collected and analysed. The primary outcome remained an objective measure of physical activity obtained through seven-day accelerometry using Actiheart. However, the chair of the Trial Steering Committee recommended that we change the way this was calculated from the use of physical activity counts to energy expenditure. On the request of the NIHR HTA monitors the rationale for this change was externally peer reviewed and approved by the NIHR HTA (please see ‘Letter from HTA 04 Aug 2010.pdf’). The changes to the protocol reflected the decision of the funding body that the method of calculating the primary outcome should change. There was no difference to participants in terms of how data is collected | HTA instructed team to make change (correspondence: 04 Aug 2010); not reported due to an oversight | – | Sheffield REC |
| Protocol Version 5 (09 Mar 2011), given a favourable opinion by Sheffield REC on 06 Jun 2011, refers to a new piece of study documentation, the study introduction letter. This letter is sent to study candidates before the participant information sheet and delivers key information in simple language | 05 | 01 Aug 2011 | Sheffield REC |
Appendix 3 Motivational interviewing
Motivational interviewing: principles and key elements
Ground work and typical day
Using key skills in MI including Open ended questions, Affirmations of change talk, Reflective listening and Summarising (OARS) to elicit client attitudes towards adopting lifestyle change. This opening exchange will also ensure client centredness through empathetic listening and respectfulness. The main outcome is a greater understanding (for both the client and therapist) of the client’s current situation and their relevant health history and envisaging the impact of changing and not changing. TYPICAL DAY: Exploration (guiding and supporting the client) to understand a typical day and what ‘high risk’ scenario’s exist as well as opportunities for change and adapting current behaviours. Key skills used are again OARS.
Assess importance and readiness
A. How important is the change? (e.g. physical activity) (1–10) ( ) Why X Not Y?
B. How ready are you to change? (1–10) ( ) Why X Not Y?
C. How confident are you to maintain the change? (1–10) ( ) Why X Not Y?
This measure is mapped (conceptually) onto the transtheoretical model (TTM) of behaviour change,147 although, unlike most other applications, the MI interview integrates more than stages of change and incorporates ‘decisional balance’, ‘self-efficacy/temptation’ and ‘processes of change’. Many other interventions in health settings wrongly assume that stages of change and TTM are the same thing when stages of change is just one of four dimensions. This measure helps both parties understand the client readiness to change across his or her own selected health behaviour change aspects (i.e. smoking, diet, exercise, alcohol).
Assessing ambivalence
Whenever a client is considering change (from a position of contemplation, for example), ambivalence is a normal consequence. Eliciting all aspects of this position is important and can be done in MI using a four-cell decisional balance grid.
When we think about making changes, most of us don’t really consider all ‘sides’ in a complete way. Instead, we often do what we think we ‘should’ do, avoid doing things we don’t feel like doing, or just feel confused or overwhelmed and give up thinking about it at all.
Thinking through the pros and cons of both changing and not making a change is one way to help us make sure we have fully considered a possible change. This can help us to ‘hang on’ to our plan in times of stress or temptation. Below, write in the reasons that you can think of in each of the boxes.
| Benefits/pros | Costs/cons | |
|---|---|---|
| Making a change | ||
| Not changing |
Summary
Again, the key counselling skill applied here is reflective listening, in which the therapist draws together the main facets of the conversation and provides a reflective summary for the client. This enables the client to ‘listen back’ to the points they have raised themself and provides an opportunity to clarify points, adjust the inference or level of importance or merely have it verbalised from an objective source. This is an important phase in preparation for action planning and will include a recapitulation and asking key questions about subsequent stages.
Action plan
When there are clear signs of preparatory change talk,84 which are Desire, Ability, Reasons, Need (DARN), then greater elicitation and strengthening is required to increase the likelihood of predictor change talk, which is Commitment, Activation, Taking steps (CAT). Use the ‘ten strategies for eliciting and strengthening change talk’ worksheet to achieve this.
Motivational interviewing practice resources pack
Motivational interviewing practice resources pack (PDF download)
Appendix 4 Questionnaire for participants in the full booster arm
Questionnaire for participants in the full booster arm (PDF download)
Appendix 5 Interview topic guide
Introduction: Just like to talk to you about your experiences of taking part in the telephone/face-to-face ‘booster’/advice and whether this influenced you being physically active.
Prompts:
Firstly, how long did your session last?
Where did it take place? Was that convenient for you
What did you think of the advice session?
Can you tell us if you felt the advice session helped in any way to keep you active? How/why do you think that was? (Probe: attitude change, reaffirm message)
What else influenced you in being able to stay active or deterred you from staying active? (e.g. costs, facilities, personal circumstances, people to be active with, priorities, others who support you/discourage you from being active, motivation for being active). How/why?
Can you describe the things that motivate you the most to be physically active? (e.g. external/internal; why feel the need to do or not do something active?)
Can you describe if and how physical activity fits into your lifestyle? (i.e. explore habits/routines)
What type of activity/activities did you participate in and why? (Interviewer refer to survey and follow up answers)
What were you expecting from the study? Was it what you expected?
Prompt: have you received any advice like this before?
What benefits do you feel there are to staying active?
Prompts:
Physical/medical?
Mood/well-being?
Social?
Other behaviours, e.g. eating habits, smoking?
Saving money? (e.g. by walking/cycling as transport)
What disadvantages do you think there are to staying active?
Prompts:
Time
Cost
Planning
Was this a good way to give you advice/help you needed? Why?
Prompts:
What would you have preferred? What, if anything, would you change about the ‘booster’/support and advice for keeping active?
If a friend was thinking of becoming/staying active, how would you advise them to go about this?
Appendix 6 Results tables from the main trial
| Mail-out | Neighbourhoods | Letters sent, n | Replies received, n (%) | Randomised, n (%) |
|---|---|---|---|---|
| 1 | High Green | 3300 | 329 (10.0) | 48 (1.5) |
| 2 | Southey Green, Longley, New Parsons Cross, Old Parsons Cross, Shirecliffe, Tinsley, Darnall, Flower, Shiregreen, Stubbin, Brushes, Acres Hill, Winn Gardens | 15,366 | 1045 (6.8) | 88 (0.6) |
| 3 | Gleadless Valley, Hemsworth, Lowedges, Batemoor, Jordanthorpe, Manor, Woodthorpe, Park Hill, Norfolk Park, Arbourthorne, Tinsley, Darnall, Acres Hill | 18,784 | 974 (5.2) | 60 (0.3) |
| 4 | Broomhall, Sharrow (including the Abbeydale corridor), Highfield, Burngreave, Abbeyfield, Fir Vale, Firshill, Woodside, Netherthorpe, Upperthorpe, Langsett, Wybourn, City Centre | 16,072 | 1185 (7.4) | 52 (0.3) |
| 5 | Brightside, Firth Park, Fox Hill, Richmond, Wincobank | 6862 | 385 (5.6) | 8 (0.1) |
| 6 | Base Green, Beighton, Charnock, Gleadless, Halfway, Handsworth, Hollins End, Mosborough, Owlthorpe, Sothall | 10,000 | 836 (8.4) | 26 (0.3) |
| Variable | Control (n = 96) | Booster (n = 186) | Total (n = 282) |
|---|---|---|---|
| Gender, n (%) | |||
| Male | 37 (38.5) | 93 (50.0) | 130 (46.1) |
| Female | 59 (61.5) | 93 (50.0) | 152 (53.9) |
| Employment status, n (%) | |||
| Part-time | 18 (18.8) | 34 (18.3) | 52 (18.4) |
| Full-time | 34 (35.4) | 59 (31.7) | 93 (33.0) |
| Not employed | 44 (45.8) | 90 (48.4) | 134 (47.5) |
| Missing | 0 (0.0) | 3 (1.6) | 3 (1.1) |
| Ethnicity, n (%) | |||
| White British | 83 (86.5) | 163 (87.6) | 246 (87.2) |
| Other | 13 (13.5) | 20 (10.8) | 33 (11.7) |
| Missing | 0 (0.0) | 3 (1.6) | 3 (1.1) |
| Marital status, n (%) | |||
| Single | 16 (16.7) | 29 (15.6) | 45 (16.0) |
| Married | 50 (52.1) | 101 (54.3) | 151 (53.5) |
| Co-habiting | 5 (5.2) | 15 (8.1) | 20 (7.1) |
| Divorced/separated | 20 (20.8) | 35 (18.8) | 55 (19.5) |
| Widowed | 5 (5.2) | 6 (3.2) | 11 (3.9) |
| Stage of change, n (%) | |||
| Contemplation | 1 (1.0) | 11 (5.9) | 12 (4.3) |
| Preparation | 39 (40.6) | 86 (46.2) | 125 (44.3) |
| Action | 36 (37.5) | 55 (29.6) | 91 (32.3) |
| Maintenance | 18 (18.8) | 32 (17.2) | 50 (17.7) |
| Missing | 2 (2.1) | 2 (1.1) | 4 (1.4) |
| Age (years) | |||
| n (%) | 96 (100.0) | 186 (100.0) | 282 (100.0) |
| Mean (SD) | 54.5 (6.8) | 54.6 (7.6) | 54.6 (7.3) |
| Median (IQR) | 54.9 (50.3 to 60.0) | 55.6 (48.1 to 61.8) | 55.3 (48.8 to 61.4) |
| Min. to max. | 40.5 to 65.5 | 40.4 to 65.1 | 40.4 to 65.5 |
| Height (m) | |||
| n (%) | 95 (99.0) | 186 (100.0) | 281 (99.6) |
| Mean (SD) | 1.7 (0.1) | 1.7 (0.1) | 1.7 (0.1) |
| Median (IQR) | 1.7 (1.6–1.7) | 1.7 (1.6–1.8) | 1.7 (1.6–1.8) |
| Min. to max. | 1.5 to 1.9 | 1.5 to 1.9 | 1.5 to 1.9 |
| Weight (kg) | |||
| n (%) | 96 (100.0) | 186 (100.0) | 282 (100.0) |
| Mean (SD) | 84.5 (19.2) | 85.5 (18.5) | 85.2 (18.7) |
| Median (IQR) | 82.8 (70.1 to 95.0) | 83.0 (73.0 to 96.8) | 82.9 (72.5 to 96.6) |
| Min. to max. | 50.8 to 160.0 | 46.9 to 145.8 | 46.9 to 160.0 |
| BMI (kg/m2) | |||
| n (%) | 95 (99.0) | 186 (100.0) | 281 (99.6) |
| Mean (SD) | 30.3 (6.3) | 30.3 (5.7) | 30.3 (5.9) |
| Median (IQR) | 28.9 (25.7 to 33.0) | 29.8 (26.4 to33.2) | 29.8 (26.3 to 33.0) |
| Min. to max. | 20.3 to 53.4 | 17.1 to 49.4 | 17.1 to 53.4 |
| SPAQ changea (3 months post randomisation) | |||
| n (%) | 96 (100.0) | 186 (100.0) | 282 (100.0) |
| Mean (SD) | 199.8 (261.6) | 215.3 (416.1) | 210.0 (370.4) |
| Median (IQR) | 120.0 (75.0 to 255.0) | 120.0 (50.0 to 255.0) | 120.0 (60.0 to 255.0) |
| Min. to max. | −1010.0 to 1240.0 | −1840.0 to 3360.0 | −1840.0 to 3360.0 |
| SF-12v2 plus 4 (PCS) | |||
| n (%) | 90 (93.8) | 180 (96.8) | 270 (95.7) |
| Mean (SD) | 46.8 (11.2) | 46.2 (10.6) | 46.4 (10.8) |
| Median (IQR) | 51.8 (39.9 to 54.8) | 49.1 (39.6 to 53.9) | 49.9 (39.7 to 54.5) |
| Min. to max. | 13.9 to 60.7 | 13.0 to 66.7 | 13.0 to 66.7 |
| SF-12v2 plus 4 (MCS) | |||
| n (%) | 90 (93.8) | 180 (96.8) | 270 (95.7) |
| Mean (SD) | 47.8 (10.2) | 49.2 (9.6) | 48.7 (9.8) |
| Median (IQR) | 50.4 (41.7 to 54.9) | 51.0 (41.9 to 57.1) | 50.8 (41.9 to 56.2) |
| Min. to max. | 20.8 to 65.8 | 15.9 to 68.5 | 15.9 to 68.5 |
| BREQ-2 (RAI) | |||
| n (%) | 93 (96.9) | 181 (97.3) | 274 (97.2) |
| Mean (SD) | 5.4 (3.7) | 5.2 (3.7) | 5.3 (3.7) |
| Median (IQR) | 5.8 (3.3 to 7.9) | 6.3 (3.3 to 8.0) | 6.0 (3.3 to 8.0) |
| Min. to max. | −3.3 to 12.0 | −9.1 to 11.5 | −9.1 to 12.0 |
| Variable | Control (n = 61) | Booster (n = 99) | Total (n = 160) |
|---|---|---|---|
| Gender, n (%) | |||
| Male | 26 (42.6) | 42 (42.4) | 68 (42.5) |
| Female | 35 (57.4) | 57 (57.6) | 92 (57.5) |
| Employment status, n (%) | |||
| Part-time | 14 (23.0) | 19 (19.2) | 33 (20.6) |
| Not employed | 27 (44.3) | 55 (55.6) | 82 (51.3) |
| Ethnicity, n (%) | |||
| White British | 54 (88.5) | 89 (89.9) | 143 (89.4) |
| Any other | 7 (11.5) | 8 (8.1) | 15 (19.4) |
| Missing | 0 (0.0) | 2 (2.0) | 2 (1.3) |
| Marital status, n (%) | |||
| Single | 11 (18.0) | 13 (13.1) | 24 (15.0) |
| Married | 30 (49.2) | 55 (55.6) | 85 (53.1) |
| Co-habiting | 5 (8.2) | 6 (6.1) | 11 (6.9) |
| Divorced/separated | 11 (18.0) | 20 (20.2) | 31 (19.4) |
| Widowed | 4 (6.6) | 5 (5.1) | 9 (5.6) |
| Stage of change, n (%) | |||
| Contemplation | 0 (0.0) | 5 (5.1) | 5 (3.1) |
| Preparation | 24 (39.3) | 40 (40.4) | 64 (40.0) |
| Action | 23 (37.7) | 34 (34.3) | 57 (35.6) |
| Maintenance | 13 (21.3) | 19 (19.2) | 32 (20.0) |
| Missing | 1 (1.6) | 1 (1.0) | 2 (1.3) |
| Age (years) | |||
| n (%) | 61 (100.0) | 99 (100.0) | 160 (100.0) |
| Mean (SD) | 54.3 (7.0) | 55.3 (7.7) | 54.9 (7.4) |
| Median (IQR) | 54.0 (50.4 to 60.6) | 57.0 (48.5 to 62.3) | 56.0 (49.4 to 61.8) |
| Min. to max. | 40.5 to 65.5 | 40.5 to 65.1 | 40.5 to 65.5 |
| Height (m) | |||
| n (%) | 61 (100.0) | 99 (100.0) | 160 (100.0) |
| Mean (SD) | 1.7 (0.1) | 1.7 (0.1) | 1.7 (0.1) |
| Median (IQR) | 1.7 (1.6 to 1.7) | 1.6 (1.6 to 1.7) | 1.7 (1.6 to 1.7) |
| Min. to max. | 1.5 to 1.9 | 1.5 to 1.9 | 1.5 to 1.9 |
| Weight (kg) | |||
| n (%) | 61 (100.0) | 99 (100.0) | 160 (100.0) |
| Mean (SD) | 81.7 (15.1) | 82.1 (17.9) | 82.0 (16.9) |
| Median (IQR) | 82.0 (70.1 to 92.0) | 80.6 (70.1 to 91.1) | 80.6 (70.1 to 91.5) |
| Min. to max. | 50.8 to 115.0 | 47.1 to 142.2 | 47.1 to 142.2 |
| BMI (kg/m2) | |||
| n (%) | 61 (100.0) | 99 (100.0) | 160 (100.0) |
| Mean (SD) | 29.2 (5.0) | 29.6 (5.7) | 29.4 (5.4) |
| Median (IQR) | 27.9 (25.6 to 32.2) | 29.2 (25.5 to 32.2) | 28.9 (25.6 to 32.2) |
| Min. to max. | 20.3 to 43.7 | 17.1 to 49.4 | 17.1 to 49.4 |
| SPAQ change (3 months post randomisation) | |||
| n (%) | 61 (100.0) | 99 (100.0) | 160 (100.0) |
| Mean (SD) | 216.9 (243.1) | 188.6 (316.4) | 199.4 (290.2) |
| Median (IQR) | 120.0 (90.0 to 255.0) | 110.0 (45.0 to 225.0) | 120.0 (60.0 to 237.5) |
| Min. to max. | −210.0 to 1240.0 | −480.0 to 1740.0 | −480.0 to 1740.0 |
| SF-12v2 plus 4 (PCS) | |||
| n (%) | 58 (95.1) | 95 (96.0) | 153 (95.6) |
| Mean (SD) | 48.4 (9.3) | 47.3 (9.8) | 47.7 (9.6) |
| Median (IQR) | 52.0 (42.0 to 54.8) | 49.9 (42.8 to 54.3) | 50.7 (42.6 to 54.5) |
| Min. to max. | 17.5 to 60.6 | 20.7 to 60.9 | 17.5 to 60.9 |
| SF-12v2 plus 4 (MCS) | |||
| n (%) | 58 (95.1) | 95 (96.0) | 153 (95.6) |
| Mean (SD) | 47.7 (9.8) | 50.0 (8.7) | 49.1 (9.2) |
| Median (IQR) | 50.1 (41.1 to 54.9) | 51.2 (44.2 to 57.1) | 51.0 (43.2 to 56.3) |
| Min. to max. | 20.8 to 63.8 | 21.7 to 65.5 | 20.8 to 65.5 |
| BREQ-2 (RAI) | |||
| n (%) | 59 (96.7) | 96 (97.0) | 155 (96.9) |
| Mean (SD) | 5.6 (3.6) | 5.6 (3.6) | 5.6 (3.6) |
| Median (IQR) | 6.1 (3.4 to 8.0) | 6.4 (3.7 to 8.3) | 6.3 (3.5 to 8.3) |
| Min. to max. | −3.3 to 12.0 | −9.1 to 11.5 | −9.1 to 12.0 |
| Variable | Non-completers | Completers | ||||
|---|---|---|---|---|---|---|
| Control | Booster | All | Control | Booster | All | |
| Age (years) | ||||||
| n | 35 | 87 | 122 | 61 | 99 | 160 |
| Mean (SD) | 55.4 (6.6) | 54.3 (7.4) | 54.6 (7.2) | 54.7 (7.0) | 55.7 (7.7) | 55.3 (7.4) |
| Median (IQR) | 58 (50.5 to 60.3) | 52.9 (48.4 to 61.9) | 54.6 (48.9 to 60.7) | 54.7 (50.7 to 61.0) | 57.8 (49.0 to 62.7) | 56.4 (49.8 to 62.3) |
| Height (m) | ||||||
| n | 34 | 87 | 121 | 61 | 99 | 160 |
| Mean (SD) | 1.7 (0.1) | 1.7 (0.1) | 1.7 (0.1) | 1.7 (0.1) | 1.7 (0.1) | 1.7 (0.1) |
| Median (IQR) | 1.6 (1.6 to 1.7) | 1.7 (1.6 to 1.8) | 1.7 (1.6 to 1.8) | 1.7 (1.6 to 1.7) | 1.6 (1.6 to 1.7) | 1.7 (1.6 to 1.7) |
| Weight (kg) | ||||||
| n | 35 | 87 | 122 | 61 | 99 | 160 |
| Mean (SD) | 89.4 (24.1) | 89.4 (18.4) | 89.4 (20.1) | 81.7 (15.1) | 82.1 (17.9) | 82.0 (16.9) |
| Median (IQR) | 82.8 (69.0 to 105.0) | 87.7 (76.0 to 99.5) | 87.5 (75.9 to 101.9) | 82 (70.1 to 92.0) | 80.6 (70.1 to 91.1) | 80.6 (70.1 to 91.5) |
| BMI (kg/m2) | ||||||
| n | 34 | 87 | 121 | 61 | 99 | 160 |
| Mean (SD) | 32.2 (7.8) | 31.2 (5.7) | 31.5 (6.4) | 29.2 (5.0) | 29.6 (5.7) | 29.4 (5.4) |
| Median (IQR) | 30 (26.3 to 37.9) | 30.9 (26.9 to 34.0) | 30.9 (26.9 to 34.4) | 27.9 (25.6 to 32.2) | 29.2 (25.5 to 32.2) | 28.9 (25.6 to 32.2) |
| SPAQ change (3 months post randomisation) | ||||||
| n | 35 | 87 | 122 | 61 | 99 | 160 |
| Mean (SD) | 170.0 (292.2) | 245.7 (506.6) | 224.0 (455.6) | 216.9 (243.1) | 188.6 (316.4) | 199.4 (290.2) |
| Median (IQR) | 110 (60.0 to 270.0) | 120 (60.0 to 300.0) | 120 (60.0 to 275.0) | 120 (90.0 to 255.0) | 110 (45.0 to 225.0) | 120 (60.0 to 237.5) |
| SF-12v2 plus 4 (PCS) | ||||||
| n | 32 | 85 | 117 | 58 | 95 | 153 |
| Mean (SD) | 43.9 (13.6) | 45.0 (11.4) | 44.7 (12.0) | 48.4 (9.3) | 47.3 (9.8) | 47.7 (9.6) |
| Median (IQR) | 51.4 (32.1 to 54.2) | 48.0 (37.3 to 53.6) | 48.7 (35.8 to 53.9) | 52.0 (42.0 to 54.8) | 49.9 (42.8 to 54.3) | 50.7 (42.6 to 54.5) |
| SF-12v2 plus 4 (MCS) | ||||||
| n | 32 | 85 | 117 | 58 | 95 | 153 |
| Mean (SD) | 48.1 (11.1) | 48.3 (10.5) | 48.3 (10.6) | 47.7 (9.8) | 50.0 (8.7) | 49.1 (9.2) |
| Median (IQR) | 51.2 (43.1 to 55.5) | 49.8 (41.2 to 56.2) | 49.9 (41.2 to 56.2) | 50.1 (41.1 to 54.9) | 51.2 (44.2 to 57.1) | 51.0 (43.2 to 56.3) |
| BREQ-2 (RAI) | ||||||
| n | 34 | 85 | 119 | 59 | 96 | 155 |
| Mean (SD) | 5.0 (3.9) | 4.8 (3.9) | 4.8 (3.9) | 5.6 (3.6) | 5.6 (3.6) | 5.6 (3.6) |
| Median (IQR) | 5.5 (2.5 to 7.4) | 5.5 (2.0 to 7.8) | 5.5 (2.2 to 7.8) | 6.1 (3.4 to 8.0) | 6.4 (3.7 to 8.3) | 6.3 (3.5 to 8.3) |
| Gender | ||||||
| n | 35 | 87 | 122 | 61 | 99 | 160 |
| Male, n (%) | 11 (31.4) | 51 (58.6) | 62 (50.8) | 26 (42.6) | 42 (42.4) | 68 (42.5) |
| Female, n (%) | 24 (68.6) | 36 (41.4) | 60 (49.2) | 35 (57.4) | 57 (57.6) | 92 (57.5) |
| Ethnicity, n (%) | ||||||
| White British | 29 (82.9) | 74 (85.1) | 103 (84.4) | 54 (88.5) | 89 (89.9) | 143 (89.4) |
| Other | 6 (17.1) | 12 (13.8) | 18 (14.8) | 7 (11.5) | 8 (8.1) | 15 (9.4) |
| Missing | 0 (0.0) | 1 (1.1) | 1 (0.8 | 0 (0.0) | 2 (2.0) | 2 (1.3) |
| Employment status, n (%) | ||||||
| Part-time | 4 (11.4) | 15 (17.2) | 19 (15.6) | 14 (23.0) | 19 (19.2) | 33 (20.6) |
| Full-time | 14 (40.0) | 34 (39.1) | 48 (39.3) | 20 (32.8) | 25 (25.3) | 45 (28.1) |
| Not employed | 17 (48.6) | 35 (40.2) | 52 (42.6) | 27 (44.3) | 55 (55.6) | 82 (51.2) |
| Missing | 0 (0.0) | 3 (3.4) | 3 (2.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Variable | Completers (n = 160), n (%) | Non-completers (n = 122), n (%) | OR (95% CI)a | p-value | Interaction | |
|---|---|---|---|---|---|---|
| OR (95% CI)b | p-value | |||||
| Gender | ||||||
| Female | 92 (57.5) | 60 (49.2) | 1.40 (0.87 to 2.24) | 0.165 | 0.3 (0.1 to 0.9) | 0.034 |
| Male | 68 (42.5) | 62 (50.8) | 1.00 | |||
| Treatment group | ||||||
| Booster | 99 (61.9) | 87 (71.3) | 0.65 (0.39 to 1.08) | 0.098 | ||
| Control | 61 (38.1) | 35 (28.7) | 1.00 | NA | ||
| Treatment group | ||||||
| Full | 52 (33) | 42 (34) | 0.71 (0.40 to 1.27) | 0.249 | ||
| Mini | 47 (29) | 45 (37) | 0.60 (0.33 to 1.07) | 0.085 | NA | |
| Control | 61 (38) | 25 (27) | 1.00 | |||
| Employment status | ||||||
| Yes | 78 (48.8) | 70 (57.4) | 0.71 (0.44 to 1.14) | 0.151 | 0.5 (0.2 to 1.3) | 0.127 |
| No | 82 (51.3) | 52 (42.3) | 1.00 | |||
| Ethnicityc | ||||||
| n | 158 | 121 | ||||
| White British | 143 (90.5) | 103 (85.1) | 1.67 (0.80 to 3.46) | 0.171 | NA | |
| Any other | 15 (9.5) | 18 (14.9) | 1.00 | |||
| Variable | Non-completers | Completers | Mean difference (95% CI)a | p-valuea | Interaction | |||
|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | Effect (95% CI)b | p-valueb | |||
| Age (years) | 122 | 54.6 (7.2) | 160 | 55.3 (7.4) | 0.7 (−1.0 to 2.4) | 0.426 | 2.1 (−1.6 to 5.8) | 0.267 |
| BMI (kg/m2) | 121 | 31.5 (6.4) | 160 | 29.4 (5.4) | −2.0 (−3.4 to −0.6) | 0.005 | 1.5 (−1.5 to 4.5) | 0.320 |
| Weight (kg) | 122 | 89.4 (20.1) | 160 | 82.0 (16.9) | −7.4 (−11.7 to −3.1) | 0.001 | 0.5 (−8.9 to 9.8) | 0.924 |
| SF-12v2 plus 4 (PCS) | 117 | 44.7 (12.0) | 153 | 47.7 (9.6) | 3.1 (0.5 to 5.7) | 0.019 | −2.2 (−7.8 to 3.5) | 0.452 |
| SF-12v2 plus 4 (MCS) | 117 | 48.3 (10.6) | 153 | 49.1 (9.2) | 0.8 (−1.6 to 3.2) | 0.508 | 2.1 (−3.1 to 7.2) | 0.430 |
| Height (m) | 121 | 1.7 (0.1) | 160 | 1.7 (0.1) | −0.0 (−0.0 to 0.0) | 1.000 | −0.0 (−0.1 to 0.0) | 0.080 |
| SPAQ changec | 122 | 199.8 (261.6) | 160 | 215.3 (416.1) | −15.5 (−95.3 to 64.3) | 0.703 | −104.0 (−292.6 to 84.6) | 0.279 |
| BREQ-2 (RAI) | 119 | 4.8 (3.9) | 155 | 5.6 (3.6) | 0.8 (−0.1 to 1.7) | 0.077 | 0.2 (−1.7 to 2.1) | 0.843 |
| Outcome | Control (n = 61), mean (SD) | Booster (n = 99), mean (SD) | Mean difference (95% CI)a | p-valuea | Adjusted mean difference (95% CI)b | p-valuec |
|---|---|---|---|---|---|---|
| Mean TEE per day (kcal) | 2265.9 (410.8) | 2226.9 (422.6) | −39.0 (−173.4 to 95.4) | 0.567 | −40.3 (−117.2 to 36.6) | 0.302 |
| Mean TEE per day (kcal) | Control, mean (SD) | Booster, mean (SD) | Mean difference (95% CI)a | p-valuea | Adjusted mean difference (95% CI)b | p-valuec |
|---|---|---|---|---|---|---|
| Multiple imputation (≥ 4 days) | (n = 61) | (n = 99) | ||||
| 2252.3 (393.6) | 2215.1 (366.0) | −37.2 (−174.9 to 100.6) | 0.595 | −34.5 (−118.1 to 49.0) | 0.415 | |
| Regression imputation (≥ 4 days) | (n = 58) | (n = 95) | ||||
| 2263.1 (398.6) | 2245.2 (409.4) | −18.0 (−151.5 to 115.5) | 0.790 | −27.0 (−97.3 to 43.3) | 0.449 | |
| Complete cases (7 complete days) | (n = 44) | (n = 68) | ||||
| 2241.7 (393.6) | 2222.5 (366.0) | −19.2 (−163.8 to 125.4) | 0.793 | −31.2 (−114.3 to 51.9) | 0.458 | |
| Per protocold | (n = 61) | (n = 96) | ||||
| 2265.9 (410.8) | 2231.3 (426.6) | −34.6 (−170.6 to 101.4) | 0.616 | −35.6 (−112.5 to 41.3) | 0.362 | |
| Multiple imputation (≥ 1 day) | (n = 70) | (n = 113) | ||||
| 2194.0 (393.6) | 2215.3 (366.0) | 21.3 (−133.1 to 175.7) | 0.786 | 9.9 (−111.3 to 131.1) | 0.872 | |
| Mean TEE per day (kcal)e | (n = 61) | (n = 98) | ||||
| 2265.9 (410.8) | 2211.4 (395.5) | −54.5 (−183.8 to 74.8) | 0.406 | −43.6 (−119.9 to 32.7) | 0.260 |
| Outcome | Control (n = 61), mean (SD) | Full booster (n = 52), mean (SD) | Mean difference (95% CI)a | p-valuea | Adjusted mean difference (95% CI)b | p-valuec |
|---|---|---|---|---|---|---|
| Mean TEE per day (kcal) | 2265.9 (410.8) | 2279.9 (425.6) | 14.0 (−142.2 to 170.2) | 0.859 | −6.7 (−96.6 to 83.2) | 0.883 |
| Outcome | Control (n = 61), mean (SD) | Mini booster (n = 47), mean (SD) | Mean difference (95% CI)a | p-valuea | Adjusted mean difference (95% CI)b | p-valuec |
|---|---|---|---|---|---|---|
| Mean TEE per day (kcal) | 2265.9 (410.8) | 2168.2 (415.8) | −97.7 (−256.6 to 61.2) | 0.226 | −69.6 (−165.4 to 26.2) | 0.153 |
| Outcome | Mini booster, mean (SD) | Full booster, mean (SD) | Mean difference (95% CI)a | p-valuea | Adjusted mean difference (95% CI)b | p-valuec |
|---|---|---|---|---|---|---|
| Mean TEE per day (kcal) | (n = 47) | (n = 52) | ||||
| 2168.2 (415.8) | 2279.9 (425.6) | 111.7 (−56.5 to 279.9) | 0.190 | 55.5 (−38.2 to 149.2) | 0.242 | |
| Mean TEE per day (kcal)d | (n = 47) | (n = 51) | ||||
| 2168.2 (415.8) | 2251.2 (375.6) | 82.9 (−75.8 to 241.6) | 0.302 | 47.0 (−45.2 to 139.2) | 0.314 |
| Outcome | Control, mean (SD) | Booster, mean (SD) | Mean difference (95% CI)a | p-valuea | Adjusted mean difference (95% CI)b | p-valuec |
|---|---|---|---|---|---|---|
| Mean TEE per day (kcal) | (n = 36) | (n = 55) | ||||
| 2177.2 (390.7) | 2308.2 (646.3) | 131.0 (−107.5 to 369.5) | 0.278 | 51.5 (−137.2 to 240.2) | 0.589 | |
| Mean TEE per day (kcal)d | (n = 36) | (n = 54) | ||||
| 2177.2 (390.7) | 2239.1 (397.1) | 61.9 (−106.8 to 230.6) | 0.468 | −7.1 (−115.8 to 101.6) | 0.897 |
| Mean TEE per day (kcal) | Control, mean (SD) | Booster, mean (SD) | Mean difference (95% CI)a | p-valuea | Adjusted mean difference (95% CI)b | p-valuec |
|---|---|---|---|---|---|---|
| Multiple imputation (≥ 4 days) | (n = 36) | (n = 55) | ||||
| 2163.0 (298.9) | 2235.2 (395.5) | 72.3 (−101.8 to 246.3) | 0.411 | 18.1 (−102.9 to 139.1) | 0.766 | |
| Regression imputation (≥ 4 days) | (n = 34) | (n = 52) | ||||
| 2202.0 (371.3) | 2281.7 (379.8) | 79.6 (−85.5 to 244.7) | 0.341 | 13.9 (−80.1 to 107.9) | 0.769 | |
| Complete cases | (n = 21) | (n = 39) | ||||
| 2118.1 (298.9) | 2315.5 (726.2) | 197.5 (−134.8 to 529.8) | 0.239 | 118.6 (−152.7 to 389.9) | 0.384 | |
| Complete casesd | (n = 21) | (n = 38) | ||||
| 2118.1 (298.9) | 2217.5 (395.5) | 99.4 (−99.1 to 297.9) | 0.320 | 31.7 (−88.7 to 152.1) | 0.599 | |
| Multiple imputation (≥ 1 days) | (n = 37) | (n = 61) | ||||
| 2168.4 (298.9) | 2215.9 (395.5) | 47.5 (−122.5 to 217.5) | 0.581 | 14.5 (−105.6 to 134.6) | 0.811 | |
| Per protocol | (n = 36) | (n = 55) | ||||
| 2177.2 (390.7) | 2308.2 (646.3) | 131.0 (−107.5 to 369.5) | 0.278 | 51.5 (−137.2 to 240.2) | 0.589 | |
| Per protocold | (n = 36) | (n = 54) | ||||
| 2177.2 (390.7) | 2239.1 (397.1) | 61.9 (−106.8 to 230.6) | 0.468 | −7.1 (−115.8 to 101.6) | 0.897 |
| Outcome | Mini booster, mean (SD) | Full booster, mean (SD) | Mean difference (95% CI)a | p-valuea | Adjusted mean difference (95% CI)b | p-valuec |
|---|---|---|---|---|---|---|
| Mean TEE per day (kcal) | (n = 25) | (n = 30) | ||||
| 2204.0 (415.9) | 2395.1 (785.7) | 191.1 (−159.3 to 541.5) | 0.279 | 100.9 (−203.8 to 405.6) | 0.508 | |
| Mean TEE per day (kcal)d | (n = 25) | (n = 29) | ||||
| 2204.0 (415.9) | 2269.4 (384.9) | 65.4 (−153.4 to 284.2) | 0.551 | −9.3 (−175.2 to 156.6) | 0.911 |
| Outcome | Control, mean (SD) | Booster, mean (SD) | Mean difference (95% CI)a | p-valuea | Adjusted mean difference (95% CI)b | p-valuec |
|---|---|---|---|---|---|---|
| PACs per week (×1000) | (n = 61) | (n = 99) | ||||
| 339.0 (146.0) | 331.7 (169.4) | −7.2 (−59.0 to 44.5) | 0.783 | 0.4 (−49.9 to 50.6) | 0.988 | |
| PACs per week (×1000)d | (n = 61) | (n = 98) | ||||
| 339.0 (146.0) | 324.4 (154.0) | −14.5 (−63.1 to 34.1) | 0.556 | −7.1 (−53.3 to 39.2) | 0.763 |
| PACs per week (×1000) | Control, mean (SD) | Booster, mean (SD) | Mean difference (95% CI)a | p-valuea | Adjusted mean difference (95% CI)b | p-valuec |
|---|---|---|---|---|---|---|
| Multiple imputation (≥ 4 days) | (n = 61) | (n = 99) | ||||
| 379.4 (135.0) | 368.5 (155.6) | –10.9 (–77.6 to 55.8) | 0.747 | –2.7 (–67.1 to 61.8) | 0.935 | |
| Regression imputation (≥ 4 days) | (n = 58) | (n = 95) | ||||
| 332.5 (133.8) | 328.1 (157.1) | –4.4 (–53.4 to 44.6) | 0.859 | –1.6 (–48.0 to 44.9) | 0.946 | |
| Complete cases (7 complete days) | (n = 44) | (n = 68) | ||||
| 325.1 (134.9) | 322.6 (155.6) | –2.6 (–59.3 to 54.1) | 0.929 | –12.3 (–65.1 to 40.4) | 0.644 | |
| Per protocol | (n = 61) | (n = 96) | ||||
| 339.0 (146.0) | 335.6 (170.5) | –3.3 (–55.6 to 48.9) | 0.900 | 2.9 (–47.8 to 53.6) | 0.910 | |
| Per protocold | (n = 61) | (n = 95) | ||||
| 339.0 (146.0) | 328.2 (154.9) | –10.8 (–59.9 to 38.3) | 0.665 | –4.9 (–51.6 to 41.8) | 0.836 |
| Outcome | Control, mean (SD) | Booster, mean (SD) | Mean difference (95% CI)a | p-valuea | Adjusted mean difference (95% CI)b | p-valuec |
|---|---|---|---|---|---|---|
| PACs per week (×1000) | (n = 36) | (n = 55) | ||||
| 319.0 (158.9) | 315.4 (202.1) | –3.6 (–83.0 to 75.7) | 0.928 | –14.5 (–90.8 to 61.7) | 0.705 | |
| PACs per week (×1000)d | (n = 36) | (n = 54) | ||||
| 319.0 (158.9) | 302.5 (179.6) | –16.6 (–90.0 to 56.8) | 0.655 | –29.4 (–100.0 to 41.4) | 0.411 |
| PACs per week (×1000) | Control, mean (SD) | Booster, mean (SD) | Mean difference (95% CI)a | p-valuea | Adjusted mean difference (95% CI)b | p-valuec |
|---|---|---|---|---|---|---|
| Multiple imputation (≥ 4 days) | (n = 36) | (n = 55) | ||||
| 351.2 (102.0) | 353.2 (176.0) | 19.8 (–120.0 to 123.8) | 0.974 | –1.3 (–120.0 to 117.5) | 0.982 | |
| Regression imputation (≥ 4 days) | (n = 34) | (n = 52) | ||||
| 323.0 (151.6) | 319.8 (188.2) | –3.3 (–79.9 to 73.4) | 0.933 | –13.5 (–83.3 to 56.3) | 0.702 | |
| Complete cases (7 complete days) | (n = 21) | (n = 39) | ||||
| 299.4 (102.0) | 295.0 (176.0) | –4.4 (–88.1 to 79.3) | 0.916 | –15.2 (–110.0 to 74.9) | 0.735 | |
| Complete cases (7 complete days)d | (n = 21) | (n = 37) | ||||
| 299.4 (102.0) | 265.8 (123.7) | –33.7 (–97.4 to 30.0) | 0.294 | –37.3 (–110.0 to 31.6) | 0.282 | |
| Per protocol | (n = 36) | (n = 55) | ||||
| 319.0 (158.9) | 315.4 (202.1) | –3.6 (–83.0 to 75.7) | 0.928 | –14.5 (–90.8 to 61.7) | 0.705 | |
| Per protocold | (n = 36) | (n = 54) | ||||
| 319.0 (158.9) | 302.5 (179.6) | –16.6 (–90.0 to 56.8) | 0.655 | –29.4 (–100.0 to 41.4) | 0.411 |
| Follow-up (months) | Outcome | Control | Booster | Mean difference (95% CI)a | p-valuea | Adjusted mean difference (95% CI)b | p-valuec | ||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | ||||||
| 3 | PCS score | 57 | 49.3 (8.9) | 89 | 48.8 (9.9) | 0.7 (–1.4 to 2.7) | 0.509 | 0.9 (–1.2 to 2.9) | 0.394 |
| MCS score | 57 | 47.2 (9.5) | 89 | 49.5 (9.1) | 0.8 (–1.5 to 3.1) | 0.499 | 0.8 (–1.6 to 3.1) | 0.525 | |
| SF-6D utility score | 56 | 0.644 (0.064) | 88 | 0.648 (0.070) | 0.002 (–0.017 to 0.021) | 0.826 | 0.005 (–0.014 to 0.024) | 0.623 | |
| 9 | PCS score | 38 | 46.1 (9.4) | 62 | 48.3 (10.4) | 1.8 (–1.2 to 4.7) | 0.244 | 1.7 (–1.2 to 4.6) | 0.260 |
| MCS score | 38 | 48.3 (10.4) | 62 | 52.3 (8.7) | 2.0 (–0.7 to 4.8) | 0.149 | 1.7 (–1.1 to 4.5) | 0.237 | |
| SF-6D utility score | 38 | 0.651 (0.083) | 62 | 0.665 (0.080) | –0.000 (–0.028 to 0.028) | 0.989 | 0.001 (–0.027 to 0.030) | 0.921 | |
| Follow-up (months) | Outcome | Control | Booster | Mean difference (95% CI)a | p-valuea | Adjusted mean difference (95% CI)b | p-valuec | ||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | ||||||
| 3 | PCS score | 70 | 48.6 (9.7) | 118 | 48.8 (9.6) | 0.7 (–1.1 to 2.5) | 0.449 | 0.8 (–0.9 to 2.6) | 0.354 |
| MCS score | 70 | 47.9 (9.4) | 118 | 48.8 (10.2) | –0.2 (–2.4 to 2.0) | 0.849 | –0.3 (–2.6 to 1.9) | 0.770 | |
| SF-6D utility score | 71 | 0.645 (0.071) | 117 | 0.649 (0.075) | –0.001 (–0.017 to 0.016) | 0.920 | 0.001 (–0.016 to 0.018) | 0.934 | |
| 9 | PCS score | 46 | 46.2 (9.0) | 81 | 48.6 (10.1) | 2.2 (–0.5 to 4.9) | 0.102 | 1.8 (–0.9 to 4.5) | 0.191 |
| MCS score | 46 | 49.1 (10.2) | 81 | 50.4 (9.3) | –0.2 (–3.0 to 2.6) | 0.892 | –0.4 (–3.3 to 2.6) | 0.804 | |
| SF-6D utility score | 47 | 0.651 (0.083) | 81 | 0.655 (0.077) | –0.004 (–0.028 to 0.021) | 0.755 | –0.005 (–0.030 to 0.020) | 0.709 | |
| Outcome | Control (n = 61), mean (SD) | Booster (n = 99), mean (SD) | Mean difference (95% CI)a | p-valuea | Adjusted mean difference (95% CI)b | p-valuec |
|---|---|---|---|---|---|---|
| At least moderate activity (minutes) | 48.4 (42.9) | 46.8 (39.6) | –1.6 (–14.7 to 11.5) | 0.810 | –1.9 (–15.1 to 11.3) | 0.776 |
| Moderate activity (minutes) | 47.1 (42.1) | 44.7 (37.9) | –2.3 (–15.0 to 10.4) | 0.721 | –2.4 (–15.1 to 10.3) | 0.710 |
| Vigorous activity (minutes) | 1.3 (2.4) | 2.0 (4.2) | 0.7 (–0.5 to 1.9) | 0.236 | 0.5 (–0.7 to 1.7) | 0.409 |
| Outcome | Control, mean (SD) | Booster, mean (SD) | Mean difference (95% CI)a | p-valuea | Adjusted mean difference (95% CI)b | p-valuec |
|---|---|---|---|---|---|---|
| Main analysis | (n = 36) | (n = 55) | ||||
| At least moderate activity (minutes) | 36.6 (22.7) | 50.8 (71.9) | 14.2 (–10.4 to 38.8) | 0.255 | 17.0 (–7.9 to 41.9) | 0.177 |
| Moderate activity (minutes) | 35.7 (22.2) | 44.9 (47.9) | 9.1 (–7.9 to 26.1) | 0.289 | 11.8 (–5.1 to 28.7) | 0.168 |
| Vigorous activity (minutes) | 0.9 (2.2) | 6.0 (29.0) | 5.1 (–4.6 to 14.8) | 0.297 | 5.2 (–4.8 to 15.2) | 0.306 |
| Sensitivity analysisd | (n = 36) | (n=54) | ||||
| At least moderate activity (minutes) | 36.6 (22.7) | 42.5 (36.7) | 5.8 (–7.8 to 19.4) | 0.400 | 8.9 (–5.0 to 22.8) | 0.208 |
| Moderate activity (minutes) | 35.7 (22.2) | 40.3 (34.6) | 4.6 (–8.3 to 17.5) | 0.482 | 7.6 (–5.5 to 20.7) | 0.252 |
| Vigorous activity (minutes) | 0.9 (2.2) | 2.1 (4.7) | 1.2 (–0.5 to 2.9) | 0.159 | 1.3 (–0.5 to 3.1) | 0.150 |
| Follow-up (months) | Outcome | Control, n (%) | Booster, n (%) | Difference in proportion maintaining or increasing physical activity (95% CI) (%)a | p-valuea |
|---|---|---|---|---|---|
| 3 | Maintained or increased physical activity | (n = 60) | (n = 96) | ||
| 30 (50.0) | 55 (57.3) | 7.3 (–8.8 to 23.3) | 0.374 | ||
| 9 | Maintained or increased physical activity | (n = 44) | (n = 66) | ||
| 23 (52.3) | 41 (62.1) | 9.8 (–9.0 to 28.7) | 0.305 |
| Follow-up (months) | Outcome | Control, n (%) | Booster, n (%) | Difference in proportion maintaining or increasing physical activity (95% CI) (%)a | p-valuea |
|---|---|---|---|---|---|
| 3 | Maintained or increased activity | (n = 76) | (n = 125) | ||
| 41 (53.9) | 71 (56.8) | 2.9 (–11.0 to 17.1) | 0.693 | ||
| 9 | Maintained or increased activity | (n = 54) | (n = 85) | ||
| 25 (46.3) | 49 (57.6) | 11.4 (–5.6 to 28.3) | 0.191 |
| Outcome | Control | Booster | Mean difference (95% CI)a | p-valueb | Adjusted mean difference (95% CI)c | p-valued | ||
|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | |||||
| Amotivation | 75 | 0.3 (0.5) | 123 | 0.2 (0.5) | –0.0 (–0.2 to 0.1) | 0.515 | –0.0 (–0.2 to 0.1) | 0.762 |
| External regulation | 75 | 0.3 (0.5) | 123 | 0.4 (0.6) | 0.1 (–0.1 to 0.2) | 0.406 | 0.1 (–0.1 to 0.2) | 0.408 |
| Introjected regulation | 76 | 1.1 (1.0) | 123 | 1.4 (1.2) | 0.3 (0.1 to 0.6) | 0.018 | 0.3 (–0.0 to 0.6) | 0.053 |
| Identified regulation | 75 | 2.9 (0.7) | 123 | 3.0 (0.6) | 0.2 (0.0 to 0.3) | 0.047 | 0.1 (–0.0 to 0.3) | 0.092 |
| Intrinsic regulation | 73 | 2.8 (1.0) | 120 | 2.9 (1.0) | 0.2 (–0.0 to 0.4) | 0.077 | 0.2 (–0.0 to 0.4) | 0.103 |
| RAI | 73 | 6.2 (3.4) | 120 | 6.2 (3.3) | 0.3 (–0.5 to 1.0) | 0.478 | 0.2 (–0.5 to 0.9) | 0.606 |
| Outcome | Control | Booster | Mean difference (95% CI)a | p-valueb | Adjusted mean difference (95% CI)c | p-valued | ||
|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | |||||
| Amotivation | 54 | 0.3 (0.6) | 84 | 0.2 (0.5) | −0.0 (−0.2 to 0.1) | 0.751 | −0.0 (−0.2 to 0.1) | 0.785 |
| External regulation | 54 | 0.3 (0.6) | 84 | 0.3 (0.5) | 0.0 (−0.2 to 0.2) | 0.895 | 0.0 (−0.2 to 0.2) | 0.939 |
| Introjected regulation | 54 | 1.2 (1.1) | 84 | 1.4 (1.1) | 0.1 (−0.2 to 0.4) | 0.479 | 0.1 (−0.2 to 0.4) | 0.657 |
| Identified regulation | 55 | 2.9 (0.7) | 84 | 3.0 (0.6) | 0.1 (−0.1 to 0.3) | 0.412 | 0.1 (−0.1 to 0.3) | 0.515 |
| Intrinsic regulation | 54 | 2.8 (1.1) | 81 | 2.8 (1.0) | 0.2 (−0.1 to 0.4) | 0.239 | 0.2 (−0.1 to 0.4) | 0.210 |
| RAI | 53 | 6.0 (3.8) | 81 | 6.1 (3.5) | 0.2 (−0.8 to 1.2) | 0.711 | 0.2 (−0.8 to 1.3) | 0.649 |
| Outcome | Control | Booster | Mean difference (95% CI)a | p-valueb | Adjusted mean difference (95% CI)c | p-valued | ||
|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | |||||
| Amotivation | 59 | 0.3 (0.5) | 95 | 0.2 (0.5) | −0.1 (−0.2 to 0.1) | 0.510 | −0.0 (−0.2 to 0.1) | 0.735 |
| External regulation | 59 | 0.3 (0.5) | 95 | 0.4 (0.6) | 0.0 (−0.1 to 0.2) | 0.514 | 0.0 (−0.1 to 0.2) | 0.565 |
| Introjected regulation | 60 | 1.1 (1.1) | 95 | 1.4 (1.2) | 0.2 (−0.1 to 0.5) | 0.175 | 0.1 (−0.2 to 0.5) | 0.434 |
| Identified regulation | 59 | 2.9 (0.8) | 95 | 3.1 (0.6) | 0.2 (0.1 to 0.4) | 0.011 | 0.2 (0.0 to 0.4) | 0.020 |
| Intrinsic regulation | 57 | 2.8 (1.0) | 92 | 3.0 (0.9) | 0.3 (0.0 to 0.5) | 0.028 | 0.2 (0.0 to 0.5) | 0.049 |
| RAI | 57 | 5.8 (3.4) | 92 | 6.4 (3.4) | 0.6 (−0.3 to 1.5) | 0.168 | 0.6 (−0.3 to 1.4) | 0.192 |
| Outcome | Control | Booster | Mean difference (95% CI)a | p-valueb | Adjusted mean difference (95% CI)c | p-valued | ||
|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | |||||
| Amotivation | 44 | 0.3 (0.6) | 65 | 0.2 (0.3) | −0.1 (−0.2 to 0.1) | 0.386 | −0.1 (−0.2 to 0.1) | 0.454 |
| External regulation | 44 | 0.3 (0.6) | 65 | 0.2 (0.4) | −0.1 (−0.2 to 0.1) | 0.328 | −0.1 (−0.3 to 0.1) | 0.340 |
| Introjected regulation | 44 | 1.3 (1.1) | 65 | 1.4 (1.1) | −0.0 (−0.4 to 0.3) | 0.894 | −0.1 (−0.4 to 0.2) | 0.527 |
| Identified regulation | 44 | 2.9 (0.7) | 65 | 3.0 (0.6) | 0.1 (−0.1 to 0.3) | 0.483 | 0.1 (−0.2 to 0.3) | 0.558 |
| Intrinsic regulation | 43 | 2.8 (1.1) | 62 | 3.0 (0.9) | 0.2 (−0.0 to 0.5) | 0.072 | 0.3 (0.0 to 0.6) | 0.043 |
| RAI | 43 | 5.9 (3.9) | 62 | 6.8 (2.6) | 0.7 (−0.4 to 1.7) | 0.218 | 0.8 (−0.3 to 1.9) | 0.169 |
| Follow-up (months) | Outcome | Control | Booster | Mean difference (95% CI)a | p-valueb | Adjusted mean difference (95% CI)c | p-valued | ||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | ||||||
| 3 | BMI (kg/m2) | 75 | 29.6 (5.9) | 124 | 30.1 (5.5) | −0.2 (−0.5 to 0.1) | 0.194 | −0.1 (−0.4 to 0.2) | 0.385 |
| 9 | BMI (kg/m2) | 55 | 28.4 (5.4) | 85 | 29.1 (5.1) | 0.1 (−0.3 to 0.6) | 0.601 | 0.1 (−0.4 to 0.6) | 0.594 |
| Follow-up (months) | Outcome | Control | Booster | Mean difference (95% CI)a | p-valueb | Adjusted mean difference (95% CI)c | p-valued | ||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | ||||||
| 3 | BMI (kg/m2) | 59 | 29.0 (4.5) | 95 | 29.6 (5.5) | −0.2 (−0.6 to 0.1) | 0.160 | −0.2 (−0.5 to 0.1) | 0.300 |
| 9 | BMI (kg/m2) | 44 | 28.4 (4.9) | 66 | 28.6 (5.2) | 0.1 (−0.5 to 0.6) | 0.844 | 0.1 (−0.5 to 0.6) | 0.839 |
| Follow-up (months) | Outcome | Control | Booster | Mean difference (95% CI)a | p-valuea | Adjusted mean difference (95% CI)b | p-valuec | ||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | ||||||
| 3 | Total minutes of physical activity | 60 | 383.8 (404.5) | 96 | 419.8 (527.9) | 49.5 (−109.4 to 206.3) | 0.545 | 32.1 (−131.1 to 195.3) | 0.698 |
| 9 | Total minutes of physical activity | 44 | 461.0 (457.4) | 66 | 535.0 (560.2) | 74.0 (−128.2 to 276.2) | 0.470 | 120.0 (−92.1 to 332.1) | 0.264 |
| Follow-up (months) | Outcome | Control | Booster | Mean difference (95% CI)a | p-valuea | Adjusted mean difference (95% CI)b | p-valuec | ||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | ||||||
| 3 | Total minutes of physical activity | 76 | 458.0 (681.2) | 125 | 411.2 (498.3) | −35.4 (−200.0 to 129.2) | 0.672 | −52.5 (−222.6 to 117.6) | 0.543 |
| 9 | Total minutes of physical activity | 54 | 446.1 (472.4) | 81 | 508.6 (597.4) | 62.4 (−128.4 to 253.3) | 0.519 | 88.4 (−114.3 to 291.1) | 0.390 |
| Follow-up (months) | Outcome | Control, mean (SD) | Booster, mean (SD) | Mean difference (95% CI)a | p-valuea | Adjusted mean difference (95% CI)b | p-valuec |
|---|---|---|---|---|---|---|---|
| 3 | Distance walked (m) on 12-minute walk test | (n = 75) | (n = 118) | ||||
| 898.9 (257.5) | 962.4 (227.5) | 63.5 (−6.3 to 133.3) | 0.074 | 80.3 (13.8 to 146.8) | 0.018 | ||
| 9 | Distance walked (m) on 12-minute walk test | (n = 51) | (n = 82) | ||||
| 992.2 (292.7) | 1077.4 (301.0) | 85.2 (−19.9 to 190.3) | 0.111 | 80.6 (−26.8 to 188.0) | 0.140 |
| Follow-up (months) | Outcome | Control, mean (SD) | Booster, mean (SD) | Mean difference (95% CI)a | p-valuea | Adjusted mean difference (95% CI)b | p-valuec |
|---|---|---|---|---|---|---|---|
| 3 | Distance walked (m) on 12-minute walk test | (n = 59) | (n = 91) | ||||
| 917.6 (254.9) | 984.2 (226.2) | 66.6 (−12.0 to 145.2) | 0.096 | 90.8 (14.5 to 167.1) | 0.020 | ||
| 9 | Distance walked (m) on 12-minute walk test | (n = 42) | (n = 64) | ||||
| 1000.2 (308.8) | 1102.4 (285.4) | 102.2 (−13.9 to 218.3) | 0.084 | 115.9 (1.1 to 230.7) | 0.048 |
| Follow-up (months) | Outcome | Control, n (%) | Mini booster, n (%) | Full booster, n (%) | Mini + full booster, n (%) | Difference in proportion maintaining or increasing physical activity (95% CI) (%) | p-value |
|---|---|---|---|---|---|---|---|
| 3 | Meeting current physical activity recommendations | (n = 73) | (n = 57) | (n = 59) | (n = 116) | ||
| 25 (34.2) | 12 (21.1) | 27 (45.8) | 39 (33.6) | −0.6 (−14.5 to 13.2) | 0.929 | ||
| 9 | Meeting current physical activity recommendations | (n = 39) | (n = 27) | (n = 35) | (n = 62) | ||
| 11 (28.2) | 7 (25.9) | 12 (34.3) | 19 (30.6) | 2.4 (−15.7 to 20.6) | 0.794 |
| Outcome measure | Subgroup | Control, mean (SD) | Booster, mean (SD) | Mean difference (95% CI) | p-value |
|---|---|---|---|---|---|
| Mean TEE per day (kcal) | Male | (n = 26) | (n = 42) | ||
| 2483.0 (421.8) | 2455.2 (416.3) | –27.8 (–236.3 to 180.6) | 0.791 | ||
| Female | (n = 35) | (n = 57) | |||
| 2104.6 (322.7) | 2058.6 (343.0) | –46.0 (–189.1 to 97.1) | 0.525 | ||
| All | (n = 61) | (n = 99) | |||
| 2265.9 (410.8) | 2226.9 (422.6) | –39.0 (–173.4 to 95.4) | 0.567 | ||
| Interaction test | NA | NA | –18.2 (–260.6 to 224.3)a | 0.882 |
| Outcome measure | Subgroup | Control, mean (SD) | Booster, mean (SD) | Mean difference (95% CI) | p-value |
|---|---|---|---|---|---|
| Mean TEE per day (kcal) | Male | (n = 13) | (n = 27) | ||
| 2425.9 (402.6) | 2409.2 (382.1) | –16.8 (–282.4 to 248.9) | 0.899 | ||
| Female | (n = 23) | (n = 27) | |||
| 2036.6 (311.9) | 2069.1 (339.6) | 32.4 (–154.2 to 219.1) | 0.728 | ||
| Alla | (n = 36) | (n = 54) | |||
| 2177.2 (390.7) | 2239.1 (397.1) | 61.9 (–106.8 to 230.6) | 0.468 | ||
| Interaction test | NA | NA | 49.2 (–262.6 to 361.0)b | 0.755 |
| Outcome measure | Subgroup | Control, mean (SD) | Booster, mean (SD) | Mean difference (95% CI) | p-value |
|---|---|---|---|---|---|
| Mean TEE per day (kcal) | Yes | (n = 16) | (n = 22) | ||
| 2097.5 (244.0) | 2181.1 (358.8) | 83.6 (–127.0 to 294.2) | 0.426 | ||
| No | (n = 45) | (n = 76) | |||
| 2325.8 (442.5) | 2249.2 (435.7) | –76.6 (–239.8 to 86.6) | 0.355 | ||
| All | (n = 61) | (n = 98) | |||
| 2265.9 (410.8) | 2233.9 (418.9) | –32.0 (–166.0 to 101.9) | 0.638 | ||
| Interaction test | NA | NA | –160.2 (–469.1 to 148.7)a | 0.307 |
| Outcome measure | Subgroup | Control, mean (SD) | Booster, mean (SD) | Mean difference (95% CI) | p-value |
|---|---|---|---|---|---|
| Mean TEE per day (kcal) | Yes | (n = 11) | (n = 14) | ||
| 2084.8 (398.5) | 2079.7 (230.0) | –5.1 (–267.2 to 257.1) | 0.968 | ||
| No | (n = 25) | (n = 39) | |||
| 2217.9 (388.4) | 2313.1 (419.0) | 95.2 (–113.4 to 303.9) | 0.365 | ||
| Alla | (n = 36) | (n = 53) | |||
| 2177.2 (390.7) | 2251.5 (390.3) | 74.2 (–93.4 to 241.9) | 0.381 | ||
| Interaction test | NA | NA | 100.3 (–264.8 to 465.3)b | 0.586 |
| Outcome measure | Subgroup | Control, mean (SD) | Full booster, mean (SD) | Mean difference (95% CI) | p-value |
|---|---|---|---|---|---|
| Mean TEE per day (kcal) | Summer/spring | (n = 48) | (n = 33) | ||
| 2275.8 (417.6) | 2315.2 (444.9) | 39.4 (–153.7 to 232.4) | 0.686 | ||
| Winter/autumn | (n = 13) | (n = 19) | |||
| 2229.3 (398.3) | 2218.6 (394.0) | –10.8 (–301.6 to 280.1) | 0.940 | ||
| All | (n = 61) | (n = 52) | |||
| 2265.9 (410.8) | 2279.9 (425.6) | 14.0 (–142.2 to 170.2) | 0.860 | ||
| Interaction test | NA | NA | –50.1 (–404.0 to 303.7)a | 0.779 |
| Outcome measure | Subgroup | Control, mean (SD) | Mini booster, mean (SD) | Mean difference (95% CI) | p-value |
|---|---|---|---|---|---|
| Mean TEE per day (kcal) | Summer/spring | (n = 48) | (n = 35) | ||
| 2275.8 (417.6) | 2154.5 (411.9) | –121.4 (–305.0 to 62.3) | 0.192 | ||
| Winter/autumn | (n = 13) | (n = 12) | |||
| 2229.3 (398.3) | 2208.4 (442.8) | –20.9 (–368.8 to 327.0) | 0.902 | ||
| All | (n = 61) | (n = 47) | |||
| 2265.9 (410.8) | 2168.2 (415.8) | –97.7 (–256.6 to 61.2) | 0.226 | ||
| Interaction test | NA | NA | 100.4 (–277.6 to 478.5)a | 0.599 |
Appendix 7 Results tables from the process evaluation
| Variable | Non-responders (n = 207) | Responders (process evaluation participants) | All participants (n = 282) | |||
|---|---|---|---|---|---|---|
| All (n = 75) | Control (n = 26) | Mini booster (n = 25) | Full booster (n = 24) | |||
| Gender, n (%) | ||||||
| Male | 98 (47) | 32 (43) | 11 (42) | 7 (28) | 14 (58) | 130 (46) |
| Female | 109 (53) | 43 (57) | 15 (58) | 18 (72) | 10 (42) | 152 (54) |
| Employment status, n (%) | ||||||
| Part-time | 35 (17) | 17 (23) | 5 (19) | 9 (36) | 3 (13) | 52 (18) |
| Full-time | 69 (33) | 24 (32) | 12 (46) | 6 (24) | 6 (25) | 93 (33) |
| Not employed | 100 (48) | 34 (45) | 9 (35) | 10 (40) | 15 (63) | 134 (48) |
| Missing | 3 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (1) |
| Ethnicity, n (%) | ||||||
| White British | 179 (87) | 67 (89) | 23 (88) | 22 (88) | 22 (92 | 246 (87) |
| Any other | 25 (13) | 8 (11) | 3 (12) | 3 (12) | 2 (8%) | 33 (12) |
| Missing | 3 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (1) |
| Stage of change, n (%) | ||||||
| Contemplation | 11 (5.3) | 1 (1.3) | 0 (0) | 0 (0.0) | 1 (4.2) | 12 (4.3) |
| Preparation | 91 (44.0) | 34 (45.3) | 13 (50.0) | 13 (52.0) | 8 (33.3) | 125 (44.3) |
| Action | 66 (31.9) | 25 (33.3) | 8 (30.8) | 6 (24.0) | 11 (45.8) | 91 (32.3) |
| Maintenance | 37 (17.9) | 13 (17.3) | 4 (15.4) | 6 (24.0) | 3 (12.5) | 50 (17.7) |
| Missing | 2 (1.0) | 2 (2.7) | 1 (3.8) | 0 (0.0) | 1 (4.2) | 4 (1.4) |
| Marital status, n (%) | ||||||
| Single | 33 (16) | 12 (16) | 5 (19) | 6 (24) | 1 (4) | 45 (16) |
| Married | 112 (54) | 39 (52) | 12 (46) | 10 (40) | 17 (71) | 151 (54) |
| Co-habiting | 14 (7) | 6 (8) | 1 (4) | 3 (12) | 2 (8) | 20 (7) |
| Divorced/separated | 40 (19) | 15 (20) | 7 (27) | 5 (20) | 3 (13) | 55 (20) |
| Widowed | 8 (4) | 3 (4) | 1 (4) | 1 (4) | 1 (4) | 11 (4) |
| Age (years) | ||||||
| n (%) | 207 (100) | 75 (100) | 26 (100) | 25 (100) | 24 (100) | 282 (100) |
| Mean (SD) | 54.1 (7.4) | 56.0 (7.0) | 54.9 (5.9) | 55.6 (8.1) | 57.5 (6.8) | 54.6 (7.3) |
| Median (IQR) | 54.2 (47.9 to 60.8) | 56.0 (50.4 to 62.7) | 52.7 (50.5 to 60.9) | 59.1 (49.9 to 62.0) | 59.5 (51.2 to 63.8) | 55.3 (48.8 to 61.4) |
| Min. to max. | 40.5 to 65.5 | 40.4 to 65.0 | 46.3 to 64.8 | 40.4 to 65.0 | 44.0 to 64.9 | 40.4 to 65.5 |
| Height (m) | ||||||
| n (%) | 206 (100) | 75 (100) | 26 (100) | 25 (100) | 24 (100) | 281 (100) |
| Mean (SD) | 1.7 (0.1) | 1.7 (0.1) | 1.7 (0.1) | 1.6 (0.1) | 1.7 (0.1) | 1.7 (0.1) |
| Median (IQR) | 1.7 (1.6 to 1.7) | 1.7 (1.6 to 1.8) | 1.7 (1.6 to 1.7) | 1.6 (1.6 to 1.7) | 1.7 (1.6 to 1.8) | 1.7 (1.6 to1.8) |
| Min. to max. | 1.5 to 1.9 | 1.5 to 1.9 | 1.5 to 1.9 | 1.5 to 1.8 | 1.5 to 1.9 | 1.5 to 1.9 |
| Weight (kg) | ||||||
| n (%) | 207 (100) | 75 (100) | 26 (100) | 25 (100) | 24 (100) | 282 (100) |
| Mean (SD) | 84.7 (19.2) | 86.5 (17.3) | 86.4 (17.1) | 82.6 (17.9) | 90.7 (16.7) | 85.2 (18.7) |
| Median (IQR) | 82.0 (71.7 to 97.3) | 84.3 (74.5 to 95.2) | 84.4 (72.6 to 92.5) | 83.0 (73.0 to 89.8) | 87.7 (76.9 to 102.3) | 82.9 (72.5 to 96.6) |
| Min. to max. | 46.9 to 160.0 | 54.7 to 141.0 | 65.3 to 135.0 | 54.7 to 141.0 | 67.7 to 124.0 | 46.9 to 160.0 |
| BMI (kg/m2) | ||||||
| n (%) | 206 (100) | 75 (100) | 26 (100) | 25 (100) | 24 (100) | 281 (100) |
| Mean (SD) | 30.2 (6.1) | 30.7 (5.5) | 31.1 (6.7) | 30.3 (5.5) | 30.8 (3.9) | 30.3 (5.9) |
| Median (IQR) | 29.7 (25.7 to 33.0) | 29.8 (27.3 to 33.2) | 28.8 (27.2 to 33.1) | 29.9 (26.9 to 32.6) | 29.9 (28.2 to 34.2) | 29.8 (26.3 to 33.0) |
| Min. to max. | 17.1 to 48.3 | 21.6 to 53.4 | 21.6 to 53.4 | 22.5 to 49.4 | 25.0 to 39.1 | 17.1 to 53.4 |
| SPAQ change (3 months post randomisation) | ||||||
| n (%) | 207 (100) | 75 (100) | 26 (100) | 25 (100) | 24 (100) | 282 (100) |
| Mean (SD) | 227.2 (401.0) | 162.7 (265.0) | 159.8 (191.6) | 143.0 (245.6) | 186.3 (349.4) | 210.0 (370.4) |
| Median (IQR) | 125.0 (70.0 to 260.0) | 90.0 (40.0 to 210.0) | 117.5 (65.0 to 180.0) | 90.0 (40.0 to 260.0) | 80.0 (35.0 to 182.5) | 120.0 (60.0 to 255.0) |
| Min. to max. | –1840.0 to 3360.0 | –480.0 to 1500.0 | –210.0 to 690.0 | –480.0 to 795.0 | –150.0 to 1500.0 | –1840.0 to 3360.0 |
| BREQ-2 (RAI) | ||||||
| n (%) | 201 (97) | 73 (97) | 24 (92) | 25 (100) | 24 (100) | 274 (97) |
| Mean (SD) | 5.2 (3.6) | 5.4 (4.0) | 5.8 (3.9) | 3.9 (4.5) | 6.4 (3.1) | 5.3 (3.7) |
| Median (IQR) | 5.8 (3.3 to 7.9) | 6.4 (2.9 to 8.3) | 6.5 (4.3 to 8.0) | 5.8 1.3 to 7.3) | 6.9 (3.7 to 9.0) | 6.0 (3.3 to 8.0) |
| Min. to max. | –9.1 to 11.5 | –5.3 to 12.0 | –2.6 to 12.0 | –5.3 to 9.2 | –0.2 to 11.0 | –9.1 to 12.0 |
| Survey question | Scoring | Control (n = 26), n (%) | Mini (n = 25), n (%) | Full (n = 24), n (%) | Total (n = 75), n (%) |
|---|---|---|---|---|---|
| Q1: Over the last 3 months have you taken part in any of the following? | |||||
| Recreational/leisure activities (e.g. gardening, cycling) | Not ticked | 6 (23.1) | 2 (8.0) | 4 (16.7) | 12 (16.0) |
| Ticked | 20 (76.9) | 23 (92.0) | 20 (83.3) | 63 (84.0) | |
| Competitive sports/exercise | Not ticked | 22 (84.6) | 22 (88.0) | 22 (91.7) | 66 (88.0) |
| Ticked | 4 (15.4) | 3 (12.0) | 2 (8.3) | 9 (12.0) | |
| Structured exercise (e.g. exercise class) | Not ticked | 16 (61.5) | 18 (72.0) | 15 (62.5) | 49 (65.3) |
| Ticked | 10 (38.5) | 7 (28.0) | 9 (37.5) | 26 (34.7) | |
| Active commuting (e.g. walking/cycling to work) | Not ticked | 11 (42.3) | 7 (28.0) | 4 (16.7) | 22 (29.3) |
| Ticked | 15 (57.7) | 18 (72.0) | 20 (83.3) | 53 (70.7) | |
| Q2: Please specify the places where you have done your chosen activities over the last 3 months | |||||
| Home | Not ticked | 12 (46.2) | 12 (48.0) | 14 (58.3) | 38 (50.7) |
| Ticked | 14 (53.8) | 13 (52.0) | 10 (41.7) | 37 (49.3) | |
| Local open space (e.g. park) | Not ticked | 14 (53.8) | 12 (48.0) | 8 (33.3) | 34 (45.3) |
| Ticked | 12 (46.2) | 13 (52.0) | 16 (66.7) | 41 (54.7) | |
| Facility (e.g. gym, pool, community centre, track) | Not ticked | 16 (61.5) | 12 (48.0) | 14 (58.3) | 42 (56.0) |
| Ticked | 10 (38.5) | 13 (52.0) | 10 (41.7) | 33 (44.0) | |
| As part of daily activities (e.g. in work, shopping, walking the dog, commuting) | Not ticked | 8 (30.8) | 8 (32.0) | 7 (29.2) | 23 (30.7) |
| Ticked | 18 (69.2) | 17 (68.0) | 17 (70.8) | 52 (69.3) | |
| Other places | Not ticked | 23 (88.5) | 21 (84.0) | 20 (83.3) | 64 (85.3) |
| Ticked | 3 (11.5) | 4 (16.0) | 4 (16.7) | 11 (14.7) | |
| Q3: Why have you chosen to stay physically active? | |||||
| To improve my health | Not at all | 1 (3.8) | 1 (4.0) | 0 (0.0) | 2 (2.7) |
| Neutral | 0 (0.0) | 2 (8.0) | 1 (4.2) | 3 (4.0) | |
| Slightly | 4 (15.4) | 3 (12.0) | 4 (16.7) | 11 (14.7) | |
| Very much | 20 (76.9) | 19 (76.0) | 19 (79.2) | 58 (77.3) | |
| To get fitter/stay fit | Not at all | 0 (0.0) | 1 (4.0) | 0 (0.0) | 1 (1.3) |
| Slightly | 6 (23.1) | 5 (20.0) | 4 (16.7) | 15 (20.0) | |
| Very much | 19 (73.1) | 19 (76.0) | 20 (83.3) | 58 (77.3) | |
| To lose weight | Not at all | 4 (15.4) | 2 (8.0) | 0 (0.0) | 6 (8.0) |
| Not really | 0 (0.0) | 0 (0.0) | 1 (4.2) | 1 (1.3) | |
| Neutral | 1 (3.8) | 1 (4.0) | 0 (0.0) | 2 (2.7) | |
| Slightly | 7 (26.9) | 8 (32.0) | 10 (41.7) | 25 (33.3) | |
| Very much | 14 (53.8) | 14 (56.0) | 13 (54.2) | 41 (54.7) | |
| To look better | Not at all | 0 (0.0) | 0 (0.0) | 1 (4.2) | 1 (1.3) |
| Not really | 3 (11.5) | 2 (8.0) | 2 (8.3) | 7 (9.3) | |
| Neutral | 6 (23.1) | 3 (12.0) | 2 (8.3) | 11 (14.7) | |
| Slightly | 6 (23.1) | 12 (48.0) | 7 (29.2) | 25 (33.3) | |
| Very much | 10 (38.5) | 7 (28.0) | 12 (50.0) | 29 (38.7) | |
| To encourage my family to be more active | Not at all | 6 (23.1) | 8 (32.0) | 6 (25.0) | 20 (26.7) |
| Not really | 6 (23.1) | 5 (20.0) | 3 (12.5) | 14 (18.7) | |
| Neutral | 8 (30.8) | 7 (28.0) | 4 (16.7) | 19 (25.3) | |
| Slightly | 4 (15.4) | 2 (8.0) | 9 (37.5) | 15 (20.0) | |
| Very much | 1 (3.8) | 2 (8.0) | 2 (8.3) | 5 (6.7) | |
| To make new friends | Not at all | 6 (23.1) | 8 (32.0) | 7 (29.2) | 21 (28.0) |
| Not really | 8 (30.8) | 10 (40.%) | 5 (20.8) | 23 (30.7) | |
| Neutral | 7 (26.9) | 5 (20.0) | 7 (29.2) | 19 (25.3) | |
| Slightly | 3 (11.5) | 1 (4.0) | 4 (16.7) | 8 (10.7) | |
| Very much | 2 (7.7) | 1 (4.0) | 1 (4.2) | 4 (5.3) | |
| To have fun/enjoyment | Not at all | 2 (7.7) | 3 (12.0) | 0 (0.0) | 5 (6.7) |
| Not really | 2 (7.7) | 2 (8.0) | 1 (4.2) | 5 (6.7) | |
| Neutral | 6 (23.1) | 5 (20.0) | 5 (20.8) | 16 (21.3) | |
| Slightly | 10 (38.5) | 7 (28.0) | 13 (54.2) | 30 (40.0) | |
| Very much | 5 (19.2) | 8 (32.0) | 5 (20.8) | 18 (24.0) | |
| To spend time with family | Not at all | 9 (34.6) | 9 (36.0) | 7 (29.2) | 25 (33.3) |
| Not really | 8 (30.8) | 5 (20.0) | 3 (12.5) | 16 (21.3) | |
| Neutral | 6 (23.1) | 7 (28.0) | 5 (20.8) | 18 (24.0) | |
| Slightly | 2 (7.7) | 2 (8.0) | 7 (29.2) | 11 (14.7) | |
| Very much | 0 (0.0) | 1 (4.0) | 1 (4.2) | 2 (2.7) | |
| To spend time with friends | Not at all | 6 (23.1) | 9 (36.0) | 7 (29.2) | 22 (29.3) |
| Not really | 10 (38.5) | 8 (32.0) | 3 (12.5) | 21 (28.0) | |
| Neutral | 8 (30.8) | 4 (16.0) | 6 (25.0) | 18 (24.0) | |
| Slightly | 1 (3.8) | 1 (4.0) | 5 (20.8) | 7 (9.3) | |
| Very much | 0 (0.0) | 2 (8.0) | 3 (12.5) | 5 (6.7) | |
| For competition/to win | Not at all | 19 (73.1) | 16 (64.0) | 17 (70.8) | 52 (69.3) |
| Not really | 4 (15.4) | 3 (12.0) | 1 (4.2) | 8 (10.7) | |
| Neutral | 2 (7.7) | 5 (20.0) | 4 (16.7) | 11 (14.7) | |
| Very much | 0 (0.0) | 0 (0.0) | 2 (8.3) | 2 (2.7) | |
| It’s part of my job | Not at all | 18 (69.2) | 17 (68.0) | 16 (66.7) | 51 (68.0) |
| Not really | 2 (7.7) | 1 (4.0) | 4 (16.7) | 7 (9.3) | |
| Neutral | 2 (7.7) | 3 (12.0) | 2 (8.3) | 7 (9.3) | |
| Slightly | 2 (7.7) | 2 (8.0) | 0 (0.0) | 4 (5.3) | |
| Very much | 1 (3.8) | 1 (4.0) | 1 (4.2) | 3 (4.0) | |
| Missing | 1 (3.8) | 1 (4.0) | 1 (4.2) | 3 (4.0) | |
| It gives me a sense of achievement | Not at all | 0 (0.0) | 1 (4.0) | 1 (4.2) | 2 (2.7) |
| Not really | 0 (0.0) | 0 (0.0) | 1 (4.2) | 1 (1.3) | |
| Neutral | 3 (11.5) | 4 (16.0) | 2 (8.3) | 9 (12.0) | |
| Slightly | 9 (34.6) | 7 (28.0) | 9 (37.5) | 25 (33.3) | |
| Very much | 14 (53.8) | 13 (52.0) | 11 (45.8) | 38 (50.7) | |
| To reduce the risks of health problems (e.g. diabetes, heart disease) | Not at all | 1 (3.8) | 1 (4.0) | 0 (0.0) | 2 (2.7) |
| Not really | 2 (7.7) | 0 (0.0) | 0 (0.0) | 2 (2.7) | |
| Neutral | 0 (0.0) | 3 (12.0) | 1 (4.2) | 4 (5.3) | |
| Slightly | 3 (11.5) | 3 (12.0) | 5 (20.8) | 11 (14.7) | |
| Very much | 19 (73.1) | 18 (72.0) | 18 (75.0) | 55 (73.3) | |
| Missing | 1 (3.8) | 0 (0.0) | 0 (0.0) | 1 (1.3) | |
| I haven’t stayed active | Not at all | 16 (61.5) | 11 (44.0) | 18 (75.0) | 45 (60.0) |
| Not really | 2 (7.7) | 4 (16.0) | 1 (4.2) | 7 (9.3) | |
| Neutral | 3 (11.5) | 4 (16.0) | 3 (12.5) | 10 (13.3) | |
| Slightly | 3 (11.5) | 3 (12.0) | 0 (0.0) | 6 (8.0) | |
| Very much | 1 (3.8) | 1 (4.0) | 0 (0.0) | 2 (2.7) | |
| Missing | 1 (3.8) | 2 (8.0) | 2 (8.3) | 5 (6.7) | |
| A health professional recommended that I should | Not at all | 11 (42.3) | 12 (48.0) | 14 (58.3) | 37 (49.3) |
| Not really | 2 (7.7) | 1 (4.0) | 0 (0.0) | 3 (4.0) | |
| Neutral | 0 (0.0) | 2 (8.0) | 1 (4.2) | 3 (4.0) | |
| Slightly | 5 (19.2) | 1 (4.0) | 3 (12.5) | 9 (12.0) | |
| Very much | 1 (3.8) | 2 (8.0) | 1 (4.2) | 4 (5.3) | |
| Missing | 7 (26.9) | 7 (28.0) | 5 (20.8) | 19 (25.3) | |
| Other reasons | Not at all | 4 (15.4) | 2 (8.0) | 2 (8.3) | 8 (10.7) |
| Neutral | 0 (0.0) | 0 (0.0) | 1 (4.2) | 1 (1.3) | |
| Missing | 22 (84.6) | 23 (92.0) | 21 (87.5) | 66 (88.0) | |
| Q4: What influences whether or not you are able to perform your chosen activity? | |||||
| Value for money | Not at all | 6 (23.1) | 4 (16.0) | 10 (41.7) | 20 (26.7) |
| Not really | 5 (19.2) | 5 (20.0) | 3 (12.5) | 13 (17.3) | |
| Neutral | 0 (0.0) | 6 (24.0) | 1 (4.2) | 7 (9.3) | |
| Slightly | 8 (30.8) | 5 (20.0) | 3 (12.5) | 16 (21.3) | |
| Very much | 6 (23.1) | 4 (16.0) | 5 (20.8) | 15 (20.0) | |
| Missing | 1 (3.8) | 1 (4.0) | 2 (8.3) | 4 (5.3) | |
| Activity is available when I want | Not at all | 0 (0.0) | 1 (4.0) | 2 (8.3) | 3 (4.0) |
| Not really | 3 (11.5) | 1 (4.0) | 2 (8.3) | 6 (8.0) | |
| Neutral | 3 (11.5) | 0 (0.0) | 0 (0.0) | 3 (4.0) | |
| Slightly | 4 (15.4) | 7 (28.0) | 6 (25.0) | 17 (22.7) | |
| Very much | 16 (61.5) | 15 (60.0) | 12 (50.0) | 43 (57.3) | |
| Missing | 0 (0.0) | 1 (4.0) | 2 (8.3) | 3 (4.0) | |
| Childcare available | Not at all | 21 (80.8s) | 21 (84.0) | 21 (87.5) | 63 (84.0) |
| Neutral | 2 (7.7) | 1 (4.0) | 0 (0.0) | 3 (4.0) | |
| Very much | 0 (0.0) | 1 (4.0) | 1 (4.2) | 2 (2.7) | |
| Missing | 3 (11.5) | 2 (8.0) | 2 (8.3) | 7 (9.3) | |
| Within walking distance of home/work | Not at all | 6 (23.1) | 5 (20.0) | 7 (29.2) | 18 (24.0) |
| Not really | 2 (7.7) | 6 (24.0) | 5 (20.8) | 13 (17.3) | |
| Neutral | 3 (11.5) | 3 (12.0) | 2 (8.3) | 8 (10.7) | |
| Slightly | 6 (23.1) | 3 (12.0) | 4 (16.7) | 13 (17.3) | |
| Very much | 8 (30.8) | 7 (28.0) | 2 (8.3) | 17 (22.7) | |
| Missing | 1 (3.8) | 1 (4.0) | 4 (16.7) | 6 (8.0) | |
| Within easy reach on public transport | Not at all | 7 (26.9) | 9 (36.0) | 6 (25.0) | 22 (29.3) |
| Not really | 2 (7.7) | 6 (24.0) | 5 (20.8) | 13 (17.3) | |
| Neutral | 1 (3.8) | 4 (16.0) | 2 (8.3) | 7 (9.3) | |
| Slightly | 9 (34.6) | 2 (8.0) | 5 (20.8) | 16 (21.3) | |
| Very much | 5 (19.2) | 3 (12.0) | 2 (8.3) | 10 (13.3) | |
| Missing | 2 (7.7) | 1 (4.0) | 4 (16.7) | 7 (9.3) | |
| If I feel I’m getting something out of it | Not at all | 2 (7.7) | 0 (0.0) | 0 (0.0) | 2 (2.7) |
| Not really | 1 (3.8) | 1 (4.0) | 1 (4.2) | 3 (4.0) | |
| Neutral | 1 (3.8) | 1 (4.0) | 1 (4.2) | 3 (4.0) | |
| Slightly | 7 (26.9) | 6 (24.0) | 6 (25.0) | 19 (25.3) | |
| Very much | 15 (57.7) | 16 (64.0) | 13 (54.2) | 44 (58.7) | |
| Missing | 0 (0.0) | 1 (4.0) | 3 (12.5) | 4 (5.3) | |
| Whether there is someone to do it with | Not at all | 11 (42.3) | 8 (32.0) | 10 (41.7) | 29 (38.7) |
| Not really | 6 (23.1) | 7 (28.0) | 1 (4.2) | 14 (18.7) | |
| Neutral | 3 (11.5) | 4 (16.0) | 3 (12.5) | 10 (13.3) | |
| Slightly | 4 (15.4) | 4 (16.0) | 6 (25.0) | 14 (18.7) | |
| Very much | 1 (3.8) | 1 (4.0) | 0 (0.0) | 2 (2.7) | |
| Missing | 1 (3.8) | 1 (4.0) | 4 (16.7) | 6 (8.0) | |
| It’s a habit | Not at all | 3 (11.5) | 7 (28.0) | 8 (33.3) | 18 (24.0) |
| Not really | 5 (19.2) | 3 (12.0) | 3 (12.5) | 11 (14.7) | |
| Neutral | 3 (11.5) | 8 (32.0) | 6 (25.0) | 17 (22.7) | |
| Slightly | 9 (34.6) | 4 (16.0) | 1 (4.2) | 14 (18.7) | |
| Very much | 6 (23.1) | 2 (8.0) | 2 (8.3) | 10 (13.3) | |
| Missing | 0 (0.0) | 1 (4.0) | 4 (16.0) | 5 (6.6) | |
| Whether I can make time to do it | Not at all | 1 (3.8) | 5 (20.0) | 3 (12.5) | 9 (12.0) |
| Not really | 4 (15.4) | 4 (16.0) | 1 (4.2) | 9 (12.0) | |
| Neutral | 4 (15.4) | 1 (4.0) | 4 (16.7) | 9 (12.0) | |
| Slightly | 12 (46.2) | 7 (28.0) | 7 (29.2) | 26 (34.7) | |
| Very much | 4 (15.4) | 7 (28.0) | 5 (20.8) | 16 (21.3) | |
| Missing | 1 (3.8) | 1 (4.0) | 4 (16.7) | 6 (8.0) | |
| My own health | Not at all | 1 (3.8) | 1 (4.0) | 0 (0.0) | 2 (2.7) |
| Not really | 1 (3.8) | 1 (4.0) | 0 (0.0) | 2 (2.7) | |
| Neutral | 2 (7.7) | 1 (4.0) | 0 (0.0) | 3 (4.0) | |
| Slightly | 2 (7.7) | 2 (8.0) | 4 (16.7) | 8 (10.7) | |
| Very much | 18 (69.2) | 19 (76.0) | 18 (75.0) | 55 (73.3) | |
| Missing | 2 (7.7) | 1 (4.0) | 2 (8.3) | 5 (6.7) | |
| Other influences | Not at all | 0 (0.0) | 1 (4.0) | 1 (4.2) | 2 (2.7) |
| Very much | 0 (0.0) | 0 (0.0) | 1 (4.2) | 1 (1.3) | |
| Missing | 26 (100.0) | 24 (96.0) | 22 (91.7) | 72 (96.0) | |
| Q5: Do you do physical activity with anyone else? | |||||
| No | 17 (65.4) | 14 (56.0) | 8 (33.3) | 39 (52.0) | |
| Yes | 9 (34.6) | 10 (40.0) | 14 (58.3) | 33 (44.0) | |
| Q6: If you answered ‘yes’ to Q5, who do you usually do physical activity with? | |||||
| Spouse/partner | Not ticked | 4 (15.4) | 5 (20.0) | 8 (33.3) | 17 (22.7) |
| Ticked | 5 (19.2) | 6 (24.0) | 8 (33.3) | 19 (25.3) | |
| Not applicable | 17 (65.4) | 14 (56.0) | 8 (33.3) | 39 (52.0) | |
| Other family member(s) | Not ticked | 6 (23.1) | 8 (32.0) | 12 (50.0) | 26 (34.7) |
| Ticked | 3 (11.5) | 3 (12.0) | 4 (16.7) | 10 (13.3) | |
| Not applicable | 17 (65.4) | 14 (56.0) | 8 (33.3) | 39 (52.0) | |
| Friend(s) | Not ticked | 7 (26.9) | 7 (28.0) | 8 (33.3) | 22 (29.3) |
| Ticked | 2 (7.7) | 4 (16.0) | 8 (33.3) | 14 (18.7) | |
| Not applicable | 17 (65.4) | 14 (56.0) | 8 (33.3) | 39 (52.0) | |
| Other adult(s) | Not ticked | 7 (26.9) | 9 (36.0) | 12 (50.0) | 28 (37.3) |
| Ticked | 2 (7.7) | 2 (8.0) | 4 (16.7) | 8 (10.7) | |
| Not applicable | 17 (65.4) | 14 (56.0) | 8 (33.3) | 39 (52.0) | |
| Child or children under 16 years | Not ticked | 7 (26.9) | 7 (28.0) | 14 (58.3) | 28 (37.3) |
| Ticked | 2 (7.7) | 4 (16.0) | 2 (8.3) | 8 (10.7) | |
| Not applicable | 17 (65.4) | 14 (56.0) | 8 (33.3) | 39 (52.0) | |
| As part of a group/class (including walking group) | Not ticked | 6 (23.1) | 9 (36.0) | 13 (54.2) | 28 (37.3) |
| Ticked | 3 (11.5) | 2 (8.0) | 3 (12.5) | 8 (10.7) | |
| Not applicable | 17 (65.4) | 14 (56.0) | 8 (33.3) | 39 (52.0) | |
| Other | Not ticked | 9 (34.6) | 11 (44.0) | 15 (62.5) | 35 (46.7) |
| Ticked | 0 (0.0) | 0 (0.0) | 1 (4.2) | 1 (1.3) | |
| Not applicable | 17 (65.4) | 14 (56.0) | 8 (33.3) | 39 (52.0) | |
| Q7: If you answered ‘yes’ to Q5, how useful do you find this support in helping you stay active? | |||||
| Not applicable | 1 (3.8) | 0 (0.0) | 0 (0.0) | 1 (1.3) | |
| Very useful | 3 (11.5) | 1 (4.0) | 9 (37.5) | 13 (17.3) | |
| Fairly useful | 3 (11.5) | 8 (32.0) | 4 (16.7) | 15 (20.0) | |
| Neither useful nor useless | 1 (3.8) | 1 (4.0) | 1 (4.2) | 3 (4.0) | |
| Very useless | 1 (3.8) | 0 (0.0) | 0 (0.0) | 1 (1.3) | |
| Doesn’t do activity (‘no’ on Q5) | 17 (65.4) | 14 (56.0) | 8 (33.3) | 39 (52.0) | |
| Missing | 0 (0.0) | 1 (4.0) | 2 (8.3) | 3 (4.0) | |
| (n = 25) | (n = 25) | (n = 50) | |||
| Q8: If you were to receive further activity ‘booster’ counselling/advice in the future, how would you prefer it to be delivered? | |||||
| Over the telephone | Not ticked | NA | 13 (52.0) | 23 (95.8) | 36 (73.5) |
| Ticked | NA | 12 (48.0) | 1 (4.2) | 13 (26.5) | |
| In person (face to face) | Not ticked | NA | 16 (64.0) | 5 (20.8) | 21 (42.9) |
| Ticked | NA | 9 (36.0) | 19 (79.2) | 28 (57.1) | |
| Written advice | Not ticked | NA | 17 (68.0) | 20 (83.3) | 37 (75.5) |
| Ticked | NA | 8 (32.0) | 4 (16.7) | 12 (24.5) | |
| Q9: Did the ‘booster’ physical activity counselling/advice meet the expectations you described above? | |||||
| No | NA | 2 (8.0) | 0 (0.0) | 2 (4.1) | |
| Yes | NA | 19 (76.0) | 23 (95.8) | 42 (85.7) | |
| Missing | NA | 4 (16.0) | 1 (4.2) | 5 (10.2) | |
| Q10: The ‘booster’ counselling/advice sessions fitted easily into my daily schedule | |||||
| Strongly disagree | NA | 2 (8.0) | 2 (8.3) | 4 (8.2) | |
| Disagree | NA | 0 (0.0) | 1 (4.2) | 1 (2.0) | |
| Neutral | NA | 1 (4.0) | 2 (8.3) | 3 (6.1) | |
| Agree | NA | 12 (48.0) | 10 (41.7) | 22 (44.9) | |
| Strongly agree | NA | 7 (28.0) | 8 (33.3) | 15 (30.6) | |
| Missing | NA | 3 (12.0) | 1 (4.2) | 4 (8.2) | |
| Q11: The ‘booster’ counselling/advice sessions were conducted at a convenient location | |||||
| Strongly disagree | NA | 0 (0.0) | 1 (4.2) | 1 (2.0) | |
| Neutral | NA | 0 (0.0) | 1 (4.2) | 1 (2.0) | |
| Agree | NA | 0 (0.0) | 10 (41.7) | 10 (20.4) | |
| Strongly agree | NA | 0 (0.0) | 10 (41.7) | 10 (20.4) | |
| Missing | NA | 25 (100.0) | 2 (8.3) | 27 (55.1) | |
| Q12: Throughout the ‘booster’ counselling/advice sessions I feel I wasn't being judged by the project worker | |||||
| Strongly disagree | NA | 1 (4.0) | 2 (8.3) | 3 (6.1) | |
| Disagree | NA | 1 (4.0) | 1 (4.2) | 2 (4.1) | |
| Agree | NA | 7 (28.0) | 5 (20.8) | 12 (24.5) | |
| Strongly agree | NA | 13 (52.0) | 15 (62.5) | 28 (57.1) | |
| Missing | NA | 3 (12.0) | 1 (4.2) | 4 (8.2) | |
| Q13: The ‘booster’ counselling/advice sessions were non-confrontational in nature | |||||
| Strongly disagree | NA | 1 (4.0) | 2 (8.3) | 3 (6.1) | |
| Disagree | NA | 0 (0.0) | 1 (4.2) | 1 (2.0) | |
| Neutral | NA | 1 (4.0) | 0 (0.0) | 1 (2.0) | |
| Agree | NA | 6 (24.0) | 5 (20.8) | 11 (22.4) | |
| Strongly agree | NA | 13 (52.0) | 14 (58.3) | 27 (55.1) | |
| Missing | NA | 4 (16.0) | 2 (8.3) | 6 (12.2) | |
| Q14: Throughout the ‘booster’ counselling/advice sessions I thought the project worker understood what I was saying | |||||
| Strongly disagree | NA | 1 (4.0) | 1 (4.2) | 2 (4.1) | |
| Agree | NA | 9 (36.0) | 9 (37.5) | 18 (36.7) | |
| Strongly agree | NA | 12 (48.0) | 13 (54.2) | 25 (51.0) | |
| Missing | NA | 3 (12.0) | 1 (4.2) | 4 (8.2) | |
| Q15: How Involved did you feel in the ‘booster’ counselling advice sessions? | |||||
| I spoke more than the project worker | NA | 15 (60.0) | 7 (29.2) | 22 (44.9) | |
| I spoke less than the project worker | NA | 3 (12.0) | 0 (0.0) | 3 (6.1) | |
| I spoke roughly the same amount as the project worker | NA | 4 (16.0) | 16 (66.7) | 20 (40.8) | |
| Missing | 3 (12.0) | 1 (4.2) | 4 (8.2) | ||
| Q16: How do you feel about the amount of contact time that occurred between you and the project worker | |||||
| About right | NA | 22 (88.0) | 22 (91.7) | 44 (89.8) | |
| Slightly too short | NA | 0 (0.0) | 1 (4.2) | 1 (2.0) | |
| Missing | NA | 3 (12.0) | 1 (4.2) | 4 (8.2) | |
| Q17: During the ‘booster’ counselling advice sessions I was encouraged to set my own goals for physical activity | |||||
| Not discussed | NA | 1 (4.0) | 0 (0.0) | 1 (2.0) | |
| Neutral | NA | 2 (8.0) | 1 (4.2) | 3 (6.1) | |
| Agree | NA | 13 (52.0) | 12 (50.0) | 25 (51.0) | |
| Strongly agree | NA | 6 (24.0) | 10 (41.7) | 16 (32.7) | |
| Missing | NA | 3 (12.0) | 1 (4.%) | 4 (8.2) | |
| Q18: As a result of the ‘booster’ counselling/advice sessions I feel I have been able to resolve my barriers towards physical activity | |||||
| Disagree | NA | 1 (4.0) | 0 (0.0) | 1 (2.0) | |
| Neutral | NA | 8 (32.0) | 5 (20.8) | 13 (26.5) | |
| Agree | NA | 10 (40.0) | 12 (50.0) | 22 (44.9) | |
| Strongly agree | NA | 3 (12.0) | 6 (25.0) | 9 (18.4) | |
| Missing | NA | 3 (12.0) | 1 (4.0) | 4 (8.0) | |
| Q19: As a result of the ‘booster’ counselling/advice sessions I now know more about the benefits of physical activity | |||||
| Neutral | NA | 8 (32.0) | 5 (20.8) | 13 (26.5) | |
| Agree | NA | 11 (44.0) | 11 (45.8) | 22 (44.9) | |
| Strongly agree | NA | 3 (12.0) | 7 (29.2) | 10 (20.4) | |
| Missing | NA | 3 (12.0) | 1 (4.2) | 4 (8.2) | |
| Q20: As a result of the ‘booster’ counselling/advice sessions I now know more about the risks associated with living an inactive lifestyle | |||||
| Not discussed | NA | 1 (4.0) | 0 (0.0) | 1 (2.0) | |
| Disagree | NA | 1 (4.0) | 0 (0.0) | 1 (2.0) | |
| Neutral | NA | 7 (28.0) | 4 (16.7) | 11 (22.4) | |
| Agree | NA | 9 (36.0) | 13 (54.2) | 22 (44.9) | |
| Strongly agree | NA | 4 (16.0) | 6 (25.0) | 10 (20.4) | |
| Missing | NA | 3 (12.0) | 1 (4.2) | 4 (8.2) | |
| Q21: As a result of the ‘booster’ counselling/advice sessions I am now more aware of available physical activity facilities and opportunities | |||||
| Not discussed | NA | 1 (4.0) | 0 (0.0) | 1 (2.0) | |
| Neutral | NA | 7 (28.0) | 5 (20.8) | 12 (24.5) | |
| Agree | NA | 9 (36.0) | 13 (54.2) | 22 (44.9) | |
| Strongly agree | NA | 4 (16.0) | 5 (20.8) | 9 (18.4) | |
| Missing | NA | 4 (16.0) | 1 (4.2) | 5 (10.2) | |
| Q22: As a result of the ‘booster’ counselling/advice sessions my confidence to stay active has increased | |||||
| Neutral | NA | 5 (20.0) | 0 (0.0) | 5 (10.2) | |
| Agree | NA | 12 (48.0) | 13 (54.2) | 25 (51.0) | |
| Strongly agree | NA | 5 (20.0) | 10 (41.7) | 15 (30.6) | |
| Missing | NA | 3 (12.0) | 1 (4.2) | 4 (8.2) | |
| Finally, would you be happy to discuss some of the things mentioned in this questionnaire further in an interview with a different person from the person you have been in contact with about this study so far, either face to face or over the telephone? | |||||
| No | 1 (3.8) | 1 (4.0) | 2 (8.3) | 4 (5.3) | |
| Yes | 25 (96.2) | 21 (84.0) | 21 (87.5) | 67 (89.3) | |
| Missing | 0 (0.0) | 3 (12.0) | 1 (4.2) | 4 (5.3) | |
| Interview method: face to face | No | 6 (23.1) | 11 (44.0) | 7 (29.2) | 24 (32.0) |
| Yes | 20 (76.9) | 14 (56.0) | 17 (70.8) | 51 (68.0) | |
| Interview method: over the telephone | No | 12 (46.2) | 11 (44.0) | 14 (58.3) | 37 (49.3) |
| Yes | 14 (53.8) | 14 (56.0) | 10 (41.7) | 38 (50.7) | |
| Variable | Not interviewed (n = 256) | Interviewed | Total (n = 282) | ||
|---|---|---|---|---|---|
| All (n = 26) | Mini booster (n = 13) | Full booster (n = 13) | |||
| Gender, n (%) | |||||
| Male | 116 (45) | 14 (54) | 7 (54) | 7 (54) | 130 (46) |
| Female | 140 (55) | 12 (46) | 6 (46) | 6 (46) | 152 (54) |
| Employment status, n (%) | |||||
| Part-time | 44 (17) | 8 (31) | 5 (38) | 3 (23) | 52 (18) |
| Full-time | 89 (35) | 4 (15) | 2 (15) | 2 (15) | 93 (33) |
| Not employed | 121 (47) | 13 (50) | 5 (38) | 8 (62) | 134 (48) |
| Missing | 2 (1) | 1 (4) | 1 (8) | 0 (0) | 3 (1) |
| Ethnicity, n (%) | |||||
| White British | 222 (87) | 24 (92) | 13 (100) | 11 (85) | 246 (87) |
| Any other | 31 (12) | 2 (8) | 0 (0) | 1 (8) | 33 (12) |
| Missing | 3 (1) | 0 (0) | 0 (0) | 1 (8) | 3 (1) |
| Marital status, n (%) | |||||
| Single | 40 (16) | 5 (19) | 4 (31) | 1 (8) | 45 (16) |
| Married | 138 (54) | 13 (50) | 5 (38) | 8 (62) | 151 (54) |
| Co-habiting | 18 (7) | 2 (8) | 1 (8) | 1 (8) | 20 (7) |
| Divorced/separated | 51 (20) | 4 (15) | 2 (15) | 2 (15) | 55 (20) |
| Widowed | 9 (4) | 2 (8) | 1 (8) | 1 (8) | 11 (4) |
| Stage of change, n (%) | |||||
| Contemplation | 12 (4.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 12 (4.3) |
| Preparation | 114 (44.5) | 11 (42.3) | 7 (53.8) | 4 (30.8) | 125 (44.3) |
| Action | 84 (32.8) | 7 (26.9) | 3 (23.1) | 4 (30.8) | 91 (32.3) |
| Maintenance | 43 (16.8) | 7 (26.9) | 3 (23.1) | 4 (30.8) | 50 (17.7) |
| Missing | 3 (1.2) | 1 (3.8) | 0 (0.0) | 1 (7.7) | 4 (1.4) |
| Age (years) | |||||
| n (%) | 256 (100) | 26 (100) | 13 (100) | 13 (100) | 282 (100) |
| Mean (SD) | 54.3 (7.4) | 57.6 (6.1) | 57.0 (5.6) | 58.2 (6.6) | 54.6 (7.3) |
| Median (IQR) | 54.5 (48.3 to 61.0) | 58.1 (53.2 to 63.5) | 56.7 (53.2 to 61.8) | 61.1 (54.3 to 63.9) | 55.3 (48.8 to 61.4) |
| Min. to max. | 40.4 to 65.5 | 45.5 to 65.1 | 47.9 to 65.1 | 45.5 to 64.9 | 40.4 to 65.5 |
| Height (m) | |||||
| n (%) | 255 (100) | 26 (100) | 13 (100) | 13 (100) | 281 (100) |
| Mean (SD) | 1.7 (0.1) | 1.7 (0.1) | 1.7 (0.1) | 1.7 (0.1) | 1.7 (0.1) |
| Median (IQR) | 1.7 (1.6 to 1.7) | 1.7 (1.6 to 1.8) | 1.7 (1.6 to 1.8) | 1.7 (1.6 to 1.8) | 1.7 (1.6 to 1.8) |
| Min. to max. | 1.5 to 1.9 | 1.6 to 1.9 | 1.6 to 1.8 | 1.6 to 1.9 | 1.5 to 1.9 |
| Weight (kg) | |||||
| n (%) | 256 (100) | 26 (100) | 13 (100) | 13 (100) | 282 (100) |
| Mean (SD) | 85.2 (19.1) | 84.5 (13.9) | 80.3 (13.1) | 88.7 (13.9) | 85.2 (18.7) |
| Median (IQR) | 83.0 (72.3 to 96.5) | 80.8 (74.5 to 96.6) | 78.0 (73.5 to 89.3) | 88.0 (79.0 to 97.0) | 82.9 (72.5 to 96.6) |
| Min. to max. | 46.9 to 160.0 | 57.0 to 114.0 | 57.0 to 101.9 | 70.5 to 114.0 | 46.9 to 160.0 |
| BMI (kg/m2) | |||||
| n (%) | 255 (100) | 26 (100) | 13 (100) | 13 (100) | 281 (100) |
| Mean (SD) | 30.4 (6.1) | 29.6 (3.9) | 27.9 (2.8) | 31.3 (4.3) | 30.3 (5.9) |
| Median (IQR) | 29.8 (26.0 to 33.2) | 29.5 (26.9 to 31.5) | 27.7 (26.7 to 30.4) | 29.9 (29.0 to 35.1) | 29.8 (26.3 to 33.0) |
| Min. to max. | 17.1 to 53.4 | 22.5 to 39.3 | 22.5 to 32.0 | 25.0 to 39.3 | 17.1 to 53.4 |
| SPAQ change (3 months post randomisation) | |||||
| n (%) | 256 (100) | 26 (100) | 13 (100) | 13 (100) | 282 (100) |
| Mean (SD) | 223.1 (382.3) | 81.1 (178.6) | 48.1 (187.3) | 114.1 (170.4) | 210.0 (370.4) |
| Median (IQR) | 120.0 (60.0 to 257.5) | 65.0 (0.0 to 200.0) | 60.0 (0.0 to 150.0) | 75.0 (40.0 to 200.0) | 120.0 (60.0 to 255.0) |
| Min. to max. | –1840.0 to 3360.0 | –480.0 to 540.0 | –480.0 to 270.0 | –150.0 to 540.0 | –1840.0 to 3360.0 |
| BREQ-2 (RAI) | |||||
| n (%) | 248 (97) | 26 (100) | 13 (100) | 13 (100) | 274 (97) |
| Mean (SD) | 5.2 (3.7) | 5.7 (4.2) | 3.8 (4.8) | 7.6 (2.5) | 5.3 (3.7) |
| Median (IQR) | 5.8 (3.2 to 7.9) | 7.2 (4.2 to 8.7) | 5.8 (0.3 to 7.5) | 8.3 (6.3 to 9.7) | 6.0 (3.3 to 8.0) |
| Min. to max. | –9.1 to 12.0 | –4.7 to 11.0 | –4.7 to 9.0 | 2.9 to 11.0 | –9.1 to 12.0 |
| Participant | Days meeting guidance/accelerometry days | Median (range) moderate/vigorous activity (minutes) |
|---|---|---|
| 1064 | 0/3 | 0 (0 to 0) |
| 1087 | 0/7 | 0 (0 to 9) |
| 1094 | 0/7 | 0 (0 to 3) |
| 1096 | 0/7 | 0 (0 to 5) |
| 1152 | 3/3 | 576 (280 to 583) |
| 1217 | NA | NA |
| 1230 | 2/7 | 3 (1 to 91) |
| 1231 | 1/7 | 7 (3 to 53) |
| 1232 | 1/7 | 15 (4 to 43) |
| 1235 | NA | NA |
| 1237 | NA | NA |
| 1238 | 2/7 | 2 (0 to 47) |
| 1240 | 0/7 | 0 (0 to 0) |
| 1243 | 0/7 | 0 (0 to 26) |
| 1245 | NA | NA |
| 1252 | 0/7 | 2 (0 to 23) |
| 1253 | NA | NA |
| 1257 | 1/7 | 1 (0 to 41) |
| 1258 | 3/7 | 28 (2 to 54) |
| 1265 | 0/7 | 5 (0 to 14) |
| 1267 | 0/7 | 13 (0 to 26) |
| 1272 | 0/7 | 3 (0 to 6) |
| 1279 | 0/7 | 0 (0 to 0) |
| 1280 | NA | NA |
| 1281 | 0/7 | 2 (0 to 12) |
| 1282 | 0/4 | 0.5 (0 to 3) |
| Name | Evocation (/5) | Collaboration (/5) | Autonomy/support (/5) | Direction (/5) | Empathy (/5) | Average global rating (/5) | % Complex reflections (%CR) | % Open questions (%OQ) | Reflection to question ratio (R : Q) | % MI adherent (%MIA) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First | Second | Mean | First | Second | Mean | First | Second | Mean | First | Second | Mean | First | Second | Mean | First | Second | Mean | First | Second | Mean | First | Second | Mean | First | Second | Mean | First | Second | Mean | |
| RA1 | 4 | 3 | 3.5 | 3 | 4 | 3.5 | 4 | 3 | 3.5 | 4 | 4 | 4 | 5 | 4 | 4.5 | 3.6 | 3.3 | 3.5 | 67 | 23 | 45 | 27 | 53 | 40 | 2.5 | 2.6 | 2.6 | 100 | 75 | 88 |
| RA2 | 2 | 3 | 2.5 | 3 | 4 | 3.5 | 3 | 3 | 3 | 5 | 5 | 5 | 3 | 3 | 3 | 2.6 | 3.3 | 3 | 24 | 11 | 18 | 36 | 36 | 36 | 0.6 | 1.8 | 1.2 | 0 | 100 | 50 |
| RA3 | 4 | 4 | 4 | 3 | 4 | 3.5 | 3 | 3 | 3 | 5 | 5 | 5 | 3 | 3 | 3 | 3.3 | 3.6 | 3.5 | 20 | 16 | 18 | 38 | 51 | 45 | 1.1 | 1.2 | 1.2 | 100 | 100 | 100 |
| RA4 | 3 | 3 | 3 | 3 | 4 | 3.5 | 4 | 3 | 3.5 | 5 | 4 | 4.5 | 4 | 3 | 3.5 | 3.6 | 3.3 | 3.5 | 45 | 22 | 34 | 28 | 54 | 41 | 0.7 | 1.6 | 1.2 | 100 | 100 | 100 |
| RA5 | 4 | – | 4 | 3 | – | 3 | 4 | – | 4 | 4 | – | 4 | 4 | – | 4 | 3.6 | – | 3.6 | 38 | – | 38 | 30 | – | 30 | 1.8 | – | 1.8 | 100 | – | – |
| RA6 | 3 | – | 3 | 3 | – | 3 | 3 | – | 3 | 2 | – | 2 | 4 | – | 4 | 3 | – | 3 | 40 | – | 40 | 33 | – | 33 | 1.6 | – | 1.6 | 100 | – | – |
| Code | Parameter | Value | Assumptions/source/derivation |
|---|---|---|---|
| A | Sessions per completer | 2 | Booster trial |
| B | Average number of completer sessions per RA | 35.67 | 214 completers, six RAs |
| C | Completers at 3 months in mini booster arm | 55 | Booster trial |
| D | Completers at 3 months in full booster arm | 52 | Booster trial |
| E | Completers at 9 months in mini booster arm | 27 | Booster trial |
| F | Completers at 9 months in full booster arm | 35 | Booster trial |
| G | Participants in mini booster | 92 | Booster trial |
| H | Participants in full booster | 94 | Booster trial |
| I | Fixed costs per RA, high estimate | £1800 | Upper estimate of training/monitoring costs from booster MI expert |
| J | Fixed costs per RA, low estimate | £1000 | Lower estimate of training/monitoring costs from booster MI expert |
| K | Hourly wage per RA | £15.76 | Assuming wage of £26,000/year and 1650 hours/year |
| L | Additional cost per hour, full booster | £10 | RA estimate of room hire costs per session |
| M | Additional cost per hour, mini booster | £4.80 | Assuming telephone call costs of 8p/minute |
| N | Session duration, mini booster | 20 minutes | RA estimate |
| O | Session duration, full booster | 30 minutes | RA estimate |
| P | Participants per completer | 1.87 | 200 participants/107 completers |
| Q | Variable cost per session, mini booster | £6.85 | N × (M + K) |
| R | Variable cost per session, full booster | £12.88 | O × (L + K) |
| S | Effective sessions per completer, 3 months, mini booster | 3.35 | A × (G/C) |
| T | Effective sessions per completer, 3 months, full booster | 3.62 | A × (H/D) |
| U | Effective sessions per completer, 9 months, mini booster | 6.81 | A × (G/E) |
| V | Effective sessions per completer, 9 months, full booster | 5.37 | A × (H/F) |
| W | Variable cost per completer, 3 months, mini booster | £22.92 | Q × S |
| X | Variable cost per completer, 3 months, full booster | £46.56 | R × T |
| Y | Variable cost per completer, 9 months, mini booster | £46.70 | Q × U |
| Z | Variable cost per completer, 9 months, full booster | £49.18 | R × V |
| AA | Fixed cost per completer, 3 months, mini booster, assuming I | £168.84 | (I/C) × S |
| AB | Fixed cost per completer, 3 months, full booster, assuming I | £182.46 | (I/C) × T |
| AC | Fixed cost per completer, 9 months, mini booster, assuming I | £343.93 | (I/C) × U |
| AD | Fixed cost per completer, 9 months, full booster, assuming J | £271.08 | (I/C) × V |
| AE | Fixed cost per completer, 3 months, mini booster, assuming J | £93.80 | (J/C) × S |
| AF | Fixed cost per completer, 3 months, full booster, assuming J | £101.37 | (J/C) × T |
| AG | Fixed cost per completer, 9 months, mini booster, assuming J | £191.07 | (J/C) × U |
| AH | Fixed cost per completer, 9 months, full booster, assuming J | £150.60 | (J/C) × V |
| AI | Total cost per completer, 3 months, mini booster, assuming I | £191.76 | W + AA |
| AJ | Total cost per completer, 3 months, full booster, assuming I | £229.02 | X + AB |
| AK | Total cost per completer, 9 months, mini booster, assuming I | £390.62 | Y + AC |
| AL | Total cost per completer, 9 months, full booster, assuming I | £340.26 | Z + AD |
| AM | Total cost per completer, 3 months, mini booster, assuming J | £116.72 | W + AE |
| AN | Total cost per completer, 3 months, full booster, assuming J | £147.93 | X + AF |
| AO | Total cost per completer, 9 months, mini booster, assuming J | £237.77 | Y + AG |
| AP | Total cost per completer, 9 months, full booster, assuming J | £219.78 | Z + AH |
Glossary
- Accelerometer
- Instrument for measuring physical activity (such as Actiheart – see below).
- Actiheart
- Chest-worn device that records heart rate, interbeat interval and physical activity. It calculates and measures activity energy expenditure.
- Bootstrapping
- A simulation method for deriving non-parametric estimates of variables of interest (e.g. the variance in the incremental cost-effectiveness ratio) from a data set.
- Cost-effectiveness acceptability curve
- A graph that plots a range of possible cost-effectiveness thresholds on the horizontal axis against the probability (chance) that the intervention will be cost-effective on the vertical axis. In technology appraisals, cost-effectiveness acceptability curves are a means of representing the uncertainty surrounding the cost-effectiveness estimates in relation to the decision.
- ‘Full booster’
- Two 20- to 30-minute face-to-face physical activity consultations, delivered in a motivational interviewing style, 1 month apart. This included an exploration of barriers and motives to change, decisional balance, agenda setting, action planning and relapse prevention.
- Incremental cost-effectiveness ratio
- The ratio of the difference between the mean cost of a technology and the cost of the next best alternative technology to the difference in the mean outcomes.
- ‘Mini booster’
- Two 20-minute telephone physical activity consultations, delivered in a motivational interviewing style, 1 month apart. This aimed to promote and sustain increased physical activity levels, focusing on exploration of relevant physical activity experience and action planning, and to discuss physical activity and usage of the DVD.
- Motivational interviewing
- A directive, client-centred counselling style for eliciting behaviour change by helping clients to explore and resolve ambivalence.
- Motivational Interviewing Treatment Integrity code
- The Motivational Interviewing Treatment Integrity code is a behavioural coding system, the use of which produces a score on a global scale indicating how well or poorly a practitioner is using motivational interviewing.
- Probabilistic sensitivity analysis
- A way of representing uncertainty in the results of economic evaluations. Uncertainty may arise from missing data, imprecise estimates or methodological controversy. A sensitivity analysis repeats the main analysis using different assumptions to examine the effect on the results. In probabilistic sensitivity analysis, probability distributions are assigned to the uncertain parameters and are incorporated into evaluation models based on decision-analytical techniques (e.g. Monte Carlo simulation).
- Research assistant
- In this study research assistants were employed to conduct research activities (screening candidates for study eligibility, collecting baseline and follow-up data) and as interventionists (delivering the motivational interviewing ‘booster’ interventions).
- Self-determination theory
- A theory of motivation concerned with supporting supposedly intrinsic or natural tendencies to behave in effective and healthy ways.
- Transtheoretical model
- Defines behaviour change as a process rather than a single event and offers practical suggestions for how individuals can change behaviour. The transtheoretical model offers practitioners a common, validated framework for guiding participants through periods of change and proposes strategies for maintaining positive behaviours.
- Utility score
- A measure of the strength of a person’s preference for a specific health state in relation to alternative health states. The utility scale assigns numerical values on a scale from 0 (death) to 1 (optimal or ‘perfect’ health). Health states can be considered worse than death and thus have a negative value.
List of abbreviations
- A&E
- accident and emergency
- ANCOVA
- analysis of covariance
- ANOVA
- analysis of variance
- BMI
- body mass index
- BREQ-2
- Behavioural Regulation in Exercise Questionnaire
- CEAF
- cost-effectiveness acceptability frontier
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CTRU
- Clinical Trials Research Unit
- DMEC
- data monitoring and ethics committee
- EXERT
- Exercise Evaluation Randomised Trial
- FAB
- Feedback, Awareness and Behaviour
- FIT
- Food and Immunity Trial
- GIS
- geographical information systems
- GP
- general practitioner
- HRQoL
- health-related quality of life
- ICER
- incremental cost-effectiveness ratio
- ICH
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use
- IMD
- Index of Multiple Deprivation
- IQR
- interquartile range
- ITN
- Integrated Transport Network™
- ITT
- intention to treat
- LSOA
- lower super output area
- MCID
- minimum clinically important difference
- MCS
- mental component summary score
- MET
- metabolic equivalent of task
- MI
- motivational interviewing
- MITI
- Motivational Interviewing Treatment Integrity
- NEJM
- New England Journal of Medicine
- NICE
- National Institute for Health and Care Excellence
- OA
- output area
- OARS
- Open ended questions, Affirmations of change talk, Reflective listening and Summarising
- ONS
- Office for National Statistics
- PAC
- physical activity counts
- PARS
- Physical Activity Referral Scheme
- PCS
- physical component summary score
- PSA
- probabilistic sensitivity analysis
- QALY
- quality-adjusted life-year
- RA
- research assistant
- RAI
- Relative Autonomy Index
- RCT
- randomised controlled trial
- RR
- relative risk
- ScHARR
- School of Health and Related Research
- SD
- standard deviation
- SF-12v2 plus 4
- 16-Item Short Form health survey instrument
- SF-6D
- Short Form questionnaire-6 Dimensions
- SPAQ
- Scottish Physical Activity Questionnaire
- TEE
- total energy expenditure